Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/41. The contractual start date was in September 2010. The draft report began editorial review in June 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Bruce et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Falls and fractures are a major public health burden. Falls can be associated with loss of independence and reduced functionality and are a major contributor to premature admission to nursing home or long-term care. 1,2 The risk of falling increases with age. Half of people aged ≥ 80 years fall at least once per year. 3 Most falls result in no injury, but can lead to fear of falling and loss of confidence in mobility. Falls can cause serious injury: each year, fracture and hospitalisation occurs in about 5% of community-dwelling older adults with a history of falling. 4 In 2016, there were 255,000 falls-related emergency hospital admissions in England among people aged ≥ 65 years. Demographic change means that the number and proportion of older people in the population is rising. In 2016 there were 1.6 million people in the UK aged ≥ 85 years (2% of total population), and this is projected to double to 3.2 million by 2036. 5 Fractures in older people will become increasingly common and this will have a major impact on use of health-care resources and service provision.
Costs of fractures and fall-related injury
The health and social care costs associated with fractures and fall-related injuries are high. Fractures and falls in those aged ≥ 65 years account for 4 million bed-days per year in England alone, at an estimated cost of £2B. 6 Direct health-care and associated social costs arise from the management of these injurious falls and fractures (the majority of costs arise from hip fracture). 7 Mortality is high in people who sustain a hip fracture: 10% die within 1 month and 30% die within 1 year of fracture. 8
Falls services in the UK NHS
Falls are a hallmark of age and becoming frail. 1 Falls have a multifactorial aetiology, with many risk factors, some of which are modifiable. Risk factors include impairments or instability of gait and balance, visual problems, cardiac rhythm abnormalities and syncope, polypharmacy and certain classes of ‘culprit’ or psychotropic medication, cognitive impairment, multiple comorbidity, foot disorders, and home and environmental hazards. Early clinical trials from the USA targeted the assessment and treatment of multiple risk factors and intervention strategies, termed multifactorial falls prevention (MFFP). These early trials were promising, indicating that multiple risk factor intervention strategies reduced risk of falling among community-dwelling older people. 9 These studies from the 1990s provided the basis for the mandatory establishment of secondary prevention in the UK:10 falls services providing MFFP interventions for people with a history of falling were subsequently introduced in England. 11 Multifactorial risk assessment, followed by targeted treatment of individual risk factors, is recommended for falls prevention in the UK, supported by clinical organisations [e.g. the American Geriatrics Society, the British Geriatrics Society and the National Institute for Health and Care Excellence (NICE)]. 2,3 These UK NHS services vary considerably in terms of service design, models of delivery and professional skill mix. 12,13
Evidence of effectiveness of falls prevention interventions
At the time of preparing the Prevention of Falls Injury Trial (PreFIT), numerous small trials had investigated the effectiveness of alternative falls prevention strategies. A 2012 Cochrane review14 (59 trials, n = 13,264) of falls prevention strategies found that exercise programmes, particularly those focused on strength and balance retraining, reduced rate of falls (i.e. number of falls) by 30% and risk of falling (i.e. number of people falling) by 18%. 14 Certain strength and balance interventions were found to be effective, especially those including targeted, individualised programmes progressed over time. However, adherence to exercise remained a challenge. Among the 59 falls prevention trials investigating exercise, only six had recorded fractures outcomes, totalling 45 fracture events.
The same Cochrane review14 identified 40 trials (n = 17,195) of MFFP studies. The review identified evidence for weaker effectiveness of MFFP interventions on falls outcomes, reporting that these interventions may reduce the rate of falls but not number of fallers (falls risk). 14,15 Among the 40 falls prevention trials investigating MFFP, only 11 had recorded fracture outcomes (totalling 289 events), but some trials included non-fracture injury. 14 Typically, the interventions identified by the Cochrane review were too poorly specified to be reproducible. These Cochrane reviews have since been updated; however, the overall conclusions are unchanged. 16 The reviews continue to highlight methodological deficiencies in existing trials, with many studies being underpowered and lacking robust data on important outcomes, including quality of life (QoL), fracture, costs of intervention and cost-effectiveness.
There is a need for robust evidence, with economic evaluation, to justify NHS provision of these falls prevention services.
Rationale for the PreFIT
Falls prevention services are widely implemented throughout the NHS, yet gaps remain in evidence regarding the prevention of falls. One of the main purposes of falls prevention is to reduce fractures and other serious injuries. 17 Adequately powered studies are required to investigate the effectiveness of such initiatives on clinical and patient-reported outcomes. This cluster randomised controlled trial (RCT) was designed to compare the effectiveness of alternative falls prevention strategies, using a screen-and-treat approach embedded within primary care, to investigate the prevention of falls and fractures in older adults.
The aim of the PreFIT was to determine the comparative clinical effectiveness and cost-effectiveness of three alternative primary care-led fall and fracture prevention strategies for older people living in the community: advice only; advice with screening for falls risk, with referral to an exercise programme for those at higher risk; and MFFP. Outcomes included fractures, falls, QoL, mortality and health-care resource use.
Research objectives
To estimate the clinical effectiveness and cost-effectiveness of the three alternative falls prevention interventions (advice only; advice supplemented with risk screening and referral to either exercise; and MFFP) in older adults. Our intention was to conduct a pragmatic trial and to include a process evaluation and a within-trial cost-effectiveness analysis.
Overview of the report
This report is structured across six chapters. We present methods (see Chapter 2), intervention development and description (see Chapter 3) and trial results (see Chapter 4) and describe the findings of the economic evaluation (see Chapter 5). Finally, we provide an overarching discussion and conclusion of our findings (see Chapter 6).
Chapter 2 Methods
Trial design and setting
We conducted a three-arm, pragmatic, cluster RCT design, with a parallel economic analysis. The setting was English primary care. We have published a detailed description of the trial protocol elsewhere. 18
Eligibility criteria
Cluster level
Practice identification and eligibility
We sought general practices (GPs) via existing research networks, including the Primary Care Research Network and Local Comprehensive Research Network. We placed advertisements in regional research newsletters and displayed posters at primary care events. We recruited triads of GPs in England with the infrastructure and services to support the trial. Specifically, we required practice agreement to adhere to a predetermined treatment pathway for the allocated intervention (advice, exercise or MFFP), local resources to deliver the active interventions and the technical capacity to undertake electronic searching to identify a random sample of older adults. We reimbursed practices for their time and postage costs.
Participant level
Participant eligibility
Community-dwelling older people aged ≥ 70 years and resident in the community or in sheltered housing were eligible for invitation by their GP. Exclusions included those housed in long-term residential or nursing care homes and those with terminal illness or expected shortened lifespan (defined as < 6 months). No specific restrictions or exclusions by age, sex, cognitive functioning, comorbidity or falls history were applied.
Participant identification
We sought to recruit an average of 150 participants per GP. A study researcher and/or practice staff member searched GP electronic databases to identify all people aged ≥ 70 years. A computer program selected a random sample of 400 of these. With an uptake rate of 35–40%, this would yield 140–60 participants per GP.
Participant exclusions by general practitioner
After generation of the random sample lists, general practitioners screened lists to remove patients who should not be approached (if not already removed via electronic search criteria). Predetermined reasons included any illness with an end-of-life prognosis of < 6 months or residence in nursing or residential accommodation.
Postal invitation and participant consent
Practices posted an invitation pack containing a participant information sheet, baseline questionnaire and consent form. We sought consent for multiple levels of access to medical data, including medical records and routine data held by the NHS Health and Social Care Information Centre (HSCIC) (NHS Digital from April 2016). At the time of trial launch, the wording of consent forms was appropriate for access to HSCIC data and approved by the Research Ethics Committee and relevant monitoring committees. Ethics issues were considered in relation to the cluster trial design. 18
Allocation sequence generation and randomisation
The unit of cluster randomisation was the GP. Once we had recruited approximately 150 participants from each of the three GPs, or no further responses were being received, GPs were then randomised. To ensure allocation concealment, we did this in blocks of three (one allocated to each treatment arm). No stratification was used. Randomisation was based on a computer-generated randomisation algorithm held and controlled centrally in Warwick Clinical Trials Unit by an independent programmer. Trial administration staff members were informed of GP allocation by e-mail. Treatment allocation was coded and unavailable to the trial management team.
Blinding
We adhered to the Consolidated Standards of Reporting Trials (CONSORT) statement 2010 update extension for cluster randomised trials. 19 Owing to the nature of the interventions, it was not possible to blind therapists or services delivering exercise or MFFP. Senior members of the research team were blind to GP and treatment allocation for the duration of the trial. We undertook data cleaning and fracture adjudication of suspected and confirmed events blind to treatment allocation.
Trial interventions (advice, exercise and multifactorial falls prevention)
We describe trial interventions in Chapter 3 and in two intervention development papers. 20,21 In brief, we randomised practices to deliver one of three interventions: advice leaflet only; advice leaflet supplemented with screening for falls risk, followed by exercise; or MFFP. The Age UK (www.ageuk.org.uk; accessed 21 April 2020) Staying Steady booklet22 was used for the advice intervention. This was mailed out after practice randomisation. Those randomised to active interventions received the Age UK advice leaflet with the falls risk balance screener. The exercise intervention was based on the Otago exercise programme (OEP), targeting lower-limb strength, balance retraining and walking. 23 Practices randomised to intervention invited participants identified to be at high risk of falling to attend a 6-month PreFIT exercise programme or the MFFP programme (Table 1). The MFFP intervention comprised an individualised, 1-hour falls assessment with appropriate onward referral for treatment, including referral to the PreFIT exercise intervention if gait or balance risk factors were identified. We based the MFFP intervention on evidence-based guidelines for falls risk assessment and treatment pathways. 2,11
| Referral: exercise programme | Therapist/venue | PreFIT exercise programme (6 months’ duration) | ||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 3 | Week 6 | Month 3 | Month 4 | Month 5 | Month 6 | ||
| Participant invited to attend exercise |
Assessor: trained physiotherapist, occupational therapist or exercise specialist Venue: outpatient clinic, hospital, community clinic or participant’s home |
1-hour face-to-face baseline assessment Assess balance and strength, conduct CST and 4TBS, prescribe programme, provide ankle weights |
30-minute face-to-face appointment or 10-minute telephone call Review and progress |
30-minute face-to-face appointment or telephone call Review and progress |
10-minute telephone call Review and progress |
10-minute telephone call Review and progress |
10-minute telephone call Review and progress |
1-hour final assessment: face-to-face appointment Repeat assessment of strength and balance, repeat CST and 4TBS |
| Referral: MFFP intervention | Therapist/venue | Multifactorial Fall Prevention (MFFP) intervention | ||||||
| Falls assessment | Actions | Treatment | Checks of onward actions | |||||
| Participant receives written or telephone invitation to attend health check appointment |
Assessor: trained nurse, medical doctor or other falls specialist Venue: GP surgery, hospital falls service or community clinic |
1-hour face-to-face appointment for detailed falls assessment and screening of multiple risk factors | Any risk factor identified? | Follow recommended treatment protocol (e.g. onward referral to general practitioner/falls consultant/optician/secondary care services/occupational therapist depending on risk factor) | Searches undertaken on sample of GP systems to review documented treatment actions (e.g. medication reviews, referral to PreFIT exercise intervention) | |||
| Gait and balance problems referred to PreFIT exercise intervention | Follow as per exercise programme above | |||||||
Screening and referral to active intervention
All participants received the advice booklet by post. Advice arm GPs delivered no further trial interventions. We used a primary care screening approach to determine onward referral of participants to the active interventions of exercise or MFFP. A short self-complete falls risk screening survey, based on previous research,24 was posted from, and returned to, GPs. Participants were categorised as being at risk of falling based on responses to falls and balance questions (high risk = multiple faller; intermediate risk = one fall or balance problems). These participants were offered the opportunity to attend for further assessment and treatment, either exercise or MFFP. Participants deemed to be at low risk (no history of falls or balance problems) received no further intervention other than the advice leaflet.
Co-interventions
No restrictions were placed regarding other agencies or health-care services contacting participants about fall prevention strategies. At trial closure, participants continued with usual health care and no further ancillary care was provided.
Baseline data: practices and participants
Descriptive data collected on GPs included practice-level deprivation at randomisation, using the UK National Index of Multiple Deprivation, which scores from 1 to 10 (from most deprived to least deprived). 25 Baseline descriptive data on participants included mobility, difficulties with mobility, cognition and activities of daily living (ADL). Mobility questions, adapted from population surveys of older adults,26,27 captured difficulties when balancing on a level surface, ability to walk outside the house, average time spent walking and difficulties with balance when performing common ADL (e.g. taking a bath, dressing). A clock-drawing test cognitive screener was included at baseline only. 28 Scoring was a 6-point system according to visuospatial aspects and the correct denotation of time: normal cognition (score 6), minor visuospatial errors (score 5), mild (score 4), moderate (score 3) or severe (score 2) visuospatial disorganisation of time or no reasonable representation of a clock (score 1).
Outcomes
Selection of primary outcome
Given the burden of injury, disability and dependence associated with fractures in older people, we selected fractures, rather than falls or falls-related QoL, as the primary outcome for the trial.
Primary outcome: fracture rate
The primary outcome was the fracture rate over 18 months, expressed as per person per 100 years, from date of GP randomisation and per time period. Number of fractures per participant was based on fractures identified in Hospital Episode Statistics (HES) and GP records, confirmed by adjudication panel. The fracture rate was derived for each participant, by accounting for time as an offset in statistical modelling.
Definition of fracture
We included all fractures, defined according to an internationally agreed definition:29 any fracture to bones in the peripheral (appendicular) skeleton, thus limbs, limb girdles, ribs and cranial and facial bones. We excluded compression fractures in the vertebral column that could not be attributed to a fall. Updates to the protocol are described in Monitoring and approval. We also estimated differences in number of proximal femoral (hip) fractures and fractures involving the distal radius or wrist to allow comparison with other reports from more recent fracture prevention trials. 30 We defined hip fractures as verified fractures with a specific description of neck of femur or proximal femur [International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10)31 code S72].
Fracture data sources
Fracture data were collected from three sources: HES, GP records and participant self-report postal questionnaires. Successive waves of NHS Digital data were purchased covering years 2010/11 to 2015/16. Fractures were screened and identified from the following sources:
-
HES acute patient care – searches of fracture codes in inpatient admissions using ICD-10 S00-T88 codes for ‘Injuries, poisoning and certain other consequences of external causes’ and M/T/W codes (as reported in the statistical analysis plan).
-
HES accident and emergency (A&E) – fracture codes in A&E diagnosis and treatment codes, including diagnosis codes (three level) 052 and 053, treatment codes (two level) 05 and 33, and treatment codes (three level) 101 and 102.
-
HES outpatient data – orthopaedic and trauma clinic attendances (main specialty codes).
-
GP records – electronic searches of Read codes (clinical terms version 2 and 3: S/N/T code searches), with additional free-text searches undertaken for fracture/#. We extracted copies of any clinical records or correspondence relating to actual or suspected fractures.
-
Participant self-reports of fall-related fractures were captured via postal surveys administered at baseline (fractures in previous year) and at 4, 8, 12 and 18 months after randomisation.
We searched all data sources to identify any potential or suspected fracture report in HES acute patient care, HES A&E, GP records or self-report. Detailed searches on GP records were undertaken for all self-reported fractures. All events were identified for cross-checking against other sources by the adjudication panel. HES outpatient data were not used beyond the first wave because this data set did not yield usable fracture codes. Additional checks in the HES acute patient care data set were made for operations in the same hospital admission or adjacent time period by checking the Office of Population Censuses and Surveys’ Classification of Surgical Operations and Procedures codes.
Fracture adjudication
Two clinically qualified members of the study team adjudicated fracture events (SEL and MU). When needed, further adjudication was made by KW. Adjudicators were blind to practice allocation and all personal-identifiable data were redacted before records were presented to the adjudication panel. A fracture event was included if it corresponded to the updated, revised fracture protocol. Fractures identified on HES acute patient care were considered confirmed with or without participant self-report or GP record; any fracture identified on HES A&E supported by a GP record was confirmatory with or without participant self-report. Primary care records were considered confirmatory when supported by hospital or clinic discharge letters or radiology (X-ray) reports confirming a fracture. Whenever possible, fractures ascertained from GP record searches were assigned an event date and corresponding ICD-10 fracture code by the panel. Fractures reported using self-report alone were discounted.
Secondary fracture outcomes
Fracture episodes
Some participants experienced multiple fractures from one fall. We characterised all fractures occurring on the same date as one episode. Statistical modelling was used to account for fractures occurring in the same individual and in the same episode.
Time to first fracture
The adjudication panel dated all fractures using HES, GP data and self-report. When data were not available on the date of fracture, then date of hospital admission or attendance was taken as date of fracture. Time to first fracture was defined as the interval, in days, between randomisation and first fracture in the study period.
Proportion of people sustaining one or more fractures or fracture episodes over 18 months
As participants might have multiple fractures as a result of one or more falls during the 18-month follow-up, the proportion of participants with at least one fracture episode was calculated.
Secondary outcomes
Secondary outcomes included falls, health-related quality of life (HRQoL), frailty, mortality and health-care resource use.
Falls
Rate per 100 person-years observation over 18 months and per time period
Our primary falls outcome was the falls rate, expressed as falls per person per 100 years, over 18 months from date of practice randomisation. The definition for falls was consistent with the Prevention of Falls Network Europe (ProFaNE) consensus. 29 The number of falls per participant was based on retrospective self-report in postal questionnaires, recorded at baseline (falls in the last 12 months) and at each follow-up time point, by asking about falls in the previous 4 months. This was the primary method of falls data collection. The number of falls between each of the follow-up time points and the number of people sustaining one or more falls were also reported.
Prospective diary
All participants were asked to complete monthly prospective falls diaries for a period of 4 months during the first year of the trial. Prospective data collection is considered the gold-standard method for falls recording, but given the large sample size this was not practical. We allocated participants to one of three time periods (randomisation to 4 months, 5–8 months or 9–12 months after randomisation) using random sampling without replacement. Prepaid postal diaries were completed daily and returned to the research office every month.
Health-related quality of life: measured over 18 months and each time period
The Short-Form questionnaire-12 items (SF-12) measures physical function, engagement in usual activities and mental functioning. 32 The physical health composite scale (PCS) and mental health composite scale (MCS) combined items allow comparison with national norms [mean score 50.0, standard deviation (SD) 10.0]. The EuroQol-5 Dimensions, three-level version (EQ-5D-3L), is a standardised measure of self-reported HRQoL that includes five domains: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. 33
Frailty: measured at baseline and 18 months
Frailty was measured using the Strawbridge questionnaire, a 16-item questionnaire comprising four frailty subdomains: (1) physical (four items), (2) nutritional (two items), (3) cognitive (four items) and (4) sensory (six items). 34 Scoring is on a four-point ordinal scale (rarely or never, sometimes, often, or very often), with those scoring ≥ 3 on at least one item being considered to have a problem or difficulty in that domain. Participants were classed as ‘frail’ if they reported having problems in two or more domains. 35
Mortality: over 18 months
Date of death was obtained from multiple sources, including notifications to the study team from family members or from primary care, HES data and searches of practice medical records on completion of the trial.
Health-care resource use (baseline and at 4, 8, 12 and 18 months)
We describe these data in more detail in Chapter 5. In brief, questions addressed attendance at health-care services, including GP, the district nurse, any time spent in a nursing or residential home and contact with physiotherapy services.
Data collection: postal questionnaires
Questionnaires were printed in large font, with a freephone number on the front page of the booklet and final free-text section for comments. Draft versions were modified after feedback from older people attending a community social group. We posted questionnaires with an explanatory cover letter. We collected core outcome data on fractures, falls, mobility, EQ-5D-3L and health-care resource by telephone if no response was received after one postal reminder. Any participant who reported a fracture event in the questionnaire was sent a separate questionnaire to elicit date, site of fracture (e.g. upper arm, wrist) and details of any hospital admission and/or radiographs taken. Participants reporting more than one fracture were contacted by telephone for further details.
Process evaluation
We measured a range of process evaluation indicators relating to intervention uptake and delivery, including time from randomisation to postal risk screener administration, uptake and predictive utility of the postal screener, uptake of active interventions, and duration and ‘dose’ of treatments delivered by exercise therapists or MFFP assessors.
The response rate to postal falls risk screeners was assessed for screening yield and utility by assessment of prediction of falls over 12 months, using area under the curve (AUC) and 95% confidence intervals (CIs), as per recommended guidance for evaluation of prediction models. 36 Intervention-related process measures for exercise included changes in strength and balance over time; thus, first and final assessments using the chair stand test (CST), the 4-test balance scale (4TBS), weights (kg) and repetitions were prescribed. We defined ‘work’ done as the product of the number of repetitions and amount of weight prescribed at each contact point (weights × repetitions). 37 We recorded the number of contacts and reported participants as having either fully or partially adhered to the exercise programme (fully adhered = six contacts or until discharge by therapist; partially adhered = fewer than six contacts). We recorded the number of MFFP assessments among those invited to attend. The skill mix of assessors was recorded by region, and participant uptake of interventions was explored by region.
Process evaluation: general practice medication prescribing
We extracted prescribing data for selected medications from GP records for two time periods captured 1 year apart: 3 calendar months pre randomisation and from 9 to 12 months post randomisation. The aim was to determine the rate of prescriptions of culprit medications (i.e. those targeted in any of the interventions) over time. In addition, we estimated the use of bisphosphonates and mineral supplementation as contextual data. We planned to undertake drug data searches on a random subsample of GPs stratified by intervention arm, but pilot searches suggested that this would be unreliable owing to differences in primary care electronic systems, which resulted in variation in format and quality of drug data. Therefore, medication searches were undertaken at all GPs. We developed a medication search protocol detailing Read codes for two drug classes of interest: (1) psychotropics and psychotropic-related medication and (2) bisphosphates and mineral supplementation. Psychotropics can increase risk of falling, and we included antidepressants, psychotropics, sedatives, hypnotics, anxiolytics and antimanic medications, as per British National Formulary38 classes 4.1.1—4.3.1. Bisphosphonates (British National Formulary class 6.6.2), are often prescribed with mineral supplementation (calcium and vitamin D preparations that include calcium carbonate, British National Formulary classes 5.1 and 9.6.4), as can slow bone loss and may decrease the risk of fractures. We collected data at practice level only, on the total number of trial participants prescribed psychotropics and bone protection drugs (mean/proportion participants per practice) and changes pre–post randomisation. Completeness of data varied by practice electronic system.
Data management
Questionnaires were scanned using FORMIC FUSION™ software (Formic Ltd, Staines, UK), which includes internal system validation checks. All scanned questionnaire entries were manually checked by the research office. All other data were manually entered by data clerks onto the bespoke database designed for the trial. All data were validated using range checks, outliers, missing data and date discrepancies. Anomalies were checked against original data sources for rectification.
Data analyses
Definition of higher falls risk
We established risk of falling using two methods, based on responses to (1) the GP-administered postal falls risk screener mailed to treatment arms only and (2) the baseline questionnaire completed by all participants. Details of response options used to classify risk of falling for each data source are provided on the project webpage (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/081441/#/; accessed 16 March 2020). The risk algorithm from the baseline questionnaire used falls and balance difficulty questions analogous to the falls risk screener, but this enabled identification of risk across the full trial population. Comparisons of risk of falling were made across treatment arms.
Sample size calculation
As reported in the published protocol,18 the study was powered using the proportion of people with at least one fracture. We used a conservative method of estimating the sample size based on a comparison in the proportion of people with fractures, recognising that this would be more than adequate for comparison of both proportions and fracture rates per person over 18 months of follow-up. There were surprisingly few data available from which to draw estimates of fracture rates for the UK older population. The sample size was based on the annual fracture incidence from UK statistics, estimated to be 6 per 100 (6%) for people aged > 70 years. 39 We prespecified a target of reduction to 4% in the intervention arm.
A total of 5700 participants, thus 1900 per arm, were required to achieve 80% power for detecting a statistically (p < 0.05) and clinically relevant (2%) reduction in proportion sustaining a fracture, from 6% to 4%, for the comparisons of advice compared with exercise and advice compared with MFFP. We aimed to recruit an average of 150 participants from each GP. To adjust for varying degrees of modest clustering, intracluster correlation (ICC) set at 0.003 inflated this sample size estimate from 5700 to 7800, or 2600 per arm. The choice of ICC was driven by data reported by Smeeth and Ng,40 who, although they did not provide information relating to fractures, reported ICC related to an outcome of ‘at least one fall’. This estimate was 0.0061. We used a slightly smaller ICC of 0.003, as the number of fracture events would be lower than number of falls. Allowing for 15% loss to follow-up, this yields a minimum target sample size of 9000 participants from a minimum of 60 practices. To reduce the possible effects of variable cluster size on statistical power, we sought to keep final cluster sizes in the range of 129 to 179.
Statistical analyses
Statistical analyses were carried out using StataSE® version 15 and 16 (StataCorp LP, College Station, TX, USA). All statistical tests were two sided and performed at the 5% significance level. We undertook three levels of analysis: (1) intention to treat (ITT), (2) a nested ITT on data from those at higher risk of falling and (3) complier-average causal effect (CACE) analyses. Unadjusted and adjusted estimates were obtained for all statistical regression models. Adjustment was made for the corresponding baseline variable (participant age, sex, falls history and GP deprivation score). The main ITT analysis included all randomised participants. In a nested ITT analysis, we compared treatments just among those at higher risk of falling, classified in accordance with the baseline questionnaire.
Treatment comparisons
The primary analysis compared all participants randomised to advice with all participants randomised to exercise and, separately, all participants randomised to advice with all participants randomised to MFFP. Only if there was evidence of a statistically or clinically important difference for either of these did we plan to proceed with comparing exercise with MFFP.
Descriptive analyses
Data were summarised and reported in accordance with the CONSORT guidelines for cluster RCTs. 19 Recruitment and participant flow are reported using CONSORT flow diagrams at the level of GP and participants in Chapter 4 (see Figures 1 and 2). Participant data from randomisation to 18 months were analysed by treatment arm and by risk classification using falls and balance questions from the baseline questionnaire. Sociodemographic variables were summarised by intervention arm, with mean (SD), median (range) and missingness reported for continuous variables, and number (%) for categorical variables. Unadjusted and adjusted estimates were obtained for all statistical regression models. Adjustment was made for the corresponding baseline variables: GP deprivation score, participant falls history, age and sex.
Primary analyses
Cook and Major41 reviewed two different approaches to the analysis of events per person-years, examining estimation of the rate by (1) dividing total number of events across all participants by total duration of participant follow-up and (2) fitting a random-effect Poisson model to accommodate the expected variation in the rate between different participants. 41 They found that the events-per-person method substantially underestimated variation in the data and felt that this method was not appropriate to summarise the incidence of rate-related data. 41 Others have described different statistical models to assess count data when there is overdispersion and excess zeros. 42–44 These models include the Poisson, negative binomial, zero-inflated Poisson, zero-inflated binomial, hurdle Poisson, hurdle binomial, Andersen–Gill and marginal Cox regression models. There is consistent evidence to suggest that the negative binomial models provide the best fit. 42 We therefore used these to analyse fracture and falls rates per person time observation.
Fractures were expressed as the fracture rate per person per 100 years of observation. Using the negative binomial model, we incorporated the random effect for GP, and this provided the unadjusted and adjusted estimates of the fracture rate per person per 100 years, taking account of all data collected over 18 months. The adjusted model accounted for baseline falls, sex, age and GP deprivation score. An offset variable was incorporated to account for the time the participant was in the study. In the light of the extremely skewed distribution of the baseline falls count, the log of the baseline falls count was used. 45 The fracture rates per person per 100 years of observation (with 95% CIs) are presented by treatment arm. The rate ratio (RaR) (95% CI) is given for advice compared with exercise and advice compared with MFFP. The standard errors (SEs) for the rates were obtained using bootstrapping methods. Model fit was assessed using the likelihood ratio (LR) test, which compared the random-effect model with the standard negative binomial model.
The fracture rate based on per person per time period was also computed for each follow-up time point to 18 months. Negative binomial models were fitted similar to those described above but without an offset variable, because the time interval was standard for all participants.
Secondary analyses
The proportion of participants who sustained one or more fractures over 18 months was analysed using random-effects logistic regression models, with the random effect as GP. Responses were taken as ‘no fracture’ (0) and ‘at least one fracture’ (1) where one or more fractures were recorded. Model fit was assessed using the LR test, comparing the standard with the random-effect logistic regression model. Unadjusted and adjusted odds ratios (ORs) (95% CI) for the odds of incurring at least one fracture compared with no fracture were calculated.
Time to first fracture was analysed using survival methods, accounting for the random effect (GP) and other key predictors, as above. A Cox proportional hazards model was fitted to the data to compare time to first fracture across treatment arms; assumptions for models were checked. The total number of fracture episodes and rate of fracture per episode were summarised by treatment arm. Frequency and proportion of hip and wrist or forearm fractures were compared by treatment arms using the chi-squared test.
Secondary outcomes
Falls analyses
Using the negative binomial model with random effect for GP, unadjusted and adjusted falls rates per 100 person-years were calculated using all data collected over 18 months. The adjusted model accounted for baseline falls, sex, age and GP deprivation score. An offset variable was incorporated into the model to account for time in the study. In the light of the extremely skewed distribution of the baseline falls count, the log of the baseline falls count was used. 45 The negative binomial model provided a rate per participant per month, expressed as per 100 person-years of observation. The falls rate per 100 person-years (95% CI) was calculated by treatment arm. The RaR (95% CI) was calculated for exercise compared with advice and for MFFP compared with advice. The SEs for rates were obtained using bootstrapping methods. Model fit was assessed using the LR test, which compared the random-effect model with the standard negative binomial model.
We analysed the proportion of participants who sustained one or more fall over 18 months, similar to the fracture analyses. At 18 months, the questionnaire time period covered 6 months (from 12 to 18 months). However, the written question enquired about falls in the previous 4 months; therefore, 4 months was used for rate calculations. Only reported data were used in rate calculations. For example, when a participant returned falls data at 4, 12 and 18 months but had not responded at 8 months, then the falls rate was based on the observation periods for data returned (in this example, 4, 12 and 18 months).
The number (proportion) of fallers was calculated. In addition, the falls rate (per 100 person-years) was calculated as a 4-monthly rate for each time interval analysed using random-effect negative binomial models. The falls rate per person per month based on diary card responses was summarised and analysed using similar methods for each time interval (baseline to 4 months, 4 to 8 months and 8 to 12 months). A comparison was made by method of reporting (retrospective vs. prospective).
Other secondary outcomes and baseline measures
Other clinical outcomes and baseline measures were summarised by treatment arm (SF-12, EQ-5D-3L, frailty, mobility and difficulties with ADL, cognitive impairment and self-reported health conditions). Mean values (SD), median (range) and missingness were reported for continuous variables, and number (%) for categorical variables. For secondary analyses, we estimated treatment effects by age, sex, falls history, frailty and cognition. Frailty status was fitted using the random-effect logistic regression model, unadjusted and adjusted, with the odds of being frail compared with non-frail by each active treatment arm compared with advice modelled. We followed validated scoring guidelines for all measures. Descriptive data for the cognition test were summarised as having higher compared with lower cognitive functioning. An additional secondary analysis was undertaken on fractures occurring after the 18-month follow-up period for the entire observation period for which HES data were available. For these events, confirmed fractures were based on only hospital statistics, as GP and self-report were not gathered beyond 18 months. We report events from the extended period as per events for the main trial.
Nested intention-to-treat analysis
In a nested ITT analysis, we compared treatment just in those at higher risk of falling, classified according to responses to falls and balance questions in the baseline questionnaire, completed by all trial participants before randomisation. This provided information on those at higher risk of falling across all three treatment arms, including the advice only group, who did not receive the falls risk screener.
Complier-average causal effect analysis
In a CACE analysis we assessed treatment effect in compliers compared with non-compliers. We defined a ‘complier’ as a participant who returned a postal falls risk screener who was deemed at risk of falling and attended their first exercise or MFFP assessment or one who returned the falls risk screener but had a low risk of falling. We defined non-compliers as those who returned a falls risk screener and were at risk of falling but did not attend treatment, or who did not return a falls risk screener.
Subgroup analyses
We prespecified subgroup analyses to explore intervention effectiveness by age, sex, falls history, cognitive impairment and frailty. 46,47 We did not expect to include large numbers of community-dwelling older people with severe cognitive impairment, but mild to moderate cognitive impairment may affect ability to engage in falls prevention strategies. Subgroup effects were tested through formal interaction tests. 48 Random-effects negative binomial models were fitted using the interaction of treatment and subgroup as the covariates. We did not adjust for other baseline covariates. RaRs (95% CI) were obtained for the treatment comparisons for advice compared with exercise and advice compared with MFFP, respectively.
Missing data
As fracture data were available for all participants from combinations of HES and GP records, we had no missingness for the primary outcome. We assessed missing data for the falls outcome. It was not possible to perform multiple imputation (MI) owing to skewness in the distribution of the data. Instead, we assessed missingness (no response) by looking at the proportion of missing data across treatment arms for the falls data within each time interval.
Adverse event reporting
A safety-reporting protocol for related and unexpected serious adverse events (SAEs) and directly attributable adverse events (AEs) was developed. An AE was defined as any untoward medical occurrence in a participant that did not necessarily have a causal relationship with treatment. All participants were aged ≥ 70 years; thus, we expected common chronic diseases associated with age (e.g. osteoarthritis and musculoskeletal conditions). It was expected that some participants would experience uncomfortable effects from exercising, such as muscle or joint discomfort. These effects were anticipated and, provided they were short-lived, were not reported as AEs. AEs were reported if they occurred during any contact time with the therapist or assessor delivering an intervention, during an intervention session or when undertaking prescribed exercise, either supervised or unsupervised. All AEs were reported to the trial team and chief investigator within the required timelines, in accordance with the PreFIT safety-reporting protocol. A SAE was an AE occurring as a direct consequence of treatment that resulted in death, threat to life, hospitalisation, disability or incapacity. Any event that required professional medical attention included, but was not restricted to, serious sprains, joint dislocation, falls or other injuries occurring as a direct consequence of the intervention. All events were reported to the trials unit immediately after the therapist became aware of them, within 24 hours for SAEs. The SAEs and AEs were recorded in the trial database. Any event related to trial interventions was referred to and reviewed by the Data Monitoring and Ethics Committee (DMEC).
Pilot phase and protocol revisions
A pilot phase was undertaken in one locality from September 2010 to March 2012. Key changes during the pilot phase included to increase postal mail-out from 300 to 400 people to increase yield from initial invitation. Other changes over time included an update to the primary outcome. The original trial protocol defined peripheral fracture as any fracture to bones in the appendicular skeleton, thus limbs and limb girdles as well as cranial and facial bones, but with exclusion of compression fractures in bones constituting the vertebral column (lumbar, thoracic and cervical vertebrae, sacrum and coccyx). This definition of appendicular skeleton also excludes the thoracic cage (sternum and ribs), as per the internationally agreed definition published by the ProFaNE network. 29 However, the definition of fracture events had evolved since protocol development. There was broad recognition among the clinical community that the ProFaNE consensus, published in 2005, required updating to reflect the contemporary epidemiology of trauma-related fractures in older people. 49 As populations age, the incidence of trauma-related skull, rib, vertebral and facial fractures increases. 50–53 During the pilot phase, we had developed methods for extracting accurate fracture data from both HES and GP records. It was apparent that fracture reporting was of sufficient quality to distinguish compression fractures of the vertebral column. We updated the PreFIT protocol and trial registry to include these fractures from HES and GP records when these were clearly consistent with a trauma mechanism. Therefore, when there was a clear description of trauma or fall, or when the fracture presentation was consistent with trauma and clearly mapped to the ICD-10, falls codes were generated from HES. Reports of vertebral osteoporotic compression fractures in GP records were excluded unless clearly linked to a report of trauma or fall.
Statistical models were revised from generalised estimating equations to generalised linear mixed-method models to account for data type and dispersion. These model revisions (updated from the original application) were reported in the prespecified analysis plan, approved by external committees and described in the published trial protocol. 18
Monitoring and approval
Regional and site-specific approvals were obtained from regional NHS research and development departments. The study was approved by the National Research Ethics Service (Research Ethics Committee reference 10/H0401/36, version 3.1, 21 May 2013), with approval granted by the Derbyshire Research Ethics Committee on 29 April 2010 (Table 2). Funder-led monitoring meetings to review pilot study recruitment and intervention data were held on 4 April 2012 and 14 September 2012, and written approval to proceed to the main trial was received in October 2012. Of the amendments approved by the Research Ethics Committee, seven were substantial and one was non-substantial.
| Date amendment approved | Overview of modifications |
|---|---|
| 29 April 2010 | Ethics approval for trial |
| 16 March 2011 | Sample size refinement, edits to primary outcome data collection, refinements to postal questionnaires and related participant materials |
| 17 August 2011 | Amendments relating to HES data |
| 1 March 2012 | Participant materials and consent form |
| 7 September 2012 | Post-pilot protocol revisions. Increase in invitations from 300 to 400 per practice. Extension of follow-up to 18 months |
| 24 June 2013 | Participant materials for 18-month follow-up |
| 7 April 2017 | Protocol addendum for additional follow-up |
| 22 August 2017 | Approval for a related PhD study |
Patient and public involvement
User groups and patient public representatives were involved in the design of materials and implementation of the trial. Older people attending a community support group and social lunch group in the Warwickshire region were invited to review patient-facing materials, including questionnaires, balance screening, cover letters and patient information sheets. The Trial Steering Committee (TSC) included an independent lay member. A patient dissemination event, attended by 48 participants and their partners or carers, was held at a University of Warwick conference centre on completion of the trial.
Trial Steering Committee
The TSC was responsible for monitoring and supervision of trial progress.
Data Monitoring and Ethics Committee
The independent DMEC monitored ethics, safety and data integrity aspects of the trial. Pilot data were reviewed by the TSC and DMEC; we proceeded to the main trial after approval by the DMEC, TSC and funder.
Chapter 3 Trial interventions
Introduction
This chapter presents a description of the trial interventions; sections are based on published work describing the exercise and MFFP interventions. 20,21 Intervention development was undertaken using Medical Research Council guidance54 for the development of complex interventions and following methodology used in other rehabilitation trials supported by the National Institute for Health Research Health Technology Assessment programme. 55,56 A key consideration was the selection of interventions suitable for testing and implementation in the UK primary care setting.
Advice intervention
A scoping survey of UK falls services, referral pathways and information materials for older people had been completed before we started the PreFIT. 12 Falls prevention leaflets for older adults were reviewed for content and clarity. The Age UK’s Staying Steady booklet22 was selected for the advice intervention because of the positive emphasis on remaining steady and physically active. The Age UK leaflet contained useful advice about improving strength and balance rather than focusing on the consequences of falling. This 29-page colourful booklet provides clear information about eyesight, hearing, foot care, managing medications, checking the home environment for falls risks and dealing with anxiety about falling. It contains practical advice on seeking help from the NHS, contact details and telephone numbers for other organisations for older people and links to further reading. The leaflet was provided free of charge by Age UK. All trial participants received this booklet by post after the GP was randomised. Those randomised to the advice arm received a booklet with a covering letter only, then no further planned intervention.
Rationale and scientific principles for the exercise intervention
Evidence for exercise type and dose
Multiple systematic reviews have explored exercise type and dosage, finding that programmes that included balance training programmes and those delivering higher doses of exercise had the greatest effect on falls reduction. 57,58 Programmes of walking-only interventions and those without any balance challenge were ineffective in preventing falls. These systematic reviews led to best-practice recommendations that exercise-based falls prevention programmes should provide a moderate to high balance challenge, should be undertaken for at least 2 hours per week and should be delivered in either a group format or home-based setting. 58 Programmes should be of sufficient dose to induce changes in muscle strength and neuromuscular function and be delivered by either trained health professionals or suitably qualified exercise instructors. Other public health guidance for maintaining musculoskeletal and cardiorespiratory health in older adults from the World Health Organization59 and American College of Sports Medicine60 recommended that strength training, or resistance exercises, targeting the major muscle groups should be undertaken two or three times per week. Given these recommendations, we reviewed the theoretical principles of balance and strength exercises during the process of selecting a suitable programme for testing in the PreFIT. A detailed description of the PreFIT exercise intervention is presented elsewhere. 21 An overview of principles and definitions used is given here.
Balance control in older age
Balance control is a prerequisite for successful mobility. Balance is defined as the ability to maintain the projection of the body’s centre of mass within manageable limits of the base of support, as in standing, sitting or in transit to a new base of support. 61,62 Balance is also a generic term describing the dynamics of body posture to prevent falling,61 and it can be quantitatively assessed by measuring the body’s centre of mass in relation to the base of support (e.g. sway on a force platform). It can also be measured using self-report or by using observation and functional testing. 62
Our ability to maintain balance depends on many inter-related processes (e.g. sensory information received from visual, vestibular, proprioceptive and exteroceptive sources). 62,63 Ageing is associated with a loss of reserve capacity in several bodily systems engaged in the control of balance and gait. Walking patterns change with increasing age: steps become shorter, push-off power is decreased and landing becomes more flat-footed. 64
Balance retraining in older people
For balance to improve, it should be challenged. 65 Progression to dynamic balance exercises is recommended, as static balance training is less likely to translate into improvements in balance during functional activities and ADL. 66 Many daily activities involve balance, such as moving from a sit to stand position, turning while walking or bending to retrieve objects from the floor. Traditional balance ‘challenges’ include reducing the base of support or moving the body’s centre of gravity out of the base of support, or a combination of both. Balance retraining exercises can lead to improvements in physical activity and function. Systematic reviews have found that balance training in older adults is most effective when exercises are performed three times per week and for at least 3 months. 58,67
Evidence for strength training in older people
Evidence for strength training in older adults was also considered. Muscle strength is greatest when young, with maximum strength peaking at age 20–40 years. By the age of 50 years, about 10% of muscle mass has gone and, thereafter, the rate of decline accelerates. 60,68 This decline is thought to be due, in part, to decreasing levels of physical activity. The consequences of loss of muscle mass include increased susceptibility to falls and fractures, and inability to perform everyday tasks.
