Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/154/07. The contractual start date was in September 2017. The draft report began editorial review in October 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Cooke et al. This work was produced by Cooke et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Cooke et al.
Chapter 1 Introduction
Background
This research was undertaken in response to a commissioning brief from the National Institute for Health Research (NIHR) Health Technology Assessment programme. The brief requested an external pilot study to assess the feasibility of undertaking a substantive trial to assess the clinical effectiveness and cost-effectiveness of surgery compared with conservative management in patients with a stable thoracolumbar fracture without spinal cord injury. The brief requested inclusion of both high- and low-energy fractures.
Thoracolumbar fractures
Thoracolumbar fractures are the most common fracture of the spinal column, although limitations in the epidemiological data available have been highlighted. 1 An estimated 40–80% of these injuries are the result of a high-energy impact, such as car accidents, falls from a height and sporting activities (e.g. horse riding and skiing). 2 When the injury is high energy, thoracolumbar fractures are commonly associated with other injuries, such as rib fractures, pneumothorax (collapsed lung) and head injury. Based on a study of a Canadian population over 3 years, the incidence of spinal injuries was 64 per 100,000, with the injuries predominantly occurring in younger men and older women. 1 It is estimated that 10–30% of people with a thoracolumbar fracture also have spinal cord injury. 1
The potential consequences for people who experience a thoracolumbar fracture include pain, loss of function that has an impact on the ability to work and to undertake other activities of daily living, spinal deformity (kyphosis) and, in some cases, paralysis. 3,4 These symptoms may persist and become chronic conditions. 5
Fracture mechanisms
Knowledge of the anatomical constraints on movement within the thoracolumbar spine and how forces act on the spine helps us to understand the ways in which thoracolumbar fractures occur. The rib cage exerts a restraining effect on the thoracic spine, where intervertebral discs are thinner than in the lumbar spine, making the thoracic spine stiffer than the lumbar spine in sagittal and lateral flexion–extension. However, because of the positioning of the facets, rotation is greater in the thoracic spine than in the lumbar spine. The range of movements of the spine decreases with age.
The anatomy of the spine is described as having three columns: (1) anterior, (2) middle and (3) posterior. The anterior column consists of the anterior vertebral body, anterior annulus fibrosus and anterior longitudinal ligament. The middle column incorporates the posterior longitudinal ligament, posterior annulus fibrosus and posterior wall of the vertebral body. The posterior column consists of the posterior ligamentous complex, which is composed of the supraspinous ligaments, interspinous ligaments, articular facet capsules and ligamenta flava. 6 Where an external force is exerted on the spine will determine the type of resulting injury. These are described as:
-
compression fractures – forces act on the anterior column and bony fragments are not dispersed
-
burst fractures – axial forces act on the anterior and middle columns with or without posterior column involvement, and bony fragments are moved away from each other
-
wedge fractures – loading forces act on the anterior column while in flexion
-
fracture–dislocations – translation forces result in displacement of the adjacent vertebrae, leading to complete disruption of the spinal cord.
Stability
Fracture stability is important, as unstable fractures can lead to potential neurological issues (including paralysis, loss of sensation, loss of sphincter control) or loss of alignment and pain. Unstable fractures are those with associated disruption of the posterior ligament complex, or with movement or displacement of vertebral bodies in relation to each other.
Diagnosis require standing X-rays, which can demonstrate loss of alignment. Magnetic resonance imaging (MRI) of the spine is utilised to assess the posterior spinal ligament complex so as to assess fracture stability. Clinical assessment of neurological issues include identifying features such as weakness or numbness or sensory issues in the legs, and bladder or bowel issues (e.g. incontinence).
Stability is a key factor in the determination of fracture treatment. Kepler et al. 7 list the fracture types, in order of increasing instability, as compression, burst, flexion–distraction and fracture–dislocation. When the posterior ligamentous complex is intact, compression fractures are considered stable. Flexion–distraction and fracture–dislocation injuries are unstable as a result of disruption of the posterior column. Burst fractures can be either stable or unstable in the presence or absence of neurological injury.
Classification systems
There are a number of classification systems for thoracolumbar fractures, mainly based on fracture morphology and different concepts of stability, although there is no agreed system in use. 1 Their common aims are to categorise different injuries, to guide treatment decisions based on injury pattern, to provide a way of comparing modalities and to evaluate patient outcomes.
The Swiss Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification by Magerl et al. provides a comprehensive classification describing the nature of injury, the degree of instability and prognostic aspects that are important for choosing the most appropriate treatment. 8 Developed in 1994, the system identifies the following three major groups of fractures, based on the mechanism of injury:
-
compression (wedge, split or coronal, or burst)
-
distraction [distraction of the posterior soft tissues (subluxation), the posterior arch (extension spondylolysis) or the anterior disc (Chance fracture)]
-
multidirectional with translation [anterior–posterior (dislocation), lateral (lateral shear) or rotational (rotational burst)].
A new AO system was devised through consensus by an international team of five spine surgeons. 9 The new system is based on three main injury categories adapted from the original Magerl et al. AO concept, which are further classified into three major groups of increasing severity:
-
compression (wedge–impaction/split pincer/incomplete burst/complete burst)
-
tension band (divided into osseous and osseo-ligamentous disruptions)
-
displacement (hyperextension/translation/separation).
Based on the extent of injury to ligaments and discs, fractures are graded from definitely stable (e.g. pure impaction fractures, type A1) to definitely unstable, when there has been severe ligament and intervertebral disc damage. In this classification, incomplete burst fractures (type A3.1.1), in which there is partial damage to the ligaments and intervertebral discs, are classified as slightly unstable. 1
Another system for thoracolumbar fractures is the thoracolumbar injury classification and severity score (TLICS), also known as the thoracolumbar injury severity score. 10 Devised by the Spine Trauma Study Group, this system has been validated and shown to have good reliability. 11,12 It is a composite scoring system based on three injury components to assess spine injury and stability: (1) morphology of injury determined by radiographic appearance (between 1 and 4 points assigned), (2) integrity of the posterior ligamentous complex (between 0 and 3 points assigned) and (3) neurological status of the patient (between 0 and 3 points assigned). It is suggested that a patient with a total score of ≤ 3 is suitable for conservative management, those with a score of 5 should be with considered for surgery and those with a score of 4 can be considered for either treatment. 10
Earlier classification systems include the McCormack et al. 13 point system for load sharing classification, developed in 1994. In 1983, the Denis14 system classified compression, burst and fracture dislocations according to the direction of force causing the injury and the anatomical column involved. The 1970 Holdsworth classification15 is based on a two-column model, and the 1984 Ferguson and Allen classification16 was applied on the basis of the mechanical mode of failure of the vertebral bodies. These systems predate current imaging modalities, so are now rarely used.
High- compared with low-energy injuries
There is no single accepted definition of or method of distinguishing high- and low-energy injuries. According to National Institute for Health and Care Excellence (NICE) guideline CG176,17 high-energy injuries can result from being struck by a motor vehicle, ejection from a motor vehicle, a fall from a height of > 1 metre or more than five stairs, a diving accident, a high-speed motor vehicle collision, a rollover motor accident, an accident involving motorised recreational vehicles, a bicycle collision or any other potentially high-energy mechanism. A different study18 used a different criterion, of a fall from above or below approximately 3 metres, to distinguish high- from low-energy proximal humeral fractures. 18 In clinical practice, it is generally accepted that low-energy injuries are defined as those resulting from a fall from a standing height or lower, and high-energy injuries are any injuries sustained by a fall from above standing height or other high-energy accidents up to and including high-speed road traffic collisions. This definition has also been used in epidemiological studies of fractures. 19,20
In osteoporosis, changes in the quality and quantity of the bone at cellular level affect the structure of the surrounding tissue, thereby impeding the bone-healing process. To account for this, implants used in patients with osteoporosis are different from those used for normal fracture fixation and the screws may have to be supplemented with cement, as the implant purchase in bone is poor. A greater number of levels of vertebrae above and below the fracture may need to be included in instrumentation to account for the poor purchase. Patients may also require supplementary medicinal treatment for osteoporosis, which itself can also influence the bone-healing process. Both of the trials included in the Cochrane review of surgery compared with conservative treatment for thoracolumbar burst fractures21,22 as well as the more recent study comparing orthosis with no orthosis23 excluded this particular population.
The feasibility of including both high- and low-impact fractures was investigated in our study. Patients with osteoporosis were therefore included in the feasibility trial.
Treatment options
The key objectives of treatment are restoration of spinal alignment and spinal stability, preservation or improvement of neurological function and avoidance of collateral damage. 1 A key decision in the management of patients is whether they are treated surgically from the outset or given conservative treatment initially, with subsequent surgical intervention if clinical need arises.
Surgery
Currently in the UK, surgical treatment generally involves either open spinal surgery or minimally invasive surgery. Both procedures include placement of pedicle screws, through different surgical approaches. Open surgery sometimes includes bony fusion in addition to implant-based stabilisation. The screws can be placed through small incisions using X-ray guidance (minimally invasive technique) or a single, bigger incision (open technique) whereby the surgeon directly feels the track of the screw prior to inserting it. The segments of the spine above and below the fracture are fixed using this technique. This may involve one vertebral body up and one down, or more in the case of a long segment fixation. Short screws can also be placed at the level of the fracture if the surgeon feels that adequate purchase can be obtained at the fracture level.
Different approaches are described in the literature,24 and evidence (albeit mainly from non-randomised trials) suggests that both are effective approaches to the management of thoracolumbar fractures. 25
Conservative treatments
There are two main conservative treatments: (1) early mobilisation with bracing and (2) early mobilisation without bracing. A recent randomised controlled trial (RCT) in patients with an isolated thoracolumbar burst fracture with no spinal cord injury and a kyphotic deformity (abnormal curve of the spine) < 30° reported that patient function at 2 years, measured using the Roland–Morris Disability Questionnaire, was equivalent in those who were treated with early ambulation plus bracing with an off-the-shelf adjustable thoracolumbar sacral orthosis (TLSO) and those treated with early ambulation without a brace. 23 Currently in the UK, conservative management usually consists of early mobilisation and bracing with a TLSO, although practice does vary across centres and individual patients. Some patients’ preference and lifestyle may preclude bracing, and bracing may not be suitable for patients with a high body mass index or skin problems, or in the presence of chest injuries and rib fractures.
A retrospective cohort study26 of patients with a burst fracture without neurological involvement or osteoporotic fracture from a UK centre described conservative management. The study encouraged patients from the outset to log roll while in bed, then inclined patients to 30°, 60° and then 90° of flexion at the hip joint in a split bed over approximately 3 days to achieve truncal stability. Patients were then mobilised out of bed and undertook supervised walking without an orthosis. A Jewett brace was provided on discharge and patients were followed up 1 week and 3 months after discharge, which included radiography to assess the extent of kyphosis. 26 Another UK study27,28 refers to an aggressive physiotherapy protocol with regular bed rolling, in-bed exercises and chest protocols, with patients assessed for brace tolerance.
An important possible advantage of conservative management is avoiding the potential risks and morbidity associated with surgery. These include infection, bleeding and damage to the adjacent structures, such as nerves, blood vessels and tendons. The intervention costs are also substantially less with the conservative option. However, there is a concern that without surgical stabilisation of the spine there is the risk of late neurological complications. 2 There is also a view that surgery helps prevent later abnormal curvature of the spine (kyphosis), although there is uncertainty about this, as well as about the relationship between kyphosis and pain and functional impairment.
Patient management
Both patients being managed surgically and those managed conservatively attend a fracture clinic regularly (generally at 2 and 6 weeks, although sometimes at 12 or 26 weeks and up to 2 years if they have had a spine fusion). At these visits standing X-rays are taken to monitor progress in terms of healing and bone formation around the fracture, to detect progression of kyphosis and the presence of pain. If there is healing of the fracture and absence of pain at 12 weeks, patients are usually discharged. If this is not the case, other treatments and procedures may be required, such as physiotherapy, facet joint injections or radiofrequency thermocoagulation of the medial branch nerves to the facets, or consideration for surgical intervention (e.g. vertebroplasty or spinal fixation). Patients may be offered surgical intervention at any point if they experience progressive deformity, uncontrolled pain or neurological change. Surgical interventions are the same as those detailed above, with the additional options of anterior reconstruction through a number of surgical approaches.
Previous research
There appears to be informal consensus that simple compression fractures without neurological complications can be managed without surgery,2 and this is reflected in UK practice. Similarly, obviously unstable fractures causing neurological damage or an elevated risk of damage do require surgical fixation. However, there is variation across surgeons and centres in whether surgical or conservative approaches are used. There is no clear evidence as to the most effective treatment in terms of pain reduction, speed of recovery and return to normal activities, or prevention of kyphosis and any associated problems with chronic back pain and balance. The boundaries of this zone of uncertainty are unclear, although using the TLICS system this would probably encompass patients scoring 4 (i.e. either treatment is considered an option). In the trials included in the Cochrane review,29 comparing surgical with conservative management, patients had an AO type A compression fracture of the 10th thoracic vertebra (T10) to the fourth lumbar vertebra (L4)21 or a burst fracture of T10 to the second lumbar vertebra (L2) without neurological deficit. 22
The Cochrane review29 included two trials that, in total, reported outcomes for 79 out of 87 participants at a follow-up of at least 2 years. The two trials reported conflicting results for pain- and function-related outcomes at final follow-up, and for numbers returning to work. Based on a visual analogue scale (VAS), one trial found less pain, whereas the other trial reported more pain, in the surgical group. Based on the Roland–Morris Disability Questionnaire results, one trial reported better function, whereas the other trial reported worse function, in the surgical group. Both trials reported more participants with complications in the surgical group [21/41 vs. 6/38; risk ratio 2.85, 95% confidence interval (CI) 0.83 to 9.75; two trials] and only participants in this group had subsequent surgery, involving implant removal either because of complications or as a matter of course. One trial reported that surgery was over four times more costly than non-surgical treatment (US$49,063 vs. US$11,264, respectively). The difference in cost between the two treatment groups was highly significant (p < 0.01). 22
Both RCTs were small and were assessed as potentially biased. The review authors29 therefore felt that there was insufficient evidence to say whether surgical or non-surgical treatment yields superior pain and functional outcomes for people with thoracolumbar burst fractures without neurological deficit. The authors suggested that it is likely, however, that surgery is associated with more early complications and the need for subsequent surgery, as well as greater initial health-care costs. The authors’ conclusion was that there is a need for a large, multicentre, high-quality and adequately reported RCT of such interventions for these types of fracture. 29 Another systematic review of complication rates,30 carried out around the same time and with broader study design eligibility criteria, similarly concluded that because of the paucity of high-quality studies no conclusions could be drawn about the relative effects of the two management approaches and that RCTs are required. A more recent review of AO type A3 and A4 fractures (neurologically intact) did not identify any new RCTs. 31
Previous systematic reviews identified the need to conduct a RCT to compare the effectiveness of surgical and conservative management of stable thoracolumbar fractures. However, there is uncertainty if such a trial would be feasible in the UK, mainly because of unknown surgical and patient equipoise, variation in use of fracture classification systems and problems in defining the appropriate population.
Aims and objectives
The project aimed to address the commissioned research question and establish whether or not it is feasible to deliver a trial comparing the clinical effectiveness and cost-effectiveness of surgical fixation with initial conservative management for patients with a stable thoracolumbar fracture without spinal cord injury. We undertook a randomised controlled external feasibility trial; a national survey of spine surgeons; a qualitative study with clinicians, recruiting staff and patients; and a costing analysis to address the following specific questions:
-
Are surgeons willing to randomise eligible patients and adhere to randomisation to (1) surgical fixation or (2) initial conservative management?
-
Are patients willing to be randomised and adhere to randomisation in a trial comparing the two treatments?
-
What is the completeness of follow-up in this population?
-
Are there a sufficient number of centres and surgeons (with sufficient caseloads of eligible patients) willing to participate in a future RCT to make the trial feasible within a viable time scale?
-
What methods of establishing spinal stability and suitability for surgery or conservative management are currently used?
-
What methods of surgical fixation and conservative management are currently being used?
-
What are the barriers to successful delivery of the future trial and how can they be overcome?
-
Can the British Spine Registry (BSR) be used to collect participant data in a trial?
-
What is the most suitable primary end point for a main trial?
-
How can we accurately identify, quantify and value economic data to capture the impact of the two treatments from both an NHS and societal perspective?
The feasibility trial assessed questions 1–3 and 7–10. Questions 1, 4–7 and 9 were assessed using the survey of spine surgeons, whereas the qualitative interviews addressed questions 2, 7 and 9.
Chapter 2 Trial design and methods
Overview
To address the research questions, we undertook the Pragmatic Randomised Evaluation of Stable Thoracolumbar fracture treatment Outcomes (PRESTO) study. The study consisted of (1) a randomised controlled external feasibility trial of surgical fixation compared with initial conservative management for patients with a stable thoracolumbar fracture without spinal cord injury; (2) a qualitative study with clinicians, recruiting staff and patients; (3) a national survey of spine surgeons; and (4) a costing analysis.
Feasibility randomised controlled trial
Study participants and setting
Patients were eligible to participate in this study if they were aged ≥ 16 years and had a diagnosis of a high- or low-energy thoracolumbar vertebral body fracture, between T10 and L2, confirmed by radiography, computerised tomography (CT) or MRI, and met at least one of the following criteria:
-
a kyphotic angle > 20° on weight-bearing radiographs, or > 15° on supine radiographs or CT
-
reduction in vertebral body height of 25%
-
fracture line propagating through the posterior wall of vertebra
-
involvement of two contiguous vertebrae
-
injury to the posterior longitudinal ligament or annulus in addition to the body fracture.
Patients were excluded if they had an unstable fracture requiring surgical stabilisation, a spinal cord injury or a pathological (other than osteoporotic) fracture (e.g. tumour/infection) or if they were considered not suitable for surgery.
The eligibility criteria used for the feasibility trial were carefully constructed by consultation and agreement between surgeons who would normally recruit to the trial, to address the commissioning brief. The challenges in defining the eligibility criteria for a multicentre trial were the variation in definition of stability and lack of a universally accepted classification system for the assessment of thoracolumbar fractures. Additional information was collected when patients had been recorded as ineligible because they had an unstable fracture requiring surgical stabilisation.
Patients fulfilling the trial inclusion criteria were enrolled from three participating secondary care centres: Barts Health NHS Trust, Leeds Teaching Hospitals NHS Trust and Cardiff & Vale University Health Board. These centres were chosen as they are in different geographical regions, increasing the generalisability and applicability of any study findings across the UK. Having a range of centres also gave an insight into the differing ways in which eligible patients are managed in different hospitals.
The three participating sites were all major trauma centres (MTCs) and, via the local trauma networks, linked to other trauma units or local hospitals that may or may not be part of the same hospital trust. Patients with thoracolumbar fractures who presented to other trauma units or hospitals within the networks would be transferred to the MTC if they triggered a secondary transfer protocol; these protocols may differ across regions and systems. Patients being managed at outlying hospitals were not considered for the trial unless their clinical pathway meant that they would be transferred to one of the participating sites.
Trial interventions
Conservative management (control) group
Conservative management in the control arm consisted of mobilisation in a brace or mobilisation without a brace, as recommended by the treating surgeon in consultation with the participant. The use and discontinuation of the brace was decided, as per usual practice, by the presence or absence of pain at the fracture site on mobilisation, but it is also possible that a brace was applied for a predetermined prescribed period. The brace could be any orthotic device that supports the spine above and below the level of the fracture, such as a TLSO, as considered appropriate by the treating surgeon.
Surgical fixation (intervention) group
In accordance with current UK practice, surgical treatment could be open spinal surgery (with or without spinal fusion) or minimally invasive stabilisation surgery. Both procedures involve placement of pedicle screws, but through different surgical approaches. The objectives of surgery in the population of patients with a stable thoracolumbar fracture without neurological deficit are early mobilisation without a brace, early return to full functionality, improved quality of life (QoL) and a reduction in patient follow-up required to assess fracture collapse.
Open pedicle screw fixation
A midline approach is most commonly performed for open pedicle screw fixation. Blood loss is minimised with diathermy dissection and careful haemostasis using bipolar and haemostatic agents. The junction between the pars interarticularis, lateral hemi-facet and transverse process is identified and a starter awl, then pedicle finder, is negotiated. A pedicle screw is placed within the pedicle, not too medially (potentially encroaching the spinal canal) or too laterally (potentially breaching the lateral wall and reducing the screw pull-out strength). Confirmation of screw placement is undertaken by direct palpation using a feeler, and further confirmation can be achieved with intraoperative fluoroscopic imaging. Following screw placement, rods are locked into the screwheads, stabilising the motion segment. Screws are placed in the unfractured vertebrae on either side of the fractured one(s).
The surgeon may then decide to perform a spinal fusion by decorticating the bony surfaces and placing local bone graft, and may use bone substitutes as graft extenders. If surgical stabilisation alone is performed, decortication and graft placement is not undertaken.
Percutaneous pedicle screw fixation (minimally invasive surgery)
This follows the same principles. However, it is performed via multiple stab incisions on either side of the midline and is guided by intraoperative fluoroscopy. The advantages of percutaneous fixation are reduced trauma to the soft tissues, reduced blood loss and possibly reduced surgical time. Usually only surgical stabilisation can be undertaken with this technique, and not spinal fusion.
Surgeon preference dictates the surgical approach used on a case-by-case basis. In open pedicle screw fixation, the most common approach is a midline one. Blood loss is minimised with diathermy dissection and careful haemostasis using bipolar and haemostatic agents. The junction between the pars interarticularis, lateral hemi-facet and transverse process is identified. Once confirmed on fluoroscopy, a guide wire is placed through a cannulated needle and a pedicle screw is then sited within the pedicle over the guide wire, not too medially (potentially encroaching the spinal canal) or too laterally (potentially breaching the lateral wall and reducing the screw pull-out strength). Following screw placement, rods are locked into the screwheads, stabilising the segment. Reliable fusion cannot be performed using this approach, only stabilisation.
Rehabilitation
Patients in both the surgical and conservative groups received physiotherapy as per routine care. Details such as number of sessions and advice given by the physiotherapist were recorded.
All care (e.g. analgesia) received by participants was in line with routine practice at the participating site. Participants were requested to attend any routine clinical appointments that were scheduled outside trial visits in line with the routine care pathway at the participating site.
Imaging assessments
The routine imaging performed on patient admission was used to confirm eligibility. No trial-specific imaging was undertaken, but information on the routine imaging undertaken for trial participants was recorded.
Primary outcome
The primary outcome was recruitment rate, defined as the proportion of eligible participants who were randomised.
Secondary outcomes
To investigate feasibility, we collected data on the following.
Recruitment
-
The number of eligible patients.
-
The proportion of eligible patients approached for consent.
-
The proportion of eligible patients not approached for consent and reasons why.
-
The proportion of patients approached who provided consent.
-
The proportion of patients approached who did not provide consent and reasons why.
Randomisation
-
The proportion of patients providing consent who were randomised.
-
The proportion of patients randomised who did not receive the randomly allocated treatment and reasons why.
Crossover
-
The proportion of patients randomised to the conservative treatment who received surgical management, at what time point and reasons why.
Dropout
-
The proportion of patients dropping out between randomisation and follow-up at each time point and reasons why.
Ability to collect clinical outcome measures
-
The proportion of complete data for each outcome measure and the proportion of successfully gathered data through the BSR to assess the feasibility of gathering patient-reported outcome measures (PROMs) and other outcome measures at baseline and follow-up.
-
The proportion of complete data to assess the feasibility of gathering data on complications and adverse events (AEs).
Outcome measures relating to trial participants
Table 1 outlines the time points when the patient outcomes were assessed. Participants were followed up at 2 weeks and at 3 months post randomisation. Additionally, all patients recruited in the first 9 months of recruitment were assessed 6 months post randomisation. The outcome measures are described as follows (mode of collection is described in Data management).
| Time point | Study period | |||||
|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post allocation | ||||
| Baseline (pre randomisation) | Randomisation | Intervention delivery | Week 2 | Month 3 | Month 6a | |
| Enrolment | ||||||
| Eligibility screen | ✗ | |||||
| Informed consent | ✗ | |||||
| Demographics | ✗ | |||||
| Allocation | ✗ | |||||
| Intervention | ||||||
| Conservative | ✗ | |||||
| Surgery | ✗ | |||||
| Assessment | ||||||
| ODI | ✗ (pre and post injury) | ✗ | ✗ | |||
| VAS for pain | ✗ (post injury) | ✗ | ✗ | |||
| SF-12 | ✗ (pre injury) | ✗b | ✗b | |||
| EQ-5D-5L | ✗ (pre and post injury) | ✗ | ✗ | |||
| Patient and surgeon preferences | ✗ | |||||
| Sagittal plane kyphosis | ✗ | ✗ | ✗ | ✗ | ||
| Treatment Information | ✗ | ✗ | ✗ | |||
| Basic health economics data (i.e. health-care resource use) | ✗ | ✗ | ||||
Oswestry Disability Index
The Oswestry Disability Index (ODI) is a commonly recommended PROM for low back pain and spinal surgery,32–34 and is part of the outcome set used by the BSR. It assesses limitations across 10 aspects of daily living (pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life and travelling) and is scored on a scale from 0 to 5, where 0 indicates the least amount of disability. 35 The total score can range from 0 to 100 (higher scores indicate higher levels of disability).
At baseline, a modified version of the ODI was used (with the permission of EuroQol Research Foundation) to capture retrospectively the participant’s index score in the week prior to their injury, in addition to the validated version, which requires participants to provide a score ‘today’. Pre- and post-injury scores were obtained to enable evaluation of the extent to which patients had achieved pre-injury health status.
Visual analogue scale for pain
The VAS is a unidimensional measure of pain intensity which has been widely used in diverse adult populations. 36,37 We used a continuous 11-point scale, anchored by two verbal descriptors, with 0 representing ‘no pain’ and 10 representing ‘worst imaginable pain’, to measure average pain. Participants scored their pain on the day of completion of the questionnaire.
Short Form questionnaire-12 items
The Short Form questionnaire-12 items (SF-12) is a 12-item, generic and widely used measure of physical and mental health completed by the participant. The population norms are a mean of 50 and standard deviation (SD) of 10 (higher scores indicating better health). 38 The rationale for including the SF-12 was that it is feasible that a delay in return to work and recreational activities could have an impact on participants’ ability to perform other daily activities and their emotional well-being.
The validated version of SF-12 was used, as modifications are not permitted. The measure asks participants to recall health status over the last 4 weeks and, therefore, baseline SF-12 score represents pre-injury health status.
EuroQol 5 Dimensions, five-level version
The EuroQol 5 Dimensions, five-level version (EQ-5D-5L), is a validated, generic PROM. 39 The descriptive system has five health domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with five response options for each domain (no problems, slight problems, moderate problems, severe problems and extreme problems). In addition, it has a health status VAS that measures self-rated health, with end points of ‘the best health you can imagine’ and ‘the worst health you can imagine’.
Some patients taking part in the trial may not have had capacity to provide complete baseline data. There is a separate proxy version of the EQ-5D-5L. However, a recent systematic review suggested that proxy completion in emergency and critical care settings does not generally give an accurate estimate of patients’ EQ-5D-5L. 40,41 Therefore, to minimise bias in quality-adjusted life-year estimation, patients who lacked capacity were to complete the baseline EQ-5D-5L at the earliest opportunity once capacity was regained.
At baseline, a modified version of the EQ-5D-5L was used (with the permission of EuroQol Research Foundation) to capture the participant’s score in the week prior to their injury, in addition to the validated version, which requires participants to rate their health ‘today’. Pre- and post-injury scores were obtained to enable us to evaluate the extent to which patients had achieved their pre-injury health status.
Sagittal plane kyphosis
Kyphotic angulation is considered to be a sign of instability. Therefore, the kyphotic angle was measured using the Cobb technique. This involves measuring the angle between two lines parallel to the superior and the inferior end plates adjacent to the fractured vertebrae on digital radiographs or on CT images on the picture archiving and communication system. If CT was considered, a sagittal section was taken through the mid-axial line.
Complications and adverse events
Information on all complications, additional surgery and AEs was collected in line with a study-specific standard operating procedure. Expected complications recorded included, but were not limited to, death within 30 days of the procedure, neurological complications, deep wound infection, superficial infection, rehospitalisation, implant failure, screw pull-out, reoperation and skin problems.
Other outcomes
Other outcomes measured were resource use (e.g. length of hospital stay), time to return to work and whether individuals returned to their previous job or to a less physically demanding role or required any job modifications, such as returning on reduced hours, and time to return to normal activities (e.g. volunteering, sports, hobbies).
Data collection using the British Spine Registry
The BSR is a web-based database established by the British Association of Spine Surgeons (BASS) in 2012 to collect information about spinal surgery in the UK. The purpose of the registry is to improve patient safety and to monitor the outcomes of spinal procedures by collecting data from clinicians and patients, to better understand procedures and techniques in use, and understand a patient’s experience and their expected QoL.
Patients provide consent to allow surgeons to enter details of their diagnosis, surgical procedure, complications and outcomes after surgery. In addition, patients are asked to provide consent for specific data, collected via the BSR, to be used in research studies.
At intervals after the spinal procedure, patients are e-mailed links to online questionnaires containing PROMs to complete.
We worked with the provider of the data collection platform from the BSR to utilise their system to collect patient-reported outcome data (ODI and EQ-5D-5L patient outcomes, together with the trial-specific resource use questions) online for participants who agreed to receive questionnaires at 3 and 6 months via e-mail links. The aim was to assess the viability (measured by proportion and completeness of data) of this method of data collection in any future definitive trial with the target of maximising efficiency in data collection, as the BSR is already in routine use in hospitals to collect information on spine patients. The data obtained via this means would be used for the trial and also submitted to the spine registry.
A postal, followed by telephone back-up, system was implemented when data were not returned via the registry (see Data management).
Participant timeline
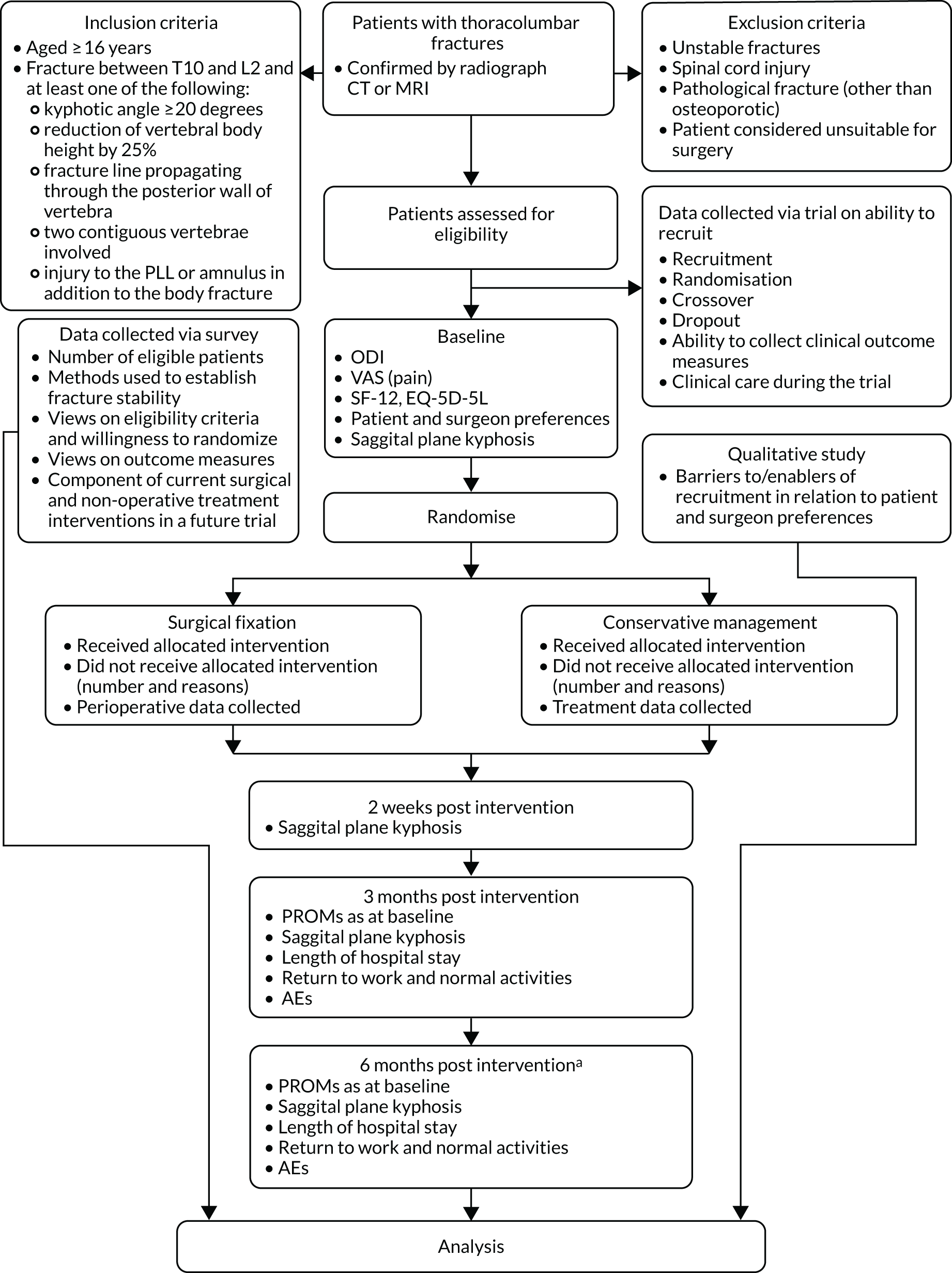
Figure 1 illustrates the process of enrolling participants into the study, the interventions compared and the timing of assessments.
FIGURE 1.
Study flow chart. a, Only those patients who reach 6-month follow-up during period. PLL, posterior longitudinal ligament.
Sample size
Based on initial discussions with the three participating centres, it was estimated that there would be at least 120 eligible patients during the 12-month recruitment period. A recruitment rate of 50% eligible patients is considered likely for trials of this type, which indicated a sample size of 60 patients. The identification of 120 eligible patients allowed an estimation of a participation rate of 50% to within a 95% CI of ± 9%. This was in line with the guidance for the sample size of feasibility and pilot studies, which suggests that there should be at least 12 participants in each arm of the study at the analysis stage. 42
Recruitment
All patients diagnosed with a thoracolumbar vertebral body fracture between T10 and L2 were assessed for eligibility. The research teams were encouraged to work closely with the surgeons at each centre to optimise the screening and recruitment procedures for their local circumstances.
Patients were provided with written information [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)] and given the opportunity to discuss and ask questions about the trial with research staff and their treating surgical team prior to making a decision on participation. Following feedback from initial interviews with trial participants as part of the qualitative research, site staff were advised to involve any relatives and friends present in these informed consent discussions. Patients did not have to provide consent immediately. If required, they were given time to discuss further with friends and family. They were given the contact details for the site principal investigator (PI), research associates and also an independent contact (e.g. Patient Advice and Liaison Service) whom they could contact for general advice on research participation. Following confirmation of eligibility by the treating surgeon, written informed consent was obtained by research staff.
The PI at each participating site was asked to confirm agreement with the assessment of eligibility for patients evaluated by other clinicians to ensure that the criteria were being applied consistently among surgeons. This additional assessment was performed following randomisation and was not to have any bearing on patient status, but performed to measure agreement.
When patients gave consent [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)], they were asked to complete a baseline form [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)], which captured demographic, mechanism of injury and the patient-reported outcomes information as well as information on any treatment preference. Surgeons were also asked to complete a baseline form that included the calculation of TLICS and AO classification scores [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. Site staff then contacted York Trials Unit (YTU), either by telephone or via the internet, to access the secure randomisation service. Participants were free to withdraw from the study at any time without affecting their care.
Throughout the study, screening logs were kept at each site to capture the number of patients assessed for eligibility and document reasons for exclusion. Patients who declined to participate or withdrew from the study were given the opportunity to discuss or inform the research team of the reason for their decision not to take part and also their treatment preference. Patients who declined to participate were invited to take part in the qualitative interviews.
Strategies for achieving adequate participant enrolment to reach the target sample size included seeking advice from patient representatives, sharing best practice with site staff, real-time feedback from qualitative interviews and regular discussion with PIs. Site research staff were encouraged to engage with other specialties, such as neurosurgery, when these staff may have had contact with potential trial participants. Posters were created for display in staff areas to encourage staff to notify appropriate research staff if they identified a potentially suitable patient [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)].
Site staff were provided with training at the site initiation visits to ensure adherence to protocol, including the delivery of the interventions in the trial, as well as discussing site-specific recruitment strategies. During the trial, training and reminders were implemented using e-mail bulletins and discussion with the PIs, and regular teleconferences were held with research associates to facilitate the sharing of good practice and to encourage staff to discuss local issues with staff from other sites so that solutions might be found. In addition, the trial co-ordinators provided support and guidance to staff as required (e.g. when new staff joined or replaced existing site staff) and sought clinical guidance from the chief investigator when necessary.
The NIHR associate PI scheme was implemented at two of the three sites to try to encourage surgical trainees or trainees from other specialties to engage with the study, and to help with identification and screening activities at the sites. The third site already utilised surgical trainees in this way.
Consent
An appropriately delegated member of the research team obtained written consent from all patients considered able to make an informed decision about their participation.
We anticipated that some potential participants would be unconscious or distracted by their injuries or have had large doses of pain relief and would, therefore, lack capacity to make an informed decision about participation. In these cases, an appropriate method, in line with the Mental Capacity Act43 and as approved by the Research Ethics Committee (REC), was used to gain prospective written agreement for the patient to be included in the trial from a consultee. This could be either a personal consultee, who could be the patient’s next of kin, relative or friend, or a nominated consultee, who could be a clinician responsible for the patient’s care but not directly involved in the running or design of the trial. A personal consultee would be sought in the first instance, but if they declined to act as a consultee or if none could be identified then a nominated consultee would be approached. The consultee (personal or nominated) was provided with an additional information sheet that explained the duties of a consultee [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)] and this was read in conjunction with the main participant information sheet.
When a personal or nominated consultee provided written agreement to participate on behalf of a participant [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)], written consent to continue in the study was to be sought from the participant at the first appropriate opportunity once they regained capacity after being given written information about their continued participation in the trial [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. In the interim, best efforts were to be made to involve participants who temporarily or permanently lacked capacity in the decision about whether or not to continue to take part in the study.
