Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/184/14. The contractual start date was in September 2017. The draft report began editorial review in December 2019 and was accepted for publication in October 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Daley et al. This work was produced by Daley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Daley et al.
Chapter 1 Background to the research
Prevalence of obesity and its consequences for health
In 2015, the Global Burden for Disease Obesity Collaboration estimated that almost 603.7 million people globally were living with obesity; this equates to a global prevalence of around 12%. 1 Approximately two-thirds of the adult population in England are classified as overweight or obese. 2 Sex differences in the prevalence of overweight and obesity have been reported, with more women than men affected. 3 Obesity is known to have negative effects on the physical, mental and social health of the population and their economic productivity. 4 Public Health England have reported that, between 2014 and 2015, obesity cost the NHS approximately £6.1B in treating associated health conditions. During 2017 and 2018, NHS hospitals in England admitted 711,000 patients with a health condition associated directly or indirectly with obesity; this is a 15% increase from 2016 and 2017. 5,6 Some people who are obese may also experience stigmatisation, which can have a detrimental effect on their mental health, quality of life and well-being. 7,8
Prevalence of obesity and overweight in women of reproductive age
The main child-bearing years of women (aged 25–34 years) hold a higher risk of weight gain than any other age for women and all ages for men. 9 A pooled analysis of global body mass index (BMI) trends estimated that the average BMI for women increased by 0.59 kg/m2 (95% credible interval 0.49 to 0.70 kg/m2) each decade between 1975 and 2014, which equates to the global population becoming, on average, 1.5 kg heavier every 10 years. 10 It has been estimated that about 20% of women in the UK aged 25–44 years are living with obesity, and approximately 50–60% of women in this age category are overweight. 11 Evidence also suggests that women living in more deprived areas of the UK are more likely to be overweight or obese. 12,13 This prevalence of overweight and obesity among women in this age category may be explained by that fact that it coincides with women’s reproductive years.
Excessive gestational weight gain
Regardless of pre-pregnancy weight status, most women gain above the recommend weight during pregnancy. 14,15 A UK-based longitudinal study found that the number of women experiencing maternal obesity at an early stage of pregnancy had increased over a 15-year period, and that this was more common among women who experienced higher levels of socioeconomic deprivation. 16 It is estimated that, on average, women gain about 14–15 kg during pregnancy and at 1 year after birth 5–9 kg is retained, with an average BMI of 29.4 kg/m2. 2,17–20 One explanation for excess weight gain during pregnancy is the traditional ideology of ‘eating for two’,21,22 which is a common explanation given by women who feel that pregnancy is a time during which they can eat what they choose without restraint. The perceived social pressures placed on women to remain slim are also relaxed during pregnancy. 23 Physical activity levels also typically decrease during pregnancy, so energy expenditure is reduced. 24
Health effects of excessive gestational weight gain
The negative health outcomes of maternal overweight and obesity for the mother and the baby have been well documented. 25–28 Many of the health risks are inter-related and include the development of gestational diabetes, pre-eclampsia and gestational hypertension in the mother. There may also be perinatal complications for the baby, such as macrosomia, newborn hypoglycaemia, jaundice, shoulder dystocia, asphyxiation and stillbirth. 29–31 A systematic review of 11 cohort studies that included data from almost 1 million women reported that an increase in BMI (by one unit or more) was associated with a 56% increased risk [95% confidence interval (CI) 1.48 to 1.96 adjusted odds ratio] of gestational diabetes in the next pregnancy. The risk of gestational diabetes was further exacerbated when maternal BMI increased by more than three BMI units between pregnancies. The review also reported that a moderate increase in BMI of two units or more increased the likelihood of a caesarean section by approximately 16%. 32 Between 2011 and 2013, approximately 50% of the women who died during pregnancy in the UK had complications associated with being overweight or obese. 33 Another systematic review and meta-analysis34 identified a 264% increase in the odds of child obesity when mothers have obesity before conception.
Postnatal weight retention
Many women report pregnancy as the critical period for the onset of excess weight, which can significantly increase the risk of later obesity and serious chronic diseases including type 2 diabetes, heart disease and cancer. 35–39 Most women will not lose all of their pregnancy-related weight gain. 17,36,40 A UK-based prospective cohort study (n = 2559) of postnatal women noted that, by 6 months postnatally, about 73% of women retained at least some of the weight gained during pregnancy. 41 Prospective observational studies have reported that, among women who have a healthy BMI prior to pregnancy, 30% are overweight 1 year after giving birth. Of women who are overweight prior to conception, 44% are obese by 1 year after giving birth, and 97% of women who are obese prior to pregnancy remain so 1 year postnatally. Studies have also suggested that women who gain excessive weight during pregnancy are more likely to retain or gain additional weight during the first 1–2 years following childbirth. 42 Of note, evidence has also indicated that women who lost all pregnancy weight within 6 months of giving birth, irrespective of breastfeeding status, were only 2.4 kg heavier 10 years after childbirth, whereas women who retained postnatal weight were 8.3 kg heavier at the 10-year follow-up. 43 Findings from a systematic review involving 17 studies44 reported that postnatal weight retention was more attributable to excessive gestational weight gain than pre-pregnancy BMI. This is important because many women will have successive pregnancies and their weight retention will pose risks to their long-term health, as well as increasing the risk of adverse outcomes for the infant during each pregnancy. 20,45,46 The National Institute for Health and Care Excellence (NICE) public health guidance47 has highlighted gaps in the evidence about acceptable and effective weight management interventions for postnatal women. Of interest here, in 2018 the Royal College of Obstetricians and Gynaecologists acknowledged that more women were conceiving when they were overweight or obese, and that women of childbearing age with a BMI of ≥ 30 kg/m2 should receive information and advice about the risks of obesity during pregnancy and childbirth, and be supported in losing weight before conception and between pregnancies, in line with NICE guidance. 47,48 More specifically, women should be supported in losing weight in the postnatal period, and women who are overweight should be offered referral to weight management services if available.
Postnatal weight retention and mental health
There is good evidence of an association between mood/postnatal depression and postnatal weight retention. 49,50 Low mood or elevated depressive symptomatology can negatively affect the bonding relationship between the mother and her child. 51 This relationship is more pronounced in women who were obese prior to pregnancy. 52 These findings are relevant because poor bonding can have long-term consequences for the child, including delays in social, cognitive and emotional development. 53
Parenthood often requires a shift in priorities. Qualitative evidence exploring the views of women has indicated that women may prioritise the care of their child over their own personal needs, and typically tend to be responsible for most child-care duties. 54–56 During the early postnatal period, there can be multiple demands and challenges associated with looking after a baby that have to be managed alongside adjustments to daily routines, changes in relationships with family and friends, recovery from childbirth, and the physical and emotional effects of recent pregnancy and childbirth. 57,58 The postnatal period can also be an emotionally delicate and demanding time for new mothers, and weight retention and/or poor self-image may be factors in the development of reduced mental health. 59,60
The postnatal period, particularly the first 6 months, is often characterised by sleep deprivation for the mother, which can consequently affect decisions about health behaviours. Evidence from systematic reviews has identified an inverse association between the amount of sleep and postnatal weight retention. 61 In addition, if women initiate poor health behaviours during the early postnatal period, there is a risk that these behaviours may become established in the mothers’ new routine with their baby and the wider family. 62,63 Moreover, studies64–66 involving a range of populations have shown that sleep deprivation is related to poor eating behaviours and weight gain. Sleep deprivation and fatigue are also likely to reduce women’s ability and motivation to engage in regular physical activity.
Weight management interventions
General populations and weight management interventions
A range of lifestyle interventions have been tested to support weight loss or prevent weight gain. The testing of interventions to facilitate weight maintenance is also a growing area of research endeavour. Lifestyle behavioural interventions usually involve asking participants to make changes to their dietary habits and/or increase their physical activity levels using cognitive and behavioural strategies, such as goal-setting, restraint of eating, self-regulation, relapse prevention and finding social support. A systematic review67 of weight loss interventions involving obese and overweight adults reported that a combination of a low-energy diet and participation in physical activity was more effective for weight loss than diet-only interventions.
Several systematic reviews have demonstrated the effectiveness of group-based commercial weight loss programmes. 68,69 The group setting of these programmes is preferred by many, as it instils group social support that can facilitate behaviour change. 70 In 2006, both NICE and the Department of Health and Social Care recommended that primary care clinicians identify people affected by overweight and obesity and offer assistance with weight management. 71,72 Despite the evidence of effectiveness and cost-effectiveness of commercial weight loss programmes, this approach is not currently offered as treatment by many Clinical Commissioning Groups. 73,74 However, NICE guidance still recommends that people living with obesity or those most at risk of developing type 2 diabetes mellitus should be referred by their general practitioner (GP) to locally available weight loss programmes. 75 Weekly attendance at these programmes may not be suitable for all patients, however, owing to the cost of attendance and the high level of commitment required to attend weekly group meetings. Furthermore, this approach might not be suitable for those who have caring responsibilities, such as postnatal women.
Postnatal weight management interventions
Research shows that, irrespective of age or ethnicity, postnatal women would prefer to weigh less, are interested in implementing strategies to lose weight and would like help to do so. 76–78 During pregnancy and soon after childbirth, women may be more open to receiving support/advice about weight management; this may, therefore, be an ideal time to encourage the development of healthy lifestyle habits. 79 Weight management interventions may not only help women to lose any weight gained during pregnancy, but also have the potential to create a healthier environment for the whole family, providing further value and benefits. 80,81 Evidence has also highlighted that women would welcome additional weight management support after having a baby, as very little support is currently offered by the NHS. 82–84
Evidence from systematic reviews of randomised controlled trials in the postnatal period
Many studies, adopting a variety of methodological designs, have tested a range of weight loss interventions during the postnatal period. 85,86 To provide a comprehensive up-to-date quantitative summary of the evidence, the authors of this report (AD and HP) completed a systematic review86 in which the specific purpose was to both descriptively and statistically (using a mega meta-analysis) summarise the findings of systematic reviews of randomised controlled trials (RCTs) that have examined the effectiveness of behavioural lifestyle interventions for weight loss in postnatal women. A mega meta-analysis can be useful because it provides a comprehensive statistical summary of all of the available evidence across eligible systematic reviews. Mega meta-analysis is also helpful when previous systematic reviews have not been able to perform meta-analysis and/or subgroup analyses because of a lack of trials.
Nine systematic reviews of RCTs were eligible for inclusion in the review and 22 unique trials from across the nine systematic reviews were eligible for inclusion in the mega meta-analysis. Table 1 shows details of the inclusion and exclusion criteria used in the review. Women who were randomised to a lifestyle intervention had a significantly lower body weight than comparators at the last follow-up visit (mean difference –1.7 kg, 95% CI –2.3 to –1.1 kg) (Figure 1). The results by subgroup are shown in Figure 2. Of interest here, the review did not find any RCTs that tested an intervention that was embedded in routine health-care appointments for postnatal women, and the review concluded that this might be a pragmatic way to offer support to all postnatal women who wish to lose weight after giving birth.
| Selection criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study type |
|
|
| Population |
|
|
| Intervention |
|
|
| Main outcome |
|
FIGURE 1.
Overall mean difference in weight change (kg) and subgroup analysis (intervention type). This figure has been reproduced with permission from Ferguson JA, Daley AJ, Parretti HM. Behavioural weight management interventions for postnatal women: a systematic review of systematic reviews of randomized controlled trials. Obesity Reviews. 86 © 2019 World Obesity Federation.
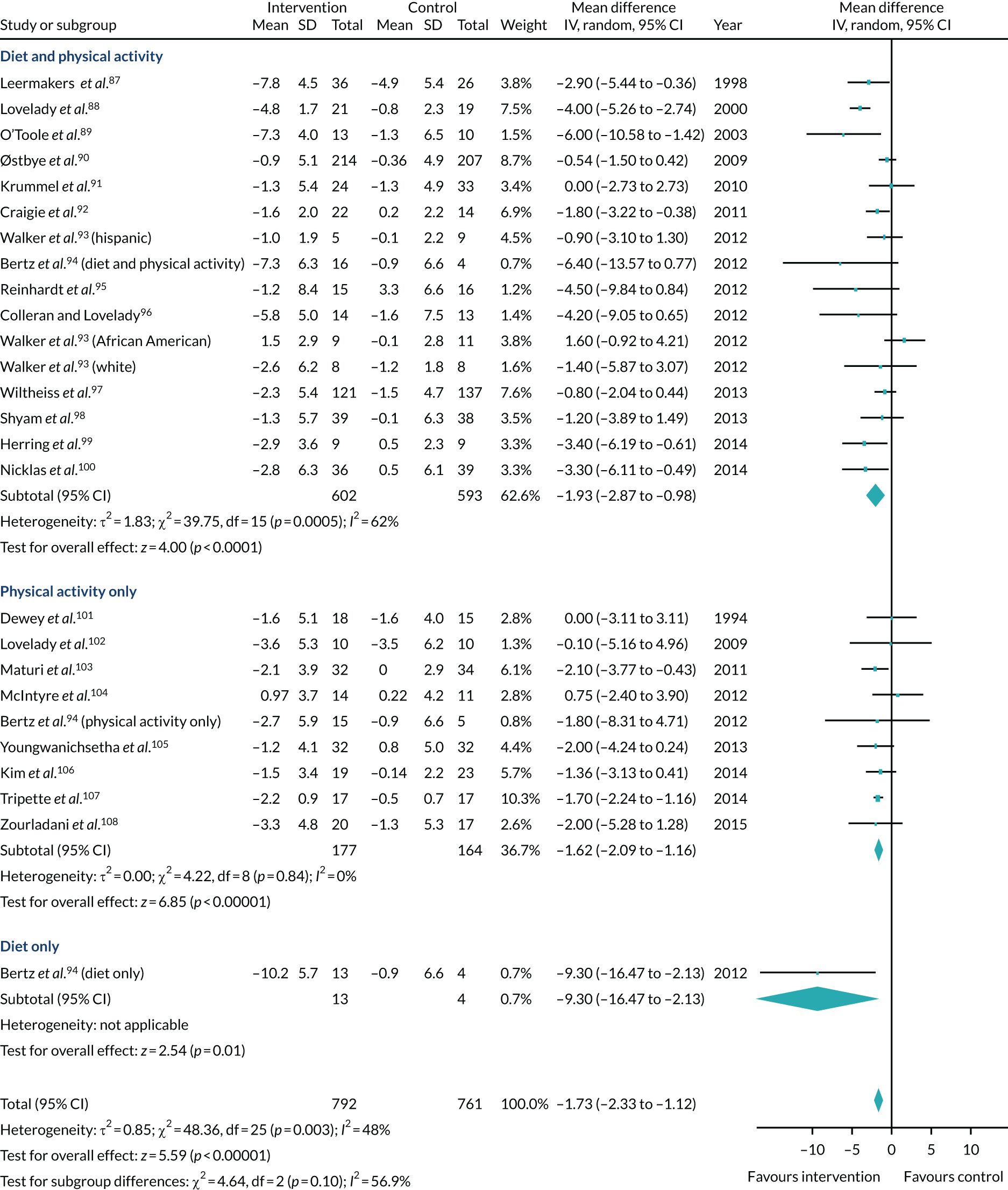
FIGURE 2.
Mean difference in weight change (kg) by subgroup analysis (length of intervention). Note that this analysis was based on end-of-intervention weights recorded in the trials and two trials90,97 were excluded (as no end of data were reported). Therefore, the figures in this forest plot differ to those shown in Figure 1. This figure has been reproduced with permission from Ferguson JA, Daley AJ, Parretti HM. Behavioural weight management interventions for postnatal women: a systematic review of systematic reviews of randomized controlled trials. Obesity Reviews. 86 © 2019 World Obesity Federation.
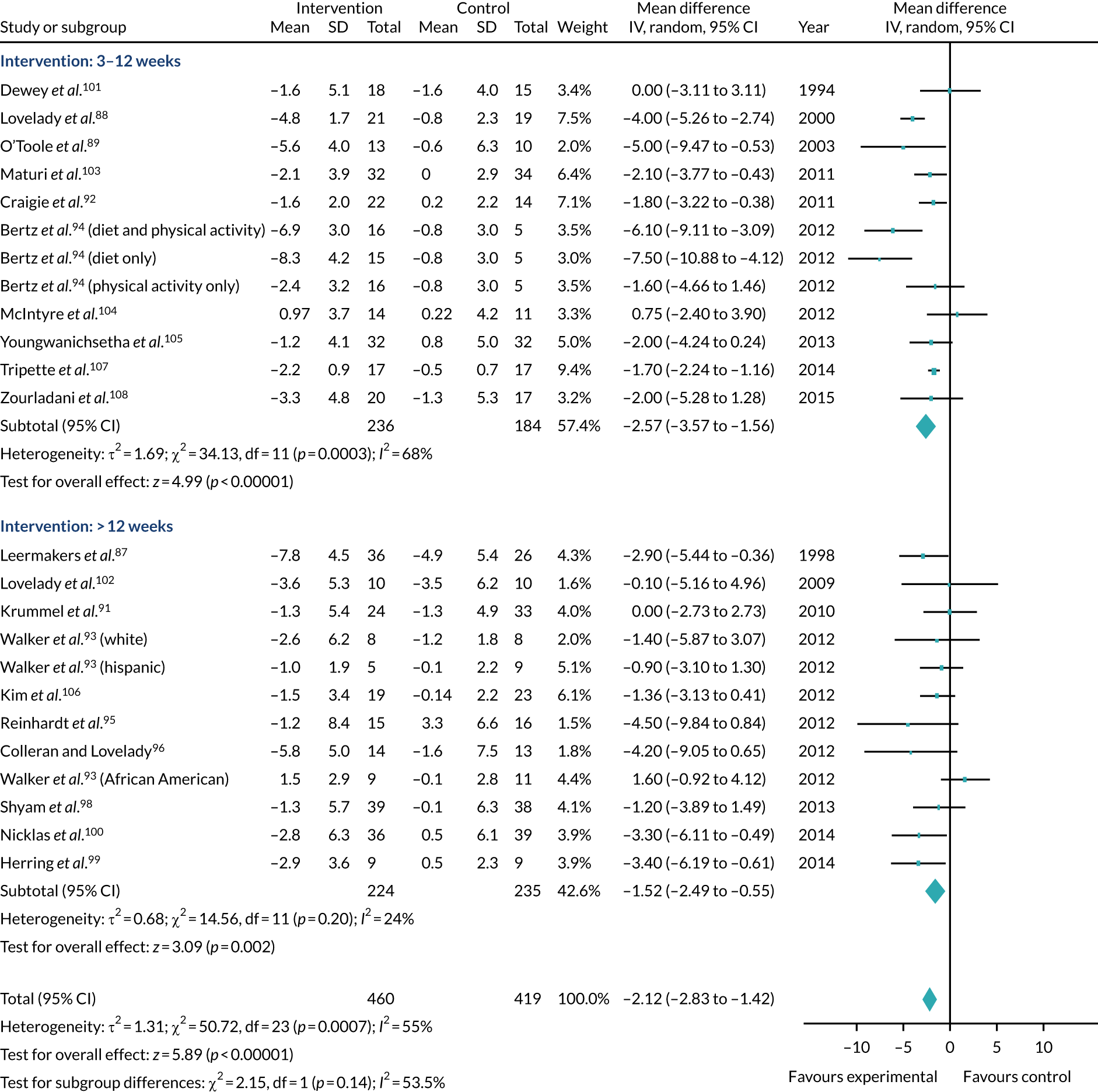
Analysis showed that interventions that involved both diet and physical activity interventions, physical activity interventions alone and dietary interventions alone were moderately effective in reducing weight relative to comparator groups, although there was only one trial in the diet-only analysis. That said, the amount of weight loss does not need to be large to bring health benefits. 109,110 Modelling has shown that even if a small amount of weight is lost, this weight loss remains cost-effective if the weight regained occurs on a lower weight trajectory. 111 Furthermore, as the relationship between obesity and mortality is linear, even small amounts of weight loss may be clinically important. 38,112,113 Clinical guidance from NICE suggests that weight loss of approximately 2 kg can contribute towards a meaningful reduction in cardiovascular disease risk and type 2 diabetes mellitus. 109
It is important to highlight, however, that many of the RCTs included in the systematic reviews recruited small samples of participants, many of whom were white and middle class. 114,115 Furthermore, many of the tested interventions were intensive lifestyle-based programmes, were tailored to each individual woman and were frequently delivered by skilled health professionals, such as psychologists and dieticians. 116–118 Despite evidence suggesting that some of these interventions were effective, these intensive interventions require substantial resources to be scaled up to offer them to every postnatal woman in the UK who would benefit from them. Resource-intensive interventions cannot be delivered to all 820,000 women who give birth each year in the UK, 520,500 of whom will be overweight. 119 Furthermore, the acceptability of some of the interventions tested could be questioned given their reported high drop-out rates and/or poor levels of intervention attendance. 87,92,120 Attempts have been made to conduct studies that might be more appealing to a broader range of women from different ethnicities and socioeconomic backgrounds; however, they have also reported poor adherence and high drop-out rates. 121 Taken together, this evidence has highlighted the need for more high-quality RCTs that examine more acceptable and effective weight management interventions, designed to be suitable for postnatal women. 122 There is also a need for data regarding the cost-effectiveness of weight management interventions for postnatal women.
Self-management and self-regulation of weight
One solution that avoids the need for intensive resources to deliver postnatal behavioural weight management interventions is the provision of interventions that encourage self-management and self-regulation of weight. Research evidence has been inconsistent regarding the preferences of postnatal women for different types of weight management interventions, with some reporting that women prefer to attend group-based sessions123,124 and others reporting that home-based self-care interventions are preferred owing to issues such as time constraints, convenience and child care requirements. 92,125 Self-help interventions may, therefore, be attractive to this population of women, given that they are a low-cost, varied, flexible option that can be tailored to their specific needs. Given that many postnatal women may find it difficult to attend formal weight loss programmes and some have expressed a preference for home-based programmes, self-help interventions for postnatal weight loss are worthy of consideration. 86 In a systematic review126 of RCTs that aimed to determine the effectiveness of self-help interventions for weight loss (self-help defined as interventions that could be delivered without person-to-person support, including formats such as smartphone, web and print), analyses showed that self-help interventions that require no human input for delivery led to a small, but significantly greater, weight loss than unsupported attempts to lose weight at 6 months compared with minimal interventions (–1.9 kg, 95% CI –2.9 to –0.8 kg). However, results were variable and the reasons for this heterogeneity were unknown. In addition, in the small number of studies providing data at 12 months weight loss was no longer significant; the effect size was comparable with that achieved at 6 months, suggesting that self-help interventions on their own may not be useful for sustaining long-term weight loss, and that additional components within weight loss interventions are required. Nonetheless, given the potential scalability and relatively low cost of this type of intervention, self-help programmes may be a useful component within a broader intervention to treat women who are overweight in primary care.
Self-monitoring of weight
Trials of self-management interventions for weight loss can inform our knowledge about which types of self-directed weight loss strategies are most effective and which may be usefully highlighted to the public and policy-makers as scalable, low-cost interventions. One such intervention that has shown promise in helping people manage their weight is regular weighing to check progress against a target: a form of self-monitoring. Self-monitoring is defined as the procedure by which individuals record their own target behaviours. 127
The potential efficacy of regular weighing (either by the individual or by someone else) has been based on the principles of self-regulation theory. 128,129 Self-regulation has been described as a process that has three distinct stages: self-monitoring, self-evaluation and self-reinforcement. Self-monitoring is a method of systematic self-observation, periodic measurement and recording of target behaviours, with the goal of increasing self-awareness. The awareness fostered during self-monitoring is considered an essential initial step in promoting and sustaining behaviour change.
Strong evidence supports the role of self-monitoring as an effective strategy that leads to decreases in unwanted behaviours and increases in desired behaviours in many health areas, including diet and physical activity. 130 Self-monitoring is often described as a central component of behavioural treatment for weight loss,131 which may include monitoring food intake, physical activity and other outcomes, such as weight, size and body shape. 132 Reviews by Michie et al. 133,134 of effective behavioural techniques for healthy eating, physical activity and reduction in alcohol consumption reported that self-monitoring was effective alone, but when combined with other techniques the effect size nearly doubled. In a systematic review of RCTs by Madigan et al.,135 to examine the effectiveness of self-weighing as a strategy for weight loss, one of the included studies examined self-weighing as a single strategy and found it to be ineffective (–0.5 kg, 95% CI –1.3 to 0.3 kg); however, adding self-weighing/self-regulation techniques to programmes resulted in a significant difference of –1.7 kg (95% CI –2.6 to –0.8 kg). Multicomponent interventions, including self-weighing compared with no/minimal control, also resulted in mean differences of –3.7 kg (95% CI –4.6 to –2.9 kg). 135 With regard to postnatal women specifically, a systematic review to determine effective behaviour change techniques in physical activity interventions for this population found that effective interventions always included self-monitoring and goal-setting. 136
Accountability/audit and feedback for weight loss
External support for weight management has been shown to be a key factor in successful weight loss because of the feeling of accountability that it can foster. 137–139 Having to explain one’s actions to another individual can change behaviour and keep individuals motivated and focused on their weight loss goals. 140,141 In practical terms, if people know that their weight is being monitored externally, they may feel more obligated to try and adhere to their weight loss goals. Bovens142 has suggested that two concepts of accountability exist: one is a virtue and the other is a mechanism. Here the focus is on accountability as a mechanism and, therefore, consists of ‘an obligation to explain and justify conduct’. 142 The concept of accountability is well versed in the areas of business and organisational change management literature, in which it has been shown to keep individuals engaged and focused on their task and ensure that goals are achieved. In recent years, the role of accountability has also been applied in the health sector, particularly in relation to weight management. For example, research has shown that couples or ‘buddies’ can positively influence the health behaviours of the other partner. 143–145 In the review of self-weighing and weight loss by Madigan et al. ,135 there was some evidence suggesting that adding accountability to a self-weighing programme improves effectiveness.
Participants in group weight loss programmes often report that it is the weekly weigh-in that is the most salient component of the programme that provides external accountability and keeps them committed to their diet and physical activity plan. 146 In practical terms, if a person knows that their weight will be monitored they are more likely to make healthy lifestyle choices and, therefore, are more motivated to stick to their weight loss goals. Related to this, Gardner et al. 147 have conducted a systematic review examining similar behaviour change techniques to accountability, called audit and feedback. They investigated whether or not audit and feedback changed health-care professionals’ behaviour and found a significant effect (odds ratio 1.43 kg, 95% CI 1.28 to 1.61 kg). Audit and feedback are similar to accountability in that participants are aware of being observed. Based on this evidence, it can be hypothesised that adding accountability/audit to self-help and self-monitoring interventions could further facilitate weight loss.
Raising the topic of weight
Health professionals working in primary care have access to a wide proportion of the population and the ability to offer a range of important information as a trusted source of advice and support; therefore, they are well positioned to address overweight and obesity in the population, as most people are registered with a general practice. NICE Clinical Guideline 189109 suggests that health-care providers should use their clinical judgement to decide when to opportunistically weigh patients, but few do. 148 NICE gives guidance on the appropriate weight management advice and treatment options to offer to patients who are overweight or obese; however, this tends to occur at the discretion of the health-care provider. Evidence has suggested that GPs and nurses are apprehensive about raising the topic of weight with their patients. 149 Some of the reasons given for this reluctance include a fear of causing offence, not feeling that they have the skills to raise the topic and a lack of appropriate treatment pathways. 150–153 Research has also identified that health professionals consider weight management unrewarding and are pessimistic about patients’ motivation to lose weight. 153
The provision of more weight management interventions in primary care settings may enable more people to receive treatment. Furthermore, it may also make it easier for the topic to be discussed because it ‘normalises’ the process of raising the topic. Having these conversations more routinely may also help to remove some of the societal stigma associated with overweight and obesity. 154,155 It is also interesting to highlight that research with GPs has suggested that, when raising the topic of weight, most patients are not offended, with such discussions being perceived as helpful by patients. 156
The use of a brief intervention by health professionals to raise a specific health topic has been shown to be 10 times more useful for initiating a behaviour change programme than leaving the responsibility exclusively to the patient. 157 The use of brief interventions involving health-care professionals signposting patients presenting in primary care to seek weight management support shows promise of effectiveness. This trial found that a simple approach of GPs raising the topic of weight and referring obese adult patients to commercial weight management programmes during routine consultations was effective in facilitating weight loss. 156 Nevertheless, postnatal women are a unique subgroup of the population who experience many challenges and barriers that may impact their ability, willingness and motivation to regularly attend commercial weight loss programmes, for example availability of child care, cost, child-feeding routines, reduced mental health and disrupted sleeping patterns.
Technology and weight management
Various health policies have identified digital technologies as a promising vehicle for health behaviour change. NHS England’s Next Steps on the NHS Five Year Forward View158 strategy highlighted that better use of information and technology to help people manage and improve their own health can help meet the health need of the growing and ageing population, and reduce pressure on services. 158 The use of technology for behaviour change has also been identified as an opportunity in Public Health England’s 5-year strategy. 159 Observational studies have reported that, irrespective of age or socioeconomic status, about 91% of new mothers use the internet as a source of information, with weight loss as the fourth most-searched topic. 160 Technology-based interventions offer self-regulatory features with the opportunity to promote self-awareness of health behaviours, and they can include a tracking system to enhance self-evaluation and self-reinforcement through monitoring devices. 161,162 Moreover, technology offers the potential to offer women a flexible approach to the management of their weight, providing the opportunity to access regular help and support.
Systematic reviews of eHealth or web-based interventions163–166 for weight loss and/or maintenance in adults have found that these interventions can result in modest weight loss in overweight/obese adults. For example, a systematic review (n = 224 studies)164 reported that internet/mobile telephone interventions improved diet, physical activity, obesity, tobacco use and alcohol use up to 1 year. Health professional involvement may also improve the effectiveness of digital or mHealth interventions. 165 More specifically, a systematic review166 reported that internet-based weight management interventions for postnatal women appeared to be beneficial in reducing weight. This evidence suggests that it would be appropriate and potentially beneficial to include the assistance of technology in the development of weight management programmes for postnatal women.
Primary care settings, child immunisations and weight management
Primary care serves as a gateway into the NHS for the population, which means that the public have regular contact with health professionals in this context. As primary care operates throughout the UK, it can provide the opportunity and framework through which large-scale weight management interventions can be delivered, with the added potential of being able to access hard-to-reach populations, thereby reducing health inequalities. Public Health England recommends that all children under the age of 5 years in the UK receive a schedule of routine immunisations that include measles, mumps, rubella, polio, tetanus, diphtheria, pertussis, Haemophilus influenzae type b, meningococcal groups B and C, hepatitis B, rotavirus gastroenteritis and pneumococcal disease (13 serotypes). 167 These immunisations are provided by general practices and are usually administered by practice nurses. During their first year, babies receive immunisations when they are 8, 12 and 16 weeks old, as well as at 1 year. In 2019, 92.1% of children had completed the primary course of immunisations by their first birthday. 167 Each child is provided with a personal child health record book, or ‘red book’ at birth, in which health-related information, including immunisations, is recorded. Parents are asked to bring this red book with them to each child immunisation appointment so that delivery of the immunisation can be recorded. Given that the vast majority of postnatal women will have regular contact with primary care services to have their child immunised, these types of contacts provide an opportunity to reach all postnatal women to offer weight management interventions. This also aligns well with the ambition of the NHS to ‘Make Every Contact Count’: to deliver health behaviour change interventions to the population during routine health-care consultations.
Trial rationale
Given the consequences of obesity, the large numbers of women having babies each year who retain weight gained during pregnancy and the NHS resource implications of later health-care needs, there is a need to evaluate pragmatic, low-cost interventions that could facilitate postnatal weight loss at a population level. Moreover, NICE has highlighted the low quality of previous research as a limitation to developing clinical guidance in this area. 47,168 There is a need to intervene routinely and early in the postnatal period, to help women to manage their excess weight after having a baby and to minimise the long-term physical and mental health risks. This may also have additional benefits by reducing weight at the start of subsequent pregnancies. Interventions to promote a healthy diet and physical activity may also raise awareness of the importance of healthy lifestyle habits that will also be of benefit to the baby. Developing and testing postnatal interventions is also important, given that lifestyle interventions during pregnancy have had only modest success to date in preventing excessive gestational weight gain. 169,170
The intervention proposed here will be delivered in the context of the national child immunisation programme in primary care to minimise the costs to the NHS and to avoid the need for additional contacts with health professionals at this busy time in women’s lives. In the UK, infants are vaccinated four times in the first year of life as part of the child immunisation programme, which has a coverage rate of 92%. 167 We propose to embed a simple and brief intervention into this national child immunisation programme. The intervention does not require additional visits or expenses for postnatal women; thus, the sustainability of the intervention is likely to be high and income and/or ethnicity will not be barriers to participation. This approach also provides the opportunity for early intervention to reduce the possibility of women gaining further weight after childbirth. Although we expect our intervention approach to result in a smaller effect than the intensive interventions tested in previous trials, because of its widespread applicability and scalability it could have a larger population-level impact.
Aims of this study
The primary aim of this study was to produce evidence that a large-scale Phase III cluster RCT of a weight management intervention in which women engage in managing their own weight, by self-monitoring their weight and by accessing an existing online weight loss programme for support, is feasible. Should the intervention be feasible and acceptable, the ambition was to undertake a large-scale Phase III cluster RCT to assess the clinical effectiveness and cost-effectiveness of the intervention in facilitating long-term weight loss.
Objectives
The objectives of this research were to:
-
assess the feasibility of delivering an intervention to promote self-management of weight loss through self-monitoring of weight and signposting to an online weight management programme by practice nurses as part of the UK child immunisation programme in women who have recently given birth
-
assess recruitment to ensure that a full-scale Phase III cluster trial is feasible
-
determine levels of adherence to the intervention (acceptability)
-
determine the extent of participant burden in completing the trial questionnaires
-
collect data on immunisation uptake rates (to check that the study does not have a detrimental effect on rates)
-
determine the potential risk for intervention contamination (whether or not participants in the control group have spontaneously accessed the online programme) to assess if the main trial sample size will need to be adjusted to account for this
-
provide estimates of the variability in the primary outcome (weight) to inform the sample size for the Phase III trial
-
assess the impact of the intervention on breastfeeding rates and psychological health in both groups
-
explore practice nurses’ views about delivering the intervention and explore any variation in intervention delivery to ascertain if any adjustment to nurse training is required using semistructured interviews
-
explore the acceptability of the intervention based on feedback from participants through semistructured interviews
-
explore the acceptability/validity of the ICEpop CAPability measure for adults (ICECAP-A) for the cost-effectiveness analysis in the Phase III trial.
