Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/144/05. The contractual start date was in May 2020. The draft report began editorial review in December 2020 and was accepted for publication in July 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimers
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 McFadden et al. This work was produced by McFadden et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 McFadden et al.
Chapter 1 Introduction
Background and context
The frequency of feeding and volume of milk intake of healthy term-born infants is generally dictated by the infant’s appetite. Term infants exhibit feeding and satiation cues and adjust their volume of intake to compensate for differences in the nutrient density of various milks. 1 In contrast, enteral feeds for preterm infants are usually given as prescribed volumes at scheduled intervals. 2 However, there is some evidence that preterm infants can self-regulate their intake. 3 Furthermore, while feeding cues may be more difficult to detect in preterm infants, they may be sufficiently evident for a parent or caregiver to recognise and respond to,4 thereby supporting safer and more successful feeding experiences. Caregivers and parents can use infants’ physiological and behavioural channels of communication to inform their feeding decisions and actions. This may also set the scene for future feeding practices and success, as well as parent–infant interaction. Although studies have shown that cue-based (also known as responsive) feeding is feasible for preterm infants, the adoption of cue-based feeding has been constrained by the ‘schedule- and volume-driven culture’ in many neonatal units (NNUs). 5
Alternatives to scheduled interval feeding regimens that aim to respond to infant feed cues have been described. 6 These are relevant to the transition from gastric tube feeding to oral feeding,7 when preterm infants are developing periods of sustained alert activity and a suck–swallow–breathe pattern8 sufficient for oral feeding to commence. 1
Cue-based feeding is a co-regulated approach. 6 The enteral feeding process starts when the caregiver recognises infant cues that indicate readiness to feed and ends when the infant demonstrates satiation. The infant, therefore, determines the timing, duration and volume of intake. At each stage during transition to oral feeding, through understanding and interpretation of their cues, infants are supported in such a way that they can achieve all they are capable of with regards to oral feeding. Cue-based feeding occurs alongside supplementary tube feeding with the understanding that, developmentally, many preterm infants are not yet ready to fully sustain themselves by oral feeding. In modifications of cue-based feeding, caregivers may pre-set a maximum permitted duration of inactivity or sleep between feeds or a maximum volume of intake or modify feeding plans to consider the reduced endurance levels of preterm infants.
The transition to oral feeding is a critical developmental stage for preterm infants. Cue-based feeding may be considered a part of an integrated approach to providing ‘developmental care’ when infants are seen as individuals and caregivers are guided by the needs and behaviours of the infant. 9
Cue-based feeding may also increase rest between feeds promoting infant-led sleep and wake patterns. Potentially, infant-led feeding patterns will facilitate development of organised behaviour states leading to earlier establishment of oral feeding and thereby shortening hospital stay for preterm infants. 1
Reducing length of hospital stay has a direct effect on hospital costs and may also decrease cot occupancy in NNUs, thus reducing the need for inter-hospital transfer of women and infants. 10 Compared with a scheduled approach, cue-based feeding may support infant stability during oral feeds as infants’ cues will be responded to, resulting in fewer episodes of physiological instability, which could cause significant harm (e.g. aspiration, desaturation and bradycardia events). 11 As feeding an infant is a primary activity over the first year of life and a major preoccupation of parents, it is anticipated that there may be other benefits for the family and caregivers of cue-based feeding, principally allowing parents to feel more directly involved with their infant’s care and better understand their infant’s communication, together with increasing parental confidence and ability to recognise and respond to their infant’s needs during their hospital stay and beyond. Enhanced parental satisfaction is a key quality indicator in measuring the effectiveness of family-integrated care in neonatal services. 12
Potential adverse effects of cue-based feeding for preterm infants are recognised. These mainly relate to whether or not such a regimen can guarantee metabolic stability, particularly normoglycaemia, in this vulnerable group. Even at the point of discharge home from hospital, some preterm infants are known to be susceptible to hypoglycaemia if a scheduled enteral feed is omitted or delayed. 13 Concern exists that repeated or prolonged episodes of hypoglycaemia may impair longer-term growth and development. 14 There may be more acute problems relating to gastrointestinal immaturity, such as feeding intolerance and a higher risk of aspiration of gastric contents into the lungs, as well as concerns that allowing unrestrained volumes of enteral intake may increase the risk of gastro-oesophageal reflux or feed intolerance. However, there is a lack of evidence of these potential adverse effects and, indeed, some problems may be exacerbated by not responding to the infant’s cues. 11 Despite these concerns, and despite a lack of evidence of benefit, cue-based feeding is established in some NNUs in other countries (e.g. Sweden15,16 and the USA17,18) and is increasingly being used in NNUs in the UK. 19 Cue-based feeding for preterm infants is now recommended as a method to increase the duration of breastfeeding in the United Nations Children’s Fund (UNICEF) Baby Friendly Initiative (BFI) ‘Ten steps to successful breastfeeding’. 20
Overall, the evidence to support cue-based feeding is limited. A recent Cochrane review1 concluded that there was low-quality evidence that cue-based feeding compared with scheduled feeding leads to earlier transition to full oral feeding. The review authors noted that this evidence should be treated with caution owing to several methodological weaknesses. 1 Furthermore, there is a lack of strong or consistent evidence of the effect of cue-based feeding compared with scheduled feeding on important outcomes for preterm infants or their families. 1 Therefore, there is a need for rigorous evaluation of cue-based feeding for preterm infants within the NHS setting, based on the most up-to-date and complete evidence, and considering stakeholders’ (including parents’) views. The first step towards this is to assess if such an intervention trial is justifiable and feasible.
Research objectives
The overall aim of this study was to develop a manualised intervention and to assess whether or not it is feasible to conduct a clinical and cost-effectiveness study of cue-based compared with scheduled feeding for preterm infants in NNUs.
The study had eight research objectives:
-
to describe the characteristics, components, theoretical basis and outcomes of approaches to feeding preterm infants transitioning from tube to oral feeding, including by feeding type (breast milk, donor breast milk, formula, combined) and method (breastfeeding, bottle feeding)
-
to identify operational policies, barriers and facilitators, and staff and parents’ education needs in NNUs implementing cue-based feeding
-
to co-produce an evidence-informed, adaptable, manualised intervention, including staff and parent educational support for feeding preterm infants at the transition from tube to oral feeding in response to feeding cues and signs of infant stability
-
to appraise the willingness of parents and staff to implement and sustain the intervention
-
to assess the associated costs of implementing cue-based feeding in NNUs
-
to determine the feasibility and acceptability of conducting a future randomised controlled trial (RCT), including views on important outcomes of parents, staff and service commissioners
-
to scope existing data-recording systems and potential short- and long-term outcome measures (e.g. feeding outcomes, length of time to transition to full oral feeding, length of stay in NNUs, adverse events, infant growth, parent–infant attachment and well-being, and parent and staff satisfaction)
-
to determine key stakeholders’ views based on the evidence from our study (objectives 1–7) of whether or not a RCT of this approach is feasible and what the components of a future study would look like.
Overview of the study and structure of the report
The study comprised four work packages (WPs) based on the Medical Research Council principles for developing and evaluating complex interventions21 and process evaluations. 22 The study flow chart is shown in Figure 1.
FIGURE 1.
Study flow chart.
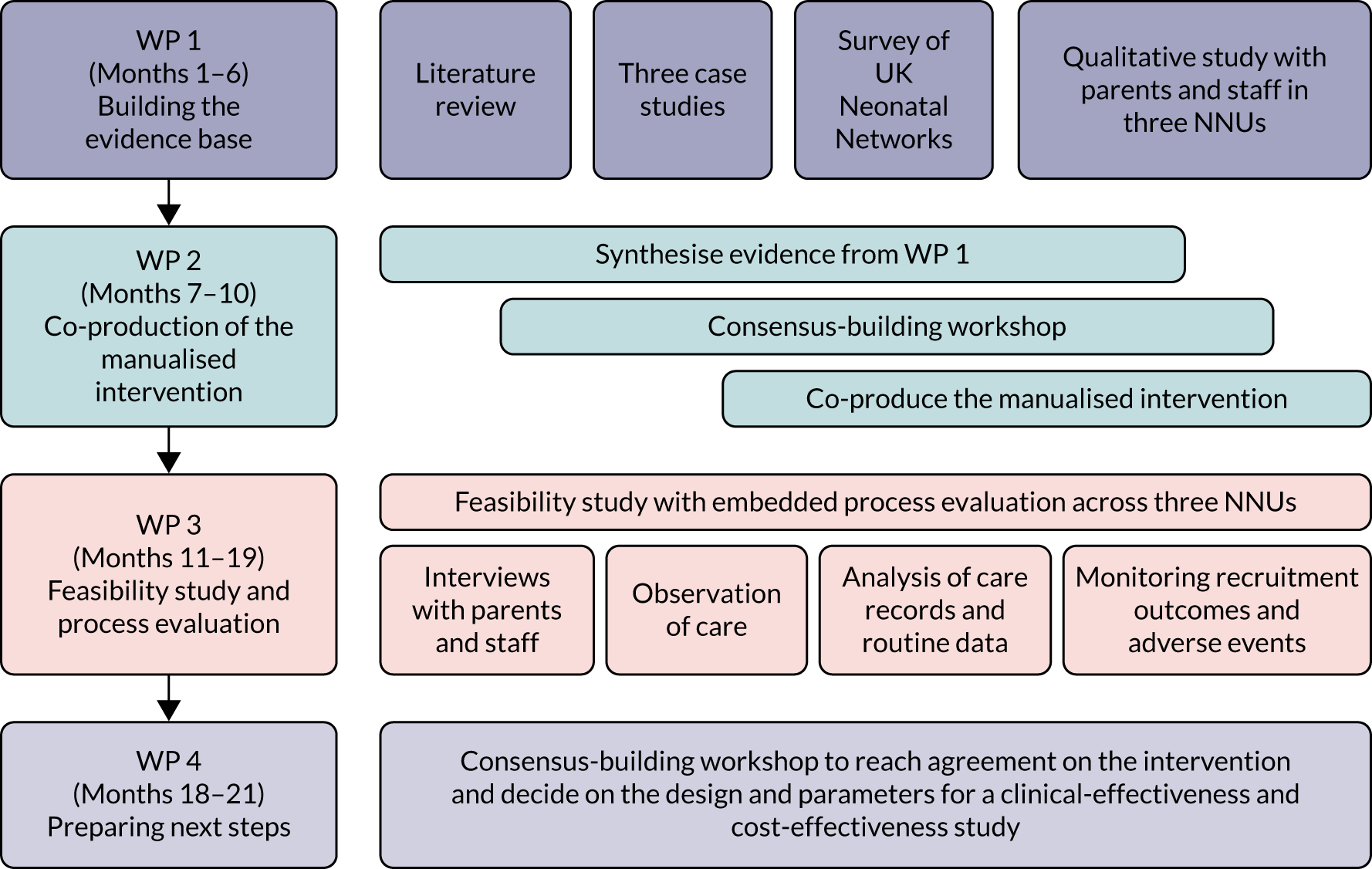
Work package 1: building the evidence base
To inform the content and methods of the manualised intervention, WP 1 comprised a systematic literature review; three case studies of NNUs with embedded cue-based feeding; telephone interviews with senior members of staff from NNUs across the UK to assess variation in approaches to the transition from tube to oral feeding; and qualitative research with parents and staff in three NNUs to gain their perspectives on cue-based feeding. The findings of this WP are presented in Chapters 3–6.
Work package 2: co-production of the intervention
To develop an evidence-informed, adaptable, manualised intervention, WP 2 comprised the synthesis of the evidence from WP 1; a consensus-building workshop with relevant stakeholders, including parents and third-sector organisations, to agree the intervention components; and co-production of the manualised intervention including behaviour change techniques (BCTs), training packages and commissioning of a short film of infant cues. A description of the methods and the final intervention are presented in Chapter 7.
Work package 3: feasibility study and process evaluation
To determine feasibility, WP 3 comprised a feasibility study with an embedded process evaluation22 to assess the willingness of parents and staff to implement the intervention, explore the design of a future study, and determine the feasibility and acceptability of conducting a future RCT. The intervention was implemented in three NNUs across the UK. The methods and findings of this WP are presented in Chapters 8–10.
Work package 4: preparing next steps
Work package 4 comprised synthesising the findings of the previous WPs and a stakeholder workshop to explore preferences, reach agreement on the manualised intervention and produce a framework for decision-making on the design, feasibility and acceptability of a future clinical and cost-effectiveness study of cue-based compared with scheduled feeding for preterm infants. We used the ADePT (A process for Decision-making after Pilot and feasibility Trials) to guide our analysis. The process and outcomes of this WP are presented in Chapter 11.
In Chapter 2 we describe how stakeholders, including parents, were involved in the study. In Chapter 12 we discuss the findings of the study, its strengths and weaknesses, and the implications of our work for taking forward the Cue-based versus scheduled feeding (Cubs) intervention.
Chapter 2 Patient, public and wider stakeholder involvement
This chapter provides an overview of our approach to patient and public involvement and wider stakeholder engagement including health-care professionals, third-sector organisations and researchers. Throughout this report, we use the term parent involvement instead of patient and public involvement. There is further detail of how parent and stakeholder involvement contributed to the implementation of the research threaded through the remainder of the report.
Pre submission of the funding application
We involved parents in preparing the application for funding to conduct the study. Through two third-sector charitable organisations [Bliss (London, UK) and TAMBA (Twins and Multiple Births Association) (now Twins Trust, Aldershot, UK)] which support parents and infants in NNUs, we discussed the study design with five women with recent experience of the transition from tube to oral feeding with a preterm infant in a NNU. The women had experienced scheduled feeding moving to cue-based feeding near discharge from the NNU. These discussions highlighted the importance of education for parents so that they understand the rationale for cue-based feeding and can identify their infant’s feeding cues. We learned that staff training is needed so that information and support for parents is consistent while also tailored to individual need. The women highlighted the need for the intervention to support breastfeeding as well as formula feeding and suggested important outcomes were time to discharge from the NNU and breastfeeding rates. The research team included a co-investigator from the neonatal charity Bliss, who contributed to the design of the study (in particular, the parent involvement component and suggestions to incorporate parents’ recording of outcome data in the intervention).
Parent involvement
Bliss was critical to facilitating parent involvement in the Cubs study. As well as having a member of Bliss on the research team, Bliss hosted the Parents’ Panel and the Bliss research team member attended all the research team meetings, Stakeholder Advisory Group meetings, and the workshops in WP 2 and WP 4.
Through an advertisement on the Bliss website, we recruited six parents with relevant experience of having an infant in a NNU to form a Parents’ Panel. Over the course of the study, three teleconferences were held with the Parents’ Panel. Activities of the Parents’ Panel included reviewing the participant information sheets prior to submission for ethics approval, co-designing the ‘Our Feeding Journey’ document that was an integral part of the intervention, and reviewing the intervention components including the posters and film. The Parents’ Panel reviewed and made many suggestions for the script of the film. The Parents’ Panel also suggested solutions to some of the challenges encountered during WP 3, for example offering alternatives to the telephone follow-up. Members of the Parents’ Panel attended the workshops in WPs 2 and 4.
Two members of the Parents’ Panel were also members of the Stakeholder Advisory Group and the Study Steering Committee. They were involved in all Stakeholder Advisory Group meetings including the final one at which they contributed to discussion of the findings, and options for optimising the intervention and research.
Stakeholder involvement
Other than parents, the main stakeholders for our work were health-care practitioners working in NNUs and researchers with an interest in neonatal care and/or intervention development and feasibility studies. We involved these stakeholders through our Stakeholder Advisory Group and the two study workshops (WPs 2 and 4). The Stakeholder Advisory Group comprised two neonatal care practitioners (one from England and one from Scotland), a representative from UNICEF UK BFI National Neonatal Network, an associate professor from Sweden with an interest in neonatal care and three researchers (a health economist, a statistician and an expert in using the ADePT framework, all from Scotland). The group met four times during the study and provided advice on the intervention and the research, including suggesting solutions to overcome challenges (e.g. slow recruitment). Wider stakeholder engagement was also achieved through the consensus-building workshops in WPs 2 and 4. The methods and findings of these workshops are reported in Chapters 7 and 11.
The parental and wider stakeholder involvement was critical to the study and had many benefits, as can be seen throughout this report. Parental engagement was better at the beginning of the study; for example, five parents attended the first workshop but only one parent attended the final workshop. However, engagement with the Parents’ Panel remained consistent.
Chapter 3 Literature review
In this chapter we report the aims, methods and findings of the systematic review of the literature and the analysis of cue-based policies and guidelines. The review was conducted to update and build on the existing Cochrane review. 1 Although Watson and McGuire1 included only RCTs, our review included studies of any design to synthesise additional information on underpinning theories, components, characteristics and outcomes of interventions, and economic evaluations.
Review aims and questions
The aims of the systematic review were to synthesise existing evidence on the components, characteristics, theoretical basis and associated BCTs23 of interventions, infant and parent outcomes and any economic evaluations.
The specific review questions were as follows.
In studies examining the effectiveness of approaches to feeding preterm infants transitioning from tube to oral feeding:
-
What are the study characteristics (participants, interventions, comparisons, context)?
-
What are the specific components of the interventions?
-
What, if any, is the theoretical basis of any intervention being tested?
-
What specific outcomes have been tested and what is the magnitude and direction of any effect?
We examined the nature of the evidence regarding the feeding type adopted (i.e. mother’s breast milk, donor breast milk, formula, any combination of these) and by feeding method (breastfeeding, bottle feeding, other methods such as cup feeding).
Review methods
The review protocol was registered on PROSPERO (registration number CRD42018097317).
Search strategy
The search strategy was informed by the existing Cochrane Review. 1 Searches were conducted in August 2018. We initially searched for existing systematic reviews from Cochrane, Campbell and Centre for Reviews and Dissemination (CRD)/Database of Abstracts of Reviews of Effects (DARE) databases. This was followed by searches of key online databases to identify other kinds of intervention studies (e.g. cohort studies; controlled trials; interrupted time series designs; controlled before-and-after studies; and programme evaluations). The following databases were searched: MEDLINE; EMBASE; Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Database of Systematic Reviews (CDSR); Health Technology Assessment (HTA) database; Cochrane Central Register of Controlled Trials (CENTRAL); Social Science Citation Index; PsycInfo® (American Psychological Association, Washington, DC, USA); Health Management Information Consortium (HMIC); Applied Social Sciences Index and Abstracts (ASSIA); Social Policy and Practice; Bibliomap; Database of Promoting Health Effectiveness Reviews (DoPHER); Trials Register of Promoting Health Interventions (TRoPHI); Social Care Online; British Nursing Index; Research Councils UK; OAIster and OpenGrey. We also carried out searches using the Google (Google Inc., Mountain View, CA, USA) advanced interface to identify relevant research on NHS and UK government sites. The search strategy used subject headings (specific to each database), key words and free-text search terms. We also used truncation and wild cards in order to increase sensitivity. No language restriction was applied to the search; however, studies were excluded at the full-text screening stage if an English translation was not available. The search architecture is shown in Box 1. The full search strategy for Ovid databases is presented in Appendix 1.
-
Pre-term/neonates OR synonyms.
-
Cue-based feeding OR synonyms.
-
1 and 2.
-
Limit to 6 years.
-
Remove duplicates from 4.
Study selection
The inclusion and exclusion criteria were set a priori and were informed by the trials included in the previous Cochrane review. 1 Publications were included if they reported empirical findings of an investigation that aimed to evaluate the effectiveness and/or cost-effectiveness of any intervention designed to support developmentally normal preterm infants born before 37 weeks’ gestation to transition from tube to oral feeding. To be included, infants had to be clinically stable, at least partially enterally fed and have an intragastric tube in place at the start of the study. Studies of multiple births were also included. We excluded studies of infants > 37 weeks’ gestation, preterm infants who had transitioned to full oral feeding, infants with major congenital anomalies, gastrointestinal disorders (e.g. necrotising enterocolitis), congenital infections and major neurological conditions (e.g. cerebral palsy, seizures, grade III–IV intracranial haemorrhage, periventricular leukomalacia) and infants whose parent(s) did not give consent for inclusion in the study. Studies were also excluded if they did not report methods and data.
Titles and abstracts were screened independently for inclusion by two reviewers. The full texts of relevant studies were retrieved and screened independently by two members of the research team. Any disagreement between reviewers was resolved through discussion with a third reviewer.
Data extraction and synthesis
All included studies were categorised based on their methodology and research questions. Data were extracted regarding (1) study country, (2) design, (3) characteristics (participants, interventions, comparisons, context), (4) intervention components, (5) theoretical basis for the intervention including BCTs and (6) outcomes including the magnitude and direction of any effect. Intervention BCTs were coded independently by two reviewers using the BCT taxonomy. 24 Coders were trained in BCT coding through an online course (URL: www.bct-taxonomy.com; accessed 9 December 2020), a 93-item taxonomy to identify BCTs within behavioural interventions. After initial coding, coders met to cross-verify coding and reach consensus when there were differences.
Quality assessment
All included studies were assessed for methodological quality according to individual elements of quality. The Cochrane Collaboration Risk of Bias tool was used to assess risk of bias in RCTs. 25 As there is no universal tool to encapsulate all domains of risk of bias in non-randomised trials, we used the recommendations of chapter 13 of the Cochrane Handbook of Systematic Reviews of Interventions. 24
Synthesis
Owing to the diversity of the evidence, the findings have been synthesised narratively.
Results
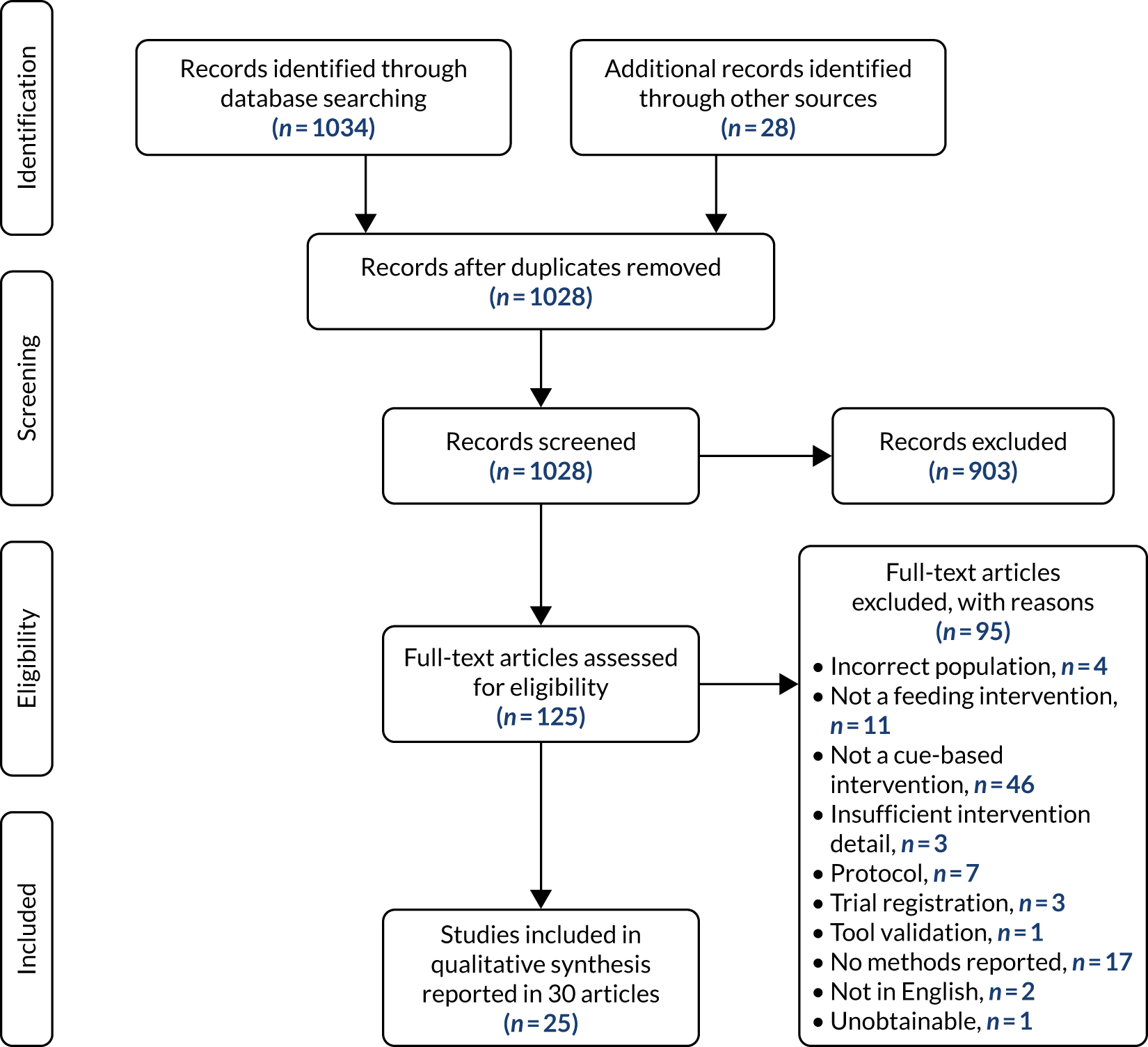
As a result of the search strategy, we screened 1028 studies and subsequently included a total of 25 unique studies reported in 30 publications (Figure 2 reports the numbers of records at each stage in the screening process).
FIGURE 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection.
Study characteristics
Twenty-five studies met the inclusion criteria and were included in this review. Studies were heterogeneous regarding methodology, inclusion/exclusion criteria and reported outcomes. All were published between 1982 and 2018, almost half (n = 12) since 2015. We found no economic evaluations.
The countries the studies were conducted in were the USA (n = 16),18,26–40 Canada (n = 4),41–44 Italy (n = 2),45,46 Australia,15,47 Sweden16 and the UK. 48 Fourteen of the intervention sites were neonatal intensive care units (NICUs) of level 3 or above;26,27,29,33,35–39,42,44–46,48 two were classified as below level 3. 28,43 The remaining studies did not specify the unit level (n = 9). 15,18,30–32,34,40,41,47
Regarding study design, 10 studies were RCTs, nine of which compared cue-based feeding with prescribed volume-driven scheduled feeding30–32,35–37,39,41,44 and one compared assessment of feeding cues at 3- and 6-hourly intervals. 29 Of the remaining 15 studies, nine reported quality improvement projects introducing cue-based feeding to protocols. 26–28,34,38,40,42,43,47 All but one study38 compared pre- and post-implementation infant outcomes. Likewise, two of the six observational studies compared intervention group outcomes with a historic cohort of preterm infants. 18,33 Of the remaining four, three did not have a comparison group15,45,46 and White and Parnell’s48 observational study documented experiences of introducing and implementing a cue-based feeding intervention.
Study participants were either preterm or very preterm infants. Infants were included in the studies based on their age either at birth or at the start of the intervention. Most studies classified the participant’s age based on gestation; however, post-conceptional age and post-menstrual age were also used. Infant characteristics such as sex, birthweight, additional medical complications and ethnicity were recorded in several studies and were tested as confounding factors in analyses. Maternal characteristics were not reported in 22 studies; among those that did report maternal characteristics,29,38,44 the information recorded was not consistent. Only nine studies reported whether infants were breastfed or bottle fed. 26,27,31–34,36,43,44
Common exclusion criteria included infants with major neurological, congenital, chromosomal and gastrointestinal complications that may affect an infant’s ability to feed. Mechanical respiratory support was also a common exclusion criterion; however, Dalgleish et al. 42 and Davidson et al. 27 included infants with respiratory complications. Only one study did not report exclusion criteria. 34
Interventions and their specific components
Over half of the studies (n = 14) implemented a specifically designed cue-based feeding protocol. Six studies27,28,33,34,40,46 used Ludwig and Waitzman’s infant-driven feeding protocol49 (or a modified version). The remaining feeding interventions included an adapted version of the Anderson Behavioural State Scale,50 Glass and Wolf’s Stepwise Oral Feeding in Infants Scale,51 the Preterm Infant Breastfeeding Behaviour Scale52 or the Co-Regulated Feeding Intervention. 53 Two of these scales had been published previously by an author of the included study. 15,38 The components of each intervention are detailed in Appendix 2.
Intervention training
Eighteen of the studies described training neonatal staff and parents to recognise infant cues. Over half of the studies (n = 14) documented examples of feeding cues; however, only nine discussed stop cues. Ten studies included recognising a successful feed. Only four studies described all three elements. 18,27,42,48 Although all studies explored the impact of cue-based feeding on preterm infant outcomes, schedules were still evident in the feeding protocols. Fourteen studies had maximum time limits, which, if exceeded, meant that gavage feeds would be used. Likewise, if a full feed was not taken within a specified time, the remaining milk was given by tube to ensure that the infant received the prescribed intake. Just over half of the studies included fidelity testing achieved through observations, and video-recordings of feeds with inter-rater reliability testing by researchers.
Assessing study quality
We classified the 25 included studies into three main types: (1) RCTs (10 studies), (2) non-randomised prospective studies with a control group (three studies) and (3) not experimental, not prospective or did not report any information about a comparison group (12 studies). We assessed RCTs using the Cochrane Risk of Bias tool25 and non-randomised prospective controlled studies using the ROBINS-I (Risk Of Bias In Non-randomised Studies – of Interventions54) tool. The remaining studies were not assessed as, by their nature, they were already considered to be at high risk of bias (Table 1).
| RCT (first author) | Domain of biasa | |||||
|---|---|---|---|---|---|---|
| Randomisation | Allocation | Blinding P | Blinding O | Incomplete O | Selective R | |
| Collinge41 | ? | ? | H | ? | L | ? |
| Gray29 | L | L | H | ? | L | L |
| Kansas30 | ? | ? | H | ? | ? | ? |
| McCain31 | L | ? | H | ? | ? | ? |
| McCain32 | L | ? | H | H | L | ? |
| Pridham35 | L | ? | H | ? | ? | ? |
| Pridham36 | L | ? | H | ? | H | ? |
| Puckett44 | L | H | H | ? | ? | ? |
| Saunders37 | ? | L | H | ? | H | ? |
| Waber39 | H | H | H | ? | H | ? |
We assessed risk of bias in the included RCTs (see Table 1). We extended the risk-of-bias assessment adopted in the original Cochrane review1 to include all elements of bias as well as assessing risk of bias in the newly included study (Gray et al. 29).
Blinding of participants and personnel was not possible in studies of this type of intervention, hence all studies were assessed as high risk of bias for this element. Limited reporting across studies made it difficult to assess all methodological elements, hence the relatively common use of the assessment ‘unsure’ (‘?’) across the data set. Although blinding was an issue in the latest study to be included in the review,29 it was assessed in all other elements to be of low risk.
We also assessed risk of bias in the non-randomised studies we included that had a control group but were not RCTs (Table 2).
| Non-RCT (first author) | Domain of biasa | ||||||
|---|---|---|---|---|---|---|---|
| Confounding | Selection | Classification | Deviations | Missing data | Outcome | Selective R | |
| Kirk18 | H | H | L | L | L | L | L |
| Ward47 | ? | ? | ? | ? | ? | ? | ? |
| Wellington40 | H | H | L | L | L | H | L |
The studies by Kirk et al. 18 and Wellington et al. 40 were potentially at high risk of bias due to confounding and selection of participants. It was difficult to assess the study by Ward et al. 47 since only a conference abstract was available from which to understand the methods applied.
We did not assess risk of bias in the 12 studies15,26–28,33,34,38,42,43,45,46,48 that were not experimental, not prospective or did not report any information about a comparison group, as, by their nature, such studies are deemed to be at risk of bias due to limitations with the methods that they employ.
