Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/23/09. The contractual start date was in April 2014. The draft report began editorial review in June 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Greenwood et al. This work was produced by Greenwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Greenwood et al.
Chapter 1 Introduction
Parts of this report are reproduced or adapted with permission from Greenwood et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Scientific background
Haemodialysis (HD) is a major treatment option for patients with end-stage kidney failure. Over 27,000 patients receive dialysis for chronic kidney disease (CKD) in the UK and 80% of these are treated with HD. 2 Improved dialysis techniques and management of co-existing disease have made HD more tolerable, and many new patients can anticipate a longer life expectancy,3 although not always with a good quality of life (QoL).
Both physical inactivity and impaired physical function are strongly associated with increased morbidity, mortality and reduced QoL in patients on HD. 3,4 Reduced QoL is also independently associated with mortality in patients on HD. Reports indicate that Short Form questionnaire-36 items (SF-36) physical component summary (PCS) scores of < 25 arbitrary units (AU) were associated with a 93% increased risk of death and a 56% increased risk of hospitalisation in HD patients, and a 10-point decrease in PCS score translated into a 25% increased risk of death within 2 years. 5 Conversely, a 1-point increase in PCS score was associated with a 3.5% improvement in the odds of death. 6 Interventions designed to increase physical function and reduce sedentary behaviour in patients on HD may mitigate cardiovascular disease risk, improve physical functioning, improve fitness for potential future kidney transplantation, lower levels of fatigue and, in turn, improve QoL. A non-randomised controlled trial (RCT) by Painter et al. 7 reported an average 4-point increase in PCS score in the intervention group (in-centre intradialytic cycling or individualised home exercise programme) and an average 6-point decrease in PCS score in the non-intervention group. A study by DeOreo8 reported that for every increase in PCS score of 5 AU there is an ≈ 10% increase in the probability of survival.
Evidence from several systematic reviews9–22 indicates that a range of exercise training interventions show potential to improve exercise capacity and physical function in patients with dialysis-dependent CKD. The greatest effects were reported after 6 months of exercise and were associated with both supervised exercise and higher intensities of exercise. However, most of the studies reviewed were small trials, many of which were not methodologically robust, and non-intradialytic interventions were occasionally included in the evidence synthesis. 16 Relatively few of the reviewed studies on intradialytic exercise training were appropriately powered to detect QoL outcomes, and none included a cost-effectiveness analysis. Moreover, adverse events (AEs) in the published literature were recorded in only a minority of studies17 or were poorly reported. 16 A recent systematic review22 on intradialytic exercise training suggests that aerobic and resistance exercise programmes, delivered alone, can improve aerobic capacity, but that a combination of both can improve a greater range of outcomes, including exercise capacity, depression and some elements of QoL. By contrast, a recent systematic review by Young et al. 18 indicated that there was insufficient evidence demonstrating whether or not cycling exercise during HD improves patient outcomes. The recommendations emerging from these systematic reviews indicated the need for (1) high-quality, adequately powered RCTs of intradialytic exercise and (2) routine collection and reporting of AE data associated with participation in these trials. The generation of this information may help to more fully identify recommendations for an exercise delivery pathway for HD patients and, ultimately, its clinical implementation. 21
The PEDAL (PrEscription of intraDialytic exercise to improve quAlity of Life in patients with chronic kidney disease) trial was designed in response to a National Institute for Health Research (NIHR)-commissioned call to evaluate the clinical benefit and cost-effectiveness of exercise during HD (intradialytic exercise) in patients with end-stage kidney disease. At the time of planning and developing the PEDAL trial (2012) there was extremely limited evidence on the effectiveness of intradialytic cycling on patient-centred outcomes such as QoL indices. In CKD stages 2–5, overall QoL was improved with exercise training. 9 Improved scores in the self-reported physical function subscales of the questionnaires appeared to be the main drivers for the overall improvement. 9 Other elements of QoL, such as vitality, social function and general health, did not show a systematic change. 9 Short-term (2–6 months), structured and supervised moderate-intensity aerobic training programmes (mainly cycling) have been reported to induce a systematic and large improvement in cardiorespiratory fitness (VO2 peak) of 17–50%, with an overall mean difference between treatment and control groups of 5.22 ml/kg/minute. Such improvements exceed the clinically important criterion of 1 metabolic equivalent (MET) (3.5 ml/kg/minute). Thus, we utilised the evidence base promoting intradialytic cycling for improved cardiorespiratory fitness to investigate whether or not the physiological benefits derived from cycling could extend to perceived enhanced QoL. Furthermore, as inconsistent improvements have been noted for objectively measured functional capacity indices (walking speed/distance, sit-to-stand 60 performance) from previous intradialytic cycling studies, we chose to evaluate key secondary outcome measures of objective physical function.
The rationale for intradialytic exercise is both intuitively and pragmatically appealing because the environment of unit-based HD provides a platform for longer-term sustainable implementation of exercise rehabilitation programmes and, thus, could promote exercise-enhancing behaviours in HD patients. The current requirement for HD patients to attend 4-hour HD sessions three times per week provides a practical opportunity to deliver a structured and supervised rehabilitation programme with an enhanced potential for participation, associated with a substantially reduced patient burden in terms of time, effort and travel cost.
Objectives
Primary objective
The primary objective of this trial was to determine, in comparison with usual care, whether or not usual care augmented by intradialytic exercise training for a period of 6 months improved Kidney Disease Quality of Life Short Form, version 1.3 (KDQoL-SF), PCS score in stage 5 CKD patients receiving maintenance HD.
Secondary objectives
The secondary objectives were to determine, in comparison to usual care, whether or not usual care augmented by intradialytic exercise training improved:
-
physical function and physical activity outcomes
-
peak aerobic capacity
-
physical fitness indicators
-
gait speed
-
lower-limb strength.
-
-
clinical measures
-
resting blood pressure
-
haemoglobin, serum phosphate and parathyroid hormone levels
-
arterial stiffness [pulse wave velocity (PWV)].
-
-
anthropometric measures
-
body mass index (BMI)
-
waist and hip circumference.
-
-
patient-reported outcomes
-
QoL and symptom burden assessments [EuroQol-5 Dimensions, five level version (EQ-5D-5L)]
-
habitual physical activity levels (International Physical Activity Questionnaire)
-
Duke Activity Status Index
-
falls confidence (Tinetti Falls Efficacy Scale).
-
Economic analysis of cost-effectiveness
-
Quality-adjusted life-years (QALYs) derived from the EQ-5D-5L.
-
Resource use associated with physiotherapy assistant time.
-
Resource use associated with delivery of exercise programme.
-
Equipment costs.
-
Hospital admissions and medication use recorded (with consent) from the patient’s clinical records.
Qualitative study
-
A nested, qualitative study investigating the experience and acceptability of the intervention for both participants and members of the renal care team.
Harms
-
Serious adverse events (SAEs).
Chapter 2 Methods
Trial design
We conducted this prospective, multicentre RCT in five regions of the UK.
Ethics approval and research governance
The trial protocol was approved by the relevant health authorities and institutional review boards, and all patients provided written informed consent. London Fulham Research Ethics Committee approved the protocol (reference 14/LO/1851). The study was prospectively registered as ISRCTN83508514. An independent data and safety monitoring committee performed regular safety surveillance. Data were entered into an electronic case report form by the investigators and were analysed at the Robertson Centre for Biostatistics, University of Glasgow.
Patient and public involvement
The planning and delivery of the PEDAL trial was facilitated by close engagement with people living with CKD and utilising HD therapy. Patients contributed directly to both the development of trial materials (e.g. participant information leaflets and consent forms) and the way the trial was conducted. INVOLVE good practice guidelines23 were followed to ensure service-user leadership in the trial delivery and dissemination of the findings. INVOLVE is a national advisory group established and funded by the NIHR to support active public involvement in NHS, public health and social care research. 23
Participants
Inclusion criteria
-
Prevalent patients with stage 5 CKD receiving maintenance HD therapy for > 3 months who were male or female, aged > 18 years and able to provide written informed consent.
Exclusion criteria
-
Patients unlikely to be receiving HD for > 6 months (this includes cachectic patients and those with severe heart failure (New York Heart Association Functional Classification ≥ 3)].
-
Patients for whom dialysis withdrawal was being considered.
-
Patients likely to receive a live donor transplant or be transferred to peritoneal dialysis in the period of time.
-
All patients within 3 months of initiation of HD. (Patients in this time frame are generally less clinically stable, many having vascular access procedures performed, and have much higher rates of intercurrent events, including death and hospitalisation).
-
Patients deemed to be clinically unstable by their treating physician.
-
Patients with bilateral lower-limb amputations.
-
Patients with dementia or severe cognitive impairment, and other patients unable to give informed consent.
-
Patients with psychiatric disorders (who are not treated and stable).
-
Patients who were pregnant.
Screening and eligibility
The majority of potential participants for the PEDAL trial were identified during routine HD clinical consultations and concurrent evaluation of clinical records to confirm eligibility for participation. Patients already established on HD for > 3 months were eligible and easily identified from hospital databases and dialysis logs. If considered eligible for the study, they were approached by a member of the renal health-care team who discussed the study and provided them with a participant information sheet with further details. After allowing the patient a minimum of 24 hours to read and consider the information in the participant information sheet, and to consult with family members, the research team approached the patient (usually during the next dialysis session) to answer any questions. If the patient agreed to proceed, an appointment was made for familiarisation and baseline outcome assessment sessions. Written informed consent was obtained by a member of the research team prior to any study assessments.
Randomisation and blinding
Randomisation was conducted using a centrally controlled, web-based randomisation system run by the Glasgow Clinical Trials Unit (GCTU). To ensure balanced assignment across critical variables, a minimisation algorithm was employed, taking into account baseline age, sex and diabetes status. It was impossible to blind the ‘treating’ physiotherapy assistants or the participants and, thus, the study implemented a blinded outcome assessment and analysis.
Intervention
Intradialytic exercise training
The intradialytic exercise prescription was based on current physical activity guidelines for the elderly24 and for people with diabetes25 and cardiovascular disease. 26 These recommend that a minimum target amount of 1000 kcal per week is expended in physical activity for health benefits, with optimal benefits associated with a weekly target physical activity accumulation of 1500–2000 kcal or at least 150 minutes per week at moderate exercise intensity and at least 2 days per week of resistance-based training for muscular endurance and strength gains. Because opportunity for structured, prescribed exercise was largely restricted to patients’ 3 days of HD, the training aim was for patients to accumulate a level as close as possible to or exceeding this minimum threshold level of 1500 kcal per week through intradialytic exercise. A target overall volume of exercise was calculated in energy expenditure units (kcal per week) using the frequency, intensity, time, type (FITT) principle and provided for all patients, with an individualised progression plan towards this goal.
The following progression plan was devised as a general guide:
-
Introductory/adoption phase: a minimum cycling duration of 21 minutes per dialysis session was used as a starting point target. By the end of the first 4 weeks, all patients were expected to cycle for 21 minutes either in two 10-minute bouts or continuously at the low end of the moderate exercise intensity range.
The progression requirements were that, by week 8, all participants should be able to cycle for ≥ 21 minutes continuously on the cycle ergometer (63 minutes per week) at the low end of the moderate exercise intensity range. The overall minimum target volume of exercise was set at 140 kcal per cycling session.
-
Progression/adoption phase: expected to last 12–14 weeks. During these weeks, emphasis was placed on progressing exercise stimulus mainly through duration. A reasonable and achievable exercise target for this group would be a minimum cycling duration of 21–30 minutes per dialysis session at moderate intensity. The overall minimum target volume of exercise was set at 1000–1200 kcal per week or ≈ 170 kcal per dialysis exercise session.
-
Behaviour development phase: expected to last up to 24 weeks. All patients should be able to achieve a target duration ranging from 30–40 minutes per dialysis session (90–120 minutes per week) at moderate to vigorous exercise intensity (55–70% VO2 reserve). The overall minimum target volume of exercise was set at ≈ 1500 kcal per week (or ≈ 214 kcal per dialysis session) and this would be achieved through adjustments to duration and intensity.
The prescribed individualised training intensity was derived from a peak aerobic capacity (VO2 peak) assessment, using a 1-minute ramp incremental protocol on a cycle ergometer. New exercise intensity ranges were established at the 3-month follow-up assessment time point. Exercise prescription was set at a workload corresponding to 40–75% of VO2 reserve. We also made a note of rate of perceived exertion, heart rate and blood pressure responses corresponding to these ranges of exercise intensity during the incremental cycle testing protocol and used these indices to guide and monitor progression until the next planned assessment point (3 months), at which time the exercise prescription was renewed.
Using a modified and custom-made Monark cycle ergometer (HaB International Ltd., Southam, UK), aerobic exercise was performed, in a semi-recumbent position, three times per week during the first 2 hours of HD. Exercise duration and intensity were recorded and monitored for each exercise session in exercise diaries. In addition, ratings of perceived exertion, BP and HR were recorded during the training sessions. The energy expenditure goals were deliverable through a progressive increase in intradialytic cycling from short bouts of 8–10 minutes to bouts of 21 minutes and bouts of 40 minutes or more, at the prescribed exercise intensity, resulting in 55%, 69% and 75%, respectively, of the target weekly minimum physical activity volume of 1000 kcal being achieved. Twice per week participants also completed lower-extremity muscular conditioning exercise, using ankle weights, after aerobic cycling exercise. Physiotherapy assistants (band 4 technical instructors) were employed in each region to deliver the intradialytic intervention. This role, supervised and quality assured by a regional co-ordinator, involved the technical implementation of the exercise prescription produced by the regional research assistant, who was blinded to treatment allocation.
Usual care: haemodialysis therapy
Usual care was based on UK Renal Association guidelines for HD2 and included management of blood pressure, treatment of anaemia, phosphate control and cardiovascular risk mitigation strategies. For the purposes of the trial, we specified that usual care, in both arms of the trial, should allow all of these treatments to continue unchanged so that we were investigating any additional benefit of the intradialytic exercise training intervention compared with usual care.
Adherence
We attempted to minimise the loss to follow-up in this study by (1) emphasising to participants the importance of their attendance at follow-up assessments even if they were no longer compliant with the intervention, (2) reducing outcome assessment appointments to a maximum of two non-dialysis day visits, (3) using a reminder protocol for non-dialysis day assessment appointments that utilised prompts by the dialysis unit staff, letters and telephone contact, (4) providing travel remuneration (including, where necessary, taxi costs), and (5) the provision of training in issues related to compliance for all study staff who came into contact with the participants.
Primary and secondary outcomes
Primary outcome
The primary end point for this study was change in KDQoL-SF PCS score between baseline and 6 months. The KDQoL-SF is a disease-specific QoL measure that includes the SF-36 as a generic core plus symptoms/problems of kidney disease scales. The SF-36 has 36 items compiled into eight scales: physical functioning, role functioning/physical, bodily pain, general health, vitality, social functioning, role functioning/emotional and mental health. These scales are scored from 0 to 100 AUs; a higher score is more positive (i.e. less pain or less limitation). Normalised scores representing overall physical functioning and mental functioning are calculated from the individual scales and are presented as PCS and mental component summary (MCS) scores. The PCS includes the dimensions of physical functioning, role functioning/physical, bodily pain, general health, vitality and social functioning.