Chapter 2 Methods
Design considerations
Before undertaking a large-scale Phase III cluster RCT to assess the clinical effectiveness and cost-effectiveness of an intervention, it is important to assess the feasibility and acceptability of such a trial. Although individually randomised trials can be less prone to selection bias and cheaper than cluster trials, a cluster design helps avoid the possibility of contamination in the comparator group. In this trial, practice nurses were trained to deliver the intervention. If an individual-randomisation design had been used, nurses could potentially use aspects of their training with participants assigned to the usual-care group. It is also possible that women registered at the same practice or living near each other (by virtue of being registered at the same practice) could potentially share information or intervention resources. Cluster randomisation helps to avoid the possibility of this contamination in usual-care participants.
Practices (clusters) were randomised to either the weight management intervention or the comparator trial group. To avoid the possibility of selection bias, which can be a concern in cluster trials, it is recommended that the randomisation of the clusters (in this case practices) occurs once the participants have been identified and recruited into the trial. In this trial, it was not possible to allocate practices to the trial groups after participants were recruited because the required number of births per practice could occur over several months, meaning that we would miss the immunisation visits in which the intervention is being delivered.
Setting
This trial took place in Birmingham, where about one-third of the population are of non-white ethnicity (compared with 13% in England). Birmingham has high levels of socioeconomic deprivation, with 40% of the population living in super output areas in the 10% most deprived areas in England.
Ethics approval and study sponsor
Favourable ethics approval for this study was obtained from Black Country Ethics Committee (reference number 236462). The University of Birmingham (UoB) was the sponsor for this trial. The day-to-day management of the trial was co-ordinated by the Birmingham Clinical Trials Unit (BCTU) at the UoB. BCTU is fully registered as a UK Clinical Research Collaboration clinical trials unit.
Trial Steering Committee
The Trial Steering Committee (TSC) met six times during the course of the study to assess its progress. The TSC included five independent members.
Patient and public involvement
As part of the Collaborations for Leadership in Applied Health Research and Care – West Midlands, a maternity Public and Researchers Involvement in Maternity and Early Pregnancy (PRIME) group was established to support and guide maternity-related research. The application proposal for this study was presented to six members of the PRIME group prior to submission and their views were incorporated into the application. Two members of the public, both of whom were married, mothers to three children, of white ethnicity and employed, were invited to join the study management group and attended all of the TSC meetings either in person or occasionally by teleconference. Their purpose and role were to help guide the research team and offer their experiences during both the trial and the qualitative study. Both patient and public involvement (PPI) representatives had recently given birth and were, therefore, able to offer feedback based on their very recent experiences of attending their general practice to have their child immunised. One of the representatives also had a personal interest in weight management after giving birth. All of the patient-facing trial documentation was viewed and commented on by the PPI representatives, and their feedback was incorporated into the documents. The PPI representatives were consulted regularly throughout all stages of the study, particularly regarding their views about different approaches to participant recruitment, their thoughts on the questions to be included in the interview topic guide and their views on planning for a subsequent Phase III trial. The research team found the feedback from the PPI representatives very useful; it kept the team ‘grounded’ and realistic in their expectations of participants. In a future trial, it would be important to invite mothers from non-white ethnic backgrounds to join the PPI group, to ensure that the views of women from a range of ethnic backgrounds are embedded in the management of the trial. The PPI representatives were reimbursed in line with the INVOLVE guidelines.
Design
A cluster randomised controlled feasibility trial design was used to assess the feasibility and acceptability of the intervention. General practice was the unit of randomisation. All women who had recently given birth and registered at participating practices were invited to take part. Group allocation was concealed from participants until baseline data were collected. To mitigate against selection bias, all women registered at general practices who gave birth at Birmingham Women’s Hospital (BWH) were sent an invitation letter inviting them to take part in the study. The trial has been reported in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for the reporting of trials.
Eligibility criteria
A complete list of all of the inclusion and exclusion criteria is detailed in the following sections, and these were applied at various stages during the recruitment process.
Inclusion criteria
-
Women aged ≥ 18 years.
-
Women who were at least 4-weeks postnatal and who had not yet attended their first child immunisation appointment.
-
Women planning to have their child immunised as part of the national immunisation programme.
-
Women with a BMI of ≥ 25kg/m2 at the time of recruitment at the baseline home visit.
-
Women able and willing to provide written informed consent.
Exclusion criteria
-
Women whose babies had died or had been removed from their care at birth.
-
Women who indicated that they were already actively involved in a weight loss programme or weight management trial to lose weight.
-
Women who were unwilling to give consent to notify their GP of their involvement in this study.
-
Women who had been diagnosed with a serious mental health difficulty requiring hospitalisation or with anorexia and/or bulimia in the past 2 years.
Methods of recruitment
Screening via Birmingham Women’s Hospital
Computerised systems at BWH allowed for systematic identification of all postnatal women who had recently given birth, regardless of socioeconomic status and ethnicity, which reduced the potential for recruitment and selection bias. Every 2 weeks, BWH conducted searches of women aged ≥ 18 years who had recently given birth and were registered at participating practices. A trial invitation letter and participant information sheet were mailed to these women from BWH asking them to contact the study researchers if they were interested in the trial. Women did not receive their letter of invitation until at least 4 weeks post delivery. BWH applied the following initial screening criteria before sending study letters of invitation to women:
-
confirmed that the participant was aged ≥ 18 years
-
confirmed that the participant had given birth at least 4 weeks previously
-
confirmed that the participant was registered at one of the participating practices
-
excluded mothers whose babies had died or had been removed from their care at birth.
The invitation letter and participant information sheet included a telephone number that potential participants could call if they were interested in the trial. Alternatively, potentially eligible participants were asked to complete a screening reply slip and return it to the research team in the post using a free-post envelope. Between September and December 2018, women who had not responded within 10 days of being sent the invitation letter and participant information sheet received a follow-up call from the research team at BWH to ask if they were interested in taking part. As the study was recruiting from practices mostly located in high-deprivation communities with ethnically diverse patient lists, it was felt that women may respond better to a telephone call in which they could talk to a researcher regarding the trial rather than by a letter alone, particularly if their literacy was low. Further screening by telephone was conducted by the research team prior to the baseline home visit. Assessment of full eligibility was completed at the baseline home visit (see Establishing full eligibility at the baseline home visit and informed consent).
Direct recruitment through general practices
Towards the end of the study recruitment period, recruitment via BWH was supplemented with recruitment strategies directly via practices. Posters advertising the trial were displayed in waiting rooms at participating practices. Posters were also made available for viewing on general practice waiting room television screens. Participants who heard about the trial through this route were asked to telephone the research team for further information, and the study invitation letter and participant information sheet were mailed to interested women, if women had not already received one. If women were still interested after receiving the invitation/participant information sheet, they contacted the research team again, either by telephone or by returning a reply slip sent to them requesting a follow-up call. During this second telephone call, initial eligibility screening checks were completed. If all initial screening eligibility criteria were met, an appointment was made for a researcher to visit potentially eligible participants at home to fully confirm eligibility and collect baseline data.
There was also the opportunity for participants to be informed about the trial directly from baby check clinics, from postnatal check-ups or at any other appointment with the GP or other health-care professional post delivery and prior to the 2-month immunisation appointment. Some GPs in participating practices were asked to give a letter of invitation and participant information sheet directly to participants whom they may have seen at postnatal check-up consultations and who may have been eligible. In practices that held baby clinics, a researcher attended these clinics on an ad hoc basis. In this instance, the researcher attending the baby clinics was not the first point of contact for participants, as these participants had already received at least one letter of invitation from BWH and/or a health-care professional at their practice. When this occurred, the researcher provided potential participants with the letter of invitation and participant information sheet directly, either to read at the practice or to take home. If, after having read these documents, women remained interested, they were screened at the practice by the researcher or telephoned at a later time to establish initial eligibility (see Screening prior to baseline home visit). If all of the initial screening criteria were met, an appointment was made for a researcher to visit their home to fully confirm eligibility and collect baseline data.
Screening prior to baseline home visit
Regardless of how women were notified about the trial or how they were approached, prior to the baseline home visit all potential participants were initially screened for eligibility by a researcher over the telephone, when verbal permission was requested to collect some screening information to establish eligibility. Screening by the researcher prior to the baseline home visit established the following:
-
reconfirmed that the participant was aged ≥ 18 years
-
reconfirmed that the participant had given birth at least 4 weeks previously
-
reconfirmed that the participant was registered at one of the participating general practices
-
collected self-reported data on participants’ height and weight to calculate an approximate BMI
-
confirmed that participants were planning to have their child immunised
-
confirmed that participants had not yet attended the first child immunisation appointment
-
confirmed that participants were not already actively involved in a weight loss programme or a weight management trial to lose weight
-
confirmed that participants were willing to give consent to notify their GP of their participation in the trial.
An appointment was arranged for a researcher to visit the home of participants who fulfilled the initial screening criteria and who were interested in taking part in the study. The baseline visit was arranged to take place between 6 and 7 weeks postnatally, no earlier than 4 weeks and before the first immunisation visit at 2 months.
Establishing full eligibility at the baseline home visit and informed consent
Written informed consent was a two-stage process. At the baseline home visit, prior to any trial measurements being undertaken, a researcher obtained written informed consent to collect further screening data to fully confirm eligibility. If women consented to screening, the researcher measured participants’ height and weight to confirm the BMI eligibility criteria. As part of the eligibility criteria, the researcher confirmed that participants had not been diagnosed with a serious mental health difficulty requiring hospitalisation or been diagnosed with anorexia and/or bulimia in the past 2 years. Participants were given the opportunity to ask questions about the trial before signing and dating the screening informed consent form. Written informed consent was obtained from participants not deemed eligible at the home visit to keep any data collected about them from the screening process.
Participants who met all of the eligibility criteria at the baseline home visit were asked if they consented to be enrolled into the main trial. The trial was explained to them again verbally, and written informed consent was obtained for enrolment. The baseline assessments were then undertaken. Participants were then notified of the trial group to which their general practice was allocated. Researchers stressed to women that participation was voluntary and that they were free to refuse to take part and could withdraw from the trial at any time.
Written informed consent was obtained for the qualitative studies with women and practice nurses/GPs after they had completed either receiving or delivering the intervention.
Randomisation
The unit of randomisation was the practice (cluster). Linked practices that shared clinical staff were considered a single practice/cluster. Linked practices that did not share staff were considered independent practices. Practices in Birmingham and Solihull were invited to participate in the trial. The practices were randomised in a 1 : 1 ratio to the weight management intervention or to no intervention (usual care), using minimisation for practice list size (large, ≥ 6000 patients; small, < 6000 patients) and Index of Multiple Deprivation (IMD) score. The IMD was based on the postcode of the practice; the IMD score ranges from 1 to 32,844 and was divided into tertiles of high, medium and low levels of deprivation. The trials unit created a computer-generated randomisation list to allocate practices to the two trial groups. The randomisation list was held securely by the clinical trials unit. Once all of the necessary approvals were in place, practices were randomised centrally at BCTU by the trial statistician, and those practices randomised to the intervention group received the required training to deliver the intervention prior to opening for the trial. To maintain allocation concealment at the start of the study, randomisation of the first practices occurred when three practices were ready to open (except for need for trial intervention training). Thereafter, practices could be randomised sequentially.
Masking
It was not possible to mask participants or those providing the intervention to group allocation. It was also not possible to mask the outcome assessor, as the researcher needed to undertake both the baseline and the follow-up home visit to collect data. We do not believe that this would have introduced bias because the aim of this study was to assess the feasibility of undertaking a large Phase III cluster RCT, and these outcomes are not affected by knowledge of group allocation, and the data relating to the feasibility outcomes were not collected during the home visits.
Intervention
Overview summary
The intervention group were offered brief support that encouraged active self-management of weight in the postnatal period when they attended their practice to have their child immunised during the first year of life. In their first year, babies are routinely immunised at 2, 3, 4 and 12 months of age; the intervention was embedded in these routine immunisation contacts, so no additional visits by participants were required. The intervention involved nurses who encouraged participants to make healthier lifestyle choices through self-monitoring of their weight and signposting to an online weight management programme [Positive Online Weight Reduction (POWeR)] for support. 171 Nurses were asked not to provide any lifestyle counselling; their role was to only provide encouragement, provide regular external accountability (i.e. so that women were mindful that their weight was being monitored by someone else) and signpost women to the POWeR programme for weight loss information. The intervention was deliberately designed to be multicomponent because evidence suggests that such interventions lead to more favourable weight management during the postnatal period. 172
The intervention behaviour change techniques were mapped in accordance with the CALO-RE taxonomy checklist (Table 2). 173 The content of the intervention has also been mapped against the Template for Intervention Description and Replication (TIDieR) checklist. 174
| Behavioural technique | Definition |
|---|---|
| Instruction on how to perform the behaviour | The practice nurse instructed participants to weigh themselves once per week. Participants were encouraged to weigh themselves on the same day and at the same time every week. The POWeR online programme also encouraged regular self-weighing, as well as giving information on how to make changes to eating and physical activity |
| Credible source | Participants were given an information leaflet at the start of the trial and were directed to the POWeR online programme |
| Social support (general) | Advice on using social support was included in the POWeR programme as were ‘POWeR stories’ from previous successful users of the programme |
| Goal-setting (behaviour) | Participants were asked to self-weigh and record their weight once per week. The POWeR online programme also allowed personalised eating and physical activity goals to be set each week |
| Goal-setting (outcome) | Participants were advised to aim for 0.5- to 1-kg weight loss per week, as advised by NICE for a general population |
| Self-monitoring of outcome(s) of behaviour | Participants were instructed to record their weight once per week on a record card that was attached to their child’s red book. Participants could also record their weight weekly on the POWeR online programme |
| Feedback of outcome(s) of behaviour | Participants were weighed at each immunisation appointment by the practice nurse and given feedback by the nurse with their weight recorded on the record card attached to their baby’s red book. The POWeR online programme also gave feedback on participant’s own review of their weekly self-weight |
| Review behaviour goal(s) | Previously set personalised eating and physical activity goals were reviewed in the POWeR online programme |
| Use of follow-up prompts | Participants were directed to the POWeR online programme, which provides weekly e-mail messages to prompt participants to access the programme. Participants were also prompted to self-weigh when seen by the nurse at the child immunisation appointments |
Women were asked to weigh themselves weekly and record this on a weight record card that was attached to their child’s health record red book, in which infant immunisations are recorded, or using the online programme. This is because nurses needed to be able to check that women were weighing themselves regularly, and the POWeR programme provides personalised information based on weight gain/loss progress. The intervention ran until the third immunisation (when the child was approximately 4 months old).
Weight loss goals
No clinical guidelines that specify rates of healthy weight loss for postnatal women are available, but for the general adult population NICE recommends 0.5–1 kg per week. 75 Participants were, therefore, advised to aim for 0.5- to 1-kg weight loss per week until they had achieved a BMI of < 25 kg/m2 and were no heavier than their pre-pregnancy weight.
Accountability
As outlined above, practice nurses did not provide any counselling about diet/physical activity; they simply weighed participants at each child immunisation visit and recorded this weight, as a source of regular external accountability for weight monitoring. Someone who is regularly weighed is more likely to maintain weight goals when they know that their progress will be monitored by another individual.
Online weight loss programme (POWeR)
Nurses signposted women to the POWeR online weight loss programme for weight loss support and assistance with goal-setting, action planning and implementation of changes to their lifestyle (https://powerpimms.lifeguidehealth.org; accessed July 2019). Women were given their own unique username and were asked to set their own secure password. The POWeR programme is an existing programme and has been shown to result in clinically effective weight loss in overweight primary care patients when combined with brief nurse support. 171 The POWeR programme is a self-guided, online, theory- and evidence-based intervention to support weight management over 12 months, and was designed to be appropriate for people in most situations, including postnatal women. Participants choose either a low-energy eating plan (a reduction of around 600 calories per day) or a low-carbohydrate eating plan. Users are also encouraged to increase their physical activity levels by choosing either a walking plan or a self-selected mixture of other physical activities. The POWeR programme focuses principally on fostering users’ self-regulation skills for autonomously self-managing their weight, rather than providing detailed dietetic advice. Users of the programme are taught active cognitive and behavioural self-regulation techniques (‘POWeR tools’) to overcome problems, such as low motivation, confidence and relapse. Evidence is provided for the effectiveness of these techniques and examples given of how others have successfully used them (‘POWeR stories’). The POWeR programme emphasises forming healthy eating and physical activity habits that should become non-intrusive and require little effort to sustain. Information about breastfeeding and weight loss was added to the programme for the purpose of this trial.
Participants were encouraged to continue to use the website weekly to track their weight, set and review eating and physical activity goals, and receive personalised advice. After entering their weight and whether or not they had achieved the goals they had set themselves the previous week, users received tailored feedback giving encouragement if they had maintained their weight loss (e.g. reminders of health benefits accrued) and met their goals. Weight gain and failing to meet goals triggered automated personalised advice, such as appropriate goal-setting and planning, boosting motivation, overcoming difficulties and recovering from lapses.
Training of practice nurses
All nurses who administered child immunisations at intervention practices were trained to deliver the intervention following a standard protocol. Training took about 20–25 minutes to complete, given that nurses’ involvement was very simple and brief. Nurses were also trained in the research trial procedures. A training manual provided information on the importance of adhering to the protocol, information on the consequences of postnatal weight retention, instructions about how to weigh and record weight in the appropriate place in the child health red book, and tips and phrases for encouraging women to weigh themselves weekly (see Report Supplementary Material 1). The nurse training also addressed any concerns nurses may have had about raising the topic of weight.
Intervention fidelity
Written informed consent was obtained from participants to audio-record their immunisation/intervention consultations so that intervention fidelity against a checklist could be assessed. Only the parts of the consultation relevant to the intervention were recorded. This process was also included to allow assessment from a practical and logistical perspective on how well the intervention fitted within immunisation visits and to also inform nurse training for any subsequent main trial. This also allowed the research team to calculate how long the intervention took nurses to deliver.
Usual-care comparator group
Women allocated to the usual-care comparator group received brief written information about following a healthy lifestyle and no other intervention at the baseline home visit.
Outcome measures and trial procedures
Primary outcome
The primary aim of the trial was to assess the feasibility of undertaking a full-scale Phase III cluster RCT. This was assessed via specific questions:
-
whether or not the trial was appealing to postnatal women (via assessment of the recruitment rate to ensure that a full Phase III trial is feasible)
-
whether or not the intervention was acceptable (via assessment of adherence to weekly self-weighing and registration with the POWeR online weight management programme)
-
whether or not the intervention had any adverse impact on infant immunisation rates (recorded attendance by practices)
-
the number of women who completed the trial and completed the trial questionnaires (follow-up).
BodyTrace weighing scales
The intervention group were given a set of real-time weight-tracking scales (BodyTrace scales, BodyTrace Inc., Palo Alto, CA, USA) as an objective process measure of adherence to weekly self-weighing (www.bodytrace.com/; accessed 12 November 2019). Each time a participant used the scales to weigh themselves, their data were sent to the research team in real time via wireless cellular data transfer. Participants who did not have wireless internet access in their homes, or did not want their weight to be transmitted to the team in real time, were given UPS ION scales (ION Health, Richmond, UK) that store 100 weight recordings on a Universal Serial Bus (USB) stick attached to the scales; with participants permission, these weight data were downloaded from the scales at the follow-up visit. The scales were delivered to participants’ homes and were set up by the research team. Women were informed that their weight data would be transferred to the research team but that they would not receive feedback regarding their weight from the research team.
Other outcomes
Although this feasibility trial was not powered to detect meaningful differences in outcome measures, it provided the opportunity to ensure that there were no issues with the completion of these measures in preparation for the main trial. All measures were assessed at baseline and follow-up in both groups unless stated otherwise. Weight and body fat were assessed using a Tanita SC-240MA analyser (Tanita Europe BV, Amsterdam, The Netherlands). Depression and anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS). 175 Body image was measured using the Body Image States Scale (BISS). 176 Self-reported physical activity was assessed using the postnatal version of the Pregnancy Physical Activity Questionnaire (PPAQ). 177 Weight control strategies were assessed at follow-up [Weight Control Strategies Scale (WCSS)]. 178 Using the revised Three-Factor Eating Questionnaire (TFEQ), the variables of conscious cognitive energy restraint of eating, uncontrolled eating and emotional eating were measured. 179 Questions from Steinberg et al. ’s180 perceptions of self-weighing questionnaire were used to measure perceptions of regular monitoring in the intervention group at follow-up only. 180
Health economics
Relative to routinely used economic quality-of-life measures, such as the EuroQol-5 Dimensions,181 the ICECAP-A has only recently been developed. 182 We assessed the acceptability of the ICECAP-A in the feasibility trial to inform the economic evaluation design in a full trial. It was seen as an important measure to include, as the benefits of weight loss are not confined to health alone and ICECAP-A offers the potential to capture well-being benefits in an economic framework.
Adverse events and serious adverse events
The collection and reporting of adverse events (AEs) was conducted in accordance with the Research Governance Framework for Health and Social Care and the requirements of the Health Research Authority (HRA). The investigator assessed the seriousness and causality (relatedness) of all AEs experienced by trial participants, and if AEs occurred they were documented in the source data with reference to the protocol. No risks were expected to arise from taking part in the trial. The intervention was considered low risk, given that it consisted only of self-monitoring of weight, goal-setting and using an online weight loss programme, all of which have been used in other populations and settings without evidence of harm. There may be certain AEs that are commonly expected in participants undergoing a weight management programme. However, as these events are well characterised, it was highly unlikely that this trial would have revealed any new safety information relating to this intervention. Therefore, AEs were not collected. AEs related to the newborn baby were not collected either.
No serious adverse events (SAEs) were anticipated as a consequence of participation in the study, but reporting requirements were outlined in the trial protocol. Safety was assessed continuously throughout the study. The following were expected SAEs and were not reported as SAEs:
-
SAEs that were related to a pre-existing condition (pre-existing conditions are medical conditions that existed before entering the trial, as we intend to monitor the safety of the intervention by capturing severe, unexpected occurrences, in relation to the intervention).
-
Death as a result of a pre-existing medical condition. The protocol stipulated that all deaths should be reported to the trials office immediately on becoming aware so that no correspondence (patient questionnaires or queries, etc.) were sent to the participant or their family.
Investigators were required to only report SAEs that were attributable to the trial intervention. The above events were not considered related to the trial intervention and were, therefore, excluded from notification to the trial office as SAEs. The protocol was that these events should be recorded in the medical records in accordance with local practice.
Demographic-, lifestyle- and pregnancy-related information (both groups)
Information on age, ethnicity, pre-pregnancy weight, timing of cessation of breastfeeding, infant feeding practices and sleeping patterns of the mother were collected. Some women resume smoking and alcohol consumption after pregnancy, which might affect their weight; therefore, these behaviours were recorded. Data on whether or not participants in both groups had attended any formal weight loss programmes during their involvement in the trial were collected, as were data on any specific weight loss strategies or diets that participants might have used. Data relating to participants’ mode of delivery, participants’ pregnancy complications and how many children they had given birth to were collected. Data on marital status were collected to ascertain participants’ general level of social support in their lives. Data on employment status and financial status were collected to provide descriptive profile data on women who agreed to participate.
Objective assessment of self-weighing (intervention group)
As an objective measure of adherence to self-weighing, the intervention group were given weighing scales (BodyTrace, www.bodytrace.com/; accessed 12 November 2019) that objectively recorded their weight every time they weighed themselves, and this information was remotely transmitted back to the research team by wireless transfer. These weighing scales were given to women at the baseline home visit by researchers. These scales were included as an objective process measure to assess adherence to frequency of self-weighing in the intervention group; we did not provide any feedback to participants, nor did we monitor fluctuations or changes in weight during the trial.
Weight record cards (intervention group)
The intervention group were asked to complete weight record cards that were collected from participants as a measure of intervention implementation at the end of the intervention. The record cards allowed us to measure how much of the intervention was delivered per protocol by practice nurses. We obtained data from the POWeR programme for participants who chose instead to record their weight on the online programme.
Use of the POWeR online programme (intervention group)
Using participants’ e-mail addresses the online POWeR software automatically recorded their usage of the website (i.e. registration, number of logins, time spent on the POWeR website, progress through the POWeR programme and the number and value of weight measurements entered).
Attendance at immunisation appointments
The intervention was delivered at child immunisation appointments at 2, 3 and 4 months postnatally. Monitoring of immunisation uptake rates in the intervention group was an explicit role of the TSC. Practices were asked to provide data on all immunisations attended by both groups, and any missed appointments were investigated and a reason allocated. We also collected patient-reported attendance at the immunisation appointments at the follow-up visit.
Intervention fidelity via audio-recording of immunisation appointments
If consent to do so was provided, immunisation appointments were audio-recorded to gauge delivery of the intervention to protocol by practice nurses. These consultations were transcribed by a researcher (NTM) and read to assess, by use of a checklist, whether or not the nurses were delivering the intervention in accordance with their training and the protocol. The specific criteria checklist for nurses were as follows:
-
weighed and recorded participants’ weight on weight record card
-
checked that participants had been weighing themselves on a weekly basis
-
asked participants if they had accessed the POWeR website
-
verbally signposted participants to the POWeR website.
Intervention contamination
The possibility of intervention contamination in the usual-care group was assessed by asking participants if they knew any other women participating in the trial and whether or not they had accessed the POWeR website.
Trial procedures
Table 3 outlines all of the assessments and outcomes measured in this trial, the time points at which they were measured and the groups that were assessed. Baseline home visits took place between 6 and 7 weeks postnatally and before the first child immunisation visit at 2 months, and took about 30–45 minutes to complete. Participants were visited at home by a researcher, where:
-
Screening consent was obtained.
-
Height, weight and percentage body fat were measured, and BMI was calculated.
-
Eligibility (inclusion/exclusion criteria) was reviewed.
-
Informed consent was obtained for eligible participants.
-
The baseline health questionnaire booklet was completed/collected. The baseline questionnaire booklet was posted prior to the baseline home visit to allow the participant to complete the booklet in their own time prior to the visit.
-
Participants were informed which group of the trial they were allocated to.
-
The usual-care group were issued with the healthy lifestyle leaflet and advised that they would receive usual care at their child immunisation appointments.
-
The intervention group were issued with the healthy lifestyles leaflet, the weight record card was attached to the red immunisation book, a trial sticker was placed on the front of the red book, and participants were given BodyTrace scales and instructed on use (issued instruction leaflet). The intervention group were provided with details instructions and individual login details for the online weight management programme.
| Assessment | Visit | ||||||
|---|---|---|---|---|---|---|---|
| Screening: 4 weeks postnatally (no earlier than 4 weeks) | Baseline home visit: 6–7 weeks postnatally (before the first immunisation) | Immunisation visits at general practice (intervention group only) (postnatally) | Follow-up home visit: 3 months post randomisation (–1 week to approximately 4 weeks) | Post follow-up | |||
| 2 months | 3 months | 4 months | |||||
| Identification of potential participants | ✗ | ||||||
| Eligibility check | ✗ | ✗ | |||||
| Valid informed consent for screening | ✗ | ||||||
| Height (cm) | ✗ | ✗ | |||||
| Weight (kg) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Per cent body fat | ✗ | ✗ | |||||
| BMI (kg/m2) | ✗ | ✗ | |||||
| Valid informed consent for full trial | ✗ | ||||||
| Pregnancy and family details | ✗ | ||||||
| Feeding of baby | ✗ | ✗ | |||||
| Sleep patterns | ✗ | ✗ | |||||
| Marital status | ✗ | ||||||
| Employment and financial status | ✗ | ||||||
| Smoking status | ✗ | ✗ | |||||
| Alcohol consumption | ✗ | ✗ | |||||
| HADS175 | ✗ | ✗ | |||||
| ICECAP-A questionnaire182 | ✗ | ✗ | |||||
| TFEQ179 | ✗ | ✗ | |||||
| BISS176 | ✗ | ✗ | |||||
| PPAQ177 | ✗ | ✗ | |||||
| WCSS178 | ✗ | ||||||
| Immunisation record from participant | ✗ | ||||||
| Weight loss resources used | ✗ | ||||||
| Intervention group only: self-weighing data | ✗ | ||||||
| Intervention group only: BodyTrace weight data | ✗ | ||||||
| Intervention group only: POWeR programme171 data | ✗ | ✗ | |||||
| Intervention group only: trial acceptability | ✗ | ||||||
| Immunisation records from GP | ✗ | ||||||
| Qualitative study interviews | ✗ | ||||||
Follow-up home visit
Follow-up visits took place 3 months after participants entered the trial and took approximately 30 minutes to complete. Participants were visited at home by a member of the research team and the following tasks were completed:
-
Weight and percentage body fat measured, BMI calculated.
-
Follow-up questionnaires collected. Questionnaires were posted to participants 5–7 days in advance (for collection by the researcher).
-
Confirmation of attendance at immunisation appointments obtained.
-
Intervention group only – willingness to participate in a semistructured interview about experiences of participating in the study; collection or photograph of the weight record card; collection of BodyTrace weighing scales.
Participant incentives
A £20 high-street shopping voucher was offered to all participants as reimbursement for any inconvenience that trial participation may have caused them. This voucher was offered at the follow-up home visit once all follow-up clinical report form (CRF) questions and health questionnaires had been completed.
Data monitoring
Given that this was a feasibility trial that was designed to test whether or not a definitive trial was feasible, a Data Monitoring and Ethics Committee was not established. Oversight of the trial was provided by the TSC.
Data management
Processes were employed to facilitate the accuracy of the data included in the final report. These processes were detailed in the trial-specific data management plan. Coding and validation were agreed between the trial manager, the statistician and the programmer, and the trial database was signed off once the implementation of these had been assured. The trial office checked incoming CRFs for compliance with the protocol, data consistency, missing data and timing. For CRFs completed by the chief investigator (or delegate) at the general practice, sites were asked for missing data or clarification of inconsistencies or discrepancies. Participant questionnaires, and patient-specific data from the baseline and follow-up CRFs, were reviewed on receipt at the trial office and inconsistent and/or missing data were queried with the participant. To ensure that participants did not feel harassed, a single letter was sent to participants outlining the discrepancy and/or missing data and requesting this information. Occasionally, participants were telephoned to request or clarify missing or ambiguous data queries (if participants consented to be telephoned). All data were entered into the trial database by suitably trained staff. Informed consent forms, CRFs and questionnaires were stored in lockable filing cabinets in a secure, swipe access part of the UoB. Trial data were kept on password-protected electronic databases, on secure UoB servers, where access was limited to staff working on the trial only. The database had ranges applied to data items if suitable and appropriate.
Data collected through the POWeR website were electronically securely transferred from the University of Southampton to the trial office at the UoB throughout the trial. This was uploaded through the trial database.
Data security
The security of the data system was governed by the policies of the UoB. The university’s Data Protection Policy and the Conditions of Use of Computing and Network Facilities set out the security arrangements under which sensitive data should be processed and stored. All studies at the UoB must be registered with the Data Protection Officer and data held in accordance with the Data Protection Act. The UoB has Data Protection Registration to cover the purposes of analysis and for the classes of data requested. The University’s Data Protection Registration number is Z6195856.
Sample size
Given that this was a feasibility trial, a formal sample size calculation was not conducted. The trial was not designed or powered to detect a statistically significant difference in efficacy between the two trial groups. Sample sizes of at least 70 participants have been recommended. 183 A recruitment target of 80 women from 10–12 practices recruited over 8 months was set.
Recruitment
The recruitment rate is presented as a percentage based on the number of participants who took part in the trial divided by the target recruitment (n = 80). BWH provided data on the number of invitation letters sent, along with data on the age and ethnicity (in summary format) of the women who were sent an invitation letter.
Adherence/acceptability
The quantitative assessment of whether or not the intervention was acceptable to participants was based on the adherence to weekly self-weighing. The trial included three sources of data regarding the frequency of self-weighing: objective recording using the BodyTrace scales, self-reported in the child health red book and recordings using the POWeR programme. In the first instance, the objective recording of weight on the BodyTrace scales was used as the authoritative source of data to assess the frequency of self-weighing/adherence. As a secondary assessment of frequency of self-weighing and adherence, weight data from all three sources were included.
Immunisation rates
To check that the intervention had no adverse impact on infant immunisation rates, practices provided data on all immunisation appointments attended during the trial. The trial took place over the first three immunisation appointments. The proportion of babies who attended all three immunisation appointments was reported. Originally, the immunisation rate for each practice in the trial was to be compared with the normal immunisation rate for that practice. However, owing to low recruitment, the immunisation data have been presented overall by trial group and compared with national uptake rates.
Decision to progress to the Phase III trial
For the Phase III trial to take place, there needed to be evidence from this feasibility trial of meeting prespecified stop–go criteria. The trial was too small to include meaningful and sensitive stop–go criteria regarding the impact of the intervention on immunisation rates. However, we checked that the intervention had not adversely affected immunisation rates. The decision to proceed to the Phase III trial was, therefore, based on three criteria using a traffic-light system: (1) the recruitment rate, (2) adherence to weekly self-weighing and (3) registration with the online weight loss programme (POWeR).
In the traffic-light system, the green-light criteria were as follows:
-
recruitment rate of ≥ 80% of the target (n = 80; i.e. recruit at least 64 women)
-
≥ 50% of the intervention group weigh themselves weekly ≥ 60% of the time
-
≥ 60% of participants registered with the online POWeR programme.
If all three criteria are met, we will proceed to an application for the full trial with the protocol unchanged (unless there is a clear message from the interviews that would improve the protocol).
The amber-light criteria were as follows:
-
recruitment rate of 50–79% of the target (n = 80; i.e. recruit between 40 and 63 women)
-
40–49% of the intervention group weigh themselves weekly 40–59% of the time
-
40–59% of the intervention group registered with the online POWeR programme.
If one or more of our amber-light criteria are met, we will plan to adapt the protocol in the light of the feedback from the interviews and our experience to improve whichever criteria are not at the ‘green-light’ level before proceeding to the application for the full trial. In discussion with the TSC, we will assess whether or not adaption of the protocol will require further assessment before progressing.
The red-light criteria were as follows:
-
recruitment rate of < 50% of the target (n = 80; i.e. recruit < 40 women)
-
< 40% of the intervention group weigh themselves weekly 40–59% of the time
-
< 40% of the intervention group registered with the online POWeR programme.
If one or more of these criteria are met, we would consider the current protocol not feasible and would not progress to an application for a full RCT with the current design. An additional red-light criterion would be concerns from the TSC that immunisation rates were adversely affected.
Data analysis
Analysis of outcome measures
A detailed statistical plan can be found in the Report Supplementary Material 2. A brief outline of these analyses is given below in relation to the proposed stop–go criteria and the outcome data collected. The stop–go criteria are based on recruitment, adherence to weekly self-weighing and registration to the POWeR programme (see above). The recruitment rate is presented as a percentage based on the number of participants who took part in the trial divided by the target recruitment (n = 80). The percentage of women in the intervention group who adhered to weekly self-weighing (according to the stop–go criteria) and who registered with the POWeR programme is also presented. The binomial normal approximation was used to calculate the corresponding 95% CIs.
