Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR129546. The protocol was agreed in August 2019. The assessment report began editorial review in July 2020 and was accepted for publication in November 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© 2021 Stinton et al. This work was produced by Stinton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: http://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Stinton et al.
Chapter 1 Introduction
Description of the health problem
Purpose of the decision to be made
Lynch syndrome is an inherited genetic condition. It is caused by mutations in genes that are involved in repairing errors that occur in deoxyribonucleic acid (DNA) when cells replicate. When mutations occur in these genes, DNA errors are not repaired. Over time, this can lead to uncontrolled cell growth. Lynch syndrome is associated with an increased risk of cancers, including colorectal, endometrial, gastric, pancreatic and kidney cancers. There is 50 : 50 chance that a person with Lynch syndrome will pass it to their children.
Recently, the National Institute for Health and Care Excellence (NICE) has recommended that people who are diagnosed with colorectal cancer (CRC) are tested for Lynch syndrome. 1 Routine testing for Lynch syndrome among people with endometrial cancer is not currently conducted. Detection of Lynch syndrome might lead to reductions in the risk of developing cancer for both the individual and their family members (through surveillance and risk-reducing strategies, such as chemoprevention) and to earlier treatment of cancers. 2,3
The external assessment group (EAG) assessed the accuracy of immunohistochemistry (IHC)-based and microsatellite instability (MSI)-based testing strategies to identify people who are at high risk of Lynch syndrome, and assessed the clinical effectiveness and cost-effectiveness of testing for Lynch syndrome among people who have endometrial cancer and their biological relatives. This will inform the NICE Diagnostics Advisory Committee guidance on whether or not testing for Lynch syndrome in people who have endometrial cancer represents a cost-effective use of NHS resources.
Population and target condition
Parts of this section have been reproduced with permission of Stinton et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to share and redistribute, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Population: people with endometrial cancer
Endometrial cancer (cancer that develops from the lining of the uterus) is the most common gynaecological cancer in the Western world. 5 Each year in the UK, there are approximately 9300 new cases of endometrial cancer and 2200 endometrial cancer-related deaths. 6,7 The incidence of endometrial cancer generally increases with age, reaching a peak of 97.3 per 100,000 population between the ages of 75 and 79 years. 6,7 The most recent estimates suggest that people with endometrial cancers have a 1-year survival rate of 89.6% and a 5-year survival rate of 75.7%. 8 Risk factors for the development of endometrial cancer include obesity, nulliparity, early age at menarche, use of hormone-replacement therapy and Lynch syndrome. 9–11
Target condition: Lynch syndrome
Lynch syndrome, formally called hereditary non-polyposis colorectal cancer (HNPCC), is a cancer-predisposition syndrome. It is estimated that there are approximately 175,000 people with Lynch syndrome in the UK. 12
Lynch syndrome is usually caused by mutations to any one of four DNA mismatch repair (MMR) genes: mutL homologue 1 (MLH1), mutS homologue 2 (MSH2), mutS homologue 6 (MSH6) or postmeiotic segregation increased 2 (PMS2). 13 A small proportion of Lynch syndrome cases are caused by deletions to the epithelial cellular adhesion molecule (EPCAM) gene, which leads to epigenetic silencing of MSH2. 13 MMR genes encode proteins that are involved in recognising and repairing errors that occur in DNA during cell division. Mutations in MMR genes prevent DNA errors from being corrected. This can lead to uncontrolled cell growth and the development of cancer. A range of cancers have been associated with Lynch syndrome, the most common of which are endometrial and colorectal. 14 Lynch syndrome accounts for 2–9% of endometrial cancers. 15,16 By the age of 75 years, approximately 57% of people with Lynch syndrome will have endometrial cancer. 14 The type and prevalence of cancer appears to vary according to which of the genes are affected. 14
Lynch syndrome has an autosomal dominant inheritance pattern, meaning that a person has a 50% chance of passing the mutated gene(s) onto their children.
Description of technologies under assessment
Three tests are considered in this assessment (see Testing strategies). There are two primary diagnostic tests (IHC and MSI), and a third test, MLH1 promoter hypermethylation testing, may be added to either or both of these two. Eleven predefined testing strategies are considered, involving varying combinations of the three tests.
Immunohistochemistry
Immunohistochemistry, in this case, uses antibodies to look for the expression of four MMR proteins (MLH1, MSH2, MSH6 and PMS2). An absence of staining for any of the proteins suggests a genetic mutation. IHC testing identifies which MMR gene is potentially affected. If MLH1 has an abnormal expression, an additional test (MLH1 promoter hypermethylation testing) can be conducted (see MLH1promoter hypermethylation testing). IHC can detect non-functional, but antibody-binding, MLH1 proteins (which would be incorrectly classified as normal);17 therefore, this may lead to a false negative result.
Microsatellite instability testing
Microsatellites are short repeats of DNA sequences. These repeats are prone to acquiring errors. When the MMR genes are not functioning, these errors are not corrected. Mutations in MMR genes lead to variations in the size of these repeats. This is called MSI. MSI testing is used to determine whether or not there are differences in the repeat numbers between tumour and non-tumour regions in a person being tested. Various markers have been described. 18 The Bethesda guidelines19 identify five markers (BAT25, BAT26, DS123, D17S250 and D5S346) for MSI for Lynch syndrome. Typically, three classifications are derived from this approach:
-
MSI-high – two or more markers show instability/> 30% of markers show instability.
-
MSI-low – one marker shows instability/< 30% of markers show instability.
-
MSI-stable – zero markers show instability [also known as microsatellite stable (MSS)].
Additional testing can be conducted to help rule out sporadic epigenetic silencing of MLH1, which might present as Lynch syndrome (see MLH1promoter hypermethylation testing).
MLH1 promoter hypermethylation testing
Hypermethylation is an epigenetic process that stops a protein being produced by a gene. MLH1 promoter hypermethylation testing is initially conducted on tumours. The test is undertaken following IHC or MSI testing, usually on patients with a MSI-high result or IHC loss in the MLH1 protein. A positive result on this test suggests that the tumour is sporadic and not a result of Lynch syndrome. However, there is some evidence that constitutional epimutations of MLH1 in normal tissue may be a cause of Lynch syndrome in a small number of cases. 20
Comparators
The comparator currently used in the UK is no diagnostic testing for Lynch syndrome in those with endometrial cancer, and therefore no subsequent cascade testing of family members.
Reference standard
Typically, Lynch syndrome is diagnosed on the basis of constitutional mutations (i.e. mutations that are present in every cell) in MMR genes, which involves sequencing [including next-generation sequencing (NGS)] to detect point mutation, small insertions or deletions in these genes and multiplex ligation-dependent probe amplification (MLPA) or NGS to detect larger structural changes (such as deletions, duplications or rearrangements) to genetic sequences that could be missed by sequencing alone. Sequencing and MLPA may be used in combination to diagnose Lynch syndrome. However, these techniques also detect novel sequence variation in MMR genes that are of unknown significance. Sequencing of tumours can be used to identify sporadic tumours (i.e. those not caused by Lynch syndrome). If a person has deficient MMR (from tumour testing), but no germline mutation is identified and no somatic cause is identified, they can be considered to have Lynch-like syndrome (also known as putative or cryptic Lynch syndrome). Additional testing has been suggested in cases for which tumour testing is positive, but no Lynch syndrome-related pathogenic variants are identified. 21,22 This includes testing for other somatic or germline pathogenic variants [e.g. biallelic mutY DNA glycosylase (MUTYH), DNA polymerase epsilon (POLE), double somatic MMR variants].
Testing strategies
The NICE has published guidance on testing for Lynch syndrome among people diagnosed with CRC. 1 Currently, there is no NICE guidance for testing for Lynch syndrome in people who have endometrial cancer. The NHS National Genomic Test Directory provides testing criteria for people who have Lynch syndrome-related cancers. 23 In brief, testing is recommended in people who have a family history of Lynch syndrome-related cancers or who have been diagnosed with endometrial cancer before the age of 50 years. The 11 proposed testing pathways for the current review are outlined in Figures 1–11. Testing strategies include all possible combinations of index tests, followed by reference standard testing.
FIGURE 1.
Strategy 1: MSI testing alone. LS, Lynch syndrome.
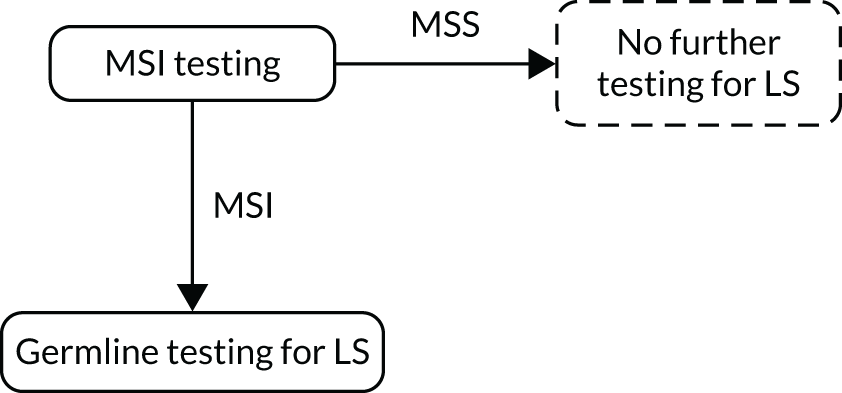
FIGURE 2.
Strategy 2: MSI testing with MLH1 promoter hypermethylation testing. a, If a germline sample is tested and is also hypermethylated, diagnose LS. LS, Lynch syndrome.
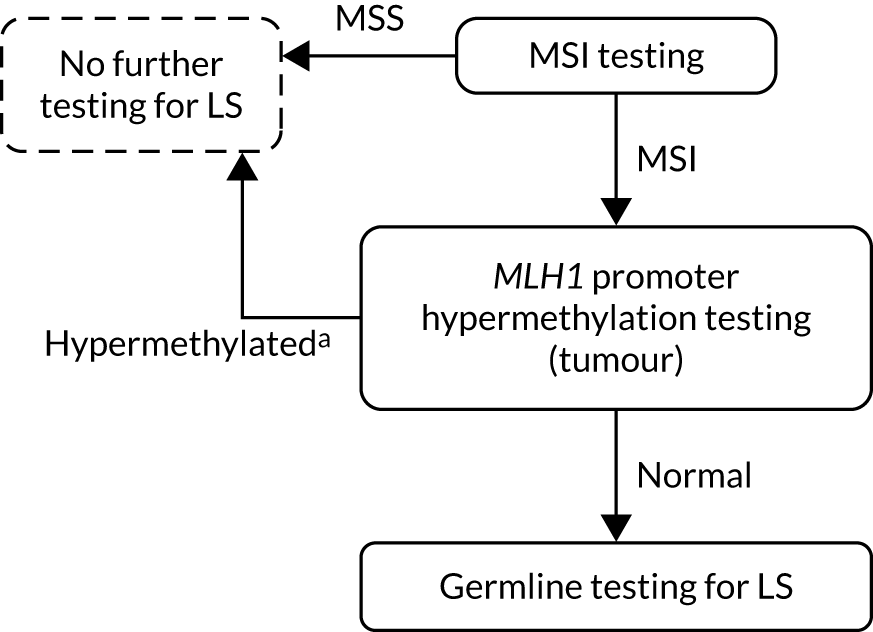
FIGURE 3.
Strategy 3: IHC-based testing. LS, Lynch syndrome.
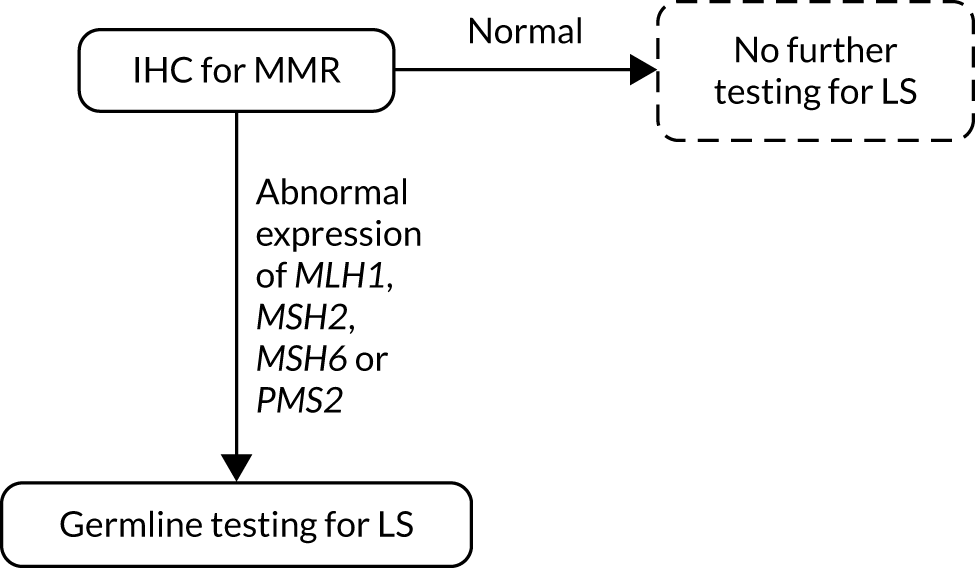
FIGURE 4.
Strategy 4: IHC testing with MLH1 promoter hypermethylation testing. a, If a germline sample is tested and is also hypermethylated, diagnose LS. LS, Lynch syndrome.
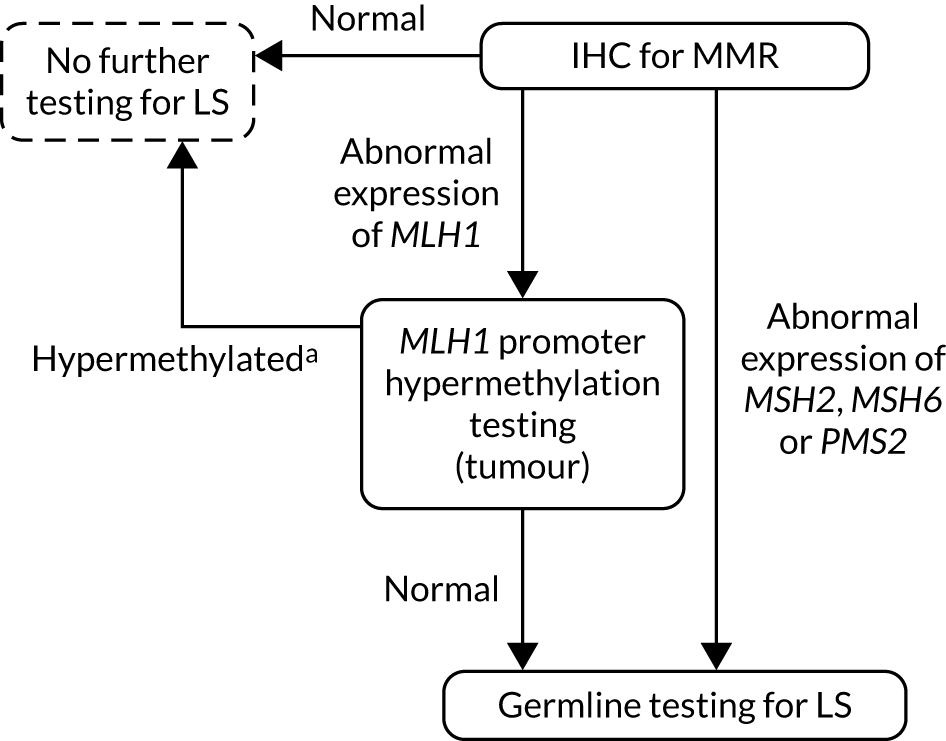
FIGURE 5.
Strategy 5: MSI testing followed by IHC testing. LS, Lynch syndrome.
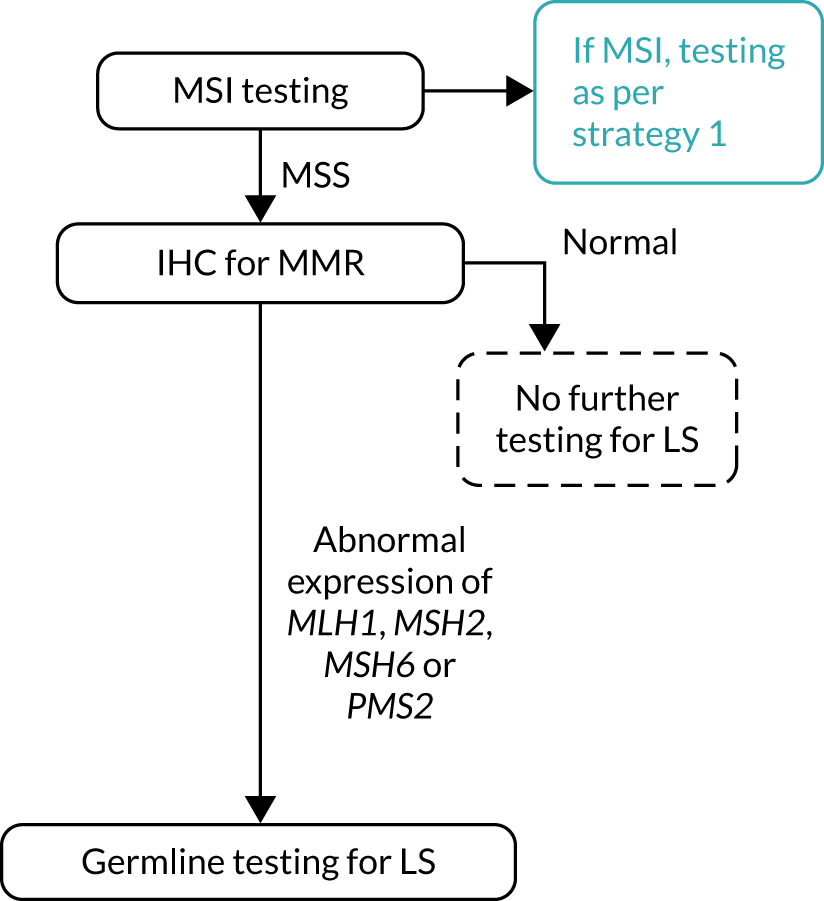
FIGURE 6.
Strategy 6: MSI testing followed by IHC testing with MLH1 promoter hypermethylation testing. a, If a germline sample is tested and is also hypermethylated, diagnose LS. LS, Lynch syndrome.
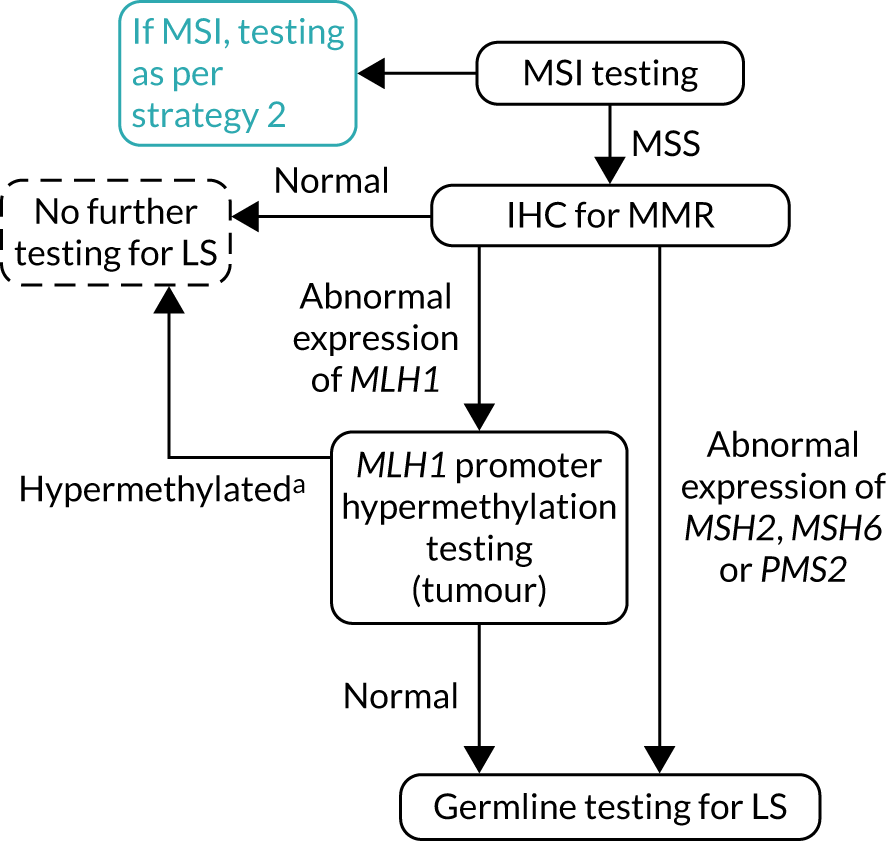
FIGURE 7.
Strategy 7: IHC followed by MSI testing. LS, Lynch syndrome.
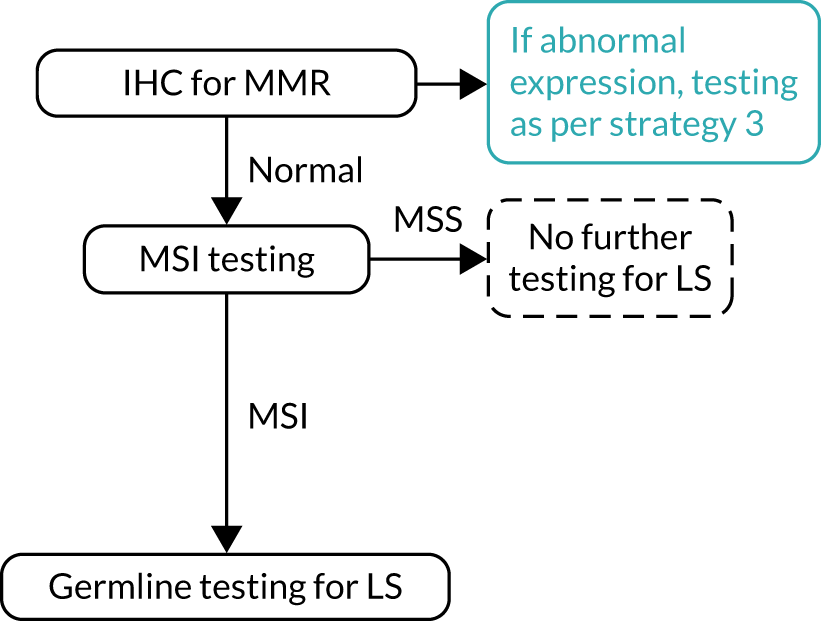
FIGURE 8.
Strategy 8: IHC testing followed by MSI testing with MLH1 promoter hypermethylation testing. a, If a germline sample is tested and is also hypermethylated, diagnose LS. LS, Lynch syndrome.
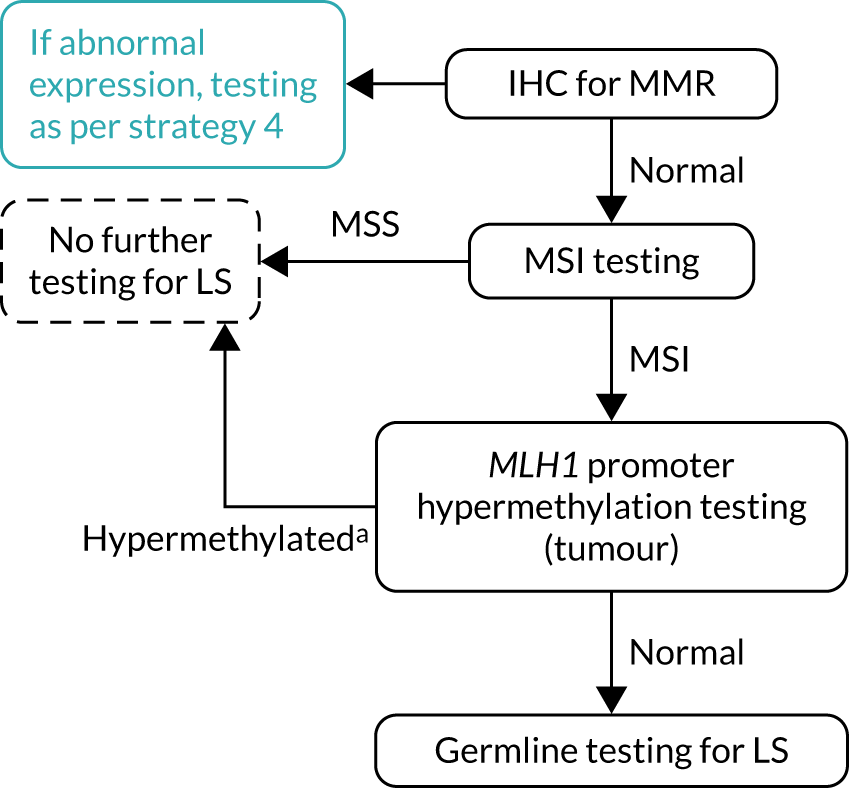
FIGURE 9.
Strategy 9: MSI and IHC testing. LS, Lynch syndrome.
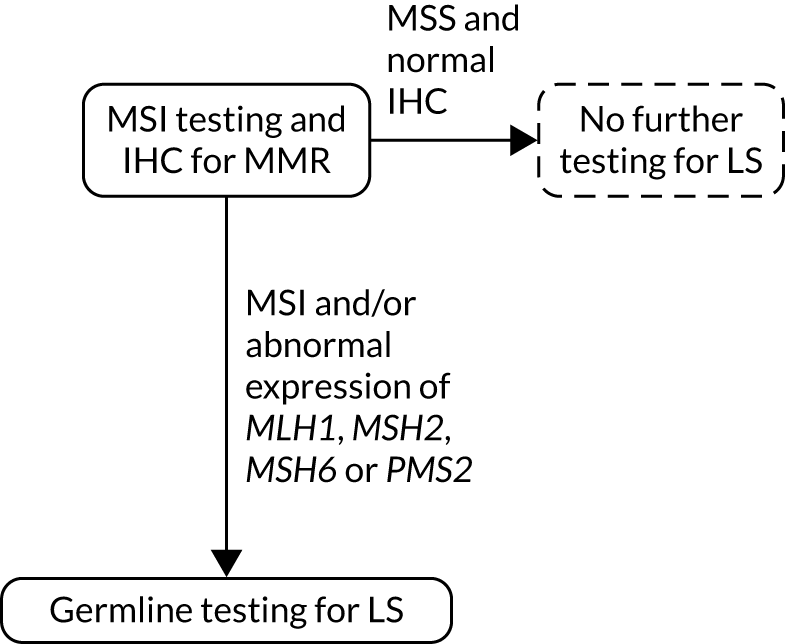
FIGURE 10.
Strategy 10: MSI and IHC testing with MLH1 promoter hypermethylation testing. a, MLH1 promoter hypermethylation testing not conducted after MSI if MLH1 expression on IHC is normal and abnormal expression of other MMR proteins is present; b, If a germline sample is tested and is also hypermethylated, diagnose LS. LS, Lynch syndrome.
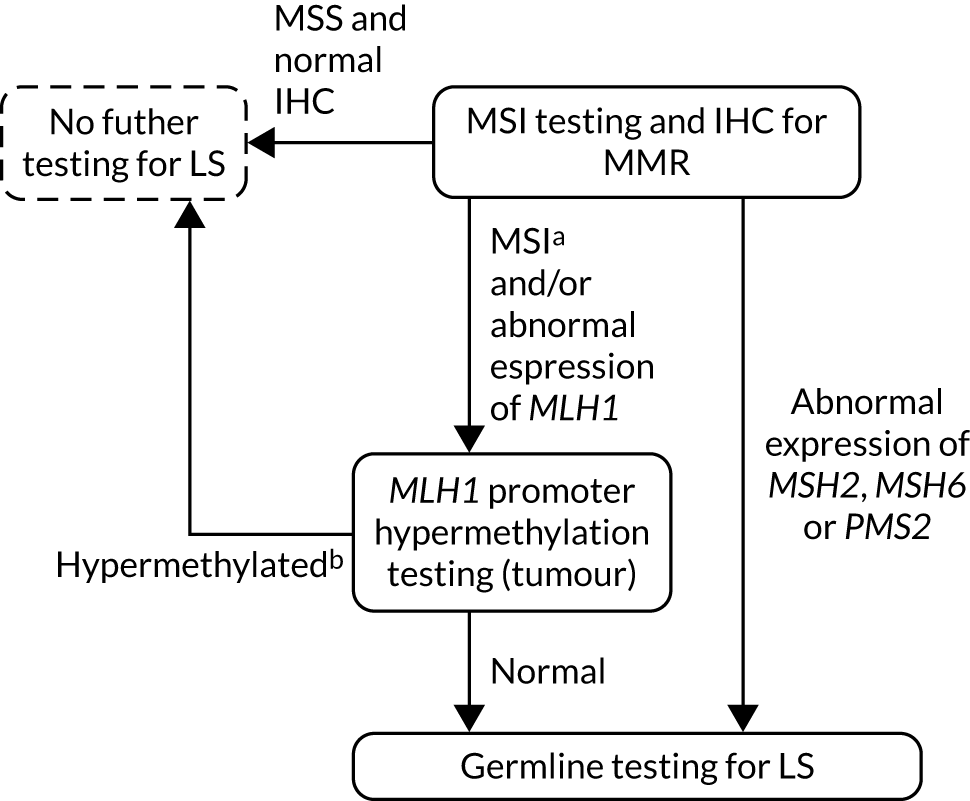
FIGURE 11.
Strategy 11: germline testing only.

Possible diagnostic pathways and approaches to the management of Lynch syndrome have been suggested by a range of societies and expert groups, including the British Gynaecological Cancer Society,24 the European HNPCC Expert group,25 the Royal College of Obstetricians and Gynaecologists,26 and the Manchester International Consensus Group. 22
Care pathways
Currently, there is no NICE guidance on the testing and management of Lynch syndrome in people with endometrial cancer. There is NICE guidance available on molecular testing strategies and a care pathway for people with CRC. 1 NHS England’s National Genomic Test Directory (Testing Criteria for Rare and Inherited Disease) specifies testing criteria for inherited MMR deficiency (Lynch syndrome). 23 Affected individuals with Lynch syndrome-related cancer should meet one of the following criteria:
-
CRC (any age, as per NICE guidance1).
-
Lynch syndrome-related cancer (aged < 50 years).
-
Two Lynch syndrome-related cancers (any age, one is colorectal or endometrial).
-
Lynch syndrome-related cancer and one or more first-degree relative has Lynch syndrome-related cancer (both occurred before the age of 60 years, one is colorectal or endometrial).
-
Lynch syndrome-related cancer and two or more relatives (first-/second-/third-degree relatives) have Lynch syndrome-related cancer (all occurring before the age of 75 years, one is colorectal or endometrial).
-
Lynch syndrome-related cancer and three or more relatives (first-/second-/third-degree relatives) have Lynch syndrome-related cancer (occurring at any age, one is colorectal or endometrial).
The recommended follow-up care for those with CRC diagnosed with Lynch syndrome is outlined in the guidelines for the management of hereditary CRC from the British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/UK Cancer Genetics Group,27 NICE diagnostics guidance 271 and the NICE draft guideline on the effectiveness of aspirin in the prevention of CRC. 28 The main follow-on care recommended includes biennial colonoscopy surveillance, daily aspirin use for those with CRC and cascade testing for CRC probands. As of August 2018, uptake of the guidance on molecular testing strategies for CRC is around 97.5%. 1
Testing for Lynch syndrome in people with endometrial cancer in the UK varies, with some NHS services testing all tumours and others doing no routine testing. The Manchester International Consensus Group,22 American College of Obstetricians and Gynecologists,29 and European Society for Medical Oncology30 clinical practice guidelines recommended a range of surveillance and preventative measures for those with gynaecological cancers, including risk-reducing total hysterectomy and bilateral salpingo-oophorectomy (H-BSO), individualised counselling, colorectal surveillance, lifestyle modifications, use of the combined oral contraceptive and daily aspirin for those with MMR pathogenic variant carriers.
Outcomes
The outcomes from the clinical effectiveness assessment were as follows:
-
prevalence of Lynch syndrome and variants of uncertain significance (VUSs)
-
test accuracy.
The outcome from the cost-effectiveness analysis is cost per quality-adjusted life-year (QALY) for each of the 11 testing strategies, compared with usual care. Other intermediate outcomes reported include the following:
-
number of probands with Lynch syndrome receiving Lynch syndrome surveillance (true positive accepting)
-
number of probands with Lynch syndrome not receiving Lynch syndrome surveillance (Lynch syndrome positive who decline and those assumed to be false negative, although without testing this cannot be confirmed)
-
number of VUSs and Lynch-assumed diagnoses.
Chapter 2 Decision questions and objectives
The overall aims of this project were to examine the test accuracy of IHC- and MSI-based strategies to detect Lynch syndrome in people who have endometrial cancer (key question 1), and to examine the clinical effectiveness (key question 2) and cost-effectiveness (key question 3) of testing for Lynch syndrome among people who have been diagnosed with endometrial cancers. The key questions for this review were as follows:
-
Key question 1 – what are the test accuracy, test failure rates, and time to diagnosis of IHC- and MSI-based strategies for detecting Lynch syndrome in people who have a diagnosis of endometrial cancer?
Subquestions:
-
1a. What is the concordance between IHC- and MSI-based strategies for detecting Lynch syndrome in people who have a diagnosis of endometrial cancer?
-
1b. What are the characteristics of discordant cases? [e.g. do people with a high risk according to MSI testing and a low risk according to IHC (or vice versa) have particular gene mutations, a family history of Lynch syndrome, different age profiles?]
-
2. What are the types and frequencies of MMR genetic mutations detected in people with endometrial cancer who are diagnosed with Lynch syndrome?
-
-
Key question 2 – what are the benefits and harms of testing for Lynch syndrome among people who have endometrial cancer, and/or their relatives?
Subquestions:
-
What are the benefits and harms of CRC surveillance for people with Lynch syndrome identified following a diagnosis of endometrial cancer, and/or their relatives?
-
What are the benefits and harms of gynaecological cancer surveillance for people with Lynch syndrome identified following a diagnosis of endometrial cancer, and/or their relatives?
-
-
Key question 3 – what is the cost-effectiveness of testing for Lynch syndrome among people diagnosed with endometrial cancer using IHC- and MSI-based strategies, compared with the current pathway for the diagnosis of Lynch syndrome?
Chapter 3 Methods
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for assessing test accuracy
What are the test accuracy, test failure rates, and time to diagnosis of IHC- and MSI-based strategies for detecting Lynch syndrome in people who have a diagnosis of endometrial cancer?
Review subquestions:
-
What is the concordance between IHC- and MSI-based strategies for detecting Lynch syndrome in people who have a diagnosis of endometrial cancer?
-
What are the characteristics of discordant cases? [e.g. do people with a high risk of Lynch syndrome according to MSI testing and a low risk according to IHC (or vice versa) have particular gene mutations, a family history of Lynch syndrome, different age profiles?]
-
What are the types and frequencies of MMR genetic mutations detected in people with endometrial cancer who have been diagnosed with Lynch syndrome?
Systematic review methods followed the principles outlined in the Cochrane Handbook for Diagnostic Test Accuracy Reviews31 and the NICE Diagnostics Assessment Programme manual. 32
Identification and selection of studies
Search strategy
The search strategy comprised the following main elements:
-
searching of electronic bibliographic databases
-
contacting experts in the field
-
scrutiny of references of included studies and relevant systematic reviews.
A comprehensive search for test accuracy and clinical effectiveness studies was developed iteratively, with reference to a previous Lynch syndrome assessment1,12 and scoping searches (Donna Barnes, NICE, 2019, personal communication). Searches were undertaken in a range of relevant bibliographic databases in August 2019. The search was developed in MEDLINE (via Ovid) and adapted appropriately for other databases. Search terms related to endometrial cancer and Lynch syndrome. No limits on study design, date or language were applied. Full details of the search strategies are provided in Appendix 1.
Searches were conducted in the following databases, from inception: MEDLINE ALL (via Ovid), EMBASE (via Ovid), Cochrane Database of Systematic Reviews (via Wiley Online Library), Cochrane Central Register of Controlled Trials (via Wiley Online Library), Database of Abstracts of Reviews of Effects [via the Centre for Reviews and Dissemination (CRD)], Health Technology Assessment (HTA) Database (via the CRD), Science Citation Index (via Web of Science), Conference Proceedings Citation Index – Science (via Web of Science) and the PROSPERO international prospective register of systematic reviews (via the CRD).
In addition, references of included studies and relevant systematic reviews were checked and experts on the team were consulted.
Records were exported to EndNote X9 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA], where duplicates were systematically identified and removed.
Study eligibility criteria
The population, intervention, comparator and outcome (PICO) framework is used in Table 1 to present the study inclusion criteria.
| PICO element | Description |
|---|---|
| Population | All test accuracy questions:
|
| Target condition | All test accuracy questions:
|
| Intervention | All test accuracy questions:
|
| Reference standard | All test accuracy questions:
|
| Comparator | Key question:
|
Subquestions 1a and 1b:
|
|
Subquestion 2:
|
|
| Outcome | Key question:
|
Subquestion 1a:
|
|
Subquestion 1b:
|
|
|
Subquestion 2: Types and frequencies of Lynch syndrome-related genetic mutations (MLH1, MSH2, MSH6, PMS2) in people newly diagnosed with Lynch syndrome after endometrial cancer, including results of MLH1 promoter hypermethylation testing |
|
| Study design | Key question:
|
Subquestions 1a and 1b:
|
|
Subquestion 2:
|
|
| Publication type | All test accuracy questions:
|
| Language | All test accuracy questions:
|
Papers that fulfilled the following criteria were excluded: non-human studies, letters, editorials and communications; qualitative studies; studies of women who have pre-cancerous conditions of the uterus (i.e. atypical endometrial hyperplasia); studies in which > 10% of the sample does not meet our inclusion criteria; studies without extractable numerical data; studies that provided insufficient information for assessment of methodological quality/risk of bias; articles not available in English; studies using index tests other than those specified in the inclusion criteria; and studies reporting the test accuracy of IHC- and MSI-based testing strategies in the general population (estimates arising from the general population are not generalisable to people who are at higher risk of Lynch syndrome because of the different risk profile). If sufficient head-to-head studies were identified that could provide meaningful analysis then other study designs were excluded.
Review strategy
Two reviewers (CS and LAK/HF) independently screened the titles and abstracts of records identified by the searches. Any disagreements were resolved through discussion or retrieval of the full publication. Potentially relevant publications were obtained, and assessed independently by two reviewers (CS and LAK/HF) with a coding tool (using inclusion/exclusion criteria) that has been piloted on a subsample of papers. Disagreements were resolved through consensus, with the inclusion of a third reviewer (HF/LAK, STP) if required. Records that were excluded at full-text stage are documented in Appendix 3, along with the reasons for their exclusion.
Extraction and study quality
Data extraction strategy
Two reviewers (CS and LAK/HF) extracted data independently using a piloted data extraction form (see Appendix 2). Disagreements were resolved through consensus, with the inclusion of a third reviewer (HF/LAK, STP) when required.
Assessment of study risk of bias
The risk of bias of test accuracy studies was assessed using a modified Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. 34,35 Two reviewers (CS and LAK/HF) independently assessed study risks of bias. Disagreements were resolved through consensus, with the inclusion of a third reviewer (HF/LAK, STP) when required. As recommended by the QUADAS-2 group, an overall quality score was not determined. 34 The results of each risk-of-bias item are presented in Tables 4–6.
Methods of analysis/synthesis
In the gold-standard study design for assessing test accuracy, an entire sample of participants receives both the index test and the reference standard. This allows direct, unbiased comparisons of the agreement between the two tests. For reasons such as cost and practicality, in many test accuracy studies only a subsample of participants receive both tests, that is individuals who are index-test positive (at higher risk for the disease or condition) receive the reference standard, whereas individuals who are index-test negative do not receive the reference standard. Although this approach accurately reflects how tests are used in clinical practice, it leads to partial verification bias (also called detection bias or workup bias); data are missing and the true diagnostic status of participants who are negative on the index is not known. Partial verification can lead to overestimation of sensitivity and underestimation (or overestimation) of specificity. 36 Inaccurate test accuracy metrics can have an impact on clinical practice in relation to referral decisions and costs.
In this report, test accuracy results are divided into ‘complete’ test accuracy studies (in which all participants receive both the index test and the reference standard) and ‘partial’ test accuracy studies (in which only participants who are index-test positive receive the reference standard). For ‘complete’ test accuracy studies, we present results on all available test accuracy metrics, that is true positives, false positives, true negatives, false negatives, sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs). For ‘partial’ test accuracy studies, we present results for only those test accuracy metrics that relate to participants who have received both the index test and the reference standard, that is true positives, false positives and PPVs. Furthermore, as there is a risk that the likelihood that someone will receive the reference standard is associated with disease status (e.g. individuals who truly have a disease may be more likely to get the reference standard than those who do not have the disease), which biases PPV upwards, we included only studies in which at least 95% of women who were eligible for germline testing (i.e. those who were index-test positive) received it. The sensitivity, specificity, PPVs and NPVs presented in this report were all calculated by the review authors and based on the true positive, false positive, false negative and true negative values that were reported in individual papers. Confidence intervals (CIs) were calculated using Wilson’s continuity correction. 37
Test accuracy results are presented for testing strategies 1–10, comparing the index tests with the eligible reference standards. Test accuracy was not assessed for strategy 11, as this approach does not include an index test. For studies that included an initial test followed by MLH1 promoter hypermethylation testing, we have analysed data at each stage of the process: (1a) IHC alone, then; (1b) IHC plus MLH1 promoter hypermethylation testing; and (2a) MSI-based testing alone, then; (2b) MSI-based testing plus MLH1 promoter hypermethylation testing. For IHC results, we have reported results together and separately for each protein. For MSI results, we have reported the panel used as per the papers, and provided a narrative summary of results on microsatellite instability-low (MSI-L) and microsatellite instability-high (MSI-H) patients. A subgroup analysis was not conducted for the different combinations of microsatellite markers because of the small number of studies and the wide range of panels used. The main analysis assumed that MSI-L was a negative test result. Owing to insufficient data, we did not conduct subgroup analyses of test accuracy by (1) age (≤ vs. > 70 years) or (2) people who have had a prior Lynch syndrome-related cancer (as defined in NHS England’s National Genomic Test Directory: Testing Criteria for Rare and Inherited Disease23). A narrative summary of the evidence is presented because meta-analysis was not possible as a result of heterogeneity.
Variants of uncertain clinical significance on germline testing are not considered to have Lynch syndrome in our test accuracy analysis. The EAG has recorded how many of these there are for a scenario analysis in the economic modelling, considering either all or none as having Lynch syndrome. In practice, patients with a negative germline test result (with no somatic cause of the tumour identified), but a positive index test, may be considered to have Lynch-like syndrome (also known as putative or cryptic Lynch syndrome) and undergo further investigation or surveillance. In particular, further investigation is undertaken if there is family history of Lynch syndrome. Because of this, the EAG descriptively recorded the characteristics of these cases such as family history, IHC results and discordant cases between the two index tests. This provides contextual information about the possibility of Lynch-like syndrome, and variants of uncertain clinical significance. However, for the reporting of test accuracy data, germline testing using sequencing with or without MLPA was considered the primary reference standard. We included studies using other diagnostic tests outlined in the Association for Clinical Genomic Science best-practice guidelines33 for genetic testing and diagnosis of Lynch syndrome, that is array-based comparative genomic hybridisation, and long-range polymerase chain reaction (PCR). The uncertainty around the effectiveness of germline testing to diagnose all cases of Lynch syndrome (see above regarding Lynch-like syndrome) is a potential weakness of the reference standard and a limitation of this review. As a subanalysis, for studies that report extra steps to the reference standard (e.g. sequencing of tumours or incorporating family history data), we recorded the additional tests that were used. Owing to the small number of studies using alternative tests, we did not compare the results of these multistage reference standards with the results of germline testing for MLH1, MSH2, MSH6, and PMS2 using sequencing with or without MLPA.
Quality assessment strategy for test accuracy studies
Quality assessment of eligible test accuracy studies was undertaken with a tailored QUADAS-2 tool. Methodological quality was assessed by two independent reviewers. Disagreements were resolved by consensus or a third reviewer.
Modifications to tailor the form of the QUADAS-2 tool to the research question in terms of the risk-of-bias assessment are outlined in Appendix 2 (the tailored QUADAS-2 form and guidance notes). No additional questions were added to the patient selection domain, the reference standard domain, flow and timing domain or any of the applicability sections. One question was added to the index test domain to assess whether or not quality assurance measures were in place.
Methods for assessing clinical effectiveness
Key question 2: what are the benefits and harms of testing for Lynch syndrome among people who have endometrial cancer, and/or their relatives?
Subquestions:
-
What are the benefits and harms of CRC surveillance for people with Lynch syndrome identified following a diagnosis of endometrial cancer, and/or their relatives?
-
What are the benefits and harms of gynaecological cancer surveillance for people with Lynch syndrome identified following a diagnosis of endometrial cancer, and/or their relatives?
This question is to identify ‘end-to-end studies’, or ‘test–treat trials’. End-to-end studies follow people from initial testing to treatment and final outcomes. These studies can remove the need for separate searches for model parameters for cost-effectiveness modelling. 32 We conducted a literature search to identify end-to-end studies of testing for Lynch syndrome among people who have been diagnosed with endometrial cancer, and/or their relatives. The same review searches and methods that were used for the test accuracy question (see Methods for assessing test accuracy) were employed to address this question. The subquestions are designed to identify the benefits and harms of the two main surveillance strategies that would be employed after identification of Lynch syndrome.
Systematic review methods followed the principles outlined in the CRD guidance for undertaking reviews in health care38 and the NICE Diagnostics Assessment Programme manual. 32
Identification and selection of studies
Search strategy
The same search strategy as described in the methods for test accuracy was used (see Identification and selection of studies).
Study eligibility criteria
Table 2 shows the study eligibility criteria.
| PICO element | Description |
|---|---|
| Population | Key question:
|
Subquestions 1 and 2:
|
|
| Target condition | Key question:
|
Subquestion 1:
|
|
Subquestion 2:
|
|
| Intervention | Key question:
|
Subquestion 1:
|
|
Subquestion 2:
|
|
| Comparator | Key question:
|
Subquestions 1 and 2:
|
|
| Outcome | Key question:
|
Subquestion 1:
|
|
Subquestion 2:
|
|
| Study design | All questions:
|
| Publication type | All questions:
|
| Language | All questions:
|
Papers that fulfilled the following criteria were excluded: non-human studies, letters, editorials and communications; qualitative studies; studies of women who have pre-cancerous conditions of the uterus (i.e. atypical endometrial hyperplasia); studies in which > 10% of the sample does not meet our inclusion criteria; studies without extractable numerical data; studies that provided insufficient information for assessment of methodological quality/risk of bias; articles not available in English; and studies using index tests other than those specified in the inclusion criteria.
Review strategy
Two reviewers (CS and LAK/HF) independently screened the titles and abstracts of records identified by the searches. Any disagreements were resolved through discussion or retrieval of the full publication. Potentially relevant publications were obtained, and were assessed independently by two reviewers (CS and LAK/HF) with a coding tool (using inclusion/exclusion criteria) that had been piloted on a subsample of papers. Disagreements were resolved through consensus, with the inclusion of a third reviewer (HF/LAK or STP) when required.
Extraction and study quality
Data extraction strategy
No studies met the inclusion criteria; therefore, no data extraction took place.
Assessment of study risk of bias
We planned to assess the risk of bias using the Cochrane Risk of Bias 2 (RoB 2) revised tool to assess risk of bias in randomised trials39 and the Cochrane Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool. 40 No studies were included, so no risk-of-bias assessments took place.
Methods of analysis/synthesis
No studies were identified that met the inclusion criteria, and so no data synthesis was undertaken.
Methods for assessing cost-effectiveness
Key question 3
What is the cost-effectiveness of testing for Lynch syndrome among people diagnosed with endometrial cancer using IHC- and MSI-based strategies, compared with the current pathway for the diagnosis of Lynch syndrome?
Review of existing cost-effectiveness models
Systematic review of existing cost-effectiveness evidence
Study identification
A comprehensive search of the literature for published economic evaluations, cost studies and health-related quality of life (HRQoL) studies was performed in a range of relevant bibliographic databases in August 2019. The database searches were developed iteratively and combined terms for Lynch syndrome and economic evaluations/cost studies/HRQoL studies, or endometrial cancer and testing and economic evaluations/cost studies/HRQoL studies. The search was informed by the strategy developed for the clinical effectiveness review and established economic and HRQoL search filters. No limits on date or language were applied. Full details of the search strategies are provided in Appendix 1.
The following databases were searched, from inception: MEDLINE ALL (via Ovid), EMBASE (via Ovid), NHS Economic Evaluation Database (NHS EED) and HTA Database (via CRD), Science Citation Index (via Web of Science), Conference Proceedings Citation Index – Science (via Web of Science), Cost-Effectiveness Analysis (CEA) Registry, EconPapers (Research Papers in Economics), and School of Health and Related Research Health Utilities Database (ScHARRHUD).
The reference lists of included studies and results of the clinical effectiveness search were also checked.
Records were exported to EndNote X9, where duplicates were systematically identified and removed.
Inclusion and exclusion of relevant studies
Inclusion criteria
To be included in the review, the following criteria were applied.
-
Population: women with endometrial cancer with no known diagnosis of Lynch syndrome, and/or their relatives.
-
Intervention: interventions used to identify women with Lynch syndrome –
-
MSI-based testing (with/without MLH1 promoter hypermethylation testing) followed by germline testing
-
IHC (with/without MLH1 promoter hypermethylation testing) followed by germline testing
-
combination of MSI-based testing and IHC (with/without MLH1 promoter hypermethylation testing) followed by germline testing
-
germline testing alone.
-
-
Comparator: no testing for Lynch syndrome.
-
Outcome measures: cost and cost-effectiveness outcomes [costs for each screening strategy, direct medical care costs, incremental cost-effectiveness ratios (ICERs), e.g. cost per QALY gained].
-
Study design: studies comprising an economic evaluation (cost analysis, cost–consequences analysis, cost-effectiveness analysis, cost–utility analysis and cost–benefit analysis), and any model-based economic evaluation involving direct comparison between strategies used to diagnose Lynch syndrome.
-
Other inclusion criteria:
-
full-text reports published in English
-
abstracts (only if they are companion publications to full-text included studies)
-
only humans.
-
Methods
The search was run by our information specialist (RC). Sifting was undertaken by two reviewers. Mary Jordan led the review sifting abstracts and titles of all identified studies, and Chris Stinton, James Keasley, Hannah Fraser and Lena Al-Khudairy acted as second reviewers. Results between the first and second reviewer were then compared, and anomalies resolved through discussion or, if this was not possible, by recourse to the full team of reviewers. Full texts of the results of the first sift were obtained and screened using the same process.
Data extraction
Information was extracted by one reviewer using a pre-piloted data extraction form (see Appendix 2) for the full economic evaluation studies. The data extraction form was developed to summarise the main characteristics of the studies and to capture useful information from the economic analysis. We extracted information about study details (title, author and year of study), baseline characteristics (PICO), methods (study perspective, time horizon, discount rate, measure of effectiveness current, assumptions and analytical methods), results (study parameters, base-case and sensitivity analyses results), discussion (study findings, limitations of the models and generalisability), other (source of funding and conflicts of interests), overall reviewer comments and conclusions (of authors and reviewers). Each completed data extraction form was cross-checked by another reviewer, with any discrepancies resolved by discussion, or recourse to a third reviewer if an agreement could not be reached.
Quality assessment
The reporting quality of the studies included in the systematic review was assessed against the Consolidated Health Economic Evaluation Reporting Standards (CHEERS)41 checklist and the Philips’ checklist. 42
The economic evaluations were appraised against a framework for best practice for reporting economic evaluation studies developed by the CHEERS task force. 41 The CHEERS assessment tool comprises six dimensions: title and abstract, introduction, methods, results, discussion and other. Under these dimensions, a series of questions check whether or not the criteria have been clearly reported. In addition, the models were critically appraised against a framework for best practice for reporting decision-analytical models developed by Philips et al. 42 The Philips’ quality assessment tool comprises two main dimensions: model structure and data used to parameterise the model. Under these dimensions, several questions assess whether or not the criteria have been clearly reported (see Appendix 2).
Study quality was assessed by one reviewer and cross-checked by a second reviewer. Any disagreements were resolved by discussion or by recourse to a third reviewer.
Data synthesis
Information extracted from the included studies was summarised narratively. Owing to the nature of economic analyses (different aims/objectives, study designs, populations and methods) these findings from individual studies were compared narratively, and recommendations for future economic models are discussed.
Model structure for independent economic assessment
A de novo economic model was developed. The model structure reflected the decision problem: to determine the costs and benefits associated with implementing a policy to offer genetic testing to identify Lynch syndrome in women newly diagnosed with endometrial cancer; to offer testing to relatives of those thereby identified as having Lynch syndrome; and to offer interventions to those identified as having Lynch syndrome (probands and cascadees) aimed at reducing the risk of them developing (further) Lynch syndrome cancers, and improving outcomes if they do.
This decision problem can be analysed in two stages. The first stage is to determine what the costs and consequences are of the initial and cascade testing strategy being considered. This stage results in estimates of the total number of individuals with Lynch syndrome identified (probands and cascadees), together with the costs incurred in identifying them. The second stage involves estimating the incremental impact of being identified with Lynch syndrome compared with not knowing this. The impact occurs as a result of various risk reduction and surveillance interventions that can be offered once it is known that a person has Lynch syndrome. The costs and consequences of these interventions need to be modelled from the point when they are offered over the lifetime of a recipient.
We adopted a modular approach involving two submodels, one for each of these stages. The first stage was modelled with a decision tree structure, as testing strategies naturally lend themselves to this approach. The second stage was modelled with a Markov cohort model structure, to analyse the lifetime incidence of CRC and endometrial cancer from the point when an individual is identified with Lynch syndrome until their death (from CRC, endometrial cancer or another cause). The outputs from this Markov model were the (mean and variance) lifetime discounted costs and QALYs resulting from risk reduction measures, surveillance and cancer. These were calculated for a range of ages at which Lynch syndrome might be identified, as a table. This table became an input for the decision tree model, hence integrating the two submodels in a unified model.
Construction of the model involved consulting the previous HTA report undertaken by Snowsill et al. 12 comparing diagnostic strategies to identify Lynch syndrome in people with CRC. This also comprised two separate stages: a diagnostic stage and a management stage. The first stage used a decision tree structure to estimate the number of probands and their relatives who would be diagnosed with Lynch syndrome, and the resource use and costs involved. The second stage used an individual patient-level model to simulate the long-term costs and benefits (life-years and QALYs accrued) associated with management and surveillance, and prophylactic treatment for probands and relatives with Lynch syndrome. In addition, data and the modelling approach used by Snowsill et al. 43 in their cost-effectiveness analysis of reflex testing for Lynch syndrome in women with endometrial cancer were drawn on, as this was the model identified as being the closest to address the current decision problem under review.
The resultant model constitutes an initial diagnostic section, a decision tree model built in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), and a subsequent Markov cohort state transition model, in R software package (The R Foundation for Statistical Computing, Vienna, Austria), to estimate the long-term benefits accrued through risk reduction and surveillance measures for both CRC and endometrial cancer as a result of Lynch syndrome identification and cascade testing of relatives.
The diagnostic pathway in the decision tree component of the model is assumed to take place within 1 year, with no discounting applied to costs. The Markov model covers a lifetime time horizon (until death or age 100 years) with annual cycles in which costs and QALYs are discounted at a rate of 3.5% per year. Both models are conducted from an NHS and Personal Social Services (PSS) perspective.
The model will now be described in greater detail.
Diagnostic decision tree
This section of the model estimates the number of endometrial cancer probands and their relatives diagnosed with Lynch syndrome using the 11 strategies for inclusion in this review against the comparative strategy of no reflex testing. Figure 12 shows an overview of the testing pathway modelled for endometrial cancer probands undergoing one of the available strategies and Figure 13 shows an overview of the testing and management pathway for relatives of probands identified with Lynch syndrome or who are assumed to have Lynch syndrome.
FIGURE 12.
Overview of diagnostic model for probands. EC, endometrial cancer; LS, Lynch syndrome.
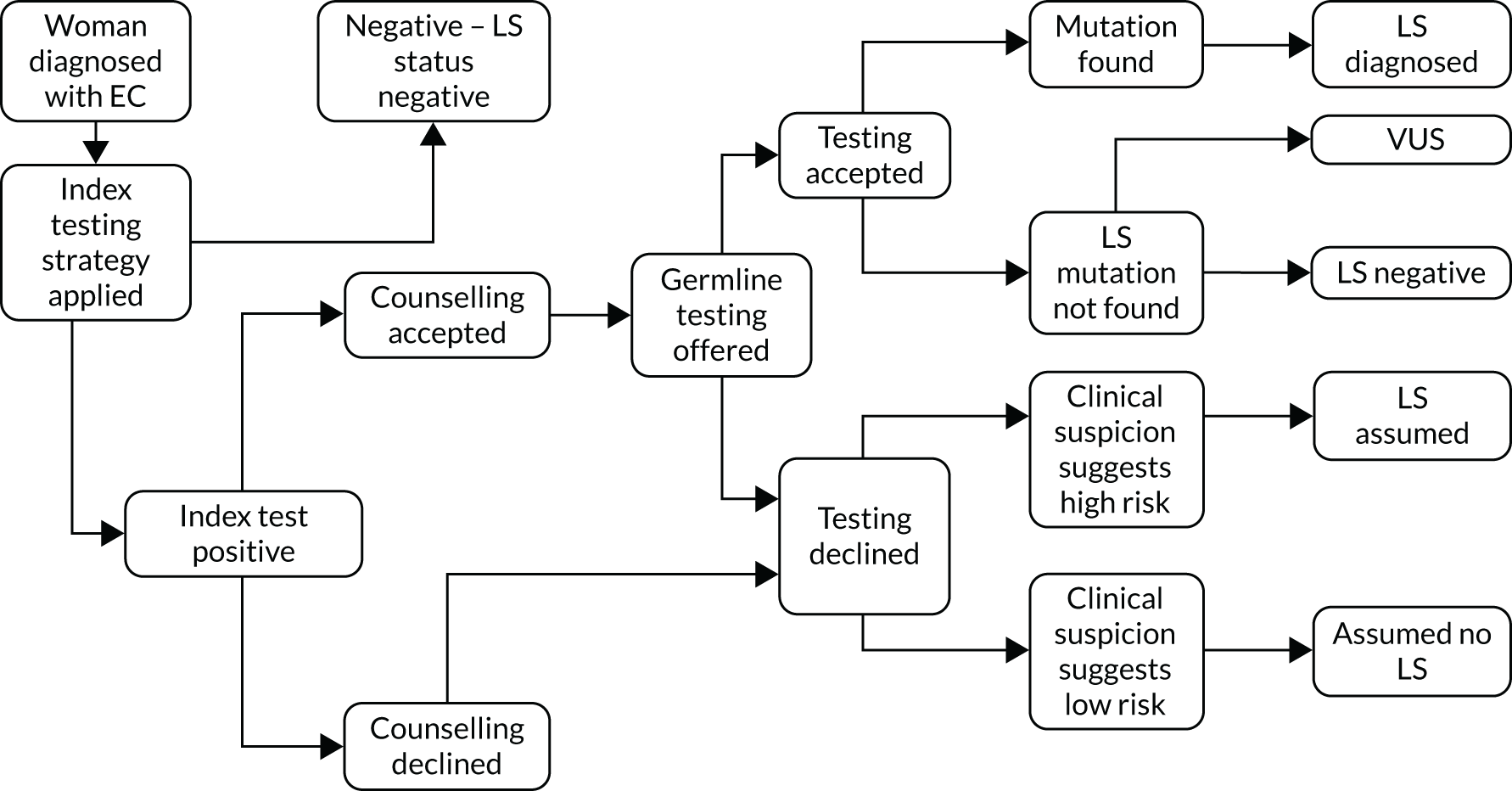
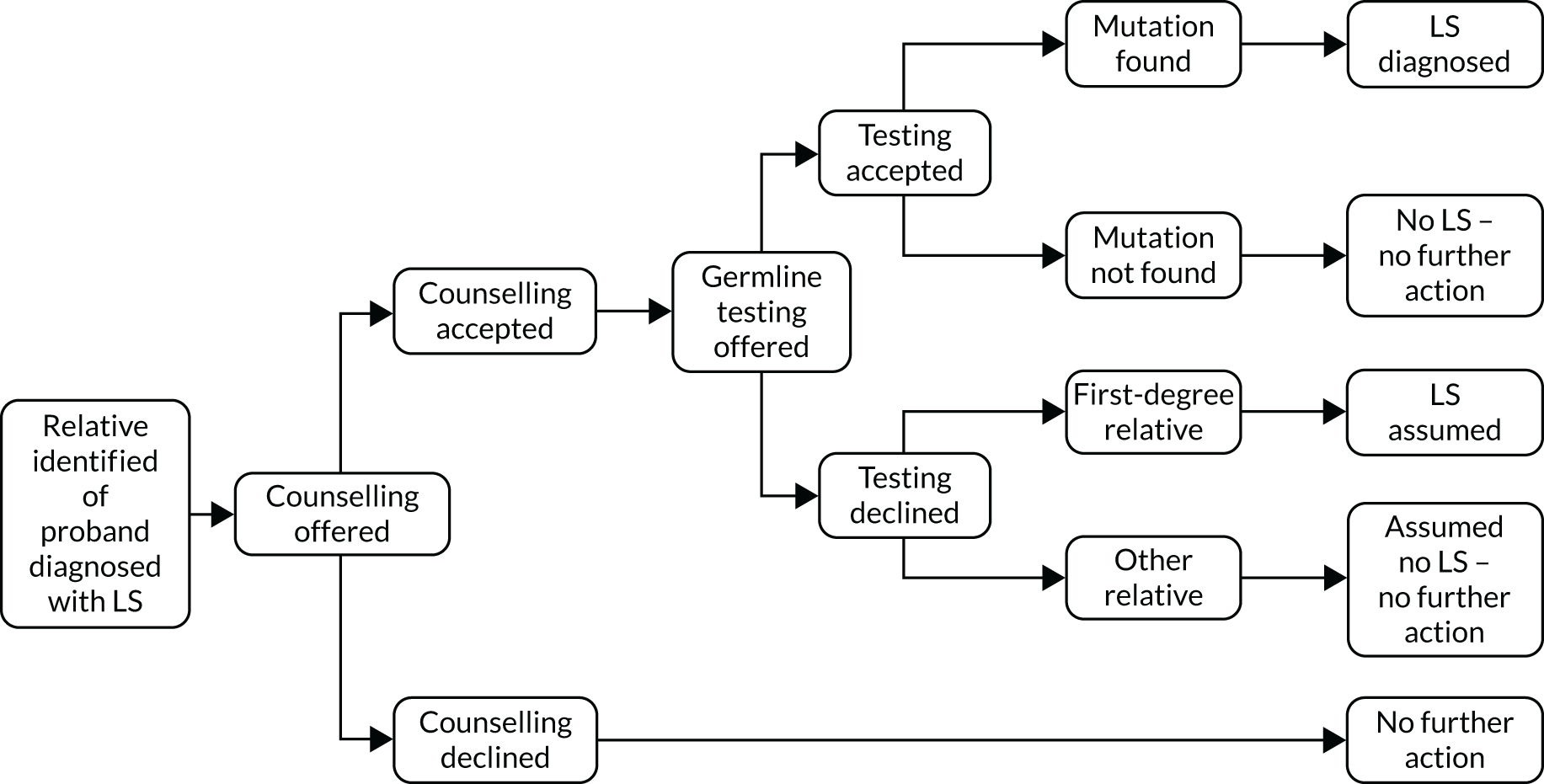
FIGURE 13.
