Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/162/02. The contractual start date was in November 2012. The draft report began editorial review in March 2020 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2021 Khunti et al. This work was produced by Khunti et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Khunti et al.
Chapter 1 Introduction: background and rationale
Parts of this chapter have been reproduced with permission from Yates et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Type 2 diabetes: prevalent but preventable
In 2019, the International Diabetes Federation estimated that 463 million people were living with diabetes. This figure is predicted to rise to 700 million by 2045. 2 Type 2 diabetes (T2D), the most common form of diabetes, has been estimated to be the third leading cause of mortality globally and its growing prevalence over recent decades, both in the UK and worldwide, is one of the greatest health challenges facing modern society. A diagnosis of T2D drastically increases lifetime health-care expenditures. 3 Health-care expenditures associated with T2D are, therefore, substantial; in the UK, diabetes currently accounts for approximately 10% of the total health resource expenditure and is projected to account for approximately 17% by 2035 as a result of the sharply increasing prevalence of T2D and its related complications. Some 80% of the costs of diabetes are attributable to complications, which include limb amputation, blindness, kidney failure and stroke. 4
This rising burden of T2D has precipitated three decades of research and health-care policies aimed at preventing diabetes in individuals deemed to be at high risk of developing the disease. High-risk status is defined as an intermediary category of glucose control that is outside the normal range but below the threshold for diagnosis of T2D. Historically, this intermediary range has been classified through impaired fasting glucose or impaired glucose tolerance following an oral glucose tolerance test (OGTT). 5 Supporting international consensus, the National Institute for Health and Care Excellence (NICE) recognises that glycated haemoglobin (HbA1c) in the range of 42–47 mmol/mol (6.0–6.4%) can also be used to identify people who are at high risk of developing T2D, alongside the traditional definitions. 6,7 This high-risk category is referred to variously as ‘intermediate hyperglycaemia’, ‘impaired glucose regulation’, ‘non-diabetic hyperglycaemia’ and ‘prediabetes’. Although we acknowledge that there is debate around these terms, we have chosen to use the term ‘prediabetes’ here to facilitate readability. Some 25–50% of individuals in these risk categories go on to develop T2D within a 10-year period and, without intervention, as many as 70% will eventually develop T2D during their lifetime. 8
Large prevention trials globally, including in Europe, the USA, China and India, have demonstrated that lifestyle interventions aimed at weight loss, a healthy diet and increased physical activity can lead to a 50% reduction in the risk of developing T2D,9 with health benefits sustained over the longer term after the intervention has ceased. 10–12 Such interventions were subsequently modelled to be cost-effective. 13 Translational research has further demonstrated that lifestyle interventions can lead to weight loss and a reduction in diabetes risk when implemented in routine clinical settings,14,15 albeit with lower effectiveness than that demonstrated in large efficacy trials. This has led to the commissioning and delivery of lifestyle diabetes prevention programme within routine care internationally. The largest national scheme is ‘Healthier You: the NHS Diabetes Prevention Programme’, which has been rolling out diabetes prevention across primary care in England16 and now aims to support 200,000 referrals per year.
However, despite the concerted efforts made to translate research into practice by enabling lifestyle interventions to be delivered to those at risk of T2D, success has been limited. Important questions remain as to how we can engage at-risk individuals with diabetes prevention programmes and sustain that engagement over time. These are questions that need to be answered if we are to optimise the prevention of diabetes in the future.
Physical activity for prevention
Physical inactivity is directly involved in the pathogenesis of prediabetes and T2D, and in observational cohort studies it has consistently been associated with an increased risk of the disease. 17 Conversely, high levels of physical activity have been associated with a lower risk of developing diabetes. Importantly, even moderate levels of physical activity have been shown to offer substantial clinical benefits. 18 For example, evidence from the Finnish Diabetes Prevention Study demonstrated that the risk of diabetes was reduced by > 60% in those with prediabetes who walked for 150 minutes per week compared with those who walked for < 60 minutes per week in their leisure time. 19 Similarly, a large international cohort study demonstrated that each 2000-steps-per-day increase in walking activity (equivalent to 20 minutes of brisk walking) decreased the risk of cardiovascular disease mortality and morbidity by 8% in people at high risk. 20 We have also demonstrated that each 30-minute increase in moderate to vigorous physical activity in adults at risk of diabetes leads to a decrease in HbA1c of 0.11%, equivalent to an 8-kg reduction in body weight. 21
Although the seminal diabetes prevention trials were successful at initiating weight loss, we have previously shown, in a systematic review of the evidence, that these same trials were unable to demonstrate clinically significant increases in physical activity over the longer term (> 12 months), and that there have not been any long-term interventions primarily focused on physical activity in those with prediabetes. 22 We therefore concluded that, at the gold standard randomised controlled trial (RCT) level of evidence, the effect of physical activity on diabetes risk is equivocal, and that strategies for effective pragmatic physical activity promotion in this population need to be researched thoroughly.
Harnessing structured education for physical activity promotion
Structured education refers to educational interventions, generally delivered in a small-group setting, that are aimed at the promotion of self-management and health behaviours and are underpinned by established health behaviour theories, a written curriculum and standardised educator training and quality assurance pathways. It is widely used as a central component of diabetes management pathways within routine care and has been recommended by NICE since 2003. 23 One of the most prominent structured education programmes for people diagnosed with T2D available to commissioning organisations nationally in the UK, and, to our knowledge, the only programme to have undergone a multicentre RCT to quantify clinical effectiveness and cost-effectiveness, is the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) programme. 24,25 The DESMOND trial reported reductions in cardiovascular disease risk profile, reduced depression, enhanced smoking cessation and weight loss, and had an incremental cost-effectiveness ratio (ICER) of £2092 per quality-adjusted life-year (QALY) gained, which makes it significantly cheaper than would normally be considered cost-effective by UK decision-makers. 24,25 The widespread existing infrastructure of the DESMOND model is now being adapted to the area of prevention, as it offers a feasible and scalable model for implementing diabetes prevention programmes in primary care and public health settings. 26
Pilot work undertaken by our group, concluding with a single-centre RCT, demonstrated that the approach used in the DESMOND programme can be successfully adapted to promote physical activity among those identified as having prediabetes and that the effectiveness of structured education can be enhanced by pedometer use. 27 The Prediabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) structured education programme was found to increase physical activity levels and substantially reduce fasting and post-challenge glucose levels in a multiethnic population over 12 months when combined with pedometer use. 27 Following this proof of principle, the PREPARE programme was subsequently developed into the Walking Away from T2D programme (referred to hereafter as ‘Walking Away’) that was broadened into an intervention for a wider high-risk population (not just those with diabetes, but those with any risk factors for T2D) for which a full educator training and quality assurance programme was also developed and piloted (see Report Supplementary Material 2). Walking Away was subsequently commissioned into routine primary care pathways throughout England as a low-resource prevention programme. A later trial of this implementation work, supported by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (now Applied Research Collaboration) infrastructure, demonstrated small changes (410 steps per day increase) to physical activity over 12 months, with evidence of greater behaviour change in those with prediabetes (513 steps per day increase). 28 Therefore, although Walking Away has been shown to be potentially effective in promoting physical activity behaviour change when delivered in primary care, particularly to those with prediabetes, work is needed to investigate whether or not greater and longer-lasting physical activity behaviour change can be achieved by integrating other low-cost pragmatic approaches enhanced with technological interventions and whether or not the intervention is effective and cost-effective when delivered to multiethnic communities that are reflective of modern Britain.
Using mobile health technologies to increase scalability
New technologies, such as the internet [electronic health (eHealth)], mobile devices and wearables [mobile health (mHealth)], have the potential to enhance behaviour change interventions by offering scalability, interactivity and reach, and have the capacity to offer highly tailored, interactive behaviour change maintenance support.
Text messaging interventions have been used to support medication adherence, physical activity, weight loss, smoking cessation and the prevention/management of chronic disease, either as a standalone intervention or in combination with other modes of delivery, such as face to face. 8,29–32 However, these interventions have tended not to be evidence based and to offer general rather than highly tailored support. A recent systematic umbrella review showed that such distal technologies in the management of people with T2D led to modest changes in HbA1c. 33 Uncertainty remained, however, about the long-term effectiveness of theory- and evidence-based physical activity interventions using mHealth, which is suitable for integration into routine and evidence-based diabetes prevention pathways and programmes in primary care. 34 Such interventions are likely to have particular salience for the promotion of physical activity given that self-monitoring interventions based on pedometers or wearables have been shown to be effective in promoting increased physical activity across different populations. 35 They were, therefore, ideally suited for integration with the eHealth or mHealth platforms. 36
Ethnicity
In industrialised societies, certain minority ethnic groups are known to have a substantially higher risk of T2D than others. Prevalence and progression rates for diabetes are up to four times higher among South Asian people, who constitute the largest ethnic minority group in the UK, than among the general population. 37 This elevated risk of chronic disease is compounded by lifestyle factors, most notably physical inactivity; South Asian people residing in the UK have been shown to be substantially less active and to have lower levels of cardiovascular fitness than the general population. 38–40 These differences in physical activity behaviour and levels of cardiorespiratory fitness have been linked to the increased prevalence of chronic disease and the higher rates of insulin resistance observed in South Asian populations;40,41 South Asian people, therefore, represent a priority target population in the prevention of T2D. However, evidence is limited that diabetes prevention programmes in a European context have been successful in changing lifestyle behaviours and improving health in minority ethnic groups. The largest trial to date, the Prevention of Diabetes and Obesity in South Asians (PODOSA) trial,42 demonstrated a small effect on weight (reduction of 1.64 kg) following a family-based intervention programme, but no significant effect on physical activity.
Principal research objectives
The principal research objectives of the PROPELS study were to:
-
develop a tailored mHealth intervention to provide follow-on support for participants referred to the Walking Away programme
-
investigate whether or not Walking Away can lead to sustained increases in physical activity after 4 years in an ethnically diverse population at high risk of T2D, when delivered at two levels of ongoing follow-on maintenance support (with and without the mHealth intervention developed in the previous objective)
-
compare the effectiveness of the tested interventions in white European and South Asian subgroups
-
conduct a within-trial and long-term economic evaluation of each tested intervention using the costs and benefits arising from the study, rates of progression to diabetes, biomedical outcomes, NHS resource use and quality of life.
Chapter 2 Trial design and methods
Parts of this chapter have been reproduced with permission from Yates et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The PRomotion Of Physical activity through structured Education with differing Levels of ongoing Support for those with prediabetes (PROPELS) study was a multicentre RCT that compared two modes of a physical activity intervention with a control condition. The RCT was centred on the established Walking Away intervention. The PROPELS trial comprised two phases: the development and piloting phase of a mHealth intervention to provide follow-on support for Walking Away, and a multicentre RCT to test the efficacy of the intervention in a diverse multiethnic population. This chapter will focus on the design and methods of the RCT, and, therefore, it closely reflects the previously published study protocol. 1
Recruitment of participants
Participants were recruited across two demographically distinct centres, Leicester and Cambridge, UK, with a required sample size of 1308 (see Sample size). The primary method of recruitment was through primary care by using data collected by the NHS Health Checks programme, a screening programme run in England designed to identify and treat vascular disease risk (heart disease, stroke, diabetes and kidney disease) in all individuals aged 40–74 years, which has led to many primary care practices recording their patients’ HbA1c or fasting glucose values. 43
In both Leicester and Cambridge, our research teams worked in collaboration with practices providing the Health Checks programme to recruit individuals who were identified as having prediabetes and were not currently on a systematic diabetes prevention pathway. To help this process, recruited practices were trained to run an established automated diabetes risk score within their practice database. 44 A function within the risk score used a Morbidity Information QUery and Export SynTax (MIQUEST) search to identify all individuals who had had a previous blood glucose or HbA1c result recorded in the prediabetes range at any point during the 5 years preceding recruitment. 44 In Cambridge, participants meeting the inclusion criteria were also recruited from existing population-level research studies (specifically the Fenland Study45).
Eligible individuals identified as having a HbA1c or blood glucose value in the prediabetes category during the previous 5 years were sent an invitation letter, a brochure about the study and a reply slip. Those recruited directly from primary care were sent the invitation letters by the primary care practice at which the search was conducted. Those recruited from existing research databases were sent the invitation by the principal investigator of that study. Individuals who were interested in taking part were asked to return the reply slip directly to the PROPELS trial research team. An appointment was then arranged for a baseline visit and the individual was sent the full study patient information sheet along with a confirmation letter.
To allow for increased generalisability and the ability to stratify results by ethnicity (see Sample size), recruitment was purposely targeted so that 25% (n = 327) of the total cohort would be of South Asian ethnic origin; therefore, we aimed to recruit 66% (n = 863) of participants from Leicester and Leicestershire, which has a more ethnically diverse population than Cambridgeshire. Leicester city is, in fact, one of the most ethnically diverse places in the UK; according to 2011 census figures,46 37% of its population are Asian/Asian British (predominantly of Indian heritage).
Eligibility/exclusion criteria
Individuals were eligible for the trial if they:
-
were aged 40–74 years, or 25–74 years if they were South Asian
-
had had a recorded plasma glucose or HbA1c value in the prediabetes range during the previous 5 years (see Table 1)
-
had access to a mobile telephone and were willing to use it as part of the study.
Individuals were excluded from the trial if they were:
-
unable to take part in ambulatory-based activity
-
pregnant
-
involved in other related intervention studies
-
diagnosed with diabetes, or diabetes was detected at baseline visit
-
unable to understand basic written and verbal English
-
unable to give informed consent.
Protocol for participants found to have type 2 diabetes at baseline
At Leicester, individuals who had a HbA1c value in the diabetes range at baseline (Table 1) were recalled for a second, confirmatory, test, and if diabetes was confirmed they were referred back to their physician for routine care. At Cambridge, the individual’s primary care clinician was informed of the need to confirm diagnosis as appropriate. Individuals found to meet the World Health Organization and NICE47,48 criteria for a diagnosis of diabetes were excluded from the study.
| Variable | Normal glycaemia | Prediabetes (inclusion criteria for PROPELS)b | T2D | |
|---|---|---|---|---|
| Upper value | Lower value | Upper value | Lower value | |
| HbA1c (%)a | < 6.0 | ≥ 6.0 | < 6.5 | ≥ 6.5 |
| HbA1c (mmol/mol) | < 42 | ≥ 42 | < 48 | ≥ 48 |
| Fasting plasma glucose (mmol/l)a | < 5.5 | ≥ 5.5 | < 7.0 | ≥ 7.0 |
| 2-hour post-challenge glucose (mmol/l) | < 7.8 | ≥ 7.8 | < 11.1 | ≥ 11.1 |
Protocol for participants found to have normal glycaemia at baseline
Individuals who were found to have normal glycaemia at baseline were included in the study, provided that they met the inclusion criterion of a historical blood glucose level in the prediabetes range during the previous 5 years.
Randomisation and blinding
Once baseline data had been collected, participants were randomised (stratified by sex and ethnicity) using an online randomisation tool hosted at the University of Leicester Clinical Trials Unit. Individuals were randomised (1 : 1 : 1) to one of three study arms:
-
control arm
-
Walking Away arm
-
Walking Away Plus arm.
The only exception to this was that individuals recruited from the same household were randomised to the same study arm. Participants were informed by letter of their allocated treatment. Study arm allocation was concealed from the study measurement teams conducting the 12- and 48-month follow-up and the research staff processing the accelerometer data.
Owing to the nature of the trial, patients and intervention providers were not blinded to study arm allocation. However, those processing the accelerometer data to generate the primary outcome were blinded to allocation.
Control study arm: detailed advice leaflet
Participants allocated to the control study arm received an advice leaflet (see Report Supplementary Material 1) detailing the likely causes, consequences, symptoms and timeline associated with prediabetes, as well as information about how physical activity can reduce the risk of developing T2D. The leaflet was informed by Leventhal’s common sense model,49 which also underpinned the structured education programme. Participants also continued to receive routine care from their GP.
Walking Away study arm: group-based behaviour change intervention with annual refresher sessions
Participants assigned to the Walking Away study arm were given the same advice leaflet that those in the control study arm received and were invited to attend, within 3 months of their baseline clinic visit, a 3-hour behaviour change intervention titled ‘Walking Away from Type 2 Diabetes’ (Walking Away), along with annual refresher sessions. The intervention is described in full in Chapter 3.
Walking Away Plus study arm: group-based behaviour change intervention, annual refresher sessions plus a mHealth intervention to provide follow-on support
Participants received the same advice leaflet and were invited to attend the Walking Away programme and annual refresher sessions as described above. In addition, they were provided with follow-on support in the form of a tailored text messaging and telephone intervention. The text messaging and telephone aspect of the intervention, hereinafter referred to as ‘the mHealth intervention’, was developed within the PROPELS programme of work; its content and the process of its development are described in full in Chapter 3.
Data collection
Data collection clinics were run by research nurses in the Leicester Diabetes Centre and in the MRC Epidemiology Unit in Cambridge and at other local community centres and clinic areas. All staff members were trained in study procedures and data were collected in accordance with the sponsor’s standardised operating procedures (see Report Supplementary Material 5). Written informed consent was obtained from each participant before data collection commenced. Details of all clinical assessments taken are provided in Table 2. After each visit, participants were sent a letter detailing selected clinical results, and the results were also sent to the participant’s general practitioner.
| Clinical assessment | Time point (months) | ||
|---|---|---|---|
| 0 | 12 | 48 | |
| 7-day step count and physical activity (accelerometer) | ✓ | ✓ | ✓ |
| Blood pressurea | ✓ | ✓ | ✓ |
| Body fat percentage | ✓ | ✓ | ✓ |
| BIPQ | ✓ | ✓ | |
| Dietary questions | ✓ | ✓ | ✓ |
| Enactment of techniques (groups 2 and 3 only) | ✓ | ✓ | |
| EQ-5D; SF-8 | ✓ | ✓ | ✓ |
| Family history of disease | ✓ | ✓ | ✓ |
| Fasting and 2-hour post-75 g challenge glucose and insulin (Leicester only) | ✓ | ✓ | |
| HbA1ca | ✓ | ✓ | ✓ |
| Heighta | ✓ | ✓ | |
| HADS | ✓ | ✓ | ✓ |
| Lipidsa | ✓ | ✓ | ✓ |
| Liver function testsa | ✓ | ✓ | ✓ |
| Medication status | ✓ | ✓ | ✓ |
| Muscular/skeletal injury | ✓ | ✓ | ✓ |
| NEWS | ✓ | ||
| Walking self-efficacy | ✓ | ✓ | ✓ |
| RPAQ | ✓ | ✓ | ✓ |
| Sleep | ✓ | ✓ | ✓ |
| Smoking status | ✓ | ✓ | ✓ |
| Urea and electrolytesa | ✓ | ✓ | ✓ |
| Use of health resources | ✓ | ✓ | ✓ |
| Waist circumferencea | ✓ | ✓ | ✓ |
| Weighta | ✓ | ✓ | ✓ |
Primary outcome measure: change in ambulatory activity at 48 months
The primary outcome measure was the change in ambulatory activity (steps per day) at 48 months, assessed using an accelerometer (Actigraph GT3X+, ActiGraph, LLC, Pensacola, FL, USA), with an intermediary assessment at 12 months. Participants were asked to wear the accelerometer on a waistband (on the right anterior axillary line) during waking hours for 7 consecutive days following their baseline and follow-up visits. At the end of the 7 days, participants were asked to return the accelerometer and log sheet to the research team in a prepaid envelope. Raw acceleration data were captured and stored at 100 Hz. For the purposes of this study, data were integrated into 60-second epochs. At least 3 days’ valid wear was required to count as a valid recording. Non-wear time was determined by ≥ 1-hour of consecutive zero counts. Data processing was undertaken on a commercially available analysis tool (Kinesoft, Saskatoon, SK, Canada).
Secondary outcomes and descriptive data
Objectively assessed time spent sedentary and in light- and moderate- to vigorous-intensity physical activity
The accelerometer that was used to measure the primary outcome detailed above also provided secondary outcome data on the number of censored steps taken per day, defined as steps taken above an intensity (500 counts per minute) used to distinguish between purposeful and incidental ambulation. 50 Commonly used Freedson cut-off points were used to distinguish between time spent sedentary and time spent in light, moderate and vigorous physical activity. 51 Compliance with the physical activity recommendations of undertaking at least 150 minutes of moderate- to vigorous-intensity physical activity per week was also assessed;52 this was calculated as (1) the accumulation of 150 minutes overall of at least moderate-intensity physical activity to meet the Chief Medical Officer’s updated physical activity guidelines52 and (2) the accumulation of 150 minutes per week of at least moderate-intensity physical activity in bouts of at least 10 minutes in duration.
Objectively assessed time spent in the postures of sitting/lying, standing and walking
Concurrently with, and in addition to, the ActiGraph accelerometer, participants were asked to wear an activPAL3™ device. This is a small, slim, monitor worn on the thigh that uses accelerometer-derived information about thigh position to determine body posture (i.e. sitting/lying, standing or stepping). The activPAL3™ was initialised using the manufacturer’s software with the default settings (i.e. 20 Hz, 10 seconds’ minimum sitting-upright period) and participants were asked to wear the device continuously (24 hours per day). The activPAL3™ was covered with a nitrile sleeve and fully wrapped in one piece of waterproof dressing [Hypafix Transparent (BSN Medical, Hamburg, Germany)] to allow participants to wear the device during bathing activities and was secured to the midline anterior aspect of the upper thigh using hypoallergenic waterproof dressing (Hypafix Transparent). Data were analysed using a bespoke open-source processing package [ProcessingPAL; URL: https://github.com/UOL-COLS/ProcessingPAL/releases/tag/V1.2 (accessed 16 November 2021)]. Data on waking wear time, time spent sedentary, time spent standing and time spent walking were utilised for this study.
Recent Physical Activity Questionnaire
Self-reported physical activity was measured using the Recent Physical Activity Questionnaire (RPAQ), which assesses physical activity across four domains (domestic, recreational, work and commuting) over the previous month. RPAQ has shown moderate to high reliability for assessing physical activity energy expenditure, and good validity in ranking individuals according to their time spent in vigorous intensity physical activity and overall physical activity energy expenditure. 53
Biochemical variables and diabetes diagnosis
Venous sampling was used to assess standard biomedical outcomes, comprising HbA1c, lipid profile (triglycerides, HDL, LDL and total cholesterol), urea and electrolytes (sodium, potassium, urea and creatinine), and liver function tests (albumin, total bilirubin, alkaline phosphatase and alanine transaminase). Assays were completed in quality-controlled clinical laboratories at University Hospitals of Leicester NHS Trust and Cambridge University Hospitals NHS Foundation Trust. At the Leicester site only, participants were assessed for fasting and 2-hour post-challenge glucose and insulin levels following a 75-g OGTT; the OGTT results will be analysed at a later date and used to provide greater clinical insight into how physical activity affects metabolic health.
For the purposes of the main trials, the classification of glycaemic status was based on HbA1c values using World Health Organization and NICE guidelines47,48 (see Table 1). During the trial, participants at the Leicester site who were found to have a HbA1c value in the diabetes range (≥ 6.5% or 48 mmol/mol) were recalled for a second, confirmatory, test, and if diabetes was confirmed they were referred to their clinician. At Cambridge, the response to a HbA1c value in the diabetes range was to send a letter to the individual’s primary care clinician informing them of the need to confirm a diagnosis.
Protocol for participants found to have type 2 diabetes during the trial
Participants diagnosed with T2D during the trial were retained and continued to be offered all study and interventional procedures, as the primary outcome measure was change in physical activity.
Standard anthropometric and demographic measurements
Height, body weight, body fat percentage and waist circumference were measured to the nearest 0.5 cm, 0.1 kg, 0.5% and 0.1 cm, respectively. Waist circumference was measured using a soft tape measure mid-way between the lowest rib and the iliac crest. Arterial blood pressure was obtained from the right arm of the participant when seated; three measurements were taken and the average of the last two measurements was used. Information on ethnicity, medication history, current smoking status, family history of diabetes in first- and second-degree relatives, and muscular/skeletal injury that prevents physical activity were obtained by self-report. Social deprivation was determined by applying the English Index of Multiple Deprivation (IMD) to participants’ postcodes. For descriptive purposes, data are categorised at the national quintile values to show that the distribution within the recruited population is generalisable to the national average.
Genetics
A blood sample for future genetic analysis was also collected from those who consented.
Cardiovascular risk
Secondary outcomes were used to estimate cardiovascular risk, calculated through the Framingham Risk Score. 54 The Framingham Risk Score has been shown to perform reasonably well in multiethnic UK populations, although it may underestimate risk in South Asian ethnic minorities. 55 However, the secondary outcomes in the PROPELS trial did not allow a more comprehensive risk score to be employed.
Sleep
Participants self-reported on two single-item questions asking about sleep duration in the last night and average sleep duration during a usual week.
Self-reported dietary behaviour
Dietary behaviour was captured in two short questionnaires used in previous research studies by our group, which were administered to participants for self-completion. The questions were based on an abbreviated dietary food frequency questionnaire developed for the European Prospective Investigation of Cancer and Nutrition (EPIC) study and a questionnaire of dietary intentions developed for the international NAVIGATOR (Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research) study. 56,57 The food frequency questionnaire captured portions per week of fresh fruit, green leafy vegetables, other vegetables, oily fish, other fish, chicken, meat, eggs, cheese and wholemeal/brown bread. In addition, alcohol intake (drinks per day) was captured. Dietary intentions captured the degree (on a 5-point Likert scale) to which each participant was actively trying to limit the amount of total fat, saturated fat, sugar or salt in their diet.
Health-related quality of life: EuroQol-5 Dimensions, SF-8 and Hospital Anxiety and Depression Scale
Health-related quality of life was measured using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L),58 and the Short Form (SF-8) Health Survey. 59 The EuroQol-5 Dimensions (EQ-5D) is a standardised questionnaire developed for use as a measure of health outcomes. It defines health in terms of five dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. It is widely used to calculate QALYs, which are essential for cost-effectiveness analysis. The SF-8 is a self-administered questionnaire that measures eight health domains (general health, physical functioning, role limitations due to physical health problems, bodily pain, vitality, social functioning, mental health and emotional roles) using eight questions. The standard (4-week) recall format was used. Data from SF-8 responses were used to derive a physical component score and a mental component score.
Depression and anxiety symptomology were measured using the Hospital Anxiety and Depression Scale to produce independent subscales for anxiety and depression. 60
Health, medication and smoking status
Medical history and medication status were measured using an interview-administered protocol. Data on family history of diabetes and cardiovascular disease, smoking status and muscular/skeletal injury were obtained by self-report. All adverse events that were reported to the study sponsor (University of Leicester) were also recorded.
Health resources
A health resources questionnaire was used to record the number of times that the participant had seen a health-care practitioner (e.g. a GP, nurse or other health worker) over the previous 12 months and the number of times that they had been to hospital. In addition, the number of contacts and costs associated with the intervention were captured by the research team and were included in the cost-effectiveness analysis of the intervention.
General practice data
The research team attempted to collect relevant biochemical data, diabetes diagnosis data and other medical event data from during the trial directly from participants’ GP practices for those lost to follow-up. Collected data were matched to the nearest follow-up time point.
Potential mediators of behaviour change
Perceptions of diabetes risk: Brief Illness Perceptions Questionnaire
The validated Brief Illness Perceptions Questionnaire (BIPQ) was used to measure perceptions and perceived knowledge of diabetes risk at 12 and 48 months. 61 BIPQ is an eight-item instrument that uses an 11-point Likert scale (where 0 = no effect and 10 = complete effect) to measure five cognitive diabetes risk representations (consequences, timeline, personal control, treatment control and identity), two emotional representations (concern and emotion) and risk comprehensibility (perceived knowledge).
Walking self-efficacy
Self-efficacy was assessed at baseline and 12 and 48 months using six items to measure participants’ confidence in their ability to walk for 10, 30 and 60 minutes each day. Items used a 100% confidence rating scale (on which 0% represented no confidence and 100% represented complete confidence).
Self-reported use of behaviour change strategies
Participants in the Walking Away and Walking Away Plus study arms reported their use of behaviour change strategies called ‘action control’ measures62 at 12 and 48 months using a 5-point Likert scale (where 1 = most of the time and 5 = never). The items assessed included how often participants set goals, formed action plans, used a pedometer, completed a physical activity log, were aware of their activity levels and were trying to be more physically active.
Uptake and adherence to Walking Away and Walking Away Plus interventions
Measures of uptake and adherence to the interventions included (1) attendance at the initial Walking Away group session, (2) attendance at the group annual refresher sessions at 12, 24 and 36 months, (3) the proportion of telephone calls completed, (4) the number of participants who registered for the text messaging service, (5) the number of STOP messages received for test messaging (i.e. number opting out of the text messaging and pedometer support), (6) the proportion of intended texts sent, and (7) the number of step count texts received from participants relative to the number of requests they were sent (engagement).
Qualitative substudies
A number of qualitative substudies were conducted to contribute to the process evaluation of the intervention:
-
Focus groups were held with the educators who delivered the programme and the telephone calls; these were held approximately 12 months after the educators had delivered the final sessions.
-
Focus groups and telephone interviews were held with participants from the two intervention arms at approximately 48 months. Purposive sampling aimed to achieve a sample that was diverse in terms of both demographic characteristics and level of engagement with the intervention [e.g. from those who attended all Walking Away group sessions (groups 2 and 3) and/or responded frequently to text messages (group 3) to those who attended fewer group sessions (groups 2 and 3) and/or requested to stop the text messages (group 3)].
These qualitative substudies focused on two novel aspects of the PROPELS intervention: its duration (4 years, compared with many previous interventions of only 1 year) and the maintenance support through telephone calls and text messaging. We were specifically interested in understanding the influences on engagement with the intervention; whether or not and how participants reported that the intervention helped them to increase and/or maintain physical activity; and how participants and educators thought that the intervention could be improved.
The procedure for conducting the interviews and focus groups and analysing the scripts is described in Chapter 5.
Sample size
For 1 – beta = 0.8, alpha = 0.025 (allowing for two a priori comparisons against control conditions), a standard deviation (SD) 4000 steps per day27,63–65 and a dropout rate of 30% (lost to follow-up and incomplete primary outcome data) over 4 years, we required 436 participants in each study arm (1308 in total) to be able to detect a difference in change in ambulatory activity of 1000 steps per day (equivalent to 10 minutes of walking per day or 70 minutes of walking per week) between the intervention study arms and the control study arm. Assuming that 25% of participants in the total cohort were South Asian, we had 80% power to detect a difference of 2000 steps per day when comparing the two intervention comparisons with the control study arm (α = 0.025) in the South Asian population.
It should be noted that this sample was intended to be updated based on relevant information that was published after the development of the protocol. Based on pooled longer-term outcome data for steps per day from two primary care-based trials that, collectively, recruited 1688 individuals, the baseline-adjusted SD at 1, 2 and 3 years post randomisation was approximately 2000 steps per day. 66 Furthermore, recent evidence has suggested that even small differences in objectively assessed physical activity are clinically important,67 with 500 steps per day defined as the minimum clinically important difference in those at risk of T2D. 66 Had the study been powered on these parameters, the overall sample size would have been unchanged, as the standardised differences (1000/4000 vs. 500/2000) are identical. Therefore, it can be noted that the study is likely to be powered to detect a smaller difference in ambulatory activity in the overall population of 500 steps per day and a difference in the South Asian population of 1000 steps per day.
Diabetes progression
We assumed a conversion rate to T2D in the control study arm of at least 24% over the entire 4 years of the study and would, therefore, have 80% power to detect a 40% reduction in the relative risk of T2D in both intervention study arms compared with the control study arm (α = 0.025). We used an estimated conversion rate at the lower level reported for traditionally defined prediabetes. 5,68
Statistical analysis
See Appendix 1 for the full statistical analysis plan (SAP) developed for this study, as well as the SAP revision history (see Table 26), SAP responsibilities (see Table 27) and SAP signatories (see Table 28). The SAP was finalised and published on the trial registry (ISRCTN83465245) before the database was locked. The trial statistician was not blinded to study arm allocation.
Analysis of the primary outcome: change in ambulatory activity (steps per day) at 48 months
The analysis involved two a priori comparisons in which each intervention arm was compared with the control study arm. Should either of these comparisons reveal a significant difference, a third a priori comparison would have been undertaken to compare the difference between intervention arms; this will be included as a secondary analysis. Data were analysed at the patient level using a modified intention-to-treat protocol; participants with complete data were analysed in the study arm to which they had been randomised, regardless of the dose of intervention they actually received. Analyses of covariance models were used, adjusting for wear time at baseline, wear time at 48 months, number of valid days at baseline, number of valid days at 48 months, the three randomisation stratification variables (centre, ethnicity and sex) and ambulatory activity at baseline as covariates. Missing data at baseline were replaced using the indicator method. 69
Ethnicity and other subgroup analyses for primary outcome
For the primary outcome only, interactions between randomised study arm and (1) sex (men/women), (2) age (< 60 years/≥ 60 years), (3) ethnicity (white European/South Asian/other), (4) family history of T2D (yes/no), (5) prediabetes status at baseline (yes/no), (6) baseline obesity status [< 30 kg/m2 (27.5 kg/m2 for South Asians), ≥ 30 kg/m2 (27.5 kg/m2 for South Asians)] and (7) baseline deprivation (split at median IMD score into high vs. low) were tested by including the relevant interaction parameters in the analysis model.
If the p-value of any of the interactions tested above was < 0.05, then the estimates and 97.5% confidence intervals (CIs) of the two intervention effects (Walking Away vs. control and Walking Away Plus vs. control) on the primary outcome were reported in the relevant subgroups.
Sensitivity and per-protocol analysis for the primary outcome
To access the possible impact of excluding those with data lost to follow-up from the analysis, the primary analysis was repeated replacing missing data using multiple imputation by chained equations, across 10 imputed data sets. In addition, to access whether or not adherence to the intervention affected the results, the primary analysis was repeated when restricted to a per-protocol data set in accordance with the following criteria:
-
control – all individuals
-
Walking Away – attended initial education and at least one follow-up annual refresher session
-
Walking Away Plus – attended initial education and at least one follow-up annual refresher session and registered with the text message service and received the initial telephone call and received at least one further telephone call during the trial.
Analysis of continuous secondary outcomes
Ambulatory activity at 12 months and all other continuous secondary outcomes at 12 and 48 months were analysed using the same strategy and at the same time points as described for the primary outcome, with the exception that accelerometer wear values were removed from non-accelerometer-derived outcomes.
Analysis of binary secondary outcomes
The odds of compliance with moderate to vigorous physical activity recommendations at 12 and 48 months were analysed using logistic regression, adjusted for the randomisation stratification variables (centre, ethnicity and sex) and compliance with moderate to vigorous physical activity recommendations at baseline as covariates.
The odds of diabetes at 12 and 48 months were analysed using logistic regression, adjusted for randomisation stratification variables (centre, ethnicity and sex). Those who were diagnosed with diabetes but had a HbA1c value subsequently recorded in the non-diabetes range were still classified as having diabetes.
Potential mediators of behaviour change and self-reported use of behaviour change strategies
To avoid multiple testing with measures that were not study outcomes, data on illness perceptions, self-efficacy and self-reported use of behaviour change strategies are reported descriptively, but were not subject to statistical testing.
Statistical significance and reporting of data
As the primary outcome involved two comparisons with the control (Walking Away and Walking Away Plus), the significance level was adjusted accordingly to account for this multiple testing. Therefore, statistical significance was considered at a p-value of < 0.025, rather than the tradition p-value of < 0.05. The results of the statistical analyses are, therefore, reported as means (97.5% CIs) to be consistent with the lower significance level.
Health economics
Two separate health economics analyses were carried out to assess the cost-effectiveness of the trial interventions. The primary analysis was a model-based analysis using the School for Public Health Research Diabetes Prevention Model (henceforth ‘the model’),70 which extrapolated trial outcomes over a lifetime horizon. The secondary analysis was an evaluation of the within-trial costs and outcomes, which assessed the costs and benefits of the interventions for the 4-year follow-up period of the trial. Both analyses took an NHS and Personal Social Services perspective, in line with current NICE guidelines. Costs for both analyses were valued in 2017/18 Great British pounds. Unit costs were obtained from nationally representative sources, such as NHS reference costs. 71 Any cost sources used from previous years were inflated to 2017/18 prices using the hospital and community services index and/or the new health services index. 72 The detailed methods of these analyses are described in Chapter 6 of this report and are summarised briefly below.
The cost of the interventions
Two different sets of intervention costs were calculated. One was the expected costs of the interventions as they would be implemented in the real world. The intention in using the costs of interventions as we expect them to be implemented is to correct for budgetary inefficiencies that are an artefact of the trial process and would not be expected to occur in a real-world setting. To estimate these, we combined data collected in the study, data from other similar educational interventions and expert opinion. These costs were used as our base-case costs in both analyses.
The other type of intervention was the cost of interventions in the PROPELS trial, regardless of whether or not these costs reflect realistic costs, if Walking Away or Walking Away Plus were to be implemented. These costs will be presented as a sensitivity analysis.
Model-based analysis
The School for Public Health Research model was used to simulate the long-term incidence of T2D, complications of T2D and other related conditions, such as cardiovascular disease, cancer and depression. The model is well validated for use in diabetes prevention interventions73 and was adapted to the specific requirements of the PROPELS trial in three ways:
-
The rates of progression to T2D in the first 4 years of the model were based on a statistical model derived from data collected in the trial.
-
Daily step count was added as a new population characteristic to incorporate the primary outcome variable of the trial.
-
The existing cardiovascular risk function was adapted to incorporate the independent effect of ambulatory activity on cardiovascular risk, as reported in the NAVIGATOR trial. 20
The model population was simulated based on an analysis of the baseline characteristics of the trial participants. Additional characteristics required for the model that were not collected in the trial were imputed using Health Survey for England data. 74 Owing to over recruitment of South Asian participants in the trial compared with the general population of the UK, analyses were performed separately for South Asian and non-South Asian populations. These results were then aggregated proportionally to the ethnic structure of the UK to provide an estimate of cost-effectiveness that was representative of the UK population as a whole.
The primary model outcomes were aggregated lifetime costs and lifetime QALYs, both of which were discounted at a rate of 3.5% per annum in line with NICE recommendations. 75 Relevant clinical outcomes, including the number of diabetes diagnoses, incidence of diabetes complications and incidence of related conditions (including cardiovascular events), are also reported.
Cost-effectiveness was reported as an ICER and net monetary benefit assuming a willingness-to-pay threshold of £20,000 per QALY, as recommended by NICE. 75 Uncertainty around these results was explored through probabilistic sensitivity analysis (PSA) and value-of-information techniques. We also conducted scenario analyses, as outlined in Chapter 6.
Within-trial analysis
The within-trial analysis was conducted in line with Ramsey et al. ’s76 2015 recommendations for cost-effectiveness analysis alongside clinical trials. Resource use was calculated using data collected as part of the trial, and the cost of this was valued using standard unit sources, including, but not limited to, the British National Formulary77 and NHS Reference Costs. 78 The outcome measure was QALYs. Utility values were calculated by mapping the EQ-5D-5L data collected in the trial to the EQ-5D-3L scores, as recommended by NICE. 75 The resulting scores were valued using the standard EQ-5D-3L tariff for the UK population. 79 The total QALYs were estimated using an area-under-the-curve method. The costs and QALYs accrued after the first year were discounted at 3.5% in line with NICE guidance. 75 As for the model-based evaluation, the results are presented as an ICER and net monetary benefit, assuming a willingness-to-pay threshold of £20,000 per QALY. 75
Research governance
The study was conducted in accordance with the Research Governance Framework for Health and Social Care. The sponsor institution responsible for verifying research governance arrangements was the University of Leicester.
Trial Steering Committee
The trial was overseen by an independent Trial Steering Committee (TSC), which was responsible for the overall management and oversight of the trial. The TSC was made up of:
-
Simon Heller, Professor of Clinical Diabetes, University of Sheffield (chairperson)
-
Richard Morris, Professor in Medical Statistics, University of Bristol
-
Des Johnston, Professor of Clinical Endocrinology, Imperial College London.
The TSC met approximately every 6 months, normally within 2 months of a meeting of the Data Monitoring and Ethics Committee (DMEC).
Data Monitoring and Ethics Committee
A fully independent DMEC reported to the TSC. Although it was highly unlikely that the non-pharmaceutical, non-invasive interventions proposed would result in any substantial negative effects on trial participants, the DMEC held responsibility for the interests of participant safety and data integrity and reviewed all reported adverse events. It also assessed data at 12 months, with predefined rules for stopping the trial for futility if specific criteria were met. These criteria were based on whether or not there was evidence that the intervention was causing harm, defined as a decrease in the primary outcome (ambulatory activity) in the intervention arms compared with the control arm, based on the 99% CI and 95% CI. If a decrease was seen based on the 99% CI in both arms, then the trial would be terminated. If a decrease was seen based on the 95% CI, then secondary outcomes would be considered in making a recommendation for termination. These criteria were not met.
In addition, the DMEC reviewed and signed off the statistical analysis plan. The DMEC comprised Graham Hitman, Professor of Molecular Medicine and Diabetes, Queen Mary, University of London (chairperson); Naveed Sattar, Professor of Cardiovascular and Medical Sciences, University of Glasgow; and Michael Campbell, Emeritus Professor of Medical Statistics, University of Sheffield.
Data integrity
The study was reviewed by the NHS National Research Ethics Service Committee East Midlands – Leicester and the Comprehensive Local Research Network (ethics number 12/EM/0151). All data were entered (through secure web-based access) and held in a specifically designed database in the Leicester Clinical Trials Unit. The database was designed with internal validity and quality control checks; potential errors were highlighted to, and corrected by, the study team. Data were released to the study statistician at predefined time points.
Ethics approval
Ethics approval was granted by the NHS National Research Ethics Service, East Midlands – Leicester Committee, which co-ordinates ethics permissions across the following study and recruitment sites:
-
University Hospitals of Leicester NHS Trust
-
Leicester City Clinical Commissioning Group
-
West Leicestershire Clinical Commissioning Group
-
East Leicestershire and Rutland Clinical Commissioning Group
-
University of Cambridge
-
MRC Epidemiology Unit
-
Cambridge and Peterborough Clinical Commissioning Group
-
Cambridge University Hospitals NHS Foundation Trust.
Amendments
The PROPELS protocol was subject to 14 amendments, none of which changed the main aims or objectives of the trial; for a list, see Appendix 3.
Trial registration
The trial was registered with ISRCTN as ISRCTN83465245: The PRomotion Of Physical activity through structured Education with differing Levels of ongoing Support for those with pre-diabetes (PROPELS) (https://doi.org/10.1186/ISRCTN83465245).
Chapter 3 Intervention description and development
This chapter details the content of Walking Away and the development work that informed the text messaging and telephone (mHealth) support element used in the Walking Away Plus study arm. The intervention description was developed in accordance with the principles of the Template for Intervention Description and Replication (TIDieR) checklist.
Walking Away: group-based behaviour change intervention with annual refresher sessions
Walking Away is an established programme that was developed within the infrastructure of NIHR CLAHRC (Collaboration for Leadership in Applied Health Research and Care) East Midlands for implementation in primary care; it has been described in detail elsewhere. 80 Walking Away went on to be implemented nationally through commissioned diabetes prevention services. Walking Away formed the core intervention programme that was evaluated within PROPELS. Based on learning generated by the development and trial work supported by the CLAHRC, minor revisions were made to Walking Away so that it could be used within PROPELS. In particular, the way that risk was communicated was broadened to emphasise that the risk of diabetes increases as the number of related risk factors increases to extend the focus beyond glycaemia.
Walking Away is underpinned by a theoretical framework focusing on linking motivational and volitional determinants of health behaviour. It draws on mutually complementary health behaviour theories and behaviour change techniques, including Bandura’s social cognitive theory,81 Gollwitzer’s implementation intentions,82 Leventhal’s common sense model83 and Chaiken’s dual process theory,84 and is modelled on the person-centred philosophy and learning techniques developed for the DESMOND programme. 24 DESMOND is a self-management programme for people living with T2D that is commissioned and delivered both nationally and internationally.
Walking Away: the initial education session
Walking Away was delivered by two trained educators to groups of up 10 participants, with participants invited to bring a family member or a guest if they wished. Sessions were delivered in a variety of settings chosen for proximity to the recruiting GP surgeries, including at the surgeries themselves, in nearby community centres and at hospital sites.
The curriculum for the initial education session, examples of activities and the underlying theories and behaviour change techniques are presented in Table 3. Walking Away was aimed at increasing participants’ knowledge of diabetes risk, changing their outcome expectations about how physical activity can lead to decreased diabetes risk, and strengthening their self-efficacy for engaging in increased physical activity. The programme was designed to harness key behaviour change techniques related to self-regulation, including goal-setting, action-planning, self-monitoring and barrier identification/problem-solving to ensure that motivation for behaviour change translated into actual behaviour change. Self-monitoring was supported through the provision of a pedometer [Yamax SW200 (Yamax, Shropshire, UK)]. Participants used their daily habitual step count (measured at baseline prior to the education programme) to set personalised steps-per-day activity goals.
| Module | Main aims | Example activity | Theoretical underpinning | Behaviour change techniques employed (mapped from Michie et al.85) | Time weighting |
|---|---|---|---|---|---|
| Introduction | Welcome/housekeeping | 5 minutes | |||
| Patient story | Give participants a chance to share their knowledge and perceptions of being identified as ‘at risk’ of T2D and highlight any concerns that they may want the programme to address | Participants are asked to share their story, how they were diagnosed as being ‘at risk’ of developing T2D and their current knowledge of being ‘at risk’ | Common sense model83 | Provide normative information about others’ behaviour | 25 minutes |
| Professional story | Use simple non-technical language, analogies, visual aids and open questions to provide participants with:
|
Individuals are helped to plot their individual risk (fasting and 2-hour blood glucose levels, cholesterol and blood pressure levels – assessed at baseline) |
Common sense model83 Dual process theory Social cognitive theory81 |
Provide information on consequences of behaviour | 35 minutes |
| Risk story |
|
Participants explored the broader risk factors for T2D beyond glucose values, including generating a list of non-modifiable (e.g. family history and age) and modifiable risk factors (e.g. overweight, blood pressure, cholesterol levels). Participants were then supported to plot their own risk factors onto a risk chart to work out their individual risk areas |
Social cognitive theory81 Dual process theory84 |
Provide information on consequences of behaviour | 25 minutes |
| Break | Refreshments and informal discussion | 10 minutes | |||
| Physical activity | Use simple non-technical language, analogies, visual aids and open questions to help participants:
|
|
Social cognitive theory81 Implementation intentions81 Dual process theory84 |
|
55 minutes |
| Diet | Increase participants’ knowledge about diet and give them an accurate understanding of the link between dietary macro-nutrients and metabolic dysfunction | Participants are asked to group models of fats and oils into saturated, polyunsaturated and monounsaturated categories |
Social cognitive theory81 Dual process theory |
Provide information on consequences of behaviour | 20 minutes |
| Conclusion | Questions and future care | Signpost to locally available groups/programmes | 5 minutes |
Participants were encouraged to increase their activity levels by up to 3000 steps per day, equivalent to around 30 minutes of walking. Goal attainment was encouraged through the behaviour change technique of setting graded tasks, whereby the use of proximal objectives, such as increasing ambulatory activity by 500 steps per day every 2 weeks, is used to work up to overall goals. Participants were encouraged to make an action plan detailing where, when and how they would reach their first proximal goal, to repeat action-planning for each new proximal goal, to wear their pedometer on a daily basis and to self-monitor their ambulatory activity using a specifically designed steps-per-day diary. Although this was primarily a physical activity intervention, a short time was allocated to covering the key dietary messages because participant groups had requested this during the intervention development process for Walking Away.
Walking Away: the annual refresher sessions
As for the original Walking Away RCT, after the initial education session, participants were offered annual group-based maintenance sessions at 12, 24 and 36 months (‘refresher sessions’). Refresher sessions each lasted 2.5 hours and were designed to revisit the key messages of the initial session, strengthen self-efficacy through sharing successes and prompt problem-solving in relation to barriers, goal-setting and self-monitoring using pedometers. The annual nature of the group sessions was designed to fit with primary care pathways, in which annual clinical follow-up is recommended for those with a high risk of chronic disease, such as prediabetes. 43,86
Walking Away Plus: with enhanced mHealth follow-on support
Participants assigned to the Walking Away Plus study arm were invited to attend the Walking Away initial education session and annual refresher sessions, as described above. In addition, they were provided with a mHealth follow-on support intervention in the form of tailored text messaging and telephone support.
Text messaging system
Text messages were used to prompt pedometer use and provide tailored feedback on meeting steps-per-day goals. The frequency of texts received varied over time to coincide with the initial and annual refresher face-to-face group sessions (Table 4).
| Time point from education attendance | Type of contact and frequency | Content (behaviour change techniques and their delivery) |
|---|---|---|
| 0 months | Initial group session (3 hours) |
|
| 1 week | First telephone call from educator (15 minutes) | The educator prompted the participant to set an action plan and personal short- and long-term goals informed by the baseline steps, and asked the participant about their confidence in achieving goals and their previous levels of physical activity. Educator recorded this information on an online form, which was saved to a database for use in tailoring subsequent text messages |
| 0–2 months | Text message contact (1–3 text messages per week) |
|
| 2–6 months | Text message contact (one per week) |
|
| 6 months | Telephone contact (15 minutes) |
|
| 7–12 months | Text message contact (once per month) |
|
| Optional | Telephone contact (15 minutes) |
|
| 12 months | Walking Away refresher session (2.5 hours) | See Walking Away study arm |
The text messaging system was hosted on a secure University of Cambridge server. The program consists of two parts:
-
a set of tables in a MySQL database
-
a set of PHP (hypertext preprocessor) scripts.
The MySQL tables contain data from participants, which were obtained in two ways:
-
data elicited by educators during telephone calls (see Telephone support)
-
data extracted from text messages participants sent to the system, such as the number of steps participants reported having taken and a STOP message sent by a participant indicating that they did not wish to receive any further text messages.
A messages table contained the bank of messages that were developed for the PROPELS trial. Schedule tables specified the days and time slots when text messages should be sent. Matrix tables specified which variables should be used to individually tailor each message.
Messages were tailored in two ways:
-
Different participants received different messages depending on the values of the relevant tailoring variables.
-
Tags were embedded in some messages enabling variable values to be inserted dynamically (e.g. #nname# to insert the participant’s nickname).
Other tables stored the educators’ identification numbers, messages sent by participants to the system and messages sent by the system to participants. Messages sent to participants included query messages, for example asking them to text in their weekly step count.
Participants registered with the system by sending a text that included the keyword ‘PROPELS’ and their nickname to a specified number. The system was fully automated except for the educator interface, which allowed manual data entry. The main PHP (hypertext preprocessor) script was set to run every 15 minutes from 06.05. The first time that it ran each day it set up the text messages to be sent that day. Then, every 15 minutes it checked whether or not the time for sending the messages had been exceeded. If it had, the messages were sent.
A message was ‘sent’ by sending a HTTP (HyperText Transfer Protocol) request to a company called FastSMS, which relayed the message over the mobile telephone network. Other scripts sent birthday and New Year messages, processed incoming texts (relayed from FastSMS) and queried the database to produce reports, for example of messages sent that day.
Telephone support
Telephone support from trained educators was used to support and tailor the text messaging system. An educator telephoned the participant approximately 1 week after the Walking Away session to confirm short- and long-term step goals and an action plan for the next 6 months, and to elicit information needed to enable the text messaging system to be tailored to variables such as confidence in increasing physical activity, previous experience of physical activity and potential mobility issues that prevented walking from being the primary activity. The collected information was captured in an online form and saved to a database for use in the text messaging programme. Participants also continued to receive two telephone calls annually to review their progress.
Structure and intensity of mHealth follow-on support
The structure and intensity of the mHealth intervention used in the PROPELS trial is detailed in Table 4.
This annual structure was repeated each year following each group education refresher session for the 4 years that constituted the intervention period.
The mHealth intervention content and the structure used were developed and piloted within the PROPELS programme of work. This development work, along with examples of the content used, is detailed below.
Development of the mHealth follow-on support intervention: a pragmatic framework for developing and piloting a text messaging intervention
The robust development of mHealth behaviour change interventions can be time-consuming, and yet in RCT protocols87,88 this is often allocated limited time. The time constraints of the PROPELS RCT protocol provided for 12 months to conceptualise, develop and test the follow-on support programme prior to the commencement of the RCT. Our methods offer a pragmatic framework for developing and piloting a text messaging intervention – drawing on relevant behaviour change theory and using rigorous qualitative methods incorporating user engagement – that lends itself well to replication and application to the development of similar interventions.
The structured, iterative process that we used to develop the PROPELS follow-on support programme involved undertaking concurrent and sequential research with the target population while maintaining a strong focus on the integration of theory and evidence. The methods that we employed combined features of published mHealth development frameworks89,90 and multiple iterative phases of qualitative research similar to a user-centered design process. 88 Our framework for intervention development and piloting was informed by the model by Dijkstra and De Vries89 for developing computer-generated tailored interventions (to conceptualise the programme) and the mHealth development and evaluation framework by Whittaker et al. 90 and Fjeldsoe et al. 91 The high level of engagement with our target population enabled us to refine the design to optimise its acceptability to users.
The four phases of the development process are described below.
Phase 1: conceptualisation
In line with Dijkstra and De Vries’89 model for developing computer-generated tailored interventions, our first step was a focused literature review to identify the key psychosocial determinants of increasing and/or maintaining physical activity levels among adults at risk of developing T2D; these determinants of physical activity were then translated into the key objectives of the PROPELS follow-on support programme.
Our literature review focused on text messaging interventions to promote physical activity, but also reviewed physical activity behaviour change interventions more broadly within our target population to identify salient behaviour change techniques. 92 The main findings from our focused literature review are summarised below.
Text messaging for physical activity promotion
Two comprehensive meta-analyses demonstrated a growing evidence base for text messaging interventions to promote health. In the first, which focused on physical activity promotion using mobile devices,93 most of the included interventions delivered through text messaging were passive, sending participants relay messages (e.g. goal intentions) or generic, non-tailored information about health benefits, and most of the participants were younger adults. However, one exception to this was a pilot study in older people with chronic obstructive pulmonary disease,94 which provided the control (self-monitoring) study arm with a pedometer and mobile telephone, prompted them to text in details about their symptoms and exercise, and responded with a standard message to thank them and encourage them to continue submitting data. Intervention (coaching) study arm participants received additional ongoing reinforcement coaching messages. Objectively measured step count increased in the self-monitoring group only. Although this intervention was feasible to deliver, delivery was not automated, as text responses were manually adjusted by a nurse, and scalability was limited by all participants being provided with a telephone. The second study of interest was a RCT in middle-aged healthy adults of a fully automated intervention, consisting of a wrist-worn device, an interactive website to provide feedback on physical activity and text messaging reminders of activity plans, which reported significant increases in objectively measured activity compared with no support. 95
The second meta-analysis investigated the efficacy of different formats of text messaging-based interventions for various health behaviours and outcomes and found that message tailoring and personalisation were significantly associated with greater intervention efficacy;96 interventions that involved decreasing frequency of messages over the course of the intervention were more effective than interventions that used a fixed message frequency;96 and text message-only physical activity interventions without tailored feedback did not increase physical activity. 97
This pointed to tailored feedback being a promising component of mHealth physical activity interventions and suggested that physical activity interventions using text messages may be more effective if they incorporate active components, such as self-monitoring, provide tailored feedback and personalised messages, and decrease the frequency of text messages over time.
Theory and behaviour change techniques
Looking beyond text messaging interventions, health behaviour change interventions that combine self-monitoring with at least one other self-regulatory behaviour change technique (e.g. goal-setting) have been found to be significantly more effective at increasing physical activity than those that do not. 98 These behaviour change techniques fit with the process of self-regulation or, more specifically, control theory,99 which proposes that setting goals, self-monitoring behaviour, receiving feedback and reviewing goals following feedback are central to behavioural self-management.
We, therefore, structured the PROPELS follow-on support programme around the use of behaviour change strategies, which informed the selection and sequencing of the primary behaviour change techniques that featured in the various components of the programme. 92 Accordingly, during the educator telephone call at week 1, physical activity goals and an action plan were established. The text messaging component drew on a range of behaviour change techniques to:
-
encourage self-monitoring of physical activity behaviour
-
provide tailored feedback regarding physical activity progress to highlight the discrepancy between goals and current behaviour
-
review behavioural goals.
A more detailed explanation of all of the behaviour change techniques employed in the PROPELS follow-on support programme has already been reported. 100
In interventions among people with or at risk of T2D, those that included a larger number of behaviour change techniques,101 or a larger number of behaviour change techniques and specific behaviour change techniques such as goal-setting,102 have been associated with greater weight loss. There is also consistent evidence demonstrating the importance across general populations and in high-risk groups of several other key determinants of physical activity behaviour change, including attitudes toward physical activity,103 intrinsic motivation104 and (maintenance) self-efficacy,105 especially when targeted in conjunction with the use of behaviour change techniques. 106 With this in mind, the text message component of the PROPELS follow-on support programme also targeted these other determinants of physical activity behaviour change. Given the uncertainty that remains about the acceptability of the aforementioned behaviour change techniques when delivered by text message, one aim of phases 2–4 was to explore the acceptability and feasibility of this approach with our target population.
Phase 2: formative research
Concurrently with phase 1, informal observations were conducted of Walking Away sessions across the diverse regions in which it had been commissioned into routine care pathways for the prevention of T2D, and discussions were held with Walking Away educators who were involved in an ongoing evaluation of Walking Away taking place in primary care. 80 The aim of this was to ensure familiarisation with the delivery of Walking Away, develop initial ideas about the possible structure and content of the PROPELS follow-on support, understand the cultural and ethnic diversity of our target population, explore educators’ views about supplementing Walking Away with text messaging and pedometer support, and inform the development of topic guides for subsequent focus groups.
Next, we formed three formative focus groups with our target population, the participants of which had all attended the Walking Away session within the previous 3 years as part of an ongoing evaluation in primary care,80 had provided consent to be contacted with regard to other research within the department, and could speak and understand spoken English. Potential participants were sent an information leaflet and an opt-in reply slip. A researcher telephoned those who expressed an interest in participating to confirm their willingness and arrange their attendance at a focus group. Written informed consent was obtained immediately before the focus groups; a total of 15 participants (five women and 10 men) aged between 39 and 76 years took part. A flexible topic guide was used that covered experiences of Walking Away (e.g. what was most and least helpful for increasing physical activity and what could be improved to facilitate sustained changes), the use of mobile telephones in everyday life and the integration of a text messaging follow-on support programme into Walking Away.
The focus groups were audio-recorded and transcribed verbatim. Our analytical approach was based on the constant comparative method,107 which involved organising the data into meaningful groups and identifying elements of interest in the data that formed the basis of repeated patterns (themes) across the data set. An initial coding framework was developed and used to code the complete data set. NVivo11 qualitative data indexing software (QSR International, Warrington, UK) was used to facilitate the analysis.
As described by Morton et al. ,100 the key findings from phase 2 that influenced intervention development and subsequent phases fell into two interlinked themes: the acceptability of text messaging for physical activity promotion and the requirements for the structure of the follow-on programme, which includes text message content.
