Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/201/10. The contractual start date was in July 2014. The draft report began editorial review in December 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Wilby et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Sciatica can be described as a symptom rather than a diagnosis. It is broadly defined as leg pain in the distribution of a lumbosacral nerve root and has already been subject to National Institute for Health and Care Excellence (NICE) review of treatment guidance. 1 Estimates of caseload vary substantially within the literature because of difficulties in definition and poor data capture. A UK epidemiological study suggests a lifetime prevalence of up to 43%, an annual incidence of 5% and a point prevalence of up to 13%. 2 Over 90% of sciatica is due to a prolapsed intervertebral disc (PID), with the average age of patients being early 40s. 3 As patients affected are typically young, working adults, it may be helpful to consider two categories of sciatica: (1) acute sciatica that lasts < 6 weeks and may be self-limiting with little or no impact on the patient’s work, and (2) persistent sciatica that persists > 6 weeks and has a tremendous impact on the patient’s working ability.
Although the duration of pain may vary considerably, and the natural history of sciatica is favourable within 1 or 2 years, many patients have pain that persists beyond 6 weeks that could have a considerable impact on their employment and lives. It is generally accepted that pain persisting beyond 6 weeks is unlikely to get better imminently and requires further patient investigation and treatment. Treatment options include drugs, injections of drug combinations into the spine and surgical techniques to remove the prolapsed disc. UK guidelines recommend non-routine magnetic resonance imaging (MRI) scanning after lifestyle modifications and simple treatments. 1
Spinal injection involves the administration of a mixture of local anaesthetic and steroid into the spine via one of three main routes: (1) through the base of the spine (i.e. a caudal epidural), (2) through the back of the spine (i.e. a interlaminar injection) or (3) through the nerve tunnel (foramen) directly adjacent to the prolapsed disc [i.e. a transforaminal epidural steroid injection (TFESI)]. The last mode (i.e. TFESI) is reported to be the most successful. 4 This specific use of a steroid (for spine injection) is outside the marketing authorisation (off-label). However, it is a commonly used and a widely accepted treatment for sciatica, although the success rate reported is highly variable because of inconsistent patient population and route, type and dose of steroid administration. Although TFESI is recommended by a number of expert review groups, including the UK NICE guideline1 (low back and radicular pain), a recent review from Danish experts on behalf of the Danish Health Authority recommends against its use for sciatica > 12 weeks’ duration. 5,6 Of the surgical techniques, microdiscectomy to remove the prolapsed disc is considered highly successful, with reported success rates of 90%. 7 However, as sciatica has a favourable natural history, there is potential that the treatment administered in the form of injection may render surgery excessive.
There is currently no care pathway in the NHS that recommends any one particular treatment over another, and no direct comparison exists between microdiscectomy to treat sciatica secondary to PID and nerve root blocks, such as TFESI. In addition, no international consensus agrees the use of TFESI for sciatica within 12 weeks’ duration. This trial aims to address that by comparing surgical microdiscectomy with steroid and local anaesthetic administered accurately to the source of leg pain against various objective outcomes.
Scientific background
Sciatica is a common condition. In the UK, in 2010/11, > 25,000 therapeutic epidural steroid injections (ESIs) were administered and > 9000 surgical procedures to remove herniated lumbar disc prolapses were performed for sciatica [Hospital Episode Statistics (HES) data]. 8 In the USA, > 200,000 microdiscectomies are performed per year. 9 In the UK, the cost to the NHS is estimated to be £700 per TFESI and approximately £4500 for surgical microdiscectomy (which requires patients to be hospitalised for 2 nights, on average).
Previous studies of surgical microdiscectomy for sciatica
Surgical removal of the PID is believed to be the treatment of choice for symptomatic PID, with > 90% success rates, return to work within 1 month in the majority of cases and complications of around 2%. 10 Several trials have attempted to compare surgical microdiscectomy with non-operative treatments, but with notable methodological flaws. Population studies have suggested that surgery is more effective than non-surgical treatments, but these studies were affected by selection bias and lacked clear definitions of the non-operative treatment arm. 3,11–13 The two most notable studies are large, prospective case series studies, the Maine Lumbar Spine Study12 and the Spine Patient Outcomes Research Trial,11 and are worthy of specific mention.
Recruiting > 500 patients treated by spinal specialist teams across Maine, almost an equal number of surgical patients were compared with non-surgical patients with sciatica. 12 Surgery was deemed effective in 71% of patients compared with 43% of non-surgical patients. The non-surgical group was, however, quite heterogeneous, with only 18% of non-surgical patients receiving ESI. In addition, the study was observational and, therefore, suffered from selection bias.
The Spine Patient Outcomes Research Trial11 had two main arms: an observational reporting group in which patients selected their treatment (surgical or not) and a randomised arm. Owing to a large degree of treatment crossover in the randomised section, intention-to-treat (ITT) analyses showed no significant difference in outcomes. However, the as-treated analysis favoured surgery. Once again, however, the non-surgical treatments being compared with surgery were heterogeneous.
To our knowledge, only one previous study14 (n = 100) has directly compared surgery with ESI (interlaminar route) and this study reported that ESI could prevent surgery in approximately 50% of cases. This study was a single practitioner cohort, but randomisation was employed in design of the study.
Previous studies of epidural steroid injections for sciatica
Epidural steroid injections are known to improve patients’ sciatica, but their efficacy varies widely throughout the literature. 15 A wide variation in practice exists across the UK in the methods of administration of the ESI.
Epidural steroid injections involve the administration of a mixture of local anaesthetic and steroid into the epidural space via one of three main routes: (1) through the base of the spine (i.e. a caudal epidural), (2) through the back of the spine (i.e. a interlaminar injection) or (3) through the nerve tunnel directly adjacent to the prolapsed disc (i.e. transforaminal injection). In the past, the most widely used injection therapy route was ESI, by either the interlaminar route or the caudal route. However, placing the needle through the bony tunnel through which the lumbar nerve root exits the spine can accurately place the drug closer to the target site (i.e. TFESI). Currently, ESI is not commissioned by all local commissioning groups within the NHS in the UK for sciatica, whereas TFESI is. This technique routinely requires X-ray guidance or computerised tomography (CT) scanning guidance and most pain clinics in the UK are able to offer this treatment.
Although randomised controlled trials (RCTs) have tested ESI for acute sciatica, these trials have not included comparisons between TFESI and interlaminar ESI. However, prospective and case–control studies have compared the two techniques and demonstrated a superior efficacy of TFESI. 13,16,17 A comprehensive review of the literature has recently been published by the Health Technology Assessment (HTA) programme. 15 Only one small RCT14 (n = 100) directly compared interlaminar ESI with surgery for sciatica secondary with PID and suggested that ESI could prevent 50% of surgical interventions. One previous UK RCT, the Wessex Epidural Steroid Trial (WEST)18 (n = 228), funded by the HTA programme, compared interlaminar injection of steroid with placebo (i.e. injection of saline between the spinous processes) in patients with sciatica ranging in duration from 4 weeks to 18 months and found no benefit of steroid injections beyond 3 weeks of follow-up. However, in this study, MRI was not undertaken as part of the trial to confirm pathology, with this relying on clinical findings alone. These possibly could be some of the factors contributing to less than promising results for ESI. Various other studies have shown that ESIs have only a small short-term effect on leg pain and disability compared with placebo, and no effect in the long term. 19 These poor medium- to long-term results have given ESIs poor perceived efficacy and hence they are not commissioned or recommended in the treatment and clinical pathway of sciatica secondary to PID management.
A prospective randomised study20,21 reported that transforaminal administration of the drug mixture into the epidural space (i.e. TFESI) under fluoroscopic guidance is the most successful route (more so than injection of saline or local anaesthetic into the epidural space, or intramuscular steroid or saline injection), and this route was used in this study. Relief of pain was corroborated by significant improvements in function and disability, and reductions in use of other health care.
Transforaminal epidural steroid injection is believed to be superior in efficacy to interlaminar administration of ESI, as the drug is delivered more accurately and closer to the site of the pathology/disc prolapse. A prospective study17 of TFESI (n = 48) for acute sciatica suggests long-term pain reduction in > 80% of patients. One RCT published in 201120 (n = 150) compared the outcomes of selective nerve root injection and local anaesthetic, local anaesthetic alone, normal saline and intramuscular injection of steroid or normal saline. The only radiological feature associated with successful outcome was the grade of nerve root compression. Of patients with low-grade root compression (n = 71), 75% responded favourably to selective nerve root injection and avoided surgery by 54 weeks’ follow-up.
Although there are few data directly comparing TFESI with interlaminar steroid injections for sciatica, during the recruitment stage of this trial a number of ongoing studies throughout the world were specifically looking at this. However, these studies were experiencing recruitment difficulties because of the lack of a surgical treatment arm. 22,23 One recent study24 (n = 238) reported that 65% of injections were effective at follow-up of > 6 months (based on patient-reported measures).
Adverse events (AEs) associated with TFESI procedures are rare, typically < 1%, but can be severe and include paraplegia, infection, haematoma, intravascular injection of medication, direct nerve trauma, subdural injection of medication, air embolism, disc entry, urinary retention, radiation exposure and hypersensitivity reactions.
The advantages of spinal injections include:
-
The injections are a relatively cheap and low-risk procedure compared with surgery.
-
Success rates have been estimated to be as high as 75%.
-
Injections are delivered as a day-case procedure and, therefore, require no hospital admission and can be easily repeated.
-
There is a range of treatment providers, including radiologists, surgeons and pain physicians.
The disadvantages of spinal injections include:
-
The true success rate of injections is largely unknown. The injections may work well in the short term, but pain may return some weeks later.
-
Injections are not able to prevent physical nerve root compression and are inappropriate for massive disc prolapses that cause motor weakness or numbness in the leg.
Economic background
Although there are a number of published economic evaluations of interest in the treatment of sciatica, they are of limited applicability, as none has directly compared microdiscectomy with TFESI. A recent systematic review25 of economic evaluations in sciatica included 16 decision-analytic models that compared a selection of management strategies. The review25 found that analyses were generally associated with poor modelling techniques, analytical methods and data quality, specifically in terms of health state representation, time horizons and utility values. Uncertainty associated with the clinical evidence populating the models was an identified contributor to these limitations.
A number of the US studies identified by Hall et al. 25 compared surgical techniques with epidural injection techniques in the treatment of sciatica. Parker et al. ,26 taking a payer perspective, including the cost of patient care (i.e. index procedures and any follow-up care or repeat procedures), reported a cost per quality-adjusted life-year (QALY) of US$16,300 (2014 US$) when comparing interspinous spacer surgery with conservative care (which could include steroid injection). Skidmore et al. 27 compared spacer surgery with conservative care (including ESI) and, in taking a broader cost perspective (i.e. patient, physician or payer, but excluding productivity losses), reported a cost per QALY of US$17,894 (2009 US$). Udeh et al. 28 estimated the cost-effectiveness of minimally invasive lumbar decompression as US$37,758 per QALY gained (2013 US$) compared with ESIs from a Medicare payer’s perspective. An evaluation29 including societal costs associated with lumbar disc herniation surgery and non-surgical treatments (including ESI) for privately insured, working patients identified that after fully accounting for the effects of disc herniation surgery on worker productivity (based on changes in earnings and missed work days) the cost per QALY gained of surgery reduced from US$52,416 to US$35,146 (2009 US$), based on a 4-year time horizon.
Economic evidence from the UK is limited. Lewis et al. 15,30 estimated the cost-effectiveness of alternative management strategies for sciatica using a deterministic model informed by an evidence synthesis based on a review of > 100 potential treatment scenarios. A 12-month time horizon was selected on the basis that patients would be managed through one of three treatment pathways: (1) primary care, (2) stepped approach or (3) immediate referral to more invasive treatments (i.e. epidural and disc surgery). Utilities derived from the EuroQol-5 Dimensions (EQ-5D) were sourced from a Dutch study31 comparing prolonged conservative care with early surgery, and costs were based on clinical opinion and derived from published UK cost sources (2008/9 prices). The results indicated that stepped-care approaches to patient management based on initial treatment with non-opioids were likely to represent the most cost-effective approach relative to strategies that involved direct referral for disc surgery.
Vertuani et al. 32 assessed the cost-effectiveness of minimally invasive surgery compared with open surgery for lumbar spinal fusion in the treatment of degenerative lumbar spinal conditions. Using published data derived from a number of sources and UK costs (subsequently converted to euros), the results indicated that minimally invasive surgery was the dominant strategy, yielding both cost savings and improved health outcomes. A cost saving of €1666 (2013 costs) per procedure was estimated, based on shorter length of hospital stay, reduced blood loss and fewer complications, and with a corresponding improvement of 0.04 QALYs over 2 years.
Price et al. 18 conducted an economic evaluation of ESI compared with placebo within a 12-month double-blind placebo-controlled randomised clinical trial in four UK hospitals. A bottom-up costing approach was applied, with resource use estimated from data on drugs, equipment, pathology and radiology services collected within the trial and supplemented by a survey of non-RCT patients for clinical staff time-based activities. QALYs were derived from Short Form questionnaire-6 Dimensions preference-based utilities from the Short Form questionnaire-36 items questionnaire over the initial 12-week period. Taking a provider perspective, an incremental cost per QALY of £44,701 was estimated for up to three injections over a 12-week period, reducing to £25,745 per QALY gained if only one injection was administered, on the basis that there are no significant health benefits beyond the first injection (2002/3 prices). An alternative perspective, reflecting the charge levied on the purchaser as opposed to the actual resource cost, yielded incremental cost-effectiveness ratios (ICERs) of £354,171 and £167,145 per QALY gained for up to three injections and only one injection, respectively.
However, by the nature of their setting, perspectives, interventions and comparators, these studies are unlikely to be directly generalisable or informative to the present decision problem concerning the cost-effectiveness of microdiscectomy compared with TFESI in the setting of the NHS in England.
The aim of the economic evaluation conducted as part of the NErve Root Block VErsus Surgery (NERVES) trial was to establish which intervention, microdiscectomy or TFESI, for the treatment of sciatica secondary to PID herniation offered greater value for money from the perspective of the NHS in England.
Rationale for research
To the best of our knowledge, there is no evidence comparing steroid injections given via the nerve foramen with any other form of treatment (i.e. surgical microdiscectomy). Neither has a robust economic analysis been performed for this condition and these treatment paradigms.
Intervention
The technologies compared are standard surgical lumbar microdiscectomy (i.e. microdiscectomy) and fluoroscopically guided TFESI of a standard combination of local anaesthetic and steroid drug.
Objectives
The NERVES trial is a pragmatic, multicentre, Phase III randomised trial comparing microdiscectomy with TFESI for persistent sciatica caused by a PID of < 12 months’ duration. An internal pilot was completed with two trial centres as part of an initial feasibility study.
Primary objective
-
To compare the clinical effectiveness of microdiscectomy with TFESI at 18 weeks post randomisation.
Secondary objectives
-
To compare the clinical effectiveness of microdiscectomy with TFESI up to 1 year post treatment.
-
To compare the cost-effectiveness of microdiscectomy with TFESI.
-
To compare quality-of-life (QoL) outcomes for both treatments.
Chapter 2 Trial design and methods
Trial design
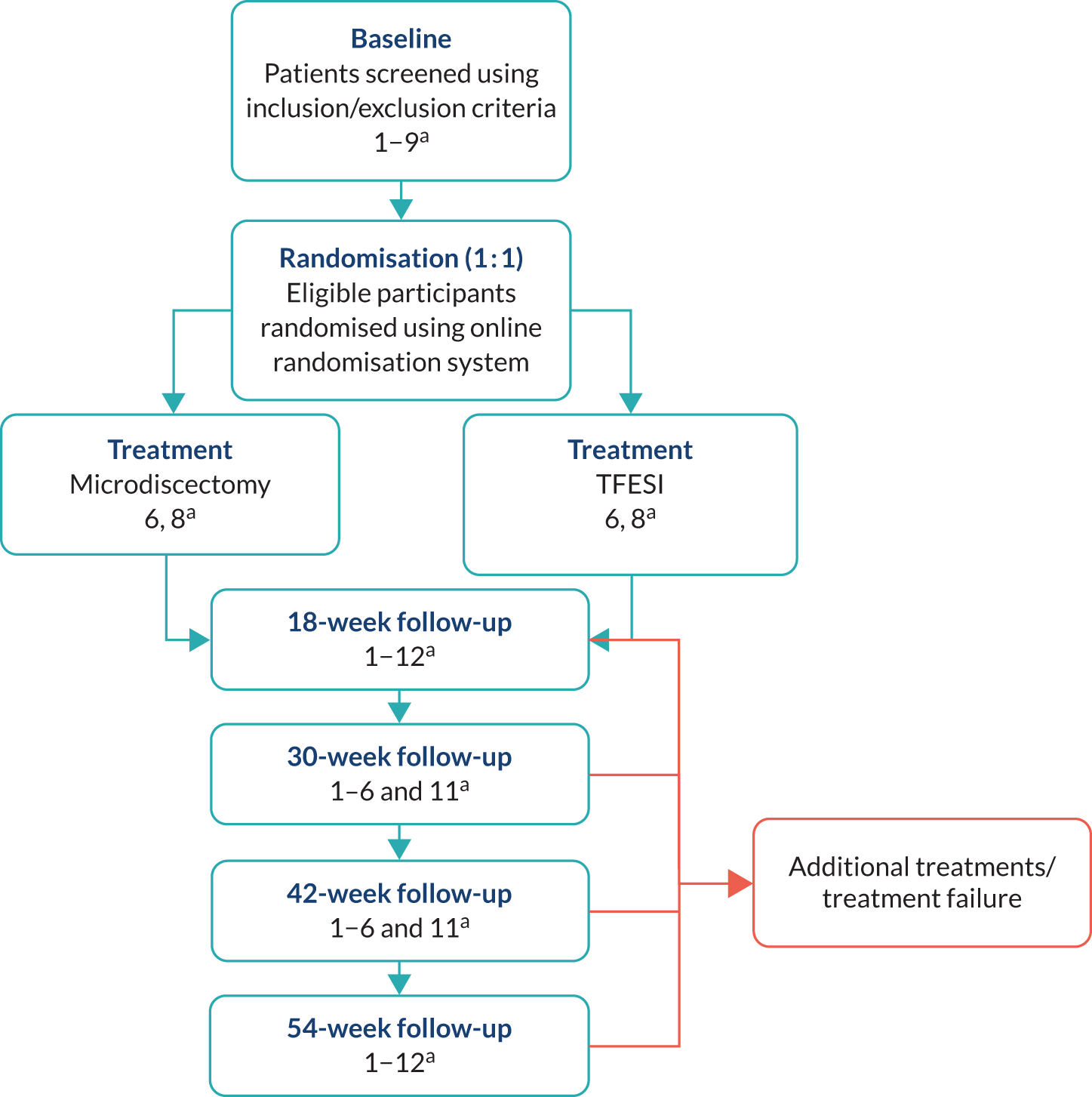
Figure 1 shows the NERVES trial design.
FIGURE 1.
The NERVES trial design. a, Trial-specific outcome assessments undertaken at each time point: (1) Oswestry Disability Questionnaire (primary outcome at the 18-week follow-up); (2) modified Roland–Morris outcome score for sciatica; (3) Core Outcome Measures Index score; (4) visual analogue scale scores for leg and back pain; (5) EuroQol-5 Dimensions, five-level version; (6) Resource Use Questionnaire; (7) physical examination; (8) pregnancy; (9) concomitant medications; (10) return to work; (11) treatment satisfaction (Likert scale); and (12) AEs. Orange indicates the possibility or option of additional treatment if primary treatment deemed unsuccessful.
Trial registration and ethics
This trial falls within the remit of the European Union Directive 2001/20/EC, transposed into UK law as the UK Statutory Instrument 2004 number 1031: Medicines for Human Use (Clinical Trials) Regulations 2004 as amended. This trial has been registered with the Medicines and Healthcare products Regulatory Agency (MHRA) and has been granted a Clinical Trial Authorisation. The Clinical Trial Authorisation reference is 21322/004/001 and the EudraCT number is 2014-002751-25.
Ethics considerations
The trial abided by the principles of the World Medical Association Declaration of Helsinki. 33
Both of the treatments offered as part of the trial are standard NHS practice. As such, there were no major ethics concerns. When treatment has been considered to be unsuccessful, participants had full access to additional treatment needed as per routine care. Participation in the trial did not prevent access to additional treatments needed.
The specific issues pertaining to this trial are:
-
requirement for an additional visit
-
patients were randomised and, therefore, were unable to choose their own treatment.
Funding was in place to allow reimbursement of financial costs incurred by the trial participant to attend an additional appointment (i.e. a 54-week follow-up appointment post randomisation).
Patients provided informed consent to participate, with information provided about the randomisation process, data collection and other trial processes.
Ethics approval
The trial protocol received the favourable opinion of a Research Ethics Committee prior to initiation at the Liverpool Clinical Trials Centre (LCTC) and underwent independent review at the research and development (R&D) offices of participating centres. Local R&D offices were sent the appropriate centre-specific information form to complete with the necessary authorisation signatures, plus any other documentation requested for review. A copy of local R&D approval was forwarded to the LCTC before the centre was initiated and patients recruited.
Consent from patients was obtained prior to participation in the trial and after a full explanation had been given of the treatment options, including the conventional and generally accepted methods of treatment. Patients were asked to read and review a patient information sheet and consent form (PISC) containing key information about the trial, and then complete, sign and date the consent form if they consented to take part in the trial. The right of the patient to refuse consent to participate in the trial without giving reasons was respected. After the patient entered the trial, the clinician remained free to give alternative treatment to that specified in the protocol, at any stage, if he/she felt it to be in the best interest of the participant. However, the reason for doing so was recorded and the participant remained within the trial for the purpose of follow-up and data analysis, according to the treatment option to which they have been allocated. Similarly, participants remained free to withdraw at any time from the protocol treatment and trial follow-up without giving reasons and without prejudicing further treatment.
Selection of centres/clinicians
The trial was run in NHS outpatient neurosurgical, pain and orthopaedic clinics and community-based services. Patients were recruited from units receiving patients from pooled tertiary referrals from general practitioners (GPs), allied health professionals and non-spinal consultants.
Participating centres were initiated once all regulatory approvals and trial-specific conditions (e.g. training requirements) had been met, and all necessary documents had been returned to the LCTC.
Centre/clinician inclusion criteria
-
TFESI performed according to protocol requirements (i.e. specified pharmaceutical agents available from pharmacy via local routine prescription routes).
-
Able to provide both treatments within 12 weeks of randomisation.
-
Principal investigator can be a representative of either neurosurgery or pain management (note that both specialties should be represented within the local research team).
-
Clinical equipoise.
-
Local R&D approval.
-
Completion and return of a ‘delegation of authority and signature log’ to the LCTC.
-
Completion and return of centre suitability assessment to the LCTC.
-
Signed contract between centre and sponsor.
-
Receipt of evidence of adherence to points (a) to (g) by the LCTC.
-
Complete progression through the green light checklist.
Centre/clinician exclusion criteria
-
Not meeting the inclusion criteria listed above.
Participant inclusion and exclusion criteria
Inclusion criteria
Patients were eligible for inclusion in the trial if they met the following criteria:
-
They had been diagnosed with lower extremity radiculopathy (sciatica).
-
They had sciatica secondary to prolapsed intervertebral disc (proven by magnetic resonance imaging).
-
The duration of their symptoms was between 6 weeks and 12 months. [Note that, if symptoms were episodic, then ‘duration of symptoms’ refers to the initial incidence of severe symptoms (i.e. the disc prolapse). It does not refer only to the most recent episode.]
-
They had leg pain non-responsive to conservative, non-invasive management.
-
They were aged 16–65 years.
-
They had previously undergone at least one form of conservative (non-operative) treatment (including but not limited to medication, physiotherapy and modification of daily activities) but this had not provided adequate relief of pain/symptoms.
-
They provided written, informed consent.
Exclusion criteria
Patients were excluded from the trial if they met any of the following criteria:
-
They had a serious neurological deficit (e.g. foot drop/possible cauda equina compression).
-
They had previously undergone spinal surgery at the level of the prolapsed intervertebral disc.
-
Their current episode of sciatica had lasted longer than 12 months.
-
They were aged < 16 years or > 65 years.
-
They had not previously undergone any form of conservative treatment.
-
Patients with a contraindication for surgery and/or injection.
-
They were known to be pregnant.
Contraindications for both groups of treatment were assessed on a case-by-case basis by the health-care team, as per routine NHS practice and according to local policy.
Changes to the eligibility criteria
During the course of the trial the following changes were made to the eligibility criteria.
Protocol v3.0, 15 December 2014, wording amended for clarity
-
Inclusion criterion: ‘Newly diagnosed sciatica secondary to PID (proven on MRI)’ changed to ‘Newly diagnosed lower extremity radiculopathy (sciatica)’.
-
Inclusion criterion: ‘Diagnosed with lower extremity radiculopathy (sciatica) secondary to a lumber disc herniation’ changed to ‘Sciatica secondary to prolapsed intervertebral disc (PID) (proven on MRI)’.
-
Exclusion criterion: ‘Pregnancy’ changed to ‘Patient known to be pregnant’.
-
Exclusion criterion: ‘Not attempted conservative non-operative treatment for a minimum of 6 weeks’ changed to ‘Patient has not attempted any form of conservative treatment’.
Protocol v4.0, 5 May 2015, wording amended for clarity
-
Inclusion criterion: ‘Newly diagnosed lower extremity radiculopathy (sciatica)’ changed to ‘Diagnosed lower extremity radiculopathy (sciatica)’.
-
Inclusion criterion: ‘Severe leg pain non-responsive to conservative, non-invasive management’ changed to ‘Leg pain non-responsive to conservative, non-invasive management’.
-
Exclusion criterion ‘Neurological deficit (foot drop/possible cauda equina compression)’ changed to ‘Serious neurological deficit (e.g. foot drop/possible cauda equina compression)’.
Protocol v6.0, 21 March 2016, wording amended to improve recruitment
-
At trial inception, 6 months was believed to be an appropriate cut-off point because the main issue governing the selection of symptom duration was in terms of getting patients back to work faster, and for this reason the shorter time point of 6 months was selected. During the course of the trial, screening logs showed that the most common reason for subject ineligibility was pain duration of > 6 months. On review, the Trial Management Group (TMG) believed that there was clinical equipoise up to 12 months of symptom duration, after which time the disc prolapse itself was unlikely to change significantly radiologically and there may not be equipoise. It was therefore agreed that it was appropriate to extend the duration of symptoms to 12 months.
-
Inclusion criterion: ‘Duration of symptoms between 6 weeks and 6 months’ changed to ‘Duration of symptoms between 6 weeks and 12 months’.
-
Exclusion criterion: ‘Sciatica presentation for longer than 6 months’ changed to ‘Sciatica presentation for longer than 12 months’.
Protocol v7.0, 25 October 2017, wording amended for clarity
-
Inclusion criterion: ‘Patient willing and able to give consent’ changed to ‘Patient has provided written, informed consent’.
Recruitment
Screening
All patients who attended a participating trial centre following referral for sciatica secondary to PID (previously proven by MRI scanning) were prospectively screened for trial eligibility. Trial information was provided to patients at, or prior to, the clinic appointment. Potentially eligible patients (i.e. those who met the eligibility criteria listed in Participant inclusion and exclusion criteria) were invited to participate in the trial. At the clinic appointment, the patient was allowed time to discuss the trial, ask questions and decide whether or not to consent to take part in the trial. Owing to the pragmatic nature of the trial, patients provided written, informed consent at the initial visit without requiring further time to consider participation. Patients requiring additional time to consider consent were managed on a case-by-case basis at a centre level and an additional visit occurred if required.
A screening log was maintained at each trial centre to record all individuals screened for the trial and the eventual outcome. Reasons for non-recruitment were documented (e.g. not eligible, declined consent) and the information was used for monitoring purposes. Patients were asked if they would like to provide a reason for non-consent, although they were not obliged to do so. Reasons for non-participation that relate to patient preference were recorded with the undesired treatment listed when possible.
Baseline and eligibility
After obtaining written, informed consent, the baseline case report form (CRF) was completed to assess and confirm eligibility. The baseline CRF included a medical and neurosurgical history based on source data in the participant’s notes and eligibility was confirmed by an appropriately qualified doctor. The details of recruitment into the NERVES trial were recorded appropriately in the participant’s notes [i.e. details of eligibility confirmation (when and by whom), consent and entry into the trial].
Participants were also asked to complete a questionnaire booklet [incorporating the Oswestry Disability Questionnaire (ODQ); modified Roland–Morris (MRM); Core Outcome Measures Index (COMI); visual analogue scale (VAS) for leg and back pain; the EuroQol-5 Dimensions, five-level version (EQ-5D-5L); and a Resource Use Questionnaire (RUQ)], with support from a health-care professional if needed. The participant-completed questionnaires were completed prior to randomisation, but after provision of consent. The ODQ collected primary outcome data for the trial and so it was important that it was completed accurately. Therefore, it was checked by centres and assistance was provided in completing it if required.
Informed consent
Informed consent is a process initiated prior to an individual agreeing to participate in a trial and continues throughout the individual’s participation. Informed consent is required for all patients participating in LCTC co-ordinated trials. In obtaining and documenting informed consent, the investigators were required to comply with applicable regulatory requirements and adhere to good clinical practice and to the ethics principles that have their origin in the Declaration of Helsinki.
There was discussion about the objectives, risks and inconveniences of the trial, and the conditions under which the trial would be conducted were provided to patients by staff with experience in obtaining informed consent. Patients were provided with a PISC describing in detail the trial interventions/products, trial procedures and risks (which was approved by an Independent Ethics Committee), and were asked to read and review it.
After the patient had read the document, the investigator explained the research trial, emphasising that participation in the trial was voluntary and that the participant could withdraw from the trial at any time and for any reason. All patients were given the opportunity to ask any questions and to discuss the trial and were given time to consider the information prior to agreeing to participate. A contact point where further information about the trial could be obtained was included in the PISC.
Patients who agreed to participate then signed and dated the informed consent document. Both the person taking consent and the patient personally signed and dated the form. A copy of the informed consent document was given to the patient for their records. The original copy was filed in the patient’s notes and a further copy of the signed consent form retained in the investigator centre file. One final copy of the consent form was sent to the co-ordinating centre (i.e. the LCTC). Centres were instructed to send the consent form within 7 days of informed consent being provided.
Patients were invited to participate in the trial at their clinical visit. Consent was sought at this initial visit, as there are no immediate routine follow-up visits. When patients requested longer to consider their decision about whether or not to participate, the local research team managed this. Potential participants could be invited to return to the clinic to provide consent at a later date, but the cost of attending the visit was not reimbursed as part of the trial. This is a reflection of current NHS practice, in which a patient would be given their treatment options and, in consultation with their health-care provider at that same appointment, would make a decision about how they wished to proceed.
The participant could, without being subject to any resulting detriment, withdraw from the trial at any time by revoking the informed consent. The rights and welfare of the patients were protected by emphasising to them that the quality of medical care would not be adversely affected if they declined to participate in this trial.
Randomisation
Patients were not randomised until:
-
fully informed written consent had been obtained from the patient
-
the baseline CRF had been accurately completed
-
full eligibility had been confirmed by a doctor.
Participants were randomised between groups in a 1 : 1 ratio, with variable block randomisation stratified by centre. Randomisation lists were created by a statistician who was not part of the main trial team.
