Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/144/09. The contractual start date was in June 2012. The draft report began editorial review in October 2020 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Marson et al. This work was produced by Marson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Marson et al.
Chapter 1 Introduction
Epilepsy is a common condition, with a prevalence of 0.5–1% and a lifetime incidence of up to 5%. 1 It is also a complex condition with many different causes and a number of seizure types and syndromes, as defined by the International Leagues Against Epilepsy. 2,3 It is uniquely stigmatising and has a negative impact on quality of life (QoL), education and employment prospects. 4,5 Anti-seizure medicines, previously called antiepileptic drugs, are the mainstay of treatment and may need to be a lifelong treatment. The aim of treatment is to maximise QoL by eliminating seizures at drug doses that do not cause adverse effects. The choice of first anti-seizure medicine is paramount if we are to maximise individuals’ educational and career prospects, their ability to return to work and their ability to drive.
Around two-thirds of people with epilepsy have focal epilepsy, in which seizures originate within networks limited to one cerebral hemisphere. Seizure types include focal aware seizures (previously called simple partial seizures), focal seizures with altered awareness (previously called complex partial seizures) and focal to bilateral tonic–clonic seizures (previously called secondary generalised tonic–clonic seizures). 2,3 Focal epilepsy can start at any age, and the incidence distribution is U-shaped, with a higher incidence in the young and the elderly. Owing to the ageing population in many countries, the incidence is higher in the elderly than in the young. 6
Although focal epilepsies can be classified according to the site of seizure onset and aetiology, there is no evidence to suggest that one syndrome or aetiology responds better to one treatment than to another. 7 Drug management is therefore generally similar whatever the aetiology or syndrome. Guidelines typically recommend lamotrigine (Lamictal®, GlaxoSmithKline plc, Brentford, UK) or carbamazepine (Tegretol®, Novartis Pharmaceuticals UK Ltd, London, UK) as first-line treatments,8 in part informed by the first SANAD trial, which identified lamotrigine as non-inferior to carbamazepine for time to 12-month remission and superior to carbamazepine, gabapentin (Neurontin®, Upjohn UK Ltd, Sandwich, UK), oxcarbazepine (Trileptal®, Novartis Pharmaceuticals UK Ltd) and topiramate (Topamax®, Janssen: Pharmaceutical Companies of Johnson & Johnson, Beerse, Belgium) for time to treatment failure. 9 Lamotrigine was therefore chosen as the standard comparator in the SANAD II trial.
Around one-third of people with epilepsy have idiopathic generalised epilepsy, also referred to as genetic generalised epilepsy, which includes several syndromes classified according to seizure type and age at onset, such as the absence epilepsies and juvenile myoclonic epilepsy. 3 Although the differing syndromes are recognised, there is currently no reliable evidence that relative treatment responses differ across syndromes. Indeed, prognostic modelling of data from the SANAD I trial indicates that relative treatment responses are consistent across syndromes. 7 In addition, at the time of epilepsy diagnosis, classification can be difficult for a proportion of people who cannot be classified as having either a focal or a generalised epilepsy, although for many a syndromic diagnosis can be made during follow-up as investigation results are received or more seizures are observed. 10,11
For many years, despite limited evidence from randomised controlled trials (RCTs), valproate (Epilim®, Sanofi SA, Paris, France) has been recommended as a first-line treatment for generalised and unclassifiable epilepsy as it has a broad spectrum of action. 12 Cochrane reviews have compared valproate with other anti-seizure medicines,13–15 but, because of problems with power and epilepsy classification, they have not shown an advantage for valproate. The SANAD I trial identified valproate as a clinically effective and cost-effective alternative to either lamotrigine or topiramate,16 and a double-blind trial of 16 weeks’ therapy in childhood and juvenile absence epilepsy found that both valproate and ethosuximide were superior to lamotrigine for the outcome time to treatment failure. 17
Valproate is not recommended for women of childbearing potential, as it is associated with a major malformation rate of around 10%,15 and up to one-third of children exposed in utero have a significant reduction in their IQ. 16 In 2017, the European Medicines Agency (EMA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) launched a pregnancy prevention programme,18 stating that women should not be prescribed valproate unless other treatments are ineffective or not tolerated. Consequently, making a treatment choice for women with idiopathic generalised epilepsy is very challenging. The two main alternatives to valproate are lamotrigine, which is less effective but safer in pregnancy, and levetiracetam (Keppra®, UCB Pharma Ltd, Slough, UK), for which we have increasing evidence of relative safety in pregnancy,19,20 but its effectiveness compared with valproate is unknown.
Although > 20 anti-seizure medicines have been licensed for use globally in the past 20 years, there is very limited evidence to inform everyday decisions, including choice of first anti-seizure medicine, because regulatory trials do not measure important longer-term outcomes (e.g. 12-month remission from seizures). In particular, very few trials have assessed the comparative clinical effectiveness or cost-effectiveness of anti-seizure medications for generalised epilepsy or epilepsy that is difficult to classify. 12 The SANAD collaborators selected levetiracetam and zonisamide (Zonegran®, Eisai Co. Ltd, Tokyo, Japan) for assessment in the SANAD II trial.
Levetiracetam is a commonly prescribed anti-seizure medication with evidence of efficacy as monotherapy in focal epilepsy. This is based on finding non-inferiority when comparing levetiracetam with carbamazepine for 6-month seizure remission, and finding similar tolerability of both medications in a regulatory trial that did not assess longer-term effectiveness. 21 A second unblinded trial compared levetiracetam with the physician’s choice of carbamazepine or valproate22 and found no significant difference between carbamazepine and levetiracetam for time to first seizure and time to treatment failure. However, this trial22 had a maximum follow-up of 12 months and could not assess the longer-term outcomes needed to inform policy. In the 2012 National Institute for Health and Care Excellence (NICE) epilepsy guideline,8 levetiracetam was not recommended as a first-line treatment based on an analysis indicating that it was not cost-effective; however, it has since become widely prescribed. Generic levetiracetam has been available in the UK since 2011, and the price of 60 × 250-mg tablets (for example) has since reduced from £29.7023 to £5.72. 24
Levetiracetam has been increasingly used as a first-line treatment in generalised epilepsy,25 particularly for women of childbearing age. Although there is RCT evidence of efficacy as an add-on treatment for some generalised seizure types,26,27 and evidence of tolerability as monotherapy when compared with valproate,22 there is currently no RCT evidence of clinical effectiveness, cost-effectiveness or economic evidence supporting the cost-effectiveness of levetiracetam when used as monotherapy or as a first-line treatment in generalised or unclassifiable epilepsy.
Zonisamide has been available for many years in Japan28 and other countries in South-East Asia, where it is commonly used both as initial monotherapy and as an add-on treatment. Its licence for use as monotherapy in focal epilepsy is based on a regulatory study demonstrating non-inferiority when compared with carbamazepine for 6-month seizure remission rates. 29 The longer-term comparative clinical effectiveness and cost-effectiveness of zonisamide in focal epilepsy are unknown, and zonisamide is not currently recommended as a first-line therapy. Zonisamide currently costs more than 12 times as much as lamotrigine on a defined daily dose basis.
The aims of the SANAD II trial were to assess the longer-term clinical effectiveness and cost-effectiveness of levetiracetam and zonisamide compared with lamotrigine in focal epilepsy, and of levetiracetam compared with valproate for generalised or unclassifiable epilepsy, in an unblinded randomised controlled trial.
Chapter 2 Trial design and methods
Parts of this chapter have been reproduced with permission from our published protocol: Balabanova et al. 30 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Study design
The SANAD II trial was a pragmatic Phase IV, multicentre, unblinded randomised controlled trial that was conducted in NHS adult neurology and paediatric services. The study was essentially two separate RCTs: the first trial recruited participants with newly diagnosed focal epilepsy who were randomised to start treatment with the ‘standard’ drug lamotrigine or with the ‘new’ drugs levetiracetam or zonisamide, and the second trial recruited participants with newly diagnosed generalised epilepsy or epilepsy that was unclassified at the time of randomisation, who were randomised to start treatment with the ‘standard’ drug valproate or with the ‘new’ drug levetiracetam. Both trials followed the previously published protocol. 30 An economic evaluation was performed to consider the cost-effectiveness of newer drugs compared with the standard drugs. A schematic of the study design is provided in Figure 1.
FIGURE 1.
Schematic of study design.
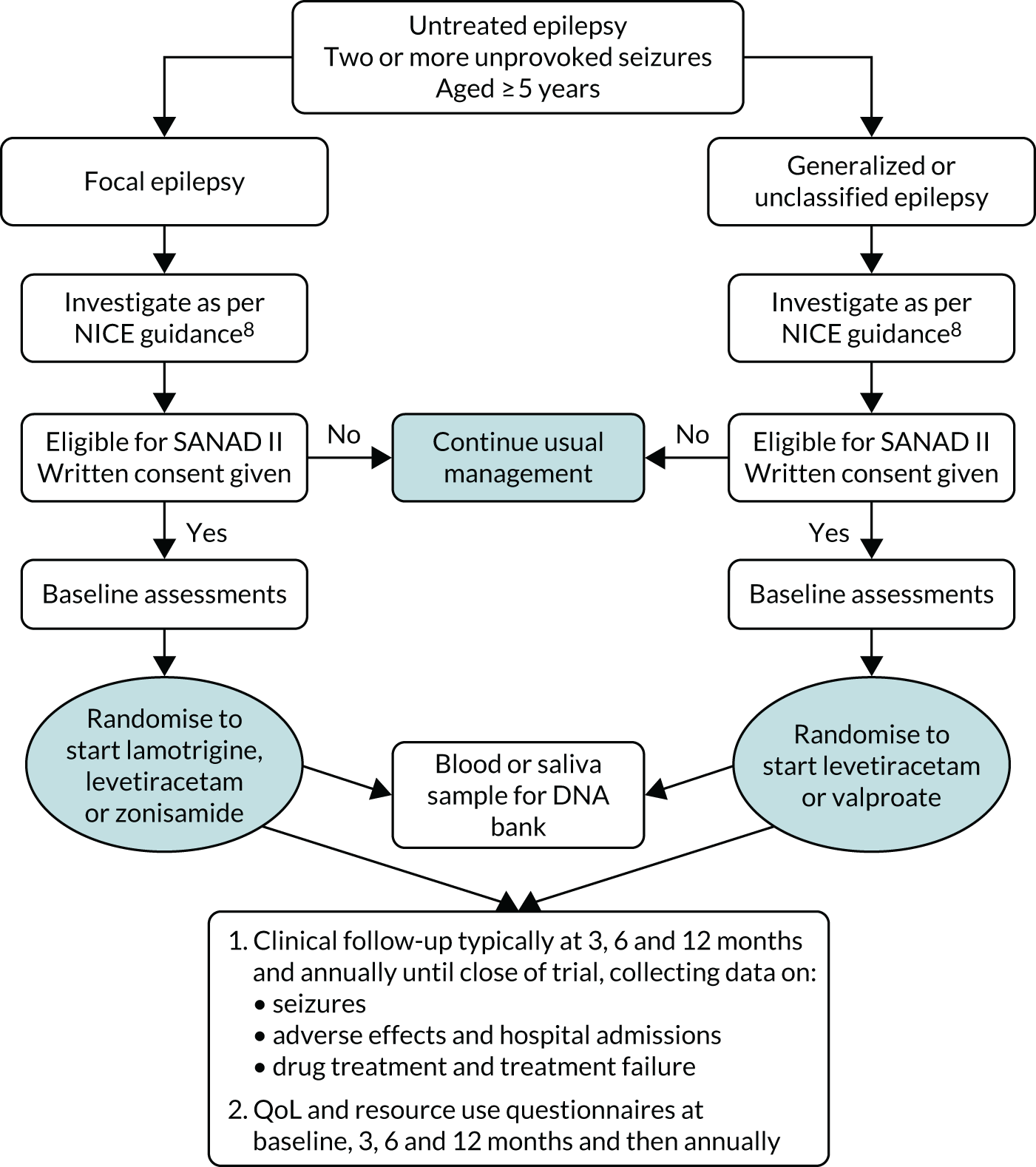
Study sites
Participants were recruited from NHS outpatient epilepsy, general neurology and paediatric (epilepsy and general) clinics in the UK. The study was co-ordinated through the UK Epilepsy Research Network, the Medicines for Children Research Network, the Wales Epilepsy Research Network and the Comprehensive Clinical Research Network. To be eligible to participate in the study, staff at the sites had to be experienced in treating epilepsy.
Participants
We aimed to recruit 1510 patients (990 with focal onset seizures and 520 with generalised onset seizures or difficult to classify seizures) with the following characteristics.
Inclusion criteria
-
Aged ≥ 5 years.
-
Previously experienced two or more spontaneous seizures that required anti-seizure medication.
-
Untreated and not previously treated with anti-seizure medication, except as emergency treatment, in the past 2 weeks.
-
Anti-seizure medication monotherapy considered the most appropriate option.
-
Willing to provide consent (patient’s parent/legal representative willing to give consent where the patient is aged < 16 years or is lacking capacity to consent).
Exclusion criteria
-
Provoked seizures only (e.g. alcohol or drug induced).
-
Acute symptomatic seizures only (e.g. within 1 month of acute brain haemorrhage, brain injury or stroke).
-
Currently treated with anti-seizure medication.
-
Progressive neurological disease (e.g. known brain tumour).
Recruitment procedure
Patients aged ≥ 5 years who had had two or more spontaneous seizures that required anti-seizure medication and had not previously been treated with anti-seizure medication were screened at the study centre sites to identify participants potentially eligible for the study. Potentially eligible patients (i.e. those meeting the eligibility criteria listed), or their parent/legally acceptable representative, where appropriate, were invited to participate in the study and were provided with a patient information sheet and consent form. The patient (or their parent/legally acceptable representative) was allowed sufficient time to discuss the trial and to decide whether or not to consent to trial entry.
Informed consent
Informed, written consent to enter the SANAD II study was obtained at the baseline visit. The original copy of the signed and dated consent form was filed in the participant’s notes. One copy of the signed consent form was given to the patient (or their parent or legal representative in the case of minors and adults with incapacity) for their records, one copy was retained in the investigator site file, and a final copy was sent to the co-ordinating centre.
If capable, and under appropriate circumstances, minors were approached to provide assent by a member of the research team with experience working with minors. The absence of assent did not exclude the patient, provided that consent had been obtained from the parent/legal representative.
For adults lacking capacity, trial participation was discussed with a personal (or professional) legal representative by a suitably experienced member of the research team. For England, Wales and Northern Ireland, a personal legal representative is someone suitable by virtue of their relationship with the adult and who is available and willing to be the personal legal representative. For Scottish sites, a personal legal representative is a welfare guardian, welfare attorney or nearest relative. They were provided with written information and asked to sign the patient representative consent form.
Informed consent for deoxyribonucleic acid collection
Deoxyribonucleic acid (DNA) collection was included as an additional option in the main SANAD II informed consent form, and the same process for obtaining informed consent was followed to obtain consent. Refusal for DNA collection did not preclude participation in the main SANAD II trial. Analysis of DNA will be funded by future applications.
Randomisation, concealment and blinding
Once eligibility criteria had been confirmed and informed consent and assent, when appropriate, had been obtained, the recruiting clinician selected the appropriate trial based on the patient’s epilepsy classification (focal vs. generalised or unclassified). Patients with focal epilepsy were then randomised in a 1 : 1 : 1 ratio to lamotrigine, levetiracetam or zonisamide; patients with generalised and unclassifiable epilepsy were randomised in a 1 : 1 ratio to levetiracetam or valproate.
Randomisation was performed using a secure (24-hour) web-based randomisation program that was controlled centrally by the Liverpool Clinical Trials Centre (LCTC). A personal login (username and password), provided by the LCTC, was required to access the randomisation system.
Patients were allocated a unique study number (randomisation number) and treatment allocation, displayed to the authorised randomiser on a secure web page, and an automated e-mail confirmation was sent to the authorised randomiser, principal investigator and the trial co-ordinator.
Randomisation used a minimisation program with a built-in random element utilising factors for centre, sex (male or female) and number of previous seizures (two, three to five, or six or more). The factors used for minimisation were not made known to the recruiting sites to avoid any risk of them predicting allocation. The recruiting clinicians were required to initiate trial treatment within 7 days of randomisation.
The SANAD II trial was unblinded, trial treatments were prescribed as per routine NHS practice and dispensed by hospital and community pharmacies, and clinicians prescribed the formulation they considered most appropriate.
Treatment group allocation
The aim of treatment was to control seizures with the minimum effective dose of drug. The trial protocol30 provided guidance on initial drug titration and maintenance doses based on the routine practice at the time that the trial was initiated, although clinicians were able to tailor this as they considered appropriate.
Focal epilepsy
For participants aged ≥ 12 years, the initial advised maintenance doses were 50 mg of lamotrigine in the morning and 100 mg in evening, 500 mg of levetiracetam twice per day or 100 mg of zonisamide twice per day. For children aged 5–12 years, the initial daily maintenance doses advised were 1.5 mg/kg lamotrigine twice per day, 40 mg/kg levetiracetam twice per day in two divided doses or 2.5 mg/kg zonisamide twice per day. The subsequent dose and treatment changes at follow-up visits were made in accordance with routine clinical practice, depending on the treatment response and adverse effects.
Generalised or unclassified epilepsy
For participants aged ≥ 12 years, the initial advised maintenance doses were 500 mg twice per day for both levetiracetam or valproate. For children aged 5–12 years, the initial daily maintenance doses advised were 25 mg/kg valproate or 40 mg/kg levetiracetam. The subsequent dose and treatment changes at follow-up visits were made in accordance with routine clinical practice, depending on the treatment response and adverse effects.
The decision to change or discontinue the allocated trial treatment was at the discretion of the treating physician and patient. Treatment could be changed or discontinued at any point during the trial period for reasons such as inadequate seizure control, unacceptable adverse events (AEs), or any change in the participant’s condition that the physician believed warranted a change in medication. Any changes in medication were documented on the appropriate follow-up case report form (CRF), along with the justification for those changes, and patients were encouraged to continue to attend follow-up visits for the remainder of the study. At the end of the trial, participation patients were to continue their treatment as per local policy.
Data collection and management
The majority of clinical data were collected using paper CRFs that were completed by personnel (usually the research nurse) during clinic visits. The paper CRFs were photocopied for local records and originals were returned to the co-ordinating centre for data entry onto a MACRO 4 database (Macro 4 Ltd, Crawley, UK). Where patients defaulted from clinic follow-up, additional information was sought from general practitioners (GPs). Patients were asked to record data on seizures in patient seizure diaries, which were used as an aide-memoire at clinic follow-up visits.
Quality-of-life and utility assessments
Patients were asked to complete questionnaires so that QoL and resource use data could be collected. Questionnaires were either issued during clinic visits or posted to patients for completion at home, with the postal service used to return completed questionnaires. The LCTC contacted non-responders by telephone, typically 3 weeks following the issue of questionnaires.
For adults, QoL outcomes were assessed using subscales of the Quality of Life in Newly Diagnosed Epilepsy Instrument (NEWQOL) battery and the Impact of Epilepsy Scale. 31 For children and adolescents aged < 16 years, QoL assessment involved both patient- and parent-based measures: children aged 8–15 years completed a generic health status measure validated for use in epilepsy [the KINDL (generic quality-of-life instrument for children)],32 as well as the ‘epilepsy impact’ and ‘attitude to epilepsy’ subscales of the QOLIE-AD (set of subscales for evaluation of health-related quality-of-life in adolescents with epilepsy). 33 Parents of all children completed proxy QoL questionnaires.
Utility scores were elicited directly from trial participants (or indirectly via parents/guardians). Adult and adolescent participants were asked to complete the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire and visual analogue scale (VAS). The EQ-5D-3L has been used previously in children, but it has not been formally validated,34 and EQ-5D-3L weights are validated for adults aged ≥ 18 years. The currently recommended approach of using parental proxy reports of QoL for this age group was used. 35 The EQ-5D-3L-Y EuroQol-5 Dimensions, three-level version (youth version) (EQ-5D-3L-Y) was also administered to children aged 8–15 years. All trial participants were also asked to complete an epilepsy-specific utility measure based on the NEWQOL-6D questionnaire. 36 QoL questionnaires were completed at baseline and annually thereafter. Adults and parents also completed a subset of QoL measures at 3 and 6 months.
Data on direct costs of health-care resources used by trial participants were collected via a modified version of the Client Service Receipt Inventory (CSRI)37 that was included in the QoL questionnaires, access to Hospital Episode Statistics (HES) data, and recording of adverse reactions (ARs) requiring hospitalisation in follow-up CRFs. Unit costs were taken from the NHS Reference Costs 2017/1838 database and other appropriate sources. 39,40
Genetic substudy
DNA was to be collected from every patient randomised in the SANAD II trial, subject to appropriate consent.
Samples, preferably in the form of whole venous blood, were collected at baseline (or at a subsequent follow-up visit, as convenient), shipped to the Department of Molecular and Clinical Pharmacology at the University of Liverpool and DNA was extracted and stored in a state-of-the-art DNA archive. Saliva samples were collected from patients who were unable to provide a blood sample. This DNA, along with the DNA stored as part of the SANAD I trial, forms a unique cohort from whom we have collected DNA linked to prospective follow-up from diagnosis that will contribute to future studies of the genetic contributions to epilepsy and treatment response.
Baseline assessment
Following consent from the patient (or parent/legal representative), the delegated member of the research team completed the baseline CRF to collect data, including seizure history, history of neurological insult or febrile seizures, family history of epilepsy, and the results of electroencephalography (EEG) or imaging [computerised tomography (CT) or magnetic resonance imaging (MRI)]. If further investigations (EEG or imaging) were requested at this visit, data on the results were collected when available, but randomisation was not delayed. If a DNA sample was provided, then the DNA sample CRF was completed. Once all eligibility criteria had been assessed, full eligibility was confirmed by a doctor who had been authorised to do so on the site delegation log; a record of this confirmation was made in the patient’s medical notes. Following the eligibility confirmation, the patient was then randomised.
Follow-up
The expected duration of follow-up for each participant was between 2 and 6.5 years, with visits planned as per routine practice: typically at 3, 6, and 12 months and annually thereafter. Patients could be seen at other times as clinically indicated. All patients were to be followed up even if allocated treatment had been withdrawn. We aimed to complete recruitment over a 4.5-year period, but a 12-month extension was required to meet the sample size target for the focal epilepsy trial, after which the trial cohort was followed up for a further 2 years, allowing a minimum follow-up of 2 years and maximum of 7.5 years for patients in the focal epilepsy trial.
Outcome measures
Primary outcome
The primary outcome was time to 12-month remission from seizures, calculated as days from randomisation to the first date at which a period of 12 months had elapsed without any seizures. For patients who did not experience a 12-month remission from seizures, observations were censored at the last follow-up visit.
Secondary outcomes
Time to 24-month remission
The time to 24-month remission from seizures was calculated as days from randomisation to the first date at which a period of 24 months had elapsed without any seizures. For patients who did not experience a 24-month remission from seizures, observations were censored at the last follow-up visit.
Time to first seizure
The time to first seizure was calculated as the number of days from randomisation to the first date at which a seizure (of any type) occurred. For patients who did not experience a seizure after randomisation, observations were censored at the last follow-up visit.
Treatment failure
Treatment failure is defined as withdrawal from randomised drug, or addition of a new anti-seizure medicine, where the reason is an unacceptable adverse reaction (UAR) or inadequate seizure control (ISC). Treatment failures, UARs and ISC are defined in Table 1, and treatment failure was measured using three outcomes:
-
Time to treatment failure overall was defined as the number of days from randomisation to a decision to withdraw the randomised drug or add a new anti-seizure medication because of ISC or a UAR. For patients who did not experience a failure due to either ISC or a UAR after randomisation, observations were censored at the last follow-up visit, or the date of treatment withdrawal, when applicable.
-
Time to treatment failure because of ISC was defined as the number of days from randomisation to a decision to withdraw the randomised drug or add a new anti-seizure medication because of ISC. For patients who did not experience a failure because of ISC after randomisation, observations were censored at the last follow-up visit, or the date of treatment withdrawal, where applicable.
-
Time to treatment failure because of UARs was defined as the number of days from randomisation to a decision to withdraw the randomised drug or add a new anti-seizure medication because of a UAR. For patients who did not experience a failure because of a UAR after randomisation, observations were censored at the last follow-up visit, or the date of treatment withdrawal, when applicable.
| Reason for withdrawal from randomised drug/addition of a new anti-seizure medication | Categorised as event or censored in ‘time to treatment failure’ | ISC/UAR |
|---|---|---|
| Inadequate seizure control | Event | ISC |
| UAR | Event | UAR |
| Remission of epilepsy categorised by clinician (regardless of length in remission) | Censored | – |
| Remission of epilepsy categorised by patient (> 12 months’ remission from seizures) | Censored | – |
| Remission of epilepsy categorised by patienta (< 12 months’ remission from seizures) | Event | UAR |
| Diagnosis no longer epilepsy | Censored | – |
| Study withdrawal – consent withdrawnb | Censored | – |
| Death (unrelated to epilepsy/anti-seizure medication)c | Censored | – |
| Death (related to epilepsy/anti-seizure medication)c | Event | Could be ISC, UAR or neither |
| Moved from area | Censored | – |
| Patient non-compliant/did not wish to continued | Event | Could be ISC, UAR or neither |
| Perceived adverse effect (e.g. pregnant or planning pregnancy) | Event | UAR |
Adverse reactions
All ARs for which the causal relationship to the trial antiepileptic treatments was assessed and judged by the investigator to be possibly, probably or almost certainly related the antiepileptic treatment were recorded at each follow-up visit. These ARs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) (www.meddra.org/) dictionary to the most appropriate lower-level term, preferred term and the higher-level System Organ Classification by the trial staff at LCTC, with clinical oversight by the chief investigator.
Sample size
The SANAD II trial was powered to detect non-inferiority of the new anti-seizure medications (levetiracetam and zonisamide) compared with standard treatments [lamotrigine (for focal epilepsy) or valproate (for generalised or unclassified epilepsy)] for the primary outcome time to 12-month remission. A new drug might become a standard first-line treatment if it is proven to be non-inferior for efficacy but superior for tolerability when compared with a standard treatment; tolerability is examined in secondary outcomes, including time to treatment failure for adverse effects. Powering the study for non-inferiority would also provide sufficient power to detect important differences between treatment policies.
The International League Against Epilepsy (ILAE) Commission on Antiepileptic Drugs defined limits of equivalence of ± 10% for the primary outcome in anti-seizure medication monotherapy studies. 41 However, the Commission was not explicit as to whether this should be on the hazard ratio (HR) or absolute scale. No empirical work had been undertaken to underpin the choice of equivalence or non-inferiority margins in epilepsy trials. The chief investigator had given numerous seminars and lectures in the UK and elsewhere about epilepsy trial methodology, and the audience had typically voted for a margin of 10% around absolute differences between anti-seizure medications for monotherapy studies when given examples of margins ranging from 20% to 5%. Communicating treatment differences to patients on a HR scale is also difficult compared with a discussion of absolute differences at specific time points. Given that the ultimate purpose of the SANAD II trial is to provide information that patients and clinicians can use to help them to make treatment decisions, the non-inferiority margin for the SANAD II trial was been chosen according to absolute differences.
Calculations were informed by the SANAD I study, which estimated the 12-month remission-free probability (at 24 months) as 0.43 (exponential hazard rate of 0.0352) for lamotrigine (focal standard), and 0.31 (exponential hazard rate of 0.0488) for valproate (generalised and unclassified epilepsy standard). The calculations assumed a HR of 1.0, 80% power, and allowance for approximately 5% losses to follow-up throughout, as observed in the SANAD I trial. For the focal epilepsy trial, two primary comparisons were of interest (i.e. levetiracetam vs. lamotrigine, and zonisamide vs. lamotrigine); therefore, the one-sided significance level was divided by 2 (one-sided alpha 0.0125). Assuming a 10% absolute difference in survival probability, the non-inferiority margin on the HR scale was:
After adjusting for 5% losses to follow-up, 330 patients were required in each of the three treatment groups (i.e. a total of 990 patients). For the generalised or unclassified epilepsy trial, there was only one comparison of interest (levetiracetam vs. valproate). Assuming a 10% absolute difference in survival probability, the non-inferiority margin on the HR scale was as follows for the trial in generalised or unclassified epilepsy:
Therefore, with a one-sided alpha of 0.025, 260 patients were required in each of the two treatment groups, allowing for 5% losses to follow-up (i.e. a total of 520 patients). The total number of patients required for both trials was 1510. The sample size was calculated using nQuery software (Statistical Solutions Ltd, Cork, Ireland).
Statistical analysis
The statistical analysis and reporting of the SANAD II trial were undertaken in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines42 and the International Conference on Harmonisation E9 guidelines. 43 Primary analyses were undertaken on an intention-to-treat (ITT) basis, including all randomised patients retained in their randomised treatment groups. The statistical and health economic analysis plans were developed before conducting final analyses. Analyses were conducted using SAS® software (version 9.4; SAS Institute, Cary, NC, USA).
All analyses were conducted separately for the trial in focal epilepsy and the trial in generalised and unclassified epilepsy. A 97.5% two-sided confidence interval (CI) was used for the primary outcome analysis for the focal epilepsy trial (see Sample size for justification). All other CIs (focal epilepsy trial and generalised or unclassified epilepsy trial) were calculated at the 95% level (two-sided), with a two-sided p-value of ≤ 0.05 used to declare statistical significance for all analyses. No formal adjustment was made for multiple testing of secondary outcomes, but conclusions drawn from the analysis of all secondary outcomes would be cautionary unless the p-value was < 0.001.
The time-to-event outcomes were summarised using Kaplan–Meier curves for each treatment group and explored using two different Cox proportional hazards regression models: (1) including the treatment effect only using an indicator variable and (2) including the treatment effect together with minimisation factors included as indicator variables for gender (male or female), number of seizures prior to randomisation (two, three to five, or six or more) and random effects for centre. The assumption of proportional hazards was investigated by examining Schoenfeld residual plots, and incorporating time-dependent covariates in all models. If the residuals were not time dependent and the parameter estimate for the time-dependent covariate was not significant at the 5% level, then the assumption of proportional hazards was assumed to hold; otherwise, an additional extended Cox model with the addition of time-dependent covariates was used. The HR and relevant CI (95% CI unless indicated otherwise) are presented for the comparison of lamotrigine with levetiracetam (focal epilepsy trial), lamotrigine with zonisamide (focal epilepsy trial) and valproate with levetiracetam (generalised or unclassified epilepsy trial). For the primary outcome (12-month remission) non-inferiority hypothesis, the upper limit of the 97.5% CI should be < 1.329 to conclude non-inferiority for the focal epilepsy trial, whereas the upper limit of the 95% CI should be < 1.314 to conclude non-inferiority for the generalised and unclassified epilepsy trial.
A per-protocol (PP) analysis of the primary outcome of time to 12-month remission was also undertaken using a Fine and Gray model,44 with treatment failure included as a competing risk, and censoring participants with drug failure (withdrawn from study or drug or other anti-seizure medication added) before achieving a period of remission. This analysis excluded participants with major protocol deviations, those subsequently given an alternative diagnosis to epilepsy and those who did not receive the drug at all.
For time to treatment failure, a competing risks analysis, using the Fine and Gray model,44 was undertaken to assess the two main reasons for treatment failure (i.e. ISC and UAR). 45 Cumulative incidence curves are presented for each treatment group.
The difference in QoL measures between treatment groups was estimated for each population (child/adult/parent–carer), and for each outcome applicable within that population. This was carried out by fitting a repeated measures random-effects model, with baseline QoL variable as a covariate, along with treatment group and time in days, using spatial power covariance structure for repeated measures (appropriate for repeated measures that can be unevenly spaced), and unstructured covariance for the random effect. 46
Analysis sets for the summary of ARs include all patients who received any dose of a study drug. All ARs and serious adverse reactions (SARs) were coded using the MedDRA dictionary to the most appropriate lower-level term, preferred term and higher-level System Organ Classification. The number (and percentage) of patients experiencing each reaction, and the number (and percentage) of occurrences of each reaction are presented with no formal statistical testing undertaken.
Interim monitoring was carried out by an Independent Data and Safety Monitoring Committee (IDSMC), meeting approximately annually. This included analyses of the primary outcome and five of the secondary outcomes (all using the Haybittle–Peto approach). 47
Health economics
The economic analysis was conducted from the perspective of the NHS and Personal Social Services in the UK. The primary economic analysis compared the costs and consequences of each anti-seizure medication over the first 24 months post randomisation. An analysis at an extended 48-month time horizon was planned for those participants followed up for ≥ 4 years.
The within-trial economic analysis was performed using individual, patient-level data from the SANAD II trial. Cost-utility analyses were conducted to estimate incremental cost-effectiveness ratios, expressed as costs per quality-adjusted life-years (QALYs) gained.
The health economic analysis was carried out in Stata® IC version 13 (StataCorp LP, College Station, TX, USA), and reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 48
Data sources
Resource use
Participants’ use of resources was considered in four broad categories: (1) resource use associated with secondary care [inpatient, outpatient, accident and emergency (A&E)], (2) other health-care and social services resource use (primary care, community services), (3) consumption of anti-seizure medication and (4) use of other medications.
The measurement of resource use was based on complementary approaches, using data collected as part of the trial and as part of routine care. Resource use postal questionnaires, completed by the parent or carer for participants aged < 16 years, included a modified CSRI based on that from the SANAD I trial. 37,49,50 This CSRI was used to collect information on participants’ use of health service resources, personal social services and medicines. The questions pertained to contacts with health professionals at the GP surgery, in the hospital and in the community; the use of emergency services; and any tests or investigations that the patients may have had. The questionnaires were initially administered at 3, 6 and 12 months and annually thereafter (up to 60 months); however, from version 7 of the protocol onwards, this questionnaire was also provided during outpatient visits to aid completeness. The questionnaires completed following visits were matched to respective time points for analysis.
In all cases, participants were asked to report their primary and secondary care and social services resource use for the 3-month period prior to completing the questionnaire, and to report their medicines use over a 4-week period prior to completing the questionnaire because of the additional complexity in the recall. The self-report questionnaires contained free-text sections that allowed participants to record any resource use that would not otherwise be captured by the questionnaire. During analysis, these records of resource use were assessed for duplication against the resources captured by the questionnaire, and any relevant, non-duplicated resources were extracted.
Self-report data were therefore available for months 0–3, 3–6, 9–12 and 21–24. Self-reported resource use for year 1 was estimated by multiplying the resource use from months 9–12 by two, and adding the resource use reported for months 0–3 and 3–6. Self-reported resource use for year 2 was estimated by multiplying resource use for months 21–24 by four, and similarly for years 3, 4 and 5. Participants’ use of concomitant medicines was multiplied by three (owing to the shorter, 4-week recall period), before estimation following the same method.
With respect to the consumption of anti-seizure medications, the type of drug and the doses taken were recorded directly within CRFs.
Routine HES data were the primary source of participants’ use of secondary care resources over the trial period. HES data were requested from NHS Digital (for patients in England)51 and from the Secure Anonymised Information Linkage databank (for patients in Wales),52 but were not obtained for patients in Scotland or Northern Ireland. HES provided Health Resource Group (HRG) data on the type of care that patients receive at a ward level, outpatient visits and A&E admissions. HES data were used as the source for baseline resource use and costs, based on the 6 months prior to randomisation. Adjustments were made when hospital episodes overlapped with randomisation dates to apportion the resource use to the periods prior, and subsequent to randomisation.
All resource use was measured, irrespective of whether or not it was epilepsy related. 53
Unit costs
Resource use was valued in monetary terms (Great British pounds) using sources of national unit costs. 24,54–56
Health Resource Groups were used as the main currency for inpatient stays, outpatient visits and A&E attendance. For data pertaining to participants from Wales, an initial mapping step was performed using the Welsh NHS Data dictionary. 57 Subsequently, HRG codes were obtained from the HES data using the NHS Digital costing grouper. 58 Unit costs were allocated based on the latest available national schedule. 54
Unit costs for primary care and community care were taken from the compendium of Unit Costs of Health and Social Care 2019. 55 Unit costs and their sources relating to items within the self-report questionnaire are presented in Appendix 4, Table 45. Unit costs relating to the most commonly reported HRGs are presented in Appendix 4, Table 46.
Total costs for resource use were calculated by multiplying the unit cost per item by the recorded number of times that each resource was used.
Medication costs were taken from the British National Formulary using drug tariff prices, when available,24 or the NHS indicative price, and the Prescription Costs Analysis for England. 56 Unit costs for trial anti-seizure medications are presented in Appendix 4, Table 47. Unless otherwise specified in the data, children aged ≥ 9 years were assumed to be prescribed tablets or capsules, whereas children aged ≤ 8 years were assumed to be prescribed an alternative form (e.g. solution, dispersible), when available.
The cost of each medicine was calculated by assessing the price per dose and multiplying this by the quantity prescribed (e.g. number of tablets, capsules, inhalers or prefilled syringes) and the number of days of treatment.
All costs are at 2019/20 prices and were discounted in the base-case analysis at the NICE-recommended rate of 3.5% per annum. 59
Health utilities
The primary health outcome measure for the economic analysis was the QALY, generated from utility data measured using the EQ-5D-3L questionnaire. 60 The secondary economic outcome measures were the EQ-VAS and an epilepsy-specific utility measure: the NEWQOL-6D. 61
The EuroQol-5 Dimensions (EQ-5D) descriptive system includes five dimensions, relating to mobility, self-care, usual activities, pain and discomfort, and anxiety. For the EQ-5D-3L and EQ-5D-3L-Y, each dimension is measured against three statements (i.e. no problems, some problems or extreme problems), scored 1, 2 and 3, respectively. The NEWQOL-6D is an epilepsy-specific measure that includes domains of worry, depression, memory, concentration, control and stigma. Responses are measured according to four categories. Utility scores are obtained from the EQ-5D-3L-Y, EQ-5D-3L, EQ-5D-3L proxy and NEWQOL-6D using UK tariff values. 61,62
For participants aged 8–15 years, self-reported responses to the EQ-5D-3L-Y or, if not available, proxy questionnaire responses (EQ-5D-3L and NEWQOL-6D) completed by a parent or carer were used. For participants aged 5–7 years, only proxy questionnaires were administered. All participants aged ≥ 8 years were administered the EQ-VAS.
All economic outcome measures were completed during the baseline visit and annually thereafter (up to 60 months), and, from version 7 of the protocol onwards, were also provided during outpatient visits to aid completeness. Utility scores at 365 days (12 months) and at 730 days (24 months) were interpolated, based on recorded utility scores and actual dates of questionnaire completion. QALY profiles were derived from these utilities, estimated based on the area under the curve (AUC), assuming the trapezoidal rule, using all available data. The QALYs derived from the secondary health economic outcomes (EQ-VAS and NEWQOL-6D) were estimated in the same way, based on AUC.
All QALYs were discounted at the NICE-recommended rate of 3.5% per annum. 59
Data analysis
Analysis consisted of all randomised participants, which is consistent with the ITT approach. All statistical tests were two-sided, with CIs and central ranges (CRs) reported at 97.5% for the trial in focal epilepsy and 95% for the trial in generalised or unclassified epilepsy.
The costs relating to secondary care were primarily sourced from HES data, but where these data were not available costs were supplemented with resource use recorded in the self-report questionnaires. Primary and community care costs and concomitant medication costs were also taken from the resource use questionnaires. If resource use questionnaires were returned but no response was provided for a given resource, then use of that resource was assumed to be zero. If participants indicated that they had used a resource but had not given a number for how many times the resource was used, then the number was assumed to be 1. The data relating to anti-seizure medications were taken from the baseline and follow-up CRFs. Missing dose data were assigned according to previous or subsequent prescriptions, based on questions relating to dose changes, or, if these were unavailable, from the British National Formulary recommended doses.
Data were examined for missingness and appropriate methods were applied depending on the level of missingness and likely mechanism of missingness. 63 Missing cost and QALY data were imputed using multiple imputation with chained equations. 63 To maximise data use, data were imputed at the level of utility scores (EQ-5D, EQ-VAS) at baseline and at 12 and 24 months; primary care, community care and concomitant medications costs at 3, 6, 12 and 24 months; and admitted patient care, outpatients, A&E and anti-seizure medication costs at 12 and 24 months. Owing to the return dates of questionnaires not coinciding exactly with 365 and 730 days, utility values for 365 and 730 days were interpolated (using linear interpolation). Baseline costs (relating to admitted patient care, outpatients, accident and emergency) were also imputed for those participants for whom HES data were not available. Imputation models were generated using predictive mean matching, and data were imputed by randomised treatment group. Variables pertaining to epilepsy classification, seizure type, age, gender, primary outcome and treatment failure were included within the imputation models. Imputation models for baseline measures omitted post-baseline outcomes to preserve randomisation. The number of imputations required was based on the level of missingness, according to the fraction of missing information. 64
Based on the imputed data, total costs and QALYs during the course of the trial were calculated, with summary statistics generated by randomised treatment group. The differences between treatment groups were compared with reference to bootstrapped CRs, based on 10,000 replications.
Total costs and QALYs (at 24 months) were adjusted for any imbalances in baseline costs and utilities respectively, and clinical or demographic variables (age, sex, epilepsy classification, with centre as random effects), using ordinary least squares regressions. 64,65 Ordinary least squares was considered to be appropriate given the large sample size. 66
Incremental analysis
Differences in estimated mean QALYs and costs by treatment group were combined to calculate incremental cost-effectiveness ratios (ICERs). Interventions were ranked according to their effectiveness (from highest to lowest QALYs), and dominance and extended dominance were determined. The ICER was calculated for non-dominated interventions as:
Net health benefits (NHB) and incremental net health benefits (INHB) were also calculated at the £20,000 per QALY and £30,000 per QALY thresholds, according to the following formulae:
where λ is the cost-effectiveness threshold. 67
The base-case was defined as being from the perspective of the NHS and Personal Social Services, adopting a 2-year time horizon, and based on the imputed data set of the ITT population, with adjusted costs and QALYs.
The protocol-specified cost-effectiveness analyses, based on the incremental cost per seizure avoided and per 12-month remission, were not conducted because there were insufficient data on likely acceptable thresholds of cost-effectiveness from other economic assessments of anti-seizure medicines.
Sensitivity analysis
Several sensitivity analyses were conducted to assess the robustness of the base-case results to key assumptions. These:
-
used discount rates of 0% and 6% per annum for costs and QALYs
-
were an unadjusted analysis (i.e. based on mean costs and QALYs, with no regression)
-
used results for complete-case cost and QALY data (i.e. those without missing data) to identify the impact of missing data and imputation
-
were based on the population as the PP cohort
-
used QALYs derived from the NEWQOL-6D and EQ-VAS
-
treated blank values in resource use questionnaires as missing, rather than zero.
A bootstrap analysis was conducted to consider the joint uncertainty in incremental costs and QALYs. This was represented as a cost-effectiveness plane and as a cost-effectiveness acceptability curve, illustrating the probability of each treatment being cost-effective for a given cost-effectiveness threshold. 68
Subgroup analysis
Subgroup analyses were conducted to investigate how cost-effectiveness varied by age, according to whether participants were adults (i.e. aged ≥ 16 years) or children (aged < 16 years).
Patient and public involvement
The SANAD II trial was designed in collaboration with Epilepsy Action (Leeds, UK), which consulted its members. A patient and public involvement representative sat on the Trial Steering Committee (TSC) and attended regular meetings during the trial. The trial team will collaborate with Epilepsy Action on dissemination of results to the public.
Protocol amendments
During the course of the SANAD II trial, a number of amendments were made to the trial protocol. These are further detailed in Appendix 2. Each amendment was assessed by the Trial Management Group (TMG), TSC, co-sponsors and funder prior to being submitted for approval. Approval for amendments was sought from the Research Ethics Committee, MHRA (if appropriate) and (post 2015) from the Health Research Authority.
Trial funder
The SANAD II trial was funded by the NIHR Health Technology Assessment programme (09/144/09).
Trial co-sponsors
The SANAD II trial was co-sponsored by the University of Liverpool and the Walton Centre NHS Foundation Trust.
Trial management and quality assurance
The SANAD II trial was managed by the LCTC. A risk assessment was performed by the LCTC in conjunction with co-sponsors and the chief investigator. The risk assessment indicated that the SANAD II trial was low risk. As such, monitoring/quality assurance was carried out centrally. This included confirming informed consent; the MACRO database containing predefined ranges that flagged data queries; and the trial statistician producing 6-monthly reports to look for errors, inconsistencies in data, assess safety and to highlight any protocol deviations.
Trial oversight
The SANAD II trial was overseen by the TMG, TSC and IDSMC.
Ethics considerations, regulatory requirements and research governance framework
The SANAD II trial was conducted in accordance with the European Clinical Trials Directive,69 ICH GCP Guidelines,70 the Declaration of Helsinki,71 UK Policy Framework for Health and Social Care Research,72 and the Medicines for Human Use (clinical trials) regulations (2004). 73 The SANAD II trial was issued a EudraCT number (2012-001884-64) and approved by the MHRA on 22 May 2012 (‘effective date’). We also sought and received approval from the North West – Liverpool East Research Ethics Committee for the SANAD II trial to proceed. This was granted on 7 June 2012.
Research Ethics Committee approval was sought for all amendments made to the protocol. MHRA approval was sought for all amendments that related to the trial investigational medicinal products. The SANAD II trial was brought under the HRA umbrella in 2016.
Chapter 3 Focal epilepsy: clinical results
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Recruitment and baseline characteristics
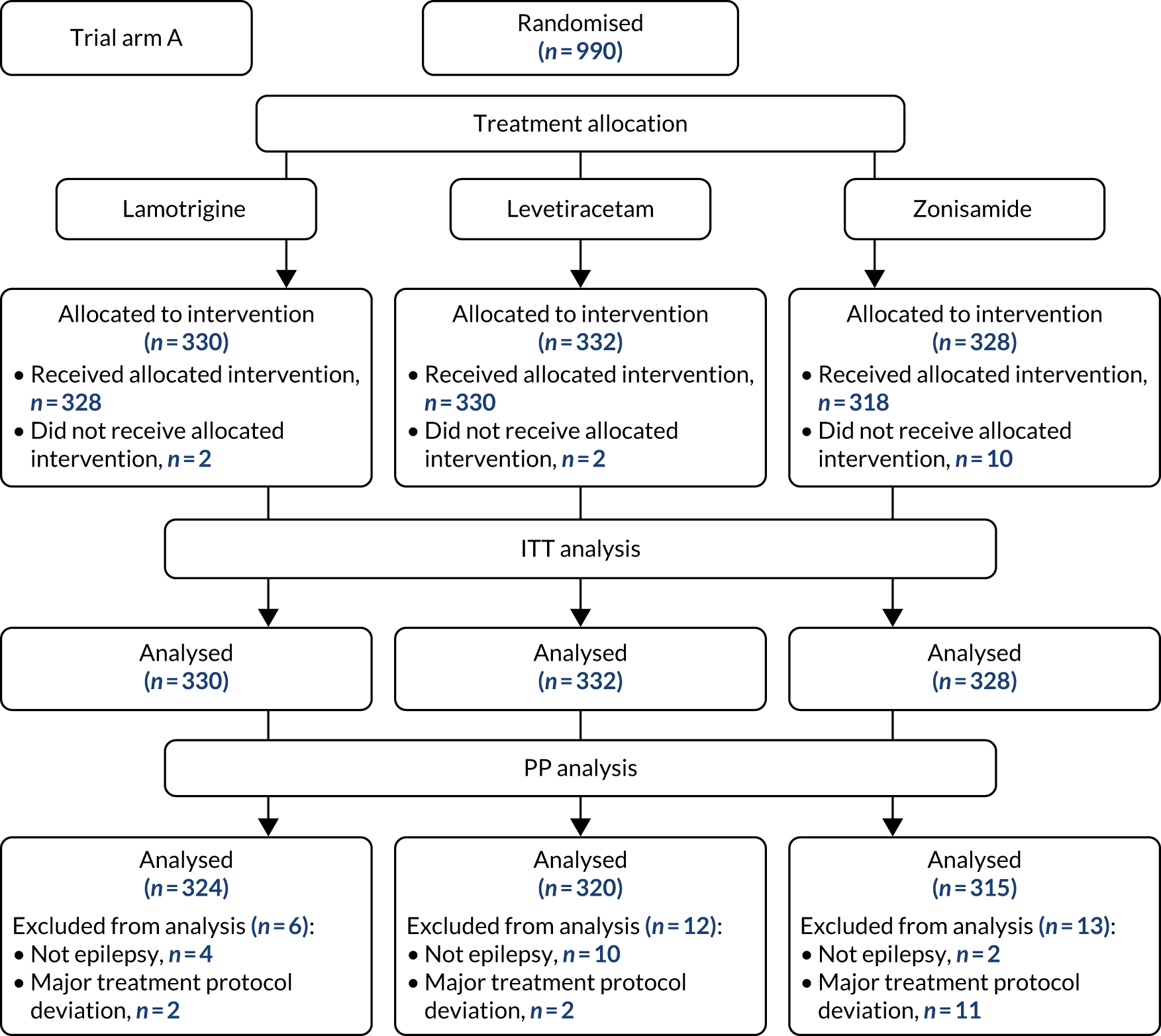
The first participant was randomised on 2 May 2013 and the last participant on 20 June 2017 (see Appendix 3, Figure 23), after which every effort was made to follow the trial cohort for a further 2 years; the last participant follow-up visit was on 17 October 2019. Sixty-five UK centres recruited between 1 and 130 patients each, and randomised a total of 990 participants: 330 to start treatment with lamotrigine, 332 to start treatment with levetiracetam and 328 to start treatment with zonisamide (Figure 2). Baseline characteristics were well balanced across treatment groups (Table 2 and see Appendix 3, Table 31). The mean age of participants was 39.3 years [standard deviation (SD) 21.2 years] and 177 (17.9%) participants were aged < 18 years. There was a predominance of males (56.7%), 4.5% of participants had a learning disability, 16.5% had a previous or current neurological disorder, 10.8% a first-degree relative with epilepsy and 4.4% had a history of febrile convulsions. A total of 35.9% of participants were classified with temporal lobe epilepsy, 6.3% with frontal lobe epilepsy, 2.1% with occipital lobe epilepsy, 2.0% with parietal lobe epilepsy and 50.4% with focal epilepsy where localisation was not specified. The median number of seizures before randomisation was 6 [interquartile range (IQR) 3–24] and participants were randomised a median of 13 days (IQR 3–36 days) after their most recent seizure.
FIGURE 2.
The CONSORT participant flow diagram: focal epilepsy trial.
| Characteristic | Lamotrigine group (N = 330) | Levetiracetam group (N = 332) | Zonisamide group (N = 328) | Total (N = 990) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 40.1 (21.7) | 37.8 (20.1) | 39.9 (21.6) | 39.3 (21.2) |
| Range | 5.1–91.9 | 5.0–87.6 | 5.0–89.1 | 5.0–91.9 |
| Gender, n (%) | ||||
| Male | 186 (56.4) | 190 (57.2) | 185 (56.4) | 561 (56.7) |
| History, n (%) | ||||
| Learning disability | 15 (4.5) | 16 (4.8) | 14 (4.3) | 45 (4.5) |
| Febrile convulsions | 10 (3.0) | 19 (5.7) | 15 (4.6) | 44 (4.4) |
| Acute symptomatic seizures | 6 (1.8) | 9 (2.7) | 4 (1.2) | 19 (1.9) |
| History of epilepsy in primary relatives | 32 (9.7) | 35 (10.5) | 40 (12.2) | 107 (10.8) |
| Neurological deficit | 12 (3.6) | 20 (6.0) | 12 (3.7) | 44 (4.4) |
| Previous or current neurological disorder, n (%) | ||||
| Stroke/cerebrovascular | 17 (5.2) | 16 (4.8) | 14 (4.3) | 47 (4.7) |
| Cerebral haemorrhage | 2 (0.6) | 5 (1.5) | 7 (2.1) | 14 (1.4) |
| Intracranial surgery | 4 (1.2) | 6 (1.8) | 10 (3.0) | 20 (2.0) |
| Head injury | 4 (1.2) | 7 (2.1) | 7 (2.1) | 18 (1.8) |
| Meningitis/encephalitis | 6 (1.8) | 5 (1.5) | 6 (1.8) | 17 (1.7) |
| Cortical dysplasia/developmental anomaly | 1 (0.3) | 3 (0.9) | (0.0) | 4 (0.4) |
| Other | 27 (8.2) | 24 (7.2) | 18 (5.5) | 69 (7.0) |
| Epilepsy syndrome, n (%) | ||||
| Benign childhood epilepsy with centrotemporal spikes | 9 (2.7) | 15 (4.5) | 10 (3.0) | 34 (3.4) |
| Childhood epilepsy with occipital paroxysms | (0.0) | 1 (0.3) | (0.0) | 1 (0.1) |
| Temporal lobe | 134 (40.6) | 110 (33.1) | 111 (33.8) | 355 (35.9) |
| Frontal lobe | 21 (6.4) | 21 (6.3) | 20 (6.1) | 62 (6.3) |
| Parietal lobe | 7 (2.1) | 8 (2.4) | 5 (1.5) | 20 (2.0) |
| Occipital lobe | 7 (2.1) | 12 (3.6) | 2 (0.6) | 21 (2.1) |
| Focal epilepsy localisation not specified | 152 (46.1) | 165 (49.7) | 182 (55.5) | 499 (50.4) |
| Other epilepsy syndrome | 3 (0.9) | 1 (0.3) | 1 (0.3) | 5 (0.5) |
| Seizure history, median (IQR) | ||||
| Total number of seizures reported | 6 (3–29) | 6 (3–22) | 6 (3–23) | 6 (3–24) |
| Days since first seizure | 333 (110–1090) | 318 (119–985) | 328 (120–1097) | 327 (114–1035) |
| Days since most recent seizure | 13 (3–41) | 13 (3–35) | 11 (3–34) | 13 (3–36) |
The median number of days of follow-up was 462.5 (IQR 365–777 days) in the lamotrigine group, 449.5 (IQR 365–824) days in the levetiracetam group and 447 (IQR 365–730) days in the zonisamide group, with completeness of follow-up statistics for the primary outcome of 77.2% in the lamotrigine group, 78.3% in the levetiracetam group and 75.6% in the zonisamide group (see Appendix 3, Table 30 and Figure 24).