Randomisation and blinding
Stratified block randomisation (permuting lengths of 2, 4, 6 and 8) with stratification by centre and type of injury (high-energy trauma or low-energy osteoporotic) was used to allocate participants on a 1 : 1 basis to surgery or conservative treatment.
The type of injury (high- or low-energy) was determined in the first instance by research staff during assessment of patient eligibility. The mechanism of injury was recorded by patient self-report. All eligibility data were confirmed by the treating surgeon prior to informed consent and randomisation.
After patients had given consent and their baseline forms had been completed, the research associate or recruiting surgeon randomised them using the YTU’s secure, web-based randomisation service and, therefore, ensured allocation concealment and immediate unbiased allocation.
As the trial compares surgery with conservative treatment, blinding of participants, surgeons and outcome assessors to treatment allocation was not possible.
Data management
Paper case report forms [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)] were used to record all the information required from the protocol, with the exception of PROMs at 3 and 6 months. ODI, EQ-5D-5L, VAS and resource use questions for patients who agreed to receive questionnaires via e-mail were obtained via the BSR and downloaded electronically at the YTU. Participants were given 3 weeks to respond via the registry platform, and during this period automated e-mail reminders were sent from the system to encourage completion. If responses were not received after 3 weeks, a postal copy of the questionnaire was sent to the participant. Questionnaires were posted to participants who did not agree to receive them by e-mail. When responses to the initial postal questionnaires were not received after 3 weeks, a postal reminder letter was sent, along with a further copy of the questionnaire. If reminder questionnaires did not elicit a response, attempts were made to contact participants by telephone to collect the outcome data.
The SF-12 measure was not collected via the registry platform, as this PROM is not collected as part of the registry data set. Furthermore, the licence holder’s requirements with regard to data ownership meant that SF-12 data collected electronically would be transferred and stored outside the European Union. This requirement could not be met within the terms of our regulatory approvals but could be considered for a main trial.
All data were completely anonymised for the analysis and subsequent reports and publications. For the purposes of ongoing data management, once randomised, individual participants were identified only by trial identification numbers, to maintain confidentiality.
All YTU data recorded electronically were held in a secure environment at the University of York, with permissions for access in line with YTU standard operating procedures. All paper study documents held at the YTU were retained in a secure location for the duration of the trial. Identifiable information was held separately from any clinical data. All essential documents, including source documents, are being retained for a minimum period of 5 years from study completion.
Statistical analysis of feasibility randomised controlled trial
A detailed statistical analysis plan was agreed with the combined Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) prior to completion of data collection. The statistical analysis plan underwent a minor revision to correct the calculation of SF-12 outcomes [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)].
A single analysis was performed at the end of the trial using Stata® v15 (StataCorp LP, College Station, TX, USA). As this was a feasibility study, no formal statistical testing was to be undertaken. Baseline data were summarised by trial arm as randomised, with no formal comparisons between the groups. Continuous data were reported descriptively (mean, SD, median, minimum, maximum and number missing), and categorical data by counts and percentages.
The recruitment rate was reported monthly and overall and broken down by hospital site. The number of eligible patients was summarised using counts and percentages.
The following were also reported: the proportion of eligible patients approached for consent; the proportion of eligible patients not approached for consent; the proportion of eligible patients approached who consented; the proportion of patients who did not provide consent; the proportion of participants providing consent who were randomised; the proportion of participants randomised who did not receive the randomly allocated treatment; the proportion of participants who crossed over from conservative treatment to surgery and at what time point; and the proportion of participants dropping out between randomisation and follow-up.
Interim analysis
There were no planned interim analyses for the feasibility trial and no stopping guidelines.
Costing analysis
The costing analysis aimed to identify data that would be needed for an economic analysis of a full-scale trial. Individual participant data from the trial were used to evaluate resource use, costs and health outcomes associated with the interventions.
The acceptability of resource use questionnaires to capture the impact of care on the NHS and productivity was assessed. The costing approach was conducted from the NHS and Personal Social Service perspectives. Health service resource use was collected prospectively during the study using self-reported questionnaires and hospital forms at 3 and 6 months. Cost components comprised all initial and subsequent inpatient episodes, outpatient hospital visits, accident and emergency (A&E) hospital admissions and primary care visits [e.g. general practitioner (GP), nurse and physiotherapy]. The total resource use was calculated for each participant in both groups for the duration of the study. Health-care resource use is presented for both arms as mean value, SD and mean difference (with 95% CI) between the groups. Unit costs were derived from established national costing sources, such as NHS reference costs,44 Personal Social Services Research Unit Unit Costs of Health and Social Care 201745 and the British National Formulary. 46
The health-related quality of life of participants was measured using the EQ-5D-5L at baseline, 3 and 6 months. The aim was to display raw EQ-5D-5L scores according to domain, to examine the movements between levels for each domain according to the trial arm. Health-related quality of life values were to be estimated using the mapping function by van Hout et al. ,47 in accordance with the recent NICE statement on the use of the EQ-5D-5L. 48
The number of missing economic data and their nature were explored to guide (1) the sources to be used for the primary and secondary analysis in a full study and (2) the imputation approach for a definitive trial.
Ethics approval and monitoring
Standard NHS cover for negligent harm was available. There was no cover for non-negligent harm.
Ethics committee approval and any changes to the project protocol
The PRESTO trial (feasibility RCT, qualitative aspects and survey of spine surgeons) was approved by North East – Newcastle & North Tyneside 1 REC on 20 March 2018 (REC reference 18/NE/0008) [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. Health Research Authority approval was also received on 20 March 2018. Confirmation of capacity and capability was given by the research and development department of each participating site.
The current study protocol has been provided via the NIHR Journals Library [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)] and a summary of the changes made to the protocol and key trial documents since the original REC approval have been listed.
Trial oversight
The day-to-day management of the trial was the responsibility of the trial manager or co-ordinator, based at YTU and supported by other relevant members of unit staff. The trial statistician and health economist were closely involved in setting up data capture systems and forms.
The Trial Management Group (TMG) was the executive decision-making body and was responsible for overseeing the day-to-day running and management of the trial. The TMG met monthly during the recruitment period and then at approximately 3-month intervals according to the needs of the study.
As this was a low-risk, feasibility study with no planned interim analyses for either futility or safety, approval was obtained from the funders to set up a combined TSC and DMEC to undertake the roles traditionally undertaken separately by the TSC and the DMEC. The combined TSC and DMEC adopted a DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics, Statistics) charter,49 which defined its terms of reference and operation in relation to independent trial oversight and advised on strategies to preserve the integrity of the trial when required. The combined committee met biannually, with the option to meet more frequently if the committee requested. Membership is listed in Acknowledgements.
Qualitative study of patient, surgeon and recruiting staff views
A qualitative study was undertaken to explore patients’, surgeons’ and local recruiting staff’s views and experiences of the interventions and trial processes. Particular attention was given to exploring perceived and experienced barriers to and facilitators of recruitment and retention that could be used to inform the design of a full-scale trial.
Design
The qualitative study had three components: (1) interviews with patients who agreed to take part in the trial, (2) interviews with patients who declined participation and (3) interviews with PRESTO trial recruiters (surgeons, physiotherapists, research nurses) and surgeons from across the UK. All interviews were semistructured and were conducted over the telephone during the trial’s 12-month recruitment phase.
Sampling
Patients
In the protocol we specified that we would purposively sample up to 25 patients: 8–10 patients from each intervention arm and five patients who declined participation in the PRESTO study. To achieve maximum variation, we proposed sampling on the basis of age, gender, trial site and treatment received. However, recruitment to the main trial, and therefore to the qualitative study, was far lower than anticipated. Approval for a protocol amendment was obtained to change to adoption of a convenience sampling frame, with selection based on those who agreed to take part in the qualitative study.
Health-care professionals and recruiting staff
Snowball sampling was used to ensure that a wide range of PRESTO trial recruitment staff and surgeons from across the UK were invited to interview. This approach is considered appropriate when the number of experts in the field is relatively small and so many surgeons are known to each other. Although snowballing techniques were used, we were mindful that, to capture a range of views on current treatment of thoracolumbar fractures and the potential barriers to and facilitators of running a full-scale trial, we would need to interview trial recruiters and surgeons who represented different geographical locations, specialties (orthopaedics, neurosurgeons) and grades (consultants, registrars). We therefore employed a number of recruitment strategies to ensure that the sample was varied.
Recruitment
Patients
After being approached to take part in the PRESTO feasibility trial, all patients, irrespective of whether they agreed or declined to participate in the PRESTO study, were invited to take part in the qualitative study and were provided with a separate participant information sheet and consent form [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. A contact details form was also completed and transferred to the qualitative research team for participants who had declined to take part in the trial. All participants who expressed an interest in the qualitative study were informed that a qualitative researcher (AS) would contact them by telephone to arrange a time for the interview to take place. Informed consent was gained at the study site, with consent forms transferred to the qualitative research team prior to the start of each interview.
Health-care professionals and recruiting staff
A range of methods were used to recruit surgeons and trial recruitment staff to the qualitative study. The PI and trial recruitment team at the three PRESTO study sites were sent an e-mail inviting them to interview. PIs were also asked to forward the initial recruitment e-mail to any surgical colleagues, both in and outside participating PRESTO study sites. Recruitment e-mails were also sent to those who expressed an interest in taking part in the qualitative study through the survey of spine surgeons (n = 19). To improve recruitment, an advertisement was placed in the BASS newsletter and PIs were asked at TMG meetings and via e-mail throughout the PRESTO study’s recruitment period to use their professional networks to identify surgeons throughout the UK who may be willing to participate. Lastly, individuals who took part in the qualitative study were asked to forward initial recruitment e-mails to colleagues. This led to an advert being placed on a regional network of spinal surgeons. Surgeons and trial recruiters were provided with written information about participating in an interview for the PRESTO study and provided written consent [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)].
When recruiting patients, trial recruiters and surgeons, a maximum of three reminder e-mails and telephone calls were placed per individual.
Data collection
All interviews were conducted via telephone, were semistructured and followed a topic guide [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. The topic guides provided a framework for the interviews and ensured that all participants were asked the same questions. However, given the semistructured nature of the interviews, topic guides were used flexibly to enable probing or other key issues to emerge. All interviews were audio-recorded and transcribed prior to analysis.
Patients
Prior to the start of each interview, participants were reminded of the study’s aims, were assured anonymity and confidentiality, and were given the opportunity to ask questions. During interviews, patients were asked how they found being approached to take part in the trial, how they felt about the randomisation process, and their views and experiences of their treatment and clinical follow-up.
Health-care professionals and recruiting staff
Interviews with trial recruiters and surgeons focused on current treatment of stable thoracolumbar fractures, paying particular attention to individuals’ treatment preferences and whether or not they felt that practice could change as a result of the findings of a main trial. Participants’ views on the barriers to and facilitators of running a full-scale trial and surgeons’ willingness to randomise were also explored. Surgeons and trial recruiters who were based at PRESTO study sites were also asked how they found approaching and consenting patients to the trial.
Data analysis
To ensure a systematic approach, thematic analysis was adopted using the stages outlined by Braun et al. :50 familiarisation, generating initial codes, searching for themes, reviewing themes, defining and naming themes, and data reporting. Following familiarisation, an initial coding framework was developed with descriptive coding based on a priori themes outlined in the topic guide. 50 The initial coding framework was then refined a number of times, to allow for emerging themes and to ensure that the framework addressed the aims of the qualitative study outlined in the study protocol. Unique identification codes were assigned to participants for the reporting of the results.
In addition to the formal analysis, initial impressions were noted following each patient interview, with any emerging issues relating to recruiting and consenting fed back to the trial manager and, when appropriate, the TMG and/or participating sites. In all instances, feedback was anonymised. However, verbal agreement was sought from participants to any issues raised being fed back to clinical and research teams.
Analysis was undertaken primarily by the qualitative researcher (AS); however, regular discussions were held with the lead qualitative researcher (JA) and trial manager (LC) throughout. Analysis and data collection were undertaken by AS, an academic research fellow with no clinical training. AS had no prior knowledge or experience of thoracolumbar fractures, but has experience of conducting qualitative research alongside RCTs. The other member of the qualitative team (JA) also has no clinical training and is a chairperson of surgical trials.
Survey of spine surgeons
We undertook an electronic survey of orthopaedic spine surgeons to determine willingness to participate in a future trial, current practice and caseload. An electronic survey was the preferred option, as surgeons working in the UK NHS have to be computer literate and have access to a range of electronic media devices and programs. Electronic surveys also offer flexibility in time and place of completion, helping maximise potential response rates.
We report the survey following as closely as possible the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). 51
Survey population
The target population was NHS spine surgeons regularly treating thoracolumbar fractures. The sampling frame was membership of the BASS or the Society of British Neurological Surgeons (SBNS). In addition, to ensure that any spinal surgeons who were not members of the BASS or the SBNS had the opportunity to complete the survey, the link was snowballed by clinical members of the research team via their personal contacts and social media. Membership of the BASS and the SBNS includes orthopaedic and neurological consultant surgeons, specialist registrars and research fellows in spinal surgery.
Although not all relevant health professionals may be members of these organisations, additional efforts to optimise the reach of the survey to relevant surgeons were made via snowballing methods.
Response rates in surveys of health-care professionals vary greatly. A systematic review and meta-analysis estimated a mean response rate to online surveys among health professionals of 38%. 52 An earlier systematic review reported response rates ranging from 9% to 94%. 53 The primary purpose of the survey was to provide descriptive information on current NHS practice.
Research ethics, informed consent and data protection
As the survey involved spine surgeons identified through professional organisations only and not through the NHS, national REC approval for the web survey was not required. A copy of the survey was submitted with the Health Research Authority application for approval of the feasibility trial.
The link to the survey was, in all circumstances, accompanied by a brief explanation of the PRESTO feasibility study and the purpose of the survey of spine surgeons. In addition, the approximate length of time for completion was given as 10–15 minutes, and details of where to find further information were given. The first page of the survey instrument included all this information again, and required participants to confirm that they agreed to complete the survey to access the survey questions.
Responses to the survey were wholly anonymous and no person-identifying information was collected within the main survey instrument. A link to a separate survey collector was presented to participants who completed the survey. In this section, participants were given the option of being acknowledged by name as a survey participant in publications which relate to the survey and/or to volunteer to take part in an in-depth interview to explore in more detail issues regarding the feasibility of a future trial. When participants were interested in taking part in an interview, their names and e-mail addresses were shared only with the qualitative researchers using the University of York secure drop-off service. In line with YTU standard operating procedures, personal information provided by participants has been stored securely, is accessible only by the research team and will be securely destroyed after 5 years.
Questionnaire development and testing
The survey was prepared in Qualtrics® November 2016 (Qualtrics, Provo, UT, USA) software. The questions were ordered in three sections and framed to collect descriptive information on:
-
current NHS management strategies for patients with thoracolumbar fractures, including methods to establish spinal stability and suitability for surgery or conservative management
-
current methods of surgical fixation
-
willingness to participate in a future trial and willingness to randomise based on the proposed inclusion criteria
-
any centre factors that would need to be overcome to make recruitment to the trial possible
-
estimated current caseload of eligible patients
-
respondent and centre characteristics.
A list of the questions and response options used is provided [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. Presentation was optimised as far as possible for completion on a laptop or desktop computer, iPad (Apple Inc., Cupertino, CA, USA), other tablet device or mobile phone. The survey was piloted by health professional members of the study team for content, and usability and technical functionality were tested by project team members and colleagues.
Survey distribution
The survey was distributed to respondents as an open survey link in a number of different ways:
BritSpine 2018, 21–23 March 2018, Leeds, UK (United Kingdom Spine Societies Board conference, approximately 450 delegates)
The link was made available on the conference web application, which could be downloaded by all attending. Members of the research team attended the conference and staffed a stand providing delegates with access to iPads or laptops for completing the survey, and distributed leaflets that included the URL and quick response code to access the survey [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)]. The launch of the survey was highlighted in a presentation given by Mr Almas Khan at the conference and delegates were encouraged to take part.
British Association of Spine Surgeons forum
Information about the survey, including the link, was posted on the BASS forum on 20 May 2018.
United Kingdom Spine Societies Board newsletter
A short article and survey link were included in the July and September 2018 editions of Spine Matters, the United Kingdom Spine Societies Board quarterly newsletter sent to all members of each of the UK spine societies.
Society of British Neurological Surgeons spring meeting, 11–13 April 2018, Torquay, UK (approximately 130 delegates)
Leaflets, including the URL and quick response code to access the survey [see www.journalslibrary.nihr.ac.uk/programmes/hta/1515407/#/ (accessed 17 April 2020)] were distributed to delegates from a stand staffed by researchers from the FORVAD (posterior cervical FORaminotomy Versus Anterior cervical Discectomy) study in a reciprocal arrangement at BritSpine (Thomson S, Sutton A, Fernandez C, Papachristofi O, Selvanathan S, Pal D, et al. , Leeds Teaching Hospitals NHS Trust, 2018, personal communication).
Southwest Spinal Club
A notice was sent to the Southwest Spinal Club, a Google Group (Google Inc., Mountain View, CA, USA). The club includes spinal surgeons from Plymouth, Southampton, Salisbury, Exeter, Taunton, Bristol, Bath, Cheltenham and Gloucester, Swindon, Newport, Swansea and Cardiff.
Snowballing
Clinical members of the research team also shared the link to the survey with their own contacts via personal social media accounts, and encouraged both participation and onward sharing.
Survey administration
The electronic link to the survey collected participant responses automatically in the secure Qualtrics application. Manual entry of data was not required.
Participation in the survey was voluntary and no incentives were offered or passwords required for completion of the questionnaire. The Qualtrics facility ‘prevent ballot box stuffing’ was activated to prevent repeat participation. Neither personal information nor contact association were collected, so we are unable to verify the effectiveness of this facility.
The survey was opened on 16 March 2018, when the link was made available for inclusion in the BritSpine 2018 conference application with its own icon, and closed on 15 November 2018.
The survey was structured in three sections: (1) current practice (eight questions), (2) attitudes to potential trial and eligibility criteria (eight questions) and (3) about participants (three questions). To reduce the number of questions and keep them relevant to a respondent, adaptive questioning was used when possible; only adaptive questions required a response to proceed. Forward and backward options were provided to enable participants to edit their responses. No consistency or completion checks were undertaken before the questionnaire was submitted.
Analysis
An analysis plan was felt unnecessary once the survey questions had been formalised, given the mechanism for recording and collating responses and the nature of the data being collected.
Response data were downloaded from Qualtrics into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). Standard checks were undertaken to identify and remove errors, such as outliers, inconsistencies and omissions. Descriptive analyses of respondent characteristics were undertaken to allow exploration of the representativeness of the sample. Descriptive analyses were undertaken of responses to questions and summary statistics are presented.
All responses collected for each question were analysed, with the response rate for each question calculated using the number of responses to individual questions as the denominator. The length of time taken by respondents to answer questions was not collected and therefore no cut-off points were used.
Patient public involvement and engagement
To gain a wide perspective from patients and the public, we included a patient co-applicant, had an independent patient or public representative on the combined TSC and DMEC, and developed a study-specific Patient Advisory Group (PAG).
The patient co-applicant provided valuable insight regarding the design of the study and important considerations for any patient who may be approached for the study. He planned to attend the monthly TMG meetings a minimum of every 3 months and was included in correspondence relating to all of the meetings. This was kept under review to minimise time burden, while also ensuring that he was able to engage with the trial team. He was also involved in the PAG review of documentation.
The patient and public involvement (PPI) representative on the combined TSC and DMEC had experienced a fractured spine and was affiliated with After Trauma (see www.aftertrauma.org; accessed July 2020), an online support service for survivors and families recovering from trauma.
Developing the Patient Advisory Group
In October 2017, an advert to recruit to the PAG was posted on the People in Research website (see www.peopleinresearch.org; accessed July 2020) and a PPI leaflet created. Calls for volunteers were sent out to the Barts Health NHS Trust musculoskeletal (MSK) resource group for PPI representatives. Clinical members of the study team also directly approached patients to publicise the initiative.
To maximise PAG representation and involvement, methods of identifying interested patients and members of the public included consulting with the head of PPI at Barts Health NHS Trust and approaching organisers of existing relevant groups, such as the ‘patient champion’ group at Leeds Teaching Hospitals NHS Trust.
One patient volunteered from the MSK group, two patients were recruited from the spinal fracture clinic and one patient from the MSK clinic, to form the PRESTO study PAG. The organisers felt that the BritSpine PAG was too small to recruit from.
Attempts to recruit to the PAG continued throughout the study.
Engagement activities
INVOLVE documentation templates and examples were used to develop the study-specific patient and public involvement and engagement (PPI&E) pack, which was then sent out to PAG members.
A meeting of the PAG was held in November 2017 to engage with members, ensure that they had all the information they needed and to ask for their comments on patient-facing documentation for the feasibility trial. Three of the four members of the public invited were present in person; the fourth agreed to provide comments by e-mail.
Facilitated by two members of the study team (PM and JK), the group were given an introduction to medical research, the trial, and the role and importance of PPI&E. Discussions also covered ethical issues, consent and comments on the patient information sheets (PISs) and consent forms. The outcomes of the PAG meeting were documented and fed back to the study team for action.
Study group responses to the PAG feedback were collated and sent to the PAG to provide group members with an update as to how their feedback had been actioned. Feedback included a request for, and assurance of, continued involvement.
Information and newsletters updating PAG members on study progress and anticipated future activities were sent out in March 2018 and June 2018.
An end of study PAG event was planned for July 2019; however, members of the original PAG were not able to attend or declined further involvement. Opportunity for involvement in the end of study PAG event was subsequently posted on the People in Research website in June 2019 (see www.peopleinresearch.org). In addition, a disability consultant cascaded information via their personal network to over 20 local groups in the Yorkshire and Humber region to recruit further members.
Representatives from After Trauma were consulted and provided assistance reviewing the study design and it is intended that it will assist with disseminating study results to the public.
Patient Advisory Group contributions
Documents reviewed by the PAG were PISs, participant consent forms, consultee/next of kin information sheets, declaration and consent forms and the draft baseline questionnaire.
Patient Advisory Group comments and the study team responses were as follows:
-
The PAG had a number of concerns regarding recruitment, in particular of patients who lack capacity to consent. The issues raised related to the diverse patient population that leads to complex issues regarding their ‘normal decision-maker’ being excluded from the decision-making process (e.g. male/female roles in some communities, potential language barriers and younger or vulnerable relatives, such as children, being put in a situation in which they have to synthesise complex information and make a decision when emotionally compromised). The group was happier with the idea of a surgeon making the call for eligibility and then next of kin being approached to say that eligibility is confirmed. However, some group members were concerned that some individual surgeons would feel pressured by their superiors to exclude or include patients, and wanted clear eligibility criteria to reduce the likelihood of this.
-
The study team addressed these issues in further written and verbal guidance on the consent process issued to recruiting sites. This needed to be balanced against ensuring that the process for consent complied with the Mental Capacity Act. 43 This legislation makes it clear that a personal consultee should be sought when possible and a professional consultee sought only if a personal consultee is not available. We proposed that no changes were made to the information presented to patients, but clarification was added to the guidance manual and addressed at site set-up training. Surgeons signed off on eligibility prior to approaching and consenting any patient. The next of kin were to be asked if they were happy to make a decision on behalf of the patient, but it was to be emphasised that it is their right to decline this. Research staff are trained in and used to conducting sensitive discussions.
-
Reassuringly, there is guidance about the nomination of any professional consultee, which ensures that they should be independent of the research team and outside any hierarchy (e.g. being junior to a member of the research team), and therefore free from potential influence when advising whether a patient is to be included or excluded.
-
The PAG suggested that amendments to documentation were recorded. When these were not in conflict with legislative or procedural requirements, the changes were accepted. Changes included, for example, amendments to the qualitative topic guides, so the researchers made it clear to interviewees at the beginning of an interview that there were no right or wrong answers, that patients did not have to answer a particular question if they did not wish to and that it was the patient’s thoughts that were of interest.
-
The PAG was happy with the main research question and planned outcomes, but suggested that the risks and benefits of each treatment arm should focus on the patient and requested that, if possible, the risks and benefits be quantified, for example adding the frequency of ‘infection’ or ‘non-union’ as a ratio or percentage. The PAG also suggested making it clear that patients may experience persistent pain post surgery, as well as in the conservative arm.
-
The risks and benefits section was revised and risks grouped under ‘common’ and ‘rare’ themes. It was not felt appropriate to use ratios or percentages, as these could not be backed up by evidence for the study patient group.
As a result of general issues raised by the PAG, the following actions were taken by the study team:
-
Further information was added regarding post-treatment care for both groups.
-
Visual information was added to the PIS.
-
Quotes from the qualitative interviews could potentially be included in the PIS for a further trial to provide the patient perspective.
-
Further information and signposting could not be added to the PIS, as the trial cannot be perceived as endorsing any publicly available information.
-
Statements were added to the PIS to emphasise that treatment received by all participants will always be delivered in the patient’s best interests.
Summary of patient and public involvement and engagement meeting: 15 July 2019
The meeting was held in an accessible venue in Leeds and was facilitated by three members of the research team (AS, LC and JA). The group were given an introduction to the trial, a summary of some of the practical issues encountered and a summary of findings. They were also given an opportunity to review the information and questionnaires that had been given to the feasibility trial participants. In total, four patients attended the meeting. Two group members had experienced a thoracolumbar fracture and two were currently receiving treatment for other spinal issues. All had prior experience of involvement in PPI&E activities.
General feedback
All group members were asked to complete a feedback form at the end of the session, which asked them to comment on the content and structure of the session and provide other comments on their treatment and experiences, and ideas for research and our research study that they may not have had a chance to voice during the session.
All feedback was positive, with patient group members reporting that the session was informative, stimulating and well structured, with a good balance of questions and answers. Group members reported that they were given enough time to consider each of the points for discussion properly and the opportunity to exchange their thoughts and ideas. Members also found it a useful opportunity to speak to other patients and share their experiences of diagnosis, treatment and postoperative support.
The group were very enthusiastic about research in this area and our research project, and made useful suggestions that could be taken forward or considered for use in a larger trial.
Summary document and patient information
Feedback from the group on the information that had been provided to patients prior to enrolment in the feasibility trial was as follows:
-
The addition of pictures of braces would be beneficial, as there are many different types (could also be included in end of study report).
-
Patient information documents should be a minimum of font size 14, Arial and not justified.
-
The glossary of terms was useful and should be included in the information given to trial patients.
-
The information should contain more flow charts and less text.
-
The group considered that the leaflet was a lengthy document, but appreciated the fact that there was summary information available that could be presented to potential participants, with the more detailed information then being presented only to patients who express an interest in participation.
-
The patient information forum is a resource that could be used. This includes templates for patient documents.
Randomisation
There were lengthy discussions about the randomisation process. There were concerns about the use of terminology such as ‘flipping a coin’ in the PRESTO study PIS, which was perceived to trivialise the injury and decision to participate. The treatment was then perceived by members of the group as ‘pot luck’.
Recruitment
The research team outlined the target population. The patient group suggested that the diagram presented to them was very helpful and could be used in the patient information and summary.
The patient group were asked what information they would want to know before making a decision on taking part.
The patient group said that if they were being recruited into the trial they would want to know a lot more about the practicalities of recovery for each of the treatments.
The patient group asked who decides on whether or not a patient enters the study. The process of determining eligibility against strict criteria for the trial was explained. The research team also explained to the group that the criteria themselves were being reviewed as part of the feasibility trial. The patient group suggested that information on the decisions that had been taken by surgeons and other care staff before approaching a potential participant could be explained to those potential patients, as there were clearly many factors that influenced this process and these were not reflected in the written trial information being given. The question ‘How do you reach the decision that each treatment is suitable for me?’ could be described via a flow chart.
The patient group asked what recovery looks like for each of the treatment arms, as they thought this was not really covered in the PRESTO study PIS. They would ask questions such as ‘Who is going to look after me if I have an operation?’ ‘If I live on my own, will I be able to put on or remove the brace without assistance?’ and ‘Will I have to travel to follow-ups?’. The research team discussed the variability found between patients because each injury is unique and the additional complication of variations in practice within and between sites. The group also asked for how long trial patients would receive support (both physical and emotional). There was a lengthy discussion about the variability of support within and between different regions and hospitals. Economic survival was also raised: ‘How will I cope financially if I have an operation and cannot work?’.
There was a suggestion from the group that neighbourhood teams had to be more flexible. It was also suggested that the information given to potential participants could include a list of questions that patients might like to ask to help inform their decision to take part, and that patients and surgeons or care teams could co-produce care plans and map patients’ individual pathways through the trial.
Suggestions were made as to the format in which information could be provided, including online or audio versions. One member reported that one rehabilitation centre had facilities to project information onto ceilings, where it can be easily read by patients who are immobilised. In addition, videos about rehabilitation to explain other elements of the trial (involving previous patients or trial participants, e.g what it is like to wake up the day after the operation) and signposts to other sources of support (e.g mental health) were proposed.
The research team presented the reasons for not wishing to take part that were given by patients approached for the trial. The group suggested that these reasons could be presented to potential trial participants, that fellow patients could be involved in the consent process and that getting backing and endorsement from groups such as the ‘spinal cord injuries owners club’ would be beneficial. A ‘Myths and misconceptions explained’ section (i.e. testimonials from other trial patients) could be included in information provided.
The group asked about whether or not patients on a private pathway could be recruited and were advised that this was not usual practice because of a lack of governance infrastructure and issues with access to data.
Consent process
The group suggested that consent should be continuously revisited because of fluctuating capacity.
In the group’s opinion, consent and reassurance are two very different things, and patients need to know explicitly what they are consenting to.
Outcomes
The group suggested the use of patient diaries to aid recollection when patient questionnaires collect up to 3 months’ data at each time point.
There was a definite split in group between mobility and pain: those who were mobile favoured pain as an outcome and those who experienced reduced mobility post injury favoured mobility as the most important outcome. There would be a shift from one to another becoming more important as patients move along their treatment pathway.
It was suggested that goals that are personal to the patient be set and that these be reviewed at periodic intervals and be used as an outcome.
Research ideas and priority areas
Take a step back: explore the role of holistic treatment that is given alongside surgery or no surgery.
Iron out variables between hospital settings and professionals, as these can have a significant impact on the brace versus surgery question.
Chapter 3 Results of the feasibility randomised controlled trial
All data in the following sections are presented under the groups to which participants were originally allocated unless specified otherwise.
CONSORT flow diagram
The flow of participants through the trial can be seen in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 2).
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. a, Multiple reasons can be given for each participant.
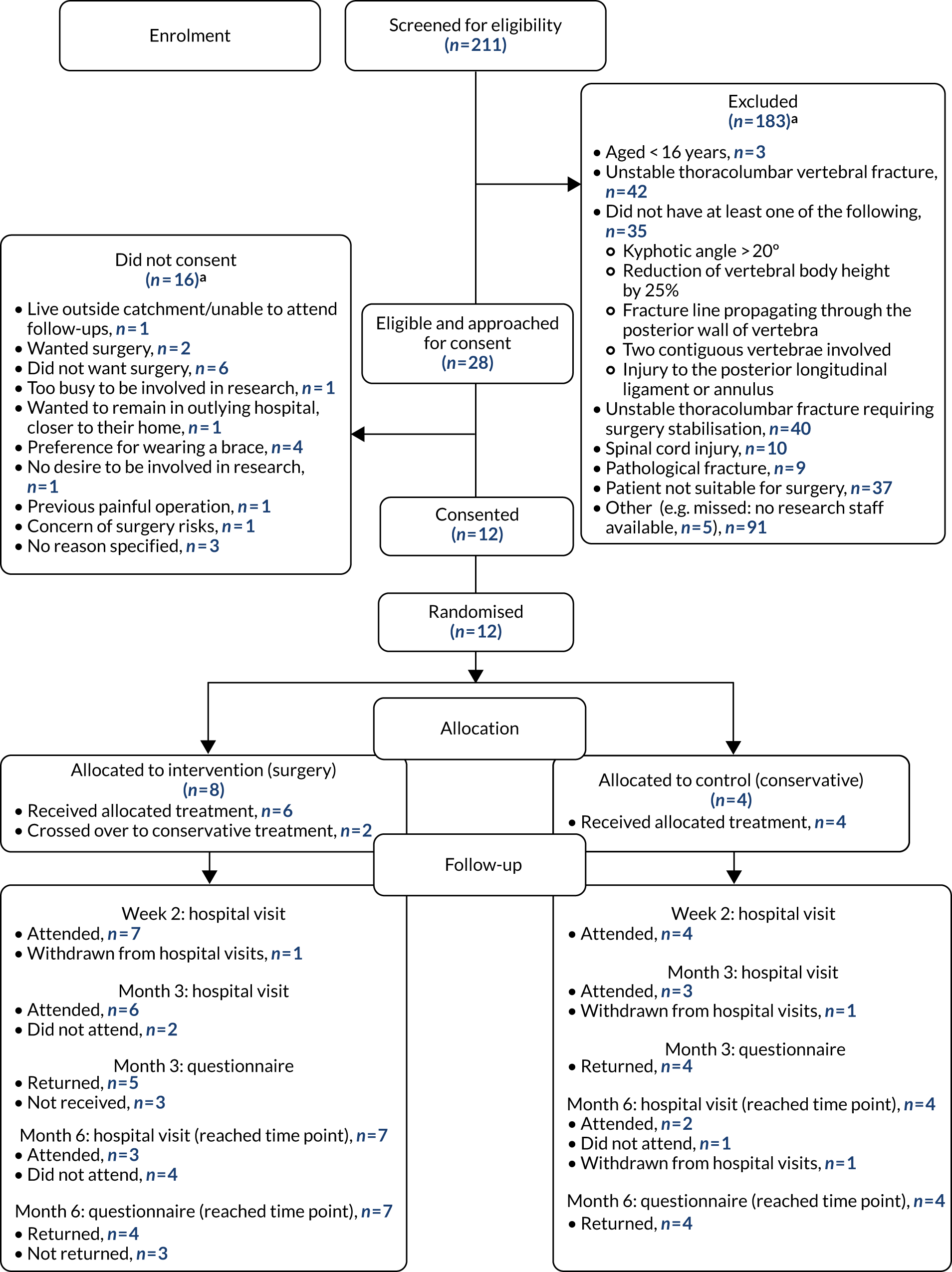
Recruitment and retention
The recruitment period of the trial was 18 April 2018 to 31 March 2019, and follow-up activities continued until 28 June 2019. The Bart’s Health NHS Trust site opened in April 2018, whereas the Leeds Teaching Hospitals NHS Trust and Cardiff & Vale University Health Board sites opened in June 2018. The sites were open for a combined total of 30.7 months.
Throughout the course of the trial, 211 patients were assessed for eligibility, of whom 28 were eligible (13.3%). Initial discussion with the participating centres indicated that there would be 120 eligible patients seen in the recruitment period; our figure was 23.3% of this number.
All three participating sites recruited at least one participant. Recruitment data broken down by month and by site are presented in Appendix 1.
Recruitment rate: primary outcome
Our primary outcome is the recruitment rate. This was defined in the trial protocol as the proportion of eligible patients who were randomised into the trial. Of 28 eligible patients, 12 were randomised; hence the proportion of patients recruited was 0.43 (95% CI 0.24 to 0.63).
Recruitment rate
Following peer review of the draft report, an alternative expression of recruitment rate was suggested. The combined total of site recruitment months was 30.7, which gives an overall average recruitment rate per month of 0.39 per site. The individual rates for each site were 0.44 for Bart’s Health NHS Trust, 0.11 for Leeds Teaching Hospitals NHS Trust and 0.61 for Cardiff & Vale University Health Board.
Participant characteristics
Table 2 summarises participant characteristics, overall and broken down by trial arm. Twelve patients were randomised into the study. The majority were male, in employment and presenting with a high-energy fracture. The mean age was higher in the conservative arm (60 years) than in the surgery arm (42 years). The majority of patients did not report any previous back problems; however, one patient reported having had a previous fracture. No patients with a formal diagnosis of osteoporosis were included.
| Characteristic | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 60.5 (10.0) | 42.3 (15.5) | 48.3 (16.2) |
| Median (minimum, maximum) | 61 (50, 70) | 45 (20, 58) | 53.5 (20, 70) |
| Gender, n (%) | |||
| Male | 3 (75.0) | 6 (75.0) | 9 (75.0) |
| Female | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, n (%) | |||
| White | 3 (75.0) | 8 (100.0) | 11 (91.7) |
| Asian | 1 (25.0) | 0 (0.0) | 1 (8.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Qualifications, n (%) | |||
| No formal qualifications | 2 (50.0) | 2 (25.0) | 4 (33.3) |
| Some qualifications | 0 (0.0) | 3 (37.5) | 3 (25.0) |
| Degree or higher | 2 (50.0) | 3 (37.5) | 5 (41.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Employment, n (%) | |||
| Full time | 1 (25.0) | 3 (37.5) | 4 (33.3) |
| Self-employed | 1 (25.0) | 1 (12.5) | 2 (16.7) |
| Retired | 2 (50.0) | 0 (0.0) | 2 (16.7) |
| Not employed: seeking work | 0 (0.0) | 2 (25.0) | 2 (16.7) |
| Other | 0 (0.0) | 2 (25.0) | 2 (16.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Smoker, n (%) | |||
| Yes | 2 (50.0) | 2 (25.0) | 4 (33.3) |
| No | 2 (50.0) | 6 (75.0) | 8 (66.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alcohol, n (%) | |||
| Yes | 3 (75.0) | 6 (75.0) | 9 (75.0) |
| No | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diabetic, n (%) | |||
| Yes | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| No | 4 (100.0) | 7 (87.5) | 11 (91.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Steroids, n (%) | |||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 4 (100.0) | 8 (100.0) | 12 (100.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Living arrangements, n (%) | |||
| Alone | 1 (25.0) | 3 (37.5) | 4 (33.3) |
| Alone, but with support | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| With partner | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| With relatives | 2 (50.0) | 2 (25.0) | 4 (33.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cause of injury, n (%) | |||
| Low-energy fall | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| High-energy fall | 2 (50.0) | 4 (50.0) | 6 (50.0) |
| Road traffic accident | 0 (0.0) | 2 (25.0) | 2 (16.7) |
| Contact sport injury | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 1 (25.0) | 0 (0.0) | 1 (8.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type of injury, n (%) | |||
| High-energy traumatic | 3 (75.0) | 6 (75.0) | 9 (75.0) |
| Low-energy osteoporotic | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| Vertebrae involved,a n (%) | |||
| T10 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| T11 | 1 (25.0) | 1 (12.5) | 2 (16.7) |
| T12 | 2 (50.0) | 4 (50.0) | 6 (50.0) |
| L1 | 1 (25.0) | 4 (50.0) | 5 (41.7) |
| L2 | 0 (0.0) | 2 (25.0) | 2 (16.7) |
| Total TLICS | |||
| Mean (SD) | 2 (1.4) | 2.9 (1.5) | 2.6 (1.4) |
| Median (minimum, maximum) | 1.5 (1, 4) | 3 (1, 5) | 2.5 (1, 5) |
| AO classification, n (%) | |||
| A1 | 1 (25.0) | 0 (0.0) | 1 (8.3) |
| A1.1 | 2 (50.0) | 0 (0.0) | 2 (16.7) |
| A1.3 | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| A2 | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| A2.1 | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| A3 | 1 (25.0) | 3 (37.5) | 4 (33.3) |
| A3.2 | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| A4 | 0 (0.0) | 1 (12.5) | 1 (8.3) |
| Kyphotic angle measurement (°) | |||
| Supine, n | 3 | 7 | 10 |
| Mean (SD) | 11.5 (6.2) | 12.9 (7.4) | 12.5 (6.7) |
| Median (minimum, maximum) | 12 (5, 17.4) | 16 (3, 23.8) | 14 (3, 23.8) |
| Standing, n | 1 | 1 | 2 |
| Mean (SD) | – | – | 23.5 (9.2) |
| Median (minimum, maximum) | 17 (–) | 30 (–) | 23.5 (17, 30) |
| Previous back problems, n (%) | |||
| Yes | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| No | 3 (75.0) | 6 (75.0) | 9 (75.0) |
| If yes, what was it? | |||
| n | 1 | 2 | 3 |
| Previous fracture, n (%) | 0 (0.0) | 1 (50.0) | 1 (33.3) |
| Diagnosis of osteoporosis, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other, n (%) | 1 (100.0) | 1 (50.0) | 2 (66.7) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Eligibility and consent
Overall, there were 28 eligible patients, all of whom were approached for consent (proportion 1.00, 95% CI 0.88 to 1.00). The proportion of eligible patients who were not approached for consent was zero (95% CI 0.00 to 0.12).
