Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/104/501. The contractual start date was in September 2015. The draft report began editorial review in September 2019 and was accepted for publication in December 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Fowler et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter are reproduced with permission from the published trial protocol. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
Scientific background
It is now widely recognised that most socially disabling chronic and non-psychotic severe and complex mental health problems begin in adolescence, with 75% of all severe and chronic mental illnesses emerging between the ages of 15 and 25 years. 2,3 A series of retrospective studies have consistently shown that severe mental illness is often preceded by social decline, that this often becomes stable, and that such premorbid social disability is predictive of the long-term course of the disorder. 4 Between 3% and 5% of adolescents present with non-psychotic severe and complex mental health problems associated with social disability. 2 The young people at highest risk of long-term social disability present with emerging signs of social decline, in association with low-level psychotic symptoms and emotional and behavioural disorder often accompanied by substance misuse problems, and risk to self and others. 2,3
Despite poor outcomes and the cost of disorders leading to social decline, young people with complex needs frequently do not access treatment, and less than 25% of young people and their families who have such needs access to specialist mental health services. 5–8 More complex cases are found in areas of social disadvantage and among those who are not in employment or education. 1 The economic costs of not addressing this disability are very high. 9 Persistent mental health problems associated with social disability in young people do not resolve naturally and may persist across the life course, resulting in severe distress and social disability, and high costs to health and a range of social and other services. 2,3 Health economic modelling of lifelong costs in this area is emerging. A recent estimate suggests that mental health problems in childhood and adolescence can result in problems across marital satisfaction, self-esteem and quality of life, and can lead to a 28% reduction in economic activity at age 50 years or a £388,000 lifetime loss per person. 10 Young people who have a combination of severe and persistent mental health needs and who are socially disabled present with problems that have the highest lifelong burden.
Several recent reports have highlighted that there is a major gap in identifying and managing the mental health problems of young people with non-psychotic severe and complex mental health problems, particularly those at risk of social disability. 5,7,8,11–13 New approaches to detection and intervention are required to meet the needs of these young people. 1 There is a gap in the evidence base for these young people. 1 Several National Institute for Health and Care Excellence (NICE) guidelines have highlighted this issue, including those for social anxiety,14 depression,15 and detection of people at risk of psychosis, and the research recommendation deriving from the NICE guideline on psychosis and schizophrenia in children and young people. 13
Young people who have non-psychotic severe and complex mental health problems and who are socially disabled are complex. Thus, they tend not to be suitable for or respond to short-term evidence-based therapies for more discrete mental health problems, such as cognitive–behavioural therapy (CBT) for anxiety, depression and conduct disorder, which are available via the Improving Access to Psychological Therapies initiative. Moreover, although this group show clear evidence of social disability, they do not meet the criteria for first-episode psychosis (FEP) and so they are not suitable for Early Intervention in Psychosis (EIP) services, for which there is now considerable evidence of benefits on social functioning. 4,16,17
Our aim in the present project is to identify and target the group of young people who are socially disabled and have non-psychotic severe and complex mental health problems and are at risk of long-term severe mental illness to offer them a new psychological intervention specifically tailored to their needs.
The most systematic service provision is often outside mental health services in statutory and voluntary sector provision for young people who are not in education, employment or training (NEET). In these services, the focus is primarily on obtaining employment and, thus, the mental health problems that present barriers to activity, work, education and training may not be recognised. 18 However, the degree to which NEET status is associated with mental health problems is increasingly recognised. 18–24 Detection of this population therefore must focus on the screening of the mental health problems of young people who have links with NEET services and who are under primary mental health care, alongside seeking referrals from those referred to Child and Adolescent Mental Health Services (CAMHS) and adult services. This group may be detected in services for those in the at-risk mental states for psychosis (ARMS), where these services are present.
Current evidence for effective interventions to address social disability among young people in the early course of severe mental illness is very limited. 4 A series of studies have been undertaken that aimed to identify people at ultra-high risk (UHR) of poor long-term outcomes associated with severe mental illness, focusing predominantly on risk of psychosis. 25–28 The success of the UHR studies is that they have shown that it is possible to set up services to identify and treat cohorts of young people who can be identified as having ARMS using defined operational criteria and structured assessment tools. 29 Furthermore, these studies have consistently identified that those who are at the highest risk are young people who present with social decline as well as subthreshold psychotic symptoms. 30,31 However, the focus of these studies has been on prevention of episodes or symptoms of psychosis, not social disability. 1 Recent studies have shown that cohorts identified using these criteria may have more transient problems than previously thought and that only a subset go on to have long-term socially disabling mental health problems. 30,31 Several prominent UHR researchers are now highlighting an alternative strategy, which is to examine functional outcome in the UHR group. This study is consistent with this strategy.
Systematic reviews of CBT for psychosis, including NICE guidelines, have consistently shown a moderate effect size on improvements in social disability where this has been assessed as a secondary outcome. 32 This has been confirmed in the recent review of the NICE guidelines for schizophrenia. 33 However, these studies have predominantly been carried out among chronic participants, not young people. 1 The feasibility of using CBT with young people who are at UHR of long-term poor outcomes has been shown in the recently completed, multicentre Early Detection and Intervention Evaluation for people at high-risk of psychosis 2 (EDIE-2) trial,34 which has shown reductions in the severity of psychotic symptoms. However, the focus of the therapy in EDIE-234 was symptom reduction,28 and this approach neither targeted nor had a significant benefit on social disability. EDIE-234 clearly demonstrated the ability of collaborating sites to recruit young people at high risk and successfully retain them in research and therapy. However, as described above, the group recruited in EDIE-234 were heterogeneous in terms of social disability. The present trial builds on EDIE-234 by focusing on a group that has a more homogeneous set of social disability problems, defined by low-activity levels, and targeting this group with a multisystemic intervention that specifically aims to address social disability.
Better outcomes on social disability and hopelessness can be obtained from a more targeted intervention specifically focused on improving social disability among those who have low functioning. We have developed a multisystemic form of CBT that targets social disability. 35,36 A successful Medical Research Council trial37 was carried out with a group of young people who had established chronic and severe social disability problems up to 8 years after a first episode of psychosis. This trial demonstrated gains in structured activity and hope, as well as reductions in symptoms,37 with evident long-term maintenance of gains in structured activity. 38 Clear indications of health economic benefits were demonstrated. 39 However, the trial was small and there was a high level of uncertainty associated with these estimates. A further, larger National Institute for Health Research (NIHR)-funded trial demonstrated gains in structured activity and reductions in symptoms for EIP service users who were experiencing their first episode of psychosis. 40
The intervention used in this study has been refined from experience in previous studies to apply to socially withdrawn young people with non-psychotic severe and complex mental health problems. 35,36 The intervention is considered developmentally appropriate for young people owing to evident gains for young people accessing EIP services. 40 Moreover, social recovery therapy (SRT) is a collaborative approach, led by the young person’s personally valued and meaningful goals. 36 This individualised goal focus means that SRT is in keeping with the adolescent and emerging adulthood stages of development, in which young people are beginning to individuate and separate from the family unit and to explore and develop their sense of self-identity. 41,42 Furthermore, SRT is in keeping with the youth perspective on social recovery, in which biographical disruption from mental health problems gives way to new meanings, identities and social connections. 43 To our knowledge, this trial was the first to specifically address both social disability and mental health problems among a high-risk population of young people presenting with social disability and non-psychotic severe and complex mental health problems.
Chapter 2 Methods
Parts of this chapter are reproduced with permission from the published trial protocol. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
Aims and objective
We aimed to undertake a definitive randomised trial to determine the clinical effectiveness and cost-effectiveness of SRT plus enhanced standard care (ESC) compared with ESC alone in young people who present with social withdrawal and non-psychotic severe and complex mental health problems, and who are at risk of long-term social disability and mental illness.
The primary hypothesis was:
-
In young people who are socially disabled and have non-psychotic severe and complex mental health problems, SRT plus ESC is superior to ESC alone in improving social recovery [as measured by hours in constructive economic activity assessed on the Time Use Survey (TUS)] over a 15-month follow-up period.
The secondary hypotheses were:
-
SRT plus ESC is superior to ESC alone in terms of cost-effectiveness.
-
SRT plus ESC is superior to ESC alone in terms of effects on mental health symptoms [attenuated psychotic symptoms (APS) and emotional disturbance].
Design
This was a pragmatic, multicentre, single-blind, superiority RCT with ascertainment of the clinical effectiveness and cost-effectiveness of SRT delivered over a 9-month period plus ESC compared with ESC alone in young people (aged 16–25 years) with non-psychotic severe and complex mental health problems and showing early signs of persistent social disability. Primary and secondary outcomes were evaluated at 15 months post randomisation (i.e. 6 months after the end of intervention or control) and limited assessment of longer-term outcomes was evaluated at 24 months post randomisation.
Procedure
Ethics approval was obtained from the former East of England Cambridgeshire and Hertfordshire National Research Ethics Service Committee for recruitment to an internal pilot (12/EE/0311) and the Preston Research Ethics Committee (REC) North West (15/NW/0590) for recruitment to the definitive trial. All participants provided written informed consent before undertaking any trial procedures.
Participants were recruited from child, adolescent and adult primary and secondary care mental health services (including youth mental health, early detection and early intervention services), and from youth, social, education and third-sector (i.e. voluntary, charitable and community) services. Potential participants were approached by their care provider and asked for agreement to be contacted by the study team. Participants were provided with verbal and written information about the study using the participant information sheet (PIS) (see Report Supplementary Material 1). Interested participants were invited to provide written consent using an informed consent form (see Report Supplementary Material 2). Under supervision and review by the Trial Management Group, research assistants (RAs) administered eligibility screening assessments. Eligible participants completed the remaining baseline assessment measures, were randomised to the intervention or control arm of the trial, and were followed up over the following 24 months. All participants were offered a thorough assessment summary report for them and their usual care provider. For those participants randomly allocated to SRT in addition to ESC, a SRT therapist made contact within 1 week to inform participants of their allocation and to arrange the first therapy appointment. Participants’ care providers (and referrers, if different) were informed of the allocation outcome.
Interventions
Social recovery therapy
The intervention was SRT plus ESC delivered by trial therapists who were clinical psychologists or qualified CBT therapists trained in the intervention. SRT is as described in a therapy manual. 36 SRT was delivered individually in face-to-face sessions, with interim telephone, text and e-mail contact. Sessions were delivered over 9 months. Sessions took place in participants’ homes, NHS premises, community and public locations. All sessions, except where conducted in public locations, were audio-recorded with participant consent.
Social recovery therapy is based on a cognitive–behavioural model that suggests that social disability evolves as a result of lifestyle patterns of low activity, which are adopted as functional behavioural patterns of avoidance and are maintained by lack of hope, a reduced sense of agency and low motivation. 36 The intervention involves promoting a sense of hopefulness, self-agency and motivation, and encouraging activity, while managing any psychotic and non-psychotic symptoms and neurocognitive problems. The approach combines multisystemic working with the use of specific CBT techniques. Multisystemic working may involve working with participants’ relatives and friends, and employment or education providers. Trial therapists adopt assertive outreach youth work principles and draw on successful social and vocational interventions, such as supported education and employment interventions.
Social recovery therapy involves three stages, which are flexibly tailored to each participant’s goals and problems:
-
Stage 1 involves assessment and developing a formulation of the person’s difficulties and barriers to social recovery. This often involves validation of real barriers, threats and difficulties, while focusing on promoting hope for social recovery.
-
Stage 2 involves identifying and working towards medium- to long-term goals guided by a multisystemic formulation of barriers to recovery. Identifying specific pathways to meaningful new activities and values is a central component of stage 2. This can include referral to appropriate vocational agencies and/or direct liaison with employers or education providers. Additional specific techniques used in stage 2 include cognitive work focused on promoting a sense of agency, consolidating a positive identity, and addressing feelings of stigma and negative beliefs about self and others.
-
Stage 3 involves the active promotion of social, work, education and leisure activities linked to personally meaningful goals, while managing symptoms. This involves specific cognitive–behavioural techniques, including behavioural experiments.
Social recovery therapy adherence and competence
Therapist adherence and competence to the SRT intervention were recorded and reviewed. All SRT therapists completed the SRT adherence checklist44 as soon as possible after each intervention session. The checklist involves indicating which components of the intervention were present during the session and briefly describing the delivery of this component to facilitate independent review. This was in addition to all SRT therapists’ recording their session notes, which further detailed the content of all SRT sessions and included additional records regarding all participant contacts beyond the sessions (including e-mail, telephone and text message contacts).
Social recovery therapy adherence was defined as follows:44 (1) full dose equated to at least six sessions that included the presence of an assessment and a social recovery formulation, and that involved at least two pieces of behavioural work conducted in the community (i.e. not in the participant’s home or a clinic room) with the therapist; (2) partial dose equated to at least six sessions, the presence of an assessment and formulation, and some behavioural work that does not meet full-dose criteria (e.g. conducted only as homework or in the clinic room, or planned but not completed); and (3) no dose reflected by fewer than six sessions and/or insufficient components to achieve a rating of partial or full dose.
Therapy cases were rated with respect to adherence (full dose vs. partial dose vs. none) by three raters. Raters reviewed the adherence checklists completed by each therapist for each session with each participant. Raters also consulted the additional session notes made by each therapist, again for each session. Inter-rater reliability of the application of adherence criteria was very high, confirming that adherence ratings were very concordant across raters [Krippendorff’s alpha = 0.9, bootstrapped 95% confidence interval (CI) 0.87 to 0.98].
The competence of the SRT therapists was recorded using the Cognitive Therapy Rating Scale Revised45 (CTS-R). Competence was rated through all SRT therapists submitting session audiotapes, which were rated by multiple raters using the CTS-R.
Enhanced standard care
The control comparator was ESC alone. The aim of ESC was to provide the optimal combinations of currently available evidence-based medical, psychological and psychosocial treatments to this group. There was no restriction on access to existing NHS standard treatment for young people with non-psychotic severe and complex mental health problems and social disability. ESC aimed to include provision of short-term individual and family psychological therapies, medication management, and support and monitoring within primary or secondary mental health services. Participants also received a range of education, social, training, vocational and youth work interventions from a variety of statutory and non-statutory service providers, including social services, voluntary agencies, and employment and education providers. ESC also involved a best practice manual (see Report Supplementary Material 3) for standard treatment, provided by the trial team to the referrer and usual care provider/case manager at referral and again at the end of the participant’s involvement in the study. This manual summarised good practice, including referral to a range of both statutory and third-sector mental health services and medication management, where appropriate. The best practice manual was produced by monitoring and mapping service contacts received across a range of services in the population of interest.
Participants and referrers and/or the usual clinical team, with participant consent, received an assessment summary report from the trial team pertaining to clinical (symptom and neurocognitive) and social problems and circumstances. Assessments identified risks to self or others and these were communicated to the referrer and/or usual care team to facilitate appropriate management. The best practice manual and the approach of the trial team were supported by service user groups and steering groups overseeing youth mental health provision in each region, and delivery was well received by participating services, with referrers keen to involve participants in both treatment and control arms.
Randomisation
Following pretrial assessments, consenting participants were randomised to trial arms stratified by age (16–19, 20–25 years); site (Sussex, East Anglia, Manchester); severity of social disability (low functioning = 16–30 hours of structured activity per week, very low functioning = 0–15 hours of structured activity per week); and whether or not they met symptomatic criteria for ARMS. A remote randomisation service allocated groups and was co-ordinated by the Norwich Clinical Trials Unit (NCTU). Allocation was by preset lists of permuted blocks with randomly distributed block sizes (agreed with the trial statistician). The lists were generated by the Data Management Team at the NCTU.
The allocation process was web based and managed as part of the trial data management system (TDMS). The allocation sequence was hidden from TDMS users. Once allocated, the details were e-mailed to nominated individuals at the trial site to enable the allocation of treatment to be implemented. The allocation was not revealed to any other users of the database or other individuals.
Blinding
Research assistants collecting baseline and follow-up data were blinded to group allocation. This was successfully maintained in the pilot using a range of procedures, which were subsequently used in the definitive trial. 1 Following allocation to the treatment or control arm, all participants in the study, as well as their care co-ordinator/referrer and clinical team (if applicable), were asked not to reveal to the research assistant (RA) the group to which the participants were randomised. Participants and family members were asked at the beginning of each assessment interview not to disclose the group to which the individual was allocated. Outside the assessments, RAs were shielded from discussion of participants in study forums where the possibility of determining the allocation group of the participants could occur. A system of web-based data entry ensured that RAs did not have access to information in the database that would reveal the allocation group. Data entered into the TDMS by trial therapists that might inadvertently lead to unblinding were hidden from non-trial therapist users.
Reported blind breaks were managed to maintain blind outcome assessments by reallocating ‘blind’ RAs to collect and score study data, and thus did not bias results. Thirty-one blind breaks occurred during the trial, but all were managed by reallocating the assessment to a blind member of trial staff. Of these blind breaks, 16 were due to the participant or a family member informing the RA of the allocation, 14 were due to referrers or other clinical staff informing the RA of allocation and one was due to a trial staff administrative error.
Patient and public involvement
Patient and public involvement (PPI) activity was led by Dr Rory Byrne. Young people with lived experience of mental health problems and NHS service use were initially recruited from EIP services in Norfolk. These young people were asked to inform the development of the original trial protocol,1 including advising on the feasibility and acceptability of the SRT intervention and the planned study assessment process.
During the internal pilot, a specific patient group [the PRODIGY Advisory Team (PAT)] was established. The PAT was fully integrated into the development and delivery of the internal pilot. The PAT contributed to the development and revisions of the trial protocol and all participant documentation. 1 Members of the PAT piloted trial assessments using role-play to inform the process of assessment selection and delivery. The PAT also provided consultation promotional materials and trial team training. The PAT improved recruitment to the internal pilot through advising on methods of engaging young people in the trial and in the provision of participant newsletters to facilitate ongoing engagement with the trial. In May 2014, the PAT reviewed the delivery of the internal pilot and contributed to the development of the funding application for the extension phase, most notably in the creation of the lay summary.
The extension phase comprised continued delivery of the trial protocol,1 as in the internal pilot, with no changes. Therefore, in the extension phase, PPI activity consisted of review of the delivery of the trial protocol by Dr Rory Byrne and Ms Ruth Chandler, lead for PPI for the extension phase sponsor, Sussex Partnership NHS Foundation Trust. Dr Rory Byrne reviewed participant documentation, such as dissemination newsletters. Dr Rory Byrne continues to consult on dissemination activities.
Outcomes
Primary outcome
The primary outcome was time use (hours per week engaged in structured activity) measured at 15 months post randomisation. This assessment was derived from the Office for National Statistics TUS interview,46 adapted for use with a clinical population. 47 Time use is an important marker of participation in structured activities that are robustly associated with reduced mental health problems and better mental health and well-being;48,49 thus, time use is a useful measure of the behavioural aspects of social recovery. 47 The TUS is validated for use with young people with mental health problems, is sensitive to discriminating between clinical and non-clinical groups,47 and shows convergent validity with measures of quality of life and social functioning. 37 Moreover, the derived outcome score is in the easily interpretable metric of weekly hours. Number of hours per week engaged in structured activity includes time spent in both constructive economic activity (e.g. paid and voluntary work, education, child care, housework and chores) and in structured leisure and sports activities. This total was also calculated without child care hours, as child care (especially of a new-born child) considerably inflates time in structured activity, but is an outlying, semirandom event that is unrelated to trial arm and is imbalanced towards female participants. In all relevant outcome models, time use was tested using the total with and without child care hours included.
Secondary outcomes
Further time use outcomes
Emotional disturbance using self-report questionnaires
-
Social anxiety [Social Interaction Anxiety Scale50 (SIAS) total score].
-
Depression (Beck Depression Inventory-II51 total score).
-
Scale for the Assessment of Negative Symptoms52 (SANS).
-
Global Assessment of Functioning53 (GAF).
-
Global Assessment of Symptoms54 (GAS).
-
Social and Occupational Functioning Assessment Scale55 (SOFAS).
Levels of attenuated psychotic symptoms and associated psychopathology using the Comprehensive Assessment of At-Risk Mental States for psychosis
-
Transition to psychosis since last assessment was recorded as a dichotomous variable (transition or no transition).
-
Comprehensive Assessment of At-Risk Mental States for psychosis (CAARMS) symptom scores were derived following the procedure used in EDIE-2:34
-
A CAARMS symptom severity score (0–144) was derived using the summed product of global severity (0–6) and frequency scores (0–6) across the four positive symptom subscales (unusual thought content, non-bizarre ideas, perceptual abnormalities and disorganised speech). Higher scores reflect more severe symptoms.
-
A CAARMS distress score was created by taking a mean average of subjective distress (0–100) across the four positive symptom subscales.
-
Change in difficulties experienced by participants in the study using the structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders-IV
-
Meeting diagnostic criteria for any mood disorders rated since last assessment point.
-
Meeting current month diagnostic criteria for up to two anxiety, somatoform or eating disorders for which diagnostic criteria were met at baseline.
Putative mediators
Putative moderators
Other outcomes
Health economic outcomes
-
Health Service Resource Use Questionnaire. 66
-
EuroQol-5D (EQ-5D) [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)]. 67
All outcomes were assessed at baseline and again at 9, 15 and 24 months post randomisation. The neurocognitive assessments were assessed only at baseline and at 15 months post randomisation. Further information regarding the timing of the assessments is shown in Appendix 2.
Participants
Sample size
The target sample size was 270 participants, providing 135 participants in each trial arm. The primary outcome was hours per week in structured activity on the TUS46,47 at 15 months, which was considered unlikely to follow a normal distribution, but potentially to have a positive skew. It was expected, therefore, that analyses would probably use logarithmically transformed data. The sample size was based on an effect size of 0.4 standard deviations (SDs) being considered a minimum clinically significant benefit. A total of 270 participants would provide > 90% statistical power to detect a 0.4 SD effect size using a two-sided 5% significance level; a total of 200 participants (i.e. accounting for > 25% loss to follow-up) would provide 80% statistical power for the same effect size. A total of 100 participants were recruited in the internal pilot phase (January 2013–February 2014) and 170 further participants were recruited in the definitive extension phase (September 2015–May 2017). The pilot participants were recruited on time and to target. A recruitment extension of 6 months was required to achieve the target of 170 participants for the extension phase. This delayed the planned end date of recruitment from November 2016 to May 2017. Follow-up assessment for the pilot 100 participants began in September 2013 and finished in March 2016. Follow-up assessment for the extension 170 participants began in June 2016 and finished in June 2019.
Inclusion criteria
-
Young people aged 16–25 years with non-psychotic severe and complex mental health problems and showing early signs of persistent social disability.
-
Presence of impairment in social and occupational function indicated by patterns of structured and constructive economic activity of < 30 hours per week and a history of social impairment problems lasting for > 6 months.
-
Presence of non-psychotic severe and complex mental health problems defined operationally as either of the following:
-
having APS that meet the criteria for ARMS
-
having non-psychotic severe and complex mental health problems which score ≤ 50 on the GAF scale (which indicates the presence of severe symptoms of at least two out of depression, anxiety, substance misuse, behavioural or thinking problems or subthreshold psychosis to the degree that they impair function), with at least moderate symptoms persisting for > 6 months.
-
Exclusion criteria
-
Active positive psychotic symptoms or history of FEP.
-
Severe learning disability problems (mild to moderate learning difficulties were not an exclusion criterion).
-
Disease or physical problems likely to interfere with ability to take part in interventions and assessments.
-
Non-English speaking to the degree that the participant is unable to fully understand and answer assessment questions or give informed consent.
Analytic plan
The analytic plan was detailed in the Statistical Analysis Plan (version 0.2, 4 July 2018) and approved by the Data Monitoring and Ethics Committee (DMEC). The primary analysis compared SRT plus ESC with ESC alone on time use at 15 months post randomisation. The primary analysis was on the intention-to-treat (ITT) principle (i.e. all participants were followed up for data collection irrespective of adherence to treatment and were analysed according to group allocation rather than intervention received). We also completed a per-protocol (PP) analysis of primary and secondary outcomes.
All hypothesis testing was at the two-sided 5% statistical significance level. CIs for parameter estimates were at the corresponding 95% level. Analyses were conducted by the trial statistician blinded to group identity (i.e. ‘subgroup’ blind). 1 Assuming a normal distribution (potentially of transformed values) for time use, a general linear model was constructed. This model included all stratification variables [i.e. recruiting site (as a random factor), age (16–19 years, 20–25 years), severity of social disability (withdrawn or extremely withdrawn) and meeting symptomatic criteria for an ARMS or not]. Time use at baseline was also included as a covariate. Logical memory at baseline was also included as a prognostic variable for long-term social recovery along with verbal fluency (total score). Treatment arm was included as a fixed effect. Estimation of model parameters was on the basis of ‘type III’ (or ‘adjusted’) least squares. The residuals from this model were examined to assess the normal distribution and homoscedastic assumptions. Transformations of the outcome were considered in the case that this assumption did not appear appropriate.
Secondary analyses were conducted in an analogous fashion through a generalised linear model, with an appropriate link and error term depending on the nature of the outcome of interest (e.g. a logistic regression model for binary outcomes). Stratification variables were again included in the model and, where available, the outcome variable at baseline was also included. Treatment group was included as a fixed factor.
The analytic plan included a consideration of the moderation of treatment effect with respect to the following baseline characteristics:
-
ARMS29 status
-
low or very low functioning according to structured activity hours per week at baseline46,47 Logical Memory I (Logical Memory Scaled Total Score61)
-
COWAT62 total score.
This was to be achieved by using an interaction term (between the putative moderator and treatment arm) to formally assess differences in treatment effect. The sample size calculation did not include reference to hypothesis testing of interaction effects and this analysis was considered exploratory.
Missing data
For both the primary and secondary outcomes, the extent and patterns of missing data were checked and full information maximum likelihood (FIML) methods were used if the degree of missing data was < 50% of those randomised for any given analysis (i.e. when considering both missing outcome and prognostic variables in a model).
Multiple imputation (MI) methods were also used as another solution for missing data and for comparison with the FIML model. Factors to include in the imputation model were all those in the analytical model plus those considered likely to be related to the missing values. Any continuous variable exhibiting a strong asymmetric distribution was transformed to produce symmetry, following recommendations for MI. 68 The imputed analysis was based on 10 imputed data sets. Standard errors for parameter estimates were constructed using Rubin’s rules. The analysis using imputed data was a secondary sensitivity analysis with complete-case analysis being the primary analysis.
Full information maximum likelihood and MI methods are based on the assumption that data are missing at random. In the present study, there is clear evidence that there are consistently more missing data in the control arm than in the treatment arm. The level of missingness of data and the bias of missingness of data increases over time. This missingness of data is likely to be due to treatment, as therapy increases the engagement of those with more severe symptoms, who tend to drop out in the control group; therefore, this is likely missing not at random. The level of missingness of data and bias at 15 months was reviewed by the statistical team and, as the total level of missingness of data was < 20% and the bias was present but not extreme, it was agreed that the ITT analysis was the appropriate test of primary and secondary outcomes at that stage. At 24 months, the interpretation of results requires care. The bias assists a conservative assessment of the superiority of SRT and, indeed, may bias in favour of ESC. FIML and MI are reported as supportive analyses but, again, such missing data analyses cannot adjust for bias due to being missing not at random.
Additional analyses
The moderation of treatment effect on the primary outcome, time use at 15 months, was considered with respect to the following baseline characteristics:
-
Logical Memory I (Logical Memory Scaled Total Score61)
-
COWAT62 total score
-
at-risk mental state versus not-at-risk mental state from the CAARMS. 29
This was achieved by using an interaction term (between the putative moderator and treatment arm) to formally assess differences in treatment effect. The sample size calculation did not include reference to hypothesis testing of interaction effects and this analysis is considered exploratory.
Software
The analyses were carried out in SAS® software (version 9.4) (SAS Institute Inc., Cary, NC, USA). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.
Chapter 3 Development and process evaluation
This report has been prepared using CHEERS (Consolidated Health Economic Evaluation Reporting Standards) (see Report Supplementary Material 4) and CONSORT (Consolidated Standards of Reporting Trials) (see Report Supplementary Material 5) guidance. The completed checklists are provided as supplementary material.