All primary analyses of outcome data were by intention to treat. Participants were analysed in the intervention group to which they were allocated (according to the randomisation of the practice), and all participants were included whether or not they received the allocated intervention. The primary comparison groups were those in the weight management intervention group and those in the usual-care group. The analysis of outcome data focused on CI estimation. Continuous outcomes (except the PPAQ; see below) were summarised using means and standard deviations (SDs). Adjusted mean differences between groups and the corresponding 95% CIs were estimated from generalised linear mixed models that included adjustment for baseline values (if available) and the minimisation variables (practice size and IMD), and practice (cluster) as a random effect. Data from the PPAQ are presented as medians with interquartile ranges (IQRs), and the unadjusted difference between the median in each group was reported along with the 95% CI that was calculated using bootstrapping methods. All estimates of differences between groups are presented with two-sided 95% CIs and no p-values are presented.
Use of the POWeR website was assessed through the number of times that participants logged on to POWeR, the number of times that participants recorded their weight on POWeR and the time spent browsing, with data presented as medians with IQRs. These data were also tabulated at each intervention week to assess usage over time. Progress through the POWeR programme is assessed by tabulating the number of stages that participants completed and the number of participants who completed each stage.
Qualitative study
Semistructured interviews with women were completed after trial follow-up to explore their views about the intervention. Practice nurses were also interviewed to understand more about their experiences of delivering the intervention during child immunisation appointments. These two interview studies are described in more detail in Chapters 5 and 6.
Changes to the protocol
Minor and substantial changes to the protocol and conduct of the trial are outlined in Table 4. One of the most important changes to note relates to the change in approach to calculating the trial recruitment rate. During the trial, we found out that BWH were sending letters to all women who had given birth at the hospital who were registered at general practices taking part in the trial, but they were unable to screen against key trial entry criteria, such as weight/BMI, because they did not have this information. This meant that letters were being sent to both eligible and ineligible women and it was, therefore, not possible to present the recruitment result as a proportion of the eligible participants invited to take part. We, therefore, changed the recruitment result so that it was presented as a proportion of the target. This was discussed and agreed with the TSC in October 2018, as well as with the Health Technology Assessment programme; this change was made to the protocol.
| Amendment number | Date of amendment | Protocol version number | Type of amendment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 5 December 2017 | 2.0 | Substantial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionInvitation letter and reply slipVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Participant information sheetVersion 2.0 (16 November 2017)Version 3.0 (5 December 2017)Screening consent formVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Full informed consent form AVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Full informed consent form BVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)GP posterVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Baseline appointment letterVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Questionnaire baselineVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Questionnaire follow-up AVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Questionnaire follow-up BVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Weight cardVersion 1.0 (12 October 2017)Version 2.0 (5 December 2017)Statement of activities (BWH)Version 1.0Version 2.0Statement of activities (GPs)Version 1.0Version 2.0Schedule of events (BWH)Version 1.0Version 2.0Schedule of events (GPs)Version 1.0Version 2.0 | Other modified documents approved | Previous version | New version | Invitation letter and reply slip | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Participant information sheet | Version 2.0 (16 November 2017) | Version 3.0 (5 December 2017) | Screening consent form | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Full informed consent form A | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Full informed consent form B | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | GP poster | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Baseline appointment letter | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Questionnaire baseline | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Questionnaire follow-up A | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Questionnaire follow-up B | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Weight card | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | Statement of activities (BWH) | Version 1.0 | Version 2.0 | Statement of activities (GPs) | Version 1.0 | Version 2.0 | Schedule of events (BWH) | Version 1.0 | Version 2.0 | Schedule of events (GPs) | Version 1.0 | Version 2.0 | |||||||||||||||||||||||||||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Invitation letter and reply slip | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant information sheet | Version 2.0 (16 November 2017) | Version 3.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Screening consent form | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full informed consent form A | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full informed consent form B | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP poster | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline appointment letter | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire baseline | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up A | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up B | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight card | Version 1.0 (12 October 2017) | Version 2.0 (5 December 2017) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (BWH) | Version 1.0 | Version 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (GPs) | Version 1.0 | Version 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (BWH) | Version 1.0 | Version 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (GPs) | Version 1.0 | Version 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.0 | 1 February 2018 | 4.0 | Substantial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionParticipant information sheetVersion 3.0 (5 December 2017)Version 5.0 (1 February 2018)Invitation letter and reply slipVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Screening consent formVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Full informed consent form AVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Full informed consent form BVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)GP letterVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)GP posterVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Healthy lifestyle leafletVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Baseline appointment letterVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Follow-up appointment letterVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Questionnaire baselineVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Questionnaire follow-up AVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Questionnaire follow-up BVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Weight record cardVersion 2.0 (5 December 2017)Version 4.0 (1 February 2018)Nurses interview participant information sheetVersion 3.0 (22 November 2017)Version 5.0 (1 February 2018)Nurses interview consent formVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Nurses interview scheduleVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Participant interview consent formVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Participant interview scheduleVersion 1.0 (12 October 2017)Version 3.0 (1 February 2018)Statement of activities (BWH)Version 2.0Version 3.0Statement of activities (GPs)Version 2.0Version 3.0Schedule of events (BWH)Version 2.0Version 3.0Schedule of events (GPs)Version 2.0Version 3.0 | Other modified documents approved | Previous version | New version | Participant information sheet | Version 3.0 (5 December 2017) | Version 5.0 (1 February 2018) | Invitation letter and reply slip | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Screening consent form | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Full informed consent form A | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Full informed consent form B | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | GP letter | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | GP poster | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Healthy lifestyle leaflet | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Baseline appointment letter | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Follow-up appointment letter | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Questionnaire baseline | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Questionnaire follow-up A | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Questionnaire follow-up B | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Weight record card | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | Nurses interview participant information sheet | Version 3.0 (22 November 2017) | Version 5.0 (1 February 2018) | Nurses interview consent form | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Nurses interview schedule | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Participant interview consent form | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Participant interview schedule | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | Statement of activities (BWH) | Version 2.0 | Version 3.0 | Statement of activities (GPs) | Version 2.0 | Version 3.0 | Schedule of events (BWH) | Version 2.0 | Version 3.0 | Schedule of events (GPs) | Version 2.0 | Version 3.0 | |||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant information sheet | Version 3.0 (5 December 2017) | Version 5.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Invitation letter and reply slip | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Screening consent form | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full informed consent form A | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full informed consent form B | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP letter | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP poster | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Healthy lifestyle leaflet | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline appointment letter | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Follow-up appointment letter | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire baseline | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up A | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up B | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight record card | Version 2.0 (5 December 2017) | Version 4.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nurses interview participant information sheet | Version 3.0 (22 November 2017) | Version 5.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nurses interview consent form | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nurses interview schedule | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant interview consent form | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant interview schedule | Version 1.0 (12 October 2017) | Version 3.0 (1 February 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (BWH) | Version 2.0 | Version 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (GPs) | Version 2.0 | Version 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (BWH) | Version 2.0 | Version 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (GPs) | Version 2.0 | Version 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.0 | 20 March 2018 | 5.0 | Minor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Non-substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionStatement of activities (BWH)Version 3.0Version 4.0Statement of activities (BWH)Version 4.0Version 5.0Statement of activities (GPs)Version 3.0Version 4.0Schedule of events (BWH)Version 3.0Version 4.0Schedule of events (GPs)Version 3.0Version 4.0 | Other modified documents approved | Previous version | New version | Statement of activities (BWH) | Version 3.0 | Version 4.0 | Statement of activities (BWH) | Version 4.0 | Version 5.0 | Statement of activities (GPs) | Version 3.0 | Version 4.0 | Schedule of events (BWH) | Version 3.0 | Version 4.0 | Schedule of events (GPs) | Version 3.0 | Version 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (BWH) | Version 3.0 | Version 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (BWH) | Version 4.0 | Version 5.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Statement of activities (GPs) | Version 3.0 | Version 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (BWH) | Version 3.0 | Version 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of events (GPs) | Version 3.0 | Version 4.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.0 | 10 May 2018 | 6.0 | Substantial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionParticipant POWeR registration cardN/AVersion 1.0 (10 May 2018)Participant instructions for the POWeR programmeN/AVersion 1.0 (10 May 2018)Participant FAQs for the POWeR programmeN/AVersion 1.0 (10 May 2018)Participant instructions for BodyTrace weighing scalesN/AVersion 1.0 (10 May 2018)Questionnaire baselineVersion 4.0 (1 February 2018)Version 5.0 (10 May 2018)Questionnaire follow-up AVersion 4.0 (1 February 2018)Version 5.0 (10 May 2018)Questionnaire follow-up BVersion 4.0 (1 February 2018)Version 5.0 (10 May 2018) | Other modified documents approved | Previous version | New version | Participant POWeR registration card | N/A | Version 1.0 (10 May 2018) | Participant instructions for the POWeR programme | N/A | Version 1.0 (10 May 2018) | Participant FAQs for the POWeR programme | N/A | Version 1.0 (10 May 2018) | Participant instructions for BodyTrace weighing scales | N/A | Version 1.0 (10 May 2018) | Questionnaire baseline | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | Questionnaire follow-up A | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | Questionnaire follow-up B | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant POWeR registration card | N/A | Version 1.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant instructions for the POWeR programme | N/A | Version 1.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant FAQs for the POWeR programme | N/A | Version 1.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant instructions for BodyTrace weighing scales | N/A | Version 1.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire baseline | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up A | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up B | Version 4.0 (1 February 2018) | Version 5.0 (10 May 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.0 | 5 November 2018 | 7.0 | Substantial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionGP flyer cover letterN/AVersion 1.0 (5 November 2018)GP flyerN/AVersion 1.0 (5 November 2018)Invitation letter and reply slip: GP versionN/AVersion 1.0 (5 November 2018) | Other modified documents approved | Previous version | New version | GP flyer cover letter | N/A | Version 1.0 (5 November 2018) | GP flyer | N/A | Version 1.0 (5 November 2018) | Invitation letter and reply slip: GP version | N/A | Version 1.0 (5 November 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP flyer cover letter | N/A | Version 1.0 (5 November 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GP flyer | N/A | Version 1.0 (5 November 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Invitation letter and reply slip: GP version | N/A | Version 1.0 (5 November 2018) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.0 | 5 February 2019 | 8.0 | Minor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Non-substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approvedPrevious versionNew versionQuestionnaire baselineVersion 5.0 (10 May 2018)Version 6.0 (5 February 2019)Questionnaire follow-up AVersion 5.0 (10 May 2018)Version 6.0 (5 February 2019)Questionnaire follow-up BVersion 5.0 (10 May 2018)Version 6.0 (5 February 2019)Participant interview scheduleVersion 3.0 (1 February 2018)Version 4.0 (5 February 2019) | Other modified documents approved | Previous version | New version | Questionnaire baseline | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | Questionnaire follow-up A | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | Questionnaire follow-up B | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | Participant interview schedule | Version 3.0 (1 February 2018) | Version 4.0 (5 February 2019) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other modified documents approved | Previous version | New version | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire baseline | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up A | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Questionnaire follow-up B | Version 5.0 (10 May 2018) | Version 6.0 (5 February 2019) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Participant interview schedule | Version 3.0 (1 February 2018) | Version 4.0 (5 February 2019) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.0 | 16 July 2019 | 9.0 | Minor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of amendment | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Non-substantial changes:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chapter 3 Results
Recruitment of practices and participants
Fourteen practices (clusters) were recruited to participate in this study: seven were randomised to deliver the weight management intervention and seven were randomised to deliver usual care. For randomisation by minimisation, practices were categorised according to list size and IMD score (Table 5).
| Variable | Trial group | Overall (N = 14) | |
|---|---|---|---|
| Intervention (N = 7) | Usual care (N = 7) | ||
| Minimisation variables (practice level, as per randomisation process) | |||
| General practice list size, n | |||
| Large (≥ 6000 patients) | 3 | 3 | 6 |
| Small (< 6000 patients) | 4 | 4 | 8 |
| IMD, n | |||
| Low | 0 | 0 | 0 |
| Medium | 1 | 0 | 1 |
| High | 6 | 7 | 13 |
| Minimisation variables (participant level) | N = 16 | N = 12 | N = 28 |
| General practice list size, n (%) | |||
| Large (≥ 6000 patients) | 8 (50) | 9 (75) | 17 (61) |
| Small (< 6000 patients) | 8 (50) | 3 (25) | 11 (39) |
| IMD, n (%)a | |||
| Low | 0 (0) | 0 (0) | 0 (0) |
| Medium | 1 (6) | 0 (0) | 1 (4) |
| High | 15 (94) | 12 (100) | 27 (96) |
A total of 368 letters were sent by BWH to potentially eligible women from the 14 participating practices. Of women who completed the initial telephone screening process, most participants were made aware of the trial via letter from BWH (87.5%). A total of 28 women consented to participate between July 2018 and April 2019 (10 months) at an average rate of 2.8 participants per month. Sixteen women were registered at practices that were delivering the weight management intervention and 12 women were registered at practices that were delivering usual care. Recruitment at each practice is detailed in Table 6. Four of the usual-care practices recruited no participants.
| Trial group | Number recruited |
|---|---|
| Intervention | |
| Practice A | 1 |
| Practice B | 1 |
| Practice C | 2 |
| Practice D | 1 |
| Practice E | 4 |
| Practice F | 2 |
| Practice G | 5 |
| Total | 16 |
| Usual care | |
| Practice H | 0 |
| Practice J | 0 |
| Practice K | 3 |
| Practice L | 8 |
| Practice M | 0 |
| Practice N | 0 |
| Practice O | 1 |
| Total | 12 |
Withdrawals, loss to follow-up and missing data
One participant who was allocated to the intervention group withdrew from the trial because they decided not to have their child immunised. All remaining participants (n = 27) completed the follow-up visit for assessment of outcomes; however, one participant allocated to the intervention group did not complete the follow-up questionnaires. Return rates for the trial CRFs and health questionnaire booklets by trial group are detailed in Table 7. All expected baseline and follow-up CRFs were returned, and only one baseline (usual care) and one follow-up (intervention) health questionnaire booklet were not returned. Data regarding attendance at child immunisation appointments were not provided by practices for three participants (intervention, n = 2; usual care, n = 1). Weight record cards were returned blank (considered not returned) for three participants (intervention group). Table 8 shows how well each outcome questionnaire was completed. Generally, ≥ 85% of received forms were completed in full. The PPAQ had the most missing items (five at baseline and three at follow-up were partially completed).
| Data | Trial group, n (%) | |||||
|---|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | |||||
| Expected | Returned | Not returned | Expected | Returned | Not returned | |
| Baseline case report form | 16 | 16 (100) | 0 (0) | 12 | 12 (100) | 0 (0) |
| Baseline health questionnaire | 16 | 16 (100) | 0 (0) | 12 | 11 (92) | 1 (8)a |
| Follow-up CRF | 15 | 15 (100) | 0 (0) | 12 | 12 (100) | 0 (0) |
| Follow-up health questionnaire | 15 | 14 (93) | 1 (7) | 12 | 12 (100) | 0 (0) |
| Immunisation data form | 15 | 13 (87) | 2 (13) | 12 | 11 (92) | 1 (8) |
| Weight record card (intervention only) | 15 | 12 (80) | 3 (20)b | |||
| Questionnaire | Baseline (expected: N = 28a), n (%) | 3-month follow-up (expected: N = 27b), n (%) | ||||
|---|---|---|---|---|---|---|
| Received | Fully completed | Partially completed | Received | Fully completed | Partially completed | |
| HADS175 | 27 | 26 (96) | 1 (4) | 26 | 25 (96) | 1 (4) |
| TFEQ179 | 27 | 23 (85) | 4 (15) | 26 | 26 (100) | 0 (0) |
| BISS176 | 27 | 26 (96) | 1 (4) | 26 | 25 (96) | 1 (4) |
| cPPAQ177 | 27 | 22 (81) | 5 (19) | 26 | 23 (88) | 3 (12) |
| ICECAP-A182 | 27 | 27 (100) | 0 (0) | 26 | 26 (100) | 0 (0) |
| WCSS178 | 26 | 22 (85) | 4 (15) | |||
| dSelf-weighing perceptions180 | 14 | 13 (93) | 1 (7) | |||
Participant trial flow
Figure 3 illustrates the flow of participants through the trial. The most common reason for non-recruitment related to potentially eligible women having already attended their first child immunisation appointment (n = 5) or having a BMI of < 25 kg/m2 (n = 4).
FIGURE 3.
Participant flow through the trial. a, The total number of general practice invitations sent is unknown. Only two sites were able to provide information on the number of invitation that they had sent: one practice sent out 12 invitations and one sent out five invitations. b, One woman withdrew from the intervention owing to the study tasks being an inconvenience, but they agreed to continue in the trial and complete the follow-up assessment.
Participant characteristics at baseline
The average age of participants was 32.1 years (SD 5.7 years). Forty-six per cent of participants (n = 13) were of white ethnicity, with the remaining 54% (n = 15) from non-white ethnic groups. Most participants were married or living with their partner (74%, n = 20). The average weight and BMI of participants at baseline were 83.6 kg (SD 17.1 kg) and 31.8 kg/m2 (SD 6.9 kg/m2), respectively. Most participants had given birth to two children (43%, n = 12) and had tried to breastfeed the baby from their most recent pregnancy (96%, n = 27). Fifteen women (53%) were exclusively breastfeeding. On average, participants reported that they had 3.2 hours (SD 1.1 hours) of uninterrupted sleep per night (Table 9).
| Characteristic | Trial group | Overall (N = 28) | |
|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | ||
| Demographic and other baseline variables | |||
| Age (years) | |||
| Mean (SD), n | 32.9 (6.1), 16 | 31.0 (5.3), 12 | 32.1 (5.7), 28 |
| Minimum–maximum | 22–41 | 24–42 | 22–42 |
| Ethnic group, n (%) | |||
| White | 5 (31) | 8 (67) | 13 (46) |
| Pakistani | 3 (19) | 0 (0) | 3 (11) |
| Other Asian | 3 (19) | 0 (0) | 3 (11) |
| Black Caribbean | 0 (0) | 1 (8) | 1 (3) |
| Black African | 1 (6) | 2 (17) | 3 (11) |
| Other | 4 (25) | 1 (8) | 5 (18) |
| Current marital status, n (%) | |||
| Single (living alone) | 1 (6) | 3 (27) | 4 (15) |
| Single (living with partner) | 3 (19) | 3 (27) | 6 (22) |
| Married | 11 (69) | 3 (27) | 14 (52) |
| Divorced/separated (living alone) | 0 (0) | 1 (9) | 1 (4) |
| Othera | 1 (6) | 1 (9) | 2 (7) |
| Missing, n | 0 | 1 | 1 |
| Current employment status, n (%) | |||
| In paid employment | 9 (56) | 6 (55) | 15 (56) |
| Unemployed | 1 (6) | 2 (18) | 3 (11) |
| Student | 1 (6) | 0 (0) | 1 (4) |
| Looking after the home/family | 5 (31) | 1 (9) | 6 (22) |
| Sick/disabled | 0 (0) | 1 (9) | 1 (4) |
| Otherb | 0 (0) | 1 (9) | 1 (4) |
| Missing, n | 0 | 1 | 1 |
| Current financial status, n (%) | |||
| Normally have enough money | 9 (56) | 0 (0) | 9 (35) |
| Enough money if I plan carefully | 6 (38) | 4 (40) | 10 (38) |
| Enough money for basic things | 1 (6) | 3 (30) | 4 (15) |
| Basic things hard to afford | 0 (0) | 3 (30) | 3 (12) |
| Missing, n | 0 | 2 | 2 |
| Clinical information | |||
| Average number of cigarettes smoked per day, n (%) | |||
| None | 16 (100) | 9 (90) | 25 (96) |
| Five or less | 0 (0) | 0 (0) | 0 (0) |
| Between 6 and 10 | 0 (0) | 1 (10) | 1 (4) |
| Missing, n | 0 | 2 | 2 |
| Drank alcohol in last week, n (%) | |||
| Yes | 4 (25) | 2 (18) | 6 (22) |
| No | 12 (75) | 9 (82) | 21 (78) |
| Missing, n | 0 | 1 | 1 |
| Mean number of units | |||
| Mean (SD), n | 5.7 (3.5), 3 | 4.0 (2.8), 2 | 5.0 (3.0), 5 |
| Minimum–maximum | 2–9 | 2–6 | 2–9 |
| Missing, n | 1 | 0 | 1 |
| Weight (kg) | |||
| Mean (SD), n | 81.6 (13.7), 16 | 86.2 (21.2), 12 | 83.6 (17.1), 28 |
| Minimum–maximum | 58.8–106.7 | 66.7–148.8 | 58.8–148.8 |
| Height (cm) | |||
| Mean (SD), n | 161.2 (9.1), 16 | 164.0 (6.2), 12 | 162.4 (8.0), 28 |
| Minimum–maximum | 147.3–178.4 | 153.0–174.9 | 147.3–178.4 |
| BMI (kg/m2) | |||
| Mean (SD), n | 31.6 (6.1), 16 | 32.1 (8.0), 12 | 31.8 (6.9), 28 |
| Minimum–maximum | 25.5–47.2 | 26.3–56.4 | 25.5–56.4 |
| Percentage body fat (%) | |||
| Mean (SD), n | 40.9 (4.0), 16 | 41.6 (6.0), 12 | 41.2 (4.8), 28 |
| Minimum–maximum | 34.6–46.8 | 32.5–56.1 | 32.5–56.1 |
| Pregnancy details | |||
| Weight before pregnancy (kg) (self-reported) | |||
| Mean (SD), n | 75.0 (14.6), 13 | 83.9 (31.0), 10 | 78.9 (23.0), 23 |
| Minimum–maximum | 55.5–104.0 | 50.0–154.6 | 50.0–154.6 |
| Missing, n | 3 | 2 | 5 |
| Number of children given birth to, n (%) | |||
| 1 | 6 (37) | 1 (8) | 7 (25) |
| 2 | 7 (44) | 5 (42) | 12 (43) |
| 3 | 3 (19) | 3 (25) | 6 (21) |
| ≥ 4 | 0 (0) | 3 (25) | 3 (11) |
| Number of children living in household, n (%) | |||
| 1 | 6 (37) | 1 (8) | 7 (25) |
| 2 | 7 (44) | 5 (42) | 12 (43) |
| ≥ 3 | 3 (19) | 6 (50) | 9 (32) |
| Health complications during this pregnancy? n (%) | |||
| Yes | 8 (50) | 3 (25) | 11 (39) |
| No | 8 (50) | 9 (75) | 17 (61) |
| If yes (n = 11) (note: not mutually exclusive), n (%) | |||
| Gestational diabetes mellitus | 1 (12.5) | 2 (67) | 3 (27) |
| Pre-eclampsia | 1 (12.5) | 0 (0) | 1 (9) |
| Gestational hypertension | 4 (50) | 0 (0) | 4 (36) |
| Pre-term delivery | 1 (12.5) | 0 (0) | 1 (9) |
| Neonatal intensive care/special care | 1 (12.5) | 0 (0) | 1 (9) |
| Otherc | 2 (25) | 2 (67) | 4 (36) |
| Type of delivery, n (%) | |||
| Normal vaginal delivery | 10 (63) | 8 (67) | 18 (64) |
| Instrumental vaginal delivery | 1 (6) | 0 (0) | 1 (4) |
| Elective caesarean section | 1 (6) | 1 (8) | 2 (7) |
| Emergency caesarean section | 4 (25) | 3 (25) | 7 (25) |
| Pregnancy and breastfeeding | |||
| Tried to breastfeed baby, n (%) | |||
| Yes | 16 (100) | 11 (92) | 27 (96) |
| No | 0 (0) | 1 (8) | 1 (4) |
| Current method of feeding, n (%) | |||
| Exclusively breastfeeding | 11 (69) | 4 (33) | 15 (53) |
| Exclusively formula feeding | 3 (19) | 5 (42) | 8 (29) |
| Both breastmilk and formula | 2 (12) | 3 (25) | 5 (18) |
| If breastfeeding, intended time to continue breastfeeding: n (%) | (N = 13) | (N = 7) | (N = 20) |
| Up to 3 months | 0 (0) | 0 (0) | 0 (0) |
| Up to 6 months | 2 (15) | 1 (14) | 3 (15) |
| Up to 9 months | 1 (8) | 0 (0) | 1 (5) |
| Up to 12 months | 1 (8) | 1 (14) | 2 (10) |
| > 1 year | 5 (38) | 3 (43) | 8 (40) |
| As long as possible | 4 (31) | 2 (29) | 6 (30) |
| Sleep | |||
| Average amount of uninterrupted sleep per night (hours) | |||
| Mean (SD), n | 3.0 (1.2), 16 | 3.5 (0.9), 12 | 3.2 (1.1), 28 |
| Minimum–maximum | 1–6 | 2–5 | 1–6 |
Adherence to self-weighing (intervention group)
Table 10 shows the number of weeks (as per the schedule of assessment) that participants weighed themselves, as recorded objectively using the BodyTrace weighing scales. Most participants (62.5%, n = 10) weighed themselves in at least eight of the weeks over the intervention period.
| Number of timesa | Weekly self-weighing according to schedule of assessments (N = 16), n (%) |
|---|---|
| 1 | 2 (12.50)b |
| 2 | 1 (6.25) |
| 3 | 1 (6.25)c |
| 4 | 1 (6.25) |
| 5 | 1 (6.25) |
| 6 | 0 (0) |
| 7 | 0 (0) |
| 8 | 1 (6.25) |
| 9 | 0 (0) |
| 10 | 1 (6.25) |
| 11 | 1 (6.25) |
| 12 | 0 (0) |
| ≥ 13 | 7 (43.75) |
Table 11 shows the number of participants who weighed themselves in each of the scheduled weeks. Follow-up was conducted until the BodyTrace scales were collected (minimum 3 months).
| Week number | Number of participants who self-weighed (N = 16), n (%) |
|---|---|
| 1 | 15 (93.75)a |
| 2 | 12 (75) |
| 3 | 12 (75) |
| 4 | 9 (56.25) |
| 5 | 11 (68.75) |
| 6 | 9 (56.25) |
| 7 | 8 (50) |
| 8 | 10 (62.5) |
| 9 | 9 (56.25) |
| 10 | 8 (50) |
| 11 | 9 (56.25) |
| 12 | 7 (43.75) |
| 13 | 9 (56.25) |
| 14 | 8 (50) |
| 15 | 4 (25) |
| 16 | 2 (12.5) |
| 17 | 0 (0) |
| 18 | 1 (6.25) |
| 19 | 0 (0) |
| 20 | 1 (6.25) |
Table 12 presents the number of participants who weighed themselves ≥ 60% of the time, between 40% and 59% of the time and < 40% of the time.
| Adherence categories | Intervention group (N = 16), n (%) |
|---|---|
| Number of participants who weighed themselves weekly ≥ 60% of the time | 10 (62.5) |
| Number of participants who weighed themselves weekly 40–59% of the time | 0 (0) |
| Number of participants who weighed themselves weekly < 40% of the time | 6 (37.5) |
Self-weighing using objective and self-reported data
Multiple sources of frequency of self-weighing data were collected. The intervention group completed weight record cards and some participants recorded their weight on the POWeR online programme. Although these additional self-reported measures of weight are not direct evidence of self-weighing, they are an indication of adherence to the intervention. As a secondary assessment of the frequency of self-weighing, the self-reported weight data were used in combination with the objective transmitted weight data from the BodyTrace scales (Tables 13–15).
| Number of timesa | Weekly self-weighing according to schedule of assessments (N = 16), n (%) |
|---|---|
| 1 | 1 (6.25) |
| 2 | 1 (6.25) |
| 3 | 1 (6.25) |
| 4 | 1 (6.25) |
| 5 | 0 (0) |
| 6 | 1 (6.25) |
| 7 | 0 (0) |
| 8 | 1 (6.25) |
| 9 | 0 (0) |
| 10 | 0 (0) |
| 11 | 0 (0) |
| 12 | 1 (6.25) |
| ≥ 13 | 9 (56.25) |
| Week number | Number of participants self-weighing (N = 16), n (%) |
|---|---|
| 1 | 16 (100) |
| 2 | 13 (81.25) |
| 3 | 13 (81.25) |
| 4 | 12 (75) |
| 5 | 13 (81.25) |
| 6 | 10 (62.5) |
| 7 | 10 (62.5) |
| 8 | 11 (68.75) |
| 9 | 10 (62.5) |
| 10 | 10 (62.5) |
| 11 | 10 (62.5) |
| 12 | 9 (56.25) |
| 13 | 11 (68.75) |
| 14 | 10 (62.5) |
| 15 | 5 (31.25) |
| 16 | 4 (25) |
| 17 | 0 (0) |
| 18 | 1 (6.25) |
| 19 | 0 (0) |
| 20 | 1 (6.25) |
| Adherence categories | Intervention group (N = 16), n (%) |
|---|---|
| Number of participants who weighed themselves weekly ≥ 60% of the time | 11 (69) |
| Number of participants who weighed themselves weekly 40–59% of the time | 1 (6) |
| Number of participants who weighed themselves weekly < 40% of the time | 4 (25) |
Use of the POWeR online weight management programme (intervention group)
A total of 9 out of 16 (56%) intervention group participants registered to use the POWeR online programme (objective data). The median number of times that these participants logged onto the POWeR website over the follow-up period was four times (IQR 2–9 times, range 1–21 times). Registered participants recorded their weight on the POWeR programme a median of two times over follow-up (IQR 1–7 times) and spent a median of 102.8 minutes in total on the POWeR programme over follow-up (IQR 58.4–189.4 minutes). Four of the nine participants who registered on the POWeR programme completed stage 1 (44%), two completed stage 2 (22%) and no participants completed all three stages (Tables 16–18). Data are reported in medians owing to skewness in the data.
| Week number | Total number of POWeR loginsa |
|---|---|
| 1 | 13 |
| 2 | 5 |
| 3 | 6 |
| 4 | 6 |
| 5 | 4 |
| 6 | 1 |
| 7 | 4 |
| 8 | 3 |
| 9 | 6 |
| 10 | 6 |
| 11 | 3 |
| 12 | 2 |
| 13 | 2 |
| 14 | 1 |
| 15 | 1 |
| 16 | 1 |
| 17 | 2 |
| 18 | 0 |
| 19 | 0 |
| 20 | 1 |
| Week number | Total number of weights recorded on the POWeR programmea |
|---|---|
| 1 | 3 |
| 2 | 5 |
| 3 | 3 |
| 4 | 4 |
| 5 | 4 |
| 6 | 1 |
| 7 | 3 |
| 8 | 3 |
| 9 | 2 |
| 10 | 3 |
| 11 | 1 |
| 12 | 1 |
| Week number | Total number of minutes that the POWeR programme was useda |
|---|---|
| 1 | 340.6 |
| 2 | 125.8 |
| 3 | 86.1 |
| 4 | 240.6 |
| 5 | 120.8 |
| 6 | 6.5 |
| 7 | 31.7 |
| 8 | 19.3 |
| 9 | 51.5 |
| 10 | 52.7 |
| 11 | 70.1 |
| 12 | 29.1 |
| 13 | 15.6 |
| 14 | 4.6 |
| 15 | 12.1 |
| 16 | 4.4 |
| 17 | 13.3 |
| 18 | 0.0 |
| 19 | 0.0 |
| 20 | 0.4 |
Stop–go criteria to proceed to a Phase III trial
Table 19 details the outcomes of the trial in relation to the prespecified traffic-light stop–go criteria. Adherence to self-weighing as per the stop–go criteria was based on the objective weight data recorded on the BodyTrace scales.
| Stop–go criteria | Estimate (95% CI) | Target met |
|---|---|---|
| Recruitment rate | 28/80, 35% (95% CI 25% to 45%) | Red |
| Adherence to intervention (self-weighing):a weighed weekly ≥ 60% of the time | 10/16,b 63% (95% CI 39% to 86%) | Green |
| Adherence to intervention: registered with the POWeR programme171 | 9/16, 56% (95% CI 32% to 81%) | Amber |
Weight management resources and support to lose weight (intervention group)
At follow-up, participants were asked about resources that they had used to help them manage their weight and whether or not they had received any support to lose weight (Table 20).
| Adherence categories | Intervention group (N = 15a), n (%) |
|---|---|
| Have you accessed or used any resources to help with your weight loss? | |
| Yes | 6 (40) |
| No | 9 (60) |
| If yes (n = 6) | |
| Online programmeb | 1 (17) |
| Exercisec | 2 (33) |
| Dietd | 3 (50) |
| Do you feel that there are people you know among your friends and family who support and encourage you with your postnatal weight loss? | |
| Yes | 11 (73) |
| No | 4 (27) |
| Self-reported accessing the POWeR programme171 | |
| Yes | 10 (67) |
| No | 5 (33) |
| Do you know anyone else taking part in the study? | |
| Yes | 1 (7) |
| No | 14 (93) |
From Table 19, nine participants registered to use the POWeR programme; however, in Table 20, 10 participants self-reported accessing the POWeR programme. Of the 10 women in Table 20 who self-reported accessing the POWeR programme, eight of these are included in the nine participants for whom there is a digital record of POWeR programme access. Two participants have self-reported access to the POWeR programme, but there is no digital record of this. Another participant had not self-reported accessing the POWeR programme, but there is a digital record of them registering to use this.