Five patients were reported as ‘missed’ owing to lack of availability of research staff; however, these patients were reported as ineligible because of the way in which data were captured for non-trial participants.
There was a wide spread of reasons why patients were not considered eligible to be entered into the trial. The number of patients who did not meet each inclusion criterion is shown in Table 3.
| Inclusion criterion | Number of ‘no’ responses/total completed answers (%) |
|---|---|
| Aged ≥ 16 years | 3/211 (1.4) |
| Stable thoracolumbar vertebral fracture | 42/210 (20.0) |
| A kyphotic angle > 20° on standing radiographs or > 15° on supine radiographs or CT scansa | 134/204 (65.7) |
| Reduction in vertebral body height of 25%a | 89/205 (43.4) |
| Fracture line propagating through the posterior wall of vertebraa | 112/205 (54.6) |
| Involvement of two contiguous vertebraea | 167/207 (80.7) |
| Injury to the posterior longitudinal ligament or annulus in addition to the body fracturea | 180/205 (87.8) |
The initial eligibility form contained one box (‘yes’) corresponding to the exclusion criteria. This was updated part-way during the trial to include both ‘yes’ and ‘no’. Hence, numbers for each criterion are simply given as counts.
The exclusion criteria are as follows:
-
unstable thoracolumbar fracture requiring surgical stabilisation (n = 40)
-
spinal cord injury (n = 10)
-
pathological fracture (n = 9)
-
patient not suitable for surgery (n = 37)
-
other reason to exclude the patient (n = 91).
Sixty-three (69%) of the 91 patients met all of the trial eligibility criteria but were deemed ineligible for ‘other’ reason alone. A breakdown of the ‘other’ reasons is below. More than one ‘other’ reason could be reported for a patient, and hence the total number of reasons presented is 105 rather than 91. Reasons are grouped and then a further breakdown is given in the square brackets (when relevant):
-
Characteristics of the fracture (n = 31) [old fracture (n = 4); transverse process fracture (n = 2); unsuitable osteoporotic fracture (n = 5); not contiguous vertebrae (n = 5); more than two contiguous vertebrae (n = 5); other vertebrae involved (n = 3); not cartigious vertebrae (n = 1); age indeterminate number of first lumbar (L1) vertebrae (n = 2); age indeterminate number of 12th thoracic (T12) vertebrae (n = 1); unsuitable kyphotic angle (n = 3)].
-
At least one of the treatments was not appropriate (n = 15) [complex operation required (n = 5); burst fracture (n = 1); fracture is not severe enough (n = 2); not suitable for short segment fixation (n = 1); unable to fit brace (n = 1); elective back surgery (n = 1); neurosurgery required (n = 2); reduced pain (n = 1); clinical decision to treat with brace (n = 1)].
-
Other conditions that prohibited further treatment (n = 12) [neurology present (n = 1); ankylosing spondylitis (n = 1); cognitive decline (n = 5); palliative care (n = 1); wheelchair bound (n = 2); polymyalgia rheumatica (n = 1); previous metalwork infected (n = 1)].
-
Problems related to alcohol or drug use (n = 11) [alcohol (n = 6); drugs (n = 5)].
-
Geographically impractical (n = 10).
-
Mental health problems (n = 9).
-
Insufficient English-language skills (n = 8).
-
Missed: no research staff available (n = 5).
-
Assessed for eligibility too long post injury (n = 3).
-
Did not meet inclusion criteria (n = 1).
A full breakdown of the ‘other’ reasons and other information that was collected about the exclusion criteria is given in Appendix 2.
Injury characteristics
Immobilisation
The types of immobilisation used for all patients screened are summarised in Table 4; patients could have more than one form of immobilisation.
| Type of immobilisation | Number of responses, n (%) |
|---|---|
| No immobilisation | 67 (31.8) |
| Brace | 45 (21.3) |
| Complete bed rest | 69 (32.7) |
| Log roll in bed with assistance | 44 (20.9) |
| Log roll in bed without assistance | 4 (1.9) |
| Bed rest and log roll | 6 (2.8) |
| Flat bed rest | 1 (0.5) |
| Sit up to 30° | 10 (4.7) |
| Flex to 30° | 3 (1.4) |
| Sit up to 45° | 5 (2.4) |
| Collar | 2 (1.0) |
| Paralysed or paraplegic | 2 (1.0) |
| Mobilise as pain allows | 1 (0.5) |
| Full spinal precaution | 3 (1.4) |
Imaging
The type of imaging that was used to diagnose the fracture is summarised in Table 5. Of the 211 patients screened, three (1.4%) underwent no imaging, 172 (81.5%) underwent one type of imaging, 34 (16.1%) underwent two types of imaging and two (1.0%) underwent three types of imaging.
| Imaging used | Number of responses, n (%) |
|---|---|
| X-ray: standing | 23 (10.9) |
| X-ray: sitting | 2 (1.0) |
| X-ray: supine | 16 (7.6) |
| CT scan: supine | 163 (77.3) |
| MRI: supine | 42 (19.9) |
Kyphotic angle measurement
The kyphotic angle was measured in 200 patients. The mean angle was 12.6° (SD 9.9°), the median was 12° and the range was –16° to 44°. Information on how the kyphotic angle was measured was included in 201 forms. In most patients (177/201, 88.1%) kyphotic angle was measured in the supine position; in a single patient (0.5%) it was measured in the sitting position and in the remaining 23 (11.4%) patients it was measured while standing. Information on the type of imaging used to measure the kyphotic angle was included in 200 forms: 151 (75.5%) forms reported the use of CT, 17 (8.5%) forms reported the use of MRI and 32 (16.0%) forms reported the use of radiography.
Mode of injury
Of the 207 injuries for which mode of injury was specified, 130 (62.8%) were due to a high-energy impact and 77 (37.2%) were caused by a low-energy impact.
Results of randomisation and crossover
Consent approach
A total of 28 patients were approached for consent. The consent process took on average 38.0 minutes (SD 26.5 minutes, based on 27 responses). The proportion of people who provided consent was 0.43 (95% CI 0.24 to 0.63) and the proportion of patients who did not provide consent was 0.57 (95% CI 0.37 to 0.76).
All patients were able to consent themselves and, hence, no consultees were required. Information on reasons for non-consent was collected from 13 of the 16 patients who declined (81.3%). Patients could give more than one reason. Six patients said that they did not want to enter the trial because they did not want to undergo surgery. Four participants said that they wanted to be treated with a brace, rather than surgery. Other reasons included surgery was wanted (n = 2), lived out of catchment or was unable to attend follow-ups (n = 1), wanted to remain in outlying hospital closer to their home (n = 1), no desire to be involved in research (n = 1), too busy to be involved in research (n = 1), had a previous painful operation (n = 1) and concern over the risks involved in surgery (n = 1).
Randomisation and crossover
A total of 12 participants were randomised into the trial, eight (66.7%) participants to surgical fixation and four (33.3%) to conservative management. Table 6 gives a breakdown of the numbers of patients randomised by site.
| Site | Randomised | Total | |
|---|---|---|---|
| Surgery | Conservative | ||
| Bart’s Health NHS Trust | 3 | 2 | 5 |
| Leeds Teaching Hospitals NHS Trust | 1 | 0 | 1 |
| Cardiff & Vale University Health Board | 4 | 2 | 6 |
| Total | 8 | 4 | 12 |
Two patients did not receive their allocated treatment. Both patients were randomised to receive surgical fixation but did not go on to receive the surgery, with the decision to cross over to conservative treatment made shortly after randomisation. The decision was made by the patient in one instance and following further discussion with the patient’s family and the wider surgical team in the other case. The proportion of patients randomised who did not receive the randomly allocated treatment was 0.17 (95% CI 0.02 to 0.48).
No patients crossed over to surgery at any time during the trial; hence, the proportion of patients randomised to the conservative treatment who received surgical management within the follow-up period was zero (95% CI 0.00 to 0.60).
Dropouts and withdrawals
One patient withdrew from hospital follow-up (in the surgery arm) between undergoing surgery and their week 2 follow-up appointment, to receive rehabilitation treatment privately, resulting in a proportion of dropout of 0.08 (95% CI 0.00 to 0.38) in this time period. Between week 2 and month 3, a further patient withdrew from hospital follow-ups (in the conservative treatment arm) because they were discharged from further hospital follow-up, giving a proportion of dropout in this time period of 0.09 (95% CI 0.00 to 0.41). At month 3, the patient who dropped out between randomisation and week 2 returned to the trial and consequently attended their non-private hospital follow-up appointment. No patients dropped out between 3 and 6 months (of the 10 patients who reached this time point and had not withdrawn), giving a proportion of 0 (95% CI 0.00 to 0.31). Both patients who withdrew continued to receive trial questionnaires.
Ability to collect clinical outcomes
Baseline forms were returned for all 12 participants (100.0%). Month 3 participant questionnaires were returned for nine (75.0%) participants (surgery, n = 5; conservative, n = 4). Month 6 questionnaires were returned by eight (72.7%) out of 11 participants who reached that time point (surgery, n = 4; conservative, n = 4). The figures and proportions about missing data are based on the forms returned. Details of the amount of missing responses for the ODI, VAS and SF-12 are given in Table 7.
| Measure | Number of missing response/total available (%) | Proportion (95% CI) | Number of scores that could not be calculated/total available (%) |
|---|---|---|---|
| ODI | 32/410 (7.8) | 0.92 (0.89 to 0.95) | 3/41 (7.3) |
| VAS | 2/29 (6.9) | 0.93 (0.77 to 0.99) | Not available |
| SF-12 | 1/312 (0.3) | 1.00 (0.98 to 1.00) | 0/26 (0.0) |
At week 2, 11 out of 12 (surgery, n = 7; conservative, n = 4) complication forms were returned by sites. At month 3, 11 out of 12 were returned (surgery, n = 8; conservative, n = 3) and at month 6, 8 out of 11 forms were returned (surgery, n = 5; conservative, n = 3). The proportion of complete complications data in the forms that were returned was 0.92 (95% CI 0.90 to 0.94). At month 3, two patients (both of whom had received surgery) did not attend their appointments, and at month 6 three patients did not attend their follow-up appointments (one patient received surgery and the other two received conservative care).
At month 6, when questionnaire data were not returned, attempts were made to contact seven participants to try and collect outcome data over the telephone. The number of attempts to collect the data ranged from one to four. Outcome data were successfully collected over the telephone for three participants.
British Spine Registry: data collection
Initially, 9 out of 12 patients [seven patients in the surgery arm and two patients in the conservative arm (75.0%)] opted in to complete questionnaires via the BSR. At 3 months, four patients [three in the surgery arm and one in the conservative arm (44.4%)] used the BSR to complete the questionnaire. At 6 months, three patients [two in the surgery arm and one in the conservative arm (33.3%)] completed the questionnaire online.
The online questionnaire could be accessed by patients at any point after receiving the initial e-mail to invite them to complete the questionnaire. This resulted in some patients completing the online version as well as the postal version at a specific time point (participants were sent a postal questionnaire if they had not completed the online one within 3 weeks). When both paper and electronic versions were received, the data collected at the earliest date were used in the descriptive summaries of the EQ-5D-5L, VAS and ODI.
Among the patients who completed measures using the BSR at both time points, the number of complete responses (for six EQ-5D-5L, one VAS and 10 ODI components) was 110 out of 119, which as a proportion is 0.92 (95% CI 0.86 to 0.96). The missing data were spread across all the measures and time points and related to all but one patient. No patients completed the health resource use questions via the BSR at either 3 or 6 months.
Table 8 summarises the method in which the outcome measures were ultimately collected (there were no withdrawals from follow-up questionnaires and 11 of 12 participants reached the 6-month time point).
| Month | Paper CRF only, n (%) | BSR only, n (%) | Both paper CRF and BSR, n (%) | Not returned, n (%) |
|---|---|---|---|---|
| 3 | 5 (41.7) | 3 (25.0) | 1 (8.3) | 3 (25.0) |
| 6 | 5 (45.5) | 0 (0.0) | 3 (27.3) | 3 (27.3) |
Outcome measure data
Oswestry Disability Index
Scores can be categorised as shown in Table 9, where a higher score indicates a higher level of disability. Data are presented graphically in Figure 3. A summary table providing descriptors of these categories can be found in Appendix 3. A score can be calculated if there are nine or 10 responses.
| ODI score | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Pre injury (collected at baseline) | |||
| n | 4 | 7 | 11 |
| Mean (SD) | 0 (0.0) | 20.0 (30.7) | 12.7 (25.8) |
| Median (minimum, maximum) | 0 (0, 0) | 0 (0, 72) | 0 (0, 72) |
| Disability category, n (%) | |||
| Minimal disability | 4 (100.0) | 5 (71.4) | 9 (81.8) |
| Moderate disability | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe disability | 0 (0.0) | 1 (14.3) | 1 (9.1) |
| Crippled | 0 (0.0) | 1 (14.3) | 1 (9.1) |
| Bed-bound | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Baseline | |||
| n | 4 | 6 | 10 |
| Mean (SD) | 46.7 (31.4) | 63.0 (25.7) | 56.5 (27.6) |
| Median (minimum, maximum) | 39 (20, 88.9) | 73.9 (14, 80) | 63.0 (14, 88.9) |
| Disability category, n (%) | |||
| Minimal disability | 1 (25.0) | 1 (16.7) | 2 (20.0) |
| Moderate disability | 1 (25.0) | 0 (0.0) | 1 (10.0) |
| Severe disability | 1 (25.0) | 1 (16.7) | 2 (20.0) |
| Crippled | 0 (0.0) | 4 (66.7) | 4 (40.0) |
| Bed-bound | 1 (25.0) | 0 (0.0) | 1 (10.0) |
| Month 3 | |||
| n | 4 | 5 | 9 |
| Mean (SD) | 30.9 (17.8) | 21.5 (9.9) | 25.7 (13.8) |
| Median (minimum, maximum) | 29 (15.6, 50) | 20 (13.3, 37.8) | 20 (13.3, 50) |
| Disability category, n (%) | |||
| Minimal disability | 2 (50.0) | 3 (60.0) | 5 (55.6) |
| Moderate disability | 0 (0.0) | 2 (40.0) | 2 (22.2) |
| Severe disability | 2 (50.0) | 0 (0.0) | 2 (22.2) |
| Crippled | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bed-bound | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 6 | |||
| n | 4 | 4 | 8 |
| Mean (SD) | 39.2 (29.4) | 12.1 (9.3) | 25.6 (24.8) |
| Median (minimum, maximum) | 37 (6.7, 76.0) | 11 (2, 24.4) | 18.2 (2, 76) |
| Disability category, n (%) | |||
| Minimal disability | 1 (25.0) | 3 (75.0) | 4 (50.0) |
| Moderate disability | 1 (25.0) | 1 (25.0) | 2 (25.0) |
| Severe disability | 1 (25.0) | 0 (0.0) | 1 (12.5) |
| Crippled | 1 (25.0) | 0 (0.0) | 1 (12.5) |
| Bed-bound | 0 (0.0) | 0 (0.0) | 0 (0.0) |
FIGURE 3.
Outcome measure data: ODI by trial arm and time point.
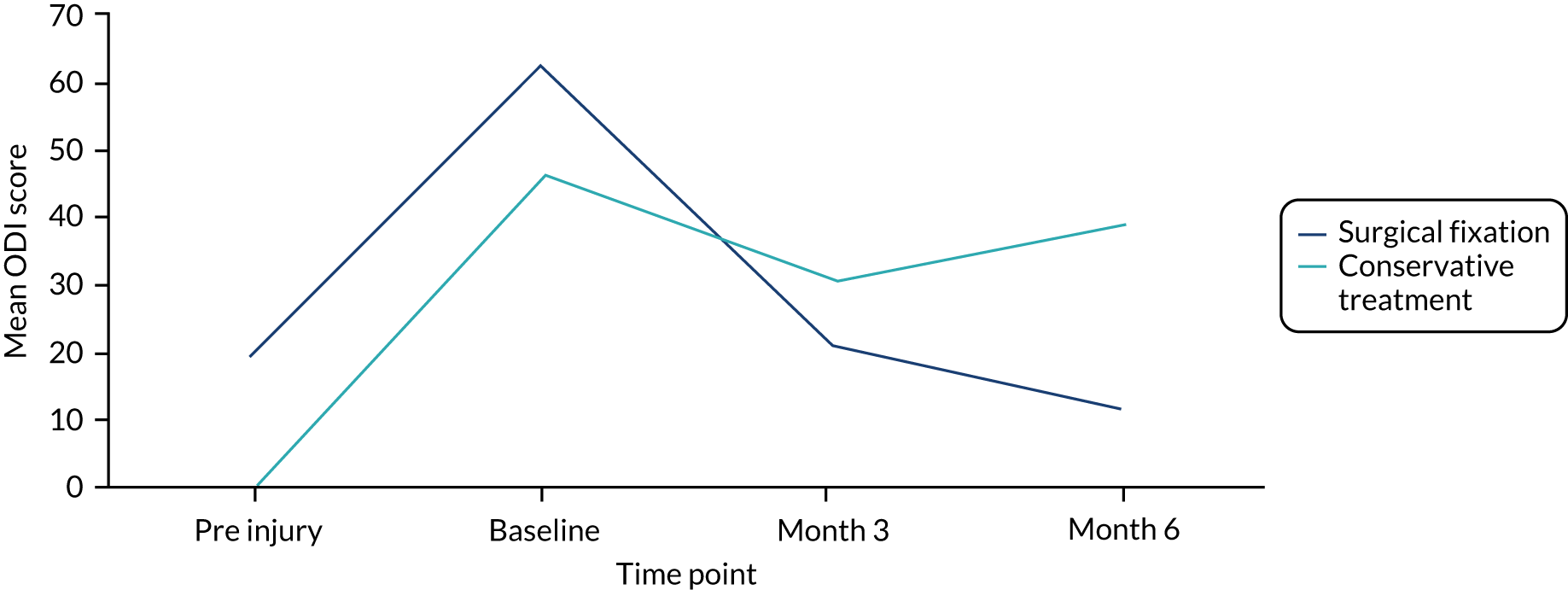
Visual analogue scale
Summaries of the VAS scores given by participants, broken down by trial arm, are given in Table 10 and presented in Figure 4. A higher score indicates increased pain.
| VAS score | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Baseline | |||
| n | 4 | 6 | 10 |
| Mean (SD) | 4.0 (3.8) | 5.1 (2.2) | 4.7 (2.8) |
| Median (minimum, maximum) | 3.7 (0.4, 8.0) | 4.8 (2.0, 8.0) | 4.8 (0.4, 8.0) |
| Month 3 | |||
| n | 4 | 5 | 9 |
| Mean (SD) | 3.2 (2.5) | 1.8 (0.6) | 2.4 (1.8) |
| Median (minimum, maximum) | 2.9 (0.8, 6.4) | 1.8 (1.0, 2.7) | 1.8 (0.8, 6.4) |
| Month 6 | |||
| n | 4 | 4 | 8 |
| Mean (SD) | 3.2 (2.9) | 2.0 (1.6) | 2.6 (2.3) |
| Median (minimum, maximum) | 3.0 (0.7, 6.2) | 1.5 (0.7, 4.3) | 1.5 (0.7, 6.2) |
FIGURE 4.
Outcome measure data: VAS by trial arm and time point.
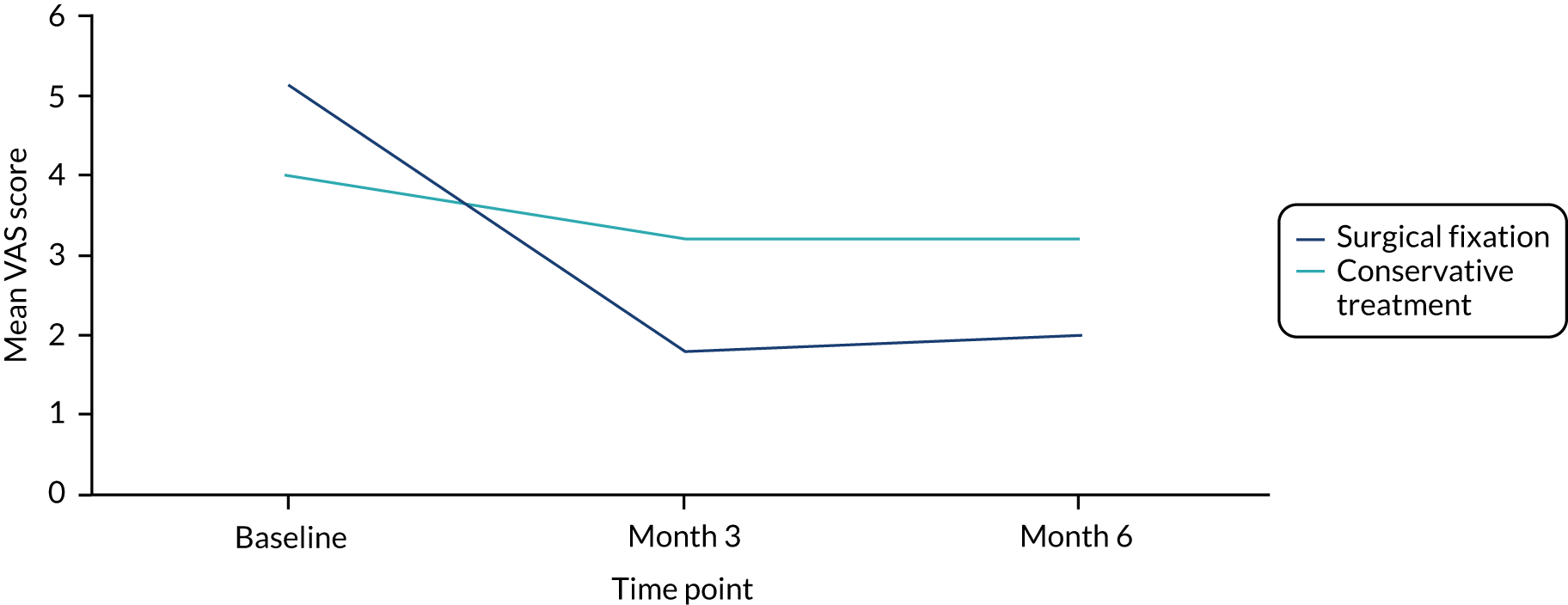
Short Form questionnaire-12 items
The SF-12 consists of 12 items, is a measure of physical and mental health and is completed by the participant. Higher scores indicate better health. The SF-12 consists of eight health domain scales that comprise either one or two questions. If a response was missing for a single question in a health domain that consists of two questions, it was replaced by the value of the other response in that domain (and hence is no longer considered missing for the score calculation). If a response was missing for a single-item domain, it remains missing. The physical and mental component scores were calculated only if there were values for all 12 items. Descriptive summaries are given in the Table 11, broken down by arm. Mean scores are presented graphically in Figures 5 and 6.
| SF-12 score | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Pre injury (collected at baseline) | |||
| n | 4 | 8 | 12 |
| PCS-12 | |||
| Mean (SD) | 40.7 (10.6) | 42.7 (11.7) | 42.0 (10.9) |
| Median (minimum, maximum) | 39.9 (28.8, 54.3) | 38.5 (31.3, 62.4) | 38.5 (28.8, 62.4) |
| MCS-12 | |||
| Mean (SD) | 54.6 (8.4) | 52.2 (16.0) | 53.0 (13.6) |
| Median (minimum, maximum) | 56.1 (43.1, 63.2) | 53.0 (23.2, 70.6) | 54.7 (23.2, 70.6) |
| Month 3 | |||
| n | 4 | 2 | 6 |
| PCS-12 | |||
| Mean (SD) | 33.3 (10.1) | 40.5 (20.6) | 35.7 (12.6) |
| Median (minimum, maximum) | 36.7 (18.6, 41.2) | 40.5 (25.9, 55.0) | 36.7 (18.6, 55.0) |
| MCS-12 | |||
| Mean (SD) | 45.1 (10.7) | 57.2 (9.7) | 49.1 (11.3) |
| Median (minimum, maximum) | 41.6 (37.0, 60.1) | 57.2 (50.4, 64.1) | 47.9 (37.0, 64.1) |
| Month 6 | |||
| n | 4 | 4 | 8 |
| PCS-12 | |||
| Mean (SD) | 32.2 (11.1) | 46.8 (13.8) | 39.5 (14.0) |
| Median (minimum, maximum) | 30.5 (21.0, 46.9) | 42.9 (35.0, 66.4) | 37.4 (21.0, 66.4) |
| MCS-12 | |||
| Mean (SD) | 43.0 (14.7) | 49.4 (16.1) | 46.2 (14.7) |
| Median (minimum, maximum) | 41.0 (27.3, 62.8) | 49.1 (34.2, 65.3) | 41.0 (27.3, 65.3) |
FIGURE 5.
Outcome measure data: SF-12 (PCS) by trial arm and time point. PCS, physical component score.
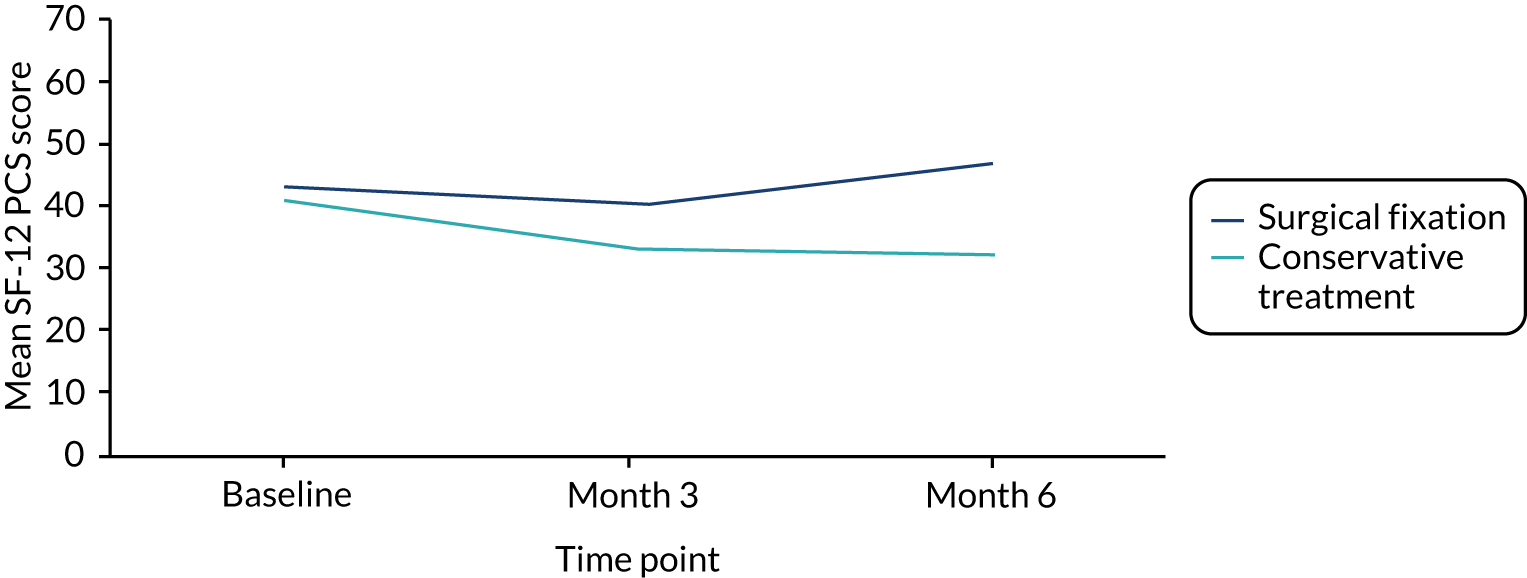
FIGURE 6.
Outcome measure data: SF-12 (MCS) by trial arm and time point. MCS, mental component score.
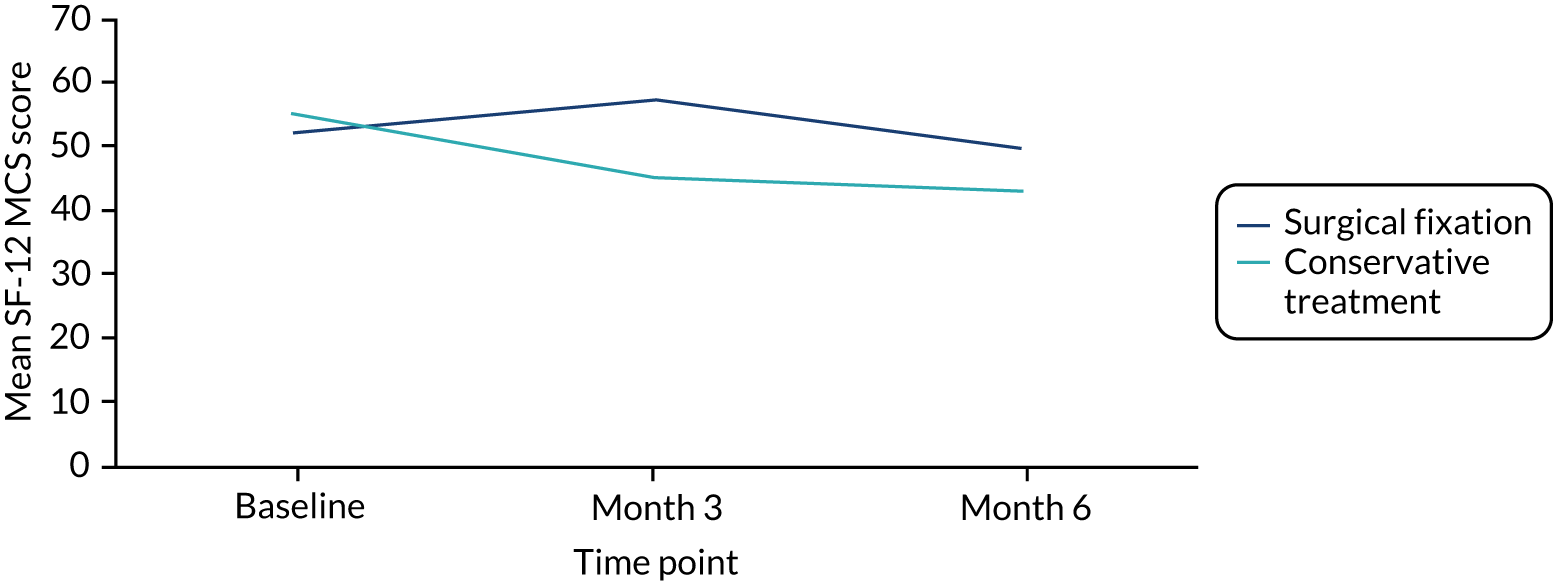
Adverse events and complications
In this trial, both complications and AEs were required to be reported, as well as details of any additional surgery. As one arm of the trial was surgery, a number of complications were to be expected and, therefore, were not deemed AEs.
No AEs were recorded in this trial. All complications recorded, broken down by category, are reported, by treatment received, in Tables 12 and 13; the two patients who crossed over from the surgical arm to conservative treatment are included in Table 13 (a total of six patients in each table).
| Complication | Week 2 (N = 5), n | Month 3 (N = 5), n | Month 6 (N= 4), n |
|---|---|---|---|
| Surgical site infection | 3 | 0 | 0 |
| Deep-wound infection | 1 | 0 | 0 |
| Delayed wound healing | 1 | 0 | 0 |
| Screw-related complication | 0 | 1 | 0 |
| Persistent pain | 2 | 0 | 1 |
| Scarring | 2 | 1 | 1 |
| Stiffness | 2 | 2 | 1 |
| Chest infection | 1 | 0 | 0 |
| Nausea and vomiting | 1 | 0 | 0 |
| Intermittent ache in spinal area | 0 | 1 | 0 |
| Complication | Week 2 (N = 6), n | Month 3 (N = 5), n | Month 6 (N = 4), n |
|---|---|---|---|
| Degenerative change | 0 | 1 | 0 |
| Persistent pain | 3 | 0 | 0 |
| Brace issues (e.g. comfort) | 2 | 0 | 0 |
| Gibbus | 0 | 1 | 0 |
| Stiffness | 0 | 1 | 0 |
Two patients who were allocated to surgery (and who had the treatment as randomised) went on to have additional surgery. The following complications were not experienced by any patient: implant problems, nerve or spinal cord injury, vascular injury, paralysis, bowel or bladder dysfunction, spinal instability, myocardial infarction, stroke, venous thromboembolism requiring treatment, non-union, neurological deterioration or skin complaints (e.g. blistering).
Patient and treatment information
Patient and surgeon preferences
Non-consenting patients
Of the 15 patients who did not consent to participate in the trial but expressed a preference for one of the two treatments, 13 (86.7%) said that they had a preference for conservative care and two (13.3%) said that they would prefer to have surgical fixation.
Surgeon preference was also requested in the case of the patients who did not provide consent. Of the 15 responses, 13 (86.7%) advised that the patient should have no surgery and the remaining two (13.3%) indicated that they were uncertain of what treatment to advise to the patient.
Randomised patients
At baseline, the 12 patients randomised to the study were asked to indicate if they would rather have surgery, no surgery or if they did not have a preference. None of the patients had a preference for no surgery, seven (58.3%) patients would have preferred surgery and 5 (41.7%) patients had no preference.
Kyphotic angle measurements
Table 14 summarises the kyphotic angle measurements at each time point for the patients randomised into the trial, broken down by arm. The baseline data are the same as is reported in Table 2, but are reported again here to allow for comparisons.
| Kyphotic angle measurement (°) | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Baseline | |||
| Supine, n | 3 | 7 | 10 |
| Mean (SD) | 11.5 (6.2) | 12.9 (7.4) | 12.5 (6.7) |
| Median (minimum, maximum) | 12 (5, 17.4) | 16 (3, 23.8) | 14 (3, 23.8) |
| Standing, n | 1 | 1 | 2 |
| Mean (SD) | – | – | 23.5 (9.2) |
| Median (minimum, maximum) | 17 (–) | 30 (–) | 23.5 (17, 30) |
| Week 2 | |||
| Standing, n | 4 | 4 | 8 |
| Mean (SD) | 18.3 (6.1) | 17.5 (11.7) | 17.9 (8.7) |
| Median (minimum, maximum) | 18.5 (11, 25) | 16.5 (7, 30) | 18.5 (7, 30) |
| Month 3 | |||
| Standing, n | 3 | 7 | 10 |
| Mean (SD) | 22.3 (6.0) | 13.3 (10.5) | 16 (10.1) |
| Median (minimum, maximum) | 23 (16, 28) | 9 (4, 34) | 14 (4, 34) |
| Month 6 | |||
| Standing, n | 1 | 1 | 2 |
| Mean (SD) | – | – | 19.5 (0.7) |
| Median (minimum, maximum) | 19 (–) | 20 (–) | 19.5 (19, 20) |
Treatment information
Methods used to establish spinal stability
Of the 12 patients randomised into the trial, nine (75.0%) had their fracture diagnosed using one type of imaging and three patients had their fracture diagnosed using two types of imaging. X-rays were used to diagnose a fracture in four patients: two standing, one sitting and one supine. One patient underwent MRI as part of the diagnosis process and 10 patients were diagnosed using CT. The kyphotic angle was measured using standing X-ray in two patients and on a CT scan in 10 patients.
Details of surgical fixation used
The average length of stay (admission to discharge) among the six patients who received surgery was 2.1 (SD 1.7) weeks. The median was 1.8 weeks, with the shortest stay being 0.3 weeks and the longest stay being 4.7 weeks.
During surgery, all patients were given general anaesthetic only. The average length of time spent in theatre was 164.8 (SD 40.5, range 132–234) minutes. The average duration of surgery was 119.7 (SD 43.9, range 80–180) minutes.
Of the six patients undergoing surgery, four underwent minimally invasive surgery (66.7%) and two had open surgery (33.3%). The stabilisation method was used in all surgeries, with one (16.7%) surgery also using the fixation method. All five reported incision types were posterior. In half of the surgeries, O-arm™ (Medtronic, Dublin, Ireland)/fluoroscopy-guided imaging was used, in two surgeries X-ray guidance was used, and in the remaining surgery both O-arm/fluoroscopy-guided imaging and X-rays were used.
