Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/20/02. The contractual start date was in October 2018. The draft report began editorial review in September 2020 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Eke et al. This work was produced by Eke et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Eke et al.
Chapter 1 Background and rationale
The acquisition of continence is an important milestone in child development. It involves planning, recognition of sensation, regulation, control, urinating and defecating in an appropriate place and cleaning and dressing afterwards. 1,2 Becoming continent involves the maturation of developmental domains, including sensory perception, cognitive and social understanding and motor planning, and there is wide variation in the age at which this occurs. Social, economic and environmental factors, parenting strategies and behaviour all affect the acquisition of continence.
Neurodisability describes a group of congenital or acquired long-term conditions that are attributed to the impairment of the brain and or neuromuscular system, creating functional limitations. 3 Impacts may include difficulties with movement, cognition, hearing, vision, communication, emotion and behaviour. Sensory disturbances may impair balance, proprioception and interoception. Children with neurodisability may often be referred to as children with special educational needs or children with disability.
Children with neurodisability have a higher incidence of delayed acquisition of continence (being clean and dry) and of incontinence (lower urinary tract and/or bowel dysfunction resulting in the involuntary leakage of urine or faeces) than other children, and may be slower to learn to manage going to the toilet, or they may not attain full independence. 4–7 Many children, however, can become continent with training. Factors that affect the ability of children with neurodisability to achieve continence include structural malformations and/or physiological impairments that can affect sensation and control, functional limitations (e.g. gross or fine movement or manual ability), learning difficulties and behavioural issues. These children may regress due to progressive impairment, psychological issues or the development of bladder or bowel dysfunction. Incontinence affects the quality of life (QoL) of the young person and that of their carers;8 the long-term physical, psychological and financial burden can be considerable. 9 There is also a cost to the NHS in terms of providing containment products for managing incontinence.
Not all children have the ability to become fully independent in toileting, but many can improve their continence. Assessing readiness for toilet training can be difficult; a child may display signs of readiness, or have the capability for readiness but not express it. Various factors can influence if and when toilet training commences. A key issue is whether or not families and professionals think that a child is ready and able to begin toilet training. Sometimes, unfortunately, assumptions are made about a child’s inability to train without a formal assessment being undertaken. There can be perceptions that the cause of any incontinence is either part of the child’s ‘condition’ or a reflection that they are ‘not ready’. This may result in the child with neurodisability not being offered the same comprehensive assessment that their typically developing peers, with similar problems, would be offered.
A variety of approaches to assessment, advice and intervention are available. 10 Children should be assessed systematically to see whether or not they are able to be trained and to identify any related medical problems that may inhibit the improvement of their continence. Toilet training strategies are complex interventions, and build on ideas initially proposed in the 1960s and 1970s. 11,12 Interventions to improve continence may include information/support, charts to monitor/feedback, scheduled drinks and toileting, cognitive–behavioural approaches, alarms, relaxation, psychotherapy, group-based programmes, medicines and surgery. 13 A systematic review14 identified limited evidence for toilet training strategies for children with physical and learning disabilities. Medication is sometimes used as part of treatment, and medications used for managing other impairments may have an impact on continence. Currently, there is uncertainty about the most effective ways to assess and promote continence in children with neurodisability.
Why this research is important
Pillar One of the National Institute for Health Research (NIHR) strategy for Adding Value in Research is to ensure that the questions being researched are those most important to patients, the public and clinicians. Research to evaluate ways to promote continence for children with neurodisability was ranked number 7 in a top 10 of topics by young people, parent carers, clinicians and charity representatives in the British Academy of Childhood Disability James Lind Alliance Research Priority Setting Partnership. 15 Improving continence can have a huge impact on the QoL of children and young people and their families, and can potentially reduce NHS expenditure in providing containment products for managing incontinence. Identifying current clinical practice in the NHS and summarising the available evidence for interventions allows us to make recommendations for research and practice for improving continence in children and young people with neurodisability.
The aim of the study was to summarise the available evidence for interventions and current practice relating to improving continence for children and young people with neurodisability. The methods in the commissioning brief were a survey of NHS practice and a systematic review. Fundamentally, we set out to examine what families and professionals were doing, and if there was any evidence that these approaches are effective. To be consistent with special education needs and disabilities (SEND) legislation,16 we considered any approaches taken to assess and promote continence for children and young people up to the age of 25 years.
Aims and objectives
We aimed to find out how NHS staff assess and treat children with neurodisability to help those children become continent. To do this, an online survey was conducted with health professionals to describe clinical practice in the NHS, addressing the following research questions:
-
How do clinicians assess the bladder and bowel health of children and young people with neurodisability, their continence capabilities and their readiness for toilet training? Which clinicians are involved in assessments?
-
Which interventions do clinicians use or recommend to improve continence for children and young people with neurodisability and how are these individualised and evaluated and/or audited? Which clinicians recommend, deliver or evaluate interventions?
We also surveyed families, school and care staff about their experiences of using interventions to improve continence, addressing the following research questions:
-
How do families, school and social care staff consider and judge children’s readiness for toilet training and need for specialist assessment and/or interventions?
-
Which factors affect the implementation of interventions to improve continence, and what is the acceptability of strategies to children and young people and their carers?
Alongside the survey, we conducted an integrated systematic review of studies evaluating the (1) effectiveness, cost-effectiveness or implementation of interventions for improving continence for children and young people with neurodisability, and (2) views, experiences and perceptions of families and/or health professionals using and delivering interventions. The systematic review aimed to answer the following research questions:
-
What is the effectiveness of interventions to improve continence in children and young people with neurodisability?
-
What is the cost-effectiveness of interventions to improve continence in children and young people with neurodisability?
-
What are the factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions to improve continence in children and young people with neurodisability?
-
What are the views, experiences and perceptions of children and young people, their families, their clinicians and others involved in their care of delivering and receiving such interventions?
Chapter 2 Overview of methods
The project was designed with four interlinked phases: preparation, consultation, review and integration. During the preparation stage (months 1–3), key issues were discussed and explored with the study’s expert Professional Advisory Group and our Family Faculty group (our public involvement group) to produce, refine and finalise the protocols for the systematic review and the surveys. We also consulted on the design of the survey questionnaires, formatting, and the application for ethics approval. In the consultation phase (months 3–13), we conducted and analysed descriptive cross-sectional surveys with health professionals, school and care staff, parent carers, and young people in England. In parallel (months 3–13), for the review phase, we carried out the systematic review. Finally, during the integration phase (months 14–16), findings from the surveys and systematic review were collated and interpreted in consultation with the Professional Advisory Group, our Family Faculty group and young people with neurodisability.
Scope
The scope of this study focused on the following:
-
Population – children and young people with non-progressive neurodisability aged up to 25 years, consistent with the Department of Health and Social Care Special Educational Needs and Disabilities code of practice17 and the Children and Families Act 2014. 18
-
Interventions – assessments and interventions to improve continence, including structured training programmes, products and assistive technology, medicines and/or surgery.
-
Outcomes – (1) effectiveness, cost-effectiveness and implementation of interventions to improve continence; and (2) views and experiences of families and health professionals.
Complete and transparent reporting
To deliver a complete and transparent report of the research, we were mindful of the Guidance for Reporting Involvement of Patients and the Public short-form;19 the Strengthening the Reporting of Observational studies in Epidemiology: cross-sectional studies;20 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 21
Throughout the report, we endeavoured to use consistent terminology for continence, assessments, interventions and outcomes. We also referred to the International Children’s Continence Society (ICCS)22 for bladder and bowel dysfunction terminology and to McComb23 for spinal cord pathology terminology.
Conceptual and theoretical frameworks
In terms of the conceptual frameworks underpinning this research, we were acutely aware of the need to take into account the complexity that comes with evaluating approaches to assess and interventions to improve continence in children and young people with neurodisability. We were mindful of the complexity of many of the relevant interventions as defined in the Medical Research Council (MRC) framework. 24 That is, salient interventions are multicomponent and context dependent, and the adoption and effectiveness of an intervention is reliant on the motivations and capabilities of children, parents and practitioners. Additionally, we considered the intricacies of the separate and combined health, education and care systems, the variable configuration of services, and the diversity of family cultures, resources and environments. 25,26
The World Health Organization’s International Classification of Functioning, Disability and Health (ICF) classifies components of health in domains of ‘body structures’, ‘body functions’, ‘activities and participation’, ‘environmental factors’ and ‘personal factors’. 1,2 The performance of activities and participation by an individual depends on their capacity and is mediated by personal and environmental factors. Thus, a health condition may involve impairments of body structures or functions, limitation in activities and/or restriction in participation; the relationships between these components are bidirectional and mediated by environmental and personal factors. Toileting is classified in the ICF as self-care (https://apps.who.int/classifications/icfbrowser/; accessed 19 October 2021). Following discussion, the team decided that menstruation was not in the scope of the commissioning brief.
Using the ICF classification, the bladder and bowel are body structures, urination and defecation are body processes, and the regulation of urination and defecation are classified as ‘self-care’ activities. Salient environmental factors include health services, systems and policies, products and technology for (1) education, (2) personal use in daily living (toilet adaptations, clothing adaptations), (3) communication, (4) personal indoor and outdoor mobility and (5) personal consumption (medicines). Not classified specifically are some medically assisted techniques and surgical approaches. Also pertinent are the designs of buildings for public and private use in terms of accessible toilets.
We sought to devise a study-specific conceptual framework diagram to inform and guide the research, and later to help integrate the findings of the systematic review and surveys (Figure 1). Initially, the framework took the form of the patient–intervention–outcome model; we identified the capabilities that individuals need to acquire for continence, the range of interventions that could be used and the ways in which the outcome of continence could be conceived. The framework outlines a logical process through which capability, needs, interventions and outcomes can be described and considered. The concepts are intended not to be linked sequentially but to be seen as interacting.
FIGURE 1.
Study-specific conceptual framework diagram to inform and guide the research. The concepts are not intended to be linked sequentially but seen as interacting.
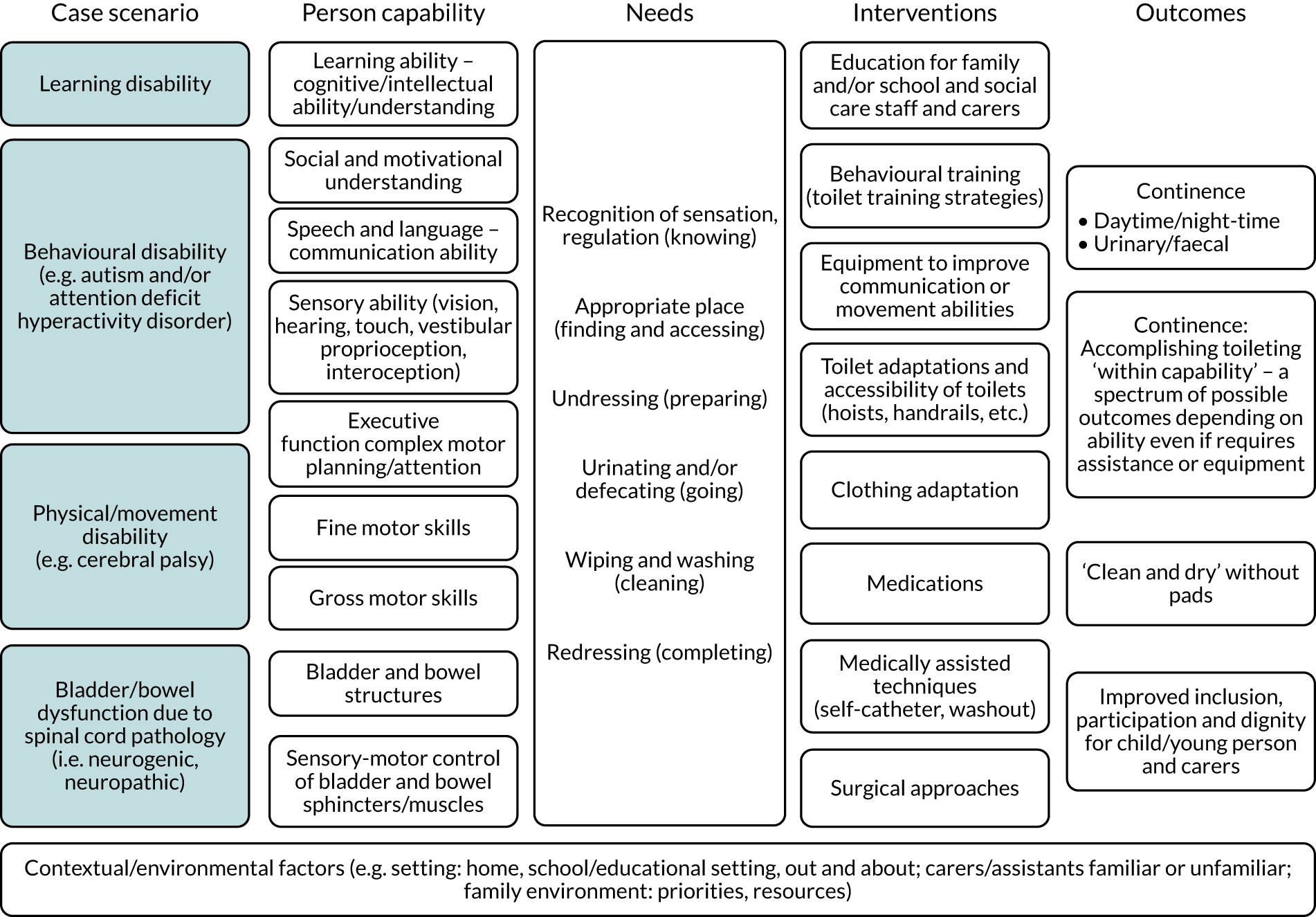
The person capabilities at the fundamental level comprise (1) intact structure and physiological functioning of the bladder and bowel, and (2) sensorimotor control of the bladder and bowel sphincters and muscles. The other aspects of capability in our framework are a summary of the toilet training skills usually acquired sequentially in typically developing children: movement and manual ability, communication, and social and motivational understanding. Additionally, we included vision as vital for negotiating toileting, although children with limited vision are amenable to familiarisation and/or adaptation.
In order that the findings of this research might be readily generalised to children and young people with neurodisability, we posited four exemplar clinical case scenarios that capture different manifestations of the person capabilities. These were (1) spinal cord pathology (e.g. spina bifida), (2) learning disability, (3) movement disability (e.g. cerebral palsy) and (4) behavioural disability [e.g. autism and attention deficit hyperactivity disorder (ADHD)]. However, in practice this was problematic, as many children have complex conditions that are affected by components of all these difficulties. Therefore, as the project developed, we moved towards the main distinction being between children with spinal cord pathology and those with non-spinal-cord-related pathology indicating potential for bladder and bowel sensorimotor control.
As we progressed, and benefiting from input from our advisers, we found that our framework was underpinned best by a needs-based approach for toileting and capabilities for achieving continence:
-
KNOWING – understanding when needing to empty bladder and/or bowel
-
FINDING – waiting until finding a toilet
-
ACCESSING – being able to enter toilet
-
PREPARING – undressing and positioning
-
GOING – emptying bladder and/or bowel
-
CLEANING – wiping
-
COMPLETING – redressing and washing hands.
In terms of outcomes, the distinction between what the concept of continence means for those individuals with and those without spinal cord pathology affecting bladder and bowel sensorimotor control is crucial. Without sphincter control, there will often be a need for assistive technology or alternative approaches to emptying the bladder or bowel. Some children with profound learning disability will always need individual assistance with toileting, and so independence may not be safely realistic. By contrast, others may be able to accomplish toileting independently, provided that adequate accessibility and/or equipment is available. This way of conceptualising continence as an outcome emphasises that toileting techniques need to be adapted to the individual’s capability, that it will vary from person to person and that the individual may require assistance or equipment. However, the aim is to be ‘clean and dry’ and, therefore, not require incontinence pads.
We organised interventions into ICF categories as follows:
-
Products and technology for education –
-
Educational approaches focusing on enabling families and carers to understand the child’s capability and necessary modifications, for example attending to toileting at regular or timed intervals.
-
Behaviour change approaches focused on the child, for example increasing awareness.
-
-
Products for personal use in daily living –
-
Environmental modifications to make toilets more accessible and easier to use, for example handrails, positioning equipment and/or hoists.
-
Adaptation of clothing to increase independence, for example hook-and-loop fasteners rather than buttons, or enabling access to self-catheterise.
-
Continence products, such as pads/nappies, used for containment.
-
-
Products and technology for communication –
-
Equipment to improve communication about toileting.
-
-
Products and technology for personal indoor and outdoor mobility –
-
Equipment to enable movement in various environments.
-
-
Products for personal consumption (subset: drugs) –
-
Medications to help regulate toileting, for example anticholinergic medications to regulate bladder emptying or laxatives to treat constipation.
-
-
Medically assisted techniques and surgical approaches –
-
Alternative ways to empty the bladder and bowel, for example catheter or washout.
-
Surgery to change structures or enable catheterisation.
-
The assessment of children and young people by families and professionals plays a crucial part. Key to this is avoiding assumptions about ability, the need to assess each individual’s capabilities for achieving continence, their likely level of independence in achieving the identified needs, and deciding systematically which interventions and approaches are likely to be helpful. Assessments can include one or more of parent/carer reports, child/young person self-report, charting regularity and events, checklists, physical examination, ultrasound or other imaging, urodynamics or direct observation.
It is important to consider that children with neurodisability and non-spinal-cord-related pathology should have a typically developing bladder and bowel. However, they will be at least as susceptible to bladder and bowel issues that can affect any child or young person more generally. Common bladder problems include enuresis, which is the involuntary discharge of urine during sleep; overactive bladder, which is characterised by frequency, urgency and/or daytime wetting; and dysfunctional voiding, which is the habitual contraction of the urethral sphincter or pelvic floor during voiding. Furthermore, the commonest bowel problem, constipation, affects up to 30% of children at any one time. Children who have a neurodisability have an increased risk of this for a variety of reasons.
Our conceptual framework highlights the crucial influence of contextual factors. Therefore, assessment should take account of all the environments where the child or young person might need a toilet, for instance home, nursery/school or college/work, or out in the community. If assistance from a carer is necessary then familiarity may be influential, in terms of communicating a need to toilet and/or the carer understanding the individual’s need for assistance.
Project management
The whole team of co-investigators and researchers working on the project met at the beginning of the study and again once the survey and systematic review were largely completed. The co-investigators consisted of consultant paediatricians, specialist continence nurses and therapists. A subset of the core team based in Exeter comprised the researchers and leads for the systematic review and survey. The systematic review was led by the Evidence Synthesis Team of the NIHR Applied Research Collaboration (ARC) South West Peninsula (known as PenARC). The survey and project management was led by the Peninsula Childhood Disability Research Unit (PenCRU). The core team met monthly and benefited from teleconferences with the whole team every 2 months. The study was also improved by our public and stakeholder engagement.
Patient and public involvement
In this study, members of the public involved as partners were predominantly parents of children and young people with neurodisability, and children and young people themselves. They were engaged in research through partnership with PenCRU. The aim of patient and public involvement in this study was to ensure that (1) the research was conducted in ways likely to be attractive and acceptable, (2) the research outputs were more likely to be perceived to be relevant and useful to families of children with neurodisability, and (3) our dissemination materials and methods were appropriate and relevant. We describe our public involvement using the elements recommended in the Guidance for Reporting Involvement of Patients and the Public (GRIPP2) short-form. 19 This section describes how parents of children affected by neurodisability were involved in the study, and discusses the impacts that parent involvement had on the research. The patient and public involvement activities in which the parents and young people participated are described and their contribution to the study is reflected on.
Family Faculty group: parent carers
PenCRU carries out public involvement through a self-styled Family Faculty group and this is facilitated by the family involvement co-ordinator. Currently, around 200 parent carers of children with neurodisability are members of the PenCRU Family Faculty group. They have all signed up to be notified of opportunities to be involved as partners in aspects of the research cycle. For substantive projects such as this study, ‘working groups’ are convened. We e-mail all members with brief information about the topic, and those who are interested and available at the time can volunteer. Parent carers in the study-specific group participate in meetings and/or input into research by e-mail or telephone. We do not train members of the Family Faculty group, but we do identify individual learning needs and provide information and encouragement consistent with any member’s personal motivation and time available.
For this study, we e-mailed the Family Faculty group when we were preparing the outline funding application, and 24 members volunteered to join the project-specific working group. Their children had various diagnoses, including movement and/or learning disabilities, such as cerebral palsy and autism, and had experience of a range of issues relating to toileting and continence. Two of the more experienced Family Faculty group members volunteered to be co-applicants on the funding bid. Our Family Faculty group met in each academic term throughout the study. There were more frequent meetings when there was more opportunity for influencing the work, especially when we were developing the survey questionnaires and recruitment processes. All the working group members were invited to each study meeting, although, owing to balancing work and family life, not all attended every meeting. In total, 16 of the 24 members attended one or more of the study meetings. On average, seven members attended every meeting.
Pre-funding application stage
The Family Faculty group first met prior to funding being granted to help develop our ideas for designing the proposed study. The group met twice in June and October 2017 to advise on the stage 1 and stage 2 applications, respectively. Their input identified key contextual factors related to toileting for children with neurodisability, and influenced our evolving strategies for advertising and consultation with families through the survey, such as how to recruit participants, how to consult with children and young people, what information should be collected in the survey to describe the sorts of families who took part, and the types of questions that would help address the research question. The frequency of future meetings was also discussed. Crucially, surveying families was not part of the commissioning brief, which focused on current NHS practice; however, members of the group felt strongly that families should be consulted.
Post-funding preparatory work
The Family Faculty group met more regularly during the preparation stage of the study, with four meetings held between November 2018 and May 2019. During these meetings, parent carers were consulted on the advertising materials, participant information forms and parent carer survey questionnaires that were all prepared for submission to the ethics committee. The Family Faculty group were consulted on ideas to engage and access potential participants for the school and care staff survey. They were keen to learn about and advise on aspects of the systematic review process.
In the earlier meetings, prior to commencement of the study, feedback from the Family Faculty group was that the response options in the survey for parent carers should be designed using mostly tick boxes or drop-down choices to enable the questionnaire to be completed quickly. Suggestions for the focus of the questions included toileting or continence interventions they had used, including any alternative therapies, rating their experience of strategies and/or whether the intervention had been successful or acceptable. They recommended providing a realistic estimate of how long it would take to complete the questionnaire, including a progress bar and ‘back button’. The Family Faculty group did not believe that a financial incentive was necessary and thought that this might lead to people taking part in the survey who did not have the correct motivation.
During the meetings between November 2018 and May 2019, the Family Faculty group assisted in revising the advertisement to share with organisations in terms of its appearance and the language used. The group provided additional suggestions of organisations or charities that could be contacted to aid recruitment. Members of the group piloted the registration process for the survey. Some members of the group felt that the registration process was an ‘extra step’ and might put people off. They noted that the process was simple and straightforward, although some had concerns that potential participants might be deterred by being asked to provide their e-mail addresses and queried whether or not a direct link to the survey might be preferable.
Members of the group commented and provided feedback on the study website design and content and made suggested changes to ensure clarity and understanding of the language used. They felt that the content was quite detailed and that a short animated video would be an easier and more accessible way for busy parents to quickly understand what the project was about and how they could get involved. Ideas for the video were developed, and suggestions included using humour and cartoon characters, keeping the video short and words limited to ensure that it was accessible to and understandable by all ages and abilities. A ‘script’ for the video narrative was proposed at a subsequent meeting and members made suggestions to add or remove content. It was agreed that a young person should narrate the video to provide an attractive voiceover to all ages. The video we produced was narrated by one of the children of a member of the Family Faculty group.
A pilot set of parent carer survey questions and response options was presented to the Family Faculty group, and members provided feedback on the questions (whether they could understand them clearly, whether the language would be readily understood) and they provided additional response options for some questions. Some felt that the parent carer questionnaire was quite long, and advocated for a shorter set of questions. Following refinements, the electronic survey questionnaire was created online. Members of the Family Faculty group piloted the survey online and provided feedback on the time they took to complete it, clarity of questions, the format and font. This further feedback was used to review and amend the questionnaire.
We developed a plain language summary protocol for the systematic review. The first draft of this was created by the researchers. Members of the Family Faculty group provided feedback on the text and format that influenced the final version. We communicated progress throughout the phases of the systematic review, asking for feedback on specific aspects such as terminology and interventions, and discussed parent carers’ views on the emerging findings.
Interpretation stage
Following completion of the survey and systematic review, the Family Faculty group met twice more during the integration stage of the study. In these meetings, the parent carers were asked to comment on and provide their interpretation of some initial findings from the systematic review and the survey, and they were consulted on potential ideas for disseminating the research findings.
Involving children and young people with neurodisability
We sought opportunities to consult children and young people with neurodisability about aspects of the study in the preparation stage, and again on the initial findings of the survey and systematic review in the integration stage of the study. A small number of PenCRU’s Family Faculty group members are young adults with neurodisability. Two of these young adults, a female group member with cerebral palsy who is profoundly deaf and a male group member with cerebral palsy, were invited to consult on various aspects of the study design. Both commented on the study website and the young person participant information sheets for the survey, and both also piloted the children and young people’s survey and offered feedback on the design, the language used and the questions asked.
The Pelican Project is a local community group in Exeter whose members work with young adults with disabilities, to assist them to make a contribution to the community. This group were approached and asked if any of their members would be willing to be consulted on their perspective of the children and young people’s survey. We met with four young adults with a range of disabilities who were assisted by their carers in the preparation stage of the study. The group were consulted on how we could access young people and encourage them to participate in the survey, and the group were also asked to review the information sheets for young people and the relevant study web pages for the survey.
Children and young people with neurodisability who attended a special school for children with severe learning difficulties were also invited to take part in the project through being consulted on our methods and findings. The group of four young people was convened with the assistance of the school advocacy lead and offered their comments and feedback regarding the questions in the survey for young people, the language used, and how they might feel about answering personal questions about their toileting ability. The advocacy lead also piloted the school and care staff survey and provided feedback.
Other stakeholder/end-user involvement
Professional Advisory Group
Our Professional Advisory Group was established in collaboration with ERIC (The Children’s Bowel & Bladder Charity). ERIC provides information and education and collaborates on research with children and young people and their families with continence challenges in the UK. With permission, we co-opted ERIC’s Professional Advisory Committee, which comprised expert professionals in the field of childhood bowel and bladder health who were keen to assist, plus one other professional who had expressed interest. Our Professional Advisory Group therefore consisted of 12 professionals who, collectively, had extensive experience in the field across medical, nursing and allied health professions.
During the preparation stage of the study, we convened the Professional Advisory Group and explained the study. Members were consulted about the study website, advertisements for the health professional survey, potential personal and organisational contacts for sharing adverts for the survey, and potential questions for consideration in the health professional survey. Following this initial meeting, the Professional Advisory Group members were consulted by e-mail regarding participant information sheets for health professionals, and they were also asked to assist with sharing advertisements for the survey once it had gone live. The Professional Advisory Group was convened again in May 2020 during the integration stage so that the members could help to interpret the findings from the survey and systematic review.
Oversight Group
The Oversight Group was approved by NIHR at the beginning of the project; it met with the core research team for the first time in May 2019. This group comprised two parent carers, a paediatrician, an allied health professional with considerable experience in neurodisability research, a psychologist with considerable experience in continence research, and another with expertise in systematic review methodology. This group met 3 months after the study commenced and provided valuable guidance, particularly in relation to adopting the needs-led approach in our conceptual framework.
Chapter 3 Systematic review of effectiveness and cost-effectiveness of interventions to improve continence for children and young people with neurodisability
Research questions
-
What is the effectiveness of interventions to improve continence in children and young people with neurodisability?
-
What is the cost-effectiveness of interventions to improve continence in children and young people with neurodisability?
-
What are the factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions to improve continence in children and young people with neurodisability?
-
What are the views, experiences and perceptions of children and young people, their families, their clinicians and others involved in their care of delivering and receiving such interventions?
Methods
This systematic review is reported in accordance with the PRISMA statement. 21 The protocol was developed in consultation with the Family Faculty group and the Professional Advisory Group. The protocol is reported in accordance with PRISMA-P reporting guidelines27 and is registered on the International Database of Prospectively Registered Systematic Reviews in Health and Social Care (CRD42018100572; www.crd.york.ac.uk/prospero/display_record.php?RecordID=100572; accessed 19 October 2021). We also produced a plain language protocol summary, which is available online (http://sites.exeter.ac.uk/iconstudy/files/2019/05/Systematic-Review-plain-language-protocol-summary.pdf; accessed 19 October 2021).
End-user involvement
In addition to consulting on our protocol, we discussed our search strategy, scoping searches and early findings with our Family Faculty group of parents who had lived experience of their children having continence issues and with the Professional Advisory Group. All discussions involved communicating progress throughout the phases of the systematic review, asking for feedback on specific aspects (e.g. terminology and interventions identified) and discussing views on findings as they emerged, such as what was surprising, what did people expect to see and how the results matched expectations.
Search strategy
The following databases were searched between 24 January 2019 and 1 February 2019: MEDLINE, EMBASE, PsycInfo®, Health Management Information Consortium, Social Policy & Practice (all via OvidSP), the Cochrane Database of Systematic Reviews and CENTRAL (via Cochrane Library), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (via EBSCOhost), British Nursing Index and ASSIA (Applied Social Sciences Index and Abstracts) (via ProQuest), Social Sciences Citation Index (via Web of Science) and ProQuest Dissertations & Theses Global. The search strategy was developed and run by an information specialist (MR), with input from our Professional Advisory Group and Family Faculty group, who reviewed and commented on the search plans. Our strategy combined search terms for continence with terms for children and terms for quantitative and qualitative study types. The search strategy as designed for MEDLINE is shown in Box 1 and the adapted strategies for the other databases are available in Appendix 1, Tables 17–21. All database searches were updated on 21 and 22 April 2020.
-
Urinary Incontinence/
-
Fecal Incontinence/
-
toilet training/
-
continence.ti.
-
incontinen*.ti.
-
exp Enuresis/
-
enure*.ti.
-
encopresis.ti.
-
toilet*.ti.
-
wetting.ti.
-
bedwetting.ti.
-
dryness.ti.
-
potty.ti,ab.
-
(dysfunction* adj voiding).ti,ab.
-
or/1-14
-
child/
-
exp Nervous System Diseases/
Annotation: includes neural tubes defects, spina bifida, etc.
-
exp Autistic Disorder/
-
exp Neurologic Manifestations/
-
exp cerebral palsy/
-
exp autism/
-
or/17-21
-
16 and 22
-
(child or childs or children or childrens or childhood).ti,ab.
-
adolescen*.ti,ab.
-
teen*.ti,ab.
-
young people.ti,ab.
-
preschool*.ti,ab.
-
toddler*.ti,ab.
-
23 or 24 or 25 or 26 or 27 or 28 or 29
-
exp clinical trial/
-
randomly.ti,ab.
-
trial.ti,ab.
-
control group.ti,ab.
-
(group* adj5 compared).ab.
-
(randomised or randomized).ti,ab.
-
systematic*.ti,ab.
-
(pubmed or medline).ab.
-
(review adj3 effectiveness).ti,ab.
-
(experiment or experimental).ti,ab.
-
(Quasi experiment* or quasi-experiment* or quasiexperiment*).ti,ab.
-
comparative study.ti,ab.
-
evaluation study.ti,ab.
-
(cross section* adj10 study).ti,ab.
-
crossover.ti,ab.
-
longitudinal study.ti,ab.
-
program* evaluation.ti,ab.
-
(control* adj5 compar*).ti,ab.
-
multicentre study.ti,ab.
-
observational study.ti,ab.
-
prospective.ti,ab.
-
retrospective.ti,ab.
-
cohort study.ti,ab.
-
qualitative research/
-
qualitative*.ti,ab.
-
interview*.ti,ab.
-
Economics/
-
exp “Costs and Cost Analysis”/
-
exp Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
cost effective*.ti,ab.
-
economic evaluation.ti,ab.
-
(cost adj2 evaluat*).ti,ab.
-
or/31-64
-
15 and 30 and 65.
Related systematic reviews were examined for other relevant studies through backwards citation searching, and forwards citation chasing was carried out via PubMed Central and Scopus using key papers. Unpublished studies were sought via conference proceedings databases (Conference Proceeding Citation Index – Science and Conference Proceeding Citation Index – Social Science & Humanities) and clinical trials websites (ClinicalTrials.gov and International Clinical Trials Registry Platform). OpenGrey and The British Library’s Explore catalogue were also searched.
Eligibility criteria
We sought to include any quantitative or qualitative study meeting the criteria below.
Population
The population was children and young people with non-progressive neurodisability aged up to 25 years, consistent with the Department of Health and Social Care Special Educational Needs and Disabilities code of practice and the Children and Families Act 2014. 18 We defined neurodisability as follows:
Neurodisability describes a group of congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations. A specific diagnosis may not be identified. Conditions may vary over time, occur alone or in combination, and include a broad range of severity and complexity. The impact may include difficulties with movement, cognition, hearing and vision, communication, emotion, and behaviour.
Interventions
Assessments including those to identify any underlying pathology and readiness for toilet training; and interventions to improve continence, including structured training programmes, products and assistive technology, medicines and/or surgery. Definitions around these categorisations are described fully in Chapter 2, Conceptual and theoretical frameworks.
Outcomes
Quantitative
Any outcome that could inform the effectiveness, cost-effectiveness or implementation of interventions to improve continence.
Qualitative
Views and experiences of families and health professionals; factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions.
Setting
Any setting.
We had intended that only studies from Organisation for Economic Co-operation and Development (OECD) countries would be included (listed in Appendices 2 and 3), but we also identified four eligible studies from the Islamic Republic of Iran that met all of the other inclusion criteria, so these were included. Particular consideration was given to the degree of transferability of findings from non-UK settings to the NHS context.
Study design
Any quantitative comparative study design, and any recognised method of qualitative data collection and analysis, including interviews, focus groups and observational techniques. This included stand-alone qualitative research, or evidence reported as part of a mixed-methods intervention evaluation. We also included process and outcome evaluations.
Study selection
At the title and abstract screening stage, we took an inclusive approach to evidence, meaning that if there was any doubt or ambiguity around whether a study was to be included or excluded, it should be included. Screening notes were created for the title and abstract screening process, which were piloted on two studies and refined following piloting. Title and abstract screening notes are available in Appendix 2. All definitions of key terms (The Children and Families Act 2014. Part 3: Children and young people with special educational needs and disabilities,17 list of OECD countries28) were included in the appendices of the screening notes (see Appendices 2 and 3). Titles and abstracts of references retrieved by the search were each screened independently by two reviewers (HH, MR, RW and JTC) using the prespecified inclusion and exclusion criteria. Screening decisions were recorded electronically in EndNote software [version X8; Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and the master database was managed by our information specialist (MR). We shared the abstract and title screening results with the Family Faculty group and with the clinical specialists in the Professional Advisory Group, discussed the relevance of the conditions and terminology identified and asked for advice on the specific clinical details found in studies.
The full texts of potentially relevant studies were obtained and independently assessed for inclusion by two reviewers (HH, MR and RW). Screening notes were refined for the full-text phase, with coding guides included for screening in EndNote software. This process was again piloted with two papers, and the screening notes were refined accordingly (see Appendix 3, Tables 22 and 23). Screening decisions were recorded electronically in EndNote and the master database, managed by our information specialist (MR). Discrepancies around data extraction were resolved by discussion; reference to a third reviewer was planned but not necessary.
Data extraction
The data extraction form was created in consultation with our project team and clinical specialists, and in line with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 29 As we were interested in interventions and describing them in as much useful detail as possible, we used the Template for Intervention Description and Replication (TIDieR) checklist30 to guide the creation of our data extraction forms. TIDieR is a guide and reporting checklist developed to improve the completeness of reporting, and, ultimately, the replicability, of interventions. A blank quantitative data extraction form is available on reasonable request.
Quantitative data were extracted by one reviewer (HH or RW) and checked by another reviewer (HH or RW). As before, reference to a third reviewer was planned but not necessary. For qualitative studies, we extracted details of the study aim, the sample, and the type and nature of the intervention/programme. We also collected data on the theoretical approach, the methods used to collect the data and the analytic processes. This process was conducted by one reviewer (HH), with a second reviewer (RW) spot-checking extractions independently and both reviewers discussing the qualitative data.
Quality appraisal
We used the Effective Public Health Practice Project (EPHPP)31 quality assessment for quantitative data studies, and quality assessment was carried out alongside data extraction by one of two reviewers (HH or RW), with assessments checked by the other. The EPHPP tool enables the assessment of selection bias, study design, blinding, level of confounding, data collection methods and data analysis, providing an overall rating of weak, moderate or strong quality. For qualitative studies, we used the Wallace criteria to determine the quality of reporting and the appropriateness of the method used. 32 The assessed criteria included theoretical perspective, appropriateness of the question, study design, context, sampling, data collection, analysis, reflexivity, appropriateness, generalisability and ethics.
Quantitative synthesis
We used the methods of quantitative synthesis outlined in the Cochrane Handbook. 33 The included quantitative studies reported a range of outcomes, which we grouped into broad categories. Intervention descriptions were extracted as reported by study authors, and grouped into broad categories identified by the principal investigator (CM) within a conceptual framework developed at the beginning of the project and refined through discussion with the co-investigator team and the Professional Advisory Group. These categories were educational interventions, equipment to improve communication or movement abilities, toilet adaptations, clothing adaptations, medically assisted techniques, behavioural training, surgical approaches and medications.
Outcomes were recorded in an overarching Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet, and terminology of outcomes, interventions and medical conditions was recorded verbatim from study author descriptions. We then consulted with our clinical experts to identify any terms obsolete in current practice, conditions outside our remit and other anomalous terminology, and we refined the Microsoft Excel spreadsheet accordingly. Outcomes relating directly to effectiveness of interventions were then grouped broadly into continence, incontinence and equipment related (e.g. diaper/nappy, anal plug use).
This research uses current accepted standardised terminology as described by the International Children’s Continence Society22 for bladder and bowel dysfunction, McComb23 for spinal cord pathology and Morris et al. 3 for neurodisability. As all terms were drawn directly from the included study literature, some of which dated back to 1965, there was a need to rationalise and redefine terms for the purposes of this paper. We therefore carried out a series of consultations with topic experts on our project team to check terminology and refine and condense these categories where it made sense to do so. This resulted in the development of two overarching groupings: one describing non-spinal-cord-related conditions and the other describing conditions with underlying spinal cord pathology. Non-spinal-cord-related conditions included autism, ADHD, developmental disability, learning disability (reworded from mental disability) and ‘mixed conditions’ (including Down syndrome, epilepsy, ‘primary genetic’, infant autism and postnatal infection). Spinal cord pathology conditions include all forms of myelodysplasia (defective development of any part of the spinal cord), including myelomeningocele, spina bifida and tethered cord syndrome, and associated neurogenic dysfunction (including neuropathic bladder and/or bowel, also referred to as neurogenic bladder and/or bowel, and neurogenic detrusor overactivity).
There were insufficient homogeneous data across studies to allow a formal meta-analysis for any outcome. As part of the synthesis process, we extracted data into tables to allow greater comparison and contrast across different features of interest, including age range of participants, type of continence (faecal/urinary/both), medical condition (Table 1) and study type (RCT, before-and-after/cohort, case–control, case reports, crossover). From a single overarching summary table, we created individual topic tables and summarised the effectiveness of results narratively, grouping outcome measures by their broad intervention category (e.g. medication), by medical condition (e.g. autism) and by study design.
| Overarching group | Summary group (where relevant) | Subgroup (where relevant) |
|---|---|---|
| Non-spinal cord related | Autism | – |
| ADHD | – | |
| Developmental disability | Angelman syndrome | |
| Learning disability | – | |
| Mixed conditions | – | |
| Spinal cord pathology | Myelodysplasia | – |
| Spina bifida | Myelomeningocele, and spina bifida with anorectal malformations, tethered cord syndrome | |
| Neurogenic dysfunction | Neuropathic bladder/bowel; neurogenic bladder/bowel; neurogenic detrusor overactivity |
Qualitative synthesis
All qualitative outcome data were extracted in the form of quotations, themes and concepts identified by study authors, and themes and concepts identified by a reviewer (HH) and reviewed by a second reviewer (RW). The data were read and re-read and the findings were organised into subthemes. The subthemes were then grouped into main themes and reported according to the condition category.
Overarching synthesis
We created a simple overarching synthesis to link the quantitative and qualitative evidence. We used the interweave method of data synthesis,34 whereby data are incorporated across multiple sources of evidence through a process of ‘interweaving’ the findings of individual studies and combining categories to produce insight and promote understanding of the evidence base in its entirety. We used the intersubjective questioning approach to interrogate the evidence and draw links between and across both the qualitative and the quantitative evidence identified in this systematic review.
Results
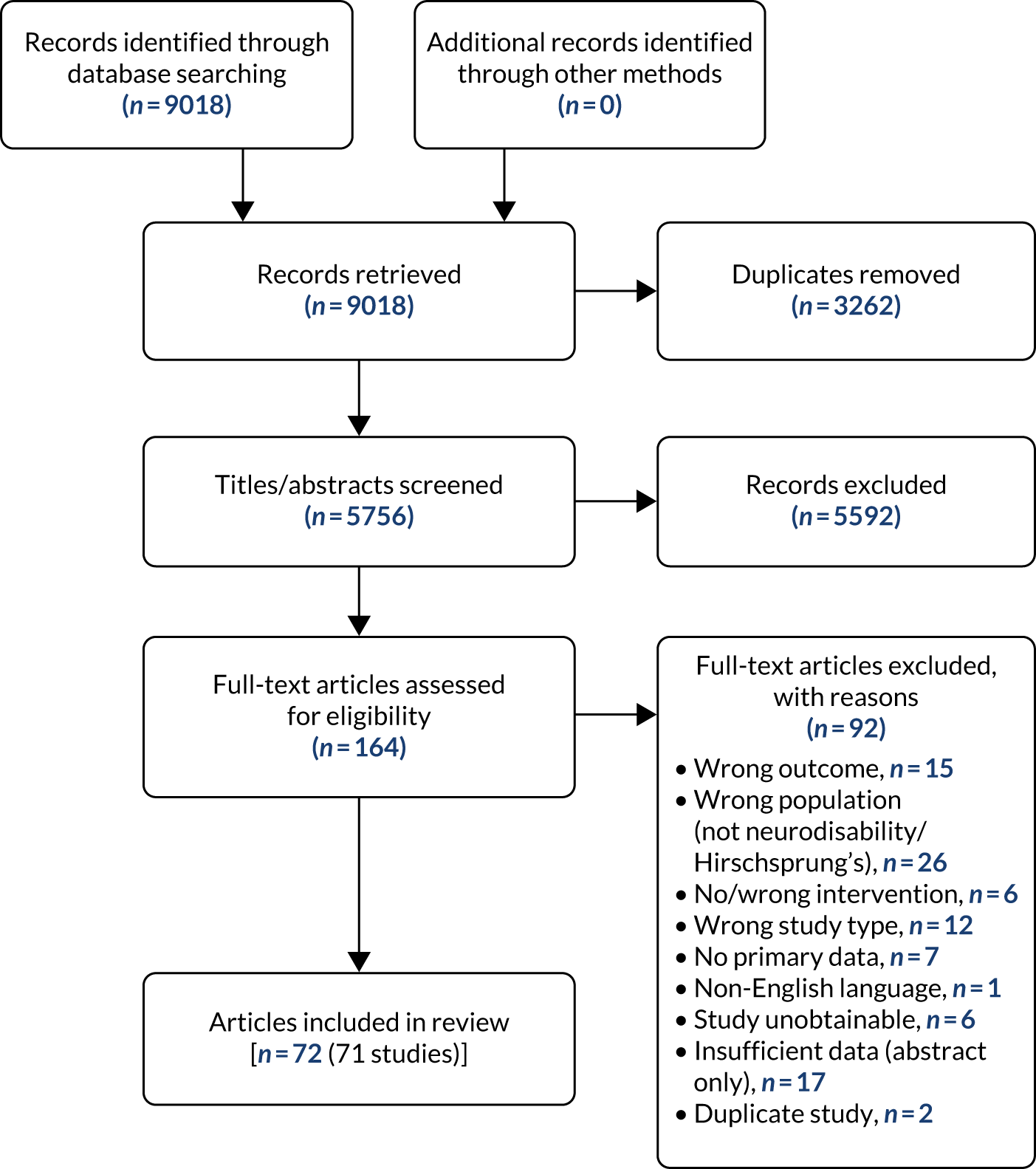
We identified 9018 records, which resulted in 5756 individual references following the removal of duplicate records. At the title and abstract screening stage, we excluded 5592 articles, and we retrieved the full texts of 164 for scrutiny at the full-text screening phase.
Following full-text screening, we included 71 studies from 72 articles in the final analysis (see Tables 2 and 3 and Appendix 4). Of these, 68 articles contained quantitative outcome data and three articles contained exclusively qualitative outcome data. The reasons for exclusion at the full-text stage are shown in the PRISMA flow diagram (Figure 2).
FIGURE 2.
The PRISMA flow diagram showing the study screening and selection process.
Summary characteristics
Summary study characteristics are shown in Tables 2 and 3, laid out by intervention type and then by study type. Further details of study results are provided in Appendix 5 (see Tables 24–28).
| First author, year (country of study), study design | Intervention group | Setting | N; age | Intervention | Duration of intervention; follow-up | EPHPP quality appraisal | Urinary/faecal/both; outcomes reported |
|---|---|---|---|---|---|---|---|
| ADHD | |||||||
| Yang,35 2015 [Taiwan (Province of China)], B&A | Medication | Paediatric clinic | 68; 5–12 years | Drug therapy – desmopressin | 4 weeks; 1 month | Moderate | Both (enuresis and lower urinary tract symptoms):
|
| Chertin,36 2007 (Israel), case–control | Medication | Paediatric clinic | 54; average 8 years | Combination therapy with desmopressin and oxybutynin vs. the tricyclic antidepressant imipramine | 1 year; NR | Weak | Urinary (enuresis):
|
| Gor,37 2012 (USA), cohort | Medication | Paediatric clinic | 671; mean 8.6 years | Desmopressin or anticholinergic treatment | 9 months; NR | Weak | Urinary (enuresis and daytime voiding symptoms):
|
| Autism | |||||||
| Lomas Mevers,38 2020 (USA), RCT | Medically assisted | Paediatric clinic | 20; 5–16 years | Liquid glycerin suppositories and reinforcement | 6 weeks; 4 weeks | Weak | Faecal:
|
| Mruzek,39 2019 (USA), RCT | Medically assisted | Paediatric clinic | 32; 3–6 years | Wireless moisture pager intervention | 12 weeks; 3 months | Moderate | Urinary:
|
| Mruzek,39 2019 (USA), RCT | Medically assisted | Paediatric clinic | 33; 36–72 months | Wireless moisture pager intervention | 12 weeks; 3 months | Moderate | Urinary:
|
| Ardic,40 2014 (Turkey), B&A | Behaviour | Special education unit | 3; 3–4 years | Azrin and Foxx adaptation (modified intensive toilet training method) | 6 hours; NR | Weak | Urinary:
|
| Keen,41 2007 (Australia), case–control | Behaviour | Kindergarten, special education unit, preschool | 5; 4 years 5 months to 6 years 9 months | Operant conditioning plus video | 7 days; 165 days | Weak | Urinary:
|
| Cicero,42 2002 (USA), case study | Behaviour | School | 3; 4–6 years | Azrin and Foxx adaptation | 22 days; 6 months and 1 year | Weak | Urinary:
|
| Developmental/learning disability | |||||||
| Edgar,43 1975 (USA), RCT | Behaviour | Special education unit | 20; 4–12 years | Behavioural training and device (electric belt, toilet with buzzer plus relaxation training) | 2 weeks; NR | Moderate | Urinary:
|
| Hundziak,44 1965 (USA), RCT | Behaviour | Special education unit | 29; 7–14 years | Operant conditioning vs. operant conditioning and conventional training vs. no treatment | 27 days; NR | Moderate | Both:
|
| Sadler,45 1977 (USA), RCT | Behaviour | Special education unit | 14; 7–12 years | Azrin and Foxx method | 4 months; NR | Weak | Both:
|
| Van Laecke,46 2009 (Belgium), B&A | Education | Special education unit | 111; 4–15 years | Adequate fluid intake | 6 weeks; NR | Weak | Urinary:
|
| Barmann,47 1981 (USA), B&A | Behaviour | Home, residential care | 3; 4–8 years | Azrin and Foxx adaptation | 10 days; 2 months | Weak | Urinary:
|
| Rinald,48 2012 (Canada), B&A | Behaviour | Home, community centre, university | 6; 3 years 3 months to 5 years 11 months | Azrin and Foxx adaptation | 4–12 days; NR | Moderate | Both:
|
| Lomas Mevers,49 2018 (USA), case study | Behaviour | Paediatric clinic | 44; 2–20 years | Behavioural intervention | 2 weeks; 6–24 months | Weak | Urinary:
|
| Angelman syndrome | |||||||
| Didden,50 2001 (the Netherlands), cohort | Behaviour | Residential facility; home | 6; 6–19 years | Azrin and Foxx adaptation | 2 days; 2.5 years | Weak | Urinary:
|
| Mixed | |||||||
| Valentine,51 1968 (UK), RCT | Medication | Hospital, at home | 16; NR | Imipramine | 3 weeks; 6 weeks | Weak | Urinary:
|
| First author, year (country of study), study design | Intervention group | Setting | N; age | Intervention | Duration of intervention; follow-up | EPHPP quality appraisal | Urinary/faecal/both; outcomes reported |
|---|---|---|---|---|---|---|---|
| Spina bifida | |||||||
| Kajbafzadeh,52 2009 (Islamic Republic of Iran), RCT | Medically assisted | Paediatric clinic | 30; 5.6 years | Pelvic floor interferential electrostimulation | 6 weeks; 18 months | Moderate | Both:
|
| Kajbafzadeh,53 2014 (Islamic Republic of Iran), RCT | Medically assisted | Paediatric clinic | 30; 6.7 years (range 3–13 years) | Transcutaneous functional electrical stimulation | 1 week; 6 months | Moderate | Urinary:
|
| Loening-Baucke,55 1988 (USA), RCT | Medically assisted | Paediatric clinic | 28; 7–21 years | Biofeedback training | Approximately 2 weeks; 12 months | Weak | Faecal:
|
| Marshall,56 2001 (UK), RCT | Medically assisted | Paediatric clinic | 77; 4–18 years | Transcutaneous electrical field stimulation – Duet Continence Stimulator | 6 weeks-5 months; NR | Moderate | Both:
|
| Steinbok,57 2016 (Canada), RCT | Surgery | Paediatric surgery | 21; 5–18 years | Filum section + medical therapy | 1 day; 3,6 and 12 months | Moderate | Urinary:
|
| Van Winckel,58 2006 (Belgium), RCT | Medically assisted | Paediatric clinic | 7; 4–13 years | Anal plug (Conveen®, Coloplast, Humlebaek, Denmark) | 3 weeks; NR | Weak | Faecal:
|
| Choi,59 2013 (Republic of Korea), B&A | Behaviour | Paediatric clinic | 53; 3–13.8 years | Stepwise bowel management programme | 3 months; NR | Moderate | Faecal:
|
| Dietrich,60 1982 (USA), B&A | Behaviour | Paediatric clinic | 55; 5.6–18.9 years | Bowel training | 1 week; 1.2 years | Weak | Faecal:
|
| Aksnes,61 2002 (Norway), B&A | Surgery | Paediatric surgery | 20; 6.3–17 years | MACE procedure | 1 day; 16 months | Weak | Faecal:
|
| Ausili,62 2010 (Italy), B&A | Medically assisted | Paediatric clinic | 62; 6–17 years | TAI; The Peristeen® Anal Irrigation System (Coloplast A/S Kokkedal, Denmark) | 3 months; NR | Weak | Faecal:
|
| Ausili,63 2018 (Italy), B&A | Medically assisted | Paediatric clinic, home | 74; 6–17 years | TAI; Peristeen | Up to 24 months; unclear | Moderate | Faecal:
|
| Choi,64 2015 (Republic of Korea), B&A | Medically assisted | Paediatric clinic | 47; 3–18 years | Irrigation cone-based transanal irrigation system (Colotip, Coloplast) or catheter-based TAI system (Peristeen anal irrigation system) | 3 months; 33 months | Weak | Faecal:
|
| Hascoet,65 2018 (France), B&A | Surgery | Paediatric clinic | 53; 8.5 years | IDBTX-A 6–8 weeks | 6–8 weeks (varied by site); 3.7 years | Weak | Urinary:
|
| Kajbafzadeh,66 2006 (Islamic Republic of Iran), B&A | Surgery | Paediatric clinic and home | 26; 6.9 ± 2.6 years | IDBTX-A | 12 hours; 4 months | Moderate | Urinary:
|
| Killam,67 1985 (USA), B&A | Medically assisted | Paediatric clinic | 8; 7–19 years | Urodynamic biofeedback treatment | 9–51 weeks; NR | Weak | Both:
|
| Ladi-Seyedian,68 2018 (Islamic Republic of Iran), B&A | Surgery | Paediatric clinic | 24; 9 years | Intravesical electromotive BoNTA ‘Dysport’ | 1 day; 1 year | Moderate | Urinary:
|
| Lima,69 2017 (Brazil), B&A | Medically assisted | Paediatric clinic | 25; 5–18 years | ISRD | 6 months; NR | Moderate |
Urinary: QoL Daily number of diapers used per day |
| Mattsson,70 2006 (Sweden), B&A | Medically assisted | Paediatric clinic | 40; 10 months–11 years | Transrectal irrigation | 1.5 years; 8 years | Moderate | Faecal:
|
| Shoshan,71 2008 (Israel), B&A | Medically assisted | Paediatric clinic | 20; 12 years | Anal plug | 4 weeks; NR | Moderate | Faecal:
|
| Horowitz,72 1997 (USA), B&A | Medication | Paediatric clinic, home | 18; 10.5 years (range 7–16 years) | Desmopressin | 6 weeks; NR | Weak | Urinary:
|
| Tarcan,73 2014 (Turkey), cohort | Surgery | Paediatric clinic | 31; 7.95 years | Intradetrusor injections of onabotulinum toxin-A | One injection; 12–42 weeks | Moderate | Urinary:
|
| Palmer,74 1997 (USA), cohort | Medically assisted | Paediatric clinic | 55; 2–14 years | Transrectal bowel stimulation | 9 weeks; NR | Weak | Faecal:
|
| Radojicic,75 2019 (Serbia), case–control | Behaviour | Paediatric clinic | 70; 4–16 years | Bowel management programme | 12 months | Weak | Both:
|
| Snodgrass,76 2009 (USA), case–control | Surgery | Paediatric surgery | 41; 3–14 years | Bladder neck sling with and without enterocytoplasty | 1 day; 1 year | Moderate | Urinary:
|
| Han,77 2004 (Republic of Korea), case study | Medically assisted | Paediatric clinic | 24; 3.9–13.2 years | Intravesical electrical stimulation | 4 weeks; 3–6 months | Weak | Faecal:
|
| King,78 2017 (Australia), case study | Medically assisted | Paediatric clinic | 20; 14.5 years | Transanal irrigation (Peristeen) | Unclear; 4.1 years | Weak | Faecal:
|
| Petersen,79 1987 (Denmark), case study | Medically assisted | Paediatric clinic | 10; 8–12 years | Transurethral intravesical electrical stimulation | 3–4 weeks; NR | Weak | Urinary:
|
| Vande Velde,80 2007 (Belgium), case study | Medically assisted | Paediatric clinic | 80; 5–18 years | Stepwise bowel management programme | 1 week; NR | Moderate | Faecal:
|
| Bar-Yosef,81 2011 (USA), case study | Surgery | Paediatric surgery | 21; 6–22 years | MACE | 1 day; 4.7 years | Weak | Both:
|
| Ibrahim,82 2017 (Nigeria), case study | Surgery | Paediatric surgery | 23; 3.5–17.8 years | ACE | 1 day; 2.6 years | Weak | Faecal:
|
| Matsuno,83 2010 (Japan), case study | Surgery | Paediatric surgery | 25; 4–23 years | Retrograde colonic enema (RCE); Malone anterograde continence enema | 1 day; 23–31 months | Weak | Faecal:
|
| Snodgrass,84 2016 (USA), case study | Surgery | Paediatric surgery | 37; 3–18 years | Bladder neck sling | 1 day; 60 months | Weak | Urinary:
|
| Van Savage,85 2000 (USA), case study | Surgery | Paediatric surgery | 16; 12 (4–21 years) | ACE | I day; NR | Weak | Faecal:
|
| Wehby,86 2004 (USA), case study | Surgery | Paediatric surgery | 60; 3–18 years | Section of the filum terminale | 1 day; 13.9 months | Weak | Both:
|
| Schletker,87 2019 (USA), case study | Behaviour | Paediatric clinic | 22; 2–24 years | Bowel management programme | 1 week; no follow-up | Weak | Faecal:
|
| Neurogenic dysfunction | |||||||
| Haddad,88 2010 (France), RCT | Medically assisted | Paediatric clinic | 33; mean age 12.22 years | Sacral neuromodulation | 6 months; 13 months | Moderate | Both:
|
| Borzyskowski,89 1982 (UK), controlled trial | Medically assisted | Hospital, possibly home | 43; 7 years | Clean intermittent catheterisation plus drug therapy | 3 months; NR | Weak | Urinary:
|
| Corbett,90 2014 (UK), B&A | Medically assisted | Paediatric clinic | 24; 4–16 years | TAI (Peristeen) | 1 week; 1 year | Weak | Faecal:
|
| Schulte-Baukloh,91 2006 (Germany), B&A | Medication | Paediatric clinic | 20; 8.9 years | Propiverine | 12 hours; 3–6 months | Weak | Urinary:
|
| Schulte-Baukloh,92 2012 (a longer-term follow-up of Schulte-Baukloh 200691) (Germany), B&A | Medication | Paediatric clinic | 17; 13 years | Propiverine | 12 hours; 36 months | Weak | Urinary:
|
| Guys,93 1999 (France), cohort | Surgery | Paediatric clinic | 33; 13 (7–17 years) | Endoscopic injection of PDMS | 6–18 months; NR | Weak | Urinary:
|
| Guys,94 2006 (France), cohort | Surgery | Paediatric clinic | 49; 14 years (SD 4.8 years) | Endoscopic injection of PDMS | 6–18 months; NR | Weak | Urinary:
|
| Silveri,95 1998 (Italy), cohort | Surgery | Paediatric clinic | 23; 6–17 years | Collagen injection | 1 hour; 19 months | Weak | Urinary:
|
| González,96 2002 (USA, Canada and Austria), case study | Surgery | Paediatric surgery | 27; 4–23 years | Artificial urinary sphincter with seromuscular colocystoplasty | 1 day; 1.1 years | Weak | Urinary:
|
| Do Ngoc Thanh,97 2009 (France), case study | Surgery | Paediatric clinic | 7; 6.5–15.5 years | Botulinum type A injections | 24 hours; 12 months | Moderate | Urinary:
|
| Myelodysplasia | |||||||
| Boone,98 1992 (USA), RCT | Medically assisted | Paediatric clinic; at home | 36; 6–12 years | Transurethral intravesical electrotherapy | 18 weeks; NR | Weak | Urinary:
|
| Åmark,99 1992 (Sweden), RCT | Medication | Paediatric clinic | 10; 6–18 years | The alpha-adrenoceptor agonist phenylpropanolamine | 1 week; 13 weeks | Weak | Urinary:
|
| Mixed | |||||||
| Åmark,100 1998 (Sweden), B&A | Medication | Paediatric clinic | 39; 0.5–18 years | Intravesical oxybutynin | 1 day; 6 months | Weak | Urinary:
|
| Naglo,101 1979 (Sweden), B&A | Medication | Paediatric clinic | 13; 6–18 years | Drug therapy plus continence training | 9 weeks; NR | Weak | Urinary:
|
| Faure,102 2017 (France), case study | Surgery | Paediatric surgery | 59; 7.6 years (1.9–17.5 years) | Young–Dees bladder neck reconstruction, with bladder neck injection as a follow-up | 1 day; 16 years | Weak | Urinary:
|
| Jawaheer,103 1999 (UK), case study | Surgery | Paediatric surgery | 18; 3–14 years | Pippi Salle bladder neck repair | 1 day; 2 years | Weak | Urinary:
|
Included quantitative studies were conducted in the USA (n = 23), France (n = 6), the UK (n = 5), Sweden (n = 4), the Islamic Republic of Iran (n = 4), Republic of Korea (n = 3), Italy (n = 3), Canada (n = 3), Belgium (n = 3), Australia (n = 2), Israel (n = 2) and Turkey (n = 2), and one was conducted in each of the following countries: Austria (combined with Canada and the USA), Brazil, Denmark, Germany, Japan, the Netherlands, Nigeria, Norway, Serbia and Taiwan (Province of China). The years studies were conducted ranged from 1977 to 2020, although this information was not reported in 32 studies. Sample population sizes ranged from 3 to 150 participants.
Of the three qualitative studies, two took place in the UK and one took place in the USA. Study years were 2004, 2009 and 2014. Sample populations included 7, 18 and 40 participants, with two studies focused on the experiences of parents and caregivers and one paper focused on the experiences of children and young people.
Intervention categories
We found no studies that met our criteria under the categories of equipment to improve communication or movement abilities, toilet adaptations or clothing adaptations. We identified 24 studies that reported on interventions relating to medically assisted techniques, such as self-catheterisation. Interventions related to behavioural training (e.g. Azrin and Foxx-style104 training approaches) were identified in 14 studies. Interventions using surgical approaches such as MACE procedures were reported in 20 studies, and nine studies (reported in 10 papers) reported interventions relating to medications such as oxybutynin. One study reported an educational intervention. Details of complex interventions can be found in Appendix 6 and further details of the recorded adaptations made to the Azrin and Foxx intervention can be obtained on request.
Faecal and urinary continence
Thirteen studies focused on both faecal and urinary incontinence, with the remaining studies focused on either urinary or faecal incontinence. Faecal and urinary intervention focus across the included studies is shown in Tables 4 and 5.
| Intervention category | Urinary (n) | Faecal (n) | Both (n) |
|---|---|---|---|
| Educational | 1 | – | – |
| Equipment | – | – | – |
| Toilet adaptations | – | – | – |
| Clothing adaptations | – | – | – |
| Behavioural training | 7 | – | 3 |
| Medically assisted techniques | 2 | 1 | – |
| Medications | 3 | – | 1 |
| Surgical approaches | – | – | – |
| Intervention category | Urinary (n) | Faecal (n) | Both (n) |
|---|---|---|---|
| Educational | – | – | – |
| Equipment | – | – | – |
| Toilet adaptations | – | – | – |
| Clothing adaptations | – | – | – |
| Behavioural training | – | 3 | 1 |
| Medically assisted techniques | 5 | 12 | 4 |
| Medications | 5 | – | – |
| Surgical approaches | 14 | 4 | 2 |
Neurological conditions
The conditions of children and young people in the studies identified by study authors included ADHD, autism, myelodysplasia, ‘developmental disability’, ‘mental disability’, spina bifida (including myelomeningocele, and spina bifida with anorectal malformations), Angelman syndrome, neurogenic dysfunction, neurogenic detrusor overactivity, neuropathic bowel/bladder, occult tethered cord syndrome and mixed conditions that appeared to fit neurodisability definitions.
Table 6 shows the number of studies reporting results for each intervention category in each condition.
| Intervention category | Condition (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | Spinal cord pathology | ||||||||
| ADHD | Autism | Angelman syndrome | Developmental/learning disability | Mixed | Spina bifida | Neurogenic dysfunction | Myelodysplasia | Mixed | |
| Educational | – | – | – | 1 | – | – | – | ||
| Equipment | – | – | – | – | – | – | – | ||
| Toilet adaptations | – | – | – | – | – | – | – | ||
| Clothing adaptations | – | – | – | – | – | – | – | ||
| Behavioural training | – | 3 | 1 | 6 | 4 | – | |||
| Medically assisted techniques | – | 3 | – | – | 17 | 3 | 1 | – | |
| Medications | 3 | – | – | – | 1 | 1 | 1 | 1 | 2 |
| Surgical approaches | – | – | – | – | – | 13 | 5 | – | 2 |
The majority of interventions identified were in the medically assisted techniques category, with the majority of populations of interest formed of children and young people with spina bifida. The next largest group of studies identified were in the surgical approaches intervention category, again with the majority of participants in the populations of children and young people with spina bifida. We identified no studies of interventions that fell into the categories of toilet adaptations, clothing adaptations or equipment.
