Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/85/01. The contractual start date was in January 2018. The draft report began editorial review in September 2020 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Hay et al. This work was produced by Hay et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Hay et al.
Chapter 1 Introduction
Funding history
Through its research prioritisation process, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme determined the need for evidence to improve the management of acute otitis media with discharge (AOMd). This resulted in the publication of two commissioning briefs (https://njl-admin.nihr.ac.uk/document/download/2010629 and https://njl-admin.nihr.ac.uk/document/download/2037271; accessed 27 October 2021).
We responded to the first brief (HTA commissioning brief 15/32) in 2015, in which we specified a two-arm trial. The stated research question was ‘[W]hat is the clinical and cost effectiveness of topical antibiotics as compared to oral antibiotics in children with acute otitis media presenting with acute ear discharge?’ and the project was called the Painful Runny EAR (PREAR) study. The stage 1 proposal was shortlisted for stage 2 but not supported and in the event no research was commissioned in response to this brief.
A further brief was issued in 2016 (HTA commissioning brief 16/85), with the research question unaltered, but now specifying two other groups in addition to topical antibiotics: immediate oral and no or delayed oral antibiotics. The present Runny Ear STudy (REST) was the successful application in response to this second brief. The commissioning board had several concerns about our PREAR proposal, which were addressed by making four changes:
-
The board was concerned that the two arms (immediate oral vs. immediate topical antibiotics) were normalising antibiotic use for this condition. We addressed this by adding the third arm, ‘delayed oral antibiotics’.
-
The board’s concern that the primary outcome was pain only, and not a broader measure of symptoms, was addressed by changing the primary outcome to ‘time to resolution of pain, fever, being unwell, disturbed sleep, otorrhoea and episodes of distress’.
-
We amended the conservative recruitment projections based on only 6% of children with acute otitis media (AOM) having otorrhoea. We revisited this assumption and, based on recent evidence, we amended this to 15%, which we believed was a more realistic estimate.
-
Finally, the board was concerned that the study did not plan to look at antimicrobial resistance (AMR) in the ear caused by topical treatment or otorrhoea virology. In the expression of interest (EoI), we had strengthened these elements, but, in response to the commissioning board’s November 2016 comment to reduce costs, we removed all microbiological elements (as they were not in HTA commissioning brief 16/85).
Structure of this report
The REST suffered from delays in set-up, followed by slow recruitment. This was the main reason for trial closure; however, the trial was actually closed at the onset of the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic, which made further recruitment unsafe, as many children were being assessed and managed using remote ‘telephone-only’ systems. The study was planned and, hence, presented as a full trial with internal pilot. In the event, only the internal pilot data were collected, and we present these results together with the qualitative data from participating site staff and parents. Formal statistical comparisons and economic analyses were not conducted because of the small numbers, but clinical and economic descriptors are presented.
Clinical background
Acute otitis media is a common childhood infection, usually presenting with the rapid onset of ear pain. Infection may follow other respiratory tract infections. In young children, the infection may present as pulling at the ear, increased crying and poor sleep. Either bacteria or viruses may be involved. Risk factors include exposure to smoke, use of pacifiers and day care attendance. The diagnosis is usually made by examination of the eardrum in those with suggestive symptoms. Signs of AOM include redness or bulging of the tympanic membrane. New ear discharge following an episode of ear pain is also suggestive of AOM.
Acute otitis media is important to children, parents and the NHS for three reasons. First, the infection causes pain and distress to the child, disrupting sleep and family routines. In around 15% of these children, a rise in middle ear pressure bursts the tympanic membrane, releasing the middle ear contents as a discharge (otorrhoea). 1 Contrary to widespread belief, children with AOMd have similar levels of pain and are more unwell at presentation than children with AOM. 2,3 Moreover, children with AOMd have a worse prognosis, and higher rates of parent-reported pain (at 1 week), repeat AOM episodes (at 3 months) and hearing problems (at 3 months). 3
Second, although estimates of parental costs (travel, over-the-counter medicines and lost earnings) vary,4–6 even the lowest estimate suggests costs of £4M in England and Wales per annum. In addition, AOMd results in health service consultations, with > 90% of UK parents attending primary care for each episode,7 which is more than the percentage for any other common symptom of acute infection, equating to > 150,000 consultations in England and Wales per annum (at an NHS cost of > £3M). 4,5
Last, in the UK,8 as in the USA,9 children with AOM or AOMd are more likely than those with any other respiratory infection to be prescribed an oral antibiotic, with three-quarters of general practitioners (GPs) prescribing oral antibiotics to at least 80% of patients. 10,11
There is good evidence that children with AOMd benefit from immediate oral antibiotics. The number needed to treat to reduce the proportion of children with pain and/or fever at 3–7 days, compared with placebo/no treatment, is three children. 9 As a result, the National Institute for Health and Care Excellence (NICE) recommends that immediate antibiotics should ‘be considered’ [reproduced with permission from NICE. 12 © NICE 2018 Otitis Media (Acute): Antimicrobial Prescribing. Available from www.nice.org.uk/guidance/ng91. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication]. However, oral antibiotics also cause side effects, are associated with subsequent eczema and hay fever,13 and are associated with population-14 and patient-level15 AMR. The UK’s Antimicrobial Resistance Action Plan16 endorses research to preserve antibiotic effects17 and, as described in the subsequent sections, there are plausible alternative treatment options to immediate antibiotic prescription: ‘delayed prescribing’ and antibiotic drops.
Delayed oral antibiotics
Current evidence for AOMd symptoms is limited to showing the (1) superiority of immediate antibiotics over placebo/no treatment2 and (2) similarity of delayed compared with immediate oral antibiotics in children with AOM. 1 Research into the clinical effectiveness and economic implications of delayed oral antibiotics is needed, as delayed prescription is likely to reduce children’s exposure to antibiotics: only around 24% of children with AOM who were given a delayed prescription in our trial were actually given the antibiotic. 1
Immediate topical antibiotics
Perforation of the tympanic membrane provides an opportunity to instil antibiotic drops directly into the middle ear, thereby reducing systemic antibiotic exposure. It has been shown that, in children with grommets (ventilation/tympanostomy tubes), topical antibiotics can reach the infected middle ear against a stream of discharge and that, compared with oral antibiotics, they are more effective for reducing otorrhoea duration, AOM recurrence and side effects. 18 This study also showed topical antibiotics to be safe8 and cost-effective (from a societal perspective). 19 However, research is needed in children with AOMd without grommets, as the tympanic membrane heals quickly and can prevent the drops from reaching the middle ear. 20 If shown to be non-inferior, we would also need to understand the acceptability of such treatment to clinicians and parents, and how to address any barriers to implementation.
Reducing systemic antibiotic exposure
Two systematic reviews found no evidence regarding the relative AMR impact of topical and systemic antibiotics. 21,22 Compared with immediate oral antibiotics, we have shown that delayed prescribing reduces antibiotic consumption, but provides similar symptom relief, for children with AOM1 (as well as adults with sore throat15 and acute lower respiratory tract infections23). Therefore, as with ciprofloxacin drops, we expect delayed antibiotics to result in fewer side effects, to reduce AMR impact and to be used in clinical practice if symptom relief is non-inferior.
Summary
Together, this evidence suggests that either topical or delayed antibiotics could be at least as effective as immediate oral antibiotics for children with AOMd, and could reduce systemic antibiotic exposure and AMR. We therefore proposed a three-arm randomised controlled trial (RCT) to investigate the clinical effectiveness and economic implications of topical or delayed oral antibiotics compared with immediate oral antibiotics, powered for the duration and severity of the symptoms most important to parents, and to also investigate adverse events, complications and AOM/AOMd recurrence.
Rationale for trial design
Trial efficiency
Acute otitis media with discharge is less common than AOM, accounting for around 15% of presentations. This means that around 15 children (aged ≥ 12 months to < 16 years) can be expected to present with AOMd per annum to larger general practices (i.e. those with ≥ 10,000 registered patients), according to Royal College of General Practitioners (RCGP) data,24 meaning that a large number of sites will be needed to meet recruitment targets. A standard approach to the set-up of such a study, that is one utilising face-to-face training and distribution of recruitment packs, would require a huge logistical effort at high cost. Therefore, to maximise trial efficiency and procedure quality, we planned to:
-
focus on larger practices
-
utilise remote training and induction of trial sites through the use of online trial procedures training
-
use simplified research governance procedures
-
train and incentivise receptionist teams to steer eligible children into appropriate appointments
-
use an embedded electronic trial platform [called TRANSFoRm (Translational Research and Patient Safety in Europe), see Participant identification] to flag participants and to simplify recruitment procedures
-
use standard FP10 NHS prescriptions in an open design
-
direct participants to address post-randomisation procedural questions to a research nurse using a telephone call on day 1.
Non-inferiority design
There is good evidence showing that immediate oral antibiotics are superior to placebo for the reduction of pain/fever in children with AOMd. 2 As a result, NICE recommends that immediate oral antibiotics ‘be considered’. 12 Our 2015 audit (available from the study authors) showed that current practice complies with NICE guidance: 88% of children with AOMd were given oral antibiotics (of which 97% were coded as immediate antibiotics). Because we expect that ciprofloxacin 0.3% ear drops (current NHS cost for 5 ml = £6.0113) will have fewer side effects18 and less of an impact on AMR than immediate oral antibiotics (NHS cost for 100 ml of amoxicillin, 250 mg per 5 ml = £1.93),13 clinical adoption of the new treatment would be likely if its clinical effectiveness is at least as good as (i.e. is not inferior to) current standard therapy14 and if it was cost-effective. We have shown that, compared with immediate oral antibiotics, delayed prescribing reduces antibiotic consumption, but provides similar symptom relief for children with AOM1 (as well as adults with acute sore throat15 and acute lower respiratory tract infections23). Therefore, as with ciprofloxacin drops, we expect delayed antibiotics to result in fewer side effects and reduced AMR impact, and also to be adopted for clinical use if symptom relief is non-inferior.
Primary outcome
In keeping with previous research,1,2,17,18,25 our patient and public involvement (PPI) group identified the most significant symptoms that should be used to judge recovery as pain, fever, being unwell, sleep disturbance, otorrhoea and episodes of distress. They also reported that they would regard their child as ‘recovered’ when they rated all of these symptoms as ‘no problem’ or a ‘very slight problem’. Our primary outcome was therefore the time to all of pain, fever, being unwell, sleep disturbance, otorrhoea and episodes of distress/crying being rated by parents as ‘no problem’ or a ‘very slight problem’, without the need for analgesia. We used a validated26 Symptom and Recovery Questionnaire (SRQ), known to be sensitive to change,1 similar to SRQs we have used in our previous studies,1,27–29 in which we achieved > 80% diary completion rates with research nurse telephone support. The presence and severity of each symptom was recorded daily using a Likert scale, in which 0 = ‘normal/none’, 1 = ‘very slight problem’, 2 = ‘slight problem’, 3 = ‘moderately bad’, 4 = ‘bad’, 5 = ‘very bad’ and 6 = ‘as bad as it could be’. The intention was for symptoms to be recorded until all symptoms had been rated 0 for two consecutive days or in the event of non-resolution for a maximum of 14 days (research has shown that the symptoms of AOM resolve in 90% of children by day 830). Symptoms were recoded using the TRANSFoRm platform31 (or using a paper SRQ), with real-time monitoring of data completion.
Secondary outcomes
Secondary outcomes also reflected the importance of symptoms to parents17 and the NHS. Those recorded in the first 14 days (on the SRQ) included time until symptoms (pain, fever, being unwell, sleep disturbance, otorrhoea, episodes of distress/crying, appetite and interference with normal activities), rated ‘moderately bad or worse’ (score ≥ 3 on our validated scale26); adverse events (diarrhoea, rash, vomiting and severe complications on days 7 and 14); parent satisfaction with treatment (on days 7 and 14); and faecal AMR profile at 2 weeks and 3 months. We measured treatment adherence, treatment crossovers at day 7 and analgesic use until symptom resolution or up until day 14 if symptoms persisted (SRQ). Finally, we asked parents to record details of NHS resource use on the SRQ up to day 14.
Longer-term outcomes measured at 3 months (using the TRANSFoRm platform or paper postal questionnaires) included AOM and AOMd recurrence, serious complications (e.g. mastoiditis) and parent-reported hearing loss at 3 months [measured using the Otitis Media Questionnaire, 14-point version (OMQ-14),32 successfully used in the recent HTA AIRS (AutoInflation Randomised Study)33,34]. Parents reporting serious complications were asked to give permission for the study team to conduct an additional review of their child’s notes.
Electronic trial platform
Previous evidence and experience
Data standards for research data collection have been formulated by the clinical trials community via The Collaborative Data Standards Interchange Consortium (CDISC) over several decades, with an established pathway for data management from source to submission for regulated clinical trials. Using CDISC standards (www.cdisc.org), there has been a steady move away from paper case report forms (CRFs) towards electronic data capture (EDC) systems. Given the rapid expansion of the use of electronic health record (EHR) systems in clinical settings, it has been proposed that EHRs could be the primary point of data entry for a clinical trial. However, direct collection of data into a digital form, referred to as electronic source (eSource), can be achieved only if the EHR is able to support research-quality data collection. Good clinical practice (GCP) principles need to be adhered to ensure that the requisite standards are in place for eSource, and changes are needed to the data collection process and governing regulations to fit this electronic context. 35
There are three models of eSource currently being explored: (1) entry into a Clinical Trial Data Management System (CTDMS) with transfer to the EHR; (2) entry into a CTDMS with copying to both the EHR and the EDC form; and (3) collection within the EHR with transfer to the EDC form. Local preferences, maturity of EHR systems and sponsor requirements are likely to maintain this heterogeneous approach, emphasising the paramount importance of adherence to standards. The Integrating the Healthcare Enterprise (IHE) collaboration36 has developed a set of profiles for eSources:37 the Retrieve Form for Data Capture (RFD) and Retrieve Process for Execution (RPE) specify forms and workflow, respectively. Several proof-of-concept studies using IHE profiles have been completed. These include integration of common data elements from the National Center for Biotechnology Information (NCBI) Cancer Biomedical Informatics Grid (CaBIG) Enterprise Vocabulary Services (EVS) into the RFD profile,38 and STARBRITE (Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide versus the Clinical Congestion Score), a single-site proof-of-concept implementation within a heart failure clinical trial. 39
Within the academic and pharmaceutical trials world, there has been a move to ‘real-world’ clinical trials as a means of gathering a larger number of data more quickly on the likely effectiveness of treatments, to satisfy increased regulatory requirements in this area. 40 It is proposed that embedding and integrating research into electronic record systems would enable automation of some elements of the trial’s screening process, with eligibility criteria matched directly with EHR-held data. 41 Potential participants who match the exclusion criteria need not be flagged. In addition, for those who are potentially eligible, data held in the EHR can be used to pre-populate the eligibility form. A similar process can be used to pre-fill electronic case report forms (eCRFs). In a reverse of this process, trial data can be added back to the EHR. 42 The ability to place trial information in routine EHRs at the point of collection would be a significant step towards safer and more efficient clinical trials. 43
Real-world trials have not yet progressed to using eSource by default, still requiring a large investment in data collection and validation. 44 Closing this gap would go a long way to providing an end-to-end ‘research and learning’ continuum for a learning health system (LHS), in which research and knowledge translation are routinely transacted using information technology (IT) systems. When interacting with EHRs, the use of robust data standards, such as the CDISC suite, is essential to the operation of the LHS to overcome the ‘silo of excellence’ culture prominent in health-care research and lower the barrier to entry for traditional clinical environments. 35,45
For the past 10 years, the US Food and Drug Administration (FDA) and the European Commission have been advocating the use of electronic platforms to manage clinical trials, with the source data obtained directly from the EHRs. 46 Advantages of this include:
-
increased data accuracy, including anonymised recording of the characteristics of eligible patients who decline to participate, thereby providing a greater understanding of final sample representativeness
-
reductions in data management
-
increased safety, by ensuring that trial data are within the clinical record
-
easier and, therefore, more efficient trial monitoring
-
EHR management of trial workflow, prompts and alerts for recruitment and follow-up, and patient-reported outcomes47
-
the use of CDISC standards for data capture. 48
Participant identification
Within-consultation ‘hot’ recruitment of patients with incident conditions is significantly more challenging than ‘cold’ recruitment of patients with prevalent conditions, as the latter group of patients can be contacted electronically or by letter. 37 The extra workload and time needed to set up and recruit within a normal consultation are major barriers to participation by GPs and general practices. 37
Participation can be increased when there is perceived clinical value and/or benefit to patients, adequate remuneration for time and streamlined recruitment processes that minimise workload. 49 One approach to overcoming these barriers is through the use of a trial platform.
REST was designed to collect quantitative data using the TRANSFoRm platform, originally developed as part of the EU FP7-funded TRANSFoRm Programme (i.e. the seventh framework programme of the European Community for research and technological development including demonstration activities). 31 The TRANSFoRm platform is designed to integrate with primary care EHR, ensuring data validity and accuracy and facilitating the nationwide engagement of the large number of primary care sites needed for REST. An additional module enables patient-reported outcome measures (PROMs) to be recorded by parents using the internet and smartphones [iOS (iPhone operating system; Apple Inc., Cupertino, CA, USA) and Android (Google Inc., Mountain View, CA, USA)]. The system was fully GCP validated as part of a European trial on gastro-oesophageal reflux diseases and registered with EudraCT (number 2014-001314-25).
The basic components of the system are:
-
a TRANSFoRm Study System (TSS) that manages projects, sites and workflow
-
middleware that manages authentication and messaging
-
a system for triggering and storing PROMs
-
data node connectors (DNCs), specific to each EHR system, that link clinical systems to the TSS via their application programming interface (API)
-
an online back-up data collection system.
For REST, relevant data elements were captured using a set of extensible markup language (XML) files, and linked to the TRANSFoRm clinical data information model (CDIM), structured in accordance with the CDISC Operational Data Model (ODM), and the timeline according to the CDISC study data model (SDM). Further ODM files containing questions for the PROMs were developed in combination with structured searches for data elements that were pre-populated by the data in the EHR.
Two areas of the added value of the TRANSFoRm platform specific to REST were (1) the triggering of ‘real-time’ eligibility reminders when potential recruits were being seen by clinicians and (2) streamlined study processes after identification, with access to REST-specific documentation, pre-populated consent forms and ‘real-time’ randomisation.
Trial intervention selection
Oral antibiotics
For the oral antibiotics (immediate and delayed), we wanted to reflect routine care and appropriate bacteriological cover. A 2010 study of 256 children with AOM recruited from primary care3 showed that 84% of the 38 children with AOMd received an immediate prescription for amoxicillin, with a further 5% receiving oral erythromycin, 3% receiving topical gentamicin and 8% receiving no antibiotic. In the majority of children [22/38 (58%)] a recognised bacterial pathogen was isolated: Streptococcus pneumoniae (n = 5), group A Streptococcus (n = 7), Staphylococcus aureus (n = 7), Pseudomonas (n = 2) and Haemophilus influenzae (n = 3). 3 A study of 177 children with AOMd isolated single pathogens in 70 (39%) samples, whereas two, three and four bacterial species were detected in 54 (30%), 20 (11%) and 7 (4%) cases, respectively. 50 Non-typeable H. influenzae was the most common and was identified in 90 children (51%), followed by Moraxella catarrhalis (35%) and S. pneumoniae (27%). 50 Children with co-infections, including non-typeable H. influenzae, had significantly more frequent recurrent AOM (adjusted odds ratio 6.6; p = 0.029). 50
Our 2015 audit (33 general practices; 56,251 children) confirmed immediate oral antibiotics as usual care for AOMd: 88% were given oral antibiotics (of which 97% were immediate), with amoxicillin being the most widely prescribed antibiotic. UK primary care practice is to prescribe amoxicillin ‘dose-by-age’. However, we used the latest British National Formulary (BNF) for Children51 prescribing guidance to prevent underdosing and overdosing the oldest and youngest children, respectively. Clarithromycin is a commonly used and well-tolerated alternative for penicillin-allergic children; hence we selected oral amoxicillin, with the option of clarithromycin in the event of recorded penicillin allergy.
Topical antibiotics
We considered there to be four main advantages to selecting ciprofloxacin 0.3% as the topical antibiotic. First, it has low potential for AMR as bloodstream absorption of ear drop quinolones has been shown to be extremely low, measured at no higher than 10 ng/ml in one study52 of children and adults given ofloxacin 0.3% solution, which is < 1% of the concentration typically seen after oral dosing. We used four drops of ciprofloxacin 0.3%, taken three times daily, and expected to see similar low blood levels to those reported for ofloxacin. Typically a drop is about 50 µl,53 giving a total daily dose of 1.8 mg. Although we are not aware of any studies measuring respiratory or gastrointestinal tract quinolone exposures following ear use, even if 100% of the ciprofloxacin was absorbed and excreted into the gut, this would be < 2 mg per day. By contrast, the typical oral dose for a child is 10 mg/kg, taken two or three times daily – equating to 300 mg daily for a 10-kg child aged 1 year. It is not surprising that evidence suggests that the risk of developing AMR post ear drop application in these locations is very low. 54
Second, ciprofloxacin ophthalmic 0.3% drops are widely available, which was a requirement for this study, as recruitment took place in a large number of primary care sites dispensed by high street pharmacies in response to standard FP10 NHS prescriptions. It is usual practice in primary and secondary care to use the eye drop formulation for ear treatment because the ophthalmic formulation is considerably less expensive and more readily available than the otic formulation. Prescribing in REST was therefore outside the licence, and the study sponsor ensured that the University of Bristol’s no-fault indemnity applied to this form of ciprofloxacin.
Third, the drops are colourless and odourless, so they did not interfere with parental assessment of otorrhoea. Finally, at the concentrations achieved in the middle ear, ciprofloxacin 0.3% drops are active against the most commonly isolated otorrhoea microbes from children presenting to primary care, namely S. pneumoniae, S. aureus, H. influenzae and Pseudomonas aeruginosa. 3
We decided against using a topical aminoglycoside, mainly because of the potential for ototoxicity. Topical antibiotics are thought to penetrate from the middle to inner ear via the round window. This results in high local concentrations in the inner ear tissues and potential for ototoxicity. One systematic review in animals reported widespread ototoxicity with topical aminoglycoside antibiotics,55 and the ototoxic properties of aminoglycosides have been intentionally used to ablate vestibular function in humans with severe middle ear disease. 56 There is also a genetic mutation, occurring in around 1% of the population, which predisposes those with the mutation to ototoxicity at low drug exposures. 57 Although the evidence for aminoglycoside ototoxicity is debated in humans, especially at the time of an active infection,58 and there are other advantages of aminoglycoside preparations (e.g. unlike ciprofloxacin, aminoglycoside–steroid combination preparations are widely available). The BNF59 states that topical aminoglycosides are contraindicated in patients with a perforation of the eardrum or grommets, and such medicolegal concerns would have significantly reduced the willingness of trial clinicians to recruit.
We also decided against an antibiotic–steroid combination drop because, although there is evidence that, for children with grommets, a topical steroid–antibiotic combination is superior to an oral antibiotic alone18 and to topical antibiotic alone60,61 in reducing the discharge, an industry study has shown that ciprofloxacin alone is more effective than a steroid only preparation. 62 This suggests that, although steroids may have additional benefit to antibiotics, antibiotics are an essential ingredient. Some studies performed in animals have shown that an antibiotic–steroid combination slows the healing of perforated tympanic membranes compared with antibiotics alone. 63,64 Although a number of aminoglycoside steroid-containing preparations are available, no combination of steroid with ciprofloxacin was widely available in the UK at the time of trial design, meaning that ciprofloxacin was not only suitable, but the only non-aminoglycoside topical antibiotic option available.
Economic evaluation
It seemed likely that intervention costs and clinical outcomes would be similar across the three arms. However, we proposed to explore costs and outcomes from the NHS perspective because we anticipated fewer side-effects and repeat consultations for delayed and topical antibiotics.
Potential harms
As an established treatment (immediate antibiotics) has been demonstrated to provide benefit, it is important that we demonstrate that the proposed treatments are non-inferior to avoid prolonged symptoms of pain in children. In November 2018, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) issued a notification regarding the safety of ciprofloxacin. The EMA’s Committee for Medicinal Products for Human Use (CHMP) subsequently endorsed the recommendations of the PRAC and concluded that the marketing authorisation of medicines containing cinoxacin, flumequine, nalidixic acid and pipemidic acid should be suspended. The CHMP review concerned only those medicines given systemically (by mouth or injection) and inhaled medicines. 65 The use of quinolones was restricted with additional warnings. As the systemic absorption of topical ciprofloxacin 0.3% is low, in consultation with the Trial Management Group (TMG) PPI member, the TMG did not think this presented any additional risks for REST participants.