Across all the studies (randomised and non-randomised) included in this review, methodological shortcomings across the data set undermine confidence in the findings generally. Only one study, the most recently found RCT published by Gray et al. ,29 was deemed to be at low risk of bias across the majority of the key bias domains (blinding of participants and personnel excepted).
Theory and behaviour change
Only two studies discuss the theoretical basis of their interventions. Thoyre et al. 38 credit two theories, namely the dynamic systems theory55 and guided participation,56,57 as the driving concepts for the extension of the co-regulated feeding intervention used in their research. Messer33 cites the synactive theory58 of Als as the theoretical base of the intervention developed for her doctoral study.
We mapped all the studies’ intervention components according to 93 hierarchically clustered techniques described in the BCT taxonomy (v1). 23 Where a technique was identified, we assessed whether the definition of the technique was fully or only partially met. Detailed assessments of all studies are available on request. Here, we summarise the main top-level findings from this analysis.
Table 3 shows that, across the included studies, 22 BCTs were identified. The majority of these were found to have been used in only one or two studies. The most commonly adopted BCT was the use of instructions to inform those delivering the intervention on how to perform the behaviour (17 studies with a total of 34 instances where this occurred). On its own, instructions to perform a behaviour that requires effort is not particularly effective. 59 The combination of BCTs is known to make interventions more effective. Of note, one study used nine BCTs,42 one used six26 and another used five. 45 This was taken into account in planning the intervention to maximise the use of BCTs that can together produce a more effective intervention.
| BCT | Number of specific components | Studies (first author) |
|---|---|---|
| Problem-solving | 2 | Thoyre38 |
| Goal-setting (outcome) | 1 | Chrupcala26 |
| Action-planning | 4 | Dalgleish,42 Giannì,45 Giannì,46 Gray29 |
| Monitoring of outcomes of behaviour without feedback | 2 | Chrupcala,26 Pridham35 |
| Biofeedback | 1 | Thoyre38 |
| Feedback on outcomes of behaviour | 1 | Gray29 |
| Social support (practical) | 3 | Giannì,45 Messer,33 Nyqvist15 |
| Instructions on how to perform the behaviour | 34 | Chrupcala,26 Collinge,41 Dalgleish,42 Davidson,27 Gelfer,28 Giannì,45 Kirk,18 Marcellus,43 McCain,31 McCain,32 Messer,33 Pridham,35 Pridham,36 Puckett,44 Saunders,37 Thoyre,38 Wellington40 |
| Information about antecedents | 2 fully, 1 partially | White48 |
| Information about health consequences | 3 fully, 2 partially | Dalgleish,42 Giannì,45 White48 |
| Salience of consequences | 1 | Thoyre38 |
| Information about social and environmental consequences | 1 fully, 1 partially | Dalgleish,42 Giannì45 |
| Information about the emotional consequences | 1 fully, 1 partially | Dalgleish,42 Giannì45 |
| Demonstration of the behaviour | 4 | Messer,33 Thoyre,38 Waber39 |
| Social comparison | 2 | Dagleish,42 Davidson27 |
| Prompts/cues | 4 | Chrupcala,26 Dagleish,42 McCain,32 Messer33 |
| Behavioural practice/rehearsal | 2 | Davidson,27 Thoyre38 |
| Credible source | 2 fully, 1 partially | Chrupcala,26 Dalgleish,42 Messer33 |
| Reducing the social environment | 1 | Chrupcala26 |
| Avoidance/reducing exposure to cues for the behaviour | 1 | White48 |
| Adding objects to the environment | 6 fully, 2 partially | Chrupcala,26 Dalgleish,42 Puckett,44 Saunders,37 Wellington,40 White48 |
| Identity associated with changed behaviour | 1 | Thoyre38 |
Outcome measures
In total, 33 outcome measures were identified during the review. As with the inclusion criteria, the definition of age differed across the studies, and when this is taken into account the number of measures increases to 41. The five most common outcome measures reported were (1) daily weight gain (n = 11), (2) total length of stay length in the NNU (n = 10), (3) length of time to full oral feed (n = 9), (4) respiratory complications or oxygen therapy requirements (n = 9) and (5) daily volume intake (n = 8). The full list of measures reported and the studies that have used them can be found in Appendix 3.
Only two papers used qualitative methods in their research. Marcellus et al. 43 explored parents’ experiences of implementing cue-based feeding through focus groups. Thoyre et al. 38 analysed video- and audio-recording and field notes of training sessions to assess the acceptability of guided participation as a method of introducing parents to cue-based feeding.
Evidence of effectiveness
This review was an update of the previous Cochrane review. 1 That review included nine small-scale, methodologically limited RCTs, leading the authors to conclude that there was a lack of strong or consistent evidence that responsive feeding affected important outcomes, and that there was low-quality evidence that feeding in response to cues may lead to earlier establishment of full oral feeding. 1
Our updated search found one further trial to include. 29 This trial was also relatively small scale (involving 55 infants), but was deemed to be at low risk of bias across the majority of the key bias domains. The study found that, among preterm infants fed when oral feeding cues are present, a 6-hour schedule did not alter the time to full oral feeding and had no effect on rate of tachypnoea or apnoea or length of hospital stay compared with a 3-hour feeding schedule. The study also found that a 6-hour oral feeding schedule led to only small reductions in the number of oral feeding attempts per day. The inclusion of this study, therefore, does not alter the conclusions of the previous Cochrane review. 1
Analysis of policies and guidelines
The aim of the guideline and policy review was to inform the development of the Cubs intervention by considering current cue-based feeding practices within NNUs worldwide.
Methods
An internet search, using the advanced Google interface, was conducted in July 2018. Search phrases were ‘cue-based feeding protocol’, ‘cue-based feeding protocol NNU’, ‘cue-based feeding guidelines UK’, ‘cue-based feeding NNU guidelines UK’ and ‘responsive feeding policy’. Potentially relevant documents were identified by reading the meta-descriptions of the generated web pages. The identified documents were read in full by a single reviewer to assess for relevance to cue-based feeding for preterm infants. The results of the search and review were discussed with the study’s chief investigator, and a final decision on inclusion or exclusion was made. All included documents were then read to identify their main points, especially around inclusion criteria, feeding methods and outcome measures, that could inform the Cubs intervention. These data were extracted and tabulated.
Results
Of the 15 documents included in the review, six were hospital guidelines and policies, five were training presentations, three were information or guidance sheets and one was a literature review. Six of the documents were from the UK (of which four were NHS guidelines and two were from UNICEF UK BFI), five were from Canada, three were guidelines from USA hospitals, and the remaining document was a guideline based on a literature review. The majority of the documents specified that the recommendations were for both breastfeeding and bottle-feeding transition (n = 11). Two did not specify this. Of the remaining two, one was written specifically for bottle-feeding transition and the other focused on bottle feeding but did have pictures of a mother breastfeeding included in the presentation.
Table 4 details the main points of each included guideline or policy and how it aided the development of the Cubs intervention. Some points have been modified based on evidence-based literature, and this is also indicated in the table. The decisions about which components of the guidelines and policies were used to develop the intervention were based on discussions within the research team and consultation with the Stakeholder Advisory Group. To be taken forward, the components had to be feasible within a UK setting and consistent with our understanding of cue-based feeding as well as the evidence base from our systematic review.
| First author and document title | Breastfeeding and/or bottle feeding | Purpose and main points |
|---|---|---|
|
Lubbe60 Clinicians’ guide for cue based transition to oral feeding in preterm infants: an easy to use clinical guide |
Breastfeeding and bottle feeding |
Published literature review producing an evidence-based guideline for cue-based feeding in pre-term babies ✓ Parent to be educated to recognise their baby’s feeding cues ✗ Babies should gain weight at a rate of 15 g/kg per day ✓ Supplementary feeding when required ✓ Babies must be physiologically stable prior to transition to oral feeds. ✗ Semi-demand feeding after discharge • Eight feeds in 24 hours ✗ Babies < 2.5 kg should be fed every 3 hours. More flexibility can be given to babies > 2.5 kg |
|
Nationwide Children’s Hospital61 Cue-based Feeding In High Risk Neonatal Intensive Care Unit Infants: Barriers, Outcomes and Opportunities |
Not specified |
A presentation introducing cue-based feeding with the aim to standardise feeding approaches and foster a cultural shift within NNUs ✓ Provide training ✓ Documentation of feeds to assist communication ✓ Audits of implementation ✓ Cue-based feeding responding to start and stop cues ✓ Supportive feeding techniques ✗ Introduces cue-based feeding scales/intervention within literature |
|
Surerus62 Cue Based Infant Driven Feedings |
Breastfeeding and bottle feeding |
Presentation of the results from implementation of a cue-based feeding protocol in a special care nursery ✓ Staff training session on cue-based infant-driven feeding ✗ Infant-driven feeding scale implemented ✓ Use of visual aids ✓ Parent education on feeding readiness, stress cues and calming a stressed baby ✗ Output was measured ✗ Daily weight measured for first 3 days of intervention ✓ Nasogastric tube remained in place to be used if baby showed signs of stress during feeding ✓ Length of stay was an outcome measure ✗ Parent confidence was measured ✗ Increase in staff knowledge was measured |
|
Baptist Health63 Cue-based Feeding |
Not specified |
Training presentation for staff to implement cue-based feeding within a NICU • Feeding-readiness assessment before every feed using scale ✗ Baby to be 32 weeks’ gestation ✓ Baby to be physiologically stable ✓ Recognition of hunger cues ✓ Monitor feeding tolerance for example negative physiological changes ✗ Monitor weight ✓ Feed via nasogastric tube, if required ✓ Assess feeding quality after every feed |
|
Southern West Midlands Newborn Network64 Bottle Feeding Guideline |
Bottle feeding |
Guideline for hospitals with the network on bottle feeding ✓ Maximise parents’ involvement in feeding and care ✗ Minimum gestational age of 34 weeks ✗ Care activities should conserve energy ✓ Observe for physiological stability prior to feed ✓ Observe for feeding cues ✗ Provide a calm environment to feed ✗ Slow flow teats ✗ Bottle feed swaddled ✗ Bottle feed in a side-lying position ✗ Paced feeding ✗ Maximum feed time of 30 minutes, with rests if required ✓ Staff training and updates |
|
Southern West Midlands Maternity and Newborn Network65 Progression from Tube to Oral Feeding (Breast or Bottle) |
Breastfeeding and bottle feeding |
Guideline introducing a modified responsive feeding flow chart for preterm infants progressing from tube to oral feeding • Cue-based feeding is slowly introduced to the feeding schedule • Flow chart for clinical staff to follow ✓ Quality of feed is defined and more important than the quantity fed ✓ Quality of each feed assessed ✓ A successful feed would be one based on start and stop cues • No longer than 4 hours between feeds but preferred to be no longer than 3 hours ✓ Nasogastric tube in place during transition ✓ Offer breastfeeding whenever cueing is seen, regardless of planned feeding schedule ✓ Encourage parent involvement in care ✓ Top-up when assessment of feed indicates it is required ✗ Weight measured every 2 days ✗ Monitor output ✗ Bottle feeding should be paced ✗ Bottle feed in an elevated side-lying position ✓ Recognise stress cues and stop feed immediately ✗ Monitor volume intake for bottle feeders and ensure prescribed volume is achieved ✓ Bottle feeding assessment chart ✓ Breastfeeding assessment charts |
|
UNICEF UK The Baby Friendly Initiative66 Responsive Feeding: Supporting Close and Loving Relationships |
Breastfeeding and bottle feeding |
Information sheet detailing the UNICEF BFI standards of responsive feeding ✓ Breastfeeding when baby shows hunger cues or signs of distress ✓ Staff training on the new UNICEF UK BFI standards ✓ Engaging conversations with mothers around their expectations of feeding |
|
Winnipeg Regional Health Authority67 Enteral Feeding and Nutrition for the Preterm and High Risk Neonate |
Breastfeeding and bottle feeding |
Clinical guidelines for enteral feeding of preterm and high-risk neonates ✗ Weekly head and length measurements ✗ Twice-weekly weights measured, adjusting volume of feed for baby accordingly ✓ When necessary, allow rest time during feed ✓ Skin to skin ✓ Kangaroo care ✗ Baby born at a gestation < 33 weeks progress through the SINC (safe individualised nipple competence) protocol ✗ First oral breastfeed should be at a pumped breast ✓ Infants must have physiological stability prior to feeding ✗ Non-nutritive sucking • Assess feeding progression every 24 hours ✓ Stop feeding at signs of physiological instability and give remaining feed by naso-gastric tube ✗ Consider weight testing for breastfeeding baby • Cue-based or semi-demand feeding when baby shows adequate progression • Feed every 3 or 4 hours ✗ Side-lying bottle feeding |
|
UNICEF UK The Baby Friendly Initiative68 Guidance for Neonatal Units |
Breastfeeding and bottle feeding |
Guidance documents from UNICEF on BFIs within NNUs ✓ Education for all clinical staff on feeding cues ✗ Promotion of breastfeeding ✓ Support and work in partnership with parents ✓ Recognition of behavioural cues by both staff and parents ✓ Written and visual aids to support conversations and information-sharing ✓ Recognising and responding to stress signals ✓ Frequent skin to skin ✓ Bottle feed in response to cues ✓ Monitor regulation of sucking and breathing when bottle feeding ✓ Nasogastric tube feeds when required ✓ Avoid force feeding • Feed in a semi-upright supported position ✗ Oral care with EBM ✗ Early expressing of breast milk and support to sustain expressing ✓ Skin to skin ✓ Educating and supporting parents to recognise and be responsive to their baby’s cues • Minimum of 8 feeds in 24 hours ✓ Update parents of care their baby received in their absence |
|
Birmingham Women’s and Children’s NHS Trust and Barts Health NHS Trust69 Every Feed Matters: Developmentally Supportive Feeding on the NICU |
Bottle feeding (although images of breastfeeding also included) |
Presentation on the role of speech and language therapists in the transition to oral feeding in NNUs and how neonates develop to oral feeding ✓ Importance of supporting neonates to develop positive emotions relating to feeding ✗ Non-nutritive sucking ✓ Skin to skin ✓ Touch ✓ Feeding should be based on quality rather than quantity ✓ Babies display readiness and stress cues ✗ Stress cues are not as well known as readiness cues ✓ Physiological and behavioural responses to stress displayed by babies ✗ Paced bottle feeding ✓ Bottle feed in an elevated side-lying position ✗ Swaddled when bottle feeding |
|
Norfolk and Norwich University Hospitals NHS Foundation Trust70 Trust Infant Feeding Policy |
Breastfeeding and bottle feeding |
NHS infant feeding policy aiming to increase breastfeeding rates and improve safe feeding among bottle fed baby ✓ Skin to skin ✗ Encourage and support expressing ✓ Recognition and responding to feeding cues for breastfeeding and bottle feeding ✓ Mothers taught to recognise effective feeding ✗ Paced bottle feeds ✓ No force feeding through the recognition of stop cues ✓ Sharing of information to enable parents to make informed decision-making |
|
Alberta Health Services71 Oral Feeding Guideline |
Breastfeeding and bottle feeding |
Clinical guideline aiming to reduce risk and enhance baby’s feeding experience ✓ Feeding commenced based on baby’s readiness to oral feed not their gestation ✗ Non-nutritive sucking to develop sucking skills ✓ Skin-to-skin care ✓ Identification of cues ✗ Bottle feeding side-lying position ✓ Feed at baby’s pace ✓ Tube feed when required ✗ Test weighing for breast-fed babies to calculate milk intake ✗ Use bottles for top-up for breastfed babies if required, after nasogastric tube removed ✗ Maximum feed time of 30 minutes • Encourage cue-driven rather than volume-driven feeding once nasogastric tube removed |
|
McMaster Children’s Hospital and St Joseph’s Healthcare72 Cue-based Feeding in the Neonatal Nurseries |
Breastfeeding and bottle feeding |
Information sheet for parents on the Infant Driven Feeding Scale ✓ Advocates cue-based feeding ✗ Infant Driven Feeding Scale used to aid transition ✓ Combines clinical planning and baby’s readiness to oral feed ✓ Quality of feed is more important than the quantity of intake |
|
Wolf 201873 FUN-damentals of feeding in the NICU |
Breastfeeding and bottle feeding |
Presentation, providing the evidence supporting a cue-based feeding approach within a NICU and offers a number of interventions available to aid transition ✗ Non-nutritive sucking ✓ Skin to skin ✗ Oral motor stimulation ✗ Oral care with EBM ✗ Step wise plan ✗ Alberta Oral Feeding Progression Plan ✗ Co-regulated feeding ✓ Describes subtle stress cues given by a baby ✓ Stop cues • Side-lying feeding position for bottle feeding |
|
BC Women’s Hospital and Health Centre74 Cue-based Feeding Guideline: NICU |
Breastfeeding and bottle feeding |
Health authority guideline for introducing cue-based feeding in their NICU ✓ Assess baby’s ability to oral feed ✓ Provide opportunities when readiness cues are shown ✓ Respond to stop cues ✓ Assess feeds based on quality being more important than quantity of intake ✓ Parents are partners in decision-making and care of their baby • Open to all babies ✗ Education for mothers on expressing ✓ Skin to skin ✓ Comforting touch encouraged ✓ Training for family members to recognise start and stop cues ✗ Pacing of bottle feeding |
Summary
The systematic review included 25 studies, of which 10 were RCTs, nine were quality improvement projects and six were observational studies. The quality of the studies was low, with all but one having a high risk of bias. Two key components of all the interventions were a cue-based feeding protocol and training for staff and parents. Only two interventions reported a theoretical basis. Across all studies, 22 BCTs were identified, of which the most common was providing instructions on how to perform the required behaviour. The studies incorporated 41 different outcomes, the most common of which were daily weight gain, length of stay in NNU, and length of time to full oral feeding. Although we found one additional small trial, our results do not change the conclusions of the Cochrane review22 that the evidence in favour of cue-based feeding is of low quality and should be treated cautiously. The analysis of policies and guidelines included 15 documents: six from the UK, five from Canada, three from the USA and one based on a literature review. The findings highlighted common features that were taken forward to the consensus-building workshop to inform the development of the Cubs intervention (see Chapter 7).
Chapter 4 Case studies of cue-based feeding
In this chapter we report three rapid organisational case studies of units that had embedded cue-based feeding, and highlight the learning for the development of the Cubs cue-based feeding intervention.
Aims
The aim was to generate a contextualised picture of cue-based feeding strategies for preterm infants in NNUs by exploring experiential knowledge and clinical or behavioural approaches that facilitated the practice. The case study goals were to confirm and detail key practices identified through literature review and consultation, with neonatal medical and nursing expertise provided by the Stakeholder Advisory Group.
The three participating NNUs were the Princess Royal Maternity (Glasgow) in Scotland, and Uppsala University Hospital (Uppsala) and Falun Hospital (Falun), both in Sweden. These units were identified by members of the Stakeholder Advisory Group as suitable for inclusion on the basis of pre-existing or embedded cue-based feeding approaches.
Method
Case studies can be focused on a group of people for a particular purpose and be designed to examine examples or phenomena that illuminate activity and behaviour within a real-life context. 75 The case studies described here involved observational visits to the clinical area, informal interviews with key informants (including senior nurses and consultant neonatologists) and access to relevant documentation, policies, guidelines and training materials.
Based on the characteristics of the trials included in Watson and McGuire,1 key features that were anticipated would inform the Cubs intervention included how to recognise infants’ readiness-to-feed cues; frequency of assessment of readiness to feed; understanding infants’ cues of physiological stability or instability during a feed; how to recognise satiation cues and when to stop an oral feed; minimum and maximum time between feeds; how to assess need for and when to give ‘top-up’ feeds by tube; stages of transition to full oral feeding; monitoring infant well-being, and mother/parent confidence and satisfaction with feeding. This information was reviewed and refined by expert neonatal medical and nursing partners to create a template for case study data collection, prior to visiting the units.
The template consisted of 46 questions grouped into broad categories to organise the collection of observational, documentary and verbal information on cue-based feeding strategies. The categories included population (infants eligible for cue-based feeding); feeding cues (how cues were defined); practicalities of feeding (‘the what’ and ‘the how’ of administering enteral nutrition); clinical markers (what guided clinical decisions or precautions were taken); written resources and policies; parent and staff experiences, and general reflections.
Each unit was visited once between July and October 2018. The visits lasted between 4 and 5 hours and were conducted by two members of the research team: a health psychologist and a neonatal nurse specialist, each experienced in their respective fields. Both researchers were always present and completed the template separately, making contemporaneous notes and observations during the visit. After visits were completed, notes were transcribed, compared and analysed based on the aims and goals of the case studies.
Findings
The results of each case study are presented based on key differences in their approach in terms of the population of infants eligible, how the cues were assessed and implementation considerations.
Unit 1 (Princess Royal Maternity, Glasgow, UK)
Population of eligible infants
Infants receiving intensive care were excluded from the study. Infants > 32 weeks’ gestation and not requiring intensive care were offered cue-based oral feeding. Infants < 32 weeks’ gestation were assessed for clinical stability and required a medical decision to be offered cue-based feeding.
Assessment of cues
Eligible infants were assessed using a five-step ‘readiness to feed’ scale. The absence or presence of recognised start or stop cues was identified by staff and/or parents and allocated the appropriate score. Oral feeding was withheld, offered or discontinued on this basis. Cue assessments were documented using the five-step tool framework before and during feeds.
Implementation
All staff were trained in the approach to cue-based feeding as a targeted quality improvement strategy. The five-step ‘readiness to feed’ scale applied to both breast- and bottle-fed infants, but an additional tool was used to guide reduction of supplementary feed volumes via gastric tube (top-ups) for breastfed infants. Infants had a prescribed volume to achieve in 24 hours which aimed for an increasing body weight trend over 1 or more days. The tool to guide reducing feeds recommended chunked reductions (i.e. by one-quarter, by half) in top-up volumes, based on quality-of-feed observations and through discussion with the mother. Kangaroo care was strongly encouraged when parents were available and wished to do this; however, infants were often dressed by the time cue-based feeding commenced so oral feeds were not necessarily pre-empted by this.
Unit 2 (Uppsala University Hospital, Uppsala, Sweden)
The population of eligible infants
A limited number of infants were excluded because they were orally ventilated (oral feeding therefore was not possible) or exclusion was medically indicated (generally infants with significant gastrointestinal disorders). The use of cue-based feeding was fully infant-driven with no minimum gestational age or formal assessment. Infants were offered the mother’s choice of oral feed as soon as they actively sought out the breast during kangaroo care. Unrestricted and prolonged kangaroo care was proactively offered in all cases, even in the case of infants born at as early as 21–22 weeks’ gestation.
Assessment of cues
Experiential, informal observation of start and stop cues was made by staff, many of whom had completed a 4-day training programme on infant neurodevelopment: Family and Infant Neurodevelopmental Education (FINE). There was also support from qualified Newborn Individualized Developmental Care and Assessment Program (NIDCAP)76 trainers. This knowledge was translated to parents and new staff through informal bedside teaching from the time of admission, regardless of the gestation of the infant. Although the quality of feeds was documented, cues were not, with responsibility for cue recognition shifting quickly to parents.
Practicalities of implementation
Pre- and post-feed test weighing was routinely used to assess the intake of breastfed infants. There were no limits on time or frequency of feeds, but a prescribed daily volume was set by medical guidance. A template specified by how much the total volume of feed could be reduced over 24 hours, which allowed flexibility from feed to feed. Many variables identified in the policy guidance (e.g. frequency, quality, sleep states, weight at feeds and over time) could influence the duration over which this occurred. Some clinical nursing judgement was required to negotiate these variables in combination with test weighing and discussion with the mother regarding top-ups.
Unit 3 (Falun Hospital, Falun, Sweden)
The population of eligible infants
The population of eligible infants was similar to that of Uppsala University Hospital; however, as this was a unit offering a lower level of intensive care facilities, complex infants or those of < 28 weeks’ gestational age were transferred to a tertiary unit. Infants who were > 28 weeks’ gestation were included unless they were ventilated or their exclusion was medically indicated (generally because of suspected gastrointestinal infection, as more serious issues would result in a transfer to higher care).
Assessment of cues
The UNICEF image chart and a ‘wheel of cues’ were used to assess when to start feeds, but an informal judgement-based approach was used to decide when to stop feeds. Any instability or issues with a specific feed informed the approach at the next feed. Documentation included quality of feed and start cues, but not stop cues. Training for most staff was informal and ad hoc (supported by one staff member with NIDCAP training). Kangaroo care was unrestricted, and parents were encouraged to learn and respond to cues early.
Practicalities of implementation
A combination of cue and scheduled approaches was in evidence; for example, very frequent feeding would see an adjustment in top-ups. A formal tool was used to guide small reductions in prescribed volumes gradually using daily weight. This assumed eight feeds a day and a 5 ml reduction in each feed, over 1 day, and further reductions (in 5-ml increments) were applied if daily weight gain was medically acceptable.
Clinical considerations
Although there were key differences in the approach to cue-based feeding, there were clinical similarities across the three sites; for example, a maximum limit of 3 hours between feeds was applied regardless of cues, and this was relaxed only when feeding was established. There was a clear focus on the importance of assessing the efficacy of breastfeeding rather than just applying the tools available to support the identification of feeding cues. All units used prescribed daily feed volumes and relied on weight and head circumference trends to ensure that nutritional, growth and development needs were achieved. This was an important feature of NNU care and a key driver in the clinical management of infants in this environment.
Facilitators
Operational approach
There were similarities in operational approach and unit ethos demonstrated by the enabling of kangaroo care, encouragement of breastfeeding and enabling of parents to be primary caregivers. Managerial support was also a principal factor in driving change, particularly for access and time for education and training for staff, the development of protocols and policy guidance, and the provision of accessible educational materials for all at the cot-side.
Education
There was recognition across all three units that the education of staff and parents is fundamental to embedding cue-based feeding approaches. Teaching was strongly orientated towards parents assuming responsibility as the primary interpreters of infant cues. A range of methods were used to increase the knowledge and practice of cue-based feeding. Educational materials such as posters or charts were available at the bedside for easy reference, and all units used the UNICEF cue guide featuring images of term-born infants. Interactional learning also took place, including bedside teaching, peer-to-peer education and reflective learning. Each unit also utilised champions or trained supporters who exhibited behavioural modelling and offered informal teaching.
Environment/equipment
The provision of rooms for parents to stay near their infants was standard, although there were differences in the type of accommodation available. In Glasgow, single rooms were available within the unit. They were relatively self-contained and situated in a designated ‘rooming-in suite’ and offered to mothers of infants transitioning to discharge. Fathers could also be accommodated. One double room within the NNU was used only for the parents of the sickest infants. The Swedish units were spacious by comparison and could accommodate adult beds in each intensive care space to enable parents to sleep adjacent to their infants. The two Swedish units did not use the traditional infant incubators (‘closed’ unit with side panel access) predominant in Glasgow; rather, they exclusively used open beds with removable hoods. As care was stepped down to high-dependency or special care, infants moved into private rooms with their parents (and sometimes siblings or extended family) for the rest of the stay. Kitchen facilities equivalent to a typical home were available to families in Sweden, including freezers, full-sized cookers and dining space. In Glasgow, the available facilities were more limited, comprising a kettle, fridge and microwave, in addition to a sitting area.
Sociocultural factors
Differences in the social care policies of Sweden and the UK resulted in a significant difference in the lived experience of staff and families in NICUs. The Swedish national insurance system facilitates parents’ presence at the NICUs. Both parents can access a benefit that covers up to 80% of their salary and allowed them to take leave from work to attend to their child in hospital. Parents are also entitled to 480 days of shared paid parental leave for every child, which does not start until infants are discharged home and can be taken up until the child is 8 years old. In Scotland, at the time, there was no equivalent to the child-in-hospital benefit support, with standard maternity/paternity leave commencing from the child’s birth. Additional unpaid parental leave amounting to a maximum of 18 weeks (90 days) can be taken at any time until the child is aged 18 years. This represents a significantly less generous package in both time and financial support. This clearly influenced the way in which staff and parents could approach time in the unit. The sites at Falun and Uppsala applied a ‘zero separation of family’ philosophy (an expectation matched by parents), with a family member in attendance 24 hours per day, whereas in Glasgow parents were strongly encouraged to be present, but any expectation was mitigated by what families could reasonably achieve.
Key learning
In Sweden, cue-based feeding practices were more established than in Glasgow, where the practice had been introduced only during the preceding year. Both Swedish units reported that, historically, there were some challenges, with older, more experienced, staff expressing a preference for scheduled feeding. However, there were key factors that helped address their concerns about risks. NNUs across Sweden collected extensive mortality and morbidity outcome comparison data for their infants. The unit in Uppsala was able to identify better outcomes than other units across several measures in these data. Staff in the unit believed that this was attributable to its approach to kangaroo care and, by extension, the fact that practices that were facilitated by this, such as cue-based feeding, became more acceptable. Staff at the unit in Falun also described similar challenges, with some staff resisting cue-based feeding, but they believed that a recent move to a purpose-built unit with better facilities to facilitate zero separation and increased kangaroo care helped to reduce resistance. Staff in Glasgow did not report any significant challenges in implementing their cue-based feeding strategy, identifying a willingness to accept the change as quality improvement.
Access to training was not perceived as sufficient in every area. Staff in Falun expressed a desire for more formalised education to be available either for or by NIDCAP trainers, particularly to help to support decision-making on the quality of feeds. Experience was seen as an important facet of managing the approach to top-ups in Falun, and there was some difficulty in articulating the clinical decision-making that guided this, suggesting that unconscious competence77 was directing the decision. It was evident across the case study sites that assessing the quality of feeds and the approach to top-ups required some degree of clinical judgement, as it was impossible to ascertain the volume an infant had swallowed at a breastfeed. This may explain the preference for scheduled feeding among more experienced staff in Sweden. Despite differences in approach, each unit had operationalised elements of top-up management to provide a degree of certainty in achieving prescribed volumes and nutritional needs by reducing reliance on interpretation or judgement. In Glasgow, this involved using minimum gestational age, increasing the infant’s likelihood of sufficiently mature reflexes, as well as a quality feed tool to guide the decision. Falun’s semi-scheduled approach meant that relatively small incremental volume reductions were of low impact and could be carefully guided by weight trends over time, and Uppsala utilised test weighing to make assessments of actual intake.
What should not be underestimated, in terms of the impact on families’ experience of cue-based feeding approaches, was the sociocultural difference evident between Scotland and Sweden. Very few infants received bottles in Falun or Uppsala and there was very little bottle-feeding equipment and very few breast milk substitutes available in the units to offer this, as breastfeeding was the accepted norm. Sweden has a very high breastfeeding rate compared with the UK. Staff here also expressed their ardent belief in preterm infants’ ability to demonstrate and follow through on cues in their own time, especially if they received unrestricted kangaroo care. Their commitment to unrestricted kangaroo care was demonstrated by having adult beds at the cot side and the use of ‘open’ incubators to reduce physical barriers. Sweden also had many advantages in terms of well-funded social welfare benefits, extensive parental leave and spacious well-facilitated ‘living’ environments available to Swedish families. There was an implicit recognition in these measures that families’ and infants’ normal state is to be together at all times. This did not just facilitate a zero-separation philosophy from staff and families, it demanded it. The feasibility of this approach is unlikely to be replicable to the same extent in the UK. Reduced family–infant interaction has an impact on the opportunity for kangaroo care and may affect opportunities for cues generally to be both recognised and responded to from a very early stage. This, arguably, suggests that a higher level of nursing input into the delivery of care and caveats for introducing cue-based approaches may be necessary.