Overview of testing and management pathway for relatives of probands. LS, Lynch syndrome.
Probands with endometrial cancer enter the model and are assigned to one of the 11 diagnostic strategies under assessment. Their path through the model is dependent on the result of the index test (combination of tests in the strategy) that they receive. Those with a positive index result are offered confirmatory germline testing. This is via a process of accepting genetic counselling and then accepting the genetic test. The proband can choose to accept or decline counselling, and those who accept counselling may then either accept or decline genetic testing. For probands who do consent to germline testing, Lynch syndrome status is confirmed.
Probands with a positive index test result and positive germline result are diagnosed with Lynch syndrome. Those with a positive index result and negative germline result are considered Lynch syndrome negative, but management of this group of individuals is subject to further investigation, as described in detail below. Probands with a positive index result who decline germline testing are assumed Lynch syndrome mutation negative, except for a specified proportion who are assumed Lynch syndrome, based on clinical suspicion.
Probands with a negative index result are not offered any further testing and are diagnosed with sporadic endometrial cancer.
In the final strategy, no index testing is performed, but probands proceed straight to genetic testing. In this case, genetic counselling and testing are offered directly to all endometrial cancer probands.
Diagnostic strategies for probands
The strategies modelled in the diagnostic component are as follows:
-
MSI testing followed by germline testing for Lynch syndrome-related mutations.
-
MSI testing followed by MLH1 promoter hypermethylation testing, followed by germline testing for Lynch syndrome-related mutations.
-
IHC MMR testing followed by germline testing for Lynch syndrome-related mutations.
-
IHC MMR testing followed by MLH1 promoter hypermethylation testing, followed by germline testing for Lynch syndrome-related mutations.
-
MSI followed by IHC then germline testing for Lynch syndrome-related mutations.
-
MSI followed by IHC plus MLH1 promoter hypermethylation testing then germline testing for Lynch syndrome-related mutations.
-
IHC followed by MSI then germline testing for Lynch syndrome-related mutations.
-
IHC followed by MSI plus MLH1 promoter hypermethylation testing then germline testing for Lynch syndrome-related mutations.
-
MSI and IHC done simultaneously, then germline testing for Lynch syndrome-related mutations.
-
MSI and IHC done simultaneously plus MLH1 promoter hypermethylation testing, then germline testing for Lynch syndrome-related mutations.
-
germline testing for Lynch syndrome-related mutations.
These strategies were compared with no testing for Lynch syndrome-related mutations and a fully incremental analysis was performed to report outcomes as ICERs based on cost per QALY.
Outcomes of diagnostic model for probands
Probands who test positive for a pathogenic mutation at germline testing are diagnosed with Lynch syndrome and offered Lynch syndrome surveillance for CRC and risk-reducing interventions, as appropriate. This is subject to the individual accepting these management options. Cascade testing is also triggered by Lynch syndrome-positive identification of the proband, whereby systematic testing of biologically at-risk relatives is undertaken. Output from the model is the number of probands with Lynch syndrome receiving Lynch syndrome surveillance and the number of probands with Lynch syndrome not receiving Lynch syndrome surveillance. As endometrial cancer probands are considered not to be at risk of further endometrial cancer, only female relatives of endometrial cancer probands who are diagnosed with Lynch syndrome are offered risk-reducing interventions for endometrial cancer.
Probands who test negative for a pathogenic mutation on index testing are diagnosed with sporadic endometrial cancer and continue with standard endometrial cancer management. They are not offered surveillance, nor is cascade testing pursued with their relatives.
Probands who decline germline testing after positive index test results are assumed a Lynch syndrome status based on clinical suspicion. Those who are assumed to not have Lynch syndrome are not offered surveillance or onward testing for their relatives. For those assumed to have Lynch syndrome (Lynch syndrome assumed), surveillance and risk reduction are offered, as well as surveillance and risk reduction for their first-degree relatives.
Probands with positive index test results on tumour tissue and negative germline results are considered Lynch syndrome negative, but, for a proportion of these, the clinical suspicion of Lynch syndrome remains. Similarly, despite negative results for currently identified pathogenic mutations for Lynch syndrome, germline testing may detect other mutation variances on these genes. These VUSs may or may not be later identified as pathogenic for Lynch syndrome, in which case status and management can be upgraded or downgraded accordingly. In these cases, it is assumed that further testing occurs on tumour tissue (somatic analysis) to either confirm sporadic cause of tumour or establish that the VUS is non-pathogenic for Lynch syndrome and management is then downgraded to that of non-Lynch syndrome individuals. Identification of new pathogenic variants is an alternative outcome of further testing, in which case individuals are modelled as being offered surveillance, as per Lynch syndrome assumed.
Probands who decline germline testing following a positive index test result are further categorised into ‘assumed non-Lynch syndrome’ or ‘Lynch syndrome assumed’, and managed accordingly.
Diagnostic strategies for relatives
Relatives follow strategy 11, straight to germline testing. This is also subject, in the model, to their acceptance of genetic counselling and acceptance of genetic testing following this counselling.
Outcomes of diagnostic model for relatives
Relatives who test positive for a pathogenic mutation at germline testing are diagnosed with Lynch syndrome and offered Lynch syndrome surveillance for CRC and risk-reducing interventions, as appropriate. This is subject to the individual accepting these management options.
Relatives who test negative for a pathogenic mutation at germline testing are not diagnosed with Lynch syndrome and no further surveillance measures are offered.
First-degree relatives who decline germline testing are diagnosed Lynch syndrome assumed and offered surveillance for CRC. Second-degree relatives and more distant relatives are subject to no further action.
Outcomes of diagnostic model summary
-
Number of probands with Lynch syndrome receiving Lynch syndrome surveillance (true positive accepting).
-
Number of probands without Lynch syndrome receiving Lynch syndrome surveillance (false positive accepting).
-
Number of probands without Lynch syndrome who do not receive Lynch syndrome surveillance (delineated as those identified as Lynch syndrome positive who decline surveillance and those diagnosed as Lynch syndrome negative) (false positive declining and true negative not offered).
-
Number of VUSs and Lynch syndrome assumed diagnoses.
Long-term outcomes model
We estimated the benefits of cascade testing by developing cohort state-transition models that simulate the incidence and mortality associated with Lynch syndrome-related cancers. We use these models to predict the benefit of being identified with Lynch syndrome through cascade testing by simulating incidence and mortality with, and without, surveillance and risk reduction measures, which we assume are adopted once Lynch syndrome has been identified. The cohort that is modelled consists of a group of individuals identical in terms of age at which they were identified as having Lynch syndrome, sex and previous Lynch syndrome cancer history (the model is repeated for a wide range of cohorts to provide the information needed for the decision tree model; this is described further in Figure 14).
FIGURE 14.
Overview of long-term model diagram. EC, endometrial cancer.
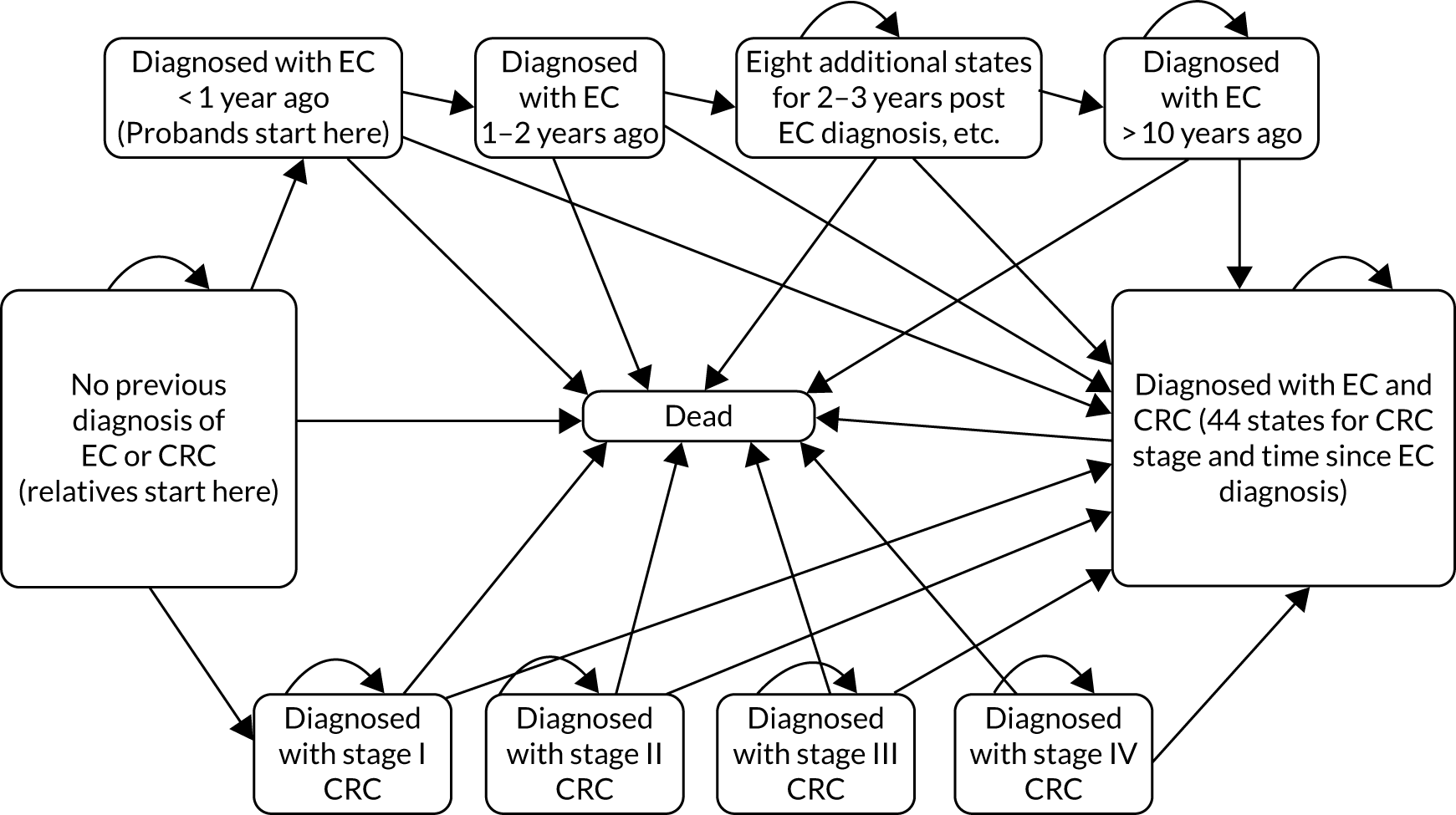
The model has five states: cancer free, CRC, endometrial cancer, both CRC and endometrial cancer, and dead. The endometrial cancer state comprises 10 ‘tunnel states’ reflecting time since incidence of endometrial cancer. These are known as tunnel states because a person in this state must move to the next state in the sequence at the end of the cycle (unless they move to death). The cohort can be of any age from 0 to 100 years and be male or female, and can start in any state. The state for women who have both endometrial cancer and CRC, therefore, has four substates, each with 10 tunnel states. For this decision problem, we simulate cohorts who are cancer free or recently diagnosed with endometrial cancer, male or female and aged in annual increments between 25 and 74 years. This gives 200 cohorts in total. We do not model outcomes for those without Lynch syndrome, on the assumption that they experience no long-term costs or benefits from Lynch syndrome testing.
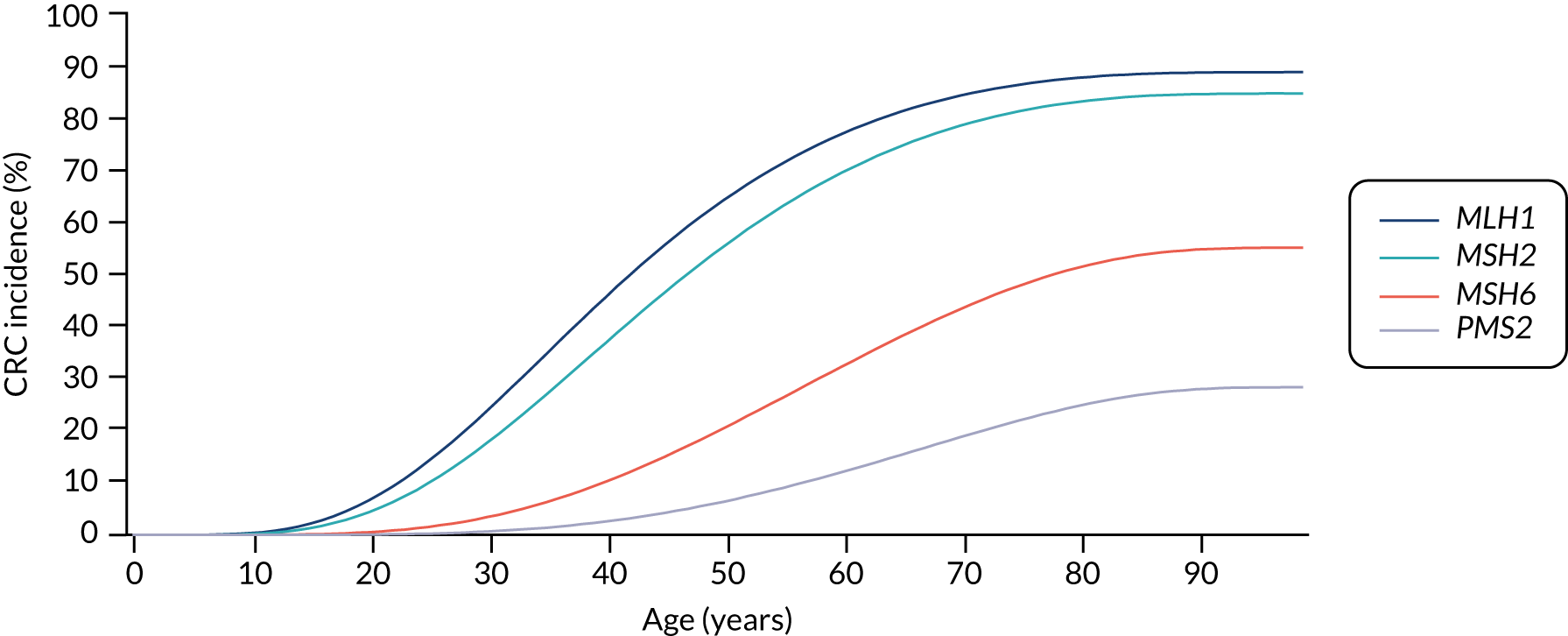
For the comparator, we assume that, as the person is unaware of their Lynch syndrome status, no surveillance or risk reduction measures are offered. We model age-related annual incidence of CRC and endometrial cancer. For CRC, we further assume that incidence is gene dependent. In line with Snowsill et al. ,43 we assume that this incidence has a log-normal distribution. Previous work in this field has drawn on data on individuals with Lynch syndrome who benefit from colonoscopic surveillance. We follow that work in assuming that, based on Järvinen et al. ,44 surveillance reduces incidence with a hazard ratio of 0.387. We apply this to the log-normal distribution to derive the incidence rates illustrated in Figures 15 and 16.
FIGURE 15.
Modelled cumulative incidence of CRC in females with Lynch syndrome, assuming no surveillance.
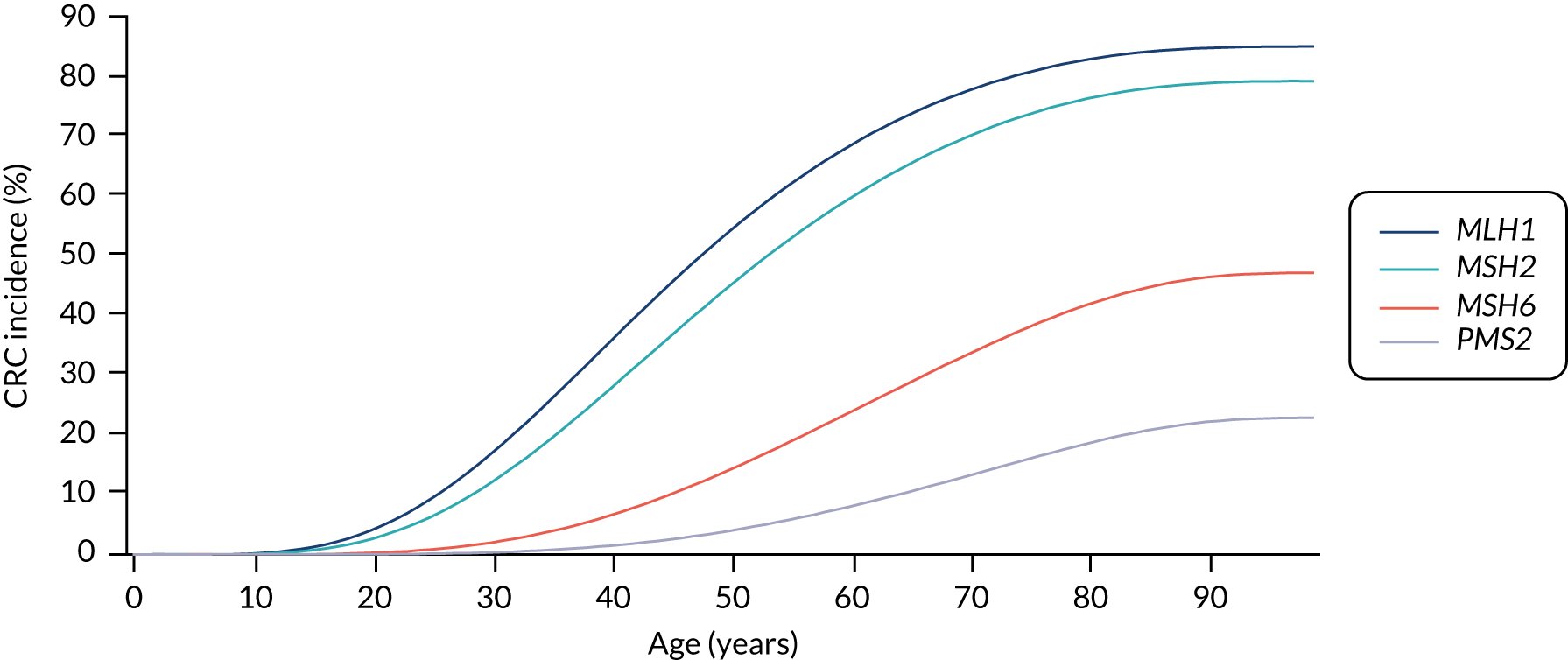
FIGURE 16.
Modelled cumulative incidence of CRC in males with Lynch syndrome, assuming no surveillance.
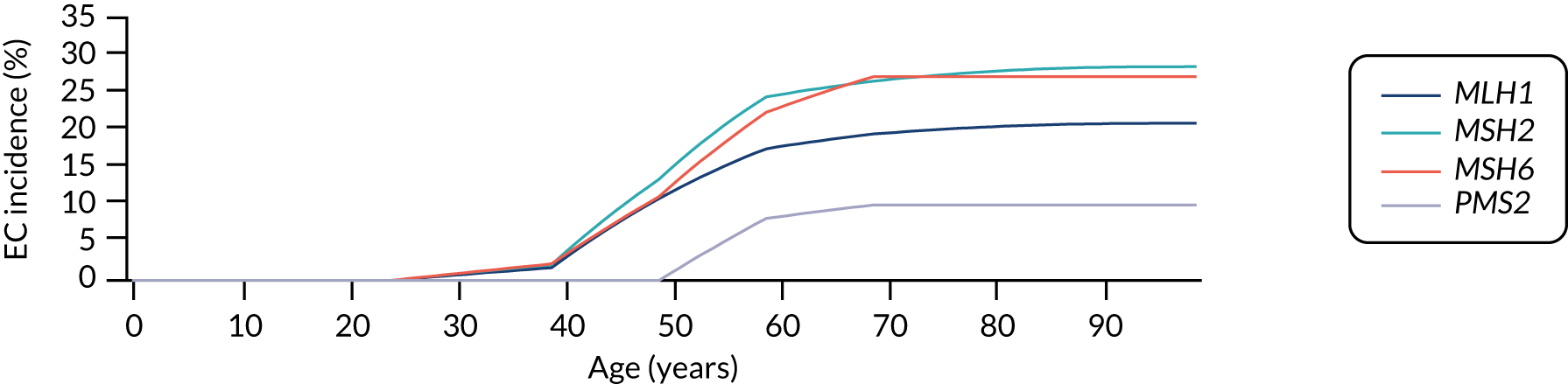
For endometrial cancer, we sourced incidence data from the Prospective Lynch Syndrome Database (PLSD). 45 This database reported gene-based risk of cancer based on 6350 individuals with Lynch syndrome. Risks are reported at ages 25, 40, 50, 60, 70 and 75 years. We fitted a piecewise linear model to these data. The cumulative lifetime incidence of endometrial cancer in the absence of preventative measures implied by this assumption is illustrated in Figure 17.
FIGURE 17.
Modelled cumulative incidence of EC in females with Lynch syndrome. EC, endometrial cancer.
For CRC, we assumed that the proportion presenting with stages I to IV were 18.8%, 48.8%, 21.3% and 11.3%, respectively. We assumed a one-off cost of treatment, dependent on age and stage at diagnosis (Table 3).
| Age (years) | Cost (£) | |||
|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | |
| 0–49 | 8754.12 | 8740.53 | 14,489.51 | 11,704.91 |
| 50–59 | 5712.39 | 7015.84 | 9691.73 | 8443.68 |
| 60–69 | 4623.22 | 5351.77 | 7259.39 | 6508.89 |
| 70–79 | 3177.62 | 3454.61 | 4485.25 | 4365.04 |
| ≥ 80 | 1379.75 | 1545.95 | 1560.59 | 806.95 |
We assumed that CRC mortality is stage dependent, with transition probabilities of 0.009, 0.035, 0.098 and 0.543 for stages I to IV, respectively. 43
For endometrial cancer, we assumed a one-off treatment cost of £6510, in line with previous work. 12 We drew on Cancer Research UK-reported statistics on endometrial cancer mortality,46 and assumed that these were the same for those with Lynch syndrome as for those without. We assumed that those who have one Lynch syndrome risk cancer are at the same risk of developing the second one as if they were cancer free, conditional on not having died from the first cancer. We also applied an age-dependent transition probability for mortality from other causes. All those still alive in the model were assigned an age-dependent quality-of-life utility weighting using accepted methodology by Ara and Brazier,47 except that those with CRC stage IV were assigned a utility of 0.178, as modelled by Snowsill et al. 12
With these assumptions, we ran the cohort model separately for a number of cohorts defined as having the same age at identification, sex and cancer history. For each cohort, we estimated the mean lifetime costs and QALYs incurred.
We then assumed that the following risk reduction and surveillance methods were offered when an individual is identified as having Lynch syndrome.
Chemoprophylaxis
We assume that, once identified with Lynch syndrome, individuals take aspirin as indicated in the Colorectal Adenoma/carcinoma Prevention Programme 2 (CAPP2) trial48 and, based on the results of that trial, their probability of developing cancer each year is reduced by a factor of 0.56 (applied equally to endometrial cancer and CRC risk).
Colorectal cancer surveillance
We assume that individuals known to have Lynch syndrome have biennial colonoscopies from age 25 years (or age at identification of Lynch syndrome if later) until age 74 years. We assume the cost of colonoscopy is £325. 49 We assume that 100,000 colonoscopies result in 8.3 deaths, 40 perforations, and 55 bleeding events necessitating hospital treatment (of which 40 are mild, 10 are moderate and five are severe). This increases the average cost of colonoscopy by £2.89. We assume that this surveillance affects both incidence and stage at presentation. For stage at presentation, the assumed proportions for those participating in surveillance are 68.6%, 10.5%, 12.8% and 8.1% for stages I to IV, respectively.
Surgical prophylaxis to prevent endometrial cancer
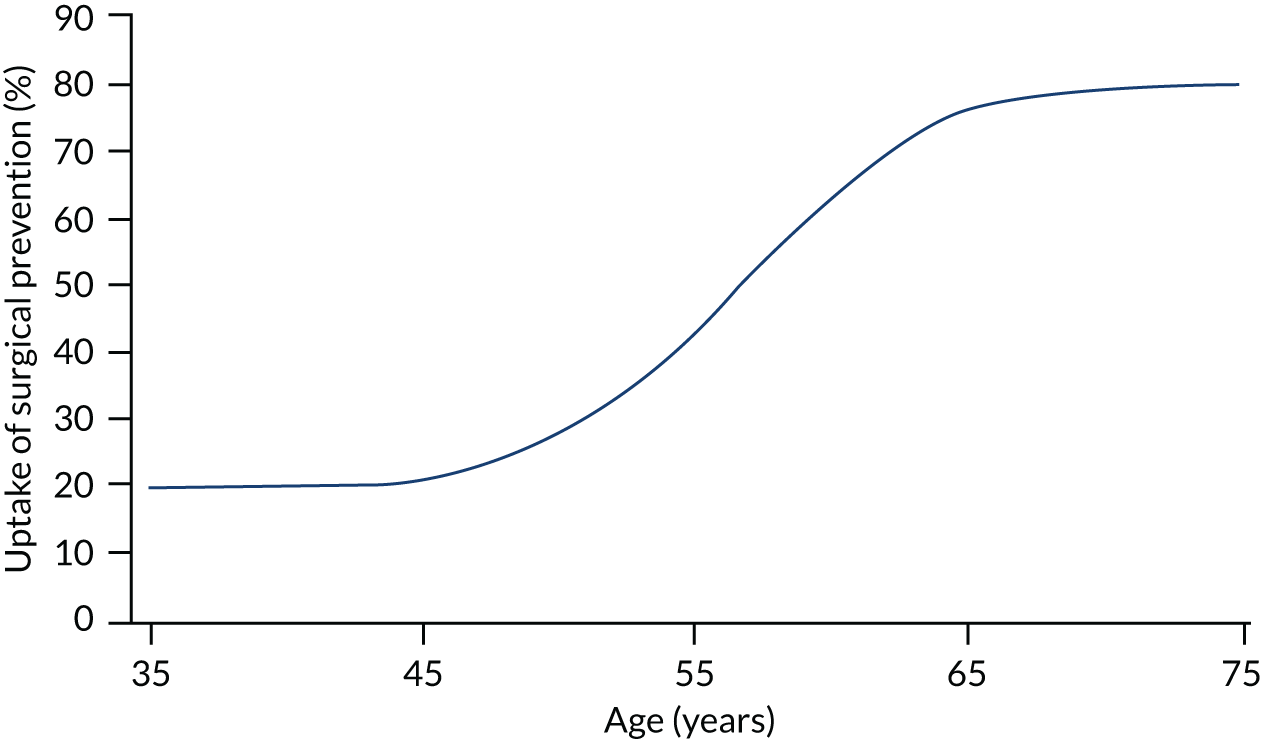
We assume that women with Lynch syndrome can opt for hysterectomy and oophorectomy (H-BSO), and that this eliminates their risk of endometrial cancer. We make the assumption that the uptake of this increases with age, based on consideration of the average age at diagnosis of probands and subsequent ages of their identified relatives, and when women might feel ready based on completion of family, menopause, etc. The cost of this is assumed to be £3428. This assumption is illustrated in Figure 18.
FIGURE 18.
Uptake of surgical prophylaxis.
Gynaecological surveillance
We assume that women who have not had surgical prophylaxis undergo annual surveillance to detect endometrial cancer. The cost is £39, plus an additional cost of £473.41 for those requiring referral for invasive surveillance. We assume that this referral occurs in 10% of cases. This does not affect the incidence of endometrial cancer, but reduces mortality by 10.2%.
Parameters
Parameter input values for both diagnostic and long-term components of the model were sourced from literature obtained during the clinical effectiveness and cost-effectiveness systematic literature review process, with the best available evidence used to inform the base case. When suitable input parameters were not obtained, targeted searches were undertaken and individual publications critiqued. Additional information was also provided by clinical experts in the field. Discussion and critique of the sources of each parameter are detailed in Chapter 6, Discussion of model input parameters.
The model runs in 1-year cycles. The starting population is of the same age and sex, in the same state, (i.e. cancer free or recent diagnosis of endometrial cancer).
Each year:
-
Transition occurs from all states to the death state based on annual mortality rates for all causes other than CRC or endometrial cancer. Death from the respective cancer state is accounted for in further transition from CRC, based on stage, and from endometrial cancer based on length of time they have spent in the endometrial cancer state. Transitions from all states to death are based on all-cause mortality.
-
Further transitions from CRC or CRC plus endometrial cancer to death based on stage (CRC) or dwell time in state (endometrial cancer).
-
Survivors in the endometrial cancer or endometrial cancer plus CRC states at the end of each cycle move on to the next tunnel state or remain in the final tunnel state prior to death. All those in the endometrial cancer or endometrial cancer plus CRC state who survive move to the next tunnel state (or stay in tunnel state 10). A quality-of-life score is assigned to the average number of individuals inhabiting each state at the start and end of each cycle.
Treatment costs of the respective cancers are assigned to an individual on entry to the cancer state and applied to the first year only (as a single, whole-disease cost). The average of the number of individuals in each state at the start and end of the cycle is assigned a quality-of-life score based on their age.
Those who move to a cancer state during the cycle are assigned treatment costs (all treatment costs are assumed to occur in the first year in the state). The model is run twice for each cohort: once assuming no Lynch syndrome-ameliorating measures (e.g. screening, prophylactic drugs), and once assuming measures are applied (as the model starts at the age at which an individual would be identified with Lynch syndrome were they to undergo genetic testing). These measures affect transition probabilities such as incidence and mortality, thereby capturing the benefit of the measures. Costs are also captured for those eligible for such measures. Colonoscopy is costed every other cycle. The number of women undergoing surgical prophylaxis is estimated from the number of women in the cancer-free or CRC states, by applying a proportion based on age, as described previously. It is assumed that the costs of aspirin, as a cheap over-the-counter medication, are not borne by the NHS.
The outputs from the model were the incremental costs and QALYs resulting from the addition of Lynch syndrome cancer-ameliorating measures. These were calculated separately by sex, for those cancer-free and those recently diagnosed with endometrial cancer, and for ages 25–74 years in 1-year intervals. These results provide an estimate of the benefit of Lynch syndrome cancer-ameliorating measures, and how these benefits vary by age and sex. To further illustrate how benefits arise in the model, results were extracted on the numbers in, and moving between, each state. These allowed the calculation of life-years gained, cancers avoided and cancer deaths prevented.
To allow these results to inform the decision tree model, we assumed that cascadees were equally likely to be any age between 25 and 74 years, and that the mean age of probands was 49 years. From this, we were able to define an output from the model as the average of the incremental results across all ages for cascadees, and the incremental results for women aged 49 years recently diagnosed with endometrial cancer for the probands. These results were used as the pay-offs for the terminal node in the decision tree model, so that the costs and QALYs per strategy could be calculated.
Quality assurance
Modelling of the independent economic assessment was conducted by two health economists, with primary development of each of the two components of the model done independently, and then checked by the second. Internal review by a senior health economist was also undertaken, with code review and cross-checking of input parameters to ensure that they originated from the described source. Furthermore, the reviewer constructed an alternative version of the diagnostic model in TreeAge (TreeAge Software, Inc., Williamstown, MA, USA) (rather than Excel) so cross-checking of results could also be carried out.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) was used to determine the impact of joint parameter uncertainty. Model parameters were assigned a distribution reflecting the amount and pattern of variation, and cost-effectiveness results were calculated by simultaneously selecting random values from each distribution. This process was repeated 10,000 times, with simulations plotted on an incremental cost-effectiveness plane, with each point representing uncertainty in the incremental mean costs and QALYs between the strategies being compared. The results from these simulations were used to obtain cost-effectiveness acceptability curves (CEACs), which illustrate the effect of sampling uncertainty, and present the probability that the testing strategy is optimal at a range of willingness-to-pay (WTP) threshold values.
To propagate uncertainty across the decision tree and lifetime cohort models, we first carried out Monte Carlo simulation for the lifetime model with distributions assigned to all stochastic parameters. This produced an output set that could be used as an input table for the pay-off nodes for probands and relatives with Lynch syndrome in the decision tree model when it was run stochastically, producing PSA outputs that reflected joint uncertainty across the two models.
Sensitivity analysis
Univariate one-way sensitivity analysis was used to explore the impact of varying one parameter at a time, while keeping all other inputs constant, to assess the robustness of the model. We varied parameter values using upper and lower limits and presented results in the form of a tornado plot.
Scenario analyses
Alternative analyses were conducted for the following scenarios:
-
Strategy-level test accuracy obtained from the Proportion of Endometrial Tumours Associated with Lynch Syndrome (PETALS) study (Dr Neil AJ Ryan, University of Manchester, 11 November 2019, personal communication).
-
Costs of testing obtained from the PETALS study (Dr Neil AJ Ryan, personal communication).
-
Strategy-level test accuracy obtained from the PETALS study (Dr Neil AJ Ryan, personal communication) and costs of testing obtained from Ryan et al. 50
-
Disutility increment as a result of having cancer increased from the value modelled in the base case.
-
Gynaecological surveillance excluded.
-
Three-year colonoscopy surveillance.
-
Excluding benefit from aspirin.
-
Excluding hazard ratio reducing incidence of CRC as a result of surveillance.
Assumptions in base case
-
Microsatellite instability-high results are treated as a positive indicator of Lynch syndrome, whereas MSI-L results are treated as a negative indicator of Lynch syndrome.
-
The sensitivity of MSI and IHC testing did not depend on which MMR gene is mutated.
-
The average number of relatives per proband was six (2.5 of whom were first-degree relatives).
-
Colorectal surveillance colonoscopies occurred every 2 years, starting age 25 years and stopping at age 75 years.
-
Surveillance colonoscopies are effective immediately on commencement of surveillance and ineffective immediately after discontinuation (i.e. no lag time).
-
Disutility is applied only to people with stage IV CRC.
-
Endometrial cancer is not modelled for women without Lynch syndrome-causing mutations.
-
Treatment for endometrial cancer is assumed to be total abdominal H-BSO with/without chemotherapy with/without radiotherapy.
-
Survival of probands with endometrial cancer is not affected by Lynch syndrome status.
-
Surveillance for endometrial cancer comprises an annual review with their general practitioner (GP), with 10% of women attending referred for invasive gynaecological surveillance, consisting of gynaecological examination, transvaginal ultrasonography, endometrial biopsy and cancer antigen-125 (CA-125) testing.
-
Gynaecological surveillance reduced the risk of mortality from endometrial cancer by 10.2%.
-
No disutility arising from prophylactic hysterectomy was assumed.
-
Prophylactic hysterectomy (total abdominal H-BSO) eliminates risk of endometrial cancer.
-
Prophylactic hysterectomy (total abdominal H-BSO) is offered to all female relatives, with no age restrictions.
Chapter 4 Clinical effectiveness results
Clinical effectiveness results
Search results
The study selection process for the clinical effectiveness review is illustrated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram in Figure 19. The search identified 6259 records through database and other searches. Following duplicate removal we screened 3308 records. One additional unpublished study, the PETALS study, was provided by NICE and included for key question 1 (Dr Neil AJ Ryan, personal communication). A total of 2981 studies were excluded by their titles and abstracts, leaving 327 full texts to be assessed for eligibility for inclusion in the review. Of these, 282 papers were subsequently excluded, leaving 45 papers (including the unpublished PETALS study). 15,16,51–92 All 45 papers were relevant for key question 1: the test accuracy of MSI- and IHC-based strategies for determining Lynch syndrome in people with endometrial cancer. The most common reasons for exclusion of test accuracy studies at this stage were that there was no eligible reference standard in the studies and that too little information was included to enable quality appraisal. The full list of excluded studies with reasons for exclusion can be found in Appendix 3.
FIGURE 19.
The PRISMA flow diagram showing the study selection process for the clinical effectiveness review.
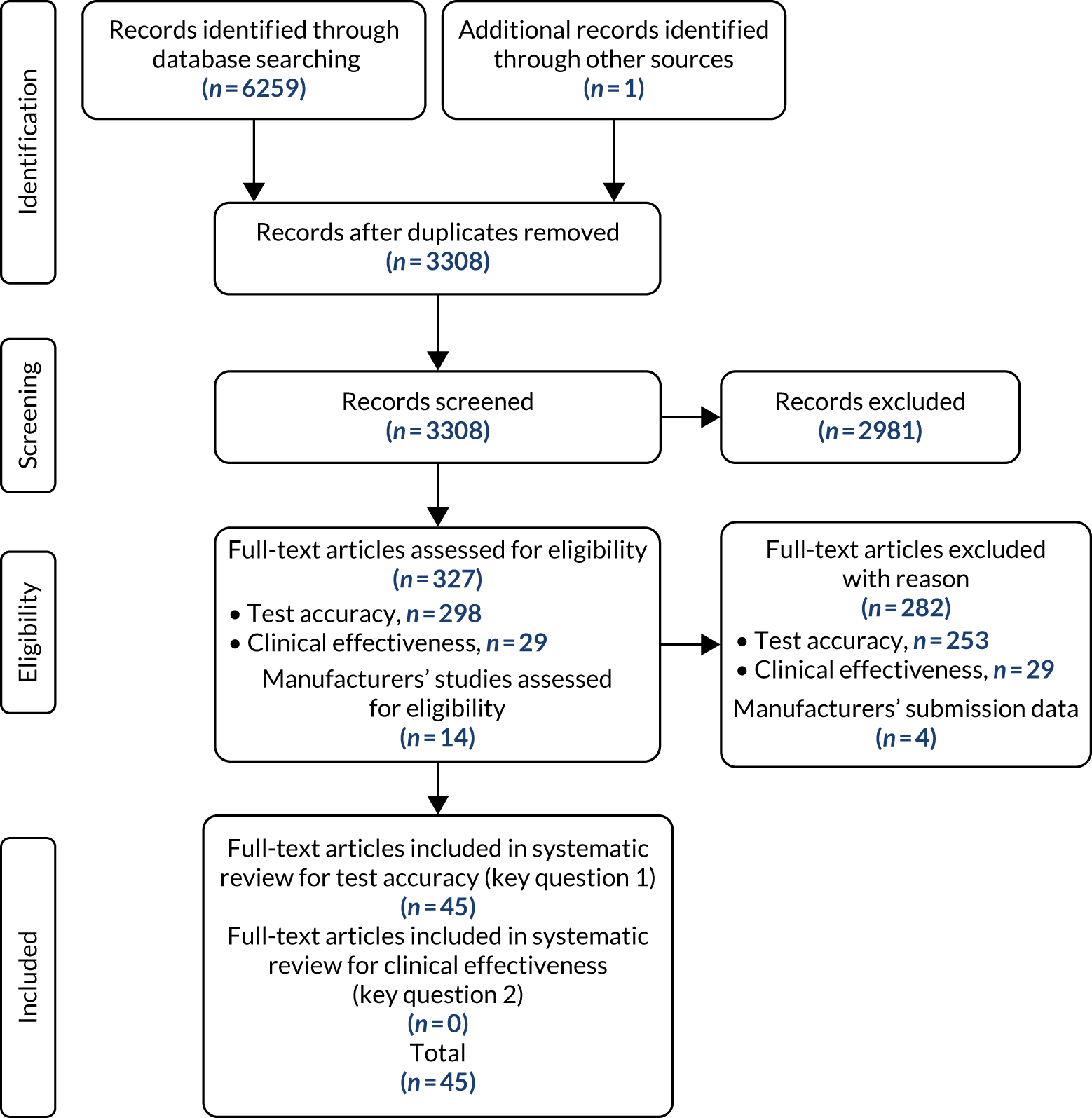
For key question 2, on the clinical effectiveness benefits and harms of testing for Lynch syndrome among people who have endometrial cancer, and/or their relatives, the search identified 29 studies that were potentially eligible for this review. We carried out the full-text assessment of the 29 records against the predefined inclusion criteria as stated in Table 2. No studies were identified that were relevant for key question 2 on the clinical effectiveness benefits and harms of testing for Lynch syndrome among people who have endometrial cancer, and/or their relatives. The most common reason for exclusion of clinical effectiveness studies at this stage was study design [not randomised controlled trials (RCTs)]. The full list of excluded studies with reasons for exclusion can be found in Appendix 3.
Study characteristics
The characteristics of the 45 studies included in the clinical effectiveness review are described in Appendix 4. ‘Unselected’ is defined in the table as including all patients in the setting over the study time period, without restrictions by age, cancer histology or family history.
Population
The 45 included papers included approximately 10,600 participants, with sample sizes ranging from 1285 to 1459 participants. 56 The results of five studies were reported in more than one paper. 53,56,57,60,65,68,70,76,87,89 These papers have been reported together (i.e. two papers are combined into one study) throughout this report. Only two studies took place in the UK [one published51 and the PETALS study (Dr Neil AJ Ryan, personal communication)], with the majority taking place in the USA (15/45; 33%) and Europe (11/45; 24%). However, ethnicity was largely unreported [32/45 (71%) did not report ethnicity]. Several studies included age as an inclusion criterion, often limiting patients to ≤ 50 years for inclusion in a study. 16,51,54,79 In the remaining studies, ages ranged from 17 to 100 years. 15,63 Only 24% of studies (10/41) were in unselected populations, meaning that all patients in particular settings were included over the study time period, without any restrictions by age, cancer histology or family history. Two studies have been classified as unselected populations but limited to all adults (all those aged > 18 years). 55,81
A total of 44% (18/41) of studies reported on patients who had previous or concurrent cancers. The number of patients included across the studies who had a history of cancer ranged from 0 to 100. 58,74,80,85 This range can be explained by studies using a history of cancer as an inclusion or exclusion criterion. For studies not using cancer history as an inclusion or exclusion criterion, the proportion ranged from 0.8% to 22.4%. 54,77,90 The types of cancer reported were ovarian, pancreatic, colon, endometrial, urinary tract, brain, breast, skin, bladder, cervical and gastric cancers.
Index tests
Nine studies (11 papers) included IHC only,52,56,59,65,69,70,76,77,84,88,90 three studies included MSI-based testing only,15,64,74 28 studies (31 papers, including the unpublished PETALS study) included both tests16,51,53–55,57,58,60–63,66–68,71–73,75,78–83,85–87,89,91,92 and 24 studies (29 papers, including the unpublished PETALS study) included MLH1 promoter hypermethylation testing in combination with IHC or MSI testing (MSI and MLH1 promoter hypermethylation testing, n = 2; IHC and MLH1 promoter hypermethylation testing, n = 6; MSI, IHC and MLH1 promoter hypermethylation testing, n = 16). 15,16,55–61,63–65,67–72,76–78,81,83,84,86,87,89,92
Comparator and reference standard
The reference standards considered appropriate in this review were sequencing in combination with MLPA, long-range PCR or targeted array comparative genomic hybridisation. Of the 33 studies (36 papers) that included a reference standard, 21 studies (24 papers, including the unpublished PETALS study) included sequencing in combination with an additional method deemed appropriate by this review to detect larger structural changes. 15,16,51,52,55,57–59,61–63,65–68,77,78,81–83,85,88–90 Two studies (three papers) reported only sequencing, and did not report any details on the method of sequencing. 60,70,92 One study did not mention sequencing, but used array comparative genomic hybridisation, PCR and MLPA in combination. 84 Two studies (three papers) did not report clearly the methods of germline testing. 56,69,76 Six of the included studies used an additional reference standard test prior to sequencing that was not an eligible reference standard in this review. Two studies used single-strand conformational variance. 64,74 The studies by Berends et al. ,54 Rubio et al. 82 and Mercado et al. 73 used denaturing gel electrophoresis, and the study reported across two papers by Baldinu et al. 53 and Strazzullo et al. 87 used denaturing high-performance liquid chromatography.
Outcomes
Data on the number of Lynch syndrome diagnoses among women with endometrial cancer were reported in 32 studies (including the unpublished PETALS study). 15,16,51–59,61–64,66,67,69,70,77,78,81–85,88–90,92 Four studies provided head-to-head test accuracy data for IHC- and MSI-based testing. 16,54,58,82 Complete test accuracy data were provided by five studies for IHC,16,54,58,82,90 four studies for MSI-based testing16,54,58,82 and four studies for IHC, MSI-based testing and MLH1 promoter hypermethylation testing. 16,58,81,83 An additional nine studies provided partial test accuracy data (true positives, false positives and PPVs) in which only women who tested positive on index tests were considered for germline testing. 16,54,58,63,66,78,82,90,92
Concordance between IHC and MSI-based testing was assessed in 23 studies. 15,16,51,54,55,58,61–63,67,68,71,72,75,78–82,85–87,91
Setting
The majority of the participants in the included studies were recruited from hospitals [26/41 (63%)]. 15,51,52,57–68,70,77–79,81–84,88,89,92 Other studies took place in cancer registries [5/41 (12%)], cancer and radiation centres/clinics [6/41 (15%)], medical centres [2/41 (5%)] and tissue biobank repositories [1/41 (2%)]. In one study, the setting was not reported. 71
Study design
All the studies in this review had a cohort design. A total of 39% of studies (16/41) were prospective cohort studies, 46% (19/41) were retrospective and 12% (6/41) had both prospective and retrospective elements. One study had a mixed design, comprising both a prospective cohort study looking at MMR assessments and a cross-sectional study comparing clinical and pathological features between Lynch syndrome and Lynch-like syndrome groups. 60,70
Quality considerations of included studies
Quality Assessment of Diagnostic Accuracy Studies-2
In the proposed testing strategies 1–10 (below), only women who tested positive on the index tests would be offered germline testing. Some studies report results from implementing the strategies of interest; however, these are partial test accuracy studies because data on true negatives and false negatives are not available. It is not, and it would not be, possible to calculate sensitivity, specificity or NPVs from these studies because of a lack of follow-up of women who were negative on the index tests. Studies in which all patients receive the reference standard provide sensitivity, specificity, PPVs and NPVs, and have been defined here as full test accuracy data studies. A total of 41 studies (45 papers) were identified, of which seven provided full test accuracy data (as all participants received both the index test and reference standard),16,54,55,58,82,83,90 26 studies (29 papers, including the PETALS study) provided partial test accuracy data (as only a subsample of participants received both the index test and reference standard)15,51–53,55–57,59–64,66–70,73,74,76–78,84,85,88,89,92 and 23 studies (including the PETALS study) provided data on concordance. 16,51,54,55,58,61,63,67,68,71,72,75,78–80,82,86,87,91
The studies providing test accuracy information were appraised using the QUADAS-2 tool and the seven complete test accuracy studies are presented prior to, and separately from, the partial test accuracy studies. Studies reporting on concordance were appraised using the quality appraisal tool for studies of diagnostic reliability (QAREL). Sixteen studies (16 papers, including the PETALS study) reported both test accuracy and concordance and were appraised using both tools. 15,16,51,54,55,58,61–64,67,68,78,82,85
Quality considerations of included studies: complete test accuracy studies
The assessment of risk of bias and applicability for the seven complete test accuracy studies using the QUADAS-2 tool are summarised in Table 4. 16,54,55,58,82,83,90 Six of the seven studies included both MSI and IHC index tests, and four included additional MLH1 promoter hypermethylation testing. 16,55,58,83 All index tests have been reported separately.
| Study and year | Risk of bias | Applicability concern level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test: MSI | Index test: IHC | Index test: MLH1 promoter hypermethylation | Reference test | Flow and timing | Role of sponsor | Patient selection | Index Test: MSI | Index test: IHC | Index test: MLH1 promoter hypermethylation | Reference standard | |
| Berends et al.54 2003 | High | Unclear | Unclear | NA | Unclear | High | Low | High | Unclear | Unclear | NA | High |
| Chao et al.58 2019 | High | Unclear | Unclear | Unclear | Unclear | High | Low | High | Low | Low | Low | Low |
| Lu et al.16 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | High | Unclear | Unclear | Unclear | Low |
| Bruegl et al.55 2016 | High | Unclear | Unclear | Unclear | Unclear | Low | high | High | Low | Low | Low | Low |
| Rubio et al.82 2016 | High | Unclear | Unclear | NA | Unclear | High | Low | High | Unclear | Unclear | NA | Low |
| Salvador et al.83 2019 | Low | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear | Unclear | Unclear | Unclear | Low |
| Tian et al.90 2019 | High | NA | Unclear | NA | Unclear | High | Unclear | High | NA | Unclear | NA | Unclear |
Risk of bias for complete test accuracy studies
In general, the methodological and reporting quality of the included studies was poor, with risk of bias considered high in two or more domains for five studies (71%). 54,55,58,82,90 One study was judged to be at high risk of bias in one domain,90 and the remaining study was rated as having an unclear risk of bias in the majority of domains [5/7 domains (71%)]. 16 No study was rated as having a low risk of bias in all domains. In 71% of studies (5/7), the risk of bias for patient selection was deemed high (domain 1: patient selection). 54,55,58,82,90 In these studies, patients were selected for inclusion by excluding patients on the grounds of age, having synchronous cancers or deemed judgement of low risk (by age and family history). In 14.5% of studies (1/7), there was not enough information to determine the risk of bias in how patients had been selected. 16 Only one study was deemed to have a low risk of bias in the patient selection: consecutive enrolment of patients in a cohort study, with no exclusions. 83
Six out of seven studies were head-to-head studies, testing patients using both MSI and IHC index tests, with one test using IHC alone. 90 In all studies, for both tests [6/6 MSI, 7/7 IHC (100%)], the risk of bias was unclear because of a lack of information around blinding between index test and reference standard results, whether thresholds were prespecified or determined pragmatically, and whether or not the laboratories performing the index tests participated in an accredited quality assessment/control scheme (domain 2: index tests). Four out of seven studies also undertook MLH1 promoter hypermethylation testing, all of which lacked information on blinding and quality assessment and so were also rated as having an unclear risk of bias. 16,55,58,90
Unclear reporting was common in the reference standard domain (domain 3: reference standard). In all studies, there was not enough information to determine whether or not the results of germline testing (reference standard) were determined without knowledge of the MSI and IHC test results (index tests). In addition, for many of the studies, it was unclear whether or not the reference standard used would correctly identify Lynch syndrome [5/7 (71%)], because there was a lack of information about the testing methods used and/or whether or not quality assurance was in place. 54,55,58,82,83 If the reference standard used in the studies does not correctly identify Lynch syndrome, this may make the index tests appear more or less accurate than they actually are. Lynch syndrome can be determined by using sequencing to detect point mutations in combination with MLPA, next-generation copy number, long-range PCR or targeted array comparative genomic hybridisation to detect larger rearrangements or for dosage analysis. One study used sequencing alongside denaturing gradient gel electrophoresis, which is not a recognised reference standard for the purpose of this study. 54 Five studies did not report information on the reference standard being carried out in accordance with best-practice guidelines (e.g. Association for Clinical Genetic Services Best Practice Guidelines for Genetic Testing and Diagnosis of Lynch Syndrome,33 American College of Medical Genetics technical standards and guidelines for genetic testing for inherited CRC18) in appropriately accredited laboratories (e.g. according to the UK Accreditation Service, the Clinical Laboratory Improvement Amendments). 54,55,58,82,83
The flow of participants through a study was rated as having a high risk of bias in 57% of studies (4/7, domain 4: flow and timing). 54,58,82,90 Three of the studies did not include all participants in their analysis,58,82,90 and one study did not give all participants the same reference standard: sequencing was given only to those with aberrant band patterns using denaturing gradient gel electrophoresis. 54 The remaining three studies were deemed to have a low risk of bias, with all participants receiving the reference standard, all participants receiving the same reference standard and all participants being included in the analysis. 16,55,83
The risk of bias associated with the role of the sponsor in four of the seven studies was deemed to be low (domain 6: role of the sponsor). 16,54,58,82 In two studies, multiple authors were employed by genetics companies who funded the studies. 55,83 In one study the funding was not specified. 90
Applicability of study findings for complete test accuracy studies
The applicability of study findings was assessed in regard to three domains: patient selection, index test (MSI, IHC and MLH1 promoter hypermethylation testing) and reference standard (germline testing). There were significant concerns regarding the applicability of the studies to UK practice for patient selection in six of the seven studies (86%; domain 1: patient selection). 16,54,55,58,82,90 In one study,83 there was not enough information to determine whether or not the population was comparable to that of the review question. Based on this review’s scope, were tests to be implemented, the test would be given to any patient with endometrial cancer, regardless of age or ethnicity. In all six studies, the populations were not ethnically comparable to the UK and/or were limited by age. None of the seven studies was undertaken in the UK.
Concerns regarding index testing (MSI and IHC) were low in 29% of studies (2/7; domain 2: index tests), with tests carried out according to best-practice guidelines and via laboratories that are participating in quality assurance programmes. 55,58 In the remaining studies, there was not enough information to ascertain the applicability of index testing [5/7 (71%) IHC and 4/6 MSI (67%)]. 16,54,82 Only four of the studies reported on MLH1 promoter hypermethylation testing; of those, 50% (2/4) had a high level of concern regarding applicability,55,58 and 50% did not report enough information to make a judgement. 16,83
Only one study was rated as having a high level of concern for the applicability with respect to the reference standard, as a non-applicable reference standard (denaturing gel electrophoresis) was used as the primary reference standard, with some patients also receiving sequencing. 54 The remainder were all rated as having a low level of concern regarding applicability, bar one study that did not report enough information for the raters to make a determination of applicability. 90
All judgements of risk of bias and applicability concerns for studies reporting complete test accuracy data can be found in Table 4.
Quality considerations of included studies: partial test accuracy studies
The assessment of risk of bias and applicability for the 26 partial test accuracy studies (29 papers, including the PETALS study) using the QUADAS-2 tool are summarised in Table 5. 15,51–53,55–57,59–64,66–70,73,74,76–78,84,85,88,89,92 Sixteen studies included both MSI and IHC index tests (62%; 16/26), one study reported only MSI64 and nine studies reported only IHC. 52,59,69,74,76,77,84,88,89 Seventeen studies (20 papers, including the PETALS study) included the additional MLH1 promoter hypermethylation testing. 15,51,55–57,59–61,63,64,67–70,76,77,84,89,92 All index tests have been reported separately.
| Study and year | Risk of bias | Applicability concern level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test: MSI | Index test: IHC | Index test: MLH1 promoter hypermethylation | Reference test | Flow and timing | Role of sponsor | Patients | Index test: MSI | Index test: IHC | Index test: MLH1 promoter hypermethylation | Reference | |
| Anagnostopoulos et al.51 2017 | High | Unclear | Unclear | Unclear | Unclear | High | Low | High | Unclear | Unclear | Unclear | Low |
| Backes et al.52 2009 | Low | NA | Unclear | NA | Unclear | High | Low | Unclear | NA | Unclear | NA | Unclear |
| Baldinu et al.53 2002 | High | Unclear | Unclear | NA | Unclear | High | Unclear | Unclear | Unclear | Unclear | NA | Low |
| Bruegl et al.55 2017 | Low | Unclear | Unclear | Unclear | Unclear | High | Low | High | Unclear | Low | Unclear | Low |
| Buchanan et al.56 2014/Nagle et al.76 2018 | High | NA | Unclear | Unclear | Unclear | High | Low | Unclear | NA | Unclear | Unclear | Low |
| Dillon et al.59 2017 | Low | NA | Unclear | Unclear | Unclear | High | Low | Unclear | NA | Unclear | Unclear | Low |
| Egoavil et al.61 2013 | Low | Unclear | Unclear | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low | Low |
| Ferguson et al.62 2014 | High | Unclear | Unclear | NA | Unclear | High | Low | Unclear | Unclear | Unclear | NA | Low |
| Goodfellow et al.64 2003 | High | Unclear | NA | Unclear | High | High | Low | Unclear | Unclear | NA | Unclear | High |
| Goodfellow et al.63 2015 | High | Unclear | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | Unclear | Unclear | Low |
| Hampel et al.15 2006 | High | Unclear | Unclear | Unclear | Unclear | High | Low | High | Unclear | Unclear | Unclear | Low |
| Latham et al.66 2019 | Unclear | Unclear | Unclear | NA | Unclear | High | Unclear | Unclear | Unclear | Unclear | NA | Low |
| Leenen et al.67 2012 | High | Unclear | Unclear | Low | Unclear | High | Low | High | Unclear | Unclear | Low | Low |
| Libera et al.68 2017/Carnevali et al.57 2017 | High | Unclear | Unclear | Low | Unclear | High | Low | High | Unclear | Unclear | Low | Low |
| Lin and Hecht69 2016 | High | NA | Unclear | Unclear | Unclear | High | Low | High | NA | Unclear | Unclear | Unclear |
| Mas-Moya et al.70 2015/Dudley et al.60 2015 | Low | Unclear | Unclear | Low | Unclear | High | Low | Unclear | Unclear | Unclear | Low | Low |
| Mercado et al.73 2012 | High | Unclear | Unclear | NA | High | High | Unclear | High | Unclear | Unclear | NA | Unclear |
| Millar et al.74 1999 | High | NA | Unclear | NA | High | High | Low | High | NA | Unclear | NA | High |
| Najdawi et al.77 2017 | High | NA | Unclear | Low | Unclear | High | Low | Unclear | NA | Unclear | Low | Low |
| Ollikainen et al.78 2005 | High | Unclear | Unclear | NA | Unclear | High | Low | High | Unclear | Low | NA | Low |
| PETALS study (Dr Neil AJ Ryan, personal communication) | Low | Low | Unclear | Low | Unclear | High | Low | Low | Low | Low | Low | Low |
| Sarode and Robinson84 2018 | Unclear | NA | Low | Unclear | High | High | Low | High | NA | Low | Low | High |
| Shin et al.85 2015 | Unclear | Unclear | Unclear | NA | Unclear | High | Low | High | Unclear | Unclear | NA | Low |
| Svampane et al.88 2014 | High | NA | Unclear | NA | Unclear | High | Low | Unclear | NA | Unclear | NA | Low |
| Takahashi et al.89 2017 | Unclear | NA | High | Unclear | Unclear | High | Low | High | NA | Unclear | Unclear | Low |
| Yoon et al.92 2008 | High | Unclear | Unclear | Unclear | Unclear | High | Low | High | Unclear | Unclear | Unclear | Low |
Risk of bias for partial test accuracy studies
Two domains were rated as having a high risk of bias. The first was patient selection (domain 1: patient selection), with 62% (16/26) of studies rated as having a high risk of bias in this domain. 15,51,53,56,57,62–64,67–69,73,74,76–78,88,92 As per the full test accuracy papers, this was because studies had strict inclusion criteria (such as age, previous/synchronous cancers or family history) that excluded many of the suitable population. The second domain in which a large proportion of studies were rated as having a high risk of bias was flow and timing, with 100% (26/26) rated as having a high risk of bias. In these studies, not all patients were given the reference standard; rather, it was usually only those believed to have the disease based on the index test result who were given the reference standard. The role of the sponsor was low in all studies bar four, in which not enough information was provided to make a determination. 53,63,66,73 In all other domains, the majority of the studies were rated as having an unclear risk of bias because of a lack of evidence provided [16/17 (94%), domain 2: index test MSI; 23/25 (92%), domain 2: index test IHC; 11/17 (65%), domain 2: index test MLH1 promoter hypermethylation testing; and 22/26 (85%), domain 3: reference standard]. The only domain with a low risk of bias was domain 5: the role of the sponsor, with 85% (22/26) of studies rated as having a low risk of bias for this domain. The remaining four studies did not report enough information to judge the risk of bias surrounding sponsor involvement. 53,63,66,73
Applicability of study findings for partial test accuracy studies
There were applicability concerns in one domain. A total of 50% (13/26) of studies had high levels of applicability concerns in the patient selection domain (domain 1: patient selection), with these studies narrowing their inclusion criteria by age and personal/familial cancer history. 15,51,55,57,67–69,73,74,78,84,85,89,92 The only other domain with a high level of applicability concern was the reference standard (domain 3: reference standard). A total of 12% of studies (3/26) were rated as having a high level of applicability concern for the reference standard, with differing methods of germline testing than were recognised in this review. Two of the studies primarily used single-strand conformational variant analysis, and the remaining study used array-based comparative genomic hybridisation/long-range PCR. 64,74,84 All other studies were considered to have a low level of applicability concern, as they provided sequencing followed by PCR or MLPA. The majority of index test ratings were unclear, with little information describing whether or not the conduct and interpretation of the tests was undertaken in accordance to best-practice guidelines and via laboratories that are participating in quality assurance programmes [16/17 (94%), domain 2: index test MSI; 21/25 (84%), domain 2: index test IHC; and 10/17 (59%), domain 2: index test MLH1 promoter hypermethylation testing]. All judgements of risk of bias and applicability concerns for studies reporting partial test accuracy data can be found in Table 5.
Quality appraisal tool for studies of diagnostic reliability
Twenty-three studies (including the unpublished PETALS study) provided data on concordance. 15,16,51,54,55,58,61–63,67,68,71,72,75,78–80,82,85–87,91 These studies were appraised using the QAREL. Two of the questions in the QAREL were deemed not applicable to the studies. Question 7, ‘were raters blinded to additional cues that were not part of the test?’, was not applicable, as this is covered by question 6 on clinical information. Question 9, ‘was the time interval between repeated measurements compatible with the stability (or theoretical stability) of the variable being measured?’, was also judged as not applicable following guidance from clinical advisors.
