Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/71/04. The contractual start date was in February 2019. The draft report began editorial review in December 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Bedford et al. This work was produced by Bedford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Bedford et al.
Chapter 1 Background
Description of the health problem
New-onset atrial fibrillation (NOAF) is defined as atrial fibrillation (AF) that occurs in a patient with no known history of chronic or paroxysmal AF. 1 It is a common arrhythmia in critically ill patients. 2 It occurs in 5–15% of all patients admitted to a general intensive care unit (ICU),3,4 rising to 23% of patients with septic shock. 5
Organised atrial activity is important for ventricular filling and cardiac output. 6 NOAF is temporally associated with a reduction in cardiac output in non-ICU patients. 7 The haemodynamic impact of NOAF in critically ill patients is poorly understood, but limited data suggest that NOAF may precede haemodynamic instability8 and may be associated with increased rates of thromboembolism. 9 NOAF during critical illness is associated with an increased risk of death in an ICU and in hospital. 10,11 There is also a significant organisational impact of NOAF because it is associated with an increased length of ICU and hospital stay, and higher health-care costs. 12
New-onset atrial fibrillation during critical illness may carry a long-term burden. Patients who develop NOAF during sepsis and survive to hospital discharge have an increased risk of heart failure and stroke, and poorer 1-year and 5-year survival. 13,14 The long-term outcomes for patients who develop NOAF in an ICU remains unclear.
It is not known whether NOAF in patients in an ICU is causally related to worse outcomes or whether NOAF may be solely a marker of disease severity. However, there is clear mechanistic plausibility behind a causal association. This demonstrates the need for optimal prevention, management and follow-up. Although a recent scoping review has broadly described studies of NOAF treatment in patients in an emergency department or an ICU, or after major surgery,10 an in-depth review of NOAF in patients in an ICU focusing on treatment efficacy is required to put current treatment practices into context and to inform future comparative studies.
Clear guidelines exist for the management of AF in patients in the community. 15 However, there is a paucity of evidence for its management in the critical care setting, for which the balance of risks and benefits associated with different treatment options is unclear. Understandably, there is significant variation within and between units in the management of this common problem. 16 Many previous studies informing NOAF treatment in ICUs are small or inadequately adjusted for confounding factors. Well-conducted, multicentre, observational studies are required to highlight candidate interventions for clinical trials.
Overall aims and objectives of the study
Scoping review
-
To evaluate the evidence for the clinical effectiveness and safety of pharmacological and non-pharmacological NOAF treatments.
-
To provide guidance for the database analysis on:
-
NOAF definitions used for patients in an ICU
-
patient subgroups who develop NOAF in an ICU
-
inclusion/exclusion of specific treatments and potential confounders
-
-
determining barriers to future research.
Database analysis: RISK-II
-
To determine how common NOAF is in critical care.
-
To determine the typical characteristics of patients with NOAF in critical care and how they compare with other patients in critical care.
-
To increase the understanding of the outcomes of patients with NOAF in critical care and how they compare with other patients in critical care.
-
To investigate how much of the difference in outcomes is explained by differences in patient characteristics and comorbidities.
Database analysis: MIMIC-III and PICRAM
-
To compare the use and clinical effectiveness of pharmacological and non-pharmacological NOAF treatments.
-
To determine the incidence of short- and long-term NOAF complications.
Patient and public involvement
Patient and public involvement was vital throughout the study. Valerie Keston-Hole and Rob Lawrence helped to develop the application, which was also reviewed by the Oxford Critical Care Patient Forum. The group strongly supported the use of existing databases for the purposes of undertaking the work. Valerie Keston-Hole sits on the group, which assesses applications for the use of the Post Intensive Care Risk-adjusted Alerting and Monitoring (PICRAM) data used in this work. Ian and Cathy Taylor provided us with a clear patient perspective when working with the expert panel and also helped us to choose research recommendations. Meetings went well and easy access to the chief investigator meant that things that were unclear could be explained by e-mail afterward and further thoughts considered. In discussion with Ian and Cathy Taylor, we identified that an area that we would improve in the future was how to present large numbers of initial data in a more comprehensible manner to our patient and public involvement (PPI) colleagues. A suggestion for the future would be to provide supplementary information that avoided technical terminology to help the understanding of the data by our PPI colleagues prior to the meetings. This would allow more spontaneous comments and discussion during the meeting. Our PPI work is not complete. We discussed our findings at the ICU patient forum. This helped us to understand how to clearly communicate our findings.
Chapter 2 Scoping review of treatments for new-onset atrial fibrillation
Parts of this chapter are adapted with permission from Drikite et al. 17 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Scoping review methods
The scoping review followed the methodological framework described by Arksey and O’Malley,18 Levac et al. 19 and Daudt et al. ,20 and the reporting complies with the recently published Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) reporting guidelines. 21
Literature searches
The search strategy was developed by an information specialist in MEDLINE (via Ovid®; Wolters Kluwer, Alphen aan den Rijn, the Netherlands) without any date or language restrictions. The search strategy included terms used to describe NOAF combined with a set of terms used for critical care.
An adapted MEDLINE search strategy was used to search the following databases in March 2019: MEDLINE, EMBASE™ (Elsevier, Amsterdam, the Netherlands), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science™ [Clarivate Analytics, Philadelphia, PA, USA; including Conference Proceedings Citation Index – Science (Clarivate Analytics)], OpenGrey, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effects (DARE; searched from 1994 to 2015). We were not able to search the National Guideline Clearinghouse, as suggested in our protocol,22 because this database was no longer available. The following clinical trial databases were searched for studies in progress or completed but not reported: International Standard Randomised Controlled Trial Number (ISRCTN), ClinicalTrials.gov, the EU Clinical Trials register, additional World Health Organization International Clinical Trials Registry Platform (ICTRP) trial databases and the National Institute for Health Research Clinical Trials Gateway.
The search results were imported into EPPI-Reviewer 4 software (Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, London, UK) and duplicates were removed. The search strategies can be found in Appendix 1. The reference lists of included review articles and studies were also reviewed to identify any relevant studies.
Eligibility criteria
The eligibility criteria used to screen titles, abstracts and full-text articles were as follows.
-
Population:
-
Studies of adults (age ≥ 16 years) with NOAF (or without any history of AF, for prevention/prophylactic studies) admitted to general medical, surgical or mixed ICUs were included.
-
Studies of cohorts defined by a single disease or narrow disease group not normally admitted to a general ICU (e.g. myocardial infarction) and studies based on service-specific ICUs (e.g. cardiothoracic or neurosurgical) were excluded.
-
Studies in which a majority (> 50%) of patients belonged to a single, specific disease or operative cohort (e.g. liver resections or lung surgery) and cohorts with a known history of chronic or paroxysmal AF were excluded.
-
Studies of disease groups commonly admitted to an ICU, such as sepsis and septic shock, were included.
-
Studies of patients with supraventricular arrhythmias if AF constituted at least 70% of arrhythmias were included. Where these data were unavailable, we included studies that grouped AF and atrial flutter together if no other arrhythmia types were included.
-
Studies reporting on populations that were a mixture of NOAF and known AF were included only if data for the NOAF subgroup were reported separately.
-
Studies that included both ICU and non-ICU patients, but which did not present results separately, were included only if > 50% of the total cohort were ICU patients and if a valid method for confounding adjustment was used with ICU status included as a covariate.
-
-
Intervention:
-
Studies investigating pharmacological, electrical and other non-pharmacological (including electrolyte) treatment strategies for treatment or prevention of NOAF were included.
-
Studies of short- or long-term anticoagulation were included.
-
Studies of ablation or surgical interventions were excluded.
-
-
Comparators:
-
Any eligible intervention could be a comparator, including no treatment or ‘standard care’.
-
Placebo was also eligible.
-
-
Outcomes – any of the following outcomes were eligible:
-
rhythm and rate control
-
length of ICU and hospital stay
-
mortality (ICU, hospital, 30 days and long term)
-
arterial thromboembolism and adverse treatment effects
-
in the case of studies of preventative/prophylactic treatments, the incidence of NOAF had to be reported.
-
-
Study design – we included quantitative studies with the following designs:
-
randomised and non-randomised trials
-
cohort studies and case series containing five or more patients
-
practitioner surveys and opinion pieces (for research recommendations and interventions not otherwise identified) were also included.
-
Two reviewers independently screened titles and abstracts and potentially relevant full-text articles. Any discrepancies between the reviewers were resolved either through discussion or by a third reviewer if necessary. Titles, abstracts and full-text articles were screened using EPPI-Reviewer 4 software. The screening of titles and abstracts was facilitated by use of the highlighting function in EPPI-Reviewer 4 (which highlights keywords associated with inclusion or exclusion criteria). This function allowed more prompt decisions to be made. After screening the titles and abstracts, all potentially relevant full-text articles were uploaded on Mendeley Reference Manager Software (1.19.5; Elsevier, Amsterdam, the Netherlands) for easy access and sharing purposes.
Full-text articles that were not published in English included papers in French, German, Czech, Chinese and Spanish. These were screened by native speakers. None of the foreign language articles was eligible for the review.
Data charting
Data-charting forms were developed for the following study designs:
-
randomised controlled trials (RCTs)
-
prospective comparative studies (non-RCTs)
-
retrospective comparative studies
-
single-group studies.
The extracted data included the following:
-
details of the study (authors, country, setting, sample size and proportion of NOAF patients included in the study)
-
population characteristics (primary diagnosis; mean age; proportion of males; severity of illness; proportion of patients on vasopressors; proportion of patients with cardiovascular disease, acute renal failure, acute respiratory failure and mechanical ventilation; mean serum potassium levels; and authors’ definition of NOAF)
-
description of intervention and comparator(s)
-
methods to address confounding (for non-randomised studies)
-
results
-
any relevant recommendations for the future research.
The data-charting forms were piloted on a small number of studies and were adapted accordingly where necessary. Decisions about which population characteristics to extract were informed by a recent systematic review on risk factors for NOAF on the ICU23 and a retrospective observational study on predictors for sustained NOAF in the critically ill. 24 All data were extracted by one reviewer and checked by another member of the team; any disagreements would be referred to a third member of the team.
Critical appraisal
Randomised trials were evaluated using version 2 of the Cochrane risk-of-bias tool (see Appendix 2). 25 Non-randomised comparative studies that fulfilled the following criteria were evaluated for risk of bias using the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool:26
-
reported as full papers
-
included at least 100 patients per treatment arm
-
reported on methods to adjust for confounding.
The ROBINS-I tool was adapted for use in this scoping review by including a stopping rule: the risk-of-bias assessment stopped if a serious or critical risk-of-bias judgement was made for the ‘bias due to confounding’ domain. For the confounding domain, decisions regarding which covariates should be reported as being controlled for in analyses were made by the clinical experts in the CAFE (Critical care Atrial Fibrillation Evaluation) study team, with supporting references where possible, and are reported in Table 1, along with the risk-of-bias judgements.
| Studya | Confounding domain | Other domains | |||
|---|---|---|---|---|---|
| Outcome | Results | Review prespecified covariates to be controlled or adjusted for | Risk-of-bias judgement | ||
| Launey 201927 | Incidence of NOAF | RD 11.9% (95% CI –23.4% to –0.5%) | Age, sex, preceding cardiovascular disease, acute renal failure, acute respiratory failure, APACHE score and the use of vasopressors23 | Serious:
|
NAc |
| RR 0.58 (95% CI 0.35 to 0.98) | |||||
| Walkey 201628 | Mortalityb | RR 0.99 (95% CI 0.86 to 1.15) | Sickness score (e.g. SOFA) or individual components of score | Serious:
|
NAc |
| RR 0.75 (95% CI 0.64 to 0.88) | |||||
| RR 0.67 (95% CI 0.59 to 0.77) | |||||
| Walkey 201629 | Stroke and bleeding | Stroke: RR 0.85 (95% CI 0.57 to 1.27) | Stroke: age, sex, heart failure, hypertension, diabetes, carotid artery disease, hypercholesterolaemia | Serious for both outcomes:
|
NAc |
| Bleeding: RR 0.97 (95% CI 0.83 to 1.14) | Bleeding: ± illness severity, systemic inflammation, type and location of surgery, nutritional status, invasive devices, and acute coagulopathy and thrombocytopenia | ||||
Collating and summarising the results
The details of the primary studies were presented in structured tables categorised by study design. For each type of study design, the extent, range and nature of the identified research were described. Study parameters and results were then described and summarised narratively.
Expert panel review
We convened a face-to-face meeting of expert panel members to review our scoping review results and to inform our subsequent database analysis. We created a list of variables identified from our scoping review that may affect NOAF treatment choice. We then circulated this list among our expert panel where it was independently added to and refined. We collated a final list of these confounding variables, which was then ratified by our expert panel. We repeated this process with definitions of NOAF, interventions of interest and outcomes of interest.
Scoping review results
Quantity and quality of the research available
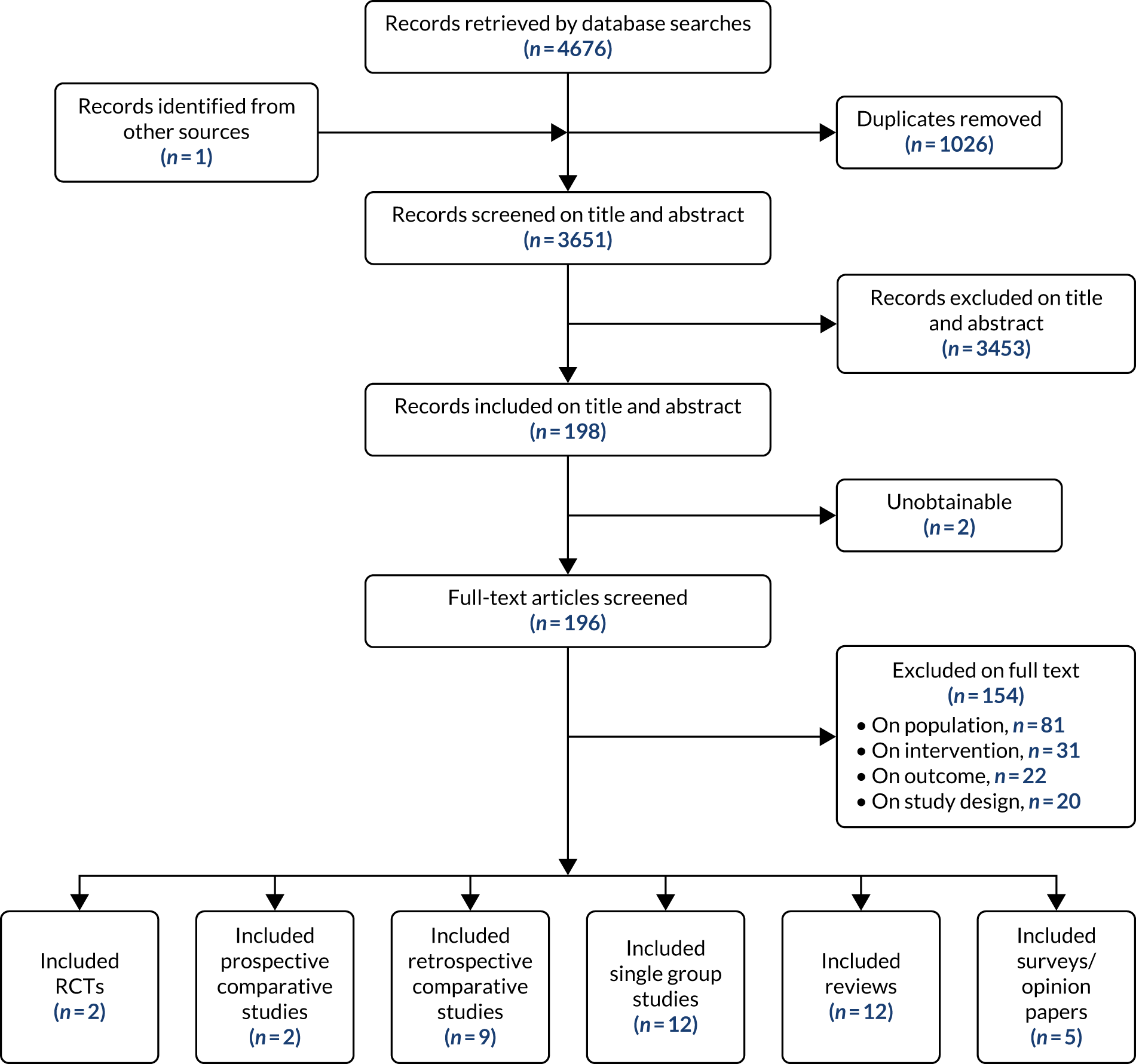
Following the removal of duplicates from the articles retrieved by database searches, 3651 articles were screened on their title and abstract. From those screened, 198 articles were identified as of potential interest and were screened on their full text. Two articles were unobtainable: a conference abstract published in 2000 and an old study from 1974 looking at amiodarone as a treatment of supraventricular tachyarrhythmias in critically ill patients. Therefore, copies of the 196 full-text articles were assessed for inclusion in the scoping review and 42 articles were included in the review. One eligible article was identified from checking the reference lists of included review articles. Figure 1 illustrates the flow of the articles throughout the review process and the number of included articles classified by study design. Studies excluded after full-text review are listed in Appendix 4, Table 19.
FIGURE 1.
Flow chart showing the number of studies identified, excluded and eligible for inclusion in the scoping review.
Risk-of-bias assessments
Randomised controlled trials
Two RCTs were included. It was judged that the Balser et al. 30 trial gave rise to some concerns about possible bias, primarily owing to the lack of reporting of randomisation methods and the lack of blinding. The Delle Karth et al. 31 trial was judged to have a high risk of bias based on the lack of reporting of randomisation methods, coupled with baseline differences in sex and age. Moreover, the trial was not blinded with respect to investigators and caregivers.
Non-randomised comparative studies
Three non-randomised studies27–29 fulfilled the criteria (see Critical appraisal) to be evaluated using the ROBINS-I risk-of-bias tool.
All three of the large, non-randomised studies were judged to have a serious risk of bias owing to confounding. This was a result of either missing covariates in the propensity score matching or the risk of residual confounding as a result of the measurement of the covariates. The two studies by Walkey et al. 28,29 stated that some key data were recorded on admission, but that these studies used enhanced administrative data that lacked the detailed sequence of events. Some data relating to the admission time point may not be representative of the time point at which a treatment decision was made. The authors noted other limitations of these two studies, adding that the findings should be ‘considered hypothesis-generating and supportive of the need for future clinical trials to investigate optimal treatment of AF during sepsis’. 28
Primary studies of clinical effectiveness and safety
Table 2 presents an overview of the primary study evidence identified in the review. Further details are reported in the following sections, according to study design.
| Intervention | Study details | ||||
|---|---|---|---|---|---|
| RCT | Prospective comparative study | Retrospective comparative study | Prospective single-group study | Retrospective single-group study | |
| Pharmacological treatments | |||||
| Amiodarone | Delle Karth 2001,31 n = 60 | Gerlach 2008,32 n = 61 |
Walkey 2016,28 n = 3174 Cho 2017,33 n = 448 Matsumoto 2015,34 n = 276 Balik 2017,35 n = 234 Mieure 2011,36 n = 126 Jaffer 2016,37 n = 65 Brown 2018,38 n = 33 |
Sleeswijk 2008,39 n = 29 Slavik 2003,40 n = not reported |
Liu 2016,41 n = 240 Mitrić 2016,42 n = 177 Kanji 2012,8 n = 139 Mayr 2004,43 n = 131 Burris 2010,44 n = 30 |
| Beta-blockers | Balser 1998,30 n = 55 | No studies |
Walkey 2016,28 n = 3174 Matsumoto 2015,34 n = 276 Balik 2017,35 n = 234 Mieure 2011,36 n = 126 Jaffer 2016,37 n = 65 Brown 2018,38 n = 33 |
Nakamura 2016,45 n = 16 |
Liu 2016,41 n = 240 Kanji 2012,8 n = 139 Burris 2010,44 n = 30 |
| Calcium channel blockers |
Delle Karth 2001,31 n = 60 Balser 1998,30 n = 55 |
Gerlach 2008,32 n = 61 |
Walkey 2016,28 n = 3174 Mieure 2011,36 n = 126 Jaffer 2016,37 n = 65 Brown 2018,38 n = 33 |
No studies |
Liu 2016,41 n = 240 Burris 2010,44 n = 30 |
| Propafenone | No studies | No studies | Balik 2017,35 n = 234 | No studies | No studies |
| Digoxin | No studies | No studies | Walkey 2016,28 n = 3174 | No studies |
Liu 2016,41 n = 240 Burris 2010,44 n = 30 |
| Ibutilide | No studies | No studies | No studies |
Hennersdorf 2002,46 n = 26 Delle Karth 2005,47 n = 17 |
No studies |
| Magnesium sulphate infusion | No studies | No studies | No studies | Sleeswijk 2008,39 n = 29 | No studies |
| Prophylactic treatments | |||||
| Hydrocortisone | No studies | Launey 2019,27 n = 261 | Kane 2014,48 n = 109 | No studies | No studies |
| Electrical treatments | |||||
| Direct-current cardioversion | No studies | No studies | No studies | Mayr 2003,49 n = 37 | No studies |
| Electrical cardioversion | No studies | No studies | No studies | No studies |
Liu 2016,41 n = 240 Kyo 2019,50 n = 85 |
| Anticoagulants | |||||
| Anticoagulants | No studies | No studies | Walkey 2016,29 n = 7522 | Slavik 2003,40 n = not reported | No studies |
Randomised controlled trials
Two small RCTs30,31 were identified as eligible and were included in the review. Both trials investigated pharmacological treatment strategies for rate control in patients with NOAF. Details of the RCTs are presented in Tables 3 and 4. Two further RCTs51,52 that studied supraventricular tachycardias were identified, but these were not eligible because < 70% of their study population were diagnosed with NOAF.
| Study details | Population characteristics | Intervention | Comparator | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
First author and year: Balser 199830 Setting: ICU Country: USA Sample size: n = 55 (esmolol, n = 28; diltiazem, n = 27) NOAF patients: n = 44 (80%) (esmolol, n = 22, 79%; diltiazem, n = 22, 81%) |
Primary diagnosis: non-cardiac surgical patients Primary diagnosisEsmolol (n)Diltiazem (n)GI/GU713Thoracic96Nonthoracic vascular43Neurosurgery23Other42No surgery20 Mean age: esmolol, 66 ± 15 years; diltiazem, 69 ± 11 years Male: esmolol, n = 14 (50%); diltiazem, n = 16 (59%) Severity of illness: APACHE III reported – esmolol, 59 ± 31; diltiazem, 65 ± 24 Patients on vasopressors: esmolol, n = 1 (3.57%); diltiazem, n = 3 (11%) Cardiovascular diseaseEsmolol, n (%)Diltiazem, n (%)Coronary artery disease10 (36)13 (48)Recent MI or ischaemia1 (3.57)2 (7)Left ventricular hypertrophy3 (11)1 (3.7) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: ‘SVT present for as long as 24 hours’ |
Primary diagnosis | Esmolol (n) | Diltiazem (n) | GI/GU | 7 | 13 | Thoracic | 9 | 6 | Nonthoracic vascular | 4 | 3 | Neurosurgery | 2 | 3 | Other | 4 | 2 | No surgery | 2 | 0 | Cardiovascular disease | Esmolol, n (%) | Diltiazem, n (%) | Coronary artery disease | 10 (36) | 13 (48) | Recent MI or ischaemia | 1 (3.57) | 2 (7) | Left ventricular hypertrophy | 3 (11) | 1 (3.7) |
Esmolol: 12.5-mg i.v. bolus, followed by additional 25- to 50-mg boluses every 3–5 minutes until the heart rate was < 110 b.p.m. or a total loading dose of 250 mg was attained. The maintenance infusion was 50 µg/kg/minute for patients receiving > 30 mg. After 15 minutes, patients whose heart rate exceeded 110 b.p.m. received 1–4 boluses of 25 mg, followed by a 50 µg/kg/minute increment in their maintenance infusion. The authors reported that this was repeated after 30 minutes for patients whose heart rate was > 100 b.p.m. Beyond 30 minutes, infusion rates were adjusted by the treating physician to maintain heart rates between 80 and 100 b.p.m. If at any time a patient had symptomatic hypotension or their systolic blood pressure was < 80 mmHg, the infusion rate was decreased by 50% or a phenylephrine infusion was administered, or both Line of NOAF treatment: second line – adenosine given before the study treatment |
Diltiazem: loading infusion of 20 mg over 2 minutes, immediately followed by a 10 mg/hour maintenance infusion. After 15 minutes, patients whose heart rate was > 110 b.p.m. received an additional loading infusion of 25 mg and a 5 mg/hour increment in their maintenance infusion. After 30 minutes, patients receiving a maintenance infusion of < 15 mg/hour with a heart rate of > 100 b.p.m. received an additional 5 mg/hour increment in their infusion rate. Beyond 30 minutes, infusion rates were adjusted by the treating physician to maintain heart rates between 80 and 100 b.p.m. If at any time a patient had symptomatic hypotension or their systolic blood pressure was < 80 mmHg, the infusion rate was decreased by 50% or a phenylephrine infusion was administered, or both Line of NOAF treatment: second line – adenosine given before the study treatment |
| Primary diagnosis | Esmolol (n) | Diltiazem (n) | ||||||||||||||||||||||||||||||||||
| GI/GU | 7 | 13 | ||||||||||||||||||||||||||||||||||
| Thoracic | 9 | 6 | ||||||||||||||||||||||||||||||||||
| Nonthoracic vascular | 4 | 3 | ||||||||||||||||||||||||||||||||||
| Neurosurgery | 2 | 3 | ||||||||||||||||||||||||||||||||||
| Other | 4 | 2 | ||||||||||||||||||||||||||||||||||
| No surgery | 2 | 0 | ||||||||||||||||||||||||||||||||||
| Cardiovascular disease | Esmolol, n (%) | Diltiazem, n (%) | ||||||||||||||||||||||||||||||||||
| Coronary artery disease | 10 (36) | 13 (48) | ||||||||||||||||||||||||||||||||||
| Recent MI or ischaemia | 1 (3.57) | 2 (7) | ||||||||||||||||||||||||||||||||||
| Left ventricular hypertrophy | 3 (11) | 1 (3.7) | ||||||||||||||||||||||||||||||||||
|
First author and year: Delle Karth 200131 Setting: ICU Country: Austria Sample size: n = 60 (diltiazem, n = 20; amiodarone bolus, n = 20; amiodarone bolus + 24 hours, n = 20) NOAF patients: n = 57 (95%) |
Primary diagnosis: Primary diagnosisDiltiazem (n)Amiodarone bolus (n)Amiodaron bolus + 24 hours (n)Congestive heart failure544Coronary artery disease22–Cardiac surgery6914Respiratory failure621Others131 Mean age: diltiazem, 64.8 ± 10 years; amiodarone bolus, 67.8 ± 9 years; amiodarone bolus + 24 hours, 71.2 ± 9 years Male: diltiazem, n = 15 (75%); amiodarone bolus, n = 17 (85%); amiodarone bolus + 24 hours, n = 11 (55%) Severity of illness: APACHE III score reported – diltiazem, 75.1 ± 35; amiodarone bolus, 76.7 ± 38; amiodarone bolus + 24 hours, 59.7 ± 8 Patients on vasopressors at the time of onset: diltiazem, n = 14 (70%); amiodarone bolus, n = 15 (75%); amiodarone bolus + 24 hours, n = 15 (75%) Patients with CVD: diltiazem, n = 13 (65%); amiodarone bolus, n = 15 (75%); amiodarone bolus + 24 hours, n = 18 (90%) Patients with acute renal failure: NR Patients with acute respiratory failure: diltiazem, n = 6 (30%); amiodarone bolus, n = 2 (10%); amiodarone bolus + 24 hours, n = 1 (5%) Mechanical ventilation at NOAF onset: diltiazem, n = 15 (75%); amiodarone bolus, n = 17 (85%); amiodarone bolus + 24 hours, n = 14 (70%) Serum potassium level: NR Definition of NOAF: recent-onset tachycardic AF was defined as ‘atrial fibrillation with a rate consistently > 120 beats/minute over a 30-minute period’ |
Primary diagnosis | Diltiazem (n) | Amiodarone bolus (n) | Amiodaron bolus + 24 hours (n) | Congestive heart failure | 5 | 4 | 4 | Coronary artery disease | 2 | 2 | – | Cardiac surgery | 6 | 9 | 14 | Respiratory failure | 6 | 2 | 1 | Others | 1 | 3 | 1 |
Diltiazem: 25 mg of diltiazem by i.v. bolus infusion over 15 minutes followed by a continuous infusion at a rate of 20 mg/hour for 24 hours Line of NOAF treatment: first line |
Amiodarone bolus: a bolus dose of 300 mg of amiodarone followed by i.v. infusion over 15 minutes Line of NOAF treatment: first line Amiodarone bolus + 24 hours: a dose of 300 mg of amiodarone followed by an i.v. bolus infusion over 15 minutes followed by a continuous infusion at a rate of 45 mg/hour over 24 hours Line of NOAF treatment: first line |
|||||||||
| Primary diagnosis | Diltiazem (n) | Amiodarone bolus (n) | Amiodaron bolus + 24 hours (n) | |||||||||||||||||||||||||||||||||
| Congestive heart failure | 5 | 4 | 4 | |||||||||||||||||||||||||||||||||
| Coronary artery disease | 2 | 2 | – | |||||||||||||||||||||||||||||||||
| Cardiac surgery | 6 | 9 | 14 | |||||||||||||||||||||||||||||||||
| Respiratory failure | 6 | 2 | 1 | |||||||||||||||||||||||||||||||||
| Others | 1 | 3 | 1 |
| Study | Results | Adverse effects | Recommendations for/barriers to future research |
|---|---|---|---|
| Balser 199830 |
|
No adverse effects | Although intuition suggests that ICU patients may benefit from accelerated conversion to sinus rhythm after operation, a much larger trial would be necessary to determine whether early conversion influences outcome |
| Delle Karth 200131 |
|
|
NR |
A RCT (n = 55)30 set in the USA compared esmolol (a beta-blocker) with diltiazem (a calcium channel blocker) in a non-cardiac surgical population. The proportion of patients diagnosed with NOAF was 79% in the esmolol group and 80% in the diltiazem group. Both esmolol and diltiazem were second-line treatments for NOAF because adenosine had been administered before the study treatments. The authors reported that loading and infusion rates were adjusted to achieve a degree of ventricular rate control similar to that achieved with standard dosing regimens used in their surgical ICU. The primary outcome that was reported was the rate of conversion to sinus rhythm. There was no statistically significant difference in conversion rate between the study groups within 2 hours for patients with NOAF: 59% in those who received esmolol and 27% in those who received diltiazem (p = 0.067). By 12 hours, 85% of patients who received esmolol had converted back to sinus rhythm, compared with 62% of patients who received diltiazem (p = 0.116). No adverse events were reported.
Delle Karth et al. 31 conducted a small RCT in Austria comparing diltiazem, an amiodarone (an anti-arrhythmic medication with multiple mechanisms of action) bolus and an amiodarone bolus in combination with 24 hours of infusion in a mixed ICU population. Ninety-five per cent of patients enrolled in the trial (n = 57) were diagnosed with NOAF. The first study group received a dose of 25 mg of diltiazem by an intravenous (i.v.) bolus infusion over 15 minutes, followed by a continuous infusion at a rate of 20 mg/hour for a total of 24 hours. The second study group was given a bolus dose of 300 mg of amiodarone, followed by an i.v. infusion over 15 minutes. The third study group was given a dose of 300 mg of amiodarone followed by an i.v. bolus infusion over 15 minutes, which was then followed by a continuous infusion at a rate of 45 mg/hour over 24 hours. Reported conversion rates within 4 hours were similar in both groups: 30% converted back to sinus rhythm in the diltiazem group, 40% in the amiodarone bolus group and 45% in the amiodarone bolus in combination with 24-hour infusion group. The authors reported a small number of adverse events (see Table 4).
Prospective comparative studies
We identified two prospective comparative studies, of which one32 investigated the effects of pharmacological treatments for NOAF and the other27 looked at prophylactic treatment to prevent NOAF in patients with septic shock. Details are presented in Tables 5 and 6.
| Study details | Population characteristics | Intervention | Comparator | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
First author and year: Gerlach 200832 Setting: surgical ICU Country: USA Sample size: n = 61 NOAF patients: n = 55 (90%) (diltiazem, n = 28; amiodarone, n = 27) |
Primary diagnosis Primary diagnosisDiltiazem (n)Amiodarone (n)Trauma59Gastrointestinal1511Vascular45Other surgery75 Mean age: diltiazem, 68.5 ± 14.6 years; amiodarone, 66.1 ± 16 years Male: diltiazem, n = 18 (58%); amiodarone, n = 21 (70%) Severity of illness: NR Patients on vasopressors at the time of onset: n = 11 (18%) (diltiazem, n = 8, 26%; amiodarone, n = 3, 10%) Patients with CVD: n = 11 (18%) (diltiazem, n = 5, 16%; amiodarone, n = 6, 20%) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: NR |
Primary diagnosis | Diltiazem (n) | Amiodarone (n) | Trauma | 5 | 9 | Gastrointestinal | 15 | 11 | Vascular | 4 | 5 | Other surgery | 7 | 5 |
Intervention treatment given to patients during the first year of the study. Diltiazem: 0.25 mg/kg i.v. bolus, followed by continuous infusion of 5–15 mg/hour titrated to a heart rate of < 120 b.p.m. Decisions to continue, discontinue or change to oral therapy after 48 hours were at the discretion of the managing physicians Line of NOAF treatment: not specifically reported; however, no other treatments reported |
Comparator treatment given to patients during the second year of the study. Amiodarone: 150 mg i.v., over at least 10 minutes, followed by continuous infusion of 1 mg per minute for 6 hours then decreased to 0.5 mg per minute. The decision to continue, discontinue or change to oral therapy after 48 hours was at the discretion of the managing physicians Line of NOAF treatment: not specifically reported; however, no other treatments reported |
|||||||||||||||||||||
| Primary diagnosis | Diltiazem (n) | Amiodarone (n) | |||||||||||||||||||||||||||||||||||||
| Trauma | 5 | 9 | |||||||||||||||||||||||||||||||||||||
| Gastrointestinal | 15 | 11 | |||||||||||||||||||||||||||||||||||||
| Vascular | 4 | 5 | |||||||||||||||||||||||||||||||||||||
| Other surgery | 7 | 5 | |||||||||||||||||||||||||||||||||||||
|
First author and year: Launey 201927 Setting: five academic ICUs Country: France Sample size: n = 261 (hydrocortisone, n = 123; no hydrocortisone, n = 138) NOAF patients: NA |
Primary diagnosis: septic shock Infection siteHydrocortisone, n (%)No hydrocortisone, n (%)Intra-abdominal72 (59)72 (52)Thoracic24 (20)32 (23)Urinary17 (14)14 (10)Other10 (8)20 (14) Mean age: hydrocortisone, 65 ± 13 years; no hydrocortisone, 63 ± 15 years Male: hydrocortisone, 61%; no hydrocortisone, 58% Severity of illness: mean SOFA score (baseline) – hydrocortisone, 10 ± 4; no hydrocortisone, 8 ± 3 Mean SAPS II (baseline): hydrocortisone, 56 ± 20; no hydrocortisone, 50 ± 20 Mean SOFA score reported (during the first 24 hours of septic shock): hydrocortisone, 13 ± 0; no hydrocortisone, 10 ± 0 Patients on vasopressors VasopressorsHydrocortisone, n (%)No hydrocortisone, n (%)Noradrenaline122 (99)135 (98)Dobutamine29 (24)6 (4)Adrenaline15 (12)10 (7) Patients with CVD CVDHydrocortisone, n (%)No hydrocortisone, n (%)Coronary disease11 (9)12 (9)Valvular disease5 (4)7 (5) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: AF was defined as 30 seconds or more of an irregular ventricular rhythm with absent P waves |
Infection site | Hydrocortisone, n (%) | No hydrocortisone, n (%) | Intra-abdominal | 72 (59) | 72 (52) | Thoracic | 24 (20) | 32 (23) | Urinary | 17 (14) | 14 (10) | Other | 10 (8) | 20 (14) | Vasopressors | Hydrocortisone, n (%) | No hydrocortisone, n (%) | Noradrenaline | 122 (99) | 135 (98) | Dobutamine | 29 (24) | 6 (4) | Adrenaline | 15 (12) | 10 (7) | CVD | Hydrocortisone, n (%) | No hydrocortisone, n (%) | Coronary disease | 11 (9) | 12 (9) | Valvular disease | 5 (4) | 7 (5) |
A hydrocortisone bolus of 100 mg followed by an infusion of 200 mg/day for 7 days followed by a short wean if the patient remained on vasopressors Line of NOAF treatment: not applicable as prophylactic treatment studied |
No prophylactic treatment |
| Infection site | Hydrocortisone, n (%) | No hydrocortisone, n (%) | |||||||||||||||||||||||||||||||||||||
| Intra-abdominal | 72 (59) | 72 (52) | |||||||||||||||||||||||||||||||||||||
| Thoracic | 24 (20) | 32 (23) | |||||||||||||||||||||||||||||||||||||
| Urinary | 17 (14) | 14 (10) | |||||||||||||||||||||||||||||||||||||
| Other | 10 (8) | 20 (14) | |||||||||||||||||||||||||||||||||||||
| Vasopressors | Hydrocortisone, n (%) | No hydrocortisone, n (%) | |||||||||||||||||||||||||||||||||||||
| Noradrenaline | 122 (99) | 135 (98) | |||||||||||||||||||||||||||||||||||||
| Dobutamine | 29 (24) | 6 (4) | |||||||||||||||||||||||||||||||||||||
| Adrenaline | 15 (12) | 10 (7) | |||||||||||||||||||||||||||||||||||||
| CVD | Hydrocortisone, n (%) | No hydrocortisone, n (%) | |||||||||||||||||||||||||||||||||||||
| Coronary disease | 11 (9) | 12 (9) | |||||||||||||||||||||||||||||||||||||
| Valvular disease | 5 (4) | 7 (5) |
| Study | Methods to address confounding | Results | Adverse effects | Recommendations for/barriers to future research |
|---|---|---|---|---|
| Gerlach 200832 | NR |
|
|
More studies including randomized controlled trials are needed to compare the use of diltiazem versus amiodarone for conversion of post-operative AF |
| Launey 201927 | The authors used inverse probability of treatment weighting using a multivariable logistic regression model to estimate the probability of treatment. Covariates included multiple measures of sepsis severity including admission severity scores and maximum doses of vasoactive medication |
|
NR | It would be interesting to study high-risk patients who develop AF and the short- and long-term outcomes in patients treated or not with hydrocortisone |
Pharmacological treatments
Gerlach et al. 32 conducted a small study (n = 61) that compared the effects of diltiazem with those of amiodarone in a surgical ICU population in the USA. 32 Ninety per cent of the included study participants were diagnosed with NOAF. Both study treatments were administered in accordance with the protocol developed by the participating surgical ICU medical team. The primary outcomes were conversion to normal sinus rhythm at 24 hours, time to conversion and adverse treatment effects. The lengths of ICU and hospital stays were also reported. The authors found no statistically significant differences between the study groups in the conversion rate at 24 hours and in the time to conversion. Similar lengths of ICU and hospital stays were also reported. One patient in the diltiazem group and two patients in the amiodarone group developed transient hypotension. This study was small and, therefore, probably underpowered to detect any treatment differences. No power calculations were reported and methods to account for confounding factors were not reported as being used in the analysis.
Prophylactic treatments
A study27 set in five French academic ICUs assessed the effect of hydrocortisone to prevent NOAF in 261 patients diagnosed with septic shock. Hydrocortisone was administered at the discretion of the attending physician, although a study treatment schedule was recommended. Patients who received hydrocortisone were more severely ill than those who did not. The unadjusted ICU and 28-day mortalities in the hydrocortisone group were higher than in the no-hydrocortisone group [37% vs. 24% (p = 0.018) and 38% vs. 26% (p = 0.036), respectively]. No relative risks (RRs) for ICU and 28-day mortality were reported in the study. However, in the propensity score-weighted analysis, patients who received hydrocortisone were less likely to develop NOAF than those who did not. The risk difference between the groups was 11.9% and the RR of developing NOAF was 0.58 [95% confidence interval (CI) 0.35 to 0.98], which indicates a benefit of hydrocortisone.
Retrospective comparative studies
Nine retrospective comparative studies28,29,33–38,48 were identified, with sample sizes ranging between 33 and 7522 patients. Seven studies28,29,33–36,48 had a sample size of > 100 patients. Six studies took place in the USA,28,29,36–38,48 two in Asia33,34 and one in Europe. 35 All studies were published after 2010. Five studies33,34,36,37,48 were available only as conference abstracts; therefore, limited data were available. None of the studies reported treatment adverse events. Details of these studies can be found in Tables 7 and 8.
| Study details | Population characteristics | Intervention | Comparator | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
First author and year: Balik 201735 Setting: 15-bed general ICU Country: Czechia Sample size: n = 234 (amiodarone, n = 177; propafenone, n = 42; metoprolol, n = 15) NOAF patients: n = 163 (69.7%) |
Primary diagnosis: septic shock Primary sources of septic shock: respiratory (57.3%), abdominal (25.2%), urosepsis (7.3%), wound/surgical (5.2%), catheter related (4.2%), maxillofacial (0.4%), neuroinfection (0.4%) Mean age: amiodarone, 67.8 ± 11.4 years; propafenone, 66.8 ± 11.3 years; metoprolol, 60.9 ± 8.3 years Male: n = 139 (59.4%) Severity of illness at the start of the anti-arrhythmic therapy: APACHE II – amiodarone, 25 ± 11.4; propafenonel, 23.2 ± 11.1; metoprolol, 19.4 ± 11.9 SOFA – amiodarone, 11.1 ± 4; propafenone, 10.2 ± 4; metoprolol, 7.0 ± 4.2 Patients on vasopressors: VasopressorAmiodarone, n (%)Propafenone, n (%)Metoprolol, n (%)Dobutamine24 (16.9)6 (7.7)–Vasopressin10 (7)2 (2.6)– Patients with CVD: n = 117 (50%) (amiodarone, n = 79, 56%; propafenone, n = 34, 43.6%; metoprolol, n = 4, 28.6%) Patients with acute renal failure: n = 64 (27.4%) Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: n = 232 (99.1%) (although does not specify whether or not this was at the onset) Serum potassium level (mmol/l): amiodarone, 4.4 ± 0.6; propafenone, 4.4 ± 0.6; metoprolol, 4.3 ± 0.5 Definition of NOAF: NR |
Vasopressor | Amiodarone, n (%) | Propafenone, n (%) | Metoprolol, n (%) | Dobutamine | 24 (16.9) | 6 (7.7) | – | Vasopressin | 10 (7) | 2 (2.6) | – |
Propafenone: the median total dose of propafenone was 2.5 g (IQR 1.0–4.0 g). The length of therapy was 5.0 days (IQR 2.0–8.5 days) Line of NOAF treatment: first and second line |
Amiodarone: median total dose of amiodarone was 3.0 g (IQR 1.8–4.6 g), given by infusion over 4 days (2–6 days) Line of NOAF treatment: first and second line Metoprolol: the median i.v. metoprolol dose was 84 mg/day (48–120 mg/day) The median length of therapy was 5 days (2–9 days) Line of NOAF treatment: first line |
||||||||||||||||||
| Vasopressor | Amiodarone, n (%) | Propafenone, n (%) | Metoprolol, n (%) | ||||||||||||||||||||||||||||||
| Dobutamine | 24 (16.9) | 6 (7.7) | – | ||||||||||||||||||||||||||||||
| Vasopressin | 10 (7) | 2 (2.6) | – | ||||||||||||||||||||||||||||||
|
First author and year: Cho 201733 (conference abstract) Setting: medical ICU Country: Republic of Korea Sample size: n = 448 NOAF patients: 100% |
Primary diagnosis: sepsis Mean age: 68.2 years Male: 68.9% Severity of illness: median CHA2DS2-VASc score, 3; median APACHE II score, 24 Patients on vasopressors: 59.9% (at the time of NOAF onset) Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: 84.5% Serum potassium level: NR Definition of NOAF: NR |
Rhythm control (43.5% patients): amiodarone used in 95.4% of rhythm control cohort Line of NOAF treatment: not specified |
Rate control (56.7% patients) Line of NOAF treatment: not specified |
||||||||||||||||||||||||||||||
|
First author and year: Jaffer 201637 (conference abstract) Setting: ICU Country: USA Sample size: n = 65 NOAF patients: 100% |
Primary diagnosis: septic shock Mean age: NR Male: 56% Severity of illness: NR Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: NR |
Amiodarone (administered to 49% of patients) Line of NOAF treatment: not specified |
Calcium channel blockers (administered to 15% of patients) and beta-blockers (administered to 12% of patients) Line of NOAF treatment: not specified |
||||||||||||||||||||||||||||||
|
First author and year: Kane 201448 (conference abstract) Setting: ICU Country: USA Sample size: n = 109 (hydrocortisone, n = 39) NOAF patients: not applicable as prophylactic treatment studied |
Primary diagnosis: septic shock Mean age: NR Male: NR Severity of illness: mean APACHE IV score reported, 97 ± 32.5 Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: NR |
Hydrocortisone (median duration 4.2 days, IQR 1.1–8.1 days) Line of NOAF treatment: not applicable as prophylactic treatment studied |
No treatment Line of NOAF treatment: not applicable |
||||||||||||||||||||||||||||||
|
First author and year: Matsumoto 201534 (conference abstract) Setting: ICU Country: Japan Sample size: n = 276 (amiodarone, n = 116; landiolol, n = 160) NOAF patients: 100% |
Primary diagnosis: NR Mean age: NR Male: NR Severity of illness: NR Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: NR |
Amiodarone: a loading infusion of 150 mg over 30 minutes followed by a continuous infusion of 20 mg/hour Line of NOAF treatment: not specified |
Landiolol: a bolus infusion of 7.5 mg followed by continuous infusion of 2.5–7.5 mg/hour Line of NOAF treatment: not specified |
||||||||||||||||||||||||||||||
|
First author and year: Brown 201838 Setting: surgical ICU Country: USA Sample size: n = 33 Initial treatment: beta-blockers, n = 22; amiodarone, n = 6; calcium channel blockers, n = 2; no treatment, n = 3 NOAF patients: 100% NOAF with rapid ventricular rate |
Primary diagnosis: oesophagectomy, n = 8; intra-abdominal surgery, n = 9; other surgery, n = 9, trauma n = 7 Sepsis at the time of onset: n = 16 (48.5%) Mean age: median age (IQR) 71 (64–80) years Male: n = 19 (58%) Severity of illness: NR for baseline or onset characteristics Patients on vasopressors (within 24 hours of NOAF onset): n = 12 (36%) Patients with CVD: coronary artery disease, 20%; stroke, 12%; peripheral vascular disease, 9% Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset (only reported for within 24 hours of onset): n = 5 (15%) Serum potassium level: patients with serum potassium of < 4 mmol/l on first laboratory after AF onset, n = 15 (45%) Definition of NOAF: AF occurring in any patient with no documented history of AF |
Beta-blockers Line of NOAF treatment: first line |
Amiodarone and calcium channel blockers Line of NOAF treatment: first, second and third Sixteen patients (48%) received a second medication owing to failure to restore sinus rhythm, with amiodarone being the most common (n = 13, 81%) |
||||||||||||||||||||||||||||||
|
First author and year: Mieure 201136 (conference abstract) Setting: ICU Country: USA Sample size: n = 126 (amiodarone, n = 61; diltiazem, n = 41; metoprolol, n = 24) NOAF patients: 100% |
Primary diagnosis: NR Mean age: NR Male: NR Severity of illness: NR Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: ‘onset 120 beats per minute’ |
Amiodarone Line of NOAF treatment: not specified |
Diltiazem Line of NOAF treatment: not specified Metoprolol Line of NOAF treatment: Not specified |
||||||||||||||||||||||||||||||
|
First author and year: Walkey 201628 Setting: 20% of hospitals in the USA Country: USA Sample size: n = 39,693 (calcium channel blockers, n = 14,202; beta-blockers, n = 11,290; digoxin, n = 7937, amiodarone, n = 6264) NOAF patients: n = 3174 Note: outcomes reported separately for NOAF patients |
Primary diagnosis: sepsis Infection siteBeta-blocker, n (%)Calcium channel blocker, n (%)Digoxin, n (%)Amiodarone, n (%)Respiratory3583 (31.7)5882 (41.4)3118 (39.3)2369 (37.8)Gastrointestinal2107 (18.7)1692 (11.9)1030 (13.0)896 (14.3)Urinary tract4173 (37.0)5439 (38.3)3008 (37.9)1980 (31.6)Skin or soft tissue982 (8.7)1217 (8.6)696 (8.8)507 (8.1)Primary bacteraemia or fungaemia140 (1.2)150 (1.1)82 (1.0)76 (1.2) Mean age: beta-blockers, 75.7 ± 11.3 years; calcium channel blockers, 75.6 ± 11.4 years; digoxin, 77.1 ± 10.7 years; amiodarone, 73.1 ± 11.7 years Male: beta-blockers, 50.4%; calcium channel blockers, 47.4%; digoxin, 48.5%; amiodarone, 55.1% Severity of illness: NR Patients on vasopressors on first hospital day: beta-blockers, 29.1%; calcium channel blockers, 26.5%; digoxin, 44.1%; amiodarone, 64.0% Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: AF that was not documented on hospital admission |
Infection site | Beta-blocker, n (%) | Calcium channel blocker, n (%) | Digoxin, n (%) | Amiodarone, n (%) | Respiratory | 3583 (31.7) | 5882 (41.4) | 3118 (39.3) | 2369 (37.8) | Gastrointestinal | 2107 (18.7) | 1692 (11.9) | 1030 (13.0) | 896 (14.3) | Urinary tract | 4173 (37.0) | 5439 (38.3) | 3008 (37.9) | 1980 (31.6) | Skin or soft tissue | 982 (8.7) | 1217 (8.6) | 696 (8.8) | 507 (8.1) | Primary bacteraemia or fungaemia | 140 (1.2) | 150 (1.1) | 82 (1.0) | 76 (1.2) |
Intravenous beta-blocker (metoprolol, esmolol, atenolol, labetalol, propranolol) Line of NOAF treatment: not specified |
Intravenous calcium channel blocker (diltiazem, verapamil) Intravenous digoxin (cardiac glycosides, digoxin, digitalis) Intravenous amiodarone Line of NOAF treatment: not specified |
| Infection site | Beta-blocker, n (%) | Calcium channel blocker, n (%) | Digoxin, n (%) | Amiodarone, n (%) | |||||||||||||||||||||||||||||
| Respiratory | 3583 (31.7) | 5882 (41.4) | 3118 (39.3) | 2369 (37.8) | |||||||||||||||||||||||||||||
| Gastrointestinal | 2107 (18.7) | 1692 (11.9) | 1030 (13.0) | 896 (14.3) | |||||||||||||||||||||||||||||
| Urinary tract | 4173 (37.0) | 5439 (38.3) | 3008 (37.9) | 1980 (31.6) | |||||||||||||||||||||||||||||
| Skin or soft tissue | 982 (8.7) | 1217 (8.6) | 696 (8.8) | 507 (8.1) | |||||||||||||||||||||||||||||
| Primary bacteraemia or fungaemia | 140 (1.2) | 150 (1.1) | 82 (1.0) | 76 (1.2) | |||||||||||||||||||||||||||||
|
First author and year: Walkey 201629 Setting: non-federal US hospitals Country: USA Sample size: n = 38,582 (pre-existing AF, n = 31,060) NOAF patients: n = 7522 (n = 5585 analysed with propensity score approach) Note: outcomes reported separately for NOAF patients |
Primary diagnosis: sepsis Mean age: anticoagulation, 73.2 ± 11.7 years; no anticoagulation, 75.8 ± 11.7 years Male: anticoagulation, n = 6941 (51%); no anticoagulation, n = 12,035 (42.2%) Severity of illness mean (SD) CHA2DS2-VASc score reported: anticoagulation, 3.4 (1.5); no anticoagulation, 3.6 (1.5) Patients on vasopressors: anticoagulation, n = 5084 (37.4%); no anticoagulation, n = 10,002 (40.1%) Patients with CVD Cardiovascular diseaseAnticoagulation, (N = 13,611) (35.3%), n (%)No anticoagulation, (N = 24,971) (64.7%), n (%)Heart failure5712 (42.0)9792 (39.2)Coronary heart disease or myocardial infarction4532 (33.3)7970 (31.9)Valvular heart disease2010 (14.8)3348 (13.4) Patients with acute renal failure: anticoagulation, n = 7612 (55.9%); no anticoagulation, n = 15,814 (63.3%) Patients with acute respiratory failure: anticoagulation, n = 5308 (39.0%); no anticoagulation, n = 9442 (37.8%) Mechanical ventilation at NOAF onset: NR Serum potassium level: NR Definition of NOAF: ‘incident AF that was not present on admission’ |
Cardiovascular disease | Anticoagulation, (N = 13,611) (35.3%), n (%) | No anticoagulation, (N = 24,971) (64.7%), n (%) | Heart failure | 5712 (42.0) | 9792 (39.2) | Coronary heart disease or myocardial infarction | 4532 (33.3) | 7970 (31.9) | Valvular heart disease | 2010 (14.8) | 3348 (13.4) |
Intravenous or subcutaneous administration of therapeutic-dose anticoagulant (including i.v. heparin, SC enoxaparin, SC dalteparin, SC fondaparinux) Patients who received oral anticoagulants as their initial anticoagulant were excluded in the primary analysis Line of NOAF treatment: not applicable as NOAF treatment not studied |
No anticoagulation Line of NOAF treatment: not applicable |
||||||||||||||||||
| Cardiovascular disease | Anticoagulation, (N = 13,611) (35.3%), n (%) | No anticoagulation, (N = 24,971) (64.7%), n (%) | |||||||||||||||||||||||||||||||
| Heart failure | 5712 (42.0) | 9792 (39.2) | |||||||||||||||||||||||||||||||
| Coronary heart disease or myocardial infarction | 4532 (33.3) | 7970 (31.9) | |||||||||||||||||||||||||||||||
| Valvular heart disease | 2010 (14.8) | 3348 (13.4) |
| Study | Methods to address confounding | Results | Recommendations for/barriers to the future research |
|---|---|---|---|
| Balik 201735 | Linear regression analysis involving univariate and multivariate testing |
|
NR |
| Cho 201733 (abstract) | Propensity matching |
|
NR |
| Jaffer 201637 (abstract) | None |
|
Further investigation into the management of arrhythmias in septic shock is needed to further elucidate the potential benefits and harms of various pharmaceutical agents |
| Kane 201448 (abstract) | Multivariate regression |
|
Given the incidence rate of AF in septic shock, a preventative study using [hydrocortisone] may be appropriate. Furthermore, future studies using [hydrocortisone] in this patient population should include AF as a secondary endpoint |
| Matsumoto 201534 (abstract) | NR |
|
NR |
| Brown 201838 | Markov chain analysis to account for patients who have achieved the outcome (sinus rhythm restoration). This analysis recognises that the outcome can be achieved by different medication. No other methods to address confounding were reported |
|
Future studies are needed to further explore this and determine many unknowns including optimal dosing and route, need for anticoagulation, and duration of treatment |
| Mieure 201136 (abstract) | NR |
|
A large randomized controlled trial designed to determine the optimal therapeutic strategy for a heterogeneous cohort of patients with new onset AF with RVR [rapid ventricular rate] is needed |
| Walkey 201628 | Propensity score matching approach using over 30 covariates covering specific patient demographics, hospital characteristics, prevalent comorbidities, type of acute organ failure and type of infection |
|
Our outcome findings should be considered hypothesis generating and supportive of the need for future clinical trials to investigate optimal treatment of AF during sepsis |
| Walkey 201629 | A propensity score approach was used to adjust for variables representing hospital characteristics, patient demographics, comorbidities, use of intensive care, measures of acute organ dysfunction, source of infection and year of hospitalisation | The authors reported RR of in-hospital ischaemic stroke associated with anticoagulation as 0.85 (95% CI 0.57 to 1.27) for patients with newly diagnosed AF. The RR of bleeding associated with parenteral anticoagulation was reported as 0.97 (95% CI 0.83 to 1.14) for patients with newly diagnosed AF | Whereas current evidence suggests that benefits may not outweigh risks of parenteral anticoagulation for AF during sepsis, further study is warranted to determine optimal timing for restarting treatment with oral anticoagulants among patients with pre-existing AF and long-term anticoagulation strategies after hospitalisation for patients with newly diagnosed AF during sepsis |
Pharmacological treatments
Seven studies28,33–38 investigated the effects of pharmacological treatments. Four studies included patients with sepsis28,33 or septic shock35,37 as their primary diagnosis. One study38 was conducted in a surgical population and two studies34,36 did not clearly specify the type of ICU and study population. A study by Walkey et al. 28 was not limited to an ICU population. A large proportion of studies did not report on the dose28,33,36–38 or the mode of administration33,36–38 of any treatment given. One study33 investigated the treatment effects of rate and rhythm control strategies, but the conference abstract did not report which specific interventions were studied. Outcomes reported included cardioversion to sinus rhythm,33–36,38 mortality28,33,35,37 and lengths of ICU and hospital stays. 37
Most of the larger studies28,34–36 (sample size > 100 patients) compared amiodarone with beta-blockers (e.g. landiolol and metoprolol). A large study28 from the USA reported that patients treated with amiodarone were more likely than patients treated with beta-blockers to be critically ill with septic shock. The RR of hospital mortality for patients who received beta-blockers compared with patients who received amiodarone was 0.67 (95% CI 0.59 to 0.77) after adjustment for confounding, which indicates a better outcome for patients with beta-blockers. However, the patient characteristics between these groups were different and the matching of groups in the NOAF cohort was not reported. Balik et al. 35 reported higher ICU mortality in patients receiving amiodarone (40%) than in patients receiving metoprolol (21%); however, this was reported as being not statistically significant. Three studies34–36 compared conversion rates between amiodarone and beta-blockers, and found that rates of conversion to sinus rhythm were slightly, but not significantly, higher in patients receiving beta-blockers. However, Balik et al. 35 did not adjust for confounding factors such as sickness score. Matsumoto et al. 34 and Mieure et al. 36 did not report the methods used for the analysis.