Measuring and mitigating threats to trial validity
External validity
Our intention was to maximise generalisability by asking clinicians to invite all potentially eligible children to participate. As with previous studies,29,66 when study invitations were declined, parents were asked if basic details (age, gender and global illness severity) could be recorded via the TRANSFoRm platform.
Internal validity
Randomisation
Concealed randomisation stratified by age (< 2 vs. ≥ 2 years) was used to ensure that treatment arms were similar with respect to both measured and unmeasured potential confounders.
Treatment crossover and adherence
In an open-label trial, which we considered necessary for REST, there is a possibility that children would not be given the treatment to which they were randomised. There is no single agreed threshold at which patients are regarded as ‘adherent’ (and it is likely to vary between diseases and medication classes), but 80% is often regarded as reasonable. 67 Higher levels of adherence than this were achieved in the previous open trial of oral versus topical antibiotics for children with grommets and ear discharge:18 88% and 93% fully adhered to oral and topical antibiotics, respectively. Minimising treatment crossover was a key clinician training element, and the TRANSFoRm platform minimised crossover by guiding clinicians to issue the ‘correct’ treatment. Finally, treatment adherence was monitored both by checking the drug prescribed at the notes review and by using the SRQ.
Performance, measurement and attrition bias
Although participants were not blinded to treatment allocation, given current treatment equipoise and the fact that all participants would receive active antibiotic treatment, we did not consider that parent knowledge of treatment allocation would significantly influence their perception of symptom severity. Members of the TMG and the statistical team remained blind to treatment allocation until analyses were completed. REST used outcome measures successfully used in our previous studies,1,27,28 which had been shown to be valid26 and sensitive to change. 1,27,28 We aimed to achieve a minimum follow-up rate of 80%, but with online data collection and telephone support from an experienced research nurse, we anticipated achieving closer to 90% for our primary outcome. 1,27,28
Previous or ongoing similar research
We reviewed the literature and trials registries in December 2016 and found no relevant published, completed or ongoing studies in AOMd (without grommets). 20 However, we are collaborating with a Dutch group [one shared applicant (AS) and two collaborators (RD, RV)] that is currently conducting another RCT68 to investigate the effect of topical antibiotics in otitis media with discharge. Although the non-inferiority design and eligibility criteria are similar, the REST and ZonMw (The Hague, the Netherlands) applications are complementary:
-
REST assessed the duration and severity of a broad range of symptoms, whereas the primary outcomes in the Dutch study are limited to pain and/or fever at 72 hours.
-
The Dutch application uses an ear drop containing two antimicrobial agents and a steroid [hydrocortisone bacitracin-colistin (Bacicoline B®, Merck Sharp & Dohme Corp., Merck & Co., Inc., Whitehouse Station, NJ, USA), not available in the UK]; we used an antimicrobial agent only.
-
The Dutch study has two arms (topical vs. immediate oral antibiotics); we had three arms.
-
The Dutch study will use ‘dose-by-weight’ amoxicillin; we used ‘dose-by-age’.
The collaboration has ensured that we harmonised our outcome definitions so that we will be able to conduct meta-analyses and, together, the two studies will strengthen generalisability, as there is evidence from other trans-European studies that UK and Dutch patients have different illness spectra.
In summary, our trial proposed to test two interventions that could reduce systemic antibiotic exposure (i.e. immediate topical and delayed oral antibiotics), placing this study at the forefront of research to improve antimicrobial stewardship in AOMd. In addition, we proposed to demonstrate efficient trial delivery using a combination of remote training and an integrated trial platform, which would provide a model for future trials of low frequency but important clinical conditions in primary care. 3
Study aim
The main aim was to investigate the clinical effectiveness and economic impact of immediate topical or delayed oral antibiotics compared with immediate oral antibiotics for symptom duration in children presenting to primary care with AOMd. 69 The research question was ‘Is either ciprofloxacin 0.3% drops or delayed oral amoxicillin (clarithromycin if penicillin allergic) non-inferior to current usual care (immediate oral antibiotics) for overall illness duration in children with AOMd presenting to primary care?’.
The secondary objectives69 were to:
-
estimate the short-term cost implications of immediate topical or delayed oral antibiotics compared with immediate oral antibiotics from the perspective of the NHS
-
compare effects on duration of ‘moderately bad or worse’ symptoms, parent satisfaction with treatment and adverse events
-
compare hearing loss and AOM/AOMd recurrence rates at 3 months
-
understand parent and clinician views of AOMd trial participation, adherence and satisfaction with allocated treatment
-
evaluate the relative AMR impact of immediate topical, delayed oral and immediate oral antibiotics.
Chapter 2 Methods
In this chapter, we describe how the REST trial was conducted, first summarising the overall trial design, then describing the intended set-up and function of the TRANSFoRm platform and then describing how the trial itself was conducted. A trial protocol has been published in full. 69
Design
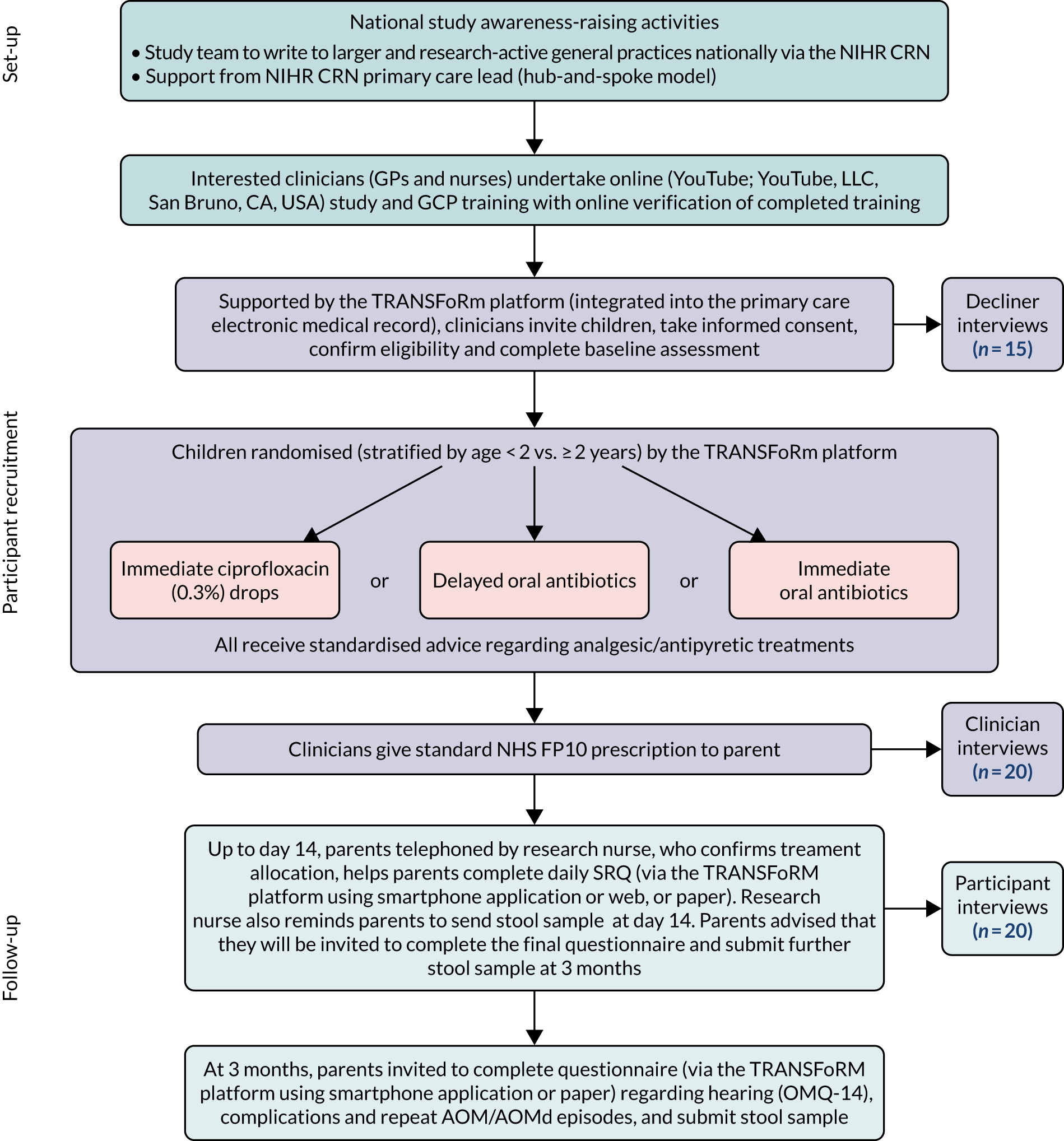
The trial was designed (Figure 1) as a pragmatic, three-arm, individually randomised (stratified by age < 2 vs. ≥ 2 years), non-inferiority, open-label trial comparing (1) immediate topical ciprofloxacin 0.3% drops with (2) delayed oral antibiotics or (3) immediate oral antibiotics.
FIGURE 1.
The REST study schema. CRN, Clinical Research Network.
Ethics
Ethics approval was granted by South Central Oxford B Research Ethics Committee on 22 May 2018 (REC reference 18/SC/0181, IRAS project ID: 229293).
Site requirement assumptions
It was clear at the design stage that recruitment would be challenging because of the (1) acute nature of AOMd requiring ‘within-consultation’ recruitment and (2) relative infrequency of AOMd.
Our detailed recruitment assumptions were based on 2011 AOM incidence data from the RCGP. 24 These data are rigorously collected and up to date (since the introduction of the 7- and 13-valent pneumococcal conjugate vaccines), and so reflect the current incidence of AOM in primary care. They suggested that the average general practice (with 7335 patients70) would see 76 AOM presentations in children aged > 12 months to ≤ 16 years per annum. Between 15%3 and 20%2 of children with AOM are thought to present with AOMd due to a spontaneous perforation of the tympanic membrane. Using the lower estimate, the average general practice will see 11 children with AOMd per annum. We therefore intended to focus site recruitment on larger (≥ 10,000 patients) and/or research-active general practices. We established that there were around 680 research-active sites in the eight Clinical Research Network (CRN) and Wales areas in which REST applicants and collaborators had previously worked, and 1958 practices with list sizes of ≥ 10,000 in England, to which we anticipate annual AOMd presentations to increase to 15 per practice per annum (or one every 3–4 weeks).
Based on this, we used the assumptions summarised in Table 1 to arrive at an estimate of the number of primary care sites needed to recruit the sample. Based on these, and a required sample of 399 children (see Sample size), we estimated that the number of primary care sites needed to recruit over two winter seasons and one summer season would be 175.
| Recruitment step | Proportion assumed to progress | Comment |
|---|---|---|
| Presentation-to-invitation ratio | 0.2 | Low because, even in sites where clinicians are aware of REST, eligible children will present at inconvenient times |
| Invitation-to-acceptance ratio | 0.67 | Based on PPI feedback |
| Invitation acceptance-to-eligibility ratio | 0.8 | |
| Eligibility-to-consent ratio | 0.8 | Some parents may change their minds about trial participation during the recruitment process |
| Product of the above (presentation to consent) | 0.08 |
Therefore, given that each trial site might see only one potentially eligible child every 3–4 weeks, and given the large number of sites required, we concluded that it would not be possible for the trial team to provide sites with in-depth support to ensure recruitment. We considered it necessary to use a ‘light-touch’, efficient-design trial method, in which clinicians would be prompted and guided through the recruitment process.
We thought that the use of an electronic trial platform fully integrated into the electronic medical record would maximise the chances of recruitment success. The key specifications we were aware of at trial outset were that full integration would facilitate:
-
trial reminder ‘pop-ups’ triggered when clinicians entered relevant diagnostic codes in children
-
autoprovision of patient information sheet (PIS) and other trial materials for potential participants
-
autopopulation of patient characteristics into trial database, reducing data entry time
-
within-consultation confirmation of eligibility
-
autoprovision of consent form (to be printed and faxed/e-mailed to study team)
-
within-consultation randomisation so that clinician could provide necessary treatment (using standard NHS prescription)
-
autoprovision of links for parents to complete symptom recovery questionnaires
-
autopopulation of EHR patient follow-up data, preventing the need for manual review of patient notes.
The TRANSFoRm platform
Overall structure
The overall architecture of the TRANSFoRm platform is summarised in Figure 2. The TRANSFoRm Clinical Trial software was initially developed as part of the EU FP7 TRANSFoRm project (2009–15) and was evaluated in a 36-site clinical trial. 31 The basic components of the system are:
-
a TSS that manages projects, sites and workflow
-
middleware that manages authentication and messaging
-
a system for triggering and storing PROMs
-
a DNC specific to each EHR system that links clinical systems to the TSS via their API
-
an online back-up data collection system.
FIGURE 2.
Schema summarising the TRANSFoRm platform’s architecture. app, application.
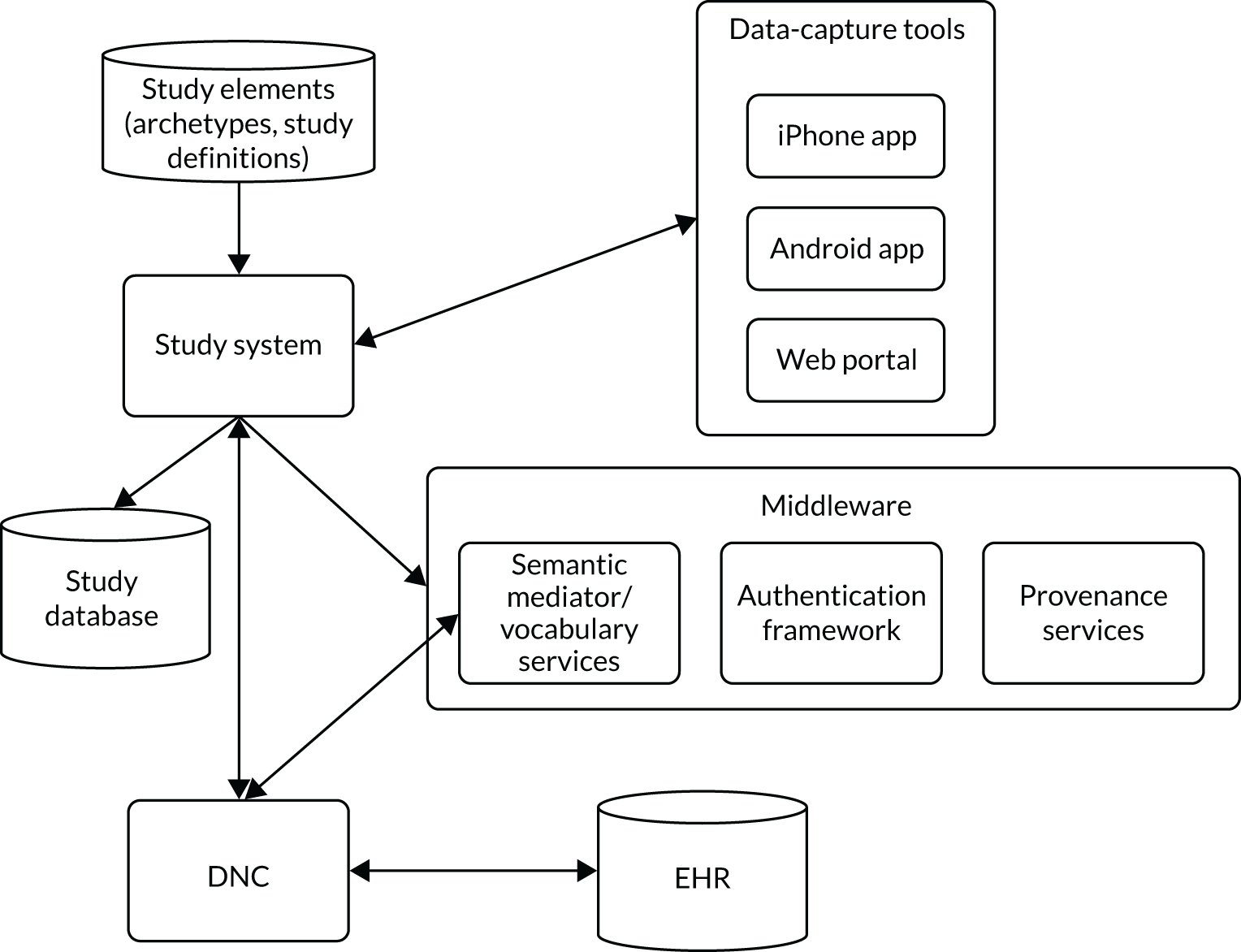
Eligibility criteria and data to be collected were specified through data elements using the TRANSFoRm platform’s CDIM, structured in accordance with the CDISC ODM and the timeline according to the CDISC SDM. Further ODM files containing questions for the PROMs were developed separately.
REST-specific functionality
Detailed specifications were agreed before development began (see Detailed TRANSFoRm technical specification for REST v1.4; this is available from the authors on request). In summary, we wanted the system to identify potentially eligible children and then ‘lead’ the clinician though the recruitment process, providing prompts and reminders throughout. REST-specific functionality is shown in Figure 3.
FIGURE 3.
Flow diagram showing the role of the TRANSFoRm platform in recruitment and data collection. CROM, clinician-reported outcome measure.
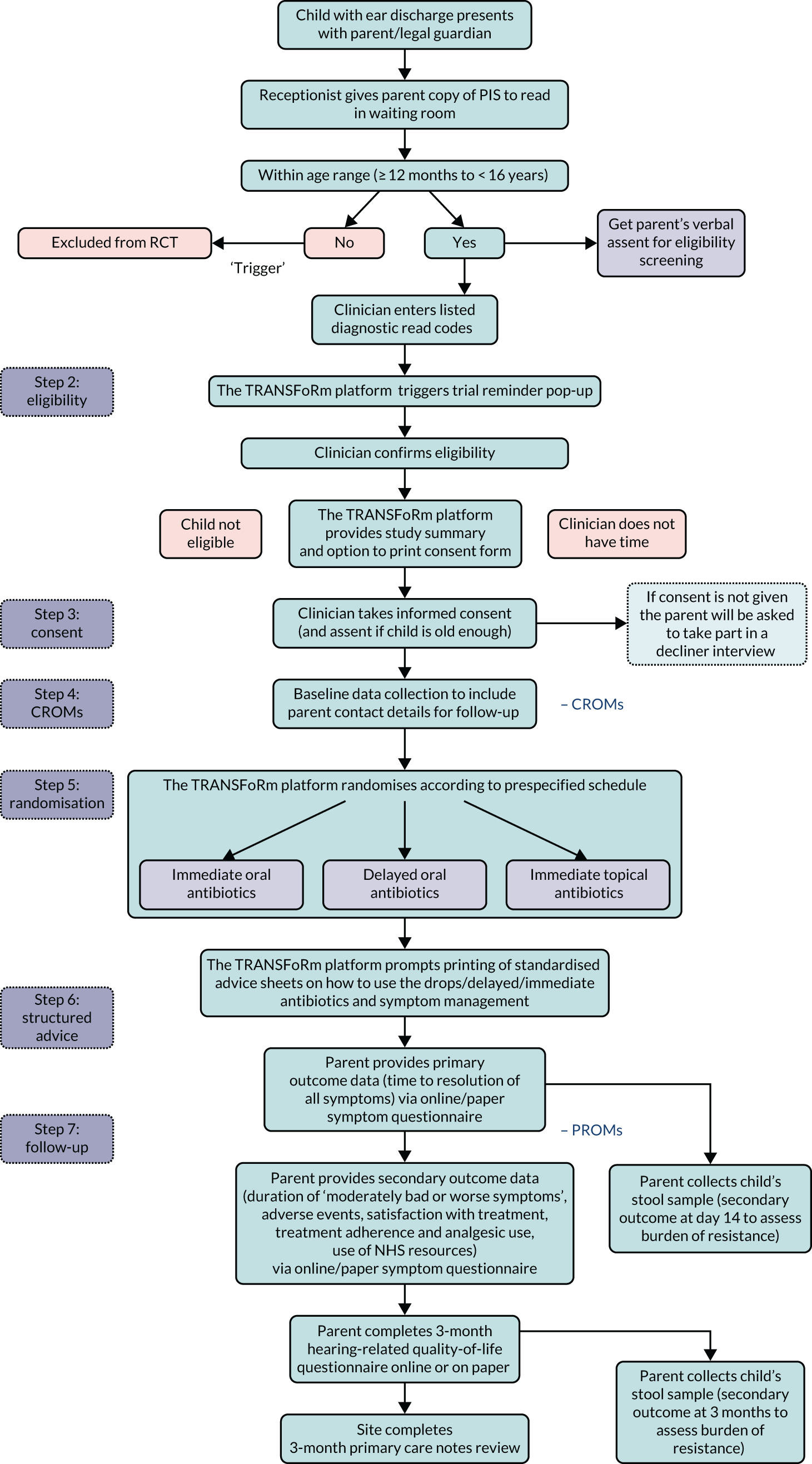
Recruitment reminders
The system was intended to respond to clinicians’ use of predetermined Read diagnostic codes by triggering a reminder ‘pop-up’ alerting the clinician that the child might be eligible for REST.
Read and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision,72 (ICD-10) clinical codes describing members of the AOM subclass (Table 2) were added to the TRANSFoRm platform’s study database. The scope was intentionally broad (i.e. sensitive) to maximise the chances of identifying potential participants using the wide range of codes used by clinicians. This was followed by application of the (specific) eligibility criteria supplied at the beginning of the (electronic) recruitment process to ensure that the patient was suitable for the clinical trial. Specifically, the steps followed were:
-
Identify open consultation. The TRANSFoRm platform’s DNC, using SystmOne®’s [The Phoenix Partnership (Leeds) Ltd, Leeds, UK] API, asked to extract information from the currently opened EHR, if any. If the request was not successful, then no consultation was in process. Otherwise, a consultation was taking place and a summarised view, containing demographic data and clinical codes inserted in the record on the current date, was temporarily stored in a local folder of the DNC. Then, a call to the TRANSFoRm platform’s TSS retrieved the given list of Read and ICD-10 clinical codes and the age comparison expression.
-
Match patient age. The system matched the patient’s age, as extracted from the summarised view, with the comparison expression. If the matching proved unsuccessful, the recruitment workflow stopped.
-
Match clinical codes. A search took place to match clinical codes from both the summarised view and the data obtained from the study database.
-
Identify prior trial entry. The algorithm searched for any trial-related clinical code that may have been already inserted in previous consultations with the same patient (e.g. Read code ‘XaN0L::Consented’ inserted into record when consent form is submitted by user) or declined (e.g. Read code ‘XaaFk::Declined’ inserted into record when decliners form is submitted by user).
-
Following the sequence 1–4. A confirmation pop-up dialog was displayed to the user before initiating the recruitment process. Simultaneously, a trial-related clinical code was inserted into the patient record to denote potential eligibility to the trial (e.g. Read code ‘XaaEl::Screened’).
-
Pop-up disposal. Disposal of the pop-up reminder window was undertaken by the clinician following Microsoft Windows® (Microsoft Corporation, Redmond, WA, USA) graphical user interface (GUI) standards, that is clicking on the red cross icon located on the top-right corner of the pop-up window. The same action to close the window was taken regardless of the outcome (user declined, consented or did not follow through with the recruitment workflow). This was because of the complexity of having more than one possible workflow path at a time and the lack of restriction on which electronic forms had to be filled in first.
| Clinical term | SNOMED CT | Read codes |
|---|---|---|
| Acute secretory otitis media | 359609001 | F510, F510z, FyuP0 |
| Acute otitis media with effusion | 270490007 | XE2QD |
| Acute transudative otitis media | 35183001 | |
| Acute otitis media | 3110003 | X00ip |
| Acute left otitis media | 194288009 | F526 |
| Acute right otitis media | 194289001 | F527 |
| Acute mucoid otitis media | 52353000 | F5102 |
| Acute serous otitis media | 194240006 | F5101 |
| Acute suppurative otitis media | 194281003 | F520, F520z |
| Acute secretory otitis media | 359609001 | |
| Acute exudative otitis media | 19399000 | |
| Acute exudative otitis media | 194287004 | |
| Acute tubotympanic catarrh | 85108007 | |
| Acute sanguinous otitis media | 77478005 | F5106 |
| Acute necrotising otitis media | 360595002 | |
| Acute seromucinous otitis media | 232251007 | |
| Recurrent acute suppurative otitis media | 232251007 | X00iq |
| Acute suppurative otitis media due to another disease | 194282005 | F5203 |
| Recurrent acute non-suppurative otitis media | 232252000 | X00ir |
| Acute suppurative otitis media without spontaneous rupture of ear drum | 14948001 | F5200 |
| Infective otitis media | 312218008 | XaDmU |
| Acute suppurative otitis media with spontaneous rupture of ear drum | 86279000 | F5201 |
| Subacute non-suppurative otitis media | 6965008 | |
| Acute otitis media with effusion | 270490007 | XE2QD |
| Recurrent acute suppurative otitis media with spontaneous rupture of ear drum | 1082561000119104 | |
| Acute otitis media of left ear with effusion | 15916831000119102 | |
| Acute otitis media of right ear with effusion | 1090731000119101 | |
| Acute persistent otitis media | 84261000119106 |
Consenting
The TRANSFoRm platform prompted and facilitated the clinician to print the REST consent form and, after the parent had signed the form, to indicate consent on the system, initiating the trial’s workflow.
