Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/38. The contractual start date was in January 2014. The draft report began editorial review in February 2020 and was accepted for publication in December 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 King et al. This work was produced by King et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 King et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from King et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Glaucoma is a pressure-related optic neuropathy that results in progressive visual field (VF) deterioration. The World Health Organization estimates that, in 2010, 4.5 million people were blind because of glaucoma,2 which accounts for 12.3% of global blindness. Glaucoma is estimated to affect around 2% of the UK population aged > 40 years, and this percentage increases with age,3–7 with as many as 10% of those in their 80s affected. Glaucoma is the second most common cause of registration as being visually impaired in the UK, accounting for 8.4–11.6% of registrations in those aged > 65 years. 8,9 However, this is likely to be an underestimate. 10
In England, there are over 1 million glaucoma-related visits to the NHS per year. The management of glaucoma patients constitutes a major part of ophthalmologists’ workload, accounting for 23% of all follow-up attendances to the UK hospital eye service11 and 13% of all new referrals. 12 The number of patients with glaucoma is predicted to increase substantially as the result of an ageing population. 13 There is currently no effective screening strategy in the UK for the early identification of all patients with glaucoma. 14
Sight loss from glaucoma is preventable; the Public Health Outcomes Framework for England 2013–1615 has made reducing the number of people living with preventable sight loss a priority. However, people are unaware of the onset of glaucoma because it is typically asymptomatic in the early stages and, as a consequence, between 10% and 39% of patients with glaucoma in the UK present with advanced disease in at least one eye. 16–20 In the most recent study, more than one-third of patients presenting to secondary care had severe disease in at least one eye. 18 Those most at risk include the socially disadvantaged with no family history of glaucoma, those with high intraocular pressure (IOP) and those who do not attend an optometrist regularly. 18,20–22
Advanced glaucoma at presentation: a risk factor for blindness
Presentation with advanced VF loss increases the risk of further progression and blindness. 23–28 Odberg et al. 23 noted in a cohort of patients with advanced glaucoma that 70% of the affected eyes had progressed after a mean of 7.6 years despite treatment. Grant and Burke25 found that eyes with a VF defect at the beginning of treatment were more likely to progress to blindness than eyes in which treatment was started when there was no VF loss. Wilson et al. 26 found that initial VF loss was the strongest determinant of the rate of further VF loss. The rate of deterioration was 11.7 times faster in eyes with more advanced VF loss at presentation. Mikelberg et al. 24 found that, when scotoma mass was small (i.e. early glaucoma), the rate of VF loss was slow, but, when scotoma mass was large (i.e. severe glaucoma), rapid linear progression of VF loss occurred. Oliver et al. 29 found that unilateral blindness owing to glaucoma more than doubled the risk of bilateral blindness.
Current treatment options
Reducing IOP is currently the only effective treatment for glaucoma. 30–33 Better control of IOP at an early stage reduces the risk of progression to blindness. The Advanced Glaucoma Intervention Study (AGIS) demonstrated that the extent of lowering of IOP was related to the progression of VF loss over an 8-year period, showing that progression was least when IOP was maintained below 18 mmHg at all follow-up visits. 34
The primary treatment options in the UK for advanced glaucoma are mainly medical or surgical interventions. Currently, most ophthalmologists treat patients medically, starting with topical drop monotherapy followed by escalating the number of drop therapies until the maximum tolerated combination therapy is achieved. 35 All frequently used eye drops (i.e. prostaglandin analogues, beta-blockers, carbonic anhydrase inhibitors, alpha-agonists and anticholinergic miotics) are now available in generic form and, therefore, cost less. In patients whose glaucoma continues to progress despite eye drop treatment or in whom the target IOP is not achieved, clinicians may opt for surgical intervention, most frequently trabeculectomy. 30–33,36 Patients have indicated that they are not concerned about the treatment that they receive as long as it is effective in the prevention of further visual loss. 37
Recently published National Institute for Health and Care Excellence (NICE) guidelines suggest that patients presenting with advanced disease should be offered augmented trabeculectomy as a primary intervention and should be offered medical management only if surgery is declined, but point out that the evidence to support this recommendation is of poor quality. 38 By using eye drops as a first-line treatment instead of surgery and by operating on patients only in whom drop therapy fails, NHS resources could be saved in the short term; however, the long-term effects on visual outcome are uncertain. Modern glaucoma eye drops lower IOP significantly more and have fewer side effects than those previously used, which may or may not reduce the need for surgery. Social resources may be saved by avoiding the need to support those becoming blind. A survey of consultant ophthalmologists indicated that most do not follow NICE guidance and prefer medical management because of the poor evidence base and concern regarding surgery complications. 35
Compared with surgery, primary eye drop treatment would save upfront surgery costs and may save other NHS costs in the short term, such as intensive follow-up, and reduce the number of patients requiring cataract surgery to restore visual function. Avoiding surgery could improve patient health and quality of life (QoL) in the short term; however, in the long term, insufficient IOP control may produce more VF loss and poorer health outcomes. A head-to-head trial of these two primary treatments is, therefore, required.
Rationale for this study
There is uncertainty about how best to manage patients diagnosed with advanced glaucoma. Such individuals have a high risk of blindness, and effective treatment is required to minimise the chances of disease progression. Currently, NICE guidelines recommend initial surgery but acknowledge the lack of evidence to support this recommendation. Surgery may be more effective in the long term but is associated with potential adverse events (AEs) and increased costs at the time of surgery. Current medical therapies (e.g. eye drops) may be able to control the disease in a proportion of patients with advanced glaucoma. Within the Treatment of Advanced Glaucoma Study (TAGS), we considered whether or not primary medical management is clinically effective and cost-effective for the management of newly diagnosed advanced glaucoma compared with the NICE-recommended treatment of augmented trabeculectomy (glaucoma surgery).
A recent Cochrane systematic review30 comparing primary medical management with surgical treatment for open-angle glaucoma (OAG) identified four relevant studies. 31,39–41 Despite methodological weaknesses and non-standard treatments, the review authors concluded that in severe OAG evidence suggested, that medication was associated with more progressive VF loss and less IOP lowering than surgery. The authors also reported that ‘risk of treatment failure was greater with medication than trabeculectomy [odds ratio (OR) 3.90, 95% confidence interval (CI) 1.60 to 9.53; hazard ratio (HR) 7.27, 95% CI 2.23 to 25.71]’. 30 Three of these four trials are now obsolete because new types of medical management have since been introduced, and the most recent study did not include patients with advanced disease.
The authors30 concluded that surgery lowers IOP more than medication; however, none of these trials specifically addressed the management of those presenting with advanced glaucoma or used modern glaucoma medications that are more effective at lowering IOP and have fewer side effects than previous generations of eye drops. The authors recommended that further randomised controlled trials (RCTs) comparing current medical managements with modern glaucoma surgery be carried out in people with advanced OAG. 30
This uncertainty has subsequently been added to the UK Database of Uncertainties about the Effects of Treatments (UK-DUETS) as an important question requiring further investigation: https://jla.nihr.ac.uk/news-and-publications/downloads/2007–2008-DUETs-Development-Report.pdf (accessed in 2014).
No previous RCT has explored the best treatment options for patients presenting with advanced glaucoma. The AGIS, for example, did not compare primary medical and surgical interventions and did not explore primary treatment options, as all patients had failed on maximum medical management prior to entry. 42 In addition, it included patients with mild glaucoma. The USA-based Collaborative Initial Glaucoma Treatment Study (CIGTS), although comparing the outcomes of primary medical with primary surgical treatment in newly diagnosed patients with glaucoma, enrolled patients presenting with mild disease (CIGTS score of 4.6 ± 4.2). 31 A recent update from the CIGTS suggests that, in a subgroup of patients presenting with more advanced disease [mean difference (MD) < –10 dB], VF progression was slower in those in whom the primary intervention was surgical. 43
We aimed to reduce the uncertainty identified by the Cochrane review,30 UK-DUETS and NICE38 by undertaking a pragmatic RCT of current best medical care in the NHS (a stepped approach of medications) compared with primary surgery. In addition, we aimed to address the concerns of the Public Health Outcomes Framework for England 2013–1615 by identifying the best treatment approach to minimise preventable sight loss in this group of vulnerable patients.
Patient and public involvement
The research topic was identified in a review of primary medical management compared with primary surgical treatment in glaucoma and was subsequently adopted by UK-DUETS. Patients fulfilling the eligibility criteria for the proposed trial agreed to participate in a focus group discussion to identify concerns that they had about the value of such a trial and their participation. Several themes were identified: patients were concerned about the real need for such a trial, that if they participated in a trial they may be going against the judgement of their clinician and that they may be randomised to the wrong group. 44 Discussion of these points in the focus group setting reassured patients both that the trial itself was important and necessary and that, currently, clinicians do not possess the evidence required to recommend one treatment above the other. Several of the patients who contributed to the focus group discussions agreed to form a patient group to develop patient-related material for the trial, particularly in relation to the consenting process, and one was a member of the Trial Steering Committee (TSC). In addition, a patient representative organisation the International Glaucoma Association (IGA) endorsed this study and its chairperson sat on the TSC (until retirement in 2018, when he continued to participate as a lay representative but not representing the IGA).
A patient representative was an active member of the Project Management Group (PMG) and, as part of this role, contributed to the development of trial materials and processes. He participated in the focus group discussions and, therefore, had insight into the experience and concerns of other glaucoma sufferers. In addition to this representative, several other patients with glaucoma who participated in the focus group discussions agreed to form a patient representative group that advised the study team on the development of patient-related study material. This was primarily aimed at the development of the patient information leaflet to provide patients with all of the information that they required to make a decision when asked to participate in the study, specifically regarding concerns about surgery.
Aims of the trial
Primary objective
The primary objective of this trial was to compare primary medical management with primary augmented trabeculectomy (glaucoma surgery) for patients presenting with advanced glaucoma [Hodapp–Parrish–Anderson (HPA) Classification severe] in terms of patient-reported health status using the National Eye Institute’s Visual Function Questionnaire-25 (VFQ-25). 45–54
Secondary objectives
-
To compare generic and vision- and glaucoma-specific patient-reported health and experiences in the short and medium term.
-
To compare the costs and benefits [quality-adjusted life-years (QALYs) gained] of surgery and medication at 2 years based on responses to the (1) EQ-5D-5L, (2) the Health Utilities Index version 3 (HUI-3) and (3) the Glaucoma Utility Index (GUI). 55
-
To compare clinical outcomes (VFMD changes, logMAR visual acuity changes, IOP, Esterman VF for driving vision, registered visual impairment).
-
To compare the need for additional cataract surgery.
-
To compare safety by comparing AEs arising from both surgical and medical interventions.
-
To employ an existing discrete choice experiment (DCE) among participants with advanced glaucoma to generate a revised scoring system for the GUI that is more sensitive and specific for those with advanced disease.
-
To compare long-term costs and benefits through a modelling evaluation.
Chapter 2 Methods
This chapter contains material reproduced with permission from King et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Some of the material in this chapter is also reproduced with permission from King AJ, Hudson J, Fernie G, Burr J, Azuara-Blanco A, Sparrow JM, et al. Baseline characteristics of participants in the Treatment of Advanced Glaucoma Study: a multicenter randomised controlled trial. Am J Ophthal 2020;213:186–94. 56
Study design
The TAGS is a pragmatic,57 two-arm, parallel-arm multicentre RCT comparing primary medical management with primary augmented trabeculectomy (standard care) (see Appendix 1). Participants were randomised to medical management or augmented trabeculectomy (1 : 1 allocation, minimised by centre and bilateral disease).
The perspective of this study was that of the NHS and the patient, with the economic perspective also reflecting Personal Social Services (PSS) and a wider perspective including patients and their families. The framework of the study was an integrated clinical and economic evaluation of the patient outcomes, costs and cost-effectiveness of the two alternative established methods of management of patients presenting with advanced glaucoma. Both treatment strategies currently have been evaluated to assess efficacy and safety. 58–63 The study protocol was published in 2017. 1
Setting
Clinical centres
Twenty-seven secondary care centres with at least one consultant who subspecialises in glaucoma recruited patients for the study.
Population
Adults with advanced (severe) glaucoma in at least one eye defined as severe according to the HPA grading64 for VF loss severity were invited to participate in the study.
Participants
Disease was classified as advanced if it met the criteria for ‘severe’ VF loss according to the HPA classification of glaucoma severity64 (the presence of any of the following):
-
VFMD < –12.00 dB
-
> 50% of points depressed below the 5% level on the pattern deviation probability plot
-
> 20 points depressed below the 1% level on the pattern deviation probability plot
-
one point in the central 5° has a sensitivity of 0 dB
-
points within 5° of fixation under 15 dB sensitivity in both upper and lower hemifields.
Consent to participate
Potential participants who were likely to be eligible for the RCT were identified at their initial consultation for glaucoma by a member of the clinical assessment team. In centres in which it was possible to vet clinic referrals prior to the patient’s attendance at clinic, potentially eligible subjects were identified from their referral letters. At the initial consultation, the consultant, research nurse or another delegated individual introduced the study and, if potential interest was expressed, provided further details of the study by means of the patient information leaflet. The contact details of all interested patients were passed on to the recruitment centre’s study research team if they were not part of the initial consultation. If the patient agreed in principle to the study, then arrangements were made for assessment and consent to be taken. This may have been at a separate appointment or at the initial visit if the patient consented to participate at that visit. Arrangements were individualised for each centre. Eligible patients were asked for their signed informed consent before being randomised. Both the patient information leaflet and the consent form referred to the possibility of long-term follow-up.
We included people who:
-
had severe glaucomatous VF loss (HPA classification)64 in one or both eyes at presentation
-
had OAG, including pigment dispersion glaucoma, pseudoexfoliative glaucoma and normal tension glaucoma
-
were willing to participate in a trial
-
were able to provide informed consent
-
were aged ≥ 18 years
-
agreed, if female and of childbearing potential, to ensure that they used effective contraception during the study and for 3 months thereafter (a negative urine pregnancy test for females of childbearing potential was required prior to randomisation).
We excluded people who:
-
were unable to undergo incisional surgery owing to an inability to lie flat or unsuitable for anaesthetic
-
had a high risk of trabeculectomy failure, such as previous conjunctival surgery or complicated cataract surgery
-
had secondary glaucomas and primary angle-closure glaucoma
-
were pregnant, nursing or planning a pregnancy, or were of childbearing potential not using a reliable method of contraception (a woman was considered to be of childbearing potential unless she was without a uterus or was post-menopausal and had been amenorrhoeic for at least 12 consecutive months).
Health technologies compared
The intervention was either primary medical management or augmented trabeculectomy. Both interventions are established and well-documented approaches to the management of glaucoma. 65 Following randomisation, care for both treatment arms followed NICE guideline recommendations. 65
Primary medical management: escalating medical therapy
Participants randomised to medical management could be prescribed a variety of licensed glaucoma medications (eye drops). These eye drops were used in accordance with NICE guidelines. 65 Escalating medical management was defined as follows: study participants may be started on one or more medications at their initial visit depending upon the judgement of the treating clinician. When monotherapy is initiated this should be with a prostaglandin analogue as directed by NICE guidelines. Subsequent addition of medications was based on clinician judgement/preference. When drops failed to control IOP adequately oral carbonic anhydrase inhibitors may be used.
Primary trabeculectomy: standard trabeculectomy augmented with mitomycin C
Standard trabeculectomy was defined as the creation of a ‘guarded fistula’ by making a small hole in the eye that is covered by a flap of partial-thickness sclera, which allows aqueous humour to egress from the eye into the subconjunctival space. The operation could be performed under either local or general anaesthetic. The dose of mitomycin C in terms of exposure time and concentration was left to the discretion of the operating surgeon and decided on a case-by-case basis.
The protocol specified that all surgery be undertaken within 3 months of randomisation by a consultant who subspecialises in glaucoma or a glaucoma fellow who has performed at least 30 trabeculectomies. Where both eyes were eligible for the study, the eye with better VFMD was allocated as the index eye. An amendment to the protocol allowed the decision about which eye would undergo trabeculectomy first to be made locally when subjects were allocated to the trabeculectomy arm. 1
To ensure that recognised standard trabeculectomy procedures66,67 were being followed by all participating glaucoma surgeons, all potential surgeons completed a questionnaire about their surgical technique that was reviewed and signed off by the chief investigator. No feedback was given because all surgeons were essentially conducting the same operation.
Compliance with study treatment
We designed TAGS as a pragmatic trial and compliance with study treatment was monitored as it would be in routine clinical practice – by asking the patient if they are using their eye drops. There is currently no practical and effective method for monitoring compliance in patients taking glaucoma medications. 68 The degree of compliance feeds into the outcome measurements, as poor compliance for medications is likely to lead to further disease progression. There was no requirement for participants to return any unused eye drops.
Accountability of the study treatment
The local clinical team used a standard hospital prescription form or asked patients’ general practitioners (GPs) to prescribe the medications required in line with standard practice for that team, pragmatically reflecting standard NHS practice.
Concomitant medication
Medications as required for normal clinical care were prescribed for the participants irrespective of their randomised allocation. Throughout the study, investigators could prescribe any concomitant medications or treatments deemed necessary to provide adequate supportive care.
Treatment allocation
All participants who agreed to enter the study were logged with the central trial office and given a unique study number. Randomisation utilised the existing proven remote automated computer randomisation application at the central trial office in the Centre for Healthcare Randomised Trials (CHaRT) (a fully registered UK Clinical Research Network clinical trials unit) in the Health Services Research Unit (HSRU), University of Aberdeen. This randomisation application was available as a telephone-based interactive voice response system and as an internet-based service.
Randomisation was computer allocated, minimised by centre and bilateral disease status. The unit of randomisation was the participant (not the eye). Participants with both eyes affected by advanced glaucoma and eligible were expected to undergo the same treatment in both eyes following randomisation. For those participants with both eyes eligible, an index eye was selected for evaluating clinical outcomes. The eye with better MD value (less severe VF damage) was nominated as the index eye.
For those randomised to the trabeculectomy arm with both eyes eligible, a period of 2–3 months would normally be allowed between operations (but this was at the discretion of the treating clinician). Prior to surgery, IOP was controlled using temporary medical management.
Study outcome measures and schedule of assessment
The TAGS outcomes and schedule of measurement are detailed in Table 1.
| Outcomes | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post randomisation (months) | |||||||
| 1 | 3 | 4 | 6 | 12 | 18 | 24 | ||
| Patient outcomes | ||||||||
| VFQ-25 | ✓ | ✓ | ✓ | ✓ | ||||
| EQ-5D-5L | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| HUI-3a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| GUIa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Patient experience questions | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Clinical outcomes | ||||||||
| Medical history | ✓ | |||||||
| VFMD | ✓ | ✓ | ✓ | ✓ | ||||
| Esterman VF | ✓ | ✓ | ||||||
| LogMAR VA | ✓ | ✓ | ✓ | ✓ | ||||
| IOP | ✓ | ✓ | ✓ | ✓ | ||||
| Standard clinical examination | ✓ | ✓ | ✓ | |||||
| Health economicsb,c | ||||||||
| Health-care utilisation | ✓ | ✓ | ✓ | |||||
| Participant cost | ✓ | ✓ | ✓ | |||||
| Participant time and traveld | ✓ | |||||||
Primary outcomes
The primary outcome was a vision-specific patient-reported outcome QoL measurement, the vision-specific health profile (VFQ-25), which was evaluated at 24 months. The VFQ-25 is a validated questionnaire that has been widely used to evaluate visual outcomes in glaucoma. 47,69–71 In addition to eliciting information about general health and vision, it specifically addresses difficulty with near vision, distance vision, driving and the effect of light conditions on vision.
Secondary outcomes
Patient centred
Patient-centred data were mainly collected through patient-completed questionnaires. The VFQ-25 was completed at baseline and 4, 12 and 24 months post randomisation. The EQ-5D-5L, HUI-3 and GUI responses were converted into health state utility values. These questionnaires were completed at baseline and at 1, 3, 6, 12, 18 and 24 months post randomisation and immediately prior to trabeculectomy.
Clinical
Visual field mean deviation
Visual field mean deviation (VFMD), a global measure of the VF, represents the amount of vision loss occurring because of glaucoma during the study period. It is a routinely measured parameter in standard care of glaucoma patients and it is the primary clinical measure on which management decisions for glaucoma are made, in accordance with NICE guidelines. 65 VF damage is the major measure of the functional impact of glaucoma with direct relevance to QoL measures. 69,72–74 The Humphrey Visual Fields test [24–2 Swedish Interactive Threshold Algorithm (SITA standard)] was performed in all participants. All VF tests were performed by VF technicians or nurses trained to carry out VF tests. VF tests eligible for analysis had to achieve predefined reliability criteria (false positives < 15%). If the VF tests were not reliable, they were repeated at the clinicians’ discretion in accordance with local clinical practice. Two baseline VF tests (24–2 SITA standard) were performed prior to randomisation to confirm eligibility. If the second VF test did not fulfil the criteria for ‘severe defect’ by the HPA criteria, a third VF test was undertaken prior to randomisation and the result of this was deemed to define whether or not the patient was eligible. These were performed at the same baseline clinic evaluation or at a separate evaluation, but had to be completed prior to randomisation. At 24 months, two reliable 24–2 SITA standard VF tests were performed and used to establish the VF outcome VFMD. In addition, a reliable Esterman VF test was performed and was used to assess driving eligibility. An independent VF reading centre assessed all the VF tests. The reading centre was masked to the treatment received by the study participant.
Intraocular pressure
Intraocular pressure was measured by Goldman tonometry at baseline and at 4, 12 and 24 months. The unit of IOP measurement is mmHg. The measurement was undertaken by two observers, and the first observer interacted directly with the patient. Without looking at the measurement dial, the investigator applied the Goldman tonometer to the eye and reached the end point for the measurement value of IOP. The second observer then recorded the values from the measurement dial. This process was repeated and both measures were recorded. If the difference between the first and the second measurements was > 3 mmHg, a third measurement was undertaken.
Visual acuity
Best-corrected log-median angle of resolution (logMAR) visual acuity (VA) was measured at baseline and at 4, 12 and 24 months post randomisation.
Ability to drive
The retention of the ability to drive is one of the most important issues to patients with glaucoma who drive. 37 All patients diagnosed with glaucoma are obliged to inform the Driver and Vehicle Licensing Agency (DVLA) of their diagnosis. Visual standards for driving are assessed on the basis of VF and VA levels. This assessment is arranged at regular intervals by the DVLA. To evaluate visual standard for driving, all participants had an Esterman VF test performed (on the Humphrey VF Analyser) at baseline and at the final visit at 24 months. Registration as visually impaired was based on VA and VF criteria. The consultant ophthalmologists were responsible for registering patients as visually disabled on the basis of these criteria. If a participant has been registered as visually impaired or severely visually impaired, this was recorded along with the date of registration in the study case report form (CRF) at 24 months.
The complications of surgery, the need for cataract surgery and therapy changes were captured from the participants’ case records. All clinical outcomes were recorded on a trial-specific CRF.
Clinical data were collected and entered onto the TAGS secure web database at the participating sites.
Economic
The objective of the economic analysis was to determine the relative cost-effectiveness of augmented trabeculectomy compared with medical management (usual care) over the trial follow-up period and extrapolated over the participant’s lifetime. Economic outcomes were:
-
incremental costs to the NHS, PSS and participants
-
incremental QALYs (based on responses to the EQ-5D-5L, HUI-3 and glaucoma utility index).
In addition, an existing DCE questionnaire with an updated design was administered to trial participants to estimate the value that individuals with advanced glaucoma place on different health states associated with glaucoma and to obtain utility scores for these health states in a population with advanced glaucoma. 55 These data were used to score the GUI responses and were incorporated into the economic evaluation reported in Chapter 6.
Details of the DCE are reported in Chapter 5, and the economic evaluation is reported in Chapter 6.
Safety reporting
We defined AEs as those events that occurred after randomisation and within the 24-month follow-up period of the trial. To be considered as an AE, an event had to be categorised as related to participation in the trial or related to glaucoma. Thus, we excluded a continuous and persistent disease or symptom, present before the trial, which failed to progress and signs or symptoms of the disease being studied (except where they deteriorated sufficiently to be considered serious).
We identified potentially expected AEs linked to medical management and trabeculectomy (with mitomycin C) as:
-
Medical management – redness, stinging, itching, transient blurred vision, eye watering, ocular discomfort, allergy, eyelash growth, change in skin colour around eye, change in iris colour, shortness of breath, unpleasant taste in mouth, dry mouth, fatigue, kidney stones, skin rash, cataract formation and retinal detachment. In some cases, some of these symptoms may have been because of preservatives in the eye drops and, if this was the case, preservative-free eye drops were used.
-
Trabeculectomy with mitomycin C – discomfort, blurred vision, corneal epithelial defect, conjunctival button hole, flap dehiscence, IOP too low, transient choroidal effusion, suprachoroidal haemorrhage, hyphaema, early bleb leak, shallow anterior chamber (grades 1–3), iris incarceration, persistent uveitis, transient or permanent ptosis, macular oedema, malignant glaucoma, corneal decompensation, cataract formation and retinal detachment, late bleb leak, bleb infection, bleb-related endophthalmitis, permanent severe loss of vision at time of surgery (< 1/500), bleeding in the eye, broad complex tachycardia while under general anaesthetic and post-operative dizziness.
In addition, a VA AE was defined as any of the following:
-
irreversible loss of 10 Early Treatment Diabetic Retinopathy Study (ETDRS) letters of logMAR VA
-
loss of two or more stages of categorical VA measurement (count fingers, hand motion, light perception, no light perception)
-
any loss to no light perception.
These definitions were based on knowledge of AEs associated with augmented trabeculectomy and the relevant product information documented in the summary of product characteristics for eye drops. The latest online version of the appropriate summary of product characteristics was considered in the assessment of an AE.
We adhered to the standard definition of serious adverse events (SAEs) as those leading to death, hospitalisation (or prolongation of existing hospitalisation), persistent or significant disability or incapacity, congenital anomaly or birth defect, as well as an event that was considered life-threatening or otherwise considered medically significant.
Pregnancy was not considered an AE or SAE; however, we put in place processes (see the trial protocol for details1) to collect pregnancy information for participants who became pregnant while participating in the study (defined as while taking or within 3 months of ceasing to take study medications).
Masking
Given that TAGS investigated medical versus surgical management, neither the participants nor the local clinical team could be masked to the randomised treatment allocation. The only masked aspect was the evaluation of VFs at the end of the study, which was undertaken by an independent reading centre masked to the allocation: Central Angiographic Resource Facility (CARF), Queen’s University Belfast.
Methods to protect against sources of bias
The allocation to treatment arms was concealed by using a central randomisation service that could be accessed only by clinical trials unit programming staff.
The evaluation of VFs collected during the study period was by CARF masked to the participant intervention. IOP was measured by two observers: one taking the reading and the other reading the IOP value to minimise the risk of measurement bias.
The expected attrition rate was low based on a previous glaucoma treatment RCT,75 but we allowed for a potential attrition rate of 13.5% over 24 months to accommodate this.
External validity was maximised by recruiting from multiple centres and participants were treated by multiple clinicians.
Statistical analysis
Ground rules for statistical analysis
The trial analysis followed a statistical analysis plan (see Report Supplementary Material 2), which was agreed in advance by the TSC. The main analyses were based on the intention-to-treat (i.e. analyse as randomised) principle and took place after the 24-month follow-up. Baseline and follow-up data were summarised using appropriate statistics and graphical summaries. Statistical significance was at the two-sided 5% level with corresponding CIs derived. All analyses were carried out using Stata® version 16 software (StataCorp LP, College Station, TX, USA).
Sample size
The primary patient-reported outcome was the health status measured by the VFQ-25 assessment at 24 months. A study with 190 participants in each arm would have 90% power at a two-sided 5% significance level to detect a difference in means of 0.33 of a standard deviation (SD), which translates to 6 points on the VFQ-25 assuming a common SD of 18 points observed in previous work that has a clinically relevant effect size in patients with advanced glaucoma. 76,77 Seven points is a likely minimally important difference based on our pilot work on VFQ-25 scores in patients with glaucoma,77 but there is uncertainty, and so we opted for a more conservative 6-point difference, which is supported by the literature for another chronic eye disease, macular degeneration. 78 Assuming a drop-out rate of 13.5% as a result of declining further follow-up and death, we had to randomise a total of 440 participants to detect this difference.
For the secondary clinical outcome (VFMD), TAGS had 90% power at 5% significance to detect a 1.3 dB difference in VFMD. This is derived from a subgroup of patients with advanced glaucoma and is a clinically significant difference in the context of advanced glaucoma and predictive further visual disability. 30,43
Centres that had a throughput of at least 20 eligible patients annually were approached to participate in the trial. This enabled the development of a recruitment projection based on 20 sites recruiting approximately 9–11 patients per year, with a staggered start of recruiting sites, and allowed 440 participants to be recruited over 3 years (assuming a reduced rate during the first month of each site set-up and 50% reduction during the holiday months of August and December).
Primary/secondary outcome analysis
The primary outcome, VFQ-25 assessed at 24 months, was analysed using a heteroscedastic partially nested repeated-measures mixed-effects linear model correcting for baseline score and bilateral disease, and time as a fixed effect. 79 The repeated measures were VFQ-25 assessed at 4, 12 and 24 months. Treatment effects were estimated from time-by-treatment interactions at each time point; the primary time of interest was 24 months. This approach uses participant data from all of the time points and incorporates a random effect for centre and surgeon using restricted maximum likelihood. We used this approach to account for the potential lack of independence of outcome data for patients treated by the same surgeon. This is known as clustering or the surgeon effect, and occurs in the trabeculectomy arm only (hence the ‘nested’ approach) in TAGS. We also adjusted for heteroscedastic errors because the residuals compared with the fitted values showed evidence that the variance of errors were different between treatment arms. We did not carry out any sensitivity analysis for missing data because < 10% of VFQ-25 data were missing. Given the pragmatic nature of TAGS, there was the potential for participants to cross over from one intervention to the other. To estimate the treatment effect in the subgroup of participants who complied with allocated treatment, we used complier-average causal effect (CACE) methods using instrumental variable regression80 and a per-protocol analysis.
For the secondary outcomes, EQ-5D-5L, HUI-3, GUI, IOP and logMAR VA, VFs were analysed following the same method as the primary outcome. The utility values for the GUI were derived from the results of the DCE (which are presented in Chapter 5). For the IOP, logMAR VA and VF tests, a sensitivity analysis was performed using data from all eligible eyes by also including a random effect at the participant level to reflect the lack of independence of eyes within participants. In addition, for the VFs a further two sensitivity analyses were performed: the first included only the VF tests performed in accordance with SITA standard and the second removed participants who had only one VF test or in whom the false positive standard was > 15%. The post hoc Dunnett’s multiplicity p-values were presented for IOP and logMAR VA. Patient experience (glaucoma getting worse) was analysed using a repeated-measures mixed-effects Poisson model adjusting for bilateral disease and including a random effect for surgeon and treatment. The need for cataract surgery, visual standards for driving and safety outcomes were analysed using the same modelling approach. Given that the number of events for patients registered as sight impaired was small, we used Fisher’s exact test. Data missing at baseline were reported as such, and for the primary/secondary outcomes continuous data were imputed using the centre-specific mean of that variable.
Subgroup analyses
Planned subgroup analyses explored the potential treatment effect moderation of sex, age (< 65 years vs. ≥ 65 years), one or both eyes affected with advanced glaucoma, Index of Multiple Deprivation (quintile) and extent of VF loss at baseline (–20 dB, ≥ –20 dB) on the primary outcome. The subgroup-by-treatment interaction was assessed by including interaction terms in the models outlined above. We used a stricter level of statistical significance (two-sided 1% significance level) and 99% CIs to reflect the exploratory nature of these analyses. A post hoc subgroup analysis of IOP at diagnosis (< 21 mmHg vs. ≥ 21 mmHg) was also explored following the same analysis method as the planned subgroup analysis.
Criteria for the termination of the trial
Owing to the staggered nature of recruitment and, therefore, the measurement of the primary outcome at 24 months, we did not anticipate that the trial would be terminated early for benefit. We proposed one main effectiveness analysis at the end of the trial. During the trial, safety and other data were monitored by reports prepared for the Data Monitoring Committee (DMC). The DMC decided to meet every 6 months until June 2018.
Economic evaluation
In this study both a ‘within-trial’ and a model-based economic evaluation were conducted. These are described in detail in Chapter 6.
Research ethics and regulatory approvals
TAGS received favourable ethics opinion from the East Midlands – Derby Research Ethics Committee (REC) on 12 December 2013 (REC reference number 13/EM/0395).
Changes to the protocol
The main changes to the protocol (see Appendix 8) since original ethics approval include the addition of further follow-up assessments (at 3, 4 and 5 years) and a genetics substudy. All amendments were reviewed by the sponsor and the independent TSC on behalf of the funder before being submitted to, and then approved by, the REC. The results of these studies are not reported in this monograph.
Management of the trial
The trial management team, based within CHaRT, University of Aberdeen, provided day-to-day support for the recruiting centres and was led by a local principal investigator (PI). The PIs, in most cases supported by research nurses, trial co-ordinators or dedicated staff, were responsible for all aspects of local organisation, including the recruitment of participants, delivery of the interventions and notification of any problems or unexpected developments during the study period.
Study oversight committees
Study Management Group
The Study Management Group (SMG) was responsible for the day-to-day management of the trial. This group consisted of the chief investigator, trial manager, senior trial manager, data co-ordinator, statistician and a sponsor representative. Members of the SMG are listed in the Acknowledgements.
Project Management Group
The Project Management Group (PMG) was responsible for overseeing the management of the trial. This group consisted of the SMG plus grant applicants, a public and patient involvement (PPI) representative and a senior programmer. Members of the PMG are listed in the Acknowledgements.
Trial Steering Committee
The Trial Steering Committee (TSC) was responsible for monitoring and supervising the progress of TAGS. The committee met seven times between April 2014 and December 2019 at agreed intervals. The TSC consisted of independent experts, a PPI member, the chief investigator and key members of the PMG. Members of the TSC are listed in the Acknowledgements.
Data Monitoring Committee
The DMC was independent of the trial and was responsible for monitoring safety and data integrity. The committee met eight times between April 2014 and August 2018, approximately every 6 months at intervals agreed by the committee. The trial statistician provided the data and analyses requested by the DMC prior to each meeting. The committee consisted of three independent experts. Members of the DMC are listed in the Acknowledgements.
Chapter 3 Participant baseline characteristics
Trial recruitment
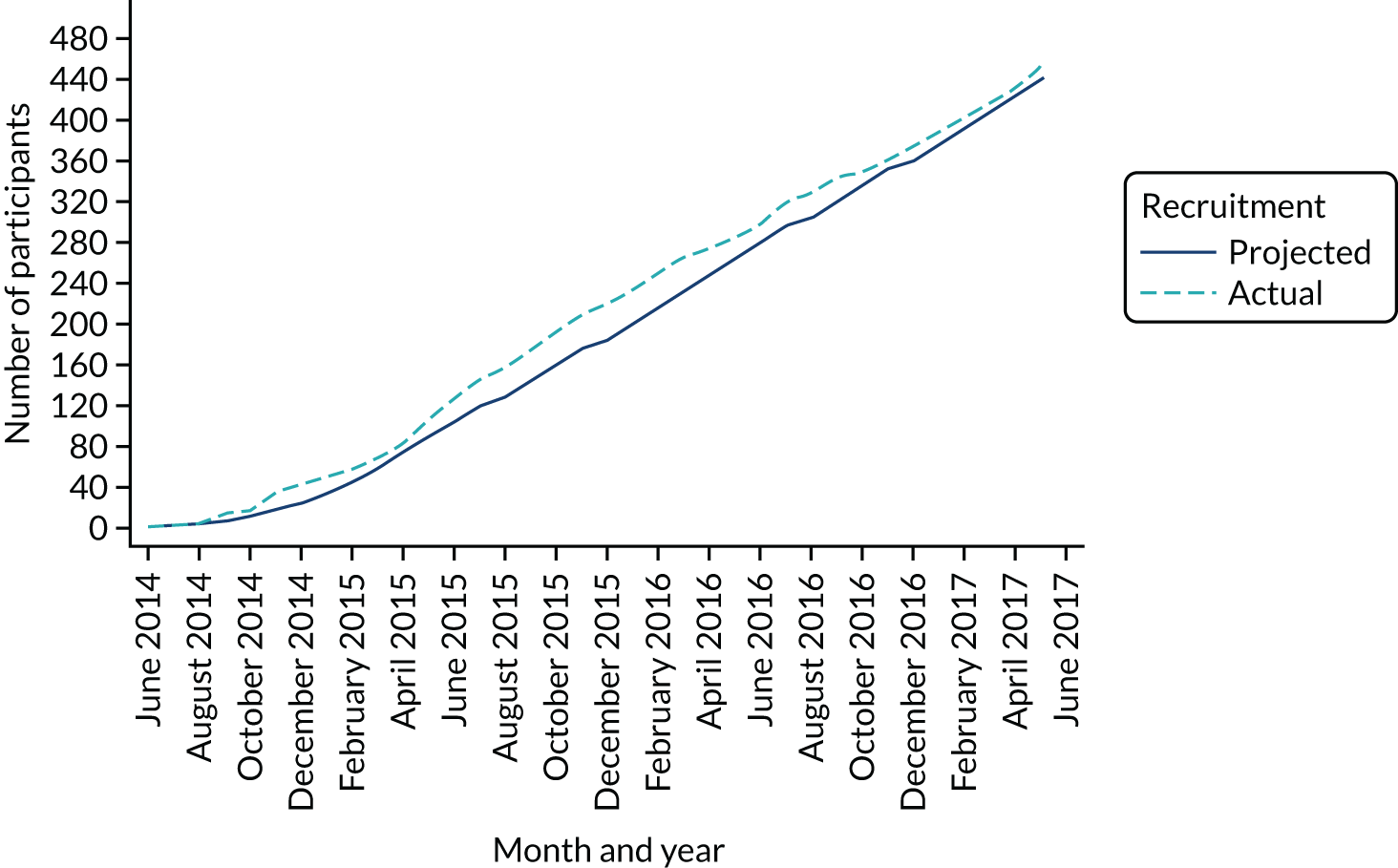
Between 3 June 2014 and 21 May 2017, we recruited 453 participants from 27 centres (Table 2): 227 were randomised to the trabeculectomy arm and 226 to the medical management arm. The trajectory of recruitment from all centres is shown in Figure 1.
| Centre | All (N = 453), n (%) | Treatment arm, n (%) | |
|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||
| Queen Alexandra Hospital, Portsmouth | 52 (11.5) | 26 (11.5) | 26 (11.5) |
| Queen’s Medical Centre, Nottingham | 46 (10.2) | 24 (10.6) | 22 (9.7) |
| Guy’s and St Thomas’, London | 36 (7.9) | 18 (7.9) | 18 (8.0) |
| Moorfields Eye Hospital, London | 26 (5.7) | 12 (5.3) | 14 (6.2) |
| Bristol Eye Hospital, Bristol | 26 (5.7) | 14 (6.2) | 12 (5.3) |
| Norfolk and Norwich University Hospital, Norwich | 24 (5.3) | 12 (5.3) | 12 (5.3) |
| Manchester Royal Eye Hospital, Manchester | 21 (4.6) | 10 (4.4) | 11 (4.9) |
| York Hospital, York | 19 (4.2) | 9 (4.0) | 10 (4.4) |
| Princess Alexandra Eye Hospital, Edinburgh | 17 (3.8) | 8 (3.5) | 9 (4.0) |
| Hinchingbrooke Hospital, Huntingdon | 17 (3.8) | 9 (4.0) | 8 (3.5) |
| Western Eye Hospital, Imperial, London | 16 (3.5) | 9 (4.0) | 7 (3.1) |
| Sunderland Eye Infirmary, Sunderland | 16 (3.5) | 8 (3.5) | 8 (3.5) |
| Royal Victoria Hospital, Belfast Health and Social Care Trust, Belfast | 15 (3.3) | 7 (3.1) | 8 (3.5) |
| Maidstone Hospital, Maidstone | 14 (3.1) | 7 (3.1) | 7 (3.1) |
| Royal Derby Hospital, Derby | 13 (2.9) | 6 (2.6) | 7 (3.1) |
| Warrington Hospital, Warrington | 13 (2.9) | 6 (2.6) | 7 (3.1) |
| James Paget Hospital, Great Yarmouth | 13 (2.9) | 7 (3.1) | 6 (2.7) |
| Birmingham and Midland Eye Centre, Birmingham | 12 (2.6) | 7 (3.1) | 5 (2.2) |
| Birmingham Heartlands Hospital, Birmingham | 12 (2.6) | 6 (2.6) | 6 (2.7) |
| University Hospital Coventry, Coventry | 10 (2.2) | 6 (2.6) | 4 (1.8) |
| Queen Margaret Hospital, Dunfermline | 9 (2.0) | 4 (1.8) | 5 (2.2) |
| Cheltenham General Hospital, Cheltenham | 8 (1.8) | 4 (1.8) | 4 (1.8) |
| Harrogate District Hospital, Harrogate | 6 (1.3) | 3 (1.3) | 3 (1.3) |
| Ninewells Hospital, Dundee | 4 (0.9) | 2 (0.9) | 2 (0.9) |
| Royal Hallamshire Hospital, Sheffield | 3 (0.7) | 1 (0.4) | 2 (0.9) |
| Hairmyres Hospital, Lanarkshire | 3 (0.7) | 1 (0.4) | 2 (0.9) |
| Gartnavel General Hospital, Glasgow | 2 (0.4) | 1 (0.4) | 1 (0.4) |
FIGURE 1.
Recruitment over time.
Participant flow
Figure 2 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for TAGS. A total of 962 potentially eligible patients were screened, of whom 509 were excluded: 233 because they were ineligible and 276 because they declined to participate. The main reasons for ineligibility were that VFs did not meet the inclusion criteria (24%) and that patients could not be randomised within 3 months of diagnosis (22.7%). Those who declined either did not give a reason (27.9%) or did not want surgery (19.6%). Further details on reasons why patients were excluded can be found in Appendix 2, Table 23.
FIGURE 2.
The CONSORT flow diagram presenting participant flow in the trial.
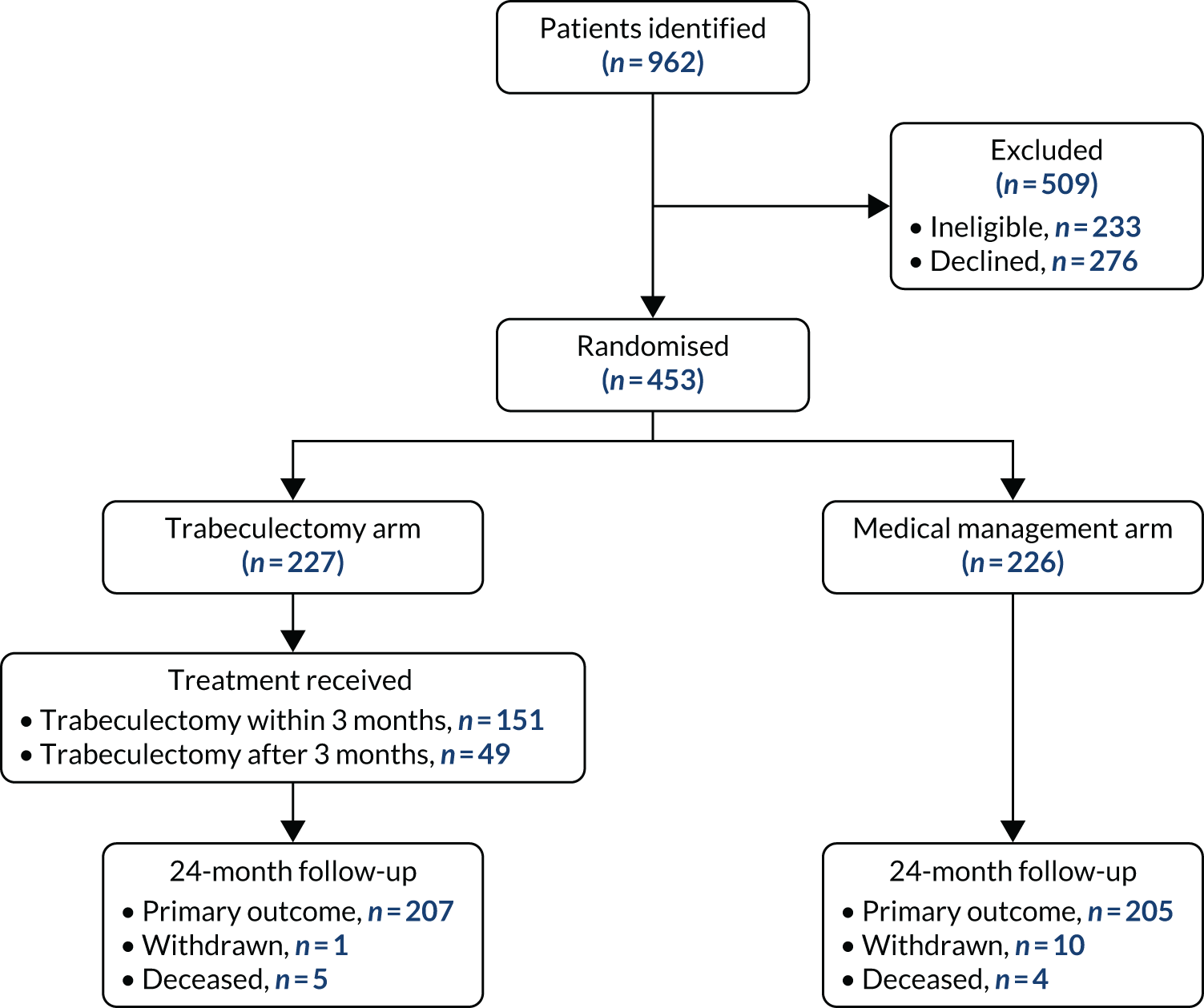
All of the participants attended a baseline clinical assessment, but one participant in the trabeculectomy arm and two participants in the medical management arm did not provide baseline VFQ-25 (primary outcome). A total of 11 participants declined further questionnaires and clinical follow-up (not including treatment clinics) from the study, and nine deaths were reported, none of which was caused by a study intervention. In the trabeculectomy arm, 201 (88.5%) participants underwent trabeculectomy in their index eye: trabeculectomy was performed within 3 months of randomisation in 151 (66.5%) participants and after 3 months in 49 (21.2%) participants. For one participant, the timing of trabeculectomy was unknown (see Figure 2). There were 16 (7.0%) participants who declined trabeculectomy, two (0.9%) died before they could receive a trabeculectomy and eight (3.5%) did not receive trabeculectomy in their index eye. Further details about the treatment received are described later in this chapter. At the 24-month follow-up, 207 (91.2%) participants in the trabeculectomy arm and 205 (90.7%) participants in the medical management arm provided primary outcome data.
Baseline characteristics
The baseline characteristics are shown in Table 3 and the treatment arms were well balanced. The mean age of the participants was 67 years in the trabeculectomy arm and 68 years in the medical management arm. Over 65% of participants were male and over 80% were white. In total, the percentage of participants who had bilateral disease was 19.4% in the trabeculectomy arm and 19.5% in the medical management arm, and over 90% in both arms had primary OAG. The mean VFQ-25 was 87.1 in both arms and the mean VFMD (dB) was –14.9 and –15.3 in the trabeculectomy arm and the medical management arm, respectively. The baseline characteristics for the non-index eye and the HPA classification of glaucoma severity are shown in Appendix 2, Tables 24 and 25, respectively.
| Characteristic | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Age (years), n; mean (SD) | 227; 67 (12.2) | 226; 68 (12.4) |
| Sex, n (%) | ||
| Male | 156 (68.7) | 147 (65.0) |
| Female | 71 (31.3) | 79 (35.0) |
| Ethnicity, n (%) | ||
| White | 182 (80.2) | 191 (84.5) |
| Afro-Caribbean | 32 (14.1) | 27 (11.9) |
| Asian – India/Pakistan/Bangladesh | 8 (3.5) | 4 (1.8) |
| Asian – Oriental | 2 (0.9) | 0 (0) |
| Mixed | 0 (0) | 1 (0.4) |
| Other | 3 (1.3) | 2 (0.9) |
| Missing | 0 (0) | 1 (0.4) |
| Advanced glaucoma in both eyes, n (%) | ||
| Yes | 44 (19.4) | 44 (19.5) |
| No | 183 (80.6) | 182 (80.5) |
| Glaucoma in both eyes, n (%) | ||
| Yes | 178 (78.4) | 169 (74.8) |
| No | 49 (21.6) | 57 (25.2) |
| Eligible to be registered as sight impaired, n (%) | ||
| No | 214 (94.3) | 212 (93.8) |
| Sight impaired | 10 (4.4) | 12 (5.3) |
| Severely sight impaired | 3 (1.3) | 2 (0.9) |
| Glaucoma diagnosis, n (%) | ||
| Primary OAG (including NTG) | 219 (96.5) | 220 (97.3) |
| Pigment dispersion syndrome | 5 (2.2) | 4 (1.8) |
| Pseudoexfoliation syndrome | 3 (1.3) | 2 (0.9) |
| Lens status, n (%) | ||
| Phakic | 212 (93.4) | 209 (92.5) |
| Pseudophakic | 15 (6.6) | 17 (7.5) |
| Central corneal thickness (µm), n; mean (SD) | 226; 539.4 (35.7) | 223; 541.4 (35.7) |
| Glaucoma eye drops, n (%) | ||
| Prostaglandin analogue | 186 (81.9) | 182 (80.5) |
| Beta-blocker | 52 (22.9) | 52 (23.0) |
| Carbonic anhydrase inhibitor | 45 (19.8) | 33 (14.6) |
| α-agonist | 7 (3.1) | 4 (1.8) |
| Diamoxa | 6 (2.6) | 2 (0.9) |
| Family history of glaucoma, n (%) | ||
| Yes | 63 (27.8) | 79 (35.0) |
| No | 152 (67.0) | 131 (58.0) |
| Missing | 12 (5.3) | 16 (7.1) |
| Number of times visited the optician in the last 10 years, n; median (IQR) | 214; 5 (2–6) | 209; 5 (3–8) |
| Index of Multiple Deprivation, n (%) | ||
| First quintile (most deprived) | 54 (23.8) | 52 (23.0) |
| Second quintile | 30 (13.2) | 37 (16.4) |
| Third quintile | 45 (19.8) | 43 (19.0) |
| Fourth quintile | 50 (22.0) | 43 (19.0) |
| Fifth quintile (least deprived) | 47 (20.7) | 49 (21.7) |
| Missing | 1 (0.4) | 2 (0.9) |
| Ocular comorbidity, n (%) | ||
| Yes | 50 (22.0) | 50 (22.1) |
| No | 177 (78.0) | 176 (77.9) |
| Ocular comorbidity details,b n (%) | ||
| AMD | 6 (12.0) | 4 (8.0) |
| Cataract | 42 (84.0) | 42 (84.0) |
| Vascular occlusion | 2 (4.0) | 1 (2.0) |
| Diabetic retinopathy | 1 (2.0) | 1 (2.0) |
| Other | 9 (18.0) | 6 (12.0) |
| VFQ-25, n; mean (SD) | 226; 87.1 (13.6) | 224; 87.1 (13.4) |
| VFQ-25 subscales, n; mean (SD) | ||
| Near activities | 225; 84.2 (18.5) | 224; 84.4 (16.9) |
| Distance activities | 226; 88.5 (16.1) | 224; 89.7 (14.4) |
| Dependency | 226; 94.0 (17.3) | 222; 94.9 (15.7) |
| Driving | 171; 85.9 (26.7) | 158; 84.8 (26.2) |
| General health | 225; 63.6 (23.4) | 223; 60.9 (22.6) |
| Role difficulties | 226; 87.1 (19.8) | 222; 87.4 (20.8) |
| Mental health | 226; 81.1 (21.2) | 224; 81.8 (19.9) |
| General vision | 223; 74.9 (14.5) | 223; 72.8 (14.2) |
| Social function | 225; 95.2 (11.9) | 224; 94.9 (12.1) |
| Colour vision | 223; 96.9 (10.9) | 222; 96.6 (11.1) |
| Peripheral vision | 224; 86.6 (20.8) | 224; 87.2 (20.2) |
| Ocular pain | 225; 84.7 (19.0) | 224; 83.9 (17.2) |
| VFMD (dB), n; mean (SD) | 227; –14.91 (6.36) | 226; –15.26 (6.34) |
| LogMAR VA, n; mean (SD) | 227; 0.15 (0.25) | 223; 0.17 (0.26) |
| Intraocular pressure (mmHg), n; mean (SD) | ||
| Diagnosis | 226; 26.9 (9.1) | 223; 25.9 (8.4) |
| Baseline | 222; 19.4 (6.2) | 221; 19.0 (5.7) |
| EQ-5D-5L, n; mean (SD) | 222; 0.844 (0.185) | 222; 0.837 (0.176) |
| HUI-3, n; mean (SD) | 214; 0.814 (0.202) | 214; 0.809 (0.208) |
| GUI, n; mean (SD) | 219; 0.884 (0.131) | 222; 0.863 (0.130) |
| Participant experience (glaucoma getting worse), n (%) | ||
| Yes | 95 (41.9) | 76 (33.6) |
| No | 113 (49.8) | 133 (58.8) |
| Missing | 19 (8.4) | 17 (7.5) |
| Visual standards for driving, n (%) | ||
| Pass | 187 (82.4) | 196 (86.7) |
| Fail | 27 (11.9) | 21 (9.3) |
| Missing | 13 (5.7) | 9 (4.0) |
Treatment received
In the trabeculectomy arm, 201 (88.5%) participants received surgery in their index eye. Of the remaining 26 participants, four had surgery in their non-index-eye only, 16 declined surgery, two died prior to having surgery and four had yet to receive surgery. Thirty-four (15.0%) participants underwent trabeculectomy in their non-index eye, of whom four did not undergo trabeculectomy in their index eye (see Appendix 3, Table 27). In the medical management arm, 39 (17.3%) participants underwent trabeculectomy (Table 4). The median time to trabeculectomy was 8.9 weeks in the trabeculectomy arm. Among the 27 participants in the medical management arm for whom the date of surgery was known, the median time to surgery was 47.7 weeks post randomisation, and in 15 cases was more than 12 months post randomisation. At the time of surgery, the mean number of eye drop agents that the patient had received pre-operatively was 1.4 (SD 1.1) in the trabeculectomy arm and 1.9 (SD 1.1) in the medical management arm (see Table 4 for the classes of eye drops prescribed.) At the time of surgery, the mean pre-operative IOP was 19.3 mmHg in the trabeculectomy arm and 21.1 mmHg in the medical management arm. Appendix 3, Table 26, gives further details of the trabeculectomy procedure. Appendix 3, Table 27, shows the results for the non-index eye.
| Trabeculectomy details | Treatment arm | |
|---|---|---|
| Trabeculectomy | Medical management | |
| Received surgery in their index eye, n/N (%) | 201/227 (88.5) | 39/226 (17.3) |
| Time to trabeculectomy (weeks), n; median (IQR) | 200; 8.9 (5.3–11.9) | 39; 47.4 (23.0–63.1) |
| Reasons for trabeculectomy,a n (%) | ||
| Uncontrolled IOP | 23 (60.5) | |
| VF progression | 6 (15.8) | |
| Drop intolerance | 4 (10.5) | |
| Missing | 11 (28.9) | |
| Trabeculectomy clinical report form provided (n) | 199 | 27 |
| Number of different types of eye drops received pre-operatively, mean (SD) | 1.4 (1.1) | 1.9 (1.1) |
| Type of pre-operative eye drop, n (%) | ||
| Carbonic anhydrase inhibitor | 57 (28.6) | 14 (51.9) |
| Prostaglandin analogue | 148 (74.4) | 21 (77.8) |
| Beta-blocker | 56 (28.1) | 12 (44.4) |
| α-agonist | 9 (4.5) | 4 (14.8) |
| Parasympathomimetic | 3 (1.5) | 0 (0) |
| Diamox, n (%) | ||
| Yes | 3 (1.5) | 1 (3.7) |
| No | 192 (96.5) | 26 (96.3) |
| Missing | 4 (2.0) | 0 (0) |
| Pre-operative IOP (mmHg), n; mean (SD) | 179; 19.3 (6.0) | 26; 21.1 (4.0) |
At the 24-month follow-up, 9 out of 181 (5.0%) participants in the trabeculectomy arm had been added to a waiting list to have a trabeculectomy in their non-index eye. In their medical management arm, 14 out of 204 (6.9%) participants had been added to a waiting list to have a trabeculectomy, in 11 of whom trabeculectomy was planned for the index eye.