Strength training is a system of physical conditioning in which muscles are exercised by being worked against an opposing force to increase strength. 62 There is good evidence to show that sedentary older adults, with support and regular training, can achieve a two- to threefold increase in muscle strength after 3 months of training. 60 In addition to effects on muscle mass, strength training can also lead to improvements in insulin action, bone density, energy metabolism, functional status and physical activity. 60,69 There is debate about the reasons why muscles become weak and atrophied over time; lack of use may be the major contributory factor rather than the ageing process alone. However, this may be a new concept for many older people and for some health-care professionals. Strength training challenges our accepted view of activity in older populations.
Selection of a suitable exercise intervention
Given the robust evidence base supporting the effectiveness of interventions targeting balance and strength, we aimed to incorporate these elements of best practice into the exercise intervention. The process for selection of the most suitable exercise intervention has been described in detail elsewhere. 70 In brief, we reviewed all exercise interventions reported in clinical trials included in systematic reviews published up to 2011. We considered all exercise interventions and programmes, although many interventions were not reported in sufficient detail to allow replication. Three established exercise programmes were shortlisted for consideration for the PreFIT: (1) the Tinetti et al. 9 exercise programme, (2) the Falls Management Exercise Programme (FaME)71 and (3) the OEP. 23,72
The Tinetti et al. 9 exercise programme, developed in the USA, includes progressive strength and balance exercises, gait and transfer training and a range of motion exercises. 9 The intervention also includes upper-limb exercises, with general recommendations for weights and progression. Starting-level exercises are predominantly chair based, and the programme also targets training in how to transfer from lying to sitting and sitting to standing, etc. This exercise programme is not widely used in the UK.
FaME, developed in the UK, is a 36-week group and home exercise programme incorporating fitness with progressive ‘chain’ exercises (movement sequences to get up and down to the floor), functional exercises and adapted tai chi. 71 The FaME intervention had been tested in one secondary prevention trial71 and was effective in reducing falls among frequent fallers when participants were provided with transport to attend classes (this encouraged attendance). The programme was not widely used in the UK setting at the time of the design stage of this trial. It has subsequently been proven to be an effective primary prevention falls intervention that also increases habitual physical activity. 73,74
The OEP, developed in New Zealand, is a programme of muscle-strengthening and balance-retraining exercises delivered at home or in the clinic setting by trained health professionals. This programme had been tested in four community-based primary prevention RCTs by the original research team,75–78 with two of these trials77,78 undertaken with those aged ≥ 80 years. The programme is individually prescribed by a physiotherapist or trained nurse and delivered via a series of home visits. It is based on robust physiological principles, incorporating progressive lower-limb strengthening exercises using ankle weights and moderate- to high-challenge balance exercises, and includes a walking plan. A meta-analysis by the Otago group of its own trials, totalling 1016 adults aged 65–97 years, randomised to either the OEP or control, reported a 35% reduction in falls (incidence RaR 0.65, CI 95% 0.57 to 0.75) and a reduction in fall-related injuries (OR 0.56, CI 95% 0.44 to 0.71). 79 A subsequent meta-analysis from an independent research group also found that the OEP reduced rate of falls compared with non-exercise control intervention (six studies; incidence RaR 0.68, CI 95% 0.56 to 0.79). 80
Rationale for selection of Otago exercise programme
In addition to the evidence base and consideration of essential components for inclusion in an exercise programme, we reviewed models and configurations of service delivery identified from a national survey of health and social care-funded UK falls services. 81 The 2007 national scoping audit of UK falls clinics reported that most localities provided group or home programmes, usually two sessions per week, over approximately 8–12 weeks. 81 As PreFIT was designed to be pragmatic rather than explanatory, we wanted to test an exercise intervention suitable for our proposed screen-and-treat approach in primary care, thus deliverable to older people living in the community. After further consultation with clinical experts in falls prevention and rehabilitation, we selected the OEP for PreFIT. In addition to the robust evidence base for clinical effectiveness, in terms of reducing falls, with clear guidance for prescription and progression, the OEP was familiar and recognised by many services and was implemented across a number of regions; in addition, established training schedules for different health-care personnel already existed (a range of national accreditations were available). We also informed the original research group from Otago, New Zealand, of our intention to test in the UK setting (approval from Professor John Campbell, University of Otago, Otago, New Zealand, 2011, personal communication). Public Health England has since recommended both OEP and FaME as cost-effective interventions for use in the UK. 82
Content of the PreFIT exercise intervention
Overview of programme
The PreFIT exercise intervention was entirely based on the OEP, with adaptions to the duration of the programme to reflect the commonly used formulations in the NHS setting. 81 It consisted of three core components: (1) strength training, (2) balance retraining and (3) a walking plan. It was a home-based programme, individually prescribed, adapted and progressed by ability. We delivered the programme over a 6-month period, with support provided by trained physiotherapists, occupational therapists, therapy assistants or exercise assistants. A menu of five strength exercises and 12 balance exercises was available, with exercises prescribed according to ability. Participants were assessed at the first appointment and then reviewed at regular intervals and progression introduced over time. We recommended six contacts over 6 months: three face-to-face appointments and three telephone contacts. Further details of the exercise plan, training intensity, progression and procedures are described below, along with minor adaptations made for trial delivery.
Prescription of strength exercises
Five strength exercises were undertaken three times per week, allowing for rest days in between. Exercises targeted the main muscle groups in the lower limbs, including the knee flexors, knee extensors, hip abductors, ankle dorsiflexors and ankle plantarflexors. Strength training was achieved using ankle cuff weights starting from 0.5 kg and body weight as resistance. The aim was for participants to achieve moderate- to high-intensity training.
Level of intensity in the PreFIT
The therapists were trained in all aspects of the programme, including how to assess intensity and progress in individual participants. The aim was for participants to work at a moderately difficult or difficult level during leg exercises. 62 For a training stimulus to be effective, completion of 10 repetitions should be moderately difficult or hard without loss of quality of contraction. If the leg exercises were too easy, then the starting weight was insufficient, and if very hard, then the weight was too high. The physiotherapist observed a participant undertaking 10 repetitions of leg exercises in a slow, controlled manner, holding the position and then returning to the start position in a controlled way. If the participant started to hurry movements, or used trick movements (compensation), then the weight was adjusted. Number of repetitions and weights prescribed were based on baseline assessment using the CST to assess lower-leg strength, as recommended in the original programme.
Principles of progression
Progression of exercise is necessary to maintain improvement and to prevent plateau or potential reversal of training effects. Progression refers to the training load or overload, with overload meaning having to work longer or harder than normal; this is required for adaptation. The body gradually adapts to exercise repetitions and increasing weights over time; thus, overload should be applied again to progress and improve further. 83 If prescribed exercises are increased too quickly, this can hamper progression, lead to demotivation and result in injury. Related to progression, the principles of rest and recovery are also important because the amount of rest between different sets of resistance exercises can affect the metabolic, hormonal and cardiovascular response to exercise. Overexercising can lead to pain and muscle injury; thus, it was important to ensure that rest days were included in the programme, to allow muscle fibres a chance to rebuild and recover.
Prescription of balance exercises
The OEP includes a menu of 12 static and progressively dynamic balance exercises of varying levels of challenge. Balance exercises are done on at least 3 days per week, although they can be done every day. Exercises at appropriate level were prescribed according to ability during the first appointment and assessment using the 4TBS. These exercises progressed from supported balance challenge movements, (e.g. tandem stand holding onto a work surface) to more complex, unsupported movements (e.g. backwards heel-to-toe walking).
Prescription of a walking plan
Research investigating the effectiveness of walking-only interventions has found no impact on falls or fall-related injuries. Indeed, some studies have reported an increased risk of falls in certain environments, such as walking outdoors on uneven pavements. 84 However, general public health guidance and the American College of Sports Medicine recommend that older people should walk 5 days per week. 60 The OEP includes a walking plan of 30 minutes at least twice per week to increase physical capacity. The PreFIT adhered to the original programme; thus, it recommended walking, but only in conjunction with the strength and balance exercises. The walking plan advice was to walk at the usual pace for up to 30 minutes at least twice per week. Outdoor walking was recommended if the physiotherapist felt that it was safe for participants. Walks could be broken up into shorter sessions (e.g. three 10-minute daily walks) and recommendations were given about how to incorporate walking into daily activities.
Procedures for delivery of the PreFIT exercise intervention
Staff expertise and training
To ensure standardisation of intervention delivery, two research physiotherapists became fully qualified OEP leaders [Vivien Nichols and Susanne Finnegan; Later Life training (Later Life Training Ltd, Killin, UK) completed 2012]. Training was supported by the research team (JB; Later Life training completed March 2011). Trial staff members provided a 5-hour structured staff training session to all therapists responsible for delivering the exercise intervention. Therapists included physiotherapists, occupational therapists, therapy assistants and exercise therapists or instructors. Training included the key skills and competencies for delivery, including correct exercise techniques, motivational and support strategies for adherence, and the roles and responsibilities expected of therapists participating in a research trial. Each therapist received a comprehensive manual containing a detailed description of all intervention procedures along with a training certificate for continuing professional development.
Location of intervention delivery
We designed the exercise intervention for completion by participants at home, but exercises could be undertaken in a group venue or exercise class led by the trained therapist if this option was available within a region. However, it was a prerequisite that any group-based session delivered the exact PreFIT programme, with individual adaption for each participant.
Recommended number of contacts throughout the intervention
In the original OEP trials every trial participant received up to five home visits (after the assessment) over 12 months. This model of multiple home visits to older people was not feasible in the UK NHS setting. For the PreFIT, six contacts were recommended, of which at least three were to be face-to-face sessions (including the assessment) in the outpatient or community clinic setting and the remainder could be telephone calls. The first and final appointments were individual clinic appointments to last for 1 hour, with interim appointments being shorter (up to 30 minutes each). The purpose of the follow-up sessions was to assess progress, to increase resistance by providing heavier ankle weights or increasing repetitions and to prescribe more challenging balance exercises.
Duration of PreFIT exercise intervention
We recommended a 6-month supported exercise programme. This is longer than current usual NHS practice: most services provide strength and balance training for between 8 and 12 weeks. 12,13 There is good evidence to suggest that exercise programmes of longer duration, that is > 3 months, are required to sustain physical benefits. 58 We considered our 6-month programme to be the most we could reasonably expect the NHS to provide.
First appointment with trial participant
The first 1-hour appointment was arranged in an outpatient clinic, community venue or at home. The purpose of the first assessment was to conduct a brief health check, undertake baseline tests of strength and balance and to prescribe the exercise programme. The therapist first assessed general health, current fitness and walking ability before undertaking strength and balance tests. General health was screened by asking about any cardiovascular disease, osteoarthritis or rheumatoid arthritis, chronic lung disease or Parkinson’s disease, cerebrovascular disease and whether or not an inhaler or angina spray was used. The baseline tests were simple and quick, and valid and reliable tests of lower-limb strength and balance were used to determine starting level of exercise prescription. 85
Assessment of strength: chair stand test
The CST, a proxy measure of lower limb strength, was used to inform the prescription of strength exercises and to determine the starting level of ankle cuff weights. The test involves timing how long it takes to perform five consecutive sit-to-stand movements starting in a sitting position in a straight-backed firm chair, preferably with no arms, placed against a wall for safety. 23 We developed a detailed trial intervention protocol to standardise all procedures and tests. 21 The findings of the CST were used to determine starting weight and/or repetitions based on performance during the test (Table 3).
| Level | CST: criteria for prescribing strength exercises | 4TBS: criteria for prescribing balance exercises | Score |
|---|---|---|---|
| 1 |
Poor strength: completed CST using arms or took > 2 minutes with arms folded (failed test). These individuals are very weak Weight: start with a light weight (e.g. 0.5 kg) and possibly no weight at all Repetitions: consider a lower number of repetitions (e.g. five to eight repetitions) |
Failed balance test: poor balance, has difficulty with feet together stand or can only achieve feet-together stand Select from only level 1 balance exercises |
1 |
| 2 |
CST successfully completed between 1 and 2 minutes: able to stand from chair but still fairly weak Weight: start with a lighter weight (e.g. 0.5 kg) Repetitions: aim for 8 to 10 repetitions if comfortable |
Managed some of balance test. Fairly good balance. Can achieve semi tandem stand Start by selecting level 2 balance exercises and moderate according to how the participant manages |
2 |
| 3 |
CST successful: good strength (e.g. five stands within 1 minute) Weight: use a reasonable starting weight (e.g. 1 kg) Repetitions: prescribe either one or two sets of 10 repetitions |
Managed most but not all of balance test. Good balance. Can achieve semi-tandem stand and can partially or completely hold the tandem stand Start by selecting both level 2 and 3 balance exercises and moderate according to how the participant manages |
3 |
| 4 |
CST successful: very good strength (e.g. five rises within 30 seconds) Weight: use heavier weights (e.g. 1 kg or possibly 1.5 kg) Repetitions: you may need to prescribe more than 10 repetitions for patients to feel that the challenge has been moderately difficult |
Balance test successful. Excellent balance that will need quite a challenge to improve it. Can achieve single-leg stand Consider starting with level 4 exercises, but moderate the prescription according to how the participant manages |
4 |
Balance assessment: 4-test balance scale
The 4TBS, used in the original programme, involves four increasingly difficult, timed, static balance challenges: (1) the feet-together stand, (2) the semi-tandem stand, (3) the tandem stand and (4) a single-leg stand. 66,85 The test is performed with the participant in bare feet, standing close to a wall or solid object for safety, but without aids. The assessor can help the participant assume the correct foot position, but progression to the next test is allowed only if a stance can be held independently for 10 seconds (see Table 3). If this is not achieved, or if support is required, then the test is then stopped and the participant is scored at the level that can be completed.
Overview of exercises
Strength and balance exercises were carried out three times per week, but balance exercises could be undertaken daily.
Warm-up exercises
Warm-up exercises comprised five gentle mobility movements of the neck, shoulders, trunk, hips, knees and ankles, and it was recommended that these be undertaken before any strength and balance exercise.
Strength exercises
Strength exercises included front and back knee strengthening (knee extensors and flexors), side strengthening (hip abductors), and calf and toe raises (ankle plantarflexors and dorsiflexors). Ankle cuff weights were used for the knee and hip exercises, with training in how to safely apply and remove weights.
Balance exercises
Twelve static and dynamic balance exercises of four levels of difficulty were included, from a tandem stand with support (level 1) to backwards heel-to-toe walking without support (level 4).
The programme took approximately 30 minutes to complete, excluding the walking plan. The therapist explained and demonstrated each prescribed exercise and observed the participant performing the exercises to ensure that participants were confident in undertaking them independently at home.
Materials given to trial participants
At the first appointment, participants received an A5-sized PreFIT exercise folder with pictures and instructions for every exercise, with supporting information written in large font. The folder included exercise diaries and general advice about physical activity and walking. The therapist could personalise each folder by adding his/her name, contact details, details of next appointments and any additional instructions about which exercises to focus on from the longer menu, according to ability. A set of ankle cuff weights were provided and these were replaced with heavier weights as people progressed over time.
Follow-up appointments and telephone calls
Follow-up appointments were recommended at 3 and 6 weeks and at 3, 4 and 5 months, with a final assessment at 6 months. The purpose of the follow-up contacts was to modify exercise prescription and to review, adapt and progress exercises when appropriate. Progression was essential to ensure that physiological challenges continued as fitness and functional ability improved. 58 Therapists were encouraged to provide additional behavioural support to encourage compliance and motivation. 23 Follow-up telephone calls were expected to last approximately 10 minutes, although actual duration varied. The Otago research team recommended a schedule of regular telephoning to enhance compliance. 66 We provided therapists with a simple checklist of points to discuss during follow-up telephone calls.
Final appointment
The final face-to-face appointment, lasting 1 hour, was arranged at approximately 6 months after the initial appointment and baseline tests were repeated. On discharge, participants were encouraged to continue with their exercise programme and were given a ‘staying active’ leaflet, which was designed for the trial. This leaflet outlined information about purchasing weights, the benefits of continued exercise and details of other opportunities for exercising in the local area.
Comparison between the PreFIT intervention and original Otago exercise programme
Differences between the trial intervention and the original OEP included a 6-month rather than 1-year intervention, and reduction from approximately six home visits to a recommended minimum of three face-to-face contacts (and three telephone calls) with a trained therapist at a suitable venue. Given the pragmatic nature of the trial, it was not feasible to investigate a highly resource-intensive intervention that would not be suitable for large-scale roll-out across the NHS. Other than these adaptations, the original OEP was delivered as recommended. We developed additional trial-related supporting materials for therapists, to aid with exercise prescription, progression and telephone calls, but no changes were made to participant materials, exercise diagrams or instructions.
Exercise adherence
Adherence to exercise can be challenging, especially over long periods. 66 Therapists were trained to encourage participants to be actively involved in the decision-making process regarding exercise selection and goal-setting. The positive outcomes associated with exercise and walking were emphasised, such as maintaining independence and remaining active, rather than reduction or prevention of falls. 86 Calendars or diaries can improve adherence to exercise. 87 These were provided in participant folders to serve as a reminder to exercise and as a prompt to self-monitor behaviour. Diaries were reviewed at follow-up appointments and therapists provided positive and constructive feedback. 88 Additional behaviour change techniques included action planning, identification of barriers to exercise and use of problem-solving strategies, including SMART (specific, measurable, achievable, relevant and timely) goal-setting to motivate participants to continue exercising. 88
Data collection
Therapists completed a detailed exercise treatment log for each participant. At the final assessment, participants and therapists were asked to report whether or not they felt that they had improved in strength, balance and walking. Complete copies of anonymised treatment logs were returned to the study office for data entry and analysis.
Exercise quality control assessments
A research physiotherapist (SF) visited intervention sites to conduct quality control assessments. Every therapist responsible for intervention delivery was visited at least once. These visits were arranged shortly after completion of training and after the therapist had undertaken one or more assessments of trial participants. The aim was to ensure that the intervention was delivered in a standardised manner, in accordance with the trial protocol and therapist training. The quality control assessor considered therapist competency in all aspects of intervention delivery. At least one appointment, either the baseline or a follow-up contact, was observed by the quality control assessor. We used a standardised checklist to monitor exercise prescription and effective and safe delivery of the programme, including progression and adherence to the protocol. Checks were made of all trial-related documentation, including appointment spreadsheets, prescription logs, treatment forms, withdrawal forms and procedures for AE reporting. Each therapist received a written, graded report (satisfactory, minor concerns or serious concerns) and follow-up visits were arranged if necessary. The research physiotherapist provided regular clinical supervision and support to therapists throughout the duration of the trial. A password-protected online forum was developed for all therapists to post questions or comments and share experiences about the trial.
Multifactorial falls prevention intervention
Connecticut multifactorial falls prevention model
For the PreFIT, we selected the original MFFP intervention developed by Tinetti et al. 9 as the model of choice. This programme was one of the first developed and reported the greatest reduction in falling, that is, by more than 30%. 9 This falls prevention programme was developed and tested by the Connecticut Collaboration for Fall Prevention, based at Yale University School of Medicine, New Haven, CT, USA. 9,89 The common problems and hazards associated with falling included difficulties with walking or moving around, multiple medications, tripping hazards, postural hypotension, visual problems, foot problems and unsafe footwear. The original 1994 protocol was developed using a consensus approach with experienced geriatricians, physical therapists, and home care and rehabilitation nurses. The core components of the ‘Tinetti MFFP model’ included assessment and treatment of different risk factors, including (1) impairments of gait, transfers or balance, (2) four or more medications or culprit medications, (3) postural hypotension or dizziness, (4) perception and sensory deficits, including of vision or hearing or decreased sense of foot positioning, (5) foot or footwear problems (pain, numbness, bunions, etc.) and (6) environmental hazards. The clinical model has been tested and found to be effective in clinical trials, with practitioners adhering to assessment and treatment components. 9 A detailed description of the PreFIT MFFP intervention is presented elsewhere. 20 An overview of principles and definitions used is given here.
Falls services in the UK
At the time of PreFIT development, many different models of MFFP services were being delivered across the UK. The 2007 national scoping audit of UK falls clinics found that most MFFP services consisted of multidisciplinary teams (92%), although these varied widely in size, format and clinical professional representation. 81 All of the 231 UK services responding to the national audit conducted multifactorial assessment, with the most common risk factors assessed being gait and balance (91%), environment hazards (76%), medications (72%), cardiovascular health (69%) and, to a lesser extent, vision (58%). Fewer than half of services assessed feet, bone health or hearing, although some clinics involved podiatrists and specialist dietetic staff. Most MFFP clinics surveyed (83%) reported matching treatment to the findings of assessments, and the most frequently provided treatments were provision of information (e.g. leaflets or education; 94%), exercise (89%) and referral for medication review (66%). 81
Adaption of Tinetti et al.9 multifactorial falls prevention model for PreFIT
The protocol and materials developed by the Connecticut research team are publicly available for use by other clinical teams. 9,90 We reviewed the risk factors and US treatment pathways, with modifications to align with current UK recommendations for falls risk assessment and treatment, and considered suitability for widespread administration within UK primary care. We adapted and updated the original Tinetti et al. 9 programme to comply with the latest evidence at time of intervention development (e.g. NICE,11 American Geriatrics Society and British Geriatrics Society2 guidelines), developed from in-depth, high-quality systematic reviews of falls prevention and treatment literature. The PreFIT MFFP intervention therefore complied with latest research evidence and policy guidance at the time of trial launch.
Overview of PreFIT multifactorial falls prevention intervention delivery
The most feasible and generalisable model, given the large trial population involved, was for MFFP delivery to be embedded within primary care services or local community, multidisciplinary falls services. Assessment and treatment by secondary care specialist services, when available, was also an acceptable model of delivery. The final PreFIT MFFP intervention comprised an assessment performed in the GP, community or general hospital, by a GP nurse, research nurse or equivalent registered health-care professional, or by a community or hospital-based falls team. The location of the falls assessment varied by region. Appointments with participants were booked for 1 hour. The core components of the MFFP intervention included assessment and treatment of seven risk factors. The assessor conducted a detailed falls history interview, with careful consideration of potential red flags, followed by screening assessment of balance and gait [timed up and go test (TUG)], vision (Snellen eye chart test), medication screen, cardiac screen (lying and standing blood pressure), feet and footwear screen, and home environment assessment. Every risk factor is assessed in every person referred to the MFFP. The PreFIT MFFP assessment is followed by recommendations or further onward referral to another service, when indicated. Risk assessment was linked to recommended treatment pathways; however, treatment or intervention was required only when a particular risk factor was identified. Participants with impaired gait and balance or fear of falling were referred to the PreFIT exercise intervention. Onward referral to exercise therapy was consistent with all models of MFFP delivery and this did not represent contamination between the intervention arms. Content of PreFIT MFFP assessment describes the individual risk factors in detail, with brief explanation about the rationale for inclusion.
Content of PreFIT MFFP assessment
Sections of text in this chapter have been published in an open access journal and adhere to the Creative Commons licence for open access articles. 20 See Report Supplementary Material 1 for an overview of MFFP.
Risk assessment
Falls history interview
The purpose of eliciting a falls history is to identify and explore any predisposing factors leading to a fall, because careful exploration of context can provide clues about causation. Eliciting a falls history involves good communication skills and systematic enquiry about fall-related events. Assessors are trained in obtaining a clear story of one specific event, usually the most recent fall. Falls can occur in those with mixtures of characteristics, some of which might increase falls risk in a specific context. The risk of falling increases as the number of these risk factors increases; thus, any context can be described in terms of the ‘falls hazards’ that they contain. The magnitude of association of a fall with any intrinsic or environmental factor is not fixed; rather, it is contingent on additional factors influencing performance of the specific activity in question. The question is not only why the participant is prone to falling, but also why they fell on that particular occasion. This approach leads to identification of intrinsic risk factors, hazardous activities and environmental challenges, any of which may then be amenable to modification.
The PreFIT health-care assessors were trained in systematic enquiry, including the elicitation of symptoms and contextual factors that occurred before, during and after any fall-related ‘event’, including trips and stumbles. Another factor they explored was fear or worry about falling. Fear of falling is common in older adults and is associated with poor balance, falls, anxiety and depression. These fears may be reasonable, and suggest that the individual has good awareness about actual falls risk, or may be exaggerated, suggesting that the person might be overly anxious. The assessment of ‘red flags’ is integrated with the taking of falls history, although it is listed as a separate risk factor on intervention materials. Training sessions covered how to follow relevant leads in a conversation and the use of open, exploratory and closed questions. Interviews were conducted at an appropriate pace and interviewers were instructed not to rush the participants and not to interrupt inappropriately while participants were explaining events.
Screen for red flags
‘Red flags’ are any warning signs from the falls assessment that warrant referral to medical care from a general practitioner or medical specialist (e.g. suspected cardiac abnormalities, history of syncope). Assessors were trained in exploration of the combination of risk characteristics, which requires clinical judgement and the ability to link different risk factors. There are no standard, validated algorithms or interview templates, despite clinical guidelines recommending that detailed falls history interviews should be taken with every older person who falls. Therefore, with input from experienced consultant geriatricians, and after observation of falls interviews with hospitalised older adults, we developed a PreFIT template of prompt questions for a falls interview. The list of prompt questions was included in the MFFP intervention manual.
Balance and gait
Mobility is assessed using the TUG, which is a simple test to assess functional mobility. 91 The TUG involves observation and measurement of the time taken for someone to stand up from a standard chair, with arms of height 40–50 cm from the seat, walk forwards a distance of 3 metres at a normal walking pace, turn and walk back to the original sitting position. Total time taken is recorded in seconds. The chair arms can be used to push off, if needed. 92 The test is undertaken while wearing shoes and using any usual walking aid. The exact distance is measured and marked on the floor using coloured tape. Assessors are trained to look for any gait problems during the test and also at other times (e.g. on entering the assessment room). Observations are made of walking, including stride length, foot clearance, veering to one side and grabbing or lunging for room furniture. Many different cut-off points on the TUG have been used. For the PreFIT, a 14-second cut-off point on the test was taken as being predictive of falls in community-dwelling older adults. 92 This threshold was then used to generate onward referral to the PreFIT exercise programme for strength and balance retraining. Any other deficits, gait or balance problems, or fear of falling, were criteria that could warrant referral to the local PreFIT exercise programme.
Postural (orthostatic) hypotension
The prevalence of postural hypotension increases with age as a result of deterioration of the postural compensatory mechanisms. Postural hypotension can occur in people with neurodegenerative disorders, Parkinson’s disease and disorders that affect the autonomic nerves. 93 Postural hypotension can be a benign, transient event (e.g. light-headedness due to dehydration, fever, infection, overexertion or from exercise). Symptoms of postural hypotension include light-headedness, dizziness, presyncope or feeling faint, and syncope (fainting)]. Some patients present with more general complaints, including fatigue, weakness, cognitive slowing, leg buckling, visual disturbances or chest pain. 93 Loss of consciousness is usually of gradual onset but may occur suddenly. Syncope refers to a transient loss of consciousness with spontaneous recovery within minutes, and can be caused by loss of blood flow to the brain. Syncope is usually a transient occurrence that resolves as soon as pulse and blood pressure return to normal. Syncope may not necessarily mean serious medical disease; however, it is important to determine the cause. Other causes of loss of consciousness may be traumatic or non-traumatic. 93
We used a standard definition for postural or orthostatic hypotension: ‘a sustained reduction of systolic blood pressure of at least 20 mmHg or a drop in systolic blood pressure to below 100 mmHg, or a reduction of diastolic blood pressure of 10 mmHg within three minutes of standing’. 93 All participants were asked about previous symptoms or episodes of dizziness or light-headedness, and lying and standing blood pressure were measured using a calibrated, manual or electronic, sphygmomanometer. The radial pulse was taken for a full minute while the participant was lying down and electrocardiography was carried out if any abnormalities were suspected. The assessor enquired about symptoms of dizziness or light-headedness during the standing phase. Symptomatic participants received a postural hypotension leaflet, which provides advice about changing position, fluid intake, etc. Any participant with postural hypotension was referred for a full general practitioner-led medication review. If postural hypotension continued after medication review and modification, then referral to a local consultant-led falls service was recommended. The intervention manual details the recommended onward referral pathways if other problems are suspected.
Polypharmacy
With increasing age, people take increasing numbers of prescription medications, over-the-counter medications and other supplements. Four in five adults aged > 75 years take at least one regular prescribed medicine, and one-third take four or more medications. 94 Multiple medications compromise adherence and increase the likelihood of adverse medication effects. Some studies report that patients take, on average, only half of their prescribed medications as intended, and the more medications prescribed, the lower the adherence. 95 Ageing also affects capacity to absorb and excrete medicines. The risk of an AE increases 10% with each additional medication, approaching 100% for persons taking 10 or more medications. 9 Many adverse reactions to medicines could be prevented, and symptom prevention and control should be carefully balanced against the AEs of multiple medications. 94 In the absence of an easy method for determining net benefit compared with the harm of a total medication regimen, we trained GP staff in both general principles and specific steps that can lessen the likelihood of AEs of multiple medications.
Culprit medications
The original Tinetti et al. 9 programme, from 1994, highlighted specific classes of high-risk or ‘culprit’ medications. These included antihypertensives, antiarrhythmics, anticonvulsants and antidepressants among many others. Others96 have since defined the main culprit medications contributing to risk of falls as those targeting the central nervous system. Other classes of drugs, although the evidence base for the causal relationship with falls is weaker, include urinary anticholinergics and alpha-blockers. 96,97 In 2010, the National Audit of Falls and Bone Health in Older People specifically highlighted psychotropic medication and night sedation as potential causes of falling. 6 We considered the available evidence to inform the format of the PreFIT medication review. We used the national audit6 and the UK Department of Health and Social Care (DHSC) policy as guidance. 94 This policy document described different levels of review (from level 0 to level 3), relating to intensity of review, skill of assessor and whether or not the review was conducted in the presence of the patient.
PreFIT medication reviews
In the PreFIT, two levels of medication reviews were carried out: first, the assessor screened all drugs prescribed to every participant attending a MFFP assessment. This involved a face-to-face discussion about prescribed drugs and use of over-the-counter drugs during the MFFP assessment, as per DHSC level-1 review. This initial screen was for high-risk medications based on our own PreFIT classification and listing of (1) psychotropics and (2) other culprit medications. Psychotropic medications included any antidepressant, antipsychotic, sedative or anxiolytic, or mood stabiliser drugs. Other culprit medications included antihypertensives, antiarrhythmics, diuretics, vestibular suppressants, analgesics, anticonvulsants, anti-Parkinson drugs and vasodilators. Any participant prescribed one or more of these drugs was then referred for a more detailed review, that is, a general practitioner-led clinical medication review, corresponding to a level-3 review. 94 This involves a separate appointment between only the participant and general practitioner, either face to face or by telephone if the medication revision is considered minor. One or more nominated general practitioners from each practice were given training on high-risk medications in older adults, risks of polypharmacy and how to conduct a falls-related medication review. A consultant geriatrician or specialist registrar, supported by a member of the trial team, delivered training to general practitioners. Every GP randomised to MFFP intervention received medication review training.
Risk assessment: visual acuity
Vision makes an important contribution to balance. Control of posture, balance and movement involves a co-ordinated set of sensory processes that continuously encodes information from visual, body-awareness (proprioception), sensorimotor and cognitive sources. 98 The impact of visual information in the role of maintaining balance can be demonstrated by standing with our eyes closed; postural sway increases by between 20% and 70%. 99 As we age, our ability to judge distances, to detect low-contrast hazards and to process moving visual information reduces. Older people take longer to adapt to multiple sensory cues, particularly moving visual information, which can increase the risk of postural instability and falls. Impaired visual acuity (sharpness or fine detail of vision) is a risk factor for falls. Impairments in other systems, such as vestibular function, increase the importance of vision for maintaining balance during movement.
Conditions affecting vision in older adults include degenerative changes and loss of ability to accommodate to close objects (the process by which the eye can focus and adjust to different distances from objects). Eye disease in older adults can include cataracts, glaucoma and age-related macular degeneration. Cataracts develop mostly in those aged > 55 years and can interfere with vision. Signs and symptoms include blurred or hazy vision, reduced intensity of colours, increased sensitivity to glare from lights, increased difficulty with nocturnal vision and changes in the eye’s refractive error. These changes can be very gradual; however, as they worsen, visual symptoms increase in severity. Early research studies examining whether or not having glaucoma or cataracts increased the risk of falls were inconsistent. However, one high-quality trial found that expedited (within 1 month) surgery in women with cataract reduced the risk of recurrent falls and fractures compared with the usual NHS 12-month wait. 100
Other common visual problems in older adults relate to the wearing of glasses with an outdated prescription. People may be unaware of their declining vision or may not perceive the benefits of regular vision assessments. 99 Cost and/or reduced access to eye care may also be a barrier to regular vision checks, although eye checks are free for adults aged > 70 years in the UK. Studies have found that wearing bifocal glasses impairs the ability of older people to negotiate obstacles and can alter normal step pattern. 57 When followed for 1 year, older adults who wore bifocal glasses were found to be twice as likely to fall as those who wore single-vision lens glasses. 98 Bifocal glasses can add to the risk of falls because near-vision lenses impair distance vision and change depth perception, affecting the ability to detect environmental hazards. 99 Therefore, older people prone to falls should avoid wearing multifocal or bifocal glasses.
The NICE clinical practice guideline11 reviewed studies of visual interventions and concluded that there was insufficient evidence that single interventions targeting vision impairment alone prevented falls, but that referral for visual correction within a multifactorial intervention was recommended. Additional benefits from visual interventions include improvements in QoL. 11,101
Vision assessment
We asked assessors to test visual acuity using a standard 3-metre Snellen eye chart test; we chose this chart because it is used more often in primary care than a 6-metre chart because of typical room size. The Snellen chart is a screening tool and is used in conjunction with questions about last eye check and changes in eyesight to detect any visual problems. 20 Participants were asked to bring their spectacles because distance vision spectacles can be worn during the sight test. The test is carried out with the individual standing or sitting 3 metres from the chart, with the distance clearly marked on the floor using tape. 20 Anyone scoring less than 6 out of 6 in either eye should be referred to an optician for an eye test. Participants were encouraged to take advantage of the free annual eye check to which they are entitled. Whether or not treatment of cataracts was recommended was based on the level of visual impairment. If vision is barely affected, no treatment is necessary, although regular check-ups are recommended. 20 When a cataract affects QoL, surgery is recommended. 102,103 However, in the PreFIT, if we suspected a cataract and that this was having an impact on functional activities, we recommended immediate referral to an optician in the first instance.
Risk assessment: foot problems
Up to one-third of older people suffer from foot problems, such as foot pain, toe deformity, weakness or restricted range of motion. 104,105 These problems are common reasons for attending primary care services. Other UK foot surveys suggest that the main foot conditions affecting older people include nail problems, corns, calluses and toe deformities. 106 Foot problems can lead to falling: studies of multiple fallers suggest that they are more likely to have foot pain or foot deformity. 104 Inappropriate footwear and the presence of a corn or bunion are also independent risk factors for falls. 107 Shoes with an elevated heel of even medium height (4.5 cm) can increase postural sway and impair overall balance performance. 108 Other types of inappropriate footwear include shoes without straps or buckles, shoes with reduced sole contact area, soft-soled shoes and those without heel support. 104,108,109
The efficacy of including foot, footwear or podiatry assessments within MFFP has been reviewed by NICE and in Cochrane systematic reviews. 11,15,57,110 These assessments found no overall conclusive evidence of benefit of a stand-alone foot assessment and treatment interventions for preventing falls. Although policy statements agree that examination of the feet and simple footwear advice should be included within any MFFP programme, these guidelines do not specify the type or composition of assessment or specific interventions. 11
One trial104 found that a multifaceted podiatry intervention reduced the rate of falls in community-dwelling older adults with disabling foot pain compared with routine podiatry care. Rate of falls and fractures were lower in the intervention group and strength, balance and foot range of motion were also significantly improved compared with those receiving usual podiatry care. 104 Given that UK policy falls assessment guidance recommends that feet and footwear examination and appropriate treatment should be undertaken, albeit unspecified, we developed a bespoke PreFIT foot assessment protocol and onward referral pathway.
Foot and footwear assessment
All participants were screened for foot problems, including pain, numbness, diabetes and regular attendance at chiropody or podiatry. A visual examination was made to check for bunions, hammertoes, calluses or toenails that may cause pain or gait disturbances. Tests were undertaken for proprioception (big-toe positioning with eyes closed) and for sensation by brushing a cotton wool ball lightly across both feet, with the sternum used as normative reference. Assessment of footwear was undertaken and an advice leaflet was given on proper-fitting shoes (e.g. wide fitting, low heel height, slightly bevelled heel, good supportive heel collar, a thin firm midsole to allow sensory input and slip-resistant sole). Referrals were made to podiatry or chiropody if these services were available in the local area.
Risk factor: environmental hazards
Policy-makers and older people often cite environmental hazards in the home as risk factors for falling. 2,11 However, it is difficult to single out the most effective intervention within any home environment assessment or modification programme. One good-quality trial111 found that home hazard assessment with a supervised modification programme reduced falls in those recently discharged from hospital. However, the association between domestic hazards and falling has been controversial. In those without a history of falls, there is no evidence of clinical effectiveness of home hazard asessments. 11 Six secondary prevention trials of home hazard modification interventions have reported effectiveness, although this observed effect is unlikely to be from the home interventions alone, because of the reductions in falls that occurred outside the home. 11,15 Home hazard removal and advice about functional activities is likely to be most effective in reducing falls in those individuals with visual impairment. 15
Benefit is achieved only if home hazard assessment includes a comprehensive functional assessment followed up with specific intervention. This, however, applies to all components of assessment and intervention programmes, not just home hazards. There is no evidence to support screening for home hazards without direct observation of the individual carrying out functional tasks in their home environment. 112,113 The American Geriatrics Society and British Geriatrics Society guidance2 states that interventions should include the adaptation or modification of the home environment to mitigate hazards as well as evaluation and intervention to promote the safe performance of daily activities. Joint problem-solving is also recommended. 114
Assessment of environmental hazards
It was not intended that all participants would have a home assessment, but if assessors had any concerns about the home situation or participants’ safety when performing activities then they made an onward referral to occupational therapy or social services. In 2004, NICE guidance specified that a suitably trained member of the health-care team (clinician, occupational therapist, nurse, physiotherapist or other trained assessor) could conduct home hazard assessments. 11 Home environment screening questions were used as per the intervention manual and in conjunction with observation during the walking test. 20 All participants in the MFFP arm who might benefit from simple advice on home safety received the home safety tip sheet. This advice sheet covers common hazards and tripping risks, putting lights on when rising to the bathroom in the middle of the night, how to seek help for installation of handrails, raised toilet seats, etc.
Factors not included in the PreFIT multifactorial falls prevention assessment
Detailed tests of urinary incontinence, hearing, osteoporosis risk and comprehensive assessment of neurological and cardiac systems are not part of the MFFP package. The falls interview included screening questions on urinary incontinence in relation to any fall or near-miss event. At the time of development, none of the published clinical trials investigating MFFP included screening of osteoporotic risk, although in many cases the description of intervention content was inadequate. 15 We purposefully excluded the addition of a protocol that included provision of bisphosphonates and bone medications to avoid the possibility of interpreting MFFP as effective when medications could have been responsible for any observed effect. We were cognisant of the different clinical backgrounds of assessors and barriers to accessing trained medical practitioners in some settings. Safety was also a consideration; for example, we did not ask assessors to undertake carotid artery stimulation to check for carotid sinus hypersensitivity.
Staff training
Health-care staff responsible for delivering the MFFP intervention received 4–5 hours of structured training, either in the GP or in hospital. Health-care staff members undertaking falls assessments ranged from experienced falls team personnel (consultant geriatricians, falls nurses, occupational therapists or physiotherapists) to GP or research staff members (e.g. advanced nurse practitioners, practice nurses and/or research nurses). Staff members were required to have a nursing or allied health-care background with professional registration. Training in medication reviews was given either to one nominated lead general practitioner or to all general practitioners in each GP randomised to MFFP as a scheduled educational session. A geriatrician with extensive experience in falls assessment (RS, KW, SR or JT) and senior researcher from the trial team jointly provided training (JB). Each assessor received a detailed intervention reference manual before training, which described risk factors, treatment pathways, trial procedures, flow charts for onward referral, etc. Laminated prompt charts and easy-to-use instruction sheets were provided (e.g. for medication assessments).
Recommended treatments
Assessors provided verbal and written advice to trial participants and also arranged onward referrals to consultant-led falls services, physiotherapy, GPs, occupational therapy, social services, etc., as per the treatment pathways we recommended for each risk factor and as per detailed manuals and the published intervention paper. 20 Trial intervention manuals will be available from the University of Warwick repository (http://wrap.warwick.ac.uk/85689/; accessed 7 October 2020).
Data collection
Assessors completed a MFFP risk assessment form for every participant randomised to MFFP. All risk factors and test findings were recorded. For onward actions or referrals, the date of referral was recorded, as was the name of person or doctor referred to. Completed copies of falls risk assessment forms with study identification number (name redacted) were returned to the study office for data entry and analysis.
Multifactorial falls prevention quality control assessments
Each trained assessor was observed while carrying out a falls assessment (with verbal permission from the participant) to ensure compliance with the intervention procedures. These quality control visits were carried out, after the assessor had been trained and carried out one or more MFFP assessments, by trial staff members (JB or SF) or by a consultant geriatrician (RS) or, in the Devon region, by a medical registrar with expertise in falls assessments (Lindsey Rohan or Meera Sritharan). A 37-item standardised checklist was developed and used to ensure that all aspects of the MFFP assessment were reviewed during each quality control visit. No input was given during the quality control assessment, unless the assessor queried a specific issue. A written, signed, graded (satisfactory, minor concerns or serious concerns) report was given to assessors, with a follow-up visit arranged if necessary. The research team provided regular supervision and support to therapists throughout the duration of the trial. Experienced medical staff members were available to deal with any complex clinical queries (KW and RS).