Walkey et al. 28 compared outcomes in patients who received digoxin and those who received beta-blockers. Following propensity score matching (n = 1932), the RR of hospital mortality for patients who received beta-blockers compared with patients who received digoxin was 0.75 (95% CI 0.64 to 0.88), which indicates a better outcome for patients treated with beta-blockers. 28 However, only around 60% of patients in the propensity score-matched cohorts were ICU patients, and the study was restricted to patients with sepsis; therefore, this study’s results should not be considered applicable to a broad ICU population. Moreover, the study was judged as being at a serious risk of bias owing to confounding (see Risk-of-bias assessments).
Four studies28,36–38 investigated the effects of calcium channel blockers (e.g. diltiazem). Walkey et al. 28 found no statistically significant difference in mortality between patients who received beta-blockers and patients who received calcium channel blockers (RR 0.99, 95% CI 86 to 1.15). Similarly, a conference abstract by Jaffer et al. 37 reported no statistically significant difference in death at discharge between patients administered beta-blockers and patients administered calcium channel blockers. Two studies36,38 compared conversion rates between patients who were administered calcium channel blockers and patients who were administered either amiodarone36 or beta-blockers. No meaningful conclusions from the results of these two studies could have been made owing to the small sample sizes. In addition, the Mieure et al. 36 study was available only as a conference abstract in which the study methods and population characteristics were not reported.
Prophylactic treatments
A small (n = 109 patients) study48 assessed the association of hydrocortisone with NOAF in patients who were diagnosed with septic shock. The authors concluded that administering hydrocortisone was associated with a reduction in the incidence of NOAF (20.5% in patients who received hydrocortisone vs. 42.9% in those who did not; p = 0.022). 48 No evidence of a difference in mortality and length of stay between the study groups was reported. This study was published as a conference abstract; therefore, limited data were available on the study population and analysis.
Anticoagulants
One large study29 (n = 7522) included a subgroup of patients who developed NOAF during sepsis in hospital, of whom just over 60% of whom were treated in an ICU. Rates of in-hospital stroke were low (n = 104, 1.9%). Given that the length of hospital stay was not reported, the duration of exposure was unclear. Following propensity score matching (n = 5585 analysed) there was no evidence of a difference in rates of in-hospital ischaemic stroke events between patients who did and those who did not receive parenteral anticoagulation (RR 0.85, 95% CI 0.57 to 1.27). Given the low event rate, the study may have had inadequate power to determine whether or not a statistically significant difference exists. There was no statistically significant difference in the risk of bleeding associated with parenteral anticoagulation between the groups (RR 0.97, 95% CI 0.83 to 1.14).
Prospective single-group studies
Six prospective single-group studies39,40,45–47,49 were included in the review. The sample sizes of the included studies39,45–47,49 ranged from 16 to 37 patients; one study40 did not report the sample size. Four studies were undertaken in Europe,39,46,47,49 one in Asia45 and one in North America. 40 Five articles39,40,46,47,49 were published between 2002 and 2008, and one article45 was published in 2016. One publication40 was available only as a conference abstract.
One study45 was conducted in a mixed ICU and one study40 was conducted in a general ICU. Two studies47,49 were conducted in specialty ICUs, such as surgical or medical ICUs. The type of ICU was not clearly specified in two studies. 39,46 Four studies39,45–47 investigated the treatment effects of pharmacological treatments and one study49 looked at electrical treatments. One study40 reported both the treatment effects of pharmacological treatments and the preventative effects of anticoagulation for stroke prophylaxis. Details of the prospective single-group studies can be found in Appendix 3, Tables 15 and 16.
Pharmacological treatments
Pharmacological treatments such as amiodarone,40 ibutilide,46,47 beta-blockers45 and MgSO4–amiodarone step-up scheme39 were investigated. Four studies39,40,46,47 reported conversion to sinus rhythm as the primary outcome and one study45 looked at mortality as an outcome.
Slavik et al. 40 investigated the treatment effects of amiodarone; however, results were not clearly reported in this conference abstract.
A study45 (n = 16) set in Japan investigated the effects of switching therapy from landiolol to the Bisoprolol patch (Bisono® tape, Toa Eiyo Corp, Tokyo, Japan) in a mixed ICU population. This study reported that survival was achieved in 81% of the patients in whom switching therapy was introduced. Another very small study39 (n = 29) investigated the effects of a new treatment protocol consisting of the infusion of magnesium sulphate (MgSO4) as a first-line therapy and amiodarone as a second-line therapy in the case of no conversion. The study population was mixed and comprised medical and surgical ICU patients who were diagnosed with NOAF. Study treatments were administered as per institutional protocol based on MgSO4–amiodarone step-up scheme, for which infusion of amiodarone was started if conversion to sinus rhythm or reduction in the ventricular rate of < 110 beats per minute (b.p.m.) within 1 hour after the start of MgSO4 infusion was not achieved. The authors reported that amiodarone was required in 13 MgSO4 non-responders, of whom 11 converted to normal sinus rhythm within 24 hours. No adverse events were reported.
Two very small studies46,47 investigated the treatment effects of ibutilide. Both studies administered i.v. ibutilide, with a maximum dose of 2 mg. Hennersdorf et al. 46 (n = 26) reported slightly lower conversion rate to sinus rhythm than Delle Karth et al. 47 (n = 17) (71% vs. 82%, respectively).
Electrical treatments
A small study49 (n = 37) assessed the effect of direct current cardioversion (DCC) in a surgical ICU population. The treatment for patients with regular supraventricular tachyarrhythmia consisted of a maximum of four consecutive cardioversions with an energy delivery of 50 J, 100 J, 200 J and 300 J. For patients with irregular supraventricular tachycardia, cardioversion was performed with an energy delivery of 100 J, 200 J and 360 J. Thirty-five per cent of patients (n = 13) primarily responded to DCC with restoration of sinus rhythm for ≥ 5 minutes, of whom 62% (n = 8) remained in sinus rhythm at 1 hour. At 24 and 48 hours, 16% and 13.5% of patients remained in sinus rhythm, respectively.
Anticoagulants
Slavik et al. 40 studied i.v. heparin as a prophylactic treatment for stroke; this was used in 36% of NOAF episodes. The authors did not report which anticoagulant was used in the other 64% of NOAF cases. It was reported that stroke prophylaxis was achieved in 91% of NOAF episodes. The authors concluded that the appropriateness of therapy for stroke prophylaxis was ‘optimal’. This was decided using prespecified study definitions. Five per cent of the study population experienced major bleeding as a side effect of i.v. heparin. 40 It must be noted that only an abstract was available for this study; therefore, limited data were obtained. Moreover, the definition of ‘appropriateness of therapy assessed as optimal, appropriate and inappropriate’ was not provided.
Retrospective single-group studies
Six retrospective single-group8,41–44,50 studies were identified, with sample sizes ranging from 30 to 240 patients. Four studies8,41–43 had a sample size of > 100 patients. Two studies were set in each of North America8,44 and Asia,41,50 one in Europe43 and one in Australia. 42 One article43 was published in 2004 and five articles8,41,42,44,50 were published between 2010 and 2019.
Four studies8,42,43,50 were conducted in mixed ICUs, one study in a surgical ICU44 and one study in a medical ICU. 41 Three studies investigated the treatment effects of pharmacological treatments,8,42,43 one study investigated electrical treatments50 and two studies looked at both pharmacological and electrical treatments. 41,44 The details of the retrospective single-group studies can be found in Appendix 3, Tables 17 and 18.
Pharmacological treatments
The following pharmacological treatments were investigated in the included studies: amiodarone,8,41–44 beta-blockers (e.g. metoprolol, esmolol and sotalol),8,41,44 calcium channel blockers (e.g. diltiazem)41,44 and digoxin. 41,44 Two studies41,44 did not report on the dose and mode of administration of the treatments studied.
Four larger studies8,41–43 (n > 100 patients) investigated the treatment effects of amiodarone. Conversion rates to normal sinus rhythm ranged from 65% to 87%. 8,41,43 Studies reported different time points for conversion rates; for example, at some point while receiving amiodarone8 and during the first 48 hours of amiodarone therapy. 43 Maintenance of normal sinus rhythm in patients who had converted back while receiving amiodarone ranged from 49% to 59% at the time of ICU discharge. 8,42 The studies reported different time points at which maintenance of sinus rhythm was achieved, such as until discharge from the ICU. 42 It should be noted that, where reported, the dose and administration of amiodarone were heterogenous between the studies. 8,42,43 One study43 reported on treatment adverse effects associated with amiodarone, finding increases in serum concentrations of creatinine and bilirubin.
A study41 undertaken in a population with sepsis reported that 76% of patients who were given beta-blockers (n = 88), 71% of those who were administered calcium channel blockers (n = 66) and 55% of those who were given digoxin glycosides (n = 27) converted back to sinus rhythm within 7 days after the onset of NOAF. Although some authors also studied the treatment effects of beta-blockers, calcium channel blockers and digoxin, the sample sizes were too small to make any meaningful conclusions8,44 or the results were not clearly reported. 44
Electrical treatments
Kyo et al. 50 investigated the effect of electrical cardioversion in a mixed ICU population (n = 85). A median of one shock per electrical cardioversion session was reported and the delivered electrical cardioversion energies in the first and second shocks were ≤ 100 J in 91% and 83% of all electrical cardioversion patients, respectively. The authors reported successful electrical cardioversion, defined as conversion to sinus rhythm for at least 5 minutes after an electrical cardioversion session, in 48% of patients, and 13% of these patients maintained sinus rhythm until ICU discharge.
Liu et al. 41 administered electrical cardioversion to eight patients and reported that 50% of these patients converted back to normal sinus rhythm. No more details on the intervention and outcome were available.
Reviews and guidelines
Twelve review articles53–64 were included in the current review. Of these, two were systematic reviews,53,56 six were narrative review articles54,57–59,62,64 and four were review articles55,60,61,63 that proposed a treatment algorithm based on available evidence. Most of the included reviews53–55,57–61,63,64 (n = 10) were published after 2012. No guidelines were identified in this scoping review.
Systematic reviews
Yoshida et al. 53 conducted a systematic review of the epidemiology, prevention and treatment of NOAF in critically ill patients. One database was searched and eligibility criteria were specified for study inclusion in the systematic review. The authors assessed the methodological quality of the included studies using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. 65 No studies on NOAF prevention were included in the systematic review and five studies8,30,39,66,67 investigating treatments for NOAF were eligible. Of the five studies identified by Yoshida et al. ,53 three 8,30,39 were eligible for this scoping review and two66,67 were excluded on outcome. The five included studies, of which one was a RCT,30 evaluated the clinical effectiveness of NOAF treatments, such as amiodarone, beta-blockers, calcium channel blockers, digoxin, magnesium sulphate and DCC. However, no conclusive findings on the treatment strategies were reported. The authors concluded that the current evidence for the management of NOAF in a general ICU population is very limited and further research is urgently required.
In 2008, Kanji et al. 56 published a systematic review of RCTs to assess the treatments of NOAF in non-cardiac ICU patients. Three databases were systematically searched, the study eligibility criteria were clearly specified and a quality assessment of each included RCT was conducted using a basic rating instrument (the Jadad scale68). Four RCTs30,51,52,69 that assessed the efficiency of procainamide (a sodium-channel blocker), flecainide (a sodium-channel blocker), esmolol, amiodarone, verapamil, diltiazem and magnesium were included in the Kanji et al. 56 review. Only one RCT 30 identified by Kanji et al. 56 was included in this scoping review. Two RCTs51,52 had a study population consisting of < 70% of NOAF patients (and so were not eligible for this scoping review). The other study69 included in the Kanji et al. 56 review investigated patients with AF; however, it was not clear whether or not these patients had NOAF (therefore, this study was excluded on population in this scoping review). The authors were not able to make evidence-based recommendations for pharmacological rhythm conversion strategies for a general ICU NOAF population owing to considerable methodological heterogeneity of the included RCTs. The authors emphasised the need for well-designed and adequately powered RCTs to evaluate treatment strategies for critically ill patients with NOAF. Moreover, they recommended that future research should address treatments of choice and goals of care using a standardised outcome measure of success.
Other types of review
The evidence on pharmacological54,55,57–63 and electrical55,59,62,63 treatment strategies for NOAF was discussed in the other review articles. Four articles54,57,59,63 discussed the management of NOAF in sepsis patients and six articles57,60–64 reviewed the literature on anticoagulation strategies for critically ill patients with NOAF. Four articles55,60,61,63 proposed an algorithm for the management of NOAF in an ICU setting based on the available evidence.
Pharmacological treatments
It is widely reported that the management of arrhythmias in critical care settings is a major problem59,62 and that research on optimal therapeutic strategies for critically ill patients with NOAF is urgently needed. 54,55,57–59,61–63 Some articles55,58,61 argued that beta-blockers may be a reasonable first-choice treatment given the current evidence of decreased mortality55 and improved heart rate control. 55,58 By contrast, some authors discussed amiodarone as being a potentially effective treatment54,57,59,60,62 based on current evidence and its widespread use; however, it was also recognised that amiodarone has potentially significant side effects. 54,60,62 Other pharmacological treatments, such as propafenone,54,62 calcium channel blockers,55,62,63 digoxin54,55,62 and ibutilide,62 were discussed, but no conclusive findings were made. Four articles55,60,61,63 proposed a treatment algorithm, but the algorithms should be interpreted cautiously because they were developed based on limited evidence that was not identified and critiqued systematically.
Electrical treatments
Reviews suggested that DCC might often be unsuccessful55 and might also be associated with a high relapse rate. 55,62 More evidence in critically ill populations is required to support this62 and the current findings should be used to guide research in therapy and mechanisms.
Anticoagulants
A review article62 concluded that there was no clear evidence of whether or not stroke risk reduction outweighs the increased risk of bleeding when using therapeutic anticoagulation in critically ill patients with NOAF. Labbe et al. 64 reported a high frequency of major bleeding events and recommended that anticoagulation therapy should be administered only in patients with the highest risk of arterial thromboembolic events. This assertion of high bleeding rates referenced one study that did not compare bleeding events between patients who received anticoagulation and patients who did not, and one study that found no significant difference in bleeding events between these two patient groups. A patient-centred single-case decision approach of whether or not to use anticoagulant therapy was also suggested in another review. 57 Sibley and Muscedere61 recommended that anticoagulation therapy should be initiated if AF persists for > 48 hours and in patients with a high risk of arterial thromboembolic events. 61 Only one review article61 discussed which drug would be appropriate to use for anticoagulation in ICU patients. Unfractionated heparin was reported to be the drug of choice for critically ill patients owing to its short half-life and reversibility with protamine; however, it must be noted that the authors of the review61 did not provide any references for this statement.
Surveys and opinion pieces
Surveys
Only one survey16 was identified in the current review. The UK-wide survey on the practice of the management of NOAF in critically ill patients was conducted in 2016 and was sent to all members of the Intensive Care Society (London, UK). A total of 3152 questionnaires were sent and 397 responses were received. The survey included questions on demographic variables of participants and their critical care unit, and a set of questions that aimed to determine the management strategies for NOAF and anticoagulation practice. In total, 72% of respondents were consultants, 46% worked in a district general hospital and 81% worked in a mixed ICU. The authors reported that 81% of respondents used amiodarone for the treatment of NOAF. Only 12% of respondents reported using beta-blockers. It was reported that 64% of respondents would not use anticoagulant therapy in critically ill patients with NOAF, whereas 31% of respondents would start anticoagulation therapy within 72 hours. The survey revealed that low-molecular-weight or high-molecular-weight heparin was considered appropriate for anticoagulant therapy.
Opinion pieces
Four opinion pieces70–73 were identified in the current review. Two72,73 were published in 2008 and are responses to a systematic review of RCTs investigating the treatments for NOAF in a critically ill, non-cardiac ICU population. 56 Both authors72,73 agree with the conclusion of Kanji et al. :56 that the evidence is lacking and that the answers still need to be provided. Walton72 believes that the best agent for use in NOAF is amiodarone because it combines rapid rate control effects and a low risk of precipitating ventricular tachyarrhythmias. 72 However, Trohman73 favours the use of beta-blockers as the initial pharmacotherapy. Both authors72,73 emphasised that treatments must be carefully studied to design an evidence-based approach to guide treatment strategies in NOAF patients in an ICU.
Walkey et al. 70 recommend beta-blockers as a reasonable first choice of initial AF therapy, given the limited and indirect evidence. The authors also commented on managing the risks of stroke, and concluded that evidence is currently lacking on risks of bleeding and estimates of stroke risk reduction associated with use of anticoagulation in critically ill patients. Therefore, the authors did not recommend using anticoagulation in NOAF patients with elevated bleeding risk because it is not currently known whether or not the benefits outweigh the risks. 70
Vieillard-Baron and Boyd71 suggest a non-anti-arrhythmic-based approach to reduce NOAF by optimising electrolytes and fluid status, limiting sympathetic activation and controlling the central venous catheter position before considering any anti-arrhythmic drugs. It must be noted that this treatment strategy was developed by the authors, and was based on the pathophysiology of AF and its risk factors present in patients with sepsis. No high-quality evidence is available to support this treatment approach.
Definitions used for new-onset atrial fibrillation
Studies varied in how they reported and defined NOAF. Four studies38,43,47,49 required NOAF to have a heart rate of > 100 b.p.m. and two studies31,36 required NOAF to have a heart rate of > 120 b.p.m. Nineteen studies8,27–30,32–35,37,39–42,44–46,48,50 did not provide a heart rate threshold for NOAF. Studies also reported different time periods for which NOAF must be sustained, ranging from 30 seconds to 24 hours. 27,30,31,41,43,47,49 Eighteen studies8,28,29,32–40,42,44–46,48,50 did not define the time period for which NOAF must be sustained. Six studies8,28,29,38,39,50 clarified in which instances AF would be considered as new onset; for example, when a patient had no prior history of AF,38 when a patient had no previous history of atrial tachyarrhythmias and anti-arrhythmic drug use,39 when AF occurred during an ICU stay,39,50 and when AF was absent on admission. 28,29 Ten studies32–35,37,40,44–46,48 did not provide any definition for NOAF.
Recommendations for and barriers to future research
Most researchers concluded that further prospective research accounting for confounding factors is required to determine the success and clinical implications of prophylactic and rhythm and rate control strategies in critically ill patients with NOAF. 8,27,28,30,32,36,37,39,41,44,45,48,53–58,61,62,70 Moreover, it has been emphasised that the optimal regimens and the best dosing strategies for treatments are yet to be established. 38,42,49 Eight primary studies31,33–35,40,43,46,47 and four review articles59,60,63,64 did not provide any recommendations for future research.
Kanji et al. 56 recognised that there are very few prospective studies conducted to evaluate the treatment strategies for NOAF in the critically ill population, given the prevalence of NOAF in this population and the associated morbidity and mortality. Kanji et al. 56 argue that the lack of prospective trials is because of the nature of this population. NOAF is considered an emergency, and enrolling these patients into prospective trials is logistically difficult because rapid treatment is often needed. Moreover, the lack of standardised outcome measures, such as the definition of successful cardioversion, was identified as a major limitation. Kanji et al. 56 also suggested that grouping AF together with other types of supraventricular tachycardias might be inappropriate because the physiology and their treatment response might be different.
Two primary studies29,40 and three review articles61,62,64 that discussed anticoagulation strategies in critically ill patients with NOAF did not provide any recommendations for the future research in this population.
Expert panel review
We convened an expert panel (see Appendix 8) to review the findings of the scoping review to inform definitions, treatments and confounders to be used in the ICU database analysis (see Chapter 4). The scoping review highlighted that definitions of NOAF in patients in an ICU and definitions of treatment success varied. In the absence of any consensus definition of NOAF, we adopted the agreed definition of AF in patients outside an ICU: any AF lasting ≥ 30 seconds. We defined time to cardioversion as the time to first reversion of sinus rhythm, and the time to rate control was defined as the time to a heart rate of < 110 b.p.m. Two studies27,41 defined AF as lasting for longer than 30 seconds. No studies provided a definition for time to cardioversion. Where studies defined a heart rate threshold for AF, it was either > 100 b.p.m. 38,43,47,49 or > 120 b.p.m. 31,36 A list of the interventions used in the studies identified in the scoping review was created and reviewed, but was not altered by the expert panel. We then screened our databases for presence of data pertaining to identified interventions. A list of identified and available interventions is shown in Appendix 8, Treatments to be included in the analysis and identified but unavailable interventions in Appendix 8, Treatments of interest, but not possible with our data. A list of confounding variables was created from those identified in studies in our scoping review. This list was then supplemented though two rounds of individual review by expert panel members, resulting in a final list of confounders that was ratified by the panel. We then screened our databases for the presence of data pertaining to identified confounders. The final list is shown in Appendix 8, Confounding/matching variables.
Chapter 3 Database analysis part 1: RISK-II database
Database analysis part 1: methods
Data sources
We analysed patient records from the RISK-II database, which includes anonymised, linked, routinely collected data from (1) the Case Mix Programme (CMP) national clinical audit of adult intensive care,74 (2) Hospital Episode Statistics (HES) for England and (3) the Office for National Statistics (ONS) mortality database.
Case Mix Programme data are collected for the purpose of service evaluation and quality improvement in critical care. 75 The CMP includes records for each admission to a participating adult high-dependency unit or ICU in England, Wales and Northern Ireland. Not all ICUs participated during the period for which data were extracted; coverage of adult general ICUs increased over the study period, reaching 100% in the final year of extracted data. Some, but not all, specialist ICUs participated (cardiothoracic ICUs were excluded from the analysis; see Inclusion and exclusion criteria). The CMP was used to identify the study sample, provide dates for the start and end of hospital admission and critical care, and to identify patient demographics.
The HES database is collected for the purpose of reimbursing NHS trusts for the provision of hospital services. The RISK-II database includes records from the admitted patient care section of HES, which contains one record for each ‘episode of care’ under one consultant during a hospital admission. One hospital admission may contain multiple episodes of care, one of which would generally correspond to the period in critical care, but there are differences between trusts in the way that these data are recorded; therefore, HES and CMP records do not align consistently. Each HES record includes up to 20 International Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), diagnosis codes and up to 24 Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures (OPCS-4) codes that were used to identify NOAF, comorbidities and diagnosis-specific rates of subsequent hospitalisation (see Identification of new-onset atrial fibrillation).
The ONS mortality database contains information about all of the deaths registered in the UK, and was used to derive indicators of mortality.
The anonymised RISK-II database is maintained by the Intensive Care National Audit and Research Centre (ICNARC) (London, UK) and was linked by NHS Digital (Leeds, UK) using a standard deterministic algorithm involving the NHS number (a unique patient identifier), date of birth, postcode and sex. The RISK-II database includes CMP records from 1 April 2009 to 31 March 2016, HES records from 1 April 2004 to 31 March 2016 and ONS records from 1 April 2009 to 31 October 2018.
Inclusion and exclusion criteria
We included the records of patients admitted to an ICU between 1 April 2009 and 31 March 2016 (5 financial years). Contiguous ICU admissions, representing transfers between ICUs and readmissions to an ICU within 1 calendar day, were combined into single records. We excluded admissions to cardiothoracic units, admissions lasting < 4 hours and patients aged < 16 years at the time of ICU admission.
Identification of new-onset atrial fibrillation
The codes used for identifying health conditions are summarised in Table 9. We defined patients as having NOAF during an ICU admission by identifying a CMP record that overlapped with a linked HES record that was the first HES record containing an ICD-10 code for AF for that patient anywhere in the database. Adopting a cautious approach to defining NOAF, we excluded patients with a linked HES record relating to the same hospital admission and contained an OPCS-4 procedure code for atrial ablation, pacemaker insertion or DCC during the same hospital admission from this group, because AF may have developed prior to ICU admission.
| Condition | ICD-10 or OPCS-4 code |
|---|---|
| Atrial fibrillation | |
| Diagnosis | I48 |
| Atrial ablation/maze procedurea | K572, K575, K578, K622 and K623 |
| Pacemaker insertiona | K601–19, K731–9, K741–3, K748–9, U311 and U318–9 |
| Direct current cardioversiona | K578, X501, X502 and K624 |
| Comorbidities | |
| Diabetes mellitus | E10–14 |
| Hypertension | I10–15 |
| Prior thromboembolism | I63–4, I74 |
| Valvular heart disease | I08–9, I33–8, Q22–3 and Q2484 |
| Dilated cardiomyopathy | I42 |
| Pulmonary hypertension | I270–2 |
| Heart failure | I50 |
| Subsequent hospitalisation with | |
| Atrial fibrillation | I48 |
| Stroke | I63 |
| Heart failure | I50 |
There are many possible ways that CMP records and HES records can overlap: the HES record may commence on the same day or on an earlier/later date than the CMP record, and similarly may finish on the same day or on an earlier/later date. Many of these scenarios result in uncertainty about whether the AF developed prior to ICU admission, in an ICU or after discharge from an ICU. We, therefore, implemented the following rules for classifying NOAF (Table 10):
-
If the HES record commenced on the same day as the CMP record, then it was considered NOAF only if there was also a prior HES record from the same hospital admission (i.e. if the patient was admitted straight to an ICU from an emergency room and, therefore, had no prior HES record from that hospital admission then we assumed that the AF developed prior to ICU admission).
-
If the HES record commenced on an earlier date than the CMP record, then the AF was assumed to have developed prior to ICU admission and not to represent NOAF.
-
If the HES record commenced after the CMP record commenced and finished before the CMP record finished, then the admission was considered to represent NOAF.
| Overlap between HES and CMP records | Classification of AF | ||
|---|---|---|---|
| HES record start date | HES record end date | Admitted to hospital on same day as the ICU? | |
| Before CMP record start | Any | No (by definition) | Pre-existing AF |
| Same as CMP record start | Any | Yes | Pre-existing AF |
| Same as CMP record start | Same as or before CMP record end | No | NOAF |
| Same as CMP record start | After CMP record end (up to 1 day) | No | NOAF |
| Same as CMP record start | After CMP record end (> 1 day) | No | Subsequent AFa |
| After CMP record start and same as or before CMP record end | Same as or before CMP record end | Either | NOAF |
| After CMP record start and same as or before CMP record end | After CMP record end (up to 1 day) | Either | NOAF |
| After CMP record start and same as or before CMP record end | After CMP record end (> 1 day) | Either | Subsequent AFa |
| After CMP record end | After CMP record end (by definition) | Either | Subsequent AF |
If the HES record continued beyond the CMP record, then it was considered NOAF only if the discrepancy was only 1 day (to allow for minor discrepancies in data entry between the systems), otherwise it was assumed that the AF developed after discharge from the ICU. As a sensitivity analysis, we allowed for any amount of discrepancy in end date provided that other rules were met. This resulted in two possible definitions of NOAF: one for use in the primary analysis and an alternative approach described above for use in the sensitivity analysis. Both, however, reflect a cautious approach to identification of NOAF and exclude many scenarios for which we cannot distinguish NOAF from prior or subsequent AF. This has implications for the interpretation of results that will be highlighted below.
Identification of comorbidities and outcomes
Comorbidities were identified from any previously linked HES record that contained an ICD-10 diagnosis code for diabetes mellitus, hypertension, thromboembolism, valvular heart disease, dilating cardiomyopathy, pulmonary hypertension or heart failure (see Table 9). The date and cause of death were obtained from the linked ONS records. Subsequent hospital admissions were identified by linked HES records and classified as involving AF, stroke or heart failure.
Selection of matched comparators
Observational research using routine data has a fixed ‘observation window’ within which the data are collected (and within the RISK-II database, this window varies across the contributing data sets, as described in Data sources). Patients admitted to an ICU at different points in time are, therefore, followed up for different amounts of time. It is also common for the quality of routine data to vary over time and between contributors to a data set (e.g. between hospitals). To ensure that follow-up and data quality are comparable between patients with NOAF and any comparison group, we selected a cohort of comparators matched on hospital and month/year of admission to ICU.
Comparator patients were selected from all available admissions that were classified as neither NOAF nor prior AF (but including admissions for patients who subsequently developed AF). To ensure that patients with multiple admissions were not over-represented among comparators, one admission was selected at random from each patient’s set of candidate comparator admissions for consideration in the matching process. Matching was then performed with the largest ratio that could be supported while ensuring that at least 99% of patients with NOAF were included in comparisons.
Statistical analysis
Patient characteristics and comorbidities are reported as median with interquartile range (IQR) or as counts and percentages. To account for varying duration of follow-up of patients admitted at different points in time, outcomes were estimated using time-to-event methods with censoring of patients at the end of the relevant data set’s observation window (31 October 2020 for mortality or 30 March 2020 for hospitalisation). Mortality was estimated using the Kaplan–Meier cumulative incidence function from the date of ICU admission and, separately, from the date of hospital discharge among hospital survivors. The cumulative incidences of subsequent hospitalisation with AF, stroke and heart failure were estimated using non-parametric methods to account for the competing risk of death. 76,77 Mortality was assessed at hospital discharge, and at 90 days, 1 year, 3 years and 5 years after hospital discharge, among hospital survivors. The cumulative incidences of hospitalisation with AF, stroke and heart failure were assessed at 1, 3 and 5 years after hospital discharge, among hospital survivors.
We estimated the associations between NOAF and outcomes before and after adjustment for patient characteristics and comorbidities using multivariable regression models adjusting for age, sex and comorbidities. Odds ratios (ORs) for hospital mortality were estimated using logistic regression. Hazard ratios (HRs) for mortality after hospital discharge were estimated using Cox proportional hazard regression. For subsequent hospitalisation with AF, stroke and heart failure, we estimated unadjusted and adjusted cause-specific hazard ratios (CHRs),78,79 censoring patients at death or the limit of follow-up. The proportional hazard assumption was tested by visual inspection of Schoenfeld residual plots. All covariates were modelled using dummy variables except for age, which was modelled continuously using a restricted cubic spline. Knot positions for the restricted cubic spline were selected in in accordance with the recommendations of Harrell. 80
Finally, results for the primary analysis were compared with a sensitivity analysis that employed an alternative operational definition for NOAF, as detailed in Identification of new-onset atrial fibrillation.
Database analysis part 1: results
Data linkage and matching
The selection of records is summarised in Figure 2. Between 1 April 2009 and 31 March 2016, there were 965,576 admissions to 248 ICUs participating in the CMP, with links available to HES and ONS. After combining multiple records representing transfers and readmissions, a total of 919,801 distinct ICU admissions were extracted. Of these, 841,005 ICU admissions met the inclusion criteria. Of 8203 records identified as NOAF or possible NOAF, 8145 were matched to 48,870 comparators. A total of 4615 (56.7%) patients with NOAF and 27,690 matched comparators were included in the primary analysis.
FIGURE 2.
Record selection and matching.
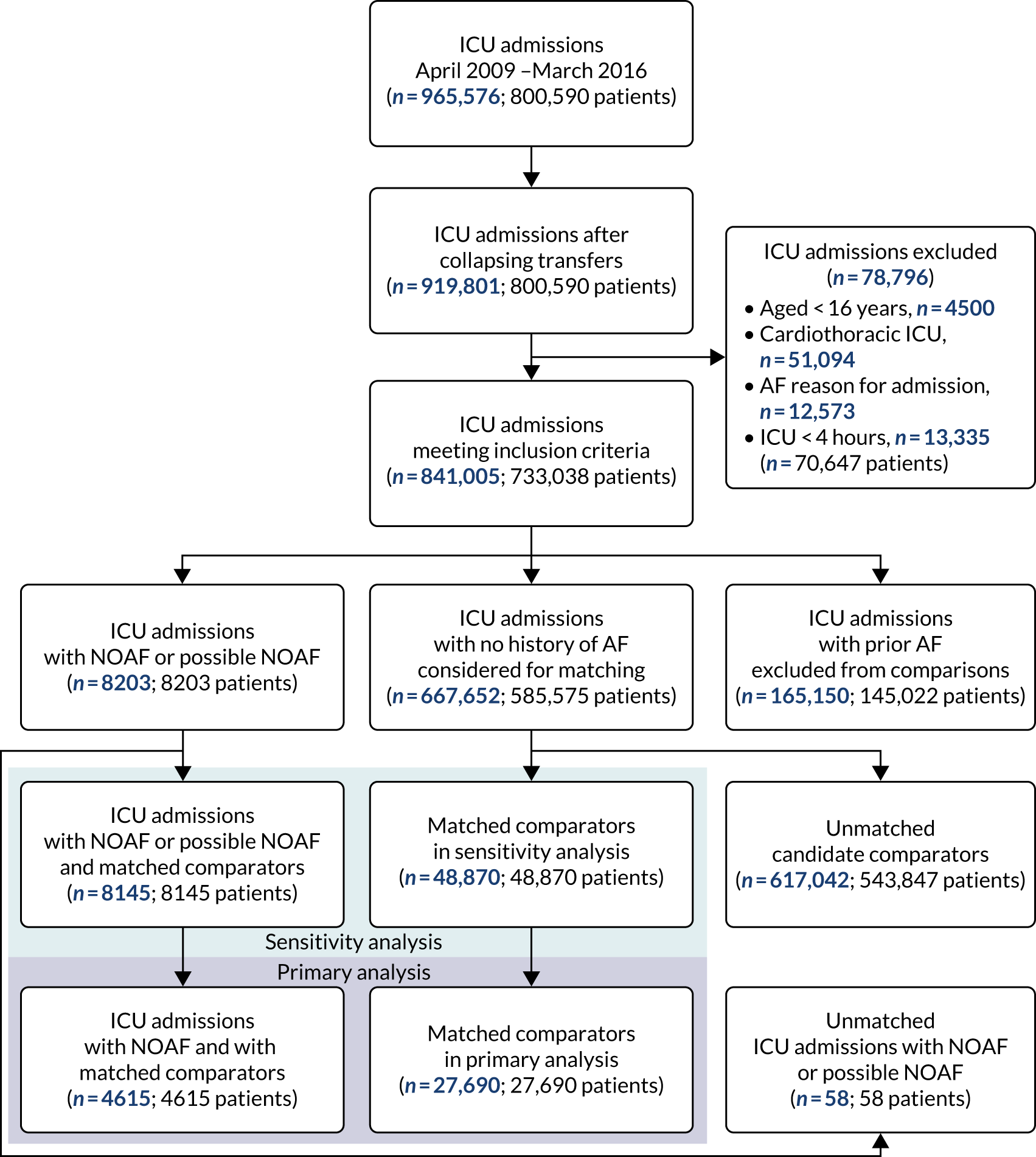
How common is new-onset atrial fibrillation in critical care?
Of the 841,005 critical care admissions examined, 4615 (0.6%) admissions had a linked HES record indicating likely NOAF. A further 3548 (0.4%) admissions had a linked HES record indicating possible NOAF but where the HES record continued for > 1 day beyond the CMP record (the latter were included in the sensitivity analysis). Although the prevalence of NOAF using either definition appeared stable over time, the prevalence of prior AF (n = 165,150, 19.6%) increased over the first 5 years of the observation window (Figure 3). A reduction in prior AF in the final year of the data partly reflects the unavailability of procedure codes for identifying atrial ablation and pacemaker insertion in that year (a structural limitation of the database, which did not appear to affect the identification of NOAF).
FIGURE 3.
Prevalence of AF in the RISK-II database.
What are the typical characteristics of patients with new-onset atrial fibrillation in critical care and how to do these compare with other patients in critical care?
Patient characteristics and comorbidities are summarised in Table 11. Patients with NOAF tended to be older and have higher levels of comorbidity, especially hypertension, heart failure and valvular heart disease, than comparator patients without NOAF.
| Characteristic | NOAF patients (N = 4615) | Comparator patients (N = 27,690) |
|---|---|---|
| Demographics | ||
| Age (years), mean (SD) | 71.5 (11.3) | 59.1 (17.8) |
| Sex (male), n (%) | 2646 (57.3) | 15,008 (54.2) |
| Ethnicity, n (%) | ||
| White | 4332 (93.9) | 25,157 (90.9) |
| Mixed | 7 (0.2) | 113 (0.4) |
| Asian | 86 (1.9) | 854 (3.1) |
| Black | 48 (1.0) | 564 (2.0) |
| Other | 35 (0.8) | 294 (1.1) |
| Not stated | 107 (2.3) | 708 (2.6) |
| Comorbidities, n (%) | ||
| Hypertension | 3050 (66.1) | 13,056 (47.2) |
| Heart failure | 1146 (24.8) | 2791 (10.1) |
| Diabetes mellitus | 1085 (23.5) | 5691 (20.6) |
| Valvular heart disease | 578 (12.5) | 1720 (6.2) |
| Prior thromboembolism | 418 (9.1) | 1715 (6.2) |
| Pulmonary hypertension | 121 (2.6) | 322 (1.2) |
| Dilated cardiomyopathy | 30 (0.7) | 141 (0.5) |
What are the outcomes for patients with new-onset atrial fibrillation in critical care and how do these compare with those for other patients in critical care?
The outcomes are summarised in Table 12 and are illustrated in Figures 4 and 5. Patients with NOAF were more likely to die, both during their hospital admission and after discharge, than comparator patients without NOAF. They were also more likely to be subsequently admitted to hospital with AF, stroke or heart failure.
| Outcome | Cumulative incidence of event (95% CI) (%) | |
|---|---|---|
| NOAF patients (N = 4615) | Comparator patients (N = 27,690) | |
| Mortality | ||
| During hospital admission, n (%) | 2000 (43.9) | 5367 (19.5) |
| Time after hospital discharge | ||
| 90 days | 8.4 (7.4 to 9.5) | 4.1 (3.9 to 4.4) |
| 1 year | 17.4 (15.9 to 18.8) | 10.6 (10.6 to 11.4) |
| 3 years | 31.8 (30.0 to 33.7) | 22.2 (21.7 to 22.8) |
| 5 years | 44.0 (42.0 to 46.2) | 30.0 (29.3 to 30.6) |
| Subsequent hospital admission for | ||
| Atrial fibrillation | ||
| 1 yeara | 25.9 (24.1 to 27.7) | 2.3 (2.1 to 2.6) |
| 3 yearsa | 36.8 (34.6 to 38.9) | 4.9 (4.6 to 5.3) |
| 5 yearsa | 42.7 (40.2 to 45.2) | 7.0 (6.6 to 7.5) |
| Stroke | ||
| 1 yeara | 1.5 (1.1 to 2.1) | 0.6 (0.5 to 0.7) |
| 3 yearsa | 2.7 (2.0 to 3.5) | 1.3 (1.2 to 1.6) |
| 5 yearsa | 4.2 (3.2 to 5.6) | 1.9 (1.7 to 2.2) |
| Heart failure | ||
| 1 yeara | 10.6 (9.4 to 11.9) | 4.1 (3.8 to 4.4) |
| 3 yearsa | 16.5 (14.9 to 18.2) | 7.2 (6.8 to 7.6) |
| 5 yearsa | 20.1 (18.8 to 23.0) | 9.3 (8.9 to 9.8) |
FIGURE 4.
Cumulative incidence of mortality from ICU admission, estimated using the Kaplan–Meier method.
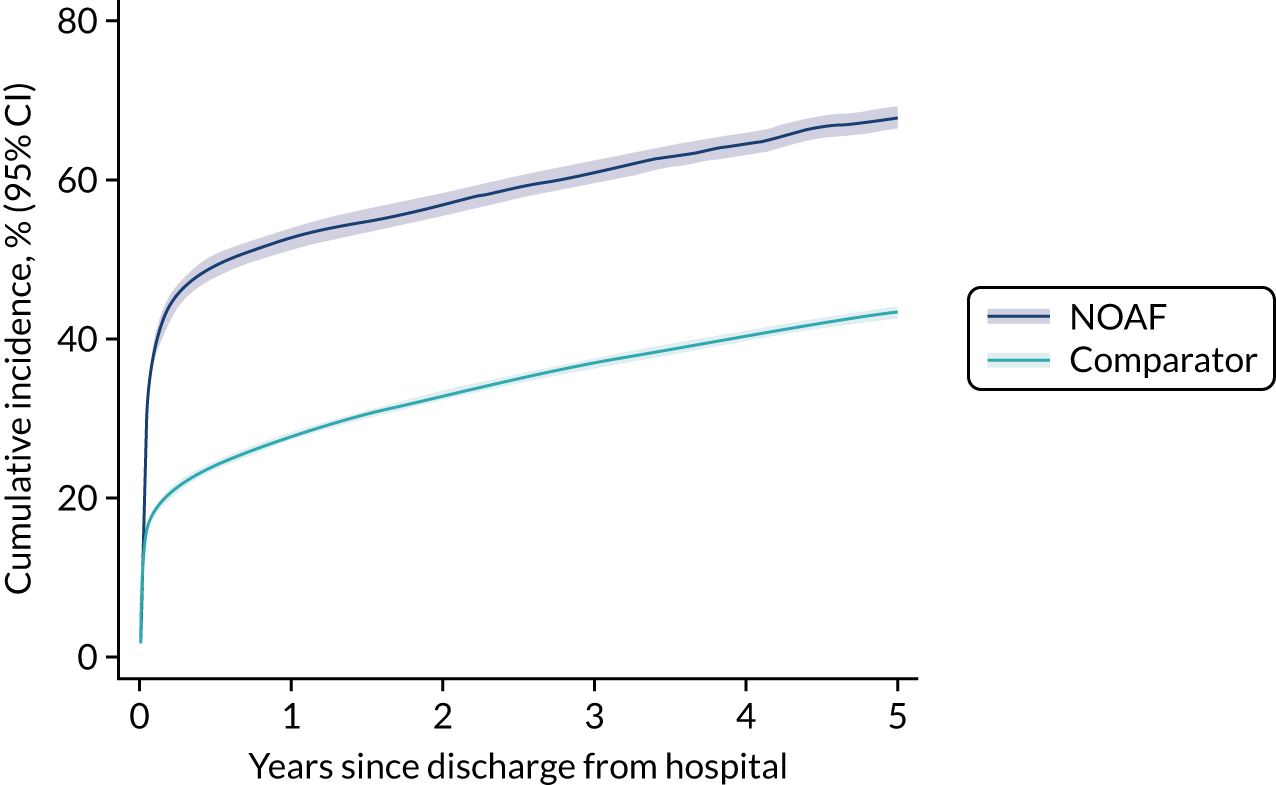
FIGURE 5.
Cumulative incidence of mortality and hospitalisation after hospital discharge. (a) Mortality after discharge; (b) hospitalisation with AF; (c) hospitalisation with stroke; (d) hospitalisation with heart failure. Cumulative incidence of mortality estimated using the Kaplan–Meier approach. The cumulative incidences of hospital admission with AF, stroke and heart failure estimated using non-parametric methods to account for competing risk of death.