Secondary outcomes
Quality of life
A documented change in EQ-5D-5L score from baseline to 6 months is a key QoL secondary outcome.
Peak aerobic capacity
VO2 peak was determined by an incremental cycling exercise tolerance protocol. Breath-by-breath gas exchange was measured using cardiopulmonary exercise testing equipment calibrated prior to each patient assessment. The exercise testing protocol started with a 3-minute unloaded cycle, followed by ramp increases in resistance of 15 watts per min until one of the following occurred: (1) plateau in oxygen uptake, (2) attainment of respiratory exchange ratio ≥ 1.15 or (3) patient request to stop. Average oxygen uptake during the final 20 seconds of the test was recorded as the VO2 peak. Electrocardiogram and heart rate were continuously monitored, and blood pressure was recorded every 1 or 2 minutes throughout the ramp incremental test. Rate of perceived exertion and angina scale were recorded every minute for safety.
Physical performance tests
Patient physical function was assessed by the sit-to-stand 60 (STS60) test27 and the 10-metre Timed Up and Go (10mTUG) test,28 both of which have been used as accurate and valid measures of lower-leg strength, balance, co-ordination, gait speed and physical function in HD patients.
Anthropometric measures
Measures of height, body mass, BMI and waist circumference were performed.
Cardiovascular risk
Carotid–femoral PWV is considered the gold standard for non-invasive arterial stiffness assessment in clinical practice29 and has been suggested as a surrogate cardiovascular end point. This was assessed with the Vicorder® system (Skidmore Medical Limited, Bristol, UK). The Vicorder system is small, portable, non-invasive and non-operator-dependant. In addition, it was available in all centres to ensure comparability of the data. Conditions for assessment, as described in the expert consensus statement by Laurent et al. ,29 were adhered to for all measurements. The measurement protocol by Hickson et al. 30 was used, mathematically removing the additional femoral segment from the Vicorder standard protocol to correct for any inherent bias at high arterial PWV. An average of three measurements (of 20 consecutive signals) was recorded at each time point. Resting predialysis blood pressure was assessed.
Physical function, physical activity and fear of falling questionnaires
Patients completed the following questionnaires to capture data about physical activity, activities of daily living and falls: the International Physical Activity Questionnaire Long Form,31 the Duke Activity Status Index (a self-reported, 12-item questionnaire that assesses activities of daily living)32 and the Tinetti Falls Efficacy Scale (measuring fear of falling). 33
Blood tests
Clinical blood tests were collected pre dialysis and included haemoglobin, serum phosphate and parathyroid hormone.
Medication
Dosages of erythropoiesis-stimulating agents were recorded.
Safety and monitoring of the intervention
Safety and monitoring data included discontinuation from the exercise intervention and permanent study withdrawals with reasons, compliance with the exercise programme and adherence to the exercise prescription and SAEs.
Engagement and fidelity to exercise prescription
General engagement with the exercise intervention was defined as the percentage of exercise sessions completed of the total prescribed. Compliance was calculated as the number of sessions completed at week 13 and 26 of the exercise programme divided by the number of expected sessions completed (39 and 78 for weeks 13 and 26, respectively) and multiplied by 100. Acceptable compliance was defined as > 70% compliance and poor compliance was defined as < 50% compliance. To monitor adherence (fidelity) to exercise prescription in terms of intensity and volume of exercise achieved, data on duration of cycling (excluding warm up and cool down), power output, number of sets and repetitions for muscle conditioning exercises were recorded in individual participant exercise diaries for each exercise session. Drop-out/temporary cessation of exercise of > 2 weeks with reasons were also recorded in the individual exercise diaries.
Sample size
Primary outcome
A sample size calculation, based on an assumed difference of 4 AU in KDQoL-SF PCS score AU and a standard deviation (SD) of 10 AU as seen in the study by Painter et al. ,7 and conservatively comparing 6-month KDQoL-SF PCS scores between groups by two sample t-test (two-sided 5% significance level) resulted in a sample size of 133 completers per group, with 83% power to detect a mean difference of 4 AU in KDQoL-SF PCS score. To allow for 30% loss to follow-up over 6 months, as seen in phase II trials,25 190 participants per group were suggested to be randomised. This sample size calculation did not take into account adjustment for baseline PCS scores. Subsequent analysis by the GCTU of within-trial change in PCS score from baseline, adjusted for baseline levels and randomisation minimisation variables, suggested that the study will have 80% power to detect a 4-point difference with only 87 participants per group with complete data at baseline and 6-month follow-up. Likewise, 115 participants per group would be needed if a zero score is imputed for deaths prior to 6 months.
Key secondary outcome
Analysis of blinded within-trial data adjusting for baseline levels and randomisation minimisation variables suggest that a study of 44 participants per group would have 90% power to detect the minimum clinically important difference in peak aerobic capacity of 3.5 ml/kg/min (1 MET).
Statistical analyses
A full statistical analysis plan was signed off prior to database lock and study unblinding. Descriptive statistics of clinical and sociodemographic variables at baseline are presented split by treatment group.
Primary outcome
The primary outcome measure (change from baseline to 6 months in KDQoL-SF PCS score) was compared between the control and intervention groups using a normal linear model, adjusting for baseline KDQoL-SF PCS score and the randomisation minimisation variables. The findings are presented as the adjusted mean difference, with 95% confidence interval (CI), between the treatment groups. The main analysis was carried out on research participants with PCS assessments at baseline and at 6 months. Two sensitivity analyses were also carried out, first imputing a score of zero for those who died prior to 6 months and, second, based on all participants with a baseline PCS score using the method of multiple imputation.
Secondary outcomes
Other continuous outcomes were analysed as for the primary outcome.
Binary outcomes were compared between treatment groups using logistic regression models, adjusting for baseline value. The results are reported as the adjusted odds ratio with 95% CI.
Time-to-event outcomes were calculated as time from randomisation and compared between treatment groups using Cox proportional hazards regression models. The results are reported as the adjusted hazard ratio for intervention versus control with 95% CI.
Data involving counts of events were compared between treatment groups using negative binomial regression models, adjusting for length of follow-up. The results are reported as the adjusted treatment effect with 95% CI.
Subgroup analyses
In the intervention group, the primary outcome was compared between those who had completed < 30% of the expected exercise sessions, those who had completed ≥ 30% but < 50% of the expected exercise sessions, those who had completed ≥ 50% but < 70% of the expected exercise sessions and those who had completed ≥ 70% of the expected exercise sessions. This was done using a linear regression model, predicting the primary outcome from the above categories for the expected exercise sessions completed, adjusting for minimisation variables.
The primary outcome was also assessed, comparing the control group with the three completer groups defined above. In addition, the relation of the percentage of sessions completed at 6 months follow-up to the primary outcome were analysed in two ways:
-
as a linear regression predicting the primary outcome from the percentage of sessions completed at 6-month follow-up, adjusting for baseline PCS score and minimisation variables, in the intervention group only
-
as a linear regression predicting the primary outcome from the percentage of sessions completed at 6-month follow-up, adjusting for baseline PCS score and minimisation variables, in all patients, setting the percentage of sessions completed to 0 in the control group.
Safety analyses
Discontinuations from the intervention and permanent study withdrawals and their reasons were tabulated, as was adherence to the exercise prescription. SAEs were tabulated by system organ class and body system.
Data monitoring and quality assurance
The trial was coordinated by a Trial Management Group (TMG), consisting of the chief investigator, GCTU Assistant Director and senior clinical trial manager and a statistician. The trial manager co-ordinated the study and was accountable to the chief investigator. The central trial office (King’s College Hospital and GCTU) provided support to each site. The central trial office was responsible for randomisation, collection of data in collaboration with the research co-ordinator, data processing and analysis. Publication and dissemination of the study results was co-ordinated by the trial office in collaboration with the chief investigator and investigators. A Trial Steering Committee was established to oversee the conduct and progress of the trial. The study’s funder – the NIHR Health Technology Assessment (HTA) programme – formally appointed the chairperson and members after the nominations from the TMG. A charter was drawn up to describe membership, roles and responsibilities of the Trial Steering Committee. The Independent Data Monitoring Committee was an independent group of experts, consisting of a nephrologist, physiotherapist, lay member and a statistician, who monitored patient safety and treatment efficacy data while the clinical trial was ongoing; the primary mandate of this committee was to protect patient safety.
Chapter 3 Results
Recruitment and retention
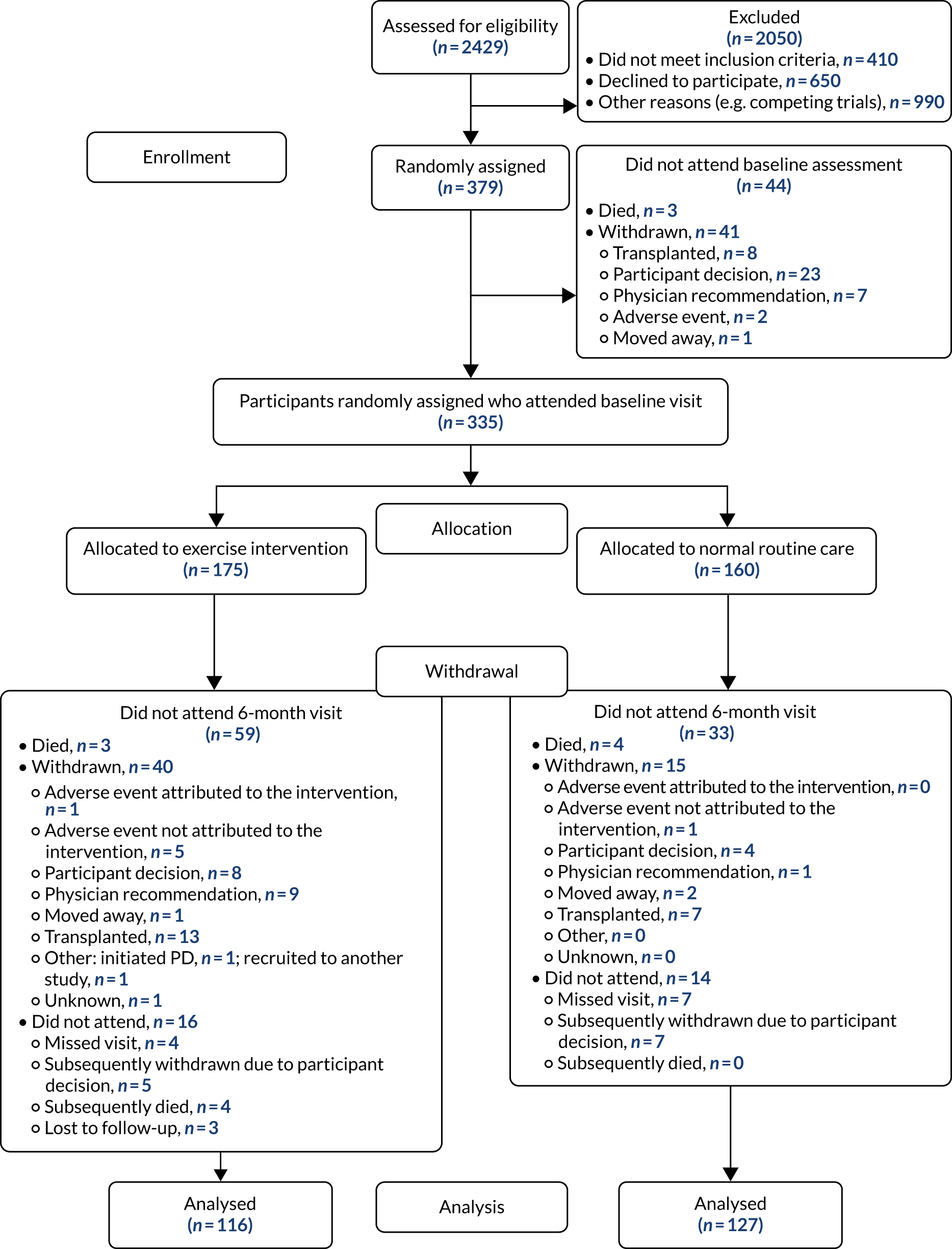
Patient flow, including recruitment to and retention in the trial, is detailed in Figure 1. The trial recruited prevalent patients with stage 5 CKD receiving HD therapy from June 2015 to June 2019 in five regional kidney units in the UK. In total, the trial recruited 379 participants. A total of 335 participants attended a baseline study visit: 175 participants who were randomised to the exercise intervention and 160 participants who were randomised to usual care. Participants were informed of group allocation only after completing all baseline assessments. Fifty-nine patients allocated to the exercise intervention and 60 participants allocated to usual care did not complete the 6-month assessment. In total, seven participants died during the study: three participants from the intervention group and four participants from the usual-care group. In the intervention group, 40 participants were withdrawn and 16 did not attend for the final 6-month assessment. In the usual-care group, 15 participants were withdrawn and 14 participants did not attend the 6-month assessment. Participants were more likely to withdraw from the trial if they were older and female. Analysis to establish how participants who withdrew differed from those who remained in the trial, stratified by arm, did not reveal significant differences in age, sex or weight, but it does appear that participants were more likely to drop out of the exercise arm if they had a history of heart failure and cerebrovascular disease (Table 1). The primary outcome was therefore known for 243 (73%) participants who attended a baseline visit: 116 (66%) participants in the intervention group and 127 (79%) participants in the usual-care group (see Figure 1).
FIGURE 1.