Acceptability of text messaging for physical activity promotion
Most participants reported that they used mobile telephones in daily life and were able and willing to use text messages, even if they did not habitually use these as their primary means of communication. Most participants agreed that text messages could serve as a useful reminder to form habits and provide additional support following an education session. Participants reported that the freedom to choose when to read a message and whether or not to act on the information it contained was a positive feature:
I think if texting had been in it [Walking Away trial] before it would have helped my motivation a lot.
FG3
. . . whereas texting is ideal. You can carry on with your normal day-to-day living but still get the motivation.
FG1-A
The opportunity to receive immediate feedback was also perceived as a benefit, with many participants thinking that the two-way interaction of reporting weekly step counts and receiving subsequent feedback would facilitate motivation and maintenance and foster a sense of accountability (i.e. having someone to report to):
It would be good knowing that we’d put the figures in at the end of the week, that you have received them and that you’ve looked at them and that you’re interested in what we’re doing.
FG2-A
Views were not unanimously positive, however; some felt simply that texting was ‘not for [their] generation’, whereas a small number reported finding text messages intrusive and/or impersonal:
No, I wouldn’t [want to receive text messages], I would find that intrusive. It’s bad enough ‘have you been mis-sold PPI,’ ‘have you done this’ . . . so you don’t even look at your text messages. If it’s not from family I block the lot so no, I wouldn’t want text messages.
FG1-C
Considerations for the mHealth follow-on support intervention
In reflecting on their experiences of Walking Away, focus group participants generally reported that they found the pedometer to be a useful monitoring tool that promoted their awareness of their activity levels. Although some participants reported still using their pedometer to monitor their physical activity 2–3 years after Walking Away, the majority reported that their engagement with the pedometer or activity diary had waned following an initial period of active engagement:
You get up at half six in the morning, you think I’ll go and get a wash and you get changed, and then you go off to work and, ‘Oh, I didn’t put it on’ . . . you start to forget about it.
FG2-C
Once I’ve got home I think, oh, I don’t think I’ll do any more, I sit on the computer or watch the telly, I need someone to push me out, get out the chair and go and do a walk.
FG3-C
The importance of feedback for facilitating behaviour change and maintenance was emphasised, with participants commonly reflecting that a lack of contact between one annual refresher session and the next had decreased their motivation to continue with the strategies discussed in the session (e.g. setting goals and wearing a pedometer). Several participants remarked that feedback on their goal-setting and progress would have been beneficial:
It would have been nice to have the results of that [physical activity measures] because we never knew about that.
FG1-D
Individuals differed greatly in their preferences about the content of the text messages; participants who described themselves as self-motivated and the sporty type (i.e. those who reported having been fairly active in the past) tended to want a different kind of message from those who self-described as sedentary and needing more of a push. Participants who reported significant mobility issues, such as osteoarthritis, found content that was focused solely on walking to be irrelevant to them. These differences led to the idea of developing follow-on support content tailored to individual characteristics that would be appeal more directly to participants.
Participants felt strongly that text messaging should supplement face-to-face contact, not replace it, especially in relation to strengthening motivation. Some suggested that telephone support, in addition to text messages, could serve to foster rapport between PROPELS educators and PROPELS participants, and provide additional support that could not be communicated over text message, and help to overcome the perception of text messages being impersonal:
But, [if] you’ve got somebody there you can speak to . . . say, ‘right I’m having a problem, I’ve done such and such and I can’t register me steps’ or whatever, it’s just about [the educator] saying ‘right, you should do this’ or ‘I’ll get somebody to ring you back and tell you what to do,’ you can’t do that on text can you?
FG1-E
Viewed in combination, the phase 2 findings indicated a need for:
-
two-way interaction (i.e. submission of step counts and provision of immediate feedback on physical activity progress)
-
timely reminders to self-monitor physical activity
-
further consideration of how perceived barriers to the use of text messaging can be overcome (i.e. presenting participants with an overview of the benefits of text messaging for follow-on support as part of the initial Walking Away session)
-
tailored and personalised text message content
-
telephone support designed to enhance rapport between educators and participants and provide additional support over and above text messages only (i.e. problem-solving and in-depth social support).
Phase 3: pretesting
Based on the findings of phases 1 and 2, we created model text messages and conducted four further focus groups with participants (n = 20; age range 52–77 years) to test these. Eligibility criteria for these were the same as in phase 2, but we also invited participants from the control arm of the Walking Away study who had not previously attended the programme;80 the recruitment and consent procedures were identical to those described for phase 2. Before attending a pretesting focus group, participants were sent a pedometer and activity diary by post and were encouraged to record their number of steps per day for 1 week. Participants were asked to bring along a mobile telephone when attending the focus group.
A topic guide covered participants’ experiences of Walking Away, as in phase 2, and also explored experiences of wearing the pedometer and recording steps. During the focus group, participants were sent example text messages (Figure 1) to provoke reactions in situ and generate think-aloud87 reactions and discussions about different message types. Data were analysed using the approach described in phase 2, with the phase 2 coding framework developed further to reflect the phase of development.
FIGURE 1.
Example text messages. Reproduced with permission from Morton et al. 100 This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR mhealth and uhealth, is properly cited. The complete bibliographic information, a link to the original publication on http://mhealth.jmir.org/, as well as this copyright and license information must be included. The figure includes minor additions and formatting changes to the original figure.
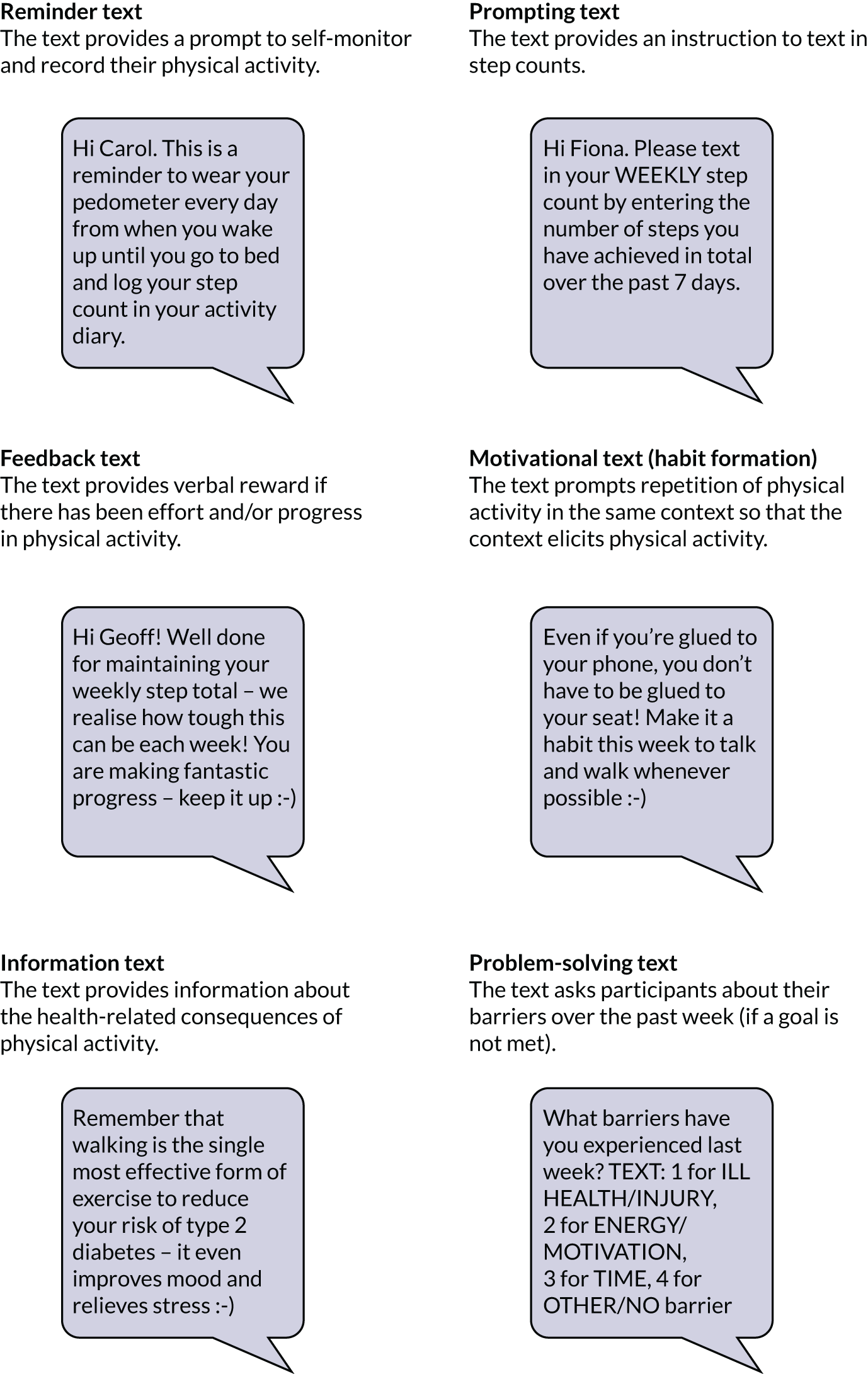
During the pretesting focus groups, participants were sent a range of text messages that were developed as a result of phases 1 and 2, which included:
-
reminder texts – reminding participants to wear the pedometer and log daily steps and instructions for texting in step counts
-
feedback texts – giving feedback on behaviour, including social reward and positive reinforcement
-
motivational texts – messages using behaviour change techniques to strengthen motivation for physical activity (e.g. habit formation, commitment, reframing physical activity beliefs)
-
information texts – information about health consequences
-
problem-solving texts – contained response options to a list of predefined barriers when a goal was not met (see Figure 1).
Depending on the response option that they chose, participants were sent a tailored motivational or information text.
The key themes emerging from the phase 3 pretesting focus groups that informed the final content of the PROPELS follow-on support programme are described in the following sections.
Self-monitoring of physical activity
Most participants reported that self-monitoring their daily steps with the pedometer had increased their awareness of their own activity and, therefore, had increased their motivation to be more active:
I found the pedometer really, really useful. I didn’t wear it all the time, but once I wear it I make sure I do 10,000 steps. If I looked at it half-way through the day and think I’ve only done 5000 then I went out for a walk purposely just to get the figures up.
FG4
Some participants reported finding the pedometer demotivating or disheartening, especially those with mobility problems, who felt that their step count was always low because they could not engage in walking as their primary activity:
I wish that it was not just dependent on the steps. Because we do all sorts of other things rather than just steps.
FG7
For such individuals, the self-monitoring process should allow for other activities to be counted (e.g. swimming and gardening).
Text message type, language and frequency
It was clear from participants’ feedback that establishing the appropriate frequency of reminder texts was critical to avoiding the intervention becoming off-putting and perceptions that it was ‘Big Brother’-like or ‘checking up on you’, especially once wearing the pedometer had become a habit. This suggested that reminders should become less frequent as the intervention progresses:
If you’ve got something constantly . . . well, not constantly, but weekly reminding you to do something then you’re still there doing it. And possibly if you’re doing it for several weeks then you’ll get actually used to wearing it and putting it on. It’s like putting your clothes on. You put your socks on, put your pants on, ‘oh I’ll put my thing [pedometer] on’. It’s all getting used to what you’re doing, like with your lifestyle.
FG5
A text message to prompt people to input their weekly step count was generally accepted to be a useful motivational tool:
I suppose the very fact that we would be doing it [texting in step counts] we are creating a certain level of discipline which we didn’t have before.
FG4
The model feedback messages sent when a step goal was achieved (i.e. positive reinforcement) were well received and helped to foster a sense of accountability:
We are all school kids in a sense, in our heads, so if someone says you did well it’s really encouraging.
FG5
We tested a variety of feedback messages to be used in the event that participants did not achieve their step goal. The consensus was that these should be fairly light-hearted, positive and encouraging. Messages emphasising discrepancy between the person’s current behaviour and their goal were well received, provided that the texts also offered encouragement and support by, for example, including positive elements along with more negative feedback:
. . . you’ve got to put in, you know, the positive that eliminates some of the negativity out of the messages. So this one was ‘thanks for the text, keep wearing your monitor and logging your steps, try to increase your activity to ensure’ . . . it’s not quite positive enough.
FG4
Several participants remarked that humour could be useful in providing feedback when a step goal was not achieved:
You can’t castigate somebody but you can try and get some laugh out of it from some point of view, saying ‘get off your bottom and go for a walk!’.
FG6
However, they also recognised that messages could be received differently and that the use of humour was risky, especially when participants’ confidence was low:
. . . if I read that and I was in the wrong mood I’d take that as you’re telling me what to do, and I’d say ‘b*****r off’.
FG4
The feedback on motivational messages was far from uniform. Overall, participants indicated that the gentle language and suggested content of the motivational messages rendered them acceptable, avoiding ‘being told you’ve got to do it’. Some of the model messages were perceived as dated (e.g. recommendations to avoid using a remote control to change the TV channel) or irrelevant (e.g. tips about using stairs at home: ‘. . . but I live in a bungalow!’). Participants tended to prefer practical tips and suggestions for increasing activity over more motivational suggestions (e.g. ‘try writing down your barriers to activity this week’). In one focus group, participants suggested using generally supportive messages not necessarily linked to physical activity or health:
I know why I’m doing it [to reduce the chances of T2D] so we don’t need reminding of it all the time.
FG7
Participants were in agreement that messages were too focused on the health consequences of inactivity and that a focus on benefits other than weight and reduced risk of T2D would be preferable:
You could just say ‘good morning, this is PROPELS, hope you have a nice day’ or whatever . . . just simple – it doesn’t need to really say anything.
FG6
. . . when you’ve got a weight problem like I’ve got, I don’t need to be reminded – I’m doing my best!
FG4
The predefined response format was viewed by some as unsuitable for problem-solving:
It’s like one of those PPI messages [spam text messages about reclaiming mis-sold insurance] — I hate those!
FG4
However, others valued the idea of being able to easily text in the reason why they had not achieved their goal. Participants generally liked the tailored and personalised texts that were triggered by their responses to the problem-solving texts (e.g. in response to selecting the illness or injury response option, participants would receive the message ‘Take it easy this week. We hope that you feel better soon’).
The concept of receiving individually tailored text messages was very popular, in particular in relation to goal progress and/or achievement:
You should get the one [text message] that’s relevant to you. If you’re doing more [steps], if you’re achieving your target or doing more, you still get one, but it should be different.
FG4
Participants observed that different people need different support, especially in terms of confidence and self-discipline in adhering to an activity plan. They suggested that people who are struggling to meet their goal and/or have mobility problems limiting the amount of walking that they could achieve should receive messages that are less direct or less pushy.
The general feedback was that the language used in texts needed to be formal, friendly and polite, with use of the participant’s name but limited use of emoticons:
I’m just warning you that it might be interpreted that you are shouting at us because in text language, capitals [letters] is shouting.
FG5
It makes it sound as though you’re talking at us, rather than a computer.
FG6
Participants felt that less is more when it comes to the frequency of messages. Overall, they perceived the approach of sending daily messages to be too heavy-handed and potentially demotivating:
. . . otherwise if you are going to get this [text message] daily you’re going ‘oh another one’ and you get fed up with it.
FG6
Phase 3 findings
-
Further emphasised the importance of personalising and tailoring messages according to key variables (e.g. previous levels of physical activity, mobility issues that limit physical activity, individuals’ confidence in increasing physical activity, goal achievement/progress).
-
Shaped the content of the messages (i.e. what type of benefits to focus on in the motivational messages).
-
Informed the frequency of messaging and sequencing of the follow-on support programme.
-
Highlighted the importance of including other activities (e.g. cycling and swimming) to maintain engagement of participants who did activities other than walking.
Summary of the changes made as a result of phases 2 and 3
We made two changes as a consequence of our findings from phases 2 and 3:
-
We incorporated a week 1 educator telephone call comprising a brief telephone-administered assessment, including key information required to tailor subsequent text messages.
-
We added a conversion chart to the activity diary to enable participants to convert other activities (e.g. for those who did not wear their pedometer or who perceived that a pedometer did not accurately assess) into steps for texting in. The chart included, for example, descriptions of other activities (e.g. swimming breaststroke, moderate effort and cycling 10 mph) and provided a conversion into a step count based on MET equivalents108 that could be added to the participant’s total activity.
Phase 4: piloting
In the final phase of development, we piloted a full set of text messages and tailoring matrices during the initial 8 weeks of the follow-on support programme. During this phase, we were able to obtain and act on participant feedback on the content and structure of the programme and on technical issues.
Drawing on the findings of phases 1–3, our research team drafted an initial set of text messages and tailoring matrices for each week of the programme; the tailoring matrices specified the individual characteristics to which each message would be adapted. A computer program was developed to automatically generate and send text messages in line with the tailoring matrices and to handle incoming messages. We then tested the content and schedule of the text messaging and pedometer programme and the processes required for its delivery (e.g. registering with the text message system, gathering information for tailoring, and receiving and replying to the messages). We also aimed to identify and resolve potential technical issues with the automated system.
The participants were 11 people (five women and six men) from the phase 2 and 3 focus groups who had indicated an interest, including participants who were less keen on the use of text messages. This 8-week pilot study mimicked the initial 8 weeks of the proposed PROPELS follow-on support programme. An instruction booklet with details on how to register and what to expect from the text messaging system was posted to participants, along with a pedometer and an activity diary. Participants were asked to wear the pedometer and self-monitor their steps using the activity diary for 1 week to determine a baseline number of steps that would inform their step goals for the next 8 weeks. A member of our research team administered the brief telephone assessment and elicited each participant’s short- and long-term step goals, their action plan for increasing physical activity and the information needed for the tailoring variables. Participants then received a weekly reminder message that prompted them to text in their weekly step count, which triggered an automated feedback message in which the content was tailored depending on goal progress. Tailored motivational messages were also sent if participants did not progress in their step counts or neglected to text in a step count.
At the end of the 8-week period, brief, semistructured telephone interviews were conducted with all available participants (n = 10) to obtain their feedback on the programme; the interviews were recorded, transcribed and analysed as in phases 2 and 3. The results are summarised in the following sections.
Programme content and structure
Most participants reported that the follow-on support had increased their awareness of their own activity, which had motivated them to be physically active. Participants found the telephone call in which the brief assessment was administered helpful in providing additional support, especially for overcoming any technical barriers:
But, you’ve got somebody there you can speak to then say, ‘right I’m having a problem, I’ve done such and such and I can’t register me steps’ or whatever, it’s just saying, ‘right, you should do this or I’ll get somebody to ring you back and tell you what to do’, you can’t do that on text can you?
R5
Participants reported finding that the system provided continued support and encouragement, and found the reminder texts to be helpful prompts to continue self-monitoring; continued goal-setting and immediate feedback provided further motivation to be active:
It’s quite nice. It keeps me sort of in the zone in the fact that I enjoy using the pedometer because it keeps my mind on exercise. I’m conscious of it, and, you know, if I haven’t done too much moving about, I go and walk some more.
R5
I usually do remember to put me pedometer on . . . but as I say it’s nice to know there’s a reminder there and when I send off my figures I get an immediate response. I think it’s all been quite encouraging actually.
R4
Participants reported finding the frequency of messages (maximum of two per week) adequate for the 8-week period, but felt that the messages could decrease in frequency over time as reminders would be needed less often:
As I say I think at the beginning you need more frequent reminders, you know I think you’ve got that right, and then as it goes on you don’t need so many.
R6
Participants were generally positive about the content, readability and clarity of the text messages and struggled to recall examples of discouraging messages. Several participants identified the feedback texts and motivational texts, which provided instructions (tips) for increasing physical activity, as particularly useful:
Do you know I’ve even started . . . this is what you have got me doing . . . when I’m on the kitchen chair, making a cup of coffee or something, I start running on the spot for 100! I count up to 100, running on the spot. So that’s another 100 steps!
R10
Participants who did not consistently increase their step counts reported receiving slightly more negative messages, but had not perceived any as chastising:
I found that very encouraging. It was good. When I’d done a good week, it’s very . . . I only missed one week, and although you didn’t down me, you didn’t say anything nasty, you just said try a little harder, I know it’s hard to get the exercise in, so I found it very encouraging.
R1
Technical issues
Of the 11 participants, nine received the full regimen of text messages as intended; minor technical glitches impeded the full delivery to the other two participants. Most participants had no difficulty registering for the text system and > 90% of all incoming messages from the participants were correctly formatted. Almost all participants responded to at least two prompting texts and received tailored feedback at least twice; three-quarters responded to all prompting texts and, therefore, received tailored feedback texts every week.
Several participants were unclear as to which messages they could respond to: some sent thank-you messages in response to the feedback texts and then received a text message about unrecognised format:
I was just replying to your request or your advice, when I didn’t do the correct steps one week, you gave me a couple of bits of helpful advice and I text back thanking you for that, and obviously it wouldn’t let me send.
R7
We also identified a need for a greater degree of flexibility in the format used for submitting step counts. Although participants were asked to text the word ‘steps’ followed by their weekly step total, some submitted only numbers, or the word ‘step’ or ‘step-count for week’, which triggered an unrecognised response text.
Finally, participants with limited experience in texting reported receiving and reading texts without problem, but utilising help from relatives (usually grandchildren) when prompted to text in their weekly step counts:
Oh, yes, I could [read all the messages] . . . it’s just getting them sent off. Because again I think this week I was late, I thought I’d sent them in twice and then I had to check with [granddaughter], and I think I had pressed some other button. I think I’ve got a handle on it now. It sounds stupid but they didn’t have all these phones back then.
R2
In summary, the piloting phase showed that (1) the structure of the follow-on support (including the brief telephone call) was acceptable; (2) the frequency of text messages over the 8-week pilot phase was acceptable but should be reduced over time; (3) the content and language used in the text messages were acceptable; (4) minor technical issues needed to be resolved; and (5) the participant instructions in both the Walking Away session and the follow-on support booklet would benefit from refinement.
See Appendix 17 for the final array of text messages used in the Walking Away Plus intervention.
Educator recruitment, training and intervention fidelity
Educators were either registered health-care professionals (e.g. nurse or dietitian) or appropriately qualified non-registered professionals (e.g. health trainer). Across both study sites, 12 educators were recruited from local health-care providers and other appropriate settings. This mix of professional backgrounds was chosen to ensure that the study was easily generalisable in routine health-care settings. All educators attended an initial 2-day training course to ensure that they understood the theories and philosophy underpinning the Walking Away programme, as well as the content and resources used. All educators were given a written curriculum to support their delivery of the programme and were given the opportunity to practise delivering the programme.
Educators received further training before delivering their first Walking Away refresher session, which was also supported by a written curriculum. In addition, specific training was provided to the individuals who delivered the telephone calls to participants in the Walking Away Plus study arm. The training was supported by an extensive curriculum that outlined the content (including behaviour change techniques and patient-centred communication skills) and included standardised scripts to guide the individual making the telephone calls, and standardised reflection sheets and checklists to promote and assess fidelity of the telephone calls (see Report Supplementary Material 4).
To enhance and assess intervention fidelity, a detailed procedure was developed by the PROPELS team (see Report Supplementary Material 3).
Quality assurance of delivery was undertaken using established tools developed to assess educational style and content used in routine care through the DESMOND collaborative. 109 Educators received structured and constructive feedback from their assessor, and key goals and action plans were developed to help them to improve their performance. Educators were asked to audio-record the telephone calls, listen back to a sample, complete the checklists and discuss these with the intervention lead. The number of calls assessed and the frequency of assessment depended on the competence level of the individual making the call, as well as the year in which the intervention was delivered. A peer feedback mechanism was also incorporated. The delivery of the text messaging system was monitored weekly by examining automatically generated lists of messages sent and received.
Chapter 4 Results
Participant recruitment commenced in December 2013 and was completed in February 2015, with data collection completed in July 2019. Overall, 12,417 individuals from 47 general practices were identified as potentially eligible to take part and were sent invitation letters, with a further 746 being identified and invited from previous research databases. Of these, 1563 individuals consented to take part and were screened for inclusion. Eighty were subsequently withdrawn because they were diagnosed with T2D at baseline and a further 117 were excluded because of ineligibility following the baseline visit, or otherwise withdrew from the trial before randomisation, leaving 1366 randomised and, therefore, included in this analysis. The overall flow of participants is highlighted in Figure 2. For the flow stratified by each site (Leicester and Cambridge), see Appendix 2, Figures 20 and 21.
FIGURE 2.
Participant flow.
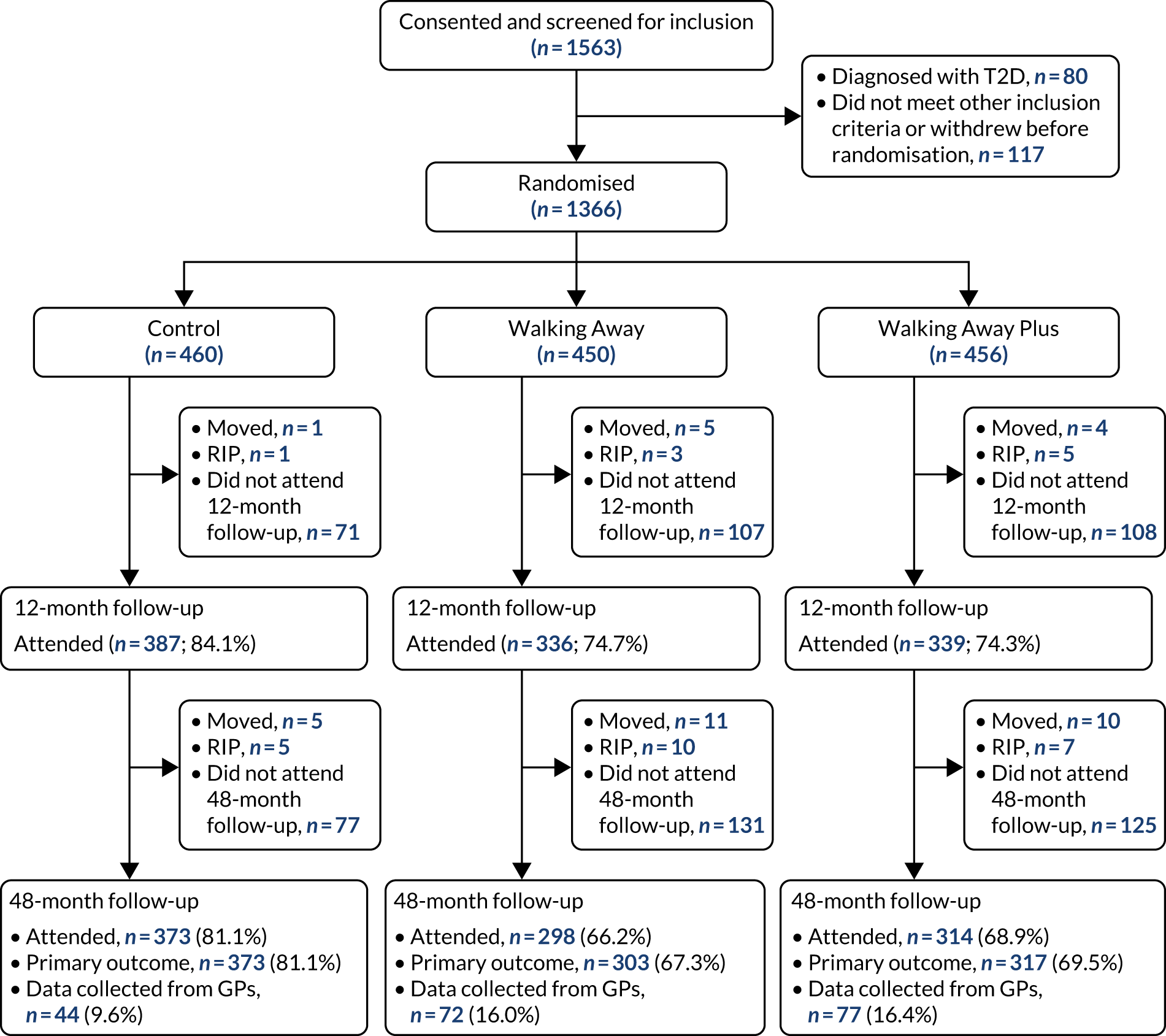
The sociodemographic and clinical characteristics of those included in the trial, stratified by randomised study arm, are presented in Table 5. Study arms were well matched. The median age ranged from 60 to 61 years across the arms.
| Participant characteristic | Study arm | ||
|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |
| Continuous data | |||
| Age (years), median (SD) | 59.4 (8.8) | 59.4 (9.4) | 59.3 (9.1) |
| BMI (kg/m2), median (IQR) | 28.5 (25.3–32.4) | 28.2 (25.3–32.5) | 28.4 (25.2–32.3) |
| Social deprivation (IMD decile), median (SD) | 5.5 (2.8) | 5.7 (3.0) | 5.7 (2.8) |
| Categorical data, % (n) | |||
| Sex | |||
| Men | 50.9 (234) | 50.4 (227) | 50.9 (232) |
| Women | 49.1 (226) | 49.6 (223) | 49.1 (224) |
| Ethnicity | |||
| White European | 71.1 (327) | 72.4 (326) | 72.1 (329) |
| South Asian | 22.4 (103) | 22.0 (99) | 22.6 (103) |
| Other | 6.5 (30) | 5.6 (25) | 5.3 (24) |
| Social deprivation (IMD national quintile) | |||
| 1 (least deprived) | 19.4 (89) | 20.7 (93) | 17.3 (79) |
| 2 | 18.7 (86) | 18.0 (81) | 18.4 (84) |
| 3 | 20.5 (94) | 17.8 (80) | 20.8 (95) |
| 4 | 22.0 (101) | 18.5 (83) | 22.1 (101) |
| 5 (most derived) | 19.4 (89) | 24.9 (112) | 21.3 (97) |
| Family history of diabetes in first-degree relatives | 43.3 (198) | 42.0 (188) | 45.3 (205) |
| Prediabetes | 37.6 (172) | 39.9 (179) | 38.7 (176) |
| Antihypertensive medication | 40.9 (169) | 44.6 (164) | 44.7 (170) |
| Lipid-lowering medication | 34.9 (144) | 37.2 (137) | 39.6 (150) |
| Steroids | 7.4 (34) | 9.1 (41) | 6.4 (29) |
| Metformin | 0.0 (0) | 0.2 (1) | 0.2 (1) |
| CVD (MI, heart failure, angina and stroke) | 8.6 (39) | 9.0 (40) | 9.9 (45) |
| Smoking status | |||
| Past | 38.3 (176) | 36.2 (163) | 38.2 (174) |
| Current | 9.8 (45) | 8.4 (38) | 11.4 (52) |
| Employment type | |||
| Full time | 37.6 (173) | 34.2 (154) | 37.1 (169) |
| Part time | 16.1 (74) | 20.4 (92) | 18.9 (86) |
| Retired | 35.0 (161) | 35.3 (159) | 33.6 (153) |
| Unemployed or other | 11.3 (52) | 10.0 (45) | 10.5 (48) |
| Educational status | |||
| Degree, higher degree or equivalent | 45.7 (205) | 45.5 (197) | 44.9 (202) |
| Marital status | |||
| Married/civil partner | 68.3 (314) | 75.6 (340) | 73.9 (337) |
| Access to the internet | 83.0 (380) | 86.2 (387) | 85.3 (388) |
| Meeting the physical activity recommendationsa | 53.7 (238) | 56.1 (245) | 57.3 (254) |
| Meeting the physical activity recommendations in 10-minute boutsa | 21.9 (97) | 25.9 (113) | 24.6 (109) |
In total, 993 (72.7%) individuals had valid primary outcome data at the 48-month follow-up and were, therefore, included in the primary analysis. Generally, those with complete data had similar characteristics to those with missing data, including baseline prediabetes status, although those with missing data in the intervention study arms were more likely to be smokers, less likely to be university educated and less likely to have access to the internet. For the specific values for the characteristics of those with and those without complete primary data, stratified by intervention study arm, see Appendix 4, Tables 29–31.
Intervention engagement for each intervention study arm is shown in Table 6. Approximately 80% attended the initial education programme in both arms and over two-thirds attended at least one annual group-based follow-on session. The majority also engaged with the key elements of the telephone and text messaging intervention in the Walking Away Plus study arm. On average, participants were sent a mean of 266 (SD 75) text messages (approximately five per month) (see Appendix 17 for the array of text messages used).
| Intervention arm, % (n) | ||
|---|---|---|
| Walking Away (N = 450) | Walking Away Plus (N = 456) | |
| Programme attendance | ||
| Attended initial education session | 79.3 (357) | 80.9 (369) |
| Attended 12-month refresher session | 57.3 (258) | 60.3 (275) |
| Attended 24-month refresher session | 49.6 (223) | 55.5 (253) |
| Attended 36-month refresher session | 48.9 (220) | 50.4 (230) |
| Attended at least one follow-up annual support session | 67.6 (304) | 69.7 (318) |
| Telephone call and text messaging intervention | ||
| Registered with text service | 77.6 (354) | |
| Received initial telephone call | 69.1 (315) | |
| Received at least one telephone call during the trial | 85.1 (388) | |
| Asked for text messaging service to be stopped | 18.9 (67) | |
Primary outcome
Over the 48 months of the trial, participants in all study arms experienced small reductions in ambulatory activity, with no difference between either of the intervention study arms and the control arm (see Figure 4). However, at 12 months, participants in the Walking Away Plus study arm had increased their total ambulatory activity by 547 (97.5% CI 211 to 882) steps per day compared with those in the control study arm (see Figure 4).
The results for total ambulatory activity were consistent with those for censored ambulatory activity, in which an increase in the Walking Away Plus study arm compared with the control arm of 531 (97.5% CI 201 to 86) steps per day was observed at 12 months. This indicates that the increase in ambulatory activity at 12 months was primarily because of purposeful movement.
When the 278 participants (62%) in the Walking Away and the 235 (52%) participants in the Walking Away Plus study arms who met the pre-protocol definition were analysed, or when missing data were replaced with multiple imputation (Table 7), the results for total ambulatory activity at 48 months – the primary end point – were not affected.
| Variable | Intervention effect 1 (Walking Away vs. control), difference (97.5% CI) | Intervention effect 2 (Walking Away Plus vs. control), difference (97.5% CI) |
|---|---|---|
| Per protocol | 30 (–359 to 419) | 427 (–63 to 916) |
| Missing data replaced by multiple imputation | 69 (–314 to 453) | 139 (–259 to 538) |
The average levels of total ambulatory activity (primary outcome) and censored ambulatory activity at each assessment time point in each study arm are shown in Table 8. The change in ambulatory activity in intervention study arms compared with the control arm at follow-up is shown in Figure 3.
| Variable | Study arm, n; mean (SD) | Intervention effect 1 (Walking Away vs. control), mean difference (97.5% CI)a | Intervention effect 2 (Walking Away Plus vs. control), mean difference (97.5% CI)a | ||
|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||
| Total ambulatory activity (steps/day) | |||||
| Baseline | 441; 6885 (3068) | 427; 7264 (3009) | 435; 7353 (3432) | ||
| 12 months | 374; 6710 (3145) | 319; 7400 (3078) | 324; 7723 (3419) | 264 (–70 to 597) | 547 (211 to 882) |
| 48 months | 373; 6534 (3168) | 303; 7038 (3101) | 317; 7235 (3728) | 91 (–282 to 463) | 121 (–290 to 532) |
| Censored ambulatory activity (steps/day) | |||||
| Baseline | 441; 5369 (2984) | 427; 5643 (2891) | 435; 5764 (3300) | ||
| 12 months | 374; 5195 (3022) | 319; 5761 (2973) | 324; 6104 (3347) | 240 (–90 to 570) | 531 (201 to 861) |
| 48 months | 373; 5062 (3015) | 303; 5459 (3003) | 317; 56 (3615) | 66 (–302 to 433) | 140 (–263 to 542) |
FIGURE 3.
Change in ambulatory activity in intervention study arms compared with control at follow-up. Data adjusted for wear time at baseline, wear time at 48 months, number of valid days at baseline, number of valid days at 48 months, randomisation stratification, variables (centre, ethnicity, sex) and baseline value.
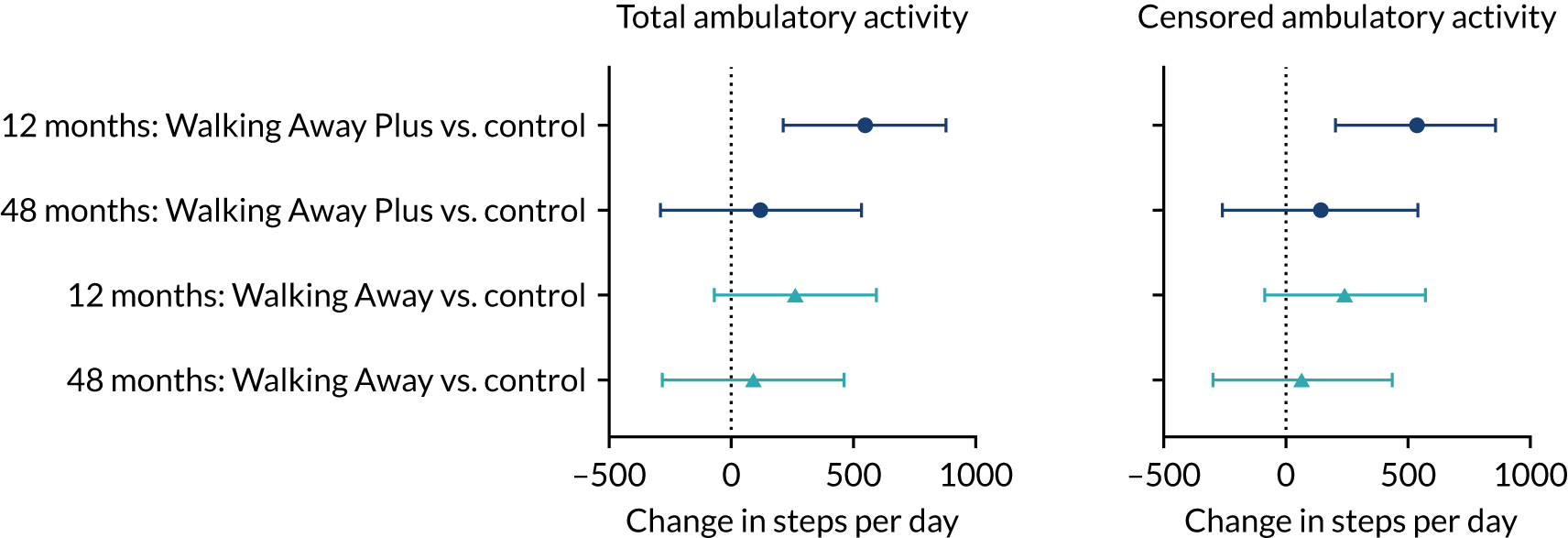
The results for the primary outcome were not modified by sex, age, ethnicity, family history of diabetes, prediabetes at baseline or obesity status, suggesting that the results for the primary outcome were consistent across these categories, including ethnicity (see Appendix 7, Table 36). However, there was evidence that the primary outcome was modified by social deprivation (p = 0.035 for interaction). In the Walking Away Plus study arm, compared with the control study arm, those above the median level of social deprivation increased their ambulatory activity (480 steps/day, 97.5% CI –73 to 1033 steps/day), whereas those below the median level had a decrease in activity level at 48 months (–370 steps/day, 97.5% CI –945 to 205 steps/day).
Physical activity and sedentary behaviour
The other objective measures of physical activity and posture are presented in Tables 9 and 10, respectively. Time in moderate to vigorous physical activity increased by 3.5 (97.5% CI 0.6 to 6.5) minutes per day and time spent walking increased by 8.5 (97.5% CI 3.3 to 13.7) minutes per day in the Walking Away Plus study arm compared with the control study arm at 12 months, but the differences were not sustained at 48 months. The self-reported measures of physical activity are presented in Appendix 8, Table 37. There was an increase in total physical activity energy expenditure in the Walking Away Plus study arm compared with the control arm of 4.4 kJ/kg/day (97.5% CI 0.0 to 8.8 kJ/kg/day) at 48 months; no other differences were detected.
| Variable | Study arm, n; mean (SD) | Intervention effect 1 (Walking Away vs. control), difference (97.5% CI)a | Intervention effect 2 (Walking Away vs. control), difference (97.5% CI)a | ||
|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||
| Time spent sedentary (minutes/day) | |||||
| Baseline | 441.0; 557.0 (92.9) | 427.0; 544.0 (91.3) | 435.0; 544.5 (97.2) | ||
| 12 months | 374.0; 562.4 (93.1) | 319.0; 550.6 (83.8) | 324.0; 545.8 (98.1) | –1.9 (–11.1 to 7.2) | –7.7 (–16.9 to 1.5) |
| 48 months | 373.0; 560.0 (86.9) | 303.0; 558.9 (87.3) | 317.0; 562.9 (101.9) | 0.1 (–10.2 to 10.4) | 4.7 (–5.7 to 15.1) |
| Time spent in light physical activity (minutes/day) | |||||
| Baseline | 441.0; 293.3 (80.7) | 427.0; 310.9 (85.7) | 435.0; 309.0 (88.9) | ||
| 12 months | 374.0; 286.7 (81.5) | 319.0; 300.2 (74.5) | 324.0; 304.0 (84.2) | 0.9 (–7.4 to 9.3) | 4.4 (–4.1 to 13.0) |
| 48 months | 373.0; 278.4 (82.7) | 303.0; 293.0 (83.7) | 317.0; 292.4 (82.4) | –0.1 (–9.8 to 9.6) | –5.7 (–15.3 to 4.0) |
| Time spent in moderate to vigorous physical activity (minutes/day) | |||||
| Baseline | 441.0; 29.8 (24.7) | 427.0; 31.4 (25.7) | 435.0; 32.1 (27.6) | ||
| 12 months | 374.0; 28.8 (25.6) | 319.0; 31.7 (24.3) | 324.0; 33.9 (28.2) | 1.3 (–1.7 to 4.3) | 3.5 (0.6 to 6.5) |
| 48 months | 373.0; 27.5 (23.7) | 303.0; 30.0 (24.9) | 317.0; 31.2 (29.6) | 0.5 (–2.8 to 3.7) | 1.6 (–1.9 to 5.0) |
| Variable | Study arm, n; mean (SD) | Intervention effect 1 (Walking Away vs. control), difference (97.5% CI)a | Intervention effect 2 (Walking Away vs. control), difference (97.5% CI)a | ||
|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||
| Time spent sitting or lying down (minutes/day) | |||||
| Baseline | 337.0; 549.3 (111.6) | 333.0; 535.7 (113.3) | 323.0; 545.6 (115.3) | ||
| 12 months | 314.0; 546.5 (107.4) | 279.0; 543.5 (112.6) | 289.0; 536.1 (108.6) | 4.3 (–10.2 to 18.9) | –8.4 (–22.9 to 6.0) |
| 48 months | 260.0; 559.1 (112.6) | 213.0; 538.8 (103.8) | 211.0; 538.0 (118.2) | –15.0 (–33.8 to 3.8) | –10.6 (–29.9 to 8.7) |
| Time spent standing (minutes/day) | |||||
| Baseline | 337.0; 288.3 (95.1) | 333.0; 306.7 (95.4) | 323.0; 294.5 (100.7) | ||
| 12 months | 314.0; 289.4 (97.0) | 279.0; 294.6 (88.3) | 289.0; 291.9 (95.3) | –6.2 (–18.5 to 6.1) | 0.3 (–11.8 to 12.5) |
| 48 months | 260.0; 281.6 (102.1) | 213.0; 300.7 (83.8) | 211.0; 292.5 (101.2) | 12.7 (–2.7 to 28.1) | 5.9 (–9.9 to 21.6) |
| Time spent walking (minutes/day) | |||||
| Baseline | 337.0; 106.0 (38.1) | 333.0; 115.3 (38.5) | 323.0; 111.5 (43.4) | ||
| 12 months | 314.0; 105.3 (38.9) | 279.0; 115.0 (39.3) | 289.0; 117.8 (41.6) | 2.4 (–2.8 to 7.6) | 8.5 (3.3 to 13.7) |
| 48 months | 260.0; 104.1 (42.1) | 213.0; 111.9 (38.2) | 211.0; 114.1 (44.2) | 2.2 (–4.3 to 8.6) | 4.8 (–2.5 to 12.0) |
At 12 months, the proportions of participants meeting the physical activity guidelines of 150 minutes per week of objectively measured physical activity of at least moderate intensity in the control, Walking Away and Walking Away Plus study arms were 50.1% (n = 190), 59.5% (n = 194) and 60.2% (n = 205), respectively. The odds ratio of meeting the physical activity guidelines was 1.61 (97.5% CI 1.05 to 2.45) higher in the Walking Away Plus study arm than in the control arm at 12 months (Figure 4), with the results maintained when considering 150 minutes accumulated in at least 10-minute bouts (see Figure 4). However, no differences were observed at 48 months.
FIGURE 4.
Odds ratio of meeting the physical activity guidelines of 150 minutes per week of at least moderate intensity in the intervention study arms compared with control at follow-up. Data are mean (97.5% CI). Adjusted for randomisation stratification variables (centre, ethnicity and sex) and baseline value.
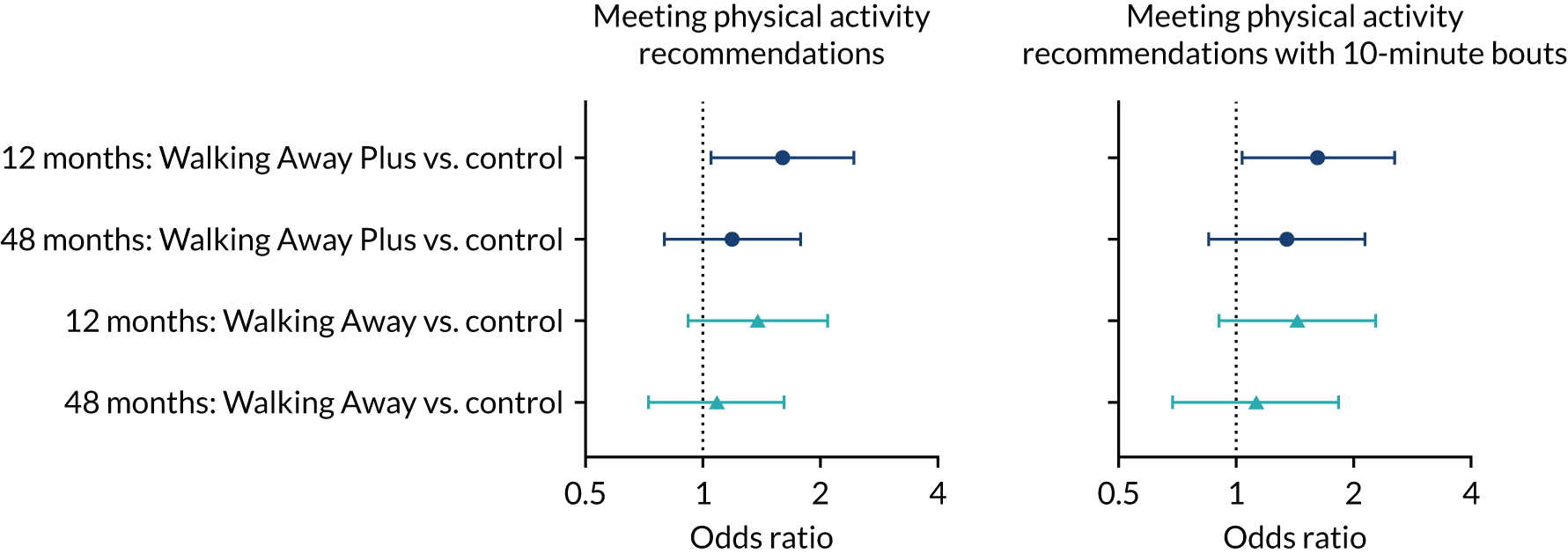
Other secondary outcomes
The anthropometric outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 38–47. There was a reduction in body mass of 0.6 kg (97.5% CI 0.03 to 1.18 kg), a reduction in waist circumference of 1.23 cm (97.5% CI 0.38 to 2.18 cm) and a reduction in body fat percentage of 0.50% (97.5% CI 0.03% to 0.98%) in the Walking Away study arm compared with the control study arm at 12 months. These effects were sustained at 48 months, with a reduction in body mass of 1.00 kg (97.5% CI 0.07 to 1.92 kg), a reduction in waist circumference of 1.57 cm (97.5% CI 0.45 to 2.70 cm) and a reduction in body fat percentage of 1.06% (97.5% CI 0.33% to 1.79%) observed in the Walking Away study arm compared with the control arm. The results for weight and waist circumference are displayed in Figure 5. There were no other changes to assessed outcomes at 12 or 48 months in the Walking Away study arm or the Walking Away Plus arm.
FIGURE 5.
Change in ambulatory activity in intervention arms compared with control at follow-up. Data are mean (97.5% CI). Adjusted for randomisation stratification variables (centre, ethnicity, sex) and baseline value.
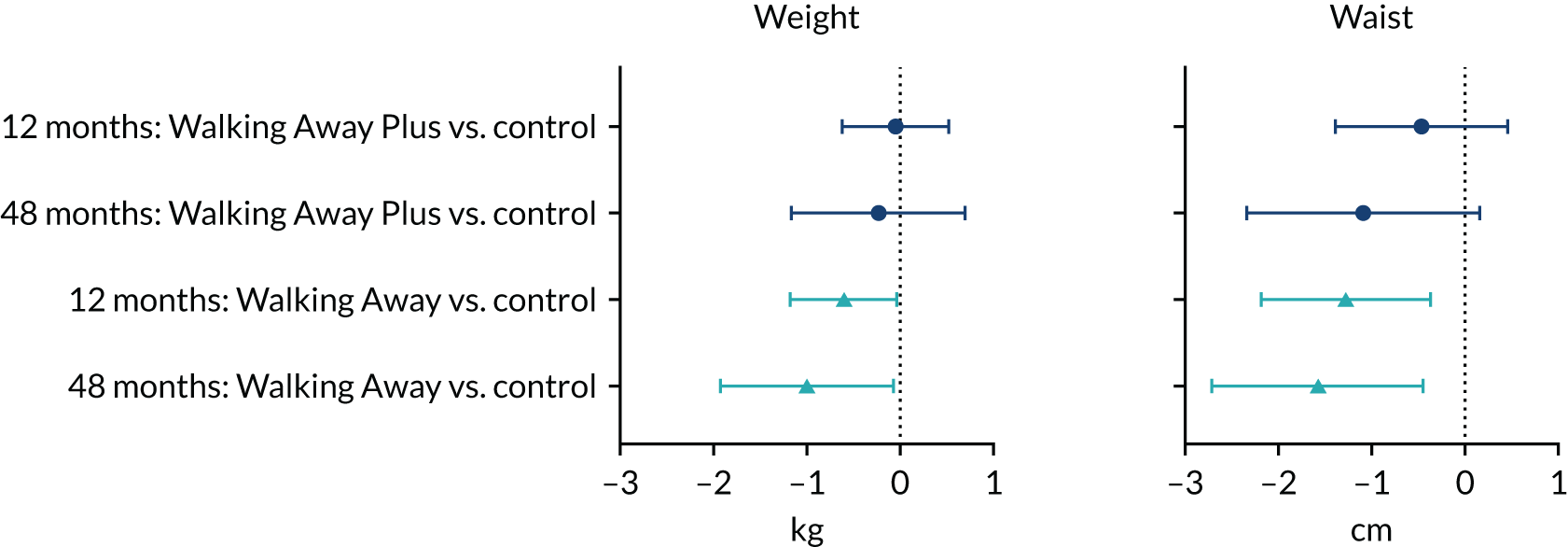
The biochemical outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 40 and 42. Triglycerides were reduced by –0.15 mmol/l (97.5% CI –0.29 to –0.01 mmol/l) in the Walking Away Plus study arm compared with the control arm at 12 months, with effects sustained at 48 months (–0.11 mmol/l, 97.5% CI –0.21 to –0.00 mmol/l). Liver enzymes alanine aminotransferase (ALT) and alkaline phosphatase (ALP) followed the pattern of weight loss in the Walking Away study arm, with reductions of 1.79 (97.5% CI 0.07 to 3.51) IU/l and 3.70 (97.5% CI 0.96 to 6.45) IU/l, respectively, compared with the control study arm observed at 48 months. There were no other changes to the assessed biochemical outcomes at 12 or 48 months.
During the trial, 39 (9.3%) individuals in the control study arm, 30 (7.8%) individuals in the Walking Away arm and 41 (10.4%) individuals in the Walking Away plus arm developed T2D. There was no difference in the odds of developing diabetes in either intervention arm compared with the control arm.
The dietary and sleep outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 44 and 46. At 12 months, both intervention arms reported an increase in fresh fruit consumption of 0.21 (97.5% CI 0.07 to 0.35) and 0.17 (97.5% CI 0.04 to 0.31) portions per week in the Walking Away and Walking Away Plus arm, respectively, compared with control. In addition, the Walking Away Plus arm reported an increase in green leafy vegetable consumption of 0.14 (97.5% CI 0.00 to 0.29) portions per week and a reduction in cheese intake of 0.16 (97.5% CI 0.1 to 0.31) portions per week. At 48 months, participants in both intervention arms also reported an increase in green leafy vegetables of 0.24 (97.5% CI 0.07 to 0.41) and 0.24 (97.5% CI 0.08 to 0.41) along with an increase in other vegetables of 0.20 (97.5% CI 0.05 to 0.35) and 0.20 (97.5% CI 0.06 to 0.35) in the Walking Away and Walking Away Plus arms, respectively, compared with the control arm. In addition, the Walking Away arm maintained their increased fruit consumption at 48 months, reporting 0.22 (97.5% CI 0.05 to 0.40) more portions per week. At 48 months, changes to fruit and vegetable consumption were matched by dietary restraint, where those in the Walking Away and Walking Away plus arms reported actively trying to limit the amount of total fat in their diet on 0.32 (97.5% CI 0.09 to 0.55) and 0.32 (97.5% CI 0.09 to 0.55) more days per week and saturated fat by 0.41 (97.5% CI 0.18 to 0.65) and 0.37 (97.5% CI 0.12 to 0.61) more days per week, respectively, than those in the control arm. No other differences in other dietary variables or sleep time were observed.
The quality-of-life, depression and anxiety outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Table 46. There was a small increase in the EQ-5D quality-of-life score of 0.02 (97.5% CI 0.00 to 0.04) units in the Walking Away arm at 12 months compared with the control arm; no other differences were observed at either time point.
See Appendix 5 for the self-efficacy and illness perception scores at baseline and follow-up in each study arm (see Tables 32–34). See Appendix 6, Table 35, for self-reported use of behaviour change strategies. Self-efficacy for walking was high in all study arms at all time points. At the 48-month follow-up, participants in the control study arm were 90% confident that they could walk for 60 minutes per day, compared with an average confidence rating of 100% in the Walking Away arm and 95% in the Walking Away Plus study arm. Illness perception scores indicated that those in Walking Away and Walking Away Plus arms increased their perceived understanding of their risk of diabetes at 12 months and 48 months following the intervention, whereas understanding remained stable in the control study arm. However, there was no consistent evidence that other key illness perceptions were systematically different between the control and the intervention study arms, including perception of the degree of agency (control) over diabetes risk or the degree to which treatment can be used to alter risk, which largely remained stable across time in all study arms.
However, the intervention study arms did differentially affect the use of behaviour change strategies, especially using pedometers over the course of the intervention. At 48 months, 64.2% of particiapnts in the Walking Away Plus arm and 49.7% in the Walking Away study arm reported using a pedometer at least some of the time, compared with 19.7% of participants in the control study arm, who had not received a pedometer as part of the study. Similarly, 40.9% and 30.6% in the Walking Away Plus and Walking Away study arms, respectively, reported keeping an exercise log at least some of the time, compared with 11.1% in the control study arm. Furthermore, 78.8% and 73.0% in the Walking Away Plus and Walking Away study arm, respectively, reported setting themselves exercise goals, compared with 64.0%% in the control study arm. Similar differences between study arms were also observed at 12 months. Full data are displayed in Appendix 6.
In the control study arm, there were seven (1.5%) serious and 47 (3.4%) non-serious adverse events. The equivalent values for Walking Away were 15 (3.3%) and 14 (3.11%), respectively, and for Walking Away Plus they were 28 (6.4%) and 16 (3.5%), respectively. Additional details and a breakdown of adverse reporting in each study arm are displayed in Appendix 15, Tables 71–73.
Chapter 5 Qualitative substudy: focus groups/interviews with educators and participants
Qualitative work was conducted to contribute to the evaluation of the intervention by observing education sessions and educator meetings around the 12-month time point, and by conducting focus groups with participants and educators at the end of the trial.
The aim was to provide in-depth qualitative data on how people engaged with the two levels of intervention over time, in particular how and why the more intense level of intervention helped (or did not help) participants to increase and sustain physical activity.
Observations
Sampling and recruitment
We purposively sampled five education sessions at the 12-month time point so that we could achieve a range in terms of educator, location and participant demographics. When booking participants into an education session that was scheduled to be observed, the booking administrator informed the participant about the observation; if a participant wanted to attend a session that was not being observed, they were offered a different session. On the day of the sessions, the researcher introduced themselves to the participants and explained the purpose of the observations; during the session, they observed from the back of the room to minimise distraction and interference.
Data capture
The researcher took anonymised handwritten field notes while not interfering with the education session. 111,112 The focus of the observation was engagement with the intervention components, including with the education session itself, and how the participants talked about their experience and levels of physical activity (including pedometer use, diary and/or text messaging, where appropriate) during the first 12 months. Key modules in the PROPELS curriculum were ‘the participants’ story’ and ‘physical activity’, which facilitated participants to talk about the goals that they had set, the challenges that they faced, the strategies that they used to overcome these and the challenges that they had not overcome. The field notes informed the topics to be explored in the qualitative interviews later.
Focus groups and interviews
Focus groups took place in Leicester Diabetes Centre, the MRC Epidemiology Unit in Cambridge and community centres. The researcher conducting the focus groups took written informed consent from participants immediately prior to the focus group.
Sampling and recruitment
Trial participants
For the first set of focus groups, we purposively sampled participants to achieve a range of participants in terms of intervention arm (groups 2 and 3), demographics and location. Further focus groups and telephone interviews were subsequently conducted to widen the sample to include experiences of participants not achieved in the first set (see below). Participants were sent an information leaflet about the focus group and interview substudy, with a reply slip to indicate willingness to be contacted to find out more. A member of the PROPELS team contacted participants who returned the reply slip to discuss the focus group study, confirm willingness to participate and, when willing, arrange their attendance at a focus group.
Educators
We invited all educators who were involved in delivering education sessions and telephone calls in the PROPELS study to participate in an end-of-trial focus group. Educators were sent the invitation and participant information sheet by e-mail, inviting them to indicate their interest by replying to the e-mail. A member of the PROPELS research team then contacted those who had replied to discuss the focus group and, if they were happy to proceed, arrange their attendance at a focus group.
Data capture
Flexible, semistructured topic guides informed the focus group schedule. With trial participants this explored their reactions to being invited to participate in PROPELS and attitudes towards being ‘at risk’ of T2D; experiences of the group educations sessions; experiences of forming and following an action plan; views about attendance and engagement over 4 years; and experiences of the telephone call and text messaging maintenance support. With educators, the topic guide explored their experience of delivering the education sessions and telephone calls. With all focus groups, when summing up discussions around the maintenance of physical activity, we referred to key theoretical explanations from Kwasnicka et al. 113 to prompt further discussions.