Time to 12-month remission
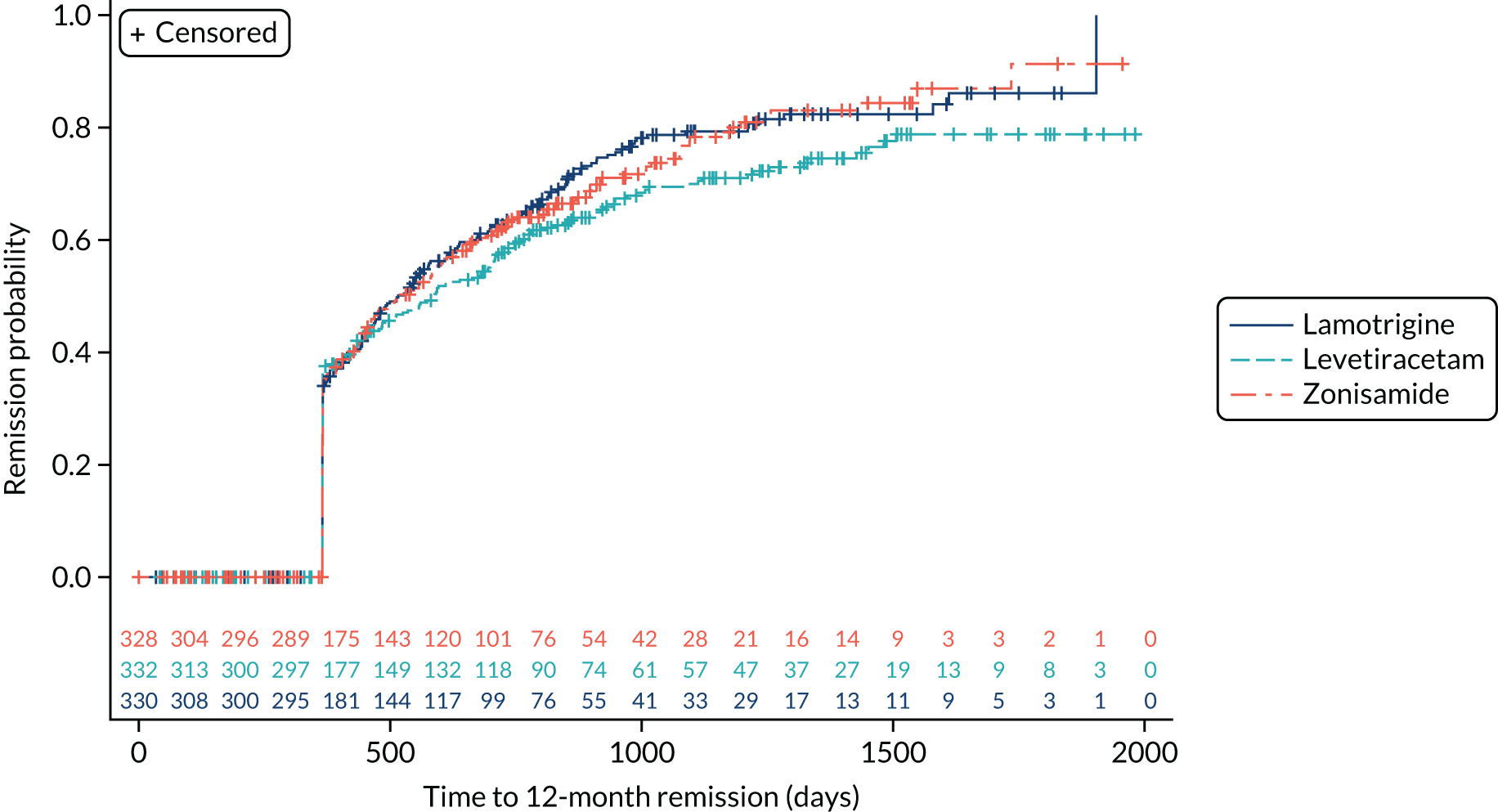
Estimates from the primary and secondary analyses are provided in Table 3. For the ITT analysis of time to 12-month remission, there is insufficient evidence to conclude non-inferiority of levetiracetam compared with lamotrigine, as the 97.5% confidence interval for the HR (1.18, 97.5% CI 0.95 to 1.47, unadjusted; 1.13, 97.5% CI 0.91 to 1.41, adjusted) includes the predefined non-inferiority margin of 1.329, but there was sufficient evidence to conclude non-inferiority of zonisamide compared with lamotrigine (HR 1.03, 97.5% 0.83 to 1.28, unadjusted; 1.01, 97.5% CI 0.81 to 1.25, adjusted). There was no evidence of violation of the assumption of proportional hazards (p = 0.90). We also present the annual 12-month remission probabilities (Table 4); for example, we estimate that, at 2 years’ follow-up, compared with the lamotrigine group, the proportion of participants who had achieved remission was 5% lower (97.5% CI –13% to 3%) in the levetiracetam group and 1% lower (97.5% CI –9% to 7%) in the zonisamide group. The Kaplan–Meier estimates of the median number of days to achieve 12-month remission were 516 (97.5% CI 457 to 577) days in the lamotrigine group, 588 (97.5% CI 472 to 706) days in the levetiracetam group and 530 (97.5% CI 453 to 601) days in the zonisamide group (Figure 3).
| Model and analysis set | Lamotrigine vs. levetiracetam HR (97.5% CI) | Lamotrigine vs. zonisamide HR (97.5% CI) |
|---|---|---|
| Primary analysis: Cox model with treatment (ITT) | 1.189 (0.96 to 1.47) | 1.03 (0.83 to 1.28) |
| Cox model with treatment (ITT), gender, number of seizures and centre as random effects | 1.13 (0.91 to 1.41) | 1.01 (0.81 to 1.25) |
| Fine and Gray model44 with treatment (PP) | 1.32a (1.05 to 1.66) | 1.37a (1.08 to 1.73) |
| Probability estimate | Events/total | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|---|
| Number at risk | ||||||
| Lamotrigine | 222/330 | 291 | 92 | 34 | 12 | 2 |
| Levetiracetam | 204/332 | 293 | 107 | 57 | 22 | 5 |
| Zonisamide | 209/328 | 284 | 92 | 29 | 10 | 2 |
| Percentage of 12-month remission (95% CI) | ||||||
| Lamotrigine | 34 (29 to 39) | 63 (58 to 69) | 79 (74 to 84) | 82 (77 to 88) | 86 (80 to 92) | |
| Levetiracetam | 37 (32 to 43) | 59 (53 to 64) | 70 (64 to 76) | 77 (71 to 82) | 79 (73 to 85) | |
| Zonisamide | 35 (29 to 40) | 63 (57 to 68) | 78 (72 to 84) | 84 (78 to 90) | 91 (83 to 100) | |
| Difference in percentage of 12-month remission compared with lamotrigine (95% CI) | ||||||
| Levetiracetam | 3 (–5 to 11) | –5 (–13 to 3) | –9 (–17 to –2) | –6 (–14 to 2) | –7 (–16 to 1) | |
| Zonisamide | 1 (–7 to 9) | –1 (–9 to 7) | –1 (–9 to 7) | 2 (–6 to 10) | 5 (–5 to 16) | |
FIGURE 3.
Kaplan–Meier plot of time to 12-month remission: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
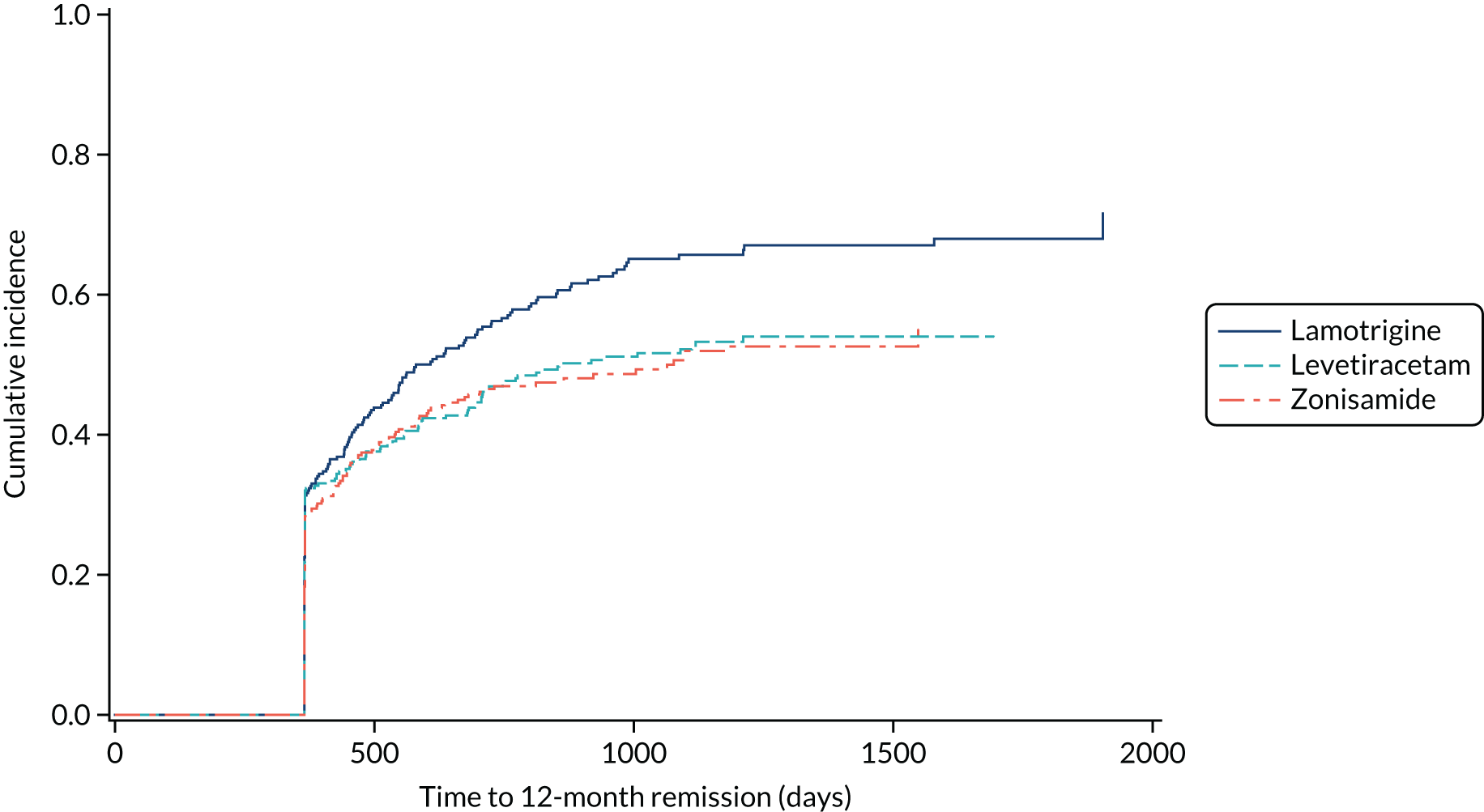
The PP analyses for time to 12-month remission excluded patients with major protocol deviations (1.5%) and patients who were later diagnosed as ‘not epilepsy’ (1.6%) and accounted for treatment failures prior to achieving 12-month remission (lamotrigine group, 24%; levetiracetam group, 35%; zonisamide group, 39%) in a competing risks analysis (Figure 4). The results indicate that lamotrigine is superior to both levetiracetam (HR 1.32, 97.5% CI 1.05 to 1.66) and zonisamide (HR 1.37, 97.5% CI 1.08 to 1.73).
FIGURE 4.
Cumulative incidence of time to 12-month remission, PP competing risks analysis: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
Additional prespecified sensitivity analyses, the results of which are shown in Appendix 3, Table 32, did not change the conclusions of the primary analyses.
Time to 24-month remission
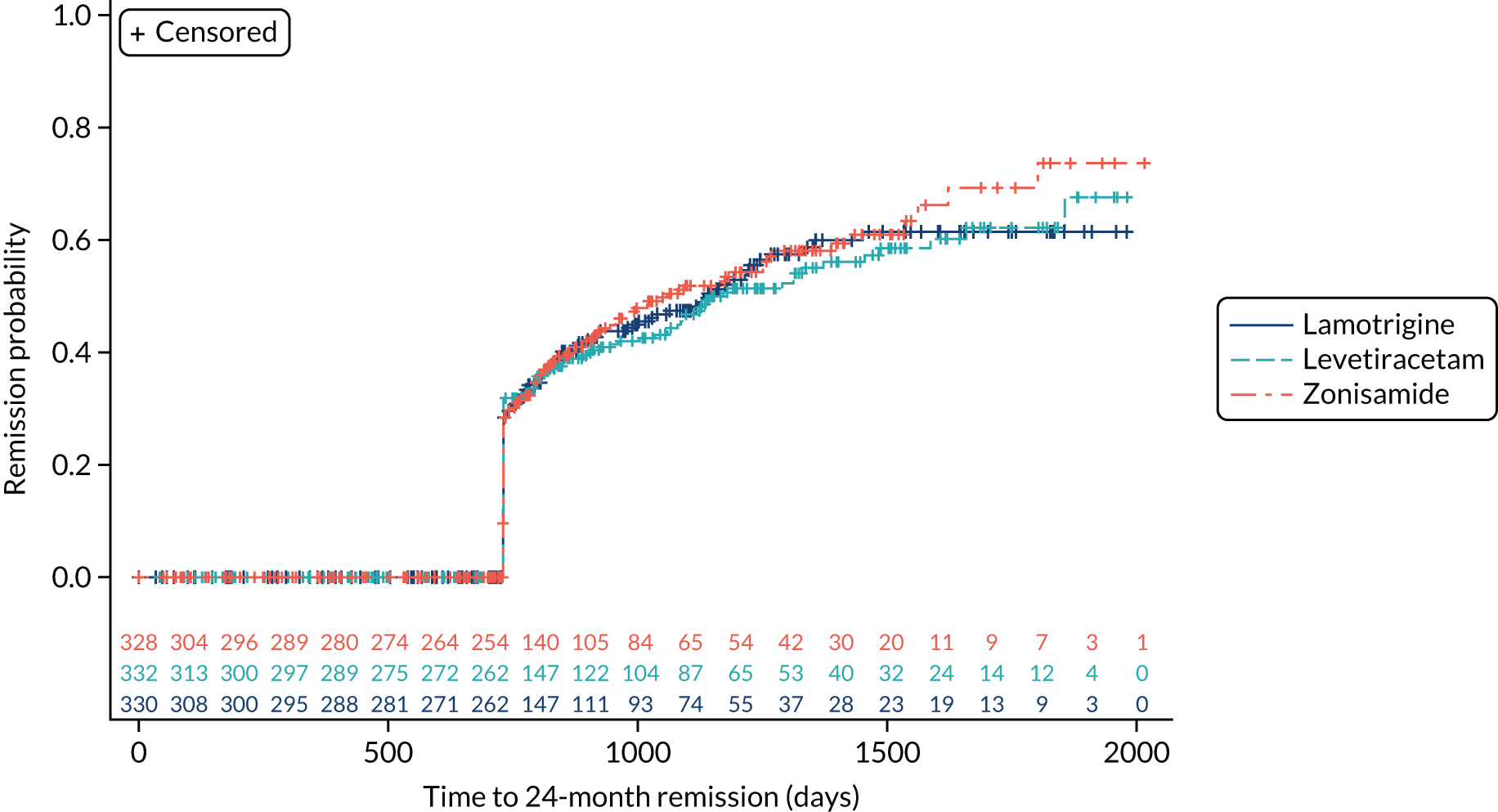
The ITT analysis of time to 24-month remission (Figure 5) indicates no significant difference between initiating treatment with lamotrigine or levetiracetam (HR 1.04, 95% CI 0.81 to 1.33) or between initiating treatment with lamotrigine of zonisamide (HR 0.96, 95% CI 0.75 to 1.23).
FIGURE 5.
Kaplan–Meier plot of time to 24 month remission: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
Time to first seizure
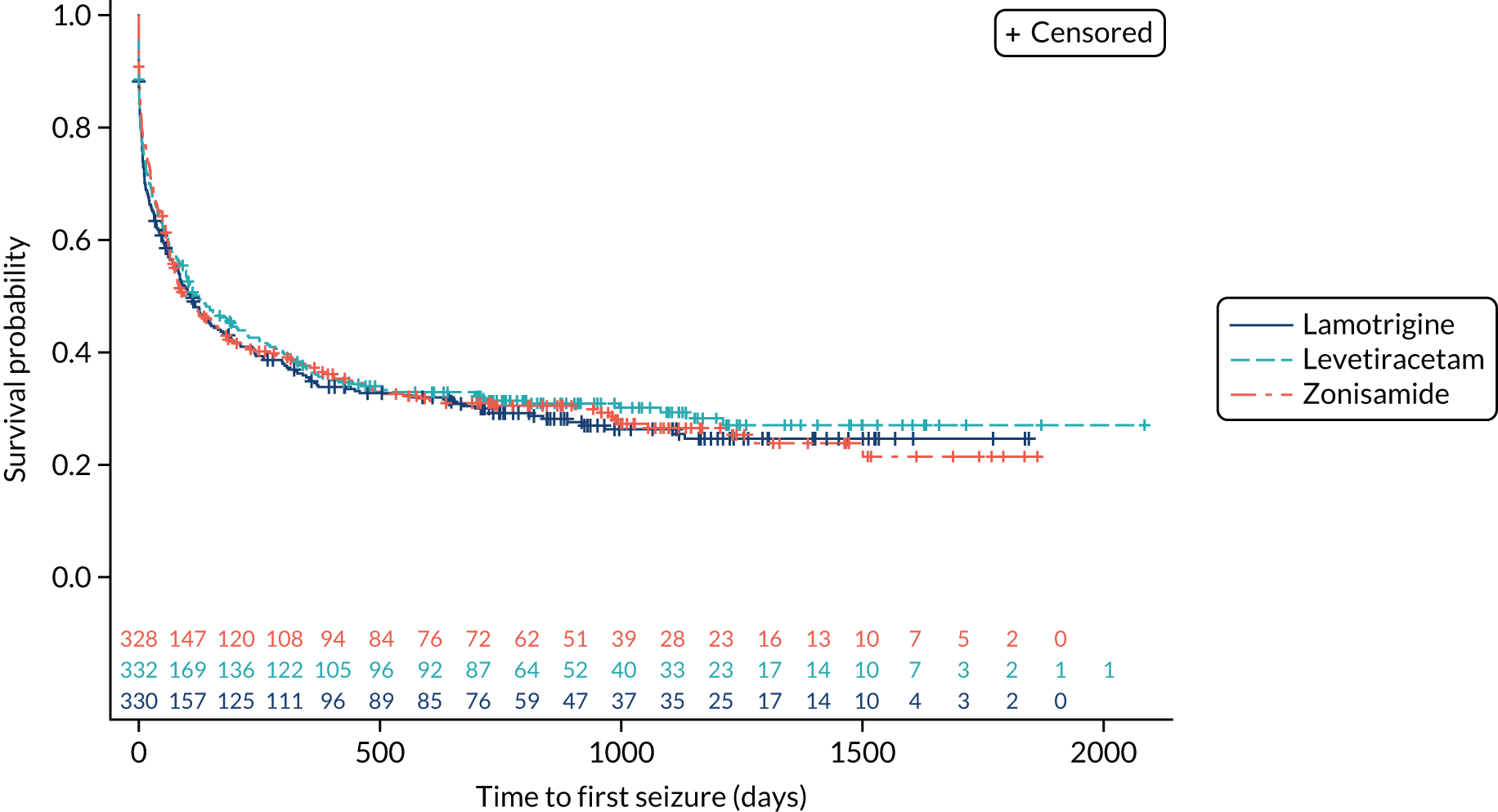
The ITT analysis of time to first seizure (Figure 6) indicates no significant difference between initiating treatment with lamotrigine or levetiracetam (HR 1.07, 95% CI 0.89 to 1.29) or between initiating treatment with lamotrigine of zonisamide (HR 1.04, 95% CI 0.86 to 1.25).
FIGURE 6.
Kaplan–Meier plot of time to first seizure: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
Time to treatment failure
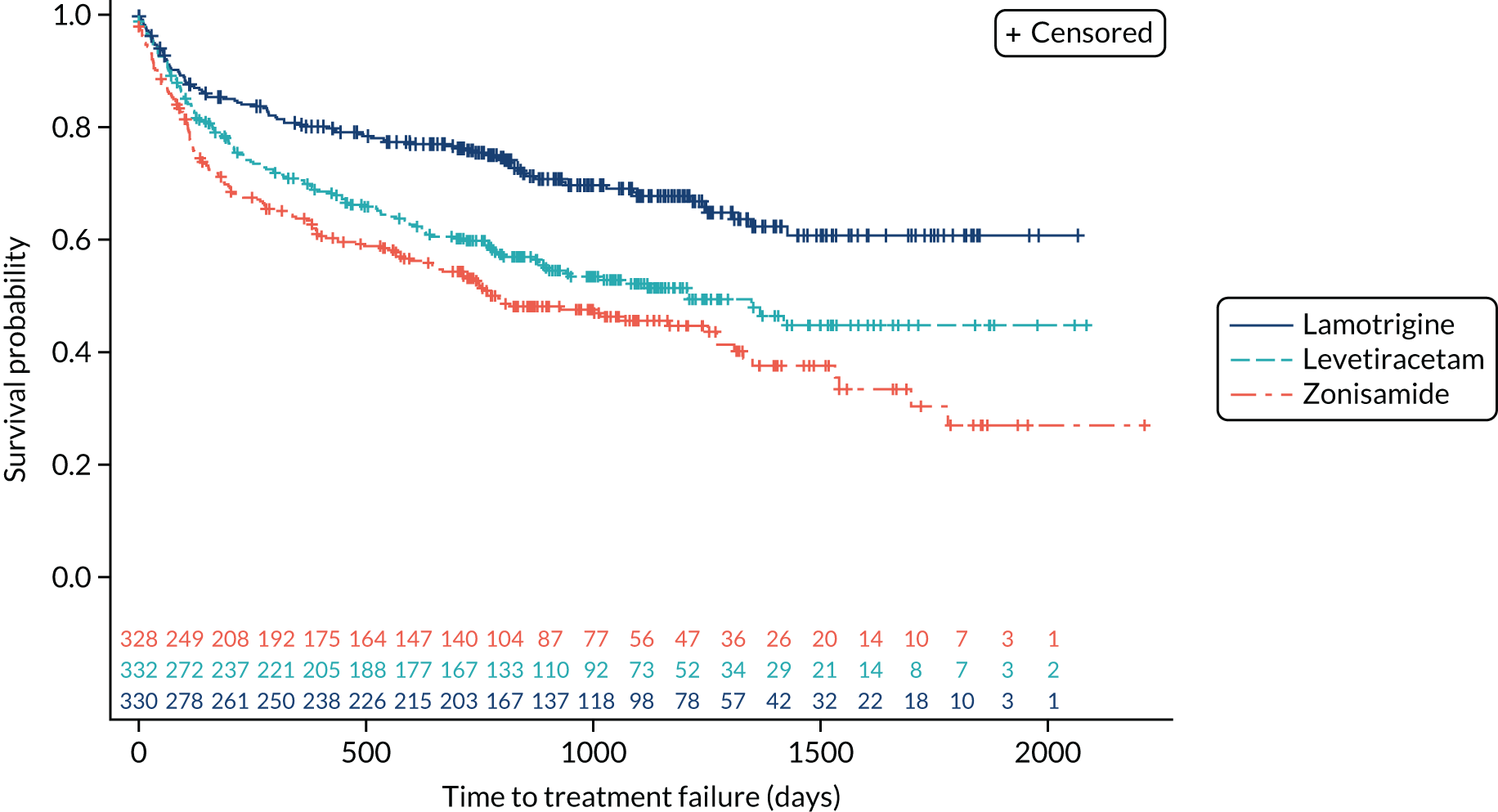
The analysis of overall time to treatment failure for any reason (Figure 7) indicates a significant advantage of lamotrigine when compared with both levetiracetam (HR 0.60, 95% CI 0.46 to 0.77) and zonisamide (HR 0.46, 95% CI 0.36 to 0.60), with no evidence to suggest violation of the assumption of proportional hazards (p = 0.77). Table 5 provides annual treatment failure rates and differences in failure rates between lamotrigine and both levetiracetam and zonisamide. At 2 years, there was a 16% (95% CI 8% to 23%) difference in the treatment failure rate on levetiracetam and lamotrigine and a 23% (95% CI 15% to 30%) difference between zonisamide and lamotrigine.
FIGURE 7.
Kaplan–Meier plot of time to treatment failure: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
| Probability estimate | Events/total | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|---|
| Number at risk | ||||||
| Lamotrigine | 97/330 | 241 | 192 | 101 | 36 | 8 |
| Levetiracetam | 146/332 | 212 | 157 | 74 | 25 | 7 |
| Zonisamide | 167/328 | 185 | 128 | 59 | 23 | 7 |
| Percentage without failure (95% CI) | ||||||
| Lamotrigine | 80 (75 to 84) | 76 (71 to 80) | 68 (62 to 73) | 61 (53 to 68) | 61 (53 to 68) | |
| Levetiracetam | 70 (65 to 75) | 60 (54 to 65) | 52 (46 to 58) | 45 (37 to 52) | 45 (37 to 52) | |
| Zonisamide | 64 (58 to 69) | 53 (47 to 59) | 45 (39 to 52) | 37 (30 to 45) | 27 (16 to 37) | |
| Difference in percentage with failure compared with lamotrigine (95% CI) | ||||||
| Levetiracetam | 10 (3 to 17) | 16 (8 to 23) | 16 (7 to 24) | 16 (5 to 27) | 16 (5 to 27) | |
| Zonisamide | 16 (9 to 23) | 23 (15 to 30) | 22 (14 to 30) | 23 (13 to 34) | 34 (21 to 47) | |
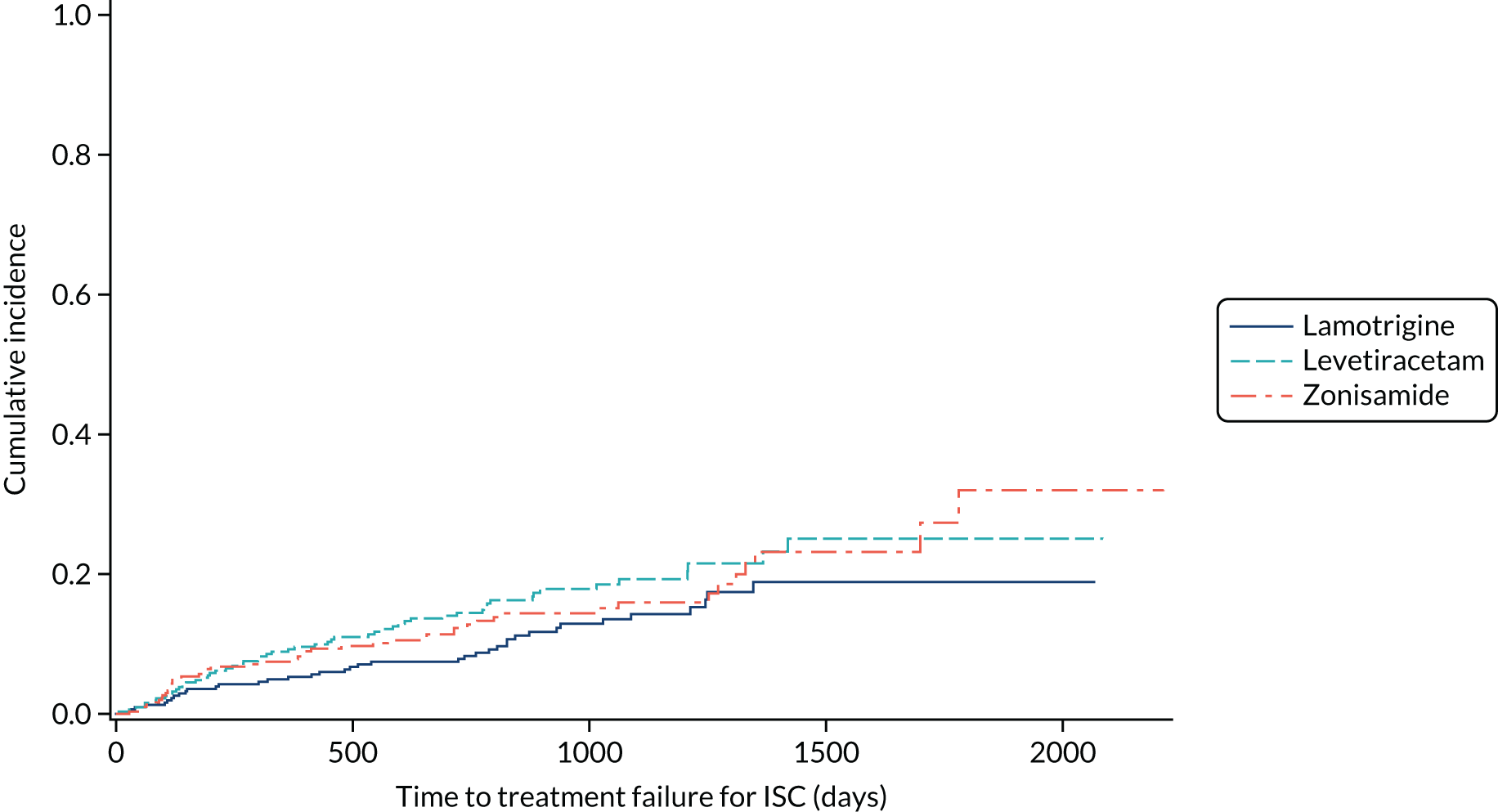
Table 6 summarises the doses taken at treatment failure or last follow-up and indicates that reasonable dose ranges were tried before deciding that failure had occurred. The competing risks analysis shows that levetiracetam treatment was significantly more likely than lamotrigine treatment to fail due to ARs (HR 0.53, 95% CI 0.35 to 0.79) (see Figure 7), but not ISC (HR 0.67, 95% CI 0.45 to 1.01) (Figure 8). Similarly, zonisamide was significantly more likely to fail than lamotrigine due to ARs (HR 0.37, 95% CI 0.25 to 0.55), but not ISC (HR 0.76, 95% CI 0.50 to 1.15) (Figure 9).
| Reason for withdrawal | Lamotrigine group | Levetiracetam group | Zonisamide group |
|---|---|---|---|
| Inadequate seizure control | n = 14 | n = 16 | n = 25 |
| First follow-up/missinga | First follow-up = 1 | First follow-up = 1, missing = 1 | First follow-up = 1 |
| Mean (SD) (mg) | 267 (152) | 2214 (955) | 277 (136) |
| Range (mg) | 75–500 | 500–3500 | 100–550 |
| Unacceptable ARs | n = 34 | n = 63 | n = 77 |
| First follow-up/missing | First follow-up = 16 | First follow-up = 18 | First follow-up = 20, missing = 3 |
| Mean (SD) (mg) | 171 (69) | 1089 (473) | 205 (101) |
| Range (mg) | 50–300 | 10–2500 | 25–500 |
| Other reason for withdrawal | n = 17 | n = 17 | n = 28 |
| First follow-up/missing | First follow-up = 6 | First follow-up = 8 | First follow-up = 9, missing = 1 |
| Mean (SD) (mg) | 164 (94) | 1188 (667) | 242 (83) |
| Range (mg) | 75–400 | 500–3000 | 150–400 |
| Remission of seizures | n = 7 | n = 7 | n = 10 |
| First follow-up/missing | 0 | First follow-up = 1 | First follow-up = 1 |
| Mean (SD) (mg) | 183 (149) | 1029 (221) | 200 (61) |
| Range (mg) | 50–500 | 800–1500 | 100–250 |
| Still on randomised drug | n = 238 | n = 188 | n = 149 |
| Missing | Missing = 11 | Missing = 10 | Missing = 17 |
| Mean (SD) (mg) | 222 (116) | 1440 (726) | 247 (112) |
| Range (mg) | 50–700 | 250–4000 | 25–600 |
FIGURE 8.
Cumulative incidence of treatment failure because of UARs from competing risks analysis: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
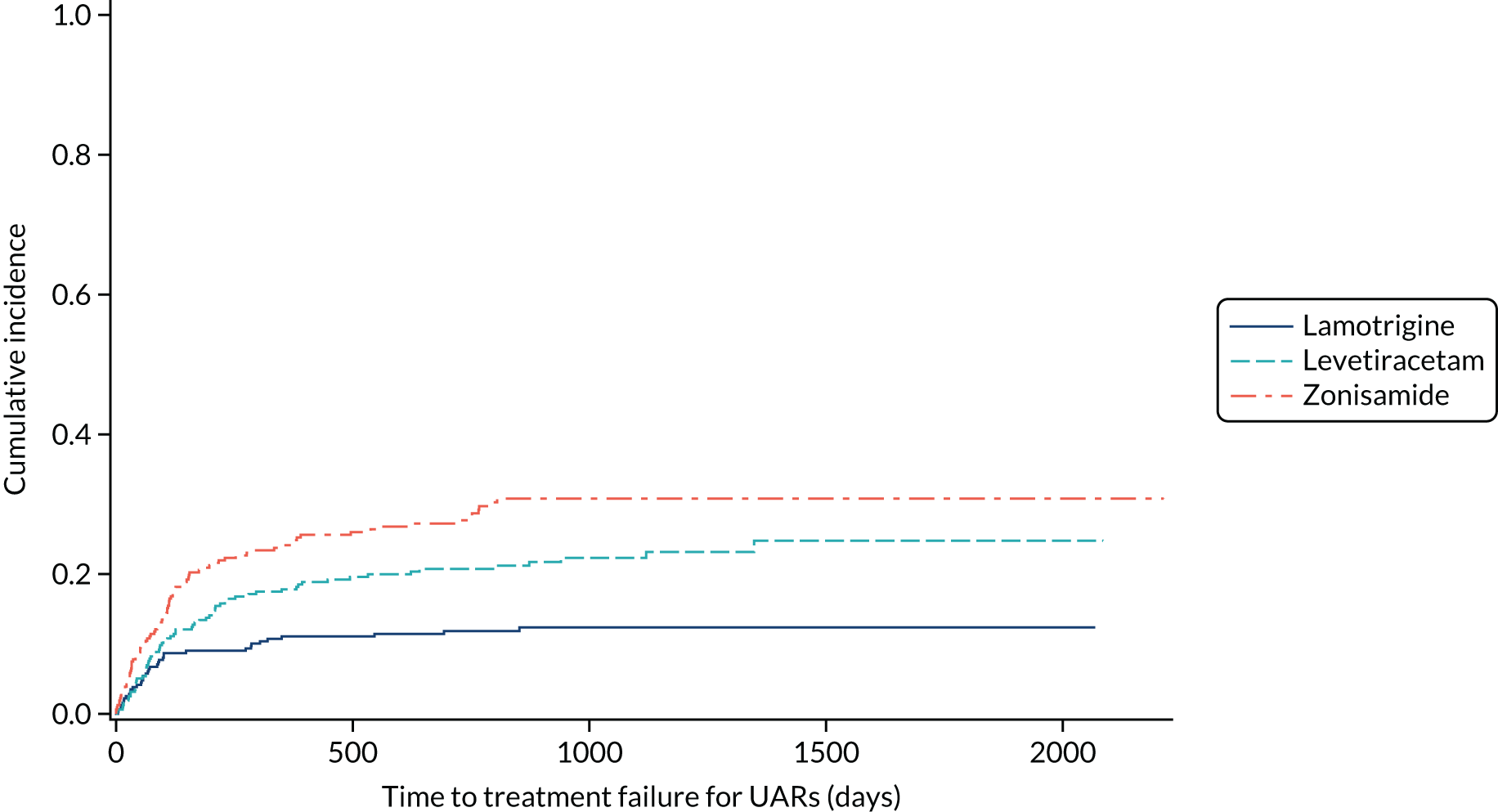
FIGURE 9.
Cumulative incidence of treatment failure because of ISC from competing risks analysis: lamotrigine vs. levetiracetam vs. zonisamide for focal epilepsy.
Safety
Data were recorded on ARs for the SANAD II trial, which were defined as AEs judged by the treating clinicians to be possibly, probably or definitely caused by anti-seizure medication. Table 7 provides an ITT (by treatment policy) summary of ARs according to the MedDRA System Organ Classification. Summaries by MedDRA-preferred term are presented in Appendix 3, Table 33.
| Event MedDRA System Organ Classification | Number of events | Number of patients (%) | ||||
|---|---|---|---|---|---|---|
| Lamotrigine group | Levetiracetam group | Zonisamide group | Lamotrigine group (N = 328) | Levetiracetam group (N = 330) | Zonisamide group (N = 324) | |
| Psychiatric disorders | 58 | 147 | 103 | 43 (13.1) | 98 (29.7) | 73 (22.5) |
| Nervous system disorders | 88 | 81 | 85 | 53 (16.2) | 55 (16.7) | 60 (18.5) |
| General disorders and administration site conditionsa | 23 | 37 | 44 | 17 (5.2) | 32 (9.7) | 39 (12.0) |
| Gastrointestinal disorders | 30 | 29 | 35 | 25 (7.6) | 22 (6.7) | 26 (8.0) |
| Skin and subcutaneous tissue disorders | 29 | 14 | 28 | 24 (7.3) | 12 (3.6) | 21 (6.5) |
| Investigations | 6 | 11 | 16 | 6 (1.8) | 11 (3.3) | 16 (4.9) |
| Metabolism and nutrition disorders | 4 | 2 | 17 | 3 (0.9) | 2 (0.6) | 16 (4.9) |
| Musculoskeletal and connective tissue disorders | 5 | 1 | 8 | 5 (1.5) | 1 (0.3) | 7 (2.2) |
| Eye disorders | 1 | 1 | 5 | 1 (0.3) | 1 (0.3) | 5 (1.5) |
| Renal and urinary disorders | 1 | 0 | 6 | 1 (0.3) | 0 | 5 (1.5) |
| Cardiac disorders | 2 | 2 | 1 | 2 (0.6) | 2 (0.6) | 1 (0.3) |
| Respiratory, thoracic and mediastinal disorders | 1 | 1 | 2 | 1 (0.3) | 1 (0.3) | 2 (0.6) |
| Injury, poisoning and procedural complications | 2 | 0 | 0 | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Ear and labyrinth disorders | 0 | 1 | 0 | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Endocrine disorders | 0 | 1 | 0 | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| Pregnancy, puerperium and perinatal conditions | 0 | 0 | 1 | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Vascular disorders | 1 | 0 | 0 | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Total number of events and patients with at least one AR | 251 | 328 | 351 | 108 (32.9) | 144 (43.6) | 146 (45.1) |
There were 251 ARs experienced by 108 (33%) participants starting treatment with lamotrigine, 328 ARs experienced by 144 (44%) participants starting treatment with levetiracetam and 351 ARs in 146 (45%) participants starting treatment with zonisamide. The main difference in adverse effect profiles was in the prevalence of psychiatric symptoms, which were reported in 13.1% of those starting on lamotrigine, 29.7% of those starting on levetiracetam and 22.5% of those starting on zonisamide.
Seven events in two participants starting on lamotrigine were classified as a SAR, compared with one event in those starting on levetiracetam and four in those starting on zonisamide; there were no suspected unexpected serious adverse reactions (SUSARs) (see Appendix 3, Table 34). There were 37 deaths during the trial: 15 (four likely to be seizure related) in participants starting on lamotrigine, 12 (two likely to be seizure related) in those starting on levetiracetam and 10 (two likely to be seizure related) in those starting on zonisamide (see Appendix 3, Table 35).
There were 11 pregnancies in 11 women starting treatment with lamotrigine (10 with normal postnatal examination and one with minor malformations), six pregnancies in five women starting on levetiracetam (five with normal postnatal examination and one termination), and 17 pregnancies in 14 women starting treatment with zonisamide [eight with normal postnatal examination, eight miscarriages (in five women) and one termination] (see Appendix 3, Table 36).
Quality of life
A total of 493 (49.8%) participants returned QoL questionnaires at baseline and at least one other time point during follow-up. A comparison of those who did and did not return questionnaires showed a similar proportion of male and females, and a similar proportion of those with learning disabilities and neurological deficits to those without, but non-responders were slightly younger (Table 8).
| Characteristic | No return | Return | Total |
|---|---|---|---|
| Age (years) (n) | 497 | 493 | 990 |
| Mean (SD) | 34.2 (18.6) | 44.5 (22.3) | 39.3 (21.2) |
| Median (IQR) | 32.2 (20.2–45.1) | 44.9 (24.8–64.2) | 37.7 (22.6–54.5) |
| Range | 5.0–88.8 | 5.0–91.9 | 5.0–91.9 |
| Missing | 0 | 0 | 0 |
| Gender (n) | 497 | 493 | 990 |
| Male, n (%) | 288 (57.9) | 273 (55.4) | 561 (56.7) |
| Female, n (%) | 209 (42.1) | 220 (44.6) | 429 (43.3) |
| Learning disability (n) | 497 | 493 | 990 |
| Yes, n (%) | 28 (5.6) | 17 (3.4) | 45 (4.5) |
| No, n (%) | 469 (94.4) | 476 (96.6) | 945 (95.5) |
| Neurological deficit (n) | 497 | 493 | 990 |
| Yes, n (%) | 28 (5.6) | 16 (3.2) | 44 (4.4) |
| No, n (%) | 469 (94.4) | 477 (96.8) | 946 (95.6) |
| Previous or current neurological disorder, n (%) | |||
| Stroke/cerebrovascular | 21 (4.2) | 26 (5.3) | 47 (4.7) |
| Cerebral haemorrhage | 10 (2.0) | 4 (0.8) | 14 (1.4) |
| Intracranial surgery | 12 (2.4) | 8 (1.6) | 20 (2.0) |
| Patients with head injury and post-traumatic amnesia for > 24 hours or a compound depressed fracture | 10 (2.0) | 8 (1.6) | 18 (1.8) |
| Meningitis/encephalitis | 9 (1.8) | 8 (1.6) | 17 (1.7) |
| Cortical dysplasia/developmental anomaly | 4 (0.8) | 0 (0.0) | 4 (0.4) |
| Other | 29 (5.8) | 40 (8.1) | 69 (7.0) |
| History, n (%) | |||
| Febrile convulsions | 27 (5.4) | 17 (3.4) | 44 (4.4) |
| Any other acute symptomatic seizures | 10 (2.0) | 9 (1.8) | 19 (1.9) |
| Family history of epilepsy in primary relatives | 71 (14.3) | 36 (7.3) | 107 (10.8) |
Overall, lamotrigine was associated with a better profile on self-reported measures than levetiracetam or zonisamide. A comparison of the treatment effects in adults (Table 9) revealed negative treatment effects for levetiracetam when compared with lamotrigine for patient-reported anxiety, depression stigma, epilepsy impact and overall QoL. Compared with lamotrigine, zonisamide had a negative treatment effect for depression, epilepsy impact and overall QoL. A comparison of the treatment effects in children is summarised in Table 10. Owing to the small sample size, it is not possible to make any reliable inference about QoL effects.
| QoL variable | Number of patients included in analysis | Treatment effect estimate (lamotrigine vs. levetiracetam)a (95% CI) | p-value | Treatment effect estimate (lamotrigine vs. zonisamide)a (95% CI) | p-value |
|---|---|---|---|---|---|
| AEs profile | 405 | –1.39 (–3.14 to 0.36) | 0.118 | –0.89 (–2.67 to 0.89) | 0.327 |
| Anxiety | 406 | –1.33 (–2.03 to –0.64) | < 0.001 | –0.22 (–0.93 to 0.49) | 0.544 |
| Depression | 406 | –1.20 (–1.83 to –0.56) | < 0.001 | –0.80 (–1.45 to –0.15) | 0.015 |
| Mastery | 364 | 0.36 (–0.20 to 0.91) | 0.206 | 0.32 (–0.25 to 0.89 | 0.276 |
| Stigma | 365 | –0.50 (–0.96 to –0.04) | 0.032 | 0.01 (–0.46 to 0.48) | 0.962 |
| Impact | 362 | 1.87 (0.73 to 3.00) | 0.001 | 1.82 (0.65 to 2.99) | 0.002 |
| Overall QoL | 358 | –0.52 (–0.77 to –0.26) | < 0.001 | –0.41 (–0.67 to –0.15) | 0.002 |
| QoL variable | Number of patients included in analysis | Treatment effect estimate (lamotrigine vs. levetiracetam)a (95% CI) | p-value | Treatment effect estimate (lamotrigine vs. zonisamide)a (95% CI) | p-value |
|---|---|---|---|---|---|
| Self-reported | |||||
| Attitude to epilepsy | 32 | –1.40 (–17.38 to 14.58) | 0.860 | –9.46 (–23.79 to 4.86) | 0.189 |
| QoL physical | 31 | –0.89 (–17.27 to 15.50) | 0.913 | –1.01 (–16.10 to 14.08) | 0.892 |
| QoL emotional | 31 | –8.01 (–19.99 to 3.97) | 0.184 | –6.31 (–17.26 to 4.65) | 0.251 |
| QoL self-esteem | 30 | –9.54 (–25.85 to 6.77) | 0.243 | 4.97 (–10.16 to 20.09) | 0.510 |
| QoL social | 31 | –1.86 (–12.87 to 9.15) | 0.734 | 1.87 (–8.56 to 12.29) | 0.718 |
| QoL family | 31 | –13.82 (–29.44 to 1.80) | 0.081 | –7.44 (–21.84 to 6.96) | 0.302 |
| QoL school | 30 | –18.75 (–32.88 to –4.62) | 0.011 | –12.43 (–25.35 to 0.50) | 0.059 |
| Impact of epilepsy | 7 | 1.82 (–27.06 to 30.70) | 0.888 | –4.81 (–27.58 to 17.95) | 0.639 |
| Parent proxy reported | |||||
| QoL physical | 62 | –4.22 (–13.93 to 5.48) | 0.391 | –6.10 (–15.49 to 3.28) | 0.201 |
| QoL emotional | 61 | 0.10 (–9.09 to 9.29) | 0.983 | 0.34 (–8.32 to 9.00) | 0.939 |
| QoL self-esteem | 60 | –5.44 (–13.58 to 2.70) | 0.189 | –2.39 (–10.15 to 5.37) | 0.544 |
| QoL social | 60 | –9.45 (–18.06 to –0.83) | 0.032 | –5.02 (–13.11 to 3.08) | 0.222 |
| QoL family | 61 | 1.28 (–7.17 to 9.73) | 0.765 | 1.36 (–6.62 to 9.34) | 0.736 |
| QoL school | 61 | –8.53 (–17.59 to 0.52) | 0.065 | –5.17 (–13.79 to 3.44) | 0.237 |
Chapter 4 Focal epilepsy results: economic
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Data completeness
The HES data were available for a total of 772 participants, relating to 266 participants randomised to start treatment with lamotrigine, 261 participants randomised to start treatment with levetiracetam and 245 participants randomised to start treatment with zonisamide. A breakdown of missing data by treatment group and outcome is provided in Appendix 4, Table 48.
A total of 789 participants completed at least one self-report questionnaire (completing the resource use, EQ-5D or both sections); 621 participants completed two or more questionnaires. In total, questionnaires were available for 3039 participant time points (once child and proxy questionnaires had been resolved).
Questionnaires returned after the change in protocol were assigned to their nearest time point for presentation purposes. Self-report resource use data were available for 550 participants at 3 months, 527 participants at 6 months, 465 participants at 12 months and 398 participants at 24 months. Resource use data were also available from 496 questionnaires returned at the later time points (36, 48 and 60 months).
Utility data (EQ-5D) were available for 616 participants at baseline; data were interpolated to 12 months for 422 participants and to 24 months for 319 participants. These are lower than the figures reported in Appendix 4, Table 48, because the 12- and 24-month questionnaires were dated less than 365 and 730 days post randomisation, respectively. For the NEWQOL-6D, fewer utility data were available because of a large number of partially completed questionnaires.
A total of 50 data sets were imputed, based on the largest fraction of missing information (0.7) and accepting < 1% reduction in power compared with 100 imputations. For the bootstrapped results, this was reduced to 10 for efficiency purposes, accepting a higher reduction in power to achieve an acceptable computation time. 64 Owing to the level of missingness, models containing the NEWQOL-6D were non-convergent; hence only complete-case results are presented for the NEWQOL-6D.
Resource use and costs
Table 11 presents the observed mean disaggregated resource use based on the self-report questionnaires. Table 12 presents the most common admitted patient care episodes, outpatient and A&E-related HRGs, and costs observed during the trial period. During the 24-month follow-up period, 339 unique HRGs were recorded in admitted patient care, including 262 in outpatients and 35 in A&E.
| Resource | Mean [range] (number of participants) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-month time point | 6-month time point | 12-month time point | 24-month time point | |||||||||
| Lamotrigine group | Levetiracetam group | Zonisamide group | Lamotrigine group | Levetiracetam group | Zonisamide group | Lamotrigine group | Levetiracetam group | Zonisamide group | Lamotrigine group | Levetiracetam group | Zonisamide group | |
| Questionnaires returned (n) | 179 | 183 | 182 | 172 | 170 | 173 | 150 | 147 | 151 | 126 | 124 | 122 |
| Primary care | ||||||||||||
| GP consultation at GP surgery | 1.02 [0–8] (90) | 1.13 [0–13] (88) | 0.98 [0–10] (92) | 0.67 [0–5] (63) | 0.87 [0–10] (72) | 0.89 [0–12] (71) | 0.76 [0–14] (65) | 1.01 [0–12] (67) | 1.10 [0–8] (76) | 0.83 [0–9] (52) | 1.09 [0–10] (56) | 1.01 [0–20] (52) |
| Nurse consultation at GP surgery | 0.58 [0–11] (46) | 0.50 [0–10] (42) | 0.46 [0–10] (47) | 0.42 [0–12] (45) | 0.38 [0–6] (35) | 0.56 [0–24] (42) | 0.63 [0–12] (48) | 0.71 [0–10] (51) | 0.73 [0–8] (52) | 0.83 [0–12] (51) | 0.85 [0–8] (47) | 0.74 [0–16] (41) |
| GP home visit | 0.01 [0–1] (1) | 0.04 [0–6] (3) | 0.05 [0–5] (5) | 0.02 [0–2] (2) | 0.04 [0–2] (5) | 0.02 [0–2] (3) | 0 | 0.02 [0–2] (2) | 0.01 [0–1] (1) | 0.02 [0–2] (1) | 0.08 [0–6] (3) | 0.02 [0–1] (3) |
| Nurse home visit | 0.10 [0–2] (14) | 0.13 [0–6] (10) | 0.05 [0–6] (4) | 0.03 [0–1] (5) | 0.37 [0–24] (11) | 0.05 [0–12] (9) | 0.01 [0–1] (1) | 0.68 [0–95] (5) | 0.01 [0–1] (1) | 0.01 [0–1] (1) | 0.19 [0–12] (10) | 0.05 [0–2] (4) |
| Community care | ||||||||||||
| Health visitor | 0.01 [0–1] (2) | 0.06 [0–6] (4) | 0.04 [0–3] (4) | 0.01 [0–1] (1) | 0.06 [0–5] (3) | 0.02 [0–3] (1) | 0.01 [0–1] (1) | 0 | 0.01 [0–2] (1) | 0.03 [0–4] (1) | 0.04 [0–3] (3) | 0.02 [0–2] (1) |
| Social worker | 0.08 [0–7] (4) | 0.04 [0–6] (3) | 0.02 [0–2] (2) | 0.06 [0–4] (3) | 0.06 [0–6] (4) | 0.03 [0–3] (3) | 0.14 [0–20] (2) | 0.07 [0–5] (4) | 0.05 [0–4] (4) | 0.02 [0–2] (2) | 0.06 [0–4] (3) | 0.06 [0–3] (4) |
| Occupational therapist | 0.09 [0–4] (9) | 0.15 [0–6] (14) | 0.09 [0–4] (9) | 0.05 [0–3] (5) | 0.10 [0–6] (7) | 0.03 [0–2] (5) | 0.17 [0–20] (5) | 0.07 [0–3] (7) | 0.03 [0–2] (3) | 0.02 [0–2] (1) | 0.29 [0–27] (5) | 0.05 [0–5] (2) |
| Psychologist | 0.07 [0–4] (8) | 0.16 [0–8] (10) | 0.09 [0–5] (7) | 0.06 [0–3] (7) | 0.20 [0–18] (10) | 0.06 [0–2] (8) | 0.03 [0–2] (4) | 0.14 [0–11] (5) | 0.07 [0–2] (7) | 0.07 [0–3] (5) | 0.21 [0–6] (8) | 0.25 [0–7] (9) |
| Counsellor | 0.02 [0–2] (2) | 0.10 [0–6] (4) | 0.18 [0–13] (6) | 0.07 [0–6] (3) | 0.20 [0–8] (7) | 0.29 [0–12] (11) | 0.09 [0–9] (4) | 0.22 [0–12] (7) | 0.15 [0–12] (5) | 0.06 [0–6] (3) | 0.21 [0–16] (8) | 0.22 [0–12] (5) |
| Physiotherapist | 0.13 [0–6] (7) | 0.16 [0–6] (10) | 0.14 [0–6] (9) | 0.09 [0–12] (4) | 0.09 [0–4] (7) | 0.13 [0–10] (7) | 0.09 [0–7] (5) | 0.32 [0–10] (11) | 0.16 [0–12] (6) | 0.13 [0–6] (6) | 0.41 [0–27] (9) | 22 [0–10] (7) |
| Secondary care | ||||||||||||
| Doctor at hospital | 0.55 [0–3] (74) | 0.79 [0–6] (86) | 0.70 [0–6] (83) | 0.68 [0–3] (86) | 1.05 [0–61] (85) | 0.79 [0–6] (92) | 0.61 [0–4] (64) | 0.63 [0–8] (56) | 0.64 [0–5] (72) | 0.53 [0–6] (49) | 0.60 [0–7] (51) | 0.61 [0–8] (44) |
| Nurse at hospital | 0.47 [0–4] (66) | 0.59 [0–6] (79) | 0.59 [0–6] (77) | 0.53 [0–16] (60) | 0.46 [0–4] (62) | 0.57 [0–6] (72) | 0.47 [0–5] (53) | 0.68 [0–13] (55) | 0.53 [0–20] (45) | 0.31 [0–5] (31) | 0.41 [0–6] (38) | 0.56 [0–10] (42) |
| Hospital overnight | 0.28 [0–18] (12) | 0.16 [0–6] (13) | 0.15 [0–7] (15) | 0.09 [0–7] (8) | 0.09 [0–5] (6) | 0.12 [0–6] (10) | 0.24 [0–16] (6) | 0.52 [0–46] (7) | 0.24 [0–10] (10) | 0.09 [0–4] (6) | 0.84 [0–77] (9) | 0.39 [0–28] (9) |
| Ambulance | 0.18 [0–7] (21) | 0.25 [0–7] (22) | 0.17 [0–4] (19) | 0.07 [0–2] (11) | 0.14 [0–6] (13) | 0.11 [0–3] (17) | 0.08 [0–3] (9) | 0.08 [0–2] (8) | 0.15 [0–5] (14) | 0.13 [0–2] (13) | 0.10 [0–3] (9) | 0.18 [0–5] (10) |
| A&E visit | 0.27 [0–7] (28) | 0.30 [0–5] (31) | 0.23 [0–4] (24) | 0.15 [0–2] (22) | 0.21 [0–4] (21) | 0.21 [0–9] (25) | 0.27 [0–8] (24) | 0.30 [0–15] (18) | 0.23 [0–6] (23) | 0.20 [0–3] (19) | 0.29 [0–4] (21) | 0.24 [0–5] (20) |
| Blood test | 0.58 [0–11] (58) | 0.36 [0–4] (51) | 0.46 [0–24] (44) | 0.34 [0–12] (42) | 0.70 [0–59] (43) | 0.46 [0–10] (44) | 0.60 [0–16] (45) | 0.48 [0–10] (41) | 0.50 [0–7] (47) | 0.73 [0–12] (47) | 0.63 [0–7] (42) | 0.52 [0–5] (40) |
| Urine test | 0.14 [0–4] (20) | 0.13 [0–3] (20) | 0.22 [0–14] (23) | 0.12 [0–2] (18) | 0.29 [0–28] (18) | 0.18 [0–3] (24) | 0.16 [0–3] (18) | 0.13 [0–2] (14) | 0.07 [0–2] (9) | 0.15 [0–3] (16) | 0.15 [0–2] (14) | 0.28 [0–9] (17) |
| Ultrasound | 0.09 [0–2] (16) | 0.09 [0–3] (13) | 0.09 [0–3] (13) | 0.06 [0–2] (9) | 0.05 [0–3] (7) | 0.13 [0–2] (18) | 0.07 [0–1] (9) | 0.05 [0–4] (5) | 0.08 [0–2] (10) | 0.04 [0–2] (4) | 0.07 [0–2] (8) | 0.14 [0–4] (12) |
| Radiography | 0.13 [0–6] (10) | 0.10 [0–3] (13) | 0.15 [0–8] (16) | 0.08 [0–3] (10) | 0.11 [0–2] (15) | 0.16 [0–4] (20) | 0.21 [0–3] (25) | 0.08 [0–3] (8) | 0.09 [0–2] 10 | 0.19 [0–6] (16) | 0.16 [0–5] (14) | 0.16 [0–3] (15) |
| CT scan | 0.07 [0–2] (11) | 0.08 [0–2] (14) | 0.08 [0–2] (14) | 0.03 [0–1] (6) | 0.04 [0–1] (7) | 0.04 [0–1] (7) | 0.05 [0–2] (7) | 0.03 [0–2] (3) | 0.01 [0–1] (2) | 0.02 [0–1] (2) | 0.02 [0–1] (3) | 0.01 [0–1] (1) |
| MRI scan | 0.21 [0–2] (36) | 0.21 [0–2] (37) | 0.24 [0–2] (41) | 0.06 [0–2] (10) | 0.06 [0–1] (11) | 0.09 [0–2] (15) | 0.07 [0–2] (9) | 0.01 [0–1] (1) | 0.05 [0–1] (7) | 0.02 [0–1] (2) | 0.02 [0–1] (2) | 0.02 [0–1] (3) |
| EEG | 0.21 [0–4] (33) | 0.15 [0–2] (26) | 0.18 [0–2] (32) | 0.04 [0–1] (7) | 0.05 [0–1] (8) | 0.03 [0–1] (6) | 0.03 [0–1] (4) | 0.01 [0–1] (2) | 0.04 [0–2] (5) | 0.01 [0–1] (1) | 0.01 [0–1] (1) | 0.01 [0–1] (1) |
| Othera | 0.11 [0–2] (18) | 0.12 [0–3] (19) | 0.16 [0–7] (19) | 0.09 [0–2] (12) | 0.12 [0–2] (19) | 0.35 [0–18] (18) | 0.09 [0–2] (11) | 0.07 [0–1] (10) | 0.10 [0–2] (14) | 0.42 [0–28] (14) | 0.17 [0–10] (10) | 0.20 [0–3] (18) |
| HRG code | Description | Attendances (n)a | |||||
|---|---|---|---|---|---|---|---|
| Lamotrigine group | Levetiracetam group | Zonisamide group | Total | ||||
| Admitted patient care | |||||||
| AA26H | Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with a CC score of 0–2 | 15 | 20 | 20 | 60 | ||
| SC97Z | Same-day radiotherapy admission or attendance (excluding Brachytherapy) | 20 | 0 | 20 | 40 | ||
| AA26G | Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with a CC score of 3–5 | b | b | b | 25 | ||
| SB97Z | Same-day chemotherapy admission or attendance | 25 | 0 | 0 | 25 | ||
| AA33C | Conventional EEG, EMG or nerve conduction studies, 19 years and over | b | b | b | 20 | ||
| PR02B | Paediatric epilepsy syndrome with a CC score of 1–5 | b | b | b | 20 | ||
| AA80Z | Complex long-term EEG monitoring | b | b | b | 15 | ||
| PR02C | Paediatric epilepsy syndrome with a CC score of 0 | b | b | b | 15 | ||
| WH50B | Procedure not carried out, for other or unspecified reasons | b | b | b | 10 | ||
| WH04E | Poisoning diagnosis without interventions, with a CC score of 0 or 1 | b | b | b | 10 | ||
| Outpatients | |||||||
| 400 | Neurology | WF01A | Non-admitted face-to-face attendance, follow-up | 800 | 840 | 825 | 2465 |
| 400 | Neurology | WF01B | Non-admitted face-to-face attendance, first | 195 | 185 | 160 | 540 |
| 420 | Paediatrics | WF01A | Non-admitted face-to-face attendance, follow-up | 120 | 155 | 145 | 420 |
| 400 | Neurology | N/A | N/A | 65 | 80 | 80 | 220 |
| 110 | Trauma and orthopaedics | WF01A | Non-admitted face-to-face attendance, follow-up | 70 | 60 | 65 | 200 |
| 650 | Physiotherapy | WF01A | Non-admitted face-to-face attendance, follow-up | 30 | 55 | 50 | 135 |
| 421 | Paediatric neurology | WF01A | Non-admitted face-to-face attendance, follow-up | 50 | 45 | 22 | 120 |
| 223 | Paediatric epilepsy | N/A | N/A | 20 | 20 | 80 | 115 |
| 110 | Trauma and orthopaedics | N/A | N/A | 40 | 45 | 30 | 115 |
| 320 | Cardiology | WF01A | Non-admitted face-to-face attendance, follow-up | 30 | 40 | 35 | 105 |
| A&E | |||||||
| N/A | N/A | ASS02 | See and treat and convey | 140 | 170 | 185 | 490 |
| T01NA | Type 01 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | 105 | 100 | 90 | 295 |
| T01NA | Type 01 non-admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | 50 | 55 | 70 | 180 |
| T01NA | Type 01 non-admitted | VB11Z | Emergency medicine, no investigation with no significant treatment | 30 | 25 | 30 | 85 |
| T01A | Type 01 admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | 25 | 30 | 25 | 75 |
| T01A | Type 01 admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | 20 | 25 | 25 | 65 |
| T01NA | Type 01 non-admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | 15 | 30 | 20 | 60 |
| T04NA | Type 01 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | b | b | b | 45 |
| T01A | Type 01 admitted | VB04Z | Emergency medicine, category 2 investigation with category 4 treatment | 15 | 15 | 15 | 45 |
| T03NA | Type 01 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | b | b | b | 35 |
Based on the imputed data, the majority of costs relate to secondary care, in particular admitted patient care and outpatient clinic attendance (Table 13). Comparing across treatment groups, zonisamide has higher secondary care costs and medicines costs than lamotrigine or levetiracetam. The total (unadjusted) costs were £5409 (97.5% CR £4584 to £6658) for participants randomised to start treatment with zonisamide, compared with £5074 (97.5% CR £4433 to £6049) for those randomised to levetiracetam and £4063 (97.5% CR £4842 to £6317) for those randomised to lamotrigine. The differences between the zonisamide and levetiracetam groups (£336, 97.5% CR –£926 to £1634) and between the levetiracetam and lamotrigine groups (£1011, 97.5% CR –£36 to £2066) were not statistically significant. However, the difference in cost between zonisamide and lamotrigine was significant (£1347, 97.5% CR £226 to £2550).
| Type of care | Totals (discounted) (£) at 24 months, mean (95% CR) | Difference (£), mean (95% CR) | ||||
|---|---|---|---|---|---|---|
| Lamotrigine group | Levetiracetam group | Zonisamide group | Levetiracetam – lamotrigine | Zonisamide – lamotrigine | Zonisamide – levetiracetam | |
| Primary and community care | 682 (551 to 1018) | 1303 (981 to 2009) | 1013 (786 to 1631) | 622 (148 to 1274) | 331 (–31 to 940) | –290 (–979 to 398) |
| Primary care | 332 (284 to 423) | 532 (416 to 724) | 411 (347 to 567) | 200 (59 to 391) | 79 (–25 to 236) | –121 (–306 to 82) |
| Community care | 350 (228 to 646) | 771 (489 to 1381) | 602 (374 to 1117) | 422 (5 to 1028) | 253 (–95 to 778) | –169 (–795 to 409) |
| Secondary care | 3025 (2606 to 3628) | 3263 (2853 to 3723) | 3882 (3140 to 4670) | 237 (–486 to 847) | 857 (–69 to 1680) | 619 (–215 to 1509) |
| Admitted patient care | 1170 (855 to 1631) | 1156 (869 to 1443) | 1663 (1153 to 2246) | –15 (–560 to 400) | 493 (–178 to 1127) | 507 (–75 to 1207) |
| Outpatient care | 1519 (1393 to 1664) | 1705 (1552 to 1876) | 1784 (1547 to 2050) | 186 (–26 to 401) | 266 (–17 to 564) | 80 (–202 to 392) |
| A&E | 336 (269 to 425) | 402 (314 to 528) | 434 (316 to 582) | 66 (–64 to 199) | 98 (–55 to 259) | 32 (–153 to 220) |
| Medicines | 356 (294 to 475) | 508 (412 to 665) | 515 (423 to 668) | 151 (–10 to 304) | 158 (15 to 316) | 7 (–154 to 193) |
| Anti-seizure medication | 125 (103 to 158) | 248 (213 to 292) | 269 (244 to 298) | 123 (75 to 171) | 144 (104 to 184) | 21 (–24 to 68) |
| Concomitant medication | 231 (175 to 348) | 260 (172 to 403) | 246 (161 to 390) | 28 (–122 to 171) | 14 (–126 to 168) | –14 (–165 to 162) |
| Total | 4063 (3617 to 4842) | 5074 (4433 to 6049) | 5409 (4584 to 6658) | 1011 (–36 to 2066) | 1347 (266 to 2550) | 336 (–926 to 1634) |
Based on imputed data, the mean baseline costs were £1215 (97.5% CR £1061 to £1375) in the zonisamide group, £1191 (97.5% CR £1035 to £1398) in the levetiracetam group and £1239 (97.5% CR £1036 to £1464) in the lamotrigine group. The base-case analysis that adjusted for baseline costs, age, gender and epilepsy type with centre as random effects yielded a mean 2-year total cost of £5400 (97.5% CR £4659 to £6770) in the zonisamide group, compared with £5104 (97.5% CR £4450 to £6141) in the levetiracetam group and £4042 (97.5% CR £3626 to £4983) in the lamotrigine group. The differences between the zonisamide and levetiracetam groups (£297, 97.5% CR –£900 to £1624) and between the levetiracetam and lamotrigine groups (£1062, 97.5% CR –£1174 to £2133) were not statistically significant. There was a significant difference of £1358 (97.5% CR £376 to £2563) between the zonisamide and lamotrigine groups.