Quality considerations in the included concordance studies are shown in Table 6. In general, the quality of the included studies was poor, with only one study (the unpublished PETALS study) having > 50% of the answers meeting the desired criteria in the questions. In particular, the representativeness of the sample was problematic in 78% of studies (18/23). 15,16,51,54,55,61,62,67,68,71,72,78–80,82,85–87 The studies were not comparable to clinical practice in the UK, with populations selected based on age, type of endometrial cancer and presence of synchronous/metachronous cancers (question 1). Only 13% of studies (3/23, including the PETALS study) were deemed representative. 55,61 Similarly, there were concerns regarding the representativeness of the raters performing the tests (question 2). In 87% of studies (20/23), there was not enough information reported to determine whether or not tests were conducted/interpreted by individuals who have undertaken the appropriate training and in laboratories that are participating in quality assurance programmes (e.g. UK National External Quality Assessment Scheme, Nordic immunohistochemical Quality Control, Clinical Laboratory Improvement Amendments). 15,16,51,54,55,58,62,67,68,71,72,75,78–80,82,85–87
| Study and year | QAREL question | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Was the sample of subjects representative? | Was the sample of raters representative? | Were raters blinded to the findings of other raters? | Were raters blinded to their own prior findings? | Were raters blinded to the accepted reference standard? | Were raters blinded to clinical information not part of test? | Were raters blinded to additional non-clinical cues? | Was the order of examination varied? | Was the time interval between repeated measures appropriate? | Was the test applied correctly and interpreted appropriately? | Were appropriate statistical measures of agreement used? | |
| Anagnostopoulos et al.51 2017 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Berends et al.54 2003 | No | Unclear | Yes | Unclear | Yes | Unclear | NA | Unclear | NA | Unclear | No |
| Bruegl et al.55 2017 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Chao et al.58 2019 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Egoavil et al.61 2013 | Yes | Yes | Yes | Unclear | Yes | Unclear | NA | Unclear | NA | Unclear | No |
| Ferguson et al.62 2014 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Goodfellow et al.63 2015 | No | Yes | Unclear | Unclear | Yes | Unclear | NA | Unclear | NA | Unclear | No |
| Hampel et al.15 2006 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Leenen 201267 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Libera et al.68 2017 | No | Unclear | Unclear | Unclear | Yes | Unclear | NA | Unclear | NA | Unclear | No |
| Lu et al.16 2007 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Masuda et al.71 2012 | No | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | NA | Unclear | No |
| McConechy et al.72 2015 | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | NA | Unclear | Yes |
| Modica et al.75 2007 | No | Unclear | Unclear | Yes | NA | Unclear | NA | No | NA | Unclear | No |
| Ollikainen et al.78 2005 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Pecorino et al.79 2017 | No | Unclear | Unclear | Unclear | NA | Unclear | NA | No | NA | Unclear | No |
| Planck et al.80 2017 | No | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | NA | Unclear | No |
| Rubio et al.82 2016 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| PETALS study (Dr Neil AJ Ryan, personal communication) | Yes | Yes | Yes | Unclear | Yes | Unclear | NA | Unclear | NA | Yes | No |
| Shin et al.85 2015 | No | Unclear | Unclear | Unclear | Yes | Unclear | NA | Unclear | NA | Unclear | No |
| Stelloo et al.86 2017 | No | Unclear | Unclear | Unclear | Unclear | Yes | NA | No | NA | Unclear | Yes |
| Strazzullo et al.87 2003 | No | Unclear | Unclear | Unclear | Unclear | Unclear | NA | Unclear | NA | Unclear | No |
| Wang et al.91 2017 | Unclear | Yes | Yes | NA | NA | Unclear | NA | No | NA | Yes | No |
There was a consistent lack of reporting regarding blinding across the studies. In 83% of studies (19/23), it was unclear whether or not raters were blinded to the findings of other raters (question 3); in 90% of studies (21/23), it was unclear whether or not raters were blinded to their own findings (question 4); in 65% of studies (11/17; six studies were concordance only studies with no reference standard, so this question was not applicable), it was unclear whether or not raters were blinded to the results from the reference standard (question 5); and, in 96% of studies (22/23, including the PETALS study), it was unclear whether or not raters were blinded to a patient’s clinical information (question 6). 16,51,54,55,58,61,63,67,68,71,72,75,78–80,82,87,91
In addition, there was a lack of reporting on how the tests were undertaken, meaning that it could not be determined whether or not the order of the testing varied [question 8; 19/23, including the PETALS study (83%)],15,16,51,54,55,58,61–63,67,68,71,72,78,80,82,85,87 or if tests had been conducted according to best-practice guidelines/via laboratories that are participating in quality assurance programmes [question 10; 21/23 (91%)]. 15,16,51,54,55,58,61–63,67,68,71,72,75,78–80,82,85–87 The majority of studies [21/23, including the PETALS study (91%)] reported raw data, but did not use any appropriate statistical measures (such as Bland–Altman plots or intraclass correlations, or between categorical/ordinal data with kappas). 15,16,51,54,55,58,61–63,67,68,71,75,78–80,82,85,87,91
Assessment of test accuracy
Parts of this section have been reproduced with permission of Stinton et al. 4 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to share and redistribute, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Number of Lynch syndrome diagnoses
Thirty-three studies provided data on Lynch syndrome diagnoses,15,16,51–59,61–64,66,67,69,70,73,77,78,81–85,88–90,92 including the unpublished PETALS study (Dr Neil AJ Ryan, personal communication). Full details of the number of women identified with Lynch syndrome and VUSs/Lynch-like syndrome, and the types and frequencies of mutations, are reported in Appendix 4. Across all 33 studies, 349 cases of Lynch syndrome were identified from 7367 women tested. The reported prevalence of Lynch syndrome ranged from 0% (0 out of 140 women tested, in a clinical experience study from the USA that included all women undergoing hysterectomies at two hospitals) to 62%. 73 The prevalence of Lynch syndrome was typically lower in studies that recruited unselected samples of women (median 3.2%, range 0–5.3%) than in studies of selected samples of women (median 7.5%, range 0.9–62%). The prevalence of Lynch syndrome in two UK studies was (confidential information has been removed) [(confidential information has been removed) women tested, including (confidential information has been removed) women with known Lynch syndrome] in an unselected sample of women (PETALS study) and 8.5% (3 out of 35 women tested) in a selected sample of women aged < 50 years. 51 The types and frequencies of MMR gene mutations varied between studies. Combining data from all studies, variants in MSH2 gene mutations were the most common (38.6% of Lynch syndrome cases), followed by MSH6 (30.4% of Lynch syndrome cases), MLH1 (23.6% of Lynch syndrome cases) and PMS2 (7.3% of Lynch syndrome cases). One study did not report which of the MMR genes were mutated,83 and 10 studies did not assess all four MMR genes. 16,53,54,64,74,78,82,85,88,92 MHL1 and MSH2 were not assessed in one study,64 MSH6 was not assessed in three studies53,74,85 and PMS2 was not assessed in 10 studies. 16,53,54,64,74,78,82,85,88,92 Combining data from studies of unselected samples of women, variants in MSH6 were the most common (39.1% of Lynch syndrome cases), followed by MSH2 (32.2% of Lynch syndrome cases), MLH1 (19.5% of Lynch syndrome cases) and PMS2 (9.2% of Lynch syndrome cases). Combining data from studies of selected samples of women, variants in MSH2 were the most common (42.1% of Lynch syndrome cases), followed by MSH6 (25.7% of Lynch syndrome cases), MLH1 (25.4% of Lynch syndrome cases) and PMS2 (6.8% of Lynch syndrome cases).
Eighty-nine VUSs were reported in 10 studies (including the PETALS study), ranging from 2–15 cases per study. 15,16,54–58,61,63,82,90 In one study (confidential information has been removed) of the VUSs were identified in women who were (confidential information has been removed). Nine women were reported to have Lynch-like syndrome from two studies, ranging from 3 to 6 cases per study. 59,70
Accuracy of screening tests
The methods, the thresholds to determine positivity of index tests and the diagnostic tests varied between studies. Results were considered positive when they exceeded the threshold as set in the individual study. Results are reported by complete test accuracy, concordance, partial test accuracy, test failures and indeterminate results. No studies reported the time from index test to result or diagnosis.
Complete test accuracy studies
Head-to-head studies
Four studies provided head-to-head test accuracy data for IHC- and MSI-based testing, although the numbers of included tumours were not identical for each of the tests because of insufficient tumour tissue being available and test failures. 16,54,58,82 Three studies had a larger number of results for IHC than for MSI: 102 versus 83,58 99 versus 9516 and 94 versus 83. 82 One study had a larger number of results for MSI than for IHC: 57 versus 51. 54 All four studies comprised selected samples of women. Two studies excluded women aged > 50 years,16,54 one study excluded women with recurrent or synchronous cancers58 and one study excluded women (1) without a personal/family history of Lynch syndrome or (2) who were aged > 50 years. 82 Two studies included an ineligible reference standard (conformational-sensitive gel electrophoresis/denaturing gradient gel electrophoresis) as part of their diagnostic process. 54,82 Three of the studies were judged to be at high risk of bias. 54,58,82 The remaining study was judged to have an unclear risk of bias, as insufficient information was presented on which to make an assessment. 16 All four studies were rated as having high applicability concerns (see Risk of bias for complete test accuracy studies and Applicability of study findings for complete test accuracy studies for further details).
For IHC, there were 28 true positives, 78 false positives, 235 true negatives and 5 false negatives; point estimates ranged from 66.7% to 100% for sensitivity, 60.9% to 83.3% for specificity, 14.3% to 37.5% for PPVs and 95.2% to 100% for NPVs. For MSI testing, there were 21 true positives, 57 false positives, 232 true negatives and 8 false negatives; point estimates ranged from 41.7% to 100% for sensitivity, 69.2% to 89.9% for specificity, 20% to 33.3% for PPVs and 88.7% to 100% for NPVs. There were no statistically significant differences (on the basis of CIs) between MSI and IHC on any of the four tests’ accuracy metrics.
Immunohistochemistry- and microsatellite instability-based testing, with MLH1 promoter hypermethylation testing
Four studies provided test accuracy data for IHC- and MSI-based testing, where a lack of expression on IHC without MLH1 methylation or MSI-H (two or more unstable markers) test was considered index-test positive. 16,58,81,83 Full details are reported in Table 7. The circumstances under which MLH1 promoter hypermethylation testing was conducted varied in the studies. In two studies, methylation testing was conducted in women who had tumours that were categorised as MSI-H or had IHC loss (MLH1 or MLH1/PMS2);16,83 in one study, methylation testing was conducted in women who had IHC MLH1 loss only. 58 In the remaining paper, the circumstances under which MLH1 promoter hypermethylation testing was conducted were not reported. 81 Three studies comprised selected samples of women,16,58,83 and one study comprised an unselected sample of women. 81 One study excluded women aged > 50 years;16 one study excluded women with recurrent or synchronous cancers;58 and one study included an unselected sample of women, but did not report data on women with uninformative MMR results or without prior tumour testing. 83 Each study used a different panel of MSI markers. There were 85 true positives, 290 false positives, 475 true negatives and 4 false negatives. Two studies reported the gene variants in Lynch syndrome cases. 16,58 The most commonly affected gene was MSH2 [9/15 cases of Lynch syndrome (60%)], followed by MSH6 [4/15 cases of Lynch syndrome (26.7%)], MLH1 [2/15 cases of Lynch syndrome (13.3%)] and PMS2 [0/15 cases of Lynch syndrome (0%)]. PMS2 was assessed in only one study. 58 In two studies, 25 VUSs were identified (median 12.5; 11–14 cases per study). 16,58 One study did not report VUSs. 83 In the remaining study, 25 VUSs were identified, but the study did not report whether or not the participants had undergone index testing. 81 Point estimates ranged from 90.5% to 100% for sensitivity, 6.6% to 92.3% for specificity, 18.3% to 56.3% for PPVs and 75.0% to 100% for NPVs. In the study with an unselected sample of women,81 there were 19 true positives, 32 false positives, 312 true negatives and 2 false negatives. Comparing CIs, there was no statistically significant difference in sensitivity, specificity, PPVs or NPVs between the studies with selected samples and those with unselected samples.
| Study and year | Number tested | Index test and cut-off point | Reference standard | 2 × 2 table (n) | % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| True positive | False positive | True negative | False negative | Sensitivity | Specificity | PPV | NPV | ||||
| Chao et al.58 2019 | 93 | IHC (MLH1, MSH2, MSH6, PMS2): negative staining of any of MMR protein | NGS, Sanger sequencing | 6 | 24 | 63 | 0 | 100.0 (51.7 to 100.0) | 72.4 (61.6 to 81.2) | 20.0 (8.4 to 39.1) | 100.0 (92.8 to 100.0) |
| MSI: MSI-H (two or more unstable markers) | |||||||||||
| Lu et al.16 2007 | 100 | IHC (MLH1, MSH2, MSH6): loss of protein expression | Sequencing, unspecified test for large deletions | 9 | 7 | 84 | 0 | 100.0 (62.9 to 100.0) | 92.3 (84.3 to 96.6) | 56.3 (30.6 to 79.2) | 100.0 (94.6 to 100.0) |
| MSI: MSI-H (two or more unstable markers) | |||||||||||
| Ring et al.81 2016 | 365 | IHC (MLH1, MSH2, MSH6, PMS2): complete absence of MMR protein expression | MLPA, NGS | 19 | 32 | 312 | 2 | 90.5 (68.2 to 98.3) | 90.7 (87.0 to 93.5) | 37.3 (24.5 to 51.9) | 99.4 (97.5 to 99.9) |
| MSI: MSI-H, but cut-off point not reported | |||||||||||
| Salvador et al.83 2019 | 296 | IHC (MLH1, MSH2, MSH6, PMS2): cut-off point not reported | MLPA, NGS | 51 | 227 | 16 | 2 | 96.2 (85.9 to 99.3) | 6.6 (3.9 to 10.7) | 18.3 (14.1 to 23.5) | 75.0 (35.6 to 88.9) |
| MSI: MSI-H (two or more unstable markers) | |||||||||||
Two studies reported the results of MLH1 promoter hypermethylation testing. 16,58 Twelve out of 13 tumours (92.3%)16 and 12 out of 15 tumours (80%) were hypermethylated. 58
A secondary analysis of test accuracy in which VUSs were considered germline positive was possible for two studies. 16,58 Estimates of test accuracy were as follows: sensitivity, 100.0% (95% CI 80.0% to 100.0%); specificity, 86.3% (95% CI 75.8% to 92.9%); PPV, 66.7% (95% CI 47.1% to 82.1%); and NPV, 100.0% (95% CI 92.8 to 100.0%);58 and sensitivity, 100.0% (95% CI 80.0% to 100.0%); specificity, 78.8% (95% CI 67.9% to 86.8%); PPV, 54.1% (95% CI 37.1 to 70.2%); and NPV, 100.0% (95% CI 92.8 to 100.0%). 16 These were similar to estimates in which VUSs were consider to be germline negative, with the exception of PPV for Chao et al. ,58 which was higher when VUSs were considered to be germline positive [66.7% (95% CI 47.1% to 82.1%) vs. 20.0% (95% CI 8.4% to 39.1%) for germline positive vs. germline negative, respectively].
Immunohistochemistry alone
Five studies provided test accuracy data for IHC. 16,54,58,82,90 Full details are provided in Table 8. All five studies comprised selected samples of women. Two studies excluded women aged > 50 years;16,54 one study excluded women with recurrent or synchronous cancers;58 one study excluded women (1) without a personal/family history of Lynch syndrome-related cancer or (2) who were aged > 50 years;82 and one study excluded women who were (1) aged > 50 years, (2) without a personal/family history of Lynch syndrome-related cancer or (3) did not have loss of expression of any MMR protein on IHC testing. 90 Four studies assessed all four MMR proteins54,58,82,90 and one study assessed MHL1, MSH2 and MSH6. 16 There were 69 true positives, 193 false positives, 243 true negatives and 6 false negatives in the five included studies. The most commonly affected gene was MSH2 [34/69 cases of Lynch syndrome (49.3%)], followed by MSH6 [18/69 cases of Lynch syndrome (26.1%)], MLH1 [14/69 cases of Lynch syndrome (20.3%)] and PMS2 [3/69 cases of Lynch syndrome (4.3%)]. PMS2 was assessed in only two studies. 58,90 In total, 33 VUSs were identified in the five studies (median 4; 3–11 cases per study). The point estimates ranged from 66.7% to 100% for sensitivity, from 6.5% to 83.3% for specificity, from 14.3% to 37.5% for PPV and from 88.9% to 100% for NPV (see Figure 23). With the exception of PPV in the study by Tian et al. ,90 CIs between studies overlapped for each of the test accuracy metrics. Test accuracy estimates for the single study that employed only MLH1, MSH2 and MSH6 were within the ranges reported by the studies using all four MMR proteins.
| Study and year | Number tested | Index test and cut-off point | Reference standard | 2 × 2 table (n) | % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| True positive | False positive | True negative | False negative | Sensitivity | Specificity | PPV | NPV | ||||
| Berends et al.54 2003 | 51 | IHC (MLH1, MSH2, and MSH6): absence of detectable nuclear staining of cancer cells | DGGE, sequencing, MLPA | 5 | 18 | 28 | 0 | 100 (46.3 to 100) | 60.9 (45.4 to 74.5) | 21.7 (8.3 to 44.2) | 100 (85 to 100) |
| Chao et al.58 2019 | 102 | IHC (MLH1, MSH2, MSH6, PMS2): negative staining of any of MMR protein | NGS, Sanger sequencing | 4 | 24 | 72 | 2 | 66.7 (24.1 to 94.0) | 75.0 (64.9 to 83.0) | 14.3 (4.7 to 33.6) | 97.3 (89.7 to 99.5) |
| Lu et al.16 2007 | 99 | IHC (MHL1, MSH2, MSH6): loss of protein expression | Sequencing, unspecified test for large deletions | 9 | 15 | 75 | 0 | 100.0 (62.9 to 100.0) | 83.3 (73.7 to 90.1) | 37.5 (19.5 to 59.2) | 100.0 (93.9 to 100.0) |
| Rubio et al.82 2016 | 94 | IHC: cut-off point not reported | CSGE, sequencing, MLPA | 10 | 21 | 60 | 3 | 76.9 (46 to 93.8) | 74.1 (62.9 to 82.9) | 32.3 (17.3 to 51.5) | 95.2 (85.8 to 98.8) |
| Tian et al.90 2019 | 165 | IHC: cut-off point not reported | Sequencing/NGS, MLPA | 41 | 115 | 8 | 1 | 97.6 (85.9 to 99.9) | 6.5 (3.1 to 12.8) | 26.3 (19.7 to 34.0) | 88.9 (50.7 to 99.4) |
Of the five studies, one presented data in sufficient detail to estimate test accuracy by individual proteins. 16 This study reported on MLH1, MSH2 and MSH6 in 99 patients. True positives were determined by the individual protein test’s ability to detect any germline mutation, not necessarily the corresponding mutation. There was wide variation in the test accuracy between the different proteins. Sensitivity was 11.1% (95% CI 0.6% to 49.3%) for MLH1, 66.7% (95% CI 30.9% to 91.0%) for MSH6 and 77.8% (95% CI 40.2% to 96.1%) for MSH2. Specificity was 87.8% (95% CI 79.2% to 93.2%) for MLH1, 95.6% (95% CI 88.4% to 98.6%) for MSH6 and 95.7% (95% CI 88.6% to 98.6%) for MSH2. PPV was 7.7% (95% CI 0.4% to 37.9%) for MLH1, 60.0% (95% CI 27.4% to 86.3%) for MSH6 and 63.6% (95% CI 31.6% to 87.6%) for MSH2. NPV was 90.7% (82.0% to 95.6%) for MLH1, 96.6% (95% CI 89.8% to 99.1%) for MSH6 and 97.6% (95% CI 91.0% to 99.6%) for MSH2. The wide variations between the test accuracy for MLH1 and the other proteins may be accounted for by the difference in the number of false positives. There were 12 false positives when testing using MLH1, compared with only four for MSH2 and MSH6.
In three of the studies, information on the IHC result by individual protein was presented only for those with a germline mutation,54,82,90 and, in the remaining study, eight IHC cases were not reported and there were discrepancies between values reported in the text and table. 58
A secondary analysis of test accuracy in which VUSs were considered germline positive was possible for four studies. 16,54,58,82 Estimates of test accuracy were as follows: sensitivity, 100.0% (95% CI 59.8% to 100.0%); specificity, 62.8% (95% CI 46.7% to 76.6%); PPV, 33.3% (95% CI 16.4% to 55.3%); and NPV, 100.0% (95% CI 84.5% to 100.0%);54 sensitivity, 90.0% (95% CI 66.9% to 98.2%); specificity, 75.6% (95% CI 64.7% to 84.1%); PPV, 47.4% (95% CI 31.3% to 64.0%); and NPV, 96.9% (95% CI 88.2% to 99.5%);58 sensitivity, 100.0% (95% CI 80.0% to 100.0%); specificity, 83.5% (95% CI 73.1% to 90.6%); PPV, 60.6% (95% CI 42.2% to 76.6%); and NPV, 100.0% (95% CI 93.1% to 100.0%);16 and sensitivity, 82.4% (55.8% to 95.3%); specificity, 75.3% (64.0% to 84.1%); PPV, 42.4% (26.0% to 60.6%); and NPV, 95.1% (85.4% to 98.7%). 82 These were similar to estimates in which VUSs were considered to be germline negative.
Microsatellite instability-based testing alone
Four studies provided test accuracy data for MSI-based testing. 16,54,58,82 Full details are provided in Table 9. All four studies comprised selected samples of women. Two studies excluded women aged > 50 years;16,54 one study excluded women with recurrent or synchronous cancers,58 and one study excluded women (1) without a personal/family history of Lynch syndrome or (2) who were aged > 50 years. 82 Three different panels of markers were used in the four studies; only two studies used the same panel of markers. 16,82 Using MSI-H (two or more unstable markers) as a cut-off point, there were 21 true positives, 57 false positive, 232 true negatives and 8 false negatives in the four included studies. The most commonly affected gene was MSH2 [13/21 cases of Lynch syndrome (61.9%)], followed by MSH6 [4/21 cases of Lynch syndrome (19%)] and MLH1 [4/21 cases of Lynch syndrome (19%)]. PMS2 was assessed in only one study;58 there were no cases of Lynch syndrome with a PMS2 mutation. In total, 29 VUSs were identified in the four studies (median 7; 3–12 cases per study). Point estimates ranged from 41.7% to 100% for sensitivity, from 69.2% to 89.9% for specificity, from 20% to 89.9% for PPV and from 88.7% to 100% for NPV. One of the included studies reported data that allowed us to calculate test accuracy using MSI-H or MSI-L (one or more unstable marker) as a cut-off point. 82 There were 5 true positives, 17 false positives, 54 true negatives and 7 false negatives. 82 The most commonly affected gene was MSH2 [3/5 cases of Lynch syndrome (60.0%)], followed by MSH6 [1/5 cases of Lynch syndrome (20%)] and MLH1 [1/5 cases of Lynch syndrome (20%)]. PMS2 was not assessed. Three VUSs were identified. Test accuracy metrics were similar to those reported using MSI-H as a cut-off point: sensitivity was 41.7%, specificity was 76.1%, PPV was 22.7% and NPV was 88.5%. Using a cut-off point of one or more stable markers changed the status of one index test result from true negative to false positive.
| Study and year | Number tested | Index test and cut-off point | Reference standard | 2 × 2 table (n) | % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| True positive | False positive | True negative | False negative | Sensitivity | Specificity | PPV | NPV | ||||
| MSI only (MSI-H vs. MSI-L/MSS) | |||||||||||
| Berends et al.54 2003 | 57 | MSI: MSI-H (two or more unstable markers) | DGGE, sequencing, MLPA | 4 | 16 | 36 | 1 | 80 (29.9 to 98.9) | 69.2 (54.7 to 80.9) | 20 (6.6 to 44.3) | 97.3 (84.2 to 99.9) |
| Chao et al.58 2019 | 83 | MSI: MSI-H (two or more unstable markers) | NGS, Sanger sequencing | 4 | 8 | 71 | 0 | 100.0 (39.6 to 100.0) | 89.9 (80.5 to 95.2) | 33.3 (11.3 to 64.6) | 100.0 (93.6 to 100.0) |
| Lu et al.16 2007 | 95 | MSI: MSI-H (two or more unstable markers) | Sequencing, unspecified test for large deletions | 8 | 17 | 70 | 0 | 100.0 (59.8 to 100.0) | 80.5 (70.3 to 87.9) | 32.0 (15.7 to 53.6) | 100.0 (93.5 to 100.0) |
| Rubio et al.82 2016 | 83 | MSI: MSI-H, number of markers not specified | CSGE, sequencing, MLPA | 5 | 16 | 55 | 7 | 41.7 (16.5 to 71.4) | 77.5 (65.7 to 86.2) | 23.8 (9.1 to 47.6) | 88.7 (77.5 to 95) |
| MSI only (MSI-H/MSI-L vs. MSS) | |||||||||||
| Rubio et al.82 2016 | 83 | MSI: MSI-H/MSI-L, number of markers not specified | CSGE, sequencing, MLPA | 5 | 17 | 54 | 7 | 41.7 (16.5 to 71.4) | 76.1 (64.2 to 85.1) | 22.7 (8.7 to 45.8) | 88.5 (77.2 to 94.9) |
A secondary analysis of test accuracy in which VUSs were considered germline positive was possible for four studies. 16,54,58,82 Estimates of test accuracy were as follows: sensitivity, 87.5% (95% CI 46.7% to 99.3%); specificity, 69.4% (95% CI 54.4% to 81.3%); PPV, 31.8% (95% CI 14.7% to 54.9%); and NPV, 97.1% (95% CI 83.4% to 99.9%);54 sensitivity, 100.0% (95% CI 75.9% to 100.0%); specificity, 91.0% (95% CI 80.9% to 96.3%); PPV, 72.7% (95% CI 49.6% to 88.4%); and NPV, 100.0% (95% CI 92.6% to 100.0%);58 sensitivity, 100.0% (95% CI 79.1% to 100.0%); specificity, 80.3% (95% CI 69.2% to 88.2%); PPV, 55.9% (95% CI 38.1% to 72.4%); and NPV, 100.0% (95% CI 92.6% to 100.0%);16 and sensitivity, 53.3% (95% CI 27.4% to 77.7%); specificity, 76.5% (95% CI 64.4% to 85.6%); PPV, 33.3% (95% CI 16.4% to 55.3%); and NPV, 88.1% (95% CI 76.5% to 94.7%). 82 These were similar to estimates in which VUSs were considered to be germline negative.
Concordance between immunohistochemistry- and microsatellite instability-based testing
Twenty-three studies, including the unpublished PETALS study (Dr Neil AJ Ryan, personal communication), provided data on concordance between IHC- and MSI-based testing. 15,16,51,54,55,58,61–63,67,68,71,72,75,78–80,82,85–87,91 Twenty studies provided complete concordance data (agreement/disagreement between IHC positive/negative and IHC negative), and three studies provided partial concordance data (IHC conducted only for MSI-H tumours,87 MSI conducted only for women with IHC loss79 and IHC conducted only for women with MSS results15). Full details of concordance are reported in Appendix 5. In the studies providing complete concordance data, there was a high level of agreement between the results of the tests (median agreement 91.8%, with the lowest level of agreement being 68.2% and the highest level of agreement being 100%) and a low level of disagreement (median disagreement 9.8%, with the lowest level of disagreement being 0% and the highest level of disagreement being 31.8%), median kappa 0.84 (range 0.32–0.97). Kappa values were calculated by the reviewers.
Few studies examined characteristics of discordant cases. Four studies reported that MLH1 promoter hypermethylation was common in discordant cases: 50% (1/2 cases),71 75% (3/4 cases),72 80% (4/5 cases)55 and 83% (10/12 cases). 86 Seven of the 23 concordance studies reported on the characteristics of discordant cases of MSI and IHC testing. 15,16,51,55,63,72,85 In two of these seven studies, it was possible to determine germline results for the discordant cases. 15,55 Bruegl et al. 55 found 5.1% disagreement, with seven out of 197 discordant cases. Of these seven, only one was found to have a germline mutation and this was in the MSH6 variant. Likewise, Hampel et al. 15 found that the only discordant case with a germline mutation was in the MSH6 variant. By contrast, Lu et al. 16 found that, of the five discordant cases, all were germline mutation negative.
Across three studies,16,55,72 20–57% (4/7, 1/5 and 2/6) of discordant results were due to MLH1 promoter hypermethylation, suggestive of epigenetic changes rather than Lynch syndrome.
For one study,51 discordance was associated with the classification of MSI-L cases. When MSI-L cases were grouped with MSS cases there were two discordant cases, whereas when MSI-H or MSI-L cases were grouped together and compared with MSS cases, there were no cases of discordance between MSI and IHC testing results. 51
It was possible to calculate the average age for discordant cases in three studies. 15,51,85 In Anagnostopoulos et al. ,51 discordant cases (n = 2) had a median age of 39.5 years, which was lower than the overall median of 48 years in the sample. Although Shin et al. 85 and Hampel et al. 15 found no real difference in age between discordant cases and the whole sample, Shin et al. 85 found two discordant cases with a mean age of 55 years at diagnosis of endometrial cancer and a mean age of 52.5 years at diagnosis of CRC, compared with the overall sample mean age of 52.5 years at endometrial cancer and 54.5 years at CRC. 85 Hampel et al. 15 found a mean age of 60.5 years in discordant cases, compared with the overall mean of 60.9 years in the whole sample.
One study85 reported on the comorbidities of other cancers in discordant cases. All cases in the study had a history of both endometrial cancer and CRC. They found that one of the two discordant cases also had a history of bladder cancer. Likewise, this was the only study to discuss family history in relation to discordant cases, and noted that both cases met the Amsterdam II criteria. 93
Testing pathways under review: partial test accuracy studies
In the proposed testing strategies 1–10, only women who test positive on the index tests would be offered germline testing. Some studies report results from implementing the strategies of interest; these are partial test accuracy studies because data on true negatives and false negatives are not available. It is not possible to calculate sensitivity, specificity or NPVs from these studies because of a lack of follow-up of women who were negative on the index tests. Studies in which full test accuracy could be extracted/calculated have already been reported (see Assessment of test accuracy), so here we report results from any studies (full or partial test accuracy) that report the numbers of true positive and false positive results for each strategy. Full details of all the strategies are provided in Table 10.
| Study and year | Number tested | Index test and cut-off point | Reference standard | 2 × 2 table (n) | % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| True positive | False positive | True negative | False negative | Sensitivity | Specificity | PPV | NPV | ||||
| Strategy 1: MSI testing alone | |||||||||||
| Berends et al.54 2003 | 57 | MSI: MSI-H – ≥ 2 unstable markers | DGGE, sequencing, MLPA | 4 | 16 | NA | NA | NA | NA | 20.0 (6.6 to 44.3) | NA |
| Chao et al.58 2019 | 83 | MSI: MSI-H – ≥ 2 unstable markers | NGS, Sanger sequencing | 4 | 8 | NA | NA | NA | NA | 33.3 (11.3 to 64.6) | NA |
| Latham et al.66 2019 | 525 | MSI: MSI sensor scores of ≥ 10 | NGS | 7 | 112 | NA | NA | NA | NA | 5.9 (2.6 to 12.2) | NA |
| Lu et al.16 2007 | 95 | MSI: MSI-H – ≥ 2 unstable markers | Sequencing, unspecified test for large deletions | 8 | 17 | NA | NA | NA | NA | 32.0 (15.7 to 53.6) | NA |
| Mercado et al.73 2012 | 24 | MSI: MSI-H – ≥ 2 unstable markers | DHPLC, sequencing | 15 | 5 | NA | NA | NA | NA | 75 (50.6 to 90.4) | NA |
| Ollikainen et al.78 2005 | 23 | MSI: MSI-H – ≥ 2 unstable markers | Sequencing & MLPA | 2 | 1 | NA | NA | NA | NA | 66.7 (12.5 to 98.2) | NA |
| PETALS study (Dr Neil AJ Ryan, personal communication) | Confidential information has been removed | MSI: MSI-H – ≥ 2 unstable markers | NGS, MLPA, long-range PCR, constitutional MLH1 promoter hypermethylation testing | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Rubio et al.82 2016 | 83 | MSI: MSI-H, number of markers not specified | CSGE, sequencing, MLPA | 5 | 16 | NA | NA | NA | NA | 23.8 (9.1 to 47.6) | NA |
| Strategy 2: MSI testing with MLH1 promoter hypermethylation testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 3: IHC alone | |||||||||||
| Berends et al.54 2003 | 51 | IHC: absence of detectable nuclear staining of cancer cells | DGGE, sequencing, MLPA | 5 | 18 | NA | NA | NA | NA | 21.7 (8.3 to 44.2) | NA |
| Chao et al.58 2019 | 102 | IHC (MLH1, MSH2, MSH6, PMS2): negative staining of any of MMR protein | NGS, Sanger sequencing | 4 | 24 | NA | NA | NA | NA | 14.3 (4.7 to 33.6) | NA |
| Lu et al.16 2007 | 99 | IHC (MHL1, MSH2, MSH6): loss of protein expression | Sequencing, unspecified test for large deletions | 9 | 15 | NA | NA | NA | NA | 37.5 (19.5 to 59.2) | NA |
| Mercado et al.73 2012 |
|
IHC (MHL1, MSH2, MSH6, PMS2): loss of protein expression | DHPLC, sequencing |
|
|
NA | NA | NA | NA |
|
NA |
| PETALS study (Dr Neil AJ Ryan, personal communication) | Confidential information has been removed | IHC (MLH1, MSH2, MSH6, PMS2): protein loss | NGS, MLPA, long-range PCR, constitutional MLH1 promoter hypermethylation testing | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Rubio et al.82 2016 | 94 | IHC: cut-off point not reported | CSGE, sequencing, MLPA | 10 | 21 | NA | NA | NA | NA | 32.3 (17.3 to 51.5) | NA |
| Tian et al.90 2019 | 165 | IHC: cut-off point not reported | Sequencing/NGS, MLPA | 41 | 115 | NA | NA | NA | NA | 26.3 (19.7 to 34.0) | NA |
| Strategy 4: IHC with MLH1 promoter hypermethylation testing | |||||||||||
| Lu et al.16 2007 | 99 | IHC (MHL1, MSH2, MSH6):loss of protein expression | Sequencing, unspecified test for large deletions | 9 | 15 | NA | NA | NA | NA | 37.5 (19.5 to 59.2) | NA |
| Ollikainen et al.78 2005 | 23 | IHC (MLH1, MSH2, MSH6): cut-off point not reported | Sequencing and MLPA | 2 | 8 | NA | NA | NA | NA | 20.0 (3.5 to 55.8) | NA |
| PETALS study (Dr Neil AJ Ryan, personal communication) | Confidential information has been removed | IHC (MHL1, MSH2, MSH6, PMS2): protein low | NGS, MLPA, long-range PCR, constitutional MLH1 promoter hypermethylation testing | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Strategy 5: MSI testing followed by IHC testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 6: MSI followed by IHC testing with MLH1 promoter hypermethylation testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 7: IHC followed by MSI-based testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 8: IHC testing followed by MSI testing with MLH1 promoter hypermethylation testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 9: MSI and IHC testing | |||||||||||
| No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data |
| Strategy 10: MSI and IHC testing with MLH1 promoter hypermethylation testing | |||||||||||
| Chao et al.58 2019 | 77 | IHC (MLH1, MSH2, MSH6, PMS2): negative staining of any of MMR protein | NGS, Sanger sequencing | 6 | 14 | NA | NA | NA | NA | 30.0 (12.8 to 54.3) | NA |
| MSI: MSI-H – ≥ 2 unstable markers | |||||||||||
| Goodfellow et al.63 2015 | 1002 | IHC (MLH1, MSH2, MSH6, plus PMS2 in subset: cut-off point not reported | NGS | 22 | 29 | NA | NA | NA | NA | 43.1 (29.6 to 57.7) | |
| MSI: MSI-H – ≥ 2 unstable markers | |||||||||||
| Lu et al.16 2007 | 100 | IHC (MHL1, MSH2, MSH6): loss of protein expression | Sequencing, unspecified test for large deletions | 9 | 24 | NA | NA | NA | NA | 27.3 (13.9 to 45.8) | NA |
| MSI: MSI-H – ≥ 2 unstable markers | |||||||||||
| Ollikainen et al.78 2005 | 23 | IHC (MLH1, MSH2, MSH6): cut-off point not reported | Sequencing and MLPA | 2 | 8 | NA | NA | NA | NA | 20.0 (3.5 to 55.8) | NA |
| MSI: MSI-H – ≥ 2 unstable markers | |||||||||||
| Salvador et al.83 2019 | 296 | IHC (MLH1, MSH2, MSH6, PMS2): cut-off point not reported | MLPA, NGS | 51 | 227 | NA | NA | NA | NA | 18.3 (14.1 to 23.5) | NA |
| MSI: MSI-H – ≥ 2 unstable markers | |||||||||||
| Yoon et al.92 2008 | 113 | MSI: MSI-H – ≥ 2 unstable markers | Sequencing | 4 | 9 | NA | NA | NA | NA | 30.8 (10.4 to 61.1) | NA |
| IHC (MHL1, MSH2, MSH6): no evidence of expression | |||||||||||
| Strategy 11: germline only | |||||||||||
| Study | Number tested | Reference standard | Number of Lynch syndrome diagnoses (%) | Notes | |||||||
| Berends et al.54 2003 | 57 | DGGE, sequencing, MLPA | 5 (8.8) | Initial reference standard was DGGE (followed, in case of aberrant band patterns, by direct sequencing of independently amplified PCR products) | |||||||
| Chao et al.58 2019 | 111 | NGS and Sanger sequencing | 6 (5.4) | – | |||||||
| Ferguson et al.62 2014 | 89 | Sequencing, MLPA | 7/89 (7.9) | – | |||||||
| Millar et al.74 1999 | 40 | SSCV, sequencing | 7 (17.5) | All of the women included had endometrial cancer and CC | |||||||
| Lu et al.16 2007 | 100 | Sequencing, unspecified test for large deletions | 9 (9) | – | |||||||
| Ring et al.81 2016 | 381 | MLPA, NGS | 22 (5.8) | Two women diagnosed with Lynch syndrome had mutations in EPCAM that extended into MSH2 | |||||||
| Rubio et al.82 2016 | 103 | CSGE, sequencing, MLPA | 14 (13.6) | – | |||||||
| Salvador et al.83 2019 | 296 | NGS, MLPA, ACGH | 51 (17.3) | – | |||||||
| Tian et al.90 2019 | 198 | Sequencing, NGS, MLPA | 45 (22.7) | – | |||||||
There is a risk that the likelihood that someone receives the reference standard is associated with disease status, for example individuals who truly have a disease may be more likely to get the reference standard than those who do not have the disease. This biases the PPV upwards. Therefore, we included only studies in which at least 95% of women who were eligible for germline testing (i.e. those who were index-test positive) received it.
Strategy 1: microsatellite instability testing alone
Eight studies, including the unpublished PETALS study (Dr Neil AJ Ryan, personal communication), provided test accuracy data for this strategy. 16,54,58,66,73,78,82
Six studies comprised selected samples of women,16,54,58,73,78,82 one study provided insufficient information on which to make an assessment of sample selection type66 and the PETALS study comprised an unselected sample of women (Dr Neil AJ Ryan, personal communication). Two studies excluded women aged > 50 years,16,54 one study excluded women with recurrent or synchronous cancers,58 one study included only women with a family history of endometrial cancer78 and one study excluded women (1) without a personal/family history of Lynch syndrome-related cancer or (2) who were aged > 50 years. 73,82
There were (confidential information has been removed) true positives and (confidential information has been removed) false positives out of (confidential information has been removed) women tested. The most commonly affected gene was MSH2 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)], followed by MSH6 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)], MLH1 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)] and PMS2 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)]. PMS2 was assessed in only four out of the eight studies (including the PETALS study). 58,66,73 In total, (confidential information has been removed) VUSs were identified in the seven studies [median (confidential information has been removed); (confidential information has been removed) cases per study]. Point estimates for PPVs ranged from (confidential information has been removed) to (confidential information has been removed).
Strategy 2: microsatellite instability testing with MLH1 promoter hypermethylation testing
No studies were identified that examined this strategy.
Strategy 3: immunohistochemistry-based testing alone
Six studies provided test accuracy data for this strategy. 16,54,58,73,82,90 All six studies comprised selected samples of women. Two studies excluded women aged > 50 years;16,54 one study excluded women with recurrent or synchronous cancers;58 one study excluded women (1) without a personal/family history of Lynch syndrome-related cancer or (2) who were aged > 50 years;82 and one study excluded women who (1) were aged > 50 years, (2) were without a personal/family history of Lynch syndrome-related cancer or (3) did not have loss of expression of any MMR protein on IHC testing. 73,90 In the five studies in which MMR genes were considered together, there were 69 true positives and 193 false positives, out of 552 women tested. The most commonly affected gene was MSH2 [34/69 cases of Lynch syndrome (49.3%)], followed by MSH6 [18/69 cases of Lynch syndrome (26.1%)], MLH1 [14/69 cases of Lynch syndrome (20.3%)] and PMS2 [3/69 cases of Lynch syndrome (3%)]. PMS2 was assessed in only three out of the six studies. 58,73,90 In total, 22 VUSs were identified in the seven studies (median 3; 2–11 cases per study). In the single study in which MMR genes were considered separately, the most commonly affected gene was MSH2 [40/80 cases of Lynch syndrome (50%)], followed by MLH1 [31/80 cases of Lynch syndrome (38.8%)] and MSH6 [9/81 cases of Lynch syndrome (11.2%)]. 73 Point estimates for PPVs ranged from 12.2% to 37.5% in the studies that reported on all four MMR genes,16,54,58,82,90 and from 77.4% to 84.6% in the study that reported each gene separately. 73
Strategy 4: immunohistochemistry testing with MLH1 promoter hypermethylation testing
Three studies provided test accuracy data for this strategy, including the PETALS study (Dr Neil AJ Ryan, personal communication). 16,78 Two studies comprised selected samples of women. 16,78 One study excluded women aged > 50 years,16 and one study included only women with a family history of endometrial cancer. 78 The PETALS study was conducted in an unselected sample of women with endometrial cancer (Dr Neil AJ Ryan, personal communication). There were (confidential information has been removed) true positives and (confidential information has been removed) false positives out of (confidential information has been removed) women tested. The most commonly affected gene was MSH2 [(confidential information has been removed) cases of Lynch syndrome, (confidential information has been removed)] followed by MSH6 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)], MLH1 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)] and PMS2 [(confidential information has been removed) cases of Lynch syndrome (confidential information has been removed)]. Only the PETALS study assessed PMS2 (Dr Neil AJ Ryan, personal communication). In total, (confidential information has been removed) VUSs were identified in the three studies [median (confidential information has been removed); (confidential information has been removed) cases per study]. Point estimates for PPVs ranged from (confidential information has been removed) to (confidential information has been removed). All three studies reported the results of MLH1 promoter hypermethylation testing. (Confidential information has been removed) out of (confidential information has been removed) tumours (confidential information has been removed),78 (confidential information has been removed) out of (confidential information has been removed) tumours (confidential information has been removed)16 and (confidential information has been removed) (PETALS study, Dr Neil AJ Ryan, personal communication) were hypermethylated.
Strategy 5: microsatellite instability testing followed by immunohistochemistry testing
No studies were identified that examined this strategy.
Strategy 6: microsatellite instability followed by immunohistochemistry testing with MLH1 promoter hypermethylation testing
No studies were identified that examined this strategy.
Strategy 7: immunohistochemistry followed by microsatellite instability testing
No studies were identified that examined this strategy.
Strategy 8: immunohistochemistry testing followed by microsatellite instability testing with MLH1 promoter hypermethylation testing
No studies were identified that examined this strategy.
Strategy 9: microsatellite instability and immunohistochemistry testing
No studies were identified that examined this strategy.
Strategy 10: microsatellite instability and immunohistochemistry testing with MLH1 promoter hypermethylation testing
Six studies provided test accuracy data for this strategy. 16,58,63,78,83,92 All six studies comprised selected samples of women. One study excluded women with recurrent or synchronous cancers;58 one study excluded women who were not considered suitable candidates for surgery, who had had prior retroperitoneal surgery or prior pelvic or abdominal radiation therapy, or who were pregnant;63 one study excluded women aged > 50 years;16 one study included only women with a family history of endometrial cancer;78 one study included an unselected sample of women, but did not report data on women with uninformative MMR results or without prior tumour testing;83 and one study included only women who answered questions about family/personal history of cancer and who had tumour and normal tissue available for analysis. 92 Four panels of markers were used in the six studies; the studies by Berends et al. 54 and Ollikainen et al. 78 used the same five-marker panel, and the studies by Lu et al. 16 and Rubio et al. 82 used the same six-marker panel. There were 94 true positives and 311 false positives out of 1627 women tested. Five studies reported the affected genes. 16,58,63,78,92 The most commonly affected gene was MSH2 [19/43 cases of Lynch syndrome (44.2%)], followed by MSH6 [16/43 cases of Lynch syndrome (37.2%)], MLH1 [5/43 cases of Lynch syndrome (11.6%)] and PMS2 [3 out of 43 cases of Lynch syndrome (7.0%)]. Only two studies assessed PMS2. 58,63 Five studies reported details of VUSs. 16,58,63,78,92 In total, 18 VUSs were identified (median 2; 0–14 cases per study). Point estimates for PPVs ranged from 18.3% to 43.1%. Five studies reported the results of MLH1 promoter hypermethylation testing. 16,58,63,78,92 From 14.3% to 92.3% of tumours were hypermethylated (368/516 tumours).
Strategy 11: germline testing only
Nine studies provided data on germline-only testing, whereby women were offered the reference standard(s), irrespective of the result of index tests. 16,54,58,62,74,81–83,90 Lynch syndrome was identified in 166 out of 1375 (12.1%) women tested (median 9; 5–51 cases of Lynch syndrome per study). In total, 47 VUSs were identified (median 3; 0–15 cases per study).
Full details on strategies 1–11 are provided in Table 10.
Test failures and indeterminate results
Data on test failures and/or indeterminate results can be found in Appendix 5.
Complete test accuracy studies
Head-to-head studies
Test failures were reported for 0–1% of tumours for IHC (1/356 tumours). No test failures were reported for MSI-based testing. No indeterminate results were reported for either of the tests. Testing was not conducted for 0–12.1% of participants (25/372 tumours) for IHC, and for 1.7–25.2% of participants (54/372 tumours) for MSI-based testing because of insufficient tumour tissue (or unspecified reasons).
Studies of immunohistochemistry alone
Test failures were reported for 0–1% of tumours for IHC (1/522 tumours). No indeterminate results were reported. Testing was not conducted for 0–16.2% of participants (57/372 tumours) for IHC because of insufficient tumour tissue (or unspecified reasons).
Studies of microsatellite instability alone
No test failures or indeterminate results were reported for MSI-based testing in any of the included studies. Testing was not conducted for 1.7–25.2% of participants (54/372 tumours) because of insufficient tumour tissue (or unspecified reasons).
Studies of immunohistochemistry/microsatellite instability and MLH1 promoter hypermethylation testing combined
Data on test failures, indeterminate results or lack of testing were reported in full for two studies. 16,58 One study did not report any data on test failures, indeterminate results or lack of testing,81 and one study did not provide these data for MLH1 promoter hypermethylation testing. 83 Test failures were reported for 0–1% of tumours for IHC (1/567 tumours). No test failures were reported for MSI-based testing or MLH1 promoter hypermethylation testing. No indeterminate results were reported for any of the three tests. Testing was not conducted for 0–8.1% of participants (9/576 tumours) for IHC, and for 0.5–25.2% of participants (39/372 tumours) for MSI-based testing because of insufficient tumour tissue (or unspecified reasons). There were no reported instances in which MLH1 promoter hypermethylation testing could not be carried out.
Partial test accuracy
Strategy 1: microsatellite instability alone
Test failures were reported in the PETALS study for (confidential information has been removed) of tumours for MSI testing [(confidential information has been removed) tumours tested]. None of the other studies reported test failures. No study reported indeterminate results. No testing was conducted for (confidential information has been removed) of participants [(confidential information has been removed) tumours] because of insufficient tumour tissue.
Strategy 3: immunohistochemistry alone
No test failures or indeterminate results were reported. No testing was conducted for 0–16.2% of participants (57/644 tumours) because of insufficient tumour tissue.
Strategy 4: immunohistochemistry with MLH1 promoter hypermethylation testing
Test failures were reported in one study, in which (confidential information has been removed) out of (confidential information has been removed) IHC tests failed (but were all successful on retesting), and (confidential information has been removed) out of (confidential information has been removed) MLH1 promoter hypermethylation tests failed multiple times (the PETALS study, Dr Neil AJ Ryan, personal communication). No other test failures or indeterminate results were reported, and all tumours had sufficient tissue for testing.
Strategy 10: microsatellite instability and immunohistochemistry with MLH1 promoter hypermethylation testing
Test failures were reported for 0–8.1% of tumours for IHC (13/1686 tumours), no tumours for MSI-based testing and 0–3.7% of tumours for MLH1 promoter hypermethylation testing (39/1163 of tumours). No indeterminate results were reported for any of the three tests. No testing was conducted for 0–8.1% of participants (9/1686 tumours) for IHC, for 0–25.2% of participants (28/1163 tumours) for MSI-based testing and for 0% (out of 173 tumours – number of tumours tested not reported for two studies83,92) of participants for MLH1 promoter hypermethylation testing because of insufficient tumour tissue.
Decline rates
A total of 33 studies reported on index test and germline testing. There were approximately (confidential information has been removed) patients included across these 32 studies. Six studies [seven papers, including the PETALS study (Dr Neil AJ Ryan, personal communication)] reported on the number of declines at baseline, prior to testing. 15,55,56,62,74,76,88 Across five of the six studies, (confidential information has been removed) people declined or failed to respond to the study invite out of approximately (confidential information has been removed) invited (incomplete reporting of denominator). From the remaining study,55 (confidential information has been removed) people failed to provide insurance to enable testing or declined, or there was insufficient tumour sample, but it was not specified precisely how many declined. Seven studies reported no declines at baseline. 61,64,65,67,77,89,90,92
Seven studies, including the PETALS study, reported on the numbers declining genetic counselling. 51,61,62,67,74,77 (Confidential information has been removed) patients declined genetic counselling out of the (confidential information has been removed) offered it.
Fifteen studies (16 papers, including the PETALS study) reported on the number declining germline testing. 16,51,52,55,60–63,67,69,70,77,81–83 Across these 15 studies, (confidential information has been removed) patients declined germline testing out of the (confidential information has been removed) patients offered the test. In addition, in two studies (including the PETALS study),61 (confidential information has been removed) patients died prior to germline testing, (confidential information has been removed) were lost to follow-up and (confidential information has been removed) were already known carriers for Lynch syndrome.
Assessment of studies of clinical effectiveness (key question 2)
No eligible studies were identified that reported on the clinical effectiveness (benefits and harms) of testing for Lynch syndrome among people who have endometrial cancer, and/or their relatives. Most studies were excluded for multiple reasons. The most common reasons for exclusion were that studies were not RCTs (and so subject to greater risk of bias) and/or they did not have any relevant outcomes. A further limitation was that most studies were in the broader Lynch syndrome population rather than among those who had endometrial cancer, which limits applicability to our question. Reasons for exclusion are given in Appendix 3.
Some of the excluded studies are discussed here. These were all considered for inclusion in the economic model, alongside other sources. de Jong et al. 94 describe reducing time trends in CRC mortality, which they associate with increasing surveillance for Lynch syndrome over time. These time trends are subject to confounding. Järvinen et al. ,95 in an observational cohort, compared Lynch mutation-positive relatives (who were offered colorectal and endometrial surveillance) with Lynch mutation-negative relatives (who received no such surveillance). They found no difference between the groups over 11 years of follow-up, although this analysis was probably underpowered, with very wide CIs, and biased because of the differences in risk profile between groups at baseline. Järvinen et al. 44 found that screening Lynch syndrome patients for CRC was associated with a reduction in CRC incidence. However, patients were not randomly allocated; they self-selected into screened and unscreened groups, so this study is subject to selection bias. HNPCC registry studies describe women’s outcomes after endometrial cancer surveillance, for example in Denmark96 and Finland. 97 These studies did not have a comparator group of unscreened women. There are RCTs of different aspirin doses in people with Lynch syndrome, in Australia98 and Israel. 99 These ongoing RCTs do not yet have any results available.
Summary of the clinical effectiveness findings and implications for the health economic model
The estimates used for prevalence, Lynch syndrome gene type and frequency, test failure and test accuracy for each strategy were taken from the clinical effectiveness analysis, as described in this section. The rest of the economic model inputs can be found in Assessment of studies of clinical effectiveness (key question 2).
Prevalence
For the health economic model, we incorporated data from the nine studies (reported in 11 papers) that assessed prevalence of Lynch syndrome in unselected samples of women with endometrial cancer; these studies were the most applicable to our population of interest, that is they did not limit on the basis of age or prior cancers. The median prevalence of Lynch syndrome in these papers [including the PETALS study (Dr Neil AJ Ryan, personal communication)] was 3.2% (range 0–5.3%). 15,52,55,56,59–61,70,76,88 This was used in our base case as overall prevalence of Lynch syndrome in endometrial cancer.
Ryan et al. 100 also systematically reviewed the evidence on the prevalence of Lynch syndrome among endometrial cancer patients. Few studies undertook germline testing in all women with endometrial cancer, so Ryan et al. 100 took a stepwise approach to estimation. They conducted a series of meta-analyses of test positivity of IHC (MLH1 specific and across all proteins), MSI and methylation. They combined data from these separate meta-analyses on overlapping, but differing, populations to estimate what proportion of women would be referred for germline analysis using a combination of these tests. They then meta-analysed the proportion of women who were positive for Lynch syndrome in germline testing in a population that was an approximation to the testing strategy positive population. They combined these analyses to estimate that 3% of women with endometrial cancer have Lynch syndrome. This approach enabled the combination of data from a large number of studies, but made assumptions about the equivalence of different populations, and was inclusive of studies that did not exactly represent the population or test of interest.
Both reviews suggest a figure for overall prevalence of around the 3% level (confidential information has been removed) [(confidential information has been removed) out of (confidential information has been removed) women tested, including (confidential information has been removed) women with known Lynch syndrome] present in their sample.
A higher base-case prevalence of 3.91% obtained through random-effects meta-analysis of results from 15 studies was used by Snowsill et al. 43 However, when studies at risk of bias as a result of high dropout rates (≥ 10%) were excluded (n = 7), the estimated prevalence obtained was reduced to 3.0%, nearer to the figure used in our base case.
When we varied our approach from using studies with unselected endometrial cancer probands to using studies with selection criteria, prevalence estimates increased to a median of 6.5% (range 0.9–36.1%). In the systematic review by Ryan et al. ,100 a subgroup analysis of studies that did not use a tumour triage stage, but proceeded directly to germline testing, also found a higher proportion of Lynch syndrome carriers, of 6%. We have therefore conducted a sensitivity analysis using an increased overall prevalence figure of 6.5%.
Prevalence by individual gene
Four studies retrieved in the systematic review [including the PETALS study (Dr Neil AJ Ryan, personal communication)] assessed all four MMR genes (MLH1, MSH2, MSH6 and PMS2) using sequencing plus MLPA as a reference standard in an unselected sample of women with endometrial cancer. 15,55,61 Data from the four studies were combined to produce prevalence estimates of MMR genes among women diagnosed with Lynch syndrome: MLH1, 17.1%; MSH2, 24.4%; MSH6, 46.4%; and PMS2, 12.2%.
When studies from our review with unselected samples were also included, which had all four genes, and any reference standard (n = 8), the results were as follows: MLH1, 16.1%; MSH2, 31.7%; MSH6, 40.5%; and PMS2, 10.1%. The model by Snowsill et al. 43 produced the following figures: MLH1, 16.9%; MSH2, 24.6%; MSH6, 47.7%; and PMS2, 10.8%. Figures do not vary substantially despite differing methodology to elicit data, although our combined estimates inflate the proportion of MHS2, while reducing MSH6 prevalence. Our preferred base-case parameters were therefore taken from the unselected studies in our review.
Test failure
Test failure rates of MSI, IHC and MLH1 promoter hypermethylation testing were all extremely low in all studies identified in the systematic literature review, with median values of 0% for all. This is likely to be explained by testing protocols in laboratories when tumours with insufficient samples were not tested. However, some test failures did occur, with the range greater for MSI (0–43.3%) than for IHC (0–11.8%); the range for MLH1 promoter hypermethylation was the lowest (0–0.03%). As all tests had a median 0% failure rate, this was used in our base case, with parameters set around this for the PSA.
Diagnostic accuracy
Initially, we attempted to identify the best test accuracy estimate per strategy from the systematic review. However, we did not use this approach in the economic model, because of issues of inconsistency described below. Instead, we used data from Lu et al. 16 to ensure consistency across strategies and to aid comparison between strategies. We undertook sensitivity analyses using estimates from the PETALS study and Snowsill et al. 43
Most applicable and least biased test accuracy per strategy approach
Although we found 45 papers describing at least partial test accuracy, only seven gave full test accuracy data from which we could extract 2 × 2 tables. Seven studies provided complete test accuracy data (i.e. sensitivity, specificity, PPVs and NPVs). 16,54,58,81–83,90 None was conducted in the UK, and most of these covered only a small subset of the strategies, so meta-analysis within each strategy was not possible because of the small number of heterogeneous studies. We identified the most applicable, least biased, study from this group. The rationale for each is outlined in the following paragraphs. Overall, data from Chao et al. 58 were considered the most applicable and least biased for strategies 1 and 3, but did not provide data for many of the other strategies. Lu et al. 16 provided data for more strategies, but for some strategies no data were available.
Strategy 1: microsatellite instability testing alone
Four studies provided data for this strategy. 16,54,58,82 Chao et al. 58 were considered to provide the most applicable and least biased data, as they included an unselected sample of women with endometrial cancer, although the study was not conducted in a country with demographics comparable to those of the UK, and had very few cases of Lynch syndrome, with an incomplete head-to-head design.
Strategy 3: immunohistochemistry alone
Five studies provided data for this strategy. 16,54,58,82,90 Chao et al. 58 were considered to provide the most applicable and least biased data, as they included an unselected sample of women with endometrial cancer, although the study was not conducted in a country with demographics comparable to those of the UK.
Strategies 4, 5, 7 and 9
No study directly assessed these strategies. One study (comprising a selected sample of women aged ≤ 50 years) presented sufficient data for us to estimate test accuracy data for this testing strategy. 16 In this study IHC, MSI-based testing and analysis of MLH1 promoter hypermethylation were employed, with the results of each test present for each of the 100 participants.
These data can be used to estimate what could have happened for strategies 4, 5, 7 and 9.
Strategy 10: microsatellite instability and immunohistochemistry testing with MLH1 promoter hypermethylation testing
Four studies provided data for this strategy. 16,58,81,83 Two studies were excluded from consideration as either they included a selected sample of women (aged < 50 years at diagnosis),16 or data were not extractable for the whole sample. 83 The most applicable and least biased accuracy data were considered to come from combining the remaining two studies, as they were similar in terms of participant selection, testing methods, choice of reference standards and sample sizes, with neither one being conducted in a country with demographics comparable to those of the UK: one was conducted in China58 and one in the USA. 81
No data were available from these papers to populate the model for the following testing strategies:
-
strategy 2: MSI testing with MLH1 promoter hypermethylation testing
-
strategy 6: MSI followed by IHC testing with MLH1 promoter hypermethylation testing
-
strategy 8: IHC testing followed by MSI testing with MLH1 promoter hypermethylation testing.
The small sample sizes, different biases, exact tests and populations between the studies means that these estimates would have made some tests spuriously appear more cost-effective than others as a result of differences between studies, rather than tests. For example, although the Chao et al. 58 study was considered to contain the best evidence for IHC and MSI accuracy, there were only four cases of Lynch syndrome for IHC and six for MSI, so, in this study, the small numbers and incomplete testing for women introduced biases suggesting a strong advantage in accuracy of MSI over IHC, which was not reflected in the rest of the literature. Furthermore, Chao et al. 58 did not give information for the pathways including MLH1 promoter hypermethylation testing, so accuracy of strategies with and strategies without MLH1 promoter hypermethylation testing would be logically inconsistent.
Consistent test accuracy estimates from Lu et al.16
The base-case estimates for test accuracy used in the model are all from Lu et al. 16 Details can be found in Table 11. This is the only paper that provides individual-level data, which can be used to estimate test accuracy for most strategies, and therefore allows some comparison of cost-effectiveness between strategies, with caveats and limitations. There are 100 cases of endometrial cancer, of which nine have Lynch syndrome, so it is a small sample with higher than expected prevalence of Lynch syndrome. It is also in a US setting, all of the participants were diagnosed with endometrial cancer before the age of 50 years and not all patients received MLH1 promoter hypermethylation testing, particularly those who were MSI-H. Furthermore, it did not include the PMS2 protein in the IHC testing panel.
| Strategy | Germline (n) | Totals (n) | Sensitivity (95% CI) (%) | Specificity (95% CI) (%) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| 1: MSI testing alone | |||||
| Index test positive | 8 | 17 | 25 | NA | NA |
| Index test negative | 0 | 70 | 70 | NA | NA |
| Totals | 8 | 87 | 95 | 100.0 (63.06 to 100) | 80.46 (70.57 to 88.19) |
| 2: MSI testing with MLH1 promoter hypermethylation testing | |||||
| Index test positive | 5 | 3 | 8 | NA | NA |
| Index test negative | 3 | 84 | 87 | NA | NA |
| Totals | 8 | 87 | 95 | 62.50 (24.49 to 91.48) | 96.55 (90.25 to 99.28) |
| 3: IHC-based testing alone | |||||
| Index test positive | 9 | 15 | 24 | NA | NA |
| Index test negative | 0 | 75 | 75 | NA | NA |
| Totals | 9 | 90 | 99 | 100.0 (66.37 to 100) | 83.33 (74.00 to 90.36) |
| 4: IHC testing with MLH1 promoter hypermethylation testing | |||||
| Index test positive | 8 | 3 | 11 | NA | NA |
| Index test negative | 0 | 87 | 87 | NA | NA |
| Totals | 8 | 90 | 98 | 100.0 (63.06 to 100) | 96.67 (90.57 to 99.31) |
| 5: MSI testing followed by IHC testing | |||||
| Index test positive | 8 | 19 | 27 | NA | NA |
| Index test negative | 0 | 68 | 68 | NA | NA |
| Totals | 8 | 87 | 95 | 100.0 (63.06 to 100) | 78.16 (68.02 to 86.31) |
| 6: MSI followed by IHC testing with MLH1 promoter hypermethylation testing | |||||
| Index test positive | 5 | 4 | 9 | NA | NA |
| Index test negative | 3 | 83 | 86 | NA | NA |
| Totals | 8 | 87 | 95 | 62.50 (24.49 to 91.48) | 95.40 (88.64 to 98.73) |
| 7: IHC followed by MSI testing | |||||
| Index test positive | 9 | 18 | 27 | NA | NA |
| Index test negative | 0 | 68 | 68 | NA | NA |
| Totals | 9 | 86 | 95 | 100.0 (66.37 to 100) | 79.07 (68.95 to 87.10) |
| 8: IHC followed by MSI testing | |||||
| Index test positive | 8 | 5 | 13 | NA | NA |
| Index test negative | 0 | 81 | 81 | NA | NA |
| Totals | 8 | 86 | 94 | 100.0 (63.06 to 100) | 94.19 (86.95 to 98.09) |
| 9: MSI and IHC testing | |||||
| Index test positive | 8 | 18 | 26 | NA | NA |
| Index test negative | 0 | 68 | 68 | NA | NA |
| Totals | 8 | 86 | 94 | 100.0 (63.06 to 100) | 79.07 (68.95 to 87.10) |
| 10: MSI and IHC testing with MLH1 promoter hypermethylation testing | |||||
| Index test positive | 8 | 5 | 13 | NA | NA |
| Index test negative | 0 | 81 | 81 | NA | NA |
| Totals | 8 | 86 | 94 | 100.0 (63.06 to 100.00) | 94.19 (86.95 to 98.09) |
Test accuracy data were extracted for strategies 1, 3–5, 7, 9 and 11 by using the individual patient data reported to calculate whether each patient was a true positive, false positive, true negative or false negative for Lynch syndrome. There were very low numbers of missing data for these strategies. When data were missing on the pathway in question, we excluded the case; when we could follow the whole strategy for that patient, we included them, even if there were missing data elsewhere. There were also incomplete data for MLH1 promoter hypermethylation testing after IHC, but, of the 13 MLH1-deficient tumours identified through IHC testing (so potentially eligible for MLH1 promoter hypermethylation testing), 12 had MLH1 promoter hypermethylation testing results. We excluded the one case without results, which was a germline-positive mutation on MLH1. There was a particular problem with lack of data on MLH1 promoter hypermethylation testing for MSI-H, affecting strategies 2, 6, 8 and 10. Overall, of the 25 cases who tested MSI-H, only 13 tested MSI status, and all 13 were in patients without a germline mutation (two were in VUSs). Excluding these would have excluded all patients with the disease. There is some evidence from an Australian study56 on the accuracy of methylation testing in cases demonstrating MLH1/PMS2 IHC loss: out of 127 cases, 111 were hypermethylated, all of whom were germline MLH1 negative. However, accuracy of methylation testing in MSI-H cases, beyond those who also have IHC MLH1 loss, is not known, so a conservative estimate was considered appropriate. 56 We pragmatically decided, for the purposes of the model, to estimate that methylation is correct 66% of the time to the nearest whole number, estimated separately for germline-positive and germline-negative cases. These estimates affected strategies 2 and 6 most acutely, with 13 cases in each (out of totals of 94 and 95, respectively) when MLH1 promoter hypermethylation testing results were assumed. This is because these strategies start with MSI-based testing, then hypermethylation of cases if instability is detected. For strategy 8, this method of estimation was applied to 3 out of 98 cases, and to 4 out of 94 cases for strategy 10. Test accuracy estimates should be viewed with extreme caution (in particular strategies 2, 6, 8 and 10).