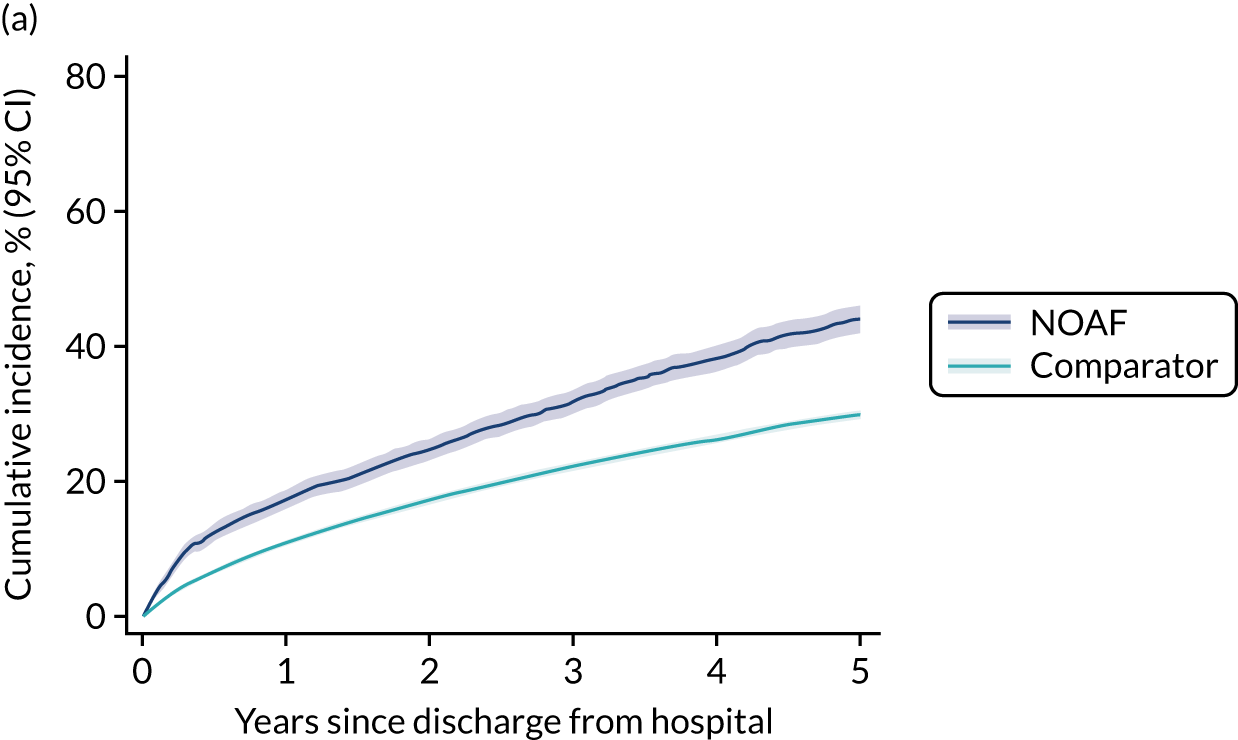
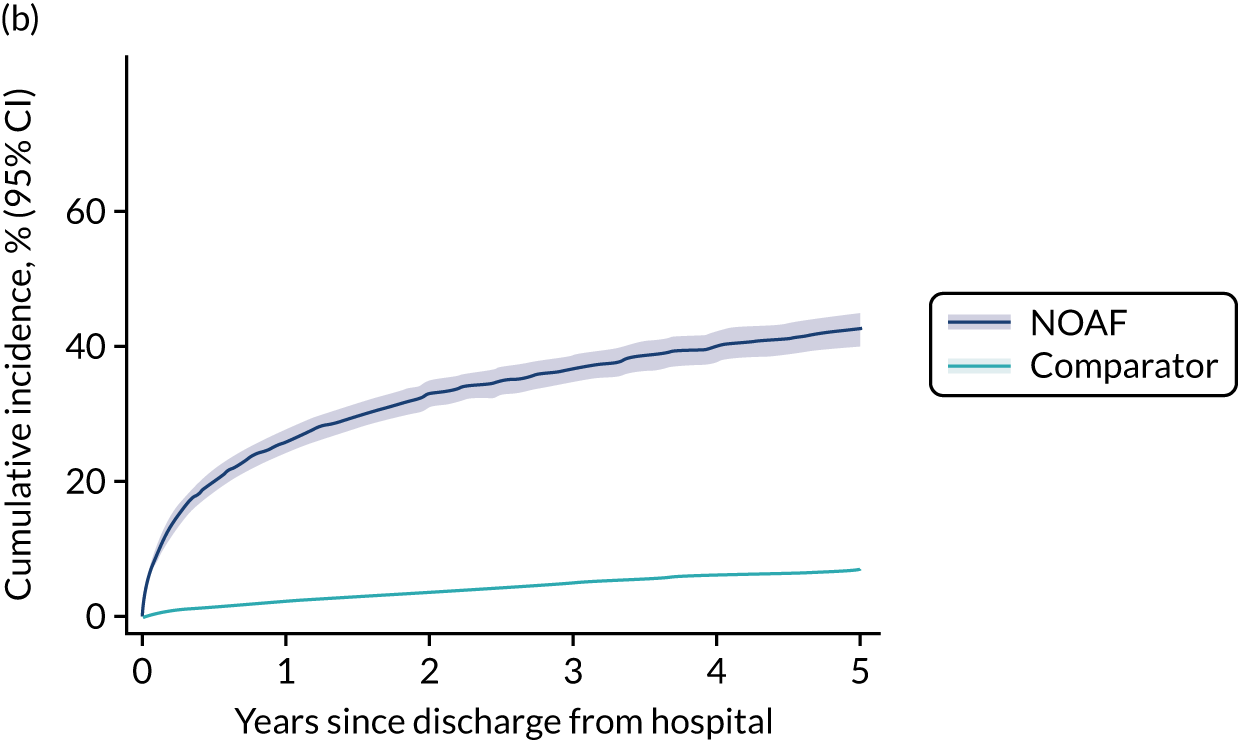
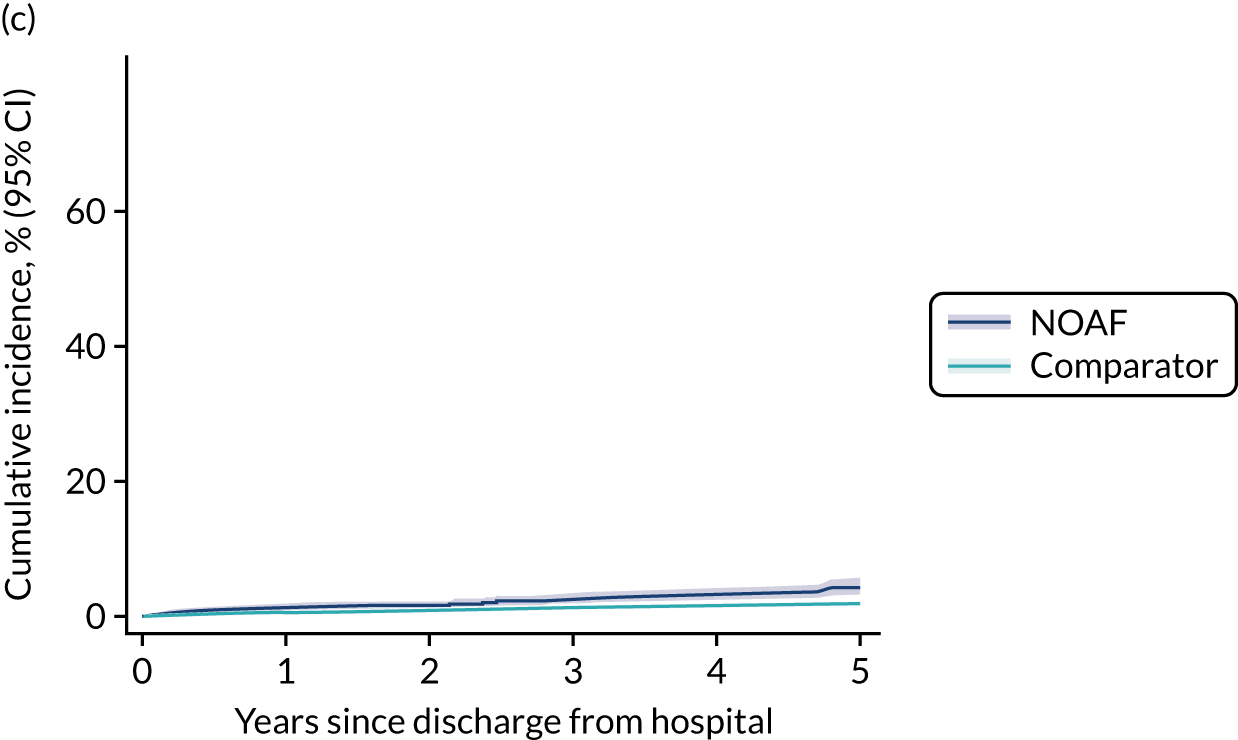
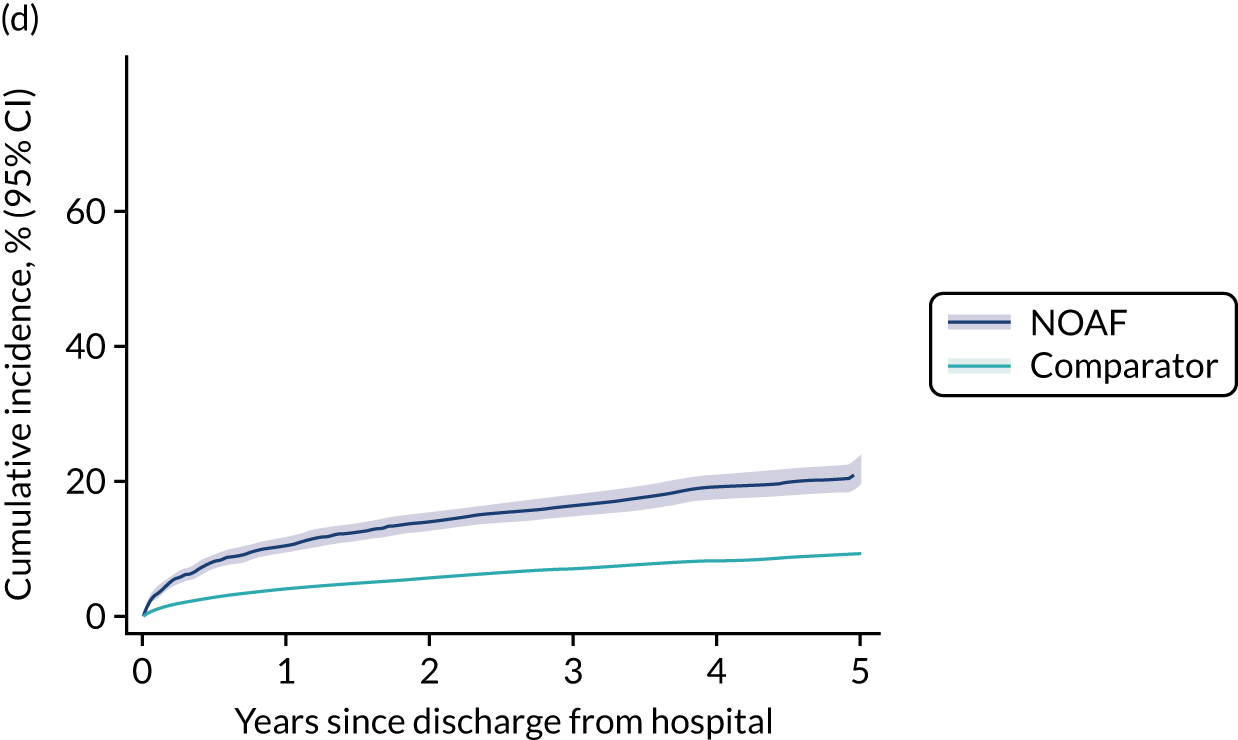
How much of the difference in outcomes is explained by differences in patient characteristics and comorbidities?
Adjusted outcomes are summarised in Table 13, with model coefficients provided in Appendix 5, Tables 20 and 21. The excess risk of hospital mortality reduced by about half when controlling for differences in patient characteristics and comorbidities (OR 3.22, 95% CI 3.02 to 3.44, before adjustment, reducing to OR 2.32, 95% CI 2.16 to 2.48, after adjustment).
| Outcome | NOAF group (N = 4615) | Comparator group (N = 27,690) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Mortality during hospital admission | 2000 | 5367 | 3.22 (3.02 to 3.44) | 2.32 (2.16 to 2.48) | ||
| Outcome | NOAF group (N = 4615) | Comparator group (N = 27,690) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Number of events | Number of person-years at risk | Number of events | Number of person-years at risk | |||
| Death 1–90 days after hospital discharge | 213 | 609 | 907 | 5400 | 2.11 (1.83 to 2.44) | 1.46 (1.26 to 1.70) |
| Death 91 days to 1 year after hospital discharge | 227 | 2250 | 1512 | 20,688 | 1.38 (1.20 to 1.59) | 0.99 (0.86 to 1.15) |
| Death > 1 year after hospital discharge | 736 | 9548 | 4675 | 96,268 | 1.66 (1.53 to 1.79) | 1.04 (0.96 to 1.12) |
| Outcome | NOAF group (N =4615) | Comparator group (N =27,690) | Unadjusted CHR (95% CI) | Adjusted CHR (95% CI) | ||
| Number of events | Number of person-years at risk | Number of events | Number of person-years at risk | |||
| Subsequent hospital admission for atrial fibrillation | 855 | 4231 | 1017 | 53,458 | 9.77 (8.91 to 10.70) | 5.86 (5.33 to 6.44) |
| Subsequent hospital admission for stroke | 68 | 5574 | 283 | 54,509 | 2.31 (1.77 to 3.02) | 1.47 (1.12 to 1.93) |
| Subsequent hospital admission for heart failure | 395 | 5087 | 1462 | 52,907 | 2.68 (2.39 to 2.99) | 1.28 (1.14 to 1.44) |
For outcomes post discharge from hospital, an examination of the Schoenfeld residuals indicated that the proportion hazards assumption was unlikely to be met for mortality or subsequent hospitalisation with AF, but was met for subsequent hospitalisation with stroke and with heart failure. In response to this, for mortality we partitioned follow-up into three time periods: 1–90 days, 90 days to 1 year and > 1 year. We then fitted separate Cox regression models for each time period (see Table 12). Results suggested that a similar proportion of the excess risk of death in the first 90 days after hospital discharge was explained by differences in patient characteristics and comorbidities as for death in hospital. After 90 days, adjustment for these factors explained all of the excess risk of death associated with NOAF (CHR ≈ 1.00, after adjustment).
The analysis of subsequent hospitalisations was complicated by the need to account for both the proportion hazards assumption and the competing risk of death. Because subsequent hospitalisation with AF exhibited the largest between-group difference, we elected to ignore the possible violation of the proportional hazards assumption and present analysis of the entire follow-up period, in keeping with the hospitalisation with stroke and heart failure. The results for hospitalisation with AF should, therefore, be interpreted as an average over the follow-up period that should not be assumed to be constant. Adjustment for patient characteristics and comorbidities indicated that about half of the excess risk of subsequent hospitalisation with each of AF, stroke and heart failure was explained by these factors.
Sensitivity analysis
For the sensitivity analysis, patients who had less-certain evidence indicating possible NOAF and their corresponding comparators were included in the analysis (n = 8145 patients with NOAF or possible NOAF and n = 48,870 comparators). Patient characteristics and comorbidities were similar between the primary and the sensitivity analysis (see Appendix 5, Table 22). However, hospital mortality fell from 43.9% among patients with NOAF in the primary analysis to 34.5% among patients using the expanded definition of NOAF in the sensitivity analysis (mortality among comparators was equivalent between the analyses) (see Appendix 5, Table 23). The results from regression models (see Appendix 5, Table 24) were consistent with the primary analysis in terms of the proportion explained by patient characteristics and comorbidities. There remained a small but statistically significant impact of NOAF on mortality > 1 year after hospital discharge; however, the CI overlapped with the equivalent interval from the primary analysis. Sensitivity analysis outcomes are illustrated in Appendix 5, Figures 11 and 12.
Chapter 4 Database analysis part 2: intensive care unit databases
Parts of this chapter are adapted with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The analysis of the RISK-II database shows that patients who develop NOAF during an ICU admission are at a significantly increased risk of mortality and hospital readmission with AF, heart failure and stroke. This highlights the importance of optimal management of this common problem.
Our scoping review has shown that the existing evidence for the best management of NOAF acquired in an ICU is limited. Common concerns included small sample sizes and inadequate adjustment for differences between treatment groups.
We, therefore, aimed to compare the clinical effectiveness of different NOAF treatments by analysing two large ICU databases after performing comprehensive adjustments for measured confounding.
Database analysis part 2: methods
Study design
We carried out a retrospective observational study of two large ICU databases from the UK (PICRAM82) and the USA [Medical Information Mart for Intensive Care III (MIMIC-III) v1.483].
The PICRAM database comprises data relating to > 12,000 patients who were treated in three general ICUs in the UK from 2008 to 2015. MIMIC-III comprises data relating to > 40,000 patients who were admitted to critical care units at a tertiary care hospital in the USA between 2001 and 2012.
All analyses were performed on each database individually given the potential for differences in case mix and interventions. Combined analyses were performed for each outcome to confirm findings. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 84
Study population
We included all adult (aged ≥ 16 years) patients. For patients who were admitted more than once to an ICU, we used their first admission. We excluded patients:
-
cared for by a coronary care or cardiac surgery team
-
with missing hospital outcome data
-
with an ICU length of stay of < 24 hours85
-
with significant arrhythmia in the first 3 hours of arrival to an ICU
-
with pre-existing arrhythmias.
We defined patients as having a pre-existing arrhythmia if an arrhythmia or a medication prescribed with an indication of heart rhythm management were identified in the patient’s medical history.
Exposure and outcomes
For all ICUs that were included in the databases, routine practice was to display three lead electrocardiograms continuously, with heart rhythm recorded by the bedside nurse at regular intervals. We defined NOAF as the documentation of AF or atrial flutter lasting for ≥ 30 seconds. 30 A documented heart rhythm was assumed to persist until the next identifiable rhythm was recorded.
The availability of data relating to the interventions of interest was assessed in the MIMIC-III and PICRAM databases. The MIMIC-III database allowed four interventions to be compared: i.v. amiodarone, i.v. beta-blockers, i.v. calcium channel blockers and electrical cardioversion. The PICRAM database allowed three interventions to be compared: i.v. amiodarone, i.v. beta-blockers and i.v. digoxin. We analysed each intervention in an intention-to-treat fashion, in which treatment groups were determined by first treatment after NOAF onset. 86
Primary outcomes
The primary end points of this study were ICU mortality, hospital mortality, rate control and rhythm control, which were analysed as time-to-event outcomes. We censored rate and rhythm control at 24 hours and censored mortality at 30 days. In the absence of a consensus definition of treatment success for ICU-acquired NOAF, we defined time to cardioversion with our expert panel as the time to first reversion of sinus rhythm42 and we defined time to rate control as the time to a heart rate of < 110 b.p.m. in the subset of patients with a heart rate of ≥ 110 b.p.m. 87
Secondary outcomes
We analysed the association of NOAF with hospital mortality. We calculated the CHR to estimate the aetiological association between NOAF and hospital outcomes, considering hospital discharge as a competing risk to mortality. We adjusted for the Oxford Acute Severity of Illness Score (OASIS),88 which was limited to the first 3 hours of ICU admission to avoid confounding post-NOAF onset. The use of early scores has been shown to remain predictive of outcome. 89
We explored the haemodynamic changes (heart rate, blood pressure and vasoactive medication dose) that are associated with NOAF. We calculated the proportion of patients receiving vasoactive medications in our cohort before and after NOAF onset. We calculated the vasoactive-inotropic score90 to quantify the change in composite dose of vasoactive medications for patients already receiving vasoactive medications prior to AF onset.
Focusing on the period 6 hours pre and post NOAF, we used smooth additive quantile regression models91 to fit the 75%, 50% and 25% quantiles of the distribution of each haemodynamic variable. We excluded the haemodynamic data recorded after each patient’s first treatment for NOAF to establish a natural history. All models included a binary covariate to indicate the onset of NOAF and allowed for changes in smoothing spline post-AF onset.
We used multilevel linear models to test whether or not there were significant changes in heart rate, blood pressure and vasoactive medication dose associated with NOAF. Each model included fixed linear segmented regression terms, with a random effect per patient to account for repeated measurements.
Adjustment for confounding
We carried out a propensity score-weighted time-to-event analysis to adjust for measured confounding in the selection of patients between treatment groups. All statistical analyses were performed using R Core v4.0.2. We generated propensity score weights that were optimised to balance the covariate distributions of the treatment groups92 using the WeightIt package. 93 The confounding variables included admission variables, laboratory variables and physiological variables adjacent to NOAF onset. The list of confounding variables was generated based on the studies identified in our scoping review. This list was then reviewed and supplemented by members of the study oversight panel. The admission variables included age; sex; the OASIS88 within the first 3 hours of ICU admission; use of preadmission beta-blocker, antipsychotic or thyroid medication; severe congestive cardiac failure; chronic obstructive pulmonary disease (COPD); liver disease; and thyroid disease. The laboratory variables at NOAF onset included the most recent (to NOAF onset) plasma sodium, potassium, magnesium, creatinine and urea concentrations; white cell count; platelet count; haemoglobin concentration; and prothrombin time. The physiological/intervention variables at NOAF onset included systolic and mean blood pressure, heart rate, body temperature, presence and dose of vasoactive agent, presence of bronchodilator therapy, mechanical ventilation, renal replacement therapy and presence of central venous access.
We assessed the balance of covariates across weighted groups by tabulating group means pre and post weighting. We calculated standardised mean differences (SMDs)94 and the maximum SMD of all pairwise treatment group comparisons for each covariate.
We carried out a weighted Cox survival analysis to determine the average treatment effect of NOAF treatments on our outcomes of interest. Missing laboratory values were handled by using multiple imputation. We generated 20 imputed data sets. To account for the uncertainty in the generated propensity scores and to allow for the estimation of 95% CIs around effect estimates, we performed resampling with replacement (bootstrapping) with recalculation of propensity score weights and effect estimates with each bootstrap sample. We obtained 1000 bootstrap samples from each imputed data set. 95 The effect estimates and CIs from each imputed data set were combined using Rubin’s rules. 96
Critical Care Health Informatics Collaborative database analysis
We also analysed the Critical Care Health Informatics Collaborative (CCHIC) database. 97 This database was created with retrospectively collected detailed data from the ICU clinical information systems from four general ICUs in London and Cambridge, in the UK, from 2014 to 2018.
Of our drugs of interest, the CCHIC database contains beta-blocker data only. We, therefore, decided to use this database to analyse only the epidemiology and characteristics of NOAF to compare with our main analyses.
We used the eligibility criteria stated in Study population. However, we were unable to exclude patients with documented pre-existing arrhythmias because these data were not available in the CCHIC database. Pre-existing arrhythmia was, therefore, determined only by the presence of arrhythmia during the first 3 hours of ICU admission. Full methods are outlined in Appendix 7.
Database analysis part 2: results
Study population
The MIMIC-III database contains data from 22,684 adult index ICU admissions. Of these patients, 220 had an ICU length of stay of < 24 hours. We identified 3905 of the remaining 22,464 patients as having pre-existing AF or AF documented within the first 3 hours of their ICU admission. Of the 18,559 patients who fulfilled our inclusion criteria, 1065 (5.7%) developed NOAF during their ICU stay. Of these patients, 742 went on to receive one of the interventions of interest. Only two patients received digoxin as their initial treatment and were, therefore, excluded, leaving 740 patients for the comparative analysis. This process is displayed in Figure 6.
FIGURE 6.
Study CONSORT flow diagrams. (a) MIMIC-III database; (b) PICRAM database. Reproduced with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
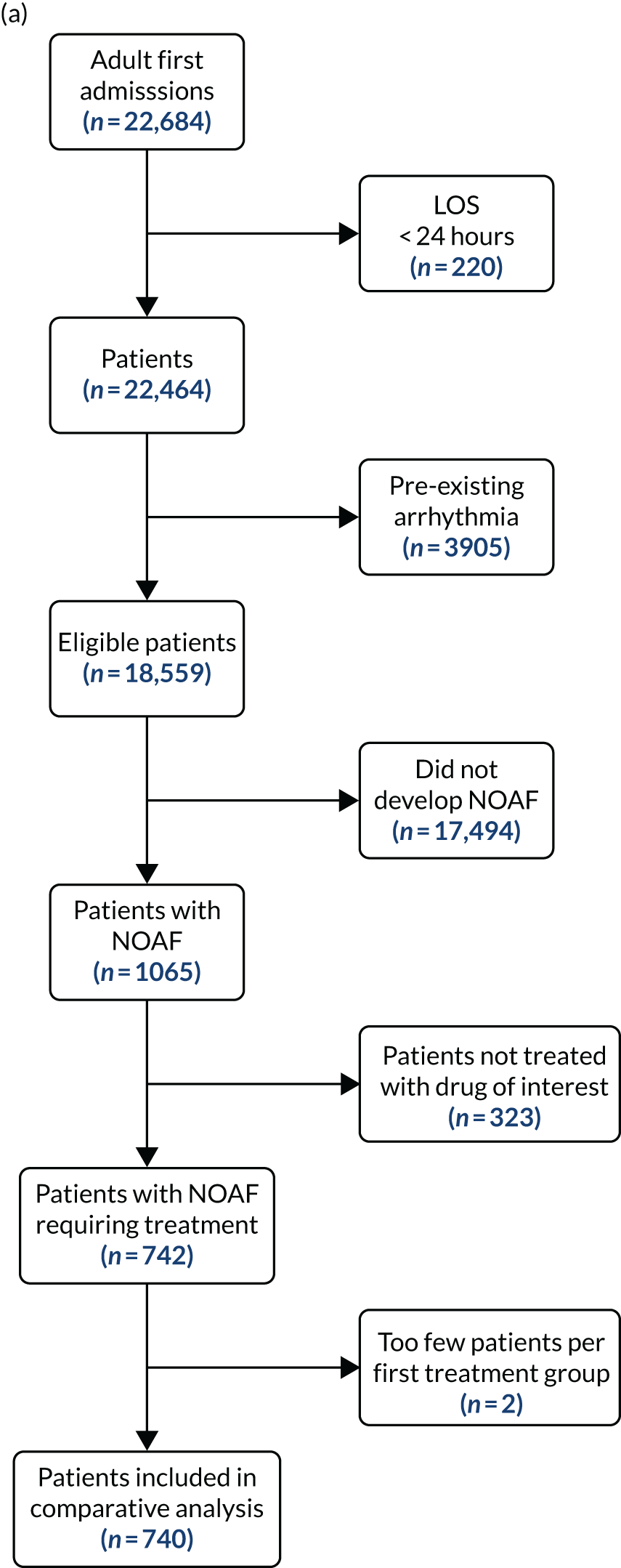
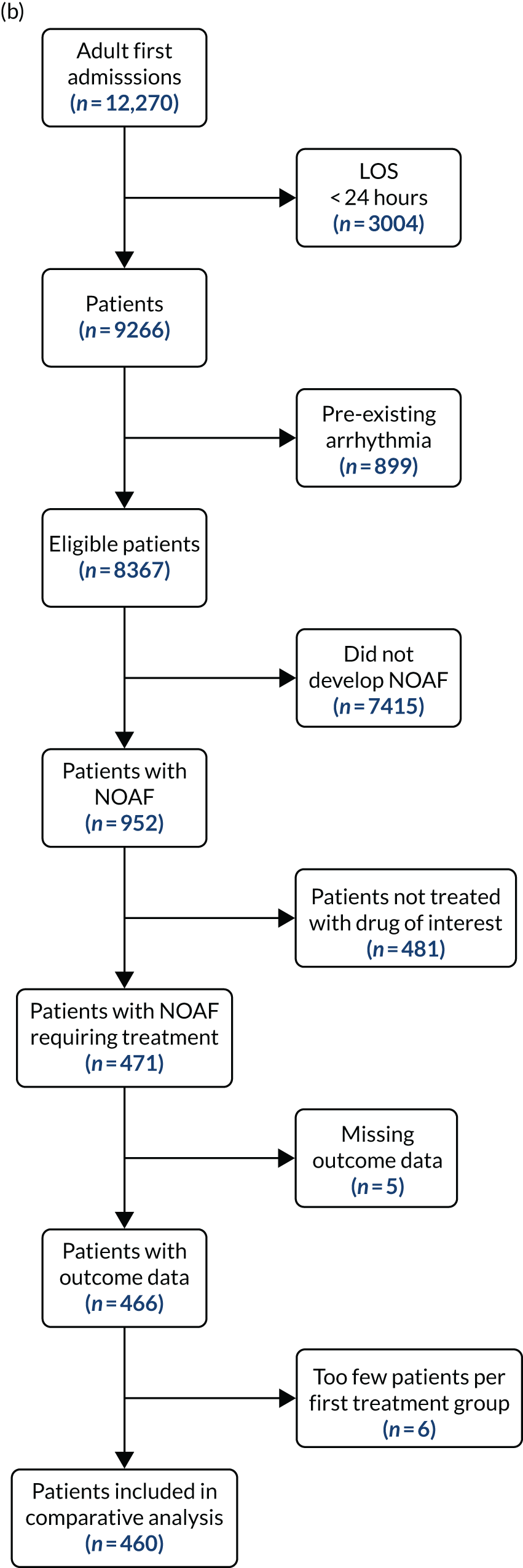
The PICRAM database contains data from 12,270 adult index ICU admissions. Of these patients, 3004 had an ICU length of stay of < 24 hours. We identified 899 of the remaining 9266 patients as having pre-existing AF or AF documented within the first 3 hours of ICU admission. Of the 8367 patients who fulfilled our inclusion criteria, 952 (11.4%) developed NOAF during their ICU stay. Of these patients, 471 went on to receive one of the interventions of interest. Five patients had missing outcome data and were, therefore, excluded from the analysis. Of those 466 patients with outcome data, only six patients received DCC or calcium channel blockers and were, therefore, excluded, leaving 460 patients for the comparative analysis. This process is displayed in Figure 6. In both databases, patients who developed NOAF were older, with a similar age difference as was identified in the RISK-II database analysis. Patients who developed NOAF also had longer ICU and hospital length of stay, and higher ICU and hospital mortality (see Appendix 6, Tables 25 and 26), than those who did not develop NOAF. The characteristics of included patients are displayed in Table 14.
| Characteristic | MIMIC-III database (N = 740) | PICRAM database (N = 460) | Overall (N = 1200) |
|---|---|---|---|
| Age (years), median (IQR) | 74 (64–82) | 70 (63–77) | 72 (64–80) |
| Sex, n (%) | |||
| Female | 372 (50) | 186 (40) | 558 (46) |
| Male | 368 (50) | 274 (60) | 642 (54) |
| COPD, n (%) | 53 (7.2) | 63 (14) | 116 (9.7) |
| Dialysis-dependent renal failure, n (%) | 1 (0.1) | 7 (1.5) | 8 (0.7) |
| NYHA class III/IV heart failure, n (%) | 0 (0) | 2 (0.4) | 2 (0.2) |
| Chronic liver disease, n (%) | 11 (1.5) | 20 (4.3) | 31 (2.6) |
| Thyroid disorder, n (%) | 33 (4.5) | 28 (6.1) | 61 (5.1) |
| Beta-blocker therapy prior to admission, n (%) | 281 (42) | 63 (14) | 344 (30) |
| Antipsychotic therapy prior to admission, n (%) | 27 (4.0) | 7 (1.5) | 34 (3.0) |
| Highest OASIS at 3 hours, median (IQR) | 36 (31–41) | 34 (26–39) | 35 (29–40) |
| Mechanical ventilation at time of NOAF, n (%) | 343 (46) | 243 (53) | 586 (49) |
| Renal replacement therapy during or < 12 hours prior to NOAF, n (%) | 47 (6.4) | 65 (14) | 112 (9.3) |
| i.v. vasoactive medication at time of NOAF, n (%) | 101 (14) | 124 (27) | 225 (19) |
| Therapeutic anticoagulation at time of NOAF, n (%) | 36 (4.9) | 48 (10) | 84 (7.0) |
| Central venous catheter at time of NOAF, n (%) | 429 (58) | 326 (71) | 755 (63) |
| Bronchodilator therapy on day of, or day preceding, NOAF, n (%) | 258 (35) | 75 (16) | 333 (28) |
| Plasma concentration, median (IQR) | |||
| Sodium (mmol/l) | 139.0 (136.0–143.0) | 137.0 (134.0–141.0) | 139.0 (136.0–142.0) |
| Potassium (mmol/l) | 4.00 (3.70–4.40) | 4.20 (3.90–4.50) | 4.00 (3.80–4.40) |
| Magnesium (mmol/l) | 0.82 (0.78–0.95) | 0.96 (0.84–1.12) | 0.86 (0.78–1.00) |
| Urea (mmol/l) | 9 (6–16) | 14 (9–20) | 11 (7–18) |
| Creatinine (µmol/l) | 97 (62–159) | 125 (78–214) | 104 (69–186) |
| White cell count (× 109/l), median (IQR) | 12 (8–16) | 11 (8–16) | 12 (8–16) |
| Haemoglobin concentration (g/l), median (IQR) | 102 (92–115) | 98 (88–113) | 101 (90–114) |
| Platelet count (× 109/l), median (IQR) | 190 (123–283) | 166 (109–234) | 181 (117–265) |
| Prothrombin time (seconds), median (IQR) | 2.65 (2.57–2.80) | 2.78 (2.71–2.94) | 2.71 (2.61–2.89) |
| Systolic blood pressure prior to AF onset (mmHg), median (IQR) | 123 (106–141) | 116 (101–133) | 120 (104–138) |
| Mean blood pressure prior to AF onset (mmHg), median (IQR) | 80 (69–91) | 77 (68–88) | 78 (69–90) |
| Heart rate prior to AF onset (b.p.m.), median (IQR) | 96 (84–112) | 115 (96–140) | 102 (87–124) |
| Treatment group (by first treatment), n (%) | |||
| Amiodarone | 94 (13) | 344 (75) | 438 (36) |
| Beta-blocker | 473 (64) | 47 (10) | 520 (43) |
| Calcium channel blocker | 144 (19) | 0 (0) | 144 (12) |
| Digoxin | 0 (0) | 69 (15) | 69 (5.8) |
| Electrical cardioversion | 29 (3.9) | 0 (0) | 29 (2.4) |
The CCHIC database included data that were related to 33,451 adult first admissions to an ICU. Of these patients, 7889 had an ICU length of stay of < 24 hours. We identified 2713 patients being paced or with another significant arrhythmia during the first 3 hours of ICU admission. Of the remaining 22,849 patients, 1003 had missing hospital mortality data. Of the remaining 21,846 eligible patients, 2618 (12%) developed NOAF (see Appendix 7, Figure 23). The characteristics of patients with and without NOAF are shown in Appendix 7, Table 34.
Characteristics of new-onset atrial fibrillation in treated patients
The time from ICU admission to the onset of NOAF in treated patients was similar between the MIMIC-III and the PICRAM databases [median 40.5 hours (IQR 21–79 hours) vs. 40.3 hours (IQR 41–75 hours), respectively]. Patients with data reported in the MIMIC-III database had, on average, shorter total durations of AF [median 11.6 hours (IQR 4–37 hours) vs. 18.1 hours (IQR 6–44 hours), respectively]. The timing of onset and AF duration data are displayed in Figures 7 and 8, respectively.
FIGURE 7.
Time from ICU admission to AF onset in treated patients. (a) MIMIC-III database; (b) PICRAM database. Data from 93 patients with time to AF onset > 168 hours (7 days) not shown.
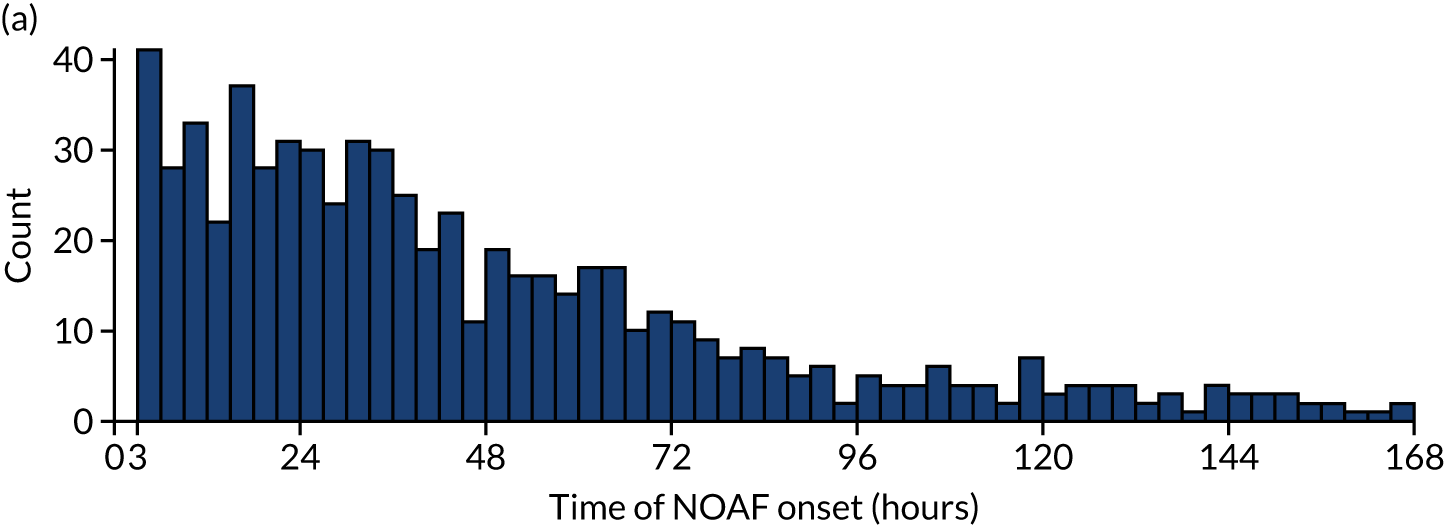
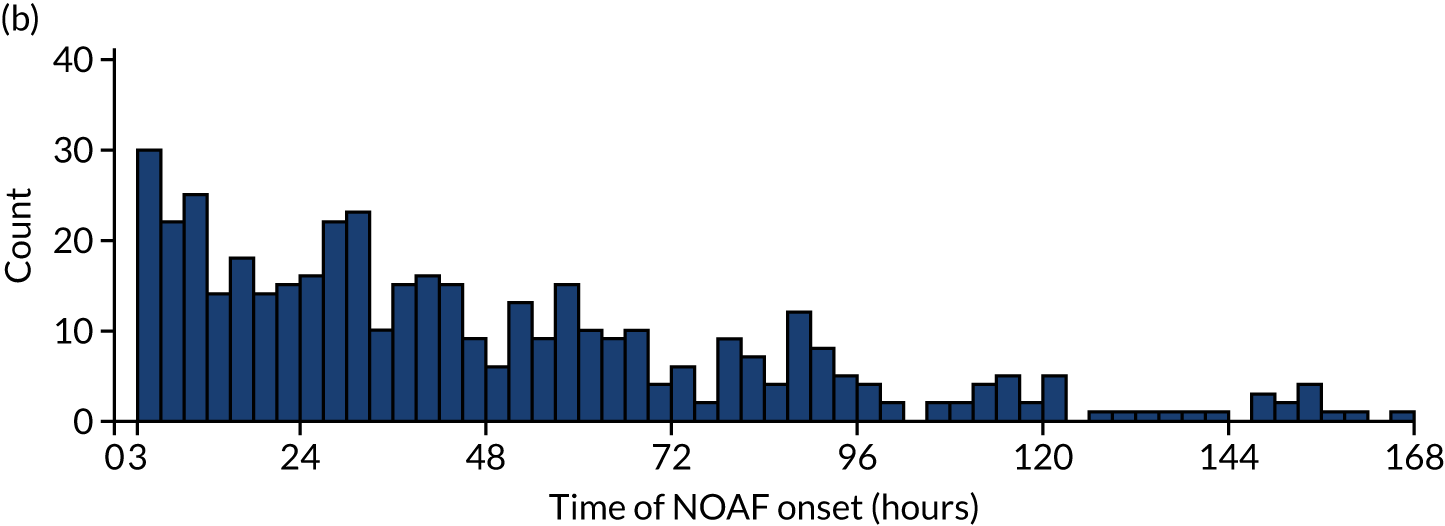
FIGURE 8.
Total duration of AF per treated patient. (a) MIMIC-III database; (b) PICRAM database. Data from 89 patients with AF duration > 120 hours (5 days) not shown.
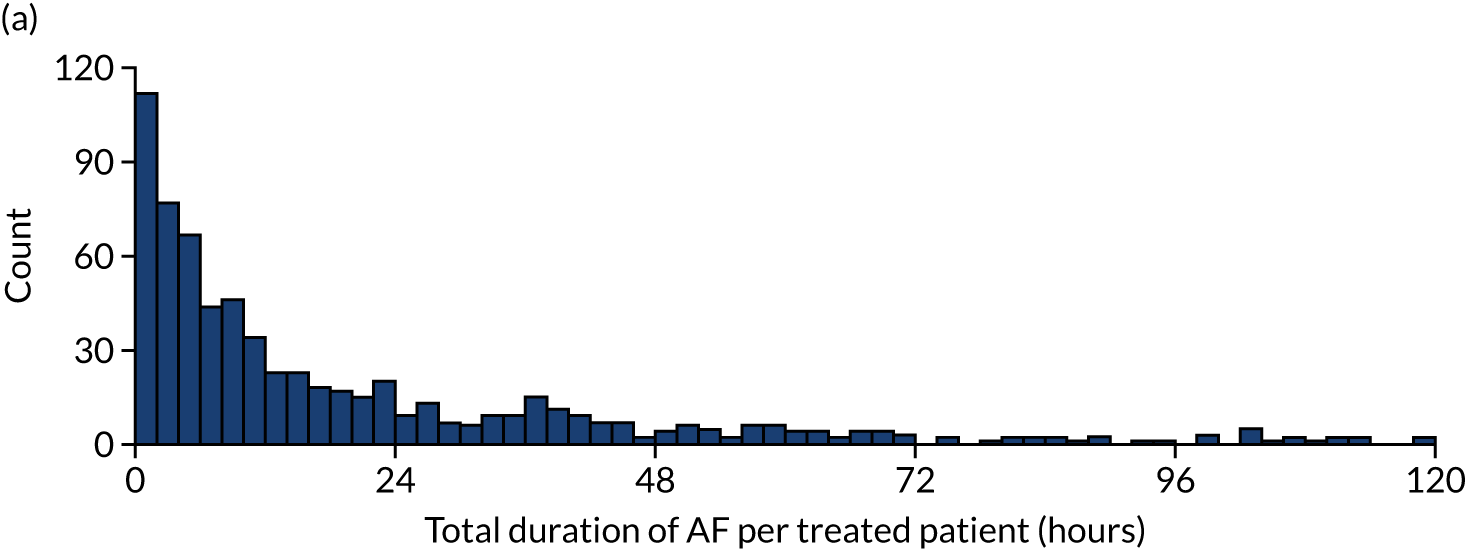
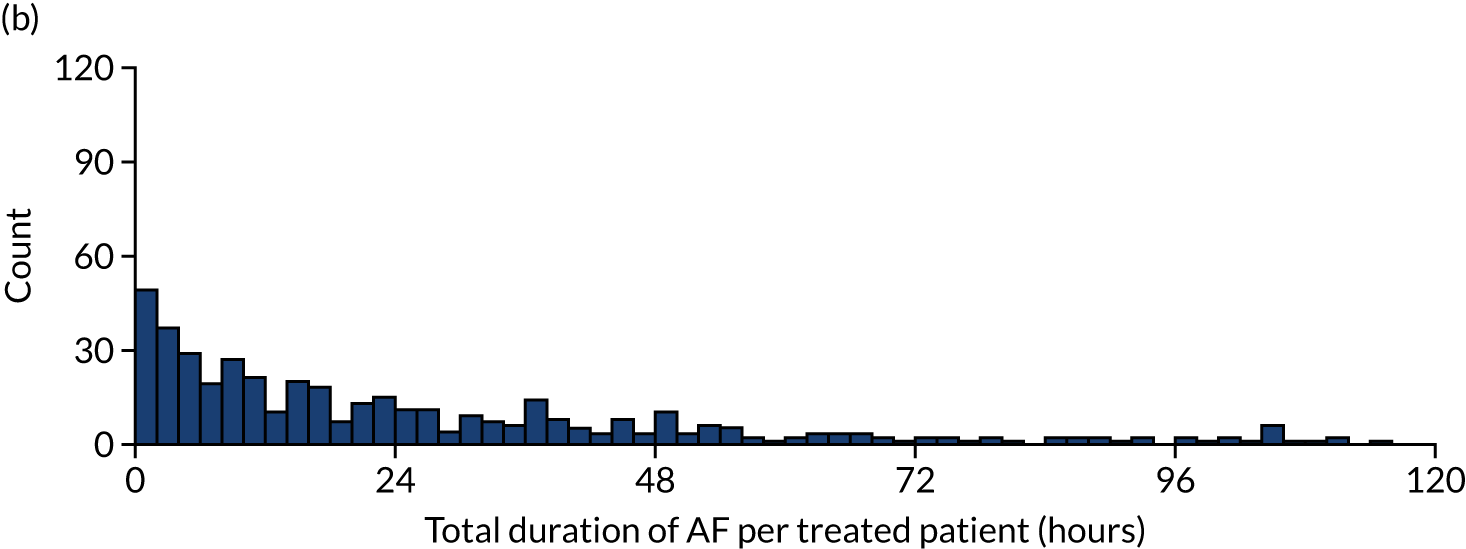
Association of new-onset atrial fibrillation with hospital mortality
In the unadjusted analysis, NOAF was associated with an almost identical increased risk of hospital mortality in the MIMIC-III and PICRAM databases [CHR 1.89 (95% CI 1.69 to 2.13) and CHR 1.89 (95% CI 1.68 to 2.13) respectively]. After adjustment for illness severity at ICU admission, this association remained evident [CHR 1.47 (95% CI 1.31 to 1.65) and CHR 1.73 (95% CI 1.54 to 1.96) respectively].
New-onset atrial fibrillation treatments
Of the patients who were identified in the MIMIC-III database, 94 received amiodarone, 473 received beta-blockers, 144 received calcium channel blockers and 29 received electrical cardioversion as their initial NOAF treatment. In the PICRAM database, 344 patients received amiodarone, 47 received beta-blockers and 69 received digoxin as their initial NOAF treatment. The characteristics of patients by treatment group are displayed in Appendix 6, Tables 27 and 28, for the MIMIC-III and PICRAM databases, respectively.
Adjustment for confounding
After propensity score weighting, covariates were well matched across all treatment groups in each database. Across 30 variables, only the mean urea concentration in the MIMIC-III database had the maximum pairwise SMD of > 0.2. Unweighted and weighted means for each treatment group can be found in Appendix 6, Tables 29 and 30.
Rate control
The MIMIC-III database
The time at which 50% of patients had achieved rate control was 60 minutes, 60 minutes, 80 minutes and 10 minutes in the amiodarone, beta-blocker, calcium channel blocker and electrical cardioversion groups, respectively. The cumulative incidence curves of rate control for each treatment group are shown in Appendix 6, Figure 13.
In the unadjusted analysis, no differences were observed between any intervention and amiodarone in the time to achieving rate control (see Appendix 6, Table 31).
After propensity score weighting, beta-blockers, calcium channel blockers and cardioversion were not associated with any significant difference in rate of achieving rate control when compared with amiodarone (HR 1.09, 95% CI 0.78 to 1.51; HR 0.81, 95% CI 0.55 to 1.19; and HR 1.59, 95% CI 0.44 to 5.75; respectively) (Figure 9; see Appendix 6, Table 31).
FIGURE 9.