Internal pilot statement
The internal pilot (NIHR reference 10/104/501) was funded in March 2012 and began recruitment in January 2013. The aims of the pilot were to (1) assess recruitment rate, quality of data collection and follow-up, (2) provide a final check on procedures in the protocol and (3) conduct a qualitative substudy to inform the objectives, using a qualitative, service user perspective. The following stop–go criteria were used to determine if it was appropriate for the pilot to be considered an internal pilot and progress to the definitive trial: (1) no necessity for substantive changes to the protocol, (2) recruitment of 80% of the planned total in the first 12 months, (3) retention of participants within the study with baseline and outcome assessments completed for > 80% of participants for the secondary and other outcomes and mediators, and for 90% of participants for the primary outcome, and (4) satisfactory delivery of competent and adherent therapy to > 80% of the treatment group.
The stop–go criteria were all satisfied. No changes to the trial protocol were necessary. The pilot recruitment was on time and to target. Primary and secondary outcome retention was excellent, achieving 92% at both 9- and 15-month assessment points. Primary and secondary outcome retention at the later-added 24-month assessment point necessitated a new consent procedure and a completion rate of 69% was achieved. Of those randomised to SRT (n = 47), 41 (87%) were deemed to have received competent and adherent therapy. The pilot DMEC and Trial Steering Committee (TSC) reviewed trial data in 2014 and supported confirmation of the pilot as an internal pilot. These two committees and the PPI representatives (the PAT) reached consensus that no changes to the trial protocol were required. The sponsor also performed a full Good Clinical Practice audit of the trial and concluded that no changes to trial procedures were needed. Additional funding was sought (NIHR reference 10/104/51) for the extension and was awarded in May 2015, with recruitment beginning in September 2015.
Process evaluation
The PRODIGY process evaluation was an evaluation of both the research and the intervention processes undertaken as three substudies, two involving patient participants and one involving SRT therapists, during the initial internal pilot phase. The patient process evaluation was conducted by a subresearch team led by a user researcher (RB) and an independent qualitative researcher (CN), neither of whom were involved in the development of SRT. Both parts of the patient process evaluation (i.e. research and intervention process substudies) were investigated using a qualitative methodology with qualitative interview data collection (see Report Supplementary Material 3) and thematic analytic methods. 69 These substudies were published as project outputs. 70,71 The final part of the process evaluation was conducted by a separate subresearch team during the non-pilot extension phase and focused exclusively on SRT therapist experiences of therapy delivery.
Research process evaluation
The research process evaluation was a qualitative exploratory substudy. All participants who had been randomised were invited to participate between April 2013 and mid-June 2013, resulting in a convenience sample of 13 participants. 71 An attempt was made to recruit substudy participants from both trial sites (East Anglia and Manchester) and both trial arms. Participant characteristics are shown in Table 1. Interviews were conducted by a user researcher (RB) and a RA between 1 month and 3 months after study randomisation. The interview schedule was semistructured and designed to probe experiences relating to study participation. Data were analysed using an inductive thematic approach72,73 with a critical realist epistemic stance. 74 Themes are shown in Table 2.
| Substudy | Characteristic, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Allocation arm | Site | Social disability | At-risk mental states | |||||||
| 16–19 | 20–25 | Male | Female | SRT + ESC | ESC | Manchester | East Anglia | Very low | Low | At risk | Not at risk | |
| Research process: substudy 171 | 7 (54) | 6 (46) | 9 (69) | 4 (31) | 6 (46) | 7 (54) | 7 (54) | 6 (46) | 11 (85) | 2 (15) | 4 (31) | 9 (69) |
| Intervention process: substudy 270 | 11 (65) | 6 (35) | 8 (47) | 9 (53) | 8 (47) | 9 (53) | 8 (47) | 9 (53) | 11 (65) | 6 (35) | 6 (35) | 11 (65) |
| Study | Theme application | Themes | ||||
|---|---|---|---|---|---|---|
| Research process: substudy 171 | Across participants | Practicalities | Acceptance | Disclosure | Altruism | Engagement |
| Intervention process: substudy 270 | Across participants | ‘It’s just the speaking to someone’: the value of talking | ‘Just do it’: the importance of activity | Motivation to change | ||
| SRT + ESC | ‘She understood me on a personal level’: the therapeutic relationship | Flexibility | ‘It’s given me tools’: the CBT toolkit | No pain, no gain: SRT as difficult | ||
| ESC alone | Allocation ambivalence | No treatment, as usual | ‘I was the one who had to do everything to help overcome it’ | |||
The themes focused around key reflections on noteworthy aspects of participating in the PRODIGY trial. 71 First, ‘practicalities’ reflected the importance of RAs offering flexible research appointments. Flexibility on behalf of the researchers was seen as a manifestation of empathy and a person-centred approach. Second, ‘acceptance’ emphasised the position of openness from which participants approached their trial participation. Participants appeared to support the process of randomisation as a ‘fair’ way to allocate the intervention; they also appreciated the scientific importance of the ‘control’ group or comparator. The assessment process was described as acceptable even if questions were sensitive, again, within the context of a positive rapport with the RA. Third, ‘disclosure’ was positioned as a useful and therapeutic process within the context of assessment completion with the RA. The extent of their own disclosure, and the positive experience thereof, seemed to be a surprise for many participants. Fourth, ‘altruism’ appeared to be a key reason for participants wanting to be involved in the trial. This related to trial involvement in general and, more specifically, to participants being in support of the randomised controlled nature of the trial. A minority of participants appeared not to have a good understanding of randomisation. Finally, ‘engagement’ was a key theme that reflected the sense of being involved in the trial as a positive experience. Despite the main focus of the substudy being the research process, participants spoke of their thoughts and experiences in relation to the SRT intervention. Engagement in SRT was seen to be helpful; participants highlighted the benefits of understanding their experiences and how things could change for the better. Nevertheless, participants also described SRT as challenging.
Intervention process evaluation
The focus of the intervention process evaluation was on both arms of the trial (i.e. SRT plus ESC, and ESC alone). Participants were purposively sampled from those involved in the trial (pilot 100 participants only) aiming to balance across sex, study site (East Anglia and Manchester), allocation and baseline ARMS status. Attempts were also made to involve participants reflecting maximum variation in age and prior service use, and to include looked-after children. 70 Nineteen people participated, and their characteristics are shown in Table 1.
Participants were interviewed by one of two researchers using a semistructured interview schedule. Questions focused on experience of psychological difficulties and previous service use, experience of trial participation and interventions received, and perceived outcomes and thoughts about future well-being. Data were analysed with an inductive thematic analysis approach69,72 taking a critical realist epistemic stance. Themes are shown in Table 2.
There were three themes common to the accounts of all participants. 70 First, “‘It’s just the speaking to someone’: the value of talking” reflected participants in both arms perceiving there to be a therapeutic value in talking to someone (RA or therapist) during their trial involvement. Many participants felt that they had avoided or not had the opportunity to do so before their trial involvement. Subthemes reflected that talking to someone helped in two main ways: ‘it’s not boiled up in me no more’ and ‘it helped me recognise the things that I wanted to change’. Second, “‘Just do it’: the importance of activity” reflected the sense of importance placed on meaningful activity by participants in both trial arms. For SRT plus ESC participants, doing these activities reflected an important part of the intervention. Yet for ESC-alone participants, increasing their occupational activity also seemed important, and several participants described making clear efforts to increase their activity levels. Finally, ‘motivation to change’ reflected a sense that determination to make positive changes in their lives was a key feature across the accounts of all participants.
There were four themes corresponding to participant experiences of the SRT intervention. 70 First, “‘She understood me on a personal level’: the therapeutic relationship” emphasised the centrality of the relationship with the SRT therapist to the participant’s experience of SRT. Participants emphasised the informal yet boundaried relationships developed with SRT therapists, which produced a sense of collaborative engagement. Second, the ‘flexibility’ theme reflected participant appreciation for the flexible way in which the intervention was delivered, for example with respect to timing and location. Third, in “‘It’s given me tools’: the CBT toolkit” participants spoke of how SRT equipped them with tools for managing their own distress and increasing their activity levels. Commonly described ‘tools’ included behavioural activation and behavioural experiments. Most participants felt that they could use the tools they had gained to their benefit beyond the intervention, although one participant felt that the intervention period was too short to allow them to use the same techniques independently. Finally, ‘No pain, no gain: SRT as difficult’ reflected that participants experienced the SRT intervention as challenging and even at times painful or overwhelming. Participants tended to emphasise that the ‘pain’ was worth it as they needed to push themselves to complete challenging exercises in order to improve their situations.
There were three themes corresponding to the experience of treatment as usual (i.e. ESC) only. 70 First, most participants expressed ‘allocation ambivalence’ and did not seem to hold negative views about having been randomised to ESC. Some expressed relief at not having to attend therapy, which they appeared to feel would have been anxiety-provoking. However, two participants did express negative views. Second, the theme ‘No treatment, as usual’ spoke to the experience of young people in the ESC-alone arm of the trial as reflecting an absence of care and support provision. Only two participants reported having received specialist mental health care since their involvement in the trial. Some participants did report support from their general practitioner (GP), although they were not particularly satisfied with the nature of this support. Finally, ‘I was the one who had to do everything to help overcome it’ reflected ESC-alone participants’ sense that they had to manage their mental health independently, in the context of not being offered SRT and the perceived lack of treatment offered by standard mental health services. Some participants did emphasise that there had been considerable improvement in their mental health despite feeling unsupported by any services, conveying a sense of pride and achievement in improving without this additional support.
Therapist experience process evaluation
The focus of the therapist experience process evaluation was to explore therapist experiences of delivering SRT, with a particular focus on therapist hopefulness in the face of engaging with patients with complex presentations. All SRT therapists involved in therapy delivery in the extension phase of the trial, including two therapists who had also been involved in therapy delivery in the internal pilot, were invited to participate. Information about participation was shared verbally and using a PIS (see Report Supplementary Material 1). Ten SRT therapists participated in a semistructured individual interview following the provision of consent using an informed consent form (see Report Supplementary Material 2). The interview guide focused on asking therapists to reflect on their experiences of delivering SRT, including experiences where therapy had gone ‘well’ and experiences where it had not gone ‘well’. Therapists were also asked about their experiences of informal and formal support and supervision. Data were analysed using interpretative phenomenological analysis. 75 Coding and analysis were performed by a SRT therapist experienced in SRT delivery but not involved in the inception or design of SRT and by a non-therapist researcher.
The finding of the therapy process evaluation was that SRT therapists emphasised the importance of working with this complex client group. In standard services, therapists reported that these young people would typically not be seen, because the typically protracted period needed to engage these young people is usually not possible within the services’ policies and practices. SRT therapists reported that, with this complex group, understanding and formulating treatment ambivalence and disengagement was essential, as was the pursuit of meaningful connection with these young people. SRT therapists also spoke of the difficulty of finding and sustaining their own hopefulness in the face of participants’ sense of ambivalence, hopelessness and ‘stuckness’. Sources of hope and scaffolds for its maintenance included access to expert and hopeful supervision, and opportunities to engage in peer supervision with other SRT therapists.
Process evaluation limitations and conclusions
The key limitations of the process evaluation substudies are that, because of the nature of the convenience sampling methods, the conclusions cannot be said to be generalisable to the whole trial sample or population beyond the trial. Nevertheless, the epistemic stance and qualitative methodologies taken were such that we sought not generalisability, but rather the unique experiences of individual participants of interest. Despite this the findings do show consistency with the wider literature, for example a previous study involving young people with ARMS. 76 Further limitations of the process evaluation substudies include the apparent weighting of the intervention arm participants towards those who had engaged with the therapy. Only one participant was included who had not received a ‘dose’ of the intervention. Although the majority of participants in the trial did receive a dose, those who did not are an under-represented group and their experiences are particularly important in understanding both research and therapy processes.
Key conclusions of the process evaluation centred around the apparent value of having someone with whom to talk and reflect on mental health and well-being. This value was identified for both the research assessments and SRT. With respect to contact with RAs, the perceived value of the research assessment process seems to reflect the notion of therapeutic assessment77 (i.e. that assessment alone can have a therapeutic effect on patients). In the first substudy, all but two participants indicated at least some degree of past mental health service involvement, ranging from CAMHS or youth mental health services to school, university, private or third-sector counselling. Nevertheless, these participants still appeared to experience their trial involvement as reflecting a novel sense of ‘opening up’. This appears to reflect the particularly in-depth nature of the research assessments and also the flexible, engaging and skilled nature of RAs’ interactions with trial participants. It is also notable that in both substudies70,71 SRT participants emphasised the difficult and challenging nature of the intervention.
Chapter 4 Results
Participant flow
Participant flow through the trial is depicted in Figure 1.
FIGURE 1.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
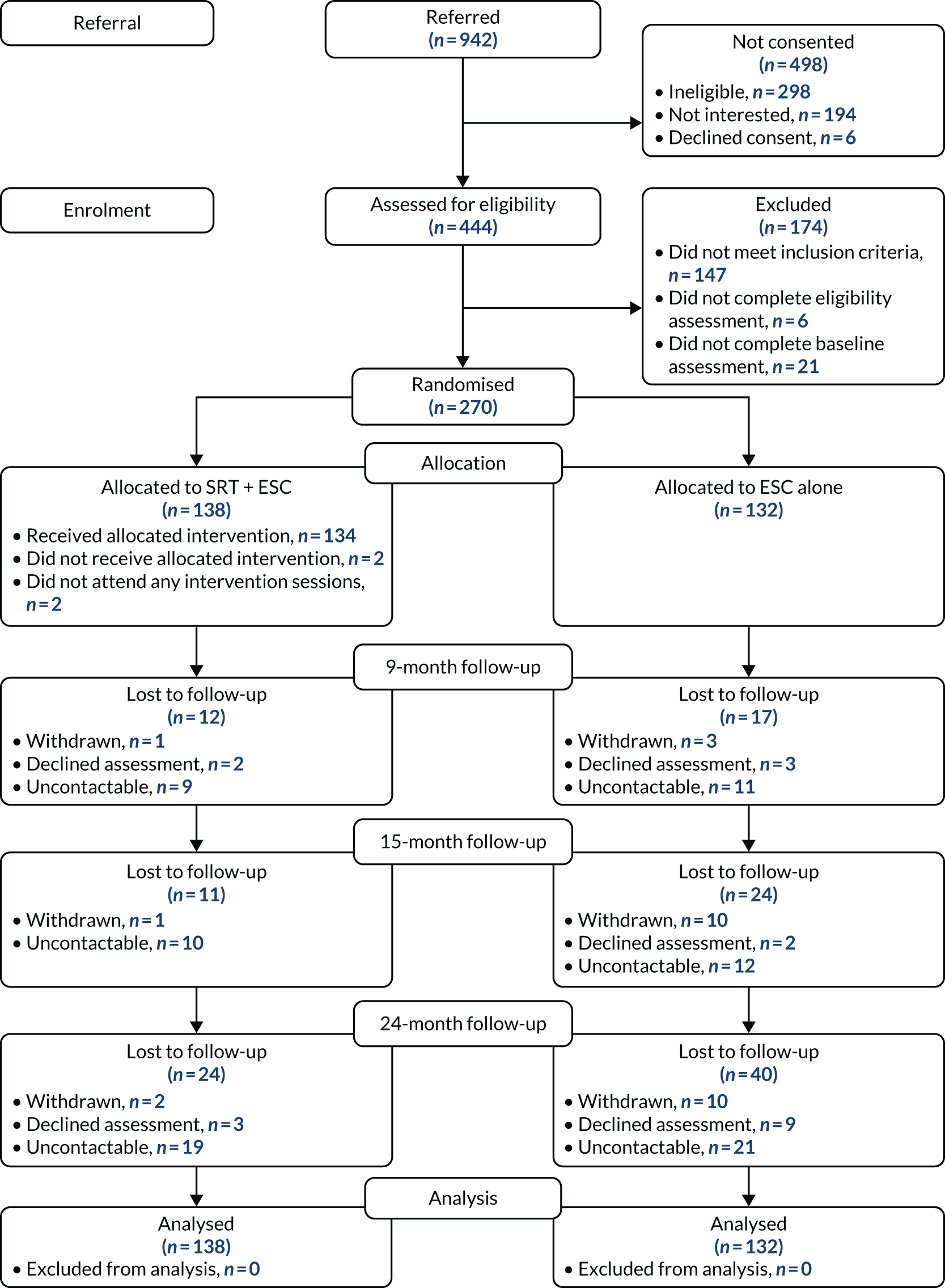
Participant non-entry
As shown in Table 3, the majority of participants who were ineligible (1) did not meet entry criteria regarding no history of or current active psychosis evidenced by symptoms and/or prescription of threshold dosage of antipsychotic medication, (2) had insufficient social impairment and/or (3) were not eligible for unknown reasons. The third group included early referrals from clinicians of potential participants who did not reach formal eligibility assessment. As shown in Table 4, the majority of participants who declined to participate for lack of interest did not give further reasons for their decision or were categorised as not interested because they and/or their referrers were not contactable (disengaged).
| Reasona | Participants (n) |
|---|---|
| Age < 16 years or > 25 years | 6 |
| + Severe learning difficulties | 1 |
| Historical or active psychotic symptoms or first episode (or taking antipsychotics at above therapeutic dose) | 63 |
| + Social impairment insufficientb | 6 |
| Severe learning difficulties | 3 |
| Disease or physical problems likely to interfere with ability to take part | 4 |
| Non-English speaking | 0 |
| Social impairment insufficientb | 76 |
| Mental health problems insufficient | 32 |
| + Social impairment insufficientb | 1 |
| Out of area | 11 |
| Risk/safety concerns | 9 |
| Re-referral of young person who had already participated | 3 |
| Reason for unsuitability unknown | 91 |
| Reasona | Participants (n) |
|---|---|
| Not interested (detail unknown) | 91 |
| Not help-seeking | 19 |
| Not interested in research participation | 10 |
| + Not interested in SRT | 9 |
| + Project too much commitment | 3 |
| + Not interested in SRT, not help-seeking | 3 |
| Not interested in SRT | 2 |
| Project too much commitment | 8 |
| Unable/unwilling to engage due to mental health problem(s) | 7 |
| Young person disengaged from referrer | 54 |
| Young person disengaged from the PRODIGY trial | 64 |
| Referrer disengaged from the PRODIGY trial | 70 |
| Recruitment ended | 4 |
Characteristics of randomised participants
Table 5 provides baseline demographic characteristics of the sample by trial arm conditions and as per the ITT and PP analyses. The aim was to recruit a sample of withdrawn young people with low activity and comorbid complex severe mental illness. There was little difference between the two groups, with both groups showing a majority of participants aged 16–19 years, with a mean age around aged 20 years, of white ethnicity, who were single, unemployed, heterosexual and living in rented accommodation. The ESC sample is evenly balanced between sexes, although there was a predominance of male participants in the SRT group.
| Characteristic | Analysis | ||
|---|---|---|---|
| ITT | PP | ||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 91) | |
| Age group (years), n (%) | |||
| 16–19 | 79 (57) | 75 (57) | 51 (56) |
| 20–25 | 59 (43) | 57 (43) | 40 (44) |
| Age (years) | |||
| Mean (SD) | 20.1 (2.5) | 20.0 (2.7) | 20.1 (2.4) |
| Missing, n | 6 | 5 | 2 |
| Sex, n (%) | |||
| Female | 54 (39) | 66 (50) | 34 (37) |
| Male | 84 (61) | 66 (50) | 57 (63) |
| Ethnicity, n (%) | |||
| White | 127 (92) | 114 (86) | 83 (91) |
| Non-white | 11 (8.0) | 18 (14) | 8 (8.8) |
| Marital status, n (%) | |||
| Partner | 18 (13) | 17 (13) | 9 (9.9) |
| Separated | 2 (1.5) | 0 | 2 (2.2) |
| Single | 118 (86) | 115 (87) | 80 (88) |
| Employment status, n (%) | |||
| Paid work | 5 (3.6) | 6 (4.6) | 3 (3.3) |
| Voluntary work | 3 (2.2) | 4 (3.0) | 3 (3.3) |
| Student | 34 (25) | 31 (24) | 22 (24) |
| Unemployed | 95 (69) | 91 (69) | 62 (69) |
| Missing, n | 1 | 0 | 1 |
| Sexual orientation, n (%) | |||
| Heterosexual | 98 (74) | 107 (82) | 64 (74) |
| Homosexual | 6 (4.5) | 6 (4.6) | 3 (3.5) |
| Bisexual | 16 (12.1) | 13 (9.9) | 9 (10.5) |
| Unsure | 6 (4.5) | 1 (0.8) | 5 (5.8) |
| Other | 6 (4.5) | 4 (3.1) | 5 (5.8) |
| Missing, n | 6 | 1 | 5 |
| Accommodation, n (%) | |||
| Accommodation with support | 8 (5.9) | 4 (3.0) | 4 (4.5) |
| Homeless/temporary accommodation | 5 (3.7) | 7 (5.3) | 2 (2.2) |
| Mobile accommodation | 0 | 1 (0.8) | 0 |
| Owner occupied | 48 (36) | 41 (31) | 32 (36) |
| Rented (local authority/housing association) | 45 (33) | 55 (42) | 27 (30) |
| Rented (private) | 29 (22) | 24 (18) | 24 (27) |
| Missing, n | 3 | 0 | 2 |
| Social functioning, n (%) | |||
| Low functioning | 40 (29.0) | 40 (30) | 21 (23) |
| Very low functioning | 98 (71) | 92 (70) | 70 (77) |
| Mental state, n (%) | |||
| At risk | 69 (50) | 64 (49) | 49 (54) |
| Not at risk | 69 (50) | 68 (52) | 42 (46) |
Of note is the severity of social disability, and psychopathology anxiety and depression present in the sample at baseline. Around half of the participants (n = 133) met the criteria for ARMS. One hundred and fourteen (86%) of the 133 participants with ARMS were categorised as experiencing APS, 11 (8.3%) were categorised as experiencing APS and vulnerability, four (3.0%) were categorised as experiencing vulnerability, three (2.3%) were categorised as experiencing APS and brief limited intermittent psychotic symptoms and one (0.8%) was categorised as experiencing brief limited intermittent psychotic symptoms. Around two-thirds of the sample entered the trial with very low functioning according to stratification. The mean structured activity level was around 11 hours, which, when compared with > 64 hours in an age-matched non-clinical sample, suggested extreme withdrawal. 47 Levels of social disability were in the severe range and > 95% of participants were unemployed. Functional status according to GAF and SOFAS similarly suggested severe functional disability. 53,55 Levels of global symptoms, depression, social anxiety and hopelessness were in the severe range,50,51,54,78 with comorbidity in the majority of cases. Alcohol and drug disorders, aggression and suicidality were also severe and prevalent. 29,63,64
Baseline scores for the outcome measures were fairly similar across both groups (see Table 6). The intervention group reported slightly greater social anxiety (SIAS) and slightly lower functioning (SOFAS) at baseline. The average scores for the CAARMS interview variables at baseline were fairly similar between the control and the intervention groups. The symptom severity and average distress scores were very similar; however, the intervention group showed a slightly lower aggression severity score and a slightly higher suicidality severity score. These results are tabulated in Table 7. The diagnostic characteristics of both groups (Tables 8 and 9) were similar at baseline, although ESC-alone participants appeared to be slightly more likely to have current panic disorder or panic with agoraphobia, and slightly less likely to report a current major depressive episode. Scores on ‘other’ outcomes at baseline were similar across groups, although the ESC-alone arm scored slightly lower for hopelessness (Table 10).
| Outcome | Population | ||
|---|---|---|---|
| ITT, mean (SD) | PP, mean (SD) | ||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 91) | |
| Primary outcome | |||
| Structured activity (hours per week) | 11.3 (8.0) | 11.3 (8.6) | 9.8 (7.4) |
| Missing, n | 0 | 0 | 0 |
| Structured activity (minus child care) (hours per week) | 11.0 (7.8) | 11.2 (8.6) | 9.7 (7.3) |
| Missing, n | 0 | 0 | 0 |
| Secondary time use outcome | |||
| Constructive economic activity (hours per week) | 8.6 (7.1) | 8.1 (7.0) | 7.3 (6.4) |
| Missing, n | 0 | 0 | 0 |
| Secondary emotional disturbance outcomes | |||
| SIAS | 52.1 (14.1) | 48.1 (16.1) | 53.9 (12.6) |
| Missing, n | 3 | 7 | 3 |
| BDI-II | 30.4 (12.8) | 30.3 (12.4) | 30.3 (11.8) |
| Missing, n | 4 | 5 | 4 |
| GAF | 37.9 (5.6) | 38.2 (5.5) | 37.8 (4.6) |
| Missing, n | 0 | 0 | 0 |
| GAS | 43.1 (7.3) | 43.2 (7.5) | 43.5 (7.0) |
| Missing, n | 0 | 0 | 0 |
| SOFAS | 41.6 (7.6) | 43.3 (7.0) | 40.5 (6.8) |
| Missing, n | 0 | 0 | 0 |
| Outcome | Allocation arm | |
|---|---|---|
| SRT + ESC, mean (SD) (N = 138) | ESC alone, mean (SD) (N = 132) | |
| CAARMS symptom severity score | 26.2 (16.5) | 26.1 (15.9) |
| Missing, n | 1 | 2 |
| CAARMS average distress score | 52.5 (27.0) | 52.1 (23.1) |
| Missing, n | 6 | 6 |
| CAARMS aggression severity score | 6.3 (5.4) | 7.7 (6.0) |
| Missing, n | 5 | 1 |
| CAARMS suicidality severity score | 6.7 (6.5) | 5.9 (6.4) |
| Missing, n | 7 | 1 |
| Outcome | Allocation arm, n (%) | |
|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |
| Current major depressive episode | 72 (52.2) | 65 (49.2) |
| Past major depressive episode | 33 (23.9) | 41 (31.1) |
| Current mania | 1 (0.7) | 1 (0.8) |
| Past mania | 2 (1.5) | 5 (3.8) |
| Current hypomania | 5 (3.6) | 2 (1.5) |
| Past hypomania | 2 (1.5) | 1 (0.8) |
| Dysthymia | 16 (11.6) | 15 (11.4) |
| Bipolar at risk | 25 (18.1) | 14 (10.6) |
| Bipolar I | 2 (1.5) | 5 (3.8) |
| Bipolar II | 4 (2.9) | 1 (0.8) |
| Major depressive disorder | 95 (68.8) | 93 (70.5) |
| Outcome | Allocation arm, n (%) | |
|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |
| Panic disordera | 6 (4.4) | 6 (4.6) |
| Panic disorder with agoraphobia | 18 (13) | 25 (19) |
| Agoraphobia without panic | 21 (15) | 31 (24) |
| Social phobia | 62 (45) | 54 (41) |
| Specific phobia | 10 (7.3) | 4 (3.0) |
| OCD | 12 (8.7) | 11 (8.3) |
| PTSD | 14 (10) | 16 (12) |
| GAD | 36 (26) | 44 (33) |
| Hypochondriasis | 4 (2.9) | 3 (2.3) |
| Body dysmorphic disorder | 14 (10) | 10 (7.6) |
| Anorexia nervosa | 1 (0.7) | 1 (0.8) |
| Bulimia nervosa | 1 (0.7) | 0 (0.0) |
| Binge-eating disorder | 2 (1.5) | 1 (0.8) |
| Anxiety disorder NOS | 4 (2.9) | 3 (2.3) |
| Outcome | Analysis | ||
|---|---|---|---|
| ITT, mean (SD) | PP, mean (SD) | ||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 91) | |
| BHS | 13.4 (5.8) | 12.7 (5.2) | 13.7 (5.6) |
| Missing, n | 9 | 10 | 7 |
| AUDIT | 5.0 (6.3) | 5.2 (6.3) | 4.3 (5.6) |
| Missing, n | 7 | 2 | 2 |
| DUDIT | 3.6 (7.2) | 3.9 (7.8) | 3.2 (7.1) |
| Missing, n | 2 | 1 | 1 |
Rates of retention
Table 11 depicts the proportion of participants assessed at follow-up in each of the three trial sites. Proportions of follow-up are broadly similar. The higher rate of 24-month follow-up in the Sussex site reflects the fact that this site participated in the extension phase only and, thus, all participants consented to this follow-up at the outset.
| Time point | Site | Proportion of participants assessed at follow-up, n (%) | |||
|---|---|---|---|---|---|
| Completed | Uncontactable | Declined | Withdrawn | ||
| 9 | Sussex | 53 (93) | 2 (3.5) | 0 (0.0) | 2 (3.5) |
| East Anglia | 102 (94) | 5 (4.6) | 1 (0.9) | 0 (0.0) | |
| Manchester | 86 (83) | 13 (13) | 4 (3.8) | 1 (1.0) | |
| Total | 241 (89) | 20 (7.4) | 5 (1.9) | 4 (1.5) | |
| 15 | Sussex | 50 (88) | 4 (7.0) | 0 (0.0) | 3 (5.3) |
| East Anglia | 98 (90) | 4 (3.7) | 1 (0.9) | 6 (5.5) | |
| Manchester | 87 (84) | 14 (14) | 1 (1.0) | 2 (1.9) | |
| Total | 235 (87) | 22 (8.1) | 2 (0.7) | 11 (4.1) | |
| 24 | Sussex | 49 (86) | 4 (7.0) | 1 (1.8) | 3 (5.3) |
| East Anglia | 84 (77) | 11 (10) | 8 (7.3) | 6 (5.5) | |
| Manchester | 73 (70) | 25 (24) | 3 (2.9) | 3 (2.9) | |
| Total | 206 (76) | 40 (15) | 12 (4.4) | 12 (4.4) | |
Intervention results
Description of enhanced standard care
Data regarding participant health and support service use were collected at each assessment point. The reporting period at baseline covered the 6 months prior to the assessment date. At each follow-up assessment, the reporting period covered the time since the previous assessment point.