Sensitivity analyses
Owing to uncertainties in the base case, a sensitivity analysis was performed, using data from a large, as yet unpublished, UK-based study (Dr Neil AJ Ryan, personal communication). (Confidential information has been removed.)
Chapter 5 Systematic literature review of other economic models
The literature search identified 4682 records through electronic database searches and other sources. After removing duplicates, 2882 records were screened for inclusion. On the basis of title and abstract, 2854 records were excluded. The remaining 28 records were included for full-text screening. A further 23 articles were excluded at the full-text stage, mainly because of being an abstract only or having an irrelevant study population. The literature search identified five studies43,101–104 that undertook an economic analysis to assess the cost-effectiveness of screening strategies used to identify Lynch syndrome in women diagnosed with endometrial cancer. The flow diagram is shown in Figure 20.
FIGURE 20.
Flow diagram of the economic model review.
Summary of the economic analyses undertaken
In this section, we summarise the economic analyses used to compare different screening strategies available to diagnose Lynch syndrome in women diagnosed with endometrial cancer.
Resnick et al.101
Resnick et al. 101 used a decision tree illustrative model structure to assess the cost-effectiveness of screening strategies for diagnosing Lynch syndrome among newly diagnosed endometrial cancer patients. The model depicted the clinical pathway that endometrial cancer patients would take while being screened for Lynch syndrome. The model started with a hypothetical cohort of 40,000 women expected to have endometrial cancer who underwent a screening strategy: Amsterdam criteria93 (full gene sequencing for women with endometrial cancer who met the revised Amsterdam criteria), sequence all (full gene sequencing for all women with endometrial cancer), sequence for all women aged < 60 years with endometrial cancer and IHC/single-gene strategy (IHC for all women with endometrial cancer after gene sequencing). After testing, women were categorised as Lynch positive or Lynch negative. With the IHC and sequencing testing strategy, women were categorised as MLH1 ≥ 60 years of age, MLH1 < 60 years of age, MSH6 or MSH2. Women with MSH6 deletion were considered to be Lynch positive, and women with MSH6 normal were categorised as MSH2 deletion (Lynch positive) or MSH2 normal (Lynch negative).
Clinical as well as cost information was required to populate the model, and this was obtained from published sources. Clinical information included the probability of fulfilling the Amsterdam criteria,93 people who do fulfil the Amsterdam criteria and have Lynch syndrome, all women with Lynch syndrome, women with Lynch syndrome stratified by age (< 60 years and ≥ 60 years) and people with normal IHC results. Resource use and costs were required for genetic consultation, full genetic sequencing, IHC and MLH1, MSH2 and MSH6 sequencing. All costs included in the model were reported in 2008 US dollars. The analysis was conducted from a third-party payer perspective, with the results presented in terms of an ICER, expressed as cost per additional Lynch syndrome case detected. Authors undertook a scenario analysis around the cost of full gene sequencing.
The base-case deterministic results showed that IHC/single-gene strategy, when compared with the Amsterdam testing strategy, had an ICER of approximately US$13,800 per Lynch syndrome case detected. The results of the scenario analysis showed that the ICER was sensitive to the cost of the full gene sequencing. Authors acknowledged and discussed the limitations of the economic analysis, then concluded that the testing strategy IHC and sequencing was the most cost-effective for identifying Lynch syndrome in women diagnosed with endometrial cancer.
The economic analysis provides a useful starting point to assess the cost-effectiveness of different testing strategies to detect Lynch syndrome in women diagnosed with endometrial cancer. Although the decision tree structure was appropriate to address the research question, the analysis was limited, as the ‘downstream’ costs and benefits associated with identifying women Lynch syndrome were not captured in the economic model. Thus, the impact of identifying these additional cases remains unanswered. In addition, the authors acknowledged that the testing strategy genotyping for the screening of MMR deficiency was not included in the economic analysis. In general, the economic evaluation was transparent and adhered to the reporting guidelines for undertaking economic analyses. Future model-based analyses could build on this simplistic model to capture the impact of including testing and treating of women with Lynch syndrome in a single cost-effectiveness analysis.
Kwon et al.102
Kwon et al. 102 used a Markov Monte Carlo simulation model to assess the cost-effectiveness of different testing strategies to identify Lynch syndrome in women diagnosed with endometrial cancer. The model started with a hypothetical cohort of women who had received treatment for endometrial cancer and were now receiving one of the following testing strategies endometrial cancer: aged < 50 years with at least one first-degree relative with a Lynch syndrome-associated cancer, endometrial cancer at < 50 years (IHC triage), endometrial cancer at < 60 years (IHC triage), endometrial cancer at any age with at least one first-degree relative with a Lynch syndrome-associated cancer (IHC triage), all endometrial cancers and any age (IHC triage), compared with Amsterdam II criteria. 93 Authors have outlined and justified why they have excluded testing strategies that include MSI.
The model was populated with clinical parameters, as well as information about resource use and costs. Clinical parameters included the prevalence, sensitivity and specificity of each testing strategy; the lifetime risk of CRC; and the 5-year mortality from CRC, all of which were obtained from the published literature. Resource use and costs included the costs for genetic counselling, gene sequencing, IHC for MMR proteins, colonoscopy and CRC treatment costs. Details of resource use and costs were provided, and references reported. All costs were reported in US dollars and reported in 2010 prices. Several simplifying assumptions were made to have a workable model structure.
The base-case analysis was undertaken from the societal perspective, with costs incurred and benefits accrued discounted based on 3% per annum. The economic analysis concluded at a lifetime time horizon, with the results reported as an ICER expressed as cost per life-year gained. It should be noted that the costs included in the analysis did not accurately reflect the viewpoint of the analysis. To our knowledge, only costs incurred by the health-care provider were included in the economic analysis, thereby reflecting a narrower perspective (third-party provider).
Deterministic results showed that IHC triage of women of any age with at least one first-degree relative with Lynch syndrome-associated cancer, when compared with women aged < 50 years and with at least one first-degree relative with Lynch syndrome-associated cancer, had an ICER of approximately US$9100 per life-year gained. Furthermore, results were reported on the number of women who would undergo IHC and, subsequently, the women diagnosed with Lynch syndrome and those who went on to develop CRC. Sensitivity and scenario analyses results showed that the ICER was robust to changes made to the model input parameters. Under the current model structure, model inputs and assumptions led the authors to conclude that IHC triage of women of any age, with at least one first-degree relative with Lynch syndrome-associated cancer, was the most cost-effective testing strategy when compared with using the Amsterdam II criteria. 93
This economic analysis adds to the existing literature about which screening strategies provide good value for money in diagnosing Lynch syndrome in women with endometrial cancer. However, there were several concerns related to this analysis. First, it is unclear about the patient pathway following testing, as no illustrative structures have been presented in the main document or online supplementary material. Second, it is unclear what assumptions are being made about the CRC mortality rate derived from the 5-year mortality obtained from the published literature. Third, care should be taken when interpreting the deterministic results, as the analysis was undertaken from the societal perspective, but the costs included in the analysis did not reflect this viewpoint.
Bruegl et al.103
Bruegl et al. 103 undertook an economic analysis alongside a clinical study to assess the cost-effectiveness of universal tissue testing (IHC for all and MLH1 methylation analysis when indicated) versus the Society of Gynaecologic Oncology (SGO) 5–10% clinical criteria105 (n = 97) for identifying Lynch syndrome in a cohort (n = 412 cases) of unselected women with endometrial cancer. Two approaches were used to assess the cost-effectiveness. First, the direct costs associated with identifying patients with probable Lynch syndrome and, second, the direct costs associated with identifying cases with probable Lynch syndrome among women with endometrial cancer, as well as their potentially affected first-degree relatives.
The analysis was conducted from a third-party payer perspective, with all costs reported in US dollars, in 2012 prices. The economic analyses included hospital and health-care professional costs associated with identifying women with probable Lynch syndrome. Costs included initial genetic counselling and follow-up visits; IHC for MLH1, MSH2, MSH6 and PMS2; MLH1 promoter hypermethylation assay for tumours with loss of MLH1; and single germline-mutation testing.
Under the SGO 5–10% clinical criteria,105 97 women would undergo further evaluation, of which 15 would be diagnosed with probable Lynch syndrome, resulting in a cost of approximately US$6100 per probable Lynch syndrome case diagnosed. Including screening for probable Lynch syndrome and their first-degree relatives under the SGO 5–10% clinical criteria105 strategy would cost approximately US$6300 per probable Lynch syndrome case diagnosed. This is based on the average number of first-degree relatives (5.3 relatives) and the estimated germline mutation rates among probable Lynch syndrome endometrial cancer patients’ first-degree relatives eligible for single-site gene mutation analysis. Under the universal tumour testing strategy, 43 women with probable Lynch syndrome would be identified, resulting in a cost of approximately US$5900 per probable Lynch syndrome case diagnosed. Including universal tumour screening for probable Lynch syndrome and screening their first-degree relatives would cost approximately US$6500 per probable Lynch syndrome case diagnosed. This is based on the average number of first-degree relatives (5.5 relatives) and the estimated germline mutation rates among probable Lynch syndrome endometrial cancer patients’ first-degree relatives eligible for single-site gene mutation analysis.
The authors concluded that, under the existing SGO 5–10% clinical criteria105 to identify Lynch syndrome in women diagnosed with endometrial cancer, this strategy is likely to miss some cases, when compared with using a universal tumour-testing strategy (IHC for DNA MMR proteins and PCR-based MLH1 methylation analysis for tumours with loss of MLH1).
The economic analysis presented here is conducted alongside a clinical trial to assess the clinical effectiveness and cost-effectiveness of different strategies that can be used to identify and diagnose women (and their first-degree relatives) with Lynch syndrome. Although the analysis adds to the existing literature, there are some concerns regarding the transferability and robustness of these results. First, as acknowledged by the authors, all potentially relevant strategies have not been included in the analysis; this is common in clinical trials. Second, the authors assumed that there is a 100% genetic counselling referral rate for endometrial cancer patients meeting the SGO 5–10% criteria,105 but referral rates are likely to be between 17% and 48%. Third, it is assumed that all patients meeting the SGO 5–10% criteria, or with tumour testing suggestive of Lynch syndrome, will accept germline counselling and/or germline testing, but this is not likely to be 100%. Fourth, the resource quantity used to derive costs is unclear, which limits the transparency about how costs were derived. Finally, the authors did not conduct sensitivity analyses to address uncertainty in the economic analysis.
Goverde et al.104
Authors undertook an economic analysis of a population-based cohort of endometrial cancer patients aged ≤ 70 years undergoing routine screening for Lynch syndrome. The economic analysis compared routine screening for Lynch syndrome by analysis of MSI and IHC for MLH1, MSH2, MSH6 and PMS2 protein expression in endometrial cancer patients up to the age of 70 years with screening for Lynch syndrome in endometrial cancer patients using an age cut-off point.
The analysis required clinical and cost information. Clinical parameters included acceptance of prophylactic gynaecological surgery, complication rate following colonoscopy, lifetime risk of developing CRC for Lynch syndrome carriers and reduction in CRC risks by Lynch syndrome surveillance. Resource use and costs included MSI analysis, genetic counselling and germline mutation analysis, IHC and MLH1 promoter hypermethylation testing, which were derived using microcosting methodology. All costs were reported in euros, in 2013 prices.
The analysis was undertaken from the third-party payer perspective, with costs incurred and benefits accrued discounted based on 3% per annum. The economic analysis concluded at a lifetime time horizon, with the results reported as an ICER, expressed as cost per life-year gained. Base-case deterministic results showed that routine screening of endometrial cancer patients up to the age of 70 years for Lynch syndrome by analysis of MSI, IHC and MLH1 promoter hypermethylation testing was cost-effective when compared with screening up to the age of 50 years, with an ICER of approximately €5300 per life-year gained. Sensitivity analysis results showed that economic analysis was sensitive to the life-years gained per female relative. The authors concluded that routine screening by analysis of MSI, IHC and MLH1 promoter hypermethylation testing for Lynch syndrome in people diagnosed with endometrial cancer up to the age of 70 years was the most cost-effective strategy, compared with an age cut-off point of 50 years.
The economic analysis builds on the current cost-effectiveness evidence of different strategies to detect Lynch syndrome in women diagnosed with endometrial cancer. In comparison to previous analyses, this analysis included the costs and benefits for first-degree relatives of probands.
Snowsill et al.43
Authors conducted an economic analysis by using a decision tree structure with Markov nodes to assess the cost-effectiveness of different testing strategies (MSI with methylation, direct mutation testing, IHC with methylation, MSI alone, IHC and a no-testing strategy) to identify Lynch syndrome in women treated for endometrial cancer. Authors clearly provided an illustrative model structure that depicted the patient pathway for endometrial cancer survivors undergoing screening for Lynch syndrome. The model started with a hypothetical cohort of women undergoing one of the screening strategies. In general, women were diagnosed as actually Lynch syndrome, actually sporadic Lynch syndrome or probable Lynch syndrome. Following diagnosis, women received CRC surveillance.
The model required clinical information (natural history, epidemiology, HRQoL, diagnostic accuracy, preventative effectiveness and utility values) and resource use and cost information (testing strategies, events and outcomes) for women undergoing screening for Lynch syndrome.
The analysis was conducted from the NHS and PSS perspective, with all costs reported in Great British pounds, in 2016/17 prices. All costs incurred and benefits accrued were discounted based on a 3.5% per annum rate. The analysis was conducted over a lifetime time horizon, with the results presented in terms of an ICER, expressed as cost per QALY. An ICER at or below the £20,000 per QALY WTP threshold was cost-effective. Several one-way sensitivity analyses, including PSAs, were undertaken based on the cost per QALY.
The base-case deterministic results showed that the IHC with methylation strategy was the most cost-effective strategy, with an ICER of approximately £14,200 per QALY. The IHC-alone strategy yielded the most QALYs and was most costly, but the results did not reach cost-effectiveness when compared with IHC with methylation, with an ICER of approximately £129,000 per QALY. The PSA results showed that there was a 0.36 probability that IHC with methylation was the most-cost-effective strategy at a WTP threshold of £20,000 per QALY. One-way sensitivity analysis results showed that the ICER was sensitive to the age of the proband and the effectiveness of colonoscopy in reducing CRC incidence. Scenario analysis results showed that, by using the effectiveness of colonoscopic surveillance to reduce the CRC incidence derived from information obtained from Arrigoni et al. ,106 none of the testing strategies was cost-effective.
The economic analysis builds on the existing cost-effectiveness evidence in this disease area, by including the diagnosis of Lynch syndrome and the benefit to probands of CRC screening. This analysis could have been improved by reporting the results in terms of the natural units, in addition to reporting the results in terms of cost per QALY alone. In addition, the model was sensitive to some model input parameters. Specific attention in the form of systematic reviews around these key parameters with a detailed critique between sources would improve the transparency in the selection of model inputs.
Characteristics of included studies
Appendix 4 summarises the characteristics of the studies included in this systematic review. Three economic analyses were undertaken in the USA,101–103 one in the Netherlands104 and one in the UK. 43 Three studies43,101,102 undertook a model-based economic analysis, and the remaining two studies103,104 conducted an economic analysis alongside observational information or a trial. Of the studies that used an economic model to depict/illustrate the patient experience, one analysis101 used a decision tree structure, one a Markov model structure102 and the other43 a combination of a decision tree structure and Markov model. All economic analyses clearly stated the research question, with all comparing strategies to identify Lynch syndrome in women diagnosed with endometrial cancer. It is notable that there was some overlap in terms of the strategies being compared between studies. It should be noted that no analysis included all strategies; however, exclusion of these testing strategies has been discussed.
The economic analyses were mainly undertaken from a third-party payer perspective, with one study102 from the societal perspective; however, the costs included did not reflect a societal viewpoint. All analyses except Snowsill et al. 43 reported their results in terms of natural units. Snowsill et al. 43 reported an ICER, expressed as cost per QALY. All studies attempted a one-way sensitivity analysis and/or a scenario analysis. One study undertook a PSA. 43
Three studies included the benefit to probands of reduction of the incidence of CRC. 43,102,104 Other ‘downstream’ cancers were not included. Surveillance was the only risk reduction measure included in these analyses. Benefit was also extended to first-degree relatives in these three studies. 43,102,104
Quality assessment of the modelling methods and economic analyses
Full details of the quality appraisal of included economic studies can be found in Appendix 6.
Structure
The structures of the models included were of judged to be of satisfactory quality. Studies clearly stated their decision problem or research questions, the viewpoint of their analysis, and the objectives of the models and economic analyses, which were coherent with the decision problem. Only one study43 provided extensive detail about pre-model analyses conducted to estimate the prevalence of Lynch syndrome in endometrial cancer patients, test performance on the sensitivity and specificity of the different testing strategies, and incidence of developing other ‘downstream’ cancers. When appropriate, all studies were conducted over a lifetime time horizon and included discounting costs incurred and the benefits accrued using appropriate rates.
Most studies that conducted a model-based analysis clearly showed the illustrative model structures, which depicted the clinical pathway for endometrial cancer patients undergoing screening for Lynch syndrome. Earlier models were simplistic, but were adequate to address the decision problem, and only included screening and diagnosis of Lynch syndrome. Subsequent models were more complex, and, in general, their model structures followed the screening → diagnosis → surveillance → treatment pathway. In general, authors assessed testing strategies that included IHC (with/without MLH1 methylation) followed by germline testing, MSI (with/without methylation) followed by germline testing, direct mutation testing (using the SGO 5–10% clinical criteria,105 Amsterdam II criteria,93 Bethesda guidelines19) or a no-testing strategy; confirmatory diagnosis by use of germline testing was included in all analyses. Studies that included risk-reducing interventions considered surveillance as a means to reduce the incidence of CRC. Other risk reduction interventions (e.g. surgery, chemoprevention and aspirin) were not included. Authors have alluded to this as a limitation and have provided reasonable justification for not including these interventions. Goverde et al. 104 included costs associated with gynaecologic surveillance for relatives.
Data
All studies required clinical as well as cost information to undertake the economic analyses. The methods used to identify relevant information were clearly stated. References were provided for all inputs, but authors were not clear about the choices made between sources of information, especially when more than one source was available. In addition, it was not clear if quality appraisal of these studies was undertaken. Information to populate the economic models was obtained mainly from published sources and supplemented with information from unpublished sources, which included clinical expert opinion. To our knowledge, no study undertook systematic reviews to identify studies reporting key inputs.
Studies clearly reported clinical (natural history, mortality, diagnostic accuracy for each testing strategy, preventative effectiveness and utility values) information and resource use and costs (testing strategy, colonoscopic surveillance for probands and relatives, treatment of CRC, genetic counselling and germline mutation analysis for relatives, and prophylactic surgical treatment for relatives) information required. Natural history information was required for the prevalence of Lynch syndrome, mutation status, lifetime risk of CRC, endometrial cancer mortality and CRC mortality. The prevalence of Lynch syndrome was required in all studies. Prevalence was reported by age of the proband101,102 and overall prevalence. 43,103,104 All studies reported the references for individual studies, but only Snowsill et al. 43 elaborated on the methods used to estimate the prevalence. The distribution of gene mutation status was reported in all studies. In general, studies reported gene mutation status for the overall population,102–104 older/younger than 60 years of age101 and by a given age. 43 The lifetime risk of CRC was required in three studies. 43,102,104 Both Kwon et al. 102 and Goverde et al. 104 provided estimates for lifetime risk of CRC, for which information was obtained from the literature. However, Kwon et al. 102 provided lifetime risk of CRC by mutation status and in the absence or presence of screening. Goverde et al. 104 provided estimates for Lynch syndrome carriers only. These two studies did not elaborate on the methods used to combine/pool the results from individual studies that reported lifetime risk of developing CRC. Conversely, Snowsill et al. 43 provided details about the methods used to estimate the lifetime risk of CRC. All economic analyses undertaken over a lifetime time horizon included mortality. People were subjected to endometrial cancer mortality, CRC mortality and age- and sex-specific mortality according to their respective locations. Snowsill et al. 43 derived transition probabilities for the risk of CRC mortality for people with/people without Lynch syndrome and by stage of the cancer. However, it was unclear if stage-specific risks of CRC mortality were derived in other analyses.
Information was required about the performance (sensitivity and specificity) of the different testing strategies included in the economic analyses. Derivation of sensitivity and specificity varied across studies, with most studies obtaining information from the literature, but authors have provided little information about how the evidence was appraised or synthesised. One study43 clearly stated the methodology used to derive pooled estimates, where appropriate. In addition, it was unclear what assumptions were made when combining the test accuracy of individual tests to form a testing strategy.
In all studies, the effectiveness of Lynch syndrome screening was based on cases of Lynch syndrome diagnosed. In addition, studies included the health benefit to women with Lynch syndrome and their first-degree relatives. 43,102,104 Economic analyses that included colonoscopic surveillance estimated the effectiveness/impact of surveillance on the incidence of CRC and mortality. 43,102,104
One study43 reported their results in terms of QALYs. Snowsill et al. 43 elaborated on the assumptions made, with justification about how utility values were estimated. First, baseline utility values were estimated from age- and sex-specific population values. Second, it was assumed that there was no disutility associated with people with stages I–III CRC. People with stage IV CRC had their utility scaled by 0.79, as opposed to 1.00 for stages I–III CRC. Finally, it was assumed that genetic counselling or testing had no impact on QALYs.
All studies reported the perspective of the analysis, but, in one study,102 these costs did not reflect the viewpoint stated. Resource use and costs were required for the costs of screening tests/strategies, genetic consultation and testing, CRC screening and treatment of CRC. All studies reported the sources of costs but, in some studies, it was difficult to decipher the resource use that was used to estimate unit costs.
Uncertainty
Most analyses43,101,102,104 included a one-way sensitivity analysis or scenario analysis by varying key input parameters to reflect lower and upper limits, or by making changes to input parameters if multiple sources of information were available to assess the impact on the base-case ICER, and/or to determine the key drivers of the economic model. It was unclear in some analyses if the sensitivity analysis was exhaustive, as no tornado plots were reported. Results were reported for all sensitivity and scenario analyses. Authors reported which input parameters were the most influential. To our knowledge, ‘best-case’ and ‘worst-case’ analyses were not undertaken. Snowsill et al. 43 explored heterogeneity and undertook a PSA. In addition, no economic analysis undertook a value-of-information assessment.
Assumptions
Authors clearly stated the assumptions made to have an executable model. In general, the assumptions made appeared to be feasible, with others being strong in some instances. There was little overlap between studies about the assumptions made. This may be because of the heterogeneous nature between the economic analyses. As expected, as model complexity increased, so did the number of assumptions. Details of the assumptions made for each study are reported in Appendix 4.
Discussion
The published economic evidence of strategies used to identify Lynch syndrome in women with endometrial cancer is limited to five studies. We identified three studies43,101,102 that undertook a model-based economic analysis and two studies103,104 that conducted an economic analysis alongside trial/observational data. Given the heterogeneous nature of economic analyses, these studies were discussed narratively and appraised using frameworks on best practice for reporting an economic evaluation and economic modelling. We found that studies were mainly transparent in the information used to undertake the analyses, but less so in the selection of inputs and the methods of evidence synthesis.
This systematic review was undertaken to identify the suitability of existing cost-effectiveness analyses, which primarily involves the comparative analysis of alternative interventions in terms of the costs and consequences. 107 To increase the transparency of the economic analyses and the confidence/robustness of the results, guidelines41,42 stipulate the importance of reporting the structure, the inputs, the assumptions and the handling of uncertainty. All studies clearly reported a statement of the decision problem, which included information about the disease/condition (Lynch syndrome), description of the patient population (women treated for endometrial cancer), strategies available to identify and diagnose Lynch syndrome (e.g. IHC and MSI) and objective(s) of the economic model. Three studies43,101,103 clearly provided definitions for people with Lynch syndrome, probable Lynch syndrome or sporadic Lynch syndrome, which increases the transparency and relevance to other settings. All analyses provided a statement of the perspective/viewpoint of the analysis, with one study102 stating that the analysis was undertaken from a societal perspective, which we later considered to be undertaken from a narrower perspective, as the costs included in the analysis did not reflect a societal viewpoint.
Understandably, the two economic analyses that were conducted alongside trial/observational data did not include all possible strategies. Likewise, no model-based economic evaluation included a comparison of all feasible strategies. However, analysts have provided justification about why models were constrained to the strategies included.
The choice of illustrative model structures appeared to be appropriate to address the decision problem. However, in one study,102 we were unclear of the illustrative structure used, which limits the transparency of the clinical course of Lynch syndrome in women with endometrial cancer and, hence, the reproducibility of the economic analysis. Philips et al. 42 reiterate that analysts should provide justification for the choice of model type and present an illustrative model structure.
Information required to parameterise the economic analyses included clinical and cost information. Despite all studies providing the sources of inputs, little information was provided about the methods used to identify inputs, details of any pre-model analysis and justification of incorporating inputs into the analyses. Inputs were mainly obtained from the literature (with no studies undertaking a systematic review) and supplemented with information from clinical experts. Studies that used clinical expert opinion have not elaborated/documented the methods used to identify and elicit information from clinicians. Philips et al. 42 provide guidance about eliciting information from clinical experts. All analyses required information about the prevalence of Lynch syndrome and the sensitivity and specificity of the different strategies. In most cases, several individual studies provided prevalence information and several reported test performance information. However, only Snowsill et al. 43 elaborated on the methods used to synthesise the evidence for prevalence, sensitivity and specificity. Deriving point estimates (as well as their confidence/credible intervals) should follow acceptable methods for synthesising the evidence. 42 These pre-model analyses should be clearly reported on or signposted. The process of data incorporation was unsatisfactory in most analyses, as authors have not provided justification when choosing between inputs, especially when more than one source of information is available, or, more so, when an input is a key driver for the economic analysis.
Snowsill et al. 43 were the exception, as the detailed outline of the model structure for the diagnostic component could easily be followed, with explanations and supplementary material available to support the use of, and methodology used to obtain, all relevant parameters. The thorough approach in reporting, as well as attention to long-term outcomes of probands and relatives, elevated this modelling study above the others reviewed.
Uncertainty is unavoidable and exists in all economic analyses. 42,107 Regardless of the type of economic evaluation, analysts should test the robustness of the results to estimate the probability that the correct decision has been made. It is common practice to undertake one-way and multivariate sensitivity analyses (e.g. deriving an ICER based on a ‘best-case’ and a ‘worst-case’ scenario) and a PSA. 108
This systematic review highlighted that none of the economic analyses undertook a value-of-information analysis. A value-of-information analysis can be used to provide a framework for analysing uncertainty in the economic model by estimating the expected costs associated with imperfect information when deciding between alternative strategies, which can be considered as uncertainty. Reducing uncertainty may lead to alternative strategies being adopted, and the value of this additional information depends on how much this additional information is likely to reduce the uncertainty. A key value-of-information measure is the expected value of perfect information, which represents the monetary value of obtaining perfect information to eliminate uncertainty for key parameters and, thus, the overall decision-making process. 109 If the costs of obtaining further information exceeds the expected value of perfect information, there is little justification for undertaking further research. 109
The economic analyses, more specifically those using an economic model to assess different strategies to identify Lynch syndrome in women with endometrial cancer, are limited to three studies. Although research in this area can be seen to be in its infancy based on the number of studies, this is not the case, as recent studies were more comprehensive by including the screening of probands and the benefit of surveillance to probands and their first-degree relatives. Development in this area may be due to the research that has been undertaken for identifying Lynch syndrome in people with CRC, and a better understanding of the natural history of endometrial cancer. To build on/develop the current modelling methodology, future advances in economic models should consider all relevant testing strategies available to identify Lynch syndrome in the jurisdiction of interest; discuss the methods used to identify inputs (preferably undertaking a systematic review for key input parameters); elaborate on meta-analysis methods, when appropriate; provide justification of choosing key inputs; include additional risk reduction methods (e.g. use of aspirin) to prevent other ‘downstream’ cancers; report cost-effectiveness results in terms of their natural units (e.g. diagnostic error avoided, cases of CRCs averted in probands, cases of endometrial cancers avoided in first-degree relatives, life-years gained) and costs per QALY; and undertake extensive sensitivity and scenario analyses, including a value-of-information analysis.
To our knowledge, this is the first systematic review of the cost-effectiveness evidence about the different strategies available to diagnose Lynch syndrome in women with endometrial cancer. This systematic review provides detail about the conduct of each economic analysis, as well as a reporting quality assessment for each study. Moreover, it provides considerations when undertaking future economic models to build on the existing evidence. There are some limitations to this systematic review. First, study selection was undertaken by Mary Jordan and James Keasley independently. However, data extraction and reporting quality appraisal were undertaken by Peter Auguste and cross-checked by Mary Jordan. Second, we have not provided details of the sources of inputs included in these economic analyses. Third, we have not discussed the transferability of these cost-effectiveness results to a specific setting or jurisdiction.
Conclusion
This systematic review highlights and summarises the studies that compared different screening strategies to identify Lynch syndrome in women treated for endometrial cancer. The results show that the evidence base is limited to five studies, with three studies using an economic model. We noticed that the modelling methodology has developed over time, with earlier models interested in identifying and diagnosing Lynch syndrome only, and more recent models including the benefit of screening to probands in reducing the incidence of other ‘downstream’ cancers, as well as benefit to first-degree relatives. These analyses all add to the existing evidence and conformed to the best-practice guidelines for the reporting of economic analyses or economic models. However, there were some concerns, which limit the transparency, robustness and, hence, transferability of these results to a specific setting/jurisdiction. Although the transferability of economic results may present challenges because of the nature of economic analyses, future economic analyses, more so those using an economic model, should be transparent in the methods used to identify data inputs, and should be clear about the methods used to synthesise clinical evidence (e.g. prevalence of Lynch syndrome, test/strategy performance and benefit of surveillance to reduce the incidence of other ‘downstream’ cancers) and the choices made between data sources. Snowsill et al. 43 achieved these key quality indicators, and as the most recent and geographically relevant (UK setting), established a comprehensive reference model on which to build our modelling approach.
Chapter 6 Economic model
Discussion of model input parameters
Although none of the cost-effectiveness studies retrieved in the systematic review answered the decision problem in full, the model by Snowsill et al. 43 proved particularly useful in terms of structure and of sourcing relevant model inputs. This work, in combination with previous reviews of Lynch syndrome testing in CRC,12,110 was drawn on to inform the modelling approach. Parameters are discussed for each section of the model in order: diagnostic decision tree, long-term CRC and long-term endometrial cancer components.
Diagnostic model
Diagnostic performance
Test accuracy was extracted from the results obtained from the clinical effectiveness systematic literature review we conducted, with test accuracy figures used in the model derived at a strategy level. Similarly, prevalence, test failure rate and prevalence by mutation were taken from our clinical effectiveness review. Extensive detail on how these figures were calculated is provided in Chapter 4, Assessment of studies of clinical effectiveness (key question 2). In addition, a parameter for the proportion of relatives tested who have positive Lynch syndrome mutations, 44% (95% CI 40.7% to 47.4), was taken from a random-effects meta-analysis of studies conducted by Snowsill et al. 110
Diagnostic mutation testing
A total of 92.5% of probands are expected to attend genetic counselling following positive index test results, irrespective of testing strategy. This figure is elicited from the clinical expert (Dr Ian M Frayling) range 90–95% in Snowsill et al. ,110 and independently corroborated more recently by a clinical expert (Demetra Georgiou, Principal Genetic Counsellor, St Mark’s Hospital, London North West University Healthcare NHS Trust, 13 December 2019, personal communication). Of those attending genetic counselling, 95% are assumed to undergo genetic testing, based on expert opinion (Demetra Georgiou, personal communication), supported by a rate of 90% assumed by Snowsill et al. 12 in their review. Unpublished data by Crosbie et al. (acceptability manuscript, Professor Emma Crosbie, University of Manchester, 19 July 2019, personal communication) supports a high acceptability of genetic testing in endometrial cancer probands, although methodology to elicit consent differed to more standard UK practice as pathway to genetic testing was gynaecologist led and did not expressly include prior genetic counselling. Consent rates were found to be (confidential information has been removed).
For strategy 11, whereby probands do not undergo an initial tumour test, the acceptance of genetic testing is assumed to be less than the acceptance in strategies whereby a positive initial test has been performed. This assumption was made in the Snowsill et al. 43 model, with acceptance of direct germline testing set at 0.500. No alternative source of data was identified to inform this parameter further; therefore, 0.500 was used in our base case.
The impact of these parameters was investigated further in one-way sensitivity analyses in which the proportion of probands accepting genetic counselling and the proportion accepting genetic testing were varied from 50% to 100%.
Predictive mutation testing
Uptake in genetic testing among relatives is a complex issue, with much variation seen in the methods used to contact ‘at-risk’ relatives, which subsequently affects the proportions of relatives accepting counselling and testing. Similarly, when patient-directed contact is ultimately reliant on the individual characteristics of the proband, it is difficult to assess the influence a female-only cohort of probands exerts over relatives with a syndrome that affects both males and females.
A combination of data from relevant literature, supplemented by clinical expert opinion, was used to determine parameters. The average number of relatives per proband who are identified through cascade testing and are assumed to be contactable was set at six per proband, in line with a 2017 CRC review,12 based on data from Snowsill et al. ,110 which was updated using Manchester regional Lynch syndrome registry results111 and unpublished data provided by Ian Frayling in previous work. 12,110
It was assumed that all six relatives made contact with their GP, with cost of GP contact attributed; of these, 77.5% were assumed to pursue referral to a genetic counsellor, based on findings of a systematic literature review of uptake of pre-symptomatic genetic testing in hereditary breast–ovarian cancer and Lynch syndrome by Menko et al. 112 Of those attending genetic counselling, 76.7% were assumed to undergo predictive testing, as reported by Barrow. 111 This figure is the recorded proportion at 12 years after relatives are informed of their ‘at-risk’ status, which, although considerably higher than the 55.7% who were tested within 3 years of being informed, may still be considered a conservative estimate. A study by Bruwer et al. 113 found that up to 97% of relatives underwent predictive testing [median 8.6 years (range 1–12 years)], and clinical expert opinion suggests that almost 90% of relatives who attend genetic counselling pursue testing at some point (Demetra Georgiou, personal communication).
To explore the impact of relative uptake further, sensitivity analyses were undertaken to vary the proportion of relatives accepting genetic counselling and the proportion accepting genetic testing from 50% to 100%. In addition, the number of relatives identified per proband was decreased from six in the base case to three, and then increased to 12 to assess sensitivity to measure.
Colorectal cancer incidence
Age-related annual incidence of CRC is sourced from the modelling work of Snowsill et al. 43 Gene-specific data from the PLSD2,14,114 were used, against which to fit parametric, non-parametric and flexible spline models with best fit resulting from the log-normal model, which we replicated in our long-term model. By applying a hazard ratio of 0.387, as used by Snowsill et al. ,12 it was assumed that any benefits associated with CRC surveillance would be countered. This served as the baseline incidence data for CRC incidence among Lynch syndrome-positive individuals who had not been identified and proceeded along the natural history pathway without risk reduction measures.
A more recent (2020) publication from the PLSD has been published since this work,45 which builds on earlier work by adding a newly recruited cohort of Lynch syndrome individuals to increase the size of the database from 2823 pathogenic mutation carriers to 6350 in total. The new cohort of 3727 was used to validate findings reported previously,2,14,114 before the merger of the two data sets occurred, which found that cumulative risk of CRC for each of the four affected genes did not differ significantly from the original (p > 0.05). The figures used by Snowsill et al. 12 were therefore considered to be valid and had been considered appropriate for use by NICE in the recent diagnostics guidance for Lynch syndrome in CRC. 1
Colorectal cancer surveillance
Surveillance for CRC is by colonoscopy performed every 2 years, which has been assumed to provide benefit by reducing the incidence of CRC through identifying and removing polyps prior to development of cancer and to detect any tumours promptly so that CRC can be diagnosed at an earlier stage, thereby improving outcomes. Similarly to Snowsill et al. ,12 we applied a hazard ratio of 0.387, from Järvinen et al. ,44 to estimate the beneficial impact that colonoscopic surveillance has on CRC incidence. It is acknowledged that this was an observational study subject to significant bias, in a cohort published in 2000. However, in the absence of more relevant recent evidence in the literature, and given that effectiveness of colonoscopic surveillance is likely to have improved over time through the introduction of clinical standards,1 the hazard ratio of 0.387 was used in our base case. To reflect the considerable uncertainty around this parameter, we conducted a scenario analysis whereby it was assumed that CRC surveillance had no impact on CRC incidence (see Table 18).
Guidelines for the management of Lynch syndrome advise that colonoscopic surveillance should be performed every 2 years. 27 This is the frequency of colonoscopy modelled in our base case, commencing for all individuals at age 25 years. However, recent reports based on reviews of findings from the PLSD115,116 suggest that intervals between colonoscopic surveillance are not correlated with decreased incidence of CRC or stage at diagnosis. Although the evidence is limited, the suggestion that biennial colonoscopy can be replaced by surveillance every 3 years with limited reduction in effectiveness was explored in scenario analysis 6. The assumption was made that benefit is unaffected.
The reduction in frequency of colonoscopy, investigated in scenario 6, also speaks towards the impact of stratified management by gene-specific mutation, as recommended in the most recent British Society of Gastroenterology guidance on CRC surveillance. 27 In our model, colonoscopy starts at age 25 years for all individuals. However, new guidelines27 state that individuals with MLH1 or MSH2 mutations should commence biennial colonoscopy at age 25 years, whereas those with MSH6 or PMS2 mutations can start surveillance later, at age 35 years (see Appendix 7).
This would result in fewer overall colonoscopies being performed, as is the case in scenario analysis 6, although the assumption that there would be no change in effectiveness is less secure, as targeted management because of known risk may be expected to improve the effectiveness of surveillance.
Endometrial cancer incidence
For endometrial cancer, we sourced incidence data from the PLSD, published in 2020. 45 This database reported gene-based risk of cancer based on 6350 individuals with Lynch syndrome. Risks are reported at ages 25, 40, 50, 60, 70 and 75 years. We fitted a piecewise linear model to these data to derive annual incidence from cumulative incidence.
Gynaecological surveillance
The benefits of gynaecological surveillance are uncertain, and clinical practice throughout the UK varies with respect to what surveillance involves and to whom it is offered. The most recent guidelines on surveillance practices have been published by the Manchester International Consensus. 22 Invasive gynaecological surveillance in females with Lynch syndrome is no longer recommended because of a lack of evidence that outcomes are improved over symptom awareness and urgent investigation of red-flag symptoms. Instead, an annual review from the age of 25 years, with an appropriate clinician to discuss red-flag symptoms and, when necessary, contraceptive and fertility needs, should be encouraged, and gynaecological referral should be made as a result of a specific need.
We follow these recommendations in our modelling by assuming that all females from the age of 25 years who have not undergone hysterectomy (for treatment of endometrial cancer or as prophylactic surgery) access non-invasive surveillance, which involves an annual review with a GP. We assume that 10% of those attending are referred onward for gynaecological review and invasive surveillance, consisting of gynaecological examination, pelvic ultrasonography, CA-125 analysis and aspiration biopsy. This is assumed to reduce mortality by 10.2%, an assumption in line with previous evaluations of Lynch syndrome screening. 12 However, the evidence for this is not completely robust. Therefore, we estimate the impact of assuming that no such surveillance is offered.
However, with uncertainty as to the benefits that may be accrued, we performed a scenario analysis removing gynaecological surveillance entirely from the model (see Appendix 7, Scenario analysis 5).
Aspirin
All probands and relatives who enter the long-term model are assumed to receive aspirin as a form of chemoprophylaxis. Based on results seen in the CAPP2 RCT,48 which show reduced incidence of CRC, we reduce the probability of individuals developing cancer each year by a factor of 0.56, applied equally to the risk of developing endometrial cancer and the risk of developing CRC.
A draft report48 on the effectiveness of aspirin in the prevention of CRC in people with Lynch syndrome finds that the balance of risks and benefits of regular aspirin use in people with Lynch syndrome supports the use of aspirin for at least 2 years in this population, and the Manchester Consensus Group22 strongly recommends that MMR pathogenic variant carriers take aspirin chemoprevention. Optimal dosage is currently unknown; the CaPP3 RCT48 is ongoing to determine this. Therefore, we assume that individuals take daily aspirin over the life course and that benefits continue over time. In scenario analysis 7, we exclude aspirin to assess the bearing this measure has on cost-effectiveness.
Variant of uncertain significance
Probands with positive index results on tumour tissue and negative germline results are considered Lynch syndrome negative, but, for a proportion of these, clinical suspicion of Lynch syndrome remains. Similarly, negative results for currently identified pathogenic mutations on germline testing may be found. These VUSs may be latterly identified as pathogenic for Lynch syndrome, or not, in which case management can either be scaled up or down accordingly. For these cases, it is assumed that further testing occurs on tumour tissue (somatic analysis) to either confirm sporadic cause of tumour or establish that VUS is non-pathogenic for Lynch syndrome and management. Clinical experts suggest that, although somatic analysis may not fully resolve the pathogenic status of VUS patients, around 50–60% of them would derive some benefit from it (Andrew Wallace, Manchester Centre for Genomic Medicine, 27 December 2019, personal communication), allowing upgrading or downgrading of VUS and influencing their associated long-term management.
Work is ongoing to reduce the number of VUSs. The International Society for Gastrointestinal Hereditary Tumors (InSiGHT) MMR Variant Interpretation Committee, which is recognised by ClinGen as the Expert Panel and is in the process of being recognised by the Food and Drug Administration as the MMR Variant Classification Expert Panel, has achieved a reduction in the number of Class-3 VUSs of 35%. 117
We used a VUS estimate of 1.2% in our model from the clinical effectiveness review, but clinical expert opinion suggests that this figure may be higher, at 2–5% (Demetra Georgiou, personal communication).
Somatic analysis may cost up to £800 (Demetra Georgiou, 16 January 2020, personal communication), which would introduce a significant extra cost to each of the test strategies. However, it is likely that, under current testing guidelines, these individuals would already qualify for somatic testing (as they have a positive index and negative germline result), so this would not be an additional cost due to VUS status alone. This cost is not included in our modelling. We use our estimated proportions of VUSs, which are then varied during sensitivity analysis, to assess the sensitivity of the ICER to this parameter. Given that any additional costs involved may be recouped by the ability to downgrade potential VUSs to lower long-term management costs, further research would be beneficial, but conclusions about the magnitude of this at the individual or national level cannot be reached in our work.
Costs
The majority of costs were obtained directly from previous work presented to NICE12 because the sources were recent, relevant and local, and, through personal communications, clinical experts confirmed the figures quoted. Hospital-related costs were obtained from the most current (2017/18) NHS reference tables. 49
Costs are reported in 2017/18 Great British pounds, with estimates for some parameters requiring adjustment using recognised methods in hospital and community health services to inflate to this cost year.
The costs of IHC, MSI and methylation testing were estimated as £210, £217 and £156, respectively, using reported costs from the UK Genetic Testing Network 2018,118 corroborated through personal communications from clinical experts. The cost of offering counselling to a proband was estimated as £28.25 (based on 15 minutes of band-6 hospital nurse time), with cost of referral for a relative estimated to be £39 (cost of a GP appointment). 6 Pre-test genetic counselling/multidisciplinary team review was estimated to cost £642.19 for probands and £514.43 for relatives; post-test genetic counselling was estimated to cost £141.44 for both. 114 Diagnostic mutation testing for Lynch syndrome was estimated to cost £755 (with testing conducted on all four genes) and predictive mutation testing for relatives was estimated to cost £165 (testing on single MMR gene under suspicion). 118
A one-off cost of CRC is incurred at the time of CRC incidence (dependent on patient age and stage at diagnosis), with no further cost being accrued as a result of time in CRC states or at time of death from CRC. These costs were sourced from Snowsill et al. ,43 who used reported data by the Economic Evaluation of Health and Social Care Interventions Policy Research Unit, based on a whole-disease model of CRC.
We assume that the cost of colonoscopy is £325.00,49 averaging across outpatient diagnostic and therapeutic colonoscopies, with an increased cost of £2.89 per colonoscopy secondary to average costs incurred from complications associated with the procedure. 12
For endometrial cancer, a one-off treatment cost of £6510 is assumed, calculated in line with previous work. 12 A cost of £3428 is assigned to prophylactic H-BSO. Women who have not had surgical prophylaxis undergo annual surveillance to detect endometrial cancer. The cost is £39.00, plus an additional cost of £473.41 for those requiring referral for invasive surveillance. We assume that this referral occurs in 10% of cases. 12 There was no cost assigned to aspirin, as it was assumed to be purchased by the individual as a low-cost over-the-counter medicine, rather than cost on prescription to the NHS. The costs involved in diagnostic testing are taken as averages of costs reported by genetic laboratories throughout the UK,118 and, as such, reflect the average national cost. The cost of DNA sequencing is decreasing. It is thought that costs of testing may be reduced in the future. A microcosting study of testing strategies for Lynch syndrome by Ryan et al. 50 showed that costs of testing at a major tertiary institution were extremely low when staff time, consumables and equipment were calculated. To illustrate the impact of reduced test costs, scenario analysis 2 was performed using these results, mindful that they were not inclusive of capital costs (electricity, rent), which are often significant. As authors also noted, the true cost associated with testing is likely to lie between sourced estimates from experts and costs calculated in their single-site specialist centre. 50 To reflect this, we also use sensitivity analysis to vary costs by 40% above and below our base-case cost, to more realistically determine price change effect.
Health-related quality of life
In our base case, we assume that those with cancer have the same utility as those without cancer, except for those with stage IV CRC and those in their first year of endometrial cancer. This assumption is line with previous work presented to NICE. 12,110 Although that previous work did cite supporting sources of evidence, it could be argued that this underestimates the impact of cancer on quality of life. For example, it seems plausible that those with stage III cancer would experience some disutility, compared with those who were disease free. Furthermore, one might expect that those who die from endometrial cancer experience a period of impaired quality of life prior to death.
To reflect this, we carried out a scenario analysis (scenario analysis 4) in which we assumed that those with stage III CRC experienced utility half-way between those for stage IV cancer and good health. We further assumed that those who died of endometrial cancer experienced 1 year in a health state equivalent to stage IV CRC prior to death.
A paucity of information is available in the literature regarding HRQoL in either Lynch syndrome, endometrial cancer or CRC patients, and efforts to find suitable information to reflect these parameters were unsuccessful. For this reason, the impacts on probands of testing are also not well understood, other than directly from patients, as there is insufficient evidence to support the implementation of a QALY detriment from the literature. Unpublished survey data, provided by University of Manchester via NICE (Thomas Walker, NICE, 19 July 2019, personal communication), recording patient responses to gynaecological surveillance in Lynch syndrome showed a range of responses to questions regarding anxiety and depression levels associated with their diagnosis. This disaggregated data appeared extremely mixed, and no patterns of psychological outcomes could be identified. This illustrates the difficulty in obtaining such data and, particularly, their transfer for use in health economic models.
Final model input parameters
This section discusses the source of inputs for all model parameters, with Tables 12 and 13 providing a summary of these.
| Parameter name | Base-case value | Source and year |
|---|---|---|
| Diagnostic parameters | ||
| Test accuracy | ||
| Strategy 1: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 1: specificity | 0.805 | Lu et al.16 2007 |
| Strategy 2: sensitivity | 0.625 | Lu et al.16 2007 |
| Strategy 2: specificity | 0.966 | Lu et al.16 2007 |
| Strategy 3: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 3: specificity | 0.833 | Lu et al.16 2007 |
| Strategy 4: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 4: specificity | 0.967 | Lu et al.16 2007 |
| Strategy 5: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 5: specificity | 0.782 | Lu et al.16 2007 |
| Strategy 6: sensitivity | 0.625 | Lu et al.16 2007 |
| Strategy 6: specificity | 0.954 | Lu et al.16 2007 |
| Strategy 7: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 7: specificity | 0.791 | Lu et al.16 2007 |
| Strategy 8: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 8: specificity | 0.942 | Lu et al.16 2007 |
| Strategy 9: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 9: specificity | 0.791 | Lu et al.16 2007 |
| Strategy 10: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 10: specificity | 0.942 | Lu et al.16 2007 |
| Strategy 11: sensitivity | 1 | Lu et al.16 2007 |
| Strategy 11: specificity | 1 | Lu et al.16 2007 |
| Test failure rate (all tests) | 0 | Median from systematic review |
| Acceptance of diagnostic tests | ||
| MSI | 1.000 | Assumption |
| IHC | 1.000 | Assumption |
| MLH1 promoter hypermethylation | 1.000 | |
| Genetic counselling: proband | 0.925 | Menko et al.112 2019, Bruwer et al.113 2013, unpublished acceptability manuscript (Professor Emma Crosbie, personal communication) |
| Genetic testing direct | 0.500 | Assumption |
| Genetic testing: proband (diagnostic) | 0.950 | Assumption |
| Genetic counselling: relative | 0.775 | Barrow111 2014 and Menko et al.112 2019 |
| Genetic testing: relative (predictive) | 0.767 | Barrow111 2014 and Menko et al.112 2019 |
| Declined diagnostic testing or no mutation found | ||
| Clinical suspicion of Lynch syndrome | Confidential information has been removed | Based on the PETALS study (number tested) |
| VUS result obtained | 0.12 | Assumption, clinical opinion |
| Costs | ||
| IHC | £210.00 | Snowsill et al.12 2017 |
| MSI | £217.00 | UK Genetic Testing Network118 2018 (average of three MSI test prices) |
| MLH1 promoter hypermethylation | £156.00 | UK Genetic Testing Network118 2018 |
| Offer of counselling | £28.25 | Band-6 nurse time |
| Pre-test clinic-related costs and genetic counselling appointment: proband | £642.19 | Slade et al.119 2016 and expert clinical opinion (Demetra Georgiou, personal communication) |
| Genetic testing on germline: proband | £755.00 | UK Genetic Testing Network118 2018 |
| Post-test clinic-related costs and follow-up: proband | £141.44 | Expert clinical opinion (Demetra Georgiou, personal communication) and Slade et al.119 2016 |
| Pre-test clinic-related costs and genetic counselling appointment: relative | £514.43 | Expert clinical opinion (Demetra Georgiou, personal communication) and Slade et al.119 2016 |
| Genetic testing on germline: relative | £165.00 | UK Genetic Testing Network118 2018 |
| Post-test clinic-related costs and follow-up: relative | £141.44 | Expert clinical opinion (Demetra Georgiou, personal communication) and Slade et al.119 2016 |
| GP appointment | £39.00 | NHS Reference Costs 2017/1849 |
| Parameter | Base-case value | Source and year |
|---|---|---|
| Population | ||
| Number of relatives per proband | 6 | Snowsill et al.12 2017 |
| Proportion of relatives who are first-degree relatives of proband | 0.424 | |
| Proportion of relatives receiving predictive testing found to have Lynch syndrome | 0.440 | |
| Proportion of relatives who are women | 0.500 | Assumption |
| Natural history | ||
| Prevalence of Lynch syndrome among all endometrial cancer | 0.032 | Nine studies (reported in 11 papers15,52,55,56,59–61,70,76,88 and the unpublished PETALS study) that assessed prevalence of Lynch syndrome in unselected samples of women with endometrial cancer |
| Gene distribution among all endometrial cancer (MLH1/MSH2/MSH6/PMS2) | 0.171 | Four unselected studies15,55,61 from our cost-effectiveness review, including the unpublished PETALS study |
| 0.244 | ||
| 0.464 | ||
| 0.122 | ||
| CRC incidence with Lynch syndrome (log-normal distribution) | Møller et al.2,14 2017, 2018 and Snowsill et al.43 2019 | |
| Mu (baseline) | 4.306 | |
| Sigma | 0.567 | |
| Beta_MSH2 | 0.100 | |
| Beta_MSH6 | 0.531 | |
| Beta_PMS2 | 0.863 | |
| Beta_male | −0.118 | |
| Beta_prevcancer | −0.230 | |
| CRC incidence for women without Lynch syndrome [by age (years), per 100,000 person-years] | Office for National Statistics8 | |
| < 25 | 3.1 | |
| 25–30 | 2.7 | |
| 30–35 | 6.5 | |
| 35–40 | 10.7 | |
| 40–45 | 11.8 | |
| 45–50 | 21.5 | |
| 50–55 | 37.6 | |
| 55–60 | 61.8 | |
| 60–65 | 91.4 | |
| 65–70 | 118.2 | |
| 70–75 | 172.1 | |
| 75–80 | 235.6 | |
| 80–85 | 309.3 | |
| 85–90 | 359.5 | |
| > 90 | 304.2 | |
| CRC incidence for men without Lynch syndrome [by age (years), per 100,000 person years] | Office for National Statistics8 | |
| < 25 | 2.3 | |
| 25–30 | 2.3 | |
| 30–35 | 5.6 | |
| 35–40 | 9.1 | |
| 40–45 | 12.0 | |
| 45–50 | 23.2 | |
| 50–55 | 42.6 | |
| 55–60 | 84.2 | |
| 60–65 | 150.3 | |
| 65–70 | 196.1 | |
| 70–75 | 276.8 | |
| 75–80 | 373.8 | |
| 80–85 | 457.5 | |
| 85–90 | 511.9 | |
| > 90 | 460.3 | |
| CRC mortality rate (without Lynch syndrome) | Snowsill et al.43 2019 | |
| Stage I | 0.014 | |
| Stage II | 0.052 | |
| Stage III | 0.148 | |
| Stage IV | 0.544 | |
| CRC mortality hazard ratio with Lynch syndrome (stages I–III) | 0.660 | Snowsill et al.43 2019 |
| Endometrial cancer mortality rate with Lynch syndrome | 0.004 | Møller et al.2 2017 |
| Endometrial cancer mortality rate without Lynch syndrome [by age (years)] | Office for National Statistics8 | |
| 15–45 | 0.026 | |
| 45–55 | 0.028 | |
| 55–65 | 0.031 | |
| 65–75 | 0.048 | |
| > 75 | 0.092 | |
| Effectiveness of risk reduction | ||
| Age range (years) for gynaecological surveillance | 25–75 | |
| Interval of surveillance | 1.000 | |
| Mortality rate decrease from gynaecological surveillance | 0.102 | |
| Aspirin risk reduction | 0.56 | Snowsill et al.12 2017 |
| Effectiveness of risk reduction | ||
| Age range (years) for surveillance colonoscopy | 25–75 | |
| Interval between colonoscopies | 2.000 | |
| Uptake of colonoscopy if diagnosed with Lynch syndrome | 1.000 | |
| Uptake of colonoscopy if diagnosed putative Lynch syndrome | 1.000 | Barrow et al.120 2015 |
| Hazard ratio for CRC incidence if undergoing colonoscopy | 0.387 | |
| CRC stage distribution in surveillance | Snowsill et al.12 2017 | |
| Stage I | 0.686 | |
| Stage II | 0.105 | |
| Stage III | 0.128 | |
| Stage IV | 0.081 | |
| CRC stage distribution not in surveillance (sporadic) | Snowsill et al.43 2019 | |
| Stage I | 0.176 | |
| Stage II | 0.270 | |
| Stage III | 0.295 | |
| Stage IV | 0.259 | |
| CRC stage distribution not in surveillance (Lynch syndrome) | Barnetson et al.121 2006 | |
| Stage I | 0.188 | |
| Stage II | 0.488 | |
| Stage III | 0.213 | |
| Stage IV | 0.113 | |
| Diagnostic MMR mutation testing | ||
| Acceptance of counselling (tumour-testing strategies) | 0.925 | Menko et al.112 2019 and Heald et al.122 2013 |
| Acceptance of counselling (direct testing) | 0.5 | Assumed |
| Acceptance of diagnostic testing (given accepted counselling) | 0.950 | Expert opinion (Demetra Georgiou, personal communication) |
| Sensitivity | 1 | Assumed |
| Specificity | 1 | Assumed |
| Predictive MMR mutation testing | Barrow et al.120 2015 | |
| Acceptance of counselling | 0.775 | |
| Acceptance of predictive testing (given accepted counselling) | 0.765 | |
| Costs (£) | ||
| Colonoscopy | 583 | |
| Stage I CRC [by age (years)] | ||
| 40–49 | 8754 | |
| 50–59 | 5712 | |
| 60–69 | 4623 | |
| 70–79 | 3178 | |
| 80–100 | 1380 | |
| Stage II CRC [by age (years)] | ||
| 40–49 | 8741 | |
| 50–59 | 7016 | |
| 60–69 | 5352 | |
| 70–79 | 3455 | |
| 80–100 | 1546 | |
| Stage III CRC [by age (years)] | ||
| 40–49 | 14,490 | |
| 50–59 | 9692 | |
| 60–69 | 7259 | |
| 70–79 | 4485 | |
| 80–100 | 1561 | |
| Stage IV CRC (by age) | ||
| 40–49 | 11,705 | |
| 50–59 | 8444 | |
| 60–69 | 6509 | |
| 70–79 | 4365 | |
| 80–100 | 807 | |
| Utilities | ||
| Baseline utility model | Ara and Brazier47 2010 | |
| Intercept | 0.9509 | |
| Male | 0.0212 | |
| Age | −0.0003 | |
| Age2 | −3.32 × 10−6 | |
| (Resulting baseline utility for proband at start) | 0.816 | |
| (Resulting baseline utility for relative at start) | 0.850 | |
| Impact of testing on HRQoL (multipliers) | ||
| Declining counselling | 1 | Assumed |
| Declining genetic testing | 1 | Assumed |
| Diagnosed with Lynch syndrome | 1 | Assumed |
| Diagnosed with putative Lynch syndrome | 1 | Assumed |
| CRC (multipliers) | ||
| Stage I | 1 | Assumed |
| Stage II | 1 | Assumed |
| Stage III | 1 | Assumed |
| Stage IV | 0.789 | Snowsill et al.12 2017 |
| Endometrial cancer (multiplier) | 1 | Assumed |
| Utility decrement on diagnosis of endometrial cancer for 1 year | 0.036 | Snowsill et al.12 2017 |
Cost-effectiveness results
The simulated population of the model consists of individual probands at a specified age diagnosed with endometrial cancer, among whom 11 different diagnostic strategies are undertaken to identify Lynch syndrome, and the relatives who would be identified in the event of diagnosis of Lynch syndrome in the proband. In the base case, the age of the proband at diagnosis of endometrial cancer is 49 years. The costs and QALYs accrued throughout each strategy are discounted at a rate of 3.5% per year, with costs reported in Great British pounds. The incremental cost per QALY when each strategy is compared with a no-testing approach in the proband is presented, followed by the pairwise comparative ICERs for all strategies.
Base-case results
Cost-effectiveness results
Table 14 summarises the base-case cost-effectiveness results (prior to rounding). IHC with MLH1 methylation is the most cost-effective strategy, with germline testing direct incurring the highest costs per QALY, compared with no testing. All 11 strategies are considered cost-effective at a WTP threshold of £20,000 per QALY.
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER vs. no testing (cost per QALY) (£) | ICER |
|---|---|---|---|---|---|---|
| No testing | 0 | – | 0 | 0 | – | – |
| MSI with MLH1 methylation | 520 | 520 | 0.0419 | 0.0419 | 12,298.41 | Extendedly dominated |
| IHC with MLH1 methylation | 630 | 630 | 0.0669 | 0.0669 | 9459.32 | £9420 |
| MSI followed by IHC with MLH1 methylation | 720 | 90 | 0.0420 | –0.0249 | 17,045.57 | Dominated |
| IHC | 790 | 160 | 0.0681 | 0.0012 | 11,628.23 | £133,330 |
| MSI | 840 | 50 | 0.0683 | 0.0002 | 12,265.95 | £250,000 |
| IHC followed by MSI with MLH1 methylation | 870 | 30 | 0.0671 | –0.0012 | 12,925.61 | Dominated |
| MSI and IHC simultaneously with MLH1 methylation | 890 | 20 | 0.0671 | 0.0000 | 13,280.76 | Dominated |
| IHC followed by MSI | 1025 | 185 | 0.0685 | 0.0002 | 14,981.99 | £925,000 |
| MSI followed by IHC | 1030 | 5 | 0.0685 | 0.0000 | 15,018.13 | Dominated |
| MSI and IHC simultaneously | 1070 | 45 | 0.0685 | 0.0000 | 15,595.83 | Dominated |
| Germline testing | 1160 | 135 | 0.0666 | –0.0019 | 17,478.16 | Dominated |
Full incremental analysis
Immunohistochemistry with MLH1 methylation was the most cost-effective testing strategy, with an ICER of approximately £9420 per QALY. All other strategies were dominated or did not reach acceptable cost-effectiveness threshold levels.
Base-case results (shown in Table 14) show that MSI with MLH1 methylation was the cheapest strategy, with expected mean costs of approximately £520, and was expected to yield 0.0419 QALYs. The comparison between no testing and IHC with MLH1 methylation extendedly dominated the comparison between no testing and MSI with MLH1 methylation (i.e. was less costly and more effective than a combination of other comparators). This demonstrated that IHC with MLH1 methylation was the most cost-effective testing strategy, with an ICER of £9420 per QALY. Although IHC, MSI and IHC followed by MSI strategies were also on the cost-effectiveness frontier, ICERs were well above the threshold levels accepted in the UK and all strategies were dominated (i.e. more costly and less effective than one or more of the comparators).
Number of people identified as having Lynch syndrome
The number of probands and relatives with Lynch syndrome identified for the 11 strategies are illustrated in Figure 21. This shows that very similar numbers of true positive Lynch syndrome individuals are identified across all testing strategies except for strategies 2 and 6 (MSI with MLH1 promoter hypermethylation testing and MSI followed by IHC with MLH1 promoter hypermethylation testing). This is expected, as sensitivity of these testing strategies are the lowest of all the strategies, at 62.5%. This is a result of our assumptions on test accuracy, which are uncertain.
FIGURE 21.
Number of probands and relatives with Lynch syndrome identified by each strategy. FN, false negative; FP, false positive; TP true positive.
