Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/04/108. The contractual start date was in January 2015. The draft report began editorial review in January 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Gray et al. This work was produced by Gray et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Gray et al.
Chapter 1 Introduction
Scale of the problem
Ischaemic heart disease, including myocardial infarction (MI), continues to be a major cause of morbidity and mortality in the UK. In 2011, 5% of deaths in England were attributable to acute MI and 13% were directly related to ischaemic heart disease. 1 In the UK and internationally, chest pain is one of the most common reasons for patients to attend the emergency department (ED), accounting for around 5% of all presentations. 2,3 The vast majority of these patients are evaluated for suspected acute coronary syndrome. In the UK, approximately 15% of these patients will receive a final diagnosis of acute coronary syndrome;4–6 however, this varies internationally, with some studies from the USA reporting that only around 4–6% of patients receive a final acute coronary syndrome diagnosis. 7,8
Diagnostic pathways for suspected acute coronary syndrome
Owing to the consequences of inadvertent discharge of a patient with acute coronary syndrome and the limitations of initial clinical assessment, most patients will be investigated with a 12-lead electrocardiogram (ECG) and cardiac troponin level measurement. Contemporary guidelines, confirmed by recent large pragmatic trials and systematic reviews of diagnostic cohorts, recommend early rule-out of acute coronary syndrome at 1–3 hours if the high-sensitivity cardiac troponin level assay is at the limit of detection, or at a very low predetermined assay-specific ‘rule-out’ threshold for either troponin level T or troponin level I. 9,10 If the troponin level is between these measurements and the 99th centile, it is normally rechecked to see if there has been a change: the delta value. 9,10 A change suggests the requirement for further evaluation in hospital, even if not above the 99th centile. Given the characteristics of modern cardiac troponin level assays, there are increasing numbers of patients who have at least one measurement above the 99th centile. These patients are diagnosed with non-ST elevation MI according to the universal definition of MI. 11 Previously, an alternative diagnosis, such as unstable angina, may have been made. Recent trial evidence suggests that there may not be an outcome benefit for more aggressive treatment strategies for this patient group. 12 Moreover, especially at lower levels above the 99th centile, the assays lack specificity for the diagnosis of MI, with a number of other conditions resulting in acute troponin release and chronically elevated concentrations seen in patients with multimorbidity. 13,14 These limitations of the troponin level assay may lead to increased investigation, including invasive angiography, for this patient group.
For the patient group with a measurable troponin level between a ‘rule-out’ threshold and the 99th centile but with acute coronary syndrome ‘ruled out’, subsequent assessment for prognostically important coronary artery disease is inconsistent in the UK, with many patients receiving limited further assessment despite subsequent MI or cardiac death rates of around 4% at 1 year. 15
Recent guidelines9,10 have suggested that patients with intermediate or mildly elevated cardiac troponin levels and limited other concerning features are at low to intermediate risk of major adverse cardiac events and may benefit from additional investigations to further delineate the diagnosis. These include non-invasive functional (e.g. exercise ECG or stress perfusion imaging) or anatomical [computerised tomography coronary angiography (CTCA)] testing and invasive coronary angiography. 4,5
The role of additional investigations
Exercise ECG testing is not widely used for the further delineation of coronary artery disease in emergency care settings in the UK. 16 Exercise ECG testing is typically used in the context of a standardised assessment alongside biomarker testing in a chest pain unit or for patients with new-onset angina with exercise-induced symptoms. These units are widespread in the USA but have been established in only a few centres in the UK following a cluster-randomised trial that failed to show evidence of benefit of these centres. 17 Current European Society of Cardiology guidelines9 recommend a variety of testing strategies, including invasive angiography, stress testing or CTCA. 4 Current National Institute for Health and Care Excellence (NICE) guidance18 recommends considering ischaemia testing in the context of acute coronary syndrome when there is a low-risk clinical prognosis. However, a systematic review of 54 observational studies incorporating 19,874 patients with clinical MI indicates that pre-discharge stress testing provides limited additional prognostic information to guide patient management. 19 All forms of non-invasive stress testing demonstrate similar sensitivities and specificities for the prediction of future cardiac events. Although the negative predictive value is high (≈ 94%), the positive predictive value is low (< 10% for cardiac death and < 20% for cardiac death or MI).
Invasive coronary angiography is recommended by both NICE and the European Society of Cardiology4,18 in patients with confirmed acute coronary syndrome or thought to be at high risk of obstructive coronary disease. However, invasive coronary angiography is expensive and associated with a small risk of significant complications, including death. 20 It often requires patients to be transferred between hospitals in the UK, as only around 35% of acute hospitals have on-site cardiac catheterisation and coronary revascularisation facilities. 20 It is not known how many patients receive inappropriate invasive coronary angiography, but the number is likely to be large and potentially increasing if all patients with an elevated troponin level and chest pain receive invasive coronary angiography. Some patients with confirmed acute coronary syndrome may not receive invasive coronary angiography owing to limited availability, belief that troponin level elevation is because of an alternative cause or other reasons for a decision to pursue non-invasive management. In the RITA-3 trial,21 of patients presenting with a non-ST elevation acute coronary syndrome, those undergoing invasive investigation were managed with medical therapy in 43% of cases, percutaneous coronary intervention in 35% of cases or coronary artery bypass graft surgery in 22% of cases. Given that CTCA has similar discriminatory value as invasive coronary angiography in determining the need for coronary revascularisation in patients with acute coronary syndrome,22 it could be used as a preliminary investigation with onward referral for percutaneous coronary intervention or coronary artery bypass graft surgery limited to patients with a clear, treatable, coronary obstruction.
Computerised tomography coronary angiography
Diagnostic accuracy
Without a doubt, there is a need for novel investigations to support the evaluation of suspected or provisionally diagnosed acute coronary syndrome that enable improved diagnosis of acute coronary syndrome and risk stratification for subsequent clinical events. Moreover, better case selection for invasive coronary angiography is important given the increasing recognition of the occurrence of MI with non-obstructive coronary artery (MINOCA) disease 23–25 and the lack of specificity (false-positive rates) with the use of high-sensitivity cardiac troponin level assays. 13,14 Ultimately, any investigation should provide information to tailor subsequent management and improve clinically important outcomes. CTCA may fulfil all of these requirements. CTCA is quicker, simpler, substantially cheaper and more readily delivered than invasive coronary angiography, and should translate into a highly effective and safe imaging strategy.
A systematic review of 21 diagnostic accuracy studies of CTCA reported a pooled sensitivity of 99% and specificity of 89% for the detection of coronary artery disease. 25 This Health Technology Assessment (HTA) comprehensive systematic review assessed the role of 64-slice multidetector computed tomography as an alternative to invasive coronary angiography. In keeping with previous analyses,26 it confirmed the excellent accuracy of multidetector computed tomography in the identification of coronary artery disease. However, this systemic review highlighted several areas that need further research and highlighted, among other things, the need to evaluate the usefulness of multidetector CTCA in patients with suspected coronary artery disease.
A more recent evidence synthesis27 evaluated the diagnostic and prognostic accuracy and cost-effectiveness of CTCA in suspected acute coronary syndrome. This evidence review of eight trials found that CTCA had good diagnostic accuracy for detecting coronary artery obstruction: sensitivity of 94% (95% predictive intervals 61% to 99%) and specificity of 87% (95% predictive intervals 16% to 100%). Economic analysis was subject to substantial uncertainty, but CTCA was likely to be cost-effective if the incidence of subsequent major adverse cardiac events was > 2% [£30,000 per quality-adjusted life-year (QALY) threshold] or > 2.9% (£20,000 per QALY threshold). Decision-analysis modelling was unable to draw reliable conclusions about the clinical effectiveness and cost-effectiveness of CTCA in suspected acute coronary syndrome. The review suggested that further research regarding the effect of testing and treatment on major adverse cardiac events is needed.
Trial evidence: acute chest pain
Four US trials28–31 investigating CTCA in patients with low-risk acute chest pain presenting to the ED promoted the use of CTCA and its widespread adoption. Meta-analyses of these trials32,33 concluded that CTCA is safe, is cost-effective and reduces the length of stay in the US health-care system. However, the event rates in these studies were extremely low, with no difference between trial arms for clinically important outcomes. Moreover, the participants had relatively long hospital stays and many additional tests compared with contemporary practice in the UK.
Since the publication of these initial trials, there have been a number of other trials from North America, Europe and Australia, with none powered to evaluate the impact of CTCA use on longer-term clinically important outcomes. 34–39 However, in a secondary analysis of the CATCH trial40 of 600 patients with suspected acute coronary syndrome and normal troponin levels and ECG, CTCA was associated with a reduction in major adverse cardiac events at 18–20 months, although the absolute number of events was small: five patients (MI, n = 2; unstable angina, n = 3) in the CTCA group compared with 14 patients (cardiac death, n = 1; MI, n = 7; unstable angina, n = 5; coronary revascularisation, n = 1) in the standard-care group [hazard ratio (HR) 0.36, 95% confidence interval (CI) 0.16 to 0.95; p = 0.04]. 40
These trials have been assimilated by a number of recent meta-analyses. 41–44 Ten acute chest pain trials of 6285 patients were synthesised to examine the benefits and risks of CTCA compared with other standard-care approaches. 42 There were no significant differences in all-cause mortality [relative risk (RR) 0.48, 95% CI 0.17 to 1.36; p = 0.17], MI (RR 0.82, 95% CI 0.49 to 1.39; p = 0.47) or major adverse cardiac events (RR 0.98, 95% CI 0.67 to 1.43; p = 0.92) between the groups. However, there were higher rates of invasive coronary angiography (RR 1.32, 95% CI 1.07 to 1.63; p = 0.01) and revascularisation (RR 1.77, 95% CI 1.35 to 2.31; p < 0.0001) with CTCA than with standard-of-care approaches. A further meta-analysis by Foy et al.,43 comparing the clinical effectiveness of CTCA with that of functional stress testing for patients with suspected coronary artery disease, included 13 trials (acute chest pain, n = 9; stable chest pain, n = 4). There were 10,315 patients in the CTCA arm and 9777 patients in the functional stress testing arm. CTCA was associated with a reduction in rates of MI (0.7% vs. 1.1%; RR 0.71, 95% CI 0.53 to 0.96). Patients undergoing CTCA were more likely than those who were not to require invasive coronary angiography (11.7% vs. 9.1%; RR 1.33, 95% CI 1.12 to 1.59) and revascularisation (7.2% vs. 4.5%; RR 1.86, 95% CI 1.43 to 2.43). The patients receiving CTCA were also more likely to receive a new diagnosis of coronary artery disease and to start new preventative therapies, such as aspirin or statin therapy, than those who were not.
The latest meta-analysis44 included data from 16 trials of both acute and stable chest pain, enrolling 21,210 participants. There was no difference in all-cause mortality (103 vs. 110; RR 0.93, 95% CI 0.71 to 1.21; p = 0.58) between the CTCA arm and the standard-care arm. There was a reduction in subsequent MI in the CTCA arm (115 vs. 156; RR 0.71, 95% CI 0.56 to 0.91; p < 0.006). This was largely because of a reduction in MI in the subgroup of patients with stable chest pain (80 vs. 120; RR 0.66, 95% CI 0.50 to 0.88; p = 0.004). There was no difference found between arms in the acute chest pain subgroup (35 vs. 36; RR 0.88, 95% CI 0.54 to 1.44; p = 0.61). The CTCA arm had higher invasive angiography rates than the standard-care arm (1044 vs. 701; RR 1.41, 95% CI 1.28 to 1.55; p < 0.00001). This was found in patients with either acute (311 vs. 205; RR 1.35, 95% CI 1.13 to 1.62; p = 0.001) or stable (733 vs. 496; RR 1.44, 95% CI 1.30 to 1.61; p < 0.00001) chest pain. This led to subsequent comparable changes in revascularisation (percutaneous coronary intervention or coronary artery bypass graft surgery) rates, again for the groups together [789 vs. 472; odds ratio (OR) 1.84, 95% CI 1.44 to 2.35; p < 0.00001] and for both acute (175 vs. 82; OR 1.95, 95% CI 1.42 to 2.69; p < 0.0001) and stable (614 vs. 390; OR 1.70, 95% CI 1.16 to 2.51; p = 0.007) chest pain. There was also a demonstrable reduction in both subsequent ED visits and hospital admissions (570 vs. 616; RR 0.75, 95% CI 0.60 to 0.94; p = 0.01) and downstream investigations in the CTCA arm (242 vs. 342; OR 0.45, 95% CI 0.22 to 0.90; p = 0.02). These findings were not significant when looking at the acute chest pain group alone.
Trial evidence: stable chest pain
The use of CTCA in the evaluation of patients with stable chest pain has been explored in several randomised controlled trials (RCTs). The two largest trials were the Scottish computed tomography of the heart (SCOT-HEART) and prospective multicentre imaging study for evaluation of chest pain (PROMISE) trials. 45–47 These trials demonstrated that CTCA was associated with improved diagnostic certainty,45 reduced rates of normal invasive coronary angiography,47,48 increases in preventative therapy and early coronary revascularisation,47,49 and reduced rates of subsequent coronary heart disease (CHD) death or non-fatal MI. 45,46 NICE guidelines recommend the use of CTCA as the first-line diagnostic test in patients with suspected CHD. 50
In a recent evaluation of a Danish country-wide registry of 86,705 stable patients being evaluated for suspected coronary artery disease, CTCA was associated with greater use of statin therapy, aspirin and invasive procedures and higher costs than functional testing. CTCA was associated with a lower risk of MI, but a similar risk of all-cause mortality. 42
Computerised tomography coronary angiography in patients with intermediate- or high-risk acute chest pain
A small, single-centre, three-arm trial investigated an imaging-first strategy of either CTCA or cardiac magnetic resonance imaging, compared with standard care, in patients with acute chest pain who had a non-diagnostic ECG and an elevated high-sensitivity cardiac troponin level. 51 An initial non-invasive imaging strategy reduced the proportion of patients referred for invasive coronary angiography during initial hospitalisation (cardiac magnetic resonance imaging 87%, CTCA 66% and routine clinical care 100%; p < 0.001 for imaging vs. routine care), with the fewest invasive coronary angiograms performed in those undergoing CTCA (p < 0.004 vs. cardiac magnetic resonance). The reduction in invasive coronary angiography persisted for at least 1 year (88%, 70% and 100%. respectively; p < 0.003 for imaging vs. routine care). Unlike previous studies, this trial showed that in higher-risk groups of patients with acute chest pain, CTCA reduced the rates of invasive coronary angiography rather than increasing it and increased the proportion of patients receiving revascularisation who had invasive coronary angiography.
The rationale for this trial
With the increasing recognition of the frequency of MINOCA disease and the issues with false-positive rates of high-sensitivity cardiac troponin level assays, CTCA could have an increasingly important role in the assessment of patients with acute coronary syndrome. In addition to the avoidance of unnecessary invasive coronary angiography, CTCA does provide a better assessment of overall plaque burden and adverse plaque characteristics that cannot be determined directly by invasive coronary angiography and assessments of luminal stenosis severity. 52 This may allow a more rigorous approach to the diagnosis of MINOCA disease and the provision of preventative therapies.
The use of early CTCA in patients with intermediate-risk acute chest pain requires investigation to establish whether or not it can enhance the diagnosis of coronary artery disease, optimise the targeting of therapeutic interventions, including coronary revascularisation and preventative therapies, and, thereby, improve long-term clinical outcomes. The clinical effectiveness and cost-effectiveness of early CTCA in suspected or provisionally diagnosed acute coronary syndrome must be clearly demonstrated before adoption of the technology into routine NHS practice given its cost, risk and uncertainty of benefit. A positive or negative trial is, therefore, equally important to the NHS.
This research is likely to have a major impact on this large and important group of patients presenting with suspected or provisionally acute coronary syndrome to NHS hospitals. If the trial is positive, those patients with coronary artery disease will receive an early and accurate anatomical characterisation of coronary arteries by CTCA, allowing targeting of invasive coronary angiography to those patients who are most likely to require revascularisation and facilitating early optimisation of preventative therapies. These interventions could save lives and reduce the burden of undiagnosed ischaemic heart disease. In patients with non-obstructive coronary artery disease or normal coronary arteries, it is likely to facilitate earlier discharge and prevent unnecessary invasive coronary angiography with the attendant risks. In terms of NHS benefit, this research is likely to lead to more optimal use of scarce and expensive resources, a reduction in duration of index hospital stay and less need for recurrent hospitalisation. The early effective use of preventative therapies could lead to lower rates of long-term cardiovascular events.
If the trial is negative, the results will prevent widespread NHS adoption of an ineffective technology that, if implemented, would substantially increase NHS costs and expose patients to unnecessary investigation with radiation exposure and potential anxiety related to a false diagnosis.
Chapter 2 Clinical effectiveness methods
Study design overview
The Rapid Assessment of Potential Ischaemic Heart Disease with CTCA (RAPID-CTCA) trial was a prospective, randomised, open, blinded end-point, parallel-group controlled trial of CTCA and standard care compared with standard care alone in patients presenting to the ED, acute medical service or cardiology service with suspected or provisionally diagnosed acute coronary syndrome who were at intermediate risk of major adverse cardiac events. 53 Recruitment was undertaken in 37 NHS tertiary and secondary care hospitals with and without on-site coronary angiography facilities. Participants allocated to receive CTCA had the scan during initial admission or, if discharged, as an ambulatory patient within 72 hours of randomisation. All participants were followed up for 1 year and were asked to complete questionnaires at baseline and 1, 6 and 12 months to assess quality of life, angina symptoms and use of NHS resources.
Clinical effectiveness and cost-effectiveness objectives
Trial aims
This trial aimed to investigate the effect of receiving an early CTCA in patients with suspected or provisionally diagnosed acute coronary syndrome presenting to the ED, acute medicine or cardiology service on health-care interventions, clinical event rates and health-care costs in a clinical trial and economic evaluation up to 1 year after the trial intervention.
The primary objective was to investigate the effect of early CTCA on all-cause death or subsequent non-fatal type 1 or type 4b MI at 1 year.
Objectives
The secondary objectives aimed to investigate the effect of early CTCA on:
-
the use of cardiovascular treatments during index hospitalisation and preventative therapies on hospital discharge
-
the proportion of patients receiving invasive coronary angiography and coronary revascularisation
-
length of stay at index hospitalisation
-
the proportion of patients representing or readmitted to hospital with suspected acute coronary syndrome or recurrent chest pain for up to 1 year
-
the use of NHS resources, including hospitalisation and other investigations and interventions for up to 1 year
-
the proportion of patients with symptoms, morbidity and mortality for up to 1 year
-
quality of life for up to 1 year
-
the incremental cost per QALY gained by providing CTCA compared with current standard practice.
Participants: eligibility criteria
Inclusion criteria
Patients aged ≥ 18 years with symptoms mandating investigation for suspected or provisionally diagnosed acute coronary syndrome with at least one of:
-
History of ischaemic heart disease (for which the clinician assessing the patient confirms history based on patient history or available health-care records).
-
Troponin level elevation above the 99th centile of the normal reference range or increase in high-sensitivity troponin levels meeting European Society of Cardiology criteria for ‘rule-in’ of MI. Troponin level assays varied from site to site (see Appendix 7, Table 32); local laboratory reference standards were used.
-
ECG abnormalities, such as ST segment depression of > 0.5 mm.
Exclusion criteria
-
Signs of, symptoms of or investigations supporting high-risk acute coronary syndrome:
-
ST elevation myocardiaI infarction (STEMI).
-
Acute coronary syndrome with signs or symptoms of arrhythmia, acute heart failure or circulatory shock.
-
Crescendo episodes of typical anginal pain.
-
Marked or dynamic ECG changes, such as ST depression of ≥ 3 mm.
-
Clinical team had scheduled an urgent invasive coronary angiography on the day of the trial eligibility assessment. This was added as an exclusion criterion on 15 January 2016.
-
-
Patient inability to undergo computerised tomography (CT):
-
severe renal failure (serum creatinine of > 250 µmol/l or estimated glomerular filtration rate of < 30 ml/minute/1.73 m2)
-
known contrast allergy
-
beta-blocker intolerance (if no alternative heart rate-limiting agent available or suitable) or allergy
-
inability to hold breath
-
atrial fibrillation for which the mean heart rate was anticipated to be > 75 beats per minute (b.p.m. ) after beta-blockade.
-
-
Patient has had invasive coronary angiography or CTCA within the last 2 years and the previous investigation revealed obstructive coronary artery disease, or patient had either investigation within the last 5 years and the result was normal.
-
Previous recruitment to the trial.
-
Known pregnancy or currently breastfeeding.
-
Inability to consent.
-
Further investigation for acute coronary syndrome would not be in the patient’s interest owing to limited life expectancy, quality of life or functional status.
-
Prisoners.
Recruitment
All potentially eligible patients were screened for eligibility by trained members of the research or clinical team using triage information and clinical or electronic records in the ED, acute medicine or cardiology services of the 37 participating centres (see Appendix 7, Table 33). The first participant was recruited on 23 March 2015 and the last on 27 June 2019, with final follow-up data collection and locking of the database on 8 September 2020.
No additional trial-specific screening tests were carried out. All potential participants completed acute clinical assessment, which included 12-lead ECG, recording of vital signs (pulse rate, non-invasive blood pressure, respiratory rate, consciousness level, oxygen saturations and blood sugar measurement) and routine blood sampling, including cardiac troponin levels and renal function. The results of this assessment informed trial eligibility. The patient was approached as soon as possible and available.
Initially, patients were recruited up to 18 hours after presentation. This time period was chosen because it allowed the longest period for recruitment in which the patient could be deemed to be receiving acute assessment, that is up to the point at which a 12-hour troponin level result was being used by clinicians for acute decision-making. The recruitment window was extended to 24 hours on 25 November 2016 after site feedback and assessment of site recruitment and reasons for non-recruitment of potentially eligible patients. Patients and clinicians were unaware of treatment allocation until after randomisation.
Consent
All eligible participants provided written informed consent after approach and discussion with appropriately trained and delegated members of the research or clinical team.
Randomisation
After assessment for eligibility and consent, the clinical research nurse or a delegated member of the clinical team collected the baseline data necessary to complete the pre-randomisation information. Randomisation was carried out using a web-based randomisation service (managed by the Edinburgh Clinical Trials Unit) to ensure allocation concealment. Randomisation was carried out within 18 hours of arrival at the hospital, extending to 24 hours after 25 November 2016. Consented patients were randomised on a 1 : 1 basis to CTCA in addition to standard care or standard care alone and were stratified by study site.
Blinding
This was an open trial. The patient, recruiting and treating clinicians, and radiologist were not blinded to the intervention, including the results. Members of the adjudication committee completing the primary outcome assessment were blinded to the intervention.
Interventions
Computerised tomography coronary angiography
The minimum technology requirement for CT was a 64-slice or greater multidetector CT scanner that was enabled to perform ECG-gated cardiac studies. The examination included a non-contrast ECG-triggered acquisition for calcium scoring (if part of local protocol) and a post-contrast ECG-gated acquisition covering the whole of the heart and the root of the aorta. Patients had to be able to hold their breath for > 20 seconds. The intervention lasted for no longer than 30 minutes and patients were observed for a period of 30 minutes afterwards. Radiation reduction techniques were employed and, when appropriate, intravenous or oral beta-blockades (or alternative heart-rate-limiting agent) were used to reduce heart rate (target of < 70 b.p.m.), enabling radiation dose-saving protocols. The use of glyceryl trinitrate for coronary artery dilatation was at the discretion of individual centres.
Given the range of conversion factors used across sites to convert dose–length product (DLP) to effective dose in mSv, radiation dose was reported as a DLP. A DLP to mSv conversion factor of 0.014 mSv/mGy/cm was calculated, similar to other recent publications. 54 A typical participant with a heart rate of < 70 b.p.m. in sinus rhythm and a body mass index of < 25 kg/m2 would be anticipated to have a DLP of ≤ 686 mGy/cm. Values exceeding this were considered to be protocol deviations. Deviations were reviewed by a radiologist or cardiologist in the central trials team, and any that were deemed to have an impact on patient safety or study outcomes were reported as protocol violations. For other participants, without the typical characteristics stated above, DLP values of > 686 mGy/cm were anticipated as part of routine clinical practice (e.g. owing to the need for continuous retrospectively gated scanning in some participants with arrhythmia). For such participants, any DLP that exceeded 1500 mGy/cm was considered to be a protocol deviation. Deviations were reviewed by a radiologist or cardiologist in the central trials team, and any that had an impact on patient safety or study outcomes were reported as protocol violations.
All participating centres were required to verify that their CTCA imaging protocol complied with the doses outlined in the research protocol prior to recruitment. Patient doses were recorded and monitored as part of the study. Iodine-based contrast media were administered intravenously using the standard local procedure at each site.
Computerised tomography coronary angiography result reporting
Computerised tomography coronary angiography results were reported by a trained radiologist or cardiologist at recruiting centres as soon as possible, ideally within 2 hours, and were immediately communicated to the treating clinician.
The clinical report detailing the results was reported in accordance with the Society of Cardiovascular CT guidelines using the American Heart Association coronary artery segment model, and included both the calcium score, if calculated, and the presence of cardiac and non-cardiac findings. 55,56 Stenoses were quantified as no significant coronary artery disease (estimated stenosis of < 10%), mild non-obstructive disease (estimated stenosis of 10–49%), moderate non-obstructive disease (estimated stenosis of 50–70%) or obstructive coronary artery disease (estimated stenosis of > 70% or > 50% for left main stem disease).
The research scan report was completed by the radiologist or cardiologist, and recorded scanner technology, acquisition protocol, DLP and patient characteristics (see Appendices 1 and 2).
Quality assurance reporting of computerised tomography coronary angiography scans
The first 10 CT scans and reports from each site were reviewed and checked by experts who were independent of the trial site and blinded to the initial report to measure interobserver reliability. This process and the reporting form are detailed in Appendices 3 and 4.