Mental health service use was recorded as the number of contacts (Table 12). Overall, high incidence of mental health service use is noted and, in most cases, incidence is highest in the ESC-alone arm. Of particular note is the especially large number of individuals in the ESC-alone arm who received psychological therapies between baseline and the 9-month assessment point: 44% of participants received, on average, almost nine therapy sessions. Furthermore, the majority of participants in both arms reported 6–8 sessions of psychological therapy in the 9-month period leading up to trial entry (see Table 12). The majority of participants in both arms reported GP contacts at baseline and throughout the trial, and the majority of participants in both arms were taking antidepressant medication (see Table 12). A sizeable minority of participants also had case management, psychiatrist input and additional medication; case management was higher for SRT plus ESC participants across the trial, and psychiatrist input and additional medication were more frequent for ESC-alone participants.
| Resource | Time point, n (%)/mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 9 months | 15 months | 24 months | |||||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 124/138) | ESC alone (N = 108/132) | SRT + ESC (N = 124/138) | ESC alone (N = 100/132) | SRT + ESC (N = 107/138) | ESC alone (N = 83/132) | |
| GP | ||||||||
| Presence | 94 (68%) | 105 (80%) | 92 (74%) | 69 (64%) | 87 (70%) | 68 (68%) | 79 (74%) | 55 (66%) |
| Contacts | 5.4 (7.7) | 4.1 (3.5) | 4.9 (5.8) | 5.3 (4.4) | 3.0 (3.5) | 4.2 (3.7) | 4.6 (7.1) | 5.3 (6.1) |
| Psychiatrist | ||||||||
| Presence | 27 (20%) | 37 (28%) | 22 (18%) | 23 (21%) | 13 (10%) | 18 (18%) | 16 (15%) | 14 (17%) |
| Contacts | 2.9 (3.6) | 2.85 (3.0) | 2.0 (1.5) | 2.6 (2.2) | 3.2 (3.2) | 1.4 (0.51) | 1.9 (1.4) | 2.6 (2.9) |
| Psychological therapiesa | ||||||||
| Presence | 71 (51%) | 73 (55%) | 33 (27%) | 47 (44%) | 26 (21%) | 25 (25%) | 30 (28%) | 26 (31%) |
| Contacts | 8.0 (9.1) | 6.3 (5.6) | 11.8 (19.8) | 8.9 (6.3) | 6.9 (10.1) | 6.8 (11.4) | 10.3 (11.9) | 8.8 (10.9) |
| Case managementb | ||||||||
| Presence | 54 (39%) | 75 (57%) | 58 (47%) | 41 (38%) | 45 (36%) | 39 (39%) | 33 (31%) | 31 (37%) |
| Contacts | 10.3 (14.4) | 9.7 (17.5) | 11.5 (13.7) | 11.3 (13.9) | 9.02 (11.3) | 10.8 (11.4) | 10.6 (12.1) | 10.0 (13.7) |
| Medication | ||||||||
| Antidepressant | 78 (57%) | 78 (59%) | 71 (57%) | 59 (55%) | 61 (49%) | 49 (49%) | 60 (56%) | 41 (49%) |
| Antipsychotic | 16 (12%) | 5 (3.8%) | 14 (11%) | 3 (2.8%) | 13 (10%) | 4 (4.00%) | 12 (11%) | 2 (2.4%) |
| Other | 24 (17%) | 35 (27%) | 18 (15%) | 18 (17%) | 20 (16%) | 22 (22%) | 26 (24%) | 17 (20%) |
| Additional anxiolytic | 12 (8.7%) | 19 (14%) | 10 (8.06%) | 15 (14%) | 11 (8.9%) | 12 (12%) | 16 (15%) | 10 (12%) |
| Benzodiazepines | 7 (5.07%) | 7 (5.3%) | 7 (5.7%) | 4 (3.7%) | 7 (5.7%) | 4 (4.00%) | 4 (3.7%) | 2 (2.4%) |
| Mood stabilisers | 0 (0.0%) | 3 (2.3%) | 1 (0.81%) | 2 (1.9%) | 1 (0.81%) | 3 (3.00%) | 1 (0.93%) | 2 (2.4%) |
| Stimulantsc | 5 (3.6%) | 11 (8.3%) | 5 (4.03%) | 5 (4.6%) | 4 (3.2%) | 5 (5.00%) | 5 (4.7%) | 3 (3.6%) |
| Psychiatric admissionsd | ||||||||
| Number of people | 11 (7.9%) | 4 (3.03%) | 5 (4.03%) | 1 (0.93%) | 6 (4.8%) | 2 (2.00%) | 7 (6.5%) | 3 (3.6%) |
| Number of visits | 1.6 (1.2) | 1.00 (0.00) | 1.8 (1.8) | 4.00 (–) | 1.3 (0.82) | 3.00 (2.8) | 1.7 (1.1) | 1.00 (0.00) |
| Total days | 11.6 (26.1) | 1.7 (1.2) | 5.00 (4.3) | 6.00 (0.00) | 23.2 (39.4) | 6.00 (4.2) | 10.4 (14.7) | 1.00 (0.00) |
| Accident and emergency contacts (non-admission) | ||||||||
| Number of people | 21 (15%) | 14 (11%) | 15 (12%) | 18 (17%) | 14 (11%) | 18 (18%) | 16 (15%) | 13 (16%) |
It is also noteworthy that a higher proportion of participants in the SRT plus ESC arm (12%) than in the ESC-alone arm (4%) were taking antipsychotic medication at trial entry (see Table 12). The slightly higher rate in the SRT plus ESC arm was notable across the follow-up points. In addition, the rate of psychiatric admission (8% vs. 3%) and length of stay (11.6 vs. 1.7 days) were slightly higher in the SRT plus ESC arm than in the ESC-alone arm at baseline, and this pattern continued across follow-ups. A higher rate of accident and emergency visits was reported in the SRT plus ESC arm than in the ESC-alone arm at baseline (15% vs. 11%); however, this pattern reversed across the follow-up periods (see Table 12).
With respect to ‘packages’ of care (Table 13), participants allocated to SRT plus ESC (38% at 9 months and 54% at 15 months) were more likely than those assigned to ESC alone (30% at 9 months and 42% at 15 months) to have no NHS mental health service provision. Where provision did occur, the ‘packages’ of care appeared similar across both trial arms during the trial [other than the ESC-alone arm being more likely than the SRT plus ESC arm to have therapy during the 9-month intervention window only (21% vs. 9%)].
| Care ‘package’ | Time point, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 9 months | 15 months | 24 months | |||||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 124/138) | ESC alone (N = 108/132) | SRT + ESC (N = 124/138) | ESC alone (N = 100/132) | SRT + ESC (N = 107/138) | ESC alone (N = 83/132) | |
| No NHS mental health provision | 18 (13) | 23 (17) | 47 (38) | 32 (30) | 66 (54) | 42 (42) | 52 (49) | 38 (46) |
| Care co-ordinator/keyworker only | 29 (21) | 23 (17) | 30 (24) | 19 (18) | 25 (20) | 21 (21) | 16 (15) | 13 (16) |
| Psychological therapy only | 33 (24) | 40 (30) | 11 (8.9) | 23 (21) | 11 (8.9) | 12 (12) | 17 (16) | 9 (11) |
| Psychological therapy plus care co-ordinator/keyworker | 21 (15) | 19 (14) | 14 (11) | 11 (10) | 8 (6.5) | 7 (7.00) | 7 (6.5) | 7 (8.4) |
| Psychiatrist only | 7 (5.07) | 6 (4.5) | 6 (4.8) | 6 (5.6) | 1 (0.81) | 3 (3.00) | 5 (4.7) | 2 (2.4) |
| Psychological therapy plus care co-ordinator/keyworker plus psychiatrist | 11 (7.9) | 6 (4.5) | 6 (4.8) | 7 (6.5) | 6 (4.9) | 2 (2.00) | 5 (4.7) | 6 (7.2) |
| Care co-ordinator/keyworker plus psychiatrist | 13 (9.4) | 8 (6.06) | 8 (6.45) | 4 (3.7) | 6 (4.9) | 9 (9.00) | 6 (5.6) | 3 (3.6) |
| Psychological therapy plus psychiatrist | 6 (4.4) | 7 (5.3) | 2 (1.61) | 6 (4.8) | 0 (0.0) | 4 (4.00) | 0 (0.0) | 3 (3.6) |
Personal and support services were recorded in terms of the number of participants accessing such services and the duration of support, in hours (Table 14). It is notable that one-quarter to one-third of participants in both arms reported multiple hours of employment support across the trial period. Large SDs for the mean average values (see Table 14) suggest that the number of hours of personal and social support that individuals receive is extremely variable, with some individuals having received very high amounts of personal and social support over the reporting periods, especially with respect to youth and statutory service provision and social support group attendance.
| Service use | Time point, n (%)/mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 9 months | 15 months | 24 months | |||||
| SRT + ESC (N = 138) | ESC alone (N = 132) | SRT + ESC (N = 124/138) | ESC alone (N = 108/132) | SRT + ESC (N = 124/138) | ESC alone (N = 100/132) | SRT + ESC (N = 107/138) | ESC alone (N = 83/132) | |
| Employment support | ||||||||
| Presence | 33 (24%) | 36 (27%) | 42 (34%) | 43 (34%) | 39 (31%) | 27 (27%) | 32 (30%) | 25 (30%) |
| Duration (hours) | 10.07 (25.2) | 5.00 (7.01) | 8.5 (13.3) | 9.0 (19.3) | 8.4 (17.6) | 11.5 (17.9) | 56.1 (173.0) | 7.9 (10.8) |
| Youth services | ||||||||
| Presence | 9 (6.5%) | 15 (11%) | 16 (13%) | 12 (11%) | 5 (4.03%) | 4 (4.00%) | 4 (3.7%) | 5 (6.02%) |
| Duration (hours) | 13.2 (24.2) | 6.3 (4.4) | 8.2 (9.8) | 36.7 (97.4) | 18.2 (20.8) | 52.2 (43.7) | 27.1 (51.9) | 16.3 (11.6) |
| Statutory services | ||||||||
| Presence | 17 (12%) | 19 (14%) | 9 (7.3%) | 9 (8.3%) | 4 (3.2%) | 4 (4.00%) | 5 (4.7%) | 4 (4.8%) |
| Duration (hours) | 4.6 (6.2) | 5.1 (6.6) | 2.1 (2.3) | 21.8 (52.32) | 5.00 (1.7) | 161.5 (320.1) | 27.1 (52.0) | 61.5 (119.0) |
| Telephone support | ||||||||
| Presence | 14 (10%) | 11 (8%) | 7 (6%) | 10 (9%) | 8 (7%) | 5 (5%) | 8 (8%) | 8 (10%) |
| Duration (hours) | 4.7 (6.3) | 1.3 (1.8) | 6.9 (8.8) | 35.1 (100.1) | 0.81 (0.5) | 1.1 (0.9) | 2.0 (2.2) | 1.7 (1.6) |
| Social support groups | ||||||||
| Presence | 4 (2.9%) | 4 (3.03%) | 8 (6.5%) | 7 (6.5%) | 6 (4.8%) | 4 (4.00%) | 3 (2.8%) | 4 (4.8%) |
| Duration (hours) | 27.3 (27.8) | 26.7 (39.1) | 22.8 (18.2) | 33.5 (58.5) | 9.8 (8.1) | 43.5 (58.7) | 5.3 (2.3) | 66.3 (53.6) |
| Housing services and support | ||||||||
| Presence | 14 (10%) | 15 (11%) | 17 (14%) | 10 (9%) | 11 (8.9%) | 6 (6.00%) | 7 (6.5%) | 8 (9.6%) |
| Duration (hours) | 4.6 (6.0) | 25.1 (66.5) | 10.7 (19.1) | 8.1 (11.6) | 10.5 (12.7) | 3.00 (1.4) | 15.8 (12.3) | 2.5 (2.4) |
| Financial services and support | ||||||||
| Presence | 11 (8.00%) | 8 (6.06%) | 12 (9.6%) | 11 (10%) | 7 (5.7%) | 8 (8.00%) | 6 (5.6%) | 8 (9.6%) |
| Duration (hours) | 1.9 (1.9) | 1.6 (1.1) | 3.1 (4.1) | 21.8 (52.3) | 2.7 (4.1) | 2.01 (2.2) | 2.5 (1.9) | 0.95 (0.96) |
| Educational services and support | ||||||||
| Presence | 6 (4.4%) | 11 (8.3%) | 16 (12%) | 14 (13%) | 12 (9.7%) | 4 (4.00%) | 4 (3.7%) | 3 (3.6%) |
| Duration (hours) | 6.1 (1.5) | 22.1 (35.6) | 68.1 (121.8) | 43.7 (113.9) | 18.7 (25.7) | 3.8 (1.8) | 2.5 (2.1) | 158.7 (267.9) |
Social recovery therapy adherence and competence
Adherence
The number of SRT sessions received by participants ranged from 0–33, with a mean of 16.8 sessions (SD = 7.9 sessions). Of the 138 participants who were randomised to receive SRT, 91 (68%) received the full dose, 23 (17%) received a partial dose and 24 (18%) received no dose. Thus, adherence was reached for 86% of participants in total (n = 113). The total number of sessions across all participants was 2298. Twenty-one (16%) participants received fewer than six SRT sessions; two participants (2%) received no sessions. On average, the no-dose group received 3.88 sessions (range 0–21 sessions, SD 4.1 sessions), the partial dose group received 16.8 sessions (range 6–32 sessions, SD 6.6 sessions) and the full-dose group received 20.2 sessions (range 7–33 sessions, SD 5.0 sessions).
Competence
Seventy-five sessions (3.3%) were rated for competence by 1–10 raters. The mean CTS-R score was 47.2 (range 33–63, SD 6.5) and 97% (n = 73) of all sessions were rated as competent, which reflects a CTS-R score of ≥ 36. Agreement among raters regarding competence status was 95%.
Main trial results
Summary of main trial results
There was no evidence of the superiority of SRT plus ESC to ESC alone. There were no consistent significant differences between the two trial arms. On the primary outcome, time spent in structured activity at 15 months, the level of missing data was 13%, with 11 (4%) participants missing in the SRT plus ESC arm and 24 (9%) participants missing in the ESC-alone arm. For the other assessments, missing data rates were higher. At 15 months, for the interview-based psychopathology assessments (SCID and CAARMS) 27% of data were missing: 30 participants (11%) in the SRT plus ESC arm and 44 participants (16%) in the ESC alone arm. For self-report measures (BDI depression, SIAS social anxiety, BHS hopelessness and SSI schizotypal experiences), 23% of data were missing at 15 months: 23 participants (9%) in the SRT plus ESC arm and 38 participants (14%) in the ESC-alone arm. There was a consistent trend for higher levels of missingness of data in the ESC-alone arm than in the SRT arm, particularly at 15 months. Rates of missingness of data were substantially less at 9 months, with less discrepancy in missingness of data between arms. The level of missingness of data and data patterns were reviewed by the statistical team and agreed to be suitable for the ITT analysis using a general linear model.
Intention-to-treat analysis of primary outcome
The primary outcome was prespecified to be levels of structured activity at 15 months. There was no evidence of any superiority of SRT over ESC or consistent differences in levels of structured activity at 15 months (both including and excluding levels of child care) (Table 15).
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 15 months | ||||||
| Structured activity (hours per week) | 22.4 (21.4) | 27.7 (26.5) | –4.44 (–10.19 to 1.31) | 0.13 | –0.12 (–0.34 to 0.10) | 0.29 |
| Missing, n | 11 | 24 | ||||
| Structured activity (minus child care) (hours per week) | 21.1 (18.1) | 24.9 (20.4) | –2.98 (–7.49 to 1.53) | 0.19 | –0.08 (–0.29 to 0.13) | 0.46 |
| Missing, n | 11 | 24 | ||||
| 9 months | ||||||
| Structured activity (hours per week) | 21.4 (16.6) | 22.3 (19.3) | –0.90 (–5.02 to 3.21) | 0.67 | 0.07 (–0.14 to 0.28) | 0.50 |
| Missing, n | 12 | 17 | ||||
| Structured activity (minus child care) (hours per week) | 20.3 (14.7) | 22.2 (19.3) | –1.71 (–5.67 to 2.26) | 0.40 | 0.06 (–0.15 to 0.27) | 0.57 |
| Missing, n | 12 | 17 | ||||
Including an interaction term for either of the mental state (ARMS vs. not at risk) or functioning (low vs. very low levels of structured activity) stratifiers did not have an impact on findings (see Appendix 3, Table 40). Therefore, there was no evidence for the superiority of SRT irrespective of participant ARMS or functioning status at baseline.
Analysis of secondary and other outcomes
There was no evidence of any superiority of SRT over ESC, or evidence for consistent differences in structured activity at 9 months or in constructive economic activity at 15 months (Table 16). There was no evidence of superiority of SRT in total levels of employment, education and voluntary work activity. As there were no consistent statistically significant differences between the intervention and the control groups in the primary or secondary outcomes, mediation and moderation analyses were not conducted.
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 15 months | ||||||
| Constructive economic activity (hours per week) | 17.4 (19.9) | 22.0 (24.5) | –4.44 (–9.88 to 1.01) | 0.11 | –0.22 (–0.46 to 0.03) | 0.085 |
| Missing, n | 11 | 24 | ||||
| Total hours paid employmentd | 75.3 (213.8) | 123.6 (275.2) | –47.03 (–111.53 to 17.47) | 0.15 | –0.30 (–0.92 to 0.31) | 0.34 |
| Missing, n | 21 | 31 | ||||
| Total hours educationd | 76.6 (174.1) | 88.8 (176.3) | –12.35 (–59.48 to 34.79) | 0.61 | –0.26 (–0.90 to 0.38) | 0.43 |
| Missing, n | 25 | 31 | ||||
| Total hours voluntary employmentd | 16.5 (46.8) | 36.8 (96.3) | –19.21 (–39.35 to 0.93) | 0.062 | –0.25 (–0.75 to 0.24) | 0.32 |
| Missing, n | 27 | 28 | ||||
| Total hours all activityd | 158.8 (252.0) | 258.8 (359.8) | –93.04 (–175.93 to –10.15) | 0.028 | –0.46 (–1.13 to 0.21) | 0.18 |
| Missing, n | 34 | 35 | ||||
| 9 months | ||||||
| Constructive economic activity (hours per week) | 15.7 (14.3) | 16.6 (15.9) | –1.14 (–4.74 to 2.45) | 0.53 | –0.01 (–0.24 to 0.23) | 0.95 |
| Missing, n | 12 | 17 | ||||
| Total hours paid employmentd | 156.9 (432.3) | 283.9 (511.4) | –106.66 (–239.19 to 25.87) | 0.11 | –0.65 (–1.45 to 0.14) | 0.11 |
| Missing, n | 25 | 48 | ||||
| Total hours educationd | 130.1 (353.3) | 108.0 (235.0) | 23.74 (–66.66 to 114.15) | 0.61 | 0.18 (–0.55 to 0.91) | 0.63 |
| Missing, n | 26 | 50 | ||||
| Total hours voluntary employmentd | 50.5 (215.4) | 50.7 (170.9) | –4.04 (–60.84 to 52.77) | 0.89 | –0.11 (–0.71 to 0.48) | 0.71 |
| Missing, n | 33 | 47 | ||||
| Total hours all activityd | 349.1 (591.2) | 454.2 (592.9) | –88.83 (–259.47 to 81.81) | 0.31 | –0.43 (–1.22 to 0.37) | 0.29 |
| Missing, n | 35 | 56 | ||||
Secondary psychopathology outcomes were analysed using general linear models, adjusting for baseline values (of the outcome variable), stratification variables and neurocognitive performance. Data for CAARMS symptom severity at 9 months, and the suicidality severity score at 9, 15 and 24 months, were found to be highly skewed and, therefore, a log-transformation on the outcome variable was used. There was no evidence of any superiority of SRT over ESC or consistent differences in levels of psychopathology, as assessed by ARMS symptom severity or distress rate (Table 17), in diagnosable depression or anxiety disorder (Tables 18 and 19), or in self-reported psychopathology (Tables 20 and 21) or other outcomes (Table 22). For all secondary psychopathology outcomes there were more missing data in the ESC-alone arm than in the SRT plus ESC arm. At 15 months, the rate of missing data in the ESC-alone arm was typically double that in the intervention arm.
| Severity and distress outcomes | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 9 months | ||||||
| CAARMS symptom severity score | 28.4 (21.5) | 27.1 (18.7) | 2.26 (–1.92 to 6.43) | 0.29 | 0.09 (–0.13 to 0.32) | 0.40 |
| Missing, n | 21 | 28 | ||||
| CAARMS average distress score | 47.5 (27.5) | 44.7 (24.3) | 2.11 (–3.73 to 7.95) | 0.48 | ||
| Missing, n | 27 | 28 | ||||
| CAARMS aggression severity score | 6.2 (5.4) | 6.2 (5.8) | 0.57 (–0.75 to 1.89) | 0.40 | ||
| Missing, n | 15 | 18 | ||||
| CAARMS suicidality severity score | 5.7 (6.9) | 4.5 (5.8) | 0.98 (–0.55 to 2.51) | 0.21 | 0.14 (–0.13 to 0.42) | 0.31 |
| Missing, n | 15 | 17 | ||||
| 15 months | ||||||
| CAARMS symptom severity score | 23.4 (21.0) | 24.3 (18.9) | 0.29 (–4.35 to 4.94) | 0.90 | ||
| Missing, n | 23 | 42 | ||||
| CAARMS average distress score | 43.4 (28.1) | 39.7 (25.8) | 4.09 (–3.52 to 11.70) | 0.29 | ||
| Missing, n | 34 | 40 | ||||
| CAARMS aggression severity score | 5.6 (5.7) | 5.5 (6.1) | 0.62 (–0.89 to 2.12) | 0.42 | ||
| Missing, n | 11 | 32 | ||||
| CAARMS suicidality severity score | 4.4 (6.1) | 3.8 (6.1) | 0.82 (–0.74 to 2.39) | 0.30 | 0.21 (–0.09 to 0.50) | 0.17 |
| Missing, n | 15 | 33 | ||||
| Mood outcome | Time point | Prevalence, n (%), n missing | Relative riska (95% CIb) | p-valuec | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Current major depressive episode | Baseline | 72 (52), 0 | 65 (49), 0 | ||
| 9 months | 26 (21), 13 | 33 (29), 19 | 0.71 (0.46 to 1.11) | 0.18 | |
| 15 months | 35 (29), 16 | 26 (26), 31 | 1.11 (0.72 to 1.72) | 0.65 | |
| Current mania | Baseline | 1 (0.7), 0 | 1 (0.8), 0 | ||
| 9 months | 1 (0.8), 13 | 2 (1.8), 20 | 0.45 (0.04 to 4.87) | 0.60 | |
| 15 months | 0 (0), 16 | 2 (2.0), 30 | – | 0.21 | |
| Current hypomania | Baseline | 5 (3.6), 0 | 2 (1.5), 0 | ||
| 9 months | 2 (1.6), 13 | 2 (1.8), 20 | 0.90 (0.13 to 6.26) | 1.00 | |
| 15 months | 1 (0.8), 16 | 2 (2.0), 30 | 0.42 (0.04 to 4.54) | 0.59 | |
| Dysthymia | Baseline | 16 (12), 0 | 15 (11), 0 | ||
| 9 months | 13 (10), 13 | 8 (7.1), 20 | 1.46 (0.63 to 3.38) | 0.49 | |
| 15 months | 15 (12), 16 | 7 (6.9), 31 | 1.77 (0.75 to 4.18) | 0.26 | |
| Bipolar at risk | Baseline | 25 (18), 0 | 14 (11), 0 | ||
| 9 months | 6 (4.8), 13 | 11 (9.8), 20 | 0.49 (0.19 to 1.28) | 0.21 | |
| 15 months | 8 (6.6), 16 | 6 (5.9), 31 | 1.10 (0.40 to 3.08) | 1.00 | |
| Bipolar I | Baseline | 2 (1.5), 0 | 5 (3.8), 0 | ||
| 9 months | 3 (2.4), 13 | 5 (4.5), 20 | 0.54 (0.13 to 2.20) | 0.48 | |
| 15 months | 2 (1.6), 16 | 5 (4.9), 30 | 0.33 (0.07 to 1.69) | 0.25 | |
| Bipolar II | Baseline | 4 (2.9), 0 | 1 (0.8), 0 | ||
| 9 months | 3 (2.4), 13 | 2 (1.8), 20 | 1.34 (0.23 to 7.90) | 1.00 | |
| 15 months | 2 (1.6), 16 | 0 (0), 30 | – | 0.50 | |
| Anxiety, eating and somatoform outcome | Time point | Prevalence, n (%), n missinga | Relative riskb (95% CIc) | p-valued | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Panic disorder with agoraphobia | Baseline | 18 (13), 0 | 25 (19), 0 | ||
| 9 months | 7 (41), 1 | 12 (55), 3 | 0.75 (0.38 to 1.50) | 0.52 | |
| 15 months | 7 (41), 1 | 12 (57), 4 | 0.72 (0.37 to 1.42) | 0.52 | |
| Agoraphobia without panic | Baseline | 21 (15), 0 | 31 (24), 0 | ||
| 9 months | 10 (53), 2 | 18 (72), 6 | 0.73 (0.45 to 1.20) | 0.22 | |
| 15 months | 11 (58), 2 | 14 (58), 7 | 0.99 (0.60 to 1.65) | 1.00 | |
| Social phobia | Baseline | 62 (45), 0 | 54 (41), 0 | ||
| 9 months | 22 (40), 7 | 24 (53), 9 | 0.75 (0.49 to 1.15) | 0.23 | |
| 15 months | 28 (52), 8 | 16 (39), 13 | 1.33 (0.84 to 2.11) | 0.30 | |
| Specific phobia | Baseline | 10 (7.3), 0 | 4 (3.0), 0 | ||
| 9 months | 3 (30), 0 | 1 (25), 0 | 1.20 (0.17 to 8.38) | 1.00 | |
| 15 months | 5 (50), 0 | 1 (25), 0 | 2.00 (0.33 to 12.18) | 0.58 | |
| OCD | Baseline | 12 (8.7), 0 | 11 (8.3), 0 | ||
| 9 months | 4 (33), 0 | 1 (10), 1 | 3.33 (0.44 to 25.23) | 0.32 | |
| 15 months | 5 (42), 0 | 0 (0), 3 | – | 0.055 | |
| PTSD | Baseline | 14 (10), 0 | 16 (12), 0 | ||
| 9 months | 5 (46), 3 | 5 (42), 4 | 1.09 (0.43 to 2.77) | 1.00 | |
| 15 months | 6 (55), 3 | 5 (46), 5 | 1.20 (0.52 to 2.79) | 1.00 | |
| GAD | Baseline | 36 (26), 0 | 44 (33), 0 | ||
| 9 months | 11 (33), 3 | 16 (44), 8 | 0.75 (0.41 to 1.37) | 0.46 | |
| 15 months | 13 (41), 4 | 10 (30), 11 | 1.34 (0.69 to 2.61) | 0.44 | |
| Body dysmorphic disorder | Baseline | 14 (10), 0 | 10 (7.6), 0 | ||
| 9 months | 8 (62), 1 | 2 (29), 3 | 2.15 (0.62 to 7.50) | 0.35 | |
| 15 months | 8 (67), 2 | 2 (29), 3 | 2.33 (0.68 to 8.04) | 0.17 | |
| Emotional disturbance | Allocation arm, mean (SD) | Adjusted differencea (95% CIb) | p-valuec | |
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| 9 months | ||||
| SIAS score | 44.1 (16.9) | 44.0 (15.6) | –2.56 (–6.13 to 1.01) | 0.16 |
| Missing, n | 23 | 29 | ||
| BDI-II score | 18.6 (15.4) | 19.9 (13.7) | –1.28 (–4.83 to 2.26) | 0.48 |
| Missing, n | 24 | 32 | ||
| 15 months | ||||
| SIAS score | 43.1 (17.7) | 42.2 (17.7) | –0.45 (–4.84 to 3.95) | 0.84 |
| Missing, n | 23 | 38 | ||
| BDI-II score | 19.2 (15.7) | 19.4 (14.9) | –0.32 (–4.06 to 3.42) | 0.87 |
| Missing, n | 25 | 38 | ||
| Emotional disturbance | Allocation arm, mean (SD) | Adjusted differencea (95% CIb) | p-valuec | |
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| 9 months | ||||
| GAF | 49.7 (15.8) | 48.6 (14.9) | 1.57 (–2.12 to 5.27) | 0.40 |
| Missing, n | 17 | 26 | ||
| GAS | 52.2 (15.2) | 51.2 (14.1) | 1.02 (–2.51 to 4.56) | 0.57 |
| Missing, n | 17 | 26 | ||
| SOFAS | 51.7 (15.5) | 53.4 (16.5) | 0.24 (–3.37 to 3.84) | 0.90 |
| Missing, n | 17 | 26 | ||
| 15 months | ||||
| GAF | 50.8 (18.0) | 51.9 (17.4) | –0.93 (–5.24 to 3.38) | 0.67 |
| Missing, n | 21 | 37 | ||
| GAS | 54.2 (16.0) | 55.6 (17.9) | –1.72 (–5.95 to 2.51) | 0.43 |
| Missing, n | 21 | 35 | ||
| SOFAS | 54.6 (17.3) | 55.8 (19.4) | 0.60 (–3.64 to 4.84) | 0.78 |
| Missing, n | 20 | 35 | ||
| Outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 9 months | ||||||
| BHS score | 9.2 (6.2) | 9.1 (5.9) | –0.17 (–1.70 to 1.36) | 0.83 | ||
| Missing, n | 28 | 33 | ||||
| AUDIT | 4.6 (6.6) | 4.4 (5.1) | 0.52 (–0.67 to 1.71) | 0.39 | 0.01 (–0.20 to 0.22) | 0.92 |
| Missing, n | 23 | 29 | ||||
| DUDIT | 3.1 (6.6) | 3.8 (7.8) | –0.71 (–2.02 to 0.61) | 0.29 | –0.08 (–0.28 to 0.12) | 0.42 |
| Missing, n | 19 | 28 | ||||
| 15 months | ||||||
| BHS score | 9.5 (6.1) | 9.5 (6.4) | –0.17 (–1.80 to 1.47) | 0.84 | ||
| Missing, n | 22 | 39 | ||||
| AUDIT | 4.6 (6.0) | 4.5 (6.0) | 0.63 (–0.69 to 1.95) | 0.35 | 0.08 (–0.14 to 0.30) | 0.46 |
| Missing, n | 22 | 35 | ||||
| DUDIT | 2.6 (5.7) | 3.4 (7.9) | –1.05 (–2.54 to 0.45) | 0.17 | –0.08 (–0.30 to 0.14) | 0.46 |
| Missing, n | 19 | 39 | ||||
Past mood and infrequently endorsed anxiety, eating and somatoform diagnoses are presented in Appendix 3, Analysis of general psychopathology diagnoses continued. The majority of participants met diagnostic criteria for a current major depressive episode (see Table 18) and almost half met criteria for current social phobia (see Table 19).