Participants were randomised using an online web randomisation system. Designated members of the trial team at site, as detailed on the delegation of authority and signature log, were given training to use the online system and then provided with unique log-in details. Data captured on the baseline CRF were entered into the online system to confirm eligibility of the participant and provide information needed for treatment allocation. Randomisation occurred at the initial clinic appointment, if possible.
The online system allocated a unique randomisation number to the participant together with their treatment allocation. The LCTC received an e-mail notification that randomisation had taken place.
Blinding
Owing to the nature of the interventions, blinding was not possible.
Trial treatments
Ionising radiation
In accordance with the Ionising Radiation (Medical Exposure) Regulations 2000,34 participants in the trial received a small exposure to ionising radiation in both groups of the trial. This was required to provide imaging for verification of the treatment level for both microdiscectomy and TFESI. The ionising radiation exposure required was part of the normal care pathway and the same exposure would be necessary outside this clinical trial context. There was no additional ionising radiation exposure to participants as a result of trial participation.
Group A: transforaminal epidural steroid injection
Standard nerve root blockade was completed, as per local policy/technique, using the lateral, foraminal portal of entry. All fluoroscopically guided techniques (e.g. CT or X-ray screening) were permitted to specify the correct level. Treating specialists included pain specialists, radiologists, anaesthetists, surgeons and other appropriately qualified medical professionals, as long as radiological level confirmation was incorporated into the procedure.
The NERVES trial is a pragmatic trial and, as such, the agents used were obtained and prescribed via normal NHS routes. The following injection regimen was followed when possible to minimise variability across the participating centres:
-
Injectate:
-
steroid [20–60 mg of triamcinolone acetonide (KENALOG™; Bristol-Myers Squibb Pharmaceuticals, Uxbridge, UK)]
-
local anaesthetic [0.25% levobupivacaine hydrochloride (2 ml) (Chirocaine®; AbbVie Inc., North Chicago, IL, USA)].
-
As the NERVES trial is a Clinical Trial of an Investigational Medicinal Product, information regarding the pharmaceutical products used was provided to the MHRA. The following active ingredients were notified to the MHRA and, therefore, are also accepted for use if appropriate:
-
Steroid:
-
dexamethasone
-
methylprednisolone acetate (Depo-Medrone®, Pfizer Inc., New York, NY, USA).
-
-
Local anaesthetic:
-
bupivacaine hydrochloride
-
lidocaine hydrochloride.
-
For the purpose of participant safety, centres were instructed to ensure that maximum doses were not exceeded (Table 1).
| Injectate | Maximum dose (mg) |
|---|---|
| Triamcinolone acetonide (e.g. KENALOG) | 80 |
| Levobupivacaine hydrochloride (e.g. Chirocaine) | 10 |
| Dexamethasone | 20 |
| Methylprednisolone acetate (Depo-Medrone) | 80 |
| Bupivacaine hydrochloride | 10 |
| Lidocaine hydrochloride | 40 |
Note that, if the maximum dose was exceeded, then a data query form was produced at the LCTC and sent to the centre with a request for justification.
All participants randomised to group A received at least one therapeutic injection. As per local policy, participants could receive another injection if there was a favourable but partial response that could be boosted by further injections. Information about any further injections was collected.
The steroid/anaesthetic combination used in the TFESI was distributed from pharmacy via routine processes and so specific trial labelling was not required as per MHRA Exemption Regulation 46 of the Medicines for Human Use (Clinical Trial) Regulations 2004. 35 It is an off-label use of steroid, but is commonly accepted practice within the NHS and in the further medical field.
Group B: microdiscectomy
Standard microdiscectomy was performed as per local treatment protocols.
Treatment specialists at centres identified the correct side (left or right) and level prior to treatment, with level localisation advised as per local treatment protocols. Information on site and level was collected.
Treatment specialists were an orthopaedic or neurosurgical consultant or consultant equivalent (i.e. associate specialist), or a specialist trainee directly supervised by a consultant.
For both groups, treatment was given within 6 weeks of randomisation when possible and centres were instructed that treatment should occur within 12 weeks of randomisation to ensure valid collection of primary outcome data at the 18-week follow-up visit.
Additional treatments
The NERVES trial protocol allocated only initial treatment for sciatica, either microdiscectomy or TFESI. During the course of follow-up, some participants required further intervention for sciatica, as per routine NHS practice. Further clinical intervention was permitted for trial participants without the participant having to withdraw from the trial.
If a participant received additional treatment, information on the type of intervention (i.e. microdiscectomy or TFESI), the details of the treatment received and the reason were collected, and they remained in the trial.
Trial participants were able to cross over prior to receiving their initial treatment allocation without withdrawing from the trial (e.g. if they became unsuitable for the treatment they were initially randomised to). This was recorded on the treatment CRF with the reason for crossover indicated.
Schedule for follow-up
All follow-up visits were scheduled from the date of randomisation.
Each participant was followed up for 54 weeks following randomisation. During this time, participants attended scheduled follow-up visits. Table 2 shows the follow-up schedule. Any additional procedures provided to the participant and completed at the trial centre during this period were documented.
| Procedure | Screening/baseline (T = 0) | Follow-up schedule | Unscheduled visitsc | ||||
|---|---|---|---|---|---|---|---|
| Intervention T = 6 weeksa | T = 18 weeks | T = 30 weeksb | T = 42 weeksb | T = 54 weeks | |||
| Signed consent form | ✓d | ||||||
| Assessment and confirmation of eligibility criteria | ✓d | ||||||
| Review of medical history | ✓d | ||||||
| Review of concomitant medications | ✓d | ✓ | ✓ | ✓ | |||
| ODQ | ✓d | ✓ | ✓b | ✓b | ✓ | ||
| RUQ | ✓d | ✓ | ✓ | ✓b | ✓b | ✓ | |
| EQ-5D-5L | ✓d | ✓ | ✓b | ✓b | ✓ | ||
| VAS scores for leg and back pain | ✓d | ✓ | ✓b | ✓b | ✓ | ||
| MRM outcome score for sciatica | ✓d | ✓ | ✓b | ✓b | ✓ | ||
| COMI score | ✓d | ✓ | ✓b | ✓b | ✓ | ||
| Trial intervention | ✓ | ||||||
| Pregnancy assessment | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Physical examination | ✓d | ✓ | ✓ | ||||
| Treatment satisfaction (Likert scale) | ✓ | ✓ | ✓ | ✓ | |||
| Return to work | ✓ | ✓ | |||||
| Assessment of related AEs | ✓ | ✓ | ✓ | ✓ | |||
| Assessment of additional interventions given to the participant during the trial period | ✓ | ✓ | ✓c | ||||
| Telephone follow-up of non-responders | (✓)e | (✓)e | |||||
Normal clinical practice would typically include a 3-month post-treatment follow-up. Therefore, participants were followed up at approximately 18 weeks post randomisation to align with routine clinical practice, and then again at 30, 42 and 54 weeks. To maintain feasibility, the 18-, 30- and 42-week visits could take place within a 2-week visit window on either side. The 54-week visit had an acceptable window of 54–62 weeks post randomisation. Participants could be seen at other times, as clinically indicated. Additional visits outside the trial protocol were recorded.
After randomisation, scheduled treatment and follow-up stages were as follows.
Treatment visit
Treatment details were recorded and the participant was presented with a RUQ booklet and asked to fill it in prior to their treatment. The centres co-ordinated provision of the RUQ booklet to the participants in preoperative assessment. Contraindications to treatment (such as pregnancy) were assessed by centres as per NHS policy and, therefore, no additional trial-specific assessments were conducted at this visit.
When a participant chose to not proceed with their allocated treatment prior to treatment being given, the participant was still expected to continue with trial follow-up and attend the follow-up visits. If a participant did not wish to continue in the trial then the date and reason for trial withdrawal were recorded.
The 18- and 54-week visits
-
A face-to-face follow-up visit (postoperative for the week 18 visit).
-
Centres were responsible for organising the follow-up within the visit window (specified in the e-mail when the participant was randomised).
-
Visits were ideally arranged to occur within the first 2 weeks of the visit window, when possible, giving the centre time within the visit window to take action if the participant did not attend their appointment.
-
Participants underwent a physical examination and data were collected, including concomitant medications, related AEs, additional treatments and work status.
-
Participants completed a questionnaire booklet (incorporating the ODQ, the MRM outcome score for sciatica, the COMI, a Likert scale for treatment satisfaction, VAS scores for leg and back pain, the EQ-5D-5L for QoL and the RUQ for costs).
-
Pregnancy was assessed when applicable.
Participants not attending visits (weeks 18 and 54)
The primary outcome data for the trial were collected at the week 18 visit and so centres were reminded of the importance of participants attending the week 18 visit throughout the recruitment period. Participants were contacted as per trust policy to urge them to attend.
When these attempts failed, centres were instructed to e-mail the LCTC to seek approval to post out the questionnaire booklet, explaining the circumstances.
Centres were instructed that visits should be arranged initially for the first 2 weeks of the visit window to give time for the questionnaire booklet to be sent out by post and completed by participants within the visit window in cases of non-attendance and then returned in a pre-paid envelope to the LCTC.
When visits did not take place, centres were instructed to telephone the participant to try and retrieve as much information as possible over the telephone.
For the week 18 visit, centres could use a telephone call to collect primary outcome data (i.e. the ODQ section within the questionnaire booklet) in exceptional circumstances, with agreement from the LCTC.
Weeks 30 and 42 (postal)
-
Centres posted the questionnaire booklet to participants at the start of the weeks 30 and 42 window, with a pre-paid envelope for the participant to return the completed booklet to the LCTC.
-
The date that the questionnaire booklet was posted was recorded by centres.
-
When a response had not been received (notified by the LCTC), the centre research nurse telephoned the participant to prompt completion and return of the questionnaire booklet and offer any help required to ensure that the questionnaire booklet was completed accurately.
Data collection and management
Data collection tools
Procedures for assessing efficacy
Efficacy of trial treatment was measured through the period of the trial using a number of outcome measures:
-
ODQ at 18 weeks after randomisation (approximately 3 months post treatment).
-
ODQ at 30, 42 and 54 weeks after randomisation.
-
VAS for leg pain at baseline and at 18, 30, 42 and 54 weeks after randomisation.
-
VAS for back pain at baseline and at 18, 30, 42 and 54 weeks after randomisation.
-
Likert scale to assess patient treatment satisfaction at 18, 30, 42 and 54 weeks after randomisation.
-
MRM score for sciatica at baseline and at 18, 30, 42 and 54 weeks after randomisation.
-
COMI score at baseline and at 18, 30, 42 and 54 weeks after randomisation.
-
work status (i.e. return to work and work days lost) at baseline and at 18, 30, 42 and 54 weeks after randomisation.
The cost-effectiveness of trial treatment, expressed as the incremental cost per QALY, was based on the following measures:
-
EQ-5D-5L questionnaire
-
RUQ
-
HES
-
concomitant medications.
Procedures for assessing safety
An assessment of AEs was undertaken at each trial clinic visit post treatment. These reviews were carried out by the principal investigator or delegated research staff.
Participant-reported outcomes
Participants were asked to complete the following participant-reported outcome measures at baseline, 18, 30, 42 and 54 weeks post randomisation:
-
ODQ
-
MRM outcome score for sciatica
-
COMI score
-
Likert scale for treatment satisfaction
-
EQ-5D-5L
-
VAS scores for leg and back pain
-
health-care resource use and participant costs (including time off work).
The questionnaire booklet was provided to the participant at the scheduled clinic visits (at baseline and at the 18- and 54-week follow-ups) and completed in clinic. The RUQ booklet was completed at the treatment visit before treatment and was provided to the participant in preoperative assessment.
Completion of these questionnaires was an important part of the trial. Particular emphasis was given to part 1 of the questionnaire booklet (i.e. the ODQ) because it was used to collect primary outcome data for the trial. It was therefore crucial that research staff at centres offered any necessary support to participants to ensure the questionnaires were completed correctly and returned to the LCTC either by centres or by the participant in accordance with the schedule for follow-up. The questionnaires took approximately 15 minutes to complete and participants were advised of the extended visit time prior to their appointment.
All questionnaires completed at baseline were completed after consent had been provided and prior to randomisation.
Sample size
The primary outcome measure of ODQ has > 30 years of validation and is supported by Outcome Measures in Rheumatology, an independent initiative of international health professionals and patient research partners interested in outcome measures and measurement methodology, especially in rheumatology. Deyo et al. 36 has recommended the use of ODQ as part of the core outcome measures for low back research, along with the use of EQ-5D. The scale ranges from 100 (extreme disability) to 0 (extreme ability). A change of 10 points has been widely regarded in the literature as the minimal clinically important difference (MCID). One study,37 specifically addressing this issue, has suggested a range of 10.5–15 points as clinically important. ODQ has > 30 years of validated, published data pertaining to low back pathology and radicular symptoms. It has formed the basis for previous HTA trials exploring sciatica and will allow useful comparisons to be made with previous data. A recent study by Chiarotto et al. 38 has sought to specify a core outcome set for low back pain (not specifically sciatica) and the authors have recommended the use of four items: (1) physical functioning (either ODQ or MRM scores), (2) numerical pain rating score for pain intensity, (3) health-related QoL assessment (although no consensus reached, the Short Form questionnaire-12 items or Patient-Reported Outcomes Measurement Information System were recommended) and (4) mortality. Although published after our trial conception, we have collected all of these outcomes apart from EQ-5D-5L for the health-related QoL and VAS for the pain intensity score.
Original trial sample size calculation
A total of 172 participants was required to detect a difference of 10 points between the two groups on the ODQ at a 5% significance level with 90% power. This assumed a standard deviation (SD) of 20 points based on similar populations in previous published trials. 18,36,37,39,40 The previous large and well-carried out WEST, based in the UK, suggested a baseline ODQ SD of between 16 and 18 points. 18 Baseline ODQ data were collected on 11 potentially eligible patients from the fast-track sciatica clinic at the Walton Centre, Liverpool, and this generated a SD of 14.4 points, well under the assumed value. The initial target sample size for the trial was 200 patients, which would allow for a 10% rate of missing outcome data. Of the originally planned seven centres involved, allowing for one to have difficulties opening, this would require recruitment of 30 patients in total from each participating centre and 50 patients from the lead centre.
Revised sample size calculation for the primary outcome
The original sample size calculation did not assume any correlation between baseline and follow-up ODQ scores, as no data were available to estimate this. The sample size calculation was revisited during the trial with the agreement of the trial oversight committees. Based on a blinded analysis of the correlation between baseline and follow-up ODQ scores in the first 47 trial participants to have outcome data available, the correlation was estimated as 0.49.
Using this estimate, our revised sample size to achieve 90% power was 66 participants per group. Allowing for 10% loss to follow-up gives a revised target of 74 participants per group (i.e. 148 participants total).
Internal pilot study
An internal pilot study was included in the trial design. The study targeted two centres to open first to collect 6 months of recruitment data before progressing to a full trial. These centres, the Walton Centre, Liverpool, and Salford Royal, Manchester, were identified to cover recruitment of participants within specialty and mixed care settings.
The aim of the internal pilot study was to assess the feasibility of recruitment and the rates of potential crossover due to patient preference or treatment failure. The criteria for progression to a full trial were:
-
at least 30 patients recruited
-
a consent rate of ≥ 40% or more
-
< 10% of patients unhappy with allocation and receive the alternative treatment
-
< 50% of patients in the injection group proceed to surgery.
Changes to the protocol
The protocol has been previously published. 41 The study opened on protocol version 3.0 and the final approved version was 8.0, which contains a detailed list of the protocol changes [see www.fundingawards.nihr.ac.uk/award/12/201/10 (accessed 16 July 2020)]. Table 3 lists the key protocol changes.
| Protocol version (date) | Key amendment |
|---|---|
| 3.0 (15 December 2014) |
Minor amendment Eligibility criteria changes: inclusion criteriaEligibility criteria changes: exclusion criteriaAdditional expected AEs associated with microdiscectomy and TFESI added Removal of requirement for investigators to assess expectedness when recording AEs ‘Do not include’ list for SAEs reporting updated Minor amendments for clarity and minor corrections |
| 4.0 (5 May 2015) |
Minor amendment Eligibility criteria changes: inclusion criteriaEligibility criteria changes: exclusion criteriaCentre inclusion criteria addedClarification of treatment timelines addedAddition of an expected TFESI regimen specifically:Addition of text clarifying AE reporting requirements specifically defining ‘reactions’ (related to IMP) vs. ‘events’ (related to procedures) Minor amendments for clarity and minor corrections |
| 5.0 (19 August 2015) |
Substantial amendment Addition of statistician sign-off and addition of IRMER |
| 6.0 (21 March 2016) |
Substantial amendment Eligibility criteria changes: inclusion criteriaEligibility criteria changes: exclusion criteriaAddition of CT scanning for guidance of the TFESI injection Addition of text outlining the procedures for follow-ups at weeks 18, 30, 42 and 54 weeks and clarification of the process for dealing with non-attendance at weeks 18 and 54 Addition of text clarifying SAE assessment of expectedness process, specifically that the chief investigator will undertake the assessment Addition of ‘Recurring prolapse of the disc’ to expected list of events for microdiscectomy table Process of reporting AEs has been reduced to one flow chart to clarify the procedure. The flow chart includes requirements to report pregnancies and any deaths Minor amendments for clarity and minor corrections |
| 7.0 (25 October 2017) |
Substantial amendment Target population changed from 200 to 148 participants Eligibility criteria changes: inclusion criteriaStudy duration changed from ‘54 weeks’ to ‘54–60 weeks’ List of accepted active ingredients for use in the TFESI group added Table of expected maximum doses for each active ingredient used in the TFESI group added Section added providing guidance on what should be done when participants do not attend the 18- and 54-week visits Option for collection of week 18 primary outcome data to be collected by telephone as ‘last resort’ added ‘Pregnancy’ section added allowing assessment of pregnancy throughout trial Statement added clarifying patients should not be withdrawn unless specifically requested ‘Revised Sample Size’ section added Pharmacovigilance section modified throughout for clarification:Definitions and responsibilities defined for assessment of expectedness: chief investigator’s responsibility (not the principal investigator) based on relevant safety information available at the time Expected AE tables reworked for clarity Addition of expected AEs ‘anaphylaxis’ and ‘low-pressure headache’ added to expected AE tables ‘Overdose of any medication without signs or symptoms’ added to non-reportable list Safety reporting period defined as ‘from intervention up to and including the week 54 follow-up visit’ Process for completing SAE forms amended as per the LCTC processes Addition of ‘urgent safety measures’ and ‘protocol deviations and serious breaches’ sections Minor amendments for clarity and minor corrections |
| 8.0 (10 April 2019) |
Substantial amendment Change in lead sponsor contact/signatory Minor amendments for clarity and minor corrections |
Patient and public involvement
The trial team collaborated with patient contributors throughout the trial, all of whom had personal experience of the condition and/or interventions being examined.
-
Prior to funding application submission, a patient representative reviewed the trial design to confirm agreement with the principle of the trial.
-
Patient representatives reviewed the full funding application prior to submission.
-
Members of the public were involved in the development of the PISC by providing feedback.
-
Patient representatives were recruited to the TMG and Trial Steering Committee (TSC), both of which were actively involved at the start of the trial.
-
Once the centres had been opened and recruitment was under way we entered a phase of the trial in which the TMG discussions focused on issues relating to governance and internal trial management. During this period, it was more difficult to engage our patient representatives and to ensure that the discussions were relevant and interesting to them. The TMG patient representative stopped attending meetings during this period and the TSC patient representative resigned in March 2017.
-
In December 2018, two patient representatives were recruited to join the TMG [via the INVOLVE website (URL: www.invo.org.uk)] with the purpose of helping us to design our end-of-trial information and ensure that the results from the trial are understandable and accessible, particularly to those who have suffered from sciatica.
-
The new TMG patient representatives have reviewed this report and we intend to involve them in producing our end-of-trial information. We hope to encourage patient representative co-authorship and co-presenting of our findings to patient and clinical audiences to ensure that the impact of the findings are maximised.
Compliance with Intervention
The LCTC monitored compliance with the randomised trial intervention through completion of CRFs at centres recording the intervention given and the allocation provided by the online randomisation system. Any deviations from the randomised intervention were explored with centres. As the NERVES trial was a pragmatic trial and the interventions were expected to reflect local NHS policy, the rates of compliance with the interventions were expected to vary between NHS sites.
Trial oversight and role of funders
Trial Management Group
The TMG was a multidisciplinary team that comprised the chief investigators, several co-investigators, patient and public involvement (PPI) representatives, a sponsor representative, health economists and members of the LCTC. The TMG was responsible for the day-to-day clinical and practical aspects of the trial.
Independent Data and Safety Monitoring Committee
The Independent Data and Safety Monitoring Committee (IDSMC) comprised an independent chairperson, an expert in the field of pain and two independent members (one was an expert in the field of neurosurgery and one was an expert in medical statistics). The main responsibilities of the IDSMC was to safeguard the interests of the NERVES trial participants, assess the safety and efficacy of the interventions during the course of the trial, and monitor the overall progress and conduct of the trial. The IDSMC met at least annually during the course of the trial and provided recommendations to the TSC. Reports to the IDSMC were produced by the statistical team at the LCTC.
Trial Steering Committee
The membership of the TSC included an independent chairperson, an independent expert in the field of pain, an independent expert in the field of neurosurgery and an independent statistician, as well as representatives from the TMG. Observers from the sponsor and the funder were also invited to meetings. The TSC met at least annually, shortly after the IDSMC met and their main role was to provide overall oversight of the trial.
Trial funder
The membership of the oversight committees was suggested by members of the TMG to the trial funders and appointed by the funders with their constitution following funder requirements.
Statistical methods
The main features of the analyses were specified in the protocol and a separate detailed statistical analysis plan (see Report Supplementary Material 1) was developed prior to randomisation codes being released.
All efficacy outcomes were analysed using the intention-to-treat principle as far as practically possible. Safety outcomes were reported based on actual treatment received. All applicable statistical tests were two-sided and used a 5% significance level. No adjustments for multiplicity were made for the secondary outcomes.
Baseline data for continuous variables are presented as both means and SDs, and medians and interquartile ranges (IQRs). Categorical variables are presented using frequencies and percentages.
The primary outcome variable (i.e. ODQ at 18 weeks) was compared between groups using a linear regression model, adjusting for the stratification variable centre and baseline ODQ. The potential impact of missing data on the primary outcome results was explored using multiple imputation analysis. An extended model was also fitted, adjusting for age, sex, duration of symptoms, body mass index (BMI) and estimated volume of canal occupied by disc prolapse as shown on MRI scan. An additional post hoc model added an adjustment for level of disc prolapse.
The secondary outcomes measured at multiple time points were analysed using repeated measures random-effects models, adjusting for baseline outcome score, time (as a continuous variable) and a time–treatment interaction. The time–treatment interaction term was dropped in models when it was found to be non-significant (p > 0.05). A post hoc analysis using joint modelling of the same longitudinal outcome data and the time to study dropout was undertaken. The aim was to assess sensitivity of the results for potentially informative dropout from the study.
The secondary outcome of participant satisfaction was compared between groups using a Mann–Whitney U-test. The satisfaction with care outcome was measured using a two-item questionnaire with a 1- to 5-point Likert scale. The average of the two items was taken, with possible values ranging from 1 to 5 and lower values indicating higher levels of satisfaction. The return-to-work status was compared using a chi-squared test.
Safety data on AEs and serious adverse events (SAEs) are presented descriptively, with no inferential statistics.
Chapter 3 Clinical effectiveness results
Screening and recruitment
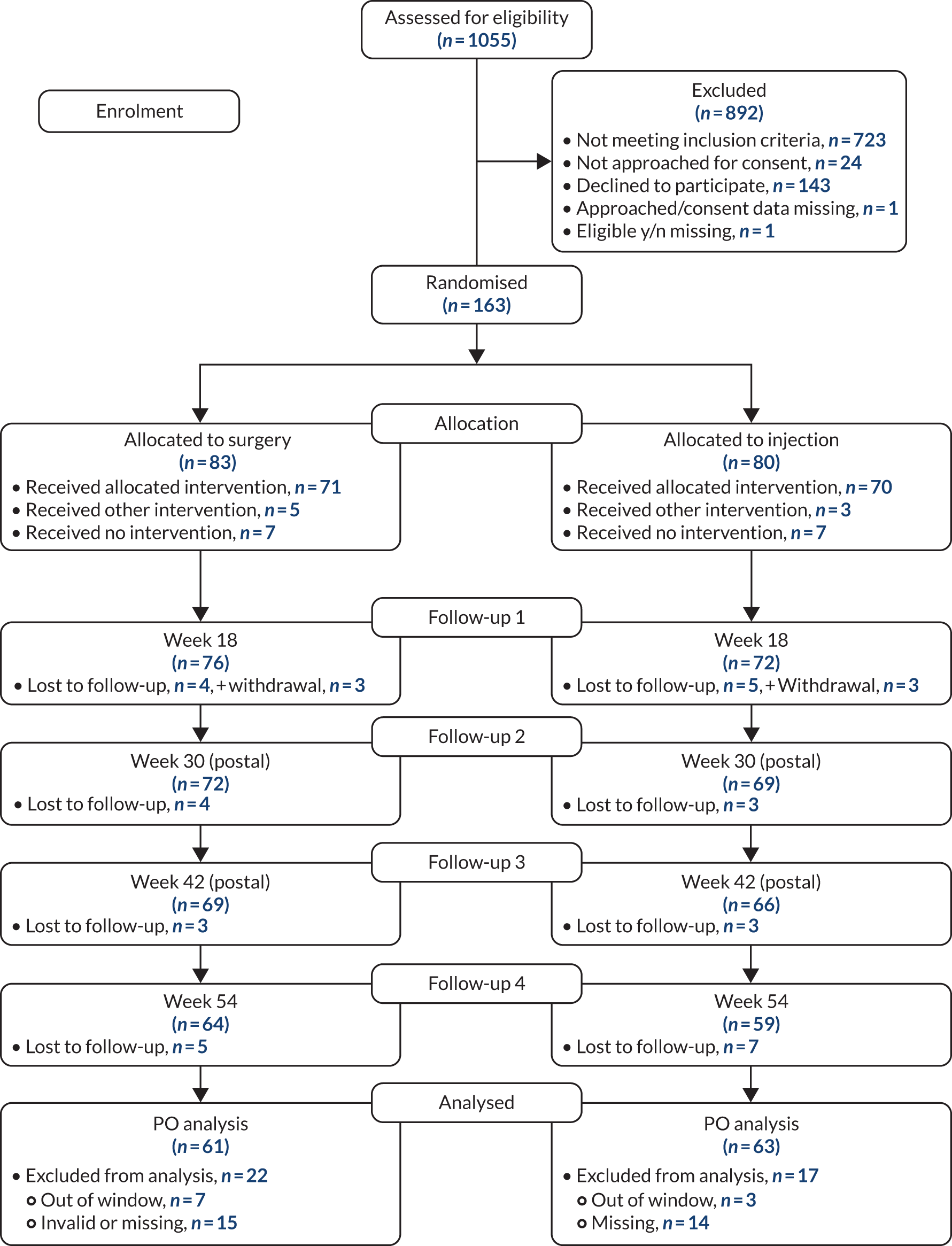
The trial opened to recruitment on 4 March 2015 and closed on 9 July 2018 after the final follow-up of all participants had been completed. A total of 1055 participants were screened for eligibility from 12 centres and a total of 163 participants were randomised from 11 of the 12 centres. Of the participants screened for eligibility, 723 (69%) were found to be ineligible. Of those eligible and approached for consent, 163 (49%) consented and were randomised into the trial.
Figure 2 shows the participant flow from screening to consent and randomisation.
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for all trial participants. PO, primary outcome; y/n, yes/no.
Internal pilot
The internal pilot phase of the trial was reviewed by the IDSMC in September 2015 after 25 participants were recruited from two centres. The committee assessed the recruitment, consent rates, treatment switches and crossover rates, and recommended that the trial continue with the remaining centres being opened.
Baseline comparability
Tables 4–6 summarise baseline participant characteristics, details of management and impact of sciatica, and relevant clinical characteristics, by randomised group. The randomised groups were generally similar in their baseline characteristics.
| Characteristic | Summary | Microdiscectomy | TFESI | Overall |
|---|---|---|---|---|
| Number of participants randomised | 83 | 80 | 163 | |
| Sex | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| Female, n (%) | 46 (55.4) | 40 (50.0) | 86 (52.8) | |
| Male, n (%) | 37 (44.6) | 40 (50.0) | 77 (47.2) | |
| Age (years) | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| Mean (SD) | 43.5 (9.9) | 41.2 (8.6) | 42.4 (9.3) | |
| Median (IQR) | 42.8 (34.9–50.5) | 41.4 (35.2–47.0) | 42.2 (35.2–48.9) | |
| Range | 23.2–65.6 | 23.3–59.8 | 23.2–65.6 | |
| Of reproductive potential (female only) | n (missing) | 46 (0) | 40 (0) | 86 (0) |
| No, n (%) | 11 (23.9) | 5 (12.5) | 16 (18.6) | |
| Yes, n (%) | 35 (76.1) | 35 (87.5) | 70 (81.4) | |
| Weight (kg) | n (missing) | 75 (8) | 71 (9) | 146 (17) |
| Mean (SD) | 83.7 (16.8) | 81.4 (20.7) | 82.6 (18.8) | |
| Median (IQR) | 82.0 (72.0–95.1) | 77.1 (67.0–94.0) | 79.3 (69.8–94.0) | |
| Range | 54.0–134.0 | 51.7–154.0 | 51.7–154.0 | |
| Height (cm) | n (missing) | 76 (7) | 71 (9) | 147 (16) |
| Mean (SD) | 171.7 (10.7) | 172.6 (9.5) | 172.2 (10.1) | |
| Median (IQR) | 170.1 (164.0–180.7) | 173.0 (167.0–180.0) | 171.5 (165.0–180.0) | |
| Range | 147.0–197.0 | 150.0–192.0 | 147.0–197.0 | |
| BMI (kg/m2) | n (missing) | 74 (9) | 68 (12) | 142 (21) |
| Mean (SD) | 28.2 (5.3) | 27.2 (6.4) | 27.7 (5.9) | |
| Median (IQR) | 26.9 (24.5–31.3) | 25.6 (22.9–29.4) | 26.4 (24.1–30.7) | |
| Range | 18.9–44.3 | 17.1–47.1 | 17.1–47.1 |
| Treatment | Summary | Microdiscectomy | TFESI | Overall |
|---|---|---|---|---|
| Number of participants randomised | 83 | 80 | 163 | |
| Taking anticoagulant medication | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| No, n (%) | 82 (98.8) | 79 (98.8) | 161 (98.8) | |
| Yes, n (%) | 1 (1.2) | 1 (1.3) | 2 (1.2) | |
| Number of weeks with symptoms | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| Mean (SD) | 21.5 (10.7) | 21.1 (11.2) | 21.3 (10.9) | |
| Median (IQR) | 17.0 (14.0–28.0) | 18.0 (13.0–27.0) | 18.0 (14.0–28.0) | |
| Previous surgery at disc level | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| No, n (%) | 82 (98.8) | 80 (100.0) | 162 (99.4) | |
| Yes, n (%) | 1a (1.2) | 0 (0.0) | 1 (0.6) | |
| Taken medication for pain and symptoms | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| Yes, n (%) | 83 (100.0) | 80 (100.0) | 163 (100.0) | |
| Modified activity | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| No, n (%) | 0 (0.0) | 1 (1.3) | 1 (0.6) | |
| Yes, n (%) | 83 (100.0) | 79 (98.8) | 162 (99.4) | |
| Attended physiotherapy | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| No, n (%) | 15 (18.1) | 16 (20.0) | 31 (19.0) | |
| Yes, n (%) | 68 (81.9) | 64 (80.0) | 132 (81.0) | |
| Other conservative treatment | n (missing) | 83 (0) | 80 (0) | 163 (0) |
| No, n (%) | 49 (59.0) | 43 (53.8) | 92 (56.4) | |
| Yes, n (%) | 34 (41.0) | 37 (46.3) | 71 (43.6) | |
| Currently employed | n (missing) | 83 (0) | 79 (1) | 162 (1) |
| No, n (%) | 21 (25.3) | 13 (16.3) | 34 (20.9) | |
| Yes, n (%) | 62 (74.7) | 66 (82.5) | 128 (78.5) | |
| Currently unable to work because of sciatica (of those employed) | n (missing) | 62 (0) | 66 (0) | 128 |
| No, n (%) | 41 (66.1) | 34 (51.5) | 75 (58.6) | |
| Yes, n (%) | 21 (33.9) | 32 (48.5) | 53 (41.4) | |
| Currently taking analgesics/steroids/anticoagulant medication | n (missing) | 83 (0) | 80 (0) | 163 |
| No, n (%) | 7 (8.4) | 7 (8.8) | 14 (8.6) | |
| Yes, n (%) | 76 (91.6) | 73 (91.3) | 149 (91.4) |
| Clinical characteristic | Summary | Microdiscectomy | TFESI | Overall |
|---|---|---|---|---|
| Number of participants randomised | 83 | 80 | 163 | |
| Estimated volume of canal occupied by disc prolapse | n (missing) | 83 (0) | 80 (0) | 163 |
| < 25%, n (%) | 43 (51.8) | 44 (55.0) | 87 (53.4) | |
| 25–49%, n (%) | 36 (43.4) | 34 (42.5) | 70 (42.9) | |
| ≥ 50%, n (%) | 4 (4.8) | 2 (2.5) | 6 (3.7) | |
| Level of disc prolapsea | n (missing) | 75 (8) | 73 (7) | 148 (15) |
| L2/3, n (%) | 1 (1.2) | 0 (0.0) | 1 (0.6) | |
| L3/4, n (%) | 1 (1.2) | 2 (2.5) | 3 (1.8) | |
| L4/5, n (%) | 27 (32.5) | 25 (31.3) | 52 (31.9) | |
| L5/S1, n (%) | 46 (55.4) | 46 (57.5) | 92 (56.4) |
Adherence to treatment, additional treatments and retention
Withdrawals
There were a total of six withdrawals from the trial (three from the microdiscectomy group and three from the TFESI group).