Chapter 4 Trial results
Primary outcome
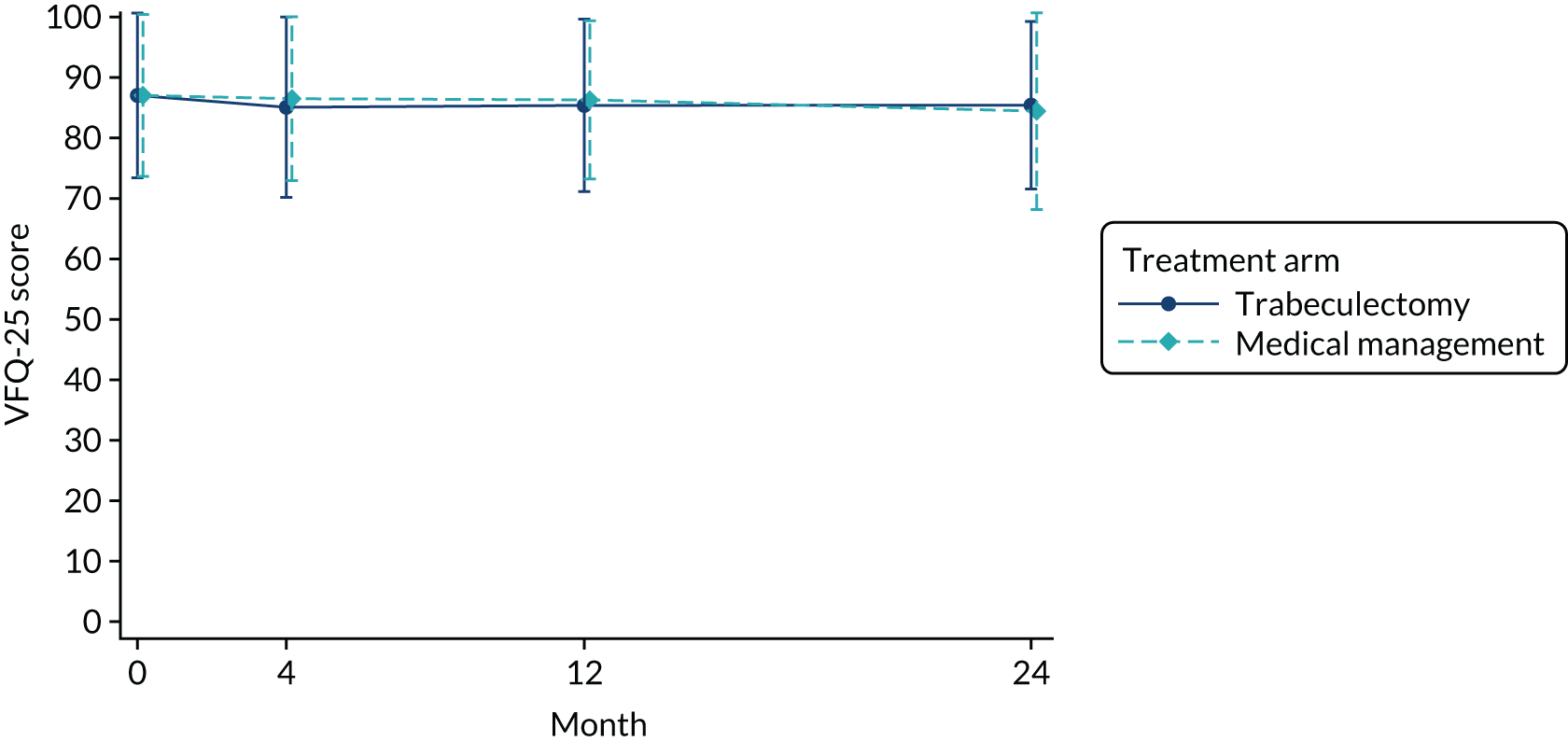
The mean VFQ-25 score in the trabeculectomy arm and the medical management arm at baseline was 87.1 (Table 5). Figure 3 shows the mean (SD) VFQ-25 score over time. At 24 months, the difference between arms was 1.06 (95% CI –1.32 to 3.43; p = 0.383). The results were similar at all other time points (see Table 5). The VFQ-25 subscales are presented in Table 5. The per-protocol analysis (see Appendix 3, Table 28) showed similar results across all time points; at 24 months, the adjusted MD was 0.95 (95% CI –1.54 to 3.45; p = 0.454). The per-protocol analysis for VFQ-25 subscales is also presented in Appendix 3, Table 28. The CACE results for VFQ-25 score were also similar at 24 months (MD 1.52, 95% CI –1.27 to 4.30; p = 0.286) (see Appendix 3, Table 29).
| Measure | Treatment arm, n; mean (SD) | Adjusted MD | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| VFQ-25 | |||||
| Baseline | 226; 87.1 (13.6) | 224; 87.1 (13.4) | |||
| 4 months | 212; 85.1 (14.9) | 216; 86.5 (13.6) | –1.24 | –3.58 to 1.11 | 0.301 |
| 12 months | 214; 85.4 (14.3) | 209; 86.3 (13.1) | –0.64 | –3.00 to 1.72 | 0.595 |
| 24 months | 207; 85.4 (13.8) | 205; 84.5 (16.3) | 1.06 | –1.32 to 3.43 | 0.383 |
| VFQ-25 subscales | |||||
| Near activities | |||||
| Baseline | 225; 84.2 (18.5) | 224; 84.4 (16.9) | |||
| 4 months | 211; 83.9 (18.0) | 214; 84.9 (17.1) | –0.56 | –4.12 to 3.01 | |
| 12 months | 214; 84.1 (18.6) | 209; 84.7 (18.4) | –0.06 | –3.63 to 3.52 | |
| 24 months | 205; 82.8 (18.4) | 204; 82.3 (19.9) | 1.11 | –2.50 to 4.73 | |
| Distance activities | |||||
| Baseline | 226; 88.5 (16.1) | 224; 89.7 (14.4) | |||
| 4 months | 211; 87.8 (16.8) | 216; 89.0 (15.4) | 0.10 | –2.92 to 3.13 | |
| 12 months | 214; 88.2 (16.3) | 209; 88.6 (15.7) | 0.94 | –2.10 to 3.99 | |
| 24 months | 207; 88.0 (15.9) | 204; 86.2 (18.9) | 2.89 | –0.18 to 5.96 | |
| Dependency | |||||
| Baseline | 226; 94.0 (17.3) | 222; 94.9 (15.7) | |||
| 4 months | 211; 91.2 (20.2) | 216; 93.4 (17.5) | –1.67 | –4.97 to 1.62 | |
| 12 months | 213; 92.1 (19.6) | 209; 94.3 (14.5) | –1.40 | –4.71 to 1.90 | |
| 24 months | 206; 93.6 (15.6) | 203; 92.7 (17.7) | 2.00 | –1.34 to 5.35 | |
| Driving | |||||
| Baseline | 171; 85.9 (26.7) | 158; 84.8 (26.2) | |||
| 4 months | 151; 82.1 (30.6) | 155; 83.0 (27.4) | –0.10 | –7.00 to 6.80 | |
| 12 months | 152; 81.3 (28.9) | 149; 79.9 (29.8) | 0.63 | –6.33 to 7.59 | |
| 24 months | 143; 81.1 (29.3) | 138; 79.9 (28.7) | 1.88 | –5.18 to 8.94 | |
| General health | |||||
| Baseline | 225; 63.6 (23.4) | 223; 60.9 (22.6) | |||
| 4 months | 211; 66.0 (23.4) | 215; 64.8 (20.9) | –0.07 | –4.68 to 4.54 | |
| 12 monthsa | 214; 66.1 (23.8) | 208; 60.5 (22.4) | 4.86 | 0.23 to 9.49 | |
| 24 months | 206; 63.3 (25.5) | 205; 59.9 (23.5) | 2.44 | –2.22 to 7.09 | |
| Role difficulties | |||||
| Baseline | 226; 87.1 (19.8) | 222; 87.4 (20.8) | |||
| 4 monthsa | 212; 82.5 (24.5) | 216; 86.3 (21.7) | –4.60 | –8.70 to –0.50 | |
| 12 months | 213; 83.2 (23.2) | 209; 84.8 (21.1) | –2.07 | –6.19 to 2.06 | |
| 24 months | 207; 83.0 (22.7) | 203; 83.4 (23.4) | –0.80 | –4.97 to 3.37 | |
| Mental health | |||||
| Baseline | 226; 81.1 (21.2) | 224; 81.8 (19.9) | |||
| 4 months | 212; 79.5 (23.3) | 216; 83.5 (19.6) | –3.76 | –7.55 to 0.02 | |
| 12 months | 214; 79.6 (22.4) | 209; 83.2 (19.7) | –3.26 | –7.06 to 0.54 | |
| 24 months | 207; 80.8 (20.7) | 205; 81.4 (21.6) | –0.55 | –4.38 to 3.28 | |
| General vision | |||||
| Baseline | 223; 74.9 (14.5) | 223; 72.8 (14.2) | |||
| 4 monthsa | 211; 71.7 (14.4) | 215; 74.0 (12.5) | –3.17 | –6.03 to –0.32 | |
| 12 months | 213; 73.1 (14.6) | 208; 73.8 (13.9) | –1.70 | –4.57 to 1.17 | |
| 24 months | 206; 73.3 (13.4) | 203; 72.2 (14.6) | –0.03 | –2.92 to 2.87 | |
| Social function | |||||
| Baseline | 225; 95.2 (11.9) | 224; 94.9 (12.1) | |||
| 4 months | 211; 95.1 (12.0) | 216; 94.6 (11.9) | 0.33 | –2.29 to 2.95 | |
| 12 months | 214; 94.3 (13.5) | 208; 94.5 (11.8) | –0.34 | –2.98 to 2.29 | |
| 24 months | 206; 95.0 (12.2) | 205; 93.2 (16.1) | 1.47 | –1.18 to 4.13 | |
| Colour vision | |||||
| Baseline | 223; 96.9 (10.9) | 222; 96.6 (11.1) | |||
| 4 months | 209; 97.4 (8.8) | 214; 96.8 (10.2) | 0.44 | –1.40 to 2.28 | |
| 12 months | 212; 96.1 (11.1) | 205; 97.4 (8.7) | –1.37 | –3.22 to 0.49 | |
| 24 months | 206; 95.6 (13.9) | 204; 95.0 (15.2) | 0.70 | –1.17 to 2.57 | |
| Peripheral vision | |||||
| Baseline | 224; 86.6 (20.8) | 224; 87.2 (20.2) | |||
| 4 months | 210; 85.4 (21.1) | 214; 85.6 (20.4) | –0.43 | –4.59 to 3.74 | |
| 12 months | 214; 86.4 (20.8) | 207; 85.6 (20.2) | 0.87 | –3.31 to 5.06 | |
| 24 months | 205; 85.1 (19.6) | 204; 83.8 (22.4) | 0.86 | –3.35 to 5.08 | |
| Ocular pain | |||||
| Baseline | 225; 84.7 (19.0) | 224; 83.9 (17.2) | |||
| 4 months | 212; 81.3 (19.2) | 216; 80.5 (18.9) | 0.22 | –2.96 to 3.40 | |
| 12 months | 214; 81.6 (20.0) | 209; 81.9 (16.3) | –0.61 | –3.80 to 2.59 | |
| 24 months | 207; 81.6 (19.7) | 205; 80.5 (18.7) | 0.98 | –2.25 to 4.21 | |
FIGURE 3.
Mean (SD) VFQ-25 by arm over time.
Subgroup analysis
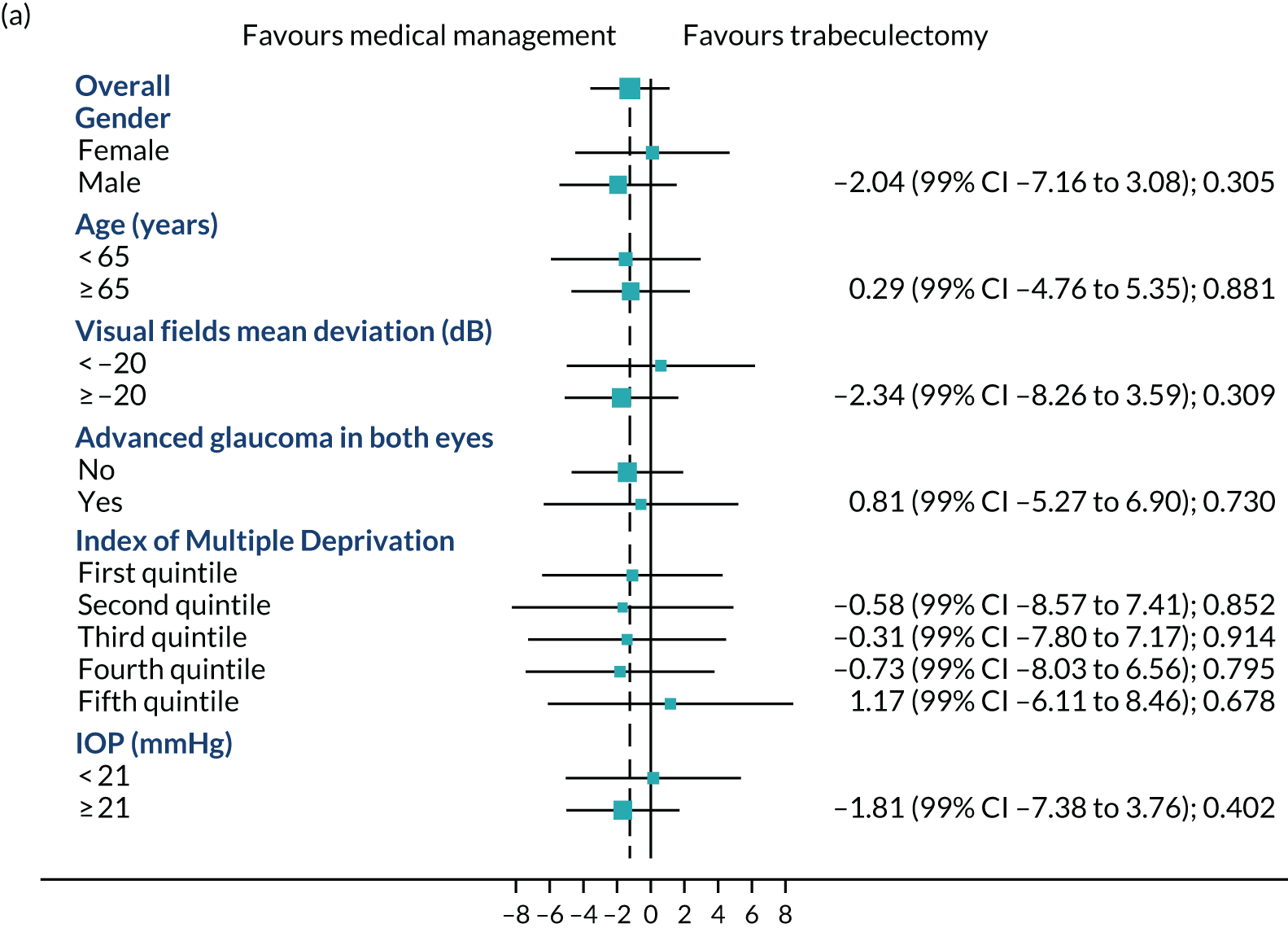
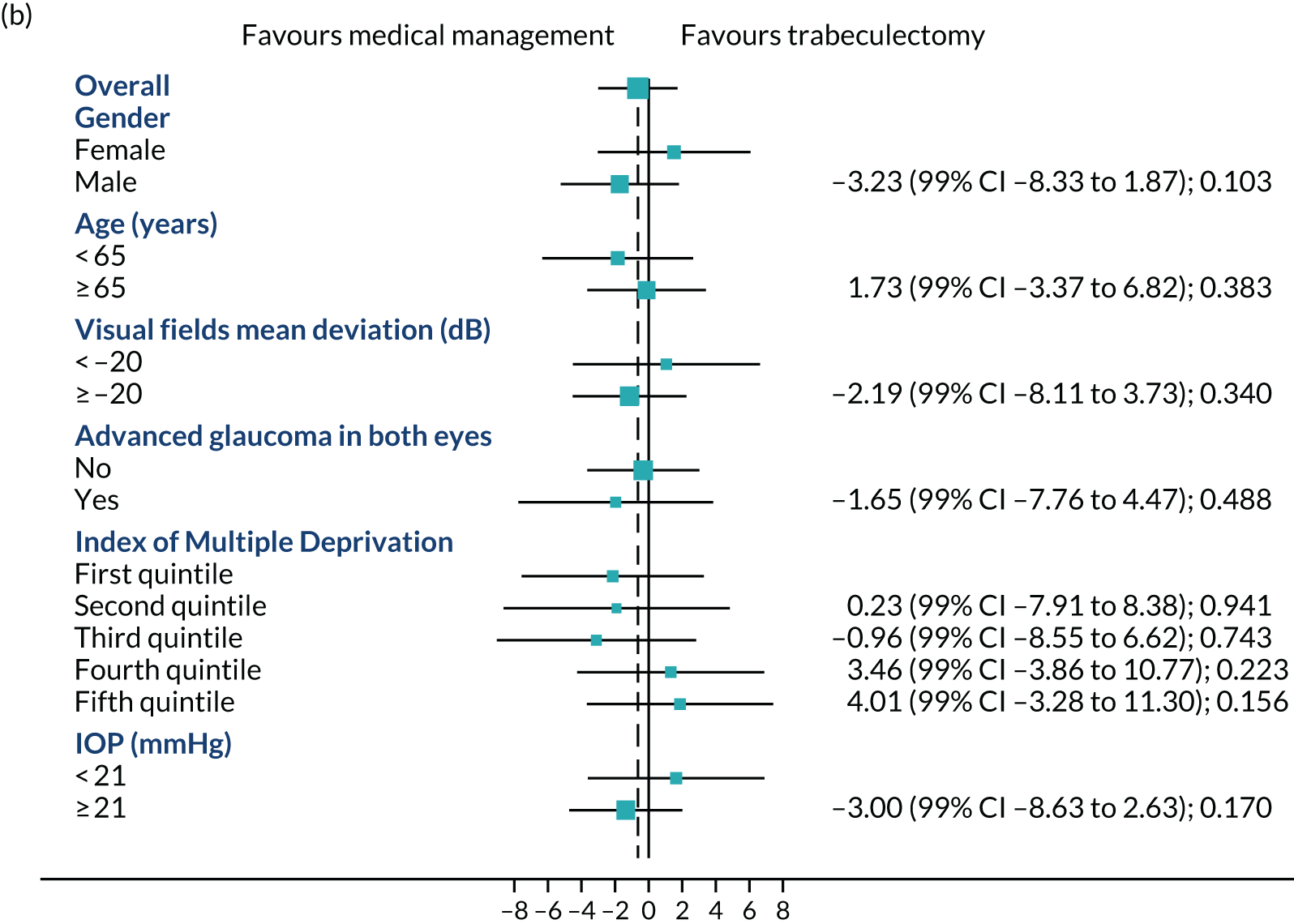
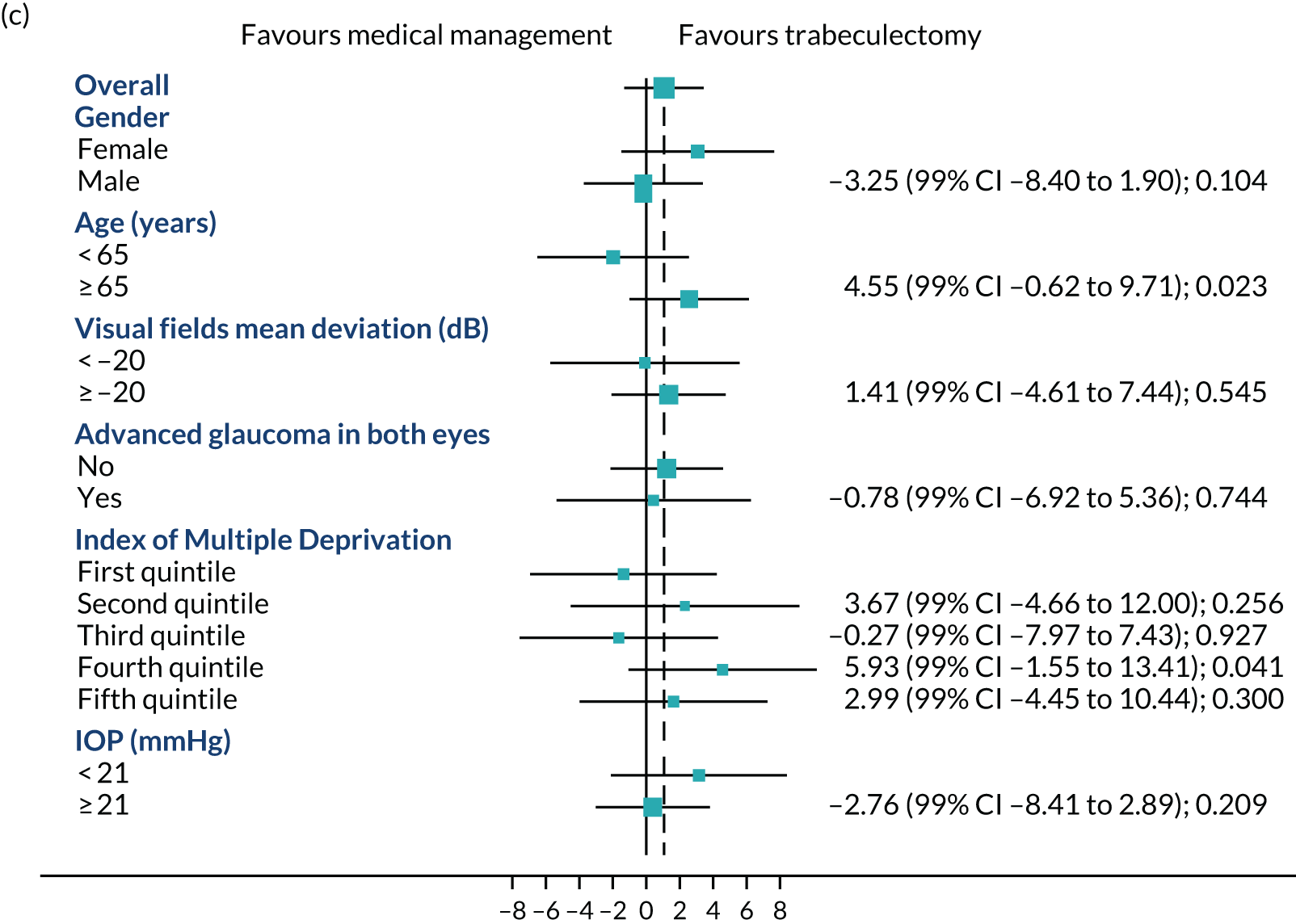
Figures 4a–c show the prespecified subgroup analyses at 4, 12 and 24 months for VFQ-25 score, sex, age (< 65 vs. ≥ 65 years), VFs (–20 dB vs. ≥ –20 dB), advanced glaucoma in both eyes, Index Multiple Deprivation quintile (most deprived to least deprived) and IOP at diagnosis (< 21 mmHg vs. ≥ 21 mmHg). There was no evidence of any treatment effect heterogeneity at 4 or 12 months. At 24 months, a potential moderating effect of age is emerging; however, there is considerable uncertainty around the estimate of the interaction effect.
FIGURE 4.
Subgroups for trabeculectomy vs. medical management: (a) 4 months, (b) 12 months and (c) 24 months. First quintile, most deprived; fifth quintile, least deprived. Boxes indicate mean differences. Solid black line indicates 99% CI. Solid vertical line indicates no effect. Dashed vertical line indicates overall effect.
Secondary outcomes
EQ-5D-5L
The mean baseline EQ-5D-5L score was 0.844 in the trabeculectomy arm and 0.837 in the medical management arm. At the follow-up time points, there was no evidence of a difference in the mean EQ-5D-5L score, and at 24 months the MD was 0.016 (95% CI –0.021 to 0.053; p = 0.405) (Table 6).
| Measure | Treatment arm | Estimatea | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| EQ-5D-5L, n; mean (SD) | |||||
| Baseline | 222; 0.844 (0.185) | 222; 0.837 (0.176) | |||
| 1 month | 194; 0.838 (0.185) | 203; 0.808 (0.203) | 0.025 | –0.012 to 0.062 | 0.189 |
| 3 months | 186; 0.836 (0.167) | 179; 0.814 (0.195) | 0.015 | –0.024 to 0.053 | 0.455 |
| 6 months | 186; 0.850 (0.184) | 195; 0.822 (0.204) | 0.016 | –0.021 to 0.054 | 0.391 |
| 12 months | 211; 0.837 (0.177) | 209; 0.823 (0.164) | 0.014 | –0.022 to 0.051 | 0.442 |
| 18 months | 181; 0.828 (0.185) | 184; 0.791 (0.219) | 0.023 | –0.016 to 0.061 | 0.244 |
| 24 months | 206; 0.810 (0.179) | 203; 0.796 (0.191) | 0.016 | –0.021 to 0.053 | 0.405 |
| HUI-3, n; mean (SD) | |||||
| Baseline | 214; 0.814 (0.202) | 214; 0.809 (0.208) | |||
| 1 month | 184; 0.791 (0.232) | 193; 0.786 (0.230) | –0.000 | –0.043 to 0.043 | 1.000 |
| 3 months | 180; 0.796 (0.223) | 179; 0.779 (0.222) | 0.007 | –0.036 to 0.051 | 0.741 |
| 6 months | 180; 0.805 (0.216) | 182; 0.782 (0.224) | 0.020 | –0.024 to 0.063 | 0.376 |
| 12 months | 204; 0.829 (0.193) | 196; 0.798 (0.199) | 0.024 | –0.018 to 0.066 | 0.262 |
| 18 months | 169; 0.802 (0.212) | 174; 0.749 (0.258) | 0.022 | –0.022 to 0.066 | 0.324 |
| 24 months | 198; 0.786 (0.227) | 193; 0.751 (0.246) | 0.036 | –0.006 to 0.078 | 0.094 |
| GUI, n; mean (SD) | |||||
| Baseline | 219; 0.884 (0.131) | 222; 0.863 (0.130) | |||
| 1 month | 194; 0.862 (0.138) | 205; 0.853 (0.156) | –0.000 | –0.028 to 0.028 | 0.984 |
| 3 months | 187; 0.849 (0.130) | 190; 0.844 (0.156) | –0.008 | –0.036 to 0.021 | 0.589 |
| 6 months | 186; 0.839 (0.159) | 191; 0.853 (0.135) | –0.024 | –0.052 to 0.005 | 0.105 |
| 12 months | 209; 0.857 (0.139) | 204; 0.860 (0.143) | –0.012 | –0.039 to 0.016 | 0.403 |
| 18 months | 181; 0.851 (0.144) | 184; 0.832 (0.157) | 0.003 | –0.026 to 0.032 | 0.832 |
| 24 months | 205; 0.849 (0.152) | 202; 0.830 (0.184) | 0.011 | –0.017 to 0.039 | 0.434 |
| Patient experience (glaucoma getting worse), n/N (%) | |||||
| Baseline | 95/208 (45.7) | 76/209 (36.4) | |||
| 1 month | 60/188 (31.9) | 50/201 (24.9) | 1.19 | 0.79 to 1.80 | 0.392 |
| 3 months | 37/182 (20.3) | 40/185 (21.6) | 0.88 | 0.55 to 1.41 | 0.591 |
| 6 months | 30/182 (16.5) | 40/189 (21.2) | 0.74 | 0.45 to 1.21 | 0.229 |
| 12 months | 38/207 (18.4) | 57/199 (28.6) | 0.59 | 0.38 to 0.91 | 0.018 |
| 18 months | 40/180 (22.2) | 38/181 (21.0) | 0.99 | 0.62 to 1.59 | 0.979 |
| 24 months | 44/196 (22.4) | 57/194 (29.4) | 0.70 | 0.46 to 1.07 | 0.099 |
| IOP (mmHg), n; mean (SD) | |||||
| Baseline | 222; 19.40 (6.15) | 221; 19.05 (5.73) | |||
| 4 months | 217; 12.39 (5.73) | 220; 16.40 (4.12) | –4.11 | –5.18 to –3.05 | < 0.001 |
| 12 months | 215; 11.90 (4.48) | 209; 16.12 (4.54) | –4.25 | –5.33 to –3.18 | < 0.001 |
| 24 months | 206; 12.40 (4.71) | 202; 15.07 (4.80) | –2.75 | –3.84 to –1.66 | < 0.001 |
| LogMAR VA, n; mean (SD) | |||||
| Baseline | 227; 0.15 (0.25) | 223; 0.17 (0.26) | |||
| 4 months | 210; 0.25 (0.31) | 217; 0.16 (0.24) | 0.10 | 0.05 to 0.14 | < 0.001 |
| 12 months | 212; 0.18 (0.23) | 209; 0.16 (0.26) | 0.03 | –0.02 to 0.08 | 0.198 |
| 24 months | 199; 0.21 (0.28) | 201; 0.16 (0.26) | 0.07 | 0.02 to 0.11 | 0.006 |
| VFMD (dB) | |||||
| Baseline, n; mean (SD) | 227; –14.91 (6.36) | 226; –15.26 (6.34) | |||
| 4 months, n; mean (SD) | 211; –14.35 (6.78) | 217; –14.84 (6.52) | –0.05 | –0.79 to 0.70 | 0.897 |
| 12 months, n; mean (SD) | 214; –14.76 (6.92) | 209; –14.95 (6.53) | 0.03 | –0.72 to 0.78 | 0.939 |
| 24 months, n; mean (SD) | 202; –15.15 (6.63) | 200; –15.42 (6.39) | 0.18 | –0.58 to 0.94 | 0.645 |
| Need for cataract surgery | |||||
| Yes, n/N (%) | 28/222 (12.6) | 27/221 (12.2) | 0.98 | 0.50 to 1.95 | 0.963 |
| Visual standards for driving (pass/no defects), n/N (%) | |||||
| Baseline | 187/214 (87.4) | 196/217 (90.3) | |||
| 24 months | 167/187 (89.3) | 168/188 (89.4) | 1.01 | 0.81 to 1.25 | 0.951 |
| Registered as sight impaired at 24 months, n/N (%) | |||||
| No | 182/186 (97.8) | 184/184 (100.0) | 0.123 | ||
| Sight impaired | 4/186 (2.2) | 0/184 (0) | |||
| Eligible to be registered at 24 months, n/N (%) | |||||
| No | 188/199 (94.5) | 187/196 (95.4) | |||
| Sight impaired | 8/199 (4.0) | 7/196 (3.6) | |||
| Severe sight impaired | 3/199 (1.5) | 2/196 (1.0) | |||
Health Utility Index version 3
The mean baseline HUI-3 score was 0.814 in the trabeculectomy arm and 0.809 in the medical management arm, with no evidence of a difference at the follow-up time points. At 24 months, the mean score was 0.786 in the trabeculectomy arm and 0.751 in the medical management arm, with a MD of 0.036 (95% CI –0.006 to 0.078; p = 0.094) (see Table 6).
Glaucoma Utility Index
The baseline mean GUI score was 0.884 in the trabeculectomy arm and 0.863 in the medical management arm. These mean scores decreased in both arms over the course of the study, but there was no evidence of a difference at any time point. At 24 months, the mean score was 0.849 in the trabeculectomy arm and 0.830 in the medical management arm, with a mean difference of 0.011 (95% CI –0.017 to 0.039; p = 0.434) (see Table 6).
Patient perception: glaucoma getting worse
At baseline, 95 out of 208 (45.7%) participants in the trabeculectomy arm and 76 out of 209 (36.4%) participants in the medical management arm perceived that their glaucoma was getting worse. At the later follow-up time points, this proportion decreased in both study arms. At 12 months, 38 out of 207 (18.4%) participants in the trabeculectomy arm and 57 out of 199 (27.3%) participants in the medical management arm perceived that their glaucoma was getting worse [relative risk (RR) 0.59, 95% CI 0.38 to 0.91; p = 0.018]. At 24 months, the RR was 0.70 (95% CI 0.46 to 1.07; p = 0.099) (see Table 6).
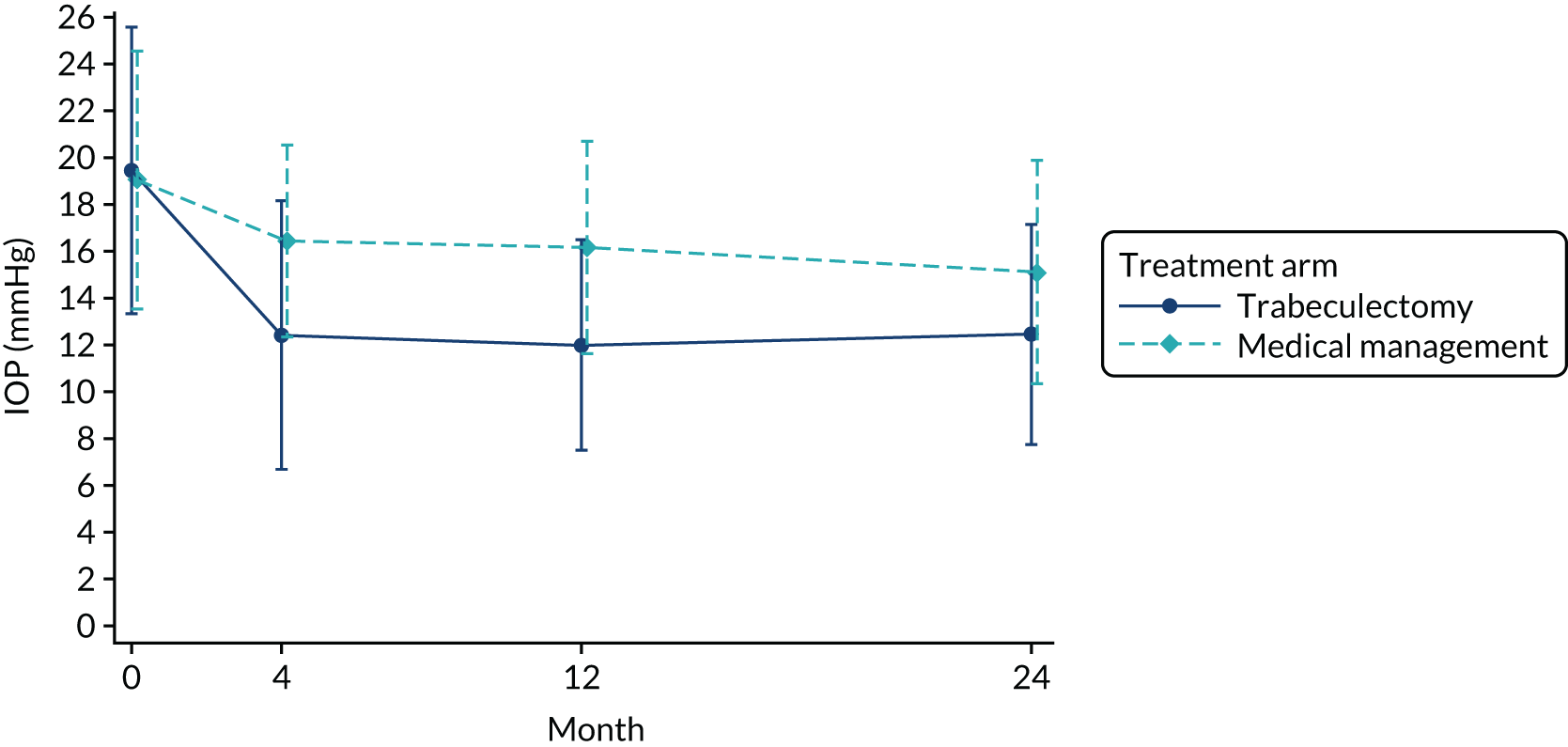
Intraocular pressure
At baseline, the mean IOP was 19.40 mmHg in the trabeculectomy arm and 19.05 mmHg in the medical management arm. At later time points, the mean IOP decreased, with lower IOP in the trabeculectomy arm at all follow-up time points. Figure 5 shows the mean and SD over time. At 24 months, the mean IOP was 12.40 mmHg in the trabeculectomy arm and 15.07 mmHg in the medical management arm (mean difference –2.75, 95% CI –3.84 to –1.66; p < 0.001) (see Table 6). The Dunnett’s multiplicity p-value was also < 0.001 for all time points The sensitivity analysis incorporating data from the non-index eye for participants with bilateral disease (see Appendix 3, Table 30) showed similar results.
FIGURE 5.
Mean (SD) IOP by arm over time. The SD values are at the end point of the vertical lines.
LogMAR visual acuity
At 4 and 24 months, there was evidence of a difference in logMAR VA in favour of medical management (MD at 24 months 0.07, 95% CI 0.02 to 0.11; p = 0.006) (see Table 6). The Dunnett’s multiplicity p-value was 0.002 for 4 months, 0.483 for 12 months and 0.061 for 24 months. The sensitivity analysis incorporating data from the non-index eye for participants with bilateral disease showed similar results (see Appendix 3, Table 30).
Visual fields mean deviation
At 24 months, the mean VFMD was –15.15 in the trabeculectomy arm and –15.42 in the medical management arm, with no evidence of a difference between arms [mean difference 0.18, 95% CI –0.58 to 0.94; p = 0.645) (see Table 6). Two sensitivity analyses were carried out. The first included only SITA standard VF (excluding SITA fast) and the second removed participants who had only one VF or for whom the false positive standard was > 15% required for reliability. Overall, the results were similar (see Appendix 3, Table 31). The sensitivity analysis incorporating data from the non-index eye for participants with bilateral disease showed similar results (see Appendix 3, Table 30).
Need for cataract surgery
Over the course of the trial, 28 out of 222 participants (12.6%) in the trabeculectomy arm and 27 out of 221 participants (12.2%) in the medical management arm needed cataract surgery, with no evidence of a difference between arms (RR 0.98, 95% CI 0.50 to 1.95; p = 0.963) at 24 months (see Table 6).
Visual standards for driving (pass/no defects)
At 24 months, 167 out of 187 participants (89.3%) in the trabeculectomy arm and 168 out of 188 participants (89.4%) in the medical management arm passed the visual driving standards, with no evidence of a difference between arms (RR 1.01, 95% CI 0.81 to 1.25; p = 0.951) (see Table 6).
Registered as sight impaired at 24 months
At 24 months, 4 out of 186 participants (2.2%) in the trabeculectomy arm and 0 out of 184 participants (0%) in the medical management arm were registered as sight impaired, with the difference between the arms having a p-value of 0.123 (see Table 6). In addition, we also recorded whether or not participants were eligible to be registered (see Table 6 and Appendix 3, Table 32, for baseline data). At baseline, 10 participants (94.3%) in the trabeculectomy arm were sight impaired and three participants (1.3%) were severely sight impaired. In the medical management arm, 12 participants (5.3%) were sight impaired at baseline and two participants (0.9%) were severely sight impaired. At 24 months, the results were similar.
Safety
A safety event is defined as either a SAE or an AE.
By allocation
The number of participants who experienced a safety event during the 24-month follow-up was 88 (38.8%) in the trabeculectomy arm and 100 (44.2%) in the medical management arm (RR 0.88, 95% CI 0.66 to 1.17; p = 0.366). The number of participants who experienced a SAE was 12 out of 226 (5.3%) participants in the trabeculectomy arm and 8 out of 226 (3.5%) participants in the medical management arm. One participant in the trabeculectomy arm had two SAEs. Table 7 provides further details of the safety events throughout the follow-up period. Appendix 3, Table 34, shows details for the non-index eye.
| Measure | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of participants with a safety event, n (%) | 88 (38.8), adjusted risk ratio 0.88 | 100 (44.2), 95% CI 0.66 to 1.17; p = 0.366 |
| SAE | ||
| Number of participants, n/N (%) | 12/226 (5.3) | 8/226 (3.5) |
| Number of events (n) | 13 | 8 |
| Details (n) | ||
| Death | 5 | 4 |
| Life-threatening | – | 1 |
| Hospitalisation | 3 | 4 |
| Significant disability | 2 | – |
| Important condition | 3 | 1 |
| Expected event | 3 | 2 |
| Classification (n) | ||
| General medical (death) | 2 | 4 |
| Unclassified (death) | 3 | – |
| General medical | 2 | 3 |
| Related to glaucoma surgery | 3 | 1 |
| General ophthalmology | 1 | – |
| Non-glaucoma vision loss | 1 | – |
| Glaucoma progression despite treatment | 1 | – |
| 4 months | ||
| Number of participants, n/N (%) | 49/217 (22.6) | 45/220 (20.5) |
| Number of events (n) | 63 | 54 |
| Details (n) | ||
| Shallow anterior chamber | 3 | – |
| Early bleb leak | 10 | – |
| Corneal epithelial defect | 2 | – |
| Conjunctival buttonhole | 2 | – |
| Hyphaema | 4 | – |
| Choroidal effusion | 6 | – |
| Suprachoroidal haemorrhage | 1 | – |
| Hypotony requiring intervention | 6 | – |
| Late bleb leak | 1 | – |
| Ptosis | 2 | – |
| Drop related | 9 | 39 |
| Ocular surface related | 9 | 11 |
| Potential AE related to surgery | 1 | – |
| Non-specific | 6 | 3 |
| Glaucoma progression | 1 | 1 |
| 12 months | ||
| Number of participants, n/N (%) | 35/216 (16.2) | 43/211 (20.4) |
| Number of events (n) | 43 | 48 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettersa | 1 | – |
| Shallow anterior chamber | 3 | – |
| Early bleb leak | 1 | 2 |
| Persistent uveitis | 1 | – |
| Conjunctival button hole | 1 | – |
| Choroidal effusion | 2 | – |
| Suprachoroidal haemorrhage | 1 | – |
| Hypotony requiring intervention | 3 | 1 |
| Late bleb leak | 1 | – |
| Blebitis | 1 | – |
| Ptosis | 3 | – |
| Non-specific unrelated uveitis | 1 | – |
| Drop related | 4 | 29 |
| Ocular surface related | 12 | 11 |
| Potential AE | 2 | – |
| Non-specific | 5 | 4 |
| Cataract | 1 | 1 |
| 24 months | ||
| Number of participants, n/N (%) | 30/211 (14.2) | 41/208 (19.7) |
| Number of events (n) | 33 | 53 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettersa | 2 | – |
| Shallow anterior chamber | 1 | 2 |
| Early bleb leak | 1 | 1 |
| Corneal epithelial defect | 1 | – |
| Macular oedema | – | 1 |
| Choroidal effusion | 1 | 2 |
| Hypotony requiring intervention | 2 | 2 |
| Late bleb leak | 2 | – |
| Blebitis | 1 | – |
| Endophthalmitisa – endogenous | 1 | – |
| Endophthalmitisa – bled related | – | 1 |
| Ptosis | – | 1 |
| Drop related | 5 | 15 |
| Ocular surface related | 8 | 15 |
| Potential AE related to surgery | – | 1 |
| Non-specific | 8 | 10 |
| Glaucoma progression | – | 1 |
| Cataract | – | 1 |
Three participants who received trabeculectomy had irreversible loss of ≥ 10 ETDRS letters:
-
Case 1 – at baseline the patient’s IOP was 62 mmHg (VFMD = –24.78 dB, logMAR = 70 letters). The patient underwent trabeculectomy and was followed up routinely afterwards. At 24 months, the logMAR VA had fallen to 16 owing to glaucoma progression. The patient had lost 54 letters of vision.
-
Case 2 – at baseline the patient’s IOP was 26 mmHg (VFMD = –21.5 dB, logMAR = 82 letters). The patient underwent trabeculectomy and was followed up routinely afterwards. At 24 months, the logMAR VA had fallen to 69 owing to glaucoma progression. The patient had lost 13 letters of vision.
-
Case 3 – at baseline the patient’s IOP was 24 mmHg (VFMD = –24.08 dB, logMAR = 80 letters). The patient underwent trabeculectomy and as followed up routinely afterwards. At 12 months, VA recorded at hand movements with IOP 12 mmHg. The participant died before the 24-month visit. The PI reported vision loss owing to central serous retinopathy unresponsive to treatment.
One participant developed a bleb-related endophthalmitis:
-
Case 1 – the patient was allocated to the medical management arm but underwent trabeculectomy for uncontrolled IOP. The patient developed a bleb-related endophthalmitis. A vitreous fluid sample obtained by vitreous tap grew Staphylococcus aureus. The patient was given intravitreal antibiotics and made a full recovery. At 24 months VFMD was –3.75 dB, VA was 78 and IOP was 13 mmHg, and the patient did not require eye drops.
One patient also developed an endogenous endophthalmitis unrelated to their glaucoma surgery:
-
Case 2 – the patient was allocated to the trabeculectomy arm and underwent left eye trabeculectomy in 2016. The patient then developed endophthalmitis in the absence of either a bleb leak or blebitis 21 months later. The patient was admitted, underwent a vitreous tap and was treated with intravitreal antibiotics. A diagnosis of endogenous endophthalmitis was made. The patient was diabetic, and a foot ulcer was believed to be the source of the infection. The patient made a full recovery with no visual loss. At 24 months, VFMD was –14.0, VA was 75 and IOP was 20 mmHg, and the patient required two types of eye drop.
By treatment received
During the 24-month follow-up period, 101 out of 240 participants (42.1%) who received trabeculectomy and 87 out of 213 participants (40.8%) who received medical management had a safety event (RR 1.03, 95% CI 0.77 to 1.37; p = 0.850).
The number of participants who had a SAE was 12 out of 239 (5.0%) in the trabeculectomy arm and 8 out of 213 (3.8%) in the medical management arm. One participant in the trabeculectomy arm had two SAEs.
At 4 months, the number of participants who had experienced an AE was 55 out of 234 (23.5%) in the trabeculectomy arm and 39 out of 203 (19.2%) in the medical management arm. At 12 months, the number of participants who had experienced an AE was 38 out of 233 participants (16.3%) who received trabeculectomy and 40 out of 194 participants (20.6%) who received medical management. At 24 months, the number of participants who had experienced an AE was 38 out of 228 participants (16.7%) who received trabeculectomy and 33 out of 191 participants (17.3%) who received medical management. Table 8 provides further details of the safety events throughout the follow-up. Appendix 3, Table 33, shows details for the non-index eye.
| Measure | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of patients receiving treatment (n) | 240 | 213 |
| Number of participants with a safety event, n (%) | 101 (42.1) adjusted RR 1.03 | 87 (40.8), 95% CI 0.77 to 1.37; p = 0.850 |
| SAE | ||
| Number of participants, n/N (%) | 12/239 (5.0) | 8/213 (3.8) |
| Number of events (n) | 13 | 8 |
| Details (n) | ||
| Death | 4 | 5 |
| Life-threatening | – | 1 |
| Hospitalisation | 4 | 3 |
| Significant disability | 2 | – |
| Important condition | 3 | 1 |
| Expected event | 4 | 1 |
| Classification (n) | ||
| General medical (death) | 3 | 3 |
| Unclassified (death) | 1 | 2 |
| General medical | 2 | 3 |
| Related to glaucoma surgery | 4 | – |
| General ophthalmology | 1 | – |
| Non-glaucoma vision loss | 1 | – |
| Glaucoma progression despite treatment | 1 | – |
| 4 months | ||
| Number of participants, n/N (%) | 55/234 (23.5) | 39/203 (19.2) |
| Number of events (n) | 71 | 46 |
| Details (n) | ||
| Shallow anterior chamber | 3 | – |
| Early bleb leak | 10 | – |
| Corneal epithelial defect | 2 | – |
| Conjunctival button hole | 2 | – |
| Hyphaema | 4 | – |
| Choroidal effusion | 6 | – |
| Suprachoroidal haemorrhage | 1 | – |
| Hypotony requiring intervention | 6 | – |
| Late bleb leak | 1 | – |
| Ptosis | 2 | – |
| Drop related | 23 | 25 |
| Ocular surface related | 3 | 18 |
| Potential AE | 1 | – |
| Non-specific | 6 | 3 |
| Glaucoma progression | 1 | 1 |
| 12 months | ||
| Number of participants, n/N (%) | 38/233 (16.3) | 40/194 (20.6) |
| Number of events (n) | 48 | 43 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettersa | 1 | – |
| Shallow anterior chamber | 3 | – |
| Early bleb leak | 3 | – |
| Persistent uveitis | 1 | – |
| Conjunctival buttonhole | 1 | – |
| Choroidal effusion | 2 | – |
| Suprachoroidal haemorrhage | 1 | – |
| Hypotony requiring intervention | 4 | – |
| Late bleb leak | 1 | – |
| Blebitis | 1 | – |
| Ptosis | 3 | – |
| Non-specific unrelated uveitis | – | 1 |
| Drop related | 5 | 28 |
| Ocular surface related | 14 | 9 |
| Potential AE related to surgery | 2 | – |
| Non-specific | 4 | 5 |
| Cataract | 1 | 1 |
| 24 months | ||
| Number of participants, n/N (%) | 38/228 (16.7) | 33/191 (17.3) |
| Number of events (n) | 47 | 39 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettersa | 2 | – |
| Shallow anterior chamber | 3 | – |
| Early bleb leak | 2 | – |
| Corneal epithelial defect | 1 | – |
| Macular oedema | 1 | – |
| Choroidal effusion | 3 | – |
| Hypotony requiring intervention | 4 | – |
| Late bleb leak | 2 | – |
| Blebitis | 1 | – |
| Endophthalmitis:a endogenous | 1 | – |
| Endophthalmitis:a bled related | 1 | – |
| Ptosis | 1 | – |
| Drop related | 3 | 17 |
| Ocular surface related | 13 | 10 |
| Potential AE related to surgery | 1 | 0 |
| Non-specific | 8 | 10 |
| Glaucoma progression | – | 1 |
| Cataract | – | 1 |
Antiglaucoma medication
Table 9 shows the prescribed antiglaucoma medication agents as allocated. The mean number of glaucoma eye drop agents at 4 months was 0.43 (SD 0.79) in the trabeculectomy arm and 1.77 (SD 0.92) in the medical management arm. At 12 months, the mean was 0.34 (SD 0.79) and 1.78 (SD 1.01) and at 24 months the mean was 0.47 (SD 0.92) and 1.64 (SD 1.15) for the trabeculectomy and medical management arm, respectively. The majority of participants used prostaglandin analogue and beta-blocker.
| Measure | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| 4 months | ||
| Number of participants, n | 217 | 220 |
| Number of glaucoma eye drops, mean (SD) | 0.43 (0.79) | 1.77 (0.92) |
| Number of participants receiving eye drops, n (%) | 61 (28.1) | 210 (95.5) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 57 (26.3) | 205 (93.2) |
| Beta-blocker | 22 (10.1) | 91 (41.4) |
| Carbonic anhydrase inhibitor | 13 (6.0) | 75 (34.1) |
| α-agonist | 1 (0.5) | 18 (8.2) |
| Pilocarpine | 1 (0.5) | 0 (0) |
| Diamox | 2 (0.9) | 1 (0.5) |
| 12 months | ||
| Number of participants, n | 216 | 211 |
| Number of glaucoma eye drops, mean (SD) | 0.34 (0.79) | 1.78 (1.01) |
| Number of participants receiving eye drops, n (%) | 41 (19.0) | 188 (89.1) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 38 (17.6) | 182 (86.3) |
| Beta-blocker | 22 (10.2) | 98 (46.4) |
| Carbonic anhydrase inhibitor | 14 (6.5) | 76 (36.0) |
| α-agonist | 0 (0) | 19 (9.0) |
| Pilocarpine | 0 (0) | 1 (0.5) |
| Diamox | 2 (0.9) | 0 (0) |
| 24 months | ||
| Number of participants, n | 211 | 208 |
| Number of glaucoma eye drops, mean (SD) | 0.47 (0.92) | 1.64 (1.15) |
| Number of participants receiving eye drops, n (%) | 53 (25.1) | 163 (78.4) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 43 (20.4) | 154 (74.0) |
| Beta-blocker | 30 (14.2) | 91 (43.8) |
| Carbonic anhydrase inhibitor | 22 (10.4) | 76 (36.5) |
| α-agonist | 4 (1.9) | 18 (8.7) |
| Pilocarpine | 0 (0) | 2 (1.0) |
| Diamox | 1 (0.5) | 1 (0.5) |
Table 10 shows the antiglaucoma medication agents prescribed by as treated. At 24 months, the mean number of antiglaucoma eye drops was 0.29 (SD 0.72) for those who received trabeculectomy and 1.96 (SD 0.98) for those who received medical management.
| Measure | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of patients receiving treatment (n) | 240 | 213 |
| 4 months | ||
| Number of participants, n | 234 | 203 |
| Number of glaucoma eye drops, n; mean (SD) | 234; 0.55 (0.95) | 203; 1.74 (0.85) |
| Number of participants receiving eye drops, n (%) | 71 (30.3) | 200 (98.5) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 65 (27.8) | 197 (97.0) |
| Beta-blocker | 32 (13.7) | 81 (39.9) |
| Carbonic anhydrase inhibitor | 29 (12.4) | 59 (29.1) |
| α-agonist | 2 (0.9) | 17 (8.4) |
| Pilocarpine | 1 (0.4) | 0 (0) |
| Diamox | 1 (0.4) | 2 (1.0) |
| 12 months | ||
| Number of participants, n | 233 | 194 |
| Number of glaucoma eye drops, n; mean (SD) | 233; 0.38 (0.89) | 194; 1.87 (0.88) |
| Number of participants receiving eye drops, n (%) | 41 (17.6) | 188 (96.9) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 35 (15.0) | 185 (95.4) |
| Beta-blocker | 24 (10.3) | 96 (49.5) |
| Carbonic anhydrase inhibitor | 26 (11.2) | 64 (33.0) |
| α-agonist | 3 (1.3) | 16 (8.2) |
| Pilocarpine | 0 (0) | 1 (0.5) |
| Diamox | 1 (0.4) | 1 (0.5) |
| 24 months | ||
| Number of participants, n | 228 | 191 |
| Number of glaucoma eye drops, n; mean (SD) | 228; 0.29 (0.72) | 191; 1.96 (0.98) |
| Number of participants receiving eye drops, n (%) | 37 (16.2) | 179 (93.7) |
| Glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 27 (11.8) | 170 (89.0) |
| Beta-blocker | 18 (7.9) | 103 (53.9) |
| Carbonic anhydrase inhibitor | 18 (7.9) | 80 (41.9) |
| α-agonist | 2 (0.9) | 20 (10.5) |
| Pilocarpine | 0 (0) | 2 (1.0) |
| Diamox | 1 (0.4) | 1 (0.5) |
Appendix 3, Table 36, shows the antiglaucoma medication prescribed for the non-index eye.