As secondary outcome measures, self-report questionnaire data were analysed using general linear models, adjusting for the stratification variables, neurocognitive performance and differences in the outcome variable at baseline (see Tables 20 and 21).
Outcomes for negative symptoms (SANS) are presented in Appendix 3, Analysis of negative symptoms. There was no consistent evidence of the superiority of SRT plus ESC over ESC alone with respect to SANS. There was no evidence for any superiority of SRT or consistent differences in levels of drug and alcohol disorders (see Table 22).
Analysis of maintenance of gains at 24 months
The 24-month assessment was designed as a specific add-on study. This was requested by the funders after completion of the internal pilot phase and recruitment of the first 100 participants. The original study was designed with a 9-month and 15-month assessment, with 15 months being the primary end point. The 24-month follow-up required recontacting the original 100 participants to reconsent and capture follow-up data, as they had not originally given consent for this longer-term assessment point. The subsequent 170 participants were then consented prospectively.
On the primary outcome of time spent in structured activity, the proportion of missing data at 24 months was 24% (65 participants). There were 25 participants in the treatment group (9%) and 40 participants in the control group (15%) with missing data. CAARMS symptom severity data were unavailable for > 50% of participants, so these data were unusable. CAARMS transition to psychosis data were missing for 34% of participants: 15% in the treatment group and 19% in the control group. SCID psychopathology data were missing for 30% of participants: 12% in the treatment group and 18% in the control group. Self-report psychopathology data were missing for 33% of participants: 14% in the treatment group and 19% in the control group.
Outcomes at 24 months suggest that the clinically important gains in both arms on structured activity (Table 23), general psychopathology (Tables 24–26) and other outcomes (Table 27) at 9 and 15 months are maintained at 24 months.
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 24.3 (18.9) | 32.4 (28.7) | –7.51 (–13.91 to –1.12) | 0.022 | –0.20 (–0.45 to 0.04) | 0.099 |
| Missing, n | 25 | 40 | ||||
| Structured activity (minus child care) (hours per week) | 23.8 (18.9) | 26.6 (20.4) | –2.37 (–7.59 to 2.84) | 0.370 | –0.09 (–0.33 to 0.16) | 0.48 |
| Missing, n | 25 | 40 | ||||
| Constructive economic activity (hours per week) | 18.6 (16.7) | 27.4 (28.0) | –8.34 (–14.41 to –2.27) | 0.007 | –0.28 (–0.55 to –0.02) | 0.038 |
| Missing, n | 25 | 40 | ||||
| Total hours educationd | 76.7 (175.3) | 93.6 (182.4) | –16.88 (–66.60 to 32.85) | 0.504 | –0.27 (–0.93 to 0.39) | 0.42 |
| Missing, n | 32 | 39 | ||||
| Total hours voluntary employmentd | 14.3 (37.7) | 38.3 (99.7) | –24.10 (–44.78 to –3.42) | 0.023 | –0.31 (–0.82 to 0.20) | 0.23 |
| Missing, n | 34 | 36 | ||||
| Total hours all activityd | 161.0 (255.0) | 258.9 (364.5) | –92.86 (–179.00 to –6.72) | 0.035 | –0.46 (–1.15 to 0.24) | 0.20 |
| Missing, n | 39 | 40 | ||||
| Total hours paid employmentd | 79.1 (218.8) | 121.7 (277.7) | –41.41 (–108.58 to 25.76) | 0.226 | –0.25(–0.88 to 0.39) | 0.439 |
| Missing, n | 27 | 37 | ||||
| Mood outcome | Time point | Prevalence, n (%), n missing | Relative riska (95% CIb) | p-valuec | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Current major depressive episode | Baseline | 72 (52.2), 0 | 65 (49.2), 0 | ||
| 24 months | 24 (22.6), 32 | 18 (21.7), 49 | 1.04 (0.61 to 1.79) | 1.00 | |
| Past major depressive episode | Baseline | 33 (23.9), 0 | 41 (31.1), 0 | ||
| 24 months | 24 (22.6), 32 | 12 (14.6), 50 | 1.55 (0.82 to 2.91) | 0.19 | |
| Current mania | Baseline | 1 (0.7), 0 | 1 (0.8), 0 | ||
| 24 months | 2 (1.9), 32 | 0 (0), 49 | – | 0.51 | |
| Past mania | Baseline | 2 (1.5), 0 | 5 (3.8), 0 | ||
| 24 months | 2 (1.9), 32 | 1 (1.2), 49 | 1.57 (0.14 to 16.98) | 1.00 | |
| Current hypomania | Baseline | 5 (3.6), 0 | 2 (1.5), 0 | ||
| 24 months | 2 (1.9), 32 | 2 (2.4), 49 | 0.78 (0.11 to 5.44) | 1.00 | |
| Past hypomania | Baseline | 2 (1.5), 0 | 1 (0.8), 0 | ||
| 24 months | 2 (1.9), 32 | 2 (2.4), 49 | 0.78 (0.11, to 5.44) | 1.00 | |
| Dysthymia | Baseline | 16 (11.6), 0 | 15 (11.4), 0 | ||
| 24 months | 20 (18.9), 32 | 7 (8.4), 49 | 2.24 (0.99 to 5.04) | 0.058 | |
| Bipolar at risk | Baseline | 25 (18.1), 0 | 14 (10.6), 0 | ||
| 24 months | 11 (10.4), 32 | 6 (7.3), 50 | 1.42 (0.55 to 3.67) | 0.61 | |
| Bipolar I | Baseline | 2 (1.5), 0 | 5 (3.8), 0 | ||
| 24 months | 4 (3.8), 32 | 1 (1.2), 50 | 3.09 (0.35 to 27.16) | 0.39 | |
| Bipolar II | Baseline | 4 (2.9), 0 | 1 (0.8), 0 | ||
| 24 months | 2 (1.9), 32 | 1 (1.2), 50 | 1.55 (0.14 to 16.77) | 1.00 | |
| Major depressive disorder | Baseline | 95 (68.8), 0 | 93 (70.5), 0 | ||
| 24 months | 45 (42.5), 32 | 28 (33.7), 49 | 1.26 (0.87 to 1.83) | 0.23 | |
| Anxiety, eating and somatoform outcome | Time point | Prevalence, n (%), n missinga | Relative riskb (95% CIc) | p-valued | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Panic disorder with agoraphobia | Baseline | 18 (13), 0 | 25 (19), 0 | ||
| 24 months | 4 (29), 4 | 4 (22), 7 | 1.29 (0.39 to 4.26) | 0.71 | |
| Agoraphobia without panic | Baseline | 21 (15), 0 | 31 (24), 0 | ||
| 24 months | 7 (44), 5 | 9 (45), 11 | 0.97 (0.47 to 2.03) | 1.00 | |
| Social phobia | Baseline | 62 (45), 0 | 54 (41), 0 | ||
| 24 months | 27 (59), 16 | 13 (36), 18 | 1.63 (0.99 to 2.67) | 0.049 | |
| Specific phobia | Baseline | 10 (7.3), 0 | 4 (3.0), 0 | ||
| 24 months | 4 (57), 3 | 1 (33), 1 | 1.71 (0.31 to 9.61) | 1.00 | |
| OCD | Baseline | 12 (8.7), 0 | 11 (8.3), 0 | ||
| 24 months | 3 (27.3), 1 | 0 (0), 3 | – | 0.23 | |
| PTSD | Baseline | 14 (10), 0 | 16 (12), 0 | ||
| 24 months | 2 (20), 4 | 4 (50), 8 | 0.40 (0.10 to 1.66) | 0.32 | |
| GAD | Baseline | 36 (26), 0 | 44 (33), 0 | ||
| 24 months | 13 (48), 9 | 11 (42), 18 | 1.14 (0.63 to 2.06) | 0.79 | |
| Body dysmorphic disorder | Baseline | 14 (10), 0 | 10 (7.6), 0 | ||
| 24 months | 4 (36.4), 3 | 0 (0), 4 | |||
| Emotional disturbance | Allocation arm, mean (SD) | Adjusted differencea (95% CIb) | p-valuec | |
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| SIAS score | 43.9 (17.6) | 41.3 (17.4) | 1.48 (–3.50 to 6.45) | 0.56 |
| Missing, n | 39 | 49 | ||
| BDI-II score | 18.0 (15.7) | 17.5 (14.8) | 1.93 (–2.22 to 6.08) | 0.36 |
| Missing, n | 41 | 51 | ||
| GAF | 50.3 (17.2) | 53.4 (16.2) | –2.87 (–7.49 to 1.76) | 0.22 |
| Missing, n | 36 | 51 | ||
| GAS | 53.3 (17.6) | 56.6 (16.6) | –3.63 (–8.40 to 1.14) | 0.14 |
| Missing, n | 38 | 50 | ||
| SOFAS | 53.3 (18.1) | 57.4 (19.4) | –2.05 (–6.91 to 2.81) | 0.41 |
| Missing, n | 38 | 50 | ||
| Outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| BHS score | 9.6 (6.1) | 7.8 (6.2) | 1.40 (–0.34 to 3.14) | 0.12 | ||
| Missing, n | 40 | 52 | ||||
| AUDIT | 3.5 (4.3) | 3.7 (3.3) | 0.24 (–0.68 to 1.16) | 0.60 | –0.12 (–0.35 to 0.10) | 0.27 |
| Missing, n | 36 | 49 | ||||
| DUDIT | 2.1 (6.0) | 3.3 (7.3) | –1.36 (–2.86 to 0.14) | 0.075 | –0.29 (–0.51 to –0.06) | 0.013 |
| Missing, n | 34 | 47 | ||||
The ITT analysis provides no evidence for superiority of SRT over ESC on any primary or secondary outcome at 24 months. There was weak evidence for superiority of SRT plus ESC over ESC alone for the other outcome of reduction of substance misuse as measured by the DUDIT at 24 months (see Table 27). On one dimension of secondary outcome (constructive economic activity) there was evidence of a consistent difference in favour of the ESC-alone arm (see Table 23). The PP analysis (Table 28) was conducted for time use variables at 24 months and supported the ITT model.
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 22.4 (18.0) | 32.4 (28.7) | –8.75 (–16.07 to –1.42) | 0.020 | –0.27 (–0.54 to 0.01) | 0.059 |
| Missing, n | 12 | 40 | ||||
| Structured activity (minus child care) (hours per week) | 21.9 (18.0) | 26.6 (20.4) | –3.94 (–9.69 to 1.82) | 0.18 | –0.16 (–0.43 to 0.12) | 0.26 |
| Missing, n | 12 | 40 | ||||
| Constructive economic activity (hours per week) | 17.9 (16.4) | 27.4 (28.0) | –7.97 (–15.02 to –0.93) | 0.027 | –0.28 (–0.59 to 0.03) | 0.073 |
| Missing, n | 12 | 40 | ||||
| Total hours paid employmentd | 42.9 (112.1) | 121.7 (277.7) | –73.53 (–141.04 to –6.02) | 0.033 | –0.55 (–1.24 to 0.15) | 0.12 |
| Missing, n | 14 | 37 | ||||
| Total hours educationd | 58.8 (141.4) | 93.6 (182.4) | –32.55 (–84.01 to 18.92) | 0.21 | –0.23 (–0.96 to 0.49) | 0.52 |
| Missing, n | 16 | 39 | ||||
| Total hours voluntary employmentd | 17.2 (41.7) | 38.3 (99.7) | –18.98 (–43.61 to 5.66) | 0.13 | –0.08 (–0.66 to 0.51) | 0.80 |
| Missing, n | 17 | 36 | ||||
| Total hours all activityd | 118.7 (172.4) | 258.9 (364.5) | –132.41 (–224.74 to –40.09) | 0.005 | –0.48 (–1.25 to 0.29) | 0.22 |
| Missing n | 21 | 40 | ||||
The missing data analysis (see Appendix 3, Analysis of missing data for primary outcome and Analysis of missing data: comparison of methods) is consistent with the ITT analysis, suggesting that there is no evidence for the superiority of SRT over ESC alone at the 24-month assessment. The missing data analyses and PP analysis are consistent with the ITT analysis in providing weak evidence of a consistent difference in favour of the ESC-alone arm. However, these results need to be interpreted with some caution. Both methods used to mitigate against bias from missing data (i.e. FIML and MI) do so only on the assumption that data are missing at random. If this assumption does not hold (i.e. when data are missing not at random), biased estimates may still occur as a result of the missing data.
Serious adverse events
All serious adverse events (SAEs) and adverse events (AEs) were categorised and recorded from randomisation until completion of the final follow-up assessment. Definitions used for delineating events are provided in Appendix 4. All events were reported to the chief investigator, sponsor, local trial site, Clinical Trials Unit, and trial oversight committees (i.e. DMEC and TSC). All SAEs were reviewed by a clinician independent of the trial for possible relatedness. SAEs would have been reported to the NHS REC, as appropriate (i.e. deemed possibly related to the trial). Zero events were considered possibly related to the trial and, thus, none was reported to the REC. The frequency and nature of SAEs and AEs are reported in Tables 29–31.
| Event type | Allocation arm, n (%) | |||
|---|---|---|---|---|
| SRT + ESC | ESC alone | |||
| Events | Individuals | Events | Individuals | |
| Any event | 105 | 48 (35) | 62 | 32 (24) |
| AEs | 52 | 35 (25) | 32 | 24 (18) |
| SAEs | 53 | 26 (19) | 30 | 16 (12) |
| Adverse reactions | 0 | 0 (0) | 0 | 0 (0) |
| Unexpected adverse reactions | 0 | 0 (0) | 0 | 0 (0) |
| Serious adverse reactions | 0 | 0 (0) | 0 | 0 (0) |
| Suspected unexpected serious adverse reactions | 0 | 0 (0) | 0 | 0 (0) |
| Reporting mechanism | Allocation arm, n (%) | |||
|---|---|---|---|---|
| SRT + ESC | ESC alone | |||
| Events | Individuals | Events | Individuals | |
| AE reported to research therapist | 17 | 14 | 0 | 0 |
| SAE reported to research therapist | 17 | 12 | 0 | 0 |
| AE reported to RA | 35 | 25 | 32 | 24 |
| SAE reported to RA | 36 | 22 | 30 | 16 |
| 9-month assessment | 6 | 6 (4.8) | 10 | 10 (8.7) |
| 15-month assessment | 17 | 13 (10) | 9 | 7 (6.5) |
| 24-month assessment | 13 | 11 (9.6) | 11 | 7 (7.6) |
| Event type | Allocation arm (n) | |||
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| Events | Individuals | Events | Individuals | |
| SAE | ||||
| Potentially/life-threatening self-harm | 1 | 1 | 2 | 2 |
| Medication or substance overdose | 16 | 10 | 10 | 7 |
| Medication/substance overdose resulting in hospital admission | 5 | 4 | 1 | 1 |
| Potentially/life-threatening road behaviour | 2 | 1 | 0 | 0 |
| Potentially/life-threatening overdose and self-harm | 1 | 1 | 0 | 0 |
| Suicidal intent | 7 | 6 | 1 | 1 |
| Suicide attempt | ||||
| Medication overdose | 7 | 7 | 3 | 3 |
| Medication overdose resulting in hospital admission | 1 | 1 | 2 | 1 |
| Self-harm, self-poisoning and/or ligature use | 4 | 4 | 3 | 3 |
| Overdose and self-harm resulting in hospital admission | 1 | 1 | 1 | 1 |
| Other | 3 | 3 | 4 | 3 |
| Assault on participant | 1 | 1 | 0 | 0 |
| Hospitalisation | ||||
| Psychiatric | 3 | 3 | 0 | 0 |
| General | 1 | 1 | 2 | 2 |
| Other | 0 | 0 | 1 | 1 |
| AE | ||||
| Suicidal ideation | 13 | 10 | 6 | 6 |
| Self-harm | 12 | 11 | 9 | 7 |
| Medication overdose | 2 | 2 | 2 | 2 |
| Assault by participant | 1 | 1 | 0 | 0 |
| Physical/sexual assault on participant | 2 | 2 | 0 | 0 |
| General medical assessment/procedure | 2 | 2 | 3 | 3 |
| Bereavement | 1 | 1 | 1 | 1 |
| Escalation in alcohol and/or substance use | 4 | 3 | 3 | 2 |
| Thoughts of harming others | 3 | 3 | 0 | 0 |
| Police cell admission | 1 | 1 | 0 | 0 |
| Acute anxiety and/or tearfulness | 0 | 0 | 3 | 3 |
| Deterioration in mental health | 1 | 1 | 2 | 2 |
Frequencies of AEs and SAEs are shown in Table 29. There were 167 reportable AEs in the trial, 83 of which were considered serious. All events were considered unrelated to the trial. It is noted that events are clustered around individuals (i.e. individuals who frequently report more than one event); therefore, the number of events exceeds the number of individuals reporting them. Moreover, an excess of both SEs and AEs in the intervention arm is noted. This excess can be explained according to the reporting mechanism (see Table 30). The number of AEs reported to RAs at assessment points was the same or very similar in both groups, but a subset of individuals in the intervention arm reported AEs to therapists during the intervention period, a mechanism that was not available to participants in the ESC-alone arm.
A discrepancy remained (see Table 30) in that more individuals in the SRT plus ESC arm than in the ESC-alone arm reported SAEs to researchers. However, it is also notable that the proportion of participants who completed all follow-ups was greater in the SRT plus ESC arm; therefore, events reported to researchers were further delineated with respect to follow-up point to allow SAEs to be calculated as proportions of completers. A smaller proportion of participants in the SRT plus ESC arm reported a lower number of SAEs to researchers at their 9-month assessment than participants in the ESC arm. At 15 and 24 months, a slightly higher percentage of SRT plus ESC participants reported SAEs to the researcher than participants in the ESC arm. In summary, apparent differences in the number of AEs appear to be due to the reporting mechanism and differences in the rates of missing data between the groups.
As shown in Table 31, the nature of AEs was similar in arms and was as expected in this vulnerable population group. The most commonly reported AEs and SAEs centred around suicidal ideation, self-harm and overdose behaviours, and suicide attempts.
Chapter 5 Health economic findings
Abstract
Objective
To estimate the cost-effectiveness of the SRT intervention, compared with ESC, a within-trial cost–utility analysis was undertaken.
Methods
A within-trial analysis was conducted, where costs and benefits were estimated over a 24-month period and discounted at 3.5% in the second year. Costs were estimated from the perspective of the NHS and Personal Social Services (PSS), at 2017/18 levels, and included intervention (training, therapy session and supervision) costs and other health professional and hospital admission costs. Total quality-adjusted life-year (QALY) scores were estimated from the EQ-5D-3L responses. Regression was undertaken to estimate the incremental cost-effectiveness ratio (ICER) (mean incremental cost/mean QALY gain), where value for money would correspond to an ICER below the cost-effectiveness threshold (λ) value of £20,000 per QALY. The level of uncertainty, according to the cost-effectiveness acceptability curve (CEAC), was also assessed at the same λ value. MI was used in the base-case analysis.
Results
Based on 270 patients, the mean incremental cost for SRT, compared with ESC, was estimated to be £3910.59 (95% CI £2708.32 to £5112.86), with a QALY gain of 0.001 (95% CI –0.099 to 0.10). Accordingly, the ICER was estimated to be > £5M, with a low level of uncertainty.
Conclusions
Social recovery therapy was estimated to be significantly more costly than ESC and there was no significant difference in outcome, and a low level of uncertainty. Consequently, SRT was not estimated to constitute value for money.
Background
Background
The protocol for this study, with associated rationale, inclusion criteria, setting and intervention descriptions, has been published. 1 In this chapter, we report the methods and results for the economic evaluation component of the study.
Objectives
In order to estimate the cost-effectiveness of the SRT intervention, compared with ESC, a within-trial (24-month) cost–utility analysis was undertaken.
Methods
Estimating costs
Costs were estimated from the perspective of the NHS and PSS at 2017/18 levels. Below, the methods used to measure and value the intervention costs and other NHS and PSS costs are outlined.
Intervention (social recovery therapy) cost
Training
All therapists who delivered the intervention in the study received training. For costing purposes, informed by our knowledge of who delivered the intervention in the study, the therapists were assumed, on average, to be NHS band 7, and thereby have a cost per hour of employment of £53.2179 (Table 32). Those who delivered the training were assumed to have a cost per hour of employment of £63.00 (grade 8a). 79 It was assumed that each therapist received 3 days of training (excluding research-related activities), with an average of four therapists receiving the training at one time, and the associated preparation/travel costs were assumed to be negligible. Total training costs (for both the trainer and therapists) were subsequently estimated, summed and equally apportioned across all intervention participants. The assumption to apportion costs to the trial participants only was in line with previous research,83 and was undertaken as we would rather be conservative and not underestimate such costs.
| Resource use | Unit cost |
|---|---|
| Intervention costs | |
| Therapists (cost per hour of employment) | £53.2179 |
| Trainer (cost per hour of employment) | £63.0079 |
| 1 hour of face-to-face contact time with therapist | £106.4279 |
| Travel cost (per session) | £32.6079 |
| Peer supervision (per session) | £106.4279 |
| Health professional visits (most commonly reported) | |
| Case managera (NHS band 5/6) | £70.9979,80 |
| Counsellor/therapista | £106.4279,80 |
| GPa | £31.0079 |
| Mental health nurse | £79.9979,80 |
| Practice nursea | £12.1066,79 |
| Psychologista | £97.5567,79 |
| Psychiatrista | £200.4179 |
| Social worker | £79.5779,81 |
| Other NHS and PSS costs | |
| Hospital admission (general ward) | £337.3682 |
| A&E visit | £160.3282 |
Therapy sessions
To inform the assumptions about cost of intervention delivery, discussions took place with two therapists who delivered the intervention (one face-to-face meeting, followed by a number of e-mail discussions). In addition, therapists were asked to record in the database the duration of all face-to-face contacts. Details of other non-contact patient-related activities (e.g. e-mails and telephone calls) could also be recorded. After examining these data and following discussions with the therapists, it was concluded that these data were likely to be under-reported and that some therapists were better at recording non-contact activities than others. Previously, it had also been estimated that for every hour of face-to-face contact time there was an associated hour of non-contact time. 80 Two therapists were asked whether or not they thought that this reflected their practice. They thought that the most appropriate assumption was that for every hour of face-to-face contact time there would be an associated hour of non-contact activity undertaken by the therapist for the same participant (example activities would include session reminders/bookings, liaison with other agencies and session preparation/planning). Travel time was not included in the 1 : 1 contact to non-contact time assumption and it was assumed that travel time would approximate to 30 minutes of therapist time for each face-to-face session. The associated travel costs were therefore assumed to be £6.00, on the assumption that the average associated mileage was 15 miles (the distance covered in 30 minutes of travelling at 30 miles per hour) at a cost of 40p per mile.
Supervision
Peer supervision was assumed to take place weekly (one-to-one with another therapist for 1 hour in total, including preparation) over the period when therapists were providing therapy. After estimating the total peer supervision cost for the study, this was equally apportioned across all intervention participants. Finally, the mean costs representing the cost of training, therapy provision and supervision per therapy participant were summed to estimate the mean total intervention cost (per intervention participant).