Chapter 4 Results
Study timeline
Trial recruitment started in September 2010, with a pilot phase conducted in 12 GPs in Devon to refine procedures. Recruitment paused from March to September 2012, while pilot data were reviewed by the funder, and restarted in September 2012. The final GPs were randomised in June 2014 and postal follow-up finished in 2016. NHS Digital provided final HES data sets including data up to the end of March 2016 in 2018. Data from all phases of the trial were combined for the analysis because there were minimal changes after the pilot.
Cluster (general practice)-level data
Information and advertisements about the trial were widely distributed via primary care newsletters and regional primary care research events. We received expressions of interest from 82 GPs. Sixty-three GPs were randomised from six localities across England: Birmingham and the Black Country (n = 2), Cambridgeshire (n = 6), Devon (n = 18), Warwickshire and Herefordshire (n = 12), Newcastle upon Tyne (n = 11) and Worcestershire (n = 14). We exceeded our GP recruitment target because we had three GPs already prepared for study entry at the time we randomised our 60th GP. The final triad of GPs was randomised and split across two different regions (Newcastle upon Tyne, and Birmingham and the Black Country). No GPs withdrew from the study. One GP closed down after recruitment and completion of intervention delivery, and the participants were registered with a new GP. This did not affect participant follow-up. We were, however, unable to obtain GP records for participants from this surgery. Figure 1 presents the cluster CONSORT flow chart for GPs. 19
FIGURE 1.
Cluster CONSORT flow diagram by GPs. a, Fall risk screener and attended treatment.
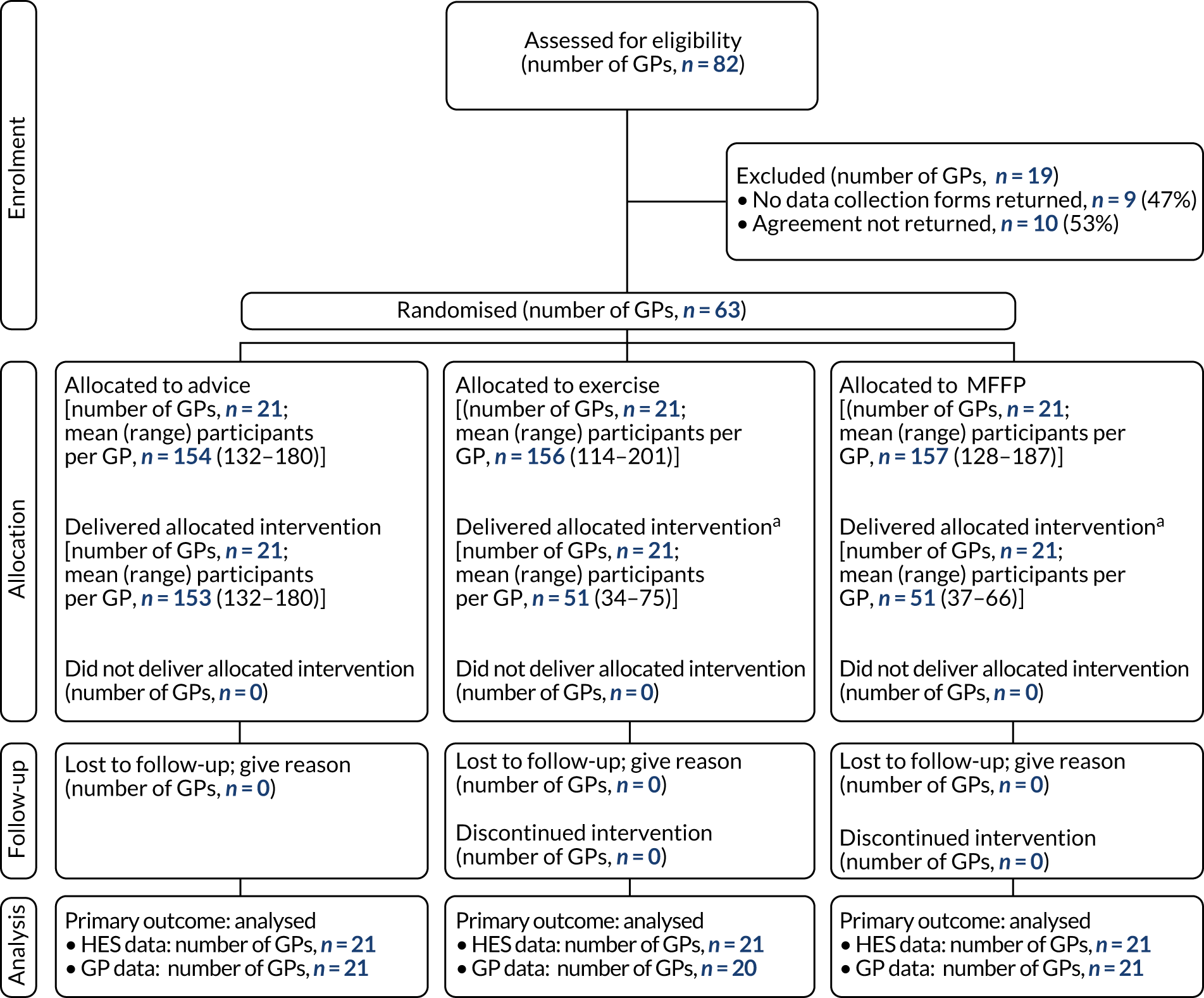
Sociodemographic characteristics of recruited general practices
Of the 63 GPs, 13 (21%) were in areas of social deprivation (score 1–3), 22 (35%) were in affluent areas (score 8–10) and the remainder were categorised as moderate [n = 28 (44%); score 4–7].
Participant recruitment and allocation
Participant-level flow is presented in the CONSORT diagram for participants (Figure 2). We invited 29,010 people to take part in the trial (see Appendix 1, Table 31). The mean number of people invited per GP was 387 (range 170–608). Out of the 29,010 participants, 9819 (33.8%) gave consent and returned baseline questionnaires. Nine people withdrew and seven died after providing consent and prior to GP randomisation. We randomised 9803 out of 29,010 participants (33.8% of those invited). The mean number of participants randomised per GP was 156 (range 115–201). Trial arms were well balanced for size, with 3223 (32.9%), 3279 (33.4%) and 3301 (33.7%) out of 9803 participants being allocated to advice, exercise and MFFP, respectively (see Appendix 1, Table 32).
FIGURE 2.
A CONSORT flow diagram by participants. a, Fall risk screener and attended treatment.
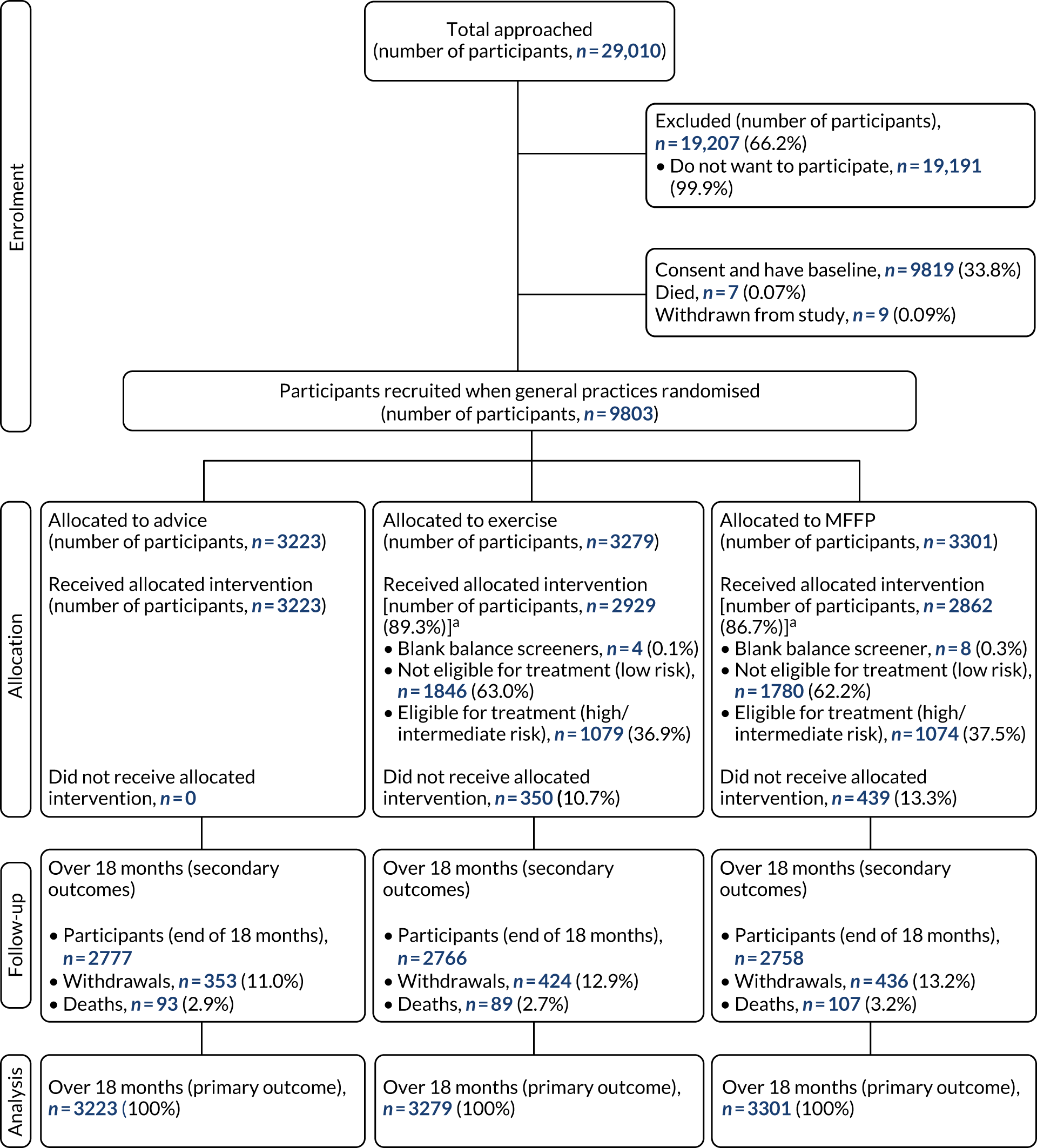
Completeness of primary outcome data
Useable fracture data were available for all participants for the whole study period. We obtained HES data for 9802 out of 9803 participants, with only one participant not providing permission for access. No participants withdrew consent for access to HES data after randomisation. Participant data were missing from one GP randomised to the exercise arm (159/9803, 1.6%), although HES data were available. There were 458 fractures confirmed by the adjudication panel during the 18-month follow-up period. Fractures were identified in both HES and GP records for 279 out of 458 (60.9%) fractures, only in HES records for 88 out of 458 (12.2%) fractures and only in GP records for 91 out of 458 (19.9%) fractures. Therefore, for 458 fracture events, source of fracture confirmation was from HES data for 367 (80.1%) participants and from GP records for the remaining 91 (19.9%) participants. The level of agreement (kappa statistic) between HES and GP records was 0.75 (95% CI 0.71 to 0.78; p < 0.001).
Completeness of secondary outcome data
Over the 18-month follow-up period, 1213 (12.4%) participants withdrew from the trial and 289 (2.9%) died. Response rates to postal questionnaires were good: 9064 (92.5%), 8578 (87.5%), 8136 (83.0%) and 7490 (76.4%) at 4, 8, 12 and 18 months’ follow-up, respectively (see Appendix 1, Table 32). There were no between-group differences in the numbers or characteristics of withdrawals and dropouts by treatment arm over the 18-month follow-up period. Participants who withdrew were more likely to be female, older, frailer and have a history of falling and poorer physical and mental health than those who remained in the trial. The characteristics of the randomised and analysed sample were very similar at baseline. Missingness in reporting of falls outcomes increased as the study progressed, similar to response rates. Missingness of falls outcome data was 10.0% at 4 months, 13.9% at 8 months, 18.1% at 12 months and 24.6% at 18 months.
Baseline characteristics of trial participants
Overall, our three groups were well balanced at baseline (Table 4). Participants’ mean age was 78 years (range 70–101 years) and just over half were female (5150/9803, 52.5%). The majority of participants were white and either married or cohabiting, and one-third lived alone. The mean age at leaving full-time education was 17 years. Sociodemographic characteristics were well balanced across treatment arms. Most participants were able to go outside unaided, although 20% (1913/9803) required a stick or support. Overall, participants were moderately active, with three-quarters self-reporting that they walked ≥ 1 hour every day. The majority of participants had no cognition impairment, although 9% (870/9803) had lower scores (0–4) on the clock-drawing test, indicating a degree of cognitive impairment.
| Characteristic | Advice (n = 3223) | Exercise (n = 3279) | MFFP (n = 3301) | Total (n = 9803) |
|---|---|---|---|---|
| Age (years), mean (SD) | 77.9 (5.7) | 78.1 (5.7) | 77.8 (5.7) | 77.9 (5.7) |
| Age (years), range | 70–101 | 70–100 | 70–101 | 70–101 |
| Age bands (years), n (%) | ||||
| 70–79 | 2140 (66.4) | 2168 (66.1) | 2247 (68.1) | 6555 (66.9) |
| 80–89 | 992 (30.8) | 990 (30.2) | 952 (28.8) | 2934 (29.9) |
| ≥ 90 | 91 (2.8) | 121 (3.7) | 102 (3.1) | 314 (3.2) |
| Female, n (%) | 1666 (51.7) | 1724 (52.6) | 1760 (53.3) | 5150 (52.5) |
| Ethnicity, n (%) | ||||
| White | 3166 (98.2) | 3225 (98.3) | 3239 (98.1) | 9630 (98.2) |
| Other | 30 (1.0) | 25 (0.8) | 39 (1.2) | 94 (1.0) |
| Missing | 27 (0.8) | 29 (0.9) | 23 (0.7) | 79 (0.8) |
| Marital status, n (%) | ||||
| Married/cohabiting | 2050 (63.6) | 2035 (62.1) | 2085 (61.3) | 6170 (62.9) |
| Widowed | 857 (26.6) | 887 (27.0) | 833 (25.2) | 2577 (26.3) |
| Divorced/separated | 175 (5.4) | 236 (7.2) | 212 (6.4) | 623 (6.4) |
| Single | 130 (4.0) | 108 (3.3) | 157 (3.8) | 395 (4.0) |
| Missing | 11 (0.4) | 13 (0.4) | 14 (0.4) | 38 (0.4) |
| Living arrangement, n (%) | ||||
| Live alone | 1048 (32.5) | 1104 (33.7) | 1065 (32.3) | 3217 (32.8) |
| Live with others | 2155 (66.9) | 2154 (65.7) | 2219 (67.2) | 6528 (66.6) |
| Missing | 20 (0.6) | 21 (0.6) | 17 (0.5) | 58 (0.6) |
| Age left FTE (years), mean (SD) | 16.8 (4.6) | 16.7 (4.6) | 16.9 (4.8) | 16.8 (4.7) |
| BMI (kg/m2), mean (SD) | 26.4 (4.7) | 26.5 (4.5) | 26.4 (4.6) | 26.5 (4.6) |
| Balance difficulties walking on level, n (%) | ||||
| Never/sometimes | 2923 (90.7) | 2994 (91.3) | 2988 (90.5) | 8905 (90.8) |
| Often/very often/always | 280 (8.7) | 268 (8.2) | 301 (9.1) | 849 (8.7) |
| Missing | 20 (0.6) | 17 (0.5) | 12 (0.4) | 49 (0.5) |
| Able to get outside, n (%) | ||||
| Unaided | 2580 (80.0) | 2599 (79.3) | 2632 (79.7) | 7811 (79.7) |
| With stick/support or help only | 611 (19.0) | 656 (20.0) | 646 (19.6) | 1913 (19.5) |
| Cannot get outside at all | 16 (0.5) | 11 (0.3) | 12 (0.4) | 39 (0.4) |
| Missing | 16 (0.5) | 13 (0.4) | 11 (0.3) | 40 (0.4) |
| On average, hours/day walking | ||||
| < 1 | 822 (25.5) | 847 (25.8) | 897 (27.2) | 2566 (26.2) |
| 1–2 | 1213 (37.7) | 1271 (38.8) | 1212 (36.7) | 3696 (37.7) |
| > 2 | 1167 (36.2) | 1145 (34.9) | 1180 (35.7) | 3492 (35.6) |
| Missing | 21 (0.6) | 16 (0.5) | 12 (0.4) | 49 (0.5) |
| HRQoL (EQ-5D-3L), mean score (SD)a | 0.76 (0.23) | 0.78 (0.23) | 0.77 (0.24) | 0.77 (0.23) |
| Missing | 143 (4.4) | 146 (4.5) | 170 (5.2) | 459 (4.7) |
| HRQoL (SF-12 PCS), mean score (SD)b | 50.3 (10.2) | 50.5 (10.3) | 50.0 (10.5) | 50.3 (10.3) |
| HRQoL (SF-12 MCS), mean score (SD)b | 50.2 (9.3) | 50.3 (8.9) | 50.1 (9.3) | 50.2 (9.2) |
| Missing | 320 (9.9) | 326 (9.9) | 338 (10.2) | 984 (10.0) |
| Clock-drawing test score, n (%)c | ||||
| 0–4 | 294 (9.1) | 271 (8.3) | 305 (9.2) | 305 (9.2) |
| 5–6 | 2882 (89.4) | 2952 (90.0) | 2917 (88.4) | 2917 (88.4) |
| Missing | 47 (1.5) | 56 (1.7) | 79 (2.4) | 79 (2.4) |
| Frailty, n (%) | ||||
| Frail | 647 (20.1) | 625 (19.1) | 733 (22.2) | 2005 (20.5) |
| Non-frail | 2535 (78.6) | 2603 (79.4) | 2528 (76.6) | 7666 (78.2) |
| Missing | 41 (1.3) | 51 (1.5) | 40 (1.2) | 132 (1.3) |
| Comorbidities, n (%) | ||||
| None | 752 (23.3) | 767 (23.4) | 792 (24.0) | 2311 (23.5) |
| One or two | 1873 (58.1) | 1902 (58.0) | 1897 (57.5) | 5672 (57.9) |
| Three or more | 598 (18.6) | 610 (18.6) | 612 (18.5) | 1820 (18.6) |
| Fallen in previous year, n (%) | ||||
| Yes | 1019 (31.6) | 1033 (31.5) | 1098 (33.3) | 3150 (32.1) |
| No | 2179 (67.6) | 2225 (67.9) | 2183 (66.1) | 6587 (67.2) |
| Missing | 25 (0.8) | 21 (0.6) | 20 (0.6) | 66 (0.7) |
| n (%) with fall-related fracture in previous year, self-report | 106 (3.3) | 112 (3.4) | 106 (3.1) | 324 (3.3) |
| n (%) at higher risk of falling, baseline questionnaire | 1382 (42.9) | 1422 (43.4) | 1487 (45.1) | 4291 (43.8) |
| Missing | 2 (0.06) | 5 (0.2) | 1 (0.03) | 8 (0.1) |
One-quarter of the sample selected no comorbidity from the list of common conditions, over half selected one or two conditions and 20% reported having three or more medical conditions diagnosed by a doctor (see Table 4). The most common self-reported comorbidity was arthritis, with almost half reporting rheumatoid arthritis or osteoarthritis (4403/9803, 44.9%). Heart troubles or angina (2679/9803, 27.5%) and diabetes (1403/9803, 14.3%) were also common. The prevalence of frailty was 21% (2005/9803). HRQoL scores indicated good levels of physical health-related and mental health-related QoL on both the SF-12 and EQ-5D-3L measures (see Table 4). Pain and discomfort was common, with 60% reporting moderate or severe pain. One-fifth of respondents reported moderate or severe anxiety and depression. The most frequently reported ADL difficulty was taking a bath, with 15% reporting a lot of difficulty or being unable to do. Most participants (> 90%) reported no difficulty or only a little difficulty performing other ADL.
History of falls and fractures in previous year
Out of 9803 people randomised, 3150 (32.1%) had fallen at least once in the previous year (see Table 4) and 4291 (43.8%) were deemed at higher risk of falling based on responses to the questions in the baseline questionnaire. A total of 324 (3.3%) participants reported having suffered a fall-related fracture in the year prior to recruitment. Although the proportions of participants falling, sustaining a fracture and being at risk of falling were similar across all three treatment groups on entry to the trial, participants in the MFFP arm reported a non-statistically significant higher falls rate in the previous year than those in the advice and exercise arms (87.3 vs. 70.6 and 73.5 falls per 100 years, respectively). This was due to five extreme fallers and, when extreme observations were removed, falls rates were very similar by treatment arm.
Primary outcome: fractures
We had a total mean follow-up period of 18.4 (SD 0.3) months, with a total of 14,853 person-years of follow-up data. There were no differences by treatment arm. The number of participants sustaining one or more fractures over 18 months’ follow-up was 379 out of 9803 (3.9%) (Tables 5 and 6). The total number of fractures by treatment arm was 133, 152, and 173 in those randomised to advice, exercise and MFFP, respectively. The unadjusted fracture rates were 2.76, 3.06 and 3.50 per person per 100 years in those randomised to advice, exercise and MFFP, respectively (see Table 5).
| Outcome | Advice | Exercise | MFFP | Total |
|---|---|---|---|---|
| From randomisation to 18-month follow-up | ||||
| Randomised, n | 3223 | 3279 | 3301 | 9803 |
| Fractures, n | 133 | 152 | 173 | 458 |
| Unadjusteda fracture rate over 18 months (95% CI) per person per 100 years | 2.76 (2.76 to 2.76) | 3.06 (3.06 to 3.06) | 3.50 (3.50 to 3.50) | 3.10 (3.10 to 3.10) |
| Adjustedb fracture rate over 18 months (95% CI) per person per 100 years | 2.59 (2.53 to 2.67) | 3.24 (3.15 to 3.33) | 3.50 (3.39 to 3.60) | 3.12 (3.06 to 3.17) |
| Participants with one or more fractures, n (%) | 110 (3.4) | 126 (3.8) | 143 (4.3) | 379 (3.9) |
| Total number of person-years of follow-up | 4868.5 | 4981.2 | 4985.3 | 14,853.0 |
| Participants with two or more fractures, n | 17 | 22 | 22 | 61 |
| Fracture episodes, n | 118 | 131 | 153 | 402 |
| Time to first fracture (months), median (IQR) | 8.5 (3.9–14.5) | 10.4 (5.1–14.1) | 9.1 (4.6–12.9) | 9.4 (4.4–13.6) |
| Time to first fracture, HRc (95% CI; p-value) | ||||
| Exercise vs. advice | 1.12 (0.87 to 1.45; 0.38) | |||
| MFFP vs. advice | 1.28 (0.99 to 1.63; 0.055) | |||
| Site of participant fracture | ||||
| Hip (S72), n (%) | 33 (1.0) | 26 (0.8) | 28 (0.9) | 87 (0.9) |
| Chi-squared test (p-value) | 0.52 | |||
| Exercise vs. advice (pairwise p-value) | 0.26 | |||
| MFFP vs. advice (pairwise p-value) | 0.52 | |||
| Wrist (S62.1, S62.8, S52.5), n (%) | 20 (0.6) | 23 (0.7) | 34 (1.0) | 77 (0.8) |
| Chi-squared test (p-value) | 0.21 | |||
| Exercise vs. advice (pairwise p-value) | 0.88 | |||
| MFFP vs. advice (pairwise p-value) | 0.13 | |||
| Fracture outcomes from randomisation to maximum follow-upd | ||||
| Fractures, n | 213 | 234 | 248 | 695 |
| Participants with one or more fracture, n (%) | 171 (5.3) | 188 (5.7) | 198 (6.0) | 557 |
| Total number of person-years of follow-up | 9089.1 | 9221.1 | 9189.9 | 27,500.0 |
| Unadjusteda fracture rate (95% CI) per person per 100 years | 2.36 (2.36 to 2.36) | 2.54 (2.54 to 2.54) | 2.73 (2.73 to 2.73) | 2.54 (2.54 to 2.55) |
| Adjustedb fracture rate (95% CI) per person per 100 years | 2.26 (2.20 to 2.31) | 2.67 (2.59 to 2.74) | 2.80 (2.72 to 2.89) | 2.58 (2.54 to 2.62) |
| Hip (S72) | 57 (1.8) | 51 (1.6) | 47 (1.4) | 155 (1.6) |
| Wrist (S62.1, S62.8, S52.5) | 31 (1.0) | 32 (1.0) | 42 (1.3) | 105 (1.1) |
| Time point (months) | Advice (N = 3223), n | Exercise (N = 3279), n | MFFP (N = 3301), n | Total (N = 9803), n |
|---|---|---|---|---|
| 0–4 | 35 | 35 | 37 | 107 |
| 4–8 | 26 | 25 | 38 | 89 |
| 8–12 | 28 | 36 | 47 | 111 |
| 12–18 | 44 | 56 | 51 | 151 |
| Total (%) fractures | 133 (4.1) | 152 (4.6) | 173 (5.2) | 458 (4.7) |
Primary analysis
For the primary ITT analyses, the fracture RaR for comparison of advice with exercise was 1.20 (95% CI 0.91 to 1.59), indicating a statistically non-significant increase in fracture rate in the exercise group (Table 7). For the comparison of advice with MFFP, the fracture RaR was 1.30 (95% CI 0.99 to 1.71), indicating an increase in fracture rate of borderline statistical significance in the MFFP group. The ICC value for between-cluster variance was almost zero (ICC 0.00001).
| Analysis | Exercise vs. advice, RaR (95% CI) | p-value | MFFP vs. advice, RaR (95% CI) | p-value |
|---|---|---|---|---|
| Primary outcome: fracture rate over 18 months (total 458 fractures), ITT analysis | ||||
| Unadjusted RaR | 1.11 (0.84 to 1.46) | 0.45 | 1.27 (0.97 to 1.66) | 0.08 |
| Adjusteda RaR | 1.20 (0.91 to 1.59) | 0.19 | 1.30 (0.99 to 1.71) | 0.06 |
| Fracture rate over 18 months (total 227 fractures), nested ITT by higher-risk strata only | ||||
| Unadjusted RaR | 0.86 (0.60 to 1.24) | 0.43 | 1.20 (0.85 to 1.68) | 0.29 |
| Adjusteda RaR | 0.94 (0.65 to 1.35) | 0.73 | 1.26 (0.89 to 1.78) | 0.20 |
| Fracture rate over 18 months (total 458 fractures), CACE | ||||
| Unadjusted RaR | 1.10 (0.64 to 1.87) | 0.73 | 1.40 (0.80 to 2.50) | 0.26 |
| Adjusteda RaR | 1.80 (0.81 to 4.05) | 0.15 | 1.64 (0.95 to 2.81) | 0.08 |
Other fracture analyses
We found no differences in time to first fracture between the exercise arm and the advice arm [hazard ratio (HR) 1.12, 95% CI 0.87 to 1.45; p = 0.38]. Time to first fracture was shorter for those randomised to MFFP than for those randomised to advice, but the difference was of borderline statistical significance (adjusted Cox regression model HR 1.28, 95% CI 0.99 to 1.63; p = 0.055). The Kaplan–Meier survival curve for time to first fracture, among all participants, by treatment group is shown in Figure 3. We found no difference in number of people with hip or wrist fractures by treatment arm (see Table 5). A total of 87 participants sustained a hip fracture over the 18-month follow-up period and, of these, one participant in the exercise arm sustained more than one hip fracture. Seventy-seven participants sustained a wrist or forearm fracture and, of these, three participants had more than one fracture (exercise, n = 1; MFFP, n = 2).
FIGURE 3.
Kaplan–Meier curve for time to first fracture over 18 months (n = 9803).
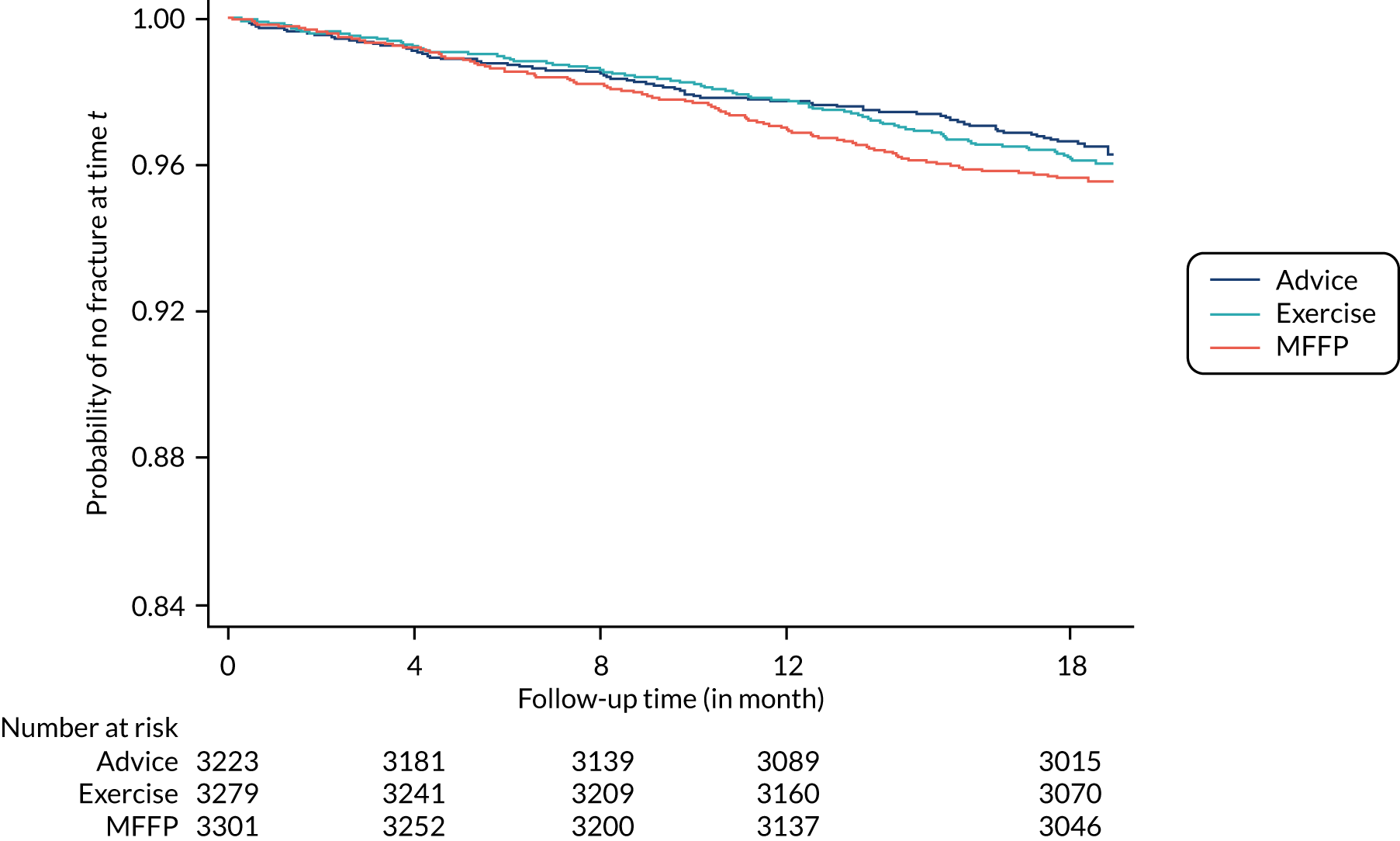
Nested intention-to-treat analysis by higher risk
Overall, more people determined from the baseline questionnaire to be at higher risk of falling sustained a fracture. A total of 4291 out of 9803 (43.8%) participants were at higher risk of falling and, out of these, 227 (5.3%) sustained one or more fractures over 18 months, compared with 152 out of 5504 (2.8%) participants considered at lower risk of falling. For the nested ITT comparison of those at higher risk of falling in the advice arm and exercise arms, the adjusted fracture RaR was 0.94 (95% CI 0.65 to 1.35) over 18 months, indicating no difference in fracture rates between treatment groups (see Table 7). For the comparison of those at higher risk in the advice arm and the higher-risk MFFP arm over 18 months’ follow-up, the fracture RaR was 1.26 (95% CI 0.89 to 1.78).
Complier-average causal effect
Out of the 6580 participants randomised to active treatment, 5085 (77.3%) complied with the postal fall risk screener and attended treatment. Compliance with screening and treatment was similar between the active intervention arms [exercise, 2543/3279 (77.6%); MFFP 2542/3301 (77.0%)]. We found no statistically significant difference in rate of fractures over 18 months by compliance status (see Table 7).
Subgroup analyses
We undertook subgroup analyses as per the prespecified analysis plan; thus, treatment effects were compared by age, sex, history of falling, frailty and cognitive impairment (Table 8). No statistically significant subgroup effects were found in the RaR of fractures over 18 months by baseline participant characteristics.
| Subgroup | Advice vs. exercise | Advice vs. MFFP | ||
|---|---|---|---|---|
| Fracture, RaR (95% CI) | p-valuea | Fracture, RaR (95% CI) | p-valuea | |
| Age (years) | ||||
| ≤ 80 (n = 7022) | 1.33 (0.92 to 1.91) | 0.16 | 1.40 (0.98 to 2.00) | 0.55 |
| > 80 (n = 2781) | 0.90 (0.59 to 1.35) | 1.19 (0.79 to 1.78) | ||
| Sex | ||||
| Male (n = 4653) | 1.13 (0.72 to 1.77) | 0.92 | 1.03 (0.65 to 1.63) | 0.33 |
| Female (n = 5150) | 1.10 (0.78 to 1.54) | 1.36 (0.98 to 1.90) | ||
| Fallen in previous year | ||||
| No (n = 6587) | 1.26 (0.89 to 1.80) | 0.41 | 1.26 (0.88 to 1.79) | 0.92 |
| Yes (n = 3150) | 1.00 (0.65 to 1.54) | 1.29 (0.85 to 1.95) | ||
| Frailty | ||||
| Non-frail (n = 7666) | 1.17 (0.85 to 1.62) | 0.44 | 1.19 (0.86 to 1.65) | 0.92 |
| Frail (n = 2005) | 0.97 (0.59 to 1.62) | 1.23 (0.76 to 1.98) | ||
| Cognition | ||||
| Score 0–4 (n = 870) | 1.20 (0.56 to 2.56) | 0.81 | 1.17 (0.56 to 2.44) | 0.90 |
| Score 5–6 (n = 8751) | 1.09 (0.81 to 1.46) | 1.23 (0.92 to 1.65) | ||
Fracture events beyond 18 months
Using HES to examine fracture events to the maximal available follow-up (27,500 person-years), total number of fractures increased from 458 to 695 events (see Table 5). Therefore, 557 participants sustained a fracture over the longer term. For the comparison of advice with exercise (ITT analysis only), the fracture RaR was 1.14 (95% CI 0.91 to 1.43; p = 0.26). For the comparison of advice with MFFP, the fracture RaR was 1.20 (95% CI 0.96 to 1.50; p = 0.11). The number of participants sustaining a hip or wrist fracture increased over time (155 and 105 participants, respectively) (see Table 5).
Secondary outcomes
Falls reported in questionnaires
We found no differences in falls rates over the 18-month follow-up by treatment group (Table 9). However, the falls rate was lower over months 4–8 in those randomised to exercise than in those randomised advice (adjusted RaR 0.78, 95% CI 0.64 to 0.96; p < 0.001). No differences were observed in fall RaRs at other interim time points, from randomisation to 4 months, months 8–12 months or months 12–18 (see Tables 9 and 10). Falls distribution by treatment arm over time is shown in Figure 4. The characteristics of participants who provided falls outcomes, that is, who returned questionnaires at 18 months, were also considered (Table 11).
| Analysis | Advice | Exercise | MFFP | Total |
|---|---|---|---|---|
| Randomised, n | 3223 | 3279 | 3301 | 9803 |
| One or more falls over 18 months, n (%) | 1276 (39.6) | 1277 (38.9) | 1301 (39.4) | 3854 (39.3) |
| Two or more falls over 18 months, n (%) | 715 (22.2) | 687 (21.0) | 743 (22.5) | 2145 (21.9) |
| Fallers, n (%) | ||||
| 0–4 months | 586 (18.2) | 605 (18.5) | 605 (18.3) | 1796 (18.3) |
| 4–8 months | 539 (16.7) | 458 (14.0) | 555 (16.8) | 1552 (15.8) |
| 8–12 months | 514 (16.0) | 500 (15.3) | 502 (15.2) | 1516 (15.5) |
| 12–18 months | 455 (14.1) | 450 (13.7) | 470 (14.3) | 1375 (14.0) |
| Unadjusted fall rate over 18 months per person per 100 years (95% CI) | 114.7 (113.8 to 115.7) | 108.0 (107.3 to 109.0) | 126.2 (125.4 to 127.1) | 116.4 (115.8 to 116.9) |
| Adjusteda falls over 18 months per person per 100 years (95% CI) | 105.6 (100.8 to 110.4) | 104.4 (98.4 to 110.4) | 127.2 (117.6 to 136.8) | 112.8 (108.0 to 116.4) |
FIGURE 4.
Plot of distribution of falls by treatment group over time (a) advice; (b) exercise; and (c) MFFP.