The researcher and assistant moderator conducting the focus groups debriefed immediately after each focus group for the purposes of preliminary analysis on both the data generated and the participant sample. This process informed the decision about when to stop data collection in terms of no new themes arising in relation to the specific research questions. A review of the data and sample from the first six focus groups indicated largely high levels of engagement with the intervention(s); thus, in the later focus groups, we actively aimed to recruit participants with lower engagement (e.g. those who had attended fewer education sessions and those who had requested to stop receiving the text messages). Furthermore, owing to the location of the focus groups, we had not reached participants in the more rural areas of the Cambridge site; therefore, we conducted telephone interviews with participants from this group.
Analysis
Focus groups were audio-recorded and transcribed verbatim. Analysis was informed by the Kwasnicka et al. 113 theoretical explanations and the constant comparative method:107 transcripts were read and re-read; five broad codes relating to the categories identified by Kwasnika et al. 113 were predefined; further open codes were generated from reading and re-reading; and all codes were subsequently refined and developed into a coding framework. Analysis was facilitated with the NVivo11 qualitative data-indexing package.
Findings
Final sample
Observations
Five 12-month education sessions were observed; these were in three different locations, delivered by 10 different educators (with two co-delivering each session) and included 40 participants with three accompanying relatives.
Focus groups and interviews
We conducted seven focus groups (n = 52) and six telephone interviews with trial participants (total trial participants, n = 58), and four focus groups and two individual interviews with educators (total educator participants, n = 16).
The key demographic and baseline characteristics of participants included in the focus groups and interviews compared with those of the overall PROPELS cohort are shown in Table 11. Those included tended to be less socially deprived, older, more likely to be white European, more likely to be male and more physically active than the general PROPELS population.
| Participant characteristic | Included in focus groups or interviews (N = 58) | Full PROPELS cohort (N = 1366) |
|---|---|---|
| Social deprivation (IMD decile), median (IQR) | 7 (5–8) | 6 (3–8) |
| Age (years), median (IQR) | 63 (56–68) | 61 (53–66) |
| Sex, n (%) | ||
| Female | 21 (36) | 673 (49) |
| Male | 37 (64) | 693 (51) |
| Ethnicity, n (%) | ||
| White European | 51 (88) | 982 (72) |
| South Asian | 6 (10) | 305 (22) |
| Other | 1 (2) | 79 (6) |
| BMI (kg/m2), median (IQR) | 28.0 (25.2–30.9) | 28.3 (25.4–32.4) |
| Ambulatory activity (steps/day), median (IQR) | 7283 (5629–10,318) | 6638 (4994–8817) |
In this chapter, we provide an overview of some of the prominent themes that emerged, with a focus on the focus group and interview data, concentrating on themes that provide some insight into the main trial results, namely the increase in physical activity levels at around 12 months (in the Walking Away Plus study arm) that was not sustained at 48 months.
Walking Away Plus
As described in Chapter 3, while both intervention study arms received a pedometer, those in the Walking Away Plus study arm also received maintenance support in the form of a telephone call from an educator (1 week after the education session), tailored text messaging and an activity diary to facilitate step-counting for reporting their weekly count by text message.
Participants from both intervention study arms spoke at length about pedometers and the ways in which these helped their awareness and activity, for example by helping them to learn first their average number of steps and how daily step-counts fluctuate, and then whether or not they met their daily goal of increased step count. Over and above this, many of those in Walking Away Plus arm were mindful of the need to send in their weekly step count, particularly early in the study:
You’re very aware, especially having the pedometers at the beginning of the study, and the monitors, you know, looking at the time and just going oh crikey, it’s 3 o’clock already and I haven’t done half of what I should be doing.
FG-P4
The activity diary was a prominent feature for many of these; a couple of participants even brought their activity diary to the focus group to demonstrate how they had maintained it for the whole study:
I have got mine; I have a record, all the [steps].
Did you do that daily?
Daily, daily, every day. I put it on my, near my bedroom, you know, chest drawer, and morning when I go, when I wear it, do zero, and then at night time I write it down, how many steps done.
FG-P2
Others made their own version of the diary when they had filled all of the pages of their first one, or produced their own version on a spreadsheet:
Focusing on action-planning and targets was really important for me – being a science-based person. In fact I created a spreadsheet, I went on a diabetic site in the States that listed all the activities and the calories that they would do. And from the PROPELS study you could see what they thought was calories relative to steps. And I linked all the activities that I was likely to do to steps and calories, and put it in the spreadsheet. So if I was doing cycling or walking or swimming or whatever, or housework or cleaning the car. I could relate that to number of steps, and I could log what I’d done during the day and come out with a steps equivalent at the end of the day, and then weekly, monthly, so on.
FG-P3
The majority of Walking Away Plus participants spoke positively about the text messages. A couple of participants had kept the messages on their telephone and read some out loud or mentioned particular messages during the focus group. In reporting how the text messages had helped, some participants explained that these served as reminders about being in the study in terms of maintaining physical activity, often acknowledging that this would help with the tendency to lapse:
I guess it was good because it reminded you in between the sessions that you were committed to a lifestyle change. [. . .] because of our inherent indolence we’re going to fall off the straight and narrow, so anything that can help bring us back and remind us of what we’re doing is probably a good thing.
The texting was really helpful, because you do sort of lapse. [. . .] if we’d gone off track, and then it was saying, ‘start texting your steps in’. [. . .] it kind of got you – certainly – back to focusing on it.
FG-P2
The text messages did not suit everyone, as evidenced by the 18.9% of participants who requested that messages stop. However, those in the focus groups who described some of the texts as annoying, amusing or irrelevant (e.g. regarding the tips for different types of activity) still appreciated their use:
And they were a sort of shotgun approach; that they shot at different bits of your lifestyle, I seem to remember. And the one that really sticks in my mind [was] that I had to do press-ups while I was cleaning my teeth!
FGP2
. . . so you could be like all day shifting bricks, then you get a text, ‘while you’re doing the hoovering, you could go this way and that way’, or ‘you could balance two tins’, and you’ve lost your temper [. . .] what do they think I’m doing, just sitting here watching TV?! I do find them a bit funny, [but] at the end of the day there’s people that probably little things like doing that, and walking to the shop rather than going in the car, is a big help to everybody. And I’m fully aware that even myself, when I went on this course, instead of just like driving now, I go to the shops and I make sure [I walk].
FG-P4
The telephone calls that Walking Away Plus participants received approximately 1 week after the education sessions appeared to be very welcome:
I mean a lot of the [text messages] were obviously just automated . . . and the responses to your steps – by my testing – were also automated and amusing. The phone calls were really interesting. Probably more encouraging in some ways than the text messages.
In particular, the telephone calls seemed to be more helpful – or additional help – for realistic goal-setting than the education sessions alone. By comparison, participants in the Walking Away study arm often mentioned struggling to think of an action plan in the session:
You can write all sorts of things down, but things happen, don’t they, you can’t . . . I don’t do action plans! I can’t.
So did you not set one in the [session]?
Well I think I probably wrote something down in the session, just to keep them happy.
FG-P5
Educators noted how many Walking Away participants commented how they wished that they were in the ‘texting group’, and this was still evident for some in the end-of-study focus groups:
I would have liked more contact myself, because it’s very easy to fall off. It could be I think. Because it’s a long time between, you actually hear from PROPELS again, if you’re on the second group. And I feel that quite a few people maybe could drop off in that time. I didn’t, fortunately. But I would love to have heard from PROPELS. Sometimes I thought maybe it had actually disappeared, or I’d dropped off the list or something.
FG-P2
Altogether, although Walking Away Plus, and the text messages in particular, did not suit the needs and preferences of all those in that study arm, the general feeling of being ‘kept on track’ and being monitored was well received, with many participants reporting that they missed receiving the messages after the trial ended.
Limited sustainability of physical activity levels
When participants (in both of the intervention arms) reflected on how their activity levels have changed (increased, decreased, fluctuated and so on) throughout the 48 months, a salient theme was the impact of a major health/illness event during the period, for example hernia or prostate operations, hip/knee replacements, musculoskeletal issues and other injuries from falls or accidents. Many spoke of associated disappointment, having increased their activity levels initially, and the difficulty of getting back to pre-incident activity levels. For example:
I had to have a hernia operation in the middle of what was the PROPELS. [. . .] I must be honest, the first 6 months after my hernia op, I couldn’t maintain what I did prior to it. But I’m back to where I was now if that makes sense. But that did come in the middle of it, yeah, you sort of drop and come back again.
Same with me. When I had my operation I was told not to do any cardiovascular for 6 months, because although it was healed up on the outside, it wasn’t quite healed up from the inside.
FG-P1
After I had my bicycle accident, I couldn’t actually get out of a chair, so I mean I really wasn’t able to do . . . I went from 28, 30 thousand steps to nothing, and there was nothing I could do about it.
FG-P5
Ageing and its associated physiological changes were referred to by many other participants when they were explaining a decrease in their activity levels, which often occurred after increased activity in the earlier years of the study:
And I can remember when we did the pedometer and first measure, I was doing 17,000 steps a day, which surprised them. But I had a dog and I gardened and did various things. But gradually over the course I got less active, and I would say I was less active now than I was when I started. Partly because my dog got old and decrepit. [Laughter] And I started getting old and decrepit, so I wasn’t doing as much gardening or walking for that reason.
FG-P5
Several participants mentioned ageing as a reason why they could no longer ‘do that extra bit’ required for increasing activity levels, preferring instead to plateau with their step count or activity goals. Reaching a plateau and being satisfied with an average step count was given as an explanation by several who had stopped monitoring and recording, and not just in the context of ageing:
I was just going to say, because we monitored ourselves for 4 years, and we did all these walks that people are doing, I can tell you, from my house to [the] park, 6.2 miles. So you don’t need to, you know exactly what you’ve walked.
So you don’t need to monitor because you know . . .
The distance.
Is that the same as what you do?
I know, the walking I do, about three-and-a-half thousand. You know, you can estimate it. I stopped after . . . I think [the pedometer] broke, I thought what do I want that on for?
FG-P1; participant C had taken part in Walking Away Plus and participant W had taken part in Walking Away
Other participants described the impact that work and other commitments had had on the fluctuation in their activity levels during the years of the study; one described the impact of this on a period in the middle of the study:
In the first year I did do a lot more exercise and watched what I eat. And then I had a dip, because I had 2 years [working away in a demanding environment]. And then it’s gone up a lot, because now I go running and I still do me steps, and when I get to 10,000 steps I don’t . . . I still carry on walking.
FG-P4
A couple of participants described how they had lost motivation after the study ended and, hence, had since reduced their activity levels:
I only did it for the study [. . .] Because I work shifts, I’ve got two young children, got a dog, I work nights, so I’m sleeping in the day, some days I did struggle to do it. So [during the study] I tried to compensate, get up half an hour early and go to the gym before I started work.
FG-P1
External influences on activity levels
Focus groups enabled comparison in situ of the experiences of participants from both Walking Away and Walking Away Plus; other than the additional support for those in the latter arm, experiences were typically similar. A number of prominent factors are worth noting for their potential influence on the activity of participants in both study arms.
The most noteworthy is the rise in popularity and ownership of smartphones and other devices for measuring and monitoring one’s physical activity from the time that the initial PROPELS education sessions were held in 2012–13 to when the post-trial focus groups were conducted with participants in 2019. At the outset, educators recalled the challenges of helping Walking Away Plus participants register with the text messaging, with many participants (and the educators themselves) being unfamiliar with the workings of their mobile telephone:
There’s that very first bit, where in the very first year when we had to keep the people behind to go through the telephone setting up with them.
Telephone set-up was an absolute nightmare.
Helping people set their mobile phone up, horrendous!
FG-E1
By comparison, in the end-of-study focus groups, the majority of participants were wearing Fitbits (Fitbit, San Francisco, CA, USA) or iPhone (Apple Inc., Cupertino, CA, USA) watches; many referred to using these or a smartphone for step-counting or similar, regardless of the intervention arm that they had been in or of their age. For example:
Yeah, one of the ladies in one of our sessions produced this Fitbit, and I thought, ‘ooh, I want one of those’ [. . .] I got one for Christmas and so I’m still wearing it now, and I really like it.
And do you measure . . . do you look at it every day?
Well it syncs with the computer every day, and then I look back and say, ‘ooh yes, that’s when I went to so and so’. You know, I find it really quite interesting to see. It sends you weekly reports.
FG-P5
It’s just a step counter on my iPhone. What it doesn’t show of course is that I’ve also done a 40-mile bike ride.
Does it not count, does it not count the bike ride?
Yeah, you can do that on something called Strava [Strava, San Francisco, CA, USA]. Strava monitors walking, running, bike riding, and every time I go on a bike ride . . . I put Strava on.
FG-P3
It appears that, during the course of the study, while Walking Away Plus participants appeared to increasingly use these newer technologies to support reporting their step count back to the PROPELS team via text, Walking Away participants were increasingly using these too. Hence, it is likely that participants in the control arm were also adopting such technology and, in turn, adapting their behaviour. Indeed, the rapid increase in self-monitoring technologies over the years when PROPELS was running should be noted as a contextual influence on participants in all three study arms.
Summary and discussion
To summarise, key themes from the focus groups provide insight into the main trial results. In terms of the 12-month increase in activity levels, the components of Walking Away Plus were described by participants as aiding physical activity increase. Pedometers and activity diaries facilitated the weekly step count to submit via text messages. Many found this self-monitoring useful and interesting, with some even taking this a step further by creating spreadsheets or other methods of long-term monitoring. Although some participants found the text messaging irritating or irrelevant, many appreciated the way that it served as a useful reminder and as a method for logging weekly step counts. When hearing about it, many Walking Away participants expressed regret at not having had the continued contact with the study that it provided.
Focus group participants reflected on how their activity levels had changed (and why) throughout the 48 months; some talked about incidents that had led them to reducing their activity levels and whether or not and how they managed to increase their levels again; in some instances, this involved changing type of activity (e.g. because of an injury). Notably, as the intervention aimed to support individuals in increasing and maintaining physical activity levels themselves, analysis focused on what participants did themselves, drawing on what they had learnt from the intervention and with the tools provided for self-monitoring, as opposed to someone or something else intervening for them to re-engage.
Participants’ accounts and discussions revealed several explanations for the limit in the sustainability of activity increase found by the trial. For some, factors related to ageing and associated health risks and conditions were prominent; falls, accidents or surgery – and associated recovery – led to long periods of reduced activity, while others spoke of a general feeling of physically not being able to do as much with their increasing age. Work and other commitments had an impact on some participants. Whereas several participants described maintaining self-monitoring activity after the study ended, others no longer saw the need, often because they were satisfied with their new habitual levels, or, for a few, had reduced motivation.
Based on an analysis of the end-of-study focus groups, recommendations for improving the intervention might include:
-
identifying an additional form of support that participants could call on in the event of a major health issues/illness, such as an extra telephone call to ‘reset’ the text messaging, and change the tailoring factors accordingly
-
identifying an additional mechanism for maintaining motivation in the long term
-
incorporating newer self-monitoring technologies that have become popular within the target age group (Fitbit, Strava, etc.).
Chapter 6 Cost-effectiveness analysis
Two cost-effectiveness analyses were undertaken. The primary analysis was a model-based analysis using the School for Public Health Research Diabetes Prevention Model (henceforth ‘the model’). 70 The secondary analysis was an alongside-trial analysis. The methods for the cost-effectiveness analyses are presented in the following order:
-
calculating the costs of the interventions
-
primary analysis – model-based evaluation
-
secondary analysis – alongside-trial analysis.
Calculating the costs of the interventions
Intervention costs
Two sets of intervention costs were calculated: one relating to the estimated costs of delivering Walking Away and Walking Away Plus, given that it is likely to be delivered in real-world practice, and the second relating to the cost of delivering Walking Away and Walking Away Plus, as per the PROPELS trial protocol. The former set was developed in consultation with personnel involved in delivering Walking Away and related interventions, and was intended to represent the most likely costs faced by the NHS were Walking Away or Walking Away Plus to be implemented. The intention is to correct for any artificially high or low implementation costs that may be an artefact of the trial process. The real-world costs were used in the base-case analyses.
The Walking Away intervention costs included educator and administrative staff time, physical resources (e.g. pedometers and teaching materials), venue hire and refreshments, staff training and participant expenses. In addition, there were further costs that applied only to the Walking Away Plus arm, namely the cost of follow-up telephone calls and text messages.
The trial management group anticipated that, in a real-world setting, staff would need to be retrained less frequently than the 4 years of the trial would suggest. Therefore, the cost of training staff was annuitised over 10 years (in line with assumptions made for similar structured education interventions114) using the following annuity calculation:
where r = discount rate (3.5%) and n = expected lifetime of the training (10 years).
In the trial, participants from both intervention arms attended the same education sessions; therefore, the common costs were totalled and divided proportionally to the number of participants randomised to each arm. The Walking Away Plus additional costs were added to this to yield a total cost per arm that was divided by the number randomised to produce a per-participant cost for each arm.
Economic analysis methods
Two analyses were undertaken: a model-based evaluation (primary analysis) and a within-trial analysis (secondary analysis). Details of both of these analyses are given below. This section details aspects of the analyses that were in common across both analyses.
Perspective, discounting and time horizon
In line with the NICE methods guide,75 the analyses took an NHS and a Personal Social Services perspective; future costs and QALYs were discounted at a rate of 3.5% per annum; and the time horizon was a lifetime horizon in the primary analysis and a 4-year time horizon in the secondary analysis.
Outcome measures
Costs and QALYs were estimated for each strategy.
The primary outcome measure was the ICER, which is change in cost/change in QALYs. As there are three strategies in this decision problem, the strategies were ordered by the total number of QALYs produced and ICERs were calculated comparing each strategy to the next least effective strategy. Any intervention that was dominated (i.e. produced fewer QALYs at a higher cost than another intervention) or was extendedly dominated (i.e. if a strategy’s ICER is higher than the ICER of the next most effective intervention) then these strategies were removed when calculating ICERs.
The value of information was calculated for this decision problem. We calculated the value of expected value of perfect information (EVPI). This statistic is the value to a decision-maker (e.g. NICE) of eliminating the uncertainty in all parameters in the economic analysis. Therefore, it provides an upper limit on any future research to reduce uncertainty on these sets of strategies. We also calculated the parameter EVPI, which is the value to the decision-maker of eliminating all uncertainty in a subset of parameters in the model, for step count, diabetes diagnoses and HbA1c.
Primary analysis: model-based evaluation
A detailed account of the methodology underlying the model is reported elsewhere. 70 In brief, it was an individual patient-level simulation in which simulated individuals were passed through a lifetime of events in annual cycles. During each annual cycle, an individual may be diagnosed with diabetes, may become unwell (from cardiovascular disease, cancer, diabetes-related complications, depression, dementia or osteoarthritis), may be prescribed antihypertensives, statins or diabetes medication, or may die (either from their illness or from background mortality). Their risk of each of these events happening is determined by a series of risk functions and the individual’s metabolic characteristics. These metabolic characteristics were set at the start of the model and updated with each annual cycle based on observations in the PROPELS trial and on modelled trajectories. 115 Each time an individual experiences a clinical event, passes into a new health state or is prescribed a new medication, this is associated with costs and health consequences. Thus, the model can extrapolate intervention effects and estimate costs and QALYs over a lifetime horizon. The model takes an NHS and Personal Social Services perspective.
The model is well validated for use in diabetes prevention interventions,73,116 but further development was required to adapt it specifically to the PROPELS trial. In particular, the following adaptations were made:
-
The model used a simulated population based on the Health Survey for England. This was changed to better represent the PROPELS trial population.
-
Step count was included as a new population characteristic. This directly affected the cardiovascular risk of modelled individuals based on the results of the NAVIGATOR trial. 20
-
During the first four annual model cycles, the pre-existing HbA1c trajectories and diabetes diagnosis mechanism were replaced by a new regression models developed using the trial data, so that diabetes diagnoses reflected those observed in the trial.
The model population
To model the cost-effectiveness of Walking Away and Walking Away Plus in the general population, we ran the model twice for each analysis: once with a population representing the South Asian trial subpopulation, and once with a population representing the non-South Asian trial subpopulation. The results from the two subgroups were weighted using data from the UK government117 on the relative size of these two populations in the UK. For example, if we found that a strategy resulted in 10 discounted QALYs being accrued in the non-south Asian population and 11 discounted QALYs being accrued in the South Asian population, and if 10% of the population was South Asian, then the total number of discounted QALYs accrued for that strategy would be 10.1, because (11 × 0.1) + (10 × 0.9) = 10.1.
Each of these subpopulations was synthesised based on an analysis of the trial participants at baseline, using the following steps:
-
assessment of the distribution of each continuous variable required by the model (e.g. height, weight and systolic blood pressure)
-
transformation of any non-normally distributed variables to generate approximately normal distributions for each variable
-
construction of a multivariate normal distribution using all variables (both continuous and categorical) simultaneously to preserve correlations between variables
-
sampling of each variable for all simulated individuals
-
for continuous variables:
-
– generation of cut-off points for each categorical variable using the proportional frequency of each category in the PROPELS data
-
– the sampled variable was converted back into the appropriate category using these cut-off points.
-
-
back-transformation of any transformed variables to the natural scale.
Step count modelling
At baseline, step count was sampled from a multivariate normal distribution as described previously. At 12 and 48 months, step count was simulated using a beta-regression. Beta-regressions predict two outcomes: a mean effect and dispersion in the mean effect. Variance can be predicted for any mean effect and dispersion using the following formula:
Furthermore, beta-regressions require that the data that are being predicted are on a scale between 0 and 1. We transformed step count so that 0 was equivalent to 1 step lower than the lowest value in the PROPELS trial (464 steps per day) and 1 was equivalent to 1 step per day more than the highest value in the PROPELS trial (25,341 steps per day).
The key advantage of this approach is that it allows heterogeneity to be included in the simulation because we know the expected number of steps per day and the expected variance in the number of steps per day for every individual. This means that we do not have to assume that an average treatment effect is appropriate, with the level of heterogeneity in identical individual outcomes being driven by the data.
In line with the statistical analysis, to estimate step count at 4 years we controlled for two indicator variables for randomised study arm (Walking Away vs. control, Walking Away Plus vs. control), the three randomisation stratification variables (centre, ethnicity and sex), ambulatory activity at baseline and ambulatory activity at 1 year as covariates. Unlike in the statistical analysis, we did not include wear time at baseline, wear time at 48 months, number of valid days at baseline or number of valid days at 48 months as covariates in the regression model, as predicting these covariates in our simulation model would have increased the computational complexity of the simulation and the causal linkages between these parameters and measured step count were unclear. We applied these control variables to both the mean effect and the dispersion parameters of the beta regression, with the exception of ethnicity for the dispersion parameter, as the models failed to converge when this covariate was included.
To estimate step count at 1 year, we controlled for two indicator variables for randomised study arm (Walking Away vs. control, and Walking Away Plus vs. control), the three randomisation stratification variables (centre, ethnicity and sex) and ambulatory activity at baseline as covariates. We applied these control variables to both the mean effect and the dispersion parameters of the beta-regression, again with the exception of ethnicity for the dispersion parameter.
As the beta-regression produces data on the mean and variance for each individual in the model, we simulated each individual’s step count by randomly sampling this from their individualised beta-distribution (based on their expected mean step count and expected dispersion). We did this separately for each individual’s step count at 1 and 4 years. After we had sampled each individual’s step counts (on the 0–1 scale), we transformed these simulated values back into steps per day. Between 0–12 and 12–48 months, step count was assumed to be linear. Beyond 48 months, step count was extrapolated based on four different possible scenarios. First, an underlying trajectory for step count was estimated by constructing an ordinary least squares linear regression model of step count and age at baseline, adjusted for sex, site, intervention arm and ethnicity from the PROPELS data. The coefficient for age from this model was used as the underlying annual change in step count in a no-intervention scenario for each individual. The observed step count was then compared with this underlying trajectory in the following four scenarios (Figure 6):
-
A. Step count increased between 0 and 12 months and then declined to 48 months, but was above the underlying trajectory at 48 months. In this case, step count would continue to decline at the same rate as between 12 and 48 months until it converged with the underlying trajectory, at which point the individual switched to the underlying trajectory.
-
B. The inverse case of (A) – step count decreased between 0 and 12 months and then increased to 48 months but remained below the underlying trajectory. In this case, the step count would continue to increase at the same rate as between 12 and 48 months until it converged with the underlying trajectory.
-
C. An individual experienced improvement during the trial such that their 48-month step count was above the underlying trajectory and step count had increased between 12 and 48 months. In such a case, the individual would not converge with the underlying trajectory were their rate of change to continue. In such a case, the annual rate of change for the underlying trajectory would be applied after 48 months such that the individual step count trajectory was parallel to the underlying trajectory.
-
D. The inverse case of (C) – the individual experienced a decline in step count from 12 to 48 months and their 48-month step count was below the underlying trajectory. In this case, the annual rate of change for the underlying trajectory was applied after 48 months such that the individual’s trajectory was parallel to the underlying trajectory.
FIGURE 6.
Example step count trajectories. In each scenario, line A represents the individual’s trajectory between years 1 and 4, line B represents the underlying trajectory, and line C represents the modelled step count.
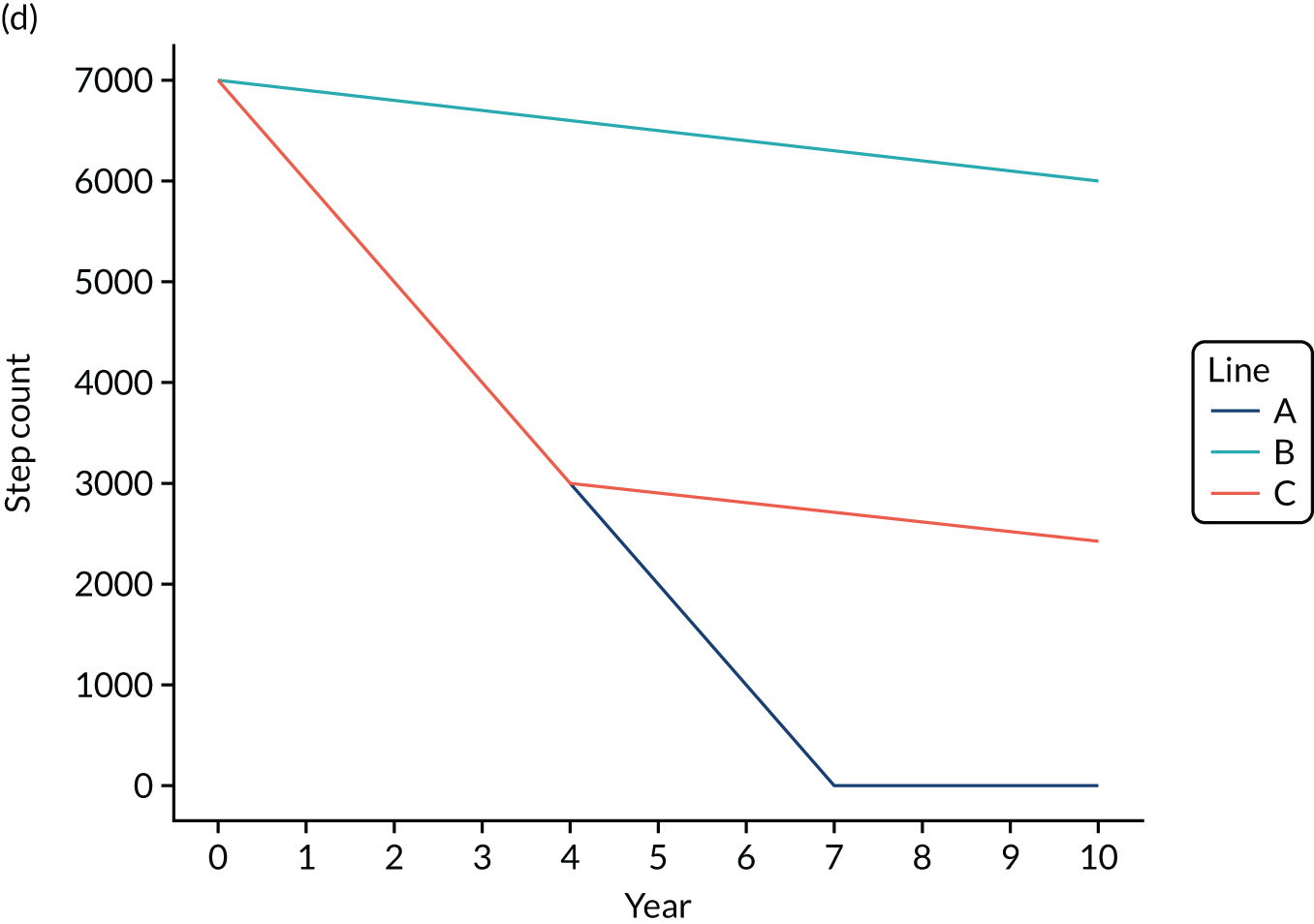
Step count and QRISK
It was assumed that the unadjusted QRISK2 algorithms currently in the model adequately described the baseline risk of cardiovascular disease (CVD) for an individual with the average baseline step count in the PROPELS trial.
A QRISK2 modifier was calculated for each individual in the model, based on model 3 (the fully adjusted model) from the NAVIGATOR analysis. 20 This analysis was a Cox’s proportional hazards model, which concluded that the hazard of cardiovascular events was reduced by 10% for every 2000-step increment at baseline and that a change from baseline of 2000 steps in 12 months was independently associated with an 8% reduction in cardiovascular event rate.
From this, per-step hazard changes were calculated by finding the 2000th root of 0.9 and 0.92, respectively. Individual hazard ratios for each patient were calculated, which adjusted for their baseline ambulatory activity and the change in step count that they experienced in the RCT. These hazard ratios were recalculated every year. The formulae for calculating each individual’s hazard ratio is as follows:
Therefore, each individual had a hazard ratio at baseline depending on their baseline ambulatory activity, which was updated every 12 months depending on their change in ambulatory activity. The individual-estimated rate of having a CVD event within the next year (as currently calculated in the existing model structure using the QRISK2 algorithm) was multiplied by the annual hazard ratio to adjust the risk of CVD events according to individual step count.
HbA1c trajectories
As for step count, we used beta-regressions to estimate HbA1c levels at 1 and 4 years due to their advantages in being able to incorporate heterogeneity. HbA1c was transformed so that 4.5% (0.1% lower than the smallest value in the PROPELS trial) was equal to 0%, and 9.2% (0.1% higher than the largest value in the PROPELS trial) was equal to 1.
To estimate HbA1c levels at 4 years, we controlled for two indicator variables for randomised study arm (Walking Away vs. control, and Walking Away Plus vs. control), HbA1c at 1 year, the three randomisation stratification variables (centre, ethnicity and sex) and HbA1c at baseline as covariates. We applied these control variables to both the mean effect and the dispersion parameters of the beta regression.
To estimate HbA1c at 1 year we controlled for two indicator variables for randomised study arm (Walking Away vs. control, and Walking Away Plus vs. control), the three randomisation stratification variables (centre, ethnicity and sex) and HbA1c at baseline as covariates. We applied these control variables to both the mean effect and the dispersion parameters of the beta regression.
Between 1 and 4 years we assumed that HbA1c levels changed linearly between the two time points. After 4 years, we assumed that HbA1c in the treatment was maintained or declined in the same way that we have assumed that step count was maintained or declined (see Step count modelling). From the fifth year onwards, the HbA1c levels of individuals are estimated using the Breeze et al. 115 trajectory models.
Modelling uncertainty
To assess uncertainty in the model, we ran both deterministic analyses and PSAs. In the deterministic case, we set all model parameters to their mean values. The model ran one fixed simulated population. In the PSA, we varied all model parameters simultaneously within their distributions and performed many different model runs. We used this set of PSA results to estimate mean costs and QALYs, and also calculated 95% CIs around these point estimates. Initially, we ran 1000 different sets of parameters. We assessed the results for stability using Hatswell et al. ’s118 criteria, and performed more PSA runs when needed.
We further explored the sources of model uncertainty using value-of-information analysis, which estimated the potential cost to the decision-maker of making the wrong decision owing to uncertainty around the results. We used Sheffield Accelerated Value of Information71 to do this. We assumed that the maximum number of people affected by the decision was equivalent to the number of people expected to be offered the National Diabetes Prevention Programme (a similar intervention that is currently being rolled out nationally), namely 100,000 per year,119 and that the decision would be relevant for 10 years.
Threshold analysis
We conducted a threshold analysis to determine the justifiable cost of the interventions for them to be cost-effective relative to usual care at a maximum acceptable ICER of £20,000 per QALY. To do this, we used a rearranged incremental net monetary benefit equation:
Secondary analysis: alongside-trial analysis
The alongside-trial analysis incorporated all of the costs incurred and health benefits accrued during the 4-year trial period. All statistical analysis was conducted in R version 3.5.3. 120
Costs
The costs that each participant incurred included the cost of the intervention (methods described previously), the cost of health-care resource use and the cost of medications taken during the trial period. Data on resource use were collected in the trial and data on unit costs were obtained from nationally representative sources. Within the trial, participants were asked about resource usage within the past 12 months at 0, 12 and 48 months. Resource use in years 2 and 3 was assumed to be the same as at 48 months. Costs incurred in years 2, 3 and 4 were discounted at 3.5% per year. 20
Resource use
Self-reported data on health-care resource use at baseline and at 12 and 48 months were taken from the PROPELS data, namely the number of times in the last 12 months that they had used each of the following health-care resources: GP, practice nurse, other health-care professional, attendance at accident and emergency, outpatient clinics, day hospital and inpatient stays. Costs of these resources were obtained from the PSSRU121 and NHS reference costs122 and are summarised in Table 12.
| Resource | Cost (£) | Source | Assumptions |
|---|---|---|---|
| Resource use | |||
| GP visit at home | 93.60 | Hernandez Alava et al.121 | |
| GP visit at surgery | 37 | Hernandez Alava et al.121 | |
| Nurse visit at home | 45 | Hernandez Alava et al.121 | One hour of band 6 nurse time |
| Nurse visit at surgery | 11.63 | Hernandez Alava et al.121 | 15.5 minutes of band 6 nurse time |
| Other health-care worker visit at home | 36 | Hernandez Alava et al.121 | One hour of band 5 staff time |
| Other health-care worker visit at surgery | 12 | Hernandez Alava et al.121 | 20 minutes of band 5 staff time |
| A&E attendance | 160 | van Hout et al.122 | |
| Outpatient clinic attendance | 134 | van Hout et al.122 | |
| Day hospital attendance | 745 | van Hout et al.122 | |
| Inpatient stay | 1864.60 | van Hout et al.122 | Weighted average of cost of inpatient stays of differing lengths |
| Medication costs | |||
| ACE inhibitors | 37.96 | Dolan et al.79 | ACE inhibitors |
| Alpha-blockers | 65.00 | Dolan et al.79 | Alpha-blockers |
| ARBs | 10.01 | Dolan et al.79 | ARBs |
| Beta-blockers | 8.45 | Dolan et al.79 | Beta-blockers |
| Calcium channel blockers | 8.45 | Dolan et al.79 | Calcium channel blockers |
| Diuretics | 6.50 | Dolan et al.79 | Diuretics |
| Aspirin | 6.76 | Dolan et al.79 | Aspirin |
| Statins | 10.01 | Dolan et al.79 | Statins |
| Fibrates | 43.55 | Dolan et al.79 | Fibrates |
| Metformin | 78.00 | Dolan et al.79 | Metformin |
Medications
Self-reported data on medication use at baseline and at 12 and 48 months were taken from the PROPELS data. It was assumed that a participant who was taking one or more of these medications was doing so long term and was expected to be taking the medication for the duration of the trial. If a participant was found to have been taking a medication at 48 months, but not at 12 months, the date of their commencing medication was assumed to be midway between these time points (i.e. 30 months). The cost of 1 year of treatment on each medication type was taken from the Regional Drug and Therapeutics Centre (Newcastle) Cost Comparison Charts. 79 It was assumed that participants had been prescribed the most commonly prescribed preparation of each medication class (as reported in the Prescription Costs Analysis120). The annual cost of treatment with the drugs included is shown in Table 12.
Utilities
The benefits of the intervention arms were estimated QALYs, calculated from EuroQol-5 Dimensions, five-level version (EQ-5D-5L), scores. 123 In line with current NICE recommendations, utility values were generated by mapping the EQ-5D-5L domain scores to EQ-5D-3L value set. Individual 5L dimension scores were mapped to 3L scores using the van Hout algorithm. 122 The mapped 3L scores were aggregated into health state indices, which were valued using the Dolan et al. 79 valuation model.
Quality-adjusted life-years
Per-participant QALYs were calculated from the utility values using the area-under-the-curve method. In line with the NICE methods guide,20 QALYs for years 2, 3 and 4 were discounted at 3.5% per year.
Cost–utility analysis
The incremental costs and benefits of the interventions were calculated using seemingly unrelated regression equations, implemented using the systemfit package in R. 119 The equation system comprised two equations:
The fitted covariates included site, age, sex, ethnicity, and baseline costs and QALYs.
Results are presented as incremental costs, incremental QALYs, ICERs and incremental net monetary benefit, assuming a willingness-to-pay threshold of £20,000 per QALY in line with NICE guidelines. 75
Scenario analyses and multiple imputation
In addition to the two sets of intervention costs described above, the analyses were performed using both complete cases and imputed data. For the latter, data were imputed using the mice (multiple imputation by chained equations) function in R. 124 Five imputed data sets were generated using the predictive mean matching option. The imputation models included all of the covariates used in the systemfit models. As it was not possible to run systemfit models directly using imputation models, the systemfit models were run separately for each imputed data set and the results were pooled manually using Rubin’s rules. 124
Results
Trial intervention costs
For reasons of practicality, participants in both the Walking Away and the Walking Away Plus arms attended the same structured education sessions. Therefore, in costing the interventions, it was assumed that the costs incurred in providing each arm were the same, except for the telephone and text message support costs, which were assigned only to the Walking Away Plus arm. The full details and unit costs used in the microcosting of the interventions are provided in Appendix 11, Tables 49 and 50.
Educators
The cost of providing an educator for 1 hour was £20.37 (including on-costs). The sessions lasted 4 hours, giving a per-session cost of £81.48. A total of 259 sessions were delivered by two educators and 123 sessions were delivered by one educator.
Delivery of the interventions was supported by a training and mentoring package, which comprised:
-
Training to deliver –
-
the initial Walking Away structured education sessions (both intervention arms)
-
the Walking Away update sessions (both intervention arms)
-
the follow-up telephone calls (Walking Away Plus only).
-
-
Mentoring to promote the fidelity of –
-
the Walking Away sessions (both intervention arms)
-
the telephone calls (Walking Away Plus only).
-
Training was delivered by two members of NHS staff: one at band 7 and one at band 8a. Fidelity promotion was carried out by the band 8a staff member only. For clarity, ‘trainers’ refers to the staff members who delivered the training sessions and ‘educators’ refers to the individuals who attended the training sessions and delivered the structured education sessions in the interventions.
Walking Away delivery training
All educators (eight in Leicester and four in Cambridge) attended the Walking Away training. One session was run in each site. Nine educators (five in Leicester and four in Cambridge) attended the update training sessions. One update training session was run in each site. Details of the total costs of running these training sessions are provided in Table 13.
| Unit cost | Quantity | Cost (£) | |
|---|---|---|---|
| Walking Away initial training | |||
| Trainer time | £53 + £63 per hour | 15 hours × two sites | 3480 |
| Plus 15 hours’ prep time | 870 | ||
| Educator time | £20.37 per hour | 15 hours × 12 educators | 3667 |
| Refreshments | £10 per head (Leicester); £9.50 per head (Cambridge) | 8 + 2; 4 + 2 | 100; 57 |
| Booklets | £25 per head | 12 | 325 |
| Stationery | £15 | 1 | 15 |
| Additional half-day top-up course for one educator (educator and band 8a trainer time only) | 292 | ||
| Total | 8805 | ||
| Walking Away update training | |||
| Trainer time | £53 + £63 per hour | 3.5 hours × two sites | 812 |
| Plus 7.5 hours’ prep time | 870 | ||
| Educator time | £20.37 per hour | 3.5 hours × nine educators | 642 |
| Refreshments | £10 per head (Leicester); £9.50 per head (Cambridge) | 5 + 2; 4 + 2 | 70; 57 |
| Booklets | £10 per head | 9 | 90 |
| Stationery | £15 | 1 | 15 |
| Total | 2556 | ||
| Telephone calls: initial training | |||
| Trainer time | £53 + £63 per hour | 3.5 hours × two sites | 812 |
| Plus 7.5 hours’ prep time | 870 | ||
| Educator time | £20.37 per hour | 3.5 hours × 12 educators | 856 |
| Refreshments | £2.50 per head (Leicester); £2 per head (Cambridge) | 8 + 2; 4 + 2 | 25; 12 |
| Total | 2575 | ||
| Telephone calls: training replacement staff | |||
| Trainer time | £63 per hour | 2 hours × eight sessions | 1008 |
| Educator time | £20.37 per hour | 2 hours × 12 trainees | 489 |
| Total | 1497 | ||
| Fidelity assessment of Walking Away in the first year | |||
| Trainer time | £63 | 4 hours × six visits | 1512 |
| Educator time | N/A | N/A | N/A |
| Total | 1512 | ||
| Fidelity assessment of Walking Away in subsequent years | |||
| Trainer time | £63 | 2 hours × six visits | £756 |
| Educator time | £20.37 | Nine educators × 2 hours × three sessions | 1100 |
| Refreshments | £2.50 per head (Leicester); £2 per head (Cambridge) | 5 + 1; 4 + 1 | 15; 10 |
| Total | 1881 | ||
| Fidelity assessment of the telephone calls | |||
| Trainer time | £63 | 2 hours × six visits | 756 |
| Educator time | £20.37 | 2 hours × three visits × eight educators | 978 |
| Refreshments | £2.50 per head (Leicester); £2 per head (Cambridge) | (5 + 1) × three visits; (3 + 1) × three visits | 45; 24 |
| Total | 1803 | ||
Telephone call delivery training
Initially, all educators were trained to deliver the telephone calls. Throughout the study, there was a high staff turnover for this part of the intervention, as educators were unwilling to make the telephone calls, and replacement staff were trained to take over. The total telephone call training costs were £4071, details of which are given in Table 13.
Walking Away fidelity training
There were two types of sessions to promote fidelity of the Walking Away education sessions: in year 1, educators were observed delivering the intervention; in years 2–4, they met with trainers for mentorship and support. The total Walking Away fidelity training cost was £3393, details of which are given in Table 14. Fidelity assessment of the telephone calls was conducted separately, and this cost £1803, details of which are provided in Table 13.
| Total | 4 years | Annual | |
|---|---|---|---|
| Walking Away | 8805 | 4235 | 1059 |
| Update | 2556 | 1229 | 307 |
| Telephone calls | 4071 | 1958 | 490 |
| Fidelity | 3393 | 1632 | 408 |
| Telephone call fidelity | 1803 | 867 | 217 |
| Totals | 20,628 | 9922 | 2480 |
| Cost of training apportioned to Walking Away arma | 7438 | 3576 | 894 |
| Cost of training apportioned to Walking Away Plus armb | 13,312 | 6401 | 1600 |
Total intervention costs
The annual cost is the total cost divided by the annuity factor. Four-year costs are the annual costs of training multiplied by 4. The total training costs for both interventions are given in Table 14.
Support staff costs
Two types of support staff, administrators and project managers were employed to assist organisation of the interventions. The trial management team estimated that it took each administrator 15 minutes to book an appointment for a participant, and 30 minutes per education session to book the venue, prepare resources and process invoices. Administrators at Cambridge were paid university grade 2 salaries and at Leicester the staff were at NHS band 3; project managers were at university grade 6 at Cambridge and NHS band 6 at Leicester. For Cambridge staff, the mean salary (including on-costs) for each grade was used to estimate annual salary. Hourly rates for grade 2 staff were calculated based on a 35-hour working week. In the case of Leicester staff, the mean salary for a band 6 hospital-based professional staff member including overheads was taken from the Personal Social Services Research Unit (PSSRU). Equivalent figures for band 3 were not available, so the hourly rate for a band 3 staff member was estimated using the ratio of hourly pay to hourly total cost for a band 4 staff member. The costs of employing these staff are shown in Table 15.
| Type of cost | Leicester | Cambridge |
|---|---|---|
| Hourly salary of administrator | £23.71 | £13.84 |
| Number of appointments booked | 2016 | 1076 |
| Total amount of administrator time (hours) | 616 | 348.5 |
| Total cost of administrator time | £14,594 | £4823 |
| Yearly salary of project manager | £69,766 | £44,481 |
| FTE of project manager hours | 0.4 | 0.2 |
| Total cost of project manager salary over 4 years | £111,625.60 | £35,584.80 |
| Total support staff cost per site | £126,491.80 | £40,407.12 |
| Total support staff cost | £162,882.49 | |
Text message system
The total number of text messages sent in the trial was 96,252. Each text cost 3.5 pence to send. In addition, there was an annual licence fee of £118 for the system, giving a total cost of text messages of £3841.
Participant expenses
Participant travel expenses were reimbursed on request at both sites. Leicester did not reimburse mileage but did pay for participants’ taxis, whereas Cambridge did reimburse mileage but did not pay for taxis. The total cost of travel expenses across the two sites was £6454.19.
Administration consumables and teaching resources
Participants in both Cambridge and Leicester were sent appointment letters by post. The Leicester site also sent participants a map showing where to go for the appointment. Costs were associated with teaching resources (paper, pens and booklets) and sets of demonstration materials. In total, the cost of administration and consumables came to £11,163.
Venue costs
In addition to the cost of staff to deliver the sessions, costs were incurred for venue hire and providing refreshments. Some venues provided refreshments in house, whereas at other sessions the refreshments were provided by the PROPELS team. The total cost of booking venues came to £9924.
Additional costs
In addition, each participant received a pedometer that cost £10.50.
Real-world cost assumptions
Following communication with personnel involved in delivering Walking Away and related interventions in a real-world setting (specifically the DESMOND National Director, Bernie Stribling, who is familiar with the intervention landscape for all Walking Away sites in the UK and Ireland), a second set of costs was estimated to reflect the expected cost of delivering the interventions outside the trial. The differences between the two sets of costs are summarised in Table 16. It is assumed that, in the real-world setting, either Walking Away or Walking Away Plus would be commissioned.
| Variable | PROPELS | Real world | ||
|---|---|---|---|---|
| Assumption | Cost | Assumption | Cost | |
| Educators | Educators were specially recruited staff | £20 per hour | Educators are band 4 or 5 non-registered health-care professionals | £31 per hour (average cost per working hour of bands 4 and 5 staff) |
| Trainers | Trainers are one band 7 and one band 8a staff member | £53 and £63 per hour, respectively | Trainers are band 7 | £53 per hour |
| Pedometers | One research-grade pedometer is provided per participant | £10.50 per participant | Pedometers are not provided to participants | £0 |
| Telephone calls | Telephone calls are made by specially recruited staff | £20 per hour | Telephone calls are made by educators | £31 per hour (average cost per working hour of bands 4 and 5 staff) |
| Expenses | Travel expenses were reimbursed | Travel expenses are not reimbursed | £0 | |
| Attendance | Nine participants per course | 10 participants per course | ||
| Number of courses over 4 years | 95.5 (382 individual sessions) | 60 (240 individual sessions) | ||
Per-participant costs
The per-participant costs are summarised in Table 17. The total within-trial cost of delivering Walking Away was £286 per participant and the cost of delivering Walking Away Plus was £338 per participant. The real-world costs of delivering the interventions are estimated to be slightly lower than the within-trial costs (Walking Away £257, Walking Away Plus £322). There are two primary reasons for the real-world costs being so close to the within-trial costs of delivering Walking Away. First, the staff members who would be expected to deliver the interventions in the real world are more expensive than the staff used in the PROPELS RCT. Second, we expect that fewer sessions per year will be delivered in real-world settings, increasing the effective per-participant cost of training the educators.
| Intervention | PROPELS | Real world |
|---|---|---|
| Walking Away | 286 | 257 |
| Walking Away Plus | 338 | 322 |
Alongside-trial analysis
Within-trial costs
Health-care resource use
Per-participant health-care resource use at baseline was slightly higher in the Walking Away (£620) and Walking Away Plus arms (£626) than in the usual care arm (£717). A detailed breakdown of the costs incurred during the trial is provided in Appendix 13, Table 69. In summary, health-care resource use was higher in the Walking Away arm than in the usual care arm, and higher still in the Walking Away Plus arm. Although primary care costs were similar across the arms, the Walking Away and Walking Away Plus arms both incurred higher hospital inpatient and outpatient costs.
Adjusted costs and quality-adjusted life-years
Table 18 shows the costs results of the systemfit models over our four scenarios, which cover imputation techniques and methods for calculating the costs of Walking Away and Walking Away Plus. estimating the difference between arms. In all scenarios, both the Walking Away and the Walking Away Plus arms incurred more total costs over the study period than the usual care arm.
| Walking Away vs. usual care, mean (SE) | Walking Away Plus vs. usual care, mean (SE) | |
|---|---|---|
| Adjusted costs from the seemingly unrelated regression (£) | ||
| Real-world costings, complete cases (n = 678) | 1193 (908) | 2149 (907) |
| Real-world costings, imputed missing costs and QALYs (n = 1366) | 949 (721) | 1648 (815) |
| Trial costings, complete cases (n = 678) | 1267 (904) | 2289 (904) |
| Trial costings, imputed missing costs and QALYs (n = 1366) | 664 (840) | 1460 (705) |
| Adjusted QALYs from the seemingly unrelated regressions | ||
| Real-world costings, complete cases (n = 678) | 0.12 (0.06) | 0.13 (0.06) |
| Real-world costings, imputed missing costs and QALYs (n = 1366) | 0.07 (0.04) | 0.02 (0.04) |
| Trial costings, complete cases (n = 678) | 0.12 (0.06) | 0.14 (0.06) |
| Trial costings, imputed missing costs and QALYs (n = 1366) | 0.07 (0.04) | 0.02 (0.03) |
Table 18 shows the QALY results of the systemfit models estimating the difference between arms. In all scenarios, both Walking Away and Walking Away Plus accrued more QALYs over the study period than the usual care arm, with the Walking Away arm accruing more QALYs than usual care. However, the incremental QALYs between either intervention and usual care are much lower than that observed in the complete-case analysis. It is unsurprising that the results may be substantially different between the imputed and the complete cases, as only 678 patients in the PROPELS data had complete cost and QALY data.
Cost-effectiveness
The ICERs from the within-trial analysis for the real-world costs and complete-case data are given in Table 19. When one QALY is valued at £20,000, in line with NICE guidelines, the within-trial analysis would indicate that Walking Away is the most cost-effective option. These results are highly volatile and are sensitive to the assumptions made about missing data and the calculation of the costs. For example, when trial costs are used and the missing data are imputed, usual care would be the most cost-effective option when one QALY is valued at £20,000. It should be noted that all within-trial results are highly uncertain.
| Incremental QALYs | Incremental costs (£) | ICER (£/QALY gained) | |
|---|---|---|---|
| Usual care | – | – | – |
| Walking Away vs. usual care | 0.124 | 1193 | 9639 |
| Walking Away Plus vs. Walking Away | 0.007 | 955 | 145,515 |
This uncertainty is illustrated on the cost-effectiveness plane in Figure 7. Interventions on the cost-effectiveness plane to the right of the dotted line are cost-effective at a willingness-to-pay threshold of £20,000, and those to the left are not. As the 95% CIs for cost and QALYs for both interventions cross the dotted line, it is not possible to be certain if either intervention is cost-effective at this threshold.
FIGURE 7.
Cost-effectiveness plane showing within-trial adjusted incremental costs and QALYs.
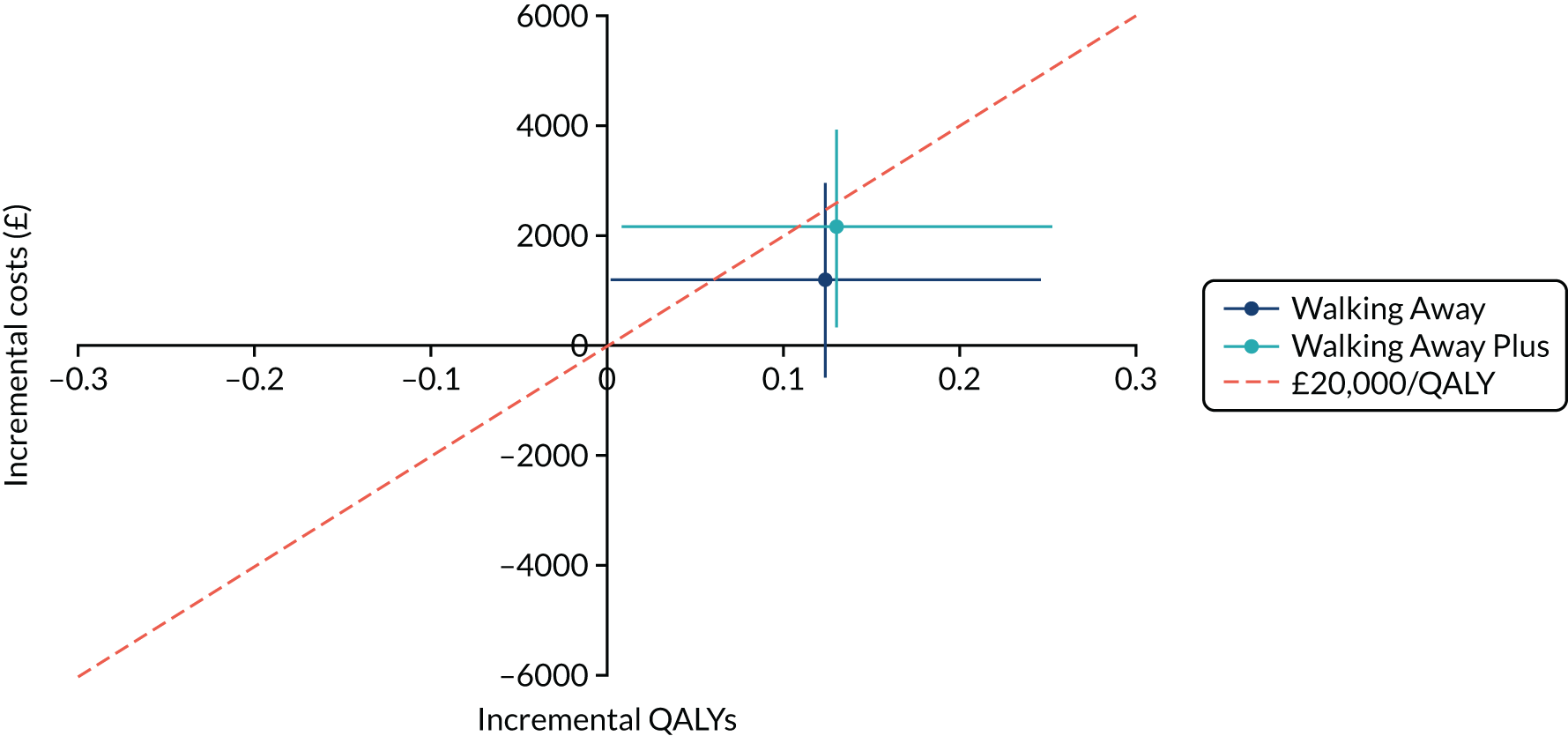
Similarly, the cost-effectiveness acceptability curve in Figure 8 demonstrates this uncertainty. The graph shows the probability that each of the three trial arms is cost-effective at varying values of a QALY. At a low willingness-to-pay threshold, usual care has the highest chance of being the most cost-effective intervention because it is the cheapest option. As the value of a QALY increases, the QALY gains in Walking Away and Walking Away Plus become more valuable and their relative chance of cost-effectiveness increases. Between the upper and lower limits of the NICE threshold, namely £20,000 and £30,000 per QALY, the three arms have a roughly even chance of being the most cost-effective option.
FIGURE 8.
Cost-effectiveness acceptability curve showing within-trial data.
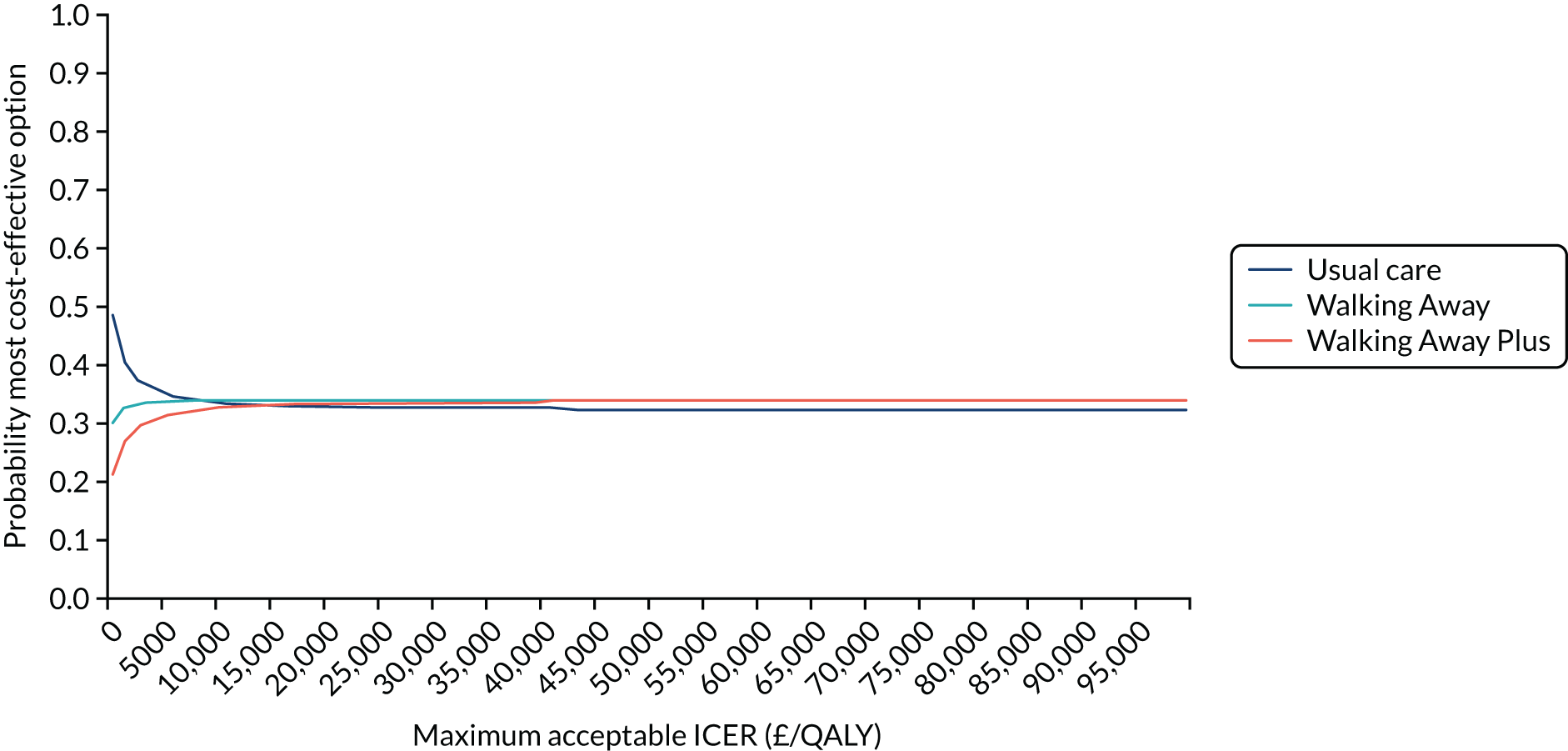
Model results
Model population
The characteristics of the simulated population compared with the real PROPELS data at baseline are shown in Appendix 10, Table 48. Overall, there was good agreement between the simulated and the real data.