Other NHS and Personal Social Services costs
A self-reported modified version of the Client Services Receipt Inventory (CSRI84) was developed, which asked participants to report any contacts with health professionals (number and place) and hospital admissions (length of stay and type of ward/unit). These methods are in keeping with a previous study39 which used a modified self-report version of the CSRI in a similar population group. Participants were asked to complete the modified CSRI at baseline (recall: previous 6 months), 9 months (recall: previous 9 months), 15 months (recall: previous 6 months) and 24 months (recall: previous 6 months); post-baseline participants were asked not to include therapy received as part of the SRT intervention (this was to enable the researchers who helped with questionnaire completion to remain blind and because this information was routinely recorded by those who provided the intervention). Control participants did not receive any specific intervention and the modified CSRI was designed to capture the standard care that they received.
Costs were assigned to each reported item of NHS and PSS resource use, with these being estimated at 2017/18 financial year levels. Subsequently, the total intervention cost (see Supervision) was added to the health professional and hospital admission costs to estimate the overall NHS and PSS costs.
Measuring outcomes
To estimate levels of health-related quality of life, participants were asked to complete the EQ-5D-3L67 at baseline, and at 9, 15 and 24 months. Use of the EQ-5D was justified on the basis that this is in keeping with NICE’s Guide to the Methods of Technology Appraisal 2013,85 that it has been used before in similar population groups,39 and that such use is considered to be appropriate. 86 Responses were then converted into a utility score (a scale where 0 is equal to death and 1 is full health) using the York A1 tariff. 81 The total area under the curve method (based on the assumption of linear interpolation)82 was then used to estimate the total QALY score for each participant over the 24-month period.
Missing data assumptions
Across all time points, five participants reported that they had contact with a particular health professional, but they did not report the number of visits. Where this was the case, the average value for those who reported the number of visits was used. Similarly, four participants reported that they had had a hospital admission, but they did not report the associated length of stay, and, again, the average value for those who reported such data was used.
In the internal pilot (n = 100), the wrong resource use questionnaire was sent to participants at the 9-month follow-up point. The baseline questionnaire was sent rather than the 9-month questionnaire, and participants were, therefore, asked about resource use in the previous 6 months rather than in the previous 9 months. To estimate costs for the missing 3-month window post baseline, reported levels of resource use for these 100 participants were inflated by 50% in this period. All other missing data were left as such and the corresponding individuals were excluded from the complete-case analysis.
Analysis
Three analyses were undertaken, where the following approaches were adopted in all analyses. In each, a within-trial analysis was conducted in which costs and benefits were estimated over a 24-month period, with costs incurred in the second year discounted at 3.5%. 85 A within-trial analysis, rather than a decision model, was undertaken as we are not aware of any previous studies that have compared SRT with ESC. The ITT approach was also followed, in which participants were analysed within the group to which they were allocated, regardless of, for example, the number of therapy sessions received. To estimate the mean incremental cost and incremental effect (QALY gain) associated with the intervention, compared with that for the control group, seemingly unrelated regression87 was undertaken to allow for any correlation between costs and effects. Baseline demographic variables [age (16–19 years, 20–25 years) and sex (male/female)] were included as covariates, along with the total baseline health professional and hospital admission cost for the overall NHS and PSS cost, and the baseline EQ-5D scores for the total QALY score. In the absence of dominance (where higher costs and lower benefits were associated with a particular intervention), the incremental cost and incremental effect would be used to estimate the ICER (mean incremental cost/mean QALY gain). 85 It was also assumed that an estimated ICER below the cost-effectiveness threshold (λ) value of £20,000 per QALY85 would suggest that an intervention constituted value for money. In addition, to estimate the level of uncertainty associated with the decision about whether or not the intervention was cost-effective, the bootstrap technique88 (with 5000 replications) was used to estimate the probability that each intervention was cost-effective according to the CEAC. 89 In particular, the probability of SRT being cost-effective was specifically estimated at the λ of £20,000 per QALY.
The first (base-case) analysis undertaken was based on overall NHS and PSS cost, and the total QALY score, where MI90 was used to estimate any missing data and enable all participants to be included. The MI model included costs (health professional and hospital admission costs at baseline, and at 9, 15 and 24 months, as well as total intervention costs) and outcomes (EQ-5D scores at baseline, and at 9, 15 and 24 months) and demographic variables [age, sex and social functioning (low functioning/very low functioning)]. EQ-5D scores were included, rather than individual dimension scores, as missing EQ-5D data were generally for the whole questionnaire. Health professional and hospital admission costs were, however, separated to allow for the possibility of different levels of missing data for these two variables. Two further complete-case91 sensitivity analyses92 were also conducted. In the first sensitivity analysis (SA1), participants were included only if they had complete cost and effect data at each time point. This enabled a comparison with the results of the base-case analysis, to assess whether or not results differed for participants who did not have missing data values imputed. A second sensitivity analysis (SA2) was also conducted, for a similar rationale, where only total intervention costs were included, as the level of missing data for this variable was anticipated to be lower.
Results
Costs
Social recovery therapy intervention cost
Training
A total of 19 therapists delivered the SRT intervention, where five group training sessions (each 3 days long) were assumed to be held. Total training costs were estimated to be £29,833.80, equating to a cost of £216.19 per participant (see Table 33).
Therapy sessions
A total of 2470 face-to-face SRT sessions were recorded across the 138 intervention participants (mean number of sessions per participant = 17.9). The mean recorded contact time per session was 63 minutes [this value was assigned to the six sessions (< 1%) where no time was recorded]. The cost of 1 hour of face-to-face contact time was estimated to be £106.42 (this includes the cost of an associated 1 hour of non-contact time). Travel costs were estimated to be £32.60 per session. Together, this meant that the mean per participant total session cost was £2571.56.
Supervision
At any one time, it was assumed that, on average over the whole study period, two SRT therapists in each of the three sites would be providing therapy and that peer supervision would, therefore, take place weekly for 1 hour between these two individuals. This was assumed to have taken place over a 5-year period in two of the sites and over a 2-year period in the third site. Over the study period, a total of 624 peer supervision sessions were thereby assumed to have taken place. At a cost of £106.42 per session, this would equate to a total cost of £66,404.68 (£481.19 per participant).
After summing the aforementioned mean cost per intervention participant training, therapy session and supervision, it was estimated that the mean total intervention cost (per SRT intervention participant) was £3268.94 (Table 33).
| Component part (totals) | Mean per SRT participant cost |
|---|---|
| Training | £216.19 |
| Therapy sessions (including travel) | £2571.56 |
| Supervision | £481.19 |
| Total | £3268.94 |
Other NHS and Personal Social Services costs
Participant response rates for the modified CSRI at baseline, and at 9, 15 and 24 months, were 270 (100%), 231 (86%), 221 (82%) and 189 (70%). Data for the participants who completed the health professional visit/hospital admission questionnaires are presented for the two groups in Table 34. It can be seen that, in contrast to the aforementioned intervention costs, there was comparatively little difference in mean health professional resource use/costs between the two arms. Mean hospital admission costs were seemingly higher in the intervention arm, but this difference was sensitive to a small number of participants [e.g. if data for the two participants with the longest length of stay (59 and 98 days) were not included, then the mean costs would be higher in the control arm].
| Resource | Allocation arm, mean number of visits/number admitted to hospital (SD) [n with available data]; mean cost (SD) | |
|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |
| Case-managera (NHS band 5/6) | 4.0 (10.9) [n = 103]; £289.66 (£786.77) | 3.0 (10.8) [n = 78]; £218.24 (£782.20) |
| Counsellor/therapista | 5.2 (10.6) [n = 103]; £541.43 (£1121.66) | 5.9 (10.7) [n = 78]; £614.38 (£1108.56) |
| GPa | 9.4 (12.7) [n = 103]; £293.81 (£394.22) | 10.6 (11.7) [n = 78]; £336.76 (£364.06) |
| Mental health nurse | 1.4 (4.7) [n = 103]; £117.12 (£389.88) | 1.8 (5.1) [n = 78]; £148.49 (£437.55) |
| Primary care nursea | 1.0 (2.4) [n = 103]; £12.91 (£29.59) | 1.6 (4.4) [n = 78]; £21.69 (£60.70) |
| Psychologista | 0.5 (3.2) [n = 103]; £52.09 (£313.82) | 0.5 (2.2) [n = 78]; £51.33 (£217.70) |
| Psychiatrista | 0.8 (1.5) [n = 103]; £160.95 (£307.25) | 0.5 (2.2) [n = 78]; £51.33 (£217.70) |
| Social worker | 0.6 (3.6) [n = 103]; £286.70 (£1566.36) | 2.3 (9.5) [n = 78]; £201.49 (£832.74) |
| Total costs | Mean cost (SD) [n with available data] | Mean cost (SD) [n with available data] |
| Total health professional visit cost | £1566.36 (£1987.75) [n = 103] | £1926.81 (£2138.89) [n = 78] |
| Total hospital admissions cost | £1023.24 (£5160.59) [n = 100] | £476.30 (£1715.98) [n = 76] |
| Total other NHS and PSS costs | £2527.27 (£6121.36) [n = 100] | £2420.61 (£2816.77) [n = 76] |
| Intervention costs | £3268.94 (£1291.75) [n = 138] | – |
| Overall NHS and PSS costs | £5927.73 (£6148.10) [n = 100] | £2420.61 (£2816.77) [n = 76] |
Finally, overall NHS and PSS costs are presented in Table 34, where it can be seen that the difference in costs between groups is comparable to the cost of the intervention itself.
Outcomes
The number of participants who completed the EQ-5D at baseline, and at 9, 15 and 24 months is shown in Table 35. It can be seen that, based on those who responded, the mean score improved for both groups over time and the total QALY score was similar in both groups.
| Score | Allocation arm, N/mean (SD) [n] | |
|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |
| Baseline EQ-5D-3L | 0.47 (0.310) [137] | 0.49 (0.275) [132] |
| 9-month EQ-5D-3L | 0.63 (0.298) [120] | 0.60 (0.308) [104] |
| 15-month EQ-5D-3L | 0.63 (0.289) [119] | 0.62 (0.322) [97] |
| 24-month EQ-5D-3L | 0.67 (0.249) [103] | 0.71 (0.243) [85] |
| QALY (discounted) | 1.22 (0.469) [93] | 1.17 (0.477) [74] |
Analysis
The results of the regression analyses are shown in Table 36. In the base case, it can be seen that the overall NHS and PSS costs were, on average, £3910.59 higher for SRT participants than for ESC participants (p < 0.01). The total QALY score was, however, not significantly different between groups. Therefore, the estimated ICER exceeded the λ value of £20,000 per QALY, at which level SRT would not be deemed cost-effective or to constitute value for money. Furthermore, according to the CEAC, it was estimated that there was a low level of uncertainty associated with that result. Table 36 shows that similar results were obtained in both sensitivity analyses that were conducted.
| Analysis (Nsrt, Nesc) | Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | CEACa |
|---|---|---|---|---|
| QALYs (truncated at 12 months) | ||||
| Base case: imputed (138, 132) | £3910.59 (£2708.32 to £5112.86) | 0.001 (–0.099 to 0.10) | £5,583,364 | 0% |
| SA1: intervention costs only: (93, 74) | £3514.31 (£3235.40 to £3793.22) | 0.064 (–0.044 to 0.17) | £59,964.25 | 2.58% |
| SA2: complete case: (88, 71) | £3876.27 (£2345.36 to £5407.19) | 0.059 (–0.052 to 0.17) | £66,222.83 | 4.8% |
Changes from protocol
The protocol1 stated that a cost-effectiveness analysis would be carried out using activity (time use) and symptoms (CAARMS); this was not conducted for the following reasons. The results of the primary analysis show that there was no evidence of any superiority of SRT over ESC for levels of structured activity at 15 months (see Table 15). Similarly, there was no evidence of any superiority of SRT over ESC in levels of psychopathology as assessed by CAARMS symptom severity scores (see Table 17). In addition to there being no statistically significant differences between groups, the numerical differences did not favour the SRT group. SRT is also more costly than ESC (see Table 34). Given these results, which show that SRT is more costly and no more effective than ESC, we considered that the proposed cost-effectiveness analysis would be of limited value.
Chapter 6 Discussion and conclusions
Discussion
The aim of this study was to undertake a definitive randomised trial to determine the clinical effectiveness and cost-effectiveness of SRT compared with ESC in young people who present with social withdrawal, and non-psychotic severe and complex mental health problems, and who are at risk of long-term social disability and mental illness. The primary hypothesis was that, in young people who are socially disabled and have non-psychotic severe and complex mental health problems, SRT will be superior to ESC in improving social recovery (as measured by hours in structured activity assessed on the TUS) over a 15-month follow-up period. There was no evidence of superiority of SRT over ESC at 15 months, nor at 9 or 24 months, with respect to time spent in structured activity. There was no evidence for the superiority of SRT over ESC in terms of cost-effectiveness or effects on secondary outcomes of mental health symptoms (APS and emotional disturbance). One secondary outcome, drug use, showed significant superiority in the SRT plus ESC arm compared with the ESC-alone arm at 24 months. The most appropriate summary of the results of this trial is that there is no evidence of superiority of SRT over ESC for young people presenting with social withdrawal and non-psychotic severe and complex mental health problems.
On some dimensions there appeared to be mean differences favouring ESC over SRT plus ESC. However, the differences on the primary outcome and the large majority of secondary outcomes did not meet the level for conventional significance, apart from social phobia and some subscales of negative symptoms at 15 months. At 24 months, mean differences favoured ESC over SRT plus ESC on structured activity. Missingness of data was consistently higher in the ESC-alone group than in the SRT plus ESC group, and the bias and total amount of missingness of data increased over time. At 24 months, > 30% of data were missing and there was a bias resulting from the fact that the amount of missingness of data in the ESC-alone group was twice that in the SRT plus ESC group. It is plausible that differential missingness of data could bias results in favour of ESC, particularly at the later assessment stages. We can be clear that there is no superiority of SRT, but, despite trends favouring ESC alone, we should be more cautious in concluding that ESC alone was superior, as most of the results did not reach conventional statistical significance levels and there was a clear bias because of unbalanced levels of missingness of data.
Participants in both arms of the trial made large gains over time from baseline on all measures. There were large effect size gains in structured and constructive economic activity of > 10 hours per week in both arms. This is more than double the 4 hours that constitutes a clinically meaningful difference. There was a > 50% improvement in the rate of participants meeting diagnostic criteria for depression, panic, agoraphobia and social phobia in both groups, and large effect gains in self-reported assessments of depression, social anxiety, hopelessness and schizotypal symptoms of paranoia and anomalous experiences. There were also marked reductions in alcohol and drug use disorders.
The ESC provided in this trial was designed and intended to maximise the delivery and availability of combinations of existing evidence-based interventions to this group of young people with non-psychotic severe and complex mental health problems. Close examination showed the ESC delivered in the trial to be highly active, involving comprehensive individualised packages of care with combinations of medication, evidence-based psychological therapy (including symptom-focused CBT) and social care (including specific employment support). Most participants in the ESC condition received case management support, a majority (in both arms) were taking antidepressant medication, around half had psychological therapy over the course of the trial, and around one-third had comprehensive employment support. The packages of care delivered outside the trial were similar in both arms, but slightly higher rates of receiving psychological therapy and packages of NHS treatment were recorded in the ESC-only group. What the trial appears to indicate is that the presence of active packages of youth mental health care, including primary care support, antidepressant medication and, where indicated, standard psychological therapy and employment and youth support, is sufficient to make relatively large effect size gains in activity and in mental health symptoms, and that the adjunctive provision of specific intensive SRT adds little to such gains. The results are possibly akin to those of the IMPACT trial93 on adolescent depression, which showed relative equivalence of the effectiveness of case management in comparison with different types of psychological therapy (psychoanalytical psychotherapy and CBT).
The results are surprising given the evidence base that gave rise to this trial. Previous research has tended to find that patients with severe and complex problems struggle to access and may be relatively unresponsive to existing interventions. 5–8 Furthermore, follow-up studies have suggested that the longer-term outcomes are poor, with social withdrawal in association with non-psychotic severe and complex mental health problems and APS predictive of poor outcomes. 2,3 In contrast, in this trial both the ESC-alone group and the SRT plus ESC groups achieved large effect size gains in structured and constructive economic activity, as assessed by time use, and in mental health symptoms, particularly in hopelessness, anxiety, paranoia and APS, over time and for up to 2 years. It is important to note that ESC in this trial was not treatment as usual. It was an active and comprehensive intensive treatment control condition comprising a combination of evidence-based interventions. As identified in our qualitative participant process evaluations,70,71 participation in the ESC arm of the trial and in the trial procedures appeared to be an enhanced intervention experience that was beneficial to participants. Future research should perhaps focus on how routine services can maximise the implementation of the optimal combination of existing evidence-based interventions for this often-neglected group of participants.
Strengths and limitations
This trial had many strengths, most notably that it is a large trial involving the recruitment of a very withdrawn population of young people with severe mental illness across diverse regions of the UK and recruited from a wide array of services. This is a difficult-to-research group for whom any consistent evidence of outcomes is difficult to obtain because of problems with non-engagement and retention. Recruitment and retention of this sample is in and of itself an achievement, and the information on outcomes for the cohort provides novel data on a group that is of increasing interest to policy-makers. The further strengths of this study were good internal and external validity for the trial on the primary outcome and on the main secondary outcomes. The study was conducted with a high degree of rigour and retention to the primary outcome was highly satisfactory. All researchers involved in the study received regular supervision and careful routine checks on inter-rater reliability throughout. All outcomes were reliable and completed blind to intervention allocation. SRT was delivered rigorously and thoroughly, with good adherence to the therapy model by a group of highly committed therapists, and was received well by participants.
A limitation of the study was that it was compromised by the level and pattern of missing data in the secondary outcomes, particularly at the 24-month assessment. However, it should be highlighted again that the primary outcome was originally designed to be assessed at 15 months and the 24-month assessment was brought in at a later stage as a specific standalone follow-up study. Although there were missing data, this was not regarded as being to a degree that compromised the use of ITT analyses as specified in the statistical analysis plan for the main study on primary and secondary outcomes at 15 months. The characteristics of the target group in this study, being by definition a difficult-to-engage and extremely withdrawn sample, represent a challenge to researchers, especially where longer-term follow-up assessments are reliant on face-to-face assessments. Notably, in the SUPEREDEN3 trial, which compared SRT plus EIP services with EIP services alone, there was also differential missingness of data with greater retention in the arm receiving SRT. 40 In the present trial, additional measures were used to improve retention across trial arms, including the provision of participant reimbursement at all assessment points, mid-point telephone contact and provision of a newsletter to participants between follow-up points; yet such measures did not appear to result in equivalent retention for those individuals not receiving SRT. Potentially, future studies in this area might maximise the rate of follow-up by focusing on hard proxy variables of engagement in services, which may be derived from health-care records rather than from face-to-face assessments, especially as engagement is itself an important outcome in this population.
A further limitation of this study was that many of the secondary outcomes have wide CIs (an indication of low statistical power), suggesting low precision or uncertainty in the estimation of treatment effects, which is probably an unavoidable consequence of undertaking research with what is a complex and heterogeneous clinical group with differing symptomatology. Furthermore, the study was, by necessity, single blind, and thus may be affected by the fact that participant were aware of their allocation and whether or not they were receiving the intervention of interest.
In line with good practice recommendations for cost-effectiveness analyses,94 we concentrated on expected large cost drivers (e.g. health professional visits). A potential limitation is, however, that certain aspects of health care (e.g. medications) were not costed. Similarly, any differences in the loss in productivity, as well as family and carer costs, were also not costed, although, given that the intervention was not estimated to be more effective than standard care, we would not expect there to be a difference in these costs between arms. Thus, the study result would have been expected to be the same, even if a wider cost perspective were adopted.
A further potential limitation is that our analysis is based on evidence from just one trial. However, if an economic model were to be created based on these trial data, then one would not expect the long-term or extrapolated results, for example, to differ from those presented here, as the intervention was not estimated to be effective.
Clinical implications
The assumption that underpinned this study was that young people who are withdrawn and have non-psychotic severe and complex health problems represent a group who are unresponsive to existing interventions and, therefore, may require a dedicated intensive psychosocial intervention. The findings of the study are important in clearly demonstrating that, contrary to prediction, this group can respond well and make clinical and social recovery if a comprehensive range of existing interventions are made available. What is needed is an enhancement of standard care to ensure the provision of the optimal combination of currently available evidence-based interventions. This includes a combination of case management and support with appropriate medication, specific symptom-focused psychological therapy, and employment and youth support. Service user feedback suggests that delivering such services in the context of hope and messages of recovery is key. 95–97 Similarly, both participants and SRT therapists in the present process evaluation emphasised the importance of hopefulness and motivation for this patient group (Briony Gee, Norfolk and Suffolk NHS Trust, 2021, personal communication). 98 The take-away message is not to do nothing; instead, it should be to highlight the need to assist services and frontline clinicians to enhance care delivery for this population and ensure that optimal care pathways are delivered for those most at need. Such care pathways must also recognise the dynamic identity exploration occurring in the those aged 14–25 years41,42 and the need for developmentally appropriate interventions for this group.
The key clinical and research implication of this study is to examine the optimal ways that services can be provided in such cases. Although the evidence here is that young people with non-psychotic severe and complex mental health problems can and do respond if the right combination of evidence-based interventions is made available, all too often such interventions are not available in routine services. The factors associated with effective delivery and implementation of the optimal combinations of interventions to this often-neglected group of young people are complex. One issue is resources and it should be noted that the present trial was conducted in services that already had a specific focus on youth mental health and the provision of interventions to young people with complex needs. In these services, an optimal combination of evidence-based interventions was available and was delivered to participants in the trial. Not all services in the NHS, let alone those in less well-resourced health-care settings, have such resources available. The present trial provides support for the provision of comprehensive youth mental health services that can offer a full range of medical, psychological and psychosocial interventions. Moreover, even where resources are available, implementation and delivery of care can be complex, as, young people may need help to be detected, to engage with services and to continue with interventions. Sometimes these young people can fall between the gap of services; for example, some of our participants had previous experience of being regarded as not having sufficiently severe symptoms to meet the criteria for EIP services, but, at the same time, their symptoms were deemed to be too severe or they were considered incapable of engaging with services for treatments to address common mental health problems. Others fall into gaps between child and adult services. This study suggests that good outcomes are possible by systematic provision of currently available evidence-based interventions. The results of this study are encouraging to all clinicians to do what is possible with the range of existing interventions to deliver optimal packages of care. If delivered in a spirit of hope for realistic recovery, good outcomes can be expected.
The qualitative research suggested that the trial procedures themselves provided a focus for optimal detection and delivery of interventions, including ESC. Possible ways to replicate such procedures to organise and implement detection and delivery of care to high-risk groups that may not otherwise engage in routine practice might be possible in more dedicated youth mental health services. These services offer open-door youth-oriented engagement and assertive case management to ensure that combinations of existing evidence-based interventions are delivered to those who need them. The qualitative research also revealed that hopelessness can potentially lead to non-engagement and lack of implementation of interventions, both within the young person and by staff who treat them, who can become overwhelmed by the complexity of clinical presentations. The results of this study, again, provide hope in suggesting that, despite initially highly complex and very severe presentations and extreme withdrawal over a period of 15 months to 2 years, many young people with complex and severe mental health and social problems improve significantly. We cannot be absolutely certain that it was the systematic detection and delivery of the right combination of existing evidence-based interventions that facilitated this; it is possible that some participants made natural recoveries. Given the chronicity, severity and complexity of problems at baseline, it seems probable that the enhancement of standard care and the systematic provision of evidence-based interventions according to need in both arms played a role in promoting recovery.
In conclusion, clinicians should be supported in their attempts to manage more complex clients with clear hope that systematic delivery of the right combination of existing interventions based on needs can be effective. Support and training to give clinicians confidence to manage such cases may also be warranted. The structure offered by the recruitment and assessment processes in this trial possibly provides systematic ways in which routine services can identify cases and offer hopefulness through a structured assessment of needs and feedback to clinicians.
Implications for detection
The evidence underpinning this study was that young people with premorbid social decline and non-psychotic severe and complex mental health problems have the poorest social outcomes, and that complex and severe psychopathology further predicts poor response to existing interventions. 4,47,99–103 This remains a good summary of the literature. How can we reconcile this evidence with the outcomes in this study that such young people appear to respond with large effect gains from the provision of ESC? What are the implications for detection and intervention? The first is that the choice of these factors (a combination of social disability and complex severe psychopathology) lacked specificity as a detection criterion; these are generic indicators. Poor social outcome is common, as is complexity in youth mental health presentations. 4,100,104
Perhaps, despite this being a combination of risk factors that are known to be associated with poor outcome, the lack of specificity and high prevalence of this presentation may result in false detection of a group at purportedly high risk. Although there is an association with poor outcome, many of these young people in fact have a good outcome, if good services are provided. It might be that this population includes a group that have a good natural course of recovery; young people are at a time of their lives when obtaining work, leaving the family home, starting higher education, or gaining a partner or friend can have a major effect in social and symptomatic presentations.
Similarly, in intervention studies, although there is an association of likelihood of poor response to specific interventions, clearly there is reserve response capacity, particularly if the service response is hopeful, wide-reaching and systematic. It is noteworthy that the service offered to this group in ESC did not reflect a single intervention, but typically involved combinations of case management, medication, psychological therapy, and employment and personal youth support. The key take-away message is that the presence of what appear to be poor prognostic factors and complexity should not lead to an assumption of non-responsiveness. Many young people can and do respond if packages of care are offered.
Finally, although the evidence from this study suggests that the present approach to high-intensive SRT is not needed, it is possible that other interventions may have a better effect. Another approach possibly worth investigating is stepped care to identify cases that do not respond to enhanced standard packages of care. In a previous study,40 the provision of SRT for young people with early psychosis was delivered only once there was no evidence of response to EIS after at least 1 year. In that study, there was clear evidence for a specific benefit of SRT. 40 In the present study, although some participants had previously been offered and had engaged with packages of care, for others SRT was provided at initial presentation to services (albeit with all participants showing evidence of at least 6 months of persistent social decline). It is possible that more specific high-intensity therapy may be best reserved for cases showing treatment resistance, perhaps after clearly being offered existing interventions for longer periods. A stepped care approach may be warranted for cases that do not respond to ESC interventions.
Further research
Capture engagement as an outcome of intervention
The differential missingness of data according to intervention allocation is notable. Although there appears to be no superiority of SRT according to the between-group data comparisons, this differential missingness of data does suggest that SRT has a clear effect on engagement. Engagement itself is an important predictor of outcome and target for intervention. Of those young people with ARMS or who have transitioned to a first episode of psychosis, approximately one in three or four young people will disengage from early intervention services. 105 Future research that evaluates SRT as a predictor of treatment and broader service engagement and patterns of continued engagement over time would be worthwhile. Furthermore, such research could explore the putative mechanisms of increased engagement and endeavour to isolate the key components of SRT that have impact.
Capture outcomes in absentia
In a population of especially withdrawn young people, the identification and operationalisation of meaningful outcomes that can be measured in the absence of face-to-face assessment would be a worthy goal. New technologies could offer a potential solution as, for example, smartphones have a high level of use among young people and could provide the means to capture behavioural data, with participant permission.
Investigating person-centred treatment for young people with emerging non-psychotic severe and complex mental health problems
The large effects in both trial conditions suggest that both ESC alone and SRT plus ESC are associated with meaningful gains across broad spectra of functioning and symptom outcomes. Nevertheless, particularly considering the substantial minority of ESC participants who dropped out of the trial, there is a need to explore what works best for whom. It is very possible that within the trial population there existed subgroups of young people for whom only certain, specific ESC and/or SRT components were necessary and sufficient. Such components may include technical, logistical and relational elements of the health-care interaction. 95 The identification of these components, using both quantitative and qualitative methods, has important clinical and economic implications for service design and delivery in the treatment of young people with emerging non-psychotic severe and complex mental health problems. Moreover, this report presents the average group-level changes and does not constitute an investigation of variation in responsiveness to either trial arm. The identification of possible subgroups of young people without psychosis, but with emerging non-psychotic severe and complex mental health problems and social disability, who may constitute a population likely to be ‘treatment resistant’ and, thus, in need of a high-intensity, specialised or social recovery intervention, would be a worthy endeavour.