The PEDAL trial Consolidated Standards of Reporting Trials (CONSORT) diagram. Reproduced with permission from Greenwood et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
| Baseline characteristic | Group | |||
|---|---|---|---|---|
| Patients not withdrawn | Patients withdrawn | |||
| Control | Intervention | Control | Intervention | |
| Age (years) | ||||
| N | 145 | 135 | 15 | 40 |
| Mean (SD) | 59.8 (14.1) | 60.5 (15.0) | 52.8 (19.9) | 56.8 (13.3) |
| Median (Q1, Q3) | 59.7 (50.5, 71.0) | 62.1 (47.9, 72.9) | 56.1 (34.7, 61.2) | 56.3 (49.6, 64.3) |
| Sex | ||||
| N | 145 | 135 | 15 | 40 |
| Female, n (%) | 55 (37.9) | 56 (41.5) | 4 (26.7) | 11 (27.5) |
| Ethnicity | ||||
| N | 145 | 135 | 15 | 40 |
| White, n (%) | 67 (46.2) | 73 (54.1) | 10 (66.7) | 19 (47.5) |
| Black Caribbean, n (%) | 26 (17.9) | 17 (12.6) | 1 (6.7) | 3 (7.5) |
| Black African, n (%) | 33 (22.8) | 24 (17.8) | 1 (6.7) | 10 (25.0) |
| South Asian, n (%) | 15 (10.3) | 16 (11.9) | 2 (13.3) | 6 (15.0) |
| Chinese, n (%) | 1 (0.7) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Other,a n (%) | 3 (2.1) | 4 (3.0) | 1 (6.7) | 2 (5.0) |
| Weight (kg) | ||||
| N | 143 | 135 | 15 | 40 |
| Mean (SD) | 80.8 (20.5) | 79.2 (18.8) | 82.5 (13.8) | 82.8 (24.8) |
| Median (Q1, Q3) | 77.0 (66.1, 92.2) | 76.4 (65.4, 90.8) | 83.0 (67.5, 91.5) | 78.5 (67.4, 90.7) |
| BMI (kg/m2) | ||||
| N | 143 | 135 | 15 | 40 |
| Mean (SD) | 28.8 (6.5) | 28.5 (6.5) | 28.8 (5.5) | 29.2 (8.8) |
| Median (Q1, Q3) | 28.0 (24.5, 32.0) | 27.0 (23.8, 32.2) | 27.8 (24.2, 32.4) | 27.6 (22.3, 32.6) |
| Smoking | ||||
| N | 145 | 135 | 15 | 40 |
| Current, n (%) | 19 (13.1) | 18 (13.3) | 0 (0.0) | 5 (12.5) |
| Former, n (%) | 45 (31.0) | 39 (28.9) | 4 (26.7) | 10 (25.0) |
| Never, n (%) | 81 (55.9) | 78 (57.8) | 11 (73.3) | 25 (62.5) |
| SBP (mmHg) | ||||
| N | 142 | 135 | 15 | 40 |
| Mean (SD) | 138.6 (23.4) | 134.4 (21.3) | 133.9 (22.6) | 134.1 (17.5) |
| Median (Q1, Q3) | 138.0 (121.8, 153.9) | 133.7 (121.3, 147.5) | 130.0 (115.0, 152.2) | 131.5 (121.0, 142.8) |
| DBP (mmHg) | ||||
| N | 142 | 135 | 15 | 40 |
| Mean (SD) | 73.4 (13.7) | 72.6 (15.4) | 75.5 (15.4) | 76.9 (10.0) |
| Median (Q1, Q3) | 73.3 (63.2, 81.7) | 71.3 (61.3, 82.7) | 74.0 (67.0, 80.7) | 76.8 (70.8, 81.5) |
| Peripheral vascular disease | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 6 (4.1) | 5 (3.7) | 0 (0.0) | 0 (0.0) |
| Diabetes | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 59 (40.7) | 52 (38.5) | 6 (40.0) | 15 (37.5) |
| Hypertension | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 116 (80.0) | 101 (74.8) | 11 (73.3) | 33 (82.5) |
| Hyperlipidaemia | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 39 (26.9) | 23 (17.0) | 4 (26.7) | 5 (12.5) |
| Previous MI | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 21 (14.5) | 14 (10.4) | 0 (0.0) | 6 (15.0) |
| Heart failure | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 17 (11.7) | 14 (10.4) | 0 (0.0) | 1 (2.5) |
| Cerebrovascular events | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 17 (11.7) | 8 (5.9) | 1 (6.7) | 0 (0.0) |
| Cardiovascular | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 25 (17.2) | 30 (22.2) | 2 (13.3) | 12 (30.0) |
| Musculoskeletal and orthopaedic condition | ||||
| N | 145 | 135 | 15 | 40 |
| Yes, n (%) | 19 (13.1) | 16 (11.9) | 1 (6.7) | 7 (17.5) |
| Hb | ||||
| N | 141 | 127 | 15 | 37 |
| Mean (SD) | 110.2 (12.1) | 109.8 (14.1) | 118.1 (14.2) | 108.9 (15.8) |
| Median (Q1, Q3) | 109.0 (103.0, 119.0) | 110.0 (102.0, 118.5) | 115.0 (109.0, 124.0) | 110.0 (100.0, 120.0) |
| CRP (mg/l) | ||||
| N | 139 | 125 | 15 | 36 |
| Mean (SD) | 15.3 (21.1) | 11.9 (15.9) | 12.5 (16.4) | 21.1 (26.6) |
| Median (Q1, Q3) | 6.6 (3.1, 18.1) | 6.0 (3.0, 14.1) | 8.0 (4.5, 11.0) | 10.9 (4.3, 28.1) |
| Dialysis efficiency (%) | ||||
| N | 141 | 125 | 15 | 37 |
| Mean (SD) | 71.2 (8.4) | 71.9 (7.3) | 71.0 (11.3) | 71.6 (7.9) |
| Median (Q1, Q3) | 72.0 (66.0, 77.0) | 73.0 (69.0, 76.5) | 74.0 (68.0, 77.8) | 71.8 (66.0, 77.0) |
Demographic and other baseline characteristics
Baseline characteristics of randomised participants
Of the 335 randomised participants, 62.4% were male (see Table 1). The median age was 59.3 years [interquartile range (IQR) 48.8–70.5 years]. Half of the randomised population was white and 34.3% was black African/black Caribbean. The mean weight was 80.5 kg (SD 20.1 kg) and most participants were classified as overweight or obese [median BMI of 27.8 kg/m2 (IQR 24.0–32.2 kg/m2)]. A total of 13% reported a musculoskeletal or orthopaedic condition, and participants had haemoglobin levels of 100–120 g/l at baseline.
Prior cardiovascular events and risk factors
A history of major adverse cardiovascular events affected the minority of participants; prior stroke and myocardial infarction were present in 8% and 12% of the cohort, respectively. A history of heart failure or peripheral vascular disease was recorded for 4% and 10% of participants, respectively. At baseline, 78% of participants had hypertension, 39% had diabetes, 21% had hyperlipidaemia and 58% had never smoked.
Adherence
A total of 175 participants received the exercise intervention. A median of 47% (IQR 28–77%) of exercise training sessions were completed by participants in the intervention group at 6 months. A total of 23% of participants had dropped out of the exercise intervention at 6 months, and 58% of participants reported temporary cessation of the intervention. At 6 months, only 18% of participants were adhering to the full exercise prescription (Table 2).
| Variable | Time point | |
|---|---|---|
| 13 weeks | 6 months | |
| Percentage of expected sessions completed | ||
| NObs (NMiss) | 162 (173) | 107 (228) |
| Mean (SD) | 46.7 (31.0) | 48.7 (29.5) |
| Median (IQR) [range]34 | 46.5 (23.0–71.8) [0.0–100.0]17 | 47.3 (28.0–77.2) [0.0–97.7] |
| Dropouts | ||
| NObs (NMiss) | 160 (175) | 119 (216) |
| No, n (%) | 123 (76.9) | 92 (77.3) |
| Yes, n (%) | 37 (23.1) | 27 (22.7) |
| Temporary cessation | ||
| NObs (NMiss) | 160 (175) | 119 (216) |
| No, n (%) | 74 (46.2) | 50 (42.0) |
| Yes, n (%) | 86 (53.8) | 69 (58.0) |
| Exercise prescription adherence | ||
| NObs (NMiss) | 160 (175) | 119 (216) |
| No, n (%) | 113 (70.6) | 98 (82.4) |
| Yes, n (%) | 47 (29.4) | 21 (17.6) |
Outcomes
The results for the primary and secondary outcome measures are presented in Tables 3 and 4. The mean difference in the change in PCS score from baseline to 6 months between the intervention group and control group was 2.4 AUs (95% CI –0.1 to 4.8 AUs; p = 0.055). In the whole sample, despite no statistical improvement in PCS score (p = 0.055), it is noteworthy that the absolute mean difference in change in PCS score from baseline to 6 months between the intervention group and control group was 2.4 AUs (95% CI –0.1 to 4.8 AUs).
| Outcome measure | Group | n | Time point, mean (SD) | Adjusted mean difference in change between exercise group and control group (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Baseline | 6 months | |||||
| Primary outcome | ||||||
| KDQoL-SF PCS score, AU | Control | 120 | 32.9 (11.3) | 31.8 (11.3) | 2.37 (–0.06 to 4.80) | 0.055 |
| Intervention | 114 | 33.8 (10.6) | 34.8 (11.6) | |||
| Secondary outcomes | ||||||
| KDQoL-SF energy/fatigue score, AU | Control | 122 | 39.8 (26.0) | 41.4 (24.9) | 0.09 (–5.58 to 5.76) | 0.97 |
| Intervention | 114 | 40.3 (27.2) | 41.4 (26.4) | |||
| KDQoL-SF burden score, AU | Control | 122 | 36.0 (28.6) | 37.3 (29.7) | –1.42 (–6.95 to 4.11) | 0.61 |
| Intervention | 113 | 37.3 (27.7) | 36.9 (29.0) | |||
| Outcome measure | Group | n | Time point, mean (SD) or median (IQR) | Adjusted treatment effect (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Baseline | 6 months | |||||
| Peak aerobic capacity (VO2 max to VO2 peak) (l/minute) | Control | 68 | 0.97 (0.38) | 0.96 (0.37) | 0.047 (–0.029 to 0.124) | 0.22 |
| Intervention | 75 | 0.95 (0.42) | 0.98 (0.43) | |||
| Peak aerobic capacity (VO2 max to VO2 peak) (ml/kg/minute) | Control | 68 | 11.9 (4.5) | 11.8 (4.2) | 0.75 (–0.20 to 1.71) | 0.12 |
| Intervention | 74 | 11.8 (5.3) | 12.4 (5.7) | |||
| Arterial stiffness via PWV (msec) [ln(x + 1)] | Control | 78 | 8.10 (6.78–9.29) | 7.78 (6.97–9.13) | 1.014 (0.971 to 1.059)a | 0.54 |
| Intervention | 78 | 7.92 (6.62–9.09) | 7.88 (6.98–9.27) | |||
| Duke Activity Status Index (points) | Control | 121 | 23.1 (13.1) | 22.7 (13.4) | 0.35 (–2.23 to 2.93) | 0.79 |
| Intervention | 112 | 24.9 (13.3) | 24.1 (14.3) | |||
| International Physical Activity Questionnaire, total physical activity (MET-minutes/week) [ln(x + 10)] | Control | 118 | 423.8 (39.0–1465.4) | 353.2 (46.1–1033.1) | 1.36 (0.84 to 2.21)a | 0.21 |
| Intervention | 106 | 709.5 (153.8–2515.1) | 591.0 (111.8–1793.2) | |||
| 10mTUG [gait speed (m/s) over 10 metres] | Control | 84 | 0.86 (0.30) | 0.87 (0.29) | 0.009 (–0.041 to 0.058) | 0.73 |
| Intervention | 79 | 0.94 (0.29) | 0.94 (0.30) | |||
| STS60 (points) | Control | 87 | 13.8 (6.6) | 14.4 (7.0) | 1.02 (–0.42 to 2.47) | 0.16 |
| Intervention | 82 | 15.8 (7.1) | 17.1 (8.1) | |||
| Tinetti Falls Efficacy Scale [ln(x)] | Control | 122 | 22.5 (10.2–46.8) | 24.5 (11.0–50.0) | 0.943 (0.796 to 1.117)a | 0.49 |
| Intervention | 112 | 23.0 (11.8–49.2) | 24.5 (11.0–46.2) | |||
| EQ-5D-5L health utility score (points) | Control | 121 | 0.688 (0.254) | 0.675 (0.261) | 0.011 (–0.044 to 0.065) | 0.69 |
| Intervention | 111 | 0.710 (0.223) | 0.697 (0.253) | |||
| EQ-5D-5L VAS score (points) | Control | 121 | 59.4 (22.7) | 59.3 (20.9) | 3.52 (–1.02 to 8.05) | 0.13 |
| Intervention | 111 | 60.7 (22.2) | 63.7 (19.3) | |||
Safety and harms
The number of hospitalisations, all-cause mortality and cardiovascular mortality were not different between the groups. Although these results should be interpreted cautiously owing to the lack of events for these secondary outcomes, there was no obvious increase in SAEs in the intervention group either (Tables 5 and 6).
| Variable | Group | n | Number of hospitalisations (hospitalisation rate per person-year) | Incident rate ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| Number of hospitalisations | Control | 160 | 84 (0.54) | 1.45 (0.98 to 2.15) | 0.064 |
| Intervention | 175 | 132 (0.85) | |||
| Variable | Group | n | Number of events (event rate per 100 person-years) | Hazard ratio (95% CI) | p-value |
| All-cause mortality | Control | 160 | 9 (5.76) | 1.19 (0.48 to 2.94) | 0.71 |
| Intervention | 174 | 10 (6.46) | |||
| Cardiovascular mortality | Control | 160 | 3 (1.92) | N/A | N/A |
| Intervention | 174 | 2 (1.29) |
| Variable | All, n (%) | Group, n (%) | |
|---|---|---|---|
| Control | Intervention | ||
| Number of randomised patients | 335 | 160 | 175 |
| Number of patients with any event | 125 | 56 | 69 |
| Blood and lymphatic system disorders | 2 (0.6) | 0 (0.0) | 2 (1.1) |
| Cardiac disorders | 15 (4.5) | 6 (3.8) | 9 (5.1) |
| Congenital, familial and genetic disorders | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Gastrointestinal disorders | 14 (4.2) | 4 (2.5) | 10 (5.7) |
| General disorders and administration site conditions | 17 (5.1) | 12 (7.5) | 5 (2.9) |
| Hepatobiliary disorders | 3 (0.9) | 1 (0.6) | 2 (1.1) |
| Infections and infestations | 47 (14.0) | 18 (11.2) | 29 (16.6) |
| Injury, poisoning and procedural complications | 28 (8.4) | 12 (7.5) | 16 (9.1) |
| Investigations | 5 (1.5) | 4 (2.5) | 1 (0.6) |
| Metabolism and nutrition disorders | 17 (5.1) | 4 (2.5) | 13 (7.4) |
| Musculoskeletal and connective tissue disorders | 4 (1.2) | 1 (0.6) | 3 (1.7) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (0.3) | 0 (0.0) | 1 (0.6) |
| Nervous system disorders | 8 (2.4) | 3 (1.9) | 5 (2.9) |
| Psychiatric disorders | 4 (1.2) | 1 (0.6) | 3 (1.7) |
| Renal and urinary disorders | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Reproductive system and breast disorders | 2 (0.6) | 1 (0.6) | 1 (0.6) |
| Respiratory, thoracic and mediastinal disorders | 13 (3.9) | 3 (1.9) | 10 (5.7) |
| Skin and subcutaneous tissue disorders | 1 (0.3) | 0 (0.0) | 1 (0.6) |
| Social circumstances | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Surgical and medical procedures | 37 (11.0) | 13 (8.1) | 24 (13.7) |
| Vascular disorders | 10 (3.0) | 6 (3.8) | 4 (2.3) |
Subgroup analyses
In patients who completed > 30% of their prescribed exercise sessions, the difference in the change in PCS score was ≈ 3 AUs and statistically significant (p = 0.039). The relationship between compliance (as percentage of sessions completed) and PCS score was not significant [estimated change in PCS score per 10% difference in compliance, 0.6 AUs (95% CI –0.1 to 1.3 AUs); p = 0.11] (Table 7). The relationship between compliance (as percentage of sessions completed) and VO2 peak was weak but statistically significant [estimated change in VO2 peak per 10% difference in compliance, 0.3 ml/kg/minute (95% CI 0.0 to 0.6 ml/kg/minute); p = 0.047] (Table 8).