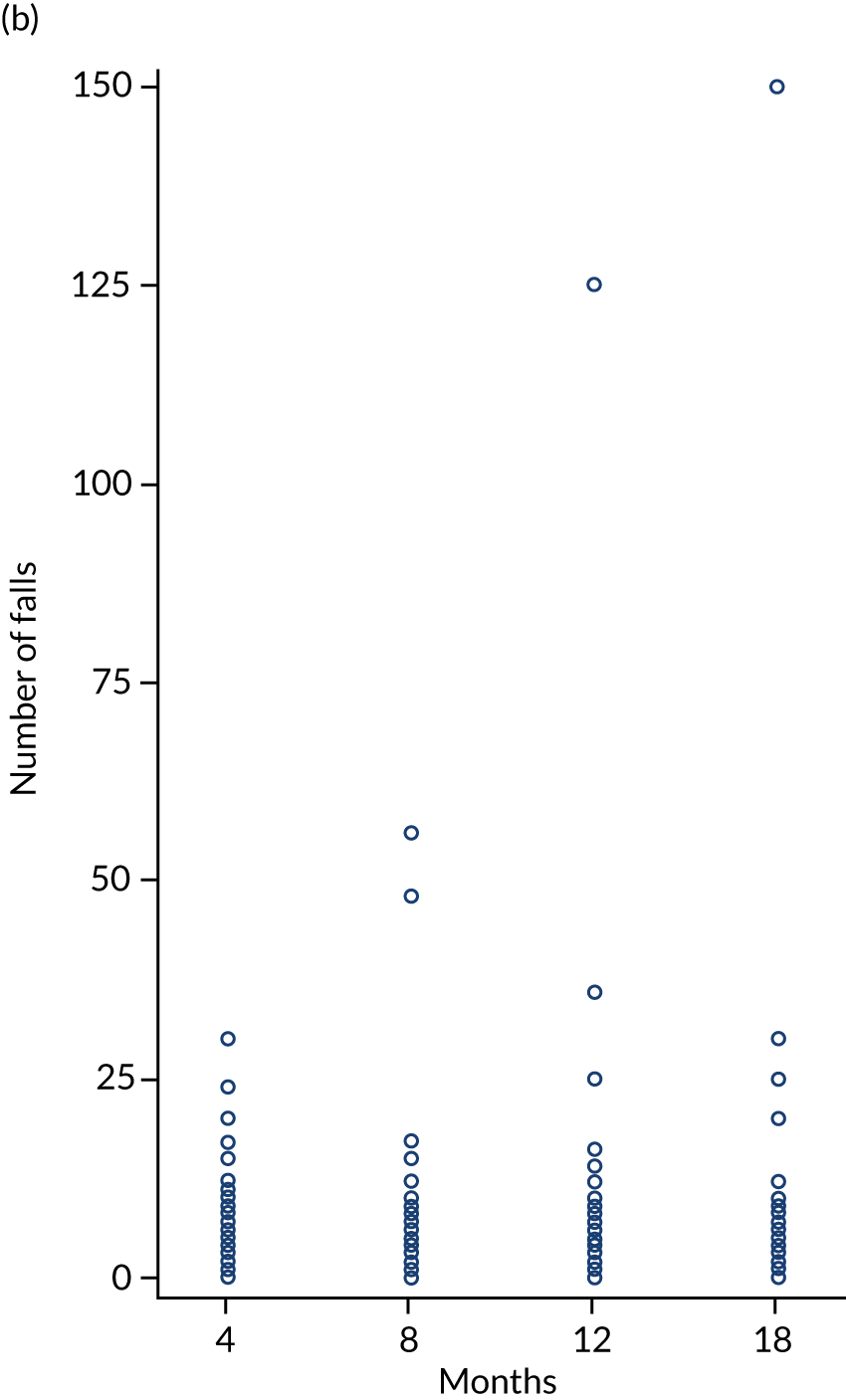
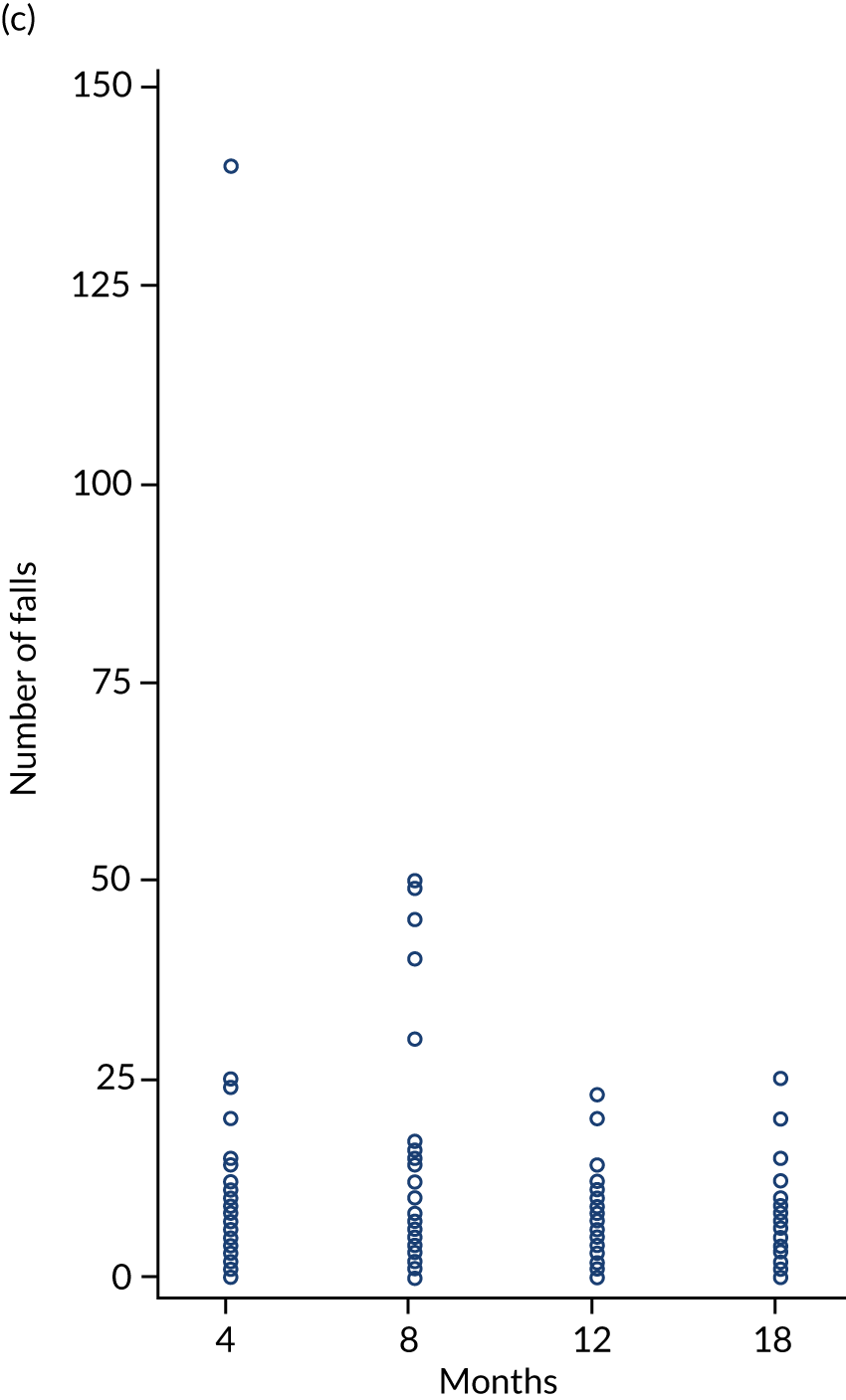
| Time period | Exercise vs. advice | MFFP vs. advice | ||
|---|---|---|---|---|
| RaR (95 CI%) | p-value | RaR (95% CI) | p-value | |
| Over 18 months | ||||
| Unadjusted RaR | 0.96 (0.80 to 1.16) | 0.69 | 1.12 (0.93 to 1.34) | 0.22 |
| Adjusted RaR | 0.99 (0.86 to 1.14) | 0.91 | 1.13 (0.98 to 1.30) | 0.10 |
| 0–4 months | ||||
| Unadjusted RaR | 1.05 (0.79 to 1.39) | 0.74 | 1.20 (0.91 to 1.58) | 0.21 |
| Adjusted RaR | 1.12 (0.87 to 1.45) | 0.47 | 1.19 (0.92 to 1.53) | 0.18 |
| 4–8 months | ||||
| Unadjusted RaR | 0.77 (0.61 to 0.98) | 0.03 | 1.11 (0.88 to 1.41) | 0.37 |
| Adjusted RaR | 0.78 (0.64 to 0.96) | 0.02 | 1.13 (0.93 to 1.37) | 0.24 |
| 8–12 months | ||||
| Unadjusted RaR | 1.05 (0.86 to 1.28) | 0.63 | 1.00 (0.82 to 1.22) | 0.12 |
| Adjusted RaRb | 1.04 (0.91 to 1.21) | 0.61 | 0.97 (0.84 to 1.13) | 0.74 |
| 12–18 months | ||||
| Unadjusted RaR | 1.11 (0.88 to 1.40) | 0.37 | 1.14 (0.90 to 1.43) | 0.28 |
| Adjusted RaR | 1.10 (0.91 to 1.34) | 0.33 | 1.13 (0.93 to 1.38) | 0.21 |
| Characteristic | Advice (N = 2493) | Exercise (N = 2500) | MFFP (N = 2497) | Total (N = 7490) |
|---|---|---|---|---|
| Age (years), mean (SD) | 77.5 (5.5) | 77.6 (5.5) | 77.3 (5.4) | 77.5 (5.5) |
| Age (years), range | 70–101 | 70–96 | 70–98 | 70–101 |
| Age band (years), n (%) | ||||
| 70–79 | 1721 (69.0) | 1739 (69.5) | 1777 (71.17) | 5237 (69.9) |
| 80–89 | 716 (28.7) | 994 (27.8) | 664 (26.6) | 2074 (27.7) |
| ≥ 90 | 56 (2.3) | 67 (2.7) | 56 (2.2) | 179 (2.4) |
| Female, n (%) | 1293 (51.9) | 1286 (51.4) | 1303 (52.2) | 3882 (51.8) |
| Ethnicity, n (%) | ||||
| White | 2455 (98.5) | 2456 (98.2) | 2456 (98.4) | 7367 (98.4) |
| Other | 20 (0.8) | 22 (0.9) | 22 (0.9) | 64 (0.8) |
| Missing | 18 (0.7) | 22 (0.9) | 19 (0.8) | 59 (0.8) |
| Marital status, n (%) | ||||
| Married/cohabiting | 1622 (65.1) | 1593 (63.7) | 1607 (64.4) | 4822 (64.4) |
| Widowed | 647 (25.9) | 632 (25.3) | 599 (24.0) | 1878 (25.0) |
| Divorced/separated | 120 (4.8) | 176 (7.0) | 159 (6.4) | 455 (6.1) |
| Single | 97 (3.9) | 88 (3.5) | 121 (4.8) | 306 (4.1) |
| Missing | 7 (0.3) | 11 (0.5) | 11 (0.4) | 29 (0.4) |
| Living arrangement, n (%) | ||||
| Live alone | 785 (31.5) | 800 (32.0) | 779 (31.2) | 2364 (31.6) |
| Live with others | 1695 (68.0) | 1684 (67.4) | 1708 (68.4) | 5087 (67.9) |
| Missing | 13 (0.5) | 16 (0.6) | 10 (0.4) | 39 (0.5) |
| Age left FTE (years), mean (SD) | 17.0 (4.7) | 16.7 (4.1) | 17.0 (4.8) | 16.9 (4.4) |
| BMI (kg/m2), mean (SD) | 26.4 (4.7) | 26.4 (4.4) | 26.4 (4.4) | 26.4 (4.5) |
| Balance difficulties walking on level, n (%) | ||||
| Never/sometimes | 2312 (92.7) | 2341 (93.6) | 2333 (93.4) | 6986 (93.3) |
| Often/very often/always | 169 (6.8) | 147 (5.9) | 156 (6.3) | 472 (6.3) |
| Missing | 12 (0.5) | 12 (0.5) | 8 (0.3) | 32 (0.4) |
| Able to get outside, n (%) | ||||
| Unaided | 2078 (83.3) | 2076 (83.0) | 2108 (84.4) | 6262 (83.6) |
| With stick/support or help only | 396 (15.9) | 409 (16.4) | 378 (15.1) | 1183 (15.8) |
| Cannot get outside at all | 10 (0.4) | 3 (0.1) | 4 (0.2) | 17 (0.2) |
| Missing | 9 (0.4) | 12 (0.5) | 7 (0.3) | 28 (0.4) |
| On average, hours/day walking | ||||
| < 1 | 583 (23.4) | 590 (23.6) | 613 (24.6) | 1786 (23.9) |
| 1–2 | 936 (37.5) | 959 (38.4) | 910 (36.4) | 2805 (37.4) |
| > 2 | 960 (38.5) | 938 (37.5) | 966 (38.7) | 2864 (38.2) |
| Missing | 14 (0.6) | 13 (0.5) | 8 (0.3) | 35 (0.5) |
| HRQoL (EQ-5D-3L), mean score (SD) | 0.79 (0.22) | 0.80 (0.21) | 0.79 (0.21) | 0.80 (0.21) |
| Missing | 88 | 102 | 108 | 298 |
| HRQoL (SF-12 PCS), mean score (SD) | 51.0 (9.9) | 51.5 (9.8) | 51.0 (10.1) | 51.1 (10.0) |
| Missing | 217 | 199 | 207 | 623 |
| HRQoL (SF-12 MCS), mean score (SD) | 50.7 (8.8) | 51.0 (8.4) | 51.0 (8.7) | 50.9 (8.6) |
| Missing | 217 | 199 | 207 | 623 |
| Clock-drawing test score, n (%) | ||||
| 0–4 | 170 (6.8) | 163 (6.5) | 162 (6.5) | 495 (6.6) |
| 5–6 | 2294 (92.0) | 2306 (92.2) | 2283 (91.4) | 6883 (91.9) |
| Missing | 29 (1.2) | 31 (1.3) | 52 (2.1) | 112 (1.5) |
| Frailty, n (%) | ||||
| Frail | 444 (17.8) | 398 (15.9) | 484 (19.4) | 1326 (17.7) |
| Non-frail | 2024 (81.2) | 2067 (82.7) | 1988 (79.6) | 6079 (81.2) |
| Missing | 25 (1.0) | 35 (1.4) | 25 (1.0) | 85 (1.1) |
| Comorbidities, n (%) | ||||
| None | 600 (24.1) | 613 (24.5) | 620 (24.8) | 1833 (24.5) |
| One or two | 1229 (49.3) | 1239 (49.6) | 1226 (49.1) | 3694 (49.3) |
| Three or more | 664 (26.6) | 648 (25.9) | 651 (26.1) | 1963 (26.2) |
| Fallen in previous year, n (%) | ||||
| Yes | 764 (30.6) | 741 (29.6) | 805 (32.2) | 2310 (30.8) |
| No | 1715 (68.8) | 1743 (69.7) | 1680 (67.3) | 5138 (68.6) |
| Missing | 14 (0.6) | 16 (0.7) | 12 (0.5) | 42 (0.6) |
| Participants with fall-related fracture in previous year, self-report, n (%) | 31 (1.2) | 31 (1.2) | 26 (1.0) | 88 (1.2) |
| Participants at higher risk of falling, baseline questionnaire, n (%) | 1028 (41.2) | 1019 (40.8) | 1063 (42.6) | 3110 (41.5) |
| Missing | 1 (0.04) | 5 (0.2) | 1 (0.04) | 8 (0.1) |
Falls reporting by diary card
Diary cards were sent to 9375 out of 9803 participants (95.6%) due to receive them [428 participants (4.4%) had either withdrawn or died before their allocated time period; Figure 5], of whom 7762 (82.8%) returned one or more completed diaries. Diary card response rate dropped slightly over each subsequent 4-month time period [randomisation to 4 months, 2758/3256 (84.7%); months 5–8, 2539/3093 (82.1%); months 9–12, 2465/3026 (81.5%)] (Table 12). Diary card non-responders were older and had poorer HRQoL scores on recruitment to the trial. Among the 6418 participants reporting falls in both data sources (diary card and questionnaire), there was substantial agreement in reporting (Cohen’s unweighted kappa test statistic 0.638). When there was lack of agreement, falls reporting was higher in diary cards than in the questionnaires. A separate manuscript reported on patterns of return (Table 13) and differences by data collection method. 115 In brief, we found an average 32% difference in the falls rates between the prospective diary cards and retrospective reporting. 115
FIGURE 5.
Flow chart for diary card data for prospective falls reporting.
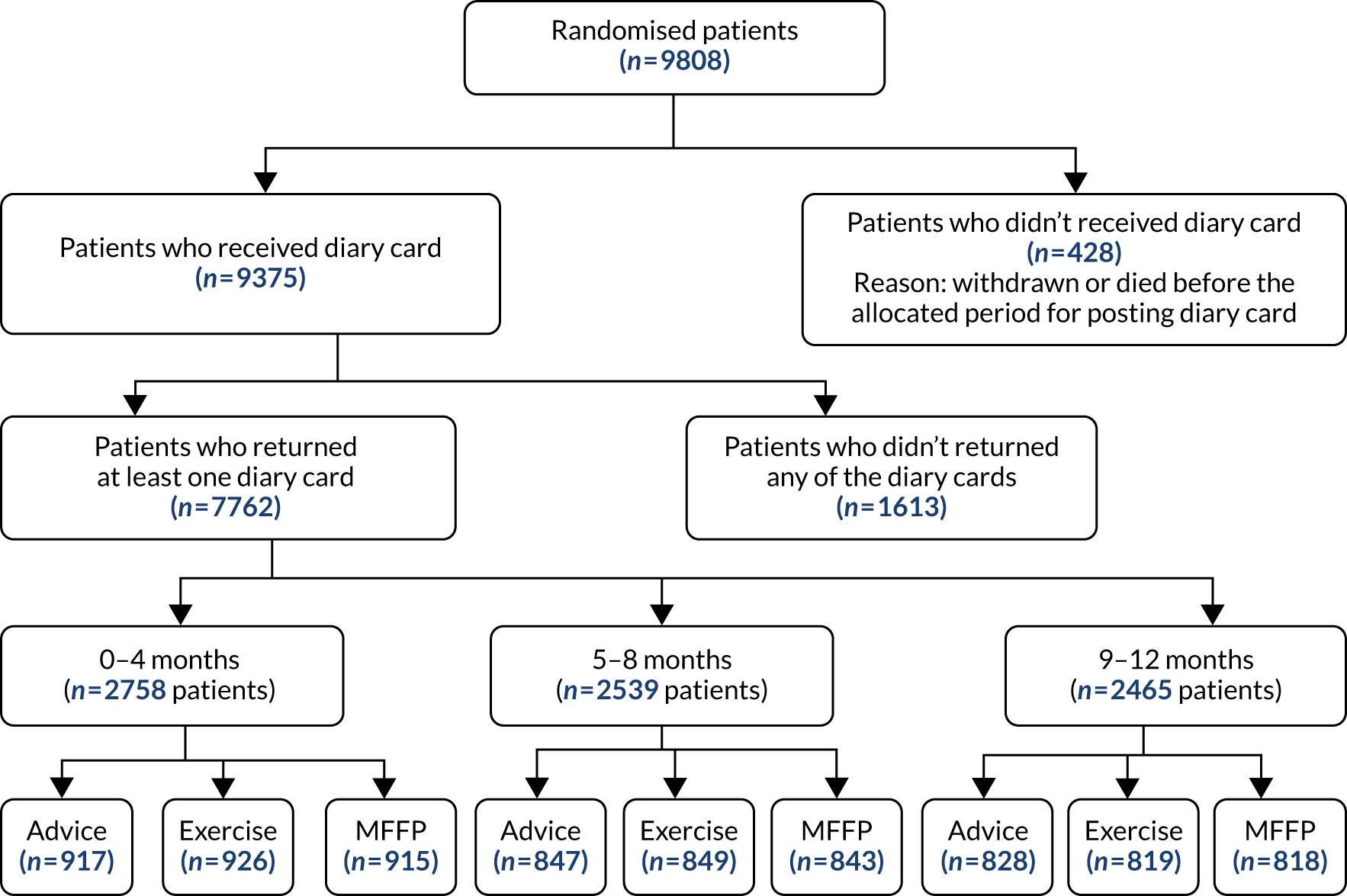
| Time period (months) | Participants returning diary cards, n (%)a | |||
|---|---|---|---|---|
| Advice | Exercise | MFFP | Total | |
| 0–4 | 917 (33.2) | 926 (33.6) | 915 (33.2) | 2758 |
| 5–8 | 847 (33.4) | 849 (33.4) | 843 (33.2) | 2539 |
| 9–12 | 828 (33.6) | 819 (33.2) | 818 (33.2) | 2465 |
| All | 2592 | 2594 | 2576 | 7762 |
| Time period (months) | Diaries returned, n (%)a | Total | |||
|---|---|---|---|---|---|
| One | Two | Three | Four | ||
| 0–4 | 62 (2.3) | 97 (3.5) | 284 (10.3) | 2315 (83.9) | 2758 |
| 5–8 | 64 (2.5) | 91 (3.6) | 319 (12.7) | 2065 (81.2) | 2539 |
| 9–12 | 49 (2.0) | 64 (2.6) | 224 (9.1) | 2128 (86.3) | 2465 |
| All | 175 | 252 | 827 | 6508 | 7762 |
Complier-average causal effect analysis: falls
A CACE analysis was conducted to estimate the treatment effect at each time point, having adjusted for non-compliance, as defined in Chapter 2. Among those participants randomised to treatment (exercise and MFFP; 6580 participants), 5085 (77.3%) complied with the screener and treatment and 1495 (22.7%) were non-compliers. Similar to the ITT findings, the CACE results found no difference in fracture rate or falls rate over 18 months by compliance status.
Subgroup analyses
We undertook subgroup analyses as per the prespecified analysis plan, that is, treatment effects were compared by age, sex, history of falling, frailty and cognitive impairment (Table 14). No statistically significant interaction effects were found in the RaR of falls by baseline participant characteristics.
| Subgroup | Exercise vs. advice | MFFP vs. advice | ||
|---|---|---|---|---|
| Falls RaR (95% CI) | p-valuea | Falls RaR (95% CI) | p-valuea | |
| Age (years) | ||||
| ≤ 80 (n = 7022) | 0.96 (0.78 to 1.16) | 0.87 | 1.11 (0.92 to 1.36) | 0.73 |
| > 80 (n = 2781) | 0.97 (0.76 to 1.25) | 1.16 (0.90 to 1.50) | ||
| Sex | ||||
| Male (n = 4653) | 1.03 (0.83 to 1.26) | 0.21 | 1.10 (0.89 to 1.35) | 0.64 |
| Female (n = 5150) | 0.90 (0.73 to 1.10) | 1.15 (0.94 to 1.41) | ||
| Fallen in previous year | ||||
| No (n = 6587) | 1.05 (0.89 to 1.24) | 0.21 | 1.24 (1.05 to 1.46) | 0.06 |
| Yes (n = 3150) | 0.92 (0.76 to 1.11) | 1.02 (0.85 to 1.23) | ||
| Frailty | ||||
| Non-frail (n = 7666) | 0.99 (0.83 to 1.16) | 0.97 | 1.09 (0.92 to 1.29) | 0.99 |
| Frail (n = 2005) | 0.99 (0.77 to 1.27) | 1.09 (0.86 to 1.38) | ||
| Cognition | ||||
| Score 0–4 (n = 870) | 1.11 (0.75 to 1.64) | 0.44 | 1.19 (0.81 to 1.75) | 0.71 |
| Score 5–6 (n = 8751) | 0.95 (0.79 to 1.15) | 1.11 (0.93 to 1.33) | ||
Health-related quality of life over time by intervention
We assessed HRQoL over time by treatment group. Table 15 presents mean (SD) SF-12 scores and missingness for PCS and MCS scores. Findings for EQ-5D-3L scores are presented in Chapter 6. Differences in mean SF-12 scores were found at interim time points; however, no differences were found in overall mean SF-12 change scores between the advice and exercise groups or between the advice and MFFP groups over time (Table 16).
| Outcome | Advice (n = 3223) | Exercise (n = 3279) | MFFP (n = 3301) | Total (n = 9803) |
|---|---|---|---|---|
| At 4 months | ||||
| SF-12 PCS | 50.2 (10.3) | 50.4 (10.2) | 50.0 (10.5) | 50.2 (10.4) |
| SF-12 MCS | 50.3 (9.0) | 50.4 (9.1) | 50.1 (9.4) | 50.3 (9.2) |
| Missing | 570 | 585 | 616 | 1771 |
| At 8 months | ||||
| SF-12 PCS | 50.3 (10.2) | 50.5 (10.2) | 50.0 (10.3) | 50.3 (10.2) |
| SF-12 MCS | 50.0 (9.1) | 50.4 (9.1) | 49.8 (9.7) | 50.1 (9.3) |
| Missing | 675 | 708 | 717 | 2100 |
| At 12 months | ||||
| SF-12 PCS | 50.3 (10.1) | 50.6 (10.1) | 49.9 (10.4) | 50.3 (10.2) |
| SF-12 MCS | 49.9 (9.3) | 50.3 (9.1) | 50.0 (9.5) | 50.1 (9.3) |
| Missing | 796 | 835 | 891 | 2522 |
| At 18 months | ||||
| SF-12 PCS | 49.9 (10.0) | 50.4 (10.0) | 49.8 (10.3) | 50.0 (10.1) |
| SF-12 MCS | 50.0 (9.0) | 50.3 (9.1) | 49.9 (9.5) | 50.1 (9.2) |
| Missing | 989 | 1065 | 1085 | 3139 |
| Outcome | Exercise vs. advice | MFFP vs. advice | ||
|---|---|---|---|---|
| MD (95% CI) | p-value | MD (95% CI) | p-value | |
| Over 18 months | ||||
| SF-12 PCS | 0.04 (–0.22 to 0.31) | 0.75 | –0.22 (–0.49 to 0.05) | 0.12 |
| SF-12 MCS | 0.13 (–0.17 to 0.43) | 0.41 | –0.21 (–0.51 to 0.09) | 0.17 |
| At 4 months | ||||
| SF-12 PCS | 0.09 (–0.24 to 0.42) | 0.59 | –0.07 (–0.42 to 0.26) | 0.67 |
| SF-12 MCS | 0.09 (–0.30 to 0.47) | 0.67 | –0.17 (–0.55 to 0.22) | 0.39 |
| At 8 months | ||||
| SF-12 PCS | 0.03 (–0.32 to 0.37) | 0.83 | –0.34 (–0.69 to 0.01) | 0.05 |
| SF-12 MCS | 0.25 (–0.15 to 0.65) | 0.23 | –0.21 (–0.61 to 0.19) | 0.30 |
| At 12 months | ||||
| SF-12 PCS | 0.02 (–0.34 to 0.38) | 0.92 | –0.38 (–0.74 to –0.02) | 0.04 |
| SF-12 MCS | 0.14 (–0.27 to 0.56) | 0.50 | –0.16 (–0.58 to 0.26) | 0.45 |
| At 18 months | ||||
| SF-12 PCS | 0.24 (–0.15 to 0.64) | 0.23 | 0.08 (–0.32 to 0.47) | 0.70 |
| SF-12 MCS | 0.02 (–0.41 to 0.45) | 0.93 | –0.19 (–0.62 to 0.25) | 0.40 |
Frailty
The proportion of responding participants classified as frail was slightly higher at 18 months (1672/7490, 22.3%) than at baseline (2005/9803, 20.5%) (Table 17). There were no differences in the odds of being frail at follow-up by treatment comparison (Table 18).
| Outcome | Advice | Exercise | MFFP | Total |
|---|---|---|---|---|
| Not frail, n (%) | 1859 (74.6) | 1906 (74.9) | 1870 (74.9) | 5635 (75.2) |
| Frail, n (%) | 576 (23.1) | 538 (21.5) | 558 (22.4) | 1672 (22.3) |
| Missing Strawbridge questionnaire, n (%) | 58 (2.3) | 56 (2.2) | 69 (2.8) | 183 (2.4) |
| Total, n | 2493 | 2500 | 3301 | 7490 |
| Analysis | Exercise vs. advice | MFFP vs. advice | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Unadjusted | 0.91 (0.80 to 1.04) | 0.17 | 0.96 (0.84 to 1.10) | 0.58 |
| Adjusteda | 0.96 (0.82 to 1.13) | 0.62 | 0.89 (0.76 to 1.05) | 0.17 |
Serious adverse events
No SAEs directly related to the interventions were reported. One participant sustained a fractured neck of femur during a trial procedure not related to the intervention [a fall sustained when returning from posting a follow-up questionnaire (exercise arm)]. Other AEs included a fall by one participant during MFFP assessment. This participant was a frequent faller and had been investigated by consultant-led services, with no cause identified. Another participant with a known history of angina reported experiencing angina when exercising at home (exercise arm). Both participants continued with interventions.
Process evaluation
This section presents overall key findings for screening utility and intervention uptake for the active interventions, that is, for those referred to exercise and MFFP and also for those in MFFP who were referred to exercise therapy. Findings are then presented in more detail for each intervention arm.
Primary care screening
Fall risk screener response rate
Referral to active treatment was determined using the primary care postal screener. Forty-two GPs, randomised to either exercise or MFFP, mailed out 6580 screeners, of which 5791 (88.0%) were returned (of which 12 were blank). Risk stratification was based on 5779 out of 6580 (87.8%) participants who returned a completed fall risk screener. There were no differences in response rate by treatment arm [exercise 2925/3279 (89.2%), MFFP 2854/3301 (86.5%)]. Over one-third of responding participants (2153/5779, 37.3%) were identified as being at higher risk of falling and eligible for invitation to treatment. The proportion of participants randomised who were at risk of falling was similar across arms [exercise, 1079/3279 (32.9%); MFFP, 1074/3301 (32.5%)]. There were no differences by age or sex between the responders and non-responders to the postal screener; however, non-responders had a slightly lower mean clock-drawing test score [responders, mean score 5.6 (SD 0.9), vs. non-responders, mean score 5.4 (SD 0.9); p < 0.001] and were more likely to be frail [responders, 1146/5779 (19.5%); non-responders, 212/801 (26.5%)].
Falls risk screener predictive utility
The postal falls risk screener performed moderately well in predicting falls over 12 and 18 months. The unadjusted AUC was 0.66 (95% CI 0.64 to 0.68; p < 0.001; n = 5438 participants) over 12 months and 0.64 (95% CI 0.63 to 0.66; p < 0.001; n = 4997 participants) over 18 months, based on participants returning a completed falls risk screener and follow-up questionnaires at 12 and 18 months, respectively. A separate analysis examined the utility of the risk screener at predicting fractures in participants with HES data who returned a risk screener. The AUC for fracture prediction was slightly lower than for falls, with AUC values of 0.60 (95% CI 0.55 to 0.64; n = 5779) and 0.59 (95% CI 0.55 to 0.63; n = 5779) over 12 and 18 months, respectively.
Staff trained
Exercise intervention
Between November 2011 and September 2014, a total of 84 therapists attended 24 training sessions: 49 (58%) physiotherapists, 14 (17%) therapy assistants, 13 (15%) exercise specialists and eight (10%) occupational therapists. All worked in NHS specialist falls prevention services, community therapy services or outpatient physiotherapy departments. Among those trained, 58 (69%) therapists delivered one or more sessions of the PreFIT intervention. The remaining 26 (31%) therapists either moved to another NHS department or left before delivering any intervention. Four therapists (4/58, 7%) received follow-up visits for minor concerns regarding protocol adherence and/or issues related to paperwork completion. The remaining quality control visits were graded as satisfactory.
Multifactorial falls prevention intervention
We trained 39 staff members at 16 MFFP training sessions between January 2012 and September 2014, of whom 16 (41%) were GP nurses or advanced nurse practitioners based in primary care, seven (18%) were nurses from the Primary Care Research Network, eight (21%) were staff members from a specialist consultant-led falls team (seven physicians and one falls nurse), six (15%) were falls prevention staff members working in community-based falls services (including occupational therapy, physiotherapy, nurse advisors for older people) and two were medical registrars (5%). Among the 39 staff members trained, eight did not undertake any MFFP assessments (five of whom were nurses). Two staff members received follow-up quality control visits. In one case this was because of major concerns and additional training was arranged and delivered by a geriatrician (Devon). Subsequent quality control checks were satisfactory. The other quality control issue was a minor concern regarding paperwork completed incorrectly (Worcestershire region). Remaining staff quality control assessments were graded as satisfactory. Medication review training was given to general practitioners in 17 out of 21 GPs randomised to MFFP. The remaining four GPs from Newcastle upon Tyne used a consultant-led falls and syncope service in which experienced physicians reviewed all medications and wrote to GPs specifying which medications were to be modified.
Uptake to exercise and multifactorial falls prevention interventions
Intervention uptake was good, with 65% (697/1079) of those invited attending their first exercise appointment and 71% (762/1074) attending MFFP assessment. These numbers constitute a relatively small proportion of the overall sample randomised to exercise and MFFP [697/3279 (21%) and 762/3301 (23%), respectively]. Five participants died before their first exercise assessment (5/1079, < 1%). Characteristics of participants attending treatment are presented in Table 19. Reasons for not attending the offered interventions included lack of time, difficulties with transport, being in poor health, already doing enough exercise and being uninterested in either exercise or having a health check.
| Characteristic | Exercise intervention | MFFP intervention | MFFP and exercisea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Declined | Attended | Total | Declined | Attended | Total | Declined | Attended | Total | |
| Participants, n (%) | 382 (35) | 697 (65) | 1079 | 312 (29) | 762 (71) | 1074 | 96 (32) | 203 (68) | 299 |
| Age (years), mean (SD) | 80.2 (6.4) | 79.3 (5.9) | 79.6 (6.1) | 80.5 (6.7) | 78.7 (5.8) | 79.2 (6.1) | 80.8 (6.1) | 79.8 (5.7) | 80.1 (5.9) |
| Age (years), n (%) | |||||||||
| 70–79 | 190 (33) | 378 (67) | 568 | 153 (25) | 455 (75) | 608 | 45 (30) | 104 (70) | 149 |
| ≥ 80 | 192 (38) | 319 (62) | 511 | 159 (34) | 307 (66) | 466 | 51 (34) | 99 (66) | 150 |
| Sex, n (%) | |||||||||
| Male | 161 (35) | 296 (65) | 457 | 112 (26) | 323 (74) | 435 | 34 (31) | 77 (69) | 111 |
| Female | 221 (36) | 401 (64) | 622 | 200 (31) | 439 (69) | 639 | 62 (33) | 126 (67) | 188 |
| Risk of falling, n (%) | |||||||||
| Intermediate | 297 (38) | 491 (62) | 788 | 209 (28) | 532 (72) | 741 | 64 (37) | 110 (63) | 174 |
| High | 85 (29) | 206 (71) | 291 | 103 (31) | 230 (69) | 333 | 32 (26) | 93 (74) | 125 |
Skill mix of delivery of multifactorial falls prevention intervention
All MFFP assessments conducted in Newcastle were completed by consultant physicians (seven doctors completed 119/762 falls assessments; 16%). For other regions, registered nurses (practice nurses or research nurses) delivered 505 out of 762 (66%) MFFP assessments and community-led falls services comprising other health-care professionals carried out 138 out of 762 (18%) assessments. The majority of assessments in Warwickshire were carried out by community-led falls services.
In summary, over 95% of the contacts delivered in each of the trial arms (MFFP and exercise) were provided by NHS practitioners who were in the usual network of care providers for participants. Five per cent of interventions were provided by research staff who were employed by the University of Warwick or on research nurse only contracts in the NHS in exceptional circumstances.
Time from randomisation to start of treatment
Median time from randomisation to the first exercise assessment was 14 weeks [interquartile range (IQR) 10–22 weeks]. This varied by region, from a median of 9 weeks in Birmingham and the Black Country to a median of 21 weeks in Devon. This was due to staffing issues within services; in some areas staff members were trained but then changed duties or departments at short notice. In the MFFP group, median time from randomisation to assessment was 16 weeks (IQR 13–23 weeks), ranging from 13 weeks in Newcastle upon Tyne to 25 weeks in Worcestershire. Delays in assessments were due to lack of availability of GP nurses to deliver assessments.
Exercise intervention
Adherence
Among the 697 participants attending exercise therapy, the overall median time spent in the programme was 25 weeks (IQR 16–27 weeks). Among the 454 [out of a total of 697 (65%)] participants who fully adhered to the recommended exercise programme, defined as having six or more contacts with the therapist, median duration in the intervention was 27 weeks (IQR 25–28 weeks) (Table 20). One-third of participants partially adhered to the intervention, defined as having fewer than six contacts with the therapist (243/697, 35%). There were no differences in adherence by region. There were no differences in adherence status by age or sex of participants.
| Treatment group | Time (weeks) spent in exercise intervention | |
|---|---|---|
| Participants, n (%) | Median (IQR) | |
| Exercise (N = 697) | ||
| Partially adhered (fewer than six contacts) | 243 (34.9) | 10 (3–18) |
| Adhered (six or more contacts) | 454 (65.1) | 27 (25–28) |
| MFFP and exercisea (N = 203) | ||
| Partially adhered (fewer than six contacts) | 79 (38.9) | 12 (4–17) |
| Adhered (six or more contacts) | 124 (61.1) | 26 (25–30) |
Contacts with therapists
The 58 trained therapists had a total of 3842 contacts with 697 participants. Over half of the contacts were face to face (2078/3842, 54%) and the remainder were by telephone (1764/3842, 46%). Although up to six contacts were recommended, 295 out of 697 (42%) participants had seven or more therapy contacts (Figure 6). Participants who fully adhered to the exercise programme had a mean of six contacts (SD 1.3 contacts) with the therapist, and those who partially adhered had a mean of four contacts (SD 2 contacts). There were no differences in mean number of contacts by region.
FIGURE 6.
Number of contacts with therapists for participants who attended the exercise intervention (n = 697).
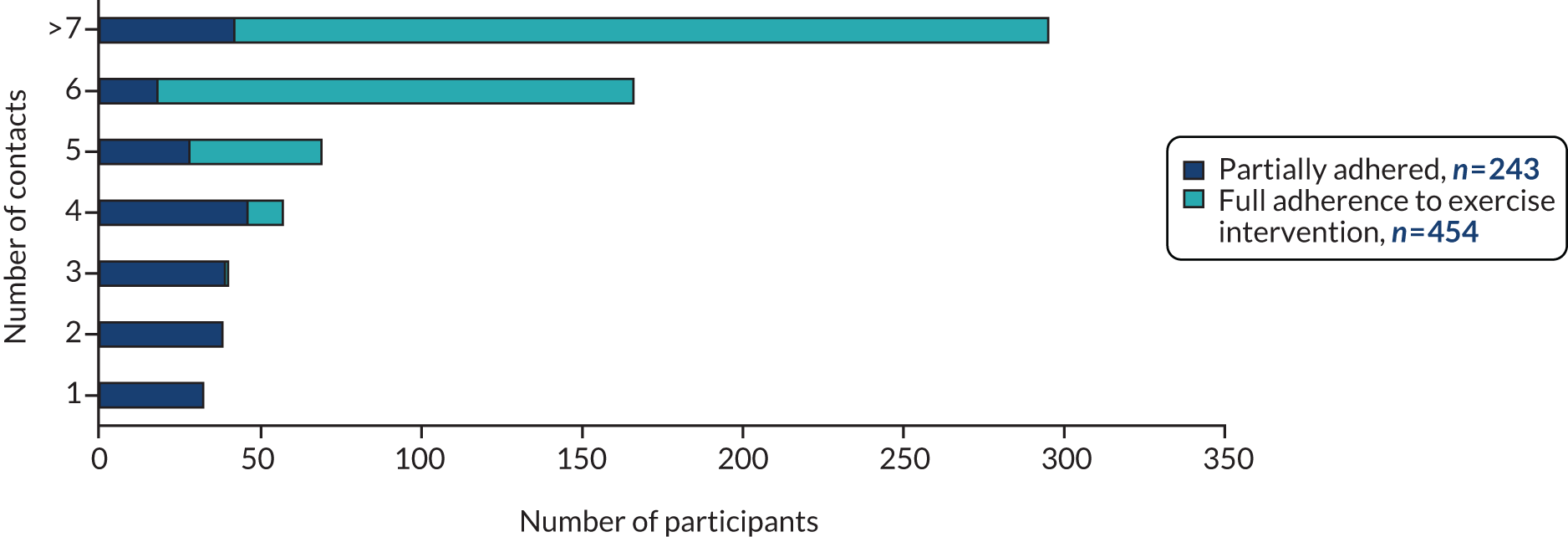
Change in leg strength over time
Among those randomised to the exercise intervention, we measured change in performance in the CST and 4TBS over time. Final test data were available for those attending the final therapy session, either session 6 or session 7. There was a statistically significant improvement in performance over time: the mean CST score was 3.5 (SD 1.1) before intervention compared with 3.7 (SD 0.9) post intervention [mean difference (MD) 0.15, 95% CI 0.08 to 0.23; p < 0.001; n = 454 participants] (Figure 7). We had incomplete data on those who partially adhered because these participants did not attend for repeat assessment of baseline tests (243/697, 35%).
FIGURE 7.
Change in CST score in participants who adhered to exercise intervention (n = 454).
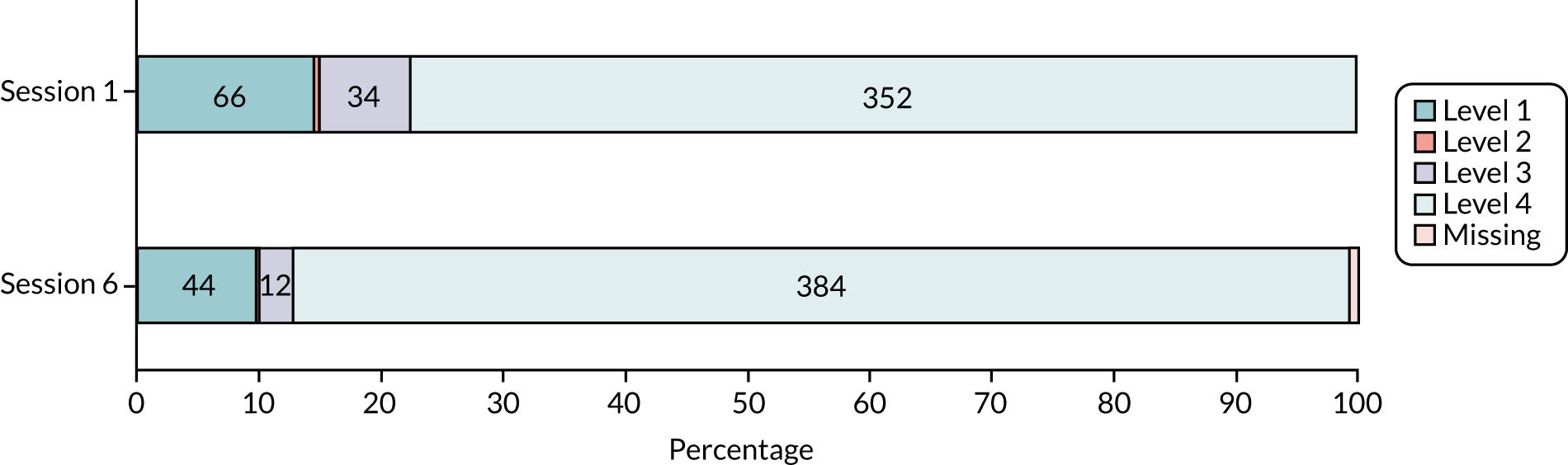
Recorded data on total weight lifted (kg) and total repetitions were used to calculate work done (weight × repetitions) over time. This was analysed by major muscle group: the quadriceps, hamstrings and abductors. Data on work carried out were based on changes between first assessment and session 6 for full adherers (399/454). Among adherers, there was good evidence of an increase in muscle function over time in the major muscle groups: quadriceps MD 5.5 (SD 15.4; paired t-test p < 0.001), hamstrings MD 5.6 (SD 13.9; paired t-test p < 0.001) and abductors MD 5.1 (SD 13.8; paired t-test p < 0.001) (Figure 8; see also Appendix 1, Table 31). Weights were progressed in 190 out of 454 (42%) participants who complied with the intervention (among these, 32 participants had two or more increases in weights).
FIGURE 8.
Change in muscle strength (mean work done) over time among participants who adhered to exercise intervention (n = 454).
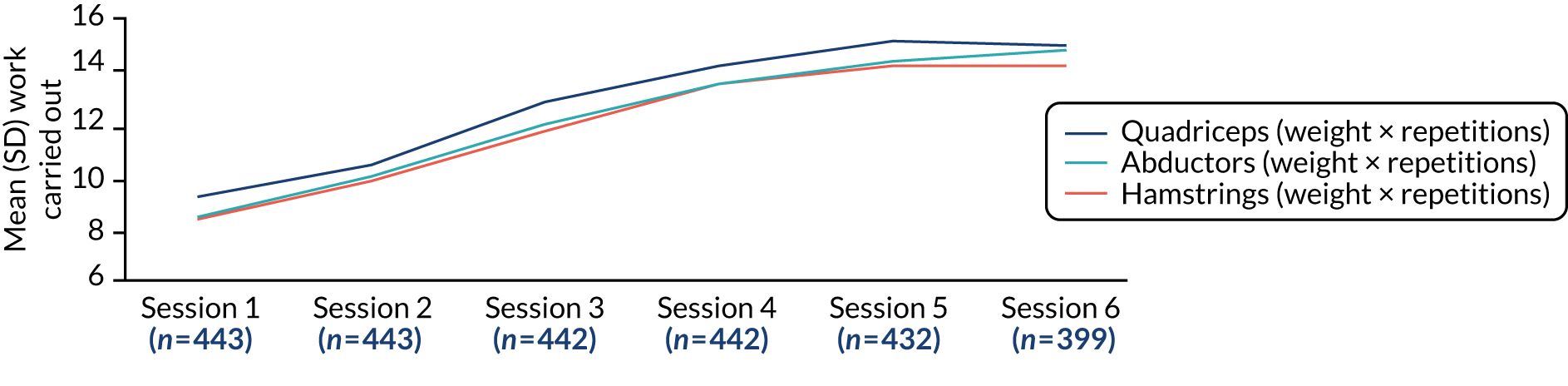
For those who partially adhered to the intervention, data on work carried out were based on changes between assessment and session 3 (159/243). A decline in muscle strength was found over time for all major muscle groups: quadriceps MD –2.4 (SD 8.4; paired t-test p < 0.001), hamstrings –2.1 (SD 7.2; paired t-test p < 0.001) and abductors MD –2.1 (SD 7.2; paired t-test p < 0.001) (Figure 9). Weights were not progressed in weaker participants.
FIGURE 9.
Change in muscle strength (mean work done) over time among participants who partially adhered to intervention (n = 243).