Conclusions
The rationale for the trial emphasised the need for detection and intervention as it had been commonly highlighted that cases of young people with non-psychotic severe and complex mental health problems tended to be neglected by services. Even though these young people often seek treatment, they were identified as falling between gaps in services and being regarded as difficult to engage and manage. This study did not find evidence for the superiority of SRT when delivered as an adjunctive to enhanced treatment as usual. This study showed that it is possible to successfully detect, recruit and retain young people with severe and complex mental health difficulties and social withdrawal into a psychological treatment trial of this type. The participants recruited demonstrated very severe withdrawal, with a mean of 11 hours per week activity compared with a population mean of > 60 hours per week for young people in the same age group;47 this was accompanied by very severe levels of depression, social anxiety, hopelessness and the presence of APS.
The study shows that it is possible for standard services to deliver enhanced packages of care to this group if resources are available and suggests that these packages can be effective. The results provide support for and are consistent with lobbying for generic youth mental health services that seek to engage young people. The results of the present study show that, in terms of interventions, offering the range of existing services available within the NHS (case management, medication, symptom-focused CBT, and employment and youth-focused support) is sufficient to obtain a clinically important effect and that a more focused, intensive and specific psychological intervention, such as SRT, may not be needed. SRT was also not estimated to be cost-effective. Potentially, it may be only young people with psychosis who need a more intensive specialist intervention and for whom interventions such as SRT show a specific effect in enhancing standard care. 40 The clinical implications of the study are therefore significant in emphasising the importance of mental health services to identify, engage and treat the types of non-psychotic severe and complex mental health problems targeted in this trial using their existing repertoire of interventions, with the confidence of obtaining good outcomes even if the initial presentations can appear somewhat intransigent, and indeed complex and overwhelming.
Acknowledgements
We wish to thank the young people who participated in the trial and all family members, friends, referring services, clinicians and others who supported their involvement. We wish to thank the PAT for their invaluable involvement in the shaping of this project. We are very grateful to our TSC and DMEC members for their invaluable support and guidance throughout this trial. We wish to acknowledge the support of our NIHR programme manager Nick Eaton. We would like to thank all staff in the sponsoring and hosting organisations for supporting the project. We are grateful to Tony Dyer and Martin Pond at NCTU for their support with data management. We thank Kelly Grant and Saffanah Al Katheer for their support with the statistical and health economic analyses.
Finally, we wish to thank all the PRODIGY therapists and RAs for their boundless enthusiasm and dedication to supporting participants in their involvement with PRODIGY; Nazire Akarsu, Helen Anderson, Karmen Au-Yeung, Samantha Bowe, Measha Bright, Kate Bristow, Nicholas Burden, Emogen Campbell, Ben Carroll, Charlotte Clark, Deborah Coton, Rachel Cranshaw, Lucie Crowter, Renata Fialho, Samantha Fraser, Brioney Gee, Adam Graham, Natasha Holden, Emma Howarth, Rebecca Ison, Christopher Jackson, Jennifer Keane, Amanada Larkin, Christine Lowen, Rebecca Lower, Elizabeth Murphy, Jasper Palmier-Claus, Heena Parmar, Katherine Pugh, Joseph Ridler, Fritha Roberts, Alice Rose, Catarina Sacadura, Verity Smith, Ann Steele, Glynis Queenan and Nick Whitehouse.
Contributions of authors
Professor David Fowler (https://orcid.org/0000-0001-5806-2659) planned the funding applications for the internal pilot and extension phase of the trial. Professor Fowler led the design of the trial, and the development of the protocol and statistical analysis plan. Professor Fowler led the trial as the Chief Investigator with Professor Paul French. Professor Fowler wrote the final report with Dr Clio Berry.
Dr Clio Berry (https://orcid.org/0000-0003-1164-9836) made a substantial contribution to the development of the trial protocol and statistical analysis plan. Dr Berry managed the trial as the trial manager (extension phase): managing the delivery of the trial, ensuring the implementation of the protocol and associated amendments, supervising and training research staff, supervising and co-ordinating recruitment, and managed the trial data, including data entry and quality appraisal, with support from NCTU. Dr Berry prepared the final data set for analysis. Dr Berry made substantial contributions to the design, delivery and analysis of the process evaluation involving research therapists. Dr Berry wrote the final report with Professor Fowler.
Dr Joanne Hodgekins (https://orcid.org/0000-0003-4124-854X) made substantial contributions to the applications for funding, design of the trial and development of the protocol. Dr Hodgekins led the trial in the East Anglia site as Principal Investigator with Dr Jon Wilson. Dr Hodgekins provided expert training and supervision for all trial staff in the assessment of time use. Dr Hodgekins made substantial contributions to the design, delivery and analysis of the process evaluations involving young people. Dr Hodgekins provided trial therapy, supervised research and therapy staff, and contributed to trial management oversight. Dr Hodgekins made substantial contributions to and critically read the final report.
Professor Robin Banerjee (https://orcid.org/0000-0002-4994-3611) made contributions to the funding applications. Professor Banerjee contributed to the design and development of the trial protocol. Professor Banerjee made substantial contributions to the development and delivery of protocol amendments. Professor Banerjee contributed to trial management oversight. Professor Banerjee made contributions to and critically read the final report.
Dr Garry Barton (https://orcid.org/0000-0001-9040-011X) was the health economist. Dr Barton made contributions to the funding applications. Dr Barton made substantial contribution to the development of the trial protocol and statistical analysis plan. Dr Barton conducted the health economic analysis and made substantial contributions to and critically read the final report.
Dr Rory Byrne was the lead for PPI. Dr Byrne made contributions to the funding applications. Dr Byrne made substantial contributions to the design of the trial and development of the protocol. Dr Byrne led the PPI activity with the PAT. Dr Byrne made substantial contributions to the design, delivery and analysis of the process evaluations involving young people. Dr Byrne made substantial contributions to the design of patient-facing materials used in the delivery of the trial. Dr Byrne contributed to trial management oversight. Dr Byrne critically read the final report.
Dr Timothy Clarke made substantial contributions to the applications for funding, design of the trial and development of the protocol. Dr Clarke led the trial as the trial manager (internal pilot phase): managing the delivery of the trial, ensuring the implementation of the protocol and associated amendments, supervising and training research staff, supervising and co-ordinating recruitment, delivering therapy and supervising research therapists, and working with NCTU to set up the trial database. Dr Clarke made substantial contributions to the design, delivery and analysis of the process evaluations involving young people. Dr Clarke made substantial contributions to and critically read the final report.
Dr Rick Fraser made contributions to the funding applications, and the design and development of the trial protocol. Dr Fraser was the Principal Investigator for the Sussex site. Dr Fraser contributed to trial management oversight. Dr Fraser critically read the final report.
Dr Kelly Grant (https://orcid.org/0000-0001-5319-8127) conducted the statistical analysis with Professor Lee Shepstone. Dr Grant critically read the final report.
Professor Kathryn Greenwood (https://orcid.org/0000-0001-7899-8980) made contributions to the funding applications, and the design and development of the trial protocol. Professor Greenwood contributed to trial management oversight. Dr Greenwood critically read the final report.
Dr Caitlin Notley (https://orcid.org/0000-0003-0876-3304) was the qualitative research lead. Dr Notley made contributions to the funding applications. Dr Notley made substantial contribution to the development of the trial protocol. Dr Notley was the overall qualitative research lead for the process evaluations and made substantial contributions to the design, delivery and analysis of the process evaluations involving young people. Dr Grant critically read the final report.
Dr Sophie Parker (https://orcid.org/0000-0001-5596-7524) made substantial contributions to the applications for funding, design of the trial and development of the protocol. Dr Parker provided site co-ordination in Greater Manchester, including supervision of research staff and co-ordination of recruitment. Dr Parker provided expert supervision and training for all trial staff in ARMS and diagnostic interview assessment. Dr Parker contributed to trial management oversight. Dr Parker made substantial contributions to and critically read the final report.
Professor Lee Shepstone was the trial statistician. Professor Shepstone made contributions to the funding applications. Professor Shepstone made a substantial contribution to the development of the trial protocol and statistical analysis plan. Professor Shepstone led the statistical analysis, which was conducted with Dr Kelly Grant. Professor Shepstone made substantial contributions to and critically read the final report.
Dr Jon Wilson (https://orcid.org/0000-0002-5279-6237) made substantial contributions to the funding applications, design of the trial and development of the protocol. Dr Wilson was the Co-Principal Investigator for the East Anglia site with Dr Hodgekins. Dr Wilson contributed to trial management oversight.
Professor Paul French made leading contributions to the applications for funding, design of the trial and development of the protocol. Professor French led the trial as Co-Chief Investigator with Professor Fowler and was the Principal Investigator for the Greater Manchester site. Professor French provided training and supervision to research and therapy staff. Professor French made substantial contributions to and critically read the final report.
Publications
Trial protocol
Fowler D, French P, Banerjee R, Barton G, Berry C, Byrne R, et al. Prevention and treatment of long-term social disability amongst young people with emerging severe mental illness with social recovery therapy (the PRODIGY trial): study protocol for a randomised controlled trial. Trials 2017;18:315.
Process evaluation
Notley C, Christopher R, Hodgekins J, Byrne R, French P, Fowler D. Participant views on involvement in a trial of social recovery cognitive–behavioural therapy. Br J Psychiatry 2015;206:122–7.
Gee B, Notley C, Byrne R, Clarke T, Hodgekins J, French P, et al. Young people’s experiences of Social recovery cognitive behavioural therapy and treatment as usual in the PRODIGY trial. Early Interven Psychiatry 2018;12:879–85.
Sacadura C. Social Recovery Therapy: An Interpretative Phenomenological Analysis of Therapists’ Experience of Hope Working with Complex Clients. Doctoral Thesis. Leicester: University of Leicester; 2018.
Data-sharing statement
We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Further information regarding SRT and associated research is available at: www.socialrecoverytherapy.co.uk.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Fowler D, French P, Banerjee R, Barton G, Berry C, Byrne R, et al. Prevention and treatment of long-term social disability amongst young people with emerging severe mental illness with social recovery therapy (the PRODIGY trial): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2062-9.
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder. Arch Gen Psychiatry 2003;60. https://doi.org/10.1001/archpsyc.60.7.709.
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593-602. https://doi.org/10.1001/archpsyc.62.6.593.
- Fowler DG, Hodgekins J, Arena K, Turner R, Lower R, Wheeler K, et al. Early detection and psychosocial intervention for young people who are at risk of developing long term socially disabling severe mental illness: should we give equal priority to functional recovery and complex emotional dysfunction as to psychotic symptom. Clin Neuropsychiatry 2010;7:63-72.
- Department of Health and Social Care . Future in Mind: Promoting, Protecting and Improving Our Children and Young People’s Mental Health and Wellbeing 2015.
- Department of Health and Social Care . Achieving Better Access to Mental Health Services by 2020 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/361648/mental-health-access.pdf (accessed 8 April 2021).
- Singh SP, Paul M, Ford T, Kramer T, Weaver T, McLaren S, et al. Process, outcome and experience of transition from child to adult mental healthcare: multiperspective study. Br J Psychiatry 2010;197:305-12. https://doi.org/10.1192/bjp.bp.109.075135.
- Department of Health and Social Care . Children and Young People in Mind: The Final Report of the National CAMHS Review 2008. https://webarchive.nationalarchives.gov.uk/20090615071556/http://publications.dcsf.gov.uk/eOrderingDownload/CAMHS-Review.pdf (accessed 8 April 2021).
- Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ 2007;10:23-41.
- Knapp M, King D, Healey A, Thomas C. Economic outcomes in adulthood and their associations with antisocial conduct, attention deficit and anxiety problems in childhood. J Ment Health Policy Econ 2011;14:137-47.
- Knapp M, Ardono V, Brimblecombe N, Evans-Lacko S, Lemmi V, King D, et al. Youth Mental Health: New Economic Evidence 2016. https://pssru.ac.uk/pub/5160.pdf (accessed 8 April 2021).
- NHS England . The Five Year Forward View for Mental Health. A Report from the Independent Mental Health Taskforce to the NHS in England 2016.
- National Institute for Health and Care Excellence (NICE) . Psychosis and Schizophrenia in Children and Young People: Recognition and Management. NICE Clinical Guidance 2013. https://nice.org.uk/guidance/cg155/resources/psychosis-and-schizophrenia-in-children-and-young-people-recognition-and-management-pdf-35109632980933 (accessed 8 April 2021).
- National Institute for Health and Care Excellence (NICE) . Social Anxiety Disorder: Recognition, Assessment and Treatment 2013. https://nice.org.uk/guidance/cg159/resources/social-anxiety-disorder-recognition-assessment-and-treatment-pdf-35109639699397 (accessed 8 April 2021).
- National Institute for Health and Care Excellence (NICE) . Depression in Children and Young People: Identification and Management 2019. https://nice.org.uk/guidance/ng134/resources/depression-in-children-and-young-people-identification-and-management-pdf-66141719350981 (accessed 8 April 2021).
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, . Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry 2008;65:762-71. https://doi.org/10.1001/archpsyc.65.7.762.
- Addington J, Gleeson J. Implementing cognitive-behavioural therapy for first-episode psychosis. Br J Psychiatry Suppl 2005;187:s72-6. https://doi.org/10.1192/bjp.187.48.s72.
- Scott J, Fowler D, McGorry P, Birchwood M, Killackey E, Christensen H, et al. Adolescents and young adults who are not in employment, education, or training. BMJ 2013;347. https://doi.org/10.1136/bmj.f5270.
- Bambra C. Yesterday once more? Unemployment and health in the 21st century. J Epidemiol Community Health 2010;64:213-15. https://doi.org/10.1136/jech.2009.090621.
- Benjet C, Hernández-Montoya D, Borges G, Méndez E, Medina-Mora ME, Aguilar-Gaxiola S. Youth who neither study nor work: mental health, education and employment. Salud Publica Mex 2012;54:410-17. https://doi.org/10.1590/S0036-36342012000400011.
- Breslau J, Miller E, Joanie Chung WJ, Schweitzer JB. Childhood and adolescent onset psychiatric disorders, substance use, and failure to graduate high school on time. J Psychiatr Res 2011;45:295-301. https://doi.org/10.1016/j.jpsychires.2010.06.014.
- Paul KI, Moser K. Unemployment impairs mental health: meta-analyses. J Vocat Behav 2009;74:264-82. https://doi.org/10.1016/j.jvb.2009.01.001.
- Power E, Clarke M, Kelleher I, Coughlan H, Lynch F, Connor D, et al. The association between economic inactivity and mental health among young people: a longitudinal study of young adults who are not in employment, education or training. Ir J Psychol Med 2015;32:155-60. https://doi.org/10.1017/ipm.2014.85.
- Sellström E, Bremberg S, O’campo P. Yearly incidence of mental disorders in economically inactive young adults. Eur J Public Health 2011;21:812-14. https://doi.org/10.1093/eurpub/ckq190.
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, et al. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull 2007;33:665-72. https://doi.org/10.1093/schbul/sbl075.
- Klosterkötter J, Ruhrmann S, Schultze-Lutter F, Salokangas RK, Linszen D, Birchwood M, et al. The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry 2005;4:161-7.
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002;59:921-8. https://doi.org/10.1001/archpsyc.59.10.921.
- Morrison AP, French P, Walford L, Lewis SW, Kilcommons A, Green J, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry 2004;185:291-7. https://doi.org/10.1192/bjp.185.4.291.
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 2005;39:964-71. https://doi.org/10.1080/j.1440-1614.2005.01714.x.
- Lin A, Wood SJ, Nelson B, Brewer WJ, Spiliotacopoulos D, Bruxner A, et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res 2011;132:1-7. https://doi.org/10.1016/j.schres.2011.06.014.
- Yung AR, Nelson B, Thompson A, Wood SJ. The psychosis threshold in ultra high risk (prodromal) research: is it valid?. Schizophr Res 2010;120:1-6. https://doi.org/10.1016/j.schres.2010.03.014.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. https://doi.org/10.1093/schbul/sbm114.
- National Institute for Health and Care Excellence (NICE) . Psychosis and Schizophrenia In Adults: Prevention and Management 2014.
- Morrison AP, Stewart SL, French P, Bentall RP, Birchwood M, Byrne R, et al. Early detection and intervention evaluation for people at high-risk of psychosis-2 (EDIE-2): trial rationale, design and baseline characteristics. Early Interv Psychiatry 2011;5:24-32. https://doi.org/10.1111/j.1751-7893.2010.00254.x.
- Fowler D, French P, Hodgekins J, Lower R, Turner R, Burton S, et al. CBT for Schizophrenia: Evidence-Based Interventions and Future Directions. Oxford: John Wiley & Sons, Ltd; 2013.
- Fowler D, Hodgekins J, Berry C, Clarke T, Palmier-Claus J, Sacadura C, et al. Social recovery therapy: a treatment manual. Psychosis 2019;11:261-72. https://doi.org/10.1080/17522439.2019.1607891.
- Fowler D, Hodgekins J, Painter M, Reilly T, Crane C, Macmillan I, et al. Cognitive behaviour therapy for improving social recovery in psychosis: a report from the ISREP MRC Trial Platform Study (Improving Social Recovery in Early Psychosis). Psychol Med 2009;39:1627-36. https://doi.org/10.1017/S0033291709005467.
- Fowler D, Hodgekins J, French P. Social Recovery Therapy in improving activity and social outcomes in early psychosis: current evidence and longer term outcomes. Schizophr Res 2019;203:99-104. https://doi.org/10.1016/j.schres.2017.10.006.
- Barton GR, Hodgekins J, Mugford M, Jones PB, Croudace T, Fowler D. Cognitive behaviour therapy for improving social recovery in psychosis: cost-effectiveness analysis. Schizophr Res 2009;112:158-63. https://doi.org/10.1016/j.schres.2009.03.041.
- Fowler D, Hodgekins J, French P, Marshall M, Freemantle N, McCrone P, et al. Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. Lancet Psychiatry 2018;5:41-50. https://doi.org/10.1016/S2215-0366(17)30476-5.
- Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000;55:469-80. https://doi.org/10.1037/0003-066X.55.5.469.
- Erikson E. Identity: Youth and Crisis. New York, NY: WW Norton & Company; 1968.
- Simonds LM, Pons RA, Stone NJ, Warren F, John M. Adolescents with anxiety and depression: is social recovery relevant?. Clin Psychol Psychother 2014;21:289-98. https://doi.org/10.1002/cpp.1841.
- Lowen C, Hodgekins J, French P, Berry C, Pugh K, Fitzsimmons M, et al. Measuring adherence in Social Recovery Therapy with people with first episode psychosis. Behav Cogn Psychother 2020;48:82-90. https://doi.org/10.1017/S1352465819000432.
- Blackburn I-M, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The Revised Cognitive Therapy Scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Short S. Review of the UK 2000 Time Use Survey. Newport: Office for National Statistics; 2006.
- Hodgekins J, French P, Birchwood M, Mugford M, Christopher R, Marshall M, et al. Comparing time use in individuals at different stages of psychosis and a non-clinical comparison group. Schizophr Res 2015;161:188-93. https://doi.org/10.1016/j.schres.2014.12.011.
- Eklund M, Leufstadius C, Bejerholm U. Time use among people with psychiatric disabilities: implications for practice. Psychiatr Rehabil J 2009;32:177-91. https://doi.org/10.2975/32.3.2009.177.191.
- Gershuny J. Time-Use Surveys and the Measurement of National Well-Being. Oxford: Centre for Time-use Research, Department of Sociology, University of Oxford; 2011.
- Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther 1998;36:455-70. https://doi.org/10.1016/S0005-7967(97)10031-6.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II). San Antonio, TX: The Psychological Corporation; 1996.
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Br J Psychiatry 1989;155:S53-8. https://doi.org/10.1192/S0007125000291502.
- Diagnostic and Statistical Manual of Mental Disorders (IV-TR). Washington, DC: American Psychiatric Association; 2000.
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976;33:766-71. https://doi.org/10.1001/archpsyc.1976.01770060086012.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 1994.
- Hayes SC, Strosahl K, Wilson KG, Bissett RT, Pistorello J, Toarmino D, et al. Measuring experiential avoidance: a preliminary test of a working model. Psychol Rec 2004;54:553-78. https://doi.org/10.1007/BF03395492.
- Steger MF, Frazier P, Oishi S, Kaler M. The meaning in life questionnaire: assessing the presence of and search for meaning in life. J Couns Psychol 2006;53:80-93. https://doi.org/10.1037/0022-0167.53.1.80.
- Snyder CR, Harris C, Anderson JR, Holleran SA, Irving LM, Sigmon ST, et al. The will and the ways: development and validation of an individual-differences measure of hope. J Pers Soc Psychol 1991;60:570-85. https://doi.org/10.1037//0022-3514.60.4.570.
- Fowler D, Freeman D, Smith B, Kuipers E, Bebbington P, Bashforth H, et al. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psychol Med 2006;36:749-59. https://doi.org/10.1017/S0033291706007355.
- Hodgekins J, Coker S, Freeman D, Ray-Glover K, Bebbington P, Garety P, et al. Assessing levels of subthreshold psychotic symptoms in the recovery phase: the Schizotypal Symptoms Inventory (SSI). J Exp Psychopathol 2012;3:582-93. https://doi.org/10.5127/jep.021211.
- Wechsler D. Wechsler Memory Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997.
- Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976.
- Babor TF, Higgins-Biddle J, Saunders J, Monteiro M. The Alcohol Use Disorders Identification Test. Guidelines For Use In Primary Care. Geneva, Switzerland: Department of Mental Health and Substance Dependence, World Health Organization; 2001.
- Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 2005;11:22-31. https://doi.org/10.1159/000081413.
- Beck AT, Steer RA. Manual for the Beck Hopelessness Scale. San Antonio, TX: The Psychological Corporation; 1988.
- Thornicroft G, Becker T, Knapp M, Knudsen H-C, Schene A, Tansella M, et al. International Outcome Measures in Mental Health: Quality of Life, Needs, Service Satisfaction, Costs and Impact On Carers. London: Gaskell; 2006.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010;171:624-32. https://doi.org/10.1093/aje/kwp425.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Gee B, Notley C, Byrne R, Clarke T, Hodgekins J, French P, et al. Young people’s experiences of Social Recovery Cognitive Behavioural Therapy and treatment as usual in the PRODIGY trial. Early Interv Psychiatry 2018;12:879-85. https://doi.org/10.1111/eip.12381.
- Notley C, Christopher R, Hodgekins J, Byrne R, French P, Fowler D. Participant views on involvement in a trial of social recovery cognitive-behavioural therapy. Br J Psychiatry 2015;206:122-7. https://doi.org/10.1192/bjp.bp.114.146472.
- Robson C. Real World Research: A Resource for Users of Social Research Methods in Applied Settings. Chichester: John Wiley & Sons Ltd; 2011.
- Notley C, Green G, Marsland L, Walker D-M. An Introduction to Health Services Research. London: SAGE Publications Ltd; 2014.
- Danermark B, Ekström M. Explaining Society: Critical Realism in the Social Sciences. London: Routledge; 2002.
- Smith JA, Osborn M, Breakwell GM. Doing Social Psychology Research. Oxford: The British Psychological Society and Blackwell Publishing Ltd; 2008.
- Byrne RE, Morrison AP. Young people at risk of psychosis: their subjective experiences of monitoring and cognitive behaviour therapy in the early detection and intervention evaluation 2 trial. Psychol Psychother 2014;87:357-71. https://doi.org/10.1111/papt.12013.
- Finn SE, Tonsager ME. Information-gathering and therapeutic models of assessment: complementary paradigms. Psychol Assess 1997;9:374-85. https://doi.org/10.1037/1040-3590.9.4.374.
- Granö N, Oksanen J, Kallionpää S, Roine M. Specificity and sensitivity of the Beck Hopelessness Scale for suicidal ideation among adolescents entering early intervention service. Nord J Psychiatry 2017;71:72-6. https://doi.org/10.1080/08039488.2016.1227370.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Kent: Personal Social Services Research Unit, University of Kent, Canterbury; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Kent: Personal Social Services Research Unit, University of Kent, Canterbury; 2017.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Barton GR, Fairall L, Bachmann MO, Uebel K, Timmerman V, Lombard C, et al. Cost-effectiveness of nurse-led versus doctor-led antiretroviral treatment in South Africa: pragmatic cluster randomised trial. Trop Med Int Health 2013;18:769-77. https://doi.org/10.1111/tmi.12093.
- Beecham J, Sleed M, Knapp M, Chiesa M, Drahorad C. The costs and effectiveness of two psychosocial treatment programmes for personality disorder: a controlled study. Eur Psychiatry 2006;21:102-9. https://doi.org/10.1016/j.eurpsy.2005.05.006.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 5 May 2021).
- Barton GR, Hodgekins J, Mugford M, Jones PB, Croudace T, Fowler D. Measuring the benefits of treatment for psychosis: validity and responsiveness of the EQ-5D. Br J Psychiatry 2009;195:170-7. https://doi.org/10.1192/bjp.bp.108.057380.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Noble SM, Hollingworth W, Tilling K. Missing data in trial-based cost-effectiveness analysis: the current state of play. Health Econ 2012;21:187-200. https://doi.org/10.1002/hec.1693.
- Drummond M, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Goodyer IM, Reynolds S, Barrett B, Byford S, Dubicka B, Hill J, et al. Cognitive behavioural therapy and short-term psychoanalytical psychotherapy versus a brief psychosocial intervention in adolescents with unipolar major depressive disorder (IMPACT): a multicentre, pragmatic, observer-blind, randomised controlled superiority trial. Lancet Psychiatry 2017;4:109-19. https://doi.org/10.1016/S2215-0366(16)30378-9.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- van Os J, Guloksuz S, Vijn TW, Hafkenscheid A, Delespaul P. The evidence-based group-level symptom-reduction model as the organizing principle for mental health care: time for change?. World Psychiatry 2019;18:88-96. https://doi.org/10.1002/wps.20609.
- Lavis A, Lester H, Everard L, Freemantle N, Amos T, Fowler D, et al. Layers of listening: qualitative analysis of the impact of early intervention services for first-episode psychosis on carers’ experiences. Br J Psychiatry 2015;207:135-42. https://doi.org/10.1192/bjp.bp.114.146415.
- Wilson J, Clarke T, Lower R, Ugochukwu U, Maxwell S, Hodgekins J, et al. Creating an innovative youth mental health service in the United Kingdom: the Norfolk Youth Service. Early Interv Psychiatry 2018;12:740-6. https://doi.org/10.1111/eip.12452.
- Sacadura C. Social Recovery Therapy: An Interpretative Phenomenological Analysis of Therapists’ Experience of Hope Working with Complex Clients. Leicester: University of Leicestershire; 2018.
- Valmaggia LR, Stahl D, Yung AR, Nelson B, Fusar-Poli P, McGorry PD, et al. Negative psychotic symptoms and impaired role functioning predict transition outcomes in the at-risk mental state: a latent class cluster analysis study. Psychol Med 2013;43:2311-25. https://doi.org/10.1017/S0033291713000251.
- Cross SPM, Scott J, Hickie IB. Predicting early transition from sub-syndromal presentations to major mental disorders. BJPsych Open 2017;3:223-7. https://doi.org/10.1192/bjpo.bp.117.004721.
- Gee B, Hodgekins J, Fowler D, Marshall M, Everard L, Lester H, et al. The course of negative symptom in first episode psychosis and the relationship with social recovery. Schizophr Res 2016;174:165-71. https://doi.org/10.1016/j.schres.2016.04.017.
- Hodgekins J, Birchwood M, Christopher R, Marshall M, Coker S, Everard L, et al. Investigating trajectories of social recovery in individuals with first-episode psychosis: a latent class growth analysis. Br J Psychiatry 2015;207:536-43. https://doi.org/10.1192/bjp.bp.114.153486.