Case report form completion
The eCRFs were presented to clinicians at appropriate points to complete. These were then automatically entered into the study database. Some information was retrieved directly from the SystmOne record and used to partially fill in the form, which could be amended by the user. Once submitted, confirmation was displayed to the user on the screen and completed forms were stored in the study database and the SystmOne record. For SystmOne, a link to the local copy of the completed record was added. To facilitate subsequent tracking of participant progress, trial-specific codes were added to the record (1) when a patient was classified as potentially eligible by the plugin, (2) after submission of the consent or decliner form and (3) on trial completion.
Recruitment process data collection
There were two approaches planned to collect process frequency data. The first involved the recording of information relevant to the submission of eCRFs by the user. Recorded data consisted of information on the closed pop-up window (see Table 2) or submitted eCRF (consented or declined eCRF), that is the identifier of the user interacting with the form, the NHS number of the patient and the current date and time. The recorded data were stored locally in a dedicated folder in the clinician’s or nurse’s computer and could be viewed or shared by them only. The patient’s NHS number was added to account for the possibility of a patient being initially discarded by the clinician as a potential candidate but recruited at a later stage by the same or a different user. This approach was, in the event, unsuccessful, as local files stored during the running of the recruitment process were overwritten each time an upgraded version of the DNC was installed. This issue was due to the architecture of the DNC and the restrictions imposed by NHS computers on installation of third-party software, such as the TRANSFoRm platform (e.g. only Windows-based administration members could permit installation).
The second approach involved auditing the EHR for trial-related clinical codes inserted by the TRANSFoRm platform’s DNC during the recruitment workflow. These clinical codes denote potentially eligible and consented participants, as well as patients who declined participation.
In November 2019, we tried to estimate the frequency of pop-up disposal by contacting 12 randomly selected sites (of the 38 open to recruitment at the time) to request data on the number of times the TRANSFoRm platform pop-up had been responded to (closed without action, declined or consented) in the previous 6 months.
Site recruitment
Site invitation
The TRANSFoRm platform was initially set up to operate with the SystmOne EHR system. If this had worked well, we intended to expand the TRANSFoRm platform to work with the EMIS® system (EMIS Health, Leeds, UK). SystmOne sites were approached in three ways:
-
Through a RCGP bulletin that was distributed to CRNs and sites. Sites would then be able to express an interest directly to the study team or through their CRN.
-
Through direct contact with CRNs who regularly promote current research studies to sites. As per standard procedures, a research information sheet for practices (RISP) was provided for CRNs to distribute to sites.
-
At networking events for CRNs where the study was promoted by members of the study team.
Interested sites completed an EoI that was passed onto the study team, triggering the set-up process. To maximise efficiency and procedural quality, we focused on larger (≥ 10,000 registered patients) practices, as well as those that were research active; however, the TRANSFoRm platform was intended to make the recruitment process streamlined and quick, so even sites that were new to research could take part. The sponsor’s (University of Bristol) ‘green light’ procedure was implemented to document preparedness to conduct recruitment.
Site approval
The green light process was intended to be ‘light touch’ from the point of view of the sponsor, which would not need to see each piece of documentation to sign off on a site. Instead, the study team entered the details of all the required documentation received (and confirmation of site staff training) onto a study database, providing a summary to the sponsor to confirm that the documents had been received.
Required paperwork included:
-
an organisation of information document
-
a completed site agreement, signed by the practice manager or principal investigator
-
a delegation log
-
signed and dated curricula vitae from all members of staff that appeared on the delegation log
-
a GCP certificate (dated within 5 years) for the principal investigator (although all staff were encouraged to submit a certificate if they had one)
-
completion of site training (see Site training).
It was agreed that eligibility could be confirmed by a nurse or nurse practitioner with GP principal investigator oversight, meaning that the site delegation log had to include a GP as principal investigator.
Once all required documentation was received and entered onto the REDcap database, a record of received documents was e-mailed to the sponsor, with a request for the site to be ‘green lighted’ and opened to recruitment.
Site training
The large number of sites with wide geographic distribution meant that face-to-face training was not feasible. Online study-specific modules were developed and recorded using professional actors, and were hosted by Health Care and Videos (see Appendix 1). Modules included (1) introduction from the joint chief investigators, (2) ‘recruiter training’, (3) ‘recruiting with TRANSFoRm’ and (4) ‘recruitment quick reminder’. If a staff member was not GCP trained, they were asked to complete the module ‘study-specific GCP and informed consent’. If a staff member was part of the reception team, they were asked to complete ‘reception staff supporting recruitment’, and if a staff member was part of the administration team, they were asked to complete ‘chief investigators introduce the REST study’.
Further detailed training videos were provided to help the recruiting clinicians to navigate the TRANSFoRm system. These can be found in Appendix 1.
Staff completed different training modules depending on their role in the study. The study team looked at staff’s roles on the delegation log to determine which training modules they should be instructed to complete. Site staff were sent the appropriate links to the training videos by e-mail. The study team could see when these modules had been completed on the study training website. Once the principal investigator had completed their training, the site could open, with only those who had completed training being permitted to download the TRANSFoRm platform software and recruit participants.
Patient eligibility
Participants
Children whose parents were seeking primary medical care for unilateral otorrhoea as the presenting symptom of AOM and observed within the previous 7 days participated.
Eligibility criteria
Inclusion
-
Aged ≥ 12 months to < 16 years.
-
Presenting with recent-onset (≤ 7 days) unilateral AOM with recent-onset (≤ 7 days) otorrhoea, currently visible or seen by parent within the last 24 hours.
-
Attending with a parent who is legally able to give consent.
-
Parent is willing and able to administer ear drops.
-
Parent is willing, able and available to complete the daily SRQ and receive regular telephone calls from the study team.
Exclusion
-
Symptoms or signs suggestive of bilateral AOM or AOMd.
-
Symptoms or signs suggestive of serious illness and/or complications (e.g. mastoiditis and/or requires immediate hospitalisation).
-
Requires immediate oral antibiotics.
-
Child is at high risk of serious complications:
-
Significant immunosuppression.
-
Heart, lung, renal, liver or neuromuscular disease comorbidities.
-
Trisomy 21, cystic fibrosis or craniofacial malformation, such as cleft palate.
-
-
Grommet tube in situ in the otorrhoea ear.
-
Currently on oral or topical antibiotics.
-
Allergy to ciprofloxacin.
-
Allergy to penicillin or anaphylactic reaction to another beta-lactam agent, and allergy to clarithromycin.
-
Child had taken part in any research involving medicines within the last 90 days.
-
Child had already participated in this trial.
Interventions
The trial had two intervention arms and a usual care comparator arm.
Intervention 1
Intervention 1 was immediate ciprofloxacin (0.3%) ear drop solution, four drops given three times per day for 7 days, with an advice sheet on how to administer the ear drops and standardised symptom management advice.
Intervention 2
Intervention 2 was delayed dose-by-age oral amoxicillin suspension, given three times per day (clarithromycin twice daily if allergic to penicillin or another suitable oral antibiotic as chosen by the GP) for 7 days, and an advice sheet containing structured delaying advice and standardised symptom management advice.
Comparator
The comparator was immediate dose-by-age oral amoxicillin (clarithromycin twice daily if allergic to penicillin or other suitable oral antibiotic as chosen by the GP), given three times per day for 7 days, and standardised symptom management advice.
Outcomes
Primary outcome
Our primary outcome was the time to resolution of all pain, fever, being unwell, sleep disturbance, otorrhoea and episodes of distress/crying being rated ‘no’ or ‘very slight’ problem by parents without need for analgesia. We used a validated self-report scale known to be sensitive to change with study research nurse telephone support. The presence and severity of each symptom was recorded daily using a Likert scale: 0 = ‘normal/none’, 1 = ‘very slight problem’, 2 = ‘slight problem’, 3 = ‘moderately bad’, 4 = ‘bad’, 5 = ‘very bad’ and 6 = ‘as bad as it could be’. We asked parents to complete the daily symptom diary each evening to cover the previous 24 hours. The intention was for symptoms to be recorded until all symptoms had been rated 0 for 2 consecutive days, or in the event of non-resolution for a maximum of 14 days. In reality, many parents continued to complete scores after 2 consecutive days of 0 scores. Symptoms were recorded using the TRANSFoRm platform (or on paper), with real-time monitoring by our research nurse to ensure data completion.
Secondary outcomes
Secondary outcomes also reflected their importance to parents and the NHS. Those recorded in the first 14 days’ SRQ included:
-
duration of ‘moderately bad or worse’ symptoms (i.e. pain, fever, being unwell, sleep disturbance, otorrhoea, episodes of distress/crying)
-
adverse events (e.g. diarrhoea, rash, vomiting and severe complications at days 7 and 14)
-
parent satisfaction with treatment (at day 14)
-
treatment adherence and analgesic use to symptom resolution up to day 14 (SRQ)
-
details of previous 7 days’ NHS resource use at days 7 and 14 on the SRQ
-
analysis of stool sample to assess burden of resistance (day 14 and month 3).
Sample size (non-inferiority)
Our previous AOM trial compared immediate antibiotics with delayed antibiotics. 1 A total of 73 children had AOMd and they (combining both randomised groups) took a median of 3 days [interquartile range (IQR) 2–4 days] to achieve the REST primary outcome. We consulted our PPI group to determine the maximum difference they regarded as unimportant, asking ‘If you were happy to take part, even if the drops took a little longer to work, how much longer would be acceptable? Please click all that apply’.
Table 3 shows the mean number of extra days considered acceptable by responders; the mean maximum unimportant difference was 1.39 [95% confidence interval (CI) 1.14 to 1.65]. This suggests that a difference of 1.5 days could be stretching the limit of acceptability to the average parent in this population. However, instead of immediate oral, half the parents were prepared to put up with > 1 extra day of symptoms. We therefore concluded that an average difference of 1.25 days is collectively acceptable. This would mean that for every five children treated with immediate ear drops (or delayed oral antibiotics) instead of immediate oral antibiotics, one fewer would have their symptoms resolved or ‘very mild’ within 3 days.
| Maximum number of days | Frequency of response |
|---|---|
| 1 | 7 |
| 1.5 | 3 |
| 2 | 4 |
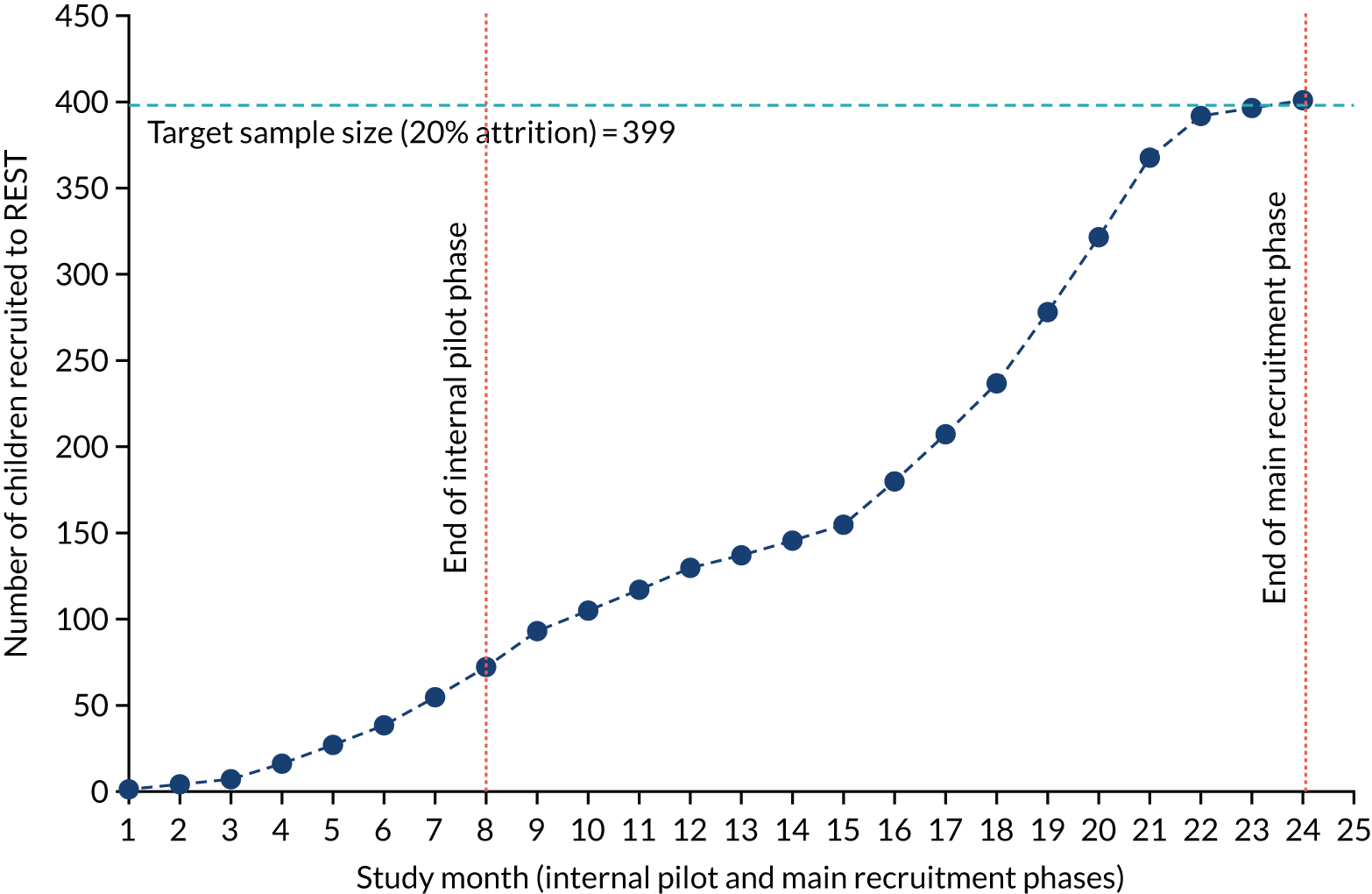
Applying survival curves to produce an increase in median survival (i.e. an increase in time to symptom resolution) of 1.25 days from a median of 3 days would be equivalent to a difference of 16.8% in the cure rate at 3 days. A two-arm non-inferiority trial normally assumes a 2.5%, one-sided, type I error. As REST was a three-arm, rather than two-arm, trial, for a 1.25% type I error to detect non-inferiority for two comparisons, with 90% power, a total sample size of 399 (which allows for 20% attrition) was required. Figure 4 shows how we intended to recruit this number over two winter seasons and one summer season.
FIGURE 4.
Planned recruitment, taking account of seasonality, including internal pilot.
Randomisation and concealment
Following eligibility confirmation and consent, children were randomised, stratified by age (< 2 and ≥ 2 years, as children < 2 years have been shown to experience longer illnesses)2 using the TRANSFoRm platform. 31 Blocks of 12 were used for allocation (four in each arm) because most practices will recruit one or two patients only. Clinicians were not able to determine treatment allocation pre randomisation. The randomisation sequence was generated by the Bristol Randomised Trials Collaboration (BRTC), supplied to the TRANSFoRm team and allocated to successive participants. A system for checking the correct randomisation allocation was built into the TRANSFoRm platform and treatment allocation was checked in the patient symptom questionnaire.
Data collection
Baseline
Eligibility criteria, baseline parent-reported symptoms and brief clinical examination findings were recorded using the embedded CRF in the EHR, managed by the TRANSFoRm platform. 31 The REST research nurse made contact by telephone on day 1 to address any questions or concerns that the parent may have had about the trial, and to ensure that the SRQ was being accessed and could be completed without difficulty using the TRANSFoRm platform. If problems occurred, responses could be noted in a paper template SRQ.
Follow-up
Days 2–14
The SRQ was provided in electronic format (for either web or iOS/Android applications) via the TRANSFoRm platform. Parents recorded the daily presence and severity of the following AOMd symptoms (until cessation of all symptoms without need for analgesia): pain, fever, being unwell, sleep disturbance, otorrhoea, episodes of distress/crying, appetite and interference with normal activities. The primary outcome was collected using the SRQ, along with a research nurse telephone call (we have achieved < 20% primary outcome attrition using this method in other similar trials). 1,27–29 The presence of adverse events, defined as new or worsening of existing symptoms, including otitis externa, rash, fungal ear infections, diarrhoea, vomiting and serious AOM complications, was recorded daily. We recorded daily measures of study medicine and analgesic/antipyretic use on the SRQ. On day 14, parents were invited to record their satisfaction with the trial treatments.
On days 7 and 14, parents were invited to record on the SRQ any use of health-care resources in the previous 7 days, including information about primary care contacts, community care and use of 111, walk-in centres and hospital services. The research nurse clarified details, such as reasons for the consultation, who was seen (e.g. GP, nurse, health-care assistant), the type of consultation (e.g. face to face, telephone, home visit) and whether it was in hours or out of hours. During the final telephone call (day 14), the research nurse reminded parents to send a stool sample [a specimen pot with research laboratory-recommended opaque polythene envelopes and label-compliant Mail Tuff™ (Antalis, London, UK) outer envelopes was sent to parents on day 7] and reminded them about the final questionnaire and stool sample at 3 months. The final questionnaire was intended to be provided in electronic format (for either web or iOS/Android applications) via the TRANSFoRm platform, but was completed on paper and sent by post because this part of the TRANSFoRm platform was not ready. In the questionnaire, parents recorded details of AOM and AOMd recurrence, audiology referrals, use of hospital services, hearing loss (using the OMQ-14)32 and any serious complications.
Statistics
Participant flow through the trial is summarised by a Consolidated Standards of Reporting Trials (CONSORT) flow chart (see Figure 6). Descriptive summary statistics of clinical and demographic characteristics are presented, both overall and separately by arm, to describe the study sample and to ascertain the comparability of the randomisation arms. Continuous data are presented as either mean and standard deviation or median and IQR, depending on data distribution. Categorical data are presented as frequency counts and percentages.
Primary analysis
The planned primary analysis was to be carried out under the intention-to-treat (ITT) principle, analysing participants in the arms to which they were randomised, without the imputation of missing data. The primary analysis of effectiveness examines whether or not immediate topical or delayed oral antibiotics are non-inferior to immediate oral antibiotics for symptom duration in children presenting to primary care with AOMd. We planned to compare symptom resolution over the 14 days of follow-up between children allocated to the immediate oral antibiotics (comparator) arm and those allocated to each of the other treatment arms using a Cox proportional hazards regression model, adjusted for age (stratification variable). The Cox regression model provides an estimate of the hazard ratio (alongside the 95% CI and p-value for the comparison), which indicates the relative likelihood of symptom resolution in intervention participants versus control participants at any given point in time. The appropriateness of the proportional hazards assumption would have been investigated. We planned to plot Kaplan–Meier survival curves to depict the probability of symptom resolution over time and the median time to symptom resolution for the three treatment arms. The planned primary analysis was not conducted because of the small number of participants recruited and early study closure.
Secondary analyses of primary outcome
Previous research has suggested that symptoms of AOM will be resolved in 90% of children by day 8. Therefore, we planned to additionally analyse the primary outcome using an accelerated failure time (AFT) model, which has previously been recommended for studies of resolution of infectious diseases. 71 The AFT model was to be adjusted for age (as in the primary analysis) and the exponentiated coefficients (alongside the associated 95% CI and p-values for the comparison) from the AFT model would have been reported. An exponentiated regression parameter from an AFT model can be interpreted as the percentage difference in time to symptom resolution between the treatment arms.
It was planned that the proportion of participants in the immediate topical and delayed oral antibiotics arms who achieved symptom resolution within 3 days would be compared (separately) with those in the immediate oral antibiotics arm. Once the absolute difference was calculated and reported alongside the associated CI, we would have reported whether or not the lower limit of the CI lay within the maximum unimportant difference. 69 The planned secondary analyses of the primary outcome were not conducted because of the small number of participants recruited and early study closure.
Secondary outcomes
We planned to repeat the primary analysis model with the outcome of symptom resolution defined as when all symptoms were rated as ‘normal/none’, ‘very slight problem’ or ‘slight problem’ (rather than the primary outcome of symptom resolution defined as all symptoms being rated as ‘normal/none’ or ‘very slight problem’).
Binary secondary outcomes (e.g. recurrence of AOMd) were to be analysed using logistic regression analysis and semicontinuous scores, such as parental/carer satisfaction and hearing loss at 3 months, were to be analysed using ordinary linear regression in which these variables conformed reasonably closely to a normal distribution; otherwise, negative binomial regression analysis or other suitable alternatives would have been chosen.
Sensitivity analyses
We planned to repeat the primary analysis and AFT models with additional adjustment for any prognostic variables demonstrating a marked imbalance at baseline (ascertained using descriptive statistics).
The primary analysis model was to be repeated under the per-protocol (PP) principle, that is the analysis being restricted to only those participants deemed to have no major protocol violations.
We planned to explore the sensitivity of the primary analysis to the impact of missing data by imputing missing primary outcome data and repeating the primary analysis model using the imputed data. The imputation model would include all variables that were part of the ITT primary analysis, as well as baseline and post-randomisation variables associated with missingness and/or prognostic of outcome.
Exploratory analyses
It was planned to explore potential treatment moderators by including treatment arms by moderator variable interaction terms into the primary analysis model (individually).
Health economics
The objective of the primary economic evaluation was to explore the relationship between cost and outcome for the three treatments for AOMd (immediate topical, delayed oral and immediate oral antibiotics) from a NHS perspective at 14 days post randomisation.
This was to take the form of a simple comparison of NHS costs and outcomes over the 2 weeks post randomisation.
A secondary cost analysis was planned to evaluate the difference in NHS secondary care costs between the trial arms in the 3 months following randomisation.
Measurement and valuation of relevant resource use
The resource use for the primary economic evaluation was collected through the SRQ. At days 7 and 14, information (related to the child’s ear problem) was collected on primary care consultations (i.e. GP and practice nurse), NHS 111 contacts and secondary care service use (i.e. accident and emergency attendances, outpatient appointments and inpatient stays). The 3-month secondary care resource use data were collected through a case note review of the general practice records. The two sources of data were compared to ensure that secondary care data were not double-counted.
All resources were valued using unit costs (2018–19 values) from established sources. Primary and community care was valued using Unit Costs of Health and Social Care 2019,73 NHS Reference Costs were used to value hospital care74 and the BNF for Children 202013 was used to value prescribed medication.
Missing data
If the questionnaire had been answered but an individual question had not been completed, it was assumed that no health-care resources had been used.
Analysis
The economic analyses were conducted using an ITT approach, that is analysing patients in the arm to which they were randomised, irrespective of any post-randomisation changes. As the follow-up period was < 1 year, costs were not discounted.
The cost of each item of resource used during the 2 weeks for primary analysis, and from week 2 to 3 months for secondary analysis of follow-up, was evaluated as the resource use multiplied by its unit cost. The total cost for each individual patient was calculated as the sum of the costs of the resource use items. The mean resource use and costs were estimated and presented by trial arm for each resource use category at 2 weeks and from week 2 to 3 months.
A cost–consequence analysis was planned in which the costs to the NHS of the three treatments at 14 days post randomisation would have been compared with the primary clinical outcome, but this was not conducted because of the small number of participants recruited.
Qualitative
The objective of the qualitative study was to understand the views and experiences of parents and primary care practice staff (including clinician recruiters) of the TRANSFoRm trial to inform recruitment strategies for this and future similar trials. Qualitative findings would also help illuminate the perceived effectiveness and acceptability of the different treatment options, explore barriers to their use within and their future uptake outside the trial.