Age ranges of included study populations
Educational interventions (one study) covered the ages of 4–15 years; behavioural training interventions (14 studies) covered the ages of 2–24 years; medically assisted interventions (24 studies) covered the ages of 36 months to 19 years; medication interventions (nine studies) covered the ages of 6 months to 18 years; and surgical interventions (20 studies) covered the ages of 3–23 years.
Study designs
Included studies used a range of study designs. These included 16 randomised controlled trials (RCTs), one controlled trial, 30 before-and-after or cohort studies, 17 case studies, four case–control studies and three qualitative studies. Where reported, the majority of before-and-after and cohort studies recruited participants prospectively. This information was not reported widely in other studies. The range of study designs used across the intervention categories is shown in Table 7.
| Intervention category | Study design (n) | ||||
|---|---|---|---|---|---|
| RCT | CT | B&A/cohort | Case–control | Case study | |
| Educational | – | – | 1 | – | – |
| Equipment | – | – | – | – | – |
| Toilet adaptations | – | – | – | – | – |
| Clothing adaptations | – | – | – | – | – |
| Behavioural training | 3 | – | 6 | 2 | 3 |
| Medically assisted techniques | 10 | 1 | 9 | – | 4 |
| Medications | 2 | – | 6 | 1 | |
| Surgical approaches | 1 | – | 8 | 1 | 10 |
Study quality
None of the included studies reporting quantitative evidence was assessed as high quality using the EPHPP quality assessment tool. 31 Quality of the quantitative studies was assessed as ‘moderate’ in 22 studies and ‘weak’ in 46 studies (Tables 8 and 9).
| First author (year); study type | Intervention group | Selection bias | Study design | Confounders | Blinding | Data collection method | Withdrawals and dropouts | Analysis | Global rating |
|---|---|---|---|---|---|---|---|---|---|
| ADHD | |||||||||
| Yang, 2015;35 B&A | Medications | Moderate | Weak | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Chertin, 2007;36 case–control | Medications | Moderate | Weak | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Gor,37 2012; cohort | Medications | Moderate | Weak | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Autism | |||||||||
| Lomas Mevers,38 2020; RCT | Medically assisted | Moderate | Moderate | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Mruzek,39 2019; RCT | Medically assisted | Moderate | Moderate | Moderate | Weak | Moderate | Moderate | Strong | Moderate |
| Mruzek,39 2019; RCT | Medically assisted | Moderate | Strong | Strong | Weak | Strong | Moderate | Strong | Moderate |
| Ardic,40 2014; B&A | Behavioural | Weak | Weak | Weak | Weak | Strong | Weak | Weak | Weak |
| Keen,41 2007; case–control | Behavioural | Weak | Weak | Weak | Weak | Moderate | Strong | Weak | Weak |
| Cicero,42 2002; case report | Behavioural | Weak | Weak | Weak | Weak | Strong | Strong | Weak | Weak |
| Developmental/learning disability | |||||||||
| Edgar,43 1975; RCT | Behavioural | Moderate | Moderate | Moderate | Weak | Strong | Strong | strong | Moderate |
| Hundziak,44 1965; RCT | Behavioural | Moderate | Moderate | Weak | Strong | Strong | Moderate | Moderate | Moderate |
| Sadler,45 1977; RCT | Behavioural | unclear | Moderate | Strong | Weak | Strong | Unclear | Moderate | Weak |
| Van Laecke,46 2009; B&A | Educational | Weak | Weak | Strong | Weak | Strong | Strong | Moderate | Weak |
| Barmann,47 1981; B&A | Behavioural | Weak | Weak | Weak | Weak | Weak | Strong | Moderate | Weak |
| Rinald,48 2012; B&A | Behavioural | Moderate | Moderate | Moderate | Weak | Strong | Moderate | Moderate | Moderate |
| Lomas Mevers,49 2018; case report | Behavioural | Moderate | Weak | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Angelman syndrome | |||||||||
| Didden50, 2001; cohort | Behavioural | Weak | Weak | Moderate | Weak | Moderate | Strong | Moderate | Weak |
| Mixed | |||||||||
| Valentine,51 1968; prospective RCT | Medications | Weak | Moderate | Weak | Weak | Moderate | Moderate | Weak | Weak |
| First author (year); study type | Intervention group | Selection bias | Study design | Confounders | Blinding | Data collection method | Withdrawals and dropouts | Analysis | Global rating |
|---|---|---|---|---|---|---|---|---|---|
| Spina bifida | |||||||||
| Kajbafzadeh,52 2009; RCT | Medically assisted | Moderate | Strong | Moderate | Moderate | Moderate | Strong | Moderate | Moderate |
| Kajbafzadeh,53 2014; RCT | Medically assisted | Moderate | Strong | Moderate | Strong | Moderate | Strong | Moderate | Moderate |
| Loening-Baucke,55 1988; RCT | Medically assisted | Weak | Weak | Weak | Weak | Moderate | Moderate | Moderate | Weak |
| Marshall,56 2001; RCT | Medically assisted | Moderate | Strong | Moderate | Strong | Moderate | Moderate | Moderate | Moderate |
| Steinbok,57 2016; RCT | Surgery | Moderate | Moderate | Strong | Weak | Strong | Moderate | Moderate | Moderate |
| Van Winckel,58 2006; RCT | Medically assisted | Moderate | Moderate | Weak | Weak | Moderate | Weak | Weak | Weak |
| Choi,59 2013; B&A | Behavioural | Moderate | Moderate | Strong | Weak | Moderate | Moderate | Strong | Moderate |
| Dietrich,60 1982; B&A | Behavioural | Moderate | Weak | Weak | Weak | Strong | Moderate | Moderate | Weak |
| Aksnes,61 2002; B&A | Surgery | Moderate | Weak | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Ausili,62 2010; B&A | Medically assisted | Strong | Moderate | Strong | Weak | Weak | Strong | Moderate | Weak |
| Ausili,63 2018; B&A | Medically assisted | Strong | Moderate | Moderate | Weak | Strong | Moderate | Moderate | Moderate |
| Choi,64 2015; B&A | Medically assisted | Moderate | Moderate | Strong | Weak | Moderate | Weak | Moderate | Weak |
| Hascoet,65 2018; B&A | Surgery | Moderate | weak | Moderate | Weak | Moderate | Strong | Moderate | Weak |
| Kajbafzadeh,66 2006; B&A | Surgery | Moderate | Strong | Strong | Weak | Moderate | Strong | Moderate | Moderate |
| Killam,67 1985; B&A | Medically assisted | Weak | Moderate | Weak | Weak | Moderate | Weak | Moderate | Weak |
| Ladi-Seyedian,68 2018; B&A | Surgery | Strong | Moderate | Moderate | Weak | Strong | Moderate | Strong | Moderate |
| Lima,69 2017; B&A | Medically assisted | Moderate | Moderate | Strong | Weak | Strong | Moderate | Strong | Moderate |
| Mattsson,70 2006; B&A | Medically assisted | Moderate | Moderate | Strong | Weak | Moderate | Strong | Moderate | Moderate |
| Shoshan,71 2008; B&A | Medically assisted | Moderate | strong | Weak | Moderate | Strong | Weak | strong | Moderate |
| Horowitz,72 1997; B&A | Medications | Weak | Weak | Weak | Moderate | Moderate | Strong | Moderate | Weak |
| Tarcan,73 2014; B&A | Surgery | Moderate | Strong | Moderate | Weak | Moderate | Strong | Strong | Moderate |
| Palmer,74 1997; B&A | Medically assisted | Moderate | Moderate | Weak | Weak | Moderate | Strong | Weak | Weak |
| Radojicic,75 2019; case–control | Behavioural | Weak | Moderate | Moderate | Weak | Moderate | Moderate | Strong | Weak |
| Snodgrass,76 2009; case–control | Surgery | Moderate | Moderate | Moderate | Moderate | Weak | Moderate | Moderate | Moderate |
| Schletker,87 2019; case report | Behavioural | Moderate | Weak | strong | Weak | Weak | Weak | Weak | Weak |
| Han,77 2004; case report; | Medically assisted | Moderate | Weak | Weak | Moderate | Strong | Moderate | Moderate | Weak |
| King,78 2017; case report | Medically assisted | Strong | Weak | Moderate | weak | Strong | Moderate | Moderate | Weak |
| Petersen,79 1987; case report | Medically assisted | Weak | Weak | Weak | Weak | Moderate | Weak | Moderate | Weak |
| Vande Velde,80 2007; case report | Medically assisted | Strong | Weak | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Bar-Yosef,81 2011; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Moderate | Weak | Weak |
| Ibrahim,82 2017; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Weak | Moderate | Weak |
| Matsuno,83 2010; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Weak | Moderate | Weak |
| Snodgrass,84 2016; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Moderate | Moderate | Weak |
| Van Savage,85 2000; case report | Surgery | Moderate | Weak | Weak | Weak | Weak | Strong | Weak | Weak |
| Wehby,86 2004; case report | Surgery | Moderate | Weak | Moderate | Weak | Moderate | Moderate | Moderate | Weak |
| Neurogenic dysfunction | |||||||||
| Haddad,88 2010; prospective RCT | Medically assisted | Moderate | Moderate | Strong | Weak | Moderate | Moderate | Strong | Moderate |
| Borzyskowski,89 1982; controlled trial | Medically assisted | Moderate | Moderate | Moderate | Weak | Moderate | Weak | Weak | Weak |
| Corbett,90 2014; B&A | Medically assisted | Moderate | Weak | Strong | Weak | Moderate | Strong | Moderate | Weak |
| Schulte-Baukloh,91,92 2006/2012; B&A | Medications | Weak | Moderate | Moderate | Weak | Moderate | Strong | Moderate | Weak |
| Guys,93 1999; cohort | Surgery | Moderate | Weak | Weak | Weak | Moderate | Strong | Moderate | Weak |
| Guys,94 2006; cohort | Surgery | Moderate | Weak | Moderate | Weak | Moderate | Strong | Strong | Weak |
| Silveri,95 1998; cohort | Surgery | Weak | Weak | Moderate | Weak | Moderate | Weak | Moderate | Weak |
| González,96 2002; case report | Surgery | Moderate | Weak | Weak | Weak | Strong | Weak | Moderate | Weak |
| Do Ngoc Thanh,97 2009; case report | Surgery | Moderate | Weak | Strong | Moderate | Strong | Strong | Moderate | Moderate |
| Myelodysplasia | |||||||||
| Boone,98 1992; RCT | Medically assisted | Weak | Weak | Unclear | Strong | Moderate | Moderate | Moderate | Weak |
| Åmark,99 1992; RCT cross-over | Medication | Weak | Moderate | Moderate | Weak | Moderate | Weak | Weak | Weak |
| Mixed group B | |||||||||
| Åmark,100 1998; B&A | Medications | Moderate | Weak | Strong | Weak | Moderate | Moderate | Moderate | Weak |
| Naglo,101 1979; B&A | Medications | Weak | Weak | Moderate | Moderate | Moderate | Strong | Weak | Weak |
| Faure,102 2017; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Weak | Moderate | Weak |
| Jawaheer,103 1999; case report | Surgery | Moderate | Weak | Weak | Weak | Moderate | Moderate | Weak | Weak |
Detailed characteristics of study quality
Educational
One study looked at an educational intervention to ensure adequate fluid intake. This study was rated as weak quality overall, with the main weaknesses linked to selection bias, study design and blinding. Some areas were rated as strong, including factors related to confounders, data collection and withdrawals and dropouts. Analysis was rated as moderate quality.
Behavioural training
Of the 14 studies looking at behavioural training interventions, 10 were classed as weak quality overall and four were classed as moderate quality overall. The studies with small samples tended to have weak quality overall ratings and weak ratings of selection bias, but this was not always related directly to study design (study design: weak, n = 8; moderate, n = 6) or accounting for confounders (confounders: weak, n = 7; moderate, n = 4; strong, n = 3). Blinding was not considered possible in this category of intervention (blinding: weak, n = 13; strong, n = 1), but in most cases this was seen to be overcome with the use of better data collection techniques (data collection: weak, n = 2; moderate, n = 5; strong, n = 7), recording of withdrawals (withdrawals and dropouts: weak, n = 2; moderate, n = 5; strong, n = 6; unclear, n = 1) and data analysis techniques (analysis: weak, n = 4; moderate, n = 7; strong, n = 3).
Medically assisted techniques
Of the 24 studies looking at medically assisted techniques, 13 were classed as weak quality overall and 11 as moderate quality overall. Studies tended to score more highly in this intervention category than in others, possibly because of the better study designs used (study design: weak, n = 7; moderate, n = 12; strong, n = 5). Most studies included larger sample sizes that were better able to account for selection bias (selection bias: weak, n = 4; moderate, n = 16; strong, n = 4) and confounding factors (confounders: weak, n = 8; moderate, n = 8; strong, n = 7; unclear, n = 1). Blinding was not always possible with these interventions (blinding: weak, n = 17; moderate, n = 4; strong, n = 3), but this was often overcome with the use of better data collection techniques (data collection: weak, n = 1; moderate, n = 17; strong, n = 6), better recording of withdrawals (withdrawals and dropouts: weak, n = 6; moderate, n = 11; strong, n = 7) and better analysis techniques (analysis: weak, n = 3; moderate, n = 16; strong, n = 5).
Medications
Of the nine studies looking at medications, eight were classed as overall weak and one was classed as moderate quality. The study design varied considerably in this category; however, all but one study had only one ‘strong’ element of quality (selection bias: weak, n = 5; moderate, n = 4; study design: weak, n = 6; moderate, n = 3; confounders: weak, n = 4; moderate, n = 4; strong, n = 1). Blinding in this intervention category may have been largely prohibited because of the nature with which the medications were administered (blinding: weak, n = 6; moderate, n = 3), but aspects of data collection, withdrawals/dropouts and analysis were able to be adjusted for some of the potential impact of the lack of blinding (data collection: moderate, n = 9; withdrawals and dropouts: weak, n = 1; moderate, n = 3; strong, n = 5; analysis: weak, n = 3; moderate, n = 6).
Surgical approaches
Of the 20 studies looking at surgical interventions, 14 were classed as overall weak and six as moderate quality, as most study designs were retrospective case reports. Case reports may be good for identifying the appropriate population (selection bias: weak, n = 1; moderate, n = 18; strong, n = 1), but, because the study design is not considered beforehand and is largely ad hoc, many elements, such as confounders, blinding and withdrawals, cannot be accounted for (study design: weak, n = 15; moderate, n = 3; strong, n = 2; confounders: weak, n = 10; moderate, n = 7; strong, n = 3; blinding: weak, n = 18; moderate, n = 2; withdrawals and dropouts: weak, n = 5; moderate, n = 7; strong, n = 8). Conversely, owing to the nature of the surgical intervention in this category, elements of data collection were based on objective measures and so data collection and analysis were stronger, despite blinding not being possible (data collection: weak, n = 2; moderate, n = 14; strong, n = 4; analysis: weak, n = 3; moderate, n = 14; strong, n = 3).
Interpretation of the results of these studies should be cautious and take into account the substantial limitations of the study quality.
Main outcomes identified
We identified 20 relevant outcomes of incontinence, which we grouped for coherence into outcomes relating directly to incontinence, continence and equipment use (e.g. pads, nappies, anal plugs). This information is summarised in Table 10. A high degree of variation was found across included studies for all these outcomes, which were measured at different time points, at various frequencies and using different reporting units and without providing sufficient information for units to be converted to common measures to allow broader synthesis across studies. This presented a substantial challenge to summarising the evidence meaningfully while maintaining fidelity to the source data.
| First-level grouping of outcomes | Subgroups of outcomes |
|---|---|
| Incontinence | Wetting clothes |
| Number of accidents at home | |
| Number of accidents at school | |
| Soiling/urination incidents per week | |
| Frequency of faecal incontinence per week | |
| Incontinent episodes (over 7 days) | |
| Accidental wetting | |
| Leakage (daily, less than once per month) | |
| DVSS | |
| Bladder and bowel dysfunction questionnaire | |
| Continence | Percentage continent at follow-up |
| Urinating in toilet | |
| Defecation in toilet | |
| Appropriate wetting | |
| Continence urinary | |
| Continence faecal | |
| Equipment | Frequency of nappy change per day |
| Number of pads and nappies used per day | |
| Fewer than eight clean intermittent catheterisation episodes per day | |
| Number of anal plugs used |
We grouped other outcomes that do not relate to effectiveness directly but may provide evidence to address factors that influence effectiveness, thereby addressing review question 3. These outcomes were frequency of bowel movements per day; Bristol Stool Form Scale; absence of urgency; bowel symptoms; bladder symptoms; anal sensation; treatment and training fidelity checklist; last incontinent episode (days); shortest continent period (days); longest continent period (days); QoL; bowel care time per day; postoperative pain; urinary tract infections (UTIs); and catheterised volume.
We identified a large number of additional outcome measures related to functional physiology. Through discussion, we decided not to synthesise these data in the text of the main synthesis below, as these outcomes were not directly relevant to the research question. All outcomes are available in Appendix 6.
Eighteen studies focused on incontinence in populations with non-spinal-cord-related disabilities, and 50 studies focused on incontinence in populations with a spinal cord-related disability. The results for these two groups are reported below and have been split into interventions designed primarily to improve urinary incontinence, faecal incontinence or both.
Non-spinal-cord-related pathology
Urinary continence
Thirteen studies focused on interventions to improve urinary continence in non-spinal cord related populations. Seven out of the 13 studies focused on children and young people with autism and/or ADHD, five studies worked with those with developmental and/or learning disability, and one study had a mixed population where the majority of conditions were non-spinal cord related. Findings were highly mixed. General improvements were observed in urinary continence within populations with ADHD and/or autism undergoing behavioural training interventions and interventions involving drug therapy (desmopressin or anticholinergic treatments). One study with a mixed population found no improvement in urinary continence with drug therapy.
In populations of children and young people with developmental or learning disability, an educational intervention focused on adequate fluid intake showed improvements in urinary continence over 6 weeks. Behavioural interventions demonstrated improvements in continence, with all three studies.
Attention deficit hyperactivity disorder and autism
Seven studies presented interventions focused on improving urinary continence in populations of children and young people with ADHD and/or autism.
Of these seven studies, three40–42 presented behavioural interventions targeted at children with autism, with one study40 using a prospective before-and-after design, one study42 using case reports and one study41 using a case–control design. Mean population ages ranged from 3 years to 6 years 9 months. Population sizes were small, with three to five children in each study. All studies used interventions based on operant conditioning, with two40,41 reporting interventions explicitly adapted from Azrin and Foxx104 behavioural training methods, one with the addition of video modelling41 and one with a reduction in intervention intensity. 40 Basic counts were reported in all cases, with simple comparison made across participants and no further analysis of results presented. All studies reported improvements favouring the intervention; one study40 reported a decrease in all children’s rates of wetting clothes. Two studies reported an increase in rates of urinating in the toilet40,41 and one study42 reported that all children (n = 3) had learned to spontaneously ask to go to the toilet, with no accidents reported after 1 year of follow-up.
Two studies39 reported outcomes of an intervention focused on the use of a wireless moisture pager as part of two prospective RCTs. One trial39 involved 33 participants with autism with ages ranging from 36 to 72 months, and the more recent trial involved 32 participants with autism with ages ranging from 3 to 6 years. In both trials, participants were allocated to the wireless moisture pager arm or to standard behavioural treatment, employing behavioural strategies for toilet training children such as scheduled ‘sits’ on toilet, visual supports and reinforcement of voiding in toilet. In both trials, all participants in the wireless moisture pager arm showed greater improvements in rates of accidents and urination in toilet success rates than those in the standard behavioural treatment arm, although these differences were not statistically significant. Parent satisfaction scores did not differ significantly between the groups. No significant difference was reported between the groups in toileting independence.
One study36 of 54 children with ADHD (mean age of 8 years) reported the outcomes of a case–control study on the use of combination therapy with desmopressin and oxybutynin compared with the tricyclic antidepressant imipramine. The study authors reported that the Dysfunctional Voiding Scoring System (DVSS) scores were significantly lower in the desmopressin and oxybutynin group than in the imipramine group (mean 6.5, SD 0.5, vs. mean 9.6, SD 0.4; p < 0.001). None of the participants in the imipramine group was completely dry during the study period.
One study37 of 150 children with ADHD (n = 130) and autism (n = 20) (mean age of 8.6 years) reported on the use of desmopressin or anticholinergic treatment. Sixty-one per cent of participants were reported to be cured of incontinence (defined as 3 consecutive dry months without medication), with a mean time to cure of 10 months for children with ADHD and 8 months for children with autism.
Developmental/learning disability
Five studies reported on interventions for children and young people with developmental or learning disability.
One study46 presented an educational intervention based around adequate fluid intake for children with developmental disability. This study used a before-and-after design, with a population of 111 children and young people aged 4–15 years. The intervention lasted 6 weeks, after which time 67% of participants were continent, compared with 39.6% before the intervention.
Three studies reported behavioural interventions for children with developmental disability. 43,47,49 One study used a RCT study design, one study used a before-and-after design and one study was a case report. 49 Population sizes ranged from 3 to 44 children and young people, with ages ranging from 2 to 20 years. A simple comparison was made across participants, with no statistical analysis of results presented. All studies reported improvements favouring the intervention. Two studies43,49 reported improvements in continence measures: 31 participants (70.45%) were classified as continent (five accidents or fewer per week);49 there were statistically significant increases in appropriate toileting and decreases in accidents. 43 One study reported decreases in the ‘number of accidents’ at home and at school. 47
One study reported a bowel training intervention using an adapted Azrin and Foxx method targeted at children with Angelman syndrome, following a cohort of six participants whose ages ranged from 6 to 19 years. 50 This study reported the mean frequency of incorrect toileting per day and mean frequencies of correct toileting per day. Incorrect toileting reduced from a mean of 1.7 (SD 1.76) times per day at baseline to 0.1 (SD 0.29) times per day at follow-up. Correct toileting increased from 0.8 (SD 0.95) times per day at baseline to 3.1 (SD 1.57) times per day at follow-up. All measures showed improvements that favoured the intervention, with toilet training taking a mean of 17.2 days across all participants (range 12–24 days).
Mixed
One study51 reported a RCT with a mixed case population, including six individuals who were classified ‘brain damaged’, four with Down syndrome, two with epilepsy, two with ‘primary genetic’, one postnatal infection, and one classified as having infant autism. The ages of participants ranged from 0.5 to 18 years. This study assessed the effectiveness of imipramine compared with placebo in a RCT crossover trial, and reported no significant difference in the number of wettings between those receiving 50 mg of imipramine and those receiving the placebo.
Faecal continence
Only one study focused on faecal incontinence alone. No studies solely reported faecal continence outcomes focused on populations with developmental/learning disability or myelodysplasia, or mixed populations.
Developmental/learning disability
One study reported findings from a RCT using liquid glycerine suppositories and reinforcement in a population of 20 children and young people aged 5–16 years with autism, with reinforcers tailored to the individual participants. 38 Participants in the intervention arm showed improvements in bowel continence, with six participants in the intervention arm reporting improvement after 6 weeks compared with no participants in the control arm (p = 0.005). Participants in the intervention arm showed improvements in bowel independence at the week 10 follow-up, with four participants reporting improved bowel independence compared with one participant in the control arm. Slight improvements were also reported according to the Clinical Global Impression scale at 6 weeks, with 1 out of 10 (10%) participants in both the intervention and the control arms reporting much improved or very much improved Clinical Global Impression scores, and 9 out of 10 (90%) participants in both arms reporting minimal improvement or no change. At 10 weeks, 5 out of 10 (50%) participants in the intervention arm reported much improved or very much improved Clinical Global Impression scores compared with 1 out of 10 (10%) in the control arm (p = 0.076).
Both urinary and faecal continence
Four studies were identified that focused on both faecal and urinary continence outcomes. In populations with developmental and learning disabilities, two studies reported improvements in all faecal and urinary continence measures following a behavioural training intervention. One study focused on children and young people with ADHD symptoms and reported an improvement in continence in just over half the participants following an intervention using medication. No studies focused on mixed populations that reported both faecal and urinary outcomes.
ADHD and autism
One study35 of 68 children aged 5–12 years with ADHD symptoms reported DVSS scores in a before-and-after study with an intervention of desmopressin. Fifty-seven per cent of participants with ADHD reported a partial or complete response to medication.
Developmental/learning disability
Three studies used behavioural training interventions and assessed both faecal and urinary outcomes in terms of frequency of defecation and urination in the toilet. 44,48 Improvements were reported across all measures post intervention, although most of these were not statistically significant. Some statistically significant differences were found in Sadler and Merkert’s 1977 study45 comparing the Azrin and Foxx method with a scheduling intervention and with a no-treatment group; the number of accidents reported was smaller in the Azrin and Foxx group than in the scheduling intervention group (p < 0.01) and the no-treatment (control) group (p < 0.01).
Spinal cord-related pathology
In Tables 2 and 3 we reported the patient groups as described by the authors: spina bifida, myelodysplasia, neurogenic dysfunction and mixed. These patient groups cover similar pathologies that can all cause neurogenic urinary and faecal incontinence. Therefore, the results of the various interventions will be considered by intervention type (instead of by population type) under the following headings: neurogenic urinary continence, neurogenic faecal continence, and both neurogenic urinary and faecal continence. One paper in the ‘mixed’ group102 reported the results for patients with neurogenic incontinence and patients with non-neurogenic incontinence related to bladder exstrophy. Only the results for those with neurogenic incontinence are relevant to our population of interest and so are reported in this review.
Neurogenic urinary continence
Twenty-four studies focused on interventions to improve urinary continence in spinal cord pathology populations. The study populations included children and young people with conditions such as spina bifida, neurogenic dysfunction and myelodysplasia, and some studies reported a ‘mixed’ study group (see Table 3). Success in continence improvement was mixed across the interventions, with some studies reporting high rates of UTIs and side effects.
Medically assisted techniques
Six studies looked at medically assisted techniques to improve urinary continence. 52,53,69,79,89,98 Three studies explored the use of neurostimulation techniques. One study assessed the effectiveness of transurethral intravesical electrical stimulation in improving continence in 10 children and young people with spina bifida. 79 Of these 10, only one achieved urinary control after 20 sessions; this lasted for 4 months, after which time the child discontinued treatment. The remaining nine children had treatment discontinued as they found the sensation unpleasant, had no observed improvement and, in one case, experienced pain.
Another study also reported on the use of transurethral intravesical electrical stimulation as part of a prospective RCT. 98 This trial had 36 participants with myelodysplasia whose ages ranged from 6 to 12 years and who received transurethral intravesical electrical stimulation or sham treatment. There was no difference between the groups in reduction of number of pads or nappies used per day. There was also no statistically significant difference found in increased bladder capacity, detrusor activation, detrusor compliance, or bladder sensation allowing timely voiding.
A later study reported the results of a RCT investigating the efficacy of transcutaneous functional electrical stimulation (FES) in children with refractory neuropathic urinary incontinence secondary to myelomeningocele. 53 Children in the treatment group (n = 15) improved their daily incontinence score (range 0–3, with 0 being most continent) from a mean of 2.7 (SD 0.4) before treatment to a mean of 1.3 (SD 0.9) after treatment, compared with a sham stimulation group (p = 0.02). Three out of 15 patients in the treatment group became completely dry between two consecutive clean intermittent catheterisations (CICs). The daily incontinence score improved from 3 to 1 at follow-up for four patients and remained unchanged for two patients. It also remained unchanged in 8 out of 15 patients in the control group. Pad-changing frequency significantly decreased in the treatment group compared with the control group, from a mean of 5.2 (SD 1.6) times per day [vs. mean 4.3 (SD 1.7) times per day] before FES therapy to a mean of 2.4 (SD 1.4) times per day [vs. mean 3.5 (SD 1.6) times per day] after FES therapy at follow-up (p = 0.03).
One study assessed the effectiveness of an intraurethral self-retaining device in 24 children and young people aged 5–18 years with myelomeningocele. 69 This study found a reduction (from a mean of eight per day to two per day) in the number of nappies used for urinary incontinence, although all participants continued to use pads for faecal incontinence. The device was ‘well tolerated’ by 21 out of 25 (85%) of participants, with no reports of complications after 6 months of use. QoL scores showed that the intraurethral self-retaining device significantly reduced the intensity of the impact of urinary incontinence on both children and adolescents (p < 0.0001).
One early study on the use of CIC89 found that 13 out of 22 (60%) children with neurogenic dysfunction (with a mean age of 7 years) in the CIC group became continent (p < 0.001) compared with 1 out of 21 participants (5%) in the control group (non-CIC).
Medication
Six studies looked at the use of medication to improve urinary continence. 72,91,92,99–101 One study reported an improvement in continence post intervention with propiverine in participants with neurogenic detrusor overactivity, with incontinence score reducing significantly (p < 0.05) at both short- and long-term follow-up. 91,92 At long-term follow-up, which ranged from 2.0 to 5.9 years,92 it was reported that monotherapy was well tolerated in 11 out of 17 patients, but comedication with intravesical oxybutynin at low doses had been applied in six cases.
One study reported the effectiveness of desmopressin in a population of 18 patients with spina bifida-related neurogenic bladder dysfunction whose ages ranged from 6 to 18 years. 72 The study authors reported a 78% success rate, with 14 out of 18 patients reporting a marked improvement in nocturnal continence. Eleven out of 18 participants were reported as ‘completely dry’, and three were reported as ‘mildly damp’ (from a nappy to an underwear liner).
One study investigated the effect of phenylpropanolamine on 10 patients aged 6–18 years with neurogenic bladder. 99 The results were mixed, with three participants reporting no improvements and seven reporting varied improvements in pseudocontinence, such as some increase in the number of 3-hour dry periods during 3 consecutive days.
Two studies reported on mixed populations. 100,101 One of the studies101 had 13 participants: 11 with myelomeningocele, one with sacral agenesis and one with spinal dysraphism. This study assessed the effectiveness of drug therapy (alprenolol) plus standard toilet training and reported an improvement in some (8/13) patients after they received the training, but there was no clear effect of the beta-blocker beyond that seen in the training programme. The other study100 assessed the effectiveness of intravesical oxybutynin in improving continence in the target population (one with vascular lumbar spinal cord insult, one with non-neurogenic bladder, one with traumatic high thoracic spinal cord injury and 36 with myelomeningocele). The authors found that continence improved following the intervention. However, UTIs were reported in 29 instances.
Surgical interventions
Fourteen studies investigated how various surgical procedures could improve urinary continence. Five studies investigated the use of injections of botulinum toxin type A. 65,66,68,73 The results were mixed, with three studies66,68,73 reporting clear improvements in continence post intervention, where 21 out of 24 (87.5%) children and young people with myelomeningocele aged between 3 and 16 years became completely dry between two consecutive CICs after 6 months. Another97 reported improved continence scores (from 3 to 0) in seven children aged 6–16 years following injection of botulinum toxin type A. A reduction in UTIs in all patients post intervention was also reported. However, one study65 reported mixed improvements, with an undefined clinical success rate of 66% (n = 53).
Two studies with children with spina bifida reported bladder neck sling procedures, with augmentation in some cases. 76,84 Both studies reported improved continence rates, and continence (dry, no pads) was achieved significantly more often with bladder neck sling than with Leadbetter/Mitchell bladder neck sling (66% vs. 37%), with bladder neck closure resulting in dryness in 65% of 85 patients84 and in improved QoL scores in one study. 76
Two studies reported findings from case reports on bladder neck reconstruction. 102,103 One study102 assessed the effectiveness of Young–Dees bladder neck reconstruction with bladder neck injection as a follow-up. The authors reported the results for two separate groups of patients: group 1 with neurogenic bladder, whom we include here, and group 2 with bladder exstrophy, who are not included in the figures below, as these patients do not fulfil our participant criteria for this review. The other study103 assessed Pippi Salle bladder neck repair. These studies were conducted in populations of 18–50 participants, with ages ranging from 1.9 to 17.5 years and conditions including neurogenic bladder, spina bifida, sacral agenesis and idiopathic neural bladder.
Among those who underwent the Pippi Salle procedure,103 daytime continence (≥ 3 hours) was achieved in 11 out of 18 children (61%). Eight out of 18 participants (44%) were continent overnight, with eight remaining completely incontinent during the night. Twelve children needed additional drug therapy (oxybutynin) to maintain continence success. In the same study, children had difficulty with catheterisations (4/5) and pelvic abscess (1/5). Seven children (39%) required further operations, and the reported complication and failure rate was high.
For those who underwent Young–Dees bladder neck reconstruction, only 6 of the 35 patients with neurogenic bladders (17%) were considered continent after undergoing treatment. 102 Owing to a lack of success with Young–Dees bladder neck reconstruction, 29 out of 35 underwent bladder neck injection with Teflon™ and collagen in cases before 1994 but only polydimethylsiloxane (PDMS; product name ‘Macroplastique’) in cases after 1994. Of these 29 patients, 13 (45%) became continent.
One study96 reported findings from an intervention using artificial urinary sphincter with seromuscular colocystoplasty to improve continence in 27 children and young people with neurogenic bladder who were aged 4–23 years (the artificial urinary sphincter increases bladder neck resistance, thus reducing leakage, and seromuscular colocystoplasty is a technique for increasing bladder capacity). Continence was achieved in 24 out of 27 patients (89%).
Three studies reported urinary continence outcomes in populations of children and young people with neurogenic dysfunction in which inert ‘bulking’ agents are injected into the bladder neck to narrow it, with the aim of reducing urinary leakage. In one study,95 56% of participants aged 6–17 years (13/23) had a mean increase in dry time (i.e. no urinary incontinence) of 2 hours post intervention, and all participants and their parents from this group were satisfied with the collagen injection intervention. The remaining 44% (10/23) of participants had an average dry time of 0.2 hours following the intervention, and none of this group or their parents were satisfied. In some participants, initial success was transient and passed after 2–4 weeks post injection.
In another study93 using PDMS, only 33.3% of participants were entirely continent following the intervention of injected PDMS, with 24% partially continent and 42% reporting no effect at all. A long-term follow-up study by the same authors94 reported success (i.e. continence) in 33% of participants and improvement in 14%, with 53% remaining unchanged, but the authors noted that ‘the outcome remained almost unchanged from 18 months of follow-up’.
Another study assessed the effectiveness of filum section and medical therapy in a population of 21 children and young people with occult tethered cord syndrome. 57 The Bowel and Bladder Dysfunction score improved by an average of 20% in the surgical arm and by an average of 24% in the medical arm. QoL improved modestly in both groups, although none of the differences was statistically significant.
Neurogenic faecal continence
Nineteen studies focused on faecal continence alone. Eighteen of these studies were in people with spina bifida, and one study was in children and young people with neuropathic bowel and anorectal malformations (ARMs). The results showed general improvements in faecal continence favouring the intervention, although not all improvements in continence were statistically significant. Self-reported satisfaction with the intervention and QoL both improved following the intervention.
Behavioural training
Three studies explored the use of an intensive bowel training intervention to improve faecal continence. One study60 of 55 patients aged 5–19 years with spina bifida suggested a significant improvement in faecal continence by the end of the intervention (p < 0.025). Similarly, another study59 of 53 patients aged 3–14 years also found significant improvement in faecal continence (p = 0.004) with a stepwise bowel management programme. The third study,87 of 22 patients aged 2–24 years also supported the success of a bowel management programme, with 17 of the patients becoming clean of stool between enemas.
Medically assisted techniques
Twelve studies reported interventions using medically assisted techniques that focused solely on faecal continence. 38,55,58,59,61–64,70,71,74,77,78,80,85,90 Seven studies looked at the use of transrectal or transanal irrigation (TAI) to improve faecal continence. Both of these interventions are the same and involve the irrigation of the rectum and lower colon using fluid inserted via a catheter that is passed through the anus into the rectum. One study70 focused on transrectal irrigation (TRI); the majority (35/40) of participants with spina bifida were still using TRI at follow-up and all participants were continent and free of constipation. Five studies focused on TAI using Peristeen;62–64,78,85,90 sample sizes ranged from 20–74 children and young people with spina bifida, aged 4–19 years. All reported improvements (some statistically significant) in continence, pseudocontinence and other measures including constipation, number of incontinence episodes, symptoms during evacuation, bowel care time, satisfaction with TAI, St Mark’s Faecal Incontinence Scale, Cleveland Clinic Constipation Scoring System and Neurogenic Bowel Dysfunction score, and QoL presenting mixed effects.
Two studies58,71 reported the outcomes of interventions using anal plugs in 18–20 children and young people with spina bifida aged 4–29 years. Both studies reported overall improvements in patient-reported outcomes relating to the impact of faecal incontinence on daily life. The median number of weekly incidents of faecal soiling reduced following the intervention (mean incidents of faecal soiling 4 vs. 0, p = 0.002;71 7/18 participants no longer needed nappies58). Post intervention, 98% of participants reported that they found the anal plug comfortable. 71
One study reported outcomes relating to an intervention of intravesical electrical stimulation in 24 children with spina bifida, with a reduction in the mean number of faecal incontinence episodes per week from 7.36 before intravesical electrical stimulation to 4.8 after intravesical electrical stimulation (p < 0.05). 77 Fifty per cent of participants reported that faecal incontinence had disappeared completely post intervention, and 25% of participants reported a reduction in faecal incontinence episodes of > 50%.
Another study74 reported the results of an intervention using transrectal bowel stimulation in children and young people with spina bifida, which showed an overall success rate of 90%.
One study80 reported a variety of techniques to achieve faecal continence or pseudocontinence in patients with spina bifida. These included a strict toilet scheme in five (all successful), retrograde enemas in 24 (21 successful), antegrade continence enemas (ACEs) in 20 (16 successful) and performing regular manual evacuation of stools in 10 (eight successful). The reported timings are unclear, but after the intervention was administered, 73% (58/80) children were faecally continent or pseudocontinent (involuntary stool loss no more than once per week). Twenty-seven per cent (22/80) of participants remained incontinent post intervention; of these, 17 participants had ceased efforts to gain pseudocontinence by the end of the study.
One study55 reported the results of a biofeedback training intervention in 28 children and young people with spina bifida aged 7–21 years. This study reported no significant difference in soiling frequency between conventional and biofeedback groups, although soiling frequency significantly reduced (p < 0.02) in the biofeedback group from pre treatment to post treatment.
Surgical interventions
Four studies looked at the use of ACE to improve faecal continence in patients with spina bifida. One case study85 reported on the effectiveness of anterograde continence enema (ACE) on faecal continence among 16 children and young people. The study authors reported resolution of constipation and faecal continence in all cases. All patients had stopped using nappies, and mothers of patients reported satisfaction with the procedure at 1.5 years’ follow-up. Another study61 reported the results of an intervention using the MACE (Malone antegrade continence enema – an alternative name for the ACE) procedure to improve continence. Self-reported independence improved, and incontinence episodes reduced in most (16 out of 19) participants. All participants reported improvement in problems with constipation. Measures of self-esteem also improved post intervention, and psychological problems reduced. One study83 also reported using the retrograde continence enema (RCE) in 25 participants aged between 4 and 23 years. The study authors reported that 10 out of 13 (76.9%) in the retrograde group and 9 out of 12 (75.0%) in the antegrade group achieved faecal continence. Another study reported on 23 case reports of patients with myelomeningocele aged 3–18 years. 82 The authors found that full continence was achieved in 13 patients (56.52%), partial continence was achieved in eight patients (34.78%) and failure occurred in two patients (8.69%).
Both urinary and faecal continence
Seven studies in total focused on outcomes related to both urinary and faecal continence. One study looked at a behavioural intervention that involved a bowel management programme in children with spina bifida. 75 Four studies looked at medically assisted interventions in children with spina bifida or other neurogenic dysfunction,67,81,86,88,105 one study looked at MACE81 and one study looked at surgery of the lumbar flavotomy and section of the filum terminale. 86 The results showed general improvements in urinary and faecal continence favouring the intervention, although not all improvements in continence were statistically significant. QoL measures showed improvements following the intervention.
Behavioural training
One study reported the results of an intervention using a bowel management programme plus drug therapy and clean CIC compared with simply drug therapy and CIC. 75 This study used a case–control design to compare 70 children and young people aged 4–16 years who had spina bifida. After 1 year, participants in group 1 (bowel management programme plus drug therapy and CIC) reported a reduction in constipation, whereas group 2 (drug therapy and CIC) reported no change. Group 1 reported a reduction in faecal incontinence (before, 82.6%; after, 28.6%), an improved mean number of dry intervals (28.9, SD 11.1, before; 150.0, SD 36.4, after) and a reduced mean number of infections (3.2, SD 1.2, before; 0.3, SD 0.5, after). The difference between groups was statistically significant for constipation, faecal incontinence, mean dry interval (all p < 0.001) and mean number of urinary infections (p = 0.005). After 1 year of follow-up, participants in group 1 reported higher QoL assessed by children (mean 84.5, SD 8.9) and parents (mean 88.9, SD 7.1) than those in group 2 (children mean 53.4, SD 12.5, and parents mean 55.4, SD 11.4). Both groups 1 and 2 demonstrated improvements in QoL following bowel management programme therapy, assessed in both children and parents (p < 0.001).
Medically assisted techniques
Four studies looked at various medically assisted techniques. One study reported results from an intervention using transcutaneous electrical field stimulation [the Duet Continence Stimulator (Dynamic Medical Instruments Ltd, Wigan, UK)] in 77 children and young people aged 4–18 years with spina bifida. 56 This study found no statistically significant difference between the intervention and placebo arms in any primary end points, including urinary continence and spontaneous defecation.
Another study reported results of a sacral neuromodulation (SNM) intervention (‘InterStim’), with improvements in urinary and faecal continence in 75% of participants with neurogenic dysfunction when SNM was activated compared with 21% of participants when the device was inactive (p = 0.001). 88 The procedure was reported to be well tolerated, with no patients dropping out because of upper urinary tract deterioration.
One study52 reported results from the use of pelvic floor interferential electrostimulation. In this study, 23 (78%) of the 30 children and young people with spina bifida aged 3–13 years became continent post intervention, with 18 (60%) retaining continence after 6 months. Improvements were reported in urinary continence and reduced enuresis immediately after treatment (p < 0.05) and after 6 months’ follow-up (although these were not statistically significant).
One study67 reported outcomes from urodynamic feedback treatment in eight children and young people aged 7–19 years with spina bifida, with some improvements reported across both faecal and urinary continence outcome measures.
Surgical interventions
One study investigated the use of a surgical procedure that included lumbar flavotomy and section of the filum terminale. 86 The procedure was carried out with 60 patients aged 3–18 years with spina bifida. The study reported complete resolution of urinary incontinence in 52% of patients, marked improvement (> 95% resolution) in 35%, moderate improvement (> 75%) in 6%, and minimal or no improvement in 8%; faecal incontinence was completely resolved in 56%, improved in 41% and unchanged in 3%.
Another study reported on the use of the MACE in 21 participants aged between 6 and 22 years with spina bifida. 81 The study reported full faecal and urinary continence post intervention in 16 patients, with 19 patients reporting faecal continence only.
Evidence on cost-effectiveness
We did not find any studies of cost-effectiveness to be able to address research question 2: what is the cost-effectiveness of interventions to improve continence in children and young people with neurodisability?
Evidence on the factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions
In this section, we anticipated identifying aspects, such as environmental factors, conducive to the effectiveness of interventions (e.g. availability of adapted toilets), potential social factors influencing effectiveness, such as a lack of parental support or teacher training, and individual factors, such as a lack of personal agency in self-care. We did not identify any such outputs in the included literature, so we have detailed outputs below that may have value in addressing this question.
We grouped other outcomes that do not relate directly to effectiveness but may provide evidence to consider factors that influence effectiveness. These outcomes, shown in Table 2 and reported across 12 different studies, were frequency of in-toilet urinations; frequency of urination accidents; frequency of incontinence (urinary or faecal); frequency of bowel movements per day; frequency of nappy changes per day; frequency of faecal incontinence per week; frequency of pad changes per day; soiling frequency; shortest continent period; longest continent period; and last incontinent episode. QoL using various scales [PEDQOL, Paediatric Enuresis Module to assess Quality Of Life (PEMQOL), Faecal Incontinence Quality of Life, HRQoL and generic QoL across different studies] was reported in six studies. Number of UTIs was reported in four studies.
Bristol Stool Form Scale, pain during procedure, postoperative pain, catheterised volume, absence of urgency, bowel symptoms, anal sensation, and treatment and training fidelity checklist were all reported in single studies, with no common studies to allow comparison.
There was insufficient common measurement or homogeneity of measures to allow for a cross-study comparison of results, so it was not possible to usefully synthesise individual study data to draw broader conclusions around the factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions to improve continence in children and young people with neurodisability.
Qualitative evidence on the views, experiences and perceptions of children and young people, their families, their clinicians and others involved in their care of delivering and receiving such interventions
All three studies identified presented qualitative evidence relating to spinal cord pathology conditions. We found no qualitative evidence relating to non-spinal-cord-related conditions. However, some of the themes, subthemes and concepts may be applicable to all children and young people with neurodisability and their families and caregivers.
Three studies reported qualitative data on experiences of interventions aimed at improving continence in children and young people with neurodisability. The results are summarised in Table 11. Two studies reported the experience of parents and one study reported the experience of children and young people. The majority of children and young people were living with spina bifida, with one paper exclusively focused on those with spina bifida, and two focusing on mixed populations in which the majority had spina bifida (31/40;106 9/18107). Two studies107,108 reported solely on experiences of faecal continence and one study106 reported solely on urinary continence.
| Study (first author and year) | Condition and population | Perspective | Based on | Themes | Subthemes | QA | Faecal/urinary/both |
|---|---|---|---|---|---|---|---|
| Sawin 2009108 | Spina bifida; 7 children (18 months–23 years) | Parent – specifically, the parent’s experience of working with their child and a HCP to find an effective bowel management programme | The ecological model of adaptation in spina bifida (Sawin et al. 2003)109 and Orem’s self-care theory (Orem 1995;110 Renpenning and Taylor 2003)111 | Theme 1: a long complicated journey |
Subtheme (a): uncertainty of accidents Subtheme (b): problems in relationships with HCPs – not being heard Subtheme (c): timing: lack of information regarding when to start a bowel programme |
Richness of data: very good Methodological rigour: 9/10 (ethics not reported) Conceptual contribution: very good |
Faecal |
| Theme 2: the impact of the journey on the child |
Subtheme (a): universal embarrassment and assault to self-esteem Subtheme (b): school issues |
||||||
| Theme 3: the family struggle |
Subtheme (a): overwhelming stress Subtheme (b): parents’ role negotiation Subtheme (c): advice to other families |
||||||
| Theme 4: the promise of the future |
Subthemes (a): the joy Subthemes (b): the frustration |
||||||
| Edwards 2004106 | Neuropathic bladder; 40 children aged 7–20 years (31/40 with spina bifida) | Children and young people | NR | Theme 5: self-perception and self-esteem |
Subthemes (a): level of understanding Subthemes (b): confidence in self-care |
Richness of data: very good Methodological rigour: 10/10 Conceptual contribution: very good |
Urinary |
| Theme 6: challenges of self catheterisation |
Subthemes (a): independence and dependence Subthemes (b): willingness to engage |
||||||
| Sanders 2014107 | Mixed conditions; 18 children; aged 3–16 years (spina bifida, n = 9; imperforate anus, n = 3; and sacral agenesis, n = 1; cloaca, n = 1; or parents did not report on an underlying condition, n = 4) | Parent | NR | Theme 7: parents’ investment in their child’s bowel management |
Subtheme (a): confidence, knowledge and experience Subtheme (b): support from professionals Subtheme (c): emotional stress and strength |
Richness of data: very good Methodological rigour: 9/10 (theoretical perspective not fully explored) Conceptual contribution: very good |
Faecal |
| Theme 8: supporting their child’s independence |
Subtheme (a): stressful experiences Subtheme (b): independence and dependence Subtheme (c): concerns for the future |
Although only three studies contributed to the qualitative analysis, all were assessed as ‘very good’ in their richness of data (on a rating scale of poor, some, good or very good), scored 9 or 10 out of 10 for methodological rigour, and assessed as ‘very good’ in their conceptual contribution (on a rating scale of poor, some, good or very good). These studies provided insight across eight themes (a long complicated journey; the impact of the journey on the child; the family struggle; the promise of the future; self-perception and self-esteem; challenges of self-catheterisation; parents’ investment in the child’s bowel management; and supporting their child’s independence) and an additional 20 subthemes.
The thematic analysis that was possible across and between papers was limited, but we were able to observe six common themes that arose in more than one study (the impact of the journey on the child; the family struggle; the promise of the future; self-perception and self-esteem; parents’ investment in their child’s bowel management; and supporting their child’s independence) and developed three new concepts not previously highlighted by study authors (understanding; peer support networks; and places of learning). Table 11 presents the themes and subthemes identified by the study authors, as well as the three new concepts. These are described below.
Concept 1: understanding
In the study by Sawin et al. ,108 one parent talked about the understanding that they experienced from a continence nurse:
I wish I had known her [nurse] when [child] was a baby, cause maybe the outcome would have been different . . . I always felt like she understood what families were going though, especially the patient, and worked hard to achieve bowel continence . . . I found her to be extremely helpful and sensitive in an area where it could be very uncomfortable to talk about.
Sawin et al. 108
The value of understanding is also reflected in the often low expectations and lack of ambition of health-care professionals. One parent remarked that if they had taken their child who did not have spina bifida and did have continence issues to the doctor, they would be referred to a specialist medical centre. In the case of a child with spina bifida, doctors say ‘it[‘s] just a part of the condition you have to live with’. Another parent commented on the lack of priority and understanding:
I don’t think most HCP understand how constricting bowel and bladder incontinence can be, especially for bowel. Nobody wants to walk around smelling bad.
Sawin et al. 108
Concept 2: peer support networks
This concept is linked with subtheme 3c, ‘advice to other families’, but was not identified by the study authors. Sanders et al. 107 described how some parents reported discovering the potential value of irrigation through peer support networks or support websites. Peer support also had the potential to influence parental attitudes and instil confidence to ask health-care professionals about trialling some procedures, such as irrigation.
Concept 3: places of learning
This concept explores the importance of places of learning and instruction. Evidence from Sanders et al. 107 suggests that although some parents were offered the opportunity to ‘come into [hospital] and stay a few days’ this was not perceived as an ‘inviting prospect’ as the hospital was a significantly different environment from home. Four parents had been taught to use irrigation at home, and at least half of those who had been taught in hospital (n = 7) believed that:
If the nurse had actually done one with us [at home] I probably would have felt less worried about some of the obstacles.
Sanders et al. 107
Evidence from Edwards et al. 106 further supports this; participants identified a clear preference for learning at home, with hospital and outpatient clinics described as ‘not private enough’ and ‘too cold and clinical’ and the process said to be ‘too rushed’.
Theme 1: a long complicated journey
Subtheme (1a): uncertainty of accidents
Sawin et al. 108 identified this theme, with parents reporting how they experienced times when continence was managed, and then their child would have an incontinence incident, which would lead to a period of uncertainty about continence and the management of incontinence:
We would go for several weeks and everything would be fine and then he’d have an accident and I think [when I saw the liquid stool], ‘Oh, gees, its diarrhoea’ . . . and you give ‘em Imodium and then they become even more impacted. So . . . we start over . . . and just never seemed to achieve any kind of [satisfactory] bowel continence.
Sawin et al. 108
This was also reflected in Sanders et al. :107
It was a case of every few weeks we’d say [trying something else but] it wasn’t working, she started being sick, [her tummy] was swollen, it was horrendous, it was absolutely horrible.
Sanders et al. 107
Subtheme (1b): problems in relationships with health-care professionals: not being heard
This was identified in Sawin et al. ,108 with parents voicing frustrations that professionals did not want to engage in conversations about achieving success in continence:
I feel like physicians in general don’t want to even talk about it. Doctors feel like it’s never going to be an achievable thing for our kids so why bother. I felt like I had to do everything on my own.
Sawin et al. 108
This was also reflected in Sanders et al. ,107 where parents reiterated the importance of professional attention and care:
When you are actually sitting in the consultant’s room, talking about it, it sounds pretty horrific, but seeing it and watching [with the nurse] how straight-forward it was gave us a bit of confidence to go and give it a try.
Sanders et al. 107
Subtheme (1c): timing: lack of information regarding when to start a bowel programme
Sawin et al. 108 reported that parents felt surprise at delays in training children, noting ‘The earlier, the better. You are training the system, not the child’. The lack of guidance from professionals on the benefits of early training was a frustration for some, and a source of stress for many:
Finding the right bowel program was a life filled with stress for the whole family . . . You know we had done everything. And it’s not that these things are bad, or that they’re completely unsuccessful, they’re not one hundred percent reliable. And one accident is one too many.
Sawin et al. 108
Theme 2: the impact of the journey on the child
Subtheme (2a): universal embarrassment and assault to self-esteem
Sawin et al. 108 reported parents feeling guilty that their children had had to deal with bowel management problems for many years, and frustrated that their children’s social development was limited as their bowel problems restricted their interactions with peers:
It’s just sad when your kid’s not able to spend the night anywhere, they’re not able to go on dates, and they’re not able to sit through biology class without having an accident. That’s no life you know.
Sawin et al. 108
Sanders et al. 107 also saw this reflected in parents’ concerns around the social difficulty of ‘soiling in the classroom’ or ‘still wearing a nappy’ for their children at nursery and school.
Parents also discussed negative experiences during holidays and continence accidents spoiling family enjoyment of time away:
Nobody wants to sit on the beach and have a big bowel accident . . . How embarrassing for us all.
Sanders et al. 107
Subtheme (2b): school issues
In Sawin et al. ,108 parents identified bowel management problems as a source of ongoing stress to the child and the school; for example, a child might need to leave class suddenly to go to the nurse’s office if they were worried that they had had an accident. Parents voiced concerns about the burden of their child’s condition on the school staff, and fatigue in their bowel care. Parents also felt uncomfortable in their perception of how school personnel viewed the family as ‘coping’ or ‘not coping’, introducing stress in relationships with the school and school staff:
School people get tired of it. You know and school nurse conveys: ‘What’s wrong with this family?’ and ‘Why can’t they get it together?’ You know they’re burned out with it. So it’s just a real vicious cycle of bad vibes.
P286 para 5
Theme 3: the family struggle
Subtheme (3a): overwhelming stress
Sawin et al. 108 reported that some parents noted that the increased friction between them and their children during adolescence was exacerbated by bowel management problems. Parents mentioned the relentless dominance of bowel management in family life:
You find yourself winding your whole life around this bowel program or fear of the accident.
Sawin et al. 108
This was also reflected in Sanders et al. ,107 where parents described an emotional resilience that was necessary in the circumstances:
We had to make a decision to trial it and keep going with it even when she’s not happy with it, emotionally it’s quite difficult because obviously it’s personal, [so] building up to doing it was quite difficult.
Sanders et al. 107
In some cases, this placed a strain on a parent’s relationship with their partner:
Irrigation became, even between my husband and I, a very, very, big thing. [We had to decide if] we could carry on.
Sanders et al. 107
Subtheme (3b): parents’ role negotiation
Sawin et al. 108 reported that parents recognised the issues around independence and self-directed care alongside the need to manage their child’s bowel care effectively. Successful moves towards independence were also mentioned, alongside the need to subtly balance support and guidance:
At one point when he became a teenager, he finally told us he couldn’t understand why we had to do the bowel program. He decided to do the bowel program himself, which meant he didn’t do anything. He thought if he didn’t do it, it would eventually happen on its own. But it doesn’t work that way.
Sawin et al. 108
This was further reflected in both Edwards et al. ,106 who reported that ‘Most young people (particularly in group 3) who were successfully using SC [self-catheterisation] could think of no good points about being catheterised as opposed to using SC’, and Sanders et al. ,107 who stated that ‘parents believed that being continent of stool and not wearing a nappy meant that children needed less help with their toilet routine, which impacted positively on their developing independence in line with their peers’. Most parents were motivated towards their child becoming independent in the future, but many described feeling ‘tied’ to undertaking their child’s irrigation:
It’s stressful when you know you are the only person who can do it. Like I’m her lifeline and if something goes wrong then it can be quite dangerous if only one person can do it.
P867 para 2
Subtheme (3c): advice to other families
In the study by Sawin et al. ,108 several parents referred to a desire to support other parents with specific goals: to prevent struggles; to pass on lessons learned; to save others from experiencing overwhelming stress; and viewing the need for bowel care in context seeing the child first and the disability second:
It’s trial and error and don’t expect it to work all the time. As they get older you have to change what they are doing. Also give them the opportunity to learn themselves.
Sawin et al. 108
Theme 4: the promise of the future
Subthemes (4a): the joy
Positive parental experiences included improvements in routine, improved self-esteem through successful bowel management such as surgery (e.g. MACE procedure) and a sense of achievement in progress.
Subthemes (4b): the frustration
Frustrating episodes were discussed, including establishing and maintaining a useful bowel programme; managing uncertainty around accidents; managing all of the factors that had an impact on continence (medication, diet, mobility, health-care professional relationships); the inadequacy of available interventions (e.g. common diet changes in terms of fibre intake; medications); and the lack of understanding from health-care professionals (with parents perceiving that bowel problems were not a priority for professionals).
A new concept identified by Sawin et al. 108 was the reliability (and lack of reliability) of management tools:
It wasn’t like the issue was taboo; it just wasn’t a priority. It seemed like the emphasis was placed on shunts, bladder, and any kind of orthopedic problem. These seemed more pressing than any kind of bowel status.
Sawin et al. 108
Theme 5: self-perception and self-esteem
Subtheme (5a): level of understanding
Edwards et al. 106 reported that most young people had good knowledge of the practical steps involved in self-catheterisation (SC), but only two had a general understanding of the reasons for using SC. The biggest gap in knowledge was about how catheterisation protects the kidneys, and there were some notable misconceptions around body function and catheter operation.
Subthemes (5b): confidence in self-care
Edwards et al. 106 also identified that children and young people voiced fears about using SC in terms of ‘doing it wrong’, but also expressed satisfaction with success, ‘feeling pleased’ when it went right. Some younger children identified benefits of having catheterisation conducted by someone else instead of undertaking SC, as they enjoyed the reassurance of having someone else there in case there was a problem. This was also reflected in Sawin et al. ,108 with one parent saying:
Since my daughter got the cecostomy, she is totally independent in managing her bowel program. She irrigates every other day before she goes to bed. She irrigates through a trapdoor device. The enema comes through and then the stool comes out. She plans for about an hour for this. She has had no accidents in a whole year! This procedure was the best thing that could have happened for my daughter.
Sawin et al. 108
Theme 6: challenges of self-catheterisation
Subthemes (6a): independence and dependence
Edwards et al. 106 reported respondents discussing the perception that self-care was a ‘burden’, alongside allowing greater independence in caring for one’s own bladder. This conflict of independence and dependence was also reflected in Sawin et al. :108
At one point when he became a teenager, he finally told us he couldn’t understand why we had to do the bowel program. He decided to do the bowel program himself, which meant he didn’t do anything. He thought if he didn’t do it, it would eventually happen on its own. But it doesn’t work that way.
Sawin et al. 108
I think [in the past] she has really isolated herself because she was concerned about continence. But as she gets older. . .like this weekend she took a friend to the game. I don’t think she would have done that a few years ago. She got up in the morning and did the bowel program so she could be extra safe.
Sawin et al. 108
This was further reflected in Sanders et al. ,107 who reported that parents also spoke about how achieving a more predictable bowel-emptying routine had increased their confidence about their child being at school. Several parents reported that their child talked positively about being clean:
It’s quite a basic thing, he feels much better about it (not soiling).
Sanders et al. 107
Sanders et al. 107 further reported that:
Several of the mothers spoke about how not soiling had allowed their daughter’s to wear ‘normal’ knickers like their school friends and as a result of no longer needing to wear pads some children were able to engage in new activities, such as swimming.
Sanders et al. 107
Theme 7: parents’ investment in their child’s bowel management
Subtheme (7a): confidence, knowledge and experience
Sanders et al. 107 reported how parents shared experiences of trying different treatments, learning how to use TAI, encouraging their child to try it and being proud of new skills acquired. There were extremely negative experiences when management went wrong, which parents described as ‘horrific’, ‘horrible’ and ‘just dreadful’. However, some positives were also reported:
A couple of parents reported being proud of their new skills, how they negotiated undertaking the irrigation with their child and the direct impact it had on their child no longer being incontinent of stool.
Sanders et al. 107
Subtheme (7b): support from professionals
Sanders et al. 107 also reported how participants discussed the value of health-care professionals’ experience in teaching parents to use TAI helped with confidence. Many parents spoke about the positive training and support they received from nurses:
We got a lot of support from the nurses, a lot of training which gave us the confidence to do it.
Sanders et al. 107
This was also reflected in the study by Sawin et al. ,108 in which a parent talked about the impact of one nurse:
I wish I had known her [nurse] when [child] was a baby, cause maybe the outcome would have been different . . . I always felt like she understood what families were going though, especially the patient, and worked hard to achieve bowel continence . . . I found her to be extremely helpful and sensitive in an area where it could be very uncomfortable to talk about.
Sawin et al. 108
Subtheme (7c): emotional stress and strength
Sanders et al. 107 reported that parents were concerned about social difficulties, such as ‘soiling in the classroom’ or ‘still wearing a nappy’ for their children at nursery and school. Similar to subtheme (3a), overwhelming stress, identified by Sawin et al. ,108 many factors were identified as influencing parental decision-making, such as parent–child conflict, the need to encourage children to become independent, the perception of positive affect on peer relationships, and the degree of emotional resilience needed to continue with TAI trials. For some parents, the decision to continue with TAI placed a strain on their relationship with their partner:
Irrigation became, even between my husband and I, a very, very, big thing. [We had to decide if] we could carry on.
Sanders et al. 107
Theme 8: supporting their child’s independence
Subtheme (8a): stressful experiences
As above, Sanders et al. 107 identified that parental stress was common among respondents, with the example given of the trial of oral treatments:
They worked extremely well but it was a nightmare, just an absolute nightmare, uncontrollable, liquid it was horrific beyond anything, it was just dreadful.
Sanders et al. 107
Subtheme (8b): independence and dependence
Independence compared with dependence was a common theme across all three papers reported here [see Subtheme (3b): parents’ role negotiation and Subthemes (6a): independence and dependence]. Sanders et al. 107 were able to reflect the discussion around parents’ perception of the ‘bureaucracy’ of getting other people to help children with TAI if they are never likely to be fully independent:
It’s stressful when you know you are the only person who can do it. Like I’m her lifeline and if something goes wrong then it can be quite dangerous if only one person can do it.
Sanders et al. 107
If you are going to use it [irrigation] and never be independent with it, it’s what do you do about other people doing it because it’s not simple? It’s the bureaucracy of getting other people to do it. He is going to respite for a week so I’ve persuaded, bless them, my district nurses to go up and do it out of their area. Well honestly I can’t tell you how hard that’s been to organize and it’s only due to their kindness that that’s happened.
Sanders et al. 107
Subtheme (8c): concerns for the future
Sanders et al. 107 found that the tension between dependence and independence was also the focus of concerns for the future and was something raised by respondents, with parents discussing the need to strike a balance between relieving children of the responsibility for their own bowel care and encouraging them to be self-reliant. This tension increased with the age of the child. Although two parents, one mother and one father, acknowledged that they should encourage their adolescent to manage TAI independently, they believed that their help resulted in the procedure being completed quickly, which left their teenagers with more free time for other activities. They were also more confident that TAI had been done correctly. On reflection, this mother wondered if routine played a part in her son ‘not managing it’ himself: ‘he is quite happy for me to do it at the moment because I’ve always done it for him.’
The reviewers also identified four new concepts: the value of understanding, the value of peer support, the importance of self-image and the relevance of the place of learning.