Computerised tomography coronary angiography images for future research
The scans sent for quality assurance (QA) reporting have been retained for future research along with all of the CT scans from the lead recruiting sites in a research repository at the University of Edinburgh (Edinburgh, UK).
Impact of computerised tomography coronary angiography on participant care
A trial guideline on the management of trial participants depending on CTCA result was developed (see Appendix 5). Its use was not mandated because the trial had a pragmatic approach and was investigating the impact of the diagnostic intervention on clinical practice and outcomes.
Compliance and withdrawal of study participants
Study participants were free to withdraw from the trial at any time. Reasons, if given, were recorded and data collected up to that time point were used in the final analyses, unless the patient specifically requested that their data were not used. If the patient withdrew consent to have their data stored, this was documented on the trial Consolidated Standards of Reporting Trials (CONSORT) flow diagram as ‘withdrawn’ and their data were not used in the final analyses. Patients were able to withdraw from participation in active follow-up, but data continued to be collected unless the patient requested otherwise. The patient may have been willing to give a reason for withdrawal, but this was not obligatory.
Crossover
Any patient in the standard-care arm who received a CTCA as part of routine care within 30 days of randomisation was defined as a crossover and was not recorded as a deviation.
Investigation guidelines and strategies for each centre were collected and the use of CTCA was monitored during the trial. Each centre was requested not to use CTCA in the routine assessment of suspected or confirmed acute coronary syndrome during trial recruitment, and was asked to inform the trials team about any changes to local practice.
Non-adherence
This was defined as any participant not receiving a reported CTCA if they had been randomised to receive it within 72 hours of the randomisation. This was recorded as a deviation. This allowed ambulatory CTCA to be delivered when appropriate. Individual site retention, crossover and non-adherence were monitored and reviewed at the Project Management Group, Trial Steering Committee and Data Monitoring Committee meetings.
Other interventions in the computerised tomography coronary angiography arm and the standard-care arm
All other management and admission or discharge decisions were at the discretion of the treating clinicians.
Standard care
All other care, except CTCA in the CTCA arm, was at the discretion of the treating clinical teams.
Trial end points
The trial end points are reported on the ISCRTN website; distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) licence. 57
Primary end point
The primary end point was all-cause death or subsequent non-fatal type 1 or 4b MI at 1 year, measured as the time to first such event. MI was defined in accordance with the third universal definition,11 and events were adjudicated by two independent cardiologists blinded to the trial intervention.
Secondary end points
Key secondary end points included:
-
CHD death or subsequent non-fatal MI
-
cardiovascular disease death or subsequent non-fatal MI
-
subsequent non-fatal MI
-
CHD death
-
cardiovascular death
-
all-cause death.
Other end points
-
Coronary heart disease death or subsequent non-fatal MI (type 1 or 4b).
-
Subsequent non-fatal MI (type 1 or 4b).
-
Non-cardiovascular death.
-
Invasive coronary angiography.
-
Coronary revascularisation.
-
Percutaneous coronary intervention.
-
Coronary artery bypass graft surgery.
-
Proportion of patients prescribed acute coronary syndrome therapies during index hospitalisation.
-
Proportion of patients discharged on preventative treatment or had alteration in dosage of preventative treatment during index hospitalisation.
-
Length of stay for index hospitalisation.
-
Re-presentation or rehospitalisation with suspected acute coronary syndrome or recurrent chest pain within 12 months after index hospitalisation.
-
Chest pain symptoms up to 12 months.
-
Patient satisfaction at 1 month.
-
Clinician certainty of presenting diagnosis after CTCA.
-
Quality of life [measured by EuroQol-5 Dimensions, five-level version (EQ-5D-5L) up to 12 months].
Adverse events and serious adverse events
-
Proportion of patients with alternative cardiovascular diagnoses identified on CTCA.
-
Proportion of patients with non-cardiovascular diagnosis identified on CTCA.
-
Radiation exposure from CTCA as trial intervention.
Sample size
The original sample size calculation was based on an estimated 1-year death or subsequent recurrent MI rate for this patient group of ≈ 20%. 58 To detect a 20% compared with a 15% difference in the rate of 1-year death or recurrent subsequent MI, 2424 evaluable patients were required (1212 per arm) to provide 90% power at two-sided p < 0.05. With a 3% drop-out rate, the sample size would have been 2500 patients. However, after recruiting and following up the first 716 participants, the overall event rate was 6.8% (95% CI 5.2% to 8.9%). In addition, the trial was recruiting at a lower rate than originally predicted. As part of an extension application, the above information was used to calculate a variety of sample sizes for a range of event rates (6%, 6.8% and 8%) and effect sizes (RR 0.5, 0.6 and 0.75) with 80% and 90% power. Given the recruitment rates with associated trial fatigue and potential for loss of clinical equipoise, event rates and funding, the only plausible sample size option was to deliver a trial of at least 1720 patients, not allowing for missing data. Given the lost to follow-up rates at that time, we would need a minimum of 1735 participants to give the trial the opportunity to detect a 3.4% absolute risk reduction at the current primary event rate of 6.8% with 80% power at two-sided p < 0.05.
Statistical methods
Parts of this section are reproduced with permission from Gray et al. 53 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The trial was reported on an intention-to-treat basis. The primary outcome was defined as the first event of all-cause death or subsequent recurrent non-fatal MI type 1 or 4b. The time to primary outcome was defined as the time from randomisation to the primary outcome. Patients discontinuing the study (for any reason) prior to reaching the primary outcome had their time to primary outcome censored at the last contact date. The relationship between the intervention and the primary outcome was analysed using Cox proportional hazard regression adjusted for study site (used to stratify the randomisation), age, baseline Global Registry of Acute Coronary Events (GRACE) score,59 previous coronary artery disease and baseline troponin level. The results were expressed as a HR with the corresponding 95% CIs and p-value. The individual elements of the composite primary outcome were reported separately. Subgroup analysis of the primary outcome was planned for age, sex, baseline GRACE score, previous coronary artery disease, baseline ECG result, baseline troponin level and admission at presentation to a study site with or without on-site invasive coronary angiography facilities. These were assessed by examining the effect of entering the treatment-by-subgroup interaction into the Cox regression model. Secondary outcomes were analysed using appropriate methods: logistic regression for binary outcomes and linear regression for normally distributed continuous outcomes, adjusted as described above. Continuous outcomes that were not normally distributed were analysed using appropriate non-parametric techniques. The primary analysis was intention to treat. Every effort was made to minimise missing data, and the primary analysis was a complete-case analysis. When there was a sufficient level of missing data for it to affect our conclusions, a multiple imputation analysis was undertaken, using clinically appropriate variables, as a sensitivity analysis. Significance testing used a hierarchical approach: for the primary outcome and the key secondary outcomes, statistical significance was declared if the outcome in question, and all prior outcomes listed, had p < 0.05. 60 The p-values for all outcomes were reported for all other outcomes but were not declared to be significant. A full statistical analysis plan was written during the trial and was finalised prior to database lock.
Ethics and governance
The trial was reviewed and approved by the Southeast Scotland Research Ethics Committee (14/SS/1096) and was conducted in accordance with the principles of good clinical practice.
Trial management and oversight
The Edinburgh Clinical Trials Unit (ECTU) was responsible for trial management, including the organisation of Trial Management Group meetings, the organisation of the Trial Steering Committee (TSC) and the Data Monitoring Committee (DMC), contracting with other organisations, the preparation of Research Ethics Committee and research and development office applications, the standard operating procedures, the provision of the randomisation system, database development, data management, data analysis, writing the report and the dissemination of findings.
Project Management Group
The trial was led by Alasdair Gray, and was co-ordinated by a trial manager from the ECTU and an emergency medicine research nurse co-ordinator, with support from the ECTU. A Project Management Group comprising the applicants and relevant members of the ECTU team oversaw trial delivery. The Academic and Clinical Central Office for Research & Development in Edinburgh provided sponsorship and monitoring oversight for the trial, which was conducted in line with relevant sponsor standard operating procedures, which are available at www.accord.ed.ac.uk/standardopprocs/CRSOPs.html (accessed January 2021). A delegation log at each site detailed the roles and responsibilities of each member of staff working on the trial.
Trial Steering Committee
A TSC was established to oversee the conduct and progress of the study. The terms of reference of the TSC, the draft template for reporting, and the names and contact details were detailed in the TSC charter.
Data Monitoring Committee
The DMC was composed of independent members, including a statistician, a cardiologist, a radiologist and an emergency or acute medicine physician. The Peto–Haybittle rule was used by the DMC as a guideline on the primary end point to trigger discussions on stopping the trial. Importantly, a decision to stop the trial did not rely on p-values alone and considered whether or not the results were convincing to the clinical community and patients.
Patient and public involvement
Patient and public involvement (PPI) representatives helped provide input to the RAPID-CTCA trial in the following ways.
Pre-funding preparation
Professor Steve Goodacre (co-applicant) met with members of the Sheffield Emergency Care Forum to consult them on the development of the study design for the grant application. The forum is a patient and public representative group that provides independent advice on emergency care-related research. They reviewed the proposal and provided advice on study design, patient procedures and ethics issues, which helped inform the final submission and subsequent study design.
Post-funding preparation
The Sheffield Emergency Care Forum was consulted again during the development of the trial information (patient information letters, consents, general practitioner letter) and the documents were amended to incorporate their feedback, which helped improve the usability of the documents.
During the trial
Patient and public involvement representatives were invited to participate in the TSC and were involved in the oversight of the trial throughout its duration. They provided valuable feedback about the patient perspective throughout the trial, which helped guide the decision-making of the trial team.
Report writing, academic paper preparation and dissemination
Patient and public involvement representatives helped to develop the Plain English summary for the final report and for dissemination of the results, which helped us ensure that this was clear and easy to interpret. The research findings will be presented at one of the Sheffield Emergency Care Forum’s regular meetings and members of the forum will help to develop material to allow us to disseminate the trial findings to the public.
Outcomes and conclusions
The inclusion of PPI representatives at each stage of the trial was beneficial because it provided continuous input throughout the project and advice when we needed it. It was helpful to have several PPI members involved because it provided a varied perspective and each member brought different strengths to the project. We received very positive feedback from the PPI members in the TSC. We received positive feedback about our level of engagement with the members and about how we had created an inclusive environment. An area for improvement is to ensure that lay language is consistently used during discussions in meetings to ensure that PPI representatives can follow complex discussions and can engage fully whenever possible. There were no negative impacts from PPI involvement in this case.
Summary of changes to the protocol
There were seven versions of the trial protocol. There were a number of changes over the duration of the trial, including changes or clarification of screening processes, duration of recruitment window, primary and secondary end points, number of recruitment sites, sample size calculation, radiation dosing reporting and assessment (see Appendix 6).
Chapter 3 Clinical effectiveness results
Patient recruitment and sites
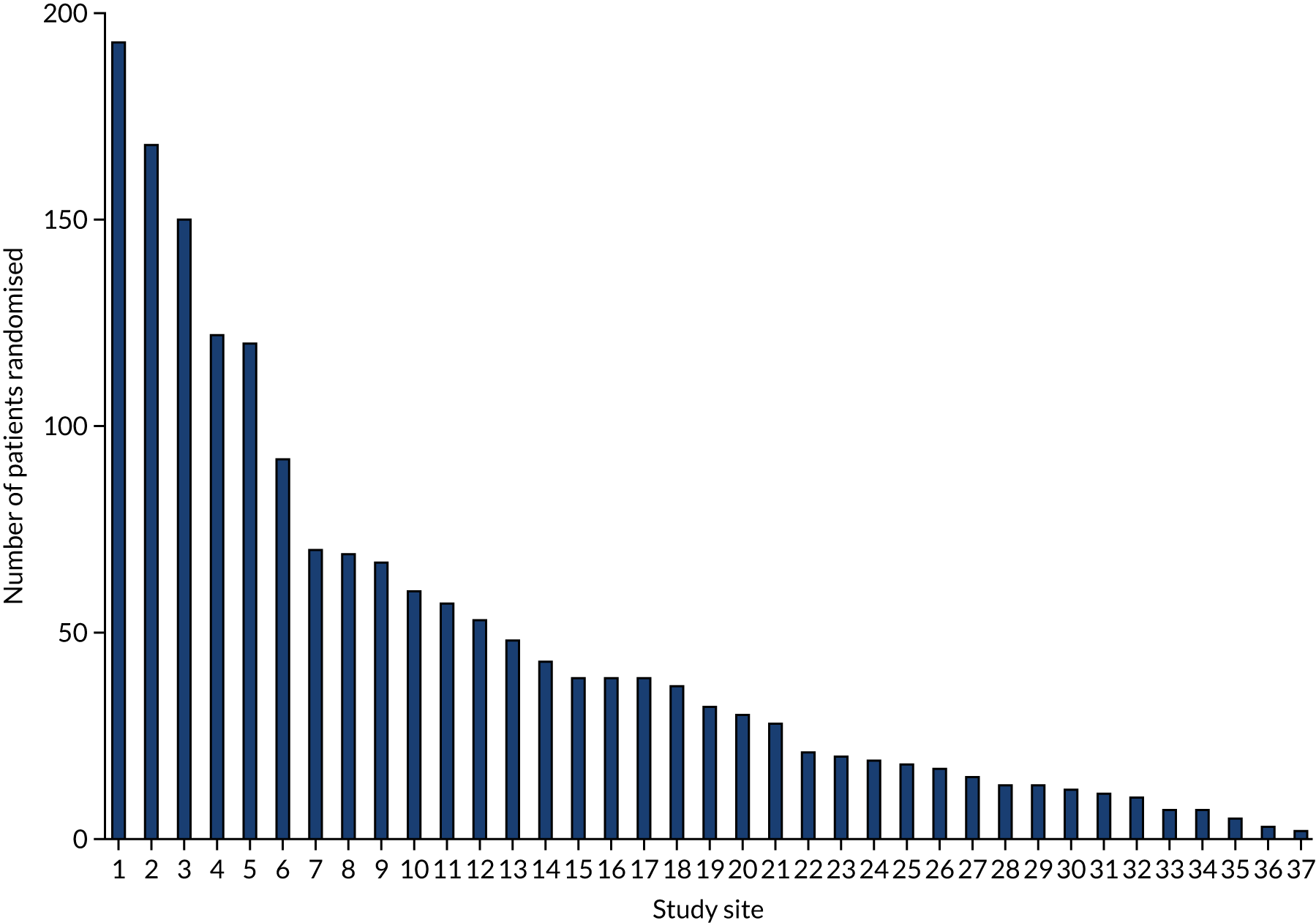
Thirty-seven hospitals participated in and recruited to the RAPID-CTCA trial. There were 27 sites in England, seven in Scotland, one in Northern Ireland, one in Wales and one in Jersey (Figure 1; see Appendix 7, Table 2). Twenty-five centres (68%) had on-site invasive angiography facilities. The recruitment target was initially 2500 participants and was revised to 1735 participants in 2018. The first patient was randomised on 23 March 2015 and the last patient was randomised on 27 June 2019 (Figure 2). Figure 3 details the relationship between the number of open sites, the number of sites actively recruiting in a given month and the number of participants recruited. The median monthly recruitment over the 50 months with a complete calendar month of recruitment was 34.5 participants (range 10 to 67 participants). Figure 4 and Appendix 7, Table 33, detail the variation in recruitment across sites, with the top six recruiting sites recruiting 844 (48%) participants. In total, 463 (26.5%) participants were recruited at sites with no on-site invasive angiography facilities.
FIGURE 1.
Distribution of recruitment sites across the UK.
FIGURE 2.
Overall trial recruitment over time.
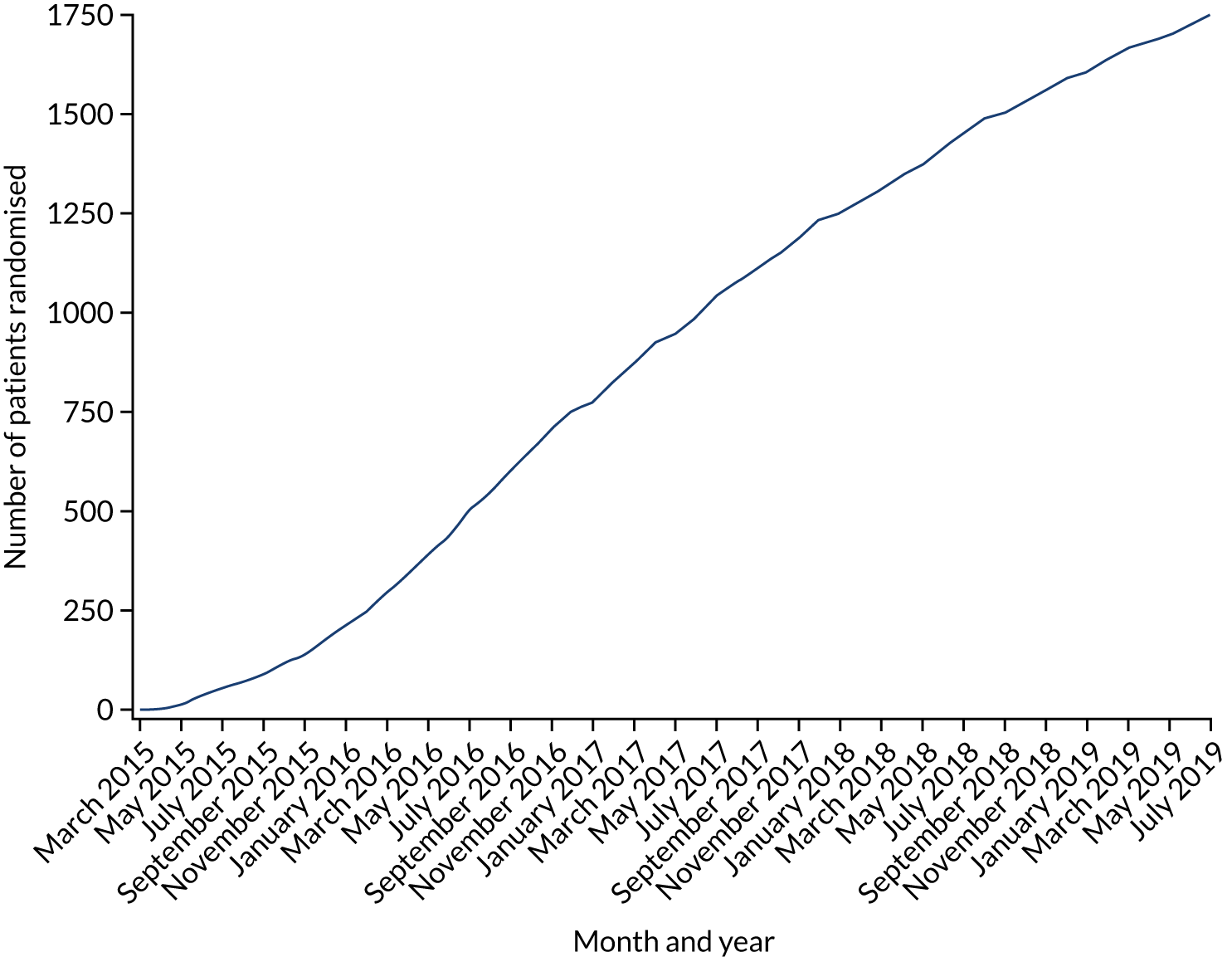
FIGURE 3.
Recruitment over time and relationship with open and active sites.
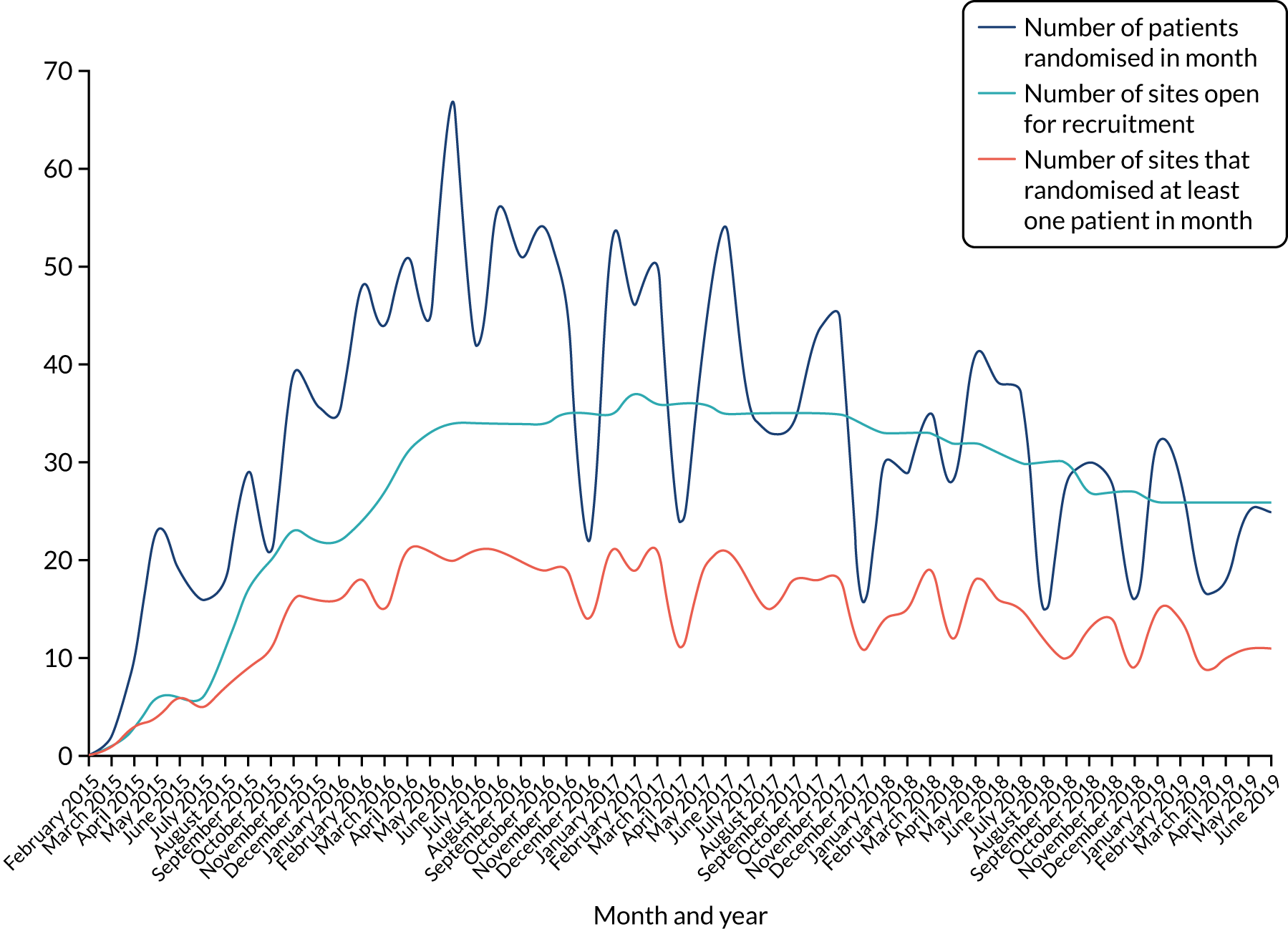
FIGURE 4.
Recruitment by study site.
Participant baseline characteristics
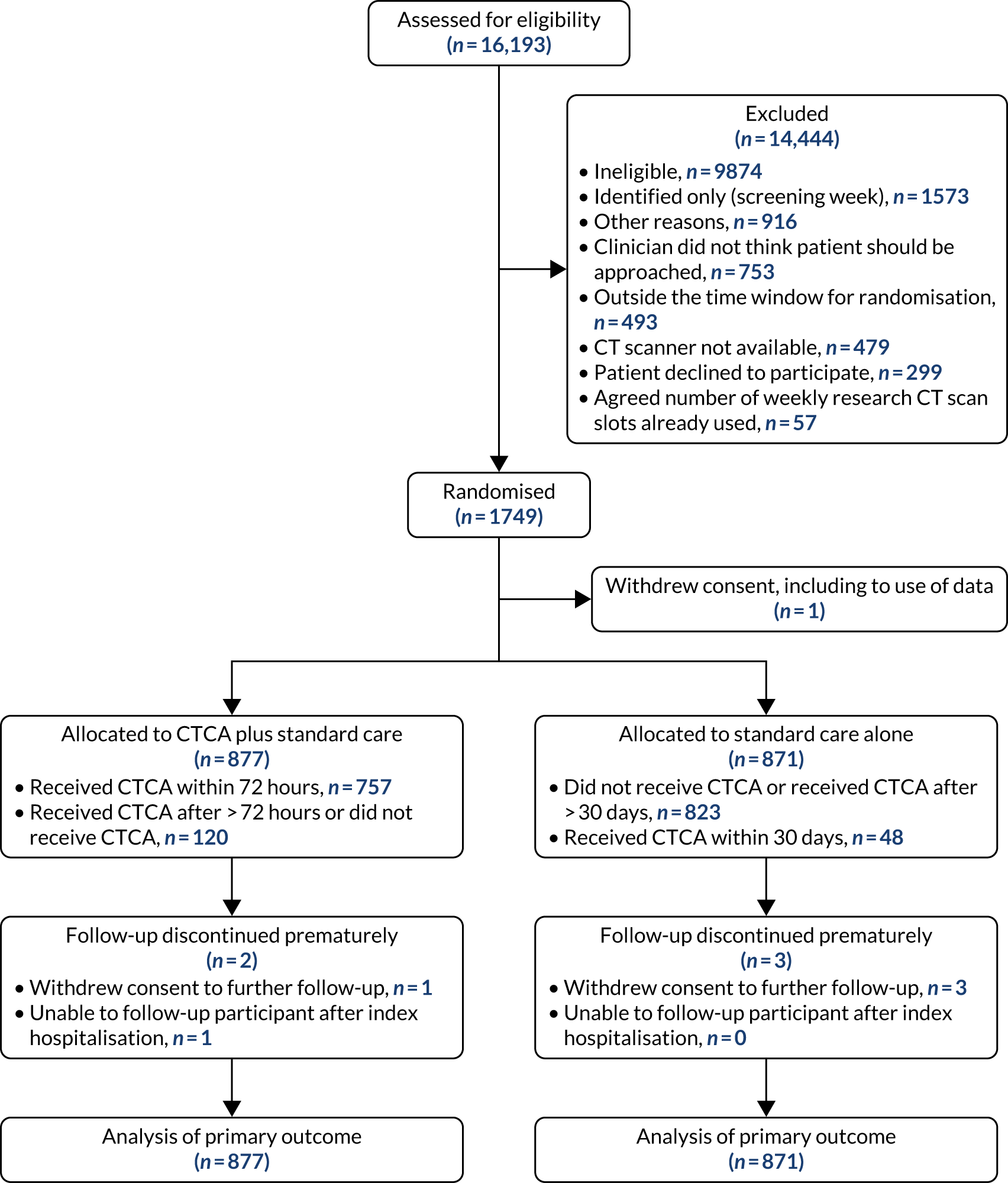
Between 23 March 2015 and 27 June 2019, 16,193 patients were screened for eligibility. In total, 1749 participants (CTCA arm, n = 877; standard care alone arm, n = 871) were recruited, with one participant withdrawal from the study, including withdrawal of consent for the use of data already collected. There was one withdrawal and one loss to follow-up in the CTCA arm and three withdrawals in the standard-care arm. Data for 1748 participants were available for the primary analysis. Figure 5 details participant flow through the trial. Baseline characteristics, enrolment, randomisation and follow-up were well matched between trial arms (Table 1; see Figure 5). Patient risk factors and comorbidities are detailed in Table 1 and routine prescriptions are reported in Table 2.