The number of vertebrae fixed ranged from 1 to 5.
All patients were given antibiotics, such as cefuroxime (n = 1), teicoplanin (n = 4), flucloxacillin (n = 1), gentamycin (n = 2) and ceftriaxone (n = 1). Some patients received more than one type of antibiotic. Deep-vein thromboprophylaxis was used for all patients. During the surgeries, no patients were given a blood transfusion and the maximum blood loss was 600 ml.
The three types of implant used were USS™ Schanz screws (DePuy Synthes, Inc., Warsaw, IN, USA) (one patient), polyaxial screws (three patients) and percutaneous screws (two patients). The screws were of diameter 6 or 6.5 mm. The rod diameter was either 5.5 or 6 mm. All the rods used were made of titanium. No cross-links were used in any of the six surgeries. Only one patient required a graft. No patients had any intraoperative complications. Post operation, five patients were instructed to mobilise as normal and one was given postoperative braces to wear. No patients experienced any unexpected events or procedures during surgery.
Details of conservative management
All participants who were assigned to conservative treatment (or crossed over to conservative treatment) wore a brace. All the braces used were non-customised. One patient who underwent surgery also wore a brace.
At the 2-week follow-up, of the six patients who received conservative treatment, three were advised to wear the brace 24 hours a day and the remaining three were instructed to wear the brace only during the day. At month 3, among the five patients treated conservatively for whom instructions were recorded, one patient was instructed to wear the brace for the same amount of time previously and four were instructed to reduce the amount of time that the brace was worn.
The end date of wearing a brace was captured for only one participant.
Length of hospital stay
Data for length of hospital stay were collected for all 12 patients. The overall average length of stay, calculated from date of treating hospital admission (if not available, date of initial hospital admission was used) to date of discharge was 1.74 (SD 1.3) weeks. The median length of stay was 1.3 (range 0.1–4.7) weeks. In the case of one patient, two different dates were reported for initial hospital admission and treating hospital admission, which were 28 days apart. This patient was not included in the calculation of mean hospital stay, as this was calculated using treating hospital admission date, when possible.
Broken down by trial arm, the mean length of stay was 1.6 (SD 0.6) weeks among the four patients randomised to conservative treatment and 1.8 (SD 1.5) weeks among the eight patients randomised to surgical treatment.
Rehabilitation
Six out of eight patients allocated to surgery reported receiving some form of physiotherapy in hospital from a physiotherapist, spinal clinical nurse specialist or orthoptist. An average of 6.6 (SD 5.5; based on data from five patients) sessions were attended. Two patients received advice or education on bracing, six received advice on mobilisation, two received advice on precautions that they should take and three received advice about exercises.
Three of the four patients allocated to conservative treatment reported some form of physiotherapy from a physiotherapist, spinal clinical nurse specialist or orthoptist in hospital. An average of 2 (SD 1; based on data from two patients) sessions were attended. Two patients received advice about bracing, three received advice about mobilisation, one received advice about precautions and one received advice about exercises.
Time to return to work
Of the eight patients who were randomised to have surgical fixation, five were in some form of employment. Of the four patients who were randomised to conservative management, two were in some form of employment. Date of return to work was reported by four patients, which was, on average, 49.3 (SD 20.2) days after their injury.
Time to return to normal activities
Of the six patients who returned their 3-month questionnaire, only one (conservative allocation) reported that they had been able to fully carry out unpaid daily activities (e.g. household chores, shopping, socialising with friends and family). Hence, five patients (three conservative allocation, two surgical allocation) reported that they had been unable to carry out some of these activities. When asked how many hours per week they had been unable to carry out unpaid activities over the previous 3-month period, they had not been able to do so for an average of 53.8 (SD 49.0) hours (based on three responses).
Of eight patients who returned their 6-month questionnaire, three (two allocated to surgery, one allocated to conservative treatment) reported that they had been unable to carry out some unpaid daily activities between 3 and 6 months. The average number of hours that they had not been able to do such activities (per week) was 31 (SD 35.4) hours, which was based on responses from two out of the three patients. The remaining five participants (three conservative allocation, two surgery allocation) reported that they had been able to fully carry out their daily activities.
Chapter 4 Economic outcomes assessment
Resource use
Physiotherapy
Information about the number and type of NHS physiotherapy sessions that patients received is outlined in Table 15.
| Physiotherapy session | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) | |||
|---|---|---|---|---|---|---|
| Month 3 (N = 4) | Month 6 (N = 4) | Month 3 (N = 2) | Month 6 (N = 4) | Month 3 (N = 6) | Month 6 (N = 8) | |
| NHS physiotherapy (hospital) | ||||||
| Missing, n (%)a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Received physiotherapy, n (%) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 1 (25.0) | 1 (16.7) | 2 (25.0) |
| Number of sessions | ||||||
| Mean (SD) | – | – | – | – | – | 8 (2.8) |
| Median (minimum, maximum) | 2 (–) | 6 (–) | – | 10 (–) | 2 (–) | 8 (6, 10) |
| Average duration of a session (minutes) | ||||||
| Mean (SD) | – | – | – | – | – | 32.5 (17.7) |
| Median (minimum, maximum) | 20 (–) | 20 (–) | – | 45 (–) | 20 (–) | 32.5 (20, 45) |
| NHS physiotherapy (community) | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Received physiotherapy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NHS physiotherapy (private) | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Received physiotherapy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (25.0) |
| Average number of sessions | ||||||
| Mean (SD) | – | – | – | 15 (1.4) | – | 15 (1.4) |
| Median (minimum, maximum) | – | – | – | 15 (14, 16) | – | 15 (14, 16) |
| Average duration of a session (minimum) | ||||||
| Mean (SD) | – | – | – | 30 (0) | – | 30 (0) |
| Median (minimum, maximum) | – | – | – | 30 (30, 30) | – | 30 (30, 30) |
Inpatient care
No inpatient care was reported by any of the six participants who completed the 3-month questionnaire or any of the eight participants who completed the 6-month questionnaire.
Outpatient care
Table 16 summarises any outpatient appointments that were attended by participants between 0 and 3 months and between 3 and 6 months.
| Outpatient appointment | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) | |||
|---|---|---|---|---|---|---|
| Month 3 (N = 4) | Month 6 (N = 4) | Month 3 (N = 2) | Month 6 (N = 4) | Month 3 (N = 6) | Month 6 (N = 8) | |
| Orthopaedic | ||||||
| Missing, n (%)a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Attended appointment, n (%) | 4 (100.0) | 1 (25.0) | 1 (50.0) | 4 (100.0) | 5 (83.3) | 5 (62.5) |
| Number of visits | ||||||
| Mean (SD) | 2 (1.4) | – | – | 1.75 (0.5) | 2 (1.2) | 1.6 (0.5) |
| Median (minimum, maximum) | 1.5 (1, 4) | 1 (–) | 2 (–) | 2 (1, 2) | 2 (1, 4) | 2 (1, 2) |
| Pathology | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Radiology | ||||||
| Missing, n (%)a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Attended appointment, n (%) | 4 (100.0) | 1 (25.0) | 1 (50.0) | 2 (50.0) | 5 (83.3) | 3 (37.5) |
| Number of visits | ||||||
| Mean (SD) | 2.3 (1.9) | – | – | – | 2.2 (1.6) | 1.5 (0.7) |
| Median (minimum, maximum) | 1.5 (1, 5) | 1 (–) | 2 (–) | 2 (–) | 2 (1, 5) | 1.5 (1, 2) |
| A&E | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| Number of visits | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | – | 2 (–) | – | – | – | 2 (–) |
| Pain clinic | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rehabilitation unit | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mental health service | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
No patients reported any usage of hospital transport on the 3-month questionnaire. A single patient reported using hospital transport once (surgery arm) between 3 and 6 months. There were no missing responses for hospital transport on either questionnaire.
Community care
Table 17 summarises all participant contact with any health professional in the community necessitated by the spine fracture. No patients reported any of the following at any time: having a GP home visit, seeing a practice nurse at a general practice or seeing a district nurse.
| Community care appointment | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) | |||
|---|---|---|---|---|---|---|
| Month 3 (N = 4) | Month 6 (N = 4) | Month 3 (N = 2) | Month 6 (N = 4) | Month 3 (N = 6) | Month 6 (N = 8) | |
| GP visit at general practice | ||||||
| Missing, n (%)a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Attended appointment, n (%) | 3 (75.0) | 2 (50.0) | 1 (50.0) | 0 (0.0) | 4 (67.7) | 2 (25.0) |
| Number of contacts | ||||||
| Mean (SD) | 1.7 (0.6) | 4 (0) | – | – | 1.5 (0.6) | 4 (0) |
| Median (minimum, maximum) | 2 (1, 2) | 4 (4, 4) | 1 (–) | – | 1.5 (1, 2) | 4 (4, 4) |
| GP home visits | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| GP telephone contacts | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| Number of contacts | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | – | 3 (–) | – | – | – | 3 (–) |
| Practice nurse at general practice | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Occupational therapist | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (25.0) |
| Number of contacts | ||||||
| Mean (SD) | – | – | – | 3 (2.8) | – | 3 (2.8) |
| Median (minimum, maximum) | – | – | – | 3 (1, 5) | – | 3 (1, 5) |
| District visits | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mental health services | ||||||
| Missing, n (%)a | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Attended appointment, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (12.5) |
| Number of contacts | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | – | – | – | 1 (–) | – | 1 (–) |
Private treatments
Participants were asked whether they had seen a private specialist for a clinical assessment or a surgical treatment between 0 and 3 months and between 3 and 6 months. A single patient, from the surgery arm, attended one session with a private specialist for an assessment between 0 and 3 months. Two patients in the surgical arm reported attending sessions for a clinical assessment between 3 and 6 months (average of two sessions). At 3 months, one patient in the surgical arm did not respond to the question about whether or not they had seen private specialist for a surgical treatment.
Medications
Table 18 reports the medications prescribed and bought in relation to the participants’ spine fracture.
| Medication | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) | |||
|---|---|---|---|---|---|---|
| Month 3 (N = 4) | Month 6 (N = 4) | Month 3 (N = 2) | Month 6 (N = 4) | Month 3 (N = 6) | Month 6 (N = 8) | |
| Paracetamol | ||||||
| Taken, n (%) | 1 (25.0) | 1 (25.0) | 1 (50.0) | 2 (50.0) | 2 (33.3) | 3 (37.5) |
| Number of days | ||||||
| Mean (SD) | – | – | – | 11.5 (12.0) | – | 11.5 (12.0) |
| Median (minimum, maximum) | 30 (–) | – | – | 11.5 (3, 20) | 30 (–) | 11.5 (3, 20) |
| Prescribed, n (%) | 1 (100.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (33.3) |
| Bought, n (%) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 2 (100.0) | 1 (50.0) | 2 (66.7) |
| Ibuprofen | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 30 (–) | – | – | – | 30 (–) | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Aspirin | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 30 (–) | – | – | – | 30 (–) | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Co-codamol | ||||||
| Taken, n (%) | 4 (100.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 4 (66.7) | 1 (12.5) |
| Number of days | ||||||
| Mean (SD) | 60 (42.4) | – | – | – | 60 (42.4) | – |
| Median (minimum, maximum) | 60 (30, 90) | – | – | – | 60 (30, 90) | – |
| Prescribed, n (%) | 4 (100.0) | 1 (100.0) | – | – | 4 (100.0) | 1 (100.0) |
| Bought, n (%) | 0 (0.0) | 0 (0.0) | – | – | 0 (0.0) | – |
| Codeine phosphate | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 30 (–) | – | – | – | 30 (–) | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Tramadol | ||||||
| Taken, n (%) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 30 (–) | – | – | – | 30 (–) | – |
| Prescribed, n (%) | 2 (100.0) | – | – | – | 2 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Diclofenac | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 30 (–) | – | – | – | 30 (–) | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Naproxen | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | 90 (–) | – | – | – | 90 (–) | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Sertraline | ||||||
| Taken, n (%) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | 90 (–) | – |
| Median (minimum, maximum) | 90 (–) | – | – | – | – | – |
| Prescribed, n (%) | 1 (100.0) | – | – | – | 1 (100.0) | – |
| Bought, n (%) | 0 (0.0) | – | – | – | 0 (0.0) | – |
| Gabapentin | ||||||
| Taken, n (%) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| Number of days | ||||||
| Mean (SD) | – | – | – | – | – | – |
| Median (minimum, maximum) | – | 90 (–) | – | – | – | 90 (–) |
| Prescribed, n (%) | – | 1 (100.0) | – | – | – | 1 (100.0) |
| Bought, n (%) | – | 0 (0.0) | – | – | – | 0 (0.0) |
Aids and adaptions
Between 0 and 3 months, four patients reported using at least one aid or adaption. Table 19 describes how many aids and adaptions patients received between months 0 and 3. No patients bought any adaptions or aids themselves during this time period. When the same question was asked about the time period 3–6 months, one patient reported buying a grab rail at the cost of £6.
| Aid/adaption | Conservative (N = 4) | Surgery (N = 2) | Overall (N = 6) |
|---|---|---|---|
| Crutches | |||
| n (%) | 2 (50.0) | 0 (0.0) | 2 (33.3) |
| Mean (SD) | 2 (0.0) | – | 2 (0.0) |
| Stick | |||
| n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | – | – | – |
| Zimmer frame | |||
| n (%) | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Mean (SD) | – | – | – |
| Grab rail | |||
| n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | – | – | – |
| Dressing aids | |||
| n (%) | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Mean (SD) | – | – | – |
| Bathing and washing aids | |||
| n (%) | 1 (25.0) | 1 (50.0) | 2 (33.3) |
| Mean (SD) | – | – | 1 (0.0) |
| Long-handle shoehorn | |||
| n (%) | 1 (25.0) | 0 (0.0) | 1 (16.7) |
| Mean (SD) | – | – | – |
EuroQol 5 Dimensions, five-level version
A summary of responses for each domain in the EQ-5D-5L is given in Table 20. Mean and SDs of the utility values corresponding to each time point are given in Table 21. Table 22 summarises the VAS component of the EQ-5D-5L. All of the patients who completed the EQ-5D-5L in the questionnaires completed the five dimensions and the health VAS in full.
| EQ-5D-5L dimension | Pre injury (collected at baseline) | Baseline | 3 months | 6 monthsa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conservative | Surgery | Conservative | Surgery | Conservative | Surgery | Conservative | Surgery | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Mobility | ||||||||||||||||
| Level 1 | 2 | 50.0 | 8 | 100.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 3 | 37.5 | 2 | 50.0 | 4 | 57.1 |
| Level 2 | 2 | 50.0 | 0 | 0.0 | 1 | 25.0 | 1 | 12.5 | 1 | 25.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 |
| Level 3 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 2 | 25.0 | 2 | 50.0 | 1 | 12.5 | 1 | 25.0 | 0 | 0.0 |
| Level 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Level 5 | 0 | 0.0 | 0 | 0.0 | 2 | 50.0 | 4 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 3 | 42.9 |
| Reporting problemsb | 2 | 50.0 | 0 | 0.0 | 4 | 100.0 | 8 | 100.0 | 3 | 75.0 | 2 | 40.0 | 2 | 50.0 | 0 | 0.0 |
| Self-care | ||||||||||||||||
| Level 1 | 4 | 100.0 | 8 | 100.0 | 0 | 0.0 | 1 | 12.5 | 1 | 25.0 | 3 | 37.5 | 1 | 25.0 | 4 | 57.1 |
| Level 2 | 0 | 0.0 | 0 | 0.0 | 2 | 50.0 | 0 | 0.0 | 2 | 50.0 | 2 | 25.0 | 2 | 50.0 | 0 | 0.0 |
| Level 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 1 | 25.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Level 4 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Level 5 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 3 | 42.9 |
| Reporting problemsb | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 | 7 | 87.5 | 3 | 75.0 | 2 | 40.0 | 3 | 75.0 | 0 | 0.0 |
| Usual activities | ||||||||||||||||
| Level 1 | 2 | 50.0 | 8 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 1 | 25.0 | 2 | 28.6 |
| Level 2 | 2 | 50.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 3 | 75.0 | 2 | 25.0 | 0 | 0.0 | 2 | 28.6 |
| Level 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 1 | 25.0 | 2 | 25.0 | 2 | 50.0 | 0 | 0.0 |
| Level 4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Level 5 | 0 | 0.0 | 0 | 0.0 | 3 | 75.0 | 6 | 75.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 3 | 42.9 |
| Reporting problemsb | 2 | 50.0 | 0 | 0.0 | 4 | 100.0 | 8 | 100.0 | 4 | 100.0 | 4 | 80.0 | 3 | 75.0 | 2 | 50.0 |
| Pain/discomfort | ||||||||||||||||
| Level 1 | 2 | 50.0 | 7 | 87.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Level 2 | 1 | 25.0 | 0 | 0.0 | 2 | 50.0 | 1 | 12.5 | 2 | 50.0 | 4 | 50.0 | 1 | 25.0 | 3 | 42.9 |
| Level 3 | 1 | 25.0 | 0 | 0.0 | 1 | 25.0 | 6 | 75.0 | 1 | 25.0 | 1 | 12.5 | 0 | 0.0 | 1 | 14.3 |
| Level 4 | 0 | 0.0 | 1 | 12.5 | 1 | 25.0 | 1 | 12.5 | 1 | 25.0 | 0 | 0.0 | 2 | 50.0 | 0 | 0.0 |
| Level 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 3 | 42.9 |
| Reporting problemsb | 2 | 50.0 | 1 | 12.5 | 4 | 100.0 | 8 | 100.0 | 4 | 100.0 | 5 | 100.0 | 3 | 75.0 | 4 | 100.0 |
| Anxiety/depression | ||||||||||||||||
| Level 1 | 2 | 50.0 | 4 | 50.0 | 1 | 25.0 | 2 | 25.0 | 1 | 25.0 | 4 | 50.0 | 1 | 25.0 | 3 | 42.9 |
| Level 2 | 2 | 50.0 | 3 | 37.5 | 1 | 25.0 | 4 | 50.0 | 2 | 50.0 | 1 | 12.5 | 1 | 25.0 | 1 | 14.3 |
| Level 3 | 0 | 0.0 | 0 | 0.0 | 4 | 25.0 | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Level 4 | 0 | 0.0 | 1 | 12.5 | 1 | 25.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Level 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 3 | 42.9 |
| Reporting problemsb | 2 | 50.0 | 4 | 50.0 | 3 | 75.0 | 6 | 75.0 | 3 | 75.0 | 1 | 20.0 | 3 | 75.0 | 1 | 25.0 |
| Time point | Conservative, mean (SD) | Surgery, mean (SD) |
|---|---|---|
| Pre injury (collected at baseline) (n = 12) | 0.83 (0.2) | 0.87 (0.2) |
| Baseline (n = 12) | 0.11 (0.4) | 0.12 (0.3) |
| 3 months (n = 9, 4 conservative, 5 surgery) | 0.52 (0.3) | 0.72 (0.1) |
| 6 months (n = 8, 4 conservative, 4 surgery ) | 0.48 (0.5) | 0.80 (0.1) |
| VAS score | Conservative (N = 4) | Surgery (N = 8) | Overall (N = 12) |
|---|---|---|---|
| Pre injury (collected at baseline) | |||
| n | 4 | 8 | 12 |
| Mean (SD) | 85 (5.8) | 86.3 (10.3) | 85.8 (8.7) |
| Median (minimum, maximum) | 85 (80, 90) | 90 (70, 100) | 90 (70, 100) |
| Baseline | |||
| n | 4 | 8 | 12 |
| Mean (SD) | 61.3 (35.2) | 46.3 (23.1) | 51.3 (27.1) |
| Median (minimum, maximum) | 72.5 (10, 90) | 47.5 (5, 90) | 50 (5, 90) |
| Month 3 | |||
| n | 4 | 5 | 9 |
| Mean (SD) | 55 (29.2) | 70.2 (20.3) | 63.4 (24.3) |
| Median (minimum, maximum) | 52.5 (30, 85) | 75 (41, 91) | 75 (30, 91) |
| Month 6 | |||
| n | 4 | 4 | 8 |
| Mean (SD) | 50 (31.6) | 81.3 (9.0) | 65.6 (27.2) |
| Median (minimum, maximum) | 45 (20, 90) | 78 (75, 94) | 75 (20, 94) |
From Table 20, we can see that a high percentage of participants across both arms experienced problems across all five EQ-5D-5L domains at baseline, as expected given the nature of the injury. Given the small number of participants, it is not possible to draw conclusions, but some responses of note are:
-
No patients in the surgical arm reported any problems with mobility or self-care at the 6-month follow-up.
-
All participants except one in the conservative arm reported having problems with pain and discomfort 6 months after their injury.
The summary scores in Table 21 indicate that patients in the surgical arm were, on average, closer to their preinjury health state at 6 months than patients in the conservative arm (means of 0.48 and 0.80, respectively).
Chapter 5 Results of the qualitative study
Patient participant characteristics
Five patients who agreed to take part in the PRESTO study were interviewed. No interviews were conducted with patients who declined to take part in the study. The sample consisted of four males and one female, with all but one participant allocated to receive surgical fixation. The causes of patients’ injuries were assault, train collision, falling, cycling accident and jumping over a railing. Further details of the PRESTO study participants are provided in Table 23.
| Participant ID | Treatment received | Gender | Other injuries sustained | Referred from local hospital | Employment status |
|---|---|---|---|---|---|
| P01 | Conservative management | Male | Not specified | No, direct to MTC | Self-employed |
| P02 | Surgical fixation | Female | Yes | No, direct to MTC | Not specified |
| P03 | Surgical fixation | Male | Yes | No, direct to MTC | Employed |
| P04 | Surgical fixation | Male | Yes | Yes | Unemployed |
| P05 | Surgical fixation | Male | None | No, direct to MTC | Employed |
Surgeon and trial recruiter participant characteristics
Twenty surgeons and trial recruiters expressed an interest in taking part in a qualitative interview. However, one participant failed to provide consent and so was not included in the analysis. Interviews were, therefore, conducted with 19 surgeons and trial recruiters. Eleven participants were surgeons, physiotherapists or research associates involved in recruiting patients to the PRESTO study. The remaining eight participants were surgeons who routinely treat patients with thoracolumbar fractures, but were from sites not involved in the PRESTO study. Participants represented 13 hospital sites, the majority of which were in England, Wales and Northern Ireland. One individual who had previously worked in England but who had relocated outside the UK was also interviewed. Twelve participants were considered to have research experience, which was because of their role (i.e. PRESTO PIs and research associates), or because they discussed having published, obtained research funding or been involved in the delivery of research projects. Further details of the PRESTO trial surgeon and recruiter interview participants are provided in Table 24.
| ID | Job role |
|---|---|
| 01 | Surgeon |
| 02 | Surgeon |
| 03 | Surgeon |
| 04 | Trial recruiter |
| 05 | Surgeon |
| 06 | Surgeon |
| 07 | Surgeon |
| 08 | Surgeon |
| 09 | Surgeon |
| 10 | Trial recruiter |
| 11 | Trial recruiter |
| 12 | Surgeon |
| 13 | Surgeon |
| 14 | Surgeon |
| 15 | Trial recruiter |
| 16 | Trial recruiter |
| 17 | Surgeon |
| 18 | Surgeon |
| 19 | Surgeon |
Qualitative interviews with surgeons, PRESTO trial recruiters and patients provide insight into their views on the intervention and trial processes, and highlight potential barriers to and facilitators of recruitment and retention that could be used to inform the design of a main trial. Findings are presented according to five main themes: (1) current treatment for stable thoracolumbar fractures; (2) factors influencing patient decision-making; (3) practicalities of approaching patients to be involved in the PRESTO study; (4) designing a full-scale trial; and (5) changing clinical practice.
Current treatment for stable thoracolumbar fractures
Surgeons’ views of the treatment that should be provided to patients with stable thoracolumbar fractures were strong, irrespective of whether they advocated conservative or surgical management (Box 1). Usual practice was known to vary throughout the UK, largely according to individual consultant preferences. The majority of surgeons in our sample reported a preference for managing stable thoracolumbar fractures conservatively, perceived by many to be the most commonly prescribed treatment for stable thoracolumbar fractures in the UK. However, what was considered conservative treatment varied. For example, some were opposed to the use of braces and preferred ‘monitoring only’, whereas others routinely prescribed braces.
06: In our unit we believe in conservative treatment for this, which is obviously the prescribed mode of treatment at this moment, but again treatment varies based on individual consultant’s preferences.
08: But there is no treatment for a stable [thoracolumbar] fracture. Everyone would agree with that.
09: So out of 100 fractures, probably 90 patients we treat conservatively, so without braces. Maybe five or six in brace and two or three by operation.
09: I’ve answered from my perspective, it doesn’t mean that it reflects off all of spinal surgeons in the UK. But the thing is you will find most of the surgeons have a one-sided view because this topic, your topic, is not a balanced topic. That’s why there’s no way, except for those surgeons you’re talking to who operate left, right and centre for all stable fractures and you will find more conservative view from most of the surgeons.
03: We’ve got more tendency to manage these non-operatively with fairly good outcomes. So, people will have more of an inclination towards non-operative medicine. We’ll also be dealing with people and we do manage these patients with vertebroplasty and kyphoplasty as well, so then you’ve got an additional procedure that people might be using rather than screw construct to stabilise.
A number of factors were reported to influence whether a surgeon recommends that a patient has surgical or conservative treatment. This was true even for surgeons who held strong preferences for a certain treatment, with each patient, fracture and situation considered unique. The number and range of factors influencing preferences and treatment for stable thoracolumbar fractures, which are discussed below, highlight the complex and nuanced nature of surgeon preferences and clinical decision-making.
Defining stability
A number of surgeons who reported routinely providing conservative treatment for stable thoracolumbar fractures felt that there was no justification for operating on stable fractures, with some claiming that only a small proportion of UK surgeons would treat these fractures surgically. For these individuals, surgical treatment would only be considered if the stable fracture showed signs of instability through kyphosis, neurological deficit or collapse at follow-up:
I don’t see how any person can justify stabilising something that’s already stable.
Right, OK.
I mean you can fuse a stable fracture for pain management but that’s not stabilising that’s fusing. That’s a different objective. That’s pain management but you’re not trying to [give] stability to something that is already stable.
The strong opinions on whether or not it is appropriate to operate on stable thoracolumbar fractures may be explained by the lack of consensus as to how stability is defined, with a number of surgeons attributing issues with defining stability to the variation in surgeon preferences and treatment of thoracolumbar fractures in the UK. One participant also commented that definitions of a thoracolumbar fracture can also vary, with some surgeons defining them as any fracture between T10 and L2 and others between 11th thoracic vertebra (T11) and L2:
I think the controversy might be that some people are treating fractures with surgery which other people might consider stable. The discrepancy is, is it stable or not.
08
Variation in defining stability may be explained by the number of methods used to determine stability in routine practice. The majority of surgeons discussed using the fracture pattern, neurological deficit, angle of kyphosis, pain and whether or not the spine collapses or moves on standing to determine spinal stability. However, whether some or all of these criteria are used varies, as do surgeons’ interpretations of the criteria and the extent to which imaging, such as MRI or CT, is used. The complexity of the decision is illustrated by the following quotation:
Our current practice is, if we have a patient who has come in with any form of injury and the CT scans show that they have a burst fracture or an A-type fracture, we will then examine them very carefully, to make sure the ligaments at the back of the spine are intact. If you feel down the back of the spine and they don’t have any tenderness or bruising or gaps between the bones, then we say it could be stable. We then get them standing, once they are comfortable standing, and that might take a day or two, and then get an X-ray, with them standing. There is a lot of, again, not very scientifically based decision-making here. Some surgeons believe that, if the spine bends more than 20 degrees in comparison to a CT scan, because the CT scan, by definition, is done with the patient lying down, and then you get them standing, and if the angle between the broken vertebrae, between the CT and the standing, is more than 20 degrees of change, then we might worry that, actually, this might not be as stable as we think and it’s best fixed. There is some evidence in the literature and, again, non-randomised trials, to say that if patients . . . the lumbar tends to be quite straight, so if your [inaudible] has got a zero-degree angle of kyphosis or forward bend, there is some evidence to suggest that, if they then develop a kyphosis more than 40 degrees, it could cause more low back pain than in the general population. I think that is one of the things people worry about. So, if the change between the CT and the X-ray within day 1 or 2 is more than 20 degrees, then the worry is that, by the time the fracture heals, in 6 months or something, they could end up with an angle that is more than 40 degrees. Using that sort of rationale, some surgeons would offer surgery . . . For ones less than 20 degrees, we would generally not operate on, but it depends on the department and their surgical threshold.
07
Surgeons also reported using various definitions and/or classification systems to define stability, with the AO spinal classification system, which categorises fractures as type A, B and C, the most frequently cited. Although there seemed to be agreement that type B and C fractures are unstable and so are likely to be treated surgically, uncertainty surrounded the ‘best’ treatment for type A fractures. Although type A fractures were generally considered stable, there were ‘some exceptions’ to this, which accounts for the variation in surgeon preferences and practice. For example, one surgeon felt that type A4s or burst fractures are where the surgical ‘grey area’ lies, whereas another felt that there was uncertainty between type A2 and A4 fractures. Participants also referred to the TLICS scoring system, which was perceived to be used mainly by ‘surgically proactive’ US surgeons and the ‘White and Punjabi’ definition of stability, which proposes that a spine is stable if there is no neurological deficit, no pain and the spine does not collapse on standing:
If you Google White and Panjabi there is a definition. These are two – I think there is a spinal surgeon and a mechanical engineer from the States, who came up with this definition, to say a spine is stable if there is not any neurological deficit, there is no severe pain, and the physiology of loading does not get deformed. So, if someone stands up and the spine collapses, then that is not stable. If it stays reasonably straight, it’s stable.
07
The difficulties of operationalising existing definitions were also reported, with many patients perceived to fall into a surgical ‘grey area’, and for whom ‘optimum’ treatment is unclear. Surgeons described how, in these cases, the determination of stability is influenced by clinical judgement and factors such as ‘the centre where you work’, ‘different perceptions of the risks and benefits of surgery’ and ‘surgeon experience’, rather than evidence. As a result, some surgeons felt that, before a full-scale trial could be conducted, consensus work is required to reach agreement on the definition of stability:
I would argue reality is you don’t see many patients that fit that [White and Punjabi] definition. With a lot of patients it’s just, well it hurts when I do this and it hurts a bit more when I do that and it hurts especially when I do that but it just hurts all the time. In my practice I don’t honestly think that I’ve seen many patients who truly fit that description apart from the ones that have already got an unstable fracture and you’re in no doubt at all that it needs fixing. I suppose depending on how firm you want to be with your lines you could argue . . . I think this is where it’s incredibly difficult because you could say, pain increases on mechanical loading but what do you define as your increase and can you have a strictly defined criteria. Because otherwise it’s incredibly subjective to what we call, does that mean sitting up in bed supported to a certain angle, does that mean sitting in a chair, sitting upright with no support, standing. It’s a difficult one and I suppose this is the issue why everyone is varied in their practice. It’s a very personal thing how you interpret the kind of definitions and how you feel you should apply them.
18
Patient preferences
Another factor that was perceived to influence clinical decision-making for stable thoracolumbar fractures was patient preference. Surgeons described how patients often have strong views on the treatment they wish to receive, with some reporting patients to be mainly pro surgery and others pro conservative management. Factors perceived to influence patient preference were pain; employment status; perceptions of a certain treatment leading to quicker recovery times; views on the risks of surgery; family members; previous experience of family members with braces or surgery; surgeon preferences; and how information is presented to them. Although one surgeon described the pressure for surgeons to ‘give people what they want’, others felt that even patients with strong preferences can be, depending on how information is presented to them, guided towards a certain treatment, which is often based on surgeon preference. For example, one surgeon described how they tell patients which treatment they would choose if they had a thoracolumbar fracture:
The concern about the risks of surgery. I think also, our population that we serve, we’re in the [location], we’ve got an older population. So, they may be more middle aged, they’re more established in their jobs, they’re less worried about losing their jobs perhaps. My previous practice [location], where you’ve got city workers and people in high-flying careers and things. They’re perhaps a little bit more worried about the impact on their employment of taking time out and things like that. They would tend to be more aggressive in trying to get back on their feet.
03
Other factors influencing clinical decision-making
Other factors influencing clinical decision-making when recommending treatment for patients with stable thoracolumbar fractures were also cited and are outlined in Table 25.
| Factor influencing clinical decision-making | Exemplar quote | |
|---|---|---|
| Evidence and training | Audits and observational research | You’ve got options, lie in bed or have an operation. What are the risks of an operation? Well it fuses your spine so it makes it stiffer. We can do damage by putting the screws in. It might not fuse. You might bleed a lot. You might even die. You might get a spinal cord injury as a result of the surgery but you will probably get out of hospital quicker. But if you lie in bed, the main thing is that you avoid surgery. Surgery is a destructive process. There is a small chance it won’t heal and you’ll end up spending time in bed and then having an operation. But the main risk that we quote is that there is a risk from lying in bed. There isn’t much information on that. I have done a retrospective study of some very difficult patients that I’ve treated with long periods of bed rest, like two or three months, and the complication rate is extremely low. Now that doesn’t prove anything but it supports my observation that actually where are all these complications08 |
| How surgeons were taught to treat stable thoracolumbar fractures | ||
| Specialty and geographical differences | Some specialties and areas in the UK are more likely than others to use surgical treatment. For example, in some hospitals, neurosurgeons are prone to prescribing braces, whereas orthopaedic surgeons are more likely to recommend surgery | Surgery has probably very little role to play in stable thoracolumbar fractures except in some areas of the UK where they operate more. Because every place is different isn’t it? Some places they operate more, some they operate less. Definitely we want better outcomes for patients09 |
| Clinical experience | ‘Journal of anecdotal medicine’: previous experiences (positive and negative) of prescribing certain treatments and observations of own practice. For example, some may have a tendency to overoperate to compensate for situations in which they did not, but feel they should have | Yeah I think in most cases it’s a product of who you trained with, what you’ve seen and actually I’m sure people have said it but one of the most powerful things is probably the ‘Journal of Anecdotal Medicine’ and burnt fingers but I think you can have one case that you get your fingers burnt with doing one thing or another and it’s very hard to look past, oh I did this and we had a really bad experience’. As a clinician it’s sometimes very hard to un-see that18 |
| Surgeons have a vested interest in surgery, ‘sometimes it is easier to do something than nothing’ | ||
| Surgeon’s ‘gut feeling’ | ||
| Risks of surgery vs. bracing | ||
| Positive views on bracing (e.g. clinical ease of prescribing a brace rather than surgery, brace less time-intensive) | ||
| Negative views on bracing (e.g. costs of a brace), particularly if patients are non-compliant, surgeons’ own experience of having a brace and compliance issues with bracing | ||
| Patient and fracture characteristics | Patients’ individual circumstances: employment, job role, frailty and age, osteoporosis, normal activity levels, mental health (whether or not taking antidepressants), smoker, diabetes | The personality of the fracture takes into account what the fracture itself looks like on an X-ray or on imaging, but also the environment that hints at how it actually occurred in the first place, the patient factors and then the ongoing patient factors. First of all, it might be a smoker. It might be a drug addict. They might be someone who is a championship motorcyclist. There are all sorts of different things and all those have different demands. All of those things feed into what we term the ‘personality of the fracture’, its likelihood of healing and the likelihood of the outcome13 |
| If patients are experiencing pain (e.g. pain on standing, long-term or chronic pain and/or pain after conservative management may indicate a need for surgery) | ||
| Polytrauma | ||
| Patient weight and height to determine amount of loading through the fracture | ||
| ‘What are people trying to avoid by offering surgery?’ For instance, if you are trying to avoid development of kyphosis or deformity then surgery may be recommended as the procedure becomes cosmetic | ||
| Outcomes | Surgeon- or patient-perceived quicker recovery time and reduced length of stay with certain treatments | Anecdotally that’s my view, is that they do often seem to get going a bit quicker. You can usually tell within the first few days. So, some fractures treated non-operatively settle down quite quickly and the pain goes away quite soon. Some of them seem to be painful for quite a bit longer and those that fall into that group definitely seem to respond to surgery. Then, you’ve got the pain from the surgery, which is a bit more predictable in terms of its improvement. Most improve over a few days following surgery. That’s based predominantly on anecdotal experience, I have to admit. I don’t know if there’s any high-level evidence to support that approach03 |
Ultimately, although surgeons, and indeed patients, may have strong preferences for a certain treatment, a range of situational, clinical and patient-specific factors influence decision-making around treatment for patients with stable thoracolumbar fractures in routine practice. These influences, along with the clinician’s experience, mean that each patient and fracture is considered unique and results in variation in practice by surgeon, specialty, hospital trust and geographical region of the UK:
I’m not saying any patient is different but it is comfort in your own practice, reading the literature, but comfort in your own practice that you’ve seen which makes you go, ‘No my experience tells me this’. That’s what we rely on, isn’t it? Of course we rely on evidence, but we rely on our own experience over the years and you go, ‘No this will be fine’, and that’s what we do there that this will work because I know this will work.
12
Factors influencing patient decision-making
The following themes highlight the challenges and complex nature of recruiting patients to RCTs and should be treated not in isolation, but rather as inter-related factors which influenced patient decision-making during recruitment to the PRESTO study. For example, surgeon equipoise may influence a patient’s willingness to be randomised.
Capacity and consent
Patients had difficulty describing, with any certainty or in detail, how they were approached about the study, which may have influenced their ability to make decisions about their treatment and entering the trial. Patients explained how the nature of their injuries, which in some cases included multiple injuries, and the medication they were prescribed meant that they found it difficult to recall recruitment and consent consultations, or to engage with discussions and written information about the study. Issues with capacity may also account for a few participants reporting having no recollection of discussions or receiving limited information about the treatment options available to them. Two participants who reported concerns regarding their capacity and who found it difficult to remember discussion surrounding treatment stated that family members were present for the majority of their inpatient stay:
But it probably would have been better if it was explained when I had someone with me, because as I say, I was very sleepy. I’d had lots of morphine, and I think I could just about keep my eyes open when they were talking to me. So it probably wasn’t ideal.