Synthesis of qualitative and quantitative evidence
Using the interweave method of data synthesis,34 we used the intersubjective questioning approach to interrogate the evidence and draw links between and across both the qualitative and the quantitative evidence identified in this systematic review. Synthesis was structured across the four review questions using a question-and-answer approach. Starting with question 1, we assessed the evidence for information that had informative potential, or might reveal areas of dissonance or silence in the identified evidence, as shown in Table 12. As qualitative evidence pertained solely to children and young people with spinal cord pathologies, we were unable to synthesise evidence between qualitative and quantitative studies on populations with non-spinal-cord-related conditions. Therefore, all of the following synthesised evidence relates solely to those with spinal cord pathologies.
| Evidence description | Review questions | Type of included evidence | Synthesis method |
|---|---|---|---|
| Effectiveness and cost-effectiveness of interventions (direct and indirect outcomes) |
Q1: What is the effectiveness of interventions to improve continence in children and young people with neurodisability? Q2. What is the cost-effectiveness of interventions to improve continence in children and young people with neurodisability? Q3. What are the factors that may enhance, or hinder, the effectiveness of interventions and/or the successful implementation of interventions to improve continence in children and young people with neurodisability? |
Quantitative evidence from 64 controlled trials, before-and-after studies, cohort studies, case–control studies and comparative case reports. No evidence to inform assessment of cost-effectiveness of interventions was identified | Narrative synthesis |
| Experiences of interventions | Q4. What are the views, experiences and perceptions of children and young people, their families, their clinicians and others involved in their care of delivering and receiving such interventions? | Qualitative evidence from three studies using qualitative data collection and synthesis | Framework analysis and narrative synthesis |
The majority of evidence was identified from 68 effectiveness studies reporting quantitative outcome data, with only three studies reporting qualitative experience of interventions. This limited the extent to which intersubjectivity could be assessed across quantitative and qualitative evidence, as, although the qualitative evidence was rich in content, the total pool of evidence was substantially smaller than the weak- to moderate-quality quantitative evidence.
The following questions arose as part of the interweave synthesis.
Is there any evidence for interventions allowing for relationships with health-care professionals?
Insufficient data were found in the quantitative evidence to address this question.
Is there any evidence for interventions taking account of the child’s experience of the intervention?
We found substantial evidence covering the use of a stepwise bowel management programme, an intraurethral self-retaining device, TAI and bladder neck sling procedures. One study59 reported interventions targeted at children with spina bifida. This was a before-and-after study design with a population of 53 participants with ages ranging from 3 to 15 years. The study reported the results of a stepwise bowel management programme using clinical efficacy and Faecal Incontinence and Constipation Quality of life (FICQOL) as outcome measures. QoL scores all improved, with impact of travel and socialisation (mean score before of 23.5 to mean score after of 9.3; p = 0.006) and caregiver support and emotional impact (mean score before of 12.7 to mean score after of 9.0; p < 0.001) showing statistically significant decreases. Non-statistically significant decreases were observed in ratings for family relationships (mean score before of 3.9 to mean score after of 2.21; p = 0.265) and financial impact (mean score before of 1.7 to mean score after of 1.0; p = 0.071).
One study69 assessed the effectiveness of an intraurethral self-retaining device with neurogenic bladder. Improved QoL scores indicated that the intraurethral self-retaining device significantly reduced how urinary incontinence affected QoL in both children and adolescents (p < 0.0001). Five studies62–64,78,90 focused on TAI using Peristeen in children and young people with spina bifida. One study90 reported results of the use of TAI (Peristeen) for faecal incontinence in 24 children and young people with neuropathic bowel and anorectal malfunctions (ARMs). The study also reported improved QoL scores from a median (aggregate) score of 40.5 before the intervention to 51.5 after (p < 0.0001). Ausili et al. 62 assessed QoL according to Neurogenic Bowel Dysfunction score and reported improvements in general satisfaction (mean score before of 3.0 to mean score after of 7.7; p < 0.001) in children and young people following the TAI intervention. Choi et al. 64 assessed the effectiveness of TAI for children with spina bifida, and reported improvements over a 3-year follow-up period in QoL scores across travel and socialisation (mean score before of 13.1 to mean score after of 8.8; p = 0.002), caregiver support (mean score before of 13.0 to mean score after of 9.1; p < 0.001), family relationships (mean score before of 3.8 to mean score after of 2.3; p = 0.035) and financial impact (mean score before of 1.6 to mean score after of 1.2; p = 0.22).
One study investigated the use of TAI in managing faecal continence in children with spina bifida, and reported QoL outcomes using the Faecal Incontinence Quality of Life questionnaire. 78 Across the four domains (i.w. lifestyle, coping/behaviour, depression/self-perception and embarrassment), no difference was found between participants who underwent the intervention and those in the control group. Individual domain data were not reported. Ausili et al. 63 investigated the effectiveness of TAI in children with spina bifida and ARMs, and reported QoL scores (using CHQ-PF50) relating to the child’s experience. The need to be assisted by a caregiver was reported by 47% of children with ARM and 69% of children with spina bifida at a mean follow-up time of 28 months. QoL scores were assessed by parents of participants, and showed improvements for children with ARM (average improvement from baseline to ≈ 24-month follow-up 18.8; p = 0.04) and spina bifida (average improvement baseline to follow-up 13.3; p = 0.664) in self-esteem, general health perception (average improvement baseline to follow-up ARM 3.3, p = 0.44; average improvement baseline to follow-up spina bifida 4.9, p = 0.154) and mental health (average improvement baseline to follow-up ARM 11.9, p = 0.09; average improvement baseline to follow-up spina bifida 14.3, p = 0.07).
One study reported bladder neck sling procedures, with augmentation in some cases, for children and young people with spina bifida. 76,84 Authors reported improved continence rates and QoL scores across overall health and increased ability to participate in social activities.
Is there any evidence for interventions accounting for the place of the family in bowel and continence management?
One study57 reported findings from a RCT assessing the effectiveness of filum section and medical therapy for a population of children and young people with occult tethered cord syndrome. The PEMQOL Child and Family Impact Scales improved modestly in both groups across all subscales of child impact, family impact, coping, child commitment and family cohesion, although all differences were not statistically significant.
One study reported on a TAI intervention targeted at children with spina bifida. 59,80 This showed statistically significant decreases in caregiver depression (mean scores 1.7 to 1.0; p = 0.001), anxiety around faecal continence (mean scores 1.9 to 1.2; p < 0.001) and ‘bothersomeness’ relating to child bowel problems (mean scores 2.2 to 1.0; p < 0.001). Note that the mean scores are assumed, as these were not reported by the authors.
Ausili et al. 63 reported QoL scores (assessed using the CHQ-PF50) in parents of children with spina bifida and ARM undergoing TAI, and reported long-term improvements across measures of parental impact – emotional (ARM mean score 9.6, p = 0.29; spina bifida mean score 21.1, p = 0.08), parental impact – time (ARM mean score 11.1, p = 0.2; spina bifida mean score 14.9, p = 0.19), family activities (ARM mean score 26.9, p = 0.19; spina bifida mean score 23.6, p = 0.05) and family cohesion (ARM mean score 2.8, p = 0.11; spina bifida mean score 1.6, p = 0.11).
When identifying evidence for interventions addressing child development and independence, one study61 reported the results of an intervention using the MACE procedure to improve continence in children and young people with spina bifida. Self-reported independence improved, and incontinence episodes reduced in most (16 out of 19) participants. All participants reported improvement in problems related to constipation. Measures of self-esteem showed improvement post intervention, and psychological problems reduced.
One study reported a TAI intervention that was targeted at children with spina bifida. 59,80 Travel and socialisation scores showed that, after children underwent a stepwise bowel management programme, there was a consistent decrease in both parents and children being concerned about leaving the house because of problems with faecal incontinence. Scores also showed reductions in the impact of faecal incontinence on children’s ability to socialise and make friends, and to take part in physical activity such as walking and sport.
Ausili et al. 63 reported on the need to be assisted by a caregiver and QoL scores relating to independence. The need to be assisted by a caregiver was reported by 47% of children with ARM and 69% of children with spina bifida at a mean follow-up time of 28 months. QoL scores (assessed using the CHQ-PF50) were assessed by parents of participants, and were reported by 47% of children with ARM and 69% of children with spina bifida at a mean follow-up time of 28 months. QoL scores (assessed using the CHQ-PF50) were assessed by parents of participants, and showed improvements across all measures for children with ARM in role/social limitations – emotional/behavioural (mean improvement in score of 3.6; p = 0.80), physical (mean improvement in score of 3.8; p = 0.28), and for children with spina bifida in role/social limitations – emotional/behavioural (mean improvement in scores of 12.0; p = 0.11) and physical (mean improvement in score of 12.1; p = 0.01).
Is there any evidence for interventions addressing the challenge of self-catheterisation?
Evidence for interventions addressing the challenge of self-catheterisation was found in three studies. One study53 reported the results of a RCT investigating the efficacy of transcutaneous functional electrical stimulation (FES) in children with refractory neuropathic urinary incontinence secondary to myelomeningocele. Children in the treatment group improved in their daily incontinence score (range 0–3, with 0 indicating most continent) from a mean of 2.7 (SD 0.4) before treatment to a mean of 1.3 (SD 0.9) after treatment compared with the sham stimulation group (p = 0.02). Three out of 15 patients in the treatment group became completely dry between two consecutive CICs. In four participants in the treatment group, their daily incontinence score improved from 3 to 1 at follow-up. Two out of 15 patients in the treatment group and 8 out of 15 patients in the control group had an unchanged daily incontinence score. Pad-changing frequency significantly decreased in the treatment group compared with the control group. Pad-changing frequency decreased from before FES therapy (mean 5.2, SD 1.6, times per day vs. mean 4.3, SD 1.7, times per day) to after FES therapy (mean 2.4, SD 1.4, times per day vs. mean 3.5, SD 1.6, times per day) at follow-up (p = 0.03).
One study compared CIC with manual expression combined with drug treatment in children and young people with congenital neuropathic bladder. 89 Thirteen out of 22 children (60%) in the CIC group became continent. In the control group, 1 out of 21 participants (5%) became continent (p < 0.001).
For children with mixed spinal pathologies who underwent the Pippi Salle procedure,103 daytime continence (≥ 3 hours) was achieved in 11 out of 18 children (61%). Eight out of 18 participants (44%) were continent overnight, with eight remaining completely incontinent during the night. Twelve children needed additional drug therapy (oxybutynin) to maintain continence success. Children had difficulty with catheterisations (4/5) and pelvic abscess (1/5). Seven children (39%) required further operations.
Is there any evidence for interventions incorporating parental investment in bladder or bowel management?
Finally, evidence for interventions incorporating parental investment in bowel management was found in two studies. One study39 reported outcomes of an intervention focused on the use of a wireless moisture pager as part of a prospective RCT addressing continence in children with autism. This trial had 33 participants with ages ranging from 36 to 72 months. Participants were allocated to the wireless moisture pager arm or the standard behavioural treatment arm, and all participants in the wireless moisture pager arm showed improvements in levels of daily training, rates of accidents and urination success rates, although these differences were not statistically significant. Parent satisfaction scores did not differ significantly between the intervention and the control groups.
One study focused on a collagen injection intervention targeted towards children and young people (n = 23, age range 6–17 years) with congenital neuropathic bladder. 95 Fifty-six per cent of participants (13/23) had a mean increase in dry time (no urinary incontinence) of 2 hours post intervention, and all participants and their parents from this group were satisfied with the intervention. The remaining 44% (10/23) of participants had an average dry time of 0.2 hours following the intervention, and none of this group or their parents were satisfied. In some participants, initial success was transient and passed after 2–4 weeks post injection.
Discussion
Summary of results
Within the quantitative data, results were mixed. Spina bifida was the most researched condition across a broad age range of 10 months to 25 years, covering behavioural, medical, medically assisted and surgical interventions. Study quality ranged from weak to moderate, with none of the included quantitative studies rated as methodologically strong. We identified three qualitative studies that were rated as methodologically strong.
Most of the evidence (68 studies) we identified related to studies assessing the effectiveness of interventions to improve continence in children and young people with neurodisability. We did not identify any studies assessing the cost-effectiveness of interventions, nor did we identify studies that reported measures in such a way that they could be used as proxy measures of cost-effectiveness, such as parental time. We identified studies reporting outcomes that could form contributory factors to enhance or hinder the effectiveness of interventions and/or the successful implementation of interventions, but it was not possible to draw clear conclusions around the direction or degree of influence of these factors from the data provided. Finally, we identified three studies reporting the views, experiences and perceptions of children and young people and their families around delivering and receiving such interventions, and these proved a rich source of qualitative data.
Comparison with previous literature
A review of evidence around toilet training neurologically able children using behavioural strategies conducted in 2008112 found that published evidence was separable into two different approaches: gradual, child-oriented training, in which caregivers respond to the child’s implicit and explicit signals for ‘readiness’ as described by Brazelton et al. ;113 and structured, end-point-oriented training, in which externally imposed toileting behaviours are taught according to a predetermined arrangement in the style of Azrin and Foxx. 104
The Azrin and Foxx approach – or adaptations of this method – features strongly in the behavioural methods identified in this systematic review as well as in the wider literature. Other authors have posited that this may, at least in part, be due to the authors’ clear description of their method in their original paper, describing a successful toilet training programme for adults with mental disabilities based in state institutions. 114 Their book Toilet Training in Less Than a Day115 has remained in print since its original publication in 1974, and this may also explain the popularity of their approach.
Strengths and limitations
The outstanding limiting feature of this systematic review is the massive heterogeneity found across studies in terms of results, reporting and methodology. With 21 outcomes directly related to effectiveness of interventions to improve continence, all reported in a variety of frequencies (hours, days, weeks) and outcome measures reported using different statistics (ranges, means, frequencies), it was not possible to find sufficiently common measures to combine study results through meta-analysis. This diversity in outcomes and the measures used to report them contributed to results that are both comprehensive and ill suited to directly answer specific effectiveness questions.
Not only are the results of this systematic review heterogeneous, but the methods of reporting are varied and inconsistent. Some included studies provide clear, specific details of interventions, allowing replication, and some studies report simple summary data. The large range of publication years may contribute to the variation in reporting, and more recently published included studies show a trend towards moderate study quality. Study design also has an influence on quality, with included RCTs generally rated as moderate rather than weak quality. Although this is not without exception, the RCTs we rated as being of weak study quality were those published less recently.
It is notable that we did not rate any of the evidence that we identified as methodologically strong. Of the 13 RCTs identified, eight were rated as moderate quality and five were rated as weak quality. Common areas of weakness were blinding and selection bias. We identified a large number of case studies during the screening phases. We had pre-stated in our published review protocol that we would include comparative data only, and, as a large number of case studies reported single cases or fewer than three cases without direct comparisons, these studies were excluded from our review. They may, however, contain insightful detail that would be of value in this area, but the place for this level of evidence is unclear.
Implications for research
It is recognised that many neurological disorders can cause difficulty in achieving urinary and faecal continence,116 but there is a paucity of research on the optimum management and improvement of continence in children and young people with neurodisability.
One review117 of toilet training in children with a diagnosis of autism, mental disability and Angelman syndrome found that most approaches used an adaptation of the Azrin and Foxx rapid toilet training method, with some aspects changed to shorten or simplify the training. The authors suggested that further studies should clarify behaviours pivotal to toilet training success, explore the limits of age and functioning, and review the prerequisite skills needed for toilet training to be successfully initiated. We found two studies focused on people with autism39,40 published since that review was conducted. Some studies included by Kroeger and Sorensen-Burnworth were excluded here, mainly because they were non-comparative single case studies and, therefore, ineligible.
A review of botulinum toxin type A found evidence for this as a reasonable alternative to surgery in the management of intractable overactive bladder in children, although evidence was lacking on dosage, injection site, delivery method and long-term follow-up. 54 Two, more recent, reviews118,119 also suggested that the administration of botulinum toxin may provide an alternative to surgery for children with neurogenic bladder, and may provide an alternative to oral anticholinergic therapy. This was supported in three out of four studies identified in this systematic review, with one study providing less conclusive support for the use of botulinum toxin type A injections.
Two reviews of the use of transanal/transrectal irrigation in children with myelomeningocele120 and children with bowel dysfunction121 supported the use of TAI when conservative methods fail, taking full care to incorporate careful management of patient selection, supervised training, clear follow-up and an individually tailored approach.
There is little published review evidence on the use of anal plugs for children and young people with neurodisability. 122 A 2015 Cochrane Review123 reported some limited evidence that anal plugs, although difficult to tolerate, were associated with reductions in incontinence if tolerated. This review included some small studies that reported benefits from the use of anal plugs as an irregular and complementary approach alongside an established bowel management programme. 58,71,124
This summary of identified evidence illustrates the difficulty of providing clear evidence-based advice about how best to improve continence in children and young people with neurodisability. The overwhelming finding from this review is the paucity of high-quality evidence to inform policy and practice. There are significant weaknesses in most existing studies that reduce their validity and hamper efforts to synthesise findings. Our findings raise a number of issues for future research, some related to generic problems with research quality and reporting and others specific to the area.
Methodological quality and reporting
We used the EPHPP tool31 so that all quantitative studies could be assessed using the same criteria, rather than specific criteria being applied for each form of study. None of the included studies was rated as ‘methodologically strong’, and we found substantial variation in the quality of reporting across all study designs. This could be improved significantly and simply through the rigorous and informed application of appropriate quality guidelines for both the conduct and the reporting of research, such as those found on the Equator network website (www.equator-network.org/).
We suggest that no further studies should be conducted unless they employ rigorous methods and can demonstrate the potential to add value to the current body of research.
Standardisation of terminology
We found broad variation in the terminology used for different conditions, interventions and symptoms. In part this is related to changes over time, and we identified many terms that were obsolete, outdated or region specific, but even recent studies used widely different terms. We encountered significant difficulty in finding common language understandable and relevant to all users of this information, and expended considerable effort simply in navigating the language, requiring repeated recourse to our specialist advisers. Future research in this area would benefit greatly from using common reference points for language and terminology, such as those used in this research, to increase consistency and comprehensibility for all stakeholders. 3,22,23
Using qualitative evidence to underpin intervention design
We found little qualitative research on the patient, parent and carer experience, although what we did find was high quality and rich in data. This type of evidence has a great deal of potential value for assessing the feasibility and acceptability of interventions for improving continence in children and young people. Further high-quality qualitative studies, particularly conducted with patients and carers, would enhance our ability to design interventions and studies likely to meet the needs of, and be valued by, children and young people and their families. For instance, concepts such as the importance of peer support and self-image are widely recognised as important clinically and were also addressed in the limited qualitative evidence base, but we saw little evidence of their incorporation into the design of interventions.
Standardisation of outcome assessment and reporting
We found wide variation in the included studies in the outcomes assessed, the way in which the outcomes were defined and measured, and the clarity of reporting, which inevitably hinders attempts to combine data from different studies. Most of the reported outcomes were clinical or functional, and few studies included patient-reported measures. This reduces our ability to assess the extent to which interventions meet the goals that are important to patients and families. We believe that a clearly defined, core outcome set for continence in children and young people with neurodisability, developed with patients, carers and other stakeholders, would enhance the ability of researchers to provide the evidence needed to enhance practice.
Conclusions
As mentioned in the implications for research above, a core outcome set, such as those supported by the COMET (Core Outcome Measures in Effectiveness Trials) initiative (www.comet-initiative.org/) for continence in children and young people with neurodisability, would bring immense added value to this area in terms of introducing consistency and standardisation to a field lacking these fundamental building blocks. Core outcome sets represent a minimum standard of items that should be measured and reported in clinical trials in this area. These core outcome sets also provide value for routine care, clinical audit and wider research using methods beyond RCTs. These agreed core outcome sets do not place restrictions on what can be measured, but form a framework that allows further work to build on agreed principles. This in turn means that common outcomes can be compared, contrasted and assessed, which would be of great value in reviews such as this.
Better research is needed in identifying qualitative evidence, and conducting quantitative evidence designed with stakeholders at the centre, to give the patient, carer and professional a clear voice. Current evidence is not always consistent in the way these individuals are represented, and there is much that can be improved. More public and patient involvement is needed to ensure that the right questions are being asked in the right way.
Specific questions that remain to be addressed are:
-
What is currently achievable with different conditions? The aim here is to help manage expectations and reduce assumptions made by all involved.
-
Where is the evidence lacking, and why?
-
Where are the cost-effectiveness data lacking?
-
Are there conditions and categories where the questions addressed were not valid (i.e. could not be answered, were not relevant or were rendered obsolete by current practice)?
Changes from the protocol
There were two deviations from the published protocol. Double data extraction was not conducted on quantitative and qualitative data owing to a lack of resource. Instead, all data extractions were checked independently by a second reviewer (BW). We included studies from the Islamic Republic of Iran, although we had stated in the protocol that we would include only OECD countries. When screening, these studies had been eligible in all other aspects and we made the judgement that excluding on the basis of OECD status would exclude potentially useful evidence, so these studies were included.
Chapter 4 Cross-sectional survey of current NHS practice
The aims of the surveys were to describe current clinical practice in the NHS, to find out how and when NHS staff assess and treat children with neurodisability to help them become continent, and to gather families’ and carers’ experiences. Four separate online surveys were conducted in parallel with (1) health professionals, (2) school and social care staff, (3) parent carers and (4) children and young people with neurodisability. The survey addressed the following research questions:
-
How do clinicians assess the bladder and bowel health of children and young people with neurodisability, their continence capabilities and their readiness for toilet training? Which clinicians are involved in assessments?
-
Which interventions do clinicians use or recommend to improve continence in children and young people with neurodisability and how are these interventions individualised and evaluated and/or audited? Which clinicians recommend, deliver or evaluate interventions?
-
How do families, school and social care staff consider and judge children’s readiness for toilet training and need for specialist assessment and/or interventions?
-
Which factors affect the implementation of interventions to improve continence, and what is the acceptability of strategies to children and young people and their carers?
Methods
Ethics approval
Ethics approval was obtained in two stages. First, we sought approval for the recruitment strategy. All recruitment and public-facing study documents and materials were produced. These included participant information sheets, website content, a video and tailored adverts. All of these materials benefited from iterative feedback from our Family Faculty group and/or Professional Advisory Group before they were finalised. Securing ethics approval for recruitment meant that we could expediently advertise the survey by making contact with professional and charitable organisations and using social media while still preparing the questionnaires.
Second, we focused on devising each of the four survey questionnaires in collaboration with members of the study team, our Family Faculty group and our Professional Advisory Group. This required considerable time and piloting with representatives of each proposed participant group. Once we were satisfied with the survey questions and response options, we were able to seek and secure ethics approval for the survey data collection to commence. Participation in the survey was voluntary and responses were pseudonymous; online registration for the survey implied that participants had read and understood the participant information. The University of Exeter Medical School Research Ethics Committee approved the study (reference UEMS REC 19/B/199). The study protocol (HTA 17/20/02 version 1.3, April 2019) was published in the NIHR Journals Library (www.journalslibrary.nihr.ac.uk/programmes/hta/172002; accessed October 2021).
Survey methods
The use of online surveys in research has developed as technology has advanced. The major strengths of an online survey lie in the potential global reach, flexibility, speed, convenience, diversity, researcher control, and ease of data entry and analysis. 125
The survey was designed with two stages; first, a registration stage and, second, a data collection stage, achieved by sending registrants the link to the questionnaires (Figure 3). This was to (1) expedite starting recruitment and (2) ensure project quality assurance, by the team knowing to whom questionnaires were sent, as opposed to an open survey link that anyone could access and input data. With this process, we were also able to the modify recruitment strategy by attempting to reach any less represented groups or areas, and send reminders to those who registered.
FIGURE 3.
Survey process.
Recruitment and registration
Recruitment was centred on the study website (http://sites.exeter.ac.uk/iconstudy/taking-part). The landing page of the survey provided links to the participant information sheets, a list of frequently asked questions and the online registration form. Easy-read versions of the information sheets were also provided. Participants were invited to register to participate in the survey by providing their name and e-mail address, which survey group they were registering for (health professional, parent/carer, school and social care staff, or young person), and their geographical region from a drop-down selection. Our geographical scope was England. However, we recorded any responses to our surveys that were received from participants in devolved UK countries or from outside the UK. Health professionals were asked to state their role as free text. The form was hosted by Wufoo (www.wufoo.com/) and embedded in the WordPress study website.
Survey adverts and e-mail messages were tailored to specific survey groups (e.g. health professionals). Wherever feasible, we sought to place a link to the registration page within adverts, and tried to place adverts appropriately to reach people directly from trusted sources. We contacted over 100 societies, charities and organisations to ask them to share the advert with members, supporters or followers (see Appendix 7). Organisations were asked to share the advert via social media, mailing lists and other mailings or newsletters; information was also shared regularly by the ICoN study team via Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) and Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com).
Some professional contacts were personally facilitated by members of our study team (e.g. British Association of Paediatric Urologists, British Association of Paediatric Urology and Continence Nurses, School And Public Health Nurses Association, British Association of Paediatric Surgeons, Bladder & Bowel UK, ERIC, Paediatric Continence Forum, British Academy of Childhood Disability, British Association for Community Child Health, Association of Paediatric Chartered Physiotherapists Neurodisability Group, Royal College of Occupational Therapists Specialist Section – Children, Young People and Families). Payment was given to the British Academy of Childhood Disability to e-mail their list of contacts in child development teams. We also used social network sites to link with health professionals through online communities (e.g. several of the WeCommunities).
To invite parent carers to register to take part in the survey, we contacted the National Network of Parent Carer Forums and many of their constituent local Parent Carer Forums, of which there is one in almost every local authority area in England. We contacted many disabled children’s charities (e.g. Council for Disabled Children, Contact) and condition-specific charities (e.g. National Autistic Society, Action Cerebral Palsy). In an effort to increase the number of participants from minority ethnic communities, we targeted Parent Carer Forums in areas with higher proportions of ethnic diversity and the Include Me TOO, a national charity supporting disabled children, young people and their families from diverse range of backgrounds. Our family involvement co-ordinator undertook considerable work using Facebook to target parent carers through online communities. Organisations were encouraged to share the opportunity to take part with their members and via e-mails, newsletters and social media.
We invited children and young people to take part through contact with PenCRU’s whole Family Faculty, national school and college contacts, via social media groups such as Health Unlocked, and through charitable organisations such as KIDS. We also asked parent carers whether their child might want to participate in a survey. We encouraged parent carers to help their child complete the young person’s survey or complete it on behalf of their child.
To reach school staff, we contacted a number of special schools, school academies and further education colleges, including independent groups of special schools such as Pace schools, the SENAD Group and the Seashell Trust. We also linked with the National Association of Independent Schools & Non-Maintained Special Schools and the Schools and Students Health Education Unit. To reach carers, we contacted 48 individual local authority special educational needs contacts, and the Social Care Institute for Excellence. Adverts were placed in the Team around the Family monthly bulletins and on the CHAIN NHS network.
Registration data were downloaded weekly from Wufoo and initially stored in a Microsoft Excel spreadsheet. These data were then uploaded into the Online Surveys (Jisc, Bristol, UK) system database, from which individualised invitations to complete one of the four questionnaires were sent. The registration process enabled any duplicated registrations to be identified, as each e-mail address has to be unique in the Online Survey software.
In terms of sample size, from a pragmatic perspective, we aimed to recruit 100–200 parent carers and around 200–300 health and care professionals. We monitored the registering participants using registration characteristics and strategically targeted advertising more purposefully to engage any particular groups that appeared under-represented. The sample we aimed to recruit was to provide an insight and help us to understand current clinical practice, but it was not expected to be representative of specific professional or demographic groups.
Survey questionnaire development
Survey questions and response options were developed for each of the four surveys in collaboration with the study team, our Professional Advisory Group and our Family Faculty group, and a small number of young people with neurodisability. Each survey was improved through a number of iterations before being piloted by members of each stakeholder group. In designing questions and response options we sought to link with the approaches described in our conceptual framework. Questionnaires were initially worked on as offline documents and only once we had produced near-final versions were they transferred to the Online Survey system to check format and functioning.
The health professional survey questions were initially developed from an activity at our first whole-team meeting and after subsequent consultation with our Professional Advisory Group. Members of our team proposed questions that they felt reflected the study research questions, and collectively discussed the potential response options or answers that they might expect to see for each question. The study researchers then created draft questionnaires. In line with the study conceptual framework, the questions were designed around the four case scenarios, with responders asked to identify which clinical case scenarios they worked with. Following piloting, it was felt that this approach created a repetitive and lengthy survey. Consequently, after discussion, and taking account of how different children with neurodisability are assessed and treated, the case scenarios were condensed into two groups, defined by whether or not there was likely to be spinal cord pathology affecting sensorimotor control of bowel and bladder function. Thus, non-spinal-cord-related conditions were those with behavioural difficulty, learning disability or physical or movement disability; and spinal cord pathology were those with a bladder and/or bowel impairment due to damage to the spinal cord, such as spina bifida. The survey structure and questions were redrafted to reflect these two groups, and participants were given the option to complete questions relating to only one or to both of the groups. Members of the Professional Advisory Group piloted the questionnaire to estimate the time required to complete the survey.
The parent carer survey questions were initially developed in a similar manner. Members of our team and the Family Faculty group were asked to consider the study research questions and to propose survey questions and potential response options. These suggestions were collated into a draft survey, which members were then able to pilot and comment on. Members of the Family Faculty group provided further feedback on the language, formatting and accessibility of the survey. Comments and suggestions were incorporated and the survey was redrafted. Three parent carers piloted the final version so that we could advise participants how long it would take to complete.
The questions for the school and social care staff survey were developed following the initial conversations among our team and with the Professional Advisory Group and Family Faculty group. Questions reflected the ‘toileting needs’ outlined in the conceptual framework and were mapped against the study research questions. The survey was piloted by members of the study team and a teacher at a local school for children with special educational needs.
The survey questions for young people were developed in collaboration with parent carer members of the Family Faculty group and two young adults with neurodisability. These discussions suggested that children and young people should be able to choose from a rated scale response option, and that, where possible, smiley face-type images were preferred over word descriptions. The draft questionnaire was presented to a group of young people at a local school for children with special educational needs who provided feedback on the structure, design, concept and language used. Once these amendments had been incorporated, the same two young adults with neurodisability piloted the survey online and provided feedback and information on the time it took to complete.
Minor amendments were made to each of the surveys following the final piloting stage to ensure that any errors were addressed and that the surveys captured as much relevant information as possible. All four surveys comprised a mix of structured rating scale response options, single answer or multiple answer response options, and some open free-text response boxes. The survey questions were then mapped against the study research questions to ensure that they were relevant to the aim of the study (see Appendix 8). The formatting of the surveys was checked across different devices (e.g. tablet, phone) and common internet browsers.
Structure of the surveys
Health professional survey
In the health professional survey, respondents were asked to choose the clinical group about which they would like to complete the survey: non-spinal cord related (children and young people with a social/communication or attention/behaviour difficulty, learning disability or physical or movement disability), spinal cord pathology (children and young people with bladder and/or bowel impairment due to damage to the spinal cord) or both (children and young people who fitted into both the non-spinal-cord-related and the spinal cord pathology group).
Those who reported for the non-spinal-cord-related and spinal cord pathology groups were clearly indicated in the screening question and answered a set of 14 clinical questions with that group in mind. Those who chose ‘both’ answered the same set of 14 questions separately for each of the two clinical groups. There were also a series of questions relating to service provision and demographic questions, which all respondents were asked to complete. Figure 4 shows a map of the health professional survey questionnaire.
FIGURE 4.
Map of health professional questionnaire.
Parent carer survey
Parent carers were also asked which clinical group the child they cared for fitted into: non-spinal-cord-related pathology, spinal cord pathology or both. Those who indicated that their child fitted into both clinical groups were asked to specify the child’s diagnosis or condition. The responses specified were then allocated by clinical members of the study co-investigator team into one of the groups. Parent carer respondents who did not specify a diagnosis were excluded from the analysis. Parent carers were asked five demographic questions, followed by 18 clinical questions relating to their child and the service provision with which they had experience.
School and social care staff survey
For school and social care staff, the questionnaire did not distinguish between clinical groups, although respondents were asked to indicate if they had experience of these groups as part of their role. School and social care staff were asked 12 questions relating to provision for children in their care, followed by four demographic questions.
Young person survey
The survey questions for children and young people also did not distinguish between the two clinical groups. Young people were asked six demographic questions, followed by five questions regarding their toileting ability and help they had received. For these questions, the respondents were asked to choose a face symbol (from smiling to sad) to represent how they felt.
Survey administration
The Online Surveys system enabled us to send each registrant a personalised invitation with a unique link to the survey relevant to them. We monitored when completed questionnaires were submitted. Up to four reminders were sent to those who had not submitted responses to encourage completion. Undelivered e-mails were most commonly a result of a misspelling at the registration stage; some of these were corrected and resent, but not all. Unique identifier numbers were allocated to every survey response submitted. The personalised link for each participant was coded so that when the participant submitted their survey responses, all of their identifiable data (e.g. e-mail address) were removed from the output when downloaded.
We used several strategies for maximising responses to surveys, including advance priming (through registration), sending reminders, confirming the confidentiality and anonymity of any data in any reports and publications, seeking to develop a rapport, ensuring a professional and well-formatted questionnaire, and offering to provide participants with a summary of findings.
Following confirmation of ethics approval of the questionnaires, all four surveys were opened on 7 July 2019. This was slightly later than our original timeline and coincided with the summer holidays. Therefore, we relaunched the surveys again in September 2019 with another wave of advertising. Registration for the survey for health professionals and parent carers closed on 31 October, although 10 days were allowed for the completion of surveys, and, therefore, registration was completely closed on 10 November 2019. Registration for the survey for school and social care staff and the survey for young people was closed on 29 November 2019, and completions were closed on 9 December 2019.
Data analyses
The numbers of registrations and completed surveys were monitored and recorded throughout the period that the surveys were open online. The total numbers and percentages of completed surveys are reported. This is based on the number of personalised links sent (denominator), and the number of completed surveys returned (numerator). The numbers and percentages of surveys that were returned after no reminders and after each of the four reminders are also reported.
For each of the surveys, numerical and free-text data were exported from the Online Survey system into a Microsoft Excel file. This file included a single row for each respondent and a column for each response option (coded numerically in the survey software) for each question in the survey, unless the question was a single-answer response from a list of options, in which case the response for that question was in a single column. The quantitative data were transferred to R (version 3.6.2; The R Foundation for Statistical Computing, Vienna, Austria) statistical software for analyses. We received a considerable number of descriptively rich free-text data; quotations from the free-text responses were selected to illustrate salient results from the quantitative analyses. Demographic characteristics of respondents for the surveys were summarised in terms of numbers and percentages for each categorical variable.
The number of respondents who completed each survey question is reported. The results are reported as the number and percentage of respondents who chose each of the response options. Where responses to questions were reported by different respondent groups, the number of respondents who completed the question in each of these groups is reported, and results are the number and percentage of these respondents who chose each of the response options. Survey questions about the respondent’s opinion or experience of methods and interventions include one or more of the four options: ‘don’t know’, ‘never used’, ‘no knowledge’ or ‘never happened to me’. For these questions, percentages reported in the tables of results (see Appendices 11–12) include all possible response options. To describe the pattern of responses among those respondents who were able to comment on their opinion or experience of the method or intervention, percentages reported in the narrative of the results are based only on those respondents with experience. The figures reported in the narrative are therefore adjusted, and those who answered one of the four options above have been removed.
Where necessary, response options and free-text responses were aggregated for analyses and reporting. For example, health professional job roles are grouped into fewer job families, and conditions or diagnoses of children in the parent carer survey are grouped into non-spinal-cord-related pathology or spinal cord pathology.
The results from the health professional survey are reported by job family (e.g. nurse, paediatrician) and for each clinical group (non-spinal-cord-related or spinal cord pathology) for each of the clinical questions, and by job family only for the service provision questions. Owing to the small numbers in the individual job roles reporting (e.g. 13 therapists for the spinal cord pathology group questions), the results are intended to provide a snapshot of current clinical practice, and should not be interpreted as representative of all professionals. The results of the parent carer survey questions are reported by clinical group. School and social care survey results are reported for all responders combined (with the exception of one question, which was separated by clinical group). Young person survey results are reported for all respondents combined.
Results
Survey responses
The responses to each of the four surveys are illustrated in the flow charts (Figure 5); 202 responses were received from 352 health professional registrations (57.4%). Among these 202 responses, 91 (45%) health professionals responded without reminders, 48 (23.8%) responded after one reminder, 23 (11.4%) responded after two reminders, 32 (15.8%) responded following three reminders and eight (4%) responded after four reminders. Among parent carers, 605 responses were received from 1028 registrations (58.9%); 340 (56.2%) parent carers responded without reminders, 128 (21.2%) responded after one reminder, 68 (11.2%) responded after two reminders, 48 (7.9%) responded following three reminders and 21 (3.5%) responded after four reminders. Among school and social care staff, 122 responses were received from 202 registrations (60.4%); 72 (59%) responded without reminders, 21 (17.2%) responded after one reminder, eight (6.6%) responded after two reminders, nine (7.4%) responded following three reminders and 12 (9.8%) responded after four reminders. Twenty responses were received from 26 registered children and young people (77%); among these, 13 (65%) responded without reminders, three (15%) responded after one reminder, two (10%) responded after two reminders, and two (10%) responded after four reminders.
FIGURE 5.
Survey response by group.
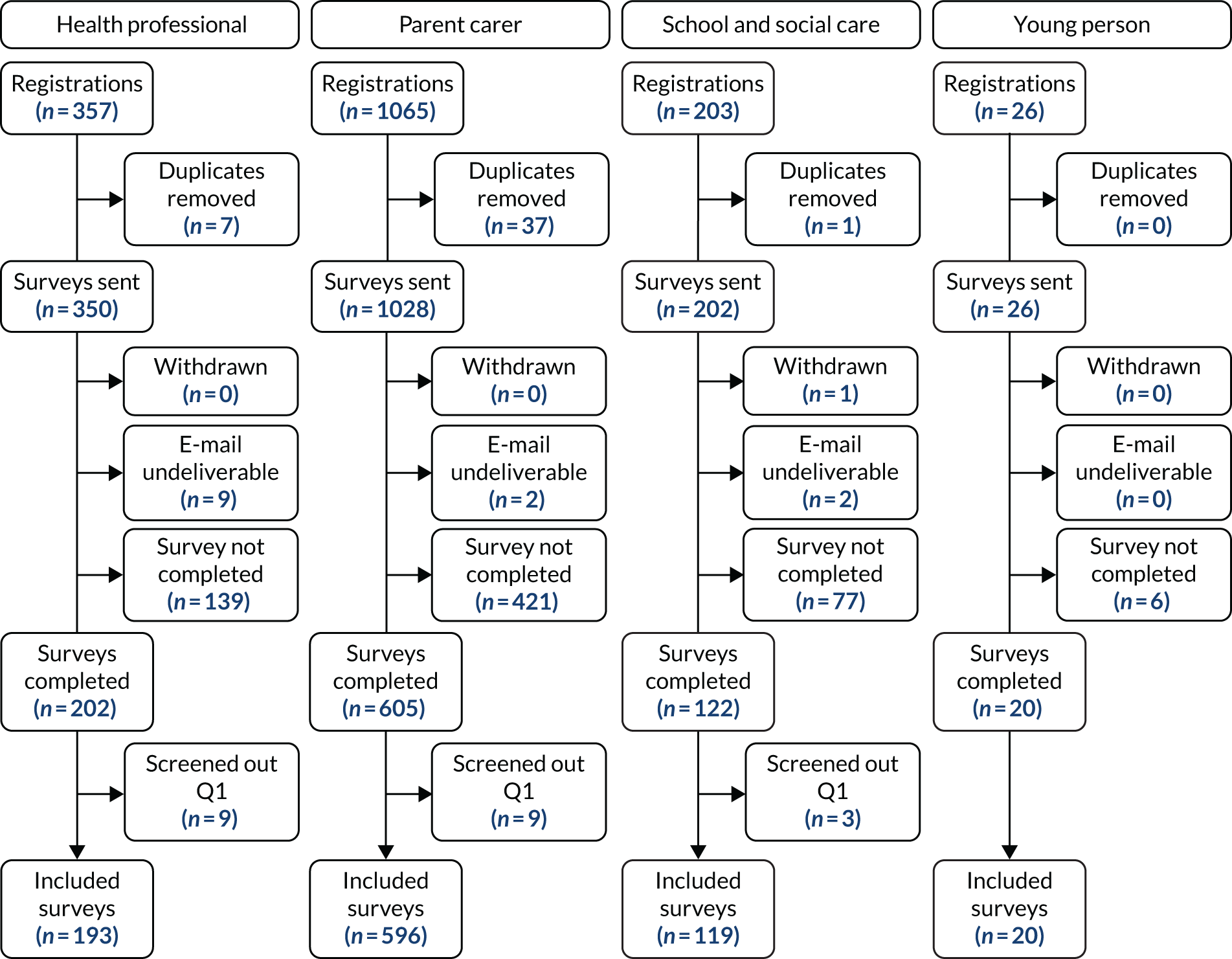
Duplicates (n = 45) were removed after registration and before the survey invitation was sent out; only one participant requested to be withdrawn from the study once they had been sent the survey. Each survey consisted of a ‘screening’ question at the beginning that asked health professionals and school and social care staff to confirm that they worked with children and young people with special educational needs and/or a disability. Similarly, parent carers were asked if they cared for a child with special educational needs and/or a disability. Across the surveys, 21 respondents answered ‘no’ to this question and were screened out of the survey.
Survey responses according to clinical group
Of the 193 health professionals who were included (see Figure 3), 96 reported working with children in the non-spinal-cord-related pathology clinical group, and nine reported that they worked with children in the spinal cord pathology clinical group. Eighty-eight reported working with both groups and answered both sets of questions. Therefore, for analysis, 184 health professionals are included for the non-spinal-cord-related pathology group and 97 health professionals are included for the spinal cord pathology group.
Of the 596 parent carer survey respondents (see Figure 3), 10 answered that their child fitted into neither of the clinical groups and were, therefore, excluded. Of the remaining 586, 518 answered that their child fitted into the non-spinal-cord-related pathology group, nine answered that their child fitted into the spinal cord group and 59 answered that their child fitted into both clinical groups. Of these 59, 52 were able to be allocated to one of the two clinical groups by clinical members of our team, based on the free-text response stating their child’s condition or diagnosis. Therefore, in total, 579 respondents were included for analyses: 559 in the non-spinal-cord-related pathology group and 20 in the spinal cord pathology group.
Descriptive characteristics
Diverse health professional job roles were listed, and a large proportion were listed as ‘other’. The health professional respondents were therefore grouped into six categories of job role for analyses. Respondents who selected ‘other’ were asked to specify their role as free text, and these roles were also grouped into the six categories (Table 13).
| Category | Roles included |
|---|---|
| Bladder and bowel specialist nurse (n = 22) | Bladder and bowel specialist nurses (n = 22) |
| Nurse (n = 79) | Children’s nurse (n = 50) |
| Learning disability nurse (n = 14) | |
| Specialist community public health nurse (school nurse) (n = 11) | |
| Adult nurse (n = 4) | |
| Paediatrician (n = 31) | Paediatrician (n = 29) |
| Paediatric neurologist (n = 2) | |
| Surgeon (n = 15) | Paediatric urologist (n = 9) |
| Paediatric surgeon (n = 5) | |
| Urological surgeon (n = 1) | |
| Therapist (n = 33) | Occupational therapist (n = 28) |
| Physiotherapist (n = 5) | |
| Other (n = 13) | Education, portage-based roles (n = 11) |
| Clinical psychologist (n = 2) |
Of the 579 included parent carer respondents, 522 (90.2%) were the child’s mother (including foster mother, adoptive mother and stepmother); 25 (4.3%) were the child’s father; 15 (2.6%) stated that they were a parent (but did not specify a gender); 10 (1.7%) were a grandparent; and two (0.3%) were a carer. Five (0.9%) did not report their relationship to the child.
Further demographic characteristics of the respondents to each of the surveys are summarised in Table 14. The majority of respondents to the health professional survey were female (81.9%). Most respondents to the parent carer survey were white British, with only 6.4% from mixed, Asian, black or other ethnic minority groups. Over two-thirds (68.4%) of the children about whom the parent carers were completing the survey were male and aged between 5 and 17 years (77%). The majority of respondents to the young person survey were female (70%) and of white ethnic origin (95%), and two-thirds (14/20, 70%) were aged > 18 years.
| A – health professionals | B – parent carers | C – school and social care staff | D – young people | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | (N = 193) | Characteristic | (N = 579) | Characteristic | (N = 119) | Characteristic | (N = 20) |
| Sex, n (%) | Sex of child, n (%) | Sex, n (%) | Sex, n (%) | ||||
| Male | 16 (8.3) | Male | 396 (68.4) | Male | 6 (5.0) | Male | 6 (30) |
| Female | 158 (81.9) | Female | 181 (31.3) | Female | 111 (93.3) | Female | 14 (70) |
| Prefer not to say | 7 (3.6) | Prefer not to say | 2 (0.3) | Prefer not to say | 1 (0.8) | Prefer not to say | 0 |
| Not answered | 12 (6.2) | Not answered | 0 | Not answered | 1 (0.8) | Not answered | 0 |
| Region of England where work, n (%) | Region of England where live, n (%) | Region of England where work, n (%) | Region of England where live, n (%) | ||||
| North West | 21 (10.9) | North West | 40 (6.9) | North West | 10 (8.4) | North West | 3 (15) |
| North East | 12 (6.2) | North East | 21 (3.6) | North East | 4 (3.4) | North East | 1 (5) |
| Yorkshire & Humberside | 9 (4.7) | Yorkshire & Humberside | 31 (5.4) | Yorkshire & Humberside | 5 (4.2) | Yorkshire & Humberside | 2 (10) |
| East Midlands | 9 (4.7) | East Midlands | 32 (5.5) | East Midlands | 2 (1.7) | East Midlands | 0 |
| West Midlands | 7 (3.6) | West Midlands | 53 (9.2) | West Midlands | 3 (2.5) | West Midlands | 0 |
| East Anglia | 9 (4.7) | East Anglia | 26 (4.5) | East Anglia | 2 (1.7) | East Anglia | 1 (5) |
| South West | 39 (20.2) | South West | 166 (28.7) | South West | 60 (50.4) | South West | 4 (20) |
| South East | 26 (13.5) | South East | 123 (21.2) | South East | 22 (18.5) | South East | 4 (20) |
| London | 38 (19.7) | London | 48 (8.3) | London | 6 (5.0) | London | 1 (5) |
| Outside England | 18 (9.3) | Outside England | 28 (4.8) | Outside England | 2 (1.7) | Outside England | 4 (20) |
| Not answered | 5 (2.6) | Not answered | 11 (1.9) | Not answered | 3 (2.5) | Not answered | 0 |
| Age of child, n (%) | Age, n (%) | ||||||
| < 5 years | 68 (11.7) | < 5 years | 0 | ||||
| 5–7 years | 136 (23.5) | 5–7 years | 0 | ||||
| 8–11 years | 153 (26.4) | 8–11 years | 1 (5) | ||||
| 12–17 years | 157 (27.1) | 12–17 years | 5 (25) | ||||
| 18–25 years | 64 (11.1) | 18–25 years | 11 (55) | ||||
| > 25 years | 0 | > 25 years | 3 (15) | ||||
| Not answered | 1 (0.2) | Not answered | 0 | ||||
| Ethnicity, n (%) | Ethnicity, n (%) | ||||||
| White | 540 (93.3) | White | 19 (95) | ||||
| Mixed/Multiple ethnic groups | 13 (2.2) | Mixed/Multiple ethnic groups | 0 | ||||
| Asian/Asian British | 10 (1.7) | Asian/Asian British | 0 | ||||
| Black/African/Caribbean/Black British | 8 (1.4) | Black/African/Caribbean/Black British | 0 | ||||
| Other ethnic group | 6 (1.0) | Other ethnic group | 1 (5) | ||||
| Not answered | 2 (0.3) | Not answered | 0 | ||||
The majority of respondents to the school and social care staff survey were female (93.3%), and over two-thirds indicated that their role was in a mainstream (25.2%) or special school (42.9%). Other settings included a short-break facility (5/119, 4.2%), community care (6/119, 5%), primary care (1/119, 0.8%) or ‘other’ setting (25/119, 21%). Job roles reported by school and social care staff included manager (19/119, 16%), assistant (28/119, 23.5%), teacher (29/119, 24.4%), enabler (5/119, 4.2%), therapist (1/119 0.8%) and other role (36/119, 30.3%).
All geographical regions of England were represented in the health professional, parent carer and school and care staff surveys, although comparatively fewer respondents were from the Midlands (< 10% in all surveys). The young person’s survey had no respondents from the Midlands.
In the sections that follow, results from the health professional and parent carer surveys are described in the context of each clinical group, either non-spinal-cord-related conditions or spinal cord pathology. The results from the school and social care staff and the young person surveys are reported separately and do not distinguish between clinical groups. For each survey, the number of respondents varies across the questions, as not all respondents answered every question in the survey. All data related to the following results can be found in Appendices 11–12.
Non-spinal-cord-related conditions: children and young people with a social/communication or attention/behaviour difficulty, learning disability or physical or movement disability
Health professionals on non-spinal-cord-related conditions
In total, 184 health professionals reported about children and young people with non-spinal cord conditions. Across all professional groups, respondents stated that children and young people with non-spinal cord-related conditions are usually referred for professional toileting advice and support via another health professional, and that they most commonly see children and young people with non-spinal cord-related conditions either weekly (102/183, 56%) or monthly (44/183, 24%) (see Appendix 9, Tables 29 and 43).
Assessment of bladder and bowel health
The most common factors that triggered a professional to assess the bladder and bowel health of children and young people with non-spinal-cord-related conditions were a perceived delay in achieving independent toileting, constipation, daytime and night-time wetting and soiling. These factors were reported as key triggers by at least 14 of the 18 bladder and bowel specialist nurses, at least 52 of the 77 nurses and at least 19 of the 31 paediatricians. These triggers were also frequently reported by surgeons (at least 8 of the 12 surgeons), apart from night-time wetting (which was reported by only 4 of the 12 surgeons). Less than one-quarter of all professionals (42/183, 23%) stated that a bladder and bowel assessment was routine for children in this group. Over half of the professionals (97/183, 53%) said that after the initial assessment they typically reassessed the patient at every visit, while one-quarter (48/183, 26%) stated that they did this at some visits. There was a mixed response as to whether the assessment was specifically for the bladder and bowel (82/183, 45%) or part of a wider assessment of the child’s health needs (96/183, 52%) (see Appendix 9, Tables 30–32).
The most common methods of assessment of bladder and bowel health used by all professionals were verbal reports from the child (139/184, 75%) and the parent carer (167/184, 91%). Nurses (82/95, 86%) also reported using charts, checklists and questionnaires in assessment. Paediatricians (30/31, 97%) and surgeons (12/12, 100%) used a physical examination as a method of assessing bladder and bowel health, but few professionals (5/77 nurses, 10/31 paediatricians, 2/12 surgeons, 2/13 other professionals) reported using blood tests. The use of more medical and surgical procedures was commonly reported by surgeons, such as urodynamics (10/12), as well as imaging methods such as ultrasound (9/12). Around three-quarters of therapists stated that they used verbal reports from the parent carer (24/33, 73%) and around two-thirds used verbal reports from the child (21/33, 64%). Therapists indicated using few other methods of assessing the child’s bladder and bowel, as assessment is not usually part of their role (see Appendix 9, Table 34):
I would not assess in a medical capacity but might offer strategies to assist in the child achieving continence, such as equipment provision.
Therapist 49811320
The most usual place for an assessment to take place varied by professional role; for therapists (20/33, 61%) and ‘other’ roles (7/12, 58%) it was at home, while for bladder and bowel specialist nurses it was more commonly in a community than clinical setting (14/18, 78%). 68% of paediatricians (21/31, 68%) and all 12 surgeons undertook assessments at the hospital (see Appendix 9, Table 33).
Assessment of capability and/or readiness for toilet training
Health professionals were asked to indicate the factors that trigger an assessment of a child’s capability and readiness for toilet training. Paediatricians (29/31, 94%), surgeons (11/12, 92%) and therapists (29/33, 88%) said that it was the developmental age of the child. For nurses, a parent carer request (62/77, 81%) was the most common trigger, and for specialist nurses a request from another health professional (14/18, 78%) or a parent (13/18, 72%) was most commonly reported. Specialist nurses indicated more frequently (13/18, 72%) that they assessed capability as they were following the local continence pathway (see Appendix 9, Table 35).
The majority (168/184, 91%) of all professionals confirmed that the parent or carer was also involved in the assessment of capability. Nurses (65/77, 84%) and specialist nurses (17/18, 94%) indicated that school staff were also involved. Paediatricians (25/31, 81%), surgeons (12/12, 100%) and therapists (27/33, 82%) suggested that health professionals other than them were involved in the assessment (see Appendix 9, Table 36).
The factor most likely to trigger the start of a formal toilet training programme was a parent carer request. This was reported by 13 out of 18 specialist nurses, 61 out of 77 nurses and 25 out of 31 paediatricians. The physical ability of the child was the most likely reason for surgeons (10/11, 91%) to begin an intervention. The developmental age of the child was reported as important by 94% of paediatricians (29/31) and 82% (9/11) of surgeons. One therapist highlighted that formal toilet training did not happen as much as it should (see Appendix 9, Table 37):
Initiation of formal toilet training happens much less than it should do with children with physical disabilities and/or learning disabilities. And, school’s capacity to support is absolutely key.
Therapist 48924085
Health professionals described a range of goals for toilet training depending on their role and the individual needs of the child. Specialist nurses and paediatricians suggested that the aim would be to develop the child’s understanding of toileting; nurses, surgeons and others considered that the main aim would be to get the child into a routine for toileting. Therapists indicated that their focus would be improving the child’s independence (see Appendix 9, Table 41):
Depends on the individual’s needs. All options used . . . but for different people.
Nurse 49902336
Any of the above [listed aims in question] could be the initial aim depending on the child’s current needs and abilities.
Therapist 49409510
Effectiveness of interventions
Health professionals told us that all categories of interventions were effective to some extent for children and young people with non-spinal-cord-related conditions. Specialist bladder and bowel nurses found behavioural interventions (17/17, 100%), simple aids (17/17, 100%) and medications (15/15, 100%) either effective or very effective. Continence products and surgical interventions in particular resulted in varied responses, as only around half (8/15 and 7/13, respectively) of specialist nurses found them effective. Specialist nurses most commonly reviewed their interventions monthly, 3-monthly or 6-monthly, and they reported that they usually judged the effectiveness of the intervention by using parent reports (18/18, 100%), child reports (16/18, 89%) or charts and checklists (15/18, 83%) (see Appendix 9, Tables 38, 39 and 42).
Nurses consiered fluid advice (71/77, 92%), simple aids (77/74, 98%) and medications (64/66, 97%) to be the most effective interventions. One-third of nurses thought that continence products were ineffective (23/67, 34%):
Continence product are sometimes essential but not effective with toileting.
Nurse 48840654
One-third of nurses found catheters (22/62, 36%), colonic enemas (21/62, 34%) or surgical interventions (19/60, 32%) effective. Nurses reported a huge variation in frequency of intervention review from weekly to annually; 99% of nurses (76/77) stated that they normally used parent report as an outcome to judge the effectiveness of the intervention (see Appendix 9, Tables 38, 39 and 42).
Paediatricians reported that medications were effective (25/26, 96%) and commonly reviewed interventions (i.e. 6-monthly) using parent reports (30/30, 100%) as the main outcome to assess effectiveness (see Appendix 9, Tables 38, 39 and 42).
Dietary advice . . . is highly effective if the parent follows the advice, but I find they rarely do.
Paediatrician 51000612
Surgeons indicated that medications (10/10, 100%) and simple aids (10/11, 91%) were effective. In particular, a large proportion of surgeons thought that diet advice was ineffective (5/12, 42%) and indicated that they themselves did not usually provide bespoke aids (4/10, 40%) or housing adaptions (4/10, 40%). Surgeons indicated that all of the interventions they used were reviewed 6-monthly, with the exception of surgical interventions that were more commonly reviewed every 3 months. They used parent reports (12/12, 100%) and child reports (11/12, 92%) as an outcome measure of effectiveness.
Therapists suggested that behavioural interventions (21/22, 95%), simple aids (21/22, 95%) and bespoke aids (20/22, 91%) were the most effective interventions they used, and they rarely used catheters (17/20, 85%), colonic enemas (17/20, 85%) or surgical interventions (18/20, 90%), as these were not part of their role:
I refer on to continence team or nurse-led team and do not recommend these interventions myself.
Therapist 49475466
In my role I would not be involved with medical appliances, issuing of pads, etc.
Therapist 49811320
The frequency which with therapists reviewed the interventions they used varied from monthly (behavioural) to annually (simple and bespoke aids) and they used parent report (31/33, 94%) as a measure of the effectiveness of the intervention (see Appendix 9, Tables 38, 39 and 42).
Generally, health professionals in all job categories suggested that they were confident in addressing at least some, if not all, of the toileting needs (knowing, finding, accessing, preparing, going, cleaning, completing), of a child or young person in their care by using an intervention. Nurses in particular were confident in addressing all of the child’s needs (> 0% for all needs), whereas surgeons were most confident in addressing ‘knowing’ that the child needs to go (see Appendix 9, Table 40).
Parent carers on non-spinal-cord-related conditions
In total, responses from 559 parent carers are reported regarding a child or young person with non-spinal-cord-related conditions.
Child/young person’s toileting needs and ability
Parent carer respondents indicated large variation in the toileting ability of the children and young people about whom they were completing the questionnaire. Approximately one-third (194/559, 35%) of parent carers stated that their child usually knew that they needed to go to the toilet without being told, but over half (312/559, 56%) said that their child could rarely or never wait to go until a toilet was found. Around one-third reported that their child could never access the cubicle on their own (199/559, 36%) or undress themselves (167/558, 30%) for toileting, and a large proportion (328/559, 59%) stated that their child could never clean themselves without help after using the toilet (see Appendix 10, Table 59).
Two-thirds of parent carers (330/558, 59%) indicated that their child using the toilet more independently was a priority for them. It was, however, one of many competing priorities and not their main priority. Only a small number (64/558, 11%) indicated that it was not a priority as they felt that their child would never be able to use the toilet independently (see Appendix 10, Table 60).
Assessment of bladder and bowel health
A large proportion of parent carers suggested that the professional help they received for their child’s toileting came from a GP (149/478, 31%) or a paediatrician (193/478, 40%). Other sources of help reported were a children’s nurse (114/478), a school nurse (115/478) and an occupational therapist (115/478). When approaching services for professional support, parent carers reported receiving mixed responses (see Appendix 10, Table 61):
[We were told] that one day he will just do it as it’s behavioural.
Parent 48838968
Told it was developmental or behavioural – no further help offered.
Parent 50504074
Some indicated feeling very supported by the professionals they approached for help, whereas others said that they had not received the support they had hoped for:
They have been very supportive, from putting in place medication, writing reports and letter, supporting in school and putting in place visual aids.
Parent 49016370
Nurse said my child will never be continent – no idea how she came to this conclusion as she asked nothing.
Parent 48839185
[They] didn’t really have any idea what to offer for a child like mine. Was a complete waste of time.
Parent 48846446
For many parent carer respondents, their child was yet to be assessed (220/544, 40%), but, among those respondents whose child had received an assessment, around one-third indicated that the assessment took place in the home (111/324, 34%) or in a community (120/324, 37%) or hospital clinic (109/324, 34%). Parent carers also indicated that the most common method of assessment was their own verbal report (398/454, 88%), and one parent also suggested:
The greatest attention was given to the verbal report of school staff.
Parent 49014529
Only 14% (62/454) of parent carers indicated that a verbal report was sought from their child. Approximately one-third of parent carers indicated that charts and checklists (172/454, 38%) and diet or fluid intake diaries (137/454, 30%) were used to assess their child, but few medical or surgical procedures such as urodynamics (35/454, 8%) and other imaging (42/454, 9%) were used (see Appendix 10, Tables 66 and 67).
Experiences of toileting interventions
Parent carers were familiar with the provision of continence products (374/534) and medications (314/522) and the majority (256/374, 68% for continence products; 290/314, 92% for medications) indicated that these were provided free of charge in their area as per NHS guidelines. A large proportion of parent carers had no experience of behavioural interventions (394/519), simple (316/523) or bespoke (411/521) aids or equipment, or housing adaptions (402/516) (see Appendix 10, Table 68).
Those parent carers who had used the interventions said that the easiest (reported as ‘very easy’ or ‘easy’) to use at home were simple aids or equipment (216/292, 74%), continence products (320/401, 80%) and medications (186/295, 63%). Similarly, when considering the most successful interventions, parent carers suggested that containment products (310/404, 77%) and simple equipment such as a toilet step (204/286, 71%) were successful (rated ‘very helpful’ or ‘helpful’). Dietary (124/256, 49%) and fluid (177/325, 54%) advice was considered helpful for around half of parent carers, but one parent indicated difficulties with implementing interventions:
The professionals told me to try dietary and behavioural things but never told me how or what to do.
Parent 49015658
A large proportion of parent carers suggested that they had never used more specific medical and surgical procedures, such as catheters (431/449), colonic enema (422/452) or surgical interventions (428/450), so could not comment on the success of these in helping with toileting problems; however, those who had used them gave mixed responses regarding success. For the parent carers who had used different methods or interventions for toileting at home, they were most commonly reviewed annually by a professional. Most parent carers (375/546, 69%) also indicated that there was no difference in toileting interventions for their child in different environments (see Appendix 10, Tables 68–71).
Sixty-one per cent of parent carers indicated that their child’s knowledge and understanding (328/536) and their child’s willingness (329/536) were the most common difficulties that they experienced in using toileting interventions at home. Nearly half (233/536, 43%) suggested that access to the appropriate help and support was also a specific difficulty. Only 6% (34/536) indicated that they experienced no difficulty at all when using different methods to help their child with toileting. Of the parent carers who responded, nearly half considered that the toileting intervention was unsuccessful because their child was not ready for toileting (221/492, 45%) or had been unable to learn to toilet independently (205/492, 42%) (see Appendix 10, Tables 72 and 73).
Many parent carers highlighted that the availability of appropriate and accessible toileting facilities was a key factor in the use, or the success or failure, of an intervention:
My son needs Changing Places toilets, with a ceiling hoist and adult sized bench. There are only 23 such toilets in my home county.
Parent 51439985
We have facilities at home to start training him, with pads when he’s out for now until better toilet facilities are available outside the home, i.e. toilets with hoists and proper seating.
Parent 50125171
Having correct facilities at pre-school has prevented being able to get started with toileting.
Parent 50885431
We are forced to choose a school based on toilet facilities. No care suite, look elsewhere. Local authority couldn’t do me a list of mainstream secondary schools filtered by toilet facilities when asked . . . lack of Changing Places toilets when out and about with a large wheelchair, a child who cannot be undressed in the wheelchair and who cannot sit or stand but needs access to a toilet is problematic all the time. Our life is ruled by toilets.
Parent 48867236
Spinal cord pathology: children and young people with a bladder and/or bowel impairment due to damage to the spinal cord
Health professionals on spinal cord pathology conditions
In total, 97 health professionals reported about improving continence in children and young people with spinal cord pathology conditions. Health professionals across all roles stated that children and young people with spinal cord pathology conditions were normally referred for professional toileting support via another health professional, and that they most commonly saw children and young people with spinal cord pathology conditions either weekly (30/96, 31%) or monthly (27/96, 28%). A similar proportion also indicated that reviews were held 3-monthly (21/96, 22%) (see Appendix 9, Tables 29 and 43).
Assessment of bladder and bowel health
The most common factor likely to trigger an assessment of bladder and bowel health in children and young people in the spinal cord pathology group was constipation, as reported by nurses (31/34, 91%) and specialist nurses (16/18, 89%), or UTIs, as reported by paediatricians (11/12, 92%). Surgeons said that the most common trigger for them was a delay in independent toileting (12/15, 80%). However, as one said:
I routinely assess bladder and bowel in all known spinal pathology, regardless of symptoms.
Surgeon 50287723
Over two-thirds (54/79, 68%) of respondents in the nursing, paediatric and surgeon professions indicated that an assessment of bladder and bowel health was routine for this group of young people. Nearly three-quarters of professionals (67/96, 70%) stated that they reassessed the child at every visit after the initial assessment, and that the assessment was most commonly part of a specific assessment of the bladder and bowel (58/97, 60%), rather than a broader assessment of the child’s health needs (39/97, 40%) (see Appendix 9, Tables 30–32).