Appendix 3, Tables 37 and 38, show the number of different bottles of medication as allocated and as treated, respectively.
Other trabeculectomy interventions
Table 11 shows details of other trabeculectomy interventions for those who received a trabeculectomy and were followed up. The total number of participants who received any intervention was 123 out of 200 (61.5%) in the trabeculectomy arm and 26 out of 39 (66.7%) in the medical management arm. The median time to the first intervention was 8 [interquartile range (IQR) 6–27] days in the trabeculectomy arm and 11 (IQR 7–19) days in the medical management arm, with the majority of participants receiving a massage or releasable release.
| Measure | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of patients who received trabeculectomy and were followed up (n) | 200 | 39 |
| Any intervention | ||
| Number of participants who received intervention, n (%) | 123 (61.5) | 26 (66.7) |
| Number of interventions received, n (%) | ||
| 0 | 77 (38.5) | 13 (33.3) |
| 1 | 29 (14.5) | 9 (23.1) |
| 2 | 23 (11.5) | 4 (10.3) |
| ≥ 3 | 71 (35.5) | 13 (33.3) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 27 (50); (1, 322) | 19 (28); (1, 112) |
| Median (IQR) | 8 (6–27) | 11 (7–19) |
| Bleb massage | ||
| Number of participants who received intervention, n (%) | 70 (35.0) | 12 (30.8) |
| Number of interventions received, n (%) | ||
| 0 | 130 (65.0) | 27 (69.2) |
| 1 | 32 (16.0) | 8 (20.5) |
| 2 | 17 (8.5) | 2 (5.1) |
| ≥ 3 | 21 (10.5) | 2 (5.1) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 10 (15); (1, 82) | 11 (9); (1, 29) |
| Median (IQR) | 7 (1–8) | 8 (7–19) |
| Suture adjustment | ||
| Number of participants who received intervention, n (%) | 5 (2.5) | 1 (2.6) |
| Number of interventions received, n (%) | ||
| 0 | 195 (97.5) | 38 (97.4) |
| 1 | 3 (1.5) | 1 (2.6) |
| 2 | 2 (1.0) | |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 64 (117); (4, 274) | |
| Median (IQR) | 15 (13–16) | |
| Suture lysis release | ||
| Number of participants who received intervention, n (%) | 10 (5.0) | 2 (5.1) |
| Number of interventions received, n (%) | ||
| 0 | 190 (95.0) | 37 (94.9) |
| 1 | 7 (3.5) | 2 (5.1) |
| 2 | 1 (0.5) | |
| ≥ 3 | 2 (1.0) | |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 28 (14); (15, 50) | 21 (–); (21, 21) |
| Median (IQR) | 24 (15–37) | 21 (21–21) |
| Releasable suture release | ||
| Number of participants who received intervention, n (%) | 63 (31.5) | 15 (38.5) |
| Number of interventions received, n (%) | ||
| 0 | 137 (68.5) | 24 (61.5) |
| 1 | 28 (14.0) | 10 (25.6) |
| 2 | 27 (13.5) | 5 (12.8) |
| ≥ 3 | 8 (4.0) | |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 22 (20); (1, 106) | 17 (12); (1, 36) |
| Median (IQR) | 15 (8–31) | 15 (8–25) |
| Unknown suture release | ||
| Number of participants who received intervention, n (%) | 3 (1.5) | |
| Number of interventions received, n (%) | ||
| 0 | 197 (98.5) | 39 (100.0) |
| 1 | 1 (0.5) | |
| 2 | 0 | 0 (–) |
| ≥ 3 | 2 (1.0) | |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 12 (8); (8, 21) | |
| Median (IQR) | 8 (8–21) | |
| 5-FU injection | ||
| Number of participants who received intervention, n (%) | 26 (13.0) | 8 (20.5) |
| Number of interventions received, n (%) | ||
| 0 | 174 (87.0) | 31 (79.5) |
| 1 | 15 (7.5) | 7 (17.9) |
| 2 | 7 (3.5) | 1 (2.6) |
| ≥ 3 | 4 (2.0) | |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 60 (86); (1, 391) | 62 (62); (21, 171) |
| Median (IQR) | 29 (15–53) | 36 (26–56) |
| Steroid injection | ||
| Number of participants who received intervention, n (%) | 19 (9.5) | 5 (12.8) |
| Number of interventions received, n (%) | ||
| 0 | 181 (90.5) | 34 (87.2) |
| 1 | 12 (6.0) | 2 (5.1) |
| 2 | 5 (2.5) | 1 (2.6) |
| ≥ 3 | 2 (1.0) | 2 (5.1) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 25 (15); (7, 60) | 19 (–); (19, 19) |
| Median (IQR) | 19 (15–34) | 19 (19–19) |
| Needling plus 5-FU injection | ||
| Number of participants who received intervention, n (%) | 31 (15.5) | 8 (20.5) |
| Number of interventions received, n (%) | ||
| 0 | 169 (84.5) | 31 (79.5) |
| 1 | 18 (9.0) | 7 (17.9) |
| 2 | 9 (4.5) | |
| ≥ 3 | 4 (2.0) | 1 (2.6) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 70 (75); (15, 322) | 70 (60); (7, 150) |
| Median (IQR) | 40 (30–67) | 71 (17–105) |
| Bleb resuturing | ||
| Number of participants who received intervention, n (%) | 5 (2.5) | 1 (2.6) |
| Number of interventions received, n (%) | ||
| 0 | 195 (97.5) | 38 (97.4) |
| 1 | 5 (2.5) | 1 (2.6) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 93 (75); (30, 188) | |
| Median (IQR) | 78 (33–154) | |
| Anterior chamber reformation | ||
| Number of participants who received intervention, n (%) | 5 (2.5) | 1 (2.6) |
| Number of interventions received, n (%) | ||
| 0 | 195 (97.5) | 38 (97.4) |
| 1 | 5 (2.5) | 1 (2.6) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 33 (32); (3, 71) | |
| Median (IQR) | 22 (6–63) | |
| Bleb revision | ||
| Number of participants who received intervention, n (%) | 7 (3.5) | 2 (5.1) |
| Number of interventions received, n (%) | ||
| 0 | 193 (96.5) | 37 (94.9) |
| 1 | 7 (3.5) | 2 (5.1) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 93 (54); (28, 156) | 112 (–); (112, 112) |
| Median (IQR) | 95 (53–134) | 112 (112–112) |
| Other interventions | ||
| Number of participants who received intervention, n (%) | 25 (12.5) | 2 (5.1) |
| Number of interventions received, n (%) | ||
| 0 | 175 (87.5) | 37 (94.9) |
| 1 | 25 (12.5) | 2 (5.1) |
| Time to first intervention (days) | ||
| Mean (SD); (min., max.) | 95 (104); (17, 331) | 33 (15); (22, 43) |
| Median (IQR) | 43 (28–126) | 33 (22–43) |
Non-index eye
A post hoc analysis looked at IOP (mmHg), logMAR and VFMD for the non-index eye (see Appendix 3, Table 35).
Chapter 5 Discrete choice experiment
Introduction
A DCE was undertaken to estimate the value that individuals with advanced glaucoma place on different health states associated with glaucoma (the approach is outlined in Methods) and to update the GUI QoL weights estimated by Burr et al. 55 The GUI QoL weights needed to be updated for this population because the preferences of those with advanced glaucoma were under-represented in the Burr et al. 55 study, with only 15% of the sample self-reporting severe glaucoma. Given that the severity of disease was not standardised, this could result in variations between participants, including some over-reporting their severity, thus diluting the preferences of those with advanced glaucoma. Furthermore, Burr et al. 55 found that individuals’ preferences changed depending on the severity of disease reported, hence the need to re-estimate the GUI utility weights based on the preferences of those with advanced glaucoma.
A selection of attributes (symptoms) and attribute levels (severity of symptoms), which can be found in the Glaucoma Profile Instrument (GPI), informed the health state profiles. The GPI is a disease-specific QoL measure that was used to develop health state profiles for glaucoma. 55 These health state profiles were used in the DCE valuation study to estimate QoL weights that could be used to estimate QALYs in the economic evaluation reported in Chapter 6.
The aim of the DCE was to identify the relative importance of each attribute and attribute level of the GPI based on the preferences of individuals with advanced glaucoma by asking participants to choose between two health state profiles. It was expected that the values for each health state profile presented to participants would vary depending on the attribute levels presented. Similar to Burr et al. ,55 the results of the model estimation were to be used to estimate preference-based weights for the GPI health state profiles in a population with advanced glaucoma. 55 These QoL weights were then incorporated into the economic evaluation to evaluate the cost-effectiveness of the surgical management of glaucoma compared with medical management (see Chapter 6).
Methods
Discrete choice experiments have been used to elicit values in different areas of economics, including transport and environmental, with increasing focus on these methods in health to elicit individuals’ preferences for products, services and health states. 55,81–83 A DCE is a type of conjoint analysis and is based on random utility theory. 84 In a DCE, individuals are asked to choose the best (or worst) alternative between at least two alternatives that have the same attributes but differing levels. Respondents are expected to act rationally when evaluating the alternatives and to choose the alternative that maximises their expected utility (i.e. satisfaction). 85 Logistic regression techniques are used to identify the relative importance of attributes and attribute levels on an individual’s utility function.
There are four stages involved in undertaking a DCE:
-
stage 1 – identification of attributes and levels
-
stage 2 – creating an efficient design
-
stage 3 – data collection
-
stage 4 – data analysis and interpretation.
Stage 1: identification of attributes and levels
The attributes and levels used in the DCE were informed by the GPI developed by Burr et al. 55 The work of Burr et al. 55 involved using a DCE to estimate the preference weights for the glaucoma utility index (GUI). 55 Briefly, Burr et al. 55 considered a wide range of potentially relevant attributes and levels that could be used to define a range of glaucoma health state profiles. These were identified using established glaucoma QoL instruments and expert opinion. These attributes and levels were refined using qualitative research methods (focus groups) with individuals with glaucoma. The attributes identified as important to individuals with glaucoma were central and near vision, lighting and glare, mobility, activities of daily living, eye discomfort, and other effects of glaucoma and its treatment. Each of these six attributes had the same four levels: no difficulty, some difficulty, a lot of difficulty or severe difficulty. 55 These six attributes with their corresponding levels were used to inform the health state profiles valued in the DCE.
Stage 2: creating an efficient design
The combinations of all attributes (n = 6) and levels (n = 4) from the GPI generates 4096 possible health state profiles (47). In the DCE, participants were asked their preference between two of these health state profiles (a choice-set question); however, asking participants to choose between all possible combinations of these health state profiles would be too onerous and not feasible. As with all DCEs, an experimental design was used so that the number of choice-set questions being valued was manageable and the main effects and higher-order interactions could still be estimated in the logistic regression. 86 The experimental design used by Burr et al. 55 was not used in the DCE conducted as part of TAGS because the design methodology has developed since that work was conducted. 55 For the current study, the experimental design followed best practice guidelines and a D-efficient design was used to identify the most efficient combination of choice-set questions. 86,87 The D-efficient design was produced using Ngene design software (ChoiceMetrics Pty Ltd, Sydney, NSW, Australia) and the design chosen was the best design that minimised the standard errors. 86
The design was split into four blocks to maximise variance in the data, and each participant was randomly allocated to one block and presented with 15 choice-set questions. Only participants who had not withdrawn from TAGS follow-up or who had not died during the study were included in the block randomisation. Figure 6 is an illustrative example of a choice-set question that participants were asked to answer.
FIGURE 6.
Example of a choice-set question.
Questionnaire design
A paper-based questionnaire was developed for each of the blocks and included the following sections (see Report Supplementary Material 1):
-
An introduction and explanation of the choice-set questions, with an illustrative example.
-
Fifteen choice-set questions, from which the participant had to choose the health state profile that they considered to be worse.
-
An ‘about you’ section that included information on level of education and annual household income, which was not collected as part of the main study.
-
A comments box where participants were asked to provide any comments they had on the questionnaire and an approximation of how long it took them to complete the questionnaire. This section was included to gauge how difficult participants found completing the choice-set questions.
Stage 3: data collection
The paper-based questionnaire and guide to completing the DCE were sent to participants, who were randomly allocated to one of the four blocks at 27 months post randomisation, 3 months after their final study visit. The randomisation was adjusted to ensure that equal numbers of respondents were randomised to each block at the end. A freepost envelope was provided for participants to return the completed questionnaire. After 3 weeks, a reminder was sent to participants who had not returned the questionnaire.
Stage 4: data analysis and interpretation
The data were analysed in Stata® version 15 (StataCorp LP, College Station, TX, USA). Descriptive statistics were used to describe the demographic characteristics of the full sample, responders and non-responders to identify any potential differences between responders and non-responders. Descriptive statistics were also used to identify any potential differences in demographic and socioeconomic characteristics (i.e. income and education) between participants in each of the four blocks.
The DCE was analysed under a random utility framework. Logistic regression techniques, specifically a conditional regression logistic model, were used to analyse participants’ stated preference for each choice-set question and quantify the relative importance of each attribute and attribute level. 84 A base category (i.e. severe difficulty) was selected for each attribute and against which the other levels of that attribute were compared; hence, 18 explanatory variables (six attributes × three levels) were included in the model. It was hypothesised that each attribute level would be positive compared with the severe difficulty level and that the coefficients would increase with improvements in difficulty. Given that all six attributes had the same levels, comparisons could be made across the attributes and the attribute levels could be ranked in order of preference.
Preference-based weights
As previously mentioned, the GPI represents 4096 possible health state profiles. Each health state profile could be classified by the levels for each attribute (1 = no difficulty, 2 = some difficulty, 3 = quite a lot of difficulty and 4 = severe difficulty), similarly to the EQ-5D-5L classification system. 88 The QoL weights were based on the QoL scale of 1 (perfect health) and 0 (severe difficulty in all of the attributes). The results of the logistic regression model were converted into preference-based weights on the assumption that the health state profile 111111 (having no difficulty with any of the attributes) was equivalent to perfect health score of 1. The coefficients associated with the other attribute levels were then anchored on this value to ensure that the ratio of preference for the different attribute levels was maintained in the QoL weights. It was also assumed that the health state profile 000000 (having severe difficulty with all of the attributes) was scored as zero.
Sensitivity analysis
A sensitivity analysis adopting the methods outlined by Burr et al. 55 was undertaken when there was no evidence of a statistically significant difference in attribute levels. 55 The levels of an attribute that were not statistically significant according to the Wald test were combined to determine whether or not the strength of preference for that attribute improved with the increased explanatory power. 55
A complete-case analysis that included participants who completed all 15 of their choice-set questions was also undertaken, as it could be argued that they had a better understanding of the task than those who only partially completed their choice-set questions.
Results
Of the 453 participants randomised into the TAGS study, 438 were assigned a random block for the DCE study (n = 15 withdrew/died). A total of 308 questionnaires (70%) were returned with the 15 choice-set questions at least partially completed. Of the 130 remaining questionnaires (30%), eight were returned with partial information provided for the demographic questions and/or with comments on why they did not complete the choice-set questions. The remaining 122 questionnaires were not returned.
Participant characteristics
Table 12 presents a summary of the demographic characteristics of the participants in the overall sample, and Appendix 4, Table 39, presents a summary of the sociodemographics of those who responded to the questionnaire and the difference in characteristics between blocks. Participants who responded to the DCE questionnaire had a mean age of 68 years. The majority of these participants were white (91%) and male (67%), and almost half (45%) had attended college or university. Each of the income brackets was equally represented across the four blocks, with nearly half of the participants who responded to the DCE questionnaire (48%) reporting an income of ≥ £20,000. The proportion of non-responders was similar across the four blocks.
| Characteristic | Complete sample (N = 438) | DCE questionnaire returned (N = 308) | DCE questionnaire not returned (N = 130) |
|---|---|---|---|
| Age (years), mean (SD) | 66.83 (12) | 68.42 (11) | 63.08 (14) |
| Male, n (%) | 293 (67) | 205 (67) | 88 (68) |
| Ethnicity, n (%) | |||
| White | 361 (82) | 280 (91) | 80 (62) |
| Asian – Oriental | 2 (< 1) | 1 (< 1) | 1 (< 1) |
| Afro-Caribbean | 57 (13) | 19 (6) | 38 (30) |
| Asian (India/Pakistan/Bangladesh) | 12 (3) | 5 (2) | 7 (5) |
| Mixed heritage | 1 (< 1) | 1 (< 1) | |
| Black British | 2 (< 1) | 2 (2) | |
| Colombian | 1 (< 1) | 1 (< 1) | |
| Nigerian | 1 (< 1) | 1 (< 1) | |
| Block allocation, n (%) | |||
| 1 | 107 (24) | 77 (25) | 30 (23) |
| 2 | 118 (27) | 74 (24) | 44 (34) |
| 3 | 107 (24) | 75 (24) | 32 (25) |
| 4 | 106 (24) | 82 (27) | 24 (18) |
Discrete choice experiment results
Of the 308 DCE questionnaires returned and used in the analysis, 283 (92%) were fully completed with all 15 choice-set questions answered. A total of 295 participants (96%) reported how long it took them to complete the DCE questionnaire, with 286 participants providing a time in minutes or hours that could be quantified. On average, it took participants 24 minutes to complete the DCE questionnaire. A descriptive summary of the number of choice-set questions completed and the time taken to complete the questionnaire is provided in Appendix 4, Table 39.
Regression model results
The Walt test determined that the explanatory variables were not equal to zero and should, therefore, be included in the model (p < 0.01). The results of the conditional logit model for the full sample are presented in Table 13. The levels for four of the attributes (i.e. central and near vision, lighting and glare, mobility, and activities of daily living) and the level ‘no difficulty’ for the attribute ‘eye discomfort’ were positive compared with the base level (i.e. severe difficulty), as expected. These results suggest that participants have a preference for health state profiles that report lower levels of difficulty in these attributes. At a 95% confidence level, only three of the attributes [i.e. central and near vision (all levels), mobility (excluding the level quite a lot of difficulty) and activities of daily living (all levels)] were statistically significant, suggesting that participants had a stronger preference for improvements in these attributes than in the other attributes.
| Attribute | Level | Coefficient | SE | p-value |
|---|---|---|---|---|
| Central and near vision | No difficulty | 0.552 | 0.072 | 0.000 |
| Some difficulty | 0.326 | 0.082 | 0.000 | |
| Quite a lot of difficulty | 0.191 | 0.082 | 0.019 | |
| Lighting and glare | No difficulty | 0.131 | 0.079 | 0.098 |
| Some difficulty | 0.021 | 0.075 | 0.782 | |
| Quite a lot of difficulty | 0.007 | 0.085 | 0.931 | |
| Mobility | No difficulty | 0.408 | 0.083 | 0.000 |
| Some difficulty | 0.290 | 0.079 | 0.000 | |
| Quite a lot of difficulty | 0.135 | 0.082 | 0.097 | |
| Activities of daily living | No difficulty | 0.352 | 0.076 | 0.000 |
| Some difficulty | 0.415 | 0.071 | 0.000 | |
| Quite a lot of difficulty | 0.188 | 0.074 | 0.011 | |
| Eye discomfort | No difficulty | 0.105 | 0.087 | 0.228 |
| Some difficulty | –0.118 | 0.088 | 0.180 | |
| Quite a lot of difficulty | –0.026 | 0.083 | 0.756 | |
| Other effects of glaucoma and its treatment | No difficulty | –0.004 | 0.079 | 0.961 |
| Some difficulty | –0.029 | 0.082 | 0.717 | |
| Quite a lot of difficulty | –0.096 | 0.080 | 0.231 | |
| Alternatives | Alternative A | 0.070 | 0.031 | 0.22 |
Counterintuitively, two of the levels for the attribute ‘eye discomfort’ and three levels for the attribute ‘other effects of glaucoma and its treatment’ had negative coefficients on average, suggesting that there was a negative preference (disutility) associated with improvements in these attributes compared with the base level (severe difficulty). However, there was no evidence that the preference for these attribute levels differed to the base level (p-values were 0.180 and 0.756, respectively).
Given that all of the attributes had the same levels, comparisons could be made across the attributes to identify the order of preference for the attributes and attribute levels. Improvements in ‘central and near vision’, ‘activities of daily living’ and ‘mobility’ yielded the most utility for participants. The attribute level with the greatest utility gain was ‘no difficulty’ with ‘central and near vision’.
Preference-based weights
Similarly to Burr et al. ,55 preference-based weights were estimated for each of the attribute levels so that the health state profiles generated by the GPI could be assigned a QoL weight that could be used to estimate QALYs. 55 These health-related quality-of-life (HRQoL) weights, based on responses to the GPI administered at baseline and at 1, 3, 6, 12, 18 and 24 months post randomisation, were used in a sensitivity analysis in the economic evaluation (further details are shown in Appendix 5, Table 45).
It was assumed that attribute levels that were negative and not statistically significant had a value of zero, indicating that there was no utility to be gained from having reduced difficulty in these attributes. This assumption was made because these results did not adhere to theoretical validity (i.e. there was a negative preference associated with an improvement in the attribute level compared with severe difficulty) and there was no evidence that this value was different from zero, based on the significance levels estimated from our model. Table 14 summaries the HRQoL weights for each attribute level and provides an illustrative example of how to estimate a value that can be used to estimate QALYs for a health profile based on the HRQoL weights.
| Attribute | Level | HRQoL weight |
|---|---|---|
| Central and near vision | No difficulty | 0.356 |
| Some difficulty | 0.211 | |
| Quite a lot of difficulty | 0.124 | |
| Severe difficulty | 0 | |
| Lighting and glare | No difficulty | 0.085 |
| Some difficulty | 0.013 | |
| Quite a lot of difficulty | 0.005 | |
| Severe difficulty | 0 | |
| Mobility | No difficulty | 0.264 |
| Some difficulty | 0.188 | |
| Quite a lot of difficulty | 0.087 | |
| Severe difficulty | 0 | |
| Activities of daily living | No difficulty | 0.227 |
| Some difficulty | 0.268 | |
| Quite a lot of difficulty | 0.121 | |
| Severe difficulty | 0 | |
| Eye discomfort | No difficulty | 0.068 |
| Some difficulty | 0 | |
| Quite a lot of difficulty | 0 | |
| Severe difficulty | 0 | |
| Other effects of glaucoma and its treatment | No difficulty | 0 |
| Some difficulty | 0 | |
| Quite a lot of difficulty | 0 | |
| Severe difficulty | 0 | |
| Profile | Health state description | QoL weight |
| 111111 | No difficulty with central and near vision, lighting and glare, mobility, activities of daily living, eye discomfort, and other effects of glaucoma and its treatment | 1 |
| (0.356 + 0.085 + 0.264 + 0.227 + 0.068 + 0) | ||
| 122321 | No difficulty with central and near vision and other effects of glaucoma and its treatment | 0.678 |
| Some difficulty with lighting and glare, mobility, and eye discomfort | (0.356 + 0.013 + 0.188 + 0.121 + 0 + 0) | |
| Quite a lot of difficulty with activities of daily living | ||
| 333223 | Some difficulty with activities of daily living and eye discomfort | 0.484 |
| Quite a lot of difficulty with central and near vision, lighting and glare, mobility, and other effects of glaucoma and its treatment | (0.124 + 0.005 + 0.087 + 0.268 + 0 + 0) |
Sensitivity analyses
A sensitivity analysis adopting the methods outlined by Burr et al. ,55 combining the levels of attribute levels that were not statistically significant, was undertaken. In this analysis, the levels of ‘lighting and glare’, ‘eye discomfort’ and ‘other effects of glaucoma and its treatment’ were each combined into one variable and compared with the base level for that attribute, ‘severe difficulty’. In this analysis, the statistical significance of these attribute levels did not improve. However, the level ‘quite a lot of problems’ for the attribute ‘mobility’ did reach statistical significance at the 5% level. There was still a negative preference for the attribute ‘other effects of glaucoma and its treatment’ despite the combination of the three levels.
The model was re-estimated on a subset of the data, including only participants who completed all 15 choice-set questions (n = 283). This analysis produced similar results to the base-case analysis in terms of the importance of the attributes and levels and the statistical significance of attributes and levels did not change in the complete-case analysis.
Chapter 6 Economic evaluation
This chapter contains material reproduced with permission from King et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The economic evaluation component of the study comprised both a within trial economic evaluation and a model-based economic evaluation to extrapolate beyond the end of the 2 years of the trial follow-up period.
Within-trial economic evaluation
Introduction
An economic evaluation is defined as a comparative analysis in terms of both costs (resource use) and consequences (outcomes and effects). 89 For this trial, the economic evaluation was to be carried out in two parts. The first part was a ‘within-trial’ economic evaluation that was conducted as part of the RCT to compare the costs and benefits of both medical and surgical pathways of glaucoma management. This within-trial analysis assessed the costs and benefits at the end of the 24-month trial period based on the trial data. The second part of the economic evaluation (discussed later in this chapter) is a model-based economic evaluation, which uses existing literature and the trial results to extrapolate the results beyond the end point of the trial.
There is evidence that, for advanced presentations of glaucoma, keeping IOP lower than 17.5 mmHg and stable has a beneficial effect on visual outcome. 34,90 However, the relative effectiveness and cost-effectiveness of medical and surgical pathways of glaucoma management are unclear. Chapter 4 describes the relative effectiveness of treatments. This section reports the methods and results of the within-trial economic evaluation (the model-based economic evaluation that extrapolated beyond the results of the trial is reported in the second part of this chapter).
The primary measures of effectiveness used in the economic evaluation are based on responses to the EQ-5D-5L, HUI-3 and GUI. The analysis was carried out from a health-care (NHS) perspective and from a patient perspective. NHS costs include the costs borne by the health service over the course of the trial and PSS costs (i.e. costs for local social services). Patient costs were estimated as well, and these included time and travel costs borne by the patient and those assisting participants to attend appointments in addition to any private health-care costs.
Methods
Data collection
Health service use costs
Data on the use of secondary care health care that each patient had accessed were collected using the CRFs completed at baseline and at 4, 12 and 24 months. Primary care costs were collected using a resource utilisation questionnaire completed at 4, 12 and 24 months. This questionnaire included visits to the ophthalmology outpatient clinic, outpatient procedures and glaucoma medications that the participants have taken. Unit costs for each item were derived from published sources including the NHS Reference Costs 2017/1891 and Unit Costs of Health and Social Care 2018. 92 The cost for medications were taken from the British National Formulary 2018. 93
Personal Social Services and patient costs
The 4-, 12- and 24-month patient questionnaires also measure the support services that the participant has accessed. This may include low-vision support services, support provided by the Royal National Institute for the Blind (RNIB) or travel assistance, such as bus passes or rail cards. Published costs were used to estimate the cost of these services.
Costs falling on the participants and any companions who accompanied the participants were estimated using the responses to the time and travel questionnaire, which was administered at 18 months, and information on use of health services. Travel costs were derived by asking participants to identify how they travelled to the appointments. If public transport or taxis were used, the fare would represent the individual’s travel costs. If the patient drove to the appointment, the participants were asked to estimate the journey in miles and a cost for each mile was taken from the TAG data book May 2018. 94 The questionnaires also included questions about the length of time that travelling to and attending one of these appointments would take. The cost of the time to attend the appointments for both the participant and any accompanying caregiver was also calculated based various activity rates published by the Office for National Statistics. 95 Any out-of-pocket private health-care costs were also included when calculating patient time and travel costs.
Derivation of costs
Inpatient costs
Participants in the trial were randomised into the trabeculectomy or medical management arm of the trial. If the participants were in the trabeculectomy arm, the baseline CRF asked details about the trabeculectomy procedure. This includes the time spent in the operating theatre, the equipment used and the staff present. Two kinds of admissions were associated with the trabeculectomy procedures in this trial: a day-case admission and an overnight inpatient admission. Costs for both kinds of procedure are available in the NHS Reference Costs 2017/1891 and are attributed to each participant who completed the surgical CRF. If the admission type was not stated, the day-case rate was assumed, as this was the most common admission for the procedure according to the NHS Reference Costs 2017/18. 91
Those who were allocated to the medical management arm of the trial were not allocated an inpatient cost at baseline, but both treatment arms were asked about any inpatient stays or procedures on each subsequent CRF form. The patient was asked about any inpatient stays associated with a list of procedures and, if appropriate, provided admission and discharge dates so that the duration of any inpatient stay could be calculated. Both the cost of the hospital and the cost of the procedure were taken from NHS Reference Costs 2017/18. 91 Further details of unit costs for hospital appointments are provided in Appendix 5, Table 40.
Outpatient costs
Secondary care outpatient costs were elicited from the CRF data collected from the 4-month visit onwards. This included both visits to the outpatient ophthalmology clinic and any necessary ophthalmological outpatient procedures. The CRF asked about outpatient-specific procedures including:
-
massage
-
adjustment/suture lysis/releasable release
-
5-FU injection
-
steroid injection
-
needling and 5-FU injections
-
bleb resuturing
-
anterior chamber reformation
-
bleb revision
-
phacoemulsification and intraocular lens.
There was also an option to include a different procedure to any of the ones named above. The number of ophthalmology outpatient appointments and the dates of attendance were collected on each CRF form. The procedures were costed using the NHS National Reference Costs 2017/18. 96 Further details of unit costs for procedures are provided in Appendix 5, Table 41.
Medication costs
Medications were also captured in the CRFs. Details on the use of the following glaucoma medications were collected:
-
prostaglandin analogues
-
beta-blockers
-
carbonic anhydrase inhibitor
-
pilocarpine
-
α-agonists
-
other.
In the trabeculectomy arm of the trial, the use of pre-operative eye drops was recorded on the CRFs and costed. Medication costs were taken from the 2018 British National Formulary. 93 Drug start dates and stop dates were reported on the CRFs. If a participant started a drug before the 2-year trial period and was still taking the medication at the end of the trial, it was assumed, for costing purposes, that they were taking the drug for the entire trial period. If, however, the participant started or stopped the medication during the course of the trial, the length of time that the participant was on the drug was calculated in months and then the cost of the drug was calculated using this duration. Further details of unit costs for medications are provided in Appendix 5, Table 42.
Primary care costs
Patients in the trial were asked to complete a participant questionnaire at baseline and at 1, 3, 4, 6 and 12 months. As part of the 4- and 12-month questionnaire, participants were also asked about the health-care resources that they utilised over the trial period, for example:
-
GP appointments in practice
-
GP appointments at home
-
GP telephone appointments
-
community optometry appointments
-
district nurse appointments
-
practice nurse appointments.
Costs for these appointments were taken from the Unit Costs of Health and Social Care 2018. 97 The number of appointments for each of the types above was multiplied by the unit costs for each appointment. Further details of unit costs for primary care are provided in Appendix 5, Table 43.
Costs to participants and their main caregiver
This study measured the travel costs that were incurred by participants when they travelled to appointments. This was measured using a dedicated time and travel questionnaire that the participant completed during the course of the study. It was originally planned to administer the questionnaire at 18 months, but, because of a change to the protocol, the questionnaire was administered at 30 months. The questionnaire asked participants to provide the average cost of a typical journey to:
-
outpatient appointment
-
GP appointment
-
community optometrist appointments
-
inpatient hospital stays.
If the journey was undertaken using public transport, the fare was used to represent travel costs. If a journey was undertaken by private car, a fuel rate of £0.45 per mile was applied based on the business and self-employed expense rate per mile. 98 Finally, if hospital transport was used, travel costs were estimated from the Unit Costs of Health and Social Care 2018. 97
The costs of the time commitments of the participants and their companions were also measured in this evaluation; this was also measured in the time and travel questionnaire. Participants were asked to choose which best described how they would spend their time if they were not attending appointments to manage their glaucoma. The participant chose from one of the following:
-
paid work
-
homemaking
-
child care
-
caring for a friend/relative
-
retired
-
full-time education
-
unemployed
-
voluntary work
-
leisure activities
-
other (please specify).
Paid work, child care, caring for a relative or friend and voluntary work were valued at £13.88 per hour, which is the average hourly wage according to the Office for National Statistics. 95 Housework and leisure activities were valued at £10.10 per hour. The time spent in unemployment, retirement or full-time education was valued at £6.04 per hour. Finally, private out-of-pockets expenses reported by participants were included in the patient time and travel costs.
Estimation of effects
Estimation of quality-adjusted life-years for the cost–utility analysis
Given that the within-trial economic evaluation is a cost–utility analysis (CUA), outcomes were measured in QALYs. QALYs were estimated from three sources: the EQ-5D-5L,99 HUI-3 (because the HUI-3 does not a have a UK value set, the existing Canadian value set was used100) and GUI. 14 The utility values for the EQ-5D-5L were mapped to an existing value set for the validated data set of results for the EQ-5D-3L. 101 During the course of the study, a UK value set for the EQ-5D-5L was produced,102 but, given that this is currently not validated, the cross-walked values from the EQ-5D-3L data set were used in the base case and the EQ-5D-5L values were examined in a sensitivity analysis. The EQ-5D-5L instrument was administered at seven time points during the trial: baseline and 1, 3, 6, 12, 18 and 24 months. The complete-case analysis included all participants who had completed the EQ-5D-5L at all seven time points. Those who had completed the EQ-5D-5L at five of the seven time points were included (as long as one of those points was baseline) with imputed data. Some participants completed an additional EQ-5D-5L before their trabeculectomy. These values are summarised in Appendix 6, Table 47.
The QALYs gained was also calculated using the scores that were derived from the responses from the HUI-3 complete cases were considered to be those who had all seven time points and values were imputed for those who had four time points or more. The utility values for the GUI were derived from the results of the DCE (which are presented in Chapter 5).
Analysis of costs and benefits
Costs
A total NHS cost per participant was calculated using data from the trial. A seemingly unrelated regression (SUREG) was used to identify any difference between the trabeculectomy and the medical management arm of the trial while controlling for any modifying factors, such as the participant’s age and their baseline utility score. In a further analysis, the SUREG was repeated with the inclusion of patient time and travel costs.
Quality-adjusted life-years
As with the costs, a total QALYs gained per participant was calculated using trial data. A SUREG was used to calculate the differences between the costs and to control for modifying factors, such as baseline utility score and treatment arm.
Missing data
Cost data were reported as missing either if the sections of the CRF reporting medications taken and procedures undertaken were completely blank (no values, positive or negative, were given in either section) or if the total costs on the CRF were reported as zero and the answer to the question ‘Has participant completed the TAGS Participant Questionnaire?’ was ‘no’, as this indicates incomplete data.
With respect to the estimation of QALYs, those who had completed four of the seven data collection surveys were included for analysis. First, to account for the missing data points, it was assumed that the previous utility remained stable. This meant that the weighted average of the two utility scores around the missing values was used to calculate the missing data. Second, multiple imputation was used to estimate missing utility values for QALY scores. No patterns were observed in the missing data, so the data were imputed randomly. 103
Sensitivity analysis
Stochastic sensitivity analysis
To assess the robustness of the study sampling, non-parametric bootstrapping was carried out. Bootstrapping is a statistical procedure that resamples a single data set to create many simulated samples to assess statistical precision. This can model the difference in net benefit if the sampling process could be repeated many times. In this example, 1000 iterations of the bootstrapping procedure were performed. This simulation process created a sample of bootstrapped means for costs and QALYs, with distributions for each. The means and other parametric statistics were then calculated for the bootstrap distribution. Bootstrapped estimates of the difference in costs and QALYs between the experimental and the control arm were used to populate the cost-effectiveness plane (the horizontal axis represents the difference in QALYs between two interventions and the vertical axis represents the corresponding difference in costs).
A cost-effectiveness acceptability curve (CEAC) quantifying the probability that an intervention is cost-effective [based on the decision-maker’s maximum willingness to pay over a range of values (e.g. £0–100,000 per QALY)] was derived from the results of the non-parametric bootstrapping.
Deterministic sensitivity analysis
Deterministic sensitivity analysis was carried out to assess the variability of different parameters on the outcomes of the economic evaluation. The QALYs were recalculated using the utility values generated from both the HUI-3 and the GUI QoL tools to see if this changed the results. Patient time and travel costs were included to assess how these contacts have an impact on potential conclusions.
Results
Response rates
The response rates for the data collection instruments used in the economic evaluation are described in Table 15. Data completion in this trial was generally very high.
| Time point | Treatment arm, % (n) | |
|---|---|---|
| Trabeculectomy | Medical management | |
| Case report form | ||
| 4 months | 99 (226) | 99 (223) |
| 12 months | 99 (226) | 100 (226) |
| 24 months | 96 (217) | 97 (219) |
| EQ-5D-5L | ||
| Baseline | 98 (222) | 98 (222) |
| 1 month | 85 (194) | 90 (203) |
| 3 months | 82 (186) | 79 (179) |
| 6 months | 82 (186) | 86 (195) |
| 12 months | 93 (211) | 92 (209) |
| 18 months | 80 (181) | 81 (184) |
| 24 months | 91 (206) | 90 (203) |
| Resource use questionnaire | ||
| 4-months | 93 (210) | 96 (216) |
| 12 months | 94 (213) | 92 (208) |
| 24 months | 92 (208) | 90 (204) |
| Time and travel questionnaire | ||
| 30 months | 68 (154) | 65 (148) |
Total resource use
The total costs for the different areas of resource use are summarised in Table 16. The largest cost drivers for total costs were ophthalmology outpatient appointments, prostaglandin analogue medications and, principally, the trabeculectomy procedure itself. The total costs are summarised in Table 17 and there was evidence of a higher cost in the first year for the trabeculectomy arm, which is largely because of the costs of the trabeculectomy procedure in the first year. In the second year, there was no evidence of a difference in the cost between the arms.
| Resource | Total cost per treatment arm (£) | |||||
|---|---|---|---|---|---|---|
| Trabeculectomy | Medical management | |||||
| Mean | Median | SD | Mean | Median | SD | |
| GP surgery consultations | 26 | 0 | 103 | 13 | 0 | 34 |
| GP home consultations | 3 | 0 | 20 | 2 | 0 | 18 |
| GP telephone consultations | 22 | 0 | 63 | 32 | 0 | 97 |
| Practice nurse consultations | 26 | 0 | 49 | 45 | 0 | 128 |
| District nurse consultations | 10 | 0 | 81 | 8 | 0 | 33 |
| Optometrist consultations | 29 | 21 | 38 | 21 | 21 | 25 |
| Ophthalmology consultations | 1313 | 1129 | 615 | 593 | 407 | 524 |
| Other consultations | 41 | 0 | 246 | 69 | 0 | 311 |
| Nights in hospital | 28 | 0 | 166 | 10 | 0 | 78 |
| Releasable release | 101 | 0 | 160 | 17 | 0 | 59 |
| Ocular massage | 97 | 0 | 192 | 5 | 0 | 30 |
| Trabeculectomy | 1821 | 1639 | 850 | 394 | 0 | 800 |
| 5-Fluorouracil injection | 30 | 0 | 95 | 8 | 0 | 37 |
| Steroid injection | 18 | 0 | 59 | 6 | 0 | 44 |
| Needling plus 5-fluorouracil injection | 37 | 0 | 99 | 10 | 0 | 54 |
| Bleb resuturing | 20 | 0 | 160 | 7 | 0 | 94 |
| Anterior chamber reformation | 3 | 0 | 20 | 1 | 0 | 9 |
| Bleb revision | 45 | 0 | 242 | 7 | 0 | 94 |
| Phacoemulsification | 63 | 0 | 309 | 81 | 0 | 374 |
| Prostaglandin analogues | 181 | 178 | 141 | 286 | 320 | 120 |
| Carbonic anhydrase inhibitor | 7 | 0 | 15 | 18 | 0 | 25 |
| Beta-blockers | 9 | 0 | 16 | 19 | 10 | 22 |
| α-agonists | 5 | 0 | 19 | 15 | 0 | 50 |
| Pilocarpine | 0 | 0 | 5 | 1 | 0 | 21 |
| Combinations | 4 | 0 | 22 | 1 | 0 | 10 |
| Diamox | 1 | 0 | 10 | 1 | 0 | 18 |
| Others | 0 | 0 | 4 | 0 | 0 | 0 |
| Total cost | Treatment arm (£) | Mean difference (p-value) | |||||
|---|---|---|---|---|---|---|---|
| Trabeculectomy | Medical management | ||||||
| Mean | Median | SD | Mean | Median | SD | ||
| Total cost to the NHS over 24 months | 3826 | 3509 | 1648 | 1685 | 1057 | 1401 | 2141 (< 0.01) |
| Total cost to the NHS between baseline and 12 months | 3157 | 2995 | 1299 | 1067 | 523 | 1299 | 2090 (< 0.01) |
| Total cost to the NHS between 12 and 24 months | 669 | 353 | 977 | 618 | 410 | 632 | 51 (0.53) |
The results of the analysis of QALYs gained using different QALY measures are summarised in Table 18. Based on the EQ-5D-5L, the QALY gain at 24 months was slightly higher in the trabeculectomy arm than in the medical management arm based on the unadjusted means. Neither the HUI-3 nor the GUI provided any evidence of a difference between arms in QALYs gained.
| Treatment | Treatment arm | Mean difference (p-value) | |||
|---|---|---|---|---|---|
| Trabeculectomy | Medical management | ||||
| Mean | SD | Mean | SD | ||
| Effectiveness | |||||
| EQ-5D-5L | |||||
| Baseline (n = 444) | 0.84 | 0.18 | 0.84 | 0.18 | |
| 1 month (n = 397) | 0.84 | 0.18 | 0.81 | 0.20 | |
| 3 months (n = 365) | 0.84 | 0.17 | 0.81 | 0.20 | |
| 6 months (n = 381) | 0.85 | 0.18 | 0.82 | 0.20 | |
| 12 months (n = 420) | 0.84 | 0.18 | 0.82 | 0.16 | |
| 18 months (n = 365) | 0.83 | 0.19 | 0.79 | 0.22 | |
| 24 months (n = 409) | 0.81 | 0.18 | 0.80 | 0.19 | |
| Complete QALYs over 24 months using EQ-5D-5L (n = 290) | 1.65 | 0.24 | 1.59 | 0.28 | 0.06 (0.04) |
| HUI-3 | |||||
| Baseline (n = 428) | 0.81 | 0.20 | 0.80 | 0.20 | |
| 1 month (n = 377) | 0.79 | 0.23 | 0.79 | 0.23 | |
| 3 months (n = 359) | 0.80 | 0.22 | 0.78 | 0.22 | |
| 6 months (n = 362) | 0.81 | 0.22 | 0.78 | 0.22 | |
| 12 months (n = 400) | 0.83 | 0.19 | 0.80 | 0.20 | |
| 18 months (n = 343) | 0.80 | 0.21 | 0.75 | 0.26 | |
| 24 months (n = 391) | 0.79 | 0.23 | 0.75 | 0.25 | |
| Complete QALYs over 24 months using HUI-3 (n = 240) | 1.61 | 0.30 | 1.54 | 0.36 | 0.07 (0.09) |
| GUI | |||||
| Baseline (n = 441) | 0.88 | 0.13 | 0.86 | 0.13 | |
| 1 month (n = 399) | 0.86 | 0.14 | 0.85 | 0.16 | |
| 3 months (n = 377) | 0.85 | 0.13 | 0.84 | 0.16 | |
| 6 months (n = 377) | 0.84 | 0.16 | 0.85 | 0.14 | |
| 12 months (n = 413) | 0.86 | 0.14 | 0.86 | 0.14 | |
| 18 months (n = 365) | 0.85 | 0.14 | 0.83 | 0.16 | |
| 24 months (n = 407) | 0.85 | 0.15 | 0.83 | 0.18 | |
| Complete QALYs over 24 months using GUI (n = 293) | 1.67 | 0.20 | 1.64 | 0.24 | 0.03 (0.25) |
Economic evaluation
Cost–utility analysis using the EQ-5D-5L
The results of the cost–utility analysis are presented in Table 19 and Figures 7 and 8. The results of the SUREG, reported in Table 19, show a small incremental QALY gain for surgery compared with medical management, but this small gain does not appears to offset by the higher costs of surgery. The effectiveness plane demonstrates that the difference in costs and QALYs for surgery compared with medical management is almost entirely in the quadrant that represents greater effect at greater cost (see Figure 8). The CEAC in Figure 7 shows that surgery is unlikely to be considered cost-effective over the range of values that society may be willing to pay for a QALY.
| EQ-5D-5L data | Treatment arm | Unadjusted cost (£) | Adjusted incremental cost (£) | Unadjusted QALY | Adjusted incremental QALY | ICER (Δcost/ΔQALY) (£) | Probability cost-effective at threshold (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £20,000 | £30,000 | £50,000 | |||||||
| Complete-case data (n = 290) | Trabeculectomy | 3686 | 2089 | 1.65 | 0.03 | 64,303 | 0 | 0 | 6 | 35 |
| Medical management | 1605 | 1.59 | 100 | 100 | 94 | 65 | ||||
| Multiple imputation data (n = 403) | Trabeculectomy | 3622 | 2013 | 1.61 | 0.04 | 45,456 | 0 | 0 | 12 | 56 |
| Medical management | 1605 | 1.56 | 100 | 100 | 88 | 44 | ||||
FIGURE 7.
Cost-effectiveness curves for the trabeculectomy arm and the medical management arm using the results from the EQ-5D-5L and multiply imputed data.
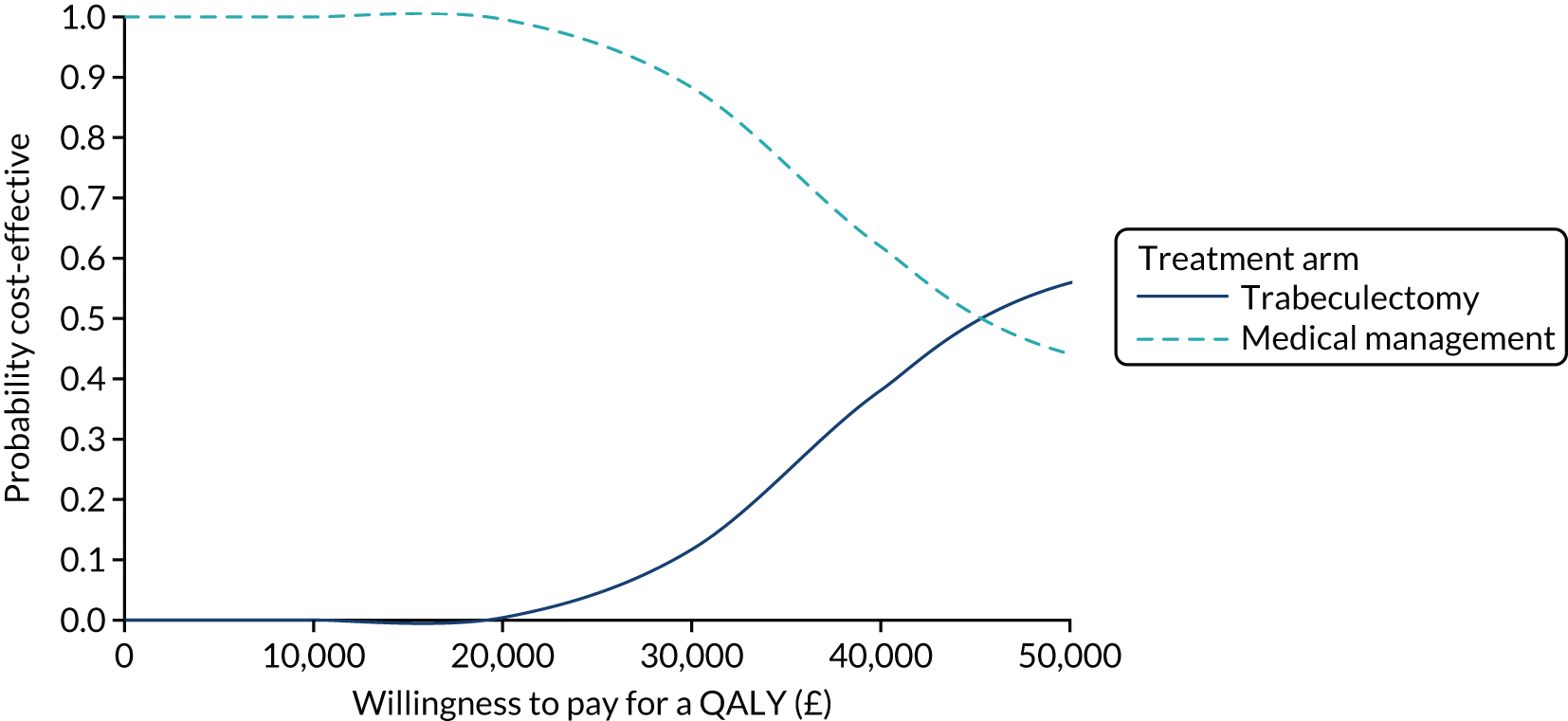
FIGURE 8.
Cost-effectiveness plane for the trabeculectomy arm vs. the medical management arm: adjusted bootstrapped replications for CUA for EQ-5D-5L results.