| Variable | Expected exercise sessions completed | ||||
|---|---|---|---|---|---|
| Control group | < 30% | ≥ 30% but < 50% | ≥ 50% but < 70% | ≥ 70% | |
| n | 120 | 25 | 28 | 21 | 29 |
| Baseline, mean (SD) | 32.9 (11.3) | 32.3 (11.8) | 36.0 (11.6) | 36.0 (13.0) | 32.2 (7.2) |
| 6 months, mean (SD) | 31.8 (11.3) | 29.1 (10.2) | 37.6 (12.2) | 37.7 (11.7) | 33.7 (11.7) |
| Change from baseline to 6 months, mean (SD) | –1.1 (10.2) | –3.3 (8.6) | 1.6 (10.6) | 1.6 (11.0) | 1.5 (10.9) |
| Variable | Adjusted mean difference (95% CI) inchange between intervention group and control group | p-value | |||
| < 30% completed vs. control completed | –2.61 (–6.64 to 1.42) | 0.20 | |||
| ≥ 30% but < 50% completed vs. control completed | 3.71 (–0.15 to 7.57) | 0.059 | |||
| ≥ 50% but < 70% completed vs. control completed | 3.67 (–0.67 to 8.01) | 0.097 | |||
| ≥ 70% completed vs. control completed | 2.88 (–0.98 to 6.73) | 0.14 | |||
| Overalla | 0.039 | ||||
| Variable | Control group | Expected exercise sessions completed | |||
|---|---|---|---|---|---|
| < 30% | ≥ 30% but < 50% | ≥ 50% but < 70% | ≥ 70% | ||
| n | 68 | 13 | 20 | 17 | 19 |
| Time point | |||||
| Baseline | |||||
| Mean (SD) | 11.9 (4.5) | 11.2 (5.1) | 13.9 (7.3) | 10.5 (3.5) | 11.1 (4.4) |
| Median (IQR) [range] | 11.6 (8.7–13.7) [4.2–30.6] | 8.9 (7.8–14.9) [6.2–20.6] | 11.4 (9.2–14.5) [6.3–32.8] | 10.4 (8.2–12.5) [5.3–17.9] | 11.3 (7.2–14.4) [3.9–19.5] |
| 6 months | |||||
| Mean (SD) | 11.8 (4.2) | 10.5 (4.5) | 14.6 (7.5) | 10.8 (4.0) | 12.7 (5.4) |
| Median (IQR) [range] | 10.8 (9.4–13.3) [5.0–26.1] | 8.8 (7.1–13.5) [5.0–20.5] | 12.3 (9.2–16.2) [7.3–33.2] | 11.0 (8.4–12.9) [4.7–20.5] | 12.7 (8.7–15.6) [3.9–22.7] |
| Change from baseline to 6 months | |||||
| Mean (SD) | –0.2 (3.1) | –0.8 (3.5) | 0.6 (3.2) | 0.3 (2.5) | 1.6 (2.6) |
| Median (IQR) [range] | –0.0 (–1.7 to 1.6) [–7.6 to 9.5] | 0.0 (–0.9 to 0.9) [–11.9 to 2.1] | 0.5 (–1.2 to 2.7) [–4.8 to 7.2] | 0.7 (–0.8 to 2.3) [–4.6 to 4.2] | 1.6 (–0.2 to 3.0) [–3.0 to 6.5] |
| Variable | Adjusted mean difference (95% CI) in change between intervention group and control group | p-value | |||
| < 30% completed vs. control completed | –1.01 (–2.73 to 0.71) | 0.246 | |||
| ≥ 30% but < 50% completed vs. control completed | 1.22 (–0.23 to 2.67) | 0.098 | |||
| ≥ 50% but < 70% completed vs. control completed | 0.09 (–1.45 to 1.62) | 0.911 | |||
| ≥ 70% completed vs. control completed | 1.79 (0.31 to 3.27) | 0.018 | |||
| Overalla | 0.037 | ||||
Chapter 4 Health economic evaluation
Background and methods
For the health economic evaluation, we compared the PEDAL intervention as an add-on to dialysis with dialysis alone. We estimated the mean between-group difference in costs of the intervention and the mean between-group difference in QALYs accrued by participants during the study, estimated as the area under the health utility curve. All costs and QALY differences were estimated over the 6-month period from randomisation; discounting future costs and effects for societal time preference is not relevant. Total costs and 1 – QALYs were modelled using a generalised linear regression model with a Gaussian distribution and a log-link, adjusting for age, sex and diabetes at baseline. The modified Park test was used to determine the distribution family for the model.
Intervention costs included costs for purchase and maintenance of exercise equipment (pro-rated to apply to the 6-month study period), initial training and supervision of staff, staff delivery of the exercise sessions and the number of sessions. Different scenarios for staff costs have been considered, varying situation on pay band, including/excluding London allowance and varying staff–patient ratios. The scenarios have been based on the staffing assumed to be required when implementing the exercise sessions in usual care, not within the trial. All costs were in Great British pounds; no currency conversions were required.
The cost of the equipment was assumed to be £1000 and the expected lifetime was assumed to be 10 years. This is the estimated cost of the modified Monark cycle ergometer if it were to be commercially available. Equipment costs over the 6-month study period were therefore assumed to be £50. The maintenance cost was £50 per year per bike. This is the cost of a call-out fee and any reasonable wear-and-tear replacements by the manufacturer. It was assumed that one bike covers four patients; therefore, equipment cost per patient was assumed to be £18.75 [(£50 + £25) ÷ 4] when doing the expected number of exercise sessions.
The level of staff required was assumed to be Agenda for Change band 4. This would be the level of staff required in the NHS to deliver this type of exercise intervention. Annual employer costs are £25,865.7180–30,114.3716 outside London and £30,065.7180–34,786.9716 in London. Cost per hour is calculated as annual staff cost ÷ 46 work weeks per year ÷ 37.5 work hours per week. The number of staff required to cover training sessions depends on the distances between satellite units of each study centre. It was assumed that 0.6 members of staff are required to cover training sessions for 12–20 patients. Values of 12 and 20 were used in the analysis. The level of staff required to supervise exercise sessions and train staff to deliver the sessions was assumed to be paid, Agenda for Change band 8. Annual employer costs are £55,078.005–64,755.960 outside London and £61,741.005–71,418.960 in London. It was assumed that one member of staff covers 80 patients.
The expected number of sessions over 6 months was calculated as 365.25 ÷ 2 ÷ 7 × 3 (three sessions per week). The number of sessions completed was calculated as number of expected sessions × percentage compliance reported in the eCRF ÷ 100. The cost per session was derived as (equipment cost + staff cost over 6 months) ÷ number of expected sessions for 6 months. The intervention cost per patient was derived as number of sessions actually attended × cost per session.
Costs in the control group were set to 0, because they received no additional intervention and, therefore, required no additional staff time or equipment.
The area under EQ-5D-5L health utility curve was derived as follows. Only the period from study entry (i.e. randomised and baseline visit complete) to 6 months was taken into account. If the baseline visit was on the date of study entry, the EQ-5D-5L health utility score collected at that visit was used in the calculation as EQ-5D-5L health utility score at entry. If the baseline visit was before randomisation, EQ-5D-5L health utility score was derived as follows:
-
if there is no follow-up visit, starting EQ-5D-5L health utility score will be the baseline EQ-5D-5L health utility score
-
if there is a follow-up visit, starting EQ-5D-5L health utility score will be determined through linear interpolation between baseline and the earliest follow-up visit thereafter.
The area under curve was derived as follows:
-
If the patient was not dead and had no follow-up beyond baseline, the 6-month EQ-5D-5L health utility score was assumed to be the same as the EQ-5D-5L health utility score at entry. The area under the EQ-5D-5L health utility curve was derived as 365.25 ÷ 2 × EQ-5D-5L health utility score at entry.
-
If the patient had their 6-month visit after 6 months from entry, EQ-5D-5L health utility score at 6 months was derived through linear interpolation between EQ-5D-5L health utility score at entry and EQ-5D-5L health utility score at 6-month visit. The area under the EQ-5D-5L health utility curve was derived as 365.25 ÷ 2 × (EQ-5D-5L health utility score at entry + EQ-5D-5L health utility score at 6 months) ÷ 2.
-
If there was no follow-up available and the patient died after 6 months, EQ-5D-5L health utility score at the time of death was set to 0 and the EQ-5D-5L health utility score at 6 months was derived through linear interpolation. The area under the curve was derived as in the point above.
-
If the 6-month visit occurred before 6 months, the EQ-5D-5L health utility score at 6 months was derived through linear interpolation between the 6-month visit and the next available information after that, either EQ-5D-5L health utility score at a following visit or at death, with EQ-5D-5L health utility score at death assumed to be 0. The area under the curve was then determined as time from entry to 6-month visit × (EQ-5D-5L health utility score at entry + EQ-5D-5L health utility score at 6-month visit) ÷ 2 + time from 6-month visit to 6 months × (EQ-5D-5L health utility score at 6-month visit + EQ-5D-5L health utility score at 6 months) ÷ 2. If there was no information after 6-month visit, EQ-5D-5L health utility score at 6 months was assumed to be the same as EQ-5D-5L health utility score at 6-month visit.
Estimated between-group differences in cost and QALYs were obtained by the method of recycled prediction in 5000 bootstrap samples. The distribution of these quantities was summarised and presented graphically in the incremental cost-effectiveness plane.
To assess whether or not the effect of treatment on QALYs differed between subgroups by age, sex and diabetes, interaction effects of these variables with treatment were added to the regression model.
Results
Table 9 shows means and CIs for costs from different sources under different scenarios for staff costs. Estimates shown assume that staff are on average pay for their pay band. Average total costs per patient over 6 months range from £231.70 (95% CI £204.30 to £259.10) to £423.90 (95% CI £373.70 to £474.10), depending on location and staff–patient ratio. The main cost factor is the staff cost for delivering the exercise sessions.
| Variable | Cost (£) per patient, mean (95% CI) | |||
|---|---|---|---|---|
| Outside London | London | |||
| Low staff–patient ratio | High staff–patient ratio | Low staff–patient ratio | High staff–patient ratio | |
| Equipment purchase and maintenance | 9.13(8.05 to 10.21) | 9.13 (8.05 to 10.21) | 9.13(8.05 to 10.21) | 9.13(8.05 to 10.21) |
| Staff delivering exercise sessions | 204.34(180.15 to 228.54) | 340.57(300.25 to 380.90) | 236.73(208.70 to 264.76) | 394.55(347.84 to 441.27) |
| Training and oversight | 18.23(16.07 to 20.38) | 18.23(16.07 to 20.38) | 20.25(17.86 to 22.65) | 20.25(17.86 to 22.65) |
| Total cost per patient over 6 months | 231.7(204.3 to 259.1) | 367.9(324.4 to 411.5) | 266.1(234.6 to 297.6) | 423.9(373.7 to 474.1) |
| Estimated difference in cost(recycled predictions) | 234.1(208.5 to 260.1) | 371.7(331.0 to 414.2) | 268.9(239.9 to 299.2) | 428.3(379.5 to 475.5) |
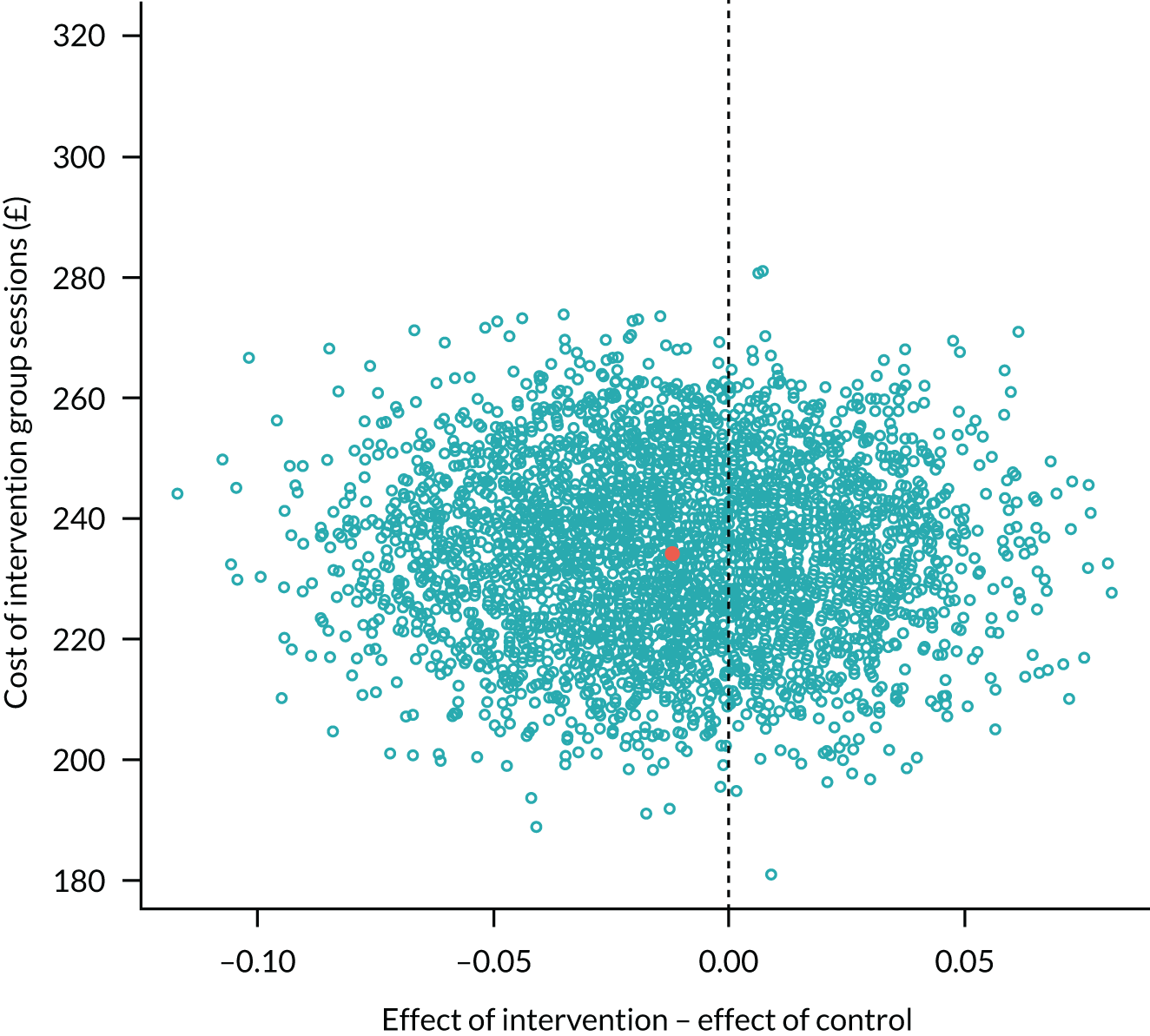
The mean of the area under the EQ-5D-5L curve was 0.665 (SD 0.248) in the control group and 0.653 (SD 0.269) in the intervention group. The mean difference between the treatment group and the intervention group, obtained using the method of recycled predictions, was –0.012, indicating lower QoL in the intervention group than in the control group, although the 95% CI (–0.069 to 0.043) included no difference. No significant subgroup effects were found for age, sex or diabetes at baseline.
Figure 2 shows the estimated differences in cost and QALYs on the ICER plane for the low staff–patient ratio outside London as an example.
FIGURE 2.
Cost-effectiveness on ICER plane, 5000 bootstrap samples, low staff–patient ratio and outside London. Reproduced with permission from Greenwood et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Discussion
The economics results suggest that, over 6 months of implementation, the PEDAL intervention resulted in higher NHS costs with no gain in QoL measured with the EQ-5D-5L. A number of factors should be borne in mind; for example, the benefit from the PEDAL intervention could be avoiding cardiovascular events and other illnesses in future years, and the 6-month time horizon would not capture this. The PEDAL protocol could be refined to target different patients on dialysis and/or to include a time point at which the benefit of the intervention was assessed, with only those reporting a good QoL gain continuing; both of these could potentially improve the cost-effectiveness.