The most common methods used to assess children with a spinal cord abnormality were verbal reports by the parent carer (77/79, 97%) and the child (72/79, 91%). Paediatricians and surgeons used a physical examination for this group. The use of other imaging and ultrasound procedures was mostly reported by surgeons (12/15 and 13/15, respectively). Therapists reported using verbal reports, checklists, diaries or observation for this group; 42% (5/12) of the therapists emphasised that an assessment of bladder and bowel health was not part of their role (see Appendix 9, Table 34).
I’m an Occupational Therapist . . . but do not assess bladder/bowel health, I assess how they access toilet, wiping skills, sensory issues around ‘holding’, environment, etc.
Therapist 48844268
I am not routinely involved in assessing bowl and bladder health – involvement is in facilitating equipment and/or environments.
Therapist 51395878
The most usual place for an assessment to take place varied by professional role; for therapists it was at home (10/12, 83%) or at school (8/12, 67%), and for bladder and bowel specialist nurses (11/17, 65%), paediatricians (9/12, 75%) and surgeons (15/15, 100%) it was a hospital setting (see Appendix 9, Table 33).
Assessment of capability and/or readiness
The most common factors that triggered an assessment of capability in a child in the spinal cord pathology group were the developmental age of the child as reported by surgeons (14/15, 93%) and the physical functioning level of the child as reported by paediatricians (11/12, 92%). Around half of the health professionals said that the child’s environment (44/96) or the parent’s capacity (50/96) was a factor in capability assessment, and fewer than half of responders (38/96) indicated that they assessed capability because it was part of the local continence protocol (see Appendix 9, Table 35).
Most health professional respondents (88/96, 92%) indicated that the parent carer was also involved in the assessment of a child’s capability for toilet training. School staff were also reported as being involved by the specialist nurses (15/18, 83%) and ‘other’ professional roles (3/3, 100%). All of the surgeons and therapists stated that other health professionals were also involved in the assessment (see Appendix 9, Table 36).
Nearly three-quarters of health professionals reported that the physical functioning level of the child (69/96) and a request from the parent carer (68/96) were most likely to initiate the start of a toilet training programme for children in the spinal cord pathology group. Over one-third of all professionals reported that a programme would be started as a result of the chronological age of the child (36/96, 37%) or because it was part of the local continence pathway or protocol (31/96, 32%). The professionals listed as ‘other’ (education, portage, psychologist) all indicated that the developmental age of the child was the trigger, with two-thirds also reporting that parent carer and other health professional requests were key triggers. For this group of children with spinal cord-related conditions, health professionals across all roles indicated that the main aim of an intervention to help with toilet training would be to improve the child’s independence in toileting or to protect the child’s bladder and bowel health. One specialist nurse emphasised that (see Appendix 9, Tables 37 and 41):
This group of children aren’t usually toilet trained, we train them to catheterise themselves.
Specialist bladder and bowel nurse 48841419
Experiences of interventions
Health professionals rated all interventions as effective for children and young people with spinal cord-related conditions. Some highlighted that interventions should not be used on their own and that individualising needed to be optimal:
Many of these methods are not effective in isolation. They should be used alongside each other and decisions about which methods to be used should be based on individual need.
Therapist 49285428
Over 75% of the bladder and bowel specialist nurses found all intervention categories to be effective, and typically reviewed them every 3 months; the only exception was continence products, but these were still considered effective by 69% of specialist nurses (11/16) and were reviewed 6-monthly. Nurses (not specialist bladder and bowel) indicated that fluid advice (30/32, 94%), simple aids (27/30, 90%) and medications (26/27, 96%) were the most effective interventions, and there was variation in the length of review, from monthly to annually. Around two-thirds of the nurses indicated that they used catheters (19/26), colonic enemas (15/25) and surgical procedures (16/24) and found them effective (see Appendix 9, Tables 38, 39 and 42).
Paediatricians reported that continence products (9/10, 90%), medications (10/10, 100%) and surgical procedures (9/10, 90%) were the most effective, and most interventions were reviewed every 6 months. Under half thought that diet advice was effective (4/11, 36%). All reporting surgeons (12/12, 100%) stated that medications, catheters, colonic enemas and surgical procedures were effective, and they typically reviewed interventions 6-monthly. All therapists (6/6, 100%) indicated that simple, bespoke, housing adaptations were effective, with reviews typically varying from monthly to annually (see Appendix 9, Tables 38, 39 and 42).
All types of professional indicated that they normally used a parent’s or child’s verbal report as an outcome to judge the effectiveness of an intervention (over 75% of all reporting professionals). Charts and checklists were also commonly used by nurses (29/36) and those in ‘other’ professional roles (3/3). Additionally, paediatricians suggested that they often used a physical examination (7/12, 58%), surgeons used other imaging (9/15, 60%), and other professionals (those in education and psychology roles) also indicated that diet and fluid diaries may be used (2/3, 67%) (see Appendix 9, Tables 38, 39 and 42).
In addressing the toileting needs (knowing, finding, accessing, preparing, going, cleaning, completing) of a child or young person in their care, all bladder and bowel specialist nurses were confident in addressing ‘finding’ and ‘going’, and two-thirds (10/15, 67%) were confident in addressing ‘knowing’. The majority (> 80%) of nurses (not specialist) were confident in addressing all steps of toileting with the exception of ‘knowing’. Therapists were confident in addressing ‘accessing’ (12/13, 92%). Surgeons (8/13, 62%) and paediatricians (5/8, 62%) were most confident about addressing ‘going’. One specialist nurse highlighted that the ability to address a child’s toileting needs depended on the availability of facilities and equipment (see Appendix 9, Table 40):
The success of addressing them depends on what is available. Parents can get a child toilet trained, but when out and about away from home and school, there is a lack of suitable facilities for toileting.
Specialist bladder and bowel nurse 49020623
Parent carers on spinal cord pathology conditions
There were 20 parent carer responses regarding a child or young person with spinal cord pathology.
Child/young person’s toileting needs and ability
Half of the parent carers (10/20, 50%) whose child had a bladder and/or bowel impairment due to spinal cord damage indicated that their child never knew that they needed to go to the toilet. Just over half reported that their child was never able to wait until they could find a toilet to use (10/19, 53%). Less than one-fifth confirmed that their child could always access the toilet themselves (4/20, 20%) or undress themselves (3/20, 15%), and two-thirds (13/20, 65%) indicated that their child could not clean themselves after using the toilet. Furthermore, one-third of parent carers indicated that their child using the toilet more independently was a priority for them (7/20, 35%), but that it was one of many competing priorities currently. Only a small number (3/20, 15%) suggested that their child would never be able to use the toilet independently because of their health condition (see Appendix 10, Tables 59 and 60).
Assessment of bladder and bowel health
Parent carers suggested that the help that they had received for their child’s toileting mostly came from a paediatric urologist (13/20, 65%) or a children’s nurse (11/20, 55%). Many also indicated that a paediatric neurologist (9/20, 45%) and a urological surgeon (8/20, 40%) were involved. Over three-quarters of parent carers of children and young people with spinal cord pathology said that the assessment of toileting ability usually took place in hospital (16/20, 80%) (see Appendix 10, Tables 65 and 66).
Around two-thirds of the parent carer responders indicated that verbal reports from them (13/19, 68%), physical examination (12/19, 63%), ultrasound (13/19, 68%) and urodynamics with a catheter (13/19, 68%) were the most frequent methods of assessment. Parent carers suggested that charts, checklists and questionnaires (1/19, 5%) and urine/faeces samples (2/19, 11%) were rarely used in assessment. Some parent carers indicated a mixed response from professionals whom they approached for help (see Appendix 10, Table 67):
It’s hard getting info (when needed) without going through a number of people first.
Parent 49013095
Once we were in the system we got all the help we needed.
Parent 49014662
We have had help with regards to catheterising, which had been great. But when it comes to seeking help with opening bowels, we have not had much help.
Parent 49320590
Effectiveness of toileting interventions
All parent carers who had experience of medications indicated that these were provided free of charge in their area (17/17, 100%). Most indicated that they had no knowledge of the provision of behavioural interventions (15/17, 88%), bespoke toileting equipment (15/18, 88%) or housing adaptions (12/19, 63%). Of those seven who did have experience of housing adaptions, five (71%) indicated that these were supplied free of charge, one said that they were subsidised, and one said that they were available to purchase. Twelve parent carers had experience of simple aids, of whom two-thirds suggested that these were free of charge (8/12), and 17 had experience of continence products, of whom just over half (9/17) suggested that these were free of charge and one-third (6/17) suggested that they were available to purchase.
All equipment (toilet seat, step, nappies) is purchased by ourselves, never been offered any support. Catheters and medication are on a free prescription.
Parent 50198593
Parent carer respondents further suggested that most interventions, if used, were usually reviewed by a professional annually. Many of the parent carer respondents for children and young people with spinal cord damage indicated that they had never used many of the interventions listed (see Appendix 10, Tables 68 and 69).
Of those parent carers with experience of using the intervention, all (5/5, 100%) reported that housing adaptions were either ‘very easy’ or ‘easy’ to use. A total of 94% (15/16) and 80% (12/15) indicated that continence products and catheters, respectively, were ‘very easy’ or ‘easy’ to use. Similarly, parent carer respondents with experience of using them indicated that continence products (15/17, 88%) and catheters (14/14, 100%) were the most successful interventions for children and young people with spinal cord impairment, while the majority of parent carer respondents indicated that they had never used behavioural interventions (9/16, 56%), bespoke equipment such as a hoist (14/17, 82%), or housing adaptions (14/19, 74%) with their child (see Appendix 10, Tables 60 and 71).
The most common difficulty parent carers experienced when using toileting interventions to help their child was their child’s willingness (14/20, 70%). The child’s lack of understanding and knowledge of what was required (9/20, 45%) was also a key difficulty to overcome. Very few parents (1/20, 5%) suggested that not enough training was provided for the methods offered. Half of the parents who responded indicated that the reason the toileting intervention had not worked was that their child had not been ready to use it (8/16, 50%), and over one-third stated that their child was unable to ever learn to toilet independently (6/16, 38%). Most parents also indicated that there was no difference in the toileting interventions that their child used in different environments (14/20, 70%) (see Appendix 10, Tables 68 and 72–74).
Service provision and access
Health professional views on provision of services and support
Generally, it was indicated that most toileting products or interventions were provided to families free of charge. Bladder and bowel specialist nurses indicated that continence products (17/18, 94%) and medications (15/18, 83%) were provided free. Nurses (60/72, 83%), paediatricians (29/29, 100%) and surgeons (13/14, 93%) all indicated that medications were provided free to families. Therapists (20/27, 74%) reported that simple and bespoke aids were also free to families (see Appendix 9, Table 44). Bladder and bowel specialist nurses (10/22, 45%) and nurses (28/79, 35%) told us that the minimum child age at which toileting aids and interventions were provided to families was 4 years. A large proportion of paediatricians (14/30, 47%), surgeons (11/15, 73%), therapists (12/33, 36%) and other professionals (6/12, 50%) said that they did not know the age at which a child would receive funding for toileting support.
The majority of specialist nurses (16/22, 73%) and nurses (39/76, 51%) considered the provision of continence pads to be a barrier to achieving continence; paediatricians (13/30, 43%), surgeons (8/15, 53%) and therapists (17/33, 52%) did not know if provision was a barrier or an enabler (see Appendix 9, Tables 45 and 46). Some professionals mentioned the complexity of using continence products:
It depends on the motivation of the family to put in place the toileting plan alongside the provision of products.
Specialist bladder and bowel nurse 48840151
Parents don’t see continence as a priority, it also helps them gain DLA [Disability Living Allowance] funding, so it discourages parents from getting out of nappies, as they fear loss of funds.
Specialist bladder and bowel nurse 48841419
The way pads are provided is problematic (e.g. not enough overall, or too many provided at once which leads to problems storing them). I think on the whole there are very low expectations for achieving independent toileting, from health and education professionals, and from parents (who will often take their lead from the professionals).
Therapist 48924085
Three-quarters of professionals (143/191, 75%) reported that if an intervention is unsuccessful then they persevere, try something different or revisit the intervention at a later date. Two-thirds of professionals (118/191, 62%) suggested that children and young people are offered different toileting interventions depending on which professional they are seen by, and nearly one-quarter (42/192, 22%) told us that they knew of toileting interventions they were unable to provide that were provided elsewhere. Half of respondents (93/191, 49%) indicated that children and young people were offered different interventions for different environments, for example school and home (see Appendix 9, Tables 47–50):
Equipment not provided for schools so have children toileted at home but not at schools as they can’t afford to buy them with budget cuts; children’s behavioural presentations are extremely different in environments therefore some toileting interventions cause high levels of stress therefore alternative approaches are taken.
Therapist 49577099
Half of the health professionals (93/192, 48%) stated that a specific bladder and bowel pathway or protocol was in place where they worked, and, of those who said that their service did have this, three-quarters indicated that there was a lead person responsible for it (70/92, 76%). The main barrier to developing a pathway, highlighted by two-thirds of health professionals, was a lack of funding or resources (94/156, 60%) and nearly one-third (52/196, 27%) indicated that commissioning or funding arrangements sometimes influenced the toileting support that they offered children (see Appendix 9, Tables 47–54):
Underfunding leads to less staff, less time for the children.
Specialist bladder and bowel nurse 48841419
We have saved circa £20K [£20,000] in 2 years by reducing containment product expenditure.
Nurse 49170511
Health professional views on access to support
Responses from professionals were mixed regarding access to toileting support for families. A large proportion of specialist nurses (17/22, 77%) and paediatricians (16/31, 52%) considered that it was ‘very easy’ or ‘easy’ for parents to access support, whereas over half of surgeons (8/15, 53%) indicated that it was ‘very difficult’ or ‘difficult’. Health professionals were split in their opinions of families’ waiting times to receive support, with 43% (83/192) indicating that the waiting time was acceptable or very acceptable, and 37% (71/192) indicating that it was unacceptable or very unacceptable. Some professionals described the reasons for waiting times for access to support (see Appendix 9, Tables 55 and 56):
Waiting times are too long for assessment, and then provision of complex equipment and/or adaptations can also take a long time.
Therapist 48880209
The wait is 18 weeks as per standard NHS outpatient referral to treatment time, under the NHS constitution.
Therapist 48840736
Not enough continence support so waiting times long and services are not funded.
Nurse 49177881
Parent carer views on access to support
For around one-third of parent carers of a child with a non-spinal-cord-related condition, the main reasons that they had initially sought professional help for toileting were the child’s age (196/546, 36%), stage of development (190/546, 35%), night-time wetting (180/546, 33%) and daytime soiling (180/546, 33%). Nearly half of the parent carers who responded also indicated that they had sought support with the expectation that they would be able to develop their child’s understanding of the basics of toileting (246/533, 46%) and ensure that their child’s bladder and bowel were healthy (244/533, 46%). For over half of the respondents in the spinal cord pathology group, parent carers stated that professional support for toileting had been started automatically from birth because of the spinal cord condition (11/20, 55%). Another common trigger for support was the issue of daytime wetting (6/20, 30%) (see Appendix 10, Table 61).
Nearly three-quarters of parent carers in the non-spinal-cord-related group suggested that it was ‘difficult’ (213/555, 38%) or ‘very difficult’ (178/555, 32%) to access support for toileting, and less than 1% (3/555) indicated that they found this ‘very easy’. Nearly one-quarter of these parent carers found the waiting time for appropriate support ‘acceptable’ (121/553, 22%). Among parent carers in the spinal cord pathology group, there was a more split response. Half of the respondents said that it was ‘easy’ (9/20, 45%) or ‘very easy’ 1/20 (5%) to access support. The main goal of these parent carers at the point of seeking support had been to ensure the bladder and bowel health of the child (14/20, 70%). Reducing the number of accidents the child had was also a key goal (8/20, 40%) (see Appendix 10, Tables 62–64).
Many parent carers described how difficult it was to access support and spoke about the impact that waiting times could have:
It is HUGELY difficult, most parents are unaware it even exists!
Parent 48839185
There just doesn’t seem to be any real practical help.
Parent 48842081
Despite being a nurse I was unable to find any service in our area when my lad was a child to support us.
Parent 48846446
Waits can be long, then even once with who you need to be, progress while waiting on further appointment for tests, etc. can be as long again, all the while the child becomes more distressed by the issues.
Parent 48913452
The waiting list is long and the service is entrenched in their ‘policies’ which are designed to fit their budget not the need of the family. They have no concern for the social or psychological impact of continence difficulties.
Parent 49014529
Provision of toileting support for young people after the age of 18 years
Professionals in all roles reported being involved in managing the transition to adult services for young people with toileting support needs, with most specialist nurses (18/22, 82%) and surgeons (12/15, 80%) reporting involvement. One-third (28/92, 30%) of those who said that they were involved in transition indicated that they started to consider the transition process when their patient was 16 years old (see Appendix 9, Tables 57 and 58).
The majority of respondents in the parent carer survey with a child aged 12–25 years reported that the transition to adult services for their child with a non-spinal-cord-related condition (137/211, 65%) or spinal cord damage (4/4, 100%) had not yet been considered. For the parent carer respondents with a child aged 12–25 years, where transition to adult services for their child with a non-spinal-cord-related condition had been considered, many of the key steps in transition outlined by the National Institute for Health and Care Excellence (NICE) had not occurred. For example, many said that having a joint meeting with a professional from child services and a professional from adult services (40/67, 60%), having a reassessment before transition (37/70, 53%) and being given a named person to co-ordinate the transition (37/69, 54%) had never been mentioned to them. Only around one-third of parent carers had been given information about transition (26/72, 36%). No parent carers with a child aged 12–25 years in the spinal cord pathology group responded regarding the steps to transition, as they had not yet considered transition (see Appendix 10, Tables 75 and 76). The comments from the parent carers who did have experience of transition illustrated the difficulty of the process:
There should be an easier way to continue to access products, than when the child reaches 18 everything stops and you have to reapply.
Parent 48841695
I had to refer my son to adult continence service as no transition.
Parent 49014529
Once my son hit 18 everyone disappeared with no transition – can’t help feeling like we’ve been deserted.
Parent 49575021
School and social care staff
In total, responses from 119 school and social care staff are reported; of these 119 staff, 76 indicated that they worked with children and young people in the non-spinal-cord-related pathology group only, and 43 worked with children and young people in both groups.
School and care staff reported that, in a large proportion of cases, they ‘always’ had to prompt the children in their care to go to the toilet (45/118, 38%). Helping the child to access the toilet (52/118, 44%), clean themselves (49/117, 42%) and return to where they had been (47/117, 40%) were also reported as tasks they always had to help children with. Staff indicated that the most common reason that would prompt them to seek specialist toileting support for children and young people with special educational needs and/or a disability would be a parent carer request (84/119, 71%). Other key triggers were the child’s functioning level (65/119, 55%), the child’s developmental age (58/119, 49%), and daytime wetting (56/119, 47%) and soiling (58/119, 49%). Many indicated that support was readily available to them in their role (see Appendix 11, Tables 77 and 78):
We have OT and school nurse on site as well as access to physio.
School and care staff 49019570
Have a nursing team on site who support with continence.
School and care staff 49033144
Once support had been sought, the most frequently reported location for an assessment of a child’s toileting ability was home (45/119, 38%) or school (54/119, 45%). No assessments were reported at a young adult’s place of work, and very few (6/119, 5%) were reported in respite care by this group of staff. The toileting assessment methods that school and care staff indicated that they had most experience with were reports from parent carers (80/110, 73%), charts, checklists or questionnaires (64/110, 58%), direct observation (52/110, 47%) and diet or fluid intake diaries (46/110, 42%). Although 110 respondents answered about their experiences of assessment, nearly three-quarters (86/119) indicated that they were not directly involved in assessing a child’s toileting ability or capability. Some illustrated the barriers they experienced in assessing children in their care (see Appendix 11, Tables 81–83):
There seems to be an ethos that children with disabilities don’t have to be toilet trained when their peers are. I talk to our school nurse and parents and do a very quick assessment. I will not toilet train a child unless their parents are going to give us their full support.
School and care staff 49045353
To access specialist services I have to notify the health visitor.
School and care staff 49200706
School and care staff said that the main aims of toileting interventions with children in the non-spinal-cord-related pathology group were to develop the child’s understanding of toileting (83/119, 70%) and get the child into a routine for toileting (77/119, 65%). For children in the spinal cord pathology group, school staff indicated that the main aim was to protect the child’s bladder and bowel health (19/35, 54%) (see Appendix 11, Table 79).
When asked about the difficulty that staff have in assisting children with specific toileting interventions, over half of those who responded indicated that the child’s knowledge and understanding (67/115, 58%), the parent carer’s capability and time (70/115, 61%) and a lack of consistency of support in different environments (69/115, 60%) were the most common difficulties they faced. Some school and care staff described the barriers they experienced in assisting with toileting:
Parental anxiety around their children having the ability to be toilet trained is a common barrier.
School and care staff 49433252
Teachers and schools require further guidance and practical support through additional adult assistance.
School and care staff 49989512
The budgeting needs of the school and the available space greatly affect the ability to meet the needs of these students.
School and care staff 50132302
Our biggest issue is accessing the correct support and parental engagement to follow the guidelines.
School and care staff 50912479
Half of the school and social care staff reported that diet (76/116, 65%) and fluid (88/116, 76%) advice were ‘easy’ or ‘very easy’ to provide, and behavioural approaches such as reward charts (94/113, 83%) were commonly considered ‘effective’ or ‘very effective’. The majority of the staff had no experience of catheters (82/113, 73%), colonic enemas (91/112, 81%) or surgical interventions (93/109, 85%) and so could not comment on the effectiveness of these (see Appendix 11, Tables 84–86).
There was variation in opinion as to whether children and young people used different toileting methods or interventions in different environments; 43% (50/116) of the respondents reported that children did have different provision in different environments, whereas 32% (37/116) said that they did not. One-quarter (29/116) of respondents had no knowledge of whether or not provision was different in different environments. Some respondents highlighted the difference in toileting provision in different environments:
Our classroom does not have clear access to the toilets. This means that children with severe communication difficulties cannot take themselves to the toilet when needed.
School and care staff 49045353
School uses hoist for manual handling and changing bed – not all parents have/use these.
School and care staff 49805938
Some of the children have specialised toilets at school but not at home (they just wear pads). It can vary.
School and care staff 49253544
There are not adequate facilities in the community to ensure consistency with toileting.
School and care staff 49317722
There was also a mixed response from school and care staff regarding ease of access to toileting support, with 35% (42/119) indicating that it was easy and 34% (40/119) indicating that it was hard. One staff member stated:
There is a lack of advice when it comes to supporting children who have sensory needs or a diagnosis of ASD [autism spectrum disorder]. I feel there needs to be more info on where to signpost parents or where staff can get more training.
School and care staff 51016052
Over half (66/119, 55%) of the school and social care staff thought that access to support for toileting was ‘very easy’ or ‘easy’ where they worked. Nearly half indicated that they knew of continence products (55/115, 48%) and medications (47/115, 41%) being provided free of charge. Almost two-thirds (77/118, 65%) had no knowledge of how support for young people at transition (i.e. aged 18 years) was managed (see Appendix 11, Tables 81, 87 and 88).
Children and young people
In total, 20 children and young people responded to the young person’s survey (see Appendix 12); 17 provided a description of their condition or diagnosis, which was classified by the study team as non-spinal cord related, and one indicated that it was part of the spinal cord pathology group. Two did not specify their condition. Seventeen of the respondents completed the survey on their own, two indicated that someone was helping them and one indicated that someone was completing it for them.
Toileting ability described by young people completing the survey was varied. Around half of respondents indicated that they could manage most aspects of toileting independently without help, which included getting to a bathroom (10/20), undressing (10/19), cleaning (11/20) and returning to their activity afterwards (11/20). Three-quarters of the respondents indicated that they independently knew they needed to go to the toilet (15/20), but only half were able to control their bladder and bowel until they reached a bathroom (10/20). One-fifth (4/20) suggested that they needed ‘a lot of help’ with physically using the toilet, and around one-quarter indicated that they could not undress themselves (5/19) for toileting or get dressed again (5/20) afterwards. Of note, all of the respondents who completed the survey were aged ≥ 8 years (see Appendix 12, Table 89). Some respondents described the help they needed with toileting:
My balance is compromised on one side, the format, layout and presentation of the toilet and associated facilities determines my success.
Young person 50626617
It’s hard to know when to go until the last minute. I find the list of steps overwhelming.
Young person 50708643
Finding it increasingly difficult to undo buttons. Then sometimes need help to fasten them again. Carers have to remind me.
Young person 50125204
When considering the methods used to assess toileting ability, about half of the young people who completed the survey indicated that they felt ‘very happy’ (4/20, 20%) or ‘OK’ (7/20, 35%) about providing verbal reports. Having a physical assessment was acceptable to nearly half of the young people (‘OK’ 9/19, 45%), but half (50%) who had experience of catheters were ‘very unhappy’. Ten out of 14 (71%) of those who had been directly observed using the toilet were ‘very unhappy’ about it, and one-quarter (5/20) stated that a professional had never checked their toilet facilities at school. There was a mixed response about how young people felt about using the toilet at school, with half of all respondents feeling ‘OK’ or ‘very happy’ about it, and half feeling ‘unhappy’ to some extent or indicating that they never used the toilet at school. A large proportion (63%) of young people were ‘very happy’ about using the toilet at home and ‘unhappy’ or ‘very unhappy’ (70%) about using the toilet when out in the community (see Appendix 12, Tables 90 and 91).
When asked about their experience of toileting interventions, over half of young people indicated that they had no experience of using alarms or timers (11/20, 55%), hoists or frames (10/20, 50%), catheters (12/20, 60%) or surgery (10/20, 50%), and < 20% indicated that they were ‘very happy’ about any of the interventions listed (see Appendix 12, Table 92).
Discussion
Key findings
The findings from all four surveys have allowed us to address the research questions of the study. Within the findings relating to each of the research questions, there were some notable differences in questionnaire responses between the four groups of survey respondents, and between the two clinical groups due to the differences in the presenting pathologies experienced. For the spinal cord pathology group, the priority was often to preserve bladder and bowel health with medically assisted or surgical interventions initiated from or soon after birth; whereas for the non-spinal-cord-related pathology group, the priority was often for them to be ‘trained’ to empty their bladder and bowels in a toilet or potty in a socially appropriate way so that they could be clean and dry without pads/nappies.
Research question 1 asked ‘how do clinicians assess bladder and bowel health of children and young people with neurodisability, their continence capabilities and readiness for toilet training, and which clinicians are involved in assessments?’.
Health professionals indicated that the main trigger of assessment and support for the non-spinal-cord-related pathology group was a delay in achieving independent toileting. This was also suggested for the spinal cord pathology group, although constipation and UTI were also key. The key methods of assessing bladder and bowel health for both clinical groups were similar across all surveys, with the most common assessments being verbal reports from the parent and the child. Across both clinical groups, the most common factors for triggering assessment of capability or the start of a toilet training programme were the developmental age and physical functioning level of the child, or a parent request.
Research question 2 asked ‘which interventions clinicians do use or recommend to improve continence for children and young people with neurodisability, how they are individualised, evaluated and/or audited, and which clinicians are recommending, delivering or evaluating interventions?’.
There were similar responses from respondents for both clinical groups of children and young people regarding the use and effectiveness of interventions. For children and young people with non-spinal-cord-related conditions, health professionals reported that behavioural interventions, simple aids and medications were the most effective and that these were commonly evaluated using charts, checklists, questionnaires, or parent and child reports. Health professionals commonly reviewed interventions either monthly, 3-monthly or 6-monthly, although there was variation by specific job role. Parent carers of children in the non-spinal cord pathology group had similar responses, also indicating the use and effectiveness of medications and simple aids, but continence products were also mentioned as effective.
For the spinal cord pathology group health professionals reported that medications, simple aids and surgical procedures were particularly effective. As with the non-spinal-cord-related pathology group, the effectiveness of interventions was evaluated using parent and child verbal reports but also by physical examination, and interventions were commonly reviewed 3-monthly. Parents rated continence products as effective for this group, but also highlighted the effectiveness of housing adaptions and catheters.
Research question 3 asked ‘how do families, school and social care staff consider and judge children’s readiness for toilet training and the need for specialist assessment and/or interventions?’.
Parent carers of children in the non-spinal-cord-related pathology group reported that the main reason they sought support for toileting was the age and developmental stage of the child, and that assessments of a child’s need for interventions mainly took place at home or in clinics. Among the spinal cord pathology group, parent carers reported that support was automatically accessed from birth, and assessments mainly occurred in the hospital. School and social care staff mainly said that the physical functioning level of the child was the reason for seeking toileting support, although the majority of school staff suggested that they were not involved in assessing a child’s ability or capability. Along with parent and child verbal reports, school staff said that charts, checklists and questionnaires were used to assess a child’s need for interventions, and indicated that assessments were commonly conducted in school. Children and young people indicated that they also found the use of verbal reports and physical assessments acceptable.
The final research question asked ‘which factors affect the implementation of interventions to improve continence, and how acceptable are strategies to children and young people and their carers?’.
Children and young people most commonly indicated that they were generally unhappy about using any interventions for toileting, but said that using the toilet at home was preferable to using toilets in other environments. There were clear differences in opinion regarding ease of access to support for families. Health professionals generally reported that access was easy for both clinical groups, although surgeons thought that it was difficult. Parent carers reported that it was difficult for the non-spinal-cord-related pathology group, but opinion was split for the spinal cord pathology group. Similarly, school and social care staff responses were roughly evenly split between whether it was easy or hard.
Both health professionals and parent carers told us that medications and continence products were the easiest interventions to use and were typically provided to families free of charge, which is in line with NHS guidelines,126 although one-third of respondents suggested that products were not free in their area. However, continence products are usually for containment and do not constitute an intervention that improves continence in the same way as toilet training to become clean and dry. School and social care staff also highlighted diet and fluid intake advice as easy for them to use, and emphasised behavioural interventions as particularly successful. Parent carers suggested that there was no difference in the interventions that their child was offered for different environments, whereas health professionals suggested that interventions were different for different environments, and were also different depending on which professional saw the child.
Reasons for failed interventions and difficulties with implementation were the same in both clinical groups and for the majority of respondents in the health professional and parent carer surveys – the child was not ready, was unable to learn or had inadequate understanding. School and social care staff told us that the capability and time of the parent carer and a lack of consistency in support in different environments were also key difficulties. A lack of adequate and accessible facilities was highlighted by many respondents as a barrier to toileting, which emphasises the need for more ‘Changing Places’ toilets, which provide the right equipment and space in a clean and safe environment to support children and young people with profound and multiple learning difficulties, or physical disabilities. 127
To take account of the complexity of children’s needs, the findings from the surveys allowed us to address the needs-led approach as outlined in our study-specific conceptual framework. Among children in the non-spinal-cord-related pathology group, parent carers told us that around half of their children had the ability to know they needed to go to the toilet, but a larger proportion could not wait until an appropriate place was found or could not clean themselves afterwards. Nursing professionals were confident that they could address all aspects of toileting with an intervention for children with non-spinal-cord-related conditions, whereas most surgeons felt confident in addressing the child ‘knowing’ they need to go. Among the spinal cord pathology group, parent carers were most likely to tell us that their children rarely or never had the ability for any aspect of toileting. Nursing professionals were generally confident in addressing finding, going and accessing, but slightly less confident in addressing the ability of the child to know they need to go.
School and care staff indicated that they always had to help children in both clinical groups to know that they needed to go and to access the toilet. On the whole, over half of the young people in the survey indicated that they could manage all aspects of toileting independently, in particular knowing that they needed to go and actually using the toilet. Dressing again afterwards was one skill that more children told us they needed help with. The main goal when accessing support was the same for both clinical groups and for parent carers, health professionals and school and care staff, which was to ensure the bladder and bowel health of the child.
Survey challenges, strengths and limitations
The online survey used in this study provided an expedient means of gathering information from a large number of diverse respondents, but also presented a number of challenges, strengths and limitations. A strength of using an online survey approach lies in its potential to reach large samples, wide geographic and demographic reach, low cost and ease of completion for participants. This has frequently been reflected in other studies and was true for this study. 128,129 In total, there were over 1600 registrations, which resulted in nearly 1000 completed questionnaires, with representation from all geographic regions of England and from all ethnic groups. Although significant time investment is involved in the design and development of a survey, there are faster response times and less data processing time with the use of an established online system.
The facility provided via the online system enabled a personalised approach to recruiting participants, including personalised invitation e-mails and reminders. When advertising the survey, efforts were made to increase the number of registrations by tailoring the advertisements to specific professional or family groups and specific regions through a large number of external organisations. Advertisements were circulated to encourage registration before the surveys were live. Multiple contacts, endorsement and tailoring the design to the population under study have previously been highlighted as effective methods of increasing response to surveys,130,131 and these are approaches that we undertook through reminders, stakeholder organisations and targeted advertising.
However, a number of limitations arose. Owing to the registration process used, there was occasionally a delay between participants registering for the survey and receiving their personalised survey link. This presented the opportunity for participants to lose interest or forget to return to the survey invitation in the e-mail they received. Additionally, the registration process relied on the participants entering their e-mail address correctly; the online survey system could not identify incorrect e-mail addresses. This meant that some participants might never have received the link or the four reminders, despite the e-mails automatically being sent via the system. The researcher managing the survey process was able to identify some incorrect e-mail addresses and correct them, but it is not possible to know how many participants never received their link to the survey. Furthermore, some participants contacted the study team to report that the personalised links were ‘broken’ and so they could not access the survey. In these cases, the links could be reset, but this relied on participants notifying the study team. These factors may have contributed to non-response by registered participants.
Partial response to the surveys also occurred. There was not a requirement within the surveys to complete every question and, therefore, participants did not always do so. Similarly, the survey responses were saved and submitted to the study team only if the participant reached the final page of the survey and clicked ‘submit’. It is possible, therefore, that some participants may have contributed many or all of the responses but failed to submit the completed survey.
We received a considerable number of free-text responses. Our protocol was that we would code and analyse these data thematically, and potentially telephone participants for further details. However, the sheer quantity of text meant that systematic analysis was beyond our capacity. Although free-text allows people to feel heard, a limitation is that analysing and presenting it is time-consuming and resource intensive, and perhaps we have not been able to do as much with these data as we might be able to do in the future. This research highlights the need for in-depth qualitative studies to explore the experiences of children and young people with neurodisability and their families, health professionals and school and care staff.
Another limitation is that an online survey may exclude those who do not use, or are not willing or able to submit their contact details to register. There is likely to be a response bias towards those who are willing and able to use online processes, and those who sought to share their views as a result of seeing the survey advertisement. Again, we would advocate well-designed qualitative research with children and young people with neurodisability, using modified means for communication where necessary, as more appropriate for gathering their views and experiences.
Although the regional locations of the respondents were collected to describe the sample, the data presented are generalised across England. We acknowledge that there is variation in service provision by locality, for example existence of a service and waiting times. This variation is not reflected in our results as this study did not seek to map or audit provision at the local level. It is also important to note that health professionals hold different roles for children and young people with neurodisability; for example, therapists may not be involved in the assessment of bladder and bowel health and surgeons would not necessarily be concerned with addressing the access to the toilet that a child has at home. Health professionals often work collaboratively as a team to address toileting and, therefore, the findings presented from the health professionals, where possible, were reported by the professional role for context, and, therefore, the numbers of respondents are small and provide a snapshot of current practice, but are not necessarily representative across the nation.
Chapter 5 Integration, stakeholder consultation and key findings
This chapter describes the integration phase of the study. The integration aimed to bring together the findings from the systematic review (see Chapter 3) and survey (see Chapter 4) phases to address the overall study research question: ‘what is the available evidence for interventions relating to improving continence for children with neurodisability?’. The following sections in this chapter highlight the integration process undertaken, and how the findings from the systematic review and surveys were explored, compared and integrated. The combined key findings are described, as is the consultation that was undertaken with our key stakeholder groups and the feedback that they provided.
Integration methods
Using the conceptual framework (see Chapter 2) as a starting point and considering evidence for children with and without spinal cord pathology separately, we approached the integration in two stages. First, we tabulated data arising from each methodology. We identified areas where there were corresponding data and where the evidence was only from one phase of the project, as the systematic review and the survey were designed to address different research questions. For example, the systematic review focused only on interventions, whereas the survey gathered data on how continence was assessed and interventions were used. The outcomes identified by the systematic review and survey also had a slightly different focus. The systematic review found measures that health professionals used to monitor a child’s progress with urinary or faecal daytime and night-time continence, whereas day and night were not distinguished in the survey.
Second, the key findings from the systematic review and the survey were discussed with our stakeholder groups and summarised under assessments, interventions and outcomes, clearly highlighting any gaps in the evidence. It is important to note that some of the gaps we identify in the evidence will be due to the different foci of the review and survey, and not necessarily because no evidence is available. Table 15 provides a summary of integration for the non-spinal-cord-related pathology group, and Table 16 provides this for the spinal cord pathology group.
| Survey findings: what are people currently doing? | Systematic review findings: what is the evidence that it is effective? | Stakeholder consultation | |
|---|---|---|---|
| Assessments | |||
|
Assessment mainly triggered by developmental age and physical functioning Most common assessments are verbal reports from parent and child, charts checklists and questionnaires, and physical examinations Most common triggers for starting toilet training programme are developmental age, physical functioning level of child or parent carer request Parent carers said assessment mainly took place at home or in clinic Health professionals and school staff said that access to support was easy; parent carers said that access was difficult |
Not within the scope of the systematic review |
FF – lack of assessments relating to fluctuation in continence over time or what else was currently happening for the child – assumptions of linear progression with continence, and this is not always the case FF – lack of assessments around menstruation and developmental life stages; this has a very direct effect on continence and incontinence FF – broader aspects of task overload (dealing with several physical health issues at one time) and ability to cope FF – lack of acknowledgement regarding mental health-related conditions, such as depression, anxiety and other stress-related measures, for both the child and the carer PAG – favoured early intervention, needs to be further worked around the developmental age and functioning level of children, particularly those with learning disabilities, as this covers such a large spectrum PAG – no consensus on when a child is ready; members stressed that this is down to perception of the parent carer or the professional. Also linked to timing: assessments (and thus interventions) not introduced early enough |
|
| Interventions | |||
|
Products and technology for education Education interventions Behavioural interventions |
Nurses and parent carers found fluid advice effective; surgeons perceived it ineffective School and social care staff found diet/fluid advice easy to implement Specialist bladder and bowel nurses, therapists and school and care staff found behavioural interventions very effective |
Intervention on adequate fluid intake for children with developmental disabilities demonstrated improvements in urinary continence over 6 weeks Studies reported post-intervention improvements in urinary and faecal continence among populations with autism, developmental and learning disabilities undergoing behavioural training interventions; one study demonstrated the effectiveness of a behavioural training intervention using an adapted Azrin and Foxx method. All measures showed improvements following the intervention |
PAG – need for better education about continence training and a need for shared decision-making. There is a need to increase research capacity among practitioners, their understanding of the value of it and how to go about it; resources, time and skills are barriers to effective use of research in practice FF – gaps identified in habit acting as a prompt (e.g. having been to an accessible toilet previously, this may act as a prompt for the participant to use that toilet again) FF – nothing in the alarm system-based interventions (behavioural) that mentioned hearing assessments; hearing is likely to have an impact on a child’s ability and understanding of toileting and on the interventions used |
|
Products for personal use in daily living Toilet adaptations Clothing adaptations Continence products |
Specialist bladder and bowel nurses, other nurses, surgeons and therapists all found simple aids effective Therapists told us that bespoke aids were most effective Parent carers reported simple aids to be effective and easy to use at home Half of the specialist nurses found continence products to be effective; one-third of other nurses suggested that these were ineffective Majority of the nurses found continence products to be a barrier to achieving continence Majority of the parent carers thought that continence products were effective and easy to use at home |
||
|
Products and technology for communication Equipment for communication |
FF – members raised the lack of experiences gathered around communication: is the child verbal, how do they communicate their needs, the impact of audio-processing delays and challenges with comprehension | ||
|
Products and technology for personal indoor and outdoor mobility Equipment for movement |
|||
|
Products for personal consumption (subset: drugs) Medications |
Nurses and surgeons found medications effective Paediatricians indicated that medications were more effective than all other interventions Parent carers found medications to be effective and easy to use at home |
General improvements were observed in urinary continence among populations with ADHD and/or autism undergoing interventions involving drug therapy (including desmopressin, oxybutynin, imipramine, and anticholinergic treatments); one study (ADHD) reported an improvement in continence in just over half of the participants following an intervention using desmopressin | |
| Medically assisted techniques and surgical approaches |
Half of specialist nurses suggested that they thought surgical approaches were effective More surgeons reported use of medically assisted and surgical approaches than any other professional |
One RCT assessing the effectiveness of a wireless moisture pager device for improving continence found that this did not improve parent satisfaction | |
| Outcomes | |||
|
Evaluation of interventions usually conducted using charts, checklists, questionnaires and parent and child reports Interventions reviewed monthly, 3-monthly or 6-monthly Children and young people were generally unhappy about using interventions, but toileting at home was preferable to toileting in other environments Health professionals said that different interventions were offered for different environments, and school and social care staff highlighted a lack of consistency in support in different environments; a lack of adequate and accessible toileting facilities was highlighted by many respondents |
FF – there was a gap identified around non-physical measures (psychological outcomes related to, for example, mental health) FF – members felt that many ‘experiences’ of toileting included social perception, including shame, embarrassment, judgement, also the experience of hidden disabilities PAG – lack of qualitative evidence in the review, but reported that this was not surprising. They felt that continence services were underfunded, resource poor and neglected in terms of research PAG – there is a greater need for research conducted with the non-spinal cord-related pathology group. There is often no care pathway for these children and young people |
||
| Survey findings: what are people currently doing? | Systematic review findings: what is the evidence that it’s effective? | Stakeholder consultation | |
|---|---|---|---|
| Assessments | |||
|
Assessment mainly triggered by delay in achieving independent toileting, constipation and UTIs Most common assessments via verbal reports from parent and child Most common trigger for starting toilet training programme was developmental age and physical functioning level of child Parent carers said that assessment mainly took place at hospital automatically from birth Health professionals and school staff said that access to support was easy |
Not within the scope of the systematic review | PAG – assessments (and thus interventions) not introduced early enough, and special schools in particular do not necessarily address the toileting needs of the child early enough | |
| Interventions | |||
|
Products and technology for education Education interventions Behavioural interventions |
Nurses found fluid advice to be effective, whereas paediatricians thought that diet advice was ineffective Health professionals indicated that behavioural interventions were effective Over half of parent carers found them unhelpful |
PAG – need for better education about continence training and a need for shared decision-making. There is a need to increase research capacity among practitioners, their understanding of the value of it and how to go about it; resources, time and skills are barriers to effective use of research in practice | |
|
Products for personal use in daily living Toilet adaptations Clothing adaptations Continence products |
Nurses and therapists found simple aids to be effective Therapists also highly rated bespoke aids and housing adaptations as effective interventions Parent carers found housing adaptations effective Specialist nurses and paediatricians found continence products to be effective Parent carers said that continence products were most effective interventions |
||
|
Products and technology for communication Equipment for communication |
|||
|
Products and technology for personal indoor and outdoor mobility Equipment for movement |
|||
|
Products for personal consumption (subset: drugs) Medications |
Nearly all nurses, paediatricians, surgeons and parent carers found medications to be effective and easy to use |
UTIs and side effects were high in some studies using drug interventions One randomised controlled crossover study found that effectiveness was mixed with active treatment (phenylpropanolamine) Within studies assessing effectiveness of medication interventions in mixed populations, the picture was mixed: one study found limited evidence of effect with intravesical oxybutynin and high rates of UTIs, one study found no effect of imipramine, and one study found limited improvement in continence for alprenolol combined with behavioural training |
|
| Medically assisted techniques and surgical approaches |
Surgeons and nurses found catheters and colonic enemas to be the most effective interventions Parent carers found catheters to be effective The majority of health professionals found surgical procedures to be effective |
One study reported effectiveness of a stepwise bowel management programme for spina bifida. Improvements post intervention were found in frequency of bowel movements, minutes spent of bowel care per day, frequency of nappy changes per day and reductions in the frequency of faecal incontinence incidents. QoL scores also improved post intervention. Similar improvements were reported in another population with continent episodes increasing One study reported 100% failure of an intraurethral self-retaining device Four studies focused on the use of antegrade enemas for faecal incontinence, and one study concentrated on the use of TAI using a Peristeen device. Results showed general improvements in faecal continence A SNM intervention (‘InterStim’) improved continence and was well tolerated One study found no evidence of improvement in urinary continence using transurethral intravesical electrotherapy as an intervention Several studies reported general and mixed improvements in continence post intervention following interventions using botulinum type A injections but parent satisfaction was mixed Studies focused on populations with neurogenic dysfunction reported mild improvements in continence post intervention (endoscopic injection of PDMS in two studies) Two studies found that surgery improved urinary continence and QoL scores Further studies found interventions generally effective in improving continence, and quality-of-life measures improved post intervention UTIs and reported side effects (e.g. difficulty in catheterisation, pelvic abscesses and failure rates) were high in some studies using surgical interventions |
|
| Outcomes | |||
|
Parent carer survey data focused on the initial aim of using an intervention and were not distinctly measurable, e.g. parent carers focused on their child developing toileting independence within their capability and to reduce the need for pads or nappy use, but specific urinary or faecal continence is not addressed Evaluations of interventions commonly conducted using parent and child verbal reports and physical examination Interventions generally reviewed 3-monthly Health professionals said that different interventions were offered for different environments, and school and social care staff highlighted a lack of consistency in support in different environments; a lack of adequate and accessible toileting facilities was highlighted by many respondents |
Focused on measures that health professionals may use to monitor a child’s progress with continence or an intervention, such as measures of urinary or faecal daytime and night-time continence No evidence |
PAG – lack of qualitative evidence in the review, but reported that this was not surprising. They felt that continence services were underfunded, resource poor and neglected in terms of research PAG – there is a greater need for research conducted with the non-spinal-cord-related pathology group. There is often no care pathway for these children and young people |
|
Consultation with stakeholders
Family Faculty group
Three parent carers participated in the meeting with two researchers, and one academic visitor observed. The three parent carers had experience of caring for children and young people with non-spinal-cord-related pathology; the feedback from this group, therefore, has been included in Table 15 only. During the meeting, we discussed and reflected on the quantitative data and then the qualitative data. In each case, we first presented the key data from the survey and systematic review and then asked the parent carers for their thoughts and reflections in terms of gaps, clear links and any unexpected findings.
Quantitative data reflection
The group were shown tables that listed the quantitative data collected in the survey and the systematic review specific to assessments, interventions and outcomes. They were given time to read and reflect on the lists presented and were asked for their thoughts, which prompted further discussion on the topic. The resulting discussions raised points relating to children’s mental health, depression and anxiety, puberty and menstruation and hearing loss.
The parent carers discussed the ‘changeability’ of or variation in continence over time. They felt that there was a need for assessments to monitor change or progress. They noted that for children and young people with conditions such as seizures or Asperger syndrome, continence can vary depending on what else is currently happening for the child. For example, poor seizure control or increased anxiety levels can result in a change in continence. The parent carers told us that in their experience with health services, there is sometimes an underlying assumption that progression with continence is linear, and emphasised that this is not always the case. This led the group to also highlight how stress and a child’s mental health, such as depression or anxiety, may have an adverse impact on continence.
The group discussed menstruation and developmental life stages. Menstruation and puberty were not in the scope of our study, and hence no data were gathered on either of these factors in the survey or the systematic review. Nevertheless, some of the group felt that it was important to note that, for some young people, these factors can have an effect on their management of continence and incontinence. Broader aspects of dealing with several physical health issues at one time that potentially affect the child’s ability to cope were also discussed.
With respect to behavioural interventions, the group felt that hearing impairment might have an impact on a child’s understanding and toileting ability, and potentially on the effectiveness of some interventions, particularly those using alarms. This led the group to discuss again how mental health may influence toileting, habit acting as a prompt (e.g. having been to an accessible toilet previously may act as a prompt for the child or young person to use that toilet again), and potentially using talking therapies such as cognitive–behavioural therapy. Furthermore, the group noted that non-physical measures (e.g. psychological outcomes related to mental health) could be used to assess progress.
Qualitative data reflection
We presented the eight qualitative themes identified in the systematic review data (see Chapter 3), which were written on flipcharts placed around the meeting room (Box 2). We asked attendees to identify which subthemes resonated with them (using a green sticker), which they did not recognise from their experiences (using a red sticker) and whether they had any additional comments (by writing on the flipcharts). The group were encouraged to allocate as many stickers and add as many comments as they wished. We also asked them to allocate some of the free-text comments from the survey to any of the themes or subthemes to which they thought that comments particularly applied.
Subthemes (a): uncertainty of accidents, (b): problems in relationships with health professionals – not being heard, (c): timing – lack of information regarding when to start a bowel programme.
Theme 2: the impact of the journey on the childSubthemes (a): universal embarrassment and assault to self-esteem, (b): school issues.
Theme 3: the family struggleSubthemes (a): overwhelming stress, (b): parents’ role negotiation, (c): advice to other families.
Theme 4: the promise of the futureSubthemes (a): the joy, (b): the frustration.
Theme 5: self-perception and self-esteemSubthemes (b): the frustration, (b): confidence in self-care.
Theme 6: challenges of self-catheterisationSubthemes (a): independence and dependence, (b): willingness to engage.
Theme 7: parents’ investment in their child’s bowel managementSubthemes (a): confidence, knowledge and experience, (b): support from professionals, (c): emotional stress and strength.
Theme 8: supporting their child’s independenceSubthemes (a): stressful experiences, (b): independence and dependence, (c): concerns for the future.
To note, the qualitative data from the systematic review came from studies of spinal cord pathology only, while the free-text responses from the survey related to either spinal cord and/or non-spinal-cord-related pathology. The parent carers present in the group only had personal experience of children with non-spinal-cord-related pathologies. Therefore, their reflections on the qualitative themes from the systematic review provide insight of the extent to which themes are shared across conditions.
The group allocated a number of green stickers to each theme, indicating that they had experience of or resonated with all of the themes. Only one red sticker was allocated, indicating that one member did not recognise, or had no experience of, ‘problems in relationships with health-care professionals’ under theme 1. One subtheme also received no indication of resonance or dissonance, and that was ‘willingness to engage’ under theme 6. Parent carers reacted the most to theme 1, ‘the impact of the journey on the child’, and its subthemes; they allocated the largest number of green stickers and most survey quotations to this theme, indicating its importance to families.
The group selected a number of quotations from the survey data that they felt reflected some of the themes from the systematic review; some also added their own quotations or comments under some of the themes. The quotations they selected are shown below in relation to the relevant theme.
Theme 2: the impact of the journey on the child
Subtheme (a): universal embarrassment and assault to self-esteem
It is not acceptable that nappies for older children are not supplied free of charge. Families are having to use ill-fitting adult incontinence products on young children which are uncomfortable, undignified and leak, causing distress.
Survey
Continence products provided we have found are not designed for active kids, they do not stay up well and are bulky and so make our 5.5yo feel very self-conscious at school.
Survey
Subtheme (b): school issues
Children with additional needs – whatever they may be, are often lost in the system once they transition to school. It is appalling.
Survey
From conversations with other families, toileting support seems to be an increasing problem in early years. Our daughter’s physical disability was so great that nursery/school made no objection to staff having to change her pads. However, for youngsters with different disabilities that impacts their toileting, they aren’t always afforded the same degree of understanding and inclusion. Also, my child’s best friend experience difficulties in mainstream school at adolescence when staff started objecting to supporting her to use the toilet once she had started her periods and suggested to her Mum that she have medication to stop her periods. They also would try to stop her drinking water in class, telling her that drinking would mean they’d have to go to the toilet and miss more class again. Despite being told she had a sensitive bladder and needed to drink because of risk of infection, and having an Education and Health Care Plan (ECHP), staff prioritised their comfort and convenience over her needs.
Survey
As my son gets older, his awareness of his own difficulties with toileting (mainly his inability to clean himself adequately) is growing and causing anxiety at school and avoidance of going to the toilet when he needs to. It is difficult to provide consistent modelling, prompting and reassurance for aspects of personal and intimate hygiene with school aged children.
Survey
[we sought support] to try and reduce anxiety around using the toilet in public areas and school.
Survey
My son has very high sensory needs and this may be why he does not realise he needs a wee until he is desperate. This has been an issue at school. I was even asked to provide evidence from his class teacher this year and she was refusing to let him go to the toilet and he nearly wet himself. I have managed to sort that issue out now thankfully. My son also had a double hernia in his groin that may have been caused by holding his bladder. This was operated on but he is at a higher risk of getting it again now. When he was younger nobody would listen when I was telling them there was something wrong with his bowel movements, not until he saw the paediatrician aged 3 and a half by which time his bowel had become ‘baggy’, in her terms. This may have been avoided by the GP (General Practitioner) giving him the Movicol at an earlier age rather than just keep telling me it will get better with age. He also still wets the bed at night when stressed.
Survey
Theme 3: the family struggle
Subtheme (a): overwhelming stress
The fact that the health service think that is acceptable for doubly incontinent children to manage on only 4 pads over a 24 hours period is truly shocking. Only parents who are prepared to fight get more. Children in wheelchairs have to sit in urine soaked pads all day. It’s neglect and an absolute disgrace. These children cannot fight for themselves for services and so if the parent isn’t doing it either because they can’t mentally cope with the stress or because they don’t know how to, no one is helping that child.
Survey
Subtheme (c): advice to other families
Never give up.
Comment by FF member
Theme 4: the promise of the future
Subtheme (a): the joy
‘The freedom!’, ‘dry nights’.
Comment by FF member
Subtheme (b): the frustration
‘Disabled toilet – judgment from others’ [in later discussion this was expanded on; others had been vocally judgmental when their child with a hidden disability was using disabled toilets, even with a radar access key].
Comment by FF member
Theme 5: self-perception and self-esteem
Subtheme (b): confidence in self-care
The parents just need to be listened to more. The children would suffer much less mental health issues if the interventions were done sooner!
Comment by FF member
Theme 6: supporting their child’s independence
Subtheme (b): independence and dependence
Daughter suggested swipe card toilets.
Comment by FF member
The group also suggested some new themes from the free-text survey responses, which they felt were not reflected within the eight existing themes. As their experience mainly related to non-spinal-cord-related conditions, they felt that different issues arose, including the following.
Social perception: from other people
-
‘Grief’: when a child with neurodisability has accidents, it is a daily reminder that they are not achieving.
-
Negative attitudes:
-
‘They are not disabled’ comments when using disabled toilets in public areas.
-
Shame and judgement – experienced by parent carers that their child is not toilet trained at an appropriate age. Parent carers suggested that they feel judged (by other parents and professionals) that their child cannot learn to toilet themselves or is delayed.
-
Embarrassment – for the child and the parent. An older child is likely to be embarrassed that they cannot manage toileting themselves.
-
Positive attitudes – often other parents have ‘handy hints’ and tips to try that are really helpful, which nurses or other support services may not suggest.
-
Communication
The following aspects of communication were highlighted as challenges in understanding the child’s needs and in providing support for toileting:
-
Is the child or young person verbal? That is, are they able to communicate their toileting needs verbally?
-
How do young people communicate their toileting needs?
-
Audio-processing delays – how these affect a child’s ability to communicate.
-
Comprehension challenges – can the child understand what is required in order to toilet?
Professional Advisory Group
The meeting was originally scheduled to be a face-to-face 2-hour meeting, but was instead convened as a 1-hour video conference because of the COVID-19 pandemic. We invited participants to consider and suggest research recommendations. We provided the Professional Advisory Group with a draft scientific summary of the study ahead of the video conference. Seven of the 12 members attended the meeting with three members of the research team; all were nurses, allied health professional or ERIC staff.
The group highlighted the paucity of qualitative research evidence in the systematic review, and particularly for children with non-spinal cord-related pathology, but reported that this was not surprising. They felt that NHS continence services were generally underfunded and resource poor, were neglected in terms of research and lacked research culture linked to practice. They were impressed with the number of parents and professionals who had participated in the surveys and thought that the fewer responses from children and young people was as they would have expected. They agreed that qualitative research adapted to suit neurodisability would be more appropriate than an online survey for ascertaining children and young people’s experiences.
The main trigger of a toileting assessment being delay in achieving independent toileting raised much discussion. The Professional Advisory Group were keen for early intervention. They felt that there needs to be further work around the developmental age and functioning level of children, particularly those with learning disabilities, as this encompasses a large spectrum of ability. For example, some children with severe learning difficulties may never meet the required developmental age considered normal for toilet training, although interventions enabling toilet training may be successful. Additionally, there is no consensus on when a child is ‘ready’ for toilet training; the group stressed that this is down to perception of the parent carer, or the professional and, therefore, needs clarity. This linked to timing, as members of the Professional Advisory Group felt that assessments and interventions were not introduced early enough leading to unnecessarily prolonged use containment products.
The need for improved education for parents, health professionals, education and care staff about continence training was emphasised by the group, as was the need for shared decision-making. The professional advisors suggested that increasing research awareness and capacity among practitioners and promoting understanding of the value of research and how to go about it, alongside the provision of resources, time and practical skills, would reduce the barriers to effective use of research in this area of practice.
The group were keen to emphasise that findings relating to children and young people with and without spinal cord pathology should not be considered together or compared. Approaches to the assessment and management of continence in each group address quite different issues. Similarly, the implications and recommendations for practice, research and policy arising from this work should be considered separately. The group advocated research to inform the care of children and young people with non-spinal-cord-related pathology. It was highlighted that there is often no evidence-based care pathway for these children and young people, whereas there is a clearer care pathway for the spinal cord pathology group.
Key findings
In Tables 15 and 16, comments and feedback received by members of the Family Faculty group are labelled with FF, and comments and feedback received by members of the Professional Advisory Group are marked with PAG. Feedback was related to assessments, interventions and outcomes as a whole (e.g. it was not necessarily specific to individual interventions such as behavioural interventions). Blank cells represent areas on which we did not gather data from the survey or the systematic review.
Summary of findings and stakeholder consultation
The findings have demonstrated that clinicians working with both non-spinal-cord pathology and spinal cord pathology groups employ a range of assessments and interventions in efforts to improve continence or independent toileting, depending on the needs of the child. Much of the evidence for children and young people with spinal cord pathology involves medicines and medically assisted and surgical techniques, whereas much of that for those with non-spinal-cord pathology is focused on behavioural interventions and making adaptations in the home. We did not identify evidence on the use of toilet and clothing adaptations in the systematic review, although the survey highlights that these types of interventions are frequently used and considered effective by the majority of professional groups, regardless of role.
The survey results illustrate the different roles that different professional groups have in assisting and enabling continence, highlighting the importance of a multidisciplinary team approach to better support children and young people and their families. Our stakeholders highlighted the need for early assessment and intervention, and the need for toileting assessments to consider the psychological aspects of toileting for young people with neurodisability, such as anxiety. The importance of assessing a child or young person’s preferred communication method and their mobility was also highlighted, as there was a distinct lack of evidence regarding communication and mobility interventions from both the survey and the systematic review.
In terms of future research, our stakeholders highlighted a number of key areas: (1) the need to involve children, young people and their families in testing and evaluating interventions in order to provide evidence that these are effective; (2) a need for more research on the effectiveness and cost-effectiveness of all interventions to improve continence for children and young people with neurodisability, particularly common conditions such as autism that have been relatively neglected in research; (3) improved education for health professionals around toileting assessments and interventions, informed by evidence and the lived experiences of children and young people and their families; and (4) the promotion of a multidisciplinary, holistic approach to improving continence.
Chapter 6 Discussion and recommendations
This research has explored what is being done to promote continence for children and young people with neurodisability and has examined whether there is any evidence that interventions are effective. The study shines a light on a neglected topic, for which clinical services are poorly resourced in many areas. There appears a paucity of research culture in terms of either routinely conducting or using applied research to inform decisions about interventions for children with neurodisability. Nevertheless, it emerged strongly throughout this research how incredibly important the subject of promoting continence is for families with children and young people who have neurodisability conditions. It was also evident that many professionals share a passion for improving continence. Hence, unsurprisingly, the topic was prioritised highly in a research prioritisation partnership in which families were able to suggest, advocate and vote for the treatment uncertainties that they thought most important to address. 15
The study-specific conceptual framework that we produced to design and interpret the research clearly distinguishes two groups of children with neurodisability with very different underlying pathology. Children with spinal cord pathology conditions, such as spina bifida, are likely to have impaired bladder and/or bowel sensorimotor control; these children require assistive technology or alternative approaches for emptying their bladder and bowels. By contrast, children with non-spinal-cord-related pathologies, such as learning disability or autism, have the potential to develop bladder and bowel sensorimotor control and to move towards becoming continent, albeit in some cases with assistance. We do not consider pads or nappies as interventions for ‘promoting’ continence; instead, these products are used for containment in both children with spinal cord pathology and children with non-spinal-cord-related pathology. We propose that promoting continence within individual capability is in the provision of training, technology and facilities to enable children and young people to be ‘clean and dry’ without the need for pads/nappies.
This research sought to establish, using a cross-sectional survey, what professionals and families were doing to improve continence, and, through a systematic review, whether or not there was any robust published evidence that interventions were effective.
Key findings
Our systematic review found a dearth of good-quality evidence for many of the interventions currently in use, and a paucity of evidence of children and young people’s or families’ experiences implementing those interventions. More evaluations have focused on medically assisted interventions and surgery for children with spinal cord pathology than on teaching children with non-spinal-cord-related pathologies how to empty their bowel and bladder into a toilet or potty. This is despite there being far fewer children with neurodisability with than without spinal cord pathology conditions.
Similarly, although a small number of qualitative studies explored the experiences of children with spinal cord pathology and toileting, we found none that explored the issues with children with non-spinal-cord-related pathology conditions. Therefore, there appears great scope for research to bring more attention to children with non-spinal-cord-related pathologies, such as autism and/or learning disability and cerebral palsy. Given the indifferent quality of evidence on the effectiveness of interventions, unsurprisingly we found no evidence at all on the cost-effectiveness of treatments for either group.
Our survey indicated how various clinicians from different professions have distinct and crucial roles to play in the assessment and treatment of continence in children with neurodisability. Early assessment and early intervention are interlinked and are crucial to success in promoting continence for children with neurodisability. Children with spinal cord pathology are typically recognised as such soon after birth and so appropriate medical or other treatments can be initiated. For children with non-spinal-cord-related pathology conditions early assessment is vital to rule out structural problems unrelated to their neurodisability. It is then crucial for early intervention to target the development of the skills necessary to improve continence and independence. Learning to use the toilet is a crucial early developmental skill and, insofar as it is possible, needs to be approached in the second year of life so that, as with other aspects of learning, the child is supported to become as independent as possible.
Overwhelmingly in the survey, child and parent reports were cited as a principal sources of information in assessment and reviews. Hence, it is important to raise expectations of parent carers and families about the potential for promoting continence and to avoid complacency and the prolonged use of pads when progress could be made. Health professionals, as well as school and care staff, have a role in raising expectations and reducing reliance on containment products. Much of the advice is based on practitioners’ experience in the absence of robust evidence, and the advice can, therefore, vary.
Although underlying bladder and bowel issues can result in symptoms that have a negative impact on the child’s and family’s QoL as well as on toilet training, health professionals may mistakenly attribute this to the child’s disability. The child may therefore not be assessed or treated appropriately. If this happens, children may not reach their potential and, in rare cases, serious underlying conditions may be overlooked. It is important that all children with delayed continence, including those with neurodisability, undergo an early and then regular holistic assessment of their bladder and bowel health and are supported with appropriate personalised treatment.
We recommend educating health professionals and school and care staff about strategies to improve continence; training must be informed by the needs and experiences of children and young people with neurodisability and their families.
Toilet training
We define toilet training as teaching a child how to empty their bowel and bladder into a toilet or potty at socially appropriate times so that nappies and pads are no longer necessary. This pertains, in the main, to those children and young people with non-spinal cord-related pathology; approaches may be tailored to children with learning disability or autism or for children with a physical disability such as cerebral palsy. All should be able to improve their continence through training, although some will require assistance with dressing or accessing or getting on and off the toilet. Most of the existing toilet training approaches involve variations on the original Azrin and Foxx approach.
We agree with Kroeger and Sorensen-Burnworth,117 who suggested that research should clarify behaviours pivotal to toilet training success, explore the limits of age and functioning, and review the prerequisite skills needed before toilet training can be successfully initiated. The factors that triggered an assessment of children in the non-spinal cord-related group were delayed toilet training and problems such as constipation. Therefore, it could be that families and professionals wait for a problem to develop before intervening. Our survey did not reveal proactive promotion of continence; no mentions were made of putting in place toilet skill development programmes. Children with certain conditions are more prone to constipation than others; hence, we need to raise awareness of potential problems among health professionals and families.