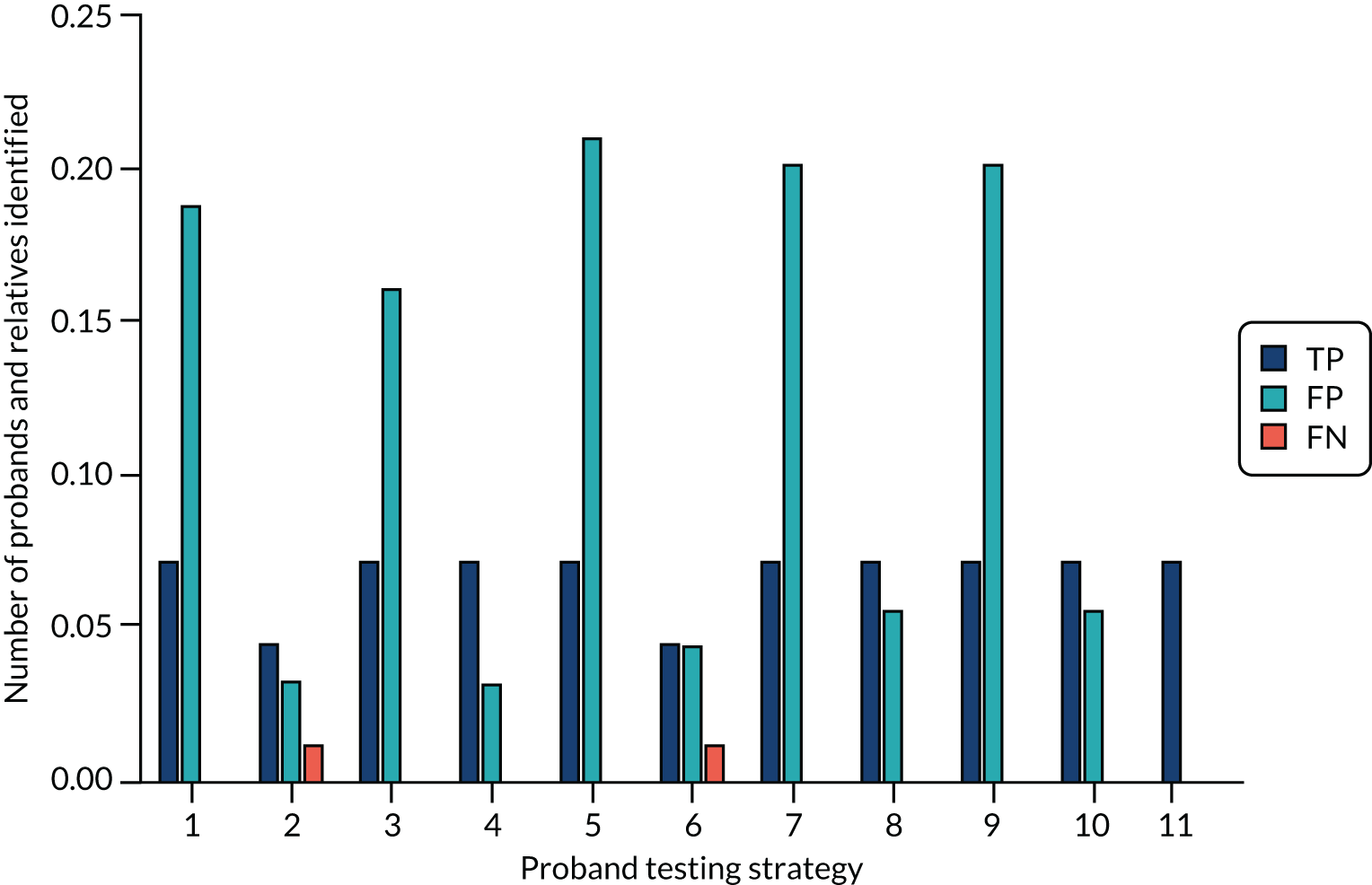
Diagnostic performance is diminished across all strategies, as some of the relatives identified decline the offer of predictive testing. In addition, if probands receive a positive diagnosis but no causative mutation is found (i.e. Lynch syndrome-assumed diagnosis), their first-degree relatives are treated as having Lynch syndrome, but second-degree relatives and beyond are treated as not having Lynch syndrome.
Long-term clinical outcomes
Results from long term-modelling of cancer outcomes
Cumulative incidence of identifying Lynch syndrome
The graphs in Figures 22–24 show model predictions of CRC and endometrial cancer incidence, by gene, from birth, illustrating our assumptions for incidence of the respective cancers in Lynch syndrome individuals without diagnosis/intervention. This is used to simulate outcomes for these individuals with and without interventions as a result of being identified as having Lynch syndrome.
FIGURE 22.
Cumulative incidence of CRC among females with Lynch syndrome.
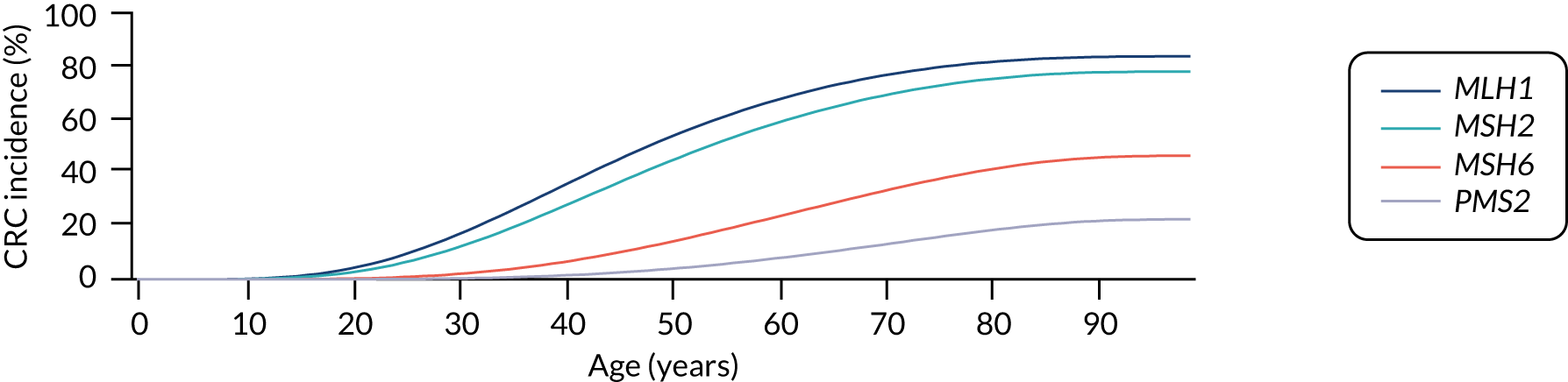
FIGURE 23.
Cumulative incidence of CRC among males with Lynch syndrome.
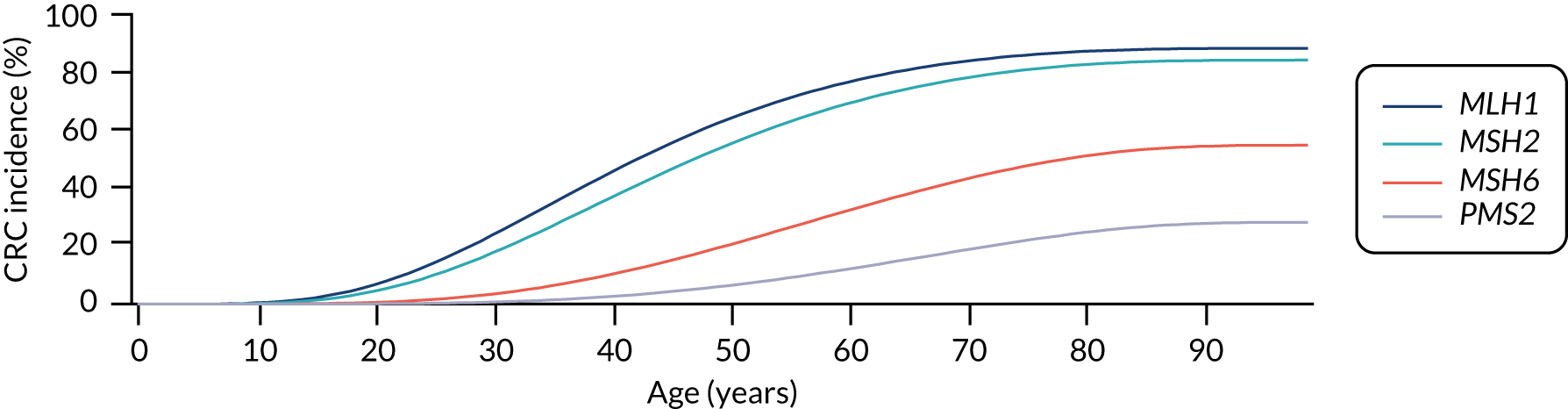
FIGURE 24.
Cumulative incidence of EC among females with Lynch syndrome. EC, endometrial cancer.
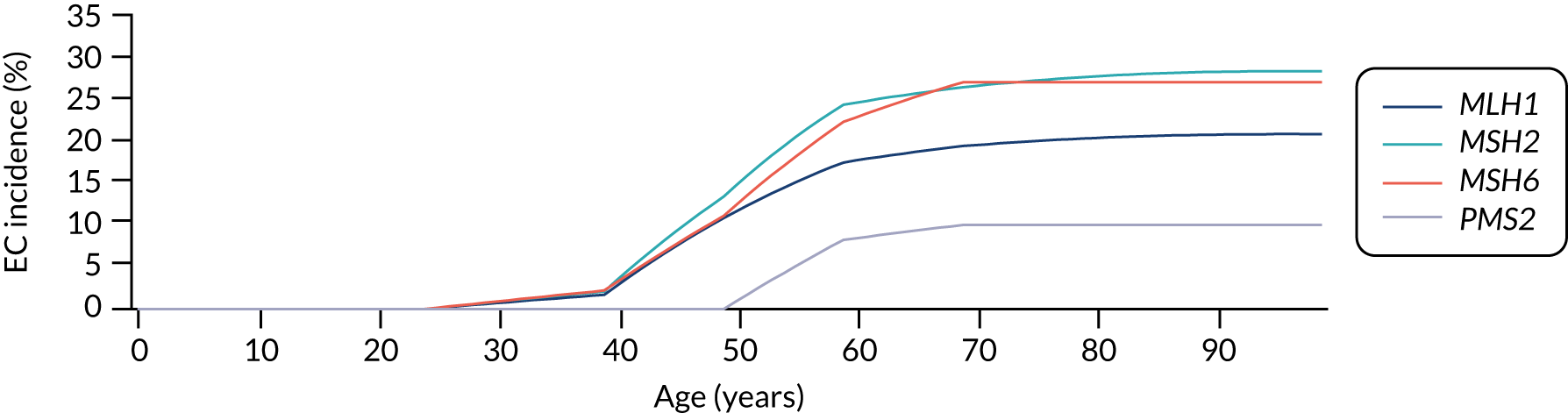
Figure 25 shows the predicted improvement in life expectancy when a person of a given age who has not previously had a Lynch syndrome-related cancer is identified with Lynch syndrome (through cascade testing) and measures are initiated to reduce their risks. For women, being identified at age 30 years through cascade testing results in an extra 6.7 years of life, falling to 0.9 years if the woman is identified at age 70 years. For men, the equivalent predicted gains are 7.4 years, falling to 0.7 years.
FIGURE 25.
Predicted improvement in life expectancy with age at identification.
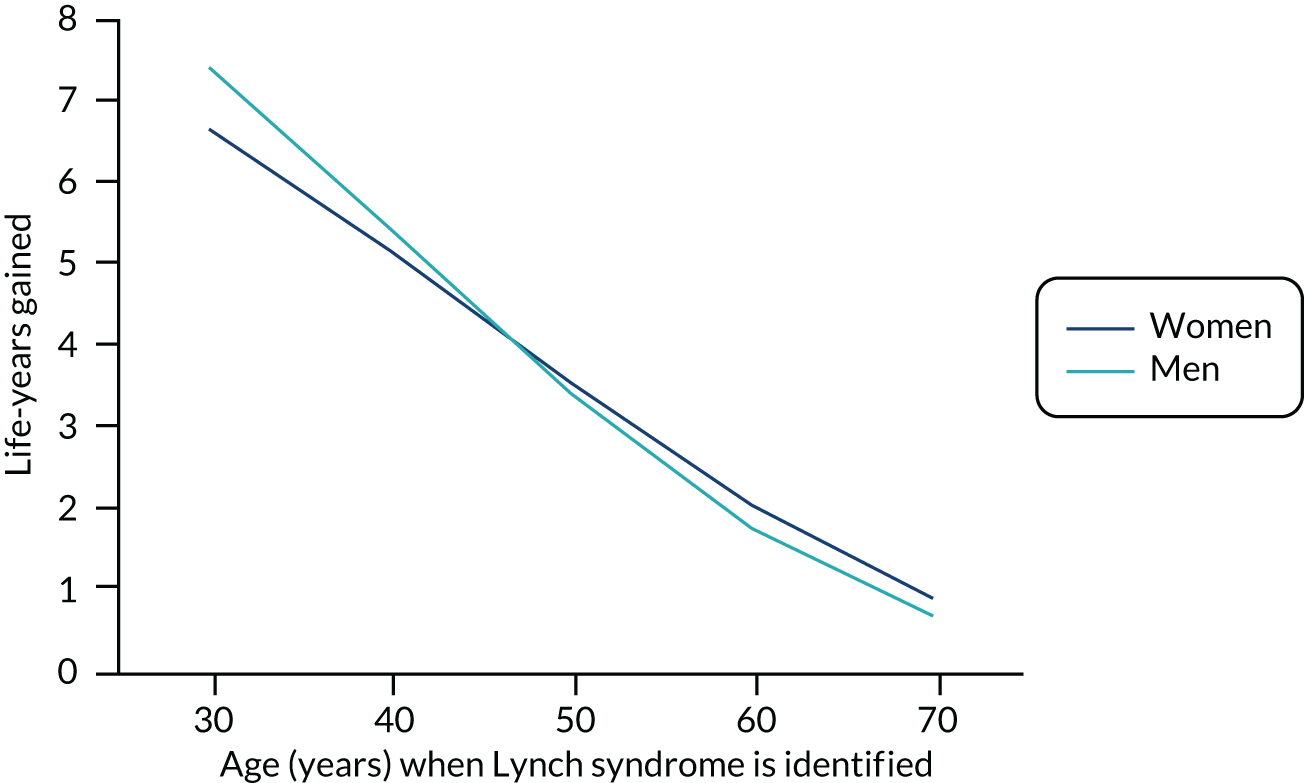
Despite the magnitude of benefits in terms of life-years gained declining as age of identification rises, this graph demonstrates that some degree of benefit is maintained through identification at any point across the life course until at least age 70 years, as modelled.
Figure 26 shows the benefits of identifying Lynch syndrome in a cohort of women of the same age in terms of cases of CRC and endometrial cancer avoided, and deaths from CRC or endometrial cancer averted, when Lynch syndrome is identified. Results are presented per 100 women identified. Figure 27 shows the CRC cases prevented and deaths averted per 100 men identified.
FIGURE 26.
Benefits of identifying 100 women with Lynch syndrome in a cohort of the same age. EC, endometrial cancer.
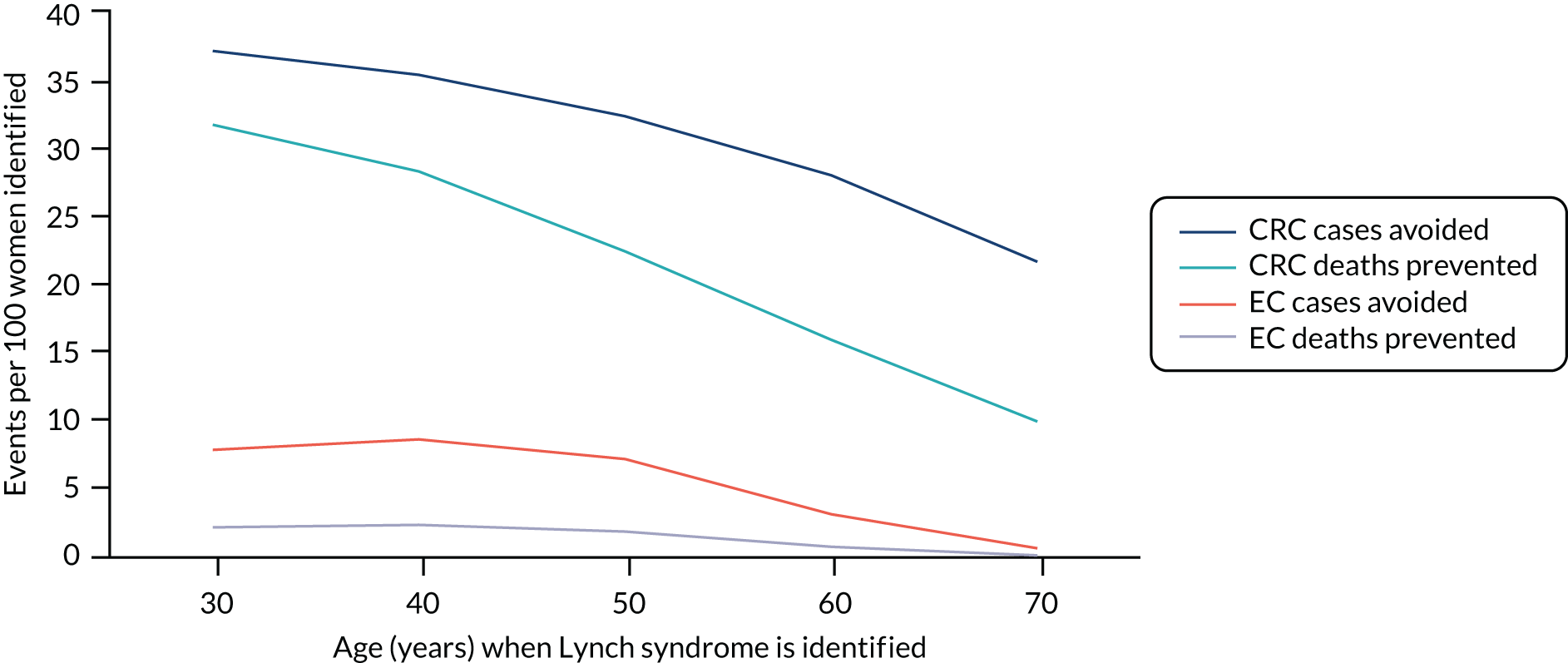
FIGURE 27.
Benefits of identifying 100 men with Lynch syndrome in a cohort of the same age.
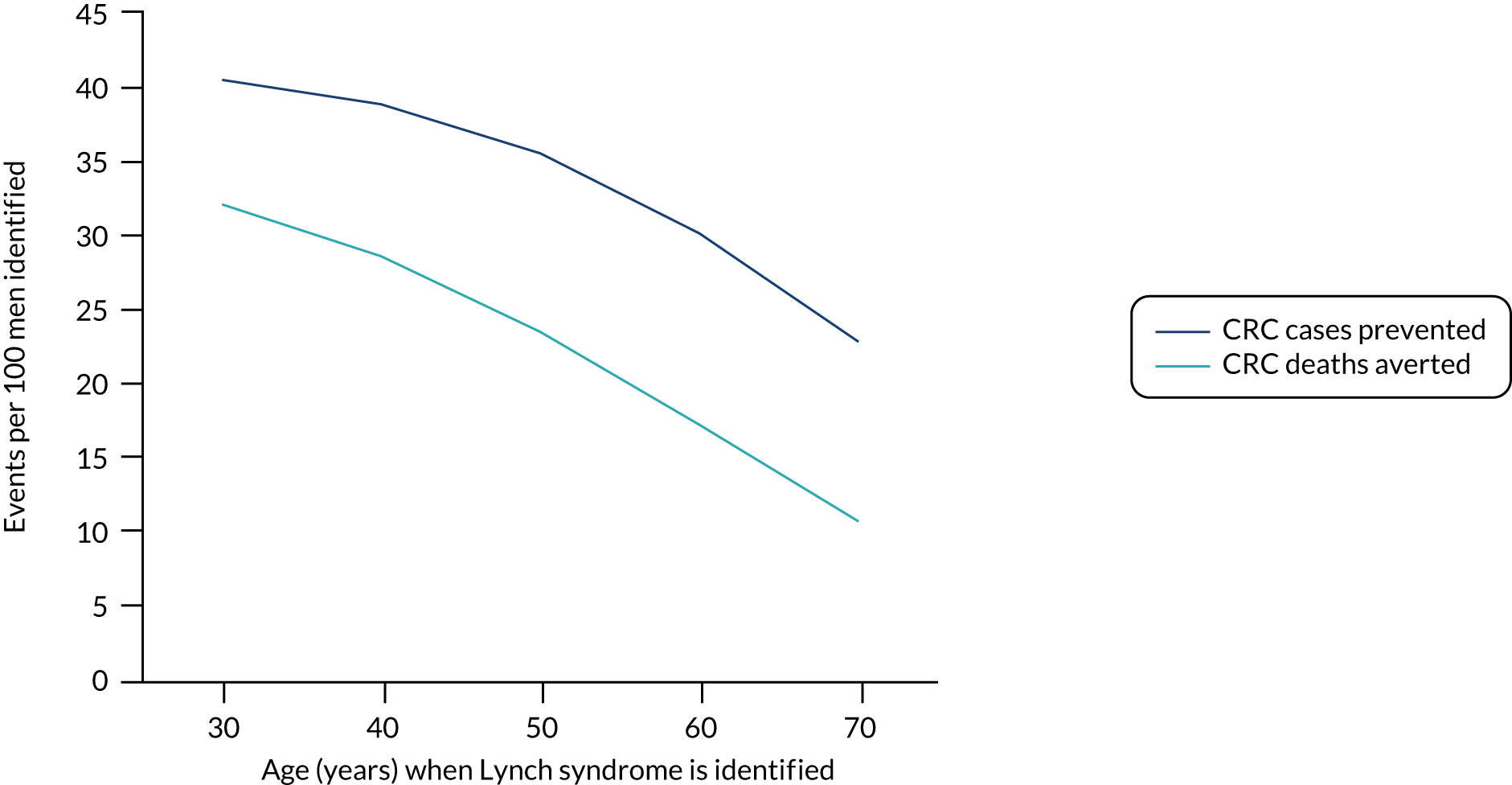
The number of CRC cases and CRC deaths prevented when 100 women of a given age who have Lynch syndrome, and have recently presented with endometrial cancer, benefit from CRC surveillance and risk reduction are presented in Figure 28. This declines with age as the relative risk of dying from other causes increases.
FIGURE 28.
Number of CRC cases and CRC deaths prevented by identification of 100 endometrial cancer probands.
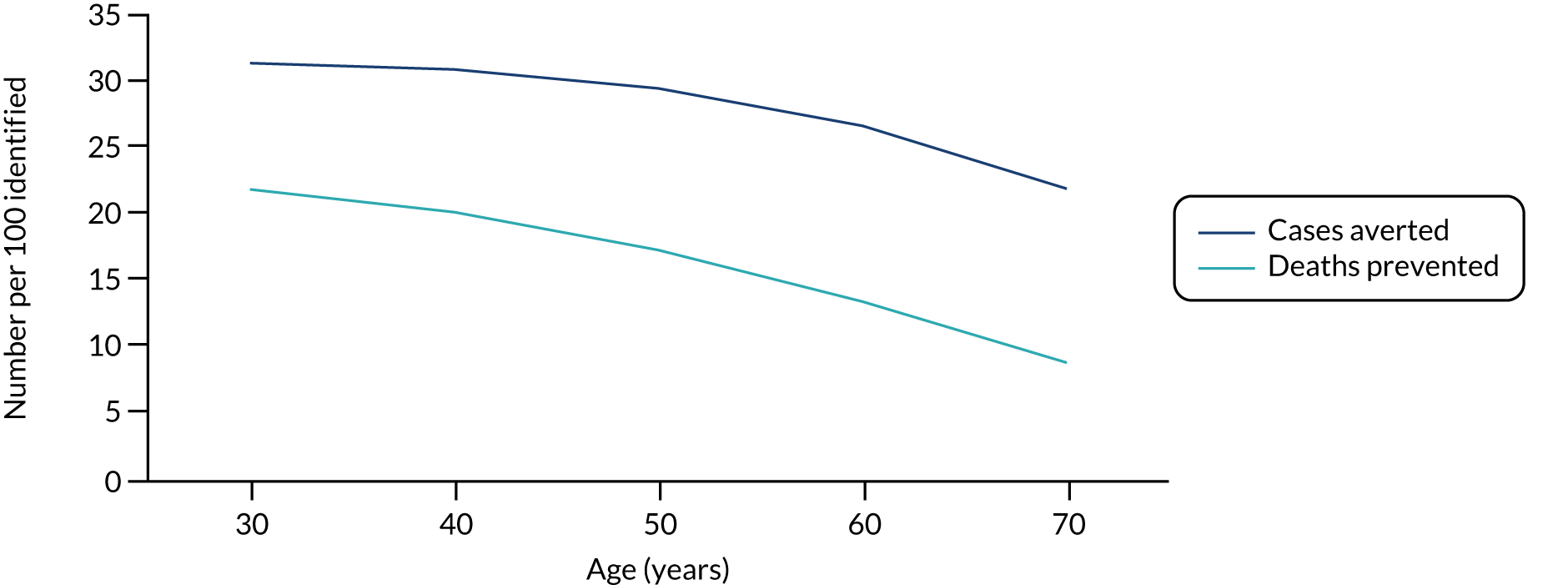
Additional outcomes
Figure 29 shows the QALY gains predicted by the lifetime cancer model, as a function of the age at which a person is identified with Lynch syndrome. As expected, these decrease with age, as the number of life-years that can be gained falls as the age at which cancer would present increases. QALY gains are similar for the three groups, except for younger men, who gain greater benefit from CRC protection as a result of their increased risk.
FIGURE 29.
The QALY gains predicted by the lifetime cancer model as a function of the age at which a person is identified as having Lynch syndrome.
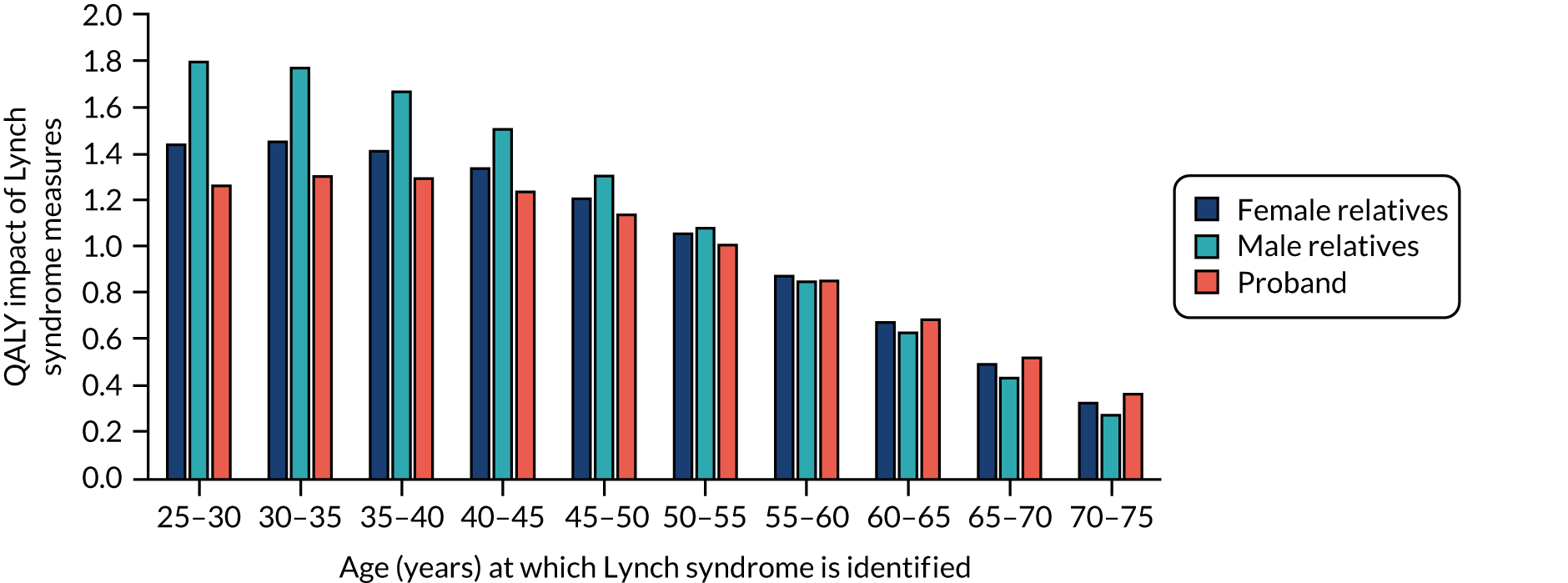
Disaggregated costs
If a person is identified with Lynch syndrome, they will incur additional costs owing to protective measures such as surveillance and prophylactic surgery. At the same time, they may incur reduced Lynch syndrome-related cancer treatment costs if the protective measures are effective. Figure 30 shows how the take-up of such measures, from the age at which a person is identified with Lynch syndrome, affects total costs. The costs for female relatives is significantly higher, largely because of the costs of prophylactic surgery to prevent endometrial cancer, which is not incurred by men or by women who have been diagnosed with endometrial cancer.
FIGURE 30.
Cost impact of Lynch syndrome management across probands and male and female relatives.
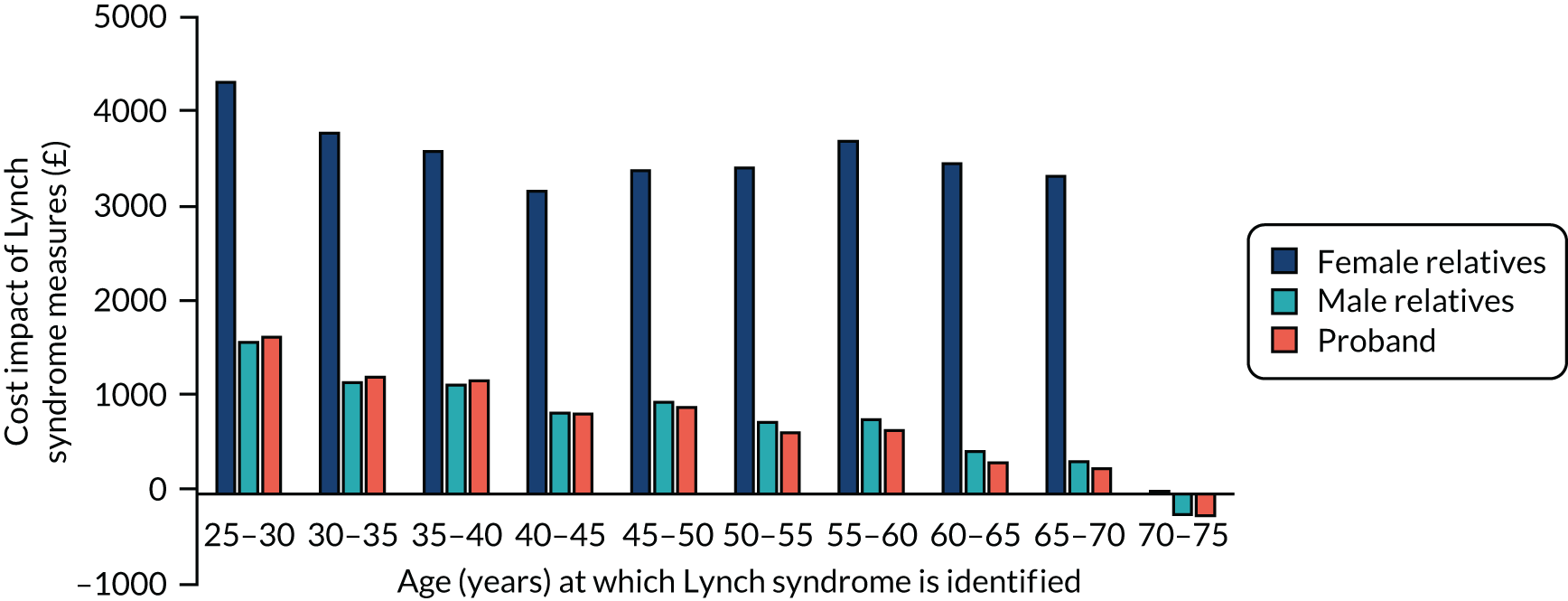
Subgroup analyses
Potential subgroup analyses of reflex testing in endometrial cancer probands aged < 70 years and probands who had previously had CRC but who did not already have a Lynch syndrome status assigned were not feasible; therefore, no results are presented.
Scenario analyses
Scenario analysis results
We undertook several scenario analyses to estimate the impact to the base case of changing key model input parameters; the full rationale for doing so is detailed in Discussion of model input parameters and Final model input parameters. The following scenario analyses were undertaken:
-
scenario analysis 1 – strategy-level test accuracy obtained from the PETALS study (Dr Neil AJ Ryan, personal communication)
-
scenario analysis 2 – costs of testing obtained from Ryan et al. 50
-
scenario analysis 3 – strategy-level test accuracy obtained from the PETALS study (Dr Neil AJ Ryan, personal communication) and costs of testing obtained from Ryan et al. 50
-
scenario analysis 4 – disutility inflated because of cancer
-
scenario analysis 5 – gynaecological surveillance excluded
-
scenario analysis 6 – 3-year colonoscopy surveillance
-
scenario analysis 7 – excluding benefit from aspirin
-
scenario analysis 8 – excluding hazard ratio that reduces incidence of CRC as a result of surveillance.
Scenario analysis 1 results
The results in Table 15 show that the most cost-effective strategy remains IHC with MLH1 methylation testing, with an ICER of approximately £9280 per QALY, in comparison with no testing. All other strategies were dominated or did not reach accepted cost-effectiveness threshold levels.
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| No testing | 0 | – | 0.0000 | – | – |
| MSI with MLH1 methylation | 480 | 480 | 0.0378 | 0.0378 | Extendedly dominated |
| IHC with MLH1 methylation | 620 | 620 | 0.0668 | 0.0668 | £9280 |
| MSI | 640 | 20 | 0.0389 | –0.0279 | Dominated |
| MSI followed by IHC with MLH1 methylation | 720 | 100 | 0.0420 | –0.0248 | Dominated |
| IHC | 820 | 200 | 0.0683 | 0.0015 | £133,330 |
| MSI and IHC with MLH1 methylation | 860 | 40 | 0.0669 | –0.0014 | Dominated |
| IHC followed by MSI with MLH1 methylation | 876 | 56 | 0.0671 | –0.0012 | Dominated |
| IHC followed by MSI | 1020 | 200 | 0.0685 | 0.0002 | £1,000,000 |
| MSI followed by IHC | 1030 | 10 | 0.0685 | 0.0000 | Dominated |
| MSI and IHC | 1060 | 40 | 0.0684 | –0.0001 | Dominated |
| Germline testing | 1160 | 40 | 0.0666 | –0.0019 | Dominated |
Using the test accuracy estimates from Ryan et al. ,100 a small decrease in the ICER of £140 per QALY from the base case was observed. This was driven by a nominal reduction in the average cost of testing of £10 while QALY gains remained static. IHC testing also followed as the next most cost-effective strategy, but equally exceeded the accepted WTP threshold.
Scenario analysis 2 results: costs of testing obtained from Ryan et al.50
Using the testing costs obtained from Ryan et al. ,50 the results (Table 16) show that the IHC with MLH1 methylation testing strategy continues to be the most cost-effective, with an ICER of approximately £5830, when compared with the MSI with MLH1 methylation testing strategy. All other strategies continue to be dominated or do not reach acceptable cost-effectiveness threshold levels.
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| No testing | 0 | – | 0.0000 | – | – |
| MSI with MLH1 methylation | 270 | – | 0.0419 | – | – |
| MSI followed by IHC with MLH1 methylation | 300 | 30 | 0.0420 | 0.0001 | Extendedly dominated |
| IHC with MLH1 methylation | 390 | 390 | 0.0669 | 0.0669 | £5830 |
| IHC followed by MSI with MLH1 methylation | 436 | 46 | 0.0671 | 0.0002 | Extendedly dominated |
| MSI and IHC with MLH1 methylation | 438 | 2 | 0.0671 | 0.0000 | Dominated |
| IHC | 500 | 110 | 0.0681 | 0.0012 | £91,670 |
| MSI | 540 | 40 | 0.0683 | 0.0002 | Extendedly dominated |
| IHC followed by MSI | 560 | 60 | 0.0685 | 0.0004 | £150,000 |
| MSI and IHC | 570 | 10 | 0.0685 | 0.0004 | Dominated |
| MSI followed by IHC | 573 | 3 | 0.0685 | 0.0000 | Dominated |
| Germline testing | 880 | 320 | 0.0666 | –0.0019 | Dominated |
The results of the microcosting study produced test cost estimates that were significantly reduced from those used in the base case; therefore, an ICER of almost half that of the base case was expected. As discussed in Discussion of model input parameters, these costs are considered grossly under-representative of the true costs involved in testing in the NHS at this point in time.
Scenario analysis 3 results: strategy-level test accuracy obtained from the PETALS study and costs of testing obtained from Ryan et al.50
The results in Table 17 show that IHC with MLH1 methylation testing continues to be the most cost-effective strategy, with an ICER of £5690 per QALY.
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| No testing | 0 | – | 0.0000 | – | – |
| MSI with MLH1 methylation | 250 | 250 | 0.0378 | 0.0378 | Extendedly dominated |
| MSI followed by IHC with MLH1 methylation | 300 | 300 | 0.0420 | 0.0042 | Extendedly dominated |
| MSI | 360 | 60 | 0.0389 | –0.0031 | Dominated |
| IHC with MLH1 methylation | 380 | 380 | 0.0668 | 0.0668 | £5690 |
| MSI and IHC with MLH1 methylation | 420 | 40 | 0.0669 | 0.0001 | Extendedly dominated |
| IHC followed by MSI with MLH1 methylation | 440 | 20 | 0.0671 | 0.0002 | Extendedly dominated |
| IHC | 530 | 150 | 0.0683 | 0.0015 | £100,000 |
| IHC followed by MSI | 560 | 30 | 0.0685 | 0.0002 | £150,000 |
| MSI and IHC | 566 | 6 | 0.0684 | –0.0001 | Dominated |
| MSI followed by IHC | 573 | 13 | 0.0685 | 0.0000 | Dominated |
| Germline testing | 880 | 320 | 0.0666 | –0.0019 | Dominated |
The results tables for scenario analyses 4–7 (see Appendix 7) illustrate that IHC with MLH1 methylation testing is the most cost-effective strategy under each scenario.
Scenario analysis 8 results: excluding hazard ratio that reduces incidence of colorectal cancer as a result of surveillance
In contrast with the base case, here we assume that colonoscopic surveillance reduces CRC incidence, with a hazard rate of 0.387, mirroring the assumptions made by Snowsill et al. 110 on CRC incidence in the presence/absence of surveillance (Table 18). The ICER increases to £20,740, exceeding the cost-effectiveness threshold of £20,000 per QALY. This is the greatest change in the ICER found across all scenario analyses.
Scenario analysis 8
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No testing | 0 | – | 0.0000 | – | – |
| MSI with MLH1 methylation | 540 | 540 | 0.0203 | 0.0203 | Extendedly dominated |
| IHC with MLH1 methylation | 670 | 670 | 0.0323 | 0.0323 | 20,740 |
| MSI followed by IHC with MLH1 methylation | 740 | 70 | 0.0204 | –0.0119 | Dominated |
| IHC | 830 | 160 | 0.0333 | 0.0010 | 160,000 |
| MSI | 870 | 40 | 0.0335 | 0.0002 | 200,000 |
| IHC followed by MSI with MLH1 methylation | 900 | 30 | 0.0325 | –0.0010 | Dominated |
| MSI and IHC simultaneously with MLH1 methylation | 930 | 60 | 0.0325 | –0.0010 | Dominated |
| IHC followed by MSI | 1060 | 190 | 0.0336 | 0.0001 | Extendedly dominated |
| MSI followed by IHC | 1070 | 200 | 0.0337 | 0.0002 | 1,000,000 |
| MSI and IHC simultaneously | 1100 | 30 | 0.0336 | –0.0001 | Dominated |
| Germline testing | 1200 | 130 | 0.0321 | –0.0016 | Dominated |
Summary
We undertook several scenario analyses to assess the impact of these changes on our base-case ICER. Under these scenarios, the results remained robust, with IHC with MLH1 methylation testing being the most cost-effective strategy.
Deterministic/probabilistic sensitivity analyses
One-way sensitivity analysis results
Deterministic sensitivity analysis results were conducted by varying key model input parameters used in the base-case analysis to assess the impact on the cost per QALY, and presented in the form of tornado plots. Figure 31 shows the tornado plot for the comparison between IHC with MLH1 methylation and a no-testing strategy. We chose this comparison because, in the base-case analysis, the incremental results showed that IHC with MLH1 methylation was the most cost-effective strategy. In addition, IHC with MLH1 methylation had the most cost-effective ICER (approximately £9460 per QALY) when each testing strategy was compared with a no-testing strategy. The sensitivity analysis results show which parameter is the key driver of the cost-effectiveness. These results show that varying the percentage of relatives accepting counselling was the most influential parameter. Decreasing the number of relatives who accept counselling by 50% led to an increase in the ICER. Likewise, increasing the percentage of relatives who accept counselling led to a decrease in the ICER. In the model, these relatives receive the germline tests if appropriate, but do not incur the costs of genetic counselling. Moreover, as expected, if there was a decrease in the prevalence of Lynch syndrome among women with endometrial cancer, the ICER increased to approximately £13,640 per QALY. Similarly, if the prevalence was increased to 6.4%, this resulted in an ICER of approximately £7350 per QALY. Based on the parameters varied, the ICER changed slightly, but remained below current WTP thresholds.
FIGURE 31.
Tornado plot for the impact of a ± 50% change in individual parameters on the ICER per QALY gained.
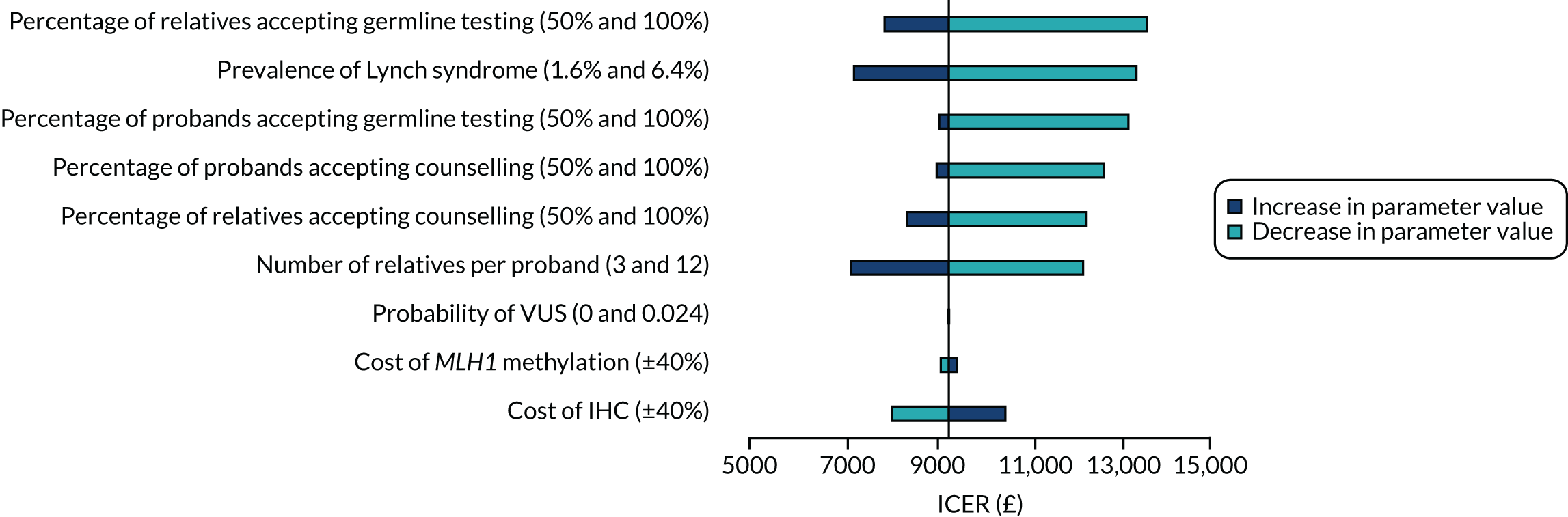
Probabilistic sensitivity analysis results
We report the PSA results that were generated by assigning distributions to key input parameters and randomly sampling from these distributions over 10,000 simulations to derive any uncertainty in the costs and outcomes. The PSA results for IHC with methylation, compared with no testing, produced an incremental cost of £600 and an incremental QALY of 0.0517, giving an ICER of £11,600 per QALY gained. Exact results have been obtained from TreeAge but were rounded by the authors. We chose this comparison because this strategy was shown to be the most cost-effective in the base-case analysis, and, across all scenario analyses, the results remained robust. Including the combined uncertainty across the parameters included in the PSA showed that the expected mean costs and QALYs yielded in the base case were underestimated, which resulted in an ICER greater than that in the deterministic results.
The probabilistic results are presented in the form of an incremental scatterplot and its corresponding CEACs. Figure 32 presents the results of the 10,000 runs of the Monte Carlo simulations; the scatterplot shows that there is some variation in the incremental costs and QALYs. Figure 33 shows the probabilistic results presented in the form of a CEAC, which shows the probability that an intervention is cost-effective at different WTP thresholds per QALY. At a WTP threshold of £20,000 per QALY, IHC has a 0.93 probability of being cost-effective when compared with no testing.
FIGURE 32.
Incremental cost-effectiveness scatterplot for the comparison between IHC with MLH1 versus no screening.
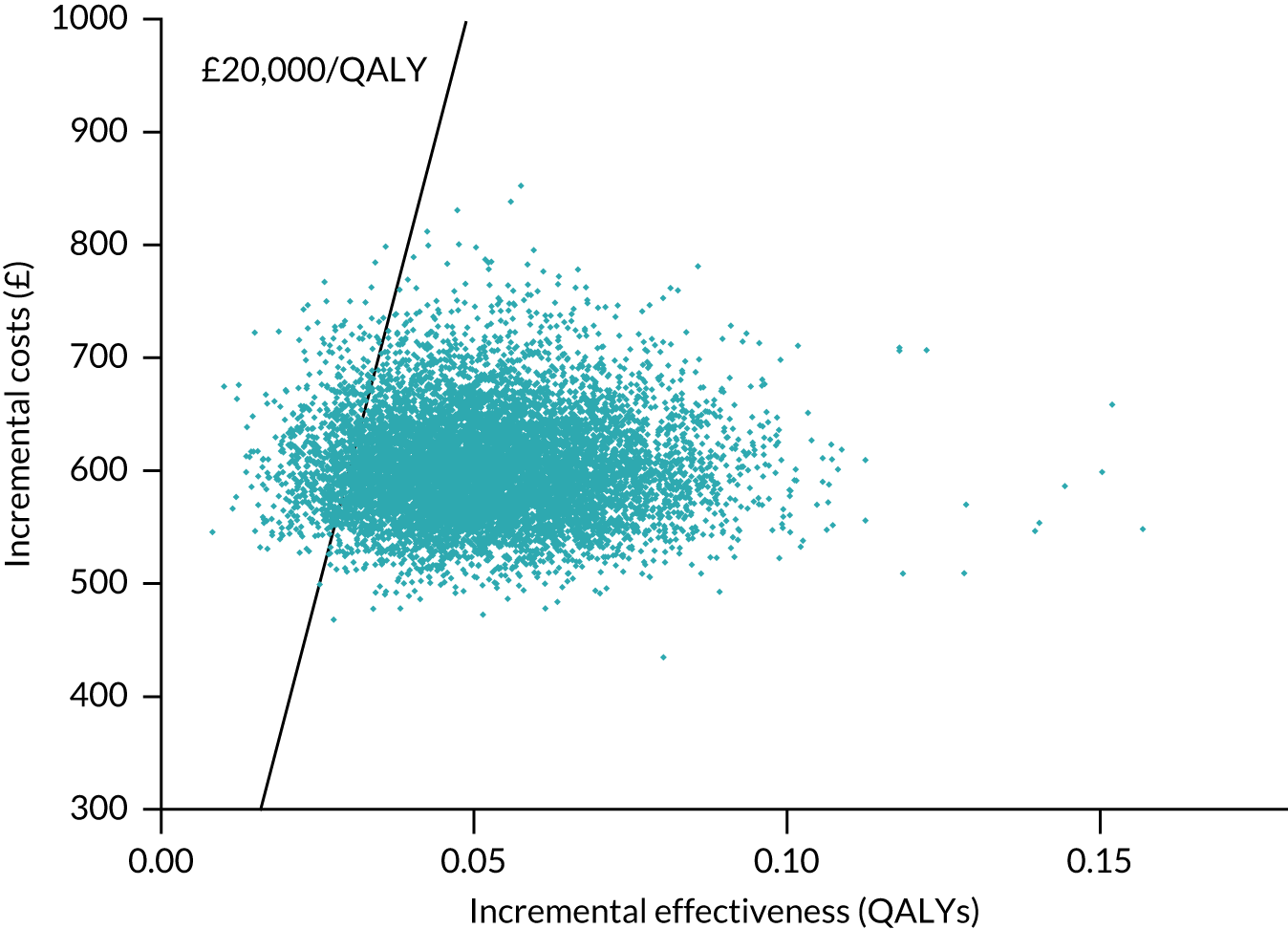
FIGURE 33.
The CEAC for the IHC with methylation strategy at different WTP thresholds.
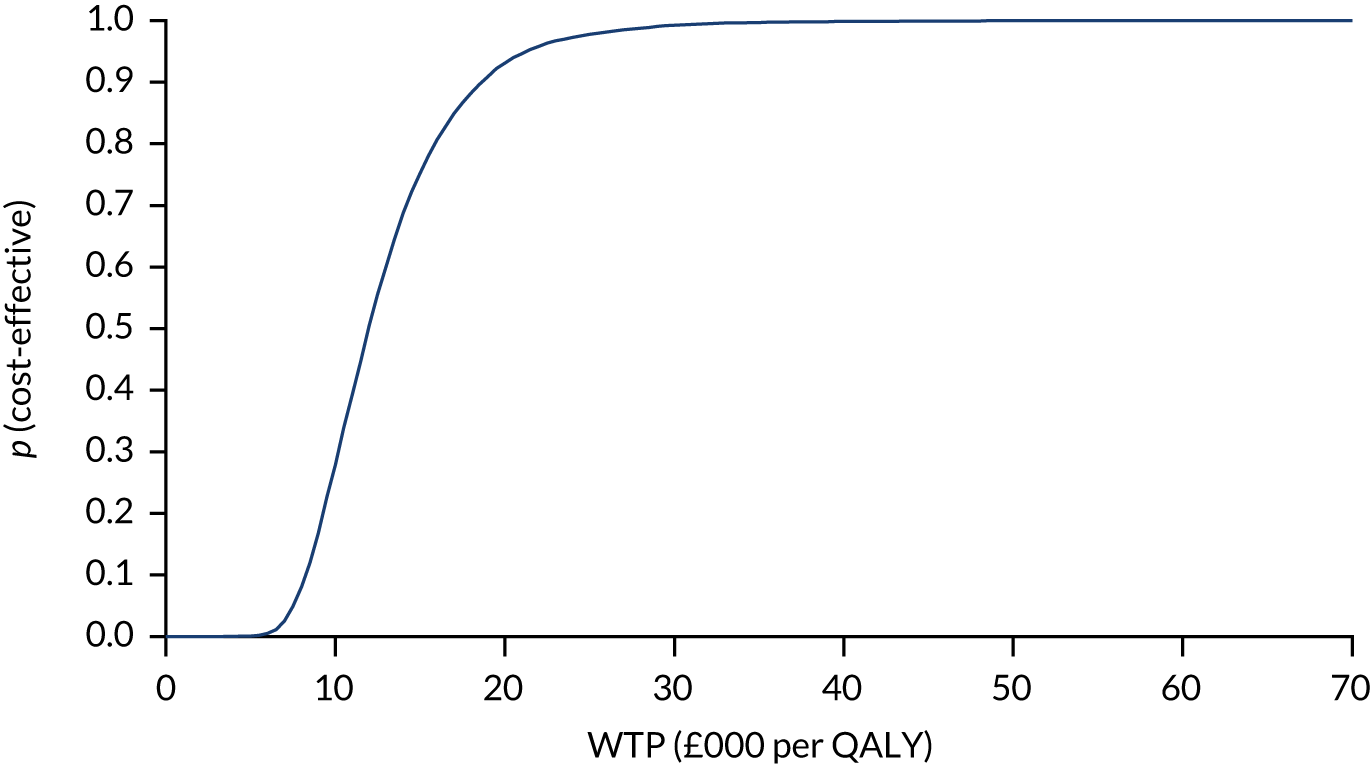
Chapter 7 Discussion
Statement of principal findings
The clinical effectiveness search identified 3308 studies; of these, 38 studies of test accuracy were included, of which seven provided full 2 × 2 data. There were four head-to-head test accuracy studies comparing MSI with IHC. None of these studies demonstrated a clear difference in accuracy between IHC and MSI. Other studies indicated that the specificity of IHC may be improved through methylation testing of patients with IHC deficiency in MLH1. There was very little evidence on accuracy of methylation testing in MSI-H patients. The test failure rate was consistently low for both tests. There was high concordance between IHC and MSI tests in most studies. No studies of clinical effectiveness of endometrial cancer surveillance met the inclusion criteria. Therefore, there were limited data on test accuracy and effectiveness of colorectal and gynaecological screening to populate the economic model, and available evidence was deemed to be at a high risk of bias. The economic model indicated that the IHC with MLH1 methylation strategy was the most cost-effective testing strategy, with an ICER of approximately £9420 per QALY. Sensitivity analyses examining different model assumptions were generally cost-effective at a WTP threshold of £20,000 per QALY.
Strengths and limitations of the assessment
The major strength of this assessment is that we followed the gold-standard methodology for conducting systematic reviews (which included independent assessment at every stage and input from expert clinicians) to identify evidence on test accuracy, disease prevalence, and the benefits and harms of gynaecologic and colorectal surveillance of women identified with Lynch syndrome. The economic model was directly informed by this systematic review.
The clinical effectiveness review had a number of limitations. First, we excluded studies for which we could not establish which reference standard(s) were used. For each study that did not explicitly state how a diagnosis of Lynch syndrome was established, we contacted the corresponding author to seek clarification. Of the authors who responded, none was able to confirm the tests used, informing us that samples were sent to commercial laboratories (sometimes multiple laboratories). We followed this up with the companies specified, but they were unable to confirm which tests had been used without us providing details of individual study participants. This was not possible; therefore, we cannot be certain if these excluded studies used the reference standards of interest for our review and if they could have provided additional information on the test accuracy of IHC and MSI for Lynch syndrome. Second, in our PICO framework, we specified that Lynch syndrome must have been diagnosed by genetic verifications of constitutional variants in the MMR genes (MHL1, MSH2, MSH6 or PMS2) using diagnostic tests outlined in the Association for Clinical Genomic Science best-practice guidelines for genetic testing and diagnosis of Lynch syndrome, prioritising sequencing with/without MLPA. 33 Variants in these four genes are thought to account for 97–99% of Lynch syndrome cases. 123 There is some evidence that variants in a fifth gene (EPCAM) may be responsible for 1–3% of Lynch syndrome cases. 13 The exclusion of EPCAM may have led to us slightly underestimating the prevalence of Lynch syndrome. Furthermore, studies that employed diagnostic tests other than the ones we specified would not have been captured in our review. Third, the number of VUS cases were reported as stated in individual studies. Over time, VUSs may be reclassified. For example, Mersch et al. 124 reported that, from a sample of 26,670 unique VUSs, 2048 (7.7%) were reclassified. In the majority of cases, these were downgraded to benign/likely benign [91.2% (1867/2048)], with only a minority being upgraded to pathogenic/likely pathogenic [8.7% (178/2048)]. 124 Data in our review came from studies published from 1999 to 2019, with the earliest cases of VUSs being reported in a study from 2003. 54 We considered VUSs to be germline negative. However, it is possible that the pathogenic status of these variants has now changed and that these individuals would now be considered to have Lynch syndrome. Fourth, we did not search for grey literature or studies published in languages other than English. It is possible that other relevant studies could have been missed by employing this approach.
In this assessment, a full systematic review was undertaken to identify evidence on test accuracy, disease prevalence, and benefits and harms of gynaecologic and colorectal surveillance in women identified with Lynch syndrome. A strength is that the economic model was directly informed by this systematic review, although articles were limited to the English language.
Conclusions from the economic model are similar to those of Snowsill et al. ,43 which is the closest equivalent review to ours in that it is constructed to review testing of endometrial cancer probands and their relatives in the UK setting, presents results in costs and QALYs, and uses a no-testing comparator. IHC with MLH1 promoter hypermethylation testing was found to be the most cost-effective strategy, with an ICER of £14,200. Although this is more expensive than our ICER of £9420 per QALY, some key differences between the base-case assumptions provide a viable explanation. First, we modelled surveillance and risk reduction interventions for both CRC and endometrial cancer, including aspirin prophylaxis, whereas the Snowsill et al. 43 model included only CRC surveillance measures. Although this is likely to reduce long-term costs in the form of surveillance, it is also likely to exclude potentially valuable benefits accrued through these practices. Second, in their base-case analysis, Snowsill et al. 43 modelled endometrial cancer probands entering the model at a specific age of 60 years, whereas proband entry to our model occurred at 49 years of age, thereby limiting comparison, as cost-effectiveness is sensitive to age of probands. However, a PSA was conducted by Snowsill et al. 43 on an alternative scenario in which probands entered their model aged 50 years, allowing more direct evaluation, and showed the probability of IHC with MLH1 methylation testing being cost-effective in 90% of the 1000 iterations.
A similar model by Snowsill et al. 12 examined optimal testing strategies for Lynch syndrome in CRC probands and their relatives. This model identified IHC plus BRAF plus MLH1 promoter hypermethylation testing as the most cost-effective strategy, with an ICER of £11,008 per QALY in their base-case analysis with CRC probands of mean age 58 years. Although the testing strategies are not relevant to the endometrial cancer population, the cost-effectiveness results are similar to our estimates for endometrial cancer probands. Cost-effectiveness was sensitive to the accuracy of tumour tests, the acceptance of genetic counselling and testing, and the number of relatives identified through cascade testing per proband. The effectiveness of surveillance colonoscopy and the lifetime risk of CRC for people with Lynch syndrome were also key determinants of cost-effectiveness. This mirrors our findings and highlights the need for further research to provide evidence for these parameters, both for robust inputs for use in economic modelling and to address the practical implications of implementation of testing and monitoring.
The main limitation of our economic model was the uncertainty in model input parameters (see Uncertainties). High-quality estimates of the effectiveness of surveillance colonoscopies are required, as the benefits of long-term effectiveness of screening for Lynch syndrome come primarily from this source. The value of offering colonoscopy in this setting needs to be ascertained so modelling in this area can be more reliable. There is even greater uncertainty about the benefits and harms of gynaecological surveillance. We have modelled only benefits, but we do not know if the benefits outweigh the harms, or even how gynaecological surveillance would be undertaken. However, our scenario analysis indicated that removing the benefits of endometrial cancer surveillance did not affect conclusions.
We also have not included any specific pathway modelling of genetic testing for somatic MMR mutations, which is sometimes used (typically in research settings) to confirm that a MMR-deficient tumour, with no constitutional pathogenic variant identified, has arisen as a result of somatic MMR mutations, rather than from Lynch syndrome. This may also be used to identify VUSs and potentially guide their long-term management. This additional layer of testing would be expected to increase total diagnostic costs, but may provide longer-term cost savings through more directed management/surveillance practices. Furthermore, it was difficult to adequately reflect the full genetic counselling process in our model, and we modelled the whole diagnostic process as occurring within 1 year, which may not represent the potentially more elongated process in practice.
Uncertainties
There was no RCT evidence on the effect of earlier detection of Lynch syndrome and intervention on long-term outcomes, only observational cohorts at high risk of bias. In particular, little is known about the balance of benefits and harms of gynaecological cancer surveillance, and there is no consensus on which tests such surveillance entails. There was only observational evidence, rated as having a high risk of bias, for the benefit of CRC screening in individuals with Lynch syndrome, with no evidence indicating whether the test should be faecal immunochemical or colonoscopy, and what the ages of eligibility or screening intervals should be in this cohort. The EAG notes recent publication of a trial of aspirin chemoprevention. 125 The results of this 10-year follow-up support the case for prevention of CRC with aspirin among people with Lynch syndrome, demonstrating a significantly reduced hazard ratio of 0.65 (95% CI 0.43 to 0.97; p = 0.035) for aspirin versus placebo in the intention-to-treat population. Significance was maintained with a hazard ratio of 0.56 (95% CI 0.34 to 0.91; p = 0.019) and an incidence rate ratio of 0.50 (95% CI 0.31 to 0.82; p = 0.0057) when restricted to a per-protocol analysis of patients who achieved 2 years' intervention.
There was limited evidence on the sensitivity of the testing strategies, because of the low disease prevalence, resulting in few cases per study, and lack of follow-up of index-test negatives to ascertain whether or not they were false negatives.
Chapter 8 Conclusions
The economic model suggests that testing women with endometrial cancer for Lynch syndrome is cost-effective. The most cost-effective testing strategy was IHC followed by methylation. However, there were limited data for test accuracy and for the benefits and harms of surveillance for colorectal and endometrial cancer surveillance once Lynch syndrome is detected. These estimates are rated as having a high risk of bias, and so model results should be interpreted with caution.
Implications for service provision
Although the concept of testing endometrial cancer patients for Lynch syndrome is cost-effective using the assumptions in the model, data were sparse and were judged to be at a high risk of bias. Therefore, were this to be implemented in the NHS, some pragmatic choices may have to be made on the details of the testing and treatment pathway. These include which exact testing strategy to use, as the economic model that indicated IHC followed by methylation was underpinned by data from a study using only three out of the four target proteins. Furthermore, decisions as to whether or not to offer gynaecological surveillance, and, if so, which specific tests and at what intervals, would need to be made.
There were consistent data suggesting that testing women with endometrial cancer for Lynch syndrome will identify a significant number of women with VUSs; pathways are required to manage these women.
Suggested research priorities
We suggest two research priorities:
-
There was no RCT evidence on the effect of earlier intervention on long-term outcomes, only observational cohorts, deemed to be at a high risk of bias. In particular, little is known about the balance of benefits and harms of gynaecological cancer surveillance. RCTs would provide evidence with a lower risk of bias.
-
The volume of test accuracy studies was significant, but most did not give the reference standard to index test-negative women. The full test accuracy studies in which all participants received the reference standard contained few cases of Lynch syndrome. Therefore, little is known about test sensitivity and false negatives. Although full test accuracy studies with large sample sizes may be prohibitively expensive because of the low prevalence of Lynch syndrome, follow-up of negative cases through disease registers could be used to determine false negative cases. Furthermore, there are very limited data on the test accuracy of MSI testing followed by MLH1 promoter hypermethylation testing in women with MSI-H.