P02
In contrast, the majority of staff did not feel that consent and capacity was an issue during the PRESTO study, or would be a problem for a future trial. This was largely due to beliefs that the trial is ‘no different to what we do now’, with surgeons making reference to how they are routinely required to obtain informed consent from patients regarding their treatment, or make decisions on the behalf of patients who lack capacity through colleagues or consultees. One health-care professional who was not involved in the PRESTO study assumed that polytrauma patients would not be included in the study owing to potential issues with their capacity:
I don’t think that [capacity is] a problem most of the time. If you think about it, to do anything you have to get informed consent, whether it’s a trial or not. If I operate on someone, I have to get their consent. If I’ve discussed surgery or not surgery, implicitly they’re consenting to have not surgery if we pursue bed rest. Things can always change. I mean you can operate further down the line. I don’t think consent is an issue for the vast majority of people.
08
Although patients’ recollection regarding the exact details of who approached them and when they were approached about the study was often patchy, the majority were able to recall being approached shortly after admission. Although this was considered to be problematic in terms of their ability to consent or have capacity during this time, a couple of participants explained how speaking to members of the research team on multiple occasions and being given time to consider the study and read study documentation was helpful. Indeed, for one individual, being given time to consider their participation led them to change their mind about participating in the PRESTO trial:
I said no at first. I had only just come in that day, and I was, you know, not with it still, but then I changed my mind.
P01
Despite the concerns raised by patients about their capacity, PRESTO trial recruiters described a range of methods that were used to ensure that patients had capacity: ensuring that patients were able to reiterate the aims of the trial and what had been discussed; leaving study documentation with patients after initial consultations; having clinical and research staff present to reiterate the trial and consent process; discussing the trial with the patient’s friends or relatives; and following up on multiple occasions with patients to discuss their participation and consent. One research nurse emphasised the importance of viewing patients holistically, and considering not only their spinal injuries, but also that they may be in shock or in pain, have polytrauma and/or other illnesses (e.g. mental health problems) or be taking medications that may have an impact on their decision-making and capacity. However, the challenges of this were acknowledged: there can be, compared with other surgical studies and depending on the patient’s circumstances and the extent of their injuries, a relatively short window between when patients are admitted and when treatment needs to be initiated. This research nurse also criticised the clinical trials unit, which, in her opinion, had branded the study as ‘just a feasibility study’, and in doing so downplayed the importance of the consent process and the implications of this for patient treatment:
I think just the realisation that this is a big operation for patients, that this is a big deal for them, and not rushing the process. I’m sure nobody would. Most new research nurses are trained in the role to ensure that they would explain to a patient that they don’t have to join anything. Then to ensure that the patient has got plenty of time and plenty of support prior to signing a consent form and to ensure that they get that time and that support prior to making a decision. I think from a study point of view, just to realise that even though I’ve been told several times, ‘Well, yes, this is just a feasibility study’. That’s fine, but it’s still a big deal for the patients that are coming through on this study. It’s a big operation and they need time to consider, even after randomisation, whether or not surgery is right for them.
11
Patient willingness to be randomised
Three participants had prior knowledge of research that may have influenced their openness to taking part in the trial and willingness to be randomised. Although a couple of patients also had prior knowledge of randomisation, and demonstrated an awareness of why research is required, for others randomisation was a new concept that was considered ‘a little bit odd at first’:
It [randomisation] doesn’t faze me. I know medicine isn’t an exact science and that sometimes choices that even experts may make can be flawed because they’ve done it on the basis of information they’ve had at their disposal, whether they’ve internalised it or whatever and it can sometimes be flawed. It didn’t worry me.
P04
Participants’ knowledge of randomisation during interviews was therefore variable. Although a couple of participants could describe randomisation clearly, others showed a lack of understanding, which was, in some cases, influenced by capacity issues. For example, one patient reported having no recollection of any discussions around randomisation and explained that they were against the idea, as they received insufficient information about the pros and cons of each treatment and wanted to be involved in decisions about their care:
They told me it was 50/50 whether they would give me surgery, or I would be in a brace, and it was chosen quite randomly by the computer. No human influence on that, at all. They obviously said that my care and everything would be exactly the same as if I wasn’t on the trial.
So when they spoke to you about the study, did they mention about how the treatment gets allocated, so did they mention the idea of randomisation to you?
No.
OK. So, when you were entered into the trial, because they know that basically both treatments work, as they explained to you, they’re not sure which treatment is sort of best, what they do is they sort of approach people and they randomly allocate them to receive either the brace or the surgery. So, it would’ve been randomly decided which treatment you were allocated. So, that wasn’t explained to you at all?
I had no recollection of that.
When discussing how randomisation is described to patients, only one member of staff was against the use of the term randomisation. A number of staff involved in recruiting patients to the PRESTO study reported informing patients that, should they agree to participate, their treatment would be chosen by a computer, without human influence. However, others felt that the potential for randomisation to be perceived as taking control away from patients and their ability to choose which treatment they have should be taken into consideration:
Some people just don’t understand randomisation. I think that’s often the crux of the issue, the fact that this decision will be left up to a computer. That process is what usually gets – people are usually very, very, receptive to the sound of the trial, until they realise they don’t get to choose which treatment they have.
04
To overcome perceptions that randomisation takes control away from the patient, staff suggested emphasising equipoise and a patient’s right to withdraw during recruitment consultations. Staff also described how patients can be ‘put at ease’ by explaining that clinicians have the final say in their treatment and by reassuring them that they will receive appropriate treatment irrespective of whether or not they choose to take part in the trial. For example, explaining to patients allocated to receive the brace that they can ‘switch’ treatments if things are not going as planned during follow-up, or that prior to operating if the surgeon feels that surgery is no longer appropriate then patients will be treated conservatively, was believed to reassure patients. As a result, some surgeons suggested that it would be important for a future trial to account for a degree of crossover to allow patients who are unhappy with their treatment or who encounter complications to change allocation:
‘We are guided by the computer, purely because both the treatment options are correct’. That makes them feel a little bit more in control, like if you say that one is better than the other, ‘Why am I being randomised then?’ When you say to them that both the options are pretty equal, then they are happy and they know that there is always that opportunity to cross over, because you do say to them that, ‘If during the follow-up process that we are treating you with a brace, if we believe that things aren’t going in the right way, then trial, no trial, we’ll switch you to whatever’s appropriate’. That is the good thing that the patients recognise, that their treatment is carrying on as it should be, whichever way the trial goes, whether you go in the trial or you don’t go in the trial, everything put together. I think that should reassure them that we are more trying to treat them appropriately rather than the trial. The trial comes second.
12
This was supported by a number of patients who, when describing randomisation, reported not being involved in deciding their treatment. Although this was not necessarily considered to be a negative by all, knowing that clinical need would override treatment allocation and that they would be able to change treatments, should this be deemed necessary, was an important factor influencing a couple of patients’ decisions to take part in the PRESTO study:
I did consciously consider the possibility of switching from one to the other. The possibility or the fact of leaving things up to a machine to decide seemed neither here nor there. I felt if medically it was deemed that one treatment was likely to give the better treatment then that would be an option.
P04
Staff also described how issues with the referral pathway and awareness of the trial among other specialties [e.g. neurosurgery and emergency department (ED)] can affect the acceptability of randomisation to patients. For example, describing randomisation can be challenging when patients have been informed by another specialty (e.g. ED) that they will receive a certain treatment. Additionally, because patients were not always admitted directly to sites involved in the PRESTO study, delays in referring patients meant that by the time patients were approached about the study some had already been told that they would be receiving a certain treatment or, depending on the length of the delay, received a brace for stabilisation or begun to heal. Challenges associated with the referral pathway and engaging staff from specialties that are not directly involved in the PRESTO study are described in more detail in Designing a full-scale trial:
What we need help with is identifying the patients that are at an early enough stage so that we don’t look like . . . well I think we’ll look a little bit silly if we see someone 3 weeks down the line, they’re obviously getting better and then we say, ‘Well actually, do you think we can randomise you to an operation’.
13
Equipoise
Ensuring that patients understand clinical equipoise was also perceived to influence their willingness to be randomised. Surgeons and trial recruiters provided a number of examples of how equipoise was described to patients, which included explaining that both treatments are effective and will lead to good outcomes and that the clinical team are happy for patients to receive either treatment. Staff also reported describing that the aim of the study is to determine the ‘absolute optimum treatment’, as although some patients clearly need surgery or conservative treatment there is a group of ‘borderline’ patients for whom both treatments are effective. Explaining to patients that both treatments are routinely provided and portraying the process as ‘random anyway’, with the care patients receive routinely dependent on the treating clinician, was believed to compensate for the perceived ‘loss of control’ some patients feel when faced with randomisation:
The main point that we put across to the patient when we discuss randomisation is that at the present moment, the debate regarding the surgery or conservative options is pretty equal among the way consultants have been practising all across the UK. So there has been no preferred treatment for either of those options. And that is what we were trying to find, and detailing the options as both an equally suggestive and successful treatment options then makes the patients more receptive to the idea of randomisation.
06
Despite emphasising the importance of presenting both treatments equally during recruitment, staff raised a number of concerns regarding surgical equipoise, partly because they perceive that it is difficult for surgeons, who are ‘opinionated’, to admit to patients that they do not know what the right answer is. However, the majority of concerns were based on the strong views clinicians have in regards to how these fractures should be treated. In particular, the lack of consensus as to how stability is defined, whether or not surgery is considered a viable method for treating stable thoracolumbar fractures and perceptions that surgery exposes patients to a level of risk that conservative management does not, caused a number of surgeons in our sample to view the treatments as unequal:
I personally don’t think that offering surgery is equally as good as non-operative treatment for such fractures because surgery is probably overkill. If you don’t offer to your own self or your own family, then you don’t offer to patients either. So if you say there are 500 spine surgeons in the UK, all 500 spine surgeons if they have stable thoracolumbar fractures, you won’t find a single spine surgeon going for operation I can tell you that, so why patients then? This is my feeling, so I’m just wondering that it may be difficult to convince patients to go for the surgery, because both are not equally viable.
09
As a result, some surgeons and trial recruiters felt that there may be difficulty recruiting sites or surgeons who would be willing to randomise patients to a full trial. These concerns were highlighted during interviews with a couple of surgeons from sites not involved in the PRESTO study, who, because they had a strong preference for conservative management and felt that it is inappropriate to operate on stable fractures, stated that they would not be willing to randomise patients in a future trial:
I don’t think there’s much variability at all for stable fractures, except for a few places in the country, nobody operates for these patients. If I asked everyone, except for 5 or 6 or 10 surgeons in the UK they operate for stable fractures, otherwise people don’t operate. So those 10 surgeons you can identify first in the UK and then run the trials using those surgeons then you can finish the trial. Otherwise, you don’t want to start the trial and then take 2 or 3 years to recruit the patients, if you still want to run this trial.
09
Others, irrespective of their treatment preferences, spoke positively of the need for a trial and considered it necessary to address the variation in current practice. This was attributed, in part, to a recent ‘sea change’ among orthopaedic surgeons, who are perceived to be increasingly open towards involvement in RCTs despite the rarity of orthopaedic surgical trials in the past. It was, however, acknowledged that there would be a proportion of surgeons at every hospital who would not be willing to be involved in a full trial, and who would be resistant to randomisation and accepting the lack of evidence. One participant raised the issue of ‘local logistics’ and emphasised the importance of ensuring strict eligibility criteria and potential issues with theatre lists ahead of a full-scale trial. For example, recruitment to a future trial could be jeopardised if a consultant who is happy to be involved in the trial randomises a patient, but the patient is then allocated to receive surgery by another consultant who is reluctant to be involved. The way in which sites are approached to take part in a future trial was therefore considered crucial, with the state of current evidence considered an important vehicle for making a clear case for equipoise. Presenting a future trial, planned protocol and eligibility criteria at key clinical meetings, such as BASS meetings, was described as one mechanism for securing buy-in from the clinical community. Others suggested that issues with surgical equipoise could be overcome in part, by conducting a cluster trial:
I suppose my immediate thought is I have no issue with it. My second thought is I know for a fact if something I felt in my normal practice I really would not put it on this, I think that’s bonkers. Once randomised to surgery there would be a bit of, you know, I don’t know how comfortably this sits with my world view, but I also think that’s the whole issue here. We’re all doing what we think sits comfortable in our world view and there isn’t a good level of research evidence that perhaps we’re just doing what we think rather than what’s been proven. So I think on a, taking a step back, I would be happy to offer things. I think there has to be and I’m sure there would be, there has to be a way of saying, actually for some reason specific to this case, there are real concerns here that I really, really don’t feel this is right for this patient, but then arguably you shouldn’t be including them in the first [place].
18
A couple of surgeons involved in the PRESTO trial reported concerns among colleagues about surgical equipoise, recounting that they experienced some ‘initial pushback’ from colleagues when presenting the PRESTO trial at departmental meetings. This was not only true of staff in their own departments, but was also experienced when trying to secure buy-in of staff from other specialties, such as neurosurgery and ED (see Navigating patient pathways and Engaging other specialties and staff groups):
When we talked a little bit about this in our department and I said, ‘Look I’m thinking of participating in this trial. What do you think?’ quite a lot of the pushback from my colleagues was, ‘Actually, if I think this is stable, why would I want to offer somebody an operation?’ OK, so if I think somebody is going to heal up, then why would I want to put something that carries with it some extra risk without necessarily a clear benefit?’ I said, ‘Well, OK, this is surgical equipoise’.
13
Patients provided a number of examples of situations in which surgeons had not been in equipoise during recruitment to the PRESTO study. For example, one patient described how, after they were allocated to receive surgery, the surgeon stated that they would have preferred them to have a brace. A number of patients also reported being told by a surgeon or member of staff from another discipline (e.g. ED) that for clinical reasons they would receive surgery. This resulted in some patients feeling that they did not receive a choice of treatment and some patients developing preferences towards surgery:
All I know is that when I was offered the two treatments I’d already been told that I was going to have an operation, and my sons had already been informed that I was going to have an operation, as I say, because a bit of the bone had broken off. So that really was the only operation, or the only treatment that I could have had because I doubt if they could have left a bit of bone floating about. So it’s sort of a bit academic to me whether . . . put it in the computer for the computer to decide. It really didn’t mean anything to me.
P02
One patient, who raised concerns about their capacity during recruitment, reported not being given a choice of treatment when approached about the PRESTO study. Given their concerns over their capacity during recruitment, they asked their partner to corroborate via text message that they were not given a choice of treatment during the interview:
My partner who was there all the time was given a choice of having the surgery for my back or a brace and he said ‘No, they said surgery’.
P03
Even those who considered themselves to be in equipoise described situations in which they felt equipoise was difficult to maintain. For example, a couple of staff involved in the PRESTO study reported that, after explaining equipoise and that the clinical team would not be able to influence treatment, some patients asked what treatment surgeons would normally recommend. Although staff reported re-emphasising equipoise, they also described how they felt that it was important to be honest with patients in these situations:
I’ve not come across it much, but potentially there could be a patient who could ask, ‘Forget the trial, ensure baseline what would you have normally done with a situation like this?’. Then we’ve got to tell them that we will do X,Y, Z, we’ve got to tell them, we’ve got to be honest with them, we’ve got to be upfront with them. We can’t say to them, ‘I don’t know’. I know and I’ve treated so far, in 15 years I have treated patients. You have to be very upfront and say, ‘This is what I would be doing’.
12
Altruism
A number of patients decided to take part in the trial for altruistic reasons, such as feeling a ‘duty to the NHS’ and wanting to help others:
For completely non-selfish reasons now. You know. That it will help you guys understand what the best treatment for this kind of injury is. If you can make someone else’s life a bit more comfortable and better, because having been in it, you know, it’s a bit of a nightmare. [Laughter] Yes. So, yes; just to give something back.
P01
However, patients’ motivations for participating were not always entirely altruistic, with participation for some being dependent on being allocated surgery. Other reasons that patients gave for participation included perceptions that they would receive better care if they were part of a trial, wanting what they considered to be best for them and wanting to ‘stick two fingers’ to their parents, (who had a preference for surgery). This, along with discussions about equipoise, led to a degree of indifference towards participation for some patients who viewed the study as an opportunity and claimed ‘I’m going to be lying on my back anyway, so what the heck’:
They were explaining to me that it would be helping other people, but I said actually at the time I was more interested in what was best for me, rather than what was best for other people. But I was happy to go ahead with the trial and that I did want an operation. And if they did come back and say that it would have been the brace and not the operation I would have said that I didn’t want to be part of the trial.
P02
Although altruism was central to many of the discussions with patients about why they decided to take part in the trial, only a small number of staff reported using altruism in recruitment consultations:
We tell them that the options are that we could treat this non-operatively or we could treat it operatively, exactly like how we feel about it. Like we believe this may be right but we don’t have the right answer, and this will help us have the right answer.
12
Patient preferences
All patients who participated in the qualitative study reported having a preference for surgery prior to randomisation, largely because they thought that recovery would be quicker. However, return to work, comfort, ease of commuting, positive experiences of previous surgery, the influence of family members having received a brace, the impact of the brace on daily life and concerns about compliance with a brace also contributed to these preferences:
I said I would rather have the operation. I know it sounds . . . I had an operation on my lower back 10 years ago which was completely different. I researched – because I’m a single dad – I mean, my daughter’s with her other dad now who lives abroad for her 6 weeks holiday, which is actually a good time. That was planned anyway. You know, I didn’t want to be limited because I am a single dad, and I run a business. So, it’s like I wanted to recover as soon as possible.
P01
In contrast, staff involved in recruiting patients to the PRESTO study perceived patients to have a strong preference for one or other treatment. For example, although some PRESTO study patients were considered pro surgery, others were reported to be daunted by surgery and the associated risks when compared with conservative treatment. This led to some concern among staff regarding the acceptability of randomisation to patients, with one member of staff overcoming this issue by describing surgery as a ‘minor spinal operation’:
What we found was, compared to other trials . . . wrist fractures, which are not related to major operations, patients are a bit more open to randomisation. Whereas with spinal fractures, obviously everyone will have strong views. Some of them feel that trials have to be less ambitious, and this is the patients we are talking about. Some of them have strong preferences not to have an operation because they think it’s dangerous.
14
Patient treatment preferences may be a barrier to recruiting patients to a full-scale trial. Some patients reported being randomised to their preferred treatment, and staff involved in recruiting patients to PRESTO also knew of patients whose allocation aligned with their treatment preferences, but reported that others were willing to be randomised only provided they did not receive surgery, with a couple of patients stating that they would have withdrawn if they had not received their preferred treatment. As a result, staff felt that they would have been able to recruit more patients to the PRESTO study if the study was designed so that patients either were not randomised or were randomised and then withdrawn if they did not receive their preferred treatment. More conservative recruitment estimates or including a ‘natural follow-up arm’ for patients who were not willing to be randomised were recommended when designing a full-scale trial:
A number of them have said, ‘Well, I’m happy to be randomised as long as I don’t have an operation’. [Laughter] On a pragmatic level, I would probably have had, probably a few more patients who would be very happy to participate in not being randomised, or being randomised and continuing with being randomised to an operation, which is obviously not randomisation. I did have a chat with [X]. I said Look I’ve got a lady. She’s not very keen on an operation, but she is quite happy to be randomised and says, ‘Well what if I say yes to randomisation and if it’s an operation then I can say no?’
13
Despite the strong views held by patients, and acknowledgement from staff that there will always be a ‘subset’ of patients who are strongly ‘pro brace’ or ‘pro surgery’, staff generally considered patients to be open to the idea of the trial and to discussing treatment options. Indeed, staff provided examples of patients changing their mind and agreeing to participate, even patients who held strong preferences towards a certain treatment initially. Staff viewed this to exemplify how patient preferences are malleable, highlighting the importance of how trial information and discussions about treatment options are presented during recruitment consultations:
I think there were, I’m [not] sure of their names now or if they were researchers, but there were like three in the room and basically, yeah [name] said, do you know what the treatment options available are? I said, oh I’m pretty much having surgery and he kind of went ‘well no’. He took me through the options. So they were very clear the way they described it and it was interesting because there was a lot of debate because my sister was there and my sister was calling my mother and my sister and mother wanted me to have surgery because they knew somebody who had a brace for scoliosis and said it was extremely uncomfortable and their entire life, you know, the period they were in a brace was pretty frustrating and so yeah there was definitely . . . from the three members of the medical team in the room and [name] was quite good at arguing, not arguing, but basically giving the other opinions to my sister and my mother who were kind of blasting me in one ear.
P05
Patient preferences may also be influenced by the information patients receive about the treatment options available to them. Despite the capacity issues reported by a couple of patients, the majority felt that they received limited information regarding their recovery and the relative pros and cons of each treatment. More specifically, patients felt that information regarding what surgical fixation entails and the impact of wearing a brace on daily life was lacking, with some attributing this to junior doctors being unable to answer questions or consultants being rushed and ‘wanting to get to the next operation’. The implications of wearing a brace and how it may have an impact on return to work, in comparison with surgery, was unclear, as there was a greater focus on the medical risks of surgery rather than the impact of a brace on the patient’s lifestyle. For example, is it possible to wear normal clothes when wearing a brace? For one patient, the limited information they received about the brace and the reassurance provided about the potential risks of surgery meant that they considered the brace more daunting than surgery. For some, the limited information they received regarding treatment led to there being a perceived imbalance between the length of time spent discussing the trial and the time spent discussing treatment options. As a result, some suggested that a dedicated clinician should be appointed to discuss treatment and recovery with patients:
So I think maybe if there was some . . . it could have been better . . . I think the brace could have been better explained. I mean they were quite good at saying it’s not a scoliosis brace it’s pretty much, I mean I came away thinking it was a spandex suit basically. Honestly that’s all I knew about it. I think maybe a picture of the potential thing you’d have to wear and also how long on average you would probably wear these things for, like just a bit more . . . yeah basic things like does it affect what you can wear? They were definitely answered in a way by the three members of the team but I can’t really recall their answers so I don’t think they were that good.
P05
Enthusiasm
One trial participant and a number of members of staff perceived the enthusiasm of trial recruiters as crucial for effective recruitment. It was acknowledged that, although clinical leads may express a willingness for their sites to be involved in a RCT and may undertake activities to promote the study within and between specialties (i.e. through presentations at trauma meetings), ensuring and maintaining staff engagement throughout a trial is challenging and something that can detrimentally affect recruitment, for example because staff forget to identify potentially eligible patients. Staff proposed involving staff with an ‘interest in spines’ and introducing inter-site competition to enhance staff engagement during recruitment to a full trial:
There are three different centres, a healthy competition might be a good idea. For example, if there was a way that we can log into a common website where there was sort of a bar graph or a chart, indicting how many patients were recruited at each centre and then the centre [that] wasn’t recruiting as much would then be spot on, to catch up with the others. I mean we as orthopaedic surgeons are usually very competitive.
06
One member of staff expressed particular concern that clinical leads in the PRESTO study had become progressively less interested in the trial as time went on and were not spending the time on the trial that had been allocated to them for that purpose. The potential impact of this on a future trial, which would have a longer duration, was also raised. However, it was recognised that lack of engagement from clinical teams is more of a problem in surgical trials than in other research studies. This was attributed to the central role of clinical teams in surgical trials, in which, in contrast to other studies, surgeons, rather than members of the research team, are responsible for ensuring that paperwork is up to date, and for checking eligibility and approaching patients in a timely manner:
It was to be a study that lasts a long time and there can be periods where there’s not much recruitment, the concern is then that people get a bit bored and disengaged with it, and I think that’s a little bit what’s happened to be honest. And I think if there was a trial that was going on for 4, 5 years, by this point, people had already started to lose interest. I think it would be really important to keep people engaged and to make sure the clinical staff are going to continue to be engaged and sort of more engaged throughout the whole thing rather than just at the beginning.
16
Practicalities of approaching patients to be involved in the PRESTO trial
Study documentation
Staff involved in the PRESTO study held mixed opinions about study documentation. Although some felt that study information sheets were appropriate and communicated key information effectively, others felt that they were too long and ‘bombarded’ patients with information. Staff also perceived it ‘burdensome’ to ask this patient population to self-report and read lengthy study documents while heavily medicated, in pain and on bed rest. Concerns were also raised about the length of baseline and follow-up questionnaires and randomisation forms, which were viewed as repetitive and asking the same information, some of which is not routinely collected (i.e. patient NHS numbers), across multiple forms. The time implications for staff to ensure that baseline questionnaires and randomisation forms were completed was also criticised, particularly for patients who are admitted on short notice and waiting to go to theatre. Despite criticisms largely relating to the length of questionnaires, one member of staff felt that other illnesses, injuries and medications should be recorded when considering a patient’s capacity and eligibility to a future trial:
I think for the patients who are processing a lot of information to then go through and ask all these questions, I don’t think it might be a little bit too much but I’d probably say that the baseline paperwork could be better, could be not so long and more appropriate questions to injury and also for the baseline not to be completed, because it’s a randomisation, you have to put in lots of information, and when the patient’s getting ready to have surgery, it makes it all very stressful and quite rushy, and I think for us it’s quite separable, so for the patient, it feels like, for them, it must feel like, why are we rushing so much? It doesn’t feel that nice when you want to get to surgery to be kind of whizzing through something which probably means they may or may not really kind of think the answer so much and just sort of say whatever they want.
16
Although acknowledging that the questionnaires used in the PRESTO trial were standardised, validated measures, staff felt that they were inappropriate for patients with spinal injuries and would need further development ahead of a main trial. For example, despite patients being on bed rest and told not to mobilise, baseline questionnaires ask patients about mobilisation. Staff also felt that some of the questions, such as those relating to patients’ sex lives, were inappropriate and merely added to the length of the questionnaire and the volume of information gathered. One member of staff also raised concerns about the specificity of some of the questions on follow-up forms. For instance, although EQ-5D-5L was considered easy to use and understand by patients, questions relating to complications and pain were viewed as not specific enough. Participants therefore suggested that study documents and questionnaires be reviewed and, when possible, streamlined ahead of a main trial. Other recommendations included adding links to online information for treatment options and procedures in study information sheets, collecting some or all information on baseline forms postoperatively or post randomisation and including a telephone number to contact a dedicated 24-hour research nurse for more information:
I think there are quite a lot of forms and a lot of them are little bit repetitive. I think the other tricky thing which we’ve had a discussion about as well is the use of the . . . I can’t remember if it’s SF-12 or 36 [Short Form questionnaire-36 items], but using both forms on baseline when . . . For example, the patients are put on bed rest, told they’re not allowed to mobilise or even sit up and then we’re asking them ‘Today, is pain preventing you from walking or is pain preventing you from washing and dressing or is pain preventing you from, whatever, your normal activities?’. It might not be that it’s pain preventing them but, actually, they’ve been put on bed rest so they’re not allowed. Some of the forms don’t always make sense in that scenario but I think that’s something that, hopefully, will be developed if it is turned into a full trial. Obviously, if someone’s on bed rest, it’s not pain that’s stopping them.
15
Two of the patients who commented on study documentation said that they found information sheets clear and felt that they used an appropriate standard of English. Discussions about study information sheets raised concerns about the consent process as two patients reported not receiving an information sheet and not being able to use it as a reference point for understanding what the trial involved when home:
I knew I was part of the trial but I really couldn’t remember what any of the details were, so it was nice to know what I’d actually agreed to.
P02
Who should approach patients about the trial?
With the exception of one surgeon who felt that recruiting patients is a ‘complex business’ that is best handled by the treating consultant, the majority of health-care professionals felt that to ‘play to the strengths of both parties’, approaching patients about the trial should be a ‘joint venture’ between a senior clinician and the research team. However, staff did acknowledge that a degree of flexibility is required with this approach, as time constraints and availability of surgeons to be present for all, or even some, recruitment conversations, as well as issues with surgeon equipoise, can prevent this from being feasible for every patient:
So I would say, if you have a unit where you’ve got surgeons who are very strongly in favour of one treatment, perhaps maybe [they] should not be the ones approaching the patient to register them for the trial. You need someone fairly independent, maybe a research nurse, if you’ve got one, or perhaps a registrar or somebody who has not yet got very strong views, to actually approach them and consent them for the trial.
03
A range of benefits to having both the clinical and research team involved in recruitment were proposed. For instance involving clinicians ensures that patient expectations are met and that conversations about their care will be discussed with a clinician, and increases patient confidence by ensuring that clinical questions are answered effectively. Involving consultants in recruitment consultations was also perceived to help overcome issues with patient preferences, particularly when preferences are based on patients being told by other specialties that they would be receiving a certain treatment. Clinical teams were also considered to be better placed to describe the ‘grey area’ surrounding treatments for thoracolumbar fractures, by building a case for both treatments, reassuring patients that both treatments are routinely provided and answering the ‘what if?’ questions that patients may have, something that was considered difficult to address in a participant information sheet. However, ensuring that research staff were present was considered important to overcome potential issues with equipoise, as surgeons may ‘knowingly or unknowingly guide patients with our views’. Equally, the research team were perceived to be best placed to communicate the ‘nitty gritty’ about the trial, as it was acknowledged that describing research is part of their daily role and that they are aware of how information should be presented to patients in a way that is not coercive. Research staff also explained how, for this study, they did not feel confident discussing the injury or proposed treatments with patients and felt unable to answer clinical questions, which may affect patient confidence in the study:
The pros are that the clinician has a very in-depth understanding and is used to presenting information about those two treatment options. I think the negative, sometimes can be in relation to knowledge of how a research trial works and the potential pitfalls of presenting information in a certain way that might lead the patient one way or the other, and how careful we need to be with that. Also, to make the patient feel very much not under pressure to join the trial. I think that’s something that, perhaps the research team is a bit better at, having more experience in that area. So I think there are pros and cons, and that obviously is clinician dependent and research team member dependent. Certainly what we found worked really well here, was to have the clinician present the clinical parts of it so that the patient knows that the clinical information is coming from a clinician who would be treating them. Then for the research team to explain participation, withdrawal procedures and all that kind of stuff.
04
Although some sites opted for research and clinical staff approaching patients together, others still involved both groups, but via separate conversations. For example, even sites that opted for a ‘dual approach’ to recruitment discussed the advantages of consultants having initial discussions with patients to gauge their interest, and discuss the patient’s injury and treatment options with the research team when holding a separate follow-up conversation. Additionally, some discussed the importance of ensuring that senior fellows or consultants are involved in recruitment, as it was perceived to be harder for patients ‘to accept these things’ from a junior member of staff, with senior staff also considered better at ensuring that the grey areas and nuances of treatment are communicated effectively:
So I speak to them first. I tell them what it entails if they’re interested to be involved in the trial. If they are interested then I will let the research nurse know, who will also go through all the details of the trial.
17
Managing patient expectations of treatment and recovery
Patients from both treatment arms reported pain and discomfort following treatment. Those who received surgical fixation reported pain and discomfort due to an infected wound and healing of the muscle tissue around the spine. One patient also reported increased discomfort and sleep problems after 12 weeks, with another experiencing discomfort because their stitches had not dissolved as expected and so were catching on clothing and bed sheets. The patient who received the brace reported it to be a ‘nightmare’ during hot weather and experienced pain as a result of the brace rubbing on a lump that had formed on their back. This patient was not satisfied with their treatment and remarked that, given the choice in future, they would opt for surgery:
I’ve been experiencing discomfort because the stitches haven’t dissolved, have actually been sticking out the back and catching on my sheets. That has actually caused me to wake up at night in some discomfort. Also, occasionally catching on shirts. If I’d gone for the brace that wouldn’t have been an issue because there wouldn’t have been stitches there to stick out.
P04
There’s sort of a lump come up, but it’s only recent and the brace sort of pushes against the lump, which causes a bit of pain as well.
P01
Two patients reported that they received information about estimated recovery times and were expecting to make a full recovery. Three participants felt that they received insufficient information regarding their expected recovery time and were unsure if they would make a full recovery. These patients expressed frustration at the lack of information they received about how to manage their recovery as an inpatient and at follow-up. In particular, patients felt that they received limited information about managing their condition and the movements that were acceptable post discharge. Junior staff, the level of engagement from clinical teams, and the quality of information received about treatment and recovery were also criticised. Particular uncertainty surrounded whether or not they should be bending, which led to concerns that further damage was being caused, or recovery was being hindered by undertaking, or not undertaking, certain movements. One patient was particularly critical of the contradictory information they received post discharge from the surgical team as to whether or not they should be undertaking physiotherapy in the first 3 months after surgery. This patient also criticised the written information provided to their GP and the information they received from their physiotherapist about post-discharge management, which contradicted advice given by the surgical team:
Towards the end of a very brief meeting with the registrar, I then said ‘Does Mr [X] do private work?’ And then the registrar disappeared, he didn’t tell me why he was disappearing but he just disappeared, and then a few minutes later, Mr [X], the consultant appeared and gave me his private card and said, ‘Well if you come and see me at [private clinic], well obviously we can discuss this in more detail’. He literally, eyelashed my X-rays and said, ‘have you been bending down? One of your rods is out of line you mustn’t bend down’. And then disappeared before I really had a chance to talk to him or ask him further questions about what he meant about ‘you can’t bend down’. I mean he did show me meaning that you have to go up and down on your knees but then what he didn’t say is that, of course it’s fine to pivot on your hips and what he meant is you can’t bend forwards, you can’t bend the middle of your back forwards, So I then had to wait for 2 weeks before I had a private appointment with him where I could actually find out what he meant.
P03
Designing a full-scale trial
Given that this was a feasibility study, there were a number of challenges and areas of uncertainty that would need to be addressed prior to a full-scale trial. Challenges included issues with the patient pathway, difficulties engaging the range of relevant specialties, sample size, eligibility criteria, resourcing and support, retention and outcome measurement.
Navigating patient pathways
Staff at participating PRESTO study sites agreed that the best time to approach patients about the trial was at admission or as soon as possible after their injury. However, the complex and idiosyncratic nature of referral pathways posed one of the main challenges to recruitment during the PRESTO study and meant that, in reality, trying to navigate these pathways to approach patients at the earliest opportunity was difficult and resulted in potentially eligible patients being missed. This was despite the various methods staff used to try to maximise their ‘capture rate’ during the PRESTO study, which included asking the PI or research manager to look through all referrals received and ‘sift out’ potential PRESTO patients, and at another site creating a ‘low-effort kink’ in the existing pathway by asking staff to call the research team directly when a patient with a fracture was admitted. These solutions were met with mixed success, as they were dependent on the engagement of clinical teams and their awareness and willingness to be involved in the study. The implications of the problems encountered with patient pathways during the PRESTO study on the design of a national trial were discussed, with a number of staff being of the opinion that, for a full scale-trial to be successful, clear and well-defined treatment pathways would be required, with individual centres responsible for recruiting around their own ‘bespoke treatment pathways’:
When it comes to national implementation, right, only problem with recruitment that you might have are the local inconsistencies. So, how the local teams go about ensuring that their capture data is the best, and that all depends upon the local level of motivation, knowledge of their systems and what is working for them and what isn’t. For example, based on this experience what we have now started to recommend is that either myself or the research manager looks at all the out of . . . referrals that we receive, which may be fractures, which may be cancers, which may be . . . and stuff like that, and out of them, sift out the potential PRESTO patients.
06
A number of examples of how referral pathways created problems for recruitment were provided by staff who were involved in the PRESTO study. Varying sources of referrals was considered to be one of the main factors affecting recruitment, with one hospital receiving referrals from up to 15 hospitals. Although sites had mechanisms in place, such as those previously described, to try and ensure that all referrals could be identified and screened for eligibility, delays in transferring patients from local hospitals to MTCs or hubs were reported. In some cases, these delays could be up to 6 weeks, meaning that patients had conservatively reached a point at which they started to heal, had started treatment or had received a brace for stabilisation, and so were no longer eligible for the PRESTO study. Therefore, although it was proposed that ahead of a main trial recruitment should be streamlined with ideally every patient with a thoracolumbar fracture transferred to the hub, the impact of this on resources, clinics and capacity was deemed too great:
We generally want to see them within a week of them sustaining the injury and a few times it has not been possible to arrange transport from the local hospital up to our centre, and that has caused patients to miss the reasonable time frame that we would like to follow these patients, for example if they reach 5 to 6 weeks following the injury by the time to come to the clinic, then it is a moot point to then discuss surgical or operative options. Because conservatively they have reached that point in their treatment, where the fracture could have reasonably been expected to heal and we wouldn’t be doing justice to either the patient or the trial to then discuss the two separate options for them.
06
Staff also discussed how potentially eligible patients were missed because outside referrals were not necessarily referred directly to staff or specialties that were involved in the PRESTO study. One participant thought that this would be a particular problem for large teaching hospitals, where, although urgent referrals or patients admitted through the ED or by neurosurgery would ideally be seen initially and then the PI be alerted, instead, patients may be stabilised or treatment initiated, even prior to referral, meaning that they are no longer eligible. Although it was not considered feasible for referring hospitals to introduce the trial to patients, as a centralised message and recruitment centre were considered important, engaging staff from other specialties at recruiting sites was thought to be crucial:
The other hospital will refer into us on something called ‘referapatient’, that’s purely digital, using the details given on that. Basically, the neurosurgeon on call will look at those images, decide on a treatment, and then reply very quickly, often within hours or minutes. So they will get a treatment plan there. It’s almost impossible to nip in between them getting the referral and replying, they reply very, very, fast. So almost everyone who would, it seems almost astounding that we haven’t managed to get anyone to come into clinic early as a result of that screening process, considering the number of people coming through there. In principle, whoever was to come through probably would have been given a treatment, so would have been given a brace probably. The only way we can get around that would be to, almost, reverse either – The best way is to get it at source, so the neurosurgeon knows, already, that this trial is going on and could treat this patient either way. At which point we would then say they would then let us know. We would then, perhaps, propose that they come to clinic to see a spinal specialist. At the end of the day, they are the people who treat these injuries most. You know, would they come across for that? That’s the best way to get . . . We have a challenge, that process requires a lot of people to do a lot of things very quickly.
04
At one site, although the trial and recruitment methods had been designed with the pathway in mind, the pathway changed after funding had been obtained. Staff at this site reported that the trial had revealed various ‘deficiencies’ in their existing pathway, which demonstrates the difficulties of designing a trial around a complex, changing system. For example, issues with resourcing and referral pathways mean that it can take up to 6 weeks for a patient to be referred to a PRESTO study surgeon at the fracture clinic, by which point patients are ‘on the path to healing’:
The thing that I’ve noticed in my unit is the definition of the pathway. If you have a really well-defined pathway, it’s easy to plug in, like ‘Where are these patients going to come from?’. Then we’ve got dedicated people to see them or space to see them. The problem that we’ve had is that someone has really messed around with the pathway in our department. That has happened in a sort of parallel with the development of the trial . . . we [used to have] this really good way where all the patients who had a fracture were referred. They were all seen by one or two consultants in terms of their imaging. They were then allocated an appropriate day and time to be seen in the fracture clinic. Then, that, as I say, just became log-jammed. Then, trying to sort that out, that then got taken from our hands. That was the reason that happened here. I don’t really have a solution for that because if I did have a solution for it, I would have instituted it. Having a really good defined current treatment pathway that people can recruit from is probably, I think, going to be the key to being able to do this successfully.