Unadjusted and adjusted HRs for rate and rhythm control. (a) MIMIC-III unadjusted; (b) PICRAM unadjusted; (c) MIMIC-III adjusted; (d) PICRAM adjusted. Adapted with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
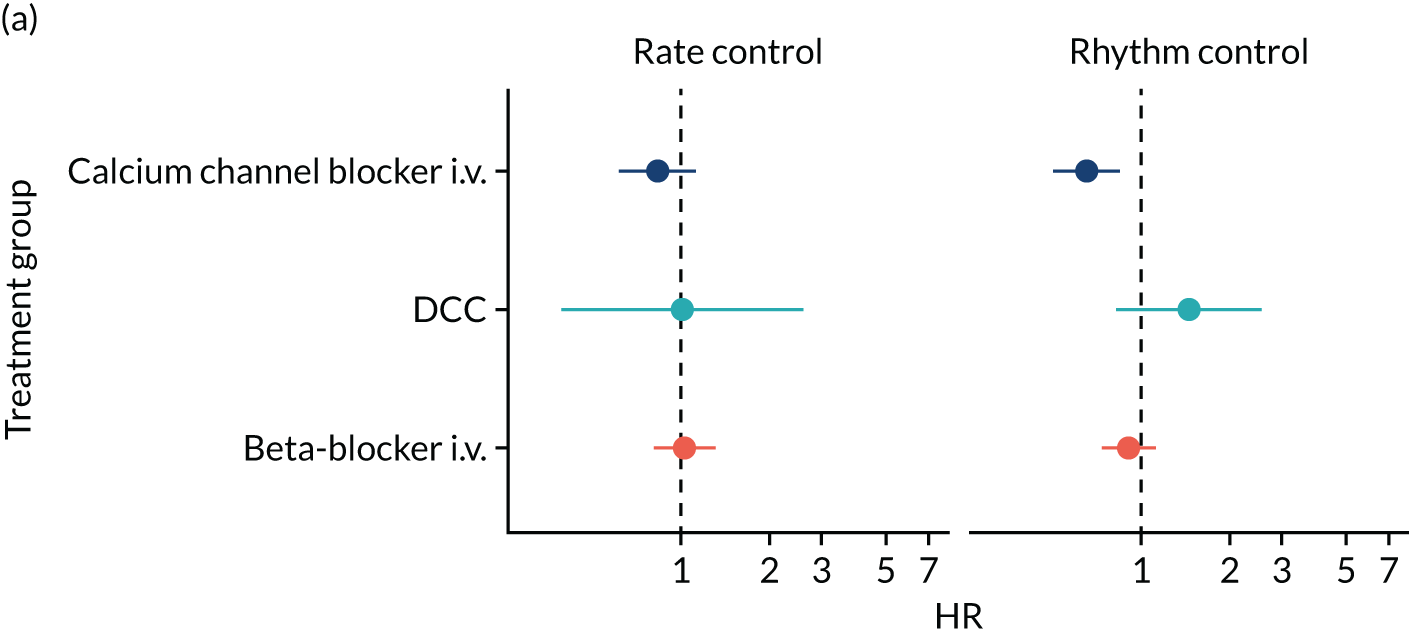
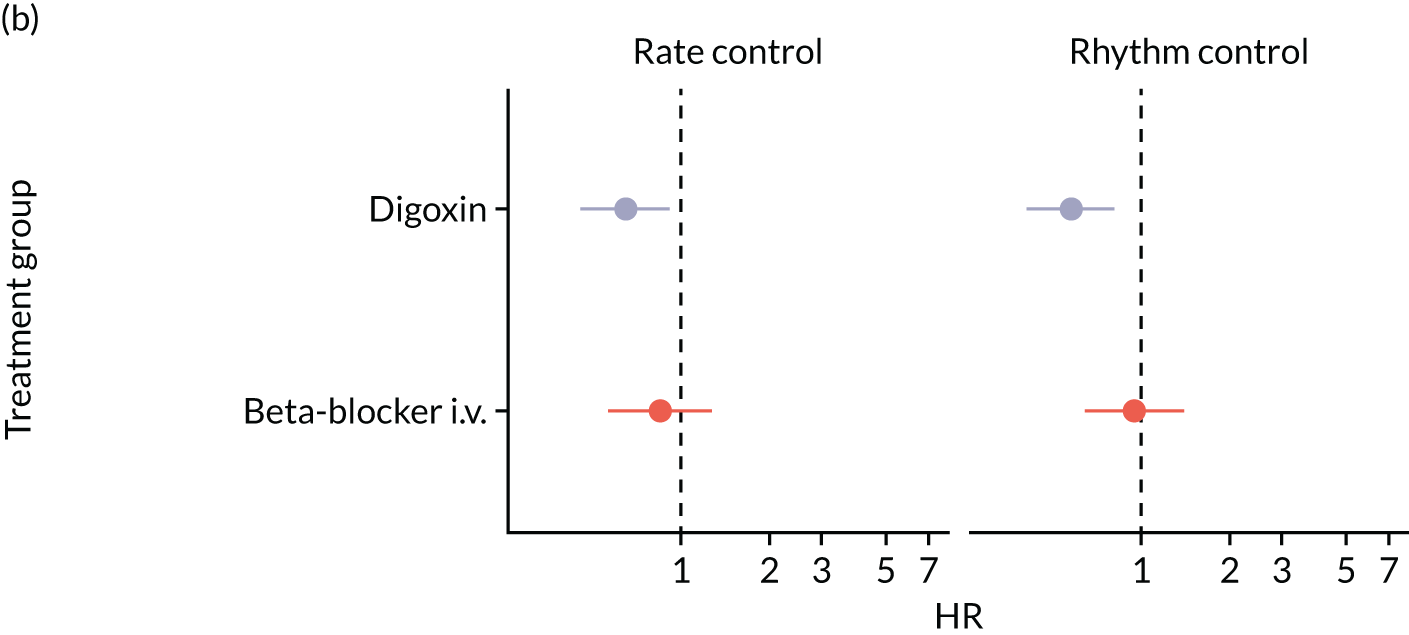
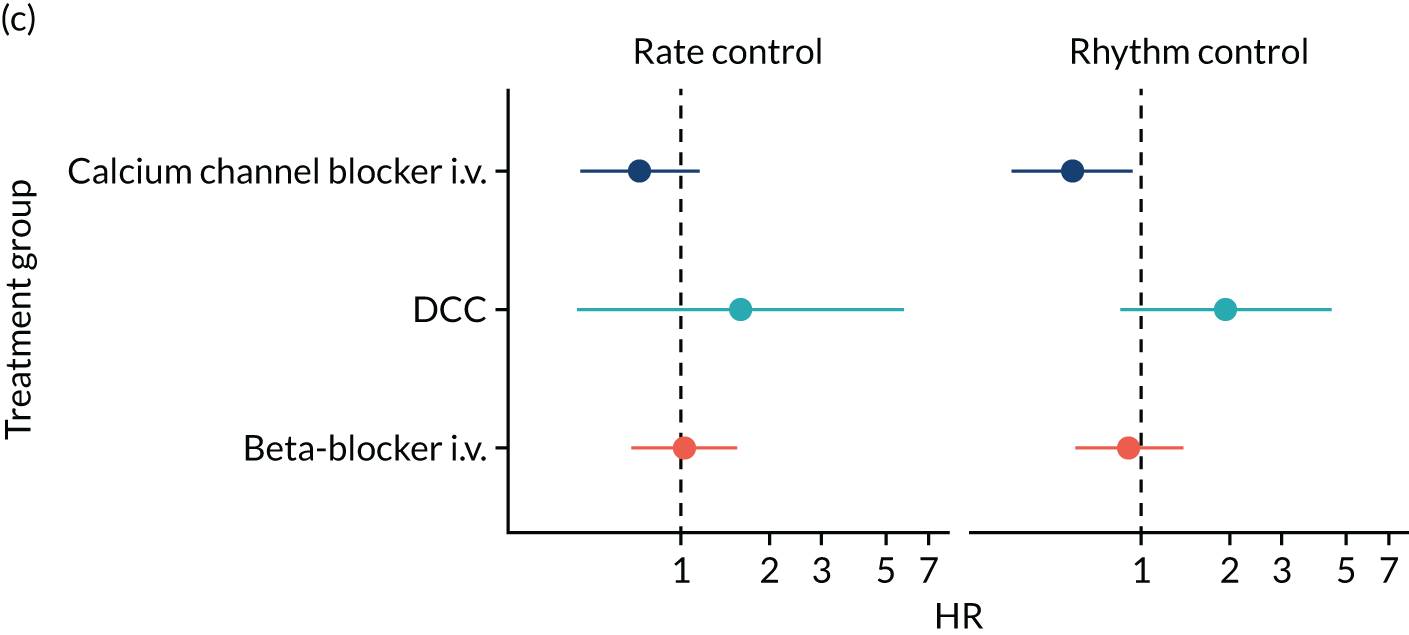
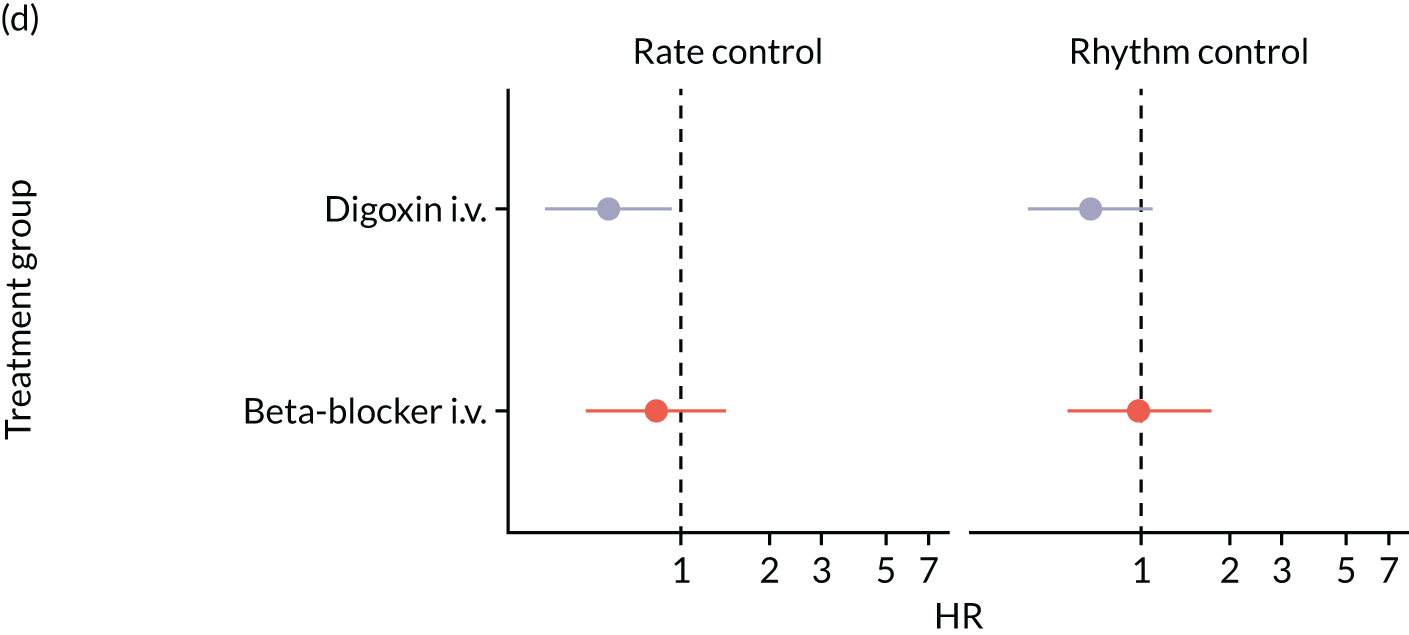
After initial rate control, reversion to a heart rate of ≥ 110 b.p.m. was common. Of those patients achieving rate control, 65%, 62%, 80% and 68% of patients in the amiodarone, beta-blocker, calcium channel blocker and electrical cardioversion groups, respectively, had at least one episode of a heart rate of ≥ 110 b.p.m. within the 24 hours after initial rate control (see Appendix 6, Figure 14).
The PICRAM database
The time at which 50% of patients had achieved rate control was 96 minutes, 115 minutes and 241 minutes in the amiodarone, beta-blocker and digoxin groups, respectively. The unadjusted cumulative incidence curves of rate control for each treatment group are displayed in Appendix 6, Figure 15.
In the unadjusted analysis, there was no evidence of a difference in achieving rate control between beta-blocker therapy and amiodarone (HR 0.85, 95% CI 0.57 to 1.27); however, digoxin appeared inferior to amiodarone in achieving rate control (HR 0.64, 95% CI 0.45 to 0.92).
After propensity score weighting, beta-blocker therapy was not associated with any significant difference in the rate of achieving rate control [adjusted hazard ratio (aHR) 0.82, 95% CI 0.48 to 1.42] compared with amiodarone. The reduced rate of achieving rate control with digoxin therapy remained evident (aHR 0.56, 95% CI 0.34 to 0.92) (see Figure 9 and Appendix 6, Table 32).
After initial rate control, reversion to a heart rate of > 110 b.p.m. was common. Of those achieving rate control, 66%, 59% and 76% of patients in the amiodarone, beta-blocker and digoxin groups, respectively, had at least one episode of a heart rate of ≥ 110 b.p.m. within the 24 hours after initial rate control. These findings are displayed in Appendix 6, Figure 16. These differences were not significant in the unadjusted or adjusted analysis.
Combined database analysis
In the unadjusted combined analysis, beta-blocker therapy was associated with a higher rate of achieving rate control (HR 1.26, 95% CI 1.10 to 1.43) and digoxin was associated with a lower rate of achieving rate control (HR 0.69, 95% CI 0.52 to 0.91) than amiodarone. After adjustment, we found no evidence of a difference between beta-blocker therapy and amiodarone in the rate of achieving rate control (aHR 1.14, 95% CI 0.91 to 1.44). Consistent with the PICRAM database analysis, we found that digoxin therapy was associated with a lower rate of achieving rate control than amiodarone (aHR 0.52, 95% CI 0.32 to 0.86) (see Appendix 6, Table 33).
In the unadjusted combined analysis, calcium channel blockers were associated with an increased rate of reversion to a heart rate of ≥ 110 b.p.m. in those patients who initially achieved rate control (HR 1.62, 95% CI 1.28 to 2.06). This finding bordered on significance after adjustment (HR 1.54, 95% CI 1.00 to 2.37).
Rhythm control
The MIMIC-III database
The time at which 50% of patients had achieved rhythm control was 159 minutes, 144 minutes, 285 minutes and 40 minutes in the amiodarone, beta-blocker, calcium channel blocker and electrical cardioversion groups, respectively (see Appendix 6, Figure 17).
In the unadjusted analysis, there was no evidence of a difference in achieving rhythm control between beta-blockers or cardioversion and amiodarone. Calcium channel blocker therapy was associated with a reduced rate of achieving rhythm control (HR 0.65, 95% CI 0.50 to 0.84).
After propensity score weighting, there remained no evidence of a difference in achieving rhythm control between beta-blockers (HR 0.91, 95% CI 0.61 to 1.35) or cardioversion (HR 2.00, 95% CI 0.86 to 4.65) and amiodarone. Calcium channel blockers remained associated with a lower rate of achieving rhythm control (HR 0.59, 95% CI 0.37 to 0.92) than amiodarone (see Figure 9 and Appendix 6, Table 31).
After initial rhythm control, reversion to AF was common. Of those patients achieving rhythm control, 36%, 39%, 47% and 46% of patients in the amiodarone, beta-blocker, calcium channel blocker and electrical cardioversion groups, respectively, had at least one episode of AF within the 24 hours after initial cardioversion (see Appendix 6, Figure 18). The differences in reversion rates were not significant in the unadjusted or adjusted analysis (see Appendix 6, Table 31).
The PICRAM database
The time at which 50% of patients had achieved rhythm control was 80 minutes, 37 minutes and 255 minutes in the amiodarone, beta-blocker and digoxin groups, respectively. Cumulative incidence curves of rhythm control for each treatment group are shown in Appendix 6, Figure 19.
In the unadjusted analysis, there was no evidence of a difference in achieving rhythm control between beta-blocker therapy and amiodarone (HR 0.95, 95% CI 0.64 to 1.40); however, digoxin appeared to be inferior to amiodarone (HR 0.57, 95% CI 0.41 to 0.81).
After propensity score weighting, beta-blocker therapy was not associated with any significant difference in the rate of achieving rhythm control (HR 0.99, 95% CI 0.57 to 1.72). Digoxin was no longer significantly associated with a lower rate of achieving rhythm control (HR 0.67, 95% CI 0.41 to 1.09) (see Figure 9 and Appendix 6, Table 32).
After initial rhythm control, reversion to AF was common. Of those patients achieving rhythm control, 54%, 51% and 70% of patients in the amiodarone, beta-blocker and digoxin groups, respectively, had at least one episode of AF within the 24 hours after initial cardioversion (see Appendix 6, Figure 20). Differences in reversion rates were not significant in the unadjusted or adjusted analysis (see Appendix 6, Table 32).
Combined analysis
Consistent with the individual database analyses, the unadjusted combined analysis suggested that calcium channel blockers (HR 0.58, 95% CI 0.47 to 0.71) and digoxin (HR 0.58, 95% CI 0.41 to 0.83) were associated with a reduced rate of achieving rhythm control compared with amiodarone. Furthermore, beta-blocker therapy was also associated with a lower rate of achieving rhythm control than amiodarone (HR 0.81, 95% CI 0.71 to 0.93).
After adjustment, we found no evidence of differences between beta-blockers (aHR 0.86, 95% CI 0.67 to 1.11), digoxin (aHR 0.64, 95% CI 0.35 to 1.17) or electrical cardioversion (aHR 1.58, 95% CI 0.71 to 3.51) and amiodarone in the rate of achieving rhythm control. We found that calcium channel blocker therapy was associated with a lower rate of achieving rhythm control than amiodarone (aHR 0.56, 95% CI 0.39 to 0.79), which was consistent with our MIMIC-III database analysis (see Appendix 6, Table 33).
Consistent with the individual database analyses, there was no evidence of a difference in the rates of reversion to AF between treatments in the unadjusted or the adjusted analyses.
Hospital survival
The MIMIC-III database
In the unadjusted analysis, beta-blocker therapy was associated with a reduced hospital mortality rate (HR 0.64, 95% CI 0.44 to 0.93). Unadjusted survival curves for each treatment group in the MIMIC-III database are displayed in Appendix 6, Figure 21.
After propensity score weighting, we found no evidence of a difference between beta-blockers (aHR 1.03, 95% CI 0.53 to 2.03), calcium channel blockers (aHR 1.30, 95% CI 0.61 to 2.76) or electrical cardioversion (HR 0.96, 95% CI 0.31 to 3.01) and amiodarone in hospital mortality (see Appendix 6, Table 31).
The PICRAM database
We found no differences in hospital survival in the unadjusted analyses. Unadjusted survival curves for each treatment group in the PICRAM database are displayed in Appendix 6, Figure 22.
After propensity score weighting, there remained no differences between beta-blockers (aHR 0.75, 95% CI 0.30 to 1.84) or digoxin (aHR 1.37, 95% CI 0.75 to 2.50) and amiodarone in hospital mortality (see Appendix 6, Table 32).
Combined analysis
Consistent with our MIMIC-III database analysis, the combined unadjusted analysis suggested that beta-blockers were associated with a reduced hospital mortality rate (HR 0.78, 0.62 to 0.99). Cardioversion appeared to be associated with a significantly increased hospital mortality rate (HR 1.92, 95% CI 1.16 to 3.17). After propensity score weighting, we found no significant difference between beta-blockers (aHR 0.97, 95% CI 0.56 to 1.68), calcium channel blockers (aHR 1.21, 95% CI 0.62 to 2.39), digoxin (aHR 1.77, 95% CI 0.77 to 4.06) or cardioversion (aHR 0.87, 95% CI 0.25 to 3.00) and amiodarone in hospital mortality (see Appendix 6, Table 33).
Haemodynamic changes associated with atrial fibrillation onset
Multilevel linear modelling revealed that NOAF was associated with a significant heart rate increase of 22 b.p.m. (p < 0.001) and 19 b.p.m. (p < 0.001) in the MIMIC-III and PICRAM databases, respectively. The average heart rate after AF onset was 122 b.p.m. and 127 b.p.m., respectively.
New-onset atrial fibrillation was associated with a significant reduction in systolic blood pressure in the MIMIC-III and the PICRAM databases of 7 mmHg and 4 mmHg, respectively (p < 0.001). This was despite significant increases in the doses of vasoactive medication after NOAF onset in those receiving vasoactive medications prior to NOAF onset [vasoactive-inotropic score increase of 2.5 (p < 0.001) and 1.8 (p = 0.001), respectively]. New hypotension (systolic blood pressure of < 90 mmHg or mean blood pressure of < 65 mmHg) occurred after NOAF in 28% and 21% of patients with a systolic blood pressure of ≥ 90 mmHg or a mean blood pressure of ≥ 65 mmHg prior to AF onset, respectively. There was a non-significant increase in the proportion of patients receiving vasoactive medications after NOAF onset in the MIMIC-III database (17.6% to 20.2%; p = 0.29). This proportion was unchanged after NOAF onset in the PICRAM database (29%).
The change in heart rate, blood pressure and vasoactive medication use over time, before and after AF onset, is displayed in Figure 10.
FIGURE 10.
Haemodynamic changes associated with AF onset. (a) Heart rate, MIMIC-III database; (b) heart rate PICRAM database; (c) systolic blood pressure, MIMIC-III database; (d) systolic blood pressure, PICRAM database; (e) vasoactive-inotropic score, MIMIC-III database; (f) vasoactive-inotropic score, PICRAM database; (g) proportion of patients on vasoactive medications, MIMIC-III database; (h) proportion of patients on vasoactive medications, PICRAM database. Vasoactive-inotropic score shown for those patients receiving vasoactive medications prior to AF onset. VIS = dopamine dose (µg/kg/minute) + dobutamine dose (µg/kg/minute) + 100 × adrenaline dose (µg/kg/minute) + 10 × milrinone dose (µg/kg/minute) + 10,000 ± vasopressin dose (units/kg/minute) + 100 × noradrenaline dose (µg/kg/minute). Reproduced with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
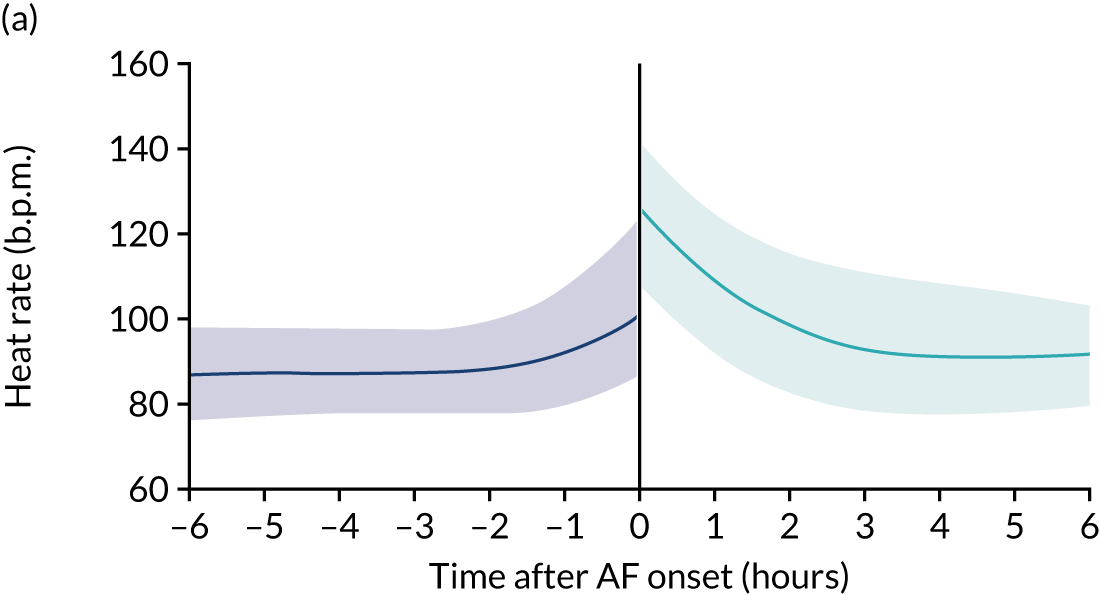
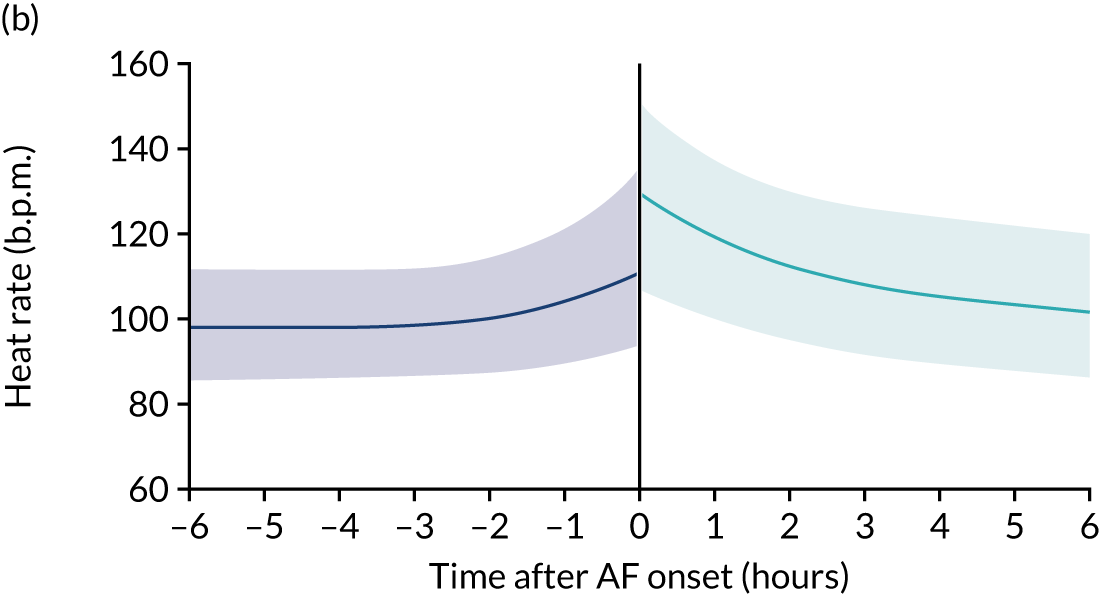
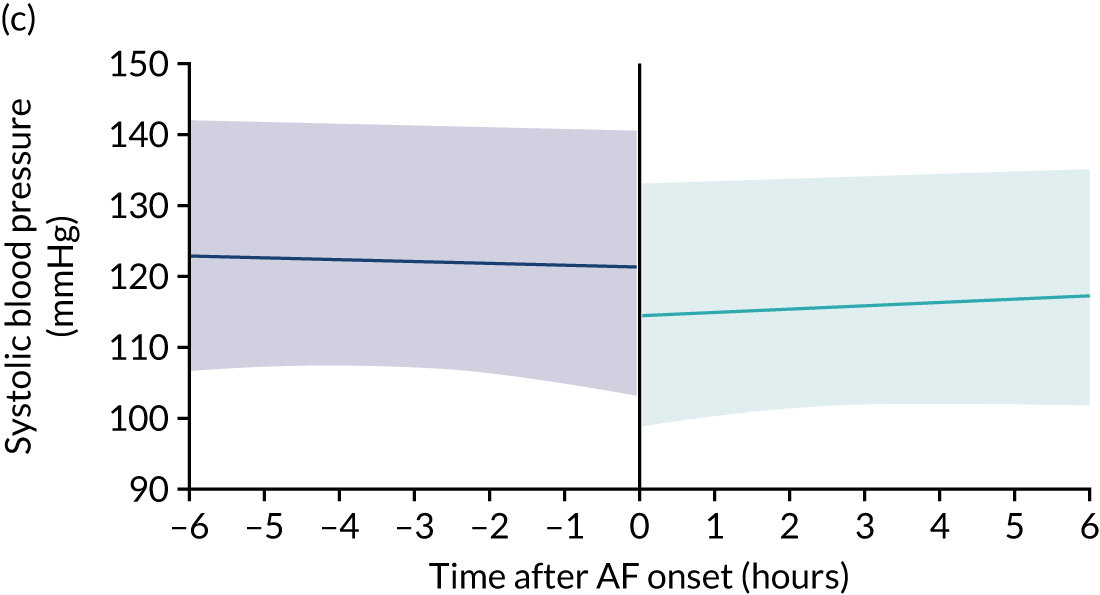
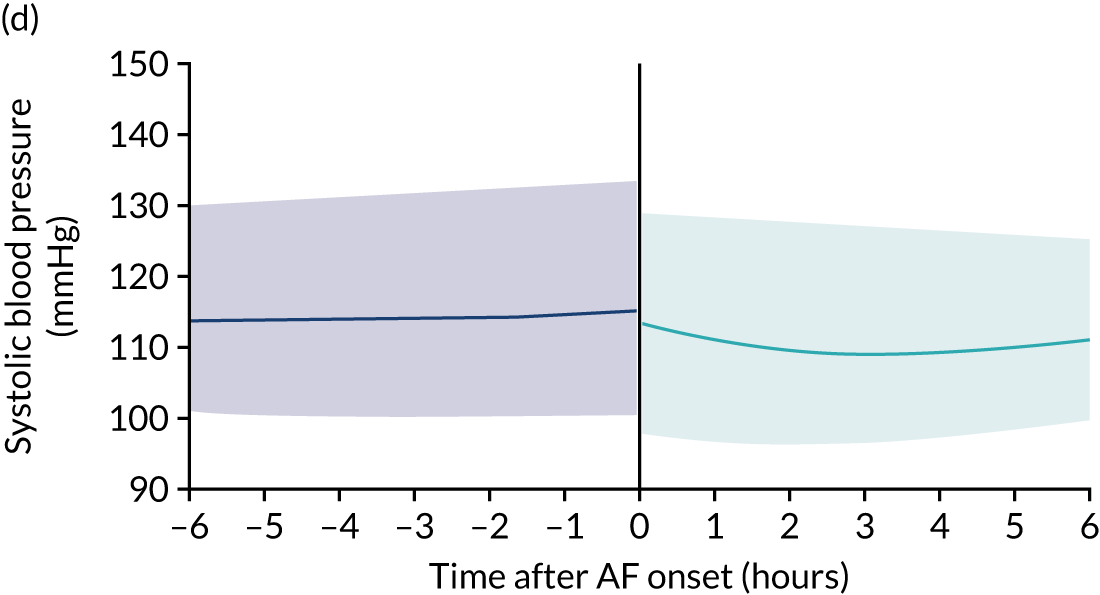
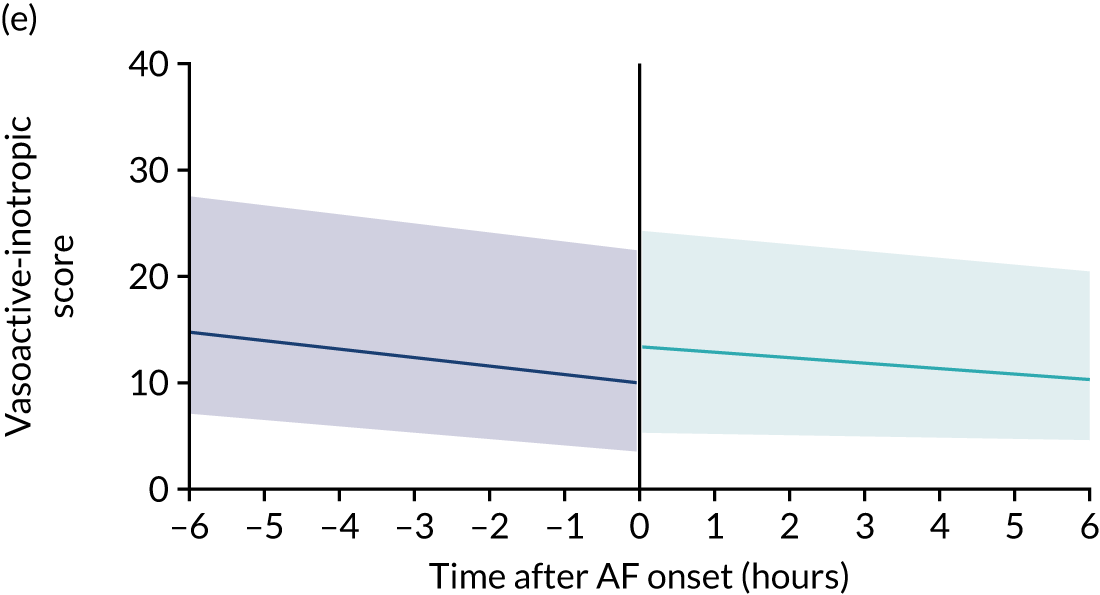
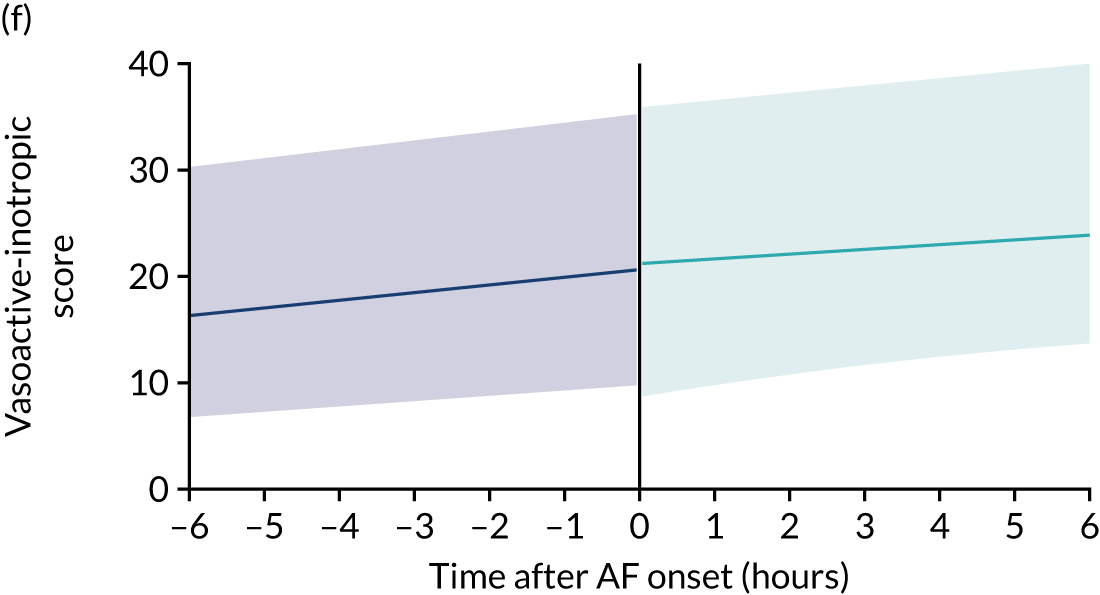
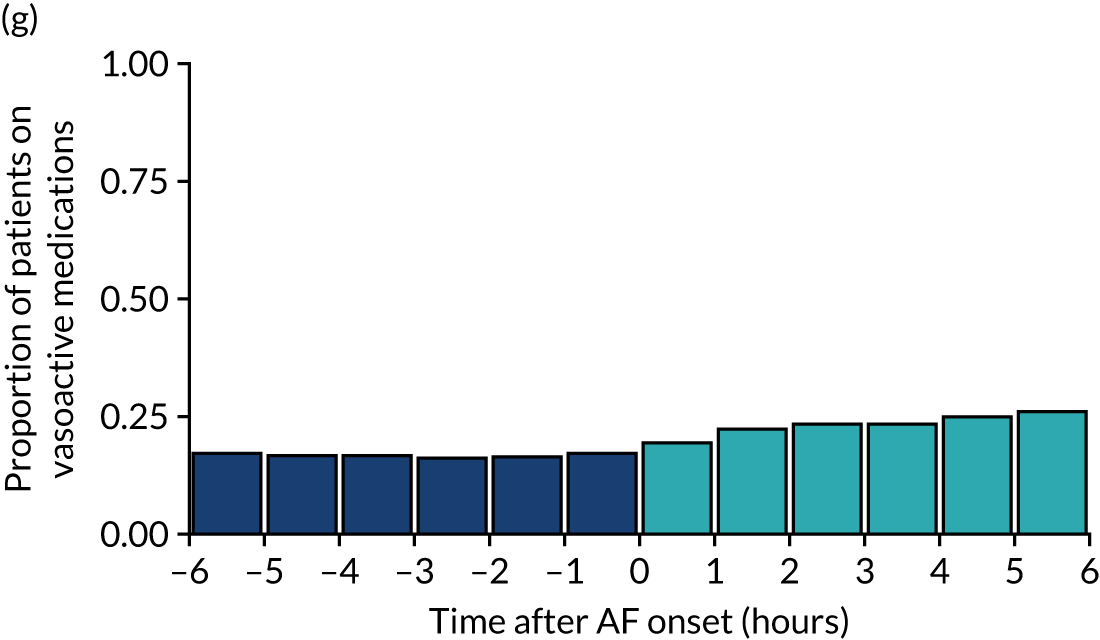
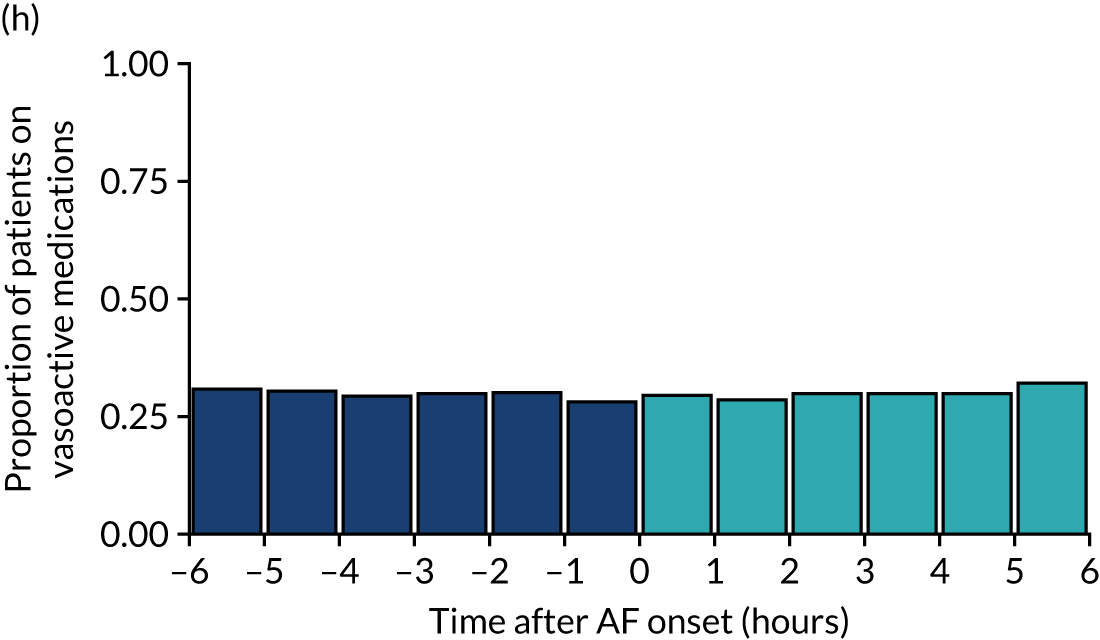
Summary
This study demonstrated that NOAF during an ICU stay is common and is associated with significant increases in heart rate, reductions in blood pressure and increases in vasoactive medication requirements. NOAF was associated with an increased rate of hospital mortality despite adjusting for variables in a validated mortality prediction model.
Beta-blocker therapy was not associated with any difference in achieving rate or rhythm control, or with any difference in hospital survival when compared with amiodarone. The hospital mortality benefit with beta-blocker therapy that was identified in the unadjusted analysis of the MIMIC-III database was no longer apparent after adjustment. This suggests that differences in survival were only because of differences in patient characteristics between treatment groups.
Digoxin therapy was associated with a lower rate of achieving rate control than amiodarone. Calcium channel blocker therapy was associated with a lower rate of achieving rhythm control than amiodarone.
Chapter 5 Expert panel
To highlight our findings, identify uncertainties and formulate research recommendations we convened an expert panel. We followed the National Institute for Health and Care Excellence Research Recommendations Process and Methods Guide. 98 Panel members are listed in Appendix 7, Table 35.
Chapter 6 Discussion
Statement of principal findings
The evidence base available for NOAF management in critically ill patients identified by our scoping review was very limited. Many studies lacked a comparator group and many of those that had a comparator group did not control for confounding factors. Only two randomised trials were identified, both of which were small and inconclusive. We identified significant heterogeneity in the definitions of both NOAF and successful treatment, making a synthesis of results difficult.
The limited evidence from our scoping review suggested that beta-blockers might be more effective than amiodarone for the conversion to sinus rhythm and mortality outcomes; however, residual bias in previous studies may explain these assertions. Whether or not and when to use anticoagulation is unknown. No conclusive findings were reported owing to the low quality of the reviewed evidence. Only one clinician survey was found, with a very low response rate. 16
Our analysis of the RISK-II database demonstrated that patients who developed NOAF in an ICU were older and had higher levels of comorbidity than those who did not. Even after controlling for these differences, patients with NOAF still had substantially higher mortality in hospital and during the first 90 days after discharge. Patients who developed NOAF in an ICU also had higher rates of subsequent hospitalisation with AF, stroke and heart failure than those who did not.
Our analysis of the detailed within-ICU MIMIC-III and PICRAM databases showed that NOAF is a common problem that occurs in 6–11% of eligible ICU patients, depending on the data source. In an ICU, NOAF is associated with a significant increase in heart rate and a significant decrease in blood pressure, despite an increase in vasoactive medication doses. Supporting our RISK-II findings, we identified a significantly increased hospital mortality rate associated with NOAF, even after adjusting for other factors that are predictive of mortality.
These findings highlight the importance of identifying optimal treatment strategies for NOAF in patients in an ICU. We found that the treatment of NOAF with digoxin or calcium channel blockers as first-line therapy is associated with poorer rate control and rhythm control, respectively. Prior to adjustment for confounding variables, we found that beta-blocker therapy was associated with improved hospital survival. We also demonstrated that patients who received beta-blockers were less unwell at admission and more stable after AF onset than those who received amiodarone. After comprehensive adjustment of these factors, there were no identifiable differences in any outcome between beta-blocker therapy and amiodarone.
Scoping review
The evidence base available for NOAF management in critically ill patients was very limited. A key problem with the studies identified in this scoping review was that many (n = 12) were single-group studies (i.e. lacking a comparator group). Of the 25 primary studies included in the review, only two were RCTs30,31 and only three of the non-randomised comparative studies27–29 attempted to control for confounding factors, which may have affected outcomes. For all of these studies, which used more robust approaches, there were still serious concerns about how bias (arising from their designs and/or analyses) might affect their results. Although the two RCTs30,31 did not find statistically significant differences in conversion rates between the treatments studied, each contained < 60 patients. Many studies27–30 concluded that more research is needed.
Heterogeneity in the treatment dose (e.g. total doses ranging from < 1 g to 8 g for amiodarone8,31,32,34,35,42,43) and administration (e.g. bolus or continuous infusion), and varying time points to assess conversion to sinus rhythm (e.g. within 2 hours,30 4 hours,31 12 hours30 and 24 hours8,32,35,36) were observed across studies. Comparing studies was, therefore, challenging. There is a need to establish optimal treatment dosing and administration regimens, as well as validated definitions of treatment success. Six studies33,34,36,37,40,48 (14%) were available only as conference abstracts. Only limited data could be extracted for these studies, making their results more difficult to interpret.
Studies varied in how they reported and defined NOAF. Different heart rate thresholds for NOAF were used. 31,36,38,43,47,49 Studies also reported different time periods for which NOAF must be sustained27,30,41,43,47,49 and for which instances AF would be considered NOAF. 8,28,29,38,39,50 Ten studies32–35,37,40,44–46,48 did not provide any definition for NOAF.
The evidence from this review34–36 suggests that beta-blockers might be more effective than amiodarone for the conversion back to normal sinus rhythm, with better outcomes for mortality reported in those who received beta-blockers than in those who received amiodarone. 28,35 A recent UK-wide survey16 found that amiodarone is the most commonly used pharmacological treatment for NOAF in UK ICUs, which suggests that these studies are not changing current practice. Calcium channel blockers appeared to be less effective than beta-blockers and amiodarone for conversion to sinus rhythm. 30,31,36 Two studies27,48 reported that hydrocortisone might be effective as a prophylactic treatment. However, a larger comparative study27 reported slightly higher (but not statistically significant) mortality associated with the use of hydrocortisone. All of the studies reporting effects of hydrocortisone had significant methodological limitations.
The evidence base for NOAF treatment strategies was reported as very limited in two systematic reviews, which agrees with our findings. 53,56 Both systematic reviews were not able to report any conclusive findings, citing the low quality of the reviewed evidence53 and methodological differences between the included studies. 56 In agreement with our findings, the review by Kanji et al. 56 concluded that a standardised outcome measure of success is needed as varying time points used to assess conversion to sinus rhythm limits recommendations on treatment efficacy. 56 Both systematic reviews emphasised the urgent need for further research studies. 53,56
The current literature29,62 suggests that it is unclear if the benefits of administering anticoagulants in critically ill patients with NOAF for stroke prevention outweigh the increased risk of bleeding. Two review articles61,64 proposed a patient-centred approach to administer anticoagulants only in patients with high risk of arterial thromboembolic events. Outside the ICU, withholding anticoagulation for a short time in the perioperative period in patients with AF undergoing elective surgery was not associated with an increased risk of arterial thromboembolism and decreased the risk of major bleeding. 99 It is, however, not known how these findings translate to critically ill patients in ICUs.
The risk assessment scores for subsequent thromboembolism following the development of NOAF have generally not been developed or validated in the ICU population. The CHA2DS2-VASc score100 has, however, once been shown to be predictive of thromboembolic event risk in the ICU setting, although with poor sensitivity and predictive value. 101 Bleeding risk scores to guide anticoagulation decisions in NOAF have been developed using population-based cohorts of patients with AF in the community. Common risk factors used in these scores include older age, renal dysfunction, hypertension and a history of bleeding. Comparative studies of these tools report varying results depending on the patient population. 102–105 Patients who develop NOAF during sepsis are at a higher risk of in-hospital and post-discharge stroke and death than those who do not, despite adjusting for confounders. 14,106 Those with NOAF are at a higher risk of thromboembolic complications than patients with pre-existing AF. 106 However, the individual risk of stroke and thromboembolic events in patients who develop NOAF during an ICU admission remains poorly understood.
Although community-based bleeding risk scores are chiefly composed of chronic comorbidities, the bleeding risk in patients in an ICU is likely to be more related to acute factors, such as illness severity, systemic inflammation, type and location of surgery, nutritional status, invasive devices, and acute coagulopathy and thrombocytopenia. 70,107–109 Bleeding risk in critically ill patients with NOAF is higher than patients in the community, with one study demonstrating that 9% of patients who received systemic anticoagulation had significant bleeding warranting cessation of anticoagulation and at least one blood transfusion. 8
A recent UK-wide survey suggested that around one-third of clinicians initiate anticoagulation in patients with NOAF during an ICU admission. 16 Once in stable AF in the community, anticoagulation can be recommended for almost all patients with AF. However, the balance of risks in patients either in an ICU or in whom AF was demonstrated only during the ICU admission is likely to be more complex and dynamic. Modified risk scores that incorporate such complexities are, therefore, required for critically ill patients with NOAF.
The RISK-II database analysis
This analysis of the RISK-II database provides clear evidence of a patient group developing NOAF in critical care with substantially worse short- and long-term outcomes than patients without any record of AF during or prior to ICU admission. We adopted a restrictive approach to ensure that patients’ CMP and HES records provided sufficient confidence in discriminating NOAF in the ICU from prior and subsequent AF. In our sensitivity analysis, we adopted a slightly broader, yet still conservative, definition of NOAF. The outcomes, although somewhat less severe, remained consistent with the primary analysis. Outcomes were substantially worse for patients with AF than for patients with no AF, even after controlling for patient characteristics and past medical history. Patients developing NOAF during an ICU admission were less likely to survive and more likely to be readmitted with AF, stroke or heart failure than those who did not develop NOAF. Comparison with other data sources suggests that our methodology identified a subset of all patients who develop NOAF in critical care. Given the inconsistent nature with which diagnoses are recorded in HES,110 the group identified as developing NOAF in this analysis might best be interpreted as representing patients for whom their AF was of sufficient clinical importance to be documented in their clinical notes (which are then used to code diagnoses in HES records).
The evidence provided by multivariable regression indicates that the impact of NOAF on mortality is not constant over time, but rather focused on the period in and immediately after discharge from hospital. Patients who developed NOAF in an ICU had increased mortality up to the limit of follow-up. However, from 90 days after hospital discharge, the increased mortality appeared to be entirely explained by their older age, sex and comorbidities. During hospital admission and the first 90 days after discharge, roughly half of the increased mortality appeared attributable to either NOAF or unobserved clinical factors that are associated with it. These findings emphasise the importance of developing strategies for both the treatment and the anticoagulation management of NOAF in the period of critical illness and its immediate aftermath, for which current evidence is lacking.
We did not break down multivariable models for subsequent hospitalisation into similar time periods, given the complexity of interpreting proportional hazards regressions with both non-proportional hazards (effects that vary over time) and competing risks. However, given that hospitalisation is closely related to mortality, it may be reasonable to assume that a similar pattern of varying effects over time would be observed.
The MIMIC-III and PICRAM database analysis
To the best of our knowledge, this is the first study to quantify the haemodynamic changes associated with NOAF onset in ICU patients accounting for patients’ pre-AF parameters at scale. We found that NOAF during an ICU admission is associated with a significant increase in heart rate and a significant decrease in blood pressure, despite an increase in vasoactive medication doses. Organised atrial activity contributes to ventricular filling, cardiac output and the closure of the atrioventricular valves. 6 New-onset atrial fibrillation precludes these mechanisms and the effects of the loss of atrial contraction on ventricular filling may be compounded by the diastolic dysfunction commonly seen during critical illness. 111 These mechanisms may explain why NOAF is temporally associated with a reduction in cardiac index in non-ICU patients with chronic heart failure. 7 Our findings are consistent with one previous study that found that haemodynamic instability developed in 37% of post-surgical ICU patients after NOAF. 8
Atrial contractile dysfunction occurs after brief episodes of AF112 and can last for several weeks after achieving rhythm control. 113 Any AF may, therefore, have considerable impact in any critically ill patient with minimal physiological reserve. Indeed, episodes of NOAF lasting for ≥ 30 minutes in the ICU are independently associated with increased hospital mortality. 11 We found that NOAF is associated with hospital mortality after adjustment for confounding variables, regardless of whether NOAF is diagnosed based on continuous monitoring (ICU databases analysis) or from hospital diagnosis codes (RISK-II database analysis). In both cases, some, but not all, of this association is explained by confounding variables. The associated mortality risk was higher in our RISK-II analysis than in our in-ICU analysis. This may be explained by the differing definitions, with NOAF in the RISK-II data probably representing AF significant enough to result in a HES code versus any AF in the in-ICU data.
Together, our findings highlight the importance of optimal treatment and follow-up of patients who develop NOAF during an ICU stay. We found that the use of digoxin is associated with lower rates of achieving heart rate control than the use of amiodarone. Digoxin may be selected to reduce heart rates without inducing hypotension; however, digoxin may be less effective during states of increased sympathetic drive,114 including critical illness. One small study of patients in the ICU with AF with rapid ventricular response (not exclusive to NOAF) demonstrated that digoxin was less effective at rate control in patients receiving catecholaminergic medication. 115
We found that the use of calcium channel blockers was associated with lower rates of achieving rhythm control than amiodarone. These findings are supported by a small, randomised study of paroxysmal AF outside the ICU,116 which reported a cardioversion proportion of 0% in patients who received verapamil compared with 77% in patients who received amiodarone. Our findings contrast with one RCT identified in our scoping review, which compared amiodarone with calcium channel blockers31 and found no difference in achieving rhythm control. However, this study included only 20 patients per treatment group, making it difficult to draw any conclusions. We did not identify any significant difference in hospital survival between any of the treatments when compared with amiodarone.
In the MIMIC-III database, the unadjusted analysis demonstrated an apparent mortality benefit in the beta-blocker treatment group. However, patients in the beta-blocker group were younger and less unwell at presentation than those in the amiodarone group. After developing NOAF, patients in the beta-blocker group had higher blood pressures, were less likely to be on vasoactive medications and had lower inflammatory markers. After our comprehensive adjustment, the difference in mortality was no longer evident. Our adjusted results conflict with one large study,28 which suggested a survival benefit with beta-blockers over amiodarone therapy in patients with sepsis. However, this study was unable to adjust for features around NOAF onset. Our study demonstrates that important differences exist between treatment groups after the onset of AF, which may influence treatment choice. Failure to adjust for these factors is likely to result in residual confounding.
Strengths and limitations
The scoping review was performed using systematic, transparent and robust methods. The bibliographic database searches were comprehensive, which allowed maximal identification of relevant studies while also minimising the possibility of publication or language biases affecting the review. We carried out the screening and data extraction processes in duplicate to reduce the risk of reviewer errors or biases affecting the review. The main limitation of the scoping review was the methodological shortcomings of the studies identified.
Our RISK-II database analysis allowed us to include > 4000 patients who developed NOAF and 27,000 matched comparators from ICUs across England, with long-term follow-up using routinely collected data. However, the analysis is limited by the sensitivity of diagnostic records. Along with the limitations in defining patients with NOAF, some outcomes also need careful interpretation. For example, ‘hospitalisation with stroke’ may miss both extremes of severity, in which mild strokes and transient ischaemic events may not result in hospital admission and catastrophic strokes may result in death without admission.