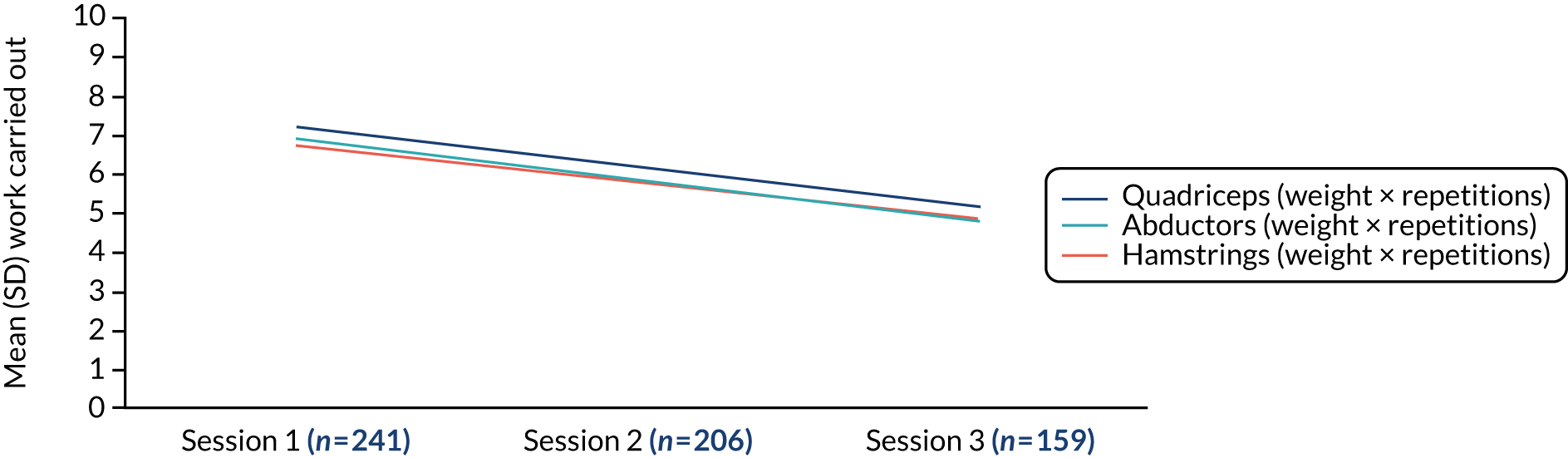
Change in balance over time
Balance problems were prevalent among those who attended exercise. Over half of participants (354/687, 52%) scored level 1 or 2 on the 4TBS on first assessment and, thus, either failed the test completely, being unable to stand with feet together, or achieved only some of the basic balance challenge (Figure 10). Mean scores improved from pre to post intervention in those who adhered to exercise [pre-intervention mean balance level 2.63 (SD 1.0); post-intervention mean balance level 3.22 (SD 0.9); MD 0.57, 95% CI 0.50 to 0.65; p = 0.001; n = 454 participants].
FIGURE 10.
Change in 4TBS levels over time in 454 participants who adhered to exercise intervention.
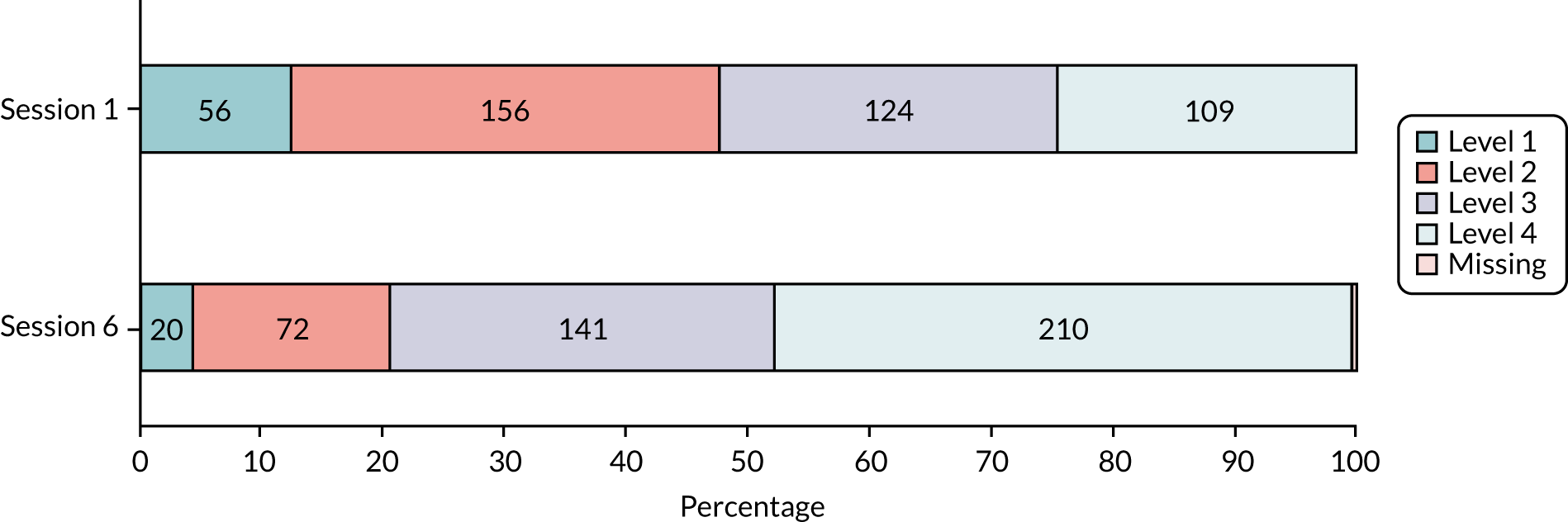
Perceived improvement at discharge
At the final assessment, therapists asked each participant about their strength, balance and walking since taking part in the programme (‘has your strength got better?’/‘has your balance improved?’/‘can you walk further or for longer?’). These data were available for participants who adhered to the exercise intervention and attended their final assessment (454/697, 65%). Two-thirds of participants reported improvements in their strength (271/454, 60%) and balance (270/454, 59%) at discharge (Figure 11). At the final appointment, therapists were asked to report their own opinion on whether or not participants had progressed. Progression was reported for 315 out of 454 (69%) participants; therapists thought that 122 out of 454 (27%) had not progressed (n = 17 missing).
FIGURE 11.
Perceived self-reported changes in strength, balance and walking among participants who adhered to the exercise intervention (n = 454).
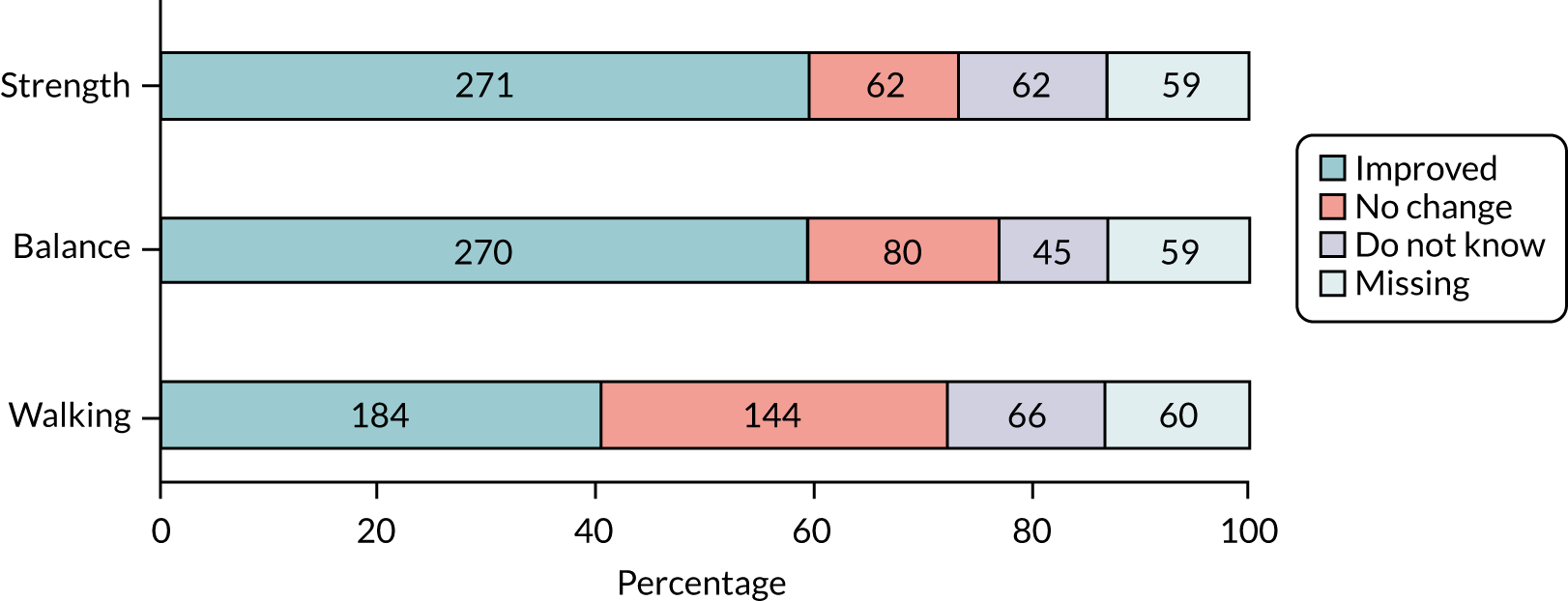
Frequency of exercise at time of discharge
At the final appointment, participants who completed the intervention were asked how regularly they were exercising: 314 out of 454 (69%) participants reported exercising three or more times per week and the remainder were exercising fewer than three times per week. At the final appointment, all participants were given an information leaflet describing opportunities for exercise classes in their local community and were encouraged to engage with these.
Multifactorial falls prevention intervention
Onward referrals to other services
All 762 participants who underwent MFFP assessment were assessed for all risk factors. A total of 432 participants were given information leaflets containing advice on how to self-manage symptoms of postural hypotension, foot care and recommended footwear, and/or tip sheets about risk management in the home environment. Among the 762 participants who returned a medication screen, 459 (60%) were referred either to their own general practitioner or to the PreFIT-nominated general practitioner in the GP for a more detailed medication review (level 3). A total of 317 out of 762 (42%) participants either failed the TUG (took ≥ 14 seconds) or had balance or gait problems or fears about falling (among whom 299 were referred for PreFIT exercise therapy and 18 were referred to other services). Fifty-eight (8%) onward referrals were made to a falls service doctor based on concerns raised in the falls history interview or the postural hypotension check. In total, 762 completed assessments resulted in 971 referrals to other health-care professionals, for example general practitioners, consultant geriatricians, PreFIT exercise therapists, podiatrists, occupational therapists or opticians, or social services (Table 21).
| Risk factor identified | Advice leaflet given | Falls service doctor | Referral to another service (n) | Total referrals (n) | |||||
|---|---|---|---|---|---|---|---|---|---|
| General practitioner | Physiotherapist | Podiatrist | Occupational therapist | Opticiana | Other | ||||
| Falls history/red flag | N/A | 50 | 28 | 17 | 0 | 0 | 0 | 4 | 99 |
| Balance/gait | N/A | 0 | 0 | 299b | 0 | 0 | 0 | 18 | 317 |
| Postural hypotension | 62 | 8 | 40 | 0 | 0 | 0 | 0 | 1 | 49 |
| Culprit medications | N/A | 0 | 459 | 0 | 0 | 0 | 0 | 0 | 459 |
| Vision | N/A | 0 | 0 | 0 | 0 | 0 | 84 | 7 | 7 |
| Feet/footwear | 143 | 0 | 0 | 0 | 11c | 1 | 0 | 5 | 17 |
| Social/home environment | 143 | 0 | 0 | 0 | 0 | 18 | 0 | 5 | 23 |
Checks of treatment referrals to other services
Based on 762 completed treatment logs returned to the research team, 459 full general practitioner-led medication reviews were recommended. GP staff members (nurses or receptionists) were required to arrange separate appointments for level 3 medication reviews, if these were not completed on the day of MFFP assessment. Based on completed treatment logs, 79 out of 459 (17%) reviews led to medication modifications by general practitioners and 339 out of 459 (74%) resulted in no changes; 41 out of 459 (9%) treatment logs had missing data.
On completion of intervention delivery, visits were made by the trial team to all 21 GPs randomised to MFFP to ascertain whether or not recommended referrals and actions had been completed and recorded in primary care electronic systems. We found that the recording of recommended actions and outcome was generally poor. We found documented evidence of action for only 36% of all treatments recommended in the MFFP (e.g. referral to, or outcome of attendance at, other services such as falls specialists, physiotherapy, consultant-led eye services). For medication reviews, there was documented evidence in GP records that among the 459 general practitioner-led reviews only 238 had been completed (52%) (Table 22).
| Referral | Falls history | Balance/gait | Medication review | Postural hypotension | Vision | Feet | Social/home | Total |
|---|---|---|---|---|---|---|---|---|
| Service referral | Falls doctor/general practitioner/other | PreFIT physiotherapist/other | General practitioner | Falls doctor/general practitioner/other | Optician/eye service | Podiatrist/OT/other | OT/social services/other | |
| Referral confirmed in GP records, n (%) | 18 (18) | 56 (18) | 238 (52) | 10 (20) | 4 (57) | 15 (88) | 10 (43) | 351 (36) |
| No confirmation found in GP records, n (%) | 81 (82) | 261 (82) | 221 (48) | 39 (80) | 3 (43) | 2 (12) | 13 (57) | 620 (64) |
| Total referrals, n (%) | 99 | 317 | 459 | 49 | 7 | 17 | 23 | 971 (100) |
It was possible to compare findings of searches of GP records with accurate treatment data obtained from therapists for 203 participants who attended PreFIT exercise. There was evidence in GP records for only 56 out of the 203 (28%) onward referrals to PreFIT exercise. Therefore, GPs had no documented record of either referral or completion of treatment for > 70% of trial participants attending exercise therapy.
Referral to exercise therapy
Uptake of multifactorial falls prevention and exercise intervention
Among the 762 participants who had a falls assessment, 299 (39%) were referred for PreFIT exercise therapy. Uptake of exercise after the MFFP assessment was good, with 203 out of the 299 (68%) participants referred attending their first appointment. The median time from MFFP assessment to the start of exercise treatment was 6 weeks (IQR 3–11 weeks), ranging from 3 weeks (IQR 2–5 weeks) in Newcastle upon Tyne to > 16 weeks (IQR 5–22 weeks) in Birmingham and the Black Country. Time from randomisation to the start of exercise treatment was longer in the MFFP arm: on average 23 weeks (IQR 17–32 weeks) from randomisation. Participant characteristics of those who attended and declined are presented in Table 19.
Adherence
Among the 203 participants attending exercise therapy, 124 (61%) adhered to the full 6-month exercise programme (mean 6.2 months, SD 1.1 months). Those who partially adhered (79/203, 39%) received less than half of the recommended programme (median 12 weeks, IQR 4–17 weeks), similar to partial adherers in the exercise treatment arm (see Table 20).
Contacts with therapists
The 28 therapists had a total of 1022 contacts with 203 participants; over half of these contacts were face to face (584/1022, 57%) and the remainder were by telephone (438/1022, 42%). Although six contacts were recommended, 51 (25%) participants had seven or more contacts with their therapist. There were no differences in mean number of therapist contacts by region. Those who fully adhered to the programme had a mean of six (SD 1.0) contacts with their therapist and those who partially adhered had a mean of three (SD 1.5) contacts with their therapist. These findings are similar to findings for participants randomised to exercise without a preceding falls assessment.
Change in leg strength over time
Final test data were available only for those attending the final therapy session, that is, those who adhered to the intervention (124/203, 61%). The mean CST score was 3.2 (SD 1.2) pre intervention and 3.3 (SD 1.4) post intervention (MD 0.1, 95% CI –0.13 to 0.32; p = 0.39; n = 124 participants) (Figure 12).
FIGURE 12.
Change in CST over time among participants who adhered to MFFP and exercise intervention (n = 124).
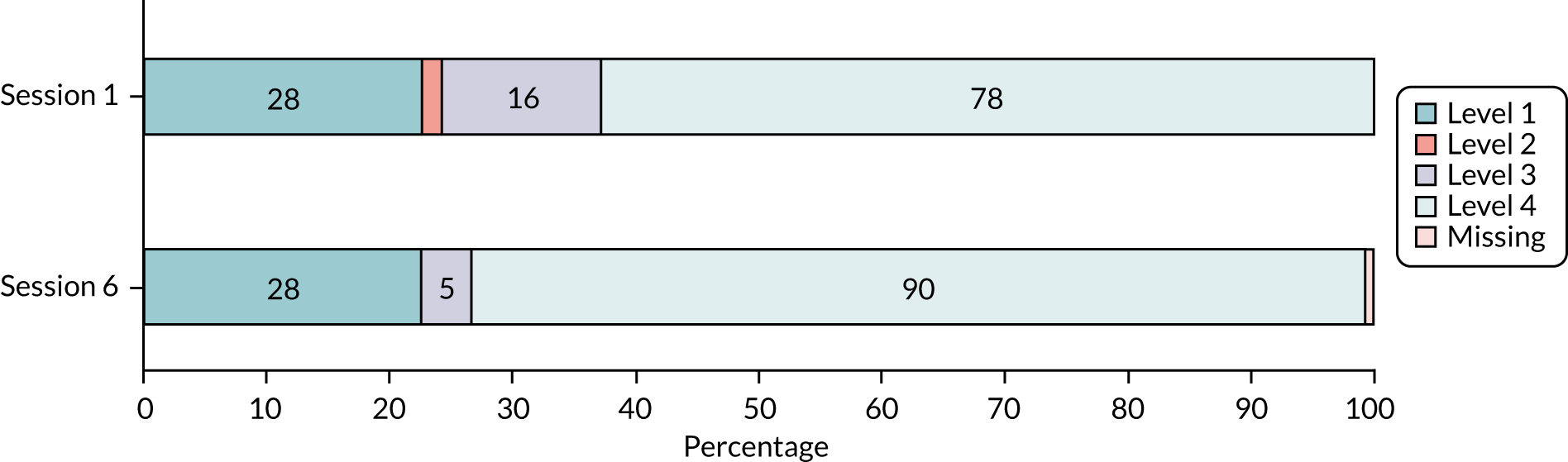
Data on work done (weights × repetitions) were based on changes between assessment and session 6 for those who fully adhered to the intervention (105/124). There was evidence of a statistically significant improvement in muscle function over time for the major muscle groups, that is, for quadriceps (paired t-test p < 0.01), hamstrings (paired t-test p < 0.002) and abductors (paired t-test p < 0.001). Weights were progressed in 48 out of 124 (39%) participants who complied with the intervention (weights were increased once in 40 participants and twice in eight participants).
Data on work done over time were available for only 48 out of 79 (61%) participants who partially adhered to the intervention, from baseline assessment to session 3. A decline in muscle strength was observed over time, similar to that seen among those who partially adhered in the exercise intervention arm, although these findings are based on a small sample: quadriceps (paired t-test p < 0.09), hamstrings (paired t-test p = 0.11) and abductors (paired t-test p = 0.40).
Change in balance over time
Balance problems were common among these participants. Over half (114/198, 58%) scored only level 1 or level 2 on the 4TBS at first assessment, thus either failing the test completely or achieving only some of the balance challenge (Figure 13). An improvement in balance was observed over time, with a higher proportion of participants achieving levels 3 and 4 of the challenge post intervention (see Figure 13).
FIGURE 13.
Change in 4TBS over time among participants who adhered to MFFP and exercise intervention (n = 124).
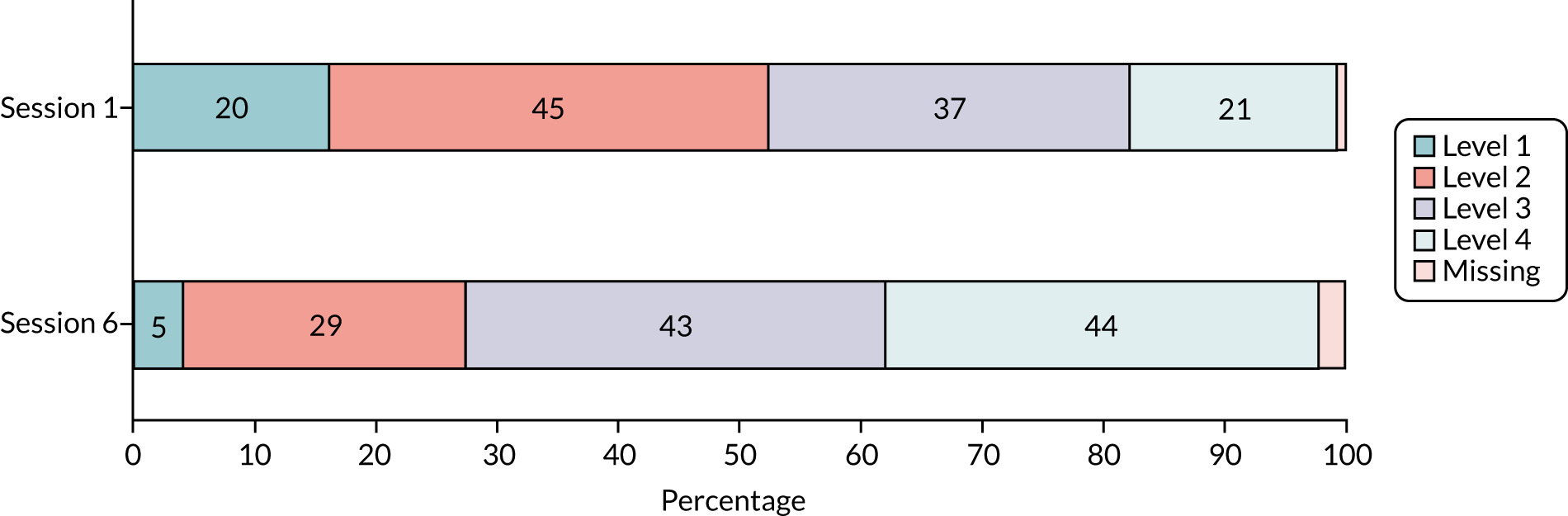
Perceived improvement at discharge
At the final assessment, therapists asked each participant about their strength, balance and walking since taking part in the intervention. These data were available for those who adhered (124/203, 61%). The proportion of participants reporting improvements in strength (65%) was similar to the proportion who adhered, although a lower proportion reported improvements in balance (52%) and walking (44%) than those randomised to the exercise arm (Figure 14). Therapists were also asked to report their own opinion on whether or not participants had progressed at the final appointment. Progression was reported in 57 out of 124 (46%) participants; therapists thought that 25 (20%) participants had not progressed (n = 42 missing).
FIGURE 14.
Change in 4TBS over time among participants who adhered to the MFFP and exercise intervention (n = 124).
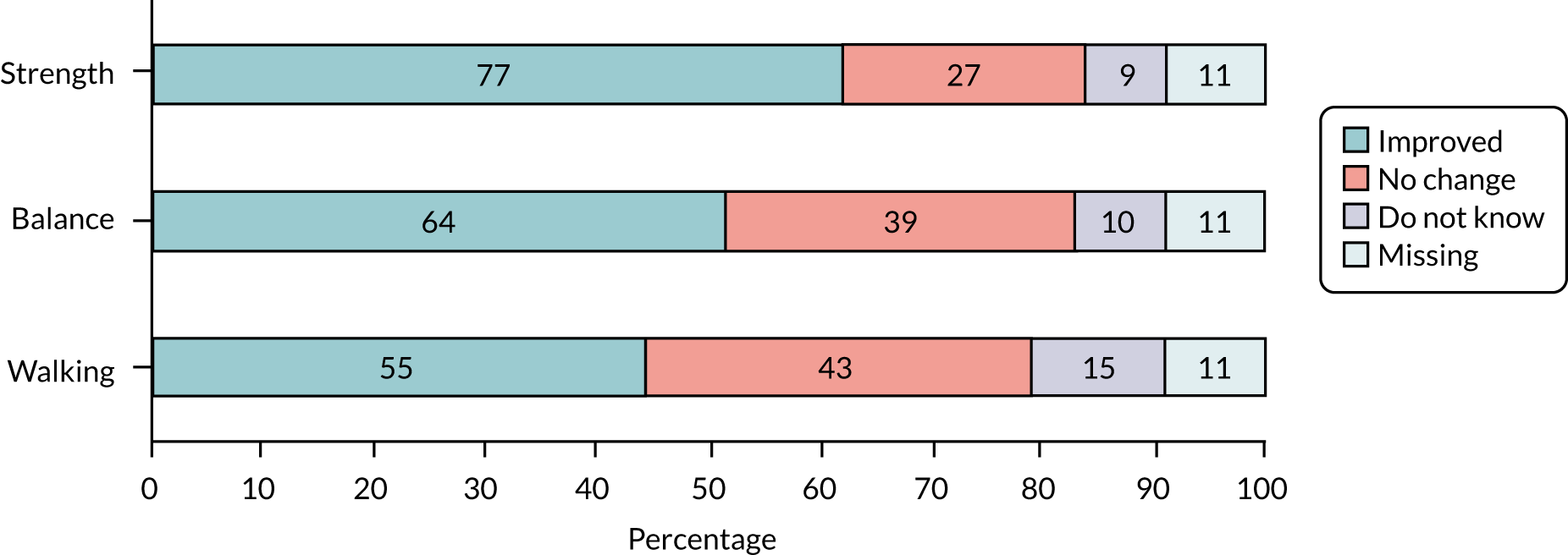
Frequency of exercise on discharge
Participants who completed the intervention were asked at their final appointment how frequently they were exercising: 77 out of 124 (62%) participants reported doing strength exercises three or more times per week and 77 (62%) participants also reported doing balance exercises three or more times per week (the remainder were exercising fewer than three times per week). As for those randomised to exercise, all participants were given an information leaflet, describing opportunities for exercise classes in their local community, at their final appointment and were encouraged to engage with these.
Process evaluation
Medication prescribing
Drug data were extracted from 59 out of 63 (94%) PreFIT GPs. The format of drug data varied by GP. At the minimum, we obtained a summary of drug name and dose per participant with or without a product issue date. For 19 out of 63 (30%) GPs, manual downloads of all prescriptions for individual trial participants were obtained as separate Microsoft Excel® spreadsheets (Microsoft Corporation, Redmond, WA, USA). For GPs using Egton Medical Information Systems (EMIS) (31/63, 49%), summary reports were obtained on total number of trial participants prescribed psychotropics, bisphosphonates and mineral supplements (e.g. 10% of PreFIT participants prescribed psychotropics). After data cleaning, we had useable medication data from 56 out of 63 (89%) GPs. Among these, 18 (32%) GPs were randomised to the advice arm, 19 (34%) GPs were randomised to the exercise arm and 19 (34%) GPs were randomised to the MFFP arm. No differences were found in data format by intervention arm.
Prescribing across general practices
From the 56 GPs with useable data, we obtained drug data for a mean of 126.3 (SD 29.4) participants per surgery pre randomisation and 127.9 (SD 26.8) participants post randomisation. Therefore, we had useable drug data for 80.8% (126/156) of trial participants per practice pre randomisation and 82.1% (128/156) per practice post randomisation. The mean number of all drug products prescribed by the 56 surgeries pre randomisation was 727.2 (SD 227.0), with a mean of 5.6 drugs (SD 1.2 drugs) prescribed per participant. Total drugs included all prescribed products for trial participants, excluding wound dressings, stockings and syringes (i.e. culprit, non-culprit and psychotropic). We found a statistically significant increase in total and mean prescribing over time, rising to a mean of 768.4 drugs (SD 218.8 drugs) per surgery (MD 41.1, 95% CI 19.4 to 62.9; paired t-test p < 0.001) and mean 5.8 drugs (SD 1.2 drugs) per patient post randomisation (MD 0.28, 95% CI 0.1 to 0.4; paired t-test p < 0.001).
General practice prescribing
All general practices
A slight increase in prescribing of psychotropic medications was observed over time across all GPs, from a mean of 18.1% of participants pre randomisation to 18.8% post randomisation (p < 0.02). The mean number of participants per practice prescribed psychotropics rose from 23.1 (SD 7.8) to 24.3 (SD 7.8) (MD –1.2, 95% CI –2.2 to –0.2; p = 0.02). A slight increase in prescribing of bisphosphonates was observed over time, from a mean of 9.7% to 10.1%; however, this increase was not statistically significant (p = 0.49). An increase in prescribing of mineral supplementation was observed across all practices over time, from 13.8% to 15.6% (p < 0.001).
Intervention arms
Although differences in prescribing were found in each intervention arm (Table 23), an independent samples analysis of covariance was used to test for MDs in prescribing across intervention arms. No differences were found in total drugs, mean drugs, psychotropics, bisphosphonates or mineral supplementation prescribed by treatment comparisons.
| Analysis | Treatment arm, mean number (SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advice (n = 18) | Exercise (n = 19) | MFFP (n = 19) | ||||||||||
| Pre | Post | MD (95% CI) | p-valuea | Pre | Post | MD (95% CI) | p-valuea | Pre | Post | MD (95% CI) | p-valuea | |
| Participants per surgery | 131.2 (19.3) | 131.6 (15.2) | 130.1 (22.2) | 132.4 (18.6) | 117.7 (40.9) | 119.9 (39.1) | ||||||
| Total drugs per surgery | 770.6 (172.2) | 808.8 (149.2) | 38.2 (5.9 to 70.5) | 0.02 | 733.3 (181.7) | 786.4 (171.8) | 53.1 (28.0 to 78.3) | < 0.001 | 680.1 (303.5) | 711.9 (300.6) | 31.8 (23.6 to 87.3) | 0.24 |
| Drugs per participant | 5.8 (0.9) | 6.1 (0.8) | 0.3 (0.1 to 0.5) | 0.007 | 5.5 (1.0) | 5.9 (1.0) | 0.4 (0.2 to 0.5) | < 0.001 | 5.5 (1.5) | 5.6 (1.5) | 0.2 (0.2 to 0.5) | 0.27 |
| Participants prescribed psychotropics | 26.1 (5.6) | 26.6 (6.2) | 0.5 (1.3 to 2.3) | 0.57 | 22.4 (8.1) | 23.8 (6.6) | 1.5 (0.3 to 3.3) | 0.10 | 21.0 (8.8) | 22.6 (10.0) | 1.6 (0.4 to 3.7) | 0.11 |
| Participants prescribed bisphosphonates | 11.9 (3.9) | 12.2 (4.0) | 0.2 (1.1 to 1.5) | 0.72 | 12.0 (4.8) | 12.1 (5.5) | 0.1 (1.5 to 1.6) | 0.94 | 10.1 (4.3) | 10.6 (4.4) | 1.5 (0.9 to 1.9) | 0.44 |
| Participants prescribed mineral supplementation | 15.8 (6.9) | 17.7 (8.2) | 1.8 (0.3 to 3.7) | 0.05 | 16.8 (7.0) | 19.2 (7.0) | 2.4 (1.2 to 3.6) | 0.001 | 15.7 (7.3) | 19.1 (9.0) | 3.4 (1.6 to 5.2) | 0.001 |
Chapter 5 Health economics
Overview of health economics analysis
We did a within-trial economic evaluation, with the objective of estimating the cost-effectiveness of exercise and MFFP, both compared with advice. The economic evaluation took the form of a cost–utility analysis, expressed in terms on incremental cost per quality-adjusted life-year (QALY) gained, incremental net health benefit (INHB) and incremental net monetary benefit (INMB). The analysis is based on an NHS and Personal Social Services (PSS) perspective, using the reference cost framework as recommended by NICE. 116
Measurement of resource use and costs
The incremental costs associated with the trial were determined using a comprehensive strategy that encompassed two approaches: estimation of (1) costs associated with the delivery of the interventions and (2) secondary care costs and broader health and PSS resource inputs and costs.
Costing of the PreFIT active interventions
A specific focus of the economic evaluation was the assessment of the cost of delivering the interventions in the active intervention arms. Participants randomised to the control arm received only the Age UK Staying Steady leaflet. 22 Those allocated to exercise or MFFP were mailed a falls risk screener, which was returned to their GP. Participants at risk of falling were invited to attend for further assessment and treatment, either exercise or MFFP, depending on GP allocation.
The exercise intervention was adapted from the OEP. 72 The OEP intervention manual is free to download online, but slight modifications were made for delivery in the trial context (with permission from the Otago research group). The PreFIT intervention consisted of individual or group sessions with a trained therapist over 6 months. Two costing perspectives were adopted for costing the intervention: one that considers delivery of the interventions but which considers adaptation of the MFFP and exercise manuals for the purpose of the intervention to be sunk costs that not occur if treatments were rolled out nationally; and one that implies consideration of both the adaptation and delivery. Our base-case scenario is to use the delivery cost perspective and not include adaptation costs, although we consider both perspectives in a sensitivity analysis. Unit costs for intervention delivery staff include employers’ National Insurance plus a percentage of salary for employers’ contribution to superannuation. Costs included minimal time for data collection; production of the manual; cost of training the PreFIT team in the formal OEP training; health-care professional training; production of participant materials, including exercise booklets and exercise equipment; and staff time for the actual delivery of the intervention (Table 24). For the sensitivity analyses, costs of revising and adapting the exercise and MFFP manuals were included.
| Identified cost | Unit of measure | Unit | Unit cost (£) | Updated cost (£) to 2015–16 using the HCHS index | Total (£) | Price source |
|---|---|---|---|---|---|---|
| Development of intervention manual and data collection forms | ||||||
| Principal research fellow (grade 8) | FTE | 3 weeks (100% FTE) | 49,539 | 53,173.41 | 3323.34 | University of Warwick 2011 prices117 |
| Trial co-ordinator, for editing | FTE | 1 week (2.2% FTE) | 37,251 | 39,983.91 | 439.82 | University of Warwick 2011 prices117 |
| Production of exercise manual | Number of manuals printed | 42 | 2.89 | 3.10 | 130.20 | Trial finance data |
| Data entry clerk for assembly (1 hour/manual) | FTE | 20 hours (1.2% FTE) | 15,353 | 16,479.37 | 98.88 | University of Warwick 2011 prices117 |
| Delivery of training to physiotherapy staff (21 sessions delivered × 6 hours = 126 hours = 7.4% FTE) | ||||||
| Principal research fellow (grade 8) | FTE | 7.4% FTE | 49,539 | 53,173.41 | 1967.40 | University of Warwick 2011 prices117 |
| Research fellow (grade 7) | FTE | 7.4% FTE | 40,280 | 43,235 | 1599.70 | University of Warwick 2011 prices117 |
| Physiotherapy training | 126 hours | 51.00 | 6426.00 | PSSRU 2015.118 Non-consultant-led physiotherapy | ||
| Mailing of screening forms to participants | ||||||
| Royal Mail postage | Number of stamps | 3301 | 0.66 | 2178.66 | Royal Mail119 | |
| Screening forms data entry (15 seconds per form) | ||||||
| Data entry clerk | FTE | 13.5 hours (0.80% FTE) | 15,353 | 16,479.37 | 135.13 | |
| Delivery of intervention | ||||||
| Participant exercise booklets | Number of booklets | 697 | 0.22 | 0.24 | 167.28 | Trial finance data |
| Ankle weights | 862a | 3.80 | 3.80 | 3275.60 | Trial finance data | |
| Labour | ||||||
| Hospital physiotherapy (outpatients) | Number of contacts | 1518 | 34 | 36 | 54,648.00 | PSSRU 2015.118 Non-consultant-led (non-admitted) follow-up physiotherapy attendance |
| Community physiotherapy (home visits) | Number of contacts | 531 | 51 | 55 | 29,205.00 | PSSRU 2014.120 Cost for a one-to-one contact with physiotherapy services for 2013/14 |
| Community physiotherapy (telephone) | Number of contacts | 1764 | 31.11 | 33 | 58,212.00 | No data on unit cost of telephone physiotherapy contact: based on GP telephone consultations, assume unit cost for physiotherapy telephone contact is 61% of face to face |
| Community physiotherapy (group) | Number of contacts | 29 | 14 | 14 | 406.00 | PSSRU 2016.121 Assume same cost (per service user) as mindfulness-based cognitive therapy: group-based intervention |
The MFFP assessment consisted of a single risk assessment session, after which participants were potentially referred to other health-care professionals and services based on their assessment results. The intervention was based on the Tinetti et al. 9 MFFP programme and modified for the PreFIT. The delivery of the MFFP intervention consisted of an individualised risk assessment conducted within a 1-hour appointment with a trained assessor.
The total cost of confirmed referral visits emanating from the MFFP assessment were included in the intervention costs (Table 25). It was possible to confirm attendances for those referred to exercise and for general practitioner-led medication reviews based on treatment logs. A decision was made to report the cost of adaptation, manualisation and delivering the interventions separately from the resource use cost. Possible double-counting of intervention data and self-reported resource use data was dealt with in the analyses. The mean cost per participant was calculated by dividing the total cost of the intervention by the total number of participants randomised to the treatment arm (Table 26).
| Identified cost | Unit of measure | Unit | Unit cost (£) | Updated cost (£) to 2015/16 using the HCHS index | Total (£) | Price source |
|---|---|---|---|---|---|---|
| Development of MFFP manual and data collection forms | ||||||
| Principal research fellow (grade 8): MFFP manual | FTE | 3 months (100% FTE) | 49,539 | 53,173.41 | 13,293.35 | University of Warwick 2011 prices117 |
| Principal research fellow (grade 8): exercise manual | FTE | 3 weeks (100% FTE) | 49,539 | 53,173.41 | 3323.34 | University of Warwick 2011 prices117 |
| Trial co-ordinator for editing MFFP manual | 1 week (2.2% FTE) | 37,251 | 39,983.91 | 879.65 | University of Warwick 2011 prices117 | |
| Trial co-ordinator, for editing exercise manual | 0.5 week (2.2% FTE) | 37,251 | 39,983.91 | 439.82 | University of Warwick 2011 prices117 | |
| Production of MFFP manual | Number of manuals printed | 39 | 2.66 | 2.86 | 111.54 | Trial finance data |
| Data entry clerk for assembly (1 hour/MFFP manual) | 20 hours (1.2% FTE) | 15,353 | 16,479.37 | 197.75 | University of Warwick 2011 prices117 | |
| Production of exercise manual | Number of manuals printed | 42 | 2.89 | 3.10 | 130.20 | Trial finance data |
| Data entry clerk for assembly (1 hour/exercise manual) | 10 hours (0.6% FTE) | 15,353 | 16,479.37 | 98.88 | University of Warwick 2011 prices117 | |
| Mailing of screening forms to participants | ||||||
| Royal Mail postage | Number of stamps | 3301 | 0.66 | 2178.66 | Royal Mail119 | |
| Screening forms data entry (15 seconds per form) | ||||||
| Data entry clerk | FTE | 13.5 hours (0.82% FTE) | 15,353 | 16,479.37 | 135.13 | |
| Delivery of training (11 sessions delivered × 6 hours = 66 hours = 4% FTE) | ||||||
| Principal research fellow (grade 8) | FTE | 4% FTE | 49,539 | 53,173.41 | 2126.94 | University of Warwick 2011 prices117 |
| Specialist registrar in geriatrics | FTE | 4% FTE | 39,300 | 42,183 | 1687.32 | NHS Employers122 |
| Delivery of intervention | ||||||
| Practice nurse for MFFP assessment | Number of participants | 551 | 56 | 56.75 | 31,269.25 | PSSRU 2015.118 Unit costs available 2014/15 (costs including qualifications given in brackets) per hour of face-to-face contact |
| Consultant-led MFFP assessment | Number of participants | 119 | 118 | 119.57 | 14,228.83 | PSSRU 2015.118 Consultant-led outpatient attendances |
| Community physiotherapy/occupational therapy for MFFP assessment | Number of participants | 92 | 51 | 52.14 | 4796.88 | PSSRU 2014.120 Cost for a one-to-one contact with physiotherapy services for 2013/14 |
| Onward referrals | ||||||
| Participant exercise booklets | Number of booklets | 203 | 0.22 | 0.24 | 48.72 | Trial finance data |
| Ankle weights | 212a | 3.80 | 3.80 | 805.60 | Trial finance data | |
| Labour | ||||||
| Hospital physiotherapy (outpatients) | Number of contacts | 437 | 34 | 14,858.00 | PSSRU 2015.118 Non-consultant-led (non-admitted) follow-up physiotherapy attendance | |
| Community physiotherapy (home visits) | Number of contacts | 167 | 51 | 8517.00 | PSSRU 2014.120 Cost for a one-to-one contact with physiotherapy services for 2013/14 | |
| Community physiotherapy (telephone) | Number of contacts | 438 | 31.11 | 13,626.18 | No data on unit cost of telephone physiotherapy contact. Based on GP telephone consultations, assume unit cost for physiotherapy telephone contact is 61% of the cost of face-to-face contact | |
| Community physiotherapy (group) | Number of contacts | 4 | 14 | 56.00 | PSSRU 2016.121 Assume same cost (per service user) as mindfulness-based cognitive therapy group-based intervention | |
| GP medication review | Number of face-to-face contacts | 287 | 36 | 10,332.00 | PSSRU 2016121 | |
| Telephone contacts | 132 | 27 | 27.36 | 3611.52 | PSSRU 2015118 | |
| Intervention | Randomised (n) | Average cost (£) per patient (ITT population) | Average cost (£) per patient, including manual development (ITT population) |
|---|---|---|---|
| Advice | 3223 | 0.66 | 0.66 |
| Exercise | 3279 | 48.13 | 49.28 |
| MFFP | 3301 | 39.44 | 44.87 |
Collection of secondary care use data
Data on inpatient hospital spells and A&E and outpatient attendances over the duration of the trial were sourced from HES data provided by NHS Digital for financial years 2011/12 to 2015/16. As these data are obtained centrally and not self-reported, we assumed that these data were complete. The completeness of hospital data and the ability to identify when participants had prolonged periods in hospital and/or A&E visits was exploited in the MI approach used to estimate missing self-reported responses. Inpatient spells during the study and other hospital-based care costs were determined by linking HES data with 2015/16 Healthcare Resource Groups (HRGs), using 2015/16 reference cost grouper software from NHS Digital, and then costed using NHS Reference Costs 2015–2016. 123 Inpatient and outpatient spell costs included unbundled costs (such as high-cost drugs) and excess bed-day costs, when applicable. All costs were discounted using a rate of 3.5% per annum.