Sampling
Parents who consented to the trial were contacted by telephone and asked if they would take part in a qualitative interview. As there were limited numbers of recruits, instead of taking a purposive approach to sampling, all parents were approached to maximise the number of parent views obtained. Parents who declined trial participation and who consented to a qualitative interview were also contacted by text message/telephone and asked to take part in a short qualitative interview. A sequenced procedure was used to contact potential interviewees. Initial contact was by text message so that the parents knew who was calling and the number from which they were calling. If possible, the interview time was arranged by text message, but if there was no response to this message then the parents were called around three times at different times of day and on different days of week (respecting their indicated preference for call time). If there was no response after three calls, it was assumed that this constituted a withdrawal of consent and no further contact was made.
Primary care staff involved in trial processes were invited to take part in qualitative interviews. Staff were purposively sampled to capture experiences of staff with different roles (i.e. recruiting clinicians, research and IT support staff) and working at different sites (i.e. recruiting, not recruiting or withdrawn).
Sample size was informed by the concept of ‘information power’,75 with analysis and sampling conducted in parallel and continuous assessment of the suitability of the information within the sample with regard to study objectives. The narrow focus of the study aim on experiences of the trial, the specificity of the experiences and the case-based analysis all indicate that higher information power would be possible from a relatively smaller sample. 75
Data collection
Semistructured interviews76 were conducted with participating parents from all arms of the trial. Most interviews were conducted within 14 days of recruitment, but a couple were conducted 6 weeks after recruitment (because of reduced availability over the Christmas holidays). Interviews with parents who declined to participate were conducted within 3 days of declining. Interviews were conducted by telephone. Parents were contacted through either a telephone call or a text message, and asked to identify a suitable time for the interview; sometimes interviews were undertaken immediately and sometimes they were arranged for a later date.
Interviews with primary care staff were conducted after varied periods of involvement in the trial to capture those with experience of trial processes. Recruiting clinicians were interviewed after they had recruited at least one participant. Primary care staff who had been involved in setting up the TRANSFoRm platform software were interviewed up to 9 months after their first involvement in the trial.
Flexible topic guides were devised for the parent and staff interviews to ensure that the primary issues were covered across all interviews, but also allowing considerable flexibility to enable participants to introduce unanticipated issues. The researcher used open-ended questioning techniques to elicit participants’ experiences and views of key events, and participants were asked to provide examples. Primary care professional’s interviews lasted up to 45 minutes, parent interviews lasted up to 30 minutes and parent decliner interviews lasted up to 10 minutes. Interviews were recorded using a digital voice recorder, and were transcribed and anonymised to protect confidentiality.
Data analysis
Interview transcripts were imported into NVivo, version 12 (QSR International, Warrington, UK), qualitative data analysis software. Analysis began shortly after data collection started and was ongoing and iterative. Thematic analysis,77 utilising a data-driven inductive approach, was used to identify and analyse patterns and themes of salience for participants and across the data set. 77 The researcher (CC) used line-by-line coding to construct draft coding frames, each based on three transcripts. A combination of deductive coding, based on the aims of the study and the topic guide, and inductive coding, identifying themes within the data, was used. A subset of transcripts were independently coded by members of the team (CC and JH) and data interpretation was discussed to achieve coding consensus and maximal rigour. The coding frame was then modified and applied to the rest of the data set, with regular meetings with Jeremy Horwood to discuss emerging findings. Finally, Christie Cabral drafted a narrative based on the analysis, with input from Jeremy Horwood. Final themes were discussed by the interdisciplinary trial team. Emergent analysis was discussed in multidisciplinary TMG meetings to ensure that findings were trustworthy and credible.
Patient and public involvement methods
Extensive PPI was undertaken during the development of the protocol and study materials. Our PPI members provided input into the development of the primary outcome and identified the most significant symptoms that should be used to judge recovery as pain, fever, being unwell, sleep disturbance, otorrhoea and episodes of distress/crying. The PPI group reviewed the symptom recovery questionnaire and patient-facing materials and commented on their suitability for use in the study. During recruitment, our PPI contributor advised on the findings of a report published by the EMA on the safety of fluoroquinolone and quinolone drops. 65
Chapter 3 Results
This chapter summarises the recruitment of sites and participants, and describes the baseline characteristics and outcomes for the 22 children who were recruited.
Site recruitment
The first site opened on 5 April 2019 and the trial was closed on 31 March 2020, primarily because of critically low recruitment, but secondarily because of the onset of the 2019/20 SARS-CoV-2 pandemic. At study closure, 122 general practices from 12 CRNs had expressed an interest, of which 71 practices confirmed participation; 61 received sponsorship; 44 opened to recruitment, with the TRANSFoRm platform installed on 72 clinical computers; and seven sites randomised 22 children.
The trial was originally planned to open for recruitment to the internal pilot in time for winter 2018/19, and then to continue recruiting for another summer and winter, until April 2020. Figure 5 shows that delays to the TRANSFoRm platform development meant that the first participant was not recruited until March 2019, 12 months later than originally planned. New internal pilot dates (June to December 2019) were agreed with the NIHR HTA programme in May 2019, but recruitment numbers remained smaller than expected, primarily because of the poor function of the TRANSFoRm platform and the need to repeatedly reinstall the platform software.
FIGURE 5.
Participant recruitment against TRANSFoRm activities and delays. Note that the x-axis is non-linear.
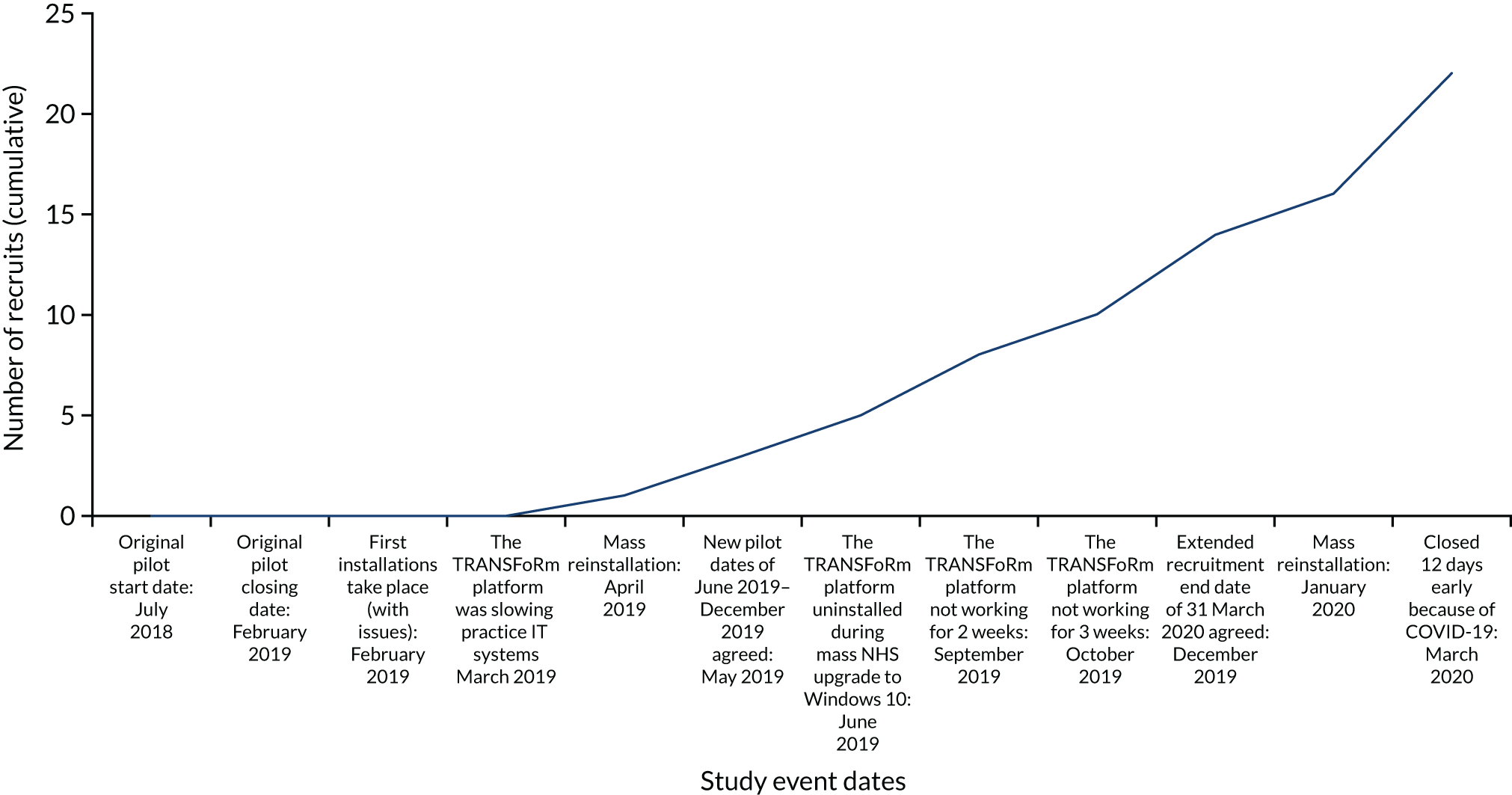
Description of general practitioner recruitment experience
Dr Claire Hombersley of Swanage Medical Centre wrote:
Recruiting for REST was relatively straightforward once the software was downloaded and working well. I identified a suitable child [from] the sit and wait surgery from the triage details on the appointment screen from our reception staff. They were all aware we were recruiting for a ‘runny ear’ trial and the waiting room had information posters displayed.
I was able to see all the children presenting with runny ears when I was working on the sit and wait surgery. If they were suitable and interested in taking part, then I added the otitis media code to the computer that launched the TRANSFoRm platform. The system then presented the required forms in an easy step wise manner starting with the patient information leaflet for the parent and/or patient to read. I then filled in the forms on the system and printed the consent forms. The system uploaded the filled in forms to the primary care record. The PIL [patient information leaflet] and the consent forms were scanned by staff later on. The trial also provided an envelope for the patient containing data protection information, the symptom and recovery questionnaire and freepost envelope, the obligatory free pen and stickers and a further parent information booklet.
The system would randomise the patient and then provide the required medication advise sheet to print off for the patient. You followed the A-J TRANSFoRm forms and then you knew everything was completed and uploaded automatically to the patient record. There was no file containing lots of bits of paper the sort through, it was streamlined and clinician friendly.
The first patient I did was time consuming as several of the documents to print out didn’t work. But the next four were quick and I could see the potential in the system. Unfortunately, the runny ears dried up and we did not see another suitable recruit for 12 months.
Reproduced with permission from Claire Hombersley, 2021, personal communication
Participant recruitment and follow-up
A total of 30 children/parents were invited to participate, of whom 22 agreed to participate and were consented and randomised (Figure 6). Of the eight who declined to participate, seven parents stated that they did not want their child to participate (no further reason was given) and two were interviewed. No further descriptors for children who declined to participate were available to assess the generalisability of the final sample.
FIGURE 6.
The REST CONSORT flow diagram.
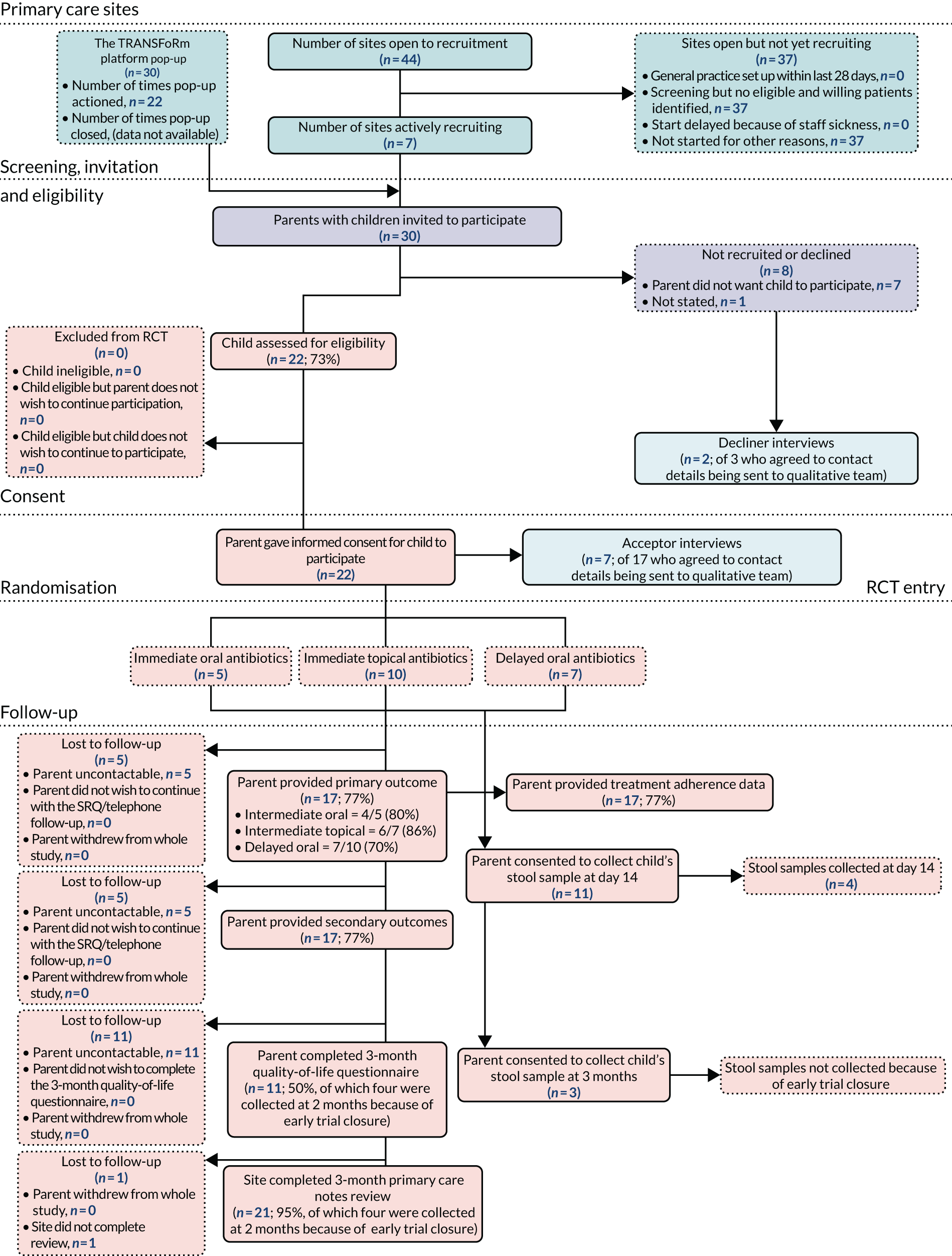
Five children were randomised to immediate oral antibiotics (the control arm), 10 were randomised to topical antibiotics and seven were randomised to delayed oral antibiotics. The parents of 17 (77%) children provided primary and secondary symptom outcomes, four (18%) provided stool samples from their child at 14 days and 11 (50%) provided OMQ-1432 quality-of-life data (planned to be undertaken at 3 months, with four parents providing this at 2 months because of early trial closure). Primary care medical record notes reviews were conducted manually by site staff for 21 (95%) children, again with four conducted early (at 2 months) because of early trial closure. The stool collection planned at 3 months was abandoned because of early trial closure.
Recruitment reminder pop-up
The TRANSFoRm platform included a ‘pop-up’ triggered by the use of AOMd-relevant diagnostic codes entered by recruiting clinicians. In November 2019, we randomly selected 12 sites (of the 38 sites open to recruitment at the time) from which to request data on the number of times the TRANSFoRm platform pop-up had been responded to (closed without action, declined or consented) in the previous 6 months. All 12 sites provided data. Table 4 shows that the pop-ups were triggered 11 times at six of the sites, with one participant’s recruitment linked to these occurrences.
| Site name | Comment |
|---|---|
| Priory Gardens Surgery | 3 patient pop-ups closed |
| Elmwood Family Doctors | 2 patient pop-ups closed |
| Glendale Surgery | 2 patient pop-ups closed |
| Well Close Medical Group | 2 patient pop-ups closed |
| The Westbank Practice | 1 patient pop-up closed |
| Woodlands Family Practice | 1 patient pop-up closed |
| Bradford-on-Avon & Melksham Health Partnership | No pop-ups closed |
| St. Augustine’s Medical Practice | No pop-ups closed |
| Chew Medical Practice | No pop-ups closed |
| Medwyn Surgery | No pop-ups closed |
| Eden Court Medical Practice | No pop-ups closed |
| Gillingham Medical Centre | No pop-ups closed |
Final sample characteristics and outcomes
Data completeness
Data were missing for the baseline characteristics of one child (Table 5), but symptom duration data were available for 17 (77%) children at day 14 and parent-reported ear-related quality of life.
| Time point | Children, n (%) |
|---|---|
| Randomised | 22 (100) |
| Baseline data | 21 (95) |
| Primary outcome | 17 (77) |
| Symptom data at day 14 | 17 (77) |
| Resource use data at day 14 | 17 (77) |
| Stool sample at day 14 | 4 (18) |
| 3-month data collection | 11 (50) |
Baseline characteristics
Of the 22 participants recruited, 13 (62%) were male; the median age of those in the sample was 5 years (IQR 2–7 years) and, on a scale of 0 to 10 (where 0 = not at all unwell and 10 = extremely unwell), clinicians rated how unwell the child was as a median of 2 (IQR 1–4) (Table 6). Approximately half of participants had a history of AOM and one-third had a history of AOMd. No comment is made regarding potential differences in participant characteristics by treatment arm because of the small numbers recruited.
| Characteristic | Immediate oral antibiotics arm (N = 5) | Delayed oral antibiotics arm (N = 7) | Immediate topical antibiotics arm (N = 10)a | Pooled across arms (N = 22)b |
|---|---|---|---|---|
| Collected at baseline appointment | ||||
| Age (years), median (IQR) | 6 (2–7) | 5 (3–11) | 5 (2–6) | 5 (2–7) |
| Sex, n (%) | ||||
| Male | 5 (100) | 5 (71) | 4 (40) | 14 (64) |
| Female | 0 (0) | 2 (29) | 6 (60) | 8 (36) |
| Day of discharge (IQR) | 4 (1–7) | 1 (1–3) | 2 (1–3) | 2 (1–4) |
| Clinician rating of how unwell child is (0 = not at all to 10 = extremely), median (IQR) | 3 (1–3) | 1 (1–4) | 2 (2–4) | 2 (1–4) |
| Temperature (°C), median (IQR) | 36.6 (36.6–36.6) | 36.7 (36.6–37.0) | 37.2 (36.6–37.3) | 36.7 (36.6–37.2) |
| Visible aural discharge, n (%) | ||||
| Yes | 3 (60) | 6 (86) | 9 (100) | 18 (86) |
| No | 2 (40) | 1 (14) | 0 (0) | 3 (14) |
| Visible perforation, n (%) | ||||
| Yes | 0 (0) | 0 (0) | 1 (11) | 1 (5) |
| No | 5 (100) | 7 (100) | 8 (89) | 20 (95) |
| History of AOM (ever), n (%) | ||||
| Yes | 4 (80) | 3 (43) | 4 (44) | 11 (52) |
| No | 1 (20) | 4 (57) | 5 (56) | 10 (48) |
| History of AOMd (ever), n (%) | ||||
| Yes | 3 (60) | 1 (14) | 3 (33) | 7 (33) |
| No | 2 (40) | 6 (86) | 6 (67) | 14 (67) |
| History of glue ear (ever), n (%) | ||||
| Yes | 2 (40) | 0 (0) | 0 (0) | 2 (10) |
| No | 3 (60) | 7 (100) | 9 (100) | 19 (90) |
| Antibiotic prescription (collected at primary care medical notes review) (n) | ||||
| Oral amoxicillin | 5 | 5 | 0 | 10 |
| Oral clarithromycin | 0 | 1 | 0 | 1 |
| Ciprofloxacin drops | 0 | 0 | 10 | 10 |
| No prescription recorded | 0 | 1 | 0 | 1 |
| Collected from SRQ at day 1 | (N = 4) | (N = 6) | (N = 7) | (N = 17) |
| Ever had grommets, n (%) | ||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No | 4 (100) | 6 (100) | 7 (100) | 17 (100) |
| Ever had ENT surgery n (%) | ||||
| Yes | 0 (0) | 1 (17) | 0 (0) | 1 (6) |
| No | 4 (100) | 5 (83) | 7 (100) | 16 (94) |
| Ever had eczema, hay fever and/or asthma, n (%) | ||||
| Yes | 1 (25) | 4 (67) | 1 (14) | 6 (35) |
| No | 3 (75) | 2 (33) | 6 (85) | 11 (65) |
| Household smoker, n (%) | ||||
| Yes | 1 (25) | 2 (33) | 1 (14) | 4 (24) |
| No | 3 (75) | 4 (67) | 6 (85) | 13 (76) |
| Level of educational qualification (parent), n (%) | ||||
| Left school before age 16 years | 0 (0) | 1 (17) | 0 (0) | 1 (6) |
| Usual examinations at age 15/16 years | 1 (25) | 1 (17) | 1 (14) | 3 (18) |
| Usual examinations at age 17/18 years | 2 (50) | 0 (0) | 0 (0) | 2 (12) |
| Further but not higher education | 0 (0) | 2 (33) | 5 (71) | 7 (41) |
| University degree | 1 (25) | 2 (33) | 1 (14) | 4 (24) |
| Not applicable | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Contamination
All antibiotics prescribed at baseline were in accordance with the protocol, that is all were one of oral amoxicillin, oral clarithromycin or topical ciprofloxacin (see Table 6). None of the children randomised to receive topical antibiotics was prescribed oral antibiotics and, vice versa, none of the children randomised to receive oral antibiotics was prescribed topical antibiotics. Of the seven children randomised to delayed oral antibiotics, six received a prescription at baseline. There was no recorded prescription for the remaining child, which is compatible with how delayed prescribing can be operationalised. 78
Outcomes
Primary outcome
Symptom duration was defined as the time to the first day on which all symptoms were rated ‘normal, no or very slight problem’, with data available for 17 (77% of those randomised) children. Overall, the median number of days to symptom resolution was 4 (IQR 3–7), and the median (IQR) number of days to symptom resolution in the immediate oral, delayed oral and immediate topical antibiotic arms was 6 (4–9), 4 (3–7) and 4 (3–6), respectively (Table 7). No formal between-arm comparative analysis was conducted because of the small numbers recruited.
| Outcome | Immediate oral antibiotics (N = 4) | Delayed oral antibiotics (N = 6) | Immediate topical antibiotics (N = 7) |
|---|---|---|---|
| Primary outcome | |||
| Median number of days (IQR) to all symptoms first resolveda | 6 (4–9) | 4 (3–7) | 4 (3–6) |
| Secondary outcomes (first 14 days) | |||
| Symptom outcomes | |||
| Number (%) with all symptoms resolveda at day 3 | |||
| Yes | 1 (25) | 3 (50) | 3 (43) |
| No | 3 (75) | 3 (50) | 4 (57) |
| Time (days) to symptoms first rated ‘normal/none’, ‘very slight problem’ or ‘slight problem’, median (IQR) | 3 (3–4) | 4 (2–6) | 3 (2–5) |
| Number (%) at day 3 with symptoms rated ‘normal/none’, ‘very slight problem’ or ‘slight problem’ | |||
| Yes | 3 (75) | 3 (50) | 5 (71) |
| No | 1 (25) | 3 (50) | 2 (29) |
| Duration (days) of moderate or worse pain, median (IQR) | 3 (2–3) | 2 (1–4) | 1 (1–3) |
| Duration (days) of moderate or worse fever, median (IQR) | 1 (1–2) | 1 (1–1) | 1 (1–1) |
| Duration (days) of moderate or worse ear discharge, n (IQR) | 3 (2–3) | 3 (2–3) | 2 (1–3) |
| Duration (days) of moderate or worse unwell, median (IQR) | 2 (2–2) | 2 (1–3) | 1 (1–1) |
| Duration (days) of moderate or worse sleep, median (IQR) | 2 (2–2) | 2 (1–2) | 1 (1–4) |
| Duration (days) of moderate or worse crying, median (IQR) | 3 (2–3) | 2 (1–3) | 1 (1–3) |
| Duration (days) of moderate or worse eating/drinking, median (IQR) | 2 (2–2) | 1 (1–1) | 1 (1–2) |
| Duration (days) of moderate or worse activities, median (IQR) | 2 (2–2) | 2 (1–2) | 1 (1–1) |
| Satisfaction with treatment at day 14, n (%) | |||
| Extremely satisfied | 2 (50) | 1 (17) | 4 (57) |
| Satisfied | 1 (25) | 4 (67) | 3 (43) |
| Neither satisfied nor dissatisfied | 0 (0) | 1 (17) | 0 (0) |
| Not satisfied | 1 (25) | 0 (0) | 0 (0) |
| Extremely dissatisfied | 0 (0) | 0 (0) | 0 (0) |
| Adverse events, n (%) | |||
| New or worsening of existing symptoms in the first week | |||
| Yes | 1 (25) | 0 (0) | 2 (29) |
| No | 3 (75) | 6 (100) | 5 (71) |
| New or worsening of existing symptoms in second week | |||
| Yes | 0 (0) | 1 (17) | 2 (29) |
| No | 4 (100) | 5 (83) | 5 (71) |
| Stool sample microbiological raw data at day 14 (N = 4) | |||
| Processed at research laboratory (n) | 1 | 0 | 3 |
| First Escherichia coli type | |||
| MALDI-TOFb raw scoresc | 2.41 | – | 2.01, 2.2, 2.45 |
| Ampicillin zone (raw data) sizes (mm)d | < 6 | – | < 6, 14, < 6 |
| Ampicillin sensitive or resistant (raw data, % resistant) | Resistant (100) | – | Resistant, sensitive, resistant (66) |
| Ciprofloxacin raw data (mean) zone size (mm)d | 32 (32) | – | 23, 35, 37 (31.7) |
| Ciprofloxacin sensitive or resistant (raw data, % resistant) | Sensitive (0) | – | Resistant, sensitive, sensitive (33) |
| Erythromycin raw data (mean) zone size (mm)d | 9 (9) | – | 11, 15, 16 (14) |
| Second E. coli type | |||
| MALDI-TOFb scorec | 2.55 | – | – |
| Ampicillin zone size (mm)d | No zone | – | – |
| E. coli ampicillin sensitive or resistant | Resistant | – | – |
| Ciprofloxacin zone size (mm)d | 27.1 | – | – |
| Ciprofloxacin sensitive or resistant | Sensitive | – | – |
| Erythromycin zone size (mm)d | 9.8 | – | – |
Secondary outcomes (first 14 days)
Symptom duration
Forty-one per cent of parents reported children’s symptoms as ‘normal/none or very slight problem’ by day 3, and 65% reported symptoms as ‘normal/none, very slight problem or slight problem’ by the same time point. The median duration until symptoms were rated as ‘normal/none, very slight problem or slight problem’ for children in all arms was 3 days (IQR 2–4 days; see Table 7); and the median number of days in the immediate oral, delayed oral and immediate topical antibiotics was 3 (3–4), 4 (2–6), and 3 (2–5), respectively. The duration of moderate or worse symptoms is reported by symptom in Table 6.