Our analysis of two within-ICU databases has several strengths. First, we carried out comprehensive adjustment that included variables around the onset of AF. We show significant differences in these peri-AF variables that have not been adjusted for in previous studies. Second, our analysis of granular health-care data allowed a detailed analysis of the haemodynamic changes associated with NOAF. Third, our analysis of routinely collected data over many years provided a sample size large enough to demonstrate differential efficacy in NOAF treatments. This analysis also has limitations. The study was retrospective in nature and the development of NOAF was not independently verified. Documentation of AF in the MIMIC-III database has, however, been shown to be accurate for determining AF onset to within 1 hour after independent review of a sample of electrocardiographic waveforms. 89 Documentation of comorbidities in the MIMIC-III database relied on hospital billing codes, which may not have identified all cases. Furthermore, although good balance of numerous confounding covariates was achieved prior to assessing treatment efficacy, we are unable to exclude bias introduced by residual unmeasured confounding. Unmeasured variables, such as echocardiographic parameters, may have contributed to the association between NOAF in ICU and outcome, and would not be represented in the propensity weights. Finally, we acknowledge the difference in case mixes between UK and USA data. Overall, patients in the MIMIC-III database were younger and had lower mortality than those in the PICRAM database, which may explain part of the difference in NOAF incidence. Identifying patients who developed NOAF reduced these differences by identifying sufficiently unwell patients in both databases. Owing to the underlying differences, we primarily analysed each database in isolation, using the combined analysis to support these primary findings.
Uncertainties
Either amiodarone or beta-blockers are commonly used in critically ill patients to control AF, but there is little evidence to support whether or not one is superior. Purported beneficial effects of beta-blocker therapy may be because of residual confounding in some studies.
In patients who develop NOAF while in an ICU, it is not clear in whom anticoagulation following hospital discharge might be beneficial.
The incidence of AF and/or left ventricular dysfunction at hospital discharge and at 3 months following the development of NOAF while in an ICU is unknown. However, readmission with heart failure and thromboembolism is increased over the 5 years following an episode of NOAF while in an ICU, particularly in the first year.
It remains unclear to what extent NOAF in patients in an ICU is causally related to worse outcomes. Evidence for causality may be supported by future randomised prevention trials, in which a reduction in AF burden is associated with better outcomes, or through the application of robust causal inference methods in observational studies.
Chapter 7 Conclusions
Implications for service provision
There are insufficient data available to make firm recommendations for service provision in the management of NOAF identified during an ICU admission.
Suggested research priorities
Research priorities were suggested by the expert panel following data review (following the National Institute for Health and Care Excellence Research Recommendations Process and Methods Guide;98 see Appendix 8). NOAF during an ICU stay is associated with substantially increased mortality, after correction for associated risk factors. Both amiodarone and beta-blockers are commonly used, but have significant side effects. Whether or not one is superior to the other has not been demonstrated. A RCT of amiodarone compared with beta-blockers for the management of NOAF in critically ill patients should be undertaken (see Appendix 8, Table 36).
The evidence for or against anticoagulation for patients who develop NOAF in an ICU is very scarce. The risk of thromboembolism is increased in those who develop NOAF compared with those who do not develop NOAF, even when corrected for known risk factors. However, current risk stratification tools have not been validated in the ‘NOAF during ICU population’ and do not take into account whether or not ICU treatments may affect future outcome. Whether or not there are subgroups of patients who develop NOAF while in an ICU who may benefit from long-term anticoagulation is unknown. Studies should be undertaken to create risk stratification tools or to investigate whether or not current tools are applicable to the ‘NOAF during ICU population’ to identify patients sufficiently at risk of future thromboembolism to merit consideration of anticoagulation (see Appendix 8, Table 37).
Readmissions with heart failure and thromboembolism increase over the 5 years following an episode of NOAF while in an ICU, particularly in the first year. Whether these events are driven by persistent left ventricular dysfunction and/or AF is unknown. A prospective cohort study to demonstrate the incidence of AF and/or left ventricular dysfunction at hospital discharge and at 3 months following development of NOAF should be undertaken (see Appendix 8, Table 38).
Acknowledgements
The authors wish to thank Mrs Valerie Keston-Hole, Mr Rob Lawrence, and Dr Ian and Mrs Cathy Taylor (public and patient representatives), and the members of the expert panel: Dr Andy Walden and Professor Ben O’Brien.
Contributions of authors
Jonathan Bedford (https://orcid.org/0000-0001-9455-022X) (Doctoral Research Fellow) extracted data for the scoping review, extracted data from the PICRAM database, performed the statistical analysis on data from the MIMIC-III and PICRAM databases, and co-wrote the report.
Laura Drikite (https://orcid.org/0000-0002-5194-4189) (Research Assistant) extracted data for the scoping review, drafted the scoping review and reviewed the data.
Mark Corbett (https://orcid.org/0000-0002-5937-1493) (Research Fellow, Systematic Reviews) contributed to the protocol, devised and supervised the scoping review, critically appraised the review evidence, and contributed to the writing of the report.
James Doidge (https://orcid.org/0000-0002-3674-3100) (Senior Statistician, Epidemiology) contributed to the conception and supervision of the analysis, and to manuscript preparation.
Paloma Ferrando-Vivas (https://orcid.org/0000-0002-2163-645X) (Statistician, Risk Modelling) contributed to the analysis and interpretation of the data, and contributed to the writing of the report.
Alistair Johnson (https://orcid.org/0000-0002-8735-3014) (Scientist) extracted data from the MIMIC-III database and harmonised data from the MIMIC-III and PICRAM databases.
Kim Rajappan (https://orcid.org/0000-0001-8996-2983) (Consultant Cardiologist, Cardiac Electrophysiology Specialist) provided expert reviews and advice on atrial fibrillation/arrhythmias.
Paul Mouncey (https://orcid.org/0000-0002-8510-8517) (Project Management at ICNARC) contributed to the study conception, study management and database analyses.
David Harrison (https://orcid.org/0000-0002-9002-9098) (Lead Statistician) contributed to study conception and contributed to the acquisition, analysis and interpretation of data.
Duncan Young (https://orcid.org/0000-0002-6838-4835) (retired Professor of Intensive Care Medicine) was involved in the study design, data extraction specifications and interpretation of the results. He was the chief investigator for the PICRAM study, which provided one of the databases for the CAFE study.
Kathryn Rowan (https://orcid.org/0000-0001-8217-5602) (Project Lead at ICNARC) contributed to study conception, design and data analysis.
Peter Watkinson (https://orcid.org/0000-0003-1023-3927) (Principal Investigator) contributed to study conception, design and data analysis, and co-wrote the manuscript.
Publications
Drikite L, Bedford JP, O’Bryan L, Petrinic T, Rajappan K, Doidge J, et al. Treatment strategies for new onset atrial fibrillation in patients treated on an intensive care unit: a systematic scoping review. Crit Care 2021;25:257.
Bedford JP, Johnson A, Redfern O, Gerry S, Doidge J, Harrison D, et al. Comparative effectiveness of common treatments for new-onset atrial fibrillation within the ICU: accounting for physiological status. J Crit Care 2022;67:14–56.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data or study materials may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Bedford J, Harford M, Petrinic T, Young JD, Watkinson PJ. Risk factors for new-onset atrial fibrillation on the general adult ICU: protocol for a systematic review. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-024640.
- Artucio H, Pereira M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit Care Med 1990;18:1383-8. https://doi.org/10.1097/00003246-199012000-00015.
- Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J 2013;165:949-55.e3. https://doi.org/10.1016/j.ahj.2013.03.020.
- Knotzer H, Mayr A, Ulmer H, Lederer W, Schobersberger W, Mutz N, et al. Tachyarrhythmias in a surgical intensive care unit: a case-controlled epidemiologic study. Intensive Care Med 2000;26:908-14. https://doi.org/10.1007/s001340051280.
- Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care 2014;18. https://doi.org/10.1186/s13054-014-0688-5.
- Samet P, Bernstein W, Levine S. Significance of the atrial contribution to ventricular filling. Am J Cardiol 1965;15:195-202. https://doi.org/10.1016/0002-9149(65)90454-6.
- Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol 1998;32:197-204. https://doi.org/10.1016/S0735-1097(98)00221-6.
- Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L. Canadian Critical Care Trials Group . Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care 2012;27:326.e1-8. https://doi.org/10.1016/j.jcrc.2011.10.011.
- Clayton B, Ball S, Read J, Waddy S. Risk of thromboembolism in patients developing critical illness-associated atrial fibrillation. Clin Med 2018;18:282-7. https://doi.org/10.7861/clinmedicine.18-4-282.
- Wetterslev M, Haase N, Hassager C, Belley-Cote EP, McIntyre WF, An Y, et al. New-onset atrial fibrillation in adult critically ill patients: a scoping review. Intensive Care Med 2019;45:928-38. https://doi.org/10.1007/s00134-019-05633-x.
- Bedford JP, Gerry S, Hatch RA, Rechner I, Young JD, Watkinson PJ. Hospital outcomes associated with new-onset atrial fibrillation during ICU admission: a multicentre competing risks analysis. J Crit Care 2020;60:72-8. https://doi.org/10.1016/j.jcrc.2020.07.009.
- Fernando SM, Mathew R, Hibbert B, Rochwerg B, Munshi L, Walkey AJ, et al. New-onset atrial fibrillation and associated outcomes and resource use among critically ill adults – a multicenter retrospective cohort study. Crit Care 2020;24. https://doi.org/10.1186/s13054-020-2730-0.
- Arrigo M, Feliot E, Gayat E, Mebazaa A. Cardiovascular events after ICU discharge in patients with new-onset atrial fibrillation: a report from the FROG-ICU study. Int J Cardiol 2018;270. https://doi.org/10.1016/j.ijcard.2018.07.080.
- Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 2014;146:1187-95. https://doi.org/10.1378/chest.14-0003.
- National Institute for Health and Care Excellence (NICE) . Atrial Fibrillation: Management 2014. www.nice.org.uk/guidance/cg180 (accessed 1 October 2020).
- Chean CS, McAuley D, Gordon A, Welters ID. Current practice in the management of new-onset atrial fibrillation in critically ill patients: a UK-wide survey. PeerJ 2017;5. https://doi.org/10.7717/peerj.3716.
- Drikite L, Bedford JP, O’Bryan L, Petrinic T, Rajappan K, Doidge J, et al. Treatment strategies for new onset atrial fibrillation in patients treated on an intensive care unit: a systematic scoping review. Crit Care 2021;25. https://doi.org/10.1186/s13054-021-03684-5.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32. https://doi.org/10.1080/1364557032000119616.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-69.
- Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-48.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467-73. https://doi.org/10.7326/M18-0850.
- Watkinson P, Johnson A, Rajappan K, Corbett M, Mouncey P, Harrison DA, et al. Critical Care Atrial Fibrillation Evaluation (CAFE): Protocol 2019. https://fundingawards.nihr.ac.uk/award/17/71/04 (accessed 1 October 2019).
- Bedford JP, Harford M, Petrinic T, Young JD, Watkinson PJ. Risk factors for new-onset atrial fibrillation on the general adult ICU: a systematic review. J Crit Care 2019;53:169-75. https://doi.org/10.1016/j.jcrc.2019.06.015.
- Yokota T, Uchino S, Yoshida T, Fujii T, Takinami M. Predictors for sustained new-onset atrial fibrillation in critically ill patients: a retrospective observational study. J Anesth 2018;32:681-7. https://doi.org/10.1007/s00540-018-2537-1.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Launey Y, Lasocki S, Asehnoune K, Gaudriot B, Chassier C, Cinotti R, et al. Impact of low-dose hydrocortisone on the incidence of atrial fibrillation in patients with septic shock: a propensity score-inverse probability of treatment weighting cohort study. J Intensive Care Med 2019;34:238-44. https://doi.org/10.1177/0885066617696847.
- Walkey AJ, Evans SR, Winter MR, Benjamin EJ. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a propensity-matched cohort study. Chest 2016;149:74-83. https://doi.org/10.1378/chest.15-0959.
- Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol 2016;1:682-90. https://doi.org/10.1001/jamacardio.2016.2181.
- Balser JR, Martinez EA, Winters BD, Perdue PW, Clarke AW, Huang W, et al. Beta-adrenergic blockade accelerates conversion of postoperative supraventricular tachyarrhythmias. Anesthesiology 1998;89:1052-9. https://doi.org/10.1097/00000542-199811000-00004.
- Delle Karth G, Geppert A, Neunteufl T, Priglinger U, Haumer M, Gschwandtner M, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med 2001;29:1149-53. https://doi.org/10.1097/00003246-200106000-00011.
- Gerlach A, Kane-Gill S, F Dasta J, Steinberg S, C Martin L, Cook C. Diltiazem versus amiodarone for new-onset atrial arrhythmias in non-cardiac post surgical patients: a cohort study. Crit Care Shock 2008;11.
- Cho MS, Nam G-B, Kim YN, Hwang J, Kim M, Lee JH, et al. Abstract 20382: comparison of outcomes after rhythm or rate control strategy for the new-onset atrial fibrillation in critically ill sepsis patients managed in the medical intensive care unit. Circulation 2017;136.
- Matsumoto Y, Shibata SC, Maeda A, Imada T, Okitsu K, Fujino Y. Comparison of the Short Acting Beta1 Blocker Landiolol With Amiodarone for Pharmacological Cardioversion for Atrial Fibrillation in Intensive Care Unit Patients n.d. https://doi.org/10.1213/01.ane.0000470325.07465.0f.
- Balik M, Kolnikova I, Maly M, Waldauf P, Tavazzi G, Kristof J. Propafenone for supraventricular arrhythmias in septic shock-comparison to amiodarone and metoprolol. J Crit Care 2017;41:16-23. https://doi.org/10.1016/j.jcrc.2017.04.027.
- Mieure KD, Moranville MP, Park JJ, Lat I, Jennings HR, Lazar S, et al. A Comparison of Intravenous Rate Control Agents for New Onset Atrial Fibrillation With Rapid Ventricular Rate n.d.
- Jaffer F, Anand S, Ajay-obe A, Parbtani R, Doraiswamy V, Malo J. Use of amiodarone in management of atrial tachyarrhythmia in septic shock. Chest 2016;150. https://doi.org/10.1016/j.chest.2016.08.374.
- Brown M, Nassoiy S, Chaney W, Plackett TP, Blackwell RH, Luchette F, et al. Impact and treatment success of new-onset atrial fibrillation with rapid ventricular rate development in the surgical intensive care unit. J Surg Res 2018;229:66-75. https://doi.org/10.1016/j.jss.2018.03.009.
- Sleeswijk ME, Tulleken JE, Van Noord T, Meertens JH, Ligtenberg JJ, Zijlstra JG. Efficacy of magnesium-amiodarone step-up scheme in critically ill patients with new-onset atrial fibrillation: a prospective observational study. J Intensive Care Med 2008;23:61-6. https://doi.org/10.1177/0885066607310181.
- Slavik RS, de Lemos J, Gorman SK, Buchkowsky S. Patterns of practice for the acute management of new-onset atrial fibrillation in critically-ill intensive care unit patients. Crit Care Med 2003;31.
- Liu WC, Lin WY, Lin CS, Huang HB, Lin TC, Cheng SM, et al. Prognostic impact of restored sinus rhythm in patients with sepsis and new-onset atrial fibrillation. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1548-2.
- Mitrić G, Udy A, Bandeshe H, Clement P, Boots R. Variable use of amiodarone is associated with a greater risk of recurrence of atrial fibrillation in the critically ill. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1252-2.
- Mayr AJ, Dünser MW, Ritsch N, Pajk W, Friesenecker B, Knotzer H, et al. High-dosage continuous amiodarone therapy to treat new-onset supraventricular tachyarrhythmias in surgical intensive care patients: an observational study. Wien Klin Wochenschr 2004;116:310-17. https://doi.org/10.1007/BF03040901.
- Burris JM, Subramanian A, Sansgiry S, Palacio CH, Bakaeen FG, Awad SS. Perioperative atrial arrhythmias in noncardiothoracic patients: a review of risk factors and treatment strategies in the veteran population. Am J Surg 2010;200:601-5. https://doi.org/10.1016/j.amjsurg.2010.07.019.
- Nakamura K, Inokuchi R, Hiruma T, Tokunaga K, Doi K, Nakajima S. Switching therapy from intravenous beta blocker to bisoprolol transdermal patch for atrial fibrillation tachycardia. J Anesth 2016;30:891-4. https://doi.org/10.1007/s00540-016-2199-9.
- Hennersdorf MG, Perings SM, Zühlke C, Heidland UE, Perings C, Heintzen MP, et al. Conversion of recent-onset atrial fibrillation or flutter with ibutilide after amiodarone has failed. Intensive Care Med 2002;28:925-9. https://doi.org/10.1007/s00134-002-1317-3.
- Delle Karth G, Schillinger M, Geppert A, Haumer M, Gwechenberger M, Meyer B, et al. Ibutilide for rapid conversion of atrial fibrillation or flutter in a mixed critically ill patient population. Wien Klin Wochenschr 2005;117:92-7. https://doi.org/10.1007/s00508-004-0297-4.
- Kane S, Hanes S. Impact of hydrocortisone on atrial fibrillation in septic shock. Crit Care Med 2014;42. https://doi.org/10.1097/01.ccm.0000458443.11317.7a.
- Mayr A, Ritsch N, Knotzer H, Dünser M, Schobersberger W, Ulmer H, et al. Effectiveness of direct-current cardioversion for treatment of supraventricular tachyarrhythmias, in particular atrial fibrillation, in surgical intensive care patients. Crit Care Med 2003;31:401-5. https://doi.org/10.1097/01.CCM.0000048627.39686.79.
- Kyo M, Hosokawa K, Ohshimo S, Kida Y, Tanabe Y, Ota K, et al. High serum potassium level is associated with successful electrical cardioversion for new-onset atrial fibrillation in the intensive care unit: a retrospective observational study. Anaesth Intensive Care 2019;47:52-9. https://doi.org/10.1177/0310057X18811815.
- Chapman MJ, Moran JL, O’Fathartaigh MS, Peisach AR, Cunningham DN. Management of atrial tachyarrhythmias in the critically ill: a comparison of intravenous procainamide and amiodarone. Intensive Care Med 1993;19:48-52. https://doi.org/10.1007/BF01709278.
- Moran JL, Gallagher J, Peake SL, Cunningham DN, Salagaras M, Leppard P. Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med 1995;23:1816-24. https://doi.org/10.1097/00003246-199511000-00005.
- Yoshida T, Fujii T, Uchino S, Takinami M. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: a systematic review. J Intensive Care 2015;3. https://doi.org/10.1186/s40560-015-0085-4.
- Balik M, Matousek V, Maly M, Brozek T. Management of arrhythmia in sepsis and septic shock. Anaesthesiol Intensive Ther 2017;49:419-29. https://doi.org/10.5603/AIT.a2017.0061.
- Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest 2018;154:1424-34. https://doi.org/10.1016/j.chest.2018.03.040.
- Kanji S, Stewart R, Fergusson DA, McIntyre L, Turgeon AF, Hébert PC. Treatment of new-onset atrial fibrillation in noncardiac intensive care unit patients: a systematic review of randomized controlled trials. Crit Care Med 2008;36:1620-4. https://doi.org/10.1097/CCM.0b013e3181709e43.
- Keller M, Meierhenrich R. New onset atrial fibrillation in patients with sepsis. Anaesthesist 2017;66:786-94. https://doi.org/10.1007/s00101-017-0334-0.
- Rehberg S, Joannidis M, Whitehouse T, Morelli A. Landiolol for managing atrial fibrillation in intensive care. Eur Heart J Supplements 2018;20:A15-18. https://doi.org/10.1093/eurheartj/sux039.
- Schwartz A, Brotfain E, Koyfman L, Klein M. Cardiac arrhythmias in a septic ICU population: a review. J Crit Care Med 2015;1:140-6. https://doi.org/10.1515/jccm-2015-0027.
- Shapiro WA, Schulze K. An algorithm for the practical management of the new onset of atrial fibrillation in patients admitted to the ICU. ICU Director 2012;3:179-84. https://doi.org/10.1177/1944451612449649.
- Sibley S, Muscedere J. New-onset atrial fibrillation in critically ill patients. Can Respir J 2015;22:179-82. https://doi.org/10.1155/2015/394961.
- Sleeswijk ME, Van Noord T, Tulleken JE, Ligtenberg JJ, Girbes AR, Zijlstra JG. Clinical review: treatment of new-onset atrial fibrillation in medical intensive care patients – a clinical framework. Crit Care 2007;11. https://doi.org/10.1186/cc6136.
- Tran B. Atrial Fibrillation in Sepsis: Should We Worry?. Relias Media; 2018.
- Labbe V, Ederhy S, Fartoukh M, Cohen A. Should we administrate anticoagulants to critically ill patients with new onset supraventricular arrhythmias?. Arch Cardiovasc Dis 2015;108:217-9. https://doi.org/10.1016/j.acvd.2015.01.001.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336. https://doi.org/10.1136/bmj.39489.470347.AD.
- Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bögelein D, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care 2010;14. https://doi.org/10.1186/cc9057.
- Seguin P, Laviolle B, Maurice A, Leclercq C, Mallédant Y. Atrial fibrillation in trauma patients requiring intensive care. Intensive Care Med 2006;32:398-404. https://doi.org/10.1007/s00134-005-0032-2.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials 1996;17:1-12. https://doi.org/10.1016/0197-2456(95)00134-4.
- Barranco F, Sanchez M, Rodriguez J, Guerrero M. Efficacy of flecainide in patients with supraventricular arrhythmias and respiratory insufficiency. Intensive Care Med 1994;20:42-4. https://doi.org/10.1007/BF02425054.
- Walkey AJ, Hogarth DK, Lip GYH. Optimizing atrial fibrillation management: from ICU and beyond. Chest 2015;148:859-64. https://doi.org/10.1378/chest.15-0358.
- Vieillard-Baron A, Boyd J. Non-antiarrhythmic interventions in new onset and paroxysmal sepsis-related atrial fibrillation. Intensive Care Med 2018;44:94-7. https://doi.org/10.1007/s00134-017-4986-7.
- Walton J. The need for adequate rate control points to amiodarone as the drug of choice for treating critically ill patients with acute onset atrial fibrillation. Crit Care Med 2008;36:3132-3. https://doi.org/10.1097/CCM.0b013e31818be8ad.
- Trohman RG. Atrial fibrillation in the critically ill: common sense for a common problem. Crit Care Med 2008;36:1681-2. https://doi.org/10.1097/CCM.0b013e31817014d2.
- Intensive Care National Audit and Research Centre . About the CMP n.d. www.icnarc.org/Our-Audit/Audits/Cmp/About (accessed 1 October 2020).
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit. Research Centre Case Mix Programme Database. Crit Care 2004;8:R99-111. https://doi.org/10.1186/cc2834.
- Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J 2004;4:103-12. https://doi.org/10.1177/1536867x0400400201.
- Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. Hoboken, NJ: John Wiley & Sons; 2004.
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244-56. https://doi.org/10.1093/aje/kwp107.
- Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978;34:541-54. https://doi.org/10.2307/2530374.
- Harrell J. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001.
- Bedford JP, Johnson A, Redfern O, Gerry S, Doidge J, Harrison D, et al. Comparative effectiveness of common treatments for new-onset atrial fibrillation within the ICU: accounting for physiological status. J Crit Care 2022;67:14-56. https://doi.org/10.1016/j.jcrc.2021.11.005.
- Johnson AE, Burgess J, Pimentel MA, Clifton DA, Young JD, Watkinson PJ, et al. Physiological trajectory of patients pre and post ICU discharge. Annu Int Conf IEEE Eng Med Biol Soc 2014;2014:3160-3. https://doi.org/10.1109/EMBC.2014.6944293.
- Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3. https://doi.org/10.1038/sdata.2016.35.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. https://doi.org/10.1016/S0140-6736(07)61602-X.
- Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients. a multicenter observational cohort study. Am J Respir Crit Care Med 2019;200:1373-80. https://doi.org/10.1164/rccm.201904-0849OC.
- Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758-64. https://doi.org/10.1093/aje/kwv254.
- Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363-73. https://doi.org/10.1056/NEJMoa1001337.
- Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med 2013;41:1711-18. https://doi.org/10.1097/CCM.0b013e31828a24fe.
- Ho KM, Dobb GJ, Knuiman M, Finn J, Lee KY, Webb SA. A comparison of admission and worst 24-hour Acute Physiology and Chronic Health Evaluation II scores in predicting hospital mortality: a retrospective cohort study. Crit Care 2006;10. https://doi.org/10.1186/cc3913.
- Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth 2019;122:428-36. https://doi.org/10.1016/j.bja.2018.12.019.
- Fasiolo M, Wood SN, Zaffran M, Nedellec R, Goude Y. Fast calibrated additive quantile regression. J Am Stat Assoc 2020;116:1402-12. https://doi.org/10.1080/01621459.2020.1725521.
- Huling JD, Mak S. Energy balancing of covariate distributions. ArXiv 2020 2004.
- Greifer N. WeightIt: Weighting for Covariate Balance in Observational Studies 2020. https://ngreifer.github.io/WeightIt/ (accessed 1 October 2010).
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. https://doi.org/10.1080/00273171.2011.568786.
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986;1:54-75. https://doi.org/10.1214/ss/1177013815.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Harris S, Shi S, Brealey D, MacCallum NS, Denaxas S, Perez-Suarez D, et al. Critical Care Health Informatics Collaborative (CCHIC): data, tools and methods for reproducible research: a multi-centre UK intensive care database. Int J Med Inform 2018;112:82-9. https://doi.org/10.1016/j.ijmedinf.2018.01.006.
- National Institute for Health and Care Excellence (NICE) . Research Recommendations Process and Methods Guide 2015.
- Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823-33. https://doi.org/10.1056/NEJMoa1501035.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263-72. https://doi.org/10.1378/chest.09-1584.
- Champion S, Lefort Y, Gaüzère BA, Drouet D, Bouchet BJ, Bossard G, et al. CHADS2 and CHA2DS2-VASc scores can predict thromboembolic events after supraventricular arrhythmia in the critically ill patients. J Crit Care 2014;29:854-8. https://doi.org/10.1016/j.jcrc.2014.05.010.
- Yao X, Gersh BJ, Sangaralingham LR, Kent DM, Shah ND, Abraham NS, et al. Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA risk scores in predicting non-vitamin k antagonist oral anticoagulants-associated bleeding in patients with atrial fibrillation. Am J Cardiol 2017;120:1549-56. https://doi.org/10.1016/j.amjcard.2017.07.051.
- Proietti M, Rivera-Caravaca JM, Esteve-Pastor MA, Romiti GF, Marin F, Lip GYH. Predicting bleeding events in anticoagulated patients with atrial fibrillation: a comparison between the HAS-BLED and GARFIELD-AF bleeding scores. J Am Heart Assoc 2018;7. https://doi.org/10.1161/JAHA.118.009766.
- Fox KAA, Lucas JE, Pieper KS, Bassand JP, Camm AJ, Fitzmaurice DA, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017157.
- Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol 2012;60:861-7. https://doi.org/10.1016/j.jacc.2012.06.019.
- Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011;306:2248-54. https://doi.org/10.1001/jama.2011.1615.
- Russell L, Holst LB, Kjeldsen L, Stensballe J, Perner A. Risks of bleeding and thrombosis in intensive care unit patients with haematological malignancies. Ann Intensive Care 2017;7. https://doi.org/10.1186/s13613-017-0341-y.
- Arnold DM, Donahoe L, Clarke FJ, Tkaczyk AJ, Heels-Ansdell D, Zytaruk N, et al. Bleeding during critical illness: a prospective cohort study using a new measurement tool. Clin Invest Med 2007;30:E93-102. https://doi.org/10.25011/cim.v30i2.985.
- Granholm A, Zeng L, Dionne JC, Perner A, Marker S, Krag M, et al. Predictors of gastrointestinal bleeding in adult ICU patients: a systematic review and meta-analysis. Intensive Care Med 2019;45:1347-59. https://doi.org/10.1007/s00134-019-05751-6.
- Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017;46:1093-1093i. https://doi.org/10.1093/ije/dyx015.
- Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J 2012;33:895-903. https://doi.org/10.1093/eurheartj/ehr351.
- Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol 1994;23:1535-40. https://doi.org/10.1016/0735-1097(94)90652-1.
- Louie EK, Liu D, Reynertson SI, Loeb HS, McKiernan TL, Scanlon PJ, et al. ‘Stunning’ of the left atrium after spontaneous conversion of atrial fibrillation to sinus rhythm: demonstration by transesophageal Doppler techniques in a canine model. J Am Coll Cardiol 1998;32:2081-6. https://doi.org/10.1016/S0735-1097(98)00508-7.
- Ang EL, Chan WL, Cleland JG, Moore D, Krikler SJ, Alexander ND, et al. Placebo controlled trial of xamoterol versus digoxin in chronic atrial fibrillation. Br Heart J 1990;64:256-60. https://doi.org/10.1136/hrt.64.4.256.
- Gritsenko D, Paris D, Aitken SL, Lee YI, Altshuler J. Amiodarone versus digoxin for rate control in critically ill patients with rapid atrial fibrillation or flutter. J Emerg Crit Care Med 2018;2. https://doi.org/10.21037/jeccm.2018.06.07.
- Noc M, Stajer D, Horvat M. Intravenous amiodarone versus verapamil for acute conversion of paroxysmal atrial fibrillation to sinus rhythm. Am J Cardiol 1990;65:679-80. https://doi.org/10.1016/0002-9149(90)91053-9.
Appendix 1 Search strategy
MEDLINE (via Ovid)
Date range searched: 1946 to present.
Date searched: 4 March 2019.
Records retrieved: 1087.
Search strategy
-
ATRIAL FIBRILLATION/
-
ATRIAL FLUTTER/
-
SUPRAVENTRICULAR TACHYCARDIA/
-
(“atrial fibrillation*” or AF).ab,ti.
-
“atrial flutter* “.ab,ti.
-
“atrial arrhythmia* “.ab,ti.
-
(“supraventricular tachycardia*” or SVT).ab,ti.
-
“NOAF*”.ab,ti.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
-
INTENSIVE CARE UNITS/
-
CRITICAL CARE/
-
SEPSIS/
-
SEPTIC SHOCK/
-
“intensive care”.ab,ti.
-
“critical care unit* “.ab,ti.
-
“intensive therapy unit* “.ab,ti.
-
“high dependenc* “.ab,ti.
-
(ICU* or ITU* or HDU* or CCU*).ab,ti.
-
“critically unwell”.ab,ti.
-
“critically ill”.ab,ti.
-
(sepsis or “septic shock”).ab,ti.
-
10 or 11 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
-
9 and 22
-
limit 23 to animals
-
limit 23 to (“newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)”)
-
“case report* “.ti.
-
24 or 25 or 26
-
23 not 27
-
“atrial fibrillation* “.ab,ti.
-
(cardiac or cardiothoracic or “cardio thoracic” or cardiopulmonary or “cardio pulmonary”).ab,ti.
-
“surg*”.ab,ti.
-
“coronary care unit* “.ab,ti.
-
29 and 30 and 31
-
29 and 32
-
33 or 34
-
28 not 35.
EMBASE (via Ovid)
Date range searched: inception to 4 March 2019.
Date searched: 4 March 2019.
Records retrieved: 3962.
Search strategy
-
ATRIAL FIBRILLATION/
-
ATRIAL FLUTTER/
-
SUPRAVENTRICULAR TACHYCARDIA/
-
(“atrial fibrillation*” or AF).ab,ti.
-
“atrial flutter* “.ab,ti.
-
“atrial arrhythmia* “.ab,ti.
-
(“supraventricular tachycardia*” or SVT).ab,ti.
-
“NOAF*”.ab,ti.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
-
INTENSIVE CARE UNITS/
-
CRITICAL CARE/
-
SEPSIS/
-
SEPTIC SHOCK/
-
“intensive care”.ab,ti.
-
“critical care unit* “.ab,ti.
-
“intensive therapy unit* “.ab,ti.
-
“high dependenc* “.ab,ti.
-
(ICU* or ITU* or HDU* or CCU*).ab,ti.
-
“critically unwell”.ab,ti.
-
“critically ill”.ab,ti.
-
(sepsis or “septic shock”).ab,ti.
-
10 or 11 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
-
9 and 22
-
limit 23 to animal studies
-
limit 23 to (infant <to one year> or preschool child <1 to 6 years> or school child <7 to 12 years>)
-
“case report* “.ti.
-
24 or 25 or 26
-
23 not 27
-
“atrial fibrillation* “.ab,ti.
-
(cardiac or cardiothoracic or “cardio thoracic” or cardiopulmonary or “cardio pulmonary”).ab,ti.
-
“surg*”.ab,ti.
-
“coronary care unit* “.ab,ti.
-
29 and 30 and 31
-
29 and 32
-
33 or 34
-
28 not 35
-
limit 36 to conference abstracts
-
36 not 37.
Cumulative Index to Nursing and Allied Health Literature
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 441.
Search strategy
-
ATRIAL FIBRILLATION/ (20,100)
-
ATRIAL FLUTTER/ (1521)
-
TACHYCARDIA, SUPRAVENTRICULAR/ (2593)
-
(“atrial fibrillation*” OR AF).ti,ab (24,117)
-
(“atrial flutter*”).ti,ab (1541)
-
(“atrial arrhythmia*”).ti,ab (867)
-
(“supraventricular tachycardia*” OR SVT).ti,ab (1683)
-
(NOAF*).ti,ab (9)
-
(1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8) (32,813)
-
INTENSIVE CARE UNITS/ (32,253)
-
CRITICAL CARE/ (19,423)
-
SHOCK, SEPTIC/ (4198)
-
(“intensive care”).ti,ab (50,295)
-
(“critical care unit*”).ti,ab (1967)
-
(“intensive therapy unit*”).ti,ab (254)
-
(“high dependenc*”).ti,ab (627)
-
(ICU* OR ITU* OR HDU* OR CCU*).ti,ab (24,659)
-
(“critically unwell”).ti,ab (17)
-
(“critically ill”).ti,ab (17,286)
-
(10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19) (92,933)
-
(9 AND 20) (480)
-
(“atrial fibrillation*”).ti,ab (21,936)
-
(cardiac OR cardiothoracic OR “cardio thoracic” OR cardiopulmonary OR “cardio pulmonary”).ti,ab (114,324)
-
(surg*).ti,ab (319,271)
-
(“coronary care unit*”).ti,ab (859)
-
(“case report*”).ti (44,822)
-
(22 AND 23 AND 24) (894)
-
(22 AND 25) (20)
-
(26 OR 27 OR 28) (45,731)
-
21 NOT 29 (361)
-
30 [Human age groups Infant∼Newborn: birth-1 month OR Infant: 1-23 months OR Child∼Preschool: 2-5 years OR Child: 6-12 years] (37)
-
ANIMAL STUDIES/ (98,956)
-
(30 AND 32) (1)
-
(31 OR 33) (38)
-
30 NOT 34 (323).
Web of Science
Includes Conference Proceedings Citation Index –Science, Science Citation Index Expanded, Social Sciences Citation Index, Arts & Humanities Citation Index, Conference Proceedings Citation Index-Science, Conference Proceedings Citation Index- Social Science & Humanities, The Book Citation Index-Science, The Book Citation Index – Social Science & Humanities, Emerging Sources Citation Index, Current chemical reactions-expanded and Index Chemicus.
Date ranged searched: 1949 to 2019.
Date searched: 6 March 2019.
Records retrieved: 1772.
Records retrieved after filtering by title: 137.
Search strategy
“TOPIC: (atrial AND fibrillat*).
Refined by: TOPIC: ((intensive OR critical) AND (care OR therapy)).
Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC”.
Cochrane Library
Includes Cochrane Reviews, Cochrane Protocols and Trials.
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved in Cochrane Reviews: 0.
Records retrieved in Cochrane Protocols: 0.
Records retrieved in Trials: 96.
Search strategy
“atrial fibrillation” AND (“intensive care” OR “critical care” OR “intensive therapy”).
Database of Abstracts of Reviews of Effects
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 4.
Search strategy
(“atrial fibrillation” AND (“critical care” OR “intensive care”).
OpenGrey
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 2.
Search strategy
“Atrial fibrillation” AND (“Intensive Care” OR “Critical Care” OR “Intensive Therapy”).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
URL: https://clinicaltrials.gov/.
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 264.
Search strategy
“Atrial fibrillation” in CONDITION field OR “Atrial flutter” in OTHER TERMS field.
International Standard Randomised Controlled Trial Number
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 292.
Search strategy
ATRIAL FIBRILLATION” in TEXT field.
EU clinical trials register
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 12.
Search strategy
“atrial fibrillation” AND (“critical care” OR “intensive care” OR “intensive therapy”).
World Health Organization International Clinical Trials Registry Platform
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 12.
Search strategy
“atrial fibrillation” AND (“critical care” OR “intensive care” OR “intensive therapy”).
National Institute for Health Research UK Clinical Trials Gateway
Date range searched: inception to 11 March 2019.
Date searched: 11 March 2019.
Records retrieved: 149.
Search strategy
“atrial fibrillation”.