The reasons given for withdrawal in the TFESI group were:
-
patient unwilling to have microdiscectomy or TFESI
-
site exhausted all means of communication with participant
-
chest infection and investigation for possible bowel cancer.
Reasons given for withdrawal from the microdiscectomy group were withdrawal of consent for follow-up, participant not happy with the process (one withdrawal) and withdrawal of consent for follow-up, no additional reason (two withdrawals).
Treatment compliance
A further 14 participants did not initially receive any treatment (seven randomised to microdiscectomy and seven to TFESI). Table 7 shows all combinations of treatments received by participants.
| Detail | Microdiscectomy (N = 83), n (%) | TFESI (N = 80), n (%) |
|---|---|---|
| Received randomised treatment initially | ||
| Single randomised treatment | 65 (78.31) | 40 (50) |
| Repeated randomised treatment | 3a (3.61) | 2 (2.5) |
| Randomised treatment then the alternative treatment at least once | 3 (3.61) | 28 (35) |
| Received alternative treatment initially | ||
| Single alternative treatment | 3 (3.61) | 3 (3.75) |
| Repeated alternative treatment | 1 (1.2) | 0 |
| Alternative treatment then the randomised treatment | 1 (1.2) | 0 |
| Lateb/no treatment | ||
| No treatment recorded during trial | 4 (4.82) | 4 (5) |
| Late randomised treatment | 0 | 1 (1.25) |
| Late alternative treatment(s) | 3 (3.61) | 2 (2.5) |
Eight participants did not receive their randomised treatment, but were given the alternative treatment instead (five randomised to microdiscectomy and three randomised to TFESI). Of the five participants randomised to microdiscectomy, three changed because of participant preference, one because of safety concerns from the anaesthetist and one because their symptoms had improved and microdiscectomy was thought to be unnecessary. Of the three participants randomised to TFESI, two changed to microdiscectomy because of participant preference and the other was reported as a surgeon decision.
Additional treatment
The rows in italics in Table 7 indicate participants who have received the alternative trial treatment after their initial treatment. The largest group (28 participants) includes those who received the TFESI initially and subsequently went on to receive microdiscectomy as an additional treatment. The first row in italics denotes those who received their allocated treatment first and then the alternative treatment. The second row in italics denotes those who initially received a treatment to which they were not allocated, but then subsequently additionally received the original planned allocation. Table 8 summarises the timing of treatments for those patients who received both TFESI and microdiscectomy.
| Timing | Microdiscectomy (N = 83), n (%) | TFESI (N = 80), n (%) |
|---|---|---|
| Received other treatment after primary outcome | 2 (2.41) | 13 (16.25) |
| Received other treatment before primary outcome | 2 (2.41) | 13 (16.25) |
| Received both treatments, but no primary outcome available | 0 | 2 (2.50) |
| Total | 4 (4.82) | 28 (35.00) |
Protocol deviations
Prespecified protocol deviations are summarised in Table 37. The most common major protocol deviations were missing primary outcome data (n = 28) and treatment compliance (i.e. receiving randomised treatment) (n = 22).
Analysis sets
Table 9 gives the number of participants in the analysis sets. The ITT data set included all randomised participants. The safety data set included every participant who received one of the trial interventions. Note that safety outcomes are analysed based on actual treatment received, rather than randomised groups.
| Population | Microdiscectomy, n | TFESI, n | Total, n |
|---|---|---|---|
| Randomised | 83 | 80 | 163 |
| ITT | 83 | 80 | 163 |
| Safetya | 105 | 82 | 155 |
Primary outcome: Oswestry Disability Questionnaire at 18 weeks
The primary outcome was the ODQ measure at 18 weeks after randomisation. Questionnaires completed within a window of 6 weeks either side of 18 weeks (i.e. 12–24 weeks) were included in this analysis. The ODQ is a 10-item questionnaire; item 8 (‘sex life’) may not be applicable. If the number of items answered is fewer than eight items then this questionnaire was excluded from the analysis and considered invalid (as specified in the statistical analysis plan, see Report Supplementary Material 1). Table 10 shows descriptive statistics of the ODQ at baseline and at week 18, including the difference between baseline and week 18 values. The differences are also categorised into those who improved by ≥ 10 points (considered clinically significant), those who improved < 10 points but at least 0 and those for whom the symptoms got worse.
| Time point | Summary | Microdiscectomy | TFESI | Overall |
|---|---|---|---|---|
| Baseline | n | 83 | 79 | 162 |
| n missing | 0 | 1 | 1 | |
| Mean (SD) | 49.39 (17.81) | 53.74 (19.35) | 51.51 (18.64) | |
| Median (IQR) | 46.67 (36.00–62.22) | 54.00 (40.00–71.11) | 50.00 (38.00–66.00) | |
| Week 18 | n | 61 | 63 | 124 |
| n missing/invalid | 22 | 17 | 39 | |
| Mean (SD) | 22.30 (19.83) | 30.02 (24.38) | 26.22 (22.51) | |
| Median (IQR) | 18.00 (6.00–36.00) | 22.22 (10.00–50.00) | 20.00 (9.00–37.89) | |
| Difference | n | 61 | 63 | 124 |
| n missing/invalid | 22 | 17 | 39 | |
| Mean (SD) | –26.74 (21.35) | –24.52 (18.89) | –25.61 (20.09) | |
| Median (IQR) | –26 (–40 to –8.89) | –26 (–38 to –6) | –26 (–39 to –8) | |
| 95% CI | –32.21 to –21.27 | –29.28 to –19.76 | –29.18 to –22.04 | |
| Difference category | n | 61 | 63 | 124 |
| Improvement of ≥ 10 points, n (%) | 45 (73.8) | 43 (68.3) | 88 (71.0) | |
| Improvement of < 10 points, n (%) | 8 (13.1) | 14 (22.2) | 22 (17.7) | |
| Deterioration in symptoms, n (%) | 8 (13.1) | 6 (9.5) | 14 (11.3) |
Table 10 shows the improvements in ODQ compared with baseline for both microdiscectomy and TFESI groups. Both groups showed similar improvements in ODQ (27 points following microdiscectomy and 25 points following TFESI). Comparable numbers of patients in each group achieved a MCID (defined as an improvement of > 10 points: 74% following microdiscectomy and 69% following TFESI). In both groups, the majority of participants’ symptoms improved from baseline to 18 weeks, with only 13% in the microdiscectomy group and 10% in the TFESI group showing a deterioration in symptoms. Figure 3 shows the distributions of the differences between baseline and 18-week scores by group.
FIGURE 3.
Distribution of differences between baseline and 18-week ODQ scores.
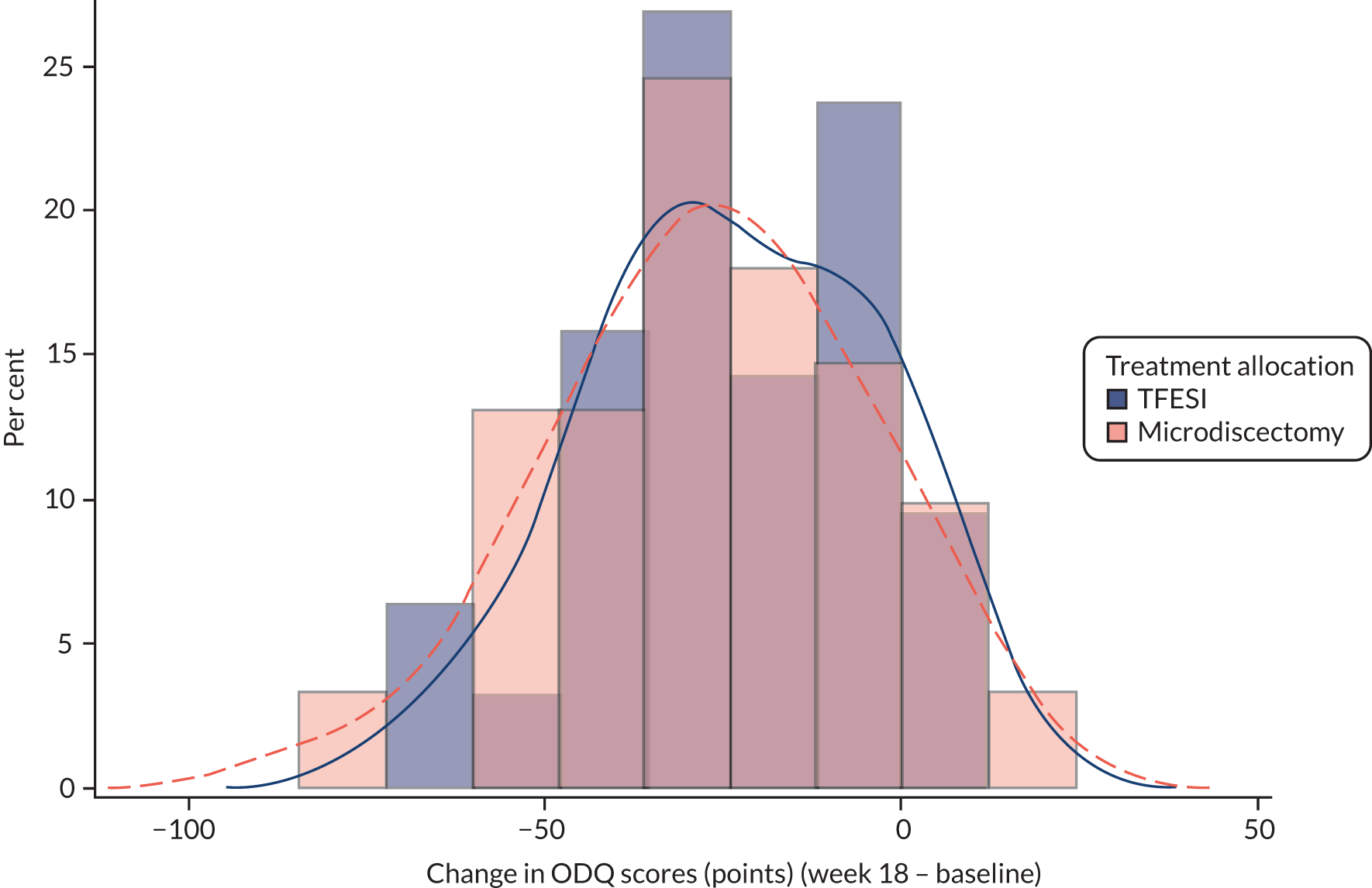
The primary outcome was compared between groups using a linear regression model, adjusting for centre and baseline ODQ score. There was no statistically significant difference in ODQ between the randomised groups, with the model estimate of the effect of microdiscectomy compared with TFESI being –4.25 points, with a 95% confidence interval (CI) of –11.09 to 2.59 points (p = 0.221). From this model, we estimate that microdiscectomy will result in an improvement in ODQ of 4.25 points more than TFESI, which is less than the MCID of 10 points (although the 95% CI narrowly includes values > 10 points, which suggests that a significant clinical difference is just plausible).
Adjustment for additional covariates
An additional model was fitted, adjusting for other prespecified variables, age, sex, duration of symptoms, BMI and estimated volume of canal. This adjusted model gave an estimate of the effect of microdiscectomy compared with TFESI of –5.03 with a 95% CI of –12.76 to 2.70. An additional variable, level of disc prolapse, was included in a post hoc analysis. This model resulted in an estimate of –4.94 (95% CI –12.81 to 2.93). Table 11 shows the full parameter estimates for both models.
| Variable | Level | Model 1a | Model 2b | ||
|---|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | ||
| Intercept | 3.40 (–29.36 to 36.17) | 5.95 (–28.76 to 40.66) | |||
| Baseline ODQ score | 0.62 (0.42 to 0.82) | 0.63 (0.43 to 0.83) | |||
| Age (years) | –0.16 (–0.56 to 0.23) | –0.20 (–0.61 to 0.21) | |||
| Sex | Female vs. male | 7.7 (0.05 to 15.34) | 5.78 (–2.06 to 13.63) | ||
| Duration of symptoms (weeks) | 0.167 (–0.26 to 0.59) | 0.22 (–0.21 to 0.65) | |||
| BMI (kg/m2) | –0.26 (–0.90 to 0.39) | –0.22 (–0.88 to 0.45) | |||
| Location | L2/3 vs. L5/S1 | –17.07 (–56.93 to 22.79) | |||
| L3/4 vs. L5/S1 | 13.81 (–25.93 to 53.55) | ||||
| L4/5 vs. L5/S1 | –5.6 (–13.48 to 2.29) | ||||
| Volume | Between 25% and 50% vs. < 25% | –0.19 (–7.85 to 7.48) | –0.82 (–8.68 to 7.04) | ||
| > 50% vs. < 25% | –10.76 (–31.03 to 9.52) | –11.84 (–32.05 to 8.36) | |||
| Allocation | Microdiscectomy vs. TFESI | –5.03 (–12.76 to 2.70) | 0.1991 | –4.94 (–12.81 to 2.93) | 0.215 |
A further post hoc analysis was conducted to assess if there was an interaction between duration of symptoms at baseline and randomised treatment. The interaction term was not statistically signficant and the estimated difference in least squares means between treatment groups for microdiscectomy compared with TFESI (when an interaction with duration of symptoms was adjusted for) was –4.18 (95% CI –11.06 to 2.70; p = 0.231). Therefore, there is no statistically significant difference between treatment groups when an interaction with the duration of symptoms at baseline is considered. This supports the conclusion of the primary outcome analysis.
Sensitivity analysis
As > 10% of data on the primary outcome were missing, a sensitivity analysis was conducted using multiple imputation. This analysis used treatment group, centre and all available measurements of ODQ at various time points to impute data to create 50 complete data sets, which were then analysed and combined to give the imputed estimate.
The estimate of the effect of microdiscectomy compared with TFESI from the imputation analysis was –3.08 (95% CI –10.16 to 3.99).
A post hoc sensitivity analysis was conducted to using multiple imputation, as above, but using only baseline ODQ to impute a week 18 score. The estimate of the effect of microdiscectomy compared with TFESI from the post hoc imputation analysis was –3.26 points (95% CI –9.91 to 3.39 points).
Table 12 summarises the estimates from the primary outcome analyses. All estimates lead to the same conclusion, that is there is no statistically significant difference between the treatments, with all estimates showing slightly better outcomes in the microdiscectomy group, although smaller than the prespecified clinically important difference.
| Analysis | Estimate | 95% CI | p-value |
|---|---|---|---|
| Primary analysis | –4.27 | –11.09 to 2.59 | 0.221 |
| Extended model | –5.03 | –12.76 to 2.70 | 0.199 |
| Post hoc extended model | –4.94 | –12.81 to 2.93 | 0.215 |
| Multiple imputation | –3.08 | –10.16 to 3.99 | 0.393 |
Secondary outcomes
Longitudinal outcomes
For the longitudinal outcomes (i.e. ODQ, VAS leg pain, VAS back pain, MRM and COMI) the questionnaires were collected at four key time points (i.e. 18, 30, 42 and 54 weeks post randomisation). The 18- and 54-week follow-up visits were conducted face to face, whereas the 30- and 42-week follow-up time points were postal questionnaires. As a result, the rates of completion are lower at these time points.
For the change from baseline summaries in the following sections the prespecified windows from the protocol for a questionnaire to be included were ± 2 weeks for the 18-, 30- and 42-week follow-ups and between 54 and 62 weeks for the 54-week follow-up. However, in the longitudinal mixed-effects models the time (in weeks) was treated as continuous and all valid and completed questionnaires were included in the analyses.
Secondary outcome 1: Oswestry Disability Questionnaire at 18, 30, 42 and 54 weeks
The ODQ data are analysed using longitudinal models and include the primary outcome time point of 18 weeks.
Table 13 shows the mean and SDs of the change from baseline for the observations at each time point. Table 13 includes observations in the prespecified time windows from the protocol. The longitudinal analysis includes all ODQ measurements taken at any point during the trial. Table 13 shows that most improvement in both groups happens between baseline and week 18, with any further improvements being relatively small.
| Time point (week) | Microdiscectomy | TFESI | Overall | |||
|---|---|---|---|---|---|---|
| n | Mean change (SD) | n | Mean change (SD) | n | Mean change (SD) | |
| 18 | 46 | –27.18 (22.31) | 51 | –24.29 (18.28) | 97 | –25.66 (20.24) |
| 30 | 40 | –26.62 (19.12) | 30 | –23.25 (17.45) | 70 | –25.17 (18.37) |
| 42 | 40 | –31.40 (17.22) | 34 | –25.51 (23.74) | 74 | –28.69 (20.54) |
| 54 | 48 | –30.38 (17.77) | 42 | –31.10 (24.35) | 90 | –30.71 (20.97) |
The longitudinal mixed-model analysis adjusted for baseline ODQ initially included a time–treatment interaction term, but as this was non-significant it was removed from the final model, as prespecified in the statistical analysis plan. The adjusted estimate for the effect of microdiscectomy compared with TFESI was –4.67 points (95% CI –10.61 to 1.28 points; p = 0.123). This estimate is consistent with that shown in the primary outcome analysis of ODQ at 18 weeks.
For the joint modelling post hoc analysis of ODQ scores, the adjusted estimate for the effect of microdiscectomy compared with TFESI was –4.62 points (95% CI –9.84 to 1.27 points; p = 0.108). The estimate is similar to the longitudinal mixed model and the conclusions remained unchanged once adjusted for informative dropout. Figure 4 shows profile plots of mean ODQ score at each scheduled time point.
FIGURE 4.
Mean profile plot of ODQ scores with standard error bars.
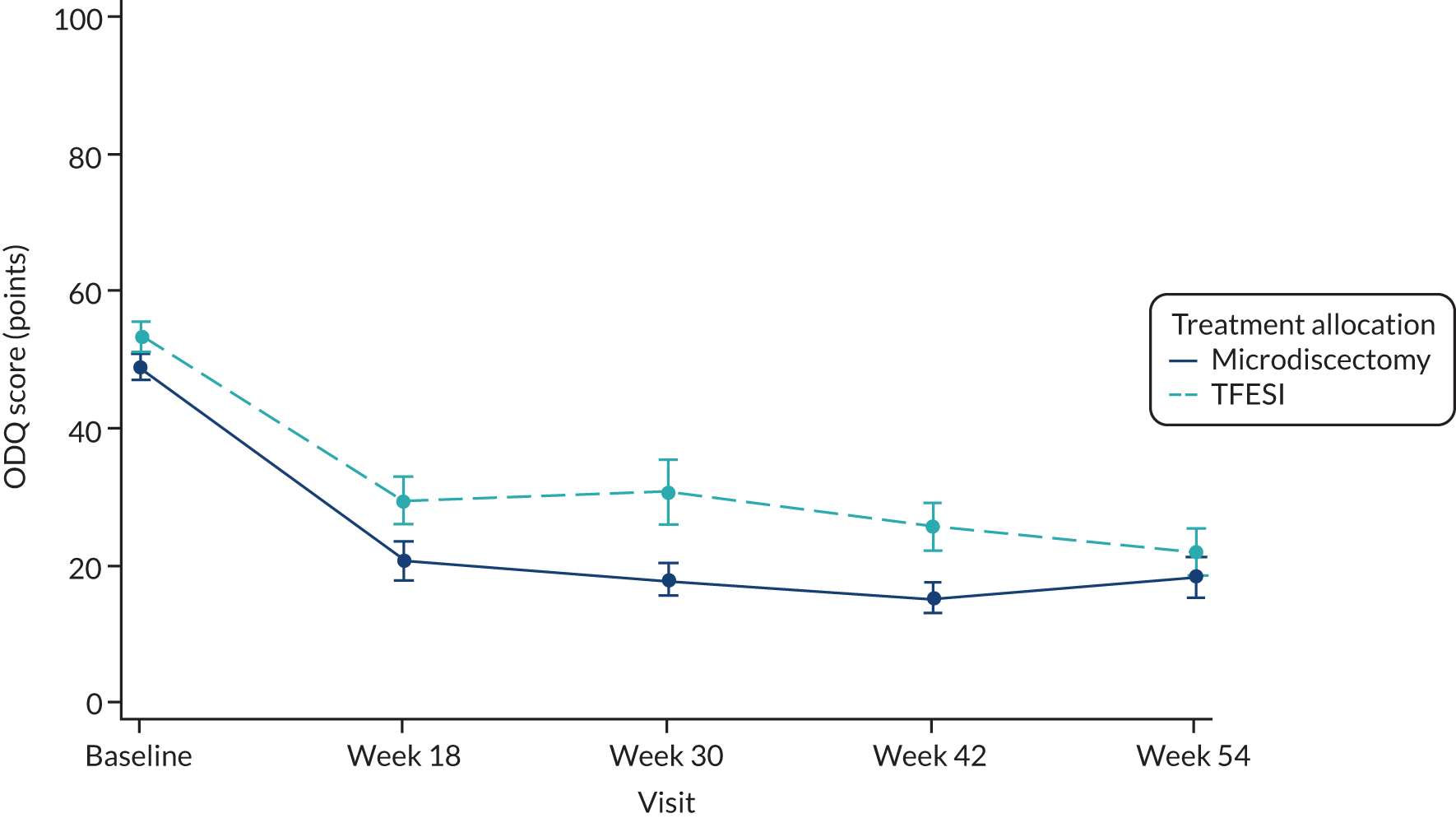
Secondary outcome 2: visual analogue scale score for leg pain at 18, 30, 42 and 54 weeks
Table 14 shows change from baseline at each time point for VAS leg pain rating, where valid measurements were available within the prespecified time window. After an initial large reduction between baseline and week 18, average VAS leg pain ratings were similar over the further follow-up visits. Figure 5 shows profile plots of mean VAS leg pain rating at each scheduled time point.
| Time point (week) | Microdiscectomy | TFESI | Overall | |||
|---|---|---|---|---|---|---|
| n | Mean change (SD) | n | Mean change (SD) | n | Mean change (SD) | |
| 18 | 45 | –58.31 (34.51) | 49 | –43.55 (32.52) | 94 | –50.62 (34.12) |
| 30 | 38 | –54.37 (27.05) | 30 | –42.70 (35.27) | 68 | –49.22 (31.25) |
| 42 | 37 | –55.81 (31.66) | 33 | –47.12 (42.28) | 70 | –51.71 (37.03) |
| 54 | 43 | –55.44 (33.57) | 39 | –47.08 (33.06) | 82 | –51.46 (33.39) |
FIGURE 5.
Mean profile plot of VAS leg pain scores with standard error bars.
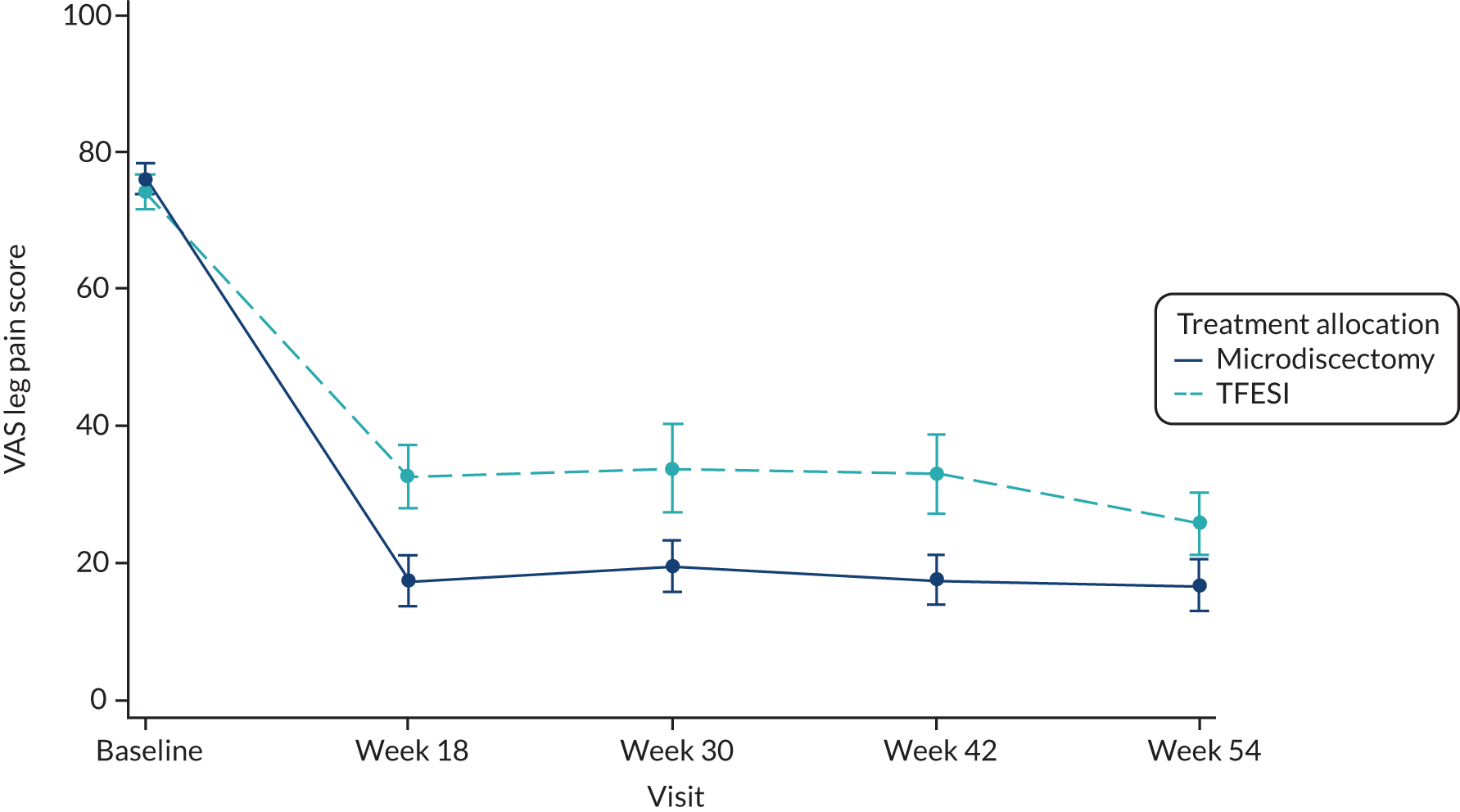
The longitudinal mixed-model analysis adjusted for baseline VAS leg pain initially included a time–treatment interaction term, but as this was non-significant it was removed from the final model, as prespecified in the statistical analysis plan. The adjusted estimate for the effect of microdiscectomy compared with TFESI was –7.04 (95% CI –15.81 to 1.73; p = 0.115).
For the joint modelling post hoc analysis of VAS leg pain scores the adjusted estimate for the effect of microdiscectomy compared with TFESI was –7.06 (95% CI –15.82 to 0.86; p = 0.098). The estimate is similar to the longitudinal mixed model and the conclusions remained unchanged once adjusted for informative dropout.
Secondary outcome 3: visual analogue scale score for back pain at 18, 30, 42 and 54 weeks
Table 15 shows change from baseline at each time point for VAS back pain rating, where valid measurements were available within the prespecified time window. The largest reduction was between baseline and week 18, with average VAS back pain ratings similar over the further follow-up visits. VAS back pain score reductions were smaller than VAS leg pain score reductions. Figure 6 shows profile plots of the mean VAS back pain rating at each scheduled time point.
| Time point (week) | Microdiscectomy | TFESI | Overall | |||
|---|---|---|---|---|---|---|
| n | Mean change (SD) | n | Mean change (SD) | n | Mean change (SD) | |
| 18 | 45 | –26.02 (32.83) | 49 | –23.41 (27.69) | 94 | –24.66 (30.12) |
| 30 | 38 | –25.00 (32.04) | 30 | –24.33 (31.95) | 68 | –24.71 (31.77) |
| 42 | 37 | –20.81 (37.43) | 33 | –23.00 (37.29) | 70 | –21.84 (37.11) |
| 54 | 42 | –23.07 (34.54) | 39 | –22.90 (29.11) | 82 | –22.99 (31.84) |
FIGURE 6.
Mean profile plot of VAS back pain scores with standard error bars.