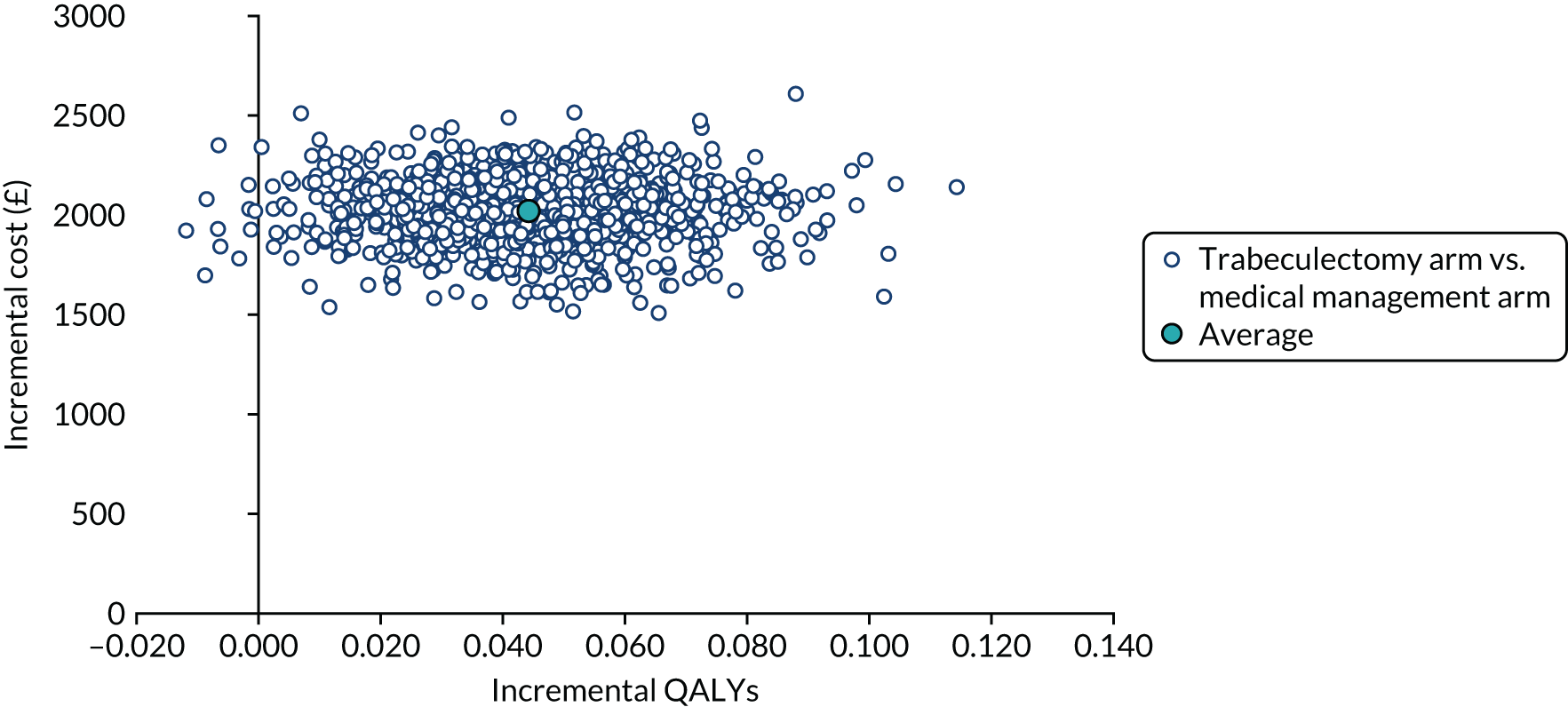
Sensitivity analysis
Appendix 5, Tables 44 and 45, and Figures 15–18 display the results of the sensitivity analysis when the HUI-3 and GUI QoL instruments are used in place of the EQ-5D-5L. Although the incremental cost-effectiveness ratios (ICERs) for the HUI-3 are smaller than that for the EQ-5D-5L, the probability of the trabeculectomy intervention being cost-effective is still very small, so the conclusions remain unchanged. A similar result was also seen for the GUI.
The inclusion of the participants’ time and travel costs in Appendix 5, Table 46, and Figures 19 and 20, demonstrate an increased difference in costs between arms and further supports the conclusion that medical management is likely to be considered more cost-effective than surgery over a 24-month period.
Model-based economic evaluation analysis
Background and rationale
Although the within-trial analysis is useful for informing decisions about cost-effectiveness in the short to medium term, it provides limited evidence to inform decisions about the cost-effectiveness of interventions in the longer term. 104 This is a very important limitation owing to the chronic nature of glaucoma,105 which can progress over time,106 and because the effects of treatment on costs and outcomes may persist into the future, with patients often living for many years after their glaucoma diagnosis. Therefore, a further analysis of the longer-term effects based on a Markov model107,108 was undertaken to extrapolate results of the trial beyond the 2-year follow-up and over the expected lifetime of patients. The results of this model can be used to inform decisions about the longer-term cost-effectiveness of trabeculectomy and medical management for people with newly diagnosed advanced glaucoma. In simple words, we tried to anticipate (extrapolate) the long-term impact of medication compared with surgery based on trial data and other related data from the literature. The logic of the modelling for this research is that the surgical procedure is expensive and, therefore, surgery is associated with higher initial costs than medical management. A longer-term time horizon would allow more time for any additional benefits of surgery to offset these initial higher costs. Essentially, a short time horizon could act as a bias against surgery.
Objective
The purpose of this model-based economic evaluation is to compare primary medical treatment with primary augmented trabeculectomy (glaucoma surgery) for patients presenting with advanced glaucoma (HPA classification severe64) in terms of costs and QALYs.
Methods
This section describes the methodology underpinning the model-based economic evaluation results. Detail is provided on the estimations of parameters used within the model and on how these values are used within the model to inform how patients move through the model. This section also describes how the model was validated to ensure that it estimates the costs of real-world glaucoma care service in the UK. Furthermore, the section describes the sensitivity analyses performed. These were carried out to analyse how uncertainty in the model’s parameters affect the final outcomes.
Model overview
A Markov model was constructed to model both treatments (trabeculectomy and primary medication)1 and disease progression that reflects the timing and allowed modelling of the logical and temporal sequence of events following the initial treatment strategies. The primary source of data for the model was the trial data set, supplemented with data from the literature where necessary. The uncertainty surrounding the model findings was assessed using probabilistic sensitivity analysis (PSA) and deterministic analysis. 107 The model was developed using TreeAge Pro 2019 (Williamstown, MA, USA).
To design the structure of the model and to classify the stages of glaucoma, we used the Enhanced Glaucoma Staging System (GSS2). 109 An external independent reading centre (CARF) reviewed and categorised TAGS visual fields into stages of VF loss based on the GSS2 system of grading. The consequences considered were the costs (of treatment and of subsequent management of patients with severe glaucoma) to the NHS and the effects on quality of life (QALYs). QALYs were estimated by assigning utility weights based on data on health state utility values derived from responses to the EQ-5D-5L (and GUI or HUI). 1 Combining these data with information on the probabilities of events occurring over time enabled cost, patient outcomes and QALYs to be estimated for a hypothetical cohort of patients undergoing each treatment strategy.
Model structure
The Markov model was developed using in-house experience of previous evaluations of glaucoma treatment,110–113 as well as the literature and advice from clinical colleagues. The model is used to simulate patient pathways from initial treatment until the end of life using data from the TAGS trial along with the best available UK relevant data to define transition probabilities for the model. All other model parameter estimates beyond 2 years were informed by the data from TAGS, other existing data sources (routine databases and the literature) and expert opinion.
Typically, Markov models have states (Markov states) in which individuals stay for a period of time. Each state reflects the subject’s level of well-being at that time. In each Markov state, the model will assign a cost and utility weight to each individual depending on the different interventions received by that individual while in that state and/or how long the individual remains a member (time spent) of that state. 107,114 The model is run for a length of time (e.g. 25 years), known as the ‘model time horizon’. This time horizon is broken down into equal parts, denoted as Markov cycles (e.g. 1-year intervals).
The cycle must be a period relevant to the condition considered (e.g. 6 months, 1 year). At the end of each cycle, individuals can remain in the state in which they started the cycle or move to a different state. Movement is dependent on the transition probability, which is defined as the probability of moving from one state to another. 115 Such models can be useful in capturing the costs and benefits of treating chronic medical conditions, such as glaucoma, over time. 116 In addition, all Markov models have at least one absorbing state, typically death, which all individuals will eventually enter if the model is given a sufficiently long time horizon. The sum of the cost in each year and the product of the utilities in each year were summed over the time horizon of the simulated patient cohort to compute total cost and quality-adjusted life-years (QALYs) for that cohort. 115
Within the model, and mirroring the trial inclusion and exclusion criteria, on entry into the model patients could have disease in one or both eyes. Again mirroring the trial inclusion criteria, one eye is defined as the index eye in terms of initial treatment and, in terms of disease severity, has advanced glaucoma, as this was also an inclusion criterion for the trial. Based on the (modified) disease classification (GSS2), we defined the stages for the economic model shown in Box 1. Other possible options for model structure are provided in Appendix 7, Tables 48–52.
Stage 1: index eye, severe (S3); non-index eye, non-severe (S0 + border + SB + S1 + S2).
Stage 2: index eye, severe (S4); non-index eye, non-severe (S0 + border + SB + S1 + S2).
Stage 3: index eye, severe (S5); non-index eye, non-severe (S0 + border + SB + S1 + S2).
Stage 4: index eye, severe (S3); non-index eye, severe (S3).
Stage 5: index eye, severe (S4); non-index eye, severe (S3).
Stage 6: index eye, severe (S5); non-index eye, severe (S3).
Stage 7: index eye, severe (S3); non-index eye, severe (S4).
Stage 8: index eye, severe (S4); non-index eye, severe (S4).
Stage 9: index eye, severe (S5); non-index eye, severe (S4).
Stage 10: index eye, severe (S3); non-index eye, severe (S5).
Stage 11: index eye, severe (S4); non-index eye, severe (S5).
Stage 12: index eye, severe (S5); non-index eye, severe (S5).
We adopted this model structure because it allows us to model both eyes independently and estimate the chance of unilateral and bilateral progression more precisely than modelling a single eye, even with a cohort Markov model. This is especially important in the case of eye disease because HRQoL and, therefore, QALYs are thought to be determined by the quality of vision in the better eye. 117 The model allows progression of the disease in both eyes over time. For the purposes of modelling, the time step over which progression could occur is 1 year because glaucoma is generally only slowly progressive. 116
Within the model, the mean age and sex distribution of the modelled cohort matches that of the trial participants at baseline. For those with binocular disease, the risk of visual loss in each eye is modelled independently. The model structure is presented in Figure 9.
FIGURE 9.
Model structure.
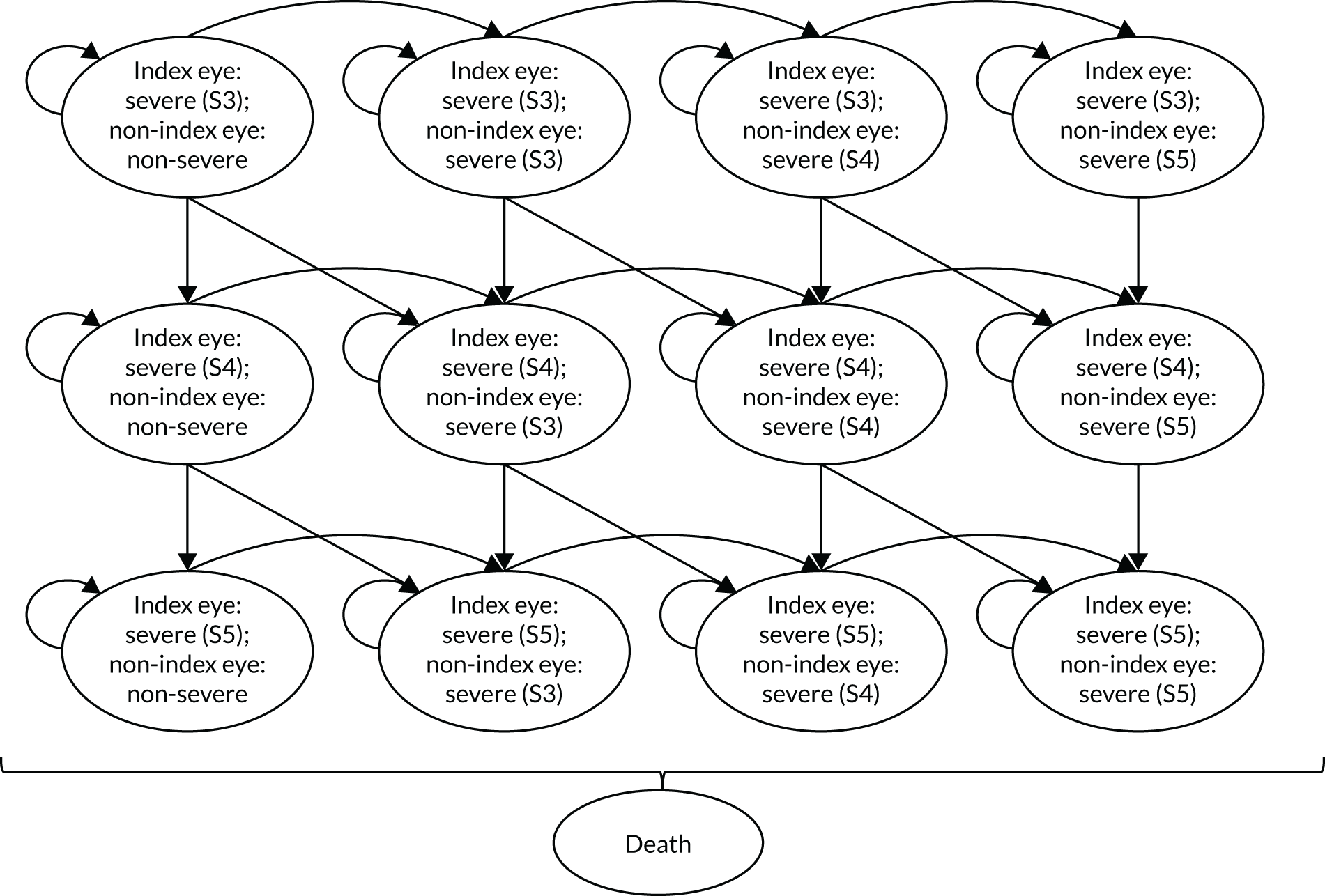
Treatment pathway
A patient starts with one of the two randomised treatments (trabeculectomy or medical management). The medical treatment strategy was chosen as the comparator and trabeculectomy as the intervention in this trial. A patient is categorised in different stages of glaucoma (based on the GSS2 classification system; see Box 1) for both the trabeculectomy arm and the medical management arm. The initial and transition probabilities of patients in each stage are based on trial data. Disease progression may occur during the trial and some patients move to more severe levels of glaucoma. Disease progression in the model is determined by a set of transition probabilities, each of which is defined in the sections below. Patients may also experience some complications that may affect related cost/utility in both arms. Therefore, complication-related cost/utility has been included in the model as well.
In the model, a patient may progress through a number of different states, each represented by increasing severity of disease (Figure 9). The Markov model used in this study (see Figure 9) considers increasing severity of glaucoma for each eye separately, as has been undertaken by others. 118 The level of the patient‘s disease on entry into the model determines the Markov model state at which the patient starts. The progress of patients through the model reflects disease progression, but it possible that patients can stay in a state and not progress further. However, they cannot move back to a less severe state because glaucoma is an irreversible disease. 117
During each model cycle, a portion of the cohort progresses based on the probabilities of progression derived from the analysis of the trial and other relevant data. In addition, it is assumed that patients move sequentially between states and cannot skip states because of the relatively slow evolution of glaucoma. 106 Finally, death from all causes is included in the model as an absorbing state (a state that someone can enter but cannot leave). Transition probabilities to this state are assumed to be independent of severity and treatment history and are derived from age-/sex-specific UK life tables. 119 Patients who are blind (in one or both eyes) are taken to have a higher risk of death than the general population and, hence, standardised mortality ratios were used to adjust the risk of death for those who are blind in one or both eyes. 120 Surgical mortality was not included in the model. All of the programming for the model was implemented in TreeAge Pro 2019.
Model assumptions
-
The model-based economic evaluation analysis took the perspective of the NHS and Personal Social Services (PSS).
-
For extrapolation, the cost and utility values (by clinical severity state and treatment allocation) beyond 24 months (beyond the duration of trial follow-up) in the model were assumed to be the same as those incurred in the second year.
-
Independent variables (e.g. progression state, have trabeculectomy treatment) and constant value of year 1 were assumed as a proxy to estimate the cost and utility for the second year.
-
All model input parameters are defined as statistical distributions in the model, allowing probabilistic sensitivity analysis to be conducted. 107
-
Ranges and distributional assumptions for input parameters were based on the trial data. We assigned gamma distributions for costs and beta distributions for utility data. 121 We have described these in more detail in Model parameters.
-
All future costs and QALYs were discounted at 3.5% per annum, the UK recommended rate, as the duration of follow-up (time horizon) was > 1 year. 122
Model parameters
Costs specified within the model
Costs were assigned to each state in the model, reflecting the costs for each 12-month period. All costs are presented in Great British pounds for the year 2018. Costs were assumed to vary according to glaucoma severity and initial treatment in both (trabeculectomy/medical management) groups. Regression techniques were applied to the data on total costs per participant obtained from the trial to identify whether or not there is a difference between those randomised to the trabeculectomy arm and those randomised to the medical management arm, for potentially modifying factors (e.g. progression rate). The objective of this is to make the model more dynamic and sensitive to main drivers of the cost over patient lifetimes. An ordinary least squares regression technique was applied:
In Equation 1, we assumed that the cost in that the second year of the model is a function of treatment (T), progression state of the disease (P) and a constant value from year 1. A dummy variable for the trial intervention arm estimates the difference in costs between the arms, controlling for all other factors in the model. Estimated beta values describe the direction and magnitude of the relationship between each variable and the dependent variable (cost). For example, if the dummy variable is specified as trabeculectomy arm = 1 and medical management arm = 0, and the beta estimate of the cost coefficient is 500. This indicates that the trabeculectomy arm (coded as 1 in the dummy variable) is £500 more costly, on average, than the medical treatment arm controlling for all other factors. If the coefficient was –500, then trabeculectomy would, on average, be £500 cheaper than medical management. We also used Cholesky decomposition and assigned multinormal distributions to these parameters to enable the model for sensitivity analysis. 121
Utilities specified in the model
Utilities associated with each health state were attached to the modelled severity states, allowing cumulative model-based QALYs to be estimated in both (trabeculectomy/medical management) arms. We assigned a zero utility weight for death. The mean QALYs for each intervention were calculated by multiplying the amount of time that patients spent in each health state by the associated health state utility values. The estimated utility used in the model was based on data retrieved from the trial using instruments such as EQ-5D-5L;123 data from the trial were also available for the HUI-3124 and the GUI,55 and these data have been used in the sensitivity analysis.
As with costs, regression techniques were carried out to derive the drivers of the difference in QALYs for a 12-month period between the arms of the trial (trabeculectomy vs. medical management) after controlling for the key predictors of QALYs. The purpose of this regression was to determine the consequences of a glaucoma state (based on GSS2 measure) and its effect on a participant’s overall QALYs:
The variables considered were:
-
dependent variable – QALYs (total QALY score across the two arms of the trial controlling for the independent factors)
-
independent variables – progression rate (P) and trabeculectomy (T).
Here, the dummy variable for the treatment arms estimates the difference in QALYs between the trabeculectomy and the medical management arms, controlling for all other factors in the model. For example, if the dummy is specified as trabeculectomy arm = 1 and medical management arm = 0 and the beta value of the coefficient is 0.50, this indicates that the trabeculectomy (coded as 1 in the dummy) provided 0.50 more QALYs over 12 months than current practice, on average, after controlling for all other factors. If the coefficient was –0.50, the QALY gain resulting from the intervention would, on average, be –0.50 lower than that achieved by medical management. As with costs, we also used Cholesky decomposition and assigned multinormal distributions to these parameters to enable the model for sensitivity analysis. 121
Progression and transition probabilities
The model structure allows a cohort of patients with advanced glaucoma to enter into the model and for disease to progress over time to eventual death. During each model cycle, a portion of the cohort progresses in severity (from no glaucoma to early, early to moderate and moderate to severe) based on the probabilities of progression derived from the analysis of the trial data.
Table 20 presents a summary of the initial and transition probabilities of progression that are used in the model. More details of initial and transition probabilities are provided in Appendix 7, Tables 53–60.
| Trabeculectomy arm | Medical management arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | Initial | Transition | Stage | Initial | Transition | ||||
| To | Year 1 | Year 2 | To | Year 1 | Year 2 | ||||
| Stage 1 | 0.14 | Stage 1 | 0.63 | 0.84 | Stage 1 | 0.13 | Stage 1 | 0.68 | 0.79 |
| Stage 2 | 0.30 | 0.16 | Stage 2 | 0.29 | 0.11 | ||||
| Stage 4 | 0.07 | 0.00 | Stage 4 | 0.00 | 0.11 | ||||
| Stage 5 | 0.00 | 0.00 | Stage 5 | 0.04 | 0.00 | ||||
| Stage 2 | 0.35 | Stage 2 | 0.86 | 0.92 | Stage 2 | 0.34 | Stage 2 | 0.80 | 0.95 |
| Stage 3 | 0.11 | 0.03 | Stage 3 | 0.04 | 0.05 | ||||
| Stage 5 | 0.01 | 0.05 | Stage 5 | 0.12 | 0.00 | ||||
| Stage 6 | 0.03 | 0.00 | Stage 6 | 0.04 | 0.00 | ||||
| Stage 3 | 0.18 | Stage 3 | 0.82 | 0.93 | Stage 3 | 0.21 | Stage 3 | 0.98 | 0.98 |
| Stage 6 | 0.18 | 0.08 | Stage 6 | 0.02 | 0.02 | ||||
| Stage 4 | 0.01 | Stage 4 | 1.00 | 0.75 | Stage 4 | 0.01 | Stage 4 | 0.33 | 1.00 |
| Stage 5 | 0.00 | 0.00 | Stage 5 | 0.33 | 0.00 | ||||
| Stage 7 | 0.00 | 0.00 | Stage 7 | 0.00 | 0.00 | ||||
| Stage 8 | 0.00 | 0.25 | Stage 8 | 0.33 | 0.00 | ||||
| Stage 5 | 0.07 | Stage 5 | 0.53 | 0.90 | Stage 5 | 0.06 | Stage 5 | 0.69 | 0.79 |
| Stage 6 | 0.13 | 0.10 | Stage 6 | 0.08 | 0.05 | ||||
| Stage 8 | 0.20 | 0.00 | Stage 8 | 0.23 | 0.16 | ||||
| Stage 9 | 0.13 | 0.00 | Stage 9 | 0.00 | 0.00 | ||||
| Stage 6 | 0.05 | Stage 6 | 0.91 | 0.84 | Stage 6 | 0.05 | Stage 6 | 0.80 | 0.85 |
| Stage 9 | 0.09 | 0.16 | Stage 9 | 0.20 | 0.15 | ||||
| Stage 7 | 0.02 | Stage 7 | 1.00 | 1.00 | Stage 7 | 0.02 | Stage 7 | 0.60 | 0.67 |
| Stage 8 | 0.00 | 0.00 | Stage 8 | 0.40 | 0.33 | ||||
| Stage 10 | 0.00 | 0.00 | Stage 10 | 0.00 | 0.00 | ||||
| Stage 11 | 0.00 | 0.00 | Stage 11 | 0.00 | 0.00 | ||||
| Stage 8 | 0.05 | Stage 8 | 0.70 | 0.70 | Stage 8 | 0.03 | Stage 8 | 0.83 | 0.92 |
| Stage 9 | 0.00 | 0.10 | Stage 9 | 0.00 | 0.08 | ||||
| Stage 11 | 0.10 | 0.20 | Stage 11 | 0.17 | 0.00 | ||||
| Stage 12 | 0.20 | 0.00 | Stage 12 | 0.00 | 0.00 | ||||
| Stage 9 | 0.02 | Stage 9 | 1.00 | 0.75 | Stage 9 | 0.02 | Stage 9 | 0.75 | 1.00 |
| Stage 12 | 0.00 | 0.25 | Stage 12 | 0.25 | 0.00 | ||||
| Stage 10 | 0 | Stage 10 | 0.00 | 0.00 | Stage 10 | 0.04 | Stage 10 | 0.88 | 1.00 |
| Stage 11 | 0.00 | 0.00 | Stage 11 | 0.12 | 0.00 | ||||
| Stage 11 | 0.05 | Stage 11 | 0.73 | 0.89 | Stage 11 | 0.03 | Stage 11 | 0.83 | 0.71 |
| Stage 12 | 0.27 | 0.11 | Stage 12 | 0.17 | 0.29 | ||||
| Stage 12 | 0.06 | Stage 12 | 1 | 1 | Stage 12 | 0.06 | Stage 12 | 1 | 1 |
Model validation
The model was validated by checking the model structure, calculations and data inputs for technical correctness. 115 The structure was reviewed by clinical experts from our TAGS team to establish that it was appropriate for the disease and its treatment. The robustness of the model to change input values was tested using sensitivity analyses to ensure that any changes to the input values produced changes to the results of the expected direction and magnitude. To establish its external consistency, the model results were compared with outcomes reported in other trials and other economic evaluations. Moreover, we assigned the same time horizon of 24 months of for the trial-based economic evaluation (reported in the first half of this chapter) to the model-based analysis (2 years) and compared the model-based with the within trial-based incremental cost-effectiveness analysis to check the accuracy of the model compared with the within trial analysis.
Base-case analysis
The base-case analysis considered a cohort of 67 year olds (65% were male) as the start of the model. Each person had severe glaucoma in at least one eye. The costs and outcomes were modelled over their estimated lifetimes (i.e. a lifetime horizon adopted). As described above, the analysis adopted a UK NHS and PSS perspective, the cycle length was set at 1 year and a discount rate of 3.5% for costs and benefits was used. 122
The results of the model are presented in ICERs. The ICER is a ratio of the difference in costs divided by the difference in the effectiveness between two alternative strategies. These data can be interpreted as how much society would have to pay for an extra unit of effectiveness. 108 Central to the assessment of cost-effectiveness is the value that society would put on gaining an additional QALY. NICE states that ‘Below a most plausible ICER of £20,000 per QALY, judgements about the acceptability of a technology as an efficient use of NHS resources are based primarily on the cost-effectiveness estimate’122 (© NICE 2018 Guide to the Methods of Technology Appraisal 2013. Available from www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication). Between £20,000 per QALY and £30,000 per QALY, judgements about the acceptability of the technology should take into account factors such as the degree of uncertainty surrounding the calculation of ICERs, the innovative nature of the technology, the particular features of the condition and population receiving the technology, and, where appropriate, the wider societal costs and benefits. Above an ICER of £30,000 per QALY, the case for supporting the technology on these factors has to be increasingly strong. In the absence of a more definitive statement, this report focuses on a willingness-to-pay threshold of £30,000 for a QALY.
To further this aim, we have also adopted the net monetary benefit (NMB) statistic. NMB represents the value of an intervention in monetary terms when a willingness-to-pay threshold for a unit of benefit (e.g. QALY) is known. 125 A net benefit in terms of NHS costs and benefits, expressed in commensurate units, was calculated for each participant in the model according to Equation 3:
where i = individual and WTP = maximum WTP threshold for a QALY.
Evaluation of uncertainty
Both probabilistic and deterministic sensitivity analysis were used to explore parameters and other forms of uncertainty surrounding model-based estimates of cost-effectiveness. 115 The deterministic sensitivity analyses were conducted to investigate the impact of varying key assumptions and/or parameter values used in the base-case analysis. A deterministic sensitivity analysis with the results presented in the form of tornado diagrams was also conducted. For the deterministic sensitivity analysis, we explored the impact of the results of changes in costs of medical management in year 1 (Cost_Year1_MD). For this analysis, the costs of medical management in year 1 were varied between £1500 and £1700. We likewise varied the cost of trabeculectomy in year 1 (Cost_Year1_TB). The cost of this was varied between £2000 and £4000. The cost of year 2 (Cost_Year2) was also varied in the range £700–10,000. Similar deterministic sensitivity analyses were conducted for utilities. For the utility of year 1 for medical management (Util_Year1_MD) and the utility of year 1 for trabeculectomy (Util_Year1_TB), utility values were varied between 0 and 1. Other deterministic sensitivity analyses included changing the time horizon for the analysis from 0 to 30 years; changing the age at which severe glaucoma started (start age) by varying the age on entry into the model from 30 to 80 years; and changing the percentage of males and females (sex ratio), with the percentage of females varying between 40% and 60%.
Alternative scenarios have been explored using PSA. PSA is a technique used in economic modelling that allows the modeller to quantify the level of confidence in the output of the analysis, in relation to uncertainty in the model inputs. 125 For the PSA, all parameter values, including costs and utilities, were defined as statistical distributions using parameters, such as a mean and SD, for a normal distribution, for example. Ranges and distributional assumptions for input parameters were based on the trial data. In a PSA, a set of input parameter values is drawn by random sampling from each distribution, and the model is ‘run’ to provide the results as probability of being cost-effective for different thresholds. This is repeated many times (typically 1000 to 10,000), resulting in a distribution of outputs that can be graphed on the cost-effectiveness plane and analysed. Different distributions are generally appropriate for different types of variable. 125 We assigned gamma distributions for costs and beta distributions for utility data. 121 We also calculated correlations between the coefficients of cost and utility for the variables included logistic regression analyses using Cholesky decomposition and assigned multinormal distributions to these parameters in the model to account uncertainty in the estimated transition probabilities.
A key output of a PSA is the proportion of results that fall favourably (i.e. considered cost-effective) in relation to a given cost-effectiveness threshold. This may be represented using a plot of costs and QALYs and a CEAC. The CEAC is a graph summarising the impact of uncertainty on the results of an economic evaluation, estimating the NMB for each strategy for each iteration of the Monte Carlo simulation so that a CEAC can be generated. The uncertainty surrounding the cost-effectiveness of the treatment is represented on a CEAC according to the probability that the intervention will be cost-effectiveness at a particular willingness-to-pay threshold.
Results
This section reports the proportion of patients in the cohort models by the level of severity of glaucoma in both eyes over time. It also reports the model-based cost-effectiveness results for primary medical treatment with primary augmented trabeculectomy (glaucoma surgery) for patients presenting with advanced glaucoma in terms of costs and QALYs. Results in terms of costs and QALYs are presented for each arm, with costs and benefits discounted at 3.5% per annum. 122
Progression from one heath state to another
In the analysis, all patients start with severe glaucoma in at least one eye (see Figure 9). Patients were assumed to progress from less severe disease states to more severe disease states. Once patients were in a more severe state, they could either remain in that state or continue to progress to the next, more severe, disease state.
For each cohort of patients with advanced glaucoma, we determined disease progression from lower stages of glaucoma to higher stages based on the TAGS trial data. We used the GSS2 progression rates to capture the progression for both the trabeculectomy arm and the medication treatment arm who progressed from less severe to more severe disease states. We generated plots of progression through disease states on a year-by-year basis for both arms for a 30-year time horizon (Figure 10).
FIGURE 10.
Markov tracings of disease states over time for (a) the trabeculectomy arm; and (b) the medical management arm.
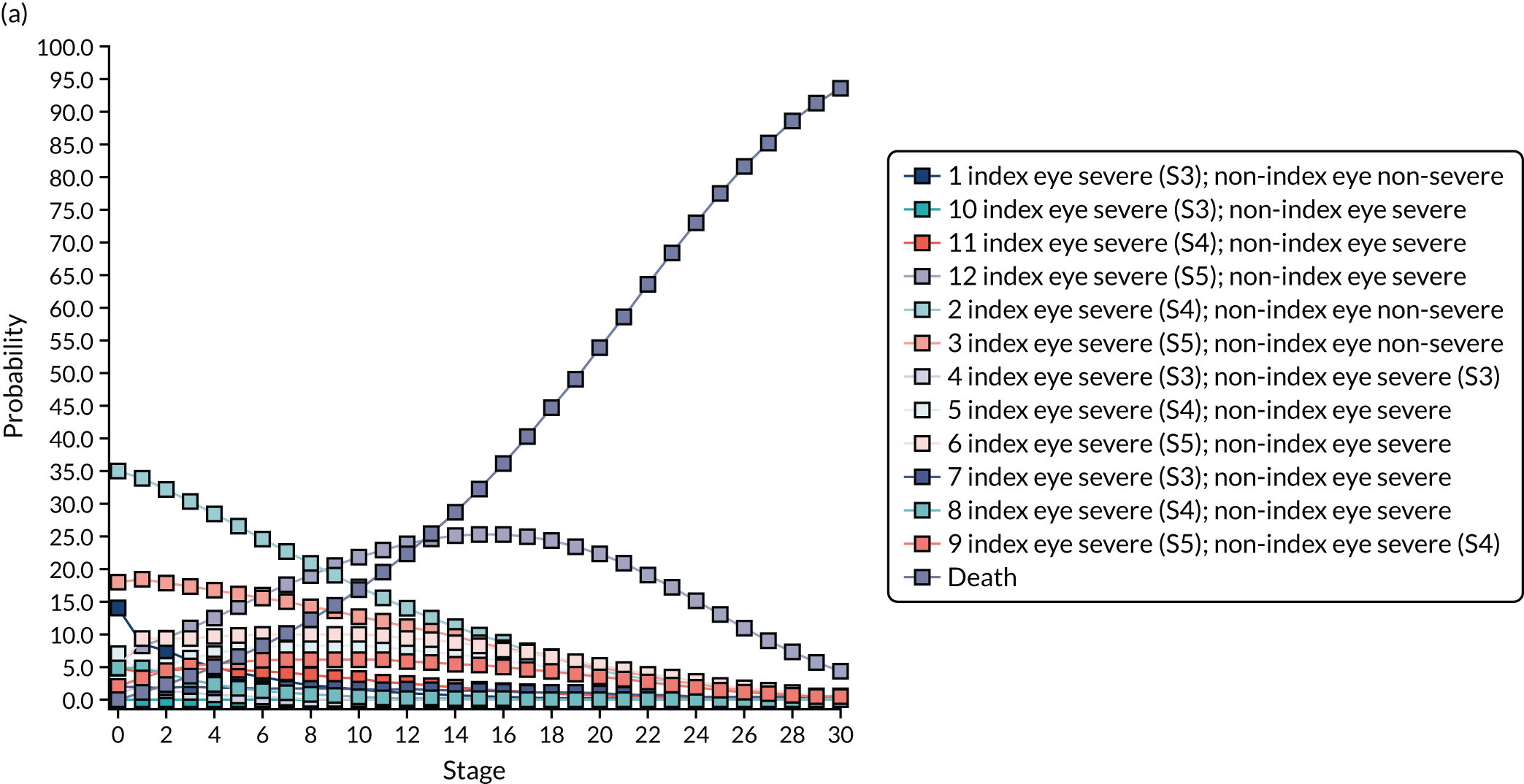
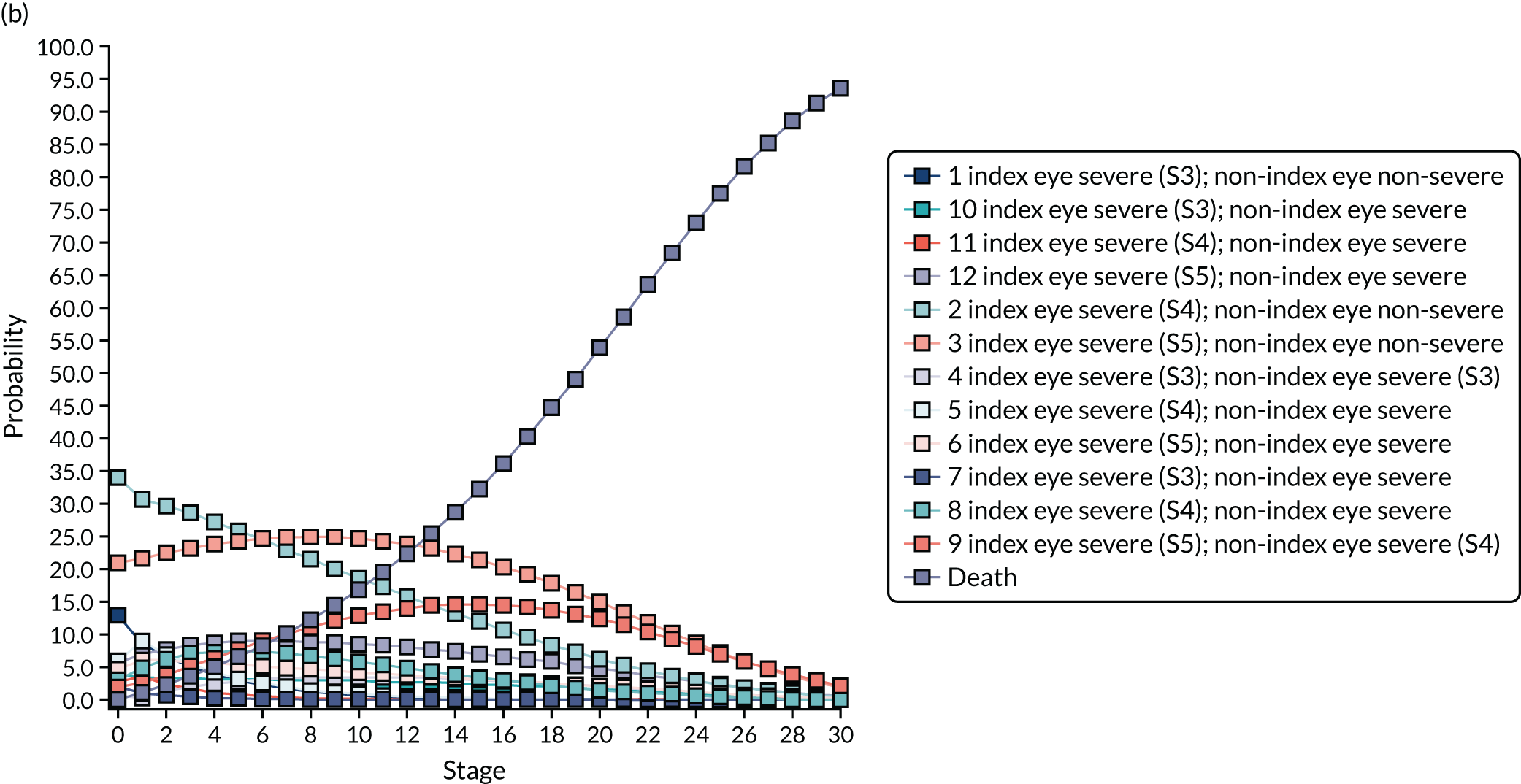
Incremental costs, quality-adjusted life-years and cost-effectiveness
Table 21 shows the QALYs (for EQ-5D-5L, HUI-3 and GUI, presented separately), total cost, incremental cost per QALY and NMB for three time horizons (and utility measures). In the base-case analysis (lifetime horizon and EQ-5D-5L-based QALYs), trabeculectomy had an additional cost of £2687, an additional 0.28 QALYs and an incremental cost per QALY gained of £9679 compared with primary medical treatment. Furthermore, the results of the PSA show that, should society be willing to pay £20,000 per QALY, the likelihood of trabeculectomy being cost-effective compared with medical treatment would be 73%.
| Time horizon | Treatment arm | Cost (£) | ΔCost (£) | QALY | ΔQALY | ICER (ΔCost/ΔQALY) (£) | NMB | Probability cost-effective at Rc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | £10,000 | £20,000 | £50,000 | ||||||||
| EQ-5D-5L-based QALYs | |||||||||||
| 2-year time horizon | Trabeculectomy | 3436 | 2106 | 1.23 | 0.04 | 47,663 | 21,108 | 0.10 | 0.26 | 0.39 | 0.05 |
| Medical management | 1330 | 1.18 | 22,330 | 0.90 | 0.74 | 0.61 | 0.50 | ||||
| 10-year time horizon | Trabeculectomy | 5421 | 2362 | 3.72 | 0.17 | 13,911 | 69,024 | 0.08 | 0.40 | 0.59 | 0.73 |
| Medical management | 3059 | 3.55 | 67,990 | 0.92 | 0.60 | 0.41 | 0.27 | ||||
| Lifetime horizon | Trabeculectomy | 7273 | 2687 | 5.92 | 0.28 | 9679 | 111,052 | 0.08 | 0.50 | 0.73 | 0.85 |
| Medical management | 4586 | 5.64 | 108,187 | 0.92 | 0.50 | 0.27 | 0.15 | ||||
| HUI-3-based QALYs | |||||||||||
| 2-year time horizon | Trabeculectomy | 3436 | 2106 | 1.17 | 0.05 | 39,724 | 19,978 | 0.09 | 0.29 | 0.40 | 0.52 |
| Medical management | 1330 | 1.12 | 21,024 | 0.91 | 0.71 | 0.60 | 0.48 | ||||
| 10-year time horizon | Trabeculectomy | 5421 | 2362 | 3.58 | 0.22 | 10,506 | 66,115 | 0.10 | 0.50 | 0.65 | 0.75 |
| Medical management | 3059 | 3.35 | 63,980 | 0.90 | 0.50 | 0.35 | 0.25 | ||||
| Lifetime horizon | Trabeculectomy | 7273 | 2687 | 5.70 | 0.38 | 7016 | 106,779 | 0.06 | 0.62 | 0.81 | 0.88 |
| Medical management | 4586 | 5.32 | 101,806 | 0.94 | 0.38 | 0.19 | 0.12 | ||||
| GUI-based QALYs | |||||||||||
| 2-year time horizon | Trabeculectomy | 3436 | 2106 | 1.24 | 0.01 | 147,247 | 21,302 | 0.09 | 0.18 | 0.28 | 0.41 |
| Medical management | 1330 | 1.23 | 23,122 | 0.91 | 0.82 | 0.72 | 0.59 | ||||
| 10-year time horizon | Trabeculectomy | 5421 | 2362 | 3.73 | 0.1 | 24,179 | 69,269 | 0.08 | 0.26 | 0.44 | 0.60 |
| Medical management | 3059 | 3.64 | 69,677 | 0.92 | 0.74 | 0.56 | 0.40 | ||||
| Lifetime horizon | Trabeculectomy | 7273 | 2687 | 5.92 | 0.16 | 16,805 | 111,165 | 0.06 | 0.32 | 0.55 | 0.74 |
| Medical management | 4586 | 5.76 | 110,655 | 0.94 | 0.68 | 0.45 | 0.26 | ||||
The model-based estimates of mean costs and QALYs at 2 years indicate that trabeculectomy for the treatment of glaucoma is expected to cost an additional £2106, on average, and to provide an average QALY gain of 0.04 compared with medical management. The corresponding incremental cost per QALY for surgery compared with medical management would be £47,663 (see Table 21). At a 10-year time horizon, the corresponding figures are £2362 and 0.17 QALYs (ICER £13,911). When running the model over 10 years, the results of the PSA show that, should society be willing to pay £20,000 per QALY, the likelihood of trabeculectomy being cost-effective compared with medical treatment would be 59% (see Table 21). Table 21 also reports the incremental cost, incremental QALY and incremental cost per QALY when QALYs are based on HUI-3 or GUI utility values. Figure 11 presents a plot of costs and QALYs in the base-case analysis. Similar results for the analyses using HUI-3- and GUI-based QALYs are presented in Appendix 7, Figures 21 and 22.
FIGURE 11.
Cost-effectiveness analysis in the base-case analysis.
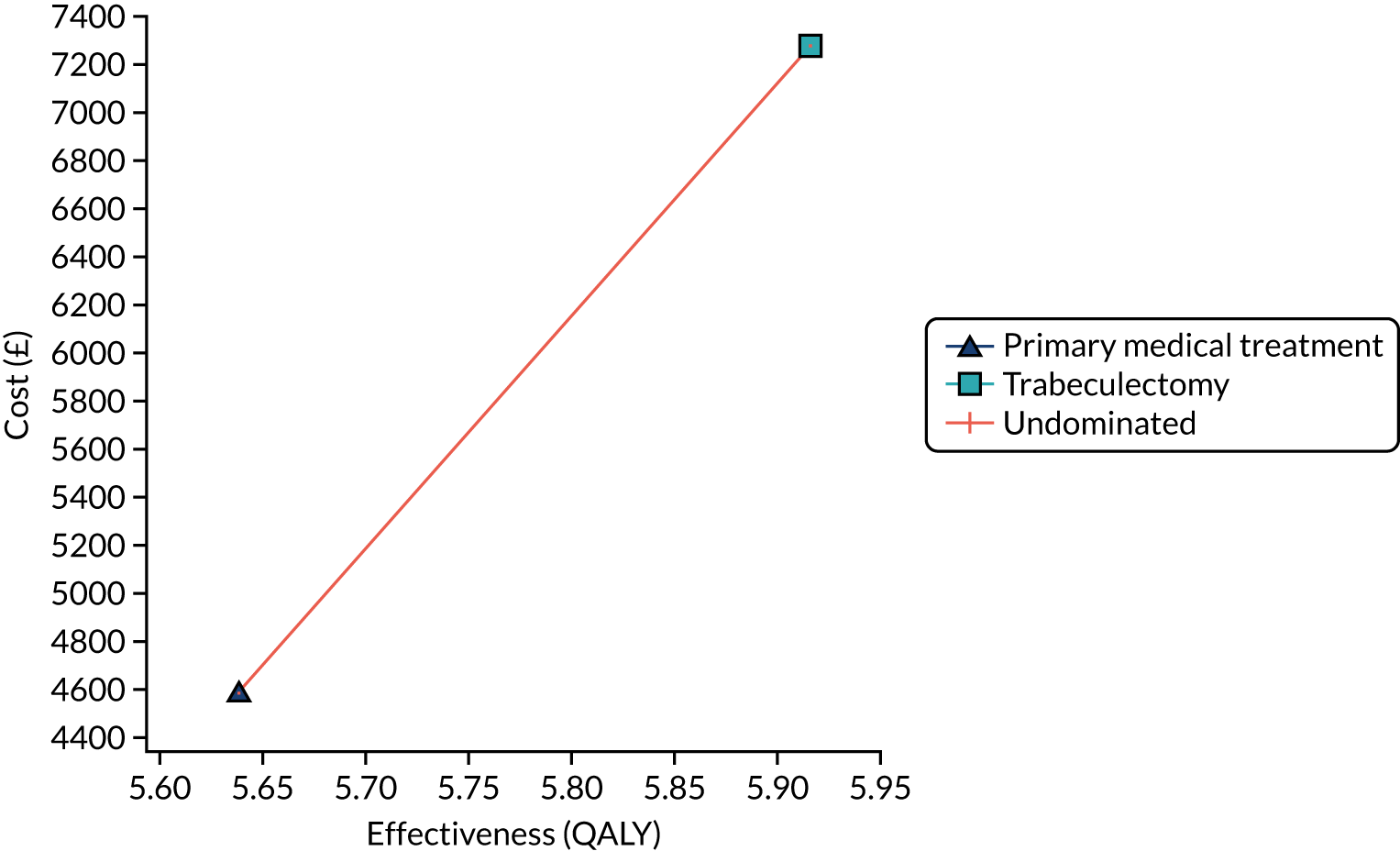
Sensitivity analysis
One-way deterministic sensitivity analysis
Deterministic sensitivity analysis shows that the model-based findings are generally robust to the changes examined. Main model inputs were varied in the sensitivity analyses to determine whether or not they may affect the ICER of trabeculectomy versus medical management (Table 22). The results of these sensitivity analyses are depicted in the form of a tornado diagram (Figure 12). The tornado diagram helped to identify main inputs that could be altered to make trabeculectomy more or less cost-effective relative to medical management. Similar analyses are shown in Appendix 7, Figures 23 and 24, for the HUI-3 and GUI, respectively.
| Parameter | Value | ICER (£ per QALY) | ||
|---|---|---|---|---|
| EQ-5D-5L | HUI-3 | GUI | ||
| Cost of year 1 (trabeculectomy) (£) | 2000 | 5594 | 4055 | 9713 |
| 3000 | 9196 | 6666 | 15,967 | |
| 4000 | 12,798 | 9277 | 22,222 | |
| Cost of year 1 (medical management) (£) | 700 | 10,986 | 7963 | 19,076 |
| 1100 | 9546 | 6919 | 16,574 | |
| 1500 | 8105 | 5875 | 14,072 | |
| Utility of year 1 (trabeculectomy) | 0.2 | Dominated | Dominated | Dominated |
| 0.6 | 70,594 | 14,483 | Dominated | |
| 1 | 6134 | 4589 | 8625 | |
| Utility of year 1 (medical management) | 0.2 | 3012 | 2819 | 3331 |
| 0.6 | 5461 | 4856 | 6606 | |
| 1 | 29,197 | 17,528 | 395,757 | |
| Cost of year 2 (£) | 700 | 7726 | 5600 | 13,415 |
| 1100 | 7878 | 5711 | 13,679 | |
| 1500 | 8031 | 5821 | 13,944 | |
| Utility of year 2 | 0.2 | 52,124 | 50,146 | 96,391 |
| 0.5 | 34,466 | 33,590 | 49,497 | |
| 1 | 20,546 | 20,231 | 25,087 | |
| Time horizon (years) | 2 | 47,663 | 39,724 | 147,247 |
| 10 | 13,910 | 10,506 | 24,179 | |
| 30 | 9679 | 7016 | 16,805 | |
| Age at (severe) glaucoma (years) | 30 | 7924 | 5614 | 13,914 |
| 42 | 8037 | 5703 | 14,099 | |
| 55 | 8406 | 5997 | 14,701 | |
| 67 | 9776 | 7093 | 16,972 | |
| 80 | 14,438 | 10,769 | 25,930 | |
FIGURE 12.
Tornado diagram for the main parameters (EQ-5D-5L measure). EV, expected value; WTP, willingness to pay.
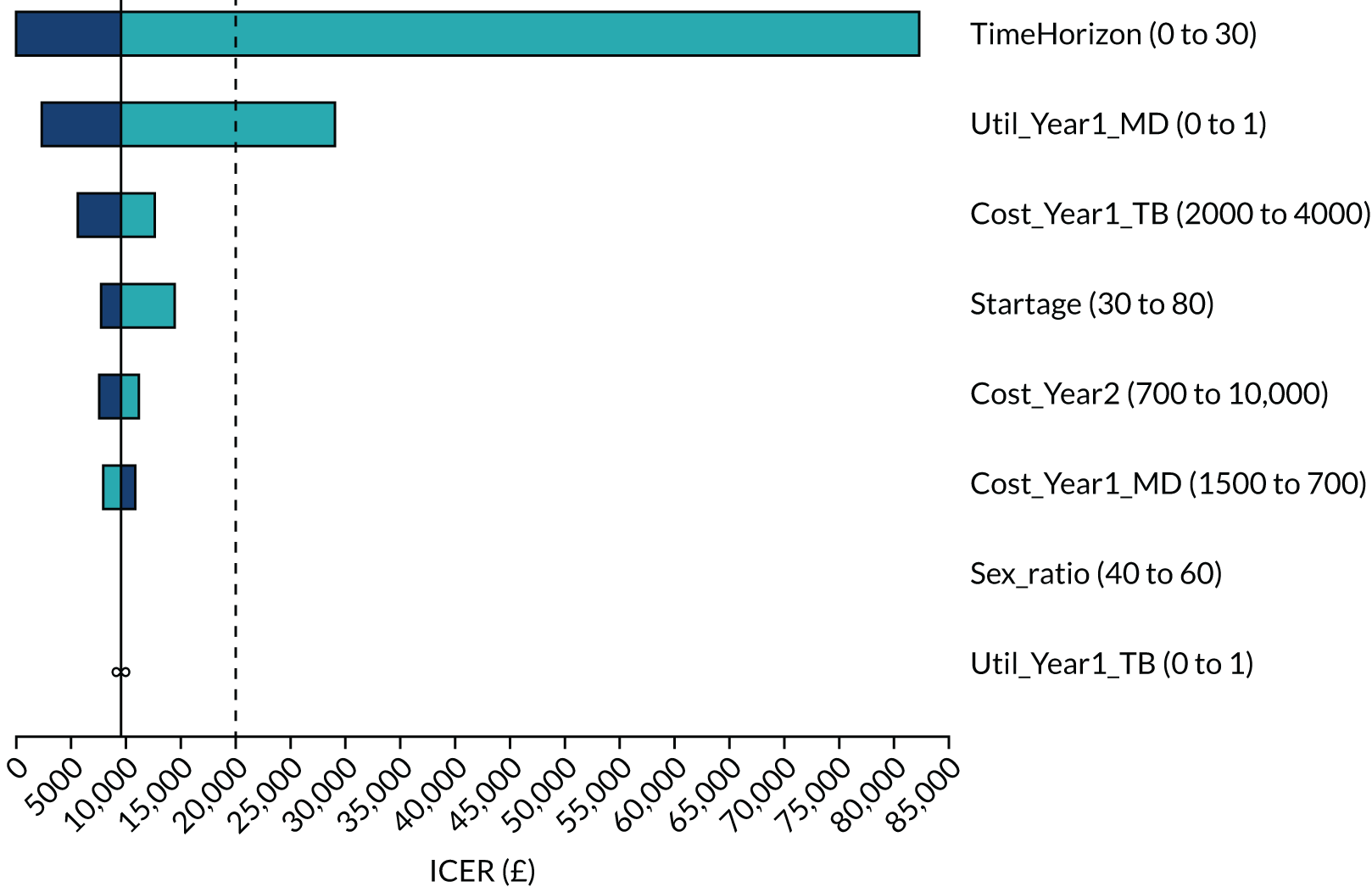
Impact of missing data
The base-case analysis made use of the EQ-5D-5L measure and lifetime horizon duration. Running multiple imputation shows a slight difference in the incremental cost value (£2707 vs. £2687) and no difference in the incremental QALYs value (0.28) compared with the base-case analysis. The input parameters of the model were based on a data set where multiple imputations (MI) were performed for missing data. These data were used to parameterise uncertainty surrounding the joint incremental costs and effects. This is presented graphically as confidence ellipses on the incremental cost-effectiveness plane (Figure 13). Moreover, the model-based CEACs based on the lifetime horizon are presented in Figure 14. The related results of all other scenario analyses (HUI-3 and GUI QALYs measures) are presented in Appendix 7; for the incremental cost-effectiveness plane these are shown in Figures 25 and 26, respectively. The CEACs are shown in Appendix 7, Figures 27 and 28, for the GUI and HUI-3, respectively.
FIGURE 13.
Incremental cost-effectiveness scatterplot: trabeculectomy vs. medical treatment in the base-case analysis. WTP, willingness to pay.