Limitations
The limitations of these case studies are that they represented an informal, time-limited snapshot of cue-based feeding approaches in three purposefully selected NNUs. A large amount of information was collected, and presentation of the results represents a rapid ethnography78 rather than an in-depth analysis of behaviours and practice. A key strength of these case studies was using two researchers with significant experience in neonatal intensive care nursing and in health psychology. The combination of the differing lenses on the clinical and behavioural aspects of cue-based feeding afforded a rounded perspective on which to inform the development of an intervention.
Chapter 5 Survey of neonatal units to determine current practice
As there is no consensus on how to manage the transition from tube feeding to full oral feeding and there was no information about current approaches and practices across the UK, we conducted a telephone survey of a purposive sample of UK NNUs.
Aim
The aims of the survey were to map and understand the range of approaches to the transition from tube feeding to full oral feeding in the UK including the scope of routine data collection systems, training needs of staff, and the variation in practice to understand ‘usual care’ that would form the control arm of a future trial.
Method
A purposive sample of 20 NNUs was selected from across the 220 units in the UK. The selection of units was based on the geographical location, designated unit level, cot capacity, population and urban–rural setting. Initially, units were contacted on behalf of the study team by regional representatives of the Neonatal Networks. Where there was no response, contact was followed up by a representative from the relevant Clinical Research Network for the trust/health board. Out of the 20 units sampled, 18 units indicated a willingness to take part; two units did not respond to the invitation.
Each unit that indicated a willingness to participate nominated an individual, or individuals, to be interviewed. Once units had agreed to be contacted, an introductory call was made by a researcher from the study team. The purpose of this call was to discuss the aim of the telephone survey, to discuss the participant information sheet and to arrange a suitable time and date for a telephone interview. Verbal consent was sought at the outset of the telephone interview.
The interviews were conducted by a member of the research team using a semistructured interview schedule based on the aims of the survey. The duration of interviews ranged from 18 to 56 minutes; the mean length was 35 minutes. The units varied from level 1 (special care) to level 3 (neonatal intensive care). The number of cots in the units ranged from 6 to 53. The job roles of interviewees included infant feeding advisor (n = 6), specialist speech and language therapist (n = 5), staff nurse (n = 4), senior charge nurse (n = 2), clinical/practice educator (n = 2), unit manager (n = 1), clinical nurse lead (n = 1), neonatologist (n = 1) and neonatal consultant (n = 1). There were four participants in one interview, two in another, and the remaining participants had individual interviews.
Interviews were recorded, transcribed and analysed using a thematic approach. 79 Initial coding was conducted independently by two researchers. The final themes were discussed and agreed with the research team.
Approvals were granted by NHS East of Scotland Research Ethics Committee (LR/18/ES/0059) and the Health Research Authority.
Findings
An overarching theme was typologies of change. This referred to the various stages in the process of change towards introducing cue-based feeding described by participants. Three typologies of change were evident in the descriptions of units’ approaches to the transition from tube to oral feeding: ‘not considering change’, ‘considering change’ and ‘making changes’. A further subtheme was ‘variations in practice’. Descriptions of these subthemes utilised the characteristics, ethos and practices of units, as well as the micro language, namely change talk,78 to categorise the stage of change of each unit at the time of the telephone survey.
Not considering change
Three of the 17 units were not contemplating changes to their protocol around the transition from tube to oral feeding. These units were typically smaller (range 10–22 cots) and were level 1 (n = 1) or level 2 units (n = 2). None had Bliss accreditation, while only one was part of a hospital which had UNICEF UK BFI accreditation. Rates of breastfeeding and kangaroo care were generally low (30–40%). The predominant model of care in these units was described as family-centred care.
Although unit policy was not to implement cue-based feeding policies, there were individual members of staff who advocated cue-based feeding:
I keep pushing [for changes] . . . I put up [information on responsive feeding] on the notice board and then I’ll say to staff ‘oh that baby is looking for a feed’ and they will do it if I say to them. But if I wasn’t there they wouldn’t do it or would just give a tube feed.
Unit 4
Within these units, the biggest barrier to implementing a cue-based feeding protocol was suggested to be the attitudes of other staff, both nursing and medical, which tended to be focused on feed volumes:
Adverse events have occurred when bottle feeding because I suppose it’s nursing staff who think no you will finish this, you will take this and just the old system . . . you have to take this amount so you will take it.
Unit 4
I think a big thing is the dependence still on the quantity that is recorded to get into a baby . . . although we wouldn’t like to still think of ourselves as medicalised it’s still mls to kg.
Unit 12
The variation in staff’s practices relating to the transition from tube to oral feeding seemed to be based on individual experience, confidence and attitudes:
. . . there can be quite a noticeable difference between staff and how they cut down on milk. It really depends on their confidence and experience . . . there is a lot of trial and error in deciding how well an infant is transitioning from tube to oral feeding and it depends on your experience and confidence in identifying this.
Unit 13
For these units, routine, in terms of sticking to a schedule, was important for staff:
I do feel we still have people who go ‘no, you’re not due for another hour’ and really the baby is showing signs . . . I do feel it’s become ingrained in neonatal units . . .
Unit 13
Considering changes
Many of the participating units (n = 13) were considering what changes could be made in their approach towards the transition from tube to oral feeding, in the form of departmental procedures, training for staff, or support for parents. These units were more likely to have Bliss and/or UNICEF UK BFI accreditation and to report an increase in breastfeeding rates in the previous year. These units tended to be small to medium-sized units and to be moving towards the implementation of family integrated care:
We are trying to bring in family integrated care and move away from medicalised types of care. We want to shift old thinking on cue-based feeding.
Unit 1
This change in the model of care the unit subscribed to was reported also to lead to changes in the overall ethos of the unit and relationship with parents. Interviewees suggested that parents were viewed as partners in care and encouraged to be actively involved in providing care alongside nursing staff:
We encourage parents to be on the unit as much as possible and encourage them to feel a valued partner in their baby’s care.
Unit 3
Although these units were considering what changes could be made, some staff were described as actively implementing cue-based feeding. However, these staff were reported to be in the minority and change at a wider systemic level was needed. As unit-wide change was yet to take place, the approach to feeding was still reported to be largely focused on volume and schedule.
In terms of the approach to the transition from tube to oral feeding, these units described using non-nutritive sucking prior to starting oral feeds, and would base the decision to transition on the infant’s gestational age. Units that had greater multidisciplinary input (e.g. speech and language therapists) appeared to be more likely to be encouraging the practice of cue-based feeding.
Making changes
Two units reported that they had actively made changes (n = 2). Both units were level 2 units with a small number of cots (10–22). One unit had level 1 UNICEF UK BFI accreditation, while the other unit had level 2 accreditation. Both units had neonatal infant feeding advisors on site. They also had dedicated input from speech and language therapists. Family integrated care was said to be embedded within the ethos of the units. Respondents from these units described actively developing specific unit guidance relating to the transition from tube to oral feeding:
We have guidance in all the nurseries about breastfeeding and babies’ cues. We do sessions as part of family integrated care to make families aware of their babies’ readiness cues. We have no set guidelines as we feel that everything is unique to the specific baby, so that’s why we provide guidance rather than guidelines.
Unit 11
Staff had access to training that was related to cue-based feeding and plans to develop more specific training and tools around cue-based feeding, such as leaflets for parents, were reported:
We put the training in first and have a lot of discussions around [cue-based feeding] before we actually start implementing [recording of feeding readiness and quality of feeds in feeding notes] . . . although the paperwork has come afterwards it really has been underpinning everything we have been trying to do over the past couple of years.
Unit 10
Areas of variation
Although there were some similarities across all the units, there were several areas of practice that differed. The minimum corrected gestational age for the transition from tube to oral feeding was reported to be anywhere from 32 to 34 weeks, while some units had no minimum corrected gestational age and instead relied on assessing each infant on a case-by-case basis. Some units used feeding support techniques such as non-nutritive sucking, or orofacial stimulation, while others did not describe using any. To assess cues, some units relied on existing tools (e.g. UNICEF UK BFI Breastfeeding Assessment Tool80), while others relied on visual observations of the infant and intuitive knowledge to help make sense of what the infants’ behaviour was communicating. The types of exclusion criteria for cue-based feeding included physiological instability, other medical intervention (e.g. high-flow or low-flow oxygen) and/or congenital disorders. In terms of assessing the need for top-up feeds, some units relied on a prescribed amount, wheras others based their decisions on a nurse-led assessment, and still others on mother-led assessment. Some units used tools to assess top-ups (UNICEF UK BFI Breastfeeding Assessment Tool80). Decision-making around when an infant should transition from tube to oral feeding could be medically led, nurse led, multidisciplinary and/or include parental involvement.
Summary
This chapter describes the findings of a telephone survey of 18 purposively selected NNUs across the UK. The findings show a wide range of practices and approaches to the transition from tube to oral feeding for preterm infants. The analysis suggested three stages in the change process towards cue-based feeding; a smaller number of units were not considering making any changes, or were actively implementing cue-based feeding, while the majority were considering but had not yet implemented systemic change to cue-based feeding. Also of interest is that in all units, regardless of where they are situated on this typology of change, there are individual staff who are enthusiastic about cue-based feeding, and those who were described as resistant to change.
Chapter 6 Qualitative research with parents and staff
To supplement the evidence from the literature review, policy and guidelines analysis, case studies and telephone survey, we conducted qualitative research in the three sites in which we intended to implement the intervention and conduct the feasibility study.
Aims
The aim of this component of the project was to understand parents’ and health-care professionals’ views and understanding of cue-based feeding to inform the intervention, particularly the training component.
Methods
We conducted focus group discussions and/or individual interviews with a convenience sample of 15 parents (12 mothers and three fathers) and 32 health-care practitioners. Health-care practitioners were invited to take part in the interview through an e-mail sent by the senior charge nurse or matron in each unit. Interviews took place during working hours. Those who were interested in participating in the study contacted the site research nurse, who arranged the time and location of the interview. Where possible, interviews were conducted as focus group discussions, but, owing to staffing constraints, it was necessary to conduct some interviews individually. Participating health-care practitioners included neonatal consultants, speech and language therapists, occupational therapists, neonatal nurses, nursery nurses, health-care assistants and infant feeding leads.
Parents were invited to an interview by a member of their infant’s care team. When parents indicated an interest, they were contacted by the site research nurse to arrange a time and date for the interview. Although these were intended to be conducted as focus group discussions, those who were interviewed indicated a preference for individual interviews. In addition, the reality of parents needing to attend to their infants at various times meant that it was not always possible to arrange group interviews that were suitable or convenient for everyone.
Interviews and focus group discussions were conducted by site research nurses guided by a semistructured interview topic guide. Staff interviews lasted 37–52 minutes and parents’ interviews 10–25 minutes. The interviews were audio-recorded. We took a pragmatic approach to analysis; each interview was listened to by a member of the research team, and notes of key points were taken. The key points were organised into common themes.
Approvals were granted by NHS East of Scotland Research Ethics Committee (LR/18/ES/0059) and the Health Research Authority.
Findings
Five themes were identified from the analysis of the data from health-care practitioners: knowledge and attitudes; concerns about cue-based feeding; variations on practice; role of medical staff; and education. Six themes were identified from the analysis of the interviews with parents: experiences, identity, getting home, feeding cues, support and suggestions.
Health-care practitioners
Knowledge and attitudes
All health-care practitioners interviewed said that they had a good understanding of cue-based feeding and were positive about it. When asked to describe feeding cues, health-care practitioners mentioned start cues only, such as head bobbing, rousing, stirring and the baby turning their head to the side. Stop cues were not mentioned.
Most interviewees indicated that cue-based feeding was already in place in their unit and that their role was to support parents to understand and implement cue-based feeding. However, some staff indicated that other staff may feel that their role is threatened by giving parents more control.
Concerns
Despite the positive attitude towards cue-based feeding, some health-care practitioners expressed concern about the clinical safety of cue-based feeding. Concerns centred on whether infants on a cue-based feeding protocol would receive adequate nutrition, or experience hypoglycaemia. Nursing staff saw it as their role to maintain the physiological health of the infant, and the perceived risks of cue-based feeding were seen to be contrary to that. One staff member perceived their role as an advocate for the infant to ensure adequate feeding.
There were also concerns mentioned about the criteria for a cue-based feeding protocol. Respondents said that, owing to the variability in current practice, it was unclear what criteria should be used, and that this could present a barrier to the development of a cue-based feeding protocol. One interviewee suggested a minimum weight to start cue-based feeding. In contrast, another health-care practitioner suggested that having defined criteria may mean that important physiological indicators of readiness to feed may be overlooked in favour of following the predefined criteria as set out in the protocol. The maturity of the baby was felt to be more important than a defined weight or gestation by this practitioner.
Variations in practice
Health-care practitioners’ descriptions of each unit’s approach to cue-based feeding varied. Areas of variance included the criteria used to assess readiness to feed; whether infants were fed to a time schedule or not; and whether or not a ratio of tube to oral feeds was followed during the transition from tube to oral feeds. One practitioner suggested focusing on an infant’s physiological stability to assess readiness to commence the transition to oral feeding, and another practitioner from the same unit suggested that the presence of suck–swallow reflexes was critical. Practitioners from another unit described a very structured approach in which the ratio of oral feeds to tube feeds was specified.
Importance of medical staff
Having medical staff on board with cue-based feeding was seen to be critically important. Although medical staff were not necessarily involved in feeding, their attitudes towards cue-based feeding and the advice that they provided to parents were perceived as being hugely influential. One medical practitioner indicated that they saw cue-based feeding as important for infants’ development across a variety of domains, and that their role was to support parents to understand this and to build their comfort in feeding their infant in accordance with cues.
Education
Education was perceived to be key to the success of implementing cue-based feeding, and it was widely believed that for education to be successful everyone (i.e. parents and all staff) needed to access it. Although health-care practitioners endorsed education for staff, this was mostly around the clinical application of the protocol, to reinforce and refresh their understanding of cue-based feeding. In contrast, health-care practitioners believed that education for parents should focus on enhancing their understanding of cues and how to respond to their infant, and could be delivered as part of wider education provided to parents within the units.
Parents
Parents’ experience
Most parents described their experiences of the transition from tube to oral feeding as focusing on the quantity and duration of feed. Decisions about feeding were reported to be led by health-care practitioners. Some parents stated that they were satisfied with this, but others found it a source of frustration. Despite most decisions around feeding being reported to be led by health-care practitioners, one parent described a collaborative and supportive approach when they made suggestions about feeding to the medical team. This parent described how nursing staff were concerned about her infant not waking up for 3-hourly feeds during the night, but were receptive when the parent suggested waiting for 4 hours. According to the parent, this strategy led to the infant being fully orally fed within 1 week.
Identity
For many parents, being able to support their infant to feed was described as an important part of their identity as a parent. It was a normal part of their caring role, which was in contrast to the medicalised care their infant had needed up to that point. For some parents, it was the first time that they really felt that they had been able to participate in their child’s care.
Parents who had previously experienced feeding a term infant described how the experience of feeding a preterm infant meant that they needed to change their perspective, as everything was different with a preterm infant.
Getting home
Parents described how feeding was the last hurdle before going home and signalled the end of their infant’s time on the unit. Although this was exciting, it was also the most frustrating part of the journey. One parent described how feeding became something that they had to do to get home, and this was stressful. For another parent this meant that they became more focused on getting the infant to feed so that they could demonstrate that the infant was ready to go home. Although focusing on cues was perceived as important, it was seen to be secondary to the process of feeding.
Cues
Paying attention to their infant’s behavioural cues and attempting to make sense of their meaning was something all parents described. However, parents did mention that there was trial and error involved in working out what the infant needed. Following a cue-based feeding approach seemed intuitive for some parents and, as a result, they felt frustrated by the adherence to a timed regime in the hospital. In contrast, others preferred the scheduled approach. For some parents, feeding according to a schedule would provide structure for the family and the infant once they were at home, whereas others felt it was safer as it would ensure that the infant was receiving enough nutrition.
Support
Parents said that nurses were a key source of support and were readily available to help parents with feeding. One parent described how the advice and support provided on the unit was much better than the support a friend received after having a term infant, and this was largely because of the amount of time spent in the NNU and in contact with staff (providing an example of a member of staff spending 1 hour supporting her to get her infant to latch onto the breast). Another parent appreciated being given advice on how to stimulate her milk supply and being provided with emotional support without being pressurised to breastfeed. Although other forms of advice and support were available, personal support from nurses was preferred by parents.
Suggestions
Parents were asked for ideas on how to encourage cue-based feeding. Most parents discussed posters they had seen around the unit and described how useful they were. Some parents suggested that a film or observing someone feeding according to cues would help their understanding. Others indicated that a way of recording achievements would help them reflect on and recognise their infant’s progress. However, parents were clear that, whatever resources were provided, they would not be a substitute for the individualised and supportive approach provided by the nursing staff.
Summary
In this chapter we describe the findings of qualitative interviews with staff and parents regarding their current experiences and approaches to the transition from tube to oral feeding. From the analysis it was evident that the health-care practitioner perspective was that most staff in their unit were implementing a cue-based approach. Although health-care practitioners reported implementing cue-based feeding in practice, their descriptions suggest that a version of cue-based feeding was being used but not as it is defined in the literature. It was also evident that staff have concerns about the safety of cue-based feeding that may hold back full implementation. A further educational issue for consideration is that staff did not appear to be familiar with stop cues. It was clear from the staff data that a multidisciplinary approach is needed to implement a change to cue-based feeding.
In contrast to the staff perspective, parents’ experiences suggest that a scheduled approach is more common. The variations in practice reported by health-care practitioners suggest inconsistency. This inconsistency could explain why parents’ experiences were different from those of health-care practitioners. From the parents’ perspectives, it also appeared that some health-care practitioners struggled to explain the reasons for different approaches (e.g. for waking an infant for feeds during the night). Discharge from the NNU was clearly a focus for parents, and this appeared to lead to some pressure around oral feeding. Parents clearly appreciated the support for feeding provided by nursing staff. Parents’ suggestions for supporting cue-based feeding included learning resources, such as posters and a film, and a means of tracking the progress of their infant.
In Chapter 7, we describe how the information reported in this chapter, together with the literature review, analysis of policies and guidelines, case studies and telephone survey, was used to inform the development of the intervention.
Chapter 7 Co-production of the intervention
Introduction
The intervention was co-produced with stakeholders and was informed by the evidence gathered in the previous stage: the systematic review; the analysis of policies and guidelines; three case studies of NNUs with embedded cue-based feeding; telephone interviews with 18 NNUs across the UK; and qualitative interviews and focus group discussions with staff and parents in three NNUs. In this chapter we describe the process of developing the intervention and the intervention components. The overall aim was to co-produce an evidence-informed, adaptable, manualised intervention that included staff and parent educational support for feeding preterm infants at the transition from tube to oral feeding in response to feeding cues and signs of infant stability.
Methods
The aim was achieved through three key activities:
-
development of a matrix of intervention options
-
a consensus-building workshop
-
co-production of the intervention.
Matrix of intervention options
Based on the synthesis of evidence from WP 1, the study team created a matrix of intervention options (see Table 5) for the components and content of the intervention underpinned by a logic model of causal assumptions (see Table 6). The matrix was formulated so that the evidence informing each option was identified, and with a series of questions to be taken forward to the consensus-building workshop.
Consensus-building workshop
The aim of the workshop was to agree the intervention components, content and format, and the approach to education and training for health-care practitioners and parents. It was attended by parents (n = 5), health-care practitioners including neonatal nurses (n = 7), a neonatologist (n = 1), speech and language therapists (n = 4), neonatal infant feeding leads (n = 5), members of the research team (n = 5) and research nurses/midwives (n = 3).
The workshop commenced with a short presentation of the methods and findings of WP 1 and was followed by activities in which small groups discussed the options for different elements of the intervention. Groups were provided with the options and the evidence on which the options were based. Each group involved mixed participants so that there were parents, clinicians and researchers in each group. At plenary sessions, each group summarised their conversations, presented their preferred options and a discussion took place until consensus was reached. The intervention components discussed were:
-
context of the Cubs intervention
-
assessment of readiness to commence transition to oral feeding
-
feeding plan
-
oral feeds, including description of start, continue and stop cues, and parameters for duration, interval and rate of transition
-
safety and monitoring
-
feeding support techniques
-
education of parents
-
education of staff.
Each group had a facilitator who was a member of the research team and a scribe who documented the main points of the discussions.
Co-production of the intervention
Based on the decisions made during the consensus-building workshop, a core co-production group, comprising members of the study team and the Parents’ Panel, co-produced the intervention. This included the intervention protocol, BCTs, training packages and the commissioning of new training material in the form of a short film of infant cues. Further resources were developed in the form of adapted assessment documentation, and a document for recording the feeding transition (‘Our Feeding Journey’). During filming, stills were also taken to be included as posters. The research team and parents co-wrote the script to accompany the film. The final version of the intervention was reviewed by members of the Stakeholder Advisory Group, the Parents’ Panel and all the research team. It was also discussed with the lead nurses at the three sites selected for implementation.
In the final stage, the research team wrote the intervention manual, designed the posters and accompanying resources and intervention packs were circulated to the sites for implementation and in preparation for the feasibility study.
Results
Matrix of intervention options
Table 5 shows the matrix of options and the evidence on which each component was based. Table 6 shows the logic model on which the intervention was developed.
| Component | Description/scope | Options/questions | Evidence from WP 1 |
|---|---|---|---|
| Context of the Cubs intervention | Importance of context to provide an enabling environment for implementing cue-based feeding: includes family integrated care, 24-hour parental access and zero separation of parents and infants, and skin-to-skin contact |
|
Policy and guideline review (UNICEF UK BFI Standards68 and Responsive Feeding Information Sheet66) |
| Assessment of readiness to commence transition to oral feeding | Assessment of readiness to commence the transition from tube feeding to oral feeding |
|
Policy and guideline review Case studies |
| Feeding plan |
Assessing parents’ feeding goals and expectations and reviewing the feeding plan based on the infant’s responses Documenting the feeding plan |
|
Policy and guideline review64 Qualitative interviews |
| Oral feeds |
Oral feed attempts: includes licking and nuzzling behaviours, highlighting start, continue and stop cues Feed duration, intervals and rate of transition |
|
Systematic review18,27–29,31,32,35,37,40,42,44–46,48,81 Policy and guideline review17,60,64,71 Case studies |
| Safety and monitoring | Assessment of the safety and effectiveness of feeds |
|
|
| Feeding support techniques | Non-nutritive sucking, pacing, positioning (elevated side lying), orofacial stimulation |
|
|
| Education and training for parents and staff | Education and training objectives, methods and resources |
|
| Problems | Intervention strategy | Intervention goals | Output | Outcomes | Impact | |
|---|---|---|---|---|---|---|
| Short term | Long term | |||||
|
Cue-based feeding is inconsistently practised within NNUs There are few training programmes relating to cue-based feeding that parents or staff can access There are no published NHS policies or protocols relating to cue-based feeding for neonatal infants transitioning from tube to oral feeding |
Design a cue-based feeding intervention that is feasible to implement in NNUs Develop a package of training for both parents and staff that incorporates a cue-based feeding protocol Intervention will be based on the COM-B model and use BCTs The causal model: |
Recruit neonatal staff of all grades and parents of infants who are transitioning from tube to oral feeds Deliver training to staff through computer-based training programme including narrated PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation, videos of cue-based feeding, posters, a feeding protocol, on-the-job learning delivered by key cascaders and tools to assess feeds Deliver training to parents through cot-side learning delivered by staff, an online narrated PowerPoint presentation, video of cue-based feeding, posters, a feeding protocol, a feeding journal and tools to assess feeds Supporting an ethos of zero separation and 24-hour access to NNU for parents Change normative beliefs about cue-based feeding by modelling cue-based feeding behaviours in videos, on posters and through key cascaders and staff Change feeding behaviour through goal-setting and action-planning |
Increased understanding of feeding cues Awareness of cue-based feeding protocol Ability to assess quality of feeds and need for top-ups Goals/actions to feed according to cues |
Increase parents’ and staff’s self-efficacy to feed according to cues Increase parents’ and staff’s motivation to feed according to cues Supportive conversations between parents and staff regarding cue-based feeding |
Maintenance of cue-based feeding in NNUs | Change in feeding culture to cue-based feeding |
Consensus-building workshop
Context of the Cubs intervention
Workshop participants felt that the ethos of the NNUs was critical to the success of the cue-based feeding intervention. Important elements to support family integrated care included access to free parking, the provision of comfortable accommodation, enabling visiting policies facilitating open access to parents, and support for siblings (e.g. play areas). Suggestions were made for communication, such as recognition that the infant belonged to the parents rather than the unit (‘your baby’ rather than ‘our baby’). Skin-to-skin contact was felt to be particularly important and could be encouraged through empowering parents and the wider family, and with visual prompts such as posters. Barriers to skin-to-skin contact for infants at the stage of transitioning from tube to oral feeding were suggested to be a focus on infants transferring from incubators to cots and the desire of staff and parents for the infants at this stage to be dressed, as a marker of progress and normality. Communication with parents should set expectations of ‘an individual feeding journey’, which may have setbacks as well as progression and that will not be the same for each infant. Specific recommendations were for NNUs to have welcome packs for parents and to encourage parents to provide feedback on their experiences throughout their stay and not just at discharge. Engaging with the UNICEF UK BFI neonatal standards68 was felt to be a good mechanism for achieving this enabling context.
The consensus of the workshop participants was that the intervention needed to refer to the importance of the ethos of the NNU.
Assessment of readiness to commence transition to oral feeding
The workshop participants felt that the assessment of readiness to commence the transition to oral feeds should not be based on a specific gestation or weight. Rather, they suggested that the assessment should be focused on respiratory stability and airway safety. It was proposed that infants requiring ventilation should be excluded from the intervention. In terms of airway safety, infants should be assessed for suck, swallow and cough/gag reflexes. Assessment should be performed initially when the infant is in an alert state and should be ongoing, especially if there are changes in the infant’s condition. It was agreed that an existing measure could be used, and a simple traffic light system was felt to be the most helpful, but it would need to be adapted so that it could be used by parents.
The consensus of the workshop participants was that there would be no minimum gestational age or weight, but that assessment should be based on respiratory stability and airway safety, using an adapted Buscot Traffic Light Tool. A contentious issue, on which there was no consensus, was whether or not infants on high-flow oxygen therapy should be included in the study. After considerable debate it was agreed that, as the issue was contentious, it was on balance best to proceed with caution and exclude these infants.
Feeding plan
It was felt by workshop participants that the use of quality feeding assessment scales, such as the UNICEF UK BFI breastfeeding assessment tool,80 would help to shift the conversation with parents about feeds towards quality rather than quantity. At the same time, this would encourage parents to express their perceptions of each feed. Conversations about feeding should start during pregnancy with midwives and, on admission to the NNU, assessing the plans made during pregnancy would be a good place to start to determine goals and expectations. Supporting women to express their breast milk or facilitating skin-to-skin contact were also felt to be good opportunities for discussing feeding plans. Documenting these conversations in the infant’s care plan was felt to be important to avoid duplication. Participants also suggested that parents’ feeding goals and expectations should be captured as a journey that takes accounts of fluctuations in progress. In addition, participants suggested that documentation of feeds should include any medical issues influencing feeding, the duration of the feed, cues exhibited and decisions regarding starting/stopping feeds, volume of any top-up given, outputs (urine, stool), assessment tool score and plans for the next feed. It was felt that all documentation and tools should be parent-friendly and accessible with good use of colour and images. Parents and staff should be encouraged to document feeds.
The consensus of the workshop participants was that the intervention should include parent-friendly assessment tools for breastfeeding and formula feeding, along with an ‘Our Feeding Journey’ record in which parents and staff could document feeds and ongoing plans.
Oral feeds
Of the lengthy list of cues extracted from the evidence from WP 1, the participants suggested that the most useful were:
-
physiological stability – respiratory stability including low-flow oxygen therapy, quiet alert state or rousing or moving towards the breast if in skin-to-skin care
-
start cues – stirring, mouthing, rooting, hands to mouth, importance of responding to the infant before crying
-
stop cues – falling asleep, coming off the breast, stopping sucking, pulling away/head turning, change in colour, loss of tone, hands splayed, sudden change in level of alertness, no interest in continuing to suck after a break.
Participants felt that no limits to duration of a feed should be set as long as the infant’s cues were continuously assessed and feeding assessment tools were used. The interval between feeds should be 1–3 hours, that is between 8 and 24 feeds per 24-hour period, depending on gestational age. It was also proposed that there be no set rates of transition (e.g. one oral feed per day increasing to alternate oral and tube feeds and then to full oral feeding), but that this should be based entirely on the infant’s cues and the assessments of feeds. Participants proposed that the approach to top-ups should be determined by the feeding assessment tools but with flexibility depending on when parents are available. For example, if a parent will be visiting soon, a smaller top-up could be given to increase the likelihood that the infant will wake for a feed while the parent is present. Feeding plans should document the parents’ wishes about how their infant is to be fed in their absence. It was felt that dividing the transition into stages was not helpful.
The consensus of the workshop participants was not to set limits to feeding duration or to the rate of transition and for top-ups to be determined by the feeding assessment tools. One contentious issue was the intervals between feeds, as some participants felt that it should be 1–4 hours rather than 1–3 hours. Consensus was reached that 4 hours would be set as the maximum interval but with an expectation that there would be at least eight feeds in 24 hours. This is supported by the findings of Gray et al. 29
Safety and monitoring
Participants suggested that monitoring should focus on a 24-hour period with growth as the key outcome. There should be a standardised approach across neonatal intensive care, high-dependency and transitional care settings. It was important for parents’ observations of their infant to be documented and listened to. Safety was mainly discussed in terms of which infants to exclude from the intervention.
The consensus of workshop participants was that monitoring should be focused on holistic assessment of the infant as well as parents’ views of feeding over a 24-hour period and be documented in the ‘Our Feeding Journey’ record.
Feeding support techniques
Participants felt that there is no place for coercive techniques such as chin lift in a cue-based feeding protocol. Other techniques such as elevated side-lying position or pacing for bottle-fed infants should be used only as part of a prescribed programme, usually following an assessment for oral aversion or need for stimulation. No other feeding support techniques should be used.
The consensus of workshop participants was that feeding support techniques should not be included in the Cubs intervention.
Education for parents
Participants felt that education for parents should include description of cues, discussion of feeding options for when parents are not in the unit and where to record their wishes, focusing on quality of feeds and using the quality assessment tools. Education should also emphasise the importance of transitioning at their infant’s own pace and not assume that a quicker transition will lead to an earlier discharge. Education should also highlight that comparisons between infants can be misleading. A variety of formats for education and training were proposed, including visual images of cues for individual parents which could also be displayed in communal areas such as waiting areas and family rooms. Other suggested formats for education and training were videos, leaflets and conversations between parents and staff and between parents. It was felt that nurses were best placed to provide the information for parents, but peer education could also be included. Modelling of cue-based feeding by staff was also suggested to be an important form of education.
The consensus of workshop participants was that training should include all elements of the intervention and be provided in accessible, user-friendly formats and be reinforced by staff at every opportunity.
Education for staff
Discussions about staff training emphasised the importance of moving away from a focus on assessing quantity/volume to quality of feeds, and changing the language used to match this focus (e.g. not talking about ‘taking a feed’), and introducing the concept of a feeding journey. It was felt that information provided for staff (e.g. description of feeding cues) and the intervention protocol should be the same as that provided for parents, to ensure consistency. The methods of providing training included being part of mandatory training and part of induction for new staff and being provided by identified champions or clinical trainers in each NNU. Flexible formats were suggested such as posters, videos, formal teaching sessions and opportunistic one-to-one discussions.
The key point of consensus was that the information given to staff and parents should be the same to ensure consistency.
Format of the intervention
Workshop participants discussed how the intervention should be presented and consensus was reached that a simple easy-to-read flow chart was preferred.