Utilities and quality-adjusted life-years
The distributions of participants’ responses to the EQ-5D-3L-Y and the NEWQOL-6D questionnaires by randomised treatment group are presented in Appendix 4, Figures 27 and 28. Based on imputed data, mean baseline utilities were 0.766 (97.5% CR 0.733 to 0.804) in the levetiracetam group, 0.800 (97.5% CR 0.760 to 0.830) in the zonisamide group and 0.779 (97.5% CR 0.751 to 0.818) in the lamotrigine group. In the base-case adjusted analysis, levetiracetam was associated with a QALY gain of 1.474 years (97.5% CR 1.393 to 1.523 years) over the 2-year time horizon, whereas zonisamide was associated with a QALY gain of 1.502 years (97.5% CR 1.418 to 1.566 years) and lamotrigine was associated with a QALY gain of 1.605 years (97.5% CR 1.547 to 1.651 years). This corresponds to a negative incremental QALY gain of –0.025 years (97.5% CR –0.058 to 0.129 years) between levetiracetam and zonisamide. The incremental QALY gains of –0.103 years (97.5% CR –0.201 to –0.015 years) between zonisamide and lamotrigine and –0.128 years (97.5% CR –0.219 to –0.065 years) between levetiracetam and lamotrigine were significant.
The QALYs based on the NEWQOL-6D were calculated for complete-case data only, over the 2-year time horizon. Levetiracetam was associated with adjusted QALY gains of 1.703 years (97.5% CR 1.678 to 1.727 years), compared with 1.712 years (97.5% CR 1.690 to 1.735 years) for zonisamide and 1.710 years (97.5% CR 1.687 to 1.733 years) for lamotrigine. Levetiracetam was, therefore, associated with a negative incremental QALY gain of –0.007 years (97.5% CR –0.035 to 0.019 years) when compared with zonisamide, and with a negative incremental QALY gain of –0.007 years (97.5% CR –0.035 to 0.019 years) when compared with lamotrigine. The incremental QALY gain between zonisamide and lamotrigine was 0.002 years (97.5% CR –0.021 to 0.025 years).
The distribution of responses to the EQ-VAS is shown in Table 14. The adjusted analysis based on the EQ-VAS resulted in a QALY gain of 1.398 years (97.5% CR 1.324 to 1.479 years) in the levetiracetam group, 1.418 years (97.5% CR 1.351 to 1.456 years) in the zonisamide group and 1.431 years (97.5% CR 1.360 to 1.476 years) in the lamotrigine gorup. The negative incremental QALY gains of –0.020 years (97.5% CR –0.094 to 0.085 years) for levetiracetam compared with zonisamide, –0.013 years (97.5% CR –0.085 to 0.060 years) for zonisamide compared with lamotrigine and –0.033 years (97.5% CR –0.112 to 0.075 years) for levetiracetam compared with lamotrigine are consistent with the base-case EQ-5D.
| Time point | Lamotrigine group | Levetiracetam group | Zonisamide group | |||
|---|---|---|---|---|---|---|
| n | Mean (97.5% CI) | n | Mean (97.5% CI) | n | Mean (97.5% CI) | |
| Baseline | 188 | 0.712 (0.681 to 0.744) | 187 | 0.707 (0.672 to 0.743) | 190 | 0.751 (0.717 to 0.784) |
| 12 months | 127 | 0.767 (0.722 to 0.812) | 124 | 0.706 (0.656 to 0.757) | 130 | 0.712 (0.664 to 0.759) |
| 24 months | 106 | 0.752 (0.701 to 0.803) | 106 | 0.715 (0.656 to 0.774) | 109 | 0.726 (0.673 to 0.780) |
Incremental analysis
Based on the point estimate mean costs and QALYs, both levetiracetam and zonisamide were more costly and less effective than lamotrigine, and were therefore dominated, meaning that they are not considered to be cost-effective. Zonisamide is associated with a negative INHB (–0.171, 97.5% CR –0.295 to –0.055) compared with lamotrigine, and levetiracetam is associated with a negative INHB compared with zonisamide (–0.010, 97.5% CR –0.142 to 0.112) at a cost-effectiveness threshold of £20,000 per QALY.
Sensitivity analyses
Table 15 presents the results of the sensitivity analyses, which are consistent with the base case for all analyses other than the NEWQOL-6D, where the NHB for levetiracetam is seen to be higher than for zonisamide at the £20,000 per QALY cost-effectiveness threshold. Table 15 also presents the complete-case analysis in which levetiracetam is associated with lower costs than lamotrigine, but lamotrigine is still associated with the higher NHB.
| Anti-seizure medication | Mean (97.5% CR) | |||||
|---|---|---|---|---|---|---|
| Total cost (£) | QALYs | NHB at £20,000 per QALY | NHB at £30,000 per QALY | INHB at £20,000 per QALY | INHB at £30,000 per QALY | |
| Base case (n = 990) | ||||||
| Lamotrigine | 4042 (3626 to 4983) | 1.605 (1.547 to 1.651) | 1.403 (1.319 to 1.458) | 1.470 (1.399 to 1.520) | ||
| Zonisamide | 5400 (4659 to 6770) | 1.502 (1.418 to 1.566) | 1.232 (1.112 to 1.307) | 1.322 (1.215 to 1.392) | –0.174 (–0.300 to –0.056) | –0.151 (–0.266 to –0.045) |
| Levetiracetam | 5104 (4450 to 6141) | 1.474 (1.393 to 1.523) | 1.222 (1.110 to 1.283) | 1.307 (1.204 to 1.361) | –0.011 (–0.146 to 0.114) | –0.016 (–0.139 to 0.091) |
| 0% discount rate (costs and QALYs) (base case 3.5%) (n = 990) | ||||||
| Lamotrigine | 4108 (3682 to 5059) | 1.633 (1.573 to 1.680) | 1.428 (1.343 to 1.484) | 1.496 (1.423 to 1.546) | ||
| Zonisamide | 5483 (4727 to 6872) | 1.528 (1.442 to 1.592) | 1.254 (1.131 to 1.330) | 1.322 (1.236 to 1.416) | –0.168 (–0.291 to –0.055) | –0.146 (–0.258 to –0.044) |
| Levetiracetam | 5189 (4517 to 6255) | 1.502 (1.417 to 1.549) | 1.243 (1.128 to 1.305) | 1.307 (1.224 to 1.385) | –0.010 (–0.139 to 0.111) | –0.014 (–0.133 to 0.091) |
| 6% discount rate (costs and QALYs) (base case 3.5%) (n = 990) | ||||||
| Lamotrigine | 3998 (3587 to 4935) | 1.586 (1.529 to 1.632) | 1.386 (1.303 to 1.440) | 1.453 (1.382 to 1.501) | ||
| Zonisamide | 5344 (4613 to 6698) | 1.485 (1.402 to 1.548) | 1.218 (1.100 to 1.291) | 1.307 (1.201 to 1.376) | –0.168 (–0.291 to –0.055) | –0.146 (–0.258 to –0.044) |
| Levetiracetam | 5046 (4405 to 6066) | 1.461 (1.378 to 1.505) | 1.208 (1.097 to 1.268) | 1.292 (1.191 to 1.346) | –0.010 (–0.139 to 0.111) | –0.014 (–0.133 to 0.089) |
| Unadjusted (no covariates) (base-case adjusted) (n = 990) | ||||||
| Lamotrigine | 4063 (3617 to 4842) | 1.600 (1.524 to 1.649) | 1.397 (1.301 to 1.450) | 1.465 (1.374 to 1.515) | ||
| Zonisamide | 5409 (4584 to 6658) | 1.521 (1.431 to 1.591) | 1.251 (1.078 to 1.278) | 1.341 (1.176 to 1.354) | –0.146 (–0.279 to –0.006) | –0.124 (–0.247 to 0.005) |
| Levetiracetam | 5074 (4433 to 6049) | 1.459 (1.362 to 1.517) | 1.205 (1.129 to 1.339) | 1.290 (1.233 to 1.421) | –0.045 (–0.195 to 0.095) | –0.051 (–0.183 to 0.076) |
| Complete-case data (cost, n = 178; EQ-5D, n = 225) (base-case imputed) | ||||||
| Lamotrigine | 3635 (2431 to 4828) | 1.628 (1.576 to 1.684) | 1.446 (1.367 to 1.537) | 1.507 (1.440 to 1.583) | ||
| Levetiracetam | 3294 (2063 to 4504) | 1.481 (1.418 to 1.545) | 1.316 (1.234 to 1.401) | 1.371 (1.299 to 1.444) | –0.131 (–0.244 to –0.024) | –0.136 (–0.233 to –0.045) |
| Zonisamide | 4704 (3375 to 6255) | 1.548 (1.483 to 1.601) | 1.313 (1.200 to 1.405) | 1.391 (1.296 to 1.466) | –0.003 (–0.146 to 0.112) | –0.020 (–0.094 to 0.109) |
| PP (n = 959) (base case all participants, ITT) | ||||||
| Lamotrigine | 4052 (3626 to 5023) | 1.605 (1.546 to 1.650) | 1.402 (1.315 to 1.456) | 1.470 (1.397 to 1.519) | ||
| Zonisamide | 5480 (4702 to 6826) | 1.503 (1.420 to 1.565) | 1.229 (1.114 to 1.304) | 1.320 (1.217 to 1.390) | –0.174 (–0.294 to –0.059) | –0.150 (–0.255 to –0.046) |
| Levetiracetam | 5118 (4465 to 6185) | 1.478 (1.394 to 1.523) | 1.221 (1.401 to 1.280) | 1.307 (1.202 to 1.361) | –0.007 (–0.137 to 0.111) | –0.013 (–0.131 to 0.088) |
| NEWQOL-6D (base-case EQ-5D) (costs as base case, NEWQOL-6D based on n = 132 complete cases) | ||||||
| Lamotrigine | 4042 (3626 to 4983) | 1.710 (1.687 to 1.733) | 1.508 (1.455 to 1.567) | 1.575 (1.536 to 1.600) | ||
| Levetiracetam | 5104 (4450 to 6141) | 1.703 (1.678 to 1.727) | 1.448 (1.390 to 1.488) | 1.533 (1.489 to 1.565) | –0.060 (–0.119 to –0.004) | –0.042 (–0.086 to –0.000) |
| Zonisamide | 5400 (4659 to 6770) | 1.712 (1.690 to 1.735) | 1.442 (1.368 to 1.483) | 1.532 (1.479 to 1.564) | –0.006 (–0.081 to 0.060) | –0.001 (–0.054 to 0.045) |
| EQ-VAS (base-case EQ-5D) (n = 990) | ||||||
| Lamotrigine | 4042 (3626 to 4983) | 1.431 (1.360 to 1.476) | 1.229 (1.127 to 1.281) | 1.296 (1.207 to 1.346) | ||
| Zonisamide | 5400 (4659 to 6770) | 1.418 (1.351 to 1.456) | 1.148 (1.044 to 1.200) | 1.238 (1.148 to 1.283) | –0.081 (–0.183 to 0.016) | –0.058 (–0.147 to 0.028) |
| Levetiracetam | 5104 (4450 to 6141) | 1.398 (1.324 to 1.479) | 1.142 (1.042 to 1.223) | 1.227 (1.138 to 1.308) | –0.006 (–0.102 to 0.121) | –0.011 (–0.093 to 0.105) |
| Treating blank responses in the questionnaire as missing (base case: as zero) | ||||||
| Lamotrigine | 4059 (3609 to 4901) | 1.605 (1.547 to 1.651) | 1.402 (1.329 to 1.449) | 1.470 (1.403 to 1.515) | ||
| Zonisamide | 5532 (4716 to 6754) | 1.502 (1.418 to 1.566) | 1.226 (1.120 to 1.299) | 1.318 (1.221 to 1.384) | –0.176 (–0.284 to –0.074) | –0.152 (–0.255 to –0.057) |
| Levetiracetam | 5100 (4430 to 6235) | 1.474 (1.393 to 1.523) | 1.222 (1.120 to 1.275) | 1.307 (1.216 to 1.355) | –0.003 (–0.127 to 0.105) | –0.010 (–0.120 to 0.086) |
The cost-effectiveness acceptability curve (Figure 10) indicates that the probability of levetiracetam being the most cost-effective treatment at a cost-effectiveness threshold of £20,000 per QALY is 0, and the probability of zonisamide being the most effective treatment is 0.001.
FIGURE 10.
Cost-effectiveness acceptability curve. Dashed lines represent cost-effectiveness thresholds of £20,000 per QALY and £30,000 per QALY.
Subgroup analyses
The results of the subgroup analysis for adults are consistent with the base-case analysis for the whole population (Table 16). For children, however, lamotrigine is associated with the highest costs (£5076, 97.5% CR £3815 to £7219) compared with levetiracetam (£4972, 97.5% CR £3739 to £6840) and zonisamide (£4638, 97.5% CR £3826 to £6974). Levetiracetam is associated with a higher QALY gain than lamotrigine and, therefore, lamotrigine is dominated. Zonisamide has a lower cost and lower QALY gain than levetiracetam, but also a lower NHB at a cost-effectiveness threshold of £20,000 per QALY, and is therefore not cost-effective at that threshold.
| Anti-seizure medication | Mean (97.5% CR) | |||||
|---|---|---|---|---|---|---|
| Total cost (£) | QALYs | NHB at £20,000 per QALY | NHB at £30,000 per QALY | INHB at £20,000 per QALY | INHB at £30,000 per QALY | |
| Base case (n = 990) | ||||||
| Lamotrigine | 4042 (3626 to 4983) | 1.605 (1.547 to 1.651) | 1.403 (1.319 to 1.458) | 1.470 (1.399 to 1.520) | ||
| Zonisamide | 5400 (4659 to 6770) | 1.502 (1.418 to 1.566) | 1.232 (1.112 to 1.307) | 1.322 (1.215 to 1.392) | –0.171 (–0.295 to –0.055) | –0.148 (–0.261 to –0.045) |
| Levetiracetam | 5104 (4450 to 6141) | 1.474 (1.393 to 1.523) | 1.222 (1.110 to 1.283) | 1.307 (1.204 to 1.361) | –0.010 (–0.142 to 0.112) | –0.015 (–0.136 to 0.089) |
| Children aged < 16 years (n = 155) | ||||||
| Levetiracetam | 4972 (3739 to 6840) | 1.556 (1.397 to 1.618) | 1.307 (1.097 to 1.394) | 1.390 (1.207 to 1.463) | ||
| Lamotrigine | 5076 (3815 to 7219) | 1.551 (1.432 to 1.638) | 1.297 (1.107 to 1.412) | 1.382 (1.221 to 1.481) | –0.010 (–0.171 to 0.191) | –0.009 (–0.148 to 0.173) |
| Zonisamide | 4638 (3826 to 6974) | 1.508 (1.381 to 1.610) | 1.277 (1.068 to 1.390) | 1.354 (1.176 to 1.460) | –0.020 (–0.242 to 0.175) | –0.028 (–0.214 to 0.143) |
| Adults aged ≥ 16 years (n = 835) | ||||||
| Lamotrigine | 3844 (3379 to 4478) | 1.612 (1.554 to 1.661) | 1.420 (1.346 to 1.475) | 1.484 (1.417 to 1.536) | ||
| Zonisamide | 5509 (4610 to 6866) | 1.508 (1.413 to 1.569) | 1.227 (1.101 to 1.320) | 1.319 (1.209 to 1.398) | –0.193 (–0.322 to –0.083) | –0.165 (–0.278 to –0.067) |
| Levetiracetam | 5178 (4435 to 6223) | 1.466 (1.381 to 1.518) | 1.207 (1.095 to 1.280) | 1.294 (1.193 to 1.359) | –0.020 (–0.158 to 0.112) | –0.025 (–0.149 to 0.090) |
Chapter 5 Focal epilepsy: discussion
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
For first-line treatment of focal epilepsy, zonisamide met our definition of non-inferiority for time to 12-month remission when compared with lamotrigine, but levetiracetam did not. No significant difference was found between lamotrigine and zonisamide in time to 24-month remission and time to first seizure. Both levetiracetam and zonisamide were significantly inferior to lamotrigine in terms of time to treatment failure.
It is important to highlight that the SANAD II trial was a pragmatic trial comparing the policies of initiating treatment with lamotrigine, levetiracetam or zonisamide, and that the primary analyses were on an ITT basis. This is particularly important when considering the outcomes of time to 12- and 24-month remission. The trial protocol recommended initial maintenance doses and titration rates, but, during follow-up, clinicians were able to make dose and drug changes as per usual clinical practice to maximise seizure control and minimise ARs. Therefore, it is reassuring to note that at 4 years the proportions achieving a 12-month remission were 82% (95% CI 77% to 88%) in those starting treatment with lamotrigine, 77% (95% CI 71% to 82%) in those starting treatment with levetiracetam and 84% (95% CI 78% to 90%) in those starting treatment with zonisamide.
Although the longer-term seizure outcomes are similar among the three treatment policies, levetiracetam and zonisamide are significantly more likely to fail than lamotrigine, resulting in treatment changes. The competing risks analysis shows that levetiracetam is more likely than lamotrigine to fail because of ARs (HR 0.53, 95% CI 0.35 to 0.79) and, although non-significant, the estimate also indicates a higher failure rate attributable to ISC (HR 0.67, 95% CI 0.45 to 1.01). Similarly, zonisamide is significantly more likely than lamotrigine to fail because of the ARs (HR 0.37, 95% CI 0.25 to 0.55) and, although non-significant, the estimate also indicates a higher failure rate attributable to ISC (HR 0.76, 95% CI 0.50 to 1.15). Treatment failures were taken into account in the PP analysis of time to 12-month remission, which took a competing risks approach and found lamotrigine to be superior to both levetiracetam (HR 1.32, 95% CI 1.05 to 1.66) and zonisamide (HR 1.37, 95% CI 1.08 to 1.73).
Initiating treatment with lamotrigine was associated with fewer ARs than initiating treatment with levetiracetam or zonisamide, and there were more psychiatric ARs in the groups starting on levetiracetam and zonisamide than in the group starting on lamotrigine. It is interesting to note that there were more pregnancies and miscarriages in the zonisamide group, but the numbers are too small to draw any conclusions.
The QoL analysis also found an overall better profile for lamotrigine than that for levetiracetam or zonisamide. Compared with lamotrigine, levetiracetam and zonisamide are associated with worse overall patient-reported QoL and depression. In addition, levetiracetam resulted in worse patient-reported anxiety, depression and stigma than lamotrigine or zonisamide.
The economic analysis indicated that neither levetiracetam nor zonisamide were cost-effective compared with lamotrigine, being both more costly and less effective and with lower NHBs over the 2-year time horizon of analysis. The mean total costs were £5400 (97.5% CR £4659 to £6770) for zonisamide and £5104 (97.5% CR £4450 to £6141) for levetiracetam, compared with £4042 (97.5% CR £3626 to £4983) for lamotrigine.
Levetiracetam and zonisamide were associated with lower QALY gains, at 1.474 years (97.5% CR 1.393 to 1.523 years) and 1.502 years (97.5% CR 1.418 to 1.566 years), respectively, than lamotrigine, at 1.605 years (97.5% CR 1.547 to 1.651 years).
Based on rank-ordering of NHBs, lamotrigine was highest at both the £20,000 and £30,000 per QALY thresholds of cost-effectiveness. This result was robust to a range of assumptions tested in sensitivity analyses, but although levetiracetam was the most cost-effective treatment in children, this subgroup analysis was limited by small numbers, and higher costs in the lamotrigine arm were principally attributable to a single participant who experienced an atypical medical journey.
As with the first SANAD trials,9,16 we have demonstrated that the NHS in the UK can deliver longer-term pragmatic epilepsy trials, collecting data from neurology and paediatric services as well as from primary care. Given the duration of the study, the quantity of follow-up data collected was high; completeness of follow-up statistic 77.2% lamotrigine, 78.3% levetiracetam and 75.6% zonisamide for the primary outcome. Nonetheless, the SANAD II trial has a number of limitations. Data on the occurrence of seizures were collected using seizure diaries and reports at clinic visits. This is a limitation of almost all outpatient clinical trials in epilepsy, as there is no other practical method for ascertaining the occurrence of seizures. It is therefore possible that seizures were missed or not reported, although there is no reason to expect systematic under- or over-reporting of seizures in any of the randomised groups. The SANAD II trial was unblinded, which was the only feasible way to collect longer-term follow-up data when knowledge of first treatment is required to inform future treatment decisions. This may have influenced decisions about dose and treatment changes, thereby biasing results for time to treatment failure and seizure outcomes. Knowledge of treatment allocated may also have influenced the reporting of ARs, and this should be taken into consideration when interpreting, for example, the higher rate of psychiatric events in the levetiracetam and zonisamide groups. In addition, only 17.9% of those recruited were aged < 18 years. The most likely explanation for this lower than expected percentage was the lack of experience with zonisamide among paediatricians, which may have introduced a reluctance to recruit patients to the trial. Clearly, this will limit the applicability of the results to the management of children with focal epilepsy. It is possible that the maintenance doses chosen introduce a systematics bias, but the similar rates of time to first seizure rates provide assurance that appropriate initial maintenance doses were chosen, and mitigates against concerns that the slower titration rate required for lamotrigine might expose individuals to risk of early seizure recurrence.
It is also important to acknowledge that there are no RCT data to inform the choice of initial maintenance dose of lamotrigine, levetiracetam, zonisamide or most other anti-seizure medications. Slightly more males than females were recruited (57% vs. 43%), and in the SANAD I trial we found that men had a higher 12-month remission rate than women, although it remains unproven as to whether this is a true treatment effect or due to under-reporting of seizures by males. 7 There was also a low return rate for QoL questionnaires and, although the rates of return were not unusually low for postal questionnaires, this will have diminished our ability to identify differences in QoL.
The economic analysis was limited by the poor return of self-report questionnaires. However, for costs this was largely mitigated by the acquisition of HES data and the use of follow-up CRFs for the costs of anti-seizure medicines, which were the main cost drivers. Owing to the AUC methodology, QALYs could be calculated provided that two or more EQ-5D questionnaires had been returned. However, for the NEWQOL-6D there were insufficient data to complete imputation; hence, only complete-case results could be presented. An additional limitation was that there is no tariff currently available for the EQ-5D-3L-Y or proxy version of the EQ-5D-3L and, therefore, the adult tariff was used throughout for estimating utilities from EQ-5D profiles. This represents a weakness in many economic evaluations of interventions in paediatric populations,75 although a valuation of children’s EQ-5D-3L-Y health states should soon be available. 76 Furthermore, given the chronic nature of epilepsy, the 2-year time horizon is somewhat limited and the planned analysis over a 4-year time horizon could not be conducted because of the limitations of missing data.
These results should be interpreted in context with previous studies that have assessed the longer-term effectiveness of treatments for focal epilepsies. One limitation is that most RCTs that have compared anti-seizure medication monotherapies in epilepsy have been undertaken to meet regulatory requirements and have not assessed longer-term outcomes. For example, the European Medicines Agency recommends assessing 6-month seizure remission rates in head-to-head trials with a standard treatment,77 whereas the Food and Drug Administration will not accept head-to-head non-inferiority trials because of concerns that interpretation as a finding of equivalence or non-inferiority could be due to treatments being similarly ineffective (assay sensitivity). 78
The SANAD I trial identified lamotrigine as a first-line treatment as it was non-inferior to carbamazepine for time to 12-month remission and superior to carbamazepine, gabapentin, oxcarbazepine and topiramate for time to treatment failure. 9 Lamotrigine was subsequently recommended as a first-line treatment8 and was chosen as the standard treatment for focal epilepsy in the SANAD II trial. An individual patient data network meta-analysis,15 which included data from the SANAD I trial and combined direct and indirect comparisons, used carbamazepine as the standard treatment comparator for focal epilepsy. The results indicated that levetiracetam was inferior to carbamazepine for time to 12-month remission, but was not significantly different to gabapentin, lamotrigine, phenobarbital, phenytoin (Epanutin®, Upjohn UK Ltd), oxcarbazepine, valproate or zonisamide. For time to treatment failure, lamotrigine and levetiracetam were superior to carbamazepine, phenobarbital was inferior to carbamazepine, and no difference was found between carbamazepine and the other assessed treatments. Therefore, these findings suggest that the SANAD II trial provides much needed longer-term head-to-head data to better inform treatment policy and guidance.
The SANAD II results have important implications for clinical practice and research. Although levetiracetam and zonisamide are licensed for use as monotherapy in focal epilepsy in Europe and elsewhere, these results suggest that their use as first-line monotherapy may not be supported. This is most relevant to levetiracetam, which has become a commonly prescribed first-line anti-seizure medication, based on easy titration, perceived good efficacy and an assumed low rate of ARs. The SANAD II trial also provides evidence that the slower titration of lamotrigine is not associated with a shorter time to first subsequent seizure and that levetiracetam has a higher withdrawal rate due to ARs. Further studies are required to assess the clinical effectiveness and cost-effectiveness of other newer anti-seizure medications, such as lacosamide (Vimpat®, UCB Pharma Ltd), brivaracetam (Briviact®, UCB Pharma Ltd) and perampanel (Fycompa®, Eisai Co. Ltd), and the design of future studies should be debated given that the SANAD I and SANAD II trials provide historical control data.
Recommendations for research
-
A network meta-analysis making both direct and indirect comparisons is required of all available anti-seizure monotherapy trials to provide an overview of the entirety of current evidence regarding clinical effectiveness for focal epilepsy. This work is under way and funded by the National Institute for Health Research (NIHR) to inform the current NICE epilepsy guidelines update. 79
-
An economic model is required, utilising the results of direct and indirect comparisons to estimate the comparative cost-effectiveness of currently available treatments for focal epilepsy.
-
Prognostic modelling of data from the SANAD I and SANAD II trials is required to explore subgroup effects and to better stratify patients for likely outcome at the time of initiating treatment for focal epilepsy.
-
Methodological work, utilising data from the SANAD I and SANAD II trials is required to inform the design of future trials assessing the clinical effectiveness and cost-effectiveness of anti-seizure medications in people with newly diagnosed focal epilepsy. This includes the possibility of designs using data from the SANAD I and SANAD II trials as historical controls.
-
Future trials are required to assess the clinical effectiveness and cost-effectiveness of other focal epilepsy treatments including lacosamide, brivaracetam, perampanel and clobazam.
In conclusion, the SANAD II results indicate that lamotrigine should remain a first-line standard treatment for focal epilepsy and that neither levetiracetam nor zonisamide should be used as routine first-line anti-seizure medications.
Chapter 6 Generalised and unclassified epilepsy: clinical results
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Recruitment and baseline characteristics
The first participant was randomised on 30 April 2013 and the last participant was randomised on 2 August 2016 (see Appendix 3, Figure 25), after which every effort was made to follow the trial cohort for a further 2 years, and the last participant visit was on 13 January 2019. Sixty-nine UK centres recruited between 1 and 40 patients each, and randomised a total of 520 participants: 260 to start treatment with levetiracetam and 260 to start treatment with valproate (Figure 11). Baseline characteristics were well balanced across treatment groups (Table 17 and see Appendix 3, Table 37). The median age of participants was 13.9 years (IQR 8.9–19.7 years) with a predominance of males (64.8%), reflecting concern about randomising females to valproate. Approximately 10% of patients had a learning disability, 3.1% a neurological deficit and 19% had a first-degree relative with epilepsy. Approximately three-quarters of the participants had generalised epilepsy and in the remainder the type of epilepsy was unclassified. Of those with generalised epilepsy (397), 26.2% had childhood absence epilepsy, 9.1% juvenile absence epilepsy, 12.8% juvenile myoclonic epilepsy, 5.8% generalised epilepsy with tonic–clonic seizures on waking and 45.3% were classified as having ‘idiopathic generalised epilepsy not specified’. Participants were randomised a median of 4 days (IQR 0–26 days) after their most recent seizure.
FIGURE 11.
The CONSORT participant flow diagram: generalised and unclassified epilepsy trial.
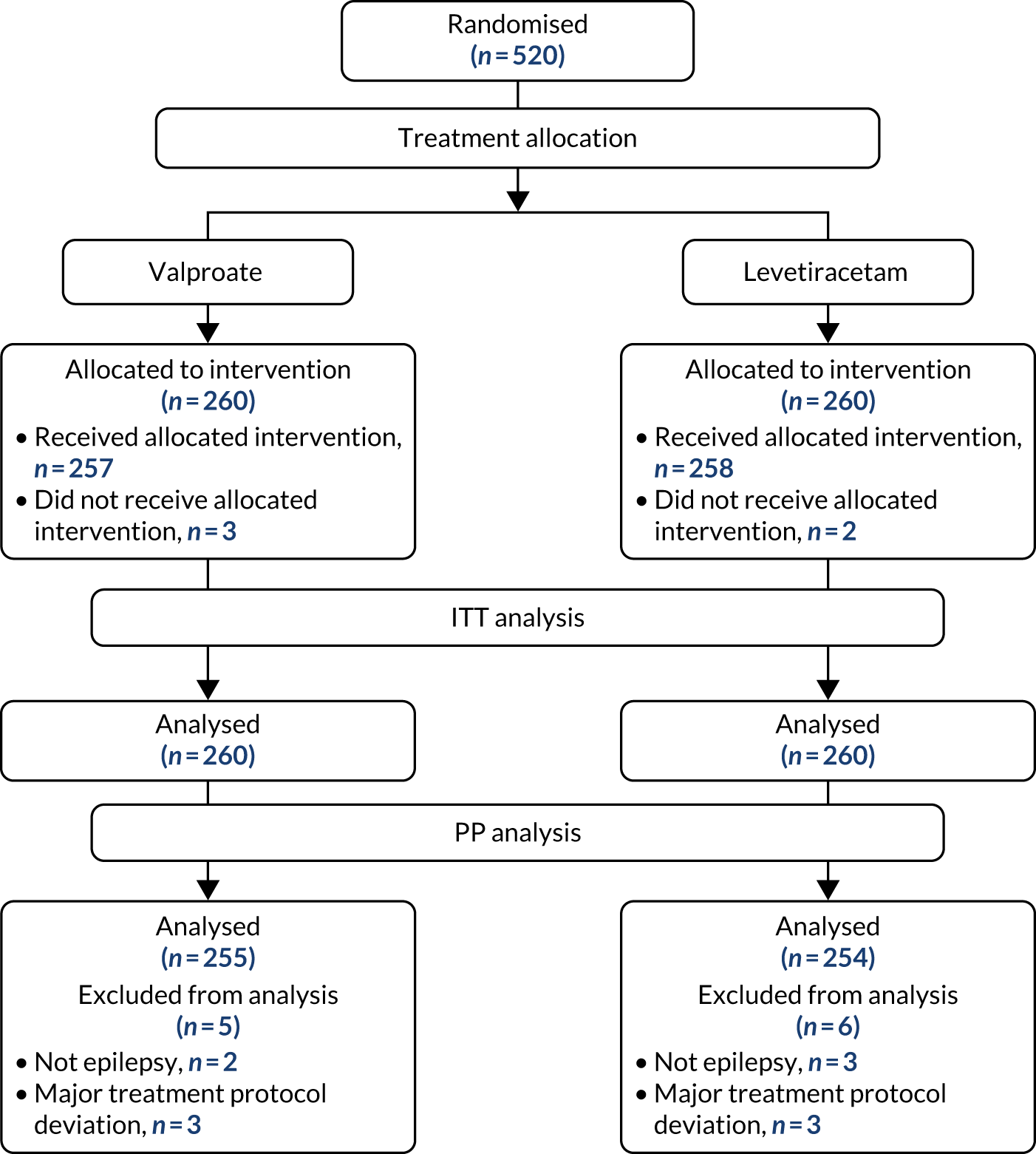
| Characteristic | Valproate group | Levetiracetam group | Total |
|---|---|---|---|
| Number of participants | 260 | 260 | 520 |
| Age (years) | |||
| Mean (SD) | 17.1 (12.9) | 16.9 (11.8) | 17.0 (12.3) |
| Median (IQR) | 13.6 (8.8–19.7) | 14.1 (9.1–19.8) | 13.9 (8.9–19.7) |
| Range | 5.0–94.4 | 5.0–83.9 | 5.0–94.4 |
| Age group (years), n (%) | |||
| 5–7 | 52 (20.0) | 48 (18.5) | 100 (19.2) |
| 8–11 | 54 (20.8) | 56 (21.5) | 110 (21.2) |
| 12–15 | 54 (20.8) | 48 (18.5) | 102 (19.6) |
| 16–29 | 70 (26.9) | 81 (31.2) | 151 (29.0) |
| ≥ 30 | 30 (11.5) | 27 (10.4) | 57 (11.0) |
| Gender, n (%) | |||
| Male | 167 (64.2) | 170 (65.4) | 337 (64.8) |
| History, n (%) | |||
| Learning disability | 22 (8.5) | 29 (11.2) | 51 (9.8) |
| Febrile convulsions | 21 (8.1) | 23 (8.8) | 44 (8.5) |
| Acute symptomatic seizures | 4 (1.5) | 10 (3.8) | 14 (2.7) |
| History of epilepsy in primary relatives | 49 (18.8) | 50 (19.2) | 99 (19.0) |
| Neurological deficit | 6 (2.3) | 10 (3.8) | 16 (3.1) |
| Previous or current neurological disorder, n (%) | |||
| Stroke/cerebrovascular | 0 | 0 | 0 |
| Cerebral haemorrhage | 0 | 2 (0.8) | 2 (0.4) |
| Intracranial surgery | 0 | 2 (0.8) | 2 (0.4) |
| Head injurya | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Meningitis/encephalitis | 4 (1.5) | 0 | 4 (0.8) |
| Cortical dysplasia/developmental anomaly | 0 | 0 | 0 |
| Other | 11 (4.2) | 13 (5.0) | 24 (4.6) |
| Epilepsy type, n (%) | |||
| Generalised | 201 (77.3) | 196 (75.4) | 397 (76.3) |
| Unclassified | 59 (22.7) | 64 (24.6) | 123 (23.7) |
| Epilepsy syndrome (generalised epilepsy only),b n (%) | |||
| Childhood absence | 52 (25.9) | 52 (26.5) | 104 (26.2) |
| Juvenile absence | 22 (10.9) | 14 (7.1) | 36 (9.1) |
| Juvenile myoclonic | 24 (11.9) | 27 (13.8) | 51 (12.8) |
| Epilepsy with tonic–clonic seizures on awakening | 11 (5.5) | 12 (6.1) | 23 (5.8) |
| Other idiopathic generalised epilepsy not specifiedc | 90 (44.8) | 90 (45.9) | 180 (45.3) |
| Other epilepsy syndrome | 10 (5.0) | 7 (3.6) | 17 (4.3) |
| Seizure history, median (IQR) | |||
| Total number of seizures reported | 10 (3–99+) | 10 (3–99+) | 10 (3–99+) |
| Days since first seizure | 203 (98–665) | 250 (110–603) | 228 (100–648) |
| Days since most recent seizure | 4 (0–26) | 4 (0–25) | 4 (0–26) |
| EEG, n (%) | |||
| EEG not done | 20 (7.7) | 24 (9.2) | 44 (8.5) |
| EEG normal | 58 (22.3) | 51 (19.6) | 109 (21.0) |
| Non-specific abnormality | 11 (4.2) | 9 (3.5) | 20 (3.8) |
| Generalised abnormality: slow wave activity with spiking | 138 (53.1) | 133 (51.2) | 271 (52.1) |
| Generalised abnormality: slow wave activity without spiking | 8 (3.1) | 7 (2.7) | 15 (2.9) |
| Focal abnormality: paroxysmal slow activity with spiking | 10 (3.8) | 8 (3.1) | 18 (3.5) |
| Focal abnormality: paroxysmal slow activity without spiking | 2 (0.8) | 7 (2.7) | 9 (1.7) |
| Other | 13 (5.0) | 21 (8.1) | 34 (6.5) |
The completeness of follow-up statistic (see Appendix 3, Table 38 and Figure 26) for the primary outcome was 87% for valproate and 83% for levetiracetam. In this analysis, the median number of days of follow-up was 427 (IQR 365–731) days for the valproate group and 550 (IQR 366–781) days for the levetiracetam group; follow-up was shorter in the valproate group, as participants allocated valproate achieved the primary outcome sooner.
Time to 12-month remission
For the ITT analysis of time to 12-month remission, there is insufficient evidence to conclude non-inferiority of levetiracetam compared with valproate, as the 95% CI for the HR (unadjusted: 1.19, 95% CI 0.96 to 1.47; adjusted: 1.23, 95% CI 0.99 to 1.52) includes the predefined non-inferiority margin of 1.314. There is crossing of the Kaplan–Meier survival curves (Figure 12) and evidence of non-equality of HRs across time (p = 0.001), violating the assumption of proportional hazards. To supplement presentation of the average treatment effect over time, we also present interval-specific HR estimates (Table 18), which indicate a significant beneficial effect of initiating treatment with valproate within the first year. Thereafter there are no statistically significant effects but the direction of benefit is in favour of valproate during the interval from 1 to 2 years and in favour of levetiracetam during the interval from 2 to 3 years. These results should be viewed cautiously, as they are sensitive to interval choice and subject to selection bias. We also present the annual difference in 12-month remission probabilities (Table 19) showing, for example, that at 1 year the proportion of patients entering 12-month remission was 9% lower in the levetiracetam group than in the valproate group. The Kaplan–Meier estimate of the median number of days to achieve 12-month remission was also shorter for valproate (445 days, 95% CI 406 to 531 days) than for levetiracetam (636 days, 95% CI 553 to 728 days).
FIGURE 12.
Kaplan–Meier plot of time to 12-month remission ITT analysis: levetiracetam vs. valproate for generalised or unclassified epilepsy.
| Model and analysis set | Time interval | HR (95% CI) | p-value for equality of HR over time |
|---|---|---|---|
| Primary analysis: Cox model with treatment (ITT) | All follow-upa | 1.19 (0.96 to 1.47) | < 0.01 |
| Cox model with treatment (ITT), gender, number of seizures and centre as random effects | All follow-upa | 1.23 (0.99 to 1.52) | < 0.01 |
| Cox model with interaction between treatment and categorical time intervals (ITT) | ≤ 1 | 1.68 (1.07 to 2.62) | 0.03 |
| (1–2) | 1.26 (0.95 to 1.66) | ||
| (2–3) | 0.57 (0.31 to 1.06) | ||
| > 3 yearsa | 0.70 (0.23 to 2.09) | ||
| Cox model with epilepsy type (generalised/unclassified) | All follow-upa | 1.19 (0.96 to 1.47) | < 0.01 |
| Fine and Gray model44 with treatment (PP) | All follow-upa | 1.68b (1.30 to 2.15) | 0.51 |
| Outcome variable | Events/total | Year 1 | Year 2 | Year 3 | Year 4 |
|---|---|---|---|---|---|
| 12-month remission, number at risk | |||||
| Valproate | 175/260 | 240 | 76 | 35 | 13 |
| Levetiracetam | 164/260 | 234 | 86 | 31 | 11 |
| Percentage 12-month remission (95% CI) | |||||
| Valproate | 36 (30 to 42) | 64 (58 to 71) | 73 (67 to 79) | 79 (72 to 85) | |
| Levetiracetam | 26 (21 to 32) | 57 (50 to 64) | 74 (67 to 80) | 82 (75 to 88) | |
| Difference in percentage of 12-month remission: levetiracetam compared with valproate (95% CI) | –9 (–18 to –1) | –7 (–16 to 2) | 0 (–8 to 9) | 3 (–6 to 12) | |
| Time to 24-month remission, number at risk | |||||
| Valproate | 103/260 | 240 | 213 | 55 | 19 |
| Levetiracetam | 76/260 | 234 | 192 | 61 | 17 |
| Percentage 24-month remission (95% CI) | |||||
| Valproate | 30 (24 to 36) | 49 (42 to 56) | 55 (47 to 63) | ||
| Levetiracetam | 18 (13 to 24) | 40 (32 to 47) | 51 (41 to 60) | ||
| Difference in percentage of 24-month remission: levetiracetam compared with valproate (95% CI) | –12 (–20 to –4) | –10 (–20 to 1) | –4 (–17 to 8) | ||
| Time to first seizure, number at risk | |||||
| Valproate | 188/260 | 86 | 64 | 22 | 4 |
| Levetiracetam | 197/260 | 62 | 35 | 11 | 3 |
| Percentage seizure free (95% CI) | |||||
| Valproate | 36 (30 to 42) | 31 (25 to 36) | 23 (17 to 29) | 21 (14 to 27) | |
| Levetiracetam | 27 (22 to 33) | 21 (16 to 26) | 18 (13 to 24) | 18 (13 to 24) | |
| Difference in percentage seizure free: levetiracetam compared with valproate (95% CI) | –8 (–17 to 0) | –9 (–17 to –2) | –5 (–13 to 4) | –2 (–11 to 6) | |
The PP analyses for time to 12-month remission (Figure 13 and see Table 18) excluded, patients with major protocol deviations (1%) and patients later diagnosed as ‘not epilepsy’ (1%) and accounted for treatment failures prior to achieving 12-month remission (32% valproate, 47% levetiracetam). This analysis indicates superiority of valproate over levetiracetam (HR 1.68, 95% CI 1.30 to 2.15). Furthermore, the assumption of constant HR across time appears reasonable in the PP analysis, suggesting that treatment failures prior to remission largely explain the non-constant effect seen in the ITT analysis.
FIGURE 13.
Cumulative incidence of time to 12-month remission from competing risks PP analysis: levetiracetam vs. valproate for generalised or unclassified epilepsy.
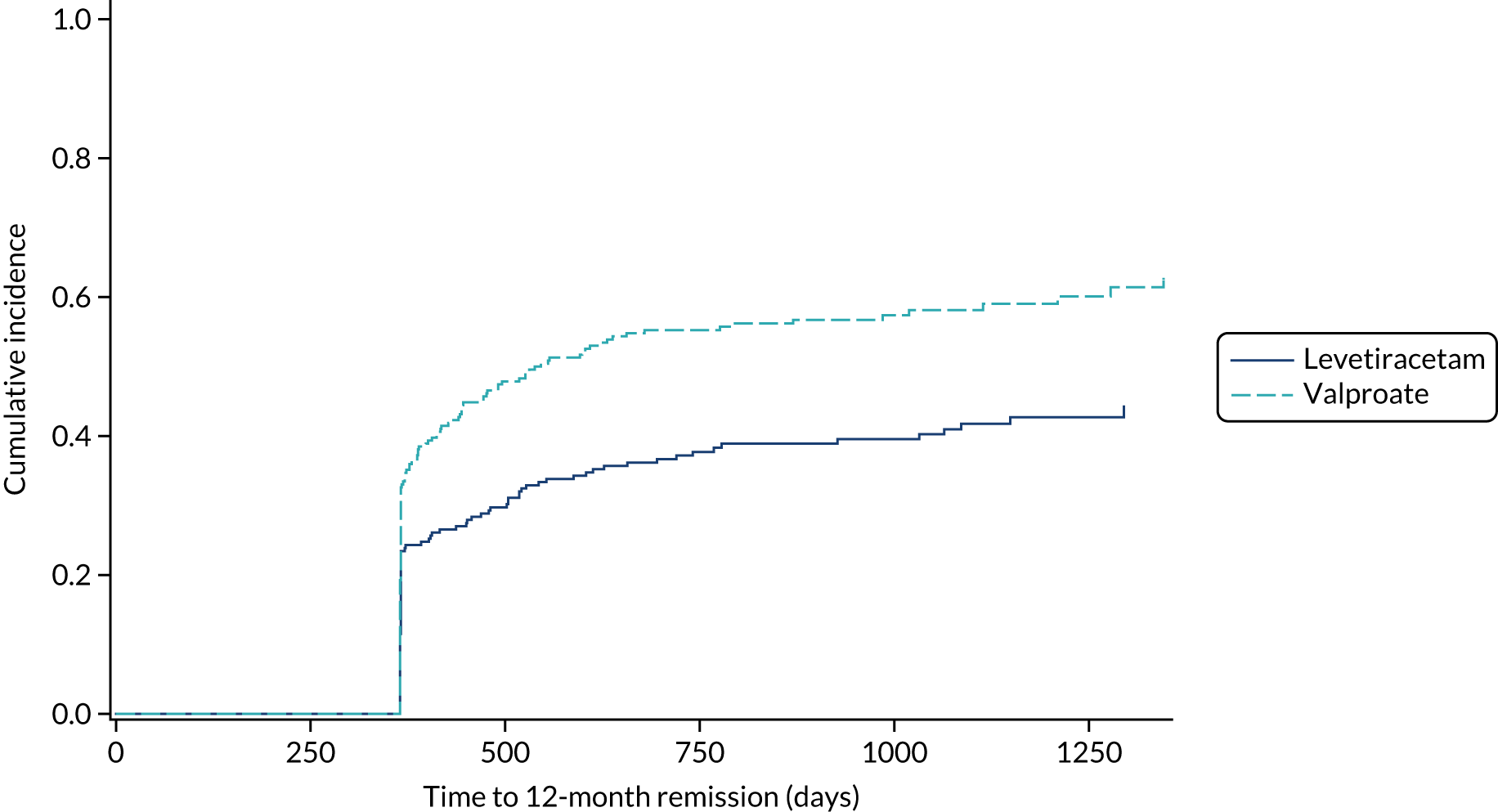
Additional prespecified sensitivity analyses, the results of which are detailed in Appendix 3, Table 39, did not change the conclusions of the primary analysis.
Subgroup effects were explored in a post hoc analysis (Figure 14) and indicate an important advantage for initiating valproate in those with other idiopathic generalised epilepsies (HR 1.55, 95% CI 1.14 to 2.11), as the difference in immediate 12-month remission rates was 19.1% (95% CI 6.6% to 31.7%), but not for absence epilepsies (HR 0.90, 95% CI 0.60 to 1.35) or unclassified epilepsy (HR 1.07, 95% CI 0.69 to 1.67).
FIGURE 14.
Kaplan–Meier plots of time to 12-month remission epilepsy type subgroup analysis: levetiracetam vs. valproate. (a) Absence epilepsy; (b) other generalised epilepsy; and (c) unclassified epilepsy.