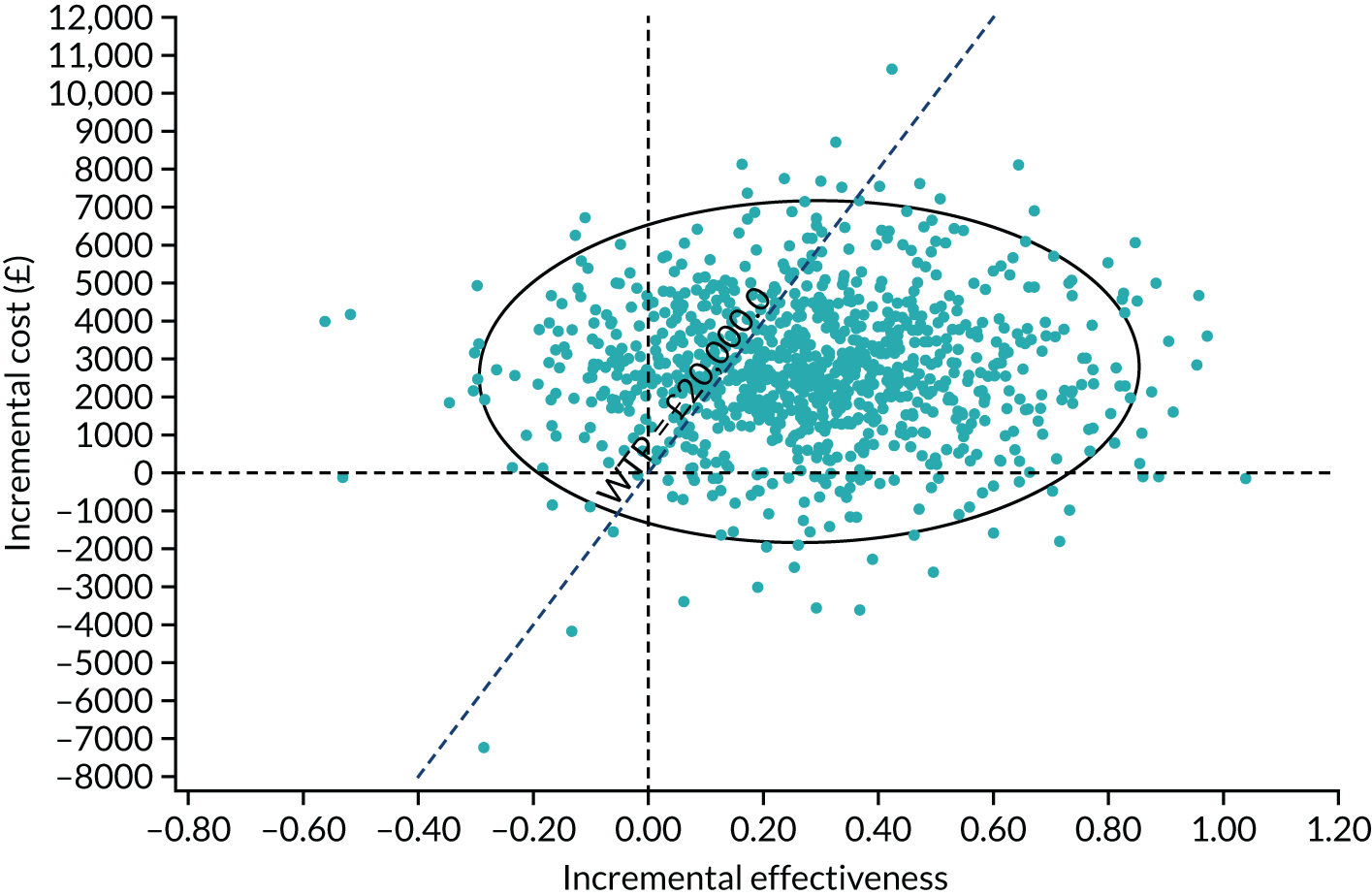
FIGURE 14.
Model-based CEAC for the base-case analysis (lifetime horizon; EQ-5D-5L measure).
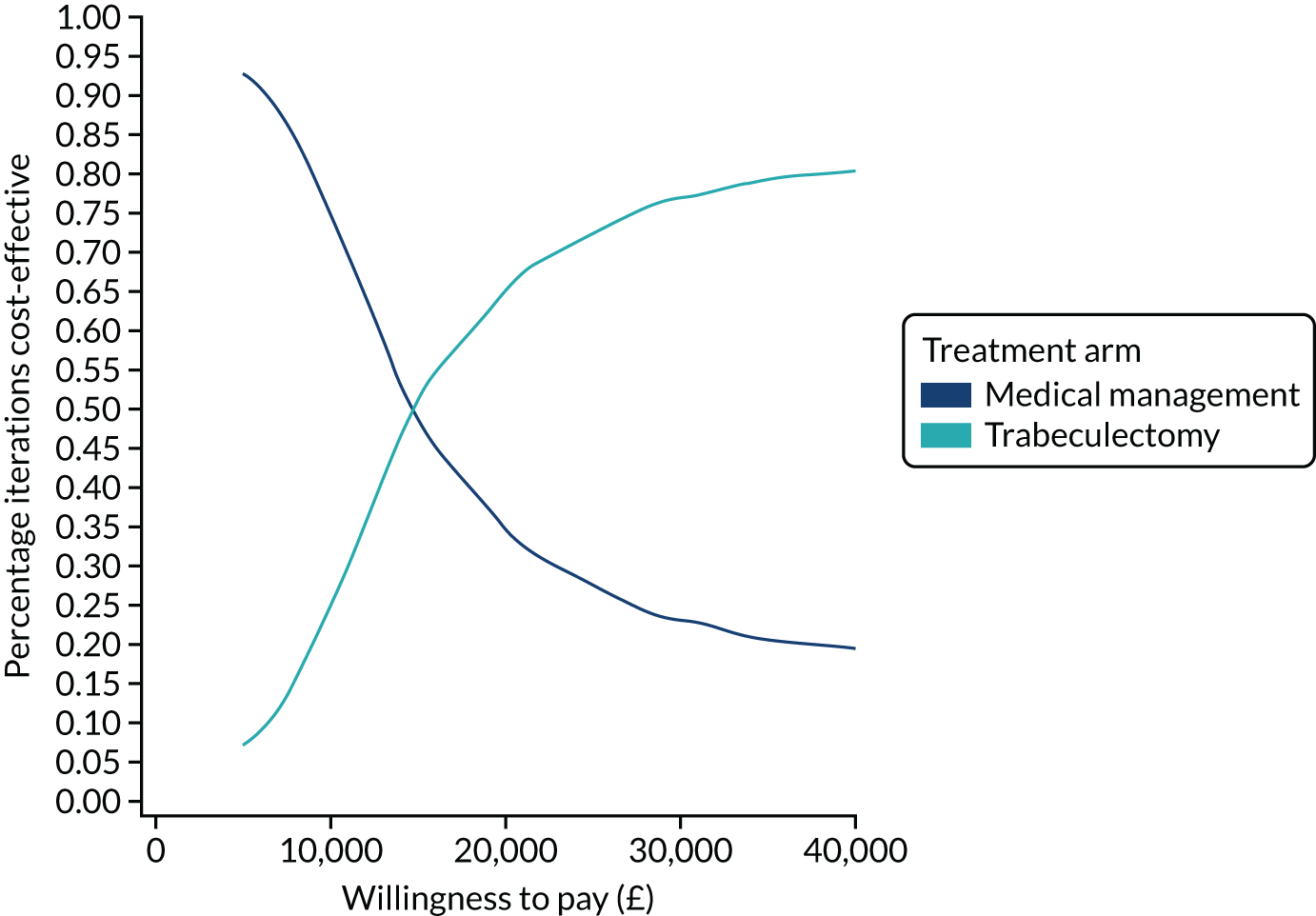
Discussion
The focus of the present model-based analysis was to perform a comparison of the cost-effectiveness of trabeculectomy and medication for treatment in patients with severe glaucoma. This analysis was completed alongside the trial-based economic evaluation and was based on TAGS data, but sought to extrapolate findings over the expected lifetime of patients treated with either trabeculectomy or medical therapy.
Chapter 7 Discussion
Summary of findings
This multicentre RCT compared initial treatment of advanced primary OAG with either augmented trabeculectomy or medical management. Patient HRQoL, clinical effectiveness and cost-effectiveness were investigated in 453 participants from 27 NHS ophthalmology departments in the UK. The duration of the trial was 24 months. The study provided no evidence of a difference in the primary outcome of vision HRQoL as measured by the VFQ-25, and a meaningful difference favouring either arm is not compatible with the data from the trial given the precision of the CIs. There was a sustained reduction in IOP in both arms, with a reduction of 21% in the medical management arm and 38% in the trabeculectomy arm compared with IOP at baseline, with a much smaller requirement for antiglaucoma eye drops in the trabeculectomy arm. The DCE questionnaire was completed by 70% of participants at 27 months post randomisation. Participants had a strong preference for reduced difficulty with central and near vision, mobility, and activities of daily living. Revised reference-based weights were estimated for the GUI based on the logistic regression results and these utility values were incorporated into the economic evaluation to estimate QALYs. In the within-trial analysis, the small increase in quality of life generated by surgery was not offset by the additional upfront costs and, therefore, trabeculectomy is unlikely to be cost-effective within the 24-month time horizon of the trial. When the results from the decision model were considered, trabeculectomy was associated with an additional cost of £2687, an additional 0.28 QALYs and an incremental cost per QALY of £9679 compared with primary medical treatment, and the likelihood of a trabeculectomy being considered cost-effective over a £0 to £20,000 range of society’s willingness to pay for a QALY was 73%. Over shorter time horizons, the incremental cost per QALY of surgery compared with medical therapy increased (£47,663 over a 24-month time horizon). The results appeared robust over the other sensitivity analyses considered.
Primary outcome
Quality of life
The outcome of management of glaucoma that is most important to patients is their ability to continue to live an independent life and maintain their QoL. 126,127
Visual Function Questionnaire-25
The primary outcome for TAGS is patient-reported vision-related QoL, as measured by the VFQ-25 at 24 months. The VFQ-25 is primarily a vision-related HRQoL instrument. Our sample size calculations indicated that a study with 190 participants in each arm would have 90% power at the 5% significance level to detect a difference in means of 0.33 of a SD; this translates to 6 points on the VFQ-25. 76–78
There was a slight reduction in VFQ-25 scores in both arms at 24 months compared with baseline. It would be expected that a reduction in HRQoL would occur as participants age. In the Berlin Ageing Study, the authors concluded that physiological reduction in vision associated with ageing materially affects QoL; however, the authors noted that the correlation with VA was small and small changes in vision do not translate into substantial impairments of daily life. 128
There was no evidence of a difference in the composite VFQ-25 scores between the trabeculectomy or the medical management arm of the study at any of the time points at which they were measured.
There was a suggestion from the subscale analysis of a reduction in the ‘role difficulties’ (MD –4.60, 95% CI –8.70 to –0.50; p = 0.028) and ‘general vision’ (MD –3.17, 95% CI –6.03 to –0.32; p = 0.029) subscales at 4 months in the trabeculectomy arm. This may reflect some reduction in visual function and activity restriction at this time, which may correspond to recovery from trabeculectomy surgery. This difference disappeared by 12 months, as would be expected following surgery recovery. At the 12-month time point, there is reduction in the ‘general health’ subscale (MD 4.86, 95% CI 0.23 to 9.49; p = 0.040) in the medical management arm. This may reflect the fact that a proportion of those in the medical management arm were aware that their glaucoma was uncontrolled or progressing (0.59, 95% CI 0.38 to 0.910; p = 0.018), resulting in the requirement for trabeculectomy or ongoing recovery from the operation. This effect disappeared by 24 months. However, care must be taken when interpreting these results owing to multiple testing.
The VFQ-25 score has been shown to be affected by disease severity in the better and also in the worse eye,46,129 and is also influenced by severity of glaucoma in both eyes, as measured by binocular VF loss. In patients with advanced glaucoma, there is a disproportionate reduction in VFQ-25 score for each additional 1-unit reduction in VFMD compared with those with less advanced disease. 130 In TAGS, there was no evidence of a difference in the VFMD score and a marginal difference in the VA score between arms; thus, it is not surprising that there was no evidence of a difference in VFQ-25.
We believe that this provides a comprehensive evaluation of vision-related QoL and is sufficient time to capture the short-term differences in effects and to accurately profile the different patient pathways associated with each intervention.
A similar result was observed in the TVT (Tube Versus Trabeculectomy) study, which compared trabeculectomy against the Bearveldt glaucoma drainage device. 51 This study also used VFQ-25. Patients were followed up for 5 years and there was no difference in VFQ-25 at any point in the study. 51 The TVT study, like TAGS, recruited patients with advanced glaucoma in the index eye. A previous primary medicine compared with trabeculectomy study (CIGTS) recruited patients with early glaucoma and used a different patient-reported outcome measure, the Vision Activities Questionnaire, but also found no difference between arms for the duration of the study. 131
Secondary outcomes
Quality of life
EQ-5D-5L
There was no evidence of a difference between arms in EQ-5D-5L score at any of the time points measured, suggesting no superiority of either intervention in terms of measures of health status. The value of the EQ-5D-5L in evaluating QoL in glaucoma, and particularly when disease severity changes, is uncertain. Only a small number of studies have evaluated EQ-5D-5L in glaucoma patients and the conclusions have been variable. 124,132–134 Two recently reported RCTs of early glaucoma patients, the LiGHT59,135 and UKGTS136 trials, both found poor sensitivity of the EQ-5D-5L to detect change. In the UKGTS study, even when comparing VF progressors with non-progressors, there was no evidence of a difference in EQ-5D-5L results. 136
Health Utility Index version 3
The HUI-3 is a generic patient-reported outcome measure that includes a vision-specific domain; however, there was no evidence of a difference between interventions using this instrument. There is little literature exploring the use of HUI-3 in glaucoma, although one study did demonstrate a difference between cases and controls. 124
Glaucoma Utility Index
The GUI is a glaucoma-specific outcome measure that is designed to quantify QoL in glaucoma patients. 14 However, once again, there was no evidence of a difference between the interventions at any time point. This is consistent with a similar observation for GUI from the LiGHT study. 59,135
Patient perception of glaucoma getting worse
There was a perception among patients in the medical management arm that their glaucoma was progressing at the 12-month time point. As suggested above, this may coincide with more active glaucoma management involving surgery in some patients in the medical management arm around this time. This effect has disappeared by 24 months. This patient perception is not picked up by any of the composite scores of the instruments used.
The QoL measurement instruments measure binocular vision and, therefore, outcomes are reported at the vision level rather than an individual eye level. This may affect the ability of changes in an eye with poor VFs to influence the QoL score if the VF in the other eye is good. In TAGS in the non-index eye VFMD was –6.10 (SD 7.7) in the trabeculectomy arm and –6.13 (SD 7.1) in the medical management arm and there was no change of MD in either arm over the 24-month follow-up period.
Clinical effectiveness
Intraocular pressure
Both of the interventions employed in TAGS are recognised to effectively lower IOP and are recognised by NICE. 65
In TAGS, the investigators were advised to use the guidance provided by the Canadian perspectives in glaucoma management: setting target IOP range to set the IOP level for individual patients; this suggests an IOP for advanced disease of < 15 mmHg, with reduction of ≥ 30% from the baseline IOP. 137
There was a clear reduction in IOP in both arms for the duration of the study from similar baseline values (holding medical management implemented at diagnosis). In the trabeculectomy arm, IOP fell to 12.4 mmHg (SD 5.73 mmHg) at 4 months and remained at around 12 mmHg for the remainder of the study. In the medical management arm, the IOP reduction was initially 4 mmHg lower than that achieved in the trabeculectomy arm [16.4 mmHg (SD 4.12 mmHg) at 4 months and 16.12 mmHg (SD 4.54 mmHg) at 12 months]. There was a further reduction in the medical management arm to 15.07 mmHg (SD 4.8 mmHg) at 24 months, but this reduction was still 3 mmHg lower than that achieved in the trabeculectomy arm. Therefore, for the duration of the study an additional 3–4 mmHg reduction in IOP was achieved in the trabeculectomy arm compared with the medical management arm.
At diagnosis, the IOP was 26.9 mmHg (SD 9.1 mmHg) in the trabeculectomy arm and 25.9 mmHg (SD 8.4 mmHg) in the medical management arm; therefore, the 24-month IOPs represent a reduction of 14.9 mmHg (55%) in the trabeculectomy arm and 10.83 mmHg (42%) in the medical management arm.
The value of medical management in prevention of VF or optic disc progression in patients with ocular hypertension, primary OAG and normal tension glaucoma is well established. 62 Two recent RCTs, both using prostaglandin analogues as primary treatment, as per NICE guidelines,65 have been undertaken. In the UKGTS,58 the baseline untreated IOP was 19.6 mmHg (SD 4.6 mmHg) in the medical management arm; by 24 months, an average reduction of 4.0 mmHg (SD 3.4 mmHg) had been achieved, resulting in an IOP of 15 mmHg, mostly on a single medication. The use of prostaglandin monotherapy had a clear benefit in terms of lowering IOP compared with no treatment, and this translated into a statistically and clinically significant reduction in VF progression rate at 24 months. 58 There was a 50% difference in VF progression in the medical management arm compared with the no treatment arm, further establishing the relationship between IOP lowering and VF progression.
The LiGHT trial59 compared selective laser trabeculoplasty with medical management in the treatment of patients presenting with ocular hypertension and mainly early primary OAG. In the primary OAG group, the baseline IOP was 23 mmHg (IQR 20–27 mmHg). In the medical management arm, IOP fell to a mean of 16.6 mmHg, and only 35% of patients required more than one type of eye drops to achieve this. Despite these results, however, 11 patients required trabeculectomy to prevent further progression.
In the medical management arm of the TAGS trial, a much greater reduction in IOP was achieved in terms of both the absolute IOP reduction and the percentage drop in IOP. This probably reflects the guidance provided for IOP lowering and the recognition that vision is more likely to be preserved in patients with advanced disease if IOP can be reduced.
The IOP lowering achieved in the trabeculectomy arm of the TAGS trial is consistent with current results61,138,139 reported from the NHS. Kirwan et al. ’s61 multicentre service evaluation of augmented trabeculectomies found that the mean IOP achieved at 24 months following augmented trabeculectomy was 12.4 mmHg (SD 4.0 mmHg). 61 Stead and King138 looked at a group of eyes with advanced glaucoma (mean deviation < –20 dB) and found that, at 24 months, the mean IOP in the cohort was 13.1 mmHg (SD 5.4 mmHg) and the mean number of different types of eye drops required was 0.6. In both of these studies, longer-term follow-up indicated a continued sustained low IOP.
Since the most recently undertaken trial of primary trabeculectomy compared with medical management, CIGTS,31 which recruited in the early 1990s, there have been significant advances in the technique of trabeculectomy surgery140 in terms of both anti-metabolite use and post-operative management, which now includes a series of routinely undertaken adjustments to improve IOP control. 141 Therefore, both the safety and the efficacy of trabeculectomy have been improved in this time. 142,143 Similarly, in CIGTS, the primary medical management was initial treatment with a beta-blocker followed by escalating medical therapy, as required. Since CIGTS recruited, several further agents have been introduced, and one type in particular, the prostaglandin analogues, has superior IOP lowering, can be applied once per day and has a low side effect profile and, therefore, is well tolerated, improving both persistence and adherence to the medication. 63 These improvements may reflect that in CIGTS the mean IOP was 15 mmHg in the trabeculectomy arm and 18 mmHg in the medical management arm post treatment; in both cases this was considerably higher than our results. One caveat, however, is that TAGS patients have advanced glaucoma and it is possible that clinicians may have undertaken a more aggressive treatment approach as indicated by consensus guidance to pursue a lower IOP and treat more aggressively, thus aiming for and achieving a lower IOP.
A sustained reduction in IOP is recognised to be the most effective method of preventing further VF loss in glaucoma. 33,34,58,144 In AGIS, long-term follow-up over 8 years indicated that lower IOP in both the predictive analysis (early IOP level behaviour) and the associate analysis (long-term IOP level behaviour) resulted in less VF loss. In the associate analysis, the cohort with the lowest sustained IOP, < 18 mmHg at all follow-up visits, had virtually no VF loss progression. For this arm, this equated to a mean IOP of 12.3 mmHg, which is virtually identical to that achieved for the trabeculectomy arm of TAGS. A difference of 3 mmHg between treatment arms is a clinically important difference and achieving a sustained IOP of 12 mmHg is a clinically important level of IOP reduction for minimising further VF progression.
Glaucoma drop usage
This profile of IOP control is also reflected in the need for glaucoma eye drops. At 4 months, only 61 participants (28%) in the trabeculectomy arm were using eye drops, equivalent to a mean of 0.43 (0.79) different types of eye drops and reflecting the fact that the majority of patients had undergone trabeculectomy and, consequently, were drop free. At the same time point, 210 (96%) participants in the medical management arm were using a mean of 1.77 (0.92) different types of eye drops. At 12 months, eye drop usage in the medical management arm remained unchanged, whereas there was further reduction in the trabeculectomy arm, probably because some patients underwent trabeculectomy as part of treatment allocation after only 4 months. It is also important to remember that 16 patients declined surgery following randomisation to the trabeculectomy arm and, therefore, remained on eye drops for IOP control. By 24 months, there was a small increase in the number of eye drops in the trabeculectomy arm, suggesting some additional medications required to maintain IOP at a sufficiently low level, and this is consistent with observations from other studies reporting trabeculectomy outcomes. 138,145 By contrast, in the medical management arm, there was a reduction in the mean number of eye drops used and the number of participants using them, reflecting the fact that, during this period, 39 patients (17.3%) required trabeculectomy to control their IOP adequately, following failure of medical management, and subsequently stopped eye drops.
Visual acuity
There is no evidence of a difference in logMAR VA at baseline between the arms. However, at 4 months the VA in the trabeculectomy arm dropped from 0.15 (SD 0.25) to 0.25 (SD 0.31), whereas it remains unchanged in the medical management arm (p < 0.001). This is likely to correspond to some visual reduction experienced by patients undergoing trabeculectomy during the postoperative recovery phase and has resolved by 12 months. At 24 months there has been some further deterioration, VA falling to 0.21 (SD 0.28) in the trabeculectomy arm compared with 0.16 (SD 0.26) in the medical management arm (p < 0.006). It is likely that this deterioration is because of the development of cataract in the trabeculectomy arm that has not yet been removed. The development of cataract is well recognised following trabeculectomy146 and in the CIGTS study cataract extraction was significantly higher in the trabeculectomy arm as opposed to the medical management arm in the first 5 years following randomisation. The hazard ratio for cataract extraction in the trabeculectomy arm compared with the medical management arm was 3.76 (95% CI 2.16 to 6.54; p < 0.001) for CIGTS. 147 This difference was clearly visible by 24 months, at which those in the trabeculectomy arm were nearly six times more likely than those in the medical management arm to have had cataract surgery.
Visual field mean deviation
At 24 months, the mean VFMD was –15.15 in the trabeculectomy arm and –15.42 in the medical management arm with no evidence of a difference (MD 0.18, 95% CI –0.58 to 0.94; p-value 0.645). There was no difference in VF progression at 24 months between the arms. To some extent, this is to be expected as 24 months is a relatively short period of time to detect change in glaucoma, especially between arms that have had a good therapeutic response to their interventions. This is particularly true when using a global measure of VF, such as VFMD. In the UKGTS, a difference was demonstrated between arms with a similar IOP difference between treatment arms; however, in UKGTS an intensive VF-testing regime requiring 20 VFs over a 24-month period was undertaken that would not be pragmatic or possible in the context of a publicly funded health system. In addition, for UKGTS they measured individual VF progression using the GPA algorithm, which is not suitable for patients with advanced VF loss. It is possible that extended follow-up for TAGS will reveal differences in VF progression, as was demonstrated in CIGTS with long-term follow-up of patients with more advanced glaucoma43 and would be expected if lower IOP is maintained in one of the treatment arms. 34
Need for cataract surgery
We have estimated cataract formation pragmatically as the need to undergo cataract surgery to improve vision. In the trabeculectomy arm, 28 participants required surgery by 24 months compared with 27 participants in the medical management arm. This is far fewer in the trabeculectomy arm than would be expected from previous studies. 147 In CIGTS, there was a six-fold difference in the need for cataract surgery at 24 months. However, in TAGS there is worsening VA recorded in the trabeculectomy arm at 24 months, which may reflect the development of subclinical cataract in this arm not yet causing sufficient visual reduction to warrant intervention. There may also be some caution on behalf of clinicians and patients to undertake cataract surgery until absolutely necessary because of the risk of compromising successful bleb functioning and causing trabeculectomy failure; therefore, in some cases cataract surgery may be delayed. 148–150
There was no evidence of a difference between arms for maintaining eligibility to drive at 24 months or eligibility for sight loss registration.
Safety
In the UK, consultants did not consider medical management to be better or equivalent to primary augmented trabeculectomy when surveyed; however, there was a concern about exposing patients to the risks associated with trabeculectomy and this was the major barrier to carrying out primary surgery. However, clinicians indicated that if robust evidence existed that supported primary surgery they would change their practise accordingly. 35 The perceived ‘high risk’ associated with trabeculectomy may stem from the National Trabeculectomy Study performed in the late 1990s, which indicated particularly high rates of early complications following trabeculectomy. 151 Improvement in surgical techniques has greatly reduced these perceived risks. 142,143 In addition, virtually all surgery is now carried out by fellowship-trained glaucoma specialists instead of primarily general ophthalmologists, as was the case at the time of the National Trabeculectomy Survey.
One of the main concerns of clinicians is the unexplained irreversible loss of central vision – ‘wipe-out’ – believed to occur in patients with advanced glaucoma undergoing trabeculectomy. 152 There is no suggestion of unexplained vision loss occurring immediately after surgery in TAGS, which indicates no episodes of wipe-out. In two prospective studies specifically exploring the development of ‘wipe-out’, no cases of severe, irreversible, unexplained central vision loss were identified, which suggests that ‘wipe-out’, if it exists, is a very infrequent occurrence. 153,154
Although visual loss is not uncommon after trabeculectomy, in the majority of cases this is reversible and explained;153–156 this is the case in TAGS, for which all episodes of visual loss have been identified.
Vision loss
There were three cases of severe VA loss reported as losing > 10 lines logMAR VA during the course of the trial. All participants were allocated to the trabeculectomy arm. For two of these patients, vision loss resulted from progressive glaucoma damage as a result of inadequately controlled glaucoma. In the third patient, who did not complete the 24-month follow-up, vision reduction was a consequence of central serous retinopathy. There were no cases of severe vision loss because of glaucoma surgery.
Endophthalmitis
Endophthalmitis is a sight-threatening complication. Following trabeculectomy, it is normally related to a bleb leak or bleb infection. Two patients were reported as having endophthalmitis. However, only one of these was a bleb-related infection. This occurred in a subject originally allocated to the medical management arm who subsequently had a trabeculectomy for uncontrolled IOP. The infection was treated with intravitreal antibiotics and the subject made a good recovery with no vision loss and a satisfactory IOP at 24 months without the need for glaucoma eye drops.
One subject allocated to the trabeculectomy arm developed an endogenous endophthalmitis believed to be related to a diabetic foot ulcer. This subject was also treated with intravitreal antibiotics and made a good visual recovery.
Bleb-related endophthalmitis is a recognised and potentially devastating consequence of trabeculectomy surgery. The frequency of bleb-related endophthalmitis varies: Luebke et al. 157 reported 0.004% (7/1816) of trabeculectomies in their consecutive case series157 and Kim et al. 158 found a risk of bleb-related endophthalmitis of 0.005% (9/1959) with a mean follow-up of 54.4 (3.5) years. 158 Several RCTs have reported bleb-related endophthalmitis rates: Wong et al. 159 reported no episodes of bleb-related endophthalmitis at 8 years in the Singapore 5-FU study159 and in CIGTS 0.1% (3/285) of trabeculectomies developed a bleb-related endophthalmitis after an average follow-up of 7.2 years. 160 In both of these studies, trabeculectomies were either unaugmented or augmented with 5-FU, which would be expected to carry a lower risk for bleb-related problems. In the UK, Kirwan et al. 61 reported two cases of bleb-related endophthalmitis within 2 years in their pragmatic multicentre service evaluation cohort of 428 eyes. 61 Alwitry and King161 undertook a national survey through the British Ophthalmic Surveillance Unit and estimated the rate of bleb-related endophthalmitis in the UK to be 0.17%. 161 In the TAGS trial, 240 trabeculectomies were performed, with one bleb-related endophthalmitis representing an incidence of 1 in 240 (0.4%) at 24 months. In the TAGS trial, the bleb-related endophthalmitis occurred in a patient who had initially been allocated to medical management and subsequently had a trabeculectomy following failed medical management.
Treatments received
In the trabeculectomy arm, 201 participants (88.5%) received surgery in their index eye and 34 participants (15.0%) received a trabeculectomy in their non-index eye (see Appendix 3, Table 27). Of these, four participants did not have a trabeculectomy in their index eye. In the medical management arm, 30 participants (17.3%) received trabeculectomy (see Table 4). The reason for participants receiving trabeculectomy in the medical management arm was mainly because of uncontrolled IOP.
Sixteen participants in the trabeculectomy arm declined to have trabeculectomy once allocated to the trabeculectomy arm. It is well recognised that some patients who agree to participate in a trial refuse to have the allocated treatment once randomisation has occurred. 162 In addition, several participants with bilateral advanced glaucoma who were allocated to the trabeculectomy arm did not have surgery performed on their index eye. For these patients, there was a reluctance on the part of patients and patients’ clinicians to perform trabeculectomy on the better eye (eye with greater mean deviation value) first and, therefore, in such cases, a decision was made to operate on the worse eye (non-index eye) first and then operate on the index eye when this had settled; however, this did not always occur.
Economic evaluation
The EQ-5D-5L has been a recommended QoL measure used to estimate QALYs in economic evaluations in the UK. However, a limitation of this generic QoL measure is that it may not be sensitive to small but clinically important changes. 111,112 The GPI and the GUI were developed by Burr et al. 55 as a health outcome measure that could be used to estimate preference-based weights in glaucoma. 104 These weights can be used as an alternative to the EQ-5D-5L to estimate QALYs to identify whether or not preference weights from a disease-specific QoL measure are more sensitive to changes in QoL than those from the EQ-5D-5L in individuals with glaucoma. The GUI QoL weight values currently available were estimated based on the responses to a DCE from individuals with self-reported mild or moderate glaucoma, with few responses from those with advanced glaucoma. 104 The aim of the DCE study was to estimate the QoL weights for the GPI in a population with advanced glaucoma and incorporate these values into the economic evaluation.
As expected, participants had a preference for reduced difficulty in the attributes central and near vision, lighting and glare, mobility, and activities of daily living compared with the base level (i.e. severe difficulty). Although some attribute values were estimated to be, on average, negative, there was no evidence that these attribute levels were any different compared with severe difficulty.
The QoL weights were estimated using the results from the logistic regression under the assumption that the health state 111111 (no difficulty with any of the attributes) was equivalent to 1 (perfect health) on the QoL scale. The values for the other attribute levels were anchored on this value to maintain the ratio between the attributes and the levels. Levels with a negative coefficient were assumed to be zero in the estimation of the QoL weights because these levels were close to zero and not statistically significant.
A sensitivity analysis was undertaken incorporating the QoL weights estimated from the DCE and participants’ responses to the GUI questionnaire administered at baseline and at 1, 3, 6, 12, 18 and 24 months post randomisation to estimate QALYs and subsequently the cost-effectiveness of surgical intervention in the management of glaucoma. On average, the GUI produced higher average total QALYs for both interventions than the EQ-5D-5L and HUI-3. There was, however, a smaller difference between treatment arms.
The results of the DCE, support the use of the EQ-5D-5L in individuals with advanced glaucoma. Out of the six attributes valued in the DCE, we found a strong preference for three attributes (central and near vision, mobility, and activities of daily living) with very little utility to be gained from reduced difficulty in the other three attributes. Two of the attributes preferred by individuals with advanced glaucoma are also included in the EQ-5D-5L (mobility and usual activities); therefore, any benefit from an intervention that targets these attributes can arguably be captured by the EQ-5D-5L.
This trial sought to address a paucity of economic evidence for medical compared with surgical interventions in the management of advanced glaucoma. 30 In the economic analysis, the trabeculectomy arm was, on average, more costly and provided more QALYs at 24 months. At 24 months, it was unlikely that trabeculectomy would be considered cost-effective compared with the medical therapy over the range of values for society’s willingness to pay for a QALY considered. The driving cost was the initial costs of the trabeculectomy procedure incurred in the in the first year of the trial in the trabeculectomy arm.
The economic evaluation model suggests that trabeculectomy is likely to be a cost-effective approach compared with medication in patients with severe glaucoma over a lifetime time horizon. The reason for this is that the initial costs (cost of year 1 treatment) are higher for patients assigned to trabeculectomy and these are partly offset in the longer term because these patients receive fewer subsequent procedures and have lower medication use.
It should be noted that there is a small difference in the estimated mean costs and QALYs when comparing the results of the within-trial analysis with those of a 2-year time horizon model-based analysis. These differences are explained partly by the way that we used the trial data to populate our model and the impact that ‘random noise’ has on the Monte Carlo simulation. A further difference between the trial and the model-based analysis is that the model used age-specific and sex-specific probabilities of death derived from UK life tables, rather than the observed data from obtained from the trial data set.
Discrete choice experiment
The aim of the DCE was to identify the preferences of individuals with advanced glaucoma and update the QoL weights estimated by Burr et al. 55 Burr et al. 55 estimated their QoL weights from a population with self-reported mild or moderate glaucoma, with only 15% of the sample reporting severe glaucoma. Participants were asked to complete 36 choice-set questions, four of which were rationality tests. The response rate (70% vs. 62%) and number of participants who attempted the choice-set questions (n = 308 vs. n = 289) were similar. Our results were comparable to Burr et al. 55 in that the strongest preferences were the attributes central and near vision, mobility, and activities of daily living. The ranking of the attribute levels was also similar. Similar to Burr et al. ,55 the remaining three attributes (i.e. lighting and glare, eye discomfort, and other effects of glaucoma and its treatment) were not statistically significant in our model but, on average, there were negative preferences. In a sensitivity analysis, we replicated their analysis by combining the levels of these attributes, however, our conclusions did not change and these attributes were not statistically significant. Hence, we can conclude that participants with advanced glaucoma do not have a strong preference for improvements in these attributes (lighting and glare, eye discomfort, and the effects of glaucoma and its treatment). A limitation of combining the attribute levels is that the ratio of preference for that attribute level compared with severe difficulty is lost in the estimation of utility scores. Second, in Burr et al. 55 all three levels of lighting and glare (no difficulty, some difficulty and quite a lot of difficulty) were combined but the level no difficulty has a different QoL weight to the other two levels, which were assumed to be zero. This is inconsistent with how they created QoL weights for the other attribute levels that were combined.
Cost-effectiveness
Within the 24-month time horizon of the trial, medical management was likely to be cost-effective over the range of values for society’s willingness to pay for a QALY considered. This recommendation changes when the benefits were extrapolated beyond the time horizon of the trial and are considered across the participant’s lifetime. This is important because the median age of glaucoma diagnosis in TAGS is 69 years. Their current average projected life expectancy is 15 years, according to published figures regarding average life expectancy. 119
For the model-based economic evaluation when a lifetime horizon is taken, trabeculectomy is likely to be considered as cost-effective compared with medical management over the range of values for society’s willingness to pay for a QALY considered compared with medication.
There are other studies that have estimated the cost-effectiveness of surgery compared with medical therapy for glaucoma treatment in the UK population. These studies were conducted in different populations of glaucoma patients and gave conflicting results. 112,163–165 Stein et al. 112 compared medications with laser trabeculoplasty for the treatment of patients with newly diagnosed mild OAG. They used a Markov model with a 25-year time horizon; the results of this suggested that medication was superior to laser trabeculoplasty, although their analysis assumed optimal medication adherence. 112 Choi et al. 113 conducted a cost-effectiveness analysis comparing medication, laser trabeculectomy and trabeculectomy in a South Korean population with mild OAG. Their analysis reported that medication was cost-effective compared with laser trabeculectomy and trabeculectomy. 113 These two studies have limited relevance to our study because they focused on treatments for patients with less severe glaucoma. Only one study was identified that also considered more severe glaucoma, as we have done in TAGS. Guedes et al. ’s166 analysis was based on Markov models and aimed to identify the most cost-effective treatment strategy for each severity of glaucoma. 166 The results of this study were similar because this study found that the surgery is cost-effective in participants who are aged < 70 years. Similarly, this study finds that patients who will live for at least 10 years after surgery are more likely to have the benefits outweigh the investment. 166
Strengths and limitations
This pragmatic study is representative of what currently happens to patients presenting with advanced glaucoma being managed in the NHS. Consequently, the concluding findings on disease progression, reduction of IOP, vision retention, safety profile of interventions and cost are highly relevant to normal clinical practice.
This trial was unmasked, which allowed the trial to capture any treatment effects on patients’ perception, which is clinical reality and reflects the patients experience of the intervention. It answers specific concerns of clinicians regarding the safety of the interventions, which was a major barrier, particularly for consideration of trabeculectomy as a primary procedure.
A common limitation of DCEs is that they can be difficult for participants to understand. However, the majority of those who responded to the DCE questionnaire (n = 283, 92%) completed all 15 choice-set questions, which suggests that they had a good understanding of the DCE exercise.
Unlike Burr et al. ,55 we found negative preferences for 5 of the 18 levels included in the regression model; however, these preferences were not statistically significant. Thus, we have no evidence that the coefficient value is different from the worst state, severe difficulty. We assigned these coefficients a QoL weight of zero where we had no evidence of any difference between these attribute levels and the most severe level (e.g. no difficulty compared with severe difficulty). This assumption potentially created a bias in our QoL weight values as positive coefficients (which were not statistically significant) were assigned a positive QoL weight, albeit close to zero, and it reduced the ratio of preference between the attributes as the negative values were given a slightly higher weighting by assuming that they were zero. However, it is unlikely that this assumption had any implications on our overall results because when the attribute levels, which were not statistically significant, were combined in a sensitivity analysis the results remained consistent, suggesting that there was no evidence of individuals having a preference for reduced difficulty in these attributes. The second possible bias is that our assumption of assigning a zero value (i.e. levels are equivalent) makes the assumption that evidence of no difference is the same as the there being evidence of no difference in levels. An alternative would be to incorporate the imprecision in estimates into the estimation of QALYs. Although this may be possible, we are unaware of any work that has undertaken this.
There was also an issue with theoretical validity in our model because we found a stronger preference for some difficulty with activities of daily living than for no difficulty with activities associated with daily living, and we had hypothesised that the coefficients would increase with reduced levels of difficulty. A reason for this could be that using a d-efficient design meant that there was no attribute level balance that may have produced this unusual finding. However, a d-efficient design is still recommended because it optimises efficiency as it reduces the standard errors (uncertainty) in the model estimation. 85 Finally, asking participants which alternative; they consider worse is more cognitively challenging than choosing the best alternative; this may explain some of the negative preferences estimated from our model. Further estimation work will be undertaken to explore these negative preferences.
For the economic evaluation, one of the key limitations of the within-trial analysis is the limited time horizon. Primary OAG is a chronic lifelong condition, so the capture of full benefit of any interventions is unlikely to be shown within this time frame. It is also possible that there have been issues with recall for individual participants when completing the resource and QoL questionnaires. A recent study that examined the use of a laser-based intervention and medical management strategy for patients presenting with earlier stage glaucoma was the LiGHT trial. 59 The LiGHT trial assessed selective laser trabeculoplasty versus medical management for glaucoma and ocular hypertension. 59 This study concludes that laser is very likely to be cost-effective within the 3-year trial outcomes. This, potentially, is for a number of reasons. First, a selective laser trabeculoplasty could be a smaller initial intervention cost (the economic results of the trial are yet to be published in full) and that the benefits were examined for an additional year. This would allow the benefits of the initial investments to accrue over a longer time period. It is for this reason that the results of the within-trial analysis should be considered alongside the decision model. Extrapolating the results beyond the initial treatment period could accrue benefits into the future and change the decision regarding which intervention is likely to be cost-effective. It is possible that trabeculectomy may be the most efficient use of resources in a patient with a greater life expectancy who will likely accrue the benefit.
Another consideration is the inclusion of the costs of the non-index eye. When considering the calculation of total costs, the costs for both the index eye and the non-index eye were included. This was the case for a number of reasons. First, the outcome measures of principle interest were not specific to the index eye and when considering vision and QoL the eyes do not work independently of one another. Second, the prognosis of one eye may affect the decisions regarding the management of the other eye. This can be a difficult consideration when carrying out work with vision-related QoL because there is a potential to under value or over value any potential costs. In this study, as shown in Table 20 within the context of the model, the spread of the severity of glaucoma in the non-index eye is equally spread between the two arms. This means that costs of including the non-index eye should be spread evenly between the two study arms and not affect the marginal difference between the costs.
The main strength of this study is that this model-based economic evaluation was carried out in parallel with the trial-based economic evaluation and was populated with data derived from the trial. All defined parameters in this model-based economic evaluation analysis were based on data collected prospectively as part of TAGS. Moreover, although other economic evaluations exist, the TAGS-based analyses (both the within trial and the model-based analyses) included patients at severe stages of glaucoma only. The results should, therefore, be generalisable across the UK NHS for patients with severe glaucoma.
Using GSS2 for classifying patients (baseline, 12 months and 24 months) into different stages of glaucoma, is another strength because it allowed our study to detect these differences in both cost and utility outcomes between trabeculectomy and medical treatment. Within the model, the severity of glaucoma has been defined based on the state of each eye separately and data on the progression of glaucoma is based on data for both eyes, allowing the modelling of the index eye and non-index eye within the model. Other studies defined health states within their model based on the condition of both eyes together and did not consider each eye separately in the model as we have. 110,111 Although HRQoL and, hence, QALYs are thought to be determined by the quality of vision in the better eye in the case of eye disease,117 the model structure estimated the chance of unilateral and bilateral progression more precisely over time.
Our evaluation has also repeated the analysis using QALYs based on different measures of utility (EQ-5D-5L, HUI-3 and GUI). The advantage of this is that the EQ-5D-5L is widely used to estimate QALYs in the UK and is the recommended approach by NICE. However, it does not include a specific vision component. The HUI-3, however, is also a generic measure of health status but unlike the EQ-5D-5L has a specific vision component. It should be noted, however, that health state utilities values are derived from a Canadian population rather than a UK population. The GUI is a condition-specific measure for which utilities have been derived directly from TAGs trial conditions. The results of our study showed that using the HUI-3 measure over a patient’s lifetime provides the largest difference in QALYs between treatment methods (0.38 additional QALYs of trabeculectomy compared with medical management); QALYs based on the EQ-5D-5L measure are ranked second (with 0.28 additional QALYs of trabeculectomy compared with medical management) whereas the GUI provides the smallest difference in QALYs (0.16 additional QALYs of trabeculectomy compared with medical management).
For the model-based economic evaluation, our analysis has some limitations that need to be considered when interpreting the results. The first limitation is the limited data to extrapolate beyond 2 years; for example, needing further surgery (cataract surgery) to manage complications was not considered in the model because the trial results provide no evidence of a difference between the two arms. Longer-term follow-up, as is planned for TAGS, may further inform the model. Medical therapy is preferred by many patients because it is non-invasive and, from the perspective of the health service, it avoids the initial cost of surgery but may be associated with higher VF loss in the longer term. In part, this may be caused because adherence to medication in the long term may be challenging for patients. 167 Finally, to address uncertainty caused by missing data, we have used multiple imputation data for the model. The results of the multiple imputations suggest that the complete-case analysis may underestimate the difference in effects between the alternatives as a result of those with poorer health outcomes being more likely to withdraw the follow-up.
Further research planned
Further funding has been secured to evaluate clinical and patient-reported outcomes at 5 years to further explore the lifetime experience, patient-reported outcomes and visual loss (VA and VF survival) in this group of participants. These data will be incorporated into an updated economic model once they become available.
Chapter 8 Conclusions
In summary, this trial shows that treating patients presenting with advanced primary OAG can be undertaken safely with primary augmented surgery. Surgery has a similar effect on patients, HRQoL but is better at reducing IOP, which is likely to reduce further VF progression during the patient’s lifetime.
The DCE found similar results to Burr et al. 55 in that an individual’s strongest preferences were for reduced difficulty in central and near vision, mobility, and activities of daily living. A revised GUI value set from a population with advanced glaucoma is now available.
The within-trial economic evaluation found that, initially, the small incremental change in HRQoL generated by surgery does not offset the additional cost to produce over the range of threshold values for society’s willingness to pay for a QALY considered when considering a societal willingness to pay for a QALY threshold. When the benefits for the surgery are extrapolated across the patient’s lifetime, the trabeculectomy becomes increasingly more likely to be considered cost-effective.
Implications for health care
The results of this RCT are widely generalisable. Twenty-seven centres recruited patients with primary OAG, which also included pigment dispersion glaucoma, pseudoexfoliation glaucoma and normal tension glaucoma, and patients from all ethnic backgrounds and adult ages were included. The trial included only patients with advanced disease who were newly diagnosed and, therefore, should be interpreted with caution for patients with less-advanced disease or those who have previously been treated for glaucoma. There are important implications for a resource-limited publicly funded health-care system and for informing future NICE guidelines that have concluded that the evidence supporting treatment options for patients presenting with advanced glaucoma is weak.
The data from this trial support primary surgery as the best primary intervention for patients presenting with advanced glaucoma. The safety of primary trabeculectomy has been demonstrated. There is a clinically important difference in the reduction of IOP achieved with primary surgery, which is likely to reduce the lifetime risk of further disease progression and blindness. Over the 2-year follow-up period, medication had the highest probability of being considered cost-effective at a £20,000 willingness-to-pay threshold. When the results of the trial were extrapolated over the patient’s lifetime, trabeculectomy had the highest probability of being considered cost-effective at a £20,000 willingness-to-pay threshold. Further randomised studies would help to confirm these findings and longer-term follow-up of patients enrolled in this study would help to verify the model-based extrapolations reported here.
Recommendations for research
Long-term follow-up is required because glaucoma is a lifelong condition and lifetime outcomes are essential to fully inform practise. This trial addresses only the first 24-month period following diagnosis; further follow-up is required to further evaluate the patient experience, clinical effectiveness, safety and cost-effectiveness of these interventions in the long term.
It is important to explore the reasons as to why so many people continue to present with advanced glaucoma and it is important to develop strategies to reduce this. Further research into suitable outcome measures to measure QoL for glaucoma is required. Further estimation work is needed to explore the negative preferences reported in the DCE. Further research into cross-walking the VFQ-25 instrument to the EQ-5D-5L is needed to allow comparison between the two measures and to potentially reduce the data collection burden of future trials. Improvements can be made to the methodology for undertaking glaucoma trials to allow differences between arms to be detected in a shorter period of time.
Acknowledgements
The authors wish to thank the members of the TSC and DMC who oversaw this study.
Trial Steering Committee:
-
independent chairperson and statistician – Professor John Thompson (chairperson from 2015), Professor of Ophthalmic Epidemiology, Department of Health Sciences, University of Leicester, Leicester, UK (retired 2019 but remains TSC chairperson)
-
chief investigator – Professor Anthony King, Honorary Professor Clinical Ophthalmology and Consultant Ophthalmologist, University of Nottingham and Queens Medical Centre, Nottingham, UK
-
trial manager – Dr Gordon Fernie, Centre for Healthcare Randomised Trials, Health Services Research Unit, University of Aberdeen, Aberdeen, UK (until January 2020)
-
independent clinician with relevant expertise – Mr Mark Batterbury, Consultant Ophthalmologist, St Paul’s Eye Unit, Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool, UK
-
independent clinician with relevant expertise – Mr Wojciech Karwatowski (from 2018), Consultant Ophthalmologist, Department of Ophthalmology, Leicester Royal Infirmary, Leicester, UK
-
patient and public involvement representative – Mr Russell Young, former Chief Executive, International Glaucoma Association (retired July 2016 but remains a TSC member)
-
Mr Faruque Ghanchi, Consultant Ophthalmologist, BRI Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK (independent clinician with relevant expertise 2015–19)
-
Professor Bal Dhillon, Professor of Ophthalmology, Princess Alexandra Eye Pavilion, Edinburgh, UK (independent clinician with relevant expertise in 2014)
-
Professor Colm O’Brien, Professor of Ophthalmology, School Of Medicine and Medical Science, Mater Hospital, Dublin, Ireland (independent chairperson in 2014).
Data Monitoring Committee:
-
independent chairperson and clinician – Professor James Morgan, Professor of Ophthalmology, School of Optometry and Vision Sciences, Cardiff University, Cardiff, UK
-
independent statistician – Professor David Crabb, Professor of Statistics and Visual Research, City University London, London, UK
-
independent triallist – Ms Sue Tebbs, Deputy Director, Birmingham Clinical Trials Unit, University of Birmingham (retired 2019), formerly Head of Operations and Deputy Director, Clinical Trials Unit, University College London, London, UK.
The authors wish to thank the members of the Study Management Group:
-
chief investigator – Anthony King
-
trial manager – Gordon Fernie
-
statistician – Jemma Hudson
-
data co-ordinator – Pauline Garden
-
senior trial manager – Alison McDonald
-
sponsor representatives – Pauline Hyman-Taylor (from February 2019), Natalie McGregor (from March 2015 to February 2019), Audrey Athlan (from July 2014 to March 2015) and Joanne Thornhill (from 2013 to July 2014).
The authors would also like to thank the members of the Project Management Group:
-
also included the members of the Study Management Group
-
grant-holders – Augusto Azuara-Blanco, Keith Barton, Jen Burr, David Garway-Heath, Graeme MacLennan, John Norrie, John Sparrow and Luke Vale
-
patient and public involvement representative – Rick Walsh
-
cHaRT trial office – Mark Forrest (from 2015) and Gladys McPherson (until 2015)
-
health economists – Eoin Maloney (until 2015) and Mehdi Javanbakht (until June 2019).
The members of the TAGS investigator group responsible for recruitment in the clinical centres were as follows:
-
Queen’s Medical Centre, Nottingham University Hospitals NHS Trust – Anthony King, Pavi Agrawal and Richard Stead.
-
Norwich & Norfolk University Hospital NHS Foundation Trust – David C Broadway.
-
Moorfields Eye Hospital NHS Foundation Trust – Nick Strouthidis, Shenton Chew, Chelvin Sng, Marta Toth, Gus Gazzard, Ahmed Elkarmouty, Eleni Nikita, Giacinto Triolo and Soledad Aguilar-Munoa.
-
St Thomas’ Hospital, Guy’s and St Thomas’s NHS Foundation Trust – Saurabh Goyal and Sheng Lim.
-
Birmingham and Midland Eye Centre, Sandwell & West Birmingham Hospitals NHS Trust – Velota Sung and Imran Masood.
-
Sunderland Eye infirmary, City Hospitals Sunderland NHS Foundation Trust – Nicholas Wride, Amanjeet Sandhu and Elizabeth Hill.
-
Bristol Eye Hospital, City Hospitals Sunderland NHS Foundation Trust – John Sparrow and Fiona Grey.
-
Hinchingbrooke Hospital, North West Anglia NHS Foundation Trust – Rupert Bourne, Gnanapragasam Nithyanandarajah and Catherine Willshire.
-
Imperial College Ophthalmology Research Group (ICORG), Western Eye Hospital, Imperial College Healthcare NHS Trust – Philip Bloom, Faisal Ahmed, Franesca Cordeiro, Laura Crawley, Eduardo Normando, Sally Ameen, Joanna Tryfinopoulou, Alistair Porteous, Gurjeet Jutley and Dimitrios Bessinis.
-
Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust – James Kirwan, Shahiba Begum, Anastasios Sepetis, Edward Rule and Richard Thornton.
-
Gloucestershire Eye Unit, Cheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust – Andrew McNaught and Nitin Anand.
-
Birmingham Heartlands Hospital, Heart of England NHS Foundation Trust – Anil Negi and Obaid Kousha.
-
Warrington Hospital, Warrington and Halton Hospitals NHS Foundation Trust – Marta Hovan.
-
Queen Margaret Hospital, Dunfermline, NHS Fife – Roshini Sanders.
-
Princess Alexandra Eye Pavilion, Edinburgh, NHS Lothian – Pankaj Kumar Agarwal and Andrew Tatham.
-
Manchester Royal Eye Hospital, Manchester University NHS Foundation Trust – Leon Au, Eleni Nikita, Cecelia Fenerty, Tanya Karaconji, Brett Drury and Duya Penmol.
-
Maidstone Hospital, Maidstone and Tunbridge Wells NHS Trust – Ejaz Ansari, Albina Dardzhikova, Reza Moosavi, Richard Imonikhe, Prodromos Kontovourikis, Luke Membrey and Goncalo Almeida.
-
The Royal Derby Hospital, Derby Hospitals NHS Foundation Trust – James Tildsley.
-
Royal Victoria Hospital, Belfast Health & Social Care Trust – Augusto Azuara-Blanco, Angela Knox, Simon Rankin and Sara Wilson.
-
James Paget University Hospitals NHS Foundation Trust – Avinash Prabhu and Subhanjan Mukherji.
-
Hairmyres Hospital, East Kilbride, NHS Lanarkshire – Amit Datta and Alisdair Fern.
-
York Teaching Hospital NHS Foundation Trust & Harrogate & District NHS Foundation Trust – Joanna Liput.
-
York Teaching Hospital NHS Foundation Trust – Tim Manners and Josh Pilling.
-
Harrogate & District NHS Foundation Trust – Clare Stemp, Karen Martin and Tracey Nixon.
-
Ninewells Hospital, Dundee, NHS Tayside – Caroline Cobb.
-
Gartnavel General Hospital, NHS Greater Glasgow and Clyde – Alan Rotchford and Sikander Sidiki.
-
University Hospital, Coventry, University Hospitals Coventry and Warwickshire NHS Trust – Atul Bansal and Obaid Kousha.
-
Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust – Graham Auger and Mary Freeman.
All participants in the trial and staff members of the TAGS investigator group responsible for recruitment in the clinical centres were as follows:
-
Princess Alexandra Eye Pavilion, Edinburgh, NHS Lothian – Margaret McDonald, Margaret Frost, Angela James, June McGhie, Doreen Stewart and Laura Hughes.
-
University Hospital, Coventry, University Hospitals Coventry and Warwickshire NHS Trust – Jeanette Allison, Margaret Dixon, Marie McCauley, Linzi Randle, Nigel Edwards, Kamaljeet Heer, Amritpal Chaggar, Vishal Thakrar and Suzanna Randen.