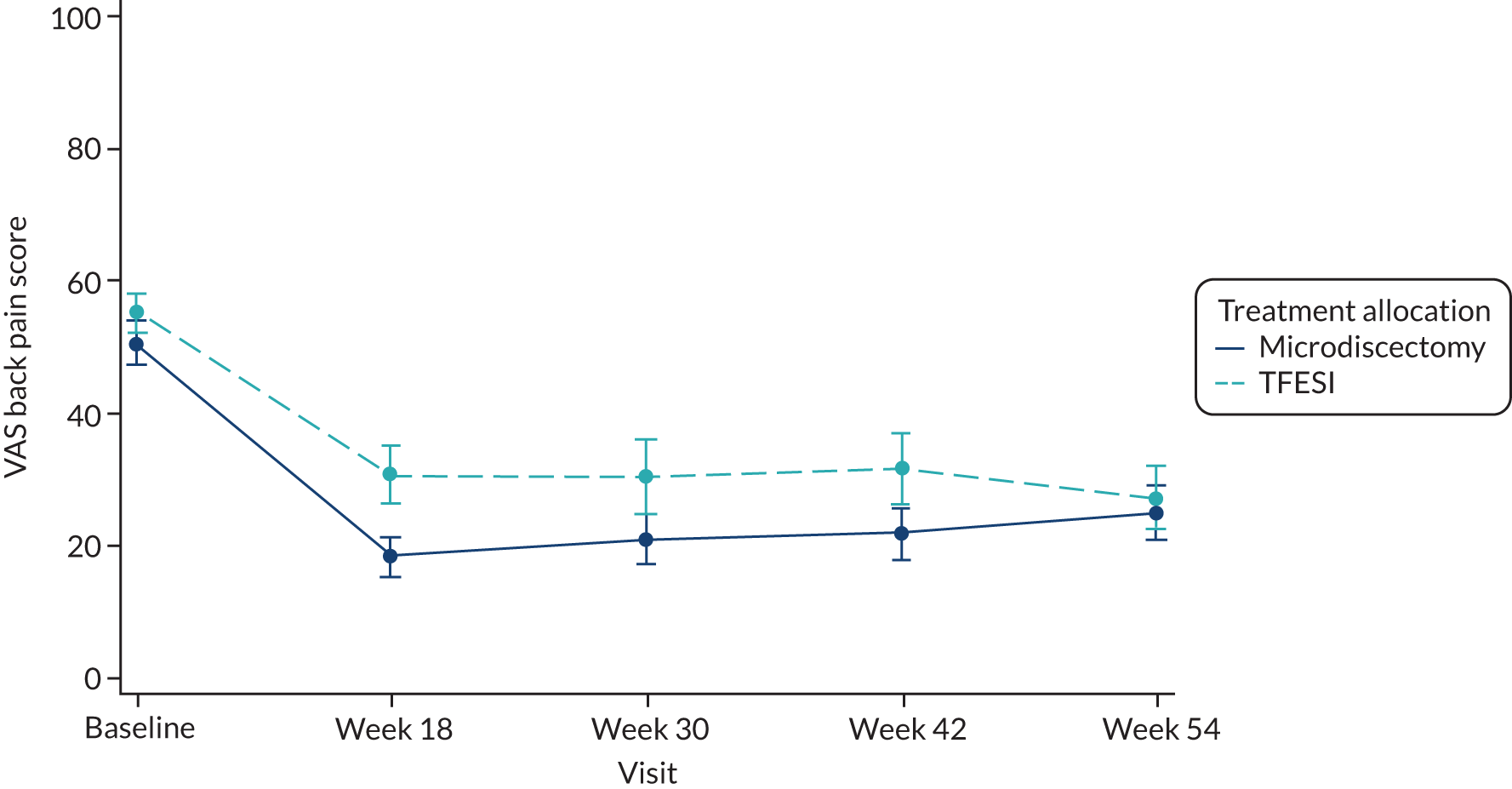
The longitudinal mixed-model analysis adjusted for baseline VAS back pain initially included a time–treatment interaction term, but as this was non-significant it was removed from the final model, as prespecified in the statistical analysis plan. The adjusted estimate for the effect of microdiscectomy compared with TFESI was –3.01 (95% CI –11.29 to 5.26; p = 0.473).
For the joint modelling post hoc analysis of VAS back pain scores, the adjusted estimate for the effect of microdiscectomy compared with TFESI was –2.87 (95% CI –10.58 to 3.16; p = 0.457). The estimate is similar to the longitudinal mixed model and the conclusions remained unchanged once adjusted for informative dropout.
Secondary outcome 4: satisfaction with care
The two questions on the satisfaction with care questionnaire were (1) ‘Over the course of treatment for your low back pain or leg pain (sciatica), how satisfied are you with your overall medical care?’ and (2) ‘How satisfied are you with the treatment outcome of your leg pain (sciatica)?’. A score of 1 on either question would be ‘very satisfied’ with care, a score of 2 ‘somewhat satisfied’, a score of 3 ‘neither satisfied nor dissatisfied’, a score of 4 ‘somewhat dissatisfied’ and a score of 5 ‘very dissatisfied’ with care. Therefore, an average score of 1 would imply being ‘very satisfied’ for both aspects of care and an average score of 5 would imply being ‘very dissatisfied’ with both aspects of care. Groups were compared using a Mann–Whitney U-test. The median score at 54 weeks in the microdiscectomy group was 1 (IQR 1–2) and in the TFESI group was 1.5 (IQR 1–3). Figure 7 shows the profile plot of median satisfaction with care scores at each scheduled time point.
FIGURE 7.
Median profile plot of satisfaction with care scores (Likert scores).
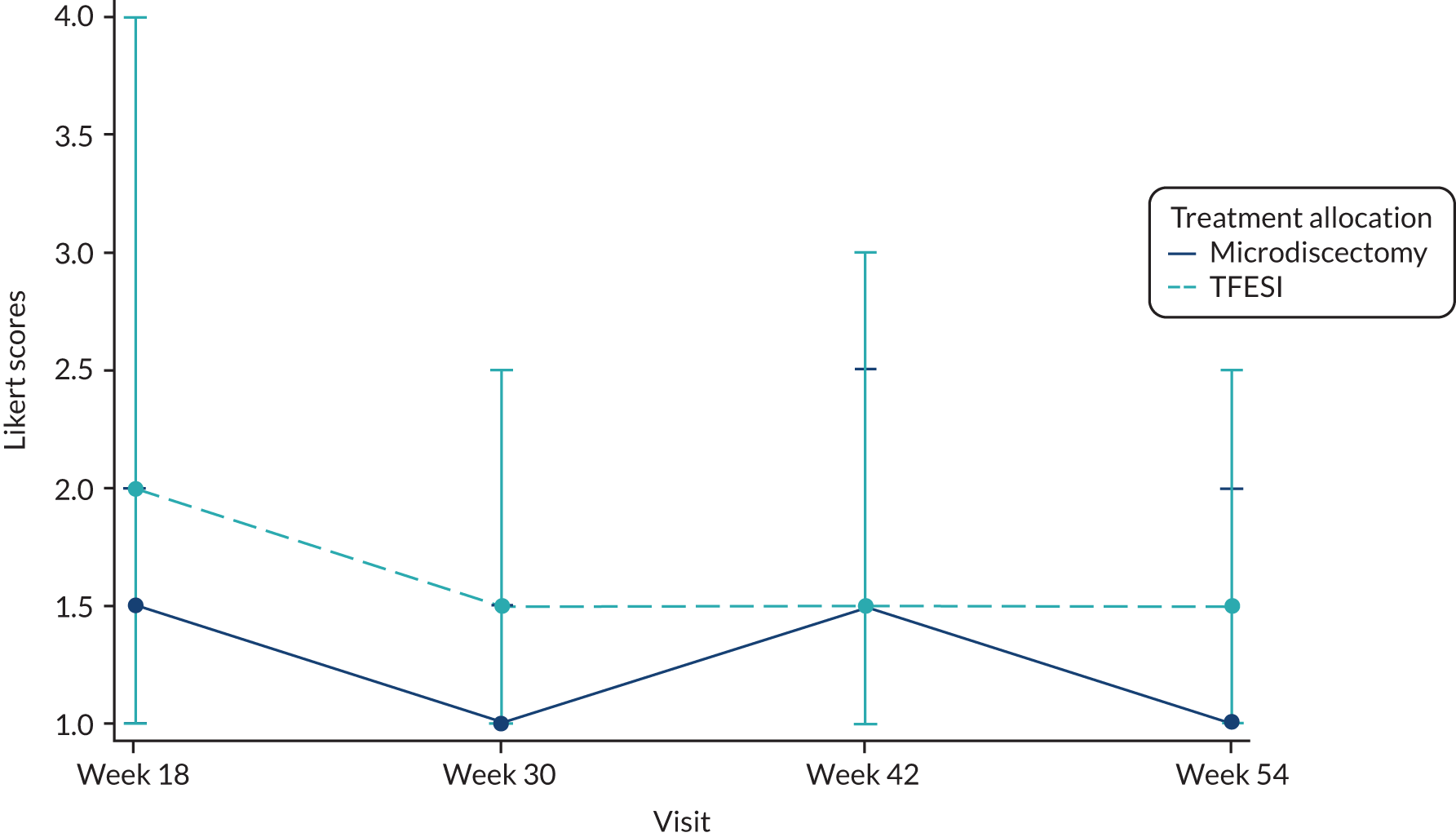
The difference between the groups was statistically significant (p = 0.021, Mann–Whitney U-test), with participants in the microdiscectomy group being more satisfied with their care than participants in the TFESI group.
Secondary outcome 5: modified Roland–Morris score at 18, 30, 42 and 54 weeks
Table 16 shows change from baseline at each time point for the MRM score, where valid measurements were available within the prespecified time window. After an initial large reduction between baseline and week 18, average MRM scores were similar over the further follow-up visits. Figure 8 shows the profile plots of mean MRM score at each scheduled time point.
| Time point (week) | Microdiscectomy | TFESI | Overall | |||
|---|---|---|---|---|---|---|
| n | Mean change (SD) | n | Mean change (SD) | n | Mean change (SD) | |
| 18 | 47 | –9.09 (6.27) | 51 | –7.73 (5.91) | 98 | –8.38 (6.09) |
| 30 | 40 | –9.58 (5.60) | 31 | –7.48 (6.68) | 71 | –8.66 (6.14) |
| 42 | 39 | –9.56 (5.86) | 34 | –8.35 (8.56) | 73 | –9.00 (7.21) |
| 54 | 47 | –9.74 (6.65) | 42 | –9.24 (6.68) | 89 | –9.51 (6.63) |
FIGURE 8.
Mean profile plot of MRM scores with standard error bars.
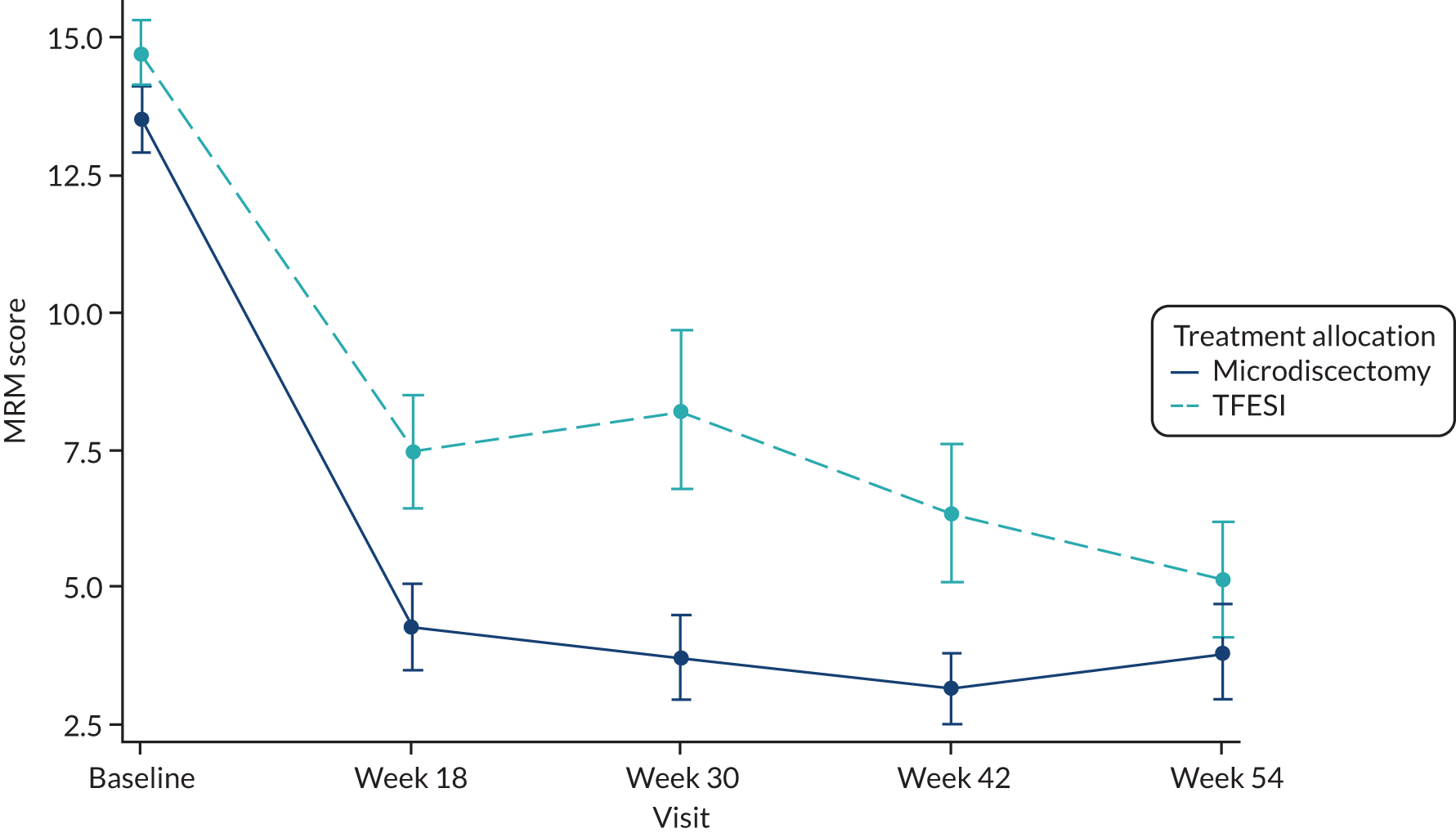
The longitudinal mixed-model analysis, adjusted for MRM initially included a time–treatment interaction term, but as this was non-significant it was removed from the final model, as prespecified in the statistical analysis plan. The adjusted estimate for the effect of microdiscectomy compared with TFESI was –1.82 (95% CI –3.67 to 0.03; p = 0.054).
For the joint modelling post hoc analysis of MRM scores, the adjusted estimate for the effect of microdiscectomy compared with TFESI was –1.72 (95% CI –3.44 to 0.10; p = 0.063). The estimate is similar to the longitudinal mixed model and the conclusions remained unchanged once adjusted for informative dropout.
Secondary outcome 6: Core Outcome Measures Index score at 18, 30, 42 and 54 weeks
Table 17 shows change from baseline at each time point for COMI score, where valid measurements were available within the prespecified time window. As with other outcomes, the largest reduction was between baseline and week 18, although the scores continued to improve in both groups between weeks 18 and 54. Figure 9 shows profile plots of the mean COMI score at each scheduled time point.
| Time point (week) | Surgery | TFESI | Overall | |||
|---|---|---|---|---|---|---|
| n | Mean change (SD) | n | Mean change (SD) | n | Mean change (SD) | |
| 18 | 42 | –3.93 (2.80) | 47 | –3.05 (2.69) | 89 | –3.46 (2.76) |
| 30 | 32 | –4.49 (2.44) | 27 | –3.33 (2.35) | 59 | –3.96 (2.45) |
| 42 | 33 | –4.92 (2.18) | 32 | –3.45 (3.14) | 65 | –4.20 (2.77) |
| 54 | 39 | –5.02 (2.32) | 37 | –3.93 (2.81) | 76 | –4.49 (2.61) |
FIGURE 9.
Mean profile plot of COMI scores with standard error bars.
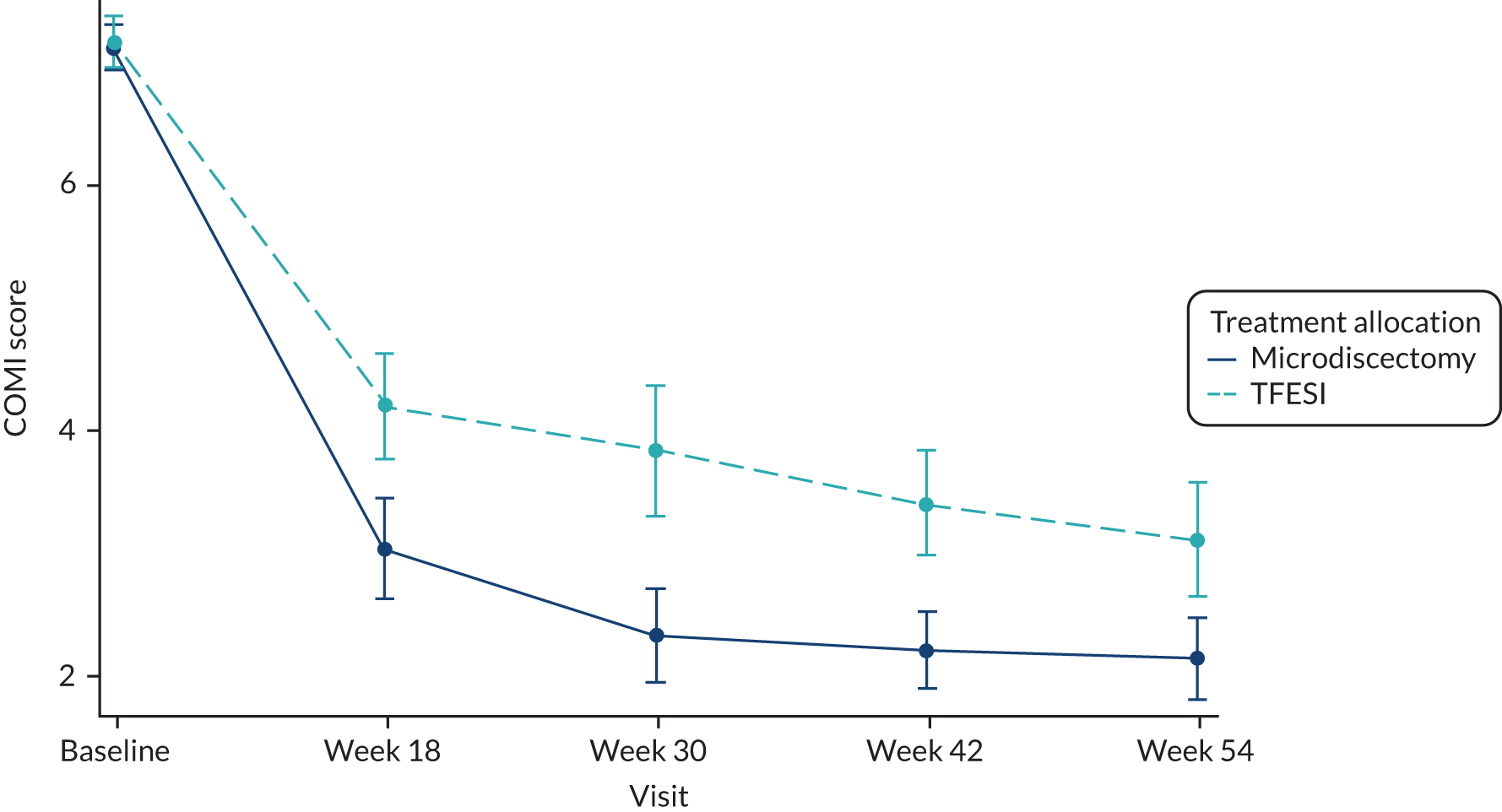
The longitudinal mixed-model analysis adjusted for baseline COMI initially included a time–treatment interaction term, but as this was non-significant it was removed from the final model, as prespecified in the statistical analysis plan. The adjusted estimate for the effect of microdiscectomy compared with TFESI was –0.77 (95% CI –1.58 to 0.03; p = 0.059).
For the joint modelling post hoc analysis of COMI scores, the adjusted estimate for the effect of microdiscectomy compared with TFESI was –0.78 (95% CI –1.54 to –0.02; p = 0.046). The estimate for microdiscectomy compared with TFESI is similar to the longitudinal mixed model; however, the p-value and CIs suggest a statistically significant treatment effect once adjusted for informative dropout (but less than the MCID of 2.2).
Secondary outcome 7: work status
Table 18 shows the number of participants who were employed/not employed at baseline. Of those participants who were employed, the numbers of participants who were off work/at work are also presented by treatment group.
| Status | Microdiscectomy (N = 83), n | TFESI (N = 80), n | Overall (N = 163), n |
|---|---|---|---|
| Unemployed | 21 | 13 | 34 |
| Employed | 62 | 66 | 128 |
| Working | 41 | 34 | 75 |
| Not working | 21 | 32 | 53 |
| Missing | 0 | 1 | 1 |
At baseline, the information collected was whether or not the participant was employed and, if so, whether or not they were able to work. However, at follow-up the information collected was whether or not the participant was currently employed and whether or not they had returned to work since the last visit. As there were extra visits between the key time points when this information could change but was not collected, based on these questions it was sometimes impossible to say whether or not the participant had returned to work and whether or not they were currently working.
Owing to the issues with the data collection we are unable to accurately calculate the number of days lost from work and the work status outcome, and so these data have not been analysed or presented.
Safety analysis
No patients died during the reporting of this trial. Safety outcomes are reported based on actual treatment received, rather than randomised group. AEs were reportable only if they were considered to be possibly related to treatment. A total of 26 AEs were reported [18 events (from 15 participants) associated with microdiscectomy and eight events (from three participants) associated with TFESI]. A summary of reported AEs is given in Table 38. The only events occurring more than once were dural tear (four events) and pseudomeningocele (two events), and all AEs were related to surgical incidental durotomy.
There were four SAEs (from four participants, 3.8%) and all were associated with microdiscectomy. None of the SAEs was unexpected. Listings of SAEs are given in Table 39. One participant developed a foot drop after an uncomplicated surgical procedure and required surgical exploration to exclude a postoperative complication. The foot drop had not improved at the end of follow-up.
Chapter 4 Economic evaluation
Objective
A within-trial economic analysis was performed using individual patient-level data from the NERVES clinical trial. The analytical approach took the form of a cost–utility analysis, using information on health-related costs and preference-based health status to calculate an ICER, expressed as cost per QALY gained.
Methods
The design and reporting of the economic analyses were specified in a separate Health Economics Analysis Plan (HEAP) (see Report Supplementary Material 2). The primary health economic analysis followed the approach of NICE’s Guide to the Methods of Technology Appraisal 201342 and adopted the perspective of the NHS in England. The economic analysis considered the costs and consequences of each intervention over the 54-week trial period and was reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards checklist. A secondary analysis that approximates a societal costing perspective was included, which additionally considered participant out-of-pocket costs and trial participants’ and their informal carers’ productivity losses arising from time off work. The analysis was conducted following a series of steps:
-
The direct medical costs associated with delivering the treatments through measuring participants’ use of health services were estimated.
-
The impact of the interventions on participants’ and informal carers’ productivity losses and out-of-pocket costs was estimated.
-
The estimated mean total cost per participant in each of the two intervention groups was compared.
-
The mean number of QALYs for each trial intervention, calculated from EQ-5D-5L questionnaire-generated utility scores, as a measure of health benefit was estimated.
-
ICERs and incremental net health benefits (INHBs) were calculated by comparing the differences between treatment groups in the mean total costs and the mean QALYs.
-
The uncertainty in the results was tested through bootstrapping and generating cost-effectiveness acceptability curves (CEACs) to estimate the probability of microdiscectomy being cost-effective compared with TFESI at NICE thresholds for cost-effectiveness.
-
Scenario analyses (SAs) were conducted to assess how assumptions in the base-case analysis can influence results.
Resource use and costs
Measurement of resource use data
Resource use categories that might differ between intervention groups were chosen a priori. These were further differentiated into sciatica related and non-sciatica related. The measurement of resource use required complementary approaches using data collected as part of the NERVES trial, as well as routine NHS data. Within-trial costs were estimated by measuring health-care resource use, including (1) microdiscectomy and TFESI procedure, and other hospitalisations (e.g. additional treatments), (2) outpatient appointments and contact with health-care professionals (e.g. GPs, physiotherapists, other health-care professionals), (3) concomitant medication use, (4) costs incurred in the management of AEs and (5) participant out-of-pocket costs. Trial participants’ use of health-care services and other costs were obtained from the following sources:
-
Routine HES data obtained from NHS Digital on secondary care admitted patient care (inpatient) and outpatient attendances.
-
CRFs completed by medical or nursing staff, relating to participant contact with hospital and health-care professionals, including treatments issued, additional treatments received and medications prescribed.
-
A RUQ43 completed by the participant at baseline and treatment visits, and at weeks 18, 30, 42 and 54 for information on the use of primary and secondary care services, visits to other health-care professionals, participant out-of-pocket expenditures on over-the-counter medications and travel costs, as well as productivity losses resulting from days off work, in the previous 3 months (6 weeks at treatment visit) or since the questionnaire was last completed.
-
AE and SAE forms completed by research staff when trial participants were admitted to hospital for events considered possibly related to the trial intervention.
Valuation of unit costs
All resource use was valued in monetary terms (Great British pounds) using appropriate UK unit costs for the cost year 2017/18. When necessary, adjustments were made for inflation using the new Hospital and Community Health Service (HCHS) Index and using the Consumer Prices Index. 44 No discount rate was applied as follow-up was approximately 1 year.
Healthcare Resource Groups (HRGs) were the main currency of secondary care attendances, including the trial interventions, additional treatments, other hospitalisations, and day case, outpatient and accident and emergency (A&E) visits (Tables 19–21). NHS National Schedule of Reference Costs 2017–1845 were applied, as these most closely reflect the actual cost of the provision of care by NHS service providers. Medication costs for steroid and local anaesthetic associated with TFESI were assumed to be included in the HRG costs, as were all costs relating to microdiscectomy. HRGs were further subcategorised according to whether the episode of care took place in the day case, elective or non-elective setting.
| Intervention | HRG codea | Description | Attendance | Cost per episode (£)b |
|---|---|---|---|---|
| Microdiscectomy | HC64C | Intermediate Extradural Spinal Procedures with CC Score 0–1c | Day case | 1971 |
| Elective | 4231 | |||
| Non-elective | 5162 | |||
| HC64B | Intermediate Extradural Spinal Procedures with CC Score 2–3 | Elective | 4782 | |
| Non-elective | 6261 | |||
| HC63C | Major Extradural Spinal Procedures with CC Score 0–1 | Elective | 4858 | |
| Non-elective | 5696 | |||
| TFESI | AB20Z | Epidural Under Image Control for Pain Management | Day case | 711 |
| Hospitalisation without surgical intervention | HC27N | Degenerative Spinal Conditions without Interventions, with CC Score 0–2 | Non-elective | 1827 |
| Other | AA26H | Muscular, Balance, Cranial or Peripheral Nerve Disorders, Epilepsy or Head Injury, with CC Score 0–2 | Day case | 523 |
| Non-elective | 1490 | |||
| WH50B | Procedure Not Carried Out, for Other or Unspecified Reasons | Day case | 338 | |
| Elective | 599 |
| Treatment area | HRG code | Description | Consultant led or non-consultant led | Cost per episode (£)a |
|---|---|---|---|---|
| Anaesthetics | WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Consultant led | 124 |
| Neurosurgery | WF01B | Non-Admitted Face-to-Face Attendance, First | Consultant led | 257 |
| WF01B | Non-Admitted Face-to-Face Attendance, First | Non-consultant led | 153 | |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Consultant led | 179 | |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Non-consultant led | 174 | |
| Pain management | WF01B | Non-Admitted Face-to-Face Attendance, First | Consultant led | 193 |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Consultant led | 147 | |
| Physiotherapy | WF01B | Non-Admitted Face-to-Face Attendance, First | Non-consultant led | 61 |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Non-consultant led and consultant led | 52 | |
| Spinal surgery service | WF01B | Non-Admitted Face-to-Face Attendance, First | Consultant led | 163 |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Consultant led | 135 | |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Non-consultant led | 81 | |
| Trauma and orthopaedics | WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Consultant led | 119 |
| WF01A | Non-Admitted Face-to-Face Attendance, Follow-up | Non-consultant led | 93 |
| Admission | HRG code | Description | Cost per episode (£)a |
|---|---|---|---|
| Emergency medicine | VB01Z, VB02Z, VB03Z, VB04Z, VB05Z, VB06Z, VB07Z, VB08Z, VB09Z, VB10Z, VB11Z, VB99Z | Weighted mean cost by proportion of attendances by code to Accident and Emergency | 160.32 |
The unit costs for all other NHS primary health-care resource use items were obtained from the Personal Social Services Research Unit44 (Table 22). Visit costs to other health-care professionals (i.e. private physiotherapists, osteopathy and chiropracty, acupuncture) were obtained from publicly available sources. 48–51
| Health-care professional attendance | Cost per episode (£)a | Costing perspective | Source |
|---|---|---|---|
| GP: surgery | 31.43 | NHS | PSSRU 201844 |
| GP: out-of-hours surgery | 109.59 | NHS | PSSRU 201844 |
| GP: home visit (call-out) | 102.10 | NHS | PSSRU 2013,47 PSSRU 201844 |
| NHS physiotherapist | 46.00 | NHS | PSSRU 201844 |
| Acupuncture | 43.10 | Wider perspective | NHS 201648 |
| Osteopath/chiropractor | 48.75 | Wider perspective | NHS 2018,49 NHS 201750 |
| Private physiotherapist | 50.70 | Wider perspective | Capital Physio 201651 |
Concomitant medications were valued in monetary terms using prescription cost analysis data from September 201752 and supplemented by the British National Formulary (BNF). 53 Over-the-counter medication costs were obtained from published retail pharmacy sources. 54 The unit costs of the 10 most commonly prescribed medications are presented in Table 23.
| Medication | Formulation | Cost per unit (£)a |
|---|---|---|
| Gabapentin | 100-mg capsule | 0.02 |
| 300-mg capsule | 0.14 | |
| 400-mg capsule | 0.03 | |
| 600-mg tablet | 0.06 | |
| 800-mg tablet | 0.30 | |
| 50-mg/ml sugar-free solution | 0.46 | |
| Paracetamol | 250-mg/5-ml oral suspension | 0.01 |
| 500-mg tablet | 0.02 | |
| Naproxen | 250-mg tablet | 0.03 |
| 500-mg tablet | 0.04 | |
| Morphine | 10-mg tablet (Sevredol®; Napp Pharmaceuticals Ltd, Cambridge, UK) | 0.09 |
| 10-mg tablet (MST Continus®; Napp Pharmaceuticals Ltd, Cambridge, UK) | 0.09 | |
| 10-mg capsule (Zomorph®; Ethypharm, UK Ltd, High Comber, UK) | 0.06 | |
| 30-mg capsule (Zomorph®; Ethypharm, UK Ltd, High Wycombe, UK) | 0.14 | |
| 100-mg capsule (Zomorph®; Ethypharm, UK Ltd, High Wycombe, UK) | 0.36 | |
| 10-mg/5-ml oral solution (Oramorph®; Boehringer Ingelheim Ltd, Bracknell, UK) | 0.02 | |
| Tramadol | 50-mg capsule | 0.03 |
| 50-mg modified-release capsule | 0.12 | |
| 100-mg modified-release capsule | 0.24 | |
| 50-mg/2-ml injection | 1.12 | |
| Co-codamol | 8-mg/500-mg tablet | 0.03 |
| 15-mg/500-mg tablet | 0.05 | |
| 30-mg/500-mg tablet | 0.04 | |
| 30-mg/500-mg tablet (Zapain®; Advanz Pharma, London, UK) | 0.03 | |
| Ibuprofen | 200-mg tablet | 0.03 |
| 400-mg tablet | 0.03 | |
| 256-mg tablet [Nurofen Express®; Reckitt Benckiser Healthcare (UK) Ltd, Slough, UK] | 0.14 | |
| 200-mg long-lasting capsules (Boots®; The Boots Company plc, Nottingham, UK) | 0.21 | |
| Gel 5% | 0.05 | |
| Gel 5% [Nurofen®; Reckitt Benckiser Healthcare (UK) Ltd, Slough, UK] | 0.09 | |
| Gel 10% | 0.05 | |
| Amitriptyline | 10-mg tablet | 0.03 |
| 20-mg tablet | 0.05 | |
| 25-mg tablet | 0.03 | |
| 35-mg tablet | 0.06 | |
| 50-mg tablet | 0.09 | |
| 10-mg/5-ml solution | 0.74 | |
| Pregabalin | 75-mg capsule (Lyrica®; Upjohn UK Ltd, Sandwich, UK) | 1.15 |
| 150-mg capsule (Lyrica®; Upjohn UK Ltd, Sandwich, UK) | 1.15 | |
| 300-mg capsule (Lyrica®; Upjohn UK Ltd, Sandwich, UK) | 1.15 | |
| 50-mg capsule | 0.14 | |
| 75-mg capsule | 0.10 | |
| 100-mg capsule | 0.12 | |
| 150-mg capsule | 0.15 | |
| 200-mg capsule | 0.15 | |
| 300-mg capsule | 0.18 | |
| Codeine phosphate | 15-mg tablet | 0.03 |
| 30-mg tablet | 0.04 | |
| 60-mg tablet | 0.05 |
Lost productivity was based on participant-reported lost earnings. In SAs, Office for National Statistics (ONS) Annual Survey of Hours and Earnings median wage values were applied based on full-time employment55,56 (Table 24). Other out-of-pocket participant costs associated with public transport to attend medical appointments were based on participant self-report. If travel was undertaken in a private car, a cost per mile of £0.68, including vehicle running costs, was applied. 57,58
| Category | Net daily earnings (£) | ||
|---|---|---|---|
| Malea | Femalea | By hospital local authority areab | |
| Age (years) | |||
| 22–29 | 75.16 | 70.18 | |
| 30–39 | 93.64 | 85.82 | |
| 40–49 | 102.63 | 84.27 | |
| 50–59 | 100.40 | 79.35 | |
| ≥ 60 | 87.87 | 71.05 | |
| Hospital local authority area | |||
| Middlesbrough | 79.39 | ||
| Cambridge | 92.02 | ||
| Sheffield | 81.85 | ||
| Preston | 79.94 | ||
| Stoke-on-Trent | 76.69 | ||
| Nottingham | 77.52 | ||
| Salford | 81.20 | ||
| Birmingham | 84.95 | ||
| Leeds | 84.13 | ||
| Liverpool | 82.41 | ||
| Southampton | 88.68 | ||
Cost analysis
Resource use data were collected from 3 months (84 days) prior to baseline to the end of the trial at 54 weeks. For completeness, all resource use was measured and costed irrespective of reason. However, to allow for SA, it was categorised as sciatica related or non-sciatica related, as reported in the CRFs or patient questionnaire. Medications were categorised in the CRFs based on reason for prescription, which allowed for identification of those prescribed for sciatica-related reasons. HES data were categorised by reason for admission, based on HRG code, to identify admissions and visits associated with sciatica. The trial design also allowed for further treatment at the discretion of the clinician in the event of incomplete symptom resolution (see Chapter 2, Trial treatments for further details) and costs for subsequent treatments within the trial period were collected via the HES data set and included in the analysis.