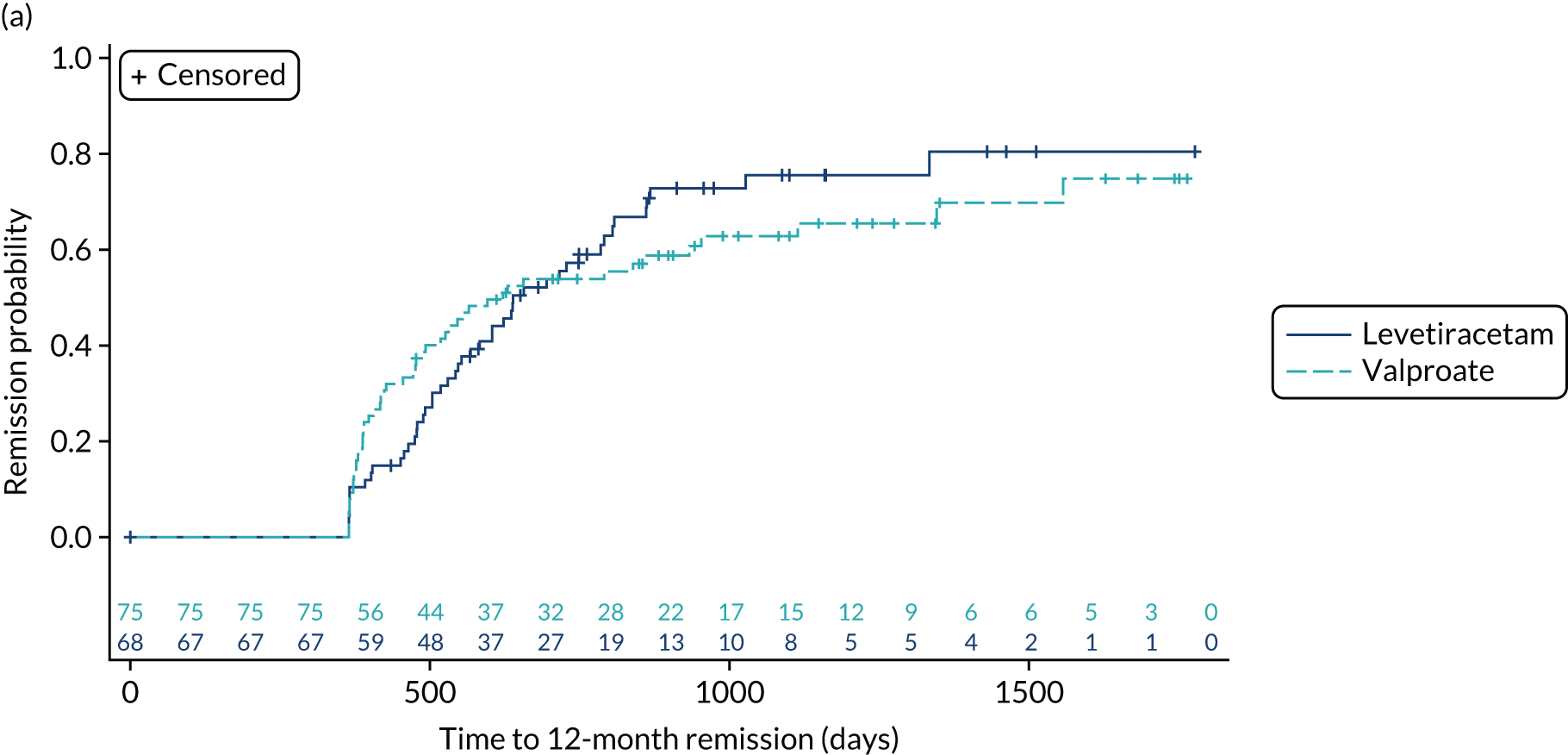
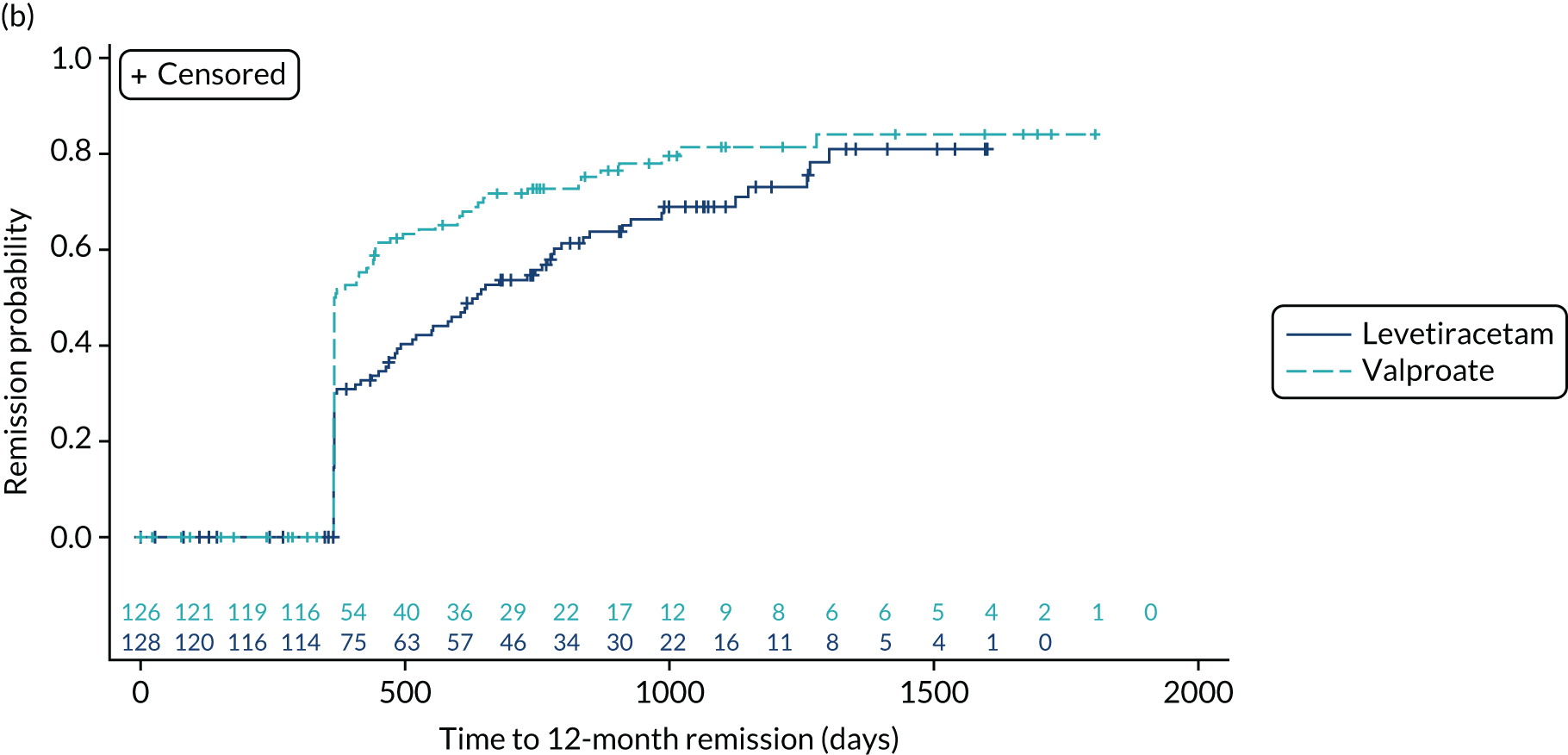
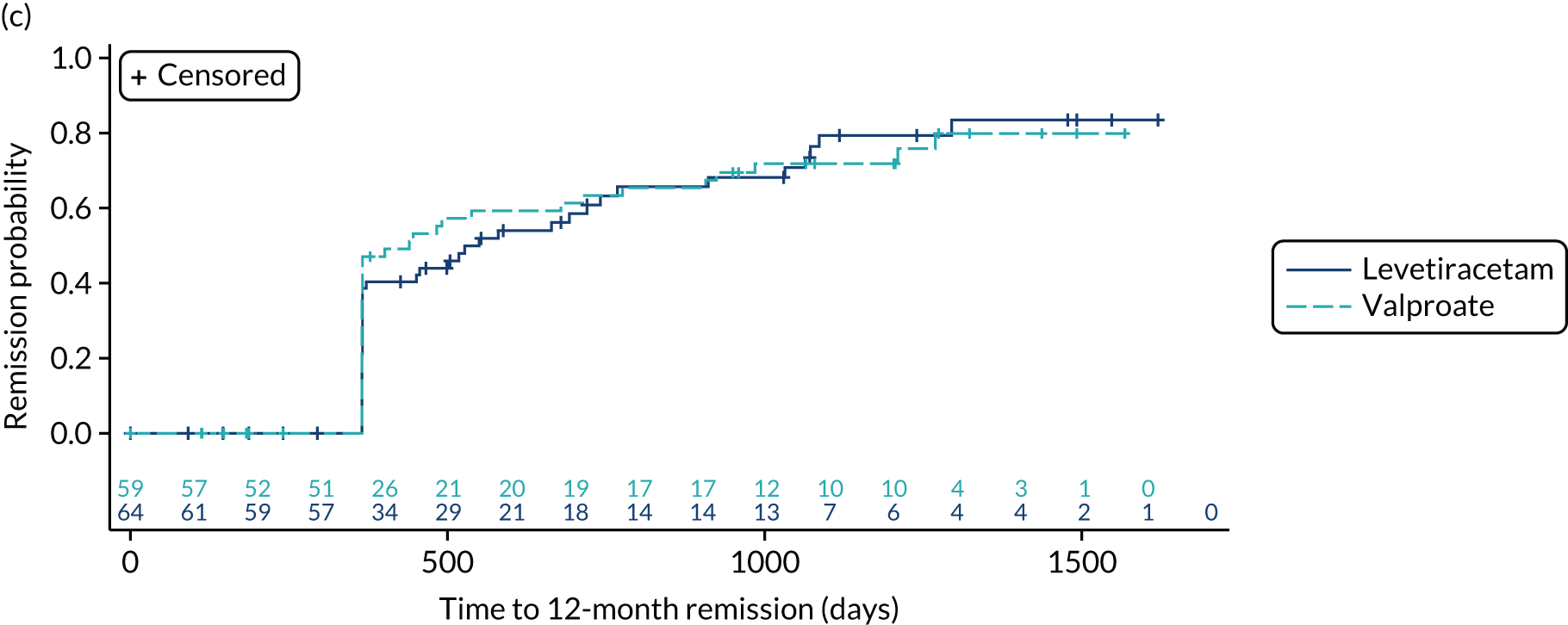
Time to 24-month remission
In the ITT analysis of time to 24-month remission, initiating treatment with valproate was superior to initiating treatment with levetiracetam (HR 1.43, 95% CI 1.06 to 1.92). As with time to 12-month remission, there is crossing of the Kaplan–Meier survival curves (Figure 15) and evidence of non-equality of HRs across time (p = 0.002), suggesting a violation of the assumption of proportional hazards. The difference in 24-month remission rates was –12% (95% CI –20% to –4%) at 24 months’ follow-up, diminishing to –4% (95% CI –17% to 8%) at 4 years.
FIGURE 15.
Kaplan–Meier plot of time to 24-month remission: levetiracetam vs. valproate for generalised or unclassified epilepsy.
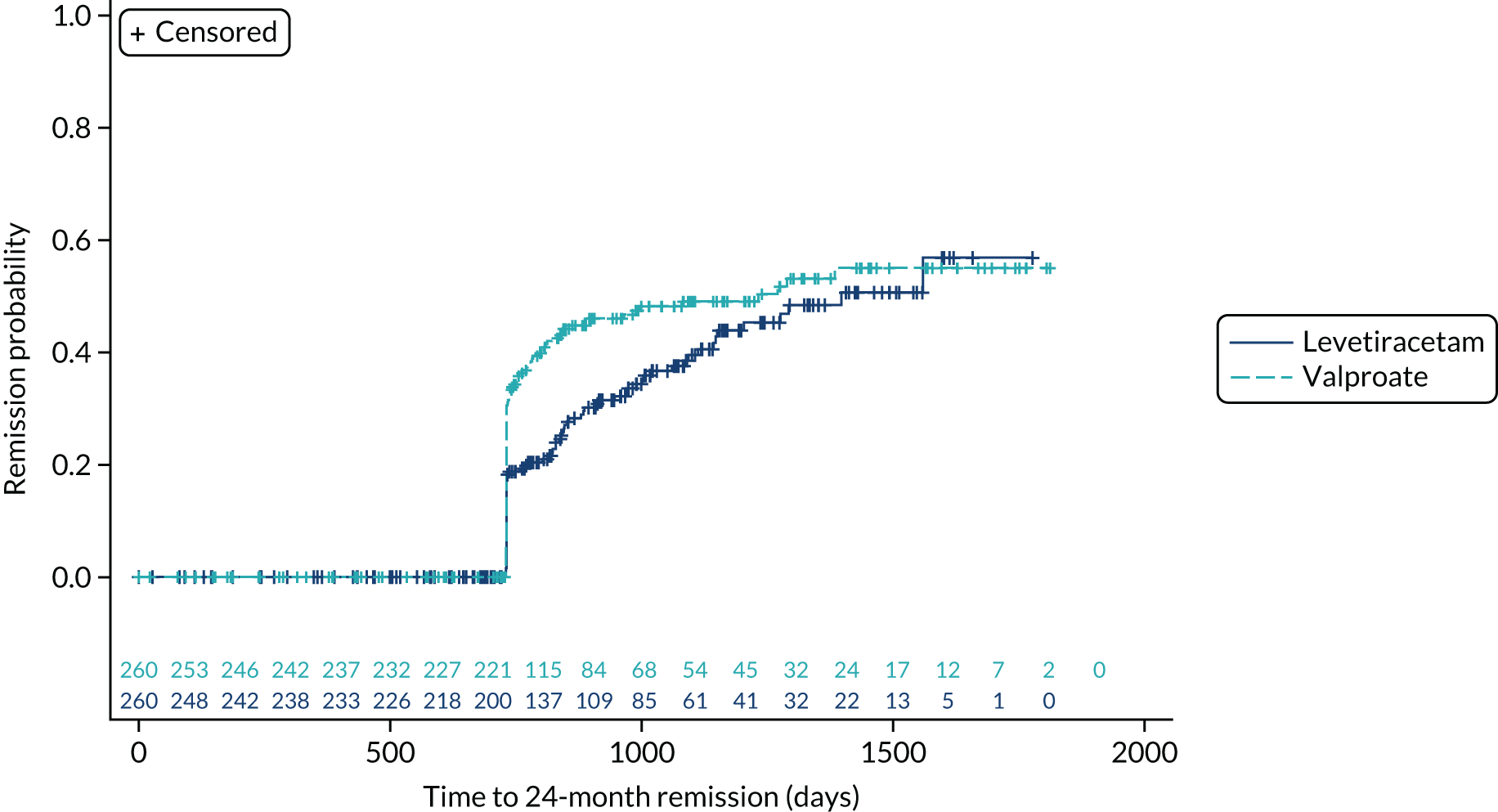
Time to first seizure
For time to first seizure (Figure 16), valproate was superior to levetiracetam (HR 0.82, 95% CI 0.67 to 1.00), and there was insufficient evidence to suggest a violation of the assumption of proportional hazards (p = 0.39) – most likely because this analysis would not be affected by treatment failures for inadequate seizure control.
FIGURE 16.
Kaplan–Meier plot of time to first seizure: levetiracetam vs. valproate for generalised or unclassified epilepsy.
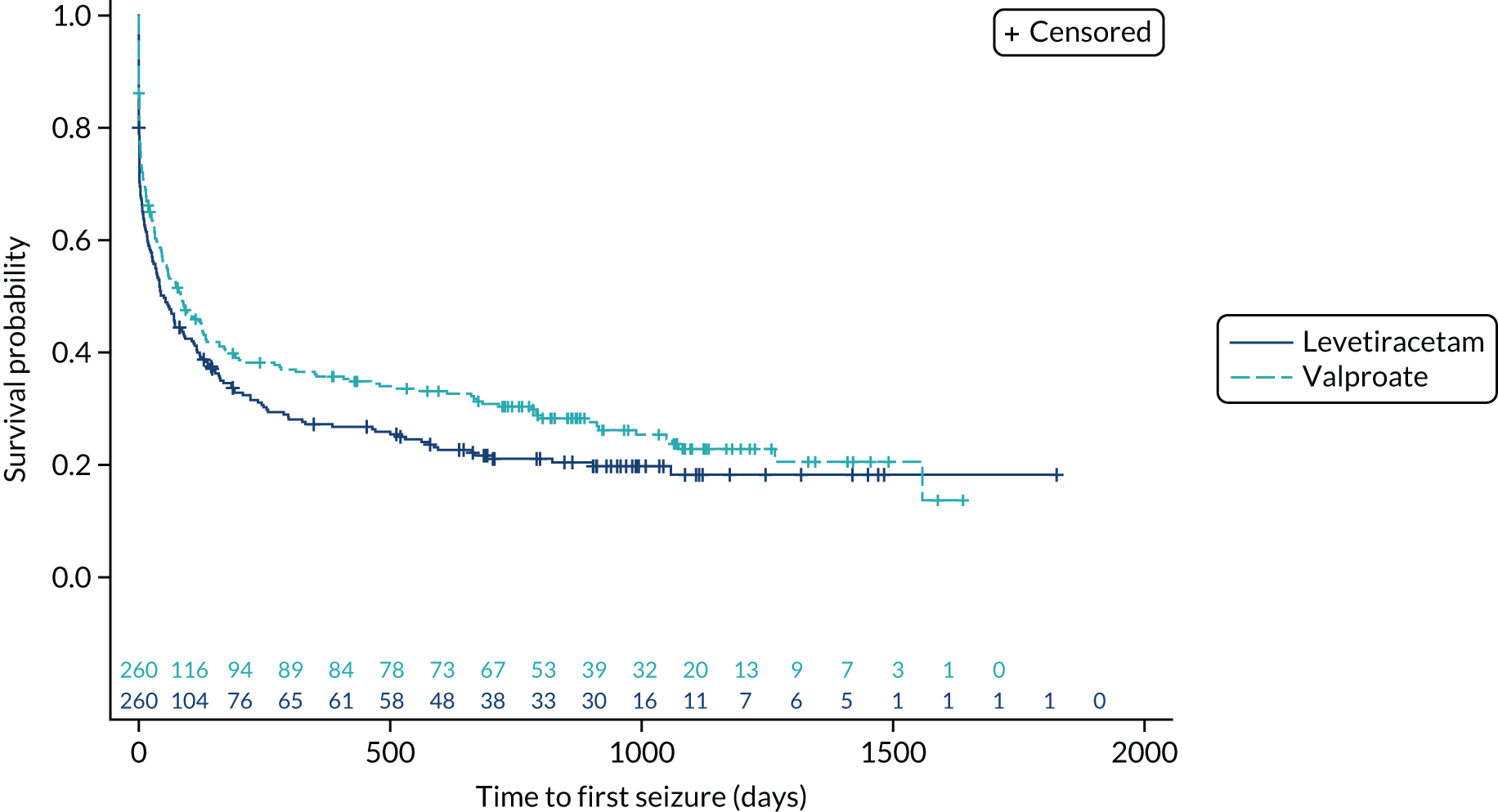
Time to treatment failure
The analysis of overall time to treatment failure for any reason (Figure 17) shows a significant benefit for valproate (HR 0.65, 95% CI 0.50 to 0.83), with no evidence to suggest a violation of the assumption of proportional hazards (p = 0.22). Table 20 provides annual treatment failure rates and differences in failure rates between valproate and levetiracetam. At 2 years there was a difference of –15% (95% CI –23% to –6%) in the treatment failure rate on levetiracetam compared with the treatment failure rate on valproate.
FIGURE 17.
Kaplan–Meier plot of time to treatment failure: levetiracetam vs. valproate for generalised or unclassified epilepsy.
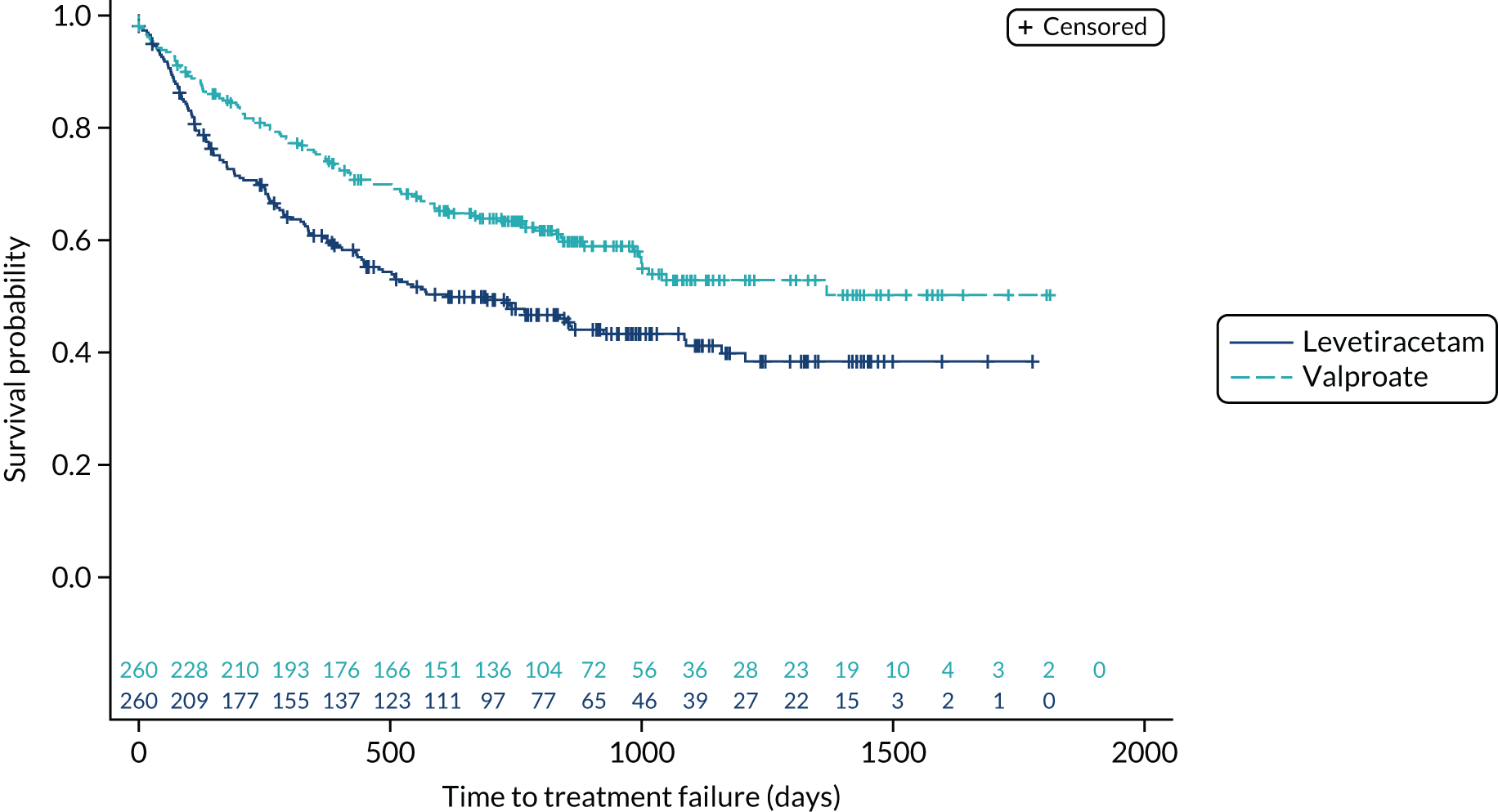
| Outcome variable | Events/total | Year 1 | Year 2 | Year 3 | Year 4 |
|---|---|---|---|---|---|
| Time to treatment failure, number at risk | |||||
| Valproate | 105/260 | 185 | 129 | 39 | 13 |
| Levetiracetam | 138/260 | 144 | 92 | 39 | 6 |
| Percentage without failure (95% CI) | |||||
| Valproate | 75 (69 to 80) | 63 (57 to 69) | 53 (45 to 60) | 50 (42 to 59) | |
| Levetiracetam | 61 (55 to 67) | 49 (42 to 55) | 41 (34 to 48) | 38 (31 to 46) | |
| Difference in percentage without failure: levetiracetam compared with valproate (95% CI) | –14 (–22 to –6) | –15 (–23 to –6) | –12 (–22 to –2) | –12 (–23 to 0) | |
| Time to treatment failure for UAR (from competing risk cumulative incidence function): percentage without failure (95% CI) | |||||
| Valproate | 87 (83 to 91) | 83 (78 to 87) | 81 (76 to 86) | 78 (70 to 86) | |
| Levetiracetam | 85 (80 to 89) | 81 (76 to 86) | 80 (75 to 85) | 80 (75 to 85) | |
| Difference in percentage without failure: levetiracetam compared with valproate (95% CI) | –3 (–9 to 4) | –2 (–9 to 5) | –1 (–8 to 6) | 2 (–7 to 11) | |
| Time to treatment failure for ISC (from competing risk cumulative incidence function): percentage without failure (95% CI) | |||||
| Valproate | 91 (87 to 94) | 86 (81 to 90) | 80 (74 to 86) | 80 (74 to 86) | |
| Levetiracetam | 78 (73 to 83) | 71 (65 to 77) | 64 (57 to 71) | 61 (53 to 69) | |
| Difference in percentage without failure: levetiracetam compared with valproate (95% CI) | –13 (–20 to –7) | –15 (–22 to –8) | –16 (–26 to –7) | –19 (–29 to –9) | |
Table 21 summarises the doses taken at treatment failure or last follow-up and indicate that reasonable dose ranges were tried before deciding failure had occurred. The competing risks analysis shows that this difference is predominantly driven by failures due to ISC with a subdistribution (HR 0.43, 95% CI 0.30 to 0.63) (Figure 18), whereas there is little difference between treatments for treatment failure due to unacceptable ARs (HR 0.93, 95% CI 0.61 to 1.40) (Figure 19).
FIGURE 18.
Cumulative incidence of treatment failure for ISC from competing risks analysis: levetiracetam vs. valproate for generalised or unclassified epilepsy.
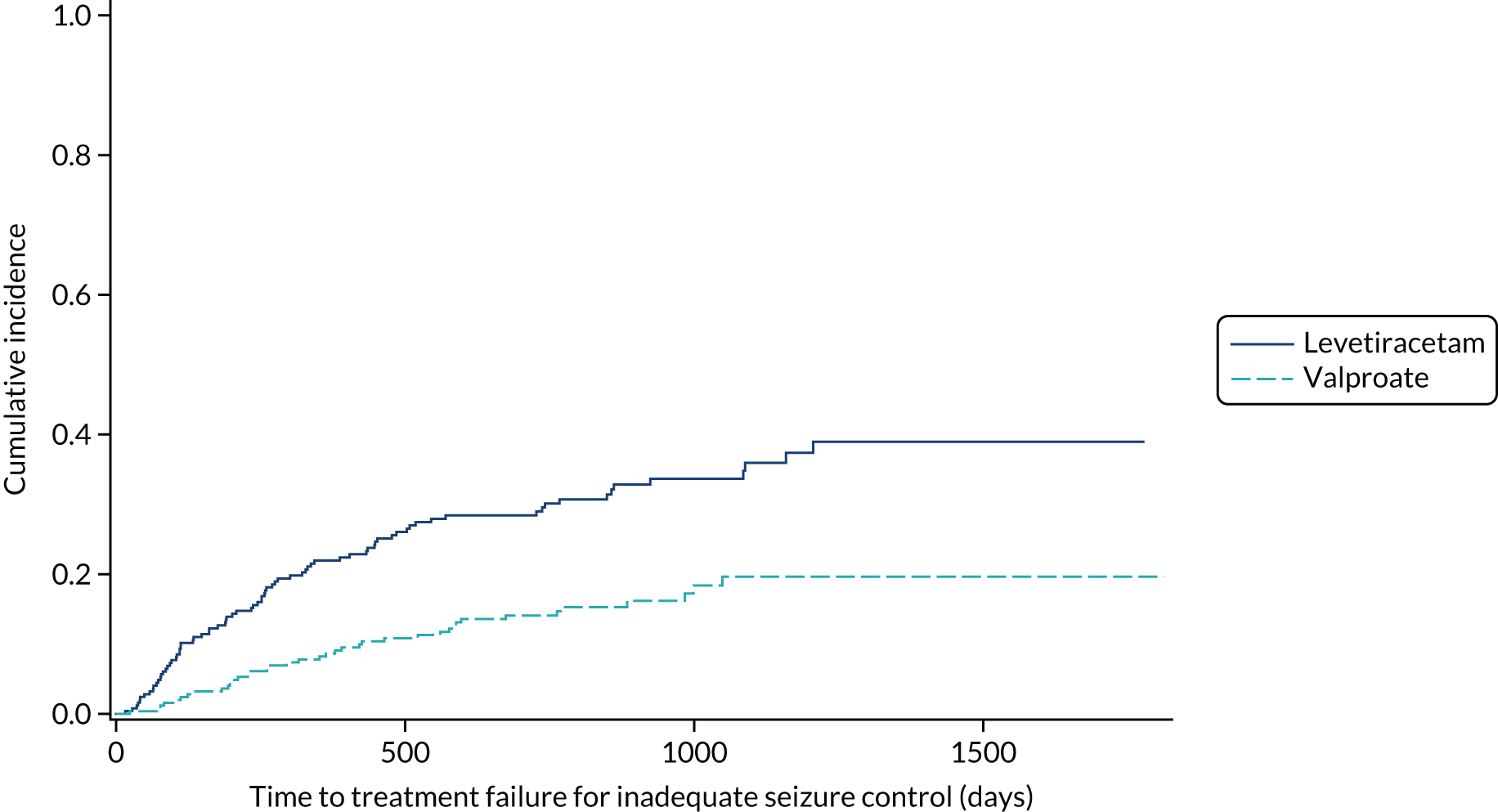
FIGURE 19.
Cumulative incidence of treatment failure due to UARs from competing risks analysis: levetiracetam vs. valproate for generalised or unclassified epilepsy.
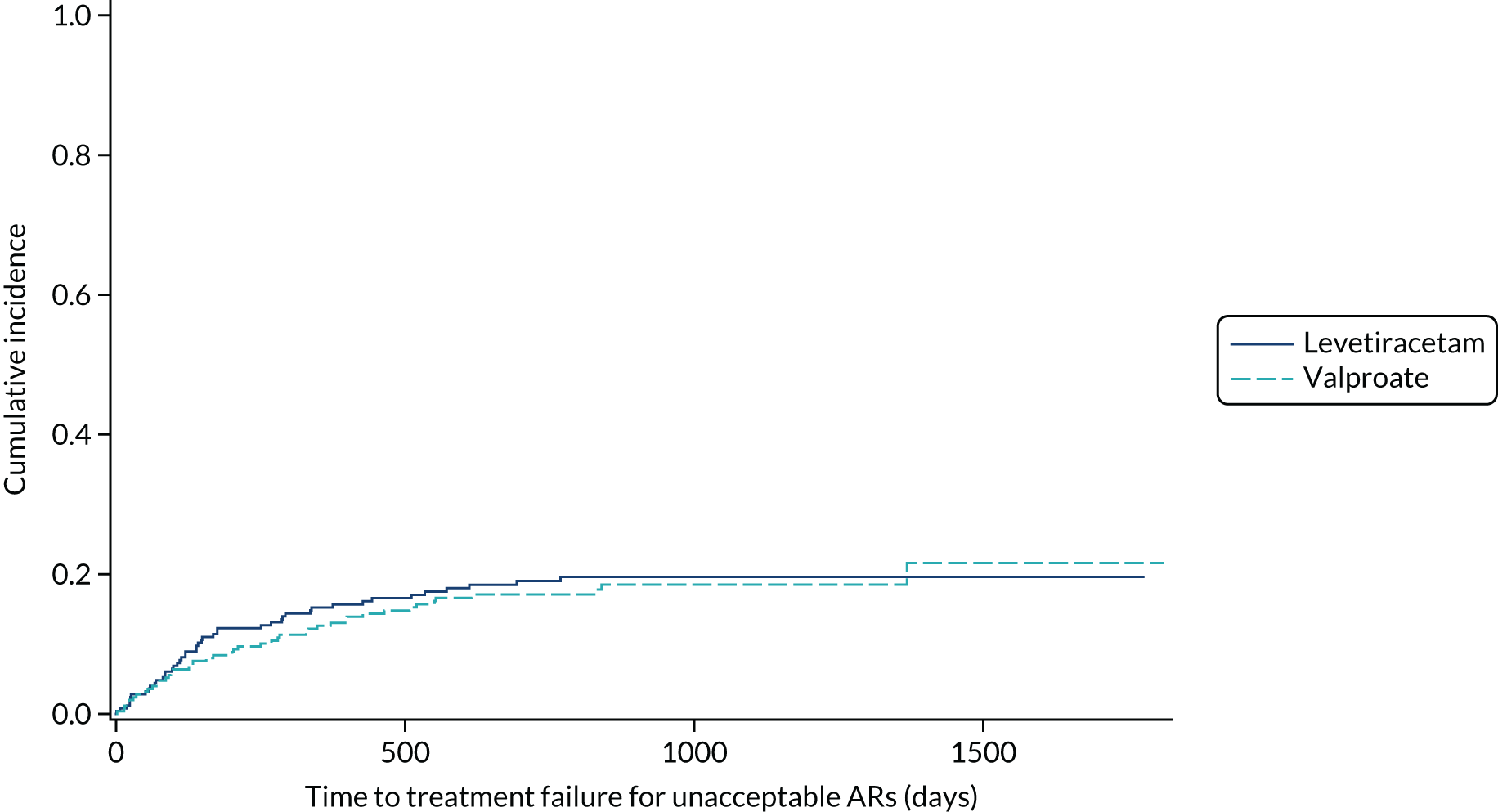
| Reason for withdrawal | Valproate group | Levetiracetam group |
|---|---|---|
| Inadequate seizure control | n = 9 | n = 27 |
| First follow-up/missinga | 0 | First follow-up = 1 |
| Mean (SD) (mg) | 1100 (397) | 2304 (866) |
| Range (mg) | 400–1700 | 1000–4000 |
| Unacceptable AEs | n = 28 | n = 28 |
| First follow-up/missing | First follow-up = 6 | First follow-up = 4 |
| Mean (SD) (mg) | 1227 (458) | 1417 (786) |
| Range (mg) | 600–2000 | 250–3000 |
| Other reason for withdrawal | n = 16 | n = 9 |
| First follow-up/missing | First follow-up = 5 | First follow-up = 6 |
| Mean (SD) (mg) | 1036 (518) | 1500 (1323) |
| Range (mg) | 500–2400 | 500–3000 |
| Remission of seizures | n = 25 | n = 15 |
| First follow-up/missing | First follow-up = 1 | 0 |
| Mean (SD) (mg) | 894 (312) | 1435 (629) |
| Range (mg) | 200–1500 | 750–3000 |
| Still on randomised drug | n = 100 | n = 96 |
| First follow-up/missing | Missing = 6 | Missing = 6 |
| Mean (SD) (mg) | 1129 (424) | 1331 (606) |
| Range (mg) | 400–3000 | 250–3000 |
The HR for overall treatment failure was not consistent across epilepsy types (Figure 20), with a significant benefit for valproate for absence (HR 0.58, 95% CI 0.37 to 0.89) and other generalised types of epilepsy (HR 0.44, 95% CI 0.30 to 0.66) but not for unclassified epilepsy (HR 1.44, 95% CI 0.85 to 2.45; test for interaction p = 0.002). The competing risks analysis shows a similar pattern of effects for failure due to ISC [absence epilepsies, HR 0.35 (95% CI 0.19 to 0.63); other generalised eplipsy, HR 0.27 (95% CI 0.14 to 0.49); and unclassified epilepsy, HR 2.15 (95% CI 0.79 to 5.86)]. There was no difference between treatments in failure due to UARs by epilepsy type.
FIGURE 20.
Hazard ratios for treatment failure by subgroups: absence epilepsies, other idiopathic generalised epilepsy and unclassified epilepsy. (a) Time to treatment failure any reason; (b) time to treatment failure for UARs; and (c) time to treatment failure for ISC. IV, inverse variance.
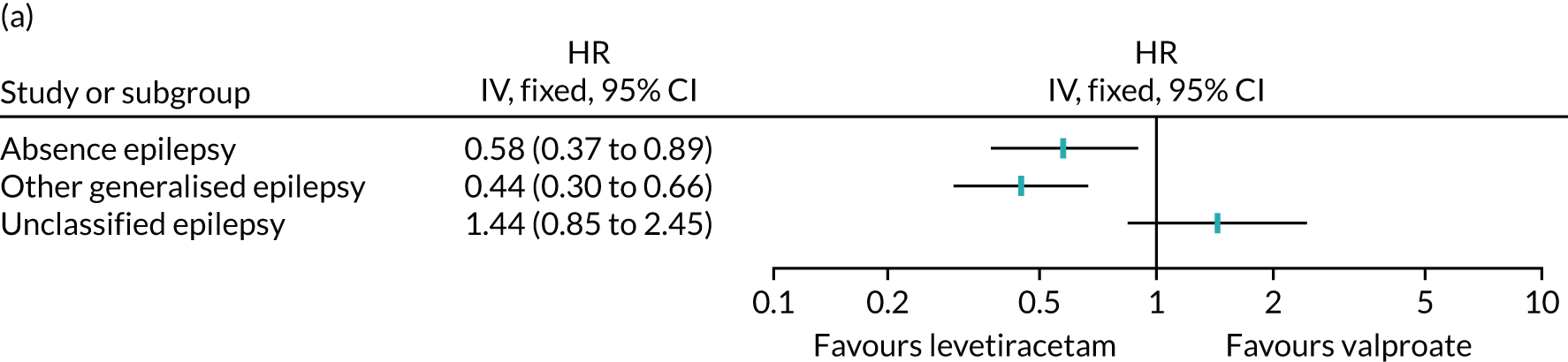
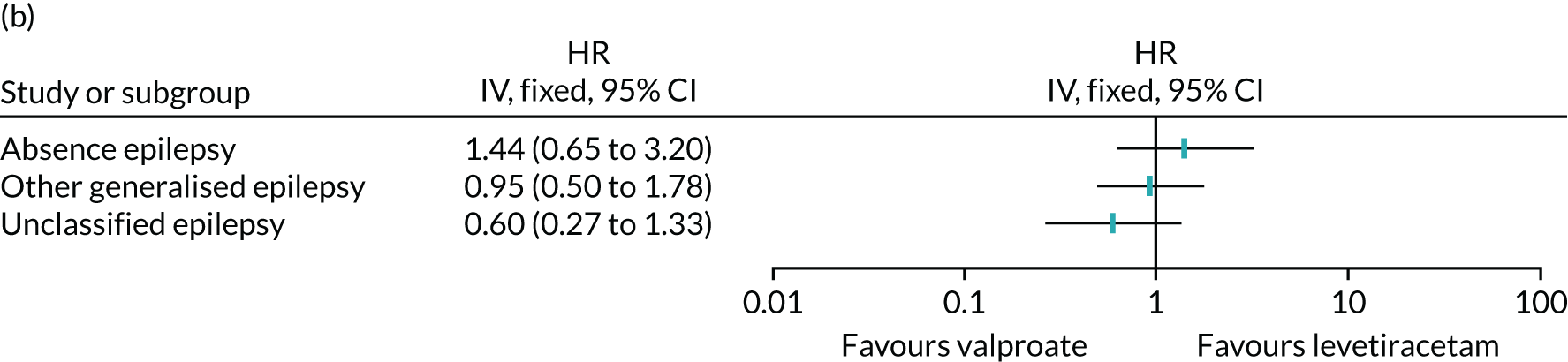
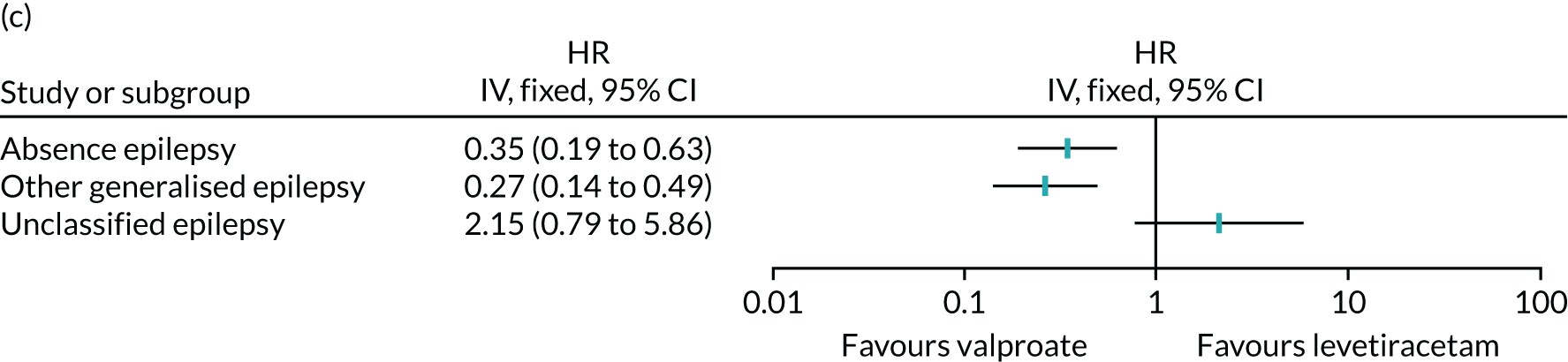
Safety
The SANAD II trial recorded data on ARs, which were AEs judged by the treating clinicians to be possibly, probably or definitely caused by anti-seizure medicines. ARs according to the MedDRA System Organ Classification are shown in Table 22, and Appendix 3, Table 40, shows ARs by MedDRA-preferred term. There were 220 ARs experienced by 96 (37.4%) patients who were randomised to start treatment with valproate and 223 ARs experienced by 107 patients (41.5%) randomised to start treatment with levetiracetam. The profile of ARs is different from most notably psychiatric symptoms reported in those allocated to levetiracetam (109 events, 66 participants) compared with those allocated to valproate (54 events, 36 participants). There were more reports of weight gain in those starting on valproate (26 participants; 10.1%) than in those starting on levetiracetam (eight participants; 3.1%). Of those randomised to start treatment with valproate, 10 (3.9%) had a total of 15 severe ARs and, of those randomised to start treatment with levetiracetam, 10 (3.5%) had a total of 16 severe ARs. For two (0.8%) patients on valproate and four (1.6%) patients on levetiracetam, the ARs were classified as serious (see Appendix 3, Table 41). None of the ARs were classified as SUSARs. There were two deaths, one in each group, that were unrelated to trial treatments (see Appendix 3, Table 42). One participant randomised to start treatment with valproate became pregnant, and the pregnancy was conceived while taking levetiracetam monotherapy and resulted in a normal healthy baby at postnatal examination. Nine participants randomised to start treatment with levetiracetam reported a pregnancy, which resulted in four normal healthy babies at postnatal examination (levetiracetam, n = 3; levetiracetam plus pregabalin being taken at the time of reporting pregnancy, n = 1), three miscarriages (all levetiracetam taken at time of reporting pregnancy), one low-birthweight baby (levetiracetam being taken at the time of reporting pregnancy) and one baby with major malformations (carbamazepine being taken at the time of reporting pregnancy) (see Appendix 3, Table 43).
| MedDRA System Organ Classification | Number of events | Number of patients (%) | ||
|---|---|---|---|---|
| Valproate group | Levetiracetam group | Valproate group (N = 257) | Levetiracetam group (N = 258) | |
| Psychiatric disorders | 54 | 109 | 36 (14.0) | 66 (25.6) |
| Nervous system disorders | 58 | 46 | 42 (16.3) | 37 (14.3) |
| Gastrointestinal disorders | 24 | 20 | 19 (7.4) | 15 (5.8) |
| Investigationsa | 31 | 11 | 29 (11.3) | 11 (4.3) |
| General disorders and administration site conditions | 20 | 17 | 16 (6.2) | 15 (5.8) |
| Metabolism and nutrition disorders | 19 | 8 | 19 (7.4) | 8 (3.1) |
| Skin and subcutaneous tissue disorders | 11 | 6 | 11 (4.3) | 5 (1.9) |
| Blood and lymphatic system disorders | 1 | 1 | 1 (0.4) | 1 (0.4) |
| Eye disorders | 1 | 1 | 1 (0.4) | 1 (0.4) |
| Respiratory, thoracic and mediastinal disorders | 0 | 2 | 0 | 2 (0.8) |
| Congenital, familial and genetic disorders | 0 | 1 | 0 | 1 (0.4) |
| Immune system disorders | 1 | 0 | 1 (0.4) | 0 |
| Injury, poisoning and procedural complications | 0 | 1 | 0 | 1 (0.4) |
Quality of life
Of the 520 randomised participants, 299 (58%) returned a baseline QoL questionnaire; the response rate was slightly higher for parents of children aged 5–15 (61% return) than for adults (50% return). At baseline, non-responders were more likely than the responders to be male (71% vs. 57%) and have unclassified epilepsy (27% vs. 19%), and were less likely to have generalised epilepsy (73% vs. 81%). Responders and non-responders were similar in terms of numbers of those with a learning disability or neurological disorder. The response rate diminished substantially after baseline for all subsequent time points, despite sending questionnaires to all participants from the trial office and intervention from the trial management team to reduce the length of the questionnaire and to encourage investigators to provide participants with questionnaires at clinical visits. Results from the repeated measures random-effects models (Table 23) suggest that there may be small differences in favour of levetiracetam for the QoL emotional (child), family (child and parent) and school (child) domains. However, because of the high level of missing data, these results cannot be considered as reliable. Imputation was also not considered reasonable because of the high level of missing data.
| QoL variable | Number of patients included in analysis,a n (%) | Treatment effect estimate (valproate vs. levetiracetam) (95% CI) | p-value |
|---|---|---|---|
| Adults (potential N = 208) | |||
| AEs profile | 67 (32) | –0.60 (–3.81 to 2.61) | 0.714 |
| Anxiety | 68 (33) | –1.06 (–2.37 to 0.25) | 0.112 |
| Depression | 68 (33) | –0.30 (–1.52 to 0.93) | 0.633 |
| Mastery | 55 (26) | 0.34 (–0.91 to 1.59) | 0.589 |
| Stigma | 56 (27) | 0.13 (–1.15 to 1.40) | 0.840 |
| Impact | 54 (26) | –0.51 (–3.31 to 2.29) | 0.718 |
| Overall QoL | 55 (26) | –0.61 (–1.28 to 0.06) | 0.073 |
| Children (self-reported) (potential N = 212) | |||
| Attitude to epilepsy | 54 (25) | –0.05 (–11.05 to 10.94) | 0.992 |
| QoL physical | 58 (27) | –2.38 (–12.06 to 7.29) | 0.622 |
| QoL emotional | 57 (27) | –10.28 (–18.23 to –2.32) | 0.012 |
| QoL self-esteem | 56 (26) | –6.91 (–16.88 to 3.06) | 0.170 |
| QoL social | 56 (26) | –6.83 (–16.38 to 2.71) | 0.156 |
| QoL family | 57 (27) | –9.66 (–17.26 to –2.05) | 0.014 |
| QoL school | 57 (27) | –11.47 (–20.95 to –1.99) | 0.019 |
| Impact of epilepsy | 26 (12) | 0.01 (–20.74 to 20.77) | 0.999 |
| Parent proxy reported (potential N = 312) | |||
| QoL physical | 141 (45) | –2.12 (–7.62 to 3.37) | 0.447 |
| QoL emotional | 147 (47) | –3.03 (–7.08 to 1.03) | 0.143 |
| QoL self-esteem | 146 (47) | –4.11 (–8.40 to 0.19) | 0.061 |
| QoL social | 147 (47) | –1.70 (–5.96 to 2.55) | 0.431 |
| QoL family | 147 (47) | –3.92 (–7.61 to –0.23) | 0.037 |
| QoL school | 146 (47) | –3.96 (–8.50 to 0.59) | 0.088 |
Chapter 7 Generalised and unclassified epilepsy results: economic
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Data completeness
Hospital Episode Statistics data were available for a total of 412 participants, relating to 202 participants in the valproate treatment group and 210 participants randomised to start treatment with levetiracetam. A breakdown of missing data by treatment group and outcome is provided in Appendix 4, Table 49.
A total of 389 participants returned at least one self-report questionnaire (completing the resource use, EQ-5D or both sections); 280 participants returned two or more questionnaires. In total, questionnaires were available for 1238 participant-time points (once child and proxy questionnaires had been resolved).
Questionnaires returned after the change in protocol were assigned to their nearest time point for presentation purposes. Self-report resource use data were available for 243 participants at 3 months, 212 at 6 months, 185 at 12 months and 148 at 24 months. Resource use data were also available from 153 questionnaires returned at the later time points (36, 48 and 60 months). It was deemed that there were insufficient data available for any meaningful analysis beyond the primary time horizon of 24 months, and thus the planned 48-month analysis was not conducted.
Utility data (EQ-5D) were available for 274 participants at baseline, and data were interpolated to 12 months for 161 participants and to 24 months for 128 participants. These are lower than the figures reported in Appendix 4, Table 49, because some 12-month questionnaires were dated before 365 days post randomisation and some 24-month questionnaires were dated before 730 days post randomisation. For the NEWQOL-6D, fewer utility data were available because of a high level of partially completed questionnaires.
A total of 50 data sets were imputed, based on the fraction of missing information 0.7 and accepting < 1% power fall-off compared with 100 imputations. For the bootstrapped results, this was reduced to 10 for efficiency purposes, accepting a larger reduction in power to achieve an acceptable computation time. 64 Owing to the level of missingness, models containing the NEWQOL-6D were non-convergent; hence only complete-case results are presented for the NEWQOL-6D.
Resource use and costs
Table 24 presents observed mean disaggregated resource use based on the self-report questionnaires. Table 25 presents the most common outpatient and A&E-related HRGs and costs observed during the trial period. During the 24-month follow-up period, 108 unique HRGs were recorded in admitted patient care, 168 in outpatients and 30 in A&E. The most common inpatient attendances were WJ11Z (disorders of immunity) (n ≈ 35), PR02C [paediatric epilepsy with a complication or comorbidity (CC) score of 0] (n ≈ 15) and PR02B (paediatric epilepsy with a CC score of 1–5) (n ≈ 15).
| Resource | Mean [range] (number of participants) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3-month time point | 6-month time point | 12-month time point | 24-month time point | |||||
| Valproate group | Levetiracetam group | Valproate group | Levetiracetam group | Valproate group | Levetiracetam group | Valproate group | Levetiracetam group | |
| Questionnaires returned (n) | 128 | 115 | 118 | 94 | 99 | 86 | 73 | 75 |
| Primary care | ||||||||
| GP consultation at GP surgery | 0.71 [0–10] (47) | 0.55 [0–6] (33) | 0.61 [0–10] (41) | 0.51 [0–6] (27) | 0.46 [0–4] (28) | 0.65 [0–10] (23) | 0.71 [0–20] (23) | 0.68 [0–9] (20) |
| Nurse consultation at GP surgery | 0.24 [0–8] (16) | 0.30 [0–6] (18) | 0.31 [0–10] (21) | 0.20 [0–2] (13) | 0.35 [0–4] (24) | 0.34 [0–10] (12) | 0.41 [0–16] (12) | 0.64 [0–14] (17) |
| GP home visit | 0.04 [0–4] (2) | 0.04 [0–3] (3) | 0.01 [0–1] (1) | 0 | 0.03 [0–1] (3) | 0.15 [0–10] (2) | 0 | 0.01 [0–10 (1) |
| Nurse home visit | 0.15 [0–5] (15) | 0.18 [0–6] (13) | 0.08 [0–4] (7) | 0.06 [0–3] (4) | 0.06 [0–2] (5) | 0.15 [0–10] (3) | 0.03 [0–1] (2) | 0.09 [0–5] (3) |
| Blood test | 0.30 [0–9] (26) | 0.30 [0–3] (27) | 0.28 [0–4] (21) | 0.23 [0–3] (15) | 0.28 [0–4] (16) | 0.22 [0–3] (16) | 0.18 [0–2] (10) | 0.15 [0–2] (8) |
| Urine test | 0.10 [0–3] (10) | 0.17 [0–3] (13) | 0.14 [0–4] (11) | 0.13 [0–3] (8) | 0.17 [0–2] (14) | 0.20 [0–10] (6) | 0.07 [0–1] (5) | 0.28 [0–12] (8) |
| Community care | ||||||||
| Health visitor | 0.03 [0–2] (3) | 0.03 [0–3] (2) | 0.02 [0–2] (1) | 0.01 [0–1] (1) | 0.01 [0–1] (1) | 0 | 0 | 0.03 [0–2] (1) |
| Social worker | 0.02 [0–2] (2) | 0.05 [0–3] (3) | 0.03 [0–2] (3) | 0.04 [0–4] (1) | 0.04 [0–3] (2) | 0 | 0 | 0.03 [0–1] (2) |
| Occupational therapist | 0.05 [0–3] (5) | 0.05 [0–2] (5) | 0.05 [0–3] (4) | 0.01 [0–1] (1) | 0.18 [0–6] (6) | 0.03 [0–2] (2) | 0.04 [0–1] (3) | 0.17 [0–10] (3) |
| Psychologist | 0.02 [0–1] (3) | 0.11 [0–7] (4) | 0.11 [0–8] (4) | 0.10 [0–8] (2) | 0.22 [0–12] (5) | 0.23 [0–12] (3) | 0.10 [0–4] (2) | 0.29 [0–10] (4) |
| Counsellor | 0.03 [0–2] (3) | 0.24 [0–8] (5) | 0.10 [0–3] (6) | 0.13 [0–6] (4) | 0.12 [0–11] (2) | 0.23 [0–6] (6) | 0.34 [0–12] (3) | 0.28 [0–12] (3) |
| Physiotherapist | 0.08 [0–4] (6) | 0.08 [0–3] (4) | 0.13 [0–9] (6) | 0.06 [0–3] (4) | 0.15 [0–5] (4) | 0.21 [0–4] (6) | 0.11 [0–5] (4) | 0.35 [0–20] (3) |
| Outpatients | ||||||||
| Doctor at hospital | 0.84 [0–5] (73) | 0.89 [0–6] (61) | 0.79 [0–4] (70) | 0.73 [0–7] (50) | 0.57 [0–3] (40) | 0.63 [0–5] (39) | 0.58 [0–5] (31) | 0.53 [0–5] (30) |
| Nurse at hospital | 0.66 [0–5] (61) | 0.65 [0–6] (54) | 0.51 [0–3] (49) | 0.56 [0–2] (45) | 0.44 [0–2] (39) | 0.50 [0–4] (31) | 0.63 [0–18] (26) | 0.37 [0–3] (24) |
| Ultrasound | 0.02 [0–10] (3) | 0.04 [0–2] (4) | 0.05 [0–2] (4) | 0.09 [0–1] (8) | 0.03 [0–1] (3) | 0.03 [0–2] (2) | 0.04 [0–2] (2) | 0.07 [0–3] (3) |
| Radiography | 0.07 [0–2] (8) | 0.09 [0–3] (8) | 0.08 [0–2] (7) | 0.07 [0–1] (7) | 0.08 [0–3] (6) | 0.15 [0–5] (9) | 0.05 [0–4] (1) | 0.12 [0–4] (5) |
| CT scan | 0.06 [0–1] (8) | 0.08 [0–3] (7) | 0.03 [0–1] (3) | 0.03 [0–1] (3) | 0.01 [0–1] (1) | 0 | 0.01 [0–1] (1) | 0 |
| MRI scan | 0.11 [0–1] (14) | 0.23 [0–6] (19) | 0.05 [0–2] (5) | 0.12 [0–1] (11) | 0.01 [0–1] (1) | 0 | 0.01 [0–1] (1) | 0.01 [0–1] (1) |
| EEG | 0.23 [0–3] (25) | 0.30 [0–6] (26) | 0.07 [0–2] (6) | 0.07 [0–1] (7) | 0.02 [0–1] (2) | 0.01 [0–1] (1) | 0.03 [0–2] (1) | 0 |
| Admitted patient care | ||||||||
| Hospital overnight | 0.11 [0–4] (8) | 0.10 [0–4] (6) | 0.02 [0–1] (2) | 0.07 [0–3] (3) | 0.05 [0–2] (4) | 0.10 [0–6] (4) | 0.04 [0–2] (2) | 0.07 [0–2] (4) |
| A&E | ||||||||
| Ambulance | 0.23 [0–4] (17) | 0.30 [0–5] (23) | 0.13 [0–5] (7) | 0.21 [0–4] (9) | 0.07 [0–1] (7) | 0.21 [0–6] (9) | 0.15 [0–6] (5) | 0.08 [0–2] (4) |
| A&E visit | 0.27 [0–4] (19) | 0.45 [0–6] (29) | 0.18 [0–3] (15) | 0.26 [0–3] (16) | 0.18 [0–3] (12) | 0.27 [0–8] (13) | 0.22 [0–6] (9) | 0.33 [0–5] (11) |
| Othera | 0.25 [0–] () | 0.34 [0–] () | 0.27 [0–] () | 0.70 [0–] () | 0.14 [0–] () | 0.44 [0–] () | 0.36 [0–] () | 0.28 [0–] () |
| Service code | Service description | HRG code | Description | Attendances (n)a | ||
|---|---|---|---|---|---|---|
| Levetiracetam group | Valproate group | Total | ||||
| Outpatients | ||||||
| 420 | Paediatrics | WF01A | Non-admitted face-to-face attendance, follow-up | 550 | 500 | 1050 |
| 400 | Neurology | WF01A | Non-admitted face-to-face attendance, follow-up | 345 | 290 | 640 |
| 421 | Paediatric neurology | WF01A | Non-admitted face-to-face attendance, follow-up | 75 | 130 | 205 |
| 400 | Neurology | WF01B | Non-admitted face-to-face attendance, first | 85 | 70 | 155 |
| 420 | Paediatrics | WF01B | Non-admitted face-to-face attendance, first | 60 | 50 | 110 |
| 110 | Trauma and orthopaedics | WF01A | Non-admitted face-to-face attendance, follow-up | 55 | 50 | 110 |
| 420 | Paediatrics | N/A | N/A | 55 | 30 | 90 |
| 110 | Trauma and orthopaedics | N/A | N/A | 25 | 30 | 55 |
| 291 | Paediatric neurodisability | N/A | N/A | 50 | 0 | 50 |
| 421 | Paediatric neurology | N/A | N/A | 25 | 25 | 50 |
| A&E | ||||||
| T01NA | Type 01 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | 100 | 75 | 170 |
| N/A | N/A | ASS02 | See and treat and convey | 95 | 75 | 170 |
| T01NA | Type 01 non-admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | 40 | 45 | 85 |
| T01NA | Type 01 non-admitted | VB11Z | Emergency medicine, no investigation with no significant treatment | 35 | 20 | 20 |
| T01NA | Type 01 non-admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | 25 | 20 | 45 |
| T01A | Type 01 admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | 10 | 15 | 25 |
| T03NA | Type 03 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | b | b | 20 |
| T04NA | Type 04 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1–2 treatment | b | b | 20 |
| T01A | Type 01 admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | b | b | 15 |
| T01A | Type 01 admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | b | b | 10 |
| T01A | Type 01 admitted | VB04Z | Emergency medicine, category 2 investigation with category 4 treatment | b | b | 10 |
| T03NA | Type 03 non-admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | b | b | 10 |
Based on the imputed data, the majority of costs relate to secondary care, in particular outpatient clinic attendance (Table 26). Anti-seizure medications also account for a high proportion of the total cost. Participants randomised to start treatment with levetiracetam produced higher costs for community care and secondary care, but lower medication costs than those randomised to start treatment with valproate. Total (unadjusted) costs for participants randomised to start treatment with levetiracetam were £4267 (95% CR £3944 to £5462), compared with valproate £4205 (95% CR £3827 to £4956). The difference of £61 (95% CR –£651 to £1230) was not statistically significant.
| Type of care | Totals (discounted) (£) at 24 months, mean (95% CR) | Difference (£) (95% CR) | |
|---|---|---|---|
| Valproate group | Levetiracetam group | ||
| Primary and community care | 1082 (719 to 1471) | 940 (843 to 2114) | 142 (–356 to 1077) |
| Primary care | 316 (233 to 383) | 255 (262 to 523) | 61 (–72 to 236) |
| Community care | 765 (436 to 1158) | 684 (531 to 1690) | 81 (–387 to 971) |
| Secondary care | 2540 (2193 to 2777) | 2447 (2275 to 2892) | 92 (–332 to 514) |
| Admitted patient care | 590 (407 to 793) | 577 (283 to 308) | 13 (–250 to 289) |
| Outpatient | 1613 (1482 to 1733) | 1594 (1489 to 1750) | 19 (–163 to 199) |
| A&E | 336 (225 to 343) | 277 (283 to 418) | 60 (–30 to 160) |
| Medicines | 646 (676 to 1001) | 818 (547 to 840) | –173 (–366 to 61) |
| Anti-seizure medication | 524 (543 to 841) | 692 (416 to 663) | –167 (–347 to 32) |
| Concomitant medication | 121 (94 to 212) | 127 (83 to 241) | –5 (–98 to 110) |
| Total | 4267 (3944 to 5462) | 4205 (3827 to 4957) | 61 (–651 to 1230) |
Based on imputed data, the mean baseline costs were £1067 (95% CI £934 to £1234) in the levetiracetam group and £1088 (95% CI £978 to £1213) in the valproate group The base-case analysis, which adjusted for baseline costs, yielded a 2-year mean total cost of £4350 (95% CR £4136 to £5623) in the levetiracetam group, compared with £4246 (95% CR £3979 to £5090) in the valproate group. This corresponds to an incremental cost of £104 (95% CR –£587 to £1234).