-
St Thomas’ Hospital, Guy’s and St Thomas’s NHS Foundation Trust – Stephanie Jones, Alba De Antonio, Andrew Ho, Elizabeth Galvis, Eme Chan, Jennifer Lee, Andrew Amon, Isabelle Chow, Hinchingbrooke Hospital, North West Anglia NHS Foundation Trust – Paula Turnbull, Jane Kean, Aarti Bahirat, Ivailo Zhekov, Chelsea Turnbull, Debra Jones, Aaron Woods and Linda Molloy.
-
Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust – Mini David, Nicola Moss, Tomaz Jazwiecki, Maureen McMahon, Kim Green, Fawne Gibb, Lisa Haines, Rashedul Hoque, Jacqueline Denham and Madhu Mamman.
-
Harrogate & District NHS Foundation Trust – Gillian Ranson, Debbie Wiggins and Caroline Clarke.
-
The Royal Derby Hospital, Derby Hospitals NHS Foundation Trust – Sue Melbourne, Charlotte Downes, Ryan Humphries, Gill Widdowson, Lynsey Havill, John Sharp, Abbie Singleton, Tracy Brear, Howard Fairey, Aariana Sohal, Dorothy Gibson and Mona Mohamed.
-
Royal Victoria Hospital, Belfast Health & Social Care Trust – Rebecca Denham, Louise Scullion, Georgina Sterrett, Deirdre Burns, Lesley Doyle, Graham Young, Paul Wright, Vittorio Silvestri, Nuala Jane Lavery, Jonathan Keenan and Katie Graham.
-
Norwich & Norfolk University Hospital NHS Foundation Trust – Heidi Cate, Sally Edmunds, Corinne Haynes, Karen Le Grys, Naomi Waller and Patricia Young.
-
Manchester University, NHS Foundation Trust – Ian Venables.
-
Kate Barugh, Monika Cien, Raisa Platt, Katie McGuiness, Stephanie Clarke, Daniel Todd, Patrick Gunn, Rosalind Creer, Leanne Richards, Catherine Park, Fatima Ahmed, Thomas Hamper, Deepali Bindal, Harriet Ndu-Melekwe, Martyn Russell, Tanya Karaconji, Brett Drury and Divya Permol.
-
Bristol Eye Hospital, University Hospitals Bristol NHS Foundation Trust – Beth Thorne, Emma Lamb, Ifan Jones, Jack Beange, Eleanor Hiscott, Jess Toole, Leila Cox, Julie Cloake, Clemence Rouquette, Gemma Bizley, Fran Paget, Carrie White, Gemma Brimson, Danielle Lee, Helen Talbot, Claire Buckland, Inderjit Chatha, Katherine Smith, Ketan Kapoor and Sebastian Howard.
-
Sunderland Eye Infirmary, City Hospitals Sunderland NHS Foundation Trust – Steve Dodds, Lauren Bell, Wenwei Woo, Jaswant Sandhu, Santy Nocon, Amanjeet Sandhu, Oliver Baylis, Ibrahim Masri, Karim El-Assal, Gemma Sloanes, Elizabeth Hill, David Lunt, Michelle Young, Shweta Singh and Sinead Connolly.
-
Gloucestershire Eye Unit, Cheltenham General Hospital, Gloucestershire Hospitals NHS Foundation Trust – Adrian Blazey, Anthony Burke and Frances Reilly.
-
Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust – Helen Pokora, Maria Edwards, Martin Rhodes, Tom Evans, Jonathan Drury and Emily Henry.
-
Nottingham University Hospitals NHS Trust – Patrick Cox, Lauren Tarr, Rebecca Nicol, Jade Higman, Zoe Bull, Laura Anderson, Renee Cammack, Jessica Kearsley, Athanasia Koumoukeli, Olivia Hay, Salina Saddiqui, Angela Skinner, Imran Jawaid, Alan Kastner, Amie Manjang, Francesco Stringa and Kostas Giannouladis.
-
Queen Margaret Hospital, Dunfermline, NHS Fife – Karen Grey, Paul Allcoat, Julie Aitken, Ruth Robertson, Julie Donaldson and Shiela Hanlon.
-
Warrington and Halton Hospitals NHS Foundation Trust – Mark Halliwell, Lynne Connell and Kerry Bunworth.
-
Maidstone Hospital, Maidstone and Tunbridge Wells NHS Trust – Ruby Einosas, Susan Lord, Laura Reid, Stephanie Tidey, Audrey Perkins, Narkirst Bahra, Lynne Holmes, Sue Crockford, Karen O’Brien and Sara Harrison.
-
Gartnavel General Hospital, NHS Greater Glasgow and Clyde – Aileen Campbell, Vicky Paterson, Geraldine Campbell and Fiona Mcintosh.
-
Moorfields Eye Hospital NHS Foundation Trust – Sophie Connor, Alexa King, Anar Shaikh, Dominic Carrington, Charles Amoah, Emerson Tingco, Jennette Elayba, Charlene Formento, Priya Dabasia, Soraya Al-Samarie, Kanom Bibi, Roel Cabellon, Christie Garcelales, Joel Real, Louis Wenigha, Martha Simango, Nafaz Illiyas, Elaina Reid, Paul Foster, Matt Kinsella, Edmore Ncube, Christine Fernandez, Hannah Williams, Evripidis Sykakis, Evangelia Gkaragkani, Roxanne Crosby-Nwaobi, Kirsty Chartier, Catherine Grigg, Panayiotis Panayiotou, Gaboitsiwe Maphango, Qin Neville, Anthony Khawaja, Karine Girard-Claudon, Nathan Kerr, Aberami Chandrakumar, Sash Y Jeetun, Hamida Begum, Hannah Hounslow, Jasmeet Bahra, Hilary Swann, Katy Barnard, Gareth Davey, Cristina Citu, Ramona Berinde, Layla Juma, Jenny Elliot, Hayley Thomas, Tran Toan Dang, Shabneez Laulloo, Lucia Gonzalez, Minara Begum, Supeetha David, Hari Jayaram, Niraapha Sivakumaran, Tina Reetun, Sapna Shah, Diana Dabrickaite, Yusrah Shweikh, Priyansha Sheel and Ashish Mashru.
-
Birmingham and Midland Eye Centre, Sandwell & West Birmingham Hospitals NHS Trust – Zain Juma, Richard Stead, Sue Southworth, Katie Atterbury, Esther Poole, Monica Hutton, Xicoli Chan, Patrick Chiam, Zoe Pilsworth, Risna Haq, Zahira Maqsood, Suaad Alasow and Melisa Fenton.
-
Hairmyres Hospital, East Kilbride, NHS Lanarkshire – Anne McMurdo, Lesley Quigley, Elizabeth Shearer, Janice Simmons, Anne Moore and Karen Murray.
-
Ninewells Hospital, Dundee, NHS Tayside – Heather Loftus and Michelle Robertson.
-
York Teaching Hospital NHS Foundation Trust – Srilakshmi Gollapothu, Alison Grice-Holt, Gillian Ranson, Gary Lamont, Sara Stringer, Carol Sarginson, Christine O’Dwyer and Joanne Wincup.
-
James Paget University Hospitals NHS Foundation Trust – Emma Stimpson, Lucy Hutchins, Tracy Duval, Lesley Parsons, Paula Head, Stephanie Cotton, Katie Riches, Amy Thanawalla, Katherine Mackintosh, Ellis Hinchley and Mya So.
-
Imperial College Ophthalmology Research Group (ICORG), Western Eye Hospital, Imperial College Healthcare NHS Trust – Altaf Mamoojee, Zena Rodrigues, Eduardo Normando, Serge Miodragovic, Seham Jeylani, Paolo Bonetti and Jessica Bonetti.
-
Heart of England NHS Foundation Trust – Carole Atkins, Clair Rea, Priyanka Divakar, Nicola Bennett, Sarita Jacob, Deepti Raina, Joseph Coombs and Farah Jabin.
The Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Contributions of authors
Anthony J King (https://orcid.org/0000-0002-3091-911X) (Honorary Professor Clinical Ophthalmology, Consultant Ophthalmic Surgeon and Chief Investigator) contributed to the conception and the design of the trial, recruitment of participants, the conduct of the trial, the interpretation of the results and the writing and editing of the report.
Gordon Fernie (https://orcid.org/0000-0002-3838-8114) (Trial Manager, Triallist) was responsible for the day-to-day management of the trial, the interpretation of the results and the writing and editing of the report.
Jemma Hudson (https://orcid.org/0000-0002-6440-6419) (Statistician) conducted the statistical analyses and the interpretation of results, and made a significant contribution to drafting and reviewing the final report.
Ashleigh Kernohan (https://orcid.org/0000-0002-5514-3186) (Health Economist) conducted the within-trial analysis of the health economics data and the writing of the health economics chapter and within-trial economic analysis of other chapters and sections of the report. She also commented on other chapters of the report.
Augusto Azuara-Blanco (https://orcid.org/0000-0002-4805-9322) (Clinical Professor of Ophthalmology) contributed to the conception and design of the trial, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
Jennifer Burr (https://orcid.org/0000-0002-9478-738X) (Reader) contributed to the conception and design of the study, interpretation of the data and provided commentary on the draft chapters of the report.
Tara Homer (https://orcid.org/0000-0002-6664-0671) (Health Economist) conducted the design and analysis of the DCE and the writing of the DCE chapter and DCE of other chapters and sections of the report. She also commented on other chapters of the report.
Hosein Shabaninejad (https://orcid.org/0000-0001-9512-1398) (Health Economist) designed and conducted the model-based economic evaluation analysis and wrote the model-based section of the health economics chapter and health economic modelling components of other chapters and sections of the report. He also commented on other chapters of the report.
John M Sparrow (https://orcid.org/0000-0003-3704-0105) (Honorary Professor of Ophthalmology) contributed to the conception and design of the trial, the recruitment of participants, interpretation of the data and provided commentary on the draft chapters of the report.
David Garway-Heath ((https://orcid.org/0000-0003-2907-6992) (Professor of Ophthalmology) contributed to the conception and design of the trial, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
Keith Barton (https://orcid.org/0000-0001-9345-7410) (Professor of Ophthalmology) contributed to the conception and design of the trial, the recruitment of participants and the interpretation of the data, and provided commentary on the draft chapters of the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) [Professor of Statistics and Director of CHaRT (until 2016)] contributed to the conception and the design of the trial, the conduct of the trial and provided commentary on the draft chapters of the report.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) (Senior Trial Manager) contributed to the conception and the design of the trial, the conduct of the trial and provided commentary on the draft chapters of the report.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Professor of Health Economics) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of results and made significant contributions to the drafting of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor, CHaRT Director, Statistician and Triallist) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of results and made significant contributions to the drafting of the report.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- King AJ, Fernie G, Azuara-Blanco A, Burr JM, Garway-Heath T, Sparrow JM, et al. Treatment of Advanced Glaucoma Study: a multicentre randomised controlled trial comparing primary medical treatment with primary trabeculectomy for people with newly diagnosed advanced glaucoma-study protocol. Br J Ophthalmol 2018;102:922-8. https://doi.org/10.1136/bjophthalmol-2017-310902.
- Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844-51.
- Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:1661-9. https://doi.org/10.1016/S0161-6420(96)30449-1.
- Wolfs RC, Borger PH, Ramrattan RS, Klaver CC, Hulsman CA, Hofman A, et al. Changing views on open-angle glaucoma: definitions and prevalences – The Rotterdam Study. Invest Ophthalmol Vis Sci 2000;41:3309-21.
- de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Hofman A, de Jong PT. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology 2005;112:1487-93. https://doi.org/10.1016/j.ophtha.2005.04.018.
- Bonomi L, Marchini G, Marraffa M, Bernardi P, De Franco I, Perfetti S, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology 1998;105:209-15. https://doi.org/10.1016/S0161-6420(98)92665-3.
- Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, Javitt J, et al. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol 1991;134:1102-10. https://doi.org/10.1093/oxfordjournals.aje.a116013.
- Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007–March 2008. Eye 2010;24:1692-9. https://doi.org/10.1038/eye.2010.122.
- Bunce C, Wormald R. Causes of blind certifications in England and Wales: April 1999–March 2000. Eye 2008;22:905-11. https://doi.org/10.1038/sj.eye.6702767.
- King AJ, Reddy A, Thompson JR, Rosenthal AR. The rates of blindness and of partial sight registration in glaucoma patients. Eye 2000;14:613-19. https://doi.org/10.1038/eye.2000.152.
- Spry PG, Spencer IC, Sparrow JM, Peters TJ, Brookes ST, Gray S, et al. The Bristol Shared Care Glaucoma Study: reliability of community optometric and hospital eye service test measures. Br J Ophthalmol 1999;83:707-12. https://doi.org/10.1136/bjo.83.6.707.
- Harrison RJ, Wild JM, Hobley AJ. Referral patterns to an ophthalmic outpatient clinic by general practitioners and ophthalmic opticians and the role of these professionals in screening for ocular disease. BMJ 1988;297:1162-7. https://doi.org/10.1136/bmj.297.6657.1162.
- Morley AMS, Murdoch I. The future of glaucoma clinics. Br J Ophthalmol 2006;90:640-5. https://doi.org/10.1136/bjo.2005.085522.
- Burr JM, Mowatt G, Hernández R, Siddiqui MA, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11410.
- Department of Health and Social Care (DHSC) . Healthy Lives, Healthy People: Improving Outcomes and Supporting Transparency 2012.
- Coffey M, Reidy A, Wormald R, Xian WX, Wright L, Courtney P. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol 1993;77:17-21. https://doi.org/10.1136/bjo.77.1.17.
- Elkington AR, MacKean J, Sargent P. A collaborative hospital glaucoma survey. Res Clin Forum 1982;4:31-40.
- Ng WS, Agarwal PK, Sidiki S, McKay L, Townend J, Azuara-Blanco A. The effect of socio-economic deprivation on severity of glaucoma at presentation. Br J Ophthalmol 2010;94:85-7. https://doi.org/10.1136/bjo.2008.153312.
- Sheldrick JH, Ng C, Austin DJ, Rosenthal AR. An analysis of referral routes and diagnostic accuracy in cases of suspected glaucoma. Ophthalmic Epidemiol 1994;1:31-9. https://doi.org/10.3109/09286589409071443.
- Sukumar S, Spencer F, Fenerty C, Harper R, Henson D. The influence of socioeconomic and clinical factors upon the presenting visual field status of patients with glaucoma. Eye 2009;23:1038-44. https://doi.org/10.1038/eye.2008.245.
- Fraser S, Bunce C, Wormald R. Risk factors for late presentation in chronic glaucoma. Invest Ophthalmol Vis Sci 1999;40:2251-7.
- Fraser S, Bunce C, Wormald R, Brunner E. Deprivation and late presentation of glaucoma: case-control study. BMJ 2001;322:639-43. https://doi.org/10.1136/bmj.322.7287.639.
- Odberg T. Visual field prognosis in advanced glaucoma. Acta Ophthalmol 1987;65:27-9. https://doi.org/10.1111/j.1755-3768.1987.tb02583.x.
- Mikelberg FS, Schulzer M, Drance SM, Lau W. The rate of progression of scotomas in glaucoma. Am J Ophthalmol 1986;101:1-6. https://doi.org/10.1016/0002-9394(86)90457-5.
- Grant WM, Burke JF, J. Why do some people go blind from glaucoma?. Ophthalmology 1982;89:991-8. https://doi.org/10.1016/S0161-6420(82)34675-8.
- Wilson R, Walker AM, Dueker DK, Crick RP. Risk factors for rate of progression of glaucomatous visual field loss: a computer-based analysis. Arch Ophthalmol 1982;100:737-41. https://doi.org/10.1001/archopht.1982.01030030741002.
- Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, et al. The probability of blindness from open-angle glaucoma. Ophthalmology 1998;105:2099-104. https://doi.org/10.1016/S0161-6420(98)91133-2.
- Parc CE, Johnson DH, Oliver JE, Hattenhauer MG, Hodge DO. The long-term outcome of glaucoma filtration surgery. Am J Ophthalmol 2001;132:27-35. https://doi.org/10.1016/S0002-9394(01)00923-0.
- Oliver JE, Hattenhauer MG, Herman D, Hodge DO, Kennedy R, Fang-Yen M, et al. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol 2002;133:764-72. https://doi.org/10.1016/S0002-9394(02)01403-4.
- Burr J, Azuara-Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD004399.pub3.
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. CIGTS Study Group . Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943-53. https://doi.org/10.1016/S0161-6420(01)00873-9.
- Collaborative Normal-Tension Glaucoma Study Group . Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126:487-97. https://doi.org/10.1016/S0002-9394(98)00223-2.
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:714-20. https://doi.org/10.1001/archopht.120.6.714.
- The Advanced Glaucoma Intervention Study Investigators . The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000;130:429-40. https://doi.org/10.1016/S0002-9394(00)00538-9.
- Stead R, Azuara-Blanco A, King AJ. Attitudes of consultant ophthalmologists in the UK to initial management of glaucoma patients presenting with severe visual field loss: a national survey. Clin Exp Ophthalmol 2011;39:858-64. https://doi.org/10.1111/j.1442-9071.2011.02574.x.
- O’Brart DP, Shiew M, Edmunds B. A randomised, prospective study comparing trabeculectomy with viscocanalostomy with adjunctive antimetabolite usage for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol 2004;88:1012-17. https://doi.org/10.1136/bjo.2003.037432.
- Bhargava JS, Patel B, Foss AJ, Avery AJ, King AJ. Views of glaucoma patients on aspects of their treatment: an assessment of patient preference by conjoint analysis. Invest Ophthalmol Vis Sci 2006;47:2885-8. https://doi.org/10.1167/iovs.05-1244.
- National Collaborating Centre for Acute Care . Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension 2009.
- Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology 1994;101:1651-6. https://doi.org/10.1016/S0161-6420(94)31120-1.
- Jay JL, Murray SB. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol 1988;72:881-9. https://doi.org/10.1136/bjo.72.12.881.
- Smith R. A comparison between medical and surgical treatment of glaucoma simplex – results of a prospective study. Trans Ophthalmol Soc Aust 1968;27:17-29.
- Ederer F, Gaasterland DE, Sullivan EK. AGIS Investigators . The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials 1994;15:299-325. https://doi.org/10.1016/0197-2456(94)90046-9.
- Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK. CIGTS Study Investigators . Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 2009;116:200-7. https://doi.org/10.1016/j.ophtha.2008.08.051.
- Leighton P, Lonsdale AJ, Tildsley J, King AJ. The willingness of patients presenting with advanced glaucoma to participate in a trial comparing primary medical vs primary surgical treatment. Eye 2012;26:300-6. https://doi.org/10.1038/eye.2011.279.
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. National Eye Institute Visual Function Questionnaire Field Test Investigators . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050-8. https://doi.org/10.1001/archopht.119.7.1050.
- McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Los Angeles Latino Eye Study Group . Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology 2008;115:941-8.e1. https://doi.org/10.1016/j.ophtha.2007.08.037.
- Hyman LG, Komaroff E, Heijl A, Bengtsson B, Leske MC. Early Manifest Glaucoma Trial Group . Treatment and vision-related quality of life in the early manifest glaucoma trial. Ophthalmology 2005;112:1505-13. https://doi.org/10.1016/j.ophtha.2005.03.028.
- Chun YS, Sung KR, Park CK, Kim HK, Yoo C, Kim YY, et al. Vision-related quality of life according to location of visual field loss in patients with glaucoma. Acta Ophthalmol 2019;97:e772-9. https://doi.org/10.1111/aos.14020.
- Daga FB, Gracitelli CPB, Diniz-Filho A, Medeiros FA. Is vision-related quality of life impaired in patients with preperimetric glaucoma?. Br J Ophthalmol 2018;103:955-9. https://doi.org/10.1136/bjophthalmol-2018-312357.
- Rulli E, Quaranta L, Riva I, Poli D, Hollander L, Galli F, et al. Visual field loss and vision-related quality of life in the Italian Primary Open Angle Glaucoma Study. Sci Rep 2018;8. https://doi.org/10.1038/s41598-017-19113-z.
- Kotecha A, Feuer WJ, Barton K, Gedde SJ. Tube Versus Trabeculectomy Study Group . Quality of life in the Tube Versus Trabeculectomy Study. Am J Ophthalmol 2017;176:228-35. https://doi.org/10.1016/j.ajo.2017.01.019.
- Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology 2016;123:552-7. https://doi.org/10.1016/j.ophtha.2015.10.046.
- Sun Y, Lin C, Waisbourd M, Ekici F, Erdem E, Wizov SS, et al. The impact of visual field clusters on performance-based measures and vision-related quality of life in patients with glaucoma. Am J Ophthalmol 2016;163:45-52. https://doi.org/10.1016/j.ajo.2015.12.006.
- Hirneiss C. The impact of a better-seeing eye and a worse-seeing eye on vision-related quality of life. Clin Ophthalmol 2014;8:1703-9. https://doi.org/10.2147/OPTH.S64200.
- Burr JM, Kilonzo M, Vale L, Ryan M. Developing a preference-based Glaucoma Utility Index using a discrete choice experiment. Optom Vis Sci 2007;84:797-808. https://doi.org/10.1097/OPX.0b013e3181339f30.
- King AJ, Hudson J, Fernie G, Burr J, Azuara-Blanco A, Sparrow JM, et al. Baseline characteristics of participants in the Treatment of Advanced Glaucoma Study: a multicenter randomized controlled trial. Am J Ophthalmol 2020;213:186-94. https://doi.org/10.1016/j.ajo.2020.01.026.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47-57. https://doi.org/10.1503/cmaj.090523.
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015;385:1295-304. https://doi.org/10.1016/S0140-6736(14)62111-5.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 2019;393:1505-16. https://doi.org/10.1016/S0140-6736(18)32213-X.
- Gedde SJ, Feuer WJ, Lim KS, Barton K, Goyal S, Ahmed IIK, et al. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 3 years of follow-up. Ophthalmology 2020;127:333-45. https://doi.org/10.1016/j.ophtha.2019.10.002.
- Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120:2532-9. https://doi.org/10.1016/j.ophtha.2013.07.049.
- Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ 2005;331. https://doi.org/10.1136/bmj.38506.594977.E0.
- Parrish RK, Palmberg P, Sheu WP. XLT Study Group . A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003;135:688-703. https://doi.org/10.1016/S0002-9394(03)00098-9.
- Hodapp E, Parrish RK, Anderson DR. Clinical Decisions in Glaucoma. St Louis, MO: Mosby Inc.; 1993.
- National Institute for Health and Care Excellence (NICE) . Glaucoma: Diagnosis and Management. NICE Guideline [NG81] 2017.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. https://doi.org/10.1016/S0140-6736(09)61116-8.
- Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, et al. Challenges in evaluating surgical innovation. Lancet 2009;374:1097-104. https://doi.org/10.1016/S0140-6736(09)61086-2.
- Cate H, Bhattacharya D, Clark A, Holland R, Broadway DC. A comparison of measures used to describe adherence to glaucoma medication in a randomised controlled trial. Clin Trials 2015;12:608-17. https://doi.org/10.1177/1740774515592636.
- Richman J, Lorenzana LL, Lankaranian D, Dugar J, Mayer JR, Wizov SS, et al. Relationships in glaucoma patients between standard vision tests, quality of life, and ability to perform daily activities. Ophthalmic Epidemiol 2010;17:144-51. https://doi.org/10.3109/09286581003734878.
- Guedes RA, Guedes VM, Freitas SM, Chaoubah A. Quality of life of medically versus surgically treated glaucoma patients. J Glaucoma 2013;22:369-73. https://doi.org/10.1097/IJG.0b013e31824ceb8b.
- Wu P, Xi S, Xia H, Lu H, Guo W. Survey on vision-related quality of life and self-management among patients with glaucoma. J Glaucoma 2014;23:75-80. https://doi.org/10.1097/IJG.0b013e318265bbf3.
- Gutierrez P, Wilson MR, Johnson C, Gordon M, Cioffi GA, Ritch R, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol 1997;115:777-84. https://doi.org/10.1001/archopht.1997.01100150779014.
- Jampel HD, Friedman DS, Quigley H, Miller R. Correlation of the binocular visual field with patient assessment of vision. Invest Ophthalmol Vis Sci 2002;43:1059-67.
- Nelson P, Aspinall P, Papasouliotis O, Worton B, O’Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma 2003;12:139-50. https://doi.org/10.1097/00061198-200304000-00009.
- Azuara-Blanco A, Burr J, Ramsay C, Cooper D, Foster PJ, Friedman DS, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet 2016;388:1389-97. https://doi.org/10.1016/S0140-6736(16)30956-4.
- Prior M, Ramsay CR, Burr JM, Campbell SE, Jenkinson DJ, Asoaka R, et al. Theoretical and empirical dimensions of the Aberdeen Glaucoma Questionnaire: a cross sectional survey and principal component analysis. BMC Ophthalmol 2013;13. https://doi.org/10.1186/1471-2415-13-72.
- Chehamzeh J. Assessment of Glaucoma: Using Patient Reported Measures in Randomised Controlled Trials 2011.
- Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci 2009;50:3629-35. https://doi.org/10.1167/iovs.08-3225.
- Candlish J, Teare MD, Dimairo M, Flight L, Mandefield L, Walters SJ. Appropriate statistical methods for analysing partially nested randomised controlled trials with continuous outcomes: a simulation study. BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0559-x.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237-70. https://doi.org/10.1177/0962280209105014.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht, the Netherlands: Springer; 2007.
- Ryan M, Netten A, Skåtun D, Smith P. Using discrete choice experiments to estimate a preference-based measure of outcome – an application to social care for older people. J Health Econ 2006;25:927-44. https://doi.org/10.1016/j.jhealeco.2006.01.001.
- McKenzie L, Cairns J, Osman L. Symptom-based outcome measures for asthma: the use of discrete choice methods to assess patient preferences. Health Policy 2001;57:193-204. https://doi.org/10.1016/S0168-8510(01)00128-2.
- McFadden D. Conditional Logit Analysis of Qualitative Choice Behaviour. Berkeley, CA: University of California at Berkeley; 1973.
- Greiner R, Bliemer M, Ballweg J. Design considerations of a choice experiment to estimate likely participation by north Australian pastoralists in contractual biodiversity conservation. J Choice Model 2014;10:34-45. https://doi.org/10.1016/j.jocm.2014.01.002.
- Bliemer MCJ, Rose JM. Experimental design influences on stated choice outputs: an empirical study in air travel choice. Transp Res Part A Policy Pract 2011;45:63-79. https://doi.org/10.1016/j.tra.2010.09.003.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013;16:3-13. https://doi.org/10.1016/j.jval.2012.08.2223.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008;115:1123-9.e. https://doi.org/10.1016/j.ophtha.2007.10.031.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017–2018 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 22 May 2020).
- Department for Transport . TAG Data Book 2019. www.gov.uk/government/publications/tag-data-book (accessed 16 December 2020).
- Office for National Statistics . Employee Earnings in the UK: 2018 2018.
- NHS Improvement . National Cost Collection for the NHS n.d. https://improvement.nhs.uk/resources/national-cost-collection/ (accessed 1 May 2018).
- Curtis L, Burns A, Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- UK Government . Simplified Expenses If You’re Self-Employed. Business and Self-Employed 2018 n.d. www.gov.uk/simpler-income-tax-simplified-expenses (accessed 1 May 2018).
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-54.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2010.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. https://doi.org/10.1002/hec.1093.
- Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet 2017;390:2183-93. https://doi.org/10.1016/S0140-6736(17)31469-1.
- Pan Y, Varma R. Natural history of glaucoma. Indian J Ophthalmol 2011;59:S19-23. https://doi.org/10.4103/0301-4738.73682.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Brusini P, Filacorda S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J Glaucoma 2006;15:40-6. https://doi.org/10.1097/01.ijg.0000195932.48288.97.
- Hernández RA, Burr JM, Vale LD. OAG Screening Project Group . Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care 2008;24:203-11. https://doi.org/10.1017/S0266462308080288.
- Javanbakht M, Azuara-Blanco A, Burr JM, Ramsay C, Cooper D, Cochran C, et al. Early lens extraction with intraocular lens implantation for the treatment of primary angle closure glaucoma: an economic evaluation based on data from the EAGLE trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013254.
- Stein JD, Kim DD, Peck WW, Giannetti SM, Hutton DW. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol 2012;130:497-505. https://doi.org/10.1001/archophthalmol.2011.2727.
- Choi JA, Song LD, Choi S, Park SM, Kwon JW, Jee D. The cost-effectiveness of medication, laser trabeculoplasty, and trabeculectomy for treatment of open-angle glaucoma in South Korea. Medicine 2019;98. https://doi.org/10.1097/MD.0000000000014026.
- Hunink MM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, et al. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge: Cambridge University Press; 2014.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2011.
- Kobelt G. Health economics, economic evaluation, and glaucoma. J Glaucoma 2002;11:531-9. https://doi.org/10.1097/00061198-200212000-00015.
- Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999;97:473-511.
- Crabb DP, Russell RA, Malik R, Anand N, Baker H, Boodhna T, et al. Frequency of visual field testing when monitoring patients newly diagnosed with glaucoma: mixed methods and modelling. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02270.
- Office for National Statistics . Interim Life Tables. National Life Tables, UK: 2016 to 2018 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/previousReleases (accessed 2019).
- Krumpaszky HG, Dietz K, Mickler A, Selbmann HK. Mortality in blind subjects. Ophthalmologica 1999;213:48-53. https://doi.org/10.1159/000027393.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment. London, UK: Springer; 2015.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Tosh J, Brazier J, Evans P, Longworth L. A review of generic preference-based measures of health-related quality of life in visual disorders. Value Health 2012;15:118-27. https://doi.org/10.1016/j.jval.2011.08.002.
- York Health Economics Consortium . Probabilistic/Stochastic/Sensitivity/Analysis;/2016 n.d. https://yhec.co.uk/glossary/probabilistic-stochastic-sensitivity-analysis/ (accessed 2020).
- Kulkarni BB, Leighton P, King AJ. Exploring patients’ expectations and preferences of glaucoma surgery outcomes to facilitate healthcare delivery and inform future glaucoma research. Br J Ophthalmol 2019;103:1850-5. https://doi.org/10.1136/bjophthalmol-2018-313401.
- Bhargava JS, Bhan-Bhargava A, Foss AJ, King AJ. Views of glaucoma patients on provision of follow-up care; an assessment of patient preferences by conjoint analysis. Br J Ophthalmol 2008;92:1601-5. https://doi.org/10.1136/bjo.2008.140483.
- Bergholz R, Dutescu RM, Steinhagen-Thiessen E, Rosada A. Ophthalmologic health status of an aging population-data from the Berlin Aging Study II (BASE-II). Graefes Arch Clin Exp Ophthalmol 2019;257:1981-8. https://doi.org/10.1007/s00417-019-04386-z.
- McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP. Los Angeles Latino Eye Study Group . Severity of visual field loss and health-related quality of life. Am J Ophthalmol 2007;143:1013-23. https://doi.org/10.1016/j.ajo.2007.02.022.
- Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122:293-301. https://doi.org/10.1016/j.ophtha.2014.08.014.
- Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE, et al. CIGTS Study Group . The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology 2001;108:1954-65. https://doi.org/10.1016/S0161-6420(01)00874-0.
- Thygesen J, Aagren M, Arnavielle S, Bron A, Fröhlich SJ, Baggesen K, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin 2008;24:1763-70. https://doi.org/10.1185/03007990802111068.
- Montemayor F, Sibley LM, Courtright P, Mikelberg FS. Contribution of multiple glaucoma medications to visual function and quality of life in patients with glaucoma. Can J Ophthalmol 2001;36:385-90. https://doi.org/10.1016/S0008-4182(01)80082-X.
- Kobelt G, Jonsson B, Bergström A, Chen E, Lindén C, Alm A. Cost-effectiveness analysis in glaucoma: what drives utility? Results from a pilot study in Sweden. Acta Ophthalmol Scand 2006;84:363-71. https://doi.org/10.1111/j.1600-0420.2005.00621.x.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23310.
- Jones L, Garway-Heath DF, Azuara-Blanco A, Crabb DP. United Kingdom Glaucoma Treatment Study Investigators . Are patient self-reported outcome measures sensitive enough to be used as end points in clinical trials?: evidence from the United Kingdom Glaucoma Treatment Study. Ophthalmology 2019;126:682-9. https://doi.org/10.1016/j.ophtha.2018.09.034.
- Damji KF, Behki R, Wang L, Target IOP. Workshop participants. Canadian perspectives in glaucoma management: setting target intraocular pressure range. Can J Ophthalmol 2003;38:189-97. https://doi.org/10.1016/S0008-4182(03)80060-1.
- Stead RE, King AJ. Outcome of trabeculectomy with mitomycin C in patients with advanced glaucoma. Br J Ophthalmol 2011;95:960-5. https://doi.org/10.1136/bjo.2010.185272.
- Chen R, King AJ. Lifetime visual outcomes of patients undergoing trabeculectomy. Br J Ophthalmol 2021;105:1566-70.
- Jones E, Clarke J, Khaw PT. Recent advances in trabeculectomy technique. Curr Opin Ophthalmol 2005;16:107-13. https://doi.org/10.1097/01.icu.0000156138.05323.6f.
- King AJ, Rotchford AP, Alwitry A, Moodie J. Frequency of bleb manipulations after trabeculectomy surgery. Br J Ophthalmol 2007;91:873-7. https://doi.org/10.1136/bjo.2006.109835.
- Khaw PT, Chiang M, Shah P, Sii F, Lockwood A, Khalili A. Enhanced trabeculectomy: the Moorfields Safer Surgery System. Dev Ophthalmol 2017;59:15-3. https://doi.org/10.1159/000458483.
- Stalmans I, Gillis A, Lafaut AS, Zeyen T. Safe trabeculectomy technique: long term outcome. Br J Ophthalmol 2006;90:44-7. https://doi.org/10.1136/bjo.2005.072884.
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268-79. https://doi.org/10.1001/archopht.120.10.1268.
- Olali C, Rotchford AP, King AJ. Outcome of repeat trabeculectomies. Clin Experiment Ophthalmol 2011;39:658-64. https://doi.org/10.1111/j.1442-9071.2011.02519.x.
- Jampel H. Trabeculectomy: more effective at causing cataract surgery than lowering intraocular pressure?. Ophthalmology 2009;116:173-4. https://doi.org/10.1016/j.ophtha.2008.11.016.
- Musch DC, Gillespie BW, Niziol LM, Janz NK, Wren PA, Rockwood EJ, et al. Collaborative Initial Glaucoma Treatment Study Group . Cataract extraction in the collaborative initial glaucoma treatment study: incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality-of-life outcomes. Arch Ophthalmol 2006;124:1694-700. https://doi.org/10.1001/archopht.124.12.1694.
- Husain R, Liang S, Foster PJ, Gazzard G, Bunce C, Chew PT, et al. Cataract surgery after trabeculectomy: the effect on trabeculectomy function. Arch Ophthalmol 2012;130:165-70. https://doi.org/10.1001/archophthalmol.2011.329.
- Longo A, Uva MG, Reibaldi A, Avitabile T, Reibaldi M. Long-term effect of phacoemulsification on trabeculectomy function. Eye 2015;29:1347-52. https://doi.org/10.1038/eye.2015.108.
- Rebolleda G, Muñoz-Negrete FJ. Effect of cataract surgery on IOP after trabeculectomy. J Cataract Refract Surg 2003;29. https://doi.org/10.1016/j.jcrs.2003.09.029.
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002;16:297-303. https://doi.org/10.1038/sj.eye.6700148.
- Moster MR, Moster ML. Wipe-out: a complication of glaucoma surgery or just a blast from the past?. Am J Ophthalmol 2005;140:705-6. https://doi.org/10.1016/j.ajo.2005.05.024.
- Topouzis F, Tranos P, Koskosas A, Pappas T, Anastasopoulos E, Dimitrakos S, et al. Risk of sudden visual loss following filtration surgery in end-stage glaucoma. Am J Ophthalmol 2005;140:661-6. https://doi.org/10.1016/j.ajo.2005.04.016.
- Balekudaru S, George R, Panday M, Singh M, Neog A, Lingam V. Prospective evaluation of early visual loss following glaucoma-filtering surgery in eyes with split fixation. J Glaucoma 2014;23:211-18. https://doi.org/10.1097/IJG.0000000000000041.
- Law SK, Nguyen AM, Coleman AL, Caprioli J. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch Ophthalmol 2007;125:1044-50. https://doi.org/10.1001/archopht.125.8.1044.
- Costa VP, Smith M, Spaeth GL, Gandham S, Markovitz B. Loss of visual acuity after trabeculectomy. Ophthalmology 1993;100:599-612. https://doi.org/10.1016/S0161-6420(93)31597-6.
- Luebke J, Neuburger M, Jordan JF, Wecker T, Boehringer D, Cakir B, et al. Bleb-related infections and long-term follow-up after trabeculectomy. Int Ophthalmol 2019;39:571-7. https://doi.org/10.1007/s10792-018-0851-0.
- Kim EA, Law SK, Coleman AL, Nouri-Mahdavi K, Giaconi JA, Yu F, et al. Long-term bleb-related infections after trabeculectomy: incidence, risk factors, and influence of bleb revision. Am J Ophthalmol 2015;159:1082-91. https://doi.org/10.1016/j.ajo.2015.03.001.
- Wong MH, Husain R, Ang BC, Gazzard G, Foster PJ, Htoon HM, et al. The Singapore 5-fluorouracil trial: intraocular pressure outcomes at 8 years. Ophthalmology 2013;120:1127-34. https://doi.org/10.1016/j.ophtha.2012.12.004.
- Zahid S, Musch DC, Niziol LM, Lichter PR. Collaborative Initial Glaucoma Treatment Study Group . Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol 2013;155:674-80. https://doi.org/10.1016/j.ajo.2012.10.017.
- Alwitry A, King AJ. Surveillance of late-onset bleb leak, blebitis and bleb-related endophthalmitis – a UK incidence study. Graefes Arch Clin Exp Ophthalmol 2012;250:1231-6. https://doi.org/10.1007/s00417-011-1920-5.
- Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-9.
- Guedes RA, Guedes VM, Chaoubah A. Cost-effectiveness comparison between non-penetrating deep sclerectomy and maximum-tolerated medical therapy for glaucoma within the Brazilian National Health System (SUS). Arq Bras Oftalmol 2012;75:11-5. https://doi.org/10.1590/S0004-27492012000100002.
- Ting NS, Li Yim JF, Ng JY. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. ClinicoEcon Outcomes Res 2014;6:523-30. https://doi.org/10.2147/CEOR.S30697.
- Bartelt-Hofer J, Ben-Debba L, Flessa S. Systematic review of economic evaluations in primary open-angle glaucoma: decision analytic modeling insights. PharmacoEcon Open 2020;4:5-12. https://doi.org/10.1007/s41669-019-0141-4.
- Guedes RAP, Guedes VMP, Gomes CEM, Chaoubah A. Maximizing cost-effectiveness by adjusting treatment strategy according to glaucoma severity. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000005745.
- Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol 2006;17:190-5. https://doi.org/10.1097/01.icu.0000193078.47616.aa.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 22 May 2020).
- Curtis L, Burns A, Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Department of Health and Social Care (DHSC) . General Ophthalmic Service Fees and Vouncher Values from April 2018 2018.
- Azuara-Blanco A, Banister K, Boachie C, McMeekin P, Gray J, Burr J, et al. Automated imaging technologies for the diagnosis of glaucoma: a comparative diagnostic study for the evaluation of the diagnostic accuracy, performance as triage tests and cost-effectiveness (GATE study). Health Technol Assess 2016;20. https://doi.org/10.3310/hta20080.