FIGURE 5.
Participant flow through the trial. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
| Characteristic | Trial arm | Overall (N = 1748) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Age (years), mean (SD) | 61.9 (12.2) | 61.2 (13.0) | 61.6 (12.6) |
| Sex: male, n (%) | 564 (64) | 550 (63) | 1114 (64) |
| Prior CHD, n (%) | 302 (34) | 299 (34) | 601 (34) |
| Elevated cardiac troponin level, n (%) | 492 (56) | 512 (59) | 1004 (57) |
| Abnormal ECG, n (%) | 549 (63) | 515 (59) | 1064 (61) |
| GRACE score, mean (SD) | 115 (36) | 114 (34) | 115 (35) |
| < 109, n (%) | 390 (44) | 384 (44) | 774 (44) |
| 109–140, n (%) | 268 (31) | 296 (34) | 564 (32) |
| > 140, n (%) | 219 (25) | 191 (22) | 410 (23) |
| Recruited at hospital with on-site invasive coronary angiography facilities, n (%) | 644 (73) | 641 (74) | 1285 (74) |
| Presenting complaint,a n (%) | |||
| Chest pain | 776 (89) | 773 (89) | 1549 (89) |
| Shortness of breath | 35 (4) | 31 (4) | 66 (4) |
| Palpitation | 17 (2) | 15 (2) | 32 (2) |
| Collapse | 10 (1) | 10 (1) | 20 (1) |
| Other | 38 (4) | 42 (5) | 80 (5) |
| Cardiovascular risk factors, n (%) | |||
| Diabetes mellitus | 153 (17) | 165 (19) | 318 (18) |
| Hypertension | 413 (47) | 404 (46) | 817 (47) |
| Hyperlipidaemia | 358 (41) | 336 (39) | 694 (40) |
| Current or ex-smoker | 530 (60) | 531 (61) | 1061 (61) |
| Family historyb | 269 (31) | 270 (31) | 539 (31) |
| Past medical history, n (%) | |||
| MIc | 180 (21) | 171 (20) | 351 (20) |
| Prior coronary angiography | 222 (25) | 214 (25) | 436 (25) |
| Prior percutaneous coronary interventiond | 115 (13) | 123 (14) | 238 (14) |
| Prior coronary artery bypass graft surgerye | 52 (6) | 48 (6) | 100 (6) |
| Cerebrovascular disease | 35 (4) | 38 (4) | 73 (4) |
| Peripheral vascular disease | 27 (3) | 28 (3) | 55 (3) |
| Medication prescribed before admission | Trial arm, n (%) | Overall (N = 1748), n (%) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Beta-blocker | 188 (21.4) | 180 (20.7) | 368 (21.1) |
| Calcium channel blocker | 109 (12.4) | 114 (13.1) | 223 (12.8) |
| ACE inhibitor or ARB | 217 (24.7) | 216 (24.8) | 433 (24.8) |
| Nicorandil | 20 (2.3) | 15 (1.7) | 35 (2.0) |
| Oral nitrate | 48 (5.5) | 47 (5.4) | 95 (5.4) |
| Ivabradine | 3 (0.3) | 4 (0.5) | 7 (0.4) |
| Ranolazine | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Buccal or sublingual nitrate | 100 (11.4) | 83 (9.5) | 183 (10.5) |
| Oral hypoglycaemic | 68 (7.8) | 51 (5.9) | 119 (6.8) |
| Insulin | 29 (3.3) | 18 (2.1) | 47 (2.7) |
| Statin | 283 (32.3) | 298 (34.2) | 581 (33.2) |
| Aspirin | 203 (23.1) | 212 (24.3) | 415 (23.7) |
| Clopidogrel | 48 (5.5) | 36 (4.1) | 84 (4.8) |
| Prasugrel | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Ticagrelor | 4 (0.5) | 1 (0.1) | 5 (0.3) |
| Warfarin | 17 (1.9) | 13 (1.5) | 30 (1.7) |
| Novel anticoagulant | 15 (1.7) | 14 (1.6) | 29 (1.7) |
| Diuretic | 73 (8.3) | 83 (9.5) | 156 (8.9) |
| Proton pump inhibitor | 214 (24.4) | 167 (19.2) | 381 (21.8) |
The mean age of participants was 62 years [standard deviation (SD) 13 years] and 1114 (63.7%) were male. At recruitment, 601 (34.4%) participants had prior CHD, 1004 (57.4%) had an elevated cardiac troponin level and 1064 (60.9%) had an ischaemic ECG (see Table 1).
Chest pain was the primary complaint in 1549 (88.7%) participants, with 857 (49.0%) having an acute coronary syndrome diagnosis (MI or unstable angina) at discharge from their index hospitalisation. The mean GRACE score was 115 (SD 35), with 410 (23.5%) participants having a GRACE score of > 140.
Participant primary symptoms, assessment and management pathways
Overall, 1549 (89%) participants reported chest pain as the primary symptom (see Table 1). The characteristics and details of the chest pain can be found in Appendix 7, Table 34. The time from presenting symptom onset to randomisation is reported in Table 3. Patients had a variety of referral pathways before attending hospital. The majority of participants (n = 957; 54.7%) telephoned emergency ambulance services. However, 521 (29.8%) patients self-presented to hospital and 188 (10.8%) were referred by a general practitioner. Most participants (n = 1557; 89.1%) were initially assessed in the ED (see Appendix 7, Table 35).
| Characteristic | Trial arm | Overall (N = 1748) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Time from onset of presenting symptom to randomisation (hours) | |||
| Median (Q1, Q3); n | 19 (10, 34); 836 | 19 (11, 31); 833 | 19 (10, 33); 1669 |
| Missing (n) | 41 | 38 | 79 |
The majority of participants (n = 1611; 92%) were in sinus rhythm at the time of their initial hospital 12-lead ECG. The characteristics of the participants’ ECG findings and troponin level results during index hospitalisation are detailed in Appendix 7, Table 36.
After initial assessment, the attending clinical team were asked how likely they thought acute coronary syndrome was as the presenting clinical diagnosis. In 642 (36.7%) patients, the clinician was highly suspicious of an acute coronary syndrome. The median level of certainty was 7 on a scale from 0 to 10, where 0 is least certain and 10 is most certain (Q1 6 to Q3 8; mean 7.1, SD 1.8) (see Appendix 7, Table 37). After initial assessment, most participants (n = 1372; 78%) were admitted to hospital. The details of the participants’ admission specialty are given in Appendix 7, Table 38.
Trial intervention: adherence and crossover
Adherence to trial allocation: computerised tomography coronary angiography plus standard care arm
The majority of participants randomised to receive CTCA (n = 767, 87.5%) underwent CTCA, with 757 (86.3%) participants receiving the CTCA within the protocol-defined 72-hour window. The other 10 (1.1%) participants received the intervention in the first 10 days. Of the 110 patients who did not receive CTCA as the allocated trial intervention, five subsequently had a CTCA scan outside the trial protocol. Six of the 767 patients who underwent CTCA as the trial intervention had a second CTCA scan during the first year of the trial. The reasons for not receiving a CTCA are detailed in Table 4.
| Reason | Total (n) |
|---|---|
| CT scanner not available | 26 |
| Clinician decision not to proceed with scan | 16 |
| Patient deterioration | 13 |
| High CAC score | 13 |
| Heart rate issue | 13 |
| Patient non-compliant in scan | 6 |
| Radiologist not available | 6 |
| Cannula issue | 5 |
| Patient declined scan | 5 |
| Exclusion criterion identified after randomisationa | 4 |
| Death | 1 |
| Other reasonb | 2 |
| Total | 110 |
Adherence to trial allocation: standard-care arm
In the standard-care arm, 48 (5.5%) participants received a CTCA within 30 days of randomisation and, therefore, met the predetermined criteria for cross over. Thirty-three of these patients received CTCA within 3 days of randomisation, eight between 4 and 10 days after randomisation, three between 11 and 20 days after randomisation, and four between 21 and 30 days after randomisation. A further 25 (2.9%) patients in the standard-care arm received a CTCA, but more than 30 days after randomisation.
Computerised tomography coronary angiography delivery and quality
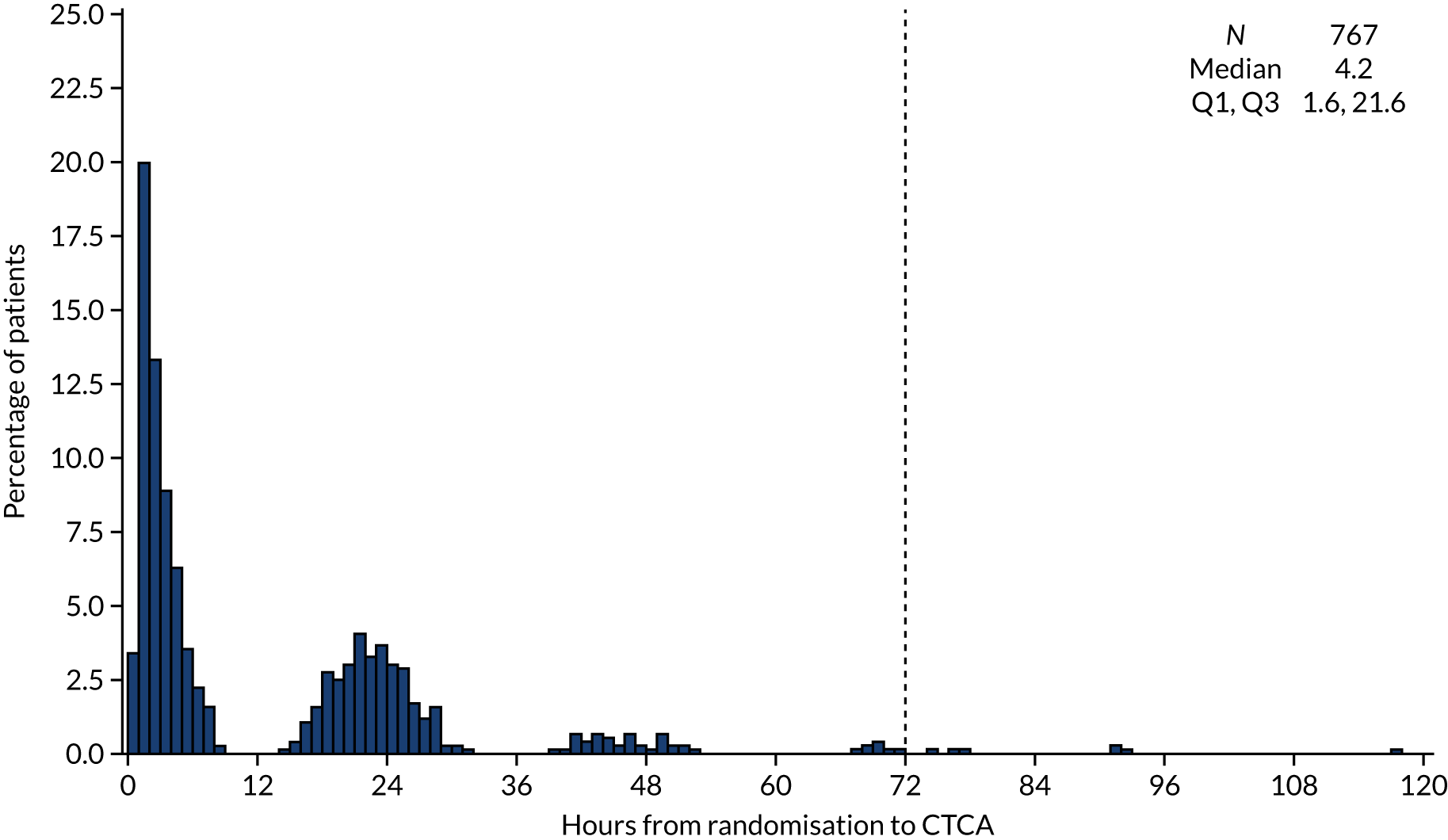
The median time from randomisation to CTCA was 4.2 [interquartile range (IQR) 1.6–21.6] hours, with CTCA being delivered on the day of randomisation in the majority of cases (Figure 6). Table 5 details the CTCA delivery and quality. CT scanners varied across the sites, from 64-slice to 320-slice CT scanners. The majority of scans were delivered during the index hospitalisation using a 64-slice (n = 358; 47.7%) or 128-slice (n = 256; 34.1%) scanner. Beta-blockade was used in 539 (70.3%) participants and sublingual glyceryl trinitrate was used in 521 (67.9%) participants as a pre treatment to optimise CT scan acquisition. The CTCA was of diagnostic quality for 700 (91.3%) participants. The first 10 CTCA scans from each site were reviewed centrally for QA purposes. The details of this process are summarised in Appendix 3.
FIGURE 6.
Time from randomisation to receiving CTCA as trial intervention for patients allocated to the CTCA arm. There were three patients allocated to the CTCA arm for whom the time from randomisation to receiving CTCA as the trial intervention was > 120 hours, and their times to undergoing CTCA of 140, 163 and 218 hours are not shown in the histogram. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
| CTCA delivery and quality | Received CTCA in the CTCA arm (N = 767), n (%) |
|---|---|
| Type of patient | |
| Inpatient | 681 (88.8) |
| Ambulatory | 86 (11.2) |
| Beta-blocker administered | |
| Yes | 539 (70.3) |
| No | 228 (29.7) |
| GTN administered | |
| Yes | 521 (67.9) |
| No | 246 (32.1) |
| Scanner detector rows/slices | |
| 64 | 358 (47.7) |
| 80 | 7 (0.9) |
| 96 | 4 (0.5) |
| 128 | 256 (34.1) |
| 256 | 37 (4.9) |
| 320 | 88 (11.7) |
| Missing | 17 |
| Scan technique | |
| Prospective (step and shoot) | 607 (79.2) |
| Retrospective | 111 (14.5) |
| Flash | 48 (6.3) |
| Missing | 1 |
| Scan quality | |
| Good (diagnostic) | 500 (65.4) |
| Moderate (diagnostic but suboptimal) | 200 (26.2) |
| Poor (limited diagnostic) | 54 (7.1) |
| Non-diagnostic (uninterpretable) | 8 (1.0) |
| Indeterminate | 2 (0.3) |
| Missing | 3 |
Computerised tomography coronary angiography findings
Computerised tomography interpretation was available for 759 out of the 767 participants who underwent CTCA. The intervention identified normal coronary arteries in 178 (23%), non-obstructive disease in 222 (29%) and obstructive disease in 359 (47%) participants. The severity of coronary artery disease was associated with increasing age, male sex, prior CHD, troponin level elevation and GRACE score, as well as the use of invasive coronary angiography and subsequent revascularisation (Table 6). Other cardiac and non-cardiac diagnoses identified on CTCA are detailed in the safety outcomes.
| Characteristic | Normal coronary arteries | Non-obstructive coronary artery disease | Obstructive coronary artery disease |
|---|---|---|---|
| Number of participants (n) | 178 | 222 | 359 |
| Age (years), mean (SD) | 54.9 (12.4) | 63.3 (11.4) | 64.0 (11.6) |
| Sex: male, n (%) | 67 (38) | 132 (59) | 279 (78) |
| Prior CHD, n (%) | 27 (15) | 85 (38) | 144 (40) |
| Elevated cardiac troponin level, n (%) | 69 (39) | 104 (47) | 249 (69) |
| Abnormal 12-lead ECG, n (%) | 114 (64) | 129 (58) | 231 (64) |
| GRACE score, n (%) | |||
| < 109 | 125 (70) | 107 (48) | 108 (30) |
| 109–140 | 34 (19) | 64 (29) | 141 (39) |
| > 140 | 19 (11) | 51 (23) | 110 (31) |
| On-site coronary angiography, n (%) | 139 (78) | 163 (73) | 254 (71) |
| Invasive coronary angiogram carried out, n (%) | 25 (14) | 83 (37) | 289 (81) |
| Acute coronary syndrome therapy, n (%) | 105 (59) | 145 (65) | 271 (75) |
| Coronary revascularisation, n (%) | 7 (4) | 26 (12) | 222 (62) |
| Preventative therapies,a n (%) | 65 (37) | 143 (64) | 274 (76) |
Primary end point
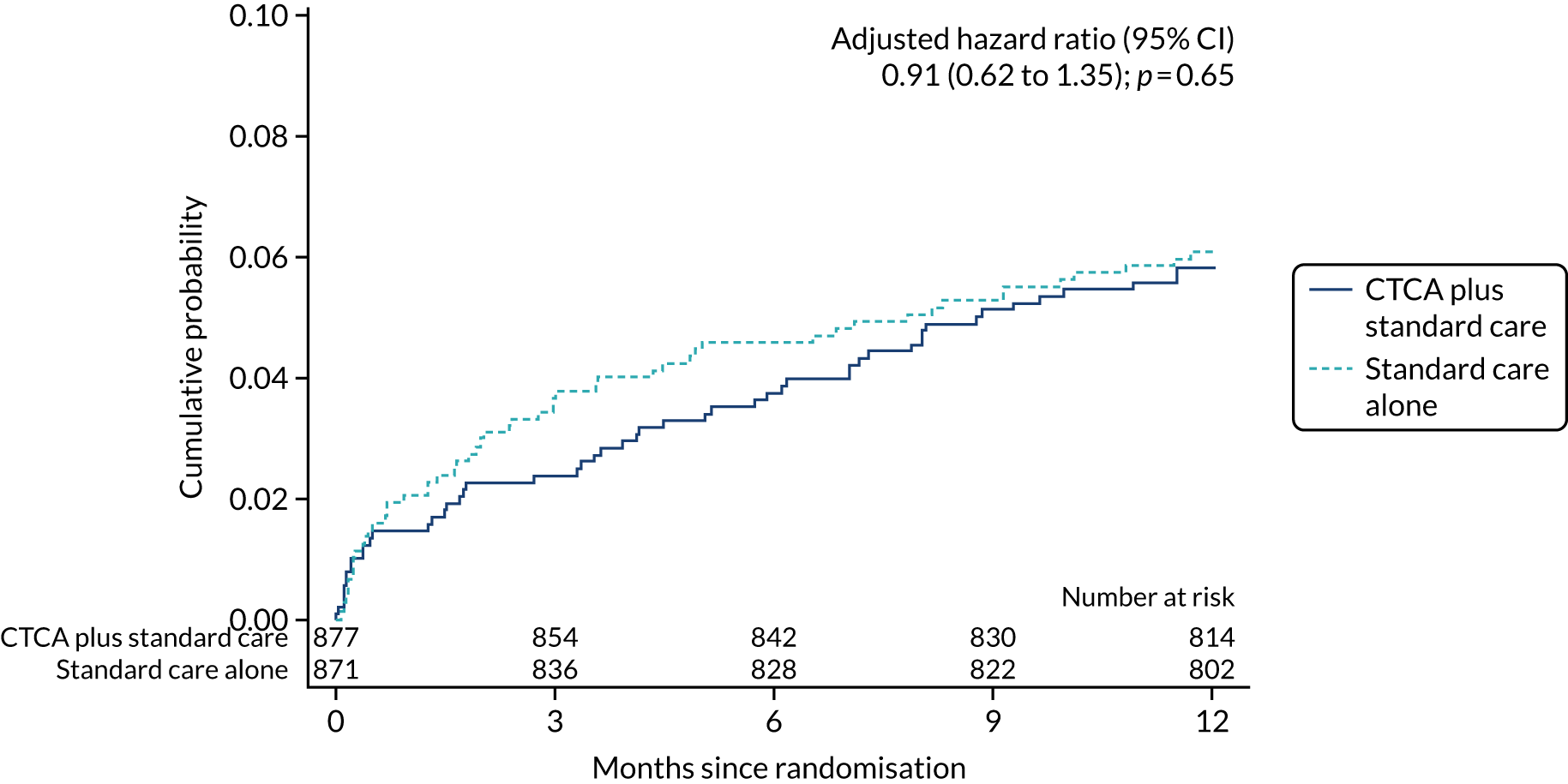
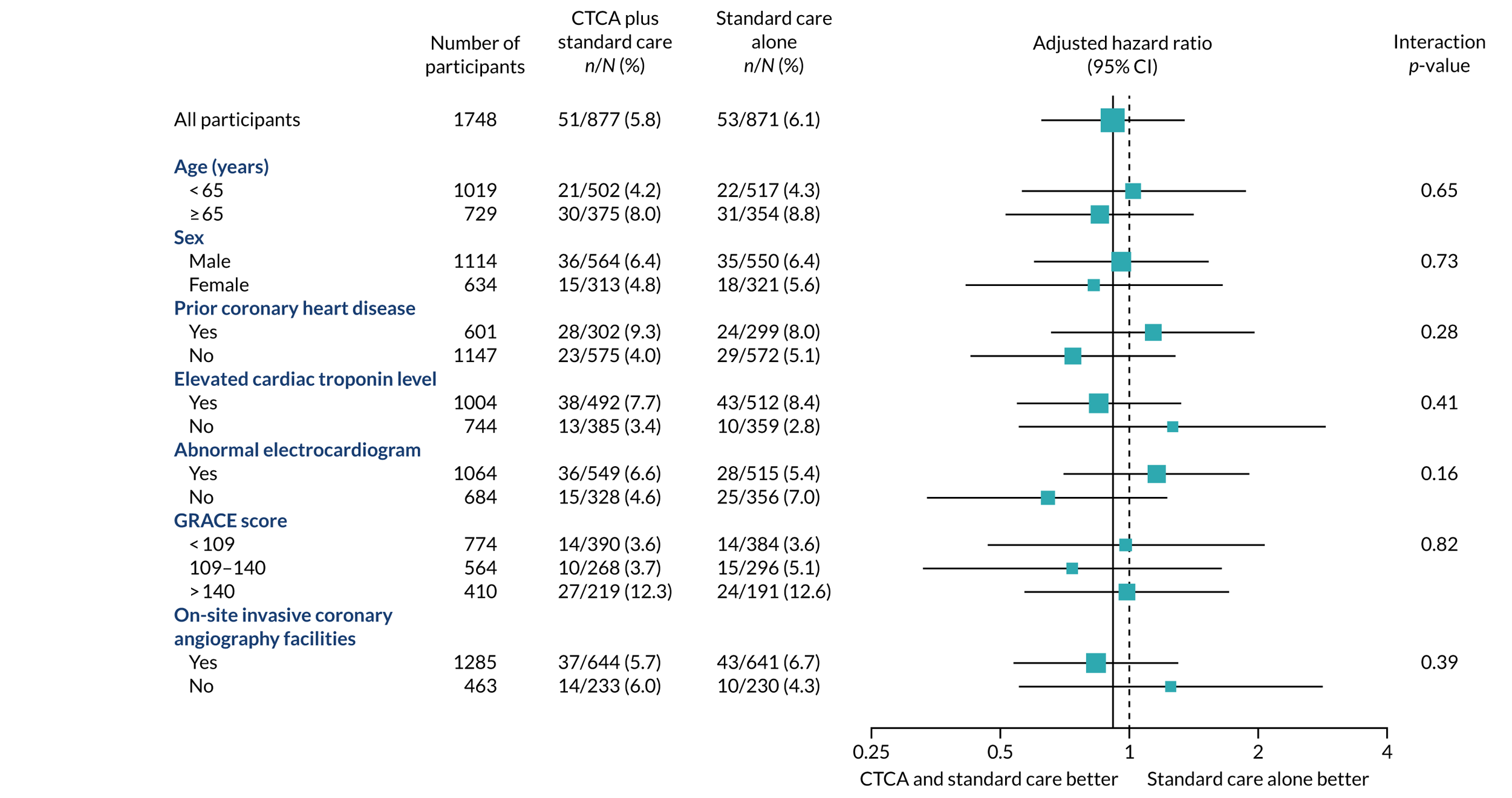
The primary end point of all-cause death or non-fatal MI (type 1 or 4b) within 12 months occurred in 51 (5.8%) out of the 877 participants in the early CTCA arm and 53 (6.1%) out of the 871 participants in the standard-care arm (adjusted HR 0.91, 95% CI 0.62 to 1.35; p = 0.65) (Figure 7 and Table 7). For the prespecified subgroup analyses for the primary outcome (age, sex, prior coronary artery disease, baseline troponin level elevation, presentation of 12-lead ECG, GRACE score and on-site invasive angiography facilities), there was no statistically significant heterogeneity for any comparison (Figure 8). Appendix 7, Table 39, details the primary outcome across the five highest recruiting centres.