When multiple sources reported the same type of resource use (e.g. hospital inpatient admissions), a hierarchical approach was applied, with priority given to routine HES data, followed by researcher-completed CRFs and, last, participant self-reported questionnaires. The different data sources were compared to ensure that all resource use was captured, but also to avoid any overlapping of data and consequent double counting.
To cost secondary care episodes, HES data were sourced from NHS Digital in the form of a series of routinely available codes and dates, including participant diagnoses, admission dates and discharge dates, and then converted into HRG codes using the NHS Digital HRG4+ 2018/19 Payment Grouper. 59 When unavailable, hospital events were assigned an appropriate HRG code based on reason for admission, condition and any complications, by reference to baseline, treatment, additional treatment, AE CRFs and patient questionnaires. Appropriate HRGs were manually applied to unassignable HRG codes (e.g. UZ01C and WA14Z) appearing in the HES data using clinical codes. Costs were assigned according to HRG code, length of stay (incorporating any excess ward bed-days) and whether the case was a day case, elective or emergency, according to the National Schedule of Reference Costs 2017–2018. 45 Locally negotiated unbundled HRGs were similarly identified through the cost grouper and costs were assigned directly from the national schedule. Privately treated participants were assigned the corresponding national schedule NHS HRG code to ensure that costs were appropriately apportioned. Visits to A&E were costed based on participant self-report, and a mean cost per visit was applied according to the national schedule. Participants admitted from A&E to hospital were further identified and costed according to the HES admitted patient care data set. If a hospital admission spanned the period preceding randomisation or beyond the trial period, an adjustment was made to apportion costs to only those incurred during the 54-week trial. To allow for follow-up visits that occurred out of the trial window, all protocol-related visits that were scheduled identically in both intervention groups were included, but all other visits outside the 54-week trial were excluded.
The RUQ collected participant-reported resource use for the preceding 3 months (or 6 weeks at the treatment visit). All visits were face to face except weeks 30 and 42 when questionnaires were issued by post with a pre-paid return envelope. Quantities of resource use for primary care services, including visits to the GP (i.e. surgery, out of hours or home visit), physiotherapist, acupuncturist, chiropractor and osteopath and any other health-care professional, were taken from participant responses and multiplied by unit costs to estimate total costs. As the base-case analysis takes the NHS perspective, all visits to the GP were included, as were NHS physiotherapist visits, assumed on the basis of a participant-reported out-of-pocket cost of < £20, which was attributed to travel costs. Physiotherapy visits with costs of > £20 were assumed to be private physiotherapy visits and were included in the secondary analysis adopting a wider perspective. As some physiotherapy visits were also identified in the HES outpatient data set, a cross-check was performed to avoid double counting and to include NHS physiotherapy visits reported by participants that were not identified in the HES data set. The costs of visits to an acupuncturist, chiropractor, osteopath and any other health-care professionals, as well as any over-the-counter medications, were considered to be participant out-of-pocket costs and included only in the secondary analysis on the basis that the NHS does not routinely fund these treatments. Questionnaires not completed within the predefined visit windows were protocol deviations but, nonetheless, were included in the analysis using appropriate time-based adjustments.
Medication costs were calculated based on CRF-recorded medication usage at baseline, treatment and follow-up visits weeks 18 and 54. All tablets, capsules, oral liquids, sprays and inhalers were costed on a unit dose basis, and creams and gels were assumed to be supplied on a one pack per month basis. If a medication administration spanned the period preceding randomisation, or beyond the trial time period, an adjustment was made to apportion costs to those administered only during the 54-week trial. For medications with missing start and/or end dates, but recorded as ongoing treatments, the start date was assumed to be 3 months (84 days) prior to baseline and the end date the trial exit date. When doses and frequencies were omitted, estimated values based on participants’ medication histories or BNF-recommended doses were applied. Medications administered on an ‘as needed’ basis were costed assuming administration based on 50% of the standard dose recommended in the BNF. In cases where prescribed doses did not match formulation doses, costs were calculated by combining different strengths to form the appropriate dose.
Out-of-pocket travel costs and participant or carer productivity losses were also obtained from the RUQ. Participants were asked to report both the number of days off work and lost earnings. When the number of days off work was reported but lost earnings values were missing, it was assumed that there were no lost earnings (i.e. participants received sick pay).
Health outcome measures
Health benefits were measured in QALYs (NICE’s preferred outcome for economic evaluations). QALYs are a generic measure of health that integrate preference-based measures of health status (utility) over time into a single index. The EQ-5D-5L descriptive measure of health status was administered to participants at baseline, and at weeks 18, 30, 42 and 54. At each time point, a five-digit code describing a participant’s health status was generated from the EQ-5D-5L participant responses. In the base-case analysis, the EQ-5D-5L to EuroQol-5 Dimensions, three-level version (EQ-5D-3L), crosswalk valuation set (i.e. the current NICE preferred value set42) was employed to generate utility scores. QALYs were calculated according to the ‘area under the curve’ approach60 for each participant’s longitudinal measures of utility, and assuming linear changes in utility values over time. In instances where EQ-5D-5L completion was subject to a trial visit time deviation, standardisation of values was achieved through adjustment to the relevant visit time point.
Missing data
Data sets for hospital admissions, outpatient care and concomitant medications were considered to be complete, but the resource use and EQ-5D questionnaires were subject to missing data. For the resource use and EQ-5D questionnaires, missing data were observed when responses were not provided or were incomplete at baseline, treatment visit and at the 18-, 30-, 42- and 54-week follow-ups. Return rates were particularly low for the postal questionnaires at weeks 30 and 42. If a participant did not return or fully complete either or both EQ-5D-5L questionnaires for weeks 30 and 42, then these utility scores were imputed by linear interpolation using observed utility values at weeks 18 and 54. Linear interpolation was also used to adjust observed utility scores based on the post-randomisation timing of the data collection relative to the scheduled collection dates. This was done to standardise the utility scores in terms of timing prior to imputing the remaining missing observations.
The utilities and costs at missing time points were then imputed via multiple imputation by chained equations (MICE) using the ‘mice’ package in the R statistical computing environment, version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria), assuming that missing data were missing at random. 61 This was applied using a data set that included utility scores at each observation and cost variables at each observation derived from the RUQ (including visits to GPs, other health-care professionals and A&E), baseline and follow-up cost of concomitant medications, cost of admitted patient care and outpatient visits according to the HES data set, and participant information (including age group, sex, duration of leg pain symptoms, treatment allocation and trial centre). The predictor matrix for the MICE procedure was specified such that all the other variables were used to impute missing values for each variable that had missing data, with the exception of baseline costs or utilities, which did not use treatment allocation.
Missing values were generated using the method of predictive mean matching and the MICE iterative procedure used a maximum of 30 iterations to achieve convergence. For the base-case analysis, and in all SAs, a total of 10 complete imputed data sets were generated. The multiple imputation was not used to obtained standard errors on subsequent regression coefficients as this was achieved via a bootstrap procedure. Therefore, 10 was considered to be a sufficient number of imputations to achieve a high level of efficiency. 62
Approach to analysis
The analysis is reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 63 The analysis was based on the ITT population (i.e. 163 participants were recruited to the trial, but six participants withdrew early on and were excluded from the cost-effectiveness analysis because no outcome data nor HES data were available). Data from 157 participants were therefore included in the economic evaluation (80 participants in the microdiscectomy group and 77 participants in the TFESI group). Participants’ use of health-care resources for the 3 months prior to randomisation was analysed (n = 163) to allow for adjustment for any imbalance in costs and outcomes observed at baseline, as well as to identify any correlation between any baseline variables and outcomes. Unadjusted mean differences between the treatment groups in utility scores, QALYs and incremental costs were reported with 95% CIs for the pre-baseline and trial periods.
Total costs at baseline were correlated with follow-up costs and this was also true on examination of baseline utilities and follow-up QALYs. Therefore, regression analyses were conducted to quantify the impact of treatment allocation on follow-up costs and QALYs while controlling for baseline costs and utilities, respectively. Plotting costs and QALYs against other potential independent variables (i.e. participant age group and sex, trial centre and duration of leg pain symptoms) did not indicate any associations, but these were included in the regression model development nonetheless. Regression models were estimated using each of the 10 imputed data sets and coefficients were averaged to obtain point estimates as per Rubin’s rules. 64
Participant QALYs were modelled using multiple linear regression, with treatment allocation and baseline utilities as covariates. Additional models were estimated, including combinations of the other potential independent variables listed above; however, these were ruled out based on F- or t-tests for nested model comparisons and inspection of residual plots. Participant follow-up costs were modelled using a generalised linear model with a log-link function and gamma probability distribution. Identity link, or log-link and Gaussian probability distribution were considered as alternatives. These were ruled out based on comparisons of the Akaike information criterion statistic and inspection of residual plots. In both models, trial centre was tested as a random-effect coefficient; however, it was not included because the estimated variance was close to zero. The analysis was performed using the R statistical computing environment, according to the regression models presented below.
Follow-up costs regression model
where C is follow-up total cost, ν is predicted follow-up total cost, α is the gamma shape parameter, γ0 is the regression intercept, γ1 is the coefficient of injection, γ2 is the coefficient of baseline total cost, T is 1 for TFESI and 0 for microdiscectomy and C0 is the baseline total cost.
Quality-adjusted life-years regression model
where Q are QALYs, µ is the predicted QALY, σ is the residual error SD, β0 is the regression intercept, β1 is the coefficient of treatment allocation, β2 is the coefficient of baseline utility score, T is 1 for TFESI and 0 for microdiscectomy and U0 is the baseline utility score.
Incremental cost-effectiveness ratio
The cost-effectiveness of microdiscectomy compared with TFESI was assessed by its ICER calculated according to the formula:
where Δcosts is the difference in mean total costs between interventions (i.e. cost microdiscectomy minus cost TFESI) and ΔQALYs is the difference in mean QALYs gained between interventions (i.e. QALYs microdiscectomy minus QALYs TFESI).
The INHB allows for further interpretation of cost-effectiveness in reporting the net health benefits that would arise further to adopting microdiscectomy rather than TFESI, conditional on a cost-effectiveness threshold of £20,000 per QALY, as specified by NICE. 42 This was calculated by:
where λ is the chosen cost-effectiveness threshold.
Uncertainties in QALYs, the incremental results and resulting cost-effectiveness metrics were evaluated using a non-parametric bootstrap of the patient-level data. The bootstrap used 10,000 replicates and empirical bootstrap CIs were obtained for the statistics of interest. The uncertainty in the ICER was represented graphically on the cost-effectiveness plane to illustrate the joint uncertainty in incremental costs and QALYs and also as a CEAC to illustrate the probability of either microdiscectomy or TFESI being cost-effective for given cost-effectiveness thresholds. 65 Estimates of ICERs were compared with a threshold of £20,000 per QALY.
Base-case analysis
The base-case analysis was defined as pertaining to the 54-week trial period, based on the imputed data set to account for missing data and subject to the regression analysis. The mean QALYs and costs and incremental values were reported for each treatment group with 95% CIs in accordance with the bootstrap analysis.
The main assumptions made in the base-case scenario are detailed in Table 25.
| Item | Description and approach |
|---|---|
| Comparator | Microdiscectomy vs. TFESI |
| Population | NERVES trial participants diagnosed with sciatica secondary to PID |
| Analysis approach | ITT, but excluding withdrawn participants |
| Model type and description | Within-trial cost-effectiveness analysis, imputed data set, subject to regression |
| Cost-effectiveness metrics | Non-parametric bootstrap, mean and 95% CI |
| Perspective | NHS |
| Time horizon | 54 weeks |
| Discount rate | Not applicable, trial limited to ≈ 1 year |
| Utility values | EQ-5D-5L mapped using a three-level crosswalk value set |
| Health outcome | QALYs measured from utility values |
| AEs | Costs only, associated with secondary care admissions |
| Resource use | |
| Secondary care | HES |
| Primary care | GP visits, GP out of hours and GP home visits |
| Physiotherapy | NHS costs associated with physiotherapy |
| A&E | A&E visits |
| Medications | Prescribed concomitant medications |
Sensitivity and scenario analyses
A number of sensitivity and SAs were conducted to assess the impact of considering alternative perspectives, inputs and costs on the ICER (Table 26). Scenario 1 reflects the wider perspective of the secondary analysis and follows the same approach as the base-case analysis, but in approximating to a societal perspective included participant costs associated with time off work and other out-of-pocket costs (Table 27).
| Scenario | Scenario description and approach | Base-case analysis |
|---|---|---|
| 1 | Approximating to a societal perspective | NHS perspective |
| 2 | Complete cases only | Imputed data set |
| 3 | QALYs from the five-level valuation set | QALYs from the three-level valuation set |
| 4 | QALYs inferred from EQ-VAS | QALYs from the three-level valuation set |
| 5 | Alternative RUQ assumption based on ‘since last completed’ approach | RUQ completed based on ‘last 3 months’ |
| 6 | Sciatica-related costs only | All costs |
| 7 | ‘When-needed’ medications assumed at 25% of BNF-recommended dose | ‘When-needed’ medications assumed at 50% of BNF-recommended dose |
| 8 | ‘When-needed’ medications assumed at 75% of BNF-recommended dose | ‘When-needed’ medications assumed at 50% of BNF-recommended dose |
| 9 | Approximating to a societal perspective, sciatica costs only | NHS perspective |
| 10 | Approximating to a societal perspective, missing cost of lost days at work assumed based on median salary by age and sex (participants) and postcode salary (carer) | NHS perspective |
| 11 | Approximating to a societal perspective, missing RUQ productivity losses obtained, when possible, using clinician-recorded employment status and median salary by age and sex | NHS perspective |
| 12 | Approximating to a societal perspective, all productivity losses based on reported days lost, where costs were median salary by age and sex (participants) and postcode salary (carer) | NHS perspective |
| Item of resourcea | Description and approach |
|---|---|
| Other health-care professional | Participant-reported out-of-pocket costs for physiotherapy, acupuncture, chiropractor, osteopath and other healthcare professionals |
| Medications | Participant-reported out-of-pocket costs for over-the-counter medications |
| Lost productivity | Participant-reported cost of lost days at work and participant-reported cost of lost carer days at work |
| Medical appointment travel | Participant-reported public transport costs and participant-reported car mileage distance |
A complete-case SA included participants presenting complete-cost data for all cost variables at all time points and outcome data at baseline and at weeks 18 and 54. To assess the impact of alternative value sets, the more recent but yet to be widely adopted value set based on the EQ-5D-5L66 was used in a SA, as was a further evaluation using the EQ-VAS, also collected in the NERVES trial, which records the respondent’s self-rated health on a vertical VAS. The EQ-VAS requires a participant to report how they are feeling on the day of questionnaire completion, by reporting a score between 0 and 100 (anchored at the respondent’s worst and best health they can imagine) and, as such, provides the participant’s own overall assessment of their health. This contrasts to the EQ-5D-3L or EQ-5D-5L utility scores, which are based on public preference valuation of EQ-5D health states.
The wording of the RUQ asked participants to report their resource use over the last 3 months or since the questionnaire was last completed (approximately 3 months). In the base case it was assumed that participants consistently reported resources use over the last 3 months. An alternative approach assumed that participants reported resource use since they last completed a questionnaire, which may exceed 3 months in cases where observations were missed. This reduced the extent of missing data in assuming that later observations include resource use that cover previous missing observations. Further analyses included evaluating the cost-effectiveness for costs related to only sciatica, as well as scenarios to account for uncertainty in dosing regimens for ‘when-needed’ medication.
A number of alternative costing approaches for societal costs associated with productivity losses were also conducted. To account for missing values in patient questionnaires relating to costs of time off work, but when the number of days off work were reported, a substitute value of a day off work was applied using ONS median gross weekly pay by sex and age for full-time employees55 and converted to daily net take-home pay. For carers, for whom age and sex were unknown, the cost of missing a day’s work was based on the median full-time gross weekly earnings by place of work, assuming that this was in the same local authority district as the hospital centre where the participant’s sciatica treatment was undertaken. 56 To supplement the RUQ data, an alternative scenario was conducted whereby missing patient questionnaires were supplemented, if feasible, with employment status as reported in the CRF. In this scenario, all patients with reported days off work but no costs were costed using ONS median wage by age and sex. Finally, a further SA accounted for implausible reported lost productivity cost values, by ignoring all participant-reported productivity losses costs and recosting based on number of reported days off work alone, using the median ONS median wage by age and sex as the cost basis for participants and median wage by postcode for carers.
Results
Data completeness
Resource use and EQ-5D-5L questionnaires were not completed by all participants. Missing data were observed when whole questionnaires were not returned or when questionnaires were returned with some questions incomplete and were therefore non-evaluable. Completion rates were highest at baseline, treatment and week 18 visits, but high levels of missingness were observed for the 30- and 42-week postal questionnaires (Table 28).
| Item | Time point, number (%) of missing observations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Treatment | Week 18 | Week 30 | Week 42 | Week 54 | |||||||
| Surgery | TFESI | Surgery | TFESI | Surgery | TFESI | Surgery | TFESI | Surgery | TFESI | Surgery | TFESI | |
| Utility scores | ||||||||||||
| EQ-5D-5L | 4 (5) | 2 (2.6) | 12 (15) | 14 (18.2) | 29 (36.3) | 34 (44.2) | 29 (36.3) | 33 (42.9) | 16 (20) | 20 (26) | ||
| Resource usea | ||||||||||||
| GP | 16 (20) | 14 (18.2) | 15 (18.8) | 10 (13) | 18 (22.5) | 20 (26) | 33 (41.2) | 38 (49.4) | 35 (43.8) | 41 (53.2) | 25 (31.2) | 26 (33.8) |
| Physiotherapy | 3 (3.8) | 3 (3.9) | 12 (15) | 7 (9.1) | 11 (13.8) | 11 (14.3) | 29 (36.2) | 34 (44.2) | 30 (37.5) | 33 (42.9) | 17 (21.2) | 18 (23.4) |
| A&E | 4 (5) | 4 (5.2) | 14 (17.5) | 7 (9.1) | 12 (15) | 12 (15.6) | 29 (36.2) | 33 (42.9) | 29 (36.2) | 33 (42.9) | 18 (22.5) | 18 (23.4) |
| OTC medication | 3 (3.8) | 3 (3.9) | 13 (16.2) | 7 (9.1) | 12 (15) | 11 (14.3) | 30 (37.5) | 33 (42.9) | 31 (38.8) | 32 (41.6) | 17 (21.2) | 19 (24.7) |
| Public transport | 4 (5) | 3 (3.9) | 12 (15) | 7 (9.1) | 11 (13.8) | 12 (15.6) | 29 (36.2) | 33 (42.9) | 29 (36.2) | 34 (44.2) | 16 (20) | 18 (23.4) |
| Private car | 4 (5) | 3 (3.9) | 12 (15) | 8 (10.4) | 12 (15) | 12 (15.6) | 30 (37.5) | 35 (45.5) | 29 (36.2) | 35 (45.5) | 18 (22.5) | 19 (24.7) |
| Lost productivity: participant | 4 (5) | 3 (3.9) | 12 (15) | 7 (9.1) | 11 (13.8) | 12 (15.6) | 30 (37.5) | 34 (44.2) | 30 (37.5) | 32 (41.6) | 16 (20) | 18 (23.4) |
| Lost productivity: carer | 3 (3.8) | 2 (2.6) | 12 (15) | 7 (9.1) | 11 (13.8) | 11 (14.3) | 29 (36.2) | 34 (44.2) | 29 (36.2) | 32 (41.6) | 16 (20) | 18 (23.4) |
Resource use and cost analysis
Observed participant use of health-care resources for all randomised patients (n = 163) and corresponding NHS HES admitted patient care and outpatient data [available only for patients who did not withdraw from the study (n = 157)] were comparable at baseline in both intervention groups for the 3 months prior to randomisation (Table 29). The main cost drivers for the pre-baseline period were related to admitted patient care (£353 for microdiscectomy vs. £422 for TFESI), outpatient visits (£208 for microdiscectomy vs. £251 for TFESI) and lost productivity (£809 for microdiscectomy vs. £614 for TFESI), accounting for 22%, 13% and 41% of all costs, respectively.
| Item | Treatment allocation, mean cost (£) (95% CI)a | Difference in mean cost (£) (95% CI) | ||||
|---|---|---|---|---|---|---|
| Microdiscectomy | TFESI | |||||
| Sciatica-related | Total | Sciatica-related | Total | Sciatica-related | Total | |
| Total NHS and societal | 1707 (1246 to 2299) | 1790 (1326 to 2358) | 1596 (1192 to 2015) | 1684 (1280 to 2155) | 111 (–583 to 795) | 106 (–609 to 783) |
| NHS | 807 (602 to 1032) | 882 (673 to 1135) | 866 (610 to 1174) | 950 (677 to 1291) | –59 (–410 to 315) | –68 (–422 to 334) |
| Admitted patient care | 327 (141 to 525) | 353 (171 to 552) | 384 (159 to 660) | 422 (182 to 699) | –56 (–357 to 282) | –69 (–376 to 276) |
| Outpatient | 185 (150 to 221) | 208 (169 to 252) | 231 (172 to 311) | 251 (187 to 330) | –47 (–127 to 42) | –43 (–125 to 51) |
| Concomitant medications | 51 (38 to 69) | 63 (48 to 83) | 34 (24 to 47) | 45 (32 to 60) | 17 (–3 to 36) | 19 (–4 to 41) |
| GP | 152 (125 to 180) | 169 (140 to 202) | 142 (108 to 183) | 153 (118 to 195) | 10 (–35 to 57) | 16 (–31 to 64) |
| Physiotherapy | 59 (37 to 84) | 59 (37 to 84) | 52 (24 to 84) | 52 (26 to 84) | 7 (–30 to 47) | 7 (–30 to 46) |
| A&E | 89 (59 to 124) | 92 (62 to 125) | 80 (57 to 108) | 89 (59 to 122) | 9 (–33 to 51) | 4 (–40 to 50) |
| Societal | 900 (495 to 1423) | 907 (497 to 1427) | 730 (473 to 1031) | 734 (480 to 1051) | 170 (–433 to 699) | 174 (–433 to 697) |
| Physiotherapy and other health-care professionals visits | 69 (35 to 112) | 69 (35 to 111) | 93 (53 to 140) | 93 (55 to 134) | –24 (–81 to 31) | –24 (–82 to 32) |
| OTC medication | 28 (19 to 37) | 28 (20 to 38) | 21 (13 to 31) | 22 (13 to 31) | 7 (–6 to 19) | 6 (–6 to 19) |
| Lost productivity: participant and carer | 804 (398 to 1363) | 809 (392 to 1388) | 611 (353 to 895) | 614 (349 to 913) | 193 (–407 to 718) | 196 (–401 to 711) |
| Medical appointment transport | 45 (24 to 76) | 46 (25 to 75) | 34 (24 to 47) | 35 (24 to 49) | 10 (–23 to 35) | 11 (–23 to 37) |
Tables 30 and 31 present the observed disaggregated health-care resource use and costs, respectively, over the 54-week trial period. HRG code HC64C was the most frequent intervention observed in the microdiscectomy group, with a reported occurrence of 66.2 per 100 participant-years, whereas HRG code AB20Z was the predominant TFESI intervention, with a rate of occurrence of 86.3 per 100 participant-years. The observation in the TFESI group of an occurrence rate for HRG code HC64C of 28.8 per 100 participant-years reflects the trial protocol, which allowed for participants to receive additional treatments as necessary. Mean observed total NHS costs were higher for microdiscectomy (£6683, 95% CI £5632 to £8074) than for TFESI (£4422, 95% CI £3682 to £5291; difference in mean £2261, 95% CI £706 to £3589), but no difference was observed in participant out-of-pockets costs for microdiscectomy (£878, 95% CI £538 to £1204) compared with TFESI (£1307, 95% CI £708 to £2092). The main cost difference between groups related to admitted patient care, with microdiscectomy costing £1926 (95% CI £467 to £3128) more than TFESI. The cost of outpatient attendances also differed between groups, with costs for microdiscectomy being £237 (95% CI £50 to £414) greater than TFESI. Total combined NHS and societal costs were higher for the microdiscectomy group (difference in mean £1832, 95% CI £53 to £3555).
| Health-care resource usea | Occurrences per 100 participant-years | Difference (95% CI) | |
|---|---|---|---|
| Microdiscectomy | TFESI | ||
| Admitted patient care HRG | |||
| HC64C, Intermediate Extradural Spinal Procedures with CC Score 0–1 | 66.2 | 28.8 | 37.4 (17.8 to 57.1) |
| HC64B, Intermediate Extradural Spinal Procedures with CC Score 2–3 | ▃ | ▃ | –2.6 (–7.5 to –0.1) |
| HC63C, Major Extradural Spinal Procedures with CC Score 0–1 | ▃ | ▃ | 9.4 (2.0 to 19.2) |
| AB20Z, Epidural Under Image Control for Pain Management | 9.6 | 86.3 | –76.7 (–91.4 to –64.4) |
| HC27N, Degenerative Spinal Conditions without Interventions, with CC Score 0–2 | ▃ | ▃ | –3.8 (–8.7 to –3.8) |
| AA26H, Muscular, Balance, Cranial or Peripheral Nerve Disorders, Epilepsy or Head Injury, with CC Score 0–2 | ▃ | ▃ | –7.6 (–12.5 to –7.6) |
| WH50B, Procedure Not Carried Out, for Other or Unspecified Reasons | ▃ | ▃ | 4.8 (4.8 to 9.7) |
| Outpatient HRG | |||
| Neurosurgery | 306.9 | 200.1 | 107 (60 to 153) |
| Physiotherapy | 158.9 | 53.8 | 105 (78 to 132) |
| Anaesthetics | 110.7 | 147.6 | –37 (–71 to –2) |
| Pain management | 45.7 | 68.8 | –23 (–45 to –1) |
| Spinal surgery service | 60.2 | 56.3 | 4 (–21 to 26) |
| GP and health-care professional visits | |||
| GP | 296.1 | 356.4 | –60.3 (–242.9 to 121.9) |
| Physiotherapy | 72.2 | 91.3 | –19.1 (–104.9 to 71.2) |
| Acupuncture | 16.9 | 6.3 | 10.6 (–17.1 to 31.2) |
| Chiropractor/osteopath | 4.8 | 2.5 | 2.3 (–6.2 to 8.5) |
| Other | 32.5 | 16.3 | 16.2 (–25.3 to 50.4) |
| A&E | 19.3 | 26.3 | –7.0 (–26.8 to 12.9) |
| Lost productivity | |||
| Days off work: participant | 1999 | 2146 | –147 (–1615 to 1353) |
| Days off work: carer | 229 | 144 | 84 (–126 to 265) |
| Itema | Treatment allocation, mean cost (£) (95% CI) | Difference in mean cost (£) (95% CI) | ||||
|---|---|---|---|---|---|---|
| Microdiscectomy | TFESI | |||||
| Sciatica | Total | Sciatica | Total | Sciatica | Total | |
| Total NHS and societal | 6522 (5839 to 7292) | 7561 (6465 to 8985) | 5171 (4193 to 6213) | 5729 (4596 to 7006) | 1351 (127 to 2573) | 1832 (53 to 3555) |
| NHS | 5780 (5191 to 6420) | 6683 (5632 to 8074) | 4150 (3440 to 4928) | 4422 (3682 to 5291) | 1630 (674 to 2580) | 2261 (706 to 3589) |
| Admitted patient care | 4523 (3975 to 5144) | 5168 (4271 to 6475) | 3110 (2452 to 3731) | 3242 (2617 to 3924) | 1413 (560 to 2275) | 1926 (467 to 3128) |
| Outpatient | 1066 (953 to 1181) | 1186 (1045 to 1327) | 885 (792 to 977) | 949 (842 to 1066) | 181 (28 to 325) | 237 (50 to 414) |
| Concomitant medications | 155 (107 to 219) | 262 (168 to 385) | 126 (80 to 181) | 183 (125 to 252) | 29 (–49 to 105) | 78 (–62 to 199) |
| GP | 44 (23 to 72) | 93 (52 to 137) | 49 (18 to 90) | 103 (56 to 166) | –5 (–45 to 39) | –10 (–77 to 62) |
| Physiotherapy | 38 (3 to 84) | 38 (3 to 88) | 18 (0 to 44) | 18 (0 to 44) | 19 (–37 to 62) | 19 (–35 to 62) |
| A&E | 15 (0 to 40) | 50 (10 to 100) | 45 (13 to 90) | 71 (26 to 128) | –30 (–74 to 20) | –20 (–94 to 50) |
| Societal | 742 (457 to 1124) | 878 (538 to 1204) | 1021 (516 to 1659) | 1307 (708 to 2092) | –279 (–884 to 407) | –429 (–1140 to 403) |
| Physiotherapy and other health-care professional visits | 10 (2 to 20) | 10 (2 to 20) | 24 (7 to 47) | 24 (7 to 47) | –14 (–34 to 10) | –14 (–34 to 10) |
| OTC medication | 27 (18 to 39) | 33 (22 to 45) | 20 (13 to 30) | 25 (16 to 35) | 7 (–7 to 21) | 8 (–7 to 23) |
| Lost productivity: participant and carer | 660 (373 to 1033) | 781 (455 to 1160) | 939 (476 to 1561) | 1128 (557 to 1850) | –279 (–887 to 425) | –347 (–1037 to 510) |
| Medical appointment transport | 45 (30 to 62) | 54 (39 to 72) | 38 (24 to 57) | 130 (29 to 319) | 7 (–16 to 31) | –76 (–184 to 123) |
Outcomes
Utility and quality-adjusted life-years
Observed participant responses by trial visit to each of the EQ-5D dimensions (i.e. anxiety and depression, mobility, pain and discomfort, self-care and usual activities) indicated less impairment (more lower scores) in the microdiscectomy group (Figures 10–14). Figure 15 presents the utility scores for the observed data using the three-level crosswalk valuation set. For participants with complete responses to the EQ-5D-5L at baseline and weeks 18 and 54, baseline mean utility values using the three-level value set were 0.328 (95% CI 0.259 to 0.392; n = 55) for the microdiscectomy group and 0.276 (95% CI 0.188 to 0.366; n = 48) for the TFESI group (Table 32), and were comparable between groups (difference in mean 0.052, 95% CI –0.060 to 0.157). Similarly, over the duration of the trial, comparable QALYs were observed in the complete-case groups (i.e. participants who completed the EQ-5D at baseline and weeks 18 and 54), with 0.654 QALYs in the microdiscectomy group and 0.591 QALYs in the TFESI group (difference in mean 0.062, 95% CI –0.033 to 0.155). The five-level value sets and VAS results exhibited a tendency to generate higher utility scores than the three-level value set, but showed minimal differences in QALY gains over the 54-week trial period, which were also not statistically different.