Appendix 2 Risk-of-bias assessment
Reproduced with permission from Sterne et al. 25 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and distribute this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Appendix 3 Data extraction tables for single-group studies
| Study details | Population characteristics | Intervention | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
First author and year: Delle Karth 200547 Setting: cardiologic ICU Note: medical patients included in the study Country: Austria Sample size: n = 17 (data extracted for medical patients only; the study also includes cardiac-surgical patients) NOAF patients: n = 15 (88.2%) |
Primary diagnosis: perspiratory failure (17.6%), heart failure (35%), sepsis (17.6%) and cardiopulmonary resuscitation (11.7%) Median (range) age reported: 63 (56.5–73) years Male: n = 11 (64.7%) Severity of illness: median (range) SAPS II reported 53 (44.5–63) Patients on vasopressors: n = 15 (88.2%) Patients with CVD: n = 6 (35%) (heart failure) Patients with acute renal failure: n = 3 (17.6%) Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: n = 13 (76.5%) Serum potassium: NR Definition of NOAF: ‘recent-onset (< 1 hour) tachycardic (> 100 beats per minute) sustained (> 10 minutes) atrial fibrillation’ |
Ibutilide: patients received up to two 10-minute i.v. infusions of 1.0 mg of ibutilide, with an interval of 10 minutes. If AF did not terminate during or within 10 minutes after the end of the first infusion, the second infusion was administered. If AF persisted at minute 60, ibutilide treatment was considered unsuccessful and further treatment (beyond minute 60) was started at the discretion of the treating physician Line of NOAF treatment: not specified |
||||||||||||
|
First author and year: Hennersdorf 200246 Setting: university hospital ICU Country: Germany Sample size: n = 26 NOAF patients: n = 7 (27%) Note: results reported separately for NOAF group |
Primary diagnosis: hypertension (31%), hypertrophic obstructive cardiomyopathy (8%), dilative cardiomyopathy (12%), cor pulmonale (15%), coronary artery disease (19%), sepsis with cardiac involvement (15%) and reduced left ventricular ejection fraction (15%) Mean age: 68 ± 11 years Male: n = 21 (80.7%) Severity of illness: APACHE II score, median (range) 8 (5–24) Patients on vasopressors: NR Patients with CVD: hypertrophic obstructive cardiomyopathy (8%), dilative cardiomyopathy (12%), cor pulmonale (15%), coronary artery disease (19%) and reduced left ventricular ejection fraction (15%) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: n = 19 (73%) (not specified if at the onset) Serum potassium: NR Definition of NOAF: NR |
Ibutilide was administered as an infusion over a period of 10 minutes. The dose of ibutilide was 1 mg i.v.; in the case of persisting arrhythmia and body weight of > 70 kg, a second infusion of 1 mg of ibutilide was administered after 30 minutes. Before the ibutilide infusion was started, all patients were given magnesium (1 g, i.v.) and potassium (if the potassium serum level was < 4.5 mmol/l). Magnesium and potassium were administered to prevent proarrhythmic effects (torsade de pointes tachycardia) All patients received heparin i.v. during the stay on the ICU Line of NOAF treatment: second line All patients received amiodarone (150 mg, i.v.) as the first-line drug. If patients failed to convert back to sinus rhythm, ibutilide was given i.v. 2 hours after the administration of amiodarone |
||||||||||||
|
First author and year: Mayr 200349 Setting: Surgical ICU Country: Austria Sample size: n = 37 NOAF patients: n = 31 (84%) |
Surgery type: abdominal (35.1%), cardiac (35.1%), vascular (2.7%), trauma (10.81%), orthopaedic (8.1%) and thoracic (5.4%) Pneumonia after surgery: 2.7% Median age (range): all patients, 72 years (34–94 years); primary responders, 67 years (34–82 years); non-responders, 73.5 years (51–94 years) Male: all patients, n = 16/37 (43.2%); primary responders, n = 7/13 (53.8%); non-responders, n = 9/24 (37.5%) Severity of illness: median (range) SAPS reported – all patients, 13 (5–29); primary responders, 14 (5–29); non-responders, 13 (9–26) Patients on vasopressors: all patients, n = 29/37 (78.37%); primary responders, n = 10/13 (76.9%); non-responders, n = 19/24 (79.16%) Patients with CVD: Cardiovascular diseaseAll patients (N = 37), n (%)Primary responders (N = 13), n (%)Non-responders (N = 24), n (%)Coronary artery disease22 (59.45)7 (53.8)15 (62.5)Heart failure13 (35)4 (30.76)9 (37.5) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium (mmol/l): all patients, 4.2 (IQR 3.5–5); primary responders, 4.2 (IQR 3.5–5); non-responders, 4.2 (IQR 3.6–4.7) Definition of NOAF: ‘SVT were defined as narrow-complex, non-sinus tachycardias with heart rate > 100 beats/min for at least 15 mins’ |
Cardiovascular disease | All patients (N = 37), n (%) | Primary responders (N = 13), n (%) | Non-responders (N = 24), n (%) | Coronary artery disease | 22 (59.45) | 7 (53.8) | 15 (62.5) | Heart failure | 13 (35) | 4 (30.76) | 9 (37.5) |
Electrical cardioversion: if required, patients were sedated with etomidate. A maximum of four consecutive shocks were administered. In patients not responding to electrical cardioversion or in recurrent arrhythmia, i.v. anti-arrhythmic therapy was started Line of NOAF treatment: not specified |
| Cardiovascular disease | All patients (N = 37), n (%) | Primary responders (N = 13), n (%) | Non-responders (N = 24), n (%) | |||||||||||
| Coronary artery disease | 22 (59.45) | 7 (53.8) | 15 (62.5) | |||||||||||
| Heart failure | 13 (35) | 4 (30.76) | 9 (37.5) | |||||||||||
|
First author and year: Nakamura 201645 Setting: medical/surgical ICU for in-hospital patients and emergency ICU for emergency outpatients Country: Japan Sample size: n = 16 NOAF patients: 100% |
Primary diagnosis: sepsis (50%) and heart failure (56.2%) Mean age: 75.0 ± 13.1 years Male: n = 7 (43.75%) Severity of illness: mean APACHE II score reported, 24.3 ± 6.0; mean SOFA score reported, 8.6 ± 3.1 Patients on vasopressors: in 62.5% of patients, noradrenaline had been given with landiolol Patients with CVD: n = 9 (56.2%) (heart failure) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: NR |
Switching therapy from landiolol to a bisoprolol patch: switching occurred where a continuous landiolol infusion was used for AF-related tachycardia, and its administration duration reached 6 days, or where long-term therapy was expected before day 6. A 4 mg/24 hour bisoprolol patch was attached and the landiolol infusion was stopped after 6 hours. Median landiolol administration time before bisoprolol patch use: 88.1 hours. Median landiolol dosage on bisoprolol patch use: 3.1 µg/kg/minute. Median noradrenaline dosage on bisoprolol patch use: 0.20 µg/kg/minute Line of NOAF treatment: second line |
||||||||||||
|
First author and year: Slavik 200340 (conference abstract) Setting: general ICU Country: Canada Sample size: NR NOAF patients: 6.6% patients developed NOAF |
Primary diagnosis: NR Mean age: NR Male: NR Severity of illness: NR Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: NR |
For NOAF rate control and conversion: i.v. amiodarone used in 74.5% of episodes For stroke prophylaxis: i.v. heparin used in 36.4% of episodes Line of NOAF treatment: not specified |
||||||||||||
|
First author and year: Sleeswijk 200839 Setting: tertiary ICU, 12 bed Country: the Netherlands Sample size: n = 29 NOAF patients: 100% |
Primary diagnosis: NR Medical patients: magnesium responders, n = 11; magnesium non-responders, n = 9 Surgery patients: magnesium responders, n = 5; magnesium non-responders, n = 4 Mean age: magnesium responders, 64 ± 16 years; magnesium non-responders 69 ± 17 years Male: n = 14 (48%) (magnesium responders, n = 7; magnesium non-responders, n = 7) Severity of illness: mean APACHE II score reported – all patients, 19 ± 7 (magnesium responders, 18 ± 7; magnesium non-responders, 21 ± 7) Patients on vasopressors: n = 17 (59%) (all patients) (magnesium responders, n = 8, 50%; magnesium non-responders, n = 9, 69%) Patients with CVD: n = 14 (48%) (all patients) (magnesium responders, n = 5, 31%; magnesium non-responders, n = 9, 69%) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: ‘New-onset AF was defined as newly developed AF during the ICU stay in patients without a previous history of atrial tachyarrhythmias and anti-arrhythmic drug use. Diagnosis was confirmed by a 12-lead electrocardiogram (ECG)’ |
Magnesium infusion: patients received MgSO4 bolus followed by continuous infusion. The infusion rate was reduced to half when plasma (Mg2+) was > 2.0 mmol/l and stopped when plasma (Mg2+) was > 3.0 mmol/l. Where sinus rhythm was achieved, the infusion was stopped at the discretion of the treating clinician Line of NOAF treatment: first line. Where no rhythm or rate control (< 110 b.p.m.) was achieved after 1 hour of starting the MgSO4 infusion, an infusion of amiodarone (loading dose of 300 mg followed by an infusion of 1200 mg/24 hours) was started. Where sinus rhythm was achieved, the amiodarone infusion was stopped at the discretion of the treating clinician Line of NOAF treatment: second line |
| Study | Results | Adverse effects | Recommendations for/barriers to the future research |
|---|---|---|---|
| Delle Karth 200547 |
|
|
NR |
| Hennersdorf 200246 |
|
Torsade de pointes (n = 3/26, 11.5%) | NR |
| Mayr 200349 |
|
NR | The optimal therapeutic regimen for effective and rapid termination of new-onset SVT in surgical ICU patients still remains to be established |
| Nakamura 201645 |
|
|
It would be worth to conduct the further study investigating the efficacy of this switching therapy in the future |
| Slavik 200340 (abstract) |
|
|
NR |
| Sleeswijk 200839 |
|
|
A randomized controlled trial is needed to investigate whether this strategy is superior to other treatment regimes |
| Study details | Population characteristics | Intervention | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
First author and year: Burris 201044 Setting: surgical ICU Country: USA Sample size: n = 30 Note: data extracted only for patients with atrial arrhythmias (total study n = 120: this includes controls that did not develop arrhythmias) NOAF patients: NR |
Primary diagnosis: general surgery (60%), vascular surgery (33%), orthopaedics (3.3%) and neurosurgery (3.3%) Mean age: 66.2 ± 7.3 years Male: NR Severity of illness: NR Patients on vasopressors: n = 14 (46.6%) (intraoperative) Patients with CVD: coronary artery disease, 40%; chronic heart failure 16.7% Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: 3.9 ± 66 mmol/l (preoperative) Definition of NOAF: NR |
Amiodarone (63%), metoprolol (26%), esmolol (13.3%), diltiazem (6.7%), digoxin (3.3%), multiple drug regimens (10%). Electrical cardioversion (only in combination with pharmacological treatment), n = 4 (13.3%) Line of NOAF treatment: not specified for each treatment |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
First author and year: Kanji 20128 Setting: three academic mixed medical/surgical ICUs Country: Canada Sample size: n = 139 NOAF patients: 100% |
Primary diagnosis: admission diagnosis reported – sepsis (18%), respiratory failure/pneumonia (19%), cardiogenic shock/cardiac arrest (4%), cerebrovascular accident (1%), rapid AF (4%), postoperative care (48%) and other (6%) Mean age: 71.6 ± 12.5 years Male: n = 83 (60%) Severity of illness: mean APACHE II score reported: 22.6 ± 9.0 Patients on vasopressors: NR Patients with CVD: coronary artery disease (29%), valvular heart disease (1%), congestive heart failure (6%) and cardiomyopathy (dilated, hypertrophic) (2%) Patients with acute renal failure: NR Patients with acute respiratory failure: n = 27 (19%) Mechanical ventilation at NOAF onset: NR Serum potassium: < 3.5 mmol/l, n = 15 (11%) Definition of NOAF: ‘Definition for NOAF cases reported as “defined as those with no previous documented history of any atrial arrhythmia documented in the physical or electronic medical record”’ |
Rhythm control attempted (n = 105) by administering i.v. amiodarone. Rate control attempted (n = 28) by administering beta-blockers, calcium channel blockers or digoxin, alone or in combination Line of NOAF treatment: not specified |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
First author and year: Kyo 201950 Setting: two mixed ICUs (emergency and medicosurgical ICU) Country: Japan Sample size: n = 85 NOAF patients: 100% |
Primary diagnosis: Type of primary diagnosisAll patients (N = 85), n (%)Successful electrical cardioversion (N = 41), n (%)Unsuccessful electrical cardioversion (N = 44), n (%)Cardiovascular33 (38)14 (34)19 (43)Pulmonary26 (31)15 (37)11 (25)Gastrointestinal10 (12)4 (10)6 (14)Neurology4 (5)1 (2)3 (7)Trauma3 (4)3 (7)–Skin and soft tissue5 (6)2 (5)3 (7)Other4 (5)2 (5)2 (5) Median age (range): all patients, 71 (64–78) years, successful electrical cardioversion, 71 (64–79) years; unsuccessful electrical cardioversion, 71 (62–77) years Male: all patients, n = 58 (68%) (successful electrical cardioversion, n = 30,73%; unsuccessful electrical cardioversion, n = 28, 64%) Severity of illness: APACHE II score at ICU admission, median (range) – all patients, 26 (17–34); successful electrical cardioversion, 26 (18–35); unsuccessful electrical cardioversion, 26 (16–31) SOFA score at the onset of AF, median (range): all patients 8 (5–11); successful electrical cardioversion, 8 (5–12); unsuccessful electrical cardioversion, 8 (5–9) Patients on vasopressors: VasopressorAll patients (N = 85), n (%)Successful electrical cardioversion (N = 41), n (%)Unsuccessful electrical cardioversion (N = 44), n (%)Noradrenaline24 (28)12 (29)12 (27)Dopamine21 (25)10 (24)11 (25)Dobutamine25 (29)14 (34)11 (25) Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: not specified whether at the time of onset – all patients, n = 71 (84%); successful electrical cardioversion, n = 37 (90%); unsuccessful electrical cardioversion, n = 34 (77%) Serum potassium (mmol/l), median (range): all patients, 4.0 (3.7–4.6); successful electrical cardioversion, 4.2 (3.9–4.8); unsuccessful electrical cardioversion, 3.9 (3.6–4.3) Definition of NOAF: ‘New-onset AF was defined as the first AF rhythm on ECG occurring during an ICU stay’ |
Type of primary diagnosis | All patients (N = 85), n (%) | Successful electrical cardioversion (N = 41), n (%) | Unsuccessful electrical cardioversion (N = 44), n (%) | Cardiovascular | 33 (38) | 14 (34) | 19 (43) | Pulmonary | 26 (31) | 15 (37) | 11 (25) | Gastrointestinal | 10 (12) | 4 (10) | 6 (14) | Neurology | 4 (5) | 1 (2) | 3 (7) | Trauma | 3 (4) | 3 (7) | – | Skin and soft tissue | 5 (6) | 2 (5) | 3 (7) | Other | 4 (5) | 2 (5) | 2 (5) | Vasopressor | All patients (N = 85), n (%) | Successful electrical cardioversion (N = 41), n (%) | Unsuccessful electrical cardioversion (N = 44), n (%) | Noradrenaline | 24 (28) | 12 (29) | 12 (27) | Dopamine | 21 (25) | 10 (24) | 11 (25) | Dobutamine | 25 (29) | 14 (34) | 11 (25) |
Electrical cardioversion: the 85 electrical cardioversion sessions included 142 shocks, with a median of one (IQR 1–2) shock per electrical cardioversion session. The delivered electrical cardioversion energy was ≤ 100 J in 91% of first shocks and 83% of second shocks in all patients Line of NOAF treatment: not specified |
||||||||||||||||||||||||||||||||||||||||
| Type of primary diagnosis | All patients (N = 85), n (%) | Successful electrical cardioversion (N = 41), n (%) | Unsuccessful electrical cardioversion (N = 44), n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cardiovascular | 33 (38) | 14 (34) | 19 (43) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pulmonary | 26 (31) | 15 (37) | 11 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gastrointestinal | 10 (12) | 4 (10) | 6 (14) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neurology | 4 (5) | 1 (2) | 3 (7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trauma | 3 (4) | 3 (7) | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Skin and soft tissue | 5 (6) | 2 (5) | 3 (7) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 4 (5) | 2 (5) | 2 (5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vasopressor | All patients (N = 85), n (%) | Successful electrical cardioversion (N = 41), n (%) | Unsuccessful electrical cardioversion (N = 44), n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noradrenaline | 24 (28) | 12 (29) | 12 (27) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dopamine | 21 (25) | 10 (24) | 11 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dobutamine | 25 (29) | 14 (34) | 11 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
First author and year: Liu 201641 Setting: medical ICU Country: Taiwan (Province of China) Sample size: n = 240 NOAF patients: 100% |
Primary diagnosis: sepsis Infection site: Infection siteNOAF to sinus rhythm (N = 165), n (%)NOAF to atrial fibrillation (AF) (N = 75), n (%)Respiratory tract112 (67.9)48 (64)Urinary tract35 (21.2)14 (18.7)Gastrointestinal9 (5.5)5 (6.7)Other9 (5.5)8 (10.7) Mean age: NOAF to sinus rhythm, 77.8 ± 10.3 years; NOAF to AF, 76.2 ± 11.0 years Male: NOAF to sinus rhythm, n = 90 (54.5%); NOAF to AF, n = 46 (61.3%) Severity of illness: mean SOFA score reported – NOAF to sinus rhythm, 7.6 ± 3.0; NOAF to AF, 9.3 ± 3.2. Mean APACHE II score: NOAF to sinus rhythm, 22.8 ± 5.8; NOAF to AF, 24.6 ± 6.1 Patients on vasopressors: VasopressorNOAF to sinus rhythm (N = 165), n (%)NOAF to AF (N = 75), n (%)Dopamine64 (38.8)49 (65.3)Noradrenaline58 (35.2)48 (64) Patients with CVD: Cardiovascular diseaseNOAF to sinus rhythm (N = 165), n (%)NOAF to AF (N = 75), n (%)Heart failure35 (21.2)15 (20)Coronary artery disease70 (42.4)37 (49.3) Patients with acute renal failure: NOAF to sinus rhythm, n = 65 (39.4%); NOAF to AF, n = 39 (52.0%) Patients with acute respiratory failure: NOAF to sinus rhythm n = 150 (90.9%); NOAF to AF, n = 71 (94.7%) Mechanical ventilation at NOAF onset: NOAF to sinus rhythm, n = 143 (86.7%); NOAF to AF, n = 69 (92.0%) Serum potassium (mmol/l): NOAF to AF, 4.2 ± 1.0; NOAF to sinus rhythm, 4.1 ± 0.9 Definition of NOAF: ‘The absence of P waves and irregular ventricular activity lasting for more than 30 seconds’. NOAF to AF defined as ‘persistent or recurrent AF 7 days after the onset of NOAF’ |
Infection site | NOAF to sinus rhythm (N = 165), n (%) | NOAF to atrial fibrillation (AF) (N = 75), n (%) | Respiratory tract | 112 (67.9) | 48 (64) | Urinary tract | 35 (21.2) | 14 (18.7) | Gastrointestinal | 9 (5.5) | 5 (6.7) | Other | 9 (5.5) | 8 (10.7) | Vasopressor | NOAF to sinus rhythm (N = 165), n (%) | NOAF to AF (N = 75), n (%) | Dopamine | 64 (38.8) | 49 (65.3) | Noradrenaline | 58 (35.2) | 48 (64) | Cardiovascular disease | NOAF to sinus rhythm (N = 165), n (%) | NOAF to AF (N = 75), n (%) | Heart failure | 35 (21.2) | 15 (20) | Coronary artery disease | 70 (42.4) | 37 (49.3) |
Amiodarone (n = 80, 33.3%), beta-blockers (n = 88, 36.7%), non-dihydropyridine calcium channel blockers (n = 66, 27.5%), digoxin glycosides (n = 27, 11.3%), electrical cardioversion (n = 8, 3.3%) Line of NOAF treatment: not specified for each treatment |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infection site | NOAF to sinus rhythm (N = 165), n (%) | NOAF to atrial fibrillation (AF) (N = 75), n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Respiratory tract | 112 (67.9) | 48 (64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Urinary tract | 35 (21.2) | 14 (18.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gastrointestinal | 9 (5.5) | 5 (6.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 9 (5.5) | 8 (10.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vasopressor | NOAF to sinus rhythm (N = 165), n (%) | NOAF to AF (N = 75), n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dopamine | 64 (38.8) | 49 (65.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noradrenaline | 58 (35.2) | 48 (64) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cardiovascular disease | NOAF to sinus rhythm (N = 165), n (%) | NOAF to AF (N = 75), n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heart failure | 35 (21.2) | 15 (20) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coronary artery disease | 70 (42.4) | 37 (49.3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
First author and year: Mayr 200443 Setting: 12-bed general and surgical ICU in a university teaching hospital Country: Austria Sample size: n = 131 NOAF patients: 93% |
Primary diagnosis: type of surgery Type of surgeryAll patients (N = 131), n (%)Responders (N = 98), n (%)Non-responders (N = 33), n (%)Cardiac61 (46.56)46 (46.9)15 (45.45)General53 (40.45)41 (41.8)12 (36.36)Vascular7 (5.3)5 (5.1)2 (6.2)Trauma5 (3.8)2 (2)3 (9.1)Orthopaedic5 (3.8)4 (4.1)1 (3) Sepsis: all patients, 23.9%; responders, 24%; non-responders, 23.6% Mean age: all patients, 68 ± 12 years (responders, 68 ± 12 years; non-responders, 67 ± 14 years) Male: all patients, n = 82 (62.6%) (responders, n = 58, 59.2%; non-responders, n = 24, 72.7%) Severity of illness: mean MODS reported: all patients, 7.5 ± 3.4 (responders, 7.4 ± 3.4; non-responders, 7.9 ± 3.5) Patients on vasopressors: NR Patients with CVD: NR Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium: NR Definition of NOAF: ‘New-onset supraventricular tachyarrhythmias were defined as “narrow-complex non-sinus tachyarrhythmias with heart rates ≥ 100 bpm lasting for longer than 30 minutes”’ |
Type of surgery | All patients (N = 131), n (%) | Responders (N = 98), n (%) | Non-responders (N = 33), n (%) | Cardiac | 61 (46.56) | 46 (46.9) | 15 (45.45) | General | 53 (40.45) | 41 (41.8) | 12 (36.36) | Vascular | 7 (5.3) | 5 (5.1) | 2 (6.2) | Trauma | 5 (3.8) | 2 (2) | 3 (9.1) | Orthopaedic | 5 (3.8) | 4 (4.1) | 1 (3) |
Amiodarone infusion: amiodarone was infused via central venous catheter at 90 mg/hour for a maximum of 12 hours, followed by a weaning regimen (initially 40–60 mg/hour for a maximum of 3 days, then 20 mg/hour for another 5–7 days). Amiodarone was continued orally (200 mg TDS) in some patients. The amiodarone infusion was stopped when the heart rate dropped below 60 b.p.m. Line of NOAF treatment: first line |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of surgery | All patients (N = 131), n (%) | Responders (N = 98), n (%) | Non-responders (N = 33), n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cardiac | 61 (46.56) | 46 (46.9) | 15 (45.45) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | 53 (40.45) | 41 (41.8) | 12 (36.36) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vascular | 7 (5.3) | 5 (5.1) | 2 (6.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trauma | 5 (3.8) | 2 (2) | 3 (9.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Orthopaedic | 5 (3.8) | 4 (4.1) | 1 (3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
First author and year: Mitrić 201642 Setting: medical-surgical trauma ICU Country: Australia Sample size: n = 177 (no recurrence of AF, n = 86; recurrence of AF, n = 91) NOAF patients: 100% |
Primary diagnosis: NR Mean age, median (range): all patients, 69 (60–75) years [no recurrence of AF, 65 (57–75) years; recurrence of AF, 71 (61–76)] Male: all patients, n = 113 (64%) (no recurrence of AF, n = 53, 61%; recurrence of AF, n = 60, 66%) Severity of illness, median (range): APACHE II score reported – all patients, 22 (17–28) [no recurrence of AF, 21 (17–26); recurrence of AF, 23 (17–29)]. SAPS II reported: all patients, 41 (31–53) [no recurrence of AF, 39 (30–49); recurrence of AF, 44 (31–58)]. Charlson Comorbidity Index Score reported: all patients, 2 (1–4) [no recurrence of AF, 2 (1–4); recurrence of AF, 3 (2–5)] Patients on vasopressors: NR Patients with CVD: Cardiovascular diseaseAll patients (N = 177), n (%)No recurrent AF (N = 86), n (%)Recurrent AF (N = 91), n (%)Myocardial infarction43 (24)17 (20)26 (29)Congestive cardiac failure22 (12)6 (7)16 (18)Ischaemic heart disease58 (33)23 (27)35 (38)Rheumatic heart disease2 (1)2 (2)0 (0)Mitral valve disease9 (5)7 (8)2 (2) Patients with acute renal failure: NR Patients with acute respiratory failure: NR Mechanical ventilation at NOAF onset: NR Serum potassium (mmol/l): reported as median (IQR) for the recurrent AF group only ParameterOn the day AF initially reverted (n = 81)aOn day AF recurred (n = 73)aKmin4.2 (3.8–4.5)4.2 (4.0–4.6)Kmax4.3 (4.1–4.7)4.4 (4.1–4.7)a Definition of NOAF: ‘a rhythm on the electrocardiogram (ECG) with replacement of P waves with rapid oscillations or fibrillatory waves that vary in size, shape and timing, associated with an irregular, frequently rapid, ventricular response when atrioventricular conduction is intact’ |
Cardiovascular disease | All patients (N = 177), n (%) | No recurrent AF (N = 86), n (%) | Recurrent AF (N = 91), n (%) | Myocardial infarction | 43 (24) | 17 (20) | 26 (29) | Congestive cardiac failure | 22 (12) | 6 (7) | 16 (18) | Ischaemic heart disease | 58 (33) | 23 (27) | 35 (38) | Rheumatic heart disease | 2 (1) | 2 (2) | 0 (0) | Mitral valve disease | 9 (5) | 7 (8) | 2 (2) | Parameter | On the day AF initially reverted (n = 81)a | On day AF recurred (n = 73)a | Kmin | 4.2 (3.8–4.5) | 4.2 (4.0–4.6) | Kmax | 4.3 (4.1–4.7) | 4.4 (4.1–4.7) | a |
Amiodarone: a bolus dose was defined as a fixed dose of > 150 mg given over 20 minutes to an hour, a continuous infusion was a fixed dose of amiodarone delivered hourly by a syringe pump for > 2 hours and delay to an infusion was a gap of 1 hour in the fluid administration record for the administration of a bolus and the start of an infusionParameterAll patients (N = 177)No AF recurrence (N = 86)AF recurrence (N = 91)No. of amiodarone boluses, n (%) 062 (35)43 (42)19 (25) 198 (55)51 (50)47 (61) 212 (7)5 (5)7 (9) 35 (3)2 (2)3 (4)Amiodarone dosing, n (%) Bolus only23 (13)3 (3)20 (23) Infusion only62 (35)43 (50)19 (25) Bolus and infusion92 (52)40 (47)52 (57)Delay to infusion after bolus, hours, median (IQR); n2 (1–4); 742 (1–3); 292 (1–6); 45Total dose (mg), median (IQR)905 (488–1651)702 (300–1117)1366 (752–2711)Infusion time (hours), median (IQR)24 (16–40)20 (12–28)31 (20–58) Line of NOAF treatment: first line |
Parameter | All patients (N = 177) | No AF recurrence (N = 86) | AF recurrence (N = 91) | No. of amiodarone boluses, n (%) | 0 | 62 (35) | 43 (42) | 19 (25) | 1 | 98 (55) | 51 (50) | 47 (61) | 2 | 12 (7) | 5 (5) | 7 (9) | 3 | 5 (3) | 2 (2) | 3 (4) | Amiodarone dosing, n (%) | Bolus only | 23 (13) | 3 (3) | 20 (23) | Infusion only | 62 (35) | 43 (50) | 19 (25) | Bolus and infusion | 92 (52) | 40 (47) | 52 (57) | Delay to infusion after bolus, hours, median (IQR); n | 2 (1–4); 74 | 2 (1–3); 29 | 2 (1–6); 45 | Total dose (mg), median (IQR) | 905 (488–1651) | 702 (300–1117) | 1366 (752–2711) | Infusion time (hours), median (IQR) | 24 (16–40) | 20 (12–28) | 31 (20–58) | ||||||||
| Cardiovascular disease | All patients (N = 177), n (%) | No recurrent AF (N = 86), n (%) | Recurrent AF (N = 91), n (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Myocardial infarction | 43 (24) | 17 (20) | 26 (29) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Congestive cardiac failure | 22 (12) | 6 (7) | 16 (18) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ischaemic heart disease | 58 (33) | 23 (27) | 35 (38) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rheumatic heart disease | 2 (1) | 2 (2) | 0 (0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mitral valve disease | 9 (5) | 7 (8) | 2 (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter | On the day AF initially reverted (n = 81)a | On day AF recurred (n = 73)a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kmin | 4.2 (3.8–4.5) | 4.2 (4.0–4.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kmax | 4.3 (4.1–4.7) | 4.4 (4.1–4.7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter | All patients (N = 177) | No AF recurrence (N = 86) | AF recurrence (N = 91) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. of amiodarone boluses, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 62 (35) | 43 (42) | 19 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 98 (55) | 51 (50) | 47 (61) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 12 (7) | 5 (5) | 7 (9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 5 (3) | 2 (2) | 3 (4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amiodarone dosing, n (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bolus only | 23 (13) | 3 (3) | 20 (23) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infusion only | 62 (35) | 43 (50) | 19 (25) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bolus and infusion | 92 (52) | 40 (47) | 52 (57) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Delay to infusion after bolus, hours, median (IQR); n | 2 (1–4); 74 | 2 (1–3); 29 | 2 (1–6); 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total dose (mg), median (IQR) | 905 (488–1651) | 702 (300–1117) | 1366 (752–2711) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infusion time (hours), median (IQR) | 24 (16–40) | 20 (12–28) | 31 (20–58) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study | Results | Adverse effects | Recommendations for/barriers to the future research |
|---|---|---|---|
| Burris 201044 |
|
NR | Randomized prospective studies are required to determine the success of alternative treatments and should provide the evidence needed to streamline management of this problem |
| Kanji 20128 |
|
NR | In the general adult critically ill population, more research is required to determine (1) whether or not attempting rhythm control is more effective at restoring sinus rhythm than attempting rate control alone, (2) whether or not attempting rhythm control improves clinical outcomes, and (3) what is the optimal anticoagulation strategy in patients who develop NOAF |
| Kyo 201950 |
|
NR | Further studies are needed to investigate the potential factors associated with the maintenance of SR [sinus rhythm] to establish a better understanding of new-onset AF in critically ill patients |
| Liu 201641 |
|
NR | A larger, prospective comparative study is needed to elucidate the clinical implications between a rate control and a rhythm control strategy in patients with sepsis and NOAF |
| Mayr 200443 |
|
Increases in serum concentrations of creatinine and bilirubin were observed | NR |
| Mitrić 201642 |
|
NR | A clear dosing guide is not available and further research is required to elicit the best dosing strategy |
Appendix 4 Excluded studies
| Study | Excluded based on | |||
|---|---|---|---|---|
| Population | Study design | Outcome | Intervention | |
| American Association of Critical Care Nurses (AACN). Preventing new-onset atrial fibrillation in the ICU. AACN Bold Voices 2018;10:20 | ✗ | |||
| Agnihotri K, Patel P, Charilou P, Patel NJ, Badheka A, Noseworthy P, et al. Impact of Atrial Fibrillation on Mortality, Length of Stay and Cost in Patients with Sepsis. Proceedings of the 38th Annual Scientific Sessions of the Heart Rhythm Society, Heart Rhythm, 10–13 May 2017, Chicago, IL, USA | ✗ | |||
| Akella K, Akella S, Akella SL, Chendrasekhar A. Atrial Fibrillation in Elderly (Age > 65 Years) Trauma Patients is Associated with Increased Mortality and Morbidity. Paper presented at the CHEST 2017 Annual Meeting, Canada, 2017. Cardiovasc Dis 2017;152:A66 | ✗ | |||
| Akhtar MI, Ullah H, Hamid M. Magnesium, a drug of diverse use. J Pak Med Assoc 2011;61:1220–5 | ✗ | |||
| Al-Hashimi M, Thompson JP. Drugs acting on the heart: anti-arrhythmics. Anaesth and Intensive Care Med 2012;13:374–7 | ✗ | |||
| Al-Khafaji A, Cho Su M. Atrial fibrillation in critical care. (Comment on: Intensive Care Med 2006 Mar;32:398–404) 2006;32:1099–100 | ✗ | |||
| Ambrus DB, Benjamin EJ, Bajwa EK, Hibbert KA, Walkey AJ. Risk factors and outcomes associated with new-onset atrial fibrillation during acute respiratory distress syndrome. J Crit Care 2015;30:994–7 | ✗ | |||
| Anane C, Owusu IK, Attakorah J. Monitoring amiodarone therapy in cardiac arrthythmias in the intensive care unit of a teaching hospital in Ghana. Int J Cardiol 2011;10 | ✗ | |||
| Ando G, Di Rosa S, Rizzo F, Carerj S, Bramanti O, Giannetto M, et al. Ibutilide for cardioversion of atrial flutter: efficacy of a single close in recent-onset arrhythmias. Minerva Cardioangiologica 2004;52:37–42 | ✗ | |||
| Arita Y, Segawa T, Yamamoto S, Hasegawa S. Landiolol is effective for the treatment of tachycardia-induced cardiogenic shock in patients during septic shock therapy. BMJ Case Rep 2017;2017:bcr–2017–222268 | ✗ | |||
| Arnautovic J, Mazhar A, Souther B, Mikhjian G, Huda N. New-Onset Atrial Fibrillation in Patients with Septic Shock. Proceedings of the 47th Society of Critical Care Medicine Critical Care Congress (SCCM), 25–28 February 2018, San Antonio, TX, USA, abstract number 184 | ✗ | |||
| Arrigo M, Bettex D, Rudiger A. Management of atrial fibrillation in critically ill patients. Crit Care Res Pract 2014;2014:840615 | ✗ | |||
| Arrigo M, Bettex D, Rudiger A. [Treatment of atrial fibrillation in intensive care units and emergency departments.] Med Klin Intensivmed Notfmed 2015;110:614–20 | ✗ | |||
| Arrigo M, Bettex D, Rudiger A. Response to: comment on ‘Management of Atrial Fibrillation in Critically Ill Patients’. Crit Care Res Pract 2016;2016:9724504 | ✗ | |||
| Arrigo M, Feliot E, Gayat E, Mebazaa A. Cardiovascular events after ICU discharge in patients with new-onset atrial fibrillation: a report from the FROG-ICU study. Int J Cardiol 2018;270:203 | ✗ | |||
| Arrigo M, Ishihara S, Feliot E, Rudiger A, Deye N, Cariou A, et al. New-onset atrial fibrillation in critically ill patients and its association with mortality: a report from the FROG-ICU study. Int J Cardiol 2018;266:95–9 | ✗ | |||
| Arsura EL, Solar M, Lefkin AS, Scher DL, Tessler S. Metoprolol in the treatment of multifocal atrial tachycardia. Crit Care Med 1987;15:591–4 | ✗ | |||
| Aydogdu M, Hanazay C, Aldag Y, Baha A, Bilgin S, Gursel G. Effects of atrial fibrillation on intensive care unit outcomes in patients with respiratory failure. J Med Surg Intensive Care Med 2017;8:32–8 | ✗ | |||
| Badheka AO, Tuliani T, Rathod A, Shenoy M, Afonso L, Jacob S. Role of lipid lowering therapy and renin angiotensin blockade in outcomes of patients with atrial fibrillation. Am J Cardiol 2012;109:1238 | ✗ | |||
| Balik M. New-onset atrial fibrillation in critically ill patients – implications for rhythm rather than rate control therapy? Int J Cardiol 2018;266:147–8 | ✗ | |||
| Balik M, Kolnikova I, Maly M, Waldauf P, Tavazzi G, Kristof J. Antiarrhythmic Therapy for Supraventricular Arrhythmias in Septic Shock. Proceedings of the 29th Annual Congress of the European Society of Intensive Care Medicine (ESICM), 1–5 October 2016, Milan, Italy, abstract number A793 | ✗ | |||
| Barranco F, Sanchez M, Rodriguez J, Guerrero M. Efficacy of flecainide in patients with supraventricular arrhythmias and respiratory insufficiency. Intensive Care Med 1994;20:42–4 | ✗ | |||
| Bender JS. Supraventricular tachyarrhythmias in the surgical intensive care unit: an under-recognized event. Am Surg 1996;62:73–5 | ✗ | |||
| Bernal E, Wolf S, Cripps M. New-onset, postoperative tachyarrhythmias in critically ill surgical patients. Burns 2018;44:249–55 | ✗ | |||
| Bernard EO, Schmid ER, Schmidlin D, Scharf C, Candinas R, Germann R. Ibutilide versus amiodarone in atrial fibrillation: a double-blinded, randomized study. Crit Care Med 2003;31:1031–4 | ✗ | |||
| Bowles HF, Thangathurai D, Morgan GE, Mikhail M. Management of supraventricular tachycardia in septic patients. Anaesthesia 1990;45:787–8 | ✗ | |||
| Carrera P, Thongprayoon C, Cheungpasitporn W, Iyer VN, Moua T. Epidemiology and outcome of new-onset atrial fibrillation in the medical intensive care unit. J Crit Care 2016;36:102–6 | ✗ | |||
| Champion S. Comment on ‘management of atrial fibrillation in critically ill patients’. Crit Care Res Pract 2015;2015:732598 | ✗ | |||
| Champion S. An overlook of new-onset atrial fibrillation in the critically ill using automated detection: have we over looked at it? Crit Care Med 2017;45:e1195 | ✗ | |||
| Champion S, Gaüzère BA, Vandroux D, Lefort Y. [Is it worth delivering Direct-Current Counter shock to critically ill patients with supra-ventricular tachyarrhythmia?] Ann Cardiol Angeiol 2018;67:260–3 | ✗ | |||
| Chapman MJ, Moran JL, O’Fathartaigh MS, Peisach AR, Cunningham DN. Management of atrial tachyarrhythmias in the critically ill: a comparison of intravenous procainamide and amiodarone. Intensive Care Med 1993;19:48–52 | ✗ | |||
| Clayton B, Ball S, Read J, Waddy S. Risk of thromboembolism in patients developing critical illness-associated atrial fibrillation. Clin Med 2018;18:282–7 | ✗ | |||
| Clemo HF, Wood MA, Gilligan DM, Ellenbogen KA. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol 1998;81:594–8 | ✗ | |||
| Crawford TC, Oral H. Cardiac arrhythmias: management of atrial fibrillation in the critically ill patient. Crit Care Clin 2007;23:855–72, vii | ✗ | |||
| Darwish OS, Strube S, Phan A, Tanios M. The Safety and Efficacy of Anticoagulation for Atrial Fibrillation in Patients with Severe Sepsis in the Medical Intensive Care Unit. Proceedings of the American Heart Association Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke 2010 Scientific Sessions, 19–21 May 2010, Washington, DC, USA | ✗ | |||
| Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother 2013;47:1266–71 | ✗ | |||
| Davies GE, Cudworth P, Lawler PG. Intravenous magnesium therapy in critically ill patients. Anaesthesia 1992;47:1104 | ✗ | |||
| Delle Karth G, Reinelt P, Buberl A, Geppert A, Huelsmann M, Berger R, Heinz G. Circadian variation in ventricular tachycardia and atrial fibrillation in a medical-cardiological ICU. Intensive Care Med 2003;29:963–8 | ✗ | |||
| Duarte PAD, Leichtweis GE, Andriolo L, Delevatti YA, Jorge AC, Fumagalli AC, et al. Factors associated with the incidence and severity of new-onset atrial fibrillation in adult critically ill patients. Crit Care Res Pract 2017;2017:8046240 | ✗ | |||
| Duarte PAD, Leichtweis GE, Andriolo L, Delevatti YA, Jorge AC, Fumagalli AC, et al. Corrigendum to ‘Factors Associated with the Incidence and Severity of New-Onset Atrial Fibrillation in Adult Critically Ill Patients’. Crit Care Res Pract 2019;2019:5710734 | ✗ | |||
| Duby JJ, Heintz SJ, Bajorek SA, Heintz BH, Durbin-Johnson BP, Cocanour CS. Prevalence and course of atrial fibrillation in critically ill trauma patients. J Intensive Care Med 2017;32:140–5 | ✗ | |||
| Eckardt L. Innovations to the therapy of atrial fibrillations in intensive care medicine. Medizinische Klinik-intensivmedizin Und Notfallmedizin 2014;109:564–5 | ✗ | |||
| Edwards JD, Kishen R. Significance and management of intractable supraventricular arrhythmias in critically ill patients. Crit Care Med 1986;14:280–2 | ✗ | |||
| Edwards JD, Wilkins RG. Atrial fibrillation precipitated by acute hypovolaemia. BMJ (Clin Res Ed) 1987;294:283–4 | ✗ | |||
| Faniel R, Schoenfeld P. Intravenous amiodarone: a successful treatment for rapid atrial fibrillation in intensive care patients. Eur Heart J 1981;2:115 | ✗ | |||
| Flato Uri Adrian P, Buhatem T, Merluzzi T, Bianco Antonio Carlos M. New anticoagulants in critical care settings. Rev Bras Ter Intensiva 2011;23:68–77 | ✗ | |||
| Friedman HZ, Goldberg SF, Bonema JD, Cragg DR, Hauser AM. Acute complications associated with new-onset atrial fibrillation. Am J Cardiol 1991;67:437–9 | ✗ | |||
| Fu J, Bi H, Xia Y, Fang H, Liu X, Tang Y, Wang D. [Risk factors of atrial fibrillation in critical ill patients.] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018;30:337–41 | ✗ | |||
| Gandhi S, Litt D, Narula N. New-onset atrial fibrillation in sepsis is associated with increased morbidity and mortality. Neth Heart J 2015;23:82–8 | ✗ | |||
| Ganguly S, Brown T, Pritchett C, Edie S, Allan P, Spivey M. Atrial fibrillation in intensive care. Intensive Care Med Exp 2015;3:A209 | ✗ | |||
| Gibb S, Rehberg S. The role of the ultra short-acting beta1-adrenoreceptor antagonist Landiolol in the treatment of atrial fibrillation: pharmacology, clinical application and current evidence in anaesthesiology, intensive care and emergency medicine. Clinical Anaesthesia 2018;59:407–21 | ✗ | |||
| Goodman S, Weiss Y, Weissman C. Update on cardiac arrhythmias in the ICU. Curr Opin Crit Care 2008;14:549–54 | ✗ | |||
| Gray RJ. Managing critically ill patients with esmolol. An ultra short-acting beta-adrenergic blocker. Chest 1988;93:398–403 | ✗ | |||
| Gray J, Haydock P, Wong A, Pierce JMT. Cardiac arrhythmias in the critically ill. Anaesth Intensive Care 2016;17:38–47 | ✗ | |||
| Guenancia C, Laurent G, Bruyère R, Quenot JP. New-onset atrial fibrillation in sepsis: so common, but so different. Crit Care Med 2016;44:e306–7 | ✗ | |||
| Guenancia C, Dargent A, Large A, Andreu P, Quenot JP. New-onset atrial fibrillation in ICU: a FROG in the throat. Int J Cardiol 2018;270:189 | ✗ | |||
| Guha K. Atrial fibrillation. Clin Med (Lond) 2019;19:90 | ✗ | |||
| Guillot M, Diouf M, Harlay ML, Janssen-Langenstein R, Lutun P, Schenck M, et al. [Supraventricular rhythm disorders of the rate/rhythm in critically ill patients.] Réanimation 2009;18:246–53 | ✗ | |||
| Hadjizacharia P, O’Keeffe T, Brown CV, Inaba K, Salim A, Chan LS, et al. Incidence, risk factors, and outcomes for atrial arrhythmias in trauma patients. Am Surg 2011;77:634–9 | ✗ | |||
| Hampden-Martin A, Miller N, Johnston B, Welters I. A Retrospective Observational Cohort Study of Patients Admitted to a Mixed Medical and Surgical Intensive Care Unit in the United Kingdom with Atrial Fibrillation: Incidence, Risk Factors and Treatment Efficacy. Proceedings of the 31st European Society of Intensive Care Medicine Annual Congress (ESICM), 20–24 October 2018, Paris, France | ✗ | |||
| Harazim M, Karvunidis T, Radej J, Horak J, Novak I, Matejovic M. [Atrial fibrillation in critically ill patients.] Anesteziol Intenzivní Med 2017;28:248–54 | ✗ | |||
| Hayashi M, Tanaka K, Kato T, Morita N, Sato N, Yasutake M, et al. Enhancing electrical cardioversion and preventing immediate reinitiation of hemodynamically deleterious atrial fibrillation with class III drug pretreatment. J Cardiovasc Electrophysiol 2005;16:740–7 | ✗ | |||
| Heinz G. Atrial fibrillation in the intensive care unit. Intensive Care Med 2006;32:345–8 | ✗ | |||
| Heinz G. Arrhythmias in the ICU: what do we know? Am J Respir Crit Care Med 2008;178:1–2 | ✗ | |||
| Heinz G. [Atrial fibrillation in the ICU. Distinct entity--special treatment?] Leitthema 2013;108:549–54 | ✗ | |||
| Hilkens M, Pickkers P, Peters WH, van der Hoeven JG. No elevation of glutathione S-transferase-a1-1 by amiodarone loading in intensive care unit patients with atrial fibrillation. Anaesth Intensive Care 2009;37:281–5 | ✗ | |||
| Holt AW. Hemodynamic responses to amiodarone in critically ill patients receiving catecholamine infusions. Crit Care Med 1989;17:1270–6 | ✗ | |||
| Hughes M, Binning A. Intravenous amiodarone in intensive care. Time for a reappraisal? Intensive Care Med 2000;26:1730–9 | ✗ | |||
| Iberti TJ, Benjamin E, Paluch TA, Gentili DR, Gabrielson GV. Use of constant-infusion verapamil for the treatment of postoperative supraventricular tachycardia. Crit Care Med 1986;14:283–4 | ✗ | |||
| Ito H, Sobue K, So M, Hirate H, Sugiura T, Azami T, et al. Use of landiolol in the perioperative management of supraventricular tachycardia. J Anesth 2006;20:253–4 | ✗ | |||
| Jelliffe R. Comment on ‘Management of Atrial Fibrillation in Critically Ill Patients’. Crit Care Res Pract 2016:1–2 | ✗ | |||
| Jenkins SA, Griffin R. New-Onset Atrial Fibrillation in Intensive Care: Incidence, Management and Outcome. Proceedings of the 36th International Symposium on Intensive Care and Emergency Medicine, 15–18 March 2016, Brussels, Belgium | ✗ | |||
| Kanjanahattakij N, Rattanawong P, Krishnamoorthy P, Horn B, Chongsathidkiet P, Garvia V, et al. New-onset atrial fibrillation is associated with increased mortality in critically ill patients: a systematic review and meta-analysis. Acta Cardiol 2019;74:162–9 | ✗ | |||
| Karth GD, Geppert A, Haumer M, Neunteufl T, Innerhofer P, Priglinger U, et al. Tachycardial atrial fibrillation and atrial flutter in intensive-care patients: comparison of diltiazem and amiodarone for control of heart rate – preliminary results. J Kardiol 2000;7:50 | ✗ | |||
| Kerton M, Wiggins J, Purkiss M. Cardiac arrhythmias in the critically ill. Anaesth Intensive Care 2018;19:298–307 | ✗ | |||
| Khoguli SS, Hopkinson RB, Beattie JM, Parmar M, Grant IS. Supraventricular tachydysrhythmias in the ICU. Br J Intensive Care 1994;4:44 | ✗ | |||
| Khoury J, Azzam ZS. Propafenone for supraventricular arrhythmias in septic shock – comparison to amiodarone and metoprolol. J Crit Care 2018;45:247 | ✗ | |||
| Klein Klouwenberg PM, Frencken JF, Kuipers S, Ong DS, Peelen LM, van Vught LA, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med 2017;195:205–11 | ✗ | |||
| Krumpl G, Domanovits H, Stix G, Heinz G, Hasenohrl N. Esmolol in cardiology, emergency and critical-care medicine. J Kardiol 2012;19:2–8 | ✗ | |||
| Kumar A. Intravenous amiodarone for therapy of atrial fibrillation and flutter in critically ill patients with severely depressed left ventricular function. South Med J 1996;89:779–85 | ✗ | |||
| Labakis M, Makrygiannis S, Margariti A, Rizikou D, Labakis S, Tselioti P, et al. Prognostic Impact of New-Onset Atrial Fibrillation in Intensive Care Unit Patients. Proceedings from the 26th Annual Congress of the European Society of Intensive Care Medicine (ESICM), 5–9 October 2013, Paris, France | ✗ | |||
| Leelathanalerk A, Dongtai W, Huckleberry Y, Kopp B, Bloom J, Alpert J. Evaluation of deprescribing amiodarone after new-onset atrial fibrillation in critical illness. Am J Med 2017;130:864–6 | ✗ | |||
| Lewis O, Ngwa J, Gillum RF, Thomas A, Davis W, Poddar V, et al. Incidence, risk factors and outcomes of new onset supraventricular arrhythmias in African American patients with severe sepsis. Ethn Dis 2016;26:205–12 | ✗ | |||
| Lim HS, Hamaad A, Lip GY. Clinical review: clinical management of atrial fibrillation – rate control versus rhythm control. Crit Care 2004;8:271–9 | ✗ | |||
| Makrygiannis SS, Margariti A, Rizikou D, Lampakis M, Vangelis S, Ampartzidou OS, et al. Incidence and predictors of new-onset atrial fibrillation in noncardiac intensive care unit patients. J Crit Care 2014;29:697.e1–5 | ✗ | |||
| Malik A, Candilio L, Hausenloy DJ. Atrial fibrillation in the intensive care setting. J Intensive Care Soc 2013;14:141–9 | ✗ | |||
| Marik PE, Zaloga GP. The management of atrial fibrillation in the ICU. J Intensive Care Med 2000;15:181–90 | ✗ | |||
| Marinheiro AR, Amador P, Ribeiro R, Alves I, Domingos G, Praxedes V, et al. Incidence, management and impact of atrial fibrillation in septic shock. Acute Cardiovasc Care 2016;5:60–1 | ✗ | |||
| Marque S, Launey Y. Traitement de la fibrillation atriale en reanimation (hors anticoagulation). Réanimation 2012;21:180–7 | ✗ | |||
| Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bögelein D, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care 2010;14:R108 | ✗ | |||
| Miller N, Hampden-Martin A, Johnston B, Welters I. Retrospective Observational Cohort Study of Anticoagulation Practices, Thromboembolic Events and Bleeding Events in Patients that Develop Atrial Fibrillation Admitted to a Mixed Surgical and Medical ICU in the United Kingdom. Proceedings of the 31st European Society of Intensive Care Medicine Annual Congress (ESICM), 20–24 October 2018, Paris, France | ✗ | |||
| Mirhoseini MF, Hamblin SE, Moore WP, Pouliot J, Jenkins JM, Wang W, et al. Antioxidant supplementation and atrial arrhythmias in critically ill trauma patients. J Surg Res 2018;222:10–16 | ✗ | |||
| Moran JL, Gallagher J, Peake SL, Cunningham DN, Salagaras M, Leppard P. Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med 1995;23:1816–24 | ✗ | |||
| Moskowitz A, Chen K, Cooper A, Chahin A, Ghassemi M, Celi L. Management of atrial fibrillation with rapid ventricular response in the intensive care unit: a secondary analysis of electronic health record data. Shock 2017;48:436–40 | ✗ | |||
| Moss TJ, Ruminski C, Lake DE, Calland JF, Enfield KB, Moorman JR. The Impact of Incident Atrial Fibrillation in the Intensive Care Unit. Proceedings of the 65th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention (ACC16), 2–4 April 2016, Chicago, IL, USA | ✗ | |||
| Murcia Llacer B, Broch Porcar MJ, Olivares Toledo D, Alvarez Cebrian F, Tejeda Adell M, Valentin Segura V. Intravenous amiodarone in the treatment of supraventricular tachyarrhytmias in critically ill patients with different diseases. Medicina Intensiva 1995;19:118–24 | ✗ | |||
| Nadler S. What is the best rate control agent for patients with sepsis and atrial fibrillation? Crit Care Alert 2016;24:4–5 | ✗ | |||
| Nair GM, Morillo CA. Magnesium in the acute management of atrial fibrillation: noise or music? Pol Arch Med Wewn 2007;117:446–7 | ✗ | |||
| Nakos G, Roustanis E, Zikou X, Saranti M, Zylis G, Papathanakos G, et al. Prevalence of Atrial Fibrillation in the Intensive Care Unit (ICU) Setting and Challenges in its Management. An Observational, Prospective, Single-Center Study. Proceedings of the American Thoracic Society International Conference, ATS 2015, May 15–20 2015, Denver, CO, USA | ✗ | |||
| Nallapareddy S, Naik M, Nortje J. Audit on management of new onset atrial fibrillation in general intensive care unit at university teaching hospital. Intensive Care Med 2010;36:S123–S | ✗ | |||
| Needleman JS, Yeh DD, Belcher DM, Quraishi SA. Vitamin D Status is Associated with New-Onset Atrial Fibrillation in Critically Ill Surgical Patients. Proceedings of Clinical Nutrition Week (CNW) 2017, 18–21 February 2017, Orlando, FL, USA | ✗ | |||
| Niazi OT, Alkhalil A, Olusanya A, Jain S, Golbari S, Banerjee R, et al. Comparison of In-Hospital Outcomes Of Septic Shock Patients with and Without Atrial Fibrillation: a Retrospective Propensity-Matched Analysis. Proceedings of the 66th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC17, 17–19 March 2017, Washington, DC, USA | ✗ | |||
| Nietupski R, Bellamy C, Miano T, Mikkelsen M, Candeloro C. Continuation of Amiodarone at Discharge For New-Onset Atrial Fibrillation in Critically Ill Patients. Proceedings of the Society of Critical Care Medicine (SCCM) 44th Critical Care Congress, 17–21 January 2015, Phoenix, AZ, USA, abstract no. 178 | ✗ | |||
| Nitti C, Falsetti L, Rucco M, Gentili T, Nobili L, Zaccone V, et al. CHA2DS2-VASc and HAS-BLED Scores do not Predict NVAF-related Events in a Population of Critically Ill Patients. Proceedings of 23rd Congresso Nazionale della Societa Scientifica FADOI, 12–15 May 2018, Bolonga, Italy | ✗ | |||
| Nowakowski PA. Supraventricular tachyarrhythmias in the intensive care unit. Postgrad Med 1992;92:34 | ✗ | |||
| Okajima M, Takamura M, Taniguchi T. Landiolol, an ultra-short-acting β1-blocker, is useful for managing supraventricular tachyarrhythmias in sepsis. World J Crit Care Med 2015;4:251–7 | ✗ | |||
| Papathanasiou A, Roustanis E, Zikou X, Galiatsou E, Papathanakos G, Zilis G, et al. The burden of new onset atrial fibrillation in the intensive care unit (ICU). An observational, prospective, single-center study. Intensive Care Med Exp 2015;3:A212 | ✗ | |||
| Personett HA, Smoot DL, Stollings JL, Sawyer M, Oyen LJ. Intravenous metoprolol versus diltiazem for rate control in noncardiac, nonthoracic postoperative atrial fibrillation. Ann Pharmacother 2014;48:314–19 | ✗ | |||
| Philip I, Allou N, Mouren S, Bourel P. Atrial fibrillation and anaesthesia-intensive care: an update. Fibrillation auriculaire et anesthesie-reanimation: Mise au point. Sang Thrombose Vaisseaux 2010;22:311–20 | ✗ | |||
| Pinski SL. Atrial fibrillation in the surgical intensive care unit: common but understudied. Crit Care Med 2004;32:890–1 | ✗ | |||
| Poveda-Jaramillo R, Monaco F, Zangrillo A, Landoni G. Ultra-short-acting β-blockers (esmolol and landiolol) in the perioperative period and in critically ill patients. J Cardiothorac Vasc Anesth 2018;32:1415–25 | ✗ | |||
| Pravinkumar E. Rate and rhythm are important in critically ill patients. BMJ 2003;327:931–2 | ✗ | |||
| Pye M, Camm AJ. Use of antiarrhythmic drugs in intensive care. Clin Intensive Care 1994;5:303–10 | ✗ | |||
| Quasim T, Riddell L, Kinsella J. Atrial fibrillation in the intensive care unit. Crit Care Med 2010;38:U124–U | ✗ | |||
| Quon MJ, Behlouli H, Pilote L. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC Clin Electrophysiol 2018;4:386–93 | ✗ | |||
| Ratnaparkhi AB, Walton J. The practice of the use of anticoagulation for acute-onset atrial fibrillation in the intensive care units of the north east region of UK; a regional audit. Intensive Care Med 2010;36:S124–S | ✗ | |||
| Richards KJC, Cohen AT. Cardiac arrhythmias in the critically ill. Anaesth Intensive Care Med 2006;7:289–93 | ✗ | |||
| Rudiger A, Breitenstein A, Arrigo M, Salzberg S, Bettex D. Suitability, Safety and Efficacy of Vernakalant for New Onset Atrial Fibrillation in Critically Ill Patients. Proceedings of the 26th Annual Congress of the European Society of Intensive Care Medicine (ESICM), 5–9 October 2013, Paris, France, abstract no. 0275 | ✗ | |||
| Salem JE, El-Aissaoui M, Alazard M, Hulot JS, Aissaoui N, Le-Heuzey JY, et al. Modeling of amiodarone effect on heart rate control in critically ill patients with atrial tachyarrhythmias. Clin Pharmacokinet 2016;55:991–1002 | ✗ | |||
| Salem JE, Dureau P, Funck-Brentano C, Hulot JS, El-Aissaoui M, Aissaoui N, et al. Effectiveness of heart rate control on hemodynamics in critically ill patients with atrial tachyarrhythmias managed by amiodarone. Pharmacol Res 2017;122:118–26 | ✗ | |||
| Salerno DM. Supraventricular tachyarrhythmias in the intensive care unit. Postgrad Med 1992;91:293–6, 299–303, 307–10 | ✗ | |||
| Salman Salam S, Bajwa Abubakr A, Afessa B. Paroxysmal atrial fibrillation in patients with sepsis admitted to the intensive care unit (ICU). Chest 2006;130:225S | ✗ | |||
| Samarin MJ, Mohrien KM, Oliphant CS. Continuous intravenous antiarrhythmic agents in the intensive care unit: strategies for safe and effective use of amiodarone, lidocaine, and procainamide. Crit Care Nurs Q 2015;38:329–44 | ✗ | |||
| Sanai L, Armstrong IR. Supraventricular tachydysrhythmias in the critically ill. A review of antidysrhythmic therapy in patients with SVT. J Intensive Care 1993;3:358 | ✗ | |||
| Sargent DA. Using ibutilide to convert atrial fibrillation and flutter. Dimens Crit Care Nurs 1999;18:2–12 | ✗ | |||
| Schoaps R, Donovan J, Hazard S, Karamchandani K. Anticoagulation on Hospital Discharge in Critically Ill Patients with New-Onset Atrial Fibrillation. Proceedings of the 46th Critical Care Congress of the Society of Critical Care Medicine (SCCM), 21–25 January 2017, Honolulu, HI, USA, abstract no. 204 | ✗ | |||
| Seemann A, Boissier F, Razazi K, Carteaux G, de Prost N, Brun-Buisson C, Mekontso Dessap A. New-onset supraventricular arrhythmia during septic shock: prevalence, risk factors and prognosis. Ann Intensive Care 2015;5:27 | ✗ | |||
| Seguin P, Laviolle B, Maurice A, Leclercq C, Mallédant Y. Atrial fibrillation in trauma patients requiring intensive care. Intensive Care Med 2006;32:398–404 | ✗ | |||
| Sekhri A, Aronow WS, Sekhri V, Palaniswamy C, Chandy D. Treatment of patients with supraventricular tachyarrhythmias in a medical intensive care unit. Compr Ther 2009;35:188–91 | ✗ | |||
| Sekhri A, Aronow WS, Sekhri V, Palaniswamy C, Chandy D. Treatment of Supraventricular Tachyarrhythmias in a Medical Intensive Care Unit Supervised by a Pulmonary Critical Care Specialist. Proceedings of the American College of Chest Physicians Annual Meeting, CHEST 2009, 31 October to 5 November 2009, San Diego, CA, USA, abstract number 136 | ✗ | |||
| Sen P, Kundu A, Sardar P, Chetterjee S, Nairooz R, Amin H, et al. Outcomes After Cardioversion in Patients Treated with Non Vitamin-K Antagonist Oral Anti Coagulants (NOACS)-Insights from a Meta Analysis. Proceedings of the American Thoracic Society International Conference, ATS 2015, 15–20 May 2015, Denver, CO, USA | ✗ | |||
| Shah A, Pillai MV, Gandhi U, Eden E, Shapiro J. New-onset atrial fibrillation/atrial flutter in Medical Intensive Care Unit (MICU) Patients with severe sepsis is associated with stroke and increased mortality. Am J Respir Crit Care Med 2014;189:A3114 | ✗ | |||
| Shaver CM, Chen W, Janz DR, May AK, Darbar D, Bernard GR, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit Care Med 2015;43:2104–11 | ✗ | |||
| Shibata SC, Uchiyama A, Ohta N, Fujino Y. Efficacy and safety of landiolol compared to amiodarone for the management of postoperative atrial fibrillation in intensive care patients. J Cardiothorac Vasc Anesth 2016;30:418–22 | ✗ | |||
| Smith H, Yeung C, Gowing S, Sadek M, Maziak D, Gilbert S, et al. A review and analysis of strategies for prediction, prevention and management of post-operative atrial fibrillation after non-cardiac thoracic surgery. J Thorac Dis 2018;10:S3799–S808 | ✗ | |||
| Joseph TT, DiMeglio M, Huffenberger A, Laudanski K. Behavioural patterns of electrolyte repletion in intensive care units: lessons from a large electronic dataset. Sci Rep 2018;8:11915 | ✗ | |||
| Tober K, Quasim T, Kinsella J. Atrial fibrillation; haemodynamics and mortality in post-operative patients on a general intensive care unit. Intensive Care Med 2012;38:S159–S | ✗ | |||
| Tracy C, Boushahri A. Managing arrhythmias in the intensive care unit. Crit Care Clin 2014;30:365–90 | ✗ | |||
| Trappe HJ. Treating critical supraventricular and ventricular arrhythmias. J Emerg Trauma Shock 2010;3:143–52 | ✗ | |||
| Trappe H-J, Brandts B, Weismueller P. Arrhythmias in the intensive care patient. Curr Opin Crit Care 2003;9:345–55 | ✗ | |||
| Trohman RG. Supraventricular tachycardia: implications for the intensivist. Crit Care Med 2000;28(Suppl. 10):N129–35 | ✗ | |||
| Trottier S, Bowie D, Stovall M. Acute Atrial Fibrillation in the ICU: Therapeutic Interventions and Hospital Course. Proceedings of the 42nd Critical Care Congress of the Society of Critical Care Medicine, SCCM 2013, 19–23 January 2013, San Juan Puerto Rico | ✗ | |||
| Van der Does WFB, De Groot NMS. Prophylaxis with amiodarone for postoperative atrial fibrillation: when and who? J Thorac Dis 2018;10:S3831-S3 | ✗ | |||
| Varriale P, Sedighi A. Acute management of atrial fibrillation and atrial flutter in the critical care unit: should it be ibutilide? Clin Cardiol 2000;23:265–8 | ✗ | |||
| Walkey AJ, Benjamin EJ, Lubitz SA. New-onset atrial fibrillation during hospitalization. J Am Coll Cardiol 2014;64:2432–3 | ✗ | |||
| Walkey AJ, Evans SR, Winter M, Benjamin E. Comparative Effectiveness of Heart Rate Control Medications for Atrial Fibrillation During Sepsis: A Propensity-Matched Cohort Study. Proceedings of the American Thoracic Society International Conference, ATS 2015, 15–20 Mat 2015, Denver, CO, USA | ✗ | |||
| Walkey AJ, McManus D. When rhythm changes cause the blues: new-onset atrial fibrillation during sepsis. Am J Respir Crit Care Med 2017;195:152–4 | ✗ | |||
| Willich T, Hammwohner M, Goette A. Therapie des Vorhofflimmern beim kritisch Kranken. Leitthema 2012;107:368–76 | ✗ | |||
| Wong A, Pierce T. Cardiac arrhythmias in the critically ill. Anaesth Intensive Care Med 2012;13:360–8 | ✗ | |||
| Wood M, Thompson J. Drugs acting on the heart: anti-arrhythmics. Anaesth Intensive Care Med 2009;10:388–91 | ✗ | |||
| Yokota T, Uchino S, Yoshida T, Fujii T, Takinami M. Predictors for sustained new-onset atrial fibrillation in critically ill patients: a retrospective observational study. J Anesth 2018;32:681–7 | ✗ | |||
| Yoshida T, Uchino S, Yokota T, Fujii T, Uezono S, Takinami M. The impact of sustained new-onset atrial fibrillation on mortality and stroke incidence in critically ill patients: a retrospective cohort study. J Crit Care 2018;44:267–72 | ✗ | |||
| Yucel E, Hollenberg S. Atrial fibrillation in critical illness: innocent bystander or guilty party? Crit Care Med 2015;43:2254–5 | ✗ | |||
| Zochios VA, Wilkinson J. Correspondence regarding: atrial fibrillation in the intensive care setting. J Intensive Care Soc 2013;14:276–7 | ✗ | |||
Appendix 5 RISK-II supplementary material
| Independent variable | Coefficient (95% CI) in outcome model for mortality | ||
|---|---|---|---|
| 1 to 90 days after discharge | 91 days to 1 year after discharge | > 1 year after discharge | |
| NOAF | 0.380 (0.231 to 0.529) | –0.005 (–0.148 to 0.138) | 0.037 (–0.043 to 0.116) |
| Age (years) (RCS) | |||
| Spline base variable 1 | 0.060 (0.039 to 0.081) | 0.076 (0.058 to 0.093) | 0.060 (0.050 to 0.070) |
| Spline base variable 2 | –0.047 (–0.079 to –0.015) | –0.068 (–0.094 to –0.042) | –0.032 (–0.047 to –0.017) |
| Spline base variable 3 | 0.233 (0.069 to 0.397) | 0.273 (0.138 to 0.409) | 0.151 (0.072 to 0.229) |
| Male sex (vs. female) | 0.080 (–0.035 to 0.196) | 0.159 (0.063 to 0.255) | 0.108 (0.053 to 0.163) |
| Hypertension | –0.099 (–0.229 to 0.031) | –0.091 (–0.198 to 0.015) | 0.036 (–0.025 to 0.098) |
| Heart failure | 0.303 (0.137 to 0.469) | 0.498 (0.359 to 0.637) | 0.350 (0.266 to 0.433) |
| Diabetes mellitus | 0.190 (0.054 to 0.326) | 0.064 (–0.052 to 0.180) | 0.218 (0.154 to 0.283) |
| Prior thromboembolism | 0.326 (0.127 to 0.525) | 0.099 (–0.084 to 0.283) | 0.219 (0.118 to 0.320) |
| Pulmonary hypertension | 0.604 (0.231 to 0.977) | 0.297 (–0.084 to 0.679) | 0.458 (0.235 to 0.681) |
| Valvular heart disease | 0.212 (0.017 to 0.407) | –0.015 (–0.196 to 0.166) | 0.075 (–0.027 to 0.178) |
| Independent variable | Coefficient (95% CI) in model for subsequent hospitalisation with: | ||
|---|---|---|---|
| Atrial fibrillation | Stroke | Heart failure | |
| NOAF | 1.767 (1.672 to 1.862) | 0.384 (0.112 to 0.656) | 0.247 (0.132 to 0.362) |
| Age (years) (RCS) | 0.041 (0.037 to 0.044) | 0.028 (0.020 to 0.036) | 0.026 (0.022 to 0.030) |
| Male sex (vs. female) | 0.227 (0.134 to 0.320) | 0.063 (–0.149 to 0.276) | 0.102 (0.010 to 0.194) |
| Hypertension | 0.282 (0.174 to 0.390) | 0.483 (0.228 to 0.738) | 0.477 (0.360 to 0.594) |
| Heart failure | 0.484 (0.365 to 0.602) | 0.160 (–0.154 to 0.475) | 2.005 (1.904 to 2.107) |
| Diabetes mellitus | 0.142 (0.036 to 0.248) | 0.151 (–0.093 to 0.395) | 0.386 (0.287 to 0.485) |
| Prior thromboembolism | 0.202 (0.042 to 0.362) | 1.425 (1.173 to 1.677) | 0.024 (–0.144 to 0.191) |
| Pulmonary hypertension | 0.236 (–0.100 to 0.572) | 0.705 (–0.011 to 1.421) | 0.465 (0.214 to 0.716) |
| Valvular heart disease | 0.346 (0.205 to 0.487) | 0.255 (–0.104 to 0.613) | 0.410 (0.285 to 0.536) |
| Variable | NOAF patients (sensitivity) (N = 8145) | Comparator patients (N = 48,870) |
|---|---|---|
| Demographics | ||
| Age (years), mean (SD) | 71.6 (11.5) | 59.0 (17.9) |
| Sex (male), n (%) | 4684 (57.5) | 26,445 (54.1) |
| Ethnicity, n (%) | ||
| White | 7634 (93.7) | 44,365 (90.8) |
| Mixed | 17 (0.2) | 236 (0.5) |
| Asian | 149 (1.8) | 1573 (3.2) |
| Black | 92 (1.1) | 945 (1.9) |
| Other | 57 (0.7) | 534 (1.1) |
| Not stated | 196 (2.4) | 1217 (2.5) |
| Comorbidities, n (%) | ||
| Hypertension | 5329 (65.4) | 22,917 (46.9) |
| Heart failure | 2049 (25.2) | 4999 (10.2) |
| Diabetes mellitus | 1946 (23.9) | 9998 (20.5) |
| Valvular heart disease | 1107 (13.6) | 3011 (6.2) |
| Prior thromboembolism | 722 (8.9) | 3053 (6.2) |
| Pulmonary hypertension | 213 (2.6) | 574 (1.2) |
| Dilating cardiomyopathy | 73 (0.9) | 229 (0.5) |
| Outcome | Cumulative incidence of event (95% CI) (%) | |
|---|---|---|
| NOAF patients (sensitivity) (N = 8145) | Comparator patients (N = 48,870) | |
| Mortality | ||
| During hospital admission, n (%) | 2774 (34.5) | 9595 (19.7) |
| Time after hospital discharge | ||
| 90 days | 8.8% (8.0 to 9.6) | 4.2% (4.0 to 4.4) |
| 1 year | 18.6% (17.6 to 19.7) | 11.1% (10.8 to 11.5) |
| 3 years | 34.1% (32.8 to 35.4) | 22.4% (22.0 to 22.8) |
| 5 years | 46.3% (44.9 to 47.8) | 30.1% (29.6 to 30.6) |
| Subsequent hospital admission fora | ||
| Atrial fibrillation | ||
| 1 year | 28.2 (26.8 to 29.4) | 2.3 (2.2 to 2.5) |
| 3 years | 39.8 (38.3 to 41.3) | 5.0 (4.7 to 5.3) |
| 5 years | 46.2 (44.4 to 47.9) | 7.1 (6.7 to 7.5) |
| Stroke | ||
| 1 year | 1.6 (1.3 to 2.0) | 0.6 (0.6 to 0.7) |
| 3 years | 3.1 (2.6 to 3.8) | 1.4 (1.2 to 1.5) |
| 5 years | 4.5 (3.8 to 5.4) | 2.0 (1.8 to 2.2) |
| Heart failure | ||
| 1 year | 11.1 (10.2 to 12.0) | 4.3 (4.0 to 4.5) |
| 3 years | 17.4 (16.3 to 18.6) | 7.5 (7.2 to 7.8) |
| 5 years | 21.8 (20.3 to 23.3) | 9.7 (9.3 to 10.0) |
FIGURE 11.