Step count trajectories
The figures below show the step count trajectories in the South Asian (Figure 9) and non-South Asian (Figure 10) populations in the deterministic model runs. The average per-day step count at baseline in the non-South Asian population was 7164 (in all three arms). At 12 months, both Walking Away and Walking Away Plus arms were given increases in step count to reflect the treatment effect of the interventions, resulting in average daily step counts of 7342 and 7563, respectively. By 48 months, the average daily step count had declined to 6981 and 7174, respectively.
FIGURE 9.
Step count trajectories in the South Asian population.
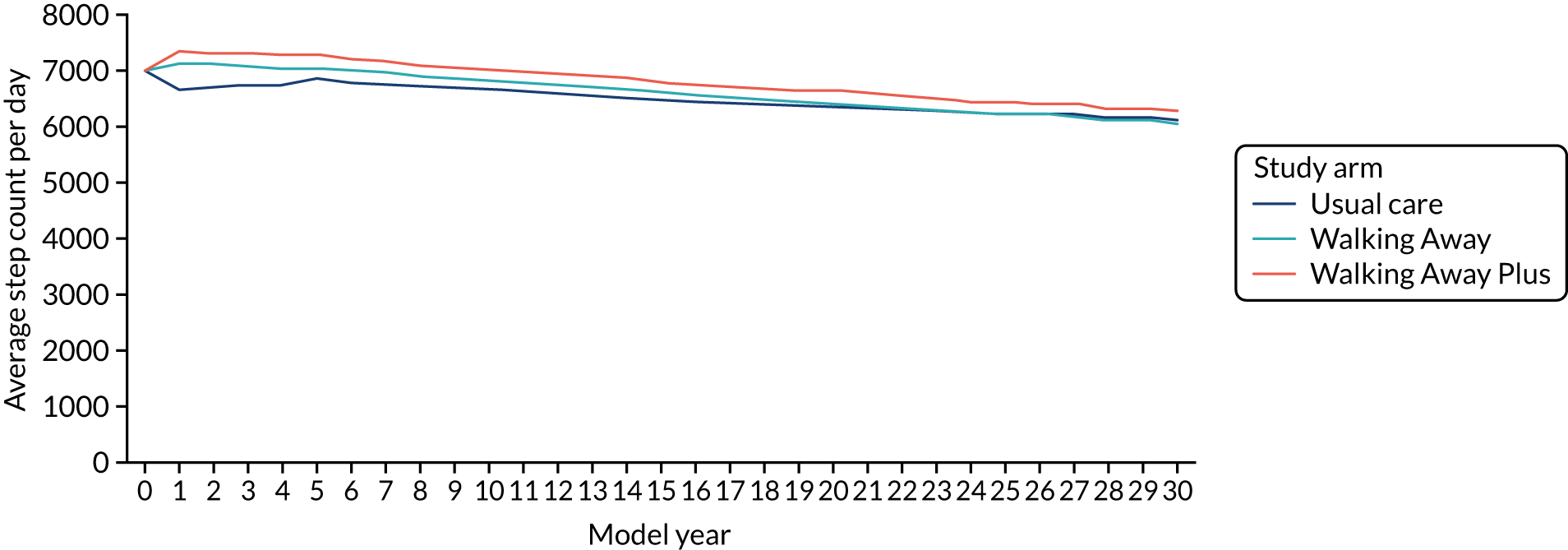
FIGURE 10.
Step count trajectories in the non-South Asian population.
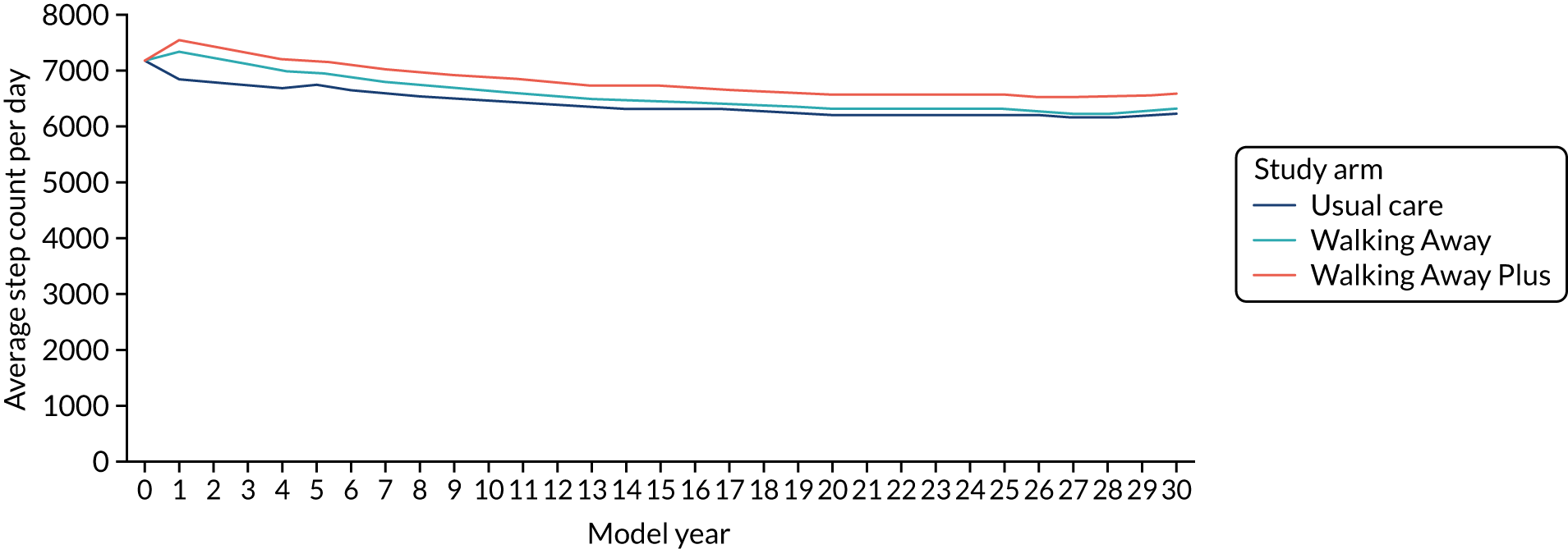
In the Asian population, the baseline step count was 7042 steps per day. At 12 months, it rose to 7178 and 7399 steps per day in the Walking Away and Walking Away Plus arms, respectively. Over the next 3 years, to the end of the trial period at 48 months, step count declined in both groups, to 7075 and 7301 steps per day, respectively.
Beyond 4 years in both subpopulations, both Walking Away and Walking Away Plus individuals converged with their individual underlying trajectory, and then continued to decline in daily step count at a rate of 67 steps per day per year, as predicted by the ordinary least squares regression model. At an aggregate level, there is some noise to this effect as individuals die, and those with a low step count (and higher cardiovascular risk) are at higher risk of mortality. Note that average step count beyond 30 years is omitted from these graphs as the small numbers of individuals make the figures unstable.
HbA1c and diabetes diagnoses
Table 20 shows the rates of diagnosis of T2D in the deterministic model runs in the non-South Asian and South Asian populations. In both subpopulations, the total number of within-trial diagnoses was largest in the Walking Away arm, followed by the usual care arm, followed by the Walking Away Plus arm. This was expected because our logistic regressions (see Appendix 12, Tables 51–68) produced odds ratios for rates of diabetes diagnosis comparing Walking Away with usual care of 1.55 (97.5% CI 0.52 to 4.63) at year 1 and 1.58 (97.5% CI 0.74 to 3.39) at year 4. Comparing Walking Away Plus with usual care, the odds ratios for the rates of diabetes diagnoses were 0.72 (97.5% CI 0.19 to 2.68) at year 1 and 1.25 (97.5% CI 0.57 to 2.74) at year 4. In all three arms, rates of diabetes diagnosis over a lifetime were higher in the South Asian population than the in the non-South Asian population.
| Study arm | |||
|---|---|---|---|
| Usual care | Walking Away | Walking Away Plus | |
| Non-South Asian population (%) | |||
| Year 1 | 0.75 | 1.65 | 1.16 |
| Year 4 | 8.60 | 10.91 | 7.75 |
| Total within-trial diagnoses | 9.35 | 12.56 | 8.90 |
| Total lifetime | 45.94 | 46.62 | 45.66 |
| South Asian population (%) | |||
| Year 1 | 0.95 | 1.31 | 0.65 |
| Year 4 | 6.83 | 9.67 | 7.62 |
| Total within-trial diagnoses | 7.78 | 10.98 | 8.27 |
| Total lifetime | 51.39 | 52.26 | 51.41 |
As shown in Figures 11 and 12, the average HbA1c level (%) was very similar across all three trial arms. The trajectory for the first 4 years (i.e. the data generated from analysis of the PROPELS trial) was not notably different from that for the subsequent years of the model (which was extrapolated using equations based on an analysis of the Whitehall data set125).
FIGURE 11.
The HbA1c trajectory in the South Asian population.
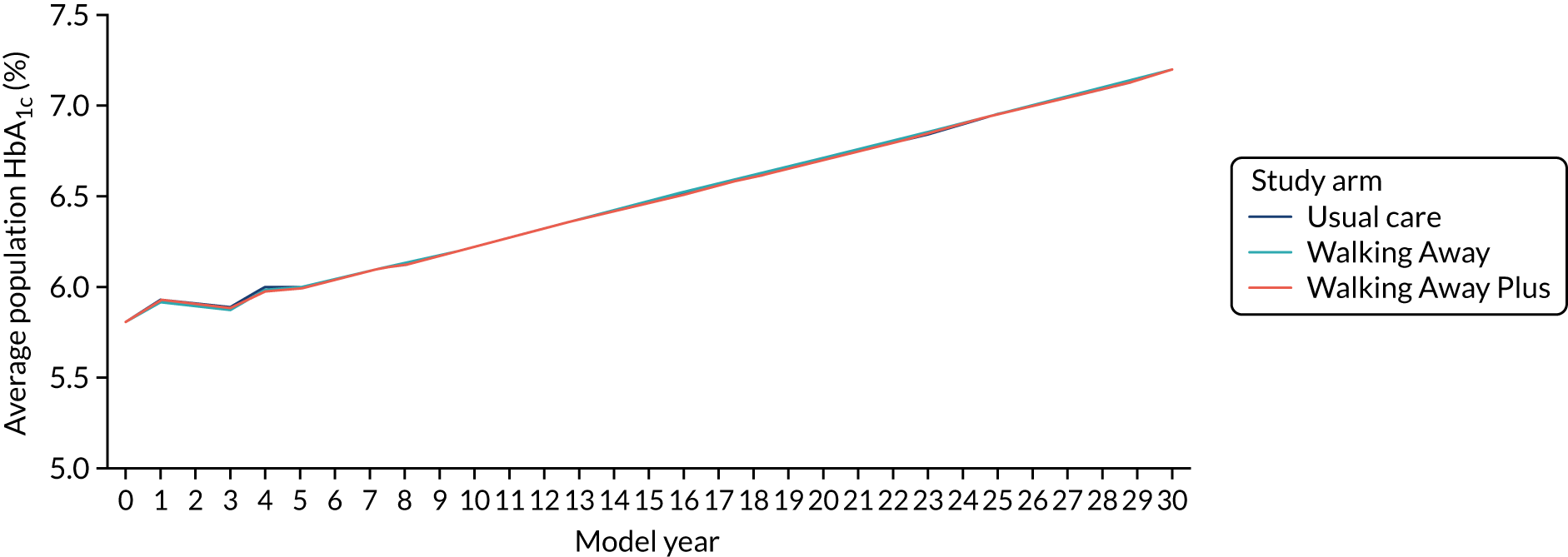
FIGURE 12.
The HbA1c trajectory in the non-South Asian population.
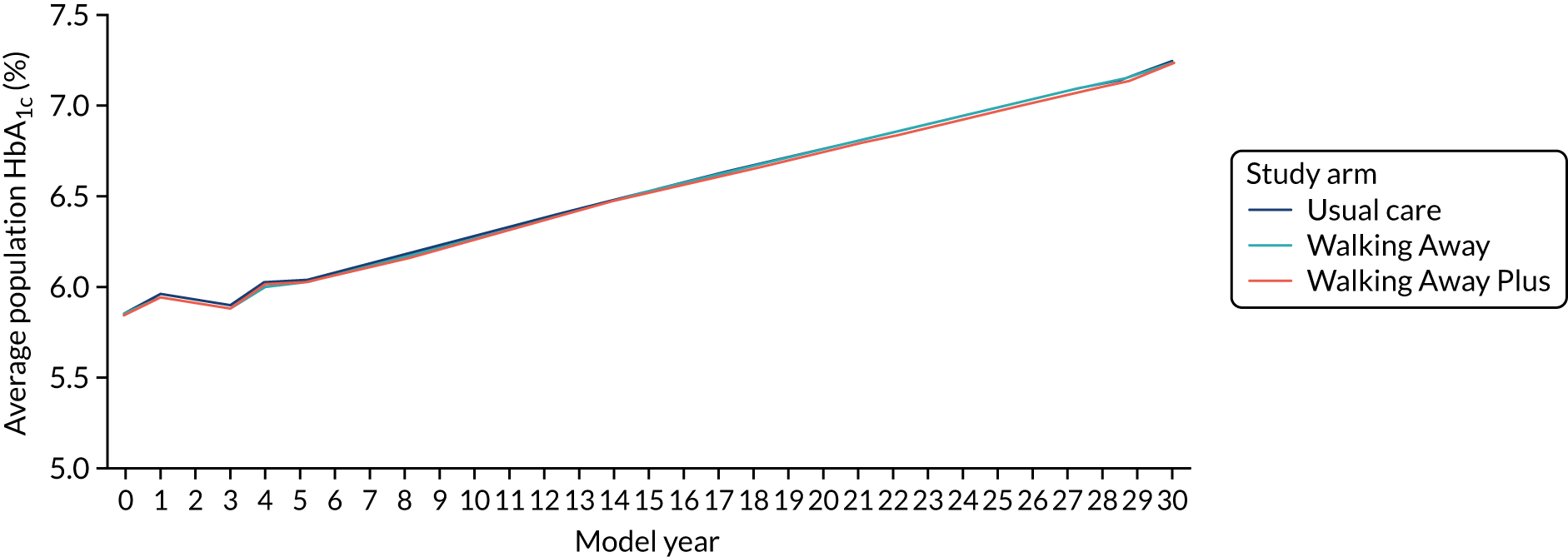
Deterministic results
In the deterministic analysis, mean parameter values were used and the population was fixed. The costs and QALYs for each subpopulation and the weighted population are given in Table 21. All results are presented per person. In both the South Asian and the non-South Asian subpopulations, Walking Away is both more expensive and less effective than usual care, that is it is dominated. In both subpopulations, Walking Away Plus is more expensive and slightly more effective than usual care, giving ICERs that are well in excess of the £20,000-per-QALY threshold.
| Population | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| South Asian population | |||||
| Usual care | 23,007 | 10.823 | – | – | – |
| Walking Away | 23,408 | 10.808 | – | – | Dominated by usual care |
| Walking Away Plus | 23,359 | 10.824 | 352 | 0.000686 | 513,242 |
| Non-South Asian population | |||||
| Usual care | 21,248 | 9.362 | – | – | – |
| Walking Away | 21,633 | 9.354 | – | – | Dominated by usual care |
| Walking Away Plus | 21,581 | 9.371 | 333 | 0.00848 | 39,224 |
| Weighted population | |||||
| Usual care | 21,341 | 9.440 | – | – | – |
| Walking Away | 21,727 | 9.432 | – | – | Dominated by usual care |
| Walking Away Plus | 21,675 | 9.448 | 334 | 0.00806 | 64,347 |
Probabilistic sensitivity analysis
The PSA results for each subpopulation are given in Table 22. In both the South Asian and the non-South Asian subpopulations, Walking Away is both more expensive and less effective than usual care, that is it is dominated by usual care. In both subpopulations, Walking Away Plus is more expensive and slightly more effective than usual care, giving ICERs that are well in excess of the £20,000-per-QALY threshold.
| Population | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| South Asian population | |||||
| Usual care | 24,489 | 10.712 | – | – | – |
| Walking Away | 24,994 | 10.697 | – | – | Dominated by usual care |
| Walking Away Plus | 24,907 | 10.713 | 418 | 0.0014 | 306,971 |
| Non-South Asian population | |||||
| Usual care | 22,494 | 9.245 | – | – | – |
| Walking Away | 22,910 | 9.235 | – | – | Dominated by usual care |
| Walking Away Plus | 22,838 | 9.252 | 344 | 0.0074 | 46,163 |
| Weighted population | |||||
| Usual care | 22,598 | 9.323 | – | – | – |
| Walking Away | 23,018 | 9.312 | – | – | Dominated by usual care |
| Walking Away Plus | 22,945 | 9.330 | 347 | 0.00705 | 49,273 |
The cost-effectiveness planes (Figures 13–15) for both subpopulations and the weighted population show that the PSA mean ICERs for both Walking Away (dark blue crosses) and Walking Away Plus (light-blue crosses) are to the left of the dotted line that indicates a cost-effectiveness threshold of £20,000 per QALY; that is, they are not cost-effective at this threshold. However, the purple and light-blue clouds of points that indicate individual PSA runs show that, for some combinations of parameters, the results are to the right of the line, indicating that both or either of the interventions could be cost-effective at this threshold.
FIGURE 13.
Cost-effectiveness plane for the model results in the South Asian population.
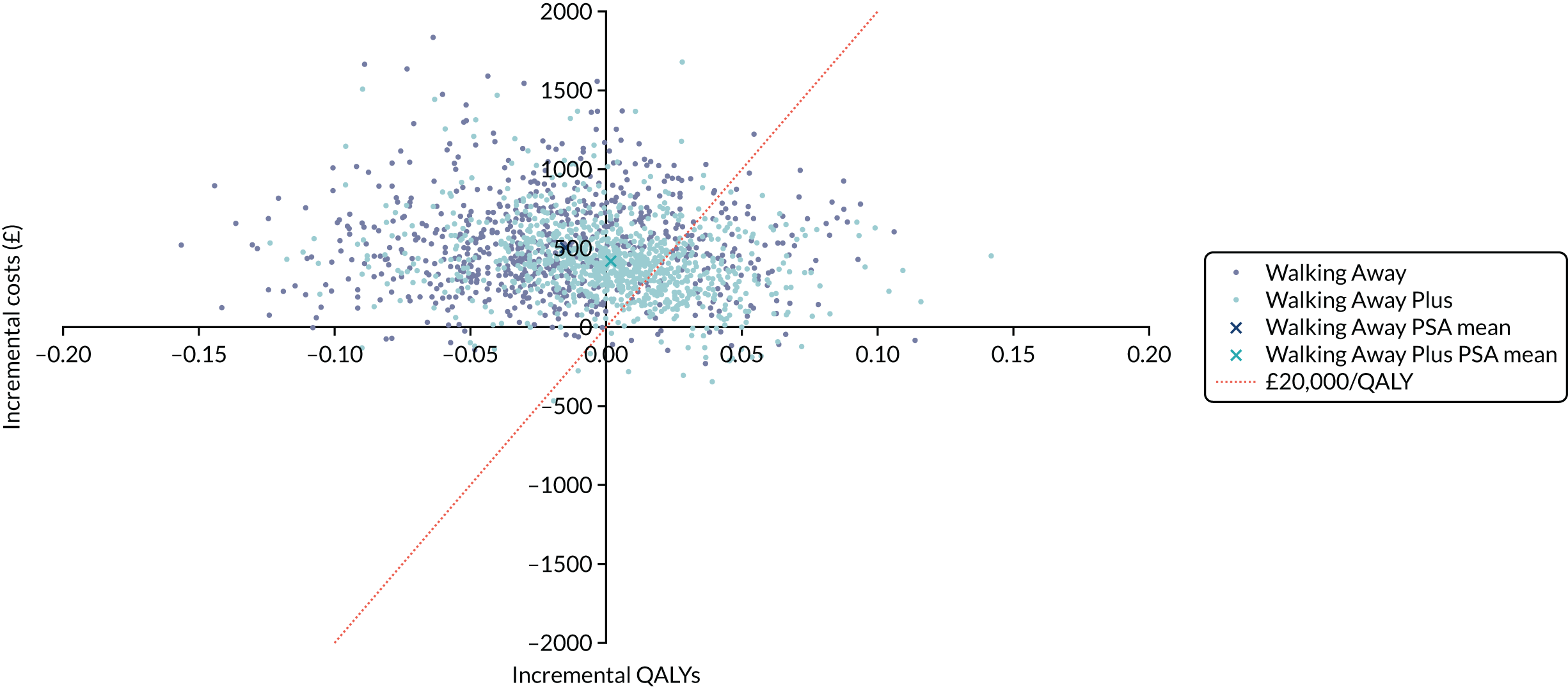
FIGURE 14.
Cost-effectiveness plane for the model results in the non-South Asian population.
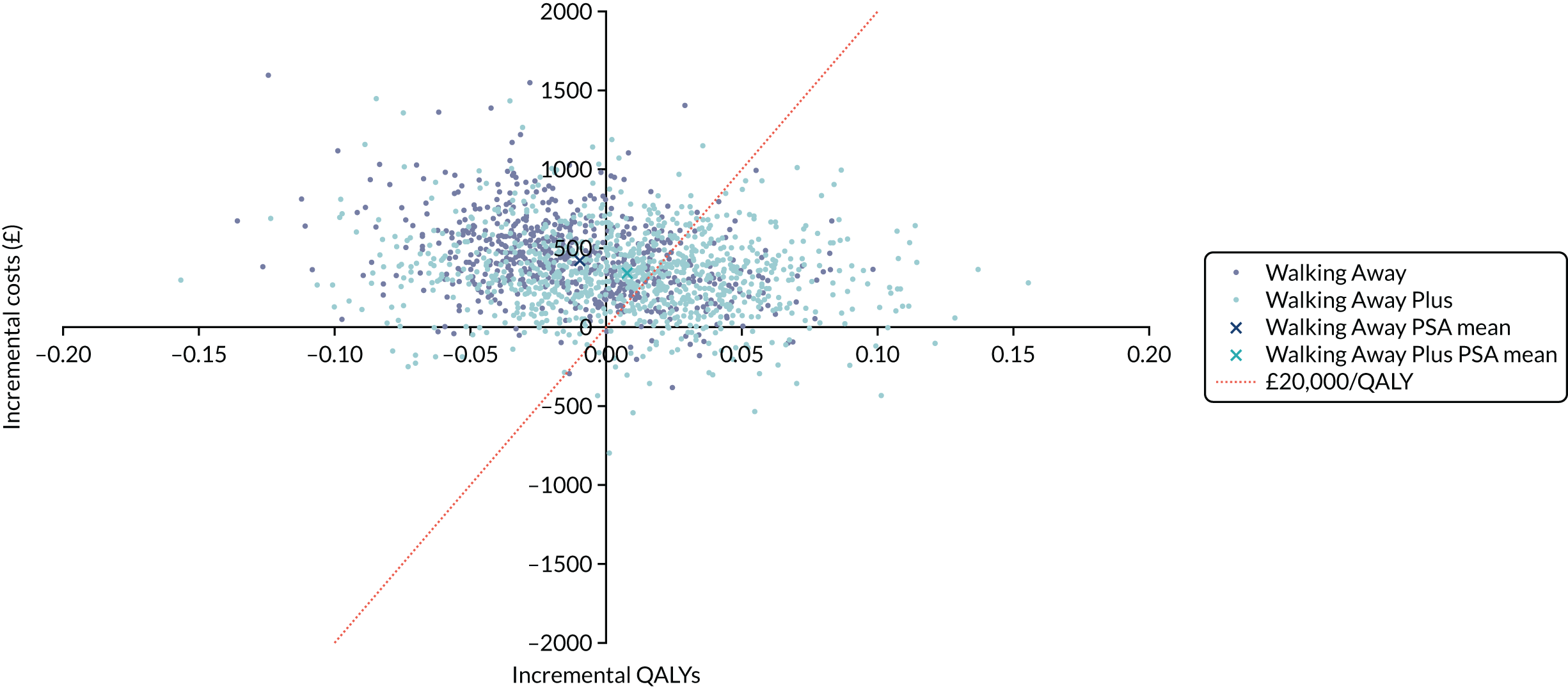
FIGURE 15.
Cost-effectiveness plane for the model results in the weighted population.
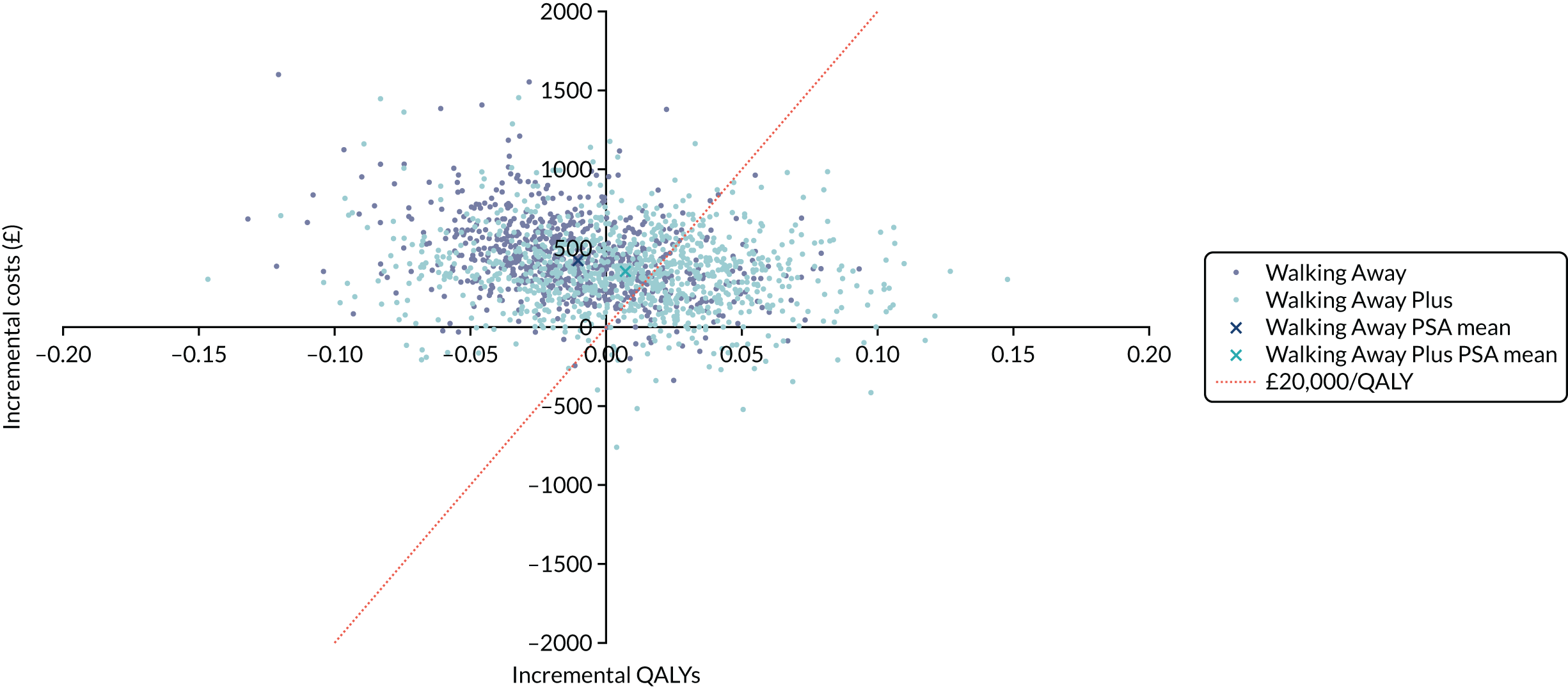
As shown by the cost-effectiveness acceptability curve in Figure 16, among the non-South Asian population, at a threshold of £20,000 per QALY there is a 52.0% chance that usual care is the most cost-effective option, a 9.8% chance that Walking Away is the most-cost-effective option and a 38.2% chance that Walking Away Plus is the most cost-effective option. At a threshold of £30,000 per QALY, the corresponding probabilities are 45.5%, 11.9% and 42.5%.
FIGURE 16.
Cost-effectiveness acceptability curve (non-South Asian population).
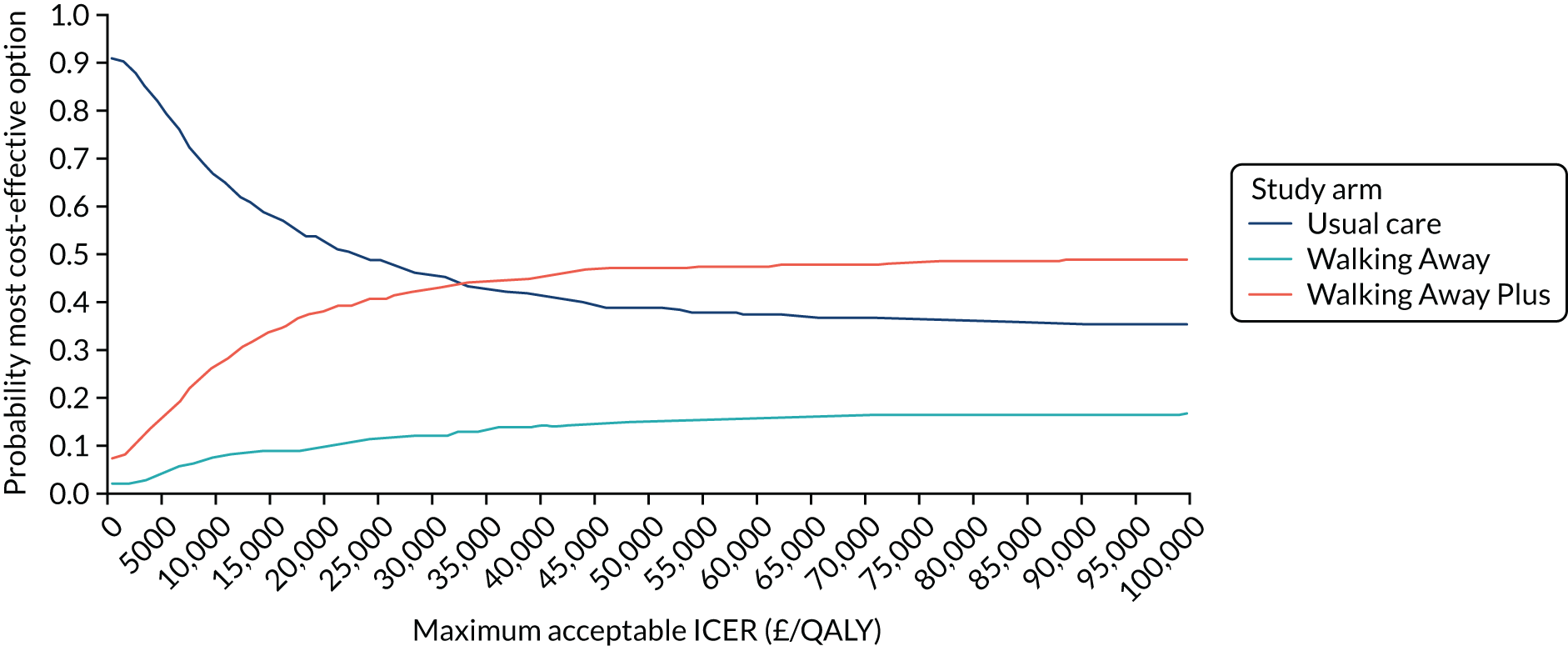
Among the Asian population (Figure 17), at a threshold of £20,000 per QALY there is a 61.8% chance that usual care is the most cost-effective option, an 11.7% chance that Walking Away is the most cost-effective option and a 26.4% chance that Walking Away Plus is the most cost-effective option. At a threshold of £30,000 per QALY, the corresponding probabilities are 54.6%, 14.0% and 31.4%, respectively.
FIGURE 17.
Cost-effectiveness acceptability curve (South Asian population).
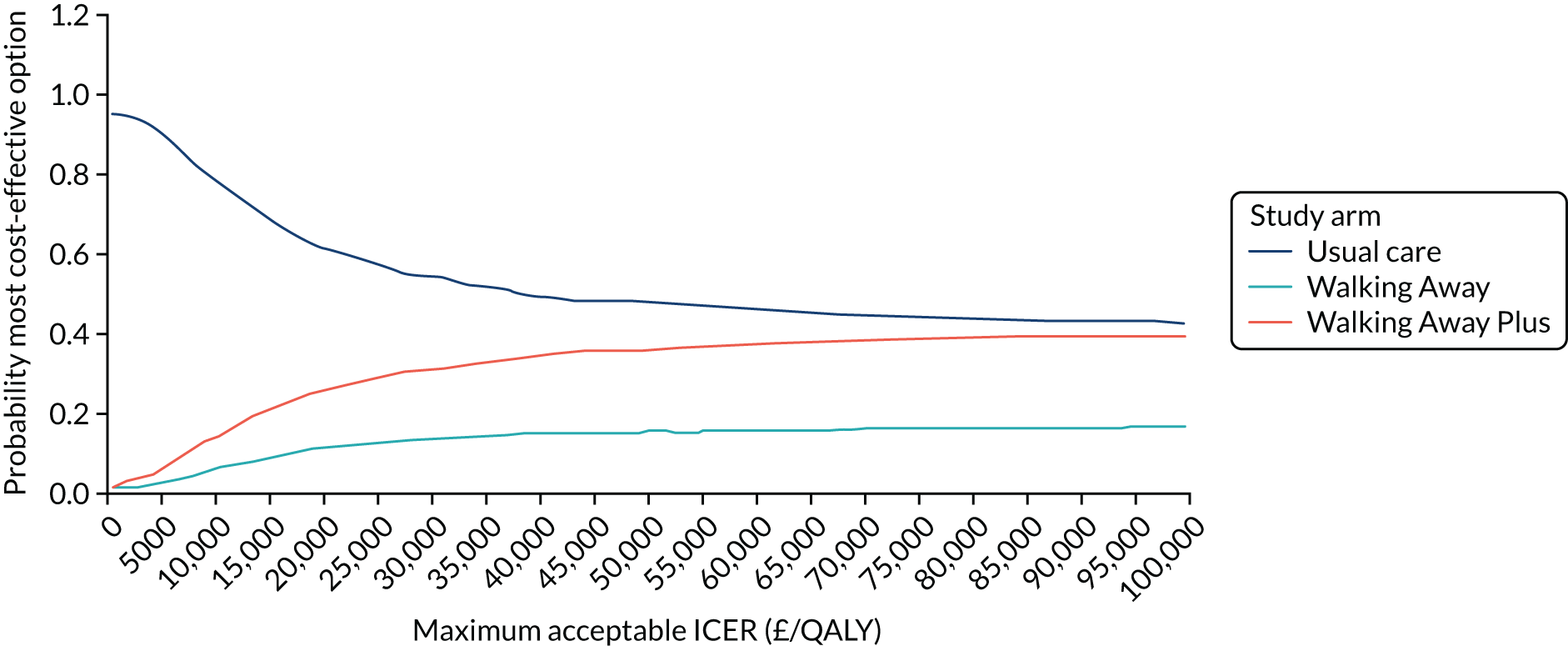
Among the weighted population (Figure 18), there is a 55.6% chance that usual care is the most cost-effective option at £20,000 per QALY, a 9.5% chance that Walking Away is the most cost-effective and a 34.9% chance that Walking Away Plus is the most cost-effective. At a threshold of £30,000 per QALY, the corresponding probabilities are 48.6%, 11.4% and 40.0%, respectively.
FIGURE 18.
Cost-effectiveness acceptability curve (weighted population).
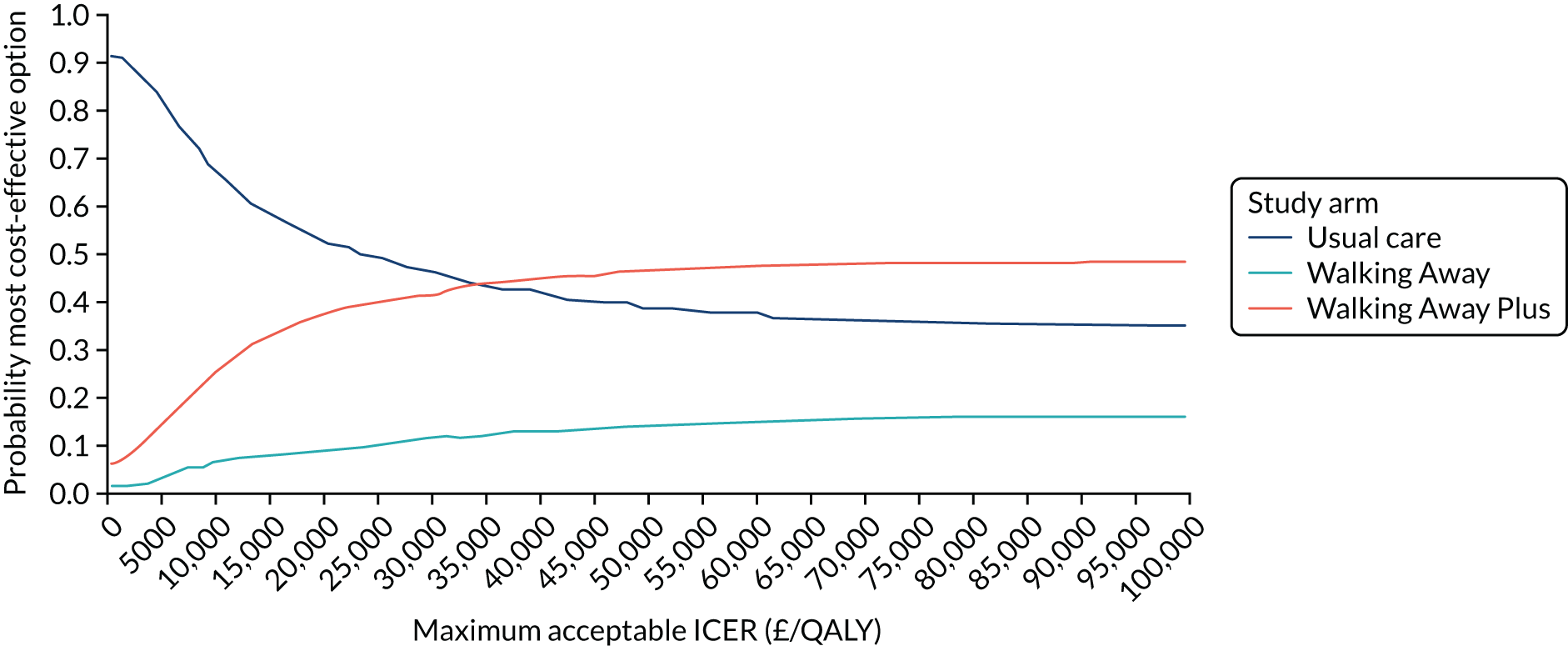
Clinical events
Table 23 shows the PSA average lifetime per-person incidence of T2D, CVD and T2D complications in each of the two subpopulations.
| Population | Usual care | Walking Away | Walking Away Plus | |||
|---|---|---|---|---|---|---|
| Number of diagnoses | Cost of treating (£) | Number of diagnoses | Cost of treating (£) | Number of diagnoses | Cost of treating (£) | |
| South Asian population | ||||||
| T2D | 0.523 | 2022 | 0.532 | 2131 | 0.524 | 2076 |
| CVD eventsa | 0.490 | 7450 | 0.489 | 7460 | 0.487 | 7432 |
| T2D complicationsb | 0.243 | 1499 | 0.245 | 1574 | 0.244 | 1533 |
| Other clinical eventsc | N/A | 13,518 | N/A | 13,572 | N/A | 13,544 |
| Intervention | – | 257 | 322 | |||
| Total cost | 24,489 | 24,994 | 24,907 | |||
| Non-South Asian population | ||||||
| T2D | 0.461 | 1522 | 0.471 | 1600 | 0.461 | 1548 |
| CVD eventsa | 0.407 | 8495 | 0.405 | 8491 | 0.403 | 8465 |
| T2D complicationsb | 0.211 | 1318 | 0.213 | 1371 | 0.211 | 1328 |
| Other clinical eventsc | 11,159 | 11,192 | 11,175 | |||
| Intervention | – | 257 | 322 | |||
| Total cost | 22,494 | 22,910 | 22,838 | |||
Intervention cost threshold analyses
Table 24 shows the results of the intervention cost threshold analysis. Walking Away Plus would need to be delivered at a cost of £116 per person to be cost-effective at a threshold of £20,000 per QALY. Because Walking Away is less effective than usual care, it would have to be less expensive than usual care by £370 to be cost-effective at £20,000 per QALY.
| Intervention | Mean value of incremental QALYs (£) | Mean incremental costs (excluding intervention cost) (£) | Maximum cost of intervention for cost-effectiveness (£) |
|---|---|---|---|
| Walking Away | –206 | 164 | –370 |
| Walking Away Plus | 141 | 25 | 116 |
Value of information
The total per-person EVPI was found to be £279.56. Assuming that the maximum number of interventions that would be delivered per year was 100,000, and that the decision relevance horizon was 10 years, the total EVPI for the UK is £279,559,484. This can be interpreted as the total value to the UK of research to eliminate all uncertainty about the cost-effectiveness of Walking Away and Walking Away Plus.
The expected value of perfect parameter information (EVPPI) for the key groups of parameters taken from the PROPELS trial are presented in Table 25 and Figure 19. The sources of uncertainty that have the largest potential impact on the decision are the parameters required to predict HbA1c and T2D diagnoses.
| Parameter group | Per-person EVPPI (£) | Whole population (n = 100,000 per annum)a | Non-South Asian population (n = 94,700 per annum) | South Asian population (n = 5300 per annum) | ||
|---|---|---|---|---|---|---|
| EVPPI for the UK over 10 years (£M) | Per-person EVPPI (£) | EVPPI for the UK over 10 years (£M) | Per-person EVPPI (£) | EVPPI for the UK over 10 years (£M) | ||
| Total EVPI | 280 | 279.6 | 298.90 | 283.1 | 182.80 | 9.7 |
| HbA1c overall | 162 | 161.6 | 193.25 | 183.0 | 89.37 | 4.7 |
| HbA1c at 12 months | 36 | 136.1 | 149.46 | 141.5 | 68.19 | 3.6 |
| HbA1c at 48 months | 154 | 153.6 | 180.43 | 170.9 | 46.34 | 2.5 |
| T2D diagnoses overall | 161 | 161.1 | 159.82 | 151.3 | 51.10 | 2.7 |
| T2D diagnoses at 12 months | 111 | 110.7 | 123.26 | 116.7 | 4.24 | 0.2 |
| T2D diagnoses at 48 months | 52 | 52.0 | 55.71 | 52.8 | 15.44 | 0.8 |
| Step count overall | 135 | 135.1 | 167.32 | 158.5 | 71.08 | 3.8 |
| Step count at 12 months | 95 | 95.0 | 145.04 | 137.4 | 62.58 | 3.3 |
| Step count at 48 months | 121 | 120.6 | 126.57 | 119.9 | 41.53 | 2.2 |
FIGURE 19.
The EVPPI of parameters gathered from PROPELS data.
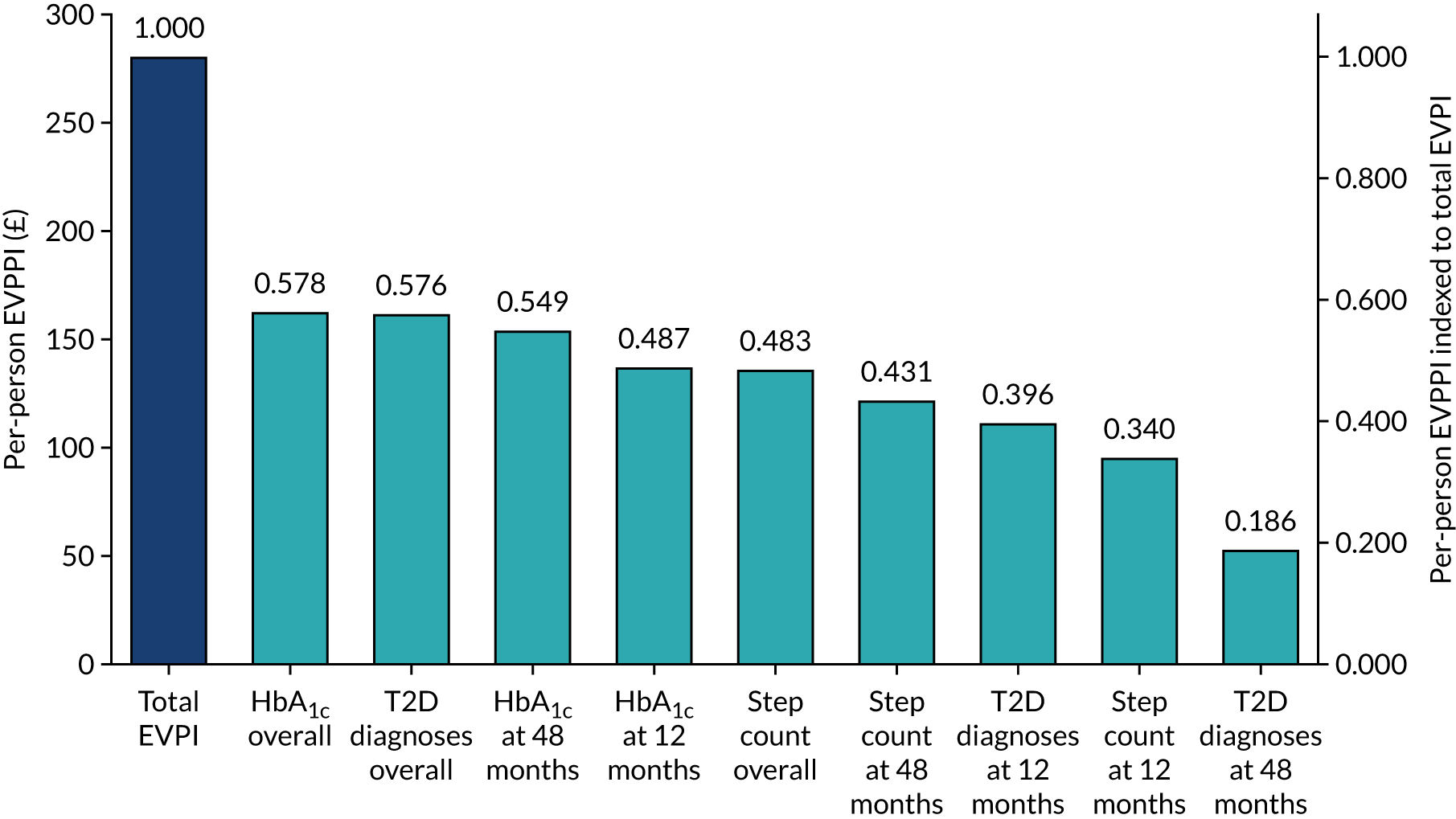
Chapter 7 Discussion and conclusions
The PROPELS study found that a pragmatic, 3-hour group-based behavioural intervention, when combined with tailored text messages and telephone calls, can be used to initiate increased ambulatory activity of over 500 steps per day during the first 12 months of intervention delivery in people with prediabetes in primary care. However, the effects were not sustained at 48-month follow-up and the results were consistent across ethnic groups. Secondary outcomes were also largely unchanged. However, there were small but sustained reductions in triglycerides in the Walking Away Plus study arm, as well as in body weight and waist circumference in the Walking Away study arm, which were accompanied by improvements in liver function at 48 months.
It is increasingly recognised that even small changes to physical activity may have important health benefits. It has recently been proposed, using a triangulation of different data sources, that 500 steps per day or 5–6 minutes of brisk walking is the minimum clinically meaningful difference in ambulatory activity, conveying a relative reduction in the risk of cardiovascular disease and all-cause mortality of between 2% and 9% for inactive populations or a difference in life expectancy of around 4 years. 126 These findings are reflected in the recently updated Physical Activity Guidelines for Americans,127 which acknowledges that even 5 minutes of moderate-intensity physical activity – equivalent to around 500 steps of brisk walking – has health benefits. The increase in ambulatory activity seen in the Walking Away Plus study arm relative to controls at 12 months, although modest, is, therefore, likely to be clinically meaningful.
Although, in this study, changes to physical activity in the Walking Away Plus study arm were not accompanied by changes to most of the assessed cardiometabolic risk factors, triglycerides were reduced, with the effects sustained after 48 months. Triglycerides are one of most consistently observed lipid profile factors that are associated with levels of physical activity. 128 The change in triglycerides (0.15 mmol/l) observed in the Walking Away Plus study arm at 12 months has previously been associated with a 2% reduction in the relative risk of CVD-related mortality. 129
Although there was no evidence of changes in objectively assessed physical activity at 48 months, there was an increase in self-reported energy expenditure of 4.4 (97.5% CI 0.0 to 8.8) kJ/kg/day. It has previously been reported that each additional 1 kJ/kg/day per year increase in physical activity energy expenditure (equivalent to the 4 kJ/kg/day increase seen in the Walking Away Plus study arm seen over 4 years) is associated with a 24% reduction in all-cause mortality, a 29% reduction in cardiovascular disease mortality and an 11% reduction in cancer mortality. 130 Although the objective measures of physical activity suggest that overall physical activity volume was no different between the study arms at 48 months, the context in which the activity occurred may have varied. Objective measures of physical activity record all movement, including movement undertaken in incidental habitual activities, such as household chores, shopping and walking between meetings at work. Self-reported questionnaires, on the other hand, are more likely to capture purposeful physical activity that the individual has cognitively assigned as physical activity, such as choosing to go for a walk. Therefore, participants in the Walking Away Plus study arm may have engaged in greater amounts of structured physical activity. This would be consistent with the findings that self-monitoring behaviours continued to be well adhered to after 48 months in the Walking Away Plus study arm, with over three-quarters of participants reporting that they set themselves physical activity goals and almost two-thirds reporting using a pedometer at least some of the time. Nevertheless, the high levels of self-reported energy expenditure could also be a result of response bias. The added health benefits of undertaking greater levels of self-reported physical activity, without accompanying changes to objectively assessed physical activity, are, therefore, uncertain and require further research.
To our knowledge, this is the first trial designed to assess the effectiveness of a pragmatic physical activity intervention over 4 years in a population with prediabetes. However, the results are consistent with other recent pragmatic physical activity trials that were published after the commencement of PROPELS (see Appendix 16, Table 74).
A previous evaluation of the standard Walking Away programme in those with risk factors for T2D demonstrated an increase in ambulatory activity of 411 steps per day at 12 months, but with no longer-term effects observed at 24 or 36 months. 28 Similarly, the Let’s Prevent Programme, which incorporated the key components of Walking Away into a broader lifestyle diabetes prevention programme, demonstrated an increase in ambulatory activity of 552 steps per day in the intervention study arm compared with the control study arm at 12 months, with an increase of 535 steps maintained at 36 months. 131 A clinician-led physical activity intervention in those with established T2D reported similar findings to the Walking Away-based prevention studies, with a 6.8-minutes-per-day increase in moderate to vigorous physical activity after 12 months, but with effects reducing to 3.6 minutes after 36 months. The PACE-UP pedometer intervention for inactive adults demonstrated increases in ambulatory activity of between 600 and 700 steps per day over 36 months, but the effect for ambulatory activity was not sustained when the intervention was evaluated in older adults aged 60–75 years over 48 months. 132 The recently published EuroFit intervention, which employed a more intensive intervention structure than Walking Away, reported a 678 steps/day increase in the intervention study arm, relative to controls after 12 months in overweight and obese men. 133 These studies are in contrast to the earlier ProActive intervention, which found no effect after 12 months of a behavioural intervention designed to increase physical activity in those with a family history of diabetes,134 while the Early ACTID dietitian- and nurse-led physical activity and dietary intervention for those with T2D reported a 5.6-minute increase in moderate or vigorous physical activity after 12 months relative to standard care. 135
This mounting evidence from trials evaluating pragmatic physical activity interventions with objective measures of physical activity are also consistent with the wider diabetes prevention and management literature. An earlier review of the seminal diabetes prevention programmes concluded that either no or only small changes to physical activity were seen when the trials were evaluated using self-reported physical activity. Even the highly intensive LookAHEAD lifestyle intervention for those with T2D, which was designed to maximise behaviour change rather than to be implemented into routine care, reported a difference of only 4 minutes per day in moderate to vigorous physical activity after 4 years in the intervention study arm compared with the control arm, with no difference seen in the overall volume of physical activity undertaken. 136 Taken together, these results suggest that small, but nevertheless clinically meaningful, increases in physical activity are possible at 12 months after receipt of a behavioural intervention designed for inactive adults or those with metabolic dysfunction within primary care, but that such changes may be difficult for individuals to sustain in the longer term.
The findings from the process measures and embedded qualitative study highlighted important themes that help provide context for the difficulties in promoting sustained physical activity behaviour change. Importantly, the results did not seem to be explained by intervention attendance or engagement with behaviour change techniques. The majority (80%) of those randomised to Walking Away and Walking Away Plus attended the initial group-education session, with 78% of those in the Walking Away Plus arm also registering with the text messaging service. In addition, the interpretation of the results was not affected by a per-protocol analysis, suggesting that engagement with all elements of the intervention did not lead to substantially increased effectiveness. In addition, self-reported rate of engagement with the key behaviour change techniques centred on pedometer use remained relatively high in the intervention arms compared with the control arm, even after 48 months. However, our qualitative research highlighted a key theme whereby major illnesses, injury or life events that occurred over the 4-year trial period in this older population caused large reductions in physical activity levels and the ability to move around freely, from which individuals found it hard to fully recover. This suggests that to be sustained, a focus on rehabilitation from illness or injury could be considered in future physical activity programmes in older populations with a high risk of chronic disease.
As far as we are aware, no longer-term (i.e. longer than 2 years), objectively assessed, physical activity interventions have incorporated mobile technology. A recent review of the literature137 in adults aged ≥ 50 years, reflective of the PROPELS population, identified 52 relevant physical activity, sedentary behaviour or sleep studies that had employed mobile technology, of which text messaging was the most common component. Among these studies, the largest sample size was 710 and the longest follow-up duration was 12 months. The authors concluded that, although there was some evidence for the effectiveness of mobile health interventions, higher-quality trials were needed. PROPELS suggests that incorporating mobile health technologies into a low-resource, pragmatic, group-based behaviour change intervention can moderately enhance the degree of behaviour change after the first 12 months, but it may not help maintain behaviour change in the longer term. Successful behaviour change maintenance has been suggested to require at least one sustained motivator. 113 Whether or not the greater short-term effectiveness in the Walking Away Plus study arm at 12 months helped to provide this sustained motivation is not investigated by the current statistical analysis plan. Future post hoc analysis using the PROPELS data will address these questions further.
Although the Walking Away programme was focused on physical activity, the programme did include a short dietary component that was designed to introduce participants to broad dietary concepts, including macronutrient composition and different types of fats. Participants in both study arms reported making modest dietary changes by increasing fruit and vegetables intake at both 12 and 48 months and actively trying to limit the amount of total and saturated fat consumed on more days of the week. In addition, participants in the Walking Away study arm lost 1 kg in weight and reduced their waist circumference by 1.6 cm compared with those in the control arm at 48 months. Although sustained, these changes were relatively modest, with smaller effects than interventions that are specifically aimed at long-term weight loss. 138 Although the impact of this degree of weight loss on mortality outcomes is uncertain,138 the Diabetes Prevention Programme reported that each additional kilogram of weight loss was associated with a 16% reduction in diabetes risk,139 suggesting that this degree of weight loss may have conferred some cardiometabolic benefits to those in the Walking Away study arm. Indeed, there was evidence that markers of liver function (ALT and ALP) were improved at 48 months in the Walking Away study arm, which is consistent with previous research showing a strong effect of weight loss on markers of liver function. 140 Interestingly, no such changes were observed in the Walking Away Plus study arm, in which markers of weight and adiposity were unchanged compared with control throughout the trial period. In Walking Away Plus, the mHealth follow-on support was specifically focused on physical activity only, which may have acted to deflect a focus on diet.
The real-world costs of delivering Walking Away and Walking Away Plus were estimated as £257 and £322 per person, respectively. The probabilistic lifetime costs of Walking Away and Walking Away Plus (£22,945 and £23,018, respectively) remained higher than those for standard care (£22,598). Lifetime cost-effectiveness modelling suggested that standard care had the highest probability of being cost-effective below a willingness-to-pay threshold of £20,000 per QALY. It was further estimated that, to reach a threshold of £20,000 per QALY, the Walking Away Plus study arm would have to be delivered at a maximum cost of £116 per person. However, there was a high level of uncertainty in these estimates, with the total value to the UK of research to eliminate all uncertainty estimated at £279,559,484. Few other studies have conducted lifetime cost-effectiveness modelling for behavioural interventions. A study141 of brief behavioural interventions suggested that those that incorporated pedometer use, although not cost saving overall, did have a high probability of being cost-effective at a threshold of £20,000 per QALY. However, consistent with our study, the value of eliminating uncertainty was estimated at £1.85B. 141 The PACE-UP study142 found that simply sending pedometers with accompanying motivational material by post was likely to be cost saving over a lifetime.
At the other end of the spectrum, intensive lifestyle interventions for diabetes prevention have also been reported to have a high probability of being cost saving. 143 It is likely that our results differ from these results for three key reasons. First, the only clinical effect of Walking Away Plus was on step count and triglycerides, without associated changes in other variables (e.g. HbA1c and weight), which were included in the brief interventions study. 141 Second, the PROPELS study showed that the effect of Walking Away Plus on physical activity had diminished by 4 years post intervention, so a constant improvement in physical activity was not included in our modelling. Third, the observed data from the PROPELS study suggested that the incidence of diabetes diagnoses was higher, but statistically insignificant, in the Walking Away and Walking Away Plus arms. The relationship between step count and diabetes diagnoses is in the opposite direction of those used the other analyses. 141,142 The inconsistent finding and the high value of eliminating future uncertainly for the cost-effectiveness of physical activity intervention suggest that further research is needed to inform commissioning decisions, especially around any assumed surrogate relationships based on change in physical activity alone and on the duration of effect of any clinical benefit. The within-trial analysis results mirrored these findings (see Appendix 14, Table 70). Although the ICER for Walking Away was estimated at £9639, which is largely consistent with previous group-based interventions for those with or at high risk of diabetes,25,144 there was a high level of uncertainty associated with it, whereas all study conditions had a near equal chance of being cost-effective at a threshold of £20,000 per QALY.