FIGURE 7.
Cumulative probability of the primary end point of 1-year all-cause death or non-fatal MI (type 1 or 4b). Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
| Outcome | Trial arm, n (%) | Estimate, HR (95% CI); p-valuea | ||
|---|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |||
| Unadjusted | Adjustedb | |||
| Primary outcome | ||||
| All-cause death or non-fatal MI (type 1 or 4b) | 51 (5.8) | 53 (6.1) | 0.95 (0.65 to 1.40); 0.79 | 0.91 (0.62 to 1.35); 0.65 |
| Secondary outcomes | ||||
| Coronary heart disease death or non-fatal MI | 47 (5.4) | 45 (5.2) | 1.03 (0.69 to 1.55); 0.88 | 1.02 (0.67 to 1.53); 0.94 |
| Cardiovascular disease death or non-fatal MI | 48 (5.5) | 46 (5.3) | 1.03 (0.69 to 1.54); 0.88 | 1.01 (0.68 to 1.52); 0.95 |
| Non-fatal MI | 39 (4.4) | 40 (4.6) | 0.96 (0.62 to 1.50); 0.87 | 0.95 (0.61 to 1.47); 0.81 |
| Coronary heart disease death | 11 (1.3) | 6 (0.7) | 1.82 (0.67 to 4.92); 0.24 | 1.78 (0.66 to 4.82); 0.26 |
| Cardiovascular death | 12 (1.4) | 8 (0.9) | 1.49 (0.61 to 3.64); 0.38 | 1.39 (0.57 to 3.42); 0.47 |
| All-cause death | 19 (2.2) | 17 (2.0) | 1.11 (0.58 to 2.13); 0.76 | 1.03 (0.53 to 1.99); 0.94 |
FIGURE 8.
Prespecified subgroup analyses of 1-year all-cause death or non-fatal MI (type 1 or 4b). The p-value is from test of interaction between the allocated treatment arm and the subgroup variable. GRACE, global registry of acute coronary events. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Key secondary outcomes
Key secondary outcomes relating to causes of death (all-cause, CHD and cardiovascular death) and non-fatal MI were also similar between the trial arms (see Table 7). There was no evidence of a difference between allocated treatment arms for any of the comparisons. The detailed data for the cumulative probability up to 1 year of an event for each of the key secondary outcomes are found in Appendix 8, Figure 18.
Other secondary outcomes
Other clinical outcomes
Other clinical outcomes reported were CHD death or non-fatal MI (type 1 or 4b), non-fatal MI (type 1 or 4b) and non-cardiovascular death. Once again, there was no evidence of a difference in outcome between trial arms (Table 8) (see Appendix 8, Figure 24).
| Outcome within 12 months | Trial arm, n (%) | Estimate, HR (95% CI); p-value | ||
|---|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | Unadjusted | Adjusteda | |
| Coronary heart disease death or non-fatal MI (type 1 or 4b) | 43 (4.9) | 43 (4.9) | 0.99 (0.65 to 1.51); 0.95 | 0.97 (0.63 to 1.48); 0.88 |
| Non-fatal MI (type 1 or 4b) | 35 (4.0) | 38 (4.4) | 0.91 (0.57 to 1.44); 0.68 | 0.89 (0.56 to 1.41); 0.62 |
| Non-cardiovascular death | 7 (0.8) | 9 (1.0) | 0.77 (0.29 to 2.07); 0.61 | 0.67 (0.24 to 1.85) 0.44 |
Treatment during index hospitalisation and secondary preventative treatment
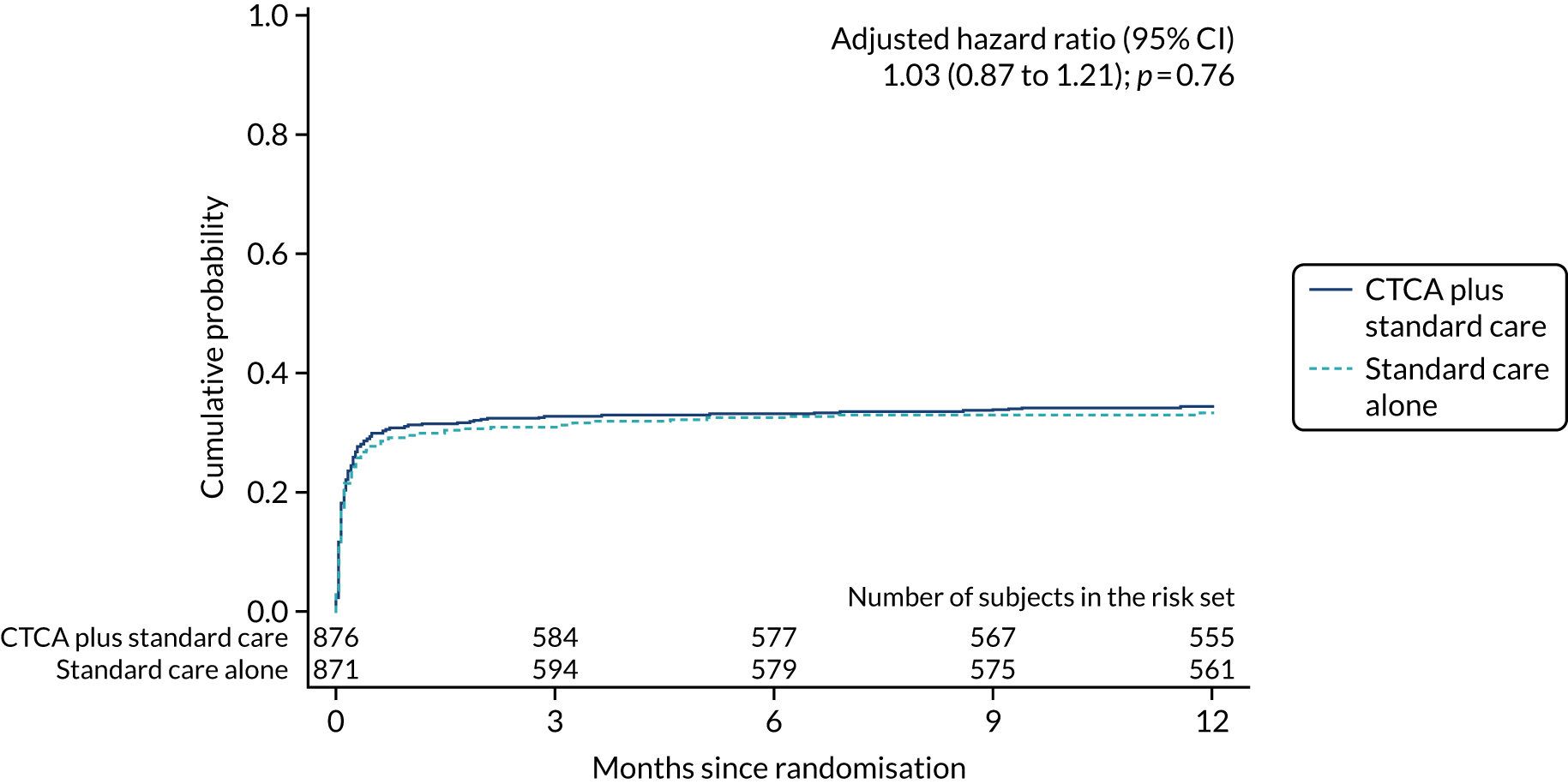
Fewer participants in the CTCA arm than in the standard-care arm received invasive coronary angiography: 474 (54.0%) compared with 530 (60.8%), respectively (adjusted HR 0.81, 95% CI 0.72 to 0.92; p = 0.001) (Table 9 and Figure 9). Despite there being relatively less invasive coronary angiography in the CTCA arm, there was no evidence of a difference in the rates of coronary revascularisation by trial allocation (adjusted HR 1.03, 95% CI 0.87 to 1.21; p = 0.76) (Figure 10). There was also no evidence of a difference in percutaneous intervention or coronary artery bypass surgery between arms (see Table 9) (see Appendix 8, Figures 27 and 28). The proportion of participants receiving revascularisation following invasive angiography was 63.3% in the CTCA arm (300/474 who received invasive angiography) compared with 54.3% in the standard-care arm (288/530 who received invasive angiography).
| Outcome within 12 months | Trial arm, n (%) | Estimate, HR (95% CI); p-value | ||
|---|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | Unadjusted | Adjusteda | |
| Invasive coronary angiography | 474 (54.0) | 530 (60.8) | 0.83 (0.74 to 0.94); 0.004 | 0.81 (0.72 to 0.92); 0.001 |
| Coronary revascularisation | 300 (34.2) | 288 (33.1) | 1.03 (0.88 to 1.22); 0.68 | 1.03 (0.87 to 1.21); 0.76 |
| Percutaneous coronary intervention | 260 (29.6) | 240 (27.6) | 1.08 (0.90 to 1.28); 0.42 | 1.08 (0.90 to 1.28); 0.42 |
| Coronary artery bypass graft surgery | 52 (5.9) | 55 (6.3) | 0.94 (0.64 to 1.37); 0.73 | 0.91 (0.62 to 1.33); 0.63 |
FIGURE 9.
Cumulative probability of invasive coronary angiography. The date of invasive coronary angiography was not known for one patient in the CTCA arm and for one patient in the standard care alone arm, and these participants are not included in the estimates of cumulative probability. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
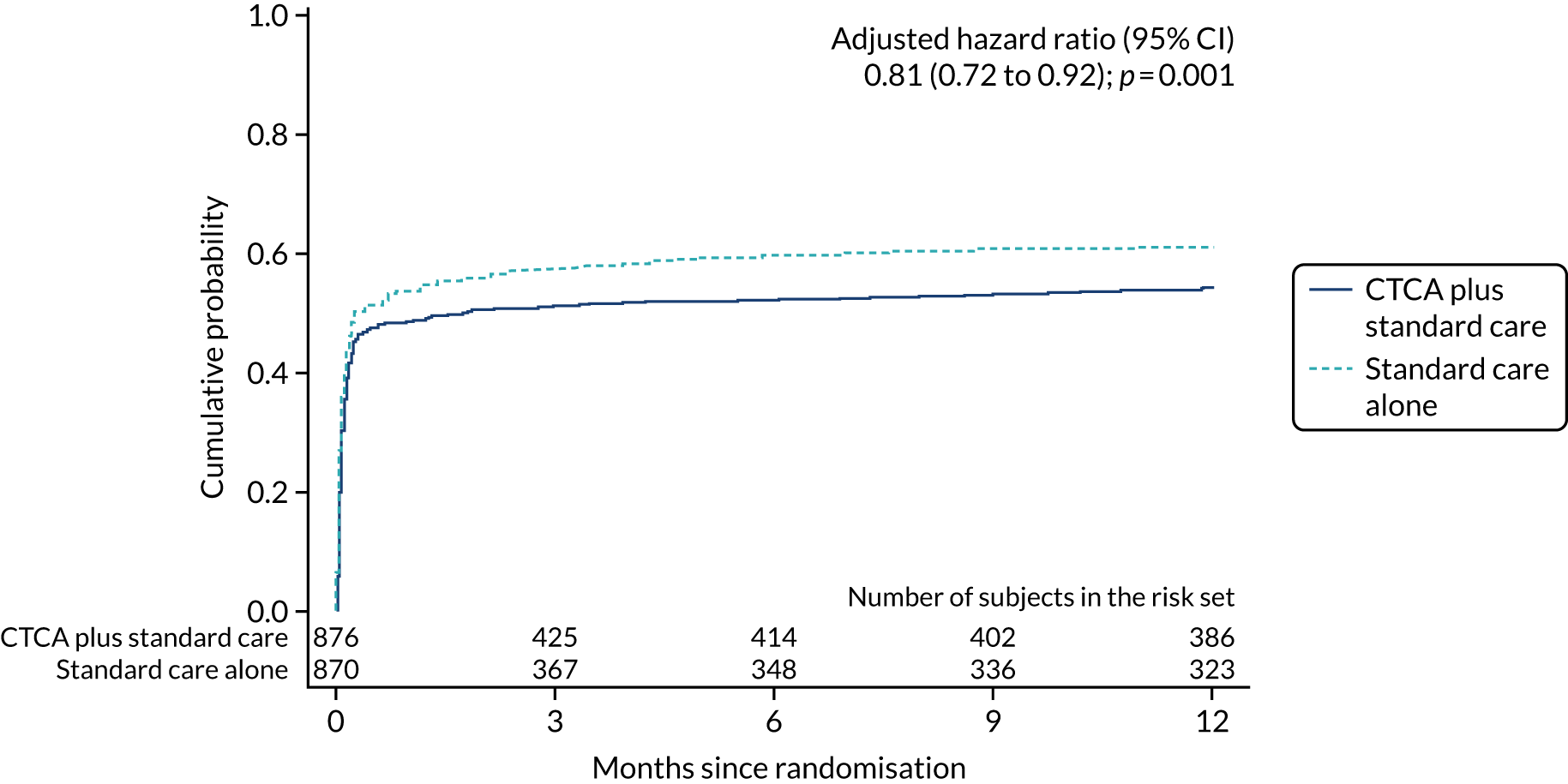
FIGURE 10.
Cumulative probability of coronary revascularisation. The date of coronary revascularisation was not known for one patient in the CTCA arm, and this participant is not included in estimates of cumulative probability. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Overall, there was no evidence of a difference in the in-hospital prescription of medications for acute coronary syndrome treatment (adjusted OR 1.06, 95% CI 0.85 to 1.32; p = 0.63). At hospital discharge, the change in prescription (increased or decreased dose, treatment started or stopped) of preventative therapies (adjusted OR 1.07, 95% CI 0.87 to 1.32; p = 0.52) was similar between trial arms (Table 10). Each component of prescription change (start, stop, increase or decrease dose) was also similar between trial arms (Table 11).
| Outcome | Trial arm, n (%) | Estimate, HR (95% CI); p-value | ||
|---|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | Unadjusted | Adjusteda | |
| Acute coronary syndrome therapy prescribed during index hospitalisation | 595 (67.8) | 580 (66.6) | 1.06 (0.87 to 1.29); 0.58 | 1.06 (0.85 to 1.32); 0.63 |
| Change in prevention treatment during index hospitalisation | 554 (63.2) | 539 (61.9) | 1.06 (0.87 to 1.28); 0.58 | 1.07 (0.87 to 1.32); 0.52 |
| Prevention treatment change during index hospitalisation | Trial arm, n (%) | |
|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |
| Started | 526 (60.0) | 509 (58.4) |
| Stopped | 71 (8.1) | 61 (7.0) |
| Dose altered | 91 (10.4) | 100 (11.5) |
Length of hospital stay
The median length of hospital stay was longer in the CTCA arm than in the standard-care arm: 2.2 (IQR 1.1–4.1) days compared with 2.0 (IQR 1.0–3.8) days, respectively (Hodges-Lehmann estimator of location shift 0.21, 95% CI 0.05 to 0.40 days; p = 0.009).
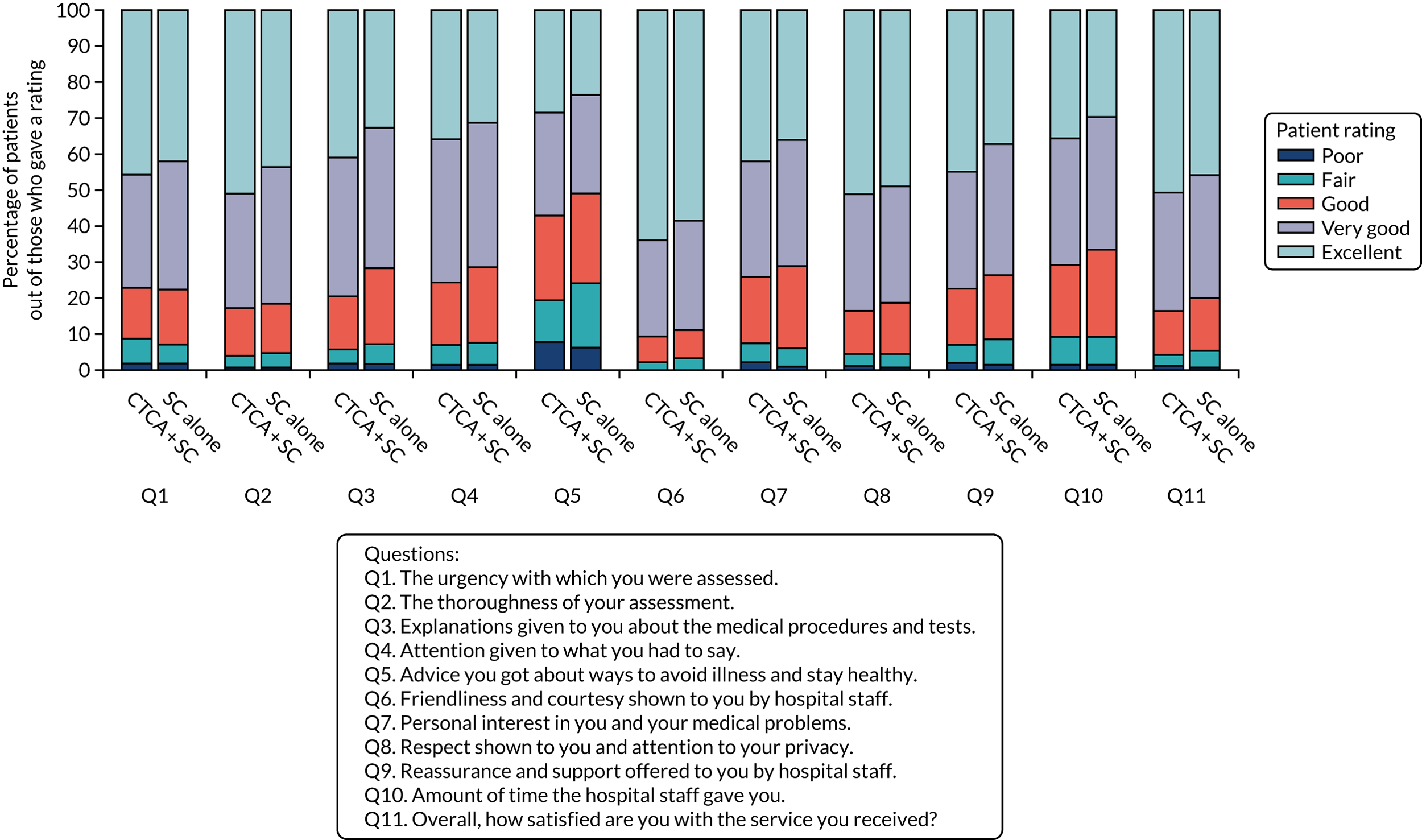
Patient satisfaction
Overall, 1322 (75.6%) participants responded to the trial patient satisfaction questionnaire at 1 month (see Appendix 7, Table 40). Participant satisfaction (rated excellent or very good on a five-point Likert scale) was higher in the CTCA arm than in the standard-care arm: 83.3% compared with 79.7%, respectively (Figure 11).
FIGURE 11.
Patient satisfaction with the care that they received when they attended hospital. Q, question; SC, standard care.
Clinician diagnostic certainty in the computerised tomography coronary angiography group
The attending clinician reported increased diagnostic certainty following CTCA. The mean increase was 1.4 (2.2) on a 10-point scale, from 7.1 (diagnostic certainty before CTCA scan) to 8.5 (diagnostic certainty after CTCA scan). The scale was from 0 to 10, with 10 being the most certain (Table 12).
| Clinician certainty | CTCA arm and received CTCA (N = 767) |
|---|---|
| Clinician certainty at time of randomisationa | |
| Mean (SD); n | 7.1 (1.8); 767 |
| Median (Q1, Q3) | 7 (6, 8) |
| Minimum, maximum | 0, 10 |
| Clinician certainty after CTCAa | |
| Mean (SD); n | 8.5 (1.6); 748 |
| Median (Q1, Q3) | 9 (8, 10) |
| Minimum, maximum | 0, 10 |
| Missing | 19 |
| Change in clinician certaintyb | |
| Mean (SD); n | 1.4 (2.2); 748 |
| Median (Q1, Q3) | 1 (0, 3) |
| Minimum, maximum | –10, 10 |
| Missing | 19 |
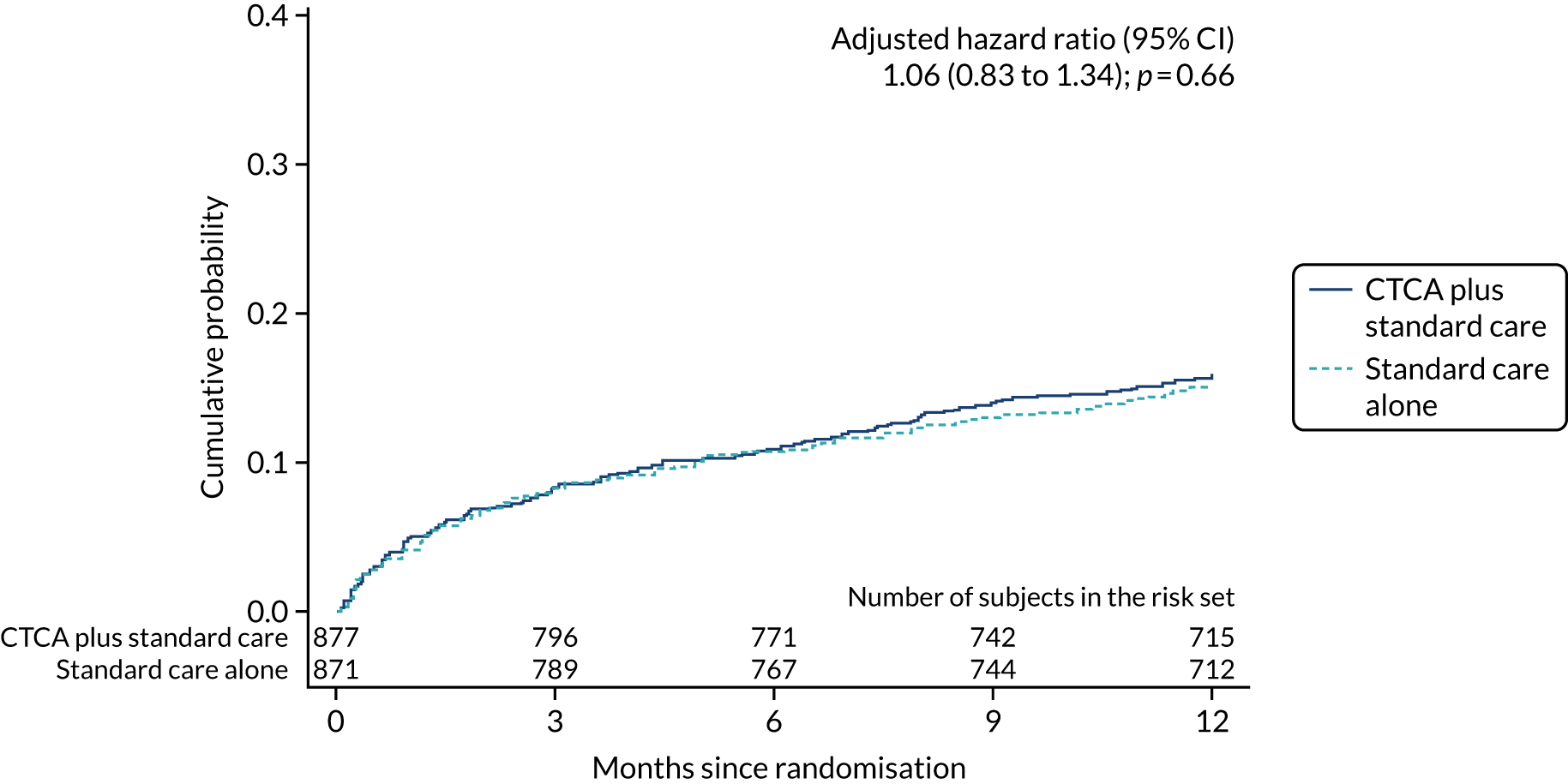
Symptoms and hospital presentations during follow-up
During 1 year of follow-up, 268 (15.3%) participants presented to hospital with suspected acute coronary syndrome. The rate of re-presentation was similar in both trial arms (adjusted HR 1.06, 95% CI 0.83 to 1.34; p = 0.66) (Figure 12). In addition, there was no evidence of a difference in chest pain symptoms between trial arms at 1, 6 and 12 months (Table 13).
FIGURE 12.