13
The majority of concerns related to the challenges of navigating referral pathways. However, for one study site, the practicalities of having the research and clinical teams on separate hospital sites caused logistical issues for recruitment and follow-up, which raised important issues that would need to be considered ahead of a main trial in the case of large teaching hospitals based across multiple sites:
I wouldn’t say it affects the recruitment per se, but certainly in relation to follow-ups and randomisation and the consent process, and in dealing with anybody who is recruited to the study, then yes, because I’m on a completely different side of the city to the main hospital.
11
Engaging other specialties and staff groups
Patients who are admitted to trial sites may initially be seen by specialties not directly engaged in the PRESTO trial, for instance in the ED, neurosurgery or general orthopaedics. Given the lack of awareness of the PRESTO study in some specialties, and the fact that different specialties and surgeons have their own preferences for how stable thoracolumbar fractures should be treated, this resulted in some patients being told that they would be prescribed a brace or, in some cases, be stabilised on admission, before they were approached about the PRESTO study. The conversations with other specialties about treatment may, for those who are still eligible, mean that patients have developed expectations or preferences for treatment that mean that randomisation is no longer considered acceptable and poses a major challenge for recruitment. The implications of this on recruiting patients to a full-scale trial were also mentioned, as it is likely that, to overcome this issue, staff from all three specialties would need to be involved in recruitment in some capacity, which was not considered feasible:
I think because of these sort of patients that are coming in and ones that would be identified for the study, obviously, they are orthopaedic patients but they’re also patients that could be seen in neurosurgery and also can be seen in the emergency department. And I think because it’s only led by the orthopaedic, a PI is an orthopaedic consultant and then there’s no involvement from other teams at all, even if we’re trying to promote it in the neurosurgery meetings or we can speak to the neurosurgery teams, . . . registrars, maybe see these patients in A&E to let us to know they don’t really have any investment in the study. So, it’s likely they’re going to forget about it. So, I think it’s a big problem that really it should’ve been a collaborative study potentially with the other teams that would be seeing these patients.
16
The influence of other specialties on patient preferences prior to recruitment consultations was exemplified by one patient, who described feeling ‘biased’ towards surgery after discussions with an ED doctor:
Well interestingly when I came into A&E and I actually saw one of the surgeons team, she was on duty in A&E and she basically said it’s 99% chance for this type of thing you’ll have screws, so you’ll have surgery. So I was kind of almost biased in a way assuring that I was going to have surgery until they came and said, OK there’s actually two options. My preference, obviously surgery is always scary but I’ve had general anaesthetic before, so I was particularly scared about it and to be honest it was my preference. I didn’t really like the idea of wearing a brace, you know, pretty much, well 24 hours a day.
P05
At some sites, patients are often admitted through neurosurgery, and so the lack of engagement from neurosurgical colleagues during the PRESTO study was a particular problem. Insufficient ‘buy-in’ from neurosurgery was attributed, in part, to a lack of collaboration in routine practice between neurosurgery and orthopaedics, but also to perceptions that neurosurgeons ‘look after themselves’. For example, some staff reported that the only way in which they were able to hear about potential PRESTO study patients was to attend morning neurosurgery meetings or to screen patients from neurosurgery handover. Despite multiple presentations about the PRESTO study, staff described the challenges of asking for a culture change ‘ahead of the evidence’, particularly when considering issues with surgical equipoise and that neurosurgery routinely provides conservative treatment, which is less resource intensive. The attitude of some neurosurgeons towards research was also perceived as a problem, with some neurosurgery departments being less research active and not having the same incentive to take part in research as other specialties:
The neurosurgery team here are not the most helpful. [Laughter] I don’t know whether that’s because we haven’t communicated the study very well or whether that’s just their mentality to research. We have decided that we will do a presentation to them again to make sure that it’s not a fault on our side. You’ll probably hear [name of staff] talk about this as well, but we did notice a couple of the patients that we were screening were being reviewed or presented in A&E and then neurosurgery registrars would be going down to review them on the request of A&E doctors. Then they would discharge them from there, so they weren’t getting admitted, which meant that those patients who have been told that they don’t need to be managed with an- they essentially just get discharged with a brace. A couple of those patients we, potentially, could have included in PRESTO, but because we missed them due to– I guess the registrars were completely unaware of the trial. Then I think once we- or at least hopefully, if we eventually present the trial to them again and ask them to contact us, that might help avoid missing more patients. It’s tricky. I get the idea that neurosurgery aren’t really research active here, so getting them involved, they can be a bit stubborn, I feel.
10
The challenges of approaching patients who have already been told by ED doctors that they will be receiving a certain treatment was also discussed by a number of participants. In particular, it was considered to be providing a poor standard of care and a cause of anxiety for patients to be discharged with no treatment, or prescribed a brace, and then receive a telephone call from the hospital asking them to attend an appointment to discuss the possibility of surgery and being part of a research study. Specific challenges with engaging ED staff in a future trial were also discussed and included the preference of ED doctors to prescribe conservative treatment and the pressure on the ED to discharge patients to help meet performance targets. The timing of ED referrals was also challenging, as patients are often admitted ‘out of hours’ and seen by doctors with a low awareness of the study. A couple of members of staff also described the ‘dangers of junior doctors’ during recruitment, who can be difficult to engage because of high turnover and their tendency to inform patients that they will be receiving a certain treatment before recruitment consultations:
Unfortunately these fractures come in late at night or early in the morning and they’re already seen by a junior member of the team. The junior staff change over so frequently, they’re not aware of the trial, and they just go by . . . meaning for stable fractures don’t fix, for unstable fractures, fix, because that’s what you’ve been taught traditionally. Once they’ve told them in A&E that they don’t need an operation, because this trial is all about stable fractures, then it is quite difficult to convince the patients otherwise.
14
Given that a number of challenges were a result of busy clinical teams, who often have strong preferences towards treatment and were perceived to lack an incentive to be involved in the PRESTO study, it was considered important for a future trial to be a collaboration between the ED, neurosurgery and orthopaedics. Spinal fellows were suggested as a more practical way of ensuring inter-specialty collaboration than asking busy clinical teams to ring PIs when a potentially eligible patient is admitted. Other suggestions for overcoming issues with referral pathways and engagement of other specialties that were encountered during the PRESTO study included:
-
ensuring that neurosurgeons and, particularly, registrars are on delegation logs to ‘intercept the pathway’ and account for the fact that treatment decisions are often made quickly
-
refresher presentations and regular briefings, as opposed to one-off presentations, about the study to account for staffing changes and junior doctor rotation
-
creating a WhatsApp (Menlo Park, CA, USA) group for clinical and research staff to improve communication and ensure that patients are approached as soon as is appropriate after admission
-
research staff attending handover meetings in person or receiving daily handovers from neurosurgery and orthopaedics to ensure that no potentially eligible patients are missed
-
asking specialties to directly call the PI or study team as soon as a patient is identified, so that they can be screened and an appointment arranged immediately
-
involving ED staff in recruitment to ensure that the ED is being screened daily and avoiding missing potentially eligible patients
-
out-of-hours support for research staff, for units that ‘encroach on the ED’ to accommodate for out-of-hours admissions
-
having an open clinic for patients to be booked into from the ED.
Fracture population size
The number of issues with recruitment experienced during the PRESTO study, and the low volume of stable thoracolumbar fractures treated at participating sites, led to the feasibility of recruiting the required number of patients for the PRESTO study being questioned. The implications of this for a full trial were also discussed. It was suggested that, even with a large number of centres achieving the required recruitment rate, recruitment would still be challenging:
A surgeon will tend to see only about . . . 5 to 10 thoracolumbar fractures a year, and maybe one of each type. So, it’s impossible to get the numbers to actually randomise them and study them . . . They are relatively rare fractures.
14
Eligibility criteria
One of the main factors hindering recruitment to the PRESTO study was that, even when patients with thoracolumbar fractures were admitted to PRESTO study sites, a large proportion did not meet the study’s eligibility criteria. The eligibility criteria for the study were criticised by individuals involved in recruiting patients to the PRESTO study and those from the wider surgical community for being both too narrow and too broad. Various examples of factors that affected a patient’s eligibility were provided by those involved in recruiting patients to the PRESTO study, which highlight the complexities of these patients and of defining eligibility criteria in this context:
The feasibility element to this trial was the fact that, on the face of it, even if you look at the data in a moderate level of detail, without doing the trial, beforehand you would still have seen a very large number of patients coming through. It’s only when you get to the real nitty-gritty of each person, you look in there and, ‘Oh, this person has metastases’. Or ‘This person is very old and frail, and has a pacemaker, and just wouldn’t be good to go under general anaesthetic’. You really dive into the notes and you find these reasons that they aren’t appropriate for the trial. It may just be an unlucky year, but there is so much data now for that, it does seem like it’s a bit of a pattern.
04
A number of difficulties with operationalising the eligibility criteria were reported and were to some extent the result of the lack of consensus as to what constitutes a stable thoracolumbar fracture, with what is considered stable varying for different patients. Associated with this was the fact that the target population was smaller than originally anticipated because the majority of patients were considered either ‘very stable’ or ‘very unstable’, and only a small number of patients met the eligibility criteria in terms of the number of vertebrae fractured and the fracture location. There was also a perceived need for more specific criteria for patients with neurological deficit:
A real challenge, actually, recently has been that people often don’t meet the criteria in terms of the number of vertebrae fractured or where the vertebrae are fractured. I’ll just elaborate on that. For example, if we had someone who had a T12 and an L1 fracture that’s fine because those two fractures are contiguous. If we just had an L1 fracture on its own then that would be fine. If we have someone who has a T12 and then an L2 fracture, that person is not eligible. That’s because that fracture requires a much longer fixation which is associated with greater morbidity. The consultant body here felt that was not a comparable operation to having a brace. It’s bound to have worse results, essentially, is what they were saying. That applies again for those who have three fractures in a row. So say T12, T11 and T10, for example. Those are coming up quite a lot. Obviously, it also applies for people who, say for example, have a T9 and then a T11 fracture because that would still mess with the fixation. That has ended up being quite a big thing. I think we’ve screened over 90 patients now, and obviously only have . . . It might be pushing up towards 100, actually, at this site, and we’ve obviously only recruited three people.
04
Thoracolumbar fractures are referred to as ‘jumpers’ fractures’ and so of additional concern was the proportion of patients who were deemed ineligible for mental health reasons. Staff also reported that a number of patients had not met the study’s eligibility criteria as they were too frail or had fallen from a height and so were not considered good candidates for surgery. Other factors which were perceived to affect the number of eligible patients during the PRESTO study included the difficulty of obtaining consent and completing study documentation for patients whose first language is not English, patients who lived in a different area from the treating hospital and so wanted to be followed up locally, and patients at high risk of infection or of encountering wound problems and wound breakdown:
The recruitment has been a really big issue, for a few reasons. One of them is the questionnaire is all– We don’t have a specific validated questionnaire in other languages, if you have a non-English-speaking patient. For example, in London or Leeds, we tend to have a lot of foreign population, who are unable to do the questionnaire fully, so that was an issue.
14
Although one participant suggested that the eligibility criteria be reviewed ahead of a main trial, along with the numbers of patients screened, a couple of participants did acknowledge that staff may be ‘hiding behind personal biases’ rather than excluding patients for ‘objective reasons’:
Whether there are any other chinks in that inclusion/exclusion criteria that would allow us to include more patients? I guess, perhaps, that will be much more useful to look at, at the end, when we see the trends really clearly and you can say, ‘Well, OK, this was a huge number of people we excluded for this reason’. Was that actually justified, in terms of the science that we have out there at the moment, to say, ‘This group of patients should have been managed as they were, and excluded?’.
04
Retention
Retention rates during the PRESTO study or a full-scale trial were not considered to be a problem, beyond what would be reasonable to expect in other RCTs, due to the severity of stable thoracolumbar fractures:
I don’t think we have had many dropouts because, like I said, this is a serious fracture and people tend to take this quite seriously. So I don’t think follow-up or dropout has been an issue.
14
Despite this, a number of practical issues with collecting data at the proposed 2-week, 3- and 6-month follow-up points during the PRESTO study were reported. For example, some patients were discharged at 2 or 12 weeks, preventing completion of follow-up questionnaires. Although PRESTO study follow-up time points were selected to deliberately coincide with routine appointments, surgeons described how, in reality, ‘every consultant does something different for follow-up’ and how patient follow-up letters routinely ‘go astray’, so that follow-up appointments were missed or were too late. One surgeon reported that these difficulties were supported by an audit of their fracture patients over a 5-year period, during which it was found that only 50% of patients were followed up for > 6 months as a result of patients being discharged without complications, with 30% of patients not seen after 8 weeks:
I think the tricky thing with this is that 2 weeks doesn’t seem to be a time point that everybody uses and again, 12 weeks . . . I think . . . yes. We had someone who was discharged after their 2-week one, so we couldn’t get a 3 month or a 6 month. I think those are the kinds of things that would be . . . I suppose those are the things that would be done anyway when you’re turning something into a trial, is making sure that you’ve got time points that you can change.
15
The challenges of collecting follow-up data during the PRESTO study were supported by one patient who described how they were ‘lost to the NHS’ after discharge, after returning to their family home in a different part of the country to where they received treatment:
I was living in [place name], had the accident in [place name] and then I went home to [place name] where my parents live and the medical services in [place name] are like pretty bad, you know, compared to [place name] and we’re out in the sticks. So I was supposed to have a 2-week medical follow-up just to check my wounds and have the dressing changed which I had with the local GP but I understand there were other check-ups at something like 1 month, 6 weeks, you know, 3-month mark. When I went to see my consultant the other day, 2 weeks ago today actually. Apparently I’m listed as lost on the NHS. For some reason they’ve been booking appointments but I haven’t received any letters in the post at any address that I’ve lived at. So I have not had any NHS follow-ups at all. I should have had three, so I’m listed as lost and I’m actually going after this to the NHS to have a proper clinic follow-up, which will be my first.
P05
A number of strategies for improving retention to a future trial were proposed, and were based on surgeons’ and trial recruiters’ experiences of the PRESTO study and being part of other RCTs. To maximise engagement, the importance of using a range of methods, such as e-mail, text message, telephone calls, and online and postal questionnaires, to collect follow-up data and communicate with patients were suggested, particularly given that the population is likely to be young and able to ‘cope with technology’. To counteract delays to follow-up and/or problems with patients not receiving letters, one consultant proposed using e-mail and/or text message automation to alert patients and consultants when a follow-up appointment is due, and to prompt clinicians to make sure that patients have an appointment scheduled. The phrasing of clinic reminders and the language used when discussing the importance of follow-up and returning questionnaires in a timely manner was also emphasised. Other methods for improving retention included allowing only a small timeframe for reminders and chasing outstanding data from patients; using incentives; ensuring sufficient resources within research teams and at sites to support chasing patients for follow-up data and visits; informing patients of the time it takes to collect data; having flexible follow-up time points for each patient; and using BSR data, which include routine questions asked to postoperative patients:
It’s good to have different options and the option of, yes, basically doing phone, doing e-mail, and doing post. I think those three options give people a choice.
16
Ensuring that patients are well supported during research studies to maximise their engagement and the completion of follow-up data was also discussed. Comparisons were made with other RCTs, in which staff felt more support was given to patients, with surgeons and trial recruiters suggesting that a main trial would need more support provided than was provided during the PRESTO study. Although it was recognised that patients were encouraged either verbally or through study documents to contact the clinical trials unit, the PI or ward staff should they have any questions during the PRESTO study, it was felt that contact information for research staff at sites should also be provided:
Going back to this being a big deal for patients, quite a big involvement rather than just a paperwork exercise-type study, I just think that, whilst they can contact the clinical trials unit and the information is given to them, if you’ve got any problems you can contact the CTU [clinical trials unit], personally I find that if they have telephone contact numbers of the research team that’s dealing with them, they tend to use those numbers and ring those numbers. That helps to support them or help them feel supported on the journey through the study. I think this study could perhaps develop that a little bit more for bigger studies taken forward. They need to be able to contact the research team. They need numbers of where they can contact somebody if they have got questions and queries.
11
Resources and support
The resources and infrastructure that are available at sites to support research were considered a significant challenge to conducting a full-scale trial. Although one of the surgeons involved in the PRESTO study felt that more time should be dedicated to PIs to ensure that they are involved in recruiting every patient, others recognised the importance of research nurse support. Given that patients are often admitted out of hours and at weekends, staff recommended that a dedicated research nurse be allocated to support a future trial. A couple of participants suggested that research nurse support be available 24 hours a day, 7 days a week; however, the majority of participants were conscious of the resourcing implications of this for sites. The potential value of involving associate PIs and/or additional consultants and registrars during recruitment was also recognised. Although this was partly viewed as a mechanism for supporting PIs and overcoming staffing issues by ensuring clinical support is available at different times during recruitment, it was also proposed to prevent issues of equipoise by maximising engagement of different staff:
I’ve recruited two associate PIs, two associate investigators, at trainee level, who are NIHR associate PIs; that’s what they’re called. They are on the rota and their job basically, every week, is just to reinforce, with their colleagues and peers that in the following week, ‘You guys are on call on these days. You’re on nights, you’re on this, you’re on that. Can you keep an eye out for any of the patients and make sure that you’re not telling them that they can’t be treated with an operation or something like that?’.
13
Although it was felt that more support was required for a full-scale trial, the practicalities of obtaining this support were acknowledged. For example, a surgeon from one of the PRESTO study sites described some of the challenges of involving trainees in recruitment due to the limited time they have available to support research, with this site also being required to appoint a senior trainee to provide extra support and motivate staff. It was also recognised that, although large teaching hospitals, or those that are research active, will have good infrastructure, smaller sites may not have the resources in place to support a trial. Staff also commented on the fact that even if sites have dedicated trial resources, such as research nurses, in place to support research, these resources are often split across multiple trials. The impact of this was felt by some of the staff involved in recruiting to the PRESTO study, who reported feeling ‘thin on the ground’, with a member of the research team at one site stating that they may have ‘skewed’ the feasibility of recruitment and site set-up at their site by working overtime and undertaking extra activities, such as attending trauma meetings to maximise recruitment. Despite these concerns, there was acknowledgement from a small number of participants that resourcing is often a problem during feasibility trials, and that more funding, or top-up funding, through local clinical research networks would be available to support a larger trial, to offset the costs of clinical time being spent on recruitment:
I’m employed for 2 hours a day, technically, to do this trial. In terms of the effort I’ve put in to engage and the effort I’ve put in to set up all these online systems, it’s potentially more than most sites would be able to do. We were saying, ‘We need to be careful in terms of how much I would be able to skew the data by overworking here’.
04
The importance of site initiation visits, administration support and a ‘good trial management team’ to support the day-to-day running of the trial and assist with uploading electronic data and resolving queries was also recognised. The support provided by the clinical trials unit during the PRESTO study was praised by a couple of PRESTO study staff at sites. However, one participant suggested that in the case of a full-scale trial a dedicated lead contact should be appointed at each site to promote discussion between sites, to prevent always having to communicate via the clinical trials unit:
In general it’s been really good, really smooth. I think, maybe the challenges have been having . . . It might be better, a bit more direct contact with the sites. Sometimes it feels like we’re contacting them or hearing about things through the trials unit, which is fine and really useful. Maybe just having a lead contact at each site that’s very clearly defined at all times. If that person changes, which it has of course, then that just being really clearly defined.
04
In addition to discussing research resourcing issues, one consultant also mentioned the importance of ensuring that research studies do not increase clinical workloads. More specifically, the need to take into account the fact that not every hospital will have dedicated trauma lists was important because a trial may increase the number of operations that could have implications for elective lists and hospital finances. A research nurse also described the importance of ensuring adequate support is in place for patients who are ‘even in feasibility studies’ making significant and sometimes life-changing decisions:
It’s their life, it’s a big thing, and it’s going to affect the way they recover, it’s going to affect GP and community services once they’re at home if anything goes wrong, it’s going to affect hospital outpatient visits, it’s going to affect the families. If they don’t recover quickly enough, both physically and psychologically, it affects lots of people and lots of services, and the support needs to be there. It’s not just an outpatient visit with a PI. The support needs to be [there], it’s a big thing.
11
Outcome measurement
Surgeons and trial recruiters proposed a wide range of outcomes to be collected during a main trial (Table 26). Aside from the range of outcomes proposed, a future trial may also need to consider how to account for the inter-related nature of many of the outcomes that were suggested:
It’s pain, neurological deficit and deformity. I guess deformity and pain are interlinked, in a way. But the key question is, if we don’t fix these fractures do they all end up with neurological problems, as in nerve-related pain?
07
| Outcome | Details | Suggested measure |
|---|---|---|
| Pain | May be dependent on whether or not the patient has an earlier injury and/or degree of kyphosis | Validated pain scales (e.g. ODI, VAS) |
|
Persistent pain Long-term back pain Back pain, leg pain Back pain |
May need to distinguish between back and leg pain. Pain that is manageable | |
|
Function Return to normal activities/previous function Return to work Length of inpatient stay |
Returning to ‘pre-injury status’ and previous activity levels. Potential link between pain and return to work, but this was not agreed on. Achieving pre-injury status with few complications and in short time. Depends on factors such as employment history and age as to what this means for each patient | Validated scores, EQ-5D, SF-16, SF-36 |
| Neurological deficit | Linked to deformity. Only considered to affect a small proportion of patients | |
| Deformity and curvature | Is the patient’s spine straight, loss of height and angulation of the fracture, disruption to the vertebral bodies. Degree of angulation can be linked to pain. Uncertainty as to level of kyphosis that is of clinical significance | X-rays and erect imaging |
| Cost-effectiveness | Linked to compliance. If prescribed a brace but it is not worn, then it is not cost-effective. Default compliance with surgery | |
| Complications | AEs and side effects from treatment. Difficult for patients to know what is normal or abnormal, particularly regarding wound management | |
| Compliance | Extent to which the patient has worn the brace. In addition, look at if they are using the brace for longer than necessary | Research nurse to photograph brace when prescribed and assess at follow-ups whether or not it looks worn |
| Predictable fracture healing | To be achieved without significant risk of complications. Return to function and pain indicators of this | |
| Depression and mental health | Linked to pain and coping | |
| BSR data | Using BSR data and including this in a future trial, includes questions routinely asked to postoperative patients |
Pain and return to normal function were considered by many to be the most important indicators of recovery. However, the challenges of collecting these ‘subjective’ outcomes in a meaningful way were acknowledged. For example, participants explained how what is considered ‘return to normal function’ varies for different individuals and may be more important for younger patients than for those who are elderly or unemployed. Despite validated pain scores being available, difficulties with quantifying pain were also acknowledged and highlighted by the fact that some surgeons were referring to general pain, whereas others suggested more specific pain outcomes, such as persistent and long-term pain or back and leg pain:
The pain questions are not very specific so I think, I can’t remember now off the top of my head, but I think there’s one that says persistent pain but there’s no, it doesn’t say what’s the score out of 10 or does it stop you sleeping, there’s no guides as to what persistent pain is because obviously someone, it’s still going to be painful after 3 months and that’s considered persistent pain but the person’s managing so there’s not enough guidelines on what that means so people could be ticking and it could not be not, one person’s persistent pain would be different from another’s persistent pain. It’s not very objective.
16
Changing clinical practice
Despite the strong opinions held towards treatment of stable thoracolumbar fractures, the majority of clinical participants felt that the surgical community would be receptive to the findings of the PRESTO study and of a future full-scale trial, particularly if one treatment was found to be more effective. This was partly due to a perceived increased awareness of the value of evidence and the growing number of spinal surgeons who are involved in research, either through direct involvement in research studies or through research positions and fellowships. Participants also felt that the community would be receptive to a ‘well-designed RCT’, particularly given the lack of RCTs in the area, and cited the importance of working to current evidence for personal development and to ensure that their practice corresponds with good clinical practice and General Medical Council standards:
If you definitely show that one treatment is superior to the other, then I think lots of people will have an open mind and then they will, just like your findings, direct their treatment calls. I think one of the main problems you’ll find is there’s not a lot of evidence based, you know, which absolutely states ‘this is the right thing to do’. And I think, because everything is retrospective or prospective, there’s never been any randomised trials, or there’s very few . . . So I think this will be very, very helpful.
17
Participants suggested that the research team adopt a varied dissemination strategy to ensure engagement with and uptake of the findings from the PRESTO study and a future trial. Although publication in high-impact journals and emphasising study quality were viewed as important, this was not considered to be enough in isolation. The majority of participants suggested disseminating findings via relevant society meetings, and national and international conferences, such as those organised by BritSpine and BASS. The way in which information is ‘marketed’ was also considered important, to ensure that findings are not misinterpreted. To achieve this, holding in-depth discussions at relevant meetings, publishing bulletins of key messages or converting key trial information into a tool that can be used to inform practice were suggested. Distributing key messages through websites and/or via society mailing lists and networks (e.g. the United Kingdom Spine Societies Board) was also recommended, as was, when possible, asking for ‘bulletins’ to be endorsed by key individuals or societies. When discussing the findings of a full-scale trial, the importance of ensuring that findings are adopted by NICE and BASS guidance was considered crucial. Although one individual cited the importance of discussing cost-effectiveness to maximise the impact it has through NHS England, Clinical Commissioning Groups and NICE guidance, the economic argument was not considered to be enough to influence surgical practice:
I think the message needs to be a simple one. I think people don’t want too much complexity. I think you need to prove and make the case that your study is well designed and robust and well supported. Once you’ve got your evidence, then you present it in a very simple formula that people can apply to their practice.
03
Chapter 6 Results of the survey of spine surgeons
Number of respondents
Ninety participants agreed to take part in the survey, 86 participants responded to at least one question and 65 (72%) respondents completed the whole survey. This means that not all participants provided answers to all of the questions. It is not possible to calculate a response rate given the variety of approaches to recruitment and because there is no definitive way to determine the number of people the survey reached. The total responding to each individual question was used as the denominator to calculate percentages.
The majority of respondents heard about the survey at BritSpine, either through the conference application or via the PRESTO study stand/flyer (n = 50, 77%). Eleven (17%) respondents responded to the notice on the BASS discussion forum. Two respondents completed the survey from the link in a BASS e-mail and one respondent via the Southwest Spinal Club Google Group.
Demographics of respondents
Of the 65 respondents who completed the question, 55 (85%) were consultant spine surgeons regularly treating thoracolumbar fractures and seven (11%) were specialist registrars or spinal fellows in spinal surgery. Of the three (5%) respondents who selected ‘other’, one was a spinal surgeon and one a retired spinal surgeon with personal experience of a fractured spine.
Experience as a spinal consultant ranged from 1 to 30 years, with an average of 9.4 years and median of 7 years. Specialist registrars and spinal fellows in spinal surgery had an average of 17 months’ experience of spinal surgery, with a range of 1 to 36 months and a median of 18 months. Six of the seven respondents had treated thoracolumbar fractures as a spinal fellow and one had not.
The majority of respondents (34, 55%), worked in trusts serving a population of between 600,000 and 900,000, and 19 (31%) respondents worked in trusts serving a population of 300,000–600,000. Seven (11%) respondents worked in a trust covering a population of 200,000–300,000 and two respondents worked in trusts serving a population of < 200,000.
The respondents stated that the number of spinal consultants responsible for ongoing management of thoracolumbar fractures in their trusts ranged from 2 to 14 (mean 5.4, median 5).
In the previous 12 months, the number of patients with a T10 to L2 thoracolumbar fracture whom the respondents had treated conservatively ranged from 0 to 60 (mean 19.1, median 15).
The number of patients treated surgically in the same period ranged from 0 to 40 (mean 7.8, median 5). This suggests that, over the previous 12 months, substantially more patients were treated conservatively than surgically by the respondents.
When asked if they would be willing to participate in a future trial, 54 (84%) of the 64 respondents said ‘yes’, eight (13%) said ‘no’ and two (3%) were unsure. Reasons given for saying no were retirement or working only with private patients (n = 3) and ‘bureaucracy’ (n = 1). One of the ‘unsure’ respondents said that it depended on time constraints.
Current practice
Imaging
There was close concordance between the 86 respondents over the frequency of use of imaging techniques to confirm a thoracolumbar fracture between T10 and L2 in the presence of neurological injury. Eighty-two (95%) respondents always or frequently requested CT (in particular, a CT trauma series), 75 (87%) always or frequently requested MRI, and 59 (78%) always or frequently requested plain radiographs. Other imaging occasionally requested were an ‘erect lateral’ (one respondent) and a CT myelogram (one respondent).
To confirm a thoracolumbar fracture between T10 and L2 in the absence of neurological injury, 79 (95%) of respondents said that they always or frequently request CT, 64 (83%) respondents use plain radiographs, 41 (49%) respondents use MRI and three (11%) respondents said ‘other’ but did not specify what.
Establishing spinal stability
Participants were asked to score the importance of a number of factors considered when establishing spinal stability on a scale from 1 (least important) to 5 (most important). To detect when participants gave a valid response to each factor, that is moved the slide scale, a starting score of 0 was included. There were between 60 and 75 responses to each of the factors, and the scores were averaged to determine which was deemed most important.
The importance of different factors when establishing spinal stability were rated by participants as follows (average score):
-
neurological deficit (4.6)
-
clinical symptoms (3.7)
-
pain when standing up (3.6)
-
spinal cord compression with normal neurology (3.1)
-
pain when lying down (2.47).
Similarly, participants rated which imaging or measures were most important to them when establishing spinal stability (average score):
-
CT (4.2)
-
standing radiograph (4.1)
-
segmental kyphosis (3.7)
-
MRI (3.9)
-
supine radiograph (2.5).
Other factors that individual respondents reported taking into account when establishing spinal stability were AO/Orthopaedic Trauma Association (OTA) 2018 classification, mechanism of injury, trunk control when lying down, trunk control and ability to roll comfortably and evidence that 20% of older patients develop neurological deficit.
Suitability for treatment options
Participants were then asked how influential six factors were in establishing suitability for surgical or conservative management. Again, response averages were calculated. The factor with the highest score (and therefore rated most important) was ‘spinal cord involvement: neurological deficits’, with a score of 4.5. Clinical symptoms, pain, spinal cord compression with normal neurology and segmental kyphosis were all rated to be of similar importance, with an average rating of 3.7, 3.5, 3.4 and 3.6, respectively.
Individual respondents also reported taking other factors into account when establishing suitability for surgical or conservative management: patient wishes; multiple injuries; age; canal compromise, retropulsion and vertebral collapse; profile of patient; and core strength.
Surgical fixation methods in current use were open spinal surgery, reported by 71 (92%) respondents, and minimally invasive surgery, reported by 62 (81%) respondents. Seven (9%) respondents reported using ‘other’ methods, of whom six specified type: vertebroplasty (n = 2), kyphoplasty (n = 2), kyphoplasty/vertebroplasty minimally invasive surgery anterior reconstruction (n = 1) and bracing (n = 1).
Open spinal surgery
Those using open spinal surgery were asked which approach they used when placing pedicle screws in unfractured vertebrae either side of the fractured one(s) and rods locked into the screwheads. Sixty-six (99%) respondents said that they used a mid-line approach and 49 (80%) respondents said that they used a lateral or anterior approach.
X-ray guidance was always or frequently used by 65 (91%) respondents and only occasionally or never by six (8%) respondents.
Fusion (decortication and graft placement) was always included in the procedure by 21 (30%) respondents, never by seven (10%) respondents and sometimes by 43 (61%) respondents. Reasons given for occasional use were in young and high-functioning patients when loss of movement will affect QoL (n = 6); for disc or ligamentous injury (n = 5); delayed surgery (n = 1); fracture morphology or multifragmented fractures (n = 2); depending on plan to remove or leave metal work (n = 2); in distraction or translation injuries (n = 1); and in patients who are judged unlikely to comply with instructions (n = 1). Respondents said that they would not perform a fusion if they were carrying out minimally invasive surgery (n = 1) or if there was joint involvement (n = 1). Another said that they mainly performed open surgery for decompression and so rarely performed a fusion. Three respondents said that they decided on a case-by-case basis.
One respondent said that they used X-ray screening guidance for positioning screws in open surgery only if they were having difficulty, but plain radiographs were always requested before and after rods to check screws and to check correction. One respondent said that it was easier to achieve reduction with open surgery and another respondent that the instrumentation for restoration of anatomy is better with open surgery.
Minimally invasive surgery
All 59 (100%) respondents reported use of X-ray guidance when placing pedicle screws in unfractured vertebrae either side of the fractured one(s) and rods locked into the screwheads, when using minimally invasive surgery. Navigation was used by 10 (19%) respondents, O-arm by seven (13%) respondents and robot-assisted surgery by one (2%) respondent.
Short screws were always or frequently used at the fracture site by 23 (38%) respondents to this question, and occasionally or never by 38 (63%) respondents.
Additional comments relating to minimally invasive surgery were about costs of surgery and equipment, one surgeon needing to use two C-arm image intensifiers for screening during surgery, and technical difficulties.
Conservative management for potentially stable fractures
When asked about conservative management of patients with potentially stable fractures, 48 (63%) respondents always or frequently used bracing with off-the-shelf adjustable TLSO, 27 (36%) respondents always or frequently used bracing with custom-made orthoses and 22 (29%) respondents did not use bracing. Thirteen (17%) customised orthoses always or frequently used bed rest.
Other conservative methods reported by individual respondents related to the use of pharmacological measures, physiotherapy (as tolerated) and incremental approaches to mobilisation. Bracing was not considered for osteoporotic patients who were stable on erect X-rays.
The purposes of a brace were ranked by participants, with preventing further kyphosis ranked the most important purpose (n = 16, 39%), followed by pain relief (n = 12, 29%), enabling the patient to mobilise (n = 9, 22%) and restricting patient movement at the fracture site (n = 4, 10%).
The frequency of use of spinal precautions in patients with stable fractures is shown for patients managed conservatively in Figure 7 and for patients managed surgically in Figure 8. The most common precautions taken in the case of conservatively managed patients were no lifting and no hyperflexion, reported as ‘always’ used by 42% and 41% of respondents, respectively. Similarly, these were the most always used precautions for those managed surgically too (reported by 35% and 36%, respectively).
FIGURE 7.
Use of spinal precautions in patients managed conservatively.
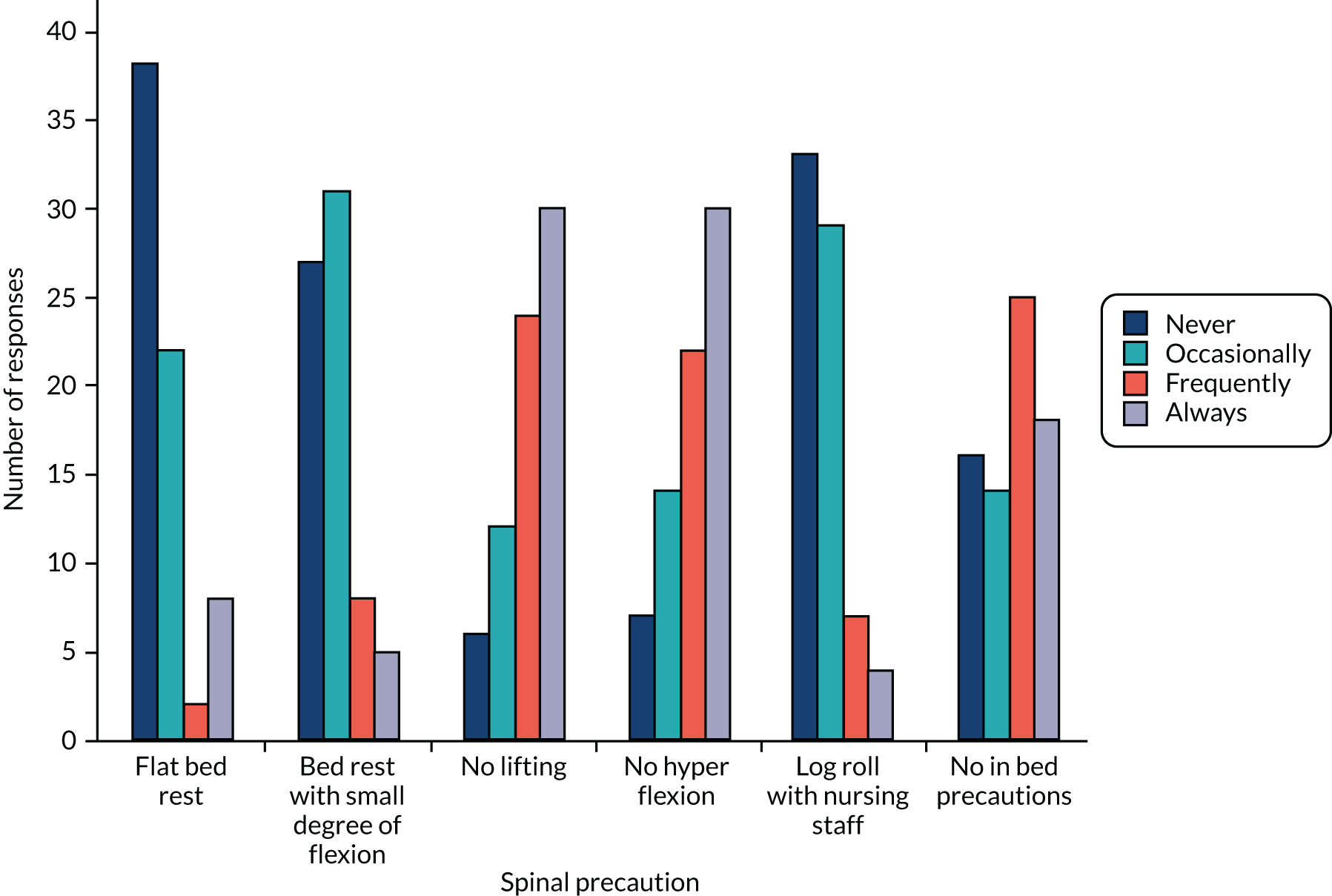
FIGURE 8.