The PROPELS trial has notable strengths and limitations. Its strengths include a population that was predominantly recruited from primary care and had coded HbA1c or glucose values in the prediabetes range, making the population reflective of those referred to currently available diabetes prevention programmes. The large, diverse multiethnic population recruited from urban and rural locations with large variations in social deprivation is also reflective of the modern UK. Indeed, the distribution of material deprivation within our sample almost exactly mirrored the national average, with an equal spread of individuals across nationally defined deprivation quintiles. To the best of our knowledge, PROPELS is also the largest and longest-running physical activity or lifestyle intervention in those with prediabetes that has been evaluated using an objective measure of physical activity.
These strengths also come with potential limitations. The length and the nature (RCT) of the trial may have acted to discourage some from taking part, thereby limiting the generalisability of the sample. For example, the relatively high levels of ambulatory activity and physical activity self-efficacy at baseline may have limited the effectiveness of the intervention in promoting further behaviour change. It is also of note that only around 40% of participants were confirmed to have prediabetes based on HbA1c values at baseline. It has previously been shown that there are high levels of regression to normal glycaemia between paired HbA1c tests when the first is within the prediabetes range,145 with a recommendation that two HbA1c tests are needed to confirm prediabetes status. Along with the possibility of regression to normoglycaemia between measurement and inclusion, it is possible that this relatively small number of participants with prediabetes based on HbA1c was because inclusion was based not only on previous HbA1c values, but also on post-challenge and fasting glucose levels. This is important as it has been shown that HbA1c, fasting glucose and post-challenge values identify discordant high-risk populations. 145,146 However, the fact that only HbA1c was measured within this trial and that participants were informed of their HbA1c status may have influenced the trial results. For example, for individuals whose HbA1c was found to be within normal ranges, this information may have acted to de-emphasise their need for behaviour change. However, the sensitivity analysis for the primary outcome did not find that results were modified by baseline HbA1c prediabetes status, suggesting that similar levels of behaviour change at follow-up were observed in those with and those without prediabetes at baseline. Furthermore, the strategy for HbA1c assessment within this study is consistent with that specified in Healthier You, the NHS Diabetes Prevention Programme, in which the HbA1c assessment has to be repeated by the provider if the original referral value is older than 3 months. 147 However, a normal value on the repeat test is not an exclusion criterion and such individuals are still accepted on the programme.
Nevertheless, the degree of engagement with interventions in this study (52% and 62% compliance with the per-protocol definition of the Walking Away Plus and Walking Away interventions, respectively) is consistent with previous implementation studies. For example, in the DEPLOY study148 conducted in the USA, participants attended 57% of available sessions, and in Finland 56% of individuals reported attending all group-based sessions in the GOAL Implementation Trial. 149 Although using objective measures of physical activity risks biasing the results as a result of the Hawthorn effect (measurement reactivity) and poor compliance, recent evidence has suggested that the Hawthorn effect is likely to be minimal in adults for moderate or vigorous physical activity. 150 In addition, the randomised design makes the results more robust, given that the Hawthorne effect by itself would not be enough to bias results for change; rather, a further assumption would be needed, namely that the Hawthorne effect differed across randomised study arms.
At 48 months, 993 (73%) participants in the sample had valid accelerometer data, which is consistent with other well-conducted RCTs over the longer term (see Appendix 16, Table 74). For example, the PACE-UP study, which recruited 1023 inactive adults from primary care, reported 67% compliance with the primary outcome, namely objectively assessed physical activity, after 36 months. 132 Given that participants in PROPELS were followed up only at 12 and 48 months, the trajectory of change between these time points was not evaluated, making it unclear whether or not the clinically meaningful change in the Walking Away Plus study arm was maintained beyond 12 months. Given that a specific aim of the trial was to explore how best to promote physical activity in South Asian communities, it was disappointing that there was not greater participation by South Asian individuals in the focus groups investigating the uptake and experience of the mHealth intervention. Finally, the rapid and continuing evolution of potential mHealth technologies and the changing landscape in terms of their take-up by the general public complicated the task of selecting the optimal technology for our purposes. Smartphone use became increasingly common during the study; when PROPELS was in development, smartphone ownership was lower. Moreover, access to communications technologies is not distributed equally: lower income households and over 54s continue to be less likely to have smartphones, laptops and tablets. Only mobile telephones and televisions have near-universal reach in the UK (96% of households). This suggested that interventions integrating SMS text messaging technology would have the greatest potential for maximal reach. However, this will change over time and other technologies will need to be considered in the future.
Conclusions
In conclusion, the PROPELS study demonstrated that combining a pragmatic physical activity intervention with text messaging and telephone support can result in modest, but clinically meaningful, changes to ambulatory activity over 12 months, but that such changes were not maintained at 48 months.
The economic evaluation showed that a pragmatic intervention for promoting physical activity to those at risk of T2D was not cost-effective and did not lead to longer-term behaviour change, even when combined with mHealth technology.
Implications for decision-makers
One interpretation of the findings from the PROPELS study suggests that individual-level approaches to physical activity behaviour change may not be effective over the longer term, however good the intervention. It is possible that, in the long term, the environmental drivers of inactivity will always triumph over efforts to address individual motivation unless these are integrated with wider systems-level interventions and policies that target the wider built environment and policies that help make physical activity more accessible, enjoyable and safe.
However, given that the population in this trial was already physical active, which may have limited generalisability to those referred to diabetes prevention programmes within routine care, these findings do not in and of themselves constitute sufficient evidence to support a change in direction for diabetes prevention policy in England or elsewhere. Rather, they support the need for routinely delivered diabetes prevention to be subject to rigorous ongoing evaluation, and for policies to be revised if they are found to have limited longer-term benefits.
Recommendations for future research
Further research is needed to:
-
Investigate which methods for maintaining physical activity behaviour change are effective over the long term in different ethnic populations. This includes investigating which intervention types, components and features – such as behaviour change techniques, real-time support and environmental change – can help maintain physical activity behaviour change and are cost-effective over the longer term and whether or not different solutions are needed for different populations or ethnic groups.
-
Evaluate the long-term effectiveness and cost-effectiveness of routinely delivered national diabetes prevention programmes. Given the results of the PROPELS programme, it is important that research frameworks are wrapped around routinely delivered diabetes prevention programmes to determine their effectiveness and cost-effectiveness over the longer term.
-
Test a stepped prevention programme of initial lifestyle intervention before offering pharmacological interventions (e.g. metformin) to those who do not adhere to or are unable to take up lifestyle interventions.
-
The PROPELS trial demonstrated that up to half of individuals offered a pragmatic behavioural physical activity intervention will not adhere to all elements, with around 20% not attending even the first educational session. This indicates a need to investigate whether or not offering alternative pharmacological therapies to those who cannot or do not want to engage with lifestyle interventions is effective or cost-effective compared with no intervention or either intervention alone.
-
Explore the importance of risk status and risk communication to behaviour change. This includes investigating how feeding back biochemical data in the normal ranges influences participants’ perception of their own risk, and whether or not this discourages behaviour change.
-
Illuminate the importance of integrating rehabilitation from illness or injury as a core intervention component to sustain long-term physical activity behaviour change.
The findings from this qualitative research aspects of this study suggest that future interventions should consider integrating rehabilitation from illness or injury as a core intervention component in order that initial behaviour change success is more resilient to derailment by injury or illnesses and so is better sustained over time.
Acknowledgements
We acknowledge the support and contribution of the study management team who worked tirelessly for the successful completion of this programme of research. We acknowledge the contribution of Rebekka Yates in helping draft and edit the report.
We thank our patient and public involvement groups for working with us for this programme of work. Both the Walking Away and Walking Away Pus interventions evaluated in this trial were developed using iterative development cycles with patient and public involvement embedded throughout. In particular, patient and public involvement facilitated through the NIHR ARC East Midlands (formerly CLAHRC) contributed to how diabetes risk was communicated in Walking Away for use within multi-ethnic populations. The dietary section was also based on patient and public involvement suggesting that the focus on physical activity should be complimented by also communicating the key dietary factors related to diabetes risk. Through the work funded within this grant, the text message content in Walking Away Plus was informed by involving those within the community who had previously attended Walking Away programme. We worked with these groups to explore the types of messaging that may best motivate behaviour change following attending a Walking Away course.
Finally, we would like to acknowledge and thank the research participants, without whom the research could not have taken place.
Contributions of authors
Kamlesh Khunti (https://orcid.org/0000-0003-2343-7099) (Professor of Primary Care Diabetes and Vascular Medicine, Chief Investigator) conceived or designed the work and was involved in the interpretation of data.
Simon Griffin (https://orcid.org/0000-0002-2157-4797) (Professor of General Practice) was involved in the interpretation of data.
Alan Brennan (https://orcid.org/0000-0002-1025-312X) (Professor of Health Economics and Decision Modelling) was involved in the analysis and the interpretation of data.
Helen Dallosso (https://orcid.org/0000-0002-6732-0864) (Senior Research Associate) was involved in the acquisition of data for the work.
Melanie Davies (https://orcid.org/0000-0002-9987-9371) (Professor of Diabetes Medicine) conceived or designed the work.
Helen Eborall (https://orcid.org/0000-0002-6023-3661) (Lecturer in Social Science Applied to Health) conceived or designed the work, was involved in intervention development, training and quality assurance, and acted as lead author for Chapter 5.
Charlotte Edwardson (https://orcid.org/0000-0001-6485-9330) (Associate Professor in Physical Activity, Sedentary Behaviour and Health) was involved in the acquisition of data for the work, and in the analysis of data.
Laura Gray (https://orcid.org/0000-0002-9284-9321) (Professor of Medical Statistics) conceived or designed the work.
Wendy Hardeman (https://orcid.org/0000-0002-6498-9407) (Senior Lecturer in Health Psychology) conceived or designed the work and was involved in intervention development, training and quality assurance.
Laura Heathcote (https://orcid.org/0000-0001-8063-7447) (Health Economics and Decision Scientist) was involved in the analysis of data and the interpretation of data, and acted as lead author for Chapter 6.
Joseph Henson (https://orcid.org/0000-0002-3898-7053) (Research Associate) was involved in the analysis of data.
Katie Morton (https://orcid.org/0000-0002-9961-6491) (Health-related Behaviour Change Researcher) was involved in intervention development, training and quality assurance.
Daniel Pollard (https://orcid.org/0000-0001-5630-0115) (Health Economics and Decision Scientist) was involved in the analysis and the interpretation of data.
Stephen Sharp (https://orcid.org/0000-0003-2375-1440) (Senior Statistician) was involved in the analysis of data.
Stephen Sutton (https://orcid.org/0000-0003-1610-0404) (Professor of Behavioural Science) conceived or designed the work.
Jacqui Troughton (https://orcid.org/0000-0003-3690-9534) (Senior Dietitian and Structured Education Specialist) was involved in intervention development, training and quality assurance.
Thomas Yates (https://orcid.org/0000-0002-5724-5178) (Professor of Physical Activity, Sedentary Behaviour and Health) conceived or designed the work, was involved in the analysis and the interpretation of data, was involved in intervention development, training and quality assurance, and acted as lead author for Chapters 1–4 and 7.
All authors were involved in final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publications
Yates T, Griffin S, Bodicoat DH, Brierly G, Dallosso H, Davies MJ, et al. PRomotion Of Physical activity through structured Education with differing Levels of ongoing Support for people at high risk of type 2 diabetes (PROPELS): study protocol for a randomized controlled trial. Trials 2015;16:289.
Khunti K, Griffin, S, Brennan A, Dallosso H, Davies MJ, Eborall HC, et al. Promoting physical activity in a multi-ethnic population at high risk of diabetes: the 48-month PROPELS randomised controlled trial. BMC Med 2021;19:130.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration; access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Yates T, Griffin S, Bodicoat DH, Brierly G, Dallosso H, Davies MJ, et al. PRomotion Of Physical activity through structured Education with differing Levels of ongoing Support for people at high risk of type 2 diabetes (PROPELS): study protocol for a randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0813-z.
- IDF Diabetes Atlas 2011.
- Leung MY, Pollack LM, Colditz GA, Change SH. Life years lost and lifetime health care expenditures associated with diabetes in the United States. Value Health 2014;17:A245-6. https://doi.org/10.1016/j.jval.2014.03.1433.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO IDF Consultation 2006.
- National Institute for Health and Care Excellence (NICE) . Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk 2012.
- International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-34. https://doi.org/10.2337/dc09-9033.
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279-90. https://doi.org/10.1016/S0140-6736(12)60283-9.
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334. https://doi.org/10.1136/bmj.39063.689375.55.
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783-9. https://doi.org/10.1016/S0140-6736(08)60766-7.
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677-86. https://doi.org/10.1016/S0140-6736(09)61457-4.
- Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673-9. https://doi.org/10.1016/S0140-6736(06)69701-8.
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. https://doi.org/10.1136/bmj.39545.585289.25.
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922-33. https://doi.org/10.2337/dc13-2195.
- Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 2018;41:1526-34. https://doi.org/10.2337/dc17-2222.
- Valabhji J, Barron E, Bradley D, Bakhai C, Fagg J, O’Neill S, et al. Early outcomes from the English National Health Service Diabetes Prevention Program. Diabetes Care 2020;43:152-60. https://doi.org/10.2337/dc19-1425.
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 2005;99:1193-204. https://doi.org/10.1152/japplphysiol.00160.2005.
- Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744-52. https://doi.org/10.2337/dc06-1842.
- Laaksonen DE, Lindström J, Lakka TA, Eriksson JG, Niskanen L, Wikström K, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 2005;54:158-65. https://doi.org/10.2337/diabetes.54.1.158.
- Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet 2014;383:1059-66. https://doi.org/10.1016/S0140-6736(13)62061-9.
- McCarthy M, Edwardson CL, Davies MJ, Henson J, Gray L, Khunti K, et al. Change in sedentary time, physical activity, bodyweight, and Hba1c in high-risk adults. Med Sci Sports Exerc 2017;49:1120-5. https://doi.org/10.1249/MSS.0000000000001218.
- Yates T, Khunti K, Bull F, Gorely T, Davies MJ. The role of physical activity in the management of impaired glucose tolerance: a systematic review. Diabetologia 2007;50:1116-26. https://doi.org/10.1007/s00125-007-0638-8.
- National Institute for Health and Care Excellence (NICE) . Guidance on the Use of Patient-Education Models for Diabetes 2003.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4093.
- Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, Yates T, et al. Let’s prevent diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovasc Diabetol 2012;11. https://doi.org/10.1186/1475-2840-11-56.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10. https://doi.org/10.2337/dc09-0130.
- Yates T, Edwardson CL, Henson J, Gray LJ, Ashra NB, Troughton J, et al. Walking Away from Type 2 diabetes: a cluster randomized controlled trial. Diabet Med 2017;34:698-707. https://doi.org/10.1111/dme.13254.
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert H, Prevost AT, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text- based facilitation of smoking cessation in primary care (iQuit in Practice). Addiction 2014;109:1184-93. https://doi.org/10.1111/add.12556.
- Lin PH, Wang Y, Levine E, Askew S, Lin S, Chang C, et al. A text messaging-assisted randomized lifestyle weight loss clinical trial among overweight adults in Beijing. Obesity 2014;22:E29-37. https://doi.org/10.1002/oby.20686.
- Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013;1:191-8. https://doi.org/10.1016/S2213-8587(13)70067-6.
- Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ. Randomised trial of text messaging on adherence to cardiovascular preventive treatment (INTERACT trial). PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0114268.
- Chakranon P, Lai YK, Tang YW, Choudhary P, Khunti K, Lee SWH. Distal technology interventions in people with diabetes: an umbrella review of multiple health outcomes. Diabet Med 2020;37:1966-76. https://doi.org/10.1111/dme.14156.
- Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database Syst Rev 2012;12. https://doi.org/10.1002/14651858.CD007457.pub2.
- Baskerville R, Ricci-Cabello I, Roberts N, Farmer A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2017;34:612-20. https://doi.org/10.1111/dme.13331.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. https://doi.org/10.1001/jama.298.19.2296.
- Barnett AH, Dixon AN, Bellary S, Hanif MW, O’hare JP, Raymond NT, et al. Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia 2006;49:2234-46. https://doi.org/10.1007/s00125-006-0325-1.
- Fischbacher CM, Hunt S, Alexander L. How physically active are South Asians in the United Kingdom? A literature review. J Public Health 2004;26:250-8. https://doi.org/10.1093/pubmed/fdh158.
- Yates T, Davies MJ, Gray LJ, Webb D, Henson J, Gill JM, et al. Levels of physical activity and relationship with markers of diabetes and cardiovascular disease risk in 5474 white European and South Asian adults screened for type 2 diabetes. Prev Med 2010;51:290-4. https://doi.org/10.1016/j.ypmed.2010.06.011.
- Ghouri N, Purves D, McConnachie A, Wilson J, Gill JM, Sattar N. Lower cardiorespiratory fitness contributes to increased insulin resistance and fasting glycaemia in middle-aged South Asian compared with European men living in the UK. Diabetologia 2013;56:2238-49. https://doi.org/10.1007/s00125-013-2969-y.
- Williams ED, Stamatakis E, Chandola T, Hamer M. Physical activity behaviour and coronary heart disease mortality among South Asian people in the UK: an observational longitudinal study. Heart 2011;97:655-9. https://doi.org/10.1136/hrt.2010.201012.
- Bhopal RS, Douglas A, Wallia S, Forbes JF, Lean ME, Gill JM, et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family-cluster randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:218-27. https://doi.org/10.1016/S2213-8587(13)70204-3.
- Gillett M, Brennan A, Watson P, Khunti K, Davies M, Mostafa S, et al. The cost-effectiveness of testing strategies for type 2 diabetes: a modelling study. Health Technol Assess 2015;19.
- Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 2012;55:959-66. https://doi.org/10.1007/s00125-011-2432-x.
- Medical Research Council Epidemiology Unit, University of Cambridge . Fenland Study: Information for Researchers n.d. www.mrc-epid.cam.ac.uk/research/studies/fenland/information-for-researchers/ (accessed 19 November 2021).
- Leicester City Council . Diversity and Migration: Community Insights Including Facts from Census 2011 2012.
- World Health Organization . Use of Glycatedhaemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation 2011. www.who.int/diabetes/publications/diagnosis_diabetes2011/en/ (accessed 2 August 2016).
- National Institute for Health and Care Excellence (NICE) . Type 2 Diabetes: Prevention in People at High Risk 2012.
- Leventhal H, Meyer D, Nerenz D. Contributions to Medical Psychology. New York, NY: Pergamon Press; 1980.
- Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US adults. Med Sci Sports Exerc 2009;41:1384-91. https://doi.org/10.1249/MSS.0b013e318199885c.
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. https://doi.org/10.1097/00005768-199805000-00021.
- UK Chief Medical Officer (CMO) Guidelines Writing Group . UK Chief Medical Officers’ Physical Activity Guidelines 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed 17 November 2021).
- Besson H, Brage S, Jakes RW, Ekelund U, Wareham NJ. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am J Clin Nutr 2010;91:106-14. https://doi.org/10.3945/ajcn.2009.28432.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
- Gopal DP, Usher-Smith JA. Cardiovascular risk models for South Asian populations: a systematic review. Int J Public Health 2016;61:525-34. https://doi.org/10.1007/s00038-015-0733-4.
- Panter J, Jones AP, van Sluijs EMF, Griffin SJ, Wareham NJ. Environmental and psychological correlates of older adult’s active commuting. Med Sci Sports Exerc 2011;43:1235-43. https://doi.org/10.1249/MSS.0b013e3182078532.
- McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477-90. https://doi.org/10.1056/NEJMoa1001121.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- Ware JE. How to Score and Interpret Single-item Health Status Measures: A Manual for Users of the SF-8 Health Survey: (With a Supplement on the SF-6 Health Survey). Lincoln: RI: QualityMetric, Inc.; 2001.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Sniehotta FF, Nagy G, Scholz U, Schwarzer R. The role of action control in implementing intentions during the first weeks of behaviour change. Br J Social Psychol 2006;45:87-106. https://doi.org/10.1348/014466605X62460.
- Tudor-Locke C, Bassett DR. How many steps/day are enough?. Sports Med 2004;34:1-8. https://doi.org/10.2165/00007256-200434010-00001.
- Chan CB, Ryan DA, Tudor-Locke C. Health benefits of a pedometer-based physical activity intervention in sedentary workers. Prev Med 2004;39:1215-22. https://doi.org/10.1016/j.ypmed.2004.04.053.
- Merom D, Rissel C, Phongsavan P, Smith BJ, Van Kemenade C, Brown WJ, et al. Promoting walking with pedometers in the community: the step-by-step trial. Am J Prev Med 2007;32:290-7. https://doi.org/10.1016/j.amepre.2006.12.007.
- Yates T, Gray LJ, Henson J, Edwardson CL, Khunti K, Davies MJ. Impact of depression and anxiety on change to physical activity following a pragmatic diabetes prevention program within primary care: pooled analysis from two randomized controlled trials. Diabetes Care 2019;42:1847-53. https://doi.org/10.2337/dc19-0400.
- Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366. https://doi.org/10.1136/bmj.l4570.
- Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, et al. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose. Evid Rep Technol Assess 2005;128:1-11.
- White IR, Carpenter J, Horton NJ. Including all individuals is not enough: lessons for intention-to-treat analysis. Clin Trials 2012;9:396-407. https://doi.org/10.1177/1740774512450098.
- Breeze PR, Thomas C, Squires H, Brennan A, Greaves C, Diggle P, et al. SPHR Diabetes Prevention Model: Detailed Description of Model Background, Methods, Assumptions and Parameters. Sheffield: School of Health and Related Research, University of Sheffield; 2015.
- NHS Improvement . Reference Costs n.d. www.england.nhs.uk/national-cost-collection/ (accessed November 2021).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Breeze PR, Thomas C, Squires H, Brennan A, Greaves C, Diggle PJ, et al. The impact of Type 2 diabetes prevention programmes based on risk-identification and lifestyle intervention intensity strategies: a cost-effectiveness analysis. Diabet Med 2017;34:632-40. https://doi.org/10.1111/dme.13314.
- NHS Digital . Health Survey for England 2014 2015.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 17 December 2019).
- Department of Health and Social Care . NHS Reference Costs n.d. www.england.nhs.uk/national-cost-collection/ (accessed 17 December 2019).
- Dolan P, Gudex C, Kind P, Williams A. Working Papers. York: Centre for Health Economics, University of York; 1995.
- Yates T, Davies MJ, Henson J, Troughton J, Edwardson C, Gray LJ, et al. Walking away from type 2 diabetes: trial protocol of a cluster randomised controlled trial evaluating a structured education programme in those at high risk of developing type 2 diabetes. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-46.
- Bandura A. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Pearson; 1986.
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. Am Psychol 1999;54. https://doi.org/10.1037/0003-066X.54.7.493.
- Leventhal H, Meyer D, Nerenz DR. The common sense representation of illness danger. Contributions to Medical Psychology 1980;2:7-30.
- Chaiken S, Zanna MP, Olson JM, Herman CP. Social Influence: The Ontario Symposium, Volume 5. Hillsdale, NJ: Lawrence Erlbaum; 1987.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- National Institute for Health and Care Excellence (NICE) . Preventing Type 2 Diabetes: Population and Community-Level Interventions 2012.
- Fonteyn ME, Kuipers B, Grobe SJ. A description of think aloud method and protocol analysis. Qual Health Res 1993;3:430-41. https://doi.org/10.1177/104973239300300403.
- Abras C, Maloney-Krichmar D, Preece J. Encyclopedia of Human-Computer Interaction. Thousand Oaks, CA: SAGE Publications Ltd; 2004.
- Dijkstra A, De Vries H. The development of computer-generated tailored interventions. Patient Educ Couns 1999;36:193-20. https://doi.org/10.1016/S0738-3991(98)00135-9.
- Whittaker R, Merry S, Dorey E, Maddison R. A development and evaluation process for mHealth interventions: examples from New Zealand. J Health Commun 2012;17:11-2. https://doi.org/10.1080/10810730.2011.649103.
- Fjeldsoe BS, Miller YD, O’Brien JL, Marshall AL. Iterative development of MobileMums: a physical activity intervention for women with young children. Int J Behav Nutr Phys Act 2012;9. https://doi.org/10.1186/1479-5868-9-151.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Fanning J, Mullen SP, McAuley E. Increasing physical activity with mobile devices: a meta-analysis. J Med Internet Res 2012;14. https://doi.org/10.2196/jmir.2171.
- Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis 2009;4:301-13. https://doi.org/10.2147/COPD.S6643.
- Hurling R, Catt M, Boni MD, Fairley BW, Hurst T, Murray P, et al. Using internet and mobile phone technology to deliver an automated physical activity program: randomized controlled trial. J Med Internet Res 2007;9. https://doi.org/10.2196/jmir.9.2.e7.
- Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med 2013;97:41-8. https://doi.org/10.1016/j.socscimed.2013.08.003.
- Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care 2009;32:813-15. https://doi.org/10.2337/dc08-1974.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Carver CS, Scheier MF. On the Self-regulation of Behavior. Cambridge: Cambridge University Press; 2001.
- Morton K, Sutton S, Hardeman W, Troughton J, Yates T, Griffin S, et al. A text-messaging and pedometer program to promote physical activity in people at high risk of type 2 diabetes: the development of the PROPELS follow-on support program. JMIR Mhealth Uhealth 2015;3. https://doi.org/10.2196/mhealth.5026.
- Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care 2012;35:2681-9. https://doi.org/10.2337/dc11-2452.
- Hankonen N, Sutton S, Prevost AT, Simmons RK, Griffin SJ, Kinmonth AL, et al. Which behavior change techniques are associated with changes in physical activity, diet and body mass index in people with recently diagnosed diabetes?. Ann Behav Med 2014;49:7-17. https://doi.org/10.1007/s12160-014-9624-9.
- Hagger M, Chatzisarantis NLD, Biddle SJH. A meta-analytic review of the theories of reasoned action and planned behavior in physical activity: predictive validity and the contribution of additional variables. J Sport Exercise Psychol 2002;24:3-32. https://doi.org/10.1123/jsep.24.1.3.
- Vallerand RJ, Hagger MS, Chatzisarantis NLD. Intrinsic Motivation and Self-determination in Exercise and Sport. Washington, DC: American Psychological Association; 2007.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. https://doi.org/10.1037//0033-295x.84.2.191.
- Anderson ES, Wojcik JR, Winett RA, Williams DM. Social-cognitive determinants of physical activity: the influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychol 2006;25:510-20. https://doi.org/10.1037/0278-6133.25.4.510.
- Charmaz K. Constructing Grounded Theory. Thousand Oaks, CA: SAGE Publications Ltd; 2014.
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575-81. https://doi.org/10.1249/MSS.0b013e31821ece12.
- Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. ‘Educator talk’ and patient change: some insights from the DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed) randomized controlled trial. Diabet Med 2008;25:1117-20. https://doi.org/10.1111/j.1464-5491.2008.02492.x.
- Khunti K, Griffin S, Brennan A, Dallosso H, Davies MJ, Eborall HC, et al. Promoting physical activity in a multi-ethnic population at high risk of diabetes: the 48-month PROPELS randomised controlled trial. BMC Med 2021;19.
- Brewer JT. Ethnography (Understanding Social Research). Philadelphia, PA: Open University Press; 2000.
- Wolfinger NH. On writing fieldnotes: collection strategies and background expectancies. Qual Res 2002;2:85-93. https://doi.org/10.1177/1468794102002001640.
- Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev 2016;10:277-96. https://doi.org/10.1080/17437199.2016.1151372.
- Winkley K, Upsher R, Stahl D, Pollard D, Gillet M, Brennan A, et al. A systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in adults with Type 2 diabetes. Diabetic Med 2018;35:16-. https://doi.org/10.2139/ssrn.3413604.
- Breeze P, Squires H, Chilcott J, Stride C, Diggle PJ, Brunner E, et al. A statistical model to describe longitudinal and correlated metabolic risk factors: the Whitehall II prospective study. J Public Health 2016;38:679-87. https://doi.org/10.1093/pubmed/fdv160.
- Thomas C, Sadler S, Breeze P, Squires H, Gillett M, Brennan A. Assessing the potential return on investment of the proposed UK NHS diabetes prevention programme in different population subgroups: an economic evaluation. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014953.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82. https://doi.org/10.1136/bmj.39609.449676.25.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Regional Drug and Therapeutics Centre (RDTC) (Newcastle) . Cost Comparisons Charts 2019.
- NHS Digital . Prescription Cost Analysis – England, 2018 2018.
- Hernandez Alava M, . Methods for Mapping Between the EQ-5D-5L and the 3L for Technology Appraisal: Report by the Decision Support Unit 2017.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Henningsen A, Hamann J. Systemfit: a package for estimating systems of simultaneous equations. J Stat Software 2007;23:1-40. https://doi.org/10.18637/jss.v023.i04.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. USA: John Wiley & Sons; 1987.
- Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251-6. https://doi.org/10.1093/ije/dyh372.
- Rowlands A, Davies M, Dempsey P, Edwardson C, Razieh C, Yates T. Wrist-worn accelerometers: recommending ∼1.0 mg as the minimum clinically important difference (MCID) in daily average acceleration for inactive adults. Br J Sports Med 2021;55:814-15. https://doi.org/10.1136/bjsports-2020-102293.
- US Department of Health and Human Services . Physical Activity Guidelines for Americans 2018.
- Ki M, Pouliou T, Li L, Power C. Physical (in)activity over 20 y in adulthood: associations with adult lipid levels in the 1958 British birth cohort. Atherosclerosis 2011;219:361-7. https://doi.org/10.1016/j.atherosclerosis.2011.07.109.
- Liu J, Zeng FF, Liu ZM, Zhang CX, Ling WH, Chen YM. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis 2013;12. https://doi.org/10.1186/1476-511X-12-159.
- Mok A, Khaw KT, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: population based cohort study. BMJ 2019;365. https://doi.org/10.1136/bmj.l2323.
- Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: The Let’s Prevent Diabetes cluster randomised controlled trial. Prev Med 2016;84:48-56. https://doi.org/10.1016/j.ypmed.2015.12.012.
- Harris T, Kerry SM, Limb ES, Furness C, Wahlich C, Victor CR, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: long-term follow-up of participants from two randomised controlled trials in UK primary care. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002526.
- Wyke S, Bunn C, Andersen E, Silva MN, van Nassau F, McSkimming P, et al. The effect of a programme to improve men’s sedentary time and physical activity: The European Fans in Training (EuroFIT) randomised controlled trial. PLOS Med 2019;16. https://doi.org/10.1371/journal.pmed.1002736.
- Kinmonth AL, Wareham NJ, Hardeman W, Sutton S, Prevost AT, Fanshawe T, et al. Efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care (ProActive UK): a randomised trial. Lancet 2008;371:41-8. https://doi.org/10.1016/S0140-6736(08)60070-7.
- Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011;378:129-39. https://doi.org/10.1016/S0140-6736(11)60442-X.
- Unick JL, Gaussoin SA, Hill JO, Jakicic JM, Bond DS, Hellgren M, et al. Four-year physical activity levels among intervention participants with type 2 diabetes. Med Sci Sports Exerc 2016;48:2437-45. https://doi.org/10.1249/MSS.0000000000001054.
- Elavsky S, Knapova L, Klocek A, Smahel D. Mobile health interventions for physical activity, sedentary behavior, and sleep in adults aged 50 years and older: a systematic literature review. J Aging Phys Act 2019;27:565-93. https://doi.org/10.1123/japa.2017-0410.
- Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029966.
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102-7. https://doi.org/10.2337/dc06-0560.
- Uslan I, Acarturk G, Karaca E, Albayrak R, Yuksel S, Colbay M, et al. The effects of weight loss on normal transaminase levels in obese patients. Am J Med Sci 2007;334:327-30. https://doi.org/10.1097/MAJ.0b013e3181557702.
- Gc VS, Suhrcke M, Hardeman W, Sutton S, Wilson ECF. Very Brief Interventions Programme Team . Cost-effectiveness and value of information analysis of brief interventions to promote physical activity in primary care. Value Health 2018;21:18-26. https://doi.org/10.1016/j.jval.2017.07.005.
- Anokye N, Fox-Rushby J, Sanghera S, Cook DG, Limb E, Furness C, et al. Short-term and long-term cost-effectiveness of a pedometer-based exercise intervention in primary care: a within-trial analysis and beyond-trial modelling. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-021978.
- Breeze PR, Thomas C, Squires H, Brennan A, Greaves C, Diggle P, et al. Cost-effectiveness of population-based, community, workplace and individual policies for diabetes prevention in the UK. Diabet Med 2017;34:1136-44. https://doi.org/10.1111/dme.13349.
- Leal J, Ahrabian D, Davies MJ, Gray LJ, Khunti J, Yates T, et al. Cost-effectiveness of a pragmatic structured education intervention for the prevention of type 2 diabetes: economic evaluation of data from the Let’s Prevent Diabetes cluster-randomised controlled trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013592.
- Sampson M, Elwell-Sutton T, Bachmann MO, Clark A, Dhatariya KK, Ferns C, et al. Discordance in glycemic categories and regression to normality at baseline in 10,000 people in a Type 2 diabetes prevention trial. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-24662-y.
- Mostafa SA, Khunti K, Srinivasan BT, Webb D, Gray LJ, Davies MJ. The potential impact and optimal cut-points of using glycated haemoglobin, HbA1c, to detect people with impaired glucose regulation in a UK multi-ethnic cohort. Diabetes Res Clin Pract 2010;90:100-8. https://doi.org/10.1016/j.diabres.2010.06.008.
- NHS England and NHS Improvement . Diabetes Prevention Programme Information Governance and Data Flows Framework 2016. www.england.nhs.uk/wp-content/uploads/2019/09/diabetes-prevention-programme-information-governance-and-data-flows-framework.pdf (accessed 17 November 2021).
- Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY pilot study. Am J Prev Med 2008;35:357-63. https://doi.org/10.1016/j.amepre.2008.06.035.
- Absetz P, Valve R, Oldenburg B, Heinonen H, Nissinen A, Fogelholm M, et al. Type 2 diabetes prevention in the ‘real world’: one-year results of the GOAL Implementation Trial. Diabetes Care 2007;30:2465-70. https://doi.org/10.2337/dc07-0171.
- Baumann S, Groß S, Voigt L, Ullrich A, Weymar F, Schwaneberg T, et al. Pitfalls in accelerometer-based measurement of physical activity: the presence of reactivity in an adult population. Scand J Med Sci Sports 2018;28:1056-63. https://doi.org/10.1111/sms.12977.
- Balducci S, D’Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 randomized clinical trial. JAMA 2019;321:880-90. https://doi.org/10.1001/jama.2019.0922.
Appendix 1 Statistical analysis plan
Appendix 2 Site-specific participant flow
FIGURE 20.
Participant flow at the Leicester site. Reproduced with permission from Khunti et al. 110 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. The figure includes minor additions and formatting changes to the original figure.
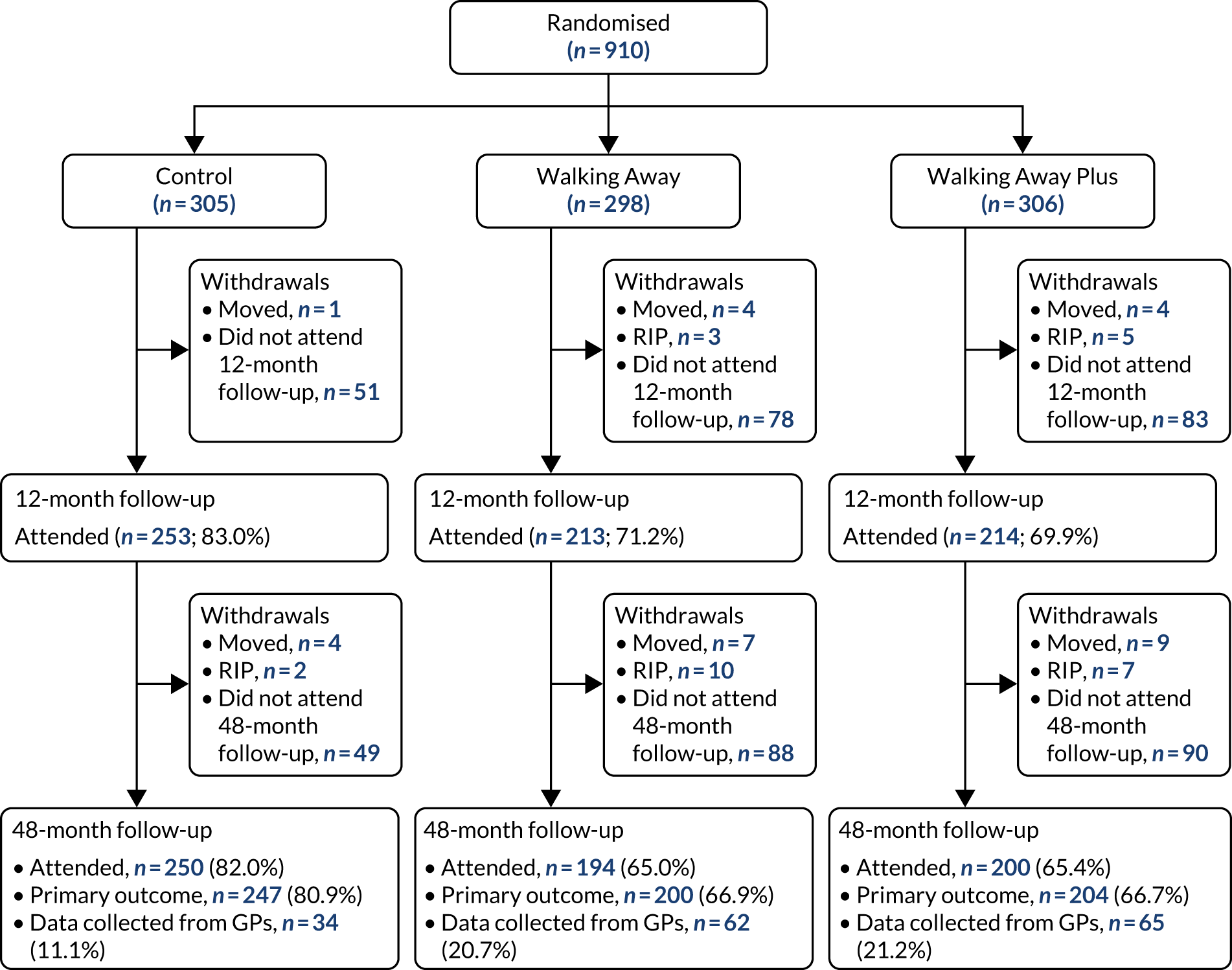
FIGURE 21.
Participant flow at the Cambridge site.
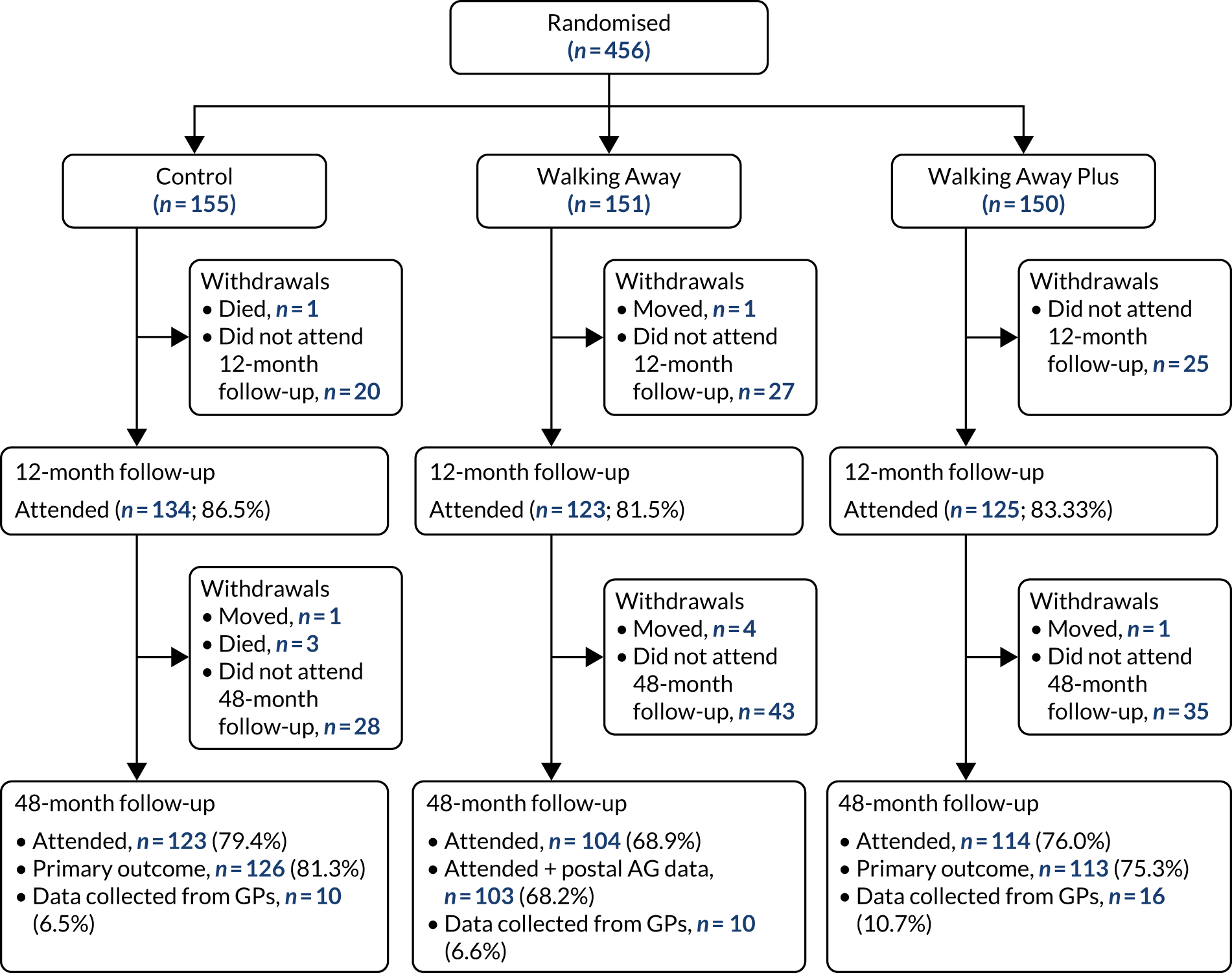
Appendix 3 Summary of PROPELS substantial amendments
| Amendment number | Date of approval | Details of amendment |
|---|---|---|
| 1 | 26 March 2013 |
|
| 2 | 8 August 2013 |
|
| 3 | 26 September 2013 |
|
| 4 | 10 December 2013 |
|
| 5 | 26 March 2014 |
|
| 6 | 20 June 2014 |
|
| 7 | 18 December 2014 |
|
| 8 | 10 March 2015 |
|
| 9 | 9 December 2015 |
|
| 10 | 28 March 2016 |
|
| 11 | 11 August 2016 |
|
| 12 | 14 June 2017 |
|
| 13 | 25 October 2017 |
|
| 14 | 8 January 2019 |
|
Appendix 4 Participants with and without primary outcome data
| Complete data (N = 373) | Missing data (N = 87) | |
|---|---|---|
| Age (years), n; mean (SD) | 373; 59.3 (8.7) | 87; 59.7 (9.1) |
| BMI (kg/m2), n; mean (SD) | 373; 29.3 (5.9) | 87; 29.6 (5.1) |
| Social deprivation (IMD decile) | 373; 5.5 (2.8) | 86; 5.5 (3.0) |
| Sex, % (n) | ||
| Men | 52.8 (197) | 42.5 (37) |
| Women | 47.2 (176) | 57.5 (50) |
| Ethnicity, % (n) | ||
| White European | 70.2 (262) | 74.7 (65) |
| South Asian | 23.1 (86) | 19.5 (17) |
| Other | 6.7 (25) | 5.7 (5) |
| Family history of diabetes in first-degree relatives, % (n/N) | 43.2 (160/370) | 43.7 (38/87) |
| Prediabetes, % (n/N) | 37.2 (138/371) | 39.1 (34/87) |
| Antihypertensive medication, % (n/N) | 42.5 (154/362) | 29.4 (15/51) |
| Lipid lowering medication, % (n/N) | 35.6 (129/362) | 29.4 (15/51) |
| Steroids, % (n) | 5.9 (22) | 13.8 (12) |
| Metformin, % (n) | 0.0 (0) | 0.0 (0) |
| CVD (MI, heart failure, angina, stroke), % (n/N) | 7.9 (29/369) | 11.5 (10/87) |
| Smoking status, % (n) | ||
| Past | 35.7 (133) | 49.4 (43) |
| Current | 9.9 (37) | 9.2 (8) |
| Employment type, % (n) | ||
| Full time | 39.4 (147) | 29.9 (26) |
| Part time | 15.3 (57) | 19.5 (17) |
| Retired | 34.3 (128) | 37.9 (33) |
| Unemployed or other | 11.0 (41) | 12.6 (11) |
| Educational status, % (n/N) | ||
| Degree, higher degree or equivalent | 46.6 (170/365) | 41.7 (35/84) |
| Marital status, % (n) | ||
| Married/civil partner | 69.4 (259) | 63.2 (55) |
| Access to the internet, % (n/N) | 83.4 (311/373) | 81.2 (69/85) |
| Complete data (N = 303) | Missing data (N = 147) | |
|---|---|---|
| Age (years), n; mean (SD) | 303; 59.8 (9.0) | 147; 58.7 (10.1) |
| BMI (kg/m2), n; mean (SD) | 303; 28.9 (5.1) | 147; 29.6 (6.3) |
| Social deprivation (IMD decile), n; mean (SD) | 302; 6.0 (3.0) | 147; 5.1 (3.0) |
| Sex, % (n) | ||
| Men | 52.8 (160) | 45.6 (67) |
| Women | 47.2 (143) | 54.4 (80) |
| Ethnicity, % (n) | ||
| White European | 73.9 (224) | 69.4 (102) |
| South Asian | 20.1 (61) | 25.9 (38) |
| Other | 5.9 (18) | 4.8 (7) |
| Family history of diabetes in first-degree relatives, % (n/N) | 42.5 (128/301) | 40.8 (60/147) |
| Prediabetes, % (n/N) | 41.1 (124/302) | 37.4 (55/147) |
| Antihypertensive medication, % (n/N) | 42.7 (122/286) | 51.2 (42/82) |
| Lipid-lowering medication, % (n/N) | 33.9 (97/286) | 48.8 (40/82) |
| Steroids, % (n) | 8.3 (25) | 10.9 (16) |
| Metformin, % (n) | 0.0 (0) | 0.7 (1) |
| CVD (MI, heart failure, angina, stroke), % (n/N) | 9.0 (27/299) | 8.8 (13/147) |
| Smoking status % (n) | ||
| Past | 36.3 (110) | 36.1 (53) |
| Current | 6.6 (20) | 12.2 (18) |
| Employment type, % (n) | ||
| Full time | 33.7 (102) | 35.4 (52) |
| Part time | 20.8 (63) | 19.7 (29) |
| Retired | 37.0 (112) | 32.0 (47) |
| Unemployed or other | 8.6 (26) | 12.9 (19) |
| Educational status, % (n/N) | ||
| Degree, higher degree or equivalent | 48.3 (141/292) | 39.7 (56/141) |
| Marital status, % (n) | ||
| Married/civil partner | 76.6 (232) | 73.5 (108) |
| Access to the internet, % (n/N) | 89.1 (269/302) | 80.3 (118/147) |
| Complete data (N = 317) | Missing data (N = 139) | |
|---|---|---|
| Age (years), n; mean (SD) | 317; 60.0 (8.5) | 139; 57.8 (10.2) |
| BMI (kg/m2), n; mean (SD) | 317; 29.0 (5.5) | 139; 29.7 (5.8) |
| Social deprivation (IMD decile), n; mean (SD) | 317; 5.9 (2.7) | 139; 5.2 (2.9) |
| Sex, % (n) | ||
| Men | 48.6 (154) | 56.1 (78) |
| Women | 51.4 (163) | 43.9 (61) |
| Ethnicity, % (n) | ||
| White European | 74.4 (236) | 66.9 (93) |
| South Asian | 20.8 (66) | 26.6 (37) |
| Other | 4.7 (15) | 6.5 (9) |
| Family history of diabetes in first-degree relatives, % (n/N) | 44.3 (140/316) | 47.4 (65/137) |
| Prediabetes, % (n/N) | 39.9 (126/316) | 36.0 (50/139) |