Overview of the Cubs intervention
The overall aim of the intervention was to support staff and parents to feed infants in response to their cues, specifically when infants are transitioning from tube to oral feeding. The components of the intervention were a training package covering the approach to cue-based feeding and study procedures, a feeding protocol, feeding assessment tools, supplementary training materials in the form of posters, a film, a narrated PowerPoint presentation, and the ‘Our Feeding Journey’ document for recording each feed (see Appendix 4). The intervention is described using the Template for Intervention description and Replication (TIDieR) framework82 in Table 7.
| Item | Description |
|---|---|
| 1. Brief name: provide the name or a phrase that describes the intervention | Cubs: cue-based feeding for preterm infants in NNUs |
| 2. Why: describe any rationale, theory, or goal of the elements essential to the intervention | The overall aim of the intervention is to support staff and parents to feed infants in response to their cues, specifically when infants are transitioning from tube to oral feeding. This will be achieved by:
|
| 3. What (materials): Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers |
Intervention manual Training plan for staff Training plan for parents Quick reference guide (shortened version of the manual) Narrated PowerPoint presentation Cubs film Posters of cues: poster 1 – infant in a cot; poster 2 – infant in skin-to-skin contact Feeding flow chart Cubs Traffic Light Readiness to Feed Chart Breastfeeding (A-F) Assessment Chart Bottle feeding (A-E) Assessment Chart Our Feeding Journey record |
| 4. Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities |
All staff in the NNU will be trained using the training plan for staff via:Parents of infants receiving the intervention will be trained at the cot-side by care staff according to the parent training plan Staff will model cue-based feeding for parents Parents and staff will have access to the supplementary teaching resources Staff will discuss with parents their feeding goals and develop a feeding plan Each infant will be monitored by parents and staff for cues for readiness to feed orally: no minimum corrected gestational age for attempts at oral feeding will be applied Feeds will be given according to the infant’s cues guided by the Traffic Light Assessment Chart: no set rate of transition Intervals between feeds will be normally 1–3 hours with a maximum interval of 4 hours (clinical judgement of staff will be used) The giving of tube top-ups will be determined by the feeding assessment charts Parents or staff will document each feed in ‘Our Feeding Plan’ The plan for the next feed agreed between parents and staff |
| 5. Who provided: for each category of intervention provider (such as psychologist, nursing assistant), describe their expertise, background, and any specific training given |
Training for staff provided by key cascaders who are trained by the study team Parents trained by care staff who have received the staff training Intervention provided by parents supported by NNU staff including nurses, doctors and allied health professionals |
| 6. How: describe the modes of delivery (such as face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group |
Staff training provided face to face in groups or one to one Parent training provided face to face and one to one at the cot-side Supplementary training materials provided online, in paper copy and posters displayed around the NNU |
| 7. Where: describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | In the NNU or transitional care ward |
| 8. When and how much: describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | The intervention was delivered over the period of time from the infant showing signs of readiness to commence oral feeding until transition to full oral feeding |
| 9. Tailoring: if the intervention was planned to be personalised, titrated or adapted, then describe what, why, when and how | The intervention is predicated on individual infant’s feeding cues and therefore is tailored to each infant and parents |
| 10. Changes: if the intervention was modified during the course of the study, describe the changes (what, why, when and how) |
No formal modifications were made One site modified the intervention by applying a minimum corrected gestational age for infants fed by bottle (34 weeks) to align to the unit’s feeding protocols |
| 11. How well – planned: if intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them | Fidelity was assessed through qualitative interviews with parents and staff, observations of practice, and analysis of feeding documentation. Assessment was conducted by research nurses in each site |
| 12. How well – actual: if intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned | Results of the feasibility and acceptability study are reported in Chapters 9 and 10 |
Summary
The Cubs cue-based infant feeding intervention was developed based on evidence from a literature review, analysis of polices and guidelines, case studies and primary qualitative research, as well as the expert opinion of the research team and stakeholders including parents. The intervention was developed using an iterative process of synthesising the evidence base, gaining consensus from stakeholders (including parents) at a workshop, co-producing the intervention with parents, and having discussions with the research team, Stakeholder Advisory Group and the nurse managers of the three sites in which the intervention was implemented. The final version of the intervention was manualised (i.e. written as a manual). One area of contention was the exclusion of infants on high-flow oxygen; however, it was felt best to adopt a cautious approach. Although there was consensus that there should be no minimum gestational age for commencing the intervention, one implementation site modified the intervention for bottle-fed infants, applying a 34 weeks’ gestational age limit to align with the site feeding protocols.
In the following chapters, the methods (see Chapter 8) and results of the feasibility study are described, incorporating quantitative results including recruitment and retention (see Chapter 9) and qualitative findings (see Chapter 10).
Chapter 8 Methods of the feasibility study of the cue-based feeding intervention
This chapter describes the methods used for the feasibility study of the intervention described in Chapter 7. The primary aim of the study was to determine the feasibility and acceptability of progressing to a full-scale comparative evaluation of the Cubs intervention. The objectives of the feasibility study were:
-
to appraise the willingness of parents and staff to implement and sustain the intervention
-
to assess the associated costs of implementing cue-based feeding in NNUs
-
to determine the feasibility and acceptability of conducting a future RCT, including views on important outcomes
-
to scope existing data-recording systems and potential short- and long-term outcome measures.
Study design and theoretical framework
The mixed-methods feasibility study comprised quantitative and qualitative approaches to meet the above objectives. The study involved implementation of the intervention in three NNUs. The study was designed as a single-arm feasibility study; no comparison group was included. Feasibility outcomes of acceptability, adoption, appropriateness and fidelity were based on the model by Proctor et al. 83 We judged feasibility as whether the intervention was practicable to implement based on the views of the participants, as well as the expert opinions of the research team and Stakeholder Advisory Group. We used the Proctor et al. 83 definition of acceptability (i.e. that the intervention was ‘agreeable, palatable or satisfactory’).
Setting
The intervention was implemented in three NNUs, two in England and one in Scotland. The sites were selected to provide different sizes and levels of unit, and diversity of populations. For pragmatic reasons, units where members of the research team had established links were selected. Although all three units have full accreditation for the UNICEF UK BFI neonatal standards,68 at the time of selecting the units only site 2 had achieved this status. Table 8 shows details of the three units.
| Characteristic | Site 1 | Site 2 | Site 3 |
|---|---|---|---|
| Type of unit | Level 3: NICU | Level 2: local NNU | Level 3: NICU |
| Number of cots | 32 | 26 | 21 |
| Transitional care | Yes | Yes | Yes |
| Location | England | England | Scotland |
| Population | Urban, ethnically diverse, high levels of social deprivation | Urban and rural | Urban and rural, high levels of social deprivation |
| Approach to transition to oral feeding | Modified scheduled approach | A form of cue-based feeding | Scheduled feeding |
| Any breastfeeding rates on discharge | 62% | 75% | 56% |
| UNICEF UK BFI neonatal standards68 | Full accreditation | Gold award | Full accreditation |
Implementation
Implementation of the intervention commenced with a site visit by the research team members, who provided an initial 2-hour training session for the research nurse and the key cascaders on the intervention and the study procedures. Each unit was provided with paper and electronic copies of the intervention manual and resources, a pack for the parents of each infant containing the intervention information and resources, and a set of posters to be displayed around the unit. Links to the Cubs film and narrated PowerPoint were also included. It was planned that the intervention would commence once training had been cascaded to most staff. Commencement of the study was delayed for several reasons, including gaining approval from the study sponsor (University of Dundee) taking longer than anticipated and a delay in being able to circulate the film (one of the infants featured in the film died and it was felt that permission should be sought from the parents to retain that section of the film). In one unit, a planned move to temporary accommodation was delayed meaning that instead of taking place before the study started, it took place just as the study was starting. The principal investigator (PI) asked to delay the start of the study until the move was completed and the staff and parents were accustomed to their new environment. Recruitment took place over 7 months (August 2019 to February 2020). All follow-up was stopped in early March 2020, as NHS research was stopped because of the COVID-19 pandemic.
Quantitative methods
Quantitative methods were used to assess recruitment and retention, and clinical outcome data on infants included in the study.
Sample and eligibility criteria
The target population was preterm infants (< 37 weeks’ gestation), including infants of multiple births, who were at least partially enterally fed, in NNUs or transitional care settings. The sample size for the feasibility study of 20 infants from each unit was based on the expert opinion of the study team and discussion with the PIs at each site. This required the recruitment of three or four infants each month, at each site, over the 6-month planned recruitment period, which the PIs assessed as achievable. This would provide enough data on the outcomes, including estimation of recruitment rate, rates of completion of the intervention and follow-up, and clinical outcomes for the infants.
The eligibility criteria were developmentally normal preterm infants born before 37 weeks’ gestation, who were clinically stable and at least partially enterally fed, had an intragastric tube in place at the start of the study, and whose parent(s) consented to their inclusion in the study. Singleton and multiple births were eligible for inclusion and any planned feeding type or method [i.e. at the breast, expressed breast milk (EBM), formula or a combination]. The exclusion criteria were infants born after 37 completed weeks of gestation, infants who were not at least partially enterally fed, preterm infants who had transitioned to full oral feeding, and infants with major congenital anomalies, gastrointestinal disorders (e.g. necrotising enterocolitis), congenital infections and major neurological conditions (e.g. cerebral palsy, seizures, grade III–IV intracranial haemorrhage, periventricular leukomalacia). Infants on high-flow oxygen were also excluded from the study.
Recruitment procedures
Infants eligible to be included in the study were identified by the care team, usually a neonatal nurse, who introduced the parents to the study and provided them with the participant information sheet. Parents interested in participating in the study completed a reply slip, which was passed to the site research nurse. The research nurse discussed with the parent the information in the participant information sheet, responded to any questions and gained written consent from parents for their infant to be included in the study. Consent for the qualitative aspects of the study was taken at the same time.
Data collection and outcome measures
For recruitment and retention assessment, the site research nurses kept a log of the total number of infants screened, number eligible, number recruited and reasons for non-recruitment. Baseline and follow-up data were collected by research nurses from the infant’s care records, as shown in Table 9.
| Outcome/characteristic | Birth | Start of intervention | Transition to full oral feeding | Discharge from NNU | 2-week follow-up |
|---|---|---|---|---|---|
| Gestational age | ✓ | ✓ | ✓ | ||
| Singleton/multiple birth | ✓ | ||||
| Age in weeks since birth | ✓ | ✓ | |||
| Days from start of intervention | ✓ | ✓ | |||
| Weight | ✓ | ✓ | ✓ | ✓ | ✓ |
Data on feeding outcomes were taken from the ‘Our Feeding Journey’ record designed specifically for the study. Outcomes included cues observed, interval between feeds, type of feed (breast milk, formula, combination) and method of feeding (at breast, bottle, tube, other). The research nurses scanned the documents for transfer to the research team and parents could keep the document if they wished. The 2-week follow-up data were collected via a telephone call or e-mail by the site research nurse.
Data analysis
Analysis took place after all data were entered in the database and the database had been locked. The number of missing data were examined to inform decisions about outcome measure selection for a definitive trial. Outcomes were summarised as means, medians and standard deviations for quantitative variables, and percentages and denominator for categorical variables. The distributions of outcomes were explored and transformations used where appropriate. Baseline characteristics for infants were tabulated in total and by site. The primary analysis consisted of descriptive measures at baseline, during intervention, discharge and at the 2-week follow-up post discharge. The changes from the start of the intervention to discharge included age at discharge, days from intervention to full oral, days from intervention to discharge, weight at transition to full oral feeding (g), weight at discharge (g) and weight at the 2-week follow-up (g). The mean weight per day of the intervention was also calculated. All analyses were performed using IBM SPSS Statistics version 26 (IBM Corporation, Armonk, NY, USA). The results of the quantitative data are presented in Chapter 9.
Feeding data from the ‘Our Feeding Journey’ documents were entered into a database. Owing to the large number of data collected, we entered data from a 24-hour period each week, starting on day 2 of the intervention (to account for infants starting the intervention at different times on the first day) and then each following seventh day until the infant was discharged. In addition, to capture the last full 24 hours on the intervention, data from the day before discharge were also included in the analysis. Missing data (i.e. where not all feeds were documented every day) made it difficult to achieve an accurate analysis of cue-based compared with scheduled feeding based on times of feeds. Therefore, on the advice of the Stakeholder Advisory Group, we selected three infants (one from each site) with the most comprehensive data to provide more detailed case studies. Each infant’s transition to oral feeding journey is unique; therefore, the case study approach was used to provide insight to the experience of transitioning to oral feeding. The case studies were not intended to be representative of the full range of participant experience. For each individual case study, we summarised data collected at baseline, during the intervention and at follow-up. Start and stop cues were analysed using percentages to identify the most common cues recognised by staff and parents.
Qualitative methods
The quantitative data were supplemented with qualitative data as part of a process evaluation to provide a more in-depth understanding of implementation outcomes of acceptability, feasibility, adoption and fidelity. 83 The study also included qualitative data from interviews with parents of infants receiving the intervention and staff involved in its implementation. Further data on fidelity were collected through observations.
Sample and recruitment
We aimed to conduct semistructured interviews with a subset of a total of 30 parents from the 60 infants included in the study, comprising 10 from each site. For the observation we did not define a sample size but aimed to conduct 18 hours of observation (six 3-hour observations) in each site. The consent for the semistructured interviews and observations was taken at the same time as the consent for the infants to be included in the study.
We aimed also to include a range of health-care practitioners involved in caring for infants as they transitioned from tube to oral feeding and supporting parents. Our target sample was 10–15 staff members from each unit, including neonatal nurses, nursery nurses, infant feeding co-ordinators, doctors, and speech and language therapists. Members of staff were recruited through an e-mail invitation to all staff members, which included the participant information sheet as an attachment. Staff members who were willing to be interviewed were requested to contact the site research nurse to ask any questions and arrange a convenient time and venue for the interview. Written consent was taken at the outset of the interviews. For the non-participant observation of practice, the site research nurse identified a day and time when there was at least one infant enrolled in the study on the unit. At least 24 hours prior to a planned, non-participant observation session, the research nurse contacted (either face to face or by e-mail) all staff who were rostered to be on duty during the observation to inform them that it was planned and to provide the participant information sheet. Written informed consent was taken immediately prior to the planned observation.
Data collection
The purpose of the interviews with parents was to explore in depth their experiences and views of cue-based feeding, the acceptability of the intervention, whether or not it was implemented as intended, parental satisfaction with care and support for infant feeding, how parents would feel about a future randomised trial and their views on important outcomes for such a trial. A semistructured topic guide was developed based on the above aims, the objectives of the study and a framework of implementation outcomes. 83 Interviews were conducted face to face by the research nurses on the NNU. As the study progressed and it became clear that arranging the interviews with parents while they were on the NNU was challenging, an amendment to the protocol was made to offer a telephone interview following discharge. However, no parents took up this option. The interviews lasted 30–60 minutes and were audio-recorded with the consent of the participants.
The purpose of the interviews with staff was to explore their views and experiences of the training provided, of implementing cue-based feeding in the context of their unit (including tailoring or modification of the intervention), and to assess acceptability, adoption, appropriateness and fidelity. A further purpose of the interviews was to assess willingness to support a future randomised trial and to identify important outcomes for such a trial. An interview topic guide was developed based on the above aims, the study objectives and implementation outcomes. 83 Interviews were conducted at a time and place that was convenient for the interviewee. Interviews lasted 40–60 minutes and were audio-recorded with the consent of the participants.
The purpose of the observations was to assess fidelity to the intervention. The observations were guided by a checklist and field notes with a focus on fidelity, and interactions between staff and parents concerning feeding. Observations were conducted by the site research nurses.
In terms of gathering data on costs, on the advice of the health economist member of the Stakeholder Advisory Group, we included questions in the interview topic guides to assess any additional time or resource needed by staff or parents, and any out-of-pocket expenses incurred by parents and families.
Data analysis
The audio-recordings of interviews were transferred securely from the sites to the University of Dundee and were transcribed verbatim. The transcripts were checked and anonymised by a member of the research team. We used a framework approach to analyse the interview and observational data. 84 This comprised a seven-stage approach: transcription, familiarisation, coding, developing a working analytical framework, applying the analytical framework, charting data into the framework matrix, and interpreting the data. The analysis was supported by qualitative data analysis software (NVivo; QSR International, Warrington, UK). The analytical framework was derived deductively using the implementation outcomes described by Proctor et al. 83 and inductively (i.e. incorporating new themes in the data through open coding). This approach to analysis enabled comparison by themes across multiple accounts as well as retaining the context of individual experience. To enhance reliability of the coding, two researchers independently coded the first few transcripts before agreeing a set of codes to apply to all transcripts. The research team met regularly during the analysis to discuss interpretation of research material. As well as using the Proctor et al. 83 implementation outcomes across staff and parent interviews, the staff transcripts were analysed using a normalisation process theory85 framework to assess context, attitudes, organisational support and barriers to the intervention.
Ethics approvals
Approval for the feasibility study was given by the North of Scotland NHS Ethics Committee (19/NS/0055), the Health Research Authority and NHS Research Scotland. Research and development approval was given by the relevant NHS trusts in England and health board in Scotland.
Summary
In this chapter we have described the methods used for the feasibility study. This includes the study objectives, design and theoretical framework, the implementation process, the research settings and the methods for the quantitative and qualitative components of the study. The following two chapters present the findings of the quantitative analysis (Chapter 9) and the qualitative analysis (Chapter 10). These findings were then taken forward to the final stage of the research (WP 4), as described in Chapter 11.
Chapter 9 Quantitative findings of the feasibility study
In this chapter, the results of the quantitative analysis described in Chapter 8 are presented. First, we address recruitment and retention relating to the recruitment of infants to the feasibility study, including rate of recruitment in total and by each site, as well as retention to the 2-week follow-up visit. We discuss specific challenges to recruitment and retention. Second, the characteristics of the infants at baseline (birth and start of the intervention) and on transition to full oral feeding and discharge are presented. This includes the following outcomes: number of days from start of intervention to full oral feeding and to discharge, and change in weight from start of intervention to full oral feeding and discharge. Finally, we present data on feeding outcomes and an analysis of the information provided in the Our Feeding Journey documents.
Recruitment and retention
The flow of infants in the study and the reasons for non-recruitment are shown in the Consolidated Standards of Reporting Trials (CONSORT) flow chart86 (Figure 3).
FIGURE 3.
The CONSORT flow chart.
Recruitment overall was about one-third of those screened, but varied by site from a low of 20% in site 2 to 47% in site 3 and 42% in site 1 (Table 10).
| Screening and recruitment | Number of participants (%) | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Total | |
| Total number screened | 48 | 78 | 30 | 156 |
| Recruited | 20 (41.6) | 16 (20.5) | 14 (46.7) | 50 (32.1) |
| Not eligible | 11 (22.9) | 8 (10.3) | 1 (3.3) | 20 (12.8) |
| Declined | 7 (14.6) | 21 (26.9) | 10 (33.3) | 38 (24.4) |
| Not consented | 1 (2.1) | 10 (12.8) | 2 (6.7) | 13 (8.3) |
| Reason unknown | 9 (18.8) | 23 (29.5) | 3 (10.0) | 35 (22.4) |
Overall, 13% of those screened were not eligible. The main reasons for this were that oral feeding was already established (n = 16) and medical conditions (n = 4). The fact that some infants were ineligible because they had already transitioned to full oral feeding suggested that screening took place too late in the infant’s feeding journey. Only 37% (n = 50) of eligible infants were recruited to the study. This included 28% (n = 38) whose parents declined to participate and 10% (n = 13) who were not consented (owing to either the research nurse or the parent not being available); for 26% (n = 35) of infants, the reason for non-recruitment was not documented. Although reasons for declining were not recorded, feedback from study research nurses suggested that many parents felt too overwhelmed at this vulnerable time to think about participating in research. Site 3 experienced a particularly quiet period when there were few infants in the unit, and an extremely busy period when staff reported that they lacked time to approach parents about the study. This affected the recruitment rate.
Table 11 shows recruitment in relation to the target of n = 60, with overall recruitment of 83%. The mean recruitment rate was seven infants per month, with a range of 3–12.
| Site | Number of participants | Percentage of target | |
|---|---|---|---|
| Target | Recruited | ||
| 1 | 20 | 20 | 100 |
| 2 | 20 | 16 | 80 |
| 3 | 20 | 14a | 70 |
| Total | 60 | 50 | 83 |
Of the 50 infants recruited to the study, 49 received the intervention (one was withdrawn, the reason was not documented) and 48 were followed up until discharge from the NNU (one was transferred to another NNU). Follow-up rates at 2 weeks post discharge were much lower with only 38% (n = 19) followed up giving a 62% drop-out rate. Non-response to telephone calls was the main reason for loss to follow-up. There was wide variation between sites with the lowest drop-out rate at site 1 (30%), followed by site 3 (77%), and the highest at site 2 (87.5%). Why there was such variance across sites is unknown, but feedback from the research nurses suggested that it may be partially related to how much effort was made to contact parents. For example, one research nurse described making multiple attempts to reach parents. Another research nurse had informed parents in advance that the follow-up telephone call would come from a ‘withheld number’. This advance knowledge may have increased the likelihood of parents responding to the telephone calls.
Evaluation of outcomes
Table 12 shows the baseline characteristics of the infants in the study including weight and gestational age at birth and at the start of the intervention.
| Characteristic | N | Mean (SD) or n (%) | Median (range) |
|---|---|---|---|
| Gestational age at birth (weeks) | 49 | 30.3 (3.0) | 30 (23–36) |
| Birth weight (g) | 49 | 1530 (548) | 1380 (540–3018) |
| Singleton | 49 | 38 (77.6) | |
| Multiple | 49 | 11 (22.4) | |
| Corrected gestational age at start of intervention (weeks) | 49 | 34.6 (1.7) | 35.0 (31–40) |
| Weight at start of intervention (g) | 46 | 1990 (454) | 1975 (1310–3120) |
Among the 49 infants with evaluable data, the mean gestation at birth was 30.3 weeks and the mean birthweight was 1530 g. Of these infants, 78% were singleton births and 22% were multiple births. The mean gestational age at the start of the intervention was 34.6 weeks, with a mean weight of 1990 g.
Table 13 shows the characteristics of the infants at transition to full oral feeding and at discharge from hospital.
| Characteristic | N | Mean (SD) or n (%) | Median (range) |
|---|---|---|---|
| Age at discharge (weeks from birth) | 46 | 6.2 (5.3) | 5.5 (0–31) |
| Corrected gestational age at discharge (weeks) | 46 | 37.4 (2.1) | 36.6 (34–44) |
| Days from intervention to full oral feeding | 43 | 10.8 (9.5) | 9.0 (0–43) |
| Days from intervention to discharge | 43 | 14.4 (10.4) | 12.0 (0–43) |
| Weight at full oral feeding (g) | 44 | 2341 (558) | 2222 (1665–4293) |
| Weight at discharge (g) | 47 | 2434 (588) | 2330 (1505–4293) |
At time of discharge from the NNU, the mean corrected gestational age was 37 weeks and 4 days (range 34+3–44+2 weeks) with a mean time of 14.4 days from the start of the intervention to discharge. The mean number of days from the start of the intervention to transition to full oral feeding was 10.8. Mean weight increased from 1990 g at the start of intervention to 2434 g at discharge. The mean daily change in weight from intervention start to discharge was 25 g. The change in weight from birth to 2-week follow-up is illustrated in Figure 4.
FIGURE 4.
Weight from birth to weight at 2-week follow-up.
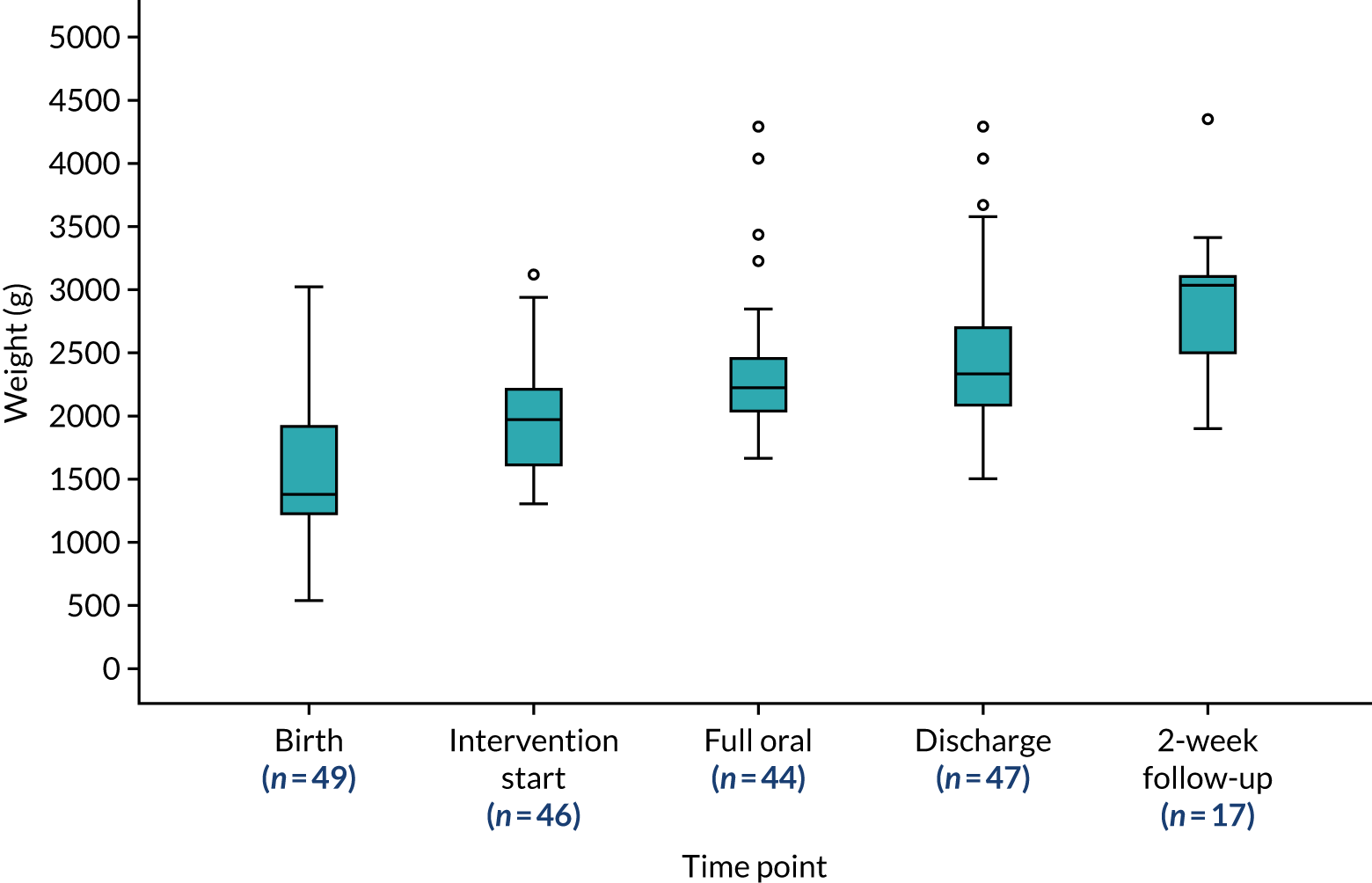
The mean age at the 2-week follow-up was 71 days from birth, with a mean corrected gestational age of 40 weeks and 2 days. The mean weight was 2842 g (Table 14).
| Characteristic | n | Mean (SD) | Median (range) |
|---|---|---|---|
| Age from birth at 2-week follow-up (days) | 19 | 73.7 (30.3) | 76.0 (22–134) |
| Corrected gestational age at 2-week follow-up (weeks) | 19 | 40.2 (2.41) | 39.1 (37–45) |
| Weight at 2-week follow-up (g) | 18 | 2842 (571) | 2959 (1900–4350) |
Feeding outcomes
Prior to their infant starting the Cubs intervention, most of the parents (n = 37) indicated that their feeding preference would be to exclusively breastfeed their infant. Figure 5 shows the feeding outcomes for these infants. On discharge, 21 of the 37 infants were being breast fed exclusively or were receiving EBM only or were fed with a combination of breastfeeding and EBM. A further six were breastfeeding and/or receiving EBM supplemented by infant formula and seven were fed exclusively with infant formula. One infant who was discharged was breastfeeding with top-ups of EBM via nasogastric tube. The remaining two infants were transferred to another unit before discharge, and were therefore lost to the study. Thirteen of the 37 infants were followed up at 2 weeks. At this time point, five infants were breastfeeding and/or receiving EBM exclusively, four were breastfeeding and/or receiving EBM supplemented by infant formula and four were fed exclusively with infant formula.
FIGURE 5.
Feeding outcomes for infants of parents who intended to breastfeed exclusively.
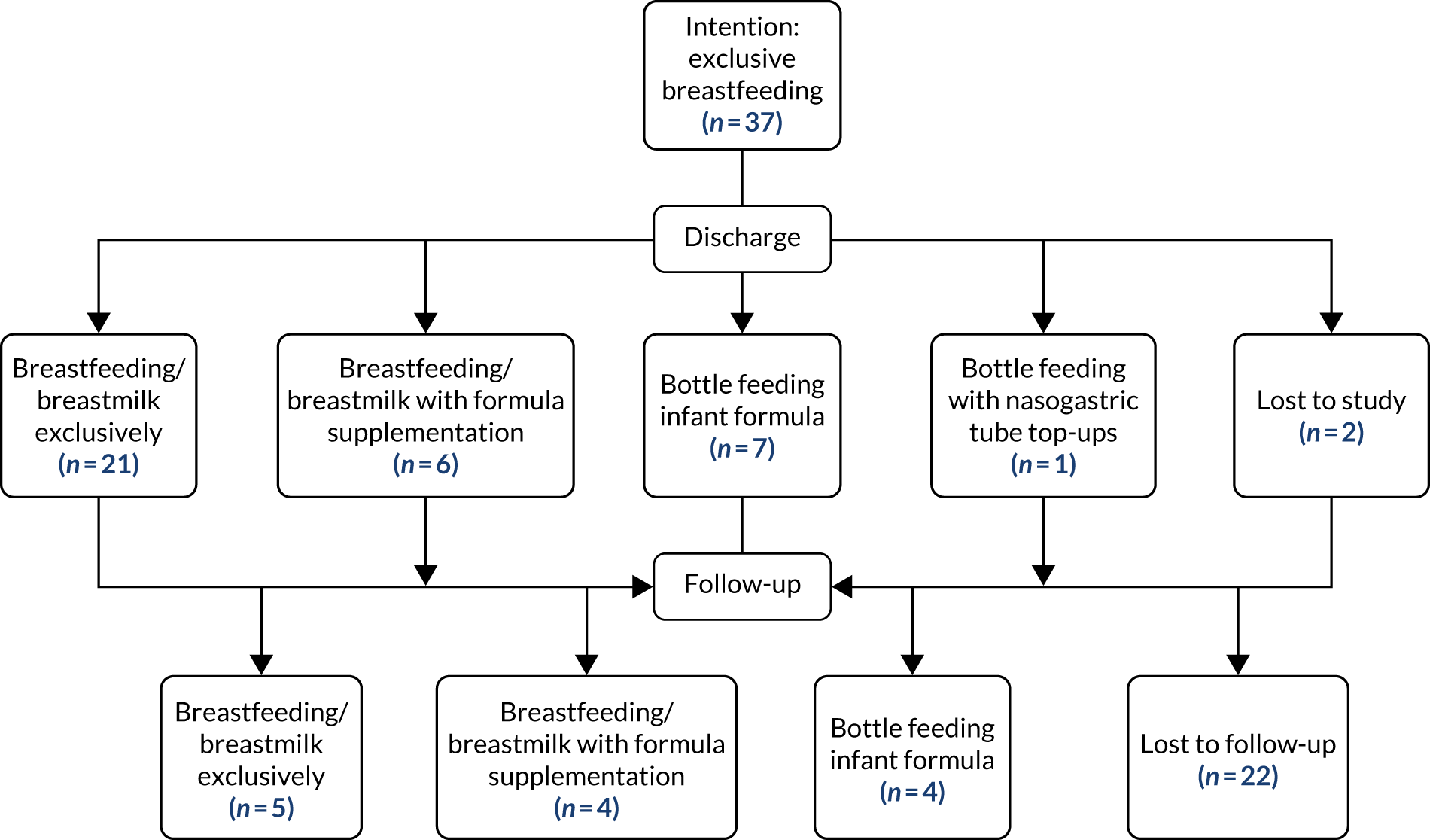
Of the remaining parents who joined the study, two planned to combine breast and infant formula feeding. On discharge one parent was feeding their baby infant formula exclusively and the other baby was transferred to another unit prior to discharge and was therefore lost to the study. The remaining nine parents stated that they planned to feed their baby with infant formula and were doing so at discharge. Two parents’ preferences were not documented.