Satisfaction with treatment
Although numbers are too small for definitive comment, 88% of parents were either ‘extremely satisfied’ or ‘satisfied’ with treatment, and higher rates of satisfaction with treatment were observed in the immediate topical antibiotic arm than in both the immediate and the delayed oral antibiotic arms.
Use of oral analgesics
Use of paracetamol and ibuprofen was poorly reported, with only one participant responding to whether or not analgesia medication was used (yes/no) over the 14 days, with the remainder leaving the ‘yes/no’ response option blank.
Adverse events
There were three reports of new or worsening of existing symptoms within the first 7 days of follow-up: one (‘scratching ear’) in the immediate oral antibiotics arm and two (‘swollen painful eye with headaches’ and ‘eye discharge’) in the immediate topical antibiotics arm (see Table 7). There were three reports of new or worsening of existing symptoms in the second 7 days of follow-up: one (‘ear leaking again’) in the delayed oral antibiotics arm and two (‘sore throat with temperature’ and ‘eye discharge’) in the immediate topical antibiotic arms. The reports of ‘eye discharge’ from the first and second 7 days were from the same participant.
Serious adverse events
No serious adverse events were reported. One child attended the emergency department but did not require hospital admission.
Stool sample microbiological data
Only four (18%) of 22 parents sent stool samples. The research laboratory processed three samples within 48 hours of receipt, with one sample, received on 30 December 2019, taking 8 days to be processed.
Adherence
In total, seven (32%) children were fully adherent to treatment allocation: two (40%) in the immediate oral antibiotic arm (Table 8), two (29%) in the delayed oral antibiotic arm (Table 9) and three (30%) in the immediate topical antibiotic arm (Table 10). Ten (67%) of the children in the immediate antibiotic arms (oral and topical) were given antibiotics within 24 hours of randomisation, compared with three (50%) children in the delayed oral antibiotic arm.
| Adherence measure | Children (n) | ||
|---|---|---|---|
| Yes | No | Missing | |
| Prescribed oral antibiotic | 5 | 0 | 0 |
| Took first dose on day of randomisation or following day | 4 | 0 | 1 |
| Then took at least 50% of doses as prescribed | 2 | 2 | 1 |
| Adherence measure | Children (n) | ||
|---|---|---|---|
| Yes | No | Missing | |
| Prescribed oral antibiotic | 6 | 0 | 1 |
| Did not start at all | 2 | 4 | 1 |
| Started after waiting until at least 2 days following randomisation | 2a | 4 | 1 |
| Adherence measure | Children (n) | ||
|---|---|---|---|
| Yes | No | Missing | |
| Prescribed drops | 10 | 0 | 0 |
| First dose on day of randomisation or following day | 6 | 1 | 3 |
| Then took at least 50% of doses as prescribed | 3 | 4 | 3 |
Secondary outcomes (ear-related quality of life at 3 months)
A total of 17 (77%) parents reported ear-related quality of life at 3 months using the OMQ-14 questionnaire. 32 Numbers were, therefore, too small to make definitive comment (Table 11).
| Secondary outcome at 3 months | Immediate oral antibiotics arm (N = 4) | Delayed oral antibiotics arm (N = 6) | Immediate topical antibiotics arm (N = 7) |
|---|---|---|---|
| Parent-reported ear-related quality of life at 3 months (OMQ-14 questionnaire)32 | n = 3 | n = 5 | n = 3 |
| Physical suffering, n (%) | |||
| Not present/no problem | 2 (67) | 4 (80) | 1 (33) |
| Hardly a problem at all | 0 (0) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 1 (20) | 1 (33) |
| Moderate problem | 1 (33) | 0 (0) | 1 (33) |
| Quite a bit of a problem | 0 (0) | 0 (0) | 0 (0) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
| Hearing loss, n (%) | |||
| Not present/no problem | 2 (67) | 3 (60) | 3 (100) |
| Hardly a problem at all | 0 (0) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 1 (20) | 0 (0) |
| Moderate problem | 0 (0) | 1 (20) | 0 (0) |
| Quite a bit of a problem | 1 (33) | 0 (0) | 0 (0) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
| Speech impairment, n (%) | |||
| Not present/no problem | 2 (67) | 5 (100) | 3 (100) |
| Hardly a problem at all | 0 (0) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 0 (0) | 0 (0) |
| Moderate problem | 0 (0) | 0 (0) | 0 (0) |
| Quite a bit of a problem | 1 (33) | 0 (0) | 0 (0) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
| Emotional distress, n (%) | |||
| Not present/no problem | 2 (67) | 4 (80) | 2 (67) |
| Hardly a problem at all | 0 (0) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 1 (20) | 0 (0) |
| Moderate problem | 1 (33) | 0 (0) | 0 (0) |
| Quite a bit of a problem | 0 (0) | 0 (0) | 1 (33) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
| Activity limitations, n (%) | |||
| Not present/no problem | 2 (67) | 5 (100) | 3 (100) |
| Hardly a problem at all | 0 (0) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 0 (0) | 0 (0) |
| Moderate problem | 1 (33) | 0 (0) | 0 (0) |
| Quite a bit of a problem | 0 (0) | 0 (0) | 0 (0) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
| Caregiver concerns, n (%) | |||
| Not present/no problem | 1 (33) | 3 (60) | 1 (33) |
| Hardly a problem at all | 1 (33) | 0 (0) | 0 (0) |
| Somewhat of a problem | 0 (0) | 0 (0) | 1 (33) |
| Moderate problem | 0 (0) | 1 (20) | 1 (33) |
| Quite a bit of a problem | 1 (33) | 1 (20) | 0 (0) |
| Very much of a problem | 0 (0) | 0 (0) | 0 (0) |
| Extreme problem | 0 (0) | 0 (0) | 0 (0) |
Health economics
A total of 17 (77%) parents provided resource use information for the 2 weeks following recruitment. There was little resource use in any arm (Table 12) and, because of the small numbers, one patient in the immediate topical antibiotics arm contributed nearly all the 14-day resource use and costs for that arm (Table 13). Two out of the six patients who had a delayed prescription did not use antibiotics during the 14 days’ follow-up. Excluding these costs from the analysis reduced the trial medicine costs for this by one-third.
| Resource use | Treatment arm, mean (SD) | ||
|---|---|---|---|
| Immediate oral antibiotics (n = 4) | Delayed oral antibiotics (n = 6) | Immediate topical antibiotics (n = 7) | |
| Number of GP face-to-face appointments | 0.25 (0.50) | 0.33 (0.50) | 0 (0) |
| Number of GP telephone consultations | 0 | 0 | 0 |
| Number of practice nurse contacts | 0 | 0 | 0.14 (0.38) |
| Number of NHS 111 contacts | 0 | 0 | 0 |
| Number of A&E attendances | 0 | 0 | 0.14 (0.38) |
| Number of outpatient attendances | 0 | 0 | 0.29 (0.76) |
| Number of overnight stays in hospital | 0 | 0 | 0 |
| Number of prescriptions for paracetamol | 0 | 0 | 0.29 (0.49) |
| Number of prescriptions for ibuprofen | 0 | 0 | 0.14 (0.38) |
| Number of prescriptions for another painkiller | 0 | 0 | 0 |
| Number of prescriptions for other medication | 0.25 (0.50) | 0 | 0.14 (0.38) |
| Resource use | Treatment arm, mean (SD) | ||
|---|---|---|---|
| Immediate oral antibiotics (n = 4) | Delayed oral antibiotics (n = 6) | Immediate topical antibiotics (n = 7) | |
| GP | 9.81 (19.62) | 13.08 (20.26) | 0 (0) |
| Practice nurse | 0 | 0 | 1.55 (4.10) |
| Emergency department | 0 | 0 | 9.71 (25.70) |
| Outpatient | 0 | 0 | 30.57 (80.88) |
| Prescribed medications | 0.54 (1.07) | 0 | 1.42 (3.11) |
| Trial medicine | 1.93 (0) | 1.86 (0.18) | 5.45 (0.70) |
| Trial medicine, excluding unused prescription costs | 1.93 (0) | 1.29 (1.00) | 5.45 (0.70) |
| Total cost | 12.27 (20.69) | 14.93 (20.32) | 48.70 (108.73) |
| Total cost, excluding unused prescription costs | 12.27 (20.69) | 14.36 (20.03) | 48.70 (108.73) |
A case note review was conducted for 21 patients. As with the 14-day follow-up, there was little resource use between week 3 and month 3, and the small sample size means it is not meaningful to compare the costs between trial arms (Table 14).
| Resource use/cost | Treatment arm, mean (SD) | ||
|---|---|---|---|
| Immediate oral antibiotics (n = 4) | Delayed oral antibiotics (n = 6) | Immediate topical antibiotics (n = 7) | |
| Resource use | |||
| Number of hearing assessments | 0 | 0 | 0.10 (0.32) |
| Number of outpatient attendances | 0.25 (0.50) | 0.29 (0.76) | 0 (0) |
| Number of A&E attendances | 0.25 (0.50) | 0 | 0 |
| Number of overnight stays in hospital | 0 | 0 | 0 |
| Costs (£) | |||
| Hearing clinic | 0 | 0 | 8.40 (26.60) |
| Outpatient | 25.25 (50.50) | 28.86 (76.35) | 0 |
| Emergency department | 17.00 (34.00) | 0 | 0 |
| Total cost | 42.25 (50.61) | 28.86 (76.35) | 8.40 (26.60) |
Qualitative
Sixteen primary care staff were interviewed: nine GPs and seven non-clinical staff from recruiting and non-recruiting practices, including one practice that withdrew from the study (Table 15). Some of the GPs had experience of recruiting to the trial and some had experience of getting the TRANSFoRm platform software to work. All of the interviewed GPs were partners and research leads for their practice, with time in practice ranging from 4 to 33 years. The non-clinical staff included practice managers, practice IT leads, a research co-ordinator and a research nurse (with no clinical role), who had experience of installing the TRANSFoRm platform software and the processes involved in getting it to work.
| Staff role | Recruiting practices, examinations (n) | Non-recruiting practices (n) | Total (n) |
|---|---|---|---|
| GPs | 5 | 4 | 9 |
| Non-clinical staff | 2 | 5 | 7 |
| Total | 7 | 9 | 16 |
Nine parents (all mothers) were interviewed: seven mothers participated in the trial and two mothers declined to take part. The parent sample captures some diversity with respect to treatment arm, and child age and gender, but most parent interviewees were from more affluent areas rather than deprived areas (Table 16).
| Characteristic | Parents (n) |
|---|---|
| Treatment | |
| Immediate ear antibiotics | 2 |
| Delayed oral antibiotics | 3 |
| Immediate oral antibiotics | 2 |
| Home IMD | |
| 1 (most deprived) | 0 |
| 2 | 0 |
| 3 | 1 |
| 4 | 3 |
| 5 (most affluent) | 3 |
| Child’s gender | |
| Female | 3 |
| Male | 4 |
| Child’s age (years) | |
| < 5 | 3 |
| 5–11 | 3 |
| > 11 | 1 |
Eight key themes were developed from the analysis:
-
whether or not the trial addressed an important clinical question
-
clinicians’ views of training materials
-
frustrations of trying to get the TRANSFoRm platform to function
-
effects of the study on primary care practices
-
primary care IT context – key challenges
-
barriers to trial recruitment
-
experiences of trial recruitment
-
reasons for parents declining.
Findings are illustrated below using anonymised verbatim quotations; P denotes quotations from parents.
The trial addressed an important clinical question
General practitioners felt that the trial addressed an important clinical question that was relevant to their practice and to their patients. GPs described being unsure about when antibiotics were needed and (in different practices) facing pressure from parents to both prescribe and not prescribe antibiotics. GPs felt that evidence about the effectiveness of the treatment options would support better treatment and communication with parents:
I liked the fact that it’s something that we see a lot and, like I say, there’s always this kind of unsureness whether we should be giving these kids antibiotics or not.
GP08
Parents don’t want to give their children antibiotics unless they absolutely have to, so they are quite willing to try some alternatives.
GP01
[W]e do see a lot of children with ear problems and there is always quite a lot of pressure to provide antibiotics from parents for them . . . having either data that says ‘You don’t need to do it’, [or] giving topical drops worked . . . would have the advantage . . . But it’s currently an off-licence use. So if we could get evidence that that was more supportive of its use, then that would be really helpful for ongoing care for patients.
GP09
Normal practice varied between GPs, reflecting a lack of clarity in relation to optimal treatment. Different GPs described normal practice as watch and wait, immediate oral antibiotics and, in some cases, delayed antibiotics:
I think I’ve probably been more likely not to give anything actually, just watch and waiting.
GP02
. . . so, normally, if they’ve got ear discharge present then we would be starting, erm, immediate oral antibiotics.
GP06
. . . if they were completely systemically well and they’ve got a runny ear then I think there might be a bit more of a negotiation about ‘well, is it getting better’, you know, ‘do you want to hang on for a day or two and see what happens, do you want a delayed prescription’.
GP07
Views of treatment options also varied between GPs, and not all were in equipoise, as not all necessarily viewed the different treatment arms as equally valid. Some GPs liked the ciprofloxacin drops because they are applied topically and, therefore, the GPs believed, less likely to cause systemic side effects. Some were familiar with the use of ciprofloxacin drops with adult patients. One GP raised concerns about the risk of toxicity and another about how quickly patients would be able to access the ear drops. Those GPs whose normal practice was to prescribe immediate antibiotics were concerned about delaying antibiotic treatment. Several GPs expressed a desire for clear evidence about the best treatment, to both guide practice and ‘validate’ their practice ‘to the powers that be’ (GP02):
. . . personally, I think [ciprofloxacin drops] are good . . . I have seen patients with recurrent [infections] who have had treatment with antibiotic drops, on occasions recommended by ENT [ear, nose and throat] . . . so I am sort of aware of it as a practice and I’m sort of comfortable with that . . .
GP06
[I]f there was data that said actually giving topical drops worked, then that would have the advantage that you don’t have the systemic side effects.
GP09
I was always a little bit hesitant to use [ciprofloxacin drops] to be honest, just because of the risk of toxicity . . . generally, if I thought there was perforation I’d stay away from drops, generally.
GP03
[S]ometimes it’s a problem actually getting them, so sometimes we’ve sent them to the chemist and they’ve commented that the chemist hasn’t had them in stock and, therefore, we’ve been rung up to say, you know, we want an alternative and I think then we’ve gone to oral antibiotics again.
GP02
I think it’s risky because . . . my fear is, is that what if a patient can’t then get in and if they can’t re-present or if their situation’s changed and . . . so I just feel uncomfortable with this delayed concept.
GP04
. . . it validates to the sort of powers that be that actually antibiotics, topically, are indicated so we are treating appropriately . . . and we wouldn’t get the pressure to, you know, from the kind of prescribing committee, we’d be able to validate the fact that, actually, we are better treating this with something rather than nothing.
GP02
The parents who participated in the trial were happy with the treatment options. The topical ear drops were seen as a good option by both parents who did want and parents who did not want antibiotic treatment:
I’m quite intrigued, I quite [like] the idea of the ear drop antibiotics for ear infections, I think they [. . .] might work.
P02
I was happy to do it if it helps, but also because he’s had so many antibiotics and [I] was happy for him to not have them . . . to be involved in something which would almost limit the use of them. Because I have been worried about the amount that he’s had to have for his ears over the years. So, yeah, the idea that there might be another option eventually . . . I think that would be really good.
P05
I thought it was good. I did want her to have some kind of treatment, I didn’t want just to leave it because I think it had been like 5 days or something at this point . . . I thought . . . there was a two out of three chance that she get a medicine. So I thought that was probably a good idea and yeah it was really easy to do and I was really pleased. And I got the drops . . . it was really simple.
P04
Clinician views of training materials
Clinicians described the training videos as detailed and comprehensive, if quite long. The length of the training videos may have meant that fewer clinicians were willing to complete the training to become recruiters, particularly as the expected number of recruits per practice, and, therefore, the recruitment-based payment to the practice, was quite small. Because of delays due to difficulties in getting the TRANSFoRm platform software to work, some clinicians reported a significant period between completing the training and actually being in a position to recruit:
. . . they were very detailed and comprehensive, I seem to remember at least one of them was quite long . . . but they were good in terms of guiding me through both the study and the sort of use of the app[lication], so what I was doing was watching little bits of it and doing little bits . . . and then pausing the video rather than watching it in one whole lot, so I watched it in piecemeal fashion as I was going through, sort of thing . . . so it did work quite well.
GP06
[T]here were quite a few . . . anything like that is going to put off some people who don’t have protected time for research . . . in a practice, you’re expecting maybe five or six doctors . . . to be recruiting, then that is going to limit because, you know, we just don’t have the time to do that . . . the problem as well is that it’s not a high recruiting study so if, for example, the practice was going to be recruiting 20 or more patients, then it would be worth watching those videos because you would get, you know, more money for doing that sort of number, but I think that you’re only expected to get quite a small number per practice so you might watch videos and do one or two patients and then that . . . you know forget really.
GP01
. . . certainly between the time that I think the training for the online software, so the integrated software rather, and then the, erm, the time the study actually went live, it seemed to be a sort of significant gap.
GP07
Frustrations of trying to get the TRANSFoRm platform to function
General practitioners and non-clinical staff involved in installing the TRANSFoRm platform and getting it to work described a long and frustrating process of troubleshooting and multiple reinstallations. Many were initially keen on the idea of the TRANSFoRm platform, as they hoped it would make the recruitment process quicker and easier. However, participants described encountering multiple problems and spending a considerable amount of time troubleshooting, seeking support from the trial team and reinstalling the programme:
I think as an idea it’s brilliant . . . it means you haven’t got piles and piles of paperwork and . . . paper forms that you’ve then got to somehow get scanned to e-mail through . . . it self-populates . . . it puts the details in, which is time saving. ‘Cause time is one of the big things in general practice.
GP09
I thought it was really interesting, I was really excited and then it’s made me a bit despondent really.
GP02
[T]he software didn’t work the first time for us, so I had quite a lot of communication with [the trial team] . . . they’d telephone and speak on the phone about working through different ways of doing it . . . to get that to actually work was quite hard.
Practice data manager, ITA01
[T]o be brutally honest [. . .] it was quite a nightmare . . . I’ve probably spent about 10 hours in total trying to install the piece of software on one computer. Um, quite often there would be loads of errors with it installing, with it not working. Um, I’d then have to send e-mails to the people that were dealing with it . . . it’s definitely taken so much longer than what we thought it was going to take.
Practice operations manager, ITA02
[I]t’s been very frustrating . . . communication [with the trial team] has been very good, but the problem has been with the REST TRANSFoRm platform . . . upset’s not the word, but it has taken an awful lot of time, to the point where the practices are saying ‘we don’t want to do this’.
Research nurse, ITA03
The software had to be installed individually on each recruiting clinician’s computer, and limited access to these computers led to delays. It was difficult to find time with a clinical computer, particularly for the early installations, which took several hours. If troubleshooting was also needed, then finding a time when the clinical computer and someone from the trial team were available led to delays in addressing problems:
[T]he major problem, um, of the installation is actually getting time to get into the GP’s room . . . we’re really limited on space so if that GP isn’t in, there’ll be a locum in their room . . . from 8.00 in the morning ‘til 6.30 or 7 o’clock at night. So actually trying to get in to have . . . 2 hours . . . is virtually impossible.
Research nurse, ITA03
[T]he software was only being installed on one particular PC [personal computer] at our practice . . . The time we had when his room was available and getting IT involved was always quite tricky. . . . we’d have to come back to the office, e-mail, wait for a time when we could speak to somebody . . . then you’re reliant on having to go back into a clinician’s room to go in and . . . use their PC and . . . they are seeing patients all day, every day so you’re trying to combine your REST study person with me . . . and then the clinician that’s in the room, to try and combine the three . . . how long these things take.
ITA04
Those who were involved early in the study and who were, therefore, installing an early version of the software felt that it was not sufficiently developed for use in practices. Participants were concerned about the time it was taking away from normal duties and the potential risk posed to practice computers:
The problem is it was rolled out far . . . too early with far too many problems and hadn’t been tested widely enough.
GP09
It basically felt like it was a prototype; it weren’t like the finished product.
Practice operations manager, ITA02
. . . it has got certainly the admin staff and the practice managers to the point of saying ‘do we want this on our practice computers? . . . is it going to cause more problems?’ . . . we have not got the time to be spending installing, take hours out installing this platform . . . it’s a very difficult situation and I’m getting everyone whingeing at me.
Research nurse, ITA03
In addition, during the trial, there was a widespread transition to Windows 10 in primary care practices and this caused some problems. Practices that had installed the early version of the TRANSFoRm platform software reported that it did not work properly after the transition to Windows 10. It also made troubleshooting more difficult as the person in the practice was using a different version of Windows to that used by the trial staff providing technical support:
. . . halfway through signing up for the REST study all our computers were then transferred over to Windows 10, so that caused a bit of an issue apparently . . . [trial staff] said that . . . [the trial had] programmed all the software for Windows 7 . . . I think the knowledge on Windows 10 wasn’t there because their systems are Windows 7 . . . so, therefore, it was very difficult for me to tell them what wasn’t working on our system because of the difference in the systems.
Practice data manager, ITA01
. . . when we went onto Windows 10, which was after the first recruitment, it’s not really settled down . . .
GP02
Some participants reported that later versions of the software worked well. The IT support manager in a practice that installed the final version of the software described the installation process as quick and easy. A GP who had not been involved in the installation process felt that the final version of the TRANSFoRm platform software worked well and had found it straightforward to recruit patients:
And now it nearly works. As in as long as you do this trick to get it to load properly, it works.