Chapter 5 Qualitative substudy
Background
A secondary aim of the PEDAL trial was to explore and document the views of participants and members of the renal care teams in relation to their experiences of usual care and usual care augmented with intradialytic exercise. The qualitative substudy used a constructivist phenomenological approach35 to provide insights into the progress and conduct of the trial in all five study regions. Views and experiences of service providers and study participants were explored, including control group participants and those who did not complete the intervention. This report describes the positive and negative impacts of participating in intradialytic exercise (where a person chose to and was able to participate). It explains individual and contextual influences on decision-making and experiences as well as influences of the RCT context.
Qualitative substudy aims and objectives
The aim was to explore participants’ and renal care teams’ expectations and experiences of exercise training, with the particular aims of understanding:
-
the beliefs and attitudes of participants and of the renal care teams towards exercise training as a treatment and how this might affect QoL
-
the acceptability and experience of the intradialytic exercise training intervention as well as how being in the usual-care group affected behaviour.
Methods
Study design and methodology
The qualitative substudy of the multiregion PEDAL RCT used a constructivist phenomenological approach. 35 This aimed for ‘empathic neutrality’ through researcher reflexivity36 and was led by a researcher (CB) who was not involved with the rest of the trial. Face-to-face interviews were conducted using a semistructured topic guide. The substudy was included in the initial RCT ethics submission, and major amendments were submitted to the relevant NHS Research Ethics Committee to include relevant documentation.
Participants and study context
Anyone who met the criteria for and participated in the PEDAL trial in the intervention group or usual-care group, or who had dropped out of the intervention but not the study, was invited to participate as long as they could communicate sufficiently to be understood in English. To ensure representation of diverse views, people from each of these ‘categories’ were invited to participate at each site. Potential interviewees were provided with an invitation letter and response form.
‘Providers’ were also recruited, with the aim of including a range of people involved in providing the study intervention and whose working roles might be impacted by the trial. Representation was sought from people in various medical, nursing and support roles in the renal unit and from the PEDAL trial team.
Selected and willing individuals were interviewed once during the life of the study. Trial participants had consented to the substudy as part of the PEDAL RCT; however, it was considered appropriate to issue an invitation with more specific information at the point of qualitative recruitment at each site. It was also important to give renal care providers information. At each site, potential participants were given the invitation letter by a member of the PEDAL trial team based at that site at least 1 week before the day of data collection. The interviewer was introduced to a potential participant by the same person and started by providing an opportunity for the potential participant to ask any further questions and accept or decline interview. All PEDAL trial participants who were approached were happy to be interviewed.
Data collection procedure
Face-to-face interviews involved primarily one researcher and one participant (patient or provider). On one occasion, two providers wished to be interviewed together for pragmatic reasons, and on one occasion two patients in a side room wished to be interviewed together. Patients were interviewed when they came in for HD and providers were interviewed opportunistically to minimise disruption to their days. The majority of participants were interviewed by the same person (CB), who has expertise in health-related qualitative research, with a minority of interviews conducted by a supervised research assistant (who was trained by CB).
Topic guides were designed to focus on the study aims and were informed by review of relevant literature. Questions of interest were designed and sequenced using guidelines from Kreuger37 to move from more general to more specific information and to enable an ‘easing’ into the interview process. Finally, participants are asked for the most important aspect of the discussion in their view. Questions were reframed as needed to ensure that it was possible to elicit both the required topics or information as well as unexpected views and experiences, without implying values or demonstrating expectations in relation to responses. Draft topic guides were reviewed and approved by the PEDAL trial project team prior to ethics review. The final topic guides are provided on the NIHR project page (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/122309#; accessed 14 April 2021). Interviews were audio-recorded. General patient characteristics emerged through interviews, with more specific details extracted after unblinding of the PEDAL trial. Profession and role were noted for providers.
Data processing and analysis
Data protection guidelines were carefully adhered to for all transportation of data through download of audio-recordings to an encrypted laptop. Study rigour was enhanced through purposive sampling, multiple coding of a sample of data, ensuring an audit trail during analysis, and triangulation of data from different sites and regions, as well as through inclusion of people with a diversity of roles in relation to the PEDAL trial and intervention. 35 Data processing and subsequent framework analysis aimed at increasing abstraction while reflecting nuances, with development of theory to explain views and experiences in this study38 (Table 10). Analysis of all data collected in two different regions was carried out by the lead researcher (CB), who created the initial thematic framework. Subsequently, a research assistant (Jane Scullion) analysed all remaining data and coded this in NVivo version 10 (QSR International, Warrington, UK), applying and modifying the thematic framework through regular discussion with the lead researcher (CB). Mapping and interpretation, with triangulation and development of overarching theory, were carried out by the lead researcher (CB).
| Stage | Description |
|---|---|
| Intelligent verbatim transcription | Professional transcription without false starts and vocalised hesitations (e.g. ‘emm’); use of pseudonyms and removal or appropriate modification of any identifiable information |
| Transcript modification | Appropriate additions from researcher’s reflexive field notes |
| Upload to NVivoa | Processed transcripts were uploaded to NVivoa for data and analysis management |
| Familiarisation with the text | Reading and re-reading of transcripts; annotation of key ideas with summary labels |
| Theme development | Synthesis of labels to link similar ideas, views or concepts and define these as subthemes, subsequently grouped into themes |
| Thematic framework development | Organisation of themes into a logical framework, with allocation of codes to enable organisation of information using NVivo v10 software |
| Application of thematic framework | Re-reading and analysis of each transcript using the new thematic framework, with incorporation of any new themes |
| Coding in NVivoa | Coding of themes emerging in text using NVivoa to ensure an audit trail |
| Mapping and interpretation | Descriptive relationships between themes were sought, for example where a participant connected two ideas with ‘. . . happened because . . .’, enabling development of a map of interconnected ideas and explanatory theory for the findings |
| Triangulation | Analysing patterns and differences between results for different geographical regions and renal units, providers and participants |
| Development of overarching theory | Theory generation, with the aim of explaining the data in the study |
Results
Summary of data and participant characteristics
Interviews took place in all five regions: two sites in London, two in Central Scotland and one in each of the other regions. Owing to the staggered start to recruitment at different sites, and recruitment over time within sites, people were interviewed at different points in their trial journey. Interviews took place over 16 months, between May 2016 and September 2017.
Tables 11 and 12 summarise participant recruitment numbers in each region (patients and providers, respectively) for the qualitative substudy. According to baseline numbers, 17% of participants in the trial were interviewed, which increased to 22% when considering numbers at the 6-month follow-up. When looking at the 6-month follow-up time point, 16% of all intervention participants who completed participation, 13% of control group participants and 40% of those who did not or could not continue participating in the intervention were interviewed.
| Region | Control group (n) | Intervention group (n) | Drop-outs from intervention group (n) | Total interviewed (n) | Total per region at baseline (n) | Percentage of baseline numbers interviewed per region |
|---|---|---|---|---|---|---|
| London (two sites: Dulwich Community Hospital main unit and satellite unit) | 6 | 6 | 4 | 16 | 154 | 10 |
| Central Scotland (two sites: Queen Elizabeth University Hospital, Glasgow, and Inverclyde Royal Hospital, Greenock) | 2 | 6 | 14 | 22 | 29 | 76 |
| North Wales and North West (one site: Bolton Renal Satellite Unit, Farnworth) | 4 | 2 | 1 | 7 | 73 | 10 |
| East Midlands (one site: Royal Derby Hospital, Derby) | 3 | 7 | 1 | 11 | 52 | 21 |
| West Midlands (one site: Castle Vale Satellite Renal Unit, Sutton Coldfield) | 3 | 6 | 0 | 9 | 14 | 64 |
| Total interviewed | 18 | 27 | 20 | 65 | 322 | 20 |
| Region | Consultant (n) | Pedal employee (n) | Nurse manager/advanced practitioner (n) | Nurse (n) | Health-care assistants (n) | Total (n) |
|---|---|---|---|---|---|---|
| London (two sites: Dulwich Community Hospital main unit and satellite unit) | 1 | 2 | 2 | 3 | 3 | 11 |
| Central Scotland (two sites: Queen Elizabeth University Hospital, Glasgow, and Inverclyde Royal Hospital, Greenock) | 1 | 2 | 3 | 1 | 2 | 9 |
| North Wales and North West (one site: Bolton Renal Satellite Unit, Farnworth) | 1 | 1 | 1 | 2 | 1 | 6 |
| East Midlands (one site: Royal Derby Hospital, Derby) | 1 | 3 | 0 | 1 | 4 | 9 |
| West Midlands (one site: Castle Vale Satellite Renal Unit, Sutton Coldfield) | 1 | 1 | 0 | 2 | 0 | 4 |
| Total interviewed | 5 | 9 | 6 | 9 | 10 | 39 |
Trial participants included in the qualitative substudy included 26 women (46%) and 31 men (54%). There was substantial diversity in terms of age, months since starting dialysis and ethnicity. There was a mean age of 60 years, with a mean HD vintage of 43 months (data unavailable for 14 participants). Most people were receiving HD for 4 hours (range 3.5–5.0 hours) three times per week. Ethnic diversity among participants was greatest in London, the West Midlands, and North Wales and North West; it was lowest in Central Scotland and at the site of interviewing in the East Midlands. Where ethnicity was noted, there was representation from Asian British (Pakistani and Indian), black Caribbean and white British people.
People were interviewed at different points in their trial journey, at a mean of 11 months from consenting to participate in the trial and 8 months from their baseline assessment.
Explanatory theory and supporting thematic analysis
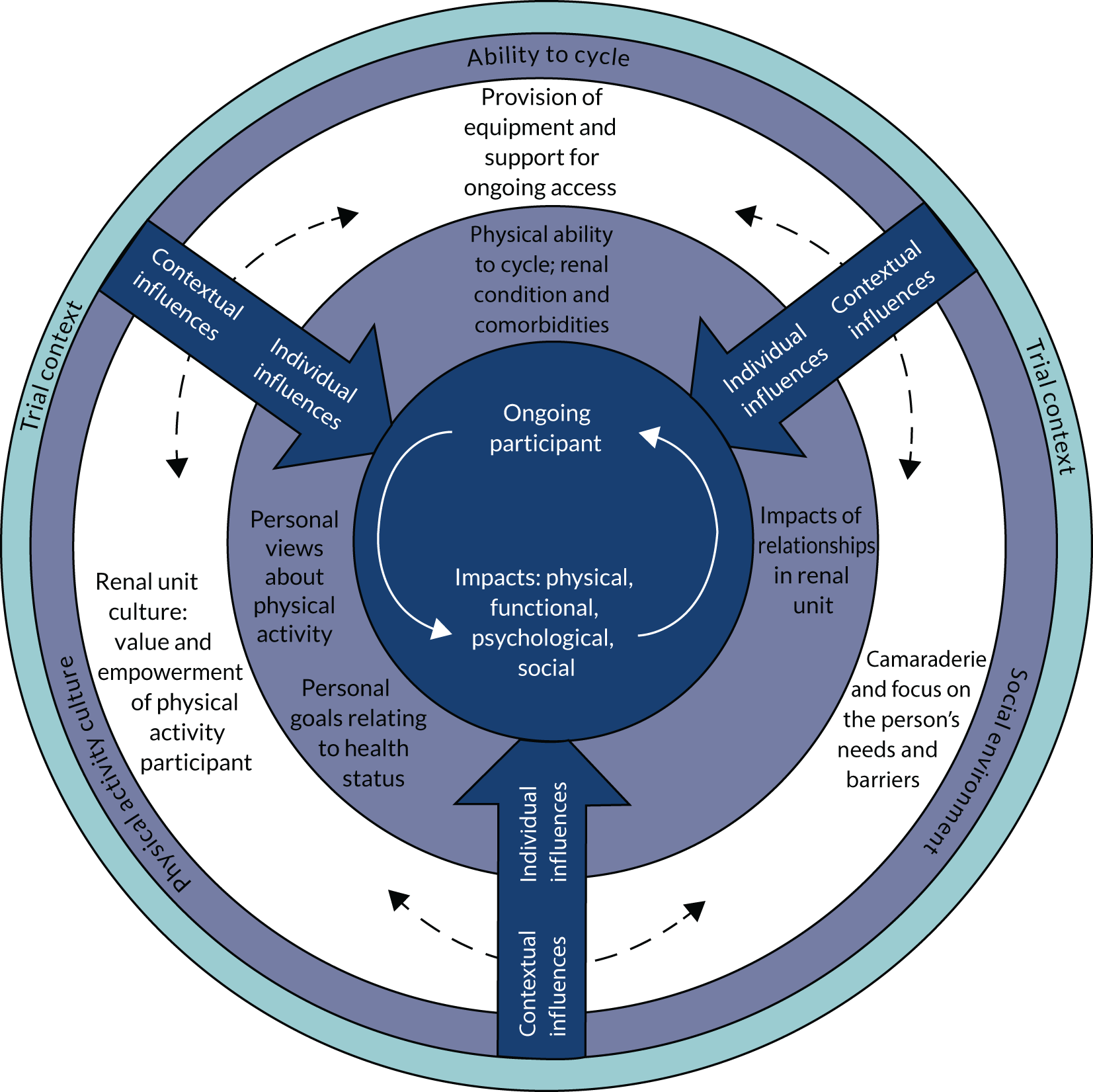
Analysis of the substantial quantity of data resulted in an explanatory model, depicted in Figure 3. This will be both explained and justified, supported by illustrative data. Explanation will move from the centre of the figure outwards, focusing first on the outcomes when people sustained participation in the intervention over time. The individual influences on decision-making about participation over time will then be explored, followed by contextual influences. Finally, an explanation of how the RCT context of participation affected decision-making will be addressed. Table 13 provides theme names and definitions.
FIGURE 3.