At follow-up, seven mothers reported that they felt their infant was having feeding complications. This was cited as the reason for introducing infant formula feeding after discharge (e.g. they were concerned that their infant was not getting enough milk at the breast). However, 17 out of 19 mothers reported that they were confident and satisfied with their infant’s feeding.
Feeding journeys
The ‘My Feeding Journey’ document enabled parents and staff to chart an infant’s journey from tube to oral feeding. They were asked to detail the start and stop cues shown by the infant; to provide detail of feeds (such as breast or bottle feed, type of milk fed and duration of feed); to provide detail of top-ups given; and to score the feed using feeding assessment charts. Free-text boxes enabled comments to be made on the current feed and any plans for the next feed.
Of the 50 infants recruited to the study, journals were returned for 36. Others may have been completed but they were taken home by parents. Data for 483 feeding days were collected. Owing to the variation in completion of the journals, we have selected those with the most complete information as case studies. Feedback from the Stakeholder Advisory Group suggested that this would be the most informative way to present the data. We present case studies of three infants, one from each site, to show variations in how the intervention was implemented and to include breastfeeding and bottle feeding experiences.
Case studies
Infant 17, site 1
This infant was from a singleton pregnancy born at 25+2 weeks’ gestation, weight 970 g; this infant was one of the most preterm in the study. Prior to birth the mother had intended to breastfeed.
The infant started on the intervention at 35 weeks’ corrected gestational age and weight 1710 g. The infant’s first breastfeed was on day 1 of the intervention in response to start cues of rooting and hand sucking and took place 90 minutes after a tube feed.
By day 7, the infant was awake and showing cues at five out of the eight feeds documented. The infant was tube fed overnight when the mother was not present, but breastfed throughout the day. The infant was said to tire at the breast and half top-ups were given in line with breastfeeding assessment scores. This feeding pattern continued until day 15, after which no feeding data were documented until the infant was recorded to have achieved full oral feeding on day 18. Discharge was on day 26, when the infant was 13 weeks old, with a gestational age of 38+5 weeks, and weighed 2090 g. When the infant went home, they were fully cue-based breastfeeding.
At follow-up the infant was described to be cue-based breastfeeding frequently, with additional breast milk given by bottle to aid medication administration. The infant had gained a further 490 g over the 2 weeks since discharge and the parents reported feeling confident and satisfied with feeding.
Infant 14, site 3
This infant was from a singleton pregnancy born at 27+4 weeks’ gestation, and weighing 726 g. The parent’s pre-birth intention was to bottle feed infant formula.
Oral feeding was introduced when the infant was just over 6 weeks old at corrected gestational age 34+2 weeks, weighing 1310 g. This was the lowest weight of all infants in the study at commencement of oral feeding. The first oral feed documented was a bottle feed given when the infant was showing cues of mouthing and putting hands to mouth. The infant was described to have taken the bottle well and the feed was scored as D, indicating that the infant was alert and fed with strong co-ordinated sucking and breathing. However, after a desaturation episode at the next feed attempt, the remaining feeds on day 1 were full tube feeds.
By day 9 of the intervention, the infant was fed at 3-hourly intervals. Half of the infant’s bottle feeds were given when the infant was showing start cues and half top-ups were given regularly at this stage. By day 16 the infant was fully orally fed by bottle and received only one small top-up. Feeding intervals ranged between 2.5 and 3.5 hours. Feeding cues were recognised for every feed, although sometimes the late cue ‘crying’ was documented. The infant was described as feeding slowly but all feeds are scored as ‘E’, showing that feeding ability had progressed well over the week.
This infant was discharged home on day 17 at 36+4 weeks’ corrected gestational age, weighing 1730 g (an increase of 1004 g since birth) and fully bottle feeding. Feeding was described as a combination of cue-based and scheduled feeding.
At the 2-week follow-up, the infant was 11 weeks and 2 days old, corrected gestational age 38+6 weeks, and weighed 2100 g, a further increase of 370 g since discharge. The infant was fully bottle fed to a schedule and the parents did not report any feeding concerns, although the infant required a surgical procedure for an inguinal hernia following discharge.
Infant 8, site 2
This infant was a singleton born at 35+1 weeks’ gestation and weighing 3018 g. The mother had documented in the feeding journal that she was very keen to breastfeed as she had felt that her feeding experience with her first infant could have been better.
The infant quickly progressed on to the intervention at 1 week and 2 days old (36+3 weeks’ corrected gestational age), weighing 2760 g. The infant progressed well, and the parent had commented that the infant ‘needed to build stamina feeding at the breast’. Feeding assessment scores and the use of top-ups were consistent with these comments.
As can be seen from Figure 6 (which is a segment of the infant’s transition), this infant was fed according to cues from the start of the intervention. The straight line in the middle of the graph is an overnight period when the mother was not present and the infant was fed by nurses on a schedule. When the mother returned the next morning, the infant was again fed in accordance with cues. The infant was discharged home after 6 days on the intervention, and was fully cue-based breastfeeding.
FIGURE 6.
Feeding journey extract.
Analysis of feeding cues
Within the journals there were over 770 documented feeding start cues. The most common cues recorded were awake and rooting. The full analysis of the range of cues is shown in Figure 7.
FIGURE 7.
Start cues.
Crying was documented 75 times across 24 infants and was the third most common feeding cue. This is a late cue, which would indicate that the infant’s early start cues had probably not been recognised. There did not appear to be a pattern to the infants who cried before feeding, although two infants transitioning to feed at the breast were both reported to cry regularly on a day that they were fed frequently.
Stop cues were not reported as often as start cues (Figure 8). The most frequently reported stop cues were ‘tires/falls asleep’ and ‘stops sucking’. More subtle cues, such as the infant raising their hands, were documented only once.
FIGURE 8.
Stop cues.
Although a large number of start cues were identified within the ‘My Feeding Journey’ record, a large number of feeds appear to have been given on a 3-hourly schedule. This may indicate that the recognition of these cues was not the driving factor for feeding the infant.
At discharge, 10 infants were fed through a combination of cue-based and scheduled feeding; at the 2-week follow-up this number was five (out of 19). Eleven infants were fully cue-based feeding at discharge and by follow-up this had dropped to six (out of 19). One infant was discharged on scheduled feeding; this increased to two infants by follow-up. For a large proportion of the infants at discharge (n = 26) and at follow-up (n = 5), the approach to feeding was not documented.
Summary
The study recruited > 80% of the target sample over 7 months, with an average recruitment rate of two infants per site per month. Retention in the study until discharge from the NNU was high, but there was a low rate of follow-up at 2 weeks after discharge. We were able to collect data on important outcomes including weight, number of days to transition to full oral feeding and time from intervention start to discharge from the NNU.
The data on infant feeding outcomes were not as comprehensive, and we had to rely on the ‘Our Feeding Journey’ documents, which varied in completeness of data. Compared with the stated feeding intentions of parents, there was a gradual overall decrease in the number of infants who were fully breastfed at discharge, and at the 2-week follow-up. The analysis of case studies suggested reasonable fidelity to the intervention, although there were indications that a 3-hourly schedule of intervals between feeds was often followed. In terms of the cues recorded, start cues were documented more frequently than stop cues.
In the next chapter we present the findings of the qualitative analysis which add depth and explanation to some of the quantitative findings.
Chapter 10 Qualitative findings
In this chapter we present the findings of the qualitative analysis focusing on the intervention context, and the acceptability, adoption, appropriateness and fidelity of the intervention. We present also the perceived benefits and mechanisms of effect and the views of participants on important outcomes for a definitive evaluation and willingness to be randomised. The findings are based on interviews with 14 parents, 16 members of staff and 21 hours (in seven episodes) of participant observation.
Recruitment and participants
Fourteen interviews with parents took place, 47% of the target of 30. Six took place in site 1, three in site 2 and five in site 3. A key challenge to conducting the interviews was finding a time and place suitable for both the parent and the research nurse. Many parents did not wish to leave the cot-side or be distracted from their infants, and it was difficult to achieve quiet and privacy on the NNUs. One research nurse reported that she had needed to make several visits to find a time when the parent was not busy with the infant. It was easier to conduct the interviews if the infant was in a room on their own. In some cases, the infant was discharged before the research nurse was able to conduct the interview with their parent. The Parents’ Panel suggested that one way to overcome this was to undertake the interview by telephone following discharge; the study protocol and approvals were amended to offer this, but no parents took up this option.
Sixteen interviews with staff took place, 53% of the lower target of 30. Three took place at site 1, five at site 2 and eight at site 3. Finding a suitable time and location for the staff interviews was challenging in the context of busy workloads and units’ layouts. Feedback from the research nurse at site 1, where there was a particularly low level of recruitment (this was the unit with the largest number of staff), suggested that time was the greatest issue, and that the unit management was not particularly supportive. It was particularly challenging for the research nurses to find a time to interview the medical staff and only one was completed.
Twenty-one hours of observation took place across the three sites on seven occasions (two each in sites 1 and 2, and three in site 3) representing 38% of the target of 54 hours on 18 occasions. The main barrier was gaining consent from staff. Feedback from the research nurse in site 1 suggested that staff felt that their practice was being judged.
The COVID-19 pandemic affected the number of interviews conducted, which otherwise would have continued throughout March and April 2020.
We present the findings in three key sections: contextual factors that influenced the implementation of the intervention, implementation outcomes and views on a future study. To preserve anonymity, as there were small numbers of parent and staff interviews, we provide only the site and whether or not the participant was a parent or staff member as contextual information for participant quotations.
Contextual factors
There were several contextual factors, (external to the intervention) identified across parent and staff perspectives with the potential to positively or negatively affect the implementation process.
Factors positively affecting the implementation process
Having a supportive, respectful environment in the NNU was an aspect of their experience that parents consistently highlighted across the three study sites:
. . . it depends on what the situation is but what I’ve gone through with losing my daughter last year, these guys are just amazing to support you and a bereavement and, welcoming a new baby as well, so they can treat you any way you need to be treated, respected, given your distance from other members and the staff that are coming in to see you, ‘Are you OK?’ and they’re really respectful. All I could really say is if your baby is born and it’s there, it’s in the best place.
Parent 13, site 3
Other supportive factors mentioned by parents included welcoming other children, having parent-led ward rounds and encouraging parents to do as much as possible for their infants.
Staff noted that the ethos of their NNUs was well aligned with the fundamental principles advocated and promoted by the Cubs intervention. Most notable were comments about having open access so that parents could be with their infants as much as possible and encouraging skin-to-skin contact:
Yeah so we follow the Baby Friendly UNICEF guidelines and we really advocate that parents are at the centre of a baby’s care and we advocate that parents should be there 24/7 if they’re available and are happy to be and the baby should be skin to skin as much as possible.
Staff 4, site 2
Factors negatively affecting the implementation process
Access to the unit and ability to stay were important contextual factors that could affect the implementation process. However, there were also implications for parents, such as social isolation, nutrition and family support, associated with spending time in the unit:
The biggest thing is allowing me to stay, to have done the 24 care because it’s not realistic to have had her there and then all of a sudden, she comes home with me with the little time that I got to spend with her. I think the hardest thing about here is it’s quite lonely for a mum, my partner obviously, has to be at work.
Parent 8, site 1
Other parents reported that they were unable to be with their infant as much as they would have liked because of other responsibilities and children. For one disabled parent, there were no accessible facilities for her to be able to stay overnight with her infant.
Some staff perceived the intervention required staff to put more demands on parents:
[There is] quite a bit of a difference between the scheduled feeding and responsive feeding because if the parents are tucked away in a room they are very much on the schedule, well the baby isn’t due for another half an hour but it’s waking up early – It’s quite hard to get those parents out of that whole routine of, ‘Gosh, well she’s not due now so I’m just going to stop, I’m not going to feed her’. And it’s quite hard to implement that at times because you kind of need to say, ‘Well actually, yeah, you’re tucked away in a room but actually we want you to be in a room because we want you to be more alert and more aware of the baby’s feeding cues’.
Staff 3, site 2
Another important contextual factor noted by staff, which could negatively affect the implementation process, was associated with the extent to which staff relied on professionals with mixed clinical backgrounds and/or previous NNU experiences when they were not as used to supporting feeding practices:
I don’t think they [families] experienced the same things necessarily, because everybody works in all different areas, and if a person that’s been working up in the intense [intensive care] bit comes down to like the low care area where the babies are wanting to feed, they might not necessarily know what they need to do . . .
Staff 3, site 2
Implementation outcomes
Acceptability
Overall, the intervention procedures were perceived as acceptable, and the intervention materials were well received and perceived as useful by both staff and parents:
Yes it [my experience with Cubs] has been positive. It kind of puts you more in tune with your child so instead of waiting for them to cry and get really agitated and irate, you kind of look, say, that half an hour before that so it’s a relaxed feeding experience rather than an agitated moving around and stressful feeding.
Parent 9, site 2
Yeah, just being able to tell more of the cues that I probably wouldn’t have noticed or picked up on so much [. . .] It was more the progress from coming into low dependency, when I kind of took over more of the feeding [. . .] So I was given the pack, which explained it a bit and it had the kind of handy A5 little handout thing [quick reference guide]. I found that quite handy.
Parent 1, site 2
Staff highlighted how helpful the pictures of cues, in the posters and parent packs, were to help parents to recognise cues:
I do think pictures were really useful at supporting learning. Lots of parents really, especially after a few weeks, their first feedings, a lot of them are quite anxious about whether they were doing it quite right but they, it seemed, I do think it really did empower them because they, they’d sort of be quite, ‘Oh yeah, they did this, they did that’, and I think it does sort of focus them a bit.
Staff 4, site 2
Staff noted that some parents could feel anxious as part of the cue-based feeding learning process. However, this was not perceived as problematic in terms of the acceptability of the intervention from the parents’ perspective, but rather, as something to consider and address as part of supporting parents in their cue-based feeding learning process:
With some parents it’s been a bit nerve-racking for them because they’re used to organising their time and if we’re just waiting for the baby to sort of wake up and feed, they’re almost sort of looking at the clock and thinking, ‘Oh, they should have had a feed, they’re not going to be putting on weight’ and getting a bit anxious.
Staff 1, site 2
Some staff found it challenging to fit some of the added tasks of the intervention, such as the recording procedures, into an already busy workload:
I did find that sometimes it was just a bit too much writing. Like, too much stuff to fill in and I think there was a box for input and output, which I can see is slightly relevant, but it was just an awful lot of stuff to put down and I think if it had been quite basic, this is how much we’re having, this is how we’re having it, you know, and maybe I think that was just a bit of repetitiveness with what you were documenting.
Staff 5, site 2
Staff commented on the shared documentation with parents as being important to developing a shared communication about their practices:
The quick reference guide with pictures on it. That was really helpful. We’ve got posters all over the unit to be honest and as I say, we were doing it before so it’s engrained into us, really, cue-based feeding. But, yeah, I think, yeah, we know how to read their signs and we’ve got a speech and language therapist as well so she helped us so yeah . . .. Yeah, definitely, team work.
Staff 2, site 1
Adoption
Uptake was uneven among members of staff, mainly owing to a limited reach in terms of the communication and engagement activities required to make all members of staff aware of the intervention:
I think it’s really important, it’s really, really easy to miss part-time staff. I don’t think that’s a new problem, I don’t think it’ll ever go away, and I think it is a challenge if you’re rolling anything out. You can’t guarantee that you’re going to see, you know, roll it out to every member of staff consistently, I think that’s an impossible target to set but I think that’s one big consideration that needs to be in place, you know, right at the beginning of how we’re going to implement something, how are we going to include the part-time members of staff.
Staff 2, site 2
In some instances, the uneven uptake of the intervention among members of staff was perceived by parents:
It [support received from staff about cue-based feeding] was really quite mixed, to be honest, it was quite mixed. There were some who were really keen, who had obviously done their research, they knew what they were doing; and some who were a bit more ‘I’m not too sure’, you know, it was always kind of left that if they weren’t sure they did go and find – they went and asked somebody else, and they did – so I was always given the information, but staff-wise, I think it was very mixed.
Parent 15, site 3
One key argument provided by members of staff to explain uneven uptake was extent of direct training (cascade training), and roll-out of the study. This was perceived as not comprehensive enough and often leading to over-reliance on those members of staff interested in the intervention:
So, did I receive training on Cubs intervention? No. I was directed, sort of self-directed, to the films, PowerPoint . . . I haven’t been told where they are, I’ve looked in the pack and that sort of thing, but I didn’t have training per se, it was sort of I found the paperwork [and] ‘can you tell me about it?’ and then self-, you know, reading it, because it’s fairly straightforward but that was all I sort of had really.
Staff 2, site 3
And even, I know they tried to do it [training], I think really, trained up for this study, for maybe eight staff or something was it, approximately? I could only probably say one, [nurse’s name] and actually, yourself [research nurse] has probably been the most informative people.
Staff 4, site 3
Medical staff awareness of the intervention was also perceived as limited and was seen as a potential route to improve the adoption and embedding of the intervention:
I would say probably the one improvement would be to increase the awareness in the medical staff of exactly what’s going on because actually probably it would be quite helpful for me to have a look at that feeding journey booklet and know how the baby is progressing and how the parents feel the baby is progressing and how close they are to being ready to move on. It’s obviously my fault, I haven’t been looking at it but it hasn’t been at the forefront of my mind. So I think having that kind of approach established in the unit and having everybody very aware of what documents there are and where to look for them is probably a good idea.
Staff 8, site 3
Similarly, some parents perceived that medical staff were not as supportive of the intervention as nursing staff:
I think the nurses specifically [supported the intervention] because I still, I think the doctors are very, ‘Oh, she’s had three feeds in a row’ whereas the nurses are a bit like, ‘Yeah, but that’s what cue-based feeding is. If she’s looking for a feed then we want to try her, whether it’s 5 minutes, 20 minutes, 1 minute, if she’s looking we’re going to give her a good’. [. . .] [nursing staff] they’re more, they’ll more stick up for you as well [. . .]. In the ward round when the doctors are maybe like asking why.
Parent 11, site 3
Appropriateness
The Cubs intervention was perceived as fitting, relevant and compatible with both routine practice and service users’ expectations of the care provided for them in NNUs:
I don’t think I dislike it [cue-based feeding] now that I understand it. I thought it, before I, I’d come and read all and kind of got my head around it, it felt a bit like it was another thing that I, you know, but now I understand it, it makes sense.
Parent 9, site 2
It [the Cubs intervention] validates the kind of care that we give anyway, I like that it recognises the parents as primary care givers, I like that the baby is allowed to say, I want some food and actually I really don’t want some food because all babies are individual.
Staff 2, site 2
Parents’ experiences showed how cue-based feeding felt less clinical and more family centred to them:
It’s been easier because I’ve been less panicking about what time is it; what time is it? And obviously, the feeds that I have done with her because she actually wanted them, they’ve been really sort of smooth. Whereas obviously, the timed one, although it’s great because she’s getting regular food, she doesn’t want it, you’re forcing food down her and it’s probably not a nice experience for her.
Parent 8, site 1
Although this was consistent with staff experiences, they also highlighted that there was an element of resistance to change from other staff that had to be overcome:
. . . it’s much more fluid in its application. It isn’t quite so regimented, and because of the newness, it was still an area of uncertainty with staff. We have had lots of changes and I recognise the pattern as a pattern of something coming in new, a bit of resistance, a bit of uncertainty [. . .] when we’re suddenly challenging the way that we’ve always done something is, it’s personally and professionally, it makes you very vulnerable.
Staff 2, site 2
However, the staff interviews showed many positive comments about the appropriateness of cue-based feeding, suggesting the rationale had been internalised:
I think it’s more natural to cue base. When you’re discharging a premature baby and they’ve been fed 3-hourly on the 2 or 3 months they’ve been alive, but it’s more natural to cue base and respond to their needs. It’s more acknowledging the parents as carers, not nurses’ schedules at the end of the day.
Staff 4, site 2
Further, staff commented on the added benefit of having an explicit shared feeding approach and materials, which legitimised the role of staff in providing support to parents:
. . . [it] legitimises what you’re telling them and gives them some back-up information as well, and consistency because not all members of staff have the same amount of experience of, you know, supporting parents in this way.
Staff 2, site 3
Staff also commented that the intervention built confidence between staff and between staff and parents on their contribution to successful feeding:
The Cubs has really prioritised the role of parents in the feeding and observation of the development of their baby. With the cues, they’re even, prior to beginning to cue, they’re sitting with a purpose at the cot side, or the incubator, with a sick baby, they’re looking for those cues.
Staff 1, Site 3
There was also a benefit in having a guide that gave everyone clarity on what their part in the process was:
The whole general process needed to be teased apart so it could be a step-by-step approach that was easy to communicate to all members of staff, bearing in mind we have 70 members of staff.
Staff 2, site 2
Fidelity
Sometimes staff found it challenging to fit the added tasks of the intervention into their usual workload, with the potential for implications in terms of the degree to which some intervention procedures could be implemented as prescribed in the original protocol:
Most of our staff are used to, they’re all taught to be aware of baby’s feeding cues and when they’re transitioning from scheduled feeds to responsive feeding, they all should be able to recognise baby’s feeding cues and however, when we document things in our current nursing records, there isn’t anywhere to record those behaviours so when babies have been recruited, the staff are told to use the log to give more detailed information of the babies and it’s been variable as to whether staff have remembered to document in the appropriate log or not so sometimes, we’ve had to remind staff to use the log and also remind them to use the scoring if babies are bottle feeding, to give them a qualitative assessment of the feed but as the trial has gone on, and more babies have recruited, I think compliance has improved.
Staff 1, site 1
However, the delivery of the intervention was largely perceived as consistent from the parents’ perspective:
Nobody’s going to say any different, I feel like everyone’s said the same thing about the cues, and what cues to look for. So, there was no misinformation, it was all the same information from the staff.
Parent 26, site 3
Yes [the support received from staff was] very helpful and 99% of the people all said the same. So if I had a question about like, mine was why was she putting her hands into her mouth quite a lot, and every nurse or every health-care assistant I asked, they near enough said the same, which was really reassuring.
Parent 9, site 2
Although, for some aspects of the support provided by nursing staff, variation was perceived by parents in the criteria employed, particularly in relation to top-up feeds:
I think the only challenge is I never know how much to top her up, if she has been feeding. I find that quite tricky and some nurses have different opinions to others, even though this is half top-up, people do it in between and kind of – yeah, I find it quite tricky to know whereabouts on the scale she is and what top-up she’s requiring.
Parent 1, site 2
Intervention materials were generally used as intended, although specific forms of engagement with the intervention materials were developed by some participants according to a range of individual needs and preferences:
There was the posters on the walls originally and there was the books that you gave us, which were really helpful and there was understanding from the staff of what to look for, the stuff maybe we’d read but hadn’t understood as well, so it was all three.
Parent 9, site 2
Parents also consistently reported that staff discussed their feeding preferences for when they were not able to be present in the unit and, upon their return to the unit, discussed any feeds that their infant had had in their absence, including any cues they had shown:
There’d be times when like he’d show cues but I wasn’t here but I was going to be here and they’d sort of hold off and like pacify him for a little bit [. . .]. Until I got here, yeah, yeah, but the cues already there ’cos I’d like ring up and I’d be like, ‘I’m nearly there!’ like don’t feed him yet, and they were like, ‘Oh, he’s showing these cues’. Yeah, so yeah and that’s helped me understand them as well so.
Parent 13, site 1
Members of staff felt the same way about this, overwhelmingly suggesting a good degree of adherence to intervention procedures, although they were also hesitant to state that this was always ensured:
Myself, yes, I do [feed the baby according to cues when the parents of a study baby are not present] but I can’t say for everyone, I’m not here 24/7. I think the majority of people do when they see that they’re on the Cubs study but obviously, I can’t guarantee that.
Staff 4, site 3
There were examples of parents noticing the difference between cue-based feeding in the study and a previous experience of scheduled feeding. This suggests that the intervention was being implemented as intended. In this example, the parent noted that the infant could have more attempts at the breast on showing cues rather than a regimented alternate breast and tube feed schedule:
You get a better choice and chance to breastfeed rather than, OK, you’ve breastfed this time, tube feed her next time. They let you try more if she’s awake and alert.
Parent 11, site 3
This was supported by a senior member of staff at the same site:
So as far as the Cubs intervention has been concerned, I’ve found that it very much decreases what intervention I need to make, in terms of moving the baby’s feeding on. So, an example of that would be that we would tend to introduce, first of all, one suck feed a day and then move to two suck feeds a day and then maybe try one suck feed, two bottles and then try alternate bottles. People would wait for the ward round and wait for me to say, OK move onto alternate treatment bottle or move onto two bottles to a tube.
Staff 8, site 3
One staff participant provided a good example of how implementing the intervention had changed her view that infants are not capable of starting oral feeds until 34 weeks:
One experience I did have, which was having been asked to support a mum with tube feeding, who was going to be on the study [. . .]. I just got into nurse mode and went straight to do the aspirate for the baby’s intragastric tube and it was only as I stood there and physically looked at the baby and realised this child was showing every picture alert sign, mouthing, rooting. We got the little one out and it was just amazing, the little one was — just did every single thing that you imagine a baby by the book would do to the point that eventually I just said to the mum, ‘Can we please video this on your phone because nobody will believe me?’ They will not believe that your child at just under 34 weeks is feeding with such competency, is feeding with such comfort and just relaxed and cueing and stopping and pausing. It was just phenomenal.
Staff 2, site 3
However, in terms of the intervals between feeds, there was evidence in the data suggesting that elements of scheduled feeding remained, albeit the cues were noted when the feeds were due. This aligns with the quantitative data suggesting that most feeds were given in accordance with a 3-hour schedule:
So you have to schedule feeds up to a certain point because babies won’t, can’t be relied upon but once they start to show us [cues] around feed times because that’s what they’ll do, their tummies will empty and just before their feed times, they’ll wake up . . ..
Staff 1, site 1
Another member of staff spoke about how, in the context of the study, they might wait a little longer than 3 hours for an infant to show cues:
Often if the baby was asleep at bang-on your 3 hours, we would tube feed that baby. Whereas sometimes giving them an extra 5 or 10 minutes, then they’re awake, they’re alert, they’re looking to feed, so, it’s just having that confidence.
Staff 5, site 3
Although limited, the observational data supported the interview data suggesting that there was good fidelity to the intervention in that there were no examples of staff or parents either starting or stopping feeds in accordance with a schedule. The most frequently observed behaviours were staff reviewing feeding plans with parents, and parents initiating and stopping oral feeding in response to cues. The least frequently observed behaviours were staff discussing parents’ expectations and feeding goals, staff discussing with parents their infants’ cues and criteria for assessing readiness to start the transition to oral feeding, and staff giving a top-up following an assessment of the quality of an oral feed.
Cost
In terms of cost, participants were asked if cue-based feeding required additional resources compared with scheduled feeding. Overall, both staff and parents suggested that this was not the case. However, staff reported that they had to shift the time allocation or organisation of some of their usual tasks to enable them to address the intervention procedures. In particular, staff noted a shift towards needing more time with parents in the early stage of the transition to oral feeding, with less time needed later as parents became confident in recognising cues. This was not perceived as problematic. Sometimes it was difficult to extrapolate whether the additional time related to implementing the intervention or to the study procedures:
So I would say probably, dependent on those things that I’ve said, I would like at least an extra half an hour to talk through how, what they’re seeing relates to the paperwork. So let’s say for example half an hour, 45 minutes, you might generally talk about cue-based feeding, an extra half an hour to go through that properly as well I think, and that would include your visit back just to check that they’re OK with it and understand and are managing to fill it out.
Staff 1, site 2
From the parents’ perspective, there were no major issues associated with implementation costs. Although some parents reported an emerging need to buy new bottle-feeding equipment to better support cue-based feeding, this was not perceived as a barrier to engaging in cue-based feeding:
The only thing I’ve had to buy is different kind of bottles, purely because the ones I’ve got were too big to start with so I had to go and get the smaller teat ones which then really, it’s almost like, they showed more cues knowing that they were able to feed from a different bottle. So yeah, I went and bought a set of bottles.
Parent 9, site 2
Other costs mentioned by parents, such as parking charges, taxi fares and buying meals in the hospital, were not related to the intervention. Parents and most staff thought that parents did not spend any more time with their infants for cue-based feeding than for scheduled feeding. However, a few staff participants commented that they thought that it had encouraged parents to stay longer at the unit:
I think the parents that have been on the Cubs study tended to be here a bit more often and would stay for maybe a few feeds in a row rather than just coming in for the one feed they knew and then going home.
Staff 6, site 3
Nursing staff noted the importance of having appropriate resources to overcome the limitations they experienced in their ability to train other members of staff, highlighting the implications that this may have had in terms of uptake and consistency in the delivery of the intervention:
. . . train more nurses to get the information across to the parents, so that then we can be then a bit more thorough, because obviously they’ve built up a relationship with us, so if someone has come in and given them this information, the research and then they show it to the nurse, ‘Oh, such and such, has just come in, and do you think this is a good idea?’ At least then we can then promote that and say, ‘Oh, yeah, this is actually really, really good, this will fit in with what I’ve just discussed with you about baby led.
Staff 3, site 2
A doctor noted that the intervention reduced the time he needed to be involved:
I suppose as a consultant doing the ward round or being in charge of the unit, my role would be to flag at ward round times to think about whether the baby is ready to feed orally yet or not. [. . .] So as far as the Cubs intervention has been concerned, I’ve found that it very much decreases what intervention I need to make, in terms of moving the baby’s feeding on. People would wait for the ward round and wait for me to say, OK move onto alternate treatment bottle or move onto two bottles to a tube.
Staff, site 3
Mechanisms of impact
We analysed the data to find any information on the mechanisms that might lead to the outcomes and assessed whether they supported our logic model (see Chapter 7). The main themes were building parents’ knowledge, confidence and skills, the supportive relationship between staff and parents, the perceived consistency between the intervention and current staff practices, and increased opportunities for parents to understand their infant’s behaviour leading to enhanced opportunity for bonding.
The intervention procedures and materials allowed parents to build up their knowledge and confidence to identify and act on cues:
My confidence has grown loads over the time. My confidence to start with, to identify the cues was I doubted myself because I was never sure whether I was seeing the right cues or was I trying to see something that wasn’t a cue so yeah, it took me a while to build my confidence but now I’m pretty confident that I know exactly what the kind of cues they’re going to show me.