Utilities and quality-adjusted life-years
The distributions of participants’ responses to the EQ-5D-3L-Y and the NEWQOL-6D questionnaires by randomised treatment group are presented in Appendix 4, Figures 29 and 30. Based on imputed data, baseline utilities were 0.831 (95% CR 0.779 to 0.850) in the levetiracetam group and 0.811 (95% CR 0.772 to 0.840) in the valproate group. In the base-case adjusted analysis, levetiracetam was associated with a QALY gain of 1.603 years (95% CR 1.500 to 1.631 years), whereas valproate was associated with a QALY gain of 1.637 years (95% CR 1.565 to 1.673 years) over the 2-year time horizon. This corresponds to an incremental QALY gain of –0.035 years (95% CR –0.137 to 0.032 years).
The QALYs based on the NEWQOL-6D were calculated for complete-case data only, over the 2-year time horizon. Levetiracetam was associated with an adjusted QALYs gain of 1.741 years (95% CR 1.695 to 1.784 years), compared with 1.727 years (95% CR 1.697 to 1.779 years) for valproate. This shows levetiracetam to be associated with an incremental QALY gain of 0.015 years (95% CR –0.051 to 0.056 years).
The distribution of responses to the EQ-VAS is presented in Table 27. The adjusted analysis based on the EQ-VAS resulted in a QALY gain of 1.453 years (95% CR 1.358 to 1.495 years) for levetiracetam and of 1.464 years (95% CR 1.369 to 1.502 years) for valproate; the incremental QALY of –0.011 years (95% CR –0.103 to 0.077 years) is consistent with the base-case EQ-5D-3L analysis.
| Time point | Valproate group | Levetiracetam group | ||
|---|---|---|---|---|
| n | Mean (95% CI) | n | Mean (95% CI) | |
| Baseline | 138 | 0.7934 (0.7625 to 0.8243) | 132 | 0.7510 (0.7146 to 0.7874) |
| 12 months | 87 | 0.8061 (0.7605 to 0.8516) | 69 | 0.7484 (0.6866 to 0.8102) |
| 24 months | 65 | 0.7929 (0.7326 to 0.8533) | 58 | 0.7893 (0.7327 to 0.8459) |
Incremental analysis
Based on the point estimate mean costs and QALYs, levetiracetam was both more costly and less effective than valproate, and, therefore, dominated, meaning that it is not considered to be cost-effective. Levetiracetam is associated with a negative incremental NHB (–0.040, 95% CR –0.175 to 0.037) at a cost-effectiveness threshold of £20,000 per QALY.
Sensitivity analysis
Table 28 presents the results of the sensitivity analyses, which are consistent with the base case for all analyses other than the complete-case analysis and NEWQOL-6D analysis, both of which are limited by missing data.
| Sensitivity analysis | Mean (95% CR) | ICER (£ per QALY) | |||
|---|---|---|---|---|---|
| Cost (£) | QALYs | NHB at £20,000 per QALY | NHB at £30,000 per QALY | ||
| Base case (n = 520) | |||||
| Valproate | 4246 (3979 to 5090) | 1.637 (1.565 to 1.673) | 1.425 (1.323 to 1.464) | 1.496 (1.407 to 1.534) | |
| Levetiracetam | 4350 (4136 to 5623) | 1.603 (1.500 to 1.631) | 1.385 (1.236 to 1.410) | 1.458 (1.328 to 1.481) | |
| Incremental | 104 (–587 to 1234) | –0.035 (–0.137 to 0.032) | –0.040 (–0.175 to 0.037) | –0.038 (–0.158 to 0.034) | Dominated |
| 0% discount rate (costs and QALYs) (base case 3.5%) (n = 520) | |||||
| Valproate | 4310 (4037 to 5171) | 1.666 (1.592 to 1.702) | 1.450 (1.346 to 1.490) | 1.522 (1.432 to 1.560) | |
| Levetiracetam | 4421 (4203 to 5721) | 1.630 (1.526 to 1.659) | 1.409 (1.257 to 1.434) | 1.483 (1.350 to 1.507) | |
| Incremental | 111 (–596 to 1264) | –0.035 (–0.140 to 0.033) | –0.041 (–0.180 to 0.037) | –0.039 (–0.162 to 0.034) | Dominated |
| 6% discount rate (costs and QALYs) (base case 3.5%) (n = 520) | |||||
| Valproate | 4202 (3939 to 5038) | 1.618 (1.547 to 1.654) | 1.408 (1.307 to 1.447) | 1.478 (1.391 to 1.515) | |
| Levetiracetam | 4302 (4091 to 5556) | 1.584 (1.483 to 1.612) | 1.369 (1.222 to 1.393) | 1.441 (1.313 to 1.464) | |
| Incremental | 99 (–584 to 1210) | –0.034 (–0.135, 0.032) | –0.039 (–0.172 to 0.036) | –0.037 (–0.155 to 0.034) | Dominated |
| Unadjusted (no covariates) (base case adjusted) (n = 520) | |||||
| Valproate | 4205 (3827 to 4956) | 1.629 (1.553 to 1.671) | 1.419 (1.320 to 1.461) | 1.489 (1.399 to 1.530) | |
| Levetiracetam | 4267 (3944 to 5462) | 1.610 (1.501 to 1.640) | 1.396 (1.247 to 1.426) | 1.467 (1.335 to 1.496) | |
| Incremental | 61 (–651 to 1230) | –0.020 (–0.132 to 0.050) | –0.023 (–0.162 to 0.065) | –0.022 (–0.149 to 0.057) | Dominated |
| Complete data (base case imputed) (cost n = 58; EQ-5D n = 109) | |||||
| Valproate | 5489 (3163 to 8881) | 1.666 (1.600 to 1.716) | 1.391 (1.200 to 1.523) | 1.483 (1.345 to 1.578) | |
| Levetiracetam | 4894 (1727 to 6703) | 1.675 (1.640 to 1.750) | 1.430 (1.343 to 1.625) | 1.512 (1.450 to 1.654) | |
| Incremental | –595 (–6920 to 3252) | 0.009 (–0.039 to 0.116) | 0.039 (–0.140 to 0.393) | 0.029 (–0.098 to 0.286) | Dominated |
| PP cohort (n = 509) (base case all participants, ITT) (n = 520) | |||||
| Valproate | 4240 (3963 to 5081) | 1.640 (1.568 to 1.674) | 1.428 (1.324 to 1.465) | 1.499 (1.409 to 1.534) | |
| Levetiracetam | 4362 (4137 to 5637) | 1.603 (1.502 to 1.632) | 1.385 (1.236 to 1.410) | 1.458 (1.328 to 1.481) | |
| Incremental | 121 (–587 to 1219) | –0.037 (–0.138 to 0.029) | –0.043 (–0.174 to 0.034) | –0.041 (–0.157 to 0.031) | Dominated |
| NEWQOL-6D (base case EQ-5D) (costs as base case, NEWQOL-6D based on n = 53 complete cases) | |||||
| Valproate | 4246 (3979 to 5090) | 1.727 (1.697 to 1.779) | 1.514 (1.453 to 1.565) | 1.585 (1.535 to 1.634) | |
| Levetiracetam | 4350 (4136 to 5623) | 1.741 (1.695 to 1.784) | 1.524 (1.428 to 1.560) | 1.596 (1.520 to 1.633) | |
| Incremental | 104 (–587 to 1234) | 0.015 (–0.051 to 0.056) | 0.009 (–0.085 to 0.064) | 0.011 (–0.069 to 0.060) | £7159 |
| EQ-VAS (base case EQ-5D) (n = 520) | |||||
| Valproate | 4246 (3979 to 5090) | 1.464 (1.369 to 1.502) | 1.252 (1.134 to 1.283) | 1.323 (1.213 to 1.356) | |
| Levetiracetam | 4350 (4136 to 5623) | 1.453 (1.358 to 1.495) | 1.235 (1.102 to 1.260) | 1.308 (1.190 to 1.335) | |
| Incremental | 104 (–587 to 1234) | –0.011 (–0.103 to 0.077) | –0.016 (–0.135 to 0.077) | –0.015 (–0.121 to 0.076) | Dominated |
| Treating blank responses in the questionnaire as missing (base case: as zero) | |||||
| Valproate | 4210 (3938 to 5152) | 1.637 (1.565 to 1.673) | 1.427 (1.330 to 1.453) | 1.497 (1.410 to 1.525) | |
| Levetiracetam | 4517 (4206 to 5791) | 1.603 (1.500 to 1.631) | 1.377 (1.241 to 1.396) | 1.452 (1.329 to 1.473) | |
| Incremental | 307 (–473 to 1424) | –0.035 (–0.137 to 0.032) | –0.050 (–0.171 to 0.018) | –0.045 (–0.158 to 0.019) | Dominated |
The cost-effectiveness plane, which depicts the joint uncertainty in costs and QALYs, is presented in Figure 21. It shows that 62% of simulations were more costly and less effective, 23% were less costly but less effective, 8% were less costly and more effective, and 7% were more costly and more effective.
FIGURE 21.
Cost-effectiveness plane based on 1000 simulations. Simulations that were cost-effective only at the lower £20,000 per QALY threshold are coloured orange; those which were cost-effective only at the higher £30,000 per QALY threshold are in light purple; those cost-effective at both the £20,000 and £30,000 per QALY thresholds are in light blue; and those that were not cost-effective at either threshold are in dark blue.
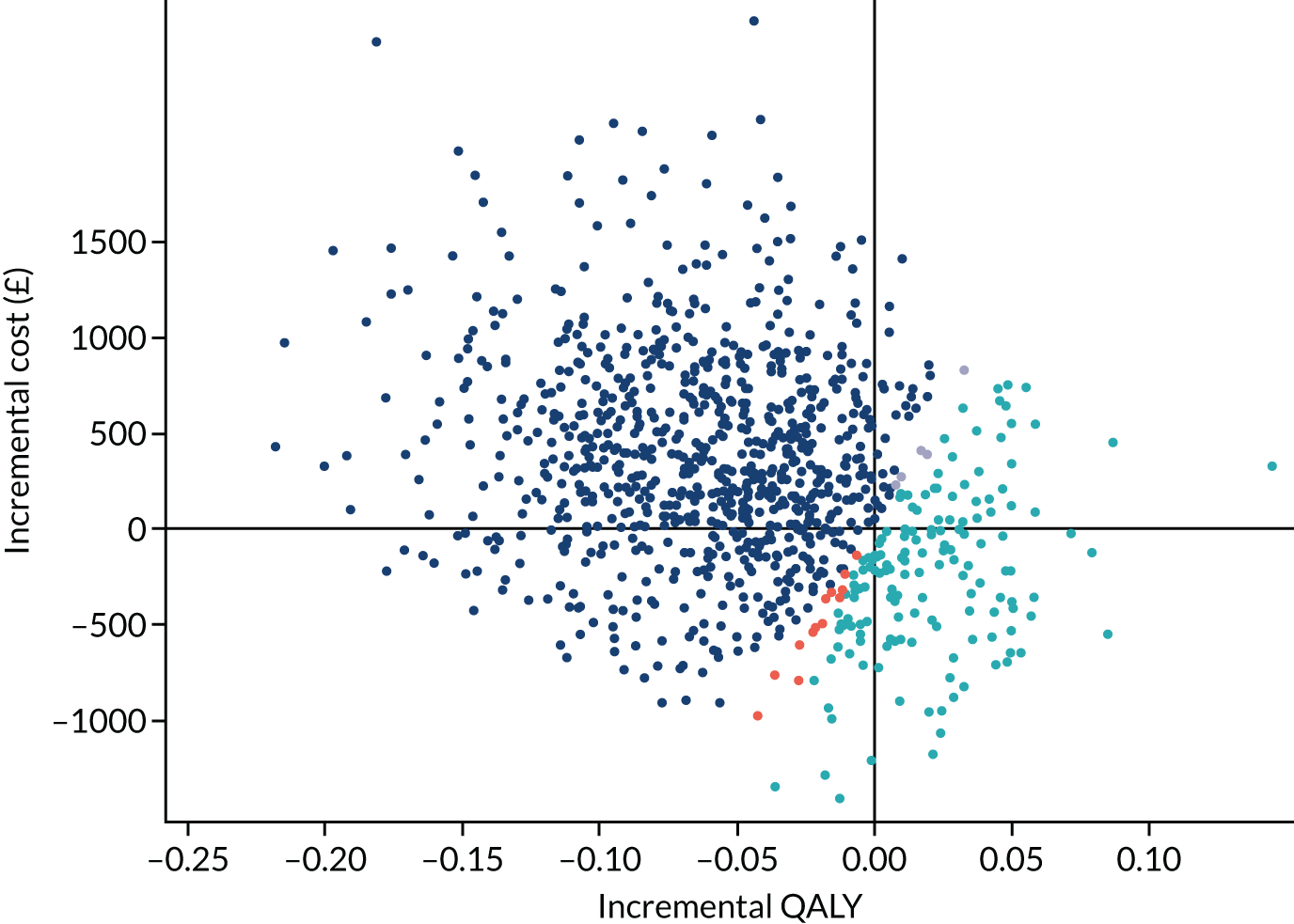
The cost-effectiveness acceptability curve (Figure 22) indicates that the probability of levetiracetam being cost-effective at a cost-effectiveness threshold of £20,000 per QALY is 0.17.
FIGURE 22.
Cost-effectiveness acceptability curve.
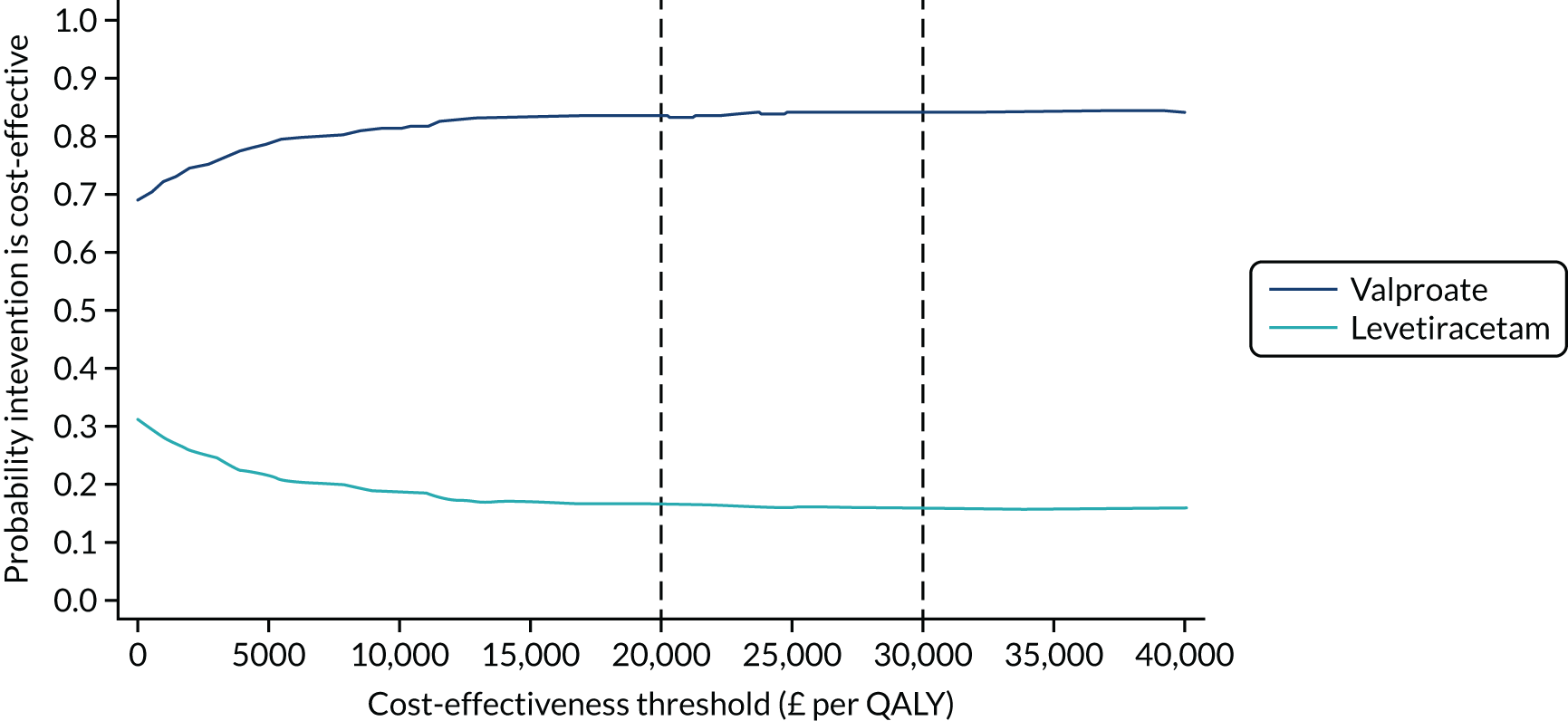
Subgroup analyses
The results of the subgroup analysis for both children and adults are consistent with the base-case analysis (all participants) and are presented in Table 29.
| Sensitivity analysis | Mean (95% CR) | ICER (£ per QALY) | |||
|---|---|---|---|---|---|
| Total cost (£) | QALYs | NHB at £20,000 per QALY | NHB at £30,000 per QALY | ||
| Base case: all participants (n = 520) | |||||
| Valproate | 4246 (3979 to 5090) | 1.637 (1.565 to 1.673) | 1.425 (1.323 to 1.464) | 1.496 (1.407 to 1.534) | |
| Levetiracetam | 4350 (4136 to 5623) | 1.603 (1.500 to 1.631) | 1.385 (1.236 to 1.410) | 1.458 (1.328 to 1.481) | |
| Incremental | 104 (–587 to 1234) | –0.035 (–0.103 to 0.077) | –0.040 (–0.175 to 0.037) | –0.038 (–0.158 to 0.034) | Dominated |
| Subgroup: children aged < 16 years (n = 312) | |||||
| Valproate | 4360 (4046 to 5149) | 1.626 (1.554 to 1.667) | 1.408 (1.307 to 1.455) | 1.481 (1.392 to 1.525) | |
| Levetiracetam | 4336 (4017 to 5516) | 1.624 (1.506 to 1.646) | 1.407 (1.254 to 1.430) | 1.479 (1.340 to 1.502) | |
| Incremental | –24 (–752 to 1065) | –0.002 (–0.123 to 0.054) | –0.001 (–0.151 to 0.069) | –0.002 (–0.136 to 0.062) | Dominated |
| Subgroup: adults aged ≥ 16 years (n = 208) | |||||
| Valproate | 3957 (3525 to 5161) | 1.654 (1.563 to 1.693) | 1.456 (1.330 to 1.497) | 1.522 (1.409 to 1.560) | |
| Levetiracetam | 4316 (3842 to 5898) | 1.576 (1.474 to 1.636) | 1.360 (1.200 to 1.425) | 1.479 (1.291 to 1.492) | |
| Incremental | 359 (–644 to 1640) | –0.078 (–0.175 to 0.015) | –0.096 (–0.232 to 0.025) | –0.090 (–0.208 to 0.018) | Dominated |
Chapter 8 Generalised and unclassified epilepsy: discussion
Parts of this chapter have been reproduced from Marson et al. 74 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Levetiracetam did not meet our definition of non-inferiority for the primary outcome of time to 12-month remission from seizures for newly diagnosed generalised epilepsy or epilepsy that was unclassified. Levetiracetam was inferior to valproate in terms of time to treatment failure, time to 24-month remission and time to first subsequent seizure. These results are particularly important when considering treatment choices for women of childbearing potential who have newly diagnosed generalised epilepsy, for whom a pregnancy prevention plan has been put in place by the EMA and MHRA18 because of concerns about teratogenic effects80–83 and developmental delay/learning difficulties following intrauterine valproate exposure. Levetiracetam has become a common first-choice treatment despite the absence of reliable evidence of its clinical effectiveness compared with valproate.
It is important to highlight that the SANAD II trial was a pragmatic trial that compared the policies of starting treatment with either levetiracetam or valproate, and that drug and dose changes were made during follow-up as per routine clinical practice to maximise seizure control and to minimise ARs. The results for time to 12-month remission indicate an immediate remission rate that is lower with levetiracetam than with valproate (difference of –9%, 95% CI –18% to –1%), but the difference between treatment policies diminishes over time. The higher treatment failure rate associated with levetiracetam is the likely explanation for finding non-proportional hazards. The PP analysis for time to 12-month remission, which took treatment failure into account using a competing risks approach, found superiority of valproate over levetiracetam (HR 1.68, 95% CI 1.30 to 2.15).
For time to treatment failure, valproate was superior to levetiracetam (HR 0.65, 95% CI 0.50 to 0.83). A competing risks analysis shows that the rate of failure due to ARs was similar in the valproate and levetiracetam groups (HR 0.93, 95% CI 0.61 to 1.40), but levetiracetam treatment was significantly more likely to fail because of ISC (HR 0.43, 95% CI 0.30 to 0.63).
To explore the treatment effects further, the cohort was split into three groups: those with absence epilepsies, those with other generalised epilepsies and those with unclassified epilepsy. For time to 12-month remission, survival curves suggest an important disadvantage for starting levetiracetam in the ‘other generalised epilepsy’ subgroup where the difference in immediate remission rate is –19.1% (95% CI –6.6% to –31.7%). In contrast, there was no clear advantage seen in the absence or unclassified epilepsy subgroups. Those with ‘other generalised epilepsies’ were mainly those with generalised tonic–clonic seizures, among whom seizure rates are low and in whom many months of observation are typically required to record seizures and make incremental changes to dose and drug. Conversely, absence seizures typically occur at a high rate, enabling more rapid decisions about dose and drug changes in order to gain early seizure control.
For time to treatment failure due to inadequate seizure control, valproate was superior to levetiracetam in patients with absence epilepsies (HR 0.35, 95% CI 0.19 to 0.63) and other generalised epilepsies (HR 0.27, 95% CI 0.14 to 0.49). Conversely, there may be an advantage for starting levetiracetam in those with unclassified epilepsy (HR 2.15, 95% CI 0.79 to 5.86). The rate of ARs was similar in both treatment arms although the profile was different; the rate of psychiatric coded events was higher in those starting on levetiracetam and weight increase was more common in those starting on valproate.
The SANAD II trial did not exclude the recruitment of females of childbearing potential, who could be recruited following appropriate consent and counselling. The number of females between the age of 12 and 50 years recruited (n = 80) was lower than the number of males recruited (n = 218), and the EMA and MHRA pregnancy prevention scheme was implemented following the commencement of the study and during most of its recruitment and follow-up period. There were 10 pregnancies during the study, none of which was exposed to valproate. Only one pregnancy occurred among those randomised to start treatment with valproate, suggesting an important impact on fertility choices if women are treated with valproate, choices that may persist after valproate is withdrawn.
Analysis of QoL outcomes does not indicate benefit for either drug, but the return rate of questionnaires was disappointingly low.
The economic analysis indicated that levetiracetam was not cost-effective compared with valproate, being both more costly and less effective. The mean total costs were £4350 (95% CR £4136 to £5623), in the levetiracetam group and £4246 (95% CR £3979 to £5090) in the valproate group. Levetiracetam was associated with a smaller mean QALY gain, at 1.603 years (95% CR 1.500 to 1.631 years), than valproate (mean 1.637 years, 95% CR 1.565 to 1.673 years). The resulting negative INHB (–0.040, 95% CR –0.175 to 0.037) responded with a low probability (0.17) of cost-effectiveness at the NICE threshold of £20,000 per QALY. This result was consistent for both the adult and child subgroups, and robust to some of the modelling assumptions tested in sensitivity analysis. Different results were apparent when considering NEWQOL-6D utilities and complete cases, although these analyses were less reliable because of data missingness.
As with the first SANAD trials,9,16 we have demonstrated that the NHS in the UK can deliver longer-term pragmatic epilepsy trials, collecting data from neurology services and from primary care. Given the duration of the study, the number of follow-up data available was large; the completeness of the follow-up statistic was 87% in the valproate group and 83% in the levetiracetam group. Nonetheless, the SANAD II trial has a number of limitations. First, it was unblinded, as that was the only feasible way to collect longer-term follow-up data when knowledge of first treatment is required to inform future treatment decisions. This may have influenced decisions about dose and treatment changes, biasing results for time to treatment failure and seizure outcomes. Knowledge of treatment allocated may also have influenced reporting of ARs, and this should be taken into consideration when interpreting, for example, the higher rate of psychiatric events in the levetiracetam group. Initial maintenance doses recommended in the protocol reflected clinical practice at the time that the SANAD II trial was undertaken, which included higher relative doses of levetiracetam in children than in adults. It is possible that the initial maintenance doses chosen introduced a systematic bias, but the similar treatment failure rates due to ARs provide some reassurance. It is also important to acknowledge that there is no RCT evidence to inform choice of the initial maintenance dose of levetiracetam, valproate or, indeed, most other anti-seizure medications. Although 76% of participants were classified as having a generalised epilepsy, only 52% had generalised spike and wave changes on EEG, indicating that some of the remaining 24% may have been misclassified. It is not possible to state whether this might have increased or diminished the treatment effects observed, but it is interesting to note that, in the subgroup analysis for 12-month remission, the estimate in unclassified patients favours levetiracetam. In addition, other than for absence epilepsies, the number of participants classified with a specific generalised epilepsy syndrome at the time of randomisation was small, precluding subgroup analyses for syndromes such as juvenile myoclonic epilepsy. More males than females were recruited (64.8% vs. 35.2%), although there is no reason to expect important differences in response by gender. 7 There was also a low return rate for QoL questionnaires, which diminished our ability to identify the QoL consequences of either policy, which also had an impact on our health economic analysis.
The economic analysis was limited by the poor return of self-report questionnaires. However, for costs this was largely mitigated by the acquisition of HES data and the use of follow-up CRFs for the costs of anti-seizure medicines, which were the main cost drivers. Owing to the AUC methodology, QALYs could be calculated provided two or more EQ-5D questionnaires had been returned. However, for the NEWQOL-6D there were insufficient data to complete imputation; hence only complete-case results could be presented. An additional limitation is that there is no tariff currently available for the EQ-5D-3L-Y or proxy version of the EQ-5D-3L and, therefore, the adult tariff was used throughout for estimating utilities from EQ-5D profiles. This represents a weakness in many economic evaluations of interventions in paediatric populations,75 although a valuation of children’s EQ-5D-3L-Y health states should soon be available. 76 Furthermore, given the chronic nature of epilepsy, the 2-year time horizon was somewhat limited, and the planned analysis over a 4-year time horizon could not be conducted because of limitations resulting from missing data.
These results should be put into context with previous studies, although few studies have assessed the longer-term effectiveness of treatments for generalised epilepsies. SANAD I16 identified valproate as a first-line treatment, as it was superior to lamotrigine for seizure control and superior to topiramate for treatment failure. 12 An individual patient data network meta-analysis,15 which included data from the SANAD I trial, failed to show superiority for 12-month remission in generalised epilepsy of any drug among valproate, levetiracetam, gabapentin, phenytoin, carbamazepine, oxcarbazepine, topiramate or phenobarbital, but the results were heavily confounded by classification errors; the original trials probably included a significant number of people whose focal epilepsy was misclassified as generalised epilepsy. Valproate was superior to carbamazepine, topiramate and phenobarbital for treatment failure. To our knowledge, the SANAD II trial is the only trial to date that provides much needed head-to-head data on the longer-term effectiveness of valproate compared with levetiracetam.
The recommendations for research are as follows:
-
A network meta-analysis making both direct and indirect comparisons is required of all available anti-seizure monotherapy trials to provide an overview of the entirety of current evidence regarding clinical effectiveness for generalised epilepsy. This work is under way and funded by NIHR to inform the current NICE epilepsy guidelines update. 79
-
An economic model is required, utilising results of direct and indirect comparisons, to estimate the comparative cost-effectiveness of currently available treatments for generalised epilepsy.
-
An assessment of women’s preferences using economic methods such as discrete choice experiments is required, taking into account clinical effectiveness as well as risk in pregnancy.
-
Prognostic modelling of data from the SANAD I and SANAD II trials is required to explore subgroup effects and to better stratify patients for likely outcome at the time of initiating treatment for generalised epilepsy.
-
Methodological work, utilising data from the SANAD I and SANAD II trials, is required to inform the design of future trials assessing the clinical effectiveness and cost-effectiveness of anti-seizure medications in people with newly diagnosed generalised epilepsy. This includes the possibility of designs using data from the SANAD I and SANAD II trials as historical controls.
In conclusion, these results have important implications for clinical practice and research. These results suggest that, for males with generalised onset seizures, first-line treatment should continue to be valproate. The results also suggest that, for women of childbearing potential, levetiracetam is inferior to valproate, as is lamotrigine (the other commonly prescribed alternative). Regulators, guideline developers, clinicians and patient groups could now consider the benefit-to-risk ratio, particularly for those in our subgroup ‘other generalised epilepsy’. Some women, particularly those in whom seizures present a particular hazard, may prefer a drug with greater efficacy notwithstanding the risk of teratogenicity. However, women may prefer a first-line drug that that is safer in pregnancy despite lower efficacy, as indicated in a discrete choice experiment that found women would accept a 5% reduction in 12-month remission probability for 1% reduction in fetal abnormality. 84 Therefore, given that our ITT results show no differences in 12-month remission rates in the longer term, levetiracetam could be a reasonable first-line treatment for women of childbearing potential with newly diagnosed idiopathic generalised epilepsy. For people with unclassified seizures, no significant difference was found but estimates favour levetiracetam. Future studies should not group generalised and unclassified epilepsy together and the international epilepsy community should identify a better strategy for assessing treatment policies in those with unclassified seizures.
Acknowledgements
We would like to thank the external members of the TSC for their advice and support on the project: Graham Venables (TSC chairperson, Royal Hallamshire Hospital, Sheffield), Carl Clarke (University of Birmingham), Kathy Bairstow (Epilepsy Action), Denise Howel (Newcastle University) and Richard Newton (Royal Manchester Children’s Hospital).
We would also like to thank the IDSMC members: Charles Warlow (Western General Hospital, Edinburgh), Tony Johnson (University of Cambridge), Simon Shorvon (University College London) and Carl Counsell (University of Aberdeen).
Thanks also go to the TMG members Sir Munir Pirmohamed (University of Liverpool), Michael Johnson (Imperial College, London) and Tracy Moitt (University of Liverpool), and sponsor representatives Dave Watling, Maria Thornton, Gillian Williams (Walton Centre), Karen Wilding (University of Liverpool), Alex Astor (University of Liverpool) and Lara Lavelle-Langham (University of Liverpool).
We would like to thank the following staff members, past and present, at LCTC: Andrew McKay (quality control statistician), Barbara Arch (statistician and statistical analysis plan author), Helen Hickey (head of trial management), Helen Hind (senior trial manager), Joanne Eatock and Clare Jackson (senior data managers), Stephanie Roberts, Briony Macdonald-Macmillan and Paul Tate (data managers), Sion Kenyon, Nadia Al-Najjar, Celeste Abbott, Gloria Nkhoma, Anna Kearney, Steven Pollock (trial co-ordinator assistants), Duncan Appelbe (information systems manager), Janet Harrison (clinical data management systems team leader), and Alex Rigby (work experience student).
We are grateful for the support of the NIHR Clinical Research Network North West Coast, the Welsh Epilepsy Research Network and, previously. The Dementias & Neurodegenerative Diseases Research Network (DeNDRoN) North West and the Medicines for Children Research Network.
Walton Centre Foundation NHS Trust (WCFT) and University of Liverpool (UoL) are both members of Liverpool Health Partners (LHP).
The authors wish to acknowledge Colin Ridyard and Yankier Pijeira Perez for their assistance with the analysis of the cost data.
We are grateful to all the participants for their commitment to the trial. We would like to thank all of the principal investigators (see Appendix 1), research nurses and other members of the team who recruited patients and supported them during the trial.
Contributions of authors
Anthony G Marson (https://orcid.org/0000-0002-6861-8806) (Chief Investigator, Professor of Neurology and Honorary Consultant Neurologist) developed the trial protocol in collaboration with co-investigators. He oversaw the delivery of the trial, and oversaw clinical aspects of the statistical analysis plan and clinical interpretation of the trial data. He led the preparation of the final report (drafting, reviewing and editing). He was chairperson of the TMG.
Girvan Burnside (https://orcid.org/0000-0001-7398-1346) (Trial Statistician and Senior Lecturer) contributed to protocol development and data capture methods, undertook the final statistical analysis, prepared data for reports throughout the trial, prepared data tables and figures for the final report, contributed to the final report (drafting reviewing and editing) and was a member of the TMG.
Richard Appleton (https://orcid.org/0000-0002-0742-2113) (Consultant Paediatric Neurologist and Honorary Professor) contributed to the trial protocol development, interpretation of the trial data and the preparation (reviewing and editing) of the final report, and was a member of the TMG.
Dave Smith (https://orcid.org/0000-0003-3449-4777) (Consultant Neurologist) contributed to the clinical interpretation of the trial data and the preparation (reviewing and editing) of the final report.
John Paul Leach (https://orcid.org/0000-0003-2086-9937) (Professor and Consultant Neurologist) contributed to the trial protocol development, interpretation of the trial data and the preparation (reviewing and editing) of the final report, and was a member of the TMG.
Graeme Sills (https://orcid.org/0000-0002-3389-8713) (Senior Lecturer in Pharmacology) co-ordinated the collection and archiving of DNA samples, contributed to the trial protocol development, interpretation of the trial data and the preparation (reviewing and editing) of the final report, and was a member of the TMG.
Catrin Tudur-Smith (https://orcid.org/0000-0003-3051-1445) (Statistical Lead, Professor of Statistics) contributed to trial design, trial protocol and data capture methods in collaboration with co-investigators. She led the blind review of the data and supervised the final analysis. She also contributed to the preparation of the final report (drafting, reviewing and editing) and was a member of the TMG.
Catrin O Plumpton (https://orcid.org/0000-0003-2710-9199) (Study Health Economist) contributed to protocol development and data capture methods, undertook the economic analysis under the supervision of Dyfrig Hughes and contributed to the final report (drafting and editing).
Dyfrig A Hughes (https://orcid.org/0000-0001-8247-7459) (Senior Health Economist) led the economic evaluation, contributed to protocol development and data capture methods, contributed to the writing of the report (drafting, reviewing and editing), and was a member of the TMG.
Paula R Williamson (https://orcid.org/0000-0001-9802-6636) (Senior Statistician, Professor of Statistics) contributed to protocol development and data capture methods, contributed to the drafting (reviewing and editing) of the final report, and was a member of the TMG.
Gus Baker (https://orcid.org/0000-0002-5736-605X) (Professor of Neuropsychology) led on the QoL assessments, contributed to protocol development and QoL data capture methods, contributed to the drafting (reviewing and editing) of the final report, and was a member of the TMG.
Silviya Balabanova (https://orcid.org/0000-0001-6989-8099) (Trial Manager) contributed to protocol development, supported the preparation of progress reports, contributed to the final report (drafting and reviewing) and was a member of the TMG.
Claire Taylor (https://orcid.org/0000-0003-4746-9730) (Trial Manager) contributed to protocol development, supported the preparation of progress reports and contributed to the final report (drafting and reviewing).
Richard Brown (https://orcid.org/0000-0002-1591-3959) (Consultant Paediatrician) contributed to clinical interpretation of the trial data and the preparation of the final report.
Dan Hindley (https://orcid.org/0000-0002-4128-6995) (Consultant Community Paediatrician) contributed to clinical interpretation of the trial data and the preparation of the final report.
Stephen Howell (https://orcid.org/0000-0001-8504-9644) (Consultant Neurologist) contributed to clinical interpretation of the trial data and the preparation of the final report.
Melissa Maguire (https://orcid.org/0000-0002-5351-6743) (Consultant Neurologist) contributed to clinical interpretation of the trial data and the preparation of the final report.
Rajiv Mohanraj (https://orcid.org/0000-0002-2559-148X) (Consultant Neurologist) contributed to clinical interpretation of the trial data and the preparation of the final report.
Philip EM Smith (https://orcid.org/0000-0003-4250-2562) (Consultant Neurologist and Honorary Professor) contributed to the trial protocol development, interpretation of the trial data and preparation of the final report, and was a member of the TMG.
Publications
Balabanova S, Taylor C, Sills G, Burnside G, Plumpton C, Smith PEM, et al. Study protocol for a pragmatic randomised controlled trial comparing the effectiveness and cost-effectiveness of levetiracetam and zonisamide versus standard treatments for epilepsy: a comparison of standard and new antiepileptic drugs (SANAD-II). BMJ Open 2020;10:e040635.
Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide or lamotrigine for newly diagnosed focal epilepsy: an open label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021;397:1363–74.
Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021;397:1375–86.
Data-sharing statement
All requests for data (pseudoanonymised and fully anonymous) should be sent to the corresponding author. For data which are not fully anonymous, the decision for data sharing also lies with the trial joint data controllers (University of Liverpool, Walton Centre NHS Foundation Trust and Bangor University). Access to available data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. New York, NY: Demos Medical Publishing; 1990.
- Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1981;22:489-501. https://doi.org/10.1111/j.1528-1157.1981.tb06159.x.
- Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389-99. https://doi.org/10.1111/j.1528-1157.1989.tb05316.x.
- Holland P, Lane S, Whitehead M, Marson AG, Jacoby A. Labor market participation following onset of seizures and early epilepsy: findings from a UK cohort. Epilepsia 2009;50:1030-9. https://doi.org/10.1111/j.1528-1167.2008.01819.x.
- Schachter SC. Quality of life for patients with epilepsy is determined by more than seizure control: the role of psychosocial factors. Expert Rev Neurother 2006;6:111-18. https://doi.org/10.1586/14737175.6.1.111.
- Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 2017;88:296-303. https://doi.org/10.1212/WNL.0000000000003509.
- Bonnett L, Smith CT, Smith D, Williamson P, Chadwick D, Marson AG. Prognostic factors for time to treatment failure and time to 12 months of remission for patients with focal epilepsy: post-hoc, subgroup analyses of data from the SANAD trial. Lancet Neurol 2012;11:331-40. https://doi.org/10.1016/S1474-4422(12)70018-2.
- National Institute for Health and Care Excellence . Epilepsies: Diagnosis and Management 2012.
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1000-15. https://doi.org/10.1016/S0140-6736(07)60460-7.
- Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorffer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol 2005;4:627-34. https://doi.org/10.1016/S1474-4422(05)70172-1.
- Zarrelli MM, Beghi E, Rocca WA, Hauser WA. Incidence of epileptic syndromes in Rochester, Minnesota: 1980–1984. Epilepsia 1999;40:1708-14. https://doi.org/10.1111/j.1528-1157.1999.tb01587.x.
- National Institute for Health and Care Excellence . The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care 2004.
- Marson AG, Williamson PR, Hutton JL, Clough HE, Chadwick DW. Carbamazepine versus valproate monotherapy for epilepsy. Cochrane Database Syst Rev 2000;3. https://doi.org/10.1002/14651858.CD001030.
- Tudur Smith C, Marson AG, Williamson PR. Phenytoin versus valproate monotherapy for partial onset seizures and generalized onset tonic–clonic seizures. Cochrane Database Syst Rev 2001;4. https://doi.org/10.1002/14651858.CD001769.
- Nevitt SJ, Sudell M, Weston J, Tudur Smith C, Marson AG. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev 2017;6. https://doi.org/10.1002/14651858.CD011412.pub2.
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1016-26. https://doi.org/10.1016/S0140-6736(07)60461-9.
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med 2010;362:790-9. https://doi.org/10.1056/NEJMoa0902014.
- European Medicines Agency . New Measures to Avoid Valproate Exposure in Pregnancy Endorsed. Member State Representatives Agree New Restrictions and Pregnancy Prevention Programme 2018. www.ema.europa.eu/en/news/new-measures-avoid-valproate-exposure-pregnancy-endorsed (accessed 16 September 2021).
- Blotière PO, Miranda S, Weill A, Mikaeloff Y, Peyre H, Ramus F, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-034829.
- Mawhinney E, Craig J, Morrow J, Russell A, Smithson WH, Parsons L, et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology 2013;80:400-5. https://doi.org/10.1212/WNL.0b013e31827f0874.
- Brodie MJ, Perucca E, Ryvlin P, Ben-Menachem E, Meencke HJ. Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology 2007;68:402-8. https://doi.org/10.1212/01.wnl.0000252941.50833.4a.
- Trinka E, Marson AG, Van Paesschen W, Kälviäinen R, Marovac J, Duncan B, et al. KOMET: an unblinded, randomised, two parallel-group, stratified trial comparing the effectiveness of levetiracetam with controlled-release carbamazepine and extended-release sodium valproate as monotherapy in patients with newly diagnosed epilepsy. J Neurol Neurosurg Psychiatry 2013;84:1138-47. https://doi.org/10.1136/jnnp-2011-300376.
- Joint Formulary Committee . British National Formulary 2010.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 18 March 2021).
- Powell G, Logan J, Kiri V, Borghs S. Trends in antiepileptic drug treatment and effectiveness in clinical practice in England from 2003 to 2016: a retrospective cohort study using electronic medical records. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-032551.
- Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 2007;69:1751-60. https://doi.org/10.1212/01.wnl.0000268699.34614.d3.
- Noachtar S, Andermann E, Meyvisch P, Andermann F, Gough WB, Schiemann-Delgado J. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology 2008;70:607-16. https://doi.org/10.1212/01.wnl.0000297512.18364.40.
- Seino M. Review of zonisamide development in Japan. Seizure 2004;13:2-4. https://doi.org/10.1016/j.seizure.2004.04.015.
- Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol 2012;11:579-88. https://doi.org/10.1016/S1474-4422(12)70105-9.
- Balabanova S, Taylor C, Sills G, Burnside G, Plumpton C, Smith PEM, et al. Study protocol for a pragmatic randomised controlled trial comparing the effectiveness and cost-effectiveness of levetiracetam and zonisamide versus standard treatments for epilepsy: a comparison of standard and new antiepileptic drugs (SANAD-II). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-040635.
- Jacoby A, Baker G, Smith D, Dewey M, Chadwick D. Measuring the impact of epilepsy: the development of a novel scale. Epilepsy Res 1993;16:83-8. https://doi.org/10.1016/0920-1211(93)90042-6.
- Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res 1998;7:399-407. https://doi.org/10.1023/a:1008853819715.
- Cramer JA, Westbrook LE, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the Quality of Life in Epilepsy Inventory for Adolescents: the QOLIE-AD-48. Epilepsia 1999;40:1114-21. https://doi.org/10.1111/j.1528-1157.1999.tb00828.x.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14. https://doi.org/10.1542/peds.2004-2127.
- Prosser LA, Hammitt JK, Keren R. Measuring health preferences for use in cost-utility and cost-benefit analyses of interventions in children: theoretical and methodological considerations. PharmacoEconomics 2007;25:713-26. https://doi.org/10.2165/00019053-200725090-00001.
- Abetz L, Jacoby A, Baker GA, McNulty P. Patient-based assessments of quality of life in newly diagnosed epilepsy patients: validation of the NEWQOL. Epilepsia 2000;41:1119-28. https://doi.org/10.1111/j.1528-1157.2000.tb00317.x.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- NHS Improvement . User Guide: Reference Costs 2017 18 2018. https://improvement.nhs.uk/documents/1978/6_-_Reference_costs_2017-18_A_Guide_to_using_the_data.pdf (accessed 18 March 2021).
- Joint Formulary Committee . British National Formulary 2019.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Chadwick D, Beghi E, Callaghan N, de Bittencourt P, Dulac O, Gram L, et al. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39:799-803. https://doi.org/10.1111/j.1528-1157.1998.tb01167.x.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
- European Medicines Agency . Topic E 9 Statistical Principles for Clinical Trials (CPMP ICH 363 96) 1998.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Williamson PR, Smith CT, Sander JW, Marson AG. Importance of competing risks in the analysis of anti-epileptic drug failure. Trials 2007;8. https://doi.org/10.1186/1745-6215-8-12.
- Davis JW. Linear Mixed Models With Repeated Effects. Introduction and Examples Using SAS STAT® Software 2017. https://site.caes.uga.edu/expstatgrif/files/2018/07/RepeatedMixedFinal1.pdf (accessed 18 March 2021).
- Pocock SJ. When (not) to stop a clinical trial for benefit. JAMA 2005;294:2228-30. https://doi.org/10.1001/jama.294.17.2228.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Database of Instruments for Resource Use Management . SANAD-II RUM 2015. www.dirum.org/instruments/details/93 (accessed 18 March 2021).
- Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al. A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11370.
- NHS Digital . Data Linkage &Amp; Extract Service 2021. https://digital.nhs.uk/ (accessed 18 March 2021).
- SAIL Databank . The Secure Anonymised Information Linkage Databank 2021. https://saildatabank.com/ (accessed 18 March 2021).
- Lomas J, Asaria M, Bojke L, Gale CP, Richardson G, Walker S. Which costs matter? Costs included in economic evaluation and their impact on decision uncertainty for stable coronary artery disease. PharmacoEcon Open 2018;2:403-13. https://doi.org/10.1007/s41669-018-0068-1.
- Department of Health and Social Care . 2018/19/National/Cost/Collection/Data/Publication 2021. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 16 September 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- NHS Business Services Authority . Prescription Cost Analysis (PCA) Data September 2019 2019. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data (accessed 18 March 2021).
- NHS Wales . NHS Wales Data Dictionary 2020. www.datadictionary.wales.nhs.uk/#!WordDocuments/livedataitemsaz.htm (accessed 18 March 2021).
- NHS Digital . National Casemix Office HRG4+ 2018 19 Payment Grouper 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/payment---hrg4-2018-19-local-payment-grouper (accessed 18 March 2021).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott Williams & Wilkins; 1996.
- Mulhern B, Rowen D, Jacoby A, Marson T, Snape D, Hughes D, et al. The development of a QALY measure for epilepsy: NEWQOL-6D. Epilepsy Behav 2012;24:36-43. https://doi.org/10.1016/j.yebeh.2012.02.025.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Gabrio A, Mason AJ, Baio G. Handling missing data in within-trial cost-effectiveness analysis: a review with future recommendations. PharmacoEcon Open 2017;1:79-97. https://doi.org/10.1007/s41669-017-0015-6.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007;8:206-13. https://doi.org/10.1007/s11121-007-0070-9.
- van Asselt AD, van Mastrigt GA, Dirksen CD, Arntz A, Severens JL, Kessels AG. How to deal with cost differences at baseline. PharmacoEconomics 2009;27:519-28. https://doi.org/10.2165/00019053-200927060-00007.
- Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916. https://doi.org/10.1002/hec.1653.
- Paulden M. Calculating and interpreting ICERs and net benefit. PharmacoEconomics 2020;38:785-807. https://doi.org/10.1007/s40273-020-00914-6.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet 2002;9:12-9.
- European Medicines Agency (EMA) . Guideline for Good Clinical Practice E6(R2): Step 5 2016.
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Health Research Authority (HRA) . UK Policy Framework for Health and Social Care Research 2017.
- Great Britain . The Medicines for Human Use (Clinical Trials) Regulations 2004 2004.
- Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide or lamotrigine for newly diagnosed focal epilepsy: an open label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021;397:1363-74. https://doi.org/10.1016/S0140-6736(21)00247-6.
- Hill H, Rowen D, Pennington B, Wong R, Wailoo A. A review of the methods used to generate utility values in NICE technology assessments for children and adolescents. Value Health 2020;23:907-17. https://doi.org/10.1016/j.jval.2020.02.011.
- Ramos-Goñi JM, Oppe M, Stolk E, Shah K, Kreimeier S, Rivero-Arias O, et al. International valuation protocol for the EQ-5D-Y-3L. PharmacoEconomics 2020;38:653-63. https://doi.org/10.1007/s40273-020-00909-3.
- European Medicines Agency . Guideline on Clinical Investigation of Medicinal Products in the Treatment of Epileptic Disorders 2010. www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-epileptic-disorders-revision-2_en.pdf (accessed 18 March 2021).
- Perucca E. From clinical trials of antiepileptic drugs to treatment. Epilepsia Open 2018;3:220-30. https://doi.org/10.1002/epi4.12239.
- Nevitt SJ, Sudell M, Tudur Smith C, Marson AG, Cividini S. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev n.d.
- Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193-8. https://doi.org/10.1136/jnnp.2005.074203.
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597-605. https://doi.org/10.1056/NEJMoa0803531.
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609-17. https://doi.org/10.1016/S1474-4422(11)70107-7.
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 2018;17:530-8. https://doi.org/10.1016/S1474-4422(18)30107-8.
- Holmes EAF, Plumpton C, Baker GA, Jacoby A, Ring A, Williamson P, et al. Patient-focused drug development methods for benefit-risk assessments: a case study using a discrete choice experiment for antiepileptic drugs. Clin Pharmacol Ther 2019;105:672-83. https://doi.org/10.1002/cpt.1231.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Department of Health and Social Care and NHS England . Out-of-Hours GP Services in England 2014. www.nao.org.uk/wp-content/uploads/2014/09/Out-of-hours-GP-services-in-England1.pdf (accessed 18 March 2021).
- NHS . NHS Voucher Values for Glasses and Lenses 2020. www.nhs.uk/using-the-nhs/help-with-health-costs/nhs-voucher-values-for-glasses-and-lenses/ (accessed 18 March 2021).
- Gray E, Donten A, Karssemeijer N, van Gils C, Evans DG, Astley S, et al. Evaluation of a stratified national breast screening program in the United Kingdom: an early model-based cost-effectiveness analysis. Value Health 2017;20:1100-9. https://doi.org/10.1016/j.jval.2017.04.012.
- Bains I, Choi YH, Soldan K, Jit M. Clinical impact and cost-effectiveness of primary cytology versus human papillomavirus testing for cervical cancer screening in England. Int J Gynecol Cancer 2019;29:669-75. https://doi.org/10.1136/ijgc-2018-000161.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014815.
Appendix 1 Trial sites and principal investigators
Note that some hospitals have at least two principal investigator names because they either had principal investigator change(s) or recruited both adult and paediatric patients. The principal investigator names within one hospital site are listed in reverse chronological order.
*Recruited both adults and children.
-
Aberdeen Royal Infirmary, Aberdeen, UK (Karen Lanyon).
-
Addenbrooke’s Hospital,* Cambridge, UK (Mark Manford, Manali Chitre and Alasdair Parker).
-
Alder Hey Children’s Hospital, Liverpool, UK (Nina Swiderska and Richard Appleton).
-
Arrowe Park Hospital, Upton, UK (James Pauling and Adrian Hughes).
-
Birmingham Children’s Hospital, Birmingham, UK (Rajat Gupta).
-
Heartlands Hospital, Birmingham, UK (Sadia Hanif and Mostafa Awadh).
-
Blackpool Victoria Hospital, Blackpool, UK (Sharmini Ragunathan and Nicola Cable).
-
Breightmet Health Centre,* Bolton, UK (Paul Cooper and Dan Hindley).
-
Burnley General Teaching Hospital, Burnley, UK (Karl Rakshi).
-
Central Middlesex Hospital, London, UK (Sophie Molloy).
-
Charing Cross Hospital, London, UK (Michael Johnson).
-
Chesterfield Royal Hospital, Chesterfield, UK (Kunle Ayonrinde).
-
Countess of Chester Hospital,* Chester, UK (Martin Wilson, Satyanarayana Saladi and John Gibb).
-
Craigavon Area Hospital, Craigavon, UK (Lesley-Ann Funston, Damhait Cassidy and Jonathan Boyd).
-
Derbyshire Children’s Hospital, Derby, UK (Mal Ratnayaka and Hani Faza).
-
Derriford Hospital, Plymouth, UK (Martin Sadler).
-
Diana Princess of Wales Hospital, Grimsby, UK (Hassan Al-Moasseb).
-
Frimley Park Hospital, Frimley, UK (Clare Galtrey and Damien Wren).
-
Furness General Hospital, Barrow-in-Furness, UK (Anas Olabi).
-
Gloucestershire Royal Hospital, Gloucester, UK (Geraint Fuller).
-
Good Hope Hospital, Birmingham, UK (Muhammed Khan and Chetana Kallappa).
-
Great Western Hospital, Swindon, UK (Ravi Chinthapalli).
-
Gwynedd Hospital,* Bangor, UK (Baba Aji, Rhys Davies and Kathryn Foster).
-
The James Cook University Hospital, Middlesbrough, UK (Nikolas Hitiris).
-
Leeds General Infirmary, Leeds, UK (Melissa Maguire).
-
Leicester Royal Infirmary, Leicester, UK (Nahin Hussain).