Cumulative probability of re-presentation or rehospitalisation with suspected acute coronary syndrome or recurrent chest pain. Reproduced with permission from Gray et al. 61 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
| Participant chest pain symptoms | Trial arm, n (%) | |
|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |
| Symptoms at 1 month | ||
| Sample size | 625 | 583 |
| No chest pain | 213 (34.1) | 205 (35.2) |
| Non-exertional chest pain | 180 (28.8) | 152 (26.1) |
| Chest pain on exertion | 232 (37.1) | 226 (38.8) |
| Symptoms at 6 months | ||
| Sample size | 607 | 558 |
| No chest pain | 278 (45.8) | 264 (47.3) |
| Non-exertional chest pain | 142 (23.4) | 119 (21.3) |
| Chest pain on exertion | 187 (30.8) | 175 (31.4) |
| Symptoms at 12 months | ||
| Sample size | 587 | 525 |
| No chest pain | 287 (48.9) | 248 (47.2) |
| Non-exertional chest pain | 121 (20.6) | 107 (20.4) |
| Chest pain on exertion | 179 (30.5) | 170 (32.4) |
Discharge diagnosis from index hospitalisation
At discharge from the index hospitalisation, 857 (49%) participants had a diagnosis of acute coronary syndrome [non-ST elevation myocardial infarction (NSTEMI), STEMI, unstable angina]. There was no difference in the discharge diagnosis of acute coronary syndrome (MI or unstable angina) between trial arms: 440 (50.2%) in the CTCA arm compared with 417 (47.9%) in the standard-care arm. Other discharge diagnoses are detailed in Table 14.
| Discharge diagnosis | Trial arm, n (%) | Overall (N = 1748), n (%) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| NSTEMI | 350 (39.9) | 339 (38.9) | 689 (39.4) |
| Chest pain: no clear diagnosis | 208 (23.7) | 218 (25.0) | 426 (24.4) |
| Unstable angina | 81 (9.2) | 70 (8.0) | 151 (8.6) |
| Stable angina | 64 (7.3) | 64 (7.3) | 128 (7.3) |
| Musculoskeletal pain | 36 (4.1) | 35 (4.0) | 71 (4.1) |
| Other gastrointestinal pain | 29 (3.3) | 21 (2.4) | 50 (2.9) |
| Pericarditis/myocarditis/myopericarditis | 20 (2.3) | 28 (3.2) | 48 (2.7) |
| Arrhythmia | 19 (2.2) | 20 (2.3) | 39 (2.2) |
| Cardiomyopathy | 4 (0.5) | 15 (1.7) | 19 (1.1) |
| STEMI | 9 (1.0) | 8 (0.9) | 17 (1.0) |
| Oesophageal pain | 9 (1.0) | 7 (0.8) | 16 (0.9) |
| Pneumonia/pleurisy | 9 (1.0) | 5 (0.6) | 14 (0.8) |
| Pulmonary embolism | 5 (0.6) | 6 (0.7) | 11 (0.6) |
| Heart failure | 5 (0.6) | 5 (0.6) | 10 (0.6) |
| Anxiety | 6 (0.7) | 3 (0.3) | 9 (0.5) |
| Syncope | 3 (0.3) | 5 (0.6) | 8 (0.5) |
| Coronary artery spasm | 3 (0.3) | 4 (0.5) | 7 (0.4) |
| Costochondritis | 2 (0.2) | 3 (0.3) | 5 (0.3) |
| Valvular heart disease | 1 (0.1) | 2 (0.2) | 3 (0.2) |
| Acute aortic syndrome | 2 (0.2) | 0 (0.0) | 2 (0.1) |
| Symptomatic anaemia | 0 (0.0) | 2 (0.2) | 2 (0.1) |
| Other | 12 (1.4) | 11 (1.3) | 23 (1.3) |
Investigations during index hospitalisation and up to 1 year
There was no evidence of a difference between trial arms and the completion of other cardiac investigations. The most common additional cardiac investigation was an echocardiogram, which was performed in 932 (53.3%) participants (Table 15) (see Appendix 8, Figure 29). The details of additional non-cardiac investigations are reported in Appendix 7, Table 41.
| Investigation | Trial arm, n (%) | |
|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |
| Echocardiogram | 462 (52.7) | 470 (54.0) |
| ECG rhythm monitoringa | 43 (4.9) | 61 (7.0) |
| Exercise ECG | 45 (5.1) | 64 (7.3) |
| Stress echocardiogram | 36 (4.1) | 42 (4.8) |
| Cardiac magnetic resonance imaging | 15 (1.7) | 15 (1.7) |
| Stress magnetic resonance imaging perfusion scanb | 64 (7.3) | 55 (6.3) |
| Radionuclide myocardial perfusion scan | 13 (1.5) | 17 (2.0) |
Patient safety
There were 32 adverse events reported in 29 (1.7%) participants. There were three non-serious adverse events definitely related to the CTCA: three problems associated with the intravenous cannula. There was one serious adverse event possibly related to the CTCA: an admission to hospital with a non-cardiac condition (Table 16).
| Adverse event | Trial arm, N; n (%) | |
|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |
| All adverse events | 22; 19 (2.2) | 10; 10 (1.1) |
| Serious adverse events | 19; 18 (2.1) | 10; 10 (1.1) |
| Possibly related | 1; 1 (0.1) | 0; 0 (0.0) |
| Re-admission with non-cardiac condition | 1; 1 (0.1) | 0; 0 (0.0) |
| Not related | 18; 17 (1.9) | 10; 10 (1.1) |
| Re-admission with non-cardiac condition | 6; 5 (0.6) | 2; 2 (0.2) |
| Re-admission with chest pain: not CHD related | 2; 2 (0.2) | 4; 4 (0.5) |
| Re-admission with chest pain: CHD related | 3; 3 (0.3) | 2; 2 (0.2) |
| Re-admission with chest pain: unclear | 1; 1 (0.1) | 2; 2 (0.2) |
| Not related: other | 5; 5 (0.6) | 0; 0 (0.0) |
| Patient deterioration prior to CTCA | 1; 1 (0.1) | 0; 0 (0.0) |
| Non-serious adverse events | 3; 3 (0.3) | 0; 0 (0.0) |
| Definitely related | 3; 3 (0.3) | 0; 0 (0.0) |
| IV cannula issue | 3; 3 (0.3) | 0; 0 (0.0) |
Alternative diagnoses on computerised tomography coronary angiography
Alternative cardiovascular and non-cardiovascular diagnoses identified on CTCA are delineated in Tables 17 and 18. Other cardiac findings were found in 225 (29.3%) participants, but very few were directly related to the participant’s presenting complaint. An alternative non-cardiac finding was found in 237 (30.9%) participants. Again, very few had alternative findings directly related to the participant presentation, such as pulmonary emboli (n = 5) or thoracic aortic dissection (n = 1).
| Other cardiac finding | CTCA arm and received CTCA (N = 767), n (%) |
|---|---|
| Other cardiac finding(s) | 225 (29.3) |
| Aortic valve calcification | 68 (8.9) |
| Cardiomegaly | 46 (6.0) |
| Left ventricular wall thinning | 45 (5.9) |
| Left ventricular hypertrophy | 39 (5.1) |
| Mitral valve calcification | 24 (3.1) |
| Patent foramen ovale | 16 (2.1) |
| Hypertrophic obstructive cardiomyopathy | 11 (1.4) |
| Previous MI | 10 (1.3) |
| Pericardial disease | 9 (1.2) |
| Anomalous vessel | 8 (1.0) |
| Aortic dilatation | 7 (0.9) |
| Device | 5 (0.7) |
| Atrial septal defect | 2 (0.3) |
| Ventricular septal defect | 1 (0.1) |
| Cardiomyopathy | 1 (0.1) |
| Myocardial hypoperfusion or non-enhancement | 1 (0.1) |
| Significant other finding | 2 (0.3) |
| Non-cardiac findings | CTCA arm and received CTCA (N = 767), n (%) |
|---|---|
| Non-cardiac finding(s) | 237 (30.9) |
| Parenchymal lung disease | 83 (10.8) |
| Hiatus hernia | 53 (6.9) |
| Pleural disease | 43 (5.6) |
| Pulmonary mass or nodule | 33 (4.3) |
| Bone pathology | 17 (2.2) |
| Liver pathology | 16 (2.1) |
| Significant lymphadenopathy | 11 (1.4) |
| Pulmonary hypertension | 8 (1.0) |
| Pulmonary emboli | 5 (0.7) |
| Pneumonia | 4 (0.5) |
| Pulmonary oedema | 4 (0.5) |
| Mediastinal masses | 2 (0.3) |
| Thoracic/aortic dissection | 1 (0.1) |
| Abdominal aortic aneurysm | 0 (0.0) |
| Significant other finding | 4 (0.5) |
Radiation exposure from computerised tomography coronary angiography in the CTCA arm during index hospitalisation
The median effective radiation dose was 3.1 (IQR 1.9–5.5) mSv (0.014 mSv/mGy/cm conversion factor). Calcium scores, when calculated and available, are categorised in Table 19.
| Total coronary artery calcium score (AU) | CTCA arm and received CTCA (N = 767), n (%) |
|---|---|
| 0 | 134 (26.0) |
| 1–100 | 143 (27.7) |
| 101–400 | 101 (19.6) |
| 401–1000 | 66 (12.8) |
| > 1000 | 72 (14.0) |
| Missing | 251 |
Radiation doses were monitored during the trial, and doses that were higher than those specified in the protocol were reviewed by a radiologist and cardiologist in the central trial team to determine whether these should be reported as a protocol deviation or, in the case that the dose may have had an impact on patient safety, a protocol violation. This process was introduced in protocol version 4 (implemented on 29 June 2016) after it was identified that high radiation doses were frequently occurring at two of the trial sites and universal definitions were needed in the protocol. There were 48 (6.3%) participants who were allocated to the CTCA arm and received CTCA as the trial intervention had their radiation dose reported as either a protocol deviation (n = 31) or a protocol violation (n = 17).
Chapter 4 Health economics
Introduction
This section details the methods, assumptions and results of the health economic analysis to evaluate the cost-effectiveness of CTCA compared with standard care for patients with suspected acute coronary syndrome in the UK. The health economic analysis, estimating the incremental costs and QALYs of CTCA compared with standard care, was conducted in two parts: trial-based economic evaluation and long-term modelling. A within-trial cost-effectiveness analysis was carried out that compared the observed costs and QALYs of the intervention and control arms during the trial period, and an analysis of the long-term cost-effectiveness of CTCA was conducted by adapting a previous decision-analytic model. 27,62 A brief overview of the aims and objectives, as well as the health economics approach, is presented in this section and described in further detail in the next sections.
Aims and objectives
The objectives of the cost-effectiveness analysis were to:
-
estimate the within-trial cost-effectiveness of CTCA compared with standard care for patients with suspected acute coronary syndrome, in terms of the costs and QALYs gained by each strategy
-
estimate the long-term cost-effectiveness of CTCA compared with standard care for patients with suspected acute coronary syndrome, in terms of the costs and QALYs gained by each strategy
-
identify the strategy that is most likely to be cost-effective for patients with suspected acute coronary syndrome, defined as the most cost-effective strategy at a willingness-to-pay threshold of £20,000–30,000 per QALY gained.
Overview of the health economics approach
In the within-trial cost-effectiveness analysis, the incremental cost per QALY gained by using CTCA compared with standard care was estimated by calculating the area under the curve for health utility using the EQ-5D-5L scores and health service costs up to 1 year. Summary EQ-5D-5L values (mean, SD) at baseline and each follow-up point, and items of resource use (mean number per patient and/or proportion) at each time point, were estimated for the two trial arms. QALYs within the trial were estimated using the area-under-the-curve technique, and costs within the trial period were estimated by applying national unit costs to the resource use.
Long-term cost-effectiveness was estimated by adapting an existing model, which was developed as part of a previous HTA evidence synthesis project. 27,62 The costs and QALYs for the first year were based on the within-trial analysis and the costs per QALY beyond the first year (for the survivors) were estimated using decision-analytic modelling. At the end of the trial period, the patients in each arm were classified according to whether they experienced a non-fatal MI during the follow-up period (MI at presentation were not included) or death. The long-term costs and QALYs are estimated based on the proportion of patients with non-fatal MI or death at 1 year. Long-term cost-effectiveness results are used to identify the strategy that is most likely to be cost-effective for patients with suspected acute coronary syndrome, defined as the most cost-effective strategy at a willingness-to-pay threshold of £20,000–30,000 per QALY gained. The modelling approach reflected the assumption that any long-term costs or effects from CTCA are likely to arise from reducing mortality or non-fatal MI over the initial 1-year follow-up period. The model, therefore, estimates the value of the lives saved and MIs avoided, and the associated costs incurred.
Methods for the within-trial analysis
The within-trial cost-effectiveness analysis was performed by comparing the observed costs and QALYs of the CTCA arm with those of the control arm during the trial period. The QALYs and costs for each arm of the trial (i.e. for each strategy) during the follow-up were used to estimate the incremental cost-effectiveness ratio (ICER) of the CTCA arm compared with the standard-care arm. Confidence intervals for the within-trial ICER were estimated to capture the sampling uncertainty.
Of the 1748 participants, who had a mean age of 62 years (64% male), 877 were randomised to receive early CTCA and 871 were randomised to receive standard care. Non-fatal MI within 12 months occurred in 39 out of the 877 participants in the early CTCA arm and in 40 out of the 871 participants in the standard-care arm. In the 12 months, there were 19 deaths among the 877 participants in the early CTCA arm and 17 deaths among the 871 participants in the standard-care arm. Along with the clinical end points, a range of data from questionnaires and case report forms were also collected. These data were used to estimate the within-trial costs and QALYs. These data sources are briefly described in the following sections.
Description of trial data
The data used for the within-trial analysis primarily related to quality of life (measured using EQ-5D-5L questionnaires) and health-care resource use (estimated from case report forms and patient questionnaires), as described below. Appendix 9, Tables 42–46, present information of the data collected in the trial.
EuroQol-5 Dimensions, five-level version
The EQ-5D-5L is the most widely used instrument used to estimate patients’ quality of life (i.e. utility). These EQ-5D-5L questionnaires were administered using paper format to the patients at baseline and 1, 6 and 12 months after index admission. The responses from the patients were then collated into electronic format for health economic analysis.
Health-care resource use
Hospital records and patient self-reported questionnaires were used to estimate the health-care resource use. Hospital resource use was primarily determined from items recorded from the hospital records by the research nurse in the case report form, whereas the patient questionnaires were used to estimate other resource use (such as general practitioner surgery visits, general practitioner home visits, nurse home visits and social worker visits) that was not captured within the case report form. In these questionnaires, the patients were asked for items of resource use in the last month (at month 1) or last 3 months (at months 6 and 12).
Methods for estimating within-trial quality-adjusted life-years
Methods for estimating quality-adjusted life-years from EuroQol-5 Dimensions, five-level version
The EQ-5D-5L questionnaire responses at baseline and 1, 6 and 12 months after index admission were used to estimate the patients’ quality of life (i.e. utility) at each time point. In line with the NICE recommendations, utility scores were estimated using the mapping algorithm by van Hout et al. 63 rather than the EQ-5D-5L value set for England. Given that the baseline mean utility values were similar between treatment arms, there was no need to adjust utilities using regression techniques. The curve of utility over different time points was constructed for each patient (accounting for missing data using the approach mentioned in Dealing with missing data in the EuroQol-5 Dimensions, five-level version, questionnaires) and the QALYs for each arm were estimated by calculating the area under the curve for health utility over the 1-year period. The patients who died during the trial were included with zero utility from the time of death and the average of all of the patients was used to estimate the overall within-trial QALYs.
Dealing with missing data in the EuroQol-5 Dimensions, five-level version, questionnaires
The primary analysis included all patients who had any follow-up data, and we used multiple imputation techniques for estimating the missed utilities for patients with missing data in the follow-up points. For cases with only interim time point(s) missing (e.g. 3 months), we used the average value from the previous time point (i.e. utility at baseline) and the next time point (i.e. 6 months). This allowed us to include all cases with at least some follow-up data, that is only those with no utility values at any follow-up point were excluded from the primary analysis. Multiple imputation was performed using the R package MICE (multiple imputation using chain equations) (The R Foundation for Statistical Computing, Vienna, Austria).
We estimated the baseline utilities for patients without missing follow-up data and patients with missing follow-up data (i.e. those who did not respond to any questionnaires) to identify if there were any systematic differences in utility values between the two arms (i.e. responders and non-responders to questionnaires). Given that the non-responder cases had slightly different baseline values to the responders, we used only the data for responders in the base-case analysis. We also undertook a secondary analysis in which we included all patients and used multiple imputation to estimate the utilities for non-responders. The results of this scenario analysis are presented in Appendix 11, Tables 49 and 50.
Methods for estimating within-trial costs
Methods for estimating health-care resource use
All health-care consumption and costs within the trial period were estimated from a health-care perspective using hospital records and from patient self-reported questionnaires. Resource use data were primarily determined from items recorded in the case report form from the case notes; data from the health service resource use questionnaire was used only for additional items not recorded in the case report form or notes (which included telephone consultations, general practitioner surgery visits, general practitioner home visits, nurse home visits and social worker visits).
All cardiac-related resource use, including the need for continued hospitalisation, additional invasive or non-invasive imaging, drug therapy and rehospitalisation for myocardial ischaemia, was captured. The difference in use of CTCA between the two arms of the trial was translated into the costs of CTCA included in the within-trial analysis. The differences in the rates of related procedures (invasive coronary angiography, other tests for coronary artery disease, coronary interventions and coronary artery bypass grafting) were captured from the case report form and were included in the estimation of costs. Other items of resource use (ED visits, outpatient visits and inpatient stays) were also captured from the case report form, as it is considered to be more accurate than a patient questionnaire. The medication costs were estimated according to their dosage, which was either just for the hospital stay (assumed as an average duration of 5 days) or for the whole year based on expert clinical input (see Appendix 10).
The general practitioner surgery visits, general practitioner home visits, nurse home visits and social worker visits were captured from the patient questionnaire. In the questionnaires, the patients were asked for items of resource use in the last month (at month 1) or last 3 months (at months 6 and 12). For this reason, we estimated costs incurred in the ‘missing’ months (e.g. months 2 and 3) using the average monthly resource use carried back (e.g. assuming resource use in months 2 and 3 were the same as the average of months 4, 5 and 6).
Dealing with missing data in resource use questionnaires
The case report form data were complete, so there were no missing data, whereas the patient questionnaire data had the same missing pattern as the EQ-5D-5L data. We, therefore, included the same cases in the primary analysis for costs and outcomes (i.e. those with at least one follow-up point) and used multiple imputation techniques for missing time points. Multiple imputation was performed using the R package MICE.
Estimating the within-trial costs
The overall resource use for each patient over the 12 months was multiplied with the unit costs (i.e. national average costs) to provide the estimated cost for each patient in the trial. Full details of the unit costs used are presented in Appendix 10, Tables 47 and 48. In brief, NHS reference costs64 were used to estimate the health-care resource use (see Table 47) and the British National Formulary and electronic market information tool65 were used to estimate the drug costs (see Table 48). The patients who died during the trial were included with zero costs from the time of death, and the costs for all of the patients in each arm were averaged to estimate the overall within-trial costs.
Estimating within-trial cost-effectiveness
The QALYs and costs for each arm of the trial (i.e. for each strategy) during follow-up were used to estimate the ICER of the CTCA arm compared with the standard-care arm. Confidence intervals for the within-trial ICER were estimated to capture the sampling uncertainty.
Results of within-trial analysis
Within-trial quality-adjusted life-years
All patients (877 randomised to early CTCA and 871 randomised to standard care) completed the EQ-5D-5L questionnaire at baseline. The mean utility at baseline was 0.752 in the CTCA arm and 0.760 in the standard-care arm. However, there were 113 patients in the CTCA arm and 158 patients in the standard-care arm without any follow-up data (i.e. did not respond to any EQ-5D-5L questionnaires). For this reason, these patients were excluded from the analysis estimating the QALYs. The scenario analysis using all patients (i.e. including those with no follow-up data) is presented in Appendix 11, Tables 49 and 50.
For patients with follow-up EQ-5D-5L data, the average baseline utility was 0.765 in the CTCA arm (n = 764) and 0.768 in the standard-care arm (n = 713), suggesting similar baseline values. In the CTCA arm, there were 185, 215 and 243 patients who did not respond to the EQ-5D-5L questionnaires at 1, 6 and 12 months, respectively. Similarly, in the standard-care arm, there were 250, 275 and 301 patients who did not respond to the EQ-5D-5L questionnaires at 1, 6 and 12 months, respectively. The responses on the individual items of the EQ-5D-5L questionnaire were used to estimate utility scores using the mapping algorithm by van Hout et al. ,63 as recommended by NICE. The value set for the UK was used to estimate the utilities using the Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) crosswalk calculator. The patients who died during the trial were included with zero utility from the time of death and the average of all of the patients was used to estimate the mean values of the utilities at different time points in both arms. These are presented in Table 20.
| Trial arm | Baseline | 1 month | 6 months | 12 months |
|---|---|---|---|---|
| CTCA | 0.765 | 0.739 | 0.758 | 0.761 |
| Standard care | 0.768 | 0.738 | 0.769 | 0.765 |
Multiple imputation techniques were used to impute the utility values for patients with missing data at 1, 6 and 12 months. This multiple imputation was performed using the package MICE in R software. The missing utility values were estimated as the average of values of data sets estimated using multiple imputations.
The QALYs were estimated using the trapezoidal rule for calculating the area under the curve. The utility values at baseline and 1, 6 and 12 months were multiplied with the corresponding time that patients spent in these utilities. It was assumed that the patients stay in the same utility until the midpoint of the time difference to the next follow-up point. For example, utility in month 1 is assumed to last until 3.5 months (i.e. the midpoint of the follow-ups at months 1 and 6) and the utility at month 6 is used for 3.5 to 9 months (i.e. the midpoint of the follow ups at months 6 and 12). Mean QALYs and 95% CIs estimated using bootstrapping are presented in Table 21.
| Trial arm | Mean QALYs | 95% CI |
|---|---|---|
| CTCA | 0.7488 | 0.7353 to 0.7621 |
| Standard care | 0.7577 | 0.7456 to 0.7699 |
Within-trial costs
Resource use data (determined from items recorded in the case report form from the case notes and patient questionnaires) were multiplied with corresponding unit costs to estimate the within-trial costs in each arm. Given the large number of resource use items, for ease of presentation, summary costs for the index hospital stay and costs in the 12 months (i.e. post index hospital stay) are presented in this section. Detailed resource use and unit costs for each of the items can be found in Appendix 10, Tables 47 and 48.
Costs in the index hospital stay
The mean resource use during the index hospital stay for each arm was estimated from the case report form and included costs of hospital stay, costs associated with MI, costs of CTCA, costs of invasive coronary angiography, cost of percutaneous coronary intervention, costs of coronary artery bypass graft surgery, costs of diagnostic tests (including echocardiogram, radionuclide scan, 24-hour tape, exercise test, magnetic resonance imaging angiography, stress echocardiogram, other ECG monitoring, cardiac magnetic resonance imaging and stress magnetic resonance imaging) and the cost of drugs in the index hospital stay. Detailed resource use and unit costs for diagnostic tests and drug costs can be found in Appendix 10, Tables 47 and 48.
The average of the resource use of all patients in each corresponding arm was multiplied with the unit costs (i.e. national average costs) to provide the mean costs for the index hospital stay. The mean resource use in the CTCA arm and standard-care arm during the index hospital stay is presented in Table 22. Unit costs derived from the NHS reference costs64 are also presented here, and more detail about the Healthcare Resource Group codes used to estimate these costs is presented in Appendix 10, Table 47.
| Trial arm | Hospital stay | MI | CTCA | Invasive coronary angiograms | Percutaneous coronary interventions | Coronary artery bypass graft surgeries | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (days) | Cost per day (£) | Proportion | Cost (£) | Total (n) | Cost (£) | Total (n)a | Cost (£) | Total (n) | Cost (£) | Total (n) | Cost (£) | |
| CTCA | 3.934 | 402 | 0.044 | 2360 | 0.888 | 195 | 0.602 | 1685 | 0.315 | 2930 | 0.059 | 11,760 |
| Standard care | 3.504 | 0.046 | 0.084 | 0.648 | 0.295 | 0.063 | ||||||
The mean costs in the CTCA arm and the standard-care arm for the index hospital stay are estimated as £4646 and £4394, respectively. A detailed breakdown of these costs is presented in Figure 13 and Table 22, which are estimated by multiplying the mean resource use during the index hospital stay for each arm (Table 23) with the unit costs derived from the NHS reference costs. 64 Detailed resource use and unit costs for diagnostic tests and drug costs can be found in Appendix 10, Tables 47 and 48. As observed in Figure 13 and Table 23, most of the costs are similar between the two arms, but the higher costs in the CTCA arm are mainly a result of the increased hospital length of stay (3.93 days in the CTCA arm vs. 3.50 days in the standard-care arm) and the additional CTCA use (average of 0.888 scans in the CTCA arm vs. 0.084 scans in the standard-care arm).
FIGURE 13.
Breakdown of the costs for index hospital stay for the CTCA and standard-care arms. CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
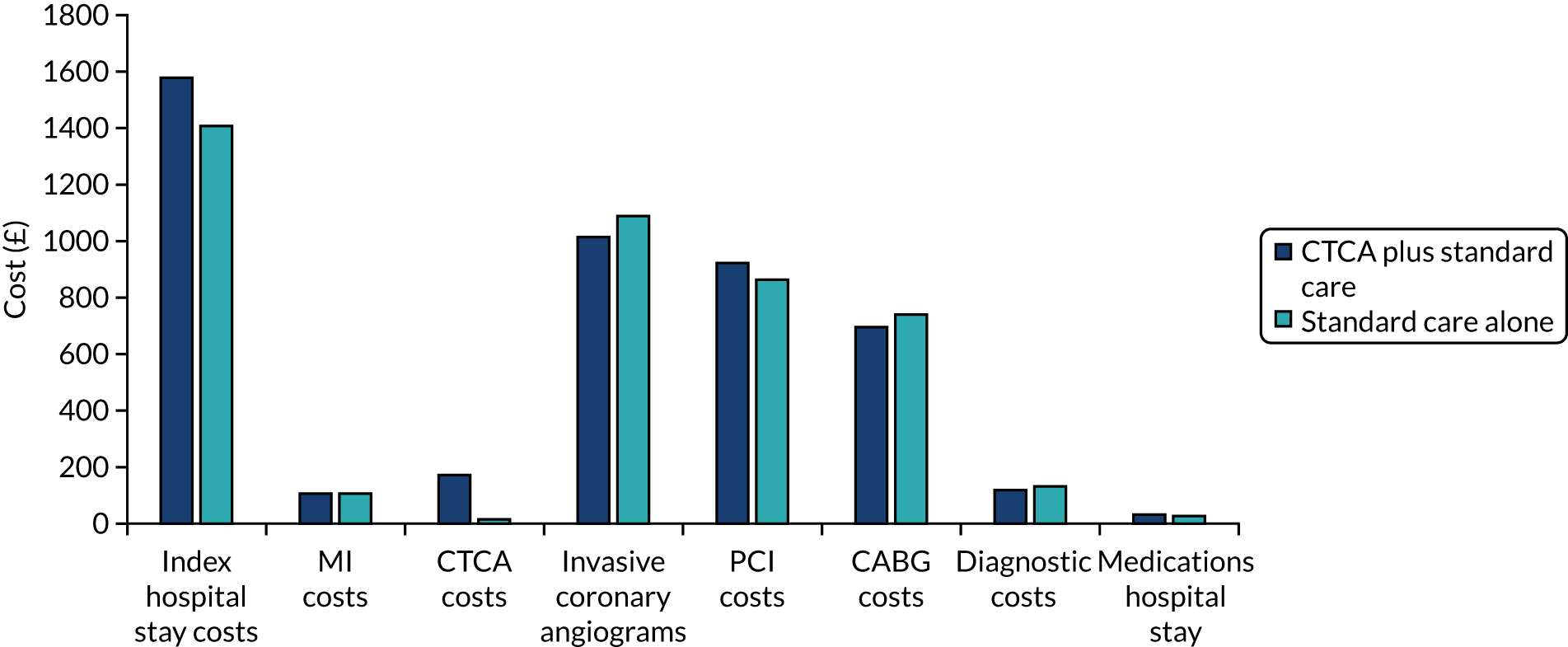
| Trial arm | Cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| Hospital stay | MI | CTCA | Invasive coronary angiogram | Percutaneous coronary intervention | Coronary artery bypass graft surgery | Diagnostic tests | Drugs in hospital stay | |
| CTCA | 1581.41 | 104.95 | 173.21 | 1014.46 | 922.10 | 697.29 | 121.19 | 31.45 |
| Standard care | 1408.62 | 108.38 | 16.34 | 1091.09 | 864.54 | 742.59 | 135.23 | 27.26 |
Costs in the 12 months post index hospitalisation
The mean resource use during the 12 months post index hospital admission was estimated for each arm from both the case report form and the patient questionnaire data.