Use of spinal precautions in patients managed surgically.
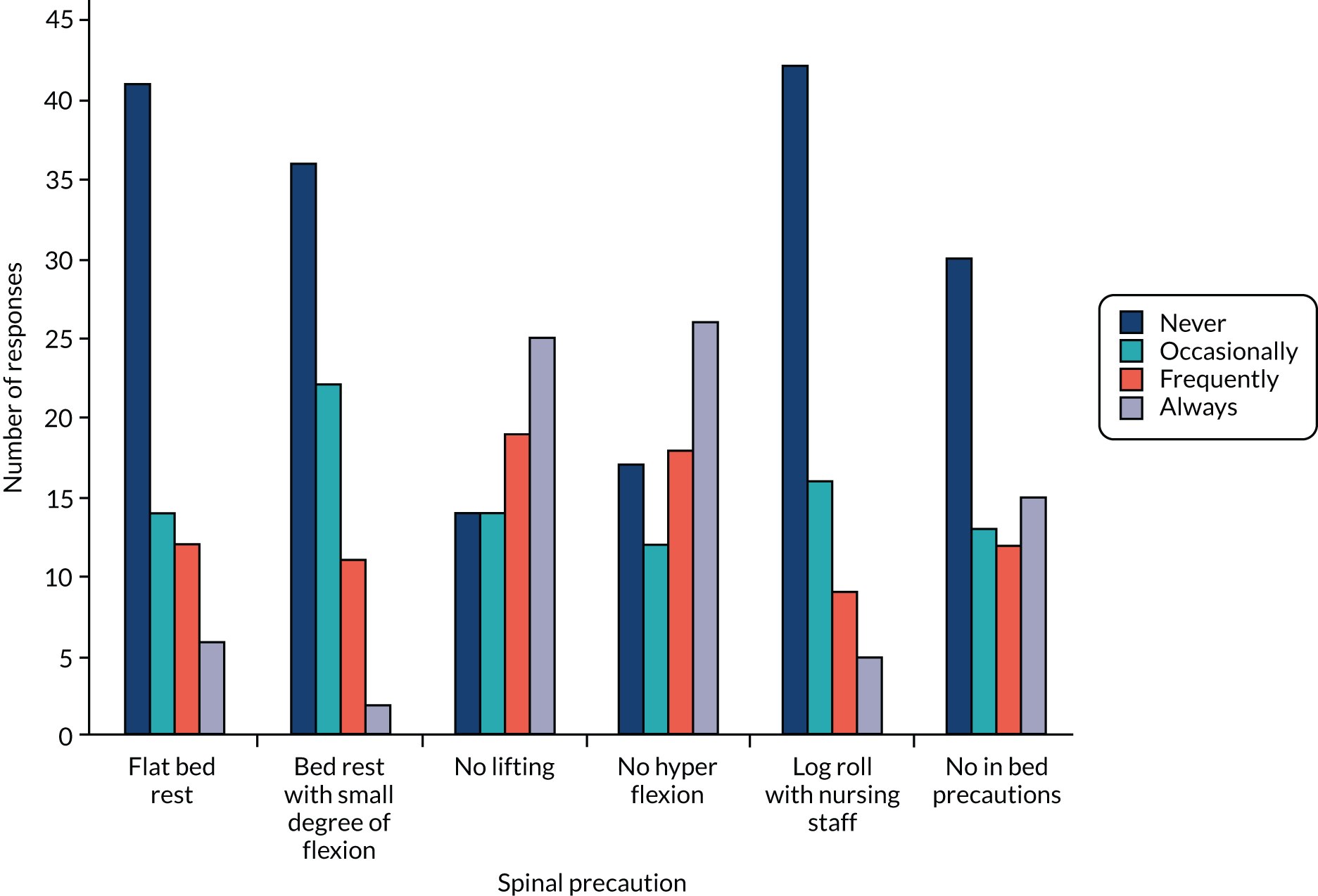
Surgeon participation in a future trial
Eligibility criteria
For inclusion
Participants were presented with proposed eligibility criteria for a future RCT, based on the criteria being used for the feasibility study, and asked to indicate whether or not they found each one acceptable by selecting ‘yes’ or ‘no’. Not all participants gave a response to each of the proposed eligibility criteria. Thirteen respondents provided comments. The majority of respondents agreed that participants should be aged ≥ 16 years (n = 62, 91%) and have a diagnosis of a high- or low-energy impact thoracolumbar vertebral fracture, between T10 and L2, and confirmed by radiography, CT or MRI (n = 62, 94%). There was slightly less agreement about the remaining options presented to participants.
Fifty-three (82%) respondents agreed with the criteria of a kyphotic angle > 20° on standing radiographs, or > 15° on supine radiographs or CT. One respondent who did not agree said that a standing radiograph should always be taken. Another suggested that the kyphotic angle should be > 20° on CT or MRI and > 25° on a standing radiograph.
Forty-five (74%) respondents agreed that a reduction in vertebral body height of 25% should be an inclusion criterion. One of the 16 (26%) respondents who disagreed felt that collapse of > 50% of the post-vertebral body wall was just an incomplete burst and that these could be managed conservatively. Another respondent felt that a small loss of height was a poor indicator of instability.
The largest difference of opinion related to the optional inclusion criterion of a fracture line propagating through the posterior wall of vertebra, with 44 (68%) respondents believing that it was appropriate and 21 (32%) respondents that it was not.
Forty-nine (78%) respondents agreed that the involvement of two contiguous vertebrae was an appropriate inclusion criterion. One respondent commented that patients with minor fractures of two vertebra should not necessarily be included.
Injury to the posterior longitudinal ligament or annulus in addition to the body fracture was considered an appropriate inclusion criterion by 52 (83%) respondents, and inappropriate by 11 (17%) respondents.
For exclusion
As seen with the majority of the proposed inclusion criteria, there was largely agreement for the proposed exclusion criteria. Fifty-four (79%) respondents agreed that it was appropriate to exclude patients with unstable fractures that obviously needed surgical stabilisation and that the decision should be made by the treating surgeon.
Exclusion of spinal cord injury patients was agreed by 53 (80%) respondents. One respondent commented that in the case of a spinal cord injury but a relatively stable-looking fracture there would be no need to stabilise. Another respondent stated that numbness is not an exclusion factor. One-third of respondents noted that a fragment in the canal depends on the degree of comminution of the vertebral body.
The majority of respondents (n = 59, 88%) agreed that pathological (other than osteoporotic) fracture (e.g. tumour or infection) was an appropriate exclusion criterion. However, three respondents felt that patients with osteoporotic fractures should be excluded.
The exclusion of patients not considered suitable for surgery was agreed as appropriate by 53 (80%) respondents. One respondent suggested being more specific, for example, when medical comorbidities make risk of surgical management too high.
A number of respondents made some suggestions for a future study. One suggested that, rather than excluding participants on the proposed criteria, it might be worth considering the use of observational data. Another suggested capturing images pre- and postoperatively, with a review of the images providing some quality control. A third suggested that surgeons be asked to indicate why they had chosen to operate.
Four respondents identified issues with defining stability and justifying surgery in patients whose fractures were judged to be stable or relatively stable. It was felt that the proposed criteria did not confirm instability unless they were clinically relevant.
Classification systems
The classification system for thoracolumbar fractures used routinely by 39 (65%) respondents was the TLICS; 23 (38.33%) respondents used the new AO Spine Thoracolumbar Classification System and 16 (28%) respondents used the original Magerl AO system. Five (11%) respondents reported using the Holdsworth classification,15 Denis classification14 and/or the McCormack et al. comminution. 13
When asked which fracture classification systems they would be prepared to use in a future trial, 30 (68%) respondents said that they would use the TLICS, 24 (57%) respondents that they would use the new AO Spine Thoracolumbar Classification System and 15 (38%) respondents that they would use the Magerl AO system. One respondent commented that the AO system is too complex and not intuitive.
Randomising patients
Fifty out of 65 (77%) respondents confirmed that they would be willing to randomise patients with a stable high-energy fracture to either surgical or conservative management. Reasons given by some of the 15 respondents (23%) who said that they would not be prepared to randomise this group of patients were related to necessary trial permissions and procedures being in place; belief that the question had already been answered; TLICS and belief that ‘osteoporotic [is] much more relevant’; belief that a trial should start with ‘low risk groups’; the role of surgery being debatable in stable fractures; and belief that a trial would be inappropriate when only involving private patients.
When asked about willingness to randomise patients with a stable low-energy fracture (e.g. fall from standing height in osteoporotic or osteopenic patients) to either surgical or conservative management, 46 out of 66 (70%) respondents said that they would be willing. Comments made by participants were again about the correct trial permissions and procedures being in place, and a trial being inappropriate for private patients. One surgeon believed that ‘most should be managed non-op [non-operatively] and most too frail for surgery’. Another surgeon felt that surgery is not needed in this group.
Outcomes for a future trial
Participants were ask to rank in order of importance (1 = most, 5 = least) five outcome domains for a future trial of patients with a relatively stable thoracolumbar fracture and add any other outcomes. Pain (n = 15, 35%), then mobility (n = 13, 30%) and return to pre-injury activity levels (n = 13, 30%) were considered the most important, but with little difference between them. Speed of return to pre-injury levels of activity (n = 2, 5%) and anxiety or depression (n = 0) were the factors ranked of least importance by the participating surgeons. Additional outcomes suggested were kyphosis and deformity, and sagittal balance.
Overcoming barriers to site participation in a trial
Respondents were asked what factors, if any, would need to be overcome to make recruitment to the proposed trial at their centre possible. Fourteen respondents listed staffing as an issue, including insufficient research nurse and administrative staff, and lack of resource for data collection and clinical time for consenting. Infrastructure issues were identified by five respondents. Two linked staffing issues to research infrastructure needs; one said that this was not insurmountable and the other said that they were currently enhancing their research infrastructure. Another identified the need for ‘national trial co-ordinator supporting centres with site visits’. Five were concerned about funding and resources.
Three respondents did not see or treat the relevant patient group. Two respondents who did treat these fractures said that there were few cases that they would consider were borderline between surgery and conservative treatment. One respondent felt that patients with a stable fracture may not consent to surgery.
One respondent required ethics approval, one respondent said that ‘early discharge is a critical factor’ and another respondent just wrote ‘Brexit!’.
Four participants who were willing to randomise patients with a stable high-energy fracture and three who were willing to randomise those with stable low-energy fractures said that there were no factors to be overcome to make recruitment to the proposed trial at their centres possible.
Chapter 7 Discussion
The PRESTO study used a range of methods to investigate the feasibility of delivering a multicentre RCT comparing surgical fixation with initial conservative management for adults with a high- or low-energy thoracolumbar vertebral body fracture between T10 and L2. The following discussion draws on data gathered from the randomised feasibility trial across three sites, in-depth interviews with the recruiting teams at the sites and surgeons external to the trial, as well as the national survey of spine surgeons.
Recruitment and retention
The primary outcome for the feasibility trial was recruitment rate, defined as the proportion of eligible patients randomised into the trial. It was predicted that 120 eligible patients would be identified during the 12-month recruitment period, but in the pilot only 28 patients from a total of 211 patients screened were found to be eligible – substantially fewer than anticipated.
Twelve patients were randomised into the feasibility trial recruited from three sites over a period of 12 months. The proportion of eligible patients randomised was 0.43 (95% CI 0.24 to 0.63). The predicted participation rate was 50%, given that the two treatments are very different and patients were expected to have strong preferences for one or the other treatment. The recruitment rate was lower than expected and the CI is wide, given the low number of eligible patients, adding to uncertainty.
Arguably the mean recruitment rate was not so low as to rule out a future trial if there were a large number of patients with this fracture. However, a substantially lower than anticipated number of patients were considered eligible for the trial during the 12-month recruitment period and this finding, together with the recruitment rate, suggests that a future trial with the current eligibility criteria is unlikely to be feasible within a reasonable timeframe. The eligibility criteria used for the feasibility trial were carefully constructed by consultation and agreement between surgeons who would normally recruit to the trial.
Once participants entered the feasibility trial, retention did not appear to be an issue of concern. One participant withdrew from hospital follow-up (in the surgery arm) between randomisation and their week 2 follow-up appointment, and a further patient withdrew from hospital follow-ups (in the conservative treatment arm) between week 2 and month 3. No patients dropped out between 3 and 6 months. Both patients who withdrew continued to receive trial questionnaires.
The context and challenges experienced during the feasibility trial in terms of the feasibility of a full-scale trial are discussed as follows, according to each step in the recruitment pathway: identification of patients with thoracolumbar fracture, assessing eligibility, conversion of eligible patients into trial participants, treatment received and trial follow-up.
Identification of patients with thoracolumbar fracture
It was predicted that 240 patients would be identified for screening during the 12-month recruitment period (expected number of eligible patients was 120, with 50% participation rate); however, 211 patients were screened (88% of the anticipated number). Reasons why this was slightly lower than expected are discussed in the following sections.
Patient pathway
The patient pathway was different at each of the sites participating in the feasibility trial, and this had an impact on the ability to recruit. This would need to be factored into individualised site recruitment strategies for a larger multicentre trial.
For example, recruiting staff at one site reported the patient pathway to be problematic, as most patients presented at one of the feeder hospitals. Only those patients who were transferred to the MTC as part of their routine pathway could be identified for screening and potentially recruited. By the time this transfer had occurred, the patients were generally being managed conservatively and therefore equipoise was also an issue. This could potentially be overcome in a main trial by the use of participant identification centres.
In another site, teams from disciplines other than spinal orthopaedics (e.g. neurosurgery) were often the first point of contact with potential patients. Information on PRESTO study recruitment was delivered to the neurosurgery team prior to commencing recruitment and a researcher presence was maintained in team meetings to improve engagement with screening and recruitment. However, decisions regarding treatment would often fall to busy on-call staff outside normal working hours; therefore, the protocol was not always adhered to.
Research support infrastructure
One of the sites had only one surgeon actively identifying and recruiting patients. The research staff were based at a different hospital site in the same trust, which introduced some logistical difficulties with regard to the identification and screening of patients, involvement in consent discussions and conducting follow-up visits. In addition, research staff were not from orthopaedic or surgical specialties and this also introduced difficulties with access to records and completion of data. A change in research staff left the PI unsupported in recruitment activity for approximately a 4-month period. The associate PI scheme was implemented at this site; however, this had no impact on recruitment as the trainee surgeons did not seem to engage with the process, despite the success of this scheme in other orthopaedic trials registered with the NIHR scheme.
During the pilot trial, various strategies were implemented at individual sites or across all sites to address some of these issues, such as regular recruitment staff attendance at spinal audit meetings. Regular meetings were held between the central trial team and recruiting staff from all sites to encourage networking and to share effective recruitment practices. However, it was not possible during the 12-month recruitment period to address some of the more structural issues, such as setting up participant identification centres and addressing the lack of research support at sites. In a three-site trial, the impact of some of these issues on recruitment may have been amplified because there was no flexibility to put a site on hold when resource issues were being addressed and to get other sites involved in the trial, as there would be in a larger trial. Equally, there is a possibility that we may have underestimated the number of eligible patients, as patients were offered treatment options and were being treated before they could be screened and approached about the trial by the research staff.
Any future trial would need to have strategies in place to handle the different patient pathways or prioritise sites that have pathways that are more likely to be feasible for recruitment; for example, engagement and close collaboration with wider specialties from the early stage of the trial, which could be achieved by selection of co-PIs in the neurosurgery team. Throughout the trial, the wider trial team emphasised the importance of ongoing and regular ‘PR [public relation]’ activities to maintain awareness about the trial. Surgical staff suggested that a monthly e-mail update from the site PI might encourage surgical colleagues to notify the PI of potential participants.
Based on the trial team’s experience of undertaking orthopaedic trials in other conditions, the research infrastructure in the area of spinal orthopaedics does not yet seem to be as developed as some other areas of orthopaedics.
Assessing eligibility
During the 12-month recruitment period for the feasibility trial, 13.3% (28/211) of patients screened were assessed as eligible. There appeared to be no issues with the practical implementation of the feasibility trial inclusion and exclusion criteria. In addition, survey respondents confirmed broad agreement with the trial inclusion and exclusion criteria.
However, the most frequent reason for exclusion from the trial was uncertainty surrounding the definition of fracture stability (n = 40). A small number of survey respondents identified issues with defining stability, and the qualitative interviews with surgeons confirmed a lack of consensus as to how stability is defined. This was attributed to variation in surgeon preference and the number of methods that could be used to define stability. Fracture pattern, neurological deficit, angle of kyphosis, pain and whether the spine collapses or moves on standing were all considered determinants of spinal stability. Other factors influencing the definition of stability were reported to be clinical judgement, a centre’s normal practice, perceptions of the risks and benefits of surgery, and surgeon experience, rather than evidence.
A number of different systems of classifying thoracolumbar fractures are available and aim to categorise different injuries and to guide treatment decisions. However, there is no agreed system in use and no classification systems was used as part of the feasibility trial eligibility criteria. As part of the PRESTO study, we explored what methods of establishing spinal instability are currently being used, and could be implemented as part of a future trial. In addition, the TLICS was calculated at baseline for trial participants to assess the feasibility of using this measure to define the population for a definitive trial. This is one of the more recent classification systems. It is a composite scoring system based on three injury components to assess spine injury and stability: (1) morphology of injury determined by radiographic appearance (between 1 and 4 points assigned); (2) integrity of the posterior ligamentous complex (between 0 and 3 points assigned); and (3) neurological status of the patient (between 0 and 3 points assigned). One prior study10 suggested that patients with a total score of ≤ 3 would be suitable for conservative management, those with a score of 5 should be considered for surgery and those with a score of 4 can be considered for either treatment.
Mean TLICS across the 12 trial participants was 2.6 at baseline and according to the definition above would be suitable for conservative management of their fracture. Using a TLICS of 4 in a future trial, rather than the current eligibility criteria, may result in a population different from the current study and an even smaller pool of patients may be eligible for inclusion in the trial.
Although assessment of fracture stability was considered the most important factor, there were a wide range of reasons why patients were deemed as not eligible to be entered into the trial; most commonly they were assessed as ‘unsuitable for surgery’. Among the population in whom this fracture is most common, unsuitability is often due to frailty or multimorbidity. However, within such broad eligibility criteria, the scope for individual interpretation is high. For example, mental health was another common exclusion criterion cited and, although the inclusion of osteoporotic patients in the feasibility study was permitted, there were instances when osteoporosis was used as a reason for exclusion. There would be scope to explore the effectiveness of stating with greater specificity which patients should not be excluded unnecessarily; however, this may enhance the complexity of running the trial and may have other unintended negative consequences, including non-participation among sites and surgeons.
Overall, there was support in the survey for the broad eligibility criteria used for the pilot study. However, we have seen from their implementation that there is variability in how these criteria are interpreted between and within sites. In particular, uncertainty around the definition of stability was the primary reason why some patients were excluded from the pilot trial. The inclusion of TLICS in the eligibility criteria for future trials is not consistent with current practice and would be likely to decrease the number of eligible patients. Findings suggest that achieving consensus regarding what constitutes a stable fracture is unlikely; therefore, a formal consensus approach to devise eligibility on the basis of stability may not yield criteria that would be acceptable across the surgical community.
Clinical equipoise
Findings from both the survey and the qualitative study show that there is a lack of clinical consensus regarding the appropriateness of surgical intervention for stable thoracolumbar fractures. Some surgeons and trial recruiters felt that there may be difficulty recruiting sites or surgeons who are willing to randomise patients to a full trial. However, the survey did suggest that these surgeons were in the minority, with 77% and 70% of surgeons stating that they would be prepared to randomise for high- and low-energy fractures, respectively. Although the majority of surgeons may be willing to state that they would randomise in principle, this may change according to individual circumstances in the real situation of recruiting a patient into a trial. For example, interviews conducted with participants taking part in the feasibility trial provided examples of situations when the recruiting surgeons had clearly expressed their preference or recommendation for a particular treatment.
Pulling all the data together, it is clear that there are a minority of surgeons (yet still a significant proportion of up to 30%) who clearly have a strong preference for conservative management and feel that it is inappropriate to operate on stable fractures, as defined by the eligibility criteria used in the feasibility trial. For surgeons who considered surgery to be an appropriate treatment option for stable fractures, their decisions to operate in routine practice were based on the eligibility criteria used in this study, their definitions of a stable fracture and their clinical judgement.
The broad range of reasons given for exclusion of patients from the feasibility trial under the criteria ‘other reason to exclude the patient’ further allowed the recruiting surgeons to potentially exclude otherwise suitable patients. A degree of ‘gatekeeping’ was apparent, based on the screening and eligibility data obtained from the feasibility trial.
As we have seen, this interplay of factors has significantly compromised the recruitment rate of the pilot RCT and is likely to do so in a full-scale trial.
Conversion of eligible patients into trial participants
The proportion of eligible patients recruited was 0.43 (95% CI 0.24 to 0.63). The predicted participation rate was 50%; therefore, the conversion rate was close to the initial expectation. Issues pertinent to the conversion process are discussed below.
Consent process
The feasibility trial was set up with the required regulatory approvals and study documentation in place to be able to recruit patients who lacked capacity to provide informed consent. The process of consultee agreement was not tested, as all recruited participants were able to consent for themselves.
The qualitative interviews with trial participants highlighted concerns with the consent process early on. Although patients were clinically assessed as having capacity at the time of consent, some reported having difficulty recalling both how they were approached about the study and their subsequent involvement in recruitment and consent discussions.
This emphasises the importance of revisiting with trial participants their involvement in the trial and what the trial is about beyond the initial consenting process. Staff were reminded that, when possible, they should involve relatives in the discussions about the study and in the informed consent process to enable additional support for patients in their decision-making. This recommendation is in line with good clinical practice guidance and other published literature on informed consent. 54
Patient preference
Given that the two arms of the pilot study entailed very different treatment options, it was expected that patients would have strong preferences. Among the PRESTO feasibility trial population, the preference was for surgery. Of the 12 patients randomised to the feasibility trial, at baseline seven expressed a preference for surgery and five had no preference. The screening data collected as part of the feasibility trial element showed that 6 out of 16 patients who declined to enter the trial cited a strong preference for avoiding surgery as a reason.
Trial participants interviewed as part of the qualitative study indicated that the appeal of surgery was a strong motivational factor for taking part in the feasibility trial. All patients who participated in the qualitative study interviews reported having a preference for surgery prior to randomisation, perceiving that recovery time would be quicker. Other influencing factors included the desire for a prompt return to work, comfort, ease of commuting, positive experiences of previous surgery, the influence of family members with experience of using a brace, the impact of the brace on daily life and concerns about adherence to instruction on wearing a brace.
It is important to consider the basis for patient preferences, given that the injury is relatively rare. Our data did call into question whether or not some of the patients who expressed preferences had come to these views from a thoroughly informed position. Written information about the trial treatments provided to patients could have been improved in both content and medium used. The patients interviewed reported that information regarding what surgical fixation entails and the impact of wearing a brace on daily life was lacking. The public involvement event in July 2019 corroborated this view, with attendees further commenting on the lack of information about recovery expectations and availability of physical and emotional support beyond the trial in each of the treatment arms.
In addition, two patients randomised to receive surgical fixation did not go on to receive surgery [the proportion not receiving the randomly allocated treatment was 0.17 (95% CI 0.02 to 0.48)], with the decision to cross over to conservative treatment made by the patients shortly after randomisation. Because the sample was small, there is considerable uncertainty in the estimate; however, given this and the findings from the qualitative study indicating that participants would have withdrawn if they had not received their preferred treatment, patient treatment preferences should be carefully addressed in the design of any future trial. In addition, surgeon biases and lack of equipoise (expressed either consciously or subconsciously) towards a particular treatment and the impact of this on patient preference cannot be ruled out. Our findings suggest that a need for information relating to all aspects of treatment and recovery for both treatment arms to be presented as a fair comparison. This should be based on patient information priorities or ‘frequently asked questions’. Patient preferences will not be eliminated; however, patients will be fully informed about all options and able to assess which fits best with their individual priorities.
This information exchange is important for the purposes of any future trial, but also in everyday clinical practice. Patient preferences are influenced by the ways in which information is presented to them by the clinical team, which in turn is shaped by their own preferences and usual practices, which vary across sites. For example, as noted above, in one pilot trial site, teams from disciplines other than spinal orthopaedics (e.g. neurosurgery) were often the first point of contact for potential patients. This had an impact on recruitment, as research staff found that patients had often been advised that they would be treated conservatively – an assumption made by the neurology team – therefore participation in a trial in which surgery was the alternative treatment option was not perceived as appealing and contradicted the previous clinical information presented.
Further consideration, therefore, should be given to the suggestions made as to the format of information being given for both patients and clinicians. The creation of online or audio versions of information could be explored. Written information could be supplemented with videos about rehabilitation and explanations around other elements of the trial. The projection of information onto ceilings where it can be easily read by immobilised patients was another option that could be investigated for use in a larger trial.
Treatments received
This was a pragmatic feasibility study that was expected to reflect current variation in practice across the NHS, within defined boundaries. We sought to describe the interventions provided to inform decisions regarding the design of a future trial and the extent to which the design would be pragmatic.
The average length of stay (admission to discharge) for the six patients who received surgery was 2.1 (SD 1.7) weeks. The median was 1.8 weeks, with the shortest stay being 0.3 weeks and the longest being 4.7 weeks.
During surgery, all patients were given only general anaesthetic. The average length of time spent in theatre was 164.8 (SD 40.5, range 132–234) minutes. The average duration of surgery was 119.7 (SD 43.9, range 80–180) minutes.
Four of the six patients undergoing surgery underwent minimally invasive surgery and two patients had open surgery. The number of vertebrae fixed ranged from one to five. The fusion method was used in all surgeries, with one surgery also using the stabilisation method.
The three types of implant used were USS Schanz screws (one patient), polyaxial screws (three patients) and percutaneous screws (two patients).
Post surgery, five patients were instructed to mobilise as normal and two patients were given postoperative braces to wear. No patients experienced any unexpected events or procedures during surgery.
All participants who were assigned to conservative treatment (or crossed over to conservative treatment) wore a brace. All the braces used were non-customised. One patient who underwent surgery was discharged with a brace.
At the 2-week follow-up, of the six patients who received conservative treatment, three were advised to wear the brace 24 hours a day and the remaining three were instructed to wear the brace only during the day. At month 3, of the five instructions recorded for conservative treatment patients, one patient was instructed to wear the brace for the same amount of time as the previous time point and four had been instructed to reduce the amount of time that the brace was worn.
Follow-up (mechanism/time points)
The follow-up clinics that were organised at 2 and 12 weeks as part of the feasibility trial were designed to be consistent with routine clinical practice. The last follow-up clinic at 26 weeks, which was the proposed primary end point for a main trial, was intended to ensure that participants in both treatment groups had the time to complete the treatment pathway being delivered. During the protocol-writing phase of the feasibility trial, it became apparent that the timing of clinic visits varied between the participating sites to a greater extent than originally expected.
When clinic visits conducted as part of routine clinical practice differed from the trial time points, site staff were asked to schedule visits in line with routine practice and record as the nearest trial time point. The qualitative interviews revealed that recruiting staff perceived difficulty in collecting outcome information from patients on questionnaires, but this is not reflected in the reasonably high proportion (> 72%) of completed outcome data achieved at all time points in the feasibility trial.
Month 3 attendance at clinic was 75% and month 6 attendance was 50%. Although the rate of follow-up, as measured by attendance at a clinic visit, is below the minimum of 80% that would be required in a future trial, this could be explained by the variability in the timing of patient follow-up at the different participating sites. Several patients had been discharged from clinical care before the 6-month visit. The method of follow-up in a larger trial should be further explored. It may be possible to conduct trial visits via telephone or other means if the outcome measures chosen do not require patients to attend a clinic for imaging or other clinical investigation.
Outcome measures
ODI, VAS, SF-12 and EQ-5D-5L questionnaires were completed at baseline and at month 3 and month 6 (if this was reached within the follow-up period). Patients could choose to complete these questionnaires online via the BSR or on paper copies sent to them through the post, except for the SF-12, which cannot be completed through the registry because of licensing issues.
Baseline forms were returned for all 12 participants (100.0%). Month 3 participant questionnaires were returned for nine (75.0%) participants and month 6 questionnaires were returned by eight (72.7% of the 11 participants who reached the time point). The return rates are lower than the 80% that would be required in a main trial. However, it is likely that the feasibility study underestimated this, as we did not fully utilise our standard methods (such as pre-notification letters, short message service reminders or financial incentives) to maximise response rates. To test the feasibility of the online data collection (via the BSR platform), patients were not offered the opportunity to complete the questionnaire at the clinic visits. In addition, the trial team had no control over reminders issued to participants on the BSR platform. Postal copies were sent only when there was no response after several weeks to the online request to participants who received questionnaires via the spine registry.
Despite the majority of participants agreeing to receive online questionnaires, only four participants completed them online and, even then, not at all required time points. This finding is consistent with recently presented findings of a trial embedded within the UK Study of Tendo Achilles Rehabilitation. 55 None of the participants completing questionnaires online filled in the resource use section. It was not possible to establish the reason for this; however, in the case of the first participants this was because the system was not working properly. There was a financial cost associated with the set-up of the data collection via the BSR. A specific pathway had to be created on the registry platform and there was an annual licence fee per participating site. Furthermore, the BSR is not routinely used for patients being managed conservatively and, although an exception was made to allow this for the feasibility trial, it is unclear whether or not this would be the case for a larger trial. Postal questionnaires were the most effective means of collecting patient-reported outcome data in this small study. Data collection via the BSR could not be the sole mechanism for collection of outcomes in a larger trial.
The number of missing responses for the ODI was 32 of 410; therefore, the overall proportion of complete data for the ODI was 0.92 (95% CI 0.89 to 0.95). For the VAS, the overall proportion of complete data was 0.93 (95% CI 0.77 to 0.99). The overall proportion of complete data for the SF-12 was 1.00 (95% CI 0.98 to 1.00).
Collection of the outcome measures chosen for the feasibility trial proved to be successful for all measures, suggesting their suitability for inclusion in a future trial from this perspective. Concerns expressed by staff taking part in the qualitative interviews regarding the length of the questionnaires and burden on participants were, therefore, unfounded.
In terms of performance (extent of floor and ceiling effects) and completion rates, none of the outcome measures stood out as being a primary outcome for a future trial. There was a broad consensus among surgeons that patient-reported outcomes were the most important consideration. Pain and return to normal function were considered by surgeons and recruiting staff to be the most important indicators of recovery. The survey respondents considered pain, then mobility and return to pre-injury activity levels to be the most important outcomes, but with little difference between them. Speed of return to pre-injury levels of activity and anxiety or depression were the factors receiving the lowest ranking for importance by the participating surgeons. Attendees at the PPI event at the end of the study suggested that the outcome of importance to patients would depend on where they were in the recovery process. For example, early on pain (relief from) was likely to be the prominent consideration for patients; however, later on in recovery pathway, and assuming an impact on mobility, it would be mobility (return to pre-injury state) that would be of greater importance to the patient. Surgeons and recruiting staff participants in the qualitative interviews proposed a wide range of outcomes to be collected during a main trial.
Consideration should be given to the exclusion of polytrauma patients in a larger trial owing to the impact of other injuries on potential outcome measures. Recruiting staff, during discussions with the trial team and during interviews, highlighted the difficulties of collecting complications and AEs when trying to distinguish those relating to the thoracolumbar fracture from complications resulting from other injuries.
Further work is required to develop a core outcome set using consensus methods and with substantial patient involvement. It is unclear whether or not outcome measures such as the ODI, which was developed for use in a patient population with chronic low back pain, fully covers the issues important to patients in terms of their recovery from a thoracolumbar fracture. None of the scales exhibited floor or ceiling effects, which suggests that they are suitable for use in a large trial from this perspective.
Importance was placed by both patients and surgeons on the patients’ return to pre-injury state, so this would need to be captured as part of trial data, as we did with the PRESTO study in the collection of both pre- and post-injury ODI and EQ-5D-5L at baseline.
Economic data
The health economic outcomes are based on the completed case report forms and comprise a QoL indicator (i.e. the EQ-5D-5L) and a bespoke resource use questionnaire. Data were gathered on treatments, length of hospital stay, rehabilitation, time to return to work and time to return to normal activities.
All participants (n = 12) completed the EQ-5D-5L before injury and at baseline. At 3 months, nine participants (four in the conservative group and five in the surgery group) completed the EQ-5D-5L, and at 6 months eight participants, four in each arm, did so. All patients who completed the EQ-5D-5L responded in full to the five dimensions and the health VAS.
Overall, little health-care resource use was reported across both trial arms, in part because this information was not completed by patients returning data via the BSR. No inpatient care was reported by any of the six participants (four in the conservative group and two in the surgery group) who completed the 3-month questionnaire health-care resource use section or any of the eight (four in each arm) participants who completed the 6-month resource use section. No patients reported any of the following at any time: having a GP home visit, seeing a practice nurse at a general practice or seeing a district nurse.
Regarding private care, a single patient attended one session with a private specialist between 0 and 3 months and a further two patients in the surgery arm reported attending sessions between 3 and 6 months (average of two sessions). One patient in the surgery arm did not answer the question on the 3-month questionnaire about seeing a private specialist for a surgical treatment.
Of the six patients who returned their 3-month questionnaire, only one patient (in the conservative treatment group) reported that they had been able to fully carry out unpaid daily activities. Hence, five patients (three allocated to conservative treatment and two allocated to surgery) reported that they had not been able to carry out some of these activities. Of the eight patients who returned their 6-month questionnaire, three (two allocated to surgery, one allocated to conservative treatment) reported that they had not been able to carry out unpaid daily activities between 3 and 6 months. The remaining five participants (three allocated to conservative treatment and two allocated to surgery) reported that they had been able to fully carry out their daily activities.
No patients reported any usage of hospital transport on the 3-month questionnaire. A single patient reported using hospital transport once (surgery arm) between 3 and 6 months. There were no missing responses for hospital transport on either questionnaire.
The EQ-5D-5L appears to be sensitive to problems in the study population. Based on the results of the feasibility study, we would make a number of changes to the resource use questionnaire. For example, health-care resource use could be compressed to include services that are typically provided by the NHS only (i.e. by removing items such as hospital transport, as this was generally not used). Similarly, given the low levels of medication uptake, we would be unlikely to benefit from conducting a micro-costing exercise in a full trial.
Postoperative pathway/rehabilitation
Variability was apparent in the postoperative pathways of patients taking part in the feasibility trial: one participating site did not refer any patient for physiotherapy, with a further site apparently actively encouraging patients to seek physiotherapy via private practice.
Five out of the eight patients allocated to surgery reported that since their surgery they had received physiotherapy in hospital from a physiotherapist, spinal clinical nurse specialist or orthoptist. A further patient reported receiving only advice from one of the aforementioned specialists. Patients reported receiving advice or education on bracing, mobilisation, precautions that they should take and exercises. Patients who reported attending sessions had attended five sessions, on average, within 2 weeks of surgery. At 3 months, the mean number of sessions attended since randomisation was seven (reported in four patients). Sessions were discontinued or became less frequent following discharge.
Of the four patients allocated to conservative treatment, three reported receiving physiotherapy sessions following randomisation. Patients reported that they had received advice about bracing, mobilisation, precautions and exercises. Patients who reported attending sessions had attended 1.5 sessions, on average, within 2 weeks of randomisation.
Three out of the five participants who were interviewed reported frustration, as they felt that they had received insufficient information regarding how they should manage their recovery, both as an inpatient and once discharged, and their expected recovery time. In particular, participants highlighted the limited information received about managing their condition and the movements that were acceptable post discharge. Furthermore, the PAG suggested that informing potential participants in greater detail about the rehabilitation pathways for each of the trial treatments would lead to patients being fully informed when making a decision about trial participation. Although the standardisation of rehabilitation therapies (e.g. physiotherapy across multiple sites) might not be feasible, the study team should consider production of further leaflets with suggested exercises. This would not be without issue, as each thoracolumbar injury is unique and rehabilitation advice is personalised.
Table 27 summarises the answers to the research questions arising from the aims and objectives of the study.
| Research question | Answer |
|---|---|
| Are surgeons willing to randomise eligible patients and adhere to randomisation to (1) surgical fixation or (2) initial conservative management? | In theory, a large proportion of surgeons are willing to randomise. In practice, only a relatively small proportion of patients are deemed eligible, possibly related to a lack of consensus around what constitutes a stable fracture and the appropriateness of surgery for stable fractures. The small number of eligible candidates identified is the main reason why a full-scale trial is not feasible |
| Are patients willing to be randomised and adhere to randomisation in a trial comparing the two treatments? | There is evidence to suggest that patients may be willing to be randomised, as half of those in the trial had a preference for surgery. However, there is a risk of resentful demoralisation among those being randomised to treatment that is not of their preference (two crossover patients) and there were concerns that clinician preferences may have influenced patients |
| What is the completeness of follow-up in this population? | Completeness of follow-up was good. Two patients (17%) withdrew from hospital follow-up over the course of the feasibility trial (one in each arm) and no patients withdrew completely. At 3 months, 75% of questionnaires were returned and at 6 months 73% were returned. The 2-week hospital follow-up had 100% attendance, the 3-month follow-up had 82% attendance and the 6-month follow-up had 50% attendance. The follow-up rates (questionnaire return and visit attendance) were lower than the 80% that would be required in a main trial. However, it is likely that the feasibility study underestimated this and the rate could be increased |
| Are there a sufficient number of centres and surgeons (with sufficient caseloads of eligible patients) willing to participate in a future RCT to make the trial feasible within a viable time scale? | There was a high level of support for a future trial. However, owing to the small proportion of patients screened who met the current trial eligibility criteria, a future trial is unlikely to be feasible |
| What methods of establishing spinal stability and suitability for surgery or conservative management are currently used? | Participants in the survey rated spinal cord involvement and neurological deficit as the most important factor when establishing spinal stability. Participants also rated both CT and standing radiograph as the most important imaging measures used to establish spinal stability, with segmental kyphosis and MRI also being of importance. Other important factors included mechanism of injury, AO/OTA 2018 classification and trunk control |
| What methods of surgical fixation and conservative management are currently being used? |
Of the six patients who underwent surgery in the feasibility trial, four (66.7%) underwent minimally invasive surgery and two had open surgery (33.3%). All surgeries involved the fusion method, with one also using the stabilisation method Survey respondents indicated current use of open spinal surgery (92%) and minimally invasive surgery (81%), together with other methods such as vertebroplasty and kyphoplasty All participants who received conservative treatment in the feasibility trial wore a non-customised brace. The majority of survey respondents (63%) always or frequently used off-the-shelf bracing, 36% always or frequently used bracing with customised orthoses and 29% did not use bracing |
| What are the barriers to successful delivery of the future trial and how can they be overcome? | There were multiple barriers identified related to the care pathway, infrastructure and suitable outcomes. Most of these were not unique to this study and could potentially be successfully addressed to allow a full trial. However, the lack of clinical consensus regarding the implementation of the eligibility criteria in practice and what constitutes a stable fracture suitable for inclusion in a trial of surgery compared with conservative management, is such that a full-scale trial is unlikely to be successful without substantial change in the extent of surgeon consensus on how the eligibility criteria would be implemented and a change in the balance of equipoise |
| Can the BSR be used to collect participant data in a trial? | The cost, logistics and incompleteness of data suggest that the registry is not feasible for use as the sole mechanism of data collection in a full-scale study |
| What is the most suitable primary end point for a main trial? | In terms of performance, none of the measures used stood out as the best measure. Pain and return to normal function were considered the most important indicators of recovery by surgeons and recruiting staff who were interviewed, whereas survey respondents considered pain, then mobility and return to pre-injury activity levels, to be the most important outcomes. Attendees at the PPI event at the end of the study suggested that the outcome of importance to patients would depend on where they were in the recovery process. Early on, pain (relief from) was likely to be the prominent consideration for patients, but later in the recovery pathway, and assuming an impact on mobility, it would be mobility (return to pre-injury state) that would be of greater importance to the patient |
| How can we accurately identify, quantify and value economic data to capture the impact of the two treatments on the NHS and productivity? | The EQ-5D-5L appears to be sensitive to problems in the study population. Data were successfully gathered on treatments, length of hospital stay, rehabilitation, time to return to work and time to return to normal activities for all trial patients who returned postal questionnaires or attended the follow-up visits |
Strengths and limitations of the research
The PRESTO study combines data generated from three elements: (1) a feasibility RCT, (2) a qualitative study involving trial participants, recruiting clinicians and research staff, as well as surgeons external to the trial, and (3) a national survey of spine surgeons. The findings are strengthened by being able to draw on the different sources of information.