Acknowledgements
The authors would like to thank Professor Dimitris Grammatopoulos and Dr Mark Arends for their expert clinical advice, and Professor Aileen Clarke and Dr Lazaros Andronis for comments on the draft report. The authors would like to thank James Keasley (Academic Foundation 2 doctor) who helped conduct the clinical effectiveness systematic review; this included screening and retrieving papers, assessing against the inclusion criteria, appraising the quality of papers and abstracting data from papers for synthesis. The authors would also like to thank Kate Evans for helping with the co-ordinating of the report and Sarah Abrahamson for formatting and checking the report.
Contributions of authors
Chris Stinton (https://orcid.org/0000-0001-9054-1940) (Senior Research Fellow) led the clinical effectiveness review. Chris Stinton, Hannah Fraser (https://orcid.org/0000-0002-7050-9684) (Research Associate) and Lena Al-Khudairy (https://orcid.org/0000-0003-0638-583X) (Senior Research Fellow) conducted the clinical effectiveness systematic review, which included screening and retrieving papers, assessing against the inclusion criteria, appraising the quality of papers and abstracting data from papers for synthesis.
Mary Jordan (https://orcid.org/0000-0002-0497-8634) (Research Fellow), Peter Auguste (https://orcid.org/0000-0001-5143-3218) (Research Fellow) and Jason Madan (https://orcid.org/0000-0003-4316-1480) (Professor in Health Economics) contributed to the cost-effectiveness review and undertook the health economic modelling.
Rachel Court (https://orcid.org/0000-0002-4567-2586) (Information specialist) developed the search strategy and undertook searches.
Dimitris Grammatopoulos (https://orcid.org/0000-0002-6296-8290) (Professor of Molecular Medicine) provided clinical guidance and helped develop the model structures.
Sian Taylor-Phillips (https://orcid.org/0000-0002-1841-4346) (Associate Professor), led the project, and contributed to all stages for clinical and cost-effectiveness.
All authors were involved in writing draft and final versions of the report.
Publication
Stinton C, Fraser H, Al-Khudairy L, Court R, Jordan M, Grammatopoulos D, Taylor-Phillips S. Testing for Lynch syndrome in people with endometrial cancer using immunohistochemistry and microsatellite instability-based testing strategies – a systematic review of test accuracy. Gynecol Oncol 2021;160:148–60.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Institute for Health and Care Excellence . Molecular Testing Strategies for Lynch Syndrome in People With Colorectal Cancer n.d. www.nice.org.uk/guidance/dg27 (accessed 2 August 2019).
- Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66:464-72. https://doi.org/10.1136/gutjnl-2015-309675.
- Lu KH, Loose DS, Yates MS, Nogueras-Gonzalez GM, Munsell MF, Chen LM, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res 2013;6:774-81. https://doi.org/10.1158/1940-6207.CAPR-13-0020.
- Stinton C, Fraser H, Al-Khudairy L, Court R, Jordan M, Grammatopoulos D, et al. Testing for Lynch syndrome in people with endometrial cancer using immunohistochemistry and microsatellite instability-based testing strategies – a systematic review of test accuracy. Gynecol Oncol 2021;160:148-60. https://doi.org/10.1016/j.ygyno.2020.10.003.
- Roett MA. Genital cancers in women: uterine cancer. FP Essent 2015;438:11-7.
- Cancer Research UK. Uterine Cancer Incidence Statistics 2016. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence#heading-One (accessed 2 August 2019).
- Cancer Research UK . Uterine Cancer Mortality Statistics 2016. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/mortality (accessed 2 August 2019).
- Office for National Statistics . Cancer Survival in England: National Estimates for Patients Followed Up to 2017 n.d. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalinengland/nationalestimatesforpatientsfollowedupto2017 (accessed 2 August 2019).
- Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer 2014;24:384-93. https://doi.org/10.1097/IGC.0000000000000075.
- Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk?. Int J Cancer 2013;132:417-26. https://doi.org/10.1002/ijc.27623.
- Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer 2019;145:1719-30. https://doi.org/10.1002/ijc.31961.
- Snowsill T, Coelho H, Huxley N, Jones-Hughes T, Briscoe S, Frayling IM, et al. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21510.
- Kuiper RP, Vissers LE, Venkatachalam R, Bodmer D, Hoenselaar E, Goossens M, et al. Recurrence and variability of germline EPCAM deletions in Lynch syndrome. Hum Mutat 2011;32:407-14. https://doi.org/10.1002/humu.21446.
- Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306-16. https://doi.org/10.1136/gutjnl-2017-314057.
- Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 2006;66:7810-17. https://doi.org/10.1158/0008-5472.CAN-06-1114.
- Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol 2007;25:5158-64. https://doi.org/10.1200/JCO.2007.10.8597.
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 2008;10:293-300. https://doi.org/10.2353/jmoldx.2008.080031.
- Hegde M, Ferber M, Mao R, Samowitz W, Ganguly A. Working Group of the American College of Medical Genetics and Genomics (ACMG) Laboratory Quality Assurance Committee . ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis). Genet Med 2014;16:101-16. https://doi.org/10.1038/gim.2013.166.
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. https://doi.org/10.1093/jnci/djh034.
- Castillejo A, Hernández-Illán E, Rodriguez-Soler M, Pérez-Carbonell L, Egoavil C, Barberá VM, et al. Prevalence of MLH1 constitutional epimutations as a cause of Lynch syndrome in unselected versus selected consecutive series of patients with colorectal cancer. J Med Genet 2015;52:498-502. https://doi.org/10.1136/jmedgenet-2015-103076.
- Cho KR, Cooper K, Croce S, Djordevic B, Herrington S, Howitt B, et al. International Society of Gynecological Pathologists (ISGyP) Endometrial Cancer Project: guidelines from the Special Techniques and Ancillary Studies Group. Int J Gynecol Pathol 2019;38:114-22. https://doi.org/10.1097/PGP.0000000000000496.
- Crosbie EJ, Ryan NAJ, Arends MJ, Bosse T, Burn J, Cornes JM, et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med 2019;21:2390-400. https://doi.org/10.1038/s41436-019-0489-y.
- NHS England . National Genomic Test Directory: Testing Criteria for Rare and Inherited Disease 2019. www.england.nhs.uk/wp-content/uploads/2018/08/rare-and-inherited-disease-eligibility-criteria-march-19.pdf (accessed 2 August 2019).
- Sundar S, Balega J, Crosbie E, Drake A, Edmondson R, Fotopoulou C, et al. BGCS uterine cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol 2017;213:71-97. https://doi.org/10.1016/j.ejogrb.2017.04.015.
- Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812-23. https://doi.org/10.1136/gutjnl-2012-304356.
- Kulkarni A, Brady AF. Management of Women with a Genetic Predisposition to Gynaecological Cancers: Scientific Impact Paper No. 48. Royal College of Obstetricians and Gynaecologists; 2015.
- Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) [published online ahead of print November 28 2019]. Gut 2019. https://doi.org/10.1136/gutjnl-2019-319915.
- National Guideline Alliance hosted by the Royal College of Obstetricians and Gynaecologist . [A1] Effectiveness of Aspirin in the Prevention of Colorectal Cancer in People With Lynch Syndrome: Evidence Review for Colorectal Cancer (Update) n.d. www.nice.org.uk/guidance/GID-NG10060/documents/evidence-review (accessed 10 January 2020).
- American College of Obstetricians and Gynecologists . Lynch Syndrome. Practice Bulletin No. 147. Obstet Gynecol 2014;124:1042-54. https://doi.org/10.1097/01.Aog.0000456325.50739.72.
- Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol 2016;27:v103-10. https://doi.org/10.1093/annonc/mdw327.
- Cochrane Screening and Diagnostic Tests Methods Group . Handbook for Diagnostic Test Accuracy Reviews n.d. https://methods.cochrane.org/sdt/handbook-dta-reviews (accessed 2 August 2019).
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 2 August 2019).
- Frayling I, Berry I, Wallace A, Payne S, Norbury G. ACGS Best Practice Guidelines for Genetic Testing and Diagnosis of Lynch Syndrome n.d. www.acgs.uk.com/media/10774/ls_bpg_approved.pdf (accessed 6 August 2019).
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- University of Bristol . QUADAS-2 2021. www.bristol.ac.uk/media-library/sites/quadas/migrated/documents/quadas2.pdf (accessed 4 May 2021).
- Whiting PF, Rutjes AW, Westwood ME, Mallett S. QUADAS-2 Steering Group . A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol 2013;66:1093-104. https://doi.org/10.1016/j.jclinepi.2013.05.014.
- Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209-12. https://doi.org/10.1080/01621459.1927.10502953.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care n.d. www.york.ac.uk/crd/guidance/ (accessed 19 October 2019).
- Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. Cochrane Methods 2016. Cochrane Database Syst Rev. 2016.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. https://doi.org/10.1007/s10198-013-0471-6.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Snowsill TM, Ryan NAJ, Crosbie EJ, Frayling IM, Evans DG, Hyde CJ. Cost-effectiveness analysis of reflex testing for Lynch syndrome in women with endometrial cancer in the UK setting. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0221419.
- Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. https://doi.org/10.1016/S0016-5085(00)70168-5.
- Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med 2020;22:15-2. https://doi.org/10.1038/s41436-019-0596-9.
- Cancer Research UK . Uterine Cancer Survival Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/survival (accessed 2 January 2020).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081-7. https://doi.org/10.1016/S0140-6736(11)61049-0.
- NHS Improvement . National Schedule of Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed 2 January 2020).
- Ryan NAJ, Davison NJ, Payne K, Cole A, Evans DG, Crosbie EJ. A micro-costing study of screening for Lynch syndrome-associated pathogenic variants in an unselected endometrial cancer population: cheap as NGS chips?. Front Oncol 2019;9. https://doi.org/10.3389/fonc.2019.00061.
- Anagnostopoulos A, McKay VH, Cooper I, Campbell F, Greenhalgh L, Kirwan J. Identifying Lynch syndrome in women presenting with endometrial carcinoma under the age of 50 years. Int J Gynecol Cancer 2017;27:931-7. https://doi.org/10.1097/IGC.0000000000000962.
- Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol 2009;114:486-90. https://doi.org/10.1016/j.ygyno.2009.05.026.
- Baldinu P, Cossu A, Manca A, Satta MP, Pisano M, Casula M, et al. Microsatellite instability and mutation analysis of candidate genes in unselected sardinian patients with endometrial carcinoma. Cancer 2002;94:3157-68. https://doi.org/10.1002/cncr.10606.
- Berends MJ, Wu Y, Sijmons RH, van der Sluis T, Ek WB, Ligtenberg MJ, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 2003;21:4364-70. https://doi.org/10.1200/JCO.2003.04.094.
- Bruegl AS, Ring KL, Daniels M, Fellman BM, Urbauer DL, Broaddus RR. Clinical challenges associated with universal screening for Lynch syndrome-associated endometrial cancer. Cancer Prev Res 2017;10:108-15. https://doi.org/10.1158/1940-6207.CAPR-16-0219.
- Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol 2014;32:90-100. https://doi.org/10.1200/JCO.2013.51.2129.
- Carnevali I, Libera L, Chiaravalli A, Sahnane N, Furlan D, Viel A, et al. Somatic testing on gynecological cancers improve the identification of Lynch syndrome. Int J Gynecol Cancer 2017;27:1543-9. https://doi.org/10.1097/IGC.0000000000001010.
- Chao X, Li L, Wu M, Ma S, Tan X, Zhong S, et al. Comparison of screening strategies for Lynch syndrome in patients with newly diagnosed endometrial cancer: a prospective cohort study in China. Cancer Commun 2019;39. https://doi.org/10.1186/s40880-019-0388-2.
- Dillon JL, Gonzalez JL, DeMars L, Bloch KJ, Tafe LJ. Universal screening for Lynch syndrome in endometrial cancers: frequency of germline mutations and identification of patients with Lynch-like syndrome. Hum Pathol 2017;70:121-8. https://doi.org/10.1016/j.humpath.2017.10.022.
- Dudley B, Brand RE, Thull D, Bahary N, Nikiforova MN, Pai RK. Germline MLH1 mutations are frequently identified in Lynch syndrome patients with colorectal and endometrial carcinoma demonstrating isolated loss of PMS2 immunohistochemical expression. Am J Surg Pathol 2015;39:1114-20. https://doi.org/10.1097/PAS.0000000000000425.
- Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sánchez-Heras AB, et al. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0079737.
- Ferguson SE, Aronson M, Pollett A, Eiriksson LR, Oza AM, Gallinger S, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer 2014;120:3932-9. https://doi.org/10.1002/cncr.28933.
- Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol 2015;33:4301-8. https://doi.org/10.1200/JCO.2015.63.9518.
- Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A 2003;100:5908-13. https://doi.org/10.1073/pnas.1030231100.
- Kato A, Sato N, Sugawara T, Takahashi K, Kito M, Makino K, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol 2016;40:770-6. https://doi.org/10.1097/PAS.0000000000000606.
- Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol 2019;37:286-95. https://doi.org/10.1200/JCO.18.00283.
- Leenen CH, van Lier MG, van Doorn HC, van Leerdam ME, Kooi SG, de Waard J, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer ≤ 70 years. Gynecol Oncol 2012;125:414-20. https://doi.org/10.1016/j.ygyno.2012.01.049.
- Libera L, Sahnane N, Carnevali IW, Cimetti L, Cerutti R, Chiaravalli AM, et al. Microsatellite analysis of sporadic and hereditary gynaecological cancer in routine diagnostics. J Clin Pathol 2017;70:792-7. https://doi.org/10.1136/jclinpath-2017-204348.
- Lin DI, Hecht JL. Targeted screening with combined age- and morphology-based criteria enriches detection of Lynch syndrome in endometrial cancer. Int J Surg Pathol 2016;24:297-305. https://doi.org/10.1177/1066896916629782.
- Mas-Moya J, Dudley B, Brand RE, Thull D, Bahary N, Nikiforova MN, et al. Clinicopathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum Pathol 2015;46:1616-25. https://doi.org/10.1016/j.humpath.2015.06.022.
- Masuda K, Banno K, Hirasawa A, Yanokura M, Tsuji K, Kobayashi Y, et al. Relationship of lower uterine segment cancer with Lynch syndrome: a novel case with an hMLH1 germline mutation. Oncol Rep 2012;28:1537-43. https://doi.org/10.3892/or.2012.2008.
- McConechy MK, Talhouk A, Li-Chang HH, Leung S, Huntsman DG, Gilks CB, et al. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol 2015;137:306-10. https://doi.org/10.1016/j.ygyno.2015.01.541.
- Mercado RC, Hampel H, Kastrinos F, Steyerberg E, Balmana J, Stoffel E, et al. Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med 2012;14:670-80. https://doi.org/10.1038/gim.2012.18.
- Millar AL, Pal T, Madlensky L, Sherman C, Temple L, Mitri A, et al. Mismatch repair gene defects contribute to the genetic basis of double primary cancers of the colorectum and endometrium. Hum Mol Genet 1999;8:823-9. https://doi.org/10.1093/hmg/8.5.823.
- Modica I, Soslow RA, Black D, Tornos C, Kauff N, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol 2007;31:744-51. https://doi.org/10.1097/01.pas.0000213428.61374.06.
- Nagle CM, O’Mara TA, Tan Y, Buchanan DD, Obermair A, Blomfield P, et al. Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol 2018;29. https://doi.org/10.3802/jgo.2018.29.e39.
- Najdawi F, Crook A, Maidens J, McEvoy C, Fellowes A, Pickett J, et al. Lessons learnt from implementation of a Lynch syndrome screening program for patients with gynaecological malignancy. Pathology 2017;49:457-64. https://doi.org/10.1016/j.pathol.2017.05.004.
- Ollikainen M, Abdel-Rahman WM, Moisio AL, Lindroos A, Kariola R, Jarvela I, et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome?. J Clin Oncol 2005;23:4609-16. https://doi.org/10.1200/JCO.2005.06.055.
- Pecorino B, Rubino C, Guardalà VF, Galia A, Scollo P. Genetic screening in young women diagnosed with endometrial cancer. J Gynecol Oncol 2017;28. https://doi.org/10.3802/jgo.2017.28.e4.
- Planck M, Rambech E, Möslein G, Müller W, Olsson H, Nilbert M. High frequency of microsatellite instability and loss of mismatch-repair protein expression in patients with double primary tumors of the endometrium and colorectum. Cancer 2002;94:2502-10. https://doi.org/10.1002/cncr.10501.
- Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, et al. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol 2016;29:1381-9. https://doi.org/10.1038/modpathol.2016.135.
- Rubio I, Ibáñez-Feijoo E, Andrés L, Aguirre E, Balmaña J, Blay P, et al. Analysis of Lynch syndrome mismatch repair genes in women with endometrial cancer. Oncology 2016;91:171-6. https://doi.org/10.1159/000447972.
- Salvador MU, Truelson MRF, Mason C, Souders B, LaDuca H, Dougall B, et al. Comprehensive paired tumor/germline testing for Lynch syndrome: bringing resolution to the diagnostic process. J Clin Oncol 2019;37:647-57. https://doi.org/10.1200/JCO.18.00696.
- Sarode VR, Robinson L. Screening for Lynch syndrome by immunohistochemistry of mismatch repair proteins: significance of indeterminate result and correlation with mutational studies. Arch Pathol Lab Med 2019;143:1225-33. https://doi.org/10.5858/arpa.2018-0201-OA.
- Shin SH, Yu EJ, Lee YK, Song YS, Seong MW, Park SS. Characteristics of hereditary nonpolyposis colorectal cancer patients with double primary cancers in endometrium and colorectum. Obstet Gynecol Sci 2015;58:112-16. https://doi.org/10.5468/ogs.2015.58.2.112.
- Stelloo E, Jansen AML, Osse EM, Nout RA, Creutzberg CL, Ruano D, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol 2017;28:96-102. https://doi.org/10.1093/annonc/mdw542.
- Strazzullo M, Cossu A, Baldinu P, Colombino M, Satta MP, Tanda F, et al. High-resolution methylation analysis of the hMLH1 promoter in sporadic endometrial and colorectal carcinomas. Cancer 2003;98:1540-6. https://doi.org/10.1002/cncr.11651.
- Svampane L, Strumfa I, Berzina D, Svampans M, Miklasevics E, Gardovskis J. Epidemiological analysis of hereditary endometrial cancer in a large study population. Arch Gynecol Obstet 2014;289:1093-9. https://doi.org/10.1007/s00404-013-3074-7.
- Takahashi K, Sato N, Sugawara T, Kato A, Sato T, Shimizu D, et al. Clinical characteristics of Lynch-like cases collaterally classified by Lynch syndrome identification strategy using universal screening in endometrial cancer. Gynecol Oncol 2017;147:388-95. https://doi.org/10.1016/j.ygyno.2017.08.016.
- Tian W, Bi R, Ren Y, He H, Shi S, Shan B, et al. Screening for hereditary cancers in patients with endometrial cancer reveals a high frequency of germline mutations in cancer predisposition genes. Int J Cancer 2019;145:1290-8. https://doi.org/10.1002/ijc.32389.
- Wang Y, Shi C, Eisenberg R, Vnencak-Jones CL. Differences in microsatellite instability profiles between endometrioid and colorectal cancers: a potential cause for false-negative results?. J Mol Diagn 2017;19:57-64. https://doi.org/10.1016/j.jmoldx.2016.07.008.
- Yoon SN, Ku JL, Shin YK, Kim KH, Choi JS, Jang EJ, et al. Hereditary nonpolyposis colorectal cancer in endometrial cancer patients. Int J Cancer 2008;122:1077-81. https://doi.org/10.1002/ijc.22986.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. https://doi.org/10.1016/S0016-5085(99)70510-X.
- de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665-71. https://doi.org/10.1053/j.gastro.2005.11.032.
- Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 2009;27:4793-7. https://doi.org/10.1200/JCO.2009.23.7784.
- Ketabi Z, Gerdes AM, Mosgaard B, Ladelund S, Bernstein I. The results of gynecologic surveillance in families with hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2014;133:526-30. https://doi.org/10.1016/j.ygyno.2014.03.012.
- Renkonen-Sinisalo L, Bützow R, Leminen A, Lehtovirta P, Mecklin JP, Järvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer 2007;120:821-4. https://doi.org/10.1002/ijc.22446.
- Macrae F. A Randomised Double Blind Dose Non-Inferiority Trial of a Daily Dose of 600 mg Versus 300 mg Versus 100 mg of Enteric Coated Aspirin As a Cancer Preventive in Carriers of a Germline Pathological Mismatch Repair Gene Defect. Lynch Syndrome. Project 3 in the Cancer Prevention Programme (CaPP3) n.d. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000804381 (accessed 2 January 2020).
- Arber N. A Randomised Double Blind Dose Non-Inferiority Trial of a Daily Dose of 600 mg Versus 300 mg Versus 100 mg of Enteric Coated Aspirin As a Cancer Preventive in Carriers of a Germline Pathological Mismatch Repair Gene Defect. Lynch Syndrome n.d. https://clinicaltrials.gov/ct2/show/nct02497820 (accessed 2 January 2020).
- Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med 2019;21:2167-80. https://doi.org/10.1038/s41436-019-0536-8.
- Resnick KE, Backes F, Hampel H, Matthews KS, Straughn JM, Cohn DE. A cost-effectiveness analysis of lynch syndrome screening among newly diagnosed endometrial cancer patients. Gynecol Oncol 2009;112.
- Kwon JS, Scott JL, Gilks CB, Daniels MS, Sun CC, Lu KH. Testing women with endometrial cancer to detect Lynch syndrome. J Clin Oncol 2011;29:2247-52. https://doi.org/10.1200/JCO.2010.32.9979.
- Bruegl AS, Djordjevic B, Rajyalakshmi L, Lu KH, Sun CCL, Broaddus R. Cost analysis comparing universal tumor testing to clinically based criteria in the evaluation of endometrial adenocarcinomas for Lynch syndrome. Gynecol Oncol 2014;133. https://doi.org/10.1016/j.ygyno.2014.03.128.
- Goverde A, Spaander MC, van Doorn HC, Dubbink HJ, van den Ouweland AM, Tops CM, et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol Oncol 2016;143:453-9. https://doi.org/10.1016/j.ygyno.2016.10.008.
- Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 2007;107:159-62. https://doi.org/10.1016/j.ygyno.2007.09.031.
- Arrigoni A, Sprujevnik T, Alvisi V, Rossi A, Ricci G, Pennazio M, et al. Clinical identification and long-term surveillance of 22 hereditary non-polyposis colon cancer Italian families. Eur J Gastroenterol Hepatol 2005;17:213-9. https://doi.org/10.1097/00042737-200502000-00013.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programme. Oxford: Oxford University Press; 2005.
- Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S. Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8120.
- Thorn J, Coast J, Andronis L. Interpretation of the expected value of perfect information and research recommendations: a systematic review and empirical investigation. Med Decis Making 2016;36:285-95. https://doi.org/10.1177/0272989X15586552.
- Snowsill T, Huxley N, Hoyle M, Jones-Hughes T, Coelho H, Cooper C, et al. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18580.
- Barrow P. Hereditary Colorectal Cancer: Registration, Screening and Prognostic Biomarker Analysis 2014.
- Menko FH, Ter Stege JA, van der Kolk LE, Jeanson KN, Schats W, Moha DA, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer 2019;18:127-35. https://doi.org/10.1007/s10689-018-0089-z.
- Bruwer Z, Futter M, Ramesar R. Communicating cancer risk within an African context: experiences, disclosure patterns and uptake rates following genetic testing for Lynch syndrome. Patient Educ Couns 2013;92:53-60. https://doi.org/10.1016/j.pec.2013.02.001.
- Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 2017;66:1657-64. https://doi.org/10.1136/gutjnl-2016-311403.
- Seppälä TT, Ahadova A, Dominguez-Valentin M, Macrae F, Evans DG, Therkildsen C, et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered Cancer Clin Pract 2019;17. https://doi.org/10.1186/s13053-019-0106-8.
- Seppälä T, Pylvänäinen K, Evans DG, Järvinen H, Renkonen-Sinisalo L, Bernstein I, et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: a Prospective Lynch Syndrome Database report. Hered Cancer Clin Pract 2017;15. https://doi.org/10.1186/s13053-017-0078-5.
- Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet 2014;46:107-15. https://doi.org/10.1038/ng.2854.
- UK Genetic Testing Network . Colorectal Cancer, Hereditary Nonpolyposis and Lynch Syndrome 2018. https://ukgtn.nhs.uk/find-a-test/search-by-disorder-gene/colorectal-cancer-hereditary-nonpolyposis-and-lynch-syndrome-534/ (accessed 14 December 2019).
- Slade I, Hanson H, George A, Kohut K, Strydom A, Wordsworth S, et al. MCG programme . A cost analysis of a cancer genetic service model in the UK. J Community Genet 2016;7:185-94. https://doi.org/10.1007/s12687-016-0266-4.
- Barrow P, Green K, Clancy T, Lalloo F, Hill J, Evans DG. Improving the uptake of predictive testing and colorectal screening in Lynch syndrome: a regional primary care survey. Clin Genet 2015;87:517-24. https://doi.org/10.1111/cge.12559.
- Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med 2006;354:2751-63. https://doi.org/10.1056/NEJMoa053493.
- Heald B, Plesec T, Liu X, Pai R, Patil D, Moline J, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing Lynch syndrome in a large academic medical center. J Clin Oncol 2013;31:1336-40. https://doi.org/10.1200/JCO.2012.45.1674.
- Tutlewska K, Lubinski J, Kurzawski G. Germline deletions in the EPCAM gene as a cause of Lynch syndrome – literature review. Hered Cancer Clin Pract 2013;11. https://doi.org/10.1186/1897-4287-11-9.
- Mersch J, Brown N, Pirzadeh-Miller S, Mundt E, Cox HC, Brown K, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA 2018;320:1266-74. https://doi.org/10.1001/jama.2018.13152.
- Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet 2020;395:1855-63. https://doi.org/10.1016/S0140-6736(20)30366-4.
- Watkins JC, Yang EJ, Muto MG, Feltmate CM, Berkowitz RS, Horowitz NS, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch syndrome and Lynch-like syndrome. Int J Gynecol Pathol 2017;36:115-27. https://doi.org/10.1097/PGP.0000000000000312.
Appendix 1 Literature search strategies
Clinical effectiveness
The following table presents a summary of bibliographic database searches.
| Database | Date of search | Number of records |
|---|---|---|
| MEDLINE (via Ovid) | 7 August 2019 | 1557 |
| EMBASE (via Ovid) | 7 August 2019 | 2775 |
| The Cochrane Library | 8 August 2019 | 36 |
| DARE and HTA Database | 8 August 2019 | 7 |
| Science Citation Index and Conference Proceedings Citation Index – Science (via Web of Science) | 8 August 2019 | 1874 |
| PROSPERO | 28 August 2019 | 10 |
Total number of records from database searches: 6259.
MEDLINE (via Ovid)
Database: MEDLINE(R) ALL (via Ovid).
Date range searched: 1946 to 6 August 2019.
Date searched: 7 August 2019.
Search strategy
-
uterine neoplasms/ (40,281)
-
exp endometrial neoplasms/ (20,609)
-
((uter* or endomet* or womb) adj4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)).ti,ab,kf. (66,373)
-
1 or 2 or 3 (92,254)
-
exp Colorectal Neoplasms, Hereditary Nonpolyposis/ (4407)
-
(lynch* adj3 syndrome*).ti,ab,kf. (2951)
-
((lynch* adj3 famil*) and (cancer* or neoplasm*)).ti,ab,kf. (360)
-
(((familial or hereditary or inherit*) adj3 (colon* or colorectal*)) and (cancer or neoplasm*)).ti,ab,kf. (4589)
-
(((hereditary or familial) adj3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)).ti,ab,kf. (3199)
-
((hereditary adj3 (cancer or neoplasm*)) and (colon* or colorectal*)).ti,ab,kf. (2886)
-
(familial adj3 (colon* or colorectal*)).ti,ab,kf. (1169)
-
HNPCC.ti,ab,kf. (2234)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (8122)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6).ti,ab,kf. (9664)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary).ti,ab,kf. (613,827)
-
14 and 15 (4480)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) adj3 (germline or DNA* or gene* or mutation* or deficienc*)).ti,ab,kf. (8308)
-
Amsterdam criteria.ti,ab,kf. (413)
-
13 or 16 or 17 or 18 (14,227)
-
4 and 19 (1557)
EMBASE (via Ovid)
Database: EMBASE Classic plus EMBASE.
Date range searched: 1947 to 6 August 2019.
Date searched: 7 August 2019.
Search strategy
-
uterus cancer/or exp endometrium cancer/or uterus carcinoma/or uterus sarcoma/ (70,395)
-
((uter* or endomet* or womb) adj4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)).ti,ab,kw. (93,982)
-
1 or 2 (117,308)
-
exp hereditary nonpolyposis colorectal cancer/ (5996)
-
(lynch* adj3 syndrome*).ti,ab,kw. (5263)
-
((lynch* adj3 famil*) and (cancer* or neoplasm*)).ti,ab,kw. (606)
-
(((familial or hereditary or inherit*) adj3 (colon* or colorectal*)) and (cancer or neoplasm*)).ti,ab,kw. (6147)
-
(((hereditary or familial) adj3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)).ti,ab,kw. (4026)
-
((hereditary adj3 (cancer or neoplasm*)) and (colon* or colorectal*)).ti,ab,kw. (4065)
-
(familial adj3 (colon* or colorectal*)).ti,ab,kw. (1590)
-
HNPCC.ti,ab,kw. (3206)
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (12,444)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6).ti,ab,kw. (16,365)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary).ti,ab,kw. (852,547)
-
13 and 14 (7503)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) adj3 (germline or DNA* or gene* or mutation* or deficienc*)).ti,ab,kw. (12,188)
-
Amsterdam criteria.ti,ab,kw. (627)
-
12 or 15 or 16 or 17 (21,665)
-
3 and 18 (2775)
Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (both via Wiley Online Library)
Date searched: 8 August 2019.
Date range searched: inception to 8 August 2019.
Search strategy
#1 MeSH descriptor: [Uterine Neoplasms] this term only (708)
#2 MeSH descriptor: [Endometrial Neoplasms] explode all trees (537)
#3 ((uter* or endomet* or womb) near/4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)):ti,ab (3139)
#4 #1 or #2 or #3 (3791)
#5 MeSH descriptor: [Colorectal Neoplasms, Hereditary Nonpolyposis] explode all trees (50)
#6 (lynch* near/3 syndrome*):ti,ab (100)
#7 ((lynch* near/3 famil*) and (cancer* or neoplasm*)):ti,ab (6)
#8 (((familial or hereditary or inherit*) near/3 (colon* or colorectal*)) and (cancer or neoplasm*)):ti,ab (118)
#9 (((hereditary or familial) near/3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)):ti,ab (48)
#10 ((hereditary near/3 (cancer or neoplasm*)) and (colon* or colorectal*)):ti,ab (51)
#11 (familial near/3 (colon* or colorectal*)):ti,ab (63)
#12 HNPCC:ti,ab (43)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 (227)
#14 (EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6):ti,ab (173)
#15 (colon* or colorectal* or lynch* or HNPCC or hereditary):ti,ab (36,712)
#16 #14 and #15 (83)
#17 ((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) near/3 (germline or DNA* or gene* or mutation* or deficienc*)):ti,ab (955)
#18 “Amsterdam criteria”:ti,ab (10)
#19 #13 or #16 or #17 or #18 (1175)
#20 #4 and #19 (36)
Total: 36 –
-
Cochrane Reviews (Cochrane Database of Systematic Reviews): 0.
-
Cochrane Protocols (Cochrane Database of Systematic Reviews): 0.
-
Trials (Cochrane Central Register of Controlled Trials): 36.
Database of Abstracts of Reviews of Effects and Health Technology Assessment Database (both via Centre for Reviews and Dissemination)
Date searched: 8 August 2019.
Date range searched: inception to 8 August 2019.
Search strategy
-
MeSH DESCRIPTOR uterine neoplasms (106)
-
MeSH DESCRIPTOR endometrial neoplasms EXPLODE ALL TREES (138)
-
((uter* or endomet* or womb) ADJ4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) (931)
-
#1 OR #2 OR #3 (931)
-
MeSH DESCRIPTOR Colorectal Neoplasms, Hereditary Nonpolyposis EXPLODE ALL TREES (37)
-
(lynch* ADJ3 syndrome*) (20)
-
((lynch* ADJ3 famil*) and (cancer* or neoplasm*)) (1)
-
(((familial or hereditary or inherit*) ADJ3 (colon* or colorectal*)) AND (cancer or neoplasm*)) (37)
-
(((hereditary or familial) ADJ3 (nonpolyposis or non-polyposis)) AND (colon* or colorectal*)) (50)
-
((hereditary ADJ3 (cancer or neoplasm*)) AND (colon* or colorectal*)) (33)
-
(familial ADJ3 (colon* or colorectal*)) (4)
-
(HNPCC) (16)
-
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (61)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) (15)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary) (3070)
-
#14 AND #15 (13)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) ADJ3 (germline or DNA* or gene* or mutation* or deficienc*)) (17)
-
(Amsterdam criteria) (6)
-
#13 OR #16 OR #17 OR #18 (68)
-
#4 AND #19 (14)
Total: 14 –
-
Database of Abstracts of Reviews of Effects: 1
-
HTA Database: 6
-
NHS EED: 7.
Science Citation Index and Conference Proceedings Citation Index – Science (via Web of Science)
Date searched: 8 August 2019.
Date range searched: inception to 8 August 2019.
| Number | Hits (n) | Search strategy |
|---|---|---|
| # 16 | 1874 |
#15 AND #1 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 15 | 17,327 |
#14 OR #13 OR #12 OR #9 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 14 | 426 |
TS = “Amsterdam criteria” Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 13 | 10,712 |
TS = ((“mismatch repair*” or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) NEAR/3 (germline or DNA* or gene* or mutation* or deficienc*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 12 | 5532 |
#11 AND #10 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 11 | 830,834 |
TS = (colon* or colorectal* or lynch* or HNPCC or hereditary) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 10 | 8611 |
TS = (EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 9 | 9323 |
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 8 | 2875 |
TS = HNPCC Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 7 | 1394 |
TS = (familial near/3 (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 6 | 4493 |
TS = (((hereditary) near/3 (cancer or neoplasm*)) and (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 5 | 3198 |
TS = (((hereditary or familial) near/3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 4 | 4967 |
TS = (((familial or hereditary or inherit*) near/3 (colon* or colorectal*)) and (cancer or neoplasm*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 3 | 434 |
TS = ((lynch* near/3 famil*) and (cancer* or neoplasm*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 2 | 4474 |
TS = (lynch* near/3 syndrome*) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
| # 1 | 58,489 |
TS = ((uter* or endomet* or womb) near/4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = All years |
PROSPERO International Prospective Register of Systematic Reviews (via Centre for Reviews and Dissemination)
Date searched: 28 August 2019.
Date range searched: inception to 8 August 2019.
#1 MeSH DESCRIPTOR uterine neoplasms (41)
#2 MeSH DESCRIPTOR endometrial neoplasms EXPLODE ALL TREES (48)
#3 ((uter* or endomet* or womb) ADJ4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) (231)
#4 #1 OR #2 OR #3 (256)
#5 MeSH DESCRIPTOR Colorectal Neoplasms, Hereditary Nonpolyposis EXPLODE ALL TREES (13)
#6 (lynch* ADJ3 syndrome*) (28)
#7 ((lynch* ADJ3 famil*) and (cancer* or neoplasm*)) (6)
#8 (((familial or hereditary or inherit*) ADJ3 (colon* or colorectal*)) AND (cancer or neoplasm*)) (29)
#9 (((hereditary or familial) ADJ3 (nonpolyposis or non-polyposis)) AND (colon* or colorectal*)) (24)
#10 ((hereditary ADJ3 (cancer or neoplasm*)) AND (colon* or colorectal*)) (26)
#11 (familial ADJ3 (colon* or colorectal*)) (6)
#12 (HNPCC) (17)
#13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (53)
#14 (EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) (14)
#15 (colon* or colorectal* or lynch* or HNPCC or hereditary) (1756)
#16 #14 AND #15 (14)
#17 ((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) ADJ3 (germline or DNA* or gene* or mutation* or deficienc*)) (15)
#18 (Amsterdam criteria) (3)
#19 #13 OR #16 OR #17 OR #18 (60)
#20 #4 AND #19 (10)
Cost-effectiveness
The following table presents a summary of the bibliographic database searches.
| Database | Date of search | Number of records |
|---|---|---|
| MEDLINE (via Ovid) | 28 August 2019 | 1105 |
| EMBASE (via Ovid) | 29 August 2019 | 2209 |
| NHS EED and HTA | 30 August 2019 | 49 |
| Science Citation Index and Conference Proceedings Citation Index – Science (via Web of Science) | 30 August 2019 | 1267 |
| CEA Registry | 30 August 2019 | 30 |
| EconPapers (RePEc) | 30 August 2019 | 13 |
| ScHARRHUD | 30 August 2019 | 8 |
Total number of records from database searches: 4681.
MEDLINE (via Ovid)
Database: MEDLINE(R) ALL (via Ovid).
Date range searched: 1946 to 27 August 2019.
Date searched: 28 August 2019.
Search strategy
-
uterine neoplasms/ (40,333)
-
exp endometrial neoplasms/ (20,699)
-
((uter* or endomet* or womb) adj4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)).ti,ab,kf. (66,492)
-
1 or 2 or 3 (92,409)
-
exp Colorectal Neoplasms, Hereditary Nonpolyposis/ (4418)
-
(lynch* adj3 syndrome*).ti,ab,kf. (2974)
-
((lynch* adj3 famil*) and (cancer* or neoplasm*)).ti,ab,kf. (363)
-
(((familial or hereditary or inherit*) adj3 (colon* or colorectal*)) and (cancer or neoplasm*)).ti,ab,kf. (4594)
-
(((hereditary or familial) adj3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)).ti,ab,kf. (3204)
-
((hereditary adj3 (cancer or neoplasm*)) and (colon* or colorectal*)).ti,ab,kf. (2896)
-
(familial adj3 (colon* or colorectal*)).ti,ab,kf. (1169)
-
HNPCC.ti,ab,kf. (2239)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (8147)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6).ti,ab,kf. (9697)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary).ti,ab,kf. (615,131)
-
14 and 15 (4492)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) adj3 (germline or DNA* or gene* or mutation* or deficienc*)).ti,ab,kf. (8336)
-
Amsterdam criteria.ti,ab,kf. (412)
-
13 or 16 or 17 or 18 (14,276)
-
exp Immunohistochemistry/ (588,192)
-
(immunohistochemistry or (IHC adj3 test*)).ti,ab,kf. (178,647)
-
Microsatellite Instability/ (2896)
-
((microsatellite adj3 instabilit*) or (msi adj3 test*)).ti,ab,kf. (7390)
-
20 or 21 or 22 or 23 (692,837)
-
exp Economics/ (582,592)
-
exp “Costs and Cost Analysis”/ (227,344)
-
Health Status/ (77,617)
-
exp “Quality of Life”/ (180,175)
-
exp Quality-Adjusted Life Years/ (11,281)
-
(pharmacoeconomic* or pharmaco-economic* or economic* or cost* or price or prices or pricing).ti,ab,kf. (790,706)
-
(expenditure$ not energy).ti,ab,kf. (28,328)
-
(value adj1 money).ti,ab,kf. (33)
-
budget*.ti,ab,kf. (27,980)
-
(health state* or health status).ti,ab,kf. (60,417)
-
(qaly* or ICER or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or short-form 36 or shortform 36 or SF-36 or SF36 or SF-6D or SF6D or SF-12 or SF12 or health utilities index or HUI).ti,ab,kf. (235,497)
-
(markov or time trade off or TTO or standard gamble or SG or hrql or hrqol or disabilit* or disutilit* or net benefit or contingent valuation).ti,ab,kf. (226,798)
-
(quality adj2 life).ti,ab,kf. (262,642)
-
(decision adj2 model).ti,ab,kf. (6437)
-
(visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).ti,ab,kf. (58,078)
-
resource*.ti,ab,kf. (312,093)
-
(well-being or wellbeing).ti,ab,kf. (82,618)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 (2,166,732)
-
19 and 42 (880)
-
4 and 24 and 42 (277)
-
43 or 44 (1105)
EMBASE (via Ovid)
Database: EMBASE Classic+EMBASE.
Date range searched: 1947 to 2019 Week 34.
Date searched: 29 August 2019.
Search strategy
-
uterus cancer/ (20,062)
-
exp endometrium cancer/ (48,235)
-
((uter* or endomet* or womb) adj4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)).ti,ab,kw. (94,282)
-
1 or 2 or 3 (116,223)
-
exp hereditary nonpolyposis colorectal cancer/ (6076)
-
(lynch* adj3 syndrome*).ti,ab,kw. (5337)
-
((lynch* adj3 famil*) and (cancer* or neoplasm*)).ti,ab,kw. (615)
-
(((familial or hereditary or inherit*) adj3 (colon* or colorectal*)) and (cancer or neoplasm*)).ti,ab,kw. (6160)
-
(((hereditary or familial) adj3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)).ti,ab,kw. (4028)
-
((hereditary adj3 (cancer or neoplasm*)) and (colon* or colorectal*)).ti,ab,kw. (4083)
-
(familial adj3 (colon* or colorectal*)).ti,ab,kw. (1593)
-
HNPCC.ti,ab,kw. (3210)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (12,545)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6).ti,ab,kw. (16,510)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary).ti,ab,kw. (855,173)
-
14 and 15 (7564)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) adj3 (germline or DNA* or gene* or mutation* or deficienc*)).ti,ab,kw. (12,290)
-
Amsterdam criteria.ti,ab,kw. (630)
-
13 or 16 or 17 or 18 (21,837)
-
exp immunohistochemistry/(591,817)
-
(immunohistochemistry or (IHC adj3 test*)).ti,ab,kw. (285,471)
-
microsatellite instability/(12,199)
-
((microsatellite adj3 instabilit*) or (msi adj3 test*)).ti,ab,kw. (10,785)
-
20 or 21 or 22 or 23 (644,776)
-
exp health economics/ (829,976)
-
exp health status/ (230,300)
-
exp “quality of life”/ (475,637)
-
exp quality adjusted life year/ (24,485)
-
(pharmacoeconomic* or pharmaco-economic* or economic* or cost* or price or prices or pricing).ti,ab,kw. (1,044,110)
-
(expenditure* not energy).ti,ab,kw. (39,410)
-
(value adj2 money).ti,ab,kw. (2333)
-
budget*.ti,ab,kw. (37,547)
-
(health state* or health status).tw. (79,129)
-
(qaly* or ICER or utilit* or EQ5D or EQ-5D or euroqol or euro-qol or short-form 36 or shortform 36 or SF-36 or SF36 or SF-6D or SF6D or SF-12 or SF12 or health utilities index or HUI).ti,ab,kw. (342,112)
-
(markov or time trade off or TTO or standard gamble or SG or hrql or hrqol or disabilit* or disutilit* or net benefit or contingent valuation).ti,ab,kw. (331,686)
-
(quality adj2 life).tw. (411,142)
-
(decision adj2 model).tw. (9764)
-
(visual analog* scale* or discrete choice experiment* or health* year* equivalen* or (willing* adj2 pay)).tw. (83,448)
-
resource*.ti,ab,kw. (401,756)
-
(well-being or wellbeing).tw. (107,606)
-
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (3,041,975)
-
19 and 41 (1824)
-
4 and 24 and 41 (541)
-
42 or 43 (2209)
NHS Economic Evaluation Database (via the Centre for Reviews and Disseminations)/Health Technology Assessment database (via the Centre for Reviews and Disseminations)
Date searched: 30 August 2019.
Date range searched: inception to 8 August 2019.
Search strategy
-
MeSH DESCRIPTOR uterine neoplasms (106)
-
MeSH DESCRIPTOR endometrial neoplasms EXPLODE ALL TREES (138)
-
((uter* or endomet* or womb) ADJ4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) (931)
-
#1 OR #2 OR #3 (931)
-
MeSH DESCRIPTOR Colorectal Neoplasms, Hereditary Nonpolyposis EXPLODE ALL TREES (37)
-
(lynch* ADJ3 syndrome*) (20)
-
((lynch* ADJ3 famil*) and (cancer* or neoplasm*)) (1)
-
(((familial or hereditary or inherit*) ADJ3 (colon* or colorectal*)) AND (cancer or neoplasm*)) (37)
-
(((hereditary or familial) ADJ3 (nonpolyposis or non-polyposis)) AND (colon* or colorectal*)) (50)
-
((hereditary ADJ3 (cancer or neoplasm*)) AND (colon* or colorectal*)) (33)
-
(familial ADJ3 (colon* or colorectal*)) (4)
-
(HNPCC) (16)
-
#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 (61)
-
(EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) (15)
-
(colon* or colorectal* or lynch* or HNPCC or hereditary) (3070)
-
#14 AND #15 (13)
-
((mismatch repair* or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) ADJ3 (germline or DNA* or gene* or mutation* or deficienc*)) (17)
-
(Amsterdam criteria) (6)
-
#13 OR #16 OR #17 OR #18 (68)
-
MeSH DESCRIPTOR Immunohistochemistry EXPLODE ALL TREES (248)
-
((immunohistochemistry or (IHC adj3 test*))) (123)
-
MeSH DESCRIPTOR Microsatellite Instability (8)
-
(((microsatellite adj3 instabilit*) or (msi adj3 test*))) (22)
-
#20 OR #21 OR #22 OR #23 (294)
-
#4 AND #24 (15)
-
#19 OR #25 (75)
-
(#26) IN NHSEED, HTA (49)
HTA Database: 22.
NHS EED: 27.
Science Citation Index and Conference Proceedings Citation Index – Science (via Web of Science)
Date searched: 30 August 2019.
Date range searched: inception to 8 August 2019.
| Number | Hits (n) | Search strategy |
|---|---|---|
| # 22 | 1267 |
#21 AND #20 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 21 | 3,347,032 |
TS = (“quality of life” or qol or hrql or hrqol or (“quality adjusted life” NEAR/0 year*) or qaly* or icer or cost* or economic* or pharmacoeconomic* or pharmaco-economic* or price or prices or pricing or (expenditure* not energy) or (value NEAR/1 money) or budget* or euro-qol or utilit* or disutilit* or (net NEAR/0 benefit*) or (contingent NEAR/0 valuation*) or euroqol or “euro qol” or eq5d or eq-5d or “short-form 36” or “shortform 36” or sf-36 or sf36 or sf-6d or sf6d or sf-12 or sf12 or “health utilities index” or hui or (time NEAR/0 trade*) or tto or “standard gamble” or sg or markov or (decision NEAR/1 model*) or (visual NEAR/0 analog*) or “discrete choice” or ((health* NEAR/0 year*) NEAR/0 equivalen*) or (health NEAR/0 stat*) or (willing* NEAR/1 pay) or resource* or wellbeing or well-being) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 20 | 21,297 |
#19 OR #15 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 19 | 4936 |
#18 AND #1 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 18 | 203,352 |
#17 OR #16 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 17 | 14,378 |
TS = ((microsatellite NEAR/3 instabilit*) or (msi NEAR/3 test*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 16 | 190,931 |
TS = (immunohistochemistry or (IHC NEAR/3 test*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 15 | 17,441 |
#14 OR #13 OR #12 OR #9 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 14 | 426 |
TS = “Amsterdam criteria” Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 13 | 10,788 |
TS = ((“mismatch repair*” or MMR or EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) NEAR/3 (germline or DNA* or gene* or mutation* or deficienc*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 12 | 5549 |
#11 AND #10 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 11 | 833,058 |
TS = (colon* or colorectal* or lynch* or HNPCC or hereditary) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 10 | 8655 |
TS = (EPCAM? or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 9 | 9375 |
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 8 | 2876 |
TS = HNPCC Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 7 | 1399 |
TS = (familial near/3 (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 6 | 4501 |
TS = (((hereditary) near/3 (cancer or neoplasm*)) and (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 5 | 3203 |
TS = (((hereditary or familial) near/3 (nonpolyposis or non-polyposis)) and (colon* or colorectal*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 4 | 4977 |
TS = (((familial or hereditary or inherit*) near/3 (colon* or colorectal*)) and (cancer or neoplasm*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 3 | 439 |
TS = ((lynch* near/3 famil*) and (cancer* or neoplasm*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 2 | 4520 |
TS = (lynch* near/3 syndrome*) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
| # 1 | 58,807 |
TS = ((uter* or endomet* or womb) near/4 (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900–2019 |
Cost-Effectiveness Analysis Registry
Date searched: 30 August 2019.
Date range searched: inception to 8 August 2019.
-
Basic search: Methods: Lynch Syndrome (10)
-
Basic search: Methods: hereditary non-polyposis (1) (0 unique)
-
Basic search: Methods: Endometrial (24) (20 unique)
Total: 30.
EconPapers (Research Papers in Economics)
Date searched: 30 August 2019.
Date range searched: inception to 8 August 2019.
“lynch syndrome” OR “hereditary non-polyposis” OR “hereditary nonpolyposis” OR HNPCC OR “familial non-polyposis” OR “familial nonpolyposis” OR “familial colorectal” OR “hereditary colorectal” OR “familial colon” OR “hereditary colon” OR ((“mismatch repair” or MMR or EPCAM* or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6) AND (germline or DNA or gene or genetic or genetics or mutation* or deficienc*)) OR “Amsterdam criteria” OR ((endometri* OR uter* OR womb) AND (microsatellite OR MSI OR immunohistochemistry OR IHC)) (13)
School of Health and Related Research Health Utilities Database
Date searched: 30 August 2019.
Date range searched: inception to 8 August 2019.