Explanatory theory relating to individual-decision-making about starting and continuing participation in exercise while on HD.
| Theme/subtheme | Definition |
|---|---|
| Reciprocal influences between impacts of participation and ongoing participation in cycling while receiving HD | |
| Impacts of participation | |
| Positive physical impacts | Positive physical impacts of intervention participation, including more energy, increased time on bike, increased strength, reduced weight, increased walking time, improved appetite, improved circulation, feeling fitter, improved stamina, reduced sickness, less breathlessness and help with restless leg syndrome |
| Positive functional impacts | Positive functional impacts of intervention participation, including increased mobility, improved sleep pattern and improved balance |
| Positive psychological impacts | Positive psychological impacts of intervention participation, including hope, feeling happier, increased self-esteem, passing the time and distraction from negative thoughts, enjoyment of routine, feeling normal again, increased confidence, feelings of improving health, sense of pride and becoming more independent |
| Positive impacts on participation in life | Positive impacts of the intervention on participation in life, including increased physical activity, increased social participation, increased interaction and competition with others, and perception of a safe space to exercise |
| Positive impacts on physical activity when outside the renal unit | Incorporating home exercise and other activity into daily routines attributed to intervention participation, including greater walking distance, cycling, getting out and about, sports participation, feeling more energy and stamina, and being able to take on more roles in the family |
| Enjoyment of intervention participation | Enjoying the experience of participation in the intervention |
| Negative experiences of intervention participation | Barriers to participation, including negative experiences of the intervention, such as exacerbation of fatigue and depression, reduced blood pressure, feeling that exercise is a ‘shock to the system’, lack of consistency in accessing the bike, feeling a lack of progression in fitness, lack of interaction with others while on the bike, musculoskeletal pain, and uncomfortable/unsafe bike |
| Individual influences on decision-making: physical activity culture, ability to cycle and social environment | |
| Physical activity culture: personal views and goals and social support relating to physical activity and health status | |
| Positive influence on continuing to cycle | |
| Personal drive for physical activity and exercise | Motivators for ongoing participation, including a drive to participate in physical activity or exercise because of previous and/or current participation, interest in improving health, and self-discipline relating to participation (note that this was influenced by feeling that participation is making a difference and seeing improvements in the health of other participants) |
| Family support | Person having family support for involvement in the intervention, positive attitudes towards exercise, and feedback about positive perceptions of intervention impacts |
| Ability to cycle: physical ability to cycle, renal condition and comorbidities | |
| Barriers to continuing to cycle | |
| Renal condition: ‘bad days’ | ‘Bad days’, during which symptoms of the person’s renal condition feel worse and the person requires more sleep, rest and support from others |
| Comorbidities | Having comorbidities |
| Became unwell | A change in health status, for example involving blood pressure, fluid overload or musculoskeletal pain |
| Social environment: impacts of relationships in the renal unit | |
| Positive impacts on continuing to cycle | |
| Motivational role of staff | Motivational influences of staff affected by their attitudes to exercise, the trial and skills |
| Camaraderie in the unit relating to cycling | Camaraderie, competition and encouragement between people within the renal unit, including patients and staff |
| Contextual influences on decision-making: social environment, physical activity culture and ability to cycle | |
| Social environment: supportive, proactive culture in the renal unit | |
| Positive impacts on continuing to cycle: supportive social environment | Relationships with staff and patients in the renal unit that demonstrate a supportive culture |
| Attitudes towards empowering and enabling renal patients | Contrasting attitudes, approaches and barriers to empowering and enabling renal patients in relation to self-care |
| Renal unit proactive about change | A renal unit having a culture of being proactive about change |
| Physical activity culture in the renal unit: different degrees of expectation, value and empowerment of physical activity participation | |
| Staff enthusiasm about physical activity and exercise for patients | A positive attitude to physical activity/the intervention for renal patients owing to justification/rationale, prior experiences, beliefs that it is necessary for management of renal disease, personal beliefs about physical activity/the intervention, and noticing positive impacts for people |
| Expectations of renal unit staff about engagement of patients with exercise | Beliefs that people who are exercising will lose interest or prefer sedentary activities |
| Ability to cycle: provision of cycling equipment and support for ongoing access | |
| Barriers to ongoing participation: Lack of opportunities to cycle | Structural barriers meaning that the exercise bike has not been available at a suitable time |
| Themes relating to the trial context | |
| Motives to consent: reasons that people give for their decision to consent to the trial | |
| Giving back (positive for continuing trial participation unless allocated to intervention group) | Decisions to consent were influenced by the desire to ‘give back’ – improving knowledge and helping others, including the next generation |
| Social influences (positive for continuing unless allocated to intervention group) | Decisions to consent were influenced by the community in the dialysis unit and trust in the people promoting the study and family |
| Invested in physical activity and exercise (positive for continuing trial participation unless allocated to control group) | Decisions to consent were influenced by having been active (cycling and other) in the past and being currently invested in achieving health-related benefits, including those relating to renal condition (e.g. preparation for life-saving transplant, losing weight, reducing breathlessness) and broader needs such as increasing mobility, circulation and playing a more active role in treatment |
| To pass the time | Decisions to consent were influenced by a desire to find a different/more useful way to pass the time, or as a distraction |
| Affordable exercise (positive for trial participation unless in control group) | Decisions to consent were influenced by the fact that it presented an opportunity to exercise with no cost |
| Barriers to consent: explanations of negative influences on decisions to consent to trial participation | |
| Conflicting priorities | Negative influences from conflicting priorities on a decision to consent, including a desire to protect ‘good days’ away from the renal unit and concerns about whether or not cycling would negatively affect HD |
| Disinterest in exercise | Negative influences from disinterest in exercise on a decision to consent, including disinterest that may have been influenced by a lack of education about exercise or a belief that exercise is not an important part of life |
| Not fit to exercise | Negative influences from fitness to exercise on a decision to consent (or ability to be included), including being too overloaded with fluids, having comorbidities, reduced strength or reduced mobility |
| Psychological barriers | Negative influences from psychological barriers on a decision to exercise, including patient perception that their condition means that they cannot exercise |
| Experiences of the control group: descriptions of experiences and views relating to being allocated to the control group in the trial | |
| Little change to activities | Having made no obvious changes to physical activity levels during the trial |
| Willing to help | Positive descriptions of willingness/pleasure in being able to contribute because of perceptions of the trial’s value/importance, and ability to continue with prior routine physical activity |
| Barriers to continuing trial participation: descriptions of barriers to ongoing trial participation | |
| Disappointment about being in the control group | Disappointment and sadness about being allocated to the control group, including because the participant was invested in increasing their physical activity, exercise and/or mobility |
| End of exercise period | Frustration at having to stop cycling at the end of the specified intervention period |
| Renal unit engagement with the trial: descriptions of influences on the renal unit’s engagement with the PEDAL trial | |
| Negative views about the PEDAL trial/barriers to engagement described by renal unit staff | Feeling too busy or short staffed to deliver the intervention, or the intervention not being a priority owing to conflicting priorities, or there being a lack of physical space |
| Positive views about the PEDAL trial/facilitators of engagement described by renal unit staff | Interest in the study question and intervention and in research more generally, and hope for better patient outcomes |
Impacts of pedalling while on dialysis
The final explanatory model (see Figure 3) provides the key outcomes in the centre; these were the aim of the intervention and were described very well by multiple participants who were able to sustain participation in both the trial and the intervention. Impacts included positive physical effects, such as more energy; increased fitness, stamina and strength; weight reduction; improved circulation; and reduced shortness of breath, symptoms from dialysis and restless leg:
Well, a bit more energy about me, certainly, than what I had a year ago. I’m getting less of the . . . problems you might get after you dialyse. When I was doing it [dialysis] first I was still getting . . . quite sick . . . but as this has gone on it’s improved. And I’m sure that the exercise must be part of that, you know, it must be helping.
Central Scotland, intervention participant 1
This resulted in functional improvements for some, such as increased mobility and balance and better sleep patterns. This was described both by people who completed the intervention and by people who did not or could not continue for the whole period for different reasons such as comorbidities:
Noticing the change in me . . . walking, going up the stairs, coming down the stairs. It’d become so difficult for me. But after exercise it became easier and easier.
North Wales and North West, participant who did not continue intervention 3
A variety of positive psychological impacts were described, including feeling happier and more hopeful, having feelings of pride and having increased confidence and self-esteem. Participants felt that time passed faster, less time was spent with negative thoughts and they enjoyed the routine, and they felt more ‘normal’ and independent again. One participant explained:
It’s been a really, really good journey. Should I say, I’ve had many ups and downs in that study. But, I mean, now I’m feeling myself, so I’m much more confident and much quicker . . . because I know if I can go on to the bike for so long and on a certain resistance . . . I’m getting a bit stronger. So that has basically boosted my confidence.
North Wales and North West, intervention participant 3
Participants and providers described increased participation in physical activity when outside the renal unit and greater participation in life more generally, which they attributed to intervention participation:
We’ve got a treadmill at home, hardly used it. And now I’m on it, ‘cause I asked [a PEDAL Trial employee] what’s the best way to . . . so he did explain and that’s helping me a lot as well.
North Wales and North West, intervention participant 1
It was also evident that there was a feedback loop between the experience of these positive impacts, enjoyment of the experience overall and the motivation to continue participating in exercise during dialysis. Some people who did not continue the intervention felt this and were disappointed to have to stop:
Yes, I felt it was doing me good. I was actually enjoying it.
Central Scotland, participant who did not continue intervention 1
Impacts of cycling were not all positive, with some describing feeling uncomfortable or unsafe on the bike, that there was a lack of interaction with others and that exercise exacerbated fatigue and depression and other co-existing conditions such as low blood pressure and musculoskeletal pain. For some people this was a barrier to continuing intervention participation, whereas others completed the intervention.
People in the control group did not describe these positive or negative feelings or experiences purely from taking part in the trial. Some had participated to give back and experienced related satisfaction but did not discuss the kinds of physical, functional, psychological and social impacts of participating in the trial that were discussed by those experiencing the intervention.
Individual influences on decision-making: physical activity culture, ability to cycle and social environment
In the explanatory theory (see Figure 1) there is an individual layer of influence on decision-making and a further contextual layer in the overall background of the RCT scenario. In the individual and contextual layers there are interacting aspects of the beliefs and culture relating to physical activity, ability to cycle and social influences.
The patient’s physical activity culture – or personal views, goals and social support relating to physical activity and their health status – affected their decisions to continue cycling. Motivators included a drive to participate in physical activity or exercise due to previous and/or current participation and interest in improving personal health outcomes or goals:
Well, I used to do a lot, I used to go to the gym quite frequently, but because I’ve got polycystic kidneys and pressure on my lungs it makes it harder for me to do . . . I try to find alternative ways to exercise, so cycling and walking’s helping quite a bit.
East Midlands, intervention participant 4
Ability to cycle mattered as well, and most frequently manifested as a barrier to participation (i.e. as a lack of ability to cycle). The highly variable nature of renal symptoms, influenced by dialysis days, affected whether or not people were having ‘good days’ or ‘bad days’. This was more likely to affect the degree of exercise participation over time during dialysis sessions. By contrast, comorbidities and changes in renal health status, such as fluid overload, blood pressure changes and musculoskeletal pain, were more likely to affect a person’s ability to continue the intervention:
When it got to the time to do it I discovered I had the problems with my hips, which is why I couldn’t do the cycling.
East Midlands, participant who did not continue intervention 3
Relationships with other patients, renal unit staff and PEDAL trial staff also affected decisions to continue cycling. The motivational role of the person directly supporting cycling was very important on a day-to-day basis:
I talk to them about the benefits of cycling and I remind them of how they felt last time, because I find generally patients after they’ve cycled do feel good and they enjoyed it and they’re feeling happier with themselves – and then it’s kind of negotiating . . . I do try to be as encouraging as I can but not pushy . . . showing a bit of empathy.
London, provider 1
Camaraderie, competition and encouragement between patients in the renal unit influenced some people, more often where there were multiple people situated together in a bay or in a longer ‘ward’ layout. This was described as a gap where people were in side-rooms:
They make jokes about it and we’ll say, ‘oh, are you on your bike today?’ You know, ‘where have you been today?’ and things like that . . . The rest of the group were quite encouraging . . . people were encouraging her.
Central Scotland, provider 4
This camaraderie linked strongly with the contextual layer of the theory: a supportive, proactive culture in the renal unit.
Contextual influences on decision-making: social environment, physical activity culture and ability to cycle
A supportive social environment in the renal unit was described at some sites, with positive relationships within the unit and culture of empowerment:
We’ve done lots of things down here that we’ve tried to motivate the patients with. I mean, we’ve done art in hospital, we’ve done lots of things, staff here are quite motivated.
Central Scotland, provider 2
A patient explained how important relationships are in the renal unit:
We sort of all really get to know each other, don’t we? And the staff are really supportive, really good. You’re trusting people with your life, because your fistula’s your life, isn’t it?
West Midlands, control participant 1
Another provider explained that, from her experience, she thought renal unit staff could empower or disempower patients unintentionally, through beliefs about ensuring safety, efficiency and patients’ capabilities:
Of course you can come in and do it yourself, and the biggest resistance I had was not the patients but the nurses and a lot of it came from . . . that maternal or paternalistic attitude of ‘I’ll do it and I know it’s done well, or it’s done properly, or I can get done quicker’ . . . you know, you just want to do it right for them, so I do think that creates an awful lot of it, because they’re with us for a long time, and if we don’t instil that confidence in them that you can do it, then the patients aren’t going to believe that they can do as well.
North Wales and North West, provider 1
Some renal units described having a proactive attitude to change:
We like embracing new things on the unit . . . It’s new at first but you adjust to it, no trouble – we seem to do that here.
North Wales and North West, provider 5
The social culture of the renal unit also linked with attitudes towards physical activity participation and engagement with the intervention. Staff enthusiasm about physical activity and exercise as necessary for renal patients was important and related to their awareness of the rationale for it, prior experiences of exercise for renal patients, personal beliefs about physical activity and exercise as beneficial for all, and noticing positive changes in participants during the trial. One provider explained:
I would love to see a lot more exercise on the unit. I want to see my patients feel better and have better outcomes. I certainly see it as one of those things that could be good for that.
East Midlands, provider 9
Some providers described having initial scepticism about whether or not renal patients would engage with the intervention:
Prior to starting I’d say that I had . . . if anything, sceptical as to what the impact would be . . . They’re not the most active bunch, renal patients.
East Midlands, provider 5
This may also have interacted with participants’ ability to cycle, owing to structural barriers affecting whether or not the exercise bike was available at a suitable time:
Sometimes it does get forgotten, and part of that is being busy as well, and short staffed.
Central Scotland, provider 3
One participant explained:
Well, everybody just encouraged me . . . patients and staff. Oh aye. Most of the staff managed to put me on. But there was a few just didn’t know how to and they kept saying I need to learn how to do this.
Central Scotland, intervention participant 2
Influences of the randomised controlled trial context on decision-making
It is important to remember that the context of participating in a RCT underlies the decision-making about both starting and continuing to participate in the intervention group and control group.
It was reassuring from a trial perspective that the evidence suggested that participants in the control group did not increase or decrease their physical activity or exercise during the trial period. However, allocation to either the control group or the intervention group had an impact on people in other ways that affected their willingness to continue participating. People gave different reasons for consenting to participate in the trial. Some people wished to ‘give back’ because they felt that they had received so much from the health system and wished to improve knowledge and help people receiving dialysis in the future. Others wished to participate to pass the time, as they found being in the renal unit boring. Both of these motivators were valuable if a person was allocated to the control group, because they might be less invested than someone in the intervention group of the study. For example, as one control group participant explained:
It’s good to be doing something rather than just sitting here doing nothing. The daytime telly’s not very good, you know.
East Midlands, control participant 2
If allocated to the intervention group, however, a person may have had less motivation to exercise than others in the intervention group. Some people consented to the trial because of social influences, for example seeing others in the unit benefitting from participation. This again was positive if the person was allocated to the intervention, but less so if allocated to the control group:
So one or two of my co-patients, they volunteered, and they went ahead. So I said, why should I be left out?
East Midlands, intervention participant 3
A strong motivation to consent involved interest in physical activity and exercise due to having been active in the past, and currently being invested in achieving health-related benefits, including those relating to renal condition and broader health and mobility needs. Some people wished to lose weight specifically to increase their likelihood of transplant. These were emotionally loaded reasons for participation and meant that a person was very affected by their randomisation:
It’s unfortunate the randomisation of the exercise to control or exercise, and it disappoints, that’s another thing that we have to take into consideration is somebody’s just been turned down and you’re exercising somebody in the next bed to them. How do you keep them interested in the trial for 9 months now?