Parent 9, site 2
The support and guidance provided by members of staff during the intervention was perceived as an integral part of the intervention by both staff and parents, suggesting that this relationship was a likely key factor to ensure successful implementation and intervention outcomes. A helpful, positive relationship between staff and parents during the intervention process was greatly valued by parents:
Yeah [the staff support with cue-based feeding was] amazing. They honestly, they’re so good and they’re good in that if you want their help, they’re there but they’re also good enough to give you space to let you just do it yourself and learn yourself.
Parent 8, site 1
Staff’s perceived consistency between the intervention and their current practices supported uptake and engagement with the intervention. However, in some instances, this resulted in a reinterpretation of the principles of the intervention whereby cue-based feeding was undertaken within the confines of scheduled feeding, thus avoiding the challenges associated with changing well-established practices. This links to the previous points made about fidelity to the intervention:
We kind of do it [support parents with cue-based feeding] anyway. We talk to parents, even though we do feed scheduled, shall we say, like, every 3 hours, I think we talk about — does he want a feed, is he looking, is he awake or is he not? So, I think we do that anyway so, I don’t think it was much of a change.
Staff 4, site 3
The intervention procedures and materials, alongside the support provided by members of staff during the intervention, enabled parents to master and embed cue-based feeding skills into their interactions with their infants:
At first, I didn’t know kind of what I was looking for as such. I had an idea in my head but overall, I didn’t entirely know what I was looking for. But now I do. [. . .] it gives parents an idea as to what you need to look for, you know, when you’re at home, for example, because that’s what you’re going to be doing when you get home. So, that’s given me an understanding of that, in that respect.
Parent 26, site 3
During this process, parents noted that their journey through the intervention and their experience of cue-based feeding allowed them to learn about a range of cues that went beyond their previous knowledge, building up their confidence to identify and act on cues, and ultimately enabling them to better understand and meet their infant’s needs in a more responsive way:
I didn’t realise there was so many cues that babies do before they want to feed. I thought it was just like looking around and crying generally, which is then you’re too late but, yeah, it’s been interesting to pick up the other cues that they have. [. . .] I feel much more confident to know when she’s ready.
Parent 1, site 2
I think it’s a great way of doing it, rather than get to the point of her screaming and then you’re behind them, aren’t you because you’re rushing around trying to get the food ready. I think this way, you get an inkling and then we’ve got a little — mainly because of the way they’ve shown me here, but we’ve got into a routine now that when she starts doing it, we change her nappy, so, she knows that. Then she sits out in her cot for a little bit to get awake and a bit more alert and by that point, she’s ready but not distressed. So, it is a nice experience, it’s not like, oh, my God, she’s hungry, I’ve got to feed her.
Parent 8, site 1
Alongside this, parents also noted that their journey through the intervention and experience of cue-based feeding afforded them increased opportunities to engage with breastfeeding and bond with their infant:
It’s quite a helpful thing for parents. I think most staff are that kind of busy with so many other things that that’s how the way it is in most jobs but as an overall experience, it has been very helpful. It helps you get to know your baby a bit more so.
Parent 9, site 2
I think it’s really helped with bonding because she’s needed something, and I’ve been able to give it to her.
Parent 15, site 3
Perceived benefits of the intervention
We asked parents and staff about their views of the benefits of the intervention and cue-based feeding. This theme overlapped considerably with those already reported, such as parents being more aware of their infant’s behaviour, finding cue-based feeding more relaxed and feeling closer to their infants. Some parents felt that it helped them to feel in control:
I like how it gives my baby and I the power to kind of know when we should be feeding because everything else is so kind of schedules and kind of procedure based in here, it’s quite nice to go back to that, yeah, just being a bit more natural and cue-based.
Parent 1, site 2
Staff concurred with this view:
I think parents that were part of the intervention felt more empowered, more in control and felt like the baby was more theirs than ours. I think it helped with them getting to know and understand their babies. The attachment, just everything, I thought it was really, really, really important.
Staff 1, site 2
In contrast to some of the views reported above about the completion of the documentation being onerous, or there being over-reliance on written information for parents, there were also positive comments in relation to being able to see an infant’s progress:
I think we do cue-based feeding anyway but perhaps we don’t write it down and it’s not then as obvious, it’s something that we, we, because we do it automatically we don’t make a note of it. And so perhaps if we focused and we’re recording it, we can look back and see an improvement for example, you know, in the baby’s ability to sort of feed.
Staff 2, site 2
There’s lots of evidence. It’s very visual. It gives parents the information that they need to make decisions about their baby to understand their baby, to understand why we have done what we’ve done when they’ve not been here.
Staff 1, site 1
Staff interviews also suggested that the study was leading to earlier discharge with some saying that they felt more confident discharging the infant from hospital:
I think in some ways, I actually feel more confident sending parents home following the study because I feel like they’re, they’re really in tune with their baby even more perhaps that they would have been.
Staff 4, site 2
One mum said that she was really happy to actually be doing it because it would prepare her for going home, for learning how to make those decisions when she was at home on her own about bottle feeding and when to feed and when not to feed. She was really quite pleased to have some documentation that would remind her of the progress her baby had made.
Staff 2, site 3
Some staff also perceived that infants were transitioning more quickly to full oral feeding although they acknowledged that this was based on only a few infants on the study:
Based on very few, so very difficult to say with any certainty but my feeling is that we’ve probably seen babies getting onto full oral feeds a little bit more quickly and certainly moving from the unit into transitional care more quickly because they have been showing cues and then their families have come and that’s all happened a little bit more quickly than it would have done had we been dictating when they were ready to move on.
Staff 8, site 3
Staff expressed uncertainty about outcomes, because there was no collective presentation of data on all infants in the unit, allowing them to collectively judge whether or not outcomes had improved:
I don’t know the results actually I have to be honest. My feeling is they transition more smoothly and you’ll have to ask the specialists but I would imagine there might be less oral aversion from following their leads but I don’t know for sure, I can’t really answer it because I don’t have any data to back up what I’m feeling.
Staff 1, site 3
Staff views were mixed about whether or not the intervention had an impact on infants’ weight gain, with many saying that they had not noticed any difference. One staff member felt that although infants might not gain as much weight, they were more comfortable:
I almost feel like maybe it might have even, because they’re not getting these set volumes as frequently, it might not, they might not weight gain too quickly, but they look a lot more comfortable when you feed them.
Staff 4, site 2
One parent provided an example of her infant gaining more weight than anticipated:
Because he’s so small, cue-based feeding they thought would affect his weight but when he had his tube removed and he was cue based just bottle and breast, we were expecting him to have lost weight but he’s actually gained 40 grams.
Parent 13, site 3
Views on a future study
We asked participants for their views on the important outcomes for a future trial and whether they would be willing to be randomised to a cue-based compared with scheduled feeding trial.
Important outcomes
Unsurprisingly, the outcome most frequently mentioned by parents and staff was weight gain, with some parents also suggesting other growth parameters, such as head circumference and length, in addition to weight. Almost as important, especially to parents, was the time to discharge or length of stay in the NNU. The time taken from the first oral feed attempt to full oral feeding was mentioned more often by staff than by parents. Other short-term outcomes mentioned by staff were numbers of infants being breastfed or fed with breast milk, parental satisfaction with the feeding experience and parents’ perceptions of cues. Some staff participants also felt that longer-term outcomes were important, mentioning oral aversion, obesity at 5 years and eating behaviours throughout childhood.
Willingness to be randomised
The majority opinion among staff and parents was that they did not support randomisation because they felt that cue-based feeding was the best approach. Staff gave more nuanced responses, suggesting that practice had moved so far towards cue-based feeding that there would be little difference between the intervention and control group, with some saying that it would be unethical to randomise to scheduled feeding. Those members of staff who supported a randomised approach to compare outcomes suggested that it would not be possible or would be confusing to randomise individual infants, and, instead, randomisation should be by hospital. A few parents said that they would be willing to be randomised but would hope that they received the intervention. One parent said that it was important to find out what was best for infants.
Summary
In this chapter we have presented analysis of qualitative interview and observational data. Recruitment to these components of the research was challenging owing to a variety of factors, including time and location for staff and parents, and processes for gaining informed consent from staff for observations. However, the data from the interviews with 14 parents and 16 staff are rich and provide an insight into how cue-based feeding was implemented in accordance with the views and experiences of staff and parents.
The context of the NNUs facilitated implementation of cue-based feeding. Barriers related to parents’ other responsibilities and the wide variation in experience of staff. In terms of implementation outcomes, the intervention was acceptable, with the resources well received, although there was some dissatisfaction among staff about the amount of documentation. The main issue in adoption of the intervention was the failure of the cascade approach to training to reach all staff in a timely manner. The intervention was perceived to fit well with current neonatal care practice, although resistance to change from some staff was mentioned. There were a considerable number of data relating to fidelity and, in general, there was good evidence that the intervention was implemented as intended, especially in relation to not applying a lower gestational age to the start of oral feeding and not having a set rate of transition. However, there was evidence that there was a 3-hourly feeding schedule for most infants. There was little information on which to assess cost but the main perception was that the intervention did not require additional staff and parent time compared with scheduled feeding, and may have reduced the time required by medical staff.
Several mechanisms of effect were suggested, including building parents’ knowledge, confidence and skills, relationships between staff and parents, consistency between the intervention and current practice, and increased opportunities for parents to understand their infant’s behaviour leading to enhanced bonding. The key perceived benefits related to empowering parents, providing evidence that underpinned decision-making and some tentative views that the intervention may lead to earlier discharge from hospital and faster transition to oral feeding.
For a future definitive evaluation, measuring infant growth and weight, and time to full oral feeding and discharge were the most important outcomes to parents and staff. Staff would also like to see longer-term follow-up of infants and the impact of cue-based feeding on feeding and obesity. There were mixed views on randomisation, suggesting that many parents and staff were not in equipoise for cue-based feeding, believing it to be the best approach. In the next chapter we describe stakeholder views on how to optimise the intervention and their views on options for the next steps for research.
Chapter 11 Options for optimising and evaluating the Cubs cue-based feeding intervention
In this chapter we describe the final stages of the study in which we explored stakeholder preferences for optimising the intervention. In addition, we discuss stakeholder preferences for the design, feasibility and acceptability of a future effectiveness study of cue-based feeding for preterm infants transitioning from tube to oral feeding. We describe how we used the process for decision-making after pilot and feasibility trials (ADePT)87 to structure this stage and how we involved stakeholders.
Methods
The primary aim of this stage of the project was to determine stakeholder views, based on the evidence of the feasibility study described in Chapters 8–10, of whether or not a RCT of the Cubs cue-based feeding intervention is feasible and what the components of a future study might look like. A secondary aim was to explore stakeholder views on how to optimise the intervention.
This stage of the work was guided by the ADePT process, which aims to provide transparency in decision-making processes after feasibility studies, and involves systematic identification and appraisal of problems and potential solutions. The process involves three key steps: (1) decisions about the type of problem (is it an issue for a future trial, an issue for the real world, or for both?); (2) assessment of all potential solutions; and (3) solutions are ranked, combined and refined. 87
We adapted this process to include stakeholder engagement in proposing potential solutions through a stakeholder workshop and in refining the final solutions through discussions with the Stakeholder Advisory Group.
Identifying challenges
In the first stage, the study team identified the key methodological and implementation challenges using the evidence from the feasibility study. We used the methodological issues for feasibility research reported by Shanyinde et al. 88 as a starting point. As our feasibility study did not involve randomisation or a control group, some of these issues such as sample size calculation, successful randomisation, and blinding procedures were not applicable. We followed the approach presented by Bugge et al. 87 and developed a table of the issues alongside the findings and evidence (Table 15). These issues formed the basis of the next stage in the process, the stakeholder workshop.
Stakeholder workshop
The aim of the workshop was to add contextual detail to the methodological issues and propose potential solutions. Workshop participants represented parents (a Bliss neonatal charity representative and one parent from the Parents’ Panel), neonatal care practitioners (five neonatal nurses, one nursery nurse, one speech and language therapist and three infant feeding leads) and research methodologists (n = 3). To provide further insight and context to the challenges, we invited the research nurses and clinicians from our three sites. Three research nurses and three clinicians attended. The workshop was facilitated by members of the research team.
The workshop comprised three activities. First, following a presentation of the feasibility study findings, participants worked in groups to discuss the challenges encountered; the aim at this stage was not to propose solutions but to define and to add detail to the challenges. Second, the groups proposed potential solutions to each challenge. Finally, as a plenary session, the solutions were ranked in terms of their feasibility and acceptability. The main points of the discussions were captured on flip charts and each working group had a member of the research team identified as a note-taker. All the workshop materials (flip charts and researcher notes) were analysed, and the table of methodological issues was developed to add additional details and the proposed solutions.
Using the ADePT process
Using the analysis of the workshop discussions and the evidence from the feasibility study, the research team worked through the three steps of the ADePT process to agree the type of each problem (type A – for the trial only; type B – for the trial and the real world; type C – for the real world); to assess all the potential solutions; and to rank, combine and refine the solutions. The results were then discussed with the Stakeholder Advisory Group to inform the options and recommendations presented as the outcome of our work.
Findings
Table 15 presents the methodological challenges, findings and evidence that were taken forward to the stakeholder workshop, where the focus of the discussions was on recruitment and retention, fidelity and acceptability, appropriate outcomes and the design of a future study.
| Methodological issue | Findings | Evidence |
|---|---|---|
| What factors influenced eligibility and what proportion of those approached were eligible? | Large proportion of infants were not recruited. The main reasons were: did not meet the inclusion criteria (already commenced oral feeding) and reason not documented |
136 out of 156 infants approached were eligible (87%) 37% of the eligible infants were recruited |
| Was recruitment successful? |
Recruitment of infants was partially successful and varied by site Low recruitment to qualitative interviews and observations especially of staff in one site: main challenges were finding appropriate time for the interviews and consent procedures for the observation |
83% of target recruitment of infants achieved (range by site 70–100%) 47% of target recruitment of parents to qualitative interviews 53% of target recruitment of staff to qualitative interviews 38% of target number of hours of observation achieved |
| Did eligible participants consent? |
Reasonable conversion to consent Research nurse and parent availability were key factors |
38 parents declined to participate and a further 13 agreed to participate but were not consented |
| Did participants adhere to the intervention? |
Mixed results: good adherence to most components of the intervention Low adherence to cascade training Some evidence that elements of scheduled feeding were retained High return rate of Our Feeding Journey documents |
Qualitative evidence of fidelity to the feeding protocol: protocol followed in that no minimum gestational age applied (except in one site for bottle-fed infants), no set rate of transition applied, but some evidence that infants were still fed predominantly in accordance with a 3-hourly schedule 72% of Our Feeding Journey documents returned, although completeness of data varied Start cues documented more than stop cues Observations showed staff and parents adhering to the intervention |
| Was the intervention acceptable to the participants? |
High degree of acceptability to parents The cascade approach to training was the least acceptable component for staff Some staff and parents found the amount of documentation unacceptable Observations were unacceptable to some staff |
High proportion of positive comments from parents about the intervention in the qualitative data Staff also made many positive comments and felt that the intervention reinforced their current practice The visual resources were well received The manual was felt by staff to be too long |
| Was it possible to calculate intervention costs and duration? |
Costs: no Duration: yes |
Both staff and parents felt that the intervention did not require additional resource Duration: mean of 11 days from intervention start to full oral feeding and 14 days from intervention start to discharge from NNU |
| Were outcome assessments completed? |
High completion for weight and duration of the intervention Low completion of feeding outcomes |
Data completion rates for most outcomes were high (88–96%) Lower compliance with completion of feeding data: 72% returned but large number of missing data |
| Were the outcomes measured those that were most appropriate? |
Yes, for most outcomes Low rates of completion of feeding data suggests that this might not be appropriate |
Large number of missing data for infant feeding outcomes |
| Was retention to the study good? |
Good retention to point of discharge Low retention to 2-week follow-up: variable by site |
96% (48/50) retention until discharge (one infant was withdrawn at start of intervention and one was transferred to another unit part way through the intervention) 38% (19/50) retention to 2-week follow-up range by site (30–87.5%) |
| Were the logistics of running a multicentre trial assessed? |
Variation between sites in recruitment and retention One site applied a modification to the intervention protocol |
Range of recruitment targets reached 70–100% One site conducted interviews with only three members of staff In one site, extrinsic contextual factors influenced recruitment |
| Did all components of the protocol work together? | Components had strong synergy | Some issues in one site with the training of staff to implement the research procedures, which were not sufficiently distinct from the training to implement the intervention leaving staff confused |
Next, we present a summary of the solutions that were ranked high for acceptability and feasibility at the stakeholder workshop.
To increase recruitment of infants, it was proposed that the window of opportunity for recruitment should be longer so that infants could be recruited and consented before oral feeding started. It was also agreed that the study information (i.e. the participant information sheets) should be more attractive and easier to read, with visual images replacing some of the text. The information also needed to be translated into relevant languages so that infants are not excluded because their parents cannot read English. Training for clinical staff on the study recruitment processes should be clearer, and reinforced regularly, with minimal time lag between training and study implementation. The study could also be more visible (e.g. with stickers and visual materials in the NNUs). In terms of recruitment for the interviews and observations, there should be an ‘opt-out’ rather than an ‘opt-in’ approach for the observations, and the interviews with staff should be shorter. An online questionnaire was thought to be more feasible for staff. It was felt to be particularly important that the site PI had the necessary influence and understanding of the research to promote the study and facilitate buy-in from staff. It was suggested that the training needs of each unit be assessed, and the training tailored accordingly.
Most of the discussion around fidelity focused on the training component of the intervention. The key suggestion was that there should be someone in each unit who had dedicated time to provide the training for the intervention to support the staff in its implementation. Therefore, the person delivering the training for the intervention would be different from the person delivering the training on the study procedures; for example, the latter could be a trial manager. Other suggested solutions were including the intervention training in mandatory training days and study days, and providing online training. The second main area for discussion around fidelity was the documentation. The key solution proposed was to capture data, especially the feeding data, electronically via a mobile application (an app). It was also proposed that the training resources for parents and staff could be included in the app, which would overcome some access problems. Further important solutions were making sure that medical staff were aware of and understood the intervention. Discussions on acceptability overlapped considerably with fidelity and similar solutions were proposed.
There was significant debate about the most important outcomes for a future trial, and the consensus was that a composite outcome was needed that covered feeding and parent–infant attachment. Short-term quantitative outcomes were said to be important, but focus on weight gain was felt to ignore the quality of feeds. Longer-term outcomes, such as feeding difficulties and oral aversion, were agreed to be important.
In terms of a future trial, it was agreed that a cluster design would be the most appropriate and that stratification would be needed to account for the different stages of NNUs’ transition towards family involvement and responsive feeding. Stratification by UNICEF UK BFI accreditation status was proposed to be the most practical way to achieve this.
The ADePT process
In the next stage the research team worked through the ADePT process to identify key problems. Type A problems (for the trial only) comprised the small proportion of eligible infants recruited, challenges in engaging staff and parents with documentation and data collection, and challenges recruiting staff to the study. There was one type B problem, namely parents and staff not adhering to all intervention components. Boxes 2 and 3 show the final evaluation and ranking of the solutions with further detail of the process presented in Appendix 5.
Improve training for staff in the research protocol of the intervention and their role in the recruitment of infants in the study.
Staff training to include research procedures to increase staff confidence that their data are confidential and not shared with managers.
Recruitment procedures for infants and staffIncrease recruitment time frame to ensure timely identification of infants for inclusion in the study.
Consent infants prior to them being ready to start oral feeding.
Increase the profile of the study through advertising materials and social media.
Staff opt-out rather than opt-in for study observations.
Research ownershipIncrease research nurse availability by embedding a role on the unit that combines research and clinical duties.
PI with research experience embedded in the unit.
Negotiate how backfill funding can be best used to incentivise and release staff for interviews.
Study design and methodologyUse routine feeding data to remove duplication of documentation for staff.
Collect follow-up outcome measures from routine health visitor data or from neonatal outreach teams.
Offer options to conduct interviews with parents at home following discharge.
Reduce interview time for staff or replace with an online questionnaire.
DocumentationDigital study documentation and collection methods.
Remove unnecessary and duplicate documentation.
Replace cascade training with in-depth training for staff and parents on the intervention provided through a dedicated role on each NNU.
Staff training to be delivered across all staff and disciplines to ensure that parents feel supported and do not receive conflicting information.
Timely training for staff to ensure that it remains relevant, and provide regular updates.
More focus on staff educating parents through modelling cue-based feeding rather than reliance on supplementary materials.
Recruitment procedures for infantsAssess infants for eligibility at an earlier gestation to ensure that feeding cues are identified early.
Cultural acceptance of cue-based feedingAlign unit protocols to the intervention.
Engage all staff to ensure that feeding transition culture is changed.
Engage medical staff so that the intervention documentation informs medical decision-making.
Enhance motivation through unit-wide displays of feeding successes.
DocumentationDigitalise intervention.
Redesign intervention and study documentation to be more engaging and accessible, and to focus on quality of feeds.
Avery et al. 89 suggest that the three predominant types of progression criteria from an internal pilot to a full trial are recruitment, protocol adherence and outcome data. Although our study was not a pilot trial, working through the ADePT process highlighted similar issues from our feasibility study.
In terms of recruitment, a key issue related to the point in their feeding journey at which infants were screened for eligibility and recruited to the study. The fact that 16 infants were ineligible because they had established oral feeding suggests that they were screened too late. Screening is further complicated as it is not a one-off event; infants progress at different rates and frequently not in a linear fashion, meaning that some infants may move in and out of eligibility over time. There appeared to be a short window of opportunity for infants to be recruited to the study as, once they showed readiness to start oral feeding, their progress could be rapid. Therefore, in a future study, earlier screening should increase the number of eligible infants. Furthermore, if infants are screened earlier, there may be more opportunity for the person taking consent to meet the parents to discuss the study. This could increase the proportion of eligible infants who are recruited. Therefore, we assessed training for care staff in the trial procedures in terms of timing of screening to be an important solution. However, a NNU is a very stressful environment for parents, and feedback suggested that many feel too overwhelmed to consider participating in research; therefore, low recruitment rates as a proportion of eligible infants may be inevitable. A further solution to increasing recruitment could be to have a research nurse who is embedded in the NNU to support screening and recruitment, and to increase the visibility of the study. The role of the PI is also critical to support the research. In one site the PI had limited research experience, and in another the PI was not sufficiently embedded in the NNU. Stakeholders proposed providing funding to backfill staff so that they could be released for interviews. In fact, this was provided to each site, but it is possible that it was not used in the most effective way to incentivise participation. Feedback from the PIs suggested that there is a shortage of suitably skilled practitioners to enable the release of staff.
There were also issues with recruiting parents and staff to the qualitative elements of the study. As a future trial would need to incorporate a process evaluation, this needs to be addressed. There appeared to be three challenges: time and privacy for interviews, consent procedures for the observations and, in one site, an issue of mistrust regarding confidentiality. Of the solutions suggested by stakeholders, we assessed as important shortening the interviews with staff and/or using an online questionnaire as an alternative to interviews. For parents, more flexible options could be available, such as conducting the interviews at home following discharge.
The main challenge to protocol adherence was implementation of the cascade training, which failed to reach all staff and took a long time. Some staff experienced too long a gap between the training and the commencement of the intervention, and, for some, there was confusion between training to implement the intervention (i.e. cue-based feeding) and training for the study recruitment procedures. The key proposed solution to overcome this was that there should be a dedicated individual in each unit whose role would be to provide the intervention training across all staff and disciplines. Training related to the research protocol and procedures, such as screening and recruitment processes, could be provided by a different person, such as an external researcher, to keep this differentiated from the intervention training. Although fewer challenges were identified with the information provided for parents, stakeholders proposed that, as well as providing information about cue-based feeding and referring parents to the study audio-visual resources, staff should model cue-based feeding for parents. This would probably require a cultural shift within units from the predominantly prescribed volume and scheduled feeding that was still evident to some extent in the study findings. Solutions for addressing this included aligning unit protocols and more engagement of medical staff in the intervention. Implementation of an audit and feedback process could contribute to such a culture change. 90,91 Our feasibility study achieved good capture of outcomes that were available from routinely collected data. The challenge was to obtain data on feeding outcomes. These data were intended to demonstrate fidelity to the intervention as well as feeding outcomes. Infant feeding data are limited in routine data and, in their current form, are driven by an assumed scheduled approach with a focus on prescribed volumes taken at each feed (i.e. quantity rather than quality of feeds). The main solution that would cover both a trial context and the real world was to provide the study information and collect feeding data through an app. Stakeholders, including parent representatives, agreed that this would be an acceptable and feasible solution for parents. The only concerns were around the hygiene of mobiles phones and access for parents who do not have a smartphone. Provision of dedicated mobile devices could overcome both concerns but would require additional resource.
The Stakeholder Advisory Group discussed the above solutions and the options for a future evaluation. One issue of concern was that there are some staff and parents who believe that cue-based feeding is the best approach and that all babies should be fed in this way. This casts doubt on whether or not there is equipoise. There are contextual factors that suggest that many NNUs are moving towards cue-based feeding driven by the move towards family-centred care, UNICEF neonatal standards that encourage responsive feeding68 and the British Association of Perinatal Medicine toolkit on Optimising Early Maternal Breast Milk for Preterm Infants that alludes to a forthcoming toolkit on transitioning to responsive feeding. 92 This is further complicated by the evidence from our work that many staff believed that they were implementing cue-based feeding although parents’ experiences were not always consistent with this view.
The two options favoured by stakeholders were (1) a cluster-randomised control trial potentially using a stepped-wedge design or (2) a quality improvement project to implement and optimise cue-based feeding. Further work is probably needed before this decision can be made. The proposed next steps were (1) to co-design with parents and test an app that would incorporate both the intervention and data collection, and (2) to survey all 220 NNUs in the UK to explore the approach to transitioning to oral feeding and willingness to participate in a multicentre trial. The latter would extend and update the selective survey undertaken in the first stage of this project.
Summary
In this chapter we have described the process undertaken to co-develop with stakeholders the implications of our work for optimising the cue-based feeding intervention, and preferences for the future evaluation of cue-based feeding. A systematic approach guided by the ADePT framework was used. The key solutions to challenges relating to recruitment, protocol adherence and outcome data focused on screening infants earlier in their feeding journey, modifying and expanding staff training, and incorporating the intervention and data collection into an app. Equipoise is an issue, driven to some extent by current practice recommendations, standards and toolkits. Further work was suggested to be needed to co-design the app and assess comprehensively current practice across the UK prior to a decision on whether the intervention should proceed to be tested via a cluster RCT or be implemented using a quality improvement approach.
Chapter 12 Discussion and conclusions
In this chapter we bring together and discuss the main findings of each stage of the Cubs project against the eight study objectives. First, we summarise the key findings, followed by an appraisal of the strengths and limitations of our work. We then discuss the implications for a future evaluation.
Summary of main findings
Objective 1: describe the characteristics, components, theoretical basis and outcomes of approaches to feeding preterm infants transitioning from tube to oral feeding
Our systematic review (see Chapter 3) included 25 studies, of which 10 were RCTs, nine were quality improvement projects and six were observational studies. The quality of the studies was low, with all but one having a high risk of bias. Two key components of all the interventions were a cue-based feeding protocol and training for staff and parents. Only two interventions reported a theoretical basis, and the most common BCT was instructions on how to perform the required behaviour. The studies incorporated 41 different outcomes, the most common of which were daily weight gain, length of stay in NNU and length of time to full oral feeding. Although we found one additional small trial, our results do not change the conclusions of the Cochrane review1 that the evidence in favour of cue-based feeding is of low quality and should be treated cautiously.
Objective 2: identify operational policies, barriers and facilitators, and staff and parents’ education needs in neonatal units implementing cue-based feeding
We conducted case studies (see Chapter 4), a telephone survey (see Chapter 5) and qualitative research with parents and staff (see Chapter 6) to address this objective. Our findings suggest that contextual factors, such as the facilities provided for parents to be with their infants in NNUs and the extent to which skin-to-skin contact is practised, are key facilitators of cue-based feeding. The financial and leave policies in Sweden supported parents to be with their preterm infants constantly. In the UK, the UNICEF UK BFI neonatal standards were also a driver of cue-based feeding. 68 Barriers included some staff’s resistance to change from a volume-driven scheduled approach, safety concerns and lack of access to training. The qualitative data suggested that health-care practitioners’ views that they are implementing cue-based feeding were not always consistent with parents’ experiences. The evidence suggested that staff have good knowledge of readiness-to-feed cues although they have less knowledge of stop cues. This suggested that the intervention should address behaviour change as much as knowledge. The telephone survey showed that almost 90% (n = 15) of the participating NNUs either had started making changes or were considering changes to implement cue-based feeding. This has implications for standard of care as a comparison in a future evaluation.
Objective 3: co-produce an evidence-informed, adaptable, manualised intervention
We used the evidence from the literature review and the barriers, facilitators and educational needs, as well as stakeholder preferences, to co-produce the Cubs intervention iteratively. We first developed a matrix of options and an intervention logic model, which formed the basis of discussions at a stakeholder workshop. Consensus was reached on most intervention components with contentious issues being the maximum interval between feeds (agreement was reached on 4 hours, but with an expectation that there would be at least eight feeds per 24 hours) and whether or not to include infants on high-flow oxygen (agreement was reached to exclude them). One site modified the intervention by implementing a minimum gestational age for commencement of oral feeding bottle-fed infants.
Objective 4: appraise willingness of parents and staff to implement and sustain the intervention
There is evidence from the quantitative and qualitative data (see Chapters 9 and 10) that many components of the intervention were well received and implemented as intended. Intervention components that were implemented as intended include no minimum gestational age for commencement of oral feeding (except for bottle-fed infants in one site) and no prescribed rate of transition to oral feeding (e.g. ratio of oral feeds to tube feeds). The feeding data were incomplete, but those available suggested that there was good recognition of readiness-to-feed cues. Fewer stop cues were documented. The most contentious intervention components were the training and documentation. The use of the cascade approach to training led to failure to reach all staff and disciplines as intended. The documentation was felt to be onerous and repetitive, although some parents appreciated having a record of their infant’s progress. Staff and parents perceived several advantages to the intervention, including that it gave more control to parents and enhanced parent–infant interaction. Staff speculated that cue-based feeding may lead to less feeding aversion in the longer term. The conclusion, therefore, is that, with changes to the training and documentation, staff and parents are willing to implement and sustain the intervention.
Objective 5: assess associated costs of implementing cue-based feeding in neonatal units
We analysed the views of parents and staff on whether or not additional resources were required for cue-based feeding than for scheduled feeding. This analysis suggested there were no differences, although a minority view was that cue-based feeding may encourage parents to be with their infants for longer periods of time, which could lead to more out-of-pocket expenses. The key driver of costs is likely to be length of stay on the NNU. Other aspects that could affect costs include breastfeeding rates and feeding problems in the longer term. These will be important considerations in a future study. On reflection, this was an ambitious objective to achieve within the scope of a feasibility study that did not have comparison group.
Objective 6: determine feasibility and acceptability of conducting a future trial, including views on important outcomes
The findings of the feasibility study (see Chapters 9 and 10) suggested that staff and parents held mixed views on the feasibility and acceptability of a future trial. There was consensus that such a trial would need to have a cluster design to avoid confusion and the cross-contamination between experimental and control arms that could occur if randomisation was at the individual level. There was some doubt about equipoise, as many staff felt that the intervention reinforced current practice rather than changing it and others felt that it would be unethical to randomise infants to a scheduled approach to feeding. A few parents could compare their experiences on the Cubs study with those of feeding a previous infant in the NNU and highlighted positive differences. However, this could reflect a change in practice over time rather than being attributable to the study intervention. The important outcomes for a future trial highlighted by staff and parents were weight gain or other parameters of growth, time to full oral feeding and time to discharge. These are consistent with other trials on cue-based compared with scheduled feeding. 1 Staff highlighted the importance of following up infants beyond discharge from the NNU into the first year and beyond to evaluate the impact on feeding outcomes, such as breastfeeding rates, feeding difficulties and obesity. There was very little mention, by parents or staff, of parent outcomes such as satisfaction, feeling in control or parent–infant attachment, although as these were identified as potential benefits of the intervention they could be assumed to be important.