Environmental adaptations
Notably, none of the studies in the systematic review met our inclusion criteria relating to common interventions used by occupational therapists and others to assist children with toileting skills through environmental modifications. These interventions include (1) the provision of equipment such as rails, steps and bespoke toilet seats to enable access to and use of a toilet and (2) modifications to clothing, for example replacing buttons with Velcro® (Velcro Companies, Knutsford, UK) to make removing and fastening easier for children. There was little or no strong evidence to support educational interventions for children and parent carers. These therapy interventions fall into a category of ‘custom and practice’ because they have been used routinely by therapists and other professionals typically over a long period of time. This may explain the lack of published research and critical scrutiny of these interventions; research is perceived as not warranted, ethical or justified because interventions are ‘believed to help’ based on professional consensus and clinical experience. Many therapy interventions fall into this category of custom and practice, although there are continued uncertainties about the appropriate approaches, timing and frequency of therapy interventions. 15 A recent scoping review of speech and language, physical and occupational therapy interventions for children with neurodisability concluded that therapy interventions tend to be highly individualised and informed, or underpinned, by clinical experience. 132 However, questions remain related to these therapy interventions that demand critical enquiry. For example, we know that much assistive equipment is abandoned, at considerable cost to the NHS,133 and, hence, there is a need to evaluate cost-effectiveness, as was recently done for powered mobility for children with neurodisability. 134
The review by Beresford et al. 132 also highlighted other issues related to therapies that may throw light on the absence of published research into therapy toileting interventions. The first is that therapy interventions are regarded as complex and hence many of the potential ‘active ingredients’ offer challenges in terms of their ‘measurement’ and the measurement of change. 135 Second, the report highlighted that, despite a growing interest in research among therapists, there is currently no strong ‘culture’ of research within these allied health professions and, hence, there is a lack of skills and resources. 132
Three studies related to occupational therapy were excluded at the ‘full-text’ screening phase. The study by Donlau et al. 136 in children with myelomeningocele was excluded as the outcomes reported were related to performance of a monitoring measure, rather than measures of continence. The study by Drysdale et al. ,137 who used video modelling, incorporating animation, to teach toileting to two children with autism, was excluded because of the study type, as it was not comparative. Koshy et al. 138 examined sensory integration and toileting skills, but focused on prevalence and did not adequately define the intervention or outcome, so there were no valid effectiveness data.
The findings of our systematic review and survey can inform how we might improve research evaluating the effectiveness of physical and occupational therapy to improve continence for children with neurodisability. There is a call from leaders in the neurodisability field to focus research on what matters most to children and families. 139 Growing references within therapy discourse and practice use the language of the ICF and its potential as a harmonising conceptual framework. 1
Medical and surgical approaches
In the systematic review we identified predominantly behavioural educational and pharmacological interventions for patients with non-spinal neurodisability, whereas the studies on patients with spinal pathology also included the use of neurostimulation, other medically assisted procedures (e.g. bowel washouts) and surgery. Essentially, in children without spinal cord pathology, training, in its broadest terms, appears to be the preferred approach. However, in children with spinal cord pathology, interventions are required to try to address the lack of those abilities that are required to provide continence – specifically, an adequate capacity reservoir to allow the storage of urine and faeces, without leakage, until voluntary emptying is appropriate. In the case of the bladder, the reservoir has to store at low pressures to avoid damage to the kidneys.
To address storage problems, we identified studies showing success using medications, such as desmopressin for children and young people with non-spinal cord-related pathologies, and anticholinergic drugs predominantly in patients with spinal cord pathology. Desmopressin is solely used for the treatment of enuresis, which is night-time (bed) wetting. Children cannot be taught to be dry at night the same way that they can be toilet trained during the day. Dryness at night is the result of physiological processes that include bladder maturation and the production during sleep of sufficient arginine vasopressin, a hormone that reduces the production of urine through reabsorption of water in the renal tubules and quality of sleep/arousal. Bedwetting is a common condition in childhood, with a number of causes. Enuresis may be wrongly attributed to disability in those with neurodisability, rather than the children being offered the same assessment and treatment as their typically developing peers. Lower urinary tract symptoms can also have a negative impact on toilet training alongside enuresis. This further emphasises the importance of specialist holistic assessment for all children with delayed toilet training to ensure that issues such as overactive bladder or dysfunctional voiding are identified and treated, if present, as these conditions will have a negative impact on a child’s ability to become clean and dry.
Some studies in our review refer to treatments with anticholinergics. These drugs may be used to reduce detrusor (bladder wall muscle) spasms and contractility to increase bladder capacity. They are frequently used in children with spinal cord and non-spinal-cord-related pathologies to treat bladder overactivity, which is also a common condition in children and adults without neurodisability, which results in urinary urgency and frequency and may cause daytime and night-time wetting. If children have bladder overactivity, then this may negatively affect their ability to be toilet trained. Children who make progress with daytime urinary continence having had an anticholinergic will have had a presumptive, or proven, diagnosis of overactive bladder. They will, therefore, have had difficult toilet training regardless of their neurodisability.
Another pharmacological approach that is used to good effect is the intravesical injection of botulinum toxin. Although our search yielded only one paper on the subject, bladder augmentation (i.e. enlarging the bladder, usually with a segment of intestine) is well established and is ‘currently the gold standard surgical procedure used to increase bladder capacity and reduce storage pressures’. 140
To address the issue of emptying the bladder, CIC56 is widely used, often using a continent catheterisable channel (Mitrofanoff principle). 141 We did, however, identify a number of different operations on the bladder neck designed to reduce or prevent unwanted leakage of urine, although the results were modest and the additional injection of bulking agents into the bladder neck to narrow it and further reduce leakage was reported. Approaches to managing faecal incontinence – and constipation – mainly focused on the mechanical emptying of the bowel by retrograde (transanal/rectal irrigation) or antegrade (MACE/ACE) washouts, which generally had good results and, where reported, improved QoL.
Producing high-quality research is difficult when studying interventions in small and heterogeneous populations. For instance, randomisation and blinding present ethical and practical problems in assessing the results of surgical procedures aimed at treating small groups of children and young people with incontinence.
Methodological strengths and weaknesses
One of the strengths of this research is the positive impact on design and interpretations as a result of the involvement of families, professional advisors from the ERIC charity, our oversight group, and other stakeholders and end users. Our four-stage approach using preparation, review of evidence, consultation in the surveys and integration benefited from external ideas and scrutiny, for which we are hugely grateful. Our oversight group provided valuable advice during the preparation stage and this led to the incorporation of a needs-led approach into our conceptual framework.
Consistent with the commissioning brief, systematic review and survey methods were used to explore what is being done and whether or not any evidence of effectiveness exists. We have discussed strengths and limitations in the relevant chapters and so will not reiterate these here. However, these methods were unlikely to garner information on the experiences of young people with neurodisability. The views and experiences of children and young people with neurodisability in relation to toileting and improving continence should be investigated to enable better understanding, particularly of children and young people with non-spinal-cord-related pathology not found in our systematic review. We did find useful qualitative research with children with spinal cord pathology. 106–108 Qualitative research with teenagers who were experiencing daytime and/or night-time wetting or soiling revealed serious consequences of persistent continence problems. Young people said that they experienced depressive symptoms, peer victimisation, poor self-image, problems with relationships and negative school experiences. 142,143 Qualitative research methods can be adapted to capture the views of a wide range of children with neurodisability,144 adapted to individual communication needs, for instance using alternative augmentative communication techniques as appropriate.
An additional problem is the variety of terminologies used. The terminological issue is already being addressed by the International Children’s Continence Society, but more multicentre studies, providing larger numbers, might be encouraged, as should studies comparing different interventions for specific problems.
Implications for clinicians or policy-makers
Our survey revealed a marked contrast in professional and family views about availability and access to services. Around three-quarters of parent carers of children with non-spinal-cord-related pathology said that it was difficult or very difficult to get help and only one-quarter thought that the waiting times were satisfactory. By contrast, health professionals had more mixed views about how accessible their services were, although some said that waiting times for assessment or equipment were too long. Our consultation with the Professional Advisory Group confirmed that continence services are resourced poorly and would benefit from greater attention to increasing capacity. A firm recommendation from this research is to start assessment and intervention early. A long waiting list and any unacceptable delay can be considered potentially harmful to children with neurodisability.
There is cause for concern about how continence is managed in transition to adulthood and adult services that merits further investigation. Most parent carers of teenagers and young adults indicated that many of the key steps recommended by NICE for transition were not being achieved. Many school and care staff in the survey were unaware of procedures for transition to adult services.
Clearly, if we believe that it is worth investing in interventions and training to improve continence, and thereby promote independence, dignity and comfort, then it is beholden on society to provide accessible toilets. The increasing, but still woefully inadequate, availability of accessible toilets in the community cannot be ignored in interpreting this report. In response to the Changing Places campaign, the UK government committed to improving the availability of fully accessible toilets in existing buildings and mandatory in new public buildings. A fully accessible Changing Places specification toilet has more room than a standard accessible toilet, with specialist equipment such as an adult-sized changing bench and a hoist system.
Unanswered questions and future research
Throughout this research it has been evident that there is no ‘one size fits all’ standardised set of interventions. The underlying causes of each child’s continence problems will vary, as will their family, school and care circumstances. Instead, the particular combination and sequence of strategies to be tried will usually have to be tailored to these specific needs and circumstances. Conventional approaches to clinical trials and evaluations tend to standardise interventions and focus on estimating the average treatment effects of key outcomes, with less attention to these contextual factors. In both the non-spinal-cord-related and spinal cord pathology groups, developmental age, the cognitive functioning of the child and parents, and social circumstances all influence both the effectiveness, and also, therefore, the choice, of interventions and their timing.
This is important information to generate, but is less useful unless we also conduct parallel process evaluations that aim to understand what causes variations in effectiveness. This includes variations in adherence of children and their families, which may be linked to acceptability, and variations in implementation of the intervention by care professionals, for instance modifying to make it more feasible, but in ways that unknowingly may undermine its mechanisms of action. In contrast, the effectiveness of interventions will likely be increased when parents and professionals, preferably in close communication, monitor progress and modify it consistent with the intervention logic without compromising mechanisms of action. That’s why most surgeons work closely with clinical nurse specialists, who in turn are in regular contact with the families, especially following surgery.
For patients with spinal cord pathology, we identified evaluations predominantly of medically assisted techniques and surgical approaches, and for patients with non-spinal-cord-related pathology evaluations focused mainly on behavioural and other interventions. However, the methodological quality was generally rated as moderate to poor, using the EPHPP checklist, for all of these quantitative studies.
Evaluative research should be designed using robust methods to minimise or avoid biases. Although trials are more common in surgery, there are particular difficulties with evaluating less common procedures in rarer conditions, so it is about finding the best research design for a fair test of intervention.
As recommended from our systematic review, seeking consensus on a core outcome set of what aspects of health to measure for evaluative research on interventions to improve continence is essential to advance research in this field. Such methodological research could explore the extent to which there are overarching core outcomes for both spinal cord pathology and non-spinal-cord-related pathology conditions, and perhaps subsets of core outcomes most applicable within these groups.
Research recommendations
Specific lines of enquiry for research that we recommend based on our work are:
-
Work with families and children to seek agreement on core outcome sets with clear definitions to be used in future evaluations of interventions aiming to improve continence for children and young people with neurodisability.
-
More evidence on the effectiveness and value for money of interventions to improve continence is needed, including resource costs and QoL for children and young people with neurodisability and their families.
-
We need to understand better the personal factors that influence how and why interventions may be more successful for some children and young people with neurodisability than for others. These include social and economic circumstances of families and resources that influence success. A better understanding of these factors will enable interventions to be individualised and optimised acceptably.
-
We need better understanding about the key environmental factors that affect how interventions are implemented in different settings, particularly how school and care staff perceive their role, and access to resources to support implementing interventions.
-
We need evidence to support and inform continence care across transition to adulthood for people with neurodisability, ensuring equity of accessing services expediently, implementation and auditing of practice with reference to NICE guidance for transition, and whether it has an impact on how transition is managed in local care pathways.
Acknowledgements
We acknowledge support from the NIHR Applied Research Collaboration South West Peninsula (PenARC), parent carers in the PenCRU, Family Faculty, and colleagues at ERIC and the ERIC Professional Advisory Group. We also acknowledge involvement from the Pelican Project and Ellen Tinkham School, which provided us the opportunity to consult with young people with special educational needs or disability.
Contributions of authors
Helen Eke (https://orcid.org/0000-0003-4781-6683) (Research Fellow) was the project manager.
Helen Eke, Susan Ball (https://orcid.org/0000-0002-9937-4832) (Senior Research Fellow) and Christopher Morris (https://orcid.org/0000-0002-9916-507X) (Professor of Child Health Research) led the survey work, with input from the whole team.
Harriet Hunt (https://orcid.org/0000-0003-1254-0568) (Research Fellow), Morwenna Rogers (https://orcid.org/0000-0002-6039-238X) (Information Specialist), Rebecca Whear (https://orcid.org/0000-0002-8379-8198) (Research Fellow) and Jo Thompson Coon (https://orcid.org/0000-0002-5161-0234) (Professor of Evidence Synthesis and Health Policy) led the systematic review.
Annette Allinson (https://orcid.org/0000-0002-4003-2731) (PenCRU Family Faculty Member) and Julia Melluish (https://orcid.org/0000-0002-9115-0906) (PenCRU Family Faculty Member) are parent carers and were PPI co-investigators.
Claire Lindsay (https://orcid.org/0000-0003-2742-6610) (Clinical and Professional Lead, Paediatric Bladder and Bowel Care Service), Davina Richardson (https://orcid.org/0000-0002-5081-7866) (Children’s Specialist Continence Nurse), June Rogers (https://orcid.org/0000-0002-9752-1181) (Children’s Specialist Continence Nurse), Eve Hutton (https://orcid.org/0000-0001-9672-3075) (Reader in Children and Young People’s Health and Wellbeing), Nicholas Madden (https://orcid.org/0000-0003-2216-1166) (Consultant Paediatric Surgeon/Urologist), Anne Wright (https://orcid.org/0000-0001-5949-6387) (Consultant Paediatrician) and Stuart Logan (https://orcid.org/0000-0002-9279-261X) (Professor of Paediatric Epidemiology) all provided clinical expertise to all aspects of the work, interpretation of findings and report.
Rob Anderson (https://orcid.org/0000-0002-3523-8559) (Professor of Health Services and Implementation Research) provided expertise in health complexity and health economics.
Christopher Morris was the principal investigator.
All authors were involved in designing the study and drafting the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- World Health Organization . International Classification of Functioning, Disability and Health 2001.
- Taras ME, Matese M, Matson JL. Handbook of Behavior Modification with the Mentally Retarded. Boston, MA: Springer; 1990.
- Morris C, Janssens A, Tomlinson R, Williams J, Logan S. Towards a definition of neurodisability: a Delphi survey. Dev Med Child Neurol 2013;55:1103-8. https://doi.org/10.1111/dmcn.12218.
- Foreman DM, Thambirajah MS. Conduct disorder, enuresis and specific developmental delays in two types of encopresis: a case-note study of 63 boys. Eur Child Adolesc Psychiatry 1996;5:33-7. https://doi.org/10.1007/BF00708212.
- Järvelin MR. Developmental history and neurological findings in enuretic children. Dev Med Child Neurol 1989;31:728-36. https://doi.org/10.1111/j.1469-8749.1989.tb04068.x.
- Joinson C, Heron J, von Gontard A, Butler U, Golding J, Emond A. Early childhood risk factors associated with daytime wetting and soiling in school-age children. J Pediatr Psychol 2008;33:739-50. https://doi.org/10.1093/jpepsy/jsn008.
- von Wendt L, Similä S, Niskanen P, Järvelin MR. Development of bowel and bladder control in the mentally retarded. Dev Med Child Neurol 1990;32:515-18. https://doi.org/10.1111/j.1469-8749.1990.tb16977.x.
- von Gontard A, de Jong TP, Rantell A, Nieuwhof-Leppink A, Badawi JK, Cardozo L. Do we manage incontinence in children and adults with special needs adequately? ICI-RS 2014. Neurourol Urodyn 2016;35:304-6. https://doi.org/10.1002/nau.22823.
- Townsend J, Heng L, Thomas T, Egan M, Meade TW. Costs of incontinence to families with severely handicapped children. Community Med 1981;3:119-22. https://doi.org/10.1093/oxfordjournals.pubmed.a043412.
- Von Gontard A, Nevéus T. The Management of Disorders of Bladder and Bowel Control in Childhood. London: Mac Keith Press; 2006.
- Brazelton TB. A child-oriented approach to toilet training. Pediatrics 1962;29:121-8.
- Foxx RM, Azrin NH. Dry pants: a rapid method of toilet training children. Behav Res Ther 1973;11:435-42. https://doi.org/10.1016/0005-7967(73)90102-2.
- von Gontard A. Urinary incontinence in children with special needs. Nat Rev Urol 2013;10:667-74. https://doi.org/10.1038/nrurol.2013.213.
- Klassen TP, Kiddoo D, Lang ME, Friesen C, Russell K, Spooner C, et al. The effectiveness of different methods of toilet training for bowel and bladder control. Evid Rep Technol Assess 2006;147:1-57.
- Morris C, Simkiss D, Busk M, Morris M, Allard A, Denness J, et al. Setting research priorities to improve the health of children and young people with neurodisability: a British Academy of Childhood Disability-James Lind Alliance Research Priority Setting Partnership. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006233.
- Department for Education and Department of Health and Social Care . Special Educational Needs and Disability Code of Practice: 0 to 25 Years – Statutory Guidance for Organisations Which Work With and Support Children and Young People Who Have Special Educational Needs or Disabilities 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/398815/SEND_Code_of_Practice_January_2015.pdf (accessed 9 November 2021).
- The Special Educational Needs and Disability Regulations 2014 n.d. www.legislation.gov.uk/uksi/2014/1530/contents/made (accessed 19 October 2021).
- Great Britain . Children and Families Act 2014: Part 3 2014.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. https://doi.org/10.1136/bmj.39335.541782.AD.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. https://doi.org/10.1016/j.ijsu.2010.02.007.
- Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the Standardization Committee of the International Children’s Continence Society. J Urol 2014;191:1863-5. e13. https://doi.org/10.1016/j.juro.2014.01.110.
- McComb JG. A practical clinical classification of spinal neural tube defects. Childs Nerv Syst 2015;31:1641-57. https://doi.org/10.1007/s00381-015-2845-9.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Plsek PE, Greenhalgh T. Complexity science: The challenge of complexity in health care. BMJ 2001;323:625-8. https://doi.org/10.1136/bmj.323.7313.625.
- Shiell A, Hawe P, Gold L. Complex interventions or complex systems? Implications for health economic evaluation. BMJ 2008;336:1281-3. https://doi.org/10.1136/bmj.39569.510521.AD.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4. https://doi.org/10.1186/2046-4053-4-1.
- OECD . List of OECD Member Countries – Ratification of the Convention on the OECD n.d. www.oecd.org/about/document/ratification-oecd-convention.htm (accessed 19 October 2021).
- Turpin DL. CONSORT and QUOROM guidelines for reporting randomized clinical trials and systematic reviews. Am J Orthod Dentofacial Orthop 2005;128:681-5. https://doi.org/10.1016/j.ajodo.2005.10.010.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Jackson N, Waters E. Guidelines for Systematic Reviews in Health Promotion and Public Health Taskforce . Criteria for the systematic review of health promotion and public health interventions. Health Promot Int 2005;20:367-74. https://doi.org/10.1093/heapro/dai022.
- Wallace A, Croucher K, Quilgars D, Baldwin S. Meeting the challenge: developing systematic reviewing in social policy. Policy Politics 2004;32:455-70. https://doi.org/10.1332/0305573042009444.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2011.
- Thompson Coon J, Gwernan-Jones R, Garside R, Nunns M, Shaw L, Melendez-Torres G, et al. Developing methods for the overarching synthesis of quantitative and qualitative evidence: the interweave synthesis approach. Res Synth Methods 2020;11:507-21. https://doi.org/10.1002/jrsm.1383.
- Yang TK, Guo YJ, Chang HC, Yang HJ, Huang KH. Attention deficit-hyperactivity disorder symptoms and daytime voiding symptoms in children with primary enuresis: an observational study to evaluate the effectiveness of desmopressin treatment. Scientific World Journal 2015;2015. https://doi.org/10.1155/2015/356121.
- Chertin B, Koulikov D, Abu-Arafeh W, Mor Y, Shenfeld OZ, Farkas A. Treatment of nocturnal enuresis in children with attention deficit hyperactivity disorder. J Urol 2007;178:1744-7. https://doi.org/10.1016/j.juro.2007.03.171.
- Gor RA, Fuhrer J, Schober JM. A retrospective observational study of enuresis, daytime voiding symptoms, and response to medical therapy in children with attention deficit hyperactivity disorder and autism spectrum disorder. J Pediatr Urol 2012;8:314-17. https://doi.org/10.1016/j.jpurol.2010.10.009.
- Lomas Mevers J, Call NA, Gerencser KR, Scheithauer M, Miller SJ, Muething C, et al. A pilot randomized clinical trial of a multidisciplinary intervention for encopresis in children with autism spectrum disorder. J Autism Dev Disord 2020;50:757-65. https://doi.org/10.1007/s10803-019-04305-5.
- Mruzek DW, McAleavey S, Loring WA, Butter E, Smith T, McDonnell E, et al. A pilot investigation of an iOS-based app for toilet training children with autism spectrum disorder. Autism 2019;23:359-70. https://doi.org/10.1177/1362361317741741.
- Ardic A, Cavkaytar A. Effectiveness of the modified intensive toilet training method on teaching toilet skills to children with autism. Educ Training Autism Dev Disabil 2014;49:263-76.
- Keen D, Brannigan KL, Cuskelly M. Toilet training for children with autism: the effects of video modeling category. J Dev Physical Disabil 2007;19:291-303. https://doi.org/10.1007/s10882-007-9044-x.
- Cicero FR, Pfadt A. Investigation of a reinforcement-based toilet training procedure for children with autism. Res Dev Disabil 2002;23:319-31. https://doi.org/10.1016/S0891-4222(02)00136-1.
- Edgar CL, Kohler HF, Hardman S. A new method for toilet training developmentally disabled children. Percept Mot Skills 1975;41:63-9. https://doi.org/10.2466/pms.1975.41.1.63.
- Hundziak M, Maurer RA, Watson LS. Operant conditioning in toilet training of severely mentally retarded boys. Am J Ment Defic 1965;70:120-4.
- Sadler OW, Merkert F. Evaluating the Foxx and Azrin toilet training procedure for retarded children in a day training center. Behav Ther 1977;8:499-500. https://doi.org/10.1016/S0005-7894(77)80093-2.
- Van Laecke E, Raes A, Vande Walle J, Hoebeke P. Adequate fluid intake, urinary incontinence, and physical and/or intellectual disability. J Urol 2009;182:2079-84. https://doi.org/10.1016/j.juro.2009.05.125.
- Barmann BC, Katz RC, O’Brien F, Beauchamp KL. Treating irregular enuresis in developmentally disabled persons: a study in the use of overcorrection. Behav Modification 1981;5:336-46. https://doi.org/10.1177/014544558153003.
- Rinald K, Mirenda P. Effectiveness of a modified rapid toilet training workshop for parents of children with developmental disabilities. Res Dev Disabil 2012;33:933-43. https://doi.org/10.1016/j.ridd.2012.01.003.
- Lomas Mevers J, Muething C, Call NA, Scheithauer M, Hewett S. A consecutive case series analysis of a behavioral intervention for enuresis in children with developmental disabilities. Dev Neurorehabil 2018;21:336-44. https://doi.org/10.1080/17518423.2018.1462269.
- Didden R, Sikkema SP, Bosman IT, Duker PC, Curfs LM. Use of a modified Azrin-Foxx toilet training procedure with individuals with Angelman-Syndrome. J Appl Res Intellectual Dis 2001;14:64-70. https://doi.org/10.1046/j.1468-3148.2001.00047.x.
- Valentine A, Maxwell C. Enuresis in severely subnormal children: a clinical trial of imipramine. J Ment Subnormal 1968;14:84-90. https://doi.org/10.1179/BJMS.1968.014.
- Kajbafzadeh AM, Sharifi-Rad L, Baradaran N, Nejat F. Effect of pelvic floor interferential electrostimulation on urodynamic parameters and incontinency of children with myelomeningocele and detrusor overactivity. Urology 2009;74:324-9. https://doi.org/10.1016/j.urology.2008.12.085.
- Kajbafzadeh AM, Sharifi-Rad L, Ladi Seyedian SS, Masoumi A. Functional electrical stimulation for management of urinary incontinence in children with myelomeningocele: a randomized trial. Pediatr Surg Int 2014;30:663-8. https://doi.org/10.1007/s00383-014-3503-0.
- Schurch B, Corcos J. Botulinum toxin injections for paediatric incontinence. Curr Opin Urol 2005;15:264-7. https://doi.org/10.1097/01.mou.0000172401.92761.86.
- Loening-Baucke V, Desch L, Wolraich M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Dev Med Child Neurol 1988;30:781-90. https://doi.org/10.1111/j.1469-8749.1988.tb14640.x.
- Marshall D. Objective Assessment of Bladder and Bowel Function Following Cutaneous Electrical Field Stimulation in Children with Spina Bifida: A Randomised Controlled Trial. Belfast: Queen’s University Belfast; 2001.
- Steinbok P, MacNeily AE, Hengel AR, Afshar K, Landgraf JM, Hader W, et al. Filum section for urinary incontinence in children with occult tethered cord syndrome: a randomized, controlled pilot study. J Urol 2016;195:1183-8. https://doi.org/10.1016/j.juro.2015.09.082.
- Van Winckel M, Van Biervliet S, Van Laecke E, Hoebeke P. Is an anal plug useful in the treatment of fecal incontinence in children with spina bifida or anal atresia?. J Urol 2006;176:342-4. https://doi.org/10.1016/S0022-5347(06)00302-8.
- Choi EK, Shin SH, Im YJ, Kim MJ, Han SW. The effects of transanal irrigation as a stepwise bowel management program on the quality of life of children with spina bifida and their caregivers. Spinal Cord 2013;51:384-8. https://doi.org/10.1038/sc.2013.8.
- Dietrich S, Okamoto G. Bowel training for children with neurogenic dysfunction: a follow-up. Arch Phys Med Rehabil 1982;63:166-70.
- Aksnes G, Diseth TH, Helseth A, Edwin B, Stange M, Aafos G, et al. Appendicostomy for antegrade enema: effects on somatic and psychosocial functioning in children with myelomeningocele. Pediatrics 2002;109:484-9. https://doi.org/10.1542/peds.109.3.484.
- Ausili E, Focarelli B, Tabacco F, Murolo D, Sigismondi M, Gasbarrini A, et al. Transanal irrigation in myelomeningocele children: an alternative, safe and valid approach for neurogenic constipation. Spinal Cord 2010;48:560-5. https://doi.org/10.1038/sc.2009.186.
- Ausili E, Marte A, Brisighelli G, Midrio P, Mosiello G, La Pergola E, et al. Short versus mid-long-term outcome of transanal irrigation in children with spina bifida and anorectal malformations. Childs Nerv Syst 2018;34:2471-9. https://doi.org/10.1007/s00381-018-3860-4.
- Choi EK, Han SW, Shin SH, Ji Y, Chon JH, Im YJ. Long-term outcome of transanal irrigation for children with spina bifida. Spinal Cord 2015;53:216-20. https://doi.org/10.1038/sc.2014.234.
- Hascoet J, Peyronnet B, Forin V, Baron M, Capon G, Prudhomme T, et al. Intradetrusor injections of botulinum toxin type A in children with spina bifida: a multicenter study. Urology 2018;116:161-7. https://doi.org/10.1016/j.urology.2018.02.033.
- Kajbafzadeh AM, Moosavi S, Tajik P, Arshadi H, Payabvash S, Salmasi AH, et al. Intravesical injection of botulinum toxin type A: management of neuropathic bladder and bowel dysfunction in children with myelomeningocele. Urology 2006;68:1091-6. https://doi.org/10.1016/j.urology.2006.05.056.
- Killam PE, Jeffries JS, Varni JW. Urodynamic biofeedback treatment of urinary incontinence in children with myelomeningocele. Biofeedback Self Regul 1985;10:161-71. https://doi.org/10.1007/BF01000751.
- Ladi-Seyedian SS, Sharifi-Rad L, Kajbafzadeh AM. Intravesical electromotive botulinum toxin type ‘A’ administration for management of urinary incontinence secondary to neuropathic detrusor overactivity in children: long-term follow-up. Urology 2018;114:167-74. https://doi.org/10.1016/j.urology.2017.11.039.
- Lima SVC, Vilar FO, Lustosa ES, Aragão DCC, Calisto FCFS, Pinto FCM. New device for intermittent emptying of the bladder in female children and adolescents: a pilot study. J Pediatr Urol 2017;13:453.e1-6. https://doi.org/10.1016/j.jpurol.2016.12.030.
- Mattsson S, Gladh G. Tap-water enema for children with myelomeningocele and neurogenic bowel dysfunction. Acta Paediatr 2006;95:369-74. https://doi.org/10.1080/08035250500437507.
- Shoshan L, Ben-Zvi D, Katz-Leurer M. Use of the anal plug in the treatment of fecal incontinence in patients with meningomyelocele. J Pediatr Nurs 2008;23:395-9. https://doi.org/10.1016/j.pedn.2006.09.006.
- Horowitz M, Combs AJ, Gerdes D. Desmopressin for nocturnal incontinence in the spina bifida population. J Urol 1997;158:2267-8. https://doi.org/10.1016/S0022-5347(01)68232-6.
- Tarcan T, Akbal C, Sekerci CA, Top T, Simşek F. Intradetrusor injections of onabotulinum toxin-A in children with urinary incontinence due to neurogenic detrusor overactivity refractory to antimuscarinic treatment. Korean J Urol 2014;55:281-7. https://doi.org/10.4111/kju.2014.55.4.281.
- Palmer LS, Richards I, Kaplan WE. Transrectal electrostimulation therapy for neuropathic bowel dysfunction in children with myelomeningocele. J Urol 1997;157:1449-52. https://doi.org/10.1016/S0022-5347(01)65017-1.
- Radojicic Z, Milivojevic S, Lazovic JM, Becanovic S, Korićanac I, Milic N. The impact of bowel management on the quality of life in children with spina bifida with overactive bladder and detrusor sphincter dyssynergia. J Pediatr Urol 2019;15:457-66. https://doi.org/10.1016/j.jpurol.2019.05.005.
- Snodgrass W, Keefover-Hicks A, Prieto J, Bush N, Adams R. Comparing outcomes of slings with versus without enterocystoplasty for neurogenic urinary incontinence. J Urol 2009;181:2709-14. https://doi.org/10.1016/j.juro.2009.02.035.
- Han SW, Kim MJ, Kim JH, Hong CH, Kim JW, Noh JY. Intravesical electrical stimulation improves neurogenic bowel dysfunction in children with spina bifida. J Urol 2004;171:2648-50. https://doi.org/10.1097/01.ju.0000108542.27476.b8.
- King SK, Stathopoulos L, Pinnuck L, Wells J, Hutson J, Heloury Y. Retrograde continence enema in children with spina bifida: not as effective as first thought. J Paediatr Child Health 2017;53:386-90. https://doi.org/10.1111/jpc.13408.
- Petersen T. Management of urinary incontinence in children with myelomeningocele. Acta Neurol Scand 1987;75:52-5. https://doi.org/10.1111/j.1600-0404.1987.tb07888.x.
- Vande Velde S, Van Biervliet S, Van Renterghem K, Van Laecke E, Hoebeke P, Van Winckel M. Achieving fecal continence in patients with spina bifida: a descriptive cohort study. J Urol 2007;178:2640-4. https://doi.org/10.1016/j.juro.2007.07.060.
- Bar-Yosef Y, Castellan M, Joshi D, Labbie A, Gosalbez R. Total continence reconstruction using the artificial urinary sphincter and the Malone antegrade continence enema. J Urol 2011;185:1444-7. https://doi.org/10.1016/j.juro.2010.11.049.
- Ibrahim M, Ismail NJ, Mohammad MA, Ismail H, Ahmed MH, Femi OL, et al. Managing fecal incontinence in patients with myelomeningocele in Sub-Saharan Africa: role of antegrade continence enema (ACE). J Pediatr Surg 2017;52:554-7. https://doi.org/10.1016/j.jpedsurg.2016.08.014.
- Matsuno D, Yamazaki Y, Shiroyanagi Y, Ueda N, Suzuki M, Nishi M, et al. The role of the retrograde colonic enema in children with spina bifida: is it inferior to the antegrade continence enema?. Pediatr Surg Int 2010;26:529-33. https://doi.org/10.1007/s00383-010-2585-6.
- Snodgrass W, Granberg C. Clinical indications for augmentation in children with neurogenic urinary incontinence following bladder outlet procedures: results of a 14-year observational study. J Pediatr Urol 2016;12:46.e1-8. https://doi.org/10.1016/j.jpurol.2015.06.018.
- Van Savage JG, Yohannes P. Laparoscopic antegrade continence enema in situ appendix procedure for refractory constipation and overflow fecal incontinence in children with spina bifida. J Urol 2000;164:1084-7. https://doi.org/10.1016/S0022-5347(05)67257-6.
- Wehby MC, O’Hollaren PS, Abtin K, Hume JL, Richards BJ. Occult tight filum terminale syndrome: results of surgical untethering. Pediatr Neurosurg 2004;40:51-7. https://doi.org/10.1159/000078908.
- Schletker J, Edmonds T, Jacobson R, Ketzer J, Hall J, Trecartin A, et al. Bowel management program in patients with spina bifida. Pediatr Surg Int 2019;35:243-5. https://doi.org/10.1007/s00383-018-4403-5.
- Haddad M, Besson R, Aubert D, Ravasse P, Lemelle J, El Ghoneimi A, et al. Sacral neuromodulation in children with urinary and fecal incontinence: a multicenter, open label, randomized, crossover study. J Urol 2010;184:696-701. https://doi.org/10.1016/j.juro.2010.03.054.
- Borzyskowski M, Mundy AR, Neville BG, Park L, Kinder CH, Joyce MR, et al. Neuropathic vesicourethral dysfunction in children. A trial comparing clean intermittent catheterisation with manual expression combined with drug treatment. Br J Urol 1982;54:641-4. https://doi.org/10.1111/j.1464-410x.1982.tb13615.x.
- Corbett P, Denny A, Dick K, Malone PS, Griffin S, Stanton MP. Peristeen integrated transanal irrigation system successfully treats faecal incontinence in children. J Pediatr Urol 2014;10:219-22. https://doi.org/10.1016/j.jpurol.2013.08.006.
- Schulte-Baukloh H, Mürtz G, Henne T, Michael T, Miller K, Knispel HH. Urodynamic effects of propiverine hydrochloride in children with neurogenic detrusor overactivity: a prospective analysis. BJU Int 2006;97:355-8. https://doi.org/10.1111/j.1464-410X.2006.05953.x.
- Schulte-Baukloh H, Mürtz G, Heine G, Austin P, Miller K, Michael T, et al. Urodynamic effects of propiverine in children and adolescents with neurogenic bladder: results of a prospective long-term study. J Pediatr Urol 2012;8:386-92. https://doi.org/10.1016/j.jpurol.2011.07.014.
- Guys JM, Simeoni-Alias J, Fakhro A, Delarue A. Use of polydimethylsiloxane for endoscopic treatment of neurogenic urinary incontinence in children. J Urol 1999;162:2133-5. https://doi.org/10.1016/S0022-5347(05)68141-4.
- Guys JM, Breaud J, Hery G, Camerlo A, Le Hors H, De Lagausie P. Endoscopic injection with polydimethylsiloxane for the treatment of pediatric urinary incontinence in the neurogenic bladder: long-term results. J Urol 2006;175:1106-10. https://doi.org/10.1016/S0022-5347(05)00404-0.
- Silveri M, Capitanucci ML, Mosiello G, Broggi G, De Gennaro M. Endoscopic treatment for urinary incontinence in children with a congenital neuropathic bladder. Br J Urol 1998;82:694-7. https://doi.org/10.1046/j.1464-410x.1998.00810.x.
- González R, Jednak R, Franc-Guimond J, Schimke CM. Treating neuropathic incontinence in children with seromuscular colocystoplasty and an artificial urinary sphincter. BJU Int 2002;90:909-11. https://doi.org/10.1046/j.1464-410X.2002.03036.x.
- Do Ngoc Thanh C, Audry G, Forin V. Botulinum toxin type A for neurogenic detrusor overactivity due to spinal cord lesions in children: a retrospective study of seven cases. J Pediatr Urol 2009;5:430-6. https://doi.org/10.1016/j.jpurol.2009.06.001.
- Boone TB, Roehrborn CG, Hurt G. Transurethral intravesical electrotherapy for neurogenic bladder dysfunction in children with myelodysplasia: a prospective, randomized clinical trial. J Urol 1992;148:550-4. https://doi.org/10.1016/S0022-5347(17)36651-X.
- Åmark P, Beck O. Effect of phenylpropanolamine on incontinence in children with neurogenic bladders. A double-blind crossover study. Acta Paediatr 1992;81:345-50. https://doi.org/10.1111/j.1651-2227.1992.tb12240.x.
- Åmark P, Bussman G, Eksborg S. Follow-up of long-time treatment with intravesical oxybutynin for neurogenic bladder in children. Eur Urol 1998;34:148-53. https://doi.org/10.1159/000019701.
- Naglo AS, Boreus LO, Hellstrom B, Nergardh A. Is beta-receptor blockade of value in continence training of children?. Scand J Rehabil Med 1979;11:63-6.
- Faure A, Hery G, Mille E, Orillac P, Da Silva B, Merrot T, et al. Long-term efficacy of Young-Dees bladder neck reconstruction: role of the associated bladder neck injection for the treatment of children with urinary incontinence. Urology 2017;108:166-70. https://doi.org/10.1016/j.urology.2017.06.005.
- Jawaheer G, Rangecroft L. The Pippi Salle procedure for neurogenic urinary incontinence in childhood: a three-year experience. Eur J Pediatr Surg 1999;9:9-11. https://doi.org/10.1055/s-2008-1072303.
- Azrin NH, Sneed TJ, Foxx RM. Dry-bed training: rapid elimination of childhood enuresis. Behav Res Ther 1974;12:147-56. https://doi.org/10.1016/0005-7967(74)90111-9.
- Marshall DF, Boston VE. Altered bladder and bowel function following cutaneous electrical field stimulation in children with spina bifida – interim results of a randomized double-blind placebo-controlled trial. Eur J Pediatr Surg 1997;7:41-3. https://doi.org/10.1055/s-2008-1071209.
- Edwards M, Borzyskowski M, Cox A, Badcock J. Neuropathic bladder and intermittent catheterization: social and psychological impact on children and adolescents. Dev Med Child Neurol 2004;46:168-77. https://doi.org/10.1017/s0012162204000301.
- Sanders C, Bray L, Driver C, Harris V. Parents of children with neurogenic bowel dysfunction: their experiences of using transanal irrigation with their child. Child Care Health Dev 2014;40:863-9. https://doi.org/10.1111/cch.12117.
- Sawin KJ, Thompson NM. The experience of finding an effective bowel management program for children with spina bifida: the parent’s perspective. J Pediatric Nurs 2009;24:280-91. https://doi.org/10.1016/j.pedn.2008.03.008.
- Sawin KJ, Buran CF, Brei TJ, Fastenau PS. Correlates of functional status, self-management, and develop-mental competence outcomes in adolescents with spina bifida. SCI Nurs 2003;20:72-85. https://doi.org/10.1177/089801010202000307.
- Orem DE. Nursing: Concepts of Practice. St. Louis, MO: Mosby; 1995.
- Renpenning KM, Taylor SG. Self-care Theory in Nursing: Selected Papers of Dorothea Orem. New York, NY: Springer; 2003.
- Vermandel A, Van Kampen M, Van Gorp C, Wyndaele JJ. How to toilet train healthy children? A review of the literature. Neurourol Urodyn 2008;27:162-6. https://doi.org/10.1002/nau.20490.
- Brazelton TB, Christophersen ER, Frauman AC, Gorski PA, Poole JM, Stadtler AC, et al. Instruction, timeliness, and medical influences affecting toilet training. Pediatrics 1999;103:1353-8.
- Azrin N, Foxx R. A rapid method of toilet training the institutionalized retarded 1. Journal of Applied Behavior Analysis 1971;4:89-9. https://doi.org/10.1901/jaba.1971.4-89.
- Azrin N, Foxx RM. Toilet Training In Less Than A Day. New York, NY: Gallery Books; 2019.
- Wu HY. Achieving urinary continence in children. Nat Rev Urol 2010;7:371-7. https://doi.org/10.1038/nrurol.2010.78.
- Kroeger K, Sorensen-Burnworth R. Toilet training individuals with autism and other developmental disabilities: a critical review. Res Autism Spectrum Dis 2009;3:607-18. https://doi.org/10.1016/j.rasd.2009.01.005.
- Kroll P. The current role of Botox in a pediatric neurogenic bladder condition. Curr Bladder Dysfunction Rep 2019;14:115-23. https://doi.org/10.1007/s11884-019-00516-9.
- Hascoet J, Manunta A, Brochard C, Arnaud A, Damphousse M, Menard H, et al. Outcomes of intra-detrusor injections of botulinum toxin in patients with spina bifida: a systematic review. Neurourol Urodyn 2017;36:557-64. https://doi.org/10.1002/nau.23025.
- Alhazmi H, Trbay M, Alqarni N, Alyami F, Khatab A, Almannie R, et al. Long-term results using a transanal irrigation system (Peristeen®) for treatment of stool incontinence in children with myelomeningocele. J Pediatr Urol 2019;15:34.e1-5. https://doi.org/10.1016/j.jpurol.2018.08.013.
- Mosiello G, Marshall D, Rolle U, Crétolle C, Santacruz BG, Frischer J, et al. Consensus review of best practice of transanal irrigation in children. J Pediatr Gastroenterol Nutr 2017;64:343-52. https://doi.org/10.1097/MPG.0000000000001483.
- Ambartsumyan L, Rodriguez L. Bowel management in children with spina bifida. J Pediatr Rehabil Med 2018;11:293-301. https://doi.org/10.3233/PRM-170533.
- Deutekom M, Dobben AC. Plugs for containing faecal incontinence. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD005086.pub4.
- Cazemier M, Felt-Bersma RJ, Mulder CJ. Anal plugs and retrograde colonic irrigation are helpful in fecal incontinence or constipation. World J Gastroenterol 2007;13:3101-5. https://doi.org/10.3748/wjg.v13.i22.3101.
- Evans JR, Mathur A. The value of online surveys. Internet Res 2005;15:195-219. https://doi.org/10.1108/10662240510590360.
- Department of Health and Social Care . Good Practice in Continence Services 2000. www.nhs.uk/chq/documents/2015%20uploads/dh%20-%20good%20practice%20in%20continence%20services.pdf (accessed 24 September 2020).
- Changing Places Consortium . Changing Places: The Practical Guide 2000. https://toiletmap.s3.amazonaws.com/site-content-images/f4779a01-d00a-4720-918b-0204a0092435/86fbfeb08ddc117335cfbf646b1eea32.pdf (accessed 15 November 2021).
- Lyons AC, Cude B, Lawrence FC, Gutter M. Conducting research online: challenges facing researchers in family and consumer sciences. Family Consumer Sci Res J 2005;33:341-56. https://doi.org/10.1177/1077727X04274116.
- Solomon D. Conducting web-based surveys. Pract Assess Res Eval 2000;7.
- Ziegler SJ. Increasing response rates in mail surveys without increasing error: a research note. Criminal Justice Policy Rev 2006;17:22-31. https://doi.org/10.1177/0887403405277788.
- Dillman D. Mail and Internet Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons; 2007.
- Beresford B, Clarke S, Maddison J. Therapy interventions for children with neurodisabilities: a qualitative scoping study. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22030.
- Cruz D, Emmel MLG, Manzini MG, Braga Mendes PV. Assistive technology accessibility and abandonment: challenges for occupational therapists. Open J Occupational Therapy 2016;4. https://doi.org/10.15453/2168-6408.1166.
- Bray N, Kolehmainen N, McAnuff J, Tanner L, Tuersley L, Beyer F, et al. Powered mobility interventions for very young children with mobility limitations to aid participation and positive development: the EMPoWER evidence synthesis. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24500.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Donlau M, Imms C, Glad Mattsson G, Mattsson S, Sjörs A, Falkmer T. Children and youth with myelomeningocele’s independence in managing clean intermittent catheterization in familiar settings. Acta Paediatr 2011;100:429-38. https://doi.org/10.1111/j.1651-2227.2010.02044.x.
- Drysdale B, Lee CYQ, Anderson A, Moore DW. Using video modeling incorporating animation to teach toileting to two children with autism spectrum disorder. J Developmental Physical Disabil 2015;27:149-65. https://doi.org/10.1007/s10882-014-9405-1.
- Koshy NM, Sugi S, Rajendran K. A study to identify prevalence and effectiveness of sensory integration on toilet skill problems among sensory processing disorder. Indian J Occupational Therapy 2018;50. https://doi.org/10.4103/0445-7706.244548.
- Rosenbaum P, Gorter J. The ‘F-words’ in childhood disability: I swear this is how we should think!. Child Care Health Dev 2012;38:457-63. https://doi.org/10.1111/j.1365-2214.2011.01338.x.
- Roth JD, Cain MP. Neuropathic Bladder and Augmentation Cystoplasty. Urol Clin North Am 2018;45:571-85. https://doi.org/10.1016/j.ucl.2018.06.005.
- Stein R, Bogaert G, Dogan HS, Hoen L, Kocvara R, Nijman RJM, et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part II operative management. Neurourol Urodyn 2020;39:498-506. https://doi.org/10.1002/nau.24248.
- Joinson C, Whale K, Randall J. Young People With Continence Problems Need Better Support at Secondary School 2018. www.bristol.ac.uk/media-library/sites/policybristol/PolicyBristol-Report-Oct18-young-people-continence-schools.pdf (accessed 24 September 2020).
- Whale K, Cramer H, Joinson C. Left behind and left out: the impact of the school environment on young people with continence problems. Br J Health Psychol 2018;23:253-77. https://doi.org/10.1111/bjhp.12284.
- Allard A, Fellowes A, Shilling V, Janssens A, Beresford B, Morris C. Key health outcomes for children and young people with neurodisability: qualitative research with young people and parents. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004611.
- Schuster MM, Hookman P, Hendrix TR, Mendeloff AI. Simultaneous manometric recording of internal and external anal sphincteric reflexes. Bull Johns Hopkins Hosp 1965;116:79-88.
Appendix 1 Search strategies for all databases
EMBASE via OvidSP
-
exp incontinence/dt, pc, rh, su, th [Drug Therapy, Prevention, Rehabilitation, Surgery, Therapy]
-
toilet training/
-
continence.ti.
-
incontinen*.ti.
-
exp Enuresis/
-
enure*.ti.
-
encopresis.ti.
-
toilet*.ti.
-
wetting.ti.
-
bedwetting.ti.
-
dryness.ti.
-
potty.ti,ab.
-
(dysfunction* adj voiding).ti,ab.
-
or/1-13
-
child/
-
exp neurologic disease/
-
developmental disorder/
-
learning disorder/
-
exp Neurologic Manifestations/
-
cerebral palsy/
-
autism/
-
or/16-21
-
15 and 22
-
(child or childs or children or childrens or childhood).ti,ab.
-
adolescen*.ti,ab.
-
teen*.ti,ab.
-
young people.ti,ab.
-
preschool*.ti,ab.
-
toddler*.ti,ab.
-
23 or 24 or 25 or 26 or 27 or 28 or 29
-
exp clinical trial/
-
“systematic review (topic)”/
-
randomly.ti,ab.
-
trial.ti,ab.
-
control group.ti,ab.
-
(group* adj5 compared).ab.
-
(randomised or randomized).ti,ab.
-
systematic*.ti,ab.
-
(pubmed or medline).ab.
-
(review adj3 effectiveness).ti,ab.
-
(experiment or experimental).ti,ab.
-
(Quasi experiment* or quasi-experiment* or quasiexperiment*).ti,ab.
-
comparative study.ti,ab.
-
evaluation study.ti,ab.
-
(cross section* adj10 study).ti,ab.
-
crossover.ti,ab.
-
longitudinal study.ti,ab.
-
program* evaluation.ti,ab.
-
(control* adj5 compar*).ti,ab.
-
multicentre study.ti,ab.
-
observational study.ti,ab.
-
prospective.ti,ab.
-
retrospective.ti,ab.
-
cohort study.ti,ab.
-
qualitative research/
-
qualitative*.ti,ab.
-
interview*.ti,ab.
-
exp health economics/
-
“cost effectiveness analysis”/
-
cost effective*.ti,ab.
-
economic evaluation.ti,ab.
-
(cost adj2 evaluat*).ti,ab.
-
or/31-62
-
14 and 30 and 63
| # | Searches |
|---|---|
| 1 | Urinary Incontinence/ |
| 2 | Fecal Incontinence/ |
| 3 | toilet training/ |
| 4 | continence.ti. |
| 5 | incontinen*.ti. |
| 6 | enure*.ti. |
| 7 | encopresis.ti. |
| 8 | toilet*.ti. |
| 9 | wetting.ti. |
| 10 | bedwetting.ti. |
| 11 | dryness.ti. |
| 12 | potty.ti,ab. |
| 13 | (dysfunctional adj voiding).ti,ab. |
| 14 | or/1-13 |
| 15 | (child or childs or children or childrens or childhood).ti,ab. |
| 16 | adolescen*.ti,ab. |
| 17 | teen*.ti,ab. |
| 18 | young people.ti,ab. |
| 19 | preschool*.ti,ab. |
| 20 | toddler*.ti,ab. |
| 21 | or/15-20 |
| 22 | exp experimental design/ |
| 23 | (group or groups or grouped).ti,ab. |
| 24 | study.ti,ab. |
| 25 | randomly.ti,ab. |
| 26 | trial.ti,ab. |
| 27 | (randomised or randomized).ti,ab. |
| 28 | systematic*.ti,ab. |
| 29 | (pubmed or medline).ab. |
| 30 | (review adj3 effectiveness).ti,ab. |
| 31 | (experiment or experimental).ti,ab. |
| 32 | (Quasi experiment* or quasi-experiment* or quasiexperiment*).ti,ab. |
| 33 | comparative study.ti,ab. |
| 34 | evaluation study.ti,ab. |
| 35 | (cross section* adj10 study).ti,ab. |
| 36 | crossover.ti,ab. |
| 37 | longitudinal study.ti,ab. |
| 38 | program* evaluation.ti,ab. |
| 39 | (control* adj5 compar*).ti,ab. |
| 40 | multicentre study.ti,ab. |
| 41 | observational study.ti,ab. |
| 42 | prospective.ti,ab. |
| 43 | retrospective.ti,ab. |
| 44 | cohort study.ti,ab. |
| 45 | qualitative research/ |
| 46 | interviewing/ |
| 47 | observation methods/ |
| 48 | qualitative*.ti,ab. |
| 49 | interview*.ti,ab. |
| 50 | (acceptability or usability or efficacy or appropriateness or effectiveness or suitability).ti,ab. |
| 51 | Economics/ |
| 52 | exp “Costs and Cost Analysis”/ |
| 53 | cost*.ti,ab. |
| 54 | economic*.ti,ab. |
| 55 | or/22-54 |
| 56 | 14 and 21 and 55 |
| # | Searches |
|---|---|
| 1 | Urinary Incontinence/ |
| 2 | toilet training/ |
| 3 | continence.ti. |
| 4 | incontinen*.ti. |
| 5 | exp Enuresis/ |
| 6 | enure*.ti. |
| 7 | encopresis.ti. |
| 8 | toilet*.ti. |
| 9 | wetting.ti. |
| 10 | bedwetting.ti. |
| 11 | dryness.ti. |
| 12 | potty.ti,ab. |
| 13 | (dysfunction* adj voiding).ti,ab. |
| 14 | or/1-13 |
| 15 | (child or childs or children or childrens or childhood).ti,ab. |
| 16 | (boy or boys or girl or girls).ti,ab. |
| 17 | adolescen*.ti,ab. |
| 18 | teen*.ti,ab. |
| 19 | young people.ti,ab. |
| 20 | preschool*.ti,ab. |
| 21 | toddler*.ti,ab. |
| 22 | or/15-21 |
| 23 | 14 and 22 |
| Search ID# | Search terms |
|---|---|
| S42 | S13 AND S22 AND S41 |
| S41 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 |
| S40 | TI interview* OR AB interview* |
| S39 | TI qualitative* OR AB qualitative* |
| S38 | TI “economic evaluation” OR AB “economic evaluation” |
| S37 | TI cost* OR AB cost* |
| S36 | (MH “Costs and Cost Analysis+”) |
| S35 | TI program* N2 evaluation OR AB program* N2 evaluation |
| S34 | TI study OR AB study |
| S33 | TI experiment* OR AB experiment* |
| S32 | TI (effective or effectiveness) OR AB (effective or effectiveness) |
| S31 | AB Pubmed or medline |
| S30 | TI systematic* OR AB systematic* |
| S29 | TI randomi?ed OR AB randomi?ed |
| S28 | TI group* OR AB group* |
| S27 | TI trial OR AB trial |
| S26 | AB randomly |
| S25 | (MH “Crossover Design”) OR (MH “Empirical Research”) |
| S24 | (MH “Clinical Trials+”) OR (MH “Nonexperimental Studies+”) OR (MH “Qualitative Studies+”) OR (MH “Quantitative Studies”) OR (MH “Quasi-Experimental Studies+”) |
| S23 | S13 AND S22 |
| S22 | S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 |
| S21 | TI toddler* OR AB toddler* |
| S20 | TI preschool* OR AB preschool* |
| S19 | TI (boys or girls) OR AB (boys or girls) |
| S18 | TI “young people” OR AB “young people” |
| S17 | TI teen* OR AB teen* |
| S16 | TI adolesc* OR AB adolesc* |
| S15 | TI (child or children or childs or childrens or childhood) OR AB (child or children or childs or childrens or childhood) |
| S14 | (MM “Child, Disabled”) |
| S13 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 |
| S12 | TI dysfunctional W1 voiding OR AB dysfunctional W1 voiding |
| S11 | TI potty OR AB potty |
| S10 | TI dryness |
| S9 | TI bedwetting |
| S8 | TI wetting |
| S7 | TI toilet* |
| S6 | TI encopresis |
| S5 | TI enure* |
| S4 | TI continence or incontinen* |
| S3 | (MM “Enuresis+”) |
| S2 | (MM “Toilet Training”) |
| S1 | (MM “Incontinence+”) |
CDSR and CENTRAL via the Cochrane Library
-
#1 MeSH descriptor: [Urinary Incontinence] explode all trees
-
#2 MeSH descriptor: [Fecal Incontinence] this term only
-
#3 MeSH descriptor: [Toilet Training] explode all trees
-
#4 MeSH descriptor: [Enuresis] this term only
-
#5 enure*:ti,ab
-
#6 encopresis:ti,ab
-
#7 toilet*:ti,ab
-
#8 wetting:ti,ab
-
#9 bedwetting:ti,ab
-
#10 dryness:ti,ab
-
#11 potty:ti,ab
-
#12 (dysfunctional NEXT voiding):ti,ab
-
#13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
-
#14 MeSH descriptor: [Child] this term only
-
#15 (child or childs or children or childrens or childhood):ti,ab
-
#16 adolescen*:ti,ab
-
#17 teen*:ti,ab
-
#18 young people:ti,ab
-
#19 preschool*:ti,ab
-
#20 toddler*:ti,ab
-
#21 #14 or #15 or #16 or #17 or #18 or #19 or #20
-
#22 #13 and #21
ASSIA via ProQuest
(MAINSUBJECT.EXACT.EXPLODE(“Incontinence”) OR TI,AB(continence or incontinen* or enure* or encopresis or toilet* or wetting or bedwetting or dryness or potty or “dysfunctional voiding”)) AND ((MAINSUBJECT.EXACT.EXPLODE(“Developmentally disabled children”) OR MAINSUBJECT.EXACT.EXPLODE(“Developmentally delayed children”)) OR Ti,ab(child or childs or children* or childhood or adoles* or teenager* or boy or boys or girl or girls or preschool or toddler* or “young people”))
BNI via ProQuest
(((MAINSUBJECT.EXACT(“Urinary incontinence”) OR MAINSUBJECT.EXACT(“Fecal incontinence”)) OR TI,AB(continence or incontinen* or enure* or encopresis or toilet* or wetting or bedwetting or dryness or potty or “dysfunctional voiding”)) AND (MAINSUBJECT.EXACT(“Disabled children”) OR Ti,ab(child or childs or children* or childhood or adoles* or teenager* or boy or boys or girl or girls or preschool or toddler* or “young people”))) AND (TI,AB(trial* or study or experiment* or systematic* or randomi?ed or group* or control* or qualitative or review or interview* or effectiveness or program* or intervention* or assessed) OR MAINSUBJECT.EXACT(“Clinical trials”))
| Set | Save History/Create AlertOpen Saved History |
|---|---|
| # 7 |
#6 AND #5 Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 6 |
TS = (study or trial* or experiment* or systematic* or randomi?ed or group* or control* or qualitative or review or interview* or effectiveness or program* or intervention* or assessed) Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 5 |
#4 AND #3 Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 4 |
TI = (child or childs or childhood or children* or adolesc* or teen* or girl or girls or boy or boys or “young people” or toddler* or preschool*) Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 3 |
#2 OR #1 Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 2 |
TI = (toilet* or enuresis or encopresis or wetting or bedwetting or dryness) Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # 1 |
TS = (continence or incontinence) Indexes = CPCI-S, CPCI-SSH Timespan = 1900-2019 |
| # | Searches |
|---|---|
| 1 | continence.ti. |
| 2 | incontinen*.ti. |
| 3 | enure*.ti. |
| 4 | encopresis.ti. |
| 5 | toilet*.ti. |
| 6 | wetting.ti. |
| 7 | bedwetting.ti. |
| 8 | dryness.ti. |
| 9 | potty.ti,ab. |
| 10 | (dysfunction* adj voiding).ti,ab. |
| 11 | or/1-10 |
| 12 | (child or childs or children or childrens or childhood).ti,ab. |
| 13 | (boy or boys or girl or girls).ti,ab. |
| 14 | adolescen*.ti,ab. |
| 15 | teen*.ti,ab. |
| 16 | young people.ti,ab. |
| 17 | preschool*.ti,ab. |
| 18 | toddler*.ti,ab. |
| 19 | or/12-18 |
| 20 | 11 and 19 |
ProQuest Dissertations & Theses
(TI,AB(continence or incontinen* or enure* or encopresis or toilet* or wetting or bedwetting or dryness or potty or “dysfunctional voiding”) AND Ti,ab(child or childs or children* or childhood or adoles* or teenager* or boy or boys or girl or girls or preschool or toddler* or “young people”)) AND TI,AB(trial or study or experiment* or systematic* or randomi?ed or group* or control* or qualitative or review or interview* or effectiveness or “program* evaluation”)
Health Technoloy Assessment database via CRD database
Incontinence and child* (Any field) or Continence and child* (any field)
Appendix 2 Screening notes: title/abstract stage
Improving continence for children and young people with neurodisability: systematic review.
Neurodisability describes a group of congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations. A specific diagnosis may not be identified. Conditions may vary over time, occur alone or in combination, and include a broad range of severity and complexity. The impact may include difficulties with movement, cognition, hearing and vision, communication, emotion, and behaviour.
Outcomes
Quantitative evidence
Any outcome describing harms, benefits and costs to children and young people or their parent carers or value-for-money for health services.
Outcomes of interest will be discussed and agreed with the Professional Advisory Group and the PenCRU Family Faculty public involvement working group and are likely to include:
-
measures of urinary and/or faecal continence
-
night-time and/or daytime continence/dryness
-
health-related quality of life
-
social functioning
-
treatment burden (on the child or young person)
-
carer burden (time, cost, psychological).
‘Economic outcomes’ will be collected from evaluation studies of all designs (whether ostensibly an effectiveness study/RCT, an observational study, a cost/outcome analysis or an economic evaluation) that reports on the costs or resource implications or related consequences/benefits for the included interventions and comparators. For example, changes in informal care, frequency of service use or numbers of referrals will be included as economic outcomes, and better support an integrated assessment of effectiveness and cost-effectiveness.
Qualitative evidence
Evaluation:
-
Attitudes, experiences, perceptions and understanding of children and young people with neurodisability of receiving interventions aimed at improving continence in this group.
-
Attitudes, experiences, perceptions and understanding of the families and carers of children and young people with neurodisability of receiving interventions aimed at improving continence in this group.
-
Attitudes, experiences, perceptions and understanding of health-care professionals involved in the care of children and young people with neurodisability who have delivered interventions aimed at improving continence in this group.
Eligibility criteria
Studies will be included if they describe harms, benefits and costs to children and young people or their parent carers or value-for-money for health services as detailed above. The title/abstract or keywords need to indicate neurodisability, or neuropathic/neurogenic bladder.
Study criteria
Study design
Quantitative evidence
It is unlikely we will find many or any RCTs in this area. All quantitative study designs reporting comparative data prioritising evidence from more robust study designs will be included in the synthesis where possible. For the assessment of cost-effectiveness, we will include economic analyses and comparative cost studies of interventions meeting the inclusion criteria.
Qualitative evidence
We will include any recognised method of qualitative data collection and analysis, including interviews, focus groups and observational techniques. This may be stand-alone qualitative research, or reported as part of a mixed-methods intervention evaluation. We will include process and outcome evaluations.
Research type
Qualitative research and process evaluations related to specific interventions aimed at improving continence in children and young people with neurodisability. We will carefully seek to identify qualitative research that is associated with the programmes included in the effectiveness review, through targeted searches for ‘sibling’ studies, although will not be confined to these.
Abstracts
Authors of studies published only as abstracts will be contacted and asked to provide further detail. If no further detail is available, the study will be excluded.
Language
Qualitative papers written in English will be included to avoid loss or distortion by translation from studies written in another language. Qualitative papers written in any language other than English will be excluded. Non-English-language quantitative papers will be translated.
Participants
Quantitative evidence: participants will be children and young people with non-progressive neurodisability (indicated by neurodisability, or neuropathic/neurogenic bladder within keywords or title/abstract) aged up to 25 years, consistent with Department of Health and Social Care Special Educational Needs and Disabilities policy and Children and Families Act 2014.
Qualitative evidence: we will seek research with –
-
children and young people with neurodisability (indicated by neurodisability, or neuropathic/neurogenic bladder within keywords or title/abstract)
-
their families and carers
-
health-care professionals providing care.
Interventions
Quantitative evidence: assessments including identification of any underlying pathology and readiness for toilet training; interventions to improve continence including structured training programmes, products and assistive technology, medicines and/or surgery; or care pathways/programmes involving combinations of continence assessment/monitoring and treatment/management interventions.
Qualitative evidence: phenomenon of interest – the factors that may enhance or hinder the effectiveness of interventions and/or the successful implementation of interventions for improving continence in children and young people with neurodisability.
Comparator(s)/control
Quantitative evidence: comparators. Any control or comparator.
Qualitative evidence: not applicable.
Context
Location: only studies from OECD countries will be included. Consideration will be given to the degree of transferability of findings from non-UK settings to the NHS context.
Time period
All time periods (no limits).
Coding
Harriet Hunt using custom 8; Jo Thompson Coon, Morwenna Rogers and Helen Eke using custom 7. For title/abstract screening, 1 = exclude and 0 = include. Coding fields for full-text screening are shown in Table 22.
| Exclusion category | Criteria | Specification: for exclusion | Notes |
|---|---|---|---|
| 2. Wrong intervention | Intervention | No aimed at improving continence in children and young people with a neurodisability. Children without neurodisability | Conditions such as:
|
| 3. Wrong outcomes | Outcomes | Effectiveness: items that do not report measures of:
|
|
| 4. Wrong population | Population | Not children or young people; people over 25 years old | |
| 5. Wrong study type: | Study design |
Editorial Opinion piece/commentary Letter Case study |
|
| 6. No primary data | Data |
Review or SR for follow-up or other paper that may be useful for background Protocol |
|
| 7. Language | Language (qualitative) |
Only qualitative papers must be in English Quantitative papers will be translated |
|
| 8. Insufficient data | Abstract only | That is, only abstract with insufficient data | |
| Duplicate publication | Duplicate | ||
| Unobtainable |
Children and Families Act 2014
The Children and Families Act 2014. Part 3: Children and young people with special educational needs and disabilities.