North Wales and North West, provider 4
A participant reinforced this:
. . . if I’m honest I’ve been a bit disappointed because I wasn’t selected to do the cycling and a lot of people who were selected don’t seem to want to do it. I’m one person who actually did and I was keen to do it, but I just wasn’t picked, so I’ve been really disappointed about that.
London, control participant 2
In addition, it is important to remember that the trial context formed a barrier for some people who were interested in exercising but did not wish to attend assessments, particularly on non-dialysis days. This was complex in relation to conflicting priorities, because people valued their ‘good days’ when not in the renal unit and were sometimes living at a substantial distance from the location for trial assessments:
The difficulty with the unit here is that we have a fair number of patients who have extended travel times to get here because they don’t live on the mainland and they have to catch the ferry. And what the study protocol dictates is that they have to come on a non-dialysis day to have their assessment. And during the study it was particularly challenging trying to explain this to the patients. Particularly those who were, you know, there were a fair number who were unhappy about having to attend for assessment if they didn’t get the intervention.
Central Scotland, provider 4
One provider stated:
A lot of patients are asking can they not be on the study and still use the bikes, so it’s like a gym on dialysis . . . There are a few that have said they don’t want to be on the study, but can they use the bike?
East Midlands, provider 6
Another issue relating to the trial context is that in all but one site the intervention was supported by a dedicated PEDAL trial employee. In the site at which the renal unit supported intervention provision, a provider described difficulties in prioritising intervention provision at busier times. This could affect the ability of the person to cycle, owing to access. It is important to note, however, that multiple providers at other sites explained that, although often enthusiastic about the intervention, they would not be able to provide it without dedicated staff after completion of the trial:
My ideal scenario would be to have a physio[therapist] that would come in and work with patients, not just doing PEDAL but doing maybe other stretches and things for renal, that would benefit renal patients while they’re on dialysis and that would take the nurses out of it.
Central Scotland, provider 3
Another provider stated:
We have got quite a lot of work coming in . . . and sometimes there’s a staff shortage and . . . I don’t think we’ll be able to support them . . . and if there is nobody to initiate that programme then I don’t think it’s going to go very far.
North Wales and North West, provider 6
Interlinkages between influences and impacts: explanatory theory
Data clearly support positive impacts from cycling during HD where a person is fit to do so and able to continue, and these impacts motivated people to continue participation. The trial context affected whether or not some people decided to take part because, although interested in exercise, they did not wish to attend assessments on non-dialysis days. The trial context also influenced whether or not people continued participation in either the control group or intervention group depending on their reason for consenting to participate.
Where people participated in exercise, their willingness and ability to continue was affected by their own individual capability to do so, their attitudes, beliefs and family support and their relationships with those around them in the renal unit. The motivational roles of other patients and PEDAL trial and renal unit staff cannot be underestimated, and availability of this support was influenced by the culture and beliefs in the unit. A positive culture of involvement and empowerment was important. This was particularly well harnessed by one PEDAL trial employee who was able to enlist the support of all staff in the unit, including medical, nursing and support staff. The impact of this was a unit where all were encouraging those cycling, resulting in a sense of enthusiasm and camaraderie. This person was also highly strategic in motivating others to consent to the trial, for example by building relationships with patients and asking the person who volunteered first to move bays and influence others into participating through role modelling.
Discussion
Results of the qualitative substudy are largely consistent, with the few existing qualitative studies exploring health-care provider and patient experiences of intradialytic exercise. The current study findings go further in relation to theory generation; therefore, it is possible to make some more in-depth suggestions for further development. The literature search conducted early in the trial was repeated in June 2020 [searching PubMed, MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycInfo® (American Psychological Association, Washington, DC, USA) and SPORTDiscus (EBSCO) using the search terms ‘exercise’ and ‘haemodialysis’ and ‘qualitative’, with no limiters] and no further studies were found.
Young et al. 39 conducted a study in the UK of patient and staff perceptions of intradialytic exercise. They carried out focus groups with 24 patients and 9 staff before implementation and semistructured interviews with 11 exercising patients and 8 staff members 6 months later. Thompson et al. 40 interviewed 25 patients and 11 staff in Canada to explore perceptions of intradialytic exercise. Heiwe and Tollin41 conducted individual interviews in Sweden with six men and four women who were experiencing implementation of intradialytic cycling. There was a focus in the studies of Young et al. 39 and Heiwe and Tollin41 on identifying barriers and motivators in the data; both looked at the stage of deciding whether or not to take part, and the former also looked at experiences 6 months later.
There were multiple common themes between the PEDAL trial themes and those in previous studies. In particular, Young et al. 39 found similar functional, psychological and physiological benefits, and viewed these as providing positive feedback that enhanced ongoing participation. Thompson et al. 40 found social benefits but did not focus on analysis of the impacts of exercise. Heiwe and Tollin41 conducted their interviews in the early stages of implementation and found that participants were motivated partly by a desire to pass the time and felt that this was a good opportunity for exercise. In addition, they noted that the positive experiences of well-being benefits fed into ongoing participation, which was evident in our study.
There were common barriers that revolved around staffing and workload, limited resources and concerns over roles and appropriate training or expertise. 39,40 Interestingly, these three studies delivered the intervention with existing staffing: kinesiologist and nursing staff,40 nursing staff41 and physiotherapist staff. 41 By contrast, in all but one of the renal units represented in the current study, a dedicated PEDAL trial employee delivered the intervention. The renal unit in the PEDAL trial that provided its own support for the intervention shared the workload of study implementation between staff and discussed similar barriers to the previously published studies. Staff in the other renal units commented on the necessity of dedicated staff time. This may relate also to a theme that was common to all studies: the importance of motivation, encouragement, reinforcement, feedback and support from staff to facilitate ongoing participation.
In the context of qualitative data collection and analysis, evidence of support from similar, previous studies provides further evidence of credibility and trustworthiness of the results, so the similarities in themes from different countries and contexts are encouraging. Our study was, to our knowledge, the largest of its kind by far, and had quite diverse participants, which provides valuable triangulation.
The results of this qualitative substudy clearly support the value of exercising while on dialysis for those who can. We also have useful insights about how to enable people to continue participation over time, both from feed forward of the positive impacts and through complex social and cultural dynamics in the renal unit. It is important to consider the insights of analysis for ongoing implementation of the intervention. The results suggest that a renal unit is an integrated system, with complex interdependencies and mutual influences on expectations, beliefs and attitudes. Staff and patients are intertwined as a community and, when considering changing complex behaviours, it is important to remember this. It would be useful to analyse the findings of this study in relation to current behaviour change theory as a secondary analysis, for example using the capability, opportunity, motivation, behaviour (COM-B) behaviour change model, which emerged from the behaviour change wheel of Mitchie et al. 42 Interestingly, Young et al. 39 considered the importance of influencing behaviours of staff as well as of patients; they recommended staff training and patient education to increase engagement. We suggest that the need for change goes beyond knowledge to culture; renal units with proactive and relationship-focused cultures appeared to engage people in the intervention in different ways, which may be more sustainable in the long term. In the future it would be valuable to explore ideas around ways in which providers empower and enable, how this is influenced by leadership and context and how this affects changes in the behaviours of people engaging in and sustaining interventions such as exercising while on dialysis.
Conclusions
The explanatory theory developed through the qualitative substudy of the PEDAL trial has provided in-depth insights into the influences on people’s decision-making relating to participation in both the trial and the intervention over time. Themes are supported by the few previous qualitative studies; however, our data are more extensive, and the resulting theoretical explanation goes further to enable suggestions for future implementation and intervention sustainability. A novel finding relates to the importance of culture, and leadership to enable this, in the renal unit. Providers interviewed felt that the trial did not impinge much on their jobs, which supported trial implementation; however, this may not be conducive to sustainability of the intervention beyond the trial. Moving forward beyond the PEDAL trial, it will be most important to influence the attitudes, beliefs and behaviours of providers. Where providers are engaged and empowered, they are much more likely to engage and empower patients in participating. Once a patient is participating (where capable), the numerous physical, functional, psychological and social benefits are likely to support their ongoing participation. This in itself may then feed forward into a positive, proactive culture in the renal unit. Further research is needed to explore the complexities of intervention implementation in the longer term, with a view to aspects of behaviour change and insights from implementation science.
Chapter 6 Discussion and conclusions
Summary of main findings
The aim of the PEDAL trial was to assess clinical effectiveness and cost-effectiveness of a 6-month intradialytic exercise programme on QoL, compared with usual care for HD in the UK. The PEDAL trial is novel in that it is the first, to our knowledge, to evaluate intradialytic exercise as would most likely be delivered nationally, should NHS commissioning include exercise training as part of the service specification for in-centre HD. Exercise training was delivered by physiotherapy assistants (band 4 technical instructors), supported by regional co-ordinators who were physiotherapists or exercise physiologists. Thus, the PEDAL trial evaluated the most realistic yet safe exercise rehabilitation intervention possible in this population.
The baseline demographic data of the PEDAL trial cohort broadly aligns with the ‘real-world’ dialysis population in the UK as detailed in the latest data from the UK Renal Registry43 and also the PIVOTAL (Proactive IV iron Therapy in haemodiALysis patients) trial, a recent, large interventional study conducted in UK renal units. 44 Baseline age, sex, BMI, blood pressure, diabetes prevalence and the proportion of current smokers are all similar to UK Renal Registry data. 43 However, the current study included a larger proportion of black African and black Caribbean participants, and a lower proportion of white participants, than is representative of the broader prevalent dialysis population in the UK. The baseline data are similar to most dialysis populations worldwide. 45–48 There are some differences in rates of diabetes prevalence, with lower rates in our patient cohort than in dialysis populations in the USA and Asia, which may limit application of the results to all other dialysis populations.
As delivered, the PEDAL programme did not improve the QoL of the whole sample, as assessed by the KDQoL-SF PCS (p = 0.055), nor did it improve QoL as assessed by the secondary outcomes of the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) or KDQoL-SF multi-item scales. In the whole sample there were no statistical or absolute changes in functional capacity, physiological variables, anthropometric measures or clinical measures. More than half (58%) of patients temporarily halted receiving their exercise intervention for a continuous period of > 2 weeks during the study; in total, patients completed less than half [47%, interquartile range 28–77%] of exercise sessions that were prescribed. These data suggest that the HD patients had poor compliance with the exercise intervention. The nested qualitative study revealed that patient views relating to change in health status, having comorbidities and having days where there was an increase in symptoms such as tiredness were possible reasons for not engaging with the exercise intervention. Because predominant themes were the ‘exercise culture’ and camaraderie that results from exercising with others, a cluster randomisation design may perhaps have fostered this camaraderie on a whole renal unit level, preventing the perception of participants feeling ‘left out’ if they were allocated to the control group.
We chose the KDQoL-SF instrument because of its validity in CKD patients49 and inclusion of a generic core that has been widely used in CKD and other populations. The lack of effectiveness of the PEDAL programme in the whole sample on PCS score warrants comparison with previous studies. A Cochrane review9 completed in 2011 concluded that exercise was beneficial for QoL in CKD patients but, unfortunately, no meta-analysis or risk-of-bias assessment was performed on this outcome measure. Furthermore, many of the included studies were not representative of the HD population because they investigated transplant,50 pre dialysis51 or ambulatory peritoneal dialysis52 patients. More recent meta-analyses have concluded positive effects of exercise but have shown significant effects on the role physical subscale only,53 showing no effect on PCS score,18 or did not analyse this outcome owing to insufficient studies. 13,54 Other meta-analyses have included non-randomised studies53 or rated studies as at high risk of bias and showing considerable heterogeneity. 14,17,19,54 Previous meta-analyses have also included extradialytic exercise programmes19,54,55 or studies that have delivered progressive resistance training as opposed to aerobic cycling alone. 10,15,16,19,20,56 In this regard, one meta-analysis22 usefully compared aerobic exercise, progressive resistance training and combined exercise; only progressive resistance training increased PCS score. Detailed analysis of the very few empirical studies included in reviews that do show positive effects of aerobic intradialytic exercise on QoL reveals that they have often utilised unrealistic interventions that would be difficult to implement in routine care. For example, Ouzouni et al. 57 investigated an exercise programme similar to the PEDAL programme [intradialytic cycling plus TheraBands (TheraBand, Akron, OH, USA) and ankle weights] but three physiologists were employed to intensely supervise only 10 patients for 10 months to obtain a significant effect. Taken together with the results reported herein, it is highly unlikely that clinically implementable intradialytic aerobic exercise training per se increases QoL, particularly at a whole-population level.
Nevertheless, there was some evidence of benefits to KDQoL-SF-assessed QoL and aerobic capacity in patients who completed > 30% of prescribed exercise sessions. Participants, clinicians and researchers continue to advocate intradialytic aerobic exercise. The PEDAL trial uniquely assessed the cost of delivery of its intervention using careful recording of harms and using health economic methods. The number of hospitalisations, all-cause mortality and cardiovascular mortality were not different between the groups. Although these results should be interpreted cautiously because of the lack of events for these secondary outcomes, there was no obvious increase in SAEs in the intervention group. The economic cost of delivering the PEDAL intervention ranged from £882 to £1866 per patient per year (depending on pay band of the physiotherapy assistant, whether or not London weighting was applied and staff–patient ratio). This calculation assumed that intradialytic exercise would be offered as part of a general physiotherapy service (with enough capacity to provide absence cover), with costs profiled throughout the programme (e.g. exercise equipment cost is shared throughout its service life), with patients who are 100% compliant and with physiotherapy assistants supervising between 6 and 10 patients per dialysis session without incurring any travel costs. For context, the cost of delivery of HD in the UK is approximately £35,023. 58 Because the PEDAL trial was not clinically effective at a whole-sample level, whether or not the cost of delivery of intradialytic exercise is justified to enhance patient choice remains a matter for debate. Because the observed benefits to KDQoL-SF-assessed QoL may be due to an experimenter effect, a similar improvement in QoL to that achieved herein could arguably be achieved with less cost and less effort through one-to-one patient interactions and provision of patient education resources on physical activity.
The PEDAL trial aimed to address a criticism of previous exercise studies that recruited only patients with high physical health status by having more inclusive inclusion criteria. Perhaps including patients with lower physical health status prevented benefits of the exercise intervention being realised. However, this explanation is unlikely because our sample is like other pragmatic exercise studies in CKD patients (e.g. in the seminal study of Painter,59 baseline PCS score was 36 AUs). Besides, poor baseline physical health status is not a reason for lack of response to exercise intervention, because patients with poorer PCS scores respond better to intervention than those with higher PCS scores. 7
Health economic evaluation
The economics evaluation suggests that, over 6 months of implementation, the PEDAL intervention resulted in higher NHS costs with no gain in QoL as measured by the EQ-5D-5L. A number of factors should be borne in mind; for example, the benefit from the PEDAL intervention could be avoiding cardiovascular events and other illnesses in future years, and the 6-month time horizon would not capture this. In addition, the PEDAL protocol could be refined to target different patients on dialysis and/or to include a time point at which the benefit of the intervention was assessed with only those reporting a good QoL gain continuing. Both of these could potentially improve the cost-effectiveness of the intervention.