Single items relating to the acceptability of the intervention to participants
The intervention group were asked a series of single questions to assess their views on the acceptability of the study/intervention, for which scores could range from 1 to 8, with higher scores being more favourable. The mean score for the question ‘would you recommend this study to your friends?’ was 6.2 out of 8. On average, the usefulness of being weighed by the practice nurse was scored 5.3 out of 8 and the usefulness of weekly self-weighing was scored 5.8 out of 8 by participants. Overall, participants felt that it was appropriate for the nurse to weigh them at child immunisation visits (6/8). To assess the impact of the intervention on participants’ mental health, a question that assessed whether or not the intervention made women anxious about their weight was included; the average response score was 3.8 out of 8 (Table 21).
| Adherence categories | Intervention group (N = 15a,b) |
|---|---|
| Would you recommend this study to a friend? | |
| Mean (SD), n | 6.2 (1.9), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| How helpful has being weighed by the nurse been for managing your weight? | |
| Mean (SD), n | 5.3 (1.8), 13 |
| Minimum–maximum | 2–8 |
| Missing, n | 2 |
| How helpful has weighing yourself weekly been for managing your weight? | |
| Mean (SD), n | 5.8 (2.2), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| How appropriate was it for the nurse to weigh you at your baby immunisation appointment? | |
| Mean (SD), n | 6.1 (2.3), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| How anxious did the study make you feel about your weight? | |
| Mean (SD), n | 3.8 (2.5), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
Delivery of the intervention by practice nurses
Table 22 provides data on the delivery of the intervention by practice nurses at each immunisation appointment, as reported on the weight record card in the child health red book. Weight record cards were available for 12 of the 16 participants in the intervention group. Delivery of the intervention components (weighing the women at the immunisation appointment, asking the women if they were weekly self-weighing and reminding the women about using the POWeR website) by practice nurses was high across all immunisation appointments.
| Adherence categories | Intervention group (N = 12a,b), n (%) |
|---|---|
| 2-month immunisation appointment | |
| Appointment attended by participant | |
| Yes | 12 (100) |
| No | 0 (0) |
| When participant was weighed in relation to the immunisation of the child | |
| Before | 5 (71) |
| After | 2 (29) |
| Declined | 0 (0) |
| Missing | 5 |
| Weight recorded by nurse at immunisation appointment | |
| Yes | 12 (100) |
| No | 0 (0) |
| Participant reminded by nurse about the POWeR programme | |
| Yes | 12 (100) |
| No | 0 (0) |
| Participant asked by nurse if they were following weekly self-weighing | |
| Yes | 12 (100) |
| No | 0 (0) |
| Missing | 0 (0) |
| 3-month immunisation appointment | |
| Appointment attended by participant | |
| Yes | 11 (100) |
| No | 0 (0) |
| Missing | 1c |
| When participant weighed in relation to the immunisation of the child | |
| Before | 4 (100) |
| After | 0 (0) |
| Declined | 0 (0) |
| Missing | 8 |
| Weight recorded by nurse at immunisation appointment | |
| Yes | 11 (100) |
| No | 0 (0) |
| Missing | 1 |
| Participant reminded by nurse about the POWeR programme | |
| Yes | 11 (100) |
| No | 0 (0) |
| Missing | 1 |
| Participant asked by nurse if they were following weekly self-weighing | |
| Yes | 11 (100) |
| No | 0 (0) |
| Missing | 1 |
| 4-month immunisation appointment | |
| Appointment attended by participant | |
| Yes | 10 (100) |
| No | 0 (0) |
| Missing | 2d |
| When participant weighed in relation to the immunisation of the child | |
| Before | 2 (100) |
| After | 0 (0) |
| Declined | 0 (0) |
| Missing | 10 |
| Weight recorded by nurse at immunisation appointment | |
| Yes | 10 (100) |
| No | 0 (0) |
| Missing | 2 |
| Participant reminded by nurse about the POWeR programme | |
| Yes | 9 (100) |
| No | 0 (0) |
| Missing | 3 |
| Participant asked by nurse if they were following weekly self-weighing | |
| Yes | 9 (100) |
| No | 0 (0) |
| Missing | 3 |
Attendance at immunisation visits
To check that the intervention did not adversely affect attendance at immunisation appointments (rates), practices provided data on attendance from participants’ medical records. Table 23 shows the number of immunisation appointments attended in each trial group and for each immunisation appointment. Practices provided immunisation data on 24 participants (expected data on 27 participants, as one woman withdrew from the study because they decided not to get their child immunised). There was no evidence that the intervention deterred participants from attending their child immunisation appointments, with 12 out of the 13 women (92%) in the intervention group for whom these data were provided attending all three child immunisation appointments and having their baby immunised.
| Adherence categories | Trial group, n (%) | |
|---|---|---|
| Intervention (N = 15a) | Usual care (N = 12) | |
| Number of participants for whom data on immunisation attendance were not provided by practices | 2 | 1 |
| Immunisation appointment | (N = 13) | (N = 11) |
| 2 months | 13 (100) | 11 (100) |
| 3 months | 13 (100) | 11 (100) |
| 4 months | 12 (92) | 11 (100) |
Intervention contamination
Intervention contamination in the usual-care group was assessed through usual-care participants’ self-reporting of whether they had accessed the POWeR website or used other resources to help with weight loss. Table 24 shows the weight management questions asked at follow-up to usual-care participants. Only one participant reported using portion-control methods to help them lose weight and no participants reported accessing the POWeR website. Three participants reported knowing someone else taking part in the study.
| Adherence categories | Usual-care group (N = 12), n (%) |
|---|---|
| Have you accessed or used any resources to help with your weight loss? | |
| Yes | 1 (8) |
| No | 11 (92) |
| If yes (n = 1): | |
| Portion controla | 1 (100) |
| Do you feel that there are people you know, among your friends and family, who support and encourage you with your postnatal weight loss? | |
| Yes | 10 (83) |
| No | 2 (17) |
| Self-reported accessing the POWeR website171 | |
| Yes | 0 (0) |
| No | 12 (100) |
| Do you know anyone else taking part in the study? | |
| Yes | 3b (25) |
| No | 9 (75) |
Clinical and participant-reported outcomes
Descriptive data relating to clinical and questionnaire outcomes are detailed in Weight and body composition. Given that this was a feasibility study, no hypothesis testing was conducted and no inferences from these data can be made regarding the success of the intervention.
Weight and body composition
The usual-care group were 7.5 kg heavier (adjusted mean difference) in weight than the intervention group (95% CI –13.8 to –1.3 kg) at follow-up. The within-group profile of weight over time showed that the intervention group, on average, lost weight (unadjusted mean change –3.3 kg), whereas the usual-care group gained weight (unadjusted mean change 1.9 kg). The intervention group had lower BMI and percentage body fat scores than the usual-care group at follow-up (Table 25).
| Adherence categories | Baseline | 3-month follow-up | Adjusted mean differenceb (95% CI) | ||
|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | ||
| Weight (kg) | |||||
| Mean (SD), n | 81.6 (13.7), 16 | 86.2 (21.2), 12 | 78.3 (13.5), 15 | 88.1 (23.9), 12 | –7.5 (–13.8 to –1.3) |
| Minimum–maximum | 58.8–106.7 | 66.7–148.8 | 60.5–106.7 | 64.1–154.3 | |
| Percentage body fat | |||||
| Mean (SD), n | 40.9 (4.0), 16 | 41.6 (6.0), 12 | 39.6 (4.7), 15 | 42.4 (7.1), 12 | –3.2 (–6.3 to –0.1) |
| Minimum–maximum | 34.6–46.8 | 32.5–56.1 | 34.5–48.9 | 30.5–57.0 | |
| BMI (kg/m2)c | |||||
| Mean (SD), n | 31.6 (6.1), 16 | 32.1 (8.0), 12 | 30.2 (6.0), 15 | 32.8 (8.8), 12 | –3.1 (–5.8 to –0.3) |
| Minimum–maximum | 25.5–47.2 | 26.3–56.4 | 24.5–47.2 | 24.0–58.4 | |
| Healthy (18.5–24.9), n (%) | 0 (0) | 0 (0) | 2 (13) | 1 (8) | |
| Overweight (25–29.9), n (%) | 8 (50) | 6 (50) | 8 (53) | 5 (42) | |
| Obese (30–39.9), n (%) | 6 (37.5) | 5 (42) | 4 (27) | 5 (42) | |
| Morbidly obese (> 40), n (%) | 2 (12.5) | 1 (8) | 1 (7) | 1 (8) | |
Mental health outcomes
Table 26 shows the data for the assessment of anxiety and depression. The intervention group reported higher anxiety scores than the usual-care group at follow-up (adjusted mean difference 3.7, 95% CI 0.9 to 6.4). The intervention group reported marginally higher depression scores than the usual-care group at follow-up (adjusted mean difference 0.5, 95% CI –1.9 to 2.9). The intervention group reported a more favourable body image score than the usual-care group at follow-up (adjusted mean difference 0.9, 95% CI –0.5 to 2.4) (Table 27).
| Adherence categories | Baseline | 3-month follow-up | Adjusted mean differenceb (95% CI) | ||
|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | ||
| HADS: depressionc | |||||
| Mean (SD), n | 5.9 (4.9), 16 | 5.0 (3.0), 11 | 6.3 (4.0), 14 | 5.5 (2.5), 12 | 0.5 (–1.9 to 2.9) |
| Minimum–maximum | 0–15 | 1–10 | 0–15 | 2–10 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| HADS: anxiety | |||||
| Mean (SD), n | 6.1 (3.8), 16 | 6.3 (3.7), 11 | 8.4 (4.1), 14 | 5.2 (3.6), 12 | 3.7 (0.9 to 6.4) |
| Minimum–maximum | 0–11 | 1–13 | 1–14 | 1–13 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| Adherence categories | Baseline | 3-month follow-up | Adjusted mean dfferenceb (95% CI) | ||
|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | ||
| BISS: overall measurec | |||||
| Mean (SD), n | 3.5 (0.9), 15 | 3.3 (1.0), 11 | 3.9 (1.4), 13 | 3.0 (1.5), 12 | 0.9 (–0.5 to 2.4) |
| Minimum–maximum | 1.8–4.8 | 1.8–4.8 | 1.2–6.0 | 1.0–5.2 | |
| Missing, n | 1 | 1 | 2 | 0 | |
Eating behaviours
The intervention group reported higher cognitive restraint of eating and uncontrolled eating scores than the usual-care group at follow-up. The usual-care group reported more favourable emotional eating scores than the intervention group at follow-up. Data and results for these outcomes are detailed in Table 28.
| Adherence categories | Baseline | 3-month follow-up | Adjusted mean differenceb (95% CI) | ||
|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | ||
| TFEQ: cognitive restraint domainc | |||||
| Mean (SD), n | 38.7 (15.0), 16 | 44.1 (28.1), 11 | 47.6 (12.7), 14 | 48.6 (23.3), 12 | 5.4 (–8.9 to 19.6) |
| Minimum–maximum | 16.7–66.7 | 11.1–77.8 | 22.2–72.2 | 0–72.2 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| TFEQ: uncontrolled eating domain | |||||
| Mean (SD), n | 47.9 (26.3), 16 | 43.0 (23.7), 11 | 50.3 (25.6), 14 | 41.0 (27.9), 12 | –0.03 (–15.4 to 15.4) |
| Minimum–maximum | 7.4–88.9 | 7.4–88.9 | 14.8–85.2 | 3.7–81.5 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| TFEQ: emotional eating domain | |||||
| Mean (SD), n | 47.9 (26.5), 16 | 48.5 (32.7), 11 | 56.3 (34.4), 14 | 43.5 (32.3), 12 | 9.1 (–25.9 to 44.0) |
| Minimum–maximum | 0–100 | 0–88.9 | 0–100 | 0–88.9 | |
| Missing, n | 0 | 1 | 1 | 0 | |
Self-reported physical activity and sedentary behaviour
Physical activity-related outcomes and time engaged in sedentary behaviour are reported in Table 29. Data are reported in medians owing to skewness in the data, and unadjusted differences in medians are reported. The intervention group reported participating in more moderate-intensity physical activity [difference 22.3 metabolic equivalent of task (MET) hours per week, 95% CI –71.4 to 116.0 MET hours per week] but less light-intensity physical activity (difference –19.6 MET hours per week, 95% CI –84.9 to 45.7 MET hours per week) than the usual-care group at follow-up. The intervention group spent more time sedentary than the usual-care group (difference 8.4 MET hours per week, 95% CI –23.7 to 40.5 MET hours per week). Full details of all of the physical activity subdomains can be found in Table 29.
| Adherence categories | Baseline | 3-month follow-up | Difference in mediansb (95% CI) | ||
|---|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | ||
| Intensity domains | |||||
| PPAQ: sedentary activity (MET hours/week) | |||||
| Median (IQR) | 60.2 (30.6–88.5) | 66.0 (28.0–97.1) | 48.1 (21.0–56.9) | 39.7 (22.4–60.8) | 8.4 (–23.7 to 40.5) |
| Minimum–maximum | 9.5–108.2 | 22.4–146.3 | 5.1–98.7 | 11.6–109.9 | |
| Missing, n | 2 | 1 | 2 | 0 | |
| PPAQ: light-intensity activity (MET hours/week) | |||||
| Median (IQR) | 119.2 (87.7–154.9) | 154.9 (135.5–166.1) | 110.8 (81.2–178.0) | 130.4 (107.8–183.9) | –19.6 (–84.9 to 45.7) |
| Minimum–maximum | 49.5–198.1 | 110.4–206.3 | 48.8–193.6 | 73.9–229.8 | |
| Missing, n | 1 | 1 | 2 | 1 | |
| PPAQ: moderate-intensity activity (MET hours/week) | |||||
| Median (IQR) | 116.4 (58.5–168.6) | 141.5 (70.8–188.7) | 150.8 (82.3–199.4) | 128.5 (56.5–167.0) | 22.3 (–71.4 to 116.0) |
| Minimum–maximum | 10.6–210.4 | 58.6–206.5 | 50.2–266.1 | 55.1–361.3 | |
| Missing, n | 0 | 1 | 1 | 1 | |
| PPAQ: vigorous-intensity activity (MET hours/week) | |||||
| Median (IQR) | 0 (0–5.8) | 0 (0–0) | 1.6 (0–9.8) | 3.3 (0–7.5) | –1.6 (–8.2 to 4.9) |
| Minimum–maximum | 0–9.8 | 0–30.0 | 0–10.1 | 0–37.0 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| Activity-type domains | |||||
| PPAQ: household/caregiving activity (MET hours/week) | |||||
| Median (IQR) | 202.6 (121.8–241.5) | 224.2 (169.6–272.8) | 181.0 (134.4–283.6) | 219.5 (150.9–309.1) | –38.5 (–159.3 to 82.3) |
| Minimum–maximum | 22.4–371.4 | 146.5–353.9 | 72.5–425.8 | 119.6–401.8 | |
| Missing, n | 1 | 1 | 2 | 0 | |
| PPAQ: occupational activity (MET hours/week) | |||||
| Median (IQR) | 0 (0–69.9) | 0 (0–71.1) | 0 (0–35.9) | 0 (0–18.0) | 0 (–19.9 to 19.9) |
| Minimum–maximum | 0–138.8 | 0–158.6 | 0–239.8 | 0–109.4 | |
| Missing, n | 0 | 1 | 0 | 1 | |
| PPAQ: sports/exercise activity (MET hours/week)c | |||||
| Median (IQR) | 6.3 (0–20.3) | 2.4 (0.8–22.0) | 18.0 (5.3–29.6) | 9.1 (6.4–17.0) | 8.9 (–5.0 to 22.8) |
| Minimum–maximum | 0–34.2 | 0–53.4 | 0–43.2 | 2.3–37.8 | |
| Missing, n | 0 | 1 | 1 | 0 | |
| Total activity | |||||
| PPAQ: total activity (MET hours/week) | |||||
| Median (IQR) | 289.7 (224.2–416.2) | 345.6 (328.1–421.3) | 265.4 (224.8–434.6) | 278.6 (212.8–409.7) | –13.2 (–209.1 to 182.7) |
| Minimum–maximum | 114.2–456.9 | 265.4–438.6 | 123.2–498.6 | 178.0–652.8 | |
| Missing, n | 3 | 1 | 3 | 1 | |
| PPAQ: total activity (excluding work domaind) (MET hours/week) | |||||
| Median (IQR) | 289.7 (181.0–323.0) | 328.1 (271.1–362.4) | 237.3 (178.9–396.2) | 301.5 (217.0–401.4) | –64.2 (–213.1 to 84.6) |
| Minimum–maximum | 71.2–456.9 | 261.0–423.6 | 123.2–498.6 | 178.0–543.4 | |
| Missing, n | 3 | 1 | 3 | 0 | |
Weight control strategies (intervention group)
At follow-up, average scores for engagement in individual item weight control strategies were comparable across the groups (Table 30).
| Adherence categories | Trial group | Adjusted mean differenceb (95% CI) | |
|---|---|---|---|
| Intervention (N = 15a) | Usual care (N = 12) | ||
| WCSS: total WCSS scorec | |||
| Mean (SD), n | 1.6 (0.6), 13 | 1.5 (0.6), 9 | –0.04 (–0.7 to 0.6) |
| Minimum–maximum | 0.8–2.7 | 0.5–2.2 | |
| Missing, n | 2 | 3 | |
| WCSS: dietary choices scorec | |||
| Mean (SD), n | 2.3 (0.7), 13 | 2.5 (0.8), 12 | –0.2 (–0.8 to 0.3) |
| Minimum–maximum | 1.2–3.7 | 0.6–3.4 | |
| Missing, n | 2 | 0 | |
| WCSS: self-monitoring strategies scorec | |||
| Mean (SD), n | 0.9 (0.9), 14 | 0.6 (0.7), 12 | 0.4 (–0.7 to 1.5) |
| Minimum–maximum | 0–2.7 | 0–2.1 | |
| Missing, n | 1 | 0 | |
| WCSS: physical activity scorec | |||
| Mean (SD), n | 1.4 (0.8), 14 | 1.2 (0.6), 11 | 0.2 (–0.5 to 0.9) |
| Minimum–maximum | 0–2.7 | 0.3–2.2 | |
| Missing, n | 1 | 1 | |
| WCSS: psychological coping scorec | |||
| Mean (SD), n | 1.5 (0.5), 13 | 1.5 (0.7), 10 | –0.03 (–0.5 to 0.4) |
| Minimum–maximum | 0.7–2.7 | 0.6–2.6 | |
| Missing, n | 2 | 2 | |
Perceptions of self-weighing (intervention group)
Overall, participants in the intervention group reported positive perceptions of regular self-weighing at 3 months, with an average score of 5.1 out of 8. Individual item scores for perceptions of self-weighing ranged from 4.2 to 5.8 out of 8 (Table 31).
| Adherence categories | Intervention group (N = 15a,b) |
|---|---|
| Over the past 3 months, how difficult was it to weigh yourself regularly? | |
| Mean (SD), n | 5.1 (2.6), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| Over the past 3 months, how difficult was it to remember to weigh yourself regularly? | |
| Mean (SD), n | 5.2 (2.6), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| Over the past 3 months, how helpful did you find regular self-weighing? | |
| Mean (SD), n | 5.8 (2.3), 13 |
| Minimum–maximum | 2–8 |
| Missing, n | 2 |
| Over the past 3 months, how frustrating was it to weigh yourself regularly? | |
| Mean (SD), n | 5.2 (2.5), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| Over the past 3 months, how anxious did you feel because of weighing yourself regularly? | |
| Mean (SD), n | 4.5 (2.6), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| Over the past 3 months, how self-conscious did you feel because of weighing yourself regularly? | |
| Mean (SD), n | 4.2 (2.6), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
| Over the past 3 months, I found weighing myself regularly to be a positive/negative experience | |
| Mean (SD), n | 5.7 (1.7), 13 |
| Minimum–maximum | 4–8 |
| Missing, n | 2 |
| How likely are you to weigh yourself regularly after this study ends? | |
| Mean (SD), n | 5.2 (2.8), 13 |
| Minimum–maximum | 1–8 |
| Missing, n | 2 |
Infant feeding and sleeping
Information relating to rates of breastfeeding and sleep patterns are presented in Tables 32 and 33, respectively. At follow-up, rates of breastfeeding were higher in the intervention group (67%, n = 10) than in the usual-care group (33%, n = 4). Hours of uninterrupted sleep per night were similar in both groups at follow-up.
| Adherence categories | Baseline, n (%) | 3-month follow-up, n (%) | ||
|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | |
| Current feeding method | ||||
| Exclusively breastfeeding | 11 (69) | 4 (33) | 10 (67) | 4 (33) |
| Exclusively formula feeding | 3 (19) | 5 (42) | 4 (27) | 7 (59) |
| Both breastmilk and formula | 2 (12) | 3 (25) | 1 (6) | 1 (8) |
| Have you been breastfeeding then stopped? | ||||
| Yes | 4 (100) | 6 (86) | ||
| No | 0 (0) | 1 (14) | ||
| Missing | 11 | 5 | ||
| Adherence categories | Baseline | 3-month follow-up | ||
|---|---|---|---|---|
| Intervention (N = 16) | Usual care (N = 12) | Intervention (N = 15a) | Usual care (N = 12) | |
| Average hours of uninterrupted sleep per night | ||||
| Mean (SD), n | 3.0 (1.2), 16 | 3.5 (0.9), 12 | 4.2 (1.8), 15 | 4.8 (1.4), 12 |
| Minimum–maximum | 1–6 | 2–5 | 2–8 | 3–8 |
Intervention fidelity assessed by audio-recordings of consultations
A total of 17 audio-recordings from immunisation appointments were completed, involving 10 mothers from six intervention practices. The aim was to audio-record as many appointments as possible; these data reflect those appointments for which both participants and nurses consented to having the consultation audio-recorded. Data from the audio-recordings indicated that the intervention took < 2 minutes to deliver in 11 consultations, between 2 and 3 minutes in five consultations and between 3 and 4 minutes in one consultation. The results show evidence of a high level of intervention fidelity by practice nurses against the intervention checklist. In one case, it was clear from the conversation that the participant had forgotten their child’s red book and, therefore, the nurse was not able to record that they had delivered the trial protocol. The overall results are displayed in Table 34.
| Adherence categories | Completed by nurse/GP, n (%) | Not completed by nurse/GP, n (%) | Not clear from recording,a n (%) | N/A, n (%) |
|---|---|---|---|---|
| Weighed and recorded weight in child health red book | 15 (88.2) | 1 (5.9) | 0 | 1 (5.9) |
| Checked participant was weighing weekly | 15 (88.2) | 2 (11.8) | 0 | 0 |
| Asked if accessed the POWeR171 website | 15 (88.2) | 1 (5.9) | 1 (5.9) | 0 |
| Signposted to the POWeR171 website | 13 (76.5) | 3 (17.6) | 1 (5.9) | 0 |
Safety
No SAEs were reported during the study.
Chapter 4 Discussion of trial findings
Main findings
This study examined the feasibility and acceptability of a multicomponent brief weight management intervention delivered to women at child immunisation appointments in primary care. The quantitative assessment of feasibility and acceptability of the intervention was based on prespecified traffic-light stop–go criteria. The recruitment target was not met (red), highlighting that changes to the methods of recruitment are required before proceeding to a Phase III trial. The target for adherence to regular self-weighing was met (green), albeit with wide CIs. Participants regularly recorded their weight on the weight record card, demonstrating that they adhered well to the main intervention component. The stop–go criteria for use of the POWeR website were categorised as amber; therefore, some additional strategies may need to be considered to assist participants in engaging with an online weight management programme. There was also an indication that the intervention may help women to successfully lose weight.
One participant withdrew from the study because they decided not to have their child immunised (intervention) and another participant withdrew from the intervention after deciding that the study tasks were an inconvenience, but agreed to remain in the trial and complete follow-up. No participants were lost to follow-up. The intervention did not have an adverse effect on attendance at immunisation appointments. This provides evidence of the feasibility and acceptability on the intervention. Most participants said that they would recommend the study to their friends. The intervention took practice nurses, on average, 2 minutes to deliver and the intervention fidelity by nurses was high, suggesting that the intervention can be delivered during child immunisations appointments in primary care.
Recruitment
Slower than expected recruitment rates are not uncommon in postnatal weight management studies184,185 and recruitment proved difficult in this study; there may be several explanations for this. A total of 13 out of 14 practices were located in areas of high deprivation (based on IMD) serving a high proportion of ethnic minority patients. This is also evidenced by the number of participants who reported ‘difficult financial status’ (65%) and the high proportion of participants from non-white ethnic backgrounds (54%). Recruitment to clinical trials from these communities is known to be difficult; therefore, our recruitment experiences are likely to present a ‘worst-case scenario’. Information about the trial was sent to participants in English; in any subsequent trial, it will be important to translate the trial documentation into other languages (e.g. Urdu and Punjabi) so that information is accessible to all women from a range of ethnic backgrounds. Recruitment was also hampered by a change in the computer system at BWH in the last 6 weeks of the recruitment period. This computer system change made it difficult for the hospital to continue to systematically send invitation letters to potentially eligible women, and there was insufficient time for the research team to introduce new recruitment methods prior to the end of the recruitment phase.
Participants received their study invitation letter around 4–6 weeks after giving birth. This is a time during which mothers and their families are adjusting to life with a small baby and, therefore, weight loss may not be considered a priority at this time. There was a maximum period of 4 weeks available between women receiving their invitation letter and women being able to complete the baseline assessment. Women could not be recruited prior to 4 weeks postnatally and the baseline visit had to be completed before the first immunisation (when the child is about 2 months old). This short time framework may have deterred some women from participating in the trial at this busy time in their lives. Therefore, rather than recruiting women postnatally, an alternative approach may be to consider recruiting women antenatally towards the end of pregnancy, when women do not have the same distractions and demands on their time. Recruiting antenatally may also be useful because it provides time for women to start to think about weight loss and to mentally prepare for changes to their lifestyle behaviours before the baby arrives and the intervention commences postnatally. In a trial (n = 656) conducted by the authors of this report (AD, SJ and KJ) that assessed a weight management intervention during pregnancy and in which women were recruited at routine antenatal appointments in the community, a recruitment rate of 80.4% was reported, demonstrating that there is an appetite among women to be approached to participate in weight management trials prior to giving birth. 186
Given the short window of opportunity available to recruit women and the cluster randomised research design adopted, it may be that an ‘opt-out’ approach to recruitment would be a more efficient method and would allow the trial to be better embedded into routine health-care practice. This may also allow for more timely translation and implementation should the intervention be shown to be effective. Evidence has suggested that higher response and recruitment rates may be obtained when studies employ opt-out methods. 187 Although acknowledging the ethics challenges that may arise in an ‘opt-out’ recruitment approach, such an approach would fit well with the cluster randomisation design; cluster trials more readily allow for an ‘opt-out’ recruitment process because a whole practice is allocated to one group allocation.
It might be the case that recruitment via other health-care professionals involved in the care of postnatal women and young babies might be useful in aiding recruitment, such as community midwives and health visitors. These routes of recruitment should be considered in future research studies.
Based on the recruitment response to this study, it is also possible that women do not want a weight management intervention shortly after giving birth; however, this is not consistent with literature that has reported that women do want early intervention. For example, longitudinal evidence has reported that, by 8 weeks postnatally, 84% of women were already attempting to, or were ready to start, managing their weight. 188 Similarly, an online survey that recruited 1015 women who had given birth in the previous 2 years and had joined Slimming World (Alfreton, UK) for the first time after having their baby, reported that approximately 45.6% (n = 463) of women began attending meetings between 1 and 7 months postnatally,189 the time frame over which this intervention was delivered. The research team discussed recruitment methods with the PPI contributors throughout the trial; further engagement with contributors would be completed to optimise the recruitment strategy to the Phase III trial.
Adherence to self-weighing
Regular self-weighing has been shown to be an important strategy in facilitating weight loss, particularly when part of multicomponent weight loss interventions. 90,190–192 Once participants are recruited, engagement and later adherence are the most important determinants of effectiveness in lifestyle interventions. Low engagement rates in weight loss interventions may result in a non-significant outcome, despite the number of contacts offered/delivered. 193 In this trial, adherence to weekly self-weighing was good, demonstrating that women are keen to engage in this behaviour to manage their weight. Data collected via the objective recording BodyTrace scales showed that 63% of the intervention group weighed themselves weekly ≥ 60% of the time during the intervention, meeting the green stop–go criteria. When all available data (self-report and objective) from the BodyTrace scales, the weight record cards and the weight data reported in the POWeR programme were combined, adherence to weekly self-weighing increased to 69% over the scheduled intervention weeks. Collectively, these data show that postnatal women are motivated to engage in regular self-weighing soon after childbirth, a strategy that has been shown to be instrumental in facilitating weight loss in other populations and contexts. 130–135 One of the attractions of self-management-based interventions is that they are flexible, are individualised and can be engaged in by women at a time that suits their daily lives. Although evidence shows that women engaged very well with self-weighing and recording of their weight, the CIs for frequency of self-weighing were wide; strategies to enhance this outcome could be considered to further increase engagement with this behaviour. Technology, such as text message reminders, may be useful in this regard.
Use of the POWeR online weight management programme
The use of technology to deliver lifestyle interventions has grown exponentially over the past decade. Technology-based interventions offer some key advantages over traditional behaviour change interventions: they have the ability to reach a large number of people at a relatively low cost; they offer increased access to the public at a time and place that suits their preferences, including the ability to overcome the need to attend face-to-face sessions to receive the intervention; and they are a way of encouraging self-care and self-management of health, which reduces NHS costs.
Participant engagement is critical to the success of any technology intervention. In total, 56% of participants registered to use the POWeR website and the amber stop–go criteria for progression was met. To have met the green stop–go criteria, ≥ 60% of participants needed to have registered with POWeR (56% achieved), which suggests that, prior to a subsequent Phase III trial, women would benefit from some additional support in using technology to support weight loss. Ideas for achieving this were raised in the qualitative interviews, such as making the POWeR website available as a mobile telephone application (hereafter referred to as app) and including more pages that are specifically relevant to postnatal women (see Chapter 5). It might also be the case that branded online weight loss programmes developed by commercial companies, such as those developed by Weight Watchers (New York, NY, USA) and Slimming World, may be useful. 194
The small number of times that participants recorded a weight on the POWeR website is likely to be related to the weight record card being used instead. Given that weights on the record card were reviewed at the immunisation appointment (external accountability), participants were, therefore, more likely to record their weight on the record card than on the POWeR website. Engagement with the POWeR programme reduced over time and any future digital programme would probably need some additional strategies to sustain engagement. However, it should also be noted that engagement with the POWeR programme varied, with some women continuing to use it after the end of follow-up.
The clinical effectiveness of the overall intervention in any future trial will depend on the uptake, the ongoing engagement with the programme and the effectiveness of the programme. Here, we have data on only the first two of these elements. A previous trial has shown that the POWeR intervention was associated with weight loss at 1 year, but that this was not statistically greater than the control. 171 There have been considerable developments in digital weight management programmes since the initial decision to use the POWeR programme as the specific weight loss tool. Prior to a definite trial, an up-to-date review of the available resources will be conducted to identify the programme that is likely to best meet the needs of women, for example available in app format, and that also has evidence of effectiveness at least in general population samples of people who are overweight. Given the proportion of women recruited from ethnic minorities, it will also be important to consider programmes that are available in several languages.
Adverse effects from self-weighing
There has been debate in the literature on whether or not encouraging regular self-weighing might lead to adverse psychological health outcomes, including eating disorders and obsessive thoughts about body weight. 195–198 However, systematic reviews and studies do not support such a view. 199–201 Nevertheless, some consideration should be given to a small amount of evidence that has suggested that self-weighing could have an impact on psychological health. A systematic review by Pacanowski et al. 202 reported that self-weighing was associated with negative outcomes in young women. Of relevance here, Hartmann-Boyce et al. 203 reported a polarisation of views in relation to participants’ attitudes and beliefs regarding the long-term use of self-monitoring techniques; some expressed experiencing a reduction in attentiveness, shame and even fear over time, whereas others claimed that self-monitoring increased levels of self-accountability, self-control and self-efficacy. Although participants in this trial were broadly supportive of encouraging women to self-weigh themselves regularly, there was also some evidence that it may lead to some participants feeling anxious about their weight; this will need to be investigated in greater depth in a subsequent larger trial and strategies put in place to mitigate occurrence.
Delivery of the intervention by nurses at child immunisation appointments
The UK guidelines advise all health-care professionals to screen for obesity and encourage weight loss via the provision of information and signposting to available weight management services. 71,75 However, evidence has shown that health-care professionals are reluctant to raise the topic of weight with their patients for fear of negative consequences, such as causing offence. 155,204 Kaplan et al. 205 have suggested that health-care providers were comfortable raising the topic of weight, but that time constraints prevented this during routine appointments. This study has provided data to show that practice nurses were able to ‘raise the topic of weight’ and deliver the intervention as per protocol. 205 Nurses delivered all components of the intervention with high fidelity. Furthermore, audio-recordings of the immunisation appointments demonstrated that, overall, nurses delivered the intervention well and in accordance with the protocol. This provides reassurance that the nurse training methods worked well and that the intervention can be delivered as intended during child immunisation appointments.
Intervention contamination
A cluster RCT methodology was adopted to minimise the potential for intervention contamination in usual care. Nevertheless, it is still possible for contamination to occur, and this trial aimed to assess this possibility of mitigating any potential effect(s) in a subsequent trial. In the usual-care group, only one participant (8%) reported that they had accessed or used resources to help them lose weight, none had accessed the POWeR website and three (25%) indicated that they knew another participant in the study. These data have indicated that the risk of intervention contamination is low, but that some consideration should be given to the possibility that contamination may occur.
Clinical outcomes
Several clinical and patient-reported outcome measures were included in this study to assess their completion rates for use in the subsequent effectiveness trial. However, this study was not powered to detect meaningful differences between the groups for these outcomes. All participants (except the participant who withdrew) provided weight data at follow-up and, similarly, there were few missing data for questionnaire-based outcomes, ranging from 0% to 15%. Missing data were primarily because of participants inadvertently not completing a specific item on questionnaires, rather than because they declined to complete them or found them difficult to understand. These findings are encouraging and provide confidence to include these questionnaires/outcomes in a larger trial. With respect to the health economics measures, the ICECAP-A was fully completed and, therefore, would form part of the economic evaluation design alongside the larger trial.
Assessments of weight, anxiety, depression, eating habits (TFEQ) and self-reported physical activity were included. Data regarding weight, body composition, participation in moderate-intensity physical activity and body image generally favoured the intervention group compared with the usual group at follow-up. The intervention group reported higher anxiety and depression scores (marginal) and lower participation in light-intensity physical activity than the usual-care group at follow-up.
Implications
The intervention was deliberately developed with the ambition of the NHS to ‘Make Every Contact Count’ in mind. 206 The implementation of a multicomponent intervention that was embedded into routine health care may ensure accessibility to all women who give birth. This approach to intervention delivery seeks to ensure that hard-to-reach women, for example those with low levels of education, those living in deprived communities and ethnic minorities, who are perhaps more likely to be affected by obesity, are given the opportunity to receive weight management support after having a baby. Embedding a weight management intervention into routine child immunisation appointments presents an opportunity to routinely identify and treat more women with obesity to improve their potential weight trajectories during their reproductive years and beyond. The intervention tested here is brief and simple to deliver, which, if proven effective in a Phase III trial, may be cost-effective for the NHS.
Most people who lose weight will regain their weight loss within 1–3 years, highlighting the difficulties of sustaining health behaviour changes once active intervention is completed. 207–209 Related to this, in postnatal women, the needs of their child change during the first year, which can mean that barriers shift and new strategies are needed over time. Weight management interventions, therefore, need to be dynamic and flexible to address the barriers that this population of women may face over time. Such approaches might be to consider including text message support and/or further support at routine health-care contacts, given that many women will continue to engage with the NHS throughout the early years of their children’s lives.
Most of the women recruited had more than one child; therefore, it is possible that the intervention is more appealing to these women who may be less likely than new mothers to be overwhelmed by the impact of a new child on their lives.