The case report form reported data on the number of ED attendances, number of outpatient visits, days spent on a coronary care unit (CCU), days spent on an intensive care unit (ICU) and total days spent in hospital (any location, excluding CCU/ICU). Patient questionnaires reported the number of telephone consultations, general practitioner surgery visits, general practitioner home visits, nurse home visits and social worker visits in the last month (at month 1) or last 3 months (at months 6 and 12). Multiple imputation techniques were used to estimate the missing data in the patient questionnaires. Resource use was also incurred in the ‘missing’ months (e.g. months 2 and 3) using the average monthly resource use carried back (e.g. assuming that resource use in months 2 and 3 was the same as the average of that in months 4, 5 and 6).
The mean resource use in the CTCA arm and standard-care arm in the 12 months post index hospitalisation, from the case report form and patient questionnaires, respectively, is presented in Tables 24 and 25. Unit costs derived from the NHS reference costs64 are also presented here, and more detail about the Healthcare Resource Group codes used to estimate these costs is presented in Appendix 10, Table 47.
| Trial arm | ED visits | Outpatient visits | Days in CCU | Days in ICU | Days in hospital | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Cost per visit (£) | Total (n) | Cost per visit (£) | Total (n) | Cost per day (£) | Total (n) | Cost per day (£) | Total (n) | Cost per day (£) | |
| CTCA | 0.75 | 168 | 4.57 | 148 | 0.41 | 917 | 0.12 | 1340 | 2.39 | 402 |
| Standard care | 0.74 | 4.44 | 0.35 | 0.08 | 1.97 | |||||
| Trial arm | Telephone consultations | General practitioner surgery visits | General practitioner home visits | Nurse home visit | Social care home visit | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n) | Cost per consultation (£) | Total (n) | Cost per visit (£) | Total (n) | Cost per visit (£) | Total (n) | Cost per visit (£) | Total (n) | Cost per visit (£) | |
| CTCA | 2.52 | 12 | 6.57 | 32 | 0.38 | 133 | 0.94 | 20 | 0.24 | 20 |
| Standard care | 2.26 | 6.34 | 0.30 | 0.87 | 0.16 | |||||
The mean costs in the CTCA arm and the standard-care arm for the 12 months post index hospitalisation are estimated as £2768 and £2451, respectively. A detailed breakdown of these costs, which are estimated by multiplying the mean resource use with the unit costs derived from the NHS reference costs, is presented in Figure 14 and Table 25. 64
FIGURE 14.
Breakdown of costs 12 months post index hospital stay in the CTCA and standard-care arms. GP, general practitioner.
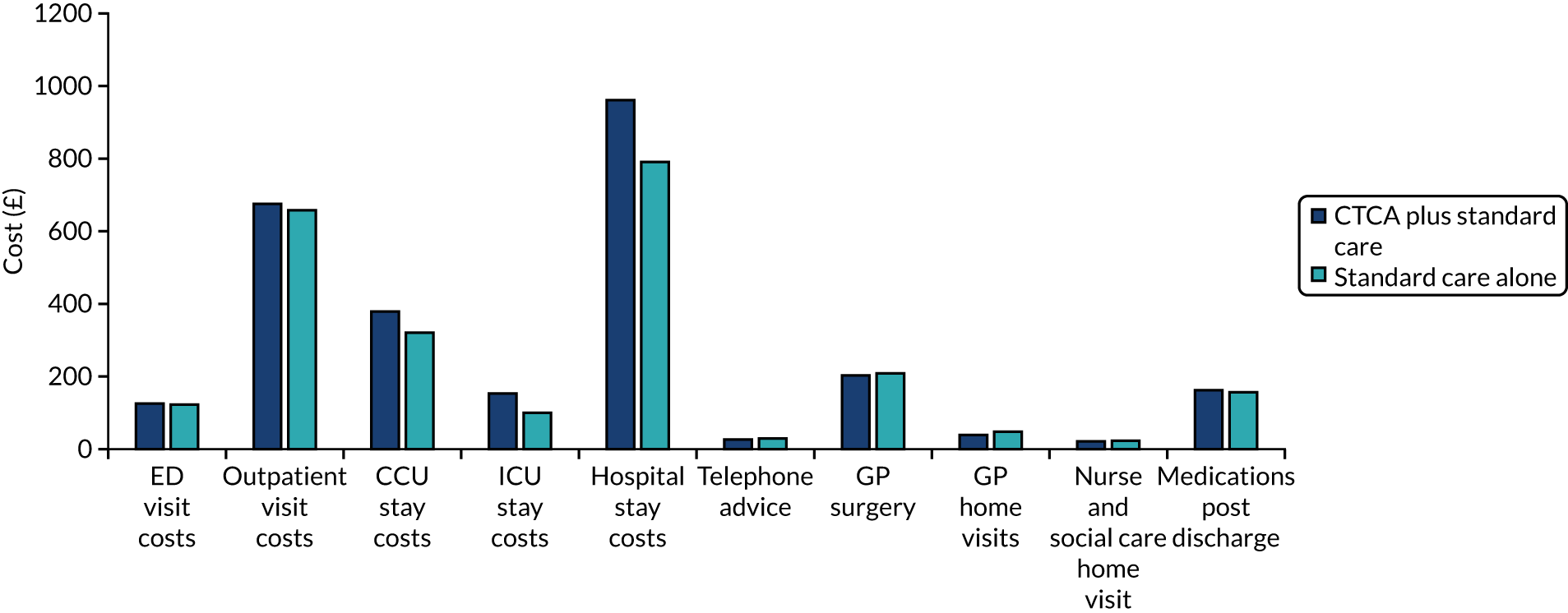
The total within-trial costs are estimated as £7414.13 and £6845.11 for the CTCA arm and the standard-care arm, respectively. The mean costs and bootstrapped CIs are presented in Table 26.
| Trial arm | Mean costs (£) (95% CI) |
|---|---|
| CTCA | 7414.13 (6840.20 to 7988.07) |
| Standard care | 6845.11 (6337.42 to 7352.79) |
Within-trial cost-effectiveness
Table 27 shows the results for the within-trial cost-effectiveness analyses. The total within-trial costs for the CTCA arm are higher than for the standard-care arm, and the total within-trial QALYs are lower in the CTCA arm than in the standard-care arm, resulting in CTCA being dominated by the standard-care arm. The greater costs in the CTCA arm are mainly a result of longer hospital length of stay (3.93 days in the CTCA arm vs. 3.50 days in the standard-care arm) and the additional CTCA use (average of 0.888 scans in the CTCA arm vs. 0.084 scans in the standard-care arm).
| Trial arm | Total costs (£) (95% CI) | Total QALYs (95% CI) | ICER |
|---|---|---|---|
| CTCA | 7414.13 (6840.20 to 7988.07) | 0.7488 (0.7353 to 0.7621) | – |
| Standard care | 6845.11 (6337.42 to 7352.79) | 0.7577 (0.7456 to 0.7699) | Dominant |
Methods for long-term modelling
The costs and QALYs for the first year were based on the within-trial analysis, and the costs/QALYs beyond the first year (for the survivors) were estimated using decision-analytic modelling. Long-term cost-effectiveness was estimated by adapting an existing model, developed as part of a previous HTA evidence synthesis project. 27,62 At the end of the trial period, the patients in each arm were classified according to whether they experienced a non-fatal MI during the follow-up period or death. MI occurring before or at recruitment to the trial was not used in this classification because this could not have been influenced by CTCA. The long-term costs and QALYs were estimated based on whether or not the patients had non-fatal MI. The model structure is presented in Figure 15.
FIGURE 15.
Structure of the long-term model.
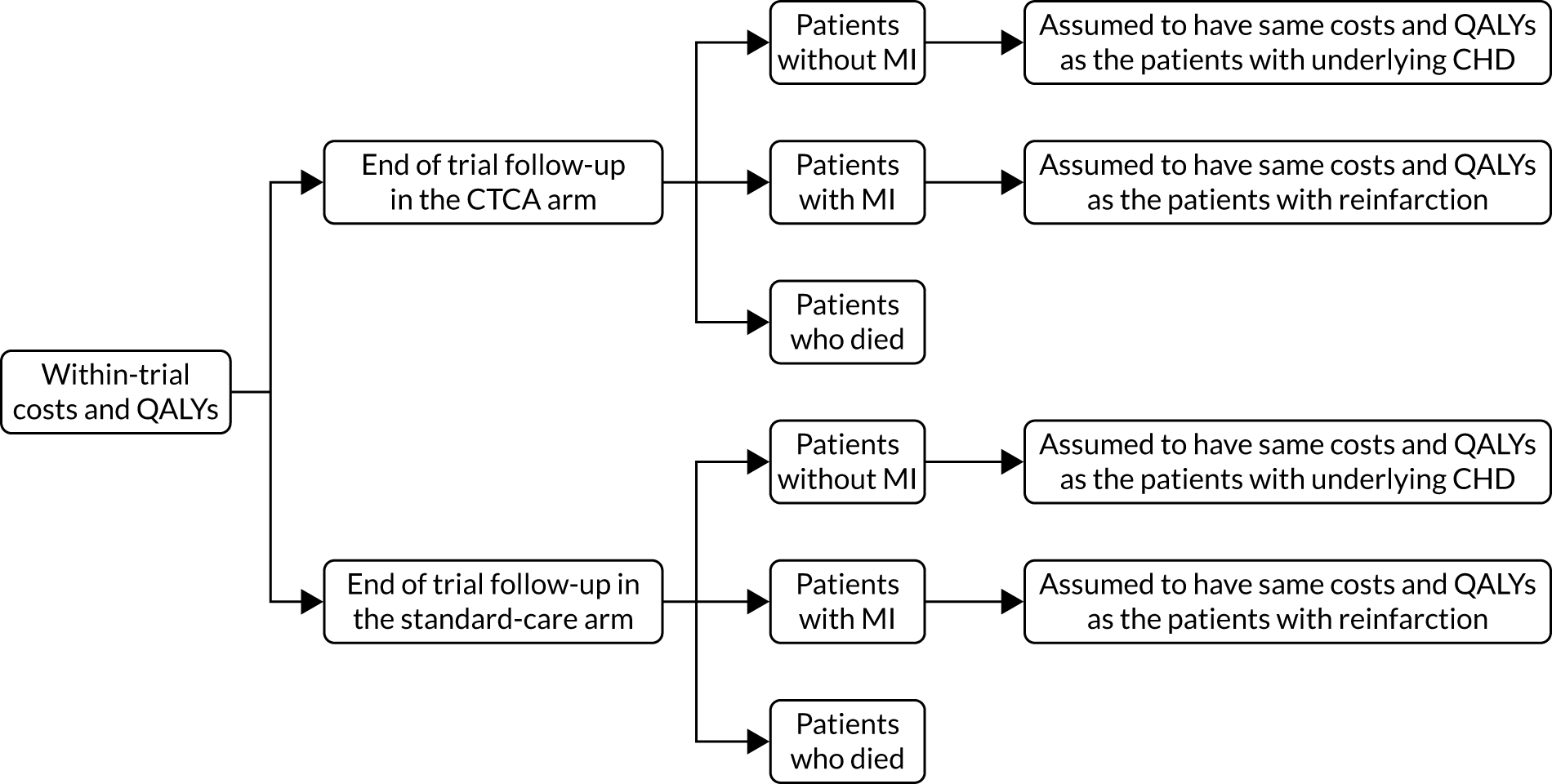
Patient status at the end of 12-month follow-up in the RAPID-CTCA trial (i.e. dead, alive with non-fatal MI during follow-up or alive without non-fatal MI during follow-up) was used to determine the proportion of patients in each of these categories entering the model from each arm of the trial (i.e. for each strategy). Patients with non-fatal MI accrued the costs and QALYs of those with CHD and reinfarction. Patients without non-fatal MI accrued the costs and QALYs of those with CHD but no reinfarction. This approach involves some simplifying assumptions (specifically that all patients without MI have CHD) but ensures that the incremental costs and QALYs associated with survival without non-fatal MI on follow-up, compared with survival with non-fatal MI, reflect the incremental costs and effects of avoiding non-fatal MI. Patients who died during the trial accrued no costs or QALYs.
We used data from the recent NICE guidelines18 to capture the life expectancy, annual costs and corresponding annual utilities based on whether or not the patients suffered reinfarction. The patients without MI in the trial period were assumed to have costs, quality of life and life expectancy associated with CHD patients. If the patients experienced a MI within the trial follow-up, they were assumed to have costs, quality of life and life expectancy associated with patients with reinfarction (i.e. reduced quality of life and life expectancy, and higher annual costs).
It is clearly not true that all patients without non-fatal MI have CHD, but attempting to model those with and without CHD separately would increase complexity without having any effect on the results, given that the proportion of patients without CHD would be the same in both strategies and would accrue the same costs and QALYs. This is because we assumed that only those with CHD could suffer non-fatal MI.
Estimating life expectancy
Based on the data from the recent NICE guidelines,18 the life expectancy was estimated by applying relevant standardised mortality ratios (SMRs) to the general population mortality rates. Age- and sex-adjusted general population mortality were based on national life tables for the UK from 2017 to 2019. The average age of the population entering the model was 61.6 years and the proportion of males was 64%, which was estimated as the average of the mean values in the CTCA and standard-care arms. SMRs were sourced from the economic analysis in the recent acute coronary syndrome NICE guidelines,18 which suggested a SMR of 2.00 (95% CI 1.99 to 2.01) for patients without reinfarction and a SMR of 3.00 (95% CI 2.95 to 3.05) for patients with reinfarction. These SMRs were combined with age- and sex-adjusted general population mortality to estimate the proportions of patients alive over time. This resulted in mean (undiscounted) life-years of 17.69 for patients without reinfarction and 14.83 for patients with reinfarction.
Estimating long-term quality-adjusted life-years
Long-term QALYs were estimated by multiplying the age-adjusted utility values of the different health states with the proportion of patients alive over time in the respective health states.
The utility values at the start of the model for patients without and with reinfarction were extracted as 0.842 and 0.821, respectively, from the NICE acute coronary syndrome guidelines. 18
These utility values were adjusted for age to account for the decreasing quality of life over time; this adjustment was performed by using the ratio of age- and sex-specific general population EQ-5D-5L utilities at different ages compared with the general population utility at the start of the mode. The age- and sex-specific general population utility values were derived using the formula from Ara and Brazier,66 which states:
Multiplicative method was used to estimate the utility values over time, that is the utility values at the start of the model were multiplied with utility decrements.
This resulted in mean undiscounted QALYs of 13.97 for patients without reinfarction and 11.52 for patients with reinfarction. Using the NICE recommended discount rate of 3.5% per annum67 resulted in discounted QALYs of 10.10 and 8.67 for patients without with reinfarction, respectively.
Estimating long-term costs
Long-term costs were estimated by multiplying the annual costs of the different health states with the proportion of patients alive over time in the respective health states. The annual costs for patients without and with reinfarction were extracted as £943 and £1415, respectively, from the NICE acute coronary syndrome guidelines. 18 Using these annual costs, along with the life expectancy estimated earlier, resulted in mean undiscounted costs of £16,685 for patients without reinfarction and £20,979 for patients with reinfarction. Using the NICE recommended discount rate of 3.5% per annum67 resulted in discounted long-term costs of £11,929 and £15,650 for patients without and with reinfarction, respectively.
Summary of modelling input parameters
The decision-analytic model assigned patients in the CTCA arm and standard-care arm with probability of death and MI. Each patient who was alive then accumulated costs and QALYs based on the cost parameters, life expectancy and utility values based on whether or not they suffered MI. A summary of the model parameters along with the distributions and sources is provided in Table 28.
| Parameter | Mean | Distribution | Source |
|---|---|---|---|
| Within-trial costs (£) | |||
| CTCA arm | 7414 | Normal (7414, 293) | RAPID-CTCA |
| Standard-care arm | 6845 | Normal (6845, 259) | RAPID-CTCA |
| Within-trial QALYs | |||
| CTCA arm | 0.7488 | Normal (0.7488, 0.01) | RAPID-CTCA |
| Standard-care arm | 0.7577 | Normal (0.7577, 0.01) | RAPID-CTCA |
| MI and death in the CTCA arm | |||
| Risk of MI | 4.4% | Beta (39, 838) | RAPID-CTCA |
| Risk of death | 2.2% | Beta (19, 858) | RAPID-CTCA |
| MI and death in the standard-care arm | |||
| Risk of MI | 4.6% | Beta (40, 831) | RAPID-CTCA |
| Risk of death | 2.0% | Beta (17, 854) | RAPID-CTCA |
| Discounted long-term QALYs | |||
| Patients without reinfarction | 10.10 | Normal (10.10, 0.05) | NICE acute coronary syndrome guidelines18 |
| Patients with reinfarction | 8.67 | Normal (8.67, 0.05) | NICE acute coronary syndrome guidelines18 |
| Discounted long-term costs (£) | |||
| Patients without reinfarction | 11,928 | Gamma (500, 24) | NICE acute coronary syndrome guidelines18 |
| Patients with reinfarction | 15,650 | Gamma (500, 31) | NICE acute coronary syndrome guidelines18 |
Estimating long-term cost-effectiveness
The cost-effectiveness of the different interventions was estimated and uncertainty was incorporated in the modelling by performing a probabilistic sensitivity analysis.
The lifetime QALYs and costs for each arm of the trial (i.e. for each strategy) were used to estimate the ICER of the CTCA arm compared with the usual care arm. An ICER measures the relative value of two strategies and is calculated as the mean incremental cost divided by the mean incremental benefits. A strategy is dominated when another strategy accrues more QALYs for less cost. The willingness-to-pay threshold is the amount of money that the decision-maker is willing to pay to gain 1 additional QALY. The usual threshold for decision-making at NICE is considered to be around £20,000–30,000 per QALY.
Parameter uncertainty was included in a probabilistic sensitivity analysis (PSA) based on Monte Carlo simulation. PSA model results include the effects of accounting for uncertainty in the model parameters (the costs, utilities, risk of mortality and MI), characterised as probability distributions. PSA is undertaken whereby the model is rerun (1000 times), each time with a different value for the risks, costs and utilities, which are independently sampled from the probability distributions.
The cost-effectiveness plane shows the incremental costs (y-axis) and incremental QALYs (x-axis) compared with usual care. In this chart, if a model run for a strategy had exactly the same costs and QALYs as usual care, the ‘sample’ for that model run would appear at the origin. Samples plotted to the right of the y-axis have more QALYs than usual care, and samples plotted above the x-axis have more costs. Samples plotted to the right of a straight line with slope lambda passing through the origin are cost-effective whereas those plotted to the left are not.
Cost-effectiveness acceptability curves (CEACs) were plotted to identify the probability of the CTCA arm being cost-effective compared with the standard-care arm for a range of threshold values for an additional QALY. A CEAC shows the proportion of model runs for which each strategy is cost-effective over a range of potential willingness-to-pay thresholds (i.e. lambda).
Results of long-term modelling
This section details the results of the deterministic cost-effectiveness results and results estimated as mean values of 1000 PSA runs, each run with a different estimate for the risks, costs and utilities sampled from the probability distributions reported in Table 29. The expected estimates of cost-effectiveness and the uncertainty around them are presented, along with the probability that each of the strategies is the most cost-effective.
Deterministic long-term cost-effectiveness results
Table 29 shows the deterministic results for the long-term cost-effectiveness analyses. The total costs for the CTCA arm are higher than for the standard-care arm, and the total QALYs are lower in the CTCA arm than in the standard-care arm, resulting in the CTCA arm being dominated by the standard-care arm. The greater costs in the CTCA arm are mainly a result of the higher within-trial costs, and the lower QALYs are a result of the larger number of deaths in the CTCA arm than in the standard-care arm.
| Trial arm | Total costs (£) | Total QALYs | ICER |
|---|---|---|---|
| CTCA | 19,251 | 10.566 | – |
| Standard care | 18,713 | 10.595 | Dominant |
Probabilistic long-term cost-effectiveness results
The model was re-run 1000 times, each time with a different value for the risks of MI/death, costs and utilities sampled from the probability distributions. In the cost-effectiveness plane shown in Figure 16, the samples fall almost equally on either side of the y-axis, suggesting that there is uncertainty in stating that CTCA is effective compared with standard care. On average, CTCA results in lower QALYs than standard care because, on average, more patients are alive in the standard-care arm than in the CTCA arm. On the other hand, almost all of the samples fall above the x-axis line, suggesting that CTCA almost always has higher costs than standard care. This is primarily because of the higher within-trial costs in the CTCA arm, which are not offset by the lifetime costs of care for excess survivors in the standard-care arm.
FIGURE 16.
Cost-effectiveness plane comparing the CTCA arm with the standard-care arm.
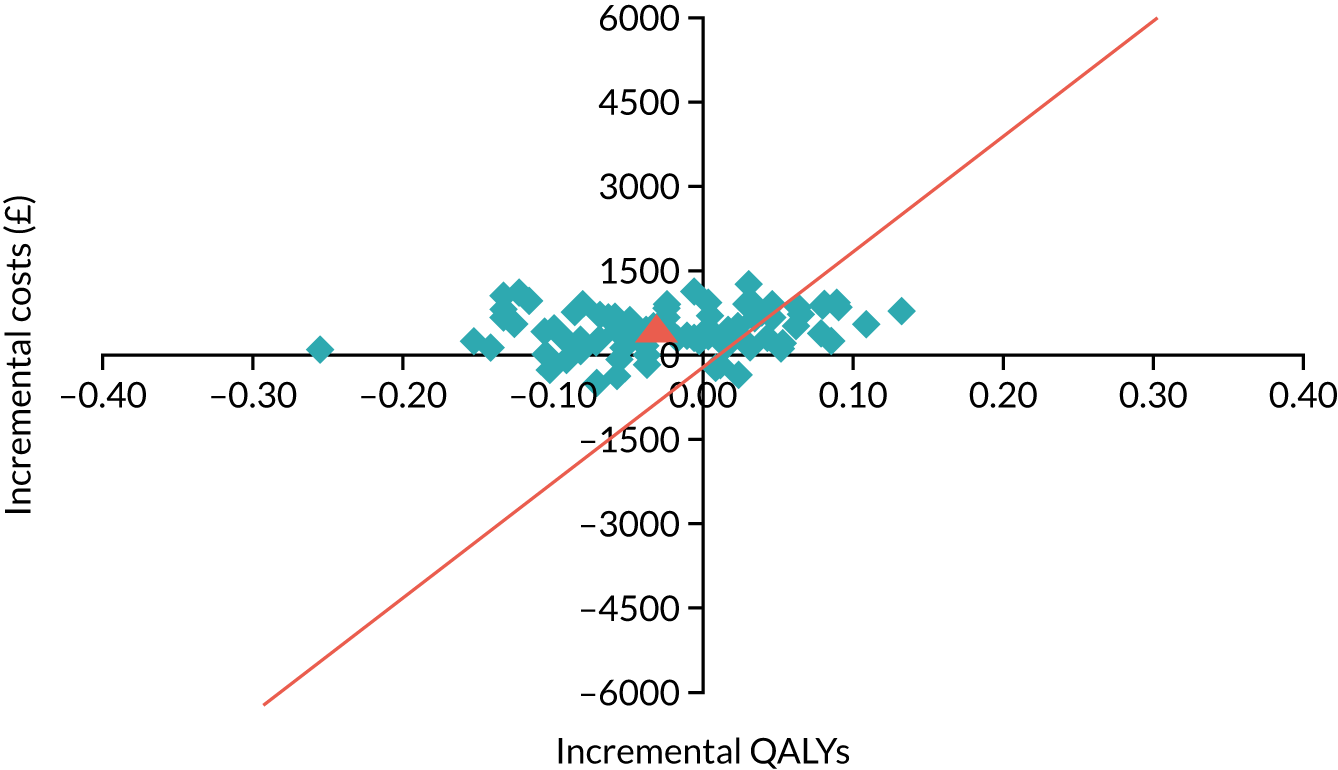
Table 30 shows the probabilistic results for the long-term cost-effectiveness analyses. As in the deterministic analysis, the total costs for the CTCA arm are higher than for the standard-care arm, and the total QALYs are lower in the CTCA arm than in the standard-care arm, resulting in the CTCA arm being dominated by the standard-care arm. The mean cost difference between the CTCA arm and the standard-care arm is £481 (higher CTCA costs) and the mean QALY difference is –0.025 (higher QALYs in the standard-care arm), suggesting that CTCA is dominated by standard care. The greater costs in the CTCA arm are mainly a result of higher within-trial costs and the lower QALYs are a result of the larger number of deaths in the CTCA arm than in the standard-care arm.
| Trial arm | Total costs (£) | Total QALYs | ICER |
|---|---|---|---|
| CTCA | 18,755 | 10.553 | – |
| Standard care | 18,274 | 10.578 | Dominant |
The CEAC in Figure 17 shows the proportion of model runs for which each strategy was cost-effective over a range of potential willingness-to-pay thresholds. As seen in Figure 17, at all of the thresholds less than £50,000 per QALY, standard care was the most cost-effective strategy in the majority of model runs. At the threshold of £20,000 per QALY, standard care had 76% probability of being cost-effective, and the percentage of model runs in which standard care was the most cost-effective strategy at a £30,000 per QALY threshold was 73%.