| Antihypertensive medication, % (n/N) | 45.7 (137/300) | 41.3 (33/80) |
| Lipid-lowering medication, % (n/N) | 39.7 (119/300) | 39.2 (31/79) |
| Steroids, % (n) | 6.0 (19) | 7.2 (10) |
| Metformin, % (n) | 0.3 (1) | 0.0 (0) |
| CVD (MI, heart failure, angina, stroke), % (n/N) | 8.9 (28/316) | 12.3 (17/138) |
| Smoking status, % (n) | ||
| Past | 39.7 (126) | 34.5 (48) |
| Current | 7.9 (25) | 19.4 (27) |
| Employment type, % (n) | ||
| Full time | 36.9 (117) | 37.4 (52) |
| Part time | 19.2 (61) | 18.0 (25) |
| Retired | 35.3 (112) | 29.5 (41) |
| Unemployed or other | 8.5 (27) | 15.1 (21) |
| Educational status, % (n/N) | ||
| Degree, higher degree or equivalent | 49.7 (156) | 33.8 (46) |
| Marital status, % (n) | ||
| Married/civil partner | 77.0 (244) | 66.9 (93) |
| Access to the internet, % (n/N) | 87.4 (277/317) | 80.4 (111/138) |
Appendix 5 Self-efficacy and illness perception scores at baseline and follow-up in each study arm
| Time point | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 48 months | ||||||||||
| N | Median | p25 | p75 | N | Median | p25 | p75 | N | Median | p25 | p75 | |
| Walking self-efficacy | ||||||||||||
| Confidence to walk for 10 minutes per day (%) | 459 | 100 | 100 | 100 | 397 | 100 | 100 | 100 | 379 | 100 | 100 | 100 |
| Confidence to walk for 30 minutes per day (%) | 458 | 100 | 80 | 100 | 397 | 100 | 80 | 100 | 379 | 100 | 90 | 100 |
| Confidence to walk for 60 minutes per day (%) | 459 | 90 | 50 | 100 | 397 | 90 | 50 | 100 | 379 | 90 | 50 | 100 |
| Illness perception | ||||||||||||
| How much does your risk of diabetes affect your life? | 310 | 4.0 | 1.0 | 7.0 | 395 | 4.0 | 1.0 | 6.0 | 379 | 4.0 | 1.0 | 6.0 |
| How long do you think your risk of diabetes will continue? | 310 | 5.0 | 3.0 | 10.0 | 393 | 7.0 | 4.0 | 10.0 | 380 | 7.0 | 3.0 | 10.0 |
| How much control do you feel you have over your risk of diabetes? | 310 | 7.0 | 5.0 | 8.0 | 394 | 7.0 | 5.0 | 8.0 | 380 | 7.0 | 5.0 | 8.0 |
| How much do you think treatment can help your risk of diabetes? | 310 | 8.0 | 6.0 | 10.0 | 393 | 8.0 | 5.0 | 10.0 | 378 | 7.0 | 5.0 | 9.0 |
| How much do you experience symptoms from your risk of diabetes? | 312 | 0.0 | 0.0 | 2.0 | 394 | 0.0 | 0.0 | 2.0 | 379 | 0.0 | 0.0 | 2.0 |
| How concerned are you about your risk of diabetes? | 311 | 7.0 | 3.0 | 9.0 | 396 | 6.0 | 3.0 | 9.0 | 380 | 6.0 | 3.0 | 8.0 |
| How well do you feel you understand your risk of diabetes? | 312 | 7.0 | 4.5 | 9.0 | 394 | 7.0 | 5.0 | 10.0 | 380 | 8.0 | 5.0 | 10.0 |
| How much does your risk of diabetes affect you emotionally? | 312 | 3.0 | 0.0 | 6.0 | 394 | 2.0 | 0.0 | 5.0 | 380 | 2.0 | 0.0 | 5.0 |
| Time point | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 48 months | ||||||||||
| N | Median | p25 | p75 | N | Median | p25 | p75 | N | Median | p25 | p75 | |
| Walking self-efficacy | ||||||||||||
| Confidence to walk for 10 minutes per day (%) | 450 | 100 | 100 | 100 | 347 | 100 | 100 | 100 | 315 | 100 | 100 | 100 |
| Confidence to walk for 30 minutes per day (%) | 450 | 100 | 80 | 100 | 346 | 100 | 90 | 100 | 315 | 100 | 80 | 100 |
| Confidence to walk for 60 minutes per day (%) | 449 | 90 | 50 | 100 | 346 | 90 | 50 | 100 | 315 | 100 | 50 | 100 |
| Illness perception | ||||||||||||
| How much does your risk of diabetes affect your life? | 303 | 3.0 | 1.0 | 6.0 | 346 | 3.0 | 1.0 | 6.0 | 315 | 5.0 | 1.0 | 7.0 |
| How long do you think your risk of diabetes will continue? | 303 | 6.0 | 3.0 | 10.0 | 344 | 7.0 | 4.0 | 10.0 | 314 | 7.0 | 3.0 | 10.0 |
| How much control do you feel you have over your risk of diabetes? | 303 | 7.0 | 5.0 | 8.0 | 344 | 7.0 | 5.0 | 8.0 | 315 | 7.0 | 6.0 | 9.0 |
| How much do you think treatment can help your risk of diabetes? | 303 | 8.0 | 6.0 | 10.0 | 342 | 7.0 | 5.0 | 9.0 | 314 | 8.0 | 5.0 | 10.0 |
| How much do you experience symptoms from your risk of diabetes? | 301 | 0.0 | 0.0 | 2.0 | 346 | 0.0 | 0.0 | 2.0 | 315 | 0.0 | 0.0 | 3.0 |
| How concerned are you about your risk of diabetes? | 302 | 7.0 | 4.0 | 10.0 | 347 | 7.0 | 4.0 | 9.0 | 312 | 7.0 | 4.0 | 9.0 |
| How well do you feel you understand your risk of diabetes? | 302 | 7.0 | 5.0 | 9.0 | 345 | 8.0 | 6.0 | 10.0 | 313 | 9.0 | 7.0 | 10.0 |
| How much does your risk of diabetes affect you emotionally? | 302 | 3.0 | 0.0 | 5.0 | 347 | 2.0 | 0.0 | 5.0 | 314 | 2.0 | 0.0 | 5.0 |
| Time point | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 48 months | ||||||||||
| N | Median | p25 | p75 | N | Median | p25 | p75 | N | Median | p25 | p75 | |
| Walking self-efficacy | ||||||||||||
| Confidence to walk for 10 minutes per day (%) | 455 | 100 | 100 | 100 | 346 | 100 | 100 | 100 | 324 | 100 | 100 | 100 |
| Confidence to walk for 30 minutes per day (%) | 455 | 100 | 80 | 100 | 345 | 100 | 90 | 100 | 324 | 100 | 80 | 100 |
| Confidence to walk for 60 minutes per day (%) | 452 | 100 | 50 | 100 | 345 | 100 | 50 | 100 | 324 | 95 | 50 | 100 |
| Illness perception | ||||||||||||
| How much does your risk of diabetes affect your life? | 308 | 3.0 | 1.0 | 5.0 | 342 | 4.0 | 1.0 | 7.0 | 325 | 5.0 | 1.0 | 8.0 |
| How long do you think your risk of diabetes will continue? | 309 | 5.0 | 3.0 | 10.0 | 342 | 7.0 | 4.0 | 10.0 | 323 | 8.0 | 5.0 | 10.0 |
| How much control do you feel you have over your risk of diabetes? | 310 | 6.0 | 5.0 | 8.0 | 342 | 7.0 | 5.0 | 8.0 | 325 | 7.0 | 5.0 | 9.0 |
| How much do you think treatment can help your risk of diabetes? | 309 | 8.0 | 6.0 | 10.0 | 341 | 8.0 | 6.0 | 10.0 | 325 | 7.0 | 5.0 | 9.0 |
| How much do you experience symptoms from your risk of diabetes? | 308 | 0.0 | 0.0 | 2.0 | 341 | 0.0 | 0.0 | 2.0 | 324 | 0.0 | 0.0 | 2.0 |
| How concerned are you about your risk of diabetes? | 308 | 7.0 | 4.0 | 9.5 | 342 | 7.0 | 4.0 | 10.0 | 325 | 7.0 | 5.0 | 9.0 |
| How well do you feel you understand your risk of diabetes? | 310 | 6.0 | 4.0 | 9.0 | 343 | 8.0 | 7.0 | 10.0 | 325 | 9.0 | 7.0 | 10.0 |
| How much does your risk of diabetes affect you emotionally? | 309 | 3.0 | 0.0 | 5.0 | 342 | 2.0 | 0.0 | 5.0 | 325 | 2.0 | 0.0 | 5.0 |
Appendix 6 Self-reported use of behaviour change strategies at baseline and follow-up in each study arm
| Self-regulation | Study arm, % (n) | |||||
|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | ||||
| 12 months | 48 months | 12 months | 48 months | 12 months | 48 months | |
| Set regular goals detailing amount of exercise you would do each day | ||||||
| Most of the time | 30.0 (119) | 30.6 (116) | 34.2 (118) | 39.0 (123) | 40.3 (139) | 38.5 (125) |
| Some of the time | 34.0 (135) | 31.1 (118) | 37.4 (129) | 34.0 (107) | 38.6 (133) | 40.3 (131) |
| Rarely | 17.9 (71) | 20.8 (79) | 18.3 (63) | 16.8 (53) | 13.9 (48) | 12.0 (39) |
| Never | 18.1 (72) | 17.4 (66) | 10.1 (35) | 10.2 (32) | 7.2 (25) | 9.2 (30) |
| Regularly set plan detailing where, when and how you would exercise | ||||||
| Most of the time | 25.4 (101) | 25.6 (97) | 25.9 (89) | 34.6 (109) | 27.9 (96) | 30.8 (100) |
| Some of the time | 31.0 (123) | 30.3 (115) | 38.1 (131) | 31.7 (100) | 43.9 (151) | 36.3 (118) |
| Rarely | 21.2 (84) | 22.4 (85) | 23.8 (82) | 22.5 (71) | 18.0 (62) | 22.8 (74) |
| Never | 22.4 (89) | 21.6 (82) | 12.2 (42) | 11.1 (35) | 10.2 (35) | 10.2 (33) |
| Worn a pedometer | ||||||
| Most of the time | 2.5 (10) | 9.0 (34) | 22.7 (78) | 25.2 (79) | 36.4 (126) | 32.4 (105) |
| Some of the time | 10.1 (40) | 10.6 (40) | 28.5 (98) | 24.5 (77) | 36.7 (127) | 31.8 (103) |
| Rarely | 11.9 (47) | 13.6 (51) | 26.2 (90) | 17.8 (56) | 15.0 (52) | 18.5 (60) |
| Never | 75.4 (298) | 66.8 (251) | 22.7 (78) | 32.5 (102) | 11.8 (41) | 17.3 (56) |
| Kept an exercise log recording your activity levels | ||||||
| Most of the time | 4.8 (19) | 7.7 (29) | 17.1 (59) | 15.6 (49) | 24.1 (83) | 19.7 (64) |
| Some of the time | 6.3 (25) | 9.8 (37) | 18.0 (62) | 15.0 (47) | 30.2 (104) | 21.2 (69) |
| Rarely | 8.3 (33) | 11.9 (45) | 17.7 (61) | 24.2 (76) | 22.4 (77) | 23.7 (77) |
| Never | 80.6 (319) | 70.7 (268) | 47.2 (163) | 45.2 (142) | 23.3 (80) | 35.4 (115) |
| Been aware of your activity levels | ||||||
| Most of the time | 41.1 (163) | 40.1 (152) | 55.8 (193) | 61.9 (195) | 66.7 (230) | 64.6 (210) |
| Some of the time | 30.0 (119) | 34.8 (132) | 26.9 (93) | 22.2 (70) | 24.9 (86) | 24.9 (81) |
| Rarely | 12.1 (48) | 11.1 (42) | 9.8 (34) | 9.8 (31) | 4.9 (17) | 5.2 (17) |
| Never | 16.9 (67) | 14.0 (53) | 7.5 (26) | 6.0 (19) | 3.5 (12) | 5.2 (17) |
| Tried to exercise regularly | ||||||
| Most of the time | 41.8 (166) | 42.0 (159) | 53.0 (183) | 53.8 (169) | 56.1 (194) | 56.6 (184) |
| Some of the time | 35.0 (139) | 32.7 (124) | 34.8 (120) | 31.2 (98) | 33.5 (116) | 32.9 (107) |
| Rarely | 13.6 (54) | 15.3 (58) | 7.5 (26) | 10.2 (32) | 8.4 (29) | 7.1 (23) |
| Never | 9.6 (38) | 10.0 (38) | 4.6 (16) | 4.8 (15) | 2.0 (7) | 3.4 (11) |
Appendix 7 Treatment-by-factor interactions
| Factor | Interaction p-value |
|---|---|
| Sex (men/women) | 0.321 |
| Age (< 60 years/≥ 60 years) | 0.420 |
| Ethnicity (white European/South Asian/other) | 0.114 |
| Family history of T2D (no/yes) | 0.216 |
| Prediabetes at baseline (no/yes) | 0.474 |
| Baseline obesity status (< 30 kg/m2/≥ 30 kg/m2 in white Europeans/other; < 27.5 kg/m2/≥ 27.5 kg/m2 in South Asians) | 0.734 |
| Baseline deprivation (below/above median IMD decile) | 0.035 |
Appendix 8 Self-reported physical activity outcomes and intervention effects
| Variable | Study arm, n; mean (SD) | Intervention effect 1 (Walking Away vs. control), difference (97.5% CI)a | Intervention effect 2 (Walking Away vs. control), difference (97.5% CI)a | ||
|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||
| Overall physical activity expenditure (kJ/kg/day) | |||||
| Baseline | 459; 33.5 (38.4) | 450; 32.9 (33.3) | 455; 30.2 (32.4) | ||
| 12 months | 397; 30.3 (31.5) | 347; 32.0 (30.1) | 345; 32.8 (33.4) | 0.3 (–3.9 to 4.4) | 2.5 (–1.9 to 6.8) |
| 48 months | 380; 28.8 (29.2) | 314; 31.2 (33.5) | 326; 32.1 (32.8) | 2.3 (–2.3 to 6.9) | 4.4 (0.0 to 8.8) |
| Time spent sedentary (minutes/day) | |||||
| Baseline | 459; 366.3 (189.9) | 450; 368.1 (180.1) | 455; 387.5 (201.3) | ||
| 12 months | 405; 338.9 (180.0) | 354; 326.5 (180.1) | 363; 327.5 (197.2) | –8.4 (–31.0 to 14.3) | –16.2 (–40.3 to 7.8) |
| 48 months | 383; 332.3 (177.8) | 318; 314.3 (164.0) | 333; 330.3 (191.9) | –18.7 (–42.1 to 4.7) | –13.0 (–38.2 to 12.2) |
| Time spent in light physical activity (minutes/day) | |||||
| Baseline | 459; 40.8 (100.0) | 450; 50.5 (108.8) | 455; 36.6 (88.4) | ||
| 12 months | 405; 43.9 (103.7) | 354; 44.4 (102.6) | 363; 36.7 (96.6) | –2.6 (–18.0 to 12.8) | –3.8 (–17.9 to 10.3) |
| 48 months | 383; 50.2 (106.3) | 318; 37.3 (88.5) | 333; 39.0 (96.6) | –13.9 (–28.6 to 0.9) | –7.3 (–22.8 to 8.1) |
| Time spent in moderate to vigorous physical activity (minutes/day) | |||||
| Baseline | 459; 117.8 (161.1) | 450; 111.9 (142.4) | 455; 101.6 (133.5) | ||
| 12 months | 405; 101.6 (133.5) | 354; 112.7 (139.1) | 363; 110.3 (139.7) | 6.2 (–11.6 to 24.1) | 9.1 (–8.9 to 27.0) |
| 48 months | 383; 96.7 (122.7) | 318; 116.0 (144.6) | 333; 109.4 (134.9) | 18.3 (–0.7 to 37.3) | 16.7 (–1.5 to 34.9) |
Appendix 9 Secondary outcome tables
| Variable | Study arm, n; mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||||||
| Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | |
| Weight (kg) | 460; 82.3 (17.8) | 387; 81.9 (18.3) | 411; 81.8 (18.6) | 450; 81.2 (17.7) | 336; 80.1 (17.3) | 364; 79.7 (17.4) | 456; 81.7 (18.6) | 340; 81.2 (17.4) | 379; 80.8 (18.3) |
| BMI (kg/m2) | 460; 29.3 (5.7) | 387; 29.2 (5.9) | 370; 29.2 (6.0) | 450; 29.1 (5.6) | 336; 28.7 (5.5) | 298; 28.5 (5.4) | 456; 29.2 (5.6) | 340; 28.9 (5.1) | 313; 28.6 (5.1) |
| Waist circumference (cm) | 459; 98.9 (13.6) | 386; 98.9 (13.9) | 368; 100.6 (14.7) | 448; 98.7 (13.9) | 335; 97.3 (13.7) | 290; 98.5 (13.3) | 454; 98.8 (14.4) | 338; 98.4 (14.3) | 312; 99.5 (13.8) |
| Body fat percentage (%) | 457; 33.6 (9.5) | 381; 33.8 (9.3) | 363; 33.5 (9.2) | 447; 33.6 (9.1) | 330; 32.9 (9.3) | 294; 32.6 (9.7) | 454; 33.5 (8.9) | 340; 33.6 (8.5) | 309; 33.2 (9.0) |
| Fat mass (kg) | 457; 28.6 (12.6) | 381; 28.1 (12.2) | 364; 28.2 (12.7) | 447; 27.9 (11.6) | 330; 27.1 (11.7) | 294; 26.6 (11.9) | 454; 28.1 (11.8) | 340; 27.7 (10.8) | 309; 27.2 (10.6) |
| Fat-free mass (kg) | 457; 53.5 (10.8) | 381; 53.5 (11.2) | 364; 53.8 (11.8) | 447; 53.2 (11.1) | 330; 53.0 (10.8) | 294; 52.3 (10.8) | 454; 53.6 (11.6) | 340; 53.5 (11.4) | 309; 52.8 (12.4) |
| Variable | 12 months, mean (97.5% CI) | 48 months, mean (97.5% CI) | ||
|---|---|---|---|---|
| Walking Away vs. control | Walking Away Plus vs. control | Walking Away vs. control | Walking Away Plus vs. control | |
| Weight (kg) | –0.60 (–1.18 to –0.03) | –0.05 (–0.62 to 0.52) | –1.00 (–1.92 to –0.07) | –0.23 (–1.16 to 0.70) |
| BMI (kg/m2) | –0.20 (–0.41 to 0.01) | –0.01 (–0.21 to 0.20) | –0.42 (–0.77 to –0.07) | –0.23 (–0.57 to 0.12) |
| Waist circumference (cm) | –1.28 (–2.18 to –0.38) | –0.47 (–1.39 to 0.45) | –1.57 (–2.70 to –0.45) | –1.09 (–2.33 to 0.15) |
| Body fat percentage (%) | –0.50 (–0.98 to –0.03) | –0.31 (–0.80 to 0.17) | –1.06 (–1.79 to –0.33) | –0.47 (–1.15 to 0.22) |
| Fat mass (kg) | –0.24 (–0.87 to 0.39) | 0.08 (–0.58 to 0.75) | –0.90 (–1.96 to 0.17) | –0.31 (–1.40 to 0.79) |
| Fat-free mass (kg) | –0.40 (–0.93 to 0.14) | –0.06 (–0.61 to 0.49) | –0.72 (–1.61 to 0.17) | –0.66 (–1.55 to 0.24) |
| Variable | Study arm, n; mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||||||
| Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | |
| HbA1c (mmol/mol) | 458; 40.0 (3.7) | 385; 41.2 (3.5) | 397; 41.1 (4.7) | 449; 40.5 (3.5) | 334; 41.4 (3.3) | 352; 41.6 (5.2) | 455; 40.4 (3.5) | 340; 41.3 (3.4) | 357; 41.8 (5.5) |
| HbA1c (%) | 460; 5.8 (0.3) | 385; 5.9 (0.3) | 389; 5.9 (0.4) | 450; 5.9 (0.3) | 335; 5.9 (0.3) | 332; 6.0 (0.4) | 456; 5.9 (0.3) | 340; 5.9 (0.3) | 341; 6.0 (0.4) |
| Total cholesterol (mmol/l) | 459; 5.2 (1.1) | 383; 5.1 (1.0) | 389; 4.8 (1.0) | 449; 5.2 (1.1) | 333; 5.1 (1.0) | 322; 4.9 (1.0) | 455; 5.2 (1.1) | 340; 5.1 (1.1) | 338; 4.8 (1.1) |
| HDL cholesterol (mmol/l) | 455; 1.4 (0.4) | 380; 1.5 (0.5) | 397; 1.5 (0.4) | 443; 1.4 (0.4) | 330; 1.4 (0.5) | 342; 1.4 (0.4) | 455; 1.4 (0.4) | 340; 1.5 (0.4) | 356; 1.5 (0.5) |
| LDL cholesterol (mmol/l) | 453; 3.0 (0.9) | 376; 2.9 (0.9) | 391; 2.6 (0.8) | 436; 3.1 (0.9) | 326; 2.9 (0.9) | 339; 2.7 (0.9) | 452; 3.1 (1.0) | 337; 2.9 (0.9) | 352; 2.7 (0.9) |
| Triglycerides (mmol/l) | 459; 1.5 (0.8) | 383; 1.7 (1.3) | 398; 1.6 (0.9) | 449; 1.6 (1.0) | 333; 1.7 (1.0) | 342; 1.6 (0.9) | 455; 1.5 (0.8) | 340; 1.5 (0.8) | 355; 1.5 (0.8) |
| Vitamin D (nmol/l) | 305; 45.8 (23.1) | 251; 44.8 (20.1) | 243; 48.5 (18.8) | 299; 44.1 (20.9) | 212; 45.6 (23.1) | 186; 49.3 (20.0) | 304; 43.8 (22.6) | 214; 45.8 (20.7) | 195; 50.7 (20.1) |
| Variable | 12 months, mean (97.5% CI) | 48 months, mean (97.5% CI) | ||
|---|---|---|---|---|
| Walking Away vs. control | Walking Away Plus vs. control | Walking Away vs. control | Walking Away Plus vs. control | |
| HbA1c (mmol/mol) | –0.14 (–0.47 to 0.20) | –0.10 (–0.43 to 0.23) | –0.13 (–0.68 to 0.42) | –0.01 (–0.63 to 0.61) |
| HbA1c (%) | –0.02 (–0.05 to 0.01) | –0.01 (–0.04 to 0.02) | –0.02 (–0.07 to 0.03) | –0.03 (–0.07 to 0.02) |
| Total cholesterol (mmol/l) | –0.04 (–0.15 to 0.06) | –0.08 (–0.18 to 0.03) | 0.02 (–0.11 to 0.15) | –0.02 (–0.16 to 0.11) |
| HDL cholesterol (mmol/l) | 0.00 (–0.03 to 0.04) | 0.01 (–0.03 to 0.05) | 0.00 (–0.03 to 0.04) | 0.04 (–0.01 to 0.08) |
| LDL cholesterol (mmol/l) | –0.02 (–0.12 to 0.07) | –0.04 (–0.13 to 0.05) | 0.03 (–0.08 to 0.15) | 0.00 (–0.11 to 0.12) |
| Triglycerides (mmol/l) | –0.09 (–0.25 to 0.06) | –0.15 (–0.29 to –0.01) | –0.07 (–0.18 to 0.03) | –0.11 (–0.21 to 0.00) |
| Vitamin D (nmol/l) | 1.52 (–2.50 to 5.53) | 0.42 (–3.20 to 4.04) | 1.17 (–2.79 to 5.12) | 1.63 (–2.26 to 5.52) |
| Variable | Study arm, n; mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||||||
| Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | |
| Other biochemistry outcomes | |||||||||
| Sodium (mmol/l) | 460; 140.4 (2.0) | 384; 140.6 (2.0) | 398; 140.6 (2.4) | 450; 140.5 (2.0) | 335; 140.5 (2.0) | 348; 140.5 (2.2) | 455; 140.3 (2.1) | 340; 140.6 (1.9) | 355; 140.6 (2.5) |
| Potassium (mmol/l) | 459; 4.3 (0.4) | 382; 4.3 (0.4) | 398; 4.2 (0.4) | 449; 4.3 (0.4) | 330; 4.3 (0.4) | 346; 4.3 (0.4) | 454; 4.3 (0.4) | 336; 4.3 (0.4) | 355; 4.2 (0.4) |
| Urea (mmol/l) | 427; 5.8 (1.5) | 384; 5.9 (1.5) | 398; 6.0 (1.7) | 420; 5.8 (1.5) | 333; 6.0 (1.4) | 348; 6.0 (1.7) | 422; 5.9 (1.6) | 340; 5.9 (1.5) | 354; 6.0 (1.7) |
| eGFR (ml/minute/1.73 m2) | 449; 85.6 (19.9) | 370; 82.9 (12.8) | 392; 83.7 (13.1) | 431; 85.5 (12.1) | 326; 82.7 (12.9) | 343; 83.1 (13.3) | 439; 85.5 (12.6) | 330; 82.5 (13.2) | 349; 84.1 (13.1) |
| Total bilirubin (µmol/l) | 459; 10.3 (5.0) | 382 (5.3) | 396; 10.3 (5.2) | 448; 10.3 (5.1) | 334; 10.3 (4.8) | 339; 10.3 (4.4) | 453; 10.5 (5.5) | 339; 10.5 (5.1) | 349; 10.4 (5.4) |
| ALP (IU/l) | 458; 82.7 (22.0) | 10.3; 82.6 (24.0) | 398; 81.5 (28.0) | 448; 82.8 (23.5) | 333; 81.6 (23.2) | 342; 78.1 (22.0) | 454; 79.8 (22.5) | 339; 78.7 (20.7) | 348; 77.6 (22.5) |
| ALT (IU/l) | 459; 26.3 (13.0) | 382; 27.0 (12.9) | 396; 26.4 (15.1) | 448; 26.6 (13.4) | 334; 25.3 (12.1) | 340; 25.0 (10.3) | 454; 26.8 (16.1) | 339; 25.5 (11.1) | 349; 27.7 (28.6) |
| GGT (IU/l) | 347; 34.7 (31.4) | 380; 34.9 (31.3) | 354; 37.9 (62.4) | 346; 35.1 (34.3) | 322; 32.6 (27.3) | 287; 33.1 (32.8) | 339; 37.3 (42.6) | 331; 33.5 (31.2) | 306; 34.3 (35.9) |
| Urine albumin-to-creatinine ratio (mg/mmol) | 439; 1.5 (3.3) | 377; 1.8 (5.8) | 270; 2.7 (6.3) | 429; 1.3 (2.9) | 324; 1.4 (3.1) | 221; 2.3 (4.9) | 429; 1.4 (3.8) | 332; 1.8 (3.8) | 243; 2.0 (3.9) |
| Cardiovascular risk | |||||||||
| 10-year cardiovascular risk (Framingham) (%) | 407; 13.8 (10.2) | 354; 14.4 (11.0) | 367; 14.9 (11.4) | 362; 14.4 (9.4) | 307; 14.0 (9.9) | 289; 15.2 (11.3) | 379; 14.5 (10.6) | 313; 14.4 (10.6) | 303; 14.8 (10.6) |
| Variable | 12 months, mean (97.5% CI) | 48 months, mean (97.5% CI) | ||
|---|---|---|---|---|
| Walking Away vs. control | Walking Away Plus vs. control | Walking Away vs. control | Walking Away Plus vs. control | |
| Sodium (mmol/l) | –0.12 (–0.39 to 0.15) | 0.03 (–0.24 to 0.29) | –0.06 (–0.38 to 0.25) | 0.09 (–0.25 to 0.42) |
| Potassium (mmol/l) | –0.03 (–0.09 to 0.02) | –0.04 (–0.10 to 0.01) | 0.02 (–0.04 to 0.08) | –0.03 (–0.09 to 0.02) |
| Urea (mmol/l) | 0.05 (–0.13 to 0.23) | –0.08 (–0.26 to 0.09) | 0.04 (–0.17 to 0.25) | –0.07 (–0.27 to 0.13) |
| eGFR (ml/minute/1.73 m2) | 0.29 (–1.64 to 2.23) | 0.26 (–1.72 to 2.23) | –0.40 (–2.36 to 1.56) | 0.42 (–1.54 to 2.38) |
| Total bilirubin (µmol/l) | 0.17 (–0.36 to 0.70) | –0.11 (–0.61 to 0.40) | –0.09 (–0.60 to 0.42) | –0.19 (–0.77 to 0.38) |
| ALP (IU/l) | –0.85 (–2.87 to 1.18) | –1.32 (–3.10 to 0.46) | –3.70 (–6.45 to –0.96) | –1.08 (–3.65 to 1.49) |
| ALT (IU/l) | –1.33 (–2.92 to 0.26) | –0.89 (–2.40 to 0.62) | –1.79 (–3.51 to –0.07) | 1.55 (–1.99 to 5.08) |
| GGT (IU/l) | –2.93 (–6.60 to 0.74) | –1.99 (–5.24 to 1.26) | –4.67 (–12.35 to 3.00) | –3.87 (–11.47 to 3.72) |
| Urine albumin to creatinine ratio (mg/mmol) | –0.29 (–0.95 to 0.38) | –0.09 (–0.77 to 0.60) | –0.10 (–1.11 to 0.91) | –0.77 (–1.70 to 0.17) |
| 10-year cardiovascular risk (Framingham) (%) | –0.86 (–1.69 to –0.04) | –0.73 (–1.57 to 0.11) | –0.26 (–1.31 to 0.78) | –0.54 (–1.65 to 0.57) |
| Diet variable | Study arm, n; mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||||||
| Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | |
| Frequency (portions/week) of | |||||||||
| Fresh fruit | 459; 4.7 (1.3) | 396; 4.7 (1.4) | 379; 4.7 (1.4) | 450; 4.8 (1.3) | 347; 5.0 (1.2) | 315; 5.1 (1.2) | 455; 4.8 (1.3) | 345; 4.9 (1.2) | 325; 5.0 (1.2) |
| Green leafy vegetables | 459; 4.3 (1.2) | 396; 4.4 (1.2) | 380; 4.2 (1.2) | 449; 4.5 (1.1) | 347; 4.5 (1.1) | 315; 4.6 (1.2) | 453; 4.4 (1.2) | 345; 4.6 (1.1) | 325; 4.5 (1.2) |
| Other vegetables | 457; 4.7 (1.0) | 392; 4.6 (1.0) | 378; 4.5 (1.1) | 447; 4.8 (1.0) | 347; 4.9 (1.0) | 314; 4.8 (1.0) | 454; 4.8 (1.0) | 344; 4.9 (1.0) | 325; 4.8 (1.0) |
| Oily fish | 459; 2.6 (1.1) | 396; 2.7 (1.1) | 380; 2.7 (1.2) | 450; 2.7 (1.2) | 346; 2.8 (1.2) | 315; 2.8 (1.2) | 455; 2.7 (1.2) | 346; 2.8 (1.1) | 326; 2.8 (1.1) |
| Other fish | 457; 2.7 (1.0) | 395; 2.8 (1.0) | 379; 2.8 (1.1) | 449; 2.7 (1.0) | 347; 2.7 (1.1) | 313; 2.8 (1.1) | 455; 2.7 (1.0) | 343; 2.8 (1.0) | 325; 2.8 (1.1) |
| Chicken | 457; 3.4 (1.1) | 396; 3.3 (1.1) | 380; 3.3 (1.1) | 450; 3.3 (1.1) | 346; 3.4 (1.1) | 315; 3.3 (1.2) | 455; 3.4 (1.1) | 345; 3.5 (1.0) | 326; 3.4 (1.0) |
| Meat | 459; 3.1 (1.2) | 395; 3.0 (1.2) | 377; 3.0 (1.2) | 450; 3.2 (1.2) | 347; 3.1 (1.2) | 315; 3.0 (1.2) | 455; 3.3 (1.2) | 345; 3.2 (1.1) | 326; 3.1 (1.1) |
| Eggs | 458; 3.3 (1.1) | 395; 3.3 (1.1) | 379; 3.4 (1.2) | 450; 3.4 (1.2) | 345; 3.4 (1.1) | 314; 3.5 (1.2) | 455; 3.4 (1.1) | 346; 3.4 (1.1) | 326; 3.5 (1.2) |
| Cheese | 458; 3.6 (1.2) | 395; 3.7 (1.2) | 379; 3.6 (1.2) | 447; 3.6 (1.1) | 346; 3.5 (1.2) | 314; 3.6 (1.2) | 454; 3.7 (1.2) | 346; 3.6 (1.2) | 325; 3.6 (1.2) |
| Wholemeal/brown bread | 459; 4.1 (1.6) | 395; 4.1 (1.6) | 378; 4.0 (1.6) | 450; 4.2 (1.6) | 346; 4.2 (1.6) | 314; 4.2 (1.6) | 455; 4.3 (1.6) | 345; 4.2 (1.6) | 326; 4.2 (1.5) |
| Alcohol (drinks/day) | 450; 2.0 (0.9) | 393; 1.9 (0.8) | 375; 1.9 (0.8) | 443; 1.9 (0.8) | 346; 1.9 (0.8) | 307; 1.9 (0.7) | 449; 2.0 (0.8) | 343; 1.9 (0.8) | 321; 1.9 (0.8) |
| Number of days/week on which individual reported limiting intake of | |||||||||
| Total fat | 458; 4.4 (1.6) | 396; 4.5 (1.6) | 380; 4.4 (1.6) | 450; 4.5 (1.5) | 347; 4.7 (1.4) | 315; 4.7 (1.4) | 454; 4.5 (1.6) | 346; 4.7 (1.4) | 326; 4.7 (1.4) |
| Saturated fat | 458; 4.5 (1.7) | 394; 4.6 (1.7) | 380; 4.4 (1.6) | 449; 4.5 (1.6) | 346; 4.8 (1.3) | 314; 4.9 (1.4) | 454; 4.6 (1.6) | 345; 4.8 (1.4) | 325; 4.8 (1.5) |
| Sugar | 459; 4.7 (1.7) | 395; 4.9 (1.5) | 380; 4.7 (1.5) | 450; 4.7 (1.5) | 345; 5.0 (1.4) | 314; 5.1 (1.3) | 454; 4.7 (1.5) | 345; 5.0 (1.4) | 326; 5.0 (1.3) |
| Salt | 459; 4.5 (1.8) | 394; 4.7 (1.7) | 380; 4.6 (1.8) | 450; 4.6 (1.7) | 347; 4.8 (1.5) | 315; 4.8 (1.6) | 453; 4.5 (1.8) | 346; 4.8 (1.6) | 326; 4.8 (1.6) |
| Diet variable | 12 months, mean (97.5% CI) | 48 months, mean (97.5% CI) | ||
|---|---|---|---|---|
| Walking Away vs. control | Walking Away Plus vs. control | Walking Away vs. control | Walking Away Plus vs. control | |
| Frequency (portions/week) of | ||||
| Green leafy vegetables | 0.23 (0.07 to 0.39) | 0.12 (–0.04 to 0.29) | 0.22 (0.05 to 0.40) | 0.13 (–0.04 to 0.30) |
| Other vegetables | 0.12 (–0.03 to 0.26) | 0.14 (0.00 to 0.29) | 0.24 (0.07 to 0.41) | 0.24 (0.08 to 0.41) |
| Oily fish | 0.21 (0.07 to 0.35) | 0.17 (0.04 to 0.31) | 0.20 (0.05 to 0.35) | 0.20 (0.06 to 0.35) |
| Other fish | 0.02 (–0.12 to 0.16) | 0.09 (–0.05 to 0.22) | 0.00 (–0.16 to 0.15) | 0.04 (–0.12 to 0.20) |
| Chicken | –0.06 (–0.20 to 0.08) | 0.03 (–0.11 to 0.16) | –0.02 (–0.17 to 0.14) | 0.06 (–0.10 to 0.22) |
| Meat | 0.05 (–0.06 to 0.16) | 0.06 (–0.05 to 0.18) | –0.01 (–0.16 to 0.13) | 0.02 (–0.12 to 0.15) |
| Eggs | –0.02 (–0.15 to 0.11) | 0.00 (–0.14 to 0.14) | –0.14 (–0.29 to 0.00) | –0.12 (–0.27 to 0.03) |
| Cheese | –0.02 (–0.15 to 0.11) | –0.01 (–0.14 to 0.12) | 0.07 (–0.09 to 0.23) | 0.05 (–0.11 to 0.21) |
| Wholemeal/brown bread | –0.15 (–0.30 to 0.00) | –0.16 (–0.31 to –0.01) | 0.00 (–0.16 to 0.17) | –0.06 (–0.24 to 0.11) |
| Alcohol (drinks/day) | 0.05 (–0.17 to 0.26) | 0.03 (–0.19 to 0.25) | 0.07 (–0.17 to 0.31) | 0.06 (–0.18 to 0.30) |
| Number of days/week on which individual reported limiting intake of | ||||
| Total fat | 0.11 (–0.11 to 0.32) | 0.09 (–0.12 to 0.31) | 0.32 (0.09 to 0.55) | 0.32 (0.09 to 0.55) |
| Saturated fat | 0.17 (–0.06 to 0.39) | 0.08 (–0.14 to 0.31) | 0.41 (0.18 to 0.65) | 0.37 (0.12 to 0.61) |
| Sugar | 0.09 (–0.11 to 0.29) | 0.06 (–0.17 to 0.29) | 0.34 (0.12 to 0.55) | 0.29 (0.07 to 0.51) |
| Salt | 0.05 (–0.16 to 0.27) | 0.06 (–0.18 to 0.30) | 0.11 (–0.14 to 0.36) | 0.21 (–0.03 to 0.46) |
| Variable | Study arm, n; mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Walking Away | Walking Away Plus | |||||||
| Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | Baseline | 12 months | 48 months | |
| Depression and anxiety | |||||||||
| Depression score | 455; 4.0 (3.3) | 393; 4.0 (3.3) | 379; 4.0 (3.4) | 446; 3.7 (3.2) | 346; 3.3 (3.0) | 313; 3.5 (3.1) | 455; 4.2 (3.7) | 342; 3.4 (3.3) | 325; 3.8 (3.9) |
| Anxiety score | 454; 6.1 (4.2) | 390; 5.8 (4.2) | 380; 5.9 (4.2) | 445; 5.8 (4.0) | 344; 5.2 (3.8) | 313; 5.5 (4.0) | 455; 6.0 (4.0) | 343; 5.5 (3.8) | 326; 5.3 (4.2) |
| Health-related quality of life | |||||||||
| Summary mental component score (SF-8) | 456; 50.4 (9.6) | 390; 50.7 (9.7) | 375; 50.2 (10.0) | 446; 51.2 (8.8) | 341; 51.8 (8.3) | 312; 51.4 (9.1) | 453; 50.6 (9.2) | 345; 51.6 (9.0) | 325; 51.2 (9.6) |
| Summary physical component score (SF-8) | 456; 48.3 (9.5) | 390; 48.3 (9.7) | 375; 48.5 (8.7) | 446; 48.7 (9.0) | 341; 49.9 (8.9) | 312; 48.5 (9.1) | 453; 48.5 (9.4) | 345; 49.3 (9.0) | 325; 48.1 (9.7) |
| Summary index (EQ-5D-5L) | 459; 0.8 (0.2) | 396; 0.8 (0.2) | 380; 0.8 (0.2) | 448; 0.8 (0.2) | 347; 0.8 (0.2) | 313; 0.8 (0.2) | 454; 0.8 (0.2) | 346; 0.8 (0.2) | 325; 0.8 (0.2) |
| Self-related health (VAS) | 459; 81.0 (16.5) | 396; 81.1 (15.4) | 380; 79.9 (15.9) | 450; 81.9 (16.3) | 347; 82.3 (15.0) | 315; 80.8 (16.6) | 454; 79.9 (17.1) | 346; 82.3 (14.1) | 326; 79.9 (17.8) |
| Sleep outcome | |||||||||
| Time spent asleep last night (hours) | 459; 6.6 (1.3) | 396; 6.8 (1.2) | 378; 6.6 (1.2) | 449; 6.5 (1.4) | 346; 6.6 (1.4) | 315; 6.6 (1.3) | 455; 6.5 (1.3) | 345; 6.7 (1.2) | 325; 6.6 (1.3) |
| Average sleep duration (hours/night) | 459; 7.4 (1.5) | 395; 7.5 (1.5) | 378; 7.4 (1.6) | 448; 7.4 (1.4) | 343; 7.4 (1.5) | 314; 7.4 (1.5) | 455; 7.4 (1.6) | 345; 7.4 (1.4) | 325; 7.3 (1.6) |
| Variable | 12 months, mean (97.5% CI) | 48 months, mean (97.5% CI) | ||
|---|---|---|---|---|
| Walking Away vs. control | Walking Away Plus vs. control | Walking Away vs. control | Walking Away Plus vs. control | |
| Depression and anxiety | ||||
| Depression score | –0.21 (–0.56 to 0.13) | –0.34 (–0.70 to 0.02) | 0.05 (–0.37 to 0.47) | –0.09 (–0.54 to 0.37) |
| Anxiety score | –0.21 (–0.63 to 0.20) | –0.14 (–0.59 to 0.32) | 0.19 (–0.30 to 0.68) | –0.31 (–0.84 to 0.23) |
| Health-related quality of life | ||||
| Summary mental component score (SF-8) | 0.49 (–0.72 to 1.70) | 0.56 (–0.72 to 1.83) | 0.43 (–0.97 to 1.83) | 0.54 (–0.89 to 1.97) |
| Summary physical component score (SF-8) | 1.07 (–0.11 to 2.25) | 0.57 (–0.65 to 1.80) | –0.31 (–1.61 to 0.99) | –0.59 (–1.94 to 0.76) |
| Summary index (EQ-5D-5L) | 0.02 (0.00 to 0.04) | 0.00 (–0.02 to 0.02) | 0.00 (–0.03 to 0.03) | 0.01 (–0.02 to 0.03) |
| Self-related health (VAS) | 0.19 (–1.75 to 2.14) | 1.19 (–0.78 to 3.16) | –0.17 (–2.45 to 2.12) | –0.26 (–2.66 to 2.14) |
| Sleep outcome | ||||
| Time spent asleep last night (hours) | –0.15 (–0.33 to 0.02) | –0.09 (–0.27 to 0.08) | 0.03 (–0.16 to 0.23) | 0.09 (–0.11 to 0.28) |
| Average sleep duration (hours/night) | –0.12 (–0.34 to 0.10) | –0.05 (–0.27 to 0.16) | –0.02 (–0.26 to 0.23) | –0.03 (–0.28 to 0.22) |
Appendix 10 Comparison of PROPELS and simulated populations
| South Asian | Non-South Asian | |||
|---|---|---|---|---|
| Simulated | PROPELS | Simulated | PROPELS | |
| Site (%) | ||||
| Leicester | 97 | 97 | 58 | 58 |
| Cambridge | 3 | 3 | 42 | 42 |
| Arm (%) | ||||
| Usual care | 35 | 34 | 34 | 34 |
| Walking Away | 32 | 32 | 32 | 33 |
| Walking Away Plus | 34 | 34 | 33 | 33 |
| Sex (%) | ||||
| Male | 58 | 58 | 49 | 49 |
| Female | 42 | 42 | 51 | 51 |
| Ethnicity (%) | ||||
| Indian | 85 | 86 | 0 | 0 |
| Pakistani | 5 | 5 | 0 | 0 |
| Bangladeshi | 1 | 1 | 0 | 0 |
| Other Asian (excluding Chinese) | 9 | 9 | 0 | 0 |
| White British | 0 | 0 | 88 | 88 |
| White Irish | 0 | 0 | 1 | 1 |
| Other white | 0 | 0 | 4 | 4 |
| White and black Caribbean | 0 | 0 | 0 | 0 |
| White and black African | 0 | 0 | 0 | 0 |
| White and Asian | 0 | 0 | 0 | 0 |
| Other mixed race | 0 | 0 | 1 | 1 |
| Chinese | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 |
| Black Caribbean | 0 | 0 | 2 | 2 |
| Black African | 0 | 0 | 3 | 3 |
| Other black | 0 | 0 | 0 | 0 |
| Smoking (%) | ||||
| Never | 74 | 73 | 47 | 47 |
| Ex-smoker | 18 | 18 | 44 | 43 |
| Current smoker | 8 | 9 | 10 | 10 |
| Atrial fibrillation (%) | ||||
| No | 100 | 99 | 94 | 95 |
| Yes | 0 | 1 | 5 | 4 |
| Unknown | 0 | 0 | 1 | 1 |
| Statins (%) | ||||
| No | 75 | 75 | 72 | 71 |
| Yes | 24 | 25 | 28 | 28 |
| Unknown | 0 | 0 | 0 | 0 |
| Antihypertensives (%) | ||||
| No | 69 | 69 | 59 | 59 |
| Yes | 31 | 31 | 41 | 40 |
| Diabetes (%) | ||||
| No | 100 | 100 | 100 | 100 |
| Yes | 0 | 0 | 0 | 0 |
| Angina (%) | ||||
| No | 99 | 98 | 93 | 93 |
| Yes | 1 | 2 | 6 | 6 |
| Unknown | 0 | 0 | 1 | 1 |
| MI (%) | ||||
| No | 99 | 97 | 97 | 95 |
| Yes | 1 | 3 | 3 | 5 |
| Unknown | 0 | 0 | 0 | 0 |
| Stroke (%) | ||||
| No | 100 | 99 | 95 | 97 |
| Yes | 0 | 1 | 3 | 2 |
| Unknown | 0 | 0 | 1 | 0 |
| IMD centile (%) | ||||
| 1 | 9 | 10 | 10 | 10 |
| 2 | 15 | 15 | 7 | 7 |
| 3 | 13 | 12 | 7 | 7 |
| 4 | 18 | 18 | 8 | 8 |
| 5 | 14 | 13 | 10 | 9 |
| 6 | 10 | 10 | 9 | 9 |
| 7 | 10 | 10 | 12 | 11 |
| 8 | 5 | 5 | 11 | 11 |
| 9 | 5 | 5 | 14 | 14 |
| 10 | 1 | 2 | 12 | 12 |
| Height (m) | 1.64 | 1.64 | 1.68 | 1.68 |
| BMI (kg/m2) | 27.44 | 27.45 | 29.72 | 29.72 |
| Total cholesterol (mmol/l) | 4.96 | 4.96 | 5.24 | 5.25 |
| Systolic blood pressure (mmHg) | 128.63 | 128.74 | 132.39 | 132.38 |
| Waist circumference (cm) | 95.91 | 95.81 | 99.79 | 99.66 |
| Drinks per occasion | 1.61 | 1.60 | 2.07 | 2.07 |
| Drinking occasions per week | 2.07 | 2.06 | 3.08 | 3.08 |
| Steps per day at baseline | 7042 | 7038 | 7164 | 7196 |
| HDL (mmol/l) | 1.34 | 1.34 | 1.46 | 1.47 |
| Age (years) | 54.69 | 54.61 | 60.78 | 60.74 |
| HbA1c at baseline (mmol/mol) | 5.81 | 5.81 | 5.86 | 5.85 |
| GP visits in last year at baseline | 4.05 | 5.12 | 3.20 | 4.20 |
Appendix 11 Microcosting details for Walking Away and Walking Away Plus
| Number | Unit cost (£) | Total cost (£) | |
|---|---|---|---|
| One-educator sessions | 123 | 81.48 | 10,022 |
| Two-educator sessions | 259 | 162.96 | 42,207 |
| Total educator cost | 52,229 | ||
| Leicester | Cambridge | |
|---|---|---|
| Costs of travel expenses | ||
| Number of sessions attended | 1418 | 766 |
| Taxi | 2.6% | – |
| Taxi (average cost of return journey) | £23.50 | – |
| Total cost of taxis | £866.40 | £0 |
| Bus fare | 2.3% | 1% |
| Bus fare (average cost) | £3.70 | £3 |
| Total cost of buses | £120.67 | £22.98 |
| Parking ticket | 79% | 30% |
| Parking ticket (average cost) | £3.80 | £4 |
| Total cost of parking | £4256.84 | £919.20 |
| Mileage | – | 5% |
| Mileage (average cost) | – | £7 |
| Total cost of mileage | £0 | £268.10 |
| Total cost of travel expenses per site | £5243.91 | £1210.28 |
| Total cost of travel expenses | £6454.19 | |
| Administration consumables | ||
| Number of letters sent | 2016 | 1076 |
| Cost per letter | £0.64 | £0.64 |
| Number of maps sent | 2016 | 0 |
| Cost per map | £0.16 | – |
| Total cost of administration consumables per site | £1613 | £689 |
| Booklets | ||
| Number of booklets | 606 | 301 |
| Cost per booklet | £7.32 | £7.32 |
| Total cost of booklets per site | £4436 | £2203 |
| Teaching resources | ||
| Number of sets of teaching resources | 1 | 3 |
| Cost per set of teaching resources | £500 | £500 |
| Total cost of paper (based on 10 sheets per session) | £63.24 | £42.16 |
| Number of packs of pens | 12 | 6 |
| Cost per pack of pens | £6.47 | £6.47 |
| Total cost of teaching resources | £641 | £1581 |
| Total cost of administration consumables and teaching resources per site | £6690 | £4473 |
| Total cost of administration consumables and teaching resources | £11,163 | |
Appendix 12 Regressions used to estimate diabetes diagnoses, step count and HbA1c in the School for Public Health Research model version 3.2
Regressions used to estimate diabetes diagnoses at 1 year
| Mean | 97.5% CI | |
|---|---|---|
| Walking Away (Walking Away = 1, 0 otherwise) | 1.55 | 0.52 to 4.63 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | 0.72 | 0.19 to 2.68 |
| Cambridge (1 = at the Cambridge site, 0 = otherwise) | 4.11 | 1.32 to 12.77 |
| Female (1 = female, 0 otherwise) | 0.54 | 0.2 to 1.44 |
| HbA1c (% scale) at baseline | 5.15 | 0.44 to 60.15 |
| HbA1c (% scale) at 1 year | 1.52 | 0.24 to 9.58 |
| Number of objectively measured steps per day/2000 at baseline | 1.00 | 0.6 to 1.67 |
| Number of objectively measured steps per day/2000 at 1 year | 0.80 | 0.49 to 1.32 |
| Mean | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –15.92 | 5.16 | –27.49 to –4.36 |
| Walking Away (Walking Away = 1, 0 otherwise) | 0.44 | 0.49 | –0.65 to 1.53 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | –0.33 | 0.59 | –1.64 to 0.99 |
| Cambridge (1 = at the Cambridge site, 0 = otherwise) | 1.41 | 0.51 | 0.28 to 2.55 |
| Female (1 = female, 0 otherwise) | –0.61 | 0.44 | –1.6 to 0.37 |
| HbA1c (% scale) at baseline | 1.64 | 1.10 | –0.82 to 4.1 |
| HbA1c (% scale) at 1 year | 0.42 | 0.82 | –1.42 to 2.26 |
| Number of objectively measured steps per day/2000 at baseline | 0.00 | 0.23 | –0.5 to 0.51 |
| Number of objectively measured steps per day/2000 at 1 year | –0.22 | 0.22 | –0.71 to 0.28 |
| Intercept | Walking Awaya | Walking Away Plusb | Cambridgec | Femaled | HbA1c (%) at baseline | HbA1c (%) at 12 months | Steps per day/2000 at baseline | Steps per day/2000 at 12 months | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 26.6279 | 0.0276 | –0.0493 | 0.5344 | –0.0596 | –3.6608 | –0.6545 | –0.1835 | –0.0141 |
| Walking Awaya | 0.0276 | 0.2370 | 0.1355 | 0.0054 | 0.0101 | –0.0459 | 0.0227 | –0.0101 | 0.0010 |
| Walking Away Plusb | –0.0493 | 0.1355 | 0.3436 | –0.0041 | 0.0010 | –0.0166 | 0.0077 | –0.0019 | –0.0068 |
| Cambridgec | 0.5344 | 0.0054 | –0.0041 | 0.2558 | –0.0097 | –0.1515 | 0.0390 | –0.0114 | 0.0003 |
| Femaled | –0.0596 | 0.0101 | 0.0010 | –0.0097 | 0.1918 | –0.0297 | 0.0248 | 0.0061 | –0.0009 |
| HbA1c (%) at baseline | –3.6608 | –0.0459 | –0.0166 | –0.1515 | –0.0297 | 1.2024 | –0.5813 | 0.0322 | –0.0179 |
| HbA1c (%) at 12 months | –0.6545 | 0.0227 | 0.0077 | 0.0390 | 0.0248 | –0.5813 | 0.6734 | –0.0090 | 0.0146 |
| Steps per day/2000 at baseline | –0.1835 | –0.0101 | –0.0019 | –0.0114 | 0.0061 | 0.0322 | –0.0090 | 0.0513 | –0.0369 |
| Steps per day/2000 at 12 months | –0.0141 | 0.0010 | –0.0068 | 0.0003 | –0.0009 | –0.0179 | 0.0146 | –0.0369 | 0.0493 |
Regressions used to estimate diabetes diagnoses at 4 years
| Mean | 97.5% CI | |
|---|---|---|
| Walking Away (Walking Away = 1, 0 otherwise) | 1.58 | 0.74 to 3.39 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | 1.25 | 0.57 to 2.74 |
| Cambridge (1 = at the Cambridge site, 0 = otherwise) | 1.08 | 0.53 to 2.18 |
| Female (1 = female, 0 otherwise) | 0.84 | 0.44 to 1.58 |
| HbA1c (% scale) at baseline | 4.92 | 1.26 to 19.3 |
| HbA1c (% scale) at 4 years | 5.94 | 2.54 to 13.88 |
| Number of objectively measured steps per day/2000 at baseline | 0.89 | 0.66 to 1.21 |
| Number of objectively measured steps per day/2000 at 4 years | 0.96 | 0.71 to 1.3 |
| Mean | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –22.59 | 3.37 | –30.15 to –15.04 |
| Walking Away (Walking Away = 1, 0 otherwise) | 0.46 | 0.34 | –0.3 to 1.22 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | 0.23 | 0.35 | –0.55 to 1.01 |
| Cambridge (1 = at the Cambridge site, 0 = otherwise) | 0.07 | 0.31 | –0.63 to 0.78 |
| Female (1 = female, 0 otherwise) | –0.18 | 0.28 | –0.81 to 0.46 |
| HbA1c (% scale) at baseline | 1.59 | 0.61 | 0.23 to 2.96 |
| HbA1c (% scale) at 1 year | 1.78 | 0.38 | 0.93 to 2.63 |
| Number of objectively measured steps per day/2000 at baseline | –0.11 | 0.14 | –0.42 to 0.19 |
| Number of objectively measured steps per day/2000 at 1 year | –0.04 | 0.13 | –0.34 to 0.26 |
| Intercept | Walking Awaya | Walking Away Plusb | Cambridgec | Femaled | HbA1c (%) at baseline | HbA1c (%) at 12 months | Steps per day/2000 at baseline | Steps per day/2000 at 12 months | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 11.3644 | –0.0409 | 0.0222 | 0.2312 | –0.0951 | –1.5575 | –0.2736 | –0.0320 | –0.0480 |
| Walking Awaya | –0.0409 | 0.1153 | 0.0613 | 0.0046 | –0.0004 | –0.0082 | 0.0057 | –0.0004 | –0.0020 |
| Walking Away Plusb | 0.0222 | 0.0613 | 0.1214 | 0.0005 | 0.0009 | –0.0075 | –0.0043 | –0.0010 | –0.0028 |
| Cambridgec | 0.2312 | 0.0046 | 0.0005 | 0.0990 | –0.0032 | –0.0722 | 0.0294 | –0.0003 | –0.0050 |
| Femaled | –0.0951 | –0.0004 | 0.0009 | –0.0032 | 0.0804 | –0.0082 | 0.0160 | 0.0009 | 0.0021 |
| HbA1c (%) at baseline | –1.5575 | –0.0082 | –0.0075 | –0.0722 | –0.0082 | 0.3712 | –0.1070 | 0.0104 | –0.0064 |
| HbA1c (%) at 12 months | –0.2736 | 0.0057 | –0.0043 | 0.0294 | 0.0160 | –0.1070 | 0.1433 | –0.0083 | 0.0121 |
| Steps per day/2000 at baseline | –0.0320 | –0.0004 | –0.0043 | –0.0003 | 0.0009 | 0.0104 | –0.0083 | 0.0185 | –0.0128 |
| Steps per day/2000 at 12 months | –0.0480 | –0.0020 | –0.0028 | –0.0050 | 0.0021 | –0.0064 | 0.0121 | –0.0128 | 0.0181 |
Regressions used to estimate HbA1c at 1 year
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –5.808 | 0.108 | –6.05 to –5.566 |
| Walking Away (1 = Walking Away, 0 = otherwise) | –0.018 | 0.014 | –0.048 to 0.013 |
| Walking Away Plus (1 = Walking Away Plus, 0 = otherwise) | –0.009 | 0.014 | –0.04 to 0.021 |
| HbA1c (%) at baseline | 0.854 | 0.019 | 0.812 to 0.896 |
| Cambridge (1 = Cambridge, 0 = Leicester) | –0.049 | 0.015 | –0.082 to –0.015 |
| White Irish (1 = white Irish, 0 = otherwise) | –0.062 | 0.076 | –0.232 to 0.109 |
| Any other white background (1 = Any other white background, 0 = otherwise) | 0.024 | 0.032 | –0.048 to 0.096 |
| White and black Caribbean (1 = white and black Caribbean, 0 = otherwise) | 0.017 | 0.142 | –0.302 to 0.336 |
| White and black African (1 = white and black African, 0 = otherwise) | –0.220 | 0.211 | –0.693 to 0.253 |
| White and Asian (1 = white and Asian, 0 = otherwise) | –0.240 | 0.248 | –0.796 to 0.317 |
| Any other mixed race (1= any other mixed race, 0 = otherwise) | 0.015 | 0.091 | –0.188 to 0.219 |
| Indian (1 = Indian, 0 = otherwise) | 0.018 | 0.015 | –0.015 to 0.051 |
| Pakistani (1 = Pakistani, 0 = otherwise) | 0.006 | 0.053 | –0.112 to 0.125 |
| Bangladeshi (1 = Bangladeshi, 0 = otherwise) | 0.420 | 0.169 | 0.041 to 0.798 |
| Any other Asian background (1 = any other Asian background, 0 = otherwise) | 0.029 | 0.041 | –0.064 to 0.121 |
| Chinese (1 = Chinese, 0 = otherwise) | 0.065 | 0.108 | –0.177 to 0.307 |
| Any other (1 = any other, 0 = otherwise) | 0.099 | 0.142 | –0.22 to 0.417 |
| Black Caribbean (1 = black Caribbean, 0 = otherwise) | 0.022 | 0.039 | –0.066 to 0.11 |
| Black African (1 = black African, 0 = otherwise) | 0.016 | 0.036 | –0.065 to 0.097 |
| Any other black background (1 = any other black background, 0 = otherwise) | 0.000 | 0.095 | –0.213 to 0.212 |
| Female (1 = female, 0 = otherwise) | –0.017 | 0.011 | –0.043 to 0.008 |
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | 6.902 | 0.826 | 5.051 to 8.752 |
| Walking Away (1 = Walking Away, 0 = otherwise) | –0.290 | 0.105 | –0.527 to –0.054 |
| Walking Away Plus (1 = Walking Away Plus, 0 = otherwise) | –0.290 | 0.105 | –0.526 to –0.055 |
| HbA1c (% scale) at baseline | –0.276 | 0.143 | –0.596 to 0.045 |
| Cambridge (1 = Cambridge site, 0 = otherwise) | –0.541 | 0.097 | –0.758 to –0.324 |
| Female (1 = female, 0 = otherwise) | 0.008 | 0.087 | –0.186 to 0.203 |
| Mean effect | Dispersion | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | Cambridge | White Irish | Any other white background | White and black Caribbean | White and black African | White and Asian | Any other mixed race | Indian | Pakistani | Bangladeshi | Any other Asian background | Chinese | Any other | Black Caribbean | Black African | Any other black background | Female | Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | Cambridge | Female | |
| Intercept | 0.01168105 | 1.27 × 10–6 | –1.87145 × 10–5 | –0.00201328 | 0.00045436 | –0.00048038 | –9.4 × 10–5 | –0.00037 | –5.5 × 10–5 | –0.00039 | 3.32 × 10–5 | –2.6 × 10–5 | 4.4 × 10–5 | 0.000441 | 0.000294 | 0.000269 | 0.000468 | 0.000124 | 3.53 × 10–5 | –0.00021 | –4.4 × 10–5 | –0.0049 | 3.79 × 10–6 | 1.04 × 10–5 | 0.000832 | –0.00017 | 2.59 × 10–5 |
| Walking Away | 1.274 × 10–6 | 0.000189 | 7.44238 × 10–5 | –1.3393 × 10–5 | 4.56248 × 10–7 | –6.2896 × 10–5 | –1.1 × 10–5 | –4.7 × 10–5 | 7.83 × 10–5 | –3 × 10–6 | –3.9 × 10–6 | 5.79 × 10–6 | 6.22 × 10–6 | –0.00011 | –3.2 × 10–6 | 2.25 × 10–5 | 7.95 × 10–5 | –1.1 × 10–5 | –9 × 10–6 | 5.23 × 10–5 | 4.55 × 10–6 | –1.6 × 10–5 | –7.3 × 10–5 | –2.9 × 10–5 | 7.63 × 10–6 | 3.14 × 10–7 | –1.2 × 10–6 |
| Walking Away Plus | –1.871 × 10–5 | 7.44 × 10–5 | 0.000186963 | –1.0079 × 10–5 | 1.33188 × 10–6 | –2.1033 × 10–5 | –4 × 10–7 | –7.2 × 10–5 | 7.76 × 10–5 | –0.00011 | –1.9 × 10–5 | 1.05 × 10–5 | –2.8 × 10–5 | 2.72 × 10–6 | –1.1 × 10–5 | 2.26 × 10–5 | 7.92 × 10–5 | 1.34 × 10–5 | 6.76 × 10–6 | 2.3 × 10–5 | 2.05 × 10–6 | –7.5 × 10–6 | –2.9 × 10–5 | –7.2 × 10–5 | 6.24 × 10–6 | 3.36 × 10–7 | –9.7 × 10–7 |
| HbA1c (%) at baseline | –0.0020133 | –1.3 × 10–5 | –1.00794 × 10–5 | 0.000351439 | –9.0674 × 10–5 | 8.22148 × 10–5 | 8.17 × 10–6 | 6.75 × 10–5 | –4.8 × 10–6 | 7.89 × 10–5 | –1.7 × 10–5 | –9.9 × 10–6 | –2.1 × 10–5 | –7.8 × 10–5 | –5.9 × 10–5 | –5 × 10–5 | –9 × 10–5 | –3.2 × 10–5 | –1.9 × 10–5 | 1.84 × 10–5 | –3.9 × 10–6 | 0.000835 | 4.26 × 10–6 | 3.11 × 10–6 | –0.00014 | 3.23 × 10–5 | –3 × 10–7 |
| Cambridge | 0.00045436 | 4.56 × 10–7 | 1.33188 × 10–6 | –9.0674 × 10–5 | 0.000221747 | –1.8629 × 10–5 | 3.39 × 10–6 | –2.1 × 10–5 | –0.00013 | –0.00015 | 4.17 × 10–5 | 6.75 × 10–5 | 7.23 × 10–5 | 9.07 × 10–5 | 5.51 × 10–5 | –0.00012 | –0.00011 | 7.64 × 10–5 | 7.24 × 10–5 | 6.15 × 10–5 | –1.5 × 10–6 | –0.00021 | 6.27 × 10–7 | 6.23 × 10–7 | 3.82 × 10–5 | –7.2 × 10–5 | 2.99 × 10–6 |
| White Irish | –0.0004804 | –6.3 × 10–5 | –2.10333 × 10–5 | 8.22148 × 10–5 | –1.8629 × 10–5 | 0.005781883 | 5.42 × 10–5 | 9.39 × 10–5 | 5.67 × 10–6 | 7.99 × 10–5 | 3.99 × 10–5 | 4.15 × 10–5 | 3.66 × 10–5 | 8.66 × 10–5 | 4.35 × 10–5 | 2.96 × 10–5 | 3.61 × 10–6 | 5.23 × 10–5 | 4.51 × 10–5 | 3.2 × 10–5 | –3.7 × 10–5 | –3.7 × 10–5 | –2.6 × 10–6 | –2.1 × 10–6 | 6.68 × 10–6 | 1.81 × 10–6 | –4.5 × 10–6 |
| Any other white background | –9.366 × 10–5 | –1.1 × 10–5 | –4.00544 × 10–7 | 8.16684 × 10–6 | 3.38596 × 10–6 | 5.42122 × 10–5 | 0.001033 | 5.17 × 10–5 | 4.12 × 10–5 | 4.48 × 10–5 | 4.88 × 10–5 | 4.99 × 10–5 | 4.87 × 10–5 | 5.67 × 10–5 | 4.86 × 10–5 | 4.31 × 10–5 | 4 × 10–5 | 5.1 × 10–5 | 5.06 × 10–5 | 4.79 × 10–5 | –1.5 × 10–6 | 4.25 × 10–6 | –3.5 × 10–7 | –3.4 × 10–7 | –3.7 × 10–7 | 1.25 × 10–6 | –3.5 × 10–6 |
| White and black Caribbean | –0.0003684 | –4.7 × 10–5 | –7.18814 × 10–5 | 6.75379 × 10–5 | –2.0932 × 10–5 | 9.3904 × 10–5 | 5.17 × 10–5 | 0.02028 | –1.6 × 10–5 | 0.000136 | 4.05 × 10–5 | 3.42 × 10–5 | 4.27 × 10–5 | 7.61 × 10–5 | 5.45 × 10–5 | 3.26 × 10–5 | 6.55 × 10–7 | 4.98 × 10–5 | 3.68 × 10–5 | 2.84 × 10–5 | –6.6 × 10–5 | 2.28 × 10–5 | –9.9 × 10–6 | –9.7 × 10–8 | –2.3 × 10–6 | –3.3 × 10–6 | –1.1 × 10–5 |
| White and black African | –5.493 × 10–5 | 7.83 × 10–5 | 7.75578 × 10–5 | –4.7795 × 10–6 | –0.00013156 | 5.67286 × 10–6 | 4.12 × 10–5 | –1.6 × 10–5 | 0.044524 | 6.79 × 10–5 | 2.8 × 10–5 | 1.73 × 10–5 | 6.94 × 10–6 | –6.3 × 10–5 | 4.18 × 10–6 | 0.000148 | 0.000182 | –8 × 10–6 | 8.6 × 10–6 | 3.56 × 10–5 | 6.86 × 10–5 | 6.64 × 10–5 | 2.35 × 10–5 | 2.33 × 10–5 | –1.5 × 10–5 | –2.5 × 10–6 | 1.21 × 10–5 |
| White and Asian | –0.0003921 | –3 × 10–6 | –0.000112439 | 7.88793 × 10–5 | –0.00014909 | 7.98558 × 10–5 | 4.48 × 10–5 | 0.000136 | 6.79 × 10–5 | 0.061634 | 2.21 × 10–5 | –9 × 10–6 | 1.24 × 10–5 | –1.4 × 10–5 | 3 × 10–5 | 0.000113 | 8.04 × 10–5 | –3.3 × 10–6 | –1.2 × 10–5 | –2 × 10–6 | –6.4 × 10–5 | –0.00013 | –1.1 × 10–6 | –5.2 × 10–5 | 2.86 × 10–5 | –4.6 × 10–5 | –3.7 × 10–5 |
| Any other mixed race | 3.3198 × 10–5 | –3.9 × 10–6 | –1.92029 × 10–5 | –1.7135 × 10–5 | 4.1678 × 10–5 | 3.99295 × 10–5 | 4.88 × 10–5 | 4.05 × 10–5 | 2.8 × 10–5 | 2.21 × 10–5 | 0.00822 | 6.27 × 10–5 | 6.89 × 10–5 | 5.19 × 10–5 | 5.57 × 10–5 | 2.45 × 10–5 | 2.13 × 10–5 | 5.72 × 10–5 | 6.31 × 10–5 | 6.18 × 10–5 | 2.17 × 10–5 | 2.26 × 10–5 | 8.43 × 10–6 | 2.83 × 10–6 | –4.2 × 10–6 | 1.84 × 10–7 | –2.1 × 10–6 |
| Indian | –2.554 × 10–5 | 5.79 × 10–6 | 1.05123 × 10–5 | –9.8789 × 10–6 | 6.75188 × 10–5 | 4.15499 × 10–5 | 4.99 × 10–5 | 3.42 × 10–5 | 1.73 × 10–5 | –9 × 10–6 | 6.27 × 10–5 | 0.000218 | 7.19 × 10–5 | 6.53 × 10–5 | 5.97 × 10–5 | 8.38 × 10–6 | 1.28 × 10–5 | 6.97 × 10–5 | 7.27 × 10–5 | 7.38 × 10–5 | 1.38 × 10–5 | 1.05 × 10–5 | 6.87 × 10–7 | 6.61 × 10–7 | –1.7 × 10–6 | –4 × 10–7 | –9.2 × 10–7 |