-
Leighton Hospital, Crewe, UK (Simon Dowson and Julie Ellison).
-
Lincoln County, Lincoln, UK (Basil Sharrack).
-
Luton & Dunstable University Hospital, Luton, UK (Vandna Gandhi).
-
Morriston Hospital, Swansea, UK (Rob Powell).
-
New Cross Hospital,* Wolverhampton, UK (Phil Tittensor, Beatrice Summers, Sastry Shashikiran and Penelope J Dison).
-
Queen Elizabeth Hospital, Birmingham, UK (Shanika Samarasekera and Doug McCorry).
-
Ninewells Hospital, Dundee, UK (Kathleen White).
-
Northampton General Hospital, Northampton, UK (Kannan Nithi).
-
Peterborough City Hospital, Peterborough, UK (Martin Richardson and Richard Brown).
-
Poole General Hospital, Poole, UK (Rupert Page).
-
Prince Charles Hospital, Merthyr Tydfil, UK (David Deekollu).
-
Queen Alexandra Hospital, Portsmouth, UK (Sean Slaght and Stephen Warriner).
-
Queen’s Hospital Burton, Burton on Trent, UK (Mansoor Ahmed).
-
Queen’s Hospital, Romford, UK (Abhijit Chaudhuri).
-
Queen’s Medical Centre, Nottingham, UK (Gabby Chow).
-
Raigmore Hospital, Inverness, UK (Javier Artal and Danute Kucinskiene).
-
Royal Albert Edward Infirmary, Wigan, UK (Harish Sreenivasa, Singara Velmurugan and Christos S Zipitis).
-
Royal Cornwall Hospital, Truro, UK (Brendan McLean).
-
Royal Derby Hospital, Derby, UK (Vaithianathar Lal, Angelous Gregoriou and Paul Maddison).
-
Royal Glamorgan Hospital, Ynysmaerdy, UK (Trevor Pickersgill).
-
Royal Gwent Hospital, Newport, UK (Joseph Anderson and Charlotte Lawthom).
-
Royal Hallamshire Hospital, Sheffield, UK (Steve Howell).
-
Royal Hampshire County Hospital, Winchester, UK (Gabriel Whitlingum, Wotjek Rakowicz and Lucy Kinton).
-
Royal Hospital for Sick Children Edinburgh, Edinburgh, UK (Alisa McLellan and Nitish Vora).
-
Royal Hospital for Children, Glasgow, UK (Sameer Zuberi).
-
Royal London Hospital, London, UK (Andrew Kelso).
-
Royal Manchester Children’s Hospital, Manchester, UK (Imelda Hughes and John Martland).
-
Royal Preston Hospital,* Preston, UK (Hedley Emsley and Christian de Goede).
-
Royal Stoke University Hospital,* Stoke-on-Trent, UK (R P Singh and Carl-Christian Moor).
-
Royal Sussex County Hospital, Brighton, UK (Julia Aram).
-
Salford Royal Hospital,* Salford, UK (Rajiv Mohanraj and Kumar Sakthivel).
-
Scunthorpe General Hospital, Scunthorpe, UK (Suresh Nelapatla).
-
Sheffield Children’s Hospital, Sheffield, UK (Chris Rittey).
-
Southampton General Hospital, Southampton, UK (Ashwin Pinto).
-
Southern General Hospital, Glasgow, UK (John Paul Leach).
-
St George’s Hospital, London, UK (Hannah Cock).
-
Stepping Hill Hospital,* Stockport, UK (Anna Richardson, Erika Houston and Christopher Cooper).
-
Sunderland Royal Hospital, Sunderland, UK (Geoff Lawson).
-
Tameside General Hospital, Ashton-under-Lyne, UK (Albert Massarano).
-
The Walton Centre, Liverpool, UK (Tony Marson).
-
Torbay Hospital, Torquay, UK (Indranil Dey).
-
University Hospital of North Tees, Stockton-on-Tees, UK (Puthuval Sivakumar).
-
University Hospital of South Manchester, Manchester, UK (Lap-Kong Yeung).
-
University Hospital of Wales, Cardiff, UK (Philip Smith).
-
Warrington Hospital, Warrington, UK (Richard Briggs).
-
Whiston Hospital, Prescot, UK (Hemalata Bentur).
-
Worcestershire Royal Hospital, Worcester, UK (Tom Heafield).
-
Worthing Hospital, Worthing, UK (Anna Mathew).
-
Wrexham Maelor Hospital,* Wrexham, UK (Dave Smith and Praveen Jauhari).
The following sites opened but did not recruit:
-
Forth Valley Royal Hospital, Larbert, UK (Malcolm Macleod).
-
Glan Clwyd Hospital, Bodelwyddan, UK (Mark Doran).
-
King’s College Hospital, London, UK (Robert Elwes).
-
The Robert Jones and Agnes Hunt Orthopaedic Hospital, Oswestry, UK (Richa Kulshrestha).
-
Royal Devon & Exeter Hospital, Exeter, UK (Richard Tomlinson).
-
Royal Liverpool Hospital, Liverpool, UK (Tony Marson).
-
Royal Surrey County Hospital, Guildford, UK (Charlie Moss).
-
Royal Victoria Hospital, Belfast, UK (John Craig).
-
Southport and Formby District General Hospital, Southport, UK (Udo Wieshmann).
Appendix 2 Key protocol amendments
| Protocol version and date | Key amendments |
|---|---|
| 1.0 (30 March 2012) | Original approved protocol |
| 2.0 (4 January 2013) |
Sections 1 and 5: some of the exclusion criteria were clarified Section 2: added new subsection ‘Definitions’ Section 6: screening section amended Section 7: dose modifications section amended Section 8: QoL and utility assessments section updated Section 10: pharmacovigilance section updated to reflect the difference in the reporting procedures for trial and non-trial ASMs |
| 3.0 (7 March 2013) |
Contact details for the University of Liverpool updated Section 7: text deleted to allow all licensed drug formulations to be used and the initial target maintenance dose for zonisamide amended in Table 1 |
| 4.0 (13 June 2014) |
Sections 1 and 5: two inclusion criteria were amended:Sections 5 and 6: text amended to include patients who lack capacity to consent for themselves Section 6: the randomisation table was reformatted for clarity and the back-up randomisation system was changed from randomisation envelopes to replica of the randomisation system based on a stand-alone personal computer at CTU Section 7: Table 1 (Arm A. Aged > 12 years) updated by adding ‘for 2 weeks’ after 50 mg a.m. 100 mg p.m. in the zonisamide column Table 2 (Arm A. Children aged 5–12 years) amended for the titration steps and the initial maintenance dose for zonisamide Section 8: Table 6 was updated to include group names in brackets to the age ranges in the column for participant age Section 10: the flow chart was updated to reflect the verbatim description by removing unexpected/expected step, as it is done centrally by the chief investigator Section 11: updated to include references to the informed consent process in incapacitated adults |
| 5.0 (22 July 2015) |
Title page: the NIHR logo updated and a funding statement added Protocol approval, contact details and glossary sections were updated Section 7: error corrected in the titrating regimen for lamotrigine Section 8: to appropriately describe the trial management, the following text: ii) ‘collected by Research Nurses from each hospital’s patient administration system (PAS)’ was replaced with ‘accessed as Hospital Episode Statistics (HES) data via the Health and Social Care Information Centre’ iii) ‘PAS’ was replaced with ‘HES’ Section 11: text amended to specify the return time frame for consent forms |
| 6.0 (19 May 2017) |
Protocol approval and contact details updated Section 1: exclusion criteria clarified and study duration updated Section 5: new section about withdrawal from the randomised drug added Section 6: text about eligibility confirmation was amended for clarity Section 10: definition for SUSAR added. Reporting flow chart amended |
| 7.0 (23 August 2017) | Section 8: Table 5 (trial assessments) updated to allow the follow-up questionnaires issue at site during routine clinic visits |
| 8.0 (28 November 2018) |
Signatories and contact details: change to UoL sponsor representative signatory because of retirement of Professor Walley and change to Walton Centre sponsor representative signatory Section 4: change to secondary outcomes Section 8: QoL and utility assessments updated Section 9: updates to text throughout section because of change in secondary outcomes in section 4.2 |
Appendix 3 Further details of results
Additional tables and figures for the focal epilepsy trial
FIGURE 23.
Recruitment graph for the focal epilepsy trial.
| Follow-up statistic | Lamotrigine group | Levetiracetam group | Zonisamide group |
|---|---|---|---|
| Completeness of follow-up statistic (%) | 77.2 | 78.3 | 75.6 |
| Maximum follow-up time (days) | |||
| Median | 1482 | 1492 | 1488 |
| IQR | 1185–1802 | 1185–1801 | 1191–1808 |
| Potential follow-up time (days) | |||
| Median | 616.5 | 718 | 654 |
| IQR | 366–1096 | 366–1096 | 366–1095 |
| Observed follow-up time (days) | |||
| Median | 462.5 | 449.5 | 447 |
| IQR | 365–777 | 365–824 | 365–730 |
| Reverse Kaplan–Meier estimatea | |||
| Median | 1096 | 1124 | 968 |
| IQR | 730–1370 | 730–1461 | 730–1398 |
| Number (%) withdrawn or lost to follow-upb | 53 (16.0) | 53 (16.0) | 59 (18.0) |
| Number (%) lost to follow-up without treatment failure | 38 (11.2) | 30 (9.0) | 32 (9.8) |
Additional baseline tables and figures for focal epilepsy trial
| Characteristic | Lamotrigine group | Levetiracetam group | Zonisamide group | Total |
|---|---|---|---|---|
| Age group (deciles) (years), n (%) | 330 | 332 | 328 | 990 |
| 0–9 | 25 (7.6) | 36 (10.8) | 28 (8.5) | 89 (9.0) |
| 10–19 | 48 (14.5) | 33 (9.9) | 40 (12.2) | 121 (12.2) |
| 20–29 | 59 (17.9) | 62 (18.7) | 48 (14.6) | 169 (17.1) |
| 30–39 | 43 (13.0) | 54 (16.3) | 59 (18.0) | 156 (15.8) |
| 40–49 | 43 (13.0) | 60 (18.1) | 56 (17.1) | 159 (16.1) |
| 50–59 | 39 (11.8) | 36 (10.8) | 28 (8.5) | 103 (10.4) |
| 60–69 | 38 (11.5) | 23 (6.9) | 33 (10.1) | 94 (9.5) |
| 70–79 | 25 (7.6) | 22 (6.6) | 20 (6.1) | 67 (6.8) |
| 80–89 | 9 (2.7) | 6 (1.8) | 16 (4.9) | 31 (3.1) |
| 90–99 | 1 (0.3) | 0 | 0 | 1 (0.1) |
| Weight in kg (if aged ≤ 12 years) (n) | 35 | 42 | 41 | 118 |
| Mean (SD) | 32 (10) | 30 (8) | 32 (14) | 31 (11) |
| Median (IQR) | 30 (26–35) | 29 (24–35) | 28 (23–41) | 28 (23–37) |
| Range | 12–55 | 15–51 | 15–76 | 12–76 |
| Missing | 5 | 3 | 3 | 11 |
| MRI, n (%) | ||||
| MRI not done | 90 (27.3) | 82 (24.7) | 86 (26.2) | 258 (26.1) |
| MRI normal | 154 (46.7) | 176 (53.0) | 162 (49.4) | 492 (49.7) |
| Head injury | 5 (2.0) | 4 (1.5) | 3 (1.2) | 12 (1.6) |
| Tumour | 4 (1.6) | 1 (0.4) | 3 (1.2) | 8 (1.1) |
| Cortical dysplasia | 1 (0.4) | 3 (1.1) | 4 (1.6) | 8 (1.1) |
| Hippocampal sclerosis | 5 (2.0) | 4 (1.5) | 3 (1.2) | 12 (1.6) |
| AVM or other vascular malformation (e.g. cavernoma) | 7 (2.8) | 7 (2.6) | 4 (1.6) | 18 (2.3) |
| Infarct | 12 (4.7) | 11 (4.1) | 4 (1.6) | 27 (3.5) |
| Haemorrhage | 4 (1.6) | 3 (1.1) | 2 (0.8) | 9 (1.2) |
| Previous infection (e.g. encephalitis/abscess) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 5 (0.7) |
| Other | 53 (17.8) | 44 (14.5) | 58 (19.0) | 155 (17.1) |
| CT scan, n (%) | ||||
| CT scan not carried out | 213 (64.5) | 222 (66.9) | 211 (64.3) | 646 (65.3) |
| CT scan normal | 80 (24.2) | 79 (23.8) | 90 (27.4) | 249 (25.2) |
| Head injury | 2 (0.7) | 1 (0.3) | 3 (1.0) | 6 (0.7) |
| Tumour | 1 (0.3) | 1 (0.3) | 2 (0.7) | 4 (0.4) |
| Cortical dysplasia | 1 (0.3) | 1 (0.3) | 0 | 2 (0.2) |
| Hippocampal sclerosis | 0 | 1 (0.3) | 0 | 1 (0.1) |
| AVM or other vascular malformation (e.g. cavernoma) | 3 (1.0) | 2 (0.7) | 1 (0.3) | 6 (0.7) |
| Infarct | 5 (1.7) | 8 (2.6) | 5 (1.6) | 18 (2.0) |
| Haemorrhage | 3 (1.0) | 3 (1.0) | 2 (0.7) | 8 (0.9) |
| Previous infection (e.g. encephalitis/abscess) | 3 (1.0) | 2 (0.7) | 4 (1.3) | 9 (1.0) |
| Porencephalic cyst | 1 (0.3) | 0 | 0 | 1 (0.1) |
| Other | 16 (5.1) | 12 (3.8) | 11 (3.5) | 39 (4.2) |
FIGURE 24.
Completeness of follow-up scatterplots of observed follow-up time by date of randomisation: focal epilepsy trial. (a) Lamotrigine; (b) levetiracetam; and (c) zonisamide.
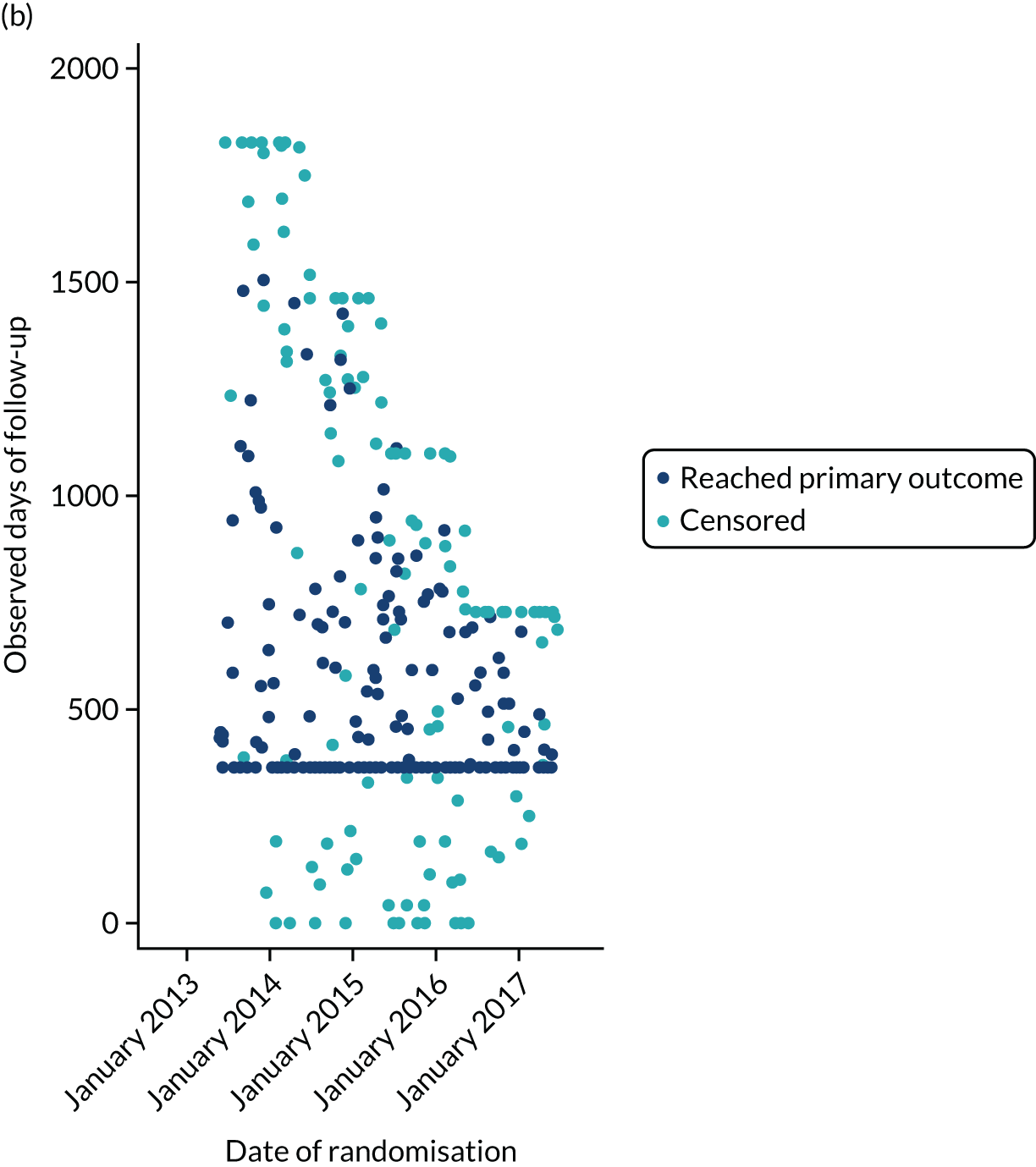
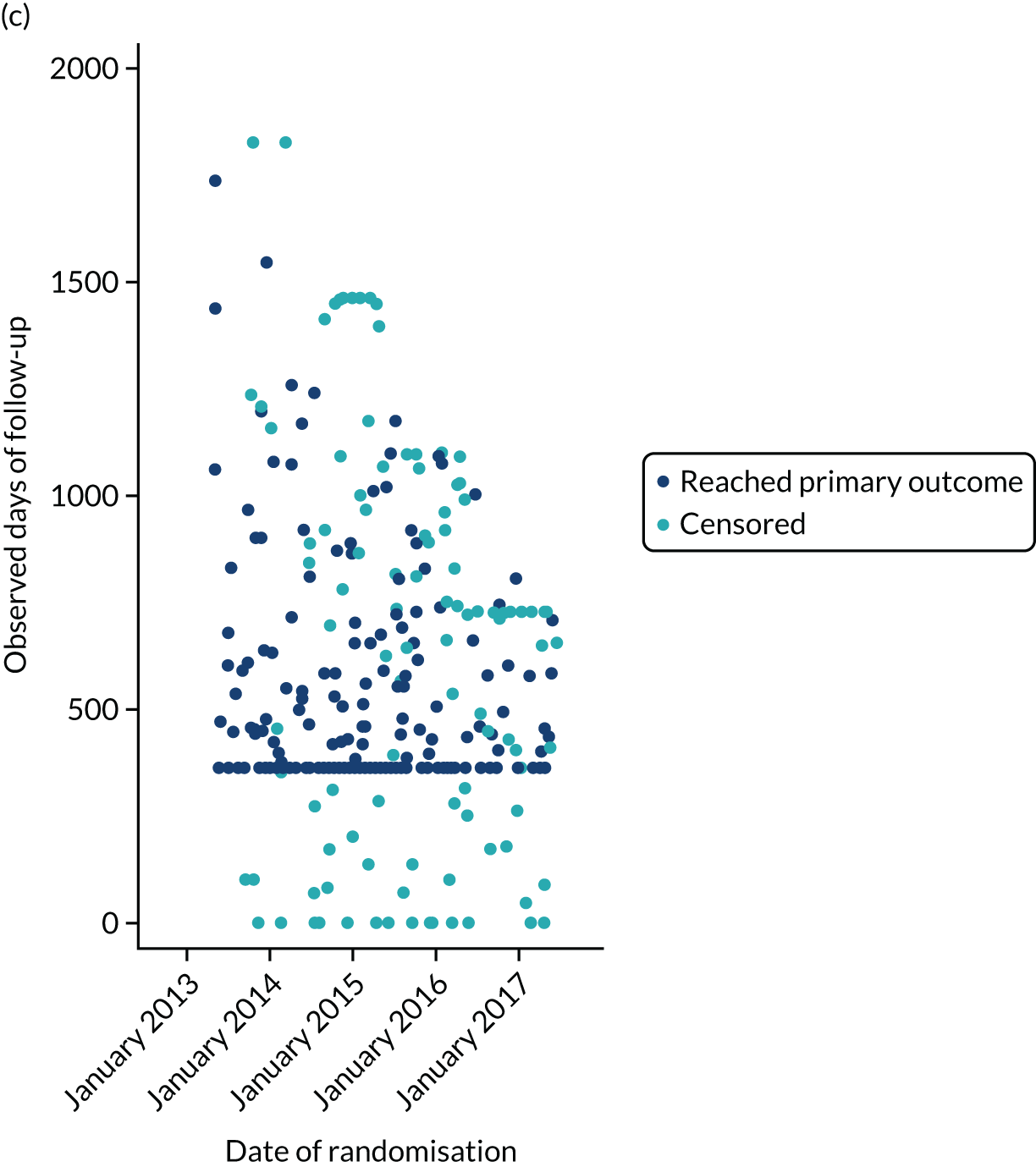
| Model and analysis set | Comparison | Time interval | HR (97.5% CI) |
|---|---|---|---|
| Alternative imputation rules | Lamotrigine vs. levetiracetam | All follow-up | 1.18 (0.98 to 1.42) |
| Misdiagnoses excluded (withdrawal reason ‘not epilepsy’) | Lamotrigine vs. levetiracetam | All follow-up | 1.18 (0.95 to 1.47) |
| Alternative imputation rules | Lamotrigine vs. zonisamide | All follow-up | 1.03 (0.86 to 1.23) |
| Misdiagnoses excluded (withdrawal reason ‘not epilepsy’) | Lamotrigine vs. zonisamide | All follow-up | 1.03 (0.83 to 1.28) |
| Event MedDRA-preferred term | Number of events | Number of patients (%) | ||||
|---|---|---|---|---|---|---|
| Lamotrigine group | Levetiracetam group | Zonisamide group | Lamotrigine group (n = 328) | Levetiracetam group (n = 330) | Zonisamide group (n = 324) | |
| Fatigue | 20 | 31 | 37 | 16 (4.9) | 29 (8.8) | 34 (10.5) |
| Depressed mood | 7 | 23 | 17 | 7 (2.1) | 20 (6.1) | 16 (4.9) |
| Irritability | 6 | 29 | 11 | 6 (1.8) | 29 (8.8) | 11 (3.4) |
| Headache | 15 | 13 | 17 | 13 (4.0) | 13 (3.9) | 16 (4.9) |
| Memory impairment | 10 | 14 | 16 | 9 (2.7) | 13 (3.9) | 15 (4.6) |
| Dizziness | 13 | 16 | 9 | 13 (4.0) | 13 (3.9) | 9 (2.8) |
| Insomnia | 14 | 9 | 11 | 12 (3.7) | 9 (2.7) | 11 (3.4) |
| Mood altered | 6 | 15 | 11 | 6 (1.8) | 14 (4.2) | 10 (3.1) |
| Nausea | 9 | 11 | 10 | 9 (2.7) | 10 (3.0) | 10 (3.1) |
| Rash | 17 | 5 | 8 | 16 (4.9) | 5 (1.5) | 7 (2.2) |
| Somnolence | 11 | 17 | 2 | 10 (3.0) | 17 (5.2) | 2 (0.6) |
| Weight decreased | 4 | 5 | 14 | 4 (1.2) | 5 (1.5) | 14 (4.3) |
| Anxiety | 4 | 9 | 9 | 4 (1.2) | 9 (2.7) | 9 (2.8) |
| Depression | 3 | 13 | 5 | 3 (0.9) | 11 (3.3) | 5 (1.5) |
| Aggression | 1 | 12 | 5 | 1 (0.3) | 12 (3.6) | 5 (1.5) |
| Decreased appetite | 2 | 2 | 14 | 2 (0.6) | 2 (0.6) | 14 (4.3) |
| Mood swings | 2 | 8 | 6 | 2 (0.6) | 7 (2.1) | 6 (1.9) |
| Tremor | 9 | 1 | 5 | 9 (2.7) | 1 (0.3) | 5 (1.5) |
| Vomiting | 5 | 5 | 4 | 5 (1.5) | 5 (1.5) | 4 (1.2) |
| Anger | 2 | 4 | 7 | 2 (0.6) | 3 (0.9) | 7 (2.2) |
| Abdominal pain | 1 | 4 | 6 | 1 (0.3) | 4 (1.2) | 6 (1.9) |
| Disturbance in attention | 4 | 2 | 5 | 4 (1.2) | 2 (0.6) | 3 (0.9) |
| Pruritus | 3 | 2 | 6 | 2 (0.6) | 2 (0.6) | 6 (1.9) |
| Sedation | 2 | 2 | 7 | 2 (0.6) | 2 (0.6) | 7 (2.2) |
| Agitation | 1 | 4 | 5 | 1 (0.3) | 4 (1.2) | 5 (1.5) |
| Amnesia | 3 | 2 | 5 | 3 (0.9) | 2 (0.6) | 5 (1.5) |
| Diarrhoea | 2 | 3 | 5 | 2 (0.6) | 3 (0.9) | 5 (1.5) |
| Weight increased | 2 | 6 | 2 | 2 (0.6) | 6 (1.8) | 2 (0.6) |
| Dry mouth | 6 | 0 | 2 | 6 (1.8) | 0 | 2 (0.6) |
| Lethargy | 3 | 2 | 3 | 3 (0.9) | 2 (0.6) | 3 (0.9) |
| Abnormal behaviour | 1 | 4 | 2 | 1 (0.3) | 4 (1.2) | 2 (0.6) |
| Arthralgia | 3 | 0 | 4 | 3 (0.9) | 0 | 4 (1.2) |
| Balance disorder | 2 | 2 | 3 | 2 (0.6) | 2 (0.6) | 3 (0.9) |
| Affect lability | 1 | 3 | 2 | 1 (0.3) | 3 (0.9) | 2 (0.6) |
| Alopecia | 1 | 2 | 3 | 1 (0.3) | 2 (0.6) | 3 (0.9) |
| Constipation | 1 | 3 | 2 | 1 (0.3) | 3 (0.9) | 1 (0.3) |
| Dysarthria | 3 | 2 | 0 | 2 (0.6) | 2 (0.6) | 0 |
| Feeling abnormal | 1 | 2 | 2 | 1 (0.3) | 1 (0.3) | 2 (0.6) |
| Paraesthesia | 3 | 1 | 1 | 3 (0.9) | 1 (0.3) | 1 (0.3) |
| Abdominal discomfort | 0 | 1 | 3 | 0 | 1 (0.3) | 2 (0.6) |
| Ataxia | 3 | 1 | 0 | 3 (0.9) | 1 (0.3) | 0 |
| Cognitive disorder | 1 | 0 | 3 | 1 (0.3) | 0 | 3 (0.9) |
| Eczema | 2 | 0 | 2 | 1 (0.3) | 0 | 1 (0.3) |
| Palpitations | 2 | 2 | 0 | 2 (0.6) | 2 (0.6) | 0 |
| Poor-quality sleep | 0 | 3 | 1 | 0 | 3 (0.9) | 1 (0.3) |
| Abnormal dreams | 3 | 0 | 0 | 3 (0.9) | 0 | 0 |
| Dry skin | 0 | 1 | 2 | 0 | 1 (0.3) | 2 (0.6) |
| Gait disturbance | 1 | 1 | 1 | 1 (0.3) | 1 (0.3) | 1 (0.3) |
| Hyperhidrosis | 0 | 1 | 2 | 0 | 1 (0.3) | 2 (0.6) |
| Muscle twitching | 1 | 0 | 2 | 1 (0.3) | 0 | 1 (0.3) |
| Nephrolithiasis | 0 | 0 | 3 | 0 | 0 | 2 (0.6) |
| Nightmare | 3 | 0 | 0 | 3 (0.9) | 0 | 0 |
| Vision blurred | 1 | 0 | 2 | 1 (0.3) | 0 | 2 (0.6) |
| Apathy | 1 | 1 | 0 | 1 (0.3) | 1 (0.3) | 0 |
| Aphasia | 0 | 0 | 2 | 0 | 0 | 2 (0.6) |
| Burning sensation | 1 | 0 | 1 | 1 (0.3) | 0 | 1 (0.3) |
| Confusional state | 0 | 1 | 1 | 0 | 1 (0.3) | 1 (0.3) |
| Defiant behaviour | 0 | 2 | 0 | 0 | 2 (0.6) | 0 |
| Drug intolerance | 0 | 1 | 1 | 0 | 1 (0.3) | 1 (0.3) |
| Dysgeusia | 1 | 0 | 1 | 1 (0.3) | 0 | 1 (0.3) |
| Dyspepsia | 0 | 1 | 1 | 0 | 1 (0.3) | 1 (0.3) |
| Emotional distress | 0 | 2 | 0 | 0 | 2 (0.6) | 0 |
| Gastrointestinal disorder | 2 | 0 | 0 | 2 (0.6) | 0 | 0 |
| Hallucination | 0 | 2 | 0 | 0 | 2 (0.6) | 0 |
| Hallucination, auditory | 0 | 0 | 2 | 0 | 0 | 2 (0.6) |
| Increased appetite | 2 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Mouth ulceration | 2 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Pain in extremity | 1 | 0 | 1 | 1 (0.3) | 0 | 1 (0.3) |
| Rash generalised | 0 | 2 | 0 | 0 | 2 (0.6) | 0 |
| Rash pruritic | 1 | 0 | 1 | 1 (0.3) | 0 | 1 (0.3) |
| Rosacea | 2 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Social avoidant behaviour | 1 | 0 | 1 | 1 (0.3) | 0 | 1 (0.3) |
| Suicidal ideation | 0 | 2 | 0 | 0 | 2 (0.6) | 0 |
| Thinking abnormal | 0 | 0 | 2 | 0 | 0 | 2 (0.6) |
| Visual impairment | 0 | 1 | 1 | 0 | 1 (0.3) | 1 (0.3) |
| Abdominal pain upper | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Accidental overdose | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Acne | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Acute kidney injury | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Adverse drug reaction | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Bruxism | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Contusion | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Conversion disorder | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Dermatitis allergic | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Diplopia | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Dissociation | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Drooling | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Drug eruption | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Dry eye | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Emotional disorder | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Epistaxis | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Extrasystoles | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Feeling drunk | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Frustration tolerance decreased | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Gout | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Haematemesis | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Hair texture abnormal | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Head discomfort | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Hyperventilation | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Hypoaesthesia | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Hypohidrosis | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Hypothyroidism | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Influenza-like illness | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Limb discomfort | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Lip pain | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Loss of libido | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Malaise | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Migraine | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Mouth swelling | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Myalgia | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Nasal valve collapse | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Obsessive–compulsive disorder | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Orthostatic hypotension | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Panic attack | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Parkinson’s disease | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Parosmia | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Peripheral swelling | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Personality change | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Pollakiuria | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Polydipsia | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Polyuria | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Premature delivery | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Presyncope | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Psychomotor hyperactivity | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Pyrexia | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Rash papular | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Restless legs syndrome | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Seizure | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Sleep disorder | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Speech disorder | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Stevens–Johnson syndrome | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Suicide attempt | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Swelling face | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Swollen tongue | 1 | 0 | 0 | 1 (0.3) | 0 | 0 |
| Tearfulness | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Tinnitus | 0 | 1 | 0 | 0 | 1 (0.3) | 0 |
| Urinary incontinence | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Weight gain poor | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Wheezing | 0 | 0 | 1 | 0 | 0 | 1 (0.3) |
| Total number of events and patients with at least one AR | 251 | 328 | 351 | 108 (32.9) | 144 (43.6) | 146 (45.1) |
| Description | Seriousness | Severity | Suspect anti-seizure medication | Expectedness | Relationship: principal investigator assessment | Relationship: chief investigator assessment | Withdrawn from study drug | Outcome |
|---|---|---|---|---|---|---|---|---|
| Randomised to start treatment with lamotrigine | ||||||||
| 1: vomiting | Required hospitalisation | Severe | Valproate | Expected | Probably | Possibly | Yes | Resolved |
| Lamotrigine | Expected | Unlikely | Possibly | No | ||||
| 2: ataxia | Required hospitalisation | Moderate | Lamotrigine | Expected | Possibly | Possibly | No | Resolved |
| Randomised to start treatment with levetiracetam | ||||||||
| 3: vomiting | Required hospitalisation | Moderate | Zonisamide | Expected | Possibly | Possibly | Yes | Resolved |
| Randomised to start treatment with zonisamide | ||||||||
| 4: nephrolithiasis | Required hospitalisation | Moderate | Zonisamide | Expected | Probably | Probably | Yes | Resolved |
| 5: suicide attempt | Medically significant/Important | Moderate | Zonisamide | Expected | Possibly | Possibly | Temporary interruption | Resolved |
| 6: acute kidney injury | Required hospitalisation | Severe | Zonisamide | Expected | Possibly | Possibly | Yes | Resolved |
| 7: premature delivery | Medically significant/Important | Severe | Zonisamide | Expected | Possibly | Possibly | No | Resolved with sequelae |
| Days from randomisation to death | Age at death (years) | Cause of death | Possibly related to trial treatments? |
|---|---|---|---|
| Randomised to start treatment with lamotrigine | |||
| 338 | 51 | Cardiac arrest; epileptic seizure | No |
| 7 | 14 | Sudden unexpected death in epilepsy | No |
| 589 | 47 | Status epilepticus; natural causes | No |
| 771 | 67 | Fall down stairs, found at bottom of stairs, blood on face and ear | No |
| 1126 | 69 | Metastatic cancer of kidney | No |
| 39 | 83 | Congestive heart failure | No |
| 697 | 82 | Alzheimer’s disease | No |
| 41 | 88 | Severe aortic stenosis | No |
| 1500 | 64 | Cardiorespiratory arrest; end-stage renal failure | No |
| 291 | 28 | Sudden unexpected death in epilepsy; abnormal blood levels of prescribed medications (levetiracetam not detected, elevated sertraline levels) | No |
| 183 | 53 | Large haemorrhagic stroke; hypertension | No |
| 1115 | 54 | Acute myocardial infarction; coronary artery disease; severe fatty liver | No |
| 1779 | 83 | Vascular dementia | No |
| 1355 | 70 | Bronchopneumonia | No |
| 413 | 72 | Progressive metastatic neuroendocrine malignancy | No |
| Randomised to start treatment with levetiracetam | |||
| 461 | 44 | Glioblastoma | No |
| 274 | 66 | Cancer of the pancreas and metastatic spread | No |
| 177 | 54 | Sudden expected death in epilepsy | No |
| 1221 | 88 | Pneumonia | No |
| 254 | 53 | Ischaemic event probably coronary | No |
| 1815 | 66 | Bladder cancer | No |
| 112 | 51 | Glioblastoma multiforme | No |
| 34 | 39 | Hypoxic brain injury; asystolic cardiac arrest (unwitnessed) | No |
| 256 | 65 | Pneumonia; anterior circulation stroke | No |
| 749 | 24 | Intracerebral haemorrhage due to rupture of arteriovenous malformation | No |
| 424 | 39 | Sudden unexplained death in epilepsy | No |
| 311 | 24 | Patient died at home. Referred to the coroner. Had been admitted to hospital on 17 August 2016 via A&E. Breathlessness. Self-discharged before respiratory review. Working diagnosis in A&E was infected, exacerbation of asthma | No |
| Randomised to start treatment with zonisamide | |||
| 644 | 84 | Lower respiratory tract infection; immobility; cervical myelopathy | No |
| 264 | 60 | Pulmonary embolism | No |
| 1600 | 85 | Multiorgan failure; hypoperfusion; status epilepticus | No |
| 547 | 75 | Hypertensive heart disease | No |
| 1746 | 87 | Old age; vascular dementia | No |
| 352 | 83 | Colon cancer | No |
| 509 | 81 | Pneumonia; acute renal failure; malignant neoplasm of rectum | No |
| 216 | 67 | Found dead at home | No |
| 341 | 48 | Epilepsy | No |
| 530 | 66 | Chronic obstructive pulmonary disease | No |
| Patient ID | Day of pregnancy reporta | Age at date of report (years) | Estimated day of deliverya | Day of delivery/miscarriagea | Outcome | Randomised drug | Drug regimen when pregnancy reported |
|---|---|---|---|---|---|---|---|
| 1 | 113 | 28 | 308 | 344 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 2 | 239 | 26 | 341 | 302 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 3 | 1416 | 36 | 1604 | 1580 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 4 | 826 | 30 | 1002 | 995 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 5 | 1113 | 26 | 1290 | 1287 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 6 | 1272 | 31 | 1472 | 1470 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 7 | 464 | 30 | 704 | 678 | Minor malformations | Lamotrigine | Carbamazepine and clobazam |
| 8 | 1246 | 20 | 1484 | 1476 | Normal postnatal examination | Lamotrigine | Levetiracetam |
| 9 | 497 | 27 | 650 | 649 | Normal postnatal examination | Lamotrigine | Lamotrigine |
| 10 | 1351 | 31 | 1582 | 1585 | Normal postnatal examination | Lamotrigine | Levetiracetam |
| 11 | 1245 | 19 | 1436 | 1439 | Normal postnatal examination | Lamotrigine | No anti-seizure medication |
| 12 | 863 | 35 | 1046 | 1051 | Normal postnatal examination | Levetiracetam | Lamotrigine |
| 13 | 351 | 29 | 545 | 534 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 14 | 2041 | 30 | Missing | 2024 | Termination | Levetiracetam | Levetiracetam |
| 15 | 679 | 33 | 908 | 905 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 16 | 407 | 38 | 480 | 471 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 16 | 1023 | 40 | 1198 | 1185 | Normal postnatal examination | Levetiracetam | Lamotrigine |
| 17 | 362 | 38 | 512 | 504 | Normal postnatal examination | Zonisamide | Levetiracetam |
| 18 | 30 | 34 | 154 | 38 | Planned abortion | Zonisamide | Lamotrigine |
| 19 | 356 | 34 | 640 | 419 | Miscarriage | Zonisamide | Zonisamide |
| 19 | 811 | 35 | Missing | 811 | Miscarriage | Zonisamide | Zonisamide |
| 20 | 1148 | 27 | 1298 | 1301 | Normal postnatal examination | Zonisamide | Zonisamide |
| 21 | 664 | 39 | Missing | 706 | Miscarriage | Zonisamide | Zonisamide |
| 21 | 1099 | 41 | 1318 | 1115 | Miscarriage | Zonisamide | Lamotrigine |
| 22 | 170 | 24 | 429 | 180 | Miscarriage | Zonisamide |
Zonisamide Lamotrigine |
| 23 | 366 | 44 | Missing | 275 | Miscarriage | Zonisamide | Zonisamide |
| 23 | 559 | 44 | 798 | 576 | Miscarriage | Zonisamide | Zonisamide |
| 24 | 1091 | 25 | 1302 | 1306 | Normal postnatal examination | Zonisamide | Lamotrigine |
| 25 | 119 | 38 | Missing | 82 | Miscarriage | Zonisamide | Levetiracetam, carbamazepine and topiramate |
| 26 | 930 | 29 | 1168 | 1158 | Normal postnatal examination | Zonisamide | Carbamazepine |
| 27 | 538 | 26 | 785 | 780 | Normal postnatal examination | Zonisamide | No anti-seizure medication |
| 28 | 1252 | 30 | 1434 | 1362 | Normal postnatal examination | Zonisamide | Levetiracetam |
| 29 | 219 | 36 | 335 | 335 | Normal postnatal examination | Zonisamide | Zonisamide |
| 30 | 1099 | 28 | 1207 | 1188 | Normal postnatal examination | Zonisamide | Zonisamide |
Additional tables and figures for the generalised and unclassified epilepsy trial
FIGURE 25.
Recruitment graph for the generalised and unclassified epilepsy trial.
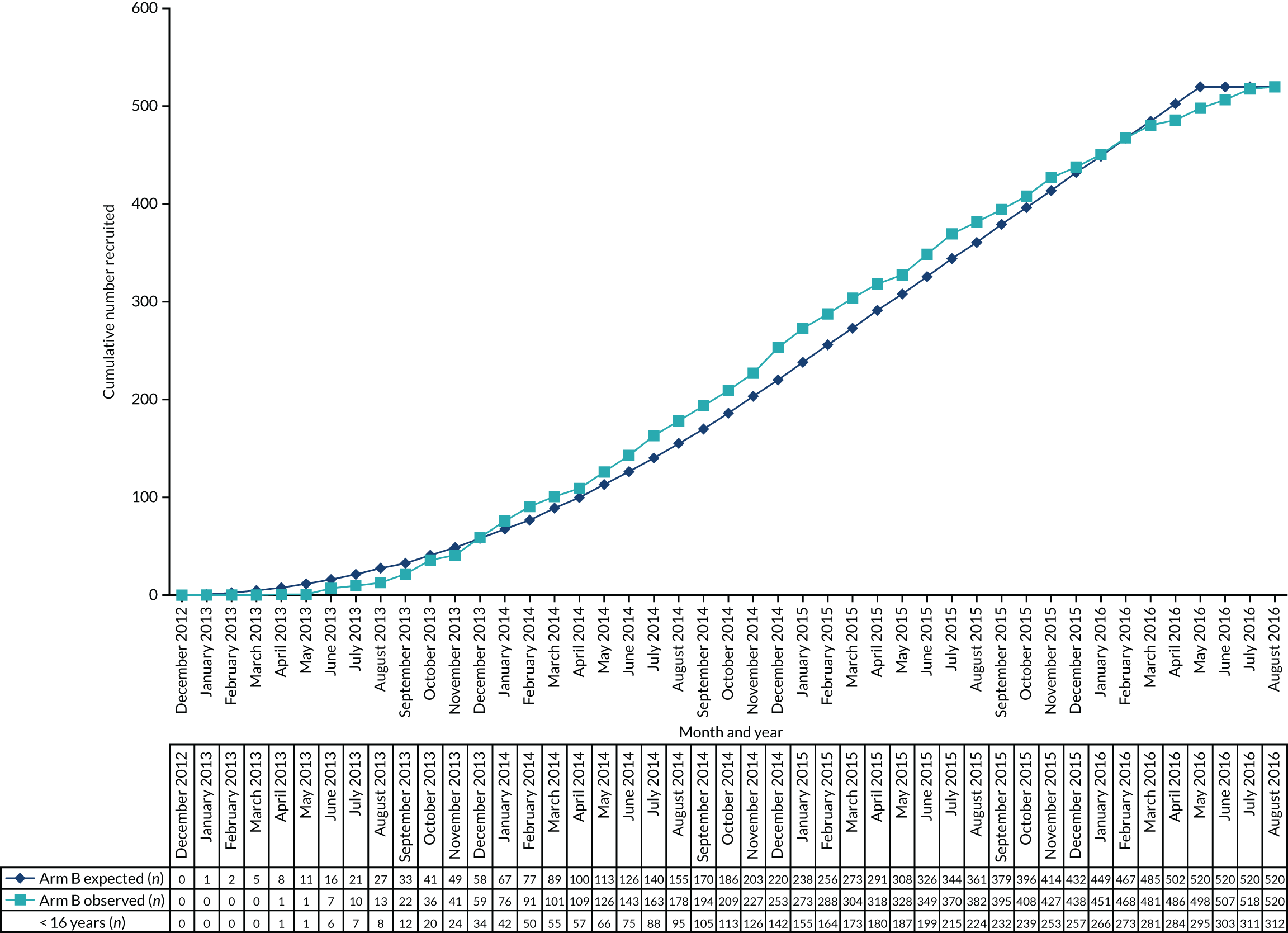
Additional baseline tables and figures for generalised and unclassified trial
| Characteristic | Valproate group | Levetiracetam group | Total |
|---|---|---|---|
| Age group (years), n (%) | 260 | 260 | 520 |
| 5–7 | 52 (20.0) | 48 (18.5) | 100 (19.2) |
| 8–11 | 54 (20.8) | 56 (21.5) | 110 (21.2) |
| 12–15 | 54 (20.8) | 48 (18.5) | 102 (19.6) |
| 16–29 | 70 (26.9) | 81 (31.2) | 151 (29.0) |
| ≥ 30 | 30 (11.5) | 27 (10.4) | 57 (11.0) |
| Weight in kg (if aged ≤ 12 years) (n) | 105 | 104 | 209 |
| Mean (SD) | 31 (11) | 32 (12) | 31 (11) |
| Median (IQR) | 29 (23–36) | 30 (24–37) | 29 (23–37) |
| Range | 15–66 | 16–81 | 15–81 |
| Missing | 16 | 12 | 28 |
| EEG, n (%) | |||
| EEG not done | 20 (7.7) | 24 (9.2) | 44 (8.5) |
| EEG normal | 58 (22.3) | 51 (19.6) | 109 (21.0) |
| Non-specific abnormality | 11 (4.2) | 9 (3.5) | 20 (3.8) |
| Generalised abnormality: slow wave activity with spiking | 138 (53.1) | 133 (51.2) | 271 (52.1) |
| Generalised abnormality: slow wave activity without spiking | 8 (3.1) | 7 (2.7) | 15 (2.9) |
| Focal abnormality: paroxysmal slow activity with spiking | 10 (3.8) | 8 (3.1) | 18 (3.5) |
| Focal abnormality: paroxysmal slow activity without spiking | 2 (0.8) | 7 (2.7) | 9 (1.7) |
| Other | 13 (5.0) | 21 (8.1) | 34 (6.5) |
| MRI, n (%) | |||
| MRI not done | 155 (59.6) | 163 (62.7) | 318 (61.2) |
| MRI normal | 78 (30.0) | 81 (31.2) | 159 (30.6) |
| Head injury | 0 | 0 | 0 |
| Tumour | 0 | 2 (0.8) | 2 (0.4) |
| Cortical dysplasia | 0 | 2 (0.8) | 2 (0.4) |
| Hippocampal sclerosis | 0 | 0 | 0 |
| AVM or other vascular malformation (e.g. cavernoma) | 2 (0.9) | 1 (0.4) | 3 (0.6) |
| Infarct | 0 | 1 (0.4) | 1 (0.2) |
| Haemorrhage | 0 | 0 | 0 |
| Previous infection (e.g. encephalitis/abscess) | 0 | 0 | 0 |
| Other | 25 (9.7) | 10 (3.9) | 35 (6.8) |
| CT scan, n (%) | |||
| CT scan not carried out | 223 (85.8 | 216 (83.1) | 439 (84.4) |
| CT scan normal | 32 (12.3) | 44 (16.9) | 76 (14.6) |
| Head injury | 0 | 0 | 0 |
| Tumour | 0 | 0 | 0 |
| Cortical dysplasia | 0 | 0 | 0 |
| Hippocampal sclerosis | 0 | 0 | 0 |
| AVM or other vascular malformation (e.g. cavernoma) | 0 | 0 | 0 |
| Infarct | 0 | 0 | 0 |
| Haemorrhage | 0 | 0 | 0 |
| Previous infection (e.g. encephalitis/abscess) | 0 | 0 | 0 |
| Porencephalic cyst | 0 | 0 | 0 |
| Other | 5 (1.9) | 0 | 5 (1.0) |
| Follow-up statistic | Valproate group | Levetiracetam group |
|---|---|---|
| Completeness of follow-up statistic | 87.2% | 82.6% |
| Maximum follow-up time (days) | ||
| Median | 1296 | 1293 |
| IQR | 1051.5–1523.5 | 1053.5–1516.5 |
| Potential follow-up time (days) | ||
| Median | 494.5 | 730 |
| IQR | 366–928.5 | 397–1096 |
| Actual follow-up time (days) | ||
| Median | 427 | 550 |
| IQR | 365–731 | 366–781 |
| Reverse Kaplan–Meier estimatea | ||
| Median | 1096 | 1084 |
| IQR | 731–1461 | 730–1412 |
| Number withdrawn or lost to follow-upb | 23 (8.9%) | 40 (15.4%) |
| Number lost to follow-up without treatment failure | 12 (4.6%) | 26 (10.0%) |
FIGURE 26.
Completeness of follow-up scatterplots of observed follow-up time by date of randomisation: generalised and unclassified epilepsy trial. (a) Levetiracetam; and (b) valproate.