The difference between ‘children’ and ‘young people’
A child is a person under compulsory school age. A young person is a person over compulsory school age but under 25. A person is no longer of compulsory school age after the last day of summer term during the year in which they become 16 (Section 83(2)). This distinction is important because once a child becomes a young person they are entitled to take decisions in relation to the Act on their own behalf, rather than having their parents take the decisions for them. This is subject to a young person ‘having capacity’ to take a decision under the Mental Capacity Act 2005.
OECD countries
Australia, Austria, Belgium, Canada, Chile, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Latvia, Lithuania, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, the UK and the USA.
Appendix 3 Screening notes: full-text stage
Improving continence for children and young people with neurodisability: systematic review.
Neurodisability describes a group of congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations. A specific diagnosis may not be identified. Conditions may vary over time, occur alone or in combination, and include a broad range of severity and complexity. The impact may include difficulties with movement, cognition, hearing and vision, communication, emotion, and behaviour.
Outcomes
Quantitative evidence
Any outcome describing harms, benefits and costs to children and young people or their parent carers or value-for-money for health services.
Outcomes of interest will be discussed and agreed with the Professional Advisory Group and the PenCRU Family Faculty public involvement working group and are likely to include:
-
measures of urinary and/or faecal continence
-
night-time and/or daytime continence/dryness
-
health-related quality of life
-
social functioning
-
treatment burden (on the child or young person)
-
carer burden (time, cost, psychological).
‘Economic outcomes’ will be collected from evaluation studies of all designs (whether ostensibly an effectiveness study/RCT, an observational study, a cost/outcome analysis or an economic evaluation) that reports on the costs or resource implications or related consequences/benefits for the included interventions and comparators. For example, changes in informal care, frequency of service use or numbers of referrals will be included as economic outcomes, and better support an integrated assessment of effectiveness and cost-effectiveness.
Qualitative evidence
Evaluation
-
Attitudes, experiences, perceptions and understanding of children and young people with neurodisability of receiving interventions aimed at improving continence in this group.
-
Attitudes, experiences, perceptions and understanding of the families and carers of children and young people with neurodisability of receiving interventions aimed at improving continence in this group.
-
Attitudes, experiences, perceptions and understanding of health-care professionals involved in the care of children and young people with neurodisability who have delivered interventions aimed at improving continence in this group.
Eligibility criteria
Studies will be included if they describe harms, benefits, and costs to children and young people or their parent carers or value-for-money for health services as detailed above. The title/abstract or keywords need to indicate neurodisability, or neuropathic/neurogenic bladder.
Study criteria
Study design
Quantitative evidence
It is unlikely we will find many or any RCTs in this area. All quantitative study designs reporting comparative data prioritising evidence from more robust study designs will be included in the synthesis where possible. For the assessment of cost-effectiveness, we will include economic analyses and comparative cost studies of interventions meeting the inclusion criteria.
Qualitative evidence
We will include any recognised method of qualitative data collection and analysis, including interviews, focus groups and observational techniques. This may be stand-alone qualitative research, or reported as part of a mixed-methods intervention evaluation. We will include process and outcome evaluations.
Research type
Qualitative research and process evaluations related to specific interventions aimed at improving continence in children and young people with neurodisability. We will carefully seek to identify qualitative research that is associated with the programmes included in the effectiveness review, through targeted searches for ‘sibling’ studies though will not be confined to these.
Abstracts
Authors of studies published only as abstracts will be contacted and asked to provide further detail. If no further detail is available, the study will be excluded.
Language
Qualitative papers written in English will be included to avoid loss or distortion by translation from studies written in another language. Qualitative papers written in any language other than English will be excluded. Non-English language quantitative papers will be translated.
Participants
Quantitative evidence: participants will be children and young people with non-progressive neurodisability (indicated by neurodisability, or neuropathic/neurogenic bladder within keywords or title/abstract) aged up to 25 years, consistent with Department of Health and Social Care Special Educational Needs and Disabilities policy and Children and Families Act 2014.
Qualitative evidence: we will seek research with –
-
children and young people with neurodisability (indicated by neurodisability, or neuropathic/neurogenic bladder within keywords or title/abstract)
-
their families and carers
-
health-care professionals providing care.
Interventions
Quantitative evidence: assessments including identification of any underlying pathology and readiness for toilet training; interventions to improve continence including structured training programmes, products and assistive technology, medicines and/or surgery; or care pathways/programmes involving combinations of continence assessment/monitoring and treatment/management interventions.
Qualitative evidence: phenomenon of interest – the factors that may enhance, or hinder the effectiveness of interventions and/or the successful implementation of interventions for improving continence in children and young people with neurodisability.
Comparator(s)/control
Quantitative evidence: comparators. Any control or comparator.
Qualitative evidence: not applicable.
Context
Location: only studies from OECD countries will be included. Consideration will be given to the degree of transferability of findings from non-UK settings to the NHS context.
Time period
All time periods (no limits).
Coding
Harriet Hunt using custom 8; Jo Thompson Coon, Morwenna Rogers and Helen Eke using custom 7. For full-text screening, 0 = include and 2–8 = exclude (with reasons in Table 23). Coding fields for full-text screening are shown in Table 23.
| Exclusion category | Criteria | Specification – for exclusion | Notes |
|---|---|---|---|
| 2. Wrong intervention | Intervention | No aimed at improving continence in children and young people with a neurodisability. Children without neurodisability | Conditions such as:
|
| 3. Wrong outcomes | Outcomes | Effectiveness: items that do not report measures of:
|
|
| 4. Wrong population | Population | Not children or young people; people over 25 years old | |
| 5. Wrong study type | Study design |
Editorial Opinion piece/commentary Letter Case study |
|
| 6. No primary data | Data |
Review or SR for follow-up or other paper that may be useful for background Protocol |
|
| 7. Language | Language (qualitative) |
Only qualitative papers must be in English Quantitative papers will be translated |
|
| 8. Insufficient data | Abstract only | That is, only abstract with insufficient data | |
| Duplicate publication | Duplicate | ||
| Unobtainable |
The Children and Families Act 2014
The Children and Families Act 2014. Part 3: Children and young people with special educational needs and disabilities.
The difference between ‘children’ and ‘young people
A child is a person under compulsory school age. A young person is a person over compulsory school age but under 25. A person is no longer of compulsory school age after the last day of summer term during the year in which they become 16 (Section 83(2)). This distinction is important because once a child becomes a young person they are entitled to take decisions in relation to the Act on their own behalf, rather than having their parents take the decisions for them. This is subject to a young person ‘having capacity’ to take a decision under the Mental Capacity Act 2005.
OECD countries
Australia, Austria, Belgium, Canada, Chile, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Latvia, Lithuania, Luxembourg, Mexico, the Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, the UK and the USA.
Appendix 4 Included studies
| 1. | 61Aksnes G, Diseth TH, Helseth A, Edwin B, Stange M, Aafos G, Emblem R. Appendicostomy for antegrade enema: effects on somatic and psychosocial functioning in children with myelomeningocele. Pediatrics 2002;109:484–9. https://doi.org/10.1542/peds.109.3.484 |
| 2. | 99Amark P, Beck O. Effect of phenylpropanolamine on incontinence in children with neurogenic bladders. A double-blind crossover study. Acta Paediatr 1992;81:345–50. https://doi.org/10.1111/j.1651-2227.1992.tb12240.x |
| 3. | 100Amark P, Bussman G, Eksborg S. Follow-up of long-time treatment with intravesical oxybutynin for neurogenic bladder in children. Eur Urol 1998;34:148–53 |
| 4. | 40Ardic A, Cavkaytar A. Effectiveness of the modified intensive toilet training method on teaching toilet skills to children with autism. Educ Training Autism Dev Disabil 2014;49:263–76 |
| 5. | 62Ausili E, Focarelli B, Tabacco F, Murolo D, Sigismondi M, Gasbarrini A, Rendeli C. Transanal irrigation in myelomeningocele children: an alternative, safe and valid approach for neurogenic constipation. Spinal Cord 2010;48:560–5. https://doi.org/10.1038/sc.2009.186 |
| 6. | 63Ausili E, Marte A, Brisighelli G, Midrio P, Mosiello G, La Pergola E, et al. Short versus mid-long-term outcome of transanal irrigation in children with spina bifida and anorectal malformations. Childs Nerv Syst 2018;34:2471–9. https://doi.org/10.1007/s00381-018-3860-4 |
| 7. | 47Barmann BC, Katz RC, O’Brien F, Beauchamp KL. Treating irregular enuresis in developmentally disabled persons: a study in the use of overcorrection. Behavior Modification 1981;5:336–46 |
| 8. | 81Bar-Yosef Y, Castellan M, Joshi D, Labbie A, Gosalbez R. Total continence reconstruction using the artificial urinary sphincter and the Malone antegrade continence enema. J Urol 2011;185:1444–7. https://doi.org/10.1016/j.juro.2010.11.049 |
| 9. | 98Boone TB, Roehrborn CG, Hurt G. Transurethral intravesical electrotherapy for neurogenic bladder dysfunction in children with myelodysplasia: a prospective, randomized clinical trial. J Urol 1992;148(2 II):550–4 |
| 10. | 89Borzyskowski M, Mundy AR, Neville BG, Park L, Kinder CH, Joyce MR, et al. Neuropathic vesicourethral dysfunction in children. A trial comparing clean intermittent catheterisation with manual expression combined with drug treatment. Br J Urol 1982;54:641–4. https://doi.org/10.1111/j.1464-410x.1982.tb13615.x |
| 11. | 36Chertin B, Koulikov D, Abu-Arafeh W, Mor Y, Shenfeld OZ, Farkas A. Treatment of nocturnal enuresis in children with attention deficit hyperactivity disorder. J Urol 2007;178(4 Pt 2):1744–7 |
| 12. | 64Choi EK, Han SW, Shin SH, Ji Y, Chon JH, Im YJ. Long-term outcome of transanal irrigation for children with spina bifida. Spinal Cord 2015;53:216–20. https://doi.org/10.1038/sc.2014.234 |
| 13. | 59Choi EK, Shin SH, Im YJ, Kim MJ, Han SW. The effects of transanal irrigation as a stepwise bowel management program on the quality of life of children with spina bifida and their caregivers. Spinal Cord 2013;51:384–8. https://doi.org/10.1038/sc.2013.8 |
| 14. | 42Cicero FR, Pfadt A. Investigation of a reinforcement-based toilet training procedure for children with autism. Res Dev Disabil 2002;23:319–31 |
| 15. | 90Corbett P, Denny A, Dick K, Malone PS, Griffin S, Stanton MP. Peristeen integrated transanal irrigation system successfully treats faecal incontinence in children. J Pediatr Urol 2014;10:219–22. https://doi.org/10.1016/j.jpurol.2013.08.006 |
| 16. | 50Didden R, Sikkema SP, Bosman IT, Duker PC, Curfs LM. Use of a modified Azrin-Foxx toilet training procedure with individuals with Angelman-Syndrome. J Appl Res Intellect Disabil 2001;14:64–70 |
| 17. | 60Dietrich S, Okamoto G. Bowel training for children with neurogenic dysfunction: a follow-up. Arch Phys Med Rehabil 1982;63:166–70 |
| 18. | 97Do Ngoc Thanh C, Audry G, Forin V. Botulinum toxin type A for neurogenic detrusor overactivity due to spinal cord lesions in children: a retrospective study of seven cases. J Pediatr Urol 2009;5:430–6. https://doi.org/10.1016/j.jpurol.2009.06.001 |
| 19. | 43Edgar CL, Kohler HF, Hardman S. A new method for toilet training developmentally disabled children. Percept Mot Skills 1975;41:63–9. https://doi.org/10.2466/pms.1975.41.1.63 |
| 20. | 112Edwards M, Borzyskowski M, Cox A, Badcock J. Neuropathic bladder and intermittent catheterization: social and psychological impact on children and adolescents. Dev Med Child Neurol 2004;46:168–77. https://doi.org/10.1017/s0012162204000301 |
| 21. | 102Faure A, Hery G, Mille E, Orillac P, Da Silva B, Merrot T, et al. Long-term efficacy of Young–Dees bladder neck reconstruction: role of the associated bladder neck injection for the treatment of children with urinary incontinence. Urology 2017;108:166–70 |
| 22. | 96González R, Jednak R, Franc-Guimond J, Schimke CM. Treating neuropathic incontinence in children with seromuscular colocystoplasty and an artificial urinary sphincter. BJU Int 2002;90:909–11 |
| 23. | 37Gor RA, Fuhrer J, Schober JM. A retrospective observational study of enuresis, daytime voiding symptoms, and response to medical therapy in children with attention deficit hyperactivity disorder and autism spectrum disorder. J Pediatr Urol 2012;8:314–17. https://doi.org/10.1016/j.jpurol.2010.10.009 |
| 24. | 94Guys JM, Breaud J, Hery G, Camerlo A, Le Hors H, De Lagausie P. Endoscopic injection with polydimethylsiloxane for the treatment of pediatric urinary incontinence in the neurogenic bladder: long-term results. J Urol 2006;175(3 Pt 1):1106–10 |
| 25. | 93Guys JM, Simeoni-Alias J, Fakhro A, Delarue A. Use of polydimethylsiloxane for endoscopic treatment of neurogenic urinary incontinence in children. J Urol 1999;162:2133–5 |
| 26. | 88Haddad M, Besson R, Aubert D, Ravasse P, Lemelle J, El Ghoneimi A, et al. Sacral neuromodulation in children with urinary and fecal incontinence: a multicenter, open label, randomized, crossover study. J Urol 2010;184:696–701. https://doi.org/10.1016/j.juro.2010.03.054 |
| 27. | 77Han SW, Kim MJ, Kim JH, Hong CH, Kim JW, Noh JY. Intravesical electrical stimulation improves neurogenic bowel dysfunction in children with spina bifida. J Urol 2004;171:2648–50 |
| 28. | 65Hascoet J, Peyronnet B, Forin V, Baron M, Capon G, Prudhomme T, et al. Intradetrusor injections of botulinum toxin type A in children with spina bifida: a multicenter study. Urology 2018;116:161–7 |
| 29. | 72Horowitz M, Combs AJ, Gerdes D. Desmopressin for nocturnal incontinence in the spina bifida population. J Urol 1997;158:2267–8 |
| 30. | 44Hundziak M, Maurer RA, Watson LS. Operant conditioning in toilet training of severely mentally retarded boys. Am J Ment Defic 1965;70:120–4 |
| 31. | 82Ibrahim M, Ismail NJ, Mohammad MA, Ismail H, Ahmed MH, Femi OL, Suwaid MA. Managing fecal incontinence in patients with myelomeningocele in Sub-Saharan Africa: Role of antegrade continence enema (ACE). J Pediatr Surg 2017;52:554–7 |
| 32. | 106Jawaheer G, Rangecroft L. The Pippi Salle procedure for neurogenic urinary incontinence in childhood: a three-year experience. Eur J Pediatr Surg 1999;9(Suppl. 1):9–11. https://doi.org/10.1055/s-2008-1072303 |
| 33. | 66Kajbafzadeh AM, Moosavi S, Tajik P, Arshadi H, Payabvash S, Salmasi AH, et al. Intravesical injection of botulinum toxin type A: management of neuropathic bladder and bowel dysfunction in children with myelomeningocele. Urology 2006;68:1091–6 |
| 34. | 52Kajbafzadeh AM, Sharifi-Rad L, Baradaran N, Nejat F. Effect of pelvic floor interferential electrostimulation on urodynamic parameters and incontinency of children with myelomeningocele and detrusor overactivity. Urology 2009;74:324–9. https://doi.org/10.1016/j.urology.2008.12.085 |
| 35. | 53Kajbafzadeh AM, Sharifi-Rad L, Ladi Seyedian SS, Masoumi A. Functional electrical stimulation for management of urinary incontinence in children with myelomeningocele: a randomized trial. Pediatr Surg Int 2014;30:663–8. https://doi.org/10.1007/s00383-014-3503-0 |
| 36. | 41Keen D, Brannigan KL, Cuskelly M. Toilet training for children with autism: the effects of video modeling category. J Dev Physical Disabil 2007;19:291–303 |
| 37. | 67Killam PE, Jeffries JS, Varni JW. Urodynamic biofeedback treatment of urinary incontinence in children with myelomeningocele. Biofeedback Self Regul 1985;10:161–71. https://doi.org/10.1007/BF01000751 |
| 38. | 78King SK, Stathopoulos L, Pinnuck L, Wells J, Hutson J, Heloury Y. Retrograde continence enema in children with spina bifida: Not as effective as first thought. J Paediatr Child Health 2017;53:386–90. https://doi.org/10.1111/jpc.13408 |
| 39. | 68Ladi-Seyedian SS, Sharifi-Rad L, Kajbafzadeh AM. Intravesical electromotive botulinum toxin type ‘A’ administration for management of urinary incontinence secondary to neuropathic detrusor overactivity in children: long-term follow-up. Urology 2018;114:167–74 |
| 40. | 69Lima SVC, Vilar FO, Lustosa ES, Aragão DCC, Calisto FCFS, Pinto FCM. New device for intermittent emptying of the bladder in female children and adolescents: a pilot study. J Pediatr Urol 2017;13:453.e1–e6 |
| 41. | 55Loening-Baucke V, Desch L, Wolraich M. Biofeedback training for patients with myelomeningocele and fecal incontinence. Dev Med Child Neurol 1988;30:781–90. https://doi.org/10.1111/j.1469-8749.1988.tb14640.x |
| 42. | 56Marshall D. Objective Assessment of Bladder and Bowel Function Following Cutaneous Electrical Field Stimulation in Children with Spina Bifida: A Randomised Controlled Trial. Belfast: Queen’s University Belfast; 2001 |
| 43. | 83Matsuno D, Yamazaki Y, Shiroyanagi Y, et al. The role of the retrograde colonic enema in children with spina bifida: is it inferior to the antegrade continence enema? Pediatr Surg Int 2010;26:529–33 |
| 44. | 70Mattsson S, Gladh G. Tap-water enema for children with myelomeningocele and neurogenic bowel dysfunction. Acta Paediatr 2006;95:369–74 |
| 45. | 49Lomas Mevers J, Muething C, Call NA, Scheithauer M, Hewett S. A consecutive case series analysis of a behavioral intervention for enuresis in children with developmental disabilities. Dev Neurorehabil 2018;21:336–44. https://doi.org/10.1080/17518423.2018.1462269 |
| 46. | 38Lomas Mevers J, Call NA, Gerencser KR, Scheithauer M, Miller SJ, Muething C, et al. A pilot randomized clinical trial of a multidisciplinary intervention for encopresis in children with autism spectrum disorder. J Autism Dev Disord 2020;50:757–65. https://doi.org/10.1007/s10803-019-04305-5 |
| 47. | 39Mruzek DW, McAleavey S, Loring WA, Butter E, Smith T, McDonnell E, et al. A pilot investigation of an iOS-based app for toilet training children with autism spectrum disorder. Autism 2019;23:359–70. https://doi.org/10.1177/1362361317741741 |
| 48. | 39Mruzek DW, McAleavey S, Loring WA, Butter E, Smith T, McDonnell E, et al. A pilot investigation of an iOS-based app for toilet training children with autism spectrum disorder. Autism 2019;23:359–70. https://doi.org/10.1177/1362361317741741 |
| 49. | 101Naglo AS, Boreus LO, Hellstrom B, Nergardh A. Is beta-receptor blockade of value in continence training of children? Scand J Rehabil Med 1979;11:63–6 |
| 50. | 74Palmer LS, Richards I, Kaplan WE. Transrectal electrostimulation therapy for neuropathic bowel dysfunction in children with myelomeningocele. J Urol 1997;157:1449–52 |
| 51. | 79Petersen T. Management of urinary incontinence in children with myelomeningocele. Acta Neurol Scand 1987;75:52–5. https://doi.org/10.1111/j.1600-0404.1987.tb07888.x |
| 52. | 75Radojicic Z, Milivojevic S, Lazovic JM, Becanovic S, Korićanac I, Milic N. The impact of bowel management on the quality of life in children with spina bifida with overactive bladder and detrusor sphincter dyssynergia. J Pediatr Urol 2019;15:457–66 |
| 53. | 49Rinald K, Mirenda P. Effectiveness of a modified rapid toilet training workshop for parents of children with developmental disabilities. Res Dev Disabil 2012;33:933–43. https://doi.org/10.1016/j.ridd.2012.01.003 |
| 54. | 45Sadler OW, Merkert F. Evaluating the Foxx and Azrin toilet training procedure for retarded children in a day training center. Behav Ther 1977;8:499–500 |
| 55. | 116Sanders C, Bray L, Driver C, Harris V. Parents of children with neurogenic bowel dysfunction: their experiences of using transanal irrigation with their child. Child Care Health Dev 2014;40:863–9. https://doi.org/10.1111/cch.12117 |
| 56. | 117Sawin KJ, Thompson NM. The experience of finding an effective bowel management program for children with spina bifida: the parent’s perspective. J Pediatr Nurs 2009;24:280–91 |
| 57. | 87Schletker J, Edmonds T, Jacobson R, Ketzer J, Hall J, Trecartin A, et al. Bowel management program in patients with spina bifida. Pediatr Surg Int 2019;35:243–5. https://doi.org/10.1007/s00383-018-4403-5 |
| 58. | 92Schulte-Baukloh H, Mürtz G, Heine G, Austin P, Miller K, Michael T, et al. Urodynamic effects of propiverine in children and adolescents with neurogenic bladder: results of a prospective long-term study. J Pediatr Urol 2012;8:386–92. https://doi.org/10.1016/j.jpurol.2011.07.014 |
| 59. | 91Schulte-Baukloh H, Mürtz G, Henne T, Michael T, Miller K, Knispel HH. Urodynamic effects of propiverine hydrochloride in children with neurogenic detrusor overactivity: a prospective analysis. BJU Int 2006;97:355–8 |
| 60. | 71Shoshan L, Ben-Zvi D, Katz-Leurer M. Use of the anal plug in the treatment of fecal incontinence in patients with meningomyelocele. J Pediatr Nurs 2008;23:395–9. https://doi.org/10.1016/j.pedn.2006.09.006 |
| 61. | 95Silveri M, Capitanucci ML, Mosiello G, Broggi G, De Gennaro M. Endoscopic treatment for urinary incontinence in children with a congenital neuropathic bladder. Br J Urol 1998;82:694–7. https://doi.org/10.1046/j.1464-410x.1998.00810.x |
| 62. | 84Snodgrass W, Granberg C. Clinical indications for augmentation in children with neurogenic urinary incontinence following bladder outlet procedures: results of a 14-year observational study. J Pediatr Urol 2016;12:46.e1–8. https://doi.org/10.1016/j.jpurol.2015.06.018 |
| 63. | 76Snodgrass W, Keefover-Hicks A, Prieto J, Bush N, Adams R. Comparing outcomes of slings with versus without enterocystoplasty for neurogenic urinary incontinence. J Urol 2009;181:2709–14. https://doi.org/10.1016/j.juro.2009.02.035 |
| 64. | 57Steinbok P, MacNeily AE, Hengel AR, et al. Filum section for urinary incontinence in children with occult tethered cord syndrome: a randomized, controlled pilot study. J Urol 2016;195(4 Pt 2):1183–8 |
| 65. | 73Tarcan T, Akbal C, Sekerci CA, Top T, Simşek F. Intradetrusor injections of onabotulinum toxin-A in children with urinary incontinence due to neurogenic detrusor overactivity refractory to antimuscarinic treatment. Korean J Urol 2014;55:281–7. https://doi.org/10.4111/kju.2014.55.4.281 |
| 66. | 51Valentine A, Maxwell C. Enuresis in severely subnormal children: a clinical trial of imipramine. J Ment Subnormal 1968;14:84–90 |
| 67. | 46Van Laecke E, Raes A, Vande Walle J, Hoebeke P. Adequate fluid intake, urinary incontinence, and physical and/or intellectual disability. J Urol 2009;182(Suppl. 4):2079–84. https://doi.org/10.1016/j.juro.2009.05.125 |
| 68. | 85Van Savage JG, Yohannes P. Laparoscopic antegrade continence enema in situ appendix procedure for refractory constipation and overflow fecal incontinence in children with spina bifida. J Urol 2000;164:1084–7 |
| 69. | 58Van Winckel M, Van Biervliet S, Van Laecke E, Hoebeke P. Is an anal plug useful in the treatment of fecal incontinence in children with spina bifida or anal atresia? J Urol 2006;176:342–4 |
| 70. | 80Vande Velde S, Van Biervliet S, Van Renterghem K, Van Laecke E, Hoebeke P, Van Winckel M. Achieving fecal continence in patients with spina bifida: a descriptive cohort study. J Urol 2007;178:2640–4 |
| 71. | 86Wehby MC, O’Hollaren PS, Abtin K, Hume JL, Richards BJ. Occult tight filum terminale syndrome: results of surgical untethering. Pediatr Neurosurg 2004;40:51–7. https://doi.org/10.1159/000078908 |
| 72. | 35Yang TK, Guo YJ, Chang HC, Yang HJ, Huang KH. Attention deficit-hyperactivity disorder symptoms and daytime voiding symptoms in children with primary enuresis: an observational study to evaluate the effectiveness of desmopressin treatment. Scientific World Journal 2015;2015:356121. https://doi.org/10.1155/2015/356121 |
Appendix 5 Systematic review results tables showing effectiveness of interventions for improving continence in children and young people with neurodisability
| Reference | n; age | Procedure | Effect | EPHPP quality appraisal | Faecal/urinary/both | |
|---|---|---|---|---|---|---|
| Developmental/learning disability | ||||||
| B&A | ||||||
| A | Van Laecke,46 2009 | 111; 4–15 years | Adequate fluid intake | Urinary continence
|
Weak | Urinary |
| Reference | n; age | Based on | Effect | EPHPP quality appraisal | Faecal/urinary/both | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autism | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B&A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Ardic,40 2014 | 3; 3–4 years | Azrin and Foxx adaptation (modified intensive toilet training method) | Intervention arm:
|
Weak | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Case–control | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Keen,41 2007 | 5; 4 years 5 months–6 years 9 months | Operant conditioning plus video |
Number of in-toilet urinations: Intervention armComparator arm |
Weak | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 70; 4–16 years | Bowel management programme |
Faecal incontinence:
|
Weak | Both | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | 3; 4–6 years | Azrin and Foxx adaptation |
Overall comparison:
|
Weak | Urinary | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Developmental/learning disability | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RCT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Edgar,43 1975 | 20; 4–12 years | Behavioural training and device (electric belt, toilet with buzzer plus relaxation training) |
Incidents per week at 2 weeks Intervention arm:Comparator arm |
Moderate | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | 29; 7–14 years | Operant conditioning vs. conventional training vs. control group |
Urination and defecation in toilet measured over 3 consecutive days (5 hours each day before/after training) Intervention arm: change from baseline (median and IQR)Comparator arm: |
Moderate | Both | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Sadler,45 1977 | 14; 7–12 years | Azrin and Foxx method |
Mean number of accidents at 4 months (taken over 1 week) Intervention arm:Comparator arm: |
Weak | Both | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B&A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Barmann,47 1981 | 3; 4–8 years | Azrin and Foxx adaptation (shortened version) | Intervention arm: follow-up (4 months)
|
Weak | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Rinald,48 2012 | 6; 3 years 3 months–5 years 11 months | Azrin and Foxx adaptation (rapid toilet training with parent inclusion and workshop format) |
The range of daily urination episodes was reported for each child; all six participants showed an increase in daily urination episodes, both in and out of the toilet, after the intervention Non-toilet defecation: % of non-toilet-initiated defecations at: BaselineInterventionFollow-upR10037.50L1000NAAm10000An100400Jac81.800Jam10033.30 Adult initiated: % of adult-initiated defecations at: BaselineInterventionFollow-upR062.5100L0100NAAm083.316.7An023.50Jac18.27075Jam066.70 Child initiated: % of child-initiated defecations at: BaselineInterventionFollow-upR000L00NAAm05050An036.5100Jac03025Jam00100 |
Baseline | Intervention | Follow-up | R | 100 | 37.5 | 0 | L | 100 | 0 | NA | Am | 100 | 0 | 0 | An | 100 | 40 | 0 | Jac | 81.8 | 0 | 0 | Jam | 100 | 33.3 | 0 | Baseline | Intervention | Follow-up | R | 0 | 62.5 | 100 | L | 0 | 100 | NA | Am | 0 | 83.3 | 16.7 | An | 0 | 23.5 | 0 | Jac | 18.2 | 70 | 75 | Jam | 0 | 66.7 | 0 | Baseline | Intervention | Follow-up | R | 0 | 0 | 0 | L | 0 | 0 | NA | Am | 0 | 50 | 50 | An | 0 | 36.5 | 100 | Jac | 0 | 30 | 25 | Jam | 0 | 0 | 100 | Moderate | Both | |||
| Baseline | Intervention | Follow-up | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| R | 100 | 37.5 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| L | 100 | 0 | NA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Am | 100 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| An | 100 | 40 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jac | 81.8 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jam | 100 | 33.3 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | Intervention | Follow-up | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| R | 0 | 62.5 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| L | 0 | 100 | NA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Am | 0 | 83.3 | 16.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| An | 0 | 23.5 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jac | 18.2 | 70 | 75 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jam | 0 | 66.7 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | Intervention | Follow-up | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| R | 0 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| L | 0 | 0 | NA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Am | 0 | 50 | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| An | 0 | 36.5 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jac | 0 | 30 | 25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jam | 0 | 0 | 100 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Case report | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Lomas Mevers,49 2018 | 44; 2–20 years | Behavioural intervention |
Overall comparison (at 6–24 months):
|
Weak | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spina bifida | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B&A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B | Choi,59 2013 | 53; 3–13.8 years | Stepwise bowel management programme | Intervention arm:
|
Moderate | Faecal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B | Dietrich,60 1982 | 55; 5.6–18.9 years | Bowel training | Intervention arm:
|
Weak | Faecal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Case report | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schletker,87 2019 | 22; 2–24 years | Bowel management programme |
Overall comparison
|
Weak | Faecal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Angelman syndrome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cohort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A | Didden,50 2001 | 6; 6–19 years | Azrin and Foxx adaptation (prompting rather than self-initiated) |
Toilet training took a median of 14.5 (mean 17.2, range 12–24) days across participants Intervention arm:Comparator arm: baseline |
Weak | Urinary | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reference | n; age | Based on/device/drug | Effect | EPHPP quality appraisal | Faecal/urinary/both | |
|---|---|---|---|---|---|---|
| Autism | ||||||
| RCT | ||||||
| A | Mruzek,39 2019 | 33; 36–72 months | Wireless moisture pager intervention | Comparison of wireless moisture pager and SBT intervention efficacy
|
Moderate | Urinary |
| Mruzek,39 2019 | 32; 3–6 years | Wireless moisture pager intervention | Comparison of wireless moisture pager and SBT intervention efficacy
|
Moderate | Urinary | |
| Myelodisplasia | ||||||
| RCT | ||||||
| B | Boone,98 1992 | 36; 6–12 years | Transurethral intravesical electrotherapy |
Overall comparison:
|
Weak | Urinary |
| Spina bifida | ||||||
| RCT | ||||||
| B | Kajbafzadeh,52 2009 | 30; 5.6 years; (range 3–12.5 years) | Pelvic floor interferential electrostimulation |
Overall comparison:
|
Moderate | Both |
| B | Kajbafzadeh,53 2014 | 30; 6.7 years (range 3–13 years) | Transcutaneous functional electrical stimulation (FES) |
Intervention arm:
|
Moderate | Urinary |
| B | Loening-Baucke,55 1988 | 28; 7–21 years | Biofeedback training |
Overall comparison:
|
Weak | Faecal |
| B | Marshall,56 2001 | 77; 4–18 years | Transcutaneous electrical field stimulation – Duet Continence Stimulator |
Overall comparison:
|
Moderate | Both |
| Lomas Mevers,38 2020 | 20; 5–16 years | Liquid glycerine suppositories and reinforcement |
Overall comparison:
|
Weak | Faecal | |
| B | Van Winckel,58 2006 (spina bifida or anal atresia) | 7; 4–13 years | Anal plug (Conveen) |
Overall comparison:
|
Weak | Faecal |
| B&A | ||||||
| B | Ausili,62 2010 | 62; 6–17 years | TAI; Peristeen anal irrigation system (TAI) | Intervention arm: mean (SD)
|
Weak | Faecal |
| B | Ausili,63 2018 (spina bifida with ARMs) | 74; 6–17 years | TAI; Peristeen | Intervention arm: T1 3 months, T2 24 months
|
Moderate | Faecal |
| B | Choi,64 2015 | 47; 3–18 years | TAI (plastic bag with irrigation cone-based TAI (Colotip, Coloplast) or catheter-based TAI (Peristeen system) | Intervention arm:
|
Weak | Faecal |
| B | Hascoet,65 2018 | 53; 8.5 years (range 1–15 years) | IDBTX-A |
Overall comparison:
|
Weak | Urinary |
| B | Killam,67 1985 | 8; 7–19 years | Urodynamic biofeedback treatment |
Overall comparison: Urinary incontinence Faecal incontinence |
Weak | Both |
| B | Lima,69 2017 | 25; 5–18 years | Intraurethral self-retaining device |
Overall comparison:
|
Moderate | Urinary |
| B | Mattsson,70 2006 | 40; 10 months–11 years | TRI |
Overall comparison:
|
Moderate | Faecal |
| B | Shoshan,71 2008 | 20; 12 years (4–29 years) | Anal plug | Intervention arm:
|
Moderate | Faecal |
| Cohort | ||||||
| B | Palmer,74 1997 | 55; 2–14 years | Transrectal bowel stimulation |
Overall comparison:
|
Weak | Faecal |
| Case report | ||||||
| B | Han,77 2004 | 24; 3.9–13.2 years | Intravesical electrical stimulation | Intervention arm: mean follow-up 15.8 months
|
Weak | Faecal |
| B | King,78 2017 | 20; 14.5 years | TAI (Peristeen) |
Overall comparison:
|
Weak | Faecal |
| B | Petersen,79 1987 | 10; 8–12 years | Transurethral intravesical electrical stimulation |
Overall comparison:
|
Weak | Urinary |
| B | Vande Velde,80 2007 | 80; 5–18 years | Self-catheterisation and self-administration of enemas by the age of 12 years (stepwise bowel management programme) |
‘Faecal continence’ (no involuntary stool loss in the absence of treatment), ‘pseudocontinence’ (involuntary stool loss no more than once per week), ‘incontinence’ (involuntary stool loss more than once per week) Intervention arm: |
Moderate | Faecal |
| Neuropathic bowel and ARMs | ||||||
| B&A | ||||||
| A | Corbett,90 2014 | 24; 4–16 years | TAI (Peristeen) | Overall comparison:
|
Weak | Faecal |
| Neuropathic bladder | ||||||
| Controlled trial | ||||||
| A | Borzyskowski,89 1982 (vesicourethral dysfunction) | 43; 7 years | CIC plus drug therapy |
Intervention arm:
|
Weak | Urinary |
| Neurogenic dysfunction | ||||||
| RCT | ||||||
| B | Haddad88 2010 (spina bifida, n = 10; sacral agenesis, n = 8; miscellaneous neurological abnormalities, n = 7; congenital coloanal and urinary malformations, n = 5) | 33; mean age 12.22 years | SNM (InterStim) |
Overall comparison:
|
Moderate | Both |
| Reference | n; age | Based on | Effect | EPHPP quality appraisal | Faecal/urinary/both | |
|---|---|---|---|---|---|---|
| ADHD and autism | ||||||
| B&A | ||||||
| A | Yang,35 2015 (ADHD symptoms) | 68; 5–12 years | Drug therapy – desmopressin |
Overall comparison:
|
Moderate | Both |
| Case–control | ||||||
| A | Chertin,36 2007 (ADHD) | 54; 8 years average | Combination therapy with desmopressin and oxybutynin vs. the tricyclic antidepressant imipramine |
Overall comparison:
|
Weak | Urinary |
| Observation | ||||||
| A | Gor,37 2012 (ADHD and autism) | 671; mean of 8.6 years | Desmopressin or anticholinergic treatment |
Overall comparison:
|
Weak | Urinary |
| Myelodysplasia and neurogenic bladder | ||||||
| RCT | ||||||
| A | Åmark,99 1992 (myelodysplasia and neurogenic bladder) | 10; 6–18 years | The alpha-adrenoceptor agonist phenylpropanolamine |
Overall comparison: Three participants reported no change in continence from placebo to administration of phenylpropanolamine. Five participants reported a one-point improvement in continence (from D to C, C to B, or B to A) from placebo to phenylpropanolamine therapy, and two participants reported a two-point improvement (from C to A) from placebo to phenylpropanolamine therapy Nine out of 10 participants reported increases in the number of 3-hour dry periods during 3 consecutive days. Eight participants reported a reduction in the mean number of leakage episodes per day from placebo to phenylpropanolamine therapy Active treatment was regarded as ineffective by five patients and of moderate value by three patients, while two reported a good effect Increase in incontinence was never reported. All patients reported that the placebo had no effect on incontinence and caused no side effects |
1 | Urinary |
| Spina bifida | ||||||
| B&A | ||||||
| B | Horowitz,72 1997 | 18; 10.5 years (range 7–16 years) | Desmopressin |
Overall comparison:
|
Weak | Urinary |
| Neurogenic dysfunction | ||||||
| B&A | ||||||
| A | Schulte-Baukloh,91 2006 (detrusor overactivity) | 20; 8.9 years | Propiverine |
Intervention arm:
|
Weak | Urinary |
| A | Schulte-Baukloh,92 2012 (a longer-term follow-up of Schulte-Baukloh 200691) | 17; 13 years | Propiverine |
Overall comparison:
|
Weak | Urinary |
| Mixed presentation | ||||||
| RCT | ||||||
| A | Valentine,51 1968 (6 brain damaged, 4 with Down syndrome, 2 with epilepsy, 2 ‘primary genetic’, 1 postnatal infection, 1 infant autism) | 16; NR | Imipramine |
Overall comparison:
|
Weak | Urinary |
| B&A | ||||||
| B | Åmark,100 1998 (one case of vascular lumbar spinal cord insult, one case of non-neurogenic neurogenic bladder and one case of traumatic high thoracic spinal cord injury; all other patients had myelomeningocele) | 39; 0.5–18 years | Intravesical oxybutynin | 37 patients. Four groups of continence described:
|
Weak | Urinary |
| B | Naglo,101 1979 [myelomeningocele (11/13), sacral agenesia (1/13) or spinal dysraphism (1/13)] | 13; 6–18 years | Drug therapy plus continence training |
Overall comparison:
|
Weak | Urinary |
| Reference | n; age | Procedure | Effect | EPHPP quality appraisal | Faecal/urinary/both | |
|---|---|---|---|---|---|---|
| Spina bifida | ||||||
| B&A | ||||||
| B | Aksnes,61 2002 | 20; 6.3–17 years | MACE |
Overall comparison: at 16 months Faecal incontinence – episodes reduced (16/19 – never/very rarely) |
Weak | Faecal |
| Kajbafzadeh,66 2006 | 26; 6.9 ± 2.6 years (range 3.5 to 13) | IDBTX-A |
Intervention arm: at 4 months Mean incontinence score had improved to 0.3 (p < 0.001) Of the 26 patients, 19 (73%) became completely dry between two consecutive CICs and four of the remaining seven patients had improved from score 3 to 1 The total improvement rate was 88% Comparator arm: baseline Mean incontinence score 2.5 |
Moderate | Urinary | |
| Ladi-Seyedian,68 2018 | 24; 9 years (3–16 years) | Intravesical electromotive BoNTA ‘Dysport’ |
Overall comparison: Twenty-one of 24 (87.5%) patients became completely dry between two consecutive CICs after 6 months, which was maintained in 18 of 24 (75%) patients at 1-year follow-up During the follow-up, 11 of 24 (45.5%), 9 of 24 (37.5%), 8 of 24 (33%), and 7 of 24 (29.1%) of the patients were completely dry between two consecutive CICs after the once BoNTA-EMDA (electromotive drug administration) treatment at 2, 3, 5 and 6 years’ follow-up, respectively |
Moderate | Urinary | |
| Cohort | ||||||
| Tarcan,73 2014 | 31; 7.95 years (5–13 years) | Intradetrusor injections of onabotulinum toxin-A |
Overall comparison: A total of 30 of 31 patients reported continnce between CIC intervals We noticed improvement in terms of continence within 2–4 weeks after onabotulinum toxin-A injection. The mean duration of effects was 28 weeks for a single injection and 36 weeks for repeated injections (minimum, 16 weeks; maximum, 52 weeks) |
Moderate | Urinary | |
| Case–control | ||||||
| B | Snodgrass,76 2009 | 41; 3–14 years | Bladder neck sling with and without enterocytoplasty |
Overall comparison: 18 patients had sling with augmentation and 23 with sling alone Improved continence rate was 83% with no differences in patients in the two groups |
Moderate | Urinary |
| Case report | ||||||
| B | Bar-Yosef,81 2011 | 21; 6–22 years | MACE |
Overall comparison: Full faecal continence in 19/21 patients following the MACE procedure Two patients reported soiling 1–2 times per week 16 patients reported full faecal and urinary continence |
Weak | Both |
| B | Ibrahim,82 2017 | 23; 3.5–17.8 years | ACE |
Overall comparison: 0.5–6.9 years, median 2.6 years Full continence in 13 (56.52%), partial continence in 8 (34.78%) and failure in 2 (8.69%) |
Weak | Faecal |
| B | Matsuno,83 2010 | 25; 4–23 years | RCE; Malone anterograde continence enema |
Overall comparison: Faecal continence for 10 of 13 (76.9%) in the retrograde group and 9 of 12 (75.0%) in the antegrade group Achievement of faecal continence did not differ between the groups |
Weak | Faecal |
| B | Snodgrass,84 2016 | 37; 3–18 years | Bladder neck sling |
Overall comparison: 60 months after bladder neck sling, 38 months after Leadbetter/Mitchell bladder neck revision, and 29 months after bladder neck closure Continence (dry, no pads) was achieved significantly more often with Leadbetter/Mitchell bladder neck revision v.s. bladder neck sling (66% vs. 37%) Bladder neck closure resulted in dryness in 65% of patients, with most incontinence occurring from the Mitrofanoff stoma associated with filling pressures > 40 cm |
Weak | Urinary |
| B | Van Savage,85 2000 | 16; 12 (4–21 years) | ACE |
Overall comparison: Constipation and faecal continence was resolved in all cases. All patients stopped using nappies |
Weak | Faecal |
| B | Wehby,86 2004 | 60; 3–18 years | Lumbar flavotomy and section of the filum terminale, a Foley catheter, placed after induction of anaesthesia |
Overall comparison: Urinary incontinence/retention Complete resolution in 52%, marked improvement (> 95% resolution) in 35%, moderate improvement (> 75%) in 6%, minimal improvement (> 50%) in 6%, and no improvement (< 50%) in 2% Faecal incontinence Completely resolved in 56%, improved in 41%, and was unchanged in 3% |
Weak | Both |
| Neurogenic bladder | ||||||
| Cohort | ||||||
| Guys,93 1999 | 33; 13 (7–17 years) | Injection of PDMS for endoscopic treatment |
Overall comparison: 33.3% patients entirely continent 24% patients partially continent 42% patients no effect at all |
Weak | Urinary | |
| Guys,94 2006 | 49; 14 years (SD 4.8 years) | Endoscopic injection of PDMS |
Overall comparison: at 73 months Success achieved in 16 patients (33%) and improvement in 7 (14%) |
Weak | Urinary | |
| Silveri,95 1998 | 23; 10.9 (6–17 years) | Collagen injection |
Overall comparison: 13 patients had a mean increase in dry time of 2 hours (range 0.5–3 hours). The remaining 10 patients had a mean average dry time of 0.2 hours (range 0–1 hours). In some patients, initial success was transient and passed after 2–4 weeks post injection |
Weak | Urinary | |
| Case report | ||||||
| A | González,96 2002 | 27; 4–23 years | Artificial urinary sphincter with seromuscular colocystoplasty |
Overall comparison: Continence achieved in 24 of the 27 (89%) patients |
Weak | Urinary |
| Neurogenic dysfunction | ||||||
| Case report | ||||||
| Do Ngoc Thanh,97 2009 (detrusor overactivity) | 7; 6.5–15.5 years | Botulinum type A injections |
Overall comparison: Continence scores improved from 3 before treatment to 0 after one injection of botulinum type A |
Moderate | Urinary | |
| Occult tethered cord syndrome | ||||||
| RCT | ||||||
| B | Steinbok,57 2016 | 21; 5–18 years | Filum section plus medical therapy |
Overall comparison: Bowel and Bladder Dysfunction score improved in the surgical and medical arms (20% and 24%) |
Moderate | Urinary |
| Mixed | ||||||
| Case report | ||||||
| A | Faure,102 2017 (neurogenic bladder, bladder extrophy) | 59; 7.6 years (1.9–17.5) | Young–Dees bladder neck reconstruction, with bladder neck injection as a follow-up |
Overall comparison: Only 10 patients (18%) were continent Owing to lack of success with YNBR, 45/55 (81.8%) underwent bladder neck reconstruction; 15/55 (31%) became continence as a result of this procedure |
Weak | Urinary |
| B | Jawaheer,103 1999 (12 – spina bifida; 5 – sacral agenesis; 1 – idiopathic neural bladder) | 18; 3–14 years | Pippi Salle bladder neck repair |
Overall comparison: Daytime continence (≥ 3 hours) achieved in 11/18 children (61%); 5 remained incontinent 8/18 children (44%) were continent at night, with 8 remaining incontinent at night 12 children needed additional drug therapy (Oxybutynin) to maintain continence success 7 children (39%) required further operations |
Weak | Urinary |
Appendix 6 Details of complex interventions
| Author, year | Brief name | Why | What materials | What procedures | Who | How | Where | When and how much |
|---|---|---|---|---|---|---|---|---|
| Non-spinal-cord-related conditions | ||||||||
| ADHD | ||||||||
| Yang,35 2015 | Desmopressin | Not reported | None | Desmopressin administered orally, starting with a standard dose of 0.2 mg and increasing after 2 weeks to 0.4 mg if symptoms persisted; dose reduced by half in the final 2 weeks of the study; bladder training programme (4 weeks) prior to treatment (evenly distributed fluid intake, avoidance of drinks causing bladder overactivity, timed voiding, fluid restriction in the evening, and emptying bladder before bedtime) | Not clearly reported | Face to face | Clinic, home | 12 weeks |
| Chertin,36 2007 | Desmopressin and oxybutynin | To compare the effectiveness of combined treatment with desmopressin and oxybutynin vs. the tricyclic antidepressant imipramine on nocturnal enuresis in children with ADHD | Not reported | An initial bladder training programme designed to decrease bladder overactivity and engage the child in the treatment process. It included an increase in daytime fluid intake, the need to respond to any sense of urgency in urination, timed voiding every 2–3 hours or during school breaks to establish cognitive control over voiding and treatment of encopresis, if it existed. Followed by treatment with drugs – desmopressin at an initial dose of 0.2 mg with an increase to 0.4 mg if the episodes of night-time wetting did not decrease at least 50%. In addition, oxybutynin was given at 0.2 mg/kg three times during the day | Not clearly reported | Face to face | Home | Desmopressin at 0.2 mg with an increase to 0.4 mg if the episodes of night-time wetting did not decrease at least 50%. Oxybutynin at 0.2 mg/kg three times during the day |
| Autism | ||||||||
| Lomas Mevers,38 2020 | Multidisciplinary intervention for encopresis | To evaluate the preliminary efficacy of this multidisciplinary intervention of encopresis in children with autism spectrum disorder using a clinical trial | Suppository and chosen reinforcer | Each session consisted of a 32-minute sequence of scheduled sitting on the toilet (i.e. sits). The sequence included 10 minutes on the toilet, followed by 1 minute of standing, repeated with 10 minutes on, 1 minute off sequence for three rounds. If a participant had a continent urination, the therapist delivered praise and the participant remained on the toilet. Participants who had a continent bowel movement were allowed to leave the bathroom, given enthusiastic praise, and access to a positive reinforcer. A continent or incontinent bowel movement ended that day’s appointment. If no continent bowel movement occurred during the first 32-minute sequence, the participant received a 5-minute break Following the break, the therapist administered a full dose of liquid glycerin suppository to promote a bowel movement and the sit sequence resumed. If still no bowel movement, then participant was given a 30-minute break followed by a second suppository and a third sit sequence | Trained research staff | Face to face | Clinic | 10 sessions (one session per day) lasting 1–4 hours |
| Mruzek,39 2019 | Wireless moisture pager [an iOS-based app (Apple Inc., Cupertino, CA, USA) with transmitter/disposable sensor] and a corresponding manualised training programme | To test the feasibility of a multisite RCT of the intervention | Caregiver and clinician manuals | An iPod-based application (Apple Inc., Cupertino, CA, USA) connected to a disposable transmitter in the child’s underwear. Is capable of time-stamped data available for e-mailing via Bluetooth by parents to study interventionists for monitoring and feedback, messaging between study interventionists and participating parents, a picture-based reinforcement menu to aid reinforcement for successful toilet use, and a timer to remind parents to reinforce intervals of continence. Connectivity between the iPod and the disposable sensor is maintained up to a distance of 30 feet | Research staff individually trained parents in a 1.5-hour centre based training using the training manual to develop an individualised toilet training programme. Four 1-hour centre-based booster sessions were also delivered to parents throughout the intervention | Face to face | Home | 12 weeks |
| Mruzek,39 2019 | Wireless moisture pager (an iOS-based app with transmitter/disposable sensor) and a corresponding manualised training programme | To develop and prepare the intervention for large-scale testing | Caregiver and clinician manuals | An iPod-based application connected to a disposable transmitter in the child’s underwear | Research staff trained parents to use the intervention in five face-to-face training sessions; parents delivered the intervention | Face to face | Clinic; home | 12 weeks |
| Ardic,40 2014 | Modified Intensive Toilet Training (adapted from Azrin and Foxx104) | To test an adaptation of the Azrin and Foxx method104 | None | Adapted from Azrin and Foxx104 | Teacher; parents | Face to face | Education centre; home | Not clearly reported; tailored to each child |
| Keen,41 2007 | Video modelling and operant conditioning | Not reported | 30-minute timer, a video player and television, recording sheets, and a 6-minute animated toilet training video; set of A4 cue cards; written toilet training procedures and instructions | Children were required to watch the video on each occasion prior to toilet use; children were prompted to request toileting using pictures, language or signs. Reinforcement was given initially for any and all of the following: walking to the toilet, undressing, sitting on the toilet, eliminating in the toilet, redressing, flushing the toilet and hand-washing | Parents, carers and teachers | Face to face; telephone advice | Kindergarten, special education unit, preschool, or a combination of these | 11 hours per day; 7 days per week; intervention duration tailored to each child |
| Cicero,42 2002 | Reinforcement-based toilet training programme (adapted from Azrin and Foxx104) | Not reported | None | Adapted from Azrin and Foxx104 | Classroom teachers; speech and language therapists | Face to face | School | 5.5 hours per day for 3 days |
| Developmental/learning disability | ||||||||
| Edgar,43 1975 | Toilet training | Encouraging response to internal cues should lead to greater mastery of self-initiation than response to external cues alone | Electric belt, toilet with buzzer; progress charts kept in the toilet training room | Fluid given hourly; 15 minutes later, children practised 10 minutes of relaxation/tension training exercises with audio cues; children were rewarded for in-toilet urination; when ‘accident’ happened the technician sharply told the child to ‘stop’, turned off the buzzer and led them to the toilet | Technicians | Face to face in small groups | Residential care setting | 8 hours per day; 4 days per week; no more than 2 weeks; tailored to each child |
| Hundziak,44 1965 | Operant conditioning | Not reported | Reinforcement device to reward eliminative responses | Children were placed on the commode every 2 hours, whenever they self-initiated and if signs of approaching elimination were ‘intercepted’. Elimination on the commode was rewarded by the attendant pressing a button to release a candy treat – at the same time a light would flash and a noise would sound | Attendants | Face to face | Special training unit | 7 hours per day; 5 days per week; 27 days |
| Sadler,45 1977 | Toilet training programme (adapted from Azrin and Foxx104) | Not reported | None | Adapted from Azrin and Foxx104 | Not reported | Face to face | Day training centre | Details not reported |
| Van Laecke,46 2009 | Personalised fluid intake | Not reported | Toilet adapted to needs of child; personalised fluid intake schedule | Adequate fluid intake calculated using a mean of 1500 ml/m2 body surface – equally divided during the day in to 4–6 portions; guidance provided to parents on fluid quantity and quality | Researcher provided instruction; parents delivered intervention | Face to face | Home | 6 weeks |
| Barmann,47 1981 | Toilet training programme (adapted from Azrin and Foxx104) | To test an adaptation of the Azrin and Foxx method104 | None | Adapted from Azrin and Foxx104 | Researcher trained parents; parents at home; teacher/carer at school | Face to face | Home; school | Training period ranged from 4 to 12 days; tailored to each child |
| Rinald,48 2012 | Rapid toilet training workshop | To evaluate the impact parent involvement in the teaching of continence using an adaptation of Azrin and Foxx104 | Printed materials to support information provided in the workshops | Workshop for parents to demonstrate the rapid toilet training method;104 toilet training implemented by parents at home; ongoing support provided by researcher | Researcher trained in parent training and toilet training for people with disabilities | Face to face; ongoing support for provided face to face or by telephone | Community centre/classroom; home | 8 hours per day; duration tailored to each child |
| Angelman syndrome | ||||||||
| Didden,50 2001 | Toilet training programme (adapted from Azrin and Foxx104) | Not reported | None | Adapted from Azrin and Foxx104 | Researchers; direct care providers | Face to face | Residential setting or day-care centre | Between 5.5 and 7 hours per day; study period not clearly reported; tailored to each child |
| Mixed populations | ||||||||
| Valentine,51 1968 | Imipramine | To support the assumption that imipramine is of value in mentally subnormal children with eneuresis | None | Imipramine given in doses of 50 mg tablets at night for 3 weeks | Parent | Face to face | Home | 3 weeks |
| Spinal cord pathology | ||||||||
| Spina bifida | ||||||||
| Kajbafzadeh,52 2009 | Transcutaneous interferential electrostimulation | Not reported | None | Pelvic floor interferential electrical stimulation | Physiotherapist | Face to face | Clinic | 18 courses over 6 weeks (20 minutes in each session 3 times per week) |
| Kajbafzadeh,53 2014 | Functional electrical stimulation | Not reported | None | Functional electrical stimulation | Not reported | Face to face | Clinic | 15 sessions (15 minutes in each session three times per week) |
| Loening-Baucke,55 1988 | Biofeedback training | To test the efficacy of biofeedback training for faecal incontinence in patients with myelomeningocele | For practice at home, a 2.5 × 3 cm balloon, small and large tubing, an upright, water-filled measuring device, which was graded | Children were instructed to try to defecate for 5 minutes four times a day after meals and an afternoon snack. Children also received three biofeedback training sessions; exercises on the perineum every other day using a three-balloon system developed by Schuster (1965).145 Sphincter exercises lasted 60–90 minutes. These children were asked to practise techniques at home for 20–30 minutes at least every other day | Not clearly reported | Face to face | Clinic (with practising at home) | Three sessions lasting 60–90 minutes. Unclear when sessions were delivered, but follow-up was at 11–12 months |
| Marshall,56 2001 | Transcutaneous electrical stimulation | As the bladder and bowel and electrically dissociated from the central nervous system in children with spina bifida, imitating this absent input with electrostimulation may reverse the defective intrinsic responses of these viscera | Electrical stimulation performed using a Duet Continence Stimulator | Electrical stimulation was performed at home by the patient, or by parents, for approximately 1 hour daily for at least 6 weeks. Compliance was encouraged by regular telephone calls to families | Parent | Face to face | Home | 6 weeks to 5 months |
| Choi,59 2013 | Bowel management programme | Not reported | Cone or Peristeen anal irrigation system (depending on child’s age); educational pocketbook | Transanal irrigation; advice on normal healthy diet | Training provided by a specialist nurse practitioner | Face to face; ongoing advice available over telephone | Clinic; home | Initially daily; if successful, frequency was reduced to once every 2–3 days; study period was not clearly reported; tailored to each child |
| Ausili,62 2010 | Transanal irrigation | Transanal irrigation is known to improve bowel function in adult patients with faecal incontinence or constipation | Peristeen anal irrigation system | Transanal irrigation | Training provided by a doctor | Self-administered | Clinic; home | 3 months |
| Ausili,63 2018 | Transanal irrigation | To evaluate the long-term effects of transanal irrigation | Peristeen anal irrigation system | TAI; 10/20 ml/kg every day for the first week; increasing the amount of water as needed to a maximum of 1 l | Training provided by specialised nurses and a medical doctor | Self-administered | Clinic; home | Not clear; follow-up data were collected at 3 and 24 months |
| Choi,64 2015 | Transanal irrigation | To evaluate the long-term effects of TAI | Irrigation cone-based system (Colotip, Coloplast) or catheter-based system (Peristeen Coloplast), depending on the child’s age; educational pocketbook | Initially, enemas were administered daily. If successful, the frequency was reduced to once every 2–3 days.3 Enema volume was initially 300–500 ml, but was increased to 500–700 ml depending on age and need | Training was provided by a specialist nurse; training included educational pocketbook, demonstration and practice by parents; advice was provided over telephone at least once; nurse was available to advise parents throughout the study | Self-administered | Clinic; home | Not clear; follow-up data were collected at 3 months and 3 years |
| Killam,67 1985 | Urodynamic biofeedback | To investigate the application of urodynamic biofeedback procedures in children with congenital neurogenic urinary incontinence | Baseline testing and feedback training was provided using a Life-Tech 6-channel Urolab kit | Biofeedback training sessions lasting 1–1.5 hours undertaken as outpatients at the orthopaedic hospital. Home programmes were developed for each participant and used throughout the training period | Urodynamic technician in clinic and parent at home | Face to face | Clinic; home | Weekly or biweekly for 9–51 weeks. Number of sessions ranged from 6 to 21 |
| Lima,69 2017 | Intraurethral self-retaining device | To evaluate the safety and efficacy of a new ISRD in female children and adolescents as an alternative to CIC | The ISRD was made of medical grade silicone. Manufactured by Medicone Innovation for Health Ltd (Cachoeirinha, Brazil) | The ISRD was made of medical-grade silicone and is available from 10 to 20 Fr in diameter with four different sizes, 3, 3.5, 4 and 4.5 cm of distance between disks. The structure of the new device was formed by two disks (one proximal or fixed and the other mobile or distal), six collectors of urine and a cover connected to the lumen of the catheter. The fixed disk was positioned at the bladder neck from the inside. The mobile disk was positioned at the level of the external urethral meatus. The device had a specific pusher to provide adequate resistance at the moment of introduction. Patients underwent local anaesthesia with lidocaine gel 20 mg/ml applied intraurethrally. The urethra was dilated up to 26 Fr when necessary to facilitate introduction of the proximal disk that was positioned in the inner portion of the bladder neck. The mobile disk was adjusted to the external meatus by sliding it along the ISRD. No systematic antibiotic prophylaxis was administered | After initial training, patients or caregivers were advised to empty the bladder at regular intervals according to bladder capacity | Face to face | Clinic; home | Follow-up 6 months |
| Mattsson,70 2006 | Tap-water enema | Not reported | Transrectal irrigation was given by Stoma Cone Irrigation Set (Hollister Ostomy Products, Libertyville, IL, USA) or Colotip (Coloplast) | Enemas were given with the child in supine position on the potty or toilet by inserting the cone into the anus and hanging the irrigation bag about 1 m above the potty or toilet. The bag was filled with a small volume (200/600 ml; median 300 ml) of lukewarm tap water without added salt. Infusion time ranged from 2/10 minutes. Most children remained on the potty/toilet for another 10/20 minutes to completely empty their bowels; some required longer times of up to 60 minutes; parents and children were instructed to perform the procedure every day | Training provided | All children over the age of 6 years were asked to self-administer the procedure | Clinic; home | Unclear. Follow-up was conducted after between 1 and 3 years |
| Horowitz,72 1997 | Desmopressin | Experience of using of desmopressin in the spina bifida population with enuresis | Not reported | Patients were given 20–40 µg of desmopressin before bed. All were started on 40 µg with fluid restriction and bedtime catheterisation. Doseage was tapered by 10 µg every 3 weeks and patients were kept on the minimum doseage to keep them dry | Parent | Face to face | Home | Initial dose was 40 µg at bedtime, decreased by intervals of 10 µg every 3 weeks |
| Palmer,74 1997 | Transrectal electrostimulation | To evaluate the efficacy of transrectal bowel stimulation for neurogenic bowel dysfunction in children with myelodysplasia | Not reported | Daily sessions of transrectal electrostimulation were performed on an outpatient basis for 2–3 weeks. If benefits were noted, 5–10 additional daily sessions were performed | Clinic staff | Face to face | Clinic | Over a 9-week period; 3 weeks of intervention (30 minutes, 5 days per week) and 3 weeks of placebo; therapy given over 24-hour period |
| Radojicic,75 2019 | Bowel management with anticholinergic medication and clear intermittent catheteristation | To study the impact of bowel management on the QoL of children with spina bifida with overactive bladder and detrusor sphincter dyssynergia | Not reported | Patients were prescribed anticholinergic medication therapy (oxybutynin) 0.2 mg/kg/dose three times daily. All patients were also regularly administered CIC every 3 hours through a continent vesicostomy or through a native urethra. Bowel management therapy, which encompassed procedures to treat constipation and faecal incontinence. Diet was altered | Parent/child | Face to face; self administered | Home | 12 months |
| Neurogenic dysfunction | ||||||||
| Haddad,88 2010 | Sacral neuromodulation | To evaluate SNM for management of urinary and faecal incontinence | S3 root a neuromodulator InterStim® (Medtronic, Dublin, Ireland) | Beginning of session (t1) InterStim implanted, end of session 7 months later InterStim removed (t2); 45-day wash-out period, then beginning of session at 9 months (t3) and end of session at 15 months (t4). The patients’ S3 roots were stimulated using an implanted device while they were under general anaesthetic. Patients were discharged home after 48 hours | Unclear | Face to face | Unclear | 15 months |
| Borzyskowski,89 1982 | CIC (plus medication) | To show that urinary diversion could be avoided and that conservative methods of treatment were effective in most children | Not reported | Children in the CIC group were managed with passage of a catheter two or three times each day. Children who were still incontinent after 3 months were given drug therapy | Not reported | Face to face | Not reported | Unclear follow-up at 3, 6, 18 months up to 4 years |
| Corbett,90 2014 | Peristeen TAI | To assess any change in bowel function and QoL of patients and their carers using TAI system, as well as compliance and complications | Not reported | The Peristeen TAI system. The carers (and child, if competent) are taught by a specialist nurse how to use the system | Training for carers provided by specialist nurse | Face to face; carer/self-administered | Clinic; home | The volume and frequency of the instillations are decided on a case-by-case basis; follow-up ranged from 2 to 48 months |
| Schulte-Baukloh,91,92 2006 and 2012 | Propiverine hydrochloride | Not reported | None | Propiverine at 0.4 mg/kg body weight (available in Germany as 5 mg coated tablet) every 12 hours as recommended; dosage was increased if considered appropriate | Clinician | Self-administered | Clinic; home | Unclear; follow-up was conducted at 3–6 months |
| Myelodysplasia | ||||||||
| Boone,98 1992 | Transurethral intravesical electrotherapy | To evaluate the efficacy of transurethral intravesical electrotherapy for neurogenic bladder problems in children with myelooidysplasia | Electrocatheter and electrode |
Filled to half capacity with saline solution and electrocatheter inserted transurethrally. The indifferent electrode was placed on the thigh. Bladder stimulation was given in exponential wave forms with parameters set for individual patients Each session lasted 90 minutes; administered five times per week for 3 weeks; then a 3-month gap from treatment; then another 3 weeks of treatment |
Clinician | Face to face | Clinic | 4–5 months |
| Åmark,99 1992 | Phenylpropanolamine | Not reported | None | Little information was provided on dose or administration other than the dose was based on pharmacokinetic data | Not reported | Self-administered | Clinic; home | Medication was given for 2 weeks; following a study visit the treatment was modified for the following 2 weeks |
| Mixed | ||||||||
| Åmark,100 1998 | Intravesical oxybutynin | To improve continence | None | Children were treated with CIC; intravesical oxybutynin 0.1 mg/kg twice daily was added and administered as a sterile pharmacy-produced solution | Not reported | Self-administered | Home | Varied; followed for 0.66–5 years (mean 2.25) with the exception of seven patients who stopped treatment after 1 week to 8 months |
| Naglo,101 1979 | Alprenolol | Not reported | None | Children received alprenolol or placebo (no dose details provided) in addition to ‘established toilet training programme’ (no details provided) | Specially trained nurse | Face to face | Paediatric hospital | One dose per 24 hours for 3 weeks |
Appendix 7 Organisations contacted to advertise surveys
List of organisations and charities that were contacted and asked to advertise the surveys, and were confirmed to have shared the survey information with their members via mailing lists, websites or social media:
-
Action Cerebral Palsy
-
Association for Continence Advice (ACA) newsletter
-
Association of Directors of Children’s Services (ADCS)
-
Association of Paediatric Chartered Physiotherapists (ACPC) – Neurodisability
-
BHTA (British Healthcare Trades Association) – Children’s equipment
-
Bladder and Bowel UK
-
British Academy of Childhood Disability
-
British Association of Paediatric Nephrology
-
British Association of Paediatric Surgeons
-
British Association of Paediatric Urology and Continence Nurses
-
Cerebra
-
CHAIN (Contact, Help, Advice and Information Network)
-
Changing Places
-
Chatterpack
-
Clear Autism
-
Contact
-
Council for Disabled Children
-
Devon County Council
-
Devon Early Years & Childcare Service
-
Devon Information Advice and Support
-
Down Syndrome Association
-
ERIC
-
Evelina’s Children’s Hospital
-
Family Faculty – PenCRU
-
Fragile X Society
-
Headway
-
Health Unlocked
-
James Lind Alliance
-
KIDS – giving disabled children a bright future
-
Lambeth Autism Group
-
Multiple Sclerosis Society
-
Mumsnet
-
NADP (National Association of Disability Practitioners)
-
National Association of Independent Schools & Non-Maintained Special Schools
-
National Day Nurseries Association
-
National Network of Parent Carer Forums
-
Netmums
-
Pace schools
-
Paediatric Continence Forum
-
Paediatric Psychology Network (PPN) – part of British Psychological Society
-
Parallel Parents Fostering
-
Royal College of Nursing – Bladder and Bowel Forum (previously Continence Care Forum)
-
Royal College of Occupational Therapists Specialist Section – Children, Young People and Families
-
School and Public Health Nurses Association (SAPHNA)
-
Seashell Trust
-
Sentient Trust
-
Schools and Students Health Education Unit
-
Social Care Institute for Excellence (SCIE)
-
Special Educational Needs contacts by county in the England
-
TACTYC – the association for professional development in early years
-
Team Around the Family – monthly bulletin
-
The CLAHRC network (Collaboration for Leadership in Applied Health Research and Care)
-
The SENAD group
-
Together for Short Lives
-
Together Gateshead
-
Transverse Myelitis Society.