FIGURE 17.
Cost-effectiveness acceptability curve. MAICER, maximum incremental cost-effectiveness ratio.
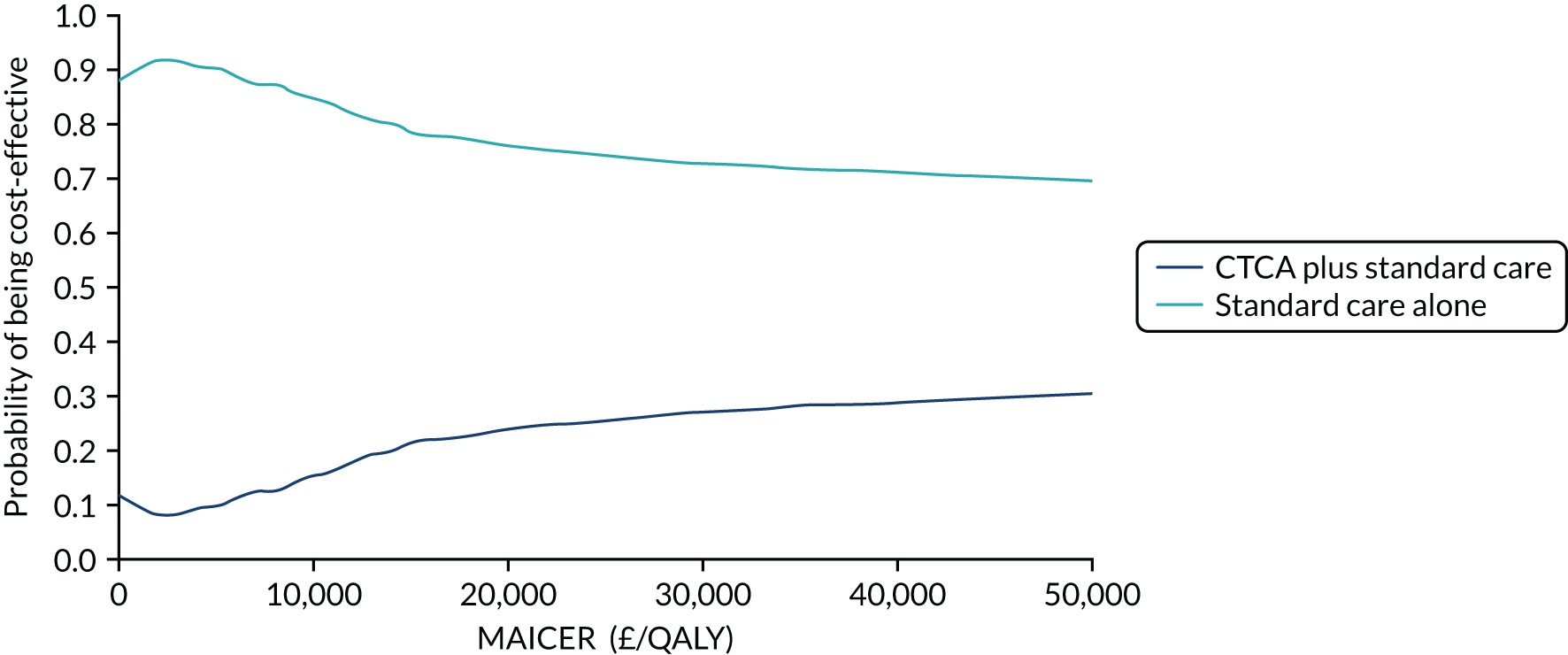
Discussion
Statement of principal findings
In the within-trial analysis, the mean costs for the CTCA arm (£7414.13) were higher than for the standard-care arm (£6845.11). The greater costs in the CTCA arm were mainly a result of the longer hospital length of stay and the additional CTCA use, despite slightly lower costs of invasive coronary angiography. The QALYs are lower in the CTCA arm (0.7488) than in the standard-care arm (0.7577). Given that standard care is more effective and less expensive than CTCA, standard care is dominant (i.e. CTCA is dominated by the standard-care arm).
The long-term economic analysis suggested that CTCA was slightly less effective than standard care, with 0.025 QALYs lost per patient treated, and was more expensive, with additional costs of £481 per patient treated. Given that standard care is more effective and less expensive than CTCA, standard care is dominant, with 76% probability of being cost-effective at the £20,000 per QALY willingness-to-pay threshold. The greater costs in the CTCA arm are mainly a result of the higher within-trial costs, and the lower QALYs are a result of the larger number of deaths in the CTCA arm than in the standard-care arm.
Strengths and limitations of the assessment
Our within-trial analysis was based on resource use and EQ-5D-5L data from a large pragmatic trial with a representative population, and included multiple imputation for missing data and bootstrapping analysis for estimating the mean costs and QALYs. Other strengths in the within-trial analysis included detailed costing based on resource use data in the trial to estimate per-patient costs, and valuing outcomes as QALYs based on EQ-5D-5L questionnaires completed by the patients.
Our long-term analysis took the economic perspective of the NHS in England and Wales, was based on an established model, used robust sources for input parameters (effectiveness estimates were from the large pragmatic trial, with a representative population, and the long-term costs and QALYs were estimated from recent NICE acute coronary syndrome guidelines), valued outcomes as QALYs, used a lifetime horizon and included a PSA.
Despite these strengths, our analysis had some limitations. Within the trial, standard-care patients were less inclined to return their questionnaires than CTCA patients (158 patients in the standard-care arm did not respond to any EQ-5D-5L questionnaires compared with 113 patients in the CTCA arm). Given that these patients without any follow-up data were excluded from the analysis, this differential response to EQ-5D-5L questionnaires could result in bias in the estimation of within-trial QALYs. In the scenario analysis using all patients (i.e. including those with no follow-up data), the QALY difference between the CTCA arm and the standard-care arm is approximately half of the difference using the base-case analysis for responders only (see Appendix 11, Table 50). However, this is unlikely to affect overall conclusions around cost-effectiveness. In addition, our analysis was not able to incorporate short-term benefits related to patient satisfaction and diagnostic certainty, as these may not be captured in the EQ-5D-5L questionnaires.
The long-term modelling assumed benefits related only to reducing deaths and non-fatal MI by 1 year, and other benefits, such as reduced rates of life-threatening arrhythmia or new-onset heart failure outcomes, were not included. The event rate for heart failure or life-threatening arrhythmia in the standard-care arm did not exceed 5% and a meaningful effect from CTCA was not observed; therefore, including the long-term costs and effects of these events in the model would not have significantly changed the results. We assumed that all patients without non-fatal MI have CHD in the model and, although this is not true, the proportion without CHD would be the same across the standard-care and CTCA arms, suggesting that it is reasonable to assume that this will not markedly affect overall cost-effectiveness. Incorporating those with and without CHD separately would increase model complexity without having any effect on the incremental results.
Conclusions
Both the within-trial analysis and the long-term cost-effectiveness modelling suggested that CTCA results in higher costs and lower QALYs than standard care, making routine use of CTCA for suspected acute coronary syndrome unlikely to be cost-effective use of NHS resources.
Chapter 5 Discussion
This pragmatic multicentre RCT aimed to establish the clinical effectiveness and cost-effectiveness of early CTCA in the management and outcome of patients presenting to the ED with suspected or provisionally diagnosed acute coronary syndrome. Importantly, it is the first trial to investigate the role of CTCA in intermediate-risk patients, unlike previous trials of low-risk acute chest pain. 28–31,34–39 Moreover, to the best of our knowledge, it is the first trial of CTCA in acute chest pain designed to investigate the impact of CTCA use on clinically important long-term outcomes.
Principal findings
Impact on clinical outcomes
There was no evidence that early CTCA had an effect on the 1-year rate of all-cause death or subsequent non-fatal type 1 (spontaneous) or 4b (stent thrombosis) MI. The trial design deliberately included a broad and representative group of patients focusing on those at intermediate risk of acute coronary syndrome. To provide increased precision around the impact of CTCA in specific risk categories, the prespecified analyses included several subgroup analyses. These included the individual inclusion criteria of troponin level elevation, previous diagnosis of coronary artery disease and ECG abnormality, as well as important risk categories such as age and sex. The GRACE score,59 an internationally accepted prognostic risk score in acute coronary syndrome and recommended in contemporary guidelines,9,10 was categorised into low, intermediate and high risk to mirror the groupings in these guidelines. Furthermore, an analysis comparing the primary outcome between sites with and without on-site invasive angiography facilities was included, as this may affect thresholds for invasive management. However, there was no evidence of benefit of CTCA for any of the prespecified subgroup analyses.
A number of key secondary end points were also investigated to ensure that all potentially important cardiovascular and CHD outcomes were examined. Once again, there was no evidence of benefit of CTCA for any of these key outcomes. In line with previous trials, we explored the impact of CTCA on all non-fatal MIs only, irrespective of subtype. Further exploration of all of the subtypes of MI, as defined in the universal definition,11 may have some value, but we did not assess this here.
It could be argued that we recruited too many patients who were at high risk of disease and, therefore, candidates for inpatient invasive coronary angiography. However, over three-quarters of participants had a low or an intermediate GRACE risk score, most did not have obstructive coronary artery disease, and, in the prespecified subgroup analyses, there was no evidence of heterogeneity of effect for the primary outcome according to the level of risk. As previously stated, these subgroups are the focus of contemporary guidelines9,10 that attempt to define intermediate-risk groups suitable for observation and additional testing, including CTCA. We were unable to demonstrate an effect of CTCA on 1-year clinical outcomes in any of these subgroups or risk categories.
Impact on treatment
The use of early CTCA had no impact on the overall rates of treatments for acute coronary syndrome, with similar rates of coronary revascularisation, prescription of acute coronary syndrome medications and discharge preventative therapies. This was similar between trial allocation whether the treatment was started, the treatment was stopped or the dose was altered. This is likely to reflect the high sensitivity of contemporary clinical assessment combining cardiac troponin level testing with a 12-lead ECG. Contemporary practice, therefore, provided limited opportunity for CTCA to identify unrecognised cases of acute coronary syndrome owing to CHD. Moreover, for CTCA to improve adverse coronary events, it would need to alter patient management. Such changes have been seen in prior studies of patients with stable chest pain in which CTCA improved the detection of unrecognised coronary artery disease, increasing the use of both preventative therapies and coronary revascularisation. These changes in management were associated with reduced rates of subsequent CHD death or non-fatal MI. 45,46 In our population of patients with acute chest pain and relatively high rates of invasive coronary angiography and coronary revascularisation, we observed no overall changes in rates of treatment or outcome. The most likely explanation for the difference between RAPID-CTCA and previous studies showing benefit from CTCA in patients with stable chest pain is that troponin level testing and the 12-lead ECG are powerful predictors of the need for intervention in acute chest pain, leaving less potential for CTCA to detect clinically important unrecognised pathology. They have much less value in stable chest pain.
Impact on processes of care
Computerised tomography coronary angiography was associated with a reduction in invasive coronary angiography, contrasting with previous acute chest pain trials in which CTCA was associated with increased rates of invasive angiography. 28–31 This disparity is likely to be a result of the differences in trial populations, especially the baseline risk and the prevalence of CHD. We recruited patients with a high prevalence of disease (50–75% with coronary artery disease) in contrast to the low prevalence (< 10%) in prior studies. 28–31
While recent adoption of high-sensitivity troponin level testing has supported earlier clinical decision-making,9,10 it has led to the misidentification of many patients who do not have MI. 13,14 The strength of CTCA is its very high negative predictive value for coronary artery disease, which is applicable to all risk groups, including those patients with non-ST segment elevation acute coronary syndrome. 68 Indeed, we found that 40–50% of patients with normal or non-obstructive coronary artery disease had an elevated cardiac troponin level. Our finding of a 19% relative reduction in the hazard for invasive coronary angiography is likely to reflect the exclusion of obstructive coronary artery disease, thereby avoiding unnecessary invasive coronary angiography, especially in patients with elevations in cardiac troponin levels not attributable to MI or obstructive coronary artery disease. Importantly, this reduction in invasive coronary angiography was not associated with differences in overall rates of coronary revascularisation or clinical events, suggesting excellent diagnostic accuracy and continued appropriate coronary revascularisation for those with obstructive coronary artery disease.
Previous meta-analysis of low-risk acute chest pain trials has shown that CTCA reduces hospital length of stay and subsequent rehospitalisation with suspected acute coronary syndrome. 44 In addition, CTCA was associated with a downstream reduction in cardiovascular investigation. 44 Once again, there was no evidence of this effect in the RAPID-CTCA trial, with no difference between arms in rehospitalisation with chest pain or subsequent cardiovascular or non-cardiovascular investigation rates. Unlike previous data, CTCA in the RAPID-CTCA trial increased rather than decreased the length of hospital stay. These are all likely to reflect the much higher prevalence of underlying coronary artery disease and consequentially high invasive angiography rates in our population than with previous trials. In addition, previous trials in low-risk populations were undertaken in other settings in which standard care for this group involves high rates of admission and intervention. Our finding that CTCA increased length of hospital stay in the intermediate-risk group suggests that the relatively low rate of admission and short time in the NHS leaves little scope for CTCA to achieve the reductions in resource use that have been shown elsewhere and used to support its use in low-risk patients.
Early CTCA was associated with some benefits. Patients valued the use of CTCA, possibly reflecting a more rapid evaluation of their clinical condition. In addition, there was enhanced diagnostic certainty for the attending clinician.
Impact on cost-effectiveness
Economic analysis showed that CTCA was not cost-effective compared with standard care. The lifetime analysis suggested that CTCA was associated with 0.025 fewer QALYs and £481 additional costs per patient treated. Standard care, therefore, dominated CTCA, with 76% probability of being cost-effective at the £20,000 per QALY threshold. The within-trial analysis showed a similar difference in mean costs per patient (£7414 vs. £6845) to the lifetime analysis, suggesting that the difference in costs in the lifetime analysis was driven by the difference in within-trial costs, which were mainly owing to longer hospital length of stay and the additional CTCA use. The cost-effectiveness plane (see Figure 28) showed that most of the estimates of the incremental cost per QALY were in the upper-left quadrant (CTCA less effective and more expensive) or upper-right quadrant (CTCA more effective and more expensive). This suggests that, although there is uncertainty around the effectiveness estimates in the probabilistic lifetime analysis, the cost estimates are more certain. A reasonable overall conclusion is, therefore, that CTCA increases costs without improving effectiveness.
Trial internal validity
We recruited a large population of patients with suspected or provisionally diagnosed acute coronary syndrome who had a spectrum of risk reflected in the range of GRACE scores and proportion of patients with troponin levels above the 99th centile at recruitment. Approximately half of the centres did not have on-site invasive angiography facilities. Our trial population was equally composed of those who did or those who did not have a final diagnosis of acute coronary syndrome, and those who did or those who did not have obstructive coronary artery disease, a population that was truly representative of patients with an intermediate level of risk. The majority of patients randomised to CTCA received the investigation and there was very limited crossover from the standard-care arm. Finally, there were data for the primary analysis on all but one of the participants recruited.
External validity and generalisability
Previous trials28–31 have employed CTCA as an approach for early and safe discharge of low-risk patients from the ED. These studies were mostly designed to look at length of stay, but meta-analyses32,33,41–44 of these previous trials indicated that early CTCA not only was associated with shorter lengths of stay, but also increased rates of invasive angiography and coronary revascularisation. However, these findings were not replicated in a subsequent multicentre trial of 500 low-risk patients in which rates of coronary angiography, revascularisation and clinical events were unchanged by CTCA. 37 Our trial now provides the data for those at higher risk than these prior studies and establishes that the influence of CTCA is distinctly different in this population of patients. Indeed, we show that CTCA can avoid unnecessary coronary angiography without affecting rates of coronary revascularisation. For hospitals without on-site or ready access to invasive coronary angiography facilities, CTCA may, therefore, be a useful approach to help to identify those patients who do not require interhospital transfer or further evaluation with invasive coronary angiography.
Prior comparisons of an initial CTCA strategy with invasive coronary angiography have demonstrated that up to 80% of invasive angiography can be avoided in patients with a low prevalence of coronary artery disease. 69–71 We observed a more modest reduction in angiography, reflecting the greater prevalence of obstructive CHD in our study population. Moreover, many patients underwent invasive angiography and, for these participants, CTCA increased radiation and contrast exposure, albeit at low median effective radiation doses and without serious adverse reactions. Although undertaking CTCA in acutely unwell patients can be challenging, we found that over 90% of the CT coronary angiograms were of diagnostic quality, comparing favourably with prior studies of CTCA in patients with stable45,46 and acute chest pain. 18 Moreover, the CT coronary angiograms clearly identified those with and without obstructive coronary artery disease, and this is reflected in the differences in the selection of those who went on to have invasive coronary angiography and coronary revascularisation.
With the increasing recognition of the prevalence of MINOCA disease,23,24 CTCA may have an increasingly important role in the assessment of patients with acute coronary syndrome. In addition to the avoidance of unnecessary invasive coronary angiography, CTCA does provide a better assessment of overall plaque burden and adverse plaque characteristics that cannot be determined directly by invasive coronary angiography and assessments of luminal stenosis severity. 51 This may allow a more rigorous approach to the diagnosis of MINOCA disease and the provision of preventative therapies. In our trial population, only half of those undergoing invasive coronary angiography proceeded to coronary revascularisation. This suggests that there is a large and substantial population of patients with MINOCA disease who will benefit from preventative therapies. Whether or not CTCA is able to better target these therapies and will have an impact on clinical outcomes in the longer term remains to be established.
Limitations
The trial had a number of limitations. As an open trial, there is concern regarding the potential for bias. However, by design, the primary end point was adjudicated by an independent clinical end-point committee who were blinded to trial allocation. In addition, the trial intervention did not affect overall rates of clinician-directed acute coronary syndrome treatments, despite improving diagnostic certainty and reducing invasive coronary angiography use. We would also acknowledge that during trial conduct we had to compromise on accepting a larger relative effect size estimate for the trial intervention. Greater relative effect size estimates have been reported in previous trials;29,30,35 although our point estimate is very similar to a recent meta-analysis44 that indicated a HR for subsequent MI of 0.88 when CTCA is used in patients with acute chest pain. However, the lower confidence boundary of the primary end point does include a clinically meaningful reduction in events. The lack of effect on treatment interventions reinforces our view that a strategy of early CTCA is very unlikely to influence subsequent MI, and a larger trial with greater power would be unlikely to detect a more modest clinically meaningful effect on 1-year outcomes. Finally, we cannot exclude that longer-term follow-up may identify further benefits in outcomes, especially if preventative therapies are more accurately targeted.
Chapter 6 Conclusions
Overall conclusions
In conclusion, early CTCA in intermediate-risk patients presenting to the ED with suspected or provisionally diagnosed acute coronary syndrome has no effect on the overall treatment and prevention of acute coronary syndromes or 1-year outcomes, and is associated with an increased length of hospital stay and health-care costs. These findings do not support the routine use of early CTCA in intermediate-risk patients with acute chest pain.
Implications for health care
Computerised tomography coronary angiography has the capability to exclude obstructive coronary artery disease, avoiding unnecessary invasive coronary angiography. Importantly, the reduction in invasive coronary angiography seen in the RAPID-CTCA trial was not associated with differences in overall rates of coronary revascularisation or clinical events, suggesting excellent diagnostic accuracy and continued appropriate coronary revascularisation for those with obstructive coronary artery disease. Therefore, the use of CTCA in patients who have acute chest pain and troponin level elevation that is unlikely to be attributable to MI or for whom there is low risk of obstructive coronary artery disease could be considered, especially if interhospital transfer is required to facilitate invasive coronary angiography.
Recommendations for research
-
A RCT to determine whether or not CTCA can reduce long-term cardiac events in patients presenting with acute chest pain when the highly sensitive troponin level assay is of intermediate level (i.e. between the rule-out and rule-in thresholds).
-
A RCT to determine whether or not CTCA can reduce rates of invasive coronary angiography and long-term cardiac events in patients with troponin levels between the 99th centile and 3 times the upper limit of normal.
-
A RCT to determine whether or not CTCA can reduce overall length of hospital stay and rates of invasive coronary angiography in hospitals without on-site invasive angiography facilities for patients with provisionally diagnosed acute coronary syndrome.
Acknowledgements
The authors would like to thank all of the participants who gave up their time to take part in the study. We would also like to thank all of the staff who supported the study in the 37 sites, in particular the principal investigators, co-investigators, research nurses and co-ordinators. The authors acknowledge the support of the staff at ECTU, TSC, DMC, Adjudication Committee, QA team and Sheffield Emergency Care Forum PPI group in delivering the trial. The full RAPID-CTCA investigator group are listed in the following section.
The RAPID-CTCA Investigator Group
Chief investigator
Professor Alasdair Gray.
Grant co-applicants/Project Management Group
Dr Alan Fletcher (Sheffield Teaching Hospitals NHS Foundation Trust), Professor Steve Goodacre, Professor Alasdair Gray, Mr Robert Lee, Professor Steff Lewis, Dr Graham McKillop (NHS Lothian), Professor David Newby, Professor Carl Roobottom, Surgeon Captain Jason Smith, Professor Robert Storey and Dr Praveen Thokala.
Trial manager
Ms Katherine Oatey.
Research nurse co-ordinator
Ms Rachel O’Brien.
Principal investigators
Professor Alasdair Gray, Professor Steve Goodacre, Professor Carl Roobottom, Dr Jason Smith, Dr Dirk Feldman, Dr Andrew Kinnon, Dr Ajay Yerramasu, Dr Robert Huggett, Dr Liza Keating, Dr Sudantha Bulugahapitiya, Dr Jehangir Din, Dr Andrew Mitchell, Dr Anne Scott, Dr Anna Beattie, Dr Khalid Alfakih, Dr Adrian Brady, Dr Atilla Kardos, Dr Hefin Jones, Dr Derek Connolly, Dr Ronak Rajani, Dr Rangasamy Muthusamy, Dr Simon Smith, Dr Abdel-Rahman Saif-El-Dean, Dr Ben Holloway, Dr Ansuman Saha, Professor Nick Curzen, Dr Matthias Schmitt, Dr Christopher Travill, Dr Ceri Davies, Professor Tim Harris, Dr Will Roberts, Dr Patrick Donnelly, Dr Justin Carter, Dr John Irving, Dr Chris Vorwerk, Dr Ash Basu, Dr Jason Dungu, Dr Elisa McAlindon, Dr Sandeep Hothi, Dr David Rosewarne, Dr Arivalagan Bapusamy, Dr Jonathan Watt and Dr Claire McGroarty.
Trial research team
Royal Infirmary of Edinburgh: Polly Black, Caroline Blackstock, Julia Grahamslaw, Mark Jones, Collette Keanie, Margaret MacLeod, Siobhan McLaughlin, Rachel O’Brien, Fergus Perkes, Alyson Phillips, Ewan Pirie, Janet Summerside, Gordon Truong, Kirsty Weston and Jennifer Wooton.
Sheffield Northern General Hospital: Sarah Bird, Peter Brown, Hridesh Chatha, Alan Fletcher, Catherine Hill, Shery Mofidi, Hasan Qayyum, Rob Storey, Judith Sugden and Anna Wilson.
Derriford Hospital, Plymouth: Alison Jeffrey, Memory Mwadeyi, Rosalyn Squire and Peter Wafer.
Torbay Hospital: Dr Lesley Archer, Lisa Felmeden, Dr Guy Gribbin, Dr Sarah Harrison, Debbie Hughes, Dr Philip Keeling, Dr Ian Mahy, Allison Summerhayes, Justine Sutton and Dr Abdullah Yonis.
Victoria Hospital: Susan Fowler, Amanda McGregor, Karen Gray, Dr Tom Hartley, Dr David Szapiro, Lorraine Dinnel and Dennis Sandeman.
Russells Hall Hospital: Julie Dean and Amy Pugh.
Royal Berkshire NHS Foundation Trust: Parminder Bhuie, Dr James Briggs, Claire Burnett, Abby Gandy, Nicola Jacques, Sarah MacGill, Dr Archie Speirs and Niamh Tolan.
Bradford Teaching Hospitals: Craig Atkinson, Dr Mark Kon, Carita Krannila and Manitha Thomas.
Royal Bournemouth Hospital: Dr Russell Bull, Stephanie Horler, Nicki Lakeman, Jane McLeod, Sara Nix and Dr Sue Thomas.
Jersey General Hospital: Dr Daniel Ahlert, Dr Christopher Edmond, Dr Christopher Hare, Kelly Anne Kinsella, Dr Jessica Langtree, Dr James Speakman and Dr Ranjit Thomas.
Borders General Hospital: Gillian Donaldson, Fiona Hall, Terry Fairbairn and Christopher Rofe.
Royal Victoria Infirmary: Jennifer Adams-Hall, Ange Bailey, Dr Kris Bailey, Leslie Bremner, Dr Ifti Haq and Angela Phillipson.
Lewisham University Hospital: Saroj David, Osman Najam and Samia Pilgrim.