FIGURE 10.
Responses by EQ-5D dimension for depression/anxiety: observed data.
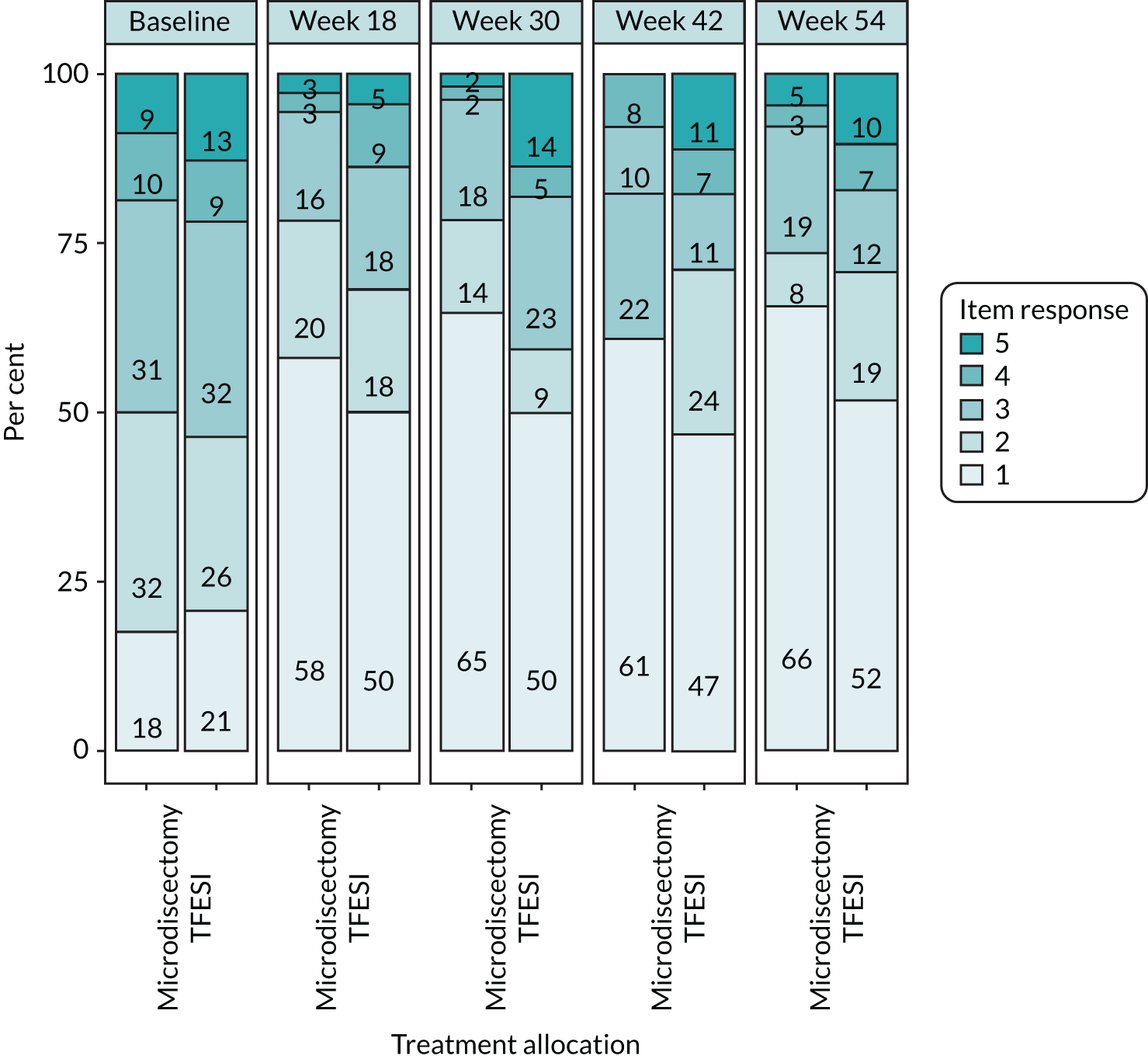
FIGURE 11.
Responses by EQ-5D dimension for mobility: observed data.
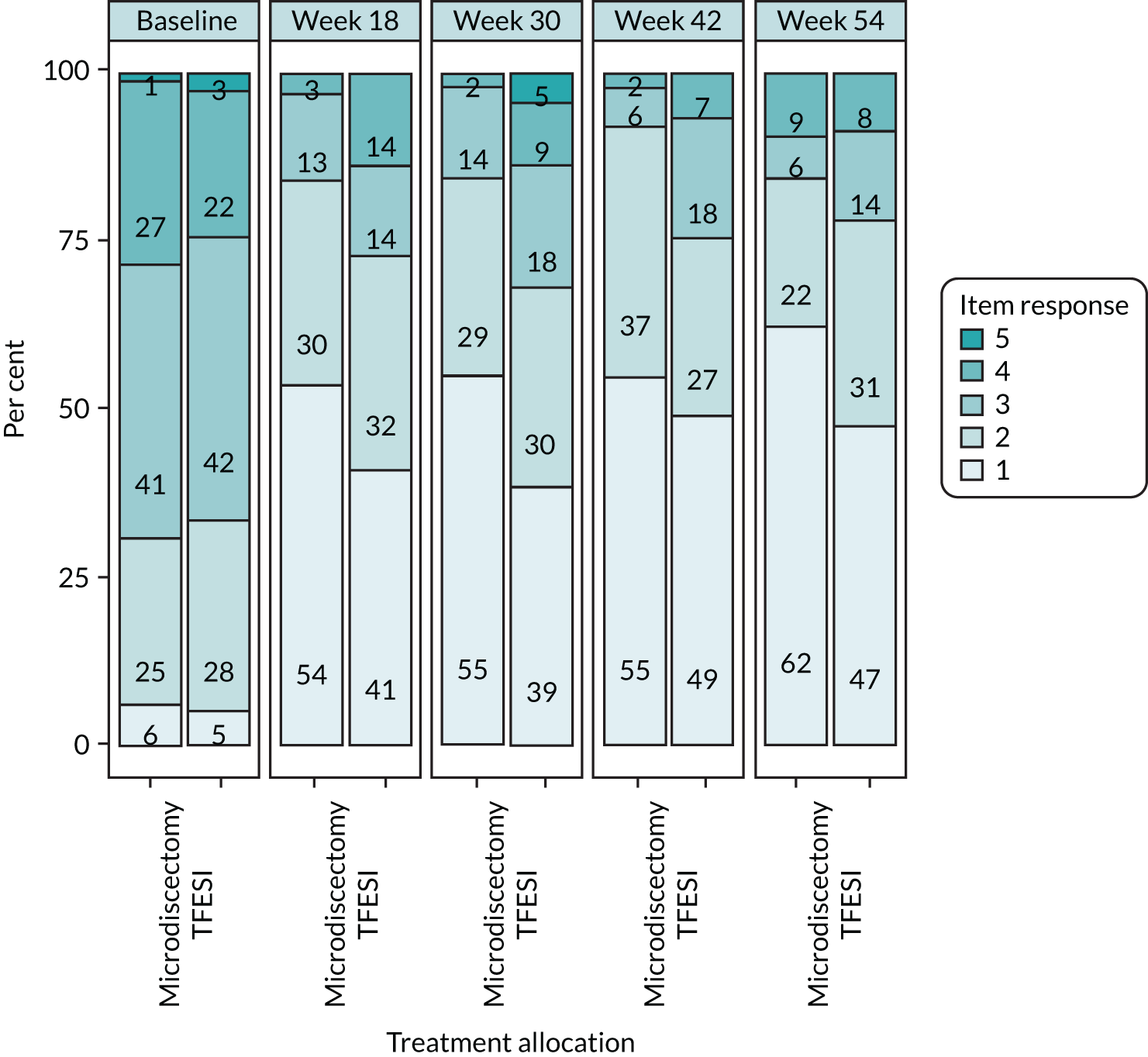
FIGURE 12.
Responses by EQ-5D dimension for pain/discomfort: observed data.
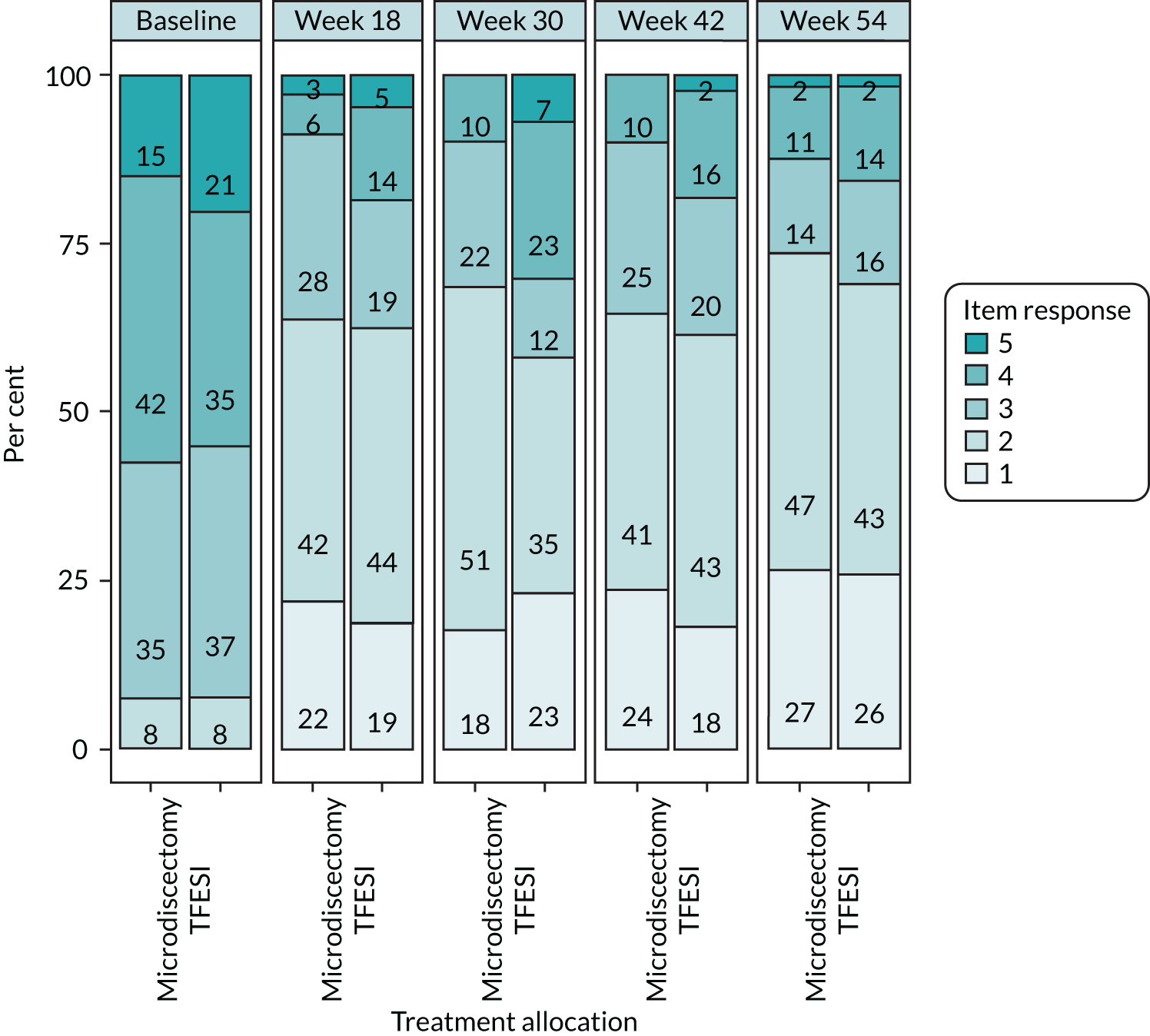
FIGURE 13.
Responses by EQ-5D dimension for self-care: observed data.
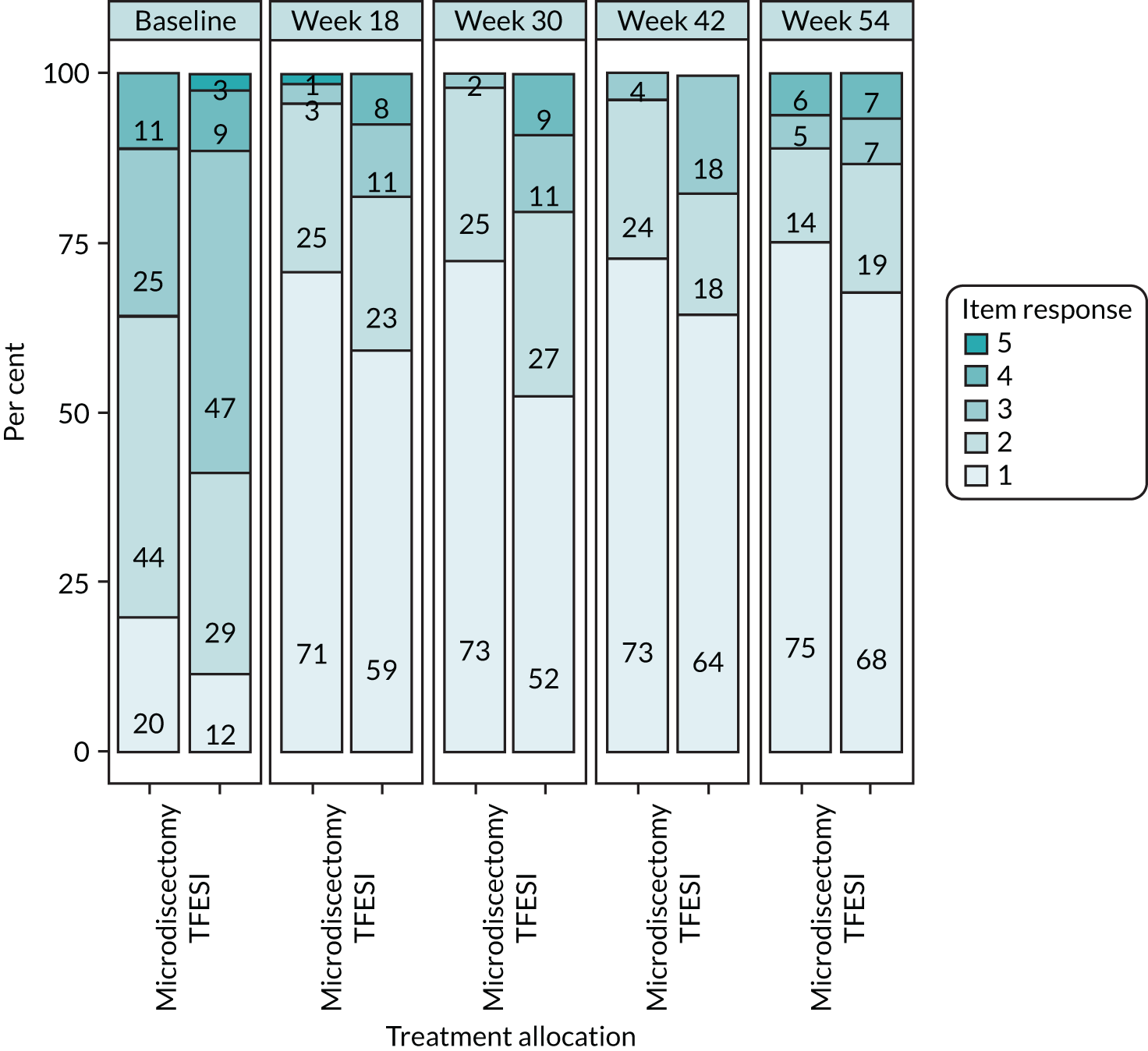
FIGURE 14.
Responses by EQ-5D dimension for usual activities: observed data.
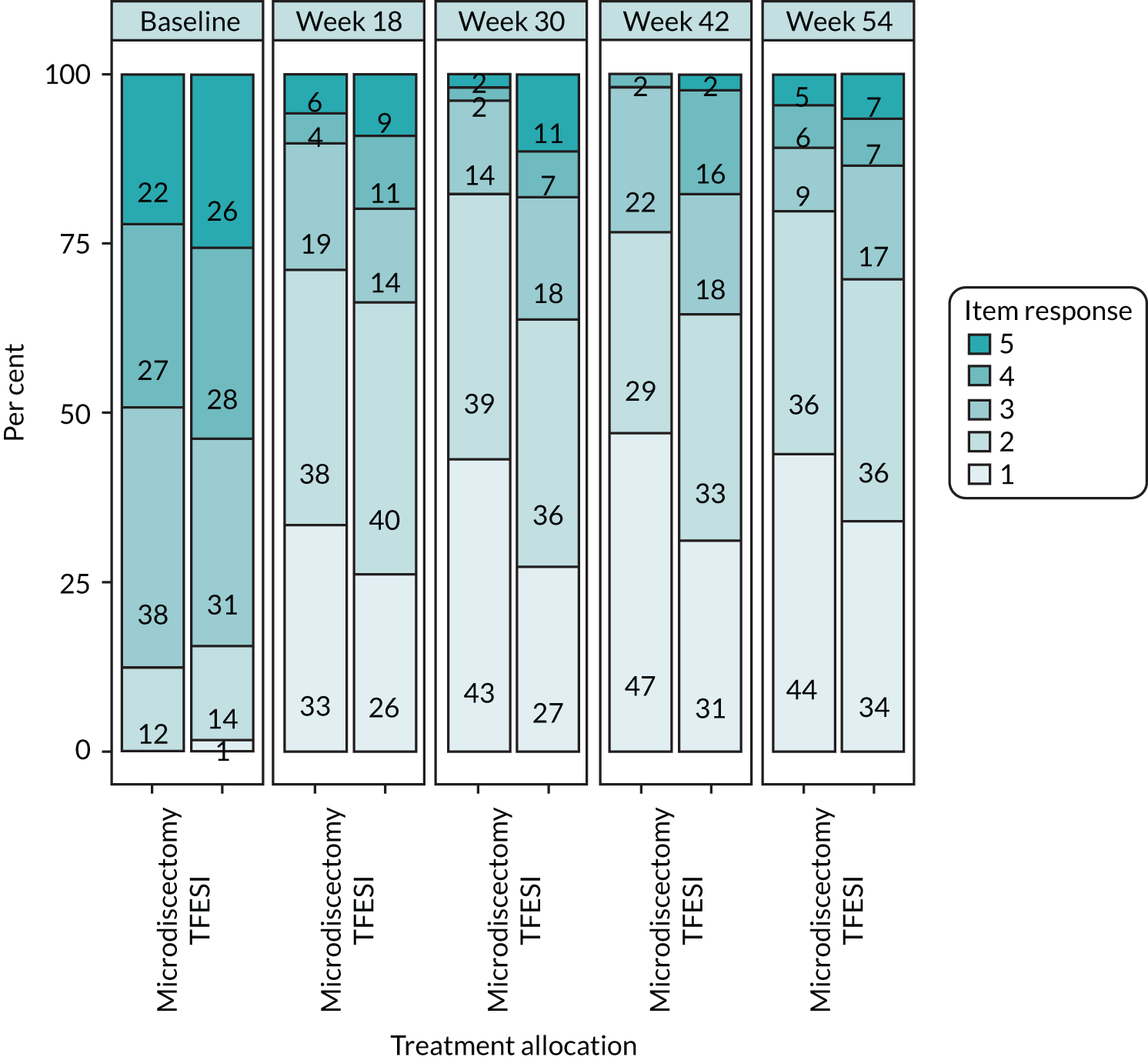
FIGURE 15.
Utility scores by visit, using three-level crosswalk value set: observed data.
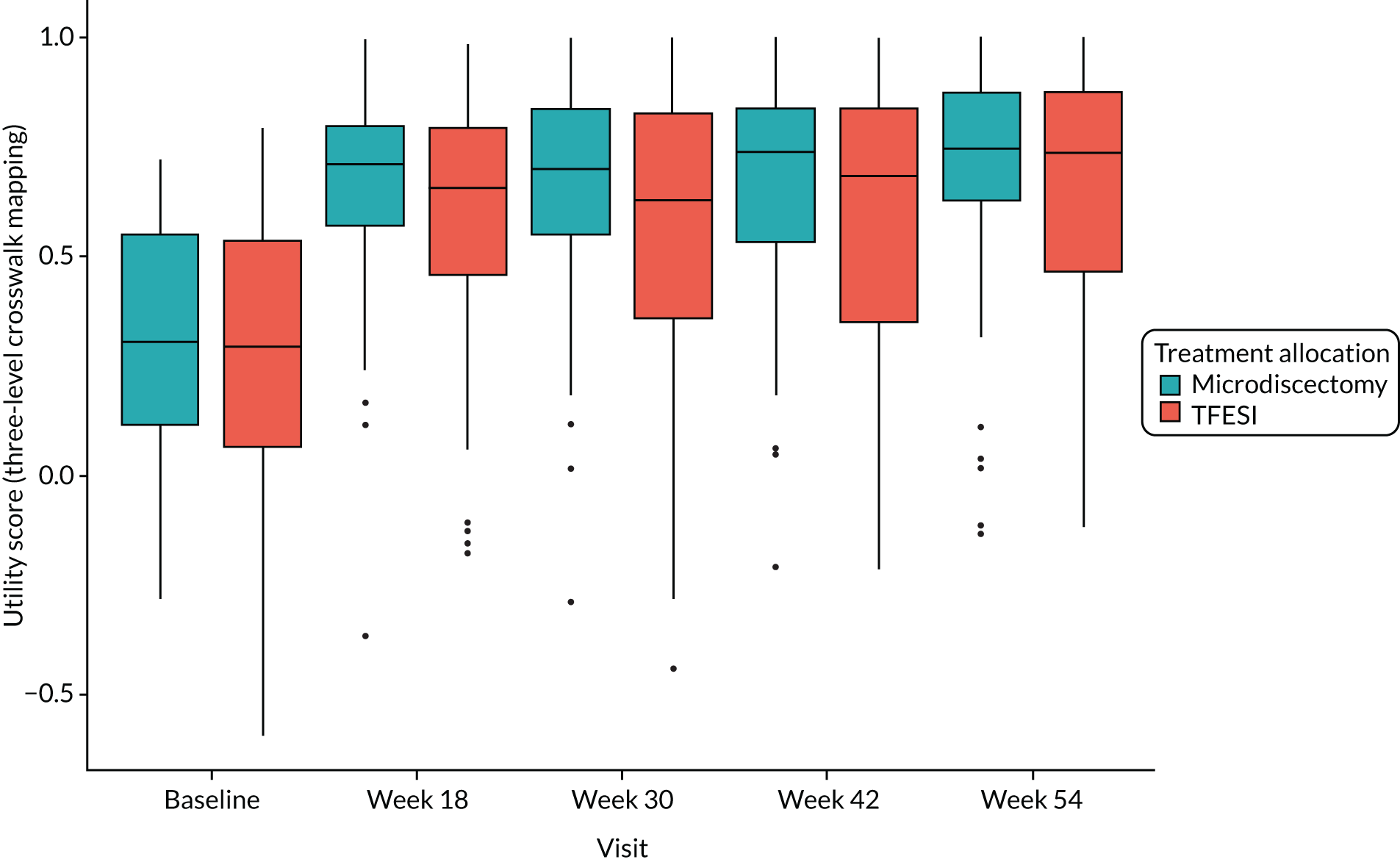
| Analysis | Health outcome | Microdiscectomy (n = 55), mean (95% CI) | TFESI (n = 48), mean (95% CI) | Difference in mean (95% CI) |
|---|---|---|---|---|
| EQ-5D-3L value set | Baseline utility | 0.328 (0.259 to 0.392) | 0.276 (0.188 to 0.366) | 0.052 (–0.060 to 0.157) |
| 54-week utility | 0.718 (0.649 to 0.784) | 0.659 (0.573 to 0.739) | 0.059 (–0.051 to 0.165) | |
| QALYs over 54 weeks | 0.654 (0.588 to 0.709) | 0.591 (0.518 to 0.658) | 0.062 (–0.033 to 0.155) | |
| EQ-5D-5L value set | Baseline utility | 0.443 (0.377 to 0.513) | 0.409 (0.322 to 0.486) | 0.034 (–0.071 to 0.137) |
| 54-week utility | 0.794 (0.728 to 0.851) | 0.737 (0.660 to 0.807) | 0.057 (–0.041 to 0.157) | |
| QALYs over 54 weeks | 0.746 (0.686 to 0.801) | 0.683 (0.614 to 0.746) | 0.063 (–0.024 to 0.148) | |
| Microdiscectomy (n = 60), mean (95% CI) | TFESI (n = 55), mean (95% CI) | |||
| EQ-VAS | Baseline utility | 0.490 (0.428 to 0.550) | 0.473 (0.414 to 0.529) | 0.017 (–0.068 to 0.099) |
| 54-week utility | 0.744 (0.682 to 0.801) | 0.677 (0.606 to 0.741) | 0.067 (–0.029 to 0.161) | |
| QALYs over 54 weeks | 0.706 (0.653 to 0.752) | 0.645 (0.593 to 0.692) | 0.061 (–0.011 to 0.130) |
Cost-effectiveness analysis results
Base-case analysis
The results following multiple imputation and statistical analysis, averaged over 10,000 bootstrap replicates and for the 54-week trial time horizon, reported mean total costs for the microdiscectomy group of £6919 (95% CI £5503 to £8046) and for the TFESI group of £4706 (95% CI £3821 to £5516). The mean total QALYs were 0.616 (95% CI 0.570 to 0.671) and 0.559 (95% CI 0.503 to 0.620) for the microdiscectomy and TFESI groups, respectively. The mean incremental costs and QALYs were £2212 (95% CI £629 to £3677) and 0.057 (95% CI –0.009 to 0.124), respectively, resulting in an ICER of £38,737 per QALY gained and, at a threshold of £20,000 per QALY, an INHB loss of 0.054 QALYs (Table 33). The cost-effectiveness plane (Figure 16) similarly indicated that the mean and the density of the joint distribution of incremental costs and QALYs was located in the north-east quadrant, indicating microdiscectomy to be more costly but with greater health benefits than TFESI. The CEAC (Figure 17) shows that the probability of microdiscectomy being cost-effective at £20,000 per QALY is 0.17 and the probability of microdiscectomy being cost-effective at a higher threshold of £30,000 per QALY is 0.37. These results are consistent with those produced in the deterministic analysis, which generated a mean incremental cost of £2240, a mean incremental QALY of 0.057 and an ICER of £39,334 per QALY gained (Table 34).
| Cost-effectiveness | Mean values (95% CI) | Cost-effectiveness threshold (95% CI) | |||
|---|---|---|---|---|---|
| Microdiscectomy | TFESI | Incremental effect | £20,000/QALY | £30,000/QALY | |
| Cost (£) | 6919 (5503 to 8046) | 4706 (3821 to 5516) | 2212 (629 to 3677) | ||
| QALY | 0.616 (0.570 to 0.671) | 0.559 (0.503 to 0.620) | 0.057 (–0.009 to 0.124) | ||
| ICER | 38,737 | ||||
| INHB (QALYs) | –0.054 (–0.166 to 0.060) | –0.017 (–0.110 to 0.077) | |||
| Probability cost-effective | 0.17 | 0.37 | |||
FIGURE 16.
Cost-effectiveness plane: base-case analysis.