Appendix 1 Trial flow diagram
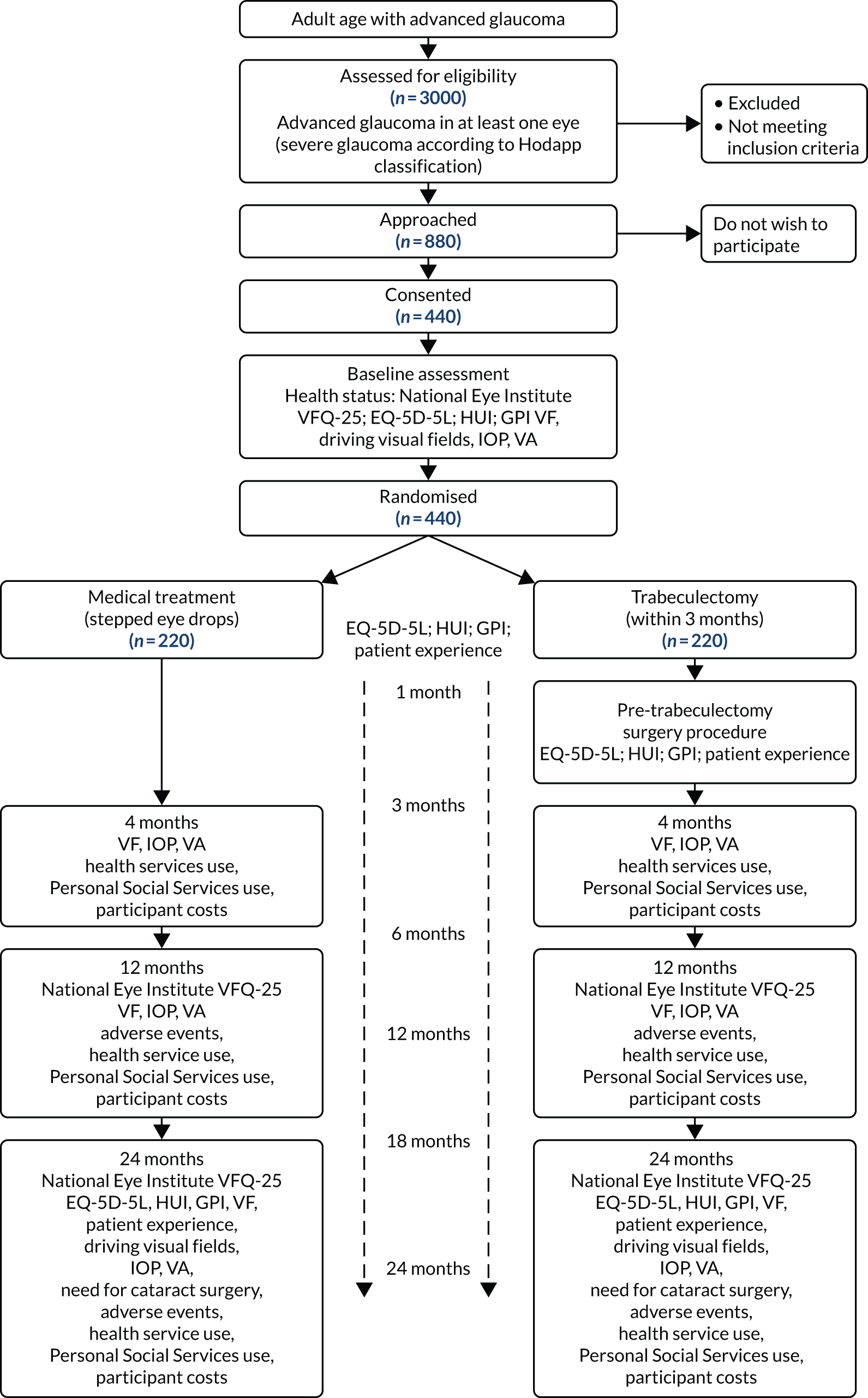
Appendix 2 Participant characteristics
| Reasona | Number of participants (n) | Percentage of total number of participants excluded |
|---|---|---|
| Reasons for ineligibility | ||
| Visual fields did not meet criteria | 56 | 24.0 |
| Could not be randomised within 3 months of diagnosis | 53 | 23.1 |
| Secondary glaucomas and primary angle-closure glaucoma | 25 | 10.7 |
| Patient unable to provide informed consent | 20 | 8.7 |
| Unreliable visual fields | 14 | 6.1 |
| Did not meet eligibility criteria | 12 | 5.2 |
| Not advanced glaucoma | 11 | 4.8 |
| Unable to undergo incisional surgery | 9 | 3.9 |
| Non-English speaker | 6 | 2.6 |
| Did not attend appointments | 1 | 0.4 |
| Female who is pregnant, nursing, planning a pregnancy or not using reliable contraception | 1 | 0.4 |
| Other clinical reason | 29 | 12.4 |
| Other non-clinical reason | 3 | 1.3 |
| Reasons for declining to take part | ||
| Patient declined to give a reason | 77 | 27.9 |
| Patient did not want surgery | 54 | 19.6 |
| Lifestyle factors (e.g. work or family commitments) | 42 | 15.2 |
| Did not wish to participate in a trial | 40 | 14.5 |
| Did not wish to be randomised | 23 | 8.3 |
| Had preference for medical or for surgery | 23 | 8.3 |
| Other reasons | 37 | 13.4 |
| Characteristic | Treatment arm | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Lens status, n (%) | ||
| Phakic | 212 (93.4) | 206 (91.2) |
| Pseudophakic | 15 (6.6) | 19 (8.4) |
| Missing | – | 1 (0.4) |
| Central corneal thickness, n; mean (SD) | 226; 540.6 (38.0) | 222; 541.2 (35.6) |
| Glaucoma eye drops, n (%) | ||
| Prostaglandin analogue | 161 (70.9) | 159 (70.4) |
| Beta-blocker | 46 (20.3) | 36 (15.9) |
| Carbonic anhydrase inhibitor | 33 (14.5) | 21 (9.3) |
| α-agonist | 5 (2.2) | 2 (0.9) |
| Ocular comorbidity, n (%) | ||
| Yes | 50 (22.0) | 48 (21.2) |
| No | 177 (78.0) | 178 (78.8) |
| Ocular comorbidity details, n (%) | ||
| AMD | 6 (12.0) | 4 (8.0) |
| Cataract | 39 (78.0) | 36 (72.0) |
| Diabetic retinopathy | 1 (2.0) | 1 (2.0) |
| Other | 8 (16.0) | 8 (16.0) |
| LogMAR VA, n; mean (SD) | 224; 0.1 (0.2) | 224; 0.1 (0.3) |
| VFMD (dB), n; mean (SD) | 227; –6.1 (7.7) | 224; –6.1 (7.1) |
| IOP (mmHg), n; mean (SD) | ||
| Diagnosis | 226; 23.3 (7.6) | 222; 22.4 (6.3) |
| Baseline | 222; 18.0 (5.1) | 220; 17.9 (4.2) |
| Classification | Treatment arm, n (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Mean deviation of < –12.00 dB | 161 (70.9) | 163 (72.1) |
| > 20 points defective at the 1% level | 198 (87.2) | 207 (91.6) |
| A point in the central 5 degrees has a sensitivity of 0 dB | 143 (63.0) | 129 (57.1) |
| > 50% of points defective in the pattern deviation probability plot at the 5% level | 193 (85.0) | 202 (89.4) |
| Points with 5 degrees of fixation under 15 dB sensitivity in both upper and lower hemifields | 42 (18.5) | 51 (22.6) |
Appendix 3 Clinical results
| Surgery details | Treatment arm, n (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Received surgery in their index eye | 201 (88.5) | 39 (17.3) |
| Surgery clinical report form provided | 199 | 27 |
| Surgeon gradea | ||
| Consultant | 176 (88.4) | 25 (92.6) |
| Fellow | 37 (18.6) | 5 (18.5) |
| Other | 11 (5.5) | 3 (11.1) |
| Missing | 3 (1.5) | 0 (0) |
| Anaesthetist gradea | ||
| Consultant | 120 (60.3) | 21 (77.8) |
| Fellow | 16 (8.0) | 3 (11.1) |
| Other | 16 (8.0) | 3 (11.1) |
| Missing | 58 (29.1) | 4 (14.8) |
| Type of anaesthesia | ||
| Regional block | 163 (81.9) | 23 (85.2) |
| General | 35 (17.6) | 4 (14.8) |
| Missing | 1 (0.5) | 0 (0) |
| Traction suture | ||
| Corneal | 184 (92.5) | 24 (88.9) |
| Superior rectus | 5 (2.5) | 1 (3.7) |
| Missing | 10 (5.0) | 2 (7.4) |
| Conjunctival flap | ||
| Fornix based | 186 (93.5) | 26 (96.3) |
| Limbal based | 12 (6.0) | 1 (3.7) |
| Missing | 1 (0.5) | 0 (0) |
| MMC dose | ||
| 0.2 mg/ml | 141 (70.9) | 23 (85.2) |
| 0.4 mg/ml | 42 (21.1) | 3 (11.1) |
| Other | 15 (7.5) | 1 (3.7) |
| Missing | 1 (0.5) | 0 (0) |
| MMC duration | ||
| 3 minutes | 151 (75.9) | 21 (77.8) |
| Other | 45 (22.6) | 5 (18.5) |
| Missing | 3 (1.5) | 1 (3.7) |
| Scleral flap sutures | ||
| Interrupted | 163 (81.9) | 24 (88.9) |
| Releasable | 86 (43.2) | 14 (51.9) |
| Adjustable | 29 (14.6) | 3 (11.1) |
| A/C maintainer | ||
| Yes | 61 (30.7) | 10 (37.0) |
| No | 137 (68.8) | 17 (63.0) |
| Missing | 1 (0.5) | 0 (0) |
| Pre-operative aproclonidine | ||
| Yes | 143 (71.9) | 20 (74.1) |
| No | 55 (27.6) | 7 (25.9) |
| Missing | 1 (0.5) | 0 (0) |
| Peri-operative miochol | ||
| Yes | 23 (11.6) | 3 (11.1) |
| No | 175 (87.9) | 24 (88.9) |
| Missing | 1 (0.5) | 0 (0) |
| Peri-operative viscoelastic | ||
| Yes | 36 (18.1) | 7 (25.9) |
| No | 162 (81.4) | 20 (74.1) |
| Missing | 1 (0.5) | 0 (0) |
| Subconjunctival antibiotic | ||
| Yes | 144 (72.4) | 23 (85.2) |
| No | 54 (27.1) | 4 (14.8) |
| Missing | 1 (0.5) | 0 (0) |
| Subconjunctival steroid | ||
| Yes | 175 (87.9) | 25 (92.6) |
| No | 23 (11.6) | 2 (7.4) |
| Missing | 1 (0.5) | 0 (0) |
| Treatment arm | ||
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Received trabeculectomy in their non-index eye, n (%) | 34 (15.0) | 13 (5.8) |
| Time to trabeculectomy (weeks), n; median (IQR) | 32; 24.1 (9.2–40.9) | 8; 54.7 (27.1–64.1) |
| Reasons for trabeculectomy,a n (%) | ||
| Uncontrolled IOP | 6 (46.2) | |
| Visual field progression | 3 (23.1) | |
| Missing | 5 (38.5) | |
| Trabeculectomy clinical report form provided | 34 | 8 |
| Pre-operation drugs, n (%) | ||
| Carbonic anhydrase inhibitor | 8 (23.5) | 2 (25.0) |
| Prostaglandin analogue | 26 (76.5) | 5 (62.5) |
| Beta-blocker | 14 (41.2) | 3 (37.5) |
| α-agonist | 2 (5.9) | 0 (0) |
| Diamox | ||
| Yes, n (%) | 1 (2.9) | 0 (0) |
| No, n (%) | 32 (94.1) | 8 (100.0) |
| Missing, n (%) | 1 (2.9) | 0 (0) |
| Pre-operative IOP, n; mean (SD) | 32; 18.3 (6.2) | 7; 20.1 (2.7) |
| Surgeon grade,a n (%) | ||
| Consultant | 32 (94.1) | 7 (87.5) |
| Fellow | 4 (11.8) | 1 (12.5) |
| Other | 1 (2.9) | 1 (12.5) |
| Missing | 1 (2.9) | 1 (12.5) |
| Anaesthetist grade,a n (%) | ||
| Consultant | 18 (52.9) | 3 (37.5) |
| Fellow | 2 (5.9) | 0 (0) |
| Other | 3 (8.8) | 0 (0) |
| Missing | 13 (38.2) | 5 (62.5) |
| Type of anaesthesia, n (%) | ||
| Regional block | 23 (67.6) | 7 (87.5) |
| General | 10 (29.4) | 0 (0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Traction suture, n (%) | ||
| Corneal | 31 (91.2) | 7 (87.5) |
| Superior rectus | 1 (2.9) | 0 (0) |
| Missing | 2 (5.9) | 1 (12.5) |
| Conjunctival flap, n (%) | ||
| Fornix based | 33 (97.1) | 7 (87.5) |
| Missing | 1 (2.9) | 1 (12.5) |
| MMC dose, n (%) | ||
| 0.2 mg/ml | 23 (67.6) | 4 (50.0) |
| 0.4 mg/ml | 8 (23.5) | 3 (37.5) |
| Other | 2 (5.9) | 0 (0) |
| Missing | 1 (2.9) | 1 (12.5) |
| MMC duration, n (%) | ||
| 3 minutes | 24 (70.6) | 6 (75.0) |
| Other | 9 (26.5) | 1 (12.5) |
| Missing | 1 (2.9) | 1 (12.5) |
| Scleral flap sutures, n (%) | ||
| Interrupted | 28 (82.4) | 6 (75.0) |
| Releasable | 13 (38.2) | 4 (50.0) |
| Adjustable | 4 (11.8) | 1 (12.5) |
| A/C maintainer, n (%) | ||
| Yes | 6 (17.6) | 5 (62.5) |
| No | 27 (79.4) | 2 (25.0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Pre-operative lopidine, n (%) | ||
| Yes | 21 (61.8) | 7 (87.5) |
| No | 12 (35.3) | 0 (0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Peri-operative miochol, n (%) | ||
| Yes | 4 (11.8) | 0 (0) |
| No | 29 (85.3) | 7 (87.5) |
| Missing | 1 (2.9) | 1 (12.5) |
| Peri-operative viscoelastic, n (%) | ||
| Yes | 6 (17.6) | 3 (37.5) |
| No | 27 (79.4) | 4 (50.0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Subconjunctival antibiotic, n (%) | ||
| Yes | 25 (73.5) | 7 (87.5) |
| No | 8 (23.5) | 0 (0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Subconjunctival steroid, n (%) | ||
| Yes | 29 (85.3) | 7 (87.5) |
| No | 4 (11.8) | 0 (0) |
| Missing | 1 (2.9) | 1 (12.5) |
| Time point | Treatment arm, n; mean (SD) | Adjusted MD | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| VFQ-25 | |||||
| Baseline | 200; 86.8 (14.1) | 185; 87.5 (12.9) | |||
| 4 months | 190; 84.8 (15.3) | 177; 87.2 (12.8) | –1.78 | –4.24 to 0.69 | 0.158 |
| 12 months | 193; 85.4 (14.3) | 171; 87.1 (12.9) | –0.86 | –3.34 to 1.62 | 0.496 |
| 24 months | 187; 85.3 (14.1) | 168; 84.9 (16.4) | 0.95 | –1.54 to 3.45 | 0.454 |
| VFQ-25 subscales | |||||
| Near activities | |||||
| Baseline | 199; 84.2 (18.6) | 185; 85.2 (16.1) | |||
| 4 months | 189; 83.7 (18.0) | 175; 85.6 (16.2) | –1.30 | –5.05 to 2.45 | 0.498 |
| 12 months | 193; 84.0 (18.6) | 171; 85.5 (17.7) | –0.74 | –4.50 to 3.02 | 0.700 |
| 24 months | 185; 82.4 (18.6) | 167; 83.2 (19.6) | –0.13 | –3.93 to 3.68 | 0.948 |
| Distance activities | |||||
| Baseline | 200; 88.0 (16.5) | 185; 89.5 (14.4) | |||
| 4 months | 189; 87.6 (16.9) | 177; 89.6 (14.3) | –0.88 | –4.13 to 2.37 | 0.594 |
| 12 months | 193; 88.5 (16.0) | 171; 89.2 (15.4) | 0.41 | –2.86 to 3.68 | 0.806 |
| 24 months | 187; 88.2 (16.0) | 167; 86.5 (18.9) | 2.51 | –0.78 to 5.80 | 0.135 |
| Dependency | |||||
| Baseline | 200; 93.4 (18.3) | 184; 95.0 (14.8) | |||
| 4 months | 189; 90.6 (20.9) | 177; 93.6 (17.2) | –2.33 | –5.86 to 1.19 | 0.194 |
| 12 months | 192; 92.2 (19.4) | 171; 94.8 (13.9) | –1.48 | –5.02 to 2.06 | 0.413 |
| 24 months | 186; 93.4 (16.1) | 167; 92.8 (18.4) | 1.73 | –1.85 to 5.31 | 0.344 |
| Driving | |||||
| Baseline | 150; 85.2 (28.0) | 130; 86.5 (23.6) | |||
| 4 months | 136; 81.8 (30.8) | 127; 84.4 (25.7) | 0.49 | –7.03 to 8.01 | 0.899 |
| 12 months | 138; 81.4 (28.9) | 121; 81.5 (28.5) | 1.58 | –6.02 to 9.17 | 0.684 |
| 24 months | 130; 81.8 (28.8) | 112; 81.8 (26.6) | 2.76 | –4.92 to 10.44 | 0.481 |
| General health | |||||
| Baseline | 199; 63.1 (23.3) | 185; 60.7 (22.2) | |||
| 4 months | 189; 66.3 (23.6) | 176; 64.2 (20.8) | –0.20 | –4.71 to 4.31 | 0.930 |
| 12 months | 193; 66.2 (23.7) | 170; 59.3 (23.2) | 5.15 | 0.61 to 9.68 | 0.026 |
| 24 months | 187; 63.5 (25.6) | 168; 59.1 (24.7) | 2.41 | –2.16 to 6.97 | 0.302 |
| Role difficulties | |||||
| Baseline | 200; 86.7 (20.2) | 184; 88.1 (19.7) | |||
| 4 months | 190; 81.8 (25.1) | 177; 88.2 (19.0) | –6.64 | –10.88 to –2.39 | 0.002 |
| 12 months | 192; 83.1 (23.2) | 171; 86.3 (19.8) | –2.97 | –7.24 to 1.30 | 0.173 |
| 24 months | 187; 83.0 (22.8) | 167; 84.1 (22.4) | –1.25 | –5.56 to 3.06 | 0.570 |
| Mental health | |||||
| Baseline | 200; 80.8 (21.8) | 185; 82.9 (19.1) | |||
| 4 months | 190; 79.0 (24.1) | 177; 84.6 (19.0) | –4.39 | –8.40 to –0.38 | 0.032 |
| 12 months | 193; 80.0 (21.8) | 171; 84.4 (19.0) | –3.29 | –7.32 to 0.74 | 0.109 |
| 24 months | 187; 80.9 (20.4) | 168; 82.5 (21.2) | –0.75 | –4.81 to 3.31 | 0.719 |
| General vision | |||||
| Baseline | 197; 74.9 (14.8) | 184; 73.2 (13.5) | |||
| 4 months | 189; 71.1 (14.6) | 176; 75.0 (12.2) | –4.46 | –7.45 to –1.47 | 0.003 |
| 12 months | 192; 72.8 (14.6) | 170; 74.2 (14.0) | –2.05 | –5.06 to 0.95 | 0.180 |
| 24 months | 187; 72.9 (13.5) | 168; 72.9 (14.9) | –0.73 | –3.76 to 2.30 | 0.637 |
| Social function | |||||
| Baseline | 199; 94.7 (12.5) | 185; 94.7 (12.1) | |||
| 4 months | 189; 94.9 (12.4) | 177; 95.0 (11.3) | –0.23 | –3.05 to 2.59 | 0.871 |
| 12 months | 193; 94.1 (13.9) | 170; 94.7 (11.8) | –0.76 | –3.60 to 2.08 | 0.602 |
| 24 months | 186; 94.7 (12.6) | 168; 92.9 (17.1) | 1.36 | –1.50 to 4.22 | 0.350 |
| Colour vision | |||||
| Baseline | 198; 96.6 (11.4) | 183; 96.4 (10.9) | |||
| 4 months | 187; 97.1 (9.2) | 175; 96.9 (10.6) | 0.26 | –1.81 to 2.33 | 0.806 |
| 12 months | 192; 96.1 (11.3) | 167; 97.3 (9.1) | –1.11 | –3.20 to 0.99 | 0.300 |
| 24 months | 186; 95.3 (14.5) | 167; 94.6 (16.0) | 0.90 | –1.20 to 3.00 | 0.401 |
| Peripheral vision | |||||
| Baseline | 199; 86.1 (21.4) | 185; 87.7 (19.9) | |||
| 4 months | 188; 85.8 (20.7) | 175; 86.1 (19.8) | –0.41 | –4.88 to 4.07 | 0.859 |
| 12 months | 193; 86.8 (20.1) | 169; 86.5 (20.6) | 0.44 | –4.06 to 4.93 | 0.849 |
| 24 months | 185; 84.9 (19.9) | 167; 84.3 (22.4) | 0.26 | –4.27 to 4.78 | 0.911 |
| Ocular pain | |||||
| Baseline | 199; 84.2 (19.3) | 185; 85.1 (16.6) | |||
| 4 months | 190; 81.1 (19.6) | 177; 81.1 (18.7) | 0.33 | –3.10 to 3.75 | 0.851 |
| 12 months | 193; 81.3 (20.3) | 171; 83.4 (15.4) | –1.46 | –4.91 to 1.98 | 0.405 |
| 24 months | 187; 81.9 (19.7) | 168; 81.0 (18.9) | 1.72 | –1.76 to 5.20 | 0.334 |
| Time point | MD | 95% CI | p-value |
|---|---|---|---|
| 4 months | –1.75 | –4.13 to 0.63 | 0.151 |
| 12 months | –0.99 | –3.74 to 1.76 | 0.479 |
| 24 months | 1.52 | –1.27 to 4.30 | 0.286 |
| Time point | Treatment arm, n; mean (SD) | Adjusted MD | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| N = 271 | N = 270 | ||||
| Intraocular pressure (mmHg) | |||||
| Baseline | 266; 19.29 (6.24) | 265; 18.97 (5.55) | |||
| 4 months | 259; 12.87 (6.00) | 262; 16.35 (4.05) | –3.74 | –4.69 to –2.79 | < 0.001 |
| 12 months | 257; 12.17 (4.50) | 248; 16.15 (4.40) | –4.21 | –5.17 to –3.25 | < 0.001 |
| 24 months | 246; 12.63 (4.65) | 240; 15.12 (4.71) | –2.74 | –3.72 to –1.77 | < 0.001 |
| LogMAR VA | |||||
| Baseline | 268; 0.15 (0.25) | 267; 0.17 (0.26) | |||
| 4 months | 250; 0.24 (0.29) | 258; 0.16 (0.23) | 0.10 | 0.06 to 0.14 | < 0.001 |
| 12 months | 253; 0.18 (0.25) | 249; 0.16 (0.25) | 0.04 | –0.00 to 0.08 | 0.077 |
| 24 months | 239; 0.21 (0.28) | 237; 0.16 (0.25) | 0.07 | 0.02 to 0.11 | 0.003 |
| Visual fields mean deviation (dB) | |||||
| Baseline | 271; –15.38 (6.93) | 270; –15.66 (6.51) | |||
| 4 months | 251; –14.68 (7.14) | 258; –15.09 (6.60) | 0.03 | –0.74 to 0.81 | 0.930 |
| 12 months | 255; –15.14 (7.28) | 249; –15.30 (6.71) | –0.17 | –0.95 to 0.60 | 0.663 |
| 24 months | 241; –15.57 (7.06) | 238; –15.84 (6.59) | 0.11 | –0.68 to 0.90 | 0.788 |
| Time point | Treatment arm, n; mean (SD) | Adjusted MD | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| SITA standard only | |||||
| Baseline | 222; –14.91 (6.33) | 219; –15.23 (6.28) | |||
| 4 months | 208; –14.37 (6.78) | 212; –14.85 (6.56) | 0.01 | –0.73 to 0.74 | 0.987 |
| 12 months | 211; –14.84 (6.93) | 204; –14.86 (6.49) | 0.03 | –0.70 to 0.77 | 0.927 |
| 24 months | 198; –15.13 (6.68) | 195; –15.54 (6.42) | 0.24 | –0.51 to 0.99 | 0.536 |
| Eligible visual field at baseline | |||||
| Baseline | 222; –14.98 (6.29) | 221; –15.24 (6.34) | |||
| 4 months | 207; –14.46 (6.45) | 212; –14.78 (6.51) | –0.22 | –0.87 to 0.43 | 0.505 |
| 12 months | 211; –14.83 (6.75) | 205; –14.90 (6.51) | –0.06 | –0.72 to 0.59 | 0.854 |
| 24 months | 199; –15.12 (6.65) | 196; –15.43 (6.32) | 0.15 | –0.52 to 0.82 | 0.658 |
| Visual impairment | Treatment arm, n/N (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| No | 214/227 (94.3) | 212/226 (93.8) |
| Sight impaired | 10/227 (4.4) | 12/226 (5.3) |
| Severely sight impaired | 3/227 (1.3) | 2/226 (0.9) |
| Treatment arm | ||
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of participants with a safety event, n (%) | 57 (23.8) | 73 (34.3) |
| SAE | ||
| Number of participants and events, n/N (%) | 1/1 (100.0) | 0 (0) |
| Details (n) | ||
| Significant disability | 1 | – |
| Expected event | 1 | – |
| Classification (n) | ||
| Glaucoma progression despite treatment | 1 | – |
| 4 months | ||
| Number of participants, n/N (%) | 23/234 (9.8) | 31/203 (15.3) |
| Number of events (n) | 30 | 39 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettera | 1 | – |
| Drop related | 22 | 19 |
| Ocular surface related | 4 | 17 |
| Non-specific | 3 | 2 |
| Glaucoma progression | – | 1 |
| 12 months | ||
| Number of participants, n/N (%) | 23/233 (9.9) | 38/194 (19.6) |
| Number of events (n) | 28 | 41 |
| Details (n) | ||
| Shallow anterior chamber | 2 | – |
| Early bleb leak | 1 | – |
| Persistent uveitis | 1 | – |
| Ptosis | – | 1 |
| Non-specific unrelated uveitis | – | 1 |
| Drop related | 13 | 25 |
| Ocular surface related | 8 | 8 |
| Potential AE related to surgery | 1 | 1 |
| Non-specific | – | 6 |
| Cataract | 1 | – |
| 24 months | ||
| Number of participants, n/N (%) | 25/228 (11.0) | 33/191 (17.3) |
| Number of events (n) | 3 | 38 |
| Details (n) | ||
| Shallow anterior chamber | 1 | 1 |
| Early bleb leak | 2 | – |
| Macular oedema | – | 1 |
| Hyphema | 2 | – |
| Choroidal effusion | 1 | – |
| Iris incarceration | 1 | – |
| Drop related | 12 | 16 |
| Ocular surface related | 8 | 7 |
| Non-specific | 1 | 11 |
| Glaucoma progression | – | 1 |
| Cataract | 1 | – |
| Related to cataract surgery | 1 | 1 |
| Treatment arm | ||
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Number of participants with a safety event, n (%) | 53 (23.3) | 77 (34.1) |
| SAE | ||
| Number of participants and events, n/N (%) | 1/1 (100.0) | 0 (0) |
| Details (n) | ||
| Significant disability | 1 | – |
| Expected event | 1 | – |
| Classification (n) | ||
| Glaucoma progression despite treatment | 1 | – |
| 4 months | ||
| Number of participants, n/N (%) | 18/217 (8.3) | 36/220 (16.4) |
| Number of events (n) | 23 | 45 |
| Details (n) | ||
| Irreversible loss of ≥ 10 ETDRS lettera | 1 | – |
| Drop related | 9 | 32 |
| Ocular surface related | 10 | 10 |
| Non-specific | 2 | 3 |
| Glaucoma progression | 1 | – |
| 12 months | ||
| Number of participants, n/N (%) | 25/216 (11.6) | 36/211 (17.1) |
| Number of events (n) | 29 | 40 |
| Details (n) | ||
| Shallow anterior chamber | 2 | – |
| Early bleb leak | 1 | – |
| Persistent uveitis | 1 | – |
| Ptosis | 1 | – |
| Non-specific unrelated uveitis | 1 | – |
| Drop related | 12 | 26 |
| Ocular surface related | 7 | 9 |
| Potential AE related to surgery | 1 | 1 |
| Non-specific | 2 | 4 |
| Cataract | 1 | – |
| 24 months | ||
| Number of participants, n/N (%) | 21/211 (10.0) | 37/208 (17.8) |
| Number of events (n) | 25 | 43 |
| Details (n) | ||
| Shallow anterior chamber | 1 | 1 |
| Early bleb leak | 1 | 1 |
| Macular oedema | – | 1 |
| Hyphema | 2 | – |
| Choroidal effusion | 1 | – |
| Iris incarceration | 1 | – |
| Drop related | 12 | 16 |
| Ocular surface related | 4 | 11 |
| Non-specific | 2 | 10 |
| Glaucoma progression | – | 1 |
| Cataract | – | 1 |
| Related to cataract surgery | 1 | 1 |
| Time point | Treatment arm, n; mean (SD) | Adjusted MD | 95% CI | p-value | |
|---|---|---|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | ||||
| Intraocular pressure (mmHg) | |||||
| Baseline | 222; 17.95 (5.13) | 220; 17.86 (4.17) | |||
| 4 months | 216; 16.77 (4.58) | 220; 16.34 (3.78) | 0.31 | –0.54 to 1.17 | 0.473 |
| 12 months | 215; 16.36 (4.89) | 209; 16.42 (3.75) | –0.13 | –0.99 to 0.74 | 0.775 |
| 24 months | 206; 16.08 (4.25) | 202; 15.58 (3.76) | 0.33 | –0.55 to 1.20 | 0.466 |
| LogMAR VA | |||||
| Baseline | 224; 0.07 (0.18) | 224; 0.09 (0.25) | |||
| 4 months | 210; 0.07 (0.18) | 217; 0.09 (0.23) | 0.00 | –0.05 to 0.05 | 0.952 |
| 12 months | 212; 0.05 (0.19) | 209; 0.09 (0.25) | –0.02 | –0.07 to 0.03 | 0.389 |
| 24 months | 200; 0.06 (0.19) | 199; 0.09 (0.23) | –0.02 | –0.07 to 0.03 | 0.455 |
| Visual fields mean deviation (dB) | |||||
| Baseline | 227; –6.10 (7.69) | 224; –6.13 (7.09) | |||
| 4 months | 212; –5.87 (7.47) | 216; –6.03 (6.83) | –0.01 | –0.73 to 0.71 | 0.979 |
| 12 months | 214; –6.34 (7.74) | 208; –6.33 (7.15) | –0.11 | –0.83 to 0.61 | 0.766 |
| 24 months | 201; –6.80 (7.83) | 200; –6.75 (7.44) | –0.17 | –0.91 to 0.56 | 0.644 |
| Time point | Treatment arm, n (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| 4 months | N = 217 | N = 220 |
| Number of glaucoma eye drops, mean (SD) | 1.01 (0.75) | 1.60 (0.92) |
| Number of participants receiving eye drops by glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 160 (73.7) | 193 (87.7) |
| Beta-blocker | 54 (24.9) | 74 (33.6) |
| Carbonic anhydrase inhibitor | 31 (14.3) | 58 (26.4) |
| α-agonist | 6 (2.8) | 11 (5.0) |
| 12 months | N = 216 | N = 211 |
| Number of glaucoma eye drops, mean (SD) | 0.95 (0.75) | 1.69 (0.95) |
| Number of participants receiving eye drops by glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 147 (68.1) | 182 (86.3) |
| Beta-blocker | 55 (25.5) | 92 (43.6) |
| Carbonic anhydrase inhibitor | 32 (14.8) | 61 (28.9) |
| α-agonist | 3 (1.4) | 16 (7.6) |
| Pilocarpine | 1 (0.5) | 0 (0) |
| 24 months | N = 211 | N = 208 |
| Number of glaucoma eye drops, mean (SD) | 1.00 (0.93) | 1.61 (1.03) |
| Number of participants receiving eye drops by glaucoma eye drop type, n (%) | ||
| Prostaglandin analogue | 131 (62.1) | 159 (76.4) |
| Beta-blocker | 54 (25.6) | 93 (44.7) |
| Carbonic anhydrase inhibitor | 40 (19.0) | 69 (33.2) |
| α-agonist | 10 (4.7) | 14 (6.7) |
| Pilocarpine | 0 (0) | 1 (0.5) |
| Time point | Treatment arm, n (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| 4 months | N = 214 | N = 218 |
| None | 156 (72.9) | 10 (4.6) |
| 1 | 36 (16.8) | 108 (49.5) |
| 2 | 21 (9.8) | 93 (42.7) |
| 3 | 1 (0.5) | 6 (2.8) |
| > 3 | 0 (0) | 1 (0.5) |
| 12 months | N = 211 | N = 205 |
| None | 173 (82.0) | 23 (11.2) |
| 1 | 17 (8.1) | 77 (37.6) |
| 2 | 20 (9.5) | 99 (48.3) |
| 3 | 1 (0.5) | 4 (2.0) |
| > 3 | 0 (0) | 2 (1.0) |
| 24 months | N = 210 | N = 201 |
| None | 158 (75.2) | 44 (21.9) |
| 1 | 19 (9.0) | 67 (33.3) |
| 2 | 31 (14.8) | 83 (41.3) |
| 3 | 1 (0.5) | 4 (2.0) |
| > 3 | 1 (0.5) | 3 (1.5) |
| Time point | Treatment arm, n (%) | |
|---|---|---|
| Trabeculectomy (N = 227) | Medical management (N = 226) | |
| Treatment received | n = 240 | n = 213 |
| 4 months | N = 232 | N = 200 |
| None | 163 (70.3) | 3 (1.5) |
| 1 | 33 (14.2) | 111 (55.5) |
| 2 | 33 (14.2) | 81 (40.5) |
| 3 | 3 (1.3) | 4 (2.0) |
| > 3 | 0 (0) | 1 (0.5) |
| 12 months | N = 231 | N = 185 |
| None | 190 (82.3) | 6 (3.2) |
| 1 | 10 (4.3) | 84 (45.4) |
| 2 | 28 (12.1) | 91 (49.2) |
| 3 | 2 (0.9) | 3 (1.6) |
| > 3 | 1 (0.4) | 1 (0.5) |
| 24 months | N = 226 | N = 185 |
| None | 190 (84.1) | 12 (6.5) |
| 1 | 11 (4.9) | 75 (40.5) |
| 2 | 22 (9.7) | 92 (49.7) |
| 3 | 1 (0.4) | 4 (2.2) |
| > 3 | 2 (0.9) | 2 (1.1) |
Appendix 4 Discrete choice experiment
| Characteristic | Total (N = 308) | Block, n (%) | |||
|---|---|---|---|---|---|
| 1 (N = 77) | 2 (N = 74) | 3 (N = 75) | 4 (N = 82) | ||
| Age (years), mean (SD) | 68.42 (11) | 68.01 (12) | 69.35 (10) | 69.01 (10) | 67.40 (11) |
| Male, n (%) | 205 (67) | 54 (70) | 45 (61) | 49 (65) | 57 (70) |
| Ethnicity, n (%) | |||||
| White | 281 (91) | 69 (90) | 65 (88) | 72 (96) | 75 (92) |
| Asian – Oriental | 1 (< 1) | 1 (1) | |||
| Afro-Caribbean | 19 (6) | 6 (8) | 6 (8) | 3 (4) | 4 (5) |
| Asian: India/Pakistan/Bangladesh | 5 (2) | 2 (3) | 1 (1) | 2 (2) | |
| Mixed | 1 (< 1) | 1 (1) | |||
| Nigerian | 1 (< 1) | 1 (1) | 1 (1) | ||
| Level of education, n (%) | |||||
| None | 18 (6) | 4 (4) | 5 (7) | 3 (4) | 6 (7) |
| Secondary school | 144 (47) | 29 (38) | 34 (46) | 42 (56) | 39 (48) |
| College | 95 (31) | 29 (38) | 24 (32) | 18 (24) | 24 (29) |
| University | 45 (14) | 12 (16) | 9 (12) | 11 (15) | 13 (16) |
| Annual income, n (%) | |||||
| < £6000 | 17 (6) | 2 (3) | 5 (7) | 3 (4) | 7 (9) |
| £6000–10,000 | 23 (7) | 2 (3) | 5 (7) | 7 (9) | 9 (11) |
| £10,001–15,000 | 52 (17) | 9 (12) | 14 (19) | 18 (24) | 11 (13) |
| £15,001–20,000 | 41 (13) | 9 (12) | 13 (18) | 8 (11) | 11 (13) |
| £20,001–25,000 | 39 (13) | 18 (23) | 8 (11) | 5 (7) | 8 (10) |
| £25,001–30,000 | 18 (6) | 5 (6) | 6 (8) | 2 (3) | 5 (6) |
| £30,001–35,000 | 27 (9) | 10 (13) | 4 (5) | 5 (7) | 8 (10) |
| > £35,000 | 62 (20) | 13 (17) | 14 (19) | 17 (23) | 18 (22) |
| Self-reported time to complete DCE | |||||
| Time (minutes), mean (SD) | 23.96 (17) | 25.84 (20) | 22.34 (14) | 25.23 (17) | 23.03 (16) |
| Time given in minutes/hours, n (%) | 286 (97) | ||||
| 1.5 days, n (%) | 1 (< 1) | ||||
| 2–3 days, n (%) | 1 (< 1) | ||||
| 3 weeks, n (%) | 1 (< 1) | ||||
| 7 days, n (%) | 1 (< 1) | ||||
| 1 week, n (%) | 1 (< 1) | ||||
| Ages, n (%) | 1 (< 1) | ||||
| Not long, n (%) | 1 (< 1) | ||||
| Too long, n (%) | 1 (< 1) | ||||
| Weeks/month, n (%) | 1 (< 1) | ||||
| Number of completed choice sets, n (%) | |||||
| 15 | 283 (92) | 72 (94) | 64 (86) | 71 (94) | 76 (93) |
| 14 | 6 (2) | 2 (3) | 3 (4) | 1 (1) | |
| 13 | 1 (< 1) | 1 (1) | |||
| 12 | 5 (2) | 2 (3) | 2 (3) | 1 (1) | |
| 11 | 4 (1) | 1 (1) | 2 (3) | 1 (1) | |
| 7 | 2 (1) | 2 (3) | |||
| 6 | 1 (< 1) | 1 (1) | |||
| 5 | 1 (< 1) | 1 (1) | |||
| 2 | 2 (1) | 1 (1) | 1 (1) | ||
| 1 | 3 (1) | 2 (3) | 1 (1) | ||
Appendix 5 Health economic results
Unit costs
The unit costs utilised in the economic evaluation are described in Tables 40–43.
| Item | Cost (£) | Unit | Reference | Notes |
|---|---|---|---|---|
| Inpatient | 448 | Per night | NHS Reference Costs 2017/18 91 | Ward costs per night |
| Outpatient appointment | 105.09 | Per appointment | NHS Reference Costs 2017/18 91 | Consultant-led ophthalmology outpatient costs |
| Ophthalmic procedure | Source | HRG code | Unit cost | Comments |
|---|---|---|---|---|
| Trabeculectomy (day case) | NHS Reference Costs 2017/18 91 | BZ92B | 1639.44 | Very major, glaucoma or iris procedures, with CC score of 0–1 |
| Trabeculectomy (inpatient) | NHS Reference Costs 2017/18 91 | BZ92B | 2184.07 | Very major, glaucoma or iris procedures, with CC score of 0–1 |
| Massage | NHS Reference Costs 2017/18 91 | BZ24G | 143.29 | Minor, glaucoma or iris procedures |
| Adjustment/suture lysis/releasable release | NHS Reference Costs 2017/18 91 | BZ95 | 143.29 | Minor, glaucoma or iris procedures |
| 5-FU injection | NHS Reference Costs 2017/18 91 | BZ95Z | 149.69 | Minor, glaucoma or iris procedures plus unit costs of fluorouracil |
| Steroid injections | NHS Reference Costs 2017/18 91 | BZ95Z | 150.16 | Minor, glaucoma or iris procedure plus unit prednisolone |
| Needling plus 5-FU injection | NHS Reference Costs 2017/18 91 | BZ94B | 149.69 | Minor, glaucoma or iris procedures plus unit costs of fluorouracil |
| Revision of bleb NEC | NHS Reference Costs 2017/18 91 | BZ91B | 1347.56 | Complex, glaucoma or iris procedures, with CC score of 0–1 |
| Reformation of anterior chamber of eye | NHS Reference Costs 2017/18 91 | BZ94B | 127.90 | Intermediate, glaucoma or iris procedures, with CC score of 0 |
| Phaco and IOL | NHS Reference Costs 2017/18 91 | BZ32B | 878.61 | Intermediate, cataract or lens procedures, with CC score of 0–1 |
| Glaucoma medication | Dose | Administration | Unit cost (£) | Source | Comments |
|---|---|---|---|---|---|
| Prostaglandin analogues | |||||
| Saflutan | 15 µg/ml | Single-dose unit eye drop | 12.20 | BNF 168 | Overall costs per box |
| Latanoprost | 50 µg/ml | Eye drop | 1.53 | BNF 168 | Overall costs per 2.5-ml bottle |
| Bimatoprost | 300 µg/ml | Eye drop | 10.30 | BNF 168 | Overall costs per 3-ml bottle |
| Travoprost | 40 µg/ml | Eye drop | 7.27 | BNF 168 | Overall costs per 2.5-ml bottle |
| Average cost | 7.83 | ||||
| Carbonic anhydrase inhibitors | |||||
| Brinzolamide | 10 mg/ml | Eye drop | 1.89 | BNF 168 | Overall costs per 5-ml bottle |
| Dorzolamide | 20 mg/ml | Eye drop | 1.55 | BNF 168 | Overall costs per 5-ml bottle |
| Average cost | 1.72 | ||||
| Alpha-2 adrenergic agonists | |||||
| Brimonidine | 0.2% | Eye drop | 1.13 | BNF 168 | Overall costs per 5-ml bottle |
| Iopidine | 5 mg/ml | Eye drop | 10.88 | BNF 168 | Overall costs per 5-ml bottle |
| Average cost | 6.01 | ||||
| Beta-blockers | |||||
| Timolol | 0.25% | Eye drop | 0.78 | BNF 168 | Overall costs per 5-ml bottle |
| Betoptic | 0.5% | Eye drop | 1.90 | BNF 168 | Overall costs per 5-ml bottle |
| Average cost | 1.34 | ||||
| Parasympathetic eye drops | |||||
| Pilocarpine hydrochloride | 1% | Eye drop | 20.78 | BNF 168 | Overall costs per 10-ml bottle |
| Average cost | 20.78 | ||||
| Oral glaucoma medications | |||||
| Acetazolamide | 250 mg | Tablet | 16.66 | BNF 168 | Overall cost per box (30) |
| Combination glaucoma medications | |||||
| Bimatoprost with timolol (AZARGA) | 10 mg/ml | Eye drops | 11.05 | BNF 168 | Overall cost per 5-ml bottle |
| Bimatoprost with timolol (Ganfort) | 5 mg/ml | Eye drops | 14.16 | BNF 168 | Overall cost per 3-ml bottle |
| Brinzolamide with brimonidine (Simbrinza) | 2 mg/ml | Eye drops | 9.23 | BNF 168 | Overall cost per 5-ml bottle |
| Dorzolamide with timolol (Cosopt) | 5 mg/ml | Eye drops | 1.50 | BNF 168 | Overall cost per 5-ml bottle |
| Dorzolamide with timolol unit dose (Cosopt) | 5 mg/ml | Single-dose unit eye drop | 28.59 | BNF 168 | Unit dose, 60 doses |
| Dorzolamide with timolol (Eylamdo) | 5 mg/ml | Eye drop | 14.29 | BNF 168 | Overall cost per 5-ml bottle |
| Travoprost with timolol (DuoTrav) | 5 mg/ml | Eye drop | 13.95 | BNF 168 | Overall cost per 5-ml bottle |
| Average cost | 13.25 | ||||
| Non-glaucoma medications | |||||
| Steroid eye drops | |||||
| Dexamethasone with hypromellose, neomycin and polymyxin B (Maxitrol) | Dexamethasone 1 mg per 1 g | Eye drop | 1.68 | BNF 168 | Overall cost per 5-ml bottle |
| Neomycin (as Neomycin sulfate) 3500 unit per 1 g | |||||
| Polymyxin B sulfate 6000 unit per 1 g | |||||
| Betamethasone sodium phosphate | 1 mg/ml | Eye drop | 2.32 | BNF 168 | Overall cost per 5-ml bottle |
| Dexamethasone (Maxidex) | 1 mg/ml | Eye drop | 1.42 | BNF 168 | Overall cost per 5-ml bottle |
| Prednisolone (Pred Forte) | 10 mg/ml | Eye drop | 1.82 | BNF 168 | Overall cost per 5-ml bottle |
| Antibiotic eye drops | |||||
| Azithromycin (azyter) | 15 mg per 1 g | Eye drop | 1.17 | BNF 168 | Unit dose, six doses |
| Choloramphenicol | 5 mg/ml | Eye drop | 1.14 | BNF 168 | Overall cost per 10-ml bottle |
| Celluvisc Unit Dose | 1% | Single-dose unit eye drop | 4.80 | BNF 168 | Unit dose, 30 doses |
| Hylo-Forte unit dose | 0.2% | Single-dose unit eye drop | 5.60 | BNF 168 | Unit dose, 30 doses |
| Sodium hyaluronate (Vismed Multi) | 0.18% | Eye drop | 6.87 | BNF 168 | Overall cost per 10-ml bottle |
| Mydriatics | |||||
| Cyclopentolate (Mydrilate) | 5 mg/ml | Eye drops | 8.08 | BNF 168 | Overall cost per 5-ml bottle |
| Atropine | 10 mg/ml | Eye drops | 15.10 | BNF 168 | Unit dose, 20 doses |
| NSAID | |||||
| Bromfenac (Yellox) | 900 µg/ml | Eye drops | 8.50 | BNF 168 | Overall cost per 5-ml bottle |
| Sympathomimetic | |||||
| Phenylephrine | 50 µg/ml | Eye drops | 11.87 | BNF 168 | Unit dose, 20 doses |
| Average cost of non-glaucoma eye drops | 7.27 | ||||
| Item | Unit | Cost (£) | Comments |
|---|---|---|---|
| GP | |||
| GP visit at their practice | Per 9.22-minute appointment | 37 | PSSRU 2018169 |
| GP home visit | 11.4 minutes per appointment | 45.98 | PSSRU 2015 (most recent information)170 |
| 11.4 minutes (2015 Health and Social Care) × 2017 hourly rate (£242) (no travel costs) | |||
| Telephone triage with GP | Cost per call | 8.10 | PSSRU 2017 (most recent information)171 |
| 15.5 minutes × 2015 hourly rate (£67) (not including travel) | |||
| Nurse | |||
| Practice nurse consultation | 15.5 minutes per consultation | 10.84 | PSSRU 2018169 |
| 15.5 minutes (length of appointment Unit Costs 2015) × 2017 hourly rate (£42) | |||
| District nurse | 25 minutes per consultation | 17.29 | PSSRU 2017171 |
| 15.5 minutes × 2015 hourly rate (£67) (not including travel) | |||
| Optician | |||
| Optometrist in practice | Per examination | 21.31 | Department of Health and Social Care172 |
| Eye examination fee (as participants have glaucoma all will be entitled to NHS eye examinations) | |||
| Optometrist at home | Per examination | 58.87 | Department of Health and Social Care172 |
| Eye examination fee plus domiciliary fee (as participants have glaucoma all will be entitled to NHS eye examinations) | |||
Sensitivity analysis use of the Health Utility Index version 3 data to calculate quality-adjusted life-years
| HUI-3 data | Treatment arm | Unadjusted cost (£) | Adjusted incremental cost (£) | Unadjusted QALY | Adjusted Incremental QALY | ICER (ΔCost/ΔQALY) (£) | Probability cost-effective at threshold (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £20,000 | £30,000 | £50,000 | |||||||
| Complete case (n = 240) | Trabulectomy | 3819 | 2129 | 1.61 | 0.06 | 33,758 | 0 | 9 | 38 | 76 |
| Medical management | 1691 | 1.54 | 100 | 91 | 62 | 24 | ||||
| Imputation (n = 382) | Trabulectomy | 3688 | 2040 | 1.54 | 0.06 | 36,130 | 0 | 4 | 32 | 72 |
| Medical management | 1640 | 1.48 | 100 | 96 | 68 | 28 | ||||
FIGURE 15.
Cost-effectiveness curves for the trabulectomy and medical management arms using the results from the imputed HUI-3 sample.
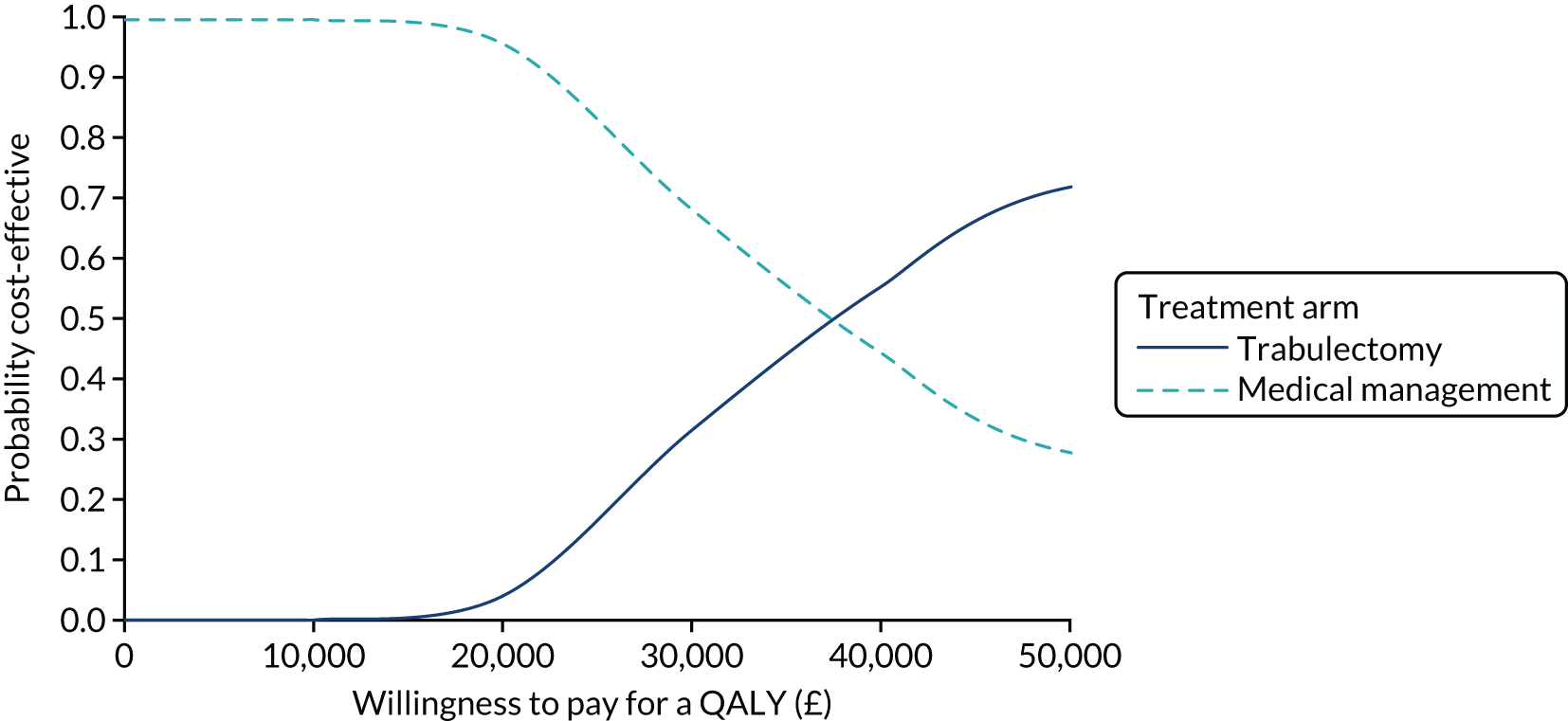
FIGURE 16.
Cost-effectiveness plane for the trabulectomy and medical management arms using the results from the imputed HUI-3 sample.
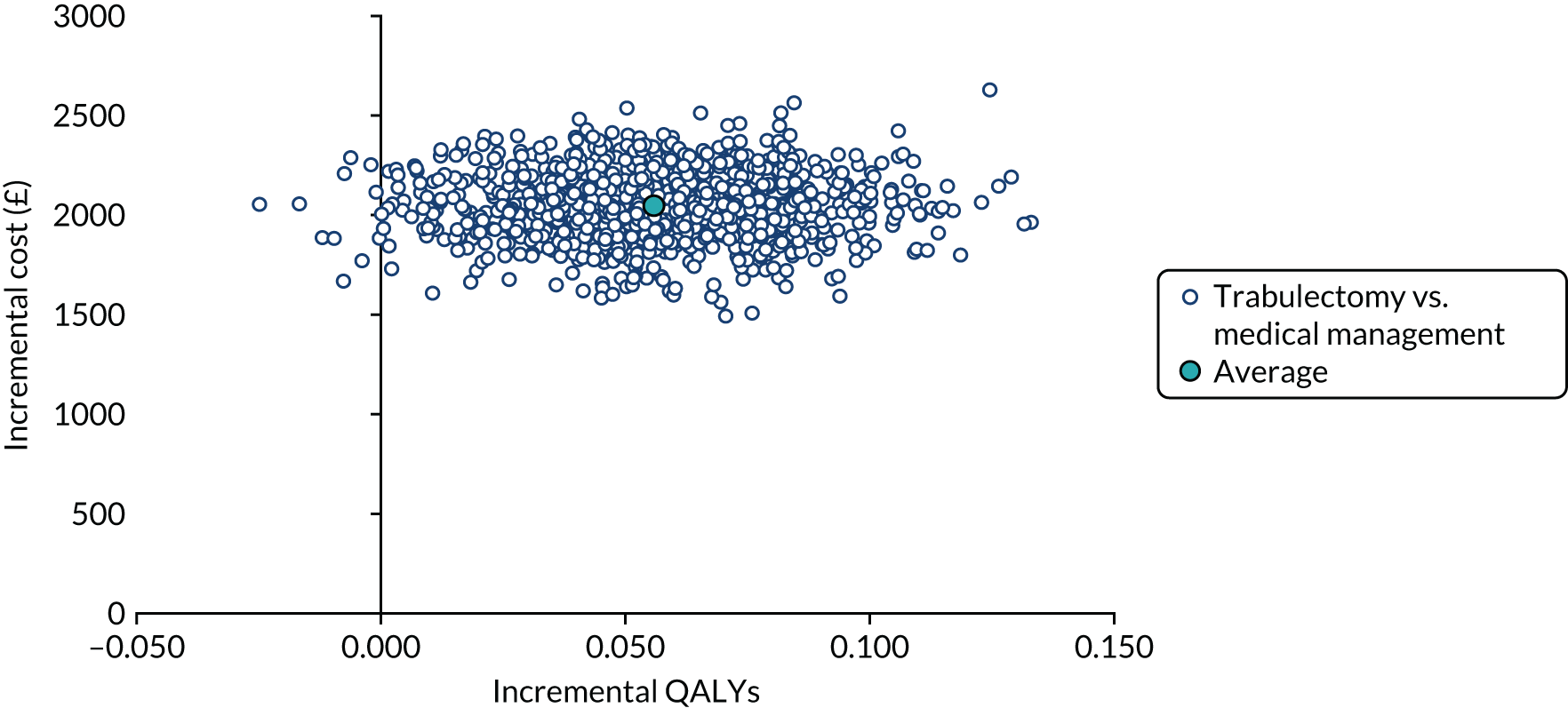
Sensitivity analysis use of the Glaucoma Utility Index data to calculate quality-adjusted life-years
| GUI data | Treatment arm | Unadjusted cost (£) | Adjusted incremental cost (£) | Unadjusted QALY | Adjusted incremental QALY | ICER (ΔCost/ΔQALY) (£) | Probability cost-effective at threshold (£) (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 20,000 | 30,000 | 50,000 | |||||||
| Complete case (n = 293) | Trabulectomy | 3683 | 2138 | 1.67 | 0.01a | 111,117 | 0 | 9 | 38 | 76 |
| Medical management | 1541 | 1.64 | 100 | 91 | 62 | 24 | ||||
| Imputation (n = 398) | Trabulectomy | 3617 | 1995 | 1.64 | 0.00 | 350,149 | 0 | 0 | 0 | 0 |
| Medical management | 1615 | 1.62 | 100 | 100 | 100 | 100 | ||||
FIGURE 17.
Cost-effectiveness curves for the trabulectomy and medical management arms using the results from the imputed GUI sample.
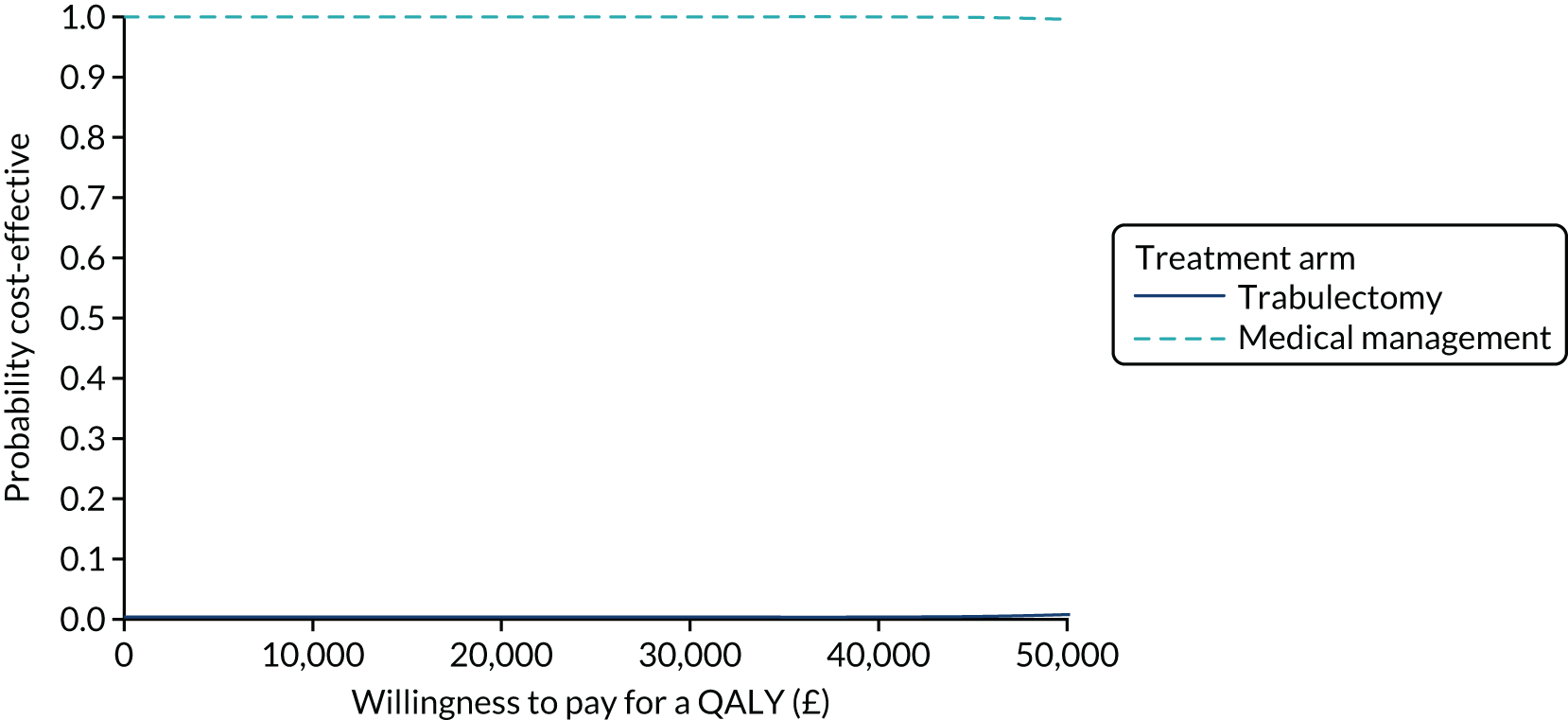
FIGURE 18.
Cost-effectiveness plane for the trabulectomy and medical management arms using the results from the imputed GUI sample.
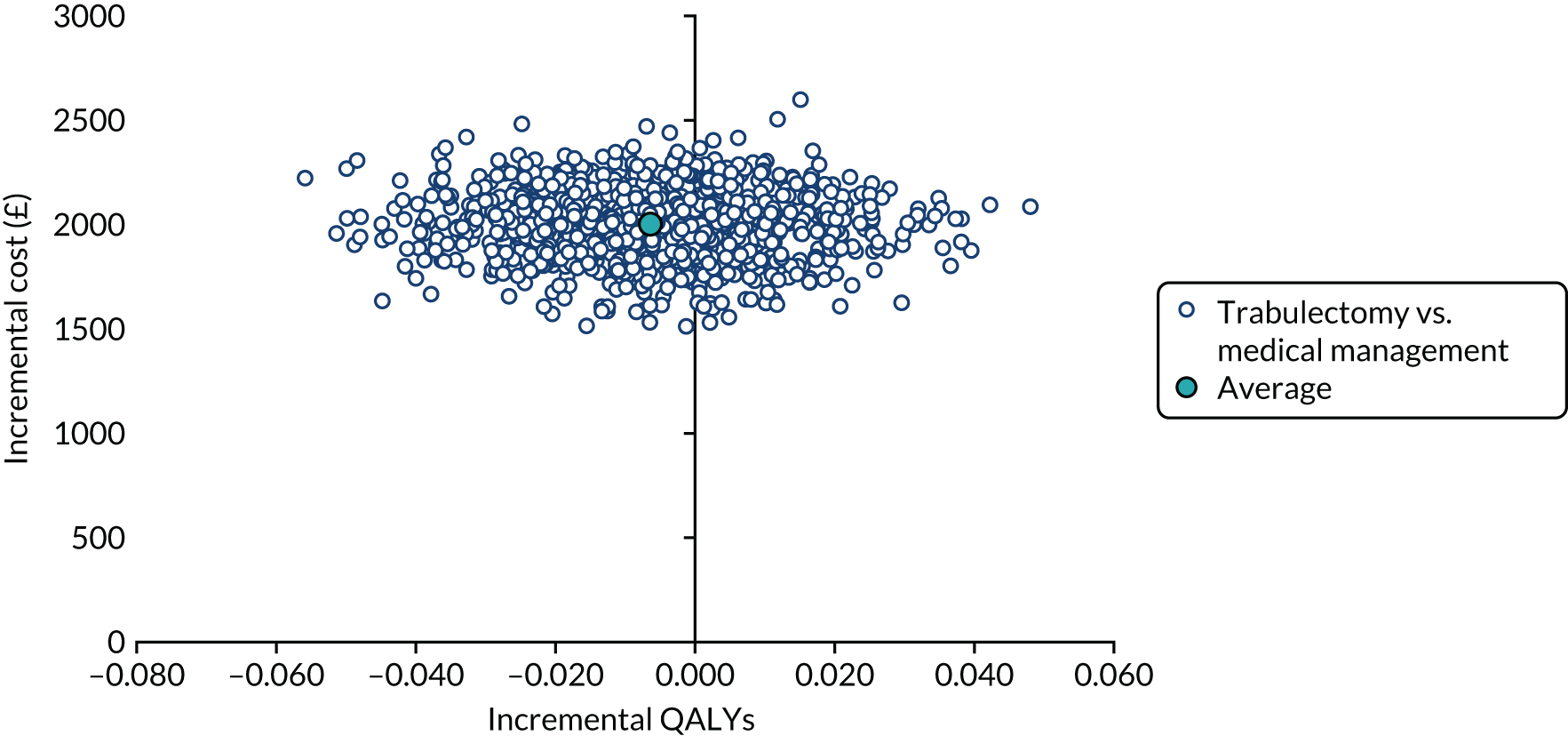
Sensitivity analysis use of the EQ-5D-5L with the participant time and travel costs
| EQ-5D-5L data with patient time and travel costs included | Treatment arm | Unadjusted cost with TT (£) | Adjusted incremental cost (£) | Unadjusted QALY | Adjusted incremental QALY | ICER (ΔCost/ΔQALY) (£) | Probability cost-effective at threshold (£) (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 20,000 | 30,000 | 50,000 | |||||||
| Complete case (n = 290) | Trabulectomy | 4453 | 2412 | 1.65 | 0.03 | 75,347 | 0 | 1 | 2 | 25 |
| Medical management | 2046 | 1.59 | 100 | 99 | 98 | 75 | ||||
| Imputation (n = 403) | Trabulectomy | 4419 | 2359 | 1.61 | 0.04 | 54,197 | 0 | 1 | 5 | 42 |
| Medical management | 2052 | 1.56 | 100 | 99 | 95 | 58 | ||||
FIGURE 19.
Cost-effectiveness curves for the trabulectomy and medical management arms using the results from the EQ-5D-5L using the time and travel data.
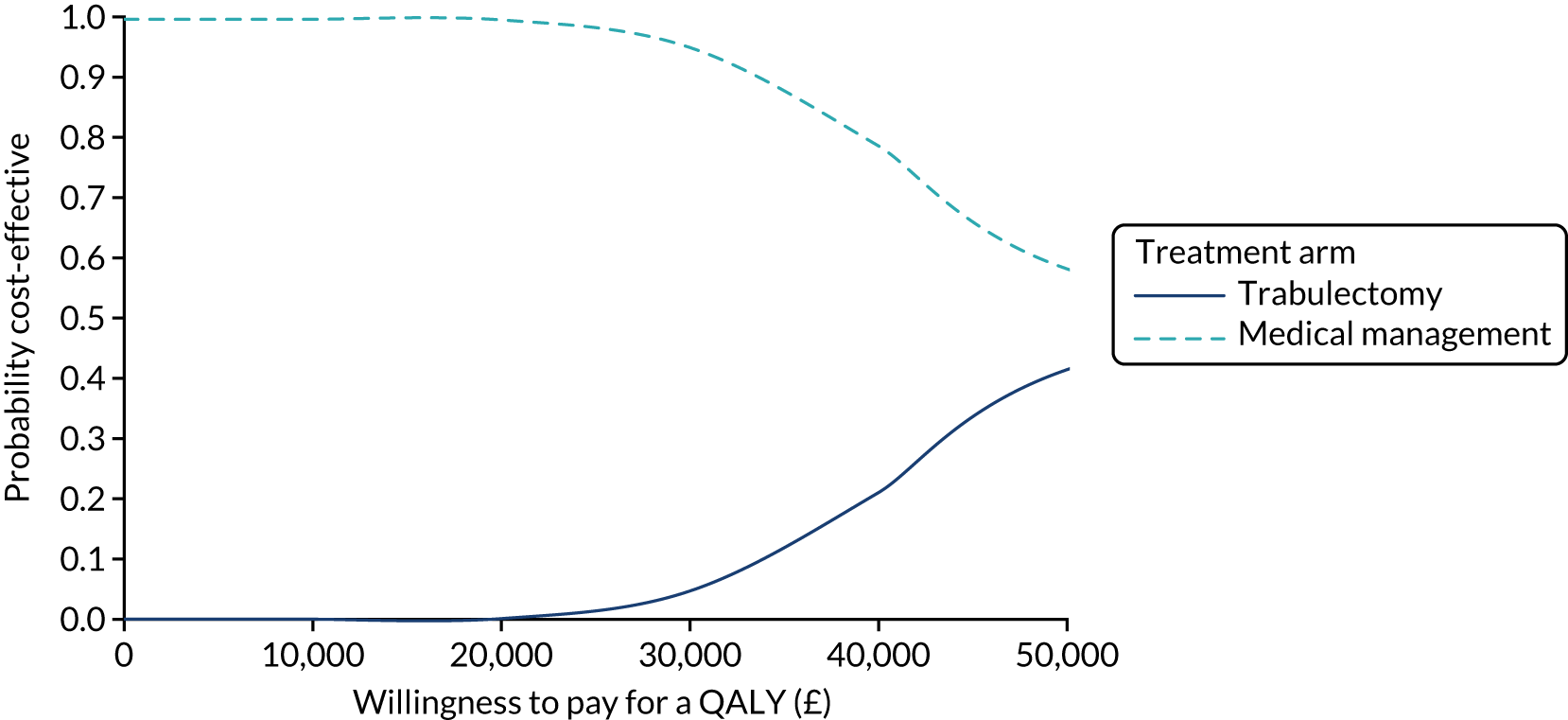
FIGURE 20.