| Pakistani | 4.399 × 10–5 | 6.22 × 10–6 | –2.83273 × 10–5 | –2.0545 × 10–5 | 7.23032 × 10–5 | 3.65705 × 10–5 | 4.87 × 10–5 | 4.27 × 10–5 | 6.94 × 10–6 | 1.24 × 10–5 | 6.89 × 10–5 | 7.19 × 10–5 | 0.002776 | 5.42 × 10–5 | 6.35 × 10–5 | 5.3 × 10–6 | 2.59 × 10–6 | 6.62 × 10–5 | 7.19 × 10–5 | 7.39 × 10–5 | 1.89 × 10–5 | –6.9 × 10–5 | –2.4 × 10–6 | –9.4 × 10–7 | 1.26 × 10–5 | –2.8 × 10–6 | –5 × 10–6 |
| Bangladeshi | 0.00044117 | –0.00011 | 2.71825 × 10–6 | –7.8058 × 10–5 | 9.07182 × 10–5 | 8.66005 × 10–5 | 5.67 × 10–5 | 7.61 × 10–5 | –6.3 × 10–5 | –1.4 × 10–5 | 5.19 × 10–5 | 6.53 × 10–5 | 5.42 × 10–5 | 0.028498 | 8.9 × 10–5 | 5.51 × 10–6 | –1 × 10–5 | 0.000104 | 7.98 × 10–5 | 2.66 × 10–5 | –6.7 × 10–5 | –6.4 × 10–5 | 3.25 × 10–5 | 7.97 × 10–7 | 9.65 × 10–6 | –1.5 × 10–5 | 1.86 × 10–5 |
| Any other Asian background | 0.00029433 | –3.2 × 10–6 | –1.10377 × 10–5 | –5.8925 × 10–5 | 5.50595 × 10–5 | 4.35291 × 10–5 | 4.86 × 10–5 | 5.45 × 10–5 | 4.18 × 10–6 | 3 × 10–5 | 5.57 × 10–5 | 5.97 × 10–5 | 6.35 × 10–5 | 8.9 × 10–5 | 0.001718 | 2.93 × 10–5 | 3.23 × 10–5 | 7.17 × 10–5 | 6.27 × 10–5 | 5.36 × 10–5 | –2.7 × 10–5 | –7 × 10–6 | 8.18 × 10–7 | 3.52 × 10–6 | 1.07 × 10–6 | –1.2 × 10–6 | 5.89 × 10–7 |
| Chinese | 0.00026907 | 2.25 × 10–5 | 2.25703 × 10–5 | –4.9943 × 10–5 | –0.00011793 | 2.96131 × 10–5 | 4.31 × 10–5 | 3.26 × 10–5 | 0.000148 | 0.000113 | 2.45 × 10–5 | 8.38 × 10–6 | 5.3 × 10–6 | 5.51 × 10–6 | 2.93 × 10–5 | 0.011652 | 0.00016 | 8.55 × 10–6 | 6.81 × 10–6 | 7.78 × 10–6 | 2.57 × 10–6 | 2.7 × 10–5 | 6.39 × 10–6 | 5.11 × 10–6 | –5.9 × 10–6 | 1.61 × 10–5 | 1.72 × 10–6 |
| Any other | 0.00046847 | 7.95 × 10–5 | 7.92104 × 10–5 | –9.0362 × 10–5 | –0.00010848 | 3.61323 × 10–6 | 4 × 10–5 | 6.55 × 10–7 | 0.000182 | 8.04 × 10–5 | 2.13 × 10–5 | 1.28 × 10–5 | 2.59 × 10–6 | –1 × 10–5 | 3.23 × 10–5 | 0.00016 | 0.020157 | 1.24 × 10–5 | 8.3 × 10–6 | 2.18 × 10–5 | 4.21 × 10–6 | –1 × 10–5 | –7.1 × 10–6 | –6.9 × 10–6 | 1.75 × 10–6 | 2.45 × 10–5 | –2.7 × 10–6 |
| Black Caribbean | 0.00012424 | –1.1 × 10–5 | 1.34193 × 10–5 | –3.2102 × 10–5 | 7.63663 × 10–5 | 5.22836 × 10–5 | 5.1 × 10–5 | 4.98 × 10–5 | –8 × 10–6 | –3.3 × 10–6 | 5.72 × 10–5 | 6.97 × 10–5 | 6.62 × 10–5 | 0.000104 | 7.17 × 10–5 | 8.55 × 10–6 | 1.24 × 10–5 | 0.001555 | 7.32 × 10–5 | 6.35 × 10–5 | –2.5 × 10–5 | 1.78 × 10–5 | 2.89 × 10–6 | 3.57 × 10–7 | –3.2 × 10–6 | –1.3 × 10–6 | 1.41 × 10–6 |
| Black African | 3.5271 × 10–5 | –9 × 10–6 | 6.75976 × 10–6 | –1.9374 × 10–5 | 7.23844 × 10–5 | 4.50839 × 10–5 | 5.06 × 10–5 | 3.68 × 10–5 | 8.6 × 10–6 | –1.2 × 10–5 | 6.31 × 10–5 | 7.27 × 10–5 | 7.19 × 10–5 | 7.98 × 10–5 | 6.27 × 10–5 | 6.81 × 10–6 | 8.3 × 10–6 | 7.32 × 10–5 | 0.001305 | 6.93 × 10–5 | 1.02 × 10–5 | 2.96 × 10–5 | 1.16 × 10–6 | 1.55 × 10–6 | –5.1 × 10–6 | 1.83 × 10–7 | –6.5 × 10–7 |
| Any other black background | –0.0002078 | 5.23 × 10–5 | 2.30185 × 10–5 | 1.84499 × 10–5 | 6.15028 × 10–5 | 3.19581 × 10–5 | 4.79 × 10–5 | 2.84 × 10–5 | 3.56 × 10–5 | –2 × 10–6 | 6.18 × 10–5 | 7.38 × 10–5 | 7.39 × 10–5 | 2.66 × 10–5 | 5.36 × 10–5 | 7.78 × 10–6 | 2.18 × 10–5 | 6.35 × 10–5 | 6.93 × 10–5 | 0.008951 | 1.82 × 10–5 | 1.36 × 10–5 | 3.72 × 10–6 | 1.02 × 10–5 | –2.7 × 10–6 | –4.9 × 10–7 | –3 × 10–6 |
| Female | –4.409 × 10–5 | 4.55 × 10–6 | 2.04903 × 10–6 | –3.8695 × 10–6 | –1.5446 × 10–6 | –3.6606 × 10–5 | –1.5 × 10–6 | –6.6 × 10–5 | 6.86 × 10–5 | –6.4 × 10–5 | 2.17 × 10–5 | 1.38 × 10–5 | 1.89 × 10–5 | –6.7 × 10–5 | –2.7 × 10–5 | 2.57 × 10–6 | 4.21 × 10–6 | –2.5 × 10–5 | 1.02 × 10–5 | 1.82 × 10–5 | 0.00013 | 2.57 × 10–5 | –1.2 × 10–6 | –9.4 × 10–7 | –3.5 × 10–7 | 3.18 × 10–6 | –5 × 10–5 |
| Intercept | –0.0049018 | –1.6 × 10–5 | –7.48844 × 10–6 | 0.000835017 | –0.0002075 | –3.7319 × 10–5 | 4.25 × 10–6 | 2.28 × 10–5 | 6.64 × 10–5 | –0.00013 | 2.26 × 10–5 | 1.05 × 10–5 | –6.9 × 10–5 | –6.4 × 10–5 | –7 × 10–6 | 2.7 × 10–5 | –1 × 10–5 | 1.78 × 10–5 | 2.96 × 10–5 | 1.36 × 10–5 | 2.57 × 10–5 | 0.681713 | –0.00081 | –0.00238 | –0.11749 | 0.026507 | –0.00171 |
| Walking Away | 3.7877 × 10–6 | –7.3 × 10–5 | –2.89186 × 10–5 | 4.25617 × 10–6 | 6.27466 × 10–7 | –2.6456 × 10–6 | –3.5 × 10–7 | –9.9 × 10–6 | 2.35 × 10–5 | –1.1 × 10–6 | 8.43 × 10–6 | 6.87 × 10–7 | –2.4 × 10–6 | 3.25 × 10–5 | 8.18 × 10–7 | 6.39 × 10–6 | –7.1 × 10–6 | 2.89 × 10–6 | 1.16 × 10–6 | 3.72 × 10–6 | –1.2 × 10–6 | –0.00081 | 0.011124 | 0.005188 | –0.00076 | 2.39 × 10–6 | 0.000162 |
| Walking Away Plus | 1.043 × 10–5 | –2.9 × 10–5 | –7.23591 × 10–5 | 3.10822 × 10–6 | 6.227 × 10–7 | –2.072 × 10–6 | –3.4 × 10–7 | –9.7 × 10–8 | 2.33 × 10–5 | –5.2 × 10–5 | 2.83 × 10–6 | 6.61 × 10–7 | –9.4 × 10–7 | 7.97 × 10–7 | 3.52 × 10–6 | 5.11 × 10–6 | –6.9 × 10–6 | 3.57 × 10–7 | 1.55 × 10–6 | 1.02 × 10–5 | –9.4 × 10–7 | –0.00238 | 0.005188 | 0.011018 | –0.00048 | –9.1 × 10–5 | 3.41 × 10–5 |
| HbA1c (%) at baseline | 0.00083196 | 7.63 × 10–6 | 6.23905 × 10–6 | –0.00014301 | 3.82398 × 10–5 | 6.67894 × 10–6 | –3.7 × 10–7 | –2.3 × 10–6 | –1.5 × 10–5 | 2.86 × 10–5 | –4.2 × 10–6 | –1.7 × 10–6 | 1.26 × 10–5 | 9.65 × 10–6 | 1.07 × 10–6 | –5.9 × 10–6 | 1.75 × 10–6 | –3.2 × 10–6 | –5.1 × 10–6 | –2.7 × 10–6 | –3.5 × 10–7 | –0.11749 | –0.00076 | –0.00048 | 0.020484 | –0.00508 | –0.00033 |
| Cambridge | –0.000173 | 3.14 × 10–7 | 3.36459 × 10–7 | 3.23157 × 10–5 | –7.2197 × 10–5 | 1.81277 × 10–6 | 1.25 × 10–6 | –3.3 × 10–6 | –2.5 × 10–6 | –4.6 × 10–5 | 1.84 × 10–7 | –4 × 10–7 | –2.8 × 10–6 | –1.5 × 10–5 | –1.2 × 10–6 | 1.61 × 10–5 | 2.45 × 10–5 | –1.3 × 10–6 | 1.83 × 10–7 | –4.9 × 10–7 | 3.18 × 10–6 | 0.026507 | 2.39 × 10–6 | –9.1 × 10–5 | –0.00508 | 0.009399 | –0.00026 |
| Female | 2.592 × 10–5 | –1.2 × 10–6 | –9.69453 × 10–7 | –3.0131 × 10–7 | 2.98605 × 10–6 | –4.5358 × 10–6 | –3.5 × 10–6 | –1.1 × 10–5 | 1.21 × 10–5 | –3.7 × 10–5 | –2.1 × 10–6 | –9.2 × 10–7 | –5 × 10–6 | 1.86 × 10–5 | 5.89 × 10–7 | 1.72 × 10–6 | –2.7 × 10–6 | 1.41 × 10–6 | –6.5 × 10–7 | –3 × 10–6 | –5 × 10–5 | –0.00171 | 0.000162 | 3.41 × 10–5 | –0.00033 | –0.00026 | 0.007521 |
Regressions used to estimate HbA1c at 4 years
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –6.428 | 0.163 | –6.795 to –6.062 |
| Walking Away (Walking Away = 1, 0 otherwise) | –0.004 | 0.019 | –0.046 to 0.038 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | –0.016 | 0.019 | –0.057 to 0.026 |
| HbA1c (%) at baseline | 0.272 | 0.042 | 0.178 to 0.365 |
| Cambridge (1 = Cambridge, 0 = Leicester) | 0.689 | 0.042 | 0.595 to 0.783 |
| White Irish (1 = white Irish, 0 = otherwise) | –0.132 | 0.019 | –0.175 to –0.089 |
| Any other white background (1 = any other white background, 0 = otherwise) | 0.246 | 0.086 | 0.054 to 0.439 |
| White and black Caribbean (1 = white and black Caribbean, 0 = otherwise) | 0.090 | 0.039 | 0.001 to 0.178 |
| White and black African (1 = white and black African, 0 = otherwise) | 0.106 | 0.141 | –0.211 to 0.423 |
| White and Asian (1 = white and Asian, 0 = otherwise) | 0.280 | 0.206 | –0.181 to 0.741 |
| Any other mixed race (1= any other mixed race, 0 = otherwise) | –0.255 | 0.189 | –0.678 to 0.168 |
| Indian (1 = Indian, 0 = otherwise) | –0.005 | 0.129 | –0.293 to 0.284 |
| Pakistani (1 = Pakistani, 0 = otherwise) | 0.020 | 0.022 | –0.03 to 0.07 |
| Bangladeshi (1 = Bangladeshi, 0 = otherwise) | –0.002 | 0.079 | –0.178 to 0.175 |
| Any other Asian background (1 = any other Asian background, 0 = otherwise) | –0.022 | 0.316 | –0.73 to 0.687 |
| Chinese (1 = Chinese, 0 = otherwise) | –0.024 | 0.056 | –0.149 to 0.102 |
| Any other (1 = any other, 0 = otherwise) | –0.061 | 0.118 | –0.326 to 0.203 |
| Black Caribbean (1 = black Caribbean, 0 = otherwise) | 0.142 | 0.166 | –0.229 to 0.514 |
| Black African (1 = black African, 0 = otherwise) | 0.044 | 0.059 | –0.089 to 0.177 |
| Any other black background (1 = any other black background, 0 = otherwise) | 0.170 | 0.054 | 0.05 to 0.29 |
| Female (1 = female, 0 otherwise) | –0.008 | 0.140 | –0.322 to 0.306 |
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | 9.624 | 0.923 | 7.555 to 11.693 |
| Walking Away (Walking Away = 1, 0 otherwise) | –0.274 | 0.112 | –0.524 to –0.024 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | –0.258 | 0.111 | –0.508 to –0.009 |
| HbA1c (% scale) at baseline | 0.658 | 0.247 | 0.104 to 1.212 |
| Cambridge (1 = Cambridge site, 0 = otherwise) | –1.571 | 0.238 | –2.103 to –1.038 |
| Female (1 = female, 0 otherwise) | 0.190 | 0.105 | –0.045 to 0.424 |
| Mean effect | Dispersion | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | Cambridge | White Irish | Any other white background | White and black Caribbean | White and black African | White and Asian | Any other mixed race | Indian | Pakistani | Bangladeshi | Any other Asian background | Chinese | Any other | Black Caribbean | Black African | Any other black background | Female | Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | HbA1c (%) at 12 months | Cambridge | Female | |
| Intercept | 0.02668525 | –2.84 × 10–5 | –4.94 × 10–5 | –0.00205223 | –0.0025428 | 0.00083728 | –0.00128 | –0.00023 | –0.00071 | –0.00046 | –0.00123 | –0.00025 | 0.000116 | –0.00033 | 0.002279 | 0.000557 | 0.000674 | 0.00144 | 0.000272 | 0.000145 | –0.00055 | –9.90 × 10–5 | 0.000637 | –0.00929 | 2.72 × 10–5 | 2.15 × 10–5 | 0.001084 | 0.000491 |
| Walking Away | –2.84 × 10–5 | 0.00035 | 0.000142219 | –8.21 × 10–5 | 6.13 × 10–5 | 8.49 × 10–6 | –9.35 × 10–5 | –1.76 × 10–5 | –0.00013 | 0.000157 | 5.18 × 10–6 | 4.06 × 10–5 | 1.14 × 10–5 | 1.14 × 10–5 | –0.00023 | 3.50 × 10–6 | 7.63 × 10–5 | 0.00014 | –5.64 × 10–6 | 1.84 × 10–6 | 0.00011 | 5.66 × 10–6 | –3.66 × 10–5 | –1.82 × 10–5 | –0.00013 | –5.28 × 10–5 | 2.69 × 10–5 | –1.45 × 10–5 |
| Walking Away Plus | –4.94 × 10–5 | 0.000142 | 0.000345322 | –6.42 × 10–5 | 4.73 × 10–5 | 5.68 × 10–6 | –5.92 × 10–5 | –1.21 × 10–5 | –8.09 × 10–5 | 0.000154 | –0.00019 | –1.29 × 10–6 | 2.23 × 10–5 | –7.40 × 10–5 | –1.43 × 10–5 | 1.68 × 10–5 | 2.19 × 10–5 | 0.000145 | 1.12 × 10–5 | 3.60 × 10–5 | 8.33 × 10–5 | –5.14 × 10–7 | 2.69 × 10–5 | –1.82 × 10–5 | –5.27 × 10–5 | –0.00013 | 2.13 × 10–5 | –9.19 × 10–6 |
| HbA1c (%) at baseline | –0.0020522 | –8.21 × 10–5 | –6.42 × 10–5 | 0.001756146 | –0.00136559 | –0.00029728 | 0.000171 | 5.23 × 10–5 | 0.000128 | –0.00027 | –9.47 × 10–5 | 5.70 × 10–5 | –2.39 × 10–5 | 8.12 × 10–5 | 0.000502 | –3.86 × 10–5 | –4.11 × 10–5 | 7.12 × 10–5 | –2.98 × 10–5 | –5.67 × 10–5 | 9.97 × 10–5 | –2.98 × 10–5 | –7.15 × 10–5 | 0.001177 | 2.57 × 10–5 | 2.05 × 10–5 | –0.00063 | 0.00042 |
| Cambridge | –0.0025428 | 6.13 × 10–5 | 4.73 × 10–5 | –0.00136559 | 0.001777184 | 0.000122123 | 4.27 × 10–5 | –2.32 × 10–5 | 5.47 × 10–6 | 0.000306 | 0.000322 | –4.06 × 10–5 | –2.74 × 10–5 | –4.89 × 10–5 | –0.00089 | –7.35 × 10–5 | –8.24 × 10–5 | –0.00033 | –4.13 × 10–5 | 2.28 × 10–7 | –4.37 × 10–5 | 1.81 × 10–5 | –5.09 × 10–5 | 0.000389 | –2.12 × 10–5 | –1.53 × 10–5 | 0.000436 | –0.00049 |
| White Irish | 0.00083728 | 8.49 × 10–6 | 5.68 × 10–6 | –0.00029728 | 0.000122123 | 0.00037068 | 7.69 × 10–6 | –3.51 × 10–6 | –6.80 × 10–5 | –0.00013 | –0.00017 | 7.51 × 10–5 | 0.000151 | 0.000131 | 0.000143 | 9.77 × 10–5 | –0.00013 | –0.00013 | 0.000163 | 0.000163 | 0.000122 | 9.50 × 10–7 | –7.41 × 10–5 | –0.00028 | –5.49 × 10–7 | 8.66 × 10–7 | 9.56 × 10–5 | –4.08 × 10–5 |
| Any other white background | –0.0012811 | –9.35 × 10–5 | –5.92 × 10–5 | 0.000171217 | 4.27 × 10–5 | 7.69 × 10–6 | 0.007397 | 9.83 × 10–5 | 0.000156 | –1.22 × 10–6 | 0.000125 | 9.78 × 10–5 | 9.54 × 10–5 | 0.000127 | 9.43 × 10–5 | 7.52 × 10–5 | 4.73 × 10–6 | –4.78 × 10–5 | 0.0001 | 9.64 × 10–5 | 0.0001 | –2.44 × 10–5 | 2.99 × 10–5 | 0.000191 | 1.22 × 10–5 | 8.39 × 10–6 | –4.48 × 10–5 | 1.29 × 10–5 |
| White and black Caribbean | –0.0002303 | –1.76 × 10–5 | –1.21 × 10–5 | 5.23 × 10–5 | –2.32 × 10–5 | –3.51 × 10–6 | 9.83 × 10–5 | 0.001557 | 9.93 × 10–5 | 5.45 × 10–5 | 9.00 × 10–5 | 8.23 × 10–5 | 8.16 × 10–5 | 8.77 × 10–5 | 0.000102 | 8.41 × 10–5 | 7.14 × 10–5 | 6.63 × 10–5 | 8.56 × 10–5 | 8.17 × 10–5 | 8.19 × 10–5 | –1.76 × 10–5 | –2.99 × 10–5 | 5.83 × 10–5 | 1.12 × 10–6 | 3.20 × 10–6 | –5.92 × 10–6 | –3.86 × 10–6 |
| White and black African | –0.0007135 | –0.00013 | –8.09 × 10–5 | 0.000128317 | 5.47 × 10–6 | –6.80 × 10–5 | 0.000156 | 9.93 × 10–5 | 0.019946 | –2.02 × 10–5 | 0.000181 | 5.52 × 10–5 | 4.81 × 10–5 | 7.21 × 10–5 | 0.000132 | 7.96 × 10–5 | 5.87 × 10–5 | 9.21 × 10–6 | 7.11 × 10–5 | 5.12 × 10–5 | 2.53 × 10–5 | –9.39 × 10–5 | 6.23 × 10–5 | 0.000133 | 1.67 × 10–5 | 4.85 × 10–6 | –3.02 × 10–5 | 6.37 × 10–6 |
| White and Asian | –0.0004594 | 0.000157 | 0.00015444 | –0.00026641 | 0.000305801 | –0.00013221 | –1.22 × 10–6 | 5.45 × 10–5 | –2.02 × 10–5 | 0.042328 | 9.84 × 10–5 | 6.76 × 10–5 | 3.53 × 10–5 | –4.71 × 10–6 | –0.00026 | 3.40 × 10–6 | 0.000183 | 0.000202 | –1.57 × 10–5 | 3.15 × 10–5 | 8.10 × 10–5 | 0.000171 | 0.000161 | 0.000137 | –1.68 × 10–5 | –1.57 × 10–5 | 7.28 × 10–6 | –2.53 × 10–5 |
| Any other mixed race | –0.0012265 | 5.18 × 10–6 | –0.000192553 | –9.47 × 10–5 | 0.000322395 | –0.00017357 | 0.000125 | 9.00 × 10–5 | 0.000181 | 9.84 × 10–5 | 0.035556 | 4.00 × 10–5 | –2.08 × 10–5 | 5.72 × 10–5 | –0.00022 | 2.52 × 10–5 | 0.000136 | 5.21 × 10–6 | –7.59 × 10–6 | –3.00 × 10–5 | –2.16 × 10–5 | –8.57 × 10–5 | 5.25 × 10–5 | –1.36 × 10–5 | –8.40 × 10–7 | 4.53 × 10–6 | –4.20 × 10–5 | 4.41 × 10–5 |
| Indian | –0.0002492 | 4.06 × 10–5 | –1.29 × 10–6 | 5.70 × 10–5 | –4.06 × 10–5 | 7.51 × 10–5 | 9.78 × 10–5 | 8.23 × 10–5 | 5.52 × 10–5 | 6.76 × 10–5 | 4.00 × 10–5 | 0.016569 | 0.000128 | 0.000139 | 9.84 × 10–5 | 9.67 × 10–5 | 4.90 × 10–5 | 5.46 × 10–5 | 0.000121 | 0.000125 | 0.000147 | 3.23 × 10–5 | 4.84 × 10–5 | 0.000187 | 1.70 × 10–6 | –1.17 × 10–5 | –2.39 × 10–5 | –7.25 × 10–6 |
| Pakistani | 0.00011563 | 1.14 × 10–5 | 2.23 × 10–5 | –2.39 × 10–5 | –2.74 × 10–5 | 0.000150831 | 9.54 × 10–5 | 8.16 × 10–5 | 4.81 × 10–5 | 3.53 × 10–5 | –2.08 × 10–5 | 0.000128 | 0.0005 | 0.00015 | 0.00017 | 0.000123 | 2.29 × 10–5 | 3.99 × 10–5 | 0.000156 | 0.000161 | 0.000159 | 2.36 × 10–5 | 4.74 × 10–5 | 1.68 × 10–5 | 2.36 × 10–6 | 1.85 × 10–6 | 1.86 × 10–6 | –4.85 × 10–6 |
| Bangladeshi | –0.0003298 | 1.14 × 10–5 | –7.40 × 10–5 | 8.12 × 10–5 | –4.89 × 10–5 | 0.000131257 | 0.000127 | 8.77 × 10–5 | 7.21 × 10–5 | –4.71 × 10–6 | 5.72 × 10–5 | 0.000139 | 0.00015 | 0.006196 | 0.000131 | 0.00011 | 1.18 × 10–5 | –9.87 × 10–6 | 0.000148 | 0.000145 | 0.000159 | 1.86 × 10–5 | 1.74 × 10–5 | 4.39 × 10–6 | –5.10 × 10–6 | –3.07 × 10–6 | –3.21 × 10–7 | 3.91 × 10–7 |
| Any other Asian background | 0.00227908 | –0.00023 | –1.43 × 10–5 | 0.000502176 | –0.00089263 | 0.000142868 | 9.43 × 10–5 | 0.000102 | 0.000132 | –0.00026 | –0.00022 | 9.84 × 10–5 | 0.00017 | 0.000131 | 0.099857 | 0.00021 | 3.17 × 10–5 | 0.000168 | 0.000215 | 0.000178 | 9.28 × 10–5 | –0.0001 | 0.000102 | 0.000146 | 1.90 × 10–5 | 4.26 × 10–6 | –6.10 × 10–6 | –1.91 × 10–5 |
| Chinese | 0.00055667 | 3.50 × 10–6 | 1.68 × 10–5 | –3.86 × 10–5 | –7.35 × 10–5 | 9.77 × 10–5 | 7.52 × 10–5 | 8.41 × 10–5 | 7.96 × 10–5 | 3.40 × 10–6 | 2.52 × 10–5 | 9.67 × 10–5 | 0.000123 | 0.00011 | 0.00021 | 0.003137 | 6.55 × 10–5 | 9.21 × 10–5 | 0.000141 | 0.000128 | 0.00011 | –6.10 × 10–5 | 6.08 × 10–5 | 2.79 × 10–5 | 5.89 × 10–7 | –6.19 × 10–6 | –8.17 × 10–6 | 3.48 × 10–6 |
| Any other | 0.00067394 | 7.63 × 10–5 | 2.19 × 10–5 | –4.11 × 10–5 | –8.24 × 10–5 | –0.00013231 | 4.73 × 10–6 | 7.14 × 10–5 | 5.87 × 10–5 | 0.000183 | 0.000136 | 4.90 × 10–5 | 2.29 × 10–5 | 1.18 × 10–5 | 3.17 × 10–5 | 6.55 × 10–5 | 0.013933 | 0.000236 | 1.97 × 10–5 | 1.72 × 10–5 | 2.56 × 10–5 | 1.04 × 10–7 | 0.000141 | –7.94 × 10–5 | 4.20 × 10–6 | 1.10 × 10–5 | –1.50 × 10–5 | 2.70 × 10–5 |
| Black Caribbean | 0.00143976 | 0.00014 | 0.000144648 | 7.12 × 10–5 | –0.00033453 | –0.00012866 | –4.78 × 10–5 | 6.63 × 10–5 | 9.21 × 10–6 | 0.000202 | 5.21 × 10–6 | 5.46 × 10–5 | 3.99 × 10–5 | –9.87 × 10–6 | 0.000168 | 9.21 × 10–5 | 0.000236 | 0.02748 | 3.56 × 10–5 | 3.53 × 10–5 | 6.09 × 10–5 | –5.90 × 10–7 | 0.000167 | –0.00013 | –2.29 × 10–5 | –2.40 × 10–5 | –5.24 × 10–6 | 2.93 × 10–5 |
| Black African | 0.00027153 | –5.64 × 10–6 | 1.12 × 10–5 | –2.98 × 10–5 | –4.13 × 10–5 | 0.000162537 | 0.0001 | 8.56 × 10–5 | 7.11 × 10–5 | –1.57 × 10–5 | –7.59 × 10–6 | 0.000121 | 0.000156 | 0.000148 | 0.000215 | 0.000141 | 1.97 × 10–5 | 3.56 × 10–5 | 0.003522 | 0.000162 | 0.000148 | –3.36 × 10–5 | 3.72 × 10–5 | 6.89 × 10–5 | 2.08 × 10–6 | 3.94 × 10–6 | –6.90 × 10–6 | –4.64 × 10–6 |
| Any other black background | 0.00014486 | 1.84 × 10–6 | 3.60 × 10–5 | –5.67 × 10–5 | 2.28 × 10–7 | 0.000162753 | 9.64 × 10–5 | 8.17 × 10–5 | 5.12 × 10–5 | 3.15 × 10–5 | –3.00 × 10–5 | 0.000125 | 0.000161 | 0.000145 | 0.000178 | 0.000128 | 1.72 × 10–5 | 3.53 × 10–5 | 0.000162 | 0.002877 | 0.000158 | 1.41 × 10–5 | 4.79 × 10–5 | 6.05 × 10–5 | –3.72 × 10–6 | –5.19 × 10–7 | –6.18 × 10–6 | –2.46 × 10–6 |
| Female | –0.0005473 | 0.00011 | 8.33 × 10–5 | 9.97 × 10–5 | –4.37 × 10–5 | 0.000121935 | 0.0001 | 8.19 × 10–5 | 2.53 × 10–5 | 8.10 × 10–5 | –2.16 × 10–5 | 0.000147 | 0.000159 | 0.000159 | 9.28 × 10–5 | 0.00011 | 2.56 × 10–5 | 6.09 × 10–5 | 0.000148 | 0.000158 | 0.019623 | 3.04 × 10–5 | 2.13 × 10–5 | 4.41 × 10–5 | 1.45 × 10–5 | 1.45 × 10–5 | –2.23 × 10–5 | 1.33 × 10–5 |
| Intercept | –9.90 × 10–5 | 5.66 × 10–6 | –5.14 × 10–7 | –2.98 × 10–5 | 1.81 × 10–5 | 9.50 × 10–7 | –2.44 × 10–5 | –1.76 × 10–5 | –9.39 × 10–5 | 0.000171 | –8.57 × 10–5 | 3.23 × 10–5 | 2.36 × 10–5 | 1.86 × 10–5 | –0.0001 | –6.10 × 10–5 | 1.04 × 10–7 | –5.90 × 10–7 | –3.36 × 10–5 | 1.41 × 10–5 | 3.04 × 10–5 | 0.000256 | 5.68 × 10–5 | 0.000151 | –1.85 × 10–6 | 4.88 × 10–7 | 3.57 × 10–6 | –1.93 × 10–5 |
| Walking Away | 0.00063747 | –3.66 × 10–5 | 2.69 × 10–5 | –7.15 × 10–5 | –5.09 × 10–5 | –7.41 × 10–5 | 2.99 × 10–5 | –2.99 × 10–5 | 6.23 × 10–5 | 0.000161 | 5.25 × 10–5 | 4.84 × 10–5 | 4.74 × 10–5 | 1.74 × 10–5 | 0.000102 | 6.08 × 10–5 | 0.000141 | 0.000167 | 3.72 × 10–5 | 4.79 × 10–5 | 2.13 × 10–5 | 5.68 × 10–5 | 0.002259 | –0.00018 | 1.08 × 10–5 | –1.15 × 10–5 | 2.83 × 10–5 | 6.51 × 10–6 |
| Walking Away Plus | –0.0092943 | –1.82 × 10–5 | –1.82 × 10–5 | 0.001177174 | 0.000389326 | –0.00028142 | 0.000191 | 5.83 × 10–5 | 0.000133 | 0.000137 | –1.36 × 10–5 | 0.000187 | 1.68 × 10–5 | 4.39 × 10–6 | 0.000146 | 2.79 × 10–5 | –7.94 × 10–5 | –0.00013 | 6.89 × 10–5 | 6.05 × 10–5 | 4.41 × 10–5 | 0.000151 | –0.00018 | 0.852306 | –0.00272 | –0.00193 | –0.07844 | –0.06705 |
| HbA1c (%) at baseline | 2.72 × 10–5 | –0.00013 | –5.27 × 10–5 | 2.57 × 10–5 | –2.12 × 10–5 | –5.49 × 10–7 | 1.22 × 10–5 | 1.12 × 10–6 | 1.67 × 10–5 | –1.68 × 10–5 | –8.40 × 10–7 | 1.70 × 10–6 | 2.36 × 10–6 | –5.10 × 10–6 | 1.90 × 10–5 | 5.89 × 10–7 | 4.20 × 10–6 | –2.29 × 10–5 | 2.08 × 10–6 | –3.72 × 10–6 | 1.45 × 10–5 | –1.85 × 10–6 | 1.08 × 10–5 | –0.00272 | 0.012435 | 0.005718 | –0.00188 | 0.001336 |
| HbA1c (%) at 12 months | 2.15 × 10–5 | –5.28 × 10–5 | –0.000129436 | 2.05 × 10–5 | –1.53 × 10–5 | 8.66 × 10–7 | 8.39 × 10–6 | 3.20 × 10–6 | 4.85 × 10–6 | –1.57 × 10–5 | 4.53 × 10–6 | –1.17 × 10–5 | 1.85 × 10–6 | –3.07 × 10–6 | 4.26 × 10–6 | –6.19 × 10–6 | 1.10 × 10–5 | –2.40 × 10–5 | 3.94 × 10–6 | –5.19 × 10–7 | 1.45 × 10–5 | 4.88 × 10–7 | –1.15 × 10–5 | –0.00193 | 0.005718 | 0.012395 | –0.0018 | 0.001151 |
| Cambridge | 0.00108424 | 2.69 × 10–5 | 2.13 × 10–5 | –0.00063212 | 0.000435711 | 9.56 × 10–5 | –4.48 × 10–5 | –5.92 × 10–6 | –3.02 × 10–5 | 7.28 × 10–6 | –4.20 × 10–5 | –2.39 × 10–5 | 1.86 × 10–6 | –3.21 × 10–7 | –6.10 × 10–6 | –8.17 × 10–6 | –1.50 × 10–5 | –5.24 × 10–6 | –6.90 × 10–6 | –6.18 × 10–6 | –2.23 × 10–5 | 3.57 × 10–6 | 2.83 × 10–5 | –0.07844 | –0.00188 | –0.0018 | 0.061058 | –0.04622 |
| Female | 0.00049063 | –1.45 × 10–5 | –9.19 × 10–6 | 0.000419803 | –0.00049193 | –4.08 × 10–5 | 1.29 × 10–5 | –3.86 × 10–6 | 6.37 × 10–6 | –2.53 × 10–5 | 4.41 × 10–5 | –7.25 × 10–6 | –4.85 × 10–6 | 3.91 × 10–7 | –1.91 × 10–5 | 3.48 × 10–6 | 2.70 × 10–5 | 2.93 × 10–5 | –4.64 × 10–6 | –2.46 × 10–6 | 1.33 × 10–5 | –1.93 × 10–5 | 6.51 × 10–6 | –0.06705 | 0.001336 | 0.001151 | –0.04622 | 0.056499 |
Regressions used to estimate step count at 1 year
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –2.390 | 0.044 | –2.488 to –2.291 |
| Walking Away (Walking Away = 1, 0 otherwise) | 0.107 | 0.033 | 0.033 to 0.18 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | 0.153 | 0.031 | 0.083 to 0.224 |
| HbA1c (%) at baseline | 0.000 | 0.000 | 0 to 0 |
| Cambridge (1 = Cambridge, 0 = Leicester) | 0.021 | 0.031 | –0.047 to 0.09 |
| White Irish (1 = white Irish, 0 = otherwise) | –0.115 | 0.167 | –0.49 to 0.259 |
| Any other white background (1 = any other white background, 0 = otherwise) | 0.044 | 0.074 | –0.121 to 0.209 |
| White and black Caribbean (1 = white and black Caribbean, 0 = otherwise) | 0.194 | 0.283 | –0.441 to 0.829 |
| White and black African (1 = white and black African, 0 = otherwise) | 0.283 | 0.383 | –0.575 to 1.142 |
| White and Asian (1 = white and Asian, 0 = otherwise) | –0.936 | 0.524 | –2.11 to 0.238 |
| Any other mixed race (1= any other mixed race, 0 = otherwise) | 0.467 | 0.209 | –0.002 to 0.937 |
| Indian (1 = Indian, 0 = otherwise) | 0.000 | 0.036 | –0.081 to 0.081 |
| Pakistani (1 = Pakistani, 0 = otherwise) | –0.086 | 0.126 | –0.368 to 0.196 |
| Bangladeshi (1 = Bangladeshi, 0 = otherwise) | 0.537 | 0.377 | –0.308 to 1.381 |
| Any other Asian background (1 = any other Asian background, 0 = otherwise) | 0.118 | 0.096 | –0.097 to 0.332 |
| Chinese (1 = Chinese, 0 = otherwise) | 0.167 | 0.207 | –0.296 to 0.63 |
| Any other (1 = any other, 0 = otherwise) | 0.361 | 0.263 | –0.228 to 0.95 |
| Black Caribbean (1 = black Caribbean, 0 = otherwise) | 0.115 | 0.099 | –0.107 to 0.338 |
| Black African (1 = black African, 0 = otherwise) | 0.042 | 0.086 | –0.151 to 0.236 |
| Any other black background (1 = any other black background, 0 = otherwise) | 0.305 | 0.210 | –0.165 to 0.775 |
| Female (1 = female, 0 otherwise) | –0.030 | 0.027 | –0.089 to 0.03 |
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | 4.086 | 0.134 | 3.786 to 4.386 |
| Walking Away (Walking Away = 1, 0 otherwise) | –0.385 | 0.107 | –0.624 to –0.147 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | –0.263 | 0.107 | –0.502 to –0.024 |
| Steps per day at baseline | 0.000 | 0.000 | 0 to 0 |
| Cambridge (1 = Cambridge site, 0 = otherwise) | –0.163 | 0.092 | –0.368 to 0.043 |
| Female (1 = Female, 0 otherwise) | 0.141 | 0.088 | –0.057 to 0.339 |
| Mean effect | Dispersion | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | Cambridge | White Irish | Any other white background | White and black Caribbean | White and black African | White and Asian | Any other mixed race | Indian | Pakistani | Bangladeshi | Any other Asian background | Chinese | Any other | Black Caribbean | Black African | Any other black background | Female | Intercept | Walking Away | Walking Away Plus | Step count at baseline | Cambridge | Female | |
| Intercept | 0.00193265 | –0.00036 | –0.000369582 | –1.5178 × 10–7 | –0.00030847 | 9.97443 × 10–5 | –3.6 × 10–5 | 0.000178 | –0.00062 | 0.001837 | –0.00047 | –0.00047 | –0.00046 | –0.0004 | –0.00024 | –9.7 × 10–5 | –3.6 × 10–5 | –0.00037 | –0.00047 | –0.00043 | –0.00047 | –0.0007 | 0.000196 | 0.000163 | 3.9 × 10–8 | 7.09 × 10–5 | 0.000204 |
| Walking Away | –0.0003645 | 0.001068 | 0.000416316 | –9.4668 × 10–9 | –2.7216 × 10–6 | –0.00044113 | –5.9 × 10–5 | –0.00029 | 0.000422 | 8.41 × 10–5 | –2.4 × 10–6 | 4.76 × 10–5 | 3.76 × 10–5 | –0.00068 | –7.8 × 10–6 | 0.000107 | 0.000431 | –5.8 × 10–5 | –6.3 × 10–5 | 0.000309 | 3.04 × 10–5 | 0.000163 | –0.00049 | –0.00021 | 5.25 × 10–9 | 9.73 × 10–6 | –7.7 × 10–6 |
| Walking Away Plus | –0.0003696 | 0.000416 | 0.000978441 | –7.1833 × 10–9 | –1.1548 × 10–5 | –0.0001514 | 2.86 × 10–5 | –0.0003 | 0.000421 | –0.00047 | –4.1 × 10–5 | 6.66 × 10–5 | –0.00016 | –1 × 10–5 | –1.1 × 10–5 | 0.000101 | 0.000442 | 8.41 × 10–5 | 5.35 × 10–5 | 0.000152 | –2.6 × 10–6 | 0.000148 | –0.00021 | –0.00044 | 6.29 × 10–9 | 1.47 × 10–5 | 8.56 × 10–6 |
| HbA1c (%) at baseline | –1.518 × 10–7 | –9.5 × 10–9 | –7.18331 × 10–9 | 2.15418 × 10–11 | –1.0852 × 10–8 | –2.1758 × 10–8 | –2.6 × 10–8 | –1.1 × 10–8 | 2.15 × 10–8 | –2.2 × 10–7 | 6.3 × 10–9 | –1.3 × 10–9 | 5.09 × 10–9 | 4.89 × 10–8 | –2 × 10–9 | 2.22 × 10–9 | –3.4 × 10–8 | –1.6 × 10–9 | 6.07 × 10–9 | –2.4 × 10–8 | 1.31 × 10–8 | 4.28 × 10–8 | 1.09 × 10–9 | 3.64 × 10–9 | –3.9 × 10–12 | 3.08 × 10–9 | –6.1 × 10–9 |
| Cambridge | –0.0003085 | –2.7 × 10–6 | –1.15484 × 10–5 | –1.0852 × 10–8 | 0.000935282 | 0.000166638 | 2.31 × 10–5 | 1.66 × 10–5 | –0.00055 | –0.0004 | 0.000328 | 0.000377 | 0.000391 | 0.000378 | 0.000243 | –0.00052 | –0.00052 | 0.000396 | 0.00039 | 0.000404 | –2.1 × 10–5 | 5.9 × 10–5 | 9.88 × 10–6 | 1.97 × 10–5 | 5.07 × 10–9 | –0.00035 | 2.12 × 10–5 |
| White Irish | 9.9744 × 10–5 | –0.00044 | –0.000151401 | –2.1758 × 10–8 | 0.000166638 | 0.027945404 | 0.000314 | 0.000452 | –0.00013 | 0.000446 | 0.00027 | 0.000276 | 0.000263 | 0.000623 | 0.000331 | 0.000114 | 2.22 × 10–5 | 0.000361 | 0.000325 | 0.000186 | –0.00018 | 7.02 × 10–5 | 2.2 × 10–5 | –1 × 10–5 | –8.9 × 10–9 | 4.33 × 10–6 | –4.5 × 10–5 |
| Any other white background | –3.567 × 10–5 | –5.9 × 10–5 | 2.86014 × 10–5 | –2.571 × 10–8 | 2.309 × 10–5 | 0.000314217 | 0.005421 | 0.000287 | 0.00018 | 0.000487 | 0.000234 | 0.000248 | 0.000225 | 0.000271 | 0.000261 | 0.000234 | 0.000269 | 0.000268 | 0.00025 | 0.000255 | –5.8 × 10–5 | 1.57 × 10–5 | –2.3 × 10–5 | –1.9 × 10–5 | 1.45 × 10–10 | 4.61 × 10–6 | –3.7 × 10–6 |
| White and black Caribbean | 0.00017763 | –0.00029 | –0.000301125 | –1.1099 × 10–8 | 1.6609 × 10–5 | 0.000452123 | 0.000287 | 0.080284 | –0.00012 | 0.00064 | 0.000204 | 0.000193 | 0.000227 | 0.000497 | 0.000337 | 0.000203 | 8.38 × 10–5 | 0.000282 | 0.000226 | 0.000123 | –0.00034 | 6.91 × 10–5 | 1.72 × 10–5 | 5.85 × 10–5 | –1.1 × 10–8 | –3 × 10–5 | 2.99 × 10–5 |
| White and black African | –0.0006218 | 0.000422 | 0.000421119 | 2.1456 × 10–8 | –0.0005474 | –0.00012505 | 0.00018 | –0.00012 | 0.146829 | –1.9 × 10–5 | 9.93 × 10–5 | 9.72 × 10–5 | 3.85 × 10–5 | –0.00029 | 5.16 × 10–6 | 0.000606 | 0.00077 | –2 × 10–5 | 4.5 × 10–5 | 0.000183 | 0.000396 | 0.000127 | –3.3 × 10–5 | –2.6 × 10–5 | –1.1 × 10–8 | 6.39 × 10–5 | –4.4 × 10–5 |
| White and Asian | 0.0018367 | 8.41 × 10–5 | –0.000468563 | –2.1616 × 10–7 | –0.00040483 | 0.000445753 | 0.000487 | 0.00064 | –1.9 × 10–5 | 0.274366 | –3.2 × 10–5 | –1.7 × 10–5 | 3.98 × 10–5 | –0.00048 | 0.000221 | 0.000481 | 0.000709 | 1.22 × 10–5 | –0.00011 | 0.0002 | –0.00044 | –0.00028 | 3.16 × 10–6 | 0.00014 | 3.44 × 10–9 | 0.000145 | 0.000142 |
| Any other mixed race | –0.0004707 | –2.4 × 10–6 | –4.12575 × 10–5 | 6.29849 × 10–9 | 0.000327689 | 0.000269742 | 0.000234 | 0.000204 | 9.93 × 10–5 | –3.2 × 10–5 | 0.043837 | 0.00039 | 0.00041 | 0.00035 | 0.000307 | 5.29 × 10–5 | 3.22 × 10–5 | 0.000371 | 0.000392 | 0.000393 | 0.0001 | –0.0003 | 9.41 × 10–6 | 0.00015 | 4.97 × 10–8 | 5.66 × 10–5 | –0.00011 |
| Indian | –0.0004653 | 4.76 × 10–5 | 6.66208 × 10–5 | –1.3299 × 10–9 | 0.00037658 | 0.000276016 | 0.000248 | 0.000193 | 9.72 × 10–5 | –1.7 × 10–5 | 0.00039 | 0.001316 | 0.000408 | 0.000362 | 0.000327 | 3.85 × 10–5 | 6.78 × 10–5 | 0.000404 | 0.000413 | 0.000434 | 6.17 × 10–5 | 3.54 × 10–6 | –6.6 × 10–6 | 5.64 × 10–6 | 1.52 × 10–10 | 2.24 × 10–6 | –8.6 × 10–6 |
| Pakistani | –0.0004641 | 3.76 × 10–5 | –0.000157389 | 5.09095 × 10–9 | 0.000391296 | 0.000263382 | 0.000225 | 0.000227 | 3.85 × 10–5 | 3.98 × 10–5 | 0.00041 | 0.000408 | 0.015819 | 0.0003 | 0.000324 | 1.25 × 10–5 | –2.7 × 10–5 | 0.000377 | 0.000402 | 0.000429 | 0.000103 | 4.17 × 10–5 | 1.55 × 10–5 | –3.2 × 10–5 | –5.4 × 10–9 | 2.51 × 10–5 | –2.9 × 10–5 |
| Bangladeshi | –0.0004025 | –0.00068 | –1.04155 × 10–5 | 4.88589 × 10–8 | 0.000377875 | 0.000623237 | 0.000271 | 0.000497 | –0.00029 | –0.00048 | 0.00035 | 0.000362 | 0.0003 | 0.141942 | 0.000433 | –2 × 10–5 | –0.00023 | 0.000528 | 0.000478 | 0.000135 | –0.00033 | 5.77 × 10–5 | 7.91 × 10–5 | 9.04 × 10–6 | –4 × 10–9 | –2.4 × 10–5 | 2.15 × 10–5 |
| Any other Asian background | –0.0002354 | –7.8 × 10–6 | –1.14241 × 10–5 | –1.9693 × 10–9 | 0.000242511 | 0.000331483 | 0.000261 | 0.000337 | 5.16 × 10–6 | 0.000221 | 0.000307 | 0.000327 | 0.000324 | 0.000433 | 0.009156 | 0.000109 | 0.000106 | 0.000372 | 0.000339 | 0.000322 | –0.00018 | 6.21 × 10–5 | 3.19 × 10–6 | 8.29 × 10–7 | –7.2 × 10–9 | 1.54 × 10–6 | 7.59 × 10–7 |
| Chinese | –9.7 × 10–5 | 0.000107 | 0.000101136 | 2.21523 × 10–9 | –0.00052423 | 0.000113654 | 0.000234 | 0.000203 | 0.000606 | 0.000481 | 5.29 × 10–5 | 3.85 × 10–5 | 1.25 × 10–5 | –2 × 10–5 | 0.000109 | 0.042662 | 0.000601 | 2.32 × 10–5 | 2.17 × 10–5 | 5.07 × 10–5 | –8.6 × 10–8 | –9.6 × 10–6 | –6.7 × 10–5 | –4 × 10–5 | 1.99 × 10–9 | 2.55 × 10–5 | 6.56 × 10–5 |
| Any other | –3.612 × 10–5 | 0.000431 | 0.000442138 | –3.4388 × 10–8 | –0.00051619 | 2.22469 × 10–5 | 0.000269 | 8.38 × 10–5 | 0.00077 | 0.000709 | 3.22 × 10–5 | 6.78 × 10–5 | –2.7 × 10–5 | –0.00023 | 0.000106 | 0.000601 | 0.069137 | 3.57 × 10–5 | 1.47 × 10–5 | 0.000189 | –3.3 × 10–6 | 0.000125 | –0.00012 | –0.00012 | –1.1 × 10–8 | 0.000149 | 4.36 × 10–6 |
| Black Caribbean | –0.000366 | –5.8 × 10–5 | 8.41236 × 10–5 | –1.6477 × 10–9 | 0.000396329 | 0.000361077 | 0.000268 | 0.000282 | –2 × 10–5 | 1.22 × 10–5 | 0.000371 | 0.000404 | 0.000377 | 0.000528 | 0.000372 | 2.32 × 10–5 | 3.57 × 10–5 | 0.009845 | 0.000425 | 0.000392 | –9.7 × 10–5 | 6.39 × 10–5 | –6 × 10–6 | –4.3 × 10–6 | –6 × 10–9 | –9.7 × 10–6 | –4.4 × 10–6 |
| Black African | –0.0004746 | –6.3 × 10–5 | 5.34539 × 10–5 | 6.06544 × 10–9 | 0.000389854 | 0.000324925 | 0.00025 | 0.000226 | 4.5 × 10–5 | –0.00011 | 0.000392 | 0.000413 | 0.000402 | 0.000478 | 0.000339 | 2.17 × 10–5 | 1.47 × 10–5 | 0.000425 | 0.007456 | 0.000399 | 3.14 × 10–5 | 2 × 10–5 | 2.21 × 10–7 | 2.15 × 10–5 | –3.2 × 10–9 | 6.16 × 10–6 | –9.3 × 10–7 |
| Any other black background | –0.0004291 | 0.000309 | 0.000151912 | –2.4315 × 10–8 | 0.000404414 | 0.000185735 | 0.000255 | 0.000123 | 0.000183 | 0.0002 | 0.000393 | 0.000434 | 0.000429 | 0.000135 | 0.000322 | 5.07 × 10–5 | 0.000189 | 0.000392 | 0.000399 | 0.043969 | 9.49 × 10–5 | 0.000116 | –6.4 × 10–5 | –3.1 × 10–5 | –7.3 × 10–9 | –5 × 10–5 | 1.11 × 10–5 |
| Female | –0.0004668 | 3.04 × 10–5 | –2.63701 × 10–6 | 1.31019 × 10–8 | –2.077 × 10–5 | –0.00018246 | –5.8 × 10–5 | –0.00034 | 0.000396 | –0.00044 | 0.0001 | 6.17 × 10–5 | 0.000103 | –0.00033 | –0.00018 | –8.6 × 10–8 | –3.3 × 10–6 | –9.7 × 10–5 | 3.14 × 10–5 | 9.49 × 10–5 | 0.00071 | 0.000207 | –8.4 × 10–6 | 8.22 × 10–6 | –7.2 × 10–9 | 2.55 × 10–5 | –0.00032 |
| Intercept | –0.0006987 | 0.000163 | 0.000148375 | 4.27813 × 10–8 | 5.89622 × 10–5 | 7.01862 × 10–5 | 1.57 × 10–5 | 6.91 × 10–5 | 0.000127 | –0.00028 | –0.0003 | 3.54 × 10–6 | 4.17 × 10–5 | 5.77 × 10–5 | 6.21 × 10–5 | –9.6 × 10–6 | 0.000125 | 6.39 × 10–5 | 2 × 10–5 | 0.000116 | 0.000207 | 0.017927 | –0.00463 | –0.00437 | –1.4 × 10–6 | –0.00183 | –0.00464 |
| Walking Away | 0.00019612 | –0.00049 | –0.000209668 | 1.08735 × 10–9 | 9.87538 × 10–6 | 2.2003 × 10–5 | –2.3 × 10–5 | 1.72 × 10–5 | –3.3 × 10–5 | 3.16 × 10–6 | 9.41 × 10–6 | –6.6 × 10–6 | 1.55 × 10–5 | 7.91 × 10–5 | 3.19 × 10–6 | –6.7 × 10–5 | –0.00012 | –6 × 10–6 | 2.21 × 10–7 | –6.4 × 10–5 | –8.4 × 10–6 | –0.00463 | 0.011345 | 0.005335 | –1 × 10–7 | –6.7 × 10–5 | 0.000204 |
| Walking Away Plus | 0.00016285 | –0.00021 | –0.000442601 | 3.6398 × 10–9 | 1.97067 × 10–5 | –1.0454 × 10–5 | –1.9 × 10–5 | 5.85 × 10–5 | –2.6 × 10–5 | 0.00014 | 0.00015 | 5.64 × 10–6 | –3.2 × 10–5 | 9.04 × 10–6 | 8.29 × 10–7 | –4 × 10–5 | –0.00012 | –4.3 × 10–6 | 2.15 × 10–5 | –3.1 × 10–5 | 8.22 × 10–6 | –0.00437 | 0.005335 | 0.011347 | –1.1 × 10–7 | –0.00027 | –8.7 × 10–5 |
| Step count at baseline | 3.8957 × 10–8 | 5.25 × 10–9 | 6.29062 × 10–9 | –3.8561 × 10–12 | 5.06603 × 10–9 | –8.8902 × 10–9 | 1.45 × 10–10 | –1.1 × 10–8 | –1.1 × 10–8 | 3.44 × 10–9 | 4.97 × 10–8 | 1.52 × 10–10 | –5.4 × 10–9 | –4 × 10–9 | –7.2 × 10–9 | 1.99 × 10–9 | –1.1 × 10–8 | –6 × 10–9 | –3.2 × 10–9 | –7.3 × 10–9 | –7.2 × 10–9 | –1.4 × 10–6 | –1 × 10–7 | –1.1 × 10–7 | 1.98 × 10–10 | –1.2 × 10–7 | 1.36 × 10–7 |
| Cambridge | 7.0941 × 10–5 | 9.73 × 10–6 | 1.47477 × 10–5 | 3.08143 × 10–9 | –0.0003492 | 4.33316 × 10–6 | 4.61 × 10–6 | –3 × 10–5 | 6.39 × 10–5 | 0.000145 | 5.66 × 10–5 | 2.24 × 10–6 | 2.51 × 10–5 | –2.4 × 10–5 | 1.54 × 10–6 | 2.55 × 10–5 | 0.000149 | –9.7 × 10–6 | 6.16 × 10–6 | –5 × 10–5 | 2.55 × 10–5 | –0.00183 | –6.7 × 10–5 | –0.00027 | –1.2 × 10–7 | 0.008392 | –0.00045 |
| Female | 0.00020359 | –7.7 × 10–6 | 8.56 × 10–6 | –6.1292 × 10–9 | 2.11866 × 10–5 | –4.4878 × 10–5 | –3.7 × 10–6 | 2.99 × 10–5 | –4.4 × 10–5 | 0.000142 | –0.00011 | –8.6 × 10–6 | –2.9 × 10–5 | 2.15 × 10–5 | 7.59 × 10–7 | 6.56 × 10–5 | 4.36 × 10–6 | –4.4 × 10–6 | –9.3 × 10–7 | 1.11 × 10–5 | –0.00032 | –0.00464 | 0.000204 | –8.7 × 10–5 | 1.36 × 10–7 | –0.00045 | 0.007778 |
Regressions used to estimate step count at 4 years
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | –2.555 | 0.052 | –2.671 to –2.438 |
| Walking Away (Walking Away = 1, 0 otherwise) | –0.013 | 0.035 | –0.091 to 0.066 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | 0.005 | 0.039 | –0.082 to 0.092 |
| HbA1c (%) at baseline | 6.59E-05 | 7.76E-06 | 0 to 0 |
| Cambridge (1 = Cambridge, 0 = Leicester) | 1.24E-04 | 7.69E-06 | 0 to 0 |
| White Irish (1 = White Irish, 0 = otherwise) | 0.124 | 0.034 | 0.047 to 0.2 |
| Any other white background (1 = any other white background, 0 = otherwise) | 0.164 | 0.169 | –0.215 to 0.542 |
| White and black Caribbean (1 = White and black Caribbean, 0 = otherwise) | 0.088 | 0.077 | –0.086 to 0.262 |
| White and black African (1 = White and black African, 0 = otherwise) | –0.481 | 0.314 | –1.185 to 0.222 |
| White and Asian (1 = White and Asian, 0 = otherwise) | –0.152 | 0.469 | –1.202 to 0.898 |
| Any other mixed race (1 = any other mixed race, 0 = otherwise) | –0.854 | 0.463 | –1.892 to 0.183 |
| Indian (1 = Indian, 0 = otherwise) | 0.032 | 0.251 | –0.53 to 0.594 |
| Pakistani (1 = Pakistani, 0 = otherwise) | 0.093 | 0.042 | –0.002 to 0.187 |
| Bangladeshi (1 = Bangladeshi, 0 = otherwise) | 0.169 | 0.162 | –0.193 to 0.532 |
| Any other Asian background (1 = any other Asian background, 0 = otherwise) | 0.413 | 0.355 | –0.384 to 1.209 |
| Chinese (1 = Chinese, 0 = otherwise) | –0.154 | 0.110 | –0.401 to 0.093 |
| Any other (1 = any other, 0 = otherwise) | 0.053 | 0.209 | –0.416 to 0.523 |
| Black Caribbean (1 = black Caribbean, 0 = otherwise) | –0.100 | 0.285 | –0.738 to 0.539 |
| Black African (1 = black African, 0 = otherwise) | 0.110 | 0.114 | –0.144 to 0.365 |
| Any other black background (1 = any other black background, 0 = otherwise) | 0.169 | 0.097 | –0.048 to 0.386 |
| Female (1 = female, 0 otherwise) | 0.136 | 0.259 | –0.445 to 0.718 |
| Coefficients | SE | 97.5% CI | |
|---|---|---|---|
| Intercept | 3.594 | 0.143 | 3.274 to 3.914 |
| Walking Away (Walking Away = 1, 0 otherwise) | 0.029 | 0.113 | –0.225 to 0.284 |
| Walking Away Plus (Walking Away Plus = 1, 0 otherwise) | –0.358 | 0.114 | –0.614 to –0.102 |
| Steps per day at baseline | –4.23E-06 | 2.39E-05 | 0 to 0 |
| Steps per day at 12 months | –6.07E-05 | 2.32E-05 | 0 to 0 |
| Cambridge (1 = Cambridge site, 0 = otherwise) | 0.072 | 0.098 | –0.148 to 0.293 |
| Female (1 = female, 0 otherwise) | 0.480 | 0.094 | 0.269 to 0.691 |
| Mean effect | Dispersion | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Walking Away | Walking Away Plus | HbA1c (%) at baseline | Cambridge | White Irish | Any other white background | White and black Caribbean | White and black African | White and Asian | Any other mixed race | Indian | Pakistani | Bangladeshi | Any other Asian background | Chinese | Any other | Black Caribbean | Black African | Any other black background | Female | Intercept | Walking Away | Walking Away Plus | Step count at baseline | Step count at 12 months | Cambridge | Female | |
| Intercept | 2.72 × 10–3 | –4.51 × 10–4 | –3.81 × 10–4 | –1.21 × 10–7 | –8.53 × 10–8 | –4.30 × 10–4 | –1.18 × 10–4 | –4.02 × 10–5 | 2.19 × 10–4 | –8.37 × 10–4 | 1.89 × 10–3 | –3.53 × 10–4 | –6.61 × 10–4 | –7.74 × 10–4 | –4.04 × 10–4 | –2.27 × 10–4 | –7.24 × 10–5 | 6.07 × 10–5 | –5.21 × 10–4 | –6.80 × 10–4 | –4.74 × 10–4 | –6.92 × 10–4 | –1.11 × 10–3 | 2.53 × 10–4 | 1.70 × 10–4 | 4.45 × 10–8 | 2.35 × 10–8 | 9.10 × 10–5 |
| Walking Away | –4.51 × 10–4 | 1.22 × 10–3 | 5.81 × 10–4 | –1.21 × 10–9 | –1.65 × 10–8 | –9.18 × 10–6 | –4.76 × 10–4 | –7.72 × 10–5 | –3.70 × 10–4 | 5.70 × 10–4 | 8.50 × 10–5 | 6.47 × 10–5 | 4.21 × 10–5 | 1.34 × 10–4 | –6.47 × 10–4 | 5.68 × 10–6 | 1.97 × 10–4 | 6.15 × 10–4 | –1.13 × 10–4 | –1.55 × 10–5 | 4.80 × 10–4 | 1.33 × 10–5 | 2.58 × 10–4 | –5.85 × 10–4 | –2.89 × 10–4 | –3.03 × 10–9 | 4.09 × 10–9 | 9.66 × 10–6 |
| Walking Away Plus | –3.81 × 10–4 | 5.81 × 10–4 | 1.51 × 10–3 | 1.03 × 10–8 | –3.45 × 10–8 | –7.53 × 10–6 | –2.15 × 10–4 | –8.11 × 10–7 | –3.02 × 10–4 | 5.55 × 10–4 | –8.19 × 10–4 | –4.02 × 10–5 | 5.79 × 10–5 | –2.00 × 10–4 | 5.21 × 10–5 | 1.55 × 10–4 | 1.27 × 10–4 | 6.57 × 10–4 | 1.10 × 10–4 | 6.90 × 10–5 | 3.25 × 10–4 | –5.30 × 10–5 | 1.26 × 10–4 | –2.96 × 10–4 | –7.10 × 10–4 | –1.28 × 10–10 | 1.75 × 10–8 | 1.62 × 10–5 |
| HbA1c (%) at baseline | –1.21 × 10–7 | –1.21 × 10–9 | 1.03 × 10–8 | 6.02 × 10–11 | –4.49 × 10–11 | –8.92 × 10–9 | –3.60 × 10–8 | –2.37 × 10–8 | 2.33 × 10–8 | 6.29 × 10–8 | –3.95 × 10–7 | 5.84 × 10–8 | –6.19 × 10–9 | –2.79 × 10–8 | 1.52 × 10–7 | 1.47 × 10–8 | 2.19 × 10–8 | 2.48 × 10–8 | 1.55 × 10–8 | –5.13 × 10–9 | 1.83 × 10–8 | 1.36 × 10–8 | 4.56 × 10–8 | –3.06 × 10–9 | –2.42 × 10–9 | –1.80 × 10–11 | 1.38 × 10–11 | 4.92 × 10–10 |
| Cambridge | –8.53 × 10–8 | –1.65 × 10–8 | –3.45 × 10–8 | –4.49 × 10–11 | 5.92 × 10–11 | –6.99 × 10–9 | 2.32 × 10–8 | –8.30 × 10–9 | –3.19 × 10–8 | –4.53 × 10–8 | 1.88 × 10–7 | –9.93 × 10–8 | 8.19 × 10–9 | 4.96 × 10–8 | –1.20 × 10–7 | –2.80 × 10–8 | –3.30 × 10–8 | –8.92 × 10–8 | –1.43 × 10–8 | 1.21 × 10–8 | –7.61 × 10–8 | 6.56 × 10–9 | 1.98 × 10–8 | 4.86 × 10–9 | 1.28 × 10–8 | 1.40 × 10–11 | –1.62 × 10–11 | 7.20 × 10–9 |
| White Irish | –4.30 × 10–4 | –9.18 × 10–6 | –7.53 × 10–6 | –8.92 × 10–9 | –6.99 × 10–9 | 1.16 × 10–3 | 4.62 × 10–4 | 4.21 × 10–5 | –9.55 × 10–5 | –6.03 × 10–4 | –4.35 × 10–4 | 4.09 × 10–4 | 5.26 × 10–4 | 5.48 × 10–4 | 5.66 × 10–4 | 2.78 × 10–4 | –5.68 × 10–4 | –5.50 × 10–4 | 5.66 × 10–4 | 5.57 × 10–4 | 5.85 × 10–4 | –4.27 × 10–5 | 1.21 × 10–4 | 1.43 × 10–5 | 1.86 × 10–5 | –9.80 × 10–10 | 5.33 × 10–9 | –4.41 × 10–4 |
| Any other white background | –1.18 × 10–4 | –4.76 × 10–4 | –2.15 × 10–4 | –3.60 × 10–8 | 2.32 × 10–8 | 4.62 × 10–4 | 2.85 × 10–2 | 3.88 × 10–4 | 4.75 × 10–4 | –2.75 × 10–4 | 4.05 × 10–4 | 3.91 × 10–4 | 4.94 × 10–4 | 4.76 × 10–4 | 7.74 × 10–4 | 4.64 × 10–4 | 3.76 × 10–6 | –1.34 × 10–4 | 5.93 × 10–4 | 5.33 × 10–4 | 3.42 × 10–4 | –1.84 × 10–4 | 6.96 × 10–5 | 8.71 × 10–5 | 5.38 × 10–6 | –6.60 × 10–9 | 1.53 × 10–9 | –7.68 × 10–5 |
| White and black Caribbean | –4.02 × 10–5 | –7.72 × 10–5 | –8.11 × 10–7 | –2.37 × 10–8 | –8.30 × 10–9 | 4.21 × 10–5 | 3.88 × 10–4 | 6.00 × 10–3 | 3.62 × 10–4 | 2.11 × 10–4 | 5.70 × 10–4 | 3.43 × 10–4 | 3.19 × 10–4 | 2.90 × 10–4 | 3.47 × 10–4 | 3.46 × 10–4 | 2.96 × 10–4 | 3.27 × 10–4 | 3.40 × 10–4 | 3.21 × 10–4 | 3.45 × 10–4 | –6.19 × 10–5 | 1.87 × 10–5 | –2.34 × 10–5 | –2.18 × 10–5 | –1.92 × 10–9 | 2.89 × 10–9 | 7.42 × 10–6 |
| White and black African | 2.19 × 10–4 | –3.70 × 10–4 | –3.02 × 10–4 | 2.33 × 10–8 | –3.19 × 10–8 | –9.55 × 10–5 | 4.75 × 10–4 | 3.62 × 10–4 | 9.84 × 10–2 | –4.45 × 10–5 | 5.27 × 10–4 | 2.56 × 10–4 | 2.09 × 10–4 | 1.95 × 10–4 | 6.39 × 10–4 | 3.99 × 10–4 | 3.20 × 10–4 | 2.10 × 10–4 | 3.28 × 10–4 | 2.27 × 10–4 | 1.20 × 10–4 | –3.63 × 10–4 | 1.71 × 10–4 | –4.85 × 10–5 | –3.28 × 10–5 | 2.96 × 10–9 | 2.22 × 10–8 | –1.66 × 10–5 |
| White and Asian | –8.37 × 10–4 | 5.70 × 10–4 | 5.55 × 10–4 | 6.29 × 10–8 | –4.53 × 10–8 | –6.03 × 10–4 | –2.75 × 10–4 | 2.11 × 10–4 | –4.45 × 10–5 | 2.20 × 10–1 | –2.38 × 10–4 | 2.85 × 10–4 | 1.14 × 10–4 | 4.16 × 10–5 | –2.79 × 10–4 | 5.61 × 10–5 | 7.37 × 10–4 | 9.70 × 10–4 | –4.23 × 10–5 | 6.72 × 10–5 | 3.25 × 10–4 | 5.79 × 10–4 | 7.73 × 10–5 | 1.07 × 10–4 | 1.24 × 10–4 | 9.42 × 10–9 | –2.61 × 10–8 | –1.42 × 10–4 |
| Any other mixed race | 1.89 × 10–3 | 8.50 × 10–5 | –8.19 × 10–4 | –3.95 × 10–7 | 1.88 × 10–7 | –4.35 × 10–4 | 4.05 × 10–4 | 5.70 × 10–4 | 5.27 × 10–4 | –2.38 × 10–4 | 2.14 × 10–1 | –5.51 × 10–5 | 1.91 × 10–5 | 2.59 × 10–4 | –1.03 × 10–3 | 1.66 × 10–4 | 4.98 × 10–4 | 5.19 × 10–4 | –1.25 × 10–4 | –4.16 × 10–5 | 1.10 × 10–4 | –4.72 × 10–4 | 4.37 × 10–5 | 3.55 × 10–5 | 1.57 × 10–4 | –2.07 × 10–8 | –1.37 × 10–8 | 3.68 × 10–5 |
| Indian | –3.53 × 10–4 | 6.47 × 10–5 | –4.02 × 10–5 | 5.84 × 10–8 | –9.93 × 10–8 | 4.09 × 10–4 | 3.91 × 10–4 | 3.43 × 10–4 | 2.56 × 10–4 | 2.85 × 10–4 | –5.51 × 10–5 | 6.29 × 10–2 | 4.99 × 10–4 | 4.54 × 10–4 | 5.53 × 10–4 | 3.88 × 10–4 | 1.85 × 10–4 | 2.77 × 10–4 | 4.91 × 10–4 | 4.90 × 10–4 | 6.91 × 10–4 | 1.87 × 10–4 | –2.07 × 10–4 | 2.70 × 10–5 | 1.87 × 10–4 | 1.03 × 10–7 | –7.46 × 10–8 | 1.02 × 10–4 |
| Pakistani | –6.61 × 10–4 | 4.21 × 10–5 | 5.79 × 10–5 | –6.19 × 10–9 | 8.19 × 10–9 | 5.26 × 10–4 | 4.94 × 10–4 | 3.19 × 10–4 | 2.09 × 10–4 | 1.14 × 10–4 | 1.91 × 10–5 | 4.99 × 10–4 | 1.78 × 10–3 | 5.85 × 10–4 | 5.21 × 10–4 | 4.19 × 10–4 | 5.29 × 10–5 | 6.77 × 10–5 | 5.69 × 10–4 | 5.85 × 10–4 | 5.92 × 10–4 | 6.72 × 10–5 | 3.52 × 10–5 | 9.38 × 10–6 | 1.22 × 10–5 | –2.74 × 10–9 | 2.34 × 10–10 | –3.04 × 10–6 |
| Bangladeshi | –7.74 × 10–4 | 1.34 × 10–4 | –2.00 × 10–4 | –2.79 × 10–8 | 4.96 × 10–8 | 5.48 × 10–4 | 4.76 × 10–4 | 2.90 × 10–4 | 1.95 × 10–4 | 4.16 × 10–5 | 2.59 × 10–4 | 4.54 × 10–4 | 5.85 × 10–4 | 2.61 × 10–2 | 3.51 × 10–4 | 3.82 × 10–4 | 2.27 × 10–5 | –4.89 × 10–5 | 5.31 × 10–4 | 5.87 × 10–4 | 5.63 × 10–4 | 6.15 × 10–5 | 8.48 × 10–5 | 3.59 × 10–5 | –3.38 × 10–5 | –1.08 × 10–8 | 6.95 × 10–9 | 1.60 × 10–5 |
| Any other Asian background | –4.04 × 10–4 | –6.47 × 10–4 | 5.21 × 10–5 | 1.52 × 10–7 | –1.20 × 10–7 | 5.66 × 10–4 | 7.74 × 10–4 | 3.47 × 10–4 | 6.39 × 10–4 | –2.79 × 10–4 | –1.03 × 10–3 | 5.53 × 10–4 | 5.21 × 10–4 | 3.51 × 10–4 | 1.26 × 10–1 | 6.43 × 10–4 | –1.32 × 10–5 | –1.07 × 10–4 | 7.89 × 10–4 | 5.92 × 10–4 | 4.02 × 10–4 | –3.79 × 10–4 | 1.02 × 10–4 | 1.00 × 10–4 | 1.35 × 10–5 | –7.30 × 10–9 | –2.86 × 10–9 | –9.24 × 10–5 |
| Chinese | –2.27 × 10–4 | 5.68 × 10–6 | 1.55 × 10–4 | 1.47 × 10–8 | –2.80 × 10–8 | 2.78 × 10–4 | 4.64 × 10–4 | 3.46 × 10–4 | 3.99 × 10–4 | 5.61 × 10–5 | 1.66 × 10–4 | 3.88 × 10–4 | 4.19 × 10–4 | 3.82 × 10–4 | 6.43 × 10–4 | 1.21 × 10–2 | 1.91 × 10–4 | 2.72 × 10–4 | 5.06 × 10–4 | 4.37 × 10–4 | 4.73 × 10–4 | –2.89 × 10–4 | 5.16 × 10–5 | 1.70 × 10–6 | –4.17 × 10–5 | 4.55 × 10–10 | –9.53 × 10–9 | 2.33 × 10–5 |
| Any other | –7.24 × 10–5 | 1.97 × 10–4 | 1.27 × 10–4 | 2.19 × 10–8 | –3.30 × 10–8 | –5.68 × 10–4 | 3.76 × 10–6 | 2.96 × 10–4 | 3.20 × 10–4 | 7.37 × 10–4 | 4.98 × 10–4 | 1.85 × 10–4 | 5.29 × 10–5 | 2.27 × 10–5 | –1.32 × 10–5 | 1.91 × 10–4 | 4.39 × 10–2 | 7.50 × 10–4 | 2.08 × 10–5 | 2.64 × 10–5 | 1.52 × 10–4 | 2.55 × 10–5 | 1.53 × 10–5 | –9.37 × 10–5 | –3.85 × 10–5 | 1.62 × 10–8 | –1.65 × 10–8 | –3.65 × 10–6 |
| Black Caribbean | 6.07 × 10–5 | 6.15 × 10–4 | 6.57 × 10–4 | 2.48 × 10–8 | –8.92 × 10–8 | –5.50 × 10–4 | –1.34 × 10–4 | 3.27 × 10–4 | 2.10 × 10–4 | 9.70 × 10–4 | 5.19 × 10–4 | 2.77 × 10–4 | 6.77 × 10–5 | –4.89 × 10–5 | –1.07 × 10–4 | 2.72 × 10–4 | 7.50 × 10–4 | 8.10 × 10–2 | 3.48 × 10–5 | 2.56 × 10–5 | 4.38 × 10–4 | –4.48 × 10–5 | 1.53 × 10–4 | –1.11 × 10–4 | –1.18 × 10–4 | –4.60 × 10–9 | –1.29 × 10–8 | 7.94 × 10–5 |
| Black African | –5.21 × 10–4 | –1.13 × 10–4 | 1.10 × 10–4 | 1.55 × 10–8 | –1.43 × 10–8 | 5.66 × 10–4 | 5.93 × 10–4 | 3.40 × 10–4 | 3.28 × 10–4 | –4.23 × 10–5 | –1.25 × 10–4 | 4.91 × 10–4 | 5.69 × 10–4 | 5.31 × 10–4 | 7.89 × 10–4 | 5.06 × 10–4 | 2.08 × 10–5 | 3.48 × 10–5 | 1.29 × 10–2 | 5.99 × 10–4 | 5.61 × 10–4 | –1.24 × 10–4 | 7.85 × 10–5 | –1.14 × 10–6 | 4.97 × 10–7 | –7.11 × 10–9 | 1.08 × 10–9 | –3.12 × 10–5 |
| Any other black background | –6.80 × 10–4 | –1.55 × 10–5 | 6.90 × 10–5 | –5.13 × 10–9 | 1.21 × 10–8 | 5.57 × 10–4 | 5.33 × 10–4 | 3.21 × 10–4 | 2.27 × 10–4 | 6.72 × 10–5 | –4.16 × 10–5 | 4.90 × 10–4 | 5.85 × 10–4 | 5.87 × 10–4 | 5.92 × 10–4 | 4.37 × 10–4 | 2.64 × 10–5 | 2.56 × 10–5 | 5.99 × 10–4 | 9.40 × 10–3 | 5.77 × 10–4 | 4.03 × 10–5 | 1.09 × 10–4 | –1.28 × 10–5 | 3.38 × 10–5 | –3.44 × 10–9 | –7.30 × 10–9 | –1.91 × 10–5 |
| Female | –4.74 × 10–4 | 4.80 × 10–4 | 3.25 × 10–4 | 1.83 × 10–8 | –7.61 × 10–8 | 5.85 × 10–4 | 3.42 × 10–4 | 3.45 × 10–4 | 1.20 × 10–4 | 3.25 × 10–4 | 1.10 × 10–4 | 6.91 × 10–4 | 5.92 × 10–4 | 5.63 × 10–4 | 4.02 × 10–4 | 4.73 × 10–4 | 1.52 × 10–4 | 4.38 × 10–4 | 5.61 × 10–4 | 5.77 × 10–4 | 6.73 × 10–2 | 4.90 × 10–5 | 1.20 × 10–4 | –3.95 × 10–5 | –7.95 × 10–6 | –5.53 × 10–9 | –6.87 × 10–9 | –6.85 × 10–5 |