Organisations and charities that were contacted via e-mail or social media and asked to disseminate information and adverts about the surveys and may have shared but did not confirm:
-
3 Dimensions Care
-
A Second Voice
-
Active Care Group
-
Autism Education Trust
-
British Association for Community Child Health
-
British Paediatric Neurology Association
-
Cambian Group – Schools, Residential, Fostering
-
Catch 22 group
-
Cavendish Education
-
CEDA (Community, Equality, Disability Action)
-
Cerebral Palsy Sport/Team
-
Children and Young People Now
-
Diversions
-
Dyspraxia Foundation
-
Early Education
-
Epilepsy Society
-
ICAN charity – the children’s communication charity
-
Include Me TOO (ethnic minority families)
-
Insight Dynamics
-
MENCAP
-
Nasen (National Association for Special Educational Needs)
-
National Association of Head Teachers
-
National Autistic Society
-
National Autistic Society academy schools
-
National Star College
-
Partners in Paediatrics (PIP)
-
Percy Hedley School
-
Pinpoint Community Services in Devon – Integrated Children’s Services
-
Primary Times
-
Priory Education and Children’s Services
-
Profound and multiple learning disabilities (PMLD – part of MENCAP)
-
RNIB (Royal National Institute of Blind People) schools
-
Royal Blind Schools
-
Royal College of General Practitioners
-
Royal College of Nursing – Children and Young People’s Forum
-
Royal College of Speech and Language Therapists
-
Ruskin Mill Trust
-
SEN (Special Educational Needs) magazine
-
SHINE – Spina Bifida, Hydrocephalus, Information, Networking, Equality
-
Special Education Consortium (part of CDC)
-
Special Needs UK
-
St Piers School (Young Epilepsy)
-
Steiner Waldorf Schools Fellowship
-
The Aurora Group
-
The Caldecott Foundation
-
The Together Trust
-
The Ups of Downs – a charity for parents of children with Down syndrome
-
Treehouse Educare
-
Treloars School and College
-
WellChild – the charity for sick children.
Appendix 8 Survey questions mapped to research questions
| Study RQ | RQ broken down | Relevant survey questions |
|---|---|---|
| RQ1: How do clinicians assess bladder and bowel health of children and young people with neurodisability, their continence capabilities, and readiness for toilet training? Which clinicians are involved in assessments? | RQ1.1: How do clinicians assess bladder and bowel health of children and young people? |
HP3: Which of the following problems would be most likely to trigger you to assess the bladder and bowel health status of a child/young person in this group? Please tick all that apply HP4: How frequently would you typically re-assess the bladder and bowel health of a child or young person in this group, following the initial assessment? HP5: Is the bladder and bowel assessment of children and young people in this group a specific toileting assessment, or part of a broader assessment of the child’s overall health needs? HP6: Where do you normally carry out a bladder and bowel assessment of a child/young person in this group? Please tick all that apply HP7: What methods do you use to assess the bladder and bowel health of a child or young person in this group? Please tick all that apply HP10: Which of these factors would be most likely to initiate the start of a formal toilet training or support programme for a child/young person in this group? Please tick all that apply HP16: Where you work, how are children/young people in this group usually referred for professional toileting advice and support? Please tick all that apply HP56: Where you work, do you have a bladder and bowel protocol/pathway for children and young people with special educational needs and/or a disability? PC14: Where has the assessment of your child’s toileting ability take place? Please tick all that apply PC15: What methods were used to assess your child’s need for professional help with toileting? Please tick all that apply SC7: Where does an assessment of a child or young person’s toileting ability usually take place? Please tick all that apply SC8: Which of the following assessment methods for children and young people with special educational needs and/or a disability have you had experience of? Please tick all that apply |
| RQ1.2: How do clinicians assess their continence capabilities? |
HP8: Which factors are most likely to trigger you to assess the capability and readiness for toilet training or support for a child/young person in this group? Please tick all that apply HP13: Which of the following toileting needs are you confident that you can address using interventions for a child/young person in this group? Please tick all that apply PC15: What methods were used to assess your child’s need for professional help with toileting? Please tick all that apply SC7: Where does an assessment of a child or young person’s toileting ability usually take place? Please tick all that apply SC8: Which of the following assessment methods for children and young people with special educational needs and/or a disability have you had experience of? Please tick all that apply |
|
| RQ1.3: How do clinicians assess readiness for toilet training? |
HP8: Which factors are most likely to trigger you to assess the capability and readiness for toilet training or support for a child/young person in this group? Please tick all that apply HP10: Which of these factors would be most likely to initiate the start of a formal toilet training or support programme for a child/young person in this group? Please tick all that apply PC15: What methods were used to assess your child’s need for professional help with toileting? Please tick all that apply SC7: Where does an assessment of a child or young person’s toileting ability usually take place? Please tick all that apply SC8: Which of the following assessment methods for children and young people with special educational needs and/or a disability have you had experience of? Please tick all that apply |
|
| RQ1.4: Which clinicians are involved in assessments? |
HP9: Who else is typically involved in the assessment of the capability and readiness for toilet training or support for a child/young person in this group (other than you)? Please tick all that apply HP16: Where you work, how are children/young people in this group usually referred for professional toileting advice and support? Please tick all that apply HP57: Where you work, is there a lead person responsible for the bladder and bowel protocol/pathway? PC10: If you or your child has received professional help or advice for toileting, please indicate from who or where from the list below. Please tick all that apply SC7b: Are you involved in assessing children and young people’s toileting ability and/or capability? If yes, please provide further information about the assessment that you conduct, or how you are involved |
|
| RQ2: Which interventions do clinicians use or recommend to improve continence for children and young people with neurodisability and how are these individualised and evaluated and/or audited? Which clinicians are recommending, delivering or evaluating interventions? | RQ2.1: Which interventions do clinicians use or recommend to improve continence? |
HP11: How effective do you find the following interventions to help with toileting for a child/young person in this group? HP12: How frequently do you typically review the following interventions if they are provided for a child/young person in this group? HP13: Which of the following toileting needs are you confident that you can address using interventions for a child/young person in this group? Please tick all that apply HP49: Where you work, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability PC16: If you and your child have experienced any of the following methods to help with toileting, please indicate how easy you found it using them at home. If you have never used the intervention, please indicate ‘never used’ PC23: Does your child use different toileting interventions for different environments? (e.g. pads at school, timed toileting at home) SC10: In your experience, how effective do you think the following methods are at helping a child or young person to manage their toileting more independently? |
| RQ2.2: How are these individualised and evaluated and/or audited? |
HP12: How frequently do you typically review the following interventions if they are provided for a child/young person in this group? HP14: What would normally be the initial aim of an intervention to help with toilet training or support for a child/young person in this group? HP15: What would you normally use as an outcome to judge the effectiveness of an intervention for a child/young person in this group? Please tick all that apply HP52: If an intervention is unsuccessful in helping a child/young person with special educational needs and/or a disability to manage their toileting, what approach do you typically take? HP53: In your opinion, do children and young people with special educational needs and/or a disability with the same problem get offered the same toileting intervention, regardless of who they are seen by? For example, would a child seen by a paediatrician be offered the same toileting intervention if they were seen by a specialist continence nurse? HP54: Where you work, are there any toileting interventions for children and young people with special educational needs and/or a disability that are not provided, which you know are provided elsewhere? HP55: Where you work, are children and young people with special educational needs and/or a disability offered different interventions for different environments? (e.g. pads for at school and timed toileting methods at home) PC18: For the methods that you and your child are using, please indicate how frequently it is currently reviewed by a professional. If you have never used the intervention, please indicate ‘never used’ PC23: Does your child use different toileting interventions for different environments? (e.g. pads at school, timed toileting at home) PC25: Please indicate which of the following steps have been planned or have already happened as part of your child’s transition to adult services in relation to toileting support SC5: Where you work, what is usually the main goal of improving toileting for a child or young person with special educational needs and/or a disability? SC13: Where you work, do children and young people with special educational needs and/or a disability use different toileting methods for different environments? (e.g. pads at school, hoist at home) SC14: Where you work, how well is transition to adult services managed for children and young people with special educational needs and/or a disability, in relation to their toileting needs? |
|
| RQ2.3: Which clinicians are recommending, delivering or evaluating interventions? |
HP11: How effective do you find the following interventions to help with toileting for a child/young person in this group? HP15: What would you normally use as an outcome to judge the effectiveness of an intervention for a child/young person in this group? Please tick all that apply HP53: In your opinion, do children and young people with special educational needs and/or a disability with the same problem get offered the same toileting intervention, regardless of who they are seen by? For example, would a child seen by a paediatrician be offered the same toileting intervention if they were seen by a specialist continence nurse? HP57: Where you work, is there a lead person responsible for the bladder and bowel protocol/pathway? PC10: If you or your child has received professional help or advice for toileting, please indicate from who or where from the list below. Please tick all that apply PC18: For the methods that you and your child are using, please indicate how frequently it is currently reviewed by a professional. If you have never used the intervention, please indicate ‘never used’ |
|
| RQ3: How do families, school and social care staff consider and judge children’s readiness for toilet training and need for specialist assessment and/or interventions? | RQ3.1: How do families, school and social care staff consider and judge children’s readiness for toilet training? |
HP9: Who else is typically involved in the assessment of the capability and readiness for toilet training or support for a child/young person in this group (other than you)? Please tick all that apply HP10: Which of these factors would be most likely to initiate the start of a formal toilet training or support programme for a child/young person in this group? Please tick all that apply PC8: Please indicate your child’s current toileting abilities using the table below. (knowing, finding, accessing, preparing, going, cleaning, completing) PC11: What prompted you as a parent/carer to seek professional support for toileting for your child? Please tick all that apply SC3: Where you work, to what extent do you have to help the children and young people with the following toileting needs? SC4: Which of the following would prompt you to seek specialist toileting support for a child or young person with special educational needs and/or a disability? Please tick all that apply SC5: Where you work, what is usually the main goal of improving toileting for a child or young person with special educational needs and/or a disability? SC7b: Are you involved in assessing children and young people’s toileting ability and/or capability? If yes, please provide further information about the assessment that you conduct, or how you are involved |
| RQ3.2: How do families, school and social care staff consider and judge children’s need for specialist assessment and/or interventions? |
HP10: Which of these factors would be most likely to initiate the start of a formal toilet training or support programme for a child/young person in this group? Please tick all that apply PC8: Please indicate your child’s current toileting abilities using the table below (knowing, finding, accessing, preparing, going, cleaning, completing) PC11: What prompted you as a parent/carer to seek professional support for toileting for your child? Please tick all that apply PC13: What were your main expectations of improving toileting for your child at the point of seeking professional support? Please tick all that apply SC3: Where you work, to what extent do you have to help the children and young people with the following toileting needs? SC4: Which of the following would prompt you to seek specialist toileting support for a child or young person with special educational needs and/or a disability? Please tick all that apply SC7b: Are you involved in assessing children and young people’s toileting ability and/or capability? If yes, please provide further information about the assessment that you conduct, or how you are involved |
|
| RQ4: Which factors affect the implementation of interventions to improve continence, and what is the acceptability of strategies to children and young people and their carers? | RQ4.1: Which factors affect the implementation of interventions to improve continence? |
HP49: Where you work, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability HP50: Where you work, what is the minimum age at which toileting aids or interventions would be funded for a child/young person with special educational needs and/or a disability that is delayed in achieving toileting? HP51: In your opinion, is the provision of continence pads for children and young people with special educational needs and/or a disability a barrier or enabler for achieving continence? HP59: In your opinion, to what extent do local commissioning/funding arrangements influence the toileting support you offer for individual children and young people with special educational needs and/or a disability? (e.g. would you recommend the use of continence pads as they are supplied free of charge for families locally?) HP60: In your opinion, how easy is it for parent/carers of children and young people with special educational needs and/or a disability to access professional toileting advice and support in your area? HP61: In your opinion, how acceptable is the waiting time in your area for families to receive the toileting support or equipment/products they require? PC12: In your opinion, how easy is it for parent/carers of children and young people with special educational needs and/or a disability to access support for toileting? PC13: What were your main expectations of improving toileting for your child at the point of seeking professional support? Please tick all that apply PC17: Now please indicate how successful you found these methods at reducing your child’s problems with toileting, or helping your child to manage their own toileting. If you have never used the intervention, please indicate ‘never used’ PC19: What difficulties have you found using methods to help with toileting at home? Please tick all that apply PC20: For any methods you have tried but that did not work, why do you think that was? Please tick all that apply PC21: Where you live, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability PC22: Where you live, how acceptable is the waiting time for families to get the equipment and products they require once an assessment has been completed? SC6: Where you work, how easy is it to access support for toileting for children and young people with special educational needs and/or a disability? SC9: Where you work, how easy is it for you to provide or use the following methods to help children and young people with toileting? SC11: Where you work, what difficulties have you found in helping children and young people to use the toileting methods (e.g. alarms or frames) provided? Please tick all that apply SC12: Where you work, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability CYP7: Please tell us about the help you need to use the toilet |
| RQ4.2: What is the acceptability of strategies to children and young people and their carers? |
HP15: What would you normally use as an outcome to judge the effectiveness of an intervention for a child/young person in this group? Please tick all that apply HP60: In your opinion, how easy is it for parent/carers of children and young people with special educational needs and/or a disability to access professional toileting advice and support in your area? HP61: In your opinion, how acceptable is the waiting time in your area for families to receive the toileting support or equipment/products they require? PC12: In your opinion, how easy is it for parent/carers of children and young people with special educational needs and/or a disability to access support for toileting? PC17: Now please indicate how successful you found these methods at reducing your child’s problems with toileting, or helping your child to manage their own toileting. If you have never used the intervention, please indicate ‘never used’ PC19: What difficulties have you found using methods to help with toileting at home? Please tick all that apply PC20: For any methods you have tried but that did not work, why do you think that was? Please tick all that apply PC21: Where you live, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability PC22: Where you live, how acceptable is the waiting time for families to get the equipment and products they require once an assessment has been completed? SC6: Where you work, how easy is it to access support for toileting for children and young people with special educational needs and/or a disability? SC9: Where you work, how easy is it for you to provide or use the following methods to help children and young people with toileting? SC11: Where you work, what difficulties have you found in helping children and young people to use the toileting methods (e.g. alarms or frames) provided? Please tick all that apply SC12: Where you work, please indicate which toileting products or equipment are provided for families of children and young people with special educational needs and/or a disability CYP7: Please tell us about the help you need to use the toilet CYP8: How do you feel about . . . |
Appendix 9 Health professional survey results
In Tables 29–43, ‘non-spinal cord pathology’ refers to children and young people with social/communication or attention/behaviour difficulty, learning disability or physical or movement disability; ‘spinal cord pathology’ refers to children and young people with bladder and/or bowel impairment due to damage to the spinal cord.
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 17) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 17) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 3) | |
| Weekly | 10 (59) | 50 (65) | 11 (35) | 6 (50) | 14 (42) | 11 (85) | 5 (29) | 13 (36) | 0 (0) | 7 (47) | 3 (23) | 2 (67) |
| Monthly | 3 (18) | 17 (22) | 9 (29) | 4 (33) | 10 (30) | 1 (8) | 5 (29) | 10 (28) | 2 (17) | 5 (33) | 5 (38) | 0 (0) |
| 3-monthly | 1 (6) | 3 (4) | 3 (10) | 1 (8) | 2 (6) | 0 (0) | 7 (41) | 5 (14) | 5 (42) | 2 (13) | 1 (8) | 1 (33) |
| 6-monthly | 1 (6) | 5 (6) | 4 (13) | 1 (8) | 2 (6) | 1 (8) | 0 (0) | 3 (8) | 3 (25) | 1 (7) | 2 (15) | 0 (0) |
| Annually | 0 (0) | 1 (1) | 2 (6) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 3 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 2 (12) | 1 (1) | 2 (6) | 0 (0) | 4 (12) | 0 (0) | 0 (0) | 1 (3) | 2 (17) | 0 (0) | 2 (15) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 34) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 3) | |
| Constipation | 16 (89) | 70 (91) | 28 (90) | 11 (92) | 10 (30) | 6 (46) | 16 (89) | 31 (91) | 11 (92) | 8 (53) | 5 (38) | 2 (67) |
| Loose stools | 13 (72) | 55 (71) | 16 (52) | 3 (25) | 4 (12) | 3 (23) | 12 (67) | 21 (62) | 7 (58) | 6 (40) | 2 (15) | 1 (33) |
| UTI | 10 (56) | 32 (42) | 17 (55) | 9 (75) | 0 (0) | 0 (0) | 13 (72) | 22 (65) | 11 (92) | 10 (67) | 2 (15) | 0 (0) |
| Urgency | 13 (72) | 34 (44) | 16 (52) | 8 (67) | 6 (18) | 1 (8) | 10 (56) | 19 (56) | 8 (67) | 9 (60) | 4 (31) | 1 (33) |
| Bowel irritability | 9 (50) | 23 (30) | 10 (32) | 2 (17) | 2 (6) | 2 (15) | 8 (44) | 15 (44) | 7 (58) | 6 (40) | 2 (15) | 0 (0) |
| Daytime wetting | 16 (89) | 63 (82) | 28 (90) | 8 (67) | 13 (39) | 6 (46) | 11 (61) | 28 (82) | 9 (75) | 10 (67) | 7 (54) | 2 (67) |
| Night-time wetting | 16 (89) | 64 (83) | 21 (68) | 4 (33) | 10 (30) | 3 (23) | 10 (56) | 25 (74) | 7 (58) | 8 (53) | 6 (46) | 1 (33) |
| Daytime soiling | 15 (83) | 60 (78) | 26 (84) | 10 (83) | 13 (39) | 4 (31) | 10 (56) | 23 (68) | 10 (83) | 10 (67) | 6 (46) | 2 (67) |
| Night-time soiling | 14 (78) | 52 (68) | 19 (61) | 8 (67) | 10 (30) | 4 (31) | 9 (50) | 23 (68) | 9 (75) | 8 (53) | 5 (38) | 1 (33) |
| A delay in achieving independent toileting | 17 (94) | 62 (81) | 24 (77) | 10 (83) | 23 (70) | 10 (77) | 11 (61) | 19 (56) | 7 (58) | 12 (80) | 10 (77) | 3 (100) |
| Assessment is undertaken routinely from birth due to spinal disability | 6 (33) | 23 (30) | 7 (23) | 2 (17) | 4 (12) | 0 (0) | 12 (67) | 22 (65) | 9 (75) | 11 (73) | 4 (31) | 0 (0) |
| Never assess in role | 0 (0) | 1 (1) | 1 (3) | 0 (0) | 7 (21) | 3 (23) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 3 (4) | 2 (6) | 0 (0) | 4 (12) | 1 (8) | 1 (6) | 1 (3) | 1 (8) | 1 (7) | 2 (15) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 12) | BBS nurses (N = 18) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 2) | |
| At every visit | 17 (94) | 45 (58) | 21 (68) | 9 (75) | 1 (3) | 4 (33) | 18 (100) | 24 (67) | 11 (92) | 13 (87) | 0 (0) | 1 (50) |
| At some visits | 1 (6) | 19 (25) | 10 (32) | 2 (17) | 12 (36) | 4 (33) | 0 (0) | 6 (17) | 1 (8) | 1 (7) | 7 (54) | 1 (50) |
| Rarely | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 4 (12) | 1 (8) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
| Never | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 7 (21) | 1 (8) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 10 (13) | 0 (0) | 1 (8) | 9 (27) | 2 (17) | 0 (0) | 3 (8) | 0 (0) | 1 (7) | 5 (38) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 30) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 3) | |
| Specific bladder and bowel assessment | 16 (89) | 44 (57) | 4 (13) | 10 (83) | 2 (6) | 6 (46) | 17 (94) | 26 (72) | 2 (17) | 11 (73) | 0 (0) | 2 (67) |
| Broader assessment of the child/young person’s health and development needs | 2 (11) | 31 (40) | 26 (87) | 1 (8) | 29 (88) | 7 (54) | 1 (6) | 10 (28) | 10 (83) | 4 (27) | 13 (100) | 1 (33) |
| Don’t know | 0 (0) | 2 (3) | 0 (0) | 1 (8) | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 12) | BBS nurses (N = 17) | Nurses (N = 35) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 12) | Other (N = 2) | |
| Home | 10 (56) | 41 (53) | 0 (0) | 0 (0) | 20 (61) | 7 (58) | 7 (41) | 20 (57) | 0 (0) | 0 (0) | 10 (83) | 1 (50) |
| Community | 14 (78) | 40 (52) | 11 (35) | 0 (0) | 7 (21) | 2 (17) | 9 (53) | 21 (60) | 3 (25) | 0 (0) | 3 (25) | 1 (50) |
| Hospital clinic | 9 (50) | 23 (30) | 21 (68) | 12 (100) | 6 (18) | 3 (25) | 11 (65) | 15 (43) | 9 (75) | 15 (100) | 2 (17) | 1 (50) |
| School/college/university | 10 (56) | 38 (49) | 5 (16) | 0 (0) | 16 (48) | 2 (17) | 4 (24) | 17 (49) | 0 (0) | 0 (0) | 8 (67) | 0 (0) |
| Young person’s place of work | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
| Respite care | 0 (0) | 7 (9) | 0 (0) | 0 (0) | 8 (24) | 1 (8) | 0 (0) | 6 (17) | 0 (0) | 0 (0) | 5 (42) | 0 (0) |
| I never assess in my role | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 8 (24) | 3 (25) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 1 (6) | 4 (5) | 1 (3) | 0 (0) | 3 (9) | 0 (0) | 1 (6) | 2 (6) | 1 (8) | 0 (0) | 1 (8) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 34) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 12) | Other (N = 3) | |
| Parent/carer verbal report | 17 (94) | 75 (97) | 29 (94) | 12 (100) | 24 (73) | 10 (77) | 18 (100) | 32 (94) | 12 (100) | 15 (100) | 9 (75) | 2 (67) |
| Child/young person verbal report | 16 (89) | 58 (75) | 27 (87) | 10 (83) | 21 (64) | 7 (54) | 17 (94) | 30 (88) | 12 (100) | 13 (87) | 9 (75) | 3 (100) |
| Chart/checklist/questionnaire | 16 (89) | 66 (86) | 10 (32) | 6 (50) | 7 (21) | 9 (69) | 15 (83) | 30 (88) | 4 (33) | 7 (47) | 3 (25) | 3 (100) |
| Physical examination | 4 (22) | 14 (18) | 30 (97) | 12 (100) | 0 (0) | 3 (23) | 6 (33) | 8 (24) | 12 (100) | 15 (100) | 0 (0) | 2 (67) |
| Ultrasound | 6 (33) | 16 (21) | 16 (52) | 9 (75) | 0 (0) | 1 (8) | 9 (50) | 9 (26) | 7 (58) | 13 (87) | 0 (0) | 1 (33) |
| Other imaging | 10 (56) | 15 (19) | 7 (23) | 8 (67) | 0 (0) | 1 (8) | 12 (67) | 10 (29) | 8 (67) | 12 (80) | 0 (0) | 1 (33) |
| Urodynamics – invasive with catheter | 1 (6) | 3 (4) | 1 (3) | 4 (33) | 0 (0) | 1 (8) | 3 (17) | 3 (9) | 2 (17) | 11 (73) | 0 (0) | 1 (33) |
| Urodynamics – non-invasive without catheter | 1 (6) | 5 (6) | 1 (3) | 6 (50) | 0 (0) | 1 (8) | 4 (22) | 5 (15) | 1 (8) | 8 (53) | 0 (0) | 1 (33) |
| Direct observation | 12 (67) | 24 (31) | 2 (6) | 4 (33) | 7 (21) | 3 (23) | 9 (50) | 13 (38) | 0 (0) | 4 (27) | 3 (25) | 1 (33) |
| Wee/poo sample | 5 (28) | 20 (26) | 12 (39) | 3 (25) | 0 (0) | 2 (15) | 6 (33) | 16 (47) | 6 (50) | 3 (20) | 0 (0) | 2 (67) |
| Diet/fluid intake diary | 15 (83) | 61 (79) | 18 (58) | 10 (83) | 3 (9) | 7 (54) | 12 (67) | 28 (82) | 6 (50) | 12 (80) | 1 (8) | 3 (100) |
| Blood test | 0 (0) | 5 (6) | 10 (32) | 3 (25) | 0 (0) | 2 (15) | 1 (6) | 3 (9) | 3 (25) | 5 (33) | 0 (0) | 2 (67) |
| I never assess in my role | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 11 (33) | 3 (23) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 5 (42) | 1 (33) |
| Other | 0 (0) | 5 (6) | 3 (10) | 1 (8) | 3 (9) | 1 (8) | 0 (0) | 2 (6) | 3 (25) | 1 (7) | 1 (8) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 35) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 3) | |
| Chronological age of child | 10 (56) | 40 (52) | 10 (32) | 7 (58) | 18 (55) | 8 (62) | 9 (50) | 19 (54) | 4 (33) | 9 (60) | 8 (62) | 2 (67) |
| Developmental age of child | 12 (67) | 53 (69) | 29 (94) | 11 (92) | 29 (88) | 11 (85) | 12 (67) | 20 (57) | 8 (67) | 14 (93) | 10 (77) | 3 (100) |
| Physical functioning level of child | 12 (67) | 60 (78) | 24 (77) | 9 (75) | 27 (82) | 8 (62) | 14 (78) | 26 (74) | 11 (92) | 12 (80) | 12 (92) | 1 (33) |
| Parent/carer request | 13 (72) | 62 (81) | 24 (77) | 6 (50) | 25 (76) | 9 (69) | 11 (61) | 25 (71) | 10 (83) | 8 (53) | 8 (62) | 3 (100) |
| Other health professional request | 14 (78) | 47 (61) | 11 (35) | 3 (25) | 16 (48) | 8 (62) | 12 (67) | 21 (60) | 3 (25) | 5 (33) | 8 (62) | 3 (100) |
| Parent/carer capacity to manage child’s toileting | 9 (50) | 40 (52) | 17 (55) | 8 (67) | 22 (67) | 8 (62) | 7 (39) | 16 (46) | 6 (50) | 10 (67) | 9 (69) | 2 (67) |
| Facilities available in the child’s environment | 9 (50) | 33 (43) | 14 (45) | 3 (25) | 20 (61) | 4 (31) | 8 (44) | 15 (43) | 6 (50) | 5 (33) | 9 (69) | 1 (33) |
| Following local continence protocol/pathway | 13 (72) | 33 (43) | 4 (13) | 2 (17) | 6 (18) | 4 (31) | 10 (56) | 17 (49) | 2 (17) | 2 (13) | 5 (38) | 2 (67) |
| I never assess in my role | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (3) | 2 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 1 (6) | 1 (1) | 1 (3) | 0 (0) | 3 (9) | 0 (0) | 2 (11) | 2 (6) | 1 (8) | 1 (7) | 2 (15) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 35) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 13) | Other (N = 3) | |
| Parent/carer | 17 (94) | 71 (92) | 29 (94) | 11 (92) | 29 (88) | 11 (85) | 16 (89) | 32 (91) | 11 (92) | 14 (93) | 12 (92) | 3 (100) |
| School staff | 17 (94) | 65 (84) | 20 (65) | 5 (42) | 23 (70) | 8 (62) | 15 (83) | 27 (77) | 5 (42) | 7 (47) | 9 (69) | 3 (100) |
| Health visitor | 6 (33) | 27 (35) | 15 (48) | 1 (8) | 14 (42) | 4 (31) | 4 (22) | 13 (37) | 4 (33) | 2 (13) | 5 (38) | 2 (67) |
| GP | 1 (6) | 9 (12) | 6 (19) | 2 (17) | 7 (21) | 2 (15) | 1 (6) | 4 (11) | 1 (8) | 3 (20) | 5 (38) | 0 (0) |
| Other health professional (e.g. paediatrician, continence nurse) | 12 (67) | 52 (68) | 25 (81) | 12 (100) | 27 (82) | 10 (77) | 14 (78) | 26 (74) | 10 (83) | 15 (100) | 13 (100) | 3 (100) |
| Don’t know | 0 (0) | 3 (4) | 1 (3) | 0 (0) | 1 (3) | 1 (8) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 4 (22) | 2 (3) | 1 (3) | 0 (0) | 1 (3) | 1 (8) | 2 (11) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 31) | Surgeons (N = 11) | Therapists (N = 32) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 14) | Therapists (N = 13) | Other (N = 3) | |
| Chronological age of child | 7 (39) | 30 (39) | 8 (26) | 4 (36) | 8 (25) | 8 (62) | 6 (33) | 18 (50) | 3 (25) | 5 (36) | 3 (23) | 1 (33) |
| Developmental age of child | 14 (78) | 48 (62) | 29 (94) | 9 (82) | 20 (62) | 10 (77) | 11 (61) | 23 (64) | 8 (67) | 10 (71) | 7 (54) | 3 (100) |
| Physical functioning level of child | 12 (67) | 49 (64) | 21 (68) | 10 (91) | 17 (53) | 6 (46) | 12 (67) | 26 (72) | 9 (75) | 11 (79) | 9 (69) | 2 (67) |
| Parent/carer request | 13 (72) | 61 (79) | 25 (81) | 5 (45) | 21 (66) | 10 (77) | 13 (72) | 27 (75) | 9 (75) | 7 (50) | 10 (77) | 2 (67) |
| Other health professional request | 10 (56) | 44 (57) | 9 (29) | 0 (0) | 13 (41) | 8 (62) | 10 (56) | 22 (61) | 3 (25) | 1 (7) | 9 (69) | 2 (67) |
| Parent/carer capacity to manage child’s toileting | 9 (50) | 38 (49) | 13 (42) | 6 (55) | 15 (47) | 8 (62) | 7 (39) | 18 (50) | 7 (58) | 6 (43) | 6 (46) | 2 (67) |
| Facilities available in the child’s environment | 8 (44) | 28 (36) | 13 (42) | 3 (27) | 16 (50) | 3 (23) | 5 (28) | 13 (36) | 6 (50) | 3 (21) | 9 (69) | 1 (33) |
| Following local continence protocol/pathway | 10 (56) | 28 (36) | 5 (16) | 1 (9) | 6 (19) | 3 (23) | 7 (39) | 15 (42) | 3 (25) | 1 (7) | 4 (31) | 1 (33) |
| Never assess this group | 0 (0) | 1 (1) | 0 (0) | 1 (9) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (8) | 0 (0) |
| Other | 2 (11) | 0 (0) | 0 (0) | 0 (0) | 2 (6) | 0 (0) | 3 (17) | 0 (0) | 0 (0) | 1 (7) | 1 (8) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses | Nurses | Paediatricians | Surgeons | Therapists | Other | BBS nurses | Nurses | Paediatricians | Surgeons | Therapists | Other | |
| Dietary advice | N = 17 | N = 77 | N = 30 | N = 12 | N = 24 | N = 12 | N = 16 | N = 32 | N = 11 | N = 15 | N = 6 | N = 3 |
| Very effective | 4 (24) | 10 (13) | 3 (10) | 0 (0) | 2 (8) | 4 (33) | 4 (25) | 5 (16) | 0 (0) | 1 (7) | 0 (0) | 1 (33) |
| Effective | 10 (59) | 44 (57) | 18 (60) | 7 (58) | 10 (42) | 5 (42) | 8 (50) | 20 (62) | 4 (36) | 8 (53) | 4 (67) | 2 (67) |
| Ineffective | 3 (18) | 22 (29) | 7 (23) | 5 (42) | 2 (8) | 2 (17) | 4 (25) | 6 (19) | 6 (55) | 6 (40) | 0 (0) | 0 (0) |
| Very ineffective | 0 (0) | 1 (1) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 10 (42) | 1 (8) | 0 (0) | 1 (3) | 1 (9) | 0 (0) | 2 (33) | 0 (0) |
| Fluid intake advice | N = 17 | N = 77 | N = 30 | N = 12 | N = 23 | N = 12 | N = 16 | N = 32 | N = 11 | N = 15 | N = 6 | N = 3 |
| Very effective | 8 (47) | 30 (39) | 3 (10) | 3 (25) | 6 (26) | 5 (42) | 8 (50) | 16 (50) | 0 (0) | 4 (27) | 0 (0) | 2 (67) |
| Effective | 8 (47) | 41 (53) | 21 (70) | 7 (58) | 9 (39) | 6 (50) | 8 (50) | 14 (44) | 9 (82) | 8 (53) | 4 (67) | 1 (33) |
| Ineffective | 1 (6) | 6 (8) | 6 (20) | 2 (17) | 2 (9) | 1 (8) | 0 (0) | 2 (6) | 1 (9) | 3 (20) | 0 (0) | 0 (0) |
| Very ineffective | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (26) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 2 (33) | 0 (0) |
| Behavioural intervention (e.g. reward chart, timer, alarms) | N = 17 | N = 76 | N = 30 | N = 12 | N = 22 | N = 12 | N = 16 | N = 32 | N = 11 | N = 15 | N = 6 | N = 3 |
| Very effective | 7 (41) | 21 (28) | 5 (17) | 1 (8) | 4 (18) | 5 (42) | 3 (19) | 8 (25) | 0 (0) | 3 (20) | 1 (17) | 2 (67) |
| Effective | 10 (59) | 45 (59) | 21 (70) | 8 (67) | 17 (77) | 6 (50) | 10 (62) | 17 (53) | 4 (36) | 5 (33) | 3 (50) | 1 (33) |
| Ineffective | 0 (0) | 4 (5) | 3 (10) | 1 (8) | 0 (0) | 1 (8) | 2 (12) | 4 (12) | 4 (36) | 4 (27) | 1 (17) | 0 (0) |
| Very ineffective | 0 (0) | 1 (1) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (13) | 0 (0) | 0 (0) |
| Never used for this group | 0 (0) | 5 (7) | 1 (3) | 1 (8) | 1 (5) | 0 (0) | 1 (6) | 3 (9) | 3 (27) | 1 (7) | 1 (17) | 0 (0) |
| Simple aid/equipment (e.g. raised step or seat) | N = 17 | N = 74 | N = 29 | N = 11 | N = 22 | N = 12 | N = 16 | N = 30 | N = 10 | N = 14 | N = 6 | N = 3 |
| Very effective | 9 (53) | 33 (45) | 3 (10) | 4 (36) | 10 (45) | 5 (42) | 2 (12) | 12 (40) | 0 (0) | 3 (21) | 3 (50) | 2 (67) |
| Effective | 8 (47) | 39 (53) | 22 (76) | 6 (55) | 11 (50) | 7 (58) | 14 (88) | 15 (50) | 5 (50) | 8 (57) | 3 (50) | 1 (33) |
| Ineffective | 0 (0) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 4 (40) | 2 (14) | 0 (0) | 0 (0) |
| Very ineffective | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 0 (0) | 2 (3) | 2 (7) | 1 (9) | 1 (5) | 0 (0) | 0 (0) | 1 (3) | 1 (10) | 1 (7) | 0 (0) | 0 (0) |
| Bespoke aid/equipment (e.g. hoist or frame) | N = 17 | N = 68 | N = 26 | N = 10 | N = 22 | N = 11 | N = 16 | N = 30 | N = 10 | N = 13 | N = 6 | N = 3 |
| Very effective | 6 (35) | 24 (35) | 1 (4) | 1 (10) | 9 (41) | 4 (36) | 3 (19) | 9 (30) | 0 (0) | 1 (8) | 3 (50) | 2 (67) |
| Effective | 10 (59) | 25 (37) | 16 (62) | 4 (40) | 11 (50) | 3 (27) | 12 (75) | 17 (57) | 7 (70) | 7 (54) | 3 (50) | 0 (0) |
| Ineffective | 0 (0) | 2 (3) | 1 (4) | 1 (10) | 0 (0) | 1 (9) | 0 (0) | 2 (7) | 0 (0) | 1 (8) | 0 (0) | 1 (33) |
| Very ineffective | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 1 (6) | 17 (25) | 8 (31) | 4 (40) | 2 (9) | 3 (27) | 1 (6) | 2 (7) | 3 (30) | 4 (31) | 0 (0) | 0 (0) |
| Housing adaptions (e.g. specialised toilet) | N = 15 | N = 68 | N = 26 | N = 10 | N = 22 | N = 11 | N = 16 | N = 28 | N = 10 | N = 13 | N = 6 | N = 3 |
| Very effective | 4 (27) | 20 (29) | 1 (4) | 3 (30) | 7 (32) | 3 (27) | 3 (19) | 8 (29) | 1 (10) | 3 (23) | 3 (50) | 1 (33) |
| Effective | 10 (67) | 29 (43) | 18 (69) | 3 (30) | 11 (50) | 3 (27) | 12 (75) | 16 (57) | 6 (60) | 6 (46) | 3 (50) | 1 (33) |
| Ineffective | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (5) | 1 (9) | 1 (6) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (33) |
| Very ineffective | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 1 (7) | 16 (24) | 7 (27) | 4 (40) | 3 (14) | 4 (36) | 0 (0) | 3 (11) | 3 (30) | 4 (31) | 0 (0) | 0 (0) |
| Continence products (e.g. pads, nappies, pull-ups) | N = 15 | N = 67 | N = 26 | N = 10 | N = 22 | N = 11 | N = 16 | N = 27 | N = 10 | N = 13 | N = 6 | N = 3 |
| Very effective | 1 (7) | 10 (15) | 3 (12) | 2 (20) | 2 (9) | 3 (27) | 0 (0) | 3 (11) | 1 (10) | 3 (23) | 0 (0) | 1 (33) |
| Effective | 7 (47) | 31 (46) | 18 (69) | 6 (60) | 9 (41) | 2 (18) | 11 (69) | 17 (63) | 8 (80) | 8 (62) | 3 (50) | 1 (33) |
| Ineffective | 4 (27) | 19 (28) | 2 (8) | 2 (20) | 2 (9) | 5 (45) | 3 (19) | 6 (22) | 0 (0) | 2 (15) | 1 (17) | 1 (33) |
| Very ineffective | 3 (20) | 4 (6) | 1 (4) | 0 (0) | 2 (9) | 1 (9) | 2 (12) | 1 (4) | 0 (0) | 0 (0) | 1 (17) | 0 (0) |
| Never used for this group | 0 (0) | 3 (4) | 2 (8) | 0 (0) | 7 (32) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 1 (17) | 0 (0) |
| Medications (e.g. laxatives) | N = 15 | N = 66 | N = 26 | N = 10 | N = 21 | N = 11 | N = 16 | N = 27 | N = 10 | N = 13 | N = 5 | N = 3 |
| Very effective | 7 (47) | 31 (47) | 6 (23) | 3 (30) | 0 (0) | 6 (55) | 9 (56) | 9 (33) | 2 (20) | 5 (38) | 0 (0) | 1 (33) |
| Effective | 8 (53) | 33 (50) | 19 (73) | 7 (70) | 8 (38) | 5 (45) | 7 (44) | 17 (63) | 8 (80) | 8 (62) | 1 (20) | 2 (67) |
| Ineffective | 0 (0) | 1 (2) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Very ineffective | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 13 (62) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 4 (80) | 0 (0) |
| Catheters | N = 13 | N = 62 | N = 23 | N = 9 | N = 20 | N = 9 | N = 15 | N = 26 | N = 10 | N = 12 | N = 5 | N = 3 |
| Very effective | 4 (31) | 8 (13) | 1 (4) | 4 (44) | 0 (0) | 0 (0) | 10 (67) | 7 (27) | 2 (20) | 7 (58) | 0 (0) | 1 (33) |
| Effective | 5 (38) | 14 (23) | 6 (26) | 3 (33) | 1 (5) | 1 (11) | 4 (27) | 12 (46) | 6 (60) | 5 (42) | 0 (0) | 1 (33) |
| Ineffective | 2 (15) | 1 (2) | 2 (9) | 0 (0) | 1 (5) | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 1 (33) |
| Very ineffective | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 2 (15) | 38 (61) | 14 (61) | 2 (22) | 17 (85) | 7 (78) | 1 (7) | 7 (27) | 2 (20) | 0 (0) | 4 (80) | 0 (0) |
| Colonic enema | N = 13 | N = 62 | N = 23 | N = 9 | N = 20 | N = 9 | N = 15 | N = 25 | N = 10 | N = 12 | N = 5 | N = 3 |
| Very effective | 3 (23) | 8 (13) | 0 (0) | 6 (67) | 0 (0) | 2 (22) | 8 (53) | 5 (20) | 1 (10) | 7 (58) | 0 (0) | 1 (33) |
| Effective | 6 (46) | 13 (21) | 5 (22) | 3 (33) | 2 (10) | 0 (0) | 4 (27) | 10 (40) | 7 (70) | 5 (42) | 1 (20) | 0 (0) |
| Ineffective | 2 (15) | 3 (5) | 3 (13) | 0 (0) | 1 (5) | 1 (11) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) |
| Very ineffective | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 2 (15) | 37 (60) | 15 (65) | 0 (0) | 17 (85) | 6 (67) | 2 (13) | 10 (40) | 2 (20) | 0 (0) | 4 (80) | 1 (33) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) | N = 13 | N = 60 | N = 23 | N = 9 | N = 20 | N = 9 | N = 15 | N = 24 | N = 10 | N = 12 | N = 5 | N = 3 |
| Very effective | 1 (8) | 6 (10) | 0 (0) | 5 (56) | 0 (0) | 1 (11) | 2 (13) | 5 (21) | 3 (30) | 7 (58) | 0 (0) | 1 (33) |
| Effective | 6 (46) | 13 (22) | 7 (30) | 3 (33) | 2 (10) | 1 (11) | 11 (73) | 11 (46) | 6 (60) | 5 (42) | 1 (20) | 0 (0) |
| Ineffective | 1 (8) | 1 (2) | 2 (9) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) |
| Very ineffective | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Never used for this group | 5 (38) | 39 (65) | 14 (61) | 1 (11) | 18 (90) | 6 (67) | 2 (13) | 8 (33) | 1 (10) | 0 (0) | 4 (80) | 1 (33) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses | Nurses | Paediatricians | Surgeons | Therapists | Other | BBS nurses | Nurses | Paediatricians | Surgeons | Therapists | Other | |||
| Dietary advice | N = 17 | N = 72 | N = 25 | N = 11 | N = 22 | N = 12 | N = 16 | N = 29 | N = 10 | N = 14 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 8 (11) | 0 (0) | 0 (0) | 3 (14) | 0 (0) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | ||
| Monthly | 4 (24) | 20 (28) | 1 (4) | 0 (0) | 0 (0) | 5 (42) | 2 (12) | 6 (21) | 1 (10) | 0 (0) | 0 (0) | 1 (50) | ||
| 3-monthly | 7 (41) | 20 (28) | 6 (24) | 4 (36) | 2 (9) | 4 (33) | 11 (69) | 8 (28) | 0 (0) | 3 (21) | 0 (0) | 1 (50) | ||
| 6-monthly | 5 (29) | 10 (14) | 13 (52) | 7 (64) | 1 (5) | 1 (8) | 2 (12) | 5 (17) | 4 (40) | 7 (50) | 0 (0) | 0 (0) | ||
| Annually | 1 (6) | 11 (15) | 3 (12) | 0 (0) | 1 (5) | 1 (8) | 1 (6) | 6 (21) | 2 (20) | 3 (21) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 0 (0) | 3 (4) | 2 (8) | 0 (0) | 15 (68) | 1 (8) | 0 (0) | 2 (7) | 3 (30) | 1 (7) | 3 (75) | 0 (0) | ||
| Fluid intake advice | N = 17 | N = 72 | N = 24 | N = 11 | N = 22 | N = 12 | N = 16 | N = 29 | N = 10 | N = 14 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 11 (15) | 0 (0) | 0 (0) | 3 (14) | 0 (0) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | ||
| Monthly | 4 (24) | 20 (28) | 1 (4) | 0 (0) | 1 (5) | 7 (58) | 2 (12) | 9 (31) | 1 (10) | 0 (0) | 0 (0) | 1 (50) | ||
| 3-monthly | 8 (47) | 20 (28) | 7 (29) | 4 (36) | 2 (9) | 2 (17) | 11 (69) | 8 (28) | 0 (0) | 3 (21) | 0 (0) | 1 (50) | ||
| 6-monthly | 5 (29) | 9 (12) | 12 (50) | 7 (64) | 1 (5) | 1 (8) | 3 (19) | 4 (14) | 6 (60) | 7 (50) | 0 (0) | 0 (0) | ||
| Annually | 0 (0) | 11 (15) | 3 (12) | 0 (0) | 1 (5) | 1 (8) | 0 (0) | 6 (21) | 2 (20) | 3 (21) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 0 (0) | 1 (1) | 1 (4) | 0 (0) | 14 (64) | 1 (8) | 0 (0) | 0 (0) | 1 (10) | 1 (7) | 3 (75) | 0 (0) | ||
| Behavioural intervention (e.g. reward chart, timer, alarms) | N = 16 | N = 70 | N = 24 | N = 11 | N = 19 | N = 12 | N = 16 | N = 29 | N = 10 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 11 (16) | 0 (0) | 0 (0) | 2 (11) | 4 (33) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 6 (38) | 22 (31) | 1 (4) | 0 (0) | 6 (32) | 4 (33) | 3 (19) | 6 (21) | 2 (20) | 0 (0) | 1 (25) | 1 (50) | ||
| 3-monthly | 7 (44) | 19 (27) | 5 (21) | 4 (36) | 3 (16) | 3 (25) | 10 (62) | 11 (38) | 0 (0) | 2 (15) | 0 (0) | 1 (50) | ||
| 6-monthly | 3 (19) | 7 (10) | 11 (46) | 7 (64) | 1 (5) | 1 (8) | 2 (12) | 3 (10) | 3 (30) | 6 (46) | 0 (0) | 0 (0) | ||
| Annually | 0 (0) | 7 (10) | 3 (12) | 0 (0) | 2 (11) | 0 (0) | 0 (0) | 5 (17) | 0 (0) | 2 (15) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 0 (0) | 4 (6) | 4 (17) | 0 (0) | 5 (26) | 0 (0) | 1 (6) | 2 (7) | 5 (50) | 3 (23) | 3 (75) | 0 (0) | ||
| Simple aid/equipment (e.g. raised step or seat) | N = 16 | N = 67 | N = 20 | N = 10 | N = 19 | N = 12 | N = 16 | N = 29 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 2 (11) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 3 (19) | 14 (21) | 0 (0) | 0 (0) | 4 (21) | 3 (25) | 1 (6) | 3 (10) | 0 (0) | 0 (0) | 1 (25) | 1 (50) | ||
| 3-monthly | 7 (44) | 20 (30) | 4 (20) | 4 (40) | 2 (11) | 5 (42) | 11 (69) | 8 (28) | 0 (0) | 2 (15) | 1 (25) | 1 (50) | ||
| 6-monthly | 2 (12) | 9 (13) | 7 (35) | 6 (60) | 2 (11) | 2 (17) | 2 (12) | 4 (14) | 5 (56) | 6 (46) | 1 (25) | 0 (0) | ||
| Annually | 4 (25) | 13 (19) | 3 (15) | 0 (0) | 7 (37) | 0 (0) | 2 (12) | 8 (28) | 0 (0) | 2 (15) | 1 (25) | 0 (0) | ||
| Never used for this group or never reviewed | 0 (0) | 7 (10) | 6 (30) | 0 (0) | 2 (11) | 2 (17) | 0 (0) | 5 (17) | 4 (44) | 3 (23) | 0 (0) | 0 (0) | ||
| Bespoke aid/equipment (e.g. hoist or frame) | N = 14 | N = 65 | N = 20 | N = 10 | N = 19 | N = 11 | N = 14 | N = 29 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 3 (5) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 4 (29) | 6 (9) | 0 (0) | 0 (0) | 5 (26) | 2 (18) | 0 (0) | 3 (10) | 0 (0) | 0 (0) | 2 (50) | 1 (50) | ||
| 3-monthly | 4 (29) | 13 (20) | 2 (10) | 1 (1) | 0 (0) | 1 (9) | 9 (64) | 7 (24) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | ||
| 6-monthly | 1 (7) | 9 (14) | 5 (25) | 5 (50) | 4 (21) | 1 (9) | 2 (14) | 4 (14) | 3 (33) | 6 (46) | 1 (25) | 0 (0) | ||
| Annually | 4 (29) | 12 (18) | 3 (15) | 1 (10) | 7 (37) | 0 (0) | 3 (21) | 7 (24) | 0 (0) | 2 (15) | 1 (25) | 0 (0) | ||
| Never used for this group or never reviewed | 1 (7) | 22 (34) | 10 (50) | 3 (30) | 2 (11) | 7 (64) | 0 (0) | 8 (28) | 6 (67) | 5 (38) | 0 (0) | 0 (0) | ||
| Housing adaptions (e.g. specialised toilet) | N = 14 | N = 65 | N = 20 | N = 10 | N = 19 | N = 11 | N = 12 | N = 28 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 3 (5) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 2 (14) | 4 (6) | 0 (0) | 0 (0) | 4 (21) | 2 (18) | 0 (0) | 3 (11) | 0 (0) | 0 (0) | 1 (25) | 1 (50) | ||
| 3-monthly | 4 (29) | 8 (12) | 3 (15) | 1 (10) | 0 (0) | 1 (9) | 7 (58) | 4 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | ||
| 6-monthly | 1 (7) | 10 (15) | 5 (25) | 5 (50) | 2 (11) | 1 (9) | 1 (8) | 5 (18) | 5 (56) | 6 (46) | 0 (0) | 0 (0) | ||
| Annually | 4 (29) | 14 (22) | 3 (15) | 1 (10) | 5 (26) | 1 (9) | 3 (25) | 6 (21) | 0 (0) | 2 (15) | 2 (50) | 0 (0) | ||
| Never used for this group or never reviewed | 3 (21) | 26 (40) | 9 (45) | 3 (30) | 7 (37) | 6 (55) | 1 (8) | 10 (36) | 4 (44) | 5 (38) | 1 (25) | 0 (0) | ||
| Continence products (e.g. pads, nappies, pull-ups) | N = 14 | N = 65 | N = 20 | N = 10 | N = 18 | N = 11 | N = 12 | N = 28 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 3 (5) | 0 (0) | 0 (0) | 3 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | (0) | 0 | (0) |
| Monthly | 0 (0) | 8 (12) | 0 (0) | 0 (0) | 1 (6) | 2 (18) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 0 | (0) | 1 | (50) |
| 3-monthly | 2 (14) | 11 (17) | 5 (25) | 3 (30) | 0 (0) | 5 (45) | 2 (17) | 6 (21) | 0 (0) | 3 (23) | 0 | (0) | 1 | (50) |
| 6-monthly | 9 (64) | 14 (22) | 7 (35) | 5 (50) | 1 (6) | 2 (18) | 8 (67) | 10 (36) | 6 (67) | 6 (46) | 0 | (0) | 0 | (0) |
| Annually | 3 (21) | 22 (34) | 3 (15) | 1 (10) | 1 (6) | 0 (0) | 2 (17) | 9 (32) | 1 (11) | 3 (23) | 0 | (0) | 0 | (0) |
| Never used for this group or never reviewed | 0 (0) | 7 (11) | 5 (25) | 1 (10) | 12 (67) | 2 (18) | 0 (0) | 1 (4) | 2 (22) | 1 (8) | 4 | (100) | 0 | (0) |
| Medications (e.g. laxatives) | N = 14 | N = 64 | N = 20 | N = 10 | N = 16 | N = 10 | N = 12 | N = 28 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 2 (14) | 15 (23) | 0 (0) | 0 (0) | 2 (12) | 1 (10) | 1 (8) | 3 (11) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | ||
| Monthly | 4 (29) | 19 (30) | 0 (0) | 0 (0) | 0 (0) | 4 (40) | 4 (33) | 8 (29) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | ||
| 3-monthly | 8 (57) | 17 (27) | 10 (50) | 4 (40) | 0 (0) | 2 (20) | 6 (50) | 9 (32) | 2 (22) | 4 (31) | 0 (0) | 1 (50) | ||
| 6-monthly | 0 (0) | 3 (5) | 8 (40) | 6 (60) | 0 (0) | 0 (0) | 1 (8) | 2 (7) | 6 (67) | 6 (46) | 0 (0) | 0 (0) | ||
| Annually | 0 (0) | 6 (9) | 2 (10) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 3 (11) | 1 (11) | 3 (23) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 0 (0) | 4 (6) | 0 (0) | 0 (0) | 13 (81) | 3 (30) | 0 (0) | 3 (11) | 0 (0) | 0 (0) | 3 (75) | 0 (0) | ||
| Catheters | N = 14 | N = 58 | N = 19 | N = 10 | N = 16 | N = 10 | N = 12 | N = 27 | N = 9 | N = 13 | N = 4 | N = 2 | ||
| Weekly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 1 (7) | 6 (10) | 0 (0) | 1 (10) | 0 (0) | 1 (10) | 3 (25) | 4 (15) | 0 (0) | 1 (8) | 0 (0) | 1 (50) | ||
| 3-monthly | 6 (43) | 4 (7) | 2 (11) | 3 (30) | 0 (0) | 0 (0) | 6 (50) | 6 (22) | 1 (11) | 3 (23) | 0 (0) | 0 (0) | ||
| 6-monthly | 1 (7) | 4 (7) | 4 (21) | 5 (50) | 0 (0) | 0 (0) | 0 (0) | 3 (11) | 6 (67) | 6 (46) | 0 (0) | 0 (0) | ||
| Annually | 1 (7) | 6 (10) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (19) | 0 (0) | 3 (23) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 5 (36) | 28 (66) | 12 (63) | 1 (10) | 16 (100) | 9 (90) | 3 (25) | 8 (30) | 2 (22) | 0 (0) | 4 (100) | 1 (50) | ||
| Colonic enema | N = 14 | N = 57 | N = 19 | N = 10 | N = 16 | N = 10 | N = 11 | N = 25 | N = 9 | N = 13 | N = 4 | N = 1 | ||
| Weekly | 0 (0) | 3 (5) | 0 (0) | 0 (0) | 1 (6) | 1 (10) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 3 (21) | 6 (11) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 2 (18) | 3 (12) | 0 (0) | 1 (8) | 0 (0) | 1 (100) | ||
| 3-monthly | 6 (43) | 4 (7) | 2 (11) | 4 (40) | 0 (0) | 0 (0) | 6 (55) | 5 (20) | 1 (11) | 3 (23) | 0 (0) | 0 (0) | ||
| 6-monthly | 0 (0) | 3 (5) | 3 (16) | 5 (50) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 5 (56) | 6 (46) | 0 (0) | 0 (0) | ||
| Annually | 1 (7) | 3 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 3 (23) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 4 (29) | 38 (67) | 14 (74) | 1 (10) | 15 (94) | 8 (80) | 3 (27) | 12 (48) | 3 (33) | 0 (0) | 4 (100) | 0 (0) | ||
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) | N = 12 | N = 55 | N = 19 | N = 9 | N = 16 | N = 10 | N = 10 | N = 24 | N = 9 | N = 13 | N = 4 | N = 1 | ||
| Weekly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Monthly | 1 (8) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | ||
| 3-monthly | 4 (33) | 4 (7) | 2 (11) | 5 (56) | 0 (0) | 1 (10) | 4 (40) | 3 (12) | 1 (11) | 5 (38) | 0 (0) | 0 (0) | ||
| 6-monthly | 1 (8) | 3 (5) | 2 (11) | 3 (33) | 0 (0) | 0 (0) | 0 (0) | 5 (21) | 4 (44) | 5 (38) | 0 (0) | 0 (0) | ||
| Annually | 0 (0) | 6 (11) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 3 (12) | 0 (0) | 3 (23) | 0 (0) | 0 (0) | ||
| Never used for this group or never reviewed | 6 (50) | 41 (75) | 14 (74) | 1 (11) | 16 (100) | 8 (80) | 3 (30) | 13 (54) | 4 (44) | 0 (0) | 4 (100) | 0 (0) | ||
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 16) | Nurses (N = 75) | Paediatricians (N = 21) | Surgeons (N = 10) | Therapists (N = 32) | Other (N = 13) | BBS nurses (N = 15) | Nurses (N = 35) | Paediatricians (N = 8) | Surgeons (N = 13) | Therapists (N = 13) | Other (N = 3) | |
| Knowing – recognition of sensation | 14 (88) | 53 (71) | 13 (62) | 7 (70) | 5 (16) | 9 (69) | 10 (67) | 20 (57) | 2 (25) | 5 (38) | 2 (15) | 3 (100) |
| Finding – an appropriate place for toileting | 15 (94) | 66 (88) | 12 (57) | 4 (40) | 23 (72) | 13 (100) | 15 (100) | 33 (94) | 4 (50) | 5 (38) | 10 (77) | 3 (100) |
| Accessing – the cubicle or toilet itself | 14 (88) | 67 (89) | 10 (48) | 4 (40) | 28 (88) | 9 (69) | 13 (87) | 30 (86) | 4 (50) | 6 (46) | 12 (92) | 2 (67) |
| Preparing – undressing | 15 (94) | 64 (85) | 9 (43) | 2 (20) | 31 (97) | 12 (92) | 14 (93) | 30 (86) | 4 (50) | 3 (23) | 11 (85) | 2 (67) |
| Going – weeing and pooing | 16 (100) | 65 (87) | 14 (67) | 5 (50) | 7 (22) | 11 (85) | 15 (100) | 30 (86) | 5 (62) | 8 (62) | 0 (0) | 2 (67) |
| Cleaning – wiping and washing | 14 (88) | 63 (84) | 12 (57) | 3 (30) | 28 (88) | 12 (92) | 14 (93) | 30 (86) | 4 (50) | 5 (38) | 10 (77) | 3 (100) |
| Completing – redressing and returning to previous activity | 13 (81) | 61 (81) | 9 (43) | 3 (30) | 27 (84) | 11 (85) | 14 (93) | 29 (83) | 3 (38) | 3 (23) | 10 (77) | 2 (67) |
| Other | 0 (0) | 4 (5) | 4 (19) | 1 (10) | 1 (3) | 2 (15) | 1 (7) | 3 (9) | 2 (25) | 2 (15) | 1 (8) | 2 (67) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 30) | Surgeons (N = 12) | Therapists (N = 32) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 12) | Other (N = 3) | |
| To develop the child/young person’s understanding of toileting | 14 (78) | 38 (49) | 20 (67) | 6 (50) | 11 (34) | 6 (46) | 10 (56) | 21 (58) | 5 (42) | 5 (33) | 8 (67) | 3 (100) |
| For the child/young person to be accident free | 9 (50) | 14 (18) | 4 (13) | 8 (67) | 6 (19) | 1 (8) | 7 (39) | 13 (36) | 4 (33) | 8 (53) | 5 (42) | 1 (33) |
| To reduce the amount of wetting/soiling | 9 (50) | 19 (25) | 9 (30) | 7 (58) | 6 (19) | 6 (46) | 9 (50) | 16 (44) | 5 (42) | 8 (53) | 5 (42) | 2 (67) |
| To improve the child/young person’s independence in using the toilet | 10 (56) | 21 (27) | 7 (23) | 7 (58) | 22 (69) | 3 (23) | 13 (72) | 17 (47) | 5 (42) | 8 (53) | 10 (83) | 3 (100) |
| To get the child/young person in a routine for toileting | 11 (61) | 39 (51) | 10 (33) | 9 (75) | 13 (41) | 7 (54) | 10 (56) | 16 (44) | 5 (42) | 5 (33) | 6 (50) | 2 (67) |
| To protect the child/young person’s bladder and bowel | 8 (44) | 18 (23) | 7 (23) | 9 (75) | 1 (3) | 0 (0) | 13 (72) | 27 (75) | 10 (83) | 11 (73) | 3 (25) | 1 (33) |
| Other | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 1 (3) | 0 (0) | 1 (7) | 2 (17) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 77) | Paediatricians (N = 30) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 36) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 12) | Other (N = 3) | |
| Parent/carer verbal report | 18 (100) | 76 (99) | 30 (100) | 12 (100) | 31 (94) | 12 (92) | 16 (89) | 36 (100) | 12 (100) | 15 (100) | 11 (92) | 3 (100) |
| Child/young person verbal report | 16 (89) | 51 (66) | 19 (63) | 11 (92) | 20 (61) | 9 (69) | 16 (89) | 31 (86) | 11 (92) | 14 (93) | 9 (75) | 3 (100) |
| Chart/checklist/questionnaire | 15 (83) | 53 (69) | 10 (33) | 3 (25) | 9 (27) | 9 (69) | 11 (61) | 29 (81) | 3 (25) | 3 (20) | 4 (33) | 3 (100) |
| Physical examination | 2 (11) | 8 (10) | 13 (43) | 5 (42) | 0 (0) | 2 (15) | 1 (6) | 7 (19) | 7 (58) | 7 (47) | 0 (0) | 1 (33) |
| Ultrasound | 0 (0) | 9 (12) | 3 (10) | 4 (33) | 0 (0) | 0 (0) | 2 (11) | 7 (19) | 3 (25) | 8 (53) | 0 (0) | 0 (0) |
| Other imaging (e.g. post-void bladder scan or isotope scan) | 4 (22) | 7 (9) | 1 (3) | 4 (33) | 0 (0) | 0 (0) | 8 (44) | 6 (17) | 3 (25) | 9 (60) | 0 (0) | 0 (0) |
| Urodynamics – invasive with catheter | 0 (0) | 2 (3) | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 2 (11) | 3 (8) | 0 (0) | 5 (33) | 0 (0) | 0 (0) |
| Urodynamics – non-invasive without catheter | 0 (0) | 2 (3) | 0 (0) | 4 (33) | 0 (0) | 0 (0) | 1 (6) | 2 (6) | 0 (0) | 4 (27) | 0 (0) | 0 (0) |
| Direct observation | 8 (44) | 18 (23) | 3 (10) | 2 (17) | 9 (27) | 3 (23) | 5 (28) | 11 (31) | 0 (0) | 2 (13) | 1 (8) | 1 (33) |
| Wee/poo sample | 0 (0) | 11 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diet/fluid intake diary | 12 (67) | 27 (35) | 5 (17) | 3 (25) | 1 (3) | 4 (31) | 8 (44) | 16 (44) | 1 (8) | 3 (20) | 1 (8) | 2 (67) |
| Blood test | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I never assess in my role | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 1 (8) | 0 (0) | 1 (8) | 0 (0) |
| Other | 1 (6) | 3 (4) | 1 (3) | 0 (0) | 2 (6) | 0 (0) | 1 (6) | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Response option | Non-spinal-cord-related pathology, n (% of responders in job family) | Spinal cord pathology, n (% of responders in job family) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBS nurses (N = 18) | Nurses (N = 76) | Paediatricians (N = 30) | Surgeons (N = 12) | Therapists (N = 33) | Other (N = 13) | BBS nurses (N = 18) | Nurses (N = 35) | Paediatricians (N = 12) | Surgeons (N = 15) | Therapists (N = 12) | Other (N = 3) | |
| Via GP | 13 (72) | 39 (51) | 18 (60) | 7 (58) | 16 (48) | 7 (54) | 10 (56) | 13 (37) | 7 (58) | 6 (40) | 7 (58) | 3 (100) |
| Via health professional (e.g. paediatrician or specialist nurse) | 18 (100) | 64 (84) | 25 (83) | 12 (100) | 28 (85) | 12 (92) | 18 (100) | 31 (89) | 12 (100) | 14 (93) | 11 (92) | 3 (100) |
| Via social care | 7 (39) | 24 (32) | 3 (10) | 0 (0) | 10 (30) | 3 (23) | 2 (11) | 8 (23) | 0 (0) | 0 (0) | 6 (50) | 1 (33) |
| Via education | 7 (39) | 41 (54) | 8 (27) | 0 (0) | 15 (45) | 6 (46) | 3 (17) | 16 (46) | 2 (17) | 0 (0) | 5 (42) | 2 (67) |
| Via parent/carer | 9 (50) | 40 (53) | 7 (23) | 0 (0) | 21 (64) | 4 (31) | 5 (28) | 14 (40) | 2 (17) | 0 (0) | 9 (75) | 2 (67) |
| Don’t know | 0 (0) | 2 (3) | 1 (3) | 0 (0) | 3 (9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 7 (9) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 3 (9) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Response option | BBS nurses, n (% of responders in job family) | Nurses, n (% of responders in job family) | Paediatricians, n (% of responders in job family) | Surgeons, n (% of responders in job family) | Therapists, n (% of responders in job family) | Other, n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Behavioural (e.g. timer, alarm) | N = 20 | N = 74 | N = 29 | N = 15 | N = 27 | N = 12 |
| Supplied free of charge | 12 (60) | 26 (35) | 14 (48) | 5 (33) | 5 (19) | 4 (33) |
| Subsidised | 0 (0) | 0 (0) | 2 (7) | 0 (0) | 1 (4) | 1 (8) |
| Available to purchase | 0 (0) | 10 (14) | 0 (0) | 1 (7) | 4 (15) | 0 (0) |
| Unavailable | 6 (30) | 26 (35) | 4 (14) | 6 (40) | 11 (41) | 6 (50) |
| Don’t know | 2 (10) | 12 (16) | 9 (31) | 3 (20) | 6 (22) | 1 (8) |
| Simple aid/equipment (e.g. raised seat or step) | N = 18 | N = 74 | N = 29 | N = 15 | N = 27 | N = 12 |
| Supplied free of charge | 7 (39) | 27 (36) | 11 (38) | 2 (13) | 20 (74) | 3 (25) |
| Subsidised | 1 (6) | 1 (1) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Available to purchase | 2 (11) | 12 (16) | 7 (24) | 1 (7) | 1 (4) | 1 (8) |
| Unavailable | 5 (28) | 25 (34) | 3 (10) | 6 (40) | 3 (11) | 5 (42) |
| Don’t know | 3 (17) | 9 (12) | 8 (28) | 5 (33) | 3 (11) | 3 (25) |
| Bespoke aid/equipment (e.g. hoist or frame) | N = 18 | N = 74 | N = 29 | N = 15 | N = 27 | N = 12 |
| Supplied free of charge | 9 (50) | 42 (57) | 18 (62) | 0 (0) | 20 (74) | 2 (17) |
| Subsidised | 2 (11) | 2 (3) | 0 (0) | 1 (7) | 1 (4) | 1 (8) |
| Available to purchase | 1 (6) | 3 (4) | 0 (0) | 0 (0) | 2 (7) | 0 (0) |
| Unavailable | 3 (17) | 15 (20) | 1 (3) | 6 (40) | 1 (4) | 4 (33) |
| Don’t know | 3 (17) | 12 (16) | 10 (34) | 8 (53) | 3 (11) | 5 (42) |
| Housing adaption (e.g. specialised toilet) | N = 18 | N = 72 | N = 29 | N = 15 | N = 27 | N = 12 |
| Supplied free of charge | 7 (39) | 26 (36) | 15 (52) | 0 (0) | 13 (48) | 3 (25) |
| Subsidised | 3 (17) | 5 (7) | 3 (10) | 1 (7) | 4 (15) | 1 (8) |
| Available to purchase | 1 (6) | 4 (6) | 0 (0) | 0 (0) | 2 (7) | 0 (0) |
| Unavailable | 3 (17) | 14 (19) | 1 (3) | 6 (40) | 4 (15) | 4 (33) |
| Don’t know | 4 (22) | 23 (32) | 10 (34) | 8 (53) | 4 (15) | 4 (33) |
| Products (e.g. pads, nappies, continence pants, pull-ups) | N = 18 | N = 72 | N = 29 | N = 14 | N = 26 | N = 12 |
| Supplied free of charge | 17 (94) | 53 (74) | 19 (66) | 8 (57) | 11 (42) | 6 (50) |
| Subsidised | 1 (6) | 5 (7) | 4 (14) | 0 (0) | 4 (15) | 1 (8) |
| Available to purchase | 0 (0) | 2 (3) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Unavailable | 0 (0) | 7 (10) | 0 (0) | 3 (21) | 2 (8) | 4 (33) |
| Don’t know | 0 (0) | 5 (7) | 5 (17) | 3 (21) | 9 (35) | 1 (8) |
| Medications (e.g. laxatives) | N = 18 | N = 72 | N = 29 | N = 14 | N = 26 | N = 12 |
| Supplied free of charge | 15 (83) | 60 (83) | 29 (100) | 13 (93) | 8 (31) | 9 (75) |
| Subsidised | 1 (6) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Available to purchase | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) |
| Unavailable | 1 (6) | 10 (14) | 0 (0) | 0 (0) | 2 (8) | 2 (17) |
| Don’t know | 1 (6) | 2 (3) | 0 (0) | 0 (0) | 16 (62) | 0 (0) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 12), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| < 2 years | 2 (9) | 3 (4) | 0 (0) | 1 (7) | 6 (18) | 0 (0) |
| 2 years | 2 (9) | 1 (1) | 1 (3) | 0 (0) | 4 (12) | 0 (0) |
| 3 years | 1 (5) | 9 (11) | 2 (7) | 0 (0) | 3 (9) | 1 (8) |
| 4 years | 10 (45) | 28 (35) | 5 (17) | 0 (0) | 3 (9) | 4 (33) |
| 5 years | 2 (9) | 20 (25) | 7 (23) | 3 (20) | 1 (3) | 1 (8) |
| 6 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 7 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ≥ 8 years | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Don’t know | 2 (9) | 15 (19) | 14 (47) | 11 (73) | 12 (36) | 6 (50) |
| Other | 3 (14) | 3 (4) | 0 (0) | 0 (0) | 4 (12) | 0 (0) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 76), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Barrier | 16 (73) | 39 (51) | 7 (23) | 1 (7) | 9 (27) | 5 (38) |
| Enabler | 4 (18) | 18 (24) | 10 (33) | 6 (40) | 7 (21) | 5 (38) |
| Don’t know | 2 (9) | 19 (25) | 13 (43) | 8 (53) | 17 (52) | 3 (23) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 78), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Persevere with intervention as it might take time | 0 (0) | 8 (10) | 1 (3) | 0 (0) | 2 (6) | 1 (8) |
| Try a different intervention | 2 (9) | 4 (5) | 2 (7) | 4 (27) | 5 (15) | 0 (0) |
| Revisit same intervention at a later date | 0 (0) | 6 (8) | 2 (7) | 0 (0) | 2 (6) | 2 (15) |
| All of the above | 19 (86) | 58 (74) | 24 (80) | 10 (67) | 22 (67) | 10 (77) |
| Other | 1 (5) | 2 (3) | 1 (3) | 1 (7) | 2 (6) | 0 (0) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 12), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 6 (27) | 13 (16) | 5 (17) | 4 (27) | 3 (9) | 2 (17) |
| No | 15 (68) | 46 (58) | 19 (63) | 9 (60) | 20 (61) | 9 (75) |
| Don’t know | 1 (5) | 20 (25) | 6 (20) | 2 (13) | 10 (30) | 1 (8) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 6 (27) | 18 (23) | 8 (27) | 3 (20) | 4 (12) | 3 (23) |
| No | 11 (50) | 26 (33) | 4 (13) | 7 (47) | 5 (15) | 4 (31) |
| Don’t know | 5 (23) | 35 (44) | 18 (60) | 5 (33) | 24 (73) | 6 (46) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 78), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 8 (36) | 39 (50) | 14 (47) | 9 (60) | 17 (52) | 6 (46) |
| No | 8 (36) | 28 (36) | 3 (10) | 2 (13) | 6 (18) | 4 (31) |
| Don’t know | 6 (27) | 11 (14) | 13 (43) | 4 (27) | 10 (30) | 3 (23) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 17 (77) | 46 (58) | 10 (33) | 10 (67) | 5 (15) | 5 (38) |
| No | 4 (18) | 21 (27) | 17 (57) | 4 (27) | 13 (39) | 4 (31) |
| Don’t know | 1 (5) | 12 (15) | 3 (10) | 1 (7) | 15 (45) | 4 (31) |
| Response option | BBS nurses (N = 16b), n (% of responders in job family) | Nurses (N = 46), n (% of responders in job family) | Paediatricians (N = 10), n (% of responders in job family) | Surgeons (N = 10), n (% of responders in job family) | Therapists (N = 5), n (% of responders in job family) | Other (N = 5), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 15 (94) | 34 (74) | 8 (80) | 6 (60) | 3 (60) | 4 (80) |
| No | 1 (6) | 6 (13) | 1 (10) | 4 (40) | 0 (0) | 0 (0) |
| Don’t know | 0 (0) | 6 (13) | 1 (10) | 0 (0) | 2 (40) | 1 (20) |
| Response option | BBS Nurses (N = 21), n (% of responders in job family) | Nurses (N = 67), n (% of responders in job family) | Paediatricians (N = 27), n (% of responders in job family) | Surgeons (N = 14), n (% of responders in job family) | Therapists (N = 18), n (% of responders in job family) | Other (N = 9), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Lack of funding and/or resources | 16 (76) | 38 (57) | 17 (63) | 10 (71) | 6 (33) | 7 (78) |
| Lack of professional interest | 1 (5) | 9 (13) | 2 (7) | 1 (7) | 1 (6) | 0 (0) |
| Time | 0 (0) | 10 (15) | 3 (11) | 2 (14) | 4 (22) | 0 (0) |
| Lack of need in local area | 0 (0) | 1 (1) | 2 (7) | 0 (0) | 1 (6) | 0 (0) |
| Don’t know | 1 (5) | 6 (9) | 1 (4) | 0 (0) | 3 (17) | 0 (0) |
| Other | 3 (14) | 3 (4) | 2 (7) | 1 (7) | 3 (17) | 2 (22) |
| Response option | BBS nurses (N = 22), n (% of responders in job family | Nurses (N = 79), n (% of responders in job family | Paediatricians (N = 31), n (% of responders in job family | Surgeons (N = 15), n (% of responders in job family | Therapists (N = 33), n (% of responders in job family | Other (N = 13), n (% of responders in job family |
|---|---|---|---|---|---|---|
| Never | 5 (23) | 12 (15) | 2 (6) | 2 (13) | 6 (18) | 1 (8) |
| Sometimes | 6 (27) | 18 (23) | 10 (32) | 5 (33) | 8 (24) | 5 (38) |
| Often | 3 (14) | 11 (14) | 10 (32) | 4 (27) | 5 (15) | 1 (8) |
| Always | 6 (27) | 19 (24) | 6 (19) | 1 (7) | 1 (3) | 1 (8) |
| Don’t know | 2 (9) | 19 (24) | 3 (10) | 3 (20) | 13 (39) | 5 (38) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 31), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Very easy | 5 (23) | 7 (9) | 0 (0) | 2 (13) | 0 (0) | 1 (8) |
| Easy | 12 (55) | 31 (39) | 16 (52) | 3 (20) | 15 (45) | 2 (15) |
| Difficult | 2 (9) | 22 (28) | 8 (26) | 8 (53) | 15 (45) | 7 (54) |
| Very difficult | 3 (14) | 12 (15) | 4 (13) | 0 (0) | 1 (3) | 1 (8) |
| Don’t know | 0 (0) | 7 (9) | 3 (10) | 2 (13) | 2 (6) | 2 (15) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 33), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Very acceptable | 4 (18) | 5 (6) | 0 (0) | 2 (13) | 2 (6) | 0 (0) |
| Acceptable | 8 (36) | 32 (41) | 11 (37) | 3 (20) | 9 (27) | 7 (54) |
| Unacceptable | 7 (32) | 17 (22) | 9 (30) | 7 (47) | 11 (33) | 4 (31) |
| Very unacceptable | 2 (9) | 9 (11) | 2 (7) | 1 (7) | 1 (3) | 1 (8) |
| Don’t know | 1 (5) | 16 (20) | 8 (27) | 2 (13) | 10 (30) | 1 (8) |
| Response option | BBS nurses (N = 22), n (% of responders in job family) | Nurses (N = 79), n (% of responders in job family) | Paediatricians (N = 30), n (% of responders in job family) | Surgeons (N = 15), n (% of responders in job family) | Therapists (N = 32), n (% of responders in job family) | Other (N = 13), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| Yes | 18 (82) | 45 (57) | 6 (20) | 12 (80) | 9 (28) | 2 (15) |
| No | 4 (18) | 34 (43) | 24 (80) | 3 (20) | 23 (72) | 11 (85) |
| Response option | BBS nurses (N = 18), n (% of responders in job family) | Nurses (N = 45), n (% of responders in job family) | Paediatricians (N = 6), n (% of responders in job family) | Surgeons (N = 12), n (% of responders in job family) | Therapists (N = 9), n (% of responders in job family) | Other (N = 2), n (% of responders in job family) |
|---|---|---|---|---|---|---|
| 14 years | 0 (0) | 8 (18) | 3 (50) | 1 (8) | 1 (11) | 0 (0) |
| 15 years | 1 (6) | 2 (4) | 0 (0) | 3 (25) | 1 (11) | 1 (50) |
| 16 years | 7 (39) | 10 (22) | 1 (17) | 7 (58) | 3 (33) | 0 (0) |
| 17 years | 1 (6) | 11 (24) | 1 (17) | 0 (0) | 2 (22) | 0 (0) |
| 18 years | 8 (44) | 13 (29) | 0 (0) | 0 (0) | 0 (0) | 1 (50) |
| Don’t know | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 1 (6) | 1 (2) | 1 (17) | 1 (8) | 2 (22) | 0 (0) |
Appendix 10 Parent carer survey results
In Tables 59–76, ‘non-spinal-cord-related pathology’ relates to children and young people with social/communication or attention/behaviour difficulty, learning disability or physical or movement disability; ‘spinal cord pathology’ relates to children and young people with bladder and/or bowel impairment due to damage to the spinal cord.