Collection of broader resource use data
We collected data on health-care resource use from participant self-report questionnaires from randomisation to 4, 8, 12 and 18 months post randomisation. Data included use of primary and community-based health care, community-based social care, residential or nursing care and aids and equipment for each time point. Broader health-care resource use costs were valued by applying unit costs from the Personal Social Services Research Unit (PSSRU) national tariffs. 120 Mean annual equipment costs include the costs of installing equipment and making adaptions. The Hospital and Community Health Services index was used to adjust costs, when necessary, to 2015/16 prices. 121 All costs were discounted using a rate of 3.5% per annum. Resource use values at the individual participant level were combined with unit costs for each resource item to estimate economic costs for resource use (Table 27).
| Resource item in questionnaire | Resource item broken down | Cost (£) | Updated cost (£) (using HCHS index) | Unit of assessment | Source |
|---|---|---|---|---|---|
| General practitioner: GP consultation | 36.00 | Per surgery consultation lasting 9.22 minutes | PSSRU 2016121 | ||
| General practitioner: home consultation | 55.86 | 57.74 | Home visit lasting 11.4 minutes |
PSSRU 2013124 £4.90 per out-of-surgery visit minute |
|
| General practitioner: telephone consultation | 27.00 | 27.36 | Per telephone consultation lasting 7.1 minutes | PSSRU 2015118 | |
| District nurse, health visitor or member of community health team | 38.00 | 38.50 | Face-to-face contact district nursing services | PSSRU 2015118 | |
| Physiotherapist/occupational therapist | 51.00 | 52.14 | Face-to-face contact in community physiotherapy services | PSSRU 2014120 | |
| Social worker | 28.50 | Assume 30-minute visit |
PSSRU 2016121 £57 cost per hour including qualification |
||
| Home help or care worker | 12.00 | Per visit lasting 30 minutes |
PSSRU 2016121 £24 per hour on a weekday |
||
| Day centre | 46.00 | Per client session lasting 3.5 hours |
PSSRU 2016121 Local authority day care for older people |
||
| Lunch or social club | 5.00 | Assumption | |||
| Food, medicine or laundry delivery service | 6.00 | 6.44 | Meals on Wheels provided by the local authority | The Information Centre (2012)125 PSS EX1 2010/11 | |
| Family or patient support, or self-help groups | 15.00 | 15.20 | 30-minute session |
PSSRU 2015118 £30 cost per hour |
|
| Nursing home | 107.14 | 110.76 | Per day |
PSSRU 2013124 £750 establishment cost per permanent resident week (private sector) |
|
| Residential care | 158.57 | 160.68 | Per day |
PSSRU 2015118 £1110 establishment cost per permanent resident week |
|
| Special aids or equipment: hospital | Level-access shower | 575.00 | Mean annual equipment cost | PSSRU 2016121 | |
| Stair lifts: straight | 232.00 | Mean annual equipment cost | PSSRU 2016121 | ||
| Stair lifts: more complex | 564.00 | Mean annual equipment cost | PSSRU 2016121 | ||
| Special aids or equipment: local authority equipment and adaptations | Hoist | 319.00 | 335.37 | Mean annual equipment cost | PSSRU 2012126 |
| Stair lift | 402.00 | 415.57 | Mean annual equipment cost | PSSRU 2012126 | |
| Grab rail | 6.00 | 6.20 | Mean annual equipment cost | PSSRU 2012126 |
Calculation of utilities and quality-adjusted life-years
The economic evaluation estimated QALY profiles for trial participants, based on participant reports of preference-based HRQoL outcomes at each follow-up time point, using the EQ-5D-3L. 127 The EQ-5D-3L uses a descriptive system that defines HRQoL across five dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. Responses in each dimension are categorised into three ordinal levels: (1) no problems, (2) some or moderate problems and (3) severe or extreme problems. For the purposes of the economic evaluation, the UK time trade-off tariff was applied to each set of responses to generate an EQ-5D-3L utility score (preference weight) for each trial participant. 127 Participants who died during the study were allocated a utility of zero at all following time points. QALYs were calculated as the area under the baseline-adjusted utility curve and were calculated using linear interpolation between utlity scores at baseline and 4, 8, 12 and 18 months. QALYs were discounted using a rate of 3.5% per annum.
Missing data
Multiple imputation using the method of chained equations was used for the base-case analysis to impute missing data. This avoids potential biases associated with complete-case analysis and is consistent with good practice guidance. 128 Data were assumed to be missing at random. MI of missing self-reported HRQoL data and self-reported costs was conducted on a full analysis data set of combined baseline data, self-reported HRQoL and costs and complete HES data. The inclusion of the HES data and the ability to condition imputation on observed and assumed complete secondary care use make the assumption that data were missing at random more plausible. The MI analysis was conducted using the PROC MI command in SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA). A total of 100 imputations were calculated.
Analyses of resource use, costs and outcome data
Multilevel linear models (MLMs) were used to identify the incremental impact of interventions over time while recognising the cluster structure of the trial and accommodating patient and practice heterogeneity. MLM addresses clustering by including random-effects parameters, which represent the differences in the cluster mean outcomes and costs from the overall mean outcome and costs in each trial arm. Three-level models were estimated with random effects for individuals and practices because data were clustered within individuals within GPs. The MLM regression-based correction is used to accommodate potential differences in mean baseline HRQoL scores and reported health and social care use between trial arms, which are expected despite randomisation. Such imbalances at baseline are important to account for when calculating the differential effects between trial arms because a participant’s HRQoL score at baseline is likely to be correlated with their subsequent HRQoL scores over the follow-up period. 129
Cost-effectiveness analyses
The incremental cost-effectiveness ratio (ICER) was estimated as the difference between the trial comparators in mean total costs divided by the difference in mean total QALYs. Value for money was determined by comparing the ICER with a cost-effectiveness threshold value, typically the NICE cost-effectiveness threshold for UK studies ranges of between £20,000 and £30,000 per QALY. This represents society’s willingness to pay for an additional QALY; when incremental utility gains are positive, lower ICER values than the threshold could be considered cost-effective for use in the NHS.
The INMB and INHB measures were also reported at a range of cost-effectiveness thresholds and represent the cost-effective argument in single metric, either as a monetary value in INMB, when the HRQoL is converted to a monetary value via the willingness to pay, or in HRQoL terms, when incremental monetary differences are converted into an expected impact on HRQoL via the use of that money elsewhere in the system. In both cases, positive values indicate that the intervention is cost-effective at the given threshold.
Sensitivity analyses
Uncertainty was assessed using a within-trial probabilistic sensitivity analysis (PSA), undertaken by drawing model parameter values from the variance–covariance matrices from the cost and utility multilevel regression models, with results presented as cost-effectiveness acceptability curves (CEACs). The CEAC illustrates the likelihood that interventions are cost-effective as the cost-effectiveness threshold varies. All analyses and cost-effectiveness modelling were conducted in SAS. Complete-case analysis was used as an additional sensitivity analysis.
Results
Cost of interventions
For the advice intervention, the only costs identified related to mailing of the Age UK Staying Steady leaflet. 22 Estimates of the total costs of delivering the exercise and MFFP interventions were summarised. The cost components are aggregated into headings for exercise as follows: (1) development of intervention manual and data collection forms (included in sensitivity analysis only); (2) mailing of screening forms and data entry on returned forms; (3) cost of training PreFIT staff; (4) delivery of training to physiotherapists; and (5) delivery of intervention, inclusive of staff time and equipment (see Table 24). For MFFP the headings are (1) development of intervention manual and data collection forms, (2) mailing of screening forms and data entry of returned forms, (3) cost of training assessors, (4) delivery of intervention and (5) onward referrals (see Table 25).
Total intervention costs were £2127 for advice, £157,823 for exercise and £130,185 for MFFP. Estimates of average cost per participant randomised to a specific intervention were £0.66 for advice, £48.13 for exercise and £39.44 for MFFP (see Table 26). Including manual development costs increased the total intervention costs for exercise and MFFP to £161,586 and £148,121, respectively, with average costs per participant of £49.28 for exercise and £44.87 for MFFP. Advice costs are unchanged. Although intervention costs per participant are measured as simple averages, all other costs for secondary care and broader resource use are measured over time and modelled in a regression framework.
Secondary care and broader resource use costs
Figures 14 and 15 show observed average complete-case secondary care costs and imputed broader resource use over time by treatment arm. Table 24 presents the corresponding average regression-corrected monthly costs, respectively, for secondary care and broader resource use costs by trial allocation and study period. Owing to the well-balanced nature of the trial, the regression analyses, which accommodate the clustering of outcomes within patients and practices over time, do not substantially change the picture that emerges from inspection of the raw and imputed data (Table 28). We see that secondary care monthly costs exceed resource use costs and both show an increasing trend over time (Figures 15 and 16). The trend appears more pronounced in the secondary care data. Inspection of the HRGs in the secondary care data demonstrates that the majority of secondary care costs are not directly related to falls, but rather to other comorbidities (e.g. cancer) (see Appendix 1, Table 33).
| Time point | Advice | Exercise | MFFP | |||
|---|---|---|---|---|---|---|
| Mean (£) | % imputed | Mean (£) | % imputed | Mean (£) | % imputed | |
| Secondary care | ||||||
| –4 months to randomisation | 115 | 0 | 108 | 0 | 100 | 0 |
| Randomisation to 4 months | 126 | 0 | 112 | 0 | 116 | 0 |
| 4 to 8 months | 152 | 0 | 137 | 0 | 147 | 0 |
| 8 to 12 months | 172 | 0 | 162 | 0 | 161 | 0 |
| 12 to 18 months | 172 | 0 | 167 | 0 | 165 | 0 |
| Resource use | ||||||
| Randomisation to 4 months | 49 | 8.2 | 43 | 8.3 | 47 | 8.9 |
| 4 to 8 months | 50 | 12.3 | 50 | 13.4 | 53 | 13.2 |
| 8 to 12 months | 52 | 15.9 | 47 | 16.5 | 55 | 17.6 |
| 12 to 18 months | 60 | 22.0 | 62 | 23.6 | 61 | 23.8 |
FIGURE 15.
Secondary care monthly costs over time, complete cases.
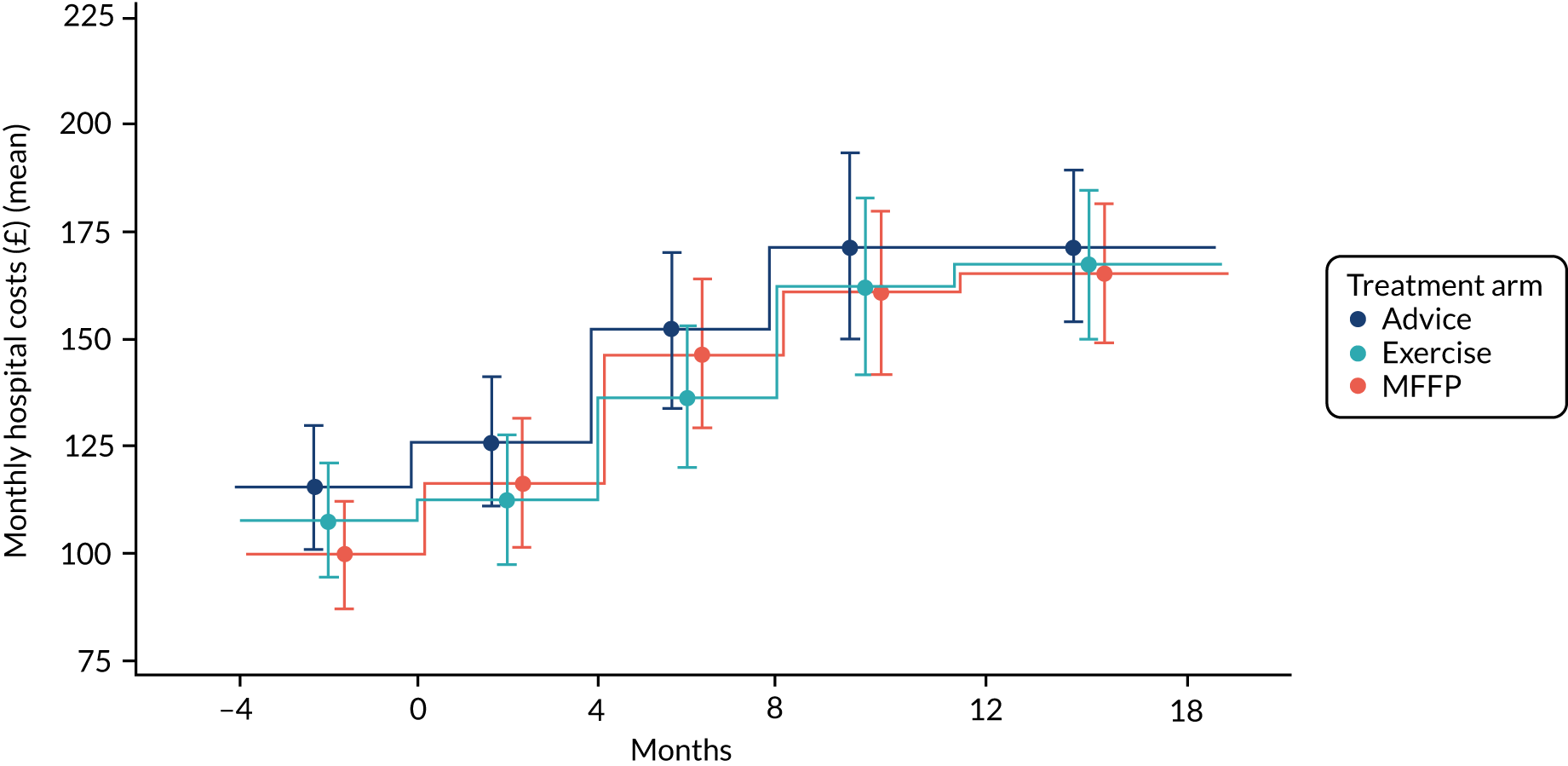
FIGURE 16.
Imputed broader resource use monthly costs over 18 months.
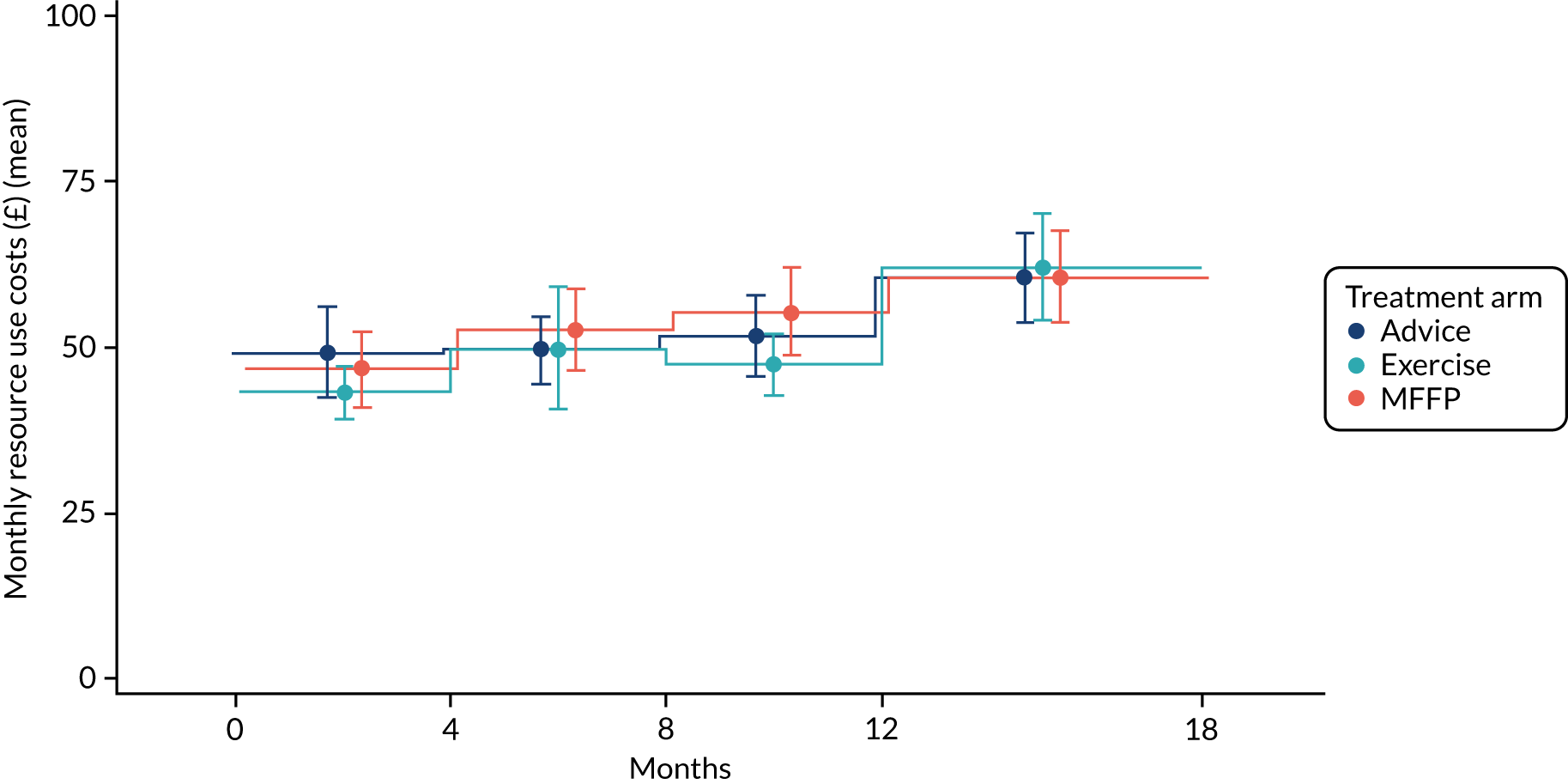
The total expected average net present value (NPV) broader resource use costs over the 18-month period are £951.10 for advice, £924.52 for exercise and £971.23 for MFFP. For secondary care, the modelled expected average NPV secondary care costs are £2785.66 for advice, £2747.79 for exercise and £2930.25 for MFFP. Although MFFP is systematically related to higher expected costs than either advice or exercise, regression results showed no statistically significant differences between the trial groups at any time point in any cost regression (see Appendix 1).
Total costs
Total costs consist of intervention, secondary care and broader resource use costs. The expected average NPV total costs for each intervention are £3737.42 for advice, £3720.44 for exercise and £3940.92 for MFFP. Resource use and secondary care costs account for by far the greatest component of total costs, and much more than intervention costs, with approximately 75% of all costs being secondary care costs (inpatient, A&E and outpatient attendance). Including the sunk costs of manual adaptation raises the expected costs to £3721.59 for exercise and £3946.35 for MFFP (relatively modest increases over the 18 months).
Inspection of the modelled regression-based expectations for participants over time shows a similar gradual increase in costs over time for all interventions, with MFFP having a small but systematically higher incremental cost at all time points. There is almost no distinguishable difference in modelled expected costs over time between advice and exercise (Figure 17).
FIGURE 17.
Modelled regression-based monthly costs over 18 months.
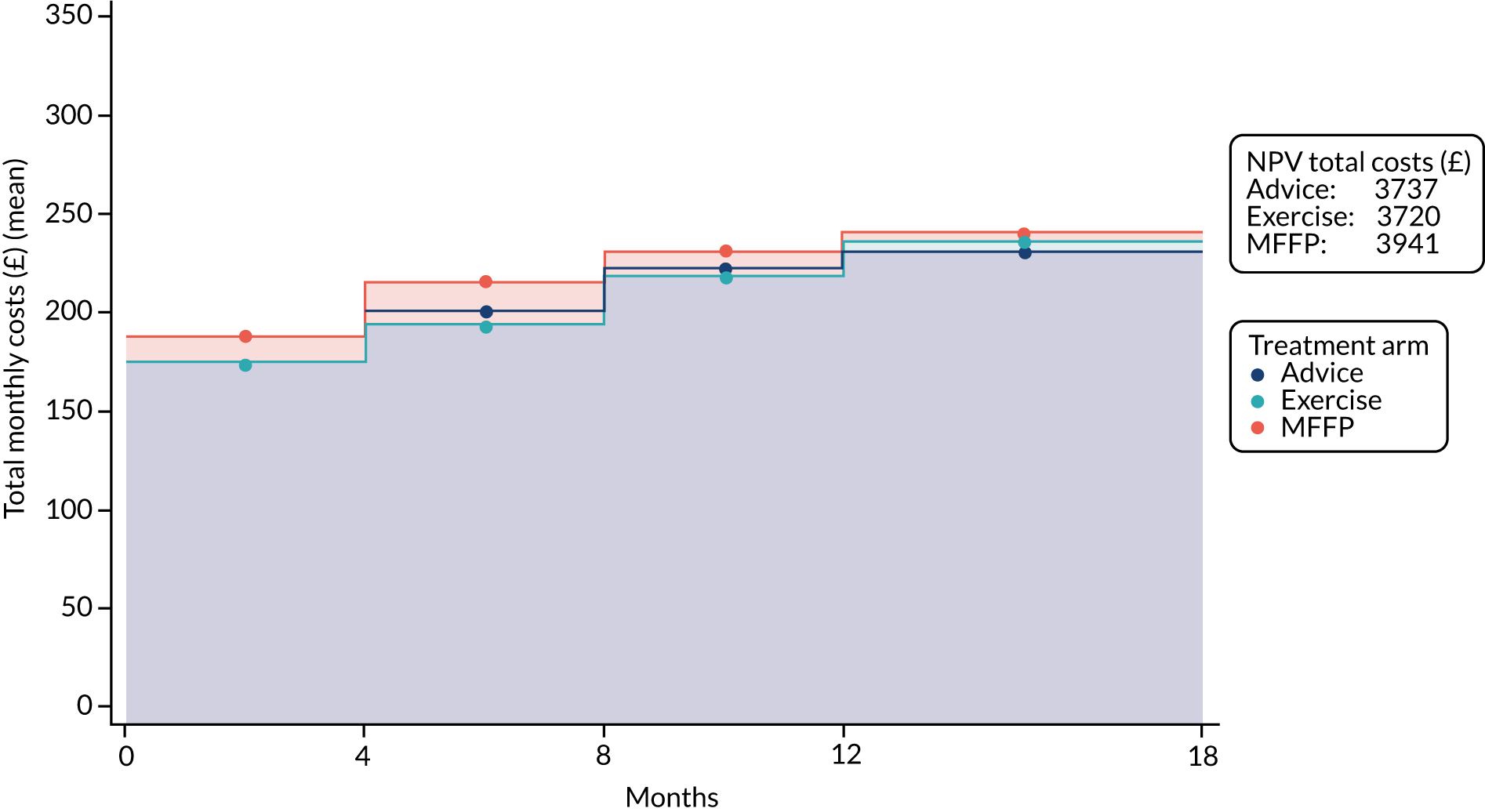
Over 18 months, a participant in the MFFP arm is expected to generate an incremental cost of £204 relative to advice and of £220 relative to exercise. The cost per participant of the advice leaflet is only £17 more than the cost of exercise (equivalent to approximately £1 per month). These are very marginal differences and, from the regression results, and we know that they are measured with a relatively large SE.
The mean expected values over the PSA simulations are £3739.83 for advice, £3713.42 for exercise and £3942.76 for MFFP, leading to almost identical incremental differences in costs as calculated in the deterministic model.
For complete-case analysis, the total costs are £3506.98 for advice, £3492.38 for exercise and £3683.12 for MFFP. These figures are lower than those obtained from the imputed data because data for self-reported resource use are more likely to be missing when there are A&E attendances or longer hospital stays. Imputation on a data set containing HES data leads to higher than overall average costs being imputed when missing; this is intuitively appealing because the expectation is that all resource use costs will be higher when A&E admission occurs. The incremental differences are similar but slightly smaller, with MFFP being the most expensive intervention, followed by advice. For example, the incremental costs of MFFP relative to advice and to exercise are now £30 smaller, at £176 and £191, respectively. Between advice and exercise, advice is more costly by £15.
Health-related quality-of-life outcomes
Figure 18 shows the pattern of EQ-5D-3L utility values over 18 months. Table 25 shows mean EQ-5D-3L values by trial allocation and by study period. The three intervention arms show EQ-5D-3L mean utility values decreasing at each time point. The regression results for the imputed EQ-5D-3L utilities show no statistically significant differences between exercise and advice, and between advice and MFFP, but there are statistically significant differences between exercise and MFFP at months 4, 12 and 18, with exercise having small but systematic incremental gains in self-reported HRQoL (0.011 at months 4 and 12 and 0.0016 at month 18). The complete-case regressions yield very similar results (Table 29).
FIGURE 18.
Pattern of EQ-5D-3L utility over 18 months.
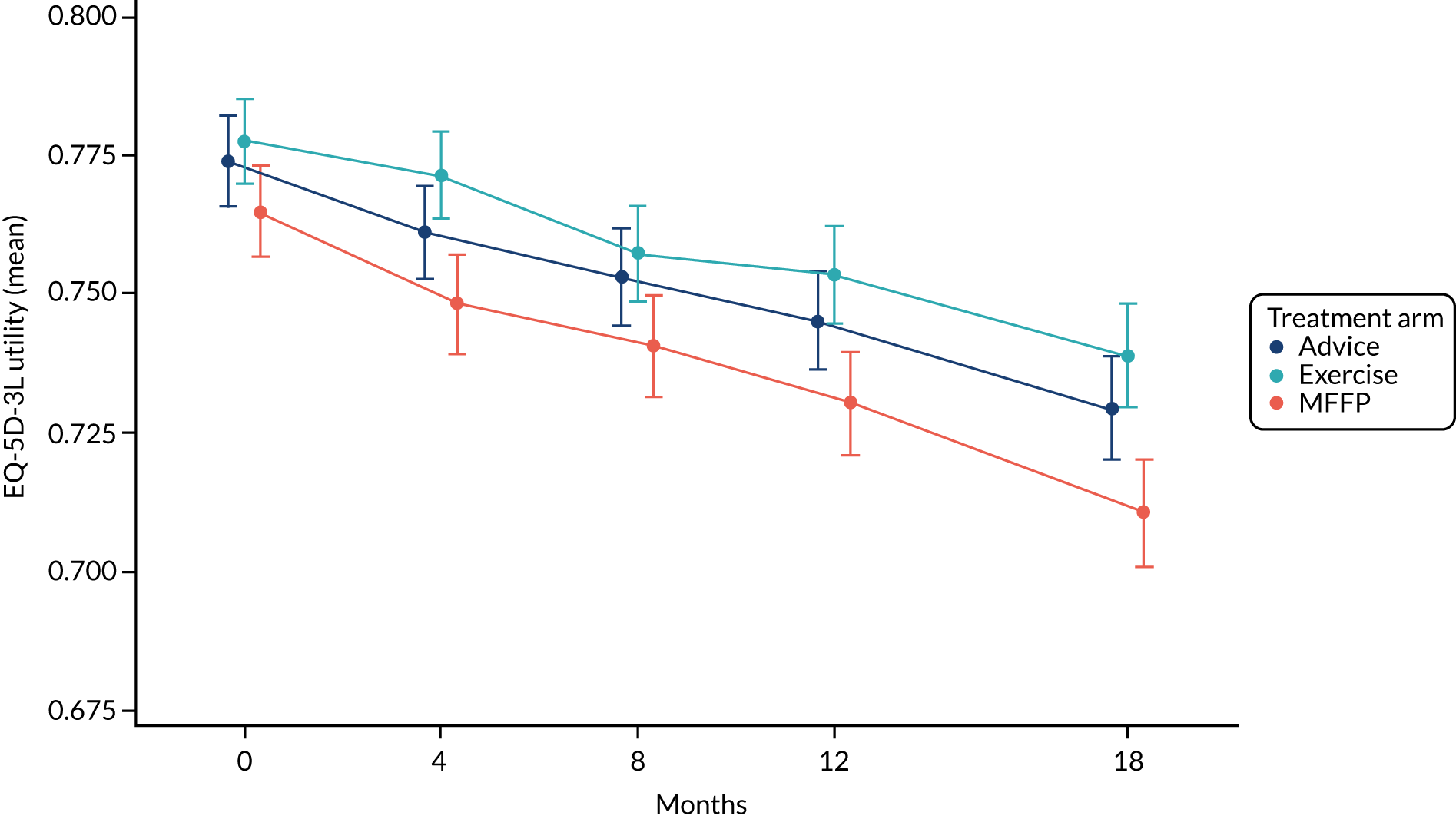
| Time point | Advice | Exercise | MFFP | |||
|---|---|---|---|---|---|---|
| Mean | % imputed | Mean | % imputed | Mean | % imputed | |
| Randomisation | 0.774 | 4.4 | 0.778 | 4.4 | 0.765 | 5.2 |
| 4 months | 0.761 | 11.7 | 0.772 | 11.7 | 0.748 | 11.8 |
| 8 months | 0.753 | 15.5 | 0.758 | 15.6 | 0.741 | 16.4 |
| 12 months | 0.745 | 18.6 | 0.754 | 19.5 | 0.730 | 20.1 |
| 18 months | 0.730 | 24.2 | 0.739 | 24.8 | 0.711 | 25.6 |
Modelling regression-based expectations over time, Figure 19 shows a consistent pattern, with exercise, followed by advice, yielding the highest HRQoL at all time points. Although it is difficult to extrapolate with within-trial models, the gap between exercise and MFFP appears to be increasing over time, leading to the expectation that if the trial period were to be extended we would see an increasing incremental difference. The NPV QALYs show an average expectation of 1.1137 for advice, 1.1195 for exercise and 1.1064 for MFFP, leading to a small incremental gain of 0.006 QALYs for exercise relative to advice and a further gain of 0.007 QALYs relative to MFFP. The incremental difference between exercise and MFFP over the 18 months of 0.013 is approximately equivalent to an additional 5 days in perfect health.
FIGURE 19.
Modelled EQ-5D-3L utility over 18 months (AUC).
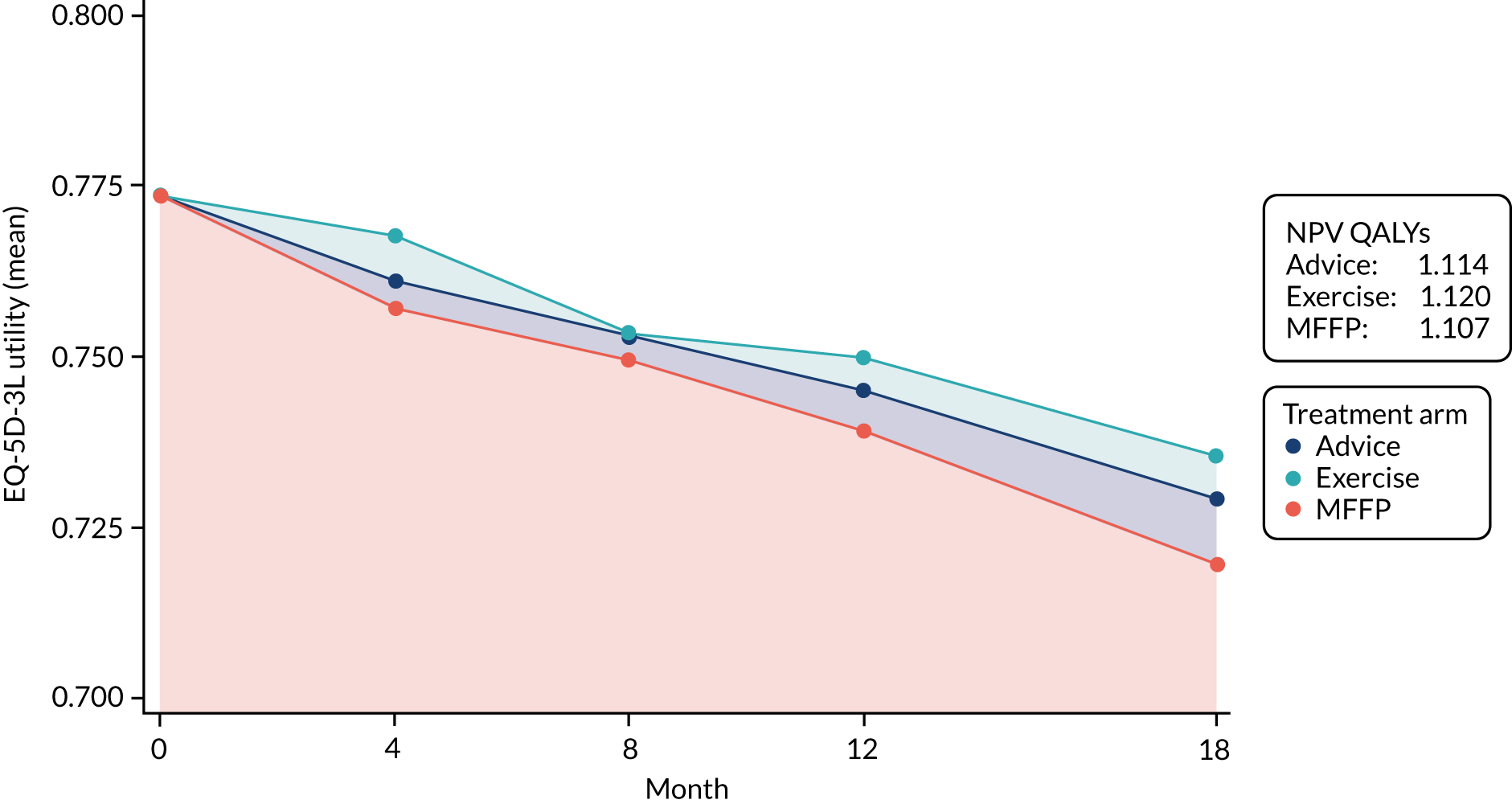
Complete-case analysis estimates expected NPV QALYs of 1.1166 for advice, 1.1206 for exercise and 1.1082 for MFFP. Although the expected NPVs are higher in all cases (a function of missing EQ-5D-3L data being most likely when hospitalisations occur, with imputation correcting for this via imputation with HES linked data), the incremental differences are similar and the substantive picture does not change. PSA-simulated means for the imputed data are 1.1136 for advice, 1.1193 for exercise and 1.1063 for MFFP, in all cases almost identical to the figures in the deterministic model.
Cost-effectiveness results
Baseline analysis
The incremental cost-effectiveness results are shown in Table 30, with costs and QALY data subject to MI. The exercise intervention predicts the highest expected QALYs and lowest expected costs, and so it dominates both advice and MFFP. As the intervention with the lowest expected QALYs and highest costs, MFFP is dominated by both advice and exercise.
| Treatment | NPV QALYs | NPV costs (£) | ICER (relative to Advice) | INMB (£) at £20,000 (relative to advice) | INMB (£) at £30,000 (relative to advice) |
|---|---|---|---|---|---|
| Cost-effectiveness, imputed data | |||||
| Advice | 1.1137 | 3737 | |||
| Exercise | 1.1195 | 3720 | Dominates | 132.98 | 190.98 |
| MFFP | 1.1064 | 3940 | Dominated | –349.50 | –422.50 |
| Sensitivity analysis: cost-effectiveness, imputed data PSA means | |||||
| Advice | 1.1136 | 3740 | |||
| Exercise | 1.1193 | 3713 | Dominates | 139.48 | 196.01 |
| MFFP | 1.1063 | 3943 | Dominated | –350.12 | –423.72 |
| Sensitivity analysis cost-effectiveness, complete case | |||||
| Advice | 1.1166 | 3507 | |||
| Exercise | 1.1206 | 3492 | Dominates | 93.96 | 133.63 |
| MFFP | 1.1083 | 3683 | Dominated | –342.97 | –426.39 |
The mean INMB associated with exercise relative to advice at cost-effectiveness thresholds of £20,000 and £30,000 per QALY was £132.98 and £190.98, respectively (see Table 30). For exercise compared with MFFP the figures are £482.48 and £613.48, respectively. For advice compared with MFFP, the figures are £349.50 and £422.50, respectively. The mean INHBs for exercise relative to advice are 0.0066 and 0.0064 for threshold values of £20,000 and £30,000, respectively. For exercise relative to MFFP the figures are 0.024 and 0.020, respectively, and for advice relative to MFFP the figures are 0.0175 and 0.0141, respectively.
In conclusion, the deterministic analysis of the imputed trial results shows that exercise dominates both advice and MFFP by providing a higher expected QALY output and lower expected costs. Similarly, advice dominates MFFP. Furthermore, because the ordering of expected costs and HRQoL is consistent across time points, this conclusion would hold if the trial had terminated at 12 months, 8 months or, indeed, at 4 months. However, the relatively low magnitudes of the INMB and INHB figures indicate that cost and QALY differences are rather small.
Sensitivity analyses
The key finding from the analysis is that exercise dominates both advice and MFFP in terms of producing higher QALYs at a lower expected cost. However, we also recognise that the practical differences between the QoL and costs estimated between treatment choices are modest, particularly between advice and exercise. For example, we expect an incremental £1 per month cost difference between advice and exercise. Given that the patient population is extremely heterogeneous in terms of underlying QoL and pre-baseline costs, and had in excess of 20% missing data for HRQoL at month 18, it is important to assess the extent to which the economic conclusions are robust to the sampling variation and variation due to MI that can occur when applied to a heterogeneous population and addressing missing data. Despite these issues, we may still be relatively certain about the substantive conclusions, because the trial size was very large, the heterogeneity was well balanced across arms and there was consistency in findings across time.
Probabilistic sensitivity analysis combined with further critical appraisal of results provides the main opportunity for assessing the robustness of the results. In PSA, alternative estimates of parameter values for utility and costs under each treatment arm are generated and repopulate the existing model structure to produce alternative possibilities of expected QALYs and costs and, hence, incremental values. As with MI, there are a set number of alternatives drawn and the incremental outcomes recorded. Inference is then drawn by looking at the results across the population of alternatives, looking at the distribution of incremental outcomes via a cost-effectiveness plane and the proportion of times each intervention looks cost-effective at various threshold values via a CEAC.
The alternative parameter values for costs and HRQoL at each time point under different treatments were drawn from the regression estimate and variance–covariance matrices, which in combination define probabilistic distributions for estimated parameters. As the variance–covariance matrices accommodate uncertainty from both sampling and imputation processes, it was considered a more efficient method than bootstrapping across all imputed data sets and more accurate than just bootstrapping across a complete-case data set. The notion of conducting PSA from the regression variance–covariance matrix was pioneered by Hoch et al. 130 Simulation via the relevant regression matrices was conducted using PROC IML of SAS and 10,000 simulations were conducted.
Figure 20 shows the simulated costs and QALYs outcomes of MFFP and exercise relative to advice. The figure in the bottom right of the composite diagram shows the standard scatterplot of costs and QALYs from the same simulation, with advice at the (0, 0) reference point and threshold lines annotated over the plot. In addition to the scatterplot, we have also included univariate histograms of the simulated incremental costs and QALYs in isolation: the cost histograms are to the right of the scatterplot and the QALY histogram is located above the scatterplot.
FIGURE 20.
Cost-effectiveness plane of MFFP and exercise (vs. advice). Purple shading indicates overlap of MFPP and exercise interventions.
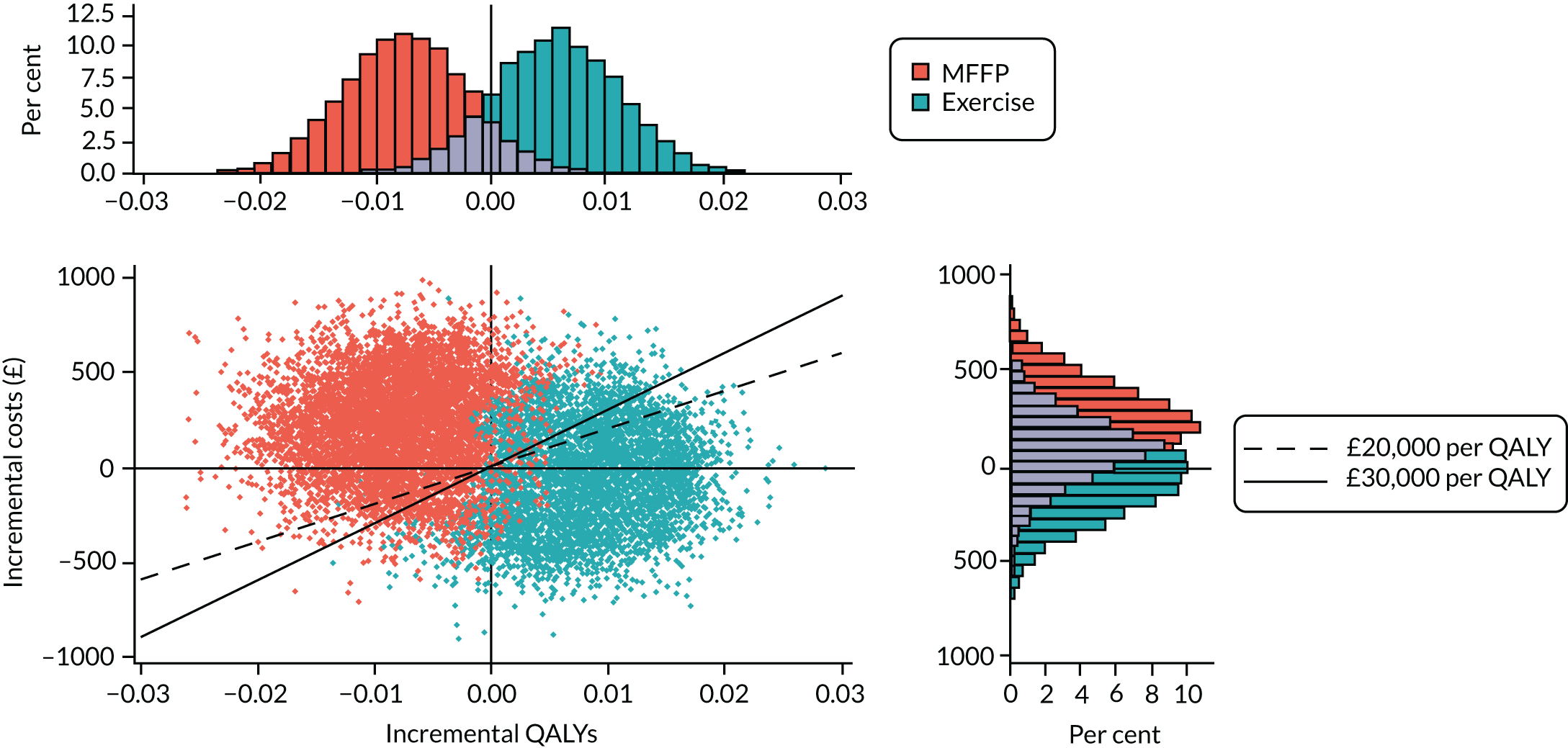
The cost-effectiveness scatterplot shows the two clouds of simulated paired incremental outcomes of MFFP and exercise relative to advice. The MFFP cloud is mainly located in the north-west quadrant, indicating that it is less effective (by being on the west side) and more expensive (by being on the north side) than advice. The clear majority of simulated points lie to the left of the threshold lines, indicating a high probability that MFFP is not cost-effective relative to advice. We note that some of the simulations lie in the south-west quadrant and to the right of the threshold lines, indicating that some simulations find MFFP cost-effective relative to advice, on the grounds that, although it is less effective, the expected cost-savings would be of a magnitude that allowed the freed-up costs to more than offset the QALY loss to the patients receiving MFFP. The cloud of simulations for exercise relative to advice is on the east side of the graph, indicating a relatively high degree of certainty regarding the incremental QALY gain, but the points are split fairly evenly over the north and south quadrants, indicating a large degree of uncertainty regarding the incremental cost estimate.