Cost-effectiveness plane for adjusted bootstrapped replications for cost–utility analysis for EQ-5D-5L results with time and travel costs.
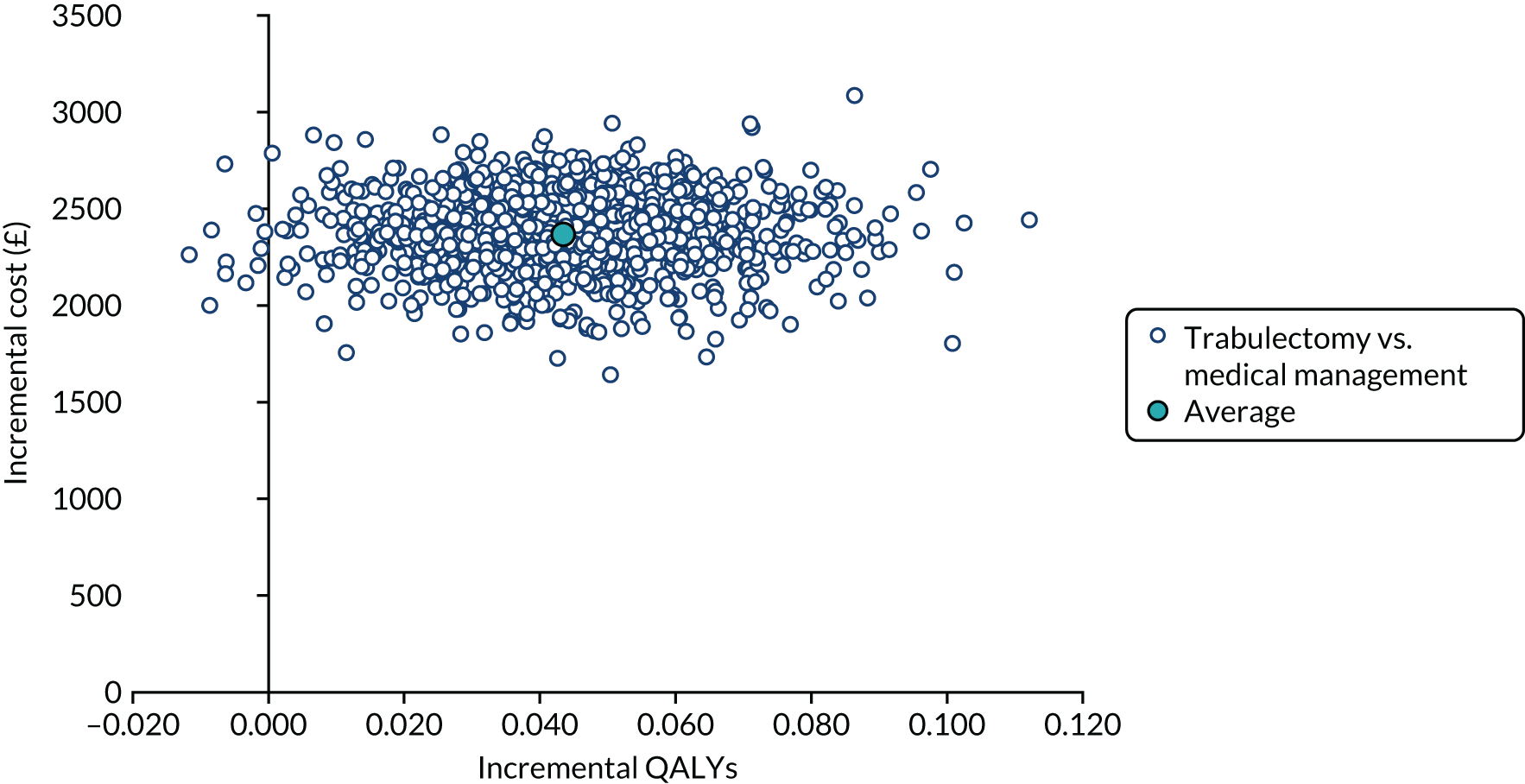
Appendix 6 Analysis of pre-trabeculectomy questionnaire utility scores
A measurement of the EQ-5D-5L questionnaire was administered to participants before they underwent a trabeculectomy. This was administered with a view to capture information about the effect of the surgical procedure on the participants. The results of those who completed the pre-trabeculectomy questionnaire in the trabulectomy arm are summarised in Table 47.
| Time point | Participants (n) | EQ-5D-5L score | |
|---|---|---|---|
| Mean | SD | ||
| Baseline | 204 | 0.84 | 0.18 |
| 1 month | 179 | 0.85 | 0.18 |
| Pre trabeculectomy | 208 | 0.85 | 0.19 |
| 3 months | 175 | 0.84 | 0.17 |
| 6 months | 176 | 0.85 | 0.19 |
Table 47 summarises the mean values for those who completed a pre-trabeculectomy questionnaire over a pre- and post-surgery period of 6 months. The utility values remain largely stable both pre and post surgery in the participants who undertook the EQ-5D-5L immediately pre surgery in the trabulectomy arm. There is a very slight decrease in utility values at the 3-month time point (0.01) that could represent post-surgical healing, but this seems to stabilise at the 6-month time point.
Appendix 7 Model parameters
Possible options for model structure
Based on GSS2 classification, we can define states of disease in patients with glaucoma variably, as were provided here in five possible options. Based on GSS2 stages (stage 0, border, stage B, stage 1, stage 2, stage 3, stage 4 and stage 5) and considering two eye health states separately, 64 (8 × 8) eye health states (conditions) are possible theoretically to define for patients. Although most of these 64 conditions (states) are irrelevant in our model because TAGS patients with severe stages of glaucoma (in at least one eye) were included in the study. This means that the indexed eye must be in a severe stage based on HPA classification. In our modified classifications of GSS2 for the model we assumed stage 0, border, stage 1 and stage 2 of GSS2 as non-severe and stage 3, stage 4 and stage 5 of GSS2 as severe. Based on this modified GSS2 classification, only 11 patients (0.02) misclassify (in 11 patients the index eye classified less than stage 3 based on GSS2), which means that the modified classification of the GSS2 for the model is most compatible.
The possible options for the model structure are provided in Tables 48–52. The differences between these options are only about the complexity that each one provides for the structure of the model. For example, the first option provides 32 states, of which not all may be necessary, and merging some of these states may provide a simpler and more reasonable structure for the model. Defining many health conditions leads to a very small initial probability of patients for each state, which makes it sensible to define fewer stages as previous related studies have done (EAGLE,111 GATE,173 etc.). Moreover, some states (conditions 7, 8 and 16 in the first option, conditions 5, 6 and 9 in the second option) are inconsistent with the assumption of the study as indicated in the TAGS protocol: ‘The eye with less severe visual field damage will be nominated the index eye in patients with both eye eligible’. The initial probabilities of these conditions would be zero and some cases may transition to these stages during trial.
The similarity between option 3 and option 4 is that both of them make the model simpler than options 1 and 2. The difference between option 3 and option 4 is that in option 3, the severity of the non-index eye is merged (stages 3–5) but the severity state of the index eye is determined separately, which makes it possible to precisely follow-up for index eye disease progression in the model, which is more consistent with the TAGS objective. On the other hand, in option 4, the severity of the index eye is merged (stages 3–5) but the severity state of the non-index eye is determined separately, which makes it possible to precisely follow-up for non-index eye disease progression, which may not be more important in this study.
| Condition number | Condition definition |
|---|---|
| 1 | Index eye (S3); non-index eye (S0) |
| 2 | Index eye (S3); non-index eye (border) |
| 3 | Index eye (S3); non-index eye (SB) |
| 4 | Index eye (S3); non-index eye (S1) |
| 5 | Index eye (S3); non-index eye (S2) |
| 6 | Index eye (S3); non-index eye (S3) |
| 7 | Index eye (S3); non-index eye (S4) |
| 8 | Index eye (S3); non-index eye (S5) |
| 9 | Index eye (S4); non-index eye (S0) |
| 10 | Index eye (S4); non-index eye (border) |
| 11 | Index eye (S4); non-index eye (SB) |
| 12 | Index eye (S4); non-index eye (S1) |
| 13 | Index eye (S4); non-index eye (S2) |
| 14 | Index eye (S4); non-index eye (S3) |
| 15 | Index eye (S4); non-index eye (S4) |
| 16 | Index eye (S4); non-index eye (S5) |
| 17 | Index eye (S5); non-index eye (S0) |
| 18 | Index eye (S5); non-index eye (border) |
| 19 | Index eye (S5); non-index eye (SB) |
| 20 | Index eye (S5); non-index eye (S1) |
| 21 | Index eye (S5); non-index eye (S2) |
| 22 | Index eye (S5); non-index eye (S3) |
| 23 | Index eye (S5); non-index eye (S4) |
| 24 | Index eye (S5); non-index eye (S5) |
| Condition number | Condition definition |
|---|---|
| 1 | Index eye: severe (S3); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 2 | Index eye: severe (S4); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 3 | Index eye: severe (S5); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 4 | Index eye: severe (S3); non-index eye: severe (S3) |
| 5 | Index eye: severe (S3); non-index eye: severe (S4) |
| 6 | Index eye: severe (S3); non-index eye: severe (S5) |
| 7 | Index eye: severe (S4); non-index eye: severe (S3) |
| 8 | Index eye: severe (S4); non-index eye: severe (S4) |
| 9 | Index eye: severe (S4); non-index eye: severe (S5) |
| 10 | Index eye: severe (S5); non-index eye: severe (S3) |
| 11 | Index eye: severe (S5); non-index eye: severe (S4) |
| 12 | Index eye: severe (S5); non-index eye: severe (S5) |
| Condition number | Condition definition |
|---|---|
| 1 | Index eye: severe (S3); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 2 | Index eye: severe (S4); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 3 | Index eye: severe (S5); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 4 | Index eye: severe (S3); non-index eye: severe (S3, S4 and S5) |
| 5 | Index eye: severe (S4); non-index eye: severe (S3, S4 and S5) |
| 6 | Index eye: severe (S5); non-index eye: severe (S3, S4 and S5) |
| Condition number | Condition definition |
|---|---|
| 1 | Index eye: severe (S3 + S4 + S5); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 2 | Index eye: severe (S3 + S4 + S5); non-index eye: severe (S3) |
| 3 | Index eye: severe (S3 + S4 + S5); non-index eye: severe (S4) |
| 4 | Index eye: severe (S3 + S4 + S5); non-index eye: severe (S5) |
| Condition number | Condition definition |
|---|---|
| 1 | Index eye: severe (S3 + S4 + S5); non-index eye: non-severe (S0 + border + SB + S1 + S2) |
| 2 | Index eye: severe (S3 + S4 + S5); non-index eye: severe (S3 + S4 + S5) |
Initial and transition probabilities of defined stages in the model
| Stage | Baseline | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 30 | 19 | 9 | 2 | 0 | ||||||||
| 2 | 76 | 65 | 8 | 1 | 2 | ||||||||
| 3 | 39 | 32 | 7 | ||||||||||
| 4 | 2 | 2 | 0 | 0 | 0 | ||||||||
| 5 | 15 | 8 | 2 | 3 | 2 | ||||||||
| 6 | 11 | 10 | 1 | ||||||||||
| 7 | 5 | 5 | 0 | 0 | 0 | ||||||||
| 8 | 10 | 7 | 0 | 1 | 2 | ||||||||
| 9 | 4 | 4 | 0 | ||||||||||
| 10 | 0 | 0 | 0 | ||||||||||
| 11 | 11 | 8 | 3 | ||||||||||
| 12 | 14 | 14 | |||||||||||
| 217 | |||||||||||||
| Stage | Baseline (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 30 | 0.633333 | 0.3 | 0.066667 | 0 | ||||||||
| 2 | 76 | 0.855263 | 0.105263 | 0.013158 | 0.026316 | ||||||||
| 3 | 39 | 0.820513 | 0.179487 | ||||||||||
| 4 | 2 | 1 | 0 | 0 | |||||||||
| 5 | 15 | 0.533333 | 0.133333 | 0.2 | 0.133333 | ||||||||
| 6 | 11 | 0.909091 | 0.090909 | ||||||||||
| 7 | 5 | 1 | 0 | 0 | |||||||||
| 8 | 10 | 0.7 | 0 | 0.1 | 0.2 | ||||||||
| 9 | 4 | 1 | 0 | ||||||||||
| 10 | 0 | 0 | |||||||||||
| 11 | 11 | 0.727273 | 0.272727 | ||||||||||
| 12 | 14 | 1 | |||||||||||
| 217 | |||||||||||||
| Stage | Baseline | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 28 | 19 | 8 | 0 | 1 | ||||||||
| 2 | 74 | 59 | 3 | 9 | 3 | ||||||||
| 3 | 46 | 45 | 1 | ||||||||||
| 4 | 3 | 1 | 1 | 0 | 1 | ||||||||
| 5 | 13 | 9 | 1 | 3 | 0 | ||||||||
| 6 | 10 | 8 | 2 | ||||||||||
| 7 | 5 | 3 | 2 | 0 | 0 | 0 | |||||||
| 8 | 6 | 5 | 0 | 1 | 0 | ||||||||
| 9 | 4 | 3 | 1 | ||||||||||
| 10 | 8 | 7 | 1 | ||||||||||
| 11 | 6 | 5 | 1 | ||||||||||
| 12 | 13 | 13 | |||||||||||
| 216 | |||||||||||||
| Stage | Baseline (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 28 | 0.678571 | 0.285714 | 0 | 0.035714 | ||||||||
| 2 | 74 | 0.797297 | 0.040541 | 0.121622 | 0.040541 | ||||||||
| 3 | 46 | 0.978261 | 0.021739 | ||||||||||
| 4 | 3 | 0.333333 | 0.333333 | 0 | 0.333333 | ||||||||
| 5 | 13 | 0.692308 | 0.076923 | 0.230769 | 0 | ||||||||
| 6 | 10 | 0.8 | 0.2 | ||||||||||
| 7 | 5 | 0.6 | 0.4 | 0 | 0 | 0 | |||||||
| 8 | 6 | 0.833333 | 0 | 0.166667 | 0 | ||||||||
| 9 | 4 | 0.75 | 0.25 | ||||||||||
| 10 | 8 | 0.875 | 0.125 | ||||||||||
| 11 | 6 | 0.833333 | 0.166667 | ||||||||||
| 12 | 13 | 1 | |||||||||||
| 216 | |||||||||||||
| Stage | 12 months (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 19 | 16 | 3 | 0 | 0 | ||||||||
| 2 | 74 | 68 | 2 | 4 | 0 | ||||||||
| 3 | 40 | 37 | 3 | ||||||||||
| 4 | 4 | 3 | 0 | 0 | 1 | ||||||||
| 5 | 10 | 9 | 1 | ||||||||||
| 6 | 19 | 16 | 3 | ||||||||||
| 7 | 5 | 5 | |||||||||||
| 8 | 10 | 7 | 1 | 2 | 0 | ||||||||
| 9 | 8 | 6 | 2 | ||||||||||
| 10 | 0 | 0 | |||||||||||
| 11 | 9 | 8 | 1 | ||||||||||
| 12 | 19 | 19 | |||||||||||
| 217 | |||||||||||||
| Stage | 12 months (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 19 | 0.842105 | 0.157895 | 0 | 0 | ||||||||
| 2 | 74 | 0.918919 | 0.027027 | 0.054054 | 0 | ||||||||
| 3 | 40 | 0.925 | 0.075 | ||||||||||
| 4 | 4 | 0.75 | 0 | 0 | 0.25 | ||||||||
| 5 | 10 | 0.9 | 0.1 | 0 | 0 | ||||||||
| 6 | 19 | 0.842105 | 0.157895 | ||||||||||
| 7 | 5 | 1 | 0 | 0 | 0 | ||||||||
| 8 | 10 | 0.7 | 0.1 | 0.2 | 0 | ||||||||
| 9 | 8 | 0.75 | 0.25 | ||||||||||
| 10 | 0 | 0 | |||||||||||
| 11 | 9 | 0.888889 | 0.111111 | ||||||||||
| 12 | 19 | 1 | |||||||||||
| 217 | |||||||||||||
| Stage | 12 months (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 19 | 15 | 2 | 2 | 0 | ||||||||
| 2 | 66 | 63 | 3 | ||||||||||
| 3 | 48 | 47 | 1 | ||||||||||
| 4 | 1 | 1 | |||||||||||
| 5 | 19 | 15 | 1 | 3 | |||||||||
| 6 | 13 | 11 | 2 | ||||||||||
| 7 | 3 | 2 | 1 | ||||||||||
| 8 | 12 | 11 | 1 | ||||||||||
| 9 | 5 | 5 | |||||||||||
| 10 | 7 | 7 | |||||||||||
| 11 | 7 | 5 | 2 | ||||||||||
| 12 | 15 | 15 | |||||||||||
| 215 | |||||||||||||
| Stage | 12 months (n) | Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 19 | 0.789474 | 0.105263 | 0.105263 | 0 | ||||||||
| 2 | 66 | 0.954545 | 0.045455 | 0 | 0 | ||||||||
| 3 | 48 | 0.979167 | 0.020833 | ||||||||||
| 4 | 1 | 1 | 0 | 0 | 0 | ||||||||
| 5 | 19 | 0.789474 | 0.052632 | 0.157895 | 0 | ||||||||
| 6 | 13 | 0.846154 | 0.153846 | ||||||||||
| 7 | 3 | 0.666667 | 0.333333 | 0 | 0 | ||||||||
| 8 | 12 | 0.916667 | 0.083333 | 0 | 0 | ||||||||
| 9 | 5 | 1 | 0 | ||||||||||
| 10 | 7 | 1 | 0 | ||||||||||
| 11 | 7 | 0.714286 | 0.285714 | ||||||||||
| 12 | 15 | 1 | |||||||||||
| 215 | |||||||||||||
FIGURE 21.
Cost-effectiveness analysis for a lifetime horizon (30 years): HUI-3 measure.
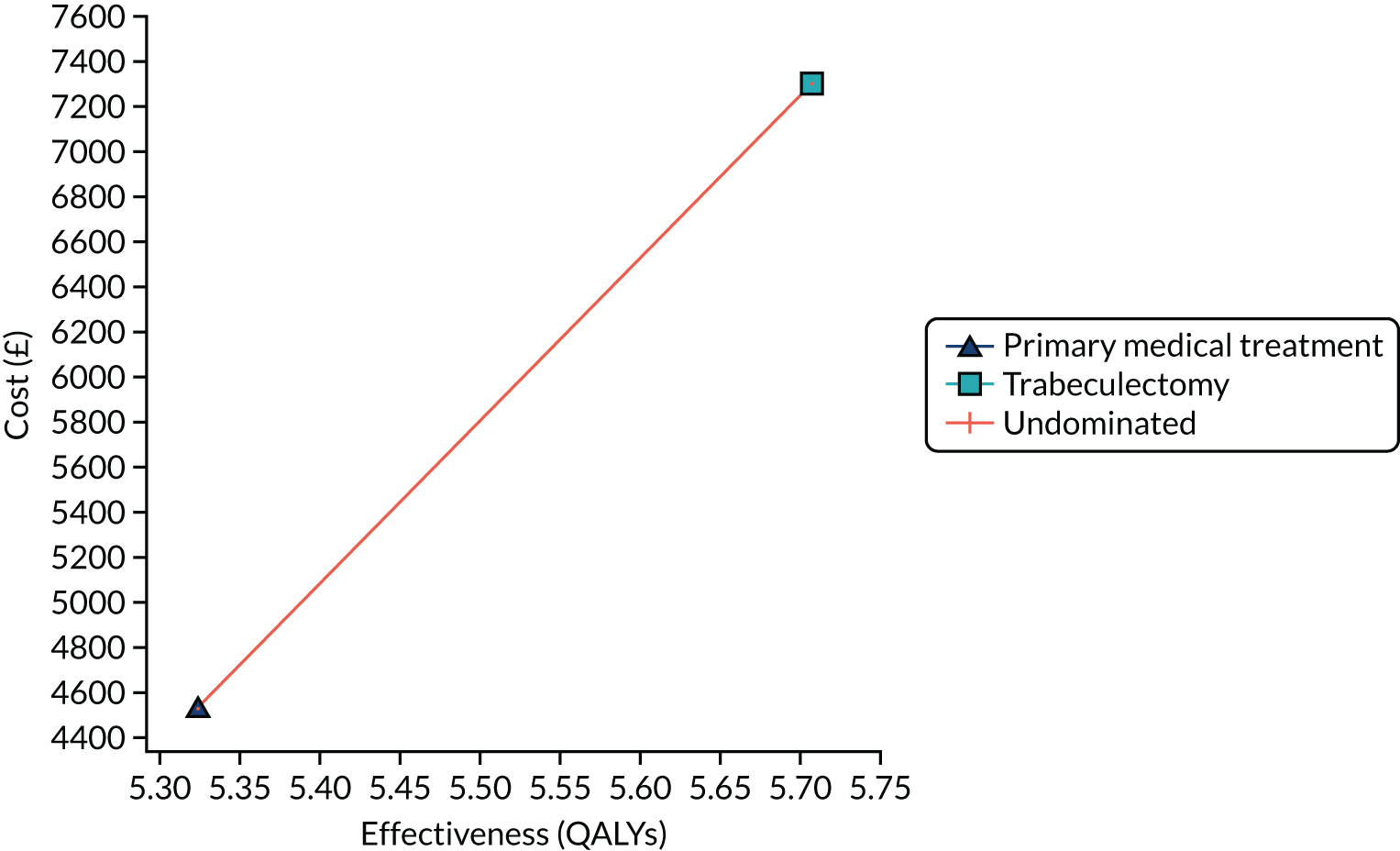
FIGURE 22.
Cost-effectiveness analysis for a lifetime horizon (30 years): GUI measure.
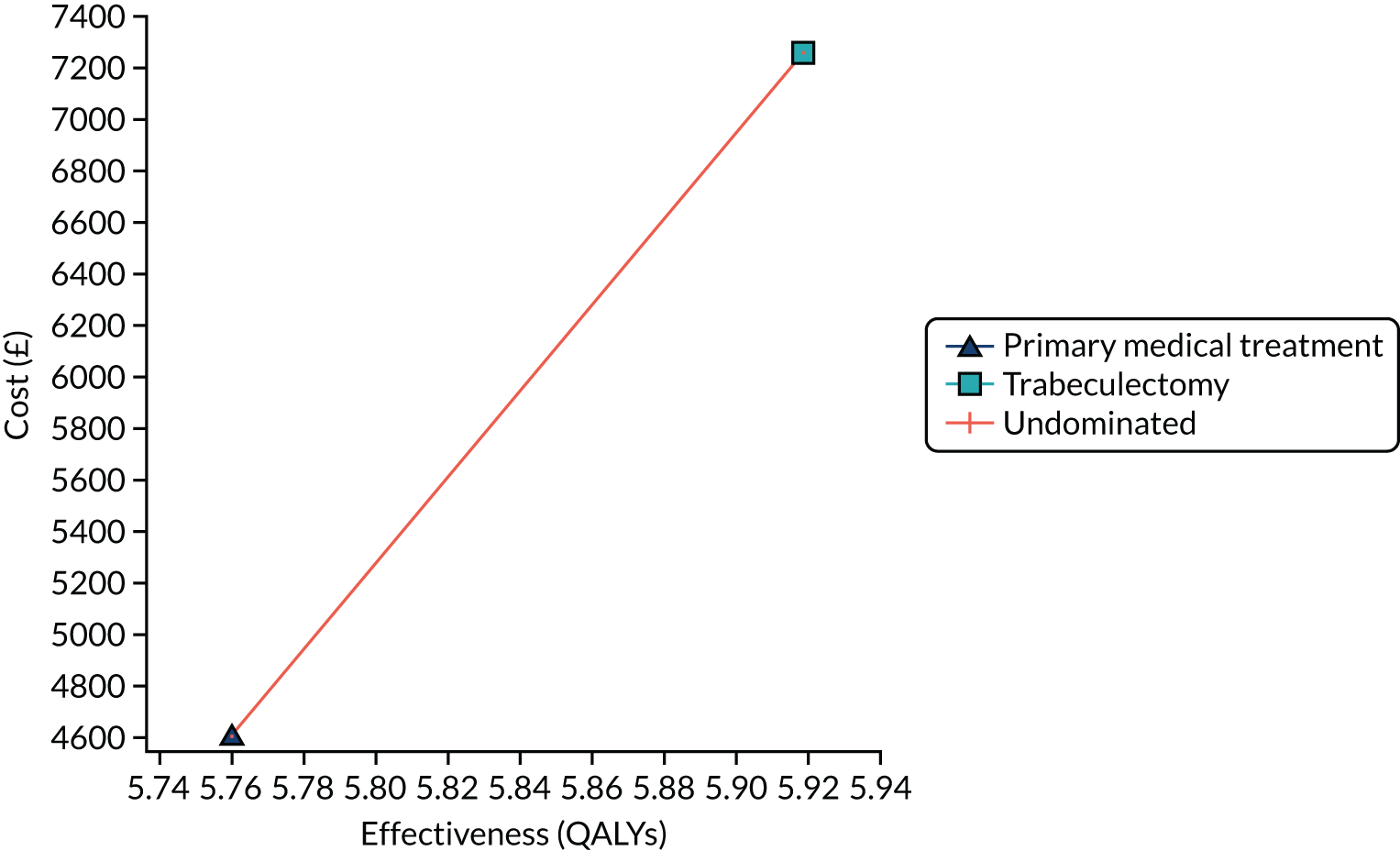
FIGURE 23.
Tornado diagram for the main parameters (HUI-3 measure).
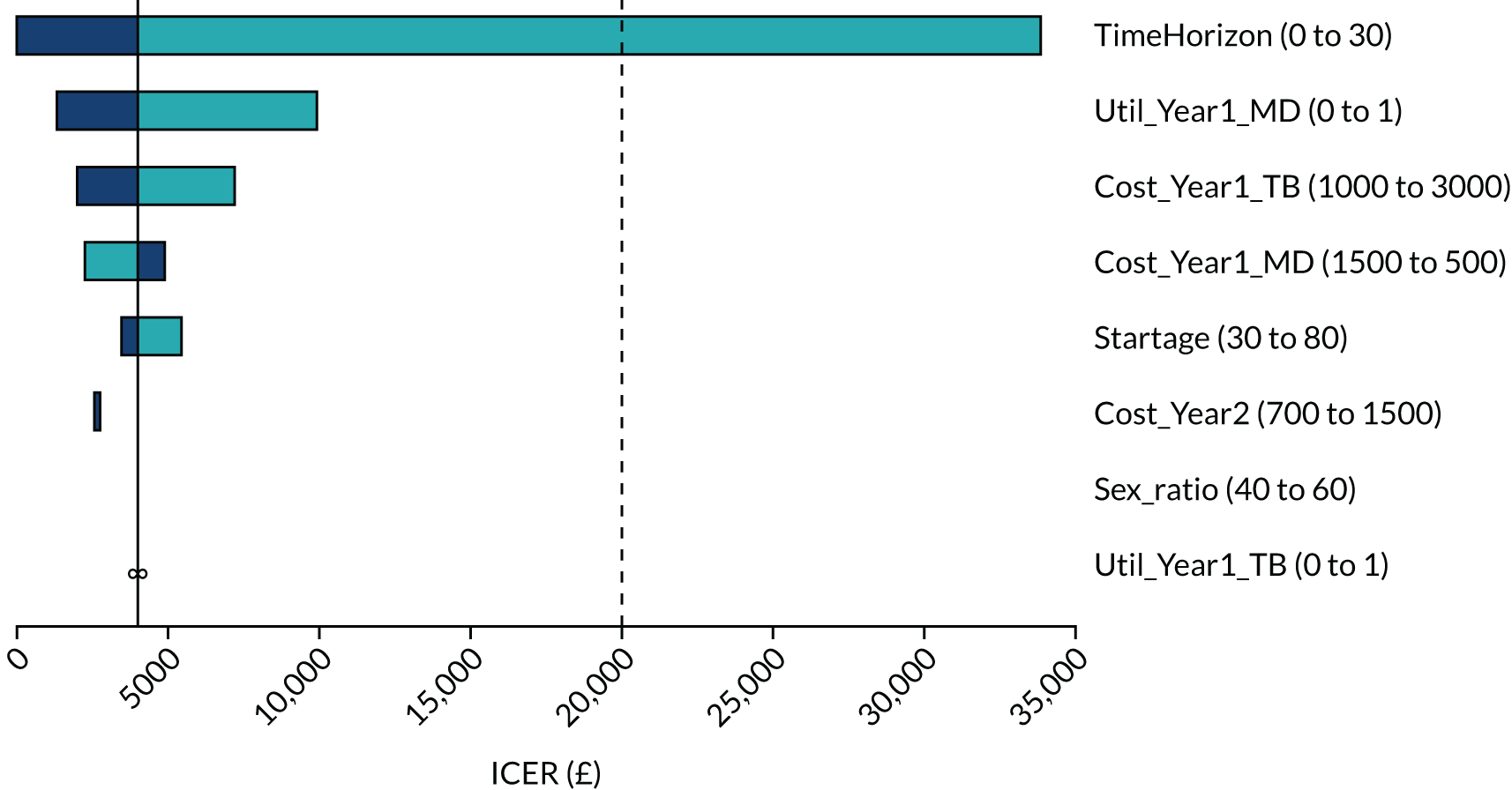
FIGURE 24.
Tornado diagram for the main parameters (GUI measure).
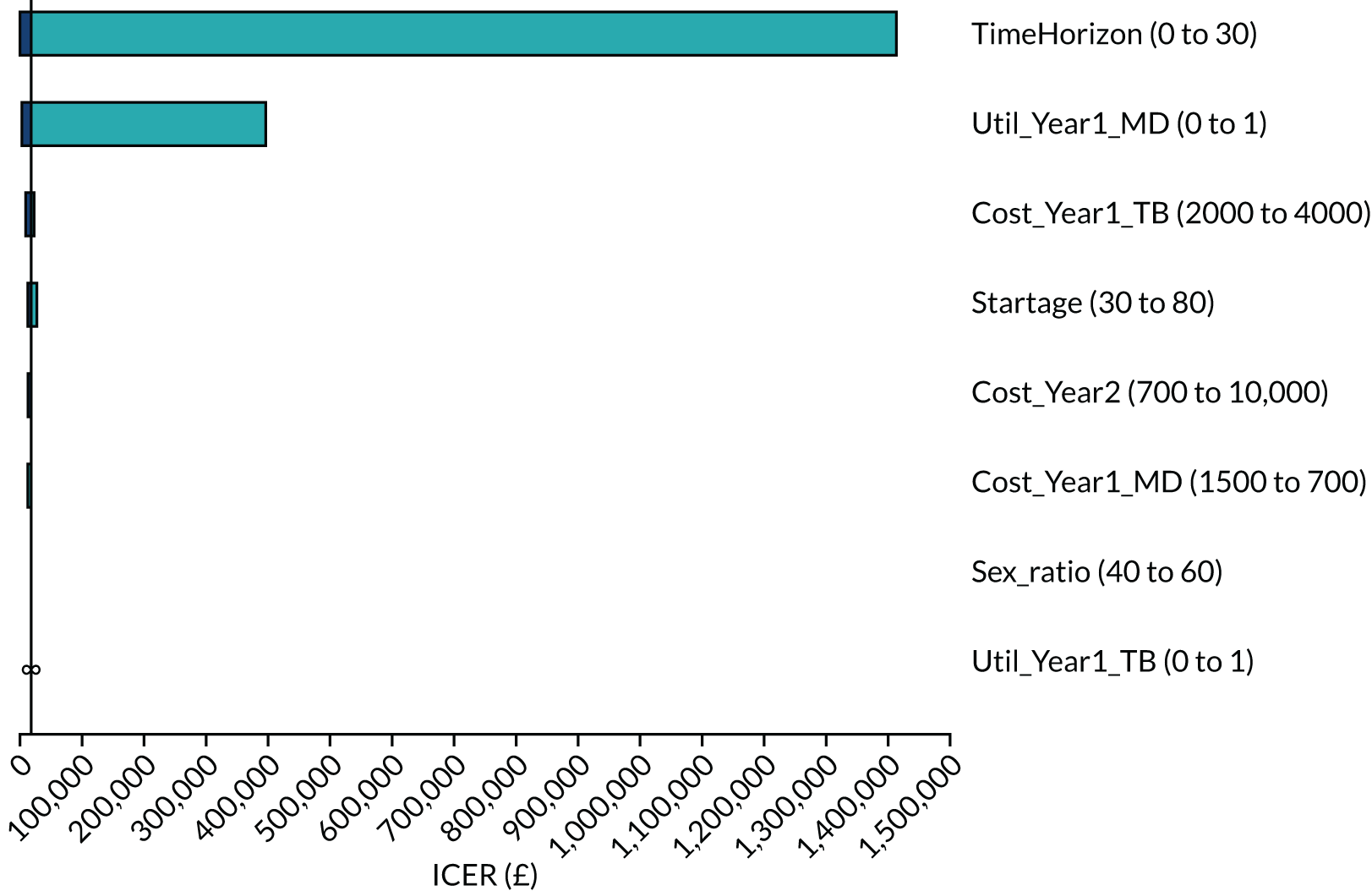
FIGURE 25.
Incremental cost-effectiveness scatterplot: trabeculectomy vs. medical treatment (HUI-3 measure). WTP, willingness to pay.
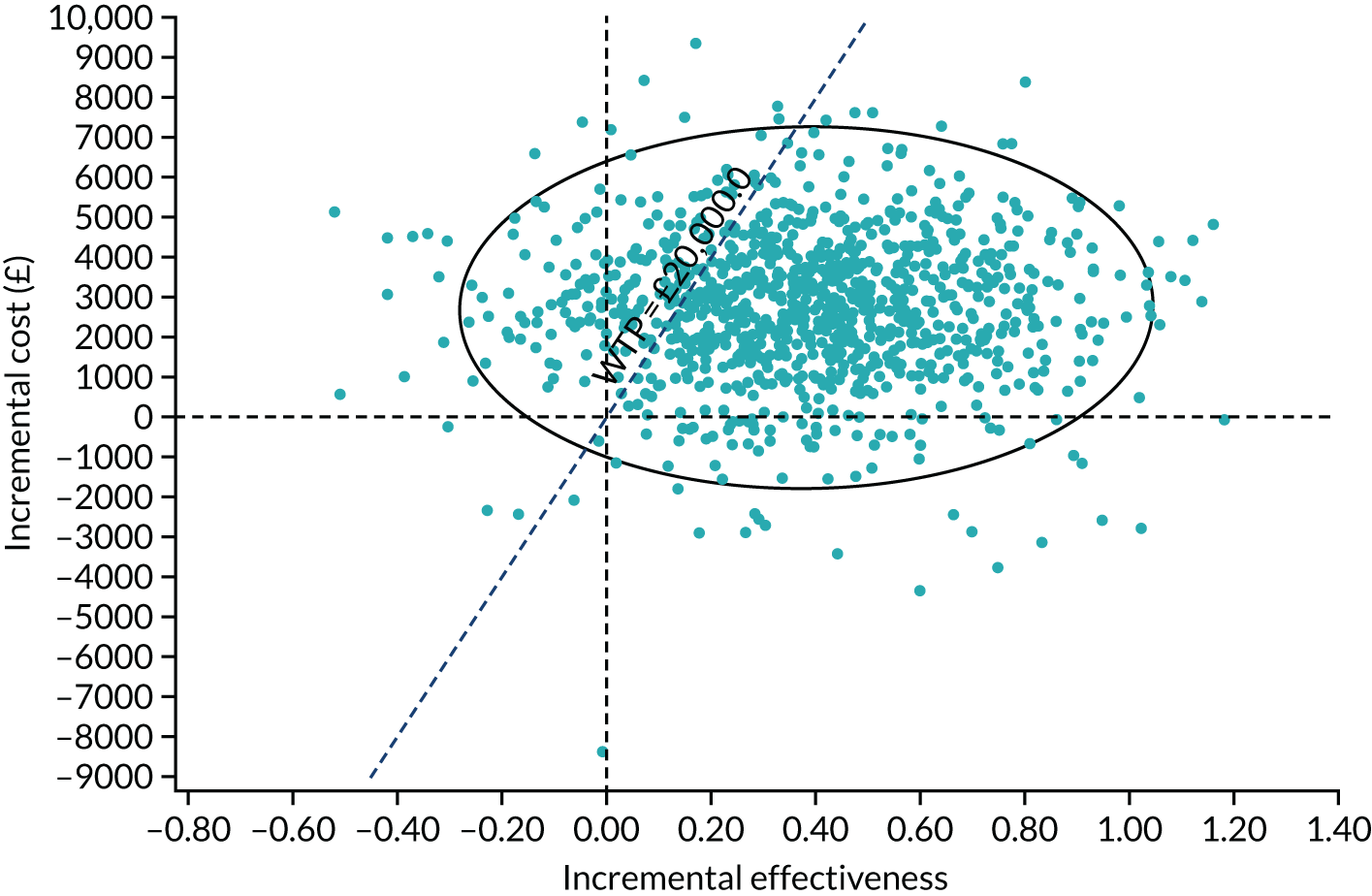
FIGURE 26.
Incremental cost-effectiveness scatterplot: trabeculectomy vs. medical treatment (GUI measure). WTP, willingness to pay.
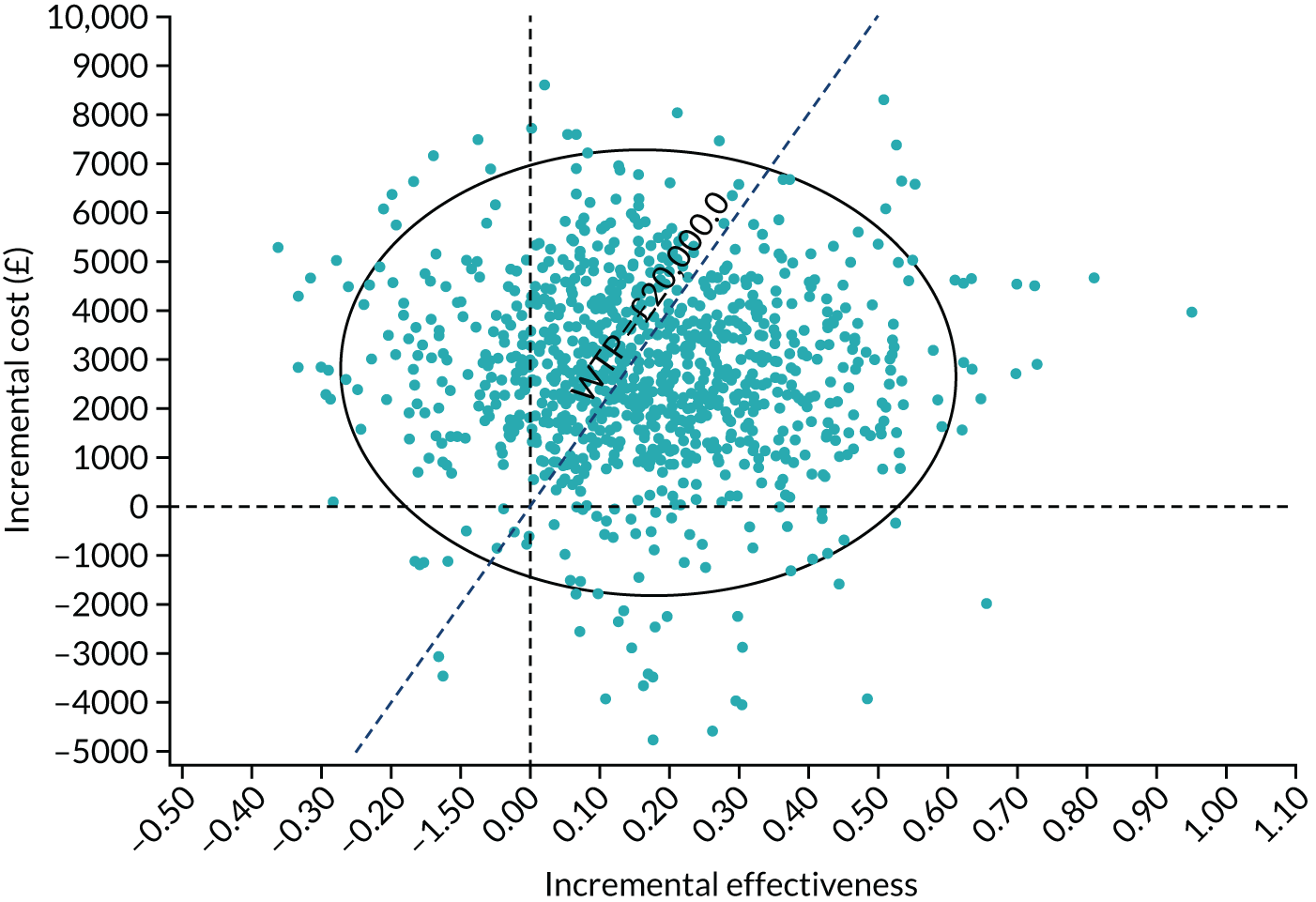
FIGURE 27.
Model-based cost-effectiveness acceptability curve for a lifetime horizon (30 years): HUI-3 measure.
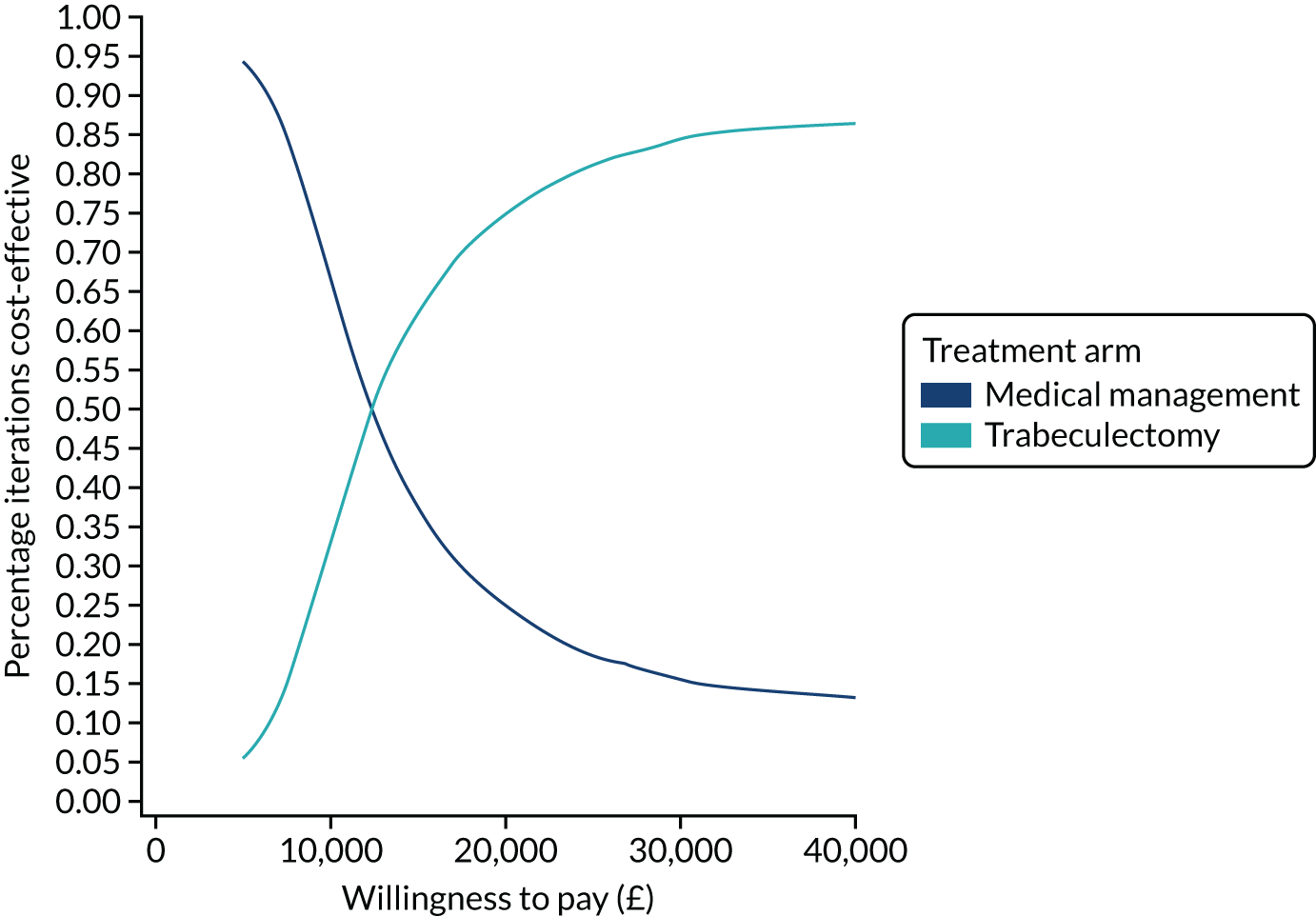
FIGURE 28.
Model-based cost-effectiveness acceptability curve for a lifetime horizon (30 years): GUI measure.
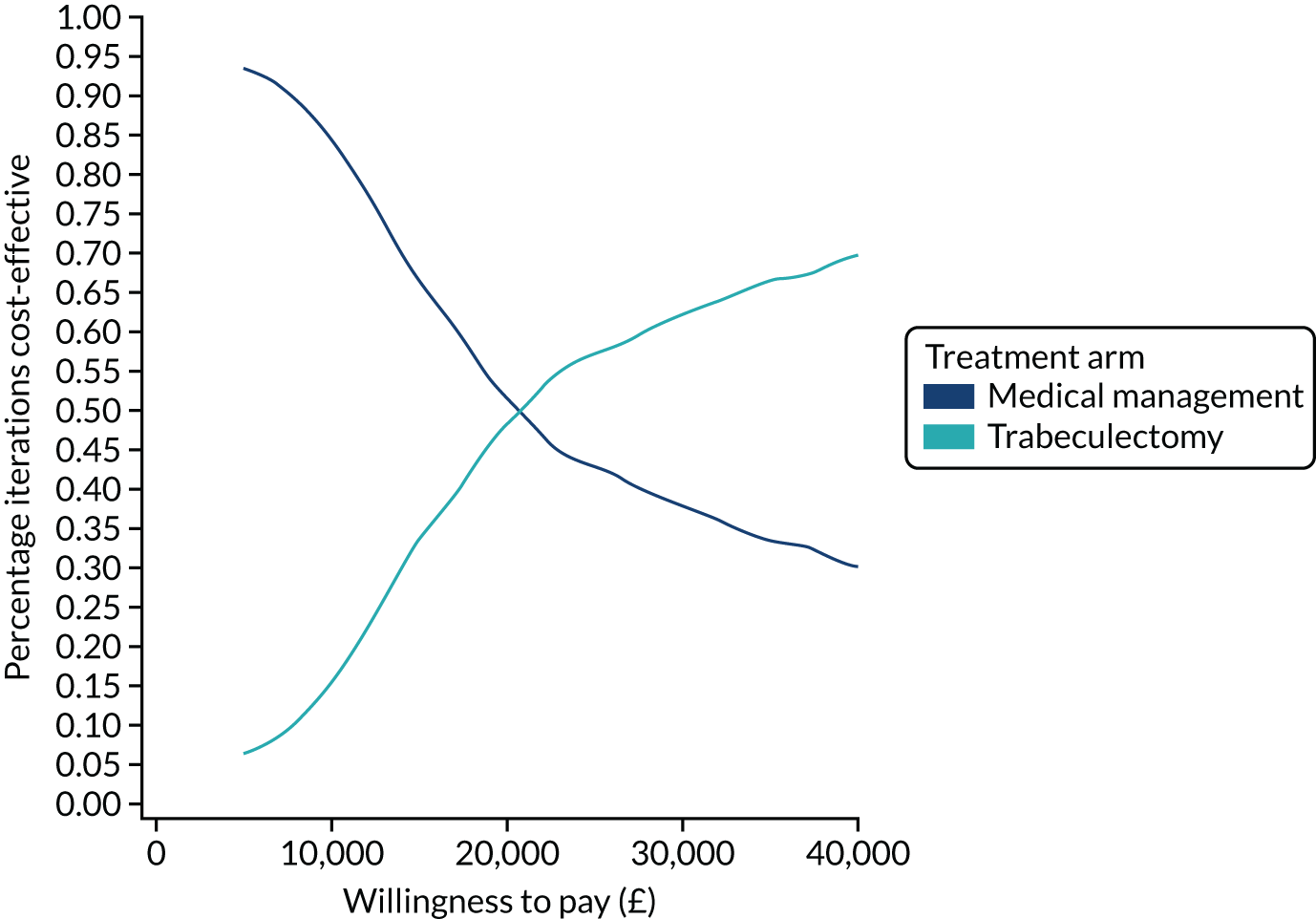
Appendix 8 Protocol changes
Amendments to the TAGS protocol during the trial.
Protocol version 1.1, date issued: 20 November 2013
5.2 Secondary outcomes – TSC requested Esterman-graded VF instead of direct question to participants on driving licence retention.
Redundant abbreviations removed from glossary of abbreviations.
Revision of eligible recruiting centres in section 6.3.1 Overall description of trial participants (see also 7.1).
Removal of reference to female partners of male participants in section 6.3.2 Inclusion criteria.
New section (6.4.3: Recruitment projection) added detailing feasibility pilot study. Subsequent sections renumbered.
Revision to section 6.4.6 (Clinical outcomes) of how eligibility determined from VF tests, Esterman VF at baseline and 24 months replace question about driving licence retention, removal of reference to pregnancies in female partners of male participants.
Revision of section 6.4.6 (Economic outcomes) rephrasing of sentence for clarity.
Revision of section 7.1 (Treatment strategies) to clarify all surgery will be undertaken by senior experienced glaucoma surgeons within 3 months of randomisation and index eye will be operated on first if both eyes eligible.
Addition of corneal decompensation to section 8.1.3 (expected AEs).
Revision of section 8.2.3 on reporting AEs and SAEs to an investigator or a delegate can update missing information following SAE notification and removal of reference to pregnancies in female partners of male participants and investigator or a delegate can notify a pregnancy.
Section 9.0 revised whereby a paragraph was removed and added to new section 6.4; redefinition of restrictions on surgeon involvement; the Statistical Analysis Plan will be available before the second, not first, TSC meeting.
In section 13.1 reference to the Declaration of Helsinki was updated.
Protocol version 1.2, date issued: 30 June 2014
In section 6.3.1 (Overall Description of Trial Participants) point 5 was corrected to refer to under 15 dB sensitivity rather than –15 dB.
Protocol version 1.3 date issued: 6 August 2014
Appendices A and B amended to highlight need for pre-surgery measures.
Protocol version 1.4, date issued: 26 August 2014
Addition of cataract formation and retinal detachment to expected AEs in trabeculectomy arm. Edit to appendix B to highlight need for VF test × 2 at each visit and Esterman at baseline and 24-month visits.
Protocol version 2, date issued: 22 October 2014
Dissociation of identification of the index eye from order of surgery following feedback from sites.
Protocol version 3, date issued: 1 March 2016
Addition of definition for VA AE for loss of vision.
Updated references in section 9.0 and reference list.
Protocol version 4, date issued: 4 August 2016
6.4.6 edited as VFQ-25 not being collected immediately prior to trabeculectomy.
Addition of broad complex tachycardia while under general anaesthetic, and post-operative dizziness to section 8.1.3 expected AEs.
9.6 long-term follow-up funding being pursued during trial.
Protocol version 5, date issued: 31 July 2018
Addition of appendix E detailing long-term follow-up study and update of study information in synopsis.
Protocol version 6, date issued: 5 March 2019
Addition of appendix F detailing substudy to collect genetic material.
List of abbreviations
- AE
- adverse event
- AGIS
- Advanced Glaucoma Intervention Study
- CACE
- complier-average causal effect
- CARF
- Central Angiographic Resource Facility
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CIGTS
- Collaborative Initial Glaucoma Treatment Study
- CRF
- case report form
- CUA
- cost–utility analysis
- DCE
- discrete choice experiment
- DMC
- Data Monitoring Committee
- DVLA
- Driver and Vehicle Licencing Agency
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- GP
- general practitioner
- GPI
- glaucoma profile instrument
- GSS2
- Enhanced Glaucoma Staging System
- GUI
- Glaucoma Utility Index
- HPA
- Hodapp–Parrish–Anderson
- HRQoL
- health-related quality of life
- HUI-3
- Health Utility Index version 3
- ICER
- incremental cost-effectiveness ratio
- IGA
- International Glaucoma Association
- IOP
- intraocular pressure
- IQR
- interquartile range
- logMAR
- logarithm of the mean angle of resolution
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OAG
- open-angle glaucoma
- PI
- principal investigator
- PMG
- Project Management Group
- PPI
- public and patient involvement
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SITA
- Swedish Interactive Threshold Algorithm
- SMG
- Study Management Group
- SUREG
- seemingly unrelated regression
- TAGS
- Treatment of Advanced Glaucoma Study
- TSC
- Trial Steering Committee
- UK-DUETS
- UK Database of Uncertainties about the Effects of Treatments
- VA
- visual acuity
- VF
- visual field
- VFMD
- visual field mean deviation
- VFQ-25
- Visual Function Questionnaire-25
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25720).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