GP09
I installed [it on GP and nurse] PCs and it went absolutely fine, there was no problems with it and it just, yeah, installed . . . literally minutes . . . it was absolutely fine. You know it was just pressing next, next, next and finish and, yeah, it was no problem at all.
IT support manager, ITA07
I didn’t really do the installing. That was done by our research nurse and my practice manager . . . I do think the actual computer recruiting system, I think, works really, really well.
GP08
In most practices, however, participants described the final version of the software as unreliable and unpredictable. Participants reported that it worked for dummy patients but not real ones, or for some patients or staff but not others, or in some sites but not others. Participants felt that they did not understand why the software appeared to work in only some circumstances and this sense of unpredictability contributed to a lack of confidence in the software:
I think one would follow all the instructions and it seemed to say, yes, it was working, erm, but then it wasn’t when you went into it the next time and lots of shutting down; you kept having to shut down Windows in order to try it again.
Research nurse, ITA06
[W]e tried doing a dummy patient this morning again and it seemed to open up, but only randomly . . . even if we have it set up, it’s going to disappear again and my admin person comes down and sets it all up again and she can’t get it to work intermittently either.
GP02
[I]t just wasn’t working on [eligible patient] . . . but it was working on most [patients] that we tried. I have no idea why.
Research nurse, ITA06
[E]ventually . . . we were able to use it, but the functionality of what the REST study app[lication] was meant to be doing wasn’t happening . . . when [GP] was trying to, like, put in the code on SystmOne, the pop-up was meant to appear . . . and apparently that pop-up was never coming up, even though it was running in the background . . . On some days, it would be behind; other days it wouldn’t come up at all . . . no apparent reason.
Practice operations manager, ITA02
I still have this background lack of confidence that its going to if I needed to.
Research Nurse, ITA06
Effects of the study on primary care practices
The biggest impact of the study was the time taken to get the TRANSFoRm platform working in the already very time-pressured context of primary care. This had an impact on the other work taking place at the general practices, including preparing data for the Quality and Outcomes Framework (QOF) and, in one case, seeing patients. When the time commitment started to impede essential work, then practices started to consider withdrawing from the study:
I mean, all of the surgeries, all of the, all of the admin staff, practice managers, the secretaries . . . GPs, there’s not a spare minute in primary care at the moment . . . Research in primary care, I think we’re, I think we’re struggling a bit . . . There’s such a burden on, on GP time for major problems that research . . . the GPs would look at the studies and say, ‘yeah, I don’t have time to do this’.
Research nurse, ITA03
I’ve spent literally hours on this trying to install the software, hours. I think our clinicians have said that, you know, enough’s enough. They don’t want me to spend any more time on it . . . they [GP partners] just [got] cross ‘cause I wasn’t doing other things . . . I do all the QOF stuff so all the quality registers and things and all the statistics, all the claims . . . all that sort of was a bit on hold really.
Data manager, IT01
At one point, I started to refuse to install stuff because we were having so many problems with what it was doing to our computers . . . We wanted to wait until they’d got it more sorted because it was just eating so much time . . . it was probably towards the end of the QOF year last year . . . that I said I wasn’t prepared to put it back on until after we finished the QOF year because I couldn’t risk the machines not working.
GP09
When practices had staff with protected research time, participation in the project was more possible. In most of the practices, the recruiting clinician was a research lead with protected time for research. Some of the practices had other research support staff, including research nurses and, in a highly research-active practice cluster, a research co-ordinator:
. . . had I not been doing [a role with protected time for research] I would not have had the time to persevere and so the trial might not have been able to recruit . . . from my practice . . . it was . . . 4, maybe 5 hours that I spent . . . I do have protected time to do it so it does enable me to do things in a slightly different way, which I don’t think might be sort of rolled out for other practices . . . if you think they’ve just got to fit it in in their lunch breaks.
GP01
. . . one of them that tried a lot was our research lead . . . the other [clinicians] have basically really given up. I can’t get their computers to work now.
Data manager, IT01
Some participants reported financial costs to the practice as a result of participating in the study. At least one had paid their IT support person for extra hours to try to get the TRANSFoRm platform to work. Several participants felt that financial support to practices did not compensate for the time spent on research activities for this study:
I’ve worked extra time to do it as well. I’ve, they’ve actually paid me extra to come in and do the REST software, so I think that’s sort of annoyed them a little bit.
Data manager, IT01
[W]e’ve asked about, erm, resources for the amount of time that it spent us to install this software and provide the feedback that they’ve asked for and answered the questions they’ve asked for . . . the practice has actually lost quite a lot of money in terms of their time by trying to engage with this and that just puts people off . . . we do expect our costs to be covered. We do research ’cos we’re interested in research. If we were interested in making money, we would do pharma, but we don’t. But we are interested in not making a loss on it and not making a significant loss on it, which is what we’ve done with this.
GP09
Primary care information technology context: key challenges
Participants identified challenges with practice, CCG and NHS IT that contributed to the difficulties in getting the TRANSFoRm platform to function.
At the practice level, there was limited IT capacity and expertise. There were issues with outdated hardware and software and with the way in which individual GPs had adjusted SystmOne settings. There was varied IT expertise and capacity in individual practices, with many reliant on a GP, manager or administrator with only modest knowledge of IT. Computer administrator rights (which are needed to install software) were usually restricted to a small number of staff and not necessarily those with the time or responsibility for setting up research studies. This meant that, in many practices, the person tasked with undertaking the work to get the TRANSFoRm platform to function often struggled with the tasks and understanding the various problems encountered:
[The TRANSFoRm platform is] designed for really up-to-date computer systems and primary care is running on . . . old equipment and it just couldn’t cope with it . . . not only is it old, but it’s very old [and] not all on the same Windows.
Research nurse, ITA06
[T]he user guide was relatively comprehensive, but I would say not written for a user . . . that had basic IT skills. I think what they were asking for [was] somebody who was fairly IT literate to install the software, which I certainly wasn’t . . . We don’t have a particular IT person that can just go along and install . . . this sort of software.
Assistant practice manager, ITA04
[W]e are a fairly small practice . . . it was pretty much . . . me on my own . . . just trying to go through the installation step by step to work out where it wasn’t working and then trying to work out why, so trial and error.
GP06
[W]e’re not all IT proficient and that’s the problem . . . large practices, they do [have IT expertise] . . . but they don’t necessarily have the admin rights . . . these [small] practices don’t [have IT expertise]. So it’s the practice manager or . . . the secretary or someone who does it.
Research nurse, ITA03
. . . we didn’t locally have full admin rights. Well, the practice manager did, but, you know, to get her to sit down for a couple of hours and set it all up was very difficult; she didn’t have a couple of hours.
Research co-ordinator, ITA05
There were issues with obtaining help from outsourced CCG-funded IT support. All practices had some IT support provided by an external body, sometimes a private provider and sometimes a CCG or NHS provider. Five different providers were mentioned by our small number of participants, and IT support arrangements varied with respect to whether the external body held exclusive administrator rights for practice computers or supported research IT. Several practices reported that their external IT support provider would not assist because the software was not on the CCG’s approved list. When they were asked to provide support, these external bodies often raised concerns about the unknown TRANSFoRm platform software, were usually unfamiliar with software for research projects and were slow in providing support because of limited capacity. Only a research co-ordinator at a very research-active practice reported getting help easily, which she attributed to a good relationship with a particular person:
[W]e’re not sort of in charge of our own IT; the IT goes out to another company . . . our IT people who are [company name A], they’re not really supposed to give admin rights to anybody in a practice . . . [company name A] will not get involved with other people’s software . . . they have a list of software that’s allowed on the system and if we’re going to put some other software onto it they will not support us installing that software.
Practice data manager, ITA01
[W]e do have [company name C], but I didn’t get them involved in it . . . ‘cause . . . [company name C] wouldn’t help with it anyway . . . because it hadn’t been signed off by our CCG so we shouldn’t be installing it on our computers.
Operations manager, ITA02
[T]here’s been problems and we’ve had to go to our CCG IT team. And I’m just looking at an e-mail that’s come in this morning and their IT team are saying ‘what is this application? Is it a trusted . . .?’
Research nurse, ITA03
. . . [company name D] so they’re IT, they’re all NHS staff, but they’re a helpline, so you ring them, erm, with an IT queries . . . they were very dubious, actually, [about the TRANSFoRm platform] . . . I’m on, I think, my third or fourth call now with our IT about it. [It] wasn’t always easy to try and co-ordinate them ringing with then trying to install the software.
Assistant practice manager, ITA04
Some practices reported that they had to obtain permission from their CCG before installing software on their practice computers. The transition to Windows 10 during the trial was linked to the loss of practice-level administrator rights over computers in some CCG areas. Whether or not practices retained some administrator rights over their computers (and, therefore, the ability to install software) varied across recruited practices. When practices had to obtain permission from their CCG to install software, this could be a lengthy process. CCGs had questions about the risk that this unknown software could corrupt NHS software or practice computers and concerns about patient data crossing the NHS firewall. The centralisation of management to the CCG was seen as supporting initiatives such as the single domain, which allows better sharing of patient notes between different types of practitioners in primary care. However, it also had the unintended consequence of restricting the installation of study-specific software. Although some practices appeared to be able to install software freely, most reported having to defer to the CCG and wait for their approval. One practice reported that their CCG had required modification of the software to increase data security before permission was given:
[W]e had a big change at our practice, erm, something called single domain, which basically means that they’ve taken a lot of admin rights away from a lot of the users, including me . . . ‘cause I think it was becoming problematic across the practices that, you know, we had free rein really. And that’s going to cause a problem with things like REST because we can’t install it; so you give us a set of instructions and we won’t be able to do it because it has to go to our localised IT who has to verify they’re OK with it first.
Assistant practice manager, ITA04
[T]he CCG took it upon themselves to be responsible for all of our hardware and software, so when Windows 10 came for the whole of the CCG, they then took charge of everything really, which in a way makes sense because they paid for it and therefore they should control it and the flow of information that’s available and try to link it all up with other bits of the NHS, but as a result things . . . fell by the wayside, unfortunately.
GP05
. . . because of the way that the NHS is set-up we had to get firewalls opened, which wasn’t something we were told about in the beginning, to enable the software to contact [trial database] and then also for them to contact back through to our software; so, basically, you had to go through the firewall through a different port. And because we, we’re not sort of in charge of our own IT, the IT goes out to another company, so that was quite complicated at the beginning, having to go through these firewalls by logging it with our IT and then our IT doing it and that took a while. So that was the first sort of big issue we came across.
Data manager, ITA01
I think [the CCG] are quite – lax might be the wrong word – but we can install software and we do install software. So we’ve installed software for other research studies with no problems.
GP09
. . . we were all set to go and then we . . . needed to change our operating system . . . [to] Windows 10 so the original . . . [the TRANSFoRm platform] downloads would no longer work . . . to get them to rework . . . we couldn’t do it ourselves any more, we had to get the CCG computer boffins in to do it for us, they didn’t want to do it because they said its software may corrupt the NHS software and they wanted more assurance from higher levels than me that it was all safe to go, so . . . I got cross the told them it was all, it had been approved at high level . . . co-ordinated at committee level and approved and was being used elsewhere and they shouldn’t be so silly . . . so they then did come and put it on for me, so it’s now up and running.
GP05 – with role in CCG
[I] installed the software once I had permission from the CCG and that took [from] July/August . . . until December . . . the CCG felt they hadn’t got enough information from the installation guys . . . to be satisfied about the security aspect of the data that’s being transferred . . . the [study] IT engineers [tested] an encryption patch . . . [and] in December 2019, it was approved for installation . . . it’s just checking the security side of things, just make sure we’re not going to get any viruses . . . it’s about data protection; you know, they want to make sure that no patient identifiable data is going to be sent over for the studies.
IT support manager, IT07
Barriers to trial recruitment
Trial recruitment was reduced or slowed by the problems with the TRANSFoRm platform software. Several practices discussed identifying eligible patients but not being able to recruit them to the study because the software did not work properly. In some practices, there was a long delay while staff struggled to get the TRANSFoRm platform to work before clinicians even started trying to recruit patients. Some practices decided to withdraw from the study before any patients had been recruited because of the time taken to try to make the software work:
. . . last month there’s been four we’ve missed and all because the software just does not open up . . . I had someone in front of me on Thursday whose mom was really interested in doing it. I couldn’t even do a decline, and we just couldn’t get it to work and I tried, honestly, I must have spent about 20 minutes, I think, trying to sort it out and I had to move on really.
GP02
We’ve missed recruiting patients to the study because we couldn’t get the software working. We’ve had them in the consulting room agreeing to do the study, but we just couldn’t get the software to work so, therefore . . . we’ve had to abandon it. So there was a possibility of at least seven or eight that we couldn’t get because I’ve gone in and sat with the clinicians as well to try and get the software to work . . . it’s not that we’re not wanting to; it’s not that we’re not trying. It’s the software that’s not working . . .
Data manager, ITA01
I think that’s probably why there’s no recruitment, because the actual software itself wasn’t doing what they needed to do and then if they’re in an appointment they obviously don’t have the time to figure it out so they just move on to the next patient. . . . the doctors . . . they don’t have the knowledge to quickly figure things like that out . . . especially if it’s software that they don’t usually use.
Operations manager, ITA02
[W]e’ve taken a massively long time to recruit four patients . . . we should have done eight in a third of the time – probably would have done if the software worked.
Research co-ordinator, ITA05
Eligible patients were sometimes missed because they were not seen by a clinician who was able to recruit to REST. The processes for channelling eligible patients towards a recruiting clinician differed across practices. This was partly due to differing processes for dealing with same-day appointment requests for acute illness. Some practices had minor illness nurses who would usually see all children with suspected ear infections. Some practices triaged all patients requesting same-day appointments, and those patients might be seen by one or two duty clinicians or any clinician with a free slot. Study recruitment problems arose when these normal processes channelled a potentially eligible patient towards a clinician who was not able to recruit to the study. Administration staff and triaging clinicians responsible for booking patients into appointment slots did not always remember to book potentially eligible patients with the few recruiting clinicians. Where an eligible patient was seen by a non-recruiting clinician, there were various ad hoc arrangements to redirect patients to recruiting clinicians, but these were probably impracticable, and there were no accounts of this happening in practice:
[W]e’ve got minor illness nurses and the minor illness nurses don’t really get involved in research as much . . . the problem is that if someone rings up with a cough or cold, all that kind of stuff, they are being put into the minor illness slot . . . we tried to actually put REST study slots into my clinic so that if anybody rings up with an earache . . . put them in here, but . . . I’ve had those slots up and running and I think it’s not even been used once . . . but we know that these guys are coming through because they have gone and seen the nursing team, yeah?
GP03
. . . reception are made aware that if they have a child with a runny ear to try and book them in with myself or the other GP in question, where possible; where not though, and this has happened . . . a couple of times, that someone else [sees an eligible patient], they send me an instant screen message to say . . . ‘do you want to see them’ . . . and then depending on . . . how my appointments are looking, I might say ‘yes that’s fine’, or I might say . . . ‘could you see patient Y for me instead?’ and then we sort of switch patients, if you see what I mean . . .
GP06
. . . whoever’s doing surgeries will have some appointments added on at the end for same day so we don’t do triage or anything else . . . the nurses will do the same . . . they might see . . . children with coughs and colds . . . the receptionists have an aide memoire to say any child with . . . earache of the right age and they’ve had any discharge at any time that they should be booked in with me.
GP05
We can’t do too much opportunistically, I mean people coming to urgent care, they struggle to get an appointment in the first place . . . our advanced practitioner might not be on the delegation log, but one of my GPs is, but they’re seeing somebody else.
ITA05
Some eligible patients were missed because recruiting clinicians were not available when the patient needed to be seen. Some recruiting clinicians worked part time and were able to protect certain time slots only, which did not necessarily coincide with when patients needed to be seen. A participant from a large, recently merged practice described the challenge of keeping triaging clinicians primed to recruit to the study when they faced long lists and multiple study reminders, and were not even sure of being able to book a patient in with a recruiting clinician:
I think there may have been a couple of kids with discharge who might have seen other GPs. You know, ‘cause I only work part time.
GP08
. . . nothing came in in the morning, and then I had a full-booked surgery in the afternoon . . . somebody came in in the afternoon . . . they came in at 4.30 which is . . . right in the middle of my surgery that was running late anyway . . . nothing’s come in this morning; I had protected time this morning . . .
GP01
. . . now we’ve merged, we’ve got 25 GPs, six partners, but . . . lots are part time . . . so trying to keep it at the forefront of their mind, those images on the computer screen that they have got them every morning, in a way you just get used to them being there, you don’t actually look at them . . . they come and face an absolutely hideous list, phone calls every morning . . . there were 2 days when there wouldn’t have been a slot, if they had remembered, they couldn’t have found a slot, which [. . .] probably make them think ‘oh, well I actually remembered and then I couldn’t book them in’.
ITA06
The number of clinicians in each practice who could recruit to the study was limited, and this contributed to the number of missed recruits. There seemed to be several reasons for the limited number of clinician recruiters per practice. In many practices, only the research lead and perhaps one other clinician completed the training to become a recruiter to minimise the training burden on practice staff. It is likely that the time it took to install the TRANSFoRm platform also contributed to practices installing it on a limited number of computers only:
I could recruit; none of the others did the training on the basis that it was most likely that the [trained] nurses would do it . . . when you’re not recruiting massive numbers, if you have too many people able to recruit, nobody does it very often, so it just takes everybody ages.
GP09
What we can’t do is install it on everybody’s system . . . we’ve got about 20 studies we might be doing at one time so you have to pick and choose your staff of who’s going to be involved.
ITA05
[W]e have it installed on mine and the other GP . . . who has the research experience . . . and the administrative machine; it’s not running on the other clinician’s machine . . . so not everyone’s recruiting, just the sort of people who know about the study.
GP06
The nature of the target patients also presented some barriers to recruitment. Acute infections are seasonal and by the time the TRANSFoRm platform software was working in most practices, the winter season, when these patients are more common, was over. Cases that fitted the REST inclusion criteria were relatively rare. One research nurse described studies evaluating a new medication for acutely unwell children who attend with a parent (and often other siblings) as one of the hardest studies to recruit to:
[I]n the summer, we don’t see that many; in the winter, probably one to two a week . . . as a surgery . . . I’m trying to think, probably about two a week, something like that.
GP02
. . . it started at a time in summer where there wasn’t very much happening [. . .] and I think that’s taken a lot of momentum away . . .
GP03
. . . anything that involves a child that’s unwell, the parents have got other children with them, you know, younger children . . . children’s studies are harder straight off ’cause the parent and the child are involved rather than just an adult and if it’s a child who’s already a bit anxious then that makes it harder; so I think the children’s ones, where its opportunistic recruitment are probably the harder ones we do . . . and if it involves medication, its more complicated straight off. I suppose it is one of the hardest scale of ones that we do in general practice. I’m afraid our current issue is time.
ITA06
Experiences of trial recruitment
When clinicians had successfully recruited, this seemed to be associated with parent interest in the study and consultations with ‘relaxed’ mothers of relatively well children who had time for recruitment. Clinicians reported positive responses to the novel treatment (ciprofloxacin ear drops) and to the study processes:
. . . well, parents were happy that something was being looked at . . . those parents that we’ve seen . . . it’s not . . . the first time they’ve been in; they’re coming in regularly with the same child saying ear pain and discharge . . . And some of them are very reluctant to have antibiotics, but all of them want some help. You know, they all seemed to like the idea of trying topical drops.
Research co-ordinator, ITA05
. . . both of [the recruited children] were . . . it made it easier . . . both children were actually incredibly well, but there was loads of gunk coming out of their ears, erm, and they both had very laid-back mums . . . I said to both of them, you know, ‘there’s this trial going on looking at the different treatments, obviously I will examine your child and if I disagree with what you’re randomised to, you know, we can decide not to go along with it’ . . . I think [the first mum] really liked the follow-up that she had with the trial team . . . all the leaflets with the advice and things like that . . . I think it was helped that they were relaxed mums and they obviously weren’t in a massive hurry and – yeah. It made it easier.
GP08
. . . mum was pretty excited to be part of it so I think that was really quite nice . . . She was randomised to cipro . . . the ear drops . . . She was fine with it, I think the patient information leaflet was very good, you know, so that was excellent.
GP03
Recruited parents described study processes as straightforward. They had a clear understanding of the study purpose and found it easy to provide follow-up data:
I think everything was really well explained and the actual medicine that she got was really easy to use; the questionnaire was really simple; it was really easy to send stuff back. No, it was absolutely fine.
P04
. . . the nurse did explain to me as much as she could but [child] was quite . . . unsettled . . . it was a bit, erm, difficult to kind of grab all the information that the nurse had to give me, but the pack was quite comprehensive . . . it did have quite a lot of information and leaflets about what the study was about so I had the chance to read a bit about it when I got home so, and I had a call from one of the ladies involved in the study as well pretty much the next day . . . which was good because then you kind of get their reassurance of the study itself.
P07
I would have been happy with . . . any of [the treatment options], because they said if you’d have had nothing then if he started to get a . . . higher temperature or got really unwell then they would . . . look at him again . . . if he became really unwell. So they said that, as it was at the moment, he was happy for him to either have antibiotics or to not have it . . . They said at any time I could sort of take him back in and have him checked.
P05
Reasons for parents declining
Of the two parent decliners who participated in an interview, one declined because she wanted her child to have antibiotic ear drop treatment and one because she did not want her child to have antibiotics. In the former case, the child had a history of ear problems and, as a result of this experience, the parent perceived ear drop antibiotics as more effective than oral antibiotics for her son’s ear infections. In the latter case, the parent was aware of the drive to reduce unnecessary antibiotics, was told by the recruiting clinician that he would not normally give antibiotics for her child’s symptoms and she did not want her child to take something that was not needed. Recruiting clinicians described parents declining to participate because they did not want to have to:
[H]e’s had some ongoing problems with his ears . . . about a year ago, he had like a runny ear for quite a long time and he went on an oral antibiotic and it didn’t clear it up. And it carried on for probably about 2 months where . . . when I went back to the doctors and they found in his records, I think from the ENT, that he should have the drops in his ear. And when he did have the drops, it cleared it up within about a week, so when they asked me to do the, you know, be part of the study they said it was a randomised, where they just leave it or have the drops or have the antibiotics. And because I knew that that had been really effective with him last time and I didn’t want it to drag on for a few months like it did last time, I just asked if they would give the drops . . . I think if it had been my daughter, who’s not had any ear problems, you know, or recommendations about what she needed, I definitely would have done the whole study.
PD01
. . . the reason that I didn’t want to participate in the study because if there was no study she wouldn’t have got any medication for it at all. So it did seem a bit odd that . . . if she participated in the study then she’d be given antibiotics. I thought the idea was that you only get antibiotics when you really, really need them . . . The doctor had said that, at this moment, she didn’t need anything . . . I didn’t want her taking something.
PD02
. . . we did start to use the software and go through some of it, sort of, and then I showed them the leaflets about it and mom just started to look more and more doubtful about the whole thing as we went on, so she showed some initial interest and sort of said ‘yeah, OK, maybe I’d be interested in that’ and then as we talked about it and what she might have to do in terms of having a phone call, doing a sort of diary and things like that, she just looked more and more doubtful about it, to the point where you thought ‘no she really doesn’t want to do this’ and I sort of said ‘look, . . . it is up to you, you don’t have to’ and she said ‘no, I think probably not’, so it was a more slow sort of build to her saying ‘no I’m not interested in it’.
GP07
Chapter 4 Challenges and recommendations
This chapter summarises the key study challenges and lessons learned. These are relevant to many primary care studies, but particularly those involving large numbers of sites and those intending to use (or develop for use) electronic trial platforms. The lessons learned are presented as ‘recommendations for future practice’ and grouped by those responsible for the activities of site identification, site set-up, site training, platform development, platform installation, troubleshooting, platform function monitoring and data management. Finally, there are two recommendations for national stakeholders, including the Department of Health and Social Care (DHSC) and NIHR.
Clinical Research Network co-ordination of site recruitment
From a study perspective, CRN facilitation of site set-up was frustrating. The CRNs were reluctant to tell the study team which practices had been invited and how many times, and there was little feedback regarding why practices did not wish to participate. The study team was clear that there was no prerequisite for sites to be ‘research active’, as the TRANSFoRm platform software was intended to guide even novice recruiters through recruitment. Indeed, for some studies, recruiting from research-naive sites could be important to ensure the generalisability of the final study sample. 79 In this case, the team sensed that the CRNs were approaching research-active sites only.