| Intercept | –6.92 × 10–4 | 1.33 × 10–5 | –5.30 × 10–5 | 1.36 × 10–8 | 6.56 × 10–9 | –4.27 × 10–5 | –1.84 × 10–4 | –6.19 × 10–5 | –3.63 × 10–4 | 5.79 × 10–4 | –4.72 × 10–4 | 1.87 × 10–4 | 6.72 × 10–5 | 6.15 × 10–5 | –3.79 × 10–4 | –2.89 × 10–4 | 2.55 × 10–5 | –4.48 × 10–5 | –1.24 × 10–4 | 4.03 × 10–5 | 4.90 × 10–5 | 9.54 × 10–4 | 3.62 × 10–4 | 7.88 × 10–6 | 3.50 × 10–5 | –1.05 × 10–8 | –5.33 × 10–9 | 3.94 × 10–5 |
| Walking Away | –1.11 × 10–3 | 2.58 × 10–4 | 1.26 × 10–4 | 4.56 × 10–8 | 1.98 × 10–8 | 1.21 × 10–4 | 6.96 × 10–5 | 1.87 × 10–5 | 1.71 × 10–4 | 7.73 × 10–5 | 4.37 × 10–5 | –2.07 × 10–4 | 3.52 × 10–5 | 8.48 × 10–5 | 1.02 × 10–4 | 5.16 × 10–5 | 1.53 × 10–5 | 1.53 × 10–4 | 7.85 × 10–5 | 1.09 × 10–4 | 1.20 × 10–4 | 3.62 × 10–4 | 2.04 × 10–2 | –4.90 × 10–3 | –4.06 × 10–3 | –1.06 × 10–6 | –6.15 × 10–7 | –1.85 × 10–3 |
| Walking Away Plus | 2.53 × 10–4 | –5.85 × 10–4 | –2.96 × 10–4 | –3.06 × 10–9 | 4.86 × 10–9 | 1.43 × 10–5 | 8.71 × 10–5 | –2.34 × 10–5 | –4.85 × 10–5 | 1.07 × 10–4 | 3.55 × 10–5 | 2.70 × 10–5 | 9.38 × 10–6 | 3.59 × 10–5 | 1.00 × 10–4 | 1.70 × 10–6 | –9.37 × 10–5 | –1.11 × 10–4 | –1.14 × 10–6 | –1.28 × 10–5 | –3.95 × 10–5 | 7.88 × 10–6 | –4.90 × 10–3 | 1.29 × 10–2 | 6.00 × 10–3 | 7.80 × 10–9 | –1.40 × 10–7 | –1.10 × 10–4 |
| Step count at baseline | 1.70 × 10–4 | –2.89 × 10–4 | –7.10 × 10–4 | –2.42 × 10–9 | 1.28 × 10–8 | 1.86 × 10–5 | 5.38 × 10–6 | –2.18 × 10–5 | –3.28 × 10–5 | 1.24 × 10–4 | 1.57 × 10–4 | 1.87 × 10–4 | 1.22 × 10–5 | –3.38 × 10–5 | 1.35 × 10–5 | –4.17 × 10–5 | –3.85 × 10–5 | –1.18 × 10–4 | 4.97 × 10–7 | 3.38 × 10–5 | –7.95 × 10–6 | 3.50 × 10–5 | –4.06 × 10–3 | 6.00 × 10–3 | 1.31 × 10–2 | 9.44 × 10–8 | –3.14 × 10–7 | –2.58 × 10–4 |
| Step count at 12 months | 4.45 × 10–8 | –3.03 × 10–9 | –1.28 × 10–10 | –1.80 × 10–11 | 1.40 × 10–11 | –9.80 × 10–10 | –6.60 × 10–9 | –1.92 × 10–9 | 2.96 × 10–9 | 9.42 × 10–9 | –2.07 × 10–8 | 1.03 × 10–7 | –2.74 × 10–9 | –1.08 × 10–8 | –7.30 × 10–9 | 4.55 × 10–10 | 1.62 × 10–8 | –4.60 × 10–9 | –7.11 × 10–9 | –3.44 × 10–9 | –5.53 × 10–9 | –1.05 × 10–8 | –1.06 × 10–6 | 7.80 × 10–9 | 9.44 × 10–8 | 5.70 × 10–10 | –4.33 × 10–10 | –7.85 × 10–8 |
| Cambridge | 2.35 × 10–8 | 4.09 × 10–9 | 1.75 × 10–8 | 1.38 × 10–11 | –1.62 × 10–11 | 5.33 × 10–9 | 1.53 × 10–9 | 2.89 × 10–9 | –2.22 × 10–8 | –2.61 × 10–8 | –1.37 × 10–8 | –7.46 × 10–8 | 2.34 × 10–10 | 6.95 × 10–9 | –2.86 × 10–9 | –9.53 × 10–9 | –1.65 × 10–8 | –1.29 × 10–8 | 1.08 × 10–9 | –7.30 × 10–9 | –6.87 × 10–9 | –5.33 × 10–9 | –6.15 × 10–7 | –1.40 × 10–7 | –3.14 × 10–7 | –4.33 × 10–10 | 5.38 × 10–10 | –8.12 × 10–8 |
| Female | 9.10 × 10–5 | 9.66 × 10–6 | 1.62 × 10–5 | 4.92 × 10–10 | 7.20 × 10–9 | –4.41 × 10–4 | –7.68 × 10–5 | 7.42 × 10–6 | –1.66 × 10–5 | –1.42 × 10–4 | 3.68 × 10–5 | 1.02 × 10–4 | –3.04 × 10–6 | 1.60 × 10–5 | –9.24 × 10–5 | 2.33 × 10–5 | –3.65 × 10–6 | 7.94 × 10–5 | –3.12 × 10–5 | –1.91 × 10–5 | –6.85 × 10–5 | 3.94 × 10–5 | –1.85 × 10–3 | –1.10 × 10–4 | –2.58 × 10–4 | –7.85 × 10–8 | –8.12 × 10–8 | 9.65 × 10–3 |
Appendix 13 Detailed breakdown of health-care resource use
| Study arm (£) | |||
|---|---|---|---|
| Usual care | Walking Away | Walking Away Plus | |
| Baseline total | 717 | 620 | 626 |
| Years 0–4 | |||
| Primary care | 484 | 474 | 541 |
| Day patient/outpatient | 859 | 1032 | 1313 |
| Inpatient | 487 | 712 | 1345 |
| A&E | 140 | 149 | 100 |
| Prescriptions | 116 | 178 | 102 |
| Intervention | 0 | 258 | 323 |
| Total years 0–4 | 2086 | 2804 | 3724 |
Appendix 14 Results of the within-trial scenario analyses
| Incremental QALYs | Incremental costs (£) | ICER (£/QALY) | |
|---|---|---|---|
| Within-trial results when real-world costs are used and missing data are imputed | |||
| Usual care | – | – | – |
| Walking Away Plus vs. usual care | 0.02 | 1648 | Dominated by WA |
| Walking Away vs. usual care | 0.07 | 949 | 13,557 |
| Within-trial results when trial costs are used and a complete-case analysis is conducted | |||
| Usual care | – | – | – |
| Walking Away vs. usual care | 0.12 | 1267 | 10,558 |
| Walking Away Plus vs. Walking Away | 0.02 | 1022 | 51,100 |
| Within trial results when trial costs are used and missing data are imputed | |||
| Usual care | – | – | – |
| Walking Away Plus vs. usual care | 0.02 | 1460 | Dominated by WA |
| Walking Away vs. usual care | 0.07 | 664 | 9486 |
Appendix 15 Adverse event reporting rates PROPELS safety data
A total of 47 non-serious adverse events (AEs) were reported, 26 of which were directly related to study procedures, namely exacerbated knee pain (one participant), musculoskeletal injury to lower back and leg while increasing physical activity (one participant), and rash from the activity monitor or its dressing (24 participants). The event of exacerbated knee pain led to the withdrawal of the participant from the intervention. The non-serious AEs were reported equally in each study arm. The non-serious AEs are as expected given the patient population, concomitant medical conditions and medication. It should be noted that because diabetes diagnosis was an outcome measure, these data are not discussed here unless the data disagree (i.e. a diagnosis of diabetes as reported by the participant and reported in the database) but the diagnosis was not confirmed on subsequent review of clinical records and biochemical data; in this case, an AE has been recorded but that patient is not discussed in the outcome section.
No hypoglycaemic episodes were reported during the study.
A total of 50 serious AEs were reported: seven in the control arm, 15 in the Walking Away arm and 28 in the Walking Away Plus arm. The increase across the arms is likely to be because of the increased contact with participants in each arm, namely clinic appointments only, clinic appointments plus face-to-face sessions, or regular telephone calls, face-to-face sessions plus clinic visits, respectively. One SAE of myocardial infarction was deemed possibly related to the intervention and led to the withdrawal of the participant from the study. The other serious AEs were deemed unrelated to the intervention; however, 21 did lead to withdrawal from the study. The most common serious AEs were cancer, myocardial infarction, stroke and hip replacement. A total of 19 serious AEs had fatal outcomes, 11 because of cancers, three because of respiratory illness, four because of heart disease and one because of motor neurone disease, all of which were unrelated to the study. The SAEs reported are as expected given the patient population, concomitant medical conditions and medication.
| Overall (N = 1366), n (%) | Study arm, n (%) | |||
|---|---|---|---|---|
| Control (N = 460) | Walking Away (N = 450) | Walking Away Plus (N = 456) | ||
| Non-serious AEs | 47 (3.44) | 17 (3.70) | 14 (3.11) | 16 (3.51) |
| Serious AEs | 50 (3.66) | 7 (1.52) | 15 (3.33) | 28 (6.14) |
| Type of AE | Overall (N = 1366), n (%) | Study arm, n (%) | ||
|---|---|---|---|---|
| Control (N = 460) | Walking Away (N = 450) | Walking Away Plus (N = 456) | ||
| Allergic reaction to penicillin | 1 (0.07) | 1 (0.22) | ||
| Allergic rhinitis | 1 (0.07) | 1 (0.22) | ||
| Arthritis | 1 (0.07) | 1 (0.22) | ||
| Broken bone | 3 (0.22) | 2 (0.44) | 1 (0.22) | |
| Cancer diagnosis | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Carpal tunnel release surgery | 1 (0.07) | 1 (0.22) | ||
| Chest pain | 1 (0.07) | 1 (0.22) | ||
| Depression: suicidal thoughts | 1 (0.07) | 1 (0.22) | ||
| Exacerbated knee paina | 1 (0.07) | 1 (0.22) | ||
| Fall | 2 (0.15) | 2 (0.44) | ||
| Head injury (concussion) | 1 (0.07) | 1 (0.22) | ||
| Hip replacement (pre-planned) | 1 (0.07) | 1 (0.22) | ||
| Musculoskeletal injury to lower back and legb | 1 (0.07) | 1 (0.22) | ||
| Panic attack | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Plantar fasciitis | 1 (0.07) | 1 (0.22) | ||
| Rash from activity monitor or dressingc | 24 (1.76) | 12 (2.61) | 5 (1.11) | 7 (1.54) |
| Tooth complaint | 1 (0.07) | 1 (0.22) | ||
| T2D | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Type of AE | Overall (N = 1366), n (%) | Study arm, n (%) | ||
|---|---|---|---|---|
| Control (N = 460) | Walking Away (N = 450) | Walking Away Plus (N = 456) | ||
| Aspiration pneumonia | 1 (0.07) | 1 (0.22) | ||
| Broken bone | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Brain haemorrhage | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Cancer | 13 (0.95) | 5 (1.09) | 2 (0.44) | 6 (1.32) |
| Cardiac surgery | 1 (0.07) | 1 (0.22) | ||
| Cataract operation | 2 (0.15) | 1 (0.22) | 1 (0.22) | |
| Cellulitis | 1 (0.07) | 1 (0.22) | ||
| Chest pain | 1 (0.07) | 1 (0.22) | ||
| Decompensated heart failure | 1 (0.07) | 1 (0.22) | ||
| Epileptic seizure | 1 (0.07) | 1 (0.22) | ||
| Fall | 1 (0.07) | 1 (0.22) | ||
| Giant cell arteritis | 1 (0.07) | 1 (0.22) | ||
| Ischaemic heart disease | 1 (0.07) | 1 (0.22) | ||
| Kidney stone | 1 (0.07) | 1 (0.22) | ||
| Hip replacement | 3 (0.29) | 3 (0.87) | ||
| Hysterectomy (planned) | 1 (0.07) | 1 (0.22) | ||
| Knee replacement | 1 (0.07) | 1 (0.22) | ||
| Myocardial infarctiona | 5 (0.37) | 1 (0.22) | 4 (0.88)b | |
| Motor neurone disease | 1 (0.07) | 1 (0.22) | ||
| Perimyocarditis | 1 (0.07) | 1 (0.22) | ||
| Pneumonia | 1 (0.07) | 1 (0.22) | ||
| Pulmonary embolus | 1 (0.07) | 1 (0.22) | ||
| Removal of cancerous prostate | 1 (0.07) | 1 (0.22) | ||
| Sinusitis | 1 (0.07) | 1 (0.22) | ||
| Sprained knee (due to traffic accident) | 1 (0.07) | 1 (0.22) | ||
| Stroke | 3 (0.29) | 1 (0.22) | 1 (0.22) | 1 (0.22) |
| Thrombophlebitis (venepuncture injury) | 1 (0.07) | 1 (0.22) | ||
Appendix 16 Recently published physical activity trials designed for delivery in primary care
| Intervention | Cohort/location | Objective measurement tool | Sample size | 12-month follow-up | 24-month follow-up | 36-month follow-up | 48-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N with valid data | Intervention effect | N with valid data | Intervention effect | N with valid data | Intervention effect | N with valid data | Intervention effect | ||||
| Walking Away from Diabetes:28 group-based structured education and pedometer use. Annual group-based follow-on support and telephone contact at 6 months offered | Risk factors for T2D defined using the Leicester Diabetes Risk Score/UK | Accelerometer (Actigraph, Pensacola, FL, USA) | 808 | 571 (71%) | 411 (117 to 704) steps/day | 559 (69%) | NS | 551 (68%) | NS | No data | |
| Let’s Prevent Diabetes:131 6-hour group-based structured education diabetes prevention programme promoting physical activity (including pedometer use), a healthy dietary and weight loss. Annual group-based follow-on support and telephone coaching every 3 months offered | Prediabetes/UK | Pedometer (New Lifestyles Inc., Lees Summit, MO, USA) | 880 | 639 (73%) | 552 (117 to 986) steps/day | 567 (64%) | NS | 487 (55%) | 536 (13 to 1059) steps/day | No data | |
| IDES_2:151 one individual physician led theoretical counselling session and eight individual theoretical and practical counselling sessions each year | Established T2D/Italy | My Wellness Key (Technogym, Cesena, Italy) | 300 | NA | 6.8 (5.2 to 8.4) MVPA minutes/day | NA | 6.5 (4.5 to 8.6) MVPA minutes/day | NA | 3.6 (1.4 to 5.9) MVPA minutes/day | No data | |
| PACE-UP132 postal or nurse-led pedometer intervention | Inactive adults/UK | Accelerometer (Actigraph) | 1023 | 956 (93%) |
Postal: 642 (329 to 955) steps/day Nurse: 677 (365 to 989) steps/day |
No data | 681 (67%) |
Postal: 627 (198 to 1056) steps/day Nurse: 670 (237 to 1102) |
No data | ||
| PACE-LIFT:132 nurse-led pedometer intervention with accelerometer feedback | Inactive older adults (aged 60–75 years)/UK | Accelerometer (Actigraph) | 298 | 273 (93%) | 610 (104 to 1117) steps/day | No data | No data | 225 (76%) | NS | ||
| EuroFIT:133 12 weekly, 90-minute sessions delivered in football grounds by football coaches | Overweight and obese men/UK, Norway, the Netherlands and Portugal | Accelerometer [ActivPAL (PAL Technologies, Glasgow, UK)] | 1113 | 921 (83%) | 678 (309 to 1048) steps/day | No data | No data | No data | |||
Appendix 17 Text messages used in the Walking Away Plus arm of the PROPELS programme
Participants in the Walking Away Plus arm of the study received support in the form of SMS text messages generated from the list below.
#fname# means insert participant’s name, #year# means insert year of programme, #ltg# means insert participant’s long-term goal etc.
| Welcome and close-out |
|---|
| Welcome to PROPELS #fname#! We are here to provide you with feedback, tips and support over the next 12 months :-) |
| Welcome back to PROPELS year #year# #fname#! |
| Thank you for participating in the PROPELS study. As the study will soon finish, text messages and phone calls will be phased out |
| Please keep up the good work as staying active benefits your health and well-being. We will contact you shortly to arrange your final clinic visit |
| Congratulations and thank you for participating in the PROPELS study. The study has now finished... |
| You will no longer receive text messages, phone calls or clinical visits with the study team. Please refer to your GP for future health checks |
| Birthday and New Year |
| Hi #fname#. We wanted to wish you a very happy birthday from the PROPELS team, have a wonderful day! |
| Hi #fname#. Wishing you a very happy new year from the PROPELS team! |
| Pedometer |
| Hi #fname#. This is a reminder to wear your pedometer every day from when you wake up until you go to bed and log your step count in your activity diary |
| Hi #fname#. Try your best to maintain your usual activity this week so we get an accurate baseline to improve on! |
| Hi #fname#, we hope that everything is going well this week and you have started to make some small changes to increase your activity :-) |
| Hi #fname# - just checking that you are wearing your pedometer and logging your steps;-) |
| Please text in your WEEKLY step count. Text STEPS followed by a space and the number of steps you have achieved in total over the past 7 days e.g., STEPS 12095 |
| Please text in your WEEKLY step count (e.g. STEPS 39473) and remember to use the converter if you have done any other activities to get an accurate number |
| Hi #fname#. Please text in your WEEKLY step count by entering the number of steps you have achieved in total over the past 7 days (e.g. STEPS 39473) |
| Hi #fname#. Just a reminder to wear your activity monitor and keep logging your daily steps :-) |
| Hi #fname#. Please text in your weekly step total when you get chance (e.g. STEPS 45372). Use your step converter to record any other activities you have done |
| Please wear your pedometer and log your steps again this week ahead of your 6 month phone call. We will ring you at some point over the next couple of weeks :-) |
| Hi #fname#! We hope you are well. Keep monitoring your steps every couple of weeks to see if you have kept up with your activity goals :-) |
| Hi #fname#! Keep monitoring your steps every few weeks to see if you can keep increasing, working towards your long-term step goal of #ltg# steps per week |
| Only one more week to go of wearing your pedometer and logging steps! Thanks again for all of your efforts so far :-) |
| You no longer need to log steps if you don’t want to. You’ll still hear from us every other week just to provide you with reminders/tips for staying active :-) |
| Thanks for the text! We’ve recorded your step count. Please remember that you are no longer required to text in every week. Keep up the good work :-) |
| If you were trying to text in your weekly steps, we didn’t recognise it-perhaps try again? The format for entering steps is STEPS XXXXX (e.g. STEPS 19874) |
| Encouragement from team |
| Hi #fname#, we hope you are getting on ok so far :-) Remember that a member of the PROPELS team will be phoning you in the next few days |
| Hi #fname#, remember that small changes (no matter how small!) add up to increase your step count :-) |
| Hi #fname#. We hope you are doing well this week and keeping up with your activity plan :-) |
| Hi #fname#. A quick hello form the PROPELS team. Stay positive and keep moving :-) Have a great day! |
| Hi #fname#. Just a quick hello from the PROPELS team. We hope you are doing well with your activity plan this week - keep up the good work! |
| Hi #fname#. Just a quick hello from the PROPELS team. We hope you are doing well with your activity plan this week - stay positive and keep moving! |
| Hi #fname#. Just a quick hello from PROPELS.We hope you are continuing to reach your step goal most days and are still enjoying your active lifestyle :-) |
| Hi #fname#.Just a quick hello from PROPELS.We hope you are continuing to increase your step count on most days to enjoy the benefits of increased activity :-) |
| Hi #fname#. We are almost 6 months into PROPELS! Praise yourself for making this commitment to your health and dramatically increasing your activity :-) |
| Hi #fname#. We are almost 6 months into PROPELS! Praise yourself for making this commitment to your health increasing your activity :-) |
| Hi #fname#. Even if you have only been able to increase activity a little, this is still a great start. Keep making small changes when you can :-) |
| Hi #fname#. Just a quick hello from the PROPELS team. We hope things are continuing to go well and you are keeping up with your activity plan :-) |
| Hi #fname#. Just a quick hello from the PROPELS team. We hope things are going well and you are continuing to make small changes to increase your walking :-) |
| Hi #fname#. Just a quick hello from the PROPELS team. We hope things are going well and you are keeping up with your activity plan :-) |
| Hi #fname#.We hope you are well and finding your activity plan ok. Remember that any increase in step count, no matter how small, is a fantastic achievement |
| Hi #fname#. We are almost 12 months in! You should feel really proud for making this commitment to your health and signing up to PROPELS :-) |
| Hi #fname#. We are almost 2 years in! You should feel really proud for making this commitment to your health and signing up to PROPELS :-) |
| Hi #fname#. We are almost 3 years in! You should feel really proud for making this commitment to your health and signing up to PROPELS :-) |
| We are almost at the end of your 4 year involvement in PROPELS. Thanks again for your hard work and commitment to this programme! |
| Thank you for your text. A member of the PROPELS team will phone you in the next few days to talk about your STEP goals for the next few weeks :-) |
| Remember that once the WA programme ends you can still use the skills you have learnt, such as setting goals and making plans, or using a pedometer :-) |
| Hi #fname#, Remember to attend your annual Walking Away Group Education session asap. The study team will contact you shortly to arrange a suitable date :-) |
| Hi #fname#-we hope that you are continuing to enjoy the benefits of increased activity! Stay positive and keep moving :-) |
| Benefits of physical activity |
| Hi #fname# :-) Remember that any increases in movement (no matter how small!) can help improve mood and relieve stress! |
| Remember that walking is the single most effective form of exercise to reduce your risk of Type 2 Diabetes - it even improves mood and relieves stress :-) |
| Hi #fname#, to try and increase your activity, remind yourself why you want to be more active-what are the benefits that are most important to you? |
| Becoming more active again is the best way to reduce your risk of Type 2 Diabetes. It will also help to give you more energy and improves concentration! |
| Moving around more has benefits you can’t always see! Even if you’re not losing weight, you’re improving your health on the inside and that’s more important! |
| Becoming more active is the best way to reduce your risk of Type 2 Diabetes. It will also help to give you more energy and improves concentration! |
| Moving more and sitting less can really help control blood pressure and even help improve your mood-remember to make small changes wherever possible! |
| Walking more has benefits that you can’t always see! Even if you’re not losing weight, you’re improving your health on the inside and that’s more important! |
| Moving around more has benefits you can’t always see! Even if you’re not losing weight, you’re improving your health on the inside and that’s more important! |
| Hi #fname#-by walking more and sitting less, you can really improve your mood and even your sleep quality-remember to make small changes wherever possible! |
| Hi #fname#-by moving more and sitting less you can really improve your mood and even your sleep quality-remember to make small changes wherever possible! |
| Hi #fname#. Did you know that regular weight-bearing exercise promotes bone formation and may prevent many forms of bone loss associated with aging? |
| Did you know that studies on the psychological effects of exercise have found that regular walking can improve your mood and the way you feel about yourself? |
| Did you know that studies on the psychological effects of exercise have found that regular activity can improve your mood and the way you feel about yourself? |
| Hi #fname#. Did you know that regular weight-bearing activity promotes bone formation and may prevent many forms of bone loss associated with aging? |
| Hi #fname#. Did you know that including some weight-bearing activities into your daily routine may prevent many forms of bone loss associated with aging? |
| Hi #fname#. By taking part in PROPELS and increasing physical activity over the next 12 months…you are significantly reducing your risk of type 2 Diabetes |
| Exercise won’t make your stress disappear, but it can help to clear your thoughts and enable you to deal with any problems more calmly |
| Remember that moderate exercise such as walking on most days of the week can help improve mood. Next time you feel a bit low, try going outside for a walk :-) |
| By maintaining increases to your activity, you’ll be setting yourself up for a lifetime of better health and more energy for everything else in your life :-) |
| By increasing your activity by even a little, you’ll be setting yourself up for a lifetime of better health and more energy for everything else in your life :-) |
| A 2011 study found that people sleep significantly better and feel more alert during the day if they get at least 150 minutes of physical activity per week |
| Remember that any activity that gets your heart beating a little faster or makes you break a slight sweat is improving the way your body handles glucose :-) |
| Don’t hold out for weight loss as a “reward”. Focus on other benefits, such as having more energy & improving your health on the inside |
| Working your muscles more often improves their ability to use insulin and absorb glucose.This puts less stress on the insulin-making cells in your body :-) |
| We realise that making time for activity is tough! Remember - a 10 min. bout of any activity that raises your heart rate a little has huge health benefits :-) |
| No matter what your weight, being active boosts good cholesterol. This keeps your blood flowing smoothly which decreases your risk of cardiovascular diseases |
| Remember the numerous benefits of moving round more: Regular physical activity helps with digestion and promotes regular bowel movements |
| Hi #fname#. By keeping up with the PROPELS program....you are continuing to lower your risk of type 2 diabetes |
| Barriers to physical activity |
| If you like, you can let us know about any barriers you had last week?TEXT:B1 for ILL HEALTH/INJURY,B2 for ENERGY/MOTIVATION,B3 for TIME,B4 for OTHER/NO barrier |
| Sorry to hear you have not been 100% - try to keep moving as much as possible if you feel up to it. Hope you feel better soon :-) |
| If you still feel unwell or you have an injury that won’t go, talk to your GP to see if you should carry on with your activity plan :-) |
| Sorry to hear you have not been 100% – try to keep moving as much as possible – even a gentle walk for 10 minutes if you feel up to it :-) |
| Keep setting yourself small goals, like taking the stairs as much as possible & reward yourself if you achieve this by doing something you enjoy :-) |
| Thanks for the text :-) It’s tough to stay motivated - why not try writing down a list of pros and cons of being more active? |
| Try doing just 10 minutes of slightly higher intensity activity each day this week instead of any longer bouts, so it can fit your schedule better :-) |
| Keep setting yourself small goals, like going for a walk before dinner, & reward yourself if you achieve this by doing something you enjoy at the end of week :-) |
| Thanks for the text :-) It’s tough to stay motivated - why not try writing down a list of pros and cons of walking more each day? |
| Moving around more has benefits you can’t always see! Even if you’re not losing weight, you’re improving your health on the inside and that’s more important! |
| We realise it can be difficult to get motivated, especially if it causes pain. Remember that even the smallest of changes add up to benefit your health :-) |
| Walking more has benefits that you can’t always see! Even if you’re not losing weight, you’re improving your health on the inside and that’s more important! |
| We realise it can be difficult to walk more, especially when things get in the way. Remember that even the smallest changes add up to improve your health :-) |
| Why not try focusing on one really small change this week instead - like always taking the stairs instead of an escalator or lift if there is a choice? |
| Spare yourself the stress of finding a good parking space and gain more energy by parking as far away from the shops as possible this week :-) |
| Reject your inner couch potato! Walk or jog on the spot while you watch your favourite 30-minute TV show at home. Try this twice this week :-) |
| Spare yourself the stress of finding a good parking space and gain more energy by parking as far away from the shops as possible this week :-) |
| Try to fit in small bouts of activity wherever you can this week - remember even small amounts of movements add up and can help you to feel better :-) |
| You don’t need to get all your activity at one time-10 minutes morning, noon and night can give you the same benefits as 30 mins in one go :-) |
| Remember-small bouts of activity are equally effective as 30 mins straight! Any movement or activity that gets your heart rate up a bit is better than nothing : |
| You don’t need to get all your walking done in one go-10 minutes morning, noon and night can give you the same benefits as 30 mins in one go :-) |
| Fitting in 3x 10 min bouts of moderate intensity activity is just as effective as 30 minutes straight. Promise yourself you’ll try this out this week :-) |
| Even if you’re glued to your phone, you don’t have to be glued to your seat! Try to move around while on the phone-even simple arm movements..it all adds up :-) |
| Even if you’re glued to your phone, you don’t have to be glued to your seat! Make it a habit this week to talk and walk whenever possible :-) |
| Remember-small bouts of activity are effective! Every time you go upstairs this week, try going up & down a few times instead |
| Make a commitment that you will turn ‘sit’ time into ‘fit’ time & move around as much as possible-even standing up and sitting down more whilst watching TV! |
| Remember-small bouts of activity are equally effective as 30 mins straight! Simply walking a bit faster than usual this week is a good thing to try :-) |
| Make a commitment that you will turn ‘sit’ time into ‘fit’ time this week & move around as much as possible-even walking on the spot whilst watching TV! |
| Don’t worry about how long it takes you to reach your goals-what’s important is that you find ways to be active that you’ll stick with :-) |
| It can be difficult to increase activity. Keep reminding yourself of why you want to increase activity? How would life be different if you became more active? |
| Thanks for the text. We’ll try to provide you with some helpful tips-This week, promise yourself that you will move around more in your home whenever possible :-) |
| Thanks for the text. We’ll try to provide you with some helpful tips-This week, why not promise yourself that you will walk instead of drive wherever possible? |
| Hints and tips |
| Hi #fname#. We realise it can be difficult to make time for activity, but remember that you can break it up into 10 minute bouts if that makes it easier? |
| To try and increase your steps this week, try to move more at home where possible. Try doing the housework or gardening a little more vigorously if you can :-) |
| Remember that the gym isn’t a necessity-if you don’t feel like going, try going for short brisk walks instead, perhaps just before dinner or after work? |
| Remember that the gym isn’t a necessity-If you don’t feel like going, try moving around more at home, such as standing up more whilst watching TV :-) |
| Make a commitment to yourself that you will leave the car at home or not get the bus for trips that are less than 2 miles this week! |
| Make a commitment to yourself that you will turn ‘sit’ time into ‘fit’ time this week and move around as much as possible whilst at home :-) |
| Reject your inner couch potato! Try walking on the spot during the ad breaks to your favourite TV show this week to help increase your step count :-) |
| Reject your inner couch potato! Try jogging on the spot during the ad breaks to your favourite TV show this week to help increase your step count :-) |
| Try to focus on reducing time spent sitting this week-it can be easier to make small changes. Think about any times last week you could have sat down less? |
| No time for activity this week? No worries! Whilst on the phone, make sure you are walking and talking….it all adds up :-) |
| Seated exercises are a great way to build strength and balance. Try marching whilst sitting, making arm circles and moving from sitting to standing this week! |
| We realise that walking can be a difficult activity if it causes pain or discomfort. Seated exercises are a great alternative for staying healthy and mobile :-) |
| No time for activity this week? No worries! Whilst on the phone, make sure you are walking and talking….it all adds up :-) |
| Seated exercises are a great way to build strength and balance. Try marching whilst sitting, making arm circles and moving from sitting to standing this week! |
| We realise that walking can be a difficult activity if it causes pain or discomfort. Seated exercises are a great alternative for staying healthy and mobile :-) |
| Hi #fname#. Remember that your body performs at its best when it’s properly hydrated - make sure you are drinking plenty of water! |
| Hi #fname# - why not promise yourself that you will walk instead of drive for journeys less than 1 mile this week? |
| Hi #fname# - why not promise yourself that you will try to take the stairs whenever there is an option this week? |
| Every time you brush your teeth-do some squats! Pretend you are sitting back towards an imaginary chair, make sure your knees don’t go in front of your toes |
| This week, every time you brush your teeth-walk on the spot. This will add a few minutes extra activity each day :-) |
| Put on a CD for 15 mins and allot a certain number of songs to complete each chore, e.g., allow 2 songs to vacuum the lounge and so on. Try this twice this week. |
| This week-put on a CD and allot a certain number of songs to complete each chore, e.g., allow 2 songs to vacuum the lounge, 3 songs to wash the dishes by hand…. |
| Stretch out your chores this week to get your step count up e.g. when hanging washing on the line, make a trip back and forth for each item. Every step counts! |
| Try to stretch out your chores this week to add an extra few minutes of activity. For example, make multiple trips to hang washing on the line instead of one. |
| Try to time yourself walking 1 mile (approx.2000 steps) and take your pulse before and after this activity. See if this changes as you increase your activity? |
| Remember to schedule your walks for times in the day or week when you feel most energetic. You’ll be more likely to stick to it! |
| Remember to schedule your activity for times in the day or week when you feel most energetic. You’ll be more likely to stick to it! |
| Schedule activity as you would an important appointment. Block off times and make sure your friends and family are aware of your commitment :-) |
| In the same way that you kept a diary of your activity for PROPELS, why not try keeping a food and drink diary to monitor your diet for a couple of weeks? |
| Before your evening meal, take a moment to relax with deep breathing or take a leisurely walk. Stress causes overeating, so chill out before you eat! |
| If you’d rather not spend a penny on exercise equipment...2 tins of beans can serve as a couple of hand weights for some arm raises while you watch TV this week |
| Hi #fname# - why not promise yourself that you will walk instead of drive for journeys less than 1 mile this week? |
| Hi #fname# - why not promise yourself that you will swap ONE journey usually made by car or public transport for walking this week? |
| Put on a CD for 15 mins and allot a certain number of songs to complete each chore e.g. allow 2 songs to vacuum the lounge and so on. Try this twice this week |
| Every time you brush your teeth-do some squats! Pretend you are sitting back towards an imaginary chair, make sure your knees don’t go in front of your toes |
| This week, every time you brush your teeth-walk on the spot. This will add a few minutes extra activity each day :-) |
| Spare yourself the stress of finding a good parking space and increase your step count further by parking as far away from the shops as possible this week :-) |
| We realise that walking is not the easiest activity, so focus on increasing the distance you can walk without taking a break, even if only by a small amount :-) |
| Spare yourself the stress of finding a good parking space and gain more energy by parking as far away from the shops as possible this week :-) |
| We realise that walking might not always be the easiest activity, so focus on other activities and use the converter in your diary to turn these into ‘steps’ :-) |
| We realise it can be difficult to make time for activity-try setting aside 15 minutes for a brisk walk, perhaps just before dinner every other day this week? |
| It can be tough to make time for a walk, but try to set aside specific days & times, making it just as much a regular part of your schedule as everything else :-) |
| We realise it can be difficult to make time for activity-try setting aside 10 minutes for a brisk walk, perhaps just before dinner every other day this week? |
| Try to focus on reducing time sitting this week-it can be easier to make small changes that way. Was there any time last week you could have sat down less? |
| Every time you are active this week, take a minute to savour the good feeling that exercise gives you. This type of internal reward can help you to stay active. |
| Each time you are active, take a minute to savour the good feelings that exercise gives you. This type of internal reward helps you make a commitment to activity. |
| If it’s hard to find time for a walk this week, don’t fall back on excuses! Schedule a brisk walk as you would any other important activity. |
| If it’s hard to find time for activity this week, don’t fall back on excuses! Schedule activity as you would any other important activity. |
| If you manage to go for a walk during the next week, try to think about how you feel after it-More energy? Less stressed? Better or worse than before you went? |
| Hit the hay! It’s difficult to be happy, let alone active, if you are tired and struggling to find enough energy to get through the day :-) |
| A 2006 study found that laughing for 15 minutes each day can help you burn 10 to 40 calories, depending on your body size and the intensity of your laughter |
| A recent study demonstrated that pedometer users lost more weight, and walked about 2,500 steps more per day than those who didn’t use a pedometer |
| Happiness researchers found that people are happy when they get what they want (not surprisingly) and when they appreciate what they already have :-) |
| Studies have shown that kindness is contagious: When people see others doing something kind, they’re more likely to give as well |
| Studies show that individuals who express gratitude on a regular basis have better health, optimism, progress toward goals, well-being, and help others more |
| Goals and goal-setting |
| Congratulations! You have achieved your long term goal of approx. #fb1# per day-that’s #fb2# more per week than when you started! Well done and keep it up :-) |
| Congratulations #fname# on achieving your short term goal. You have increased by roughly #fb3# steps per day-that’s #fb2# more per week than when you started! |
| Thanks for the text #fname#. Keep wearing your monitor and logging your steps and try to make small changes so that you reach your goal next week :-) |
| Congratulations #fname#! You have continued to increase your weekly physical activity. That’s fantastic progress..keep up the hard work :-) |
| Hi #fname# Well done for maintaining your weekly step total - we realise how tough this can be each week! You are making fantastic progress - keep it up :-) |
| Thanks for the text! You decreased slightly from last week but not to worry-keep making small changes and you will soon be achieving your goal every week :-) |
| Thanks for the text. We realise that increasing your activity is not always easy. Keep it up and hopefully you will increase a little next week :-) |
| Remember to keep setting a new goal every few weeks to keep up your hard work. If you achieve this goal, reward yourself by doing something you enjoy! |
| Remember to keep setting yourself a new goal every couple of weeks, to keep up your hard work. This might be ‘taking the stairs whenever possible’ :-) |
| Remember to keep setting yourself a new goal every couple of weeks, to keep up your hard work. This might be ‘taking a short walk once per day if possible’ :-) |
| Remember to keep setting yourself a new goal every week in order to keep making progress. This might be ‘taking the stairs whenever possible’ :-) |
| Remember to keep setting yourself a new goal every week in order to keep making progress. This might be ‘taking a short walk once per day if possible’ :-) |
| Keep setting yourself small goals like going for a 10 minute walk twice per day, every day this week. Reward yourself if you achieve this at the end of the week. |
| Hi #fname#, keep setting yourself small goals like taking the stairs as much as possible and reward yourself if you achieve this at the end of the week :-) |
| Hi #fname# Well done for maintaining your weekly step total - this is a great achievement! Keep up your hard work :-) |
| You are making really great progress on reaching your long-term step goal! Keep up the good work, stay positive and keep moving :-) |
| Well done for maintaining your weekly activity-we realise how tough this can be! You are making great progress towards achieving your long term goal-keep it up! |
| Thanks! You decreased slightly from last week but keep making small changes and you will soon be back on track and getting closer to your long term goal :-) |
| Thanks for the text. Increasing activity is a challenge. You will receive a text shortly that will help us to provide you with relevant tips for getting active |
| Thanks for the text! You decreased slightly from last week but not to worry-keep making small changes and you will soon be achieving your goal every week :-) |
| Congratulations again! You have continued to increase your weekly steps, which is brilliant - well done! |
| Congratulations! You have managed to maintain your weekly steps which is brilliant - keep making small changes to get one step closer to your long-term goal :-) |
| Thanks for the text :-) We realise that increasing your activity can be really tough. We’ll contact you shortly to try and provide some helpful suggestions |
| Thanks! You decreased a bit from last week but not to worry-you will receive a text shortly that will help us provide you with relevant tips for getting active |
| Congratulations on making more progress - you are doing great! Keep up the good work, stay positive and keep moving :-) |
| Thanks for the text. We’ll send you another text shortly so that we can try to give you further suggestions for increasing activity, even if only by a little :-) |
| Remember that small bouts of activity, such as a brisk 10-15 minute walk before dinner each day will help you to maintain your long-term step goal :-) |
| Remember that small bouts of activity throughout the day, such as a little walk before dinner, will help you to maintain your long-term step goal :-) |
| You did well to reach your long term goal so soon! Small bouts of activity, such as a 10 min. walk before dinner will help you to reach that target every week :-) |
| You did well to reach your long term goal so soon! Remember that small bouts of activity throughout the day will help you to reach that target every week :-) |
| You are making great progress! Keep setting aside specific days and times for a walk to make it just as much a part of your regular schedule as anything else :-) |
| You are making great progress! Keep setting aside specific days and times for activity to make it just as much a part of your regular schedule as anything else! |
| You have made really great progress towards your long term goal and increased your step count significantly over the past few weeks. Keep up the good work :-) |
| To help you to reach your short term goal this week - why not try setting aside 15 minutes every day before dinner for a brisk walk? |
| To help you to reach your short term goal-try setting aside 15 minutes every day to move around more in the house - that will soon get your step count up :-) |
| It can be tough to stay motivated-try to think about the pros and cons of being more active? How would things be different if you increased your walking? |
| It can be tough to stay motivated-try to think about the pros and cons of being more active? How would things be different if you were more active? |
| Studies have shown that keeping up with goal-setting (e.g.10,000 per day) is important, even if you don’t reach it every day. This goal should be a challenge:-) |
| Studies have shown that keeping up with goal-setting around your chosen activity is important for maintaining it, even if you don’t reach it every day :-) |
List of abbreviations
- AE
- adverse event
- ALP
- alkaline phosphatase
- ALT
- alanine aminotransferase
- BIPQ
- Brief Illness Perceptions Questionnaire
- CI
- confidence interval
- CLAHRC
- Collaboration for Leadership in Applied Health Research and Care
- CVD
- cardiovascular disease
- DESMOND
- Diabetes Education and Self-Management for Ongoing and Newly Diagnosed
- DMEC
- Data Monitoring and Ethics Committee
- eHealth
- electronic health
- EPIC
- European Prospective Investigation of Cancer and Nutrition
- EQ-5D
- EuroQol 5-Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- HbA1c
- glycated haemoglobin
- ICER
- incremental cost-effectiveness ratio
- mHealth
- mobile health
- NAVIGATOR
- Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OGTT
- oral glucose tolerance test
- PI
- principal investigator
- PREPARE
- Prediabetes Risk Education and Physical Activity Recommendation and Education
- PROPELS
- The Promotion of Physical Activity through structured Education with differing Levels of ongoing Support for those with prediabetes
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RPAQ
- Recent Physical Activity Questionnaire
- SAP
- statistical analysis plan
- SD
- standard deviation
- SF-8
- Short Form 8
- T2D
- type 2 diabetes
- TSC
- Trial Steering Committee
Glossary
- mHealth
- The use of mobile technologies to support medical and health practices.
- Non-diabetic hyperglycaemia
- An intermediary glucose control category that is outside the normal range but below the threshold for diagnosis of type 2 diabetes.
- Prediabetes
- A synonym for non-diabetic hyperglycaemia.
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25770).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