Strengths and limitations
This study has several methodological strengths and makes a unique contribution to the literature. To the best of our knowledge, this is the first study worldwide to assess the merits of a weight loss intervention embedded in a national child immunisation programme. This study was appealing to both first-time and multiparous mothers, suggesting that weight management during the postnatal period is a concern to women irrespective of the number of children that they have given birth to previously. Although the recruited sample was small, women varied in terms of their socioeconomic status, ethnicity and employment status, suggesting that the experiences of a wide range of women are represented in these findings. Importantly, the sample included a high proportion of women from more deprived areas. Practice nurses were trained to deliver the intervention following standardised procedures ensuring that the intervention had the best opportunity to be successful, and evidence shows that nurses adhered well to the protocol. The trial was conducted and reported in line with CONSORT guidelines to ensure that the trial methods were transparent and reproducible.
Process evaluations are often not included when evaluating complex health behaviour change interventions, but they can be very useful in helping to provide knowledge of ‘how’ the intervention is being delivered in practice. Several approaches to process evaluation were included in this study in relation to its setting, intervention delivery and the acceptability and implementation of the intervention. A selection of immunisation appointments in which the intervention was delivered were audio-recorded, and this provided objective ‘real-time’ naturalistic data on the interactions between participants and nurses to further enhance our understanding of how the intervention can be refined to maximise its effectiveness. The inclusion of the BodyTrace weighing scales allowed objective data on the frequency at which women weighed themselves each week to be collected, providing further real-time objective process evaluation data.
Many weight management studies rely on self-reported weight as outcome data at baseline and follow-up. By contrast, in this study assessments of weight were objectively measured by a researcher to ensure that these outcome data were accurate and not prone to bias or under-reporting. This approach also minimised the probability of missing data. Weight loss studies can often experience high loss to follow-up rates, but we were able to collect weight data on all participants who completed follow-up (27/28; one participant withdrew), demonstrating that the strategies used to ensure minimal loss to follow-up were effective. These strategies included conducting home visits for data collection at baseline and follow-up and providing a £20 financial incentive; these strategies should be used in subsequent research. We conducted semistructured interviews to obtain further understanding about the trial processes and the intervention components from both the participants’ and the nurses’ perspectives (see Chapters 5 and 6). The findings from the interviews provide important information in which the results of this trial can be understood and interpreted; the qualitative data collected can assist with the development of a more acceptable and potentially effective intervention in a subsequent Phase III trial.
Objective data on attendance at immunisation appointments were collected from medical records, so that the impact of the intervention on immunisation rates could be objectively assessed. This study provides reassurance that the intervention will not adversely affect immunisation uptake rates, which is critical to the integrity and safety of the intervention.
This study should also be interpreted in the light of some methodological limitations. By using a centralised hospital records system to invite all women who had recently given birth to take part in the study, the aim was to reduce the likelihood of recruiting only women who were highly motivated to lose weight, but we cannot discount the possibility that atypical women were recruited. Similarly, study procedures that involved home visits at baseline and follow-up may have resulted in recruitment of more motivated women. The characteristics of the sample may not be representative of the eligible populations, and those recruited may have responded to the intervention more favourably than might be the case for postnatal women more generally. Data collection on the views of women who declined to participate would have been useful in developing our understanding of the acceptability of the intervention. As this was a feasibility trial, the sample size was small and the findings should be interpreted with this in mind. Women self-reported their physical activity behaviour; therefore, the data may be prone to bias and over-reporting. Future studies should consider including an objective assessment of physical activity. A more detailed analysis of body composition would have been useful, as some studies have reported fluctuations in body fat percentage during the year following childbirth.
We assessed the intervention over only the first three immunisation appointments at 1, 3 and 4 months; the intervention was not delivered at the 12-month child immunisation appointment, so the longer-term effects of the intervention were not assessed. Nevertheless, it is important to consider that, as mothers become more accustomed to a routine with their new baby, they may be able to dedicate more time to their own health and well-being,209 which may translate into a greater lifestyle behaviour change over time. An opportunity to measure greater change in health behaviours may have been missed, and in any subsequent trial this later 12-month immunisation appointment should be included in the intervention.
Sleep deprivation is common for many women during the postnatal period and can affect mood and energy levels. 210 Weight loss has also been linked to sleep duration and quality. 211–213 To attain more accurate and detailed data on this variable, future studies should include an objective measure of sleep, ideally through a tracker watch that records these data in real time each day.
Conclusions
This trial has provided evidence that a brief weight loss intervention focused on promoting self-management of weight that was delivered by nurses during routine child immunisations visits was acceptable to women who were recruited. Practice nurses were able to deliver the intervention with high fidelity, indicating that the intervention is feasible to deliver during child immunisation appointments. Adherence by participants to the self-weighing component was generally good. Uptake of the weight management programme was acceptable, although there is scope for improvement. However, recruitment was a challenge and the methods used to recruit postnatal women were not successful, and alternative approaches need to be tested prior to progressing to a Phase III trial.
Chapter 5 Qualitative study (participants)
Introduction
After giving birth, a window of opportunity opens in which women may be motivated to lose weight. 214,215 Although many weight loss interventions for postnatal women have been tested and shown to have varying levels of effectiveness,216 few studies have explored the views of women who participate in such interventions as well as simultaneously exploring the experiences of those who deliver these interventions to women. The Medical Research Council have advocated the use of feasibility and acceptability studies prior to more comprehensive and costly evaluations of complex health-care interventions. 217 This process allows any potential issues that might affect the delivery and acceptance of the intervention to be identified and resolved early on in the research process. 217 The use of nested qualitative research has been shown to be beneficial when addressing issues relating to the acceptability and practicality of an intervention. 218,219 It was intended that the findings from this qualitative study and the interviews with practice nurses who delivered the intervention would be understood alongside the trial data to provide a comprehensive multiperspective assessment of the feasibility and acceptability of the intervention. This chapter will explore the views of women who participated in the intervention. Chapter 6 reports the views of practice nurses who delivered the intervention during child immunisation visits. This study (chapter) had the following objectives to:
-
explore whether or not child immunisation appointments are an appropriate setting for postnatal mother’s weight to be monitored
-
capture intervention participants’ views about how useful the intervention was in helping them manage their weight
-
determine what elements of the intervention facilitated and/or impeded its acceptability
-
investigate what aspects of the intervention were acceptable and unacceptable to participants, as well as the reasons for these feelings and opinions
-
assess which components of the intervention may need to be amended, if any, and invite feedback from participants about how the intervention might be improved
-
assess if the intervention led to participants experiencing any psychological harm relating to their weight.
Methods
Semistructured interviews were used to explore women’s views of the intervention. Interviews were held after women had completed the intervention. Interviews were used, rather than focus groups or observation, because this study was focused on exploring women’s individual views and experiences, rather than views that might be revealed in a group format or through their ‘presentation of self’ in front of others. 220 Semistructured interviews were chosen for several further reasons. Interviews provide the opportunity for key questions to be asked of participants, which allows comparison of question responses with others who have also experienced the intervention as well as the participant group as a whole. This process allows for patterns of variation to be explored while still allowing flexibility. 221 We aimed to gain a thorough understanding of and be able to describe trial participants’ and nurses’ experiences of an intervention embedded in an existing service; therefore, we used a ‘generic approach’ rather than following a specific theoretical perspective. 222 This study has been reported in line with the Consolidated Criteria for Reporting Qualitative Research (COREQ) guidelines for the reporting of qualitative studies.
Recruitment of participants
During the follow-up home visit at 3 months post enrolment, participants in the intervention group were asked if they were willing to participate in an interview to talk about their experiences of the trial intervention. Those willing to participate were asked to sign a separate written consent form prior to the interview taking place.
Interview topic guide and interview procedures
Before any interviews were conducted, topic guides with broad, open-ended questions were piloted to ensure that the questions were easily understood and coherent. The interview topic guide comprised open-ended questions and prompts to explore participants’ reflections of key elements of the intervention, including their experience of regular self-weighing; their experience of being weighed by the practice nurse during child immunisation appointments; their thoughts on being referred to a website for weight management advice and information; and their general views of the intervention, including its timing (see Appendix 1). Participants were met once and given the freedom to expand on areas of interest and concern to them. If required, the questions were modified by the researcher to allow them to be better understood by participants, and their responses clarified and expanded on should they appear to be relevant to the study. Interviews lasted between 30 and 61 minutes. Natalie Tyldesley-Marshall conducted the interviews face to face in participants’ homes, usually with the baby present. The transcripts were anonymised with unique participant identification numbers. Natalie Tyldesley-Marshall is female with many years’ experience of qualitative research and has a Master’s degree in Social Research. Participants received a £20 high-street shopping voucher to cover any out-of-pocket expenses associated with taking part in this study.
Data analysis
Data collected during the interviews were digitally recorded on an encrypted audio-recording device. Field notes were taken immediately after the interviews and integrated into the transcripts. Data were transcribed by a commercial company. A confidentiality agreement between the UoB (sponsor) and the transcription company was in place prior to any data being sent. Interviews were recorded and transcribed verbatim with the permission of participants. Interview transcripts were thematically analysed using the framework method, a recognised tool for collating data and facilitating its interpretation. 223 Data management was facilitated by using QSR International’s NVivo 12 Plus. A list of overall and individual themes for trial participants was compiled to allow for cross-group/individual comparison.
An early transcript was independently reviewed by four authors (NTM, AD, HP and SG) (each with different disciplinary backgrounds: sociology, psychological and general practice) to develop the coding frame. Interviews were read and listened to at least two or three times to allow the study lead researcher (NTM) to become familiar with the raw data. Transcripts were analysed line by line and assigned codes, derived from the data, then assigned to themes identified. A framework ‘matrix’ (or grid) was developed with one horizontal row for each participant and one code for each vertical column, and was organised with codes under each theme on a new sheet. This enabled the researcher to immerse themselves in the data. Each transcript extract was entered into the matrix and summarised, with transcript page and line reference provided to allow comparison of participants by codes and themes by the same researcher (NTM), and to provide transparency in the coding and analysis process. 223
Regular meetings were held with the qualitative study team to discuss additional codes and ideas to achieve consensus that would improve both the quality and the rigour of the study. This was useful because an experienced qualitative researcher (SG) was in the team, who guided the framework analysis process. Early thoughts about coding, themes and the direction of the analysis were also made and kept for increased transparency and rigorousness of the research. A record was also kept of the coding, themes and any changes throughout the analysis. 223 Early transcripts were read to understand whether or not newer codes could be applied to earlier transcripts, and once interviews produced no new codes it was concluded that data saturation had been reached and no new knowledge would be obtained from further interviews. 224 The coding book is available in Report Supplementary Material 2.
Results
There were 14 participants who were allocated to the intervention group, and one indicated during recruitment to the trial that she was not interested in participating in an interview. Of the 13 participants remaining, two were not available in the time frame of the study, one had no time available and one was not contactable in the time frame of the study. A total of nine participants from the intervention group took part in an interview. The sociodemographic profile of these participants is shown in Table 35. Three main themes emerged from the data with 18 subthemes (Table 36). Participants were randomly assigned numbers; these do not correspond to the order in which the interviews took place. Each quotation is followed by participants’ assigned number.
| Age (years) | Ethnicity | Baseline BMI (kg/m2) | Follow-up BMI (kg/m2) | General practice (IMD rank) | Self-reported financial status [employment status] | Number of children | Marital status | Gained/lost weight |
|---|---|---|---|---|---|---|---|---|
| 42 | White | 30.8 | 27.4 | High | I have enough money if I plan my spending carefully[in paid employment] | 1 | Married | Lost |
| 30 | White | 34.9 | 34.4 | High | I normally have enough money for whatever I want[looking after the home/family] | 2 | Married | Lost |
| 40 | White | 27.7 | 27.1 | High | I have enough money if I plan my spending carefully[in paid employment] | 3 | Single (living with partner) | Lost |
| 30 | Black African | 36.1 | 36.1 | High | I have enough money if I plan my spending carefully[in paid employment] | 3 | Single (living alone) | Stayed the same |
| 23 | Other Asian | 31.6 | 29.0 | High | I normally have enough money for whatever I want[student] | 2 | Married | Lost |
| 33 | White | 27.7 | 25.5 | High | I normally have enough money for whatever I want[in paid employment] | 1 | Married | Lost |
| 29 | Other (Iraqi) | 42.4 | 32.2 | Medium | I have enough money if I plan my spending carefully[looking after the family/home] | 2 | Married | Lost |
| 32 | White | 28.7 | 27.8 | High | I normally have enough money for whatever I want[in paid employment] | 1 | Single (living with partner) | Lost |
| 39 | Other Asian | 30.0 | 26.5 | High | I normally have enough money for whatever I want[in paid employment] | 2 | Married | Lost |
| Intervention participants’ coding framework | Practice nurses’ coding framework |
|---|---|
| Barriers to and facilitators of weight loss | |
| Barriers to weight loss | Perceived barriers to mothers’ weight loss |
| Facilitators of weight loss | Perceived facilitators of mothers’ weight loss |
| How to lose weight | How to lose weight |
| Ideal time, space and role for weight loss and intervention | |
| Potential barriers to weight loss according to nurses | |
| Evaluation of the trial | |
| Areas for improvement | Areas for improvement |
| Credibility of scales | |
| Assessment of training | |
| Identifying trial participants | |
| Impact of the trial on appointments | |
| Impact of the trial/intervention | Impact of the trial on participants |
| Importance of numbers | |
| Opt out | Rolling out the intervention |
| Positives of the intervention | Positives of the intervention |
| Recording weight | |
| Steps in appointment | Steps in appointment |
| Website content | Website |
| Website positives | |
| Feelings around weighing and weight loss | |
| Accountability: participants | |
| Emotional issues around losing weight | |
| Feelings knowing that they will be weighed | |
| Feelings when nurse weighs | Participants’ feelings while weighing |
| Reasons for starting the trial | |
| Self-weighing feelings | Nurses’ feelings when weighing participants |
Pre-pregnancy weight and alternatives to conventional approaches
All mothers had previously tried to lose weight and all mentioned having used diet and/or exercise to manage their weight, with over half trying both. No participants mentioned specific diets, such as the 5 : 2 diet and Atkins Diet, but rather they referred to calorie counting or cutting out high-calorie food, alcohol or snacks from their diet. Exercise was mentioned by most participants and physical activities included using a commercial gym, exercise classes and more ad hoc community-based exercise, such as walking:
Yeah, like there have been times before where I’ve gone through stages of kind of, of weighing myself and recording it and that kind of thing, but I’ve never done any kind of erm, like official like Weight Watchers erm, so yeah, never kind of really consistently or with any particular um kind of diet or a structure to it.
Participant 5, lost weight
However, two women reported trying to lose weight previously through ‘shortcuts’ or alternative methods, such as consumption of cider vinegar, Chinese tea and ‘fat burners’ (weight loss tablets). Both of these participants had also tried conventional dieting methods:
I was trying to diet before I got married and then it didn’t work. So I joined, this, doctor in [city] who prescribed me some medication that . . . Fat burners, something like that, and it’s meant to suppress your appetite.
Participant 7, lost weight
Reasons for participating in the trial
All of the women were motivated to join the study to lose weight; however, the reasons for this varied between non-specific reasons, such as wanting to lose weight, and more specific reasons related to amount of weight, for example wanting to return to their pre-pregnancy weight, or wanting to lose weight for a particular event, such as an upcoming holiday:
I want to lose weight. As soon as I had a baby I wanted to lose weight because I’m planning on a big holiday! So I want, I want to be able to go on the beach.
Participant 2, no weight loss
One participant was motivated to lose weight for medical reasons stemming from their current weight, and another participant recognised the health risks as a result of their weight in combination with their ethnicity.
Would I recommend the trial to other postnatal women?
All participants except one would recommend joining the study to other mothers, but with some considerations. The content of the website was the only reason that one participant would not recommend the trial:
I’d say no because of only the content of it. It wasn’t . . . There were bits there . . . but once you’ve read the same thing . . . for the last 3 weeks it’s the same again. There’s no change to it.
Participant 4, lost weight
One participant was concerned about those with eating disorders being recruited to the trial. Although most participants talked about the difficulties of being a mother to a newborn baby, only one would have started the intervention a few weeks later, so as not to place too much pressure on a mother (particularly first-time mothers) who could be going through a ‘hard’ time:
Err, yes, I, I would . . . But I would say to them though, ya know, it’s it’s . . . just . . . [slight sigh] if they’re in the right space. As I said, I never realised how hard being a, a parent would be. It’s really, really hard and um, . . . it, it I think if somebody was really struggling with, ya know, a new baby, I wouldn’t recommend it ‘cos I think it’s, it’s something they don’t need to then be ‘Oh, I’m sorry, but you’re really fat, you need to lose weight as well’.
Participant 3, lost weight
When asked what sort of things would help other new mothers lose weight after pregnancy, almost all of the participants gave answers in terms of changing diet, increased exercise and a few specific exercises or strategies, such as cooking meals at home. These answers were typically tied into the notion of social support and having significant others (especially those whom they lived with) encourage them in their new weight loss behaviours, and support them in ways such as reminding them not to buy certain foods, caring for the baby while they exercised or buying aids that allowed them to exercise with their new baby:
So I think having some kind of support system in place . . . non-judgemental support system in place that isn’t like ‘Oh my gosh, you didn’t lose weight. You must be eating too many Oreos, but instead like this is a common issue. Here are some steps you can take. Hang in there’.
Participant 1, lost weight
Barriers to weight loss for new mothers
Participants discussed several barriers to their weight loss. For most participants, their baby was a barrier to navigate around if they wanted to exercise, and it was hard to leave the house to exercise. Gyms did not always have breastfeeding facilities, crèches or mother and baby classes. If there was nowhere to ‘store’ the baby, then suitable child care (paid or unpaid) had to be found. Alternatively, ‘baby-friendly’ physical activity had to be found. Breastfeeding was seen as a barrier to weight loss, for example when gyms did not have facilities for breastfeeding and when increased hunger from breastfeeding led to increased calorie consumption:
[They are] not a once every 3-hour baby, [they’re] a once every hour baby. Which makes it hard. [They don’t] like bottles and so swimming I can do because I can run up ter [the local public swimming baths], swim fer a half hour, come back and be back for [their] next feed.
Participant 1, lost weight
The more I’m breastfeeding, I’m more hungry . . . than anything else because my [child], although now I’m trying to like give [them] solids, [they] don’t want it. [They’re] constantly on my breasts.
Participant 2, no weight loss
Three participants mentioned that tiredness from raising a newborn baby led to them making less healthy dietary choices; when fatigued, they chose less healthy options that required less effort to prepare. Similarly, having a newborn baby meant an unpredictable schedule and a necessity to plan and work around the schedule of the baby, which similarly led to participants choosing less healthy food options that were quicker to prepare:
It’s tough . . . already. You have a new baby . . . that is depriving you of sleep. Erm, you have what, 2 hours sleep, interruptions overnight at times all you want is, ya know, cake, wine, takeaway and a few indulgences.
Participant 3, lost weight
Yeah! Easier to just go to [shop] and just [quieter] get a ready meal from M&S [London, UK] and put it in the microwave than spending 2 hours cooking.
Participant 2, no weight loss
Participants commented that continuing and maintaining behavioural changes was difficult (despite the perceived effectiveness of the intervention), and most of the participants talked about the temptation to ‘fall off the wagon’ and stop maintaining their weight loss efforts in the face of alternative pressures, such as stress and tiredness, or allowing themselves an ‘indulgence’. The study was seen to help them keep ‘on track’:
It [being involved with PIMMS-WL] been good ‘cos’ it helped me to, stay in track of my weight ‘cos’ I see, weigh myself every week. So kinda like, this week if I lose weight I’m . . . happy, then next week if I put it on loads, I can’t . . . I need to watch what I’m eating.
Participant 2, no weight loss
Facilitators of weight loss
Several comments were made about potential facilitators of weight loss. Some participants reported that being a new mother had brought more of a freedom from the restrictions on exercise during pregnancy and a time to restart exercise habits that their baby could join in with. One participant (who had a supportive partner who was able to look after their baby) was able to use the time when their baby slept to exercise and was of the view that they now had more time available to exercise than when they were employed. Another participant viewed being a new mother as liberating, given that they were restricted from exercising during pregnancy and had experienced a post-pregnancy condition that had restricted their exercise for a number of weeks thereafter:
Like I know some people have a really difficult time with babies with sleep and getting out of the house and all that kind of stuff. I don’t know if I’ve been lucky because [they’re] relatively easy to do things with, but that’s allowed me to go out and do exercise.
Participant 5, lost weight
Although breastfeeding was viewed as a barrier to weight loss, in some instances it was also considered a facilitator in terms of the view that it can lead to more calories being ‘burned’:
I mean in, in my case I really do think that breastfeeding’s helped a lot [towards weight loss].
Participant 1, lost weight
Having a newborn in the house made one mother think more about the impact that her own health behaviours were having on her baby; therefore, both she and her partner tried to model healthy eating behaviours to engrain ‘good habits’:
We want [them] to eat healthy, ya know, and it’s all vegetables and fruits that we have [them] on, so that’s nice ter- We eat [their] leftovers too. They’re good for us too. We’ll show [them] like ‘Look, pear is good!’.
Participant 1, lost weight
Intervention components
Participants expressed a range of views about the intervention components and their feelings about how effective each component was in helping them to manage their weight. The following section provides a summary of the thoughts and opinions of the women; it is not an exhaustive list. Table 37 provides an overview of participants’ feelings on being weighed by practice nurses and self-weighing.
| Participant identity number | Feelings about self-weighing | Feelings when anticipating nurse weighing | Feelings during nurse weighing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Good | Indifferent | Bad | Good | Indifferent | Bad | Good | Indifferent | Bad | |
| 1 | Good/control/pride | Fear | Excited/pride | Fine | Fear | Good | Fear | ||
| 2 | Happy | Fear | Motivated | Fine | Fear | ||||
| 3 | Worry | Unfazed | Unfazed | ||||||
| 4 | Good | Unfazed | Unfazed | Unfazed | |||||
| 5 | Fine | Bad | Fine | Fine | |||||
| 6 | Control | Worry | Fine | Worry | Fine | ||||
| 7 | Excited/good/pride | Good/excited | Excited/good/motivated | ||||||
| 8 | Good/control | Worry | Fine | Happy | Worry | ||||
| 9 | Pleased/excited | Unfazed | Fine | Fine | |||||
Being weighed at child immunisations
When anticipating being weighed by nurses at child immunisation appointments, most participants were unconcerned about being weighed. Two participants felt good or excited about being weighed and two felt anxious/scared when they expected to have gained weight. One participant commented that being weighed ‘motivated’ her to lose weight (Participant 7). At the appointments, most participants reported that they continued to feel unconcerned about being weighed. Two women continued to feel anxious when they recorded weight gain on the nurse scales:
They [the nurse weighing me] didn’t bother me. I’m, I’m not bothered, yeah, so it’s fine.
Participant 4, lost weight
Most participants reported that the nurse was supportive and/or ‘non-judgemental’ if they had maintained or gained weight. Participants commented that nurses tended to congratulate them on weight loss or say something encouraging, such as they would lose weight by the next immunisation, or suggest that they go to the POWeR website for more weight loss support, which many participants found a source of encouragement or motivation:
She would always tell me how well I did. And how great I looked and tell me I’m doing a great job and that, that weight’s gonna to come right off. And um, and she was, she was always very positive.
Participant 1, lost weight
No participants thought that their baby’s immunisation appointment was an inappropriate or unsuitable time for delivery of a weight loss intervention or to discuss weight-related issues. Some participants described it as a ‘good time’ to discuss weight (Participant 9) or said that it ‘definitely worked’ (Participant 5). Some women praised the convenience that they did not have to make an ‘extra’ trip out of the house with their baby:
Yeah, it seemed to work quite well as a way of doing it. Erm, and because . . . you’re there every 4 weeks, it’s a good . . . space of time where you can . . . ya know, you’re going to be able to see a difference, in your weight, in that time, so it seemed, it seemed like a good, a good opportunity to do that that weigh in. Yeah. It was just kind of another point to check in on things.
Participant 5, lost weight
Two participants felt that there should be more support for weight loss for postnatal women from the NHS shortly after child birth and, therefore, that this intervention was ideal:
I’m happy for the attention on [them], but I feel like . . . um, postnatal care just kind of falls to the wayside fer, the mothers, and so, I was happy to get any attention at all, ya know what I mean? Like ‘How are you feeling? Ya know, get on a scale,’ instead of it just . . . Otherwise I would have just walked in, they would have given [them] a jab and I would have walked out.
Participant 1, lost weight
Because when you have a baby, you’re on your own after that, after the midwife finished doing whatever . . . Even if you go to the doctor and wanna lose weight, they’ll tell you ‘Go diet.’. That’s what they tell you. They don’t even tell you how you can do it. They just tell you . . . ‘Go diet.’. ‘Diet I tried is not working. Give me another advice!’.
Participant 2, no weight loss
Only one mother was concerned that the nurse appeared busy and had many mothers queuing outside the consultation room, so felt that if they had questions about weight loss they would not ask them:
But when the lady came to my house, the PIMMS lady, then I was quite happy to talk to her an’ . . . have a conversation, whereas I think in the, in the setting of a busy GP practice I’m not sure . . . I would have . . . felt like I could really . . . sort of talk for a long or, ya know, ask questions or anything ‘cos I feel, you just feel like, ya know, they’re so busy you don’t want ter . . . take up too much of their time.
Participant 9, lost weight
Weekly self-weighing and recording of weight
No participants objected to self-weighing and recording their weight at home. However, some participants reported problems with the reliability of the BodyTrace weighing scales, with some finding that the reported weight deviated by ≈ 1 kg, depending on the room, time of day or type of floor used when weighing. This led some women to view the scales that were used at the immunisation appointments or their own eyes (e.g. by looking at the fit of their clothes) as a more reliable measure of their weight loss progress. Almost all of the participants indicated that they were able to weigh themselves either every week or almost every week, although one participant decided to stop weighing herself after 4 weeks owing to technical problems with her scales. All of the participants found the process of self-weighing easy to remember, typically doing so on a specific day:
Yeah, it was fine. By just setting . . . the day and the time to do it it meant that I could just . . . yeah, just every Wednesday morning.
Participant 9, lost weight
Emotions and self-weighing
When weighing themselves, participants reported that their expectations of their weight affected their emotions; typically, they felt ‘good’ when they expected to see that their weight had gone down, whereas they felt worried, fearful or ‘bad’ if they expected an increase in their weight because of a ‘bad week’. However, for two participants, self-weighing made them feel in control of their weight:
[I felt] good, when it showed that I’d lost sooo much.
Participant 7, lost weight
Yeah. Ermmm, some weeks I felt a bit anxious if I hadn’t . . . done well.
Participant 6, lost weight
I guess just like on days when I weigh myself and my weight has gone down, I feel great and like, I’m in control of things. Do you know what I mean? Like I’m directing the ship [NTM laughs] and it’s going in the right direction.
Participant 1, lost weight
All of the participants reported finding self-weighing useful. Most participants reported finding the weight displayed on the scales as an important way to see that their weight loss efforts were having an effect and that they were making progress. For some participants, the scales changed their point of reference for their weight. Rather than comparing their current weight with, for example, their weight before becoming a mother, perhaps in their 20s, they compared their current weight with their weight at the start of the intervention:
I guess it [weighing yourself regularly] kind of helped you, um, know how you were progressing.
Participant 3, lost weight
And if I hadn’t . . . had that [figure] to compare against I would just always feel like I was . . . ‘I’m heavier than I used to be.’. Instead of ‘look how much I’ve lost in the last 6 weeks’.
Participant 1, lost weight
Some of the participants commented that the actual weight displayed on the scales was less important than other measures of progress, for example how their clothes fitted and their reflection in the mirror. For the most part, these views were likely to be expressed by participants who had experienced issues with the reliability of the scales:
I normally go off erm, how my clothes fit anyway. Because that’s what they normally say anyway.
Participant 4, lost weight
How do the jeans feel ya know, when I’m wearing, wearing, wearing them or how do I kind of look in the mirror? Ya know, those, those are the ways that I, tell. And I can feel it in myself as well . . . So it’s more on how I feel than necessarily a number, on the, scales, I guess.
Participant 5, lost weight
The POWeR website
All of the participants commented that they felt that it was acceptable for nurses to refer them to the POWeR website for support, rather than being given the information about weight management directly from the nurse at the immunisation appointment. The reason given for this view was that the website could be accessed more frequently from home at any time of the day, which was important because this allowed access to fit around the unpredictable schedule of the baby and while their baby was sleeping:
[It’s] easier because you haven’t gotta go out of the house to them. You know, go out of the house with a baby in tow. Um, and you can sort of do it at home. At your own pace sort of thing.
Participant 4, lost weight
Well, you don’t meet the nurses often, right? While, with the website I can go on once a week. [baby interruption] But errr . . . with the website, I could go in and I could access it more often.
Participant 1, lost weight
The website was generally well regarded and found to provide useful information. One participant, however, pointed out that the website contained information that she had ‘seen before’ (Participant 5). Another participant commented that there were few ‘bells and whistles’ to it (Participant 1), although its appearance was not as important as its function:
There’s quite a lot of info- helpful information on the, website, yeah. Gives you a list of all the um . . . ideas of if you want to do like a low-carb diet or a low-calorie diet an’, things like that and it gives you like . . . guideline that you could follow.
Participant 6, lost weight
Two participants commented that the information on the POWeR website came from a ‘trusted source’ owing to its links with the NHS, and one participant valued that the website was free to use and free from advertising. One participant had been concerned and confused by many differing opinions from the internet and friends’ opinions; therefore, they were very pleased to have advice that they could trust about which diets were safe:
Yeah. Yeah, it’s very helpful for new mums and . . . maybe you can find the best way and . . . healthy way because not everything is healthy it’s a very . . . helpful and safe website.
Participant 8, lost weight
Most of the participants were able to access the POWeR website, but one participant had technical difficulties related to their laptop computer. One participant had difficulties when they lost the password and another encountered a difficulty with the link to the POWeR website. Although most of the participants used a computer to access the website, one mother commented that the site could be more compatible with mobile telephones. Participants commented that, because they had a new baby, they were not always able to have immediate access to a laptop to access the POWeR website. By contrast, they always had access to their mobile telephone:
Ermmm, the only thing about it was I was doing it on my phone. And it’s not like . . . erm, kind of phone compat-, compatible, so you have to like zoom in . . . On stuff it’s just easier when I had like, a few minutes on my phone. ‘Cos I’ve always got my phone with me I’d say that would make it better. Definitely.
Participant 5, lost weight
I’m still weighing myself and I put that in My Fitness Pal [Under Armour, Baltimore, MD, USA] app so I can see how much I’ve gained or lost.
Participant 1, lost weight
Some of the participants mentioned that the modules did not take long to read, that reading the modules could be started and dropped easily, and that the modules fitted around their baby’s schedule. Two participants commented on the website being motivating:
It was nice that they weren’t super lengthy. I think that was . . . the best part because if they were [NTM laughs] I wouldn’t have had time to do it.
Participant 1, lost weight
Because you’ve just given birth you don’t wanna do . . . erm, you’ve got no time for, so you do it at your own pace. Erm, no, I think it’s, it’s really good. An’ it, an’ it was easy.
Participant 7, lost weight
I’ve actually made a, a concerted effort to . . . even abolish all freezer mixes like, ya know, fish and chips and just fings that are really quick, erm, to in replace of home-cooked/slow-cooked meals because now I just . . . cook batch and um, it lasts for ya know 3 or 4 days and it’s healthy. So I think yeah, but that sort of incentive from, the PIMMS-WL, erm the POWeR ya know website, is, it’s good. So that, all together has ya know helped me, lose weight.
Participant 3, lost weight
Entering a new goal every time that they logged on was found to be difficult by two participants; however, two other participants found that setting goals and regularly reviewing these goals was useful:
[My e-mail reminder] was kind of like ‘Right, do you want to review any of your targets?’ and I was kind of like ‘Well, I’ve got targets there. Can I really be bothered to review them and change them?’. So there’s probably not, that much that I’m getting out of it now.
Participant 5, lost weight
I liked the, setting goals. It had me set goals and then . . . then it would ask, you know, what was helpful and what wasn’t or ‘Were you able to hit your goals or not?’ or ‘Do you want to modify them?’. That was . . . I found the goal-setting really helpful.
Participant 1, lost weight
Two participants suggested that the content of the POWeR website could be more focused on postnatal-specific diet and exercise issues, such as having post-pregnancy complications that delay or restrict certain exercise and needing to avoid drastic calorie reductions because of breast milk production. A couple of participants thought that videos for ‘exercise with baby’ would have been useful, and one thought that other people’s ‘inspirational stories’ would provide motivation:
Maybe personalising the website, more too . . . um, to, kind of the real dilemmas of . . . motherhood in the early years, ya know, in the early weeks and early months of . . . um, because like I wasn’t physically ready to do some of it.
Participant 1, lost weight
I’m not sure if they had any like stories about other people, I can’t remember. Like, things like that that you could just like read up inspirational things that you can like . . . Think, if you’re having a bad day or read stories and it’s a bit, it helps you a bit.
Participant 6, lost weight
Some participants liked that the POWeR website allowed them to personalise information for them. They were allowed a choice (primarily between a reduced-calorie and a reduced-carbohydrate diet) and to select the modules of interest to them:
Ermm, so yeah, you, you choose your plans. You choose your goals, which was good, and then. You review your goals at the end of the week.
Participant 3, lost weight
All participants would recommend the website to others, although a couple had provisos. One stated that it would be helpful only if the mothers did not already have basic knowledge of nutrition and diet, such as knowing which foods are carbohydrates and which foods have high calories:
I’d recommend it to a mum who had no idea at all about how to lose weight or how to eat healthily. I think if you’ve got a relatively good grasp, then I don’t think it would be useful.
Participant 5, lost weight
Participants made several suggestions for improving the POWeR website, which included better compatibility with mobile telephones, inclusion of videos of exercises that could be undertaken with a baby and at home, and inspirational success stories of new mothers who had lost a lot of weight, ideally through the intervention.
Strengths of the intervention
Two participants advocated that the intervention should be rolled out to other hospitals:
Personally, I think women, would love the opportunity to be given to them, personally, but obviously I can’t speak for every women. But personally I think it’s a great opportunity to be . . . introduced . . . not, not just for the women in hospital. Across Birmingham.
Participant 2, no weight loss
Accountability, or the notion that they would have ‘an obligation to explain and justify conduct’, was mentioned by most participants:
Like even though you don’t feel like you’re being . . . I never felt like I was kind of being checked up on or pressured, but you kind of think ‘Well, I’ve set myself these 12 or 16 weeks ter try and lose weight. I’m committed to doing it.’. Just by having an external, agency there who are aware and involved in it can potentially motivate you to do, to do more than if no one’s going to know, if you’ve not, tried or not.