Recommendation
-
Clinical Research Networks (CRNs) should keep logs of which sites have been invited, when and how many times. These should be shared with study teams to populate CONSORT flow diagrams and allow a description of the generalisability of the recruiting sites.
Site set-up
We worked closely with the sponsor to use risk-adapted site set-up and study conduct approaches, aiming to reduce the burden on general practices. Despite this, there were still long delays in securing the required documentation from the general practices, particularly the delegation logs and curricula vitae, for which the sponsor required wet ink signatures. Despite clear instructions, these were regularly incomplete or incorrect, with signatures missing. As staff were inevitably busy, having to return a document because of a small mistake often resulted in several days/weeks of delays, and the paperwork was often forgotten and needed to be chased. This added to the workload of the study team, who were often chasing multiple sites for various documents/amendments to documents, and prevented the site from receiving the green light from the sponsor.
The study team kept a spreadsheet record of all documents received from each site within each of the 15 CRNs. A portable document format (PDF)/Microsoft Word (Microsoft Corporation) copy of each document was stored electronically in dedicated site folders. Although this system worked to a degree, a study of this size may have been better employing a more robust electronic document management system that could highlight missing or wrong documentation. This would have made it easier for the study team to chase sites.
Recommendations
-
Sponsors should consider accepting electronic versions of delegation logs with electronic signatures. These should be designed so that submission of incomplete logs/curricula vitae is not possible.
-
For large distributed trials with many sites, a robust electronic data management system to track documentation should be employed.
Site training
Because of the number of sites expected to be needed for REST, it was decided that online training (see Appendix 1) would be a more appropriate and efficient way of carrying out study training. It was predicted that busy GPs and nurses would complete the training at times that were convenient to them, and that this would expedite opening to recruitment. Unfortunately, this did not prove to be as an efficient method as hoped because clinician time is not ring-fenced for remote training as it is for face-to-face training, and staff sometimes took weeks or months to complete online training. Without the face-to-face engagement, it was difficult to encourage site staff (some of whom the study team had not worked with previously) to complete the training in a timely manner. With more studies employing remote training mechanisms, research-active general practices need to be encouraged to allow time for staff to complete remote training.
Recommendation
-
When online site training is used, studies should provide training using a website that provides automated reminders and notifies the sponsor and study team when training is complete.
Electronic platform
Development
The TRANSFoRm platform was a novel electronic platform that was considered necessary for the REST trial as most general practices would see only a few cases of AOMd each season. The vision was that the system would ‘take the hand of the clinician’ and guide them quickly through eligibility, randomisation, baseline data collection and randomisation, all of which would be integrated within the EHR to autopopulate relevant data, thereby saving time, minimising data entry and minimising data entry errors. The platform was considered key to facilitating recruitment and was designed to support many sites, reducing the burden on the trial team and increasing the quality of the data.
In the event, we seriously underestimated the variety of computer configurations in English general practices, the complexity of IT support arrangements, the difficulties of integrating and ensuring smooth platform function, and the resources required to overcome these obstacles. These led to significant delays to the first general practice opening to recruitment, delays in recruiting further general practices, delays in site set-up and, ultimately, reduced participant recruitment.
The failure of the TRANSFoRm platform to provide data regarding which sites were and were not managing to recruit meant that the study team was virtually blind to, and unable to support, site recruitment activity. We have minimal information regarding the frequency of pop-up triggers (see Table 3) and know of only 30 children being invited to participate (see Figure 6). Compared with our recruitment assumptions (see Table 1), 30 invitations would have resulted in two or three children recruited. We are aware of instances of potentially eligible children not being recruited because the TRANSFoRm platform was not available. Practice staff found this frustrating, with some sites withdrawing from the study.
Detailed platform specifications were drafted (see Detailed TRANSFoRm technical specification for REST v1.4; available from study authors on request). Some were specific to REST, but some could be applied to other studies. For example, some were intended to provide estimations of the generalisability of the final trial sample in relation to the characteristics of potentially eligible children invited but declining. Some of the TRANSFoRm platform’s software components had incompatibilities with certain versions of the Windows operating system, and this made automated updating of the TRANSFoRm platform’s DNC difficult. Other software, such as EHR software, would be updated without prior warning, with updates proving incompatible with the TRANSFoRm platform’s software.
Recommendations
-
Electronic trial platforms should be used to harness the unprecedented opportunities to monitor, measure and test recruitment assumptions, identifying where in the recruitment process, from presentation to consent, the key ‘drop-offs’ occur.
-
All necessary platform preparatory activities and required resources should be clearly defined, taking care not to underestimate.
-
The skills needed to set up a trial platform and to set up a trial are distinct and complementary. Ideally, teams should be co-located to ensure that platform specifications meet individual trial requirements.
-
Platform software needs to be compatible with all practice software systems.
-
Closer integration with EHR providers would prevent incompatible updates (note that this could be obviated if national criteria were agreed or if the trial platform was integral to the EHR).
Installation
Owing to the lack of a standardised set of security requirements for software in general practices, some providers requested further assurances from NHS Digital (Leeds, UK) and for additional security features to be added to the system. We engaged with NHS Digital and NHSX (Leeds/London, UK), and they confirmed that they consider our software to be safe for installation, but declared that any detailed technical audit was outside their remit and was the responsibility of EHR providers. This required us to negotiate approval with the EHR providers, The Phoenix Partnership (TPP; Leeds, UK) and Egton Medical Information Systems (EMIS) Web (EMIS Health, Leeds, UK).
Although IT support is formally handled by the CCGs, it is often outsourced to independent third parties. The installation of software such as the TRANSFoRm platform is typically not covered by the contracts governing these outsourced relationships, and the criteria for establishing the safety of new software prior to practice installation were not clear. Some third parties quoted prohibitively high costs to support installation. These issues came to light only after the practices were recruited. The REST team worked hard to obtain CCG approvals from many areas, but some approvals took months, with key areas such as the Nottinghamshire Health Informatics Service (NHIS) Change Advisory Board (CAB) and the Devon CCG arriving too late.
Recommendation
-
Project teams need to work closely with EHR providers and CCGs from the study outset to agree on the software deployment process and the validation criteria required (note that this could be obviated if national criteria were agreed).
In addition to CCG approval, installation agreement was part of the site agreement, meaning that national- and CCG-level approvals were required before practices could sign the recruitment contract, which included identifying the site personnel involved, machines and training.
Recommendations
-
A pilot installation incapable of being used for recruitment (and, therefore, not a site agreement requirement) should be performed on one computer in each practice, tested, and left to run for a week, before the software is installed on other machines.
-
Where software reinstallation is required, it must be undertaken in a way that does not disrupt the work of the practice.
Troubleshooting
To try to assist the under-resourced TRANSFoRm team (based in London), some troubleshooting tasks were reassigned to the RCT team (based in Bristol). However, this was less effective than was hoped because the Bristol team did not have the IT skills and experience to efficiently address the challenges, leading to further delays.
Recommendations
-
Electronic study platforms require teams dedicated to (1) development and (2) troubleshooting.
-
Careful consideration should be given to who is responsible for troubleshooting. Although it may seem obvious that this would be performed by the trial team (as it involves interacting with sites), it requires awareness of platform function and, therefore, may be better provided by the platform development team.
Monitoring function
No one in the team anticipated the importance of a dashboard reporting real-time platform functionality. This can be likened to the real-time status reporting of the London Underground tube system. 72 Much of the time, neither the TRANSFoRm team nor the trial team were aware that the platform had stopped working, either across all sites or at specific sites. Even now, we cannot estimate the proportion of time during which, or the proportion of practices at which, a fully functioning platform was able to facilitate recruitment. Functionality could be threatened for a number of reasons, including problems with and updates for the TRANSFoRm platform, the host EHR system, the Windows operating system or practice network configurations.
Recommendation
-
Electronic trial platforms would be best served by a dashboard function to monitor and log platform functionality in real-time, providing real-time alerts and diagnostics for reduced function, and logging functionality across time and space.
Data management
At the project outset, no agreement was made on the format and types of data to be sent from the study database to the trial team.
Recommendation
-
The format of the final data set to be extracted from the study database should be prespecified to ensure appropriate data format and avoid the submission of linked clinical and personal data.
National stakeholders, including the Department of Health and Social Care and the National Institute for Health Research
Many of the above challenges and solutions may be better addressed on a national level by a single co-ordinated management and implementation group. This would be particularly helpful for the NHS IT-related challenges, as NHS IT will continue adapting to meet future NHS needs and, unless research is considered as part of these changes, future software study platform developers could find it similarly difficult to ‘bolt on and hold on’ software to these changes.
We consider that the key national stakeholders should include the DHSC; NHS Digital; NHSX; NIHR; senior researchers with trial and observational study experience; EHR providers, such as EMIS Web and TPP; the Medicines and Healthcare products Regulatory Agency; Clinical Practice Research Datalink; and GP IT Futures framework. 80
The prize is rich and the opportunity clear: to lead the world in the delivery of pragmatic research that quickly and efficiently develops highly generalisable new knowledge to improve patient care. The onset of the SARS-CoV-2 pandemic perfectly illustrated the need for and value of infrastructure ready to quickly respond to the changing health needs of the UK population.
In our view, the NIHR and other funders need to be part of the solution, but cannot solve it alone. We are delighted that the NIHR HTA accepted our argument that REST needed to be underpinned by an electronic research platform, but in doing so it took a risk to support the time needed to develop the platform. We are deeply regretful that we were not able to fulfil this ambition.
Recommendations
-
Research funders need to formally recognise the potential of electronic study platforms if they wish to put the NHS on the leading edge of pragmatic research globally, allowing the delivery of new, near-real-time generalisable knowledge. This could also provide unprecedented opportunities to monitor, measure and test recruitment assumptions, identifying where in the recruitment process, from presentation to consent, the key ‘drop-offs’ that influence final study sample representativeness occur.
-
NIHR and research funders should consider convening a meeting of national stakeholders to define a strategy for the development, implementation and ongoing management of electronic study platform software.
Trial management
The TMG and co-chief investigators were aware throughout the study of the delays to the delivery of the TRANSFoRm platform, despite repeated reassurances that the platform was almost ready. The co-chief investigators found it challenging to manage these delays, particularly given the academic nature of the collaboration with the partners responsible for platform delivery, and the need to maintain good working relationships. Although we feel that good relationships were maintained, we reflected with our host (Bristol, North Somerset and South Gloucestershire CCG) and the sponsor (University of Bristol) on how to manage delays in future studies. This meeting resulted in the host proactively communicating their role of enforcing contractual obligations to all their hosted studies, which includes engagement with project managers between management meetings and the creation of the CCG Contractor Escalation Guidance document (see CCG Contractor Escalation Policy; available on request from study authors), which explains and guides the assistance that the host can provide to chief investigators and project managers with underperforming collaborators.
Chapter 5 Discussion
Summary of main findings
Trial
To our knowledge, this is the first time a clinical trial of an investigational medicinal product has been conducted using an electronic trial platform in the UK. We planned to establish 175 sites to recruit 399 children. By study closure, 122 general practices from 12 CRNs had expressed an interest in supporting the study, of which 71 confirmed participation; 61 received sponsorship; 44 opened to recruitment, with the TRANSFoRm platform installed on 72 clinical computers; and seven sites randomised 22 children.
Although the main reason for poor recruitment was the delayed and intermittent functioning of the electronic platform, the number of AOMd presentations was also smaller than usual seasonally adjusted averages, contributing an estimated 25% to under-recruitment.
Despite the ‘hands-off’ nature of recruitment, randomisation and the use of standard NHS FP10 prescriptions for treatment, baseline data were available for 21 (95%) children, all children were given treatment as randomised, 38% of children fully adhered to the treatment and the symptom-based primary outcome was available at the 14-day follow-up for 17 (77%) children. The number recruited was too small to perform comparative analyses and draw definitive conclusions regarding clinical effectiveness, cost-effectiveness or safety, but we observed that all symptoms (including the primary outcome) except one were of shorter duration in the immediate topical arm than in the immediate oral antibiotic arm, and that parent satisfaction with treatment was higher in the immediate topical antibiotics arm than in the immediate oral antibiotic arm.
Electronic trial platform
Delays in the set-up and functionality of the TRANSFoRm platform function led to a cycle of increasing challenges resulting in critically low recruitment and early trial closure. Key performance challenges included (1) underestimating the technical challenge of integrating platform and EHR software; (2) underestimating the resources required to troubleshoot the resulting problems; (3) the need for repeated platform reinstallations at the sites, sometimes due to unannounced changes to EHR software; (4) multiple and complex site IT security arrangements, often involving third parties without contracts covering research; (5) failure to foresee the need for a platform ‘dashboard’ function, resulting in the TMG being unaware when the platform was/was not functional; and (6) site staff’s progressively reduced motivation to reinstall and use the software. When the electronic trial platform was operational, clinicians reported strongly liking its features and also reported that it assisted recruitment as intended; however, the function of the platform was acknowledged as ‘too little, too late’.
Qualitative
Qualitative clinician interviews found that the trial addressed a question of importance to clinicians and parents, and that, when the platform functioned as intended, it was liked. However, site staff reported the software not working properly for long periods, resulting in potentially eligible patients being missed. Moreover, getting the software to work placed significant burdens on general practices, diverting staff time from core activities.
The IT arrangements in primary care practices were varied and changing, with limited capacity, expertise and support. Although some practices had employed an IT expert, many relied on non-experts who had limited knowledge or training to provide internal IT support. This limited IT expertise within practices made it difficult for them to work with the software development team to diagnose and solve problems with the software. External IT support, which was provided by experts, was often not available for research. Administrator rights over practice computers, which were needed to install the TRANSFoRm platform, were not always held by staff within practices. Practice IT arrangements changed during the trial so that practices that did have administrator rights for the first installation of the TRANSFoRm platform did not have administration rights for subsequent installations and had to apply to their CCG for permission and support. Some of the changes to the practice IT arrangements were driven by new legislation, such as the General Data Protection Regulation,81 and initiatives to allow better integration of primary care medical record systems with others, such as out-of-hours services. The changes made in service of those broader NHS objectives restricted the use of specialist project software on practice computers.
Strength and weaknesses
Trial
To our knowledge, this is the first RCT to investigate the clinical effectiveness and cost-effectiveness of immediate topical antibiotics or delayed oral antibiotics compared with immediate oral antibiotics (standard care) for children with AOMd without grommets, and one of the first to attempt this using an integrated electronic trial platform. The qualitative evaluation provided rich contextual evidence regarding the advantages of and problems with the TRANSFoRm platform.
The over-riding weakness was the failure to recruit enough children to address the research question. That said, when functioning, the electronic trial platform did assist with within-consultation recruitment, demonstrating that it (1) can work and (2) has multiple advantages.
First, the system did support the identification, baseline assessment and consenting of children. Clinicians provided treatment as randomised for all children and most parents of recruited children provided symptom duration data. One child with a visible tympanic membrane was recruited, but should have been excluded. This cannot be attributed to the trial platform, but could have been prevented if the platform alerted the clinician to the presence of an exclusion criterion. The recruitment reminder ‘pop-ups’ were triggered by Read diagnostic codes, but would have been more sensitive had we also used Read symptom codes.
Second, as previously discussed, an electronic platform could provide unprecedented evidence regarding the generalisability of the final sample, support the recruitment of a very large number of ‘real-world’ pragmatic studies, improve baseline and follow-up data entry efficiency and accuracy, and ensure that the studies meet the many regulatory requirements.
Although the main outcome data attrition rates were acceptable, we note the low rate of stool collection at day 14 and the poor recording of analgesic use. We are also aware that one child who was recruited had otorrhoea that was possibly related to a foreign body. This should have been an exclusion criterion. Regarding treatment fidelity, there were high levels of agreement with allocated arms and no crossover between arms. Although the level of adherence appears low, this is of less concern when participants are randomised to a treatment strategy in an open trial because adherence (i.e. antibiotic use) is likely to mirror actual management in real-world settings. It is, for instance, recognised that the levels of antibiotic use are higher when the prescription is supplied during a consultation and when following a delayed strategy outside the trial context. 78,82
Qualitative
The small number of recruited parents and the early shut-down of the study because of the COVID-19 (coronavirus disease 2019) lockdown constrained recruitment to the qualitative study. However, sufficient ‘information power’ was achieved for the core themes presented. 75 The qualitative interviews captured a range of views from clinicians and other primary care staff involved in the study, which could be undertaken in a relatively small sample because of the specificity of experiences. 75 It was possible to purposively sample clinicians and non-clinicians from recruiting and non-recruiting practices, which captured a good range of views and experiences with respect to the trial. The primary care staff also described considerable variety in terms of the IT arrangements for practices, although it seems likely that this study does not capture the full range of variation in UK general practice IT arrangements.
The parent sample captured experiences from all three arms of the trial and a range of experiences of children’s ear infections. It also captured a range of views from parents from medium-to-affluent neighbourhoods, but did not capture views of parents from more deprived areas. Although the experience of being involved in the trial is quite specific, which means that a small sample can still provide information power, we recognise that the experiences of parents from deprived areas may be different and we do not know how well the experiences of our sample represent the experiences of parents from these areas. If larger numbers of parents had been recruited to the trial, it could have been possible to conduct purposive sampling of parents and capture a greater range of experiences, particularly those from more deprived areas.
Results in the context of other research
There is strong evidence that children with AOMd benefit from immediate oral antibiotics,2 but, to our knowledge, there is an absence of evidence regarding clinical effectiveness and economic implications of immediate topical antibiotics and delayed oral antibiotics for AOMd in children without grommets tubes. REST has a sister trial, called PLOTS (Pijnlijk LoopOor Therapie Studie), which is still running in the Netherlands, that is investigating the role of a combined topical antibiotic and steroid preparation for children with AOMd without grommets. 68 This study has yet to publish a report. Although the non-inferiority design and eligibility criteria are similar for PLOTS and REST, the studies are complementary with regard to primary outcomes, offering the prospect of meta-analysis using the REST results.
The number of children recruited was too small for definitive comment, but we note that our sample was older [median age 5 years (IQR 2–7 years)] than the median age of 3 years (IQR 1–5 years) reported by Smith et al. 3 in an observational study investigating the natural history of AOMd in children presenting to UK primary care. We also note that there was a predominance of boys (62%) in our sample.
Thirty-eight per cent of the parents fully adhered to the treatment as prescribed. Sixty-six per cent of children who were prescribed immediate (oral or topical) antibiotics started them within 24 hours of randomisation, compared with 50% in the delayed oral antibiotic arm, which suggests that delaying advice had only marginal effects on parental behaviour.
The RCGP Research & Surveillance Centre’s Weekly Returns Service83 provides weekly notifications for communicable and respiratory disease for England graphically. For AOM, markedly smaller numbers were evident compared with the 5-year average. Over the period when REST was actively recruiting, numbers were reduced by between one-quarter and half compared with other years. We, therefore, estimate that at least one-quarter of the shortfall of recruited patients (compared with those projected) could, accordingly, be explained by this unusual drop in relevant infections.
Implications
Acute otitis media with discharge research
The clinical and research communities should wait for PLOTS to publish its report, and for any REST–PLOTS data syntheses to be completed before deciding whether or not sufficient evidence is available to change the management of children with AOMd, and whether or not further research investigating the clinical effectiveness and cost-effectiveness of immediate topical antibiotics and delayed oral antibiotics for children with AOMd is necessary.
If this question remains unanswered, the NIHR and research community will need to consider whether or not a further REST-type study is feasible. We remain convinced that the most efficient way to conduct this study would rely on a functioning electronic trial platform. Efforts should, therefore, be focused on establishing this facility, not only for this research question, but for the wider research community.
Recommendations arising from lessons learned
These are grouped by those responsible for the following activities: site identification, site set-up, site training, platform development, platform installation, troubleshooting, platform function monitoring and data management. Finally, there are two recommendations for national stakeholders, including DHSC and NIHR.
The National Institute for Health Research Clinical Research Network
-
CRNs could keep logs of which sites have been invited, when and how many times. These could be shared with study teams to populate CONSORT flow diagrams and allow a description of the generalisability of the recruiting sites.
Sponsors
-
Sponsors could consider accepting electronic versions of delegation logs with electronic signatures. These could be designed so that submission of incomplete logs/curricula vitae is not possible.
-
For large distributed trials with many sites, a robust electronic data management system to track documentation could be employed.
Trial management teams
-
When online site training is used, studies could provide training using a website that provides automated reminders and notifies the sponsor and study team when training is complete.
Electronic study platform
Developers
-
Electronic trial platforms could be used to harness the unprecedented opportunities to monitor, measure and test recruitment assumptions, identifying where in the recruitment process, from presentation to consent, the key ‘drop-offs’ occur.
-
All necessary platform preparatory activities and required resources could be clearly defined, taking care not to underestimate.
-
The skills needed to set up a trial platform and to set up a trial are distinct and complementary. Ideally, teams could be co-located to ensure that platform specifications meet individual trial requirements.
-
Platform software needs to be compatible with all practice software systems.
-
Closer integration with EHR providers could prevent incompatible updates (note that this could be obviated if national criteria were agreed or the trial platform was integral to the EHR).
Installers
-
Project teams need to work closely with EHR providers and CCGs from the study outset to agree on the software deployment process and the validation criteria required (note that this could be obviated if national criteria were agreed or the trial platform was integral to the EHR).
-
A pilot install incapable of being used for recruitment (and, therefore, not a site agreement requirement) could be performed on one computer in each practice, tested, and left to run for a week, before installing software on other machines.
-
Where software reinstallation is required, it must be undertaken in a way which does not disrupt the work of the practice.
Troubleshooters
-
Electronic study platforms require teams dedicated to (1) development and (2) troubleshooting.
-
Careful consideration could be given to who is responsible for troubleshooting. Although it may seem obvious that this is would be performed by the trial team (as it involves interacting with sites), it requires awareness of platform function and, therefore, may be better provided by the platform development team.
Function monitoring
-
Electronic trial platforms could be best served by a dashboard function to monitor and log platform functionality in real time, providing real-time alerts and diagnostics for reduced function, and logging functionality across time and space.
Data management
-
The format of the final data set to be extracted from the study database could be prespecified to ensure appropriate data format and avoid the submission of linked clinical and personal data.
National stakeholders, including the Department of Health and Social Care and the National Institute for Health Research
-
Research funders need to formally recognise the potential of electronic study platforms if they wish to put the NHS on the leading edge of pragmatic research globally, allowing the delivery of new, near-real-time generalisable knowledge. This could also provide unprecedented opportunities to monitor, measure and test recruitment assumptions, identifying where in the recruitment process, from presentation to consent, the key ‘drop-offs’ that influence final study sample representativeness occur.
-
NIHR and research funders could consider convening a meeting of national stakeholders to define a strategy for the development, implementation and ongoing management of electronic study platform software.
Conclusions
We are unable to comment on treatment effects because of the insufficient number of participants recruited. We were also unable to establish the feasibility of running a platform-supported pragmatic trial for AOMd in primary care. The late development and intermittent functioning of the TRANSFoRm platform within the SystmOne EHR system resulted in the small recruitment numbers, failure to reach the required sample size and inability to answer the main research question. Our experience has highlighted the technical issues that need to be overcome before electronic trial platform technology should be adopted in the primary care setting.
We have carefully documented our experience and presented clear recommendations in the hope that they will be used by the DHSC, NIHR and wider research community. The prize is rich and the opportunity clear: to lead the world in the delivery of pragmatic research that quickly and efficiently produces generalisable new knowledge to improve patient care. The onset of the SARS-CoV-2 pandemic perfectly illustrated the need for and value of infrastructure ready to quickly respond to the changing health needs of the UK population.
Acknowledgements
Trial Steering and Data Monitoring Committees
We wish to thank Professors Jonathan Mant and Toby Prevost (chairpersons) and Dr Gail Hayward, Dr Fiona Warren, Dr Fiona Hamilton, Catherine Hamilton, Hayley Dash and Professor Christian Mallen.
Individuals
The authors wish to thank the following collaborators for their advice and support: Professor Chris Butler, Professor Nick Francis, Dr Beth Stuart and Professor Desmond A Nunez.
We wish to thank the sponsor and CCG representatives: Rachel Davies, Adam Taylor, Katalin Bagi and Paul Roy.