The univariate histograms reinforce this perspective. If we consider the cost histogram, we notice several elements. First, the distribution of incremental costs of exercise relative to advice is virtually centred over zero and has relatively wide tails stretching from being £894 more expensive to £903 cost saving; a relatively wide distribution. Second, we also notice that the distribution of incremental costs of MFFP relative to advice is fairly wide and, although the body of the distribution indicates that MFFP is more expensive, there is some substantial proportion of the distribution indicating a potential cost saving outcome.
The QALY perspective looks more certain. The range of simulated QALY differences is quite small and, importantly, there are clear differences between the central locations of the distribution and that of zero incremental effect. The PSA shows that, although the magnitude is small, the uncertainty stemming from sampling a heterogeneous population with imputation is relatively minor, with only a small possibility that MFFP is more effective than advice and that advice is more effective than exercise.
The CEAC provides a consolidated means of summarising these findings. At the left-hand side of the graph, where we value QALYs at £0, the economic argument is solely driven by the cost argument. The uncertainty is so substantial that it is almost impossible to determine whether advice or exercise is the most cost-effective. Indeed, the uncertainty is so substantial that there is a non-zero possibility that MFFP is the most cost-effective.
However, as we move along the x-axis and afford greater value to the QALY component of the cost-effectiveness argument, the greater certainty that have that the estimates of incremental QALYs will start to have a bigger impact. Overall, the CEACs show that exercise is always expected to be the most cost-effective intervention (Figure 21), ranging from a 50% probability based on costs alone to 80.5% as QALYs dominate. Over the conventional range of amounts that decision-makers are willing to pay for an additional QALY, that is, between £20,000 and £30,000, the probability that the exercise intervention is cost-effective varies between 70% and 75%, driven by the incremental differences in QALY expectations. Over that range, MFFP is the most cost-effective option approximately 1% of the time.
FIGURE 21.
Cost-effectiveness acceptability curve baseline analysis.
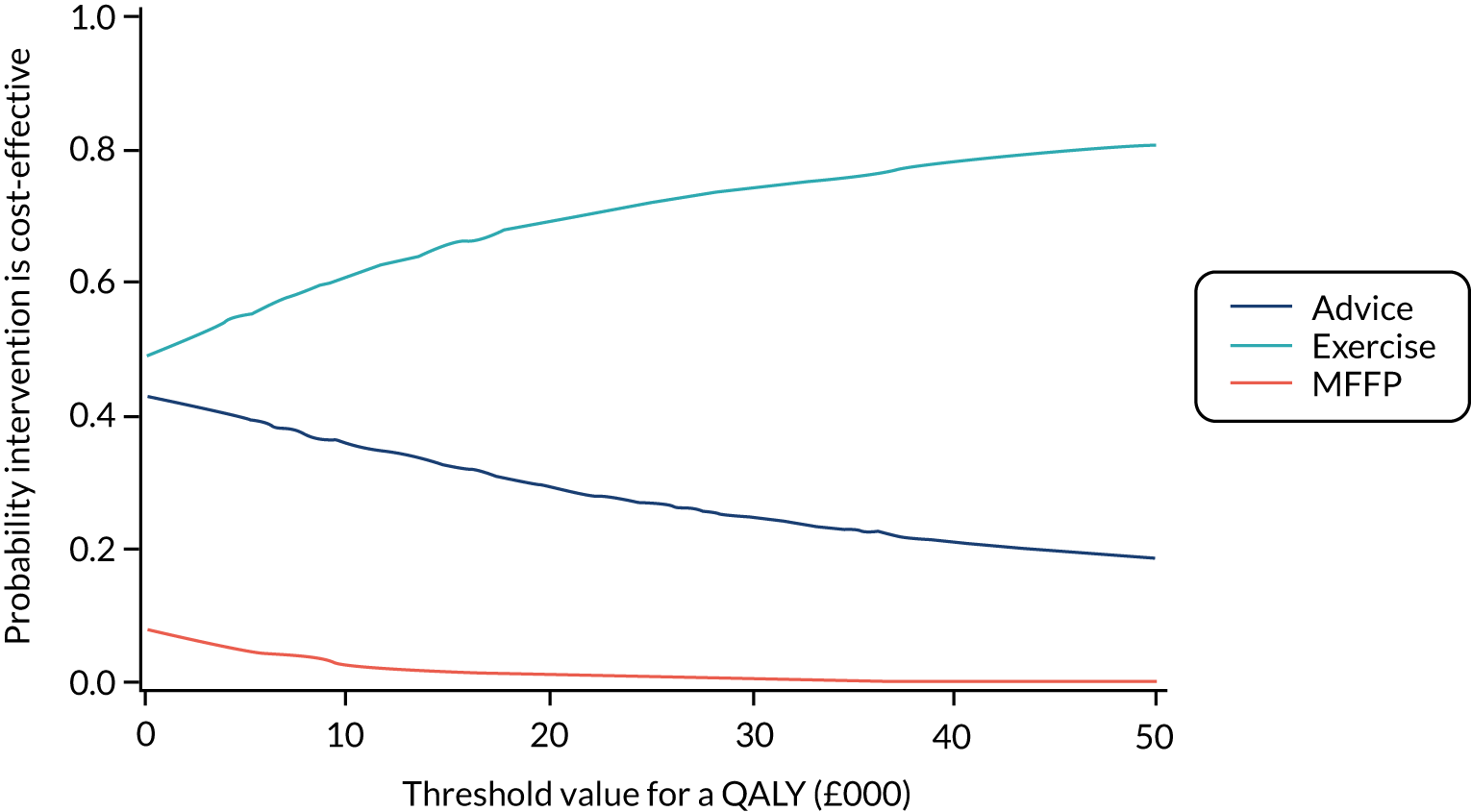
This allows us to draw a number of conclusions. First, there is some substantial uncertainty in the incremental cost differences between all treatments and at face value those uncertainties translate to reasonably substantial cost differences. Second, there is relatively more certainty within the QALY calculations. Third, and as a consequence of the first two conclusions, as the ‘value’ of a QALY increases, the more certain QALY component starts to exceed any possible differences in costs. As a result, we can conclude that the uncertainty in costs is not particularly important in establishing which is the most cost-effective treatment. The CEACs rise more steeply before the £20,000 threshold and more broadly flatten beyond this. Therefore, resolving the uncertainty we have in costs has merit only if we value QALYs < £20,000.
In addition to the imputed model, PSA was also conducted on the complete-case analysis as a sensitivity analysis. The probability that the exercise intervention is cost-effective is lower than in the baseline analysis, ranging from 64.5% to 68.5% between £20,000 and £30,000 thresholds, and asymptotes at 73% as the threshold of willingness to pay increases.
Discussion
The trial-based economic evaluation is based on a large randomised trial with well-balanced cohorts at baseline. Although there was an increasing pattern of missing self-reported data on broader resource use and HRQoL over time, the pattern was very well balanced across interventions. MI was used to address missing data and was further bolstered by the addition of what is considered complete secondary care data (using HES), which allows us to estimate broader costs and HRQoL on fully observed patterns of secondary care use. Multilevel linear regression models were used to accommodate the clustering of results of HRQoL, broader resource use and secondary care costs within participants within GPs and provided regression-corrected expectations for use in the economic modelling. Although the modelling shows substantial differences between individuals, in terms of expected costs and QALYs over time, the well-balanced nature of the cohorts and large sample size mean that simply comparing raw sample averages across interventions gives a generally valid impression of relative effectiveness. Because missing data are more likely when there are large secondary care costs and when the participant has an A&E episode, and, as observed, HRQoL is generally lower and broader resource use costs are generally higher when there is an A&E episode, imputation has the effect of lowering QALYs and increasing costs. However, as the pattern of missingness is very similar across treatment arms, the impact of imputation on the incremental differences between interventions is minimal.
We found increasing costs over time in all interventions for both broader resource use and secondary care costs. The increase is greatest in secondary care costs, which dominate the overall cost generation, with approximately 75% of costs occurring here. Examination of the HRGs associated with the secondary care costs shows that the majority of these costs are unrelated to falls, being mainly due to chemotherapy (see Appendix 1). Overall, there is an expectation that exercise generates lower costs than advice, which generates lower costs than MFFP. Although the differences are small (approximately £1 per month for exercise vs. advice, rising to £12 per month for exercise vs. MFFP) and not statistically significantly different, the pattern is consistent, with MFFP being the most costly intervention at all time points. There was virtually no difference in expected costs between advice and exercise.
There was a more observable difference in incremental QALYs between interventions, although the order is the same and as consistent, with exercise delivering the highest HRQoL over time, followed by advice and then MFFP. The incremental differences between interventions appear to be increasing over time and the regression model finds small but statistically significant differences between exercise and MFFP at months 4, 12 and 18. In all cases, HRQoL is falling over time. The incremental difference between exercise and MFFP is 0.013 QALYs (approximately an extra 5 days in perfect health spread over 18 months). For exercise compared with advice, this is 0.006 QALYs.
Applying these expectations in the NICE reference case framework finds that, as exercise produces the highest expected QALYs and the lowest expected costs, it dominates both advice and MFFP. Similarly, advice dominates MFFP. However, although the ordering and conclusions are clear, the magnitudes of the INHB and INMB are small, indicating marginal practical differences in cost and QALY outcomes between interventions. The INMB between exercise and MFFP valued at £30,000 per QALY is £613 and between exercise and advice the equivalent figure is £191. These results are robust to using complete-case analysis and the means from the PSA.
The PSA is driven by uncertainty in the regression results, as captured by the variance–covariance matrix. The impact on the imputed results of PSA is to suggest that exercise is the most cost-effective treatment approximately 67–75% of the time as the willingness to pay for a QALY rises from £20,000 to £30,000. At the extremes, when either costs (willingness to pay = £0) or QALYs (maximum willingness to pay is infinite) dominate the evaluation, the figure is 49–81%. MFFP is almost never simulated to be cost-effective as the willingness to pay increases. The probability that MFFP is cost-effective is approximately 8% when QALYs are not valued and falls to 1% when the threshold value is £20,000 per QALY. Therefore, any uncertainty about the most cost-effective treatment is between exercise and advice.
Nevertheless, because of the large size of the trial and the balance across cohorts, the economic conclusion is largely robust to PSA, despite the heterogeneity and the impact of exogenous factors. Any uncertainty about the most cost-effective treatment is between advice and exercise, because MFFP is only very possibly cost-effective when only costs are considered (and even then, it is unlikely to be cost-effective). As the value of QALYs grows, it becomes more likely that exercise is the most cost-effective treatment. It is possible that the impact of exercise on HRQoL is not just related to the likelihood of falls.
Chapter 6 Discussion
Study findings and key messages
This trial is the first large-scale, pragmatic, definitive trial to investigate the clinical effectiveness and cost-effectiveness of different falls prevention interventions embedded within UK primary care services. 131 The trial was designed to reflect a significant and contemporary dilemma in UK health policy, which is whether or not to introduce systematic screening and linked interventions from primary care for falls prevention. We aimed to provide evidence to inform UK health-care practitioners about the options for preventing falls and fractures in older people. Using this population screen-and-treat model in a sample of older people, we found no statistically significant difference in fractures between treatment arms.
We found evidence of interim benefits in rate of falls and QoL outcomes in those undertaking exercise, and these findings, along with differences in secondary care usage, led to marginal cost-effectiveness benefits in the exercise group relative to MFFP. Overall, exercise dominated advice and MFFP, suggesting that exercise might be the most cost-effective intervention for future investment.
Key findings from the PreFIT contrast with recent systematic reviews reporting that these interventions lead to a reduction in falls and fractures, and we therefore consider possible reasons for these differences. Issues relating to internal and external validity of the trial are discussed, with consideration given to the characteristics of our participant cohort. We then consider the wider clinical and public health policy implications of our findings on falls prevention services in the NHS.
Internal and external validity
We approached > 29,000 older people and recruited almost 10,000 participants, with an age range spanning 30 years. GP deprivation was representative of practices across England, with a good sociodemographic spread of GPs recruited from more deprived inner cities to affluent urban, rural and semi rural localities. GP recruitment was staggered to avoid overburdening local services and spanned several years, allowing for seasonal variation in falls and hospital admissions. We used several strategies to maximise the efficiency of the study design, including controlling cluster size through random sampling in primary care and the use of random subsampling for falls data collection. The very large sample size and robust approaches to capture fractures and falls allowed for comprehensive analyses of outcomes and secondary analyses of treatment effects by important clinical covariates.
Data collection
We triangulated multiple sources of evidence for incident fracture events over time by purchasing multiple waves of national statistics of hospital attendances and admissions and by completing comprehensive searches of primary care records (62 out of 63 GPs). Hospital discharge letters and radiological reports were obtained for fractures reported in GP records, whenever possible. Self-reported fracture events were neither necessary nor sufficient in themselves for fracture confirmation. NHS HES data are widely used for epidemiological and health services research. Data quality and accuracy is reportedly higher in the more recent data sets (from 2011 onwards), and in the more established data sets (such as the acute patient care and A&E data sets that we used in our analysis). We used ICD-10 three- and four-digit diagnostic coding and carefully screened all relevant injury and falls codes as per the prespecified analysis plan. Accuracy of ICD-10 coding for primary diagnoses in HES has been reported at 96% (IQR 89–96%). The adjudication panel were blind to treatment allocation and thus we are very confident of low risk of ascertainment or detection bias for our primary outcome.
For secondary outcomes, two methods, retrospective (with a short time frame) and prospective, were used to capture participant self-reported falls outcomes. Using a within-trial, random-sampling strategy, we restricted prospective diaries to a 4-month period over the first year of follow-up, rather than over 12 months. This revealed a higher mean rate of falling per month reported on prospective diary cards and also a small, but important, impact of diary card allocation on withdrawals from the trial over time. Extending prospective falls data collection to 12 months, as recommended by international falls prevention groups, would have had a significant impact on study attrition and would have required postal administration of 117,648 diaries. Our innovative approach reduced burden on participants and administrative burden through unnecessary follow-up of falls outcomes that did not require as much statistical power for definitive analysis. Latest research testing wearable technologies for monitoring balance and falls is being undertaken in hospitalised patients; however, these technologies are in the early stages of development and their use in large population studies is not yet feasible. 132
Uptake to trial
We found that uptake to the PreFIT (33%) was reasonable compared with other trials recruiting older adults via primary care. Recent preventative lifestyle trials using similar methods to identify and approach older people via primary care yielded lower uptakes (e.g. the UK Lifestyle Matters RCT mailed 18,331 people aged ≥ 65 years and achieved 2% uptake). 133,134 Using a similar design, the high-quality UK ProAct65+ cluster trial,74 comparing OEP and FaME with advice, recruited 6% of 20,500 people aged ≥ 65 years invited from primary care. Our invitation letters referred to a study investigating how to remain fit and active, and how to prevent falls and fractures, rather than specifically mentioning exercise per se. People aged ≥ 80 years, sometimes termed the oldest old, are also under-represented in clinical trials and are considered a hard-to-reach population, despite being the fastest-growing age group in the UK. 135 One-third of our sample were aged ≥ 80 years (n = 3247), of whom 300 were aged ≥ 90 years on recruitment. We piloted materials with older people, using larger font size and clear instructions along with provision of a freephone telephone number to encourage participants to ring for help with completion of questionnaires and falls diaries. We believe that these strategies contributed to high uptake and retention over time.
Participant characteristics
Perhaps unsurprisingly, people who did agree to participate in the trial were fairly active, with approximately 90% being cognitively intact and self-reporting high levels of activity at baseline. Very few participants had substantial problems with ADL or severe mobility restrictions and we acknowledge that there may be a risk of healthy respondent bias. Nevertheless, despite being predominantly active and mobile, one-third of participants had fallen in the year prior to recruitment, suggesting that our sample were representative of community-dwelling older people. These data have changed little over the last 30 years: early epidemiological studies9,10 reported that up to one-third of older people fall once or more per year. The risk algorithm, using responses in the baseline questionnaire for the whole cohort, found that 44% of participants were considered at risk of falling. One-fifth of participants had symptoms of frailty (20%), predominantly sensory deficit problems, although the Strawbridge questionnaire is weighted towards measurement of sensory deficits. Frailty was correlated with older age, falls history and other characteristics. As with other falls prevention trials,74 those who were older and frail were more likely to withdraw from the trial, although we found no differences in either rate of mortality or withdrawals by treatment arm. Our treatment groups were well balanced across arms, although a non-statistically significant higher falls rate in those randomised to the multifactorial intervention was observed; this was due to five extreme fallers. When extreme observations were removed, fall rates in the three treatment arms were very similar.
Our population was predominantly white (98%) and, thus, under-representative of the overall black and ethnic minority population in England (UK 2011 Census:136 86% white). However, when age is taken into account, only 4.6% of the English population aged ≥ 70 years is non-white. The mean age at leaving school was 16.8 years. We did not record highest qualification after leaving school, although the UK Office for National Statistics data5 show that over half of adults aged ≥ 65 years left school without any formal qualification.
Quality of life
We followed validated scoring guidelines for all measures. QoL in the PreFIT sample was high compared with both US and UK population norms. Population normative scores on the SF-12 scale for adults aged ≥ 75 years are lower for physical health, but mental health scores are comparable to those in our trial cohort [population mean SF-12 PCS, 38.7 (SD 11.0); population mean MCS, 50.1 (SD 10.9)]. 32,137 In the ProACT65+ trial, mean SF-12 PCS and MCS scores were 36.9 (SD 6.6) and 48.8 (SD 6.3), respectively. 74 The PreFIT trial participants, therefore, had better physical health than and comparable mental health to other research populations of similar age, despite being an older cohort than recruited to ProACT65+. 74 Missingness was low at baseline for QoL scales but increased over time. Comparison of complete-case and imputed data (n = 460) for EQ-5D-3L scores used in cost-effectiveness analysis did not change estimates.
Screening in primary care
We found that risk screening in primary care was feasible and cheap to undertake; almost 90% of people approached by their GP responded to the fall risk screener. Utility of the screener was good and prediction was comparable to accuracy values reported in studies using longer, more complex, falls risk screening tools. A short, annual screening questionnaire administered to older people in primary care will yield good-quality information about falls and balance problems. The more challenging and controversial issue is how best to intervene once those at risk have been identified.
Referrals and uptake to intervention
We extended participant follow-up to 18 months to allow time for postal screening by primary care teams and risk stratification before arranging referrals to active treatment. Mean time to first treatment was approximately 8 weeks, although some localities took longer. However, overall, this was acceptable and likely to be reflective of current NHS services. The longer follow-up period of 18 months allowed for the capture of treatment effects on fracture and falls outcomes. Uptake of and adherence to trial interventions was very good and comparable with that seen in other falls prevention clinical trials. 138
Comparison with other studies
Multifactorial falls prevention
The early trials investigating MFFP interventions were very promising,9,10 but these have proven difficult to replicate in large, multicentre, pragmatic trials. Recent Cochrane reviews have separately investigated trials testing multifactorial and exercise interventions. 16,139 The latest review16 of multifactorial interventions based on risk factor assessment and recommended treatment, most commonly to exercise, environment or assistive technologies, medication review and psychological interventions, included 44 RCTs (15,733 participants). Median trial size was 303 participants. In marked contrast to our findings, multifactorial interventions were found to reduce the rate of falls compared with control (RaR 0.77, 95% CI 0.67 to 0.87; n = 19 RCTs; n = 5853 participants; I2 = 88%), but there was considerable statistical and methodological heterogeneity, weakening confidence in the treatment effect. Most of these trials attempted to select participants at higher risk of falling. There was very little evidence for the effect of multifactorial interventions on other fall-related outcomes. Only 11 out of 44 (25%) trials testing multifactorial interventions reported fracture outcomes and data were extracted for meta-analysis from nine trials [totalling 147 people sustaining one or more fractures out of 2850 participants (5.2%)]. Subgroup analyses of trials at low risk of selection bias found no difference between treatment groups (RaR 0.78, 95% CI 0.49 to 1.23; four trials; n = 1521 participants; I2 = 0%). However, when analyses were restricted to three trials at low risk of detection bias for fractures, the results were strongly in favour of a multifactorial intervention reducing risk of fractures (RaR 0.47, 95% CI to 0.24 to 0.93; three trials; n = 1055 participants; I2 = 0%), but this finding is based on only 39 people with a fracture (3.7%).
Our primary ITT analysis found an increased rate of fractures in participants randomised to MFFP compared with participants randomised to advice, which was of borderline statistical significance (RaR 1.30, 95% CI 0.99 to 1.71; p = 0.06). These findings are based on a much larger event rate and population, with 253 people sustaining a fracture over 18 months [out of 6524 participants randomised to advice and MFFP (3.9%)]. Importantly, we have tested a screen-and-treat approach in an unselected population. This means that our findings are directly applicable to a community strategy for reducing falls injuries. 17,82 We have not, in contrast to other studies,16,138 tested our approach on a highly selected population. Our findings indicate, beyond any reasonable doubt, that our approach to screening for falls risk and offering our MFFP intervention will not reduce fractures and is not cost-effective.
Exercise
Our exercise intervention did not reduce fractures (RaR 1.20, 95% CI 0.91 to 1.59). The 2019 Cochrane review139 included 10 trials reporting fall-related fracture outcomes: smaller trials (< 100 participants) suggest a trend towards fracture reduction, whereas larger, high-quality trials find no evidence of effect. 140 Although the 2019 Cochrane review139 concluded that exercise programmes may reduce fall-related fractures, this was considered low-certainty evidence.
Exercise has been shown in many other studies to reduce both rate of falls and number of fallers. The 2019 Cochrane review included 108 trials (23,047 participants) and reported a 23% reduction in falls (RaR 0.77, 95% CI 0.71 to 0.83; n = 59 studies) based on high-certainty evidence. 139 Exercise also reduced the number of people experiencing a fall by 15% (equivalent to 72 fewer fallers over 1 year in the exercise group than in the control group; n = 63 studies). 139 Overall, we found no difference in falls rate (RaR 0.99, 95% CI 0.86 to 1.14). Nevertheless, we did find that exercise reduced the falls rate between the 4-month and 8-month follow-ups (RaR 0.78, 95% CI 0.64 to 0.96). To put this into context, the crude analysis of number of fallers (falls risk) in the PreFIT revealed a difference of 81 people between 4 and 8 months (539 fallers in the control vs. 458 fallers in exercise group). Although we cannot exclude the possibility that this is a chance finding because of multiple comparisons, it is potentially an important observation. However, this effect was transitory; very few behavioural, physical or, indeed, pharmacological interventions have sustained treatment benefits for months or years beyond end of active therapy.
Exercise to prevent falls is premised on modifying gait and balance problems. The timing of the transient reduction that we observed in the rate of falls at 8 months in the exercise arm corresponded with the completion of the 6-month exercise programme. We also found improvements in muscle strength and balance in those who adhered to the full exercise intervention. Incremental improvements in lower leg strength were largely attributed to improved ability to undertake more repetitions while wearing ankle weights rather than to an increase in weight lifted. Owing to the model of the exercise programme, physiotherapists recommended increases in repetitions much more often than increases in weight, although almost half of participants who complied with the programme transitioned to heavier weights. The ProAct65+ trial73,74 is, to the best of our knowledge, the only other UK-based study to deliver a 6-month OEP, although contacts were with peer mentors rather than therapists; a reduction in falls was observed in those who achieved 75% of the exercise intervention. 73,74 In the PreFIT, among those who complied with the exercise intervention, work done during a session increased by 60% over time. We found marked improvements in balance: half of our participants were in the poorest balance categories on starting the exercise programme, reducing to 20% of participants on completion. This substantial improvement in balance transition exceeded that observed in other community-based trials74 despite the fact that our participants were, on average, slightly older. Those who dropped out of the programme had weaker leg strength initially and over time, although attrition limited our ability to compare leg strength in non-adherers in the longer term. Overall, the reduction in rate and number of fallers would be substantial if replicated at a population level, and if efforts at exercise could be continued over time with sustained progression and the reduction in falls maintained. However, there is no indication of an effect on fracture, our primary outcome of interest. Nevertheless, our intervention is likely to be cost-effective and was designed to be implementable within the NHS.
Interpretation of study findings
There are important issues to consider when interpreting our findings. Possible issues include failure of our screening process to identify those most at risk, targeting the wrong risk factors in the multifactorial intervention, delivering diluted interventions and concerns over intervention fidelity. We failed to detect a meaningful treatment effect, yet we may have identified the true treatment effect of these interventions on fracture outcomes. It is plausible that falls prevention services do not prevent fracture outcomes. Falls prevention interventions may reasonably prevent falls, but the causal pathway from falls prevention to prevention of fractures is perhaps weak. A range of contributory factors were identified during fracture adjudication, including comorbidity and alcohol, which were not targeted by our interventions.
Our multifactorial intervention assessed for the main risk factors tested in many other trials and for which there was some suggestion of an association with increased risk of falling. Over 400 risk factors have been identified for falling. Multifactorial assessments are complex interventions and involve many dimensions of complexity, as recognised by the Medical Research Council. These include multiple interacting components, multiple behaviours required by those delivering and receiving the intervention, more than one organisational group targeted by the intervention and extent or degree of flexibility permitted (e.g. potential variability when interviewing older people, staff skill mix in different NHS settings). Our underlying assumption was that assessors, general practitioners and older participants would adhere to all prescribed behaviours and act on recommendations for onward referral and treatment. We closely monitored all referrals to exercise and to other health-care professionals and general practitioner-led medication reviews, but it was not possible to track whether or not participants acted on all other advice and recommendations for behaviour change. Despite attempts to control and standardise interventions, we recognise the influence of underlying contextual factors on the delivery of a complex intervention, such as multifactorial falls assessment. Another notable finding was that less than half of participants (42%) attending MFFP were referred for exercise therapy, despite these participants being considered at risk of falling based on their self-completed risk screener. Clinical teams were asked to assess gait balance problems using two methods: (1) the TUG and/or (2) visual assessment for any balance and gait problems or fear of falling. This is entirely consistent with the original Tinetti et al. 9 model of MFFP (observe gait and balance during transitioning). 141
Importantly, we found that the point estimate of the effect was for an increased fracture risk. For the MFFP comparison, this approached statistical significance. Therefore, a radically different approach is needed to effect a meaningful change in fracture rates. Simply using ‘stronger’ interventions or improving adherence to the interventions is likely to increase the existing trend to increased fractures in the intervention groups.
Intervention fidelity
Intervention fidelity is an important consideration in multicentre intervention trials. Complex interventions have more scope for variation in delivery and are more vulnerable than simple interventions to failure to implement components as they should be implemented. 142 However, we developed clear protocols and treatment pathways for each risk factor and had substantial expert input into developing interventions. Several elements of the falls assessments were novel for primary care staff (e.g. falls interview and the Snellen eye chart test). Every staff member received training from a geriatrician, and assessors were required to successfully undertake assessments of every risk factor before training certificates were signed. We provided clear supportive materials (e.g. laminated prompt sheets of interview questions, listings of culprit drugs to screen for during medication reviews). Quality of training delivered was assessed during the pilot phase and adaptations were made to include simple and complex case study examples. We did not undertake video-recording of face-to-face participant consultations, nor did we tape or video-record general practitioner discussions with trial participants about medication changes, which may have provided more insight into quality of delivery. Intervention staff members were observed by a trained assessor, as per usual recommended evaluations checks of fidelity for complex intervention trials.
We are confident that the MFFP intervention was delivered as recommended, albeit within the limitations of existing NHS services. Access to NHS podiatry was very limited and so participants were advised to book appointments with a private chiropodist or podiatrist in regions with excessively long waiting lists; the exception was for people with diabetes who could be directly referred to NHS diabetic podiatry services. It was not possible to trace non-health-care referrals or attendance at private podiatry or opticians for eye checks (free to those aged ≥ 70 years). One region delivered consultant-led geriatrician services and in other regions assessments were undertaken in primary care by nursing staff or by non-consultant falls teams. This non-geriatrician model is currently recommended by the British Geriatrics Society, which suggests that any member of the primary health-care team should be able to co-ordinate a comprehensive geriatric assessment; thus, nurses, general practitioners and pharmacy staff members can all undertake medication reviews. 143,144 Only complex cases should be referred to a geriatrician. 144 We undertook post hoc exploratory analysis on skill mix for multifactorial delivery, finding no effect. Therefore, the PreFIT MFFP intervention followed a service model not only recommended in 2011 at trial launch but also recommended and used in many clinical services today.
Medication reviews
Medication reviews are complex interventions in themselves and can be challenging and time-consuming to carry out. The 2017 cluster Opti-Med RCT,145 testing clinical medication reviews in older people in primary care with ‘geriatric problems’ (including mobility problems, falls and fear of falling), aimed to reduce inappropriate drug use and found no difference in QoL, geriatric problems, satisfaction with medication or self-reported medication adherence. These intensive, detailed reviews were undertaken by expert teams, and specific recommendations were made to general practitioners. However, the authors found that only 41% of medication changes (442/1084) recommended by the expert team were implemented or partially implemented by general practitioners, who were significantly more likely to implement the addition of a drug than the cessation of a drug (47% vs. 35%, respectively; p = 0.002). 146 Intervention studies aiming to reduce inappropriate prescribing have not resulted in measurable changes in patient outcomes and it has been argued that efforts should focus on high-risk patients. Willeboordse et al. 146 report a low uptake of recommended medication changes comparable to rates found in other studies (less than half of recommendations are actioned), although this can be higher if the patients’ own general practitioner is involved in the screening process. 146 Other research on polypharmacy and multimorbidity has found that general practitioners’ medication management strategies vary, leading to differences in proposed medication changes. 147
Fracture prevention
Although we have evidence of interim benefits from secondary outcomes and process evaluation to suggest that HRQoL and leg strength improved, this did not translate into a reduction in number of fractures. It is plausible that risk of harm is higher from exercise than multifactorial interventions owing to increased mobility and encouragement of activity. The ProAct65+ trial148 examined bone density changes in those who undertook two falls prevention exercise programmes (OEP and FaME) but found no effect on bone mineral density or bone structural parameters. The ProAct65+ trial148 suggested that 6 months of exercise intervention was insufficient to lead to bone mineralisation changes and that a greater magnitude of progressive loading and/or longer duration of exercise was required to achieve changes in bone density. 148 Recent expert statements for patients with osteoporosis recommend impact exercise, in addition to strength exercise, to promote bone health. 149
Strengths of the study
Undoubtedly, the major strength of PreFIT is the size and rigorous quality of the trial: it is the largest population-based fracture and falls prevention study carried out in the UK setting. The cluster trial design, in which clusters are assembled and participants randomly sampled and enrolled prior to randomisation, provides methodological rigour and avoids contamination bias. We obtained individual signed informed consent rather than gatekeeper consent, as per good practice guidelines for cluster trials. 19 We carefully tracked uptake, screening and referral to treatments at different stages. Treatment protocols were developed with experienced geriatricians and carefully piloted and tested before roll-out. We triangulated multiple data sources to ascertain the primary outcome. We achieved high follow-up rates over 12 and 18 months for all secondary outcomes and used strategies to capture falls outcomes prospectively and retrospectively. Importantly, imputation for missingness on QoL outcomes used for cost-effectiveness analyses did not change utility estimates.
Limitations of the study
We observed a lower than anticipated fracture event rate for the population, but this was offset by exceeding our planned sample size of 9000 by 9%, increasing follow-up time and achieving 99.9% data collection for the primary outcome. This allowed all prespecified statistical analyses to be completed with certainty. Our total fracture event rate, even within individual intervention arms, exceeds aggregated values reported in recent systematic reviews. The rate of observed fractures was lower than in the original sample size estimate. However, we used more efficient statistical techniques than originally planned to account for the lower fracture rate. We used linear mixed models, rather than generalised estimating equations, to account for data format and overdispersion. As with cluster randomised designs,150 there is a small risk of baseline imbalance in GP characteristics, because we did not incorporate stratification. This would have been challenging to achieve, given the requirements for inclusion (i.e. ability to provide intervention or access to services in the locality that could provide interventions). We adjusted for GP deprivation and examined GP characteristics by treatment arm.
Patient and public involvement
We included a lay member on one external committee, who provided input at all stages. Trial materials were developed and piloted in the early stages, with older volunteers attending a lunch social club in Coventry. In November 2018, we hosted a patient dissemination event at the University of Warwick to feed back results to a sample of participants. Owing to trial size, it was not possible to invite all participants and therefore invitations were sent to those resident in Warwickshire, with confirmation given to the earliest respondents. A total of 48 older people and their partners or carers attended the event. This event proved very successful and participants were very keen to learn of study findings.
Cost-effectiveness findings
We found small but relatively systematic and consistent differences between the expectations in QALYs and costs for the three interventions. Evidence across all time points suggests that exercise produces higher expected QoL and lower costs than either advice or MFFP. We noted that the population of participants was highly heterogeneous, with marked differences in expected costs and QALYs over time between individuals. Inspection of the secondary care data indicated that the majority of the secondary care costs were unrelated to falls and attributable instead to cancer treatment and cataract surgery procedures. Although it is not possible to demonstrate, one can surmise that QoL is equally highly influenced by the presence and/or treatment of cancer.
This heterogeneity was mitigated by the trial being appropriately powered and extremely well balanced across arms, such that the impact of heterogeneity is minimised. In addition, random-effects models were used to inform the economic models and accommodate patient-level heterogeneity in the health economic analysis.
The results from the cost-effectiveness analysis are consistent with a recent literature review conducted to identify cost-effective interventions to prevent falls in older people living in the community and published by Public Health England. 82 The review found that the majority of studies assessing the cost-effectiveness of multifactorial assessments reported negative results. Evidence on exercise was, however, mixed and related mainly to group-based interventions studies.
The main strength of the health economic analysis was that the trial was prospectively designed for a cost-effectiveness analysis using individual-level data on a very large number of older individuals well balanced across intervention arms. Costs and outcomes were carefully considered in the trial design, with the purpose of reaching a robust conclusion with respect to cost-effectiveness. The main limitation was that the analysis was limited to the trial horizon and the potentially large impact of exogenous factors on costs and QoL, which means that, even with a sample size this large, there is some non-marginal uncertainty left. Several factors were fundamental to our decision not to construct a decision-analytic model. The observed time period was sufficient to draw inference on the relative cost-effectiveness of each treatment and there was, therefore, no underlying need to extrapolate the results over time (i.e. the conclusions would not be changed by taking a longer-term perspective).
The underlying structure of costs and QALY generation, which we initially assumed to be mainly driven by falls and fractures, was less well established than expected and became less certain over the duration of the project. We do not believe that the differences in QALYs we observed were solely generated by an impact on falls and fractures and therefore do not believe that a model structured around falls and fractures captures the impact. As the relative pattern of QoL over time is consistent and increasing in incremental differences, we do not believe that extending the duration of observation over a longer time frame would change the results.
Future recommendations
Falls and fracture prevention remains an important target of preventative health care. Exercise remains the most promising intervention for primary care. However, future work should focus on improving uptake and adherence to strength and balance programmes within a broader framework or focus on a family of interventions to target geriatric syndromes.
Conclusions
In conclusion, the PreFIT tested the delivery of alternative fall prevention strategies embedded within the UK primary care setting and found that neither a multifactorial falls assessment intervention nor exercise reduced fractures in older people living in the community. We found no differential differences in fracture rates by sex, age or falls history in our subgroup analyses. We found an interim reduction in falls and small improvements in HRQoL in those randomised to exercise, compared with advice, but this was not sustained in the longer term. Nevertheless, the QoL benefits observed in the exercise arm dominated in the health economic analyses.
The PreFIT exercise intervention may reduce falls in the short term, but there is no evidence to support a reduction in falls over the longer term and no evidence for any reduction in fractures outcomes. The PreFIT MFFP intervention does not reduce rates of fractures or falls. The health economic results suggest that exercise therapy, when compared with advice and MFFP, is cost saving and has a small effect on overall QoL.
Acknowledgements
Trial Management Group
-
Professor Chris Bojke (trial health economist).
-
Professor Julie Bruce (research lead).
-
Dr Susanne Finnegan (research physiotherapist).
-
Mrs Susie Hennings (senior project manager).
-
Dr Anower Hossain (trial statistician).
-
Professor Claire Hulme (trial health economist).
-
Dr Chen Ji (trial statistician).
-
Professor Ranjit Lall (trial statistician).
-
Professor Sarah E Lamb (chairperson).
-
Dr Roberta Longo (trial health economist).
-
Dr Laetitia Schmitt (trial health economist).
-
Mr Craig Turner (trial administrator).
-
Professor Martin Underwood (Professor of Primary Care Research).
-
Mrs Emma Withers (trial manager/senior project manager).
Trial Steering Committee
-
Professor Ann Ashburn (independent).
-
Professor Julie Bruce (research lead).
-
Mr Alex Bush (independent).
-
Professor Ranjit Lall (trial statistician).
-
Professor Sarah E Lamb (chief investigator).
-
Professor Chris Salisbury (independent chairperson).
-
Emma Withers (trial manager).
Data Monitoring and Ethics Committee
-
Professor Opinder Sahota (independent).
-
Professor Maria Stokes (independent).
-
Professor Stephen Walter (independent chairperson).
Statisticians
-
Dr Anower Hossain (trial statistician).
-
Dr Chen Ji (trial statistician).
-
Professor Ranjit Lall (lead trial statistician).
The trial team
University of Warwick
A Andrews, D Baird, K Booth, C Bridle, J Brodie, J Bruce, S Careless, J Dodd, S Duggan, R Fearn, S Finnegan, L Hall, S Hennings, A Hossain, C Ji, R Lall, R Mant, R Potter, R Rai, G Scott, N Strickland, M Underwood, I Wall, E Withers and P Zeh.
University of Leeds
C Bojke, C Hulme, R Longo and L Schmitt.
General practices
Devon
Barnfield Hill Surgery, Barton Surgery, Bovey Tracey & Chudleigh Practice, Brunel Medical Practice, Budleigh Salterton Medical Practice, Chapel Platt Surgery, Chiddenbrook Surgery, Claremont Medical Practice, College Surgery Partnership, Honiton Practice, Leatside Surgery, Litchdon Medical Centre, Raleigh Surgery, Redfern Health Centre, Richmond Medical Centre, Rolle Medical Partnership, St Leonard’s Research Practice and Wyndham House.
Warwickshire/Herefordshire
Castle Medical Centre, Clarendon Lodge, Croft Medical Centre, Hastings House, King Street Surgery, Bobblestock Surgery, Meon Medical Centre, Mortimer Medical Practice, Priory Medical Centre, The Marches Surgery, The New Dispensary, Warwick Gates Family Health Centre and Westside Medical Centre.
Worcestershire
Elbury Moor Medical Centre, Hillview Medical Centre, Link End Surgery, New Court Surgery, Pershore Medical Practice, Severn Valley Medical Practice, Spring Gardens Group Medical Practice, St Martin’s Gate Surgery, St Stephens Surgery, Corbett Medical Practice, The Ridgeway Surgery, Thorneloe Lodge Surgery, Upton Surgery and Winyates Health Centre.
Cambridgeshire
Cherry Hinton & Brookfields Medical Practice, Doddington Medical Centre, Firs House Surgery, Lensfield Medical Practice, Staploe Medical Centre and Cornerstone Practice.
Newcastle upon Tyne
Benfield Park Healthcare and Diagnostic Centre Betts Avenue Medical Centre, Denton Turret Medical Centre, Falcon Medical Group, Gosforth Memorial Medical Centre, Holly Medical Group, Newburn Surgery, Regent Medical Centre, Roseworth Surgery, The Grove Medical Group and The Park Medical Group.
Birmingham and the Black Country
Hobs Moat Medical Centre and the Three Villages Medical Practice.
Exercise intervention therapists
Barnstaple Clinical Commissioning Trust, North Devon
B Hill.
Brookfields Hospital, Cambridge
M Darlow, M Mackay, T Nicholas and I Tripp.
Cambridgeshire Community Services NHS Trust, Cambridge
A Bewers, J Campbell, B Cirillo, A Crossman, K Everitt, B Fraser, K Golding, S Hanna, H Koch, H Mason, J Reid, J Riglin and S Spence.
Dudley Group NHS Foundation Trust, West Midlands
S Ward.
Healthworks, Newcastle upon Tyne
L Charlton, T Collingbourne, J Ducker, H English, J Firth, L Flanagan, A Grenfall, B Johnson, S Murray and D Robson.
Heart of England NHS Foundation Trust
T McLoughlin.
Lemington Centre, Newcastle upon Tyne
V Thompson.
Moor Street Clinic, Worcester
L Ambler and T Lambert.