| Always | Usually | Rarely | Never | Don’t know | |
|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||
| Knowing: does your child know when they need to go without you asking or telling them? (N = 559) | 64 (11) | 194 (35) | 128 (23) | 141 (25) | 32 (6) |
| Finding: can your child wait to go until they find a toilet to use? (N = 559) | 41 (7) | 187 (33) | 117 (21) | 195 (35) | 19 (3) |
| Accessing: can your child get in to the toilet cubicle or bathroom on their own? (N = 559) | 131 (23) | 182 (33) | 47 (8) | 199 (36) | 0 (0) |
| Preparing: can your child undress themselves? (N = 558) | 120 (22) | 187 (34) | 84 (15) | 167 (30) | 0 (0) |
| Going: can your child use the toilet on their own once they are there? (N = 559) | 123 (22) | 171 (31) | 62 (11) | 199 (36) | 4 (1) |
| Cleaning: can your child clean themselves (wipe bottom and wash hands) afterwards without any help? (N = 559) | 23 (4) | 78 (14) | 128 (23) | 328 (59) | 2 (0) |
| Completing: can your child get dressed again without help? (N = 559) | 87 (16) | 164 (29) | 98 (18) | 210 (38) | 0 (0) |
| Spinal cord pathology | |||||
| Knowing: does your child know when they need to go without you asking or telling them? (N = 20) | 1 (5) | 6 (30) | 2 (10) | 10 (50) | 1 (5) |
| Finding: can your child wait to go until they find a toilet to use? (N = 19) | 1 (5) | 3 (16) | 4 (21) | 10 (53) | 1 (5) |
| Accessing: can your child get in to the toilet cubicle or bathroom on their own? (N = 20) | 4 (20) | 4 (20) | 4 (20) | 7 (35) | 1 (5) |
| Preparing: can your child undress themselves? (N = 20) | 3 (15) | 6 (30) | 4 (20) | 6 (30) | 1 (5) |
| Going: can your child use the toilet on their own once they are there? (N = 20) | 1 (5) | 7 (35) | 0 (0) | 11 (55) | 1 (5) |
| Cleaning: can your child clean themselves (wipe bottom and wash hands) afterwards without any help? (N = 20) | 0 (0) | 4 (20) | 2 (10) | 13 (65) | 1 (5) |
| Completing: can your child get dressed again without help? (N = 20) | 3 (15) | 5 (25) | 3 (15) | 8 (40) | 1 (5) |
| Response option | Non-spinal-cord-related pathology (N = 558), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| Yes, it’s my main priority | 46 (8) | 4 (20) |
| Yes, it’s one of many competing priorities | 330 (59) | 7 (35) |
| No, my child’s other needs are more important currently | 101 (18) | 4 (20) |
| My child will never be able to use the toilet without help, so it’s not a priority | 64 (11) | 3 (15) |
| Don’t know | 6 (1) | 2 (10) |
| Other | 11 (2) | 0 (0) |
| Response option | Non-spinal-cord-related pathology (N = 546), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| Support started automatically as a baby due to spinal cord damage | 5 (1) | 11 (55) |
| I have not accessed any support for toileting | 77 (14) | 2 (10) |
| Child’s age | 196 (36) | 5 (25) |
| Child’s stage of development | 190 (35) | 4 (20) |
| Doctor/nurse recommendation | 52 (10) | 1 (5) |
| Starting school/school request or expectation | 124 (23) | 3 (15) |
| Pressure from family | 30 (5) | 0 (0) |
| Pressure from other parents | 16 (3) | 0 (0) |
| Constipation | 131 (24) | 3 (15) |
| Diarrhoea | 36 (7) | 1 (5) |
| UTI (bladder, kidneys or urethra) | 35 (6) | 5 (25) |
| Urgency (i.e. sudden urge to go) | 40 (7) | 1 (5) |
| Bowel irritability (e.g. cramps, pain, bloating, gas) | 52 (10) | 1 (5) |
| Daytime wetting | 171 (31) | 6 (30) |
| Night-time wetting | 180 (33) | 5 (25) |
| Daytime soiling (pooing in pants) | 181 (33) | 4 (20) |
| Night-time soiling (pooing in pants) | 101 (18) | 4 (20) |
| Other | 87 (16) | 1 (5) |
| Response option | Non-spinal-cord-related pathology (N = 555), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| Very easy | 3 (< 1) | 1 (5) |
| Easy | 82 (15) | 9 (45) |
| Difficult | 213 (38) | 5 (25) |
| Very difficult | 178 (32) | 4 (20) |
| Don’t know | 79 (14) | 1 (5) |
| Response option | Non-spinal-cord-related pathology (N = 533), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| To develop my child’s understanding of the basics of toileting | 246 (46) | 4 (20) |
| For my child to be accident free | 168 (32) | 7 (35) |
| To reduce the amount of accidents my child has/had | 170 (32) | 8 (40) |
| For my child to be able to use the toilet without help | 183 (34) | 7 (35) |
| To get my child in a routine for toileting | 188 (35) | 7 (35) |
| To make sure my child’s bladder and bowel are healthy | 244 (46) | 14 (70) |
| Other | 79 (15) | 0 (0) |
| Response option | Non-spinal-cord-related pathology (N = 553), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| Very acceptable | 27 (5) | 3 (15) |
| Acceptable | 121 (22) | 7 (35) |
| Unacceptable | 79 (14) | 3 (15) |
| Very unacceptable | 64 (12) | 2 (10) |
| Don’t know | 262 (47) | 5 (25) |
| Response option | Non-spinal-cord-related pathology (N = 478), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| GP | 149 (31) | 4 (20) |
| Paediatrician | 193 (40) | 7 (35) |
| Paediatric surgeon | 16 (3) | 4 (20) |
| Paediatric neurologist | 25 (5) | 9 (45) |
| Paediatric urologist | 48 (10) | 13 (65) |
| Adult neurologist | 3 (< 1) | 1 (5) |
| Neurosurgeon | 3 (< 1) | 3 (15) |
| Neurophysiologist | 0 (0) | 0 (0) |
| Neuropsychologist | 3 (< 1) | 0 (0) |
| Urological surgeon | 16 (3) | 8 (40) |
| General psychiatrist | 2 (< 1) | 0 (0) |
| Child and adolescent psychiatrist | 19 (4) | 1 (5) |
| Psychiatrist of intellectual disability or learning disability | 6 (1) | 0 (0) |
| Clinical psychologist | 15 (3) | 0 (0) |
| Adult nurse | 8 (2) | 0 (0) |
| Children’s nurse | 114 (24) | 11 (55) |
| Specialist community public health nurse (school nurse) | 115 (24) | 2 (10) |
| Health visitor | 67 (14) | 5 (25) |
| Learning disability nurse | 44 (9) | 1 (5) |
| District nurse | 5 (1) | 1 (5) |
| Dietitian | 22 (5) | 0 (0) |
| Occupational therapist | 115 (24) | 6 (30) |
| Physiotherapist | 23 (5) | 3 (15) |
| Speech and language therapist | 35 (7) | 1 (5) |
| Charity (e.g. ERIC Bladder & Bowel UK) | 83 (17) | 5 (25) |
| Other | 138 (29) | 2 (10) |
| Response option | Non-spinal-cord-related pathology (N = 544), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| My child has not been assessed | 220 (40) | 3 (15) |
| Home | 111 (20) | 4 (20) |
| Community clinic | 120 (22) | 1 (5) |
| Hospital clinic | 109 (20) | 16 (80) |
| School/college/university | 95 (17) | 2 (10) |
| Respite care | 8 (1) | 0 (0) |
| Young person’s place of work | 0 (0) | 0 (0) |
| Other | 24 (4) | 0 (0) |
| Response option | Non-spinal-cord-related pathology (N = 454), n (% of responders) | Spinal cord pathology (N = 19), n (% of responders) |
|---|---|---|
| Parent/carer verbal report | 398 (88) | 13 (68) |
| Child/young person verbal report | 62 (14) | 7 (37) |
| Chart/checklist/questionnaire | 172 (38) | 1 (5) |
| Physical examination | 105 (23) | 12 (63) |
| Ultrasound | 69 (15) | 13 (68) |
| Other imaging (e.g. bladder scan or isotope scan) | 42 (9) | 9 (47) |
| Urodynamics – invasive with catheter | 13 (3) | 13 (68) |
| Urodynamics – non-invasive without catheter (e.g. flow rate) | 22 (5) | 4 (21) |
| Direct observation | 41 (9) | 4 (21) |
| Wee/poo samples | 59 (13) | 2 (11) |
| Diet/fluid intake diaries | 137 (30) | 4 (21) |
| Blood tests | 40 (9) | 3 (16) |
| Other | 40 (9) | 0 (0) |
| Supplied free of charge, n (% of responders) | Subsidised, n (% of responders) | Available to purchase, n (% of responders) | Unavailable, n (% of responders) | Don’t know/never used, n (% of responders) | |
|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||
| Behavioural (e.g. timers, alarms) (N = 519) | 29 (6) | 1 (< 1) | 46 (9) | 49 (9) | 394 (76) |
| Simple aids or equipment (e.g. raised step or seat) (N = 523) | 91 (17) | 2 (< 1) | 69 (13) | 45 (9) | 316 (60) |
| Bespoke aids or equipment (e.g. hoist or frame) (N = 521) | 73 (14) | 5 (1) | 12 (2) | 20 (4) | 411 (79) |
| Housing adaptions (e.g. specialised toilet) (N = 516) | 61 (12) | 19 (4) | 14 (3) | 20 (4) | 402 (78) |
| Continence products (e.g. pads, nappies, pull-ups) (N = 534) | 256 (48) | 21 (4) | 55 (10) | 42 (8) | 160 (30) |
| Medications (e.g. laxatives) (N = 522) | 290 (56) | 3 (< 1) | 15 (3) | 6 (1) | 208 (40) |
| Spinal cord pathology | |||||
| Behavioural (e.g. timers, alarms) (N = 17) | 0 (0) | 0 (0) | 0 (0) | 2 (12) | 15 (88) |
| Simple aids or equipment (e.g. raised step or seat) (N = 19) | 8 (42) | 0 (0) | 2 (11) | 2 (11) | 7 (37) |
| Bespoke aids or equipment (e.g. hoist or frame) (N = 17) | 2 (12) | 0 (0) | 0 (0) | 0 (0) | 15 (88) |
| Housing adaptions (e.g. specialised toilet) (N = 19) | 5 (26) | 1 (5) | 1 (5) | 0 (0) | 12 (63) |
| Continence products (e.g. pads, nappies, pull-ups) (N = 19) | 9 (47) | 1 (5) | 6 (32) | 1 (5) | 2 (11) |
| Medications (e.g. laxatives) (N = 18) | 17 (94) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| Weekly, n (% of responders) | Monthly, n (% of responders) | 3-monthly, n (% of responders) | 6-monthly, n (% of responders) | Annually, n (% of responders) | Don’t know, n (% of responders) | Never used, n (% of responders) | |
|---|---|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||||
| Dietary advice (N = 463) | 3 (< 1) | 3 (< 1) | 34 (7) | 29 (6) | 44 (10) | 123 (27) | 227 (49) |
| Fluid intake advice (N = 461) | 6 (1) | 9 (2) | 38 (8) | 35 (8) | 53 (11) | 136 (30) | 184 (40) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 455) | 10 (2) | 9 (2) | 18 (4) | 18 (4) | 30 (7) | 136 (30) | 234 (51) |
| Simple aid/equipment (e.g. raised step or seat) (N = 453) | 7 (2) | 2 (< 1) | 15 (3) | 11 (2) | 30 (7) | 162 (36) | 226 (50) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 451) | 1 (< 1) | 0 (0) | 0 (0) | 8 (2) | 21 (5) | 69 (15) | 352 (78) |
| Housing adaptions (e.g. specialised toilet) (N = 447) | 2 (< 1) | 0 (0) | 1 (< 1) | 5 (1) | 20 (4) | 63 (14) | 356 (80) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 471) | 16 (3) | 3 (< 1) | 26 (6) | 39 (8) | 81 (17) | 161 (34) | 145 (31) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 460) | 8 (2) | 12 (3) | 45 (10) | 37 (8) | 67 (15) | 81 (18) | 210 (46) |
| Catheters (N = 443) | 1 (< 1) | 2 (< 1) | 1 (< 1) | 3 (< 1) | 4 (1) | 26 (6) | 406 (92) |
| Colonic enema (bowel washout) (N = 442) | 2 (< 1) | 0 (0) | 3 (< 1) | 2 (< 1) | 5 (1) | 31 (7) | 399 (90) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 441) | 1 (< 1) | 0 (0) | 1 (< 1) | 2 (< 1) | 3 (< 1) | 31 (7) | 403 (91) |
| Spinal cord pathology | |||||||
| Dietary advice (N = 17) | 0 (0) | 0 (0) | 3 (18) | 2 (12) | 1 (6) | 2 (12) | 9 (53) |
| Fluid intake advice (N = 17) | 0 (0) | 1 (6) | 2 (12) | 1 (6) | 4 (24) | 3 (18) | 6 (35) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 16) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 1 (6) | 3 (19) | 11 (69) |
| Simple aid/equipment (e.g. raised step or seat) (N = 17) | 0 (0) | 0 (0) | 1 (6) | 2 (12) | 4 (24) | 2 (12) | 8 (47) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 16) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (6) | 1 (6) | 13 (81) |
| Housing adaptions (e.g. specialised toilet) (N = 17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 2 (12) | 14 (82) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 18) | 0 (0) | 2 (11) | 2 (11) | 1 (6) | 4 (22) | 5 (28) | 4 (22) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 17) | 0 (0) | 1 (6) | 5 (29) | 4 (24) | 4 (24) | 1 (6) | 2 (12) |
| Catheters (N = 18) | 0 (0) | 2 (11) | 3 (17) | 3 (17) | 5 (28) | 0 (0) | 5 (28) |
| Colonic enema (bowel washout) (N = 17) | 0 (0) | 1 (6) | 3 (18) | 3 (18) | 2 (12) | 1 (6) | 7 (41) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 18) | 0 (0) | 2 (11) | 1 (6) | 1 (6) | 1 (6) | 1 (6) | 12 (67) |
| Very easy, n (% of responders) | Easy, n (% of responders) | Difficult, n (% of responders) | Very difficult, n (% of responders) | Never used, n (% of responders) | |
|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||
| Dietary advice (N = 476) | 37 (8) | 104 (22) | 64 (13) | 39 (8) | 232 (49) |
| Fluid intake advice (N = 491) | 51 (10) | 140 (29) | 101 (21) | 32 (7) | 167 (34) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 483) | 18 (4) | 72 (15) | 99 (20) | 103 (21) | 191 (40) |
| Simple aid/equipment (e.g. raised step or seat) (N = 479) | 60 (13) | 156 (33) | 54 (11) | 22 (5) | 187 (39) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 456) | 12 (3) | 31 (7) | 22 (5) | 12 (3) | 379 (83) |
| Housing adaptions (e.g. specialised toilet) (N = 456) | 16 (4) | 23 (5) | 15 (3) | 14 (3) | 388 (85) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 497) | 132 (27) | 188 (38) | 60 (12) | 21 (4) | 96 (19) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 489) | 55 (11) | 131 (27) | 73 (15) | 36 (7) | 194 (40) |
| Catheters (N = 442) | 2 (< 1) | 3 (< 1) | 5 (1) | 4 (1) | 428 (97) |
| Colonic enema (bowel washout) (N = 450) | 4 (1) | 11 (2) | 3 (< 1) | 11 (2) | 421 (94) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 447) | 1 (< 1) | 6 (1) | 4 (1) | 8 (2) | 428 (96) |
| Spinal cord pathology | |||||
| Dietary advice (N = 18) | 5 (28) | 2 (11) | 4 (22) | 0 (0) | 7 (39) |
| Fluid intake advice (N = 18) | 4 (22) | 3 (17) | 5 (28) | 1 (6) | 5 (28) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 17) | 0 (0) | 3 (18) | 2 (12) | 2 (12) | 10 (59) |
| Simple aid/equipment (e.g. raised step or seat) (N = 20) | 5 (25) | 3 (15) | 2 (10) | 2 (10) | 8 (40) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 18) | 1 (6) | 1 (6) | 2 (11) | 0 (0) | 14 (78) |
| Housing adaptions (e.g. specialised toilet) (N = 19) | 2 (11) | 3 (16) | 0 (0) | 0 (0) | 14 (74) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 19) | 7 (37) | 8 (42) | 1 (5) | 0 (0) | 3 (16) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 18) | 6 (33) | 4 (22) | 4 (22) | 3 (17) | 1 (6) |
| Catheters (N = 19) | 4 (21) | 8 (42) | 2 (11) | 1 (5) | 4 (21) |
| Colonic enema (bowel washout) (N = 18) | 1 (6) | 6 (33) | 1 (6) | 3 (17) | 7 (39) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 18) | 2 (11) | 2 (11) | 1 (6) | 1 (6) | 12 (67) |
| Very helpful, n (% of responders) | Helpful, n (% of responders) | Unhelpful, n (% of responders) | Very unhelpful, n (% of responders) | Never used, n (% of responders) | |
|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||
| Dietary advice (N = 474) | 15 (3) | 109 (23) | 92 (19) | 40 (8) | 218 (46) |
| Fluid intake advice (N = 484) | 34 (7) | 143 (30) | 111 (23) | 37 (8) | 159 (33) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 487) | 11 (2) | 67 (14) | 131 (27) | 95 (20) | 183 (38) |
| Simple aid/equipment (e.g. raised step or seat) (N = 482) | 44 (9) | 160 (33) | 61 (13) | 21 (4) | 196 (41) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 459) | 29 (6) | 34 (7) | 16 (3) | 6 (1) | 374 (81) |
| Housing adaptions (e.g. specialised toilet) (N = 456) | 30 (7) | 25 (5) | 12 (3) | 5 (1) | 384 (84) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 496) | 145 (29) | 165 (33) | 69 (14) | 25 (5) | 92 (19) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 486) | 67 (14) | 123 (25) | 60 (12) | 25 (5) | 211 (43) |
| Catheters (N = 449) | 6 (1) | 2 (< 1) | 5 (1) | 5 (1) | 431 (96) |
| Colonic enema (bowel washout) (N = 452) | 9 (2) | 8 (2) | 6 (1) | 7 (2) | 422 (93) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 450) | 7 (2) | 6 (1) | 6 (1) | 3 (< 1) | 428 (95) |
| Spinal cord pathology | |||||
| Dietary advice (N = 17) | 2 (12) | 6 (35) | 1 (6) | 1 (6) | 7 (41) |
| Fluid intake advice (N = 17) | 4 (24) | 6 (35) | 1 (6) | 1 (6) | 5 (29) |
| Behavioural intervention (e.g. reward chart, timer, alarm) (N = 16) | 0 (0) | 3 (19) | 4 (25) | 0 (0) | 9 (56) |
| Simple aid/equipment (e.g. raised step or seat) (N = 16) | 2 (13) | 5 (31) | 1 (6) | 1 (6) | 7 (44) |
| Bespoke aid/equipment (e.g. hoist or frame) (N = 17) | 0 (0) | 3 (18) | 0 (0) | 0 (0) | 14 (82) |
| Housing adaptions (e.g. specialised toilet) (N = 19) | 3 (16) | 2 (11) | 0 (0) | 0 (0) | 14 (74) |
| Continence products (e.g. nappies, pads, pull-ups) (N = 19) | 6 (32) | 9 (47) | 1 (5) | 1 (5) | 2 (11) |
| Medication (e.g. laxatives, anticholinergics, desmopressin) (N = 17) | 4 (24) | 4 (24) | 7 (41) | 0 (0) | 2 (12) |
| Catheters (N = 19) | 7 (37) | 7 (37) | 0 (0) | 0 (0) | 5 (26) |
| Colonic enema (bowel washout) (N = 17) | 5 (29) | 3 (18) | 0 (0) | 1 (6) | 8 (47) |
| Surgical intervention (e.g. botox, bladder reconstruction, Mitrofanoff) (N = 18) | 4 (22) | 1 (6) | 0 (0) | 1 (6) | 12 (67) |
| Response option | Non-spinal-cord-related pathology (N = 536), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| No difficulties experienced | 34 (6) | 4 (20) |
| My child’s knowledge and understanding of what is required | 328 (61) | 9 (45) |
| My own knowledge and understanding of what is required | 56 (10) | 4 (20) |
| My own ability and time to focus on toileting | 164 (31) | 6 (30) |
| My child’s willingness | 329 (61) | 14 (70) |
| Mine and my child’s lack of interest/motivation to change | 67 (13) | 1 (5) |
| Not enough training in how to use the methods offered | 52 (10) | 1 (5) |
| Delays in professional assessments | 149 (28) | 2 (10) |
| Access to appropriate help and support | 233 (43) | 7 (35) |
| Funding and/or resources for equipment and products | 126 (24) | 3 (15) |
| Lack of consistency in support in different environments (e.g. facility at home but not at school) | 205 (38) | 4 (20) |
| Other | 47 (9) | 1 (5) |
| Response option | Non-spinal-cord-related pathology (N = 492), n (% of responders) | Spinal cord pathology (N = 16), n (% of responders) |
|---|---|---|
| My child was not ready | 221 (45) | 8 (50) |
| I was not ready | 22 (4) | 0 (0) |
| Family/home circumstances were not ready | 55 (11) | 1 (6) |
| My child is unable to learn or manage toileting independently | 205 (42) | 6 (38) |
| My child’s needs were not considered | 111 (23) | 2 (13) |
| Inappropriate aids or equipment supplied | 41 (8) | 1 (6) |
| No appropriate aids or equipment supplied | 55 (11) | 2 (13) |
| No support has been given | 138 (28) | 2 (13) |
| Other | 55 (11) | 3 (19) |
| Response option | Non-spinal-cord-related pathology (N = 546), n (% of responders) | Spinal cord pathology (N = 20), n (% of responders) |
|---|---|---|
| Yes | 140 (26) | 6 (30) |
| No | 375 (69) | 14 (70) |
| Don’t know | 31 (6) | 0 (0) |
| Response option | Non-spinal-cord-related pathology (N = 211), n (% of responders) | Spinal cord pathology (N = 4), n (% of responders) |
|---|---|---|
| Yes | 74 (35) | 0 (0) |
| No | 137 (65) | 4 (100) |
| This has already happened, n (% of responders) | This is planned to happen, n (% of responders) | I know this should happen but it has not been planned yet, n (% of responders) | This has never been mentioned to us, n (% of responders) | Don’t know, n (% of responders) | |
|---|---|---|---|---|---|
| Non-spinal-cord-related pathology | |||||
| Someone has talked to my child about transition to adult services (N = 68) | 13 (19) | 6 (9) | 15 (22) | 22 (32) | 12 (18) |
| Someone has talked to me about my child’s transition to adult services (N = 72) | 26 (36) | 7 (10) | 17 (24) | 18 (25) | 4 (6) |
| We have been given information about adult services (N = 72) | 19 (26) | 5 (7) | 16 (22) | 26 (36) | 6 (8) |
| We have met someone from adult services (N = 71) | 24 (34) | 2 (3) | 11 (15) | 27 (38) | 7 (10) |
| We have had a joint meeting with someone from both the child and adult service (N = 67) | 10 (15) | 3 (4) | 8 (12) | 40 (60) | 6 (9) |
| My child has been reassessed for their toileting needs post 18 years (N = 70) | 14 (20) | 2 (3) | 10 (14) | 37 (53) | 7 (10) |
| A care plan has been written for my child’s future post 18 years (N = 67) | 9 (13) | 2 (3) | 12 (18) | 33 (49) | 11 (16) |
| We have a named person (doctor or nurse) to co-ordinate the transition to adult services (N = 69) | 9 (13) | 1 (1) | 12 (17) | 37 (54) | 10 (14) |
| Spinal cord pathology | |||||
| No one with a child aged 12–25 years responded ‘yes’ to Q24 | |||||
Appendix 11 Education and care staff survey results
| All | Always, n (% of responders) | Usually, n (% of responders) | Rarely, n (% of responders) | Never, n (% of responders) | Don’t know, n (% of responders) |
|---|---|---|---|---|---|
| Knowing – prompting the child that they need to go (N = 118) | 45 (38) | 46 (39) | 17 (14) | 7 (6) | 3 (3) |
| Finding – helping the child to find the toilet (N = 117) | 32 (27) | 32 (27) | 33 (28) | 18 (15) | 2 (2) |
| Accessing – helping the child to access and use the toilet (N = 118) | 52 (44) | 38 (32) | 15 (13) | 12 (10) | 1 (1) |
| Preparing – undressing the child (N = 117) | 42 (36) | 42 (36) | 17 (15) | 15 (13) | 1 (1) |
| Going – weeing and pooing (N = 117) | 26 (22) | 28 (24) | 30 (26) | 27 (23) | 6 (5) |
| Cleaning – wiping and washing the child afterwards (N = 117) | 49 (42) | 33 (28) | 21 (18) | 13 (11) | 1 (1) |
| Completing – redressing the child and helping them to return to previous activity (N = 117) | 47 (40) | 39 (33) | 16 (14) | 14 (12) | 1 (1) |
| Response option | All, n (% of responders) (N = 119) |
|---|---|
| Child’s chronological age | 47 (39) |
| Child’s developmental age | 58 (49) |
| Child’s physical functioning level | 65 (55) |
| Parent/carer request | 84 (71) |
| Doctor/nurse request or recommendation | 44 (37) |
| Parent/carer capacity | 44 (37) |
| Environmental facilities | 19 (16) |
| School request or expectation | 39 (33) |
| Constipation | 50 (42) |
| Loose stools | 33 (28) |
| UTI | 29 (24) |
| Urgency | 24 (20) |
| Bowel irritability | 20 (17) |
| Daytime wetting | 56 (47) |
| Night-time wetting | 31 (26) |
| Daytime soiling | 58 (49) |
| Night-time soiling | 34 (29) |
| I do not typically seek support for toileting on behalf of children and young people | 29 (24) |
| Other | 1 (1) |
| Response option | Non-spinal-cord-related pathology, n (% of responders) (N = 119) | Spinal cord pathology, n (% of responders) (N = 35) |
|---|---|---|
| To develop the child/young person’s understanding of toileting | 83 (70) | 16 (46) |
| For the child/young person to be accident free | 60 (50) | 10 (29) |
| To reduce the number of accidents | 62 (52) | 10 (29) |
| For the child/young person to be able to use the toilet without help | 74 (62) | 13 (37) |
| To get the child/young person in a routine for toileting | 77 (65) | 14 (40) |
| To protect the child/young person’s bladder and bowel | 33 (28) | 19 (54) |
| Never see this group | 5 (4) | 1 (3) |
| Other | 9 (8) | 2 (6) |
| Response option | All, n (% of responders) (N = 119) |
|---|---|
| Very easy | 24 (20) |
| Easy | 42 (35) |
| Hard | 40 (34) |
| Very hard | 4 (3) |
| Don’t know | 9 (8) |
| Response option | All, n (% of responders) (N = 119) |
|---|---|
| Home | 45 (38) |
| Community clinic | 31 (26) |
| Hospital clinic | 23 (19) |
| School/college/university | 54 (45) |
| Respite care | 6 (5) |
| Young person’s place of work | 0 (0) |
| Don’t know | 30 (25) |
| Other | 4 (3) |
| Response option | All, n (% of responders) (N = 119) |
|---|---|
| Yes | 33 (28) |
| No | 86 (72) |
| Response option | All, n (% of responders) (N = 110) |
|---|---|
| Parent/carer report | 80 (73) |
| Child/young person report | 37 (34) |
| Chart/checklist/questionnaire | 64 (58) |
| Physical examination | 8 (7) |
| Ultrasound | 4 (4) |
| Other imaging (e.g. bladder scanning) | 3 (3) |
| Urodynamics – invasive with catheter | 1 (1) |
| Urodynamics – non-invasive without catheter (e.g. flow rate) | 1 (1) |
| Direct observation | 52 (47) |
| Wee/poo samples | 12 (11) |
| Diet/fluid intake diaries | 46 (42) |
| Blood tests | 1 (1) |
| Other | 4 (4) |
| All | Very easy, n (% of responders) | Easy, n (% of responders) | Difficult, n (% of responders) | Very difficult, n (% of responders) | Never used, n (% of responders) |
|---|---|---|---|---|---|
| Dietary advice (N = 116) | 17 (15) | 59 (51) | 19 (16) | 1 (1) | 20 (17) |
| Fluid intake advice (N = 116) | 24 (21) | 64 (55) | 16 (14) | 2 (2) | 10 (9) |
| Behavioural intervention (e.g. reward charts, timers, alarms) (N = 115) | 46 (40) | 52 (45) | 10 (9) | 1 (1) | 6 (5) |
| Physical aids/equipment (e.g. specialised toilets, hoists, frames) (N = 116) | 30 (26) | 47 (41) | 15 (13) | 7 (6) | 17 (15) |
| Continence products (e.g. nappies, pads, pull-ups, continence pants) (N = 114) | 34 (30) | 49 (43) | 15 (13) | 4 (4) | 12 (11) |
| Medication or drugs (e.g. laxatives, anticholinergics, desmopressin) (N = 113) | 15 (13) | 37 (33) | 19 (17) | 1 (1) | 41 (36) |
| Catheterisation (N = 113) | 8 (7) | 11 (10) | 9 (8) | 3 (3) | 82 (73) |
| Colonic enema (bowel washout) (N = 112) | 1 (1) | 5 (4) | 12 (11) | 3 (3) | 91 (81) |
| Surgical intervention (e.g. cytoscopic botox, ACE, bladder reconstruction, Mitrofanoff) (N = 109) | 2 (2) | 4 (4) | 8 (7) | 2 (2) | 93 (85) |
| All | Very effective, n (% of responders) | Effective, n (% of responders) | Ineffective, n (% of responders) | Very ineffective, n (% of responders) | No knowledge/never used, n (% of responders) |
|---|---|---|---|---|---|
| Dietary advice (N = 115) | 12 (10) | 57 (50) | 18 (16) | 4 (3) | 24 (21) |
| Fluid intake advice (N = 115) | 19 (17) | 63 (55) | 16 (14) | 2 (2) | 15 (13) |
| Behavioural intervention (e.g. reward charts, timers, alarms) (N = 113) | 20 (18) | 74 (65) | 10 (9) | 1 (1) | 8 (7) |
| Physical aids/equipment (e.g. specialised toilets, hoists, frames) (N = 115) | 34 (30) | 58 (50) | 0 (0) | 0 (0) | 23 (20) |
| Continence products (e.g. nappies, pads, pull-ups, continence pants) (N = 116) | 22 (19) | 60 (52) | 18 (16) | 3 (3) | 13 (11) |
| Medication or drugs (e.g. laxatives, anticholinergics, desmopressin) (N = 112) | 7 (6) | 54 (48) | 11 (10) | 2 (2) | 38 (34) |
| Catheterisation (N = 112) | 5 (4) | 14 (13) | 0 (0) | 0 (0) | 93 (83) |
| Colonic enema (retrograde) (N = 111) | 1 (1) | 6 (5) | 1 (1) | 0 (0) | 103 (93) |
| Surgical intervention (e.g. cytoscopic botox, ACE, bladder reconstruction, Mitrofanoff) (N = 111) | 2 (2) | 7 (6) | 0 (0) | 0 (0) | 102 (92) |
| Response option | All, n (% of responders) (N = 115) |
|---|---|
| No difficulties experienced | 16 (14) |
| Child/young person’s knowledge and understanding of what is required | 67 (58) |
| Parent/carer knowledge and understanding of what is required | 54 (47) |
| Parent/carer capability and time to implement different methods | 70 (61) |
| Child/young person’s adherence to the intervention | 48 (42) |
| Delays in professional assessments | 44 (38) |
| Access to appropriate help and support | 38 (33) |
| Funding and/or resources for equipment and products | 53 (46) |
| Lack of interest/motivation to change | 40 (35) |
| Lack of consistency of support in different environments (e.g. facility at home but not at school) | 69 (60) |
| Other | 5 (4) |
| All | Supplied free of charge, n (% of responders) | Subsidised, n (% of responders) | Available to purchase, n (% of responders) | Unavailable, n (% of responders) | Don’t know/never used, n (% of responders) |
|---|---|---|---|---|---|
| Behavioural (e.g. timers, alarms) (N = 112) | 22 (20) | 3 (3) | 18 (16) | 14 (13) | 55 (49) |
| Aids or equipment (e.g. frames, steps) (N = 113) | 43 (38) | 7 (6) | 18 (16) | 13 (12) | 32 (28) |
| Housing adaptions (e.g. specialised toilets, hoists) (N = 113) | 29 (26) | 14 (12) | 9 (8) | 11 (10) | 50 (44) |
| Products (e.g. pads, nappies, continence pants, pull-ups) (N = 115) | 55 (48) | 8 (7) | 17 (15) | 13 (11) | 22 (19) |
| Medications (e.g. laxatives) (N = 113) | 47 (42) | 2 (2) | 7 (6) | 15 (13) | 42 (37) |
| Response option | All, n (% of responders) (N = 116) |
|---|---|
| Yes | 50 (43) |
| No | 37 (32) |
| Don’t know | 29 (25) |
Appendix 12 Children and young people survey results
| I can do this on my own | I need a bit of help with this | I need a lot of help with this | I cannot do this | Don’t know | |
|---|---|---|---|---|---|
| I know I need to go to the toilet without someone telling me (N = 20) | 15 (75) | 2 (10) | 1 (5) | 2 (10) | 0 (0) |
| I can hold on until I get to the toilet (N = 20) | 10 (50) | 3 (15) | 1 (5) | 4 (20) | 1 (5) |
| I can get myself in to the room or bathroom (N = 20) | 12 (60) | 4 (20) | 1 (5) | 3 (15) | 0 (0) |
| I can undress myself (N = 19) | 10 (53) | 2 (11) | 2 (11) | 5 (26) | 0 (0) |
| I can use the toilet (N = 20) | 13 (65) | 1 (5) | 4 (20) | 2 (10) | 0 (0) |
| I can wipe myself afterwards (N = 20) | 11 (55) | 4 (20) | 2 (10) | 3 (15) | 0 (0) |
| I can get dressed again (N = 20) | 9 (45) | 4 (20) | 2 (10) | 5 (25) | 0 (0) |
| I can wash my hands (N = 20) | 12 (60) | 3 (15) | 2 (10) | 3 (15) | 0 (0) |
| I can leave the toilet and go back to where I was before (N = 20) | 11 (55) | 5 (25) | 1 (5) | 2 (10) | 1 (5) |
| Very happy | OK | A bit unhappy | Very unhappy | Don’t know or this has never happened to me | |
|---|---|---|---|---|---|
| Talking to an expert about using the toilet (N = 20) | 4 (20) | 7 (35) | 5 (25) | 2 (10) | 2 (10) |
| Your parent or carer talking to an expert about how you use the toilet (N = 19) | 7 (37) | 5 (26) | 2 (11) | 2 (11) | 3 (16) |
| An expert checking your toilet at school or work (N = 20) | 7 (35) | 4 (20) | 0 (0) | 4 (20) | 5 (25) |
| Being observed using the toilet by an expert (N = 20) | 0 (0) | 4 (20) | 0 (0) | 10 (50) | 6 (30) |
| Having a physical examination (e.g. someone feeling your tummy) (N = 19) | 4 (21) | 6 (32) | 4 (21) | 1 (5) | 4 (21) |
| Being scanned (e.g. ultrasound or X-ray) (N = 20) | 4 (20) | 9 (45) | 3 (15) | 1 (5) | 3 (15) |
| Being assessed with a catheter (a tube to help you wee) (N = 20) | 2 (10) | 2 (10) | 2 (10) | 6 (30) | 8 (40) |
| Giving a wee or poo sample (N = 20) | 3 (15) | 9 (45) | 3 (15) | 3 (15) | 2 (10) |
| Having a blood test (N = 20) | 6 (30) | 5 (25) | 2 (10) | 5 (25) | 2 (10) |
| Using a food or water diary (N = 20) | 3 (15) | 10 (50) | 2 (10) | 1 (5) | 4 (20) |
| Very happy | OK | A bit unhappy | Very unhappy | Don’t know or this has never happened to me | |
|---|---|---|---|---|---|
| Using the toilet at home (N = 19) | 12 (63) | 2 (11) | 3 (16) | 1 (5) | 1 (5) |
| Using the toilet at school/college/work (N = 20) | 5 (25) | 5 (25) | 3 (15) | 5 (25) | 2 (10) |
| Using the toilet when out and about e.g. in a restaurant (N = 20) | 2 (10) | 3 (15) | 9 (45) | 5 (25) | 1 (5) |
| Very happy | OK | A bit unhappy | Very unhappy | Don’t know or this has never happened to me | |
|---|---|---|---|---|---|
| Following a special water/food diet (N = 20) | 2 (10) | 5 (25) | 7 (35) | 2 (10) | 4 (20) |
| Using an alarm or timer to remind me to wee/poo (N = 20) | 0 (0) | 6 (30) | 1 (5) | 2 (10) | 11 (55) |
| Using a hoist or a frame to help me use the toilet (N = 20) | 1 (5) | 7 (35) | 1 (5) | 1 (5) | 10 (50) |
| Using pads, nappies or pull-ups (N = 20) | 3 (15) | 3 (15) | 1 (5) | 5 (25) | 8 (40) |
| Taking medication to help me wee/poo (N = 20) | 4 (20) | 2 (10) | 5 (25) | 1 (5) | 8 (40) |
| Using a catheter or bowel washout (tubes that help you to wee or poo) (N = 20) | 1 (5) | 2 (10) | 0 (0) | 5 (25) | 12 (60) |
| Having surgery (N = 20) | 2 (10) | 0 (0) | 2 (10) | 6 (30) | 10 (50) |
List of abbreviations
- ACE
- antegrade continence enema
- ADHD
- attention deficit hyperactivity disorder
- ARM
- anorectal malformations
- CIC
- clean intermittent catheterisation
- DVSS
- Dysfunctional Voiding Scoring System
- EPHPP
- Effective Public Health Practice Project
- ERIC
- The Children’s Bowel & Bladder Charity
- ICF
- International Classification of Functioning, Disability and Health
- MACE
- Malone antegrade continence enema
- NIHR
- National Institute for Health Research
- OECD
- Organisation for Economic Co-operation and Development
- PDMS
- polydimethylsiloxane
- PEMQOL
- Paediatric Enuresis Module to assess Quality Of Life
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QoL
- quality of life
- RCE
- retrograde continence enema
- RCT
- randomised controlled trial
- SNM
- sacral neuromodulation
- TAI
- transanal irrigation
- TRI
- transrectal irrigation
- UTI
- urinary tract infection
Glossary
- Anal plug
- A device inserted into the anus or lower rectum to reduce the risk of faecal soiling.
- Anorectal malformation
- A developmental anomaly of the anus and rectum. They have a higher incidence of spinal anomalies and myelodysplasia (see Myelodysplasia).
- Antegrade continence enema
- A technique in which the bowel can be emptied by passing fluid through a surgically created tract from an opening on the abdominal wall directly into the bowel (usually the caecum, or more distally). The aim is to reduce the risk of involuntary soiling.
- Appendicostomy
- A surgically created opening of the appendix on the abdominal wall to create a passage between the skin and bowel, usually for bowel washouts (see Antegrade continence enema and Malone antegrade continence enema).
- Azrin and Foxx method
- A technique of toilet training developed in the 1970s that involves teaching a child to toilet (urinate in the potty) through repetition. It includes other approaches, such as praise, verbal disapproval and corrective action.
- Biofeedback
- A technique that involves using electronic aids to supplement visual or auditory feedback to improve the control of a normal involuntary bodily function.
- Bladder neck sling (with and without enterocytoplasty; see Enterocystoplasy)
- Endogenous or exogenous material surgically inserted at the bladder neck to reduce the risk of urinary leakage.
- Botulinum toxin
- A substance that can cause temporary paralysis (see Intradetrusor injections of Botulinum toxin type A).
- Catheter
- A tube for admitting or removing gases or liquids to and from parts of the body, such as urine from the bladder (see Foley catheter).
- Clean intermittent catheterisation
- The intermittent passage of a (usually disposable) catheter into the bladder to drain urine and empty the bladder. The catheter (see Catheter) can be passed through the urethra or via a surgically created passage such as a Mitrofanoff (see Mitrofanoff procedure), which usually uses the appendix as the conduit between the skin and the bladder. This can be performed by the patient (see Clean intermittent self-catheterisation) or by a carer. [This does not apply to the passage of a catheter via an appendicostomy (see Appendicostomy) for colonic washouts (see Antegrade continence enema and Malone antegrade continence enema).]
- Clean intermittent self-catheterisation
- Clean intermittent catheterisation (see Clean intermittent catheterisation) performed by the patient instead of a carer.
- Collagen injection
- A surgical procedure that involves cystoscopy, usually under general anaesthetic, and injection of collagen as a bulking agent into the bladder neck to narrow it to prevent leakage, thus avoiding more formal surgery to the bladder neck (see Polydimethylsiloxane and Teflon).
- Continence
- The ability to retain urine or faeces in the bladder or bowel, respectively, until the conditions are appropriate for micturition or defecation.
- Enterocystoplasy
- A surgical procedure that uses a segment of bowel to increase the capacity of the bladder. (In children with neurogenic dysfunction, this is often performed along with other surgical procedures, e.g. antegrade continence enema and bladder neck operations.)
- Family Faculty
- A public involvement panel comprising parent carers of children with neurodisability and some young adults with neurodisability who work in partnership with the PenCRU research team as experts by experience.
- Foley catheter
- A catheter (tube) passed into the bladder to allow the continuous drainage of urine, as opposed to clean intermittent catheterisation (see Clean intermittent catheterisation). The Foley catheter is a self-retaining catheter with a balloon that remains in the bladder but can be inflated and deflated using an intrinsic fine tube with an external valved opening.
- Intradetrusor injections of Botulinum toxin type A
- Injection of Botulinum toxin type A (see Botulinum toxin) into the detrusor (bladder wall muscle) to paralyse it, thus preventing avoiding leakage caused by involuntary detrusor contractions. The injections are administered via a cystoscope (telescope/endoscope inserted via the urethra to access the inside of the bladder). This can avoid more major surgical procedures.
- Intraurethral self-retaining device
- A device inserted into the urethra to reduce the risk of urinary leakage.
- Intravesical electrical stimulation
- See Transurethral intravesical electrotherapy.
- Leadbetter–Mitchell bladder neck revision
- A surgical procedure to narrow the bladder neck and reduce the involuntary leakage of urine (see Young–Dees bladder neck reconstruction and Pippi Salle bladder neck repair).
- Malone antegrade continence enema
- See Antegrade continence enema. The technique was invented by a British paediatric urologist, Mr Padraig Malone.
- Mitrofanoff procedure
- A surgical procedure in which the appendix is used to create a channel through which a catheter (see Catheter) can be passed from the skin to the bladder to allow the drainage of urine from the bladder (see Clean intermittent catheterisation).
- Modified intensive toilet training method
- A modification of the Azrin and Foxx technique (see Azrin and Foxx method).
- Myelodysplasia
- The defective development of any part of the spinal cord, especially the lower segments. This is an umbrella term that includes spina bifida,* myelomeningocele,* sacral agenesis and tethered cord syndrome. People with myelodysplasia are likely to have a neurogenic* (neuropathic) bladder and/or bowel (*see above and below).
- Myelomeningocele
- A type of spina bifida whereby the dysplastic meninges and spinal cord protrude through a defect in the posterior vertebral arches to extend beyond the spinal canal (see Myelodysplasia and Spina bifida).
- Nappy
- Any disposable product used to contain incontinence. This may include a pad with or without a fixation pant, a ‘nappy-style’ product, a belted product and pull-up pants.
- Neurogenic (bladder/bowel)
- Bladder/bowel dysfunction due to a brain, spinal cord or nerve problem.
- Neuropathic (bladder/bowel)
- Synonymous with neurogenic (see Neurogenic).
- Non-spinal-cord-related pathology
- In this context, children with continence problems associated with neurodisability but excluding children with any form of spinal cord pathology (e.g. myelodysplasia or spinal trauma; see Myelodysplasia).
- Orthograde enema
- Synonymous with antegrade continence enema.
- Pelvic floor interferential electrostimulation
- Interferential current, a form of electrical stimulation to the bladder and pelvic floor delivered by adherent skin electrodes on the abdominal wall for 20 minutes three times per week. The aim is to alter neuromuscular function of the bladder to improve continence.
- Peristeen
- A commercial device for performing transanal irrigation (see Transanal irrigation).
- Pippi Salle bladder neck repair
- A surgical procedure to narrow the bladder neck and reduce the involuntary leakage of urine (see Leadbetter–Mitchell bladder neck revision and Young–Dees bladder neck reconstruction).
- Polydimethylsiloxane
- A substance that can be injected into the bladder neck as a bulking agent to reduce the involuntary leakage of urine (see Collagen injection and Teflon).
- Professional Advisory Group
- ERIC’s Professional Advisory Committee, comprising 12 health professionals across medical, nursing and allied health professions with expertise in the field of childhood bowel and bladder health.
- Pseudocontinence
- Continence achieved using artificial methods to empty the bowel or bladder, rather than under voluntary control.
- Retrograde colonic enema
- A technique in which the bowel can be emptied by passing fluid through the anus into the bowel to evacuate faeces. The aim is to reduce the risk of involuntary soiling.
- Sacral neuromodulation
- An implanted medical device that can help improve bladder function by sending electrical signals to the nerves that control the bladder and pelvic floor.
- Spina bifida
- The embryologic failure of fusion of one or more vertebral arches; there may be associated abnormalities of the spinal cord and meninges that surround the spinal cord (see Myelodysplasia and Myelomeningocele).
- Spinal cord pathology
- An abnormality in the structure or function of the spinal cord, whether congenital or acquired.
- Stoma
- An opening of a viscus (e.g. bowel, bladder) on to the skin, usually of the abdominal wall (see Appendicostomy).
- Teflon™
- A substance that can be injected into the bladder neck as a bulking agent to reduce the involuntary leakage of urine (see Collagen injection and Polydimethylsiloxane).
- Toilet training
- The teaching of a child how to empty their bowel and bladder into a toilet (or potty) at socially appropriate times so that nappies are no longer necessary.
- Transanal irrigation
- Transanal bowel irrigation is a way of facilitating the evacuation of faeces from the bowel by introducing water or other fluids into the colon via the anus in a quantity sufficient to reach beyond the rectum. It is used as a treatment for severe chronic constipation and also for patients with neurogenic bowel dysfunction to reduce the risk of faecal incontinence. The aim is to empty the sigmoid colon and rectum, and possibly also the descending colon.
- Transcutaneous electrical nerve stimulation
- A form of electrical stimulation delivered used therapeutically aiming to improve neuromuscular function or reduce pain.
- Transrectal bowel stimulation
- Intermittent therapy using electrical stimulation administered using electrodes placed inside the rectum with the aim of improving rectal function.
- Transrectal irrigation
- Synonymous with Transanal irrigation.
- Transurethral intravesical electrotherapy
- A technique aimed at modifying bladder function by intermittently delivering a weak electrical current to the inside of the bladder using a urethral catheter.
- Urodynamic biofeedback treatment
- A technique using urodynamics (see Urodynamics) to supplement other visual, sensory or auditory feedback to improve bladder control (see Biofeedback).
- Urodynamics
- Methods of measuring bladder function using ultrasound or X-ray imaging of the bladder, urinary flow rates, electromyography to measure pelvic floor muscle activity, or catheters with pressure transducers to measure pressure within the bladder.
- Wireless moisture pager
- A moisture-sensitive device placed inside a nappy or underwear that sends a signal to a remote sound box when the sensor becomes wet with urine. The device can also be wired and the sound box attached to the child’s clothing. Also referred to as a wetting alarm.
- Young–Dees bladder neck reconstruction
- A surgical procedure to narrow the bladder neck and reduce the involuntary leakage of urine (see Leadbetter–Mitchell bladder neck revision).