We also wish to thank Aideen Ahern (junior health economist), Jennifer Bostock (lay reviewer for comments on the Plain English summary), Karen Coulman (PPI advice), Harriet Downing (supported original grant application), Ioana Fodor (for medicines advice), Sarah Gallagher (provided advice on nursing elements), Dr Claire Hombersley (for enthusiastically supporting platform troubleshooting), Martin House (for database advice), Chris Metcalfe (for methodological advice) and Alan Noel (for taking over from Andrew Lovering in overseeing microbiological elements).
The TMG agreed authorship based on the following principles:
-
Authors must meet the criteria set out by the International Committee of Medical Journal Editors Recommendations. 84
-
Chief investigators are the joint lead authors.
-
Lead authors for other sections are to be put second, third, fourth, etc. (in the order of where their sections appear).
-
The remaining authors commenting on sections are to be added in alphabetical order (by surname).
Medicines for Childrens Network
We would like to thank the medicines for children network for allowing us to use their leaflets on otitis media as part of the patient information for the trial.
Contributions of authors
Alastair D Hay (https://orcid.org/0000-0003-3012-375X) (Joint Chief Investigator) had overall responsibility for leading the TMG in the design, scientific integrity, delivery, safety and publication of the trial, on time and within budget. He also drafted the Abstract, Scientific summary, and Chapters 4 and 5; oversaw Chapter 3; and reviewed the final draft of the report.
Michael V Moore (https://orcid.org/0000-0002-5127-4509) (Joint Chief Investigator) had overall responsibility for leading the TMG in the design, scientific integrity, delivery, safety and publication of the trial, on time and within budget. He also drafted the Plain English summary, oversaw Chapters 1 and 2 and reviewed the final draft of the report.
Jodi Taylor (https://orcid.org/0000-0001-7171-8923) (Senior BRTC Trial Manager) was responsible for the trial managers and BRTC staff, oversaw Sue Harris, Kate Rowley and Annie Sadoo in drafting the methods and reviewed the final draft of the report.
Nicholas Turner (https://orcid.org/0000-0003-1591-6997) (Trial Statistician) had oversight of clinical data collection and clinical data analysis, drafted the quantitative results and reviewed the final draft of the report.
Sian Noble (https://orcid.org/0000-0002-8011-0722) (Senior Health Economist) had oversight of health economics data collection, health economics data analysis and writing, and reviewed the final draft of the report.
Christie Cabral (https://orcid.org/0000-0002-9884-0555) (Co-investigator) oversaw the design, conduct and reporting of the qualitative study and reviewed the final draft of the report.
Jeremy Horwood (https://orcid.org/0000-0001-7092-4960) (Lead Qualitative Researcher) oversaw the design, conduct and reporting of the qualitative study and reviewed the final draft of the report.
Vibhore Prasad (https://orcid.org/0000-0001-5470-276X) (Co-investigator) oversaw the trial design; Nottingham, East Midlands and East Coast recruitment; and proofread and reviewed the final draft of the report.
Kathryn Curtis (https://orcid.org/0000-0001-5367-9182) (Trial Manager) had day-to-day responsibility for trial management and helped draft Chapter 2.
Brendan Delaney (https://orcid.org/0000-0002-3518-0131) (Co-investigator) was responsible for the trial design and the TRANSFoRm platform functionality within REST, and reviewed the final draft of the report.
Roger Damoiseaux (https://orcid.org/0000-0001-8052-0302) (Collaborator) was a ZonMw-funded PLOTS (sister) trial investigator, was involved in the trial design and results interpretation, and reviewed the final draft of the report.
Jesús Domínguez (https://orcid.org/0000-0002-7372-315X) (Research Fellow) was the lead TRANSFoRm developer.
Archana Tapuria (https://orcid.org/0000-0003-3074-9832) defined the eligibility codes in the study system, created data collection forms and authored the user guide.
Sue Harris (https://orcid.org/0000-0002-9177-7987) (Trial Research Nurse) was responsible for clinical input to trial paperwork and parent follow up, and helped draft Chapter 2.
Paul Little (https://orcid.org/0000-0003-3664-1873) (Co-investigator) was responsible for the trial design and results interpretation, and reviewed the final draft of the report.
Andrew Lovering (https://orcid.org/0000-0002-7573-3220) (Co-investigator) was responsible for the trial design (microbiological elements) and results interpretation, and reviewed the final draft of the report.
Richard Morris (https://orcid.org/0000-0001-7240-4563) (Senior Statistician) provided senior statistical and methodology expertise, was involved in the trial design and results interpretation, and reviewed the final draft of the report.
Kate Rowley (https://orcid.org/0000-0003-4388-5569) (Trial Co-ordinator) was responsible for trial co-ordination and helped draft Chapter 2.
Annie Sadoo (https://orcid.org/0000-0001-5129-4596) (Trial Administrator) was responsible for trial administration and helped draft Chapter 2.
Anne Schilder (https://orcid.org/0000-0002-5496-4580) (Co-investigator) provided otolaryngology expertise, was involved in the trial design and North London CRN recruitment, and reviewed the final draft of the report.
Roderick Venekamp (https://orcid.org/0000-0002-1446-9614) (Collaborator) was a ZonMw-funded PLOTS (sister) trial investigator, was involved in the trial design and results interpretation, and reviewed the final draft of the report.
Scott Wilkes (https://orcid.org/0000-0003-2949-7711) (Co-investigator) was responsible for the trial design and North East and North West CRN recruitment.
Vasa Curcin (https://orcid.org/0000-0002-8308-2886) (Co-investigator) was the TRANSFoRm platform design and development lead, wrote the first draft of the TRANSFoRm elements and reviewed the final draft of the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Little P, Gould C, Williamson I, Moore M, Warner G, Dunleavey J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001;322:336-42. https://doi.org/10.1136/bmj.322.7282.336.
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006;368:1429-35. https://doi.org/10.1016/S0140-6736(06)69606-2.
- Smith L, Ewings P, Smith C, Thompson M, Harnden A, Mant D. Ear discharge in children presenting with acute otitis media: observational study from UK general practice. Br J Gen Pract 2010;60:101-5. https://doi.org/10.3399/bjgp10X483148.
- Hollinghurst S, Gorst C, Fahey T, Hay AD. Measuring the financial burden of acute cough in pre-school children: a cost of illness study. BMC Fam Pract 2008;9. https://doi.org/10.1186/1471-2296-9-10.
- Hollinghurst S, Redmond N, Costelloe C, Montgomery A, Fletcher M, Peters TJ, et al. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): economic evaluation of a randomised controlled trial. BMJ 2008;337. https://doi.org/10.1136/bmj.a1490.
- Wolleswinkel-van den Bosch JH, Stolk EA, Francois M, Gasparini R, Brosa M. The health care burden and societal impact of acute otitis media in seven European countries: results of an internet survey. Vaccine 2010;28:G39-52. https://doi.org/10.1016/j.vaccine.2010.06.014.
- Hay AD, Heron J, Ness A. ALSPAC study team . The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract 2005;22:367-74. https://doi.org/10.1093/fampra/cmi035.
- Williamson I, Benge S, Mullee M, Little P. Consultations for middle ear disease, antibiotic prescribing and risk factors for reattendance: a case-linked cohort study. Br J Gen Pract 2006;56:170-5.
- Finkelstein JA, Metlay JP, Davis RL, Rifas-Shiman SL, Dowell SF, Platt R. Antimicrobial use in defined populations of infants and young children. Arch Pediatr Adolesc Med 2000;154:395-400. https://doi.org/10.1001/archpedi.154.4.395.
- Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 2005;55:603-8.
- Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health 2004;26:268-74. https://doi.org/10.1093/pubmed/fdh160.
- National Institute for Health and Care Excellence (NICE) . Otitis Media (Acute): Antimicrobial Prescribing Guidance 2018. www.nice.org.uk/guidance/ng91 (accessed 14 September 2021).
- National Institute for Health and Care Excellence . BNF for Children 2020 n.d. https://bnfc.nice.org.uk/ (accessed 1 November 2020).
- Kaji AH, Lewis RJ. Noninferiority trials: is a new treatment almost as effective as another?. JAMA 2015;313:2371-2. https://doi.org/10.1001/jama.2015.6645.
- Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997;314:722-7. https://doi.org/10.1136/bmj.314.7082.722.
- Department of Health and Social Care . UK Five Year Antimicrobial Resistance Strategy 2013 to 2018 n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf (accessed October 2021).
- Barber C, Ille S, Vergison A, Coates H. Acute otitis media in young children – what do parents say?. Int J Pediatr Otorhinolaryngol 2014;78:300-6. https://doi.org/10.1016/j.ijporl.2013.11.030.
- van Dongen TM, van der Heijden GJ, Venekamp RP, Rovers MM, Schilder AG. A trial of treatment for acute otorrhea in children with tympanostomy tubes. N Engl J Med 2014;370:723-33. https://doi.org/10.1056/NEJMoa1301630.
- van Dongen TM, Schilder AG, Venekamp RP, de Wit GA, van der Heijden GJ. Cost-effectiveness of treatment of acute otorrhea in children with tympanostomy tubes. Pediatrics 2015;135:e1182-9. https://doi.org/10.1542/peds.2014-3141.
- Venekamp RP, Prasad V, Hay AD. Are topical antibiotics an alternative to oral antibiotics for children with acute otitis media and ear discharge?. BMJ 2016;352. https://doi.org/10.1136/bmj.i308.
- Bryce A, Costelloe C, Hawcroft C, Wootton M, Hay AD. Faecal carriage of antibiotic resistant Escherichia coli in asymptomatic children and associations with primary care antibiotic prescribing: a systematic review and meta-analysis. BMC Infect Dis 2016;16. https://doi.org/10.1186/s12879-016-1697-6.
- Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ 2016;352. https://doi.org/10.1136/bmj.i939.
- Little P, Rumsby K, Kelly J, Watson L, Moore M, Warner G, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA 2005;293:3029-35. https://doi.org/10.1001/jama.293.24.3029.
- Royal College of General Practitioners . Research and Surveillance Centre: Weekly Returns Service Annual Report 2011 2011.
- van Dongen TM, Schilder AG, Manders LA, van der Veen EL, van der Heijden GJ. Good agreement between parents and physician in the assessment of ear discharge in children. Pediatr Infect Dis J 2012;31:868-9. https://doi.org/10.1097/INF.0b013e318258f100.
- Watson L, Little P, Moore M, Warner G, Williamson I. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract 2001;18:553-4. https://doi.org/10.1093/fampra/18.5.553.
- Hay AD, Costelloe C, Redmond NM, Montgomery AA, Fletcher M, Hollinghurst S, et al. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial. BMJ 2008;337. https://doi.org/10.1136/bmj.a1302.
- Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract 2003;20:696-705. https://doi.org/10.1093/fampra/cmg613.
- Hay AD, Sterne JA, Hood K, Little P, Delaney B, Hollingworth W, et al. Improving the diagnosis and treatment of urinary tract infection in young children in primary care: results from the DUTY prospective diagnostic cohort study. Ann Fam Med 2016;14:325-36. https://doi.org/10.1370/afm.1954.
- Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD. TARGET Programme Team . Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013;347. https://doi.org/10.1136/bmj.f7027.
- Delaney BC, Curcin V, Andreasson A, Arvanitis TN, Bastiaens H, Corrigan D, et al. Translational medicine and patient safety in Europe: TRANSFoRm – architecture for the learning health system in Europe. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/961526.
- Dakin H, Petrou S, Haggard M, Benge S, Williamson I. Mapping analyses to estimate health utilities based on responses to the OM8-30 Otitis Media Questionnaire. Qual Life Res 2010;19:65-80. https://doi.org/10.1007/s11136-009-9558-z.
- Williamson I, Vennik J, Harnden A, Voysey M, Perera R, Kelly S, et al. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: an open randomized controlled trial. CMAJ 2015;187:961-9. https://doi.org/10.1503/cmaj.141608.
- Williamson I, Vennik J, Harnden A, Voysey M, Perera R, Breen M, et al. An open randomised study of autoinflation in 4- to 11-year-old school children with otitis media with effusion in primary care. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19720.
- Ethier JF, Curcin V, McGilchrist MM, Choi Keung SNL, Zhao L, Andreasson A, et al. eSource for clinical trials: implementation and evaluation of a standards-based approach in a real world trial. Int J Med Inform 2017;106:17-24. https://doi.org/10.1016/j.ijmedinf.2017.06.006.
- Integrating the Healthcare Enterprise n.d. www.ihe.net (accessed 27 October 2021).
- Bain L. CDISC Journal: Healthcare Link and eSource. Austin, TX: Clinical Data Interchange Standards Consortium; 2011.
- Ethier JF, Dameron O, Curcin V, McGilchrist MM, Verheij RA, Arvanitis TN, et al. A unified structural/terminological interoperability framework based on LexEVS: application to TRANSFoRm. J Am Med Inform Assoc 2013;20:986-94. https://doi.org/10.1136/amiajnl-2012-001312.
- Kush R, Alschuler L, Ruggeri R, Cassells S, Gupta N, Bain L, et al. Implementing single source: the STARBRITE proof-of-concept study. J Am Med Inform Assoc 2007;14:662-73. https://doi.org/10.1197/jamia.M2157.
- Fiore LD, Brophy M, Ferguson RE, D’Avolio L, Hermos JA, Lew RA, et al. A point-of-care clinical trial comparing insulin administered using a sliding scale versus a weight-based regimen. Clin Trials 2011;8:183-95. https://doi.org/10.1177/1740774510395635.
- Fiore M, Palassini E, Fumagalli E, Pilotti S, Tamborini E, Stacchiotti S, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45. https://doi.org/10.1016/j.ejso.2008.11.005.
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290:1624-32. https://doi.org/10.1001/jama.290.12.1624.
- Staa TP, Goldacre B, Gulliford M, Cassell J, Pirmohamed M, Taweel A, et al. Pragmatic randomised trials using routine electronic health records: putting them to the test. BMJ 2012;344. https://doi.org/10.1136/bmj.e55.
- Vestbo J, Leather D, Diar Bakerly N, New J, Gibson JM, McCorkindale S, et al. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med 2016;375:1253-60. https://doi.org/10.1056/NEJMoa1608033.
- Grossmann C, Powers B, McGinnis JM. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care: Workshop Series Summary. Washington, DC: The National Academies Press; 2011.
- Food and Drug Administration . Guidance for Industry: Electronic Source Data in Clinical Investigations 2013.
- Grossman C, Powers B, McGinnis JM. Digital Infrastructure for the Learning Health System: The Foundations for Continuous Improvement in Health and Health Care. Washington, DC: The National Academies Press; 2011.
- Bierer BE, Li R, Barnes M, Sim I. A global, neutral platform for sharing trial data. N Engl J Med 2016;374:2411-13. https://doi.org/10.1056/NEJMp1605348.
- Ngune I, Jiwa M, Dadich A, Lotriet J, Sriram D. Effective recruitment strategies in primary care research: a systematic review. Qual Prim Care 2012;20:115-23.
- Marchisio P, Esposito S, Picca M, Baggi E, Terranova L, Orenti A, et al. Prospective evaluation of the aetiology of actute otitis media with spontaneous tympanic membrance perforation. Clin Microbiol Infect 2017;23:486.e1-6. https://doi.org/10.1016/j.cmi.2017.01.010.
- BNF for Children 2017–18. London: BMJ Group, Pharmaceutical Press, and RCPCH Publications; 2017.
- Ohyama M, Furuta S, Ueno K, Katsuda K, Nobori T, Kiyota R, et al. Ofloxacin otic solution in patients with otitis media: an analysis of drug concentrations. Arch Otolaryngol Head Neck Surg 1999;125:337-40. https://doi.org/10.1001/archotol.125.3.337.
- German EJ, Hurst MA, Wood D. Reliability of drop size from multi-dose eye drop bottles: is it cause for concern?. Eye 1999;13:93-100. https://doi.org/10.1038/eye.1999.17.
- Weber PC, Roland PS, Hannley M, Friedman R, Manolidis S, Matz G, et al. The development of antibiotic resistant organisms with the use of ototopical medications. Otolaryngol Head Neck Surg 2004;130:S89-94. https://doi.org/10.1016/j.otohns.2003.12.009.
- Roland PS, Rybak L, Hannley M, Matz G, Stewart MG, Manolidis S, et al. Animal ototoxicity of topical antibiotics and the relevance to clinical treatment of human subjects. Otolaryngol Head Neck Surg 2004;130:S57-78. https://doi.org/10.1016/j.otohns.2003.12.008.
- Kaplan DM, Hehar SS, Bance ML, Rutka JA. Intentional ablation of vestibular function using commercially available topical gentamicin-betamethasone eardrops in patients with Meniere’s disease: further evidence for topical eardrop ototoxicity. Laryngoscope 2002;112:689-95. https://doi.org/10.1097/00005537-200204000-00018.
- Elstner M, Schmidt C, Zingler VC, Prokisch H, Bettecken T, Elson JL, et al. Mitochondrial 12S rRNA susceptibility mutations in aminoglycoside-associated and idiopathic bilateral vestibulopathy. Biochem Biophys Res Commun 2008;377:379-83. https://doi.org/10.1016/j.bbrc.2008.09.134.
- Phillips JS, Yung MW, Burton MJ, Swan IR. Evidence review and ENT-UK consensus report for the use of aminoglycoside-containing ear drops in the presence of an open middle ear. Clin Otolaryngol 2007;32:330-6. https://doi.org/10.1111/j.1749-4486.2007.01532.x.
- Joint Formulary Committee . British National Formulary n.d.:2017-18.
- Roland PS, Anon JB, Moe RD, Conroy PJ, Wall GM, Dupre SJ, et al. Topical ciprofloxacin/dexamethasone is superior to ciprofloxacin alone in pediatric patients with acute otitis media and otorrhea through tympanostomy tubes. Laryngoscope 2003;113:2116-22. https://doi.org/10.1097/00005537-200312000-00011.
- Roland PS, Kreisler LS, Reese B, Anon JB, Lanier B, Conroy PJ, et al. Topical ciprofloxacin/dexamethasone otic suspension is superior to ofloxacin otic solution in the treatment of children with acute otitis media with otorrhea through tympanostomy tubes. Pediatrics 2004;113:e40-6. https://doi.org/10.1542/peds.113.1.e40.
- Spektor Z, Pumarola F, Ismail K, Lanier B, Hussain I, Ansley J, et al. Efficacy and safety of ciprofloxacin plus fluocinolone in otitis media with tympanostomy tubes in pediatric patients: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2017;143:341-9. https://doi.org/10.1001/jamaoto.2016.3537.
- Yazici A, Naiboglu B, Oysu C, Toros SZ, Noseri H, Karaca CT, et al. Effect of ototopical ciprofloxacin-dexamethasone on myringotomy in a rat model. Int J Pediatr Otorhinolaryngol 2009;73:301-5. https://doi.org/10.1016/j.ijporl.2008.10.021.
- Hebda PA, Yuksel S, Dohar JE. Effects of ciprofloxacin-dexamethasone on myringotomy wound healing. Laryngoscope 2007;117:522-8. https://doi.org/10.1097/MLG.0b013e31802f3c86.
- European Medicines Agency . Quinolone- and Fluoroquinolone-Containing Medicinal Products n.d. https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed October 2021).
- Hay AD, Redmond NM, Turnbull S, Christensen H, Thornton H, Little P, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902-10. https://doi.org/10.1016/S2213-2600(16)30223-5.
- Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028-35. https://doi.org/10.1161/CIRCULATIONAHA.108.768986.
- Netherlands Trial Register . Trial NL6535 (NTR6723): Is Treatment With Eardrops As Good As Oral Treatment for Children With Acute Otitis Media Presenting With Ear Discharge? n.d. www.trialregister.nl/trial/6535 (accessed 27 October 2021).
- Curtis K, Moore M, Cabral C, Curcin V, Horwood J, Morris R, et al. A multi-centre, pragmatic, three-arm, individually randomised, non-inferiority, open trial to compare immediate orally administered, immediate topically administered or delayed orally administered antibiotics for acute otitis media with discharge in children: the Runny Ear STudy (REST): study protocol. Trials 2020;21. https://doi.org/10.1186/s13063-020-04419-7.
- NHS Digital . Numbers of Patients Registered at a GP Practice – July 2015 2015. https://digital.nhs.uk/data-and-information/publications/statistical/patients-registered-at-a-gp-practice/july-2015 (accessed 14 September 2021).
- Kay R, Kinnersley N. On the use of the accelerated failure time model as an alternative to the proportional hazards model in the treatment of time to event data: a case study in influenza. Therapeutic Innovation and Regulatory Science 2002;36:571-9. https://doi.org/10.1177/009286150203600312.
- Transport for London . Status Updates: Tube, Overground, TfL Rail, DLR &Amp; Tram n.d. https://tfl.gov.uk/tube-dlr-overground/status/ (accessed 16 September 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- NHS England . National Cost Collection for the NHS 2020 n.d. www.england.nhs.uk/national-cost-collection/ (accessed 17 September 2021).
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60. https://doi.org/10.1177/1049732315617444.
- Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3. https://doi.org/10.1136/bmj.311.6999.251.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Little P, Moore M, Kelly J, Williamson I, Leydon G, McDermott L, et al. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g1606.
- Blair PS, Turnbull S, Ingram J, Redmond N, Lucas PJ, Cabral C, et al. Feasibility cluster randomised controlled trial of a within-consultation intervention to reduce antibiotic prescribing for children presenting to primary care with acute respiratory tract infection and cough. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014506.
- NHS Digital . GP IT Futures Systems and Services n.d. https://digital.nhs.uk/services/gp-it-futures-systems (accessed 15 October 2021).
- European Parliament . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation), OJ 2016 L 119 1 2016.
- Marchetti F, Ronfani L, Nibali SC, Tamburlini G. Italian Study Group on Acute Otitis Media . Delayed prescription may reduce the use of antibiotics for acute otitis media: a prospective observational study in primary care. Arch Pediatr Adolesc Med 2005;159:679-84. https://doi.org/10.1001/archpedi.159.7.679.
- Royal College of General Practitioners . RSC Communicable and Respiratory Disease Report for England. 2020. www.rcgp.org.uk/-/media/Files/CIRC/WeeklyReport_Summer_wk32_2020.ashx (accessed 11 June 2021).
- ICMJE . Defining the Role of Authors and Contributors n.d. www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html (accessed 15 October 2021).
Appendix 1 Website links to study introduction and training videos
The study introduction video can be accessed at the following URL [reproduced with permission from Hay and Moore (2021)]:
The study training videos can be accessed at the following URLs [reproduced with permission from Vimeo, LLC (New York, NY, USA; 2021); all accessed 11 June 2021]:
List of abbreviations
- AFT
- accelerated failure time
- AMR
- antimicrobial resistance
- AOM
- acute otitis media
- AOMd
- acute otitis media with discharge
- API
- application programming interface
- BNF
- British National Formulary
- BRTC
- Bristol Randomised Trials Collaboration
- CCG
- Clinical Commissioning Group
- CDIM
- clinical data information model
- CDISC
- Collaborative Data Standards Interchange Consortium
- CHMP
- Committee for Medicinal Products for Human Use
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN
- Clinical Research Network
- CTDMS
- Clinical Trial Data Management System
- DHSC
- Department of Health and Social Care
- DNC
- data node connector
- eCRF
- electronic case report form
- EDC
- electronic data capture
- EHR
- electronic health record
- EMA
- European Medicines Agency
- EMIS
- Egton Medical Information Systems
- ENT
- ear, nose and throat
- EoI
- expression of interest
- eSource
- electronic source
- GCP
- good clinical practice
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IHE
- Integrating the Healthcare Enterprise
- iOS
- iPhone operating system
- IQR
- interquartile range
- IT
- information technology
- ITT
- intention to treat
- LHS
- learning health system
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ODM
- operational data model
- OMQ-14
- Otitis Media Questionnaire, 14-point version
- PC
- personal computer
- PIS
- patient information sheet
- PPI
- patient and public involvement
- PRAC
- Pharmacovigilance Risk Assessment Committee
- PREAR
- Painful Runny EAR
- PROM
- patient-reported outcome measure
- QOF
- Quality and Outcomes Framework
- RCGP
- Royal College of General Practitioners
- RCT
- randomised controlled trial
- REST
- Runny Ear STudy
- RFD
- Retrieve Form for Data Capture
- SDM
- study data model
- SRQ
- Symptom and Recovery Questionnaire
- TMG
- Trial Management Group
- TPP
- The Phoenix Partnership
- TRANSFoRm
- Translational Research and Patient Safety in Europe
- TSS
- TRANSFoRm Study System