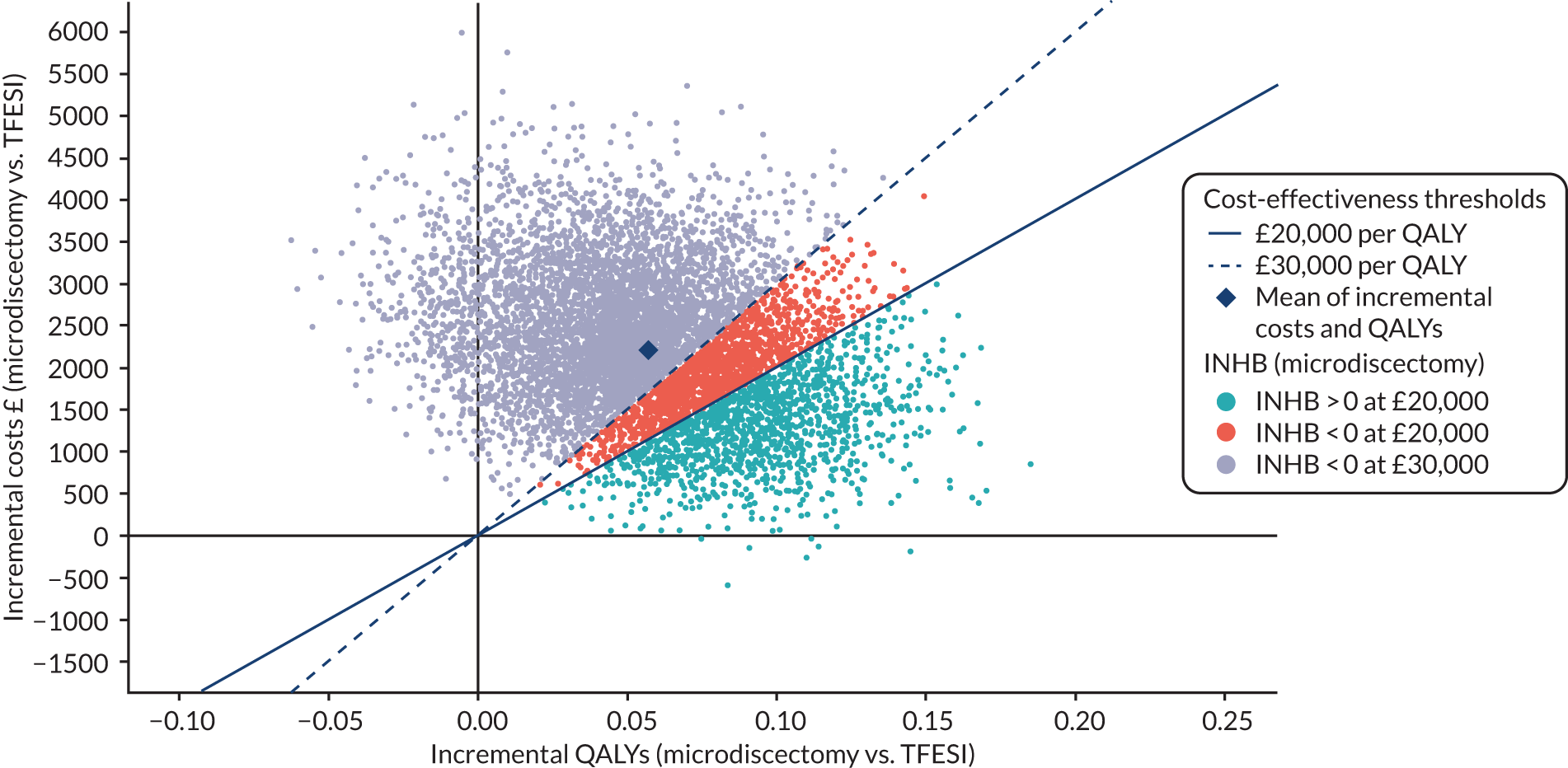
FIGURE 17.
Cost-effectiveness acceptability curve: base-case analysis. Vertical dotted lines represent the NICE threshold range of £20,000–30,000 per QALY. 42
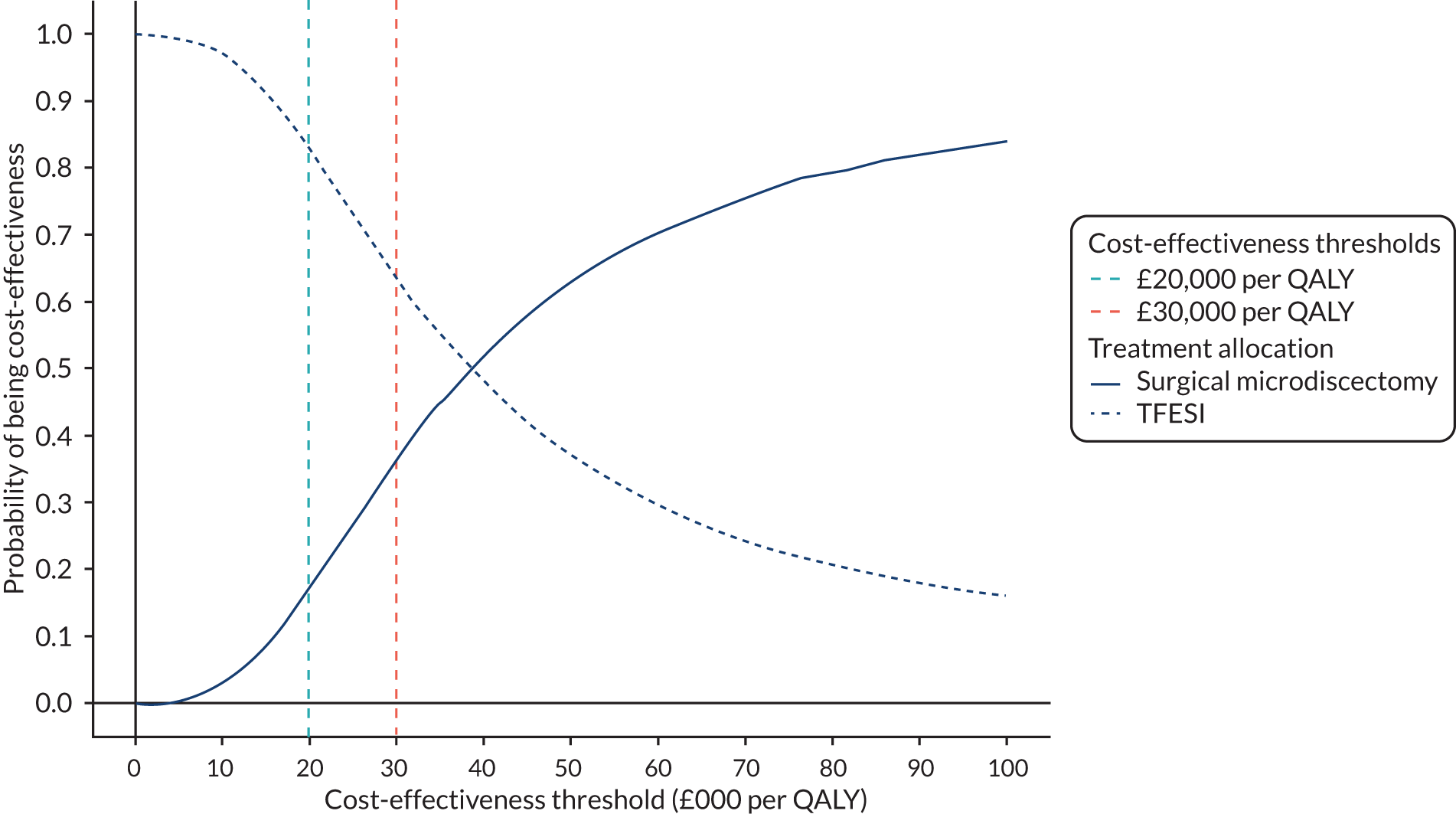
| Scenario | Treatment allocation, total cost (£) | Treatment allocation, total QALYs | Incremental | ICER (£/QALY) | INHB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Microdiscectomy | TFESI | Microdiscectomy | TFESI | Cost (£) | QALY | £20,000/QALY | £30,000/QALY | ||
| Base casea | 6919 | 4706 | 0.616 | 0.559 | 2212 | 0.057 | 38,737 | –0.054 | –0.017 |
| Base case, deterministic | 6941 | 4701 | 0.617 | 0.560 | 2240 | 0.057 | 39,344 | –0.055 | –0.018 |
| SA 1 | 8353 | 6856 | 0.611 | 0.554 | 1497 | 0.057 | 26,290 | –0.018 | 0.007 |
| SA 2 | 5816 | 3948 | 0.730 | 0.617 | 1868 | 0.113 | 16,512 | 0.020 | 0.051 |
| SA 3 | 6913 | 4689 | 0.709 | 0.653 | 2224 | 0.056 | 39,392 | –0.055 | –0.018 |
| SA 4 | 6925 | 4702 | 0.690 | 0.644 | 2222 | 0.046 | 48,113 | –0.065 | –0.028 |
| SA 5 | 6901 | 4669 | 0.615 | 0.553 | 2232 | 0.062 | 35,717 | –0.049 | –0.012 |
| SA 6 | 5920 | 4299 | 0.616 | 0.558 | 1620 | 0.057 | 28,251 | –0.024 | 0.003 |
| SA 7 | 6886 | 4693 | 0.610 | 0.557 | 2193 | 0.053 | 41,422 | –0.057 | –0.020 |
| SA 8 | 6935 | 4690 | 0.621 | 0.559 | 2245 | 0.062 | 36,163 | –0.050 | –0.013 |
| SA 9 | 7115 | 5824 | 0.608 | 0.551 | 1291 | 0.056 | 22,923 | –0.008 | 0.013 |
| SA 10 | 8566 | 6967 | 0.613 | 0.555 | 1600 | 0.057 | 27,981 | –0.023 | 0.004 |
| SA 11 | 8147 | 6363 | 0.613 | 0.559 | 1784 | 0.054 | 32,807 | –0.035 | –0.005 |
| SA 12 | 10,194 | 8239 | 0.608 | 0.555 | 1956 | 0.053 | 36,621 | –0.044 | –0.012 |
Scenario analyses
For all scenarios that utilised imputed data sets to address the missing data (i.e. all except the complete-case analysis, SA 2), none resulted in an ICER < £20,000 per QALY gained (see Table 34).
In scenarios in which only cost inputs differ, mean QALYs are also observed to differ slightly, relative to the base case. This can occur because the cost variables, among others, are used in the multiple imputation procedure to predict missing values in utility scores and, therefore, different costs can affect the value of imputed utilities. In the complete-case analysis (i.e. SA 2) an ICER of £16,512 per QALY gained was estimated, but there were only 35 participants providing complete data and, therefore, owing to this small sample of participants, these results should be interpreted with caution.
When the approximating to societal perspective was adopted (i.e. SA 1), mean total costs increased to £8353 for microdiscectomy and £6856 for TFESI, but the mean incremental cost difference reduced to £1497. As there was no difference in incremental QALYs between this scenario and the base case, including productivity losses lowers the ICER to £26,290 per QALY gained. Alternative costing approaches for productivity losses (i.e. SAs 10–12) had an impact on the results and effectively reduced the cost-effectiveness of microdiscectomy by increasing the ICER to £27,981, £32,807 and £36,621 per QALY gained, respectively.
Scenario analyses 3 and 4 considered the impact of applying alternative utility scoring methods (i.e. the EQ-5D-5L tariffs and EQ-VAS). Using the five-level valuation set had minimal effect, but applying the EQ-VAS scores increased the ICER to £48,113 per QALY gained. The EQ-VAS, however, is not generally used to inform decision-making.
Taking an alternative approach to costing resource use and assuming that respondents completed the questionnaire ‘since last completed’ (i.e. SA 5) reduced the ICER to £35,717 per QALY gained, whereas considering only sciatica-related costs (i.e. SA 6) had a larger effect, reducing the ICER to £28,251 per QALY gained. The effect of combining only sciatica-related costs in a wider perspective (i.e. SA 9) resulted in a lower ICER of £22,923 per QALY gained. Varying the dose of ‘when-needed’ medications to 25% and 75% of the time (i.e. SAs 7 and 8) resulted in ICERs of £41,422 and £36,163 per QALY gained, respectively.
Chapter 5 Discussion
Aim of the study
Guidelines exist and recommend several options of treatment for sciatica secondary to PID without recommending one form of treatment over another. ESIs have been reported to have poor efficacy for sciatica, with a high cost per QALY, and this has led to variations in commissioning of this treatment across the UK. TFESIs are recommended along with microdiscectomy for sciatica secondary to PID; however, with the exception of cohort studies, long-term evidence pertaining to safety and effectiveness of TFESI is lacking. Given that it is widely recognised that PIDs often resolve spontaneously within 12–24 months, patients may well need only a short-term solution for their radicular pain episode. We therefore presented, to the best of our knowledge, the first RCT comparing two recommended treatment options for sciatica secondary to PID (i.e. microdiscectomy and TFESI) and conducted a health economic evaluation of the two treatments.
Clinical effectiveness
There was no significant difference observed between the two treatments at the primary outcome time point of 18 weeks post randomisation. A total of 73.8% of surgical participants and 68.3% of TFESI participants achieved the MCID (i.e. a 10-point reduction in ODQ) at 18 weeks. Overall, ODQ scores from baseline in 86.9% of surgical participants compared with 90.5% of TFESI-treated patients. Microdiscectomy resulted in an average improvement in ODQ of < 5 points compared with TFESI, which is less than the MCID in ODQ. This level of benefit was sustained at longer follow-up time points.
Although point estimates suggested that microdiscectomy produced a greater reduction in scores for VAS leg and back pain, MRM and COMI, there was no significant difference between the two treatments and CIs did not include the MCIDs (where known). For VAS leg pain, TFESI resulted in a > 40-point reduction from baseline score compared with a > 50-point reduction following microdiscectomy. Similar trends were found for VAS back pain, MRM and COMI scores, with improvements from baseline after both treatments, but no significant differences between the treatments. There was an observed difference between the level of satisfaction for the two treatments, but both were deemed to be satisfactory among patients.
Cost-effectiveness
The economic evaluation indicated that the ICER for microdiscectomy, in comparison with TFESI, exceeds the threshold range usually implemented in the NHS. Standard NICE decision-making applies a ceiling ratio of £20,000 per QALY, but allows for a higher threshold of up to £30,000 per QALY when certain criteria are met, including whether or not treatments are life-extending or innovative and if QoL or broader societal issues may not be captured adequately in the QALY. However, it is unlikely, given the nature of sciatica, that microdiscectomy and TFESI would qualify for appraisal with reference to the higher threshold, and, therefore, a threshold of £20,000 per QALY was assumed. The results indicate that, although microdiscectomy generates greater health benefits than TFESI, it is more costly and, with a cost-effectiveness ratio of £38,737 per QALY gained, it would not be considered a cost-effective option for the treatment of persistent radicular pain secondary to PID herniation. This result is consistent under alternative assumptions and methods considered in the SAs. The secondary analysis, which considered an approximating to societal cost perspective, demonstrated an improvement in the cost-effectiveness of microdiscectomy, although this was sensitive to the costing method applied.
Strengths and limitations
Design
To the best of our knowledge, this study is the first to directly compare two nationally recommended treatments for persistent sciatica secondary to PID. Previous studies comparing surgery with conservative care have included multiple treatment options, including physiotherapy, ESI and pharmacological treatments, and have not been a direct comparison of two treatments. Hitherto, there is little evidence supporting ESI for > 3–4 weeks after the onset of sciatica outside single cohort studies. The pragmatic nature of this study allowed local NHS treatment and probably contributed to successful recruitment and ‘buy-in’ for the trial by regional units. Eleven centres contributed to recruitment, and this improved the representation and validity of the results. Previously, the only comparable RCT comparing surgery with ESI had been a single-centre study14 of > 100 patients that utilised a posterior interlaminar injection route.
We wanted to exclude patients with a rapid positive natural history, and therefore the minimal length of symptoms was arbitrarily taken as 6 weeks, in keeping with the NICE back pain and radiculopathy guidelines recommending investigations after 6 weeks of symptoms. Initially, the authors wanted to minimise duration of radiculopathy up to 6 months in an attempt to coincide with statutory sick pay. However, this negatively affected recruitment rates because of long NHS waiting times, hence, this inclusion criterion was modified to allow recruitment of patients with up to 12 months of radicular symptoms. Post hoc analysis of interaction between duration of symptoms and treatment allocation indicated that duration of symptoms does not appear to be a treatment effect modifier.
The ODQ was chosen as the primary outcome measure because of its long history of clinical validation and in accordance with previous recommendations of core outcome sets. 36 Although this study was devised prior to a recent publication from Chiarotto et al. ,38 the core outcome sets recommended have been collected within the NERVES trial. For physical functioning, the ODQ or MRM is recommended, and we collected both. A numerical pain intensity score and health-related QoL score is also recommended, and we recorded VAS and EQ-5D-5L scores. Finally, recording mortality rates is recommended and, again, this was captured by our safety reporting and no participants died during the trial period. The COMI has the advantage of being easily tolerated by participants because of its small number of questions and so this was included in the NERVES trial. The COMI is gaining popularity across Europe and may allow wider comparison with large registry data sets of routinely collected outcomes for sciatica. This study found that there was no significant difference between microdiscectomy and TFESI for the primary outcome. Future studies may utilise a single physical function outcome domain, perhaps COMI, for participant co-operation.
The study suffered from missing data, and patients whose data were incomplete were excluded from the primary analysis. Participants with missing data were, however, incorporated into secondary analyses and sensitivity analyses of the primary outcome indicated robustness to the assumptions made about the missing data. The number of missing data may reflect the large numbers of questionnaires being presented to participants, but this was an accepted risk to capture as much clinical data as possible. Future studies could use fewer outcome measures, such as those recommended by Chiarotto et al. 38 (i.e. ODQ, VAS and EQ-5D).
Conduct
Overall, 163 participants consented to taking part in the study; however, 168 patients declined or were not asked, giving an overall consent rate of 49%. Some units declined to take part in the study, reflecting pre-existing clinical preference for one treatment over another, with some clinicians favouring surgery and others favouring TFESI. The difficulty of obtaining consent may reflect the stark difference between the two treatments. Of those participants who were asked to provide consent, the reasons given for not providing consent were related to treatment preference. The reasons provided were not wanting to be randomised (n = 60), not wanting surgery (n = 33 patients) and not wanting TFESI (n = 22 patients). This is typical of surgical trials, but may be considered slightly lower than expected, possibly because of the difference between the two treatments (i.e. one being a day-case procedure carried out under local anaesthetic and the other a surgical procedure carried out under general anaesthetic and necessitating an overnight or short hospitalisation). Given the current study findings, it would be difficult to justify ethically comparing the same two treatments in future studies because of the lack of clinical difference and the clear difference in safety profiles of the two.
Clinical effectiveness analysis
The trial found no evidence of a significant difference in ODQ scores between the two treatment groups at 18 weeks post randomisation. Furthermore, no difference in any of the other secondary outcome domains (i.e. COMI or MRM) was found.
Overall, 28 out of 80 patients (35%) in the TFESI group received microdiscectomy as an additional treatment and, therefore, TFESI was effective in avoiding microdiscectomy in 65% of participants randomised to TFESI. Of these patients, half received the additional treatment following the primary outcome assessment, thereby having only a minimal impact on interpretation/validity of the results. The authors feel that this level of additional treatment is an improvement compared with previous studies of microdiscectomy versus conservative care, in which the rate was > 40%. 11
One additional possibility regarding the interpretation of these data is that neither treatment was effective, given the lack of a third, control, group receiving no treatment. The TMG did not think that this was an ethics design option as all participants recruited had disabling sciatica (i.e. a baseline VAS score for leg pain of > 7). A recent paper by Bailey et al. 68 explored an almost identical cohort of participants, treating sciatica secondary to PID of between 4 and 12 months’ duration either conservatively or with surgery. The fact that we included only patients with symptoms of > 4 months’ duration was important, as previous studies have focused on patients with symptoms of typically < 3 months’ duration. 11,12,14,68 The majority of symptoms may well improve naturally in this time frame, but beyond 3 months symptoms are less likely to resolve quickly and it may take up to 12–24 months for natural improvement to manifest. The NERVES trial baseline data recorded an average duration of sciatica of > 4 months at recruitment and, therefore, a similarly matched group to the study by Bailey et al. 68 In the study by Bailey et al. ,68 the conservative treatment arm received medication and physiotherapy with the possibility of also receiving ESI, but it is not clear what proportion of participants received injections. Importantly, Bailey et al. 68 found a clear difference between surgery and conservative management, with surgery improving VAS scores for leg pain more effectively than conservative treatment. This is not the same result reported by our data. We speculate that a rigorously controlled injection group, as presented in this study, accounts for the difference in reported efficacy and therefore the benefit of TFESI is real. Furthermore, the precipitous drop in outcome scores post treatment seen in both groups does suggest a treatment effect. Finally, because the participants recruited had significant sciatica (i.e. a baseline VAS score for leg pain of > 7) with an average duration of > 4 months, we do not believe that spontaneous resolution of symptoms would have occurred as quickly as it did without treatment. For these reasons, we believe that both TFESI and surgery are effective treatment options for this group of patients.
Economic analysis
The economic analysis has strengths in that it utilised routine patient-level NHS data sets and nationally reported costs collected within a pragmatic RCT that is designed to reflect current management and NHS practice for the care of the trial population. Unlike previous economic analyses, the NERVES trial used patient-level data and, therefore, avoided the use of non-trial data and any subsequent bias that might be introduced as a consequence. Participant-reported health outcomes were measured at key time points using the EQ-5D-5L questionnaire, which offered greater sensitivity than the three-level version to assess the impact of treatment on each health domain. However, because of presently unresolved issues regarding the five-level value set for deriving health state utility scores, NICE currently recommends mapping from the five-level to a three-level value set, which was the approach applied in the base case. Alternative valuation sets, explored in a SA, demonstrated that the five-level valuation set had minimal effect on the ICER and would not affect decision-making. This is contrary to other reported findings comparing the five- and three-level value sets, which suggest that in most cases ICERs are substantially higher when the five-level value set is applied. 69 Deriving EQ-5D scores from the NERVES trial primary outcome measure, the ODQ, was not deemed feasible as no robust relationship exists between these measures. 70
There were also some weaknesses to the economic analysis. Given the pragmatic nature of the trial and reliance on participant-reported outcomes, it was inevitable that there would be a degree of missing data. In particular, completion rates were lower for the two data collections that were reliant on postal questionnaires (i.e. weeks 30 and 42). When possible, assumptions around resource use, costs and QoL were made to maximise the use of data acquired within the trial. When this was not possible, multiple imputation was used to impute missing values, conditional on observed data that were considered informative to the imputation. 71 Imputing missing data associated with health state utilities and resource use may act as an additional source of uncertainty. It is also acknowledged that the participant-completed RUQ may be subject to recall bias; however, patient questionnaires are an important supplement to clinical records and provide resource use and costing data that would otherwise not be readily available. The 3-month recall period used in the NERVES trial is representative of the median recall time frame employed in UK National Institute for Health Research (NIHR) HTA programme-funded trials. 72 A further limitation was to limit the time horizon of analysis to 54 weeks, consistent with the clinical trial follow-up period. No extrapolation or modelling was performed to include a longer-term assessment of costs and consequences. Given that recurrence rates of lower back pain after discectomy of 65% at 3 years have been reported,73 a longer analytical time horizon might be more informative, but liable to bias given the absence of longer-term follow-up of NERVES trial participants.
Our estimation of productivity losses was subject to both missing data and incomplete questionnaire reporting of time off work. To address the gaps in question completion, a number of assumptions were made and alternative scenarios were constructed, including replacing partially completed questions with missing costs based on ONS data on reported median salaries, as well as applying median salary data to account for questionable reported lost productivity costs. However, the wider perspective that approximated societal costs remains subject to bias, and limited in its robustness and generalisability, but nonetheless corresponds with findings from Koenig et al. 29 (i.e. the ICER is reduced when loss of productivity costs are taken into account).
Previous economic evaluations were found not to be generalisable to the present analysis because of differences in setting, perspective and interventions tested. Although, to the best of our knowledge, this is the first UK study to compare microdiscectomy with TFESI, Price et al. ,18 in a comparison of ESI with placebo, reported an ICER of £44,701 per QALY gained from a provider perspective, increasing to £354,171 per QALY gained from a purchaser perspective. However, it is not feasible to draw comparisons between this analysis and the present NERVES trial for a number of reasons. The WEST,18 conducted between 1999 and 2002, compared blind ESI (see Chapter 1, Scientific background), whereas the NERVES trial has used the transforaminal route, which delivers the injectate much nearer to the site of pathology (i.e. the prolapsed disc and involved nerve root).
In terms of costing, Price et al. 18 utilised a bottom-up approach based on unit costs from a single NHS trust. This differs from the approach undertaken in the NERVES trial, which follows the NICE reference case,42 which recommends a HRG costing approach and utilises NHS reference costs. 45 These costs represent national average unit costs from all NHS organisations in England. Moreover, Price et al. 18 considered only the direct medical costs that were associated with the epidural technique and its follow-up, whereas the greatest cost component – resource use relating to clinical staff time – was calculated from non-RCT-based survey data. Regarding health outcomes, Price et al. 18 derived utility scores from the Short Form questionnaire-36 items, which differs from the NICE-preferred EQ-5D, and QALYs gained were based on only completed 12-week data, despite follow-up extending to 52-weeks. Small incremental differences (< 0.006) in QALYs were observed between ESI and placebo over the 12 weeks, which have the effect of making the ICER highly unstable, as reflected in the wide variation in ICERs reported. In contrast to Price et al. 18 who use a placebo control, the NERVES trial was unable to estimate a value for cost-effectiveness of TFESI per se, as we did not feel ethically justified in including a ‘no-treatment’ group in this study. From our inclusion criteria, all participants had previously exhausted simple conservative care and required further treatment.
Safety
There was a clear difference in the safety profiles of the two treatment arms, with four SAEs in the surgical group and none in the TFESI group. Two patients who experienced a SAE had to be readmitted to hospital for surgical intervention for cerebrospinal fluid-related complications: one required repeat microdiscectomy for a recurrent disc and one required exploration microdiscectomy for an unexplained foot drop post procedure. AEs were deemed minor in the TFESI group. No participant in the TFESI group required admission to hospital during the 54-week follow-up period. The authors acknowledge that the follow-up time is unlikely to capture a late discitis in this group, but theoretically this risk should diminish over time.
Implications for practice and health care
To the best of our knowledge, the NERVES trial is the first RCT comparing surgical microdiscectomy with TFESI. We feel that there is good evidence to support the use of TFESI for patients with radiculopathy not improving with simple medical treatments. Further studies would assist in the establishment and safety of TFESI as the primary treatment of choice for sciatica secondary to PID. We feel that the streamlining of patients down a TFESI pathway (with careful safety of patient selection/triage) could increase the accessibility for patients requiring TFESI and could potentially reduce waiting times for treatment, given the larger availability of TFESI skill mix NHS personnel than surgical skill mix NHS personnel. We suggest that outside emergency cases (e.g. foot weakness/cauda equina syndrome), microdiscectomy should be reserved for patients who have either declined or failed to benefit from TFESI.
Conclusions
In summary, we found there to be no significant difference in clinical outcome following the two treatments: microdiscectomy and TFESI. The results indicate that microdiscectomy is not expected to be cost-effective when compared with TFESI at usual thresholds of cost-effectiveness operating in the UK.
Implications for future research
To the best of our knowledge, the NERVES trial is the first RCT comparing microdiscectomy with TFESI for sciatica secondary to PID. Moreover, it provides valid long-term outcomes for TFESI up to 54 weeks following the onset of radicular pain. Further studies could extend the period of follow-up to assess the outcome of patients beyond 54 weeks and to see the number of patients receiving microdiscectomy in addition to TFESI over a longer time scale. We would recommend further studies addressing the streamlining of services across the UK with the aim of creating a treatment pathway for sciatica secondary to PID. Perhaps the second step in the process following on from the NERVES trial is a health economic assessment of a sciatica treatment paradigm whereby patients are triaged into a TFESI treatment pathway and then followed up for a slightly longer time (e.g. 2 years). Participants could then be referred for surgical consideration only if symptoms did not improve substantially. This could allow an accurate and real assessment of cost-effectiveness for microdiscectomy for this failed TFESI group and would lend further support to the suspected safety of this paradigm.
No difference was found between any of the outcome tools employed (i.e. ODQ, COMI and MRM). The capture of several outcome domains will allow comparison of the current data with routinely collected large data sets or registries that regularly capture outcome data in the form of COMI or ODQ. Future sciatica studies could limit the number of physical functioning outcome domains employed to one of ODQ, COMI or MRM, based on Chiarotto et al. ’s38 recommendations, although capturing COMI in addition would incorporate Deyo et al. ’s36 recommendations and also allow comparison with European registry data. In addition, Short Form questionnaire-12 items may be used instead of EQ-5D-5L.
Given the clear difference in safety profiles between surgery and TFESI (in favour of TFESI) and with no clinical difference in outcome and no health economic benefit, it is difficult to imagine ethics committees agreeing to the same trial design again. The authors believe that there is no longer clinical equipoise between the two treatments. Future studies would likely be variations of newer TFESI injectate agents or cohort studies utilising health economic analyses.
Acknowledgements
We would like to thank the external members of the TSC for their advice and support on the project: Mr Phil Sell (TSC chairperson, Queen’s Medical Centre, Nottingham), Mr Ioannis Fouyas (Western General Hospital, Edinburgh), Professor Chris Rogers (University of Bristol, Bristol), Professor Turo Nurmikko (University of Liverpool, Liverpool), Mr Jake Timothy (Leeds General Infirmary, Leeds) and Mrs Sharon Morgan (PPI representative).
Our thanks go also to the IDSMC: Mr John O’Dowd (IDSMC chairperson, Harley Street Clinic, London), Dr Simon Thomson (Orsett Hospital, Essex) and Professor John Norrie (University of Edinburgh, Edinburgh; Professor John Norrie is a member of the NIHR HTA and Efficacy and Mechanism Evaluation Editorial Board).
Our thanks also go to the following TMG members: Gillian Hamblin (sponsor representative), Dave Watling (sponsor representative), Maria Thornton (sponsor representative), Paul Sacco (University of Liverpool, Liverpool), Hoo Kee Tsang (University of Aintree, Liverpool) and Stephen Lawlor (PPI representative).
We would like to thank the following LCTC staff: Carolyn Hopkins (trial co-ordinator), Kirsty Marrow (trial co-ordinator), Kelly Davies (trial co-ordinator assistant), Beth Conroy (trial statistician), Clare Jackson (senior data manager) and Sue Howlin (senior data manager).
We would also like to thank Dr Colin Ridyard and Yankier Pijeira Perez from Bangor University for assisting with the economic evaluation.
We are grateful to all the participants for their commitment to the trial. We would like to thank the principal investigators, research nurses and other members of the team who recruited patients and supported them during the trial: The Walton Centre, Liverpool – Simon Clark (principal investigator), Kate O’Hanlon, Keren Smallwood and Louise Fasting; James Cook University Hospital, Middlesbrough – Nitin Mukerji (principal investigator), Simon Tizzard and Emanuel Cirstea; Seacroft Hospital, Leeds – Ganesan Baranidharan (principal investigator), Simon Thomson, Paulito Castino, Tracey Crowther and Sena Desmennu; Solent MSK, Southampton – Cathy Price (principal investigator), Anna Thornhill and Colin Griffiths; Addenbrooke’s Hospital, Cambridge – Richard Mannion (principal investigator), Peter Hutchinson and Karen Caldwell; Salford Royal Hospital, Manchester – John Leach (principal investigator), Lisa Hankinson and Diane Daniel; Queen’s Medical Centre, Nottingham – Nasir Quraishi (principal investigator), Lovelyn Umeloh, James Hegarty, Jacob Szolin-Jones and Carolynn Wake; Royal Hallamshire Hospital, Sheffield – Yahia Al-Tamimi (principal investigator), Rose Clegg and Grace Cole; Royal Orthopaedic Hospital, Birmingham – Adrian Gardner (principal investigator) and Claudette Jones; Royal Stoke University Hospital, Stoke-on-Trent – Nikolaos Tzerakis (principal investigator) and Joanne Hiden; and Royal Preston Hospital, Preston – Aprajay Golash (principal investigator) and Janice Birt.