Northern Devon Healthcare NHS Trust
L Butler, J Cutforth, K Edwards, R Edwards, S Markevics, N Molly, S Pearson, H Richardson, H Showell, C Thomas, S Thomas, H Willmott and J Yabsley.
Prospect View, Malvern Health Centre, Malvern
D Clarke.
South Hams Hospital, Kingsbridge
P Adams, S Cable, V Hallett, S Jarvis, H Pickard, T Rogers and S Stephens.
South Warwickshire NHS Foundation Trust
G Allibone, A Bruce, J Hack, J Harding, P Kirk, A McGregor, O Owoso, C Ritchie, P Saunders, D Taylor, H Walding, A Wale and C Williams.
Torbay and Southern Devon Health and Social Care NHS Trust
C Bailey, C Gordon, F Hussey, P Llewellyn, L Love and L Parker.
Warwick Clinical Trials Unit, Coventry
J Nussbaum.
Worcestershire Acute Hospitals NHS Trust
E Campbell, L Emett, S Oldham and V Monk.
Wye Valley NHS Trust, Hereford
C Bryan, J Burns, K Davies, K Duguid, J Hardman and E Quinn.
Other
M Dobson, H Edwards, J Lawson, A Palmer, E Parker and H Roberts.
Multifactorial falls prevention intervention personnel
Barnfield Surgery, Exeter
J Doherty, S Sparks.
Barton Surgery, Bedford
B Sheppard, P Shute and S Woodward.
Cambridgeshire Community Services NHS Trust, Cambridge
K Fell, H Jung and E Li.
Chiddenbrook Surgery, Devon
C Comont.
Corbett Medical Practice, Droitwich
A Dougherty.
Doddington Medical Centre, Cambridgeshire
B Deboys, R Friend and S Waldron.
Hillview Medical Centre, Redditch
A Cimarosit and G Stanton.
Hobs Moat Medical Centre, Solihull
L Steyn.
Link End Surgery, Malvern
J Hollman.
Litchdon Medical Centre, Barnstaple
S McDonald.
Mortimer Medical Practice, Barnstaple
G Busby, S Wallis and C Williams.
Northern Devon Healthcare NHS Trust
A Mason.
Raleigh Surgery, Exmouth
M Cornish.
Royal Devon and Exeter NHS Foundation Trust
L Rohan.
South Warwickshire NHS Foundation Trust
R Clifford, S Ducker and L Moakes.
Warwick Medical School, Coventry
P Darbyshire and S Joshi.
Provision of Age UK Leaflets
Age UK.
PreFIT Study Group
Chief investigator
Professor Sarah E Lamb.
Co-investigators (grant holders)
Professor Martin Underwood, Professor Finbarr Martin, Professor Lucy Yardley, Professor Dawn Skelton, Professor Keith Willett, Professor Sandra Eldridge, Dr Anne-Marie Slowther and Dr Sarah Duggan.
Trial research lead
Professor Julie Bruce.
Senior project managers
Susie Hennings and Emma Withers.
Trial management/administration
Agata Andrews, Duncan Baird, Katie Booth, Jonathan Brodie, Samuel Careless, Jenny Dodd, Rachael Fearn, Laura Hall, Rhys Mant, Rishpal Rai, Greg Scott, Craig Turner, Isobel Wall and Emma Withers.
Research fellows/nurses
Susanne Finnegan, Rachel Potter, Nicola Walker and Peter Zeh.
Trial statisticians
Dr Anower Hossain, Dr Chen Ji and Professor Ranjit Lall.
Health economists
Chris Bojke, Claire Hulme, Roberta Longo and Laetitia Schmitt.
Clinical intervention trainers
Susanne Finnegan, Vivien Nichols, Dr Shvaita Ralhan, Dr Ray Sheridan, Dr Jonathan Treml, Dr Katharine Westacott.
Regional principal investigators
Jo Burns (Hereford region), Dr John Davison (Newcastle upon Tyne), Dr Ruma Dutta (Worcestershire region), Harm Gordjin (Warwickshire region), Jackie Riglin (Cambridge region), Dr Fiona Shaw (Newcastle upon Tyne), Dr Ray Sheridan (Devon region) and Dr Jonathan Treml (Birmingham and the Black Country Region).
Data programming team
Henry Adjei, Chocks Muthiah and Ade Willis.
Contributions of authors
Julie Bruce (https://orcid.org/0000-0002-8462-7999) (Research Lead) was responsible for the study design, developed the protocol, developed interventions, was a member of the Trial Management Group and the TSC and was responsible for the writing and reviewing of the report.
Anower Hossain (https://orcid.org/0000-0002-1708-9940) (Research Fellow and Trial Statistician) was a member of the Trial Management Group, was responsible for the statistical analysis of the trial and was responsible for the writing and reviewing of the report.
Ranjit Lall (https://orcid.org/0000-0003-1737-2866) (Professor of Clinical Trials and Lead Statistician) was a member of the Trial Management Group, was responsible for the study design, was responsible for the statistical analysis of the trial and was responsible for the writing and reviewing of the report.
Emma J Withers (https://orcid.org/0000-0003-4832-9609) (Trial Manager and Senior Project Manager) was a member of the Trial Management Group, was responsible for the study design, was responsible for the running of the trial and was responsible for the writing and reviewing of the report.
Susanne Finnegan (https://orcid.org/0000-0001-8152-7610) (Research Physiotherapist) was a member of the Trial Management Group, was responsible for training of exercise intervention and was responsible for the writing and reviewing of the report.
Martin Underwood (https://orcid.org/0000-0002-0309-1708) (Professor of Primary Care) was a member of the Trial Management Group, was responsible for the study design and was responsible for the writing and reviewing of the report.
Chen Ji (https://orcid.org/0000-0003-4919-3299) (Research Fellow) was a member of the Trial Management Group, was responsible for the statistical analysis of the HES data and was responsible for the writing and reviewing of the report.
Chris Bojke (https://orcid.org/0000-0003-2601-0314) (Professor of Health Economics) was a member of the Trial Management Group, was responsible for the health economics analysis and was responsible for the writing and reviewing of the report.
Roberta Longo (https://orcid.org/0000-0002-9379-1627) (Research Fellow) was a member of the Trial Management Group, was responsible for the health economics analysis and was responsible for the writing and reviewing of the report.
Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Professor of Health Economics), a former member of the Trial Management Group, was responsible for the study design and for the writing and reviewing of the report.
Susie Hennings (https://orcid.org/0000-0002-8160-6658) (Senior Project Manager), a former member of the Trial Management Group, was responsible for the writing and reviewing of the report.
Ray Sheridan (https://orcid.org/0000-0001-9169-6549) (Consultant Geriatrician) was responsible for the study design and for the writing and reviewing of the report.
Katharine Westacott (https://orcid.org/0000-0002-4039-9373) (Geriatrician) was responsible for MFFP training and for the writing and reviewing of the report.
Shvaita Ralhan (https://orcid.org/0000-0003-4699-228X) (Consultant Geriatrician) was responsible for MFFP training and for the writing and reviewing of the report.
Finbarr Martin (https://orcid.org/0000-0002-6911-3498) (Consultant Physician and Geriatrician) was responsible for study design and for the writing and reviewing of the report.
John Davison (https://orcid.org/0000-0002-0697-0820) (Consultant Physician) was responsible for the writing and reviewing of the report.
Fiona Shaw (https://orcid.org/0000-0002-9744-2977) (Consultant Geriatrician) was responsible for the writing and reviewing of the report.
Dawn A Skelton (https://orcid.org/0000-0001-6223-9840) (Professor in Ageing and Health) was responsible for the writing and reviewing of the report.
Jonathan Treml (https://orcid.org/0000-0002-6024-3544) (Consultant Geriatrician) was responsible for MFFP training and for the writing and reviewing of the report.
Keith Willett (https://orcid.org/0000-0001-8615-725X) (Professor of Orthopaedic Trauma Surgery and Director for Acute Care to NHS England) was responsible for the writing and reviewing of the report.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor of Trauma Rehabilitation and chief investigator) was responsible for study conception and design and the writing and reviewing of the report and was guarantor.
Publications
Bruce J, Lall R, Withers EJ, Finnegan S, Underwood M, Hulme C, et al. A cluster randomised controlled trial of advice, exercise or multifactorial assessment to prevent falls and fractures in community-dwelling older adults: protocol for the Prevention of Falls Injury Trial (PreFIT). BMJ Open 2016;6:e009362.
Bruce J, Ralhan S, Sheridan R, Westacott K, Withers E, Finnegan S, et al. The design and development of a complex multifactorial falls assessment intervention for falls prevention: the Prevention of Falls Injury Trial (PreFIT). BMC Geriatrics 2017;17:116.
Finnegan S, Bruce J, Skelton DA, Withers EJ, Lamb SE on behalf of the PreFIT Study Group. Development and delivery of an exercise programme for falls prevention: the Prevention of Fall Injury Trial (PreFIT). Physiotherapy 2018;104:72–9.
Griffin J, Lall R, Bruce J, Withers E, Finnegan S, Lamb SE; PreFIT Study Group. Comparison of alternative falls data collection methods in the Prevention of Falls Injury Trial (PreFIT). J Clin Epidemiol 2019;106:32–40.
Lamb SE, Bruce J, Hossain A, Ji C, Longo R, Lall R, et al. Screening and intervention to prevent falls and fractures in older people. N Engl J Med 2020;383:1848–59.
Data-sharing statement
All requests for data should be sent to the corresponding author. Access to the available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Bischoff-Ferrari HA. The role of falls in fracture prediction. Curr Osteoporos Rep 2011;9:116-21. https://doi.org/10.1007/s11914-011-0059-y.
- Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society . Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2010;59:148-57. https://doi.org/10.1111/j.1532-5415.2010.03234.x.
- National Institute for Health and Care Excellence (NICE) . Falls: The Assessment and Prevention of Falls in Older People 2013.
- Rubenstein LZ, Powers CM, MacLean CH. Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann Intern Med 2001;135:686-93. https://doi.org/10.7326/0003-4819-135-8_Part_2-200110161-00007.
- Office for National Statistics (ONS) . National Population Projections: 2016-Based Statistical Bulletin 2017.
- Royal College of Physicians (RCP) . Falling Standards, Broken Promises: Report of the National Audit of Falls and Bone Health in Older People 2010 2011.
- British Orthopaedic Association (BOA) . The Care of Patients With Fragility Fracture 2007.
- Ftouh S, Morga A, Swift C. Guideline Development Group . Management of hip fracture in adults: summary of NICE guidance. BMJ 2011;342. https://doi.org/10.1136/bmj.d3304.
- Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994;331:821-7. https://doi.org/10.1056/NEJM199409293311301.
- Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet 1999;353:93-7. https://doi.org/10.1016/S0140-6736(98)06119-4.
- National Institute for Health and Care Excellence (NICE) . Clinical Practice Guideline for the Assessment and Prevention of Falls in Older People 2004.
- Lamb SE, Fisher JD, Gates S, Potter R, Cooke MW, Carter YH. A national survey of services for the prevention and management of falls in the UK. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-233.
- Royal College of Physicians (RCP) . Older People’s Experiences of Therapeutic Exercise As Part of a Falls Prevention Service – Patient and Public Involvement 2012.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. BMJ 2008;336:130-3. https://doi.org/10.1136/bmj.39412.525243.BE.
- Hopewell S, Adedire O, Copsey BJ, Boniface GJ, Sherrington C, Clemson L, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2018;7. https://doi.org/10.1002/14651858.CD012221.pub2.
- Public Health England (PHE) . Falls and Fracture Consensus Statement: Supporting Commissioning for Prevention 2017.
- Bruce J, Lall R, Withers EJ, Finnegan S, Underwood M, Hulme C, et al. A cluster randomised controlled trial of advice, exercise or multifactorial assessment to prevent falls and fractures in community-dwelling older adults: protocol for the prevention of falls injury trial (PreFIT). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009362.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . CONSORT 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Bruce J, Ralhan S, Sheridan R, Westacott K, Withers E, Finnegan S, et al. The design and development of a complex multifactorial falls assessment intervention for falls prevention: The Prevention of Falls Injury Trial (PreFIT). BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0492-6.
- Finnegan S, Bruce J, Skelton DA, Withers EJ, Lamb SE. PreFIT Study Group . Development and delivery of an exercise programme for falls prevention: the Prevention of Falls Injury Trial (PreFIT). Physiotherapy 2018;104:72-9. https://doi.org/10.1016/j.physio.2017.06.004.
- Age UK . Staying Steady 2009.
- Campbell AJ, Robertson MC. Otago Exercise Programme to Prevent Falls in Older Adults. Dunedin: University of Otago, Otago Medical School; 2003.
- Lamb SE, McCabe C, Becker C, Fried LP, Guralnik JM. The optimal sequence and selection of screening test items to predict fall risk in older disabled women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci 2008;63:1082-8. https://doi.org/10.1093/gerona/63.10.1082.
- GOV.UK . The English Indices of Deprivation 2015 n.d. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 24 May 2019).
- Activity and Health Research Limited . Allied Dunbar National Fitness Survey 1991.
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. https://doi.org/10.1016/0895-4356(93)90053-4.
- Shua-Haim J, Koppuzha G, Gross J. A simple scoring system for clock drawing in patients with Alzheimer’s disease. J Am Geriatr Soc 1996;44. https://doi.org/10.1111/j.1532-5415.1996.tb00931.x.
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Shepstone L, Lenaghan E, Cooper C, Clarke S, Fong-Soe-Khioe R, Fordham R, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet 2018;391:741-7. https://doi.org/10.1016/S0140-6736(17)32640-5.
- World Health Organization . ICD-10 Online Versions 2016. www.who.int/classifications/icd/icdonlineversions/en/ (accessed 24 May 2019).
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 HEALTH Survey. Lincoln, RI: Quality Metric Incorporated; 2002.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998;53:S9-16. https://doi.org/10.1093/geronb/53b.1.s9.
- Matthews M, Lucas A, Boland R, Hirth V, Odenheimer G, Wieland D, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004;16:34-40. https://doi.org/10.1007/bf03324529.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). Ann Intern Med 2015;162:735-6. https://doi.org/10.7326/L15-5093-2.
- Lamb SE, Mistry D, Alleyne S, Atherton N, Brown D, Copsey B, et al. Aerobic and strength training exercise programme for cognitive impairment in people with mild to moderate dementia: the DAPA RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22280.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 22 April 2020).
- Donaldson LJ, Reckless IP, Scholes S, Mindell JS, Shelton NJ. The epidemiology of fractures in England. J Epidemiol Community Health 2008;62:174-80. https://doi.org/10.1136/jech.2006.056622.
- Smeeth L, Ng ES. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC trial of the assessment and management of older people in the community. Control Clin Trials 2002;23:409-21. https://doi.org/10.1016/S0197-2456(02)00208-8.
- Cook RJ, Major P. Methodology for treatment evaluation in patients with cancer metastatic to bone. J Natl Cancer Inst 2001;93:534-8. https://doi.org/10.1093/jnci/93.7.534.
- Khan A, Ullah S, Nitz J. Statistical modelling of falls count data with excess zeros. Inj Prev 2011;17:266-70. https://doi.org/10.1136/ip.2011.031740.
- Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. J Gerontol A Biol Sci Med Sci 2005;60:530-4. https://doi.org/10.1093/gerona/60.4.530.
- Ullah S, Finch CF, Day L. Statistical modelling for falls count data. Accid Anal Prev 2010;42:384-92. https://doi.org/10.1016/j.aap.2009.08.018.
- Zheng H, Kimber A, Goodwin VA, Pickering RM. A comparison of different ways of including baseline counts in negative binomial models for data from falls prevention trials. Biom J 2018;60:66-78. https://doi.org/10.1002/bimj.201700103.
- Eldridge S, Spencer A, Cryer C, Parsons S, Underwood M, Feder G. Why modelling a complex intervention is an important precursor to trial design: lessons from studying an intervention to reduce falls-related injuries in older people. J Health Serv Res Policy 2005;10:133-42. https://doi.org/10.1258/1355819054338942.
- Pincus T, Miles C, Froud R, Underwood M, Carnes D, Taylor SJ. Methodological criteria for the assessment of moderators in systematic reviews of randomised controlled trials: a consensus study. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-14.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5330.
- Copsey B, Hopewell S, Becker C, Cameron ID, Lamb SE. Appraising the uptake and use of recommendations for a common outcome data set for clinical trials: a case study in fall injury prevention. Trials 2016;17. https://doi.org/10.1186/s13063-016-1259-7.
- Kannus P, Palvanen M, Niemi S, Parkkari J. Alarming rise in the number and incidence of fall-induced cervical spine injuries among older adults. J Gerontol A Biol Sci Med Sci 2007;62:180-3. https://doi.org/10.1093/gerona/62.2.180.
- Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ. Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res 2014;29:581-9. https://doi.org/10.1002/jbmr.2072.
- Korhonen N, Kannus P, Niemi S, Parkkari J, Sievänen H. Rapid increase in fall-induced cervical spine injuries among older Finnish adults between 1970 and 2011. Age Ageing 2014;43:567-71. https://doi.org/10.1093/ageing/afu060.
- Palvanen M, Kannus P, Niemi S, Parkkari J. Hospital-treated minimal-trauma rib fractures in elderly Finns: long-term trends and projections for the future. Osteoporos Int 2004;15:649-53. https://doi.org/10.1007/s00198-003-1585-y.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14410.
- Williams MA, Williamson EM, Heine PJ, Nichols V, Glover MJ, Dritsaki M, et al. Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19190.
- Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD007146.pub2.
- Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. N S W Public Health Bull 2011;22:78-83. https://doi.org/10.1071/NB10056.
- World Health Organization (WHO) . Global Recommendations on Physical Activity for Health 65 Years and Above 2011.
- American College of Sports Medicine . ACSM Position Stands 2010. www.acsm.org/acsm-positions-policy/official-positions/ACSM-position-stands (accessed 24 May 2019).
- Winter DA. ABC Anatomy, Biomechanics and Control of Balance During Standing and Walking. Waterloo, ON: Waterloo Biomechanics; 1995.
- Jones K, Barker K. Human Movement Explained (Physiotherapy Practice Explained). London: Butterworth-Heinemann; 1995.
- Massion J. Postural control system. Curr Opin Neurobiol 1994;4:877-87. https://doi.org/10.1016/0959-4388(94)90137-6.
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther 1990;70:340-7. https://doi.org/10.1093/ptj/70.6.340.
- Farlie MK, Robins L, Keating JL, Molloy E, Haines TP. Intensity of challenge to the balance system is not reported in the prescription of balance exercises in randomised trials: a systematic review. J Physiother 2013;59:227-35. https://doi.org/10.1016/S1836-9553(13)70199-1.
- Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001;30:77-83. https://doi.org/10.1093/ageing/30.1.77.
- Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD004963.pub3.
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine . American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510-30. https://doi.org/10.1249/MSS.0b013e3181a0c95c.
- Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226-37. https://doi.org/10.1016/j.arr.2010.03.004.
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Taxonomy Investigators. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-125.
- Skelton DA, Dinan S, Campbell M, Rutherford O. Tailored group exercise (Falls Management Exercise – FaME) reduces falls in community dwelling adults (an RCT). Age Ageing 2005;34:636-9. https://doi.org/10.1093/ageing/afi174.
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315:1065-9. https://doi.org/10.1136/bmj.315.7115.1065.
- Gawler S, Skelton DA, Dinan-Young S, Masud T, Morris RW, Griffin M, et al. Reducing falls among older people in general practice: the ProAct65+ exercise intervention trial. Arch Gerontol Geriatr 2016;67:46-54. https://doi.org/10.1016/j.archger.2016.06.019.
- Iliffe S, Kendrick D, Morris R, Griffin M, Haworth D, Carpenter H, et al. Promoting physical activity in older people in general practice: ProAct65+ cluster randomised controlled trial. Br J Gen Pract 2015;65:e731-8. https://doi.org/10.3399/bjgp15X687361.
- Campbell J. Implementation of Multifactorial Interventions for Fall and Fracture Prevention. Oxford: Oxford University Press; 2006.
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing 1999;28:513-18. https://doi.org/10.1093/ageing/28.6.513.
- Gardner MM, Phty M, Robertson MC, McGee R, Campbell AJ. Application of a falls prevention program for older people to primary health care practice. Prev Med 2002;34:546-53. https://doi.org/10.1006/pmed.2002.1017.
- Robertson MC, Gardner MM, Devlin N, McGee R, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ 2001;322:701-4. https://doi.org/10.1136/bmj.322.7288.701.
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 2002;50:905-11. https://doi.org/10.1046/j.1532-5415.2002.50218.x.
- Thomas S, Mackintosh S, Halbert J. Does the ‘Otago exercise programme’ reduce mortality and falls in older adults?: a systematic review and meta-analysis. Age Ageing 2010;39:681-7. https://doi.org/10.1093/ageing/afq102.
- Lamb S, Gates S, Fisher J, Cooke M, Carter Y, McCabe C. Scoping Exercise on Fallers’ Clinics. London: National Co-ordinating Centre for NHS Service Delivery and Organisation R&D; 2007.
- Public Health England (PHE) . A Structured Literature Review to Identify Cost-Effective Interventions to Prevent Falls in Older People Living in the Community 2018.
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2002;34:364-80. https://doi.org/10.1097/00005768-200202000-00027.
- Ebrahim S, Thompson PW, Baskaran V, Evans K. Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Ageing 1997;26:253-60. https://doi.org/10.1093/ageing/26.4.253.
- Rossiter-Fornoff JE, Wolf SL, Wolfson LI, Buchner DM. A cross-sectional validation study of the FICSIT common data base static balance measures. Frailty and injuries: cooperative studies of intervention techniques. J Gerontol A Biol Sci Med Sci 1995;50:M291-7. https://doi.org/10.1093/gerona/50a.6.m291.
- Yardley L, Beyer N, Hauer K, McKee K, Ballinger C, Todd C. Recommendations for promoting the engagement of older people in activities to prevent falls. Qual Saf Health Care 2007;16:230-4. https://doi.org/10.1136/qshc.2006.019802.
- Moseley GL. Do training diaries affect and reflect adherence to home programs?. Arthritis Rheum 2006;55:662-4. https://doi.org/10.1002/art.22086.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34:119-26. https://doi.org/10.1111/j.1532-5415.1986.tb05480.x.
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701-7. https://doi.org/10.1056/NEJM198812293192604.
- Podsiadlo D, Richardson S. The timed ‘up & go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x.
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896-903. https://doi.org/10.1093/ptj/80.9.896.
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 2011;161:46-8. https://doi.org/10.1016/j.autneu.2011.02.004.
- Task Force on Medicines Partnership and The National Collaborative Medicines Management Services Programme . Room for Review: A Guide to Medication Review – The Agenda, for Patients, Practitioners and Managers 2002.
- Royal Pharmaceutical Society of Great Britain (RPSGB) . From Compliance to Concordance: Achieving Partnership in Medicine-Taking 1997.
- Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci 2007;62:1172-81. https://doi.org/10.1093/gerona/62.10.1172.
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009;169:1952-60. https://doi.org/10.1001/archinternmed.2009.357.
- Lord SR, Dayhew J, Howland A. Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc 2002;50:1760-6. https://doi.org/10.1046/j.1532-5415.2002.50502.x.
- Lord SR, Smith ST, Menant JC. Vision and falls in older people: risk factors and intervention strategies. Clin Geriatr Med 2010;26:569-81. https://doi.org/10.1016/j.cger.2010.06.002.
- Harwood RH, Foss AJ, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol 2005;89:53-9. https://doi.org/10.1136/bjo.2004.049478.
- Brown GC, Brown MM, Stein JD, Smiddy WE. Ophthalmic Utility Research Study Group . Vision-related quality of life associated with unilateral and bilateral ocular conditions. Ophthalmology 2018;125:965-71. https://doi.org/10.1016/j.ophtha.2017.12.033.
- National Institute for Health and Care Excellence (NICE) . Implantation of Accommodating Intraocular Lens for Cataract. Interventional Procedures Programme 2007.
- Harwood RH, Conroy SP. Slow walking speed in elderly people. BMJ 2009;339. https://doi.org/10.1136/bmj.b4236.
- Spink MJ, Menz HB, Fotoohabadi MR, Wee E, Landorf KB, Hill KD, et al. Effectiveness of a multifaceted podiatry intervention to prevent falls in community dwelling older people with disabling foot pain: randomised controlled trial. BMJ 2011;342. https://doi.org/10.1136/bmj.d3411.
- Menz HB, Jordan KP, Roddy E, Croft PR. Characteristics of primary care consultations for musculoskeletal foot and ankle problems in the UK. Rheumatology 2010;49:1391-8. https://doi.org/10.1093/rheumatology/keq092.
- Farndon L, Barnes A, Littlewood K, Harle J, Beecroft C, Burnside J, et al. Clinical audit of core podiatry treatment in the NHS. J Foot Ankle Res 2009;2. https://doi.org/10.1186/1757-1146-2-7.
- Dolinis J, Harrison JE, Andrews GR. Factors associated with falling in older Adelaide residents. Aust N Z J Public Health 1997;21:462-8. https://doi.org/10.1111/j.1467-842x.1997.tb01736.x.
- Menant JC, Steele JR, Menz HB, Munro BJ, Lord SR. Effects of footwear features on balance and stepping in older people. Gerontology 2008;54:18-23. https://doi.org/10.1159/000115850.
- Menant JC, Steele JR, Menz HB, Munro BJ, Lord SR. Optimizing footwear for older people at risk of falls. J Rehabil Res Dev 2008;45:1167-81. https://doi.org/10.1682/JRRD.2007.10.0168.
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev 2003;4. https://doi.org/10.1002/14651858.CD000340.
- Cumming RG, Thomas M, Szonyi G, Salkeld G, O’Neill E, Westbury C, et al. Home visits by an occupational therapist for assessment and modification of environmental hazards: a randomized trial of falls prevention. J Am Geriatr Soc 1999;47:1397-402. https://doi.org/10.1111/j.1532-5415.1999.tb01556.x.
- Clemson L, Mackenzie L, Ballinger C, Close JC, Cumming RG. Environmental interventions to prevent falls in community-dwelling older people: a meta-analysis of randomized trials. J Aging Health 2008;20:954-71. https://doi.org/10.1177/0898264308324672.
- Pighills AC, Torgerson DJ, Sheldon TA, Drummond AE, Bland JM. Environmental assessment and modification to prevent falls in older people. J Am Geriatr Soc 2011;59:26-33. https://doi.org/10.1111/j.1532-5415.2010.03221.x.
- Clemson L, Cusick A, Fozzard C. Managing risk and exerting control: determining follow through with falls prevention. Disabil Rehabil 1999;21:531-41. https://doi.org/10.1080/096382899297189.
- Griffin J, Lall R, Bruce J, Withers E, Finnegan S, Lamb SE. PreFIT Study Group . Comparison of alternative falls data collection methods in the Prevention of Falls Injury Trial (PreFIT). J Clin Epidemiol 2019;106:32-40. https://doi.org/10.1016/j.jclinepi.2018.09.006.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- University of Warwick . Salary Costs August 2010 n.d. https://warwick.ac.uk/services/humanresources/internal/payroll/oldsalcosts/salcostsaug11penoct11 (accessed 8 October 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Royal Mail n.d. www.royalmail.com (accessed 20 March 2020).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- NHS Employers . Pay Circular (M&Amp;D) 1 2011 n.d. www.nhsemployers.org/-/media/Employers/Publications/Pay-circulars/Pay-Circular-_MD_-1-2011.pdf (accessed 20 March 2020).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015–2016 2016.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Health & Social Care Information Centre . PSS EX1 2010/11 2012.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Lamb SE, Bruce J, Hossain A, Ji C, Longo R, Lall R, et al. Screening and intervention to prevent falls and fractures in older people. N Engl J Med 2020;383:1848-59.
- Rucco R, Sorriso A, Liparoti M, Ferraioli G, Sorrentino P, Ambrosanio M, et al. Type and location of wearable sensors for monitoring falls during static and dynamic tasks in healthy elderly: a review. Sensors 2018;18. https://doi.org/10.3390/s18051613.
- Chatters R, Newbould L, Sprange K, Hind D, Mountain G, Shortland K, et al. Recruitment of older adults to three preventative lifestyle improvement studies. Trials 2018;19. https://doi.org/10.1186/s13063-018-2482-1.
- Forster SE, Jones L, Saxton JM, Flower DJ, Foulds G, Powers HJ, et al. Recruiting older people to a randomised controlled dietary intervention trial – how hard can it be?. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-17.
- Iliffe S, Walters K, Manthorpe J, Goodman C, Kharicha K. Health and well-being promotion strategies for ‘hard to reach’ older people in England: a mapping exercise. Prim Health Care Res Dev 2017;18:563-73. https://doi.org/10.1017/S1463423617000378.
- Office of National Statistics . Census Analysis: Ethnicity and Religion of the Non-UK Born Population in England and Wales 2011. www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/2011censusanalysisethnicityandreligionofthenonukbornpopulationinenglandandwales/2015-06-18 (accessed 30 April 2020).
- Pettit T, Livingston G, Manela M, Kitchen G, Katona C, Bowling A. Validation and normative data of health status measures in older people: the Islington study. Int J Geriatr Psychiatry 2001;16:1061-70. https://doi.org/10.1002/gps.479.
- Simek EM, McPhate L, Haines TP. Adherence to and efficacy of home exercise programs to prevent falls: a systematic review and meta-analysis of the impact of exercise program characteristics. Prev Med 2012;55:262-75. https://doi.org/10.1016/j.ypmed.2012.07.007.
- Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD012424.pub2.
- Gill TM, Guralnik JM, Pahor M, Church T, Fielding RA, King AC, et al. Effect of structured physical activity on overall burden and transitions between states of major mobility disability in older persons: secondary analysis of a randomized trial. Ann Intern Med 2016;165:833-40. https://doi.org/10.7326/M16-0529.
- Koch M, Gottschalk M, Baker DI, Palumbo S, Tinetti ME. An impairment and disability assessment and treatment protocol for community-living elderly persons. Phys Ther 1994;74:286-94. https://doi.org/10.1093/ptj/74.4.286.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-40.
- Turner G, Clegg A. British Geriatrics Society. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014;43:744-7. https://doi.org/10.1093/ageing/afu138.
- British Geriatrics Society . Comprehensive Geriatric Assessment Toolkit for Primary Care Practitioners 2019.
- Willeboordse F, Schellevis FG, Chau SH, Hugtenburg JG, Elders PJM. The effectiveness of optimised clinical medication reviews for geriatric patients: Opti-Med a cluster randomised controlled trial. Fam Pract 2017;34:437-45. https://doi.org/10.1093/fampra/cmx007.
- Willeboordse F, Schellevis FG, Meulendijk MC, Hugtenburg JG, Elders PJM. Implementation fidelity of a clinical medication review intervention: process evaluation. Int J Clin Pharm 2018;40:550-65. https://doi.org/10.1007/s11096-018-0615-y.
- Kwint HF, Bermingham L, Faber A, Gussekloo J, Bouvy ML. The relationship between the extent of collaboration of general practitioners and pharmacists and the implementation of recommendations arising from medication review: a systematic review. Drugs Aging 2013;30:91-102. https://doi.org/10.1007/s40266-012-0048-6.
- Duckham RL, Masud T, Taylor R, Kendrick D, Carpenter H, Iliffe S, et al. Randomised controlled trial of the effectiveness of community group and home-based falls prevention exercise programmes on bone health in older people: the ProAct65+ bone study. Age Ageing 2015;44:573-9. https://doi.org/10.1093/ageing/afv055.
- Giangregorio LM, McGill S, Wark JD, Laprade J, Heinonen A, Ashe MC, et al. Too Fit To Fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int 2015;26:891-910. https://doi.org/10.1007/s00198-014-2881-4.
- Ivers NM, Halperin IJ, Barnsley J, Grimshaw JM, Shah BR, Tu K, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-120.
Appendix 1 Supplementary tables
| Participant flow | Denominator for percentages | Advice, n (%) | Exercise, n (%) | MFFP, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Total GP list size | a | 222,051 (36.9) | 182,942 (30.4) | 197,070 (32.7) | 602,063 |
| Aged ≥ 70 years | b | 28,576 (12.9) | 28,772 (15.7) | 23,727 (12.0) | 81,075 (13.5) |
| Total excluded (nursing home/residential care) | c | 1145 (4.0) | 954 (3.3) | 736 (3.1) | 2835 (3.5) |
| Total patients eligible to approach | c | 27,431 (96.0) | 27,818 (96.7) | 22,991 (96.9) | 78,240 (96.5) |
| Total randomly selected for invitation | d | 9603 (35.0) | 9548 (34.3) | 9859 (42.9) | 29,010 (37.1) |
| Total exclusions by general practitioner | e | 1003 (10.4) | 794 (8.3) | 762 (7.7) | 2559 (8.8) |
| Total invited | e | 7782 (81.0) | 8720 (91.3) | 7886 (80.0) | 24,388 (84.1) |
| Total consented | f | 3232 (41.5) | 3284 (37.7) | 3305 (41.9) | 9821 (40.3) |
| Total consented, returned baseline | f | 3230 (41.5) | 3284 (37.7) | 3305 (41.9) | 9819 (40.3) |
| Withdrawals prior to GP randomisation | g | 4 (0.12) | 4 (0.12) | 1 (0.03) | 9 (0.09) |
| Deaths prior to GP randomisation | g | 3 (0.09) | 1 (0.03) | 3 (0.09) | 7 (0.07) |
| Total participants randomised | g | 3223 (99.8) | 3279 (99.8) | 3301 (99.9) | 9803 (99.8) |
| Participant flow | Advice, n (%) | Exercise, n (%) | MFFP, n (%) | Total, n (%) |
|---|---|---|---|---|
| Participants consented/returned baseline data | 3230 | 3284 | 3305 | 9819 |
| Withdrawals prior to randomisation of GP | 4 (0.12) | 4 (0.12) | 1 (0.03) | 9 (0.09) |
| Deaths prior to randomisation of GP | 3 (0.09) | 1 (0.03) | 3 (0.09) | 7 (0.07) |
| Total participants: GP randomised | 3223 (99.8) | 3279 (99.8) | 3301 (99.9) | 9803 (99.8) |
| Completion between GP randomisation and 4-month follow-up | ||||
| Participant contacted for follow-up | 3223 | 3279 | 3301 | 9803 |
| Withdrawals during the follow-up period | 102 (3.2) | 119 (3.6) | 98 (3.0) | 319 (3.3) |
| Died during the follow-up period | 21 (0.7) | 16 (0.5) | 27 (0.8) | 64 (0.7) |
| Non-respondents | 114 (3.5) | 101 (3.1) | 141 (4.3) | 356 (3.6) |
| Reached follow-up and responded to the 4-month CRF | 2957 (91.8) | 3006 (91.7) | 3008 (91.1) | 8971 (91.5) |
| Reached follow-up and responded to the 4-month core outcomes | 29 (0.9) | 37 (1.1) | 27 (0.8) | 93 (1.0) |
| Total (CRF + core outcomes) | 2986 (92.7) | 3043 (92.8) | 3035 (91.9) | 9064 (92.5) |
| Completion between 4- and 8-month follow-up | ||||
| Participant contacted for follow-up | 3100 | 3144 | 3176 | 9420 |
| Withdrawals during the follow-up period | 98 (3.2) | 129 (4.1) | 133 (4.2) | 360 (3.8) |
| Died during the follow-up period | 18 (0.6) | 20 (0.6) | 18 (0.6) | 56 (0.6) |
| Non-respondents | 145 (4.7) | 137 (4.4) | 144 (4.5) | 426 (4.5) |
| Reached follow-up and responded to the 8-month CRF | 2813 (90.7) | 2826 (89.9) | 2842 (89.5) | 8481 (90.0) |
| Reached follow-up and responded to the 8-month core outcomes | 26 (0.8) | 32 (1.0) | 39 (1.2) | 97 (1.0) |
| Total (CRF + core outcomes) | 2839 (91.5) | 2858 (90.9) | 2881 (90.7) | 8578 (91.0) |
| Completion between 8- and 12-month follow-up | ||||
| Participant contacted for follow-up | 2984 | 2995 | 3025 | 9004 |
| Withdrawals during the follow-up period | 96 (3.2) | 119 (4.0) | 121 (4.0) | 336 (3.7) |
| Died during the follow-up period | 21 (0.7) | 14 (0.5) | 29 (1.0) | 64 (0.7) |
| Non-respondents | 169 (5.7) | 131 (4.4) | 168 (5.5) | 468 (5.2) |
| Reached follow-up and responded to the 12-month CRF | 2674 (89.6) | 2711 (90.5) | 2678 (88.5) | 8063 (89.5) |
| Reached follow-up and responded to the 12-month core outcomes | 24 (0.8) | 20 (0.7) | 29 (1.0) | 73 (0.8) |
| Total (CRF + core outcomes) | 2698 (90.4) | 2731 (91.2) | 2707 (89.5) | 8136 (90.3) |
| Completion between 12- and 18-month follow-up | ||||
| Participant contacted for follow-up | 2867 | 2862 | 2875 | 8604 |
| Withdrawals during the follow-up period | 57 (2.0) | 57 (2.0) | 84 (2.9) | 198 (2.3) |
| Died during the follow-up period | 33 (1.2) | 39 (1.4) | 33 (1.1) | 105 (1.2) |
| Non-respondents | 284 (9.9) | 266 (9.3) | 261 (9.1) | 811 (9.4) |
| Reached follow-up and responded to the 18-month CRF | 2459 (85.8) | 2458 (85.9) | 2455 (85.4) | 7372 (85.7) |
| Reached follow-up and responded to the 18-month core outcomes | 34 (1.2) | 42 (1.5) | 42 (1.5) | 118 (1.4) |
| Total (CRF + core outcomes) | 2493 (87.0) | 2500 (87.4) | 2497 (86.9) | 7490 (87.1) |
| HRG | Number of episodes | Cost (£) |
|---|---|---|
| Advice arm | ||
| SB97Z: same day chemotherapy admission or attendance | 285 | 282,968 |
| BZ34C: phacoemulsification cataract extraction and lens implant with CC score 0–1 | 270 | 238,664 |
| HN12F: very major hip procedures for non-trauma with CC score 0–1 | 37 | 230,339 |
| HN22E: very major knee procedures for non-trauma with CC score 0–1 | 24 | 141,536 |
| HN22D: very major knee procedures for non-trauma with CC score 2–3 | 21 | 135,807 |
| AA35D: stroke with CC score 7–9 | 15 | 127,345 |
| WH09G: tendency to fall senility or other conditions affecting cognitive functions without interventions with CC score 0–1 | 31 | 114,702 |
| HN12E: very major hip procedures for non-trauma with CC score 2–3 | 17 | 112,159 |
| WD11Z: all patients ≥ 70 years with a mental health primary diagnosis treated by a non-specialist mental health service provider | 18 | 93,384 |
| LA04Q: kidney or urinary tract infections without interventions with CC score 4–7 | 14 | 86,209 |
| Exercise arm | ||
| SB97Z: same day chemotherapy admission or attendance | 331 | 349,392 |
| BZ34C: phacoemulsification cataract extraction and lens implant with CC score 0–1 | 215 | 190,047 |
| HN22E: very major knee procedures for non-trauma with CC score 0–1 | 23 | 134,700 |
| HN12E: very major hip procedures for non-trauma with CC score 2–3 | 20 | 132,834 |
| HN12F: very major hip procedures for non-trauma with CC score 0–1 | 18 | 113,178 |
| HN22D: very major knee procedures for non-trauma with CC score 2–3 | 17 | 110,751 |
| SA12K: thrombocytopenia with CC score 0–1 | 72 | 106,593 |
| BZ86B: intermediate vitreous retinal procedures 19 years and over with CC score 0–1 | 96 | 99,779 |
| LA04Q: kidney or urinary tract infections without interventions with CC score 4–7 | 21 | 96,848 |
| WJ11Z: other disorders of immunity | 56 | 86,361 |
| MFFP arm | ||
| SB97Z: same day chemotherapy admission or attendance | 231 | 296,339 |
| HN22E: very major knee procedures for non-trauma with CC score 0–1 | 32 | 194,348 |
| BZ34C: phacoemulsification cataract extraction and lens implant with CC score 0–1 | 198 | 175,020 |
| HN12F: very major hip procedures for non-trauma with CC score 0–1 | 25 | 166,471 |
| HN22D: very major knee procedures for non-trauma with CC score 2–3 | 21 | 148,434 |
| WD11Z: all patients ≥ 70 years with a mental health primary diagnosis treated by a non-specialist mental health service provider | 22 | 99,239 |
| BZ86B: intermediate vitreous retinal procedures 19 years and over with CC score 0–1 | 90 | 93,029 |
| AA35A: stroke with CC score 16+ | 5 | 80,188 |
| HN12E: very major hip procedures for non-trauma with CC score 2–3 | 12 | 78,700 |
| DZ11U: lobar atypical or viral pneumonia without interventions with CC sore 4–6 | 22 | 71,994 |
List of abbreviations
- 4TBS
- 4-test balance scale
- A&E
- accident and emergency
- ADL
- activities of daily living
- AE
- adverse event
- AUC
- area under the curve
- CACE
- complier-average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CST
- chair stand test
- DHSC
- Department of Health and Social Care
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FaME
- Falls Management Exercise Programme
- GP
- general practice
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSCIC
- Health and Social Care Information Centre
- ICC
- intracluster correlation
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- INHB
- incremental net health benefit
- INMB
- incremental net monetary benefit
- IQR
- interquartile range
- ITT
- intention to treat
- LR
- likelihood ratio
- MCS
- mental health composite scale
- MD
- mean difference
- MFFP
- multifactorial falls prevention
- MI
- multiple imputation
- MLM
- multilevel linear model
- NICE
- National Institute for Health and Care Excellence
- NPV
- net present value
- OEP
- Otago exercise programme
- OR
- odds ratio
- PCS
- physical health composite scale
- PreFIT
- Prevention of Falls Injury Trial
- ProFaNE
- Prevention of Falls Network Europe
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RaR
- rate ratio
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short-Form questionnaire-12 items
- TSC
- Trial Steering Committee
- TUG
- timed up and go test
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25340).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