Qualitative analyses of patient views
The explanatory theory developed through the qualitative substudy of the PEDAL trial has provided in-depth insights into the influences on people’s decision-making relating to participation both in the trial and in the intervention over time. Themes are supported by the few previous qualitative studies; however, our data are more extensive, and the resulting theoretical explanation goes further to enable suggestions for future implementation and intervention sustainability. A novel finding relates to the importance of culture, and leadership to enable this, in the renal unit. Providers interviewed felt that the trial did not impinge much on their jobs, which supported trial implementation; however, this may not be conducive to sustainability of the intervention beyond the trial. Moving forward beyond the PEDAL trial, it will be most important to influence the attitudes, beliefs and behaviours of providers. Where providers are engaged and empowered, they are much more likely to engage and empower patients in participating. Once a patient is participating (where capable), the numerous physical, functional, psychological and social benefits are likely to support their ongoing participation. This in itself may then feed forward into a positive, proactive culture in the renal unit. Further research is needed to explore the complexities of intervention implementation in the longer term, with a view to aspects of behaviour change and insights from implementation science.
Limitations
The study was designed to assess a pragmatic, clinically implementable intradialytic exercise intervention. By design, the study relied on a patient-reported outcome measure for its primary outcome; it is recognised that the primary limitation of this study was the lack of an attention control group. To alleviate this concern, multiple outcomes were assessed, and objective measures were obtained whenever possible; only objective measures of habitual physical activity were not of sufficient quality to be presented in this manuscript (owing to poor patient compliance with wearing accelerometers). In addition, many patients withdrew from the study (following randomisation but before baseline, and also during follow-up) or were too unwell to complete all functional capacity and physiological outcome assessments. This particularly affected the assessments of peak aerobic capacity but is to be expected in a study with inclusive inclusion criteria and arduous outcome assessments. Finally, the study was not powered to detect differences in some secondary outcomes, including mortality. Nevertheless, we reported these data to allow a balance of benefits and harms to be assessed. Future studies should address these concerns by including attention control groups and being adequately powered to investigate minimal clinically important differences of all outcome measures. Based on the findings presented herein, studies should address poor compliance by investigating exercise counselling and behaviour change interventions (such as motivational interviewing and self-determination theory) and should specifically target ways to overcome complications associated with concurrent medical events (such as fatigue). Investigators should consider alternative mechanisms of action of exercise interventions, including self-efficacy and stage of change. 43 Finally, the benefits of including progressive resistance training should be confirmed, even if extradialytic delivery is required.
Conclusion
A pragmatic intradialytic aerobic exercise programme, which could realistically be commissioned as part of routine care, did not improve QoL of this deconditioned sample of HD patients. The lack of clinical effectiveness and cost-effectiveness was probably due to poor compliance and only modest changes in aerobic capacity, particularly in a subgroup of patients with concurrent medical events and associated fatigue.
Future work
The benefits of longer interventions, including progressive resistance training, should be confirmed, even if extradialytic delivery is required. Future studies need to evaluate whether or not there are subgroups of patients that may benefit from this type of intervention, and also whether or not there is scope to optimise the exercise intervention to improve compliance and effectiveness. This should include further qualitative work.
Acknowledgements
We are grateful to the research assistants, physiotherapy assistants and research nurses who facilitated completion of this study.
Contributions of authors
Sharlene A Greenwood (https://orcid.org/0000-0001-9485-4645), Pelagia Koufaki (https://orcid.org/0000-0002-1406-3729), Jamie H Macdonald (https://orcid.org/0000-0002-2375-146X), Catherine Bulley (https://orcid.org/0000-0001-8338-5388), Ian Ford (https://orcid.org/0000-0001-5927-1823), Iain C Macdougall (https://orcid.org/0000-0001-9098-8611), Alice C Smith (https://orcid.org/0000-0002-9234-9060) and Thomas H Mercer (https://orcid.org/0000-0002-5078-4769) conceived and designed the study.
Catherine Bulley, Ian Ford and Claudia-Martina Messow (https://orcid.org/0000-0003-0082-9946) analysed the data.
Sharlene A Greenwood, Pelagia Koufaki and Jamie H Macdonald interpreted and contextualised the data and drafted the paper.
Sharlene A Greenwood, Pelagia Koufaki, Jamie H Macdonald, Catherine Bulley, Sunil Bhandari (https://orcid.org/0000-0002-0996-9622), James O Burton (https://orcid.org/0000-0003-1176-7592), Indranil Dasgupta (https://orcid.org/0000-0002-7448-2677), Kenneth Farrington (https://orcid.org/0000-0001-8862-6056), Ian Ford, Philip A Kalra (https://orcid.org/0000-0001-7652-1572), Mick Kumwenda (https://orcid.org/0000-0001-8343-1129), Iain C Macdougall, Claudia-Martina Messow, Chante Reid (https://orcid.org/0000-0003-2530-6025), Alice C Smith, Maarten W Taal (https://orcid.org/0000-0002-9065-212X), Peter C Thomson (https://orcid.org/0000-0003-4761-2648), David C Wheeler (https://orcid.org/0000-0003-0745-3478), Magdi Yaqoob (https://orcid.org/0000-0002-9904-127X) and Thomas H Mercer revised the paper.
Sandip Mitra (https://orcid.org/0000-0003-0389-636X) and Claire White (https://orcid.org/0000-0002-8908-8648) revised the paper.
All authors approved the final report.
Publication
Greenwood SA, Koufaki P, Macdonald JH, Bhandari S, Burton JO, Dasgupta I, et al. Randomized Trial - PrEscription of intraDialytic exercise to improve quAlity of Life (PEDAL) in patients receiving hemodialysis. Kidney Int Rep 2021; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Greenwood SA, Koufaki P, Macdonald JH, Bhandari S, Burton JO, Dasgupta I, et al. Randomized Trial - PrEscription of intraDialytic exercise to improve quAlity of Life (PEDAL) in patients receiving hemodialysis. Kidney Int Rep 2021. https://doi.org/10.1016/j.ekir.2021.05.034.
- Ashby D, Borman N, Burton JO, Corbett R, Davenport A, Farrington K, et al. Renal Association Clinical Practice Guideline on Haemodialysis. BMC Nephrol 2019;20. https://doi.org/10.1186/s12882-019-1527-3.
- Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 2005;45:690-701. https://doi.org/10.1053/j.ajkd.2004.12.013.
- Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 2004;65:719-24. https://doi.org/10.1111/j.1523-1755.2004.00411.x.
- Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int 2003;63:1843-51. https://doi.org/10.1046/j.1523-1755.2003.00931.x.
- Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003;41:1286-92. https://doi.org/10.1016/S0272-6386(03)00361-5.
- Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis 2000;35:482-92. https://doi.org/10.1016/S0272-6386(00)70202-2.
- DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 1997;30:204-12. https://doi.org/10.1016/S0272-6386(97)90053-6.
- Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011;10. https://doi.org/10.1002/14651858.CD003236.pub2.
- Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014;64:383-93. https://doi.org/10.1053/j.ajkd.2014.03.020.
- Cheema BS, Chan D, Fahey P, Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med 2014;44:1125-38. https://doi.org/10.1007/s40279-014-0176-8.
- Segura-Ortí E. Exercise in hemodialysis patients: a literature systematic review. Nefrologia 2010;30:236-46.
- Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 2011;16:626-32. https://doi.org/10.1111/j.1440-1797.2011.01471.x.
- Salhab N, Karavetian M, Kooman J, Fiaccadori E, El Khoury CF. Effects of intradialytic aerobic exercise on hemodialysis patients: a systematic review and meta-analysis. J Nephrol 2019;32:549-66. https://doi.org/10.1007/s40620-018-00565-z.
- Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol 2014;40:478-90. https://doi.org/10.1159/000368722.
- Chung YC, Yeh ML, Liu YM. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs 2017;26:1801-13. https://doi.org/10.1111/jocn.13514.
- Pu J, Jiang Z, Wu W, Li L, Zhang L, Li Y, et al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2017-020633.
- Young HML, March DS, Graham-Brown MPM, Jones AW, Curtis F, Grantham CS, et al. Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant 2018;33:1436-45. https://doi.org/10.1093/ndt/gfy045.
- Huang M, Lv A, Wang J, Xu N, Ma G, Zhai Z, et al. Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am J Nephrol 2019;50:240-54. https://doi.org/10.1159/000502447.
- Zhao QG, Zhang HR, Wen X, Wang Y, Chen XM, Chen N, et al. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil 2019;33:147-56. https://doi.org/10.1177/0269215518817083.
- Clarkson MJ, Bennett PN, Fraser SF, Warmington SA. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol 2019;316:F856-F872. https://doi.org/10.1152/ajprenal.00317.2018.
- Gomes Neto M, de Lacerda FFR, Lopes AA, Martinez BP, Saquetto MB. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil 2018;32:1189-202. https://doi.org/10.1177/0269215518760380.
- NIHR INVOLVE . NIHR INVOLVE 2021. www.invo.org.uk/about-involve/ (accessed 13 April 2021).
- Department of Health, Social Services and Public Safety, Scottish Government, Welsh Government, Department of Health and Social Care . Start Active, Stay Active: A Report on Physical Activity for Health from the Four Home Countries’ Chief Medical Officers 2011. http://dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_128209 (accessed 1 December 2019).
- Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Med Sci Sports Exerc 2010;42:2282-303. https://doi.org/10.1249/MSS.0b013e3181eeb61c.
- Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation 2013;128:2422-46. https://doi.org/10.1161/01.cir.0000436752.99896.22.
- Segura-Ortí E, Martínez-Olmos FJ. Test-retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther 2011;91:1244-52. https://doi.org/10.2522/ptj.20100141.
- Abe Y, Matsunaga A, Matsuzawa R, Kutsuna T, Yamamoto S, Yoneki K, et al. Determinants of slow walking speed in ambulatory patients undergoing maintenance hemodialysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0151037.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588-605. https://doi.org/10.1093/eurheartj/ehl254.
- Hickson SS, Butlin M, Broad J, Avolio AP, Wilkinson IB, McEniery CM. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res 2009;32:1079-85. https://doi.org/10.1038/hr.2009.154.
- Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-115.
- Coutinho-Myrrha MA, Dias RC, Fernandes AA, Araújo CG, Hlatky MA, Pereira DG, et al. Duke Activity Status Index for cardiovascular diseases: validation of the Portuguese translation. Arq Bras Cardiol 2014;102:383-90. https://doi.org/10.5935/abc.20140031.
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 1990;45:P239-43. https://doi.org/10.1093/geronj/45.6.P239.
- Gonzalez AM, Filho GJ, Pestana JO, Linhares MM, Silva MH, Moura RM, et al. Effects of Eurocollins solution as aortic flush for the procurement of human pancreas. Transplantation 2005;80:1269-74. https://doi.org/10.1097/01.TP.0000177640.53848.3D.
- Grbich C. Qualitative Research – An Introduction. London: SAGE Publications Ltd; 1999.
- Snape D, Spencer L, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. London: SAGE Publications Ltd; 2000.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. New York, NY: Routledge; 1994.
- Young HM, Hudson N, Clarke AL, Dungey M, Feehally J, Burton JO, et al. Patient and staff perceptions of intradialytic exercise before and after implementation: a qualitative study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0128995.
- Thompson S, Tonelli M, Klarenbach S, Molzahn A. A qualitative study to explore patient and staff perceptions of intradialytic exercise. Clin J Am Soc Nephrol 2016;11:1024-33. https://doi.org/10.2215/CJN.11981115.
- Heiwe S, Tollin H. Patients’ perspectives on the implementation of intra-dialytic cycling – a phenomenographic study. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-68.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Byrne C CF, Castledine C, Davenport A, Dawnay A, Fraser S, Maxwell H, et al. UK Renal Registry 20th Annual Report of the Renal Association. Nephron 2018;139:1-12. https://doi.org/10.1159/000490958.
- Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. Randomized trial comparing proactive, high-dose versus reactive, low-dose intravenous iron supplementation in hemodialysis (PIVOTAL): study design and baseline data. Am J Nephrol 2018;48:260-8. https://doi.org/10.1159/000493551.
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2019;73:A7-8. https://doi.org/10.1053/j.ajkd.2019.01.001.
- Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2003;14:3270-7. https://doi.org/10.1097/01.ASN.0000100127.54107.57.
- Duranton F, Kramer A, Szwarc I, Bieber B, Gayrard N, Jover B, et al. Geographical variations in blood pressure level and seasonality in hemodialysis patients. Hypertension 2018;71:289-96. https://doi.org/10.1161/HYPERTENSIONAHA.117.10274.
- Lopes AA, Bragg-Gresham JL, Satayathum S, McCullough K, Pifer T, Goodkin DA, et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2003;41:605-15. https://doi.org/10.1053/ajkd.2003.50122.
- Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994;3:329-38. https://doi.org/10.1007/BF00451725.
- Dimeo F, Wencke M, Krist L, Westhoff T, Rothermund L. Effects of a stamina training programme over 8 weeks in patients after kidney transplantation. Deutsche Zeitschrift Fur Sportmedizin 2007;58.
- Matsumoto Y, Furuta A, Furuta S, Miyajima M, Sugino T, Nagata K, et al. The impact of pre-dialytic endurance training on nutritional status and quality of life in stable hemodialysis patients (Sawada study). Ren Fail 2007;29:587-93. https://doi.org/10.1080/08860220701392157.
- Jong K, LS. J, Hyun Y, Suk P, Young J, Eun A. Effects of Walking Exercise for Health Status in Patients on Continuous Ambulatory Peritoneal Dialysis n.d.
- Afsar B, Siriopol D, Aslan G, Eren OC, Dagel T, Kilic U, et al. The impact of exercise on physical function, cardiovascular outcomes and quality of life in chronic kidney disease patients: a systematic review. Int Urol Nephrol 2018;50:885-904.
- Pei G, Tang Y, Tan L, Tan J, Ge L, Qin W. Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int Urol Nephrol 2019;51:1787-95.
- Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 2015;8:753-65. https://doi.org/10.1093/ckj/sfv099.
- Matsuzawa R, Hoshi K, Yoneki K, Harada M, Watanabe T, Shimoda T, et al. Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep 2017;2:1096-110. https://doi.org/10.1016/j.ekir.2017.06.008.
- Ouzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil 2009;23:53-6. https://doi.org/10.1177/0269215508096760.
- Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting--a multicentre study. Nephrol Dial Transplant 2008;23:1982-9. https://doi.org/10.1093/ndt/gfm870.
- Painter P, Carlson L, Carey S, Paul SM, Myll J. Low-functioning hemodialysis patients improve with exercise training. Am J Kidney Dis 2000;36:600-8. https://doi.org/10.1053/ajkd.2000.16200.
List of abbreviations
- 10mTUG
- 10-metre Timed Up and Go
- AE
- adverse event
- AU
- arbitrary units
- BMI
- body mass index
- CI
- confidence interval
- CKD
- chronic kidney disease
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCTU
- Glasgow Clinical Trials Unit
- HD
- haemodialysis
- IQR
- interquartile range
- KDQoL-SF
- Kidney Disease Quality of Life Short Form, version 1.3
- MET
- metabolic equivalent
- NIHR
- National Institute for Health Research
- PCS
- physical component summary
- PEDAL
- PrEscription of intraDialytic exercise to improve quAlity of Life in patients with chronic kidney disease
- PWV
- pulse wave velocity
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- STS60
- sit-to-stand 60
- TMG
- Trial Management Group