Participant 5, lost weight
Because obviously now I know that there’s also, a third person who’s watching me. It’s not just . . . me . . . between the closed door or the researcher who’s seeing whatever is, recording. Yak now, yeah, it was motivating.
Participant 2, no weight loss
About half of the participants commented that simply being part of the trial was a useful, regular reminder that they had to focus on losing weight and that they had to be continually mindful of their behaviour:
Yes ‘cos obviously to lose weight these days . . . is difficult to do on your own than when you’ve got somebody there nagging you in the back of your head. You know you’ve got this programme that you’re doing. It’s like just going to Weight Watchers. You know every week you have to be going to be weighed. It’s similar to dis as well. Every week you have to be weighed, so . . . you’re keeping track of your weight.
Participant 2, no weight loss
Participants felt that a particular strength of the intervention was that it did not require women to make additional visits to the practice to be weighed. A few participants mentioned how little effort it required and that it was easy to integrate the intervention components into their existing behaviour. All participants found that the intervention, or some aspect of it, increased their motivation for or commitment to losing weight. Over half of the participants commented on the perceived effectiveness of the intervention for them, and two found this to be motivating:
That would be . . . quite inconvenient. Just to go there to be weighed. Because I’d have to take the baby, and. In the early days . . . ya know . . . It’s more difficult to get out and about, so . . . I was happy to do it, ‘cos I was there anyway.
Participant 9, lost weight
It hasn’t, it hasn’t been too kind of . . . too much work or effort or anything like that, like. [. . .] Yaknow, it was very much kind of fitting it in, alongside what I was kind of doing anyway.
Participant 5, lost weight
Yeah, erm, it’s made me more focused, made me more determined . . . to get back on it. To give me like the incentive. It’s given me like a head start.
Participant 6, lost weight
It’s very helllll, helpful for us because when I check, my weight is going down and it helps me to continues.
Participant 8, lost weight
Most women reported liking the ‘non-judgemental’ delivery of the intervention, with reference to both the nurses and the POWeR website:
And um, it didn’t make you feel bad . . . Like, yaknow, you didn’t feel bad for having a bad week. It was actually very encouraging, like ‘Yaknow, you’ve had a bad week, don’t give up. Try this module on how to deal with slip-ups’.
Participant 3, lost weight
Later impact of the study
Most participants stated that they were now more confident that they would be able to manage their weight in the future and that they accepted that they could lose weight:
The fact that I did erm, is a positive thing and it shows that I do know, what I need to do to lose weight. It’s just a case of maintaining it. Erm, soo . . . yeah.
Participant 5, lost weight
Thank you for the, thank you for this research. It’s very, very helpful for me and I changed my life.
Participant 8, lost weight
One participant reported a change in perspective after the study, an increased awareness of the lifestyle choices that affected their weight. Some mothers found that taking part had instilled effective habits for weight loss:
I’m more conscience, conscious about what I eat. Brown bread. Yeah, like just changing like little things, like um, the olive oil instead of the regular and, not eating out as much.
Participant 7, lost weight
I did a lot of the stuff that, the trial encouraged has now become just a routine, so hence, erm that’s an important part, is, erm, habit. So um, yeah, I’m fairly happy and confident that I should be able to, continue all the things that I’ve learned.
Participant 3, lost weight
General suggestions for improvement
One participant thought that self-weighing could be less frequent than weekly, which would allow a longer period between weighing and a larger, more noticeable difference in weight loss:
Erm, it was better, er, using the scales, as I said, erm, e- every few weeks in the immunisation. I found that more useful because every week you get obsessed with the, the tiny numbers, whereas if you weigh yourself every, almost every month you kind of . . . you appreciate I’ve actually lost a lot of weight, um, as opposed to ‘Oh, I’ve lost a pound’, ‘I’ve lost yaknow two pounds’.
Participant 3, lost weight
A few participants believed that it would have been helpful to have someone contact them in between immunisation appointments to check their progress and encourage them to keep ‘on track’ with their weight loss targets:
I think someone should have called me or e-mailed me to like push me a little bit and threaten me a bit. Not threaten me, but like just say erm, ‘We’ve noticed you haven’t weighed yourself this week’ something like that. Or ‘Is there a problem with your scales?’.
Participant 7, lost weight
About half of the participants thought that it would have been useful to have a telephone number or an e-mail address to contact if they needed to ask something and did not want to wait until the next appointment. However, their responses suggested that this was more a need for regular contact with a supportive person to keep them motivated and continuing to adhere to their new behaviours, and all of these participants wanted this in addition to the website, not to replace it. One participant would also have liked to have something tangible, such as leaflets about weight loss, and not just website access:
I mean it is helpful um, having the website, but. I’d probably like a, maybe a bit more . . . um, personal support or sumfin. . . . If you had someone that you could call or text if you were having like a bad day or something, I think like something like that’d help just to know that you’ve got . . . a bit of extra support, to be honest.
Participant 6, lost weight
Two participants suggested that the trial could have been mentioned earlier to pregnant women to make them aware of its existence before attempting to recruit them after pregnancy:
Yeah. I fink . . . dis programme . . . like instead of just us having it after birth, I think . . . Yaknow like towards the end of the pregnancy, I think if . . . obviously I don’t know if the midwives are going to agree to do it – but if your midwife can start talking to you about it [. . .] just give you information, like the leaflet for you to read, and then, next time when you go see them, then they can just ask the question ‘What do you think about the leaflet I gave you?’.
Participant 2, no weight loss
It was also suggested that nurses could be supported by a health visitor or someone in a similar role, who would visit new mothers at home to discuss weight loss.
Changing the methods of recruitment to the trial
Participants were asked for their views in relation to changing the method of recruitment to opt-out. It was put to participants that trial practices could make the intervention routine care for all new mothers (which could be declined) and all women at practices would enter the trial. Their expectations of how new mothers would feel about this method of recruitment varied. Most thought that women would have no problem with this approach, given that they could always refuse to participate:
I don’t think people have, a major problem with it . . . It might just be, if people aren’t . . . losing the weight, because I know a lot of people struggle. I think it’s a, a bit of postnatal care sort of thing, which’d be quite helpful for some people.
Participant 4, lost weight
Some participants suggested that if an ‘opt-out’ method to recruitment was used, health-care staff should be mindful when approaching mothers because weight is a sensitive topic and women should be approached in a caring manner:
I think . . . maybe people would be alright with it. But I know people can be very sensitive about their weight, so. I think if you explain to them and just say ‘Look, we’re offering you the opportunity ter, have weigh-ins at each of your immunisations so that over the next 12 to 16 weeks if you would like to try and lose weight you could do that,’ erm, then maybe people would go ‘Oh yeah, that would be a good opportunity’.
Participant 5, lost weight
But then you have to bear in mind people with anorexia and stuff like that. That’s the only thing. Yeah.
Participant 7, lost weight
Discussion
The study explored the views of participants who experienced the trial intervention. Most participants were keen to lose weight after having a baby and were motivated to join the trial for this reason. Using child immunisation appointments as a context for delivering weight management was viewed as acceptable and some viewed it as ‘ideal’. All but one participant would recommend the intervention to their friends. Regular self-weighing and recording of weight was viewed as an acceptable and sustainable strategy for weight loss. Women also liked the use of technology to facilitate weight loss. Many participants talked about the difficulty of maintaining and persevering with their dietary and physical activity behaviours to facilitate weight loss, such as depriving themselves of their favourite ‘indulgences’ and choosing the most convenient dietary option rather than the healthy option. Social support for weight loss was considered important in terms of reminders not to buy particular foods and providing child care to allow participants to exercise. Some of these key themes are discussed in more depth in the following sections.
Weight loss after pregnancy
Studies have reported that women are motivated to lose weight after having a baby, and this was also the case for participants in this trial. 76–79 Participants expressed a strong desire to lose weight and welcomed support from the NHS via this trial to help them achieve this goal. Although women expressed several barriers to losing weight, such as lack of gym facilities, lack of child care and tiredness, the time after giving birth appears to be a good opportunity to engage by facilitating changes to their dietary practices and physical activity behaviours. This process may have additional benefits to women’s health; for example, exercise is known to improve mental health and, specifically, postnatal depression, and weight loss has been associated with improved mood and body image. 226–228
Self-weighing as a strategy for weight loss
Women were generally accepting of the instruction to weigh themselves and record their weight regularly. Participants reported that self-weighing and recording of weight was easy to do and they were able to remember to do so each week. Several women commented that they had used an implementation strategy (action plan) by weighing themselves at the same time and/or day each week. This is important because having an action plan is the cornerstone of long-term behaviour change and it can be critical to preventing relapse. These findings are also encouraging because there has been some concern in the literature that asking people to weigh themselves regularly might cause unintended negative psychological consequences, including disordered eating. 195 Consistent with these findings, several other studies have not found an association between self-weighing and negative psychological health outcomes. 197–200
Using child immunisation appointments to offer a weight loss intervention
To the knowledge of the authors, no other published study has tested an intervention embedded in child immunisation appointments and, therefore, the intervention tested here was novel. The PPI representatives were involved in its development and they expressed some apprehension about how well the intervention would be received by mothers. Most of this concern was based on child immunisations being a stressful time for mothers and, therefore, a weight management intervention may not be well received at this time. However, participants in this study did not report a similar concern. Women reported that it was acceptable for practice nurses to deliver the intervention at child immunisation appointments and for nurses to serve as a source of external accountability for their weight management (see Weighing by practice nurses and accountability). No participants reported feeling that the intervention took time away from their baby or the immunisation. Participants commented that the intervention was convenient because it did not require any extra visits and that they welcome support from the NHS and, consequently, this type of intervention was ideal for them. Participants felt that nurses were supportive and having encouragement after a ‘slip up’ enabled the participants to continue with their behaviour, rather than viewing their efforts (and themselves) as a failure and reverting to a number of past unhealthy behaviours: to ‘fall off the wagon’. All participants indicated that they would recommend the study to their friends, highlighting additional acceptability of the intervention.
Weighing by practice nurses and accountability
The intervention was deliberately designed to be brief and simple for nurses to deliver, so that it could be readily embedded in routine consultations in primary care. The centre piece of the intervention was the principle of ‘accountability’: the notion that someone other than yourself is observing and cares whether or not you reach your weight loss goals. The primary role of the nurse was to provide external accountability, not to provide detailed advice or counselling. Women reported that the nurses were supportive, and knowing that they would be weighed by nurses helped to keep them focused on their weight loss goals (external accountability) and motivated them to persevere with changes to their diet and physical activity behaviours.
Weight and emotions
Although self-weighing may help people manage their weight, there may be concerns that it will have adverse psychological consequences or lead to the adoption of unhealthy weight control behaviours. Some researchers have suggested that feedback about body size may lead to psychological distress and that self-weighing may have a negative impact on body image and/or mood by continually reinforcing to people that their current body size is not ideal, or result in unhealthy dietary behaviours, such as binge eating and skipping meals. 195,196 As highlighted earlier, there is little evidence to support these concerns, but it is still important that trials continue to monitor whether or not self-weighing leads to negative psychological events. The emotions experienced by participants when weighing themselves varied depending on the weight that they expected to see displayed on the scales: typically they felt ‘good’ when they expected to see that their weight had decreased, whereas they felt worried, ‘fearful’ or ‘bad’ if they expected an increase because of a ‘bad week’. The results of this qualitative study are consistent with Hartman-Boyce et al. ’s202 synthesis of qualitative studies of self-weighing, in which viewing weight as a way to assess success or failure towards weight loss was found to lead to feelings of guilt, shame and disappointment, including avoidance of self-weighing in those who suspected that their weight was stable or had increased. Shifting perceptions of self-weighing as a means to self-knowledge and better understanding of weight and its fluctuations/changes, however, may remove the emotional impact associated with self-weighing. It is interesting to note that more participants reported stronger negative emotional responses to weighing themselves than being weighed by the nurses. Most participants were unconcerned about being weighed by the nurse, which may be related to participants feeling that their nurse was non-judgemental and supportive.
Using technology to assist with weight loss
Technology is increasingly being used to assist with health behaviour change in public health and NHS contexts. It was important for this study to capitalise on this and consider ways in which technology could be used in the intervention to assist both participants and practice nurses. None of the participants was concerned about being directed to a website for support and advice about weight loss rather than being given support directly from a nurse during the appointment. Moreover, some participants preferred the website because it could be accessed more frequently from home and at any time of the day, especially around their new baby’s unpredictable schedule. Most participants liked the opportunity to personalise and make choices on the website; however, a few mothers thought that the content could be more tailored to postnatal mothers, taking into consideration post-pregnancy complications and providing advice on and videos of exercises that could be undertaken at home with a baby.
Using an opt-out method of recruitment
In preparation for the possibility of a subsequent Phase III trial, participants were asked for their views about a change to the recruitment process used here and adopting an a opt-out recruitment method. Most participants did not foresee any problem in ‘rolling out’ the intervention using an ‘opt-out’ approach to recruitment if the process was adequately explained to women in a sensitive manner.
Suggestions for improving the intervention
Overall, women found the intervention feasible and acceptable, while also suggesting several ways in which the intervention could be refined to make engagement and implementation easier for them. Rather than having to login to an online weight management website using a computer, it was suggested that it would be more convenient and practical if women were able to access the programme using a mobile telephone app, in which they could track their weight. Some mothers believed that it would have been helpful to have an additional contact with them to check their progress and encourage their efforts; this would also have the added benefit of providing further external accountability, which participants felt was motivating for them. It was suggested that successful case studies of women who had lost weight be included and that the research team provide a contact number or an e-mail address so that participants could ask questions about issues related to their lifestyle behaviours. Another suggestion was to provide women with information packs or leaflets about lifestyle behaviours that women could read at their leisure. Some participants suggested that it would be better to raise the topic of recruitment to this trial with women antenatally, before attempting to recruit postnatally.
Strengths and limitations
The findings from this study should be considered in the light of its methodological strengths and limitations. This nested qualitative study was important because it offered and contributed context to how the intervention was experienced and received by participants; an integral strategy when determining the feasibility and acceptability of a complex intervention. 229 This study provided additional in-depth data that contributed towards the process evaluation of the intervention. The experiences of women who had both lost and gained weight at follow-up are represented in the findings. Women from a range of backgrounds were interviewed and at least one participant from each intervention practice was interviewed. A comprehensive approach to data analysis was undertaken involving four researchers (NTM, AD, HP and SG) ensuring consensus at each stage of the process. These findings can be used to make adjustments to the design, delivery and implementation of the intervention before it is tested in a Phase III RCT. A topic guide with set questions/topics and probes also ensured that each participant was interviewed in a consistent manner. The study also has some limitations. The number of participants interviewed was small and the views reported here may represent more motivated women. The interviews were held in participants’ homes with a researcher and, therefore, they may have expressed more favourable views of the intervention than if the interviews had been conducted in a neutral environment with an independent researcher.
Conclusions
Participants were keen to lose weight after giving birth. Overall, participants reported that the intervention was acceptable to them and they welcomed the support to lose weight that was provided by the trial. Child immunisation appointments were viewed as an acceptable environment in which to offer postnatal women a weight management intervention, and this was not viewed as distracting from the health of the baby. Participants would recommend the intervention to their friends. Weekly self-weighing and recording of weight was seen as an acceptable and sustainable strategy for weight loss in postnatal women. Women also liked the use of technology to help them lose weight. Participants made several suggestions on how the intervention could be developed and refined to further enhance the applicability and longer-term implementation of the intervention in health routine care for all women who wish to lose weight after having a baby.
Chapter 6 Nurses’ experiences of delivering the intervention
Background
Health-care professionals can have an important impact on the health behaviours of the public. By raising the topic of weight loss, health professionals can provide information and support to help women lose weight. However, there are a number of barriers to health-care professionals raising the topic of weight loss during consultations, such as time and workload pressures; fear of offending; concerns about damaging ongoing relationships by raising this sensitive topic; a belief that obesity is not a medical problem or an appropriate topic for them to raise; and a lack of confidence in patients’ ability to make behaviour changes. 153,230 An intervention embedded in routine health-care appointments could help to address many of the concerns that health professionals have and help to ‘normalise’ the topic of weight loss. This study aimed to assess the views of practice nurses who delivered the intervention described in Chapter 2. The objectives were to:
-
explore nurses’ views about women’s perceptions of the intervention in practice
-
investigate nurses’ feelings about raising the topic of weight with postnatal women at child immunisation appointments
-
capture nurses’ views about delivering the intervention and the impact that it had on the structure and duration of child immunisation appointments
-
gather suggestions from nurses about how to improve the delivery and content of the intervention, including the training provided for them.
Methods
Recruitment and data collection
Practice nurses who delivered the intervention were asked by telephone or e-mail to participate in a semistructured interview about their views on postnatal weight loss and their experiences of delivering the intervention during routine child immunisation appointments after all of the participants recruited from their practice had completed the study. Practice nurses provided written informed consent to participate in the study. Interviews took place either face to face or by telephone. For interviews that took place by telephone, nurses were verbally asked the questions on the nurse interview consent form and asked to state their agreement with each statement; their responses were audio-recorded. Semistructured interviews were chosen as the method of inquiry for all of the reasons outlined in Chapter 6. Nurses delivering the intervention were chosen for interview to provide accounts of the same phenomenon from different perspectives. 231 Nurses received a £10 high-street shopping voucher for any out-of-pocket expenses. This study has been reported in line with the COREQ guidelines for the reporting of qualitative studies.
Interview topic guide and interview procedures
The semistructured interview topic guide (see Appendix 2) explored nurses’ reflections on delivering the intervention in practice. The questions covered general warm-up questions relating to nurses’ careers and typical immunisation appointments, their thoughts on referring women to the POWeR website and their feedback on the training that they had received to deliver the intervention and on delivering the intervention in practice. Interviews lasted between 22 minutes and 66 minutes. Interviews were audio-recorded and professionally transcribed verbatim. (One interview was not audio-recorded at the request of the nurse and handwritten notes were made instead.) The transcripts were anonymised, with each nurse given a unique study identification number. Field notes were taken immediately after the interviews and integrated into the transcripts. Nurse transcripts were also anonymised by excluding the name of the general practice and the name of the nurse from the transcripts.
Data analysis
Data analysis followed the same processes detailed in Chapter 5. The coding book is available in Report Supplementary Material 3.
Results
Characteristics of the nurses/general practitioners and emergent themes
A total of six nurses and one GP who delivered the intervention agreed to participate in an interview from across all of the intervention practices (they are herein referred to as nurses, as the GP responses were not distinctly different from those of the nurses). None declined to participate in this interview study. Most of the nurses had been practice nurses for over 10 years and had been delivering immunisations for all of this time. Interviews were conducted one on one: three were completed face to face with the nurses at their practice and four were completed by telephone (three at their general practice and one at home). A total of 15 themes and five subthemes emerged from the nurses’ data. Nurses were randomly assigned numbers that do not correspond to the order in which the interviews were undertaken. Each quotation is followed by the assigned number of the nurse in question and by the page number and line number from their transcript, to aid transparency.
Impact of being overweight or obese on participants
When nurses were asked about the impact that being overweight or obese had on mothers, most responded in terms of the increased risk of developing long-term conditions (diabetes or cardiovascular disease). Nurses were of the view that being overweight had an impact on mother’s self-confidence, and two nurses referred to an increased risk of developing depression:
A lack of confidence from a physical point of view . . . I would say . . . um . . . they’ve more, more chances . . . of developing chronic diseases in later life.
Nurse 3, p2, 50
Losing weight after having a baby
All nurses thought that participants did not view being overweight as a problem or important soon after pregnancy. Most nurses stated that participants had raised the issue of weight management with them during an immunisation appointment. However, one nurse noted that there was an increasing general awareness that being overweight could negatively affect health:
So now their weight is a big issue, more normal really, so they are accepting and willing to cooperate. [. . .] Recently, yaknow, the knowledge is getting more and more. The people trying to lose weight, more people are coming forward, compared to before, yaknow.
Nurse 7, p2, 69
More than half of the nurses felt that mothers expected to gain weight with pregnancy, with two even describing resistance to weight loss from mothers that needed to be overcome. One nurse also highlighted that weight loss requires change and perseverance, which most people find difficult:
If you tell someone what to do it’s not going to work. They’re going to possibly do, the opposite. It’s got to be the right time for them ter, ter want to do it.
Nurse 5, p15, 589
Most nurses commented that new mothers were focused on their baby and deprioritised other concerns, such as their own health and well-being:
As, as a new mum. I think you put your erm, priority into your baby so.
Mmmm you put yourself second.
Nurse 5, p2, 77
Pregnancy myths and misconceptions
Nearly half of the nurses believed that participants held a number of pregnancy myths, such as ‘eating for two’, as well as unrealistic expectations about weight loss because of celebrities being able to return to their previous body shape and weight a short time after pregnancy:
Yaknow, when you see these celebs on the telly, and they’ve had a baby . . . and they come out 4 weeks later . . . looking as trim as anything Yeah. You think but what have you done to get that and should you have been doing that? A week after you’ve had a baby, you know? So I just think the pressure’s on everybody.
Nurse 4, p20, 762
Post pregnancy as a vulnerable time
Just under half of the nurses described the postnatal period as a vulnerable time (especially for first-time mothers) in which mothers should not be ‘burdened’ with any ‘pressure’, such as goals to lose weight. One nurse commented that the mothers most concerned about their weight would stop bringing their child for immunisations, which would reduce immunisation rates:
Just relax for a bit. You’ve been through a really traumatic experience. You might already have two toddlers running you . . . ragged. I just think . . . sort of give them a break really, yaknow?
Nurse 4, p20, 768
Raising the topic of weight loss
Over half of the nurses were concerned about having enough time in the immunisation appointment to raise a potentially upsetting topic and then provide adequate support. Two nurses were also concerned about damaging their relationship with the mother:
In an appointment which is already quite full with the baby, you can’t offer the mum the support that you want to offer her. You haven’t got the time to say ‘Well sit down, let’s have a chat’. You know, you don’t have the time for that [. . .] because I’d just feel awful if . . . somebody walked out and I was thinking ‘Oh God, I hope they’re alright’.
Nurse 4, p5, 153
I think if you’re asking mom if she’s fine, and she’s feeling OK, to then say ‘And how is your weight going?’. It’s a little bit . . . rude I think.
Nurse 4, p12, 425
Breastfeeding was mentioned by two nurses as a facilitator of weight loss, as expressed below:
I notice myself that people who are, tend to breastfeed their babies [. . .], they’re more likely to lose their weight, to go back to normal . . . if they’re on their just normal diet. Avoiding just junk food, than somebody who’s, yaknow, not on any breast . . . they don’t do breastfeeding.
Nurse 1, p18, 735
Potential barriers to weighing women at child immunisation appointments
All nurses reported that there was ‘not enough time’ in an appointment to add more tasks (regardless of how long their slots were); therefore, additional tasks could not be easily added. Most nurses commented about the purpose of the appointment being for child immunisations or the baby, and so weighing the mother was not as high a priority:
I mean, it would be great . . . if I had time ter, yaknow, give them the encouragement and spend more time on that, but the focus of the consultation was the child.
Nurse 5, p7, 264
Legitimising weighing at consultations
Although all nurses reported feeling comfortable and confident weighing people, and more than half stated that they were already weighing mothers in their surgery, nearly half mentioned that, because participants had consented to be in the trial, this provided justification for or legitimised them weighing participants at appointments:
Ummm . . . I wouldn’t feel any concern about that [weighing PIMMS-WL mothers] because I would know, know that they’d given their consent. So . . . no concerns at all.
Nurse 3, p6, 214
I didn’t mind. It’s never uncomfortable to ask them because they obviously know what they’ve signed up for.
Nurse 6, p10, 369
Two nurses said that they would not feel comfortable approaching overweight new mothers to weigh them on an ad hoc basis, and one of these nurses stated that they would not feel comfortable weighing new mothers if it became standard practice:
I dunno whether it puts a little bit of pressure on because how quick’s too quick and how slow is too slow, d’ya know what I mean? So yeah, I don’t think I’d feel comfortable just . . . No, I don’t think I would feel comfortable [weighing as standard practice].
Nurse 4, p11, 406
Most nurses commented that weighing women was not ‘on their template’; that it is not a job-role task that they are expected to complete at immunisation appointments:
Well, personally I’ve never done it in baby imms and whenever I’ve worked with other nurses it’s never been, it’s never been part of the consultation.
Nurse 4, p13, 469
Weight loss as the mother’s responsibility
Most of the nurses felt that, at least partly, weight loss was the responsibility of the mother:
Like . . . people know that they need to lose weight if they are overweight.
Nurse 5, p15, 584
I suppose you’ve got to have the motivation in wanting to do it [lose weight] as well, haven’t you, to be fair.
Nurse 6, p11, 413
Two nurses commented that weight gain was expected and essentially unavoidable in mothers during pregnancy:
Yaknow, I realise it is a time in a woman’s life when she does put, weight on.
Nurse 5, p12, 470
Trial training
Training for the trial was administered in person and took approximately 30 minutes to deliver. The training was adapted for the situation and ease of the nurses, so sometimes it was delivered by telephone and sometimes it was summarised for the nurse’s convenience. Each nurse’s experience and description of the training varied. Although one nurse thought that the training covered ‘a lot of content’ in a short amount of time (Nurse 2), two nurses felt that the training was very basic or insufficient (one of these nurses was trained by telephone):
So I wouldn’t say I received any sort of training; it was just somebody on the phone talking through what I needed to do when the mums came in and what to look out for, what would be in the red book to suggest that they were on the trial.
Nurse 4, p2, 53
All but one nurse felt prepared to deliver the intervention after their training. Three nurses felt nervous until they had delivered the intervention to at least one mother and the training was no longer theoretical:
I would say I was, not . . . particularly prepared because . . . errrr, I tend to feel prepared when I’ve done something. After I’d seen the patient. I felt . . . better prepared to do it again, but I only saw one. So yeah. Not particularly prepared.
Nurse 3, p3, 110
Nearly half of the nurses rarely referred back to the manual: two because they did not feel the need to and the other because they did not have the time. Most nurses who referred back to the manual did so before seeing a participant or highlighted key parts in the manual for quick reference:
Obviously, ‘cos’ if you’re doin’ it all the time you don’t . . . just comes second nature, but when you’re just doing it every ad-hoc, it’s just a good refresher just to remind you of the things you need to be asking.
Nurse 6, p3, 101
Delivering the intervention
Most of the nurses were able to identify intervention participants at immunisation appointments from the trial sticker on the front of the red book and/or by the insertion of the weight record card next to the immunisation record page in the red book. However, this method was reliant on participants remembering to show the booklet in enough time to be weighed during the appointment and for the nurse to recognise the trial sticker/record card. Two nurses expressed dissatisfaction at this and would have preferred to have been alerted much earlier, while two other nurses did not find these stickers useful or did not remember seeing them. Nurses relied on additional means to identify intervention participants, with some receiving e-mail reminders from the research team or being informed by their manager:
Ermmm, when you do a child vaccination, often a parent will not. Won’t present: Mmmm. The stickers on the red book To you. It really depends on, on the mum. But . . . very often you’re focusing on . . . the baby and the vaccination.
Nurse 3, p4, 147
About half of the nurses reported immunising the child before weighing participants because they wanted the immunisation to be completed quickly as it can be stressful for some mothers. Just under half of the nurses reported weighing before immunising, giving reasons such as delaying the baby crying until the end or not identifying a trial participant until seeing their sticker later in the appointment. Almost all of the nurses reported that it was easy to access the weight record card and it was straightforward to record the participants’ weight in the red book.
Most nurses described a non-judgemental and/or supportive attitude and ensured that they did not criticise participants or tell them to try harder when they had not lost weight. By stating that weight loss was a long-term goal or they believed that the participant would lose the weight by the next appointment, most nurses felt that they encouraged participants in a constructive manner:
So I’d just be giving them kinda general encouragement that . . . it is OK and weight loss is a, a long-term . . . a long-term process. Yaknow, you’re not gonna see massive changes straightaway. And so it’s just kinda gentle . . . encouragement.
Nurse 5, p7, 277
No nurses reported any irritation or upset from participants being directed to the POWeR website. One nurse found it convenient to refer participants to a website for weight loss advice, whereas another pointed out that they would have previously referred mothers to the NHS Choices website (a website covering a variety of health topics including weight loss, although not specifically designed for postnatal mothers). Two nurses also had concerns about the information technology literacy of some of the mothers, especially in minority ethnic communities, while another two nurses suggested diversifying the approach by providing physical information, such as leaflets, or more person-to-person advice, in addition to the website:
I think it’s a good idea. But a-again . . . it really depends, on the person and whether they respond well to . . . written information . . . or whether they, they want a more personal approach . . . Some people just like . . . to have a chat with you . . . about their weight and the kind of foods that they should be eating . . .
Nurse 3, p10, 392
About half of the nurses highlighted the problems of participants accessing reliable and consistent advice on weight loss after pregnancy from the internet and were glad that participants had the POWeR website as an up-to-date source of correct information:
So I’d much rather that than them . . . just . . . Google [Google Inc., Mountain View, CA, USA] and find bits of what everyone’s. So no, that, that was fine. It’s just a shame we hadn’t the opportunity to look at it to see what it was they were looking at, but we knew it was the right stuff.
Nurse 4, p16, 606
Almost all of the nurses reported that participants did not bring up the website in their conversations, although when the nurses asked participants whether or not they had been regularly visiting the website (as directed by the training), the comments surrounding the website were mixed. Many nurses reported that participants did not comment beyond saying that they used the website and how often, that they were having trouble accessing it or that they found it useful or easy to use:
Yeah, they said they’d both been on it and they found it easy to use, so. That was good we hadn’t the opportunity to look at it to see what it was they were looking at, but we knew it was the right stuff.
Nurse 5, p10, 405
Participants’ feelings about being weighed at immunisation appointments
When asked about how participants appeared to feel while being weighed, most nurses reported that they seemed comfortable, with one nurse reporting that their mother ‘jumped on the scales’ eagerly and appeared to enjoy finding out how much weight they had lost (Nurse 2). Two of the nurses reported that some of their participants were uncomfortable with being weighed, appearing embarrassed. Nearly half of the nurses reported that participants gave reasons before they were weighed by them about why they had or had not lost weight. Only one nurse reported that any (two) of their participants declined to be weighed; both of these participants had not lost weight. Only one nurse reported mothers hesitating initially about getting on the weighing scales, but they were encouraged to do so with little difficulty:
And how comfortable do you think, the mothers were with you weighing them?
. . . It was OK. It was OK. They were quite errrr . . . alright.
Nurse 1, p10, 392
Role of health professionals in providing support for weight loss
When asked who should be providing new mothers with advice and information on how to lose weight, nurses felt that any health professional in contact with mothers would be appropriate. One nurse described their role as ideal, given that midwives or health-care visitors were focused on the baby and not the mother. One nurse referred to weight loss companies, such as Weight Watchers and Slimming World, that had more time and the ability to support new mothers in their weight loss efforts:
I think it’s, probably everybody. While they’re seeing the mother. Yeah. Who is the patient is feeling more comfortable with.
Nurse 1, p19, 773
However, just over half of the nurses stated that the ‘ideal time’ for raising the topic of weight loss with mothers was 6–8 weeks after pregnancy or the postnatal check; half of the nurses also thought that the topic should be raised during pregnancy. It was suggested that providing leaflets or information packs or couching the intervention in terms of ‘healthier eating’ or ‘lifestyle choices’ might be appropriate:
I just think . . . as a health-care provider I’d be more erm, open to having that discussion [about weight loss] at the postnatal check with the mum.
Nurse 5, p13, 516
Role of accountability in weight loss
Accountability is a sense of obligation, of not ‘letting someone down’, and of having to explain and justify conduct to another. This concept was mentioned by only two of the nurses and was linked in both cases to motivation to adhere to behaviours to lose weight:
I think it’s more of an incentive. Especially knowing that they [the recruits] was being weighed.
Nurse 6, p9, 332
‘Cos I suppose if they [the recruits] go anywhere and hand their red book over, their weight’s in there for everybody to see, isn’t it?
Nurse 4, p15, 553
Strengths of the intervention
All of the nurses commented on the intervention being a ‘good idea’ and/or likely to increase participants’ motivation to lose weight. Three nurses reported that the intervention was not onerous or difficult to deliver. Three nurses were initially concerned that they would be inundated with questions about weight loss but that this had not been their experience.
Roll-out of the intervention to all postnatal women
Nurses were asked to imagine a scenario in which the intervention was rolled out to all postnatal women who attended their immunisation appointments and to comment on how they would feel if this were to happen. Two nurses thought that this would be unproblematic. Another nurse thought that this would take more time but would be ‘manageable’. Three nurses commented on the potential logistical problem of having to electronically record the weight and logging in and out of the baby’s and the mother’s file to update both the mother and the infant records, given that immunisations are recorded in the child’s record, not the mothers’ record:
It wasn’t really taking much of my time and, errrrrr yaknow, it’s something they’re here anyway. No, I didn’t, it couldn’t . . . it really doesn’t make any difference.
Nurse 1, p15, 594
Ermmm, it would be difficult because you’ve got the baby on the, on your computer screen, so to go into the mother to record the weight in the computer . . . that, that would just take . . . too much . . . time. It’s just . . . not very logistically easy.
Nurse 5, p10, 387
Two nurses thought that it would be difficult to roll the intervention out because of the additional time that weighing and recording women’s weight would take.
We might be lucky to have 10, 15 minutes and otherwise . . . And that’s just enough to do the baby. So if it was um . . . if there was something else it would be much harder.
Nurse 6, p7, 276
Suggestions for improving the trial
One nurse suggested that a digital copy of the nurses training manual would simplify searching for information. It was also suggested that the training could be split over two sessions or that ‘refresher’ sessions could be delivered at various times during the intervention. One nurse suggested that some consideration should be given to the idea of deferring the start of intervention until 12 weeks after childbirth or giving promotional literature about the trial antenatally.
Nurses’ standard appointment slots at their surgeries varied from 10 to 20 minutes. Two nurses thought that a longer appointment time was needed to deliver the intervention. Just over half of the nurses perceived that the intervention added 5–10 minutes to their usual appointment length; however, one nurse thought that it took 2–5 minutes, another thought that it added only 1–2 minutes, and another thought that it did not take any extra time and that only reorganisation of the appointment was required:
I think the appointment should have been . . . made to be 20 minutes, but then that would mean that you’d see less, less babies, less mums. Ermm, that’s the only thing, it did take a bit extra time.
Nurse 5, p14, 551
One nurse suggested that participants have their own booklet to record their weight, rather than recording their weight in the red book, and another suggested that an appointment just for the mother, not one targeted at the baby, might be helpful:
In an appointment which is already quite full with the baby, you can’t offer the mum the support that you want to offer her. You haven’t got the time to say ‘Well sit down, let’s have a chat’. You know, you don’t have the time for that. So I think maybe it would be more appropriate if it was offered to mums in an appointment on their own.