Sensitivity analysis: mortality from ICU admission.
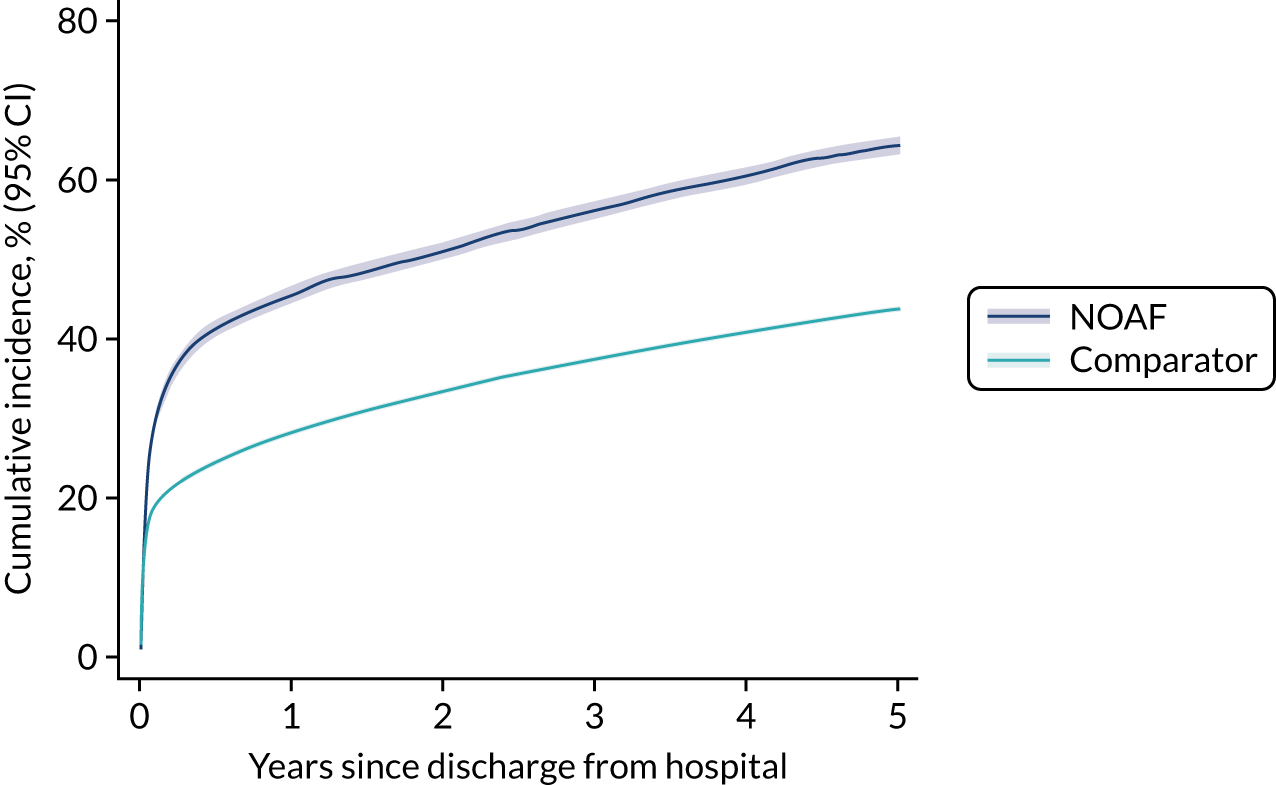
FIGURE 12.
Sensitivity analysis: outcomes after hospital discharge. (a) Mortality after discharge; (b) hospitalisation with AF; (c) hospitalisation with stroke; (d) hospitalisation with heart failure.
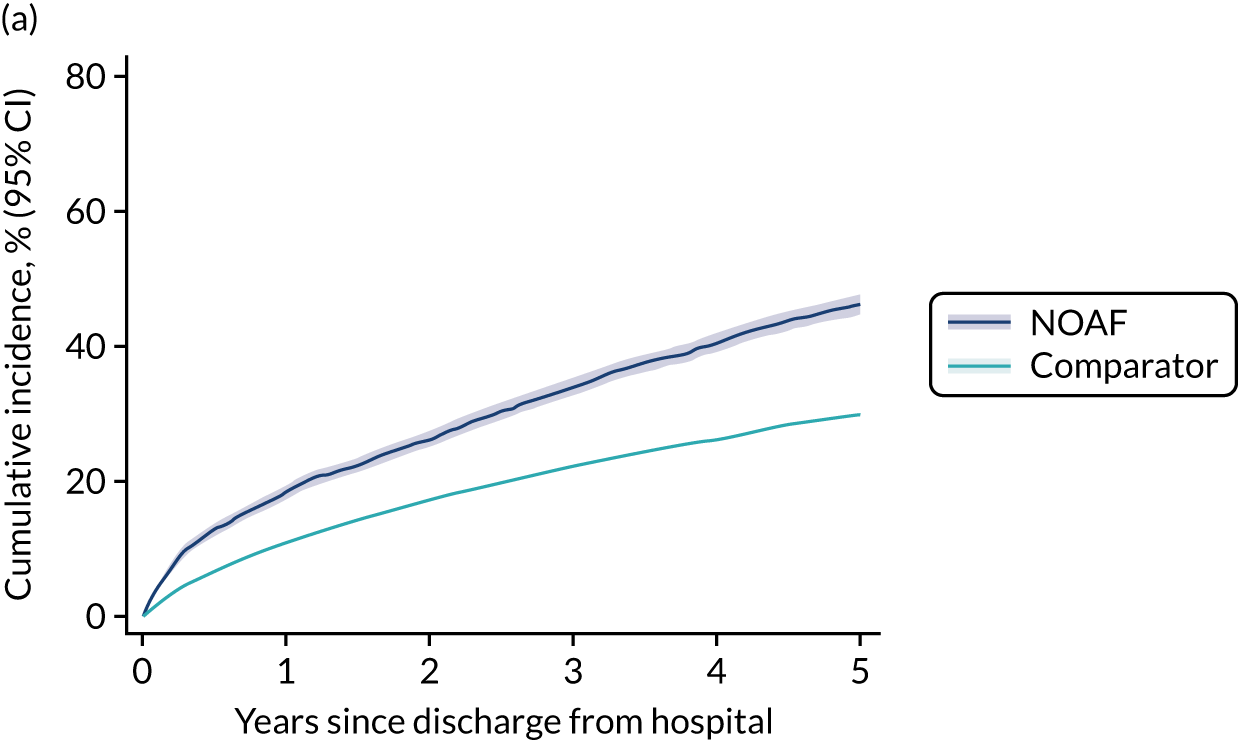
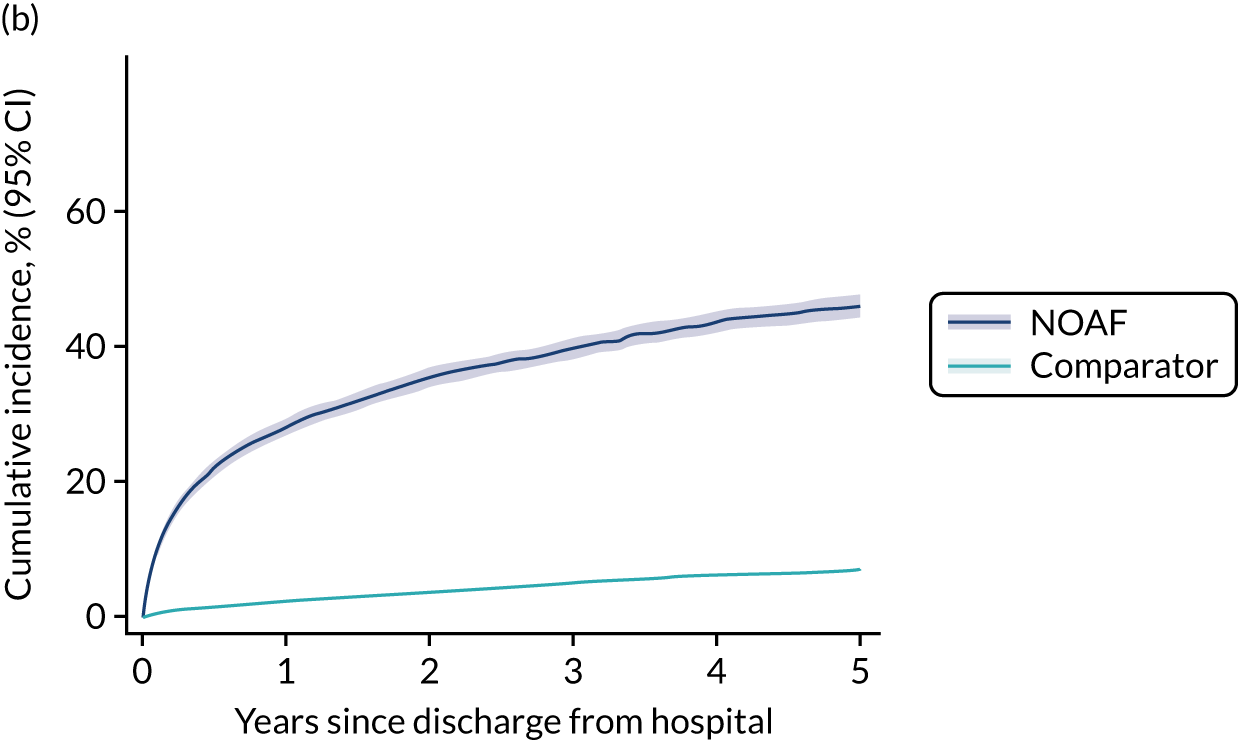
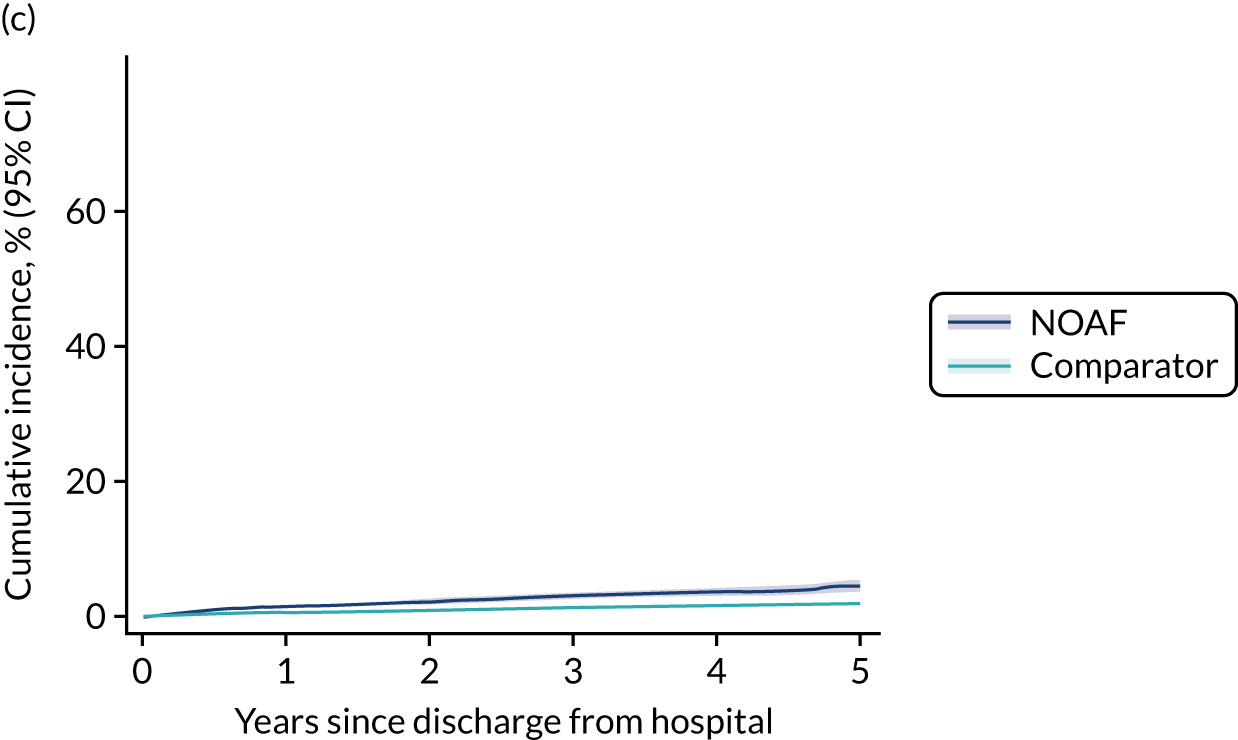
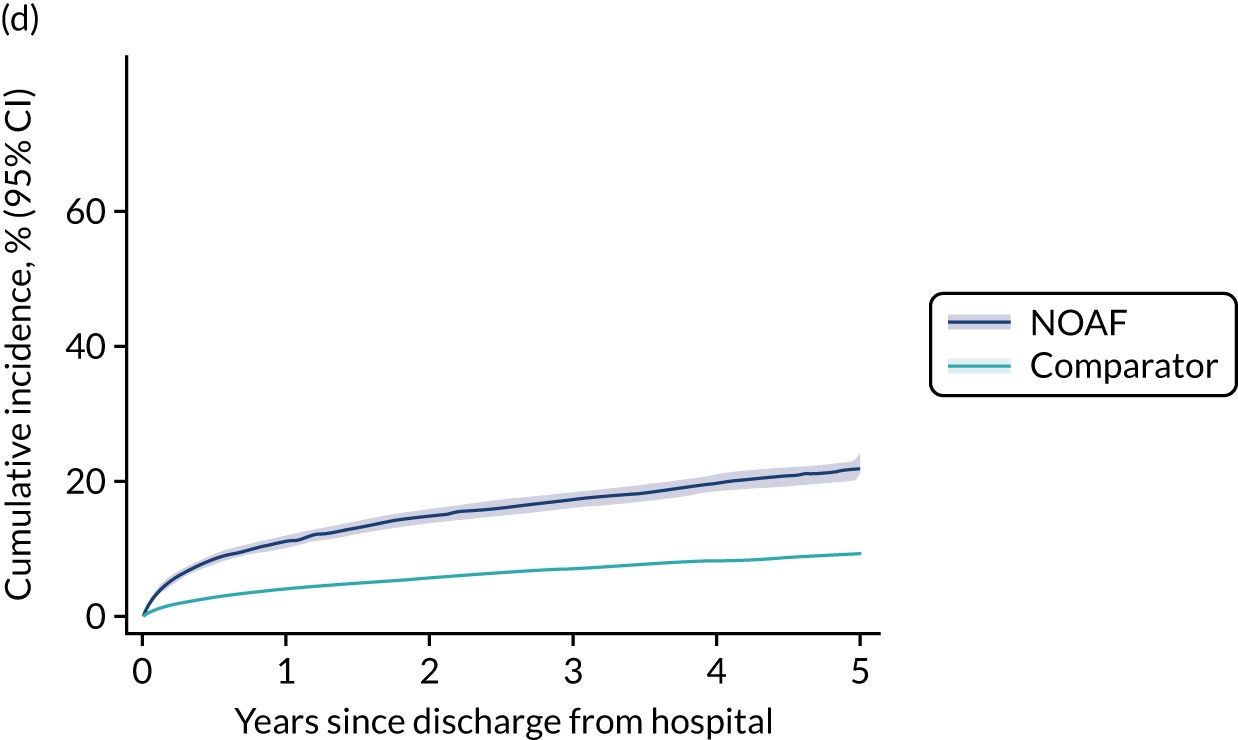
| Outcome | NOAF group (N = 8145) | Comparator group (N = 48,870) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Mortality during hospital admission | 2774 | 9595 | 2.14 (2.03 to 2.25) | 1.51 (1.43 to 1.59) | ||
| Outcome | NOAF group (N = 8145) | Comparator group (N = 48,870) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| Number of events | Number of person-years at risk | Number of events | Number of person-years at risk | |||
| Death 1–90 days after hospital discharge | 456 | 1247 | 1614 | 9497 | 2.26 (2.05 to 2.49) | 1.54 (1.38 to 1.71) |
| Death 91 days to 1 year after hospital discharge | 514 | 4595 | 2716 | 36,352 | 1.50 (1.37 to 1.65) | 1.04 (0.95 to 1.16) |
| Death > 1 year after hospital discharge | 1582 | 19,351 | 8054 | 171,327 | 1.76 (1.67 to 1.86) | 1.07 (1.01 to 1.13) |
| Outcome | NOAF group (N = 8145) | Comparator group (N = 48,870) | Unadjusted CHR (95% CI) | Adjusted CHR (95% CI) | ||
| Number of events | Number of person-years at risk | Number of events | Number of person-years at risk | |||
| Subsequent hospital admission for atrial fibrillation | 1926 | 8461 | 1865 | 96,570 | 10.67 (10.02 to 11.38) | 6.41 (5.99 to 6.85) |
| Subsequent hospital admission for stroke | 157 | 11,498 | 520 | 98,450 | 2.53 (2.12 to 3.03) | 1.59 (1.32 to 1.91) |
| Subsequent hospital admission for heart failure | 857 | 10,517 | 2718 | 95,410 | 2.71 (2.51 to 2.92) | 1.25 (1.15 to 1.35) |
Appendix 6 Intensive care unit databases supplementary material
Parts of this appendix are reproduced or adapted with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The appendix includes minor additions and formatting changes to the original appendix.
| Characteristic | Never had AF (N = 17,494) | NOAF (N = 1065) |
|---|---|---|
| Age (years), median (IQR) | 59 (47–73) | 75 (64–83) |
| Sex, n (%) | ||
| Female | 8440 (48) | 518 (49) |
| Male | 9054 (52) | 547 (51) |
| Weight (kg), median (IQR) | 77 (64–91) | 77 (65–92) |
| ICU length of stay (days), median (IQR) | 1.8 (1.0–3.5) | 5.8 (3.0–11.8) |
| ICU mortality, n (%) | 1260 (7.2) | 265 (25) |
| Hospital length of stay (days), median (IQR) | 6 (4–12) | 12 (7–21) |
| Hospital mortality, n (%) | 1887 (11) | 347 (33) |
| Characteristic | Never had AF (N = 7415) | NOAF (N = 952) |
|---|---|---|
| Age (years), median (IQR) | 61 (45–71) | 71 (64–78) |
| Sex, n (%) | ||
| Female | 3120 (42) | 368 (39) |
| Male | 4295 (58) | 584 (61) |
| Weight (kg), median (IQR) | 72 (62–83) | 75 (65–85) |
| ICU length of stay (days), median (IQR) | 2.4 (1.5–4.7) | 6.1 (3.1–12.8) |
| ICU mortality, n (%) | 628 (8.5) | 193 (20) |
| Hospital length of stay (days),a median (IQR) | 13 (7–25) | 19 (10–40) |
| Hospital mortality, n (%) | 1122 (16) | 350 (37) |
| Characteristic | Treatment group | Overall (N = 740) | |||
|---|---|---|---|---|---|
| Amiodarone (N = 94) | Beta-blocker (N = 473) | Calcium channel blocker (N = 144) | DCC (N = 29) | ||
| Age (years), median (IQR) | 76 (63–83) | 73 (64–83) | 73 (65–81) | 77 (69–85) | 74 (64–82) |
| Sex, n (%) | |||||
| Female | 51 (54) | 234 (49) | 77 (53) | 10 (34) | 372 (50) |
| Male | 43 (46) | 239 (51) | 67 (47) | 19 (66) | 368 (50) |
| COPD, n (%) | 3 (3.2) | 28 (5.9) | 19 (13) | 3 (10) | 53 (7.2) |
| NYHA class III/IV heart failure, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dialysis-dependent renal failure, n (%) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.1) |
| Chronic liver disease, n (%) | 2 (2.1) | 7 (1.5) | 2 (1.4) | 0 (0) | 11 (1.5) |
| Thyroid disorder, n (%) | 2 (2.1) | 27 (5.7) | 3 (2.1) | 1 (3.4) | 33 (4.5) |
| Beta-blocker therapy prior to admission, n (%) | 38 (46) | 193 (45) | 37 (28) | 13 (52) | 281 (42) |
| Antipsychotic medication prior to admission, n (%) | 4 (4.8) | 19 (4.4) | 4 (3.1) | 0 (0) | 27 (4.0) |
| Highest OASIS at 3 hours, median (IQR) | 38 (32–43) | 36 (31–40) | 36 (30–40) | 40 (33–43) | 36 (31–41) |
| Mechanical ventilation at time of NOAF, n (%) | 60 (64) | 206 (44) | 55 (38) | 22 (76) | 343 (46) |
| Renal replacement therapy during or < 12 hours prior to NOAF, n (%) | 5 (5.3) | 38 (8.0) | 1 (0.7) | 3 (10) | 47 (6.4) |
| i.v. vasoactive medication at time of NOAF, n (%) | 34 (36) | 45 (9.5) | 9 (6.2) | 13 (45) | 101 (14) |
| Therapeutic anticoagulation at time of NOAF, n (%) | 6 (6.4) | 23 (4.9) | 5 (3.5) | 2 (6.9) | 36 (4.9) |
| Central venous catheter at time of NOAF, n (%) | 69 (73) | 261 (55) | 75 (52) | 24 (83) | 429 (58) |
| Bronchodilator therapy on day of, or day preceding, NOAF, n (%) | 33 (35) | 155 (33) | 62 (43) | 8 (28) | 258 (35) |
| Plasma concentration, median (IQR) | |||||
| Sodium (mmol/l) | 138 (136–141) | 140 (137–143) | 140 (137–143) | 138 (136–143) | 139 (136–143) |
| Potassium (mmol/l) | 4.0 (3.7–4.5) | 3.9 (3.6–4.3) | 4.0 (3.7–4.3) | 4.1 (3.7–4.4) | 4.0 (3.7–4.4) |
| Magnesium (mmol/l) | 0.86 (0.78–0.95) | 0.82 (0.78–0.95) | 0.86 (0.78–0.95) | 0.82 (0.78–0.91) | 0.82 (0.78–0.95) |
| Urea (mmol/l) | 10.0 (6.4–15.4) | 8.9 (5.7–15.7) | 8.9 (6.1–15.4) | 19.8 (9.5–23.8) | 9.3 (6.1–16.1) |
| Creatinine (µmol/l) | 97 (71–168) | 97 (62–159) | 88 (62–139) | 159 (86–270) | 97 (62–159) |
| White cell count (× 109/l), median (IQR) | 13.6 (8.8–19.7) | 11.6 (8.8–15.5) | 11.3 (7.5–16.3) | 13.1 (10.5–16.3) | 11.8 (8.5–16.1) |
| Haemoglobin concentration (g/l), median (IQR) | 100 (88–113) | 104 (92–115) | 101 (93–116) | 99 (91–111) | 102 (92–115) |
| Platelet count (× 109/l), median (IQR) | 179 (91–258) | 190 (129–286) | 205 (137–291) | 161 (111–219) | 190 (123–283) |
| Prothrombin time (seconds), median (IQR) | 15.2 (13.7–17.8) | 14.2 (13.1–16.3) | 14.0 (12.9–15.6) | 15.0 (13.6–17.5) | 14.2 (13.1–16.4) |
| Systolic blood pressure after AF onset (mmHg), median (IQR) | 103 (93–122) | 119 (104–140) | 115 (97–132) | 93 (88–111) | 116 (100–135) |
| Mean blood pressure after AF onset (mmHg), median (IQR) | 72 (63–81) | 80 (69–92) | 76 (66–88) | 67 (61–78) | 78 (67–90) |
| Heart rate after AF onset (b.p.m.), median (IQR) | 124 (110–139) | 121 (102–136) | 124 (110–141) | 123 (98–147) | 122 (104–137) |
| Characteristic | Treatment group | Overall (N = 460) | ||
|---|---|---|---|---|
| Amiodarone (N = 344) | Beta-blockers (N = 47) | Digoxin (N = 69) | ||
| Age (years), median (IQR) | 69 (63–77) | 70 (64–76) | 75 (65–81) | 70 (63–77) |
| Sex, n (%) | ||||
| Female | 141 (41) | 20 (43) | 25 (36) | 186 (40) |
| Male | 203 (59) | 27 (57) | 44 (64) | 274 (60) |
| COPD, n (%) | 51 (15) | 1 (2.1) | 11 (16) | 63 (14) |
| NYHA class III/IV heart failure, n (%) | 2 (0.6) | 0 (0) | 0 (0) | 2 (0.4) |
| Dialysis-dependent renal failure, n (%) | 6 (1.7) | 0 (0) | 1 (1.4) | 7 (1.5) |
| Chronic liver disease, n (%) | 14 (4.1) | 1 (2.1) | 5 (7.2) | 20 (4.3) |
| Thyroid disorder, n (%) | 21 (6.1) | 3 (6.4) | 4 (5.8) | 28 (6.1) |
| Beta-blocker therapy prior to admission, n (%) | 44 (13) | 10 (21) | 9 (13) | 63 (14) |
| Antipsychotic medication prior to admission, n (%) | 5 (1.5) | 1 (2.1) | 1 (1.4) | 7 (1.5) |
| Highest OASIS at 3 hours, median (IQR) | 34 (27–40) | 34 (22–38) | 30 (25–36) | 34 (26–39) |
| Mechanical ventilation at time of NOAF, n (%) | 192 (56) | 22 (47) | 29 (42) | 243 (53) |
| Renal replacement therapy during or < 12 hours prior to NOAF, n (%) | 52 (15) | 5 (11) | 8 (12) | 65 (14) |
| i.v. vasoactive medication at time of NOAF, n (%) | 105 (31) | 6 (13) | 13 (19) | 124 (27) |
| Therapeutic anticoagulation at time of NOAF, n (%) | 37 (11) | 5 (11) | 6 (8.7) | 48 (10) |
| Central venous catheter at time of NOAF, n (%) | 262 (76) | 32 (68) | 32 (46) | 326 (71) |
| Bronchodilator therapy on day of, or day preceding, NOAF, n (%) | 57 (17) | 7 (15) | 11 (16) | 75 (16) |
| Plasma concentration, median (IQR) | ||||
| Sodium (mmol/l) | 137 (134–141) | 139 (136–144) | 138 (135–140) | 137 (134–141) |
| Potassium (mmol/l) | 4.2 (3.9–4.5) | 4.1 (4.0–4.6) | 4.2 (3.9–4.4) | 4.2 (3.9–4.5) |
| Magnesium (mmol/l) | 0.95 (0.84–1.14) | 1.01 (0.92–1.16) | 0.92 (0.82–1.08) | 0.96 (0.84–1.12) |
| Urea (mmol/l) | 13.8 (9.5–20.1) | 12.1 (7.8–17.7) | 13.2 (8.2–18.5) | 13.6 (8.8–19.5) |
| Creatinine concentration (μmol/l) | 134 (78–224) | 108 (70–151) | 112 (84–185) | 125 (78–214) |
| White cell count (× 109/l), median (IQR) | 11.1 (7.5–16.2) | 10.6 (7.6–13.4) | 12.0 (9.5–16.8) | 11.1 (7.7–16.3) |
| Haemoglobin concentration (g/l), median (IQR) | 97 (87–111) | 103 (94–112) | 101 (90–116) | 98 (88–113) |
| Platelet count (× 109/l), median (IQR) | 163 (105–231) | 192 (118–247) | 180 (136–237) | 166 (109–234) |
| Prothrombin time (seconds), median (IQR) | 16.2 (15.0–19.0) | 15.5 (14.4–17.0) | 16.6 (15.0–19.4) | 16.1 (15.0–19.0) |
| Systolic blood pressure after AF onset (mmHg), median (IQR) | 112 (97–128) | 128 (108–156) | 119 (103–135) | 116 (99–131) |
| Mean blood pressure after AF onset (mmHg), median (IQR) | 74 (67–85) | 83 (73–94) | 78 (70–88) | 75 (67–86) |
| Heart rate after AF onset (b.p.m.), median (IQR) | 128 (107–149) | 125 (110–146) | 120 (98–140) | 127 (107–147) |
| Variable | Unweighted means | Weighted means | Maximum pairwise SMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amiodarone | Beta-blocker | Calcium channel blocker | DCC | Amiodarone | Beta-blocker | Calcium channel blocker | DCC | Unweighted | Weighted | |
| Age (years) | 71.84 | 71.98 | 72.56 | 74.14 | 72.90 | 72.56 | 72.74 | 72.72 | 0.17 | 0.03 |
| Male sex | 0.46 | 0.51 | 0.47 | 0.66 | 0.51 | 0.51 | 0.51 | 0.54 | 0.20 | 0.03 |
| OASIS 3-hour score | 36.80 | 35.21 | 35.22 | 38.93 | 35.87 | 35.89 | 35.94 | 36.71 | 0.48 | 0.11 |
| Beta-blocker on admission | 0.40 | 0.45 | 0.31 | 0.52 | 0.44 | 0.44 | 0.41 | 0.49 | 0.21 | 0.08 |
| Antipsychotic medication on admission | 0.05 | 0.06 | 0.03 | 0.00 | 0.04 | 0.04 | 0.04 | 0.00 | 0.06 | 0.04 |
| Thyroid disorder | 0.02 | 0.06 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 | 0.01 |
| COPD | 0.03 | 0.06 | 0.13 | 0.10 | 0.06 | 0.08 | 0.08 | 0.11 | 0.10 | 0.05 |
| Liver disease | 0.02 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 |
| Dialysis-dependent renal failure | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Plasma sodium concentration (mmol/l) | 138.10 | 139.63 | 140.04 | 139.28 | 139.32 | 139.48 | 139.46 | 139.47 | 0.35 | 0.03 |
| Plasma potassium concentration (mmol/l) | 4.10 | 4.02 | 4.02 | 4.03 | 4.01 | 4.02 | 4.01 | 3.97 | 0.15 | 0.08 |
| Plasma magnesium concentration (mmol/l) | 0.86 | 0.86 | 0.87 | 0.84 | 0.85 | 0.85 | 0.85 | 0.83 | 0.20 | 0.17 |
| Plasma creatinine concentration (mmol/l) | 164.16 | 148.06 | 125.38 | 219.54 | 157.14 | 152.43 | 149.86 | 163.62 | 0.57 | 0.08 |
| Plasma urea concentration (µmol/l) | 11.97 | 12.00 | 12.12 | 18.48 | 12.28 | 13.02 | 13.32 | 15.14 | 0.69 | 0.30 |
| White cell count (× 109/l) | 14.92 | 12.68 | 12.63 | 15.31 | 13.52 | 13.33 | 12.88 | 13.81 | 0.32 | 0.11 |
| Haemoglobin concentration (g/l) | 102.89 | 104.00 | 104.82 | 99.52 | 102.99 | 103.07 | 103.44 | 101.20 | 0.30 | 0.13 |
| Platelet count (× 10/l) | 207 | 215 | 221 | 184 | 206 | 209 | 209 | 200 | 0.29 | 0.07 |
| Therapeutic anticoagulation at time of NOAF | 0.06 | 0.05 | 0.03 | 0.07 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.01 |
| Log-prothrombin time | 2.76 | 2.70 | 2.70 | 2.74 | 2.72 | 2.72 | 2.72 | 2.74 | 0.24 | 0.10 |
| Systolic blood pressure after AF onset (mmHg) | 105.88 | 119.20 | 116.69 | 89.62 | 113.40 | 113.60 | 113.54 | 108.28 | 0.83 | 0.15 |
| Mean blood pressure after AF onset (mmHg) | 67.26 | 74.67 | 74.45 | 58.11 | 71.23 | 71.37 | 71.92 | 67.57 | 0.76 | 0.20 |
| Heart rate after AF onset (b.p.m.) | 123.68 | 120.74 | 123.56 | 122.14 | 123.32 | 122.87 | 123.09 | 125.32 | 0.11 | 0.09 |
| Temperature (°C) | 37.04 | 37.02 | 37.06 | 36.88 | 37.02 | 37.02 | 37.06 | 37.02 | 0.23 | 0.05 |
| i.v. vasoactive medication at time of NOAF | 0.36 | 0.10 | 0.06 | 0.45 | 0.17 | 0.16 | 0.15 | 0.21 | 0.39 | 0.06 |
| Noradrenaline dose (µg/kg/minute) | 0.12 | 0.02 | 0.01 | 0.08 | 0.03 | 0.03 | 0.02 | 0.04 | 0.66 | 0.09 |
| Vasopressin dose (µg/kg/minute) | 0.17 | 0.05 | 0.02 | 0.29 | 0.07 | 0.07 | 0.05 | 0.10 | 0.48 | 0.08 |
| Bronchodilator therapy on day of, or day preceding, NOAF | 0.35 | 0.33 | 0.43 | 0.28 | 0.33 | 0.33 | 0.35 | 0.30 | 0.15 | 0.04 |
| Mechanical ventilation at time of NOAF | 0.64 | 0.44 | 0.38 | 0.76 | 0.51 | 0.50 | 0.47 | 0.58 | 0.38 | 0.11 |
| Central venous catheter at time of NOAF | 0.73 | 0.55 | 0.52 | 0.83 | 0.63 | 0.61 | 0.61 | 0.68 | 0.31 | 0.08 |
| Renal replacement therapy during or < 12 hours prior to NOAF | 0.05 | 0.08 | 0.01 | 0.10 | 0.04 | 0.05 | 0.02 | 0.04 | 0.10 | 0.03 |
| Variable | Unweighted means | Weighted means | Maximum pairwise SMD | |||||
|---|---|---|---|---|---|---|---|---|
| Amiodarone | Beta-blocker | Digoxin | Amiodarone | Beta-blocker | Digoxin | Unweighted | Weighted | |
| Age (years) | 68.60 | 68.94 | 71.78 | 69.52 | 69.23 | 70.24 | 0.28 | 0.09 |
| Male sex | 0.59 | 0.57 | 0.64 | 0.60 | 0.59 | 0.62 | 0.06 | 0.02 |
| OASIS 3-hour | 33.77 | 31.17 | 29.83 | 32.75 | 32.62 | 31.91 | 0.43 | 0.09 |
| Beta-blocker on admission | 0.13 | 0.21 | 0.13 | 0.14 | 0.16 | 0.15 | 0.08 | 0.02 |
| Antipsychotic medication on admission | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| Thyroid disorder | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.07 | 0.01 | 0.00 |
| COPD | 0.15 | 0.02 | 0.16 | 0.12 | 0.05 | 0.13 | 0.14 | 0.07 |
| Liver disease | 0.04 | 0.02 | 0.07 | 0.04 | 0.03 | 0.05 | 0.05 | 0.02 |
| NYHA class III/IV heart failure | 0.01 | NA | 0.00 | 0.00 | NA | 0.00 | 0.01 | 0.00 |
| Dialysis-dependent renal failure | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 |
| Plasma sodium concentration (mmol/l) | 137.51 | 139.79 | 138.05 | 138.13 | 138.91 | 138.31 | 0.40 | 0.14 |
| Plasma potassium concentration (mmol/l) | 4.24 | 4.30 | 4.20 | 4.24 | 4.26 | 4.24 | 0.19 | 0.05 |
| Plasma magnesium concentration (mmol/l) | 0.99 | 1.02 | 0.96 | 0.99 | 1.00 | 0.99 | 0.29 | 0.08 |
| Plasma creatinine concentration (mmol/l) | 171.42 | 142.16 | 156.38 | 164.82 | 165.09 | 164.62 | 0.25 | 0.00 |
| Plasma urea concentration (µmol/l) | 16.01 | 13.68 | 13.88 | 15.03 | 14.56 | 14.27 | 0.27 | 0.09 |
| White cell count (× 109/l) | 12.60 | 11.24 | 12.88 | 12.48 | 11.94 | 12.52 | 0.25 | 0.09 |
| Haemoglobin concentration (g/l) | 100.55 | 104.40 | 105.42 | 101.74 | 102.35 | 101.98 | 0.27 | 0.03 |
| Platelet count (× 109/l) | 179.62 | 197.59 | 207.64 | 190.48 | 196.85 | 197.06 | 0.25 | 0.06 |
| Therapeutic anticoagulation at time of NOAF | 0.11 | 0.11 | 0.09 | 0.11 | 0.13 | 0.11 | 0.02 | 0.02 |
| Log-prothrombin time | 2.86 | 2.80 | 2.92 | 2.86 | 2.82 | 2.88 | 0.41 | 0.18 |
| Systolic blood pressure after AF onset (mmHg) | 117.19 | 123.94 | 119.54 | 118.81 | 120.14 | 119.19 | 0.22 | 0.04 |
| Mean blood pressure after AF onset (mmHg) | 75.35 | 80.36 | 78.03 | 76.66 | 77.66 | 76.93 | 0.26 | 0.05 |
| Heart rate after AF onset (b.p.m.) | 125.48 | 129.06 | 117.77 | 124.50 | 125.74 | 124.48 | 0.41 | 0.05 |
| Temperature (°C) | 36.58 | 36.84 | 36.54 | 36.63 | 36.75 | 36.60 | 0.34 | 0.17 |
| i.v. vasoactive medication at time of NOAF | 0.31 | 0.13 | 0.19 | 0.26 | 0.22 | 0.24 | 0.18 | 0.04 |
| Noradrenaline dose (µg/kg/minute) | 0.06 | 0.04 | 0.04 | 0.06 | 0.05 | 0.06 | 0.23 | 0.05 |
| Vasopressin dose (µg/kg/minute) | 0.05 | NA | 0.00 | 0.02 | NA | 0.00 | 0.29 | 0.12 |
| Bronchodilator therapy on day of, or day preceding, NOAF | 0.17 | 0.15 | 0.16 | 0.17 | 0.18 | 0.16 | 0.02 | 0.02 |
| Mechanical ventilation at time of NOAF | 0.56 | 0.47 | 0.42 | 0.52 | 0.53 | 0.49 | 0.14 | 0.04 |
| Central venous catheter at time of NOAF | 0.76 | 0.68 | 0.46 | 0.69 | 0.71 | 0.64 | 0.30 | 0.07 |
| Renal replacement therapy during or < 12 hours prior to NOAF | 0.15 | 0.11 | 0.12 | 0.14 | 0.14 | 0.13 | 0.04 | 0.01 |
| Treatment | Unadjusted HR | 95% CI | Adjusted | HR 95% CI |
|---|---|---|---|---|
| Rate control | ||||
| Beta-blocker | 1.03 | 0.81 to 1.30 | 1.09 | 0.78 to 1.51 |
| Calcium channel blocker | 0.83 | 0.62 to 1.12 | 0.81 | 0.55 to 1.19 |
| Cardioversion | 1.01 | 0.39 to 2.62 | 1.59 | 0.44 to 5.75 |
| Rhythm control | ||||
| Beta-blocker | 0.91 | 0.73 to 1.12 | 0.91 | 0.61 to 1.35 |
| Calcium channel blocker | 0.65 | 0.50 to 0.84 | 0.59 | 0.37 to 0.92 |
| Cardioversion | 1.45 | 0.82 to 2.57 | 2.00 | 0.86 to 4.65 |
| Reversion to AF | ||||
| Beta-blocker | 1.18 | 0.79 to 1.78 | 1.37 | 0.67 to 2.78 |
| Calcium channel blocker | 1.44 | 0.90 to 2.31 | 1.73 | 0.78 to 3.84 |
| Cardioversion | 1.80 | 0.83 to 3.90 | 1.01 | 0.28 to 3.71 |
| Reversion to heart rate of ≥ 110 b.p.m. | ||||
| Beta-blocker | 1.08 | 0.80 to 1.46 | 0.95 | 0.59 to 1.52 |
| Calcium channel blocker | 1.44 | 1.00 to 2.07 | 1.61 | 0.93 to 2.79 |
| Cardioversion | 0.67 | 0.30 to 1.53 | 0.93 | 0.36 to 2.42 |
| Hospital mortality | ||||
| Beta-blocker | 0.64 | 0.44 to 0.93 | 1.03 | 0.53 to 2.03 |
| Calcium channel blocker | 0.77 | 0.50 to 1.20 | 1.30 | 0.61 to 2.76 |
| Cardioversion | 1.56 | 0.86 to 2.83 | 0.96 | 0.31 to 3.01 |
| Treatment | Unadjusted HR | 95% CI | Adjusted HR | 95% CI |
|---|---|---|---|---|
| Rate control | ||||
| Beta-blocker | 0.85 | 0.57 to 1.27 | 0.82 | 0.48 to 1.42 |
| Digoxin | 0.64 | 0.45 to 0.92 | 0.56 | 0.34 to 0.92 |
| Rhythm control | ||||
| Beta-blocker | 0.95 | 0.64 to 1.40 | 0.99 | 0.57 to 1.72 |
| Digoxin | 0.57 | 0.41 to 0.81 | 0.67 | 0.41 to 1.09 |
| Reversion to AF | ||||
| Beta-blocker | 0.79 | 0.50 to 1.27 | 0.84 | 0.42 to 1.65 |
| Digoxin | 1.21 | 0.78 to 1.89 | 1.32 | 0.71 to 2.47 |
| Reversion to heart rate of ≥ 110 b.p.m. | ||||
| Beta-blocker | 0.94 | 0.58 to 1.52 | 0.88 | 0.43 to 1.79 |
| Digoxin | 1.41 | 0.91 to 2.19 | 1.14 | 0.63 to 2.09 |
| Hospital mortality | ||||
| Beta-blocker | 0.74 | 0.40 to 1.38 | 0.75 | 0.30 to 1.84 |
| Digoxin | 1.21 | 0.79 to 1.86 | 1.37 | 0.75 to 2.50 |
| Treatment | Unadjusted HR | 95% CI | Adjusted HR | 95% CI |
|---|---|---|---|---|
| Rate control | ||||
| Beta-blocker | 1.26 | 1.10 to 1.43 | 1.14 | 0.91 to 1.44 |
| Calcium channel blocker | 1.06 | 0.86 to 1.29 | 0.88 | 0.63 to 1.23 |
| Digoxin | 0.69 | 0.52 to 0.91 | 0.52 | 0.32 to 0.86 |
| Electrical cardioversion | 1.74 | 0.90 to 3.36 | 2.30 | 0.87 to 6.06 |
| Rhythm control | ||||
| Beta-blocker | 0.81 | 0.71 to 0.93 | 0.86 | 0.67 to 1.11 |
| Calcium channel blocker | 0.58 | 0.47 to 0.71 | 0.56 | 0.39 to 0.79 |
| Digoxin | 0.58 | 0.41 to 0.83 | 0.64 | 0.35 to 1.17 |
| Electrical cardioversion | 1.25 | 0.77 to 2.03 | 1.58 | 0.71 to 3.51 |
| Reversion to AF | ||||
| Beta-blocker | 0.68 | 0.55 to 0.84 | 0.72 | 0.48 to 1.08 |
| Calcium channel blocker | 0.81 | 0.58 to 1.13 | 0.89 | 0.48 to 1.64 |
| Digoxin | 1.39 | 0.90 to 2.14 | 2.22 | 0.95 to 5.21 |
| Electrical cardioversion | 1.02 | 0.52 to 1.98 | 0.64 | 0.20 to 2.02 |
| Reversion to heart rate of ≥ 110 b.p.m. | ||||
| Beta-blocker | 1.00 | 0.85 to 1.17 | 0.88 | 0.65 to 1.18 |
| Calcium channel blocker | 1.62 | 1.28 to 2.06 | 1.54 | 1.00 to 2.37 |
| Digoxin | 1.24 | 0.92 to 1.66 | 1.26 | 0.75 to 2.12 |
| Electrical cardioversion | 1.24 | 0.68 to 2.26 | 0.90 | 0.32 to 2.51 |
| Hospital mortality | ||||
| Beta-blocker | 0.78 | 0.62 to 0.99 | 0.97 | 0.56 to 1.68 |
| Calcium channel blocker | 0.95 | 0.67 to 1.33 | 1.21 | 0.62 to 2.39 |
| Digoxin | 1.16 | 0.76 to 1.79 | 1.77 | 0.77 to 4.06 |
| Electrical cardioversion | 1.92 | 1.16 to 3.17 | 0.87 | 0.25 to 3.00 |
FIGURE 13.