Objective 7: scope existing data-recording systems and potential short- and long-term outcome measures
Outcome data on weight, number of days to establishment of oral feeding and length of stay in NNU were complete, suggesting that these data are readily available. Data on feeding outcomes were more challenging and, in particular, there were low rates of retention during the 2-week follow-up post discharge. The data on methods of feeding should be routinely available, for example through health visitor records; however, such data would not include whether or not cue-based feeding was continued following discharge. Routine data available from NNUs are currently based on a volume-driven approach (e.g. intake and output). No data are available on quality of feeds. We found no data collected routinely on parent outcomes or parent–infant attachment and no measure of this in our systematic review. A further step in the future could be to search more widely for relevant measures or to develop new ones.
Objective 8: determine key stakeholder views of whether or not a randomised controlled trial is feasible and what the components of a future study would look like
We elicited stakeholder views through a stakeholder workshop, where solutions to identified challenges were proposed, and through the Study Stakeholder Advisory Group. The conclusion was that further work is needed before a RCT is feasible. First, the intervention should be digitalised (i.e. an app developed that includes both the intervention and feeding outcome data collection). A minimum data set should be agreed to avoid collecting unnecessary data. For example, stakeholders felt that collecting information on cues was interesting and could inform the training (e.g. to focus more on stop cues), but probably is not necessary for the next stage of the evaluation. To further assess feasibility, a survey of all 220 UK NNUs could be conducted to provide a more complete and updated assessment of approaches to transitioning from tube to oral feeding and willingness to participate in a multicentre study. There was a suggestion that, if a significant proportion of units are moving towards implementing cue-based feeding, a quality improvement approach to implementing the intervention may be more appropriate.
Strengths and limitations
There were several strengths and limitations of this study. We applied a rigorous, systematic and comprehensive approach to developing the evidence base for the intervention. The systematic review followed established review methodology to identify and assess for risk of bias in all relevant studies. We analysed the interventions not just for their effect, but also for theories and BCTs. A limitation was that most of the 24 included studies were assessed to be at high risk of bias. We extracted critical learning from rapidly but rigorously conducted case studies of three purposively selected NNUs, each using different approaches to cue-based feeding. A limitation was that the case studies were time-limited snapshots. The telephone survey of NNUs was informative in highlighting the range of approaches to transitioning from tube to oral feeding and intentions to implement cue-based feeding. A limitation was that owing to time and resource limitations, we were only able to sample a small proportion of UK NNUs. A further strength was that we generated primary qualitative data to increase our understanding of parents’ and practitioners’ knowledge, experiences and views of the transition to oral feeding. This helped to inform the development of the Cubs intervention.
A considerable strength of the study was that we were able to use the evidence to develop the intervention using a structured co-production approach. We were able to build consensus on components and implementation with involvement of a wide range of stakeholders, including health-care practitioners and parents. The resulting intervention was well received when implemented during the feasibility study. A limitation of the intervention was that we did not have the resources to consider translation to different languages to increase inclusion. A further limitation was an underestimation of the challenge of reaching all staff with the training materials, which led to a delay in commencing the recruitment phase of the study.
A strength of our work was the multimethod feasibility study in three purposively selected NNUs of different sizes and levels, which served different populations. A limitation was that, by the time we commenced the feasibility study, all three units had gained UNICEF UK BFI accreditation. We acknowledge that further challenges may have been evident in units that were not accredited. NNUs are challenging environments in which to implement change. Although challenging, we recruited > 80% of our target number of infants with high retention in the study until discharge. A limitation was the small number of staff and parents participating in qualitative interviews and observations. The small number of medical staff who engaged in the training and interviews was a significant weakness. Nevertheless, we generated rich qualitative data. A strength was our approach to analysis using two theoretical frameworks: Proctor et al. ’s83 implementation outcomes and normalisation process theory. 85 We also used a theoretical framework, the ADePT process,87 to facilitate a structured and transparent approach to decision-making.
Stakeholder involvement and parent engagement throughout the study were key strengths of our work. Parents were engaged through the Parents’ Panel, and their feedback and advice was crucial, as described in Chapter 2. The involvement of the charity Bliss was essential to facilitate parental involvement. We retained parental involvement throughout the study, although we had much stronger engagement in the early stages. Towards the end, only one parent was able to attend the second workshop and only one attended the final Stakeholder Advisory Group meeting. An alternative could have been to seek new members for the Parents’ Panel as the study progressed. It was inevitable that parental responsibilities and commitments changed over time as their infants grew older. A further limitation of our approach to parental engagement was that the parents did not represent the diversity of parents who experience care of an infant in a NNU; for example, we did not have parents from minority ethnic backgrounds. We chose a pragmatic approach to recruiting parents through the charity Bliss, but a more targeted process is needed to optimise inclusion and diversity. We had excellent engagement from health-care practitioner stakeholders, except for medical staff. This was a limitation, and alternatives to attendance at workshops may be needed.
There is no doubt that the COVID-19 pandemic had an impact on our study. We had to close the study to recruitment earlier than intended; even a few more weeks could have increased the number of qualitative interviews and follow-up rates. It probably also had an impact on maintaining parental engagement to the end of the study.
Implications for a future evaluation
Our study developed an evidence-informed cue-based feeding intervention for the transition from tube to oral feeding for infants in NNUs. Our systematic review identified that previous studies comparing cue-based feeding with scheduled feeding are small and have methodological limitations, suggesting a need for a larger-scale rigorous evaluation. The intervention was implemented in three NNUs to assess feasibility, although, without a control group or randomisation, some questions about progression to a full trial remain unanswered. Our study differed from most studies included in our systematic review as it was an intervention development and feasibility study without a comparison group. Our study also differed in the level of stakeholder engagement in the intervention design and in developing the recommendations for a future evaluation.
There were two further related challenges. First, there was evidence that health-care practitioners and, to some extent, parents are not in equipoise, believing that cue-based feeding is the preferred approach to the transition from tube to oral feeding. The second issue is that our telephone survey, albeit based on a small sample, suggested that most NNUs are at least considering implementing cue-based feeding. This is further influenced by contextual drivers such as UNICEF UK BFI neonatal standards. 68 This suggests challenges regarding the nature of standard of care that would be the comparator in a trial. This is complex, as there was some evidence that staff believe that they are implementing cue-based feeding, albeit while retaining elements of a scheduled approach. Given that the intervention incorporates staff training, resources displayed in NNUs and changes to a feeding protocol, a cluster stepped-wedge design was considered the most appropriate approach. Further work to undertake a comprehensive assessment of the 220 NNUs in the UK would provide a stronger basis for deciding if a trial design is appropriate. An alternative approach could be a quality improvement design.
The cluster design is ideal for interventions where unit-level implementation would be more practical and viable than individual randomisation. For this intervention, for example, individual randomisation would be difficult in a NNU, as nurses would be required to continually switch between feeding regimes for different infants. The design also reduces the risk of cross-contamination, as the two feeding regimes operating side by side would be likely to lead to confusion and potentially queries from parents about why other infants have different feeding regimes. Stratification would be worthwhile, as it would increase efficiency, and could be done on size and possibly by current type of feeding approach (fixed, mixed) and by UNICEF UK BFI accreditation status. It would also reduce potential biases. Alternatively, minimisation could be employed to provide balance on a number of key factors.
Recruitment bias is an issue in cluster designs and can be reduced in several ways. First, recruitment could happen at the same time as allocation to intervention, so that the units, and potentially mothers, do not know which regime will be followed at that time. However, this has the drawback that only infants in the NNUs at that time would be enrolled. This may reduce power but in a cluster trial the design effect is reduced with a lower number of recruits per unit. This effect could be reduced by collecting data on infants’ weights, if this is the primary outcome for all infants. Of course, it would have to be explained to parents before recruitment that selection of intervention is random, and before the unit gives consent to participate in the study. Any subsequent trial would follow the Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials. 93 Intention-to-treat analysis would be utilised to reduce the potential for bias due to non-compliance. Control units could, pragmatically, be a mixture of different approaches, so including co-interventions, though clearly excluding cue-based feeding, would help to maximise recruitment and reduce bias. Statistical issues of clustering would have to be considered in sample size estimation and subsequent analyses. 94
Key weaknesses in previous research are summarised in the Cochrane review. 1 A future trial of the Cubs intervention could overcome these weaknesses through testing an intervention that has been co-designed with relevant stakeholders; focusing on infants at the transition from tube to oral feeding; ensuring that the trial has an adequate sample size and length of follow-up; selecting outcomes that are important to parents and staff; and involving stakeholders in the trial design.
Through our feasibility study we were able to identify solutions to some of the challenges encountered that have implications for a future evaluation. To optimise recruitment, infants should be screened earlier in their feeding journey so that consent can be taken before the infant progresses to start oral feeding. The study procedures will need to be clear that the intervention should start only when the infant is assessed as being ready to start attempting oral feeds. Other factors that could be considered to enhance recruitment are embedding a research nurse or midwife with additional clinical duties within the NNU to support staff, and improving the training of staff for their role in approaching parents about the study. This could be highly effective in engendering trust among other staff, and could be cost-effective. If observations are included in a future process evaluation, a more pragmatic approach to obtaining consent from clinical staff is needed.
The main challenge to protocol adherence was the cascade training, which did not reach all staff in a timely manner. Alternative approaches are needed, and identifying a dedicated trainer within each NNU could overcome this. There was also evidence in the findings that there remained elements of a prescribed volume and schedule approach. A change in NNU culture is needed, which was beyond the scope of the intervention developed in this study. This will require engagement of medical staff, alignment of unit protocols and strategies to enhance staff motivation. The documentation of feeding progress and outcomes was too onerous and requires both a reduction in the amount of information requested and to be more user-friendly and accessible through digitalisation.
We did not have a control group and therefore were unable to test randomisation procedures or calculate the sample size needed for a future trial. Pilot work is needed to address this. Pilot work is also required to assess the feasibility and acceptability of a composite primary outcome that captures not only infant growth but also a measure of parent–infant attachment or interaction. There was considerable support for the assessment of longer-term feeding outcomes for a future evaluation, and this could also be assessed in pilot work. Finally, pilot work would also provide an opportunity to refine the entry criteria.
Conclusions
Our work has demonstrated that it is feasible and acceptable to implement an evidence-informed cue-based feeding intervention for the transition from tube to oral feeding for preterm infants in NNUs. The intervention was well received, but the training element needs to be improved. Further work is needed to digitalise the intervention and feeding outcome data collection, and to assess the feasibility of a cluster RCT, noting some evidence of an existing lack of equipoise.
Acknowledgements
The Cubs team acknowledges the contribution of study team members William McGuire (who is a member of the National Institute for Health Research Health Technology Assessment and Efficacy and Mechanism Evaluation Editorial Board) and Debbie Bezalel and colleagues from the neonatal charity Bliss. We thank Emma Lamont for contributing to the analysis of qualitative data.
We acknowledge the site PIs and research nurses: Alison Findlay, Louise Rattenbury, Elizabeth Bailey, Anne McLeod, Jacqueline Tipper and Samantha Nightingale.
We acknowledge Professor Renee Flacking, Gillian Bowker and Marjorie Clarke for facilitating the case studies.
We acknowledge Magneto films (London, UK) and all the staff and parents who facilitated and participated in the Cubs film, and Helen Scharf, who kindly provided breastfeeding images for a poster.
We acknowledge all members of the Stakeholder Advisory Group and Parents’ Panel.
Finally, we are grateful to all the participants in our study.
Contributions of authors
Alison McFadden (https://orcid.org/0000-0002-5164-2025) (Professor of Mother and Infant Public Health) was the PI for the study. She designed the study, drafted the report, contributed to developing data collection tools and analysing all the data and contributed to the systematic review and developing the manual. She contributed to developing and delivering the training, facilitating the stakeholder workshops and conducting the ADePT process.
Bronagh Fitzpatrick (https://orcid.org/0000-0002-2068-2761) (Post-doctoral Researcher) project managed the study and contributed to drafting the report and conducting the case studies and telephone survey. She developed the manual and delivered the training. She contributed to the systematic review and facilitating the workshops. She chaired the Parents’ Panel.
Shona Shinwell (https://orcid.org/0000-0001-9369-9698) (Clinical Academic Research Fellow) contributed to drafting the report, developing the intervention, analysing the quantitative and qualitative data and conducting the ADePT process.
Karen Tosh (https://orcid.org/0000-0002-5724-3383) (Lecturer in Children’s Nursing) contributed to designing the study, drafting the report, conducting and analysing the case studies and contributed to the systematic review and developing the intervention and training.
Peter Donnan (https://orcid.org/0000-0001-7828-0610) (Professor of Epidemiology and Biostatistics) contributed to drafting the report, analysed the quantitative data and assessed the feasibility of a future trial.
Louise M Wallace (https://orcid.org/0000-0003-3770-0580) (Professor of Psychology and Health) contributed to drafting the final report, analysing the qualitative data and conducting the ADePT process. She chaired the Stakeholder Advisory Group.
Emily Johnson (https://orcid.org/0000-0003-4005-3169) (Specialist Speech and Language Therapist) contributed to drafting the report, conducting the case studies and developing the intervention.
Steve MacGillivray (https://orcid.org/0000-0002-1933-6431) (Reader in Mental Health) contributed to drafting the report and conducting the systematic review.
Anna Gavine (https://orcid.org/0000-0003-3750-2445) (Lecturer in Mother and Infant Health) contributed to drafting the report and conducting the systematic review.
Albert Farre (https://orcid.org/0000-0001-8970-6146) (Lecturer in Adolescent and Child Health) contributed to drafting the report and analysing the qualitative data.
Helen Mactier (https://orcid.org/0000-0001-6154-5758) (Consultant Neonatologist and Honorary Clinical Associate Professor) contributed to drafting the report, developing the intervention and assessing the implications for future research.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Watson J, McGuire W. Responsive versus scheduled feeding for preterm infants. Cochrane Database Syst Rev 2016;8. https://doi.org/10.1002/14651858.CD005255.pub5.
- Siddell EP, Froman RD. A national survey of neonatal intensive-care units: criteria used to determine readiness for oral feedings. J Obstet Gynecol Neonatal Nurs 1994;23:783-9. https://doi.org/10.1111/j.1552-6909.1994.tb01953.x.
- Tyson JE, Lasky RE, Mize CE, Richards CJ, Blair-Smith N, Whyte R, et al. Growth, metabolic response, and development in very-low-birth-weight infants fed banked human milk or enriched formula. I. Neonatal findings. J Pediatr 1983;103:95-104. https://doi.org/10.1016/S0022-3476(83)80790-2.
- Ross ES, Browne JV. Developmental progression of feeding skills: an approach to supporting feeding in preterm infants. Semin Neonatol 2002;7:469-75. https://doi.org/10.1053/siny.2002.0152.
- Shaker CS. Cue-based feeding in the NICU: using the infant’s communication as a guide. Neonatal Netw 2013;32:404-8. https://doi.org/10.1891/0730-0832.32.6.404.
- Crosson DD, Pickler RH. An integrated review of the literature on demand feedings for preterm infants. Adv Neonatal Care 2004;4. https://doi.org/10.1016/j.adnc.2004.05.004.
- Davanzo R, Strajn T, Kennedy J, Crocetta A, De Cunto A. From tube to breast: the bridging role of semi-demand breastfeeding. J Hum Lact 2014;30:405-9. https://doi.org/10.1177/0890334414548697.
- Holditch-Davis D, Brandon DH, Schwartz T. Development of behaviors in preterm infants: relation to sleeping and waking. Nurs Res 2003;52:307-17. https://doi.org/10.1097/00006199-200309000-00005.
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev 2006;2. https://doi.org/10.1002/14651858.CD001814.pub2.
- Hoogewerf M, Ter Horst HJ, Groen H, Nieuwenhuis T, Bos AF, van Dijk MWG. The prevalence of feeding problems in children formerly treated in a neonatal intensive care unit. J Perinatol 2017;37:578-84. https://doi.org/10.1038/jp.2016.256.
- Thoyre SM, Holditch-Davis D, Schwartz TA, Melendez Roman CR, Nix W. Coregulated approach to feeding preterm infants with lung disease: effects during feeding. Nurs Res 2012;61:242-51. https://doi.org/10.1097/NNR.0b013e31824b02ad.
- Nair M, Yoshida S, Lambrechts T, Boschi-Pinto C, Bose K, Mason EM, et al. Facilitators and barriers to quality of care in maternal, newborn and child health: a global situational analysis through metareview. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004749.
- Mola-Schenzle E, Staffler A, Klemme M, Pellegrini F, Molinaro G, Parhofer KG, et al. Clinically stable very low birthweight infants are at risk for recurrent tissue glucose fluctuations even after fully established enteral nutrition. Arch Dis Child Fetal Neonatal 2015;100:F126-31. https://doi.org/10.1136/archdischild-2014-306168.
- Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr 1999;134:492-8. https://doi.org/10.1016/S0022-3476(99)70209-X.
- Nyqvist KH. Early attainment of breastfeeding competence in very preterm infants. Acta Paediatr 2008;97:776-81. https://doi.org/10.1111/j.1651-2227.2008.00810.x.
- Ericson J, Flacking R. Estimated breastfeeding to support breastfeeding in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs 2013;42:29-37. https://doi.org/10.1111/j.1552-6909.2012.01423.x.
- Ross ES, Philbin MK. Supporting oral feeding in fragile infants: an evidence-based method for quality bottle-feedings of preterm, ill, and fragile infants. J Perinat Neonatal Nurs 2011;25:349-57. https://doi.org/10.1097/JPN.0b013e318234ac7a.
- Kirk AT, Alder SC, King JD. Cue-based oral feeding clinical pathway results in earlier attainment of full oral feeding in premature infants. J Perinatol 2007;27:572-8. https://doi.org/10.1038/sj.jp.7211791.
- Johnson E. Transition to Oral Feeding for Infants on UK Neonatal Units: A Survey of Current Practices n.d.
- Nyqvist KH, Häggkvist AP, Hansen MN, Kylberg E, Frandsen AL, Maastrup R, et al. Expansion of the baby-friendly hospital initiative ten steps to successful breastfeeding into neonatal intensive care: expert group recommendations. J Hum Lact 2013;29:300-9. https://doi.org/10.1177/0890334413489775.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013;50:587-92. https://doi.org/10.1016/j.ijnurstu.2012.09.010.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Reeves B, Deeks J, Higgins J, Wells G, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). Oxford: The Cochrane Collaboration; 2011.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Updated March 2011). Oxford: The Cochrane Collaboration; 2011.
- Chrupcala KA, Edwards TM, Spatz DL. A continuous quality improvement project to implement infant-driven feeding as a standard of practice in the newborn/infant intensive care unit. J Obstet Gynecol Neonatal Nurs 2015;44:654-64. https://doi.org/10.1111/1552-6909.12727.
- Davidson E, Hinton D, Ryan-Wenger N, Jadcherla S. Quality improvement study of effectiveness of cue-based feeding in infants with bronchopulmonary dysplasia in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs 2013;42:629-40. https://doi.org/10.1111/1552-6909.12257.
- Gelfer P, McCarthy A, Spruill CT. Infant driven feeding for preterm infants: learning through experience. Newborn Infant Nurs Rev 2015;15:64-7. https://doi.org/10.1053/j.nainr.2015.04.004.
- Gray MM, Medoff-Cooper B, Enlow EM, Mukhopadhyay S, DeMauro SB. Every three-hour versus every six-hour oral feeding in preterm infants: a randomised clinical trial. Acta Paediatr 2017;106:236-41. https://doi.org/10.1111/apa.13658.
- Kansas K, Mackley A, Desai S, Leef K, Paul D, Stefano P. Self-regulation of Feeding in the Premature Infant; a Randomized Trial of Ad Lib vs. Scheduled Feedings. Baltimore, MD: International Pediatric Research Foundation, Inc.; 2004.
- McCain GC, Gartside PS, Greenberg JM, Lott JW. A feeding protocol for healthy preterm infants that shortens time to oral feeding. J Pediatr 2001;139:374-9. https://doi.org/10.1067/mpd.2001.117077.
- McCain GC, Del Moral T, Duncan RC, Fontaine JL, Pino LD. Transition from gavage to nipple feeding for preterm infants with bronchopulmonary dysplasia. Nurs Res 2012;61:380-7. https://doi.org/10.1097/NNR.0b013e318268cefb.
- Messer LL. Infant-Driven Feeding Vs. Scheduled Feeding: The Effect on Hospital Length of Stay 2016.
- Murray N. Effects of Implementation of Infant Driven Feeding (IDF) Protocols Which Included Pre and Post Breast Feeding Weights in 35 Week Gestational Age Infants in the Neonatal Intensive Care Unit (NICU) n.d.
- Pridham K, Kosorok MR, Greer F, Carey P, Kayata S, Sondel S. The effects of prescribed versus ad libitum feedings and formula caloric density on premature infant dietary intake and weight gain. Nurs Res 1999;48:86-93. https://doi.org/10.1097/00006199-199903000-00007.
- Pridham KF, Kosorok MR, Greer F, Kayata S, Bhattacharya A, Grunwald P. Comparison of caloric intake and weight outcomes of an ad lib feeding regimen for preterm infants in two nurseries. J Adv Nurs 2001;35:751-9. https://doi.org/10.1046/j.1365-2648.2001.01907.x.
- Saunders RB, Friedman CB, Stramoski PR. Feeding preterm infants: schedule or demand?. J Obstet Gynecol Neonatal Nurs 1991;20:212-20. https://doi.org/10.1111/j.1552-6909.1991.tb02533.x.
- Thoyre SM, Hubbard C, Park J, Pridham K, McKechnie A. Implementing co-regulated feeding with mothers of preterm infants. MCN Am J Matern Child Nurs 2016;41. https://doi.org/10.1097/NMC.0000000000000245.
- Waber B, Hubler EG, Padden ML. A comparison of outcomes in demand versus schedule formula-fed premature infants. Nutr Clin Pract 1998;13:132-5. https://doi.org/10.1002/j.1941-2452.1998.tb03061.x.
- Wellington A, Perlman JM. Infant-driven feeding in premature infants: a quality improvement project. Arch Dis Child Fetal Neonatal Ed 2015;100:F495-500. https://doi.org/10.1136/archdischild-2015-308296.
- Collinge JM, Bradley K, Perks C, Rezny A, Topping P. Demand vs. scheduled feedings for premature infants. J Obstet Gynecol Neonatal Nurs 1982;11:362-7. https://doi.org/10.1111/j.1552-6909.1982.tb01036.x.
- Dalgleish SR, Kostecky LL, Blachly N. Eating in ‘SINC’: Safe Individualized Nipple-Feeding Competence, a quality improvement project to explore infant-driven oral feeding for very premature infants requiring noninvasive respiratory support. Neonatal Netw 2016;35:217-27. https://doi.org/10.1891/0730-0832.35.4.217.
- Marcellus L, Harrison A, Mackinnon K. Quality improvement for neonatal nurses, part II: using a PDSA quality improvement cycle approach to implement an oral feeding progression guideline for premature infants. Neonatal Netw 2012;31:215-22. https://doi.org/10.1891/0730-0832.31.4.215.
- Puckett B, Grover VK, Holt T, Sankaran K. Cue-based feeding for preterm infants: a prospective trial. Am J Perinatol 2008;25:623-8. https://doi.org/10.1055/s-0028-1090583.
- Giannì ML, Sannino P, Bezze E, Comito C, Plevani L, Roggero P, et al. Does parental involvement affect the development of feeding skills in preterm infants? A prospective study. Early Hum Dev 2016;103:123-8. https://doi.org/10.1016/j.earlhumdev.2016.08.006.
- Giannì ML, Sannino P, Bezze E, Plevani L, Esposito C, Muscolo S, et al. Usefulness of the Infant Driven Scale in the early identification of preterm infants at risk for delayed oral feeding independency. Early Hum Dev 2017;115:18-22. https://doi.org/10.1016/j.earlhumdev.2017.08.008.
- Ward B, Fan W. A cue based oral feeding clinical pathway strategy for preterm infants reduces length of nasogastric feeding. J Paediatr Child Health 2018;54:3-128. https://doi.org/10.1111/jpc.13882_339.
- White A, Parnell K. The transition from tube to full oral feeding (breast or bottle) – a cue-based developmental approach. J Neonatal Nurs 2013;19:189-97. https://doi.org/10.1016/j.jnn.2013.03.006.
- Ludwig SM, Waitzman KA. Changing feeding documentation to reflect infant-driven feeding practice. Newborn Infant Nurs Rev 2007;7:155-60. https://doi.org/10.1053/j.nainr.2007.06.007.
- Gill NE, Behnke M, Conlon M, McNeely JB, Anderson GC. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nurs Res 1988;37:347-50. https://doi.org/10.1097/00006199-198811000-00007.
- Glass RP, Wolf LS. A global perspective on feeding assessment in the neonatal intensive care unit. Am J Occup Ther 1994;48:514-26.
- Nyqvist KH, Sjödén PO, Ewald U. The development of preterm infants’ breastfeeding behavior. Early Hum Dev 1999;55:247-64. https://doi.org/10.1016/S0378-3782(99)00025-0.
- Thoyre S, Park J, Pados B, Hubbard C. Developing a co-regulated, cue-based feeding practice: the critical role of assessment and reflection. J Neonatal Nurs 2013;19:139-48. https://doi.org/10.1016/j.jnn.2013.01.002.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Goldfield EC. A dynamical systems approach to infant oral feeding and dysphagia: from model system to therapeutic medical device. Ecol Psychol 2007;19:21-48. https://doi.org/10.1080/10407410709336949.
- Pridham KF. Guided participation and development of care-giving competencies for families of low birth-weight infants. J Adv Nurs 1998;28:948-58. https://doi.org/10.1046/j.1365-2648.1998.00814.x.
- Pridham K, Limbo R. Guided Participation Certificate Program. Madison, WI: University of Wisconsin–Madison, School of Nursing; 2011.
- Als H. Toward a synactive theory of development: promise for the assessment and support of infant individuality. Infant Ment Health J 1982;3:229-43. https://doi.org/10.1002/1097-0355(198224)3:4<229::AID-IMHJ2280030405>3.0.CO;2-H.
- Schroé H, Van Dyck D, De Paepe A, Poppe L, Loh WW, Verloigne M, et al. Which behaviour change techniques are effective to promote physical activity and reduce sedentary behaviour in adults: a factorial randomized trial of an e- and m-health intervention. Int J Behav Nutr Phys Act 2020;17. https://doi.org/10.1186/s12966-020-01001-x.
- Lubbe W. Clinicians guide for cue-based transition to oral feeding in preterm infants: an easy-to-use clinical guide. J Eval Clin Pract 2018;24:80-8. https://doi.org/10.1111/jep.12721.
- Nationwide Children’s Hospital . Cue-Based Feeding In High Risk Neonatal Intensive Care Unit Infants: Barriers, Outcomes and Opportunities 2013.
- Feedings 2013. www.advocatehealth.com/assets/documents/c3.pdf (accessed 14 December 2020).
- Baptist Health . Cue-Based Feeding n.d. https://content.baptisthealth.net/healthstream/Cue-Based_Feeding/Cue-based_Feeding.pdf (accessed 14 December 2020).
- Southern West Midlands Newborn Network . Bottle Feeding Guideline 2011. www.networks.nhs.uk/nhs-networks/southern-west-midlands-newborn-network/documents/Bottle%20feeding%20Guideline%20Final%20Approved.pdf (accessed 24 November 2020).
- Southern West Midlands Maternity and Newborn Network . Progression from Tube to Oral Feeding (Breast or Bottle) 2019. http://swmnodn.org.uk/wp-content/uploads/2018/12/Progression-from-the-tube-to-oral-feeding-Nov-2018.pdf (accessed 14 December 2020).
- UNICEF UK . The Baby Friendly Initiative. Responsive Feeding: Supporting Close and Loving Relationships 2016. www.unicef.org.uk/babyfriendly/baby-friendly-resources/relationship-building-resources/responsive-feeding-infosheet/ (accessed 14 December 2020).
- Winnipeg Regional Health Authority . Enteral Feeding and Nutrition for the Preterm and High Risk Neonate 2017. https://professionals.wrha.mb.ca/old/extranet/eipt/files/EIPT-035-031.pdf (accessed 14 December 2020).
- UNICEF UK . The Baby Friendly Initiative. Guidance for Neonatal Units 2016.
- Birmingham Women’s and Children’s NHS Foundation Trust and Barts Health NHS Foundation Trust . Every Feed Matters: Developmentally Supportive Feeding on the NICU 2017. https://bcuassets.blob.core.windows.net/docs/every-feed-matters-131448503799788007.pdf (accessed 14 December 2020).
- Norfolk and Norwich University Hospitals NHS Foundation Trust . Trust Infant Feeding Policy 2017. www.nnuh.nhs.uk/publication/infant-feeding-trust-policy-p-gfn2-version-5-1/ (accessed 14 December 2020).
- Alberta Health Services . Oral Feeding Guideline 2016. https://extranet.ahsnet.ca/teams/policydocuments/1/clp-calgary-childrens-health-neonatology-nutrition-oral-feeding-2-o-2.pdf (accessed 24 November 2020).
- McMaster Children’s Hospital and St Joseph’s Healthcare . Cue-Based Feeding in the Neonatal Nurseries 2014. www.hamiltonhealthsciences.ca/wp-content/uploads/2019/08/CueBasedFeedingNN-lw.pdf (accessed 14 December 2020).
- Wolf L. FUN-Damentals of Feeding in the NICU 2018. www.neonatalcann.ca/Test/CANN_2018_National_Conference/Speaker_Handouts/Lynn_Wolf_Saturday_1300.pdf (accessed 14 December 2020).
- BC Women’s Hospital and Health Centre . Cue-Based Feeding Guideline: NICU 2014. http://policyandorders.cw.bc.ca/resource-gallery/Documents/BC%20Women’s%20Hospital%20-%20Neonatal%20Program/NN.07.01%20Cue%20Based%20Feeding%20Guideline%20NICU.pdf (accessed 14 December 2020).
- Rodgers M, Thomas S, Harden M, Parker G, Street A, Eastwood A. Developing a methodological framework for organisational case studies: a rapid review and consensus development process. Epidemiol Community Health 2016;70. https://doi.org/10.1136/jech-2016-208064.129.
- Als H, McAnulty GB. The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) with Kangaroo Mother Care (KMC): comprehensive care for preterm infants. Curr Womens Health Rev 2011;7:288-301. https://doi.org/10.2174/157340411796355216.
- Rezaee M, Farahian M. Subconscious vs. unconscious learning: a short review of the terms. Am J Psychol Behav Sci 2015;2:98-100.
- Isaacs E, Jordan B. Advancing Ethnography in Corporate Environments: Challenges and Emerging Opportunities. London: Routledge; 2013.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- The Baby Friendly Initiative . Breastfeeding Assessment Tool (Neonatal) 2019. www.unicef.org.uk/babyfriendly/baby-friendly-resources/implementing-standards-resources/breastfeeding-assessment-tools/ (accessed 24 November 2020).
- Drenckpohl D, Dudas R, Justice S, McConnell C, Macwan KS. Outcomes from an oral feeding protocol implemented in the NICU. Infant Child Adolesc Nutr 2009;1:6-10. https://doi.org/10.1177/1941406408328535.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65-76. https://doi.org/10.1007/s10488-010-0319-7.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Bugge C, Williams B, Hagen S, Logan J, Glazener C, Pringle S, et al. A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-353.
- Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-117.
- Avery KN, Williamson PR, Gamble C, O’Connell Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013537.
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6. https://doi.org/10.1002/14651858.CD000259.pub3.
- Ivers NM, Sales A, Colquhoun H, Michie S, Foy R, Francis JJ, et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-14.
- British Association of Perinatal Medicine . Optimising Early Maternal Breast Milk for Preterm Infants: A Quality Improvement Toolkit 2020. www.bapm.org/pages/196-maternal-breast-milk-toolkit (accessed 9 December 2020).
- Weijer C, Grimshaw JM, Eccles MP, McRae AD, White A, Brehaut JC, et al. The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001346.
- Taljaard M, Grimshaw JM. Concept, characteristics and implications of cluster randomization. J Clin Invest 2014;4:1-4. https://doi.org/10.4155/cli.13.115.
Appendix 1 Systematic review search strategy for Ovid databases
Databases searched
The databases searched were EMBASE (1974 to 6 August 2018), HMIC (1979 to May 2018), Ovid MEDLINE(R) ALL (1946 to 6 August 2018) and Midwives Information and Resource Service (MIDIRS): Maternity and Infant Care.
Date range searched: 1988 to 26 April 2013.
Date of search: 7 August 2018.
Search strategy
-
exp Infant, Premature/ (135,414)
-
exp Premature babies/ (157)
-
(premature infant or premature baby or premature newborn or premature babies).mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] (17,888)
-
(Cue based feeding or feeding advance or infant driven feeding or infant feeding or Feeding Behavior or Feeding Behaviour or Sucking Behavior or Sucking Behaviour or Cues or oral feeding or demand feeding or semi-demand feeding or self-regulatory feeding or ad libitum or feeding cues or satiation).mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, px, rx, an, ui, sy] (365,218)
-
1 or 2 or 3 (142,708)
-
4 and 5 (2724)
-
limit 6 to yr=“2016 -Current” (315)
-
remove duplicates from 7 (262)
Appendix 2 Systematic review table of included studies
| Study (first author) | Study design | Setting | Participants | Inclusion criteria | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Chrupcala26 | Quality improvement before and after intervention | Level 4 NNU, Children’s Hospital, Philadelphia, PA, USA | 170 neonates |
Infants in NNU between 31 and 40 weeks’ gestational age Did not include infants who were intubated, had CPAP, high-flow nasal cannula or did not have suck or gag reflexes |
In after group Phase 1: n = 79 Phase 2: n = 71 The front-line clinician ordered that all patients fed by mouth would be fed according to cues per the infant-driven feeding protocol. All clinical nurses in the NNU were required to independently complete an online mandatory education module. All neonates who were fed by mouth were assigned a feeding/swallowing goal based on infant cues |
n = 20 Not infant-driven, scheduled |
Type of feeding on discharge Length of hospital stay Surgical anomalies |
|
Type of feeding: Bottle or breast Infant formula and mother’s breast milk |
|||||||
|
Cues: For initiating feed: waking, stirring For terminating feed: disengagement and stopping cues, inconsistency, stress |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| Collinge41 | RCT | NNU, Montreal Children’s Hospital, QC, Canada | 36 preterm infants |
Birthweight between 1800 g and 2500 g In the NNU for at least 24 hours Had no major neurological disorders or gastrointestinal problems |
n = 18 Responsive feeding, defined as ‘allowing the infant to feed as frequently as they wish, and to take as much as desired at each feeding’ Infants were fed (orally or via a gastric feeding tube) in response to crying, sucking on fingers or pacifier, activity and rooting |
n = 18 Received prescribed volumes of milk (up to 160 ml/kg per day) either orally or via a feeding tube at 3- to 4-hourly intervals |
Volume intake Weight gain Duration from first oral feed to discharge Number of times requiring wakening Time to discharge Feeds per day Gavage feeds for demand schedule |
|
Type of feeding: Bottle or breast Infant formula and mother’s own breast milk |
|||||||
|
Cues: For initiating feed: crying, trying to suck fingers or a pacifier, moving actively and rooting. Not settling with position or nappy change or with a pacifier For terminating feed: not described |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| Dalgleish42 | Retrospective audit | Five level 2 or level 3 NNUs, Canada | 196 preterm infants |
< 32 weeks’ gestation Physiologically stable, with or without CPAP and able to swallow/manage secretions |
n = 105 Infant-driven, demand/semi-demand feeding Development and implementation of a safe individualised nipple-feeding competent (SINC) algorithm |
n = 91 Baseline chart audit. Infants not following the SINC algorithm. The audit noted when NF was initiated, patterns of how feeds were advanced, time from initiation to exclusive oral feeds, amount of direct breastfeeding and length of stay, as well as the type of respiratory support required at NF initiation and advancement |
Weight gain First breastfeed Frequency of breastfeeding Discharge age Length of hospital stay |
|
Type of feeding: Bottle and breast Content of bottle unclear, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: licking, rooting, mouthing, alert state, physiological stability, hunger cues For terminating feed: bradycardia, tachycardia, oxygen desaturation, colour change, loss of postural tone, loss of state |
|||||||
|
Delivered by: Parents (preferred); unclear who else |
|||||||
| Davidson27 | Retrospective study | Level 3 NNU, Children’s Hospital, Ohio, USA | 115 preterm infants | Gestational age at birth ranged from 23 to 29 weeks |
n = 55 When infants achieved scores of 4 or 5 (= hunger cues) at ≥ 50% of their feeding times, health-care providers ordered to begin cue-based oral feeds |
n = 60 Health-care provider-driven feeding group |
Weight gain Duration from first oral feed to full oral feed PMA at first oral feeding Evaluation of the scales used |
|
Type of feeding: Bottle or breast Type of food in bottle was unclear, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: hunger cues, awakens at or before scheduled care, alert or fussy during care, rooting and/or hands to mouth For terminating feed: if infant demonstrated two disengagement or distress cues (e.g. falling asleep, nasal flaring, arching, or an unco-ordinated suck–swallow–breathe pattern that resulted in bradycardia) |
|||||||
|
Delivered by: Nurses, occupational therapists, lactation consultants, licensed practical nurses and patient care assistants |
|||||||
| Gelfer28 | Before and after intervention | Level 2 NNU, Children’s Memorial Hermann Hospital, Houston, TX, USA | 124 preterm infants | Healthy premature infants born at ≥ 30 weeks’ gestation |
n = 60 Infant-driven feeding using the Infant-Driven Feeding Scales: readiness and quality; feeding and behavioural cues |
n = 64 No information on the control group |
Weight gain Initiation of oral feeds Success and difficulties of the programme Time to ad lib feeds Age at discharge Length of hospital stay Number of feeding therapist consultations needed |
|
Type of feeding: Bottle or breast Type of food in bottle was unclear, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: readiness behaviour, alert or fussy prior to care, rooting and/or hands to mouth behaviour, good tone For terminating feed: signs of disengagement or distress |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| Giannì45 | Prospective observational, no control | Tertiary NNU, Italy | 81 preterm infants | Preterm infants born at ≤ 32 weeks’ gestation and white without known congenital and/or chromosomal diseases, death during hospitalisation, and transfer to another institution |
n = 81 Oral feeding was started on the basis of infant’s feeding-readiness cues, and feeding advancement was performed according to the infants’ cardiorespiratory stability and feeding tolerance. Efforts were made by all health-care professionals to promote infant–parent interaction, including postural care, KMC and breastfeeding |
There was no control group |
Weight gain Duration from first oral feed to full oral feed Length of hospital stay Age at discharge Weight at discharge Starting and duration of enteral feeding Starting and duration of full oral feeding Number of times fed by parents Exclusive breastfeeding, breastfeeding plus bottle, breastfeeding, enteral feeding at discharge |
|
Type of feeding: Bottle or breast Unclear for bottle feeding, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: not described For terminating feed: not described |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| Giannì46 | Prospective, observational, no control | Level 3 NNU, Milan, Italy | 47 preterm infants | Infants born at a gestational age of ≤ 32 weeks |
n = 47 Enteral feeding started within 24 hours of birth using human milk or preterm formula when human milk was unavailable. Oral feeding was started based on the feeding-readiness cues and feeding progress followed medical advice, based on cardiorespiratory stability and gastrointestinal tolerance. Lactation counselling was provided At the time of the study a cue-based feeding approach was not in place in the NNU |
There was no control group |
Efficacy of the instrument Feeding type at discharge Age when feeding independently |
|
Type of feeding: Bottle or breast Bottle content was not specified, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: based on a scale: alert or fussy before care, rooting, hands to mouth, actively taking the pacifier, good muscle tone For terminating feed: not described |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| Gray29 | RCT (head to head) | Two level 3 NNUs in Philadelphia, PA, USA | 55 preterm infants | Infants born at ≤ 33-week gestation |
Intervention 1 (feed every 6 hours), n = 28 Intervention 2 (feed every 3 hours), n = 27 Infants were randomly allocated to receive 3-hour or 6-hour assessment for oral feeding cues Infants in the 6-hour group were assessed for feeding cues four times daily; those in the 3-hour group were assessed eight times daily by the bedside nurse and offered an oral feed via breast or bottle when they demonstrated stable vital signs and feeding cues |
There was no control group |
Duration from first oral feed to full oral feed Growth velocity while feeding (g/day) Length growth while feeding (cm/day) Length of hospital stay Number of attempted oral feeds (per day) Frequency of breastfeeding (feeds/day) Age at discharge (weeks) Days of respiratory support during hospitalisation |
|
Type of feeding: Bottle or breast Type of food in bottle was unclear, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: presence of feeding cues For terminating feed: desaturations, apnoea, respiratory distress or feeding refusal |
|||||||
|
Delivered by: Nurses |
|||||||
| Kansas30 | RCT | duPont Hospital for Children, Philadelphia, PA, USA | 59 preterm infants |
Born before 33 weeks’ gestational age Able to take at least half of their enteral feeds orally from a nipple (either bottle or breast) |
n = 29 At randomisation, enteral feeding tubes were removed and infants were then fed in response to cues (no maximum or minimum feeding volume or interval) via a nipple |
n = 30 Scheduled interval feeding with gavage feeding if infant did not ingest prescribed volume from nipple |
Calorie intake Volume intake Weight gain Time to full nipple feed Length of hospital stay |
|
Type of feeding: Unclear if breast nipple or bottle nipple Mother’s own breast milk and unclear if bottle |
|||||||
|
Cues: Not described |
|||||||
|
Delivered by: Unclear |
|||||||
| Kirk18 | Prospective study group vs. historic cohort controls | Not described, UT, USA | 51 neonates | Infants born between 32 and 37 weeks (PMA) and off mechanical ventilation |
n = 28 Authors designed a clinical pathway that allowed the bedside nurse to advance oral feeding through specific milestones using infant behavioural feeding-readiness signs and hunger cues rather than daily physician orders |
n = 23 Feeds initiated and advanced according to the discretion of the attending physician |
Weight gain Duration from first oral feed to full oral feed Achieving full oral feeding as discharge criteria Length of hospital stay |
|
Type of feeding: Bottle or breast Mother’s own breast milk and unclear regarding bottle contents |
|||||||
|
Cues: For initiating feed: behavioural readiness signs and hunger cues, awake, alert, stable For terminating feed: signs of stress, such as a change in colour, in state of alertness, in breathing pattern and abnormalities in swallowing |
|||||||
|
Delivered by: Nurses |
|||||||
| Marcellus43 | Quality improvement retrospective | NNU level 1, Victoria General Hospital, Canada | 89 preterm infants | Gestational age at birth of < 35 weeks |
n = 50 Not well described. A stepwise oral feeding in infants approach was developed |
n = 39 They represented the baseline or starting point for the project. How the feeding was carried out was not described |
Time between full enteral and full oral feeding Corrected age at full oral feeds Corrected age at discharge |
|
Type of feeding: Bottle or breast Type of food was unclear |
|||||||
|
Cues: Not described |
|||||||
|
Delivered by: Parents, NNU staff |
|||||||
| McCain31 | RCT | NNUs affiliated to the University of Cincinnati, Cincinnati, OH, USA | 81 preterm infants |
Born > 32 weeks’ gestation ≤ 34 weeks and appropriate for gestational age Receiving enteral feeds of fortified human milk or commercial formula at 105–130kcal/kg per day without supplemental intravenous fluids or parenteral nutrition Had no congenital anomalies, gastrointestinal conditions, neurological diagnoses, or grade III/IV intracranial haemorrhage |
n = 40 Responsive (‘semi-demand’) feeding: infants received 10 minutes of non-nutritive sucking every 3 hours to assess wakefulness and behavioural state Infants who were wakeful were offered an oral feed. If the infant was not sufficiently awake, they were left to sleep for a further 30 minutes and the process was repeated. If the infant continued to sleep at that stage, or the minimum prescribed amount was not taken, the infants were given a gavage feed of the full prescribed volume |
n = 41 Received prescribed volumes of milk either orally or via feeding tube at 3-hourly intervals. Feeding duration was restricted to a maximum of 30 minutes One infant in the control group was transferred to another hospital after completing the study protocol |
Calorie intake Volume intake Weight gain Behavioural responses Days to oral feeding |
|
Type of feeding: Unclear Infant formula and fortified human milk |
|||||||
|
Cues: For initiating feed: states 3 or higher (restlessness or wakefulness) as rated using the Anderson Behavioural State Scale (not described) For terminating feed: infant stopped sucking, fell asleep or showed clinical instability, such as apnoea or bradycardia |
|||||||
|
Delivered by: Nurses Study staff delivered the Anderson Behavioural State Scale |
|||||||
| McCain32 | RCT | NNU at Jackson Memorial Hospital, Miami, FL, USA | 96 preterm infants with bronchopulmonary dysplasia (BPD) |
Infants born before or at 34 weeks’ gestation with BPD Had no congenital anomalies, gastrointestinal conditions, neurological diagnoses, or grade III/IV intracranial haemorrhage |
n = 48 (44 completed) Responsive (‘semi-demand’) feeding regulated by using infant behavioural and cardiorespiratory signs, which determined the frequency, length and volume of nipple/oral feeds. Infants offered 3-hourly feeds if awake |
n = 48 (42 completed) Scheduled feeding (24 kcal/oz) at 3-hourly intervals. Standard care increased in number of nipple to gavage feeds per day |
Weight gain (daily) Duration from first oral feed to full oral feed (days to nipple feeding) Duration from first oral feed to discharge |
|
Type of feeding: Bottle feeding Infant formula and fortified human milk |
|||||||
|
Cues: For initiating feed: not described For terminating feed: feeds were stopped when an infant ingested the entire amount of feeding volume, refused to suck any longer, fell asleep, or had a distress event (e.g. choking, apnoea, bradycardia) |
|||||||
|
Delivered by: Nursery nurses, nurses |
|||||||
| Messer33 | Retrospective chart review before and after | Level 3 NNU, children’s hospital within a hospital, USA | 29 preterm infants |
27–32 weeks’ gestation at birth with a birthweight of 1000–1700 g, receiving oral nutrition only (gavage or nipple, but not breastfeeding) Had no neurological or gastrointestinal disorders or congenital anomalies |
n = 14 Post-intervention group Demand fed according to a readiness-to-feed protocol Under no circumstance could the bottle or nipple be manipulated to get more volume from the bottle; the infant that had fallen asleep could not be waked. At that point, the remainder of the feeding was given by gavage |
n = 15 Pre-intervention group Scheduled, volume-driven feeds |
Length of hospital stay |
|
Type of feeding: Bottle – unclear what type of food |
|||||||
|
Cues: For initiating feed: drowsy, awake or fussy prior to care, rooting or hand to mouth, keeps pacifier, good tone For terminating feed: no hunger cues, no change in tone, apnoea, bradycardia, tachypnoea |
|||||||
|
Delivered by: Nurses; the speech and language team delivered the training |
|||||||
| Murray34 | Chart review; non-randomised, before and after | NNU, Methodist Women’s Hospital, USA | 37 preterm infants | Preterm infants with a gestational age at birth between 35 weeks and 35 weeks + 6 days |
n = 19 Following the infant-driven feeding programme. Infants are allowed nipple attempts only if they show certain readiness cues. The accurate assessment of the infant’s breastfeeding attempts is key in infant-driven feeding. Infant is gavage or bottle fed the remaining amount |
n = 18 Volume-driven feeding with the clinician ordering the number of nipple feeds the infants can attempt each day, based on weight and gestational age |
Weight gain Length of hospital stay |
|
Type of feeding: Unclear; the content was mother’s breast milk and unclear as to formula content |
|||||||
|
Cues: For initiating feed: not described For terminating feed: not described |
|||||||
|
Delivered by: Unclear |
|||||||
| Nyqvist15 | Prospective descriptive study, no control | NNU, Uppsala University Children’s Hospital, Sweden | 15 preterm infants |
Gestational age at birth of 32–36 weeks Had no neurological illness, congenital anomaly or chromosomal abnormality |
n = 15 Semi-demand feeding occurred during the intermediate phase before demand feeding: nutrition prescriptions were changed from scheduled feeding; the mother was encouraged to feed on all cues. When a defined time interval had lapsed, to reach a daily breastfeeding frequency sufficient for adequate infant growth; supplementation was given when required |
No control group |
Initiation of oral = breastfeeding Initiation of cup feeding Initiation of bottle feeding Prescription of daily milk volume Initiation of (semi) demand feeding Attainment of full oral feeding Attainment of full breastfeeding Early discharge for home care Length of hospital stay |
|
Type of feeding: Breast, bottle, cup Mother’s own breast milk, donor’s breast milk, supplementation when required to cup feeding |
|||||||
|
Cues: For initiating feed: infant signs of behavioural state shifts and interest in sucking, cues of hunger For terminating feed: infant cues of satiety |
|||||||
|
Delivered by: Parents, author of study |
|||||||
| Pridham35 | RCT | Level III NNU in WI, USA | 78 preterm infants | < 35 weeks’ gestational age at birth and appropriate weight for gestational age. Infants were enrolled in the trial when taking at least 80% of enteral feeds directly from a nipple (either breast or bottle), at which point tube feeding was ceased and all feeds were offered by nipple |
n = 45 (Nursery B) Responsive feeding initiated in response to infant hunger cues and terminated in response to infant satiation Enteral feeds directly from a nipple (either breast or bottle), at which point tube feeding was ceased and all feeds were offered by nipple. Most infants received standard formula milk. As part of a factorial trial design, some infants were randomly allocated to receive calorie-enriched formula milk |
n = 33 (Nursery B) Prescribed feeding (20 kcal/oz formula or breast milk) at 4-hourly intervals |
Calorie intake Volume intake Weight gain |
|
Type of feeding: Bottle Infant formula |
|||||||
|
Cues: For initiating feed: infant stirring, rooting, sucking a fist or fingers, placing hand to mouth, being awake, crying intermittently and crying continuously For terminating feed: infant falling asleep, failure to resume sucking after release of the nipple, signs of fatigue, and signs of contentment with no indication of interest in continuing the feed |
|||||||
|
Delivered by: Nursery nurses |
|||||||
| Pridham36 | RCT | Level 3 NNU in WI, USA | 22 preterm infants |
< 35 weeks’ gestational age at birth and appropriate weight for gestational age Infants were enrolled in the trial when taking at least 80% of enteral feeds directly from a nipple (either breast or bottle), at which point tube feeding was ceased and all feeds were offered by nipple |
n = 12 (Nursery A) Responsive, initiated in response to infant hunger cues and terminated in response to infant satiation |
n = 10 (Nursery A) Prescribed feeding (20 kcal/oz formula or breast milk) at 3-hourly intervals All of the breast-milk-fed infants on the prescribed regimen took less than the prescribed amount at breast and were given the remainder of the feeding by bottle |
Calorie intake Volume intake Weight gain |
|
Type of feeding: Bottle or breast Infant formula, mother’s breast milk, or a mix of bottle and breast |
|||||||
|
Cues: Not described, but assessment for readiness to begin nipple feeding considered ‘awake state’ |
|||||||
|
Delivered by: Nurses |
|||||||
| Puckett44 | RCT | Level 3 NNU in Saskatoon, SK, Canada | 80 moderate preterm infants |
Infants with a post-conceptual age of 32 weeks and a weight of ≥ 1500 g Who tolerated oral feeding without intravenous nutritional support Had no congenital abnormalities, major gastrointestinal surgery or severe intraventricular haemorrhage. Infants mechanically ventilated were excluded |
n = 39 At study entry gavage feeds were discontinued and infants fed orally on demand in response to hunger cues. There was a 5-hour limit between feeds; if no cues, the infant was woken for feeding |
n = 40 Continued standard scheduled (schedule not reported) gavage and bottle feeding |
Volume intake (only for bottle-fed babies) Weight gain Behavioural responses (number of cues) Length of hospital stay Times wakened for feed Frequency of feeding per day Breastfeeding per day Number of adverse events during feeding |
|
Type of feeding: Bottle or breast Infant formula or breast milk at parents’ request |
|||||||
|
Cues: For initiating feed: crying, quiet alert, hand to mouth activity, sucking on fingers, fist or pacifier, rooting, inability to settle after position change, nappy change or pacifier For terminating feed: satiation cues, such as turning head away, holding hands in stop manner, falling asleep, no interest in restarting feed after burp/break in sucking, adverse events |
|||||||
|
Delivered by: Family members, nurses |
|||||||
| Saunders37 | RCT | Level 3 NNU at the Women’s Hospital, Greensboro, NC, USA | 29 preterm infants | Preterm infants (≤ 37 weeks’ gestation) without major neurological or gastrointestinal disorders. Weight of ≥ 1500 g |
n = 15 Responsive to hunger cues with a 5-hour limit between feeds Infants in either group who failed to take adequate amounts orally for two consecutive feeds were fed a prescribed volume (to achieve a daily intake of 120 ml/kg per day) via an intragastric feeding tube for the next feed |
n = 14 Prescribed feeding of set volumes at 3-hourly intervals to achieve at least 120 ml/kg per day intake |
Calorie intake Volume intake Weight gaina Weight loss Behavioural responses (hunger cues)a Accu-Chek (Roche Diabetes Care Limited, Burgess Hill, UK) Urine specific gravity Length of hospital stay |
|
Type of feeding: Bottle Infant formula |
|||||||
|
Cues: For initiating feed: rooting, sucking, hand to mouth, crying, not settling after a nappy change For terminating feed: not described |
|||||||
|
Delivered by: Nursery nurses |
|||||||
| Thoyre38 |
Descriptive – co-regulated feeding intervention Original study sequential cohort, no comparison |
Level 3 NNU, Children’s Hospital in Chapel Hill, NC, USA | 17 preterm infants | Preterm infants of ≤ 30 weeks’ gestational age at birth |
n = 17 (infants) and n = 16 (mothers) Co-regulated feeding intervention. Cues also include breathing and swallowing signals amplified by a microphone on the infant’s neck. Intervention comprised five sessions, the first one before infant was on oral feeding and the subsequent four during transition from tube to oral feeding. They focused on feeding-related issues identified by the mother or intervention nurse |
No control |
Duration from first oral feed to full oral feed Age at discharge |
|
Type of feeding: Bottle or breast Bottle’s content unclear, mother’s own breast milk |
|||||||
|
Cues: For initiating feed: breathing and pauses in breathing, cues of calmness, organisation and engagement. For terminating feed: cues of distress and disengagement |
|||||||
|
Delivered by: Parents, nurses, lactation consultant or the mother’s grandmother or sister |
|||||||
| Waber39 | RCT | Level 3 NNU, Mid-Atlanta, USA | 10 preterm infants | Preterm infants of ≤ 34 weeks’ gestational age at birth |
n = 5 Infants were fed on demand after showing at least two signs of hunger. Feeding tubes were removed. Were fed at intervals not longer than 2 hours. Were awakened gently for feeding after a maximum of 5 hours if they did not self-waken |
n = 5 Schedule fed, 3- to 4-hourly, nipple or gavage fed a prescribed amount |
Calorie intake Volume intake Weight (daily) Duration from first oral feed to discharge (recorded, only mentioned in discussion) Behavioural responses (hunger cues per day) Head circumference Length measurement Time and duration of each feed Feeding method Hunger and satiation cues at each feed |
|
Type of feeding: Unclear method of feed Infant formula |
|||||||
|
Cues: For initiating feed: hunger cues – crying; hand-to-mouth activity; sucking on fingers, fist or pacifier; rooting; or inability to settle after position change, nappy change, or pacifier For terminating feed: satiation cues – refusal to suck and sleep |
|||||||
|
Delivered by: Staff – unclear |
|||||||
| Ward47 (abstract only) | Before-and-after study, non-randomised | NNU, Northern Hospital, Melbourne, VIC, Australia | 438 preterm infants | Preterm infants, undefined gestational age |
n = 146 Oral feeds are upgraded according to infant cues, and nasogastric tubes are removed when a minimum of four full sucking feeds within a 24-hour period have been achieved |
n = 292 Schedule fed |
Duration from first oral feed to full oral feed Weight gain (no numbers) Length of hospital stay Time to discharge |
|
Type of feeding: Unclear |
|||||||
|
Cues: For initiating feed: not described For terminating feed: not described |
|||||||
|
Delivered by: Unclear |
|||||||
| Wellington40 | Before and after intervention, evaluation of intervention | NNU, New York Presbyterian Hospital, USA | 254 preterm infants | Preterm infants of < 34 weeks’ gestational age without congenital conditions, necrotising colitis or cleft palate |
n = 101 A ‘Premature Infant Feeding Assessment’ flowsheet was introduced, which allows the bedside nurse to evaluate an infant prior to each feeding using a ‘readiness score’ every 3 hours. The nurse initiates charting readiness scores at 32 weeks’ PMA but continues to gavage feed until an infant is at least 33 weeks’ PMA and is scoring 1–2 on the Readiness Scale for at least half the day for 1–2 days. Following an oral feed, the nurse documents a ‘quality score’ |
n = 153 Practitioner-driven feeding method. The approach did not incorporate feeding cues from the baby. It was outcome oriented whereby a ‘good’ feeding was equated with a finished bottle |
Age at first nipple feed Age at full nipple feed PMA at discharge Evaluation of the protocol |
|
Type of feeding: Bottle or breast Content of bottle unclear; mother’s own breast milk |
|||||||
|
Cues: For initiating feed: drowsy, awake or fussy prior to care, rooting or hand to mouth, keeps pacifier, good tone For terminating feed: no hunger cues, no change in tone, apnoea, bradycardia, tachypnea |
|||||||
|
Delivered by: Parents, nurses |
|||||||
| White48 | Intervention evaluation no comparison | UK | N/A | Preterm infants transitioning from tube to oral feeding |
Creation of an educational poster with a clear pathway on cue-based feeding in the transition from tube to oral feeding of preterm infants Creation of appropriate policy and guidelines Staff and parents were encouraged to recognise and respond to feeding cues and stress cues |
N/A | Success of the intervention |
|
Type of feeding: Bottle, breast. Type of milk not specified |
|||||||
|
Cues: For initiating feed: hand to mouth, mouth opening, rooting for the breast, responds to gentle touch to the face, alert and looking at the breast, protrudes tongue, licks at the breast For terminating feed: not described |
|||||||
|
Delivered by: Unclear |
Appendix 3 Systematic review list of outcome measures in included studies
| Outcome measure used | Studies reporting this outcome measure (first author) |
|---|---|
| Age at discharge (corrected gestational age) | Chrupcala,26 Dalgleish,42 Gray,29 Messer33 |
| Age on discharge (days since birth) | Thoyre38 |
| Age on discharge (post-conceptional age) | Puckett44 |
| Age on discharge (post-menstrual age) | Giannì,45 Giannì,46 McCain,32 Thoyre,38 White48 |
| Length of time to full oral feeds | Davidson,27 Giannì,45 Giannì,46 Gray,29 Messer,33 Murray,34 Nyqvist,15 McCain,32 Ward47 |
| Feeding method on discharge | Chrupcala,26 Dalgleish,42 Giannì,45 Giannì,46 McCain,32 Thoyre38 |
| Age when taking full oral feeds (corrected gestational age) | Messer33 |
| Age when taking oral full feeds (days since birth) | Marcellus43 |
| Age when taking full oral feeds (post-conceptional age) | Gelfer28 |
| Age when taking full oral feeds (post-menstrual age) | Giannì,45 Kirk,18 McCain,32 White48 |
| Age at first oral feed (corrected gestational age) | Dalgleish,42 Messer33 |
| Age at first oral feed (post-conceptional age) | Gelfer28 |
| Age at first oral feed (post-menstrual age) | Davidson,27 Giannì,45 McCain,32 White48 |
| Number of feeding cues | McCain,32 Puckett,44 Saunders,37 Waber39 |
| Length of time to discharge from entry to study | Nyqvist,15 Puckett,44 Saunders,37 Waber,39 Ward,47 White48 |
| Total length of stay in NNU | Chrupcala,26 Gelfer,28 Giannì,45 Giannì,46 Gray,29 Kansas,30 Kirk,18 Nyqvist,15 Marcellus,43 McCain31 |
| Impact on nursing staff | Messer,33 Puckett44 |
| Age parents feed their infant for the first time | Giannì45 |
| Daily volume intake | Collinge,41 Kansas,30 Murray,34 Pridham,35 Pridham,36 Puckett,44 Saunders,37 Waber39 |
| Percentage of volume intake per feed | Collinge41 |
| Daily weight gain | Collinge,41 Davidson,27 Gelfer,28 Gray,29 Kansas,30 Kirk,18 Pridham35 |
| Overall weight gain | Gelfer28 |
| Weight velocity | Davidson27 |
| Weight at discharge | Dalgleish,42 Giannì,45 Giannì,46 Kansas,30 Marcellus,43 Puckett,44 Saunders37 |
| Number of breast feeds per day | Gray,29 Puckett44 |
| Number of feeds per day | Collinge,41 Gray,29 Puckett,44 Saunders37 |
| Number of feeds given by parents per day | Giannì45 |
| Number of gavage feeds per day | Collinge,41 Saunders,37 Waber39 |
| Time interval between feeds | Collinge,41 Saunders,37 Waber39 |
| Length of feeds | Saunders,37 Waber39 |
| Caloric and or protein intake per day | Murray,34 Pridham,35 Pridham,36 Puckett,44 Waber39 |
| Number of times infant required wakening for feed | Collinge,41 Puckett44 |
| Blood glucose levels | Collinge41 |
| Feeding problems (e.g. refusal/slow intake) | Collinge,41 Thoyre38 |
| Growth measurements (e.g. length) | Gray,29 Waber39 |
| Medical complications/interventions required | Chrupcala,26 Dalgleish,42 Gray,29 Kirk,18 Murray,34 Nyqvist,15 McCain,32 Pridham,35 Pridham,36 Puckett44 |
| Suitability of the intervention to staff and or parents | Messer,33 McCain,32 Thoyre,38 White48 |
Appendix 4 Our Feeding Journey
Appendix 5 ADePT process
List of abbreviations
- ADePT
- A process for Decision-making after Pilot and feasibility Trials
- app
- application
- BCT
- behaviour change technique
- BFI
- Baby Friendly Initiative
- CONSORT
- Consolidated Standards of Reporting Trials
- Cubs
- Cue-based versus scheduled feeding
- EBM
- expressed breast milk
- NICU
- neonatal intensive care unit
- NIDCAP
- Newborn Individualized Developmental Care and Assessment Program
- NNU
- neonatal unit
- PI
- Principal Investigator
- RCT
- randomised controlled trial
- TIDieR
- Template for Intervention description and Replication
- UNICEF
- United Nations Children’s Fund
- WP
- work package