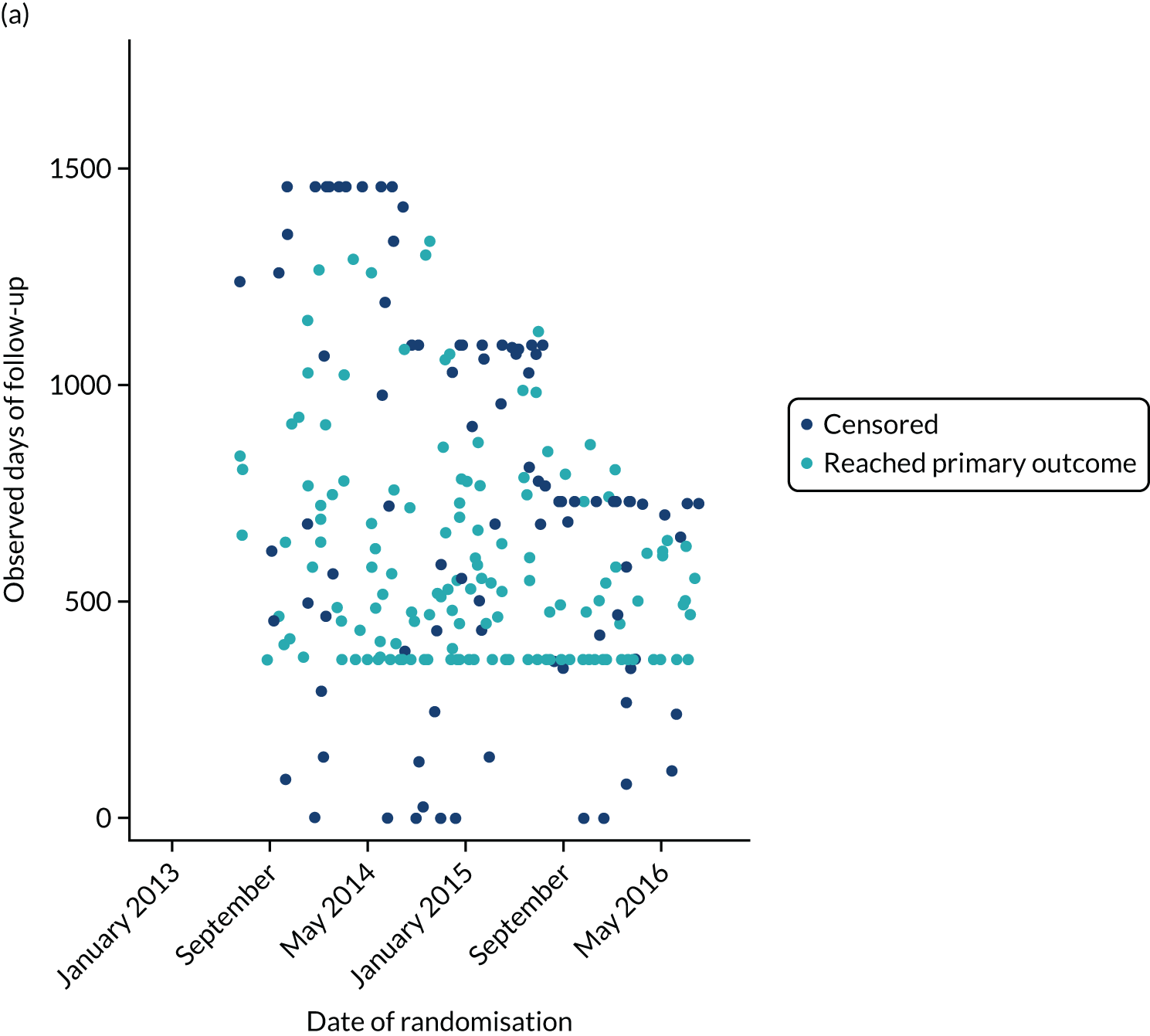
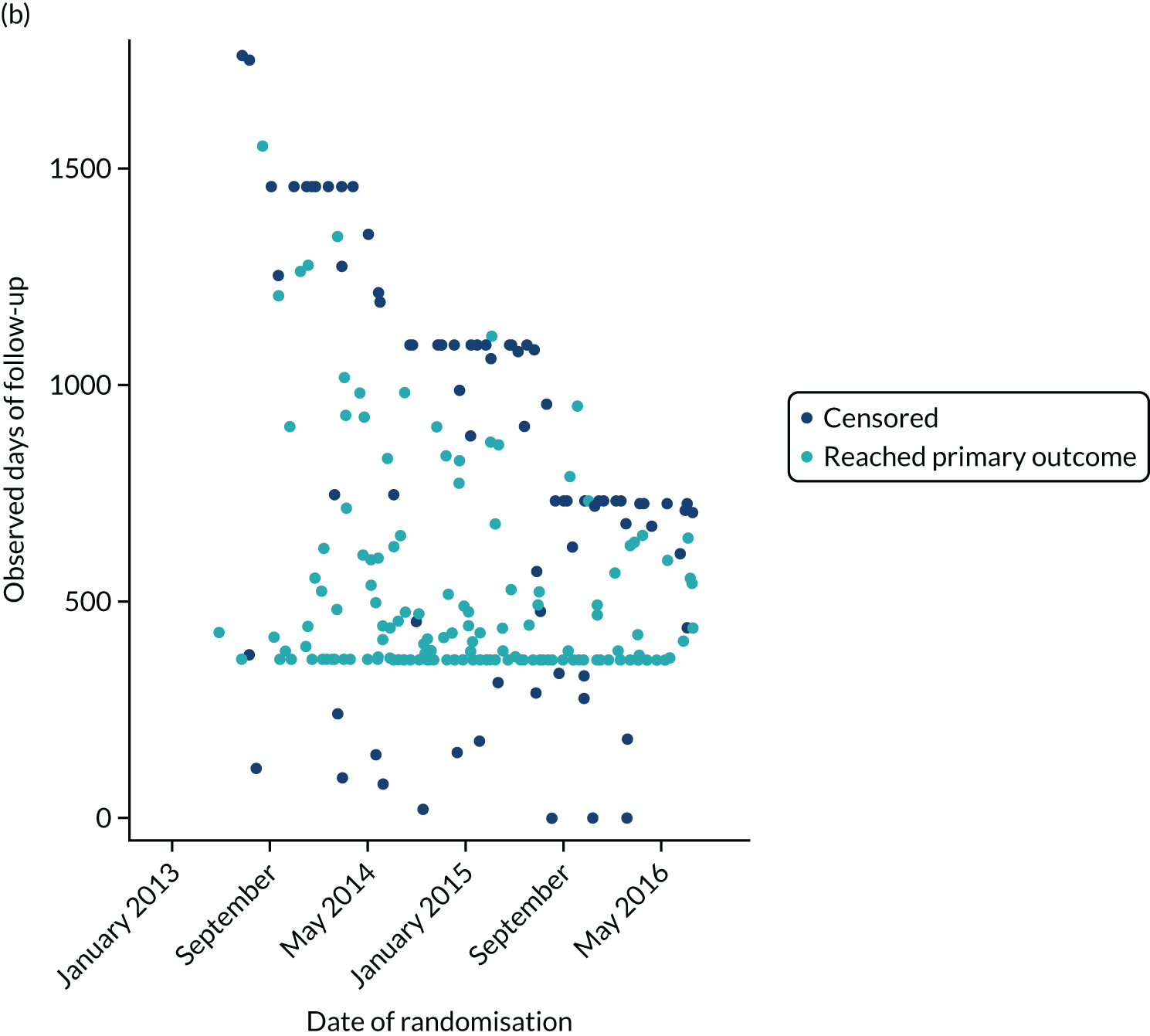
| Model and analysis set | Comparison | Time interval | HR (97.5% CI) |
|---|---|---|---|
| Alternative imputation rules | Valproate vs. levetiracetam | All follow-up | 1.24 (1.01 to 1.51) |
| Misdiagnoses excluded (withdrawal reason ‘not epilepsy’) | Valproate vs. levetiracetam | All follow-up | 1.18 (0.95 to 1.46) |
| Event MedDRA-preferred term | Number of events | Number of patients (%) | ||
|---|---|---|---|---|
| Valproate group | Levetiracetam group | Valproate group (n = 257) | Levetiracetam group (n = 258) | |
| Weight increased | 27 | 8 | 26 (10.1) | 8 (3.1) |
| Fatigue | 16 | 16 | 14 (5.4) | 15 (5.8) |
| Abnormal behaviour | 8 | 21 | 8 (3.1) | 18 (7.0) |
| Aggression | 9 | 14 | 9 (3.5) | 13 (5.0) |
| Headache | 10 | 9 | 10 (3.9) | 8 (3.1) |
| Increased appetite | 14 | 4 | 14 (5.4) | 4 (1.6) |
| Tremor | 13 | 4 | 11 (4.3) | 4 (1.6) |
| Nausea | 10 | 6 | 9 (3.5) | 6 (2.3) |
| Depressed mood | 2 | 12 | 2 (0.8) | 10 (3.9) |
| Anger | 4 | 8 | 4 (1.6) | 8 (3.1) |
| Depression | 3 | 9 | 3 (1.2) | 8 (3.1) |
| Somnolence | 7 | 4 | 7 (2.7) | 4 (1.6) |
| Alopecia | 7 | 2 | 7 (2.7) | 2 (0.8) |
| Decreased appetite | 5 | 4 | 5 (1.9) | 4 (1.6) |
| Lethargy | 4 | 5 | 4 (1.6) | 5 (1.9) |
| Insomnia | 4 | 4 | 4 (1.6) | 4 (1.6) |
| Memory impairment | 5 | 3 | 5 (1.9) | 3 (1.2) |
| Mood altered | 3 | 5 | 3 (1.2) | 5 (1.9) |
| Abdominal pain | 5 | 2 | 5 (1.9) | 2 (0.8) |
| Anxiety | 1 | 6 | 1 (0.4) | 5 (1.9) |
| Disturbance in attention | 4 | 3 | 4 (1.6) | 3 (1.2) |
| Dizziness | 2 | 5 | 2 (0.8) | 5 (1.9) |
| Irritability | 2 | 5 | 2 (0.8) | 5 (1.9) |
| Suicidal ideation | 2 | 3 | 2 (0.8) | 2 (0.8) |
| Vomiting | 4 | 1 | 4 (1.6) | 1 (0.4) |
| Weight decreased | 2 | 3 | 2 (0.8) | 3 (1.2) |
| Agitation | 0 | 4 | 0 | 3 (1.2) |
| Cognitive disorder | 2 | 2 | 2 (0.8) | 2 (0.8) |
| Enuresis | 3 | 1 | 3 (1.2) | 1 (0.4) |
| Psychomotor hyperactivity | 2 | 2 | 2 (0.8) | 2 (0.8) |
| Sedation | 2 | 2 | 2 (0.8) | 2 (0.8) |
| Sleep disorder | 3 | 1 | 3 (1.2) | 1 (0.4) |
| Amnesia | 1 | 2 | 1 (0.4) | 2 (0.8) |
| Diarrhoea | 2 | 1 | 1 (0.4) | 1 (0.4) |
| Tearfulness | 0 | 3 | 0 | 3 (1.2) |
| Acne | 2 | 0 | 2 (0.8) | 0 |
| Affect lability | 2 | 0 | 2 (0.8) | 0 |
| Anal incontinence | 0 | 2 | 0 | 1 (0.4) |
| Ataxia | 2 | 0 | 1 (0.4) | 0 |
| Constipation | 0 | 2 | 0 | 2 (0.8) |
| Dermatitis allergic | 0 | 2 | 0 | 1 (0.4) |
| Diplopia | 1 | 1 | 1 (0.4) | 1 (0.4) |
| Emotional disorder | 0 | 2 | 0 | 2 (0.8) |
| Gait disturbance | 1 | 1 | 1 (0.4) | 1 (0.4) |
| Migraine | 1 | 1 | 1 (0.4) | 1 (0.4) |
| Mood swings | 0 | 2 | 0 | 2 (0.8) |
| Personality change | 0 | 2 | 0 | 2 (0.8) |
| Rash | 2 | 0 | 2 (0.8) | 0 |
| Abdominal pain upper | 0 | 1 | 0 | 1 (0.4) |
| Abnormal dreams | 0 | 1 | 0 | 1 (0.4) |
| Alanine aminotransferase increased | 1 | 0 | 1 (0.4) | 0 |
| Apathy | 0 | 1 | 0 | 1 (0.4) |
| Aphasia | 0 | 1 | 0 | 1 (0.4) |
| Asthenia | 1 | 0 | 1 (0.4) | 0 |
| Bicytopenia | 1 | 0 | 1 (0.4) | 0 |
| Co-ordination abnormal | 1 | 0 | 1 (0.4) | 0 |
| Defaecation urgency | 1 | 0 | 1 (0.4) | 0 |
| Defiant behaviour | 0 | 1 | 0 | 1 (0.4) |
| Disorientation | 1 | 0 | 1 (0.4) | 0 |
| Distractibility | 1 | 0 | 1 (0.4) | 0 |
| Drooling | 1 | 0 | 1 (0.4) | 0 |
| Dysarthria | 0 | 1 | 0 | 1 (0.4) |
| Dyspepsia | 1 | 0 | 1 (0.4) | 0 |
| Dysphemia | 0 | 1 | 0 | 1 (0.4) |
| Eating disorder | 1 | 0 | 1 (0.4) | 0 |
| Encephalocele | 0 | 1 | 0 | 1 (0.4) |
| Epistaxis | 0 | 1 | 0 | 1 (0.4) |
| Erythema multiforme | 0 | 1 | 0 | 1 (0.4) |
| Flatulence | 0 | 1 | 0 | 1 (0.4) |
| Frustration tolerance decreased | 0 | 1 | 0 | 1 (0.4) |
| Gastritis | 1 | 0 | 1 (0.4) | 0 |
| Gingival bleeding | 0 | 1 | 0 | 1 (0.4) |
| Gingival hypertrophy | 0 | 1 | 0 | 1 (0.4) |
| Head banging | 1 | 0 | 1 (0.4) | 0 |
| Hiccups | 0 | 1 | 0 | 1 (0.4) |
| Hunger | 1 | 0 | 1 (0.4) | 0 |
| Hypersensitivity | 1 | 0 | 1 (0.4) | 0 |
| Intentional overdose | 0 | 1 | 0 | 1 (0.4) |
| Intentional self-injury | 0 | 1 | 0 | 1 (0.4) |
| Loss of libido | 1 | 0 | 1 (0.4) | 0 |
| Mouth ulceration | 0 | 1 | 0 | 1 (0.4) |
| Pancreatitis | 0 | 1 | 0 | 1 (0.4) |
| Pancytopenia | 0 | 1 | 0 | 1 (0.4) |
| Panic attack | 1 | 0 | 1 (0.4) | 0 |
| Platelet count decreased | 1 | 0 | 1 (0.4) | 0 |
| Poor-quality sleep | 1 | 0 | 1 (0.4) | 0 |
| Restlessness | 1 | 0 | 1 (0.4) | 0 |
| Seizure | 0 | 1 | 0 | 1 (0.4) |
| Self-injurious ideation | 1 | 0 | 1 (0.4) | 0 |
| Slow speech | 0 | 1 | 0 | 1 (0.4) |
| Staring | 0 | 1 | 0 | 1 (0.4) |
| Swelling face | 0 | 1 | 0 | 1 (0.4) |
| Thirst | 1 | 0 | 1 (0.4) | 0 |
| Total number of events and patients with at least one AR | 220 | 223 | 96 (37.4) | 107 (41.5) |
| Description | Seriousness | Severity | Suspect anti-seizure medication | Expectedness | Relationship: principal investigator assessment | Relationship: chief investigator assessment | Withdrawn from study drug | Outcome |
|---|---|---|---|---|---|---|---|---|
| Randomised to start treatment with valproate | ||||||||
| 1: bicytopenia | Required hospitalisation | Mild | Valproate | Expected | Probably | Possibly | Yes | Ongoing at final follow-up |
| 2: suicidal ideation | Required hospitalisation | Moderate | Valproate | Expected | Possibly | Unlikely | No | Resolved with sequelae |
| Randomised to start treatment with levetiracetam | ||||||||
| 3: dizziness | Required hospitalisation | Moderate | Valproate | Expected | Unrelated | Probably | No | Resolved |
| Lamotrigine | Expected | Probably | Probably | Yes | ||||
| 4: intentional overdose | Required hospitalisation | Mild | Levetiracetam | Expected | Possibly | Unlikely | No | Resolved |
| 5: suicidal ideation | Required hospitalisation | Moderate | Levetiracetam | Expected | Possibly | Possibly | No | Resolved with sequelae |
| 6: pancreatitis | Required hospitalisation | Severe | Valproate | Expected | Almost certainly | Probably | Yes | Resolved |
| Days from randomisation to death | Age at death (years) | Cause of death | Possibly related to trial treatments? |
|---|---|---|---|
| Randomised to start treatment with valproate | |||
| 1150 | 97 | Ruptured aortic aneurysm | No |
| Randomised to start treatment with levetiracetam | |||
| 901 | 36 | Sudden unexpected death in epilepsy | No |
| Patient ID | Day of pregnancy reporta | Age at date of report | Estimated day of deliverya | Day of delivery/miscarriagea | Outcome | Randomised drug | Drug regimen when pregnancy reported |
|---|---|---|---|---|---|---|---|
| 1 | 1113 | 21 | 1242 | 1237 | Normal postnatal examination | Valproate | Levetiracetam |
| 2 | 1001 | 17 | 1249 | 1252 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 2 | 1683 | 19 | Missing | 1647 | Miscarriage | Levetiracetam | Levetiracetam |
| 2 | 1737 | 19 | Missing | 1708 | Miscarriage | Levetiracetam | Levetiracetam |
| 3 | 195 | 17 | 340 | 321 | Other | Levetiracetam | Levetiracetam |
| 4 | 112 | 26 | 338 | 144 | Miscarriage | Levetiracetam | Levetiracetam |
| 4 | 274 | 26 | 465 | 469 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 4 | 1044 | 29 | 1245 | 1269 | Major malformations | Levetiracetam | Carbamazepine |
| 5 | 1107 | 22 | 1283 | 1271 | Normal postnatal examination | Levetiracetam | Levetiracetam |
| 6 | 686 | 26 | 903 | 891 | Normal postnatal examination | Levetiracetam | Levetiracetam and pregabalin |
| Characteristic | No return | Return | Total |
|---|---|---|---|
| Age (years) (n) | 299 | 221 | 520 |
| Mean (SD) | 16.4 (8.8) | 17.8 (15.9) | 17.0 (12.3) |
| Median (IQR) | 15.3 (9.5–21.2) | 12.7 (8.3–18.2) | 13.9 (8.9–19.7) |
| Range | 5.0–48.8 | 5.0–94.4 | 5.0–94.4 |
| Missing | 0 | 0 | 0 |
| Gender (n) | 299 | 221 | 520 |
| Male, n (%) | 211 (70.6) | 126 (57.0) | 337 (64.8) |
| Female, n (%) | 88 (29.4) | 95 (43.0) | 183 (35.2) |
| Learning disability (n) | 299 | 221 | 520 |
| Yes, n (%) | 30 (10.0) | 21 (9.5) | 51 (9.8) |
| No, n (%) | 269 (90.0) | 200 (90.5) | 469 (90.2) |
| Neurological deficit (n) | 299 | 221 | 520 |
| Yes, n (%) | 9 (3.0) | 7 (3.2) | 16 (3.1) |
| No, n (%) | 290 (97.0) | 214 (96.8) | 504 (96.9) |
| Previous or current neurological disorder, n (%) | |||
| Stroke/cerebrovascular | 0 | 0 | 0 |
| Cerebral haemorrhage | 0 | 2 (0.9) | 2 (0.4) |
| Intracranial surgery | 0 | 2 (0.9) | 2 (0.4) |
| Head injury: post-traumatic amnesia for > 24 hours or a compound depressed fracture | 2 (0.7) | 0 | 2 (0.4) |
| Meningitis/encephalitis | 3 (1.0) | 1 (0.5) | 4 (0.8) |
| Cortical dysplasia/developmental anomaly | 0 | 0 | 0 |
| Other | 12 (4.0) | 12 (5.4) | 24 (4.6) |
| History, n (%) | |||
| Febrile convulsions | 29 (9.7) | 15 (6.8) | 44 (8.5) |
| Any other acute symptomatic seizures | 8 (2.7) | 6 (2.7) | 14 (2.7) |
| Family history of epilepsy in primary relatives | 57 (19.1) | 42 (19.0) | 99 (19.0) |
| Epilepsy type (n) | 299 | 221 | 520 |
| Generalised epilepsy, n (%) | 218 (72.9) | 179 (81.0) | 397 (76.3) |
| Unclassified epilepsy, n (%) | 81 (27.1) | 42 (19.0) | 123 (23.7) |
| Epilepsy syndrome (generalised epilepsy only), n (%) | |||
| Childhood absence | 54 (24.8) | 50 (27.9) | 104 (26.2) |
| Juvenile absence | 14 (6.4) | 22 (12.3) | 36 (9.1) |
| Juvenile myoclonic | 37 (17.0) | 14 (7.8) | 51 (12.8) |
| Epilepsy with tonic–clonic seizures on awakening | 12 (5.5) | 11 (6.1) | 23 (5.8) |
| Other idiopathic generalised epilepsy not specified | 104 (47.7) | 76 (42.5) | 180 (45.3) |
| Other epilepsy syndrome | 7 (3.2) | 10 (5.6) | 17 (4.3) |
Appendix 4 Additional tables and figures for the health economic analysis
| Item of resource | Unit cost (child) | Assumption | Reference |
|---|---|---|---|
| GP consultation at GP surgery | £39.00 | 9.22 minutes | 55 |
| Nurse consultation at GP surgery | £10.85 | 15.5 minutes | 55,85 |
| GP home visit | £99.45 | 11.4 minutes, 12 minutes’ travel | 55,85 |
| Nurse home visit | £40.00 | N02AF | 54 |
| Doctor at hospital |
£185.00 (£203.00) |
Adult: service 400 Child: service 223 |
54 |
| Nurse at hospital | £29.19 | 15.5 minutes | 55 |
| Hospital overnight | £589.00 | Non-elective stay | 54 |
| Ambulance | £257.00 | ASS02 | 54 |
| A&E visit | £192.18 | (T01A, T01NA)a | 54 |
| Blood test | £3.00 | DAPS05 | 54 |
| Urine test | £2.00 | DAPS | 54 |
| Ultrasound | £54.82 | (RD40Z, RD41Z, RD42Z, RD43Z)a | 54 |
| Radiography | £31.00 | DAPF | 54 |
| CT scan |
£88.53 (£99.74) |
Adult: (RD20A, RD21A)a Child: (RD20B, RD21B)a |
54 |
| MRI scan |
£138.24 (£141.87) |
Adult: (RD01A, RD02A)a Child: (RD01B, RD02B)a |
54 |
| EEG |
£199.00 (£340.00) |
Adult: AA33C Child: AA33D |
54 |
| Health visitor | £72.00 | N03G | 54 |
| Social worker |
£50.00 (£51.00) |
1-hour visit | 55 |
| Occupational therapist |
£83.00 (£141.00) |
Adult: A06A1 Child: A06C1 |
54 |
| Psychologist | £199.00 | Service 656 | 54 |
| Counsellor |
£45.00 (£94.00) |
1-hour visit | 55 |
| Physiotherapist |
£63.00 (£101.00) |
Adult: A08A1 Child: A08C1 |
54 |
| Resources identified from free text | |||
| Telephone consultation (GP) | £15.52 | 55 | |
| GP out of hours | £72.97 | Inflated to 2018/19 | 86 |
| MMR | £7.64 | In addition to nurse appointment | 24 |
| Pharmacist | £11.00 | Band 6, 15 minutes | 55 |
| Repeat prescription | £7.30 | 55 | |
| Stool test | £2.00 | DAPS | 54 |
| MRSA swab/saliva test | £8.00 | DAPS07 | 54 |
| Psychiatrist |
£226.00 (£227.00) |
Adult: Service 713 Child: Service 711 |
54 |
| (Family) support worker | £24.00 | 55 | |
| Speech therapist |
£107.00 (£100.00) |
Adult: A13A1 Child: A13C1 |
54 |
| Dietitian | £90.00 | A03 | 54 |
| Sexual health specialist | £92.00 | 55 | |
| Podiatrist | £43.00 | A09A | 54 |
| Podiatrist minor surgery | £86.00 | A09B | 54 |
| Midwife | £58.00 | N01A | 54 |
| Assistive technology team | £123.00 | NCRT | 54 |
| Hearing test |
£101.00 (£89.00) |
Adult: CA37A Child: CA37B |
54 |
| Optician | £76.00 | Service 662 | 54 |
| NHS glasses | £39.10 | Voucher A | 87 |
| Dentist | £98.00 | M01B | 54 |
| Hygienist | £17.00 | 30-minute visit | 55 |
| Orthodontist | £121.00 | Service 143 | 54 |
| CAMHS | (£221.00) | CAMHSCC | 54 |
| School nurse/SENCO | (£68.00) | N05CO | 54 |
| Mammogram | £57.37 | Inflated to 2018/19 | 88 |
| Cervical smear | £39.76 | Inflated to 2018/19 | 89 |
| NHS Direct | £13.02 | Inflated to 2018/19 | 90 |
| Anticoagulant service | £37.00 | Service 324 | 54 |
| Radiofrequency for pain management | £699.00 | AB15Z | 54 |
| Radiotherapy | £182.00 | SC31Z | 54 |
| Venesection | £4.00 | DAPS08 | 54 |
| ECG |
£72.57 (£53.58) |
Adult: RD51A Child: RD51B |
54 |
| Video telemetry/long-term EEG monitoring | £491.00 | AA81Z | 54 |
| Cerebral angiography/contrast fluoroscopy | £170.00 | RD31Z | 54 |
| Spinal fluid test |
£617.00 (£882.00) |
Adult: HC72A Child: HC72B |
54 |
| Cystoscopy |
£250.00 (£849.00) |
Adult: LB72A Child: LB72B |
54 |
| Colonoscopy | £520.00 | FE32Z | 54 |
| Sigmoidoscopy | £386.00 | FE35Z | 54 |
| Endoscopy | £454.00 | FE22Z | 54 |
| Dual X-ray absorptiometry | £71.92 | RD50Z | 54 |
| PET scan |
£506.00 (£389.00) |
Adult: RN01A Child: RN01B |
54 |
| Peak flow test | £152.00 | DZ45Z | 54 |
| Field exercise test | £55.00 | DZ32Z | 54 |
| Cataract operation | £915.00 | BZ34C | 54 |
| Orthotics | £124.00 | Service 658 | 54 |
| Intermediate sinus procedures | £2344.00 | CA28Z | 54 |
| Insertion of grommets | £998.00 | CA35B | 54 |
| Arm fracture and CC | £1417.00 | HE51G | 54 |
| Rib fracture | £1025.00 | HE71D | 54 |
| Hand fracture | £384.00 | HE41D | 54 |
| Minor dental procedures in those aged < 19 | £153.00 | CD03B | 54 |
| Tooth extraction in those aged ≤ 18 years | £491.00 | CD07B | 54 |
| Minor skin procedures |
£215.00 (£288.00) |
Adult: JC43C Child: JC43D |
54 |
| Diabetic retinopathy screen | £108.00 | BZ88A | 54 |
| Nasal polypectomy | £1715.00 | CA14Z | 54 |
| Skin biopsy external nose | £461.00 | CA16Z | 54 |
| Percutaneous biopsy | £1491.00 | YH32A | 54 |
| Liver biopsy | £671.00 | YG11A | 54 |
| Biopsy of prostate | £504.00 | LB76Z | 54 |
| Sleep apnoea test | £309.00 | DZ50Z | 54 |
| Pelvis fracture (hip fracture) | £2117.00 | HE11H | 54 |
| Vaginal tape operation for urinary incontinence | £2020.00 | LB51B | 54 |
| Minor foot operation |
£832.00 (£580.00) |
Adult: HN35A Child: HN35B |
54 |
| Hernia repair | £2651.00 | FF60D | 54 |
| Hysterectomy | £3515.00 | MA08B | 54 |
| Triple heart bypass | £10,199.00 | ED28B | 54 |
| Hip replacement | £6057.00 | HN12F | 54 |
| Pacemaker fitted | £1085.00 | EY08E | 54 |
| Implantation of loop recorder | £1270.00 | EY12B | 54 |
| Removal of loop recorder | £693.00 | EY13Z | 54 |
| Cholecystectomy (gall bladder removal) | £2861.00 | GA10K | 54 |
| Knee replacement | £5699.00 | HN22E | 54 |
| Reconstructive surgery (chest clinic) | £5706.00 | JA30Z | 54 |
| Cardiac catheterisation | £1142.00 | EY43F | 54 |
| Walk-in centre visit | £72.07 | (T02A, T02NA, T03A, T03NA, T04A and T04NA)a | 54 |
| See and treat (no convey) | £209.00 | ASS01 | 54 |
| HRG code | Description | Elective | NEL | NES | Day case | ||
|---|---|---|---|---|---|---|---|
| Admitted patient care | |||||||
| AA26G | Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with a CC score of 3–5 | £3051 | £1924 | £416 | £549 | ||
| AA26H | Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with a CC score of 0–2 | £2358 | £1713 | £357 | £595 | ||
| AA33C | Conventional EEG, EMG or nerve conduction studies, 19 years and over | £1952 | £2993 | £827 | £807 | ||
| AA80Z | Complex long-term EEG monitoring | £2126 | £2960 | £1182 | £901 | ||
| PR02B | Paediatric epilepsy syndrome with a CC score of 1–5 | £2835 | £3242 | £602 | £998 | ||
| PR02C | Paediatric epilepsy syndrome with a CC score of 0 | £1800 | £2741 | £564 | £742 | ||
| SB97Z | Same-day chemotherapy admission or attendance | £308 | £3014 | £382 | £110 | ||
| SC97Z | Same-day radiotherapy admission or attendance (excluding brachytherapy) | £972 | – | £287 | £1389 | ||
| WH04E | Poisoning diagnosis without interventions, with a CC score of 0 or 1 | £1176 | £1347 | £383 | £362 | ||
| WH50B | Procedure not carried out, for other or unspecified reasons | £578 | £1995 | £477 | £330 | ||
| WJ11Z | Other disorders of immunity | £759 | £3258 | £454 | £437 | ||
| Service | Currency | Consultation | Procedure | ||||
| Outpatients | |||||||
| 110 | Trauma and orthopaedics | WF01A | Non-admitted face-to-face attendance, follow-up | £120 | £245 | ||
| 110 | Trauma and orthopaedics | N/A | N/A | £120 | N/A | ||
| 223 | Paediatric epilepsy | N/A | N/A | £203 | N/A | ||
| 291 | Paediatric neuro-disability | N/A | N/A | £251 | N/A | ||
| 320 | Cardiology | WF01A | Non-admitted face-to-face attendance, follow-up | £139 | £193 | ||
| 400 | Neurology | WF01A | Non-admitted face-to-face attendance, follow-up | £177 | £697 | ||
| 400 | Neurology | WF01B | Non-admitted face-to-face attendance, first | £177 | £410 | ||
| 400 | Neurology | N/A | N/A | £177 | N/A | ||
| 420 | Paediatrics | WF01A | Non-admitted face-to-face attendance, follow-up | £217 | £889 | ||
| 420 | Paediatrics | WF01B | Non-admitted face-to-face attendance, first | £217 | £321 | ||
| 420 | Paediatrics | N/A | N/A | £217 | N/A | ||
| 421 | Paediatric neurology | WF01A | Non-admitted face-to-face attendance, follow-up | £339 | £1099 | ||
| 421 | Paediatric neurology | N/A | N/A | £339 | N/A | ||
| 650 | Physiotherapy | WF01A | Non-admitted face-to-face attendance, follow-up | £58 | £80 | ||
| Service | Currency | ||||||
| A&E | |||||||
| N/A | N/A | ASS02 | See and treat and convey | £257 | |||
| T01A | Type 01 admitted | VB04Z | Emergency medicine, category 2 investigation with category 4 treatment | £318 | |||
| T01A | Type 01 admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | £251 | |||
| T01A | Type 01 admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | £220 | |||
| T01A | Type 01 admitted | VB09Z | Emergency medicine, category 1 investigation with category 1 or 2 treatment | £159 | |||
| T01NA | Type 01 non-admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | £200 | |||
| T01NA | Type 01 non-admitted | VB08Z | Emergency medicine, category 2 investigation with category 1 treatment | £179 | |||
| T01NA | Type 01 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1 or 2 treatment | £133 | |||
| T01NA | Type 01 non-admitted | VB11Z | Emergency medicine, no investigation with no significant treatment | £114 | |||
| T03NA | Type 03 non-admitted | VB07Z | Emergency medicine, category 2 investigation with category 2 treatment | £110 | |||
| T03NA | Type 03 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1 or 2 treatment | £68 | |||
| T04NA | Type 04 non-admitted | VB09Z | Emergency medicine, category 1 investigation with category 1 or 2 treatment | £53 | |||
| Formulation | Strength | N/volume | Unit cost (£) |
|---|---|---|---|
| Lamotrigine | |||
| Dispersible tablet | 2 mg | 30 | 18.81 |
| Dispersible tablet | 5 mg | 28 | 7.67 |
| Dispersible tablet | 25 mg | 56 | 4.70 |
| Dispersible tablet | 100 mg | 56 | 6.29 |
| Tablet | 25 mg | 56 | 1.89 |
| Tablet | 50 mg | 56 | 2.46 |
| Tablet | 100 mg | 56 | 3.48 |
| Tablet | 200 mg | 56 | 4.37 |
| Levetiracetam | |||
| Tablet | 250 mg | 60 | 5.72 |
| Tablet | 500 mg | 60 | 9.97 |
| Tablet | 750 mg | 60 | 8.96 |
| Tablet | 1 g | 60 | 14.97 |
| Oral solution, sugar free | 100 mg/ml | 300 | 7.71 |
| Valproate | |||
| Gastroresistant tablet | 200 mg | 100 | 10.56 |
| Gastroresistant tablet | 500 mg | 100 | 25.44 |
| Modified-release capsule | 300 mg | 100 | 13.00 |
| Modified-release granules | 250 mg | 30 | 30.00 |
| Modified-release granules [sodium valproate (Epilim®, Sanofi SA)] | 1000 mg | 30 | 30.00 |
| Modified-release granules [sodium valproate (Episenta®, Desitin Pharma Ltd, Atterbury, UK)] | 1000 mg | 100 | 41.00 |
| Modified-release tablet | 200 mg | 30 | 3.50 |
| Modified-release tablet | 300 mg | 30 | 5.24 |
| Modified-release tablet | 500 mg | 30 | 8.73 |
| Oral solution, sugar free | 40 mg/ml | 300 | 10.64 |
| Zonisamide | |||
| Capsule | 25 mg | 14 | 7.55 |
| Capsule | 50 mg | 56 | 40.01 |
| Capsule | 100 mg | 56 | 5.27 |
| Variable | Time point | Lamotrigine group (number of participants) | Levetiracetam group (number of participants) | Zonisamide group (number of participants) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete | Incomplete | Total | Complete | Incomplete | Total | Complete | Incomplete | Total | ||
| Participants | ||||||||||
| Admitted patient care | Baseline | 266 | 64 | 330 | 261 | 71 | 332 | 245 | 83 | 328 |
| Outpatients | Baseline | 266 | 64 | 330 | 261 | 71 | 332 | 245 | 83 | 328 |
| A&E | Baseline | 266 | 64 | 330 | 261 | 71 | 332 | 245 | 83 | 328 |
| Primary care | 3 months | 182 | 148 | 330 | 186 | 146 | 332 | 182 | 146 | 328 |
| Community care | 3 months | 182 | 148 | 330 | 186 | 146 | 332 | 182 | 146 | 328 |
| Concomitant medication | 3 months | 182 | 148 | 330 | 186 | 146 | 332 | 182 | 146 | 328 |
| Primary care | 6 months | 177 | 153 | 330 | 176 | 156 | 332 | 174 | 154 | 328 |
| Community care | 6 months | 177 | 153 | 330 | 176 | 156 | 332 | 174 | 154 | 328 |
| Concomitant medication | 6 months | 177 | 153 | 330 | 176 | 156 | 332 | 174 | 154 | 328 |
| Primary care | 12 months | 156 | 174 | 330 | 154 | 178 | 332 | 155 | 173 | 328 |
| Community care | 12 months | 156 | 174 | 330 | 154 | 178 | 332 | 155 | 173 | 328 |
| Admitted patient care | 12 months | 298 | 34 | 330 | 286 | 47 | 332 | 272 | 56 | 328 |
| Outpatients | 12 months | 298 | 34 | 330 | 286 | 47 | 332 | 272 | 56 | 328 |
| A&E | 12 months | 298 | 34 | 330 | 286 | 47 | 332 | 272 | 56 | 328 |
| Anti-seizure medication | 12 months | 291 | 39 | 330 | 293 | 39 | 332 | 280 | 48 | 328 |
| Concomitant medication | 12 months | 156 | 174 | 330 | 154 | 178 | 332 | 155 | 173 | 328 |
| Primary care | 24 months | 135 | 195 | 330 | 133 | 199 | 332 | 130 | 198 | 328 |
| Community care | 24 months | 135 | 195 | 330 | 133 | 199 | 332 | 130 | 198 | 328 |
| Admitted patient care | 24 months | 299 | 32 | 330 | 291 | 43 | 332 | 280 | 48 | 328 |
| Outpatients | 24 months | 299 | 32 | 330 | 291 | 43 | 332 | 280 | 48 | 328 |
| A&E | 24 months | 299 | 32 | 330 | 291 | 43 | 332 | 280 | 48 | 328 |
| Anti-seizure medication | 24 months | 257 | 73 | 330 | 260 | 72 | 332 | 239 | 89 | 328 |
| Concomitant medication | 24 months | 135 | 195 | 330 | 133 | 199 | 332 | 130 | 198 | 328 |
| Primary care | 36 months | 93 | 175 | 268 | 92 | 174 | 266 | 84 | 183 | 267 |
| Community care | 36 months | 93 | 175 | 268 | 92 | 174 | 266 | 84 | 183 | 267 |
| Admitted patient care | 36 months | 236 | 32 | 268 | 225 | 41 | 266 | 217 | 50 | 267 |
| Outpatients | 36 months | 236 | 32 | 268 | 225 | 41 | 266 | 217 | 50 | 267 |
| A&E | 36 months | 236 | 32 | 268 | 225 | 41 | 266 | 217 | 50 | 267 |
| Anti-seizure medication | 36 months | 125 | 143 | 268 | 134 | 132 | 266 | 118 | 149 | 267 |
| Concomitant medication | 36 months | 93 | 175 | 268 | 92 | 174 | 266 | 84 | 183 | 267 |
| Primary care | 48 months | 46 | 125 | 171 | 58 | 117 | 175 | 44 | 130 | 174 |
| Community care | 48 months | 46 | 125 | 171 | 58 | 117 | 175 | 44 | 130 | 174 |
| Admitted patient care | 48 months | 150 | 21 | 171 | 151 | 24 | 175 | 141 | 33 | 174 |
| Outpatients | 48 months | 150 | 21 | 171 | 151 | 24 | 175 | 141 | 33 | 174 |
| A&E | 48 months | 150 | 21 | 171 | 151 | 24 | 175 | 141 | 33 | 174 |
| Anti-seizure medication | 48 months | 62 | 109 | 171 | 66 | 109 | 175 | 52 | 122 | 174 |
| Concomitant medication | 48 months | 46 | 125 | 171 | 58 | 117 | 175 | 44 | 130 | 174 |
| Primary care | 60 months | 26 | 54 | 80 | 29 | 50 | 79 | 24 | 53 | 77 |
| Community care | 60 months | 26 | 54 | 80 | 29 | 50 | 79 | 24 | 53 | 77 |
| Admitted patient care | 60 months | 74 | 6 | 80 | 69 | 10 | 79 | 59 | 18 | 77 |
| Outpatients | 60 months | 74 | 6 | 80 | 69 | 10 | 79 | 59 | 18 | 77 |
| A&E | 60 months | 74 | 6 | 80 | 69 | 10 | 79 | 59 | 18 | 77 |
| Anti-seizure medication | 60 months | 19 | 61 | 80 | 22 | 57 | 80 | 16 | 61 | 80 |
| Concomitant medication | 60 months | 26 | 54 | 80 | 29 | 50 | 79 | 24 | 53 | 77 |
| Utilities | ||||||||||
| EQ-5D | Baseline | 209 | 121 | 330 | 202 | 130 | 332 | 205 | 123 | 328 |
| NEWQOL-6D | Baseline | 201 | 129 | 330 | 190 | 142 | 332 | 186 | 142 | 328 |
| EQ-VAS | Baseline | 188 | 142 | 330 | 187 | 145 | 332 | 190 | 138 | 328 |
| EQ-5D | 12 months | 148 | 182 | 330 | 148 | 184 | 332 | 147 | 181 | 328 |
| NEWQOL-6D | 12 months | 107 | 223 | 330 | 100 | 232 | 332 | 104 | 224 | 328 |
| EQ-VAS | 12 months | 135 | 194 | 330 | 126 | 206 | 332 | 136 | 192 | 328 |
| EQ-5D | 24 months | 121 | 209 | 330 | 124 | 208 | 332 | 122 | 206 | 328 |
| NEWQOL-6D | 24 months | 87 | 243 | 330 | 88 | 244 | 332 | 80 | 248 | 328 |
| EQ-VAS | 24 months | 116 | 214 | 330 | 111 | 221 | 332 | 114 | 214 | 328 |
| EQ-5D | 36 months | 94 | 174 | 268 | 93 | 173 | 266 | 83 | 184 | 267 |
| NEWQOL-6D | 36 months | 69 | 199 | 268 | 58 | 208 | 266 | 61 | 206 | 267 |
| EQ-VAS | 36 months | 93 | 175 | 268 | 89 | 177 | 266 | 78 | 189 | 267 |
| EQ-5D | 48 months | 50 | 121 | 171 | 58 | 117 | 175 | 46 | 128 | 174 |
| NEWQOL-6D | 48 months | 37 | 134 | 171 | 41 | 134 | 175 | 33 | 141 | 174 |
| EQ-VAS | 48 months | 48 | 123 | 171 | 55 | 120 | 175 | 43 | 131 | 174 |
| EQ-5D | 60 months | 31 | 49 | 80 | 31 | 48 | 79 | 26 | 51 | 77 |
| NEWQOL-6D | 60 months | 25 | 55 | 80 | 16 | 63 | 79 | 17 | 60 | 77 |
| EQ-VAS | 60 months | 31 | 49 | 80 | 30 | 49 | 79 | 25 | 52 | 77 |
FIGURE 27.
Distribution of participants’ responses to each EQ-5D attribute by treatment allocated and time: focal epilepsy. Levels range from 1 to 3, with 3 representing the most severe problem. (a) Mobility; (b) self-care; (c) usual activities; (d) pain or discomfort; and (e) anxiety or depression.
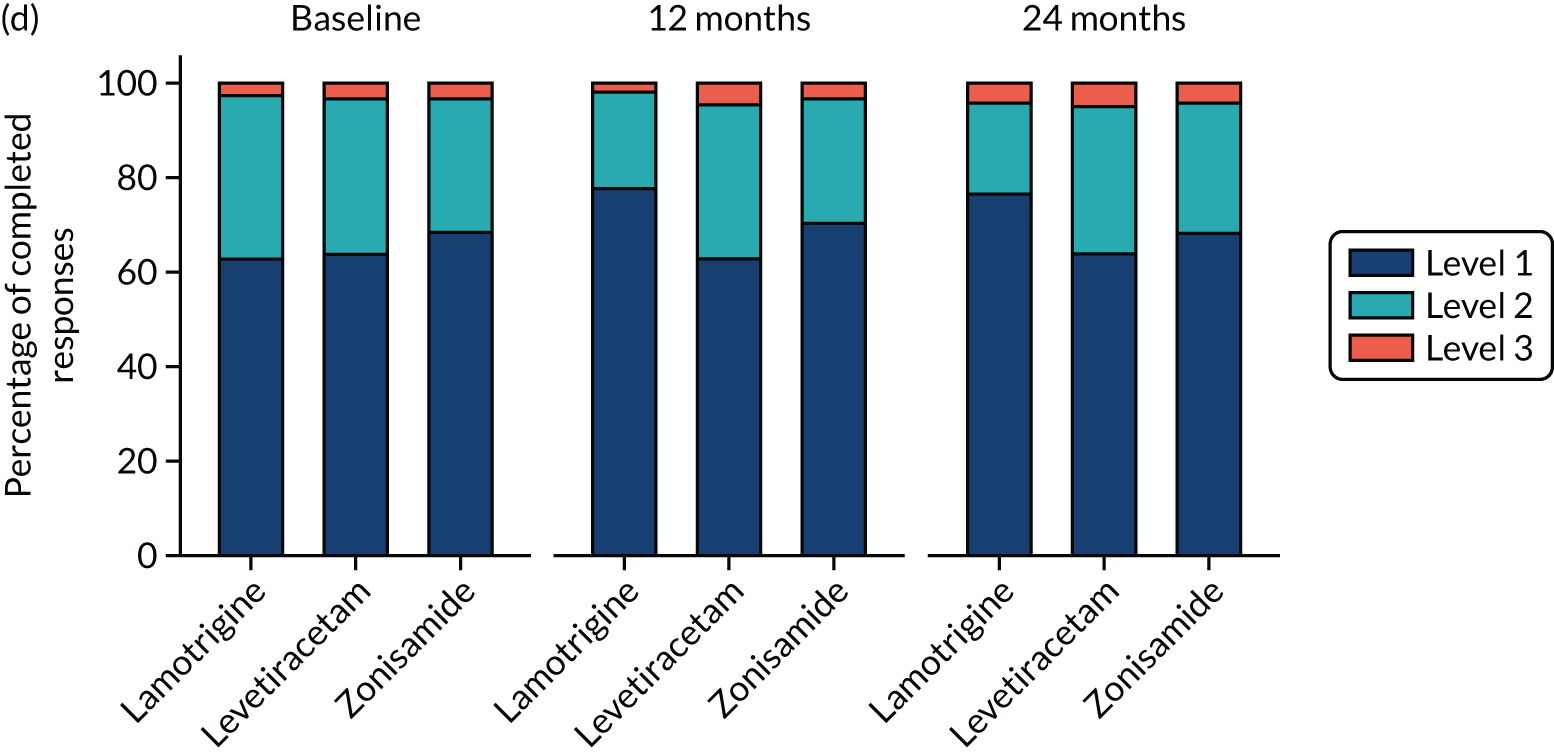
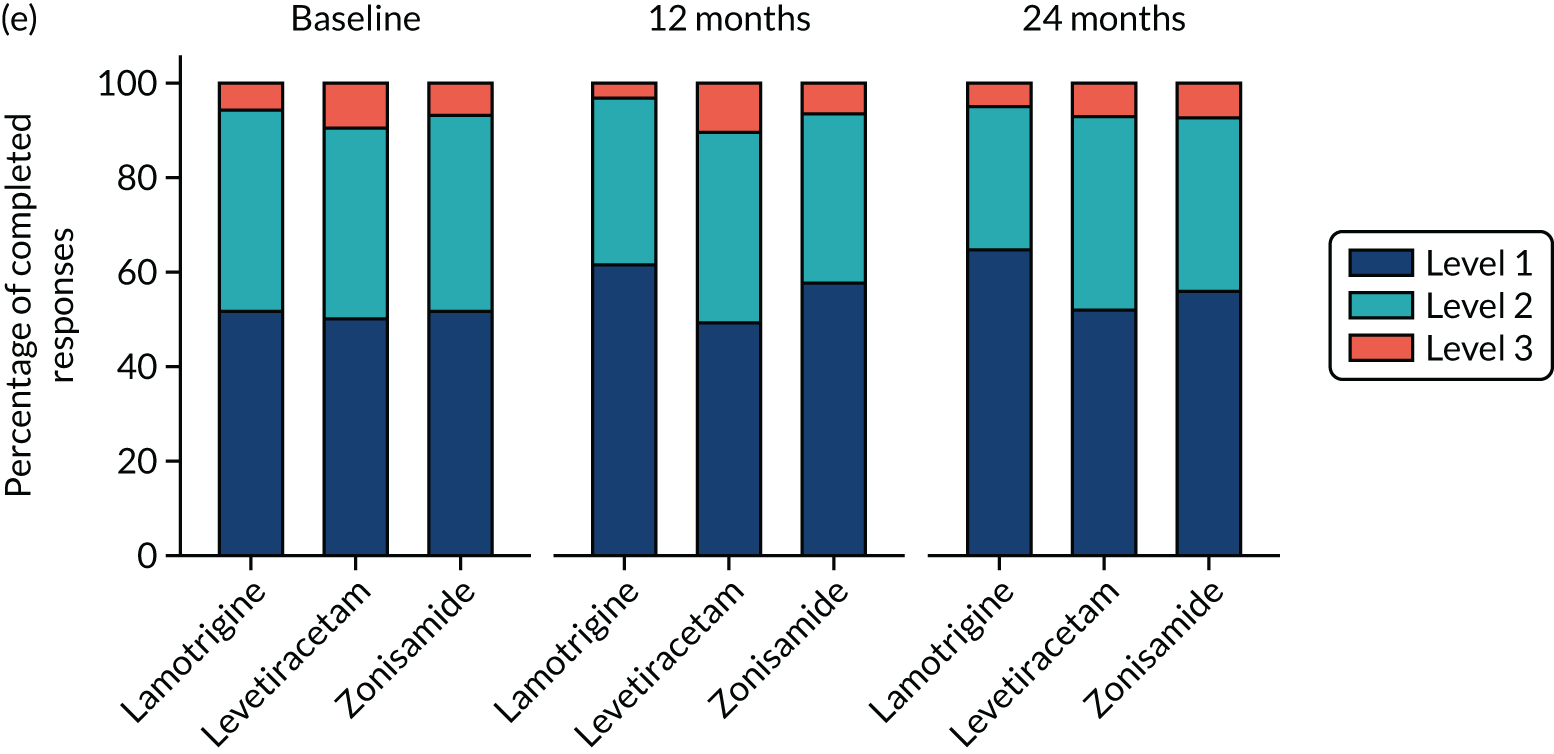
FIGURE 28.
Distribution of participants’ responses to each NEWQOL-6D attribute by treatment allocated and time: focal epilepsy. Levels range from 1 to 4, with 4 representing the most severe problem. (a) Worry; (b) depression; (c) memory; (d) concentration; (e) control; and (f) stigma.