Cumulative incidence plot of time from treatment to heart rate of < 110 b.p.m. for each treatment: MIMIC-III database.
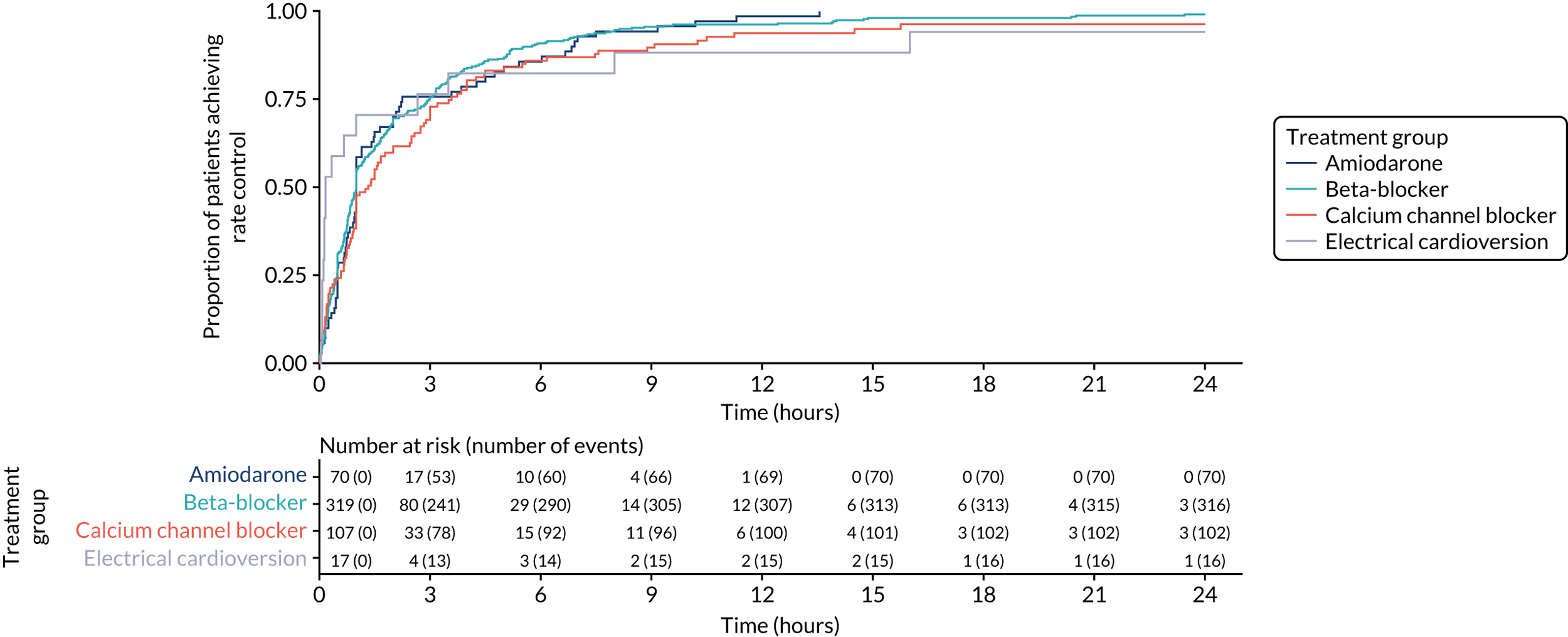
FIGURE 14.
Kaplan–Meier curves of time from achieving rate control to reversion to heart rate of ≥ 110 b.p.m.: MIMIC-III database.
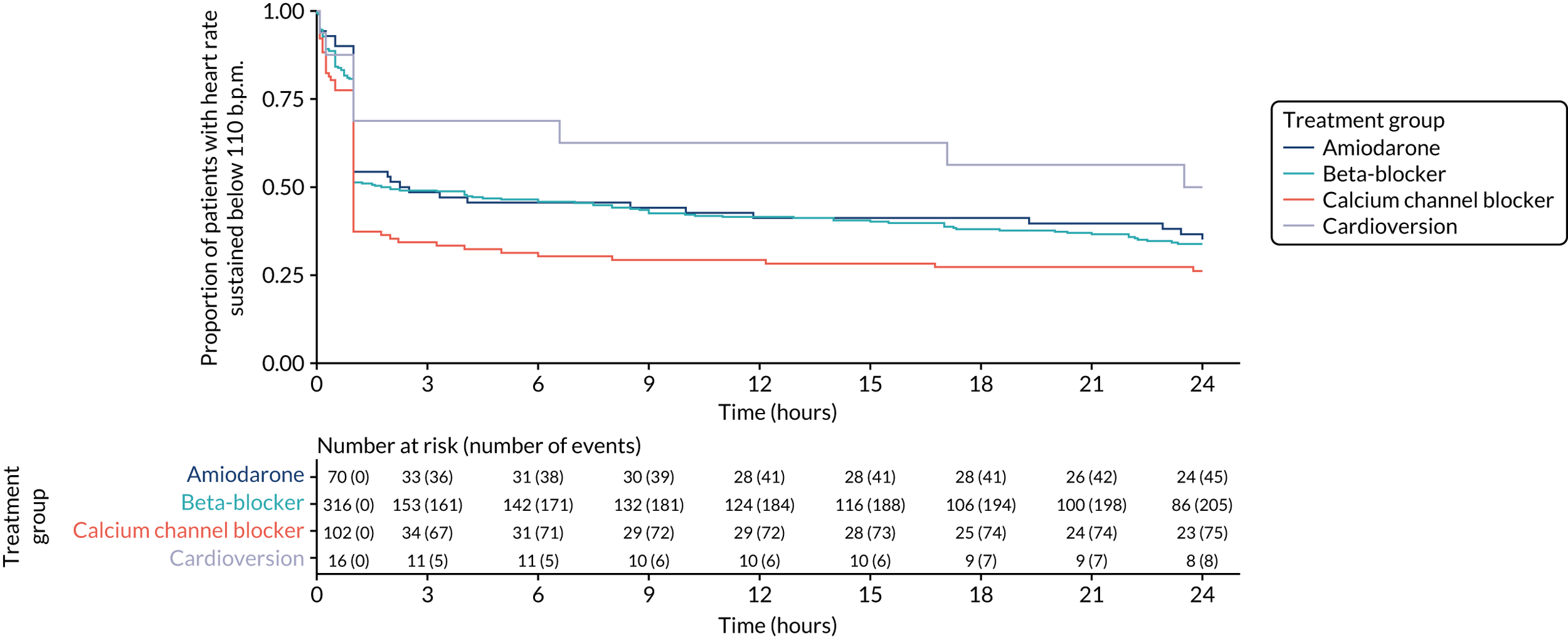
FIGURE 15.
Cumulative incidence plot of time from treatment to heart rate of < 110 b.p.m. for each treatment: PICRAM database.
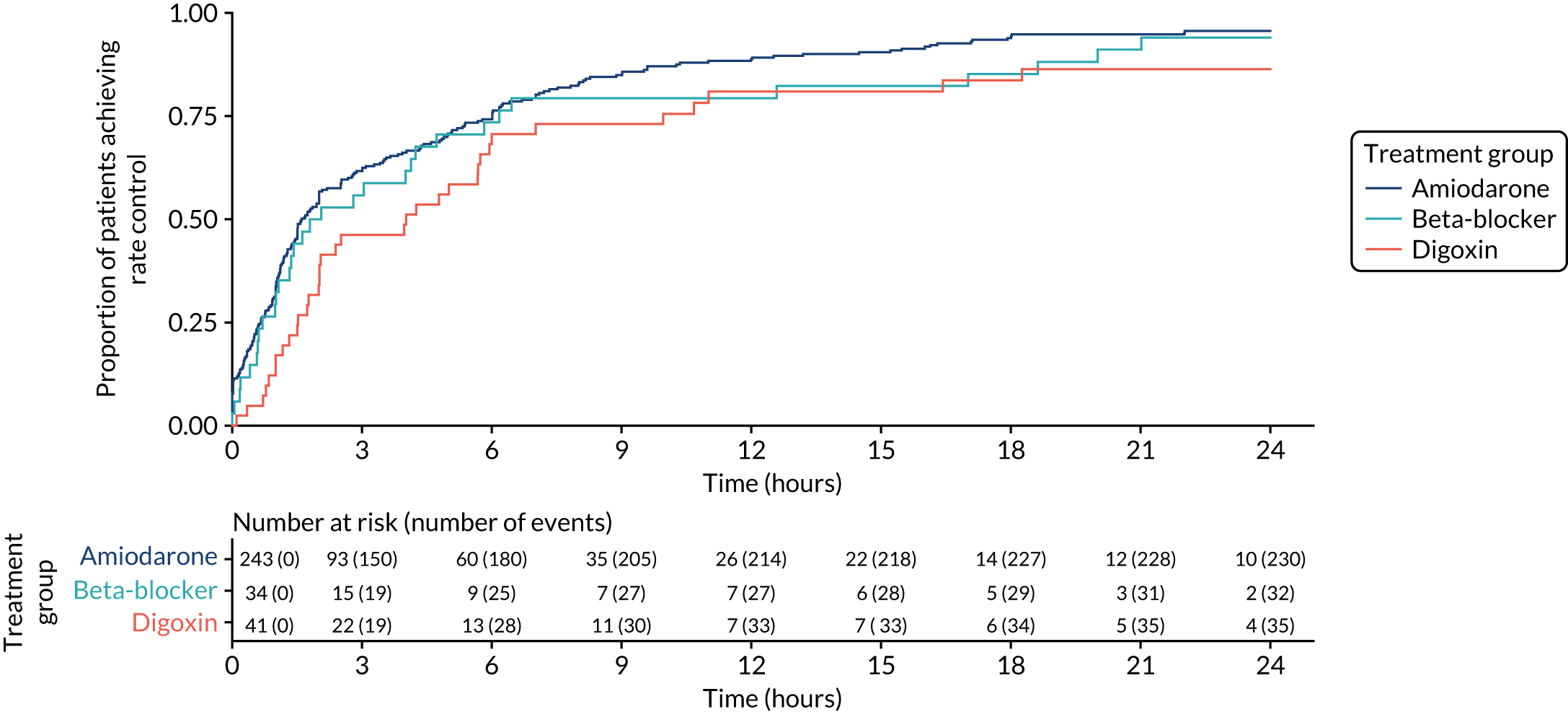
FIGURE 16.
Kaplan–Meier curves of time from achieving rate control to reversion to heart rate of ≥ 110 b.p.m.: PICRAM database.
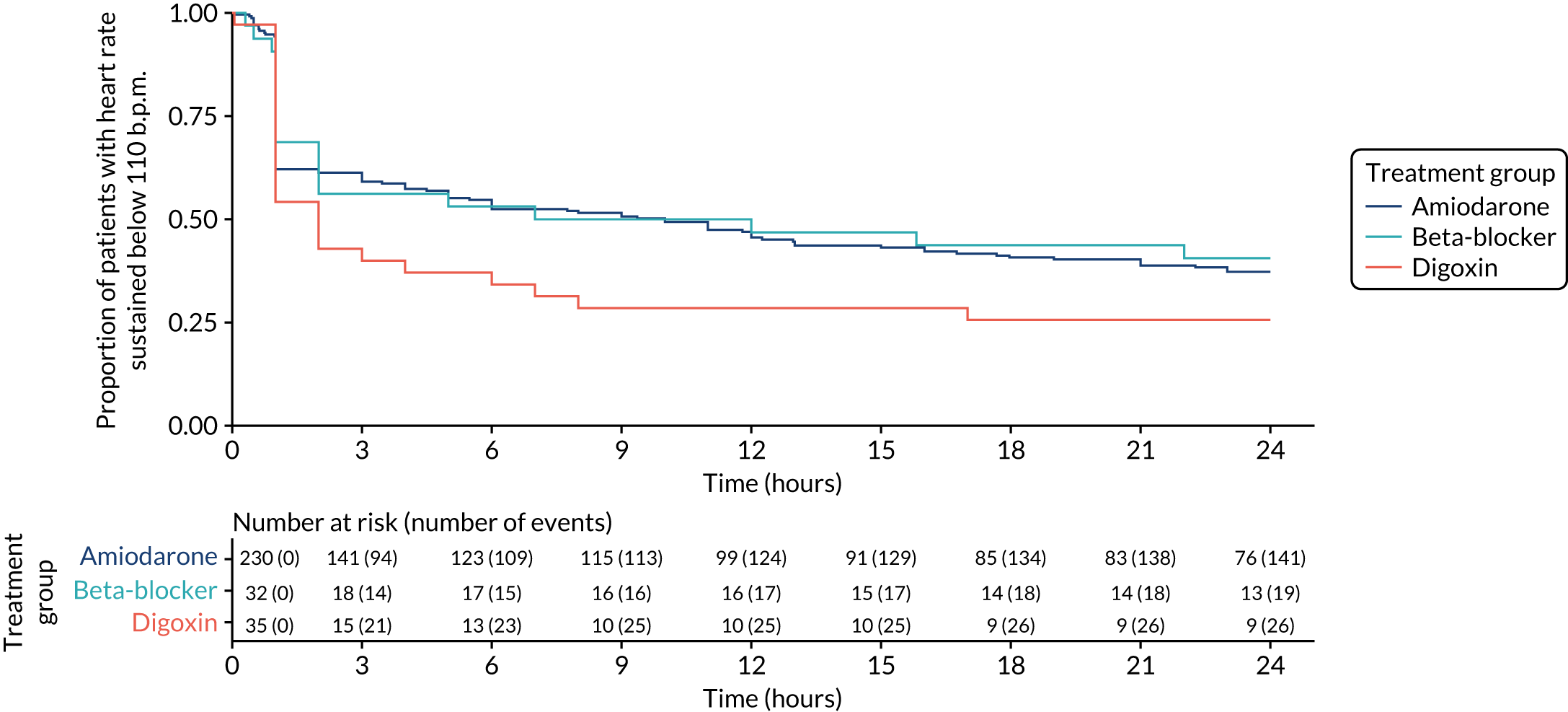
FIGURE 17.
Cumulative incidence plot of time from treatment to rhythm control for each treatment: MIMIC-III database.
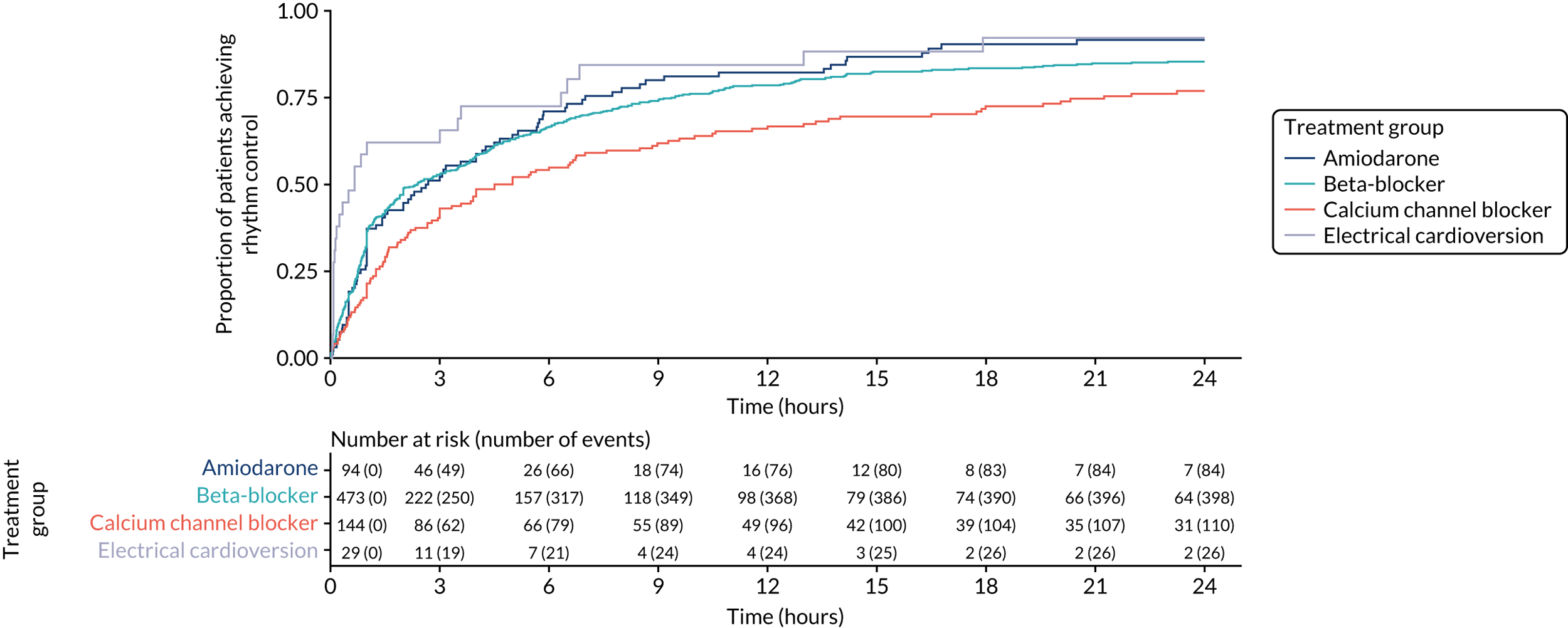
FIGURE 18.
Kaplan–Meier curves for each treatment of time from achieving rhythm control to reversion to AF: MIMIC-III database.
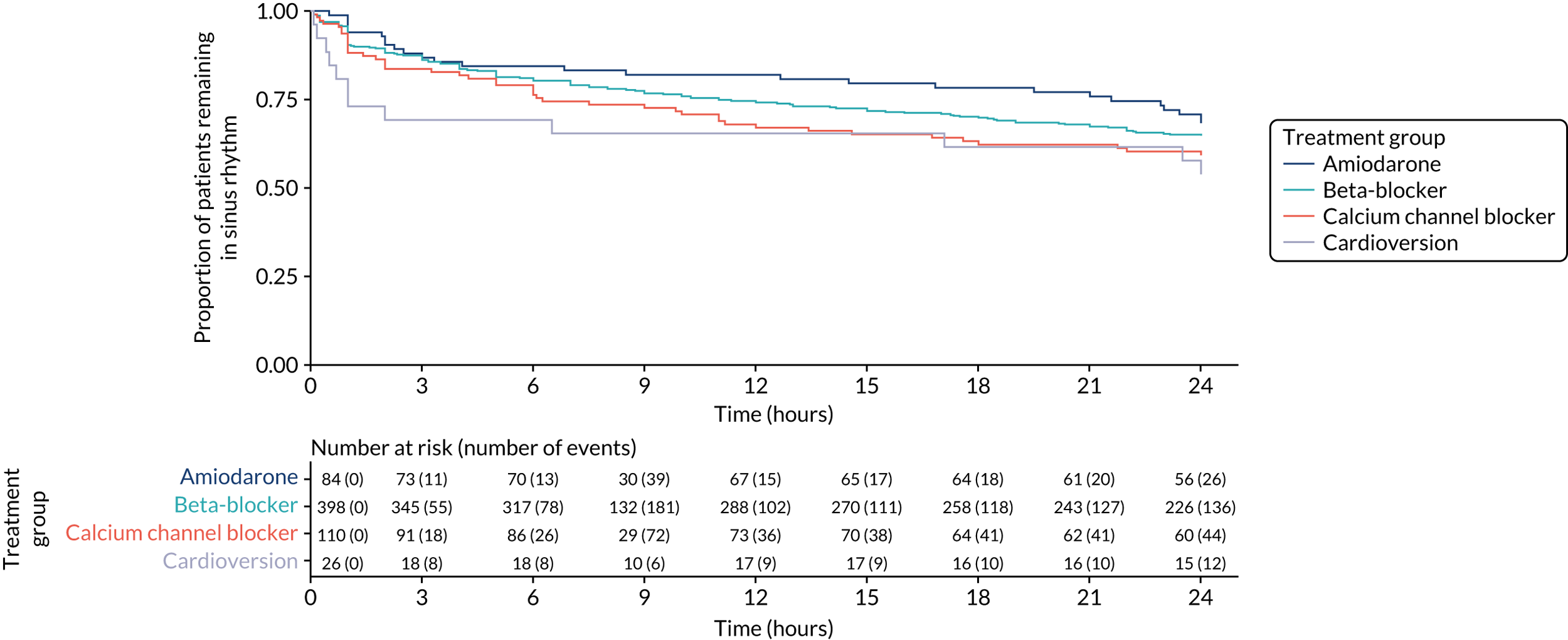
FIGURE 19.
Cumulative incidence plot of time from treatment to rhythm control for each treatment: PICRAM database.
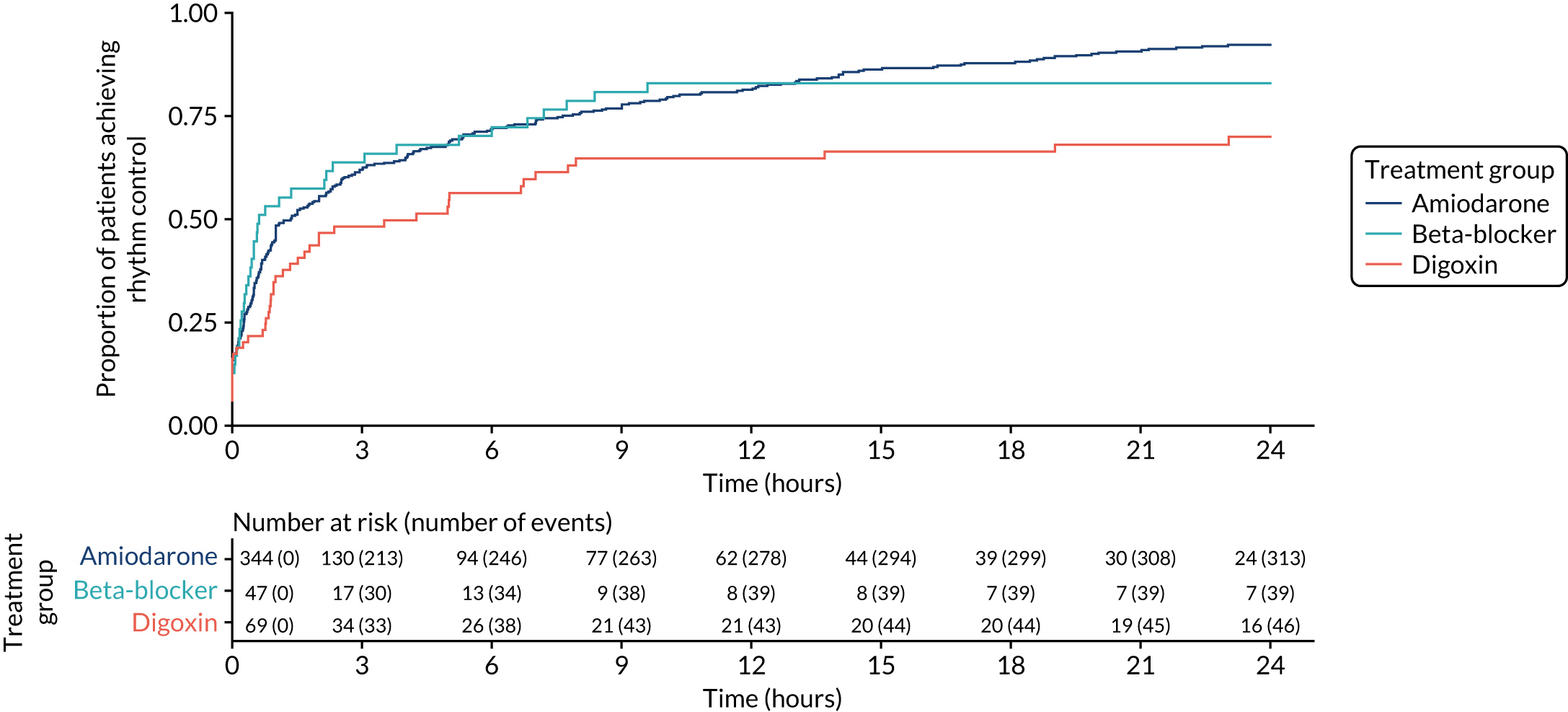
FIGURE 20.
Kaplan–Meier curves for each treatment of time from achieving rhythm control to reversion to AF: PICRAM database.
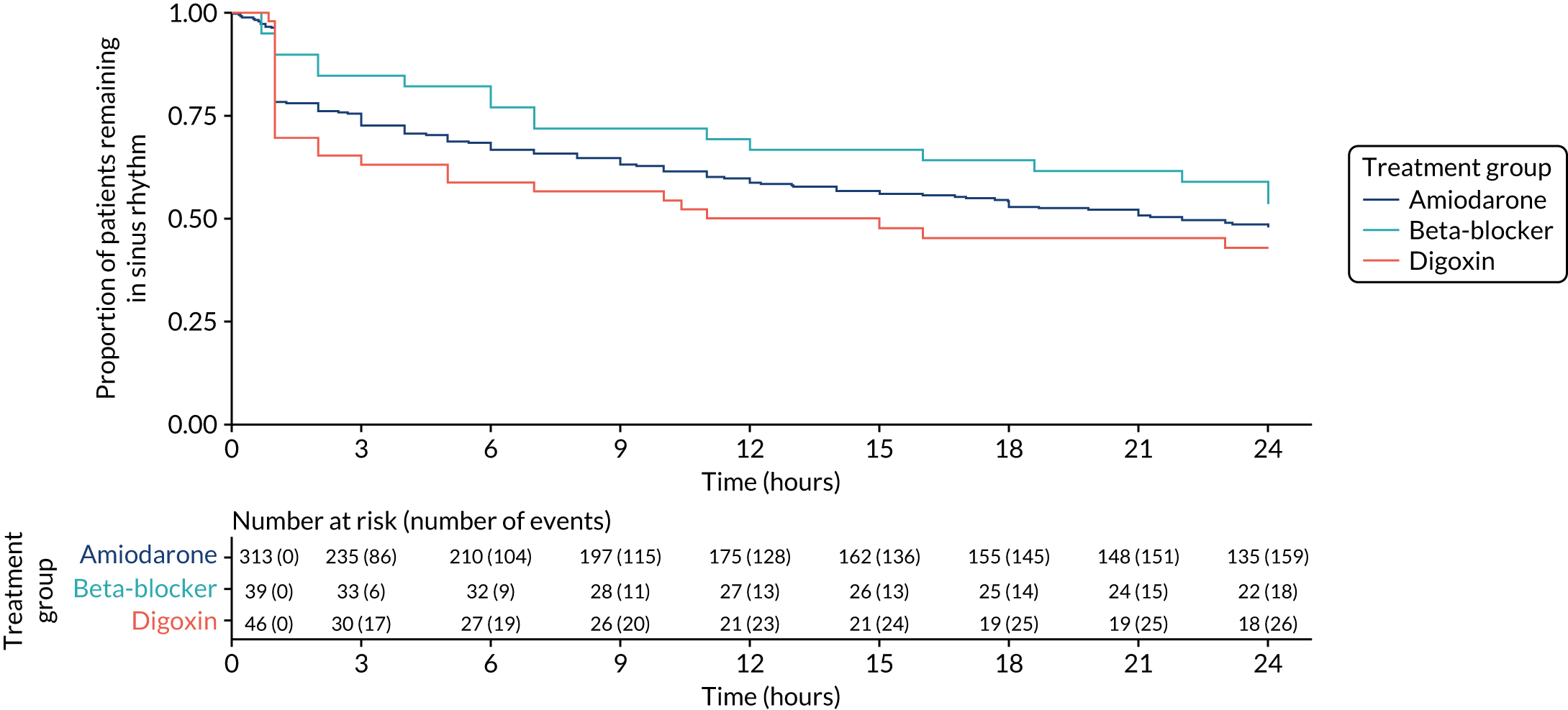
FIGURE 21.
Kaplan–Meier survival curves for each treatment group: MIMIC-III database.
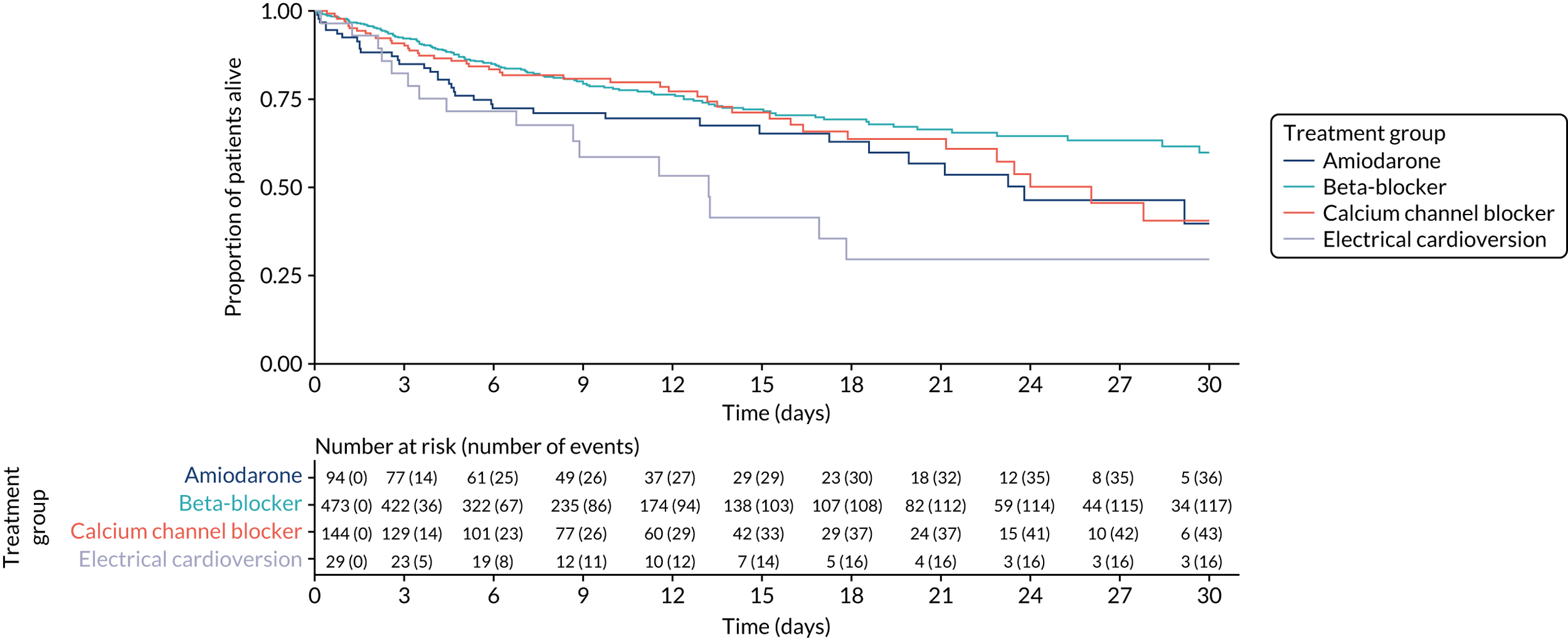
FIGURE 22.
Kaplan–Meier survival curves for each treatment group: PICRAM database.
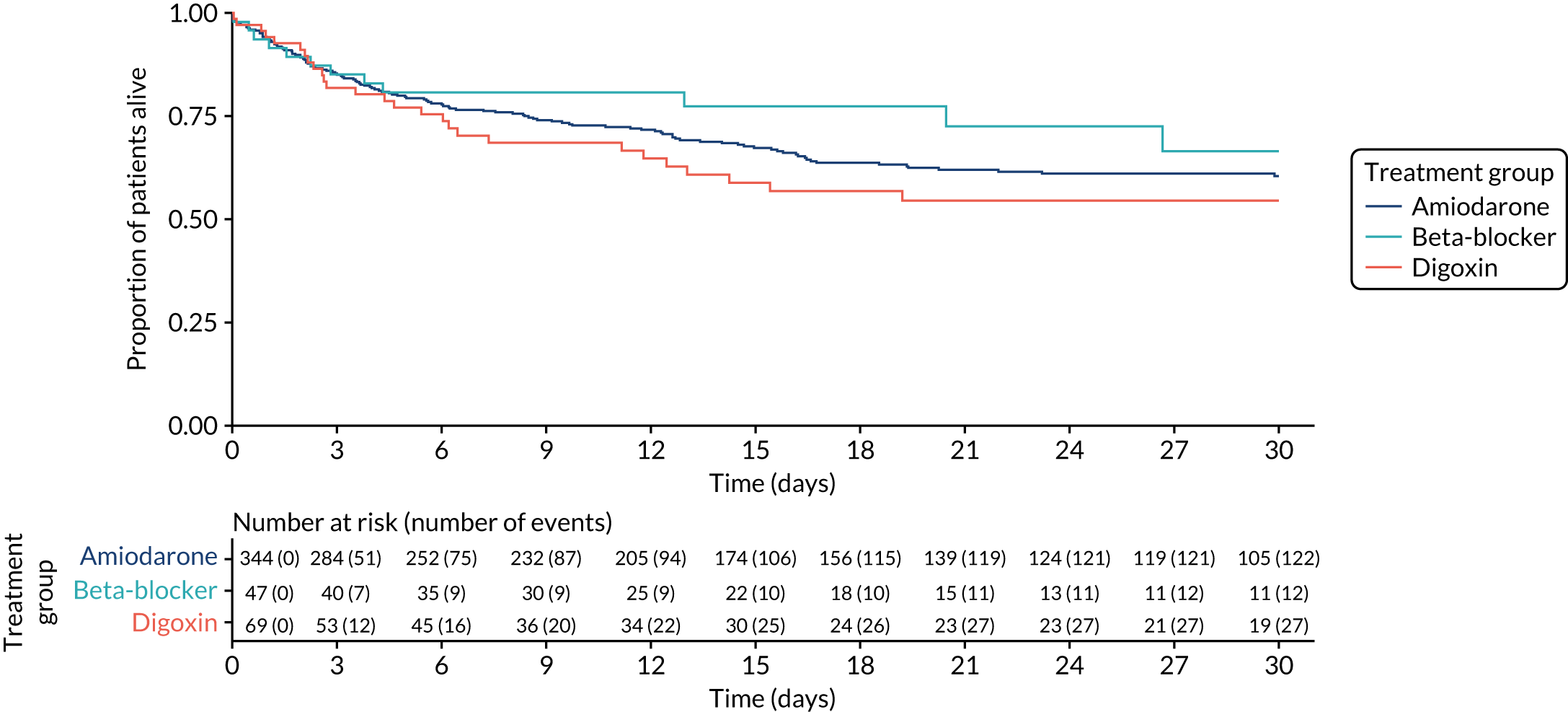
Appendix 7 Critical Care Health Informatics Collaborative database analysis
Aim
The aim of this brief report was to investigate the incidence and characteristics of NOAF in a multicentre UK-based intensive care population. The CCHIC database was not included in the main analysis because it lacks data pertaining to most anti-arrhythmic medications.
This analysis was performed to allow comparison with data extracted for the main analysis to assess consistency and generalisability of our findings in the main report.
Methods
Study design
We carried out a retrospective analysis of patient data collected for the Health Informatics Collaborative (CCHIC) database. The HIC database was created with retrospectively collected detailed data from the ICU clinical information systems relating to patients treated on four general ICUs in London and Cambridge, UK, from 2014 to 2018.
Study population
We included data relating to all adult patients during their first ICU admission. We used the eligibility criteria stated in Chapter 4, Study population. However, we were unable to exclude patients with documented pre-existing arrhythmias because these data were not available in the CCHIC database. Pre-existing arrhythmia was, therefore, determined only by the presence of arrhythmia during the first 3 hours of ICU admission.
Results
Study population
The CCHIC database included data relating to 33,451 adult first admissions to an ICU. Of these patients, 7889 had an ICU length of stay of < 24 hours. We identified 2713 patients being paced or with another significant arrhythmia during the first 3 hours of ICU admission. Of the remaining 22,849 patients, 1003 had missing hospital mortality data. Of the remaining 21,846 eligible patients, 2618 (12%) developed NOAF. This process is outlined in Figure 23. No data were missing in our cohort for baseline demographic variables. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were missing for 3635 patients. Hospital length of stay was missing for 2008 patients. Patients who developed NOAF appeared older and more unwell and more likely to be male and, interestingly, slightly more likely to have had elective surgery than those who did not develop NOAF (Table 34).
FIGURE 23.
The CCHIC database analysis flow chart.
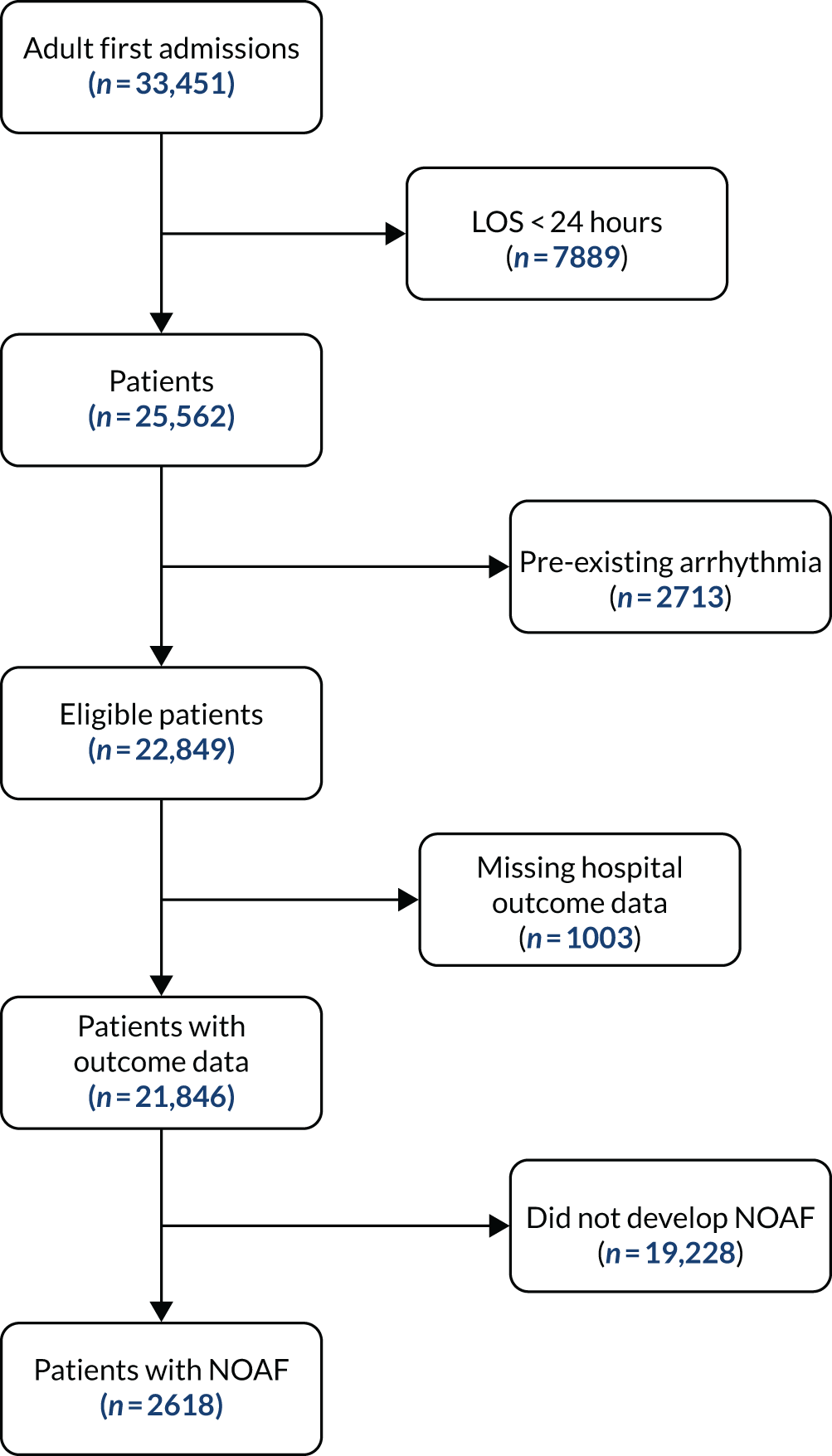
| Characteristic | Never AF (N = 19,228) | NOAF (N = 2618) |
|---|---|---|
| Age (years), median (IQR) | 60 (45–70) | 70 (65–80) |
| Sex, n (%) | ||
| Female | 8266 (43) | 885 (34) |
| Male | 10,962 (57) | 1733 (66) |
| APACHE II score, median (IQR) | 14 (11–18) | 16 (13–21) |
| Missing, n | 3237 | 398 |
| Elective surgery, n (%) | 6513 (34) | 991 (38) |
| ICU length of stay (days), median (IQR) | 2 (1–5) | 5 (3–11) |
| ICU mortality, n (%) | 845 (4.4) | 250 (9.5) |
| Hospital length of stay (days), median (IQR) | 13 (8–26) | 18 (10–36) |
| Missing, n | 1729 | 279 |
| Hospital mortality, n (%) | 1673 (8.7) | 450 (17) |
Characteristics of new-onset atrial fibrillation
The median time from ICU admission to the first episode of NOAF was 43 hours (IQR 23.5–73 hours). The median total duration of AF per patient who developed NOAF was 13.5 hours (IQR 4–37 hours).
Discussion
This analysis demonstrates that the incidence of NOAF and the time to NOAF onset among eligible patients is similar across the PICRAM and CCHIC databases. The association between NOAF and mortality was evident in the CCHIC database. The total duration of AF per patient appeared shorter in the CCHIC database than in the PICRAM database. The CCHIC database analysis included treated and untreated episodes of AF; therefore, the average duration may have been reduced by very brief episodes in which treatment was not felt to be warranted.
Conclusion
The epidemiology of NOAF identified in the CCHIC database is like that observed in the PICRAM database. Similar incidence and onset times suggest that the NOAF identified by bedside observations is a comparable phenomenon across these databases.
Appendix 8 Expert panel details
Parts of this appendix are reproduced or adapted with permission from Bedford et al. 81 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The appendix includes minor additions and formatting changes to the original appendix.
Expert panel details and research recommendations
| Name | Role | Institution |
|---|---|---|
| Professor Peter Watkinson | Associate professor of intensive care medicine | University of Oxford, Oxford, UK |
| Dr Jonathan Bedford | Clinical research fellow | University of Oxford, Oxford, UK |
| Dr Andrew Walden | Consultant in intensive care medicine | Intensive care unit, Royal Berkshire Hospital, Reading, UK |
| Professor Ben O’Brien | Professor of perioperative medicine | St Bartholomew’s Hospital & Barts Heart Centre, London, UK |
| Dr Kim Rajappan | Consultant in cardiology | Oxford University Hospitals NHS Foundation Trust, Oxford, UK |
| Dr Ian Taylor | Lay representative | NA |
| Mrs Cathy Taylor | Lay representative | NA |
Treatments and confounding variables
The following lists were drawn initially from the scoping review and were then refined and ratified by the expert panel, as outlined in Chapter 2, Expert panel review.
Treatments of interest
Treatments to be included in the analysis
-
Amiodarone i.v.
-
Beta-blockers i.v.
-
Labetalol.
-
Esmolol.
-
Metoprolol.
-
-
Calcium channel blockers i.v.
-
Diltiazem.
-
Verapamil.
-
-
Digoxin i.v.
-
Electrical cardioversion.
Treatments of interest but not possible with our data
Propafenone, ibutilide and landiolol were identified as candidate therapies in the scoping review; however, these are not available in our data sets.
Confounding/matching variables
Demographic/comorbid factors
-
Age.
-
Sex.
-
Congestive cardiac failure.
-
Severe respiratory disease/pulmonary fibrosis.
-
COPD (previous or current diagnosis).
-
Chronic liver disease (previous or current diagnosis).
-
Chronic renal failure.
-
Thyroid disorders (previous or current diagnosis, or taking relevant medications).
-
Preadmission beta-blockers.
-
Preadmission antipsychotic medication.
Admission factors
-
Illness severity in the first 3 hours (therefore, not influenced by NOAF, as we are excluding patients in AF in the first 3 hours).
Factors at the time of new-onset atrial fibrillation treatment
-
Heart rate.
-
Blood pressure.
-
Body temperature.
-
Presence and type of vasopressor/inotrope.
-
Dose of vasopressor/inotrope.
-
White cell count.
-
Plasma electrolyte (K, Mg, Na, Ca) concentrations.
-
Plasma urea and creatinine concentrations.
-
Platelet count.
-
Prothrombin time.
-
Presence of therapeutic dose anticoagulation.
-
Presence of bronchodilator therapy.
-
Mechanical ventilation.
-
Haemofiltration (current or previous 12 hours).
-
Presence of central venous access.
Research recommendations
Amiodarone versus beta-blockers
| Domain | Description |
|---|---|
| Step 2: prioritise | |
| Uncertainty identified | Either amiodarone or beta-blockers are commonly used in critically ill patients to control AF, but there is little evidence to support whether or not one is superior |
| Reason uncertain (conflicting or lack of evidence)? | Although cohort studies have suggested survival advantages to beta-blockers, the evidence is conflicting and subject to bias |
| Step 3: two-component research recommendation | |
| Structured statement | A RCT of amiodarone vs. beta-blockers for management of NOAF in critically ill patients should be undertaken |
| Structured rationale | NOAF during ICU is associated with substantially increased mortality after correction for associated risk factors. Both amiodarone and beta-blockers are commonly used but have significant side effects. Whether or not one is superior to the other has not been demonstrated |
| PICOS | Patients: patients who experience NOAF while in an ICU |
| Intervention: amiodarone | |
| Control: beta-blocker | |
Outcomes:
|
|
| Study type: RCT | |
Risk stratification tools for anticoagulation
| Domain | Description |
|---|---|
| Step 2: prioritise | |
| Uncertainty identified | It is not clear in which patients who develop NOAF while in an ICU anticoagulation following hospital discharge might be beneficial |
| Reason uncertain (conflicting or lack of evidence)? | There is very little evidence to inform practice, but the risk of thromboembolism is increased in comparison with those who do not develop NOAF even when corrected for known risk factors |
| Step 3: two-component research recommendation | |
| Structured statement | Whether or not there are subgroups of patients who develop NOAF while in an ICU who may benefit from long-term anticoagulation is unknown. Studies should be undertaken to create risk stratification tools or investigate whether or not current tools are applicable to the ‘NOAF during ICU population’ to identify patients sufficiently at risk of future thromboembolism to merit consideration of anticoagulation |
| Structured rationale | The risk of thromboembolism is increased compared with those who do not develop NOAF, even when corrected for known risk factors. However, current risk stratification tools have not been validated in the ‘NOAF during ICU population’ and do not take account if ICU treatments that may affect future outcome |
| PICOS | Patients: patients experiencing an episode of NOAF during an ICU admission |
| Intervention/control: none | |
| Outcome: thromboembolism | |
| Study type: cohort study with long-term follow-up | |
Incidence of atrial fibrillation and left ventricular dysfunction
| Domain | Description |
|---|---|
| Step 2: prioritise | |
| Uncertainty identified | The incidence of AF and/or left ventricular dysfunction at hospital discharge and at 3 months following development of NOAF while in an ICU is unknown. However, readmission with heart failure and thromboembolism is increased over the 5 years following an episode of NOAF while in an ICU, particularly in the first year |
| Reason uncertain (conflicting or lack of evidence)? | Lack of evidence |
| Step 3: two-component research recommendation | |
| Structured statement | A prospective cohort study to demonstrate the incidence of AF and/or left ventricular dysfunction at hospital discharge and at 3 months following development of NOAF should be undertaken |
| Structured rationale | Readmission with heart failure and thromboembolism is increased over the 5 years following an episode of NOAF while in an ICU, particularly in the first year. Whether or not these events are driven by persistent left ventricular dysfunction and/or AF is unknown |
| PICOS | Patients: patients who experience NOAF while in an ICU |
| Intervention: AF detection and echocardiogram at/near hospital discharge and at 3 months | |
| Control: NA | |
| Outcomes: AF, left ventricular dysfunction, heart failure, thromboembolism, CHA2DS2-VASc and anticoagulation | |
| Study type: prospective cohort | |
List of abbreviations
- AF
- atrial fibrillation
- aHR
- adjusted hazard ratio
- APACHE
- Acute Physiology and Chronic Health Evaluation
- b.p.m.
- beats per minute
- CAFE
- Critical care Atrial Fibrillation Evaluation
- CCHIC
- Critical Care Health Informatics Collaborative
- CHR
- cause-specific hazard ratio
- CI
- confidence interval
- CMP
- Case Mix Programme
- COPD
- chronic obstructive pulmonary disease
- DCC
- direct current cardioversion
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- ICD-10
- International Classification of Diseases and Related Health Problems, Tenth Revision
- ICNARC
- Intensive Care National Audit & Research Centre
- ICU
- intensive care unit
- IQR
- interquartile range
- i.v.
- intravenous
- MgSO4
- magnesium sulphate
- MIMIC-III
- Medical Information Mart for Intensive Care III
- NOAF
- new-onset atrial fibrillation
- OASIS
- Oxford Acute Severity of Illness Score
- ONS
- Office for National Statistics
- OPCS-4
- Office of Population Censuses and Surveys Classification of Surgical Operations and Procedures
- OR
- odds ratio
- PICRAM
- Post Intensive Care Risk-adjusted Alerting and Monitoring
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- ROBINS-I
- Risk Of Bias In Non-randomized Studies – of Interventions
- RR
- relative risk
- SBP
- systolic blood pressure
- SMD
- standardised mean difference