Contributions of authors
Martin J Wilby (https://orcid.org/0000-0001-6647-9040) (Chief Investigator and Consultant Spinal Neurosurgeon) developed the trial protocol in collaboration with co-investigators. He oversaw the delivery of the trial, assisted in preparing trial update reports, oversaw clinical aspects of the statistical analysis plan and clinical interpretation of the trial data. He led the preparation of the final report (drafting, reviewing and editing) and was chairperson of the TMG.
Ashley Best (https://orcid.org/0000-0002-7268-8735) (Trial Statistician) undertook the final statistical analysis under the supervision of Girvan Burnside, prepared data for reports, prepared data tables and figures for the final report, and co-led the preparation of the final report. He was a member of the TMG.
Eifiona Wood (https://orcid.org/0000-0002-2785-7325) (Trial Health Economist) undertook the economic analysis under the supervision of Dyfrig A Hughes, contributed to the final report (drafting, reviewing and editing) and was a member of the TMG.
Girvan Burnside (https://orcid.org/0000-0001-7398-1346) (Senior Statistician) contributed to protocol development and data capture methods, proposed statistical analysis methods and approved the statistical analysis plan, oversaw trial monitoring activities and co-led the preparation of the final report. He was also a member of the TMG.
Emma Bedson (https://orcid.org/0000-0001-5209-1854) (Senior Trials Manager) contributed to protocol development, gave guidance and support on all aspects of governance and trial delivery, and supported the preparation of progress reports. She contributed to the final report (drafting and reviewing) and was a member of the TMG.
Hannah Short (https://orcid.org/0000-0003-4215-9258) (Trial Co-ordinator) contributed to all aspects of governance, trial delivery and preparation of progress reports. She contributed to the final report (drafting and reviewing) and was a member of the TMG.
Dianne Wheatley (https://orcid.org/0000-0002-9230-8349) (Trial Data Manager) contributed to trial delivery and was a member of the TMG.
Daniel Hill-McManus (https://orcid.org/0000-0002-1936-3907) (Health Economist) contributed to the economic evaluation and the final report (drafting).
Manohar Sharma (https://orcid.org/0000-0002-0354-3145) (Co-investigator and Consultant in Pain Medicine) contributed to the development of the trial protocol. He contributed to trial delivery and the final report (reviewing and editing). He was also a member of the TMG.
Simon Clark (https://orcid.org/0000-0003-1225-2320) (Principal Investigator and Consultant Neurosurgeon) contributed to trial delivery and the writing of the final report (reviewing). He was also a member of the TMG.
Jennifer Bostock (https://orcid.org/0000-0001-9261-9350) (PPI Representative) contributed to the writing of the final report (reviewing) and was a member of the TMG.
Sally Hay (PPI Representative) contributed to the writing of the final report (reviewing) and was a member of the TMG.
Ganesan Baranidharan (https://orcid.org/0000-0002-1898-8243) (Consultant in Anaesthesia and Pain Medicine) contributed to trial design, trial delivery and the writing of the final report (reviewing).
Cathy Price (https://orcid.org/0000-0003-0111-9364) (Consultant in Pain Medicine) contributed to trial design, trial delivery and the writing of the final report (reviewing).
Richard Mannion (https://orcid.org/0000-0001-6010-6227) (Consultant Neurosurgeon) contributed to trial design, trial delivery and the writing of the final report (reviewing).
Peter J Hutchinson (https://orcid.org/0000-0002-2796-1835) (Co-applicant and Professor of Neurosurgery) contributed to trial design, trial delivery and the writing of the final report (reviewing).
Dyfrig A Hughes (https://orcid.org/0000-0001-8247-7459) (Co-applicant and Senior Health Economist) led the economic evaluation, contributed to development of the funding application, protocol development and data capture methods. He contributed to the final report (drafting, reviewing and editing) and was a member of the TMG.
Anthony Marson (https://orcid.org/0000-0002-6861-8806) (Co-applicant and Consultant Neurologist) contributed to the writing of the final report (reviewing) and was a member of the TMG.
Paula R Williamson (https://orcid.org/0000-0001-9802-6636) [Co-applicant, Clinical Trials Research Centre (now LCTC) Director (from 1 April 2005 to 31 December 2018) and Professor of Medical Statistics] contributed to trial design, development of the funding application, trial protocol, data capture methods and statistical analysis, in collaboration with co-investigators. She contributed to the preparation of the final report (reviewing and editing) and was a member of the TMG.
Publications
Wilby MJ, Hopkins C, Bedson E, Howlin S, Burnside G, Conroy EJ, et al. NErve Root Block VErsus Surgery (NERVES) for the treatment of radicular pain secondary to a prolapsed intervertebral disc herniation: study protocol for a multi-centre randomised controlled trial. Trials 2018;19:475.
Wilby MJ, Best A, Wood E, Burnside G, Bedson E, Short H, et al. Surgical microdiscectomy versus transforaminal epidural steroid injection in patients with sciatica secondary to herniated lumbar disc (NERVES): a phase 3, multicentre, open-label, randomised controlled trial and economic evaluation [published online ahead of print March 18 2021]. Lancet Rheumatol 2021.
Data-sharing statement
All requests for data should be sent to the corresponding author. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- de Campos TF. Low back pain and sciatica in over 16s: assessment and management NICE Guideline [NG59]. J Physiother 2017;63. https://doi.org/10.1016/j.jphys.2017.02.012.
- Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine 2008;33:2464-72. https://doi.org/10.1097/BRS.0b013e318183a4a2.
- Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine 2006;31:2409-14. https://doi.org/10.1097/01.brs.0000239178.08796.52.
- Rados I, Sakic K, Fingler M, Kapural L. Efficacy of interlaminar vs transforaminal epidural steroid injection for the treatment of chronic unilateral radicular pain: prospective, randomized study. Pain Med 2011;12:1316-21. https://doi.org/10.1111/j.1526-4637.2011.01213.x.
- Jensen RK, Kongsted A, Kjaer P, Koes B. Diagnosis and treatment of sciatica. BMJ 2019;367. https://doi.org/10.1136/bmj.l6273.
- Stochkendahl MJ, Kjaer P, Hartvigsen J, Kongsted A, Aaboe J, Andersen M, et al. National clinical guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J 2018;27:60-75. https://doi.org/10.1007/s00586-017-5099-2.
- Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 2007;356:2245-56. https://doi.org/10.1056/NEJMoa064039.
- NHS Digital . Data Access Environment (DAE) n.d. https://digital.nhs.uk/services/data-access-environment-dae (accessed September 2020).
- Taylor VM, Deyo RA, Cherkin DC, Kreuter W. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976 1994;19:1207-12. https://doi.org/10.1097/00007632-199405310-00002.
- Koebbe CJ, Maroon JC, Abla A, El-Kadi H, Bost J. Lumbar microdiscectomy: a historical perspective and current technical considerations. Neurosurg Focus 2002;13. https://doi.org/10.3171/foc.2002.13.2.4.
- Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33:2789-800. https://doi.org/10.1097/BRS.0b013e31818ed8f4.
- Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine 1996;21:1777-86. https://doi.org/10.1097/00007632-199608010-00011.
- Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW. Leiden-The Hague Spine Intervention Prognostic Study Group . Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ 2008;336:1355-8. https://doi.org/10.1136/bmj.a143.
- Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg Am 2004;86:670-9. https://doi.org/10.2106/00004623-200404000-00002.
- Lewis R, Williams N, Matar HE, Din N, Fitzsimmons D, Phillips C, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15390.
- Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: a comprehensive, evidence-based review. Reg Anesth Pain Med 2013;38:175-200. https://doi.org/10.1097/AAP.0b013e31828ea086.
- Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil 1998;79:1362-6. https://doi.org/10.1016/S0003-9993(98)90228-3.
- Price C, Arden N, Coglan L, Rogers P. Cost-effectiveness and safety of epidural steroids in the management of sciatica. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9330.
- Pinto RZ, Maher CG, Ferreira ML, Hancock M, Oliveira VC, McLachlan AJ, et al. Epidural corticosteroid injections in the management of sciatica: a systematic review and meta-analysis. Ann Intern Med 2012;157:865-77. https://doi.org/10.7326/0003-4819-157-12-201212180-00564.
- Ghahreman A, Bogduk N. Predictors of a favorable response to transforaminal injection of steroids in patients with lumbar radicular pain due to disc herniation. Pain Med 2011;12:871-9. https://doi.org/10.1111/j.1526-4637.2011.01116.x.
- Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med 2010;11:1149-68. https://doi.org/10.1111/j.1526-4637.2010.00908.x.
- El-Yahchouchi CA, Plastaras CT, Maus TP, Carr CM, McCormick ZL, Geske JR, et al. Adverse event rates associated with transforaminal and interlaminar epidural steroid injections: a multi-institutional study. Pain Med 2016;17:239-49. https://doi.org/10.1111/pme.12896.
- Wei G, Liang J, Chen B, Zhou C, Ru N, Chen J, et al. Comparison of transforaminal verse interlaminar epidural steroid injection in low back pain with lumbosacral radicular pain: a meta-analysis of the literature. Int Orthop 2016;40:2533-45. https://doi.org/10.1007/s00264-016-3220-5.
- Jeong HS, Lee JW, Kim SH, Myung JS, Kim JH, Kang HS. Effectiveness of transforaminal epidural steroid injection by using a preganglionic approach: a prospective randomized controlled study 1. Radiology 2007;245:584-90. https://doi.org/10.1148/radiol.2452062007.
- Hall JA, Konstantinou K, Lewis M, Oppong R, Ogollah R, Jowett S. Systematic review of decision analytic modelling in economic evaluations of low back pain and sciatica. Appl Health Econ Health Policy 2019;17:467-91. https://doi.org/10.1007/s40258-019-00471-w.
- Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: conservative care, laminectomy, and the superion interspinous spacer. Int J Spine Surg 2015;9. https://doi.org/10.14444/2028.
- Skidmore G, Ackerman SJ, Bergin C, Ross D, Butler J, Suthar M, et al. Cost-effectiveness of the X-STOP® interspinous spacer for lumbar spinal stenosis. Spine 2011;36:E345-56. https://doi.org/10.1097/BRS.0b013e3181f2ed2f.
- Udeh BL, Costandi S, Dalton JE, Ghosh R, Yousef H, Mekhail N. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients. Pain Pract 2015;15:107-16. https://doi.org/10.1111/papr.12160.
- Koenig L, Dall TM, Gu Q, Saavoss J, Schafer MF. How does accounting for worker productivity affect the measured cost-effectiveness of lumbar discectomy?. Clin Orthop Relat Res 2014;472:1069-79. https://doi.org/10.1007/s11999-013-3440-6.
- Fitzsimmons D, Phillips CJ, Bennett H, Jones M, Williams N, Lewis R, et al. Cost-effectiveness of different strategies to manage patients with sciatica. Pain 2014;155:1318-27. https://doi.org/10.1016/j.pain.2014.04.008.
- van den Hout WB, Peul WC, Koes BW, Brand R, Kievit J, Thomeer RT. Leiden-The Hague Spine Intervention Prognostic Study Group . Prolonged conservative care versus early surgery in patients with sciatica from lumbar disc herniation: cost utility analysis alongside a randomised controlled trial. BMJ 2008;336:1351-4. https://doi.org/10.1136/bmj.39583.709074.BE.
- Vertuani S, Nilsson J, Borgman B, Buseghin G, Leonard C, Assietti R, et al. A cost-effectiveness analysis of minimally invasive versus open surgery techniques for lumbar spinal fusion in Italy and the United Kingdom. Value Health 2015;18:810-16. https://doi.org/10.1016/j.jval.2015.05.002.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Ionising Radiation (Medical Exposure) Regulations 2000 . Statutory Instrument 2000 No. 1059 2000.
- Medicines for Human Use (Clinical Trials) Regulations . Statutory Instrument 2004 1031 2004.
- Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine 1998;23:2003-13. https://doi.org/10.1097/00007632-199809150-00018.
- Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther 2002;82:8-24. https://doi.org/10.1093/ptj/82.1.8.
- Chiarotto A, Boers M, Deyo RA, Buchbinder R, Corbin TP, Costa LOP, et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 2018;159:481-95. https://doi.org/10.1097/j.pain.0000000000001117.
- Devogelaer JP, Dreiser RL, Abadie E, Avouac B, Bouvenot G, Carbonell Abello J, et al. Guidelines for clinical studies assessing the efficacy of drugs for the management of acute low back pain. Clin Exp Rheumatol 2003;21:691-4.
- Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3.
- Wilby MJ, Hopkins C, Bedson E, Howlin S, Burnside G, Conroy EJ, et al. NErve Root Block VErsus Surgery (NERVES) for the treatment of radicular pain secondary to a prolapsed intervertebral disc herniation: study protocol for a multi-centre randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2677-5.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013.
- NERVES Resource Use Questionnaire. In Database of Instruments for Resource Use Measurement; n.d.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NHS Improvement . National Schedule of Reference Costs 2017–2018 2018.
- NHS Digital . National Casemix Office 2017. Casemix Companion HRG4+ 2017 18 Local Payment Grouper 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2013. Personal Social Services Research Unit, University of Kent, Canterbury; 2013.
- NHS . Acupuncture 2016. www.nhs.uk/conditions/acupuncture/ (accessed 18 August 2019).
- NHS . Osteopathy 2018. www.nhs.uk/conditions/osteopathy/ (accessed 28 August 2019).
- NHS . Chiropractic 2017. www.nhs.uk/conditions/chiropractic/ (accessed 28 August 2019).
- Capital Physio, London, UK . How Much Does Private Physiotherapy Cost? 2016. www.capitalphysio.com/general/how-much-does-private-physiotherapy-cost/tle (accessed 1 August 2019).
- NHS Business Services Authority . Prescription Cost Analysis (PCA) Data September 2017 2017. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data (accessed 27 August 2019).
- Joint Formulary Committee . British National Formulary 2017.
- Boots Ltd . Retail Pharmacy Prices 2019. www.boots.com/ (accessed 2 April 2019).
- Office for National Statistics . Annual Survey of Hours and Earnings 2017, Table 6 2017. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/agegroupashetable6 (accessed 28 August 2019).
- Office for National Statistics . Annual Survey of Hours and Earnings 2017, 9. Regional Earnings, Figure 13. Median Full-Time Gross Weekly Earnings by Place of Work, Great Britain, April 2017 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2017provisionaland2016revisedresults#the-make-up-of-earnings (accessed 28 August 2019).
- Royal Automobile Club . Typical Vehicle Running Costs – For a Diesel Engine Car 2016. https://media.rac.co.uk/blog_posts/typical-vehicle-running-costs-for-a-diesel-engine-car-42609 (accessed 29 August 2019).
- Royal Automobile Club . Typical Vehicle Running Costs – For a Petrol Engine Car 2016. https://media.rac.co.uk/blog_posts/typical-vehicle-running-costs-for-petrol-engine-cars-42585 (accessed 29 August 2019).
- NHS Digital . National Casemix Office HRG4+ 201819 Payment Grouper 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/payment---hrg4-2018-19-local-payment-grouper (accessed 4 July 2019).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. https://doi.org/10.1177/096228029900800102.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. https://doi.org/10.1007/s10198-013-0471-6.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- NHS Digital . NHS Digital Hospital Episode Statistics (HES) Analysis Guide 2019.
- Bailey CS, Rasoulinejad P, Taylor D, Sequeira K, Miller T, Watson J, et al. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med 2020;382:1093-102. https://doi.org/10.1056/NEJMoa1912658.
- Hernandez Alava M, Wailoo A, Grimm S, Pudney S, Gomes M, Sadique Z, et al. EQ-5D-5L versus EQ-5D-3L: the impact on cost effectiveness in the United Kingdom. Value Health 2018;21:49-56. https://doi.org/10.1016/j.jval.2017.09.004.
- Carreon LY, Bratcher KR, Das N, Nienhuis JB, Glassman SD. Estimating EQ-5D values from the Oswestry Disability Index and numeric rating scales for back and leg pain. Spine 2014;39:678-82. https://doi.org/10.1097/BRS.0000000000000220.
- Leurent B, Gomes M, Carpenter JR. Missing data in trial-based cost-effectiveness analysis: an incomplete journey. Health Econ 2018;27:1024-40. https://doi.org/10.1002/hec.3654.
- Ridyard CH, Hughes DA. DIRUM Team . Development of a database of instruments for resource-use measurement: purpose, feasibility, and design. Value Health 2012;15:650-5. https://doi.org/10.1016/j.jval.2012.03.004.
- Suri P, Pearson AM, Zhao W, Lurie JD, Scherer EA, Morgan TS, et al. Pain recurrence after discectomy for symptomatic lumbar disc herniation. Spine 2017;42:755-63. https://doi.org/10.1097/BRS.0000000000001894.
Appendix 1 Additional tables from clinical effectiveness results
| Centre code | Date centre opened | Patients screened, n | Patients eligible, n (%) | Patients ineligible, n (%) | Consent provided, n (% of eligible) | Consent not provided, n (% of eligible) | Consent provided but patient not randomised, n (% of consented) | Randomised, n (% of consented) |
|---|---|---|---|---|---|---|---|---|
| 0006 | 1 April 2016 | 28 | 27 (96.43) | 1 (3.57) | 14 (51.85) | 13 (48.15) | 0 | 14 (100.0) |
| 0007 | 14 July 2016 | 23 | 23 (100.0) | 0 | 11 (47.83) | 12 (52.17) | 0 | 11 (100.0) |
| 0093 | 15 August 2016 | 116 | 16 (13.79) | 100 (86.21) | 6 (37.50) | 10 (62.50) | 0 | 6 (100.0) |
| 0137 | 8 August 2016 | 33 | 0 | 33 (100.0) | 0 | 0 | 0 | 0 |
| 0160 | 1 December 2015 | 287 | 8 (2.787) | 279 (97.21) | 1 (12.50) | 7 (87.50) | 0 | 1 (100.0) |
| 0182 | 29 July 2016 | 5 | 5 (100.0) | 0 | 1 (20.00) | 4 (80.00) | 0 | 1 (100.0) |
| 0213 | 27 September 2017 | 8 | 8 (100.0) | 0 | 8 (100.0) | 0 (0.000) | 0 | 8 (100.0) |
| 0400 | 13 April 2015 | 52 | 20 (38.46) | 32 (61.54) | 9 (45.00) | 11 (55.00) | 0 | 9 (100.0) |
| 0428 | 7 October 2015 | 138 | 32 (23.19) | 106 (76.81) | 6 (18.75) | 26 (81.25) | 0 | 6 (100.0) |
| 0492 | 2 March 2016 | 138 | 55 (39.86) | 83 (60.14) | 14 (25.45) | 41 (74.55) | 0 | 14 (100.0) |
| 0578 | 4 March 2015 | 186 | 103 (55.38) | 82a (44.09) | 80 (77.67) | 23 (22.33) | 0 | 80 (100.0) |
| 3253 | 1 April 2016 | 41 | 34 (82.93) | 7 (17.07) | 13 (38.24) | 21 (61.76) | 0 | 13 (100.0) |
| Total | 1055 | 331 (31.37) | 723a (68.53) | 163 (49.24) | 168 (50.76) | 0 | 163 (100.0) | |
FIGURE 18.
Final recruitment graph.
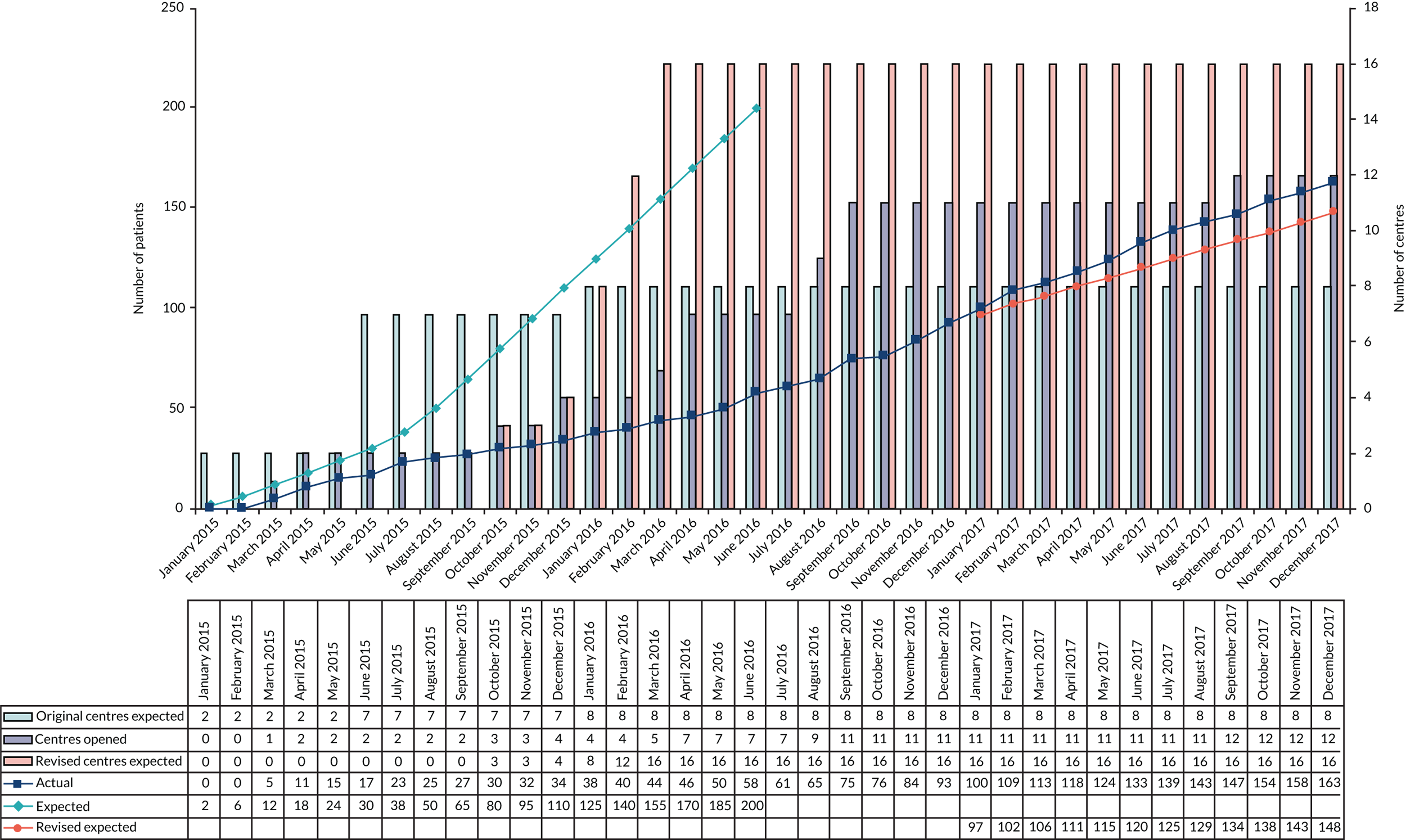
| Characteristic | Summary | Microdiscectomy | TFESI | Overall |
|---|---|---|---|---|
| Number randomised | 83 | 80 | 163 | |
| Posture | n (missing) | 83 (0) | 80 (0) | 163 |
| Abnormal, n (%) | 38 (45.8) | 41 (51.3) | 79 (48.5) | |
| Normal, n (%) | 43 (51.8) | 37 (46.3) | 80 (49.1) | |
| Not done, n (%) | 2 (2.4) | 2 (2.5) | 4 (2.5) | |
| Range of movement | n (missing) | 83 (0) | 80 (0) | 163 |
| Abnormal, n (%) | 52 (62.7) | 50 (62.5) | 102 (62.6) | |
| Normal, n (%) | 27 (32.5) | 27 (33.8) | 54 (33.1) | |
| Not done, n (%) | 4 (4.8) | 3 (3.8) | 7 (4.3) | |
| Muscle strength | n (missing) | 83 (0) | 80 (0) | 163 |
| Abnormal, n (%) | 12 (14.5) | 18 (22.5) | 30 (18.4) | |
| Normal, n (%) | 67 (80.7) | 59 (73.8) | 126 (77.3) | |
| Not done, n (%) | 4 (4.8) | 3 (3.8) | 7 (4.3) | |
| Left ankle jerks present | n (missing) | 81 (2) | 77 (3) | 158 (5) |
| No, n (%) | 13 (15.7) | 11 (13.8) | 24 (14.7) | |
| Yes, n (%) | 68 (81.9) | 66 (82.5) | 134 (82.2) | |
| Right ankle jerks present | n (missing) | 81 (2) | 79 (1) | 160 (3) |
| No, n (%) | 13 (15.7) | 13 (16.3) | 26 (16.0) | |
| Yes, n (%) | 68 (81.9) | 66 (82.5) | 134 (82.2) | |
| Left knee jerks present | n (missing) | 81 (2) | 77 (3) | 158 (5) |
| No, n (%) | 2 (2.4) | 4 (5.0) | 6 (3.7) | |
| Yes, n (%) | 79 (95.2) | 73 (91.3) | 152 (93.3) | |
| Right knee jerks present | n (missing) | 81 (2) | 79 (1) | 160 (3) |
| No, n (%) | 3 (3.6) | 5 (6.3) | 8 (4.9) | |
| Yes, n (%) | 78 (94.0) | 74 (92.5) | 152 (93.3) | |
| SLR reduction present | n (missing) | 81 (2) | 80 (0) | 161 (2) |
| No, n (%) | 6 (7.2) | 4 (5.0) | 10 (6.1) | |
| Yes, n (%) | 75 (90.4) | 76 (95.0) | 151 (92.6) | |
| Location of SLR reduction (if present) | n (missing) | 75 (0) | 76 (0) | 151 (0) |
| Bilateral, n (%) | 7 (9.3) | 9 (11.8) | 16 (10.6) | |
| Unilateral (left), n (%) | 37 (49.3) | 39 (51.3) | 76 (50.3) | |
| Unilateral (right), n (%) | 31 (41.3) | 28 (36.8) | 59 (39.1) | |
| Any other abnormalities | n (missing) | 82 (1) | 80 (0) | 162 (1) |
| No, n (%) | 63 (75.9) | 59 (73.8) | 122 (74.8) | |
| Yes, n (%) | 19 (22.9) | 21 (26.3) | 40 (24.5) |
| Protocol deviations | Microdiscectomy, n (%) | TFESI, n (%) | Total, n (%) |
|---|---|---|---|
| Total | 83 | 80 | 163 |
| Any protocol deviation | 69 (83.1) | 74 (92.5) | 143 (87.7) |
| At least one major | 26 (31.3) | 20 (25.0) | 46 (28.2) |
| Duration of symptoms between 6 and 54 weeks | 0 (1.4) | 1 (1.4) | 1 (0.6) |
| Severe leg pain non-responsive to conservative, non-invasive management | 1 (1.2) | 0 (0.0) | 1 (0.6) |
| Previous spinal surgery at the same intervertebral disc (level) | 1 (1.2) | 0 (0.0) | 1 (0.6) |
| Treatment compliance | 12 (14.5) | 10 (12.5) | 22 (13.5) |
| Treatment timeline compliance | 6 (7.2) | 1 (1.3) | 7 (4.3) |
| Missing primary outcome questionnaire | 14 (16.9) | 14 (17.5) | 28 (17.2) |
| Protocol-specified assessment tools not used | 3 (1.3) | 1 (1.3) | 4 (2.5) |
| At least one minora | 67 (80.7) | 71 (88.8) | 138 (84.7) |
| AE | Microdiscectomy (N = 105) | TFESI (N = 82) | Overall (N = 155) | ||||
|---|---|---|---|---|---|---|---|
| System Organ Class | Preferred term | Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) |
| Total | 18 | 15 (14.29) | 8 | 3 (3.66) | 26 | 18 (11.61) | |
| Nervous system disorders | Hypoaesthesia | 1 | 1 (0.95) | 5 | 2 (2.44) | 6 | 3 (1.94) |
| Cerebrospinal fluid leakage | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Peroneal nerve palsy | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Radicular pain | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Injury, poisoning and procedural complications | Dural tear | 4 | 4 (3.81) | 0 | 0 (0.00) | 4 | 4 (2.58) |
| Pseudomeningocele | 2 | 2 (1.90) | 0 | 0 (0.00) | 2 | 2 (1.29) | |
| Surgical procedure repeated | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Wound complication | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Infections and infestations | Postoperative wound infection | 2 | 2 (1.90) | 0 | 0 (0.00) | 2 | 2 (1.29) |
| Wound infection | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Musculoskeletal and connective tissue disorders | Pain in extremity | 1 | 1 (0.95) | 1 | 1 (1.22) | 2 | 2 (1.29) |
| Sciatica | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) | |
| Renal and urinary disorders | Pollakiuria | 0 | 0 (0.00) | 1 | 1 (1.22) | 1 | 1 (0.65) |
| Urinary incontinence | 0 | 0 (0.00) | 1 | 1 (1.22) | 1 | 1 (0.65) | |
| General disorders and administration site conditions | Swelling | 1 | 1 (0.95) | 0 | 0 (0.00) | 1 | 1 (0.65) |
| System Organ Class/preferred term | Onset date | Serious criteria | Severity | Expected | Related | Action | Outcome |
|---|---|---|---|---|---|---|---|
| Principal investigator/chief investigator | |||||||
| Injury, poisoning and procedural complications/surgical procedure repeated | 25 November 2015 | Required hospitalisation | Severe | Expected | Almost certainly/almost certainly | Hospital admission, other action: redo disc surgery | Resolved |
| Nervous system disorders/peroneal nerve palsy | 11 February 2017 | Prolonged existing hospitalisation, persistent or significant disability/incapacity, weakness of foot, further surgical intervention | Severe | Expected | Almost certainly/almost certainly | Treated with concomitant medication, re-explored surgically 12 February 2017 | Ongoing at final follow-up |
| Infections and infestations/postoperative wound infection | 19 September 2017 | Required hospitalisation | Severe | Expected | Almost certainly/probably | Treated with concomitant medication | Resolved |
| Injury, poisoning and procedural complications/pseudomeningocele | 5 December 2016 | Required hospitalisation | Moderate | Expected | Almost certainly/almost certainly | Treated with concomitant medication, MRI, outpatient appointments | Resolved |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- BMI
- body mass index
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COMI
- Core Outcome Measures Index
- CRF
- case report form
- CT
- computerised tomography
- ESI
- epidural steroid injection
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HCHS
- Hospital and Community Health Service
- HES
- Hospital Episode Statistics
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- INHB
- incremental net health benefit
- IQR
- interquartile range
- ITT
- intention to treat
- LCTC
- Liverpool Clinical Trials Centre
- MCID
- minimal clinically important difference
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MICE
- multiple imputation by chained equations
- MRI
- magnetic resonance imaging
- MRM
- modified Roland–Morris
- NERVES
- NErve Root Block VErsus Surgery
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ODQ
- Oswestry Disability Questionnaire
- ONS
- Office for National Statistics
- PID
- prolapsed intervertebral disc
- PISC
- patient information sheet and consent form
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- RUQ
- Resource Use Questionnaire
- SA
- scenario analysis
- SAE
- serious adverse event
- SD
- standard deviation
- TFESI
- transforaminal epidural steroid injection
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WEST
- Wessex Epidural Steroid Trial
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25240).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