Nurse 4, p5, 153
Two nurses thought that a more ‘holistic’ approach could be taken to the sessions. The focus would not be solely on the mother’s weight and weight loss; instead, blood pressure and additional health checks could be made with the aim of assessing the general health of mothers:
Yaknow, I just think . . . for some mums it’s a bit of pressure, but I think it’d be lovely if there was sort of like an 8-week check for mum . . . say a 16-week check with a nurse, to just say ‘How ya doing?’ where you would generally say to mum ‘Well let’s check your blood pressure, let’s check your weight’, because it’s more of a health check, to see how they are.
Nurse 4, p19, 724
Discussion
Summary
Practice nurses expressed a range of views about postnatal weight loss and delivering the trial intervention during routine child immunisation appointments. Nurses commented that, in their opinion, mothers did not view being overweight as a concern soon after pregnancy. Nurses felt that new mothers were focused on their baby and not on their own health and well-being, and held unrealistic expectations about weight loss after childbirth. Some nurses felt that the postnatal period was a vulnerable time, particularly for first-time mothers, in which mothers should not be burdened with any pressure to lose weight. Some nurses were concerned about raising the topic of weight; it was considered a sensitive topic, and they felt that they did not have sufficient time to address concerns women might have and that it would affect their relationship with mothers. However, nurses also commented that the trial provided a basis on which they could raise the topic of weight and provided a platform to have these conversations.
Overall, nurses felt that the intervention was easy to deliver, that the intervention was a good idea, that women engaged well with the components, that the intervention was likely to increase motivation for weight loss and that the intervention appeared to be effective in increasing postnatal weight loss. Some nurses, however, felt that, if the intervention were to be rolled out, extra time would be required. Nurses believed that mothers appeared comfortable with being weighed by them.
Although some nurses had initial reservations about the intervention associated with concerns that women would want to have lengthy conversations or ask many questions about weight, in practice this was not the case. Moreover, nurses reported that participants were accepting of being directed to the POWeR website for support and advice, although there was some concern about the computer literacy skills of ethnic minority women whose first language was not English. Nurses made a number of practical suggestions for improving the conduct and later implementation of the trial. Some of the key issues raised by nurses and relevant to this trial intervention will be explored in more depth in the following sections.
Losing weight after having a baby
Studies have highlighted that women find it difficult to lose weight after having a baby. 42–44 Similarly, nurses highlighted that motherhood was a particularly vulnerable time and discussed the pressure or burden of trying to lose weight soon after birth as undesirable. This was linked to the possibility of increasing the risk of postnatal depression by two nurses. All nurses felt that new mothers did not view being overweight or obese as a health concern and most nurses thought that new mothers prioritised their baby’s health over their own. There was a feeling among some nurses that mothers held unhelpful ‘myths’ and misconceptions about weight loss and pregnancy/childbirth that hindered their weight loss efforts.
Raising the topic of weight
Research has reported that there are many barriers to health-care professionals raising the topic of weight loss, such as difficulty in raising a sensitive topic. 153,230 Nurses commented that they felt more comfortable raising the topic of weight and weighing participants because the participants had chosen to be involved in the trial and were expecting to be weighed at their appointment. One nurse stated that she would not feel comfortable if weighing all mothers became standard care. It was interesting to note that a lack of ability to lose weight was not specifically mentioned as a reason not to raise the topic with participants, but nurses did mention resistance to change/lack of engagement.
Training to deliver the intervention
Nurses mostly felt that they were well prepared to deliver the intervention, but that this was not fully the case until they had put their training into practice with their first participant. Nurses made some suggestions about how the training could be improved (see Intervention roll-out and suggestions for developing the intervention) to ensure that they felt confident in raising the topic of weight and successfully delivering the intervention. It is of note here that delivery of the intervention per protocol by nurses was very high (see Chapter 3).
Delivering the intervention in practice
In the UK, weighing pregnant women and new mothers has swung in and out of NHS policy several times over the past century, meaning that health professionals are likely to have become uncertain about the merit and role of regular weighing in health care. In the past few years, there has been increased evidence for the role of regular weighing as part of multicomponent weight management interventions; however, very recent evidence in pregnant women showed that weighing alone by midwives during pregnancy was not effective in preventing excessive gestational weight gain. 232 In this study, all of the nurses commented that the intervention was a ‘good idea’ and/or that the intervention would help to increase women’s motivation to lose weight. When asked to recall how they delivered the intervention, most of the nurses listed most of the five steps from the training manual checklist. Interestingly, two nurses seemed to be ‘reframing’ weight loss to participants by changing their perceptions from weight loss being a short-term goal to weight loss being a more long-term ambition. This is a strategy found to be effective when used by those aiming to lose weight without professional support or a formal programme underpinning their efforts. 233
Most nurses reported that mothers were comfortable with being weighed, with only a couple of nurses reporting that some of their participants felt embarrassed. Evidence has shown that people who are overweight often feel self-conscious about their bodies, ‘ashamed and stigmatised’. 7,8,154 It is possible that such feelings might be mitigated, however, if regular weighing was integrated into routine care, as this would serve to normalise this process; overweight mothers might not feel that they are being ‘singled out’ or treated differently. This suggestion fits with another suggestion from the nurses that the intervention could be less focused on the weight and couched within a broader ‘health check’ after having a baby framework.
Most nurses were comfortable and confident in delivering the intervention, and some commented that they were already weighing some patients at their practice in relation to a practice-wide policy or on their own initiative. It is interesting to note, however, that these nurses were still uncomfortable with weighing mothers, referring to the additional ‘pressure’ or ‘burden’ on mothers at a particularly vulnerable time.
Intervention roll-out and suggestions for developing the intervention
Most nurses did not foresee any major problems related to the future roll-out of the intervention and thought that it was ‘manageable’. No nurses reported any practical issues in delivering the intervention. Nurses suggested diversifying the approach by providing physical information, such as leaflets and more person-to-person advice, in addition to the website. However, the issue of having to switch between the mothers’ and the baby’s electronic records should be considered and raised in the training.
Practice nurses offered several strategies for developing the intervention, both in terms of improving women’s experience of the intervention and in relation to their delivery of the intervention to women. It was suggested that nurses might benefit from refresher training sessions when there is a longer than expected gap between their training and their first participant. A digital copy of the training manual would also aid training before seeing a new participant.
Strengths and limitations
This study has a number of strengths and limitations that should be considered when interpreting the findings. The results provide additional information from which the results of the RCT can be interpreted. Because we tested a novel intervention for the first time, we have provided in-depth experiential data on a weight management intervention embedded in child immunisation visits in primary care. All of the nurses, from a range backgrounds and experiences as practice nurses, contributed to this study. The data presented here are based on nurses’ personal experiences of delivering the intervention in practice, within their working lives, rather than hypothetical scenarios. The nurses were recruited from practices located in areas of very high deprivation with a high proportion of ethnic minority patients, which can lead to a challenging environment in which to deliver health care. Despite these challenges, nurses felt that they were able to deliver the intervention as per protocol. The study also has some limitations that should be considered. Only six nurses and one GP were interviewed; therefore, data represent the views of a small number of nurses who delivered the intervention. One of the interviews was not audio-recorded at the request of the nurse and notes were taken instead; it is possible that some comments were not recorded accurately.
Conclusion
Overall, nurses felt that they were able to raise the topic of weight because the trial provided a framework in which they could legitimately have conversations about this topic. However, some caveats to successful implementation were raised by nurses: they felt that the intervention was easy to deliver and that it would motivate women to lose weight. If the intervention were to be rolled out and integrated into routine care, nurses felt that extra time at child immunisation appointments would be required to deliver the intervention. Participants appeared accepting of being directed by nurses to the POWeR website for support and advice about weight loss, but nurses felt that more consideration should be given to ensuring computer literacy among ethnic minority women to allow them to access and benefit from the online weight management support.
Chapter 7 Comparison of participants’ and nurses’ experiences
This brief chapter aims to draw together the findings from the interviews with participants and nurses presented in Chapters 5 and 6, to highlight the similarities and inconsistencies in their experiences and/or views about the intervention to assist with future refinements to the intervention.
Raising the topic of weight loss
The most commonly reported barriers to nurses raising the topic of weight loss were workload pressures; lack of time at immunisation appointments; fear of raising a potentially sensitive topic; viewing weight loss as the mother’s responsibility; and perceiving weight management as being outside their typical tasks as a practice nurse. Several nurses commented that motherhood was a particularly vulnerable time and they wanted to avoid putting pressure on women to lose weight. However, although most participants discussed the difficult times of motherhood, unprompted and unrelated to weight loss, no participants reported weight loss being a sensitive or difficult topic for them to discuss with the nurse or any other health professional. By contrast, some mothers commented that they wanted the topic of weight loss to be raised or the weight loss intervention to have been discussed with them sooner.
Barriers to and facilitators of weight loss
The perceived facilitators of, and barriers to, new mothers’ weight loss efforts offered by nurses were different from those offered by the participants. All of the nurses reported that, generally, new mothers did not view being overweight as a problem or viewed themselves as not being overweight. In addition, most nurses perceived a focus on the new baby to the exclusion of all else, including the mother’s own health needs. However, participants had a different view of this impact of the baby on their lifestyle, and commented that they had to think about negotiating their diet and exercise behaviours around the baby’s schedule, lack of sleep and breastfeeding, not that they did not have time to focus on these health behaviours.
Process involved in delivering the intervention
Nurses were asked to weigh participants and record this on the record card in the red book; to remind women to weigh themselves weekly and to record this in the red book; and to encourage use of the POWeR website and/or ‘signpost’ participants to do so. All of the nurses reported weighing the mothers and almost all reported that they checked whether or not participants had been self-weighing weekly by asking or checking the record card. All of the nurses reported that they signposted participants to the POWeR programme and most of the nurses had checked that the participants had visited the website. All of the participants reported being weighed by the nurse, but most did not mention being asked if they had been self-weighing weekly, and only about half of participants mentioned that they had been signposted to the POWeR website.
The nurses did not appear to view being supportive or encouraging as noteworthy, yet when prompted most nurses reported encouraging participants with weight loss. By contrast, most participants mentioned and appreciated the supportive and non-judgemental approach of the nurse to their weight loss, particularly when participants had gained weight.
Accountability
Most participants mentioned accountability, that someone other than themselves was monitoring whether or not they were weighing themselves regularly and that they wanted to avoid ‘letting down’ the nurse; this provided them with a source of motivation to continue to adhere to a healthy lifestyle and weight loss. It is interesting to note that only two nurses discussed or raised the concept of accountability in their interview conversations.
The POWeR website
Most of the nurses reported that participants did not particularly bring up conversations about the POWeR website at appointments, but most mothers commented positively about the website, finding it useful and easy to use.
General thoughts about the study
All of the nurses commented that the intervention was a ‘good idea’ and participants similarly viewed the intervention favourably. One of the nurses felt that participants should have their own appointment rather than the intervention being offered at the child immunisation appointment, yet many participants reported that one of the things that they liked about the intervention was that no extra appointments were required and that attending extra appointments would be difficult, with one participant explicitly commenting that they would not attend extra appointments. In addition, no participants considered their baby’s immunisation appointment to be an unsuitable time for them to receive a weight loss intervention.
Rolling out PIMMS-WL
Neither participants nor nurses believed that rolling out the intervention would be difficult or problematic to achieve. Women did not believe that there would be any objections from women if an ‘opt-out’ recruitment procedure was implemented, so long as it was made clear they still had the right to decline the study/intervention and nurses were tactful and sensitive when raising the topic of weight.
Chapter 8 Overall discussion
This final chapter aims to provide a broad synthesis of the study findings and includes recommendations for future research. This report has presented data from a RCT and two nested qualitative studies with participants and practice nurses. The trial tested the feasibility of a brief routine weight management intervention embedded in the national child immunisation programme and reported that adherence to the intervention was broadly acceptable, but that the primary method of recruitment was not successful.
The qualitative study with participants raised several considerations for research. Most of the participants commented that they were keen to lose weight after giving birth and were motivated to join the trial for this reason. Women liked the idea of using child immunisation appointments to receive a weight management intervention. Participants commented that they would recommend the intervention to their friends. Self-weighing and recording of weight were seen as an acceptable strategy for weight loss and participants liked the use of technology to help them lose weight. Many participants talked about the difficulty of maintaining and persevering with their dietary and physical activity behaviours to facilitate weight loss. Participants liked the idea that someone was monitoring their weight and there was someone who they were ‘accountable’ to for their weight loss progress.
Despite concerns about raising the topic of weight, most nurses felt that the intervention facilitated the conversation and could be embedded in routine child immunisation appointments. Nurses suggested that the length of child immunisation appointments could be extended to accommodate the intervention. Although some nurses in the qualitative study commented that the intervention added 10 minutes to the length of the immunisation appointments, audio-recordings of consultations indicated that the intervention took nurses/GPs approximately 2 minutes to implement. As nurses were being asked to deliver a new task, doing so may have been perceived to have taken longer.
Strengths and limitations of the programme of research
The specific strengths and limitations of each study have been previously discussed in Chapters 4–6; this chapter will take a broader perspective of the strengths and limitations of this research. The use of mixed methods was a particular strength because it provided the opportunity to collect different types of data and perspectives on the research questions. Completing the trial prior to conducting the semistructured interview studies provided information that could be fed into the interview topic guides in the qualitative studies, thus providing the opportunity to gain a deeper understanding about the feasibility and acceptability of the intervention. The inclusion of interviews with both participants and practice nurses provided simultaneous dual perspectives on the same processes of experiencing and implementing the intervention. The findings of this research can now be used to develop this intervention in a Phase III trial or to develop other research that aims to address brief interventions for postnatal women in primary care. The inclusion of the BodyTrace weighing scales as an objective process measure of frequency of self-weighing was a key methodological strength of the study, particularly in relation to providing evidence of intervention adherence.
To the best of our knowledge, the study reported here is the first to investigate the merits of the integration of a weight management intervention for postnatal women in existing primary care contacts with minimal costs to the NHS. This study, therefore, makes a unique contribution to the literature.
The study also had some wider limitations that should be considered when planning future studies. Although the study took place in Birmingham, which has a very diverse population, all of the study materials were in written in English and this may have prevented some women from participating. Although the completion rates for the questionnaires were high, the questionnaire booklet was relatively long, taking about 20 minutes to complete; this may have negatively affected the women’s responses. Participants were not asked about their diet or diet quality and such data would be useful alongside data collected on weight, although it is recognised that dietary recall can be inaccurate. 234,235 Physical activity was collected by self-report and future studies should consider collecting these data using objective measurement devices, such as accelerometers. The scales and questionnaires used in this study to assess body image and body dissatisfaction scores were designed for use with the general population and not specifically with postnatal women. Future research should also consider including additional anthropometric measurements to assess body composition (e.g. waist circumference) that may be better placed to detect changes in this outcome. Some consideration should be given to the research that has suggested that people who participate in weight loss studies may not be representative of the general population. 236 Thus, it is possible that the study attracted more health-aware or health-conscious women and future studies should consider including a measure to assess this outcome.
Implications of the research and future research recommendations
NICE has called for more high-quality research in this area and this study directly addresses this gap. This study has contributed to research into obesity prevention and management by extending the evidence available on interventions to reduce postnatal weight retention and weight gain during the postnatal period. However, more high-quality research is necessary to ensure that the consistent development of policies and guidance for maternal weight gain and postnatal weight loss can be implemented. Attempts could be made to improve the design and conduct of RCTs in this topic to increase the quality of the evidence. There are several specific ways in which research on this question should be developed and improved, and these are outlined in the following sections.
The design of this trial resulted in a short time frame in which women could be recruited; this may have deterred some women from participating at a busy time in their lives. Therefore, rather than recruiting women postnatally, an alternative approach could be to recruit women antenatally, towards the end of their pregnancy, when women do not have the same distractions and demands on their time. Recruiting antenatally may also be beneficial because it provides time for women to begin to consider making changes to their health behaviours before their baby is born; the intervention can then commence postnatally. Furthermore, given the short window of opportunity available to recruit women, it may be that an ‘opt-out’ approach to recruitment would be a more efficient method and would also allow the trial to be better embedded in routine health-care practice. This may also allow for more efficient implementation in the NHS should the intervention be shown to be effective. Such an approach to recruitment is also supported by evidence that has suggested that higher response and recruitment rates may be obtained when studies use opt-out methods. 187 Data from the qualitative study with participants also showed some evidence that an opt-out approach to recruitment may be acceptable, but it may still be important to seek the views of more postnatal women on this question before a subsequent Phase III trial. It may be the case that recruitment via other health professionals involved in the care of postnatal women and young babies might be useful in aiding recruitment, such as community midwives and health visitors. These routes of recruitment could be considered in future research studies.
This trial took place in Birmingham, a very ethnically diverse city. The trial recruitment documents and intervention materials were not translated into different languages; this may have increased both the number of women recruited and the intervention adherence. Any future study should consider making all of the study documents available in several languages, particularly when they take place in large multi-ethnic cities.
This study did not gather information on the reasons why women did not take up the offer to participate. Future research could benefit from conducting in-depth qualitative research on the reasons why women decide not to participate in studies of this type.
As a feasibility trial, the intervention was assessed over the first three child immunisation appointments when the child was 1, 3 and 4 months old. The intervention was not delivered at the 12-month child immunisation appointment; therefore, the longer-term effects of the intervention were not assessed. Trials with long(er)-term follow-up would contribute to the evidence and the quality of that evidence.
Weighing patients during routine appointments may help alleviate the fear and concerns that many health-care professionals and patients can experience when discussing weight. Interventions, such as the one tested here, showcase the need for more research testing interventions involving weighing of patients during routine health-care appointments. Research would also be worthwhile that considers different ways in which health-care professionals can support women to lose weight after having a baby, for example via community midwives and health visitors.
The role of technology in postnatal weight loss might also be a useful avenue for future research endeavours because this allows intervention support to be delivered at scale. The inclusion of commercial weight loss programmes in broader weight loss interventions might also be worthwhile, as recent research has demonstrated that such approaches may be feasible and acceptable to postnatal women. 194 Specifically, it could be that referral or signposting by practice nurses to commercial weight loss programmes, in addition to weighing women at child immunisation appointments, could improve the effectiveness of the intervention; this would be an important question for future research to pursue.
Although evidence shows that women engaged well with self-weighing and recording of their weight, the CIs for frequency of self-weighing were wide; strategies to enhance this outcome could be considered to further increase engagement with this behaviour. Technology, such as text message reminders, may be useful in this regard and future research should consider including such prompts. It might also be the case that branded online weight loss programmes developed by commercial companies may be useful and could be used to support the face-to-face intervention consultations that the practice nurses delivered; future research that examines the role of commercial weight management programmes could be worthwhile. 194
Women self-reported their physical activity and the data may be prone to bias and over-reporting. Future studies should consider including an objective assessment of physical activity. A more detailed analysis of body composition would have been useful because some studies have reported fluctuations in body fat percentage during the year following childbirth.
Conclusion
The findings of this study have demonstrated that it is possible for nurses to deliver a brief weight loss intervention to postnatal women, focused on promoting self-management of weight, during child immunisation appointments in primary care. Although women and nurses responded well to the intervention and adherence to weekly self-weighing was high, there is some scope to improve participants’ engagement with the intervention. The recruitment of participants was challenging and the study sample was small, highlighting that the recruitment methods used were not successful. Alternative approaches to recruitment need to be explored prior to a Phase III trial.
Acknowledgements
The authors would like to thank the PPI representatives who contributed to the TSC and the lay representatives who contributed to the study design. We wish to acknowledge the following people from the BCTU who assisted with the conduct of the trial: Hannah Bensoussane, Danielle Brushfield-Smith, Catherine Hewitt, Nicholas Hilken, Natalie Ives, Natalie Marchevsky, Gurmail Rai, Sophie Reckless, Sarah Tearne and Alexandra Vince. The authors would also like to thank BWH and the general practices that supported the study. The authors would like to thank the women who participated in this research. Kate Jolly is part funded and Ruth V Pritchett was fully funded by the National Institute for Health Research (NIHR) Applied Research Collaboration West Midlands. Helen M Parretti was funded by a NIHR Academic Clinical Lectureship.
Contributions of authors
Amanda J Daley (https://orcid.org/0000-0002-4866-8726) (Chief Investigator, Professor of Behavioural Medicine and NIHR Research Professor in Public Heath) developed the original idea for the study and funding application, along with Helen M Parretti and the co-investigators. She developed the research protocol with the other co-investigators, including the training manual for practice nurses. She oversaw the delivery of the trial and led the preparation of the final report (drafting, reviewing and editing).
Kate Jolly (https://orcid.org/0000-0002-6224-2115) (Co-investigator, Professor of Public Health and Primary Care) contributed to development of the funding application and research protocol in collaboration with co-investigators. She contributed to the preparation of the final report (drafting, reviewing and editing).
Natalie Ives (https://orcid.org/0000-0002-1664-7541) (Reader in Clinical Trials, Statistical Team Leader) contributed to the development of the trial protocol in collaboration with co-investigators. She provided oversight regarding the statistical analysis plan and the conduct of the statistical analyses and contributed to the preparation of the final report (drafting, reviewing and editing).
Susan A Jebb (https://orcid.org/0000-0001-9190-2920) (Co-investigator, Professor of Diet and Population Health) contributed to development of the funding application and research protocol in collaboration with co-investigators. She contributed to the preparation of the final report (drafting, reviewing and editing).
Sarah Tearne (https://orcid.org/0000-0001-7944-2917) (Trials Manager Team Leader) contributed to development of the trial protocol in collaboration with co-investigators. She was responsible for the management of the trial and contributed to the preparation of the final report (drafting, reviewing and editing).
Sheila M Greenfield (https://orcid.org/0000-0002-8796-4114) (Co-investigator, Professor of Medical Sociology) contributed to development of the funding application and research protocol in collaboration with co-investigators. She oversaw the development of the protocol for the qualitative studies and the preparation of the final report (drafting, reviewing and editing).
Lucy Yardley (https://orcid.org/0000-0002-3853-883X) (Co-investigator, Professor of Health Psychology) contributed to development of the funding application and trial protocol in collaboration with co-investigators. She contributed expertise regarding the digital technology aspects of the intervention and to the preparation of the final report (drafting, reviewing and editing).
Paul Little (https://orcid.org/0000-0003-3664-1873) (Co-investigator, Professor of Primary Care Research) contributed to development of the funding application and trial protocol in collaboration with co-investigators. He contributed to the preparation of the final report (drafting, reviewing and editing).
Natalie Tyldesley-Marshall (https://orcid.org/0000-0002-0576-3459) (Research Associate) contributed to development of the qualitative study protocols in collaboration with co-investigators. She conducted the interviews and analyses for both qualitative studies and contributed to the preparation of the final report (drafting, reviewing and editing).
Hannah Bensoussane (https://orcid.org/0000-0002-6266-8204) (Statistician) prepared the data for analysis and conducted the statistical analyses. She contributed to the preparation of the final report (drafting, reviewing and editing).
Ruth V Pritchett (https://orcid.org/0000-0002-9958-1233) (Co-investigator, Research Fellow) contributed to development of the funding application and research protocol in collaboration with co-investigators. She contributed to the preparation of the final report (drafting, reviewing and editing).
Emma Frew (https://orcid.org/0000-0002-5462-1158) (Co-investigator, Professor of Health Economics), contributed to the development of the trial protocol in collaboration with co-investigators. She provided oversight regarding the health economic data and contributed to the preparation of the final report (drafting, reviewing and editing).
Helen M Parretti (https://orcid.org/0000-0002-7184-269X) (Co-chief Investigator, Senior Clinical Lecturer in Primary Care) contributed to development of the funding application and research protocol in collaboration with co-investigators. She provided clinical oversight and contributed to the delivery of the trial and to the preparation of the final report (drafting, reviewing and editing).
Publications
Daley AJ, Jolly K, Bensoussane H, Ives N, Jebb SA, Tearne S, et al. Feasibility and acceptability of a brief routine weight management intervention for postnatal women embedded within the national child immunisation programme in primary care: randomised controlled cluster feasibility trial. Trials 2020;21:757.
Parretti HM, Ives N, Tearne S, Vince A, Greenfield SM, Jolly K, et al. Protocol for the feasibility and acceptability of a brief routine weight management intervention for postnatal women embedded within the national child immunisation programme: randomised controlled cluster feasibility trial with nested qualitative study (PIMMS-WL). BMJ Open 2020;10:e033027.
Tyldesley-Marshall N, Greenfield SM, Parretti HM, Jolly K, Jebb S, Daley AJ. The experiences of postnatal women and healthcare professionals of a brief weight management intervention embedded within the national child immunisation programme. BMC Pregnancy Childbirth 2021;21:462.
Data-sharing statement
The data sets used and analysed during this study are available from the corresponding author on reasonable request.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. New Eng J Med 2017;377:13-27. https://doi.org/10.1056/NEJMoa1614362.
- The NHS Information Centre, Lifestyles Statistics . Statistics on Obesity, Physical Activity and Diet 2012. http://content.digital.nhs.uk/catalogue/PUB05131/obes-phys-acti-diet-eng-2012-rep.pdf (accessed 23 July 2018).
- Kanter R, Caballero B. Global gender disparities in obesity: a review. Adv Nutr 2012;3:491-8. https://doi.org/10.3945/an.112.002063.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815-25. https://doi.org/10.1016/S0140-6736(11)60814-3.
- NHS Digital . Statistics on Obesity, Physical Activity and Diet, England 2019 2019. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesity-physical-activity-and-diet-england-2019 (accessed 1 October 2019).
- NHS Digital . Statistics on Obesity, Physical Activity and Diet, England 2018 2018. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/statistics-on-obesity-physical-activity-and-diet-england-2018 (accessed 5 October 2019).
- Jackson SE, Steptoe A. Association between perceived weight discrimination and physical activity: a population-based study among English middle-aged and older adults. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014592.
- Annis NM, Cash TF, Hrabosky JI. Body image and psychosocial differences among stable average weight, currently overweight, and formerly overweight women: the role of stigmatizing experiences. Body Image 2004;1:155-67. https://doi.org/10.1016/j.bodyim.2003.12.001.
- Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med 1990;150:665-72. https://doi.org/10.1001/archinte.1990.00390150135026.
- NCD Risk Factor Collaboration . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377-96. https://doi.org/10.1016/S0140-6736(16)30054-X.
- Baker C. Obesity Statistics: Briefing Paper. House of Commons Library 2018. https://researchbriefings.files.parliament.uk/documents/SN03336/SN03336.pdf (accessed 12 July 2019).
- Booth HP, Charlton J, Gulliford MC. Socioeconomic inequality in morbid obesity with body mass index more than 40kg/m2 in the United States and England. SSM Popul Health 2017;3:172-8. https://doi.org/10.1016/j.ssmph.2016.12.012.
- Devaux M, Sassi F. Social inequalities in obesity and overweight in 11 OECD countries. Eur J Public Health 2013;23:464-9. https://doi.org/10.1093/eurpub/ckr058.
- Institute for Medicine . Weight Gain in Pregnancy: Re-Examining The Guidelines 2009.
- Cohen TR, Koski KG. Limiting excess weight gain in healthy pregnant women: importance of energy intakes, physical activity, and adherence to gestational weight gain guidelines. J Pregnancy 2013;2013. https://doi.org/10.1155/2013/787032.
- Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007;114:187-94. https://doi.org/10.1111/j.1471-0528.2006.01180.x.
- Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord 2003;27:117-27. https://doi.org/10.1038/sj.ijo.0802156.
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164-70. https://doi.org/10.1016/S0140-6736(06)69473-7.
- Linné Y, Dye L, Barkeling B, Rössner S. Weight development over time in parous women – the SPAWN study – 15 years follow-up. Int J Obes Relat Metab Disord 2003;27:1516-22. https://doi.org/10.1038/sj.ijo.0802441.
- Rooney BL, Schauberger CW, Mathiason MA. Impact of peri-natal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 2005;106:1349-56. https://doi.org/10.1097/01.AOG.0000185480.09068.4a.
- Marshall E, Moon MA, Mirchandani A, Smith DG, Nichols LP, Zhao X, et al. ‘Baby Wants Tacos’: analysis of health-related facebook posts from young pregnant women. Matern Child Health J 2019;23:1400-13. https://doi.org/10.1007/s10995-019-02776-7.
- Chuang CH, Velott DL, Weisman CS. Exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions. Matern Child Health J 2010;14:713-19. https://doi.org/10.1007/s10995-009-0518-6.
- Clark A, Skouteris H, Wertheim E, Paxton SJ, Milgrom J. My baby body: a qualitative insight into women’s body-related experiences and mood during pregnancy and the postpartum. J Reprod Infant Psychol 2009;27:330-45. https://doi.org/10.1080/02646830903190904.
- Symons Downs D, Hausenblas HA. Women’s exercise beliefs and behaviours during their pregnancy and postpartum. Am Coll Nurses Midwives 2004;49:138-44. https://doi.org/10.1016/j.jmwh.2003.11.009.
- Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am 2009;36:317-32. https://doi.org/10.1016/j.ogc.2009.04.001.
- Cheney K, Farber R, Barratt AL, McGeechan K, de Vries B, Ogle R, et al. Population attributable fractions of perinatal outcomes for nulliparous women associated with overweight and obesity, 1990–2014. Med J Aust 2018;208:119-25. https://doi.org/10.5694/mja17.00344.
- Ziauddeen N, Roderick PJ, Macklon NS, Alwan NA. Is maternal weight gain between pregnancies associated with risk of large-for-gestational age birth? Analysis of a UK population-based cohort. Lancet 2018;392. https://doi.org/10.1016/S0140-6736(18)32927-1.
- Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344. https://doi.org/10.1136/bmj.e2088.
- Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess 2008;168:1-223.
- Thorsdottir I, Torfadottir JE, Birgisdottir BE, Geirsson RT. Weight gain in women of normal weight before pregnancy: complications in pregnancy or delivery and birth outcome. Obstet Gynecol 2002;99:799-806. https://doi.org/10.1097/00006250-200205000-00021.
- Ramachenderan J, Bradford J, McLean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol 2008;48:228-35. https://doi.org/10.1111/j.1479-828X.2008.00860.x.
- Oteng-Ntim E, Mononen S, Sawicki O, Seed PT, Bick D, Poston L. Interpregnancy weight change and adverse pregnancy outcomes: a systematic review and meta-analysis. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-018778.
- Knight M, Tuffnell D, Kenyon S, Shakespeare J, Gray R, Kurinczuk JJ. (editors) on behalf of MBRRACE-UK . Saving Lives, Improving Mothers’ Care-Surveillance of Maternal Deaths in the UK 2011–13 and Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–13 2015. www.npeu.ox.ac.uk/downloads/files/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202015.pdf (accessed September 2019).
- Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLOS Med 2019;16. https://doi.org/10.1371/journal.pmed.1002817.
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med 2003;26:149-59. https://doi.org/10.1207/S15324796ABM2602_07.
- Walker LO. Managing excessive weight gain during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs 2007;36:490-50. https://doi.org/10.1111/j.1552-6909.2007.00179.x.
- Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity-results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes 2007;31:1642-51. https://doi.org/10.1038/sj.ijo.0803655.
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. https://doi.org/10.1016/S0140-6736(09)60318-4.
- Linné Y. Effects of obesity on women’s reproduction and complications during pregnancy. Obes Rev 2004;5:137-43. https://doi.org/10.1111/j.1467-789X.2004.00147.x.
- Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 2015;125:144-52. https://doi.org/10.1097/AOG.0000000000000565.
- Hollis JL, Crozier SR, Inskip HM, Cooper C, Godfrey KM, Harvey NC, et al. Modifiable risk factors of maternal postpartum weight retention: an analysis of their combined impact and potential opportunities for prevention. Int J Obes 2017;41:1091-8. https://doi.org/10.1038/ijo.2017.78.
- Lipsky LM, Strawderman MS, Olson CM. Maternal weight change between 1 and 2 years postpartum: the importance of 1 year weight retention. Obesity 2012;20:1496-502. https://doi.org/10.1038/oby.2012.41.
- Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002;100:245-52. https://doi.org/10.1016/S0029-7844(02)02125-7.
- Rong K, Yu K, Han X, Szeto IM, Qin X, Wang J, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 2015;18:2172-82. https://doi.org/10.1017/S1368980014002523.
- Bogaerts A, Van den Bergh BR, Ameye L, Witters I, Martens E, Timmerman D, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol 2013;122:999-1009. https://doi.org/10.1097/AOG.0b013e3182a7f63e.
- Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord 2000;24:1660-8. https://doi.org/10.1038/sj.ijo.0801456.
- National Institute for Health and Care Excellence (NICE) . Weight Management Before, During and After Pregnancy: NICE Public Health Guidance 27 2010. www.nice.org.uk/guidance/ph27 (accessed 2 March 2018).
- Denison FC AN, Keag O, Hor K, Reynolds RM, Milne A, Diamond A. on behalf of the Royal College of Obstetricians and Gynaecologists . Care of Women With Obesity in Pregnancy. Green-Top Guideline No. 72 2018. www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg72/ (accessed 1 September 2019).
- Walker LO, Sterling BS. Weight loss, gain, or stability from 6 weeks to 6 months postpartum: associations with depressive symptoms and behavioral habits. J Womens Health 2020;29:541-9. https://doi.org/10.1089/jwh.2019.7949.
- Nagl M, Linde K, Stepan H, Kersting A. Obesity and anxiety during pregnancy and postpartum: a systematic review. J Affect Disord 2015;186:293-305. https://doi.org/10.1016/j.jad.2015.06.054.
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health 2003;6:263-74. https://doi.org/10.1007/s00737-003-0024-6.
- Ertel KA, Huang T, Rifas-Shiman SL, Kleinman K, Rich-Edwards J, Oken E, et al. Perinatal weight and risk of prenatal and postpartum depressive symptoms. Ann Epidemiol 2017;27:695-700.e1. https://doi.org/10.1016/j.annepidem.2017.10.007.
- Winston R, Chicot R. The importance of early bonding on the long-term mental health and resilience of children. London J Prim Care 2016;8:12-4. https://doi.org/10.1080/17571472.2015.1133012.
- Carter-Edwards L, Østbye T, Bastian LA, Yarnall KS, Krause KM, Simmons TJ. Barriers to adopting a healthy lifestyle: insight from postpartum women. BMC Res Notes 2009;2. https://doi.org/10.1186/1756-0500-2-161.
- Sary MP, Turnip SS. Attitude difference between fathers and mothers toward fathers involvement in child rearing activities among couples with 0-12 months Old babies. Community based study in a primary health care setting. Proc Soc Behav 2015;190:92-6. https://doi.org/10.1016/j.sbspro.2015.04.921.
- Deutsch FM. Equally shared parenting. Curr Dir Psychol Sci 2001;10:25-8. https://doi.org/10.1111/1467-8721.00107.
- Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth 2005;32:187-93. https://doi.org/10.1111/j.0730-7659.2005.00368.x.