- Cross SPM, Hermens DF, Scott J, Salvador-Carulla L, Hickie IB. Differential impact of current diagnosis and clinical stage on attendance at a youth mental health service. Early Interv Psychiatry 2017;11:255-62. https://doi.org/10.1111/eip.12319.
- Hickie IB, Scott EM, Hermens DF, Naismith SL, Guastella AJ, Kaur M, et al. Applying clinical staging to young people who present for mental health care. Early Interv Psychiatry 2013;7:31-43. https://doi.org/10.1111/j.1751-7893.2012.00366.x.
- Doyle R, Turner N, Fanning F, Brennan D, Renwick L, Lawlor E, et al. First-episode psychosis and disengagement from treatment: a systematic review. Psychiatr Serv 2014;65:603-11. https://doi.org/10.1176/appi.ps.201200570.
- Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 1982;8:470-84. https://doi.org/10.1093/schbul/8.3.470.
Appendix 1 Changes to protocol
| Phase | Protocol version | Amendment | |||
|---|---|---|---|---|---|
| Number | Classification | Date | Details | ||
| Internal pilot | Amendment 1 | Substantial | 20 December 2012 |
|
|
| Amendment 2 | Minor | 1 February 2013 | Addition of new Participant Identification Centres | ||
| Amendment 3 | Minor | 19 February 2013 | Addition of new logos to study documents | ||
| Amendment 4 | Minor | 1 May 2013 | Addition of new research sites within existing centres | ||
| Amendment 5 | Minor | 5 March 2014 | Principal Investigator activity delegation. Addition of thank-you cards to participants at trial exit. Modification to mid-point contact procedures | ||
| Amendment 6 | Substantial | 19 January 2015 |
|
||
| Amendment 7 | Minor | 27 April 2017 | Extension of trial end date to May 2019 in the light of extended recruitment period in the recruitment extension phase to reach target of 270 participants across the trial | ||
| Extension | V1.0 29 June 2015 | Amendment 1.1 | Minor | 26 February 2016 | Clarification that inclusion criterion 2 (active positive psychotic symptoms or history of FEP) referred to presence of psychosis as operationalised symptomatically or as according to therapeutic antipsychotic medication (or both) |
| V2.0 29 January 2016 | Amendment 2.0 | Substantial | 2 February 2016 |
|
|
| V2.0 29 January 2016 | Amendment 2.1 | Substantial | 1 April 2016 | Addition of new leaflet to support GP recruitment (V2, 23 March 2016) | |
| V2.0 29 January 2016 | Amendment 2.2 | Minor | 24 November 2016 | Continuation of recruitment beyond planned end date of November 2016 to reach target of 270 participants across the trial | |
| V3.0 27 July 2017 | Amendment 3.0 | Substantial | 1 September 2017 |
|
|
| V4.0 13 February 2018 | Amendment 4.0 | Substantial | 6 April 2018 |
|
|
Appendix 2 Supplementary figures
| PRODIGY | Study period | ||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post allocation | Close out | ||||
| Screening | Baseline | Intervention (months) | Follow-up (months) | Follow-up (months) | |||
| Time point | –t1a | –t2a,b | 0 | 9 | 9 | 15 | 24 |
| Enrolment | |||||||
| Informed consent | ✗ | ||||||
| Eligibility screen | ✗ | ||||||
| Randomisation | ✗ | ||||||
| Interventions | |||||||
| SRT + ESC | ♦▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬♦ | ||||||
| ESC alone | ♦▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬♦ | ||||||
| Assessments | |||||||
| Primary outcome | |||||||
| TUSc | ✗ | ✗ | ✗ | ✗ | |||
| Secondary mental health outcomes | |||||||
| GAFc | ✗ | ✗ | ✗ | ✗ | |||
| CAARMSc | ✗ | ✗ | ✗ | ✗ | |||
| SANS | ✗ | ✗ | ✗ | ✗ | |||
| SCID | ✗ | ✗ | ✗ | ✗ | |||
| SIAS | ✗ | ✗ | ✗ | ✗ | |||
| BDI-II | ✗ | ✗ | ✗ | ✗ | |||
| Health economic outcomes | |||||||
| HSRUQ | ✗ | ✗ | ✗ | ✗ | |||
| EQ-5D | ✗ | ✗ | ✗ | ✗ | |||
| Hypothesised mediators | |||||||
| AAQ-II | ✗ | ✗ | ✗ | ✗ | |||
| MLQ | ✗ | ✗ | ✗ | ✗ | |||
| THS | ✗ | ✗ | ✗ | ✗ | |||
| SSI | ✗ | ✗ | ✗ | ✗ | |||
| BCSS | ✗ | ✗ | ✗ | ✗ | |||
| Hypothesised moderators | |||||||
| Logical Memory I | ✗ | ✗ | |||||
| COWAT | ✗ | ✗ | |||||
| Other outcomes | |||||||
| AUDIT | ✗ | ✗ | ✗ | ✗ | |||
| DUDIT | ✗ | ✗ | ✗ | ✗ | |||
| BHS | ✗ | ✗ | ✗ | ✗ | |||
| AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ||
Appendix 3 Supplementary statistical analysis tables
Analysis of secondary time use outcomes
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 9 months | ||||||
| Constructive economic activity (hours per week) | 14.0 (11.8) | 16.6 (15.9) | –1.36 (–5.14 to 2.42) | 0.48 | 0.03 (–0.23 to 0.29) | 0.804 |
| Missing, n | 4 | 17 | ||||
| Total hours paid employmentd | 99.8 (311.3) | 283.9 (511.4) | –157.12 (–292.33 to –21.91) | 0.023 | –1.00 (–1.86 to –0.14) | 0.023 |
| Missing, n | 11 | 48 | ||||
| Total hours educationd | 134.5 (367.6) | 108.0 (235.0) | 36.07 (–64.20 to 136.34) | 0.48 | 0.22 (–0.61 to 1.04) | 0.602 |
| Missing, n | 13 | 50 | ||||
| Total hours voluntary employmentd | 53.2 (244.5) | 50.7 (170.9) | –2.38 (–70.81 to 66.06) | 0.95 | –0.02 (–0.70 to 0.66) | 0.96 |
| Missing, n | 19 | 47 | ||||
| Total hours all activityd | 291.8 (499.4) | 454.2 (592.9) | –107.46 (–283.94 to 69.01) | 0.23 | –0.42 (–1.30 to 0.46) | 0.35 |
| Missing, n | 19 | 56 | ||||
| 15 months | ||||||
| Constructive economic activity (hours per week) | 13.3 (13.2) | 22.0 (24.5) | –6.92 (–12.72 to –1.12) | 0.020 | –0.28 (–0.56 to –0.00) | 0.049 |
| Missing, n | 4 | 24 | ||||
| Total hours paid employmentd | 41.3 (110.2) | 123.6 (275.2) | –73.89 (–139.23 to –8.55) | 0.027 | –0.58 (–1.25 to 0.10) | 0.095 |
| Missing, n | 11 | 31 | ||||
| Total hours educationd | 54.0 (137.4) | 88.8 (176.3) | –34.56 (–83.38 to 14.26) | 0.16 | –0.33 (–1.03 to 0.37) | 0.35 |
| Missing, n | 13 | 31 | ||||
| Total hours voluntary employmentd | 17.1 (41.2) | 36.8 (96.3) | –16.58 (–40.21 to 7.04) | 0.17 | –0.03 (–0.60 to 0.54) | 0.92 |
| Missing, n | 15 | 28 | ||||
| Total hours all activityd | 113.0 (170.3) | 258.8 (359.8) | –134.51 (–224.41 to –44.60) | 0.004 | –0.52 (–1.28 to 0.24) | 0.18 |
| Missing, n | 19 | 35 | ||||
Analysis of general psychopathology diagnoses continued
| CAARMS transition | Allocation arm, n (%) | Odds ratioa (95% CIb) | p-valuec | |
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| Transition to psychosis at 9 months | 12 (9.8) | 8 (7.5) | 1.30 (0.49 to 3.44) | 0.59 |
| Missing, n | 16 | 25 | ||
| Transition to psychosis at 15 months | 6 (5.6) | 1 (1.1) | 7.33 (0.71 to 76.25) | 0.095 |
| Missing, n | 30 | 44 | ||
| CAARMS transition | Allocation arm, n (%) | Odds ratioa (95% CIb) | p-valuec | |
|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | |||
| Transition to psychosis at 24 months | 3 (3.1) | 2 (2.5) | 1.29 (0.20 to 8.19) | 0.79 |
| Missing, n | 40 | 51 | ||
| Mood outcome | Time point | Prevalence, n (%), n missing | Relative riska (95% CIb) | p-valuec | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Past major depressive episode | Baseline | 33 (23.9), 0 | 41 (31.1), 0 | ||
| 9 months | 53 (42.4), 13 | 36 (32.1), 20 | 1.32 (0.94 to 1.85) | 0.109 | |
| 15 months | 33 (27.1), 16 | 25 (25.0), 32 | 1.08 (0.69 to 1.69) | 0.76 | |
| Past mania | Baseline | 2 (1.5), 0 | 5 (3.8), 0 | ||
| 9 months | 2 (1.6), 13 | 3 (2.7), 20 | 0.60 (0.10 to 3.51) | 0.67 | |
| 15 months | 1 (0.8), 16 | 2 (2.0), 30 | 0.42 (0.04 to 4.54) | 0.59 | |
| Past hypomania | Baseline | 2 (1.5), 0 | 1 (0.8), 0 | ||
| 9 months | 2 (1.6), 13 | 3 (2.7), 20 | 0.60 (0.10 to 3.51) | 0.67 | |
| 15 months | 4 (3.3), 16 | 2 (2.0), 30 | 1.67 (0.31 to 8.94) | 0.69 | |
| Major depressive disorder | Baseline | 95 (68.8), 0 | 93 (70.5), 0 | ||
| 9 months | 66 (52.8), 13 | 55 (49.6), 21 | 1.07 (0.83 to 1.37) | 0.70 | |
| 15 months | 58 (47.5), 16 | 43 (42.2), 30 | 1.13 (0.84 to 1.51) | 0.50 | |
| Anxiety, eating and somatoform outcome | Time point | Prevalence, n (%), n missinga | Relative riskb (95% CIc) | p-valued | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Panic disorder | Baseline | 6 (4.4), 0 | 6 (4.6), 1 | ||
| 9 months | 4 (80.0), 1 | 3 (50.0), 0 | 1.60 (0.64 to 3.98) | 0.55 | |
| 15 months | 1 (25.0), 2 | 2 (40.0), 1 | 0.63 (0.08 to 4.66) | 1.00 | |
| Hypochondriasis | Baseline | 4 (2.9), 0 | 3 (2.3), 0 | ||
| 9 months | 2 (50.0), 0 | 2 (66.7), 0 | 0.75 (0.21 to 2.66) | 1.00 | |
| 15 months | 3 (75.0), 0 | 1 (33.3), 0 | 2.25 (0.41 to 12.28) | 0.47 | |
| Anorexia nervosa | Baseline | 1 (0.7), 0 | 1 (0.8), 0 | ||
| 9 months | 0 (0), 0 | 0 (0), 0 | – | ||
| 15 months | 0 (0), 0 | 0 (0), 0 | – | ||
| Bulimia nervosa | Baseline | 1 (0.7), 0 | 0 (0), 0 | ||
| 9 months | 0 (0), 1 | 0 (0), 0 | – | ||
| 15 months | 0 (0), 1 | 0 (0), 0 | – | ||
| Binge-eating disorder | Baseline | 2 (1.5), 0 | 1 (0.8), 0 | ||
| 9 months | 1 (50.0), 0 | 0 (0), 0 | – | 1.00 | |
| 15 months | 1 (50.0), 0 | 0 (0), 0 | – | 1.00 | |
| Anxiety disorder NOS | Baseline | 4 (2.9), 0 | 3 (2.3), 0 | ||
| 9 months | 1 (25.0), 0 | 2 (100), 1 | 0.25 (0.05 to 1.36) | 0.40 | |
| 15 months | 1 (25.0), 0 | 1 (50.0), 1 | 0.50 (0.06 to 4.47) | 1.00 | |
| Mood outcome | Time point | Prevalence, n (%), n missing | Relative riska (95% CIb) | p-valuec | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Past major depressive episode | Baseline | 33 (23.9), 0 | 41 (31.1), 0 | ||
| 24 months | 24 (22.6), 32 | 12 (14.6), 50 | 1.55 (0.82 to 2.91) | 0.19 | |
| Past mania | Baseline | 2 (1.5), 0 | 5 (3.8), 0 | ||
| 24 months | 2 (1.9), 32 | 1 (1.2), 49 | 1.57 (0.14 to 16.98) | 1.00 | |
| Past hypomania | Baseline | 2 (1.5), 0 | 1 (0.8), 0 | ||
| 24 months | 2 (1.9), 32 | 2 (2.4), 49 | 0.78 (0.11 to 5.44) | 1.00 | |
| Major depressive disorder | Baseline | 95 (68.8), 0 | 93 (70.5), 0 | ||
| 24 months | 45 (42.5), 32 | 28 (33.7), 49 | 1.26 (0.87 to 1.83) | 0.23 | |
| Anxiety, eating and somatoform outcome | Time point | Prevalence, n (%), n missinga | Relative riskb (95% CIc) | p-valued | |
|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | ||||
| Panic disorder | Baseline | 6 (4.4), 0 | 6 (4.6), 1 | ||
| 24 months | 0 (0), 2 | 3 (60.0), 1 | 0.17 | ||
| Specific phobia | Baseline | 10 (7.3), 0 | 4 (3.0), 0 | ||
| 24 months | 4 (57.1), 3 | 1 (33.3), 1 | 1.71 (0.31 to 9.61) | 1.00 | |
| Hypochondriasis | Baseline | 4 (2.9), 0 | 3 (2.3), 0 | ||
| 24 months | 3 (100), 1 | 0 (0), 1 | – | 0.10 | |
| Anorexia nervosa | Baseline | 1 (0.7), 0 | 1 (0.8), 0 | ||
| 24 months | 0 (0), 0 | 0 (0), 0 | – | ||
| Bulimia nervosa | Baseline | 1 (0.7), 0 | 0 (0), 0 | ||
| 24 months | 0 (0), 1 | 0 (0), 0 | – | ||
| Binge-eating disorder | Baseline | 2 (1.5), 0 | 1 (0.8), 0 | ||
| 24 months | 1 (50.0), 0 | 1 (100), 0 | 0.50 (0.13 to 2.00) | 1.00 | |
| Anxiety disorder NOS | Baseline | 4 (2.9), 0 | 3 (2.3), 0 | ||
| 24 months | 1 (25.0), 0 | 0 (0), 2 | – | 1.00 | |
Analysis of negative symptoms
| Symptom | Allocation arm, mean (SD) | |
|---|---|---|
| SRT + ESC (n = 138) | ESC alone (n = 132) | |
| Unchanging facial expressiona | 1.1 (1.5) | 0.9 (1.3) |
| Decreased spontaneous movement | 0.6 (1.1) | 0.6 (1.0) |
| Paucity of expressive gestures | 1.1 (1.5) | 0.8 (1.4) |
| Poor eye contact | 1.2 (1.4) | 0.9 (1.2) |
| Affective non-responsivity | 0.7 (1.2) | 0.5 (1.0) |
| Lack of vocal inflections | 1.2 (1.5) | 0.8 (1.2) |
| Global rating of affective flat | 1.2 (1.4) | 0.9 (1.1) |
| Inappropriate affect | 0.3 (0.7) | 0.4 (0.8) |
| Poverty of speech | 0.9 (1.4) | 0.8 (1.2) |
| Poverty of content of speech | 0.5 (1.1) | 0.5 (1.0) |
| Blocking | 0.3 (0.9) | 0.3 (0.7) |
| Increased latency of response | 0.7 (1.1) | 0.5 (1.0) |
| Global rating of alogia | 0.8 (1.0) | 0.7 (0.9) |
| Grooming and hygiene | 1.0 (1.2) | 0.9 (1.1) |
| Impersistence at work or school | 4.1 (1.2) | 4.2 (1.2) |
| Physical anergia | 3.1 (1.4) | 3.3 (1.4) |
| Global rating of avolition apathy | 3.2 (1.1) | 3.3 (1.0) |
| Recreational interest and activity | 2.8 (1.4) | 2.9 (1.4) |
| Sexual interest and activitya,b | 1.5 (1.9) | 1.7 (1.9) |
| Ability to feel intimacy and closenessa | 1.8 (1.7) | 1.8 (1.6) |
| Relationships with friends and peers | 3.1 (1.5) | 2.9 (1.5) |
| Global rating of anhedonia asocialitya | 2.9 (1.2) | 2.8 (1.1) |
| Social inattentiveness | 0.6 (1.1) | 0.8 (1.3) |
| Inattentiveness during mental state testingc | 0.9 (1.4) | 0.9 (1.3) |
| Global rating of attentionc | 0.8 (1.0) | 0.9 (0.9) |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Unchanging facial expression | 0.8 (1.2), 22 | 0.6 (1.1), 29 | 0.16 (–0.12 to 0.45) | 0.26 | 0.09 (–0.05 to 0.22) | 0.201 |
| Decreased spontaneous movement | 0.5 (0.9), 21 | 0.3 (0.9), 29 | 0.08 (–0.12 to 0.29) | 0.44 | 0.06 (–0.04 to 0.16) | 0.27 |
| Paucity of expressive gestures | 0.8 (1.4), 21 | 0.5 (0.9), 29 | 0.24 (–0.03 to 0.50) | 0.079 | 0.08 (–0.04 to 0.20) | 0.17 |
| Poor eye contact | 1.1 (1.4), 21 | 0.8 (1.1), 29 | 0.14 (–0.13 to 0.40) | 0.307 | 0.04 (–0.08 to 0.16) | 0.53 |
| Affective non-responsivity | 0.4 (0.9), 20 | 0.3 (0.6), 29 | 0.09 (–0.11 to 0.29) | 0.36 | 0.03 (–0.07 to 0.14) | 0.52 |
| Lack of vocal inflections | 0.8 (1.2), 18 | 0.5 (0.9), 29 | 0.13 (–0.13 to 0.38) | 0.33 | 0.05 (–0.07 to 0.17) | 0.408 |
| Global rating of affective flat | 0.9 (1.1), 22 | 0.7 (0.9), 29 | 0.11 (–0.12 to 0.35) | 0.33 | 0.05 (–0.07 to 0.16) | 0.43 |
| Inappropriate affect | 0.3 (0.7), 20 | 0.3 (0.6), 30 | 0.02 (–0.14 to 0.17) | 0.82 | 0.01 (–0.08 to 0.09) | 0.909 |
| Poverty of speech | 0.8 (1.3), 18 | 0.5 (1.0), 28 | 0.21 (–0.04 to 0.46) | 0.099 | 0.10 (–0.01 to 0.21) | 0.084 |
| Poverty of content of speech | 0.3 (0.7), 18 | 0.4 (0.9), 28 | –0.09 (–0.30 to 0.12) | 0.401 | –0.04 (–0.14 to 0.07) | 0.503 |
| Blocking | 0.3 (0.7), 18 | 0.3 (0.7), 28 | –0.02 (–0.19 to 0.16) | 0.87 | –0.00 (–0.10 to 0.09) | 0.95 |
| Increased latency of response | 0.5 (1.0), 18 | 0.3 (0.7), 28 | 0.09 (–0.11 to 0.30) | 0.38 | 0.04 (–0.06 to 0.15) | 0.43 |
| Global rating of alogia | 0.7 (0.9), 18 | 0.5 (0.8), 28 | 0.15 (–0.06 to 0.37) | 0.16 | 0.07 (–0.04 to 0.19) | 0.21 |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Grooming and hygiene | 0.8 (1.1), 18 | 0.7 (1.0), 28 | –0.00 (–0.25 to 0.24) | 0.98 | –0.01 (–0.13 to 0.11) | 0.85 |
| Impersistence at work or school | 2.9 (2.0), 18 | 3.0 (2.0), 28 | –0.03 (–0.50 to 0.43) | 0.89 | –0.03 (–0.19 to 0.13) | 0.68 |
| Physical anergia | 2.1 (1.6), 18 | 2.4 (1.7), 30 | –0.23 (–0.62 to 0.16) | 0.25 | ||
| Global rating of avolition apathy | 2.3 (1.5), 18 | 2.5 (1.5), 30 | –0.10 (–0.44 to 0.24) | 0.58 | ||
| Recreational interest and activity | 1.4 (1.7), 18 | 1.4 (1.6), 30 | 0.04 (–0.37 to 0.45) | 0.83 | –0.01 (–0.18 to 0.17) | 0.95 |
| Sexual interest and activity | 0.9 (1.5), 21 | 1.0 (1.6), 32 | –0.15 (–0.56 to 0.25) | 0.456 | –0.06 (–0.23 to 0.11) | 0.46 |
| Ability to feel intimacy and closeness | 1.0 (1.4), 18 | 1.1 (1.3), 29 | –0.11 (–0.46 to 0.25) | 0.56 | –0.08 (–0.23 to 0.08) | 0.33 |
| Relationships with friends and peers | 1.8 (1.7), 18 | 1.8 (1.7), 29 | –0.07 (–0.48 to 0.34) | 0.74 | –0.01 (–0.17 to 0.15) | 0.87 |
| Global rating of anhedonia asociality | 1.7 (1.4), 19 | 1.8 (1.4), 30 | –0.14 (–0.49 to 0.21) | 0.43 | ||
| Social inattentiveness | 0.3 (0.8), 22 | 0.6 (1.1), 29 | –0.17 (–0.42 to 0.08) | 0.18 | –0.07 (–0.19 to 0.05) | 0.26 |
| Inattentiveness during mental state testing | 0.7 (1.1), 20 | 0.8 (1.2), 32 | 0.04 (–0.24 to 0.31) | 0.79 | 0.02 (–0.11 to 0.15) | 0.733 |
| Global rating of attention | 0.6 (0.9), 23 | 0.7 (1.0), 31 | –0.08 (–0.31 to 0.16) | 0.54 | –0.04 (–0.16 to 0.08) | 0.527 |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Unchanging facial expression | 0.9 (1.2), 27 | 0.6 (1.0), 38 | 0.26 (–0.03 to 0.56) | 0.080 | 0.13 (–0.01 to 0.28) | 0.062 |
| Decreased spontaneous movement | 0.4 (0.8), 26 | 0.2 (0.6), 38 | 0.16 (–0.02 to 0.33) | 0.076 | 0.10 (0.00 to 0.19) | 0.044 |
| Paucity of expressive gestures | 0.8 (1.4), 26 | 0.4 (0.9), 38 | 0.22 (–0.05 to 0.49) | 0.12 | 0.09 (–0.03 to 0.21) | 0.16 |
| Poor eye contact | 0.9 (1.3), 27 | 0.7 (1.0), 38 | 0.16 (–0.11 to 0.43) | 0.24 | 0.06 (–0.07 to 0.18) | 0.39 |
| Affective non-responsivity | 0.5 (1.0), 26 | 0.3 (0.6), 38 | 0.18 (–0.03 to 0.40) | 0.094 | 0.08 (–0.02 to 0.19) | 0.12 |
| Lack of vocal inflections | 0.8 (1.2), 26 | 0.3 (0.7), 36 | 0.38 (0.13 to 0.63) | 0.003 | 0.19 (0.07 to 0.31) | 0.002 |
| Global rating of affective flat | 0.9 (1.1), 28 | 0.6 (0.8), 38 | 0.16 (–0.07 to 0.39) | 0.18 | 0.08 (–0.04 to 0.20) | 0.22 |
| Inappropriate affect | 0.2 (0.4), 26 | 0.2 (0.5), 38 | 0.01 (–0.11 to 0.13) | 0.88 | 0.02 (–0.06 to 0.10) | 0.62 |
| Poverty of speech | 0.7 (1.3), 22 | 0.5 (1.0), 36 | 0.20 (–0.05 to 0.45) | 0.12 | 0.09 (–0.03 to 0.20) | 0.15 |
| Poverty of content of speech | 0.5 (1.0), 22 | 0.3 (0.7), 36 | 0.16 (–0.06 to 0.39) | 0.15 | 0.06 (–0.05 to 0.18) | 0.27 |
| Blocking | 0.2 (0.6), 22 | 0.2 (0.6), 36 | –0.02 (–0.18 to 0.15) | 0.85 | 0.00 (–0.09 to 0.09) | 1.00 |
| Increased latency of response | 0.5 (0.9), 23 | 0.2 (0.6), 36 | 0.23 (0.02 to 0.44) | 0.028 | 0.12 (0.02 to 0.23) | 0.024 |
| Global rating of alogia | 0.7 (0.8), 22 | 0.5 (0.7), 36 | 0.17 (–0.02 to 0.37) | 0.087 | 0.10 (–0.01 to 0.21) | 0.070 |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Grooming and hygiene | 0.7 (1.1), 24 | 0.6 (1.0), 35 | 0.02 (–0.24 to 0.28) | 0.86 | 0.00 (–0.13 to 0.13) | 0.98 |
| Impersistence at work or school | 2.9 (2.0), 23 | 2.8 (2.1), 35 | 0.05 (–0.43 to 0.53) | 0.85 | ||
| Physical anergia | 2.0 (1.6), 23 | 2.0 (1.7), 35 | 0.02 (–0.38 to 0.43) | 0.905 | ||
| Global rating of avolition apathy | 2.3 (1.4), 24 | 2.2 (1.5), 36 | 0.13 (–0.23 to 0.48) | 0.48 | ||
| Recreational interest and activity | 1.4 (1.6), 24 | 1.4 (1.6), 35 | –0.02 (–0.44 to 0.39) | 0.91 | ||
| Sexual interest and activity | 1.2 (1.8), 25 | 1.1 (1.7), 36 | 0.10 (–0.35 to 0.55) | 0.65 | 0.04 (–0.14 to 0.22) | 0.68 |
| Ability to feel intimacy and closeness | 1.0 (1.3), 24 | 1.0 (1.2), 35 | 0.02 (–0.30 to 0.34) | 0.89 | 0.00 (–0.15 to 0.15) | 0.99 |
| Relationships with friends and peers | 2.0 (1.7), 24 | 1.5 (1.7), 35 | 0.46 (0.03 to 0.89) | 0.036 | ||
| Global rating of anhedonia asociality | 1.8 (1.4), 25 | 1.6 (1.3), 35 | 0.17 (–0.18 to 0.51) | 0.34 | ||
| Social inattentiveness | 0.4 (0.8), 25 | 0.3 (0.9), 36 | 0.10 (–0.13 to 0.33) | 0.40 | 0.07 (–0.05 to 0.18) | 0.25 |
| Inattentiveness during mental state testing | 0.7 (1.1), 24 | 0.8 (1.3), 36 | –0.09 (–0.37 to 0.19) | 0.54 | –0.03 (–0.16 to 0.10) | 0.66 |
| Global rating of attention | 0.6 (0.8), 25 | 0.6 (0.9), 37 | 0.04 (–0.18 to 0.26) | 0.73 | 0.03 (–0.08 to 0.15) | 0.58 |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Unchanging facial expression | 1.0 (1.3), 39 | 0.6 (1.1), 51 | 0.36 (0.02 to 0.70) | 0.041 | 0.18 (0.03 to 0.34) | 0.022 |
| Decreased spontaneous movement | 0.6 (1.1), 39 | 0.3 (0.8), 51 | 0.28 (–0.00 to 0.56) | 0.051 | 0.15 (0.01 to 0.28) | 0.033 |
| Paucity of expressive gestures | 1.1 (1.6), 39 | 0.5 (1.0), 51 | 0.52 (0.16 to 0.88) | 0.005 | 0.22 (0.06 to 0.38) | 0.007 |
| Poor eye contact | 1.0 (1.2), 39 | 0.6 (1.0), 51 | 0.34 (0.05 to 0.62) | 0.023 | 0.17 (0.03 to 0.31) | 0.014 |
| Affective non-responsivity | 0.6 (1.0), 39 | 0.3 (0.7), 51 | 0.27 (0.01 to 0.53) | 0.042 | 0.15 (0.02 to 0.27) | 0.025 |
| Lack of vocal inflections | 1.0 (1.3), 39 | 0.5 (1.0), 51 | 0.40 (0.08 to 0.73) | 0.014 | 0.23 (0.08 to 0.37) | 0.003 |
| Global rating of affective flat | 1.3 (1.2), 39 | 0.6 (1.0), 51 | 0.54 (0.25 to 0.82) | < 0.001 | 0.28 (0.15 to 0.41) | < 0.001 |
| Inappropriate affect | 0.1 (0.5), 38 | 0.2 (0.7), 51 | –0.09 (–0.25 to 0.07) | 0.26 | –0.04 (–0.12 to 0.04) | 0.35 |
| Poverty of speech | 0.7 (1.3), 39 | 0.4 (1.0), 50 | 0.27 (–0.01 to 0.55) | 0.060 | 0.13 (–0.00 to 0.26) | 0.052 |
| Poverty of content of speech | 0.4 (0.8), 38 | 0.3 (0.8), 50 | 0.03 (–0.19 to 0.26) | 0.76 | 0.02 (–0.10 to 0.13) | 0.75 |
| Blocking | 0.4 (0.8), 39 | 0.3 (0.8), 51 | 0.11 (–0.10 to 0.32) | 0.30 | 0.06 (–0.04 to 0.17) | 0.23 |
| Increased latency of response | 0.5 (0.9), 39 | 0.4 (0.7), 50 | 0.04 (–0.20 to 0.28) | 0.75 | 0.01 (–0.12 to 0.13) | 0.89 |
| Global rating of alogia | 0.8 (1.0), 39 | 0.5 (0.8), 50 | 0.23 (–0.01 to 0.47) | 0.057 | 0.12 (–0.01 to 0.25) | 0.066 |
| Symptom | Allocation arm, mean (SD), n missing | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Grooming and hygiene | 0.6 (1.1), 40 | 0.7 (1.2), 51 | –0.15 (–0.45 to 0.15) | 0.33 | –0.08 (–0.22 to 0.07) | 0.29 |
| Impersistence at work or school | 2.8 (2.1), 40 | 2.6 (2.1), 51 | 0.09 (–0.46 to 0.65) | 0.74 | ||
| Physical anergia | 2.2 (1.7), 40 | 2.2 (1.8), 51 | –0.02 (–0.50 to 0.45) | 0.92 | ||
| Global rating of avolition apathy | 2.3 (1.6), 40 | 2.2 (1.6), 51 | 0.16 (–0.26 to 0.57) | 0.45 | ||
| Recreational interest and activity | 1.4 (1.6), 40 | 1.3 (1.4), 51 | 0.16 (–0.28 to 0.60) | 0.48 | 0.02 (–0.17 to 0.21) | 0.82 |
| Sexual interest and activity | 1.3 (1.8), 45 | 1.3 (1.8), 51 | 0.07 (–0.45 to 0.59) | 0.78 | 0.02 (–0.19 to 0.22) | 0.87 |
| Ability to feel intimacy and closeness | 1.0 (1.3), 40 | 1.0 (1.3), 51 | 0.03 (–0.33 to 0.38) | 0.89 | 0.00 (–0.16 to 0.16) | 0.97 |
| Relationships with friends and peers | 2.3 (1.9), 40 | 1.5 (1.5), 51 | 0.78 (0.31 to 1.26) | 0.001 | ||
| Global rating of anhedonia asociality | 2.0 (1.5), 40 | 1.6 (1.3), 51 | 0.43 (0.03 to 0.82) | 0.034 | ||
| Social inattentiveness | 0.3 (0.8), 41 | 0.3 (0.8), 51 | –0.03 (–0.25 to 0.20) | 0.81 | –0.01 (–0.12 to 0.10) | 0.84 |
| Inattentiveness during mental state testing | 0.9 (1.3), 42 | 0.8 (1.4), 51 | 0.08 (–0.28 to 0.44) | 0.66 | 0.06 (–0.09 to 0.22) | 0.42 |
| Global rating of attention | 0.7 (1.0), 42 | 0.6 (0.9), 51 | 0.07 (–0.19 to 0.33) | 0.59 | 0.04 (–0.08 to 0.17) | 0.509 |
Per-protocol analysis of primary outcome
For the time use variables, we explored the analysis of the PP population as well as the ITT population. These results are presented in Table 53.