Although the number of recruits was significantly smaller than anticipated, the number screened was similar to that predicted and the conversion rate of eligible patients, importantly, was not much below the 50% predicted rate. The main issue was that few patients screened met the eligibility criteria. It is possible that the broad exclusion criteria allowed personal bias towards a particular treatment to be hidden, resulting in a degree of ‘gatekeeping’ by those assessing eligibility.
A number of limitations need to be considered when interpreting the findings. Although the three sites included showed diversity in their care pathways and research infrastructure, it is possible that there is further diversity that was not captured or that the sites are in some way different, which affects the generalisability of the findings. Unlike a full-scale multicentre trial, there was no scope within the feasibility trial to add additional sites or replace the sites; therefore, some of the challenges may have been amplified and we may have underestimated the feasibility of a future trial.
We were not able to successfully recruit patients who declined participation in the trial to the qualitative study. This was, therefore, a missed opportunity to gain valuable depth of insight into reasons for patients declining to take part. This has been successfully achieved in other studies and may reflect some of the reasons underlying the small number of participants recruited into the study overall.
Although a small number of patients took part in the qualitative study, they constituted a substantial proportion of trial participants and a wide range of perspectives were captured.
Overall, although there may be scope to improve the consent rate for a future trial, this does not address the key barrier to future feasibility, that is the small number of patients meeting the eligibility criteria.
Chapter 8 Conclusions
The findings of the PRESTO study demonstrate that the trial design tested in the feasibility RCT element would be unlikely to be successful in a definitive trial at this time. This was mainly related to the small number of people meeting the eligibility criteria. The recruitment and follow-up rates were slightly lower than anticipated, but there is room to increase these based on information gathered during the feasibility study and the fact that there was support in the surgical community for a future trial.
There were some contradictions among the different sources of data. Although there was support for the eligibility criteria used as part of the feasibility trial, there was a lack of consensus around the definition of fracture stability, which is central to defining the study population. Further consensus work would need to be undertaken in advance of any definitive trial.
Implications for research
It is uncertain whether or not a definitive trial comparing conservative and surgical treatments for the treatment of thoracolumbar vertebral body fracture, between T10 and L2, is feasible. The following recommendations are made should the context change to mean that a definitive trial was under consideration.
The design should be modified to include an integrated recruitment intervention to support surgeons in their decision-making to recruit participants and to aid other staff involved in the recruitment process to fully explain the trial and each treatment option in a balanced way. Trial design would need to take into account both surgeons’ and patients’ treatment preferences during treatment allocation to improve surgeon and patient participation, and willingness to randomise or to be randomised. The content of and medium used for information provided to patients would need to be reconsidered to maximise recruitment into a larger trial.
For the PRESTO study, the qualitative interviews produced some important learning points concerning the consent procedures, which were fed back to recruiting staff during the recruitment period. Based on our findings, the inclusion of qualitative interviews of both trial participants and non-consenting patients would be an effective strategy to inform recruitment practice in an ongoing manner during the trial recruitment phase. The use of methodology such as the QuinteT Recruitment Intervention56 could be explored for a definitive trial, given that recruitment was difficult in the PRESTO feasibility trial. Enhanced training and support should be considered to support clinicians and others involved in recruitment to deliver trial information in a balanced way to potential participants. For example, the use of a ‘consent hints and tips document’ in the current feasibility trial could be further developed and supplemented by the use of training and support videos.
Regardless of how the small number of eligible patients is interpreted, any future trial will need to have strategies in place to handle the different patient pathways or to prioritise sites that have pathways that are more likely to be feasible for recruitment.
The recruitment issues experienced demonstrate that multiple spinal surgeons from orthopaedic and neurosurgical backgrounds need to be involved at participating sites to support PIs, and strong team cohesion is paramount to success.
Further work should be undertaken regarding the medium or mechanism (audio, electronic) of information provision in a larger trial. Patients recruited to the feasibility trial were sometimes completely immobilised during screening and baseline measurements, and informed consent discussions.
A future trial comparing treatments for thoracolumbar fractures would need to account for variation in length and timing of clinical follow-up across sites and consider the use of only patient-reported outcomes beyond the intervention. If a future trial were to involve clinic visits, consideration should be given to flexibility about where participants attend a follow-up. The trial could be designed so that visits could be undertaken at any participating site. This potentially would result in increased recruitment, as patients declined participation in the feasibility trial because they were injured away from home and were unable to attend the recruiting hospital for follow-up.
Use of the BSR as a mechanism for the collection of patient-reported outcomes in a future trial comparing treatments for thoracolumbar fractures did not yield complete data. Further customisation of the platform (e.g. to ensure that the e-mails sent to trial participants contain the trial branding) via increased collaboration with the provider would be necessary. The cost of this additional work may be prohibitive.
Further research is also required to understand more fully patient preferences in terms of how they are followed up and variation across different groups.
Acknowledgements
Most importantly, we thank the patients participating in the feasibility trial, without whom this trial would have not been possible.
Thank you also to the surgeons and staff who gave up their time to be interviewed as part of the qualitative element and/or to complete the survey.
A general thanks also to all those people whose commitment and efforts in the design and conduct of the PRESTO trial allowed us to successfully complete this study and this final report.
A specific thanks to the following for their involvement in recruitment and data collection at participating sites, and who made a valuable contribution to the study, but who are not named as authors: Shanaz Ahmad, Elizabeth Atha, Paulito Castino, Ramsey Chammaa, Tracey Crowther, Rowanne Eeles, Heather Jarvis, Jamila Kassam, Chloe Keith-Jopp, Ahmed Sader, Martina Santarelli, Fady Sedra, Jessica Whiteman, Matthew Williams and Sophie Young.
Dr Lynne Stobbart undertook some of the qualitative interviews.
The teams in York and London contributed to the design of data collection forms, information systems and management of data.
A special thanks to Mr Adam Levene, who was our patient representative in the TMG and who, throughout the study, contributed to discussions on various trial-related materials.
Thank you also to the members of our combined TSC and DMEC: Mr Dan Perry (chairperson), Mr Ashley Cole (independent member), Mr Stuart Blagg (independent member), Ms Laura Mandefield (independent member), Dr Simon Rawlinson, (independent member), Dr Caroline Alexander (independent member) and Mr Joe Millar (sponsor representative).
We also thank the PAG who we met with in London and Leeds, and consulted with via telephone and e-mail about the progress of the study.
Thank you to the following surgeons who participated in the survey: Elmunzar Bagouri, Tony Bateman, James Casha, Derek Cawley, Mr Shashank Chitgopkar, HV Dabke, Evan Davies, Andreas Demetriades, Jeremy Fairbank, Andrew Frost, Tim Germon, Cyrus Jensen, Kevin Morris, Had Mushtaq, Ben Okafor, Himanshu Sharma and Paul Thorpe.
The investigators acknowledge the support of the British Orthopaedic Association Orthopaedic Surgery Research Centre, which supports the development of surgical trials.
Contributions of authors
Mrs Elizabeth Cook (https://orcid.org/0000-0001-6902-0235) (Research Fellow, Trial Manager) was a co-applicant and trial manager who contributed to the design and conduct of the trial throughout the duration of the study, setting up of sites and acquisition of data in all elements of the trial, writing and overseeing reports for publication, and contributed to and commented on all drafts of the report.
Dr Arabella Scantlebury (https://orcid.org/0000-0003-3518-2740) (Research Fellow, Qualitative Researcher) led on the design and conduct of the qualitative interview element, participated in trial management processes, was involved in the PPI work, and contributed to and commented on all drafts of the report.
Mrs Alison Booth (https://orcid.org/0000-0001-7138-6295) (Research Fellow, Trial Manager) led on the design and co-ordination of the survey element of the trial, participated in trial management processes, and contributed to and commented on all drafts of the report.
Mrs Emma Turner (https://orcid.org/0000-0001-8173-0248) (Trial Support Officer) was involved during the development of trial materials, setting up of sites and acquisition of data in all elements of the trial, and has read and contributed to the trial report.
Mr Arun Ranganathan (https://orcid.org/0000-0001-9260-6437) (Consultant Spinal and Orthopaedic Surgeon, chief investigator) was lead co-applicant and chief investigator of the trial, contributing to the design and conduct of the trial, with overall management of the trial throughout its duration, and contributed to and commented on all drafts of the report.
Mr Almas Khan (https://orcid.org/0000-0002-7916-6219) (Consultant Trauma and Orthopaedic Surgeon) was a co-applicant, contributing to the design and conduct of the trial as site PI, and contributed to and commented on all drafts of the report.
Mr Sashin Ahuja (https://orcid.org/0000-0001-8676-6100) (Consultant Orthopaedic Spinal Surgeon) was a co-applicant, contributing to the design and conduct of the trial as site PI, and contributed to and commented on all drafts of the report.
Mr Peter May (https://orcid.org/0000-0003-4900-227X) (Orthopaedic Research Physiotherapist) was involved in the co-ordination of the PAG, with the development of trial materials and acquisition of data in the trial, and has commented on the trial report.
Professor Amar Rangan (https://orcid.org/0000-0002-5452-8578) (Professor of Orthopaedic Surgery) was a co-applicant and mentor to the chief investigator, contributed to the design and conduct of the trial, and contributed to and commented on all drafts of the report.
Ms Jenny Roche (https://orcid.org/0000-0003-1822-9519) (Statistician) led on the preparation of the statistical analysis plan, conducted the final analysis, and contributed to and commented on the final report.
Ms Elizabeth Coleman (https://orcid.org/0000-0003-4210-1865) (Statistician) contributed to the design and development of statistical elements and data collection forms, contributed to the preparation of the statistical analysis plan, contributed to the final analysis, and contributed to and commented on the final report.
Ms Catherine Hilton (https://orcid.org/0000-0003-0410-6788) (Orthopaedic Research Physiotherapist) was a co-applicant who contributed to the study implementation at the lead site and has commented on the trial report.
Ms Belén Corbacho (https://orcid.org/0000-0002-2359-0379) (Research Fellow, Health Economist) was a co-applicant who contributed to the design and conduct of the health economics sections of the trial throughout the duration of the study, and contributed to and commented on all drafts of the report.
Professor Catherine Hewitt (https://orcid.org/0000-0002-0415-3536) (Professor of Trials and Statistics/Deputy Director YTU) was a co-applicant and had overall responsibility for the statistical analyses of the trial data, contributed to the design and analysis of the study, and commented on all drafts of the report.
Professor Joy Adamson (https://orcid.org/0000-0002-9860-0850) (Mary Kinross Charitable Trust and Royal College of Surgeons Chairperson in Surgical Trials and Health Sciences) was a co-applicant who contributed to the design and analysis of the qualitative study, and contributed to and commented on all drafts of the report.
Professor David Torgerson (https://orcid.org/0000-0002-1667-4275) (Director of YTU) was a co-applicant and helped write the study protocol, and is a trial methodologist responsible for the overall methods of the trial.
Dr Catriona McDaid (https://orcid.org/0000-0002-3751-7260) (Reader in Trials) was a co-applicant and contributed to the design and conduct of the trial, and commented on all drafts of the report.
Publication
Cook E, Booth A, Coleman E, Scantlebury A, McDaid C, Hewitt C, et al. Pragmatic randomised evaluation of stable thoracolumbar fracture treatment outcomes (PRESTO): study protocol for a randomised controlled feasibility trial combined with a qualitative study and survey. Pilot Feasibility Stud 2020;6.
Data-sharing statement
The data sets generated and/or analysed during the current study (fully anonymised) are available on request. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review by the chief investigator.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Heinzelmann M, Wanner GA, Boos N, Aebi M. Spinal Disorders. Berlin: Springer; 2008.
- Wood KB, Li W, Lebl DR, Lebl DS, Ploumis A. Management of thoracolumbar spine fractures. Spine J 2014;14:145-64. https://doi.org/10.1016/j.spinee.2012.10.041.
- Gopinath B, Harris IA, Nicholas M, Casey P, Blyth F, Maher CG, et al. A comparison of health outcomes in older versus younger adults following a road traffic crash injury: a cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0122732.
- Jagnoor J, De Wolf A, Nicholas M, Maher CG, Casey P, Blyth F, et al. Restriction in functioning and quality of life is common in people 2 months after compensable motor vehicle crashes: prospective cohort study. Inj Epidemiol 2015;2. https://doi.org/10.1186/s40621-015-0042-7.
- Platts-Mills TF, Flannigan SA, Bortsov AV, Smith S, Domeier RM, Swor RA, et al. Persistent pain among older adults discharged home from the emergency department after motor vehicle crash: a prospective cohort study. Ann Emerg Med 2016;67:166-76.e1. https://doi.org/10.1016/j.annemergmed.2015.05.003.
- Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res 1984;189:65-76. https://doi.org/10.1097/00003086-198410000-00008.
- Kepler CK, Vroome C, Goldfarb M, Nyirjesy S, Millhouse P, Lonjon G, et al. Variation in the management of thoracolumbar trauma and postoperative infection. J Spinal Disord Tech 2015;28:E212-8. https://doi.org/10.1097/BSD.0000000000000224.
- Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 1994;3:184-201. https://doi.org/10.1007/BF02221591.
- Reinhold M, Audigé L, Schnake KJ, Bellabarba C, Dai LY, Oner FC. AO spine injury classification system: a revision proposal for the thoracic and lumbar spine. Eur Spine J 2013;22:2184-201. https://doi.org/10.1007/s00586-013-2738-0.
- Vaccaro AR, Lehman RA, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine 2005;30:2325-33. https://doi.org/10.1097/01.brs.0000182986.43345.cb.
- Lenarz CJ, Place HM. Evaluation of a new spine classification system, does it accurately predict treatment?. J Spinal Disord Tech 2010;23:192-6. https://doi.org/10.1097/BSD.0b013e31819e30c1.
- Vaccaro AR, Baron EM, Sanfilippo J, Jacoby S, Steuve J, Grossman E, et al. Reliability of a novel classification system for thoracolumbar injuries: the Thoracolumbar Injury Severity Score. Spine 2006;31:62-9. https://doi.org/10.1097/01.brs.0000218072.25964.a9.
- McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine 1994;19:1741-4. https://doi.org/10.1097/00007632-199408000-00014.
- Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine 1983;8:817-31. https://doi.org/10.1097/00007632-198311000-00003.
- Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am 1970;52:1534-51. https://doi.org/10.2106/00004623-197052080-00002.
- Ferguson RL, Allen BL. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Relat Res 1984;189:77-88. https://doi.org/10.1097/00003086-198410000-00009.
- National Institute for Health and Care Excellence . Head Injury: Assessment and Early Management 2014. www.nice.org.uk/guidance/cg176/resources/head-injury-assessment-and-early-management-pdf-35109755595493 (accessed 19 June 2019).
- Hahnhaussen J, Hak DJ, Weckbach S, Ertel W, Stahel PF. High-energy proximal femur fractures in geriatric patients: a retrospective analysis of short-term complications and in-hospital mortality in 32 consecutive patients. Geriatr Orthop Surg Rehabil 2011;2:195-202. https://doi.org/10.1177/2151458511427702.
- Diamantopoulos AP, Rohde G, Johnsrud I, Skoie IM, Hochberg M, Haugeberg G. The epidemiology of low- and high-energy distal radius fracture in middle-aged and elderly men and women in Southern Norway. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0043367.
- Øyen J, Rohde G, Hochberg M, Johnsen V, Haugeberg G. Low bone mineral density is a significant risk factor for low-energy distal radius fractures in middle-aged and elderly men: a case–control study. BMC Musculoskelet Disord 2011;12. https://doi.org/10.1186/1471-2474-12-67.
- Siebenga J, Leferink VJ, Segers MJ, Elzinga MJ, Bakker FC, Haarman HJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine 2006;31:2881-90. https://doi.org/10.1097/01.brs.0000247804.91869.1e.
- Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am 2003;85:773-81. https://doi.org/10.2106/00004623-200305000-00001.
- Bailey CS, Urquhart JC, Dvorak MF, Nadeau M, Boyd MC, Thomas KC, et al. Orthosis versus no orthosis for the treatment of thoracolumbar burst fractures without neurologic injury: a multicenter prospective randomized equivalence trial. Spine J 2014;14:2557-64. https://doi.org/10.1016/j.spinee.2013.10.017.
- Pneumaticos SG, Triantafyllopoulos GK, Giannoudis PV. Advances made in the treatment of thoracolumbar fractures: current trends and future directions. Injury 2013;44:703-12. https://doi.org/10.1016/j.injury.2012.12.005.
- McAnany SJ, Overley SC, Kim JS, Baird EO, Qureshi SA, Anderson PA. Open versus minimally invasive fixation techniques for thoracolumbar trauma: a meta-analysis. Global Spine J 2016;6:186-94. https://doi.org/10.1055/s-0035-1554777.
- Jaffray DC, Eisenstein SM, Balain B, Trivedi JM, Newton Ede M. Early mobilisation of thoracolumbar burst fractures without neurology: a natural history observation. Bone Joint J 2016;98:97-101. https://doi.org/10.1302/0301-620X.98B1.36121.
- Kumar A, Aujla R, Lee C. The management of thoracolumbar burst fractures: a prospective study between conservative management, traditional open spinal surgery and minimally interventional spinal surgery. Springerplus 2015;4. https://doi.org/10.1186/s40064-015-0960-4.
- Rudol G, Gummerson NW. (ii) Thoracolumbar spinal fractures: review of anatomy, biomechanics, classification and treatment. Orthop Trauma 2014;28:70-8. https://doi.org/10.1016/j.mporth.2014.01.003.
- Abudou M, Chen X, Kong X, Wu T. Surgical versus non-surgical treatment for thoracolumbar burst fractures without neurological deficit. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD005079.pub3.
- Ghobrial GM, Maulucci CM, Maltenfort M, Dalyai RT, Vaccaro AR, Fehlings MG, et al. Operative and nonoperative adverse events in the management of traumatic fractures of the thoracolumbar spine: a systematic review. Neurosurg Focus 2014;37. https://doi.org/10.3171/2014.4.FOCUS1467.
- Rometsch ESM, Härtl R, McGuire RA, Gallo-Kopf BS, Kalampoki V, Kandziora F. AO spine injury classification system: a revision proposal for the thoracic and lumbar spine. Eur Spine J 2017;7:350-72. https://doi.org/10.1177/2192568217699202.
- Clement RC, Welander A, Stowell C, Cha TD, Chen JL, Davies M, et al. A proposed set of metrics for standardized outcome reporting in the management of low back pain. Acta Orthop 2015;86:523-33. https://doi.org/10.3109/17453674.2015.1036696.
- Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther 2002;82:8-24. https://doi.org/10.1093/ptj/82.1.8.
- Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine 1998;23:2003-13. https://doi.org/10.1097/00007632-199809150-00018.
- Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine 2000;25:2940-53. https://doi.org/10.1097/00007632-200011150-00017.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain. Arthritis Care Res 2011;63:S240-S52. https://doi.org/10.1002/acr.20543.
- McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988;18:1007-19. https://doi.org/10.1017/s0033291700009934.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- EuroQol . EQ-5D 2017. www.euroqol.org (accessed 24 September 2019).
- Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. PharmacoEconomics 2017;35:501-15. https://doi.org/10.1007/s40273-016-0485-x.
- Granja C, Teixeira-Pinto A, Costa-Pereira A. Quality of life after intensive care – evaluation with EQ-5D questionnaire. Intensive Care Med 2002;28:898-907. https://doi.org/10.1007/s00134-002-1345-z.
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91. https://doi.org/10.1002/pst.185.
- Great Britain . Mental Capacity Act 2005 2005.
- Department of Health and Social Care . NHS Reference Costs 2016 n.d. www.gov.uk/government/collections/nhs-reference-costs (accessed 24 September 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- National Institute for Health and Care Excellence . British National Formulary 2017 n.d. https://bnf.nice.org.uk/ (accessed 24 September 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 24 September 2019).
- NHS Health Technology Assessment Programme . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Braun V, Clarke V, Hayfield N, Terry G, Liamputtong P. Handbook of Research Methods in Health Social Sciences. Singapore: Springer; 2019.
- Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6. https://doi.org/10.2196/jmir.6.3.e34.
- Cho YI, Johnson TP, Vangeest JB. Enhancing surveys of health care professionals: a meta-analysis of techniques to improve response. Eval Health Prof 2013;36:382-407. https://doi.org/10.1177/0163278713496425.
- Braithwaite D, Emery J, De Lusignan S, Sutton S. Using the Internet to conduct surveys of health professionals: a valid alternative?. Fam Pract 2003;20:545-51. https://doi.org/10.1093/fampra/cmg509.
- D’Souza RS, Johnson RL, Bettini L, Schulte PJ, Burkle C. Room for improvement: a systematic review and meta-analysis on the informed consent process for emergency surgery. Mayo Clin Proc 2019;94:1786-98. https://doi.org/10.1016/j.mayocp.2019.02.026.
- Meeting abstracts from the 5th International Clinical Trials Methodology Conference (ICTMC 2019). Trials 2019;20.
- Rooshenas L, Paramasivan S, Jepson M, Donovan JL. Intensive triangulation of qualitative research and quantitative data to improve recruitment to randomized trials: the QuinteT approach. Qual Health Res 2019;29:672-9. https://doi.org/10.1177/1049732319828693.
Appendix 1 Recruitment data
| Site | Recruitment start date | 2018 | 2019 | Total number of patients recruited | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | November | December | January | February | March | |||
| Bart’s Health NHS Trust | 18 April 2018 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 5 |
| Leeds Teaching Hospitals NHS Trust | 4 June 2018 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cardiff & Vale University Health Board | 20 June 2018 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 6 |
| Total | 3 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 1 | 12 | |
| Site | 2018 | 2019 | Overall rate of patients recruited | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| June | July | August | September | October | November | December | January | February | March | ||
| Bart’s Health NHS Trust | 0.67 | 0.25 | 0 | 0 | 0 | 0.67 | 0 | 0 | 0 | 0 | 0.33 |
| Leeds Teaching Hospitals NHS Trust | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
| Cardiff & Vale University Health Board | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0.75 |
FIGURE 9.
Monthly patient recruitment by site (Bart’s Health NHS Trust opened April, Leeds Teaching Hospitals NHS Trust and Cardiff & Vale University Health Board opened June).
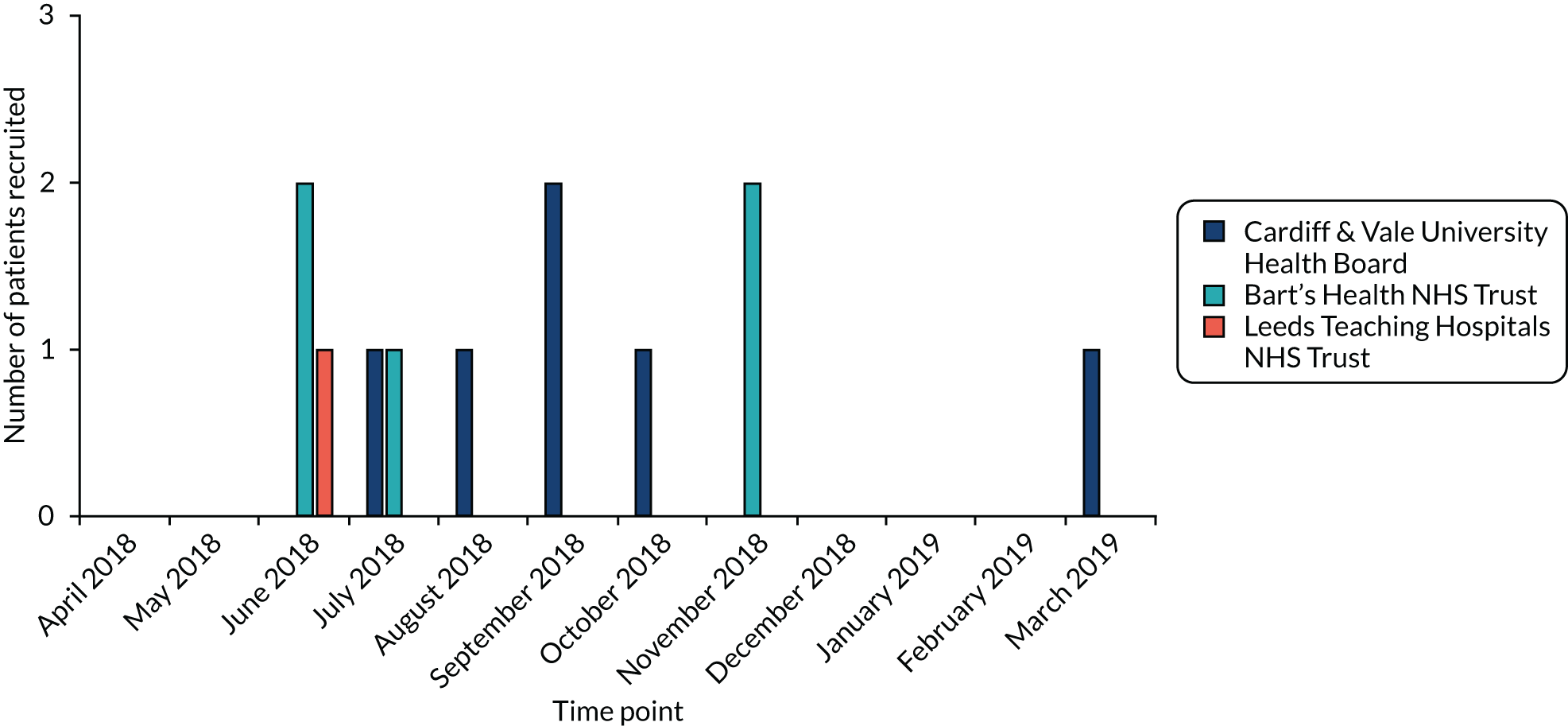
Appendix 2 Additional exclusion criteria information
Three column injury including ligamentum flavum.
Three-column injury; vertebral body; facet joint; posterior ligamentous complex.
Injury to spinal cord; three-column injury at 10; L1; T11; T10 all fractured; therefore complex OP.
Comminuted burst fracture retropulsed into cord canal. Neurology present. Posterior ligamentous complex damaged.
Chance fracture; considered unstable due to spinous process fracture.
Involving both pedicules as well as facet joints.
Retropulsion of fracture into spinal canal; burst fracture; neurology.
Chance fracture; already fixed in Southampton.
Spinal cord injury.
Three-column injury. Spinal cord signal change.
Three-column column injury including posterior elements.
Three-column injury including posterior elements.
Disruption of posterior ligamentous complex.
Three-column injury to L1; posterior translocation of vertebrae.
Chance fracture; deemed unstable by spinal MDT.
Three-column injury of T9 will need surgical fixation meaning T10 fracture will need to be fixed.
Three-column injury.
Old fracture – L1 mild retropulsion; severe angulation and posterior element widening.
L1 chance unstable #.
1. L2 burst # requiring fixation.
L1/T12 chance # three-column injury.
Burst #.
Three-column burst # T12.
Ankylosing spondylitis.
Unstable – three-column injury with PLL disruption.
T11 and L1 burst #. T11 minor retropulsion of the posterior inferior cortex but no significant canal stenosis; at L1 there is retropulsion of the posterior cortex causing thecal compression and possible canal stenosis. Unstable fractures.
Initial CT report suggested this was unstable; but a MSK radiology review the following day established that this was a stable fracture.
No available information in the medical records.
The following reasons were given when both exclusion reasons 1 and 5 (‘other reason to exclude patient’) were ticked
Failure of previous metalwork infected 13/12/2017.
Patient paraplegic from old T5–6 level spinal cord lesion.
Patient managed with a brace but not coping as still has ongoing pain. Patient to be managed surgically.
Suicide attempt; three-column T12 # chance #.
> 2 contiguous vertebrae – three-column injury and ankylosing spondylitis.
Significant head injury; physiologically unstable; traumatic cardiac arrest; both lungs decompressed; intubated and medically paralysed; evidence of hypoxic brain injury.
PLL, posterior longitudinal ligament.
MDT, multidisciplinary team; MSK, musculoskeletal; OP, operation; PLL, posterior longitudinal ligament.
Three contiguous vertebrae (L1; 2; 3) therefore would require long fixation. Complex ETOH; drug; mental health.
Determined as old injury – nil acute fracture.
Transverse process fracture.
History of COPD; diabetes; UTIs and age. Poor surgical candidate.
Old injury confirmed on MRI.
Old fracture; more than 5 years.
Frail heart failure; poor mobility; dementia; ulcers; poor surgical candidate.
Old injury; incidental finding.
80 years. Chest infection; COPD not fit for surgery.
Old fracture; not suitable for OP.
Age and comorbidities; including malnutrition and CKD. Also cognitive decline.
Old injury – not suitable for surgery.
Palliative for lung metastases.
CKD3 [chronic kidney disease]; pacemaker; 86 years old; frail; not safe for operation!
Not severe fracture does not warrant surgery.
Fracture of L1 and L3 meaning would require longer and high risk fixation (morbidity) (not contiguous).
93% pacemaker. Poor surgical candidate.
Injury minor; does not warrant fixation.
Given age – aim to avoid surgery.
Admitted with heart attack; not fit for surgery.
Patient elderly > 83 years old. Diagnosis COPD.
Past medical history of reduced vision; dementia; previous stroke and aphasia.
Patient elderly n/a for spinal surgery.
Encephalopathy (new) and confusion after fall. Not thought to be suitable to discuss implications of randomisation. Fall was a few weeks ago.
Two fractures occurred spontaneously inpatient on steroids so not a candidate for short segment fixation.
Osteoporotic fracture not suitable for short segment fixation.
Has multiple myeloma.
Osteoporotic fracture for TLSO.
The following reasons were given when both exclusion reasons 4 and 5 (‘other reason to exclude patient’) were ticked
Transverse process fracture.
Fixation of T10 and T12 would mean complex operation. Not comparable to brace.
Transverse process fracture T1–3.
13 year old fracture – not for operation unable to assess images as taken at Basildon.
Dementia; old injury.
Old T9 and T12 injury; dementia.
Appear osteopenic; low-energy injury W1 significant compression injury both 4 to 5.
Osteoporotic fracture; patient for end of life care.
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ETOH, ethanol; n/a, not applicable; OP, operation; UTI, urinary tract infection.
Not too contiguous (L1/2/3) – OP would be associated with increased mortality.
Complex mental health – suicidal.
Nil English.
ETOH – excessive. Patient jumped out of window when denied alcohol by wife.
Missed over weekend. Treatment advice given by clinical team.
Patient on unit from Canada – unable to follow-up.
Severe alcoholism/suicide/depression.
Risk of homelessness; safeguarding issues; admitted after suicide attempt; alcohol excess.
Suicide attempt; complex mental health history. Excluded due to poor life expectancy and challenge to follow-up.
Had pelvic dissociation and lives in Devon so not happy for follow up in London.
Not geographically stable.
Old fracture.
IVDU – suicide attempt.
Inadequate English to complete self-reported questionnaires.
Patient fractured three consecutive vertebral bodies which would require extensive surgery with increased morbidity.
Dementia diagnosis; three admissions to psychiatric home prior to fall from second floor. Record of depression and suicide attempt.
Only crack fracture – no collapse – no pain clinically.
Not cartigious vertebrae.
Not too contiguous (three level injury) requiring extensive fixation. Not comparable OP to TLSO.
Evasive history (police). Drug and alcohol abuse and personality disorder.
Heroin and cocaine user.
Not too contiguous.
Not contiguous vertebrae would require extensive surgery.
Dementia.
Not contiguous.
Nil English.
Patient having follow up in Scotland.
Wheelchair bound; therefore outcome measured.
Discharged before appropriate imaging taken.
Age indeterminate # of L1.
Neurosurgery team decided required surgery. Patient preference was to be treated TLSO brace.
Age indeterminate # of L1.
3 #s L1–L3.
Reduced research staff over Christmas period.
Patient does not speak English. Language not stated.
Self harms; suicidal; drug use; ETOH.
No research staff available and PT provided with brace.
Language barriers.
Patient had previous stroke with right arm weakness therefore unable to fit brace independently.
L1 burst # likely longstanding and T11# PT already advised conservative management.
Likely osteoporotic #. Patient not on site RLH.
Osteoporotic #. Patient not on site @ RLH.
PT not at RLH.
Patient not on site.
More than two vertebrae contiguous.
No research staff available – weekend.
Age indeterminate # of T12.
Can’t speak English.
Two contiguous vertebrae involved; L3 vertebrae.
(1) patient not identified as reduced research cover over weekend; (2) non-English speaking.
PT requested follow up in local hospital.
More than two contiguous vertebrae involved.
Patient returning to Romania. Doesn’t speak English.
Only 3 degrees of kyphotics.
Kyphotic angle < 15 degrees.
Referred 3/52 after initial injury.
PT presented to trial team 5/7/18. Already mobilising without brace 26/06/18.
Patient has diffuse idiopathic skeletal hyperostosis. Not a candidate for short segment fixation – none so long.
T8 and T9 #.
Not severe enough.
Polymyalgia rheumatica. Longstanding back pain following previous L2#.
Recent elective low back operation in October.
Dementia.
Four contiguous vertebrae involved. Operative option too big.
Injury occurred 3–4 weeks ago. < 20 degrees on standing XR. Drugs/alc not clear of date.
X-ray report says not possible to establish the chronicity of the X-ray. Patient not approached as not possible to identify age of fracture. Therefore not suitable to randomise to operation.
Frame pattern ineligible.
L3 # as well as L1.
Fracture reported to be chronic by radiologists. Also fracture seen on another XR (chest) in January 2019.
Minimal pain.
Conservative management – eligibility criteria not fully met. Stable fracture; brace removed before discharge.
Presentation to fracture clinic too long post injury.
Osteopenic fracture (probable old fracture).
Clinical decision to treat with TLSO.
Osteoporotic fracture – not for surgery.
Osteoporotic fracture.
alc, alcohol; ETOH, ethanol; IVDU, intravenous drug user; L3, third lumbar vertebra; OP, operation; PT, patient; RLH, Royal London Hospital; XR, X-ray.
Appendix 3 Oswestry Disability Index category descriptors
| Score | Category | Description |
|---|---|---|
| 0–20% | Minimal disability | The patient can cope with most living activities. Usually no treatment is indicated apart from advice on lifting, sitting and exercise |
| 21–40% | Moderate disability | The patient experiences more pain and difficulty with sitting, lifting and standing. Travel and social life are more difficult and they may be disabled from work. Personal care, sexual activity and sleeping are not grossly affected, and the patient can usually be managed by conservative means |
| 41–60% | Severe disability | Pain remains the main problem in this group, but activities of daily living are affected. These patients require a detailed investigation |
| 61–80% | Crippled | Back pain impinges on all aspects of the patient’s life. Positive intervention is required |
| 81–100% | Bed-bound | These patients are either bed-bound or exaggerating their symptoms |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AO
- Arbeitsgemeinschaft für Osteosynthesefragen
- BASS
- British Association of Spine Surgeons
- BSR
- British Spine Registry
- CI
- confidence interval
- CT
- computerised tomography
- DMEC
- Data Monitoring and Ethics Committee
- ED
- emergency department
- EQ-5D-5L
- EuroQol 5 Dimensions, five-level version
- GP
- general practitioner
- L1
- first lumbar vertebra
- L2
- second lumbar vertebra
- MRI
- magnetic resonance imaging
- MSK
- musculoskeletal
- MTC
- major trauma centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ODI
- Oswestry Disability Index
- OTA
- Orthopaedic Trauma Association
- PAG
- Patient Advisory Group
- PI
- principal investigator
- PIS
- patient information sheet
- PPI
- patient and public involvement
- PPI&E
- patient and public involvement and engagement
- PRESTO
- Pragmatic Randomised Evaluation of Stable Thoracolumbar fracture treatment Outcomes
- PROM
- patient-reported outcome measure
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SBNS
- Society of British Neurological Surgeons
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- T10
- 10th thoracic vertebra
- T11
- 11th thoracic vertebra
- T12
- 12th thoracic vertebra
- TLICS
- thoracolumbar injury classification and severity score
- TLSO
- thoracolumbar sacral orthosis
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- YTU
- York Trials Unit