(lynch* OR familial OR hereditary OR mismatch repair or MMR or EPCAM* or MLH1 or MSH2 or MSH6 or PMS2 or hMSH2 or hMLH1 or hPMS2 or hMSH6 or amsterdam criteria) OR ((endometri* OR uter* OR womb) and (neoplas* or cancer* or carcinom* or adenocarcinom* or tumour* or tumor* or malignan* or dysplasis* or disease* or adenocanthom* or sarcom*)) (8)
Appendix 2 Data extraction and quality appraisal forms
Data extraction form for clinical effectiveness studies
Quality appraisal tools for clinical effectiveness studies
QUADAS-2 tool reproduced with permission from the University of Bristol. 35
Data extraction for economic evaluation studies
Appendix 3 Excluded studies with rationale
| Reference | Reason for exclusion |
|---|---|
| Question 1 | |
| 1. Abbaszadegan MR, Asadzadeh H, Rastin F, Dadkhah E. Microsatellite instability in young women with endometrioid type endometrial cancer. Iran J Public Health 2009;38:24–30 | No reference standard |
| 2. Adams R, Geiersbach K, Tripp S, Samowitz W. Unusual immunohistochemistry staining patterns encountered in cancers screened for Lynch syndrome. Lab Invest 2015;1:144A | Not enough information to quality appraise – abstract |
| 3. Adán-Merino L, Aldeguer-Martínez M, Alonso-Gamarra E, Valentín-Gómez F, Zaera-De la Fuente C, Martín-Chávarri S. Diagnosis and clinical behavior in patients with Lynch-like syndrome. Rev Gastroenterol Mex 2018;83:470–4 | Wrong population |
| 4. Adar T, Rodgers LH, Shannon KM, Yoshida M, Ma T, Mattia A, et al. Enhancing the identification of Lynch syndrome through universal screening of both endometrial and colon cancers. Gastroenterology 2017;152(Suppl. 1):S178 | Not enough information to quality appraise – abstract |
| 5. Affolter K, Wilson A, Samowitz W, Geiersbach K. Base pair changes in assessing microsatellite instability and correlation to mismatch repair status by immunohistochemistry. Lab Invest 2013;1:141A | Not enough information to quality appraise – abstract |
| 6. Aguirre E, Mele M, Tuset N, Velasco A, Tarragona J, Sampayo M, et al. Screening for Lynch syndrome among endometrial cancer patients less than 60 years. Ann Oncol 2016;27:296–312 | Not enough information to quality appraise – abstract |
| 7. Alenda C, Egoavil C, Soto JL, Castillejo A, Barbera VM, Roman MJ, et al. Prevalence of Lynch syndrome among unselected endometrial cancer patients. Lab Invest 2012;1:258A | Not enough information to quality appraise – abstract |
| 8. AlHilli MM, Carr CE, Priyadarshini A, Radeva M, Marquard J. Predictors of Lynch syndrome and clinical outcomes among universally screened endometrial cancer patients. Gynecol Oncol 2017;145(Suppl. 1):92 | Not enough information to quality appraise – abstract |
| 9. Al-Nourhji O, Zhang G, Zou Y, Biscotti CV, Rose P, Yang B. PD-L1 frequently expressed in endometrial carcinoma associated with mismatch-repair deficiency. Lab Invest 2017;97(Suppl. 1):273A | Not enough information to quality appraise – abstract |
| 10. Andrade C, Mengatto M, Vieira M, Cadamuro M, Palmero E, Oliveira J, et al. Screening endometrial cancer for Lynch syndrome in a Brazilian public health care system cancer center. Gynecol Oncol 2013;130:e100 | Not enough information to quality appraise – abstract |
| 11. Anonymous. Uterine cancer could be harbinger of other cancers. An inherited mutation – Lynch syndrome – may lead to higher risk. Duke Med Health News 2006;12:9–10 | Editorial |
| 12. Anonymous. Abstracts presented for the 40th annual meeting of the Society of Gynecologic Oncologists. Gynecol Oncol 2009;112(Suppl. 1) | Not enough information to quality appraise – abstract |
| 13. Anonymous. StatBite: Lynch syndrome increases the risk of various cancers. J Natl Cancer Inst 2010;102:1383 | Not enough information to quality appraise – abstract |
| 14. Avila M, Alvarado M, Axtell AE, Goff J, Funston JR, Lentz SE. Universal immunohistochemistry testing in endometrial cancer tumors maximizes Lynch syndrome identification among affected individuals. Gynecol Oncol 2019;154:e13 | Not enough information to quality appraise – abstract |
| 15. Ayme A, Arcioni S, Membrez V, Feilchenfeldt J, Fonteneau L, Viassolo V, et al. Systematic screening for Lynch syndrome in a cohort of colorectal and endometrial cancer patients in Switzerland: the SYSSYL study. Fam Cancer 2017;16(1 Suppl. 1):S116 | Not enough information to quality appraise – abstract |
| 16. Backes FJ, Hampel H, Backes KA, Vaccarello L, Lewandowski G, Bell JA, et al. Are prediction models for Lynch syndrome valid for probands with endometrial cancer? Fam Cancer 2009;8:483–7 | Not test accuracy |
| 17. Backman AS, Walton-Bernstedt S, Bjork J. A large proportion of Lynch syndrome patients still undergo genetic screening first in connection with their diagnosis of cancer. Gastroenterology 2016;1:S364 | Not enough information to quality appraise – abstract |
| 18. Baker T, Deihimi S, Martin LP, Hall MJ, Hampel H, El-Deiry WS. Variable DNA mismatch repair-associated gene profiles in colorectal versus uterine cancers. J Clin Oncol 2017;35(Suppl. 1) | Not enough information to quality appraise – abstract |
| 19. Ballester VR, Carrera R, Blazquez C, Casalots A, Ramos MC, Vazquez J, et al. Universal screening for Lynch Syndrome detection. Virchows Arch 2016;469(Suppl. 1):S202 | Not enough information to quality appraise – abstract |
| 20. Banno K, Susumu N, Hirao T, Yanokura M, Hirasawa A, Aoki D, et al. Identification of germline MSH2 gene mutations in endometrial cancer not fulfilling the new clinical criteria for hereditary nonpolyposis colorectal cancer. Cancer Genet Cytogenet 2003;146:58–65 | Ineligible reference standard |
| 21. Banno K, Susumu N, Yanokura M, Hirao T, Iwata T, Hirasawa A, et al. Association of HNPCC and endometrial cancers. Int J Clin Oncol 2004;9:262–9 | Review |
| 22. Barinoff J, Lange J, Brandi C, Schulze C, Riener MO, Aulmann S, et al. HNPCC related endometrial carcinoma: management in the clinical routine. Int J Gynecol Cancer 2016;26(Suppl. 3):125 | Not enough information to quality appraise – abstract |
| 23. Bartley AN, Luthra R, Saraiya D, Broaddus RR. Discordance between molecular and immunohistochemical analyses for Lynch syndrome assessment. Lab Invest 2011;1:144A | Not enough information to quality appraise – abstract |
| 24. Bartosch C, Relvas S, Jeronimo C, Lopes JM. Evaluation of mismatch repair (MMR) protein immunohistochemical expression in endometrial carcinomas. Virchows Arch 2013;463:311–12 | Not enough information to quality appraise – abstract |
| 25. Bats A, Rossi L, Buecher B, Borghese B, Douay-Hauser N, Lecuru F. Clinico-pathological characteristics of endometrial cancer in Lynch syndrome. Int J Gynecol Cancer 2013;1:73 | Not enough information to quality appraise – abstract |
| 26. Batte BA, Bruegl AS, Daniels MS, Ring KL, Dempsey KM, Djordjevic B, et al. Consequences of universal MSI/IHC in screening endometrial cancer patients for Lynch syndrome. Gynecol Oncol 2014;134:319–25 | Authors contacted due to unclear reporting. Authors could not confirm information around testing |
| 27. Beneder C, Vorburger SA, Balli M, Mueller MD. [Is a screening according to the Lynch syndrome meaningful for young patients with endometrium carcinoma.] Geburtshilfe und Frauenheilkunde 2008;68:431 | Foreign-language paper |
| 28. Bennett J, Pesci A, ,Badrinarain J, Da Silva A, Oliva E. Mismatch repair protein expression in endometrioid carcinoma of the ovary: incidence and clinicopathologic associations in 77 cases. Lab Invest 2017;97(Suppl. 1):276A | Not enough information to quality appraise – abstract |
| 29. Benshushan A, Gazit N, Goldberg Y, Peretz T, Zik A. Genetics of endometrial cancer is greater than previously estimated in the our local population. Int J Gynecol Cancer 2017;27(Suppl. 4):100 | Not enough information to quality appraise – abstract |
| 30. Billingsley CC, Cohn D, Mutch DG, Broaddus R, Ramirez N, Lankes H, et al. Clinical implications for MSI, MLH1 methylation analysis and IHC in Lynch screening for endometrial cancer patients: an analysis of 940 endometrioid endometrial cancer cases from the GOG 0210 study. Gynecol Oncol 2015;137:4–5 | Not enough information to quality appraise – abstract |
| 31. Bohiltea RE, Radoi V, Turcan N, Cirstoiu MM. National genetic screening for endometrial cancer. Gineco.eu 2016;12:15–18 | No primary data. Not enough information to quality appraise |
| 32. Boyd J, Kohler MF, Berchuck A, Watson P, Lynch HT, Risinger JI. Microsatellite instability in sporadic endometrial carcinomas and those associated with HNPCC. Am J Hum Genet 1993;53:22 | Not enough information to appraise – abstract |
| 33. Brodsky AL, Lee J, Asgari S, Fehniger J, Levine DA, Pothuri B. Genetic counselor involvement with abnormal immunohistochemistry results improves genetic testing in patients with endometrial cancer. Gynecol Oncol 2019;154(Suppl. 1):282–3 | Not enough information to quality appraise – abstract |
| 34. Bruegl A, Djordjevic B, Broaddus R. Lynch syndrome screening criteria: a new approach to identifying patients at risk via clinical history and pathology (CHiP) criteria. Gynecol Oncol 2012;1:S85–6 | Not enough information to quality appraise – abstract |
| 35. Bruegl A, Djordjevic B, Fellman B, Urbauer D, Luthra R, Broaddus R. Screening by young age and family history of colon cancer misses the majority of endometrial cancer patients with Lynch syndrome. Lab Invest 2013;1:267A | Not enough information to quality appraise – abstract |
| 36. Bruegl A, Djordjevic B, Fellman B, Urbauer D, Luthra R, Lu K, et al. A population-based study to evaluate SGO criteria for the identification of Lynch syndrome among endometrial cancer patients. Gynecol Oncol 2013;130:e28 | Not enough information to quality appraise – abstract |
| 37. Bruegl A, Daniels M, Broaddus RR. Does universal tissue testing provide universal answers? Clinical challenges associated with tumor screening for Lynch syndrome associated endometrial cancer. Lab Invest 2017;97(Suppl. 1):277A | Not enough information to quality appraise – abstract |
| 38. Bruegl AS, Djordjevic B, Urbauer DL, Westin SN, Soliman PT, Lu KH, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch syndrome in women with endometrial cancer. Curr Pharm Des 2014;20:1655–63 | Not enough information to quality appraise – abstract |
| 39. Bruegl AS, Djordjevic B, Batte B, Daniels M, Fellman B, Urbauer D, et al. Evaluation of clinical criteria for the identification of Lynch syndrome among unselected patients with endometrial cancer. Cancer Prev Res 2014;7:686–97 | No reference standard |
| 40. Bruegl AS, Djordjevic B, Urbauer DL, Westin SN, Soliman PT, Lu KH, et al. Utility of MLH1 methylation analysis in the clinical evaluation of Lynch syndrome in women with endometrial cancer. Curr Pharm Des 2014;20:1655–63 | No reference standard |
| 41. Bruegl AS, Djordjevic B, Westin SN, Soliman PT, Brandt AC, Daniels MS, et al. An alternative approach to identify women at risk for colorectal cancer. J Clin Oncol 2012;30(15 Suppl. 1) | Not enough information to quality appraise – abstract |
| 42. Bruegl A, Westin S, Djordjevic B, Broaddus R. Common screening criteria for Lynch syndrome: who are we missing? Gynecol Oncol 2012;125:S195 | Not enough information to quality appraise – abstract |
| 43. Bruegl AS, Djordjevic B, Fellman BM, Urbauer DL, Luthra R, Lu KH. Poor performance of published clinical screening criteria for the population-based identification of endometrial cancer patients with Lynch syndrome. Cancer Res 2013;73(8 Suppl. 1). 104th annual meeting of the American Association for Cancer Research (AACR) | Not enough information to quality appraise – abstract |
| 44. Bruegl AS, Djordjevic B, Rajyalakshmi L, Lu KH, Sun CC, Broaddus R. Cost analysis comparing universal tumor testing to clinically based criteria in the evaluation of endometrial adenocarcinomas for Lynch syndrome. Gynecol Oncol 2014;1:45 | No reference standard |
| 45. Bruegl AS, Djordjevic B, Urbauer D, Westin SN, Soliman PT, Lu KH, et al. Can clinical criteria reliably distinguish between sporadic and Lynch syndrome-associated endometrial carcinomas with immunohistochemical loss of MLH1? Cancer Prev Res 2012;5(Suppl. 1) | Not enough information to quality appraise – abstract |
| 46. Bruegl AS, Ring KL, Daniels M, Fellman BM, Urbauer DL, Broaddus RR. Clinical challenges associated with universal screening for Lynch syndrome-associated endometrial cancer. Cancer Prev Res 2017;10:108–15 | Abstract only. Associated full paper included |
| 47. Buchanan DD, Clendenning M, Jayasekara H, Joo JE, Wong EM, Southey MC, et al. Double somatic mutations as a cause of tumor mismatch repair deficiency in population-based colorectal and endometrial cancer with Lynch-like syndrome. Cancer Res 2017;77(Suppl. 1) | Not enough information to quality appraise – abstract |
| 48. Burks RT, Kessis TD, Cho KR, Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene 1994;9:1163–6 | No reference standard. Not lynch testing |
| 49. Busmanis I, Chew I. MSI and mucinous differentiation in uterine endometrioid carcinoma. Virchows Arch 2011;1:S230 | Not enough information to quality appraise – abstract |
| 50. Busmanis I, Lim T, Tan MH. MSI in an Asian series of primary uterine endometrioid carcinoma. Virchows Arch 2013;463:307–8 | Not enough information to quality appraise – abstract |
| 51. Cadoo KA, Tran C, DeLair D, Arnold AG, Ashraf A, Jewell EL, et al. Clinical characterization of DNA mismatch repair deficiency (MMR-D) in endometrial cancer (EC). J Clin Oncol 2016;34(Suppl. 15) | Not Lynch syndrome. No reference standard. Not enough information to quality appraise – abstract |
| 52. Calkins SM, Karnezis AN, Conrad PG, Chen LM, Rabban JT. Lynch syndrome screening tests in uterine cancer patients > 50 years depends on clinical and tumor morphology criteria: evidence against universal testing. Lab Invest 2012;1:262A | Not enough information to quality appraise – abstract |
| 53. Carvalho SD, Pardal J. Universal Lynch syndrome screening in endometrial cancer: two years of experience. Virchows Arch 2018;473(Suppl. 1):s70 | Not enough information to quality appraise – abstract |
| 54. Catasus L, Machin P, Matias-Guiu X, Prat J. Microsatellite instability in endometrial carcinomas: clinicopathologic correlations in a series of 42 cases. Hum Pathol 1998;29:1160–4 | No reference standard |
| 55. Cederquist K, Emanuelsson M, Göransson I, Holinski-Feder E, Müller-Koch Y, Golovleva I, Grönberg H. Mutation analysis of the MLH1, MSH2 and MSH6 genes in patients with double primary cancers of the colorectum and the endometrium: a population-based study in northern Sweden. Int J Cancer 2004;109:370–6 | More than 10% wrong population |
| 56. Cesca C, Barcus M, Amaker B, Bach D, Gainey T, Lamb T, et al. Absence of mismatch repair gene protein in subset of endometrioid carcinomas by immunohistochemistry. Lab Invest 2001;81:134A | Not enough information to quality appraise – abstract |
| 57. Cetinkaya K, Yuce E. Lynch syndrome in patients treated for endometrial cancer. Eur J Gynaecol Oncol 2017;38:607–13 | Unclear methods and wrong study design |
| 58. Chao XP, Li L, Wu M, Ma SQ, Tan XJ, Zhong S, Lang JH. [A prospective cohort study about the screening tests of mismatch repair protein and clinical criteria for Lynch syndrome associated endometrial carcinoma.] Zhonghua Yi Xue Za Zhi 2019;99:1178–83 | Foreign-language paper |
| 59. Chao XP, Li L, Wu M, Ma SQ, Tan XJ, Zhong S, Lang JH. [A prospective cohort study about the screening tests of mismatch repair protein and clinical criteria for Lynch syndrome associated endometrial carcinoma.] Zhonghua Yi Xue Za Zhi 2019;99:1178–83 | Foreign-language paper |
| 60. Chapel DB, Yamada SD, Cowan M, Lastra RR. Immunohistochemistry for mismatch repair protein deficiency in endometrioid endometrial carcinoma yields equivalent results when performed on endometrial biopsy/curettage or hysterectomy specimens. Gynecol Oncol 2018;149:570–4 | Not Lynch syndrome testing |
| 61. Chavez JA, Suarez A, Parwani A, Li Z. Tumor infiltrating lymphocytes and PD-L1 expression in 162 endometrial carcinomas with deficient mmr: comparison between MLH1 methylation and Lynch syndrome. Lab Invest 2018;98(Suppl. 1):411 | Not enough information to quality appraise – abstract |
| 62. Chen L, Holstein J, Blanco A, Chan J, Powell C, Rabban J, et al. Identifying Lynch syndrome in women with endometrial cancer. Gynecol Oncol 2011;1:S34 | Not enough information to quality appraise – abstract |
| 63. Chen L, Holstein J, Blanco A, Chan J, Powell C, Rabban J, et al. Identifying Lynch syndrome in women with endometrial cancer. Fam Cancer 2011;1:S39–40 | Not enough information to quality appraise – abstract |
| 64. Chern JY, Madden N, Lee J, Gerber D, Cantor A, Asgari S, et al. Utility of multi-gene panel testing with next generation sequencing in women with endometrial cancer. J Clin Oncol 2017;35(15 Suppl. 1) | Not enough information to quality appraise – abstract |
| 65. Chew I, Ho PY, Ngo L, Chia J. Clinicopathologic features, DNA mismatch repair status and expression of ER and pr in endometrial adenocarcinomas in young women < = 40 years old. Proc Singapore Healthc 2010;2:S242 | Not enough information to quality appraise – abstract |
| 66. Chiaravalli AM, Furlan D, Facco C, Tibiletti MG, Dionigi A, Casati B, et al. Immunohistochemical pattern of hMSH2/hMLH1 in familial and sporadic colorectal, gastric, endometrial and ovarian carcinomas with instability in microsatellite sequences. Virchows Arch 2001;438:39–48 | No reference standard. Wrong population |
| 67. Chirasophon S, Manchana T, Teerapakpinyo C. High-risk epithelial ovarian cancer patients for hereditary ovarian cancer. J Obstet Gynaecol Res 2017;43:929–34 | No intervention |
| 68. Cimetti L, Carnevali I, Chiaravalli AM, Sahnane N, Furlan D, Libera L, Gynaecological cancer as sentinel cancer in Lynch syndrome: clinico-pathological and molecular features. Virchows Arch 2014;1:S41 | Not enough information to quality appraise – abstract |
| 69. Cohen SA, McIlvried DE. Genetic counselor review of gynecologic pathology reports improves quality of screening program for Lynch syndrome. Fam Cancer 2013;12:791–2 | Not enough information to quality appraise – abstract |
| 70. Cohn DE, Babb S, Whelan AJ, Mutch DG, Herzog TJ, Rader JS, et al. Atypical clustering of gynecologic malignancies: a family study including molecular analysis of candidate genes. Gynecol Oncol 2000;77:18–25 | Case study |
| 71. Conlon N, Soslow R, Delair D. Young patients with uterine serous carcinoma: a study of selected epidemiological and immunohistochemical features including expression of DNA MMR proteins. Lab Invest 2014;1:279A | Not enough information to quality appraise – abstract |
| 72. Cosgrove CM, Backes FJ, Hampel H, Salani R, Copeland LJ, O'Malley DM, et al. A single institution pilot study for universal Lynch syndrome screening: a key step towards statewide screening and care. Gynecol Oncol 2017;145(Suppl. 1):136 | Not enough information to quality appraise – abstract |
| 73. Cossio SL, Koehler-Santos P, Pessini SA, Mónego H, Edelweiss MI, Meurer L, et al. Clinical and histomolecular endometrial tumor characterization of patients at-risk for Lynch syndrome in South of Brazil. Fam Cancer 2010;9:131–9 | No reference standard |
| 74. Costa Trachsel I, Carrera R, Blazquez CM, Andreu FJ, Vazquez J, Ramos MC, et al. Methylation study in the universal screening of Lynch syndrome in endometrial and colorectal carcinoma. Virchows Arch 2017;471(1 Suppl. 1):S91 | Not enough information to quality appraise – abstract |
| 75. Crim A, Greenwade M, Chen S, Mannel R, McMeekin S, Moore K, et al. Prevalence of Lynch syndrome-associated tumors in minority patients with endometrial cancer. Gynecol Oncol 2016;143:212 | Not enough information to quality appraise – abstract |
| 76. Crim AK, Perkins VB, Husain S, Ding K, Holman LL. Feasibility of two-antibody vs. four-antibody mismatch repair protein immunohistochemistry as initial screening for Lynch syndrome in patients with endometrial adenocarcinoma. Gynecol Oncol 2017;145(Suppl. 1):44 | Not enough information to quality appraise – abstract |
| 77. Carneiro da Silva F, Ferreira JR, Torrezan GT, Figueiredo MC, Santos ÉM, Nakagawa WT, et al. Clinical and molecular characterization of Brazilian patients suspected to have Lynch syndrome. PLOS ONE 2015;10:e0139753 | Wrong population |
| 78. Daniels MS, Urbauer DL, Zangeneh A, Batte BA, Dempsey KM, Lu KH. Outcomes of screening endometrial cancer patients for Lynch syndrome by patient-administered checklist. Gynecol Oncol 2013;131:619–23 | No index tests |
| 79. de la Chapelle A. The incidence of Lynch syndrome. Fam Cancer 2005;4:233–7 | Review |
| 80. de Leon ED, Robinson L, Euhus D, Burstein E, Sarode VR. Evaluation of Lynch syndrome by immunohistochemistry and quantitative scoring by digital image analysis as a screening tool for the diagnosis of hereditary colon cancer and correlation with genetic analysis. Gastroenterology 2014;1:S–346 | Not enough information to quality appraise – abstract |
| 81. Dekker N, van Dorst EBL, van Gijn ME, Offerhaus JA, Ausems MGEM. Genetic counseling and testing for Lynch syndrome in unselected patients with endometrial cancer. Int J Gynecol Cancer 2011;3:S498 | Not enough information to quality appraise – abstract |
| 82. Dempsey KM, Broaddus R, You YN, Noblin SJ, Mork M, Fellman B, et al. Is it all Lynch syndrome?: An assessment of family history in individuals with mismatch repair-deficient tumors. Genet Med 2015;17:476–84 | Author contacted. Could not provide information to enable inclusion |
| 83. Devlin LA, Graham CA, Price JH, Morrison PJ. Germline MSH6 mutations are more prevalent in endometrial cancer patient cohorts than hereditary non polyposis colorectal cancer cohorts. Ulster Med J 2008;77:25–30 | No intervention |
| 84. Di Nanni DD, De Leo A, Ceccarelli C, Perrone AM, De Iaco P, Santini D. et al. Association of tumour morphology with mismatch-repair protein status in endometrial cancers: a single unit experience. Virchows Arch 2018;473(Suppl. 1):S34 | Not enough information to quality appraise – abstract |
| 85. Dillon JL, Gonzalez JL, Bloch KJ, Tafe LJ. Universal screening for Lynch syndrome in endometrial cancer: the Dartmouth–Hitchcock Medical Center experience. J Mol Diagn 2016;18:1026 | Not enough information to quality appraise – abstract |
| 86. DiMaio MA, Kwok S, Longacre TA. Analysis of epcam expression in Lynch syndrome associated neoplasia. Lab Invest 2013;1:271A | Not enough information to quality appraise – abstract |
| 87. Doghri R, Houcine Y, Sellami R, Boujelbene N, Charfi L, Kamoun S, et al. Utility of immunohistochemistry in evaluation of microsatellite instability in endometrial carcinoma. Virchows Arch 2017;471(Suppl. 1):S83–4 | Not enough information to quality appraise – abstract |
| 88. Dong F, Costigan DC, Howitt BE. Targeted next-generation sequencing in the detection of mismatch repair deficiency in endometrial cancers. Mod Pathol 2019;32:252–7 | Not Lynch testing. No reference standard. No concordance information |
| 89. Dottino JA, Lu KH. Towards value-based universal Lynch syndrome identification in endometrial cancer patients. Gynecol Oncol 2016;143:451–2 | Editorial |
| 90. Dušek M, Hadravský L, Stehlík J, Černá K, Čurčíková R, Švajdler M, et al. [Results of morphological screening for Lynch syndrome during the period 2013–2016.] Cesk Patol 2018;54:86–92 | Foreign-language paper |
| 91. Eiriksson L, Aronson M, Clarke B, Mojtahedi G, Massey C, Oza AM, et al. Performance characteristics of a brief Family History Questionnaire to screen for Lynch syndrome in women with newly diagnosed endometrial cancer. Gynecol Oncol 2015;136:311–16 | Duplicate information and unclear reference standard. Included main paper by Ferguson et al.62 |
| 92. Elvin JA, Sanford EM, Chalmers Z, Frampton G, Santin AD, Vergilio JA, et al. Evaluation of microsatellite instability (MSI) status in endometrial adenocarcinoma by comprehensive genomic profiling (CGP) identifies subset that may benefit from immunotherapy. Lab Invest 2016;1:282A | Not enough information to quality appraise – abstract |
| 93. Erbarut Seven I, Kombak FE. Evaluation of mismatch repair (MMR) protein expression for Lynch syndrome screening in endometrial cancers in Turkish women: a preliminary study. Virchows Arch 2017;471(1 Suppl. 1):S93 | Not enough information to quality appraise – abstract |
| 94. Erbarut Seven I, Kombak FE, Bozkurtlar E, Yalcinkaya C, Ates EA. Evaluation of mismatch repair (MMR) protein expression with correlation of germline mutation analysis for Lynch syndrome screening in endometrial cancers in Turkish women; preliminary results. Virchows Arch 2018;473(Suppl. 1):S36 | Not enough information to quality appraise – abstract |
| 95. Faquin WC, Fitzgerald JT, Lin MC, Boynton KA, Muto MG, Mutter GL. Sporadic microsatellite instability is specific to neoplastic and preneoplastic endometrial tissues. Am J Clin Pathol 2000;113:576–82 | Wrong disease |
| 96. Fein LA, Pinto A, McCarter K, Guido LP, Schlumbrecht MP, Slomovitz BM, et al. Mismatch repair (MMR) screening among minority women with endometrial cancer. Gynecol Oncol 2019;154(Suppl. 1):108 | Not enough information to quality appraise – abstract |
| 97. Erbarut Seven I, Kombak FE, Bozkurtlar E, Yalcinkaya C, Arslan Ates E, Güney AI, et al. Evaluation of mismatch repair (MMR) protein expression with correlation of germline mutation analysis for Lynch syndrome screening in endometrial cancers in Turkish women; preliminary results. Virchows Arch 2018;473:S36–S36 | Not enough information to quality appraise – abstract |
| 98. Ferguson SE, Aronson M, Eiriksson LR, Mojtahedi G, Pollett A, Gallinger S, et al. Screening for Lynch syndrome in unselected women with endometrial cancer. J Clin Oncol 2013;31(15 Suppl. 1) | Not enough information to quality appraise – abstract |
| 99. Fix D, Chiang S, Murali R, Park K, Soslow R, Cadoo K, et al. Simplified immunohistochemical and targeted sequencing approach reproduces classification of endometrial carcinoma into TCGA molecular subgroups. Lab Invest 2017;97(Suppl. 1):285A | Not enough information to quality appraise – abstract |
| 100. Fountzila E, Kotoula V, Pentherdoudakis G, Manousou K, Vrettou E, Poulios C, et al. Prognostic implications of mismatch repair deficiency in patients with early-stage colorectal and endometrial cancer. Ann Oncol 2018;29(Suppl. 8):viii654 | Not enough information to quality appraise – abstract |
| 101. Frankel WL, Hampel H, Lajeunesse J, Panescu J, Jones S, de la Chapelle A. Immunohistochemical staining for MLH1, MSH2 and MSH6 identifies germline mutations in mismatch repair genes in colorectal and endometrial cancers initially found to be microsatellite stable. Lab Invest 2005;85:103A | Not enough information to quality appraise – abstract |
| 102. Frimer M, Levano KS, Rodriguez-Gabin A, Wang Y, Goldberg GL, Horwitz SB, Hou JY. Germline mutations of the DNA repair pathways in uterine serous carcinoma. Gynecol Oncol 2016;141:101–7 | No index tests or outcomes of interest |
| 103. Frolova A, Babb S, Zantow E, Powell MA, Thaker AR, Mutch DG. Universal screening for Lynch syndrome in endometrial cancer results in increased acceptance of genetic counseling and testing. Gynecol Oncol 2015;1:37 | Not enough information to quality appraise – abstract |
| 104. Frolova AI, Babb SA, Zantow E, Hagemann AR, Powell MA, Thaker PH, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol 2015;137:7–13 | Author contacted due to unclear information. Author could not provide information to warrant inclusion |
| 105. Garg K, Kauff ND, Soslow RA. Endometrial carcinomas with DNA mismatch repair abnormalities: genotypic phenotypic correlations. Lab Invest 2011;1:247A | Not enough information to quality appraise – abstract |
| 106. Garg K, Leitao MM, Kauff ND, Hansen J, Kosarin K, Shia J, Soslow RA. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol 2009;33:925–33 | No outcomes of interest |
| 107. Geurts-Giele WR, Leenen CH, Dubbink HJ, Meijssen IC, Post E, Sleddens HF, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. J Pathol 2014;234:548–59 | Wrong population – participants are known germline Lynch syndrome negative |
| 108. Gleeson J, Gallagher D. Diagnosing Lynch syndrome. Ir Med J 2016;109:P487 | Not enough information to quality appraise – abstract |
| 109. González L, Ortiz AP, Suárez EL, Umpierre S, Billoch J, Marcos MJ, et al. Case-case study of factors associated to hMLH1, hMSH2, and hMSH6 protein expression among endometrial cancer patients of the University District Hospital of San Juan, Puerto Rico. Int J Gynecol Cancer 2012;22:826–9 | No reference standard |
| 110. Halvarsson B, Lindblom A, Rambech E, Lagerstedt K, Nilbert M. Microsatellite instability analysis and/or immunostaining for the diagnosis of hereditary nonpolyposis colorectal cancer? Virchows Arch 2004;444:135–141 | Wrong population |
| 111. Hampel H, De La Chapelle A. How do we approach the goal of identifying everybody with Lynch syndrome? Fam Cancer 2013;12:313–17 | Review |
| 112. Haraga J, Nagasaka T, Nakamura K, Haruma T, Nishida T, Nyuya A, et al. Significance of MSH2 promoter methylation in endometrial cancer with MSH2 deficiency. Ann Oncol 2017;28(Suppl. 5):v348 | Not enough information to quality appraise – abstract |
| 113. Haraldsdottir S, Hampel H, Tomsic J, Frankel WL, Pearlman R, de la Chapelle A, Pritchard CC. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology 2014;147:1308–16.e1 | Not testing for Lynch, no outcomes, wrong population |
| 114. Haraldsdottir S, Hampell H, Tomsic J, Pearlman R, de la Chapelle A, Pritchard CC, et al. Bi-allelic somatic tumor mutations explain the majority of colorectal and endometrial cancer cases with defective mismatch repair without an identifiable germline mutation or MLH1 epigenetic silencing. J Mol Diagn 2014;16:771 | Subgroup analysis of lynch patients without germline mutation. Main paper Hampel 200615 is included |
| 115. Hardisson D, Moreno-Bueno G, Sánchez L, Sarrió D, Suárez A, Calero F, Palacios J. Tissue microarray immunohistochemical expression analysis of mismatch repair (hMLH1 and hMSH2 genes) in endometrial carcinoma and atypical endometrial hyperplasia: relationship with microsatellite instability. Mod Pathol 2003;16:1148–58 | No reference standard. Wrong population |
| 116. Hartnett EG, Stuckey A, McCourt C. Evaluation of universal immunohistochemistry screening for diagnosing Lynch syndrome in endometrial cancer patients at a tertiary care center. Gynecol Oncol 2015;139:599 | Not enough information to quality appraise – abstract |
| 117. Joehlin-Price A, Soloman D, Rabban J. Genomic profiling of undifferentiated endometrial carcinomas reveals frequent aberrations in SWI/SNF chromatin remodeling genes, extensive microsatellite instability, and novel recurrent mutations in epigenetic regulatory genes. Lab Invest 2018;98(Suppl. 1):428 | Not enough information to quality appraise – abstract |
| 118. Jprn U. Evaluation of the Usefulness and Validity of the Expanded Criteria for Primary Screening of Lynch Syndrome. 2012. URL: www.who.int/trialsearch/trial2.aspx?Trialid=jprn-umin000008192 (accessed 26 September 2019) | Trial registry. Author contacted but no relevant information provided to warrant inclusion |
| 119. Kanaya T, Kyo S, Sakaguchi J, Maida Y, Nakamura M, Takakura M, et al. Association of mismatch repair deficiency with PTEN frameshift mutations in endometrial cancers and the precursors in a Japanese population. Am J Clin Pathol 2005;124:89–96 | More than 10% wrong population |
| 120. Kanbour-Shakir A, Elishaev E, Dudley B, Al-Hamdawi D, Huang M, Dabbs DJ, et al. Universal mismatch repair protein testing in endometrial cancer: a single institutional experience. Lab Invest 2014;1:289A | Not enough information to quality appraise – abstract |
| 121. Kast K, Dobberschütz C, Sadowski CE, Pistorius S, Wimberger P. Prevalence of Lynch syndrome in unselected patients with endometrial or ovarian cancer. Arch Gynecol Obstet 2016;294:1299–303 | No relevant information |
| 122. Kawaguchi M, Banno K, Yanokura M, Kobayashi Y, Kishimi A, Ogawa S, et al. Analysis of candidate target genes for mononucleotide repeat mutation in microsatellite instability-high (MSI-H) endometrial cancer. Int J Oncol 2009;35:977–82 | No reference standard |
| 123. Kawaguchi M, Yanokura M, Banno K, Kobayashi Y, Kuwabara Y, Kobayashi M, et al. Analysis of a correlation between the BRAF V600E mutation and abnormal DNA mismatch repair in patients with sporadic endometrial cancer. Int J Oncol 2009;34:1541–7 | No reference standard |
| 124. Kim MK. Rapid genetic screening experience among endometrial cancer about Lynch syndrome by surgeon. Ann Oncol 2016;27(Suppl. 9):ix100 | Not enough information to quality appraise – abstract |
| 125. Kim MK, Heo EJ. Surgeon’s role about genetic screening among endometrial cancer with regard to Lynch syndrome. J Obstet Gynaecol Res 2017;43:1908 | Not enough information to quality appraise – abstract |
| 126. Kim MK, Song SY, Do IG, Kim SH, Choi CH, Kim TJ, et al. Synchronous gynecologic malignancy and preliminary results of Lynch syndrome. J Gynecol Oncol 2011;22:233–8 | No reference standard |
| 127. Kip NS, Rahm AK, Guha S, Fan A, Kaspar H, Gogoi R, et al. Evaluation to include endometrial carcinoma in universal Lynch syndrome screening at Geisinger Health System. Am J Clin Pathol 2015;2:A234 | Not enough information to quality appraise – abstract |
| 128. Kiyozumi Y, Matsubayashi H, Horiuchi Y, Higashigawa S, Oishi T, Abe M, et al. Germline mismatch repair gene variants analyzed by universal sequencing in Japanese cancer patients. Cancer Med 2019;8:5534–43 | Not Lynch syndrome testing. No outcome information for the population of interest |
| 129. Kobayashi K, Matsushima M, Koi S, Saito H, Sagae S, Kudo R, Nakamura Y. Mutational analysis of mismatch repair genes, hMLH1 and hMSH2, in sporadic endometrial carcinomas with microsatellite instability. Jpn J Cancer Res 1996;87:141–5 | No reference standard. Not testing for Lynch syndrome |
| 130. Koptiuch C, Samowitz W, Jasperson K, Kohlmann W, Mooney R, Soisson A. Incorporating paired germline/tumor analyses into a universal Lynch syndrome screening program: decreasing unexplained mismatch repair deficiency incidence. Fam Cancer 2019;18(Suppl. 1):S68–S69 | Not enough information to quality appraise – abstract |
| 131. Kost ER, Valente PT, Lynch BA, Krishnegowda NK, Hertz AM, Hall KL, et al. Clinical and pathologic features of hispanic endometrial cancer patients with loss of mismatch repair expression. Int J Gynecol Cancer 2016;26:1129–36 | No reference standard |
| 132. Kowalski LD, Mutch DG, Herzog TJ, Rader JS, Goodfellow PJ. Mutational analysis of MLH1 and MSH2 in 25 prospectively-acquired RER+ endometrial cancers. Genes Chromosomes Cancer 1997;18:219–27 | No reference standard. Not testing for Lynch syndrome |
| 133. Kurnit KC, Westin SN, Coleman RL. Microsatellite instability in endometrial cancer: New purpose for an old test. Cancer 2019;125:2154–63 | Commentary |
| 134. Lautrup CK, Okkels H, Sogaard CH, Sogaard-Andersen E, Grove A. Universal screening for Lynch syndrome in patients with endometrial cancer. Fam Cancer 2017;16(Suppl. 1):S126–7 | Not enough information to quality appraise – abstract |
| 135. Lee HI, Choi GW, Choi J, Kim KY. [Microsatellite instability in endometrial adenocarcinomas of young women.] Korean J Pathol 2008;42:202–7 | Foreign-language paper |
| 136. Lee HJ, Choi MC, Jang JH, Jung SG, Park H, Joo WD, et al. Clinicopathologic characteristics of double primary endometrial and colorectal cancers in a single institution. J Obstet Gynaecol Res 2018;44:944–50 | Authors contacted due to unclear reporting. Authors could not confirm information around testing |
| 137. Lee J, Brodsky AL, Gerber D, Fehniger J, Asgari S, Cantor A, et al. Importance of genetic counseling referrals for high-risk women with endometrial cancer despite intact mismatch repair immunohistochemistry. J Clin Oncol 2018;36(Suppl. 1) | Not enough information to quality appraise – abstract |
| 138. Lee J, Gubernick LR, Brodsky AL, Fehniger JE, Levine DA, Gerber D, et al. Missed opportunities: genetic counseling and testing among an ethnically diverse cohort of women with endometrial cancer. Gynecol Oncol 2018;151:153–8 | Authors contacted because of unclear reporting. Authors could not confirm information around testing |
| 139. Lee PJ, McNulty S, Duncavage EJ, Heusel JW, Hagemann IS. Clinical targeted next-generation sequencing shows increased mutational load in endometrioid-type endometrial adenocarcinoma with deficient DNA mismatch repair. Int J Gynecol Pathol 2018;37:581–9 | Ineligible reference standard. Wrong population |
| 140. Leenen CHM, Dubbink EJ, van Lier MGF, Hulspas SM, Kuipers EJ, van Leerdam ME, et al. Challenges and pitfalls in screening for Lynch syndrome by molecular tumor tissue analysis. Gastroenterology 2011;1:S352–3 | Not enough information to quality appraise – abstract |
| 141. van Lier MGF, Leenen CHM, Wagner A, Dubbink EJ, Dinjens WNM, Kuipers EJ, et al. Routine MSI-analysis in endometrial cancer ≤ 70 years increases identification of patients at risk for Lynch syndrome. Gastroenterology 2010;1:S613 | Not enough information to quality appraise – abstract |
| 142. van Lier MGF, Leenen CHM, Wagner A, Dubbink EJ, Dinjens WNM, Kuipers EJ, et al. Routine MSI-analysis in endometrial cancer ≥ 70 years increases identification of patients at risk for Lynch syndrome. Fam Cancer 2011;1:S48 | Not enough information to quality appraise – abstract |
| 143. Lim MC, Seo SS, Kang S, Seong MW, Lee BY, Park SY. Hereditary non-polyposis colorectal cancer/Lynch syndrome in Korean patients with endometrial cancer. Jpn J Clin Oncol 2010;40:1121–7 | No index test |
| 144. Lim PC, Tester D, Cliby W, Ziesmer SC, Roche PC, Hartmann L, et al. Absence of mutations in DNA mismatch repair genes in sporadic endometrial tumors with microsatellite instability. Clin Cancer Res 1996;2:1907–11 | No reference standard |
| 145. Lin D, Hecht J. Targeted screening with combined age- and morphology-based criteria enriches detection of Lynch syndrome in endometrial cancer. Lab Invest 2015;1:295A | Not enough information to quality appraise – abstract |
| 146. Livi A, Turchetti D, Miccoli S, Godino L, Ferrari S, Zuntini R, et al. Routine mismatch repair immunohistochemistry analysis as a valuable method to improve Lynch syndrome’s diagnosis among women with endometrial cancer. Fam Cancer 2017;16(1 Suppl. 1):S100–1 | Not enough information to quality appraise – abstract |
| 147. Long B, Lilyquist J, Weaver A, Hu C, Gnanaolivu R, Lee KY, et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol Oncol 2019;152:20–5 | No intervention. Wrong population |
| 148. Long Q, Peng Y, Tang Z, Wu C. Role of endometrial cancer abnormal MMR protein in screening Lynch-syndrome families. Int J Clin Exp Pathol 2014;7:7297–303 | No reference standard |
| 149. Lu K, Schorge J, Rodabaugh K, Daniels M, Tran N, Chung L, et al. Prevalence of MLH1 or MSH2 germline mutations in young women with endometrial cancer. Cancer Epidemiol Biomarkers Prev 2003;12:1352S–3S | Not enough information to quality appraise – abstract |
| 150. Lu KH, Schorge JO, Rodabaugh KJ, Sun CC, Daniels MS, White KG, et al. Defining criteria for Lynch Syndrome/HNPCC in women under 50 with endometrial cancer: final results of a prospective, multi-center study. J Clin Oncol 2005;23:848S | Not enough information to quality appraise – abstract |
| 151. Lucas E, Chen H, Molberg K, Castrillon DH, Rivera Colon G, Li L, Hinson S, et al. Mismatch repair protein expression in endometrioid intraepithelial neoplasia/atypical hyperplasia: should we screen for Lynch syndrome in precancerous lesions? Int J Gynecol Pathol 2018;31:31 | Wrong population |
| 152. MacQueen I, Peredo J, Tangney K, Ho S, Scheuner M, Russell M. Targeted screening for Lynch syndrome: is the veteran population different? Dis Colon Rectum 2016;59:e271 | Not enough information to quality appraise – abstract |
| 153. Mafnas C, Martin B, Ford J, Longacre T. Lynch syndrome screening: discordance in MMR and germline test results. Lab Invest 2015;1:177A | Not enough information to quality appraise – abstract |
| 154. Manchana T. Lynch syndrome screening in thai endometrial cancer patients. Gynecol Oncol 2019;154(Suppl. 1):41 | Not enough information to quality appraise – abstract |
| 155. Martin B, Mafnas C, Ford J, Longacre T. Universal screening for gynecologic and colorectal cancer: a single institution experience. Lab Invest 2015;1:297A | Not enough information to quality appraise – abstract |
| 156. Matthews KS, Estes JM, Conner MG, Manne U, Whitworth JM, Huh WK, et al. Lynch syndrome in women less than 50 years of age with endometrial cancer. Obstet Gynecol 2008;111:1161–6 | No reference standard |
| 157. Miesfeldt S, Feero WG, Lucas FL, Rasmussen K. Association of patient navigation with care coordination in a Lynch syndrome screening program. Transl Behav Med 2018;8:450–5 | No relevant outcomes |
| 158. Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol 2014;38:1501–9 | Authors contacted because of unclear reporting. Authors could not confirm information around testing |
| 159. Mills AM, Sloan EA, Thomas M, Modesitt SC, Stoler MH, Atkins KA, Moskaluk CA. Clinicopathologic comparison of Lynch syndrome-associated and ‘Lynch-like’ endometrial carcinomas identified on universal screening using mismatch repair protein immunohistochemistry. Am J Surg Pathol 2016;40:155–65 | Ineligible reference standard |
| 160. Minamiguchi K, Takahama J, Uchiyama T, Taiji R, Saito N, Okada H, et al. Uterine endometrial carcinoma with DNA mismatch repair deficiency: magnetic resonance imaging findings and clinical features. Jpn J Radiol 2018;36:429–36 | Not Lynch syndrome testing |
| 161. Moir-Meyer GL, Pearson JF, Lose F, Scott RJ, McEvoy M, Attia J, et al. Rare germline copy number deletions of likely functional importance are implicated in endometrial cancer predisposition. Hum Genet 2015;134:269–78 | No index tests. Not testing for Lynch syndrome |
| 162. Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol 2013;130:121–6 | Authors contacted because of unclear reporting. Authors could not confirm information around testing |
| 163. Murali K, Scaranti M, Vroobel K, Attygalle A, George A. Prevalence and clinical implications of mismatch repair (MMR) deficiency in unselected non-serous epithelial ovarian cancer (EOC) patients. Fam Cancer 2019;18(Suppl. 1):S59 | Not enough information to quality appraise – abstract |
| 164. Musulen E, Munoz-Marmol AM, Sanz C, Carrato C, Mate JL, Ariza A. Absence of V600E B-RAF mutation in MLH1-negative endometrial carcinoma. Lab Invest 2011;1:261A | Not enough information to quality appraise – abstract |
| 165. Mutch DG, Powell MA, Mallon MA, Goodfellow PJ. RAS/RAF mutation and defective DNA mismatch repair in endometrial cancers. Am J Obstet Gynecol 2004;190:935–42 | No reference standard |
| 166. Nakagawa H, Hampel H, de la Chapelle A. Identification and characterization of genomic rearrangements of MSH2 and MLH1 in Lynch syndrome (HNPCC) by novel techniques. Hum Mutat 2003;22:258 | Wrong population |
| 167. Nakagawa H, Hampel H, de la Chapelle A. Concordance of immunohistochemistry among HNPCC heterodimers MLH1 and PMS2, and MSH2 and MSH6 in microsatellite unstable colorectal and endometrial carcinomas. Lab Invest 2003;83:128A–9A | Not enough information to quality appraise – abstract |
| 168. Nguyen TD, Delvincourt C, Gorisse MC, Penet C. When brachytherapy met genetic oncology. Can radiation oncologists improve the detection of hereditary non-polyposis colorectal cancer? Eur J Med Genet 2011;54:60–62 | Wrong study design |
| 169. Nieminen TT, Gylling A, Abdel-Rahman WM, Nuorva K, Aarnio M, Renkonen-Sinisalo L, et al. Molecular analysis of endometrial tumorigenesis: importance of complex hyperplasia regardless of atypia. Clin Cancer Res 2009;15:5772–83 | Not testing for Lynch syndrome. More than 10% wrong population |
| 170. Niessen RC, Hofstra RM, Westers H, Ligtenberg MJ, Kooi K, Jager PO, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer 2009;48:737–44 | Not testing for Lynch syndrome |
| 171. Niessen RC, Kleibeuker JH, Westers H, Jager PO, Rozeveld D, Bos KK, et al. PMS2 involvement in patients suspected of Lynch syndrome. Genes Chromosomes Cancer 2009;48:322–9 | Not testing for Lynch syndrome |
| 172. Nofech-Mozes S, Ghorab Z, Hsieh E, Ackerman I, Thomas S, Covens A, et al. Immunohistochenical analysis of mismatch repair (MMR) genes in premenopausal women with endometrial endometrioid adenocarcinoma (EEA). Lab Invest 2007;87:208A | Not enough information to quality appraise – abstract |
| 173. Nugent W, Durie N, Palaia T, Ragolia L, Hanna I, Staszewski H. Retrospective review of endometrial cancer (EC) specimens in individuals younger than age 50 for microsatellite instability (MSI) testing and DNA mismatch repair enzyme (MMR) expression. J Clin Oncol 2011;29(Suppl. 1) | Not enough information to quality appraise – abstract |
| 174. Offman SL, Liuo S, Mills AM, Longacre TA. A clinicopathologic analysis of 419 consecutive endometrial carcinomas with emphasis on lower uterine segment tumors. Lab Invest 2012;1:290A–1A | Not enough information to quality appraise – abstract |
| 175. Orbegoso Aguilar CMA, Vroobel K, Attygalle A, Lalondrelle S, Taylor A, Nobbenhuis M, et al. MMR deficiency(d) in an unselected cohort of endometrial cancer (EC) patients, the Royal Marsden experience. Ann Oncol 2018;29(Suppl. 9) | Not enough information to quality appraise – abstract |
| 176. Orbegoso Aguilar CMA, Vroobel K, Lalondrelle S, Taylor A, Nobbenhuis M, Attygalle A, et al. Prevalence and clinical implications of mismatch repair (MMR) deficiency in unselected endometrial cancer (EC) patients. Ann Oncol 2018;29(Suppl. 8):viii342 | Not enough information to quality appraise – abstract |
| 177. Orbegoso C, Vroobel K, Lalondrelle S, Taylor A, Nobbenhuis M, Attygalle A, et al. Prevalence and clinical implications of mismatch repair (MMR) deficiency in unselected endometrial cancer (EC) patients. Fam Cancer 2019;18(Suppl. 1):S61–2 | Not enough information to quality appraise – abstract |
| 178. Orfanelli T, Sokoloff L, Schwartz MA, Tomita SA, Hayes MP, Blank SV. What happens in the real world? Assessment of two screening tools for Lynch syndrome in patients with endometrial cancer: universal tumor testing versus clinical screening. Gynecol Oncol 2019;154(Suppl. 1):220–1 | Not enough information to quality appraise – abstract |
| 179. Özdemir TR, Alan M, Sancı M, Koç A. Targeted next-generation sequencing of MLH1, MSH2, and MSH6 genes in patients with endometrial carcinoma under 50 years of age. Balkan Med J 2019;36:37–42 | No intervention |
| 180. Padron MMM, Camacho-Vanegas O, Camacho SC, Martignetti JA. Use of a targeted Lynch syndrome next generation sequencing panel in women at risk for or with endometrial cancer. Obstet Gynecol 2019;133:149S–50S | Not enough information to quality appraise – abstract |
| 181. Pai RK, Plesec TP, Abdul-Karim FW, Yang B, Marquard J, Shadrach B, Roma AR. Abrupt loss of MLH1 and PMS2 expression in endometrial carcinoma: molecular and morphologic analysis of 6 cases. Am J Surg Pathol 2015;39:993–9 | Ineligible reference standard |
| 182. Palmieri G, Manca A, Cossu A, Ruiu G, Pisano M, Cherchi P, et al. Microsatellite analysis at 10q25-q26 in Sardinian patients with sporadic endometrial carcinoma: identification of specification patterns of genetic alteration. Cancer 2000;89:1773–82 | No reference standard |
| 183. Parc YR, Halling KC, Burgart LJ, McDonnell SK, Schaid DJ, Thibodeau SN, Halling AC. Microsatellite instability and hMLH1/hMSH2 expression in young endometrial carcinoma patients: associations with family history and histopathology. Int J Cancer 2000;86:60–6 | No reference standard |
| 184. Peiró G, Diebold J, Lohse P, Ruebsamen H, Lohse P, Baretton GB, Löhrs U. Microsatellite instability, loss of heterozygosity, and loss of hMLH1 and hMSH2 protein expression in endometrial carcinoma. Hum Pathol 2002;33:347–54 | No reference standard. Wrong population |
| 185. Peiró-Marqués FM, Ventero MP, Alenda C, López JA, Román MJ, García A, et al. PD-L1 expression and overall survival in high grade endometrial carcinomas. Virchows Arch 2017;471(Suppl. 1):S92 | Not enough information to quality appraise – abstract |
| 186. Peterson LM, Medeiros F, Kipp BR, Smith DI, Halling KC. Molecular genetic characterization of endometrial adenocarcinoma by microsatellite instability, KRAS, BRAF, and MLH1 promoter hypermethylation analysis, and DNA MMR protein immunohistochemistry. J Mol Diagn 2007;9:688 | Not enough information to quality appraise – abstract |
| 187. Pyatt R, Chadwick RB, Johnson CK, Niemann TH, Hampell H, Graham JS, et al. The occurrence of microsatellite instability and DNA mismatch repair gene mutations in endometrial carcinomas. Faseb J 2000;14:A789 | Not enough information to quality appraise – abstract |
| 188. Rabban JT, Calkins SM, Karnezis AN, Grenert JP, Blanco A, Crawford B, Chen LM. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. Am J Surg Pathol 2014;38:793–800 | Authors contacted because of unclear reporting. Authors could not confirm information around testing |
| 189. Ramsoekh D, Wagner A, van Leerdam ME, Dinjens WN, Steyerberg EW, Halley DJ, et al. A high incidence of MSH6 mutations in Amsterdam criteria II-negative families tested in a diagnostic setting. Gut 2008;57:1539–44 | Wrong population |
| 190. Ranola JMO, Pearlman R, Hampel H, Shirts BH. Modified capture-recapture estimates of the number of families with Lynch syndrome in Central Ohio. Fam Cancer 2019;18:67–73 | Wrong population |
| 191. Resnick KE, Frankel WL, Morrison CD, Fowler JM, Copeland LJ, Stephens J, et al. Mismatch repair status and outcomes after adjuvant therapy in patients with surgically staged endometrial cancer. Gynecol Oncol 2010;117:234–8 | No reference standard |
| 192. Ring K, Bruegl A, Allen B, Elkin E, Singh N, Hartman AR, et al. The utility of a next-generation sequencing (NGS) panel to identify germline mutations associated with endometrial cancer. Lab Invest 2016;1:306A | Not enough information to quality appraise – abstract |
| 193. Ring KL, Bruegl AS, Allen B, Elkin EP, Singh N, Hartman AR. Multi-gene panel testing in an unselected endometrial cancer cohort. J Clin Oncol 2015;33(Suppl. 1) | Not enough information to quality appraise – abstract |
| 194. Ring KL, Bruegl AS, Batte BAL, Daniels MS, Munsell FM, Lu KH. A prospective evaluation of universal tumor testing strategies for Lynch syndrome in endometrial cancer. J Clin Oncol 2014;32(Suppl. 1) | Not enough information to quality appraise – abstract |
| 195. Ring KL, Bruegl AS, Celestino J, Schmandt RE, Broaddus R, Lu KH. A new Lynch syndrome: what is the role for polymerase D1 mutations in hereditary endometrial cancer? Gynecol Oncol 2014;1:77 | Not enough information to quality appraise – abstract |
| 196. Ring KL, Connor EV, Atkins KA, Ricketts W, Kashlan B, Modesitt SC. Women 50 years or younger with endometrial cancer: the argument for universal mismatch repair screening and potential for targeted therapeutics. Int J Gynecol Cancer 2013;23:853–60 | No reference standard |
| 197. Ritterhouse L, Howitt BE, Rojas-Rujilla V, Kuo FC, Sholl LM. Molecular signatures of hypermutated endometrial carcinomas. J Mol Diagn 2016;18:1007 | Not Lynch syndrome. No reference standard. Not enough information to quality appraise – abstract |
| 198. Romero A, Garre P, Valentin O, Sanz J, Pérez-Segura P, Llovet P, et al. Frequency and variability of genomic rearrangements on MSH2 in Spanish Lynch syndrome families. PLOS ONE 2013;8:e72195 | Not enough information to quality appraise – abstract |
| 199. Romero I, Garcia-Casado Z, Martinez P, Illueca C, Zorrero Cristina, Bosch JM, et al. Prospective comparison of different methods of identifying Lynch syndrome (LS) associated endometrial cancer (EC) and prognostic factor (PF) impact. J Clin Oncol 2016;34(Suppl. 15) | Not enough information to quality appraise – abstract |
| 200. Romero I, Garcia-Casado Z, Martinez P, Illueca C, Zorrero C, Grau M, et al. Prospective correlation between clinical and pathologic criteria identification of Lynch syndrome (LS) in endometrial carcinoma (EC) patients and relapse. J Clin Oncol 2013;31(Suppl. 1) | Not enough information to quality appraise – abstract |
| 201. Roth RM, Haraldsdottir S, Hampel H, Arnold CA, Frankel WL. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol 2016;146:50–6 | Wrong population – participants with known Lynch syndrome |
| 202. Rumilla KM, Mensink KA, Brigl A, Thibodeau SN, Medeiros F. Clinical testing for defective DNA mismatch repair in 335 gynecologic neoplasms. Lab Invest 2009;1:235A | Not enough information to quality appraise – abstract |
| 203. Ruszkiewicz A, Bennett G, Moore J, Manavis J, Rudzki B, Shen L, Suthers G. Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumours. Pathology 2002;34:541–7 | Wrong disease |
| 204. Ryan N, Glaire M, Ramchander N, Davison N, Walker T, Donnelly L, et al. The case for screening all endometrial cancer patients for Lynch syndrome. BJOG 2019;126:e130 | Not enough information to quality appraise – abstract |
| 205. Salyer CV, Lentz SE, Dontsi M, Armstrong MA, Hoodfar E, Alvarado M, et al. Lynch syndrome in women with endometrial cancer: comparison of universal and age-based strategies in a California healthcare system. Gynecol Oncol 2019;154(Suppl. 1):206–7 | Wrong population, wrong test, not Lynch syndrome testing, not enough information to quality appraise – abstract |
| 206. Salyera C, Lentzb S, Dontsic M, Armstrongc MA, Buttc A, Hoodfar E, et al. Comparison of effectiveness of two strategies to identify Lynch syndrome in women with endometrial cancer. Gynecol Oncol 2019;154:e12–e13 | Not enough information to quality appraise – abstract |
| 207. Sanchez Garcia A, Balaguer F, Balmana J, Ocana T, Esteban I, Llort G, et al. Evaluation of a 25-gene panel in patients with suspected Lynch syndrome: FAMOSA study. United European Gastroenterol J 2016;4(Suppl. 1):A656 | Not enough information to quality appraise – abstract |
| 208. Sanchez Mete L, Vocaturo G, Martayn A, Diodoro M, Casini B, Mannisi E, et al. Early-onset endometrial cancer patients with or without family history are at high risk for Lynch syndrome. United European Gastroenterol J 2018;6(Suppl.):A680–1 | Not enough information to quality appraise – abstract |
| 209. Sanchez-Mete L, Vocaturo G, Martayan A, Casini B, Diodoro E, Mannisi E, et al. Early-onset endometrial cancer patients are at high risk for Lynch syndrome regardless of family history. Dig Liver Dis 2019;51:E156–7 | Not enough information to quality appraise – abstract |
| 210. Saraiya DS, Corey BA , Raymond VM, Saam J, Arnell C, Moye K, et al. Women with dual gynecologic primary cancers can have mutations in Lynch syndrome genes or BRCA1/BRCA2, reflecting the overlap in clinical histories between these syndromes. Gynecol Oncol 2014;1:47 | Not enough information to quality appraise – abstract |
| 211. Sareen P, Blandon R, Miller S, Ford W, Shoup B. Lynch syndrome: should endometrial cancer patients in the 50–60 age group be screened? Gynecol Oncol 2012;125:S193 | Not enough information to quality appraise – abstract |
| 212. Sari A, Pollett A, Eiriksson LR, Lumsden-Johanson B, Van de Laar E, Kazerouni H, et al. Interobserver agreement for mismatch repair protein immunohistochemistry in endometrial and nonserous, nonmucinous ovarian carcinomas. Am J Surg Pathol 2019;43:591–600 | No reference standard and only one index test |
| 213. Shannon C, Kirk J, Barnetson R, Evans J, Schnitzler M, Quinn M, et al. Incidence of microsatellite instability in synchronous tumors of the ovary and endometrium. Clin Cancer Res 2003;9:1387–92 | No reference standard. No outcome data |
| 214. Schiavone MB, Mueller JJ, Cadoo K, Kauff ND, Jewel E, DeLair D, et al. Mismatch repair abnormalities are associated with aggressive tumor pathology in young women with endometrial cancer. Gynecol Oncol 2016;1:125 | Wrong population, wrong test, not Lynch syndrome testing, not enough information to quality appraise – abstract |
| 215. Schofield L, Sherwood AM, Grieu F, Brennan B, Stewart C, Goldblatt J, et al. Is routine screening for Lynch syndrome in endometrial cancer using microsatellite instability and immunohistochemical tests worthwhile? Twin Res Hum Genet 2009;12:231 | Not enough information to quality appraise – abstract |
| 216. Semba S, Ouyang H, Han SY, Kato Y, Horii A. Analysis of the candidate target genes for mutation in microsatellite instability-positive cancers of the colorectum, stomach, and endometrium. Int J Oncol 2000;16:731–7 | Wrong population. No test accuracy data |
| 217. Shamsani J, Waddell N, O’Mara T, Kazakoff Stephen, Spurdle A. Identification of genetic variants associated with risk of endometrial cancer. Twin Res Hum Genet 2017;20:447 | Not enough information to quality appraise – abstract |
| 218. Shannon C, Kirk J, Barnetson R, Evans J, Schnitzler M, Quinn M, et al. Incidence of microsatellite instability in synchronous tumors of the ovary and endometrium. Clin Cancer Res 2003;9:1387–92 | No reference standard |
| 219. Shirts BH, Konnick EQ, Upham S, Walsh T, Ranola JMO, Jacobson AL, et al. Using somatic mutations to classify pathogenic Lynch syndrome variants. J Mol Diagn 2016;18:946–7 | Wrong population, wrong test, not Lynch syndrome testing, not enough information to quality appraise – abstract |
| 220. Sobczuk A, Smolarz B, Romanowicz-Makowska H, Pertynski T. [Microsatellite instability in women with endometrial cancer from the Lodz region of Poland.] Menopause Review-Przeglad Menopauzalny 2008;7:42–5 | Foreign-language paper |
| 221. Soliman PT, Broaddus RR, Schmeler KM, Daniels MS, Gonzalez D, Slomovitz BM, et al. Women with synchronous primary cancers of the endometrium and ovary: do they have Lynch syndrome? J Clin Oncol 2005;23:9344–50 | No reference standard |
| 222. Son J, Carr C, Radeva M, Priyadarshini A, Marquard J, Al Hilli M. Molecular and pathologic features of endometrial cancer in young patients. Gynecol Oncol 2019;154:e22 | Not enough information to quality appraise – abstract |
| 223. Song T, Kim MK, Lee YY, Choi CH, Kim TJ, Lee JW, et al. Women with double primary cancers of the colorectum and endometrium: do they have Lynch syndrome? Eur J Obstet Gynecol Reprod Biol 2016;199:208–12 | No reference standard and no concordance information for endometrial cancer |
| 224. Staebler A, Lax SF, Ellenson LH. Altered expression of hMLH1 and hMSH2 protein in endometrial carcinomas with microsatellite instability. Hum Pathol 2000;31:354–8 | No reference standard. Not testing for Lynch syndrome |
| 225. Stasenkoa M, Tunnageb IU, Ashleya CW, Rubinsteina M, Muellera JJ, Leitao MM Jr, et al. Clinical outcomes of patients with POLE-mutated endometrioid endometrial cancer. Gynecol Oncol 2019;154(Suppl. 1):33 | Not enough information to quality appraise – abstract |
| 226. Stelloo E, Jansen A, Osse E, Nout R, Ruano D, van Wezel T, et al. Comprehensive analysis of microsatellite instability and mismatch repair protein expression in nearly 700 endometrial cancers. Virchows Arch 2016;469(Suppl. 1):S19 | Not enough information to quality appraise – abstract |
| 227. Stewart A. Genetic testing strategies in newly diagnosed endometrial cancer patients aimed at reducing morbidity or mortality from Lynch syndrome in the index case or her relatives. PLOS Curr 2013;5 | Review |
| 228. Straubhar S, Soisson A, Dodson M, Simons E, Kohlmann W, Jarboe E, et al. Cost analysis of universal screening for Lynch syndrome in patients with endometrial cancer. Gynecol Oncol 2016;143:214 | Not enough information to quality appraise – abstract |
| 229. Strickland SV, Norquist B, Swisher E, Rendi MH, Garcia R, Kilgore MR. Evaluation of a universal mismatch repair protein immunohistochemistry screening strategy in women with endometrial carcinoma 60 years of age or younger. Lab Invest 2017;97(Suppl. 1):311A | Not enough information to quality appraise – abstract |
| 230. Suerink M, Ten Broeke SW, Nielsen M. Findings linking mismatch repair mutation with age at endometrial and ovarian cancer onset in Lynch syndrome. JAMA Oncol 2018;4:889–90 | Response to editor, not a study |
| 231. Sugawara T, Sato N, Shimizu D, Sato T, Makino K, Kito M, et al. Efficient screening strategy for Lynch syndrome in Japanese endometrial cancer. Tohoku J Exp Med 2015;235:117–25 | Ineligible reference standard |
| 232. Sugihara T. [Analysis of correlation between MMR (mismatch repair genes) expression and clinicopathological factors in endometrial cancer.] Teikyo Medical J 2016;39:61–8 | Foreign-language paper |
| 233. Swisher EM, Mutch DG, Herzog TJ, Rader JS, Kowalski LD, Elbendary A, Goodfellow PJ. Analysis of MSH3 in endometrial cancers with defective DNA mismatch repair. J Soc Gynecol Investig 1998;5:210–16 | No Lynch syndrome testing. No test accuracy data |
| 234. Takeda T, Banno K, Yanokura M, Adachi M, Iijima M, Kunitomi H, et al. Methylation analysis of DNA mismatch repair genes using DNA derived from the peripheral blood of patients with endometrial cancer: epimutation in endometrial carcinogenesis. Genes 2016;7:E86 | No reference standard and no concordance information |
| 235. Tanaka T, Takehara K, Yokoyama T, Fujimoto E, Tomono K, Sakai M, et al. The usefulness of evaluation of DNA mismatch repair protein expression as a screening for Lynch syndrome in endometrial cancer. Int J Gynecol Cancer 2018;28(Suppl. 2):1193 | Not enough information to quality appraise – abstract |
| 236. Tangjitgamol S, Kittisiam T, Tanvanich S. Prevalence and prognostic role of mismatch repair gene defect in endometrial cancer patients. Tumour Biol 2017;39:1010428317725834 | No reference standard |
| 237. Tunnage IU, Stasenko M, Ashley CW, Rubinstein M, Latham AJ, Mueller JJ, et al. Clinical outcomes of patients with pole mutated endometrioid endometrial cancer. Gynecol Oncol 2019;153:e9 | Not enough information to quality appraise – abstract |
| 238. Vargas R, et al. Lynch syndrome screening in endometrial cancer patients with immunohistochemistry: a single center experience. Gynecol Oncol 2015;136:407 | Not enough information to quality appraise – abstract |
| 239. Vasen HF, Hendriks Y, de Jong AE, van Puijenbroek M, Tops C, Bröcker-Vriends AH, et al. Identification of HNPCC by molecular analysis of colorectal and endometrial tumors. Dis Markers 2004;20:207–13 | Review |
| 240. Vassileva V, Millar A, Briollais L, Chapman W, Bapat B. Apoptotic and growth regulatory genes as mutational targets in mismatch repair deficient endometrioid adenocarcinomas of young patients. Oncol Rep 2004;11:931–7 | Ineligible reference standard |
| 241. Vierkoetter KR, Ayabe AR, VanDrunen M, Ahn HJ, Shimizu DM, Terada KY. Lynch syndrome in patients with clear cell and endometrioid cancers of the ovary. Gynecol Oncol 2014;135:81–4 | Wrong disease |
| 242. Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, Koeffler HP, Karlan BY. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol 2010;116:516–21 | Authors contacted because of unclear reporting. Authors could not confirm information around testing |
| 243. Wang H, Tian W, Bi R, Ren Y, He H, Shi S, et al. Screening for inherited cancer syndromes in Chinese patients with endometrial cancer. Ann Oncol 2018;29(Suppl. 8):viii345 | Not enough information to quality appraise – abstract |