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| 9 months | ||||||
| Structured activity (hours per week) | 19.4 (14.5) | 22.3 (19.3) | –1.23 (–5.71 to 3.25) | 0.59 | 0.09 (–0.14 to 0.32) | 0.45 |
| Missing, n | 4 | 17 | ||||
| Structured activity (minus child care; hours per week) | 18.5 (12.8) | 22.2 (19.3) | –2.04 (–6.41 to 2.34) | 0.36 | 0.08 (–0.16 to 0.31) | 0.52 |
| Missing, n | 4 | 17 | ||||
| 15 months | ||||||
| Structured activity (hours per week) | 18.0 (15.3) | 27.7 (26.5) | –7.00 (–13.22 to –0.78) | 0.028 | –0.16 (–0.41 to 0.08) | 0.19 |
| Missing, n | 4 | 24 | ||||
| Structured activity (minus child care; hours per week) | 17.6 (14.7) | 24.9 (20.4) | –4.99 (–9.89 to –0.08) | 0.046 | –0.13 (–0.37 to 0.12) | 0.31 |
| Missing, n | 4 | 24 | ||||
Time use linear models exploring interactions
When adding interaction terms (treatment arm × severity of social disability, or treatment arm × mental state risk) in turn to the linear models for the primary outcome measure (structured hours at 15 months), there is no evidence to suggest a difference between ESC-alone and ESC plus SRT arms, using either interaction term.
For the remainder of the time use outcome measures, when adding the interaction term (treatment arm × ARMS status), there is no evidence to suggest a difference between ESC alone and ESC plus SRT, for any of the time use variables. When adding the interaction term treatment arm × severity of social disability, Table 54 shows the relevant results (p < 0.1).
For the ITT population, there is moderate evidence at 24 months of a difference in structured activity hours and constructive activity hours (p = 0.032 and p = 0.048, respectively), both in favour of the ESC-alone arm. It is estimated that, at 24 months, hours of average structured acitivity is 36.3% higher in the ESC-alone arm than in the ESC plus SRT group, and hours of constructive activity is 37.7% higher in the ESC-alone group. These are slightly larger differences in averages than if not including this interaction term within the linear model.
For the PP population, there is moderate (p = 0.021) and weak (p = 0.051) evidence of a difference in structured and constructive activity hours, respectively, at 24 months in favour of the ESC-alone arm. It is estimated that, at 24 months, hours of structured activity is, on average, 44.8% higher in the ESC-alone group than in the ESC plus SRT group, and hours of constructive activity is, on average, 41.9% higher in the ESC-alone group. As with the ITT population, these average differences are slightly higher than if not including this interaction term within the model. There is also weak evidence (p = 0.070) of a difference between treatment groups of structured activity hours not including child care at 24 months, favouring the ESC-alone arm, with an average of 33.6% more hours. This outcome measure was not found to be significant when not using an interaction term within the model. It is to be noted that the CIs for these adjusted differences (for ITT and PP) include or are very close to zero, which may affect the significance of these results.
| Population | Time point (months) | Outcome measure | Log-transformed | |
|---|---|---|---|---|
| Adjusted difference (95% CI) | p-value | |||
| ITT | 24 | Structured activity hours per week | –0.31 (–0.60 to –0.03) | 0.032 |
| ITT | 24 | Constructive economic activity hours per week | –0.32 (–0.64 to –0.00) | 0.048 |
| PP | 24 | Structured activity hours per week | –0.37 (–0.69 to –0.06) | 0.021 |
| PP | 24 | Constructive economic activity hours per week | –0.35 (–0.70 to 0.00) | 0.051 |
| PP | 24 | Structured activity hours per week (minus child care) | –0.29 (–0.60 to 0.02) | 0.070 |
Analysis of missing data for primary outcome
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 21.4 (16.6) | 22.3 (19.3) | 0.89 (–3.09 to 4.88) | 0.66 | –0.07 (–0.27 to 0.13) | 0.50 |
| Missing, n | 12 | 17 | ||||
| Constructive economic activity (hours per week) | 15.7 (14.3) | 16.6 (15.9) | 1.13 (–2.35 to 4.60) | 0.53 | 0.01 (–0.22 to 0.23) | 0.94 |
| Missing, n | 12 | 17 | ||||
| Structured activity (minus child care; hours per week) | 20.3 (14.7) | 22.2 (19.3) | 1.69 (–2.15 to 5.53) | 0.39 | –0.06 (–0.26 to 0.14) | 0.56 |
| Missing, n | 12 | 17 | ||||
| Total hours paid employmentd | 156.9 (432.3) | 283.9 (511.4) | 104.22 (–22.94 to 231.37) | 0.108 | 0.64 (–0.13 to 1.40) | 0.103 |
| Missing, n | 25 | 48 | ||||
| Total hours educationd | 130.1 (353.3) | 108.0 (235.0) | –23.32 (–110.22 to 63.59) | 0.60 | –0.17 (–0.88 to 0.53) | 0.63 |
| Missing, n | 26 | 50 | ||||
| Total hours voluntary employmentd | 50.5 (215.4) | 50.7 (170.9) | 3.95 (–50.52 to 58.41) | 0.89 | 0.11 (–0.46 to 0.68) | 0.702 |
| Missing, n | 33 | 47 | ||||
| Total hours all activityd | 349.1 (591.2) | 454.2 (592.9) | 87.39 (–76.36 to 251.14) | 0.30 | 0.42 (–0.34 to 1.18) | 0.28 |
| Missing, n | 35 | 56 | ||||
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 22.4 (21.4) | 27.7 (26.5) | 4.38 (–1.18 to 9.94) | 0.12 | 0.12 (–0.10 to 0.33) | 0.28 |
| Missing, n | 11 | 24 | ||||
| Constructive economic activity (hours per week) | 17.4 (19.9) | 22.0 (24.5) | 4.36 (–0.90 to 9.62) | 0.104 | 0.21 (–0.03 to 0.45) | 0.080 |
| Missing, n | 11 | 24 | ||||
| Structured activity (minus child care; hours per week) | 21.1 (18.1) | 24.9 (20.4) | 2.94 (–1.42 to 7.30) | 0.19 | 0.08 (–0.13 to 0.29) | 0.45 |
| Missing, n | 11 | 24 | ||||
| Total hours paid employmentd | 75.3 (213.8) | 123.6 (275.2) | 46.38 (–15.98 to 108.73) | 0.15 | 0.30 (–0.30 to 0.89) | 0.33 |
| Missing, n | 21 | 31 | ||||
| Total hours educationd | 76.6 (174.1) | 88.8 (176.3) | 12.20 (–33.39 to 57.79) | 0.60 | 0.26 (–0.36 to 0.87) | 0.42 |
| Missing, n | 25 | 31 | ||||
| Total hours voluntary employmentd | 16.5 (46.8) | 36.8 (96.3) | 19.05 (–0.47 to 38.57) | 0.056 | 0.25 (–0.23 to 0.73) | 0.308 |
| Missing, n | 27 | 28 | ||||
| Total hours all activityd | 158.8 (252.0) | 258.8 (359.8) | 92.20 (172.34 to –12.05) | 0.024 | 0.46 (–0.19 to 1.11) | 0.17 |
| Missing, n | 34 | 35 | ||||
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 24.3 (18.9) | 32.4 (28.7) | 7.40 (1.25 to 13.56) | 0.018 | 0.20 (–0.03 to 0.43) | 0.092 |
| Missing, n | 25 | 40 | ||||
| Constructive economic activity (hours per week) | 18.6 (16.7) | 27.4 (28.0) | 8.22 (2.38 to 14.06) | 0.006 | 0.28 (0.02 to 0.54) | 0.034 |
| Missing, n | 25 | 40 | ||||
| Structured activity minus child care (hours per week) | 23.8 (18.9) | 26.6 (20.4) | 2.34 (–2.68 to 7.35) | 0.36 | 0.08 (–0.15 to 0.32) | 0.48 |
| Missing, n | 25 | 40 | ||||
| Total hours paid employmentd | 79.1 (218.8) | 121.7 (277.7) | 41.04 (–23.95 to 106.04) | 0.22 | 0.25 (–0.37 to 0.86) | 0.43 |
| Missing, n | 27 | 37 | ||||
| Total hours educationd | 76.7 (175.3) | 93.6 (182.4) | 16.72 (–31.33 to 64.77) | 0.50 | 0.27 (–0.37 to 0.91) | 0.42 |
| Missing, n | 32 | 39 | ||||
| Total hours voluntary employmentd | 14.3 (37.7) | 38.3 (99.7) | 23.89 (3.89 to 43.88) | 0.019 | 0.31 (–0.18 to 0.80) | 0.27 |
| Missing, n | 34 | 36 | ||||
| Total hours all activityd | 161.0 (255.0) | 258.9 (364.5) | 92.09 (8.90 to 175.28) | 0.030 | 0.45 (–0.22 to 1.12) | 0.19 |
| Missing, n | 39 | 40 | ||||
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 21.4 (16.6) | 22.3 (19.3) | –1.05 (–5.14 to 3.04) | 0.61 | 0.08 (–0.12 to 0.28) | 0.45 |
| Missing, n | 12 | 17 | ||||
| Constructive economic activity (hours per week) | 15.7 (14.3) | 16.6 (15.9) | –1.13 (–4.71 to 2.45) | 0.54 | –0.02 (–0.25 to 0.22) | 0.90 |
| Missing, n | 12 | 17 | ||||
| Structured activity (minus child care; hours per week) | 20.3 (14.7) | 22.2 (19.3) | –1.68 (–5.58 to 2.22) | 0.40 | 0.03 (–0.20 to 0.27) | 0.78 |
| Missing, n | 12 | 17 | ||||
| Total hours paid employmentd | 156.9 (432.3) | 283.9 (511.4) | –112.32 (–240.74 to 16.11) | 0.086 | –0.63 (–1.40 to 0.13) | 0.10 |
| Missing, n | 25 | 48 | ||||
| Total hours educationd | 130.1 (353.3) | 108.0 (235.0) | 17.75 (–77.29 to 112.78) | 0.71 | 0.13 (–0.60 to 0.87) | 0.72 |
| Missing, n | 26 | 50 | ||||
| Total hours voluntary employmentd | 50.5 (215.4) | 50.7 (170.9) | –7.00 (–65.89 to 51.88) | 0.82 | –0.17 (–0.77 to 0.42) | 0.57 |
| Missing, n | 33 | 47 | ||||
| Total hours all activityd | 349.1 (591.2) | 454.2 (592.9) | –102.59 (–274.95 to 69.77) | 0.24 | –0.45 (–1.26 to 0.36) | 0.28 |
| Missing, n | 35 | 56 | ||||
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 22.4 (21.4) | 27.7 (26.5) | –4.45 (–10.05 to 1.14) | 0.12 | –0.13 (–0.35 to 0.09) | 0.24 |
| Missing, n | 11 | 24 | ||||
| Constructive activity (hours per week) | 17.4 (19.9) | 22.0 (24.5) | –4.68 (–10.10 to 0.74) | 0.091 | –0.23 (–0.47 to 0.01) | 0.06 |
| Missing, n | 11 | 24 | ||||
| Structured activity (minus child care; hours per week) | 21.1 (18.1) | 24.9 (20.4) | –3.26 (–7.73 to 1.20) | 0.15 | –0.09 (–0.31 to 0.12) | 0.41 |
| Missing, n | 11 | 24 | ||||
| Total hours paid employmentd | 75.3 (213.8) | 123.6 (275.2) | –45.73 (–108.62 to 17.16) | 0.15 | –0.31 (–0.94 to 0.32) | 0.33 |
| Missing, n | 21 | 31 | ||||
| Total hours educationd | 76.6 (174.1) | 88.8 (176.3) | –9.13 (–55.47 to 37.22) | 0.70 | –0.27 (–0.91 to 0.37) | 0.41 |
| Missing, n | 25 | 31 | ||||
| Total hours voluntary employmentd | 16.5 (46.8) | 36.8 (96.3) | –20.53 (–40.54 to –0.53) | 0.044 | –0.24 (–0.73 to 0.25) | 0.34 |
| Missing, n | 27 | 28 | ||||
| Total hours all activityd | 158.8 (252.0) | 258.8 (359.8) | –98.43 (–182.08 to –14.79) | 0.021 | –0.46 (–1.18 to 0.26) | 0.21 |
| Missing, n | 34 | 35 | ||||
| Time use outcome | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132) | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Structured activity (hours per week) | 24.3 (18.9) | 32.4 (28.7) | –7.54 (–13.83 to –1.25) | 0.019 | –0.21 (–0.46 to 0.04) | 0.101 |
| Missing, n | 25 | 40 | ||||
| Constructive activity (hours per week) | 18.6 (16.7) | 27.4 (28.0) | –8.40 (–14.44 to –2.37) | 0.006 | –0.29 (–0.56 to –0.02) | 0.038 |
| Missing, n | 25 | 40 | ||||
| Structured activity (minus child care; hours per week) | 23.8 (18.9) | 26.6 (20.4) | –2.52 (–7.85 to 2.80) | 0.35 | –0.08 (–0.33 to 0.17) | 0.53 |
| Missing, n | 25 | 40 | ||||
| Total hours paid employmentd | 79.1 (218.8) | 121.7 (277.7) | –44.60 (–116.95 to 27.75) | 0.23 | –0.25 (–0.89 to 0.38) | 0.44 |
| Missing, n | 27 | 37 | ||||
| Total hours educationd | 76.7 (175.3) | 93.6 (182.4) | –17.86 (–67.12 to 31.40) | 0.48 | –0.26 (–0.92 to 0.40) | 0.44 |
| Missing, n | 32 | 39 | ||||
| Total hours voluntary employmentd | 14.3 (37.7) | 38.3 (99.7) | –24.08 (–44.50 to –3.66) | 0.021 | –0.30 (–0.86 to 0.26) | 0.29 |
| Missing, n | 34 | 36 | ||||
| Total hours all activityd | 161.0 (255.0) | 258.9 (364.5) | –100.51 (–188.96 to –12.07) | 0.026 | –0.46 (–1.18 to 0.27) | 0.22 |
| Missing, n | 39 | 40 | ||||
Analysis of missing data: comparison of methods
The proportion of CAARMS data missing at 24 months was high. For example, CAARMS average distress was missing for 64.4% of those in the ESC-alone arm and for 60.0% of those in the SRT plus ESC arm; similarly, aggression severity score was missing for 62.9% of participants in the ESC-alone arm and for 55.8% of those in the SRT plus ESC arm.
Owing to this high proportion of missing data, a missing data analysis was undertaken for these outcome measures using two different analysis methods: MI and FIML. The results are very similar to the outcomes presented in Table 55, with the CAARMS average distress score at 24 months having moderate evidence (p = 0.020) of a difference. On average, the SRT plus ESC arm scored 11.36 higher than the ESC-alone arm. There was also weak evidence of a difference in the CAARMS aggression severity score at 24 months (p = 0.059), with the SRT plus ESC arm scoring, on average, 1.80 higher than the ESC-alone arm. A comparison of methods is presented in Table 62 and shows no evidence of a difference between missing data analysis approaches.
| CAARMS score | Allocation arm, mean (SD) | Untransformed | Log-transformed | |||
|---|---|---|---|---|---|---|
| SRT + ESC (N = 138) | ESC alone (N = 132 | Adjusted differencea (95% CIb) | p-valuec | Adjusted differencea (95% CIb) | p-valuec | |
| Symptom severity score | 20.4 (21.3) | 20.2 (19.4) | 1.45 (–5.54 to 8.44) | 0.68 | ||
| Missing, n | 79 | 86 | ||||
| Average distress score | 42.8 (26.6) | 31.0 (26.5) | 11.64 (1.29 to 22.00) | 0.028 | ||
| Missing, n | 80 | 85 | ||||
| Aggression severity score | 5.0 (5.5) | 3.5 (5.2) | 1.86 (–0.16 to 3.88) | 0.071 | ||
| Missing, n | 77 | 83 | ||||
| Suicidality severity score | 4.3 (6.6) | 3.8 (5.9) | 0.74 (–1.46 to 2.95) | 0.505 | 0.11 (–0.29 to 0.52) | 0.58 |
| Missing, n | 77 | 82 | ||||
| Missing data method | CAARMS severity secondary outcome measure (at 24 months) | Adjusted differencea (95% CIb) | p-valuec |
|---|---|---|---|
| FIML | Average distress score | 11.36d (1.77 to 20.96) | 0.020 |
| MI | Average distress score | 11.83e (1.50 to 22.16) | 0.025 |
| FIML | Aggression severity score | 1.80d (–0.07 to 3.67) | 0.059 |
| MI | Aggression severity score | 1.66e (–0.26 to 3.59) | 0.090 |
Appendix 4 Adverse event definitions
| Event | Definition |
|---|---|
| AE |
Any untoward medical occurrence in a patient or clinical trial participant that does not necessarily have a causal relationship with this product AEs include an exacerbation of a pre-existing illness, an increase in the frequency or intensity of a pre-existing episodic event or condition, a condition (regardless of whether or not present prior to the start of the trial) that is detected after trial intervention administration (this does not include pre-existing conditions recorded as such at baseline), and continuous persistent disease or a symptom present at baseline that worsens following administration of the trial treatment AEs do not include medical or surgical procedures, pre-existing disease or a condition present before treatment that does not worsen, hospitalisation where no untoward or unintended response has occurred (e.g. elective cosmetic surgery), and overdose of medication without signs or symptoms |
| Adverse reaction | Any untoward and unintended response to an investigational medicinal product related to any dose administered |
| Unexpected adverse reaction | An adverse reaction, the nature or severity of which is not consistent with the applicable product information (e.g. investigator’s brochure for an unauthorised product or summary of product characteristics for an authorised product) |
| SAE or serious adverse reaction | Any AE or adverse reaction that at any dose: |
Glossary
- Acceptance and Avoidance Questionnaire II
- A brief self-report measure of the presence or absence of experiential avoidance/psychological inflexibility (unwillingness to experience one’s own negative thoughts or emotions).
- Alcohol Use Disorders Identification Test
- A brief self-report measure capturing the presence or absence of levels of harmful alcohol use.
- Assertive outreach
- A model of care for people with complex needs that emphasises flexible engagement and visiting people in community settings.
- At-risk mental states
- A state or phase in which a person is considered to have an elevated risk of developing psychosis. At-risk mental states include attenuated symptoms of psychosis and may include changes in mood, cognition, thought content and behaviours.
- Attenuated symptoms of psychosis
- Experiences such as mild confusion in thinking, suspiciousness, odd beliefs and perceptual distortions that are not quite of psychotic intensity or frequency.
- Beck Depression Inventory-II
- A brief self-report measure capturing the presence or absence of symptoms associated with depression.
- Beck Hopelessness Scale
- A brief self-report measure capturing the presence or absence of hopelessness.
- Brief Core Schema Scale
- A brief self-report measure capturing the presence or absence of positive and negative evaluations of oneself and other people.
- Child and Adolescent Mental Health Services/Children and Young People’s Services
- NHS mental health services for children and young people, generally provided until the age at which compulsory education would cease.
- Cognitive Therapy Rating Scale Revised
- A brief measure focusing on competent use of cognitive–behavioural therapy.
- Comprehensive Assessment of At-Risk Mental States
- A structured mental state interview conducted by a trained assessor that is used to assess attenuated psychotic symptoms and associated psychopathology, drug use, and risk to self and others.
- Constructive economic activity
- Scored from the Time Use Survey; a measure of hours spent in paid or voluntary work, education, child or other caring activities, and household chores.
- Controlled Oral Word Association Test
- A brief neuropsychological assessment conducted by a trained assessor in which people verbally generate words beginning with a given letter in 60-second trials.
- Drug Use Disorders Identification Test
- A brief self-report measure capturing the presence or absence of levels of harmful drug use.
- Early Intervention in Psychosis
- A model of care provision for young people (typically aged ≥ 14 years, although this is variable nationally) during and for 2–4 years after the first episode of psychosis. The model of care involves care co-ordination and medical, psychological and psychosocial intervention.
- EuroQol-5 Dimensions
- A brief generic self-report measure of quality of life.
- Global Assessment of Functioning
- A 0–100 scale rated by a trained assessor that captures the presence or absence of severe symptoms of at least two out of depression, anxiety, substance misuse, behavioural or thinking problems, or subthreshold psychosis to the degree that they impair function.
- Health Service Resource Use Questionnaire
- A brief self-report measure capturing use of physical health and mental health support services modified from the Client Service Receipt Inventory.
- Logical Memory I
- A brief neuropsychological assessment conducted by a trained assessor in which people verbally recall a short story immediately after its auditory presentation by the assessor.
- Meaning in Life Questionnaire
- Brief self-report measure assessing the perception of searching for and of experiencing meaning and purpose within one’s life.
- Multisystemic
- A model of care that focuses on working with the systems around a young person, including family, peers, school and community.
- National Pupil Database
- Contains detailed information about pupils in schools and colleges in England.
- Scale for Assessment of Negative Symptoms
- A scale scored by a trained assessor evaluating the presence or absence of symptom domains including affective blunting, apathy, impoverished thinking, asociality and disturbance of attention.
- Schizotypal Symptoms Inventory
- A brief self-report measure capturing the presence or absence of unusual and anomalous experiences, including paranoia.
- Social Interaction Anxiety Scale
- A brief self-report measure capturing the presence or absence of social anxiety.
- Structured Activity
- Scored from the Time Use Survey; a measure of hours spent in constructive economic activity plus structured leisure and sports activities.
- Structured Clinical Interview
- A structured clinical interview conducted by a trained assessor designed to categorise symptoms and experiences according to the major diagnoses from the Diagnostic and Statistical Manual of Mental Disorders.
- Time Use Survey
- Derived from the Office for National Statistics’ Time Use Survey, this is an established measure with good psychometric properties that assesses hours per week engaged in constructive economic and structured activity. Data are captured within a semistructured interview conducted by a trained assessor and scored in the metric of hours of activity.
- Trait Hope Scale
- A brief self-report measure capturing the presence or absence of the general trait hopefulness. This measure is presented as ‘The Future Scale’ to participants.
List of abbreviations
- AE
- adverse event
- APS
- attenuated psychotic symptoms
- ARMS
- at-risk mental states for psychosis
- AUDIT
- Alcohol Use Disorders Identification Test
- BHS
- Beck Hopelessness Scale
- CAARMS
- Comprehensive Assessment of At-Risk Mental States for psychosis
- CAMHS
- Child and Adolescent Mental Health Services
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COWAT
- Controlled Oral Word Association Test
- CSRI
- Client Services Receipt Inventory
- CTRS-R
- Cognitive Therapy Rating Scale Revised
- DMEC
- Data Monitoring and Ethics Committee
- DUDIT
- Drug Use Disorders Identification Test
- EDIE-2
- Early Detection and Intervention Evaluation for people at high-risk of psychosis 2 trial
- EIP
- Early Intervention in Psychosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ESC
- enhanced standard care
- FEP
- first-episode psychosis
- FIML
- full information maximum likelihood
- GAF
- Global Assessment of Functioning
- GAS
- Global Assessment of Symptoms
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- MI
- multiple imputation
- NCTU
- Norwich Clinical Trials Unit
- NEET
- not in education, employment or training
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PAT
- PRODIGY Advisory Team
- PIS
- participant information sheet
- PP
- per protocol
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RA
- research assistant
- REC
- Research Ethics Committee
- SA1
- sensitivity analysis 1
- SA2
- sensitivity analysis 2
- SAE
- serious adverse event
- SANS
- Scale for the Assessment of Negative Symptoms
- SD
- standard deviation
- SIAS
- Social Interaction Anxiety Scale
- SOFAS
- Social and Occupational Functioning Assessment Scale
- SRT
- social recovery therapy
- SSI
- Schizotypal Symptoms Inventory
- TDMS
- trial data management system
- TSC
- Trial Steering Committee
- TUS
- Time Use Survey
- UHR
- ultra-high risk
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25700).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