- Montgomery KS, Bushee TD, Phillips JD, Kirkpatrick T, Catledge C, Braveboy K, et al. Women’s challenges with postpartum weight loss. Matern Child Health J 2011;15:1176-84. https://doi.org/10.1007/s10995-010-0681-9.
- Herring SJ, Rich-Edwards JW, Oken E, Rifas-Shiman SL, Kleinman KP, Gillman MW. Association of postpartum depression with weight retention 1 year after childbirth. Obesity 2008;16:1296-301. https://doi.org/10.1038/oby.2008.71.
- Hartley E, Hill B, Bailey C, Fuller-Tyszkiewicz M, Skouteris H. The associations of weight status and body attitudes with depressive and anxiety symptoms across the first year postpartum. Womens Health Issues 2018;28:530-8. https://doi.org/10.1016/j.whi.2018.07.002.
- Xiao RS, Kroll-Desrosiers AR, Goldberg RJ, Pagoto SL, Person SD, Waring ME. The impact of sleep, stress, and depression on postpartum weight retention: a systematic review. J Psychosom Res 2014;77:351-8. https://doi.org/10.1016/j.jpsychores.2014.09.016.
- Schwarzer R. Modeling health behavior change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol 2008;57:1-29. https://doi.org/10.1111/j.1464-0597.2007.00325.x.
- Wu J. Rewarding healthy behaviors – pay patients for performance. Ann Fam Med 2012;10:261-3. https://doi.org/10.1370/afm.1334.
- Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health 2017;3:383-8. https://doi.org/10.1016/j.sleh.2017.07.013.
- Pacheco SR, Miranda AM, Coelho R, Monteiro AC, Bragança G, Loureiro HC. Overweight in youth and sleep quality: is there a link?. Arch Endocrinol Metab 2017;61:367-73. https://doi.org/10.1590/2359-3997000000265.
- Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010;11:180-4. https://doi.org/10.1016/j.sleep.2009.07.014.
- Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord 2015;14. https://doi.org/10.1186/s40200-015-0154-1.
- Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med 2015;162:501-12. https://doi.org/10.7326/M14-2238.
- Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P. Behavioural Weight Management Review Group. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obes Rev 2014;15:920-32. https://doi.org/10.1111/obr.12220.
- Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563-71. https://doi.org/10.1056/NEJMoa061883.
- National Institute for Health and Care Excellence . Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children 2014. www.nice.org.uk/guidance/cg189/evidence/obesity-update-appendix-p-pdf-6960327450 (accessed 24 August 2017).
- Reddy S. Care Pathway for the Management of Overweight and Obesity. London: Department of Health and Social Care; 2006.
- Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485-92. https://doi.org/10.1016/S0140-6736(11)61344-5.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d6500.
- National Institute for Health and Care Excellence (NICE) . Weight Management: Lifestyle Services for Overweight or Obese Adults 2014. www.nice.org.uk/guidance/ph53 (accessed 11 October 2017).
- Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-20.
- Groth S, David T. New mothers’ views of weight and exercise. Amer J Matern Child Nurs 2008;33:364-70. https://doi.org/10.1097/01.NMC.0000341257.26169.30.
- Hodgkinson EL, Smith DM, Wittkowski A. Women’s experiences of their pregnancy and postpartum body image: a systematic review and meta-synthesis. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-330.
- Godin G, Vezina L, Leclerc O. Factors influencing intentions of pregnant women to exercise after giving birth. Public Health Rep 1989;104:188-95.
- Lindsay A, Sussner K, Kim J, Kim J. Gortmaker . The role of parents in preventing childhood obesity. Future Child 2006;16:169-86. https://doi.org/10.1353/foc.2006.0006.
- Fogelholm M, Nuutinen O, Pasanen M, Myöhänen E, Säätelä T. Parent-child relationship of physical activity patterns and obesity. Int J Obes Relat Metab Disord 1999;23:1262-8. https://doi.org/10.1038/sj.ijo.0801061.
- Christenson A, Johansson E, Reynisdottir S, Torgerson J, Hemmingsson E. Women’s perceived reasons for their excessive postpartum weight retention: a qualitative interview study. PLOS ONE 2017;11. https://doi.org/10.1371/journal.pone.0167731.
- Dinsdale S, Branch K, Cook L, Shucksmith J. ‘As soon as you’ve had the baby that’s it . . . a qualitative study of 24 postnatal women on their experience of maternal obesity care pathways. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-3289-1.
- Heslehurst N, Dinsdale S, Sedgewick G, Simpson H, Sen S, Summerbell CD, et al. An evaluation of the implementation of maternal obesity pathways of care: a mixed methods study with data integration. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0127122.
- Johnson M, Campbell F, Messina J, Preston L, Buckley Woods H, Goyder E. Weight management during pregnancy: a systematic review of qualitative evidence. Midwifery 2013;29:1287-96. https://doi.org/10.1016/j.midw.2012.11.016.
- Ferguson JA, Daley AJ, Paretti HM. Behavioural weight management interventions for postnatal women: a systematic review of systematic reviews of randomized controlled trials. Obes Rev 2018;20:829-41. https://doi.org/10.1111/obr.12834.
- Leermakers EA, Anglin K, Wing RR. Reducing postpartum weight retention through a correspondence intervention. Int J Obes Relat Metab Disord 1998;22:1103-9. https://doi.org/10.1038/sj.ijo.0800734.
- Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J Med 2000;342:449-53. https://doi.org/10.1056/NEJM200002173420701.
- O’Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. J Womens Health 2003;12:991-8. https://doi.org/10.1089/154099903322643910.
- Østbye T, Krause K, Lovelady C, Morey MC, Bastian LA, Peterson BL, et al. Active mothers postpartum: a randomized controlled weight-loss intervention trial. Am J Prev Med 2009;37:173-80. https://doi.org/10.1016/j.amepre.2009.05.016.
- Krummel D, Semmens E, MacBride AM, Fisher B. Lessons learned from the mother’s overweight management study in four West Virginia WIC offices. J Nutr Educa Behav 2010;42:S52-S8. https://doi.org/10.1016/j.jneb.2010.02.012.
- Craigie AM, Macleod M, Barton KL, Treweek S, Anderson AS. on behalf of the WeighWell team . Supporting postpartum weight loss in women living in deprived communities: design implications for a randomised controlled trial. Eur J Clin Nutr 2011;65:952-8. https://doi.org/10.1038/ejcn.2011.56.
- Walker LO, Sterling BS, Latimer L, Kim SH, Garcia AA, Fowles ER. Ethnic-specific weight-loss interventions for low-income postpartum women: findings and lessons. West J Nurs Res 2012;34:654-76. https://doi.org/10.1177/0193945911403775.
- Bertz F, Brekke HK, Ellegard L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr 2012;96:698-705. https://doi.org/10.3945/ajcn.112.040196.
- Reinhardt JA, van der Ploeg HP, Grzegrzulka R, Timperley JG. lmplementing lifestyle change through phone-based motivational interviewing in rural-based women with previous gestational diabetes mellitus. Health Promot J Austr 2012;23:5-9. https://doi.org/10.1071/HE12005.
- Colleran HL, Lovelady CA. Use of MyPyramid Menu Planner for Moms in a weight-loss intervention during lactation. J Acad Nutr Diet 2012;112:553-8. https://doi.org/10.1016/j.jand.2011.12.004.
- Wiltheiss GA, Lovelady CA, West DG, Brouwer RJ, Krause KM, Ostbye T. Diet quality and weight change among overweight and obese post-partum women enrolled in a behavioral intervention program. J Acad Nutr Diet 2013;113:54-62. https://doi.org/10.1016/j.jand.2012.08.012.
- Shyam S, Arshad F, Abdul Ghani R, Wahab NA, Safii NS, Nisak MY, et al. Low glycaemic index diets improve glucose tolerance and body weight in women with previous history of gestational diabetes: a six months randomized trial. Nutr J 2013;12. https://doi.org/10.1186/1475-2891-12-68.
- Herring SJ, Cruice JF, Bennett GG, Davey A, Foster GD. Using technology to promote postpartum weight loss in urban, low-income mothers: a pilot randomized controlled trial. J Nutr Educ Behav 2014;46:610-5. https://doi.org/10.1016/j.jneb.2014.06.002.
- Nicklas JM, Zera CA, England LJ, Rosner BA, Horton E, Levkoff SE, et al. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol 2014;124:563-70. https://doi.org/10.1097/AOG.0000000000000420.
- Dewey KG, Lovelady CA, Nommsen-Rivers LA, McCrory MA, Lonnerdal B. A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. N Engl J Med 1994;330:449-53. https://doi.org/10.1056/NEJM199402173300701.
- Lovelady CA, Bopp MJ, Colleran HL, Mackie HK, Wideman L. Effect of exercise training on loss of bone mineral density during lactation. Med Sci Sports Ex 2009;41:1902-7. https://doi.org/10.1249/MSS.0b013e3181a5a68b.
- Maturi MS, Afshary P, Abedi P. Effect of physical activity intervention based on a pedometer on physical activity level and anthropometric measures after childbirth: a randomized controlled trial. BMC Pregnancy Childbirth 2011;11. https://doi.org/10.1186/1471-2393-11-103.
- McIntyre HD, Peacock A, Miller YD, Koh D, Marshall AL. Pilot Study of an individualised early postpartum intervention to increase physical activity in women with previous gestational diabetes. Int J Endocrinol 2012;2012. https://doi.org/10.1155/2012/892019.
- Youngwanichsetha S, Phumdoung S, Ingkathawornwong T. The effects of tai chi qigong exercise on plasma glucose levels and health status of postpartum Thai women with type 2 diabetes. Focus Altern Complement Ther 2013;18:182-7. https://doi.org/10.1111/fct.12064.
- Kim C, Draska M, Hess ML, Wilson EJ, Richardson CR. A web-based pedometer programme in women with a recent history of gestational diabetes. Diabet Med 2012;29:278-83. https://doi.org/10.1111/j.1464-5491.2011.03415.x.
- Tripette J, Murakami H, Gando Y, Kawakami R, Sasaki A, Hanawa S, et al. Home-based active video games to promote weight loss during the postpartum period. Med Sci Sports Exerc 2014;46:472-8. https://doi.org/10.1249/MSS.0000000000000136.
- Zourladani A, Zafrakas M, Chatzigiannis B, Papasozomenou P, Vavilis D, Matziari C. The effect of physical exercise on postpartum fitness, hormone and lipid levels: a randomized controlled trial in primiparous, lactating women. Arch Gynecol Obstet 2015;291:525-30. https://doi.org/10.1007/s00404-014-3418-y.
- National Institute for Health and Care Excellence (NICE) . Putting NICE Guidance Into Practice. Costing Report: Obesity. Implementing the NICE Guideline on Obesity (CG189) 2014. www.nice.org.uk/guidance/cg189/resources/costing-report-pdf-193304845 (accessed 23 March 2019).
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481-6. https://doi.org/10.2337/dc10-2415.
- National Institute for Health and Care Excellence (NICE) . Costing Report: Managing Overweight and Obesity in Adults: Lifestyle Weight Management Services 2014. www.nice.org.uk/guidance/ph53/resources/costing-report-pdf-69241357 (accessed 14 February 2018).
- Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591-60. https://doi.org/10.1016/j.cmet.2016.02.005.
- Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss intervention for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 2017;359. https://doi.org/10.1136/bmj.j4849.
- Kuhlmann AK, Dietz PM, Galavotti C, England LJ. Weight-management interventions for pregnant or postpartum women. Am J Prev Med 2008;34:523-8. https://doi.org/10.1016/j.amepre.2008.02.010.
- Elliott-Sale KJ, Barnett CT, Sale C. Exercise interventions for weight management during pregnancy and up to 1 year postpartum among normal weight, overweight and obese women: a systematic review and meta-analysis. Br J Sports Med 2015;49:1336-42. https://doi.org/10.1136/bjsports-2014-093875.
- Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta-analysis of randomized controlled trials. Prev Med 2013;56:351-64. https://doi.org/10.1016/j.ypmed.2013.02.021.
- Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes 2014;38:626-35. https://doi.org/10.1038/ijo.2013.183.
- Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD005627.pub3.
- Office for National Statistics . Births in England and Wales: 2018: Live Births, Stillbirths and the Intensity of Childbearing, Measured by the Total Fertility Rate 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2018 (accessed 2 February 2018).
- Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery 2011;27:257-64. https://doi.org/10.1016/j.midw.2009.06.009.
- Joshi PP, Quintiliani LM, McCarthy AC, Gilmore A, Mahesri M, Sullivan LM, et al. A randomized controlled feasibility trial in behavioral weight management for underserved postpartum african american women: the RENEW study. Prev Chronic Dis 2018;15. https://doi.org/10.5888/pcd15.170400.
- Ruchat SM, Mottola MF, Skow RJ, Nagpal TS, Meah VL, James M, et al. Effectiveness of exercise interventions in the prevention of excessive gestational weight gain and postpartum weight retention: a systematic review and meta-analysis. Br J Sports Med 2018;52:1347-56. https://doi.org/10.1136/bjsports-2018-099399.
- Setse R, Grogan R, Cooper LA, Strobino D, Powe NR, Nicholson W. Weight loss programs for urban-based, postpartum African-American women: perceived barriers and preferred components. Matern Child Health J 2008;12:119-27. https://doi.org/10.1007/s10995-007-0211-6.
- Phelan S. Pregnancy: a ‘teachable moment’ for weight control and obesity prevention. Amer J Obstet Gynecol 2010;202. https://doi.org/10.1016/j.ajog.2009.06.008.
- Atkinson L, Olander EK, French DP. Why don’t many obese pregnant and post-natal women engage with a weight management service?. J Reprod Infant Psychol 2013;31:245-56. https://doi.org/10.1080/02646838.2013.809518.
- Hartmann-Boyce J, Jebb SA, Fletcher BR, Aveyard P. Self-help for weight loss in overweight and obese adults: systematic review and meta-analysis. Am J Public Health 2015;105:e43-57. https://doi.org/10.2105/AJPH.2014.302389.
- Nelson RO, Hayes SC. Theoretical explanations for reactivity in self-monitoring. Behav Modif 1981;5:3-14. https://doi.org/10.1177/014544558151001.
- Kanfer FH. Self-monitoring: methodological limitations and clinical applications. J Consult Clin Psychol 1970;35. https://doi.org/10.1037/h0029874.
- Cameron LD, Leventhal H. The Self-regulation of Health and Illness Behavior. London: Routledge; 2003.
- Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens 2015;28:1209-21. https://doi.org/10.1093/ajh/hpv008.
- Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011;111:92-102. https://doi.org/10.1016/j.jada.2010.10.008.
- Hartmann-Boyce J, Aveyard P, Piernas C, Koshiaris C, Velardo C, Salvi D, et al. Cognitive and behavioural strategies for weight management: the Oxford Food and Activity Behaviours (OxFAB) cohort study. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0202072.
- Michie S, Whittington C, Hamoudi Z, Zarnani F, Tober G, West R. Identification of behaviour change techniques to reduce excessive alcohol consumption. Addiction 2012;107:1431-40. https://doi.org/10.1111/j.1360-0443.2012.03845.x.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Madigan CD, Daley AJ, Lewis AL, Aveyard P, Jolly K. Is self-weighing an effective tool for weight loss: a systematic literature review and meta-analysis. Int J Behav Nutr Phys Act 2015;12. https://doi.org/10.1186/s12966-015-0267-4.
- Gilinsky AS, Kirk AF, Hughes AR, Lindsay RS. Lifestyle interventions for type 2 diabetes prevention in women with prior gestational diabetes: a systematic review and meta-analysis of behavioural, anthropometric and metabolic outcomes. Prev Med Rep 2015;2:448-61. https://doi.org/10.1016/j.pmedr.2015.05.009.
- Cleo G, Hersch J, Thomas R. Participant experiences of two successful habit-based weight-loss interventions in Australia: a qualitative study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020146.
- Borek AJ, Abraham C, Greaves CJ, Tarrant M, Garner N, Pascale M. ‘We’re all in the same boat’: a qualitative study on how groups work in a diabetes prevention and management programme. Br J Health Psychol 2019;24:787-805. https://doi.org/10.1111/bjhp.12379.
- Metzgar CJ, Preston AG, Miller DL, Nickols-Richardson SM. Facilitators and barriers to weight loss and weight loss maintenance: a qualitative exploration. J Hum Nutr Diet 2015;28:593-60. https://doi.org/10.1111/jhn.12273.
- Dalle Grave R, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Major factors for facilitating change in behavioral strategies to reduce obesity. Psychol Res Behav Manag 2013;6:101-10. https://doi.org/10.2147/PRBM.S40460.
- Burke LE, Swigart V, Warziski Turk M, Derro N, Ewing LJ. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res 2009;19:815-28. https://doi.org/10.1177/1049732309335395.
- Bovens M. Two concepts of accountability: accountability as a virtue and as a mechanism. West Eur Polit 2010;33:946-67. https://doi.org/10.1080/01402382.2010.486119.
- Wooldridge JS, Ranby KW, Roberts S, Huebschmann AG. A couples-based approach for increasing physical activity among adults with type 2 diabetes: a pilot feasibility randomized controlled trial. Diabetes Educ 2019;45:629-41. https://doi.org/10.1177/0145721719881722.
- Berry E, Davies M, Dempster M. Exploring the effectiveness of couples interventions for adults living with a chronic physical illness: a systematic review. Patient Educ Coun 2017;100:1287-303. https://doi.org/10.1016/j.pec.2017.02.015.
- Khan CM, Stephens MA, Franks MM, Rook KS, Salem JK. Influences of spousal support and control on diabetes management through physical activity. Health Psychol 2013;32:739-47. https://doi.org/10.1037/a0028609.
- Ahern A, Boyland EJ, Jebb SA, Cohn SR. Participants’ explanatory model of being overweight and their experiences of two weight loss interventions. Ann Fam Med 2013;11:251-7. https://doi.org/10.1370/afm.1446.
- Gardner B, Whittington C, McAteer J, Eccles MP, Michie S. Using theory to synthesise evidence from behaviour change interventions: the example of audit and feedback. Soc Sci Med 2010;70:1618-25. https://doi.org/10.1016/j.socscimed.2010.01.039.
- Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006642.
- Blackburn M, Stathi A, Keogh E, Eccleston C. Raising the topic of weight in general practice: perspectives of GPs and primary care nurses. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008546.
- Huang H, Yu H, Marin E, Brock S, Davis T. Physicians’ weight loss counselling in two public hospital primary care clinics. Acad Med 2004;79:156-61. https://doi.org/10.1097/00001888-200402000-00012.
- American Medical Association . Talking About Weight With Your Patients 2011.
- Chisholm A, Hart J, Lam V, Peters S. Current challenges of behaviour change talk for medical professionals and trainees. Patient Educ Couns 2012;87. https://doi.org/10.1016/j.pec.2011.12.001.
- Dewhurst A, Peters S, Devereux-Fitzgerald A, Hart J. Physicians’ views and experiences of discussing weight management within routine clinical consultations: a thematic synthesis. Patient Educ Couns 2017;100:897-908. https://doi.org/10.1016/j.pec.2016.12.017.
- Drury CA, Louis M. Exploring the association between body weight, stigma of obesity, and health care avoidance. J Am Acad Nurse Pract 2002;14:554-61. https://doi.org/10.1111/j.1745-7599.2002.tb00089.x.
- Bradbury D, Chisholm A, Watson PM, Bundy C, Bradbury N, Birtwistle S. Barriers and facilitators to health care professionals discussing child weight with parents: a meta-synthesis of qualitative studies. Br J Health Psychol 2018;23:701-22. https://doi.org/10.1111/bjhp.12312.
- Aveyard P, Lewis A, Tearne S, Hood K, Christian-Brown A, Adab P, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. Lancet 2016;388:2492-500. https://doi.org/10.1016/S0140-6736(16)31893-1.
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med 2013;173:458-64. https://doi.org/10.1001/jamainternmed.2013.3751.
- Department of Health and Social Care . Next Steps on the NHS Five Year Forward View’ Reflects How NHS England Intends to Meet the Seven Overarching Objectives in the Mandate 2018. https://www.england.nhs.uk/wp-content/uploads/2017/03/NEXT-STEPS-ON-THE-NHS-FIVE-YEAR-FORWARD-VIEW.pdf (accessed 10 July 2018).
- Public Health England . From Evidence Into Action: Opportunities to Protect and Improve the Nation’s Health. Strategic Document Setting Out Public Health England’s Priorities for the Next 5 Years 2014. www.gov.uk/government/publications/from-evidence-into-action-opportunities-to-protect-and-improve-the-nations-health (accessed 21 June 2017).
- Slomian J, Bruyère O, Reginster JY, Emonts P. The internet as a source of information used by women after childbirth to meet their need for information: a web-based survey. Midwifery 2017;48:46-52. https://doi.org/10.1016/j.midw.2017.03.005.
- Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: a mixed-methods evaluation. JMIR Mhealth Uhealth 2016;4. https://doi.org/10.2196/mhealth.4225.
- Brickwood KJ, Watson G, O’Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth 2019;7. https://doi.org/10.2196/11819.
- Dounavi K, Tsoumani O. Mobile health applications in weight management: a systematic literature review. Amer J Prev Med 2019;56:894-903. https://doi.org/10.1016/j.amepre.2018.12.005.
- Afshin A, Babalola D, Mclean M, Yu Z, Ma W, Chen CY, et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc 2016;5. https://doi.org/10.1161/JAHA.115.003058.
- Zhao J, Freeman B, Li M. Can mobile phone apps influence people’s health behavior change? An evidence review. J Med Internet Res 2016;18. https://doi.org/10.2196/jmir.5692.
- Lau Y, Klainin-Yobas P, Htun TP, Wong SN, Tan KL, Ho-Lim ST, et al. Electronic-based lifestyle interventions in overweight or obese perinatal women: a systematic review and meta-analysis. Obes Rev 2017;18:1071-87. https://doi.org/10.1111/obr.12557.
- NHS Digital . Childhood Vaccination Coverage Statistics – England 2018–19 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics/england-2018-19 (accessed 18 November 2019).
- Fealy SM, Taylor RM, Foureur M, Attia J, Ebert L, Bisquera A, et al. Weighing as a stand-alone intervention does not reduce excessive gestational weight gain compared to routine antenatal care: a systematic review and meta-analysis of randomised controlled trials. BMC Pregnancy Childbirth 2017;17. https://doi.org/10.1186/s12884-016-1207-2.
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g1285.
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767-77. https://doi.org/10.1016/S2213-8587(15)00227-2.
- Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ, et al. An internet-based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:821-8. https://doi.org/10.1016/S2213-8587(16)30099-7.
- Sherifali D, Nerenberg KA, Wilson S, Semeniuk K, Ali MU, Redman LM, et al. The effectiveness of eHealth Technologies on Weight management in pregnant and postpartum women: systematic review and meta-analysis. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8006.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Cash TF, Fleming EC, Alindogan J, Steadman L, Whitehead A. Beyond body image as a trait: the development and validation of the Body Image States Scale. Eat Disord 2002;10:103-13. https://doi.org/10.1080/10640260290081678.
- Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004;36:1750-60. https://doi.org/10.1249/01.mss.0000142303.49306.0d.
- Pinto AM, Fava JL, Raynor HA, LaRose JG, Wing RR. Development and validation of the weight control strategies scale. Obesity 2013;21:2429-36. https://doi.org/10.1002/oby.20368.
- de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr 2004;134:2372-80. https://doi.org/10.1093/jn/134.9.2372.
- Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and email. Obesity 2013;21. https://doi.org/10.1002/oby.20396.
- EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16. https://doi.org/10.1016/0168-8510(90)90421-9.
- Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167-76. https://doi.org/10.1007/s11136-011-9927-2.
- Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-264.
- Stendell-Hollis NR, Laudermilk MJ, West JL, Thompson PA, Thomson CA. Recruitment of lactating women into a randomized dietary intervention: successful strategies and factors promoting enrollment and retention. Contemp Clin Trials 2011;32:505-11. https://doi.org/10.1016/j.cct.2011.03.007.
- Haste A, Adamson AJ, McColl E, Araujo-Soares V, Bell R. Problems recruiting and retaining postnatal women to a pilot randomised controlled trial of a web-delivered weight loss intervention. BMC Res Notes 2018;11. https://doi.org/10.1186/s13104-018-3305-x.
- Daley A, Jolly K, Jebb SA, Roalfe A, Mackilllop L, Lewis A, et al. Effectiveness of a behavioural intervention involving regular weighing and feedback by community midwives within routine antenatal care to prevent excessive gestational weight gain: POPS2 randomised controlled trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-030174.
- Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev 2018;2. https://doi.org/10.1002/14651858.MR000013.pub6.
- Ohlendorf JM. Stages of change in the trajectory of postpartum weight self-management. J Obstet Gynecol Neonatal Nurs 2012;41:57-70. https://doi.org/10.1111/j.1552-6909.2011.01323.x.
- Avery A, Hillier S, Pallister C, Barber J, Lavin J. Factors influencing engagement in postnatal weight management and weight and wellbeing outcomes. B J Midwifery 2016;24:806-12. https://doi.org/10.12968/bjom.2016.24.11.806.
- Shieh C, Knisely MR, Clark D, Carpenter JS. Self-weighing in weight management interventions: a systematic review of literature. Obes Res Clin Pract 2016;10:493-519. https://doi.org/10.1016/j.orcp.2016.01.004.
- Painter SL, Ahmed R, Hill JO, Kushner RF, Lindquist R, Brunning S, et al. What matters in weight loss? An in-depth analysis of self-monitoring. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.7457.
- Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med 2005;30:210-16. https://doi.org/10.1207/s15324796abm3003_5.
- Harrison CL, Teede HJ, Lombard CB. How effective is self-weighing in the setting of a lifestyle intervention to reduce gestational weight gain and postpartum weight retention?. Aust N Z J Obstet Gynaecol 2014;54:382-5. https://doi.org/10.1111/ajo.12207.
- Bick D, Taylor C, Bhavnani V, Healey A, Seed P, Roberts S, et al. Lifestyle information and commercial weight management groups to support maternal postnatal weight management and positive lifestyle behaviour: the SWAN feasibility randomised controlled trial. BJOG 2020;127:636-45. https://doi.org/10.1111/1471-0528.16043.
- Ogden J, Whyman C. The effect of repeated weighing on psychological state. Eur Eat Disord Rev 1997;5:121-30. https://doi.org/10.1002/(SICI)1099-0968(199706)5:2<121::AID-ERV167>3.0.CO;2-N.
- Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity 2015;23:256-65. https://doi.org/10.1002/oby.20946.
- Benn Y, Webb TL, Chang BP, Harkin B. What is the psychological impact of self-weighing? A meta-analysis. Health Psychol Rev 2016;10:187-203. https://doi.org/10.1080/17437199.2016.1138871.
- Mercurio A, Rima B. Watching my weight: self-weighing, body surveillance and body dissatisfaction. Sex Roles 2011;65:47-55. https://doi.org/10.1007/s11199-011-9980-x.
- Madigan CD, Jolly K, Lewis AL, Aveyard P, Daley AJ. A randomised controlled trial of the effectiveness of self-weighing as a weight loss intervention. Int J Behav Nutr Phys Act 2014;11. https://doi.org/10.1186/s12966-014-0125-9.
- Vanwormer JJ, French SA, Pereira MA, Welsh EM. The impact of regular self-weighing on weight management: a systematic literature review. Int J Behav Nutr Phys Act 2008;5. https://doi.org/10.1186/1479-5868-5-54.
- Daley AJ, Jolly K, Jebb SA, Lewis AL, Clifford S, Roalfe AK, et al. Feasibility and acceptability of regular weighing, setting weight gain limits and providing feedback by community midwives to prevent excess weight gain during pregnancy: randomised controlled trial and qualitative study. BMC Obes 2015;2. https://doi.org/10.1186/s40608-015-0061-5.
- Pacanowski CR, Linde JA, Neumark-Sztainer D. Self-weighing: helpful or harmful for psychological well-being? A review of the literature. Curr Obes Rep 2015;4:65-72. https://doi.org/10.1007/s13679-015-0142-2.
- Hartmann-Boyce J, Boylan AM, Jebb SA, Aveyard P. ‘Experiences of monitoring in self-directed weight loss and weight loss maintenance: systematic review of qualitative studies’. Qual Health Res 2018;29:124-34. https://doi.org/10.1177/1049732318784815.
- Ahern AL, Aveyard P, Boyland EJ, Halford JC, Jebb SA. WRAP trial team . Inequalities in the uptake of weight management interventions in a pragmatic trial: an observational study in primary care. Br J Gen Pract 2016;66:e258-63. https://doi.org/10.3399/bjgp16X684337.
- Kaplan LM, Golden A, Jinnett K, Kolotkin RL, Kyle TK, Look M, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity 2018;26:61-9. https://doi.org/10.1002/oby.22054.
- Health Education England . Making Every Contact Count 2019. www.makingeverycontactcount.co.uk/ (accessed 27 November 2019).
- McKinley MC, Allen-Walker V, McGirr C, Rooney C, Woodside JV. Weight loss after pregnancy: challenges and opportunities. Nutr Res Rev 2018;31:225-38. https://doi.org/10.1017/S0954422418000070.
- Wing R, Phelan S. Long term weight loss maintenance. Am J Clin Nutr 2005;82:222S-5S. https://doi.org/10.1093/ajcn.82.1.222S.
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol 2000;19:5-16. https://doi.org/10.1037/0278-6133.19.suppl1.5.
- Ohlin A, Rössner S. Trends in eating patterns, physical activity and socio-demographic factors in relation to postpartum body weight development. Br J Nutr 1994;71:457-70. https://doi.org/10.1079/bjn19940155.
- Killgore W. Effect of sleep deprivation on cognitions. Prog Brain Res 2010;185:105-29.
- Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 2010;153:435-41. https://doi.org/10.7326/0003-4819-153-7-201010050-00006.
- Thomson CA, Morrow KL, Flatt SW, Wertheim BC, Perfect MM, Ravia JJ, et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity 2012;20:1419-25. https://doi.org/10.1038/oby.2012.62.
- Brown MJ, Sinclair M, Liddle D, Hill AJ, Madden E, Stockdale J. A systematic review investigating healthy lifestyle interventions incorporating goal setting strategies for preventing excess gestational weight gain. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0039503.
- Farpour-Lambert NJ, Ells LJ, Martinez de Tejada B, Scott C. Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies. Front Endocrinol 2018;9. https://doi.org/10.3389/fendo.2018.00546.
- Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev 2013;7.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Thomas KS, Bradshaw LE, Sach TH, Cowdell F, Batchelor JM, Lawton S, et al. Randomised controlled trial of silk therapeutic garments for the management of atopic eczema in children: the CLOTHES trial. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21160.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Goffman E. Presentation of Self in Everyday Life. London: Penguin; 1990.
- Grbich C. Qualitative Research in Health: An Introduction. London: SAGE Publications Ltd; 1999.
- Smith J, Bekker H, Cheater F. Theoretical versus pragmatic design challenges in qualitative research. Nurse Res 2011;18:39-51. https://doi.org/10.7748/nr2011.01.18.2.39.c8283.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Tyldesley-Marshall N, Greenfield SM, Parretti HM, Jolly K, Jebb S, Daley AJ. The experiences of postnatal women and healthcare professionals of a brief weight management intervention embedded within the national child immunisation programme. BMC Pregnancy Childbirth 2021;21. https://doi.org/10.1186/s12884-021-03905-3.
- Department of Health and Social Care . UK Chief Medical Officers’ Physical Activity Guidelines n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_1280 (accessed 12 December 2019).
- Pritchett RV, Daley AJ, Jolly K. Does aerobic exercise reduce postpartum depressive symptoms? A systematic review and meta-analysis. Br J Gen Pract 2017;67:e684-e691. https://doi.org/10.3399/bjgp17X692525.
- Daley AJ, Foster L, Long G, Palmer C, Robinson O, Walmsley H, et al. The effectiveness of exercise for the prevention and treatment of antenatal depression: systematic review with meta-analysis. BJOG 2015;122:57-62. https://doi.org/10.1111/1471-0528.12909.
- Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: a new approach to evaluating complex public health interventions. Soc Sci Med 2012;75:2299-306. https://doi.org/10.1016/j.socscimed.2012.08.032.
- Keyworth C, Epton T, Goldthorpe J, Calam R, Armitage CJ. ‘It’s difficult, I think it’s complicated’: Health care professionals’ barriers and enablers to providing opportunistic behaviour change interventions during routine medical consultations. Br J Health Psychol 2019;24:571-92. https://doi.org/10.1111/bjhp.12368.
- Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant 2002;36:391-409. https://doi.org/10.1023/A:1020909529486.
- Daley AJ, Jolly K, Jebb SA, Roalfe AK, Mackillop L, Lewis AL, et al. Effectiveness of regular weighing, weight target setting and feedback by community midwives within routine antenatal care in preventing excessive gestational weight gain: randomised controlled trial. BMC Obes 2015;3. https://doi.org/10.1186/s40608-016-0086-4.
- Hartmann-Boyce J, Nourse R, Boylan AM, Jebb SA, Aveyard P. Experiences of reframing during self-directed weight loss and weight loss maintenance: systematic review of qualitative studies. Appl Psychol Health Well Being 2018;10:309-29. https://doi.org/10.1111/aphw.12132.
- Byers T. Food frequency dietary assessment: how bad is good enough?. Amer J Epidemiol 2001;154:1087-8. https://doi.org/10.1093/aje/154.12.1087.
- Biró G, Hulshof KF, Ovesen L, Amorim Cruz JA. EFCOSUM Group . Selection of methodology to assess food intake. Eur J Clin Nutr 2002;56:25-32. https://doi.org/10.1038/sj.ejcn.1601426.
- Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Behavioural Weight Management Review Group. Weight change among people randomized to minimal intervention control groups in weight loss trials. Obesity 2016;24:772-80. https://doi.org/10.1002/oby.21255.
Appendix 1 Participant interview topic guide
Appendix 2 Nurse interview topic guide
List of abbreviations
- AE
- adverse event
- BCTU
- Birmingham Clinical Trials Unit
- BISS
- Body Image States Scale
- BMI
- body mass index
- BWH
- Birmingham Women’s Hospital
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COREQ
- Consolidated Criteria for Reporting Qualitative Research
- CRF
- clinical report form
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRA
- Health Research Authority
- ICECAP-A
- ICEpop CAPability measure for adults
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- MET
- metabolic equivalent of task
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- POWeR
- Positive Online Weight Reduction
- PPAQ
- Pregnancy Physical Activity Questionnaire
- PPI
- patient and public involvement
- PRIME
- Public and Researchers Involvement in Maternity and Early Pregnancy
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- TFEQ
- Three-Factor Eating Questionnaire
- TSC
- Trial Steering Committee
- UoB
- University of Birmingham
- USB
- Universal Serial Bus
- WCSS
- Weight Control Strategies Scale
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25490).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