| 244. Wang M, Aldubayan S, Connor AA, Wong B, Mcnamara K, Khan T, et al. Genetic testing for Lynch syndrome in the province of Ontario. Cancer 2016;122:1672–9 | Not enough information to quality appraise. Population unclear |
| 245. Watkins J, et al. Universal Lynch screening in endometrial cancers: an examination of immunohistochemical subgroups and associated clinical and histologic features. Lab Invest 2015;1:314A | Not enough information to quality appraise – abstract |
| 246. Watkins JC, Nucci MR, Ritterhouse LL, Howitt BE, Sholl LM. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am J Surg Pathol 2016;40:909–16 | Same sample as Watkins et al.126 Author contacted because of lack of information |
| 247. Watkins JC, Yang EJ, Muto MG, Feltmate CM, Berkowitz RS, Horowitz NS, et al. Universal screening for mismatch-repair deficiency in endometrial cancers to identify patients with Lynch syndrome and Lynch-like syndrome. Int J Gynecol Pathol 2017;36:115–27 | Same sample as Watkins et al.126 Author contacted because of lack of information |
| 248. Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, Broaddus RR. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol 2008;26:5965–71 | Not testing for Lynch syndrome |
| 249. Wolf B, Henglmueller S, Janschek E, Ilencikova D, Ludwig-Papst C, Bergmann M, et al. Spectrum of germ-line MLH1 and MSH2 mutations in Austrian patients with hereditary nonpolyposis colorectal cancer. Wien Klin Wochenschr 2005;117:269–77 | Wrong population |
| 250. Wong A, Kuick CH, Aung ACL, Leong MY, Lim YK, Aggarwal I, et al. Universal endometrial carcinoma Lynch syndrome screening in Singapore. Fam Cancer 2019;18(Suppl. 1):S70–S71 | Not enough information to quality appraise – abstract |
| 251. Wu X, Thomas BC, Bakkum-Gamez JN, Swanson CL, Langstraat CL, Wick MJ, et al. Implementation of a universal endometrial cancer Lynch syndrome screening program: lessons learned. Lab Invest 2017;97(Suppl. 1):316A–317A | Not enough information to quality appraise – abstract |
| 252. Zannoni GF, Santoro A, Angelico G, Spadola S, Arciuolo D, Valente M, et al. Clear cell carcinoma of the endometrium: an immunohistochemical and molecular analysis of 45 cases. Hum Pathol 2019;92:10–17 | Wrong population. Ineligible reference standard |
| 253. Zauber P, Denehy TR, Taylor RR, Ongcapin EH, Marotta S, Sabbath-Solitare M. Strong correlation between molecular changes in endometrial carcinomas and concomitant hyperplasia. Int J Gynecol Cancer 2015;25:863–8 | Ineligible reference standard. Wrong population. No relevant outcome data |
| Question two | |
| 1. Helder-Woolderink J, de Bock G, Hollema H, van Oven M, Mourits M. Pain evaluation during gynaecological surveillance in women with Lynch syndrome. Fam Cancer 2017;16:205–10 | Participant, comparator and study design not relevant |
| 2. Tzortzatos G, Andersson E, Soller M, Askmalm MS, Zagoras T, Georgii-Hemming P, et al. The gynecological surveillance of women with Lynch syndrome in Sweden. Gynecol Oncol 2015;138:717–22 | Particpants, comparator and study design not relevant |
| 3. Moldovan R, Keating S, Clancy T. The impact of risk-reducing gynaecological surgery in premenopausal women at high risk of endometrial and ovarian cancer due to Lynch syndrome. Fam Cancer 2015;14:51–60 | Comparator and study design not relevant |
| 4. Frolova AI, Babb SA, Zantow E, Hagemann AR, Powell MA, Thaker PH, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol 2015;137:7–13 | Comparator, outcomes and study design not relevant |
| 5. Nebgen DR, Lu KH, Rimes S, Keeler E, Broaddus R, Munsell MF, Lynch PM. Combined colonoscopy and endometrial biopsy cancer screening results in women with Lynch syndrome. Gynecol Oncol 2014;135:85–9 | Intervention, comparator and study design not relevant |
| 6. Ketabi Z, Gerdes AM, Mosgaard B, Ladelund S, Bernstein I. The results of gynecologic surveillance in families with hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2014;133:526–30 | Participant, comparator and study design not relevant |
| 7. Helder-Woolderink JM, De Bock GH, Sijmons RH, Hollema H, Mourits MJ. The additional value of endometrial sampling in the early detection of endometrial cancer in women with Lynch syndrome. Gynecol Oncol 2013;131:304–8 | Comparator and study design not relevant |
| 8. Huang M, Sun C, Boyd-Rogers S, Burzawa J, Milbourne A, Keeler E, et al. Prospective study of combined colon and endometrial cancer screening in women with Lynch syndrome: a patient-centered approach. J Oncol Pract 2011;7:43–7 | Comparator, outcomes and study design not relevant |
| 9. Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 2009;27:4793–7 | Participant and study design not relevant |
| 10. Wang Y, Xue F, Broaddus RR, Tao X, Xie SS, Zhu Y. Clinicopathological features in endometrial carcinoma associated with Lynch syndrome in China. Int J Gynecol Cancer 2009;19:651–6 | Intervention, comparator and study design not relevant |
| 11. Renkonen-Sinisalo L, Bützow R, Leminen A, Lehtovirta P, Mecklin JP, Järvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer 2007;120:821–4 | Participant and study design not relevant |
| 12. de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665–71 | Comparator and study design not relevant |
| 13. Collins V, Meiser B, Gaff C, St John DJ, Halliday J. Screening and preventive behaviors one year after predictive genetic testing for hereditary nonpolyposis colorectal carcinoma. Cancer 2005;104:273–81 | Participant, comparator and study design not relevant |
| 14. Rijcken FE, Mourits MJ, Kleibeuker JH, Hollema H, van der Zee AG. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol 2003;91:74–80 | Participant, comparator and study design not relevant |
| 15. Dove-Edwin I, Boks D, Goff S, Kenter GG, Carpenter R, Vasen HF, Thomas HJ. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 2002;94:1708–12 | Participant, comparator and study design not relevant |
| 16. Adar T, Rodgers LH, Shannon KM, Yoshida M, Ma T, Mattia A, et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 2018;124:3145–53 | Comparator, outcome and study design not relevant |
| 17. Salyer C, Lentz S, Dontsi M, Armstrong MA, Butt A, Hoodfar E, et al. Comparison of effectiveness of two strategies to identify Lynch syndrome in women with endometrial cancer. Gynecol Oncol 2019;154:e12–13 | Intervention, comparator, outcome and study design not relevant |
| 18. Nebgen D, Lu K, Chisholm G, Sun C, Earles T, Soletsky B, Lynch P. Lynch Syndrome – combined endometrial and colon cancer screening results. Fam Cancer 2019;18:S1–88 | Participant, comparator and study design not relevant |
| 19. Crawford R, Newcombe B, Bolton H, Ngu SF, Freeman S, Addley H, et al. The Ten Year Experience of a Regional Specialist Gynaecology Cancer Genetics Clinic with Lynch Syndrome. The European Society of Gynaecological Oncology 20th International Meeting, Vienna, 4–7 November 2017 | Not enough information to quality appraise – abstract |
| 20. Adar T, Rodgers LH, Shannon KM, Yoshida M, Ma T, Mattia A, et al. A tailored approach to BRAF and MLH1 methylation testing in a universal screening program for Lynch syndrome. Mod Pathol 2017;30:440–7 | Participant not relevant |
| 21. Hartnett E, Stuckey A, Danilack V, McCourt C. Evaluation of universal immunohistochemistry screening for diagnosing Lynch syndrome in endometrial cancer patients at a tertiary care center. Gynecol Oncol 2015;139:599 | Comparator, outcomes and study design not relevant |
| 22. Mutch DG, Powell MA, Schmidt A, Broaddus R, Ramirez N, Tritchler D, et al. Clinicopathologic features associated with defective DNA mismatch repair (MMR): a GOG 0210 cohort study of 1041 endometrioid endometrial cancer cases. Gynecol Oncol 2015;137:20–21 | Intervention, comparator, study design not relevant |
| 23. Fu L, Sheng JQ, Li XO, Jin P, Mu H, Han M, et al. Mismatch repair gene mutation analysis and colonoscopy surveillance in Chinese Lynch syndrome families. Cell Oncol 2013;36:225–31 | Participant and study design not relevant |
| 24. Abstracts of the 13th International Meeting on Psychosocial Aspects of Hereditary Cancer (IMPAHC), Sydney, NSW, 7–8 March 2013. Fam Cancer 2013;12(Suppl. 1):1–22 | Conference preceedings. No relevant data |
| 25. Lu K, Chen L, Lynch H, Munsell M, Cornelison T, Boyd-Rogers S, et al. A prospective, multicenter randomized study of oral contraceptive versus Depo-Provera for the prevention of endometrial cancer in women with Lynch syndrome. Gynecol Oncol 2013;116:S4–5 | Participant, intervention, outcome and study design not relevant |
| 26. Wang Y, Xue F, Broaddus RR, Tao X, Xie SS, Zhu Y. Clinicopathological features in endometrial carcinoma associated with Lynch syndrome in China. Int J Gynecol Cancer 2009;19:651–6 | Intervention, comparator and study design not relevant |
| 27. Järvinen HJ. Endoscopic surveillance in hereditary nonpolyposis colorectal cancer. Tech Gastrointest Endosc 2006;8:110–13 | Participant and study design not relevant |
| 28. Macrae F. A Randomised Double Blind Dose Non-inferiority Trial of a Daily Dose of 600 mg versus 300 mg versus 100 mg of Enteric Coated Aspirin as a Cancer Preventive in Carriers of a Germline Pathological Mismatch Repair Gene Defect. Lynch Syndrome. Project 3 in the Cancer Prevention Programme (CaPP3). URL: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000804381 (accessed 2 January 2020)98 | Participants and comparator not relevant |
| 29. Arber N. A Randomised Double Blind Dose Non-inferiority Trial of a Daily Dose of 600 mg Versus 300 mg Versus 100 mg of Enteric Coated Aspirin as a Cancer Preventive in Carriers of a Germline Pathological Mismatch Repair Gene Defect. Lynch Syndrome. URL: https://clinicaltrials.gov/ct2/show/nct02497820 (accessed 2 January 2020)99 | Participants and comparator not relevant |
Appendix 4 Study characteristics of included studies
Characteristics of included clinical effectiveness studies
| Study and year | Country | Study design | Study setting | Time period | Outcomes | Sample size included | Selected/unselected sample | Age (years) (range) | Ethnicity | Previous/concurrent cancers | Relatives | Index test(s) | Reference standard tests(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anagnostopoulos et al.51 2017 | England | Cohort (prospective and retrospective) | Hospital/cancer registry | January 2005–September 2012 | Prevalence | 35 | Selected | Median 45 (31–49) | NR | NR | Not extractable | MSI and IHC | Sequencing and MLPA |
| Backes et al.52 2009 | USA | Clinical experience and prospective cohort (MMR proteins only) | Hospital and university medical centre | April 2007; end date not reported | Prevalence | 140 | Unselected | Mean 60.5 (30–91) | NR | 13 reported, unclear whether or not from whole sample:
|
NR for whole sample | IHC | Large rearrangement and deletion testing. Full gene analysis and sequencing |
| Baldinu et al.53 2002/Strazzullo et al.87 2003 | Italy | Prospective cohort | University | 1989–97 | Partial test accuracy and prevalence | 116 | Selected | Median 64 (35–88) | NR | NR | Excluded if they had first- or second-degree relatives with HNPCC | MSI and IHC | Denaturing high-performance liquid chromatography and sequencing |
| Berends et al.54 2003 | The Netherlands | Retrospective and prospective cohort | Cancer registry | Before 1989–2000 | Complete test accuracy, MSI only (MSI-H vs. MSI-L/MSS), IHC only, strategy 1, strategy 3, strategy 11, prevalence and concordance | 58 | Selected | Median 45 (27–49) | NR | 13/38 (22.4%) | 22/58 (37.9%) cancer diagnosis in first-degree relatives | MSI and IHC | DGGE and sequencing |
| Ring et al.81 2016 | USA | Prospective cohort | Cancer centre | August 2012–14 | Concordance and prevalence | 203 | Unselected but adult only |
|
For 381 (retrospective sample):
|
NR | NR for whole sample | MSI, IHC and MLH1 promoter hypermethylation testing | NGS and MLPA |
| Buchanan et al.56 2014/Nagle et al.76 2018 | Australia | Prospective cohort | Cancer registries | July 2005–December 2013 | Test accuracy by proteins and prevalence | 1459 (698 from Nagle et al.76) | Selected | IHC tested, mean 61.8 (27.1–79.9) | NR | 65/702 (9.3%) | First-degree relatives
|
IHC and MLH1 promoter hypermethylation testing | Unspecified germline testing and MLPA |
| Carnevali et al.57 2017/Libera et al.68 2017 | Italy | Retrospective cohort | Hospital | 1994–2014 | Concordance and prevalence | 88 (74 in Carnevali et al.57) | Selected | Carnevali et al.:57
|
NR | 3/74 (4%) ovarian cancer | 16/61 (31.1%) met Amsterdam criteria | MSI, IHC and MLH1 promoter hypermethylation testing | Sanger sequencing and MLPA |
| Libera et al.:68 NR | |||||||||||||
| Chao et al.58 2019 | China | Prospective cohort | Hospital | December 2017–August 2018 |
|
111 | Selected |
|
NR | 0 – excluded | 14/111 (12.6%) Amsterdam II criteria;93 two met Bethesda criteria | IHC, MSI and MLH1 promoter hypermethylation testing | NGS and Sanger sequencing |
| Dillon et al.59 2017 | Lebanon | Retrospective cohort | Hospital | May 2015–December 2016 | Prevalence | 233 | Unselected | Median 63 (30–90) | NR | NR for whole population | NR | IHC and MLH1 promoter hypermethylation testing | NGS |
| Dudley et al.60 2015/ Mas-Moya et al.70 2015 | USA | Prospective cohort and cross-sectional | Hospital | January 2008–May 2014 | Strategy 10 and prevalence | 215 | Unselected | NR for whole sample | NR | NR | NR | IHC, MSI and MLH1 promoter hypermethylation testing | Sequencing |
| Egoavil et al.61 2013 | Spain | Retrospective cohort | Hospital | 2004–9 | Concordance and prevalence | 173 | Unselected | Mean 63.3 (29–90) | NR |
|
|
MSI, IHC and MLH1 promoter hypermethylation testing | PCR, sequencing and MLPA |
| Ferguson et al.62 2014 | Canada | Prospective cohort | Hospital | July 2010–June 2011 | Prevalence, strategy 11 and concordance | 117 | Selected | Median 61 (26–91) | NR | Excluded patient with ovarian primary tumour |
|
MSI and IHC | Sequencing and MLPA |
| Goodfellow et al.63 2015 | USA | Prospective cohort | Hospital | 2003–7 | Strategy 10, concordance and prevalence | 1043 | Selected after 2007 | Mean 62 (25–100) |
|
NR | 938/1043 (90%) had Lynch syndrome-associated cancers | MSI, IHC and MLH1 promoter hypermethylation testing | NGS |
| Goodfellow et al.64 2003 | USA | Prospective cohort | University hospitals | NR | Prevalence | 441 | Unclear | Median 64.6 (26–92) | NR | NR for whole sample | NR for whole sample | MSI and MLH1 promoter hypermethylation testing | SSCV and sequencing |
| Hampel et al.15 2006 | USA | Retrospective cohort | Hospital | January 1999–December 2003 | Strategy 6, prevalence and concordance | 543 | Unselected | Mean 60.9 (17–94) | 95% white | NR | NR | MSI and MLH1 promoter hypermethylation testing | Sequencing and MLPA |
| Kato et al.65 2016/Takahashi et al.89 2017 | Japan | Retrospective cohort | Hospital | January 2003–December 2013 | Prevalence | 360 | Selected | Median 59 (28–89) | 360/360 (100%) Asian | 30/348 (8.6%) personal history of Lynch syndrome (Takahashi et al.89) |
|
IHC and MLH1 promoter hypermethylation testing | PCR, sequencing and MLPA |
| Latham et al.66 2019 | USA | Retrospective cohort | Hospital | January 2014–June 2017 | Strategy 1 and prevalence | 525 | Unclear | Median 55–60 across all MSI groups | NR for whole sample | NR | NR | MSI and IHC | NGS |
| Leenen et al.67 2012 | The Netherlands | Prospective cohort | Hospital/academic medical centre | May 2007–September 2009 | Prevalence | 179 | Selected | Median 61 (IQR 57–66) | NR | NR | NR | MSI, IHC and MLH1 promoter hypermethylation testing | Sequencing and MLPA |
| Lin and Hecht69 2016 | USA | Prospective cohort | Medical centre | July 2009–December 2013 | Prevalence | 76 | Selected | Mean 55 (23–95) | NR | 7/76 (9.2%) concurrent ovarian cancer | NR | IHC and MLH1 promoter hypermethylation testing | NR |
| Lu et al.16 2007 | USA | Prospective cohort | Gynaecologic oncology clinics | January 2000; end date NR | Complete test accuracy, MSI only (MSI-H vs. MSI-L/MSS), IHC only, test accuracy by proteins, strategy 1, strategy 3, strategy 4, strategy 10, strategy 11 and prevalence | 100 | Selected |
|
NR | 12/100 (12%)
|
21/100 (21%) Lynch syndrome-related cancer in at least one first-degree relative | MSI, IHC and MLH1 promoter hypermethylation testing | Sequencing and unclear further testing for large deletions |
| Masuda et al.71 2012 | Japan | Prospective cohort study | NR | January 2000–July 2002 | Concordance | 36 | Selected | NR overall
|
Asian | NR | One had a family history of cancer | MSI, IHC and MLH1 promoter hypermethylation testing | NA |
| McConechy et al.72 2015 | Canada | Retrospective cohort study | Tissue biobank repository | NR | Concordance | 157 | Unselected | Mean 62.6 | NR | NR | NR | MSI, IHC and MLH1 promoter hypermethylation testing | NA |
| Mercado et al.73 2012 | USA | Retrospective cohort study | Hospitals | NR | Strategy 1, strategy 3 and prevalence | 129 | Selected | Median 63 (38–89) |
|
|
|
MSI, IHC | Denaturing high-performance liquid chromatography and sequencing |
| Millar et al.74 1999 | Canada | Retrospective cohort | Cancer registry | 1971–96 | Strategy 11 and prevalence | 40 | Selected | NR | NR | 40/40 (100%) all synchronous endometrial and CRC patients | 4/40 (10%) met Amsterdam criteria | MSI | SSCV then PCR and sequencing |
| Modica et al.75 2007 | USA | Retrospective cohort | Cancer centre | 1992–2003 | Concordance | 90 | Selected |
|
NR | NR | Yes | MSI and IHC | NA |
| Najdawi et al.77 2017 | Australia | Prospective cohort (clinical experience study) | Hospital | August 2012–December 2016 | Prevalence | 124 | Selected | Mean 64.5 (31–93) | NR | Synchronous uterine and ovarian, 1/124 (0.8%) | NR for whole sample | IHC and MLH1 promoter hypermethylation testing | Sequencing and MLPA |
| Ollikainen et al.78 2005 | Finland | Cohort (retrospective and prospective) | Hospital | 1986–97 | Strategy 1, strategy 4, strategy 10 and prevalence | 23 | Selected |
|
NR | 2/23 (9%) breast cancer | 23/23 (100%) family history of endometrial cancer | MSI, IHC and MLH1 promoter hypermethylation testing | Sequencing and MLPA |
| Pecorino et al.79 2017 | Italy | Prospective cohort | Hospital | 2007–14 | Concordance | 41 | Selected | Mean 44.4 (32–50) | NR | Unclear | Unclear | MSI and IHC | NA |
| Planck et al.80 2017 | Sweden | Retrospective cohort | Population-based cancer registry | 1958–98 | Concordance | 36 | Selected | Mean 47 (37–61) | NR | 36/36 (100%) adenocarcinoma of the large bowel and uterine corpus | NR | MSI and IHC | NA |
| Ring et al.81 2016 | USA | Prospective cohort | Hospital | NR | Complete test accuracy, prevalence and strategy 11 | 381 | Unselected adult only | Mean 61 at diagnosis |
|
NR | NR for whole sample | MSI, IHC and MLH1 promoter hypermethylation testing | NGS and MLPA |
| Rubio et al.82 2016 | Spain | Retrospective and prospective cohort | Hospital | 3 years | Complete test accuracy, MSI only (MSI-H vs. MSI-L/MSS), MSI only (MSI-H/L vs. MSS), IHC only, strategy 1, strategy 3, strategy 11, prevalence and concordance | 103 | Selected | NR | NR |
|
64/99 (65%) available histories | MSI and IHC | CSGE sequencing, MLPA |
| PETALS study (Dr Neil AJ Ryan, personal communication) | UK | Prospective and retrospective cohort study | Gynaecology cancer centre | 2 years | Strategy 1, strategy 3, strategy 4, prevalence and concordance | Confidential information has been removed | Unselected | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | MSI, IHC and MLH1 promoter hypermethylation testing | Long-range PCR, NGS and MLPA |
| Salvador et al.83 2019 | USA | Retrospective cohort study | Laboratory/hospital | 2016–18 | Complete test accuracy, strategy 10, strategy 11 and prevalence | 237 | Selected | NR for endometrial cancer patients alone | NR for endometrial cancer patients alone | NR for endometrial cancer sample alone | NR for endometrial cancer sample alone | MSI, IHC and MLH1 promoter hypermethylation testing | NGS and MLPA |
| Sarode and Robinson84 2018 | USA | Retrospective cohort (including prospective analysis of tissue) | Hospital | September 2011–August 2013 | Strategy 4 and prevalence | 99 | Selected | NR for whole sample | NR for whole sample | NR for endometrial cancer patients | NR for whole sample | IHC and MLH1 promoter hypermethylation testing | ACGH, long-range PCR and MLPA |
| Shin et al.85 2015 | Republic of Korea | Retrospective cohort study | Hospital | January 2004–December 2013 | Strategy 9, synchronous cancers and prevalence | 12 | Selected | Median 52.5 at diagnosis | NR |
|
NR for whole sample | MSI and IHC | Sequencing and PCR |
| Stelloo et al.86 2017 | The Netherlands | Retrospective cohort study | Radiation centres | NR | Concordance | 686 | Selected | Mean 69 (41–88) | NR | NR | NR | MSI, IHC and MLH1 promoter hypermethylation testing | NA |
| Svampane et al.88 2014 | Latvia | Retrospective cohort | Hospital | January 2006–April 2010 | Prevalence | 704 | Unselected | Range 30–80 | NR | NR | 19 women with family history of HNPCC (meeting Amsterdam I or II criteria) | IHC | Sequencing |
| Tian et al.90 2019 | China | Prospective cohort | Cancer centre | January 2014–July 2017 | Prevalence, IHC only, strategy 3 and strategy 11 | 198 | Selected | NR in whole sample | Chinese |
|
47/196 (24%) Lynch syndrome-related tumour in a first-degree relative | IHC | Sequencing, NGS and MLPA |
| Wang et al.91 2017 | USA | Retrospective cohort study | University medical centre | June 2012–January 2015 | Concordance | 402 | Unclear | Median 61 (30–86) | NR | NR | NR | MSI and IHC | NA |
| Yoon et al.92 2008 | Republic of Korea | Prospective cohort | Hospital | January 1996–December 2004 | Prevalence and strategy 10 | 113 | Selected | NR | NR | NR | Four women met Amsterdam II criteria for HNPCC, one of whom had a sister with endometrial cancer and CRC | MSI, IHC and MLH1 promoter hypermethylation testing | Sequencing |
Characteristics of included health economics studies
| Study and country | Aim of the study | Study characteristics (study design, perspective, setting) | Intervention/comparator | Outcome(s) | Model type | Health states | Results (base-case and sensitivity analyses) |
|---|---|---|---|---|---|---|---|
| Resnick et al.101 2009, USA | To assess the cost-effectiveness of screening strategies for diagnosing Lynch syndrome among newly diagnosed endometrial cancer patients | Model-based cost-effectiveness analysis, undertaken from the viewpoint of the third-party payer | Amsterdam criteria (full gene sequencing for women with endometrial cancer who meet the revised Amsterdam criteria), sequence all (full gene sequencing for all women with endometrial cancer), sequence for all women aged < 60 years with endometrial cancer and IHC/single gene strategy (IHC for all women with endometrial cancer after gene sequencing) | Cost per additional Lynch syndrome case detected | Decision tree structure | Lynch positive, Lynch negative, MSH6 deletion (Lynch positive), MSH2 deletion (Lynch positive), MSH2 deletion (Lynch negative) | In comparison to the Amsterdam criteria strategy, IHC/single gene strategy was more costly but detected more Lynch syndrome cases from the hypothetical cohort of 40,000 women with endometrial cancer, equating to an ICER of approximately US$13,800 per Lynch syndrome case detected. The ICER was sensitive to the cost of full gene sequencing |
| Kwon et al.102 2011, USA | To assess the cost-effectiveness to compare the benefits and costs of each testing strategy | Model-based economic analysis, societal perspective |
|
Cost per life-year gained | Markov Monte Carlo simulation model, with annual cycle lengths | Well, at risk of CRC, CRC – unscreened, CRC – screened and dead | IHC triage of women at any age, with at least one first-degree relative with a Lynch syndrome-associated cancer – when compared with at age < 50 years, at least one first-degree relative had a mean incremental cost of US$22 and expected to yield an additional 0.00263 life-years, which equated to an ICER of approximately US$9,100 per life-year gained. Results from the sensitivity analysis showed that the ICER was robust to changes made to model input parameters |
| Breugl et al.103 2014, USA | To assess the cost-effectiveness of universal tissue testing versus the SGO 5–10% clinical criteria105 for identifying Lynch syndrome in a cohort of unselected women with endometrial cancer | Cost-effectiveness analysis, third-party payer | SGO 5–10% critical criteria vs. universal tissue testing | Cost per probable Lynch syndrome | NA | NA | The SGO 5–10% clinical criteria strategy identified 15 women diagnosed as probable Lynch syndrome; the universal tissue testing strategy identified 43 women with probable Lynch syndrome |
| Goverde et al.104 2016, the Netherlands | To assess the cost-effectiveness of routine screening for Lynch syndrome among endometrial cancer patients aged ≤ 70 years | Cost-effectiveness analysis | MSI; IHC for MLH1, MSH2, MSH6 and PMS2 protein expression; and the revised Bethesda guidelines | Cost per life-years gained based on the number of Lynch syndrome cases identified among probands and their relatives | NA | NA | Routine screening of endometrial cancer patients aged ≤ 70 years, compared with screening endometrial cancer patients aged ≤ 50 years, resulted in an ICER of approximately €5300 per life-year gained. Sensitivity analysis results showed that the health benefits (life-years gained) per female relative had the greatest impact on the ICER |
| Snowsill et al.43 2019, UK | To identify the relative cost-effectiveness of reflex testing for Lynch syndrome in women with endometrial cancer in the NHS | Model-based cost-effectiveness analysis, NHS and PSS perspective |
|
Cost per QALY | Decision tree and Markov model, with monthly cycle lengths |
|
Testing with IHC with methylation was the most cost-effective strategy, with an ICER of approximately £14,200 per QALY. The IHC-alone strategy was the most effective and the most costly, but the results did not reach cost-effectiveness when compared with IHC with methylation, with an ICER of approximately £129,000 per QALY Authors stated that the PSA results were in line with the deterministic results. From the 1000 iterations, there was a 0.36 probability that IHC with methylation was cost-effective at a WTP threshold of £20,000 per QALY. The ICER was sensitive to the age of the proband and the effectiveness of colonoscopy in reducing CRC incidence. When using the effectiveness results from Arrigoni et al. 106 for reducing the incidence of CRC, none of the testing strategies was cost-effective |
Appendix 5 Test accuracy results
Prevalence of Lynch syndrome
| Study (first author and year) | Country | Sample size (n) | Lynch syndrome prevalence, n (%) | Gene variant (n) | VUSs | Notes | |||
|---|---|---|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | PMS2 | ||||||
| Unselected samples | |||||||||
| Backes et al. 200952 | USA | 140 | 0 (0) | 0 | 0 | 0 | 0 | None reported | – |
| Bruegl et al. 201755 | USA | 213 | 7 (3.3) | 3 | 0 | 2 | 2 | 2 | |
| Buchanan et al. 201456/Nagle et al. 201876 | Australia | 702 | 22 (3.1) | 3 | 8 | 10 | 1 | 4 | Only included women with IHC data (702/1459 women with endometrial cancer) |
| Dillon et al. 201759 | Lebanon | 233 | 5 (2.1) | 1 | 2 | 2 | 0 | 3 Lynch like | – |
| Dudley et al. 201560/Mas-Moya et al. 201670 | USA | 215 | 11 (5.1) | 3 | 5 | 1 | 2 | 6 Lynch like | – |
| Egoavil et al. 201361 | Spain | 173 | 8 (4.6) | 1 | 3 | 3 | 1 | 2 | – |
| Hampel et al. 200615 | USA | 543 | 10 (1.8) | 1 | 3 | 6 | 0 | 13 | – |
| PETALS study (Dr Neil AJ Ryan, personal communication) | UK | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Svampane et al. 201488 | Latvia | 113 | 6 (5.3) | 3 | 3 | 2 | NA | None reported | Two women had germline mutations in both MLH1 and MSH2 |
| Selected samples | |||||||||
| Anagnostopoulos et al. 201751 | England | 35 | 3 (8.5) | 0 | 2 | 1 | 0 | None reported | Only included women diagnosed with endometrial cancer aged < 50 years |
| Baldinu et al. 200253/Strazzullo et al. 200387 | Italy | 116 | 1 (0.9) | 1 | 0 | NA | NA | None reported | Assessed only for MLH1 and MSH2 |
| Berends et al. 200354 | The Netherlands | 58 | 5 (8.6) | 1 | 3 | 1 | NA | 3 | Initial reference standard was denaturing gradient gel electrophoresis |
| Carnevali et al. 201757/Libera et al. 201768 | Italy | 61 | 22 (36.1) | 7 | 8 | 5 | 2 | 6 | Only included women with suspected Lynch syndrome on the basis of clinical criteria |
| Chao et al. 201958 | China | 93 | 6 (6.5) | 1 | 2 | 3 | 0 | 14 | – |
| Ferguson et al. 201462 | Canada | 118 | 7 (5.9) | 4 | 1 | 2 | 0 | None reported | – |
| Goodfellow et al. 201563 | USA | 1002 | 22 (2.2) | 2 | 7 | 10 | 3 | 2 | – |
| Leenen et al. 201267 | The Netherlands | 179 | 7 (3.9) | 0 | 0 | 6 | 1 | None reported | Only includes women aged < 70 years |
| Lin et al. 201669 | USA | 74 | 3 (4.21) | 1 | 0 | 2 | 0 | None reported | Study included two women with known Lynch syndrome (these have been excluded from the sample) |
| Lu et al. 200716 | USA | 100 | 9 (9) | 1 | 7 | 1 | NA | 11 VUS | – |
| Mercado et al. 201273 | USA | 129 | 80 (62) | 31 | 40 | 9 | 0 | 0 | – |
| Millar et al. 199974 | Canada | 40 | 7 (17.5) | 1 | 6 | NA | NA | None reported | All women had endometrial cancer and CRC. Only MLH1 and MSH2 assessed |
| Najdawi et al. 201777 | Australia | 124 | 3 (2.4) | 0 | 1 | 0 | 2 | None reported | Only including women undergoing surgery with curative intent |
| Ollikainen et al. 200578 | Finland | 23 | 2 (8.9) | 0 | 1 | 1 | NA | None reported | – |
| Ring et al. 201681 | USA | 365 | 21 (6.0) | 3 | 7 | 6 | 6 | 25 | Includes two EPCAM-MSH2 variants |
| Rubio et al. 201682 | Spain | 103 | 14 (13.6)
|
1 | 2 | 6 | NA | 4 | – |
| Salvador et al. 201983 | USA | 296 | 51 (17.3) | NR | NR | NR | NR | NR | Mixed endometrial cancer/CRC sample. Only partial data extractable for endometrial cancer |
| Sarode et al. 201984 | USA | 99 | 4 (4.0) | 1 | 0 | 3 | 0 | None reported | – |
| Shin et al. 201585 | Republic of Korea | 12 | 3 (25) | 2 | 1 | NA | NA | None reported | All women had endometrial cancer and CRC. Only MLH1 and MSH2 assessed |
| Takahasi et al. 201789/Kato et al. 201665 | Japan | 360 | 10 (2.8) | 3 | 4 | 2 | 1 | 2 VUSs; 15 Lynch like | Overlapping, but not identical, populations |
| Tian et al. 201990 | China | 198 | 45 (22.7) | 10 | 20 | 11 | 4 | 15 VUSs | – |
| Yoon et al. 200892 | Republic of Korea | 113 | 5 (4.4) | 1 | 2 | 6 | NA | None reported | One woman diagnosed with Lynch syndrome did not meet MSI/IHC referral criteria, but was offered germline as she met HNPCC criteria |
| Sample selection unclear | |||||||||
| Goodfellow et al. 200364 | USA | 441 | 7 (1.6) | NA | NA | 7 | NA | None reported | Only MSH6 investigated. Sample included five women with known MSH2 germline mutations |
| Latham et al. 201966 | USA | 525 | 7 (1.3) | 2 | 1 | 3 | 1 | None reported | Non-standard approach to MSI, no MSI-L |
Concordance between immunohistochemistry and microsatellite instability-based testing
| Study (first author and year) | Country | Sample size in analysis (n) | MSI thresholda | Agreement, n/N (%) | Disagreement, n/N (%) | Kappa (95% CI) | Notes |
|---|---|---|---|---|---|---|---|
| Anagnostopoulos et al. 201751 | England | 32 | NR | 30/32 (93.75) | 2/32 (6.25) | 0.86 (0.66 to 1.00) | Kappa calculated by CS using GraphPad (GraphPad Software, Inc., San Diego, CA, USA) |
| Berends et al. 200354 | The Netherlands | 51 | MSI-H | 36/51 (70.6) | 15/51 (29.4) | 0.403 (0.155 to 0.651) | Kappa calculated by CS using GraphPad |
| Bruegl et al. 201755 | USA | 197 | MSI-H (three unstable markers) | 190/197 (96.4) | 7/197 (3.6) | 0.91 (0.84 to 0.98) | Kappa calculated by CS using GraphPad |
| MSI-H/L (one or more unstable markers) | 187/197 (94.9) | 10/197 (5.1) | 0.87 (0.80 to 0.95) | ||||
| Chao et al. 201958 | China | 77 | MSI-H | 73/77 (94.8) | 4/77 (5.2) | 0.803 (0.616 to 0.989) | Kappa calculated by CS using GraphPad |
| Egoavil et al. 201361 | Spain | 173 | MSI-H | 156/173 (90.2) | 17/173 (9.8) | 0.77 (0.67 to 0.87) | Kappa calculated by CS using GraphPad |
| Ferguson et al. 201462 | Canada | 117 | MSI-H | 111/117 (94.9) | 6/117 (5.1) | 0.866 (0.762 to 0.969) | Kappa calculated by CS using GraphPad |
| Goodfellow et al. 201563 | USA | 934 | MSI-H | 907/934 (97.1) | 27/934 (2.9) | 0.94 (0.91 to 0.96) | Kappa calculated by CS using GraphPad |
| MSI-H/L | 893/934 (95.6) | 41/934 (4.4) | 0.91 (0.88 to 0.93) | Kappa calculated by CS using GraphPad | |||
| Hampel et al. 200615 | USA | 211 | NA | See ‘Notes’ column | See ‘Notes’ column | Not calculable | IHC conducted only for women with MSS results:
|
| Leenen et al. 201267 | The Netherlands | 179 | MSI-H | 179/179 (100) | 0/179 (0) | 1.00 (1.00 to 1.00) | Kappa calculated by CS using GraphPad |
| Libera et al. 201768 | Italy | 71 | MSI-H | 1. 68/71 (95.8) | 1. 3/71 (4.2) | 1. 0.91 (0.82 to 1.00) | 1. Borderline MSI = MSI-H |
| 2. 61/71 (85.9) | 2. 10/71 (10.1) | 2. 0.72 (0.57 to 0.88) |
2. Borderline MSI = MSS Kappa calculated by CS using GraphPad |
||||
| Lu et al. 200716 | USA | 100 | MSI-H | 89/94 (94.9) | 5/94 (5.3) | 0.858 (0.738 to 0.979) | Kappa calculated by CS using GraphPad |
| Masuda et al. 201271 | Japan | 9 | MSI-H | 7/9 (77.8) | 2/9 (22.2) | 0.526 (0.016 to 1.000) |
|
| MSI-H/L | 8/9 (88.9) | 1/9 (11.1) | 0.769 (0.354 to 1.000) | ||||
| McConechy et al. 201572 | Canada | 89 | MSI-H | 83/89 (93.3) | 6/89 (6.7) | 0.837 (0.711 to 0.963) | Kappa calculated by CS using GraphPad |
| Modica et al. 200775 | USA | 85 | MSI-H | 74/85 (87.1) | 11/85 (12.9) | 0.739 (0.596 to 0.883) | Samples selected for equal representation of MSI-H and MSS |
| Ollikainen et al. 200578 | Finland | 22 | MSI-H | 15/22 (68.2) | 7/22 (31.8) | 0.319 (0.014 to 0.624) | Kappa calculated by CS using GraphPad |
| MSI-H/L | 18/22 (81.8) | 4/22 (18.2) | 0. 621 (0.310 to 0.932) | ||||
| Pecorino et al. 201779 | Italy | 19 | NA | See ‘Notes’ column | See ‘Notes’ column | Not calculable | MSI conducted only for women with IHC loss:
|
| PETALS study, (Dr Neil AJ Ryan, personal communication) | UK | Confidential information has been removed | MSI-H | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Kappa calculated by CS using GraphPad |
| MSI-H/L | |||||||
| Planck et al. 200280 | Sweden | 28 | MSI-H/L | 20/28 (71.4) | 8/28 (28.6) | 0.44 (0.15 to 0.74) |
|
| Rubio et al. 201682 | Spain | 103 | NR | NR/NR (86.06) | NR/NR (13.92) | Not calculable | Percentage of agreement is reported in the paper, but no details are provided to enable checking or any further calculations |
| Shin et al. 201585 | Republic of Korea | 12 | MSI-H | 6/8 (75) | 2/8 (25) | Not calculated |
|
| Stelloo et al. 201786 | The Netherlands | 696 |
|
658/672 (97.9) | 14/672 (2.1) | 0.944 (0.915 to 0.973) |
|
|
663/678 (97.8) | 15/678 (2.2) | 0.942 (0.913 to 0.971) | ||||
| Strazzullo et al. 2003;87 same population as Baldinu et al. 200253 | Italy | 31 | MSI-H | See ‘Notes’ column | See ‘Notes’ column | Not calculated | IHC conducted only for MSI-H tumours:
|
| Wang et al. 201791 | USA | 78 | MSI-H | 77/78 (98.7) | 1/78 (1.3) | 0.965 (0.896 to 1.000) | Kappa calculated by CS using GraphPad |
Test failures and indeterminate results in index test
| Study (first author and year) | IHC, n/N (%) | MSI, n/N (%) | MLH1 promoter hypermethylation, n/N (%) | Reference standard, n/N (%) | Notes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Test failures | Indeterminate results | Test failures | Indeterminate results | Test failures | Indeterminate results | Test failures | Indeterminate results | ||
| Anagnostopoulos et al. 201751 | 0/35 (0) | 0/35 (0) | 0/35 (0) | 0/35 (0) | 0/2 (0) | 0/2 (0) | 0/9 (0) | 0/9 (0) | Included only women with both IHC and MSI data |
| Backes et al. 200952 | 0/140 (0) | 0/140 (0) | NA | NA | NA | NA | 0/2 (0) | 0/2 (0) | – |
| Baldinu et al. 200253/Strazzullo et al. 200387 | 0/39 (0) | 0/39 (0) | 0/39 (0) | 12/39 (30.8) | NA | NA | 0/9 (0) | 0/9 (0) | Assessed for MLH1 and MSH2 only |
| Berends et al. 200354 | 0/51 (0) | 0/51 (0) | 0/57 (0) | 0/57 (0) | NA | NA | 0/58 (0) | 0/58 (0) | Insufficient tumour tissue: IHC, 7/58; MSI, 1/58 |
| Bruegl et al. 201755 | NR | NR | NR | NR | NR | NR | 0/11 (0) | 0/11 (0) | ‘Insufficient tissue to perform the evaluation’ given as one of group of reasons for lack of index test. Number not reported |
| Buchanan et al. 201456/Nagle et al. 201876 | 0/702 (0), see note 1 | 0/702 (0), see note 1 | NA | NA | NR, see note 2 | NR, see note 2 | 0/170 (0) | 0/170 (0) |
1. Included only women with IHC results 2. Offered only to women with MMR deficiency and sufficient tumour tissue or random sample of MMR proficient |
| Carnevali et al. 201757/Libera et al. 201768 | 0/71 (0) | 0/71 (0) | 0/71 (0) | 13/71 (18.3) | NA | NA | 0/28 (0) | 0/28 (0) | All women met clinical criteria for Lynch syndrome |
| Chao et al. 201958 | 0/102 (0) | 0/102 (0) | 0/102 (0) | 0/102 (0) | 0/14 (0) | 0/14 (0) | 0/111 (0) | 0/111 (0) | Insufficient tumour tissue: IHC = 9/111; MSI = 28/111 |
| Dillon et al. 201759 | 0/233 (0) | 0/233 (0) | NA | NA | 0/51 (0) | 0/51 (0) | 0/8 (0) | 0/8 (0) | Insufficient tumour tissue: MLH1 promoter hypermethylation = 1/51 |
| Egoavil et al. 201361 | 0/173 (0) | 0/173 (0) | 0/173 (0) | 0/173 (0) | 0/44 (0) | 0/44 (0) | 0/19 (0) | 0/19 (0) | – |
| Ferguson et al. 201462 | 0/118 (0) | 0/118 (0) | 0/117 (0) | 0/117 (0) | NA | NA | 0/89 (0) | 0/89 (0) | Insufficient tumour tissue: MSI, 1/118 |
| Goodfellow et al. 200364 | NA | NA | 0/441 (0) | 0/441 (0) | 0/137 (0) | 0/137 (0) | 0/7 (0) | 0/7 (0) | – |
| Goodfellow et al. 201563 | 3/1043 (0.3) | 0/1043 (0) | 0/1043 (0) | 0/1043 (0) | 39/1,043 (0.3) | 0/1043 (3.7) | 2/53 (3.8) | 0/53 (0) | – |
| Hampel et al. 200615 |
15/127 (11.8) See note 1 |
0/543 (0) | 0/543 (0) | 0/543 (0) | See note 2 | 0/118 (0) | See note 2 | 0/118 (0) |
|
| Kato et al. 201665/Takahashi et al. 201789 | 0/360 (0) | 0/360 (0) | NA | NA | NA | NA | 0/27 (0) | 0/27 (0) | IHC, 12 specimens not available |
| Latham et al. 201966 | NR | NR | 0/525 (0) | 0/525 (0) | NA | NA | 0/119 (0) | 0/119 (0) | For one woman diagnosed with Lynch syndrome, IHC was ‘not available’. No further details |
| Leenen et al. 201267 | 0/179 (0) | 0/179 (0) | 0/179 (0) | 0/179 (0) | 0/42 (0) | 0/42 (0) | 0/10 (0) | 0/10 () | Four IHC tests not conducted because no tumour tissue available |
| Lin et al. 201669 | 0/74 (0) | 2/74 (2.6) | NA | NA | 0/14 (0) | 0/14 (0) | 0/3 (0) | 0/3 (0) | – |
| Lu et al. 200716 | 1/100 (1) | 0/100 (0) | 0/100 (0) | 0/100 (0) | 0/100 (0) | 0/100 (0) | 0/100 (0) | 0/100 (0) | Five MSI tests not conducted because of insufficient tumour tissue |
| Mas-Moya et al. 201670/Dudley et al. 201560 | 0/215 (0) | 0/215 (0) | 0/215 (0) | 0/215 (0) | NR | NR | 0/17 (0) | 0/17 (0) | – |
| Masuda et al. 201271 | 0/36 (0) | 0/36 (0) | 0/36 (0) | 0/36 (0) | NR | NR | NA | NA | Concordance only |
| McConechy et al. 201572 | 0/89 (0) | 0/89 (0) | 0/89 (0) | 0/89 (0) | NA | NA | NA | NA | Insufficient tumour tissue: IHC, 2/157, MSI, 0/157 (68 insufficient normal tissue) |
| Mercado et al. 201273 | 0/74 (0) | 0/74 (0) | 0/24 (0) | 0/24 (0) | NA | NA | 0/80 (0) | 0/80 (0) | IHC results reported by protein in paper, with different numbers of women tested for each protein. The denominator reported for IHC refers to the largest sample of women in the study. The denominator for germline refers to all women who received germline testing |
| Millar et al. 199974 | NA | NA | 0/40 (0) | 0/40 (0) | NA | NA | 0/40 (0) | 0/40 (0) | – |
| Modica et al. 200775 | 0/90 (0) | 5/90 (5.6) | 0/90 (0) | 0/90 (0) | NA | NA | NA | NA | Concordance only |
| Najdawi et al. 201777 | 0/124 (0) | 0/124 (0) | NA | NA | 0/26 (0) | 0/26 (0) | 0/9 (0) | 0/9) | Two IHC tests not conducted because of insufficient tumour material |
| Ollikainen et al. 200578 | 0/23 (0) | 1/23 (4.5) | 0/23 (0) | 0/23 (0) | 0/6 (0) | 0/6 (0) | 0/10 (0) | 0/10 () | Includes only women with a family history of endometrial cancer. Table 2 in the Ollikainen 200578 paper says one IHC not determined. No further details are provided in the Ollikainen 200578 paper |
| Pecorino et al. 201779 | 0/41 (0) | 0/41 () | 0/19 (0) | 0/19 (0) | NA | NA | NA | NA | MSI was conducted only for women who had loss on IHC |
| Planck et al. 200280 | 0/30 (0) | 2/30 (6.6) | 0/30 (0) | 1/30 (3.3) | NA | NA | NA | NA | All women had CRC and endometrial cancer |
| Ring et al. 201681 | 0/365 (0) | 0/365 (0) | 0/365 (0) | 0/365 (0) | NR | NR | 0/381 (0) | 0/381 (0) |
|
| Rubio et al. 201682 | NR | NR | NR | NR | NA | NA | 0/103 (0) | 0/103 (0) |
|
| PETALS study, (Dr Neil AJ Ryan, personal communication) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed. IHC had incomplete staining (women with incomplete loss were referred for germline testing) |
| Salvador et al. 201983 | NR | NR | NR | NR | NR | NR | 0/296 (0) | 0/296 (0) | Mixed endometrial cancer/CRC sample. Only partial data extractable for endometrial cancer |
| Sarode et al. 201984 | 0/99 (0) | 4/99 (4) | NA | NA | NR | NR | NR | NR | – |
| Shin et al. 201585 | 0/8 (0) | 0/8 (0) | 0/12 (0) | 0/12 (0) | NA | NA | 0/3 (0) | 0/3 (0) | All women had CRC and endometrial cancer |
| Stelloo et al. 201786 | 0/696 (0) | 18/696 (2.6) | NR | NR | NA | NA | NA | NA | 168 women excluded without reason |
| Svampane et al. 201488 | 2/111 (1.8) | 0/111 (0) | NA | NA | NA | NA | 0/8 (0) | 0/8 (0) | No cancer tissue found, 2/113 |
| Tian et al. 201990 | NR | NR | NA | NA | NA | NA | 0/198 (0) | 0/198 (0) | 32 IHC results not available; no details given |
| Wang et al. 201791 | 0/78 (0) | 0/78 (0) | 0/78 (0) | 0/78 (0) | NA | NA | NA | NA | Concordance only |
| Yoon et al. 200892 | 0/113 (0) | 0/113 (0) | 0/113 (0) | 0/113 (0) | NR | NR | 0/16 (0) | 0/16 (0) | – |
Appendix 6 Quality assessment of included studies
Quality assessment of included economic evaluation studies
| Assessment | Study and year | ||||
|---|---|---|---|---|---|
| Resnick et al.101 2009 | Kwon et al.102 2011 | Bruegl et al.103 2014 | Goverde et al.104 2016 | Snowsill et al.43 2019 | |
| Title | Yes | Yes | Yes | Yes | Yes |
| Abstract | Yes | Yes | Yes | Yes | Yes |
| Introduction | |||||
| Background and objectives | Yes | Yes | Yes | Yes | Yes |
| Methods | |||||
| Target population and subgroups | Yes | Yes | Yes | Yes | Yes |
| Setting and location | Yes | Yes | Yes | Yes | Yes |
| Study perspective | Yes | Yes | Yes | Yes | Yes |
| Comparators | Yes | Yes | Yes | Yes | Yes |
| Time horizon | Yes | Yes | NA | Yes | Yes |
| Discount rate | Yes | Yes | NA | Yes | Yes |
| Choice of health outcomes | Yes | Yes | Yes | Yes | Yes |
| Measurement of effectiveness | Yes | Yes | Yes | Yes | Yes |
| Measurement and valuation of preference-based outcomes | Yes | NA | NA | NA | Unclear |
| Estimating resources and costs | Yes | Yes | Yes | Yes | Yes |
| Currency, price date and conversion | Yes | Yes | Yes | Yes | Yes |
| Choice of model | Yes | Yes | NA | NA | Yes |
| Assumptions | Yes | Yes | Yes | Yes | Yes |
| Analytical methods | Yes | Yes | Yes | Yes | Yes |
| Results | |||||
| Study parameters | Yes | Yes | Yes | Yes | Yes |
| Incremental costs and outcomes | Yes | Yes | Yes | Yes | |
| Characterising uncertainty | Yes | Yes | Yes | Yes | Yes |
| Discussion | |||||
| Study findings | Yes | Yes | Yes | Yes | Yes |
| Limitations | Yes | Yes | Yes | Yes | Yes |
| Generalisability | Yes | NR | NR | Yes | No |
| Other | |||||
| Source of funding | Yes | Yes | Yes | Yes | Yes |
| Conflicts of interest | Yes | Yes | Yes | Yes | Yes |
| Number | Philips’ criteria | Studies | ||
|---|---|---|---|---|
| Resnick et al.101 2009 | Kwon et al.102 2011 | Snowsill et al.43 2019 | ||
| Structure | ||||
| 1. | Is there a clear statement of the decision problem? | Yes | Yes | Yes |
| 2. | Is the objective of the model specified and consistent with the stated decision problem? | Yes | Yes | Yes |
| 3. | Is the primary decision-maker specified? | Yes | Yes | Yes |
| 4. | Is the perspective of the model stated clearly? | Yes | Yes | Yes |
| 5. | Are the model inputs consistent with the stated perspective? | Yes | No | Yes |
| 6. | Has the scope of the model been stated and justified? | Yes | No | Yes |
| 7. | Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Yes | Yes | Yes |
| 8. | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Yes | Yes | Yes |
| 9. | Are the sources of the data used to develop the structure of the model specified? | Yes | Yes | Yes |
| 10. | Are the causal relationships described by the model structure justified appropriately? | Yes | Yes | Yes |
| 11. | Are the structural assumptions transparent and justified? | Yes | Yes | Yes |
| 12. | Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Yes | Yes | Yes |
| 13. | Is there a clear definition of the options under evaluation? | Yes | Yes | Yes |
| 14. | Have all feasible and practical options been evaluated? | No | No | No |
| 15. | Is there justification for the exclusion of feasible options? | No | Yes | Yes |
| 16. | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Yes | Yes | Yes |
| 17. | Is the time horizon of the model sufficient to reflect all important differences between the options? | No | Yes | Yes |
| 18. | Are the time horizon of the model and the duration of treatment described and justified? | No | Yes | Yes |
| 19. | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Yes | Yes | Yes |
| 20. | Is the cycle length defined and justified in terms of the natural history of disease? | NA | Yes | Yes |
| Data | ||||
| 21. | Are the data identification methods transparent and appropriate given the objectives of the model? | Yes | Yes | Yes |
| 22. | When choices have been made between data sources, are these justified appropriately? | No | No | No |
| 23. | Has particular attention been paid to identifying data for the important parameters of the model? | Unclear | Unclear | No |
| 24. | Has the quality of the data been assessed appropriately? | Unclear | Unclear | Unclear |
| 25. | When expert opinion has been used, are the methods described and justified? | No | NA | Yes |
| 26. | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Yes | Yes | Yes |
| 27. | Is the choice of baseline data described and justified? | Yes | Yes | Yes |
| 28. | Are transition probabilities calculated appropriately? | NA | Unclear | Yes |
| 29. | Has a half-cycle correction been applied to both costs and outcomes? | NA | No | Yes |
| 30. | If not, has the omission been justified? | No | No | NA |
| 31. | If relative treatment effects have been derived from trial data, have they been synthesised using appropriate techniques? | NA | NA | Yes |
| 32. | Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | NA | NA | Yes |
| 33. | Have alternative extrapolation assumptions been explored through sensitivity analysis? | NA | NA | Yes |
| 34. | Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | NA | NA | NA |
| 35. | Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis | NA | NA | NA |
| 36. | Are the costs incorporated into the model justified? | Yes | Yes | Yes |
| 37. | Has the source for all costs been described? | Yes | Yes | Yes |
| 38. | Have discount rates been described and justified given the target decision-maker? | Yes | Yes | Yes |
| 39. | Are the utilities incorporated in the model appropriate? | NA | NA | Yes |
| 40. | Is the source of utility weights referenced? | NA | NA | Yes |
| 41. | Are the methods of derivation for the utility weights justified? | NA | NA | Yes |
| 42. | Have all data incorporated in the model been described and referenced in sufficient detail? | Yes | No | Yes |
| 43. | Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | Yes | Yes | Yes |
| 44. | Is the process of data incorporation transparent? | No | No | Yes |
| 45. | If data have been incorporated as distributions, has the choice of distributions for each parameter been described and justified? | NA | NA | Yes |
| 46. | If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | NA | NA | Yes |
| 47. | Have the four principal types of uncertainty been addressed? | No | No | Yes |
| 48. | If not, has the omission of particular forms of uncertainty been justified? | No | No | NA |
| 49. | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | No | No | Yes |
| 50. | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | No | No | Yes |
| 51. | Has heterogeneity been dealt with by running the model separately for different subgroups? | No | Yes | Yes |
| 52. | Are the methods of assessment of parameter uncertainty appropriate? | Yes | Yes | Yes |
| 53. | If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Yes | Yes | Yes |
| 54. | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | No | No | Yes |
| 55. | Are any counterintuitive results from the model explained and justified? | NA | NA | NA |
| 56. | If the model has been calibrated against independent data, have any differences been explained and justified? | Yes | NA | Yes |
| 57. | Have the results been compared with those of previous models and any differences in results explained? | Yes | Yes | Yes |
Appendix 7 Health economic results
Model input parameters: colorectal cancer surveillance supporting information
FIGURE 34.
Gene-specific management of Lynch syndrome. Reproduced from Monahan et al. 27 © Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/. Minor amendments have been made for journal style.
Additional scenario analyses
Scenario analysis 4
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No testing | 0 | – | 0.0000 | – | – |
| MSI with MLH1 methylation | 520 | 520 | 0.0522 | 0.0522 | Extendedly dominated |
| IHC with MLH1 methylation | 630 | 630 | 0.0832 | 0.0832 | 7570 |
| MSI followed by IHC with MLH1 methylation | 720 | 90 | 0.0523 | –0.0309 | Dominated |
| IHC | 790 | 70 | 0.0849 | 0.0017 | 41,180 |
| MSI | 840 | 50 | 0.0853 | 0.0004 | 125,000 |
| IHC followed by MSI with MLH1 methylation | 870 | 30 | 0.0835 | –0.0018 | Dominated |
| MSI and IHC with MLH1 methylation | 890 | 20 | 0.0853 | 0.0000 | Dominated |
| IHC followed by MSI | 1026 | 186 | 0.0854 | 0.0001 | Extendedly dominated |
| MSI followed by IHC | 1029 | 189 | 0.0856 | 0.0003 | 630,000 |
| MSI and IHC | 1070 | 41 | 0.0854 | –0.0002 | Dominated |
| Germline testing | 1160 | 31 | 0.0828 | –0.0028 | Dominated |
Scenario analysis 5
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No testing | 0 | – | 0 | – | – |
| MSI with MLH1 methylation | 510 | 510 | 0.0413 | 0.0413 | Extendedly dominated |
| IHC with MLH1 methylation | 620 | 620 | 0.0659 | 0.0659 | 9410 |
| MSI followed by IHC with MLH1 methylation | 710 | 90 | 0.0414 | –0.0245 | Dominated |
| IHC | 780 | 160 | 0.0671 | 0.0012 | 133,330 |
| MSI | 830 | 50 | 0.0673 | 0.0002 | 250,000 |
| IHC followed by MSI with MLH1 methylation | 860 | 30 | 0.0661 | –0.0012 | Dominated |
| MSI and IHC with MLH1 methylation | 880 | 50 | 0.0661 | –0.0012 | Dominated |
| IHC followed by MSI | 1010 | 180 | 0.0675 | 0.0002 | 900,000 |
| MSI followed by IHC | 1020 | 10 | 0.0675 | 0.0000 | Dominated |
| MSI and IHC | 1060 | 50 | 0.0675 | 0.0000 | Dominated |
| Germline testing | 1150 | 140 | 0.0656 | –0.0019 | Dominated |
Scenario analysis 6
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No testing | 0 | – | 0 | – | – |
| MSI with MLH1 methylation | 475 | 475 | 0.0415 | 0.0415 | Extendedly dominated |
| IHC with MLH1 methylation | 570 | 570 | 0.0662 | 0.0662 | 8610 |
| MSI followed by IHC with MLH1 methylation | 680 | 110 | 0.0416 | –0.0246 | Dominated |
| IHC | 730 | 160 | 0.0674 | 0.0012 | 133,330 |
| MSI | 770 | 40 | 0.0677 | 0.0003 | 133,330 |
| IHC followed by MSI with MLH1 methylation | 800 | 30 | 0.0665 | –0.0012 | Dominated |
| MSI and IHC with MLH1 methylation | 830 | 60 | 0.0665 | –0.0012 | Dominated |
| IHC followed by MSI | 959 | 189 | 0.0678 | 0.0001 | Extendedly dominated |
| MSI followed by IHC | 963 | 193 | 0.0679 | 0.0002 | 965,000 |
| Germline testing | 1000 | 37 | 0.0660 | –0.0019 | Dominated |
| MSI and IHC | 1000 | 0 | 0.0678 | –0.0001 | Dominated |
Scenario analysis 7
| Strategy | Expected mean costs (£) | Incremental costs (£) | Expected mean QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No testing | 0 | – | 0 | – | – |
| MSI with MLH1 methylation | 530 | 530 | 0.0351 | 0.0351 | Extendedly dominated |
| IHC with MLH1 methylation | 660 | 660 | 0.0560 | 0.0560 | 11,790 |
| MSI followed by IHC with MLH1 methylation | 730 | 70 | 0.0352 | –0.0208 | Dominated |
| IHC | 810 | 150 | 0.0570 | 0.0010 | 150,000 |
| MSI | 860 | 50 | 0.0572 | 0.0002 | 250,000 |
| IHC followed by MSI with MLH1 methylation | 890 | 30 | 0.0562 | –0.0010 | Dominated |
| MSI and IHC with MLH1 methylation | 910 | 50 | 0.0562 | –0.0010 | Dominated |
| IHC followed by MSI | 1048 | 188 | 0.0573 | 0.0001 | Extendedly dominated |
| MSI followed by IHC | 1052 | 195 | 0.0574 | 0.0002 | 975,000 |
| MSI and IHC | 1090 | 38 | 0.0573 | –0.0001 | Dominated |
| Germline testing | 1190 | 138 | 0.0558 | –0.0016 | Dominated |
Probabilistic sensitivity analysis distributions and approach
The following tables summarise the distributions used for all model parameters. A two-stage bootstrapping approach was taken to combine uncertainty in the diagnostic and long-term models for the PSA. First, the long-term model was run probabilistically in R. This generated a set of jointly sampled (to allow for correlation between outcomes for relatives and probands) values for costs and QALYs for probands and relatives reflecting uncertainty in these parameters. The values were stored as a table and then used as a sampling frame in the diagnostic model. This meant that, for each PSA run, the number of probands and relatives identified by testing was sampled probabilistically, and then the costs and QALYs attributable to a proband and a relative were sampled from the table of PSA values generated from the long-term model. The resulting total costs and QALYs reflected uncertainty in all parameters across the two models, and were used to generate the PSA results reported.
Model input parameters required
| Variable | Base-case value | Distribution | Parameters |
|---|---|---|---|
| Test accuracy | |||
| Sensitivity IHC with MLH1 methylation | Fixed | ||
| Specificity IHC with MLH1 methylation | – | Beta | α = 56.10, β = 1.93 |
| Costs (£, 2018/19 prices) | |||
| GP visit | 39.00 | Log-normal | µ = 3.66, σ = 0.10 |
| IHC test | 210.00 | Log-normal | µ = 5.35, σ = 0.10 |
| MMR proband | 755.00 | Log-normal | µ = 6.63, σ = 0.10 |
| MMR relative | 165.00 | Log-normal | µ = 5.11, σ = 0.10 |
| Offer counselling | 28.25 | Log-normal | µ = 3.34, σ = 0.10 |
| Pre-test proband | 642.19 | Log-normal | µ = 6.46, σ = 0.10 |
| Post-test proband | 141.44 | Log-normal | µ = 4.95, σ = 0.10 |
| Pre-test relative | 514.13 | Log-normal | µ = 4.95, σ = 0.10 |
| Post-test relative | 141.44 | Log-normal | µ = 6.24, σ = 0.10 |
| CRC incidence, log-normal parameters | |||
| Constant (female with MLH1 and no previous CRC) | 4.306 | Multivariate normal | Mu = (4.306, 0.100, 0.531, 0.863, –0.118, –0.230) |
| Standard deviation | 0.567 | See Variance–covariance matrix used for colorectal cancer incidence in probablistic sensitivity analysis | |
| Coefficient for | |||
| MSH2 | 0.100 | ||
| MSH6 | 0.531 | ||
| PMS2 | 0.863 | ||
| Male | –0.118 | ||
| Previous cancer | –0.230 | ||
| CRC mortality | |||
| Stage I | 0.0090 | Log-normal | µ = –4.26, σ = 0.054 |
| Stage II | 0.0345 | Log-normal | µ = –2.95, σ = 0.014 |
| Stage III | 0.0977 | Log-normal | µ = –1.91, σ = 0.009 |
| Stage IV | 0.5440 | Log-normal | µ = –0.42, σ = 0.357 |
| Aspirin incidence rate ratio | 0.5800 | Log-normal | µ = –0.55, σ = 0.288 |
| CRC surveillance hazard ratio for incidence | 0.3870 | Uniform | 0.387, 1.000 |
| CRC stage at presentation | |||
| Without surveillance | |||
| Stage I | 68.5% | Dirichlet | 29.5, 4.5, 5.5, 3.5 |
| Stage II | 10.5% | ||
| Stage III | 12.7% | ||
| Stage IV | 8.12% | ||
| With surveillance | |||
| Stage I | 18.8% | Dirichlet | 7.5, 19.5, 8.5, 4.5 |
| Stage II | 48.7% | ||
| Stage III | 21.2% | ||
| Stage IV | 11.3% | ||
| CRC treatment costs | |||
| CRC treatment costs | See Table 3 | Gamma | Param1 = 25, Param2 = see Param2 values for Gamma distribution giving uncertainty around colorectal cancer treatment costs in probabilistics sensitivity analysis |
| Endometrial cancer incidencea | |||
| Gene | |||
| MLH1 by age (years) | |||
| 25 | 0 | Fixed | Not applicable |
| 40 | 0.019 | Beta | α = 3.4, β = 173.6 |
| 50 | 0.147 | Beta | α = 39.1, β = 226.6 |
| 60 | 0.273 | Beta | α = 62.7, β = 166.9 |
| 70 | 0.352 | Beta | α = 57.5, β = 105.9 |
| 75 | 0.370 | Beta | α = 48.9, β = 83.3 |
| MSH2 by age (years) | |||
| 25 | 0 | Fixed | Not applicable |
| 40 | 0.023 | Beta | α = 2.8, β = 119.1 |
| 50 | 0.175 | Beta | α = 32.5, β = 153.2 |
| 60 | 0.380 | Beta | α = 58.4, β = 95.3 |
| 70 | 0.465 | Beta | α = 54.4, β = 62.6 |
| 75 | 0.489 | Beta | α = 44.2, β = 46.17 |
| MSH6 by age (years) | |||
| 25 | 0 | Fixed | Not applicable |
| 40 | 0.023 | Beta | α = 0.1, β = 4.8 |
| 50 | 0.126 | Beta | α = 2.8, β = 19.7 |
| 60 | 0.283 | Beta | α = 10.7, β = 27.2 |
| 70 | 0.411 | Beta | α = 17.4, β = 24.9 |
| 75 | 0.411 | Beta | α = 13.7, β = 19.7 |
| PMS2 by age (years) | |||
| 25 | 0 | Fixed | Not applicable |
| 40 | 0 | Fixed | Not applicable |
| 50 | 0 | Fixed | Not applicable |
| 60 | 0.093 | Beta | α = 0.5, β = 5.2 |
| 70 | 0.128 | Beta | α = 1.0, β = 6.7 |
| 75 | 0.128 | Beta | α = 1.0, β = 6.8 |
Variance–covariance matrix used for colorectal cancer incidence in probabilistic sensitivity analysis
| 0.0048610 | 0.0024265 | 0.00306302 | –2.84316 × 10–5 | –0.001366422 | –0.001293855 | 0.001470131 |
| 0.002426593 | 0.016159274 | 0.006487453 | –0.000390521 | –0.00359213 | –0.000760428 | 0.005271669 |
| 0.003063026 | 0.006487453 | 0.110071236 | –0.000804802 | –0.006512378 | –2.36405 × 10–5 | 0.009192278 |
| –2.84316 × 10–5 | –0.000390521 | –0.000804802 | 0.005788262 | 0.003564862 | –0.003063665 | –0.001316882 |
| –0.001366422 | –0.00359213 | –0.006512378 | 0.003564862 | 0.009596563 | –0.003612567 | –0.006267887 |
| –0.001293855 | –0.000760428 | –2.36405 × 10–5 | –0.003063665 | –0.003612567 | 0.003639508 | 0.001641483 |
| 0.001470131 | 0.005271669 | 0.009192278 | –0.001316882 | –0.006267887 | 0.001641483 | 0.010196606 |
Param2 values for Gamma distribution giving uncertainty around colorectal cancer treatment costs in probabilistics sensitivity analysis
| 350.1648682 | 349.6213287 | 579.5803466 | 468.1965605 |
| 228.4956424 | 280.6336233 | 387.6690935 | 337.7470812 |
| 184.9288611 | 214.0709403 | 290.3755235 | 260.355419 |
| 127.1046208 | 138.1844644 | 179.4098447 | 174.6016107 |
| 55.1901643 | 61.83807046 | 62.42342068 | 32.27788397 |
Glossary
- Cascadee
- A relative of someone who presents with cancer of interest, who can be further identified as a first- or second-degree relative.
- Constitutional
- Present in every cell of the body.
- Deoxyribonucleic acid sequencing
- Gene sequencing to detect point mutations and small insertions or deletions in genes. Next-generation deoxyribonucleic acid sequencing is also used for copy number variation analysis. Next-generation sequencing is also referred to as ‘massive parallel sequencing’ or ‘second-generation sequencing’.
- Germline
- Inherited.
- Immunohistochemistry
- This is an index test performed on tumour tissue involving chemical staining of a selected panel of proteins to identify errors in these specific proteins.
- Incremental cost-effectiveness ratio
- Difference in costs divided by the difference in effects of two different treatment strategies/interventions to produce a summary ratio of cost-effectiveness.
- Lynch syndrome assumed
- Status given to probands with a positive tumour test but who have declined germline testing, or first-degree relatives who have declined germline testing.
- Lynch syndrome negative
- People who have had germline testing and have obtained a negative result. These may be probands or relatives.
- Lynch syndrome positive
- People who have had germline testing and have obtained a positive result. These may be probands or relatives.
- Lynch-like
- People who have had a negative germline test and a negative somatic tumour testing.
- Multiplex ligation-dependent probe amplification
- This is used to detect larger structural changes to genes (deletions, duplications or rearrangements); next-generation sequencing data can also identify structural variants.
- MutL homologue 1
- One of the four proteins identified leading to diagnosis of Lynch syndrome when a mismatch repair error occurs in one of these at germline level.
- MutS homologue 2
- One of the four proteins identified leading to diagnosis of Lynch syndrome when a mismatch repair error occurs in one of these at germline level.
- MutS homologue 6
- One of the four proteins identified leading to diagnosis of Lynch syndrome when a mismatch repair error occurs in one of these at germline level.
- Postmeiotic segregation increased 2
- One of the four proteins identified leading to diagnosis of Lynch syndrome when a mismatch repair error occurs in one of these at germline level.
- Probabilistic sensitivity analysis
- Modelling technique using sample distributions from across input parameters to reflect uncertainty in a decision problem.
- Proband
- A person who presents with a tumour of a cancer of interest.
- Putative Lynch syndrome
- Alternative term for people with Lynch-like diagnosis.
- Reference standard
- Germline testing of normal (non-tumour) tissue for constitutional mutations in mismatch repair genes (i.e. inherited mutations that are present in every cell). This involves both deoxyribonucleic acid sequencing and multiplex ligation-dependent probe amplification techniques.
- Selected sample
- A group of participants limited to only those with particular characteristics, for example aged < 50 years without a personal/family history of cancer.
- Somatic mutation
- Non-inherited mutations.
- Unselected sample
- A group of participants not limited to those with particular characteristics.
- Variant of uncertain significance
- People who have had a positive germline test, but the mutation found is not known to be pathogenic for Lynch syndrome.
List of abbreviations
- CA-125
- cancer antigen-125
- CAPP2
- Colorectal Adenoma/carcinoma Prevention Programme 2
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CRC
- colorectal cancer
- CRD
- Centre for Reviews and Dissemination
- DNA
- deoxyribonucleic acid
- EAG
- external assessment group
- EPCAM
- epithelial cellular adhesion molecule
- GP
- general practitioner
- H-BSO
- hysterectomy and bilateral salpingo-oophorectomy
- HNPCC
- hereditary non-polyposis colorectal cancer
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IHC
- immunohistochemistry
- MLH1
- mutL homologue 1
- MLPA
- multiplex ligation-dependent probe amplification
- MMR
- mismatch repair
- MSH2
- mutS homologue 2
- MSH6
- mutS homologue 6
- MSI
- microsatellite instability
- MSI-H
- microsatellite instability-high
- MSI-L
- microsatellite instability-low
- MSS
- microsatellite stable
- NGS
- next-generation sequencing
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- PCR
- polymerase chain reaction
- PETALS
- Proportion of Endometrial Tumours Associated with Lynch Syndrome
- PICO
- population, intervention, comparator and outcome
- PLSD
- Prospective Lynch Syndrome Database
- PMS2
- postmeiotic segregation increased 2
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QAREL
- quality appraisal tool for studies of diagnostic reliability
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies-2
- RCT
- randomised controlled trial
- SGO
- Society of Gynaecologic Oncology
- VUS
- variant of uncertain significance
- WTP
- willingness to pay
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