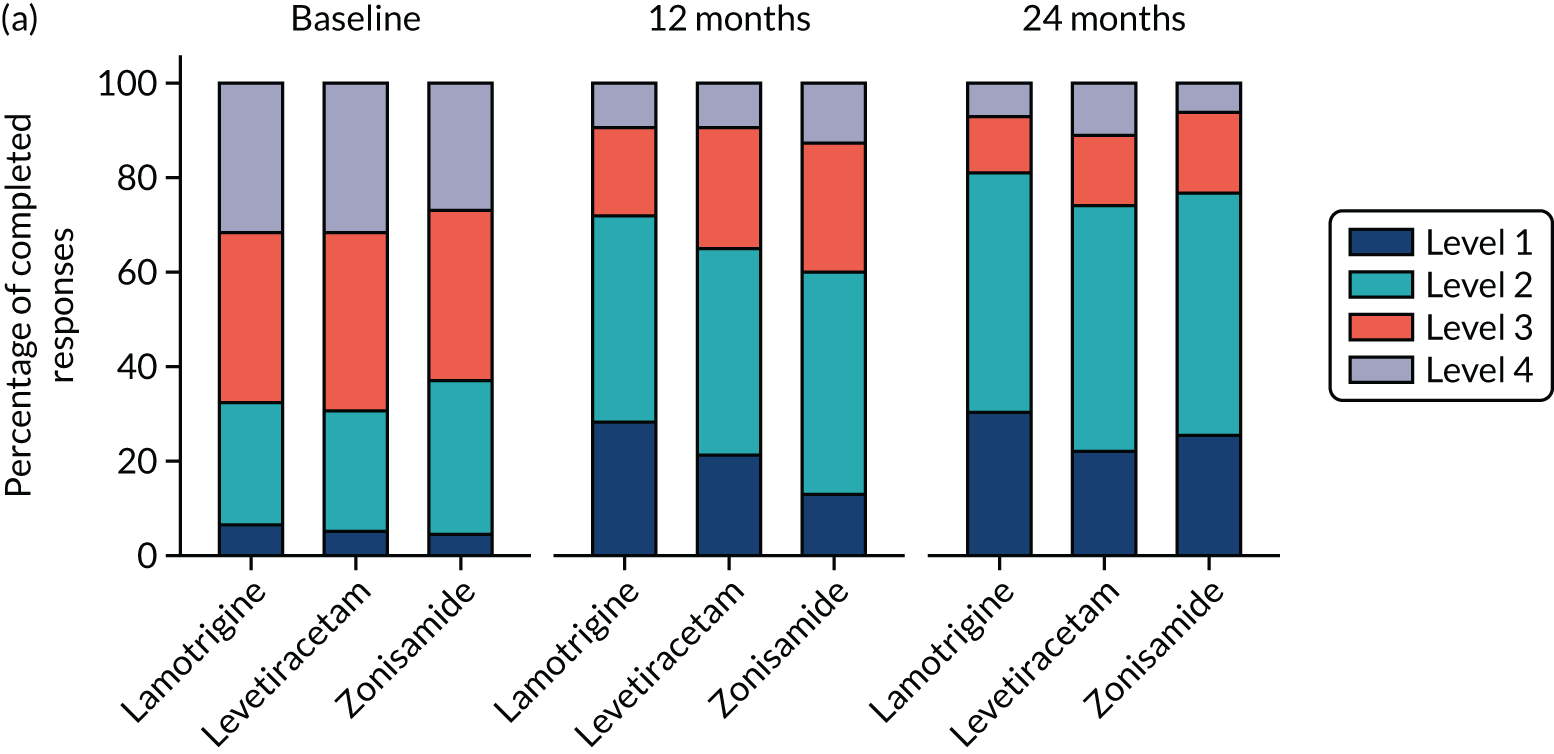
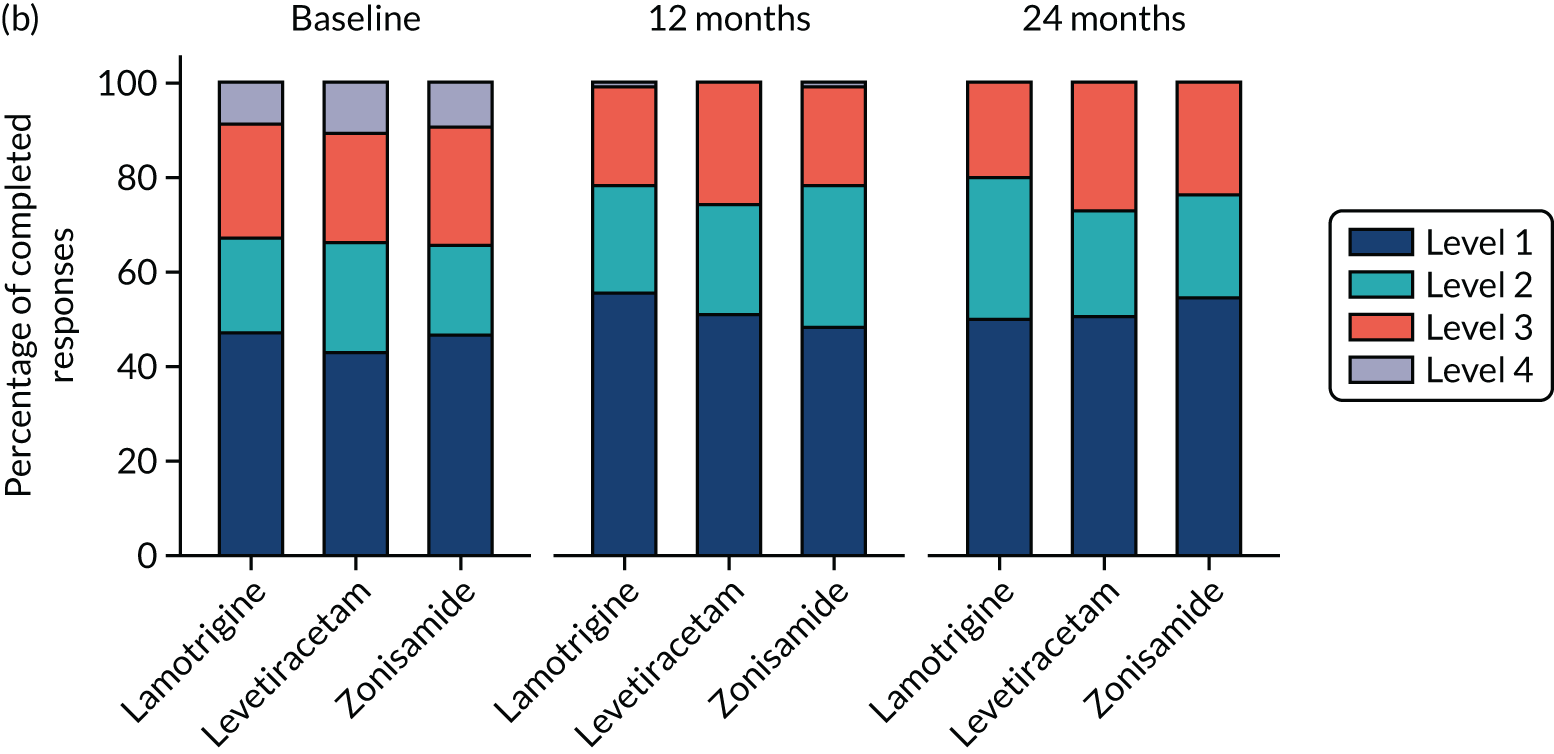
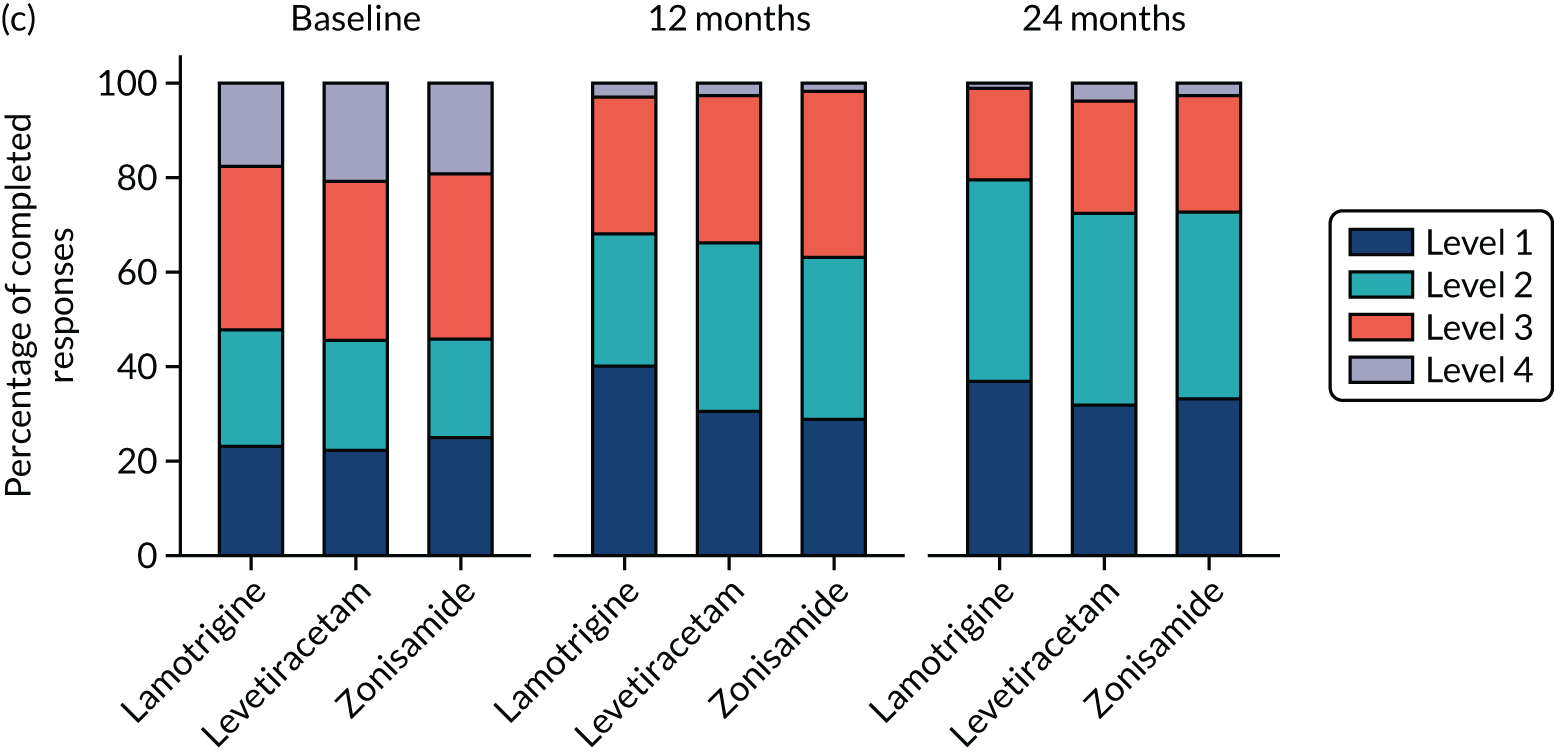
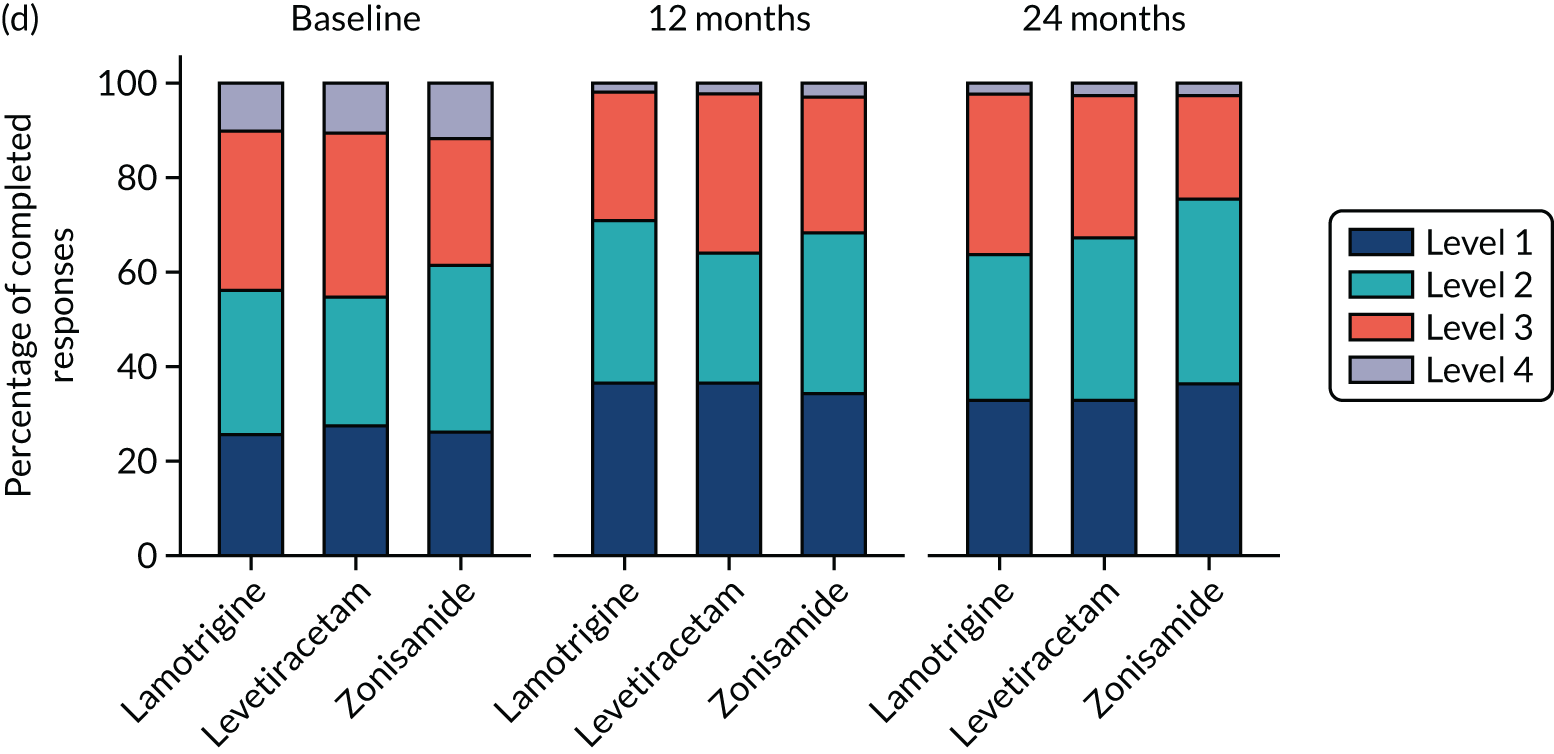
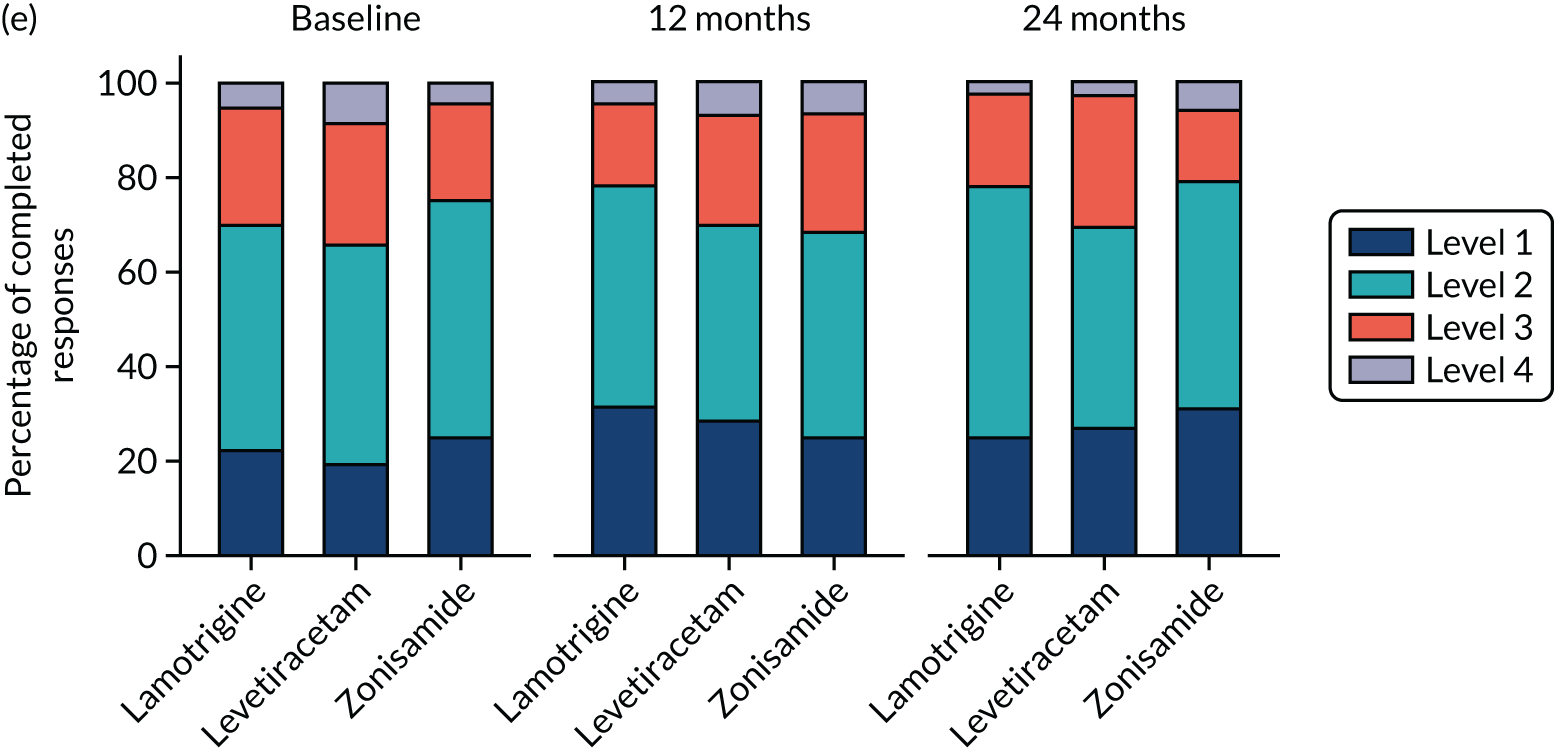
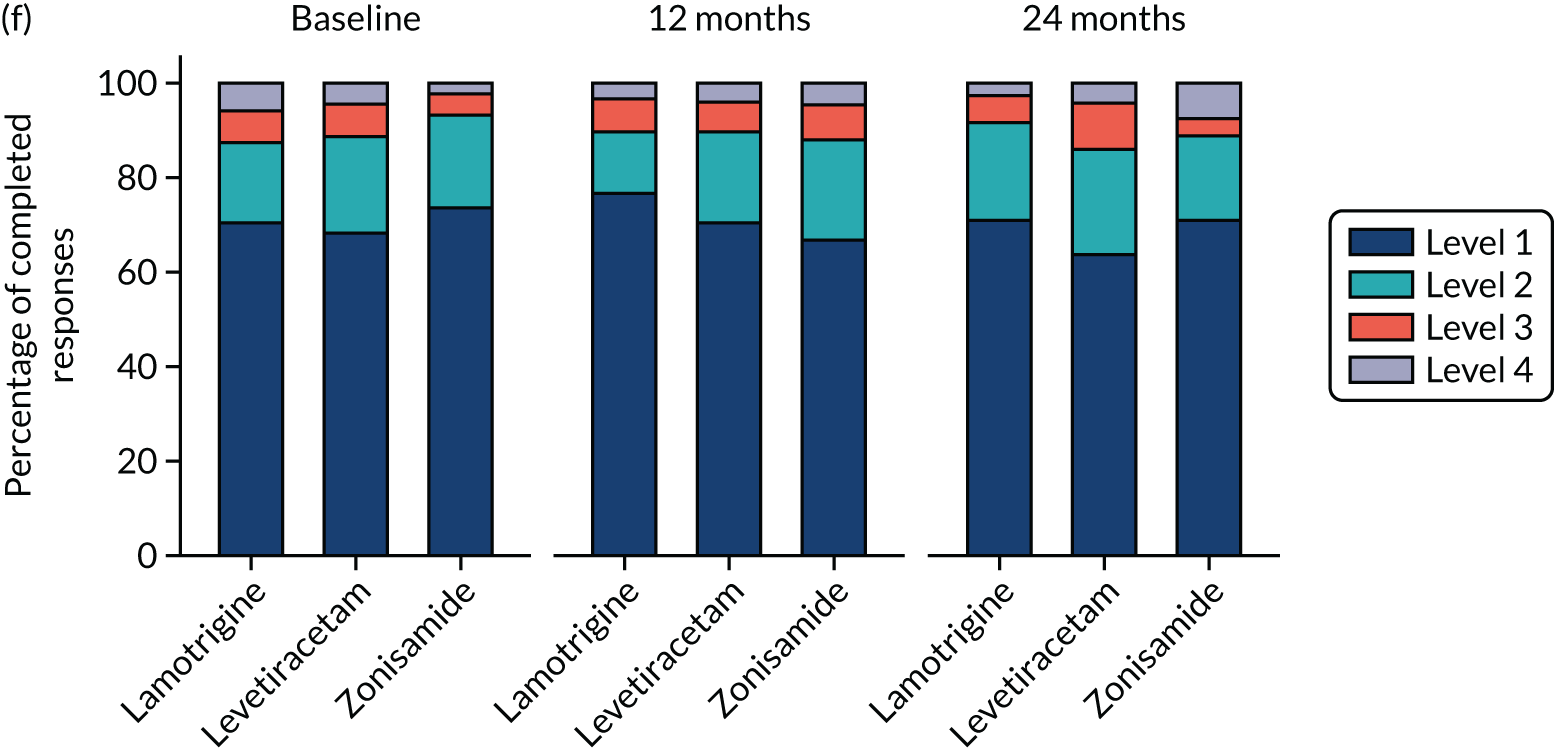
| Variable | Time point | Valproate group (number of participants) | Levetiracetam group (number of participants) | ||||
|---|---|---|---|---|---|---|---|
| Complete | Incomplete | Total | Complete | Incomplete | Total | ||
| Participants | |||||||
| Admitted patient care | Baseline | 202 | 58 | 260 | 210 | 50 | 260 |
| Outpatients | Baseline | 202 | 58 | 260 | 210 | 50 | 260 |
| A&E | Baseline | 202 | 58 | 260 | 210 | 50 | 260 |
| Primary care | 3 months | 128 | 132 | 260 | 115 | 145 | 260 |
| Community care | 3 months | 128 | 132 | 260 | 115 | 145 | 260 |
| Concomitant medication | 3 months | 128 | 132 | 260 | 115 | 145 | 260 |
| Primary care | 6 months | 118 | 142 | 260 | 94 | 166 | 260 |
| Community care | 6 months | 118 | 142 | 260 | 94 | 166 | 260 |
| Concomitant medication | 6 months | 118 | 142 | 260 | 94 | 166 | 260 |
| Primary care | 12 months | 99 | 161 | 260 | 86 | 174 | 260 |
| Community care | 12 months | 99 | 161 | 260 | 86 | 174 | 260 |
| Admitted patient care | 12 months | 218 | 42 | 260 | 221 | 39 | 260 |
| Outpatients | 12 months | 218 | 42 | 260 | 221 | 39 | 260 |
| A&E | 12 months | 218 | 42 | 260 | 221 | 39 | 260 |
| Anti-seizure medication | 12 months | 234 | 26 | 260 | 230 | 30 | 260 |
| Concomitant medication | 12 months | 99 | 161 | 260 | 86 | 174 | 260 |
| Primary care | 24 months | 73 | 187 | 260 | 75 | 185 | 260 |
| Community care | 24 months | 73 | 187 | 260 | 75 | 185 | 260 |
| Admitted patient care | 24 months | 224 | 36 | 260 | 223 | 37 | 260 |
| Outpatients | 24 months | 224 | 36 | 260 | 223 | 37 | 260 |
| A&E | 24 months | 224 | 36 | 260 | 223 | 37 | 260 |
| Anti-seizure medication | 24 months | 206 | 54 | 260 | 185 | 75 | 260 |
| Concomitant medication | 24 months | 73 | 187 | 260 | 75 | 185 | 260 |
| Primary care | 36 months | 52 | 139 | 191 | 50 | 139 | 189 |
| Community care | 36 months | 52 | 139 | 191 | 50 | 139 | 189 |
| Admitted patient care | 36 months | 160 | 31 | 191 | 158 | 31 | 189 |
| Outpatients | 36 months | 160 | 31 | 191 | 158 | 31 | 189 |
| A&E | 36 months | 160 | 31 | 191 | 158 | 31 | 189 |
| Anti-seizure medication | 36 months | 86 | 105 | 191 | 82 | 107 | 189 |
| Concomitant medication | 36 months | 52 | 139 | 191 | 49 | 140 | 189 |
| Primary care | 48 months | 23 | 61 | 84 | 19 | 64 | 83 |
| Community care | 48 months | 23 | 61 | 84 | 19 | 64 | 83 |
| Admitted patient care | 48 months | 69 | 15 | 84 | 66 | 17 | 83 |
| Outpatients | 48 months | 69 | 15 | 84 | 66 | 17 | 83 |
| A&E | 48 months | 69 | 15 | 84 | 66 | 17 | 83 |
| Anti-seizure medication | 48 months | 27 | 57 | 84 | 23 | 60 | 83 |
| Concomitant medication | 48 months | 22 | 62 | 84 | 19 | 64 | 83 |
| Primary care | 60 months | 5 | 3 | 8 | 4 | 3 | 7 |
| Community care | 60 months | 5 | 3 | 8 | 4 | 3 | 7 |
| Admitted patient care | 60 months | 8 | 0 | 8 | 5 | 2 | 7 |
| Outpatients | 60 months | 8 | 0 | 8 | 5 | 2 | 7 |
| A&E | 60 months | 8 | 0 | 8 | 5 | 2 | 7 |
| Anti-seizure medication | 60 months | 0 | 8 | 8 | 0 | 7 | 7 |
| Concomitant medication | 60 months | 5 | 3 | 8 | 4 | 3 | 7 |
| Utilities | |||||||
| EQ-5D | Baseline | 145 | 115 | 260 | 129 | 131 | 260 |
| NEWQOL-6D | Baseline | 101 | 159 | 260 | 85 | 175 | 260 |
| EQ-VAS | Baseline | 139 | 121 | 260 | 132 | 128 | 260 |
| EQ-5D | 12 months | 98 | 162 | 260 | 83 | 177 | 260 |
| NEWQOL-6D | 12 months | 55 | 205 | 260 | 44 | 216 | 260 |
| EQ-VAS | 12 months | 89 | 171 | 260 | 73 | 187 | 260 |
| EQ-5D | 24 months | 72 | 188 | 260 | 73 | 187 | 260 |
| NEWQOL-6D | 24 months | 33 | 227 | 260 | 34 | 226 | 260 |
| EQ-VAS | 24 months | 68 | 192 | 260 | 63 | 197 | 260 |
| EQ-5D | 36 months | 52 | 139 | 191 | 49 | 140 | 189 |
| NEWQOL-6D | 36 months | 32 | 159 | 191 | 30 | 159 | 189 |
| EQ-VAS | 36 months | 48 | 143 | 191 | 43 | 146 | 189 |
| EQ-5D | 48 months | 23 | 61 | 84 | 19 | 64 | 83 |
| NEWQOL-6D | 48 months | 14 | 70 | 84 | 13 | 70 | 83 |
| EQ-VAS | 48 months | 21 | 63 | 84 | 16 | 67 | 83 |
| EQ-5D | 60 months | 5 | 3 | 8 | 4 | 3 | 7 |
| NEWQOL-6D | 60 months | 4 | 5 | 8 | 1 | 6 | 7 |
| EQ-VAS | 60 months | 5 | 3 | 8 | 4 | 3 | 7 |
FIGURE 29.
Distribution of participants’ responses to each EQ-5D attribute by treatment allocated and time: generalised and unclassified epilepsy. For presentation purposes, visits within 90 days of the intended visit date are included. Levels range from 1 to 3, with 3 representing the most severe problem. The percentage of completed responses (%) are reported by intervention group. (a) Mobility; (b) self-care; (c) usual activities; (d) pain or discomfort; and (e) anxiety or depression.
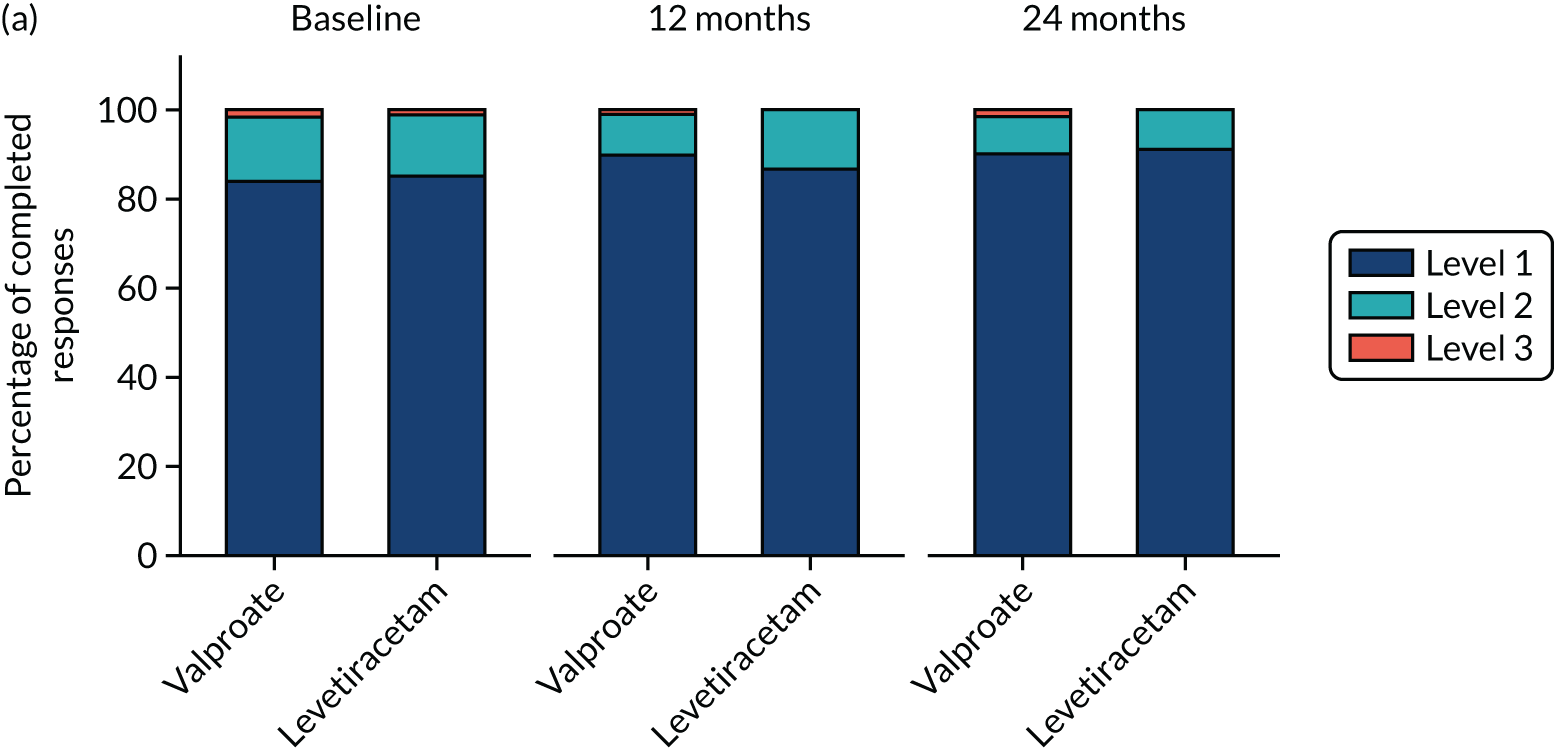
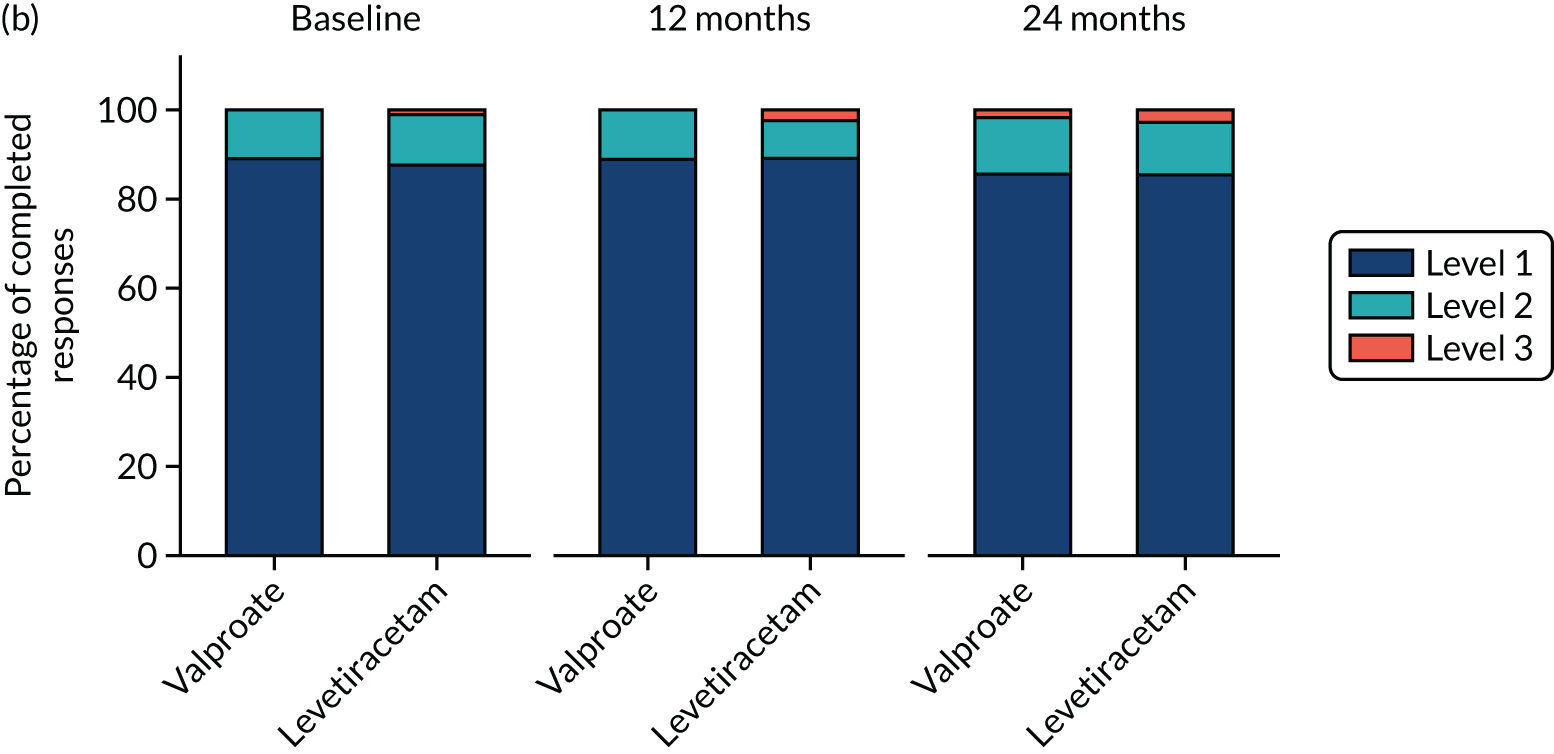
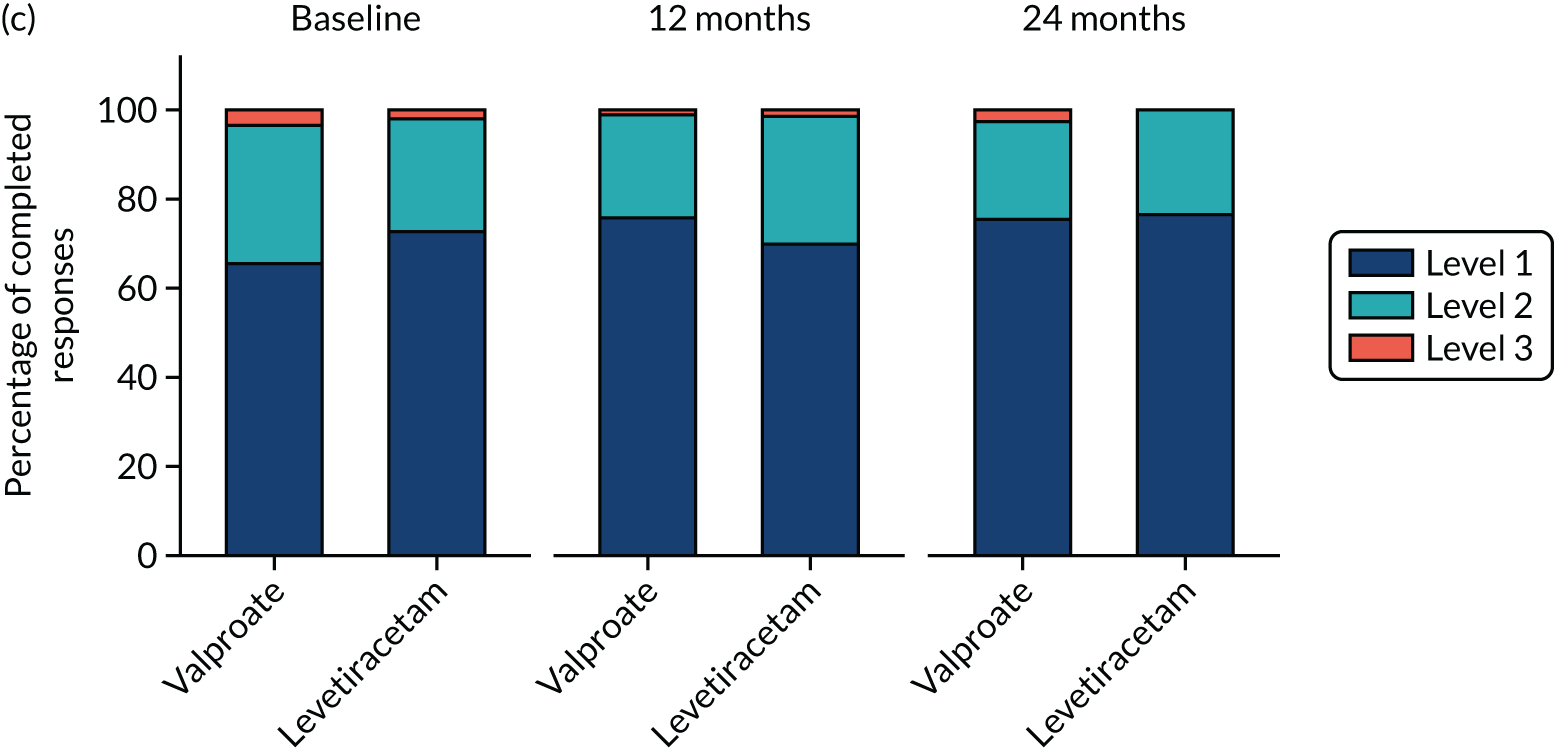
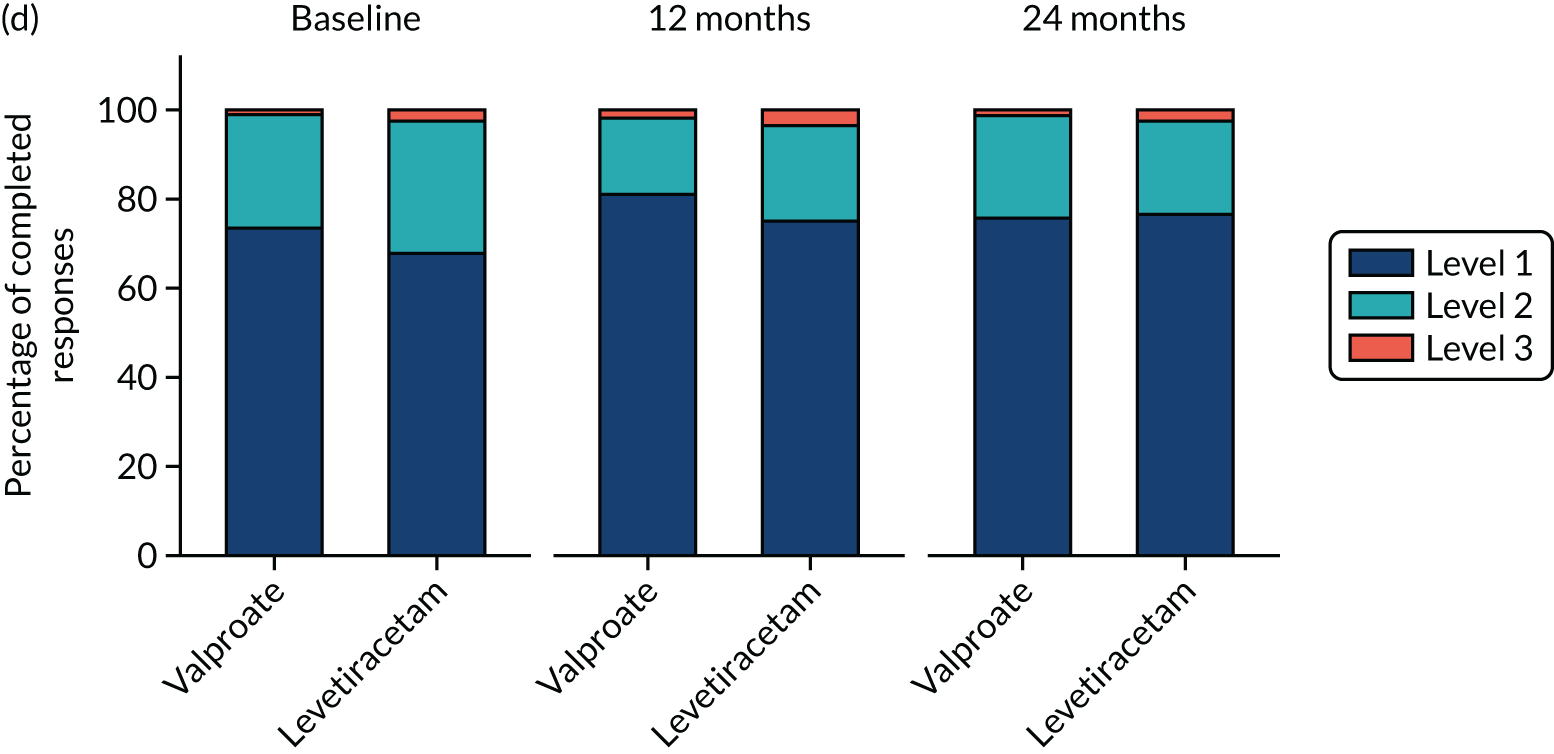
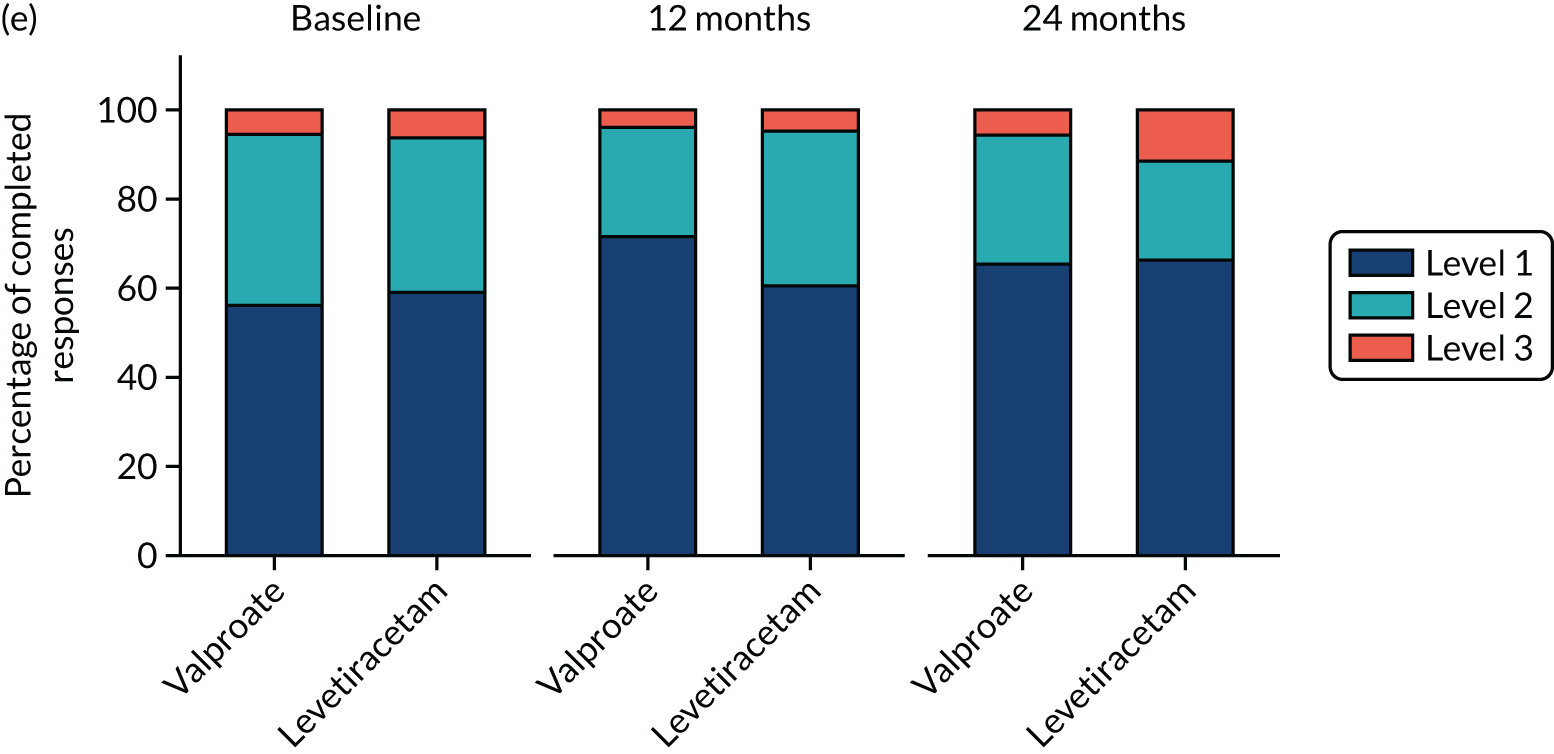
FIGURE 30.
Distribution of participants’ responses to each NEWQOL-6D attribute by treatment allocated and time: generalised and unclassified epilepsy. Levels range from 1 to 4, with 4 representing the most severe problem. The percentage of completed responses (%) are reported by intervention group. (a) Worry; (b) depression; (c) memory; (d) concentration; (e) control; and (f) stigma.
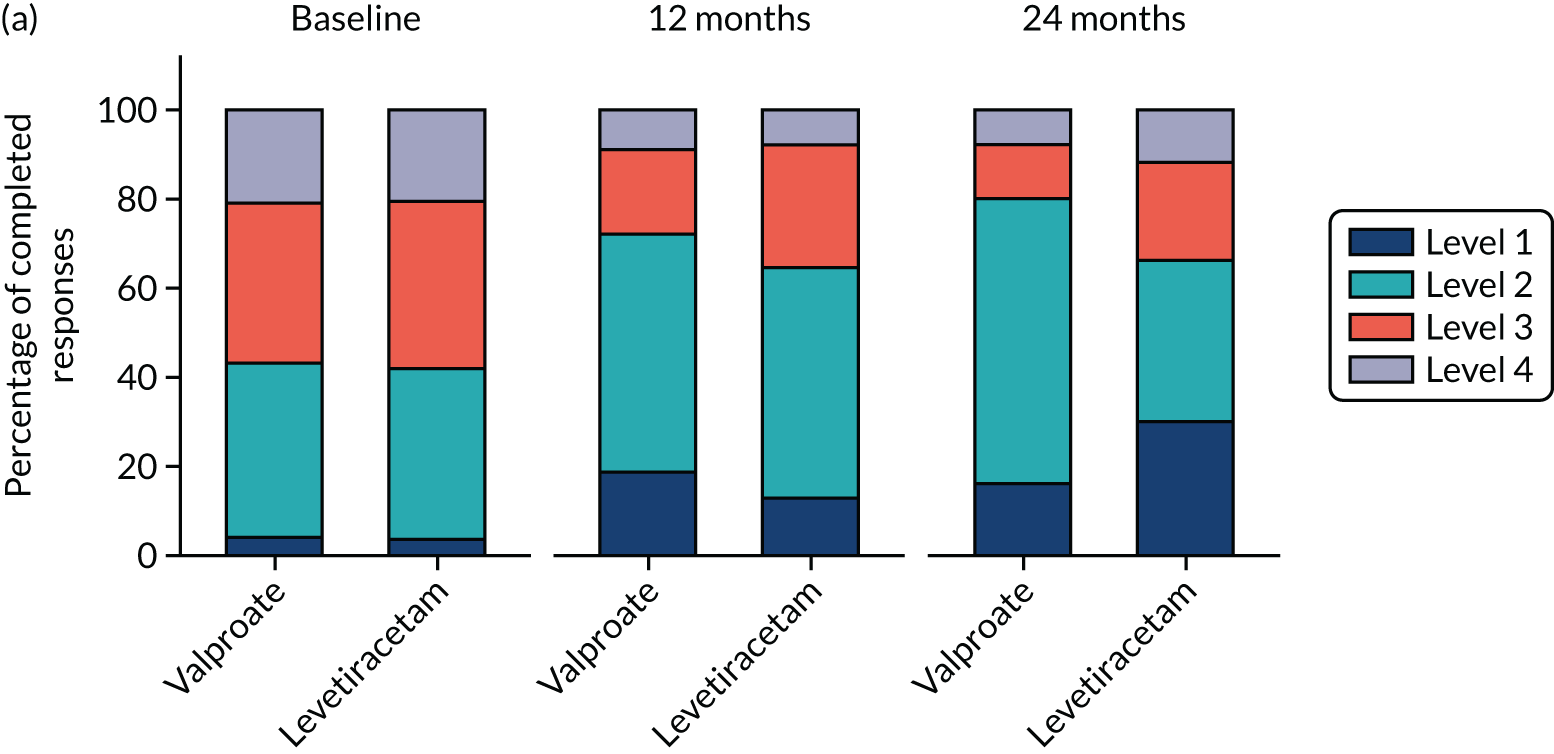
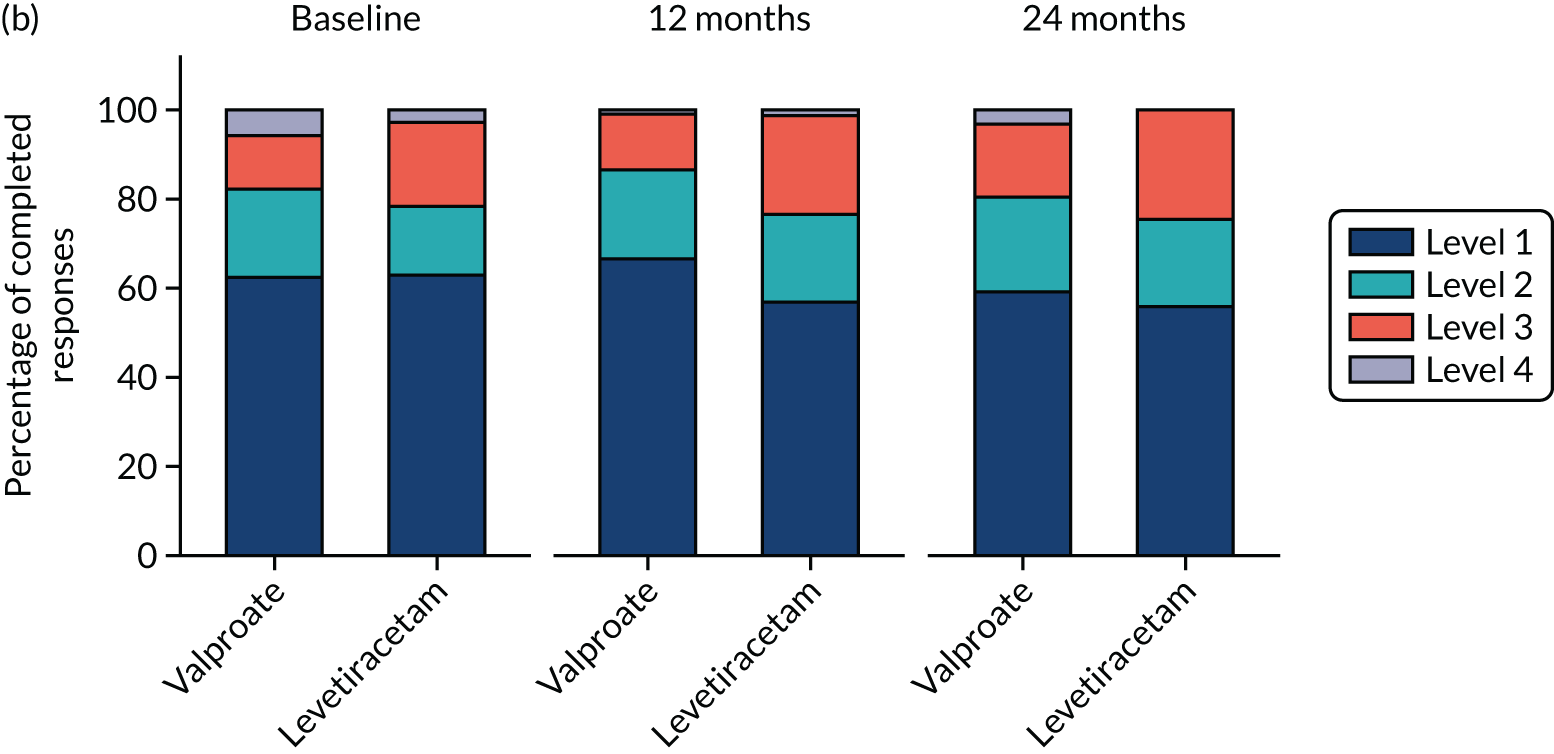
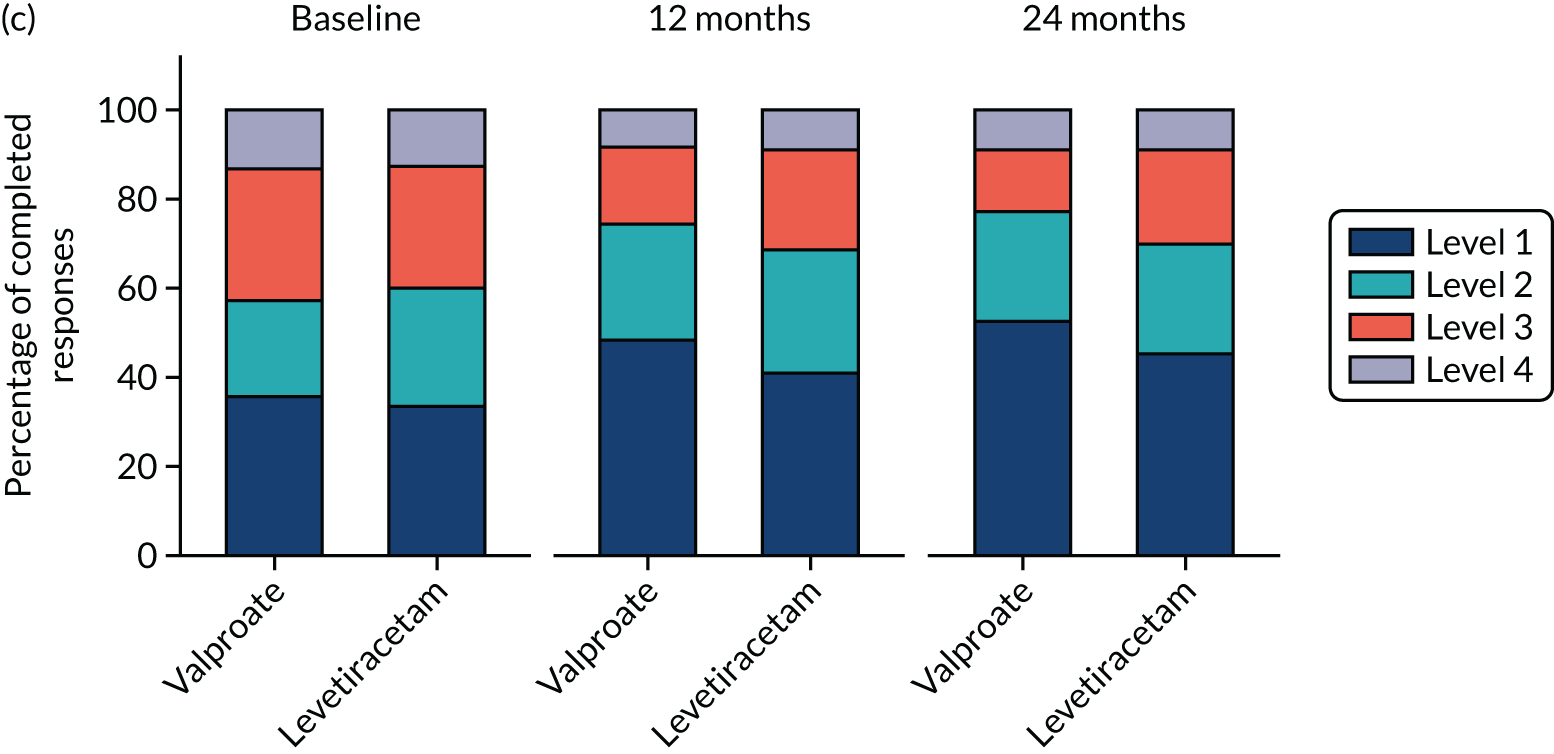
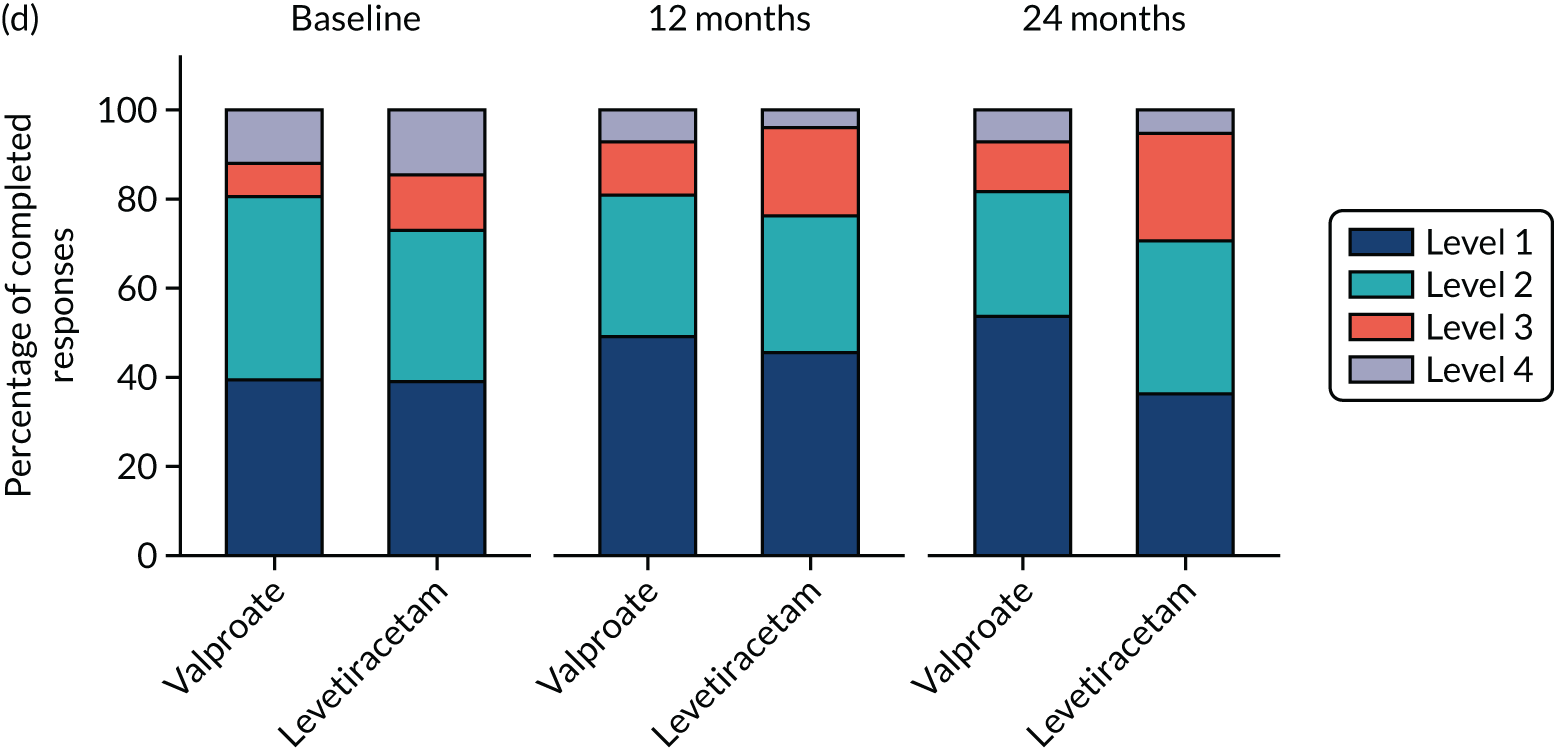
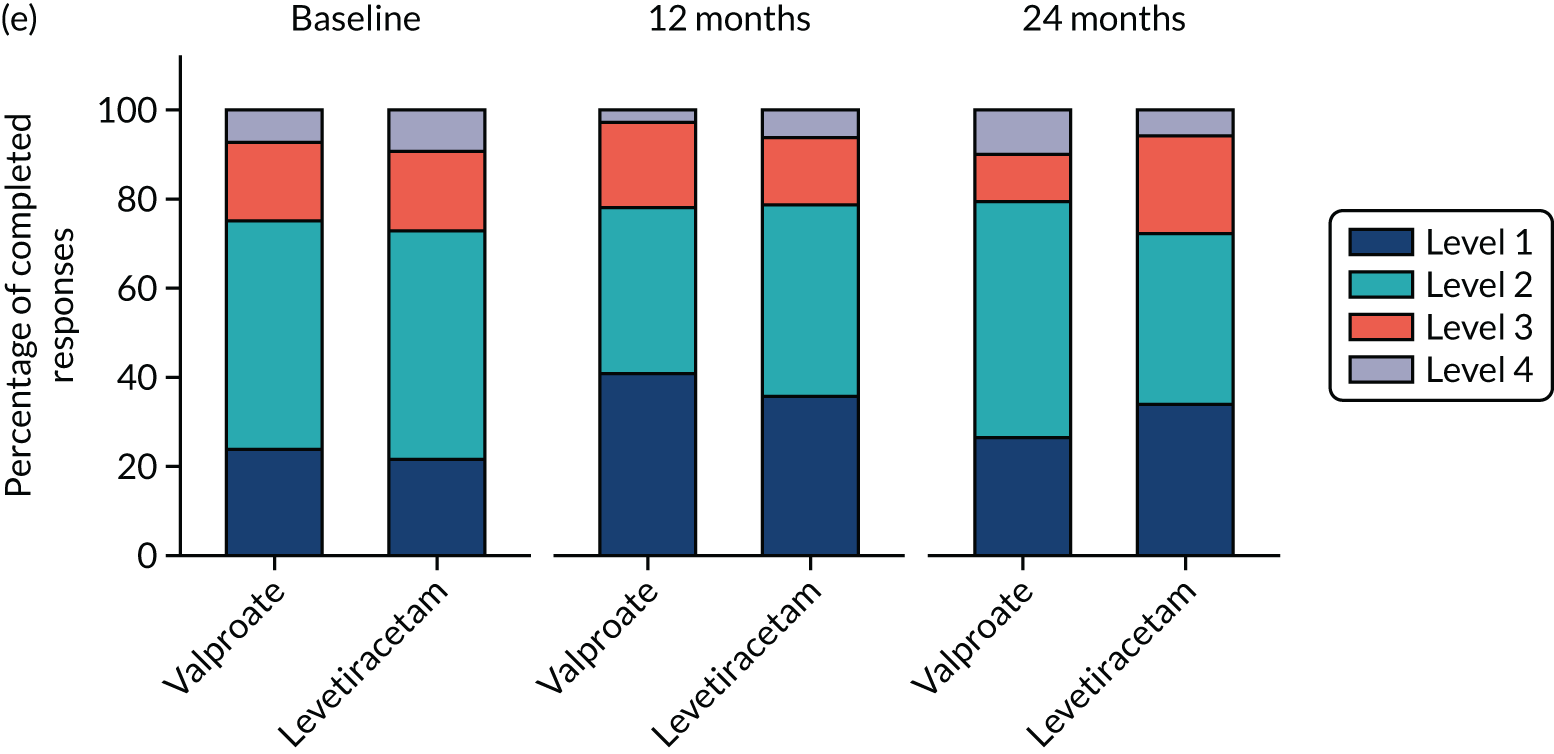
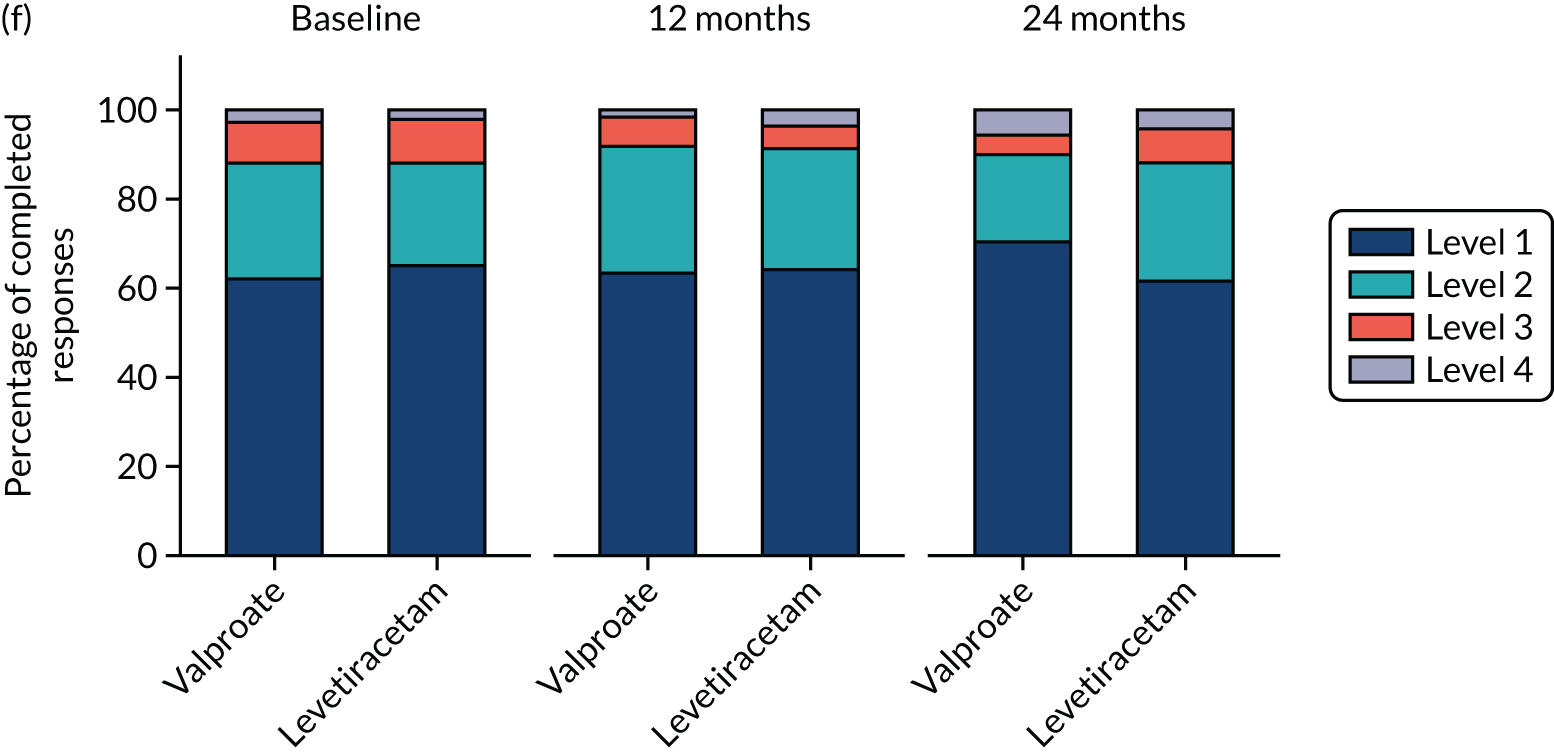
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AR
- adverse reaction
- AUC
- area under the curve
- CC
- complication and comorbidity
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CR
- central range
- CRF
- case report form
- CSRI
- Client Service Receipt Inventory
- CT
- computerised tomography
- DNA
- deoxyribonucleic acid
- EEG
- electroencephalography
- EMA
- European Medicines Agency
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-3L-Y
- EuroQol-5 Dimensions, three-level version (youth version)
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Health Resource Group
- ICER
- incremental cost-effectiveness ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- INHB
- incremental net health benefit
- IQR
- interquartile range
- ISC
- inadequate seizure control
- ITT
- intention to treat
- LCTC
- Liverpool Clinical Trials Centre
- MedDRA
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRI
- magnetic resonance imaging
- NEWQOL
- Quality of Life in Newly Diagnosed Epilepsy Instrument
- NHB
- net health benefit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PP
- per protocol
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAR
- serious adverse reaction
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UAR
- unacceptable adverse reaction
- VAS
- visual analogue scale