Glasgow Royal Infirmary: Claire Adams, Ammani Brown, Andrew Dougherty, Ailsa Geddes, Karen Lang, David Lowe, Ross MacDuff, Lorraine McGregor, Giles Roditi, Susan Thornton and Joyce Triscott.
Milton Keynes Hospital NHS Foundation Trust: Felicia Adjei, Antoanela Colda, Caitlin Chapman, Veronica Edgell, Michael Fell, Laszlo Halmai, Aarzoo Khan, John Northfield, Cheryl Padilla-Harris, Mike Pashler, Gill Richie, Diane Scaletta, Sarah-Beth Sunderland, Joanne Turner, Lois Vickery, Sonya Walia, Felicity Williams, Lynn Wren and Nicola Wright.
University Hospitals of the North Midlands: Holly Maguire and Resti Varquez.
Sandwell Hospitals: Julie Colley, Anthony D’Sa, Vinoda Sharma, Ashley Turner and Sylvia Willets.
Guy’s and St Thomas’ NHS Foundation Trust: Megan Bell, Dr Giulia Benedetti, Kirsty Gibson, Dr Sze Mun Mak, Dr Rebecca Preston, Amy Raynsford and Ruth Sanchez-Vidal.
Rotherham General Hospital: Susan Biggins, Kathryn Dixon, Peter Kraut, Mwada Lawan, Victoria Murray, Dr Tom Mwambingu, Rachel Walker and Carol Weston.
Leeds General Infirmary: Roo Byrom-Goulthorp, Dr Michael Darby, Dr Annette Eunice Ikongo, Johnstone, Alan Lin and Melanie McGinlay.
Queen Elizabeth Hospital Birmingham: Tania Albutt, Vicky Dawson, Claire Dowling, Karen Isaacs, Cheyanne Kaila, Dr Gareth Lewis, Nicky Mortimer, Sunitha San, Kelly Tabor and Kealy Wright (nee Tombs).
Surrey and Sussex Hospitals: Dr Riaz Ahmed, Sally Collins, Sarah Davies and Nokukhanya Ndlouu.
University Hospital Southampton NHS Foundation Trust: Ausami Abbas, Karen Banks, Julie Bigg, Alison Calver, Simon Corbett, Peter Cowburn, Zoe Duke, Andrew Flett, Sam Gough, Huon Gray, Stephen Harden, Paul Haydock, Michael Mahmoudi, Zoe Nicholas, John Paisey, Charles Peebles, Drew Rakhit, John Rawlins, Paul Roberts, Benoy Shah, James Shambrook, Iain Simpson, Rohit Sirohi, Wagas Ullah, Katharine Vedwan, James Wilkinson and Arthur Yue.
Manchester University NHS Foundation Trust: Sarra Giannopoulou, Melanie Greaves, Stephen McGlynn, Chris Miller, Akhila Muthuswamy, Lindsay Murray, Anie Nicholas and Matthias Schmitt.
Luton and Dunstable University Hospital: Susan Gent and Nafisa Hussain.
Barts Health NHS Trust Royal London Hospital: Raine Astin-Chamberlain, Ben Bloom, Olivia Bolton, Dan Martin, Lyrics Noba, Georgia Norman, Shelley Page, Helen Power, Imogen Skene, David Smith and Jon Walters.
Whipps Cross University Hospital: see Barts Health NHS Trust Royal London Hospital.
Worcestershire Acute Hospitals NHS Trust: Angela Doughty, Elaine Byng-Hollander and Dr Helen Routledge.
Ulster Hospital Belfast: Leah Hammond, Jayne Hutchinson, Stephanie Kelly, Susan Regan and Aileen Smith.
North Tees and Hartlepool NHS Trust: Julie Gray, Sarah Purvis and Pam Race.
Ninewells Hospital: Christine Almaden-Boyle, Kim Bissett, Carol Blues, Jackie Duff, Scot Dundas, Shirley Fawcett, Dr Graeme Houston, Emma Hutchison, Debbie Letham, Ann Mackintosh, Laura Meach, Laura Jayne Queripcz and Alan Webster.
Queen Alexandra Hospital: Julian Atchley, Zoe Daly and Kat Ellinor.
Betsi Cadwaladr University Health Board: Richard Cowell, Helen Craddock, Rachel Hughes, Lynda Sackett, Victoria Saul, Fiona Smith, Jane Stockport and Clare Watkins.
Basildon and Thurrock University Hospitals NHS Foundation Trust: Edward Barden, Jackie Colnet, Swamy Gedeza, Laura Hoskin, Lauren Kittridge, Gracie Maloney, Claire McCormick, Anne Nicholson, Stacey Pepper, Joanne Riches and Annaliza Sevillano.
The Royal Wolverhampton NHS Trust: Vincent Amoah, Stacey Aulton, Dr Arivalagan Bapusamy, Victoria Cottam, Stella Metherell, Sarah Milgate, Elizabeth Radford, Dr David Rosewarne and Andy Smallwood.
Raigmore Hospital: Charlotte Barr, Jonathan Broadie, David Eason, Ing-Marie Logie, Debbie McDonald, Laura O’Keeffe, Donna Patience and Lesley Patience.
Queen Elizabeth University Hospital: Dr Faheem Ahmad, Nicola Baxter, Ammani Brown, Dr John Byrne, Dr Damien Collison, Tracey Hopkins, Hayley King, Dr David Lowe, Evonne McLennan, Dr Giles Roditi, Dr David Stobo, Mark Wilson and Rosie Woodward.
Trial Steering Committee
Chairperson: Professor Tim Coats (University of Leicester).
Members: Mr Kenneth Archibald (Retired, Layperson), Mr Graham Bell (Retired, Layperson), Dr Russell Bull (The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust), Professor Alasdair Gray, Professor Gerry McCann (University of Leicester), Dr Tarun Mittal (Royal Brompton and Harefield NHS Foundation Trust), Mr Rodney Mycock (Retired, Layperson) and Dr James Rudd (University of Cambridge).
Data Monitoring Committee
Chairperson: Professor Carrol Gamble (University of Liverpool).
Members: Professor Simon Carley (Manchester University NHS Foundation Trust) and Professor Simon Padley (Chelsea and Westminster Hospital NHS Foundation Trust).
Adjudication committee
Professor Nick Mills (University of Edinburgh), Dr Andrew Chapman (University of Edinburgh) and Dr Anoop Shah (London School of Hygiene and Tropical Medicine).
Computerised tomography coronary angiography Quality Assurance Group
Dr Mark Jones and Dr Fergus Perks (NHS Lothian), and Dr Gareth Morgan-Hughes, Dr Vikram Raju, Ms Kym Luke and Professor Carl Roobottom (Plymouth Hospitals NHS Trust).
Edinburgh Clinical Trials Unit
Ms Ruth Armstrong, Mrs Julia Boyd, Mr David Buchanan, Mrs Christine Campbell, Mr Ronnie Harkess, Mr Robert Lee, Professor Steff Lewis, Ms Lynsey Milne, Ms Lumine Na, Ms Katherine Oatey, Mr Phillip Rayson, Ms Aryelly Rodriguez, Mrs Pamela Sinclair, Dr Lorraine Smith, Mrs Michelle Stevens, Mr Tony Wackett, Mr Allan Walker and Mr Christopher White.
Contributions of authors
All grant co-applicants were involved in the conceptualisation and design of the study. All report authors have contributed to the development of this report and commented on the final drafts. In addition, specific contributions are as detailed below.
Alasdair J Gray (https://orcid.org/0000-0003-1460-8327) (Consultant in Emergency Medicine, Honorary Professor of Emergency Medicine) was chief investigator and site lead for Edinburgh; conceived and planned the study; acted as study lead; led writing of the report and all aspects of study design, conduct and analysis; and designed and led the training of research staff.
Carl Roobottom (https://orcid.org/0000-0001-5066-7645) (Honorary Consultant – Radiology, Chairperson of Radiology) was co-site lead for Plymouth; provided expertise in radiology, including QA of the CTCA scans; and contributed to protocol design, staff training and supervision, and writing the report.
Jason E Smith (https://orcid.org/0000-0002-6143-0421) (Defence Professor of Emergency Medicine) was co-site lead for Plymouth; provided expertise in emergency medicine; and contributed to the protocol design, staff training and supervision, and writing the report.
Steve Goodacre (https://orcid.org/0000-0003-0803-8444) (National Institute for Health and Care Research Senior Investigator, School of Health and Related Research Director of Research) was site lead for Sheffield; provided expertise in emergency medicine; and contributed to the protocol design, staff training and supervision, and writing the report. He co-designed the health economics analysis.
Katherine Oatey (https://orcid.org/0000-0002-4667-9763) (Trial Manager) provided expertise in study management; contributed to protocol design; provided staff training and supervision; was project manager; and contributed to the writing, editing and critical reviewing of the report.
Rachel O’Brien (https://orcid.org/0000-0002-9885-8915) (Research Nurse Co-ordinator) contributed to protocol design, participated in patient recruitment, and provided staff training and supervision across all sites. She contributed to the writing of the report.
Robert F Storey (https://orcid.org/0000-0002-6677-6229) (Professor of Cardiology) provided expertise in cardiology and contributed to the protocol design and writing the report.
Nick Curzen (https://orcid.org/0000-0001-9651-7829) (Professor of Cardiology, University of Southampton) was the principal investigator for one of the top recruiting sites; provided cardiology and CTCA imaging expertise; and contributed to the writing, editing and critical review of the report.
Liza Keating (https://orcid.org/0000-0001-9028-2520) (Consultant in Emergency Medicine and Critical Care) was the principal investigator for one of the top recruiting sites; provided emergency medicine expertise; and contributed to the writing, editing and critical review of the report.
Attila Kardos (https://orcid.org/0000-0002-0231-7605) (Consultant Cardiologist, Honorary Professor of Cardiovascular Medicine) was the principal investigator for one of the top recruiting sites; provided cardiology and CTCA imaging expertise; and contributed to the writing, editing and critical review of the report.
Dirk Felmeden (https://orcid.org/0000-0003-3564-5055) (Consultant Cardiologist) was the principal investigator for one of the top recruiting sites; provided cardiology and CTCA imaging expertise; and contributed to the writing, editing and critical review of the report.
Robert J Lee (https://orcid.org/0000-0001-9379-3748) (Senior Statistician) was a trial statistician and contributed to the development of the statistical analysis plan, undertook the statistical analyses and drafted sections of the report.
Praveen Thokala (https://orcid.org/0000-0003-4122-2366) (Health Economist) provided expertise in health economics, co-designed the health economics analysis, carried out the analysis of the health economics data, and contributed to the writing of the report.
Steff C Lewis (https://orcid.org/0000-0003-1210-2314) (Professor of Medical Statistics) was the trial statistician, co-designed the protocol, led the design of the statistical analysis plan, oversaw the statistical analyses and contributed to the writing of the report.
David E Newby (https://orcid.org/0000-0001-7971-4628) (British Heart Foundation Duke of Edinburgh Chair of Cardiology, Consultant Cardiologist) provided expertise in cardiology and contributed to the protocol design and writing the report.
Publications
Gray AJ, Roobottom C, Smith JE, Goodacre S, Oatley K, O’Brien R, et al. The RAPID-CTCA trial (Rapid Assessment of Potential Ischaemic heart Disease with Computed Tomography Coronary Angiography) – a multicentre parallel-group randomised trial to compare early computerised tomography coronary angiography versus standard care in patients presenting with suspected or confirmed acute coronary syndrome: study protocol for a randomised controlled trial. Trials 2016;17:579.
Gray AJ, Roobottom C, Smith JE, Goodacre S, Oatley K, O’Brien R, et al. Early computed tomography coronary angiography in patients with suspected acute coronary syndrome: randomised controlled trial. BMJ 2021;374:n2106.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- NHS Digital . Hospital Episode Statistics, Admitted Patient Care – England 2011–12: Primary Diagnosis, 4 Characters Table 2012. www.hscic.gov.uk/catalogue/PUB08288 (accessed 5 January 2014).
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30. https://doi.org/10.1136/hrt.2003.027599.
- Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2017 Emergency Department Summary Tables n.d. www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf (accessed 7 January 2021).
- Body R, Carley S, McDowell G, Jaffe AS, France M, Cruickshank K, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332-9. https://doi.org/10.1016/j.jacc.2011.06.026.
- Body R, Carley S, McDowell G, Pemberton P, Burrows G, Cook G, et al. The Manchester Acute Coronary Syndromes (MACS) decision rule for suspected cardiac chest pain: derivation and external validation. Heart 2014;100:1462-8. https://doi.org/10.1136/heartjnl-2014-305564.
- Carlton EW, Cullen L, Than M, Gamble J, Khattab A, Greaves K. A novel diagnostic protocol to identify patients suitable for discharge after a single high-sensitivity troponin. Heart 2015;101:1041-6. https://doi.org/10.1136/heartjnl-2014-307288.
- Shah ASV, Sandoval Y, Noaman A, Sexter A, Vaswani A, Smith SW, et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ 2017;359. https://doi.org/10.1136/bmj.j4788.
- Mahler SA, Lenoir KM, Wells BJ, Burke GL, Duncan PW, Case LD, et al. Safely identifying emergency department patients with acute chest pain for early discharge: the HEART pathway accelerated diagnostic protocol. Circulation 2018;138:2456-68. https://doi.org/10.1161/CIRCULATIONAHA.118.036528.
- Collet J, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;00:1-79. https://doi.org/10.1093/eurheartj/ehaa624.
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e139-e228. https://doi.org/10.1016/j.jacc.2014.09.017.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. https://doi.org/10.1093/eurheartj/ehs184.
- Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. https://doi.org/10.1016/S0140-6736(18)31923-8.
- White HD, Thygesen K, Alpert JS, Jaffe AS. Clinical implications of the third universal definition of myocardial infarction. Heart 2014;100:424-32. https://doi.org/10.1136/heartjnl-2012-302976.
- Lee KK, Noaman A, Vaswani A, Gibbins M, Griffiths M, Chapman AR, et al. Prevalence, determinants, and clinical associations of high-sensitivity cardiac troponin in patients attending emergency departments. Am J Med 2019;132:110.e8-110.e21. https://doi.org/10.1016/j.amjmed.2018.10.002.
- Shah ASV, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. https://doi.org/10.1016/S0140-6736(15)00391-8.
- Dunham M, Challen K, Walter D. Risk stratification of patients with acute chest pain without a rise in troponin: current practice in England. Emerg Med J 2010;27:461-4. https://doi.org/10.1136/emj.2008.068163.
- Goodacre S, Cross E, Lewis C, Nicholl J, Capewell S. ESCAPE Research Team . Effectiveness and safety of chest pain assessment to prevent emergency admissions: ESCAPE cluster randomised trial. BMJ 2007;335. https://doi.org/10.1136/bmj.39325.624109.AE.
- National Institute for Health and Care Excellence (NICE) . Acute Coronary Syndromes 2020.
- Shaw LJ, Peterson ED, Kesler K, Hasselblad V, Califf RM. A metaanalysis of predischarge risk stratification after acute myocardial infarction with stress electrocardiographic, myocardial perfusion, and ventricular function imaging. Am J Cardiol 1996;78:1327-37. https://doi.org/10.1016/S0002-9149(96)00653-4.
- British Cardiology Interventional Society . Audit Returns: Adult Interventional Procedures 2012.
- Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet 2002;360:743-51. https://doi.org/10.1016/S0140-6736(02)09894-X.
- Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. https://doi.org/10.1056/NEJMoa0806576.
- Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143-53. https://doi.org/10.1093/eurheartj/ehw149.
- Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): the past, present, and future management. Circulation 2017;135:1490-3. https://doi.org/10.1161/CIRCULATIONAHA.117.027666.
- Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12170.
- Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J 2008;29:531-56. https://doi.org/10.1093/eurheartj/ehm544.
- Goodacre S, Thokala P, Carroll C, Stevens JW, Leaviss J, Al Khalaf M, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17010.
- Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 2007;49:863-71. https://doi.org/10.1016/j.jacc.2006.08.064.
- Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011;58:1414-22. https://doi.org/10.1016/j.jacc.2011.03.068.
- Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. https://doi.org/10.1056/NEJMoa1201161.
- Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393-403. https://doi.org/10.1056/NEJMoa1201163.
- D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Omedé P, Sciuto F, Presutti DG, et al. Coronary computed tomographic angiography for detection of coronary artery disease in patients presenting to the emergency department with chest pain: a meta-analysis of randomized clinical trials. Eur Heart J Cardiovasc Imaging 2013;14:782-9. https://doi.org/10.1093/ehjci/jes287.
- Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, et al. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol 2013;61:880-92. https://doi.org/10.1016/j.jacc.2012.11.061.
- Miller AH, Pepe PE, Peshock R, Bhore R, Yancy CC, Xuan L, et al. Is coronary computed tomography angiography a resource sparing strategy in the risk stratification and evaluation of acute chest pain? Results of a randomized controlled trial. Acad Emerg Med 2011;18:458-67. https://doi.org/10.1111/j.1553-2712.2011.01066.x.
- Linde JJ, Kofoed KF, Sørgaard M, Kelbæk H, Jensen GB, Nielsen WB, et al. Cardiac computed tomography guided treatment strategy in patients with recent acute-onset chest pain: results from the randomised, controlled trial: CArdiac cT in the treatment of acute CHest pain (CATCH). Int J Cardiol 2013;168:5257-62. https://doi.org/10.1016/j.ijcard.2013.08.020.
- Hamilton-Craig C, Fifoot A, Hansen M, Pincus M, Chan J, Walters DL, et al. Diagnostic performance and cost of CT angiography versus stress ECG – a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int J Cardiol 2014;177:867-73. https://doi.org/10.1016/j.ijcard.2014.10.090.
- Levsky JM, Spevack DM, Travin MI, Menegus MA, Huang PW, Clark ET, et al. Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med 2015;163:174-83. https://doi.org/10.7326/M14-2948.
- Dedic A, Lubbers MM, Schaap J, Lammers J, Lamfers EJ, Rensing BJ, et al. Coronary CT angiography for suspected ACS in the era of high-sensitivity troponins: randomized multicenter study. J Am Coll Cardiol 2016;67:16-2. https://doi.org/10.1016/j.jacc.2015.10.045.
- Uretsky S, Argulian E, Supariwala A, Agarwal SK, El-Hayek G, Chavez P, et al. Comparative effectiveness of coronary CT angiography vs stress cardiac imaging in patients following hospital admission for chest pain work-up: the Prospective First Evaluation in Chest Pain (PERFECT) Trial. J Nucl Cardiol 2017;24:1267-78. https://doi.org/10.1007/s12350-015-0354-6.
- Linde JJ, Hove JD, Sørgaard M, Kelbæk H, Jensen GB, Kühl JT, et al. Long-term clinical impact of Coronary CT angiography in patients with recent acute-onset chest pain: the randomized controlled CATCH trial. JACC Cardiovasc Imaging 2015;8:1404-13. https://doi.org/10.1016/j.jcmg.2015.07.015.
- Gongora CA, Bavishi C, Uretsky S, Argulian E. Acute chest pain evaluation using coronary computed tomography angiography compared with standard of care: a meta-analysis of randomised clinical trials. Heart 2018;104:215-25. https://doi.org/10.1136/heartjnl-2017-311647.
- Jørgensen ME, Andersson C, Nørgaard BL, Abdulla J, Shreibati JB, Torp-Pedersen C, et al. Functional testing or coronary computed tomography angiography in patients with stable coronary artery disease. J Am Coll Cardiol 2017;69:1761-70. https://doi.org/10.1016/j.jacc.2017.01.046.
- Foy AJ, Dhruva SS, Peterson B, Mandrola JM, Morgan DJ, Redberg RF. Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: a systematic review and meta-analysis. JAMA Intern Med 2017;177:1623-31. https://doi.org/10.1001/jamainternmed.2017.4772.
- Siddiqui WJ, Rawala MS, Abid W, Zain M, Sadaf MI, Abbasi D, et al. Is physiologic stress test with imaging comparable to anatomic examination of coronary arteries by coronary computed tomography angiography to investigate coronary artery disease? – A systematic review and meta-analysis. Cureus 2020;12. https://doi.org/10.7759/cureus.6941.
- The SCOT-HEART investigators . CT coronary angiography in patients with suspected angina due to coronary artery disease (SCOT-HEART): an open-label, parallel group multicentre trial. Lancet 2015;285:2383-91. https://doi.org/10.1016/S0140-6736(15)60291-4.
- Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924-33. https://doi.org/10.1056/NEJMoa1805971.
- Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291-300. https://doi.org/10.1056/NEJMoa1415516.
- Williams MC, Hunter A, Shah ASV, Assi V, Lewis S, Smith J, et al. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol 2016;67:1759-68. https://doi.org/10.1016/j.jacc.2016.02.026.
- Adamson PD, Williams MC, Dweck MR, Mills NL, Boon NA, Daghem M, et al. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain. J Am Coll Cardiol 2019;74:2058-70. https://doi.org/10.1016/j.jacc.2019.07.085.
- National Institute for Health and Care Excellence (NICE) . Recent-Onset Chest Pain of Suspected Cardiac Origin: Assessment and Diagnosis 2010. www.nice.org.uk/guidance/cg95 (accessed 7 January 2021).
- Smulders MW, Kietselaer BLJH, Wildberger JE, Dagnelie PC, Brunner-La Rocca HP, Mingels AMA, et al. Initial imaging-guided strategy versus routine care in patients with non-ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019;74:2466-77. https://doi.org/10.1016/j.jacc.2019.09.027.
- Williams MC, Kwiecinski J, Doris M, . Low-attenuation non-calcified plaque on coronary CT angiography predicts myocardial infarction. Circulation 2020;141:1452-62. https://doi.org/10.1161/CIRCULATIONAHA.119.044720.
- Gray AJ, Roobottom C, Smith JE, Goodacre S, Oatey K, O’Brien R, et al. The RAPID-CTCA trial (Rapid Assessment of Potential Ischaemic Heart Disease with CTCA) – a multicentre parallel-group randomised trial to compare early computerised tomography coronary angiography versus standard care in patients presenting with suspected or confirmed acute coronary syndrome: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1717-2.
- American Association of Physicists in Medicine . The Measurement, Reporting, and Management of Radiation Dose in CT 2008.
- Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342-58. https://doi.org/10.1016/j.jcct.2014.07.003.
- Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5-40. https://doi.org/10.1161/01.cir.51.4.5.
- ISRCTN Registry . Rapid Assessment of Potential Ischaemic Heart Disease With Computed Tomography Coronary Angiography (CTCA) n.d. https://doi.org/10.1186/ISRCTN19102565 (accessed 9 November 2021).
- Mills NL, Churchhouse AM, Lee KK, Anand A, Gamble D, Shah AS, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210-16. https://doi.org/10.1001/jama.2011.338.
- Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333. https://doi.org/10.1136/bmj.38985.646481.55.
- The European Agency for the Evaluation of Medicinal Products . Evaluation of Medicines for Human Use 2002. www.ema.europa.eu/en/documents/scientific-guideline/points-consider-multiplicity-issues-clinical-trials_en.pdf (accessed 12 January 2021).
- Gray AJ, Roobottom C, Smith JE, Goodacre S, Oatey K, O’Brien R, et al. Early computed tomography coronary angiography in patients with suspected acute coronary syndrome: randomised controlled trial. BMJ 2021;374. https://doi.org/10.1136/bmj.n2106.
- Thokala P, Goodacre SW, Collinson PO, Stevens JW, Mills NL, Newby DE, et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart 2012;98:1498-503. https://doi.org/10.1136/heartjnl-2012-302188.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018 2019 2019.
- Department of Health and Social Care (DHSC) . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2011. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed September 2020).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Process and Methods [PMG9] . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9 (accessed September 2020).
- Joint Formulary Committee . British National Formulary (online) n.d. https://bnf.nice.org.uk (accessed September 2020).
- Linde JJ, Kelbæk H, Hansen TF, Sigvardsen PE, Torp-Pedersen C, Bech J, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2020;75:453-63. https://doi.org/10.1016/j.jacc.2019.12.012.
- Dewey M, Rief M, Martus P, Kendziora B, Feger S, Dreger H, et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ 2016;355. https://doi.org/10.1136/bmj.i5441.
- Chang HJ, Lin FY, Gebow D, An HY, Andreini D, Bathina R, et al. Selective referral using coronary computed tomography angiography versus direct referral for individuals referred to invasive coronary angiography for suspected coronary artery disease. JACC Cardiovasc Imaging 2019;12:1303-12. https://doi.org/10.1016/j.jcmg.2018.09.018.
- The Royal College of Physicians, The British Society of Cardiovascular Imaging and the Royal College of Radiologist . Standards of Practice of Computed Tomography Coronary Angiography (CTCA) in Adult Patients 2014.
- Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10. https://doi.org/10.1007/s12410-017-9412-6.
- de Nigris E, Holmgren U, Aurivillius M, Jenkins M. The cost of a severe chronic obstructive pulmonary disease (COPD) exacerbation, estimated from recent triple therapy studies in patients with COPD: KRONOS and ETHOS. Am J Respir Crit Care Med 2020;201. https://doi.org/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A6290.
- Schroeder M, Shah D, Risebrough N, Martin A, Zhang S, Ndirangu K, et al. Cost-effectiveness analysis of a single-inhaler triple therapy for patients with advanced chronic obstructive pulmonary disease (COPD) using the FULFIL trial: a UK perspective. Respir Med: X 2019;1. https://doi.org/10.1016/j.yrmex.2019.100008.
- Driessen M, Shah D, Risebrough N, Baker T, Naya I, Briggs A, et al. Cost-effectiveness of umeclidinium as add-on to ICS/LABA therapy in COPD: a UK perspective. Respiratory Medicine 2018;145:130-7.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
Appendix 1 Guidance of practice of computerised tomography coronary angiography in adult patients: RAPID-CTCA trial
Introduction
This document has been provided to give guidance on CTCA protocols with regard to the RAPID-CTCA trial. These standards have been recommended by the British Society of Cardiovascular Imaging and form guidelines of standard practice. They are for reference and guidance. Patient protocols are not set out for the trial: these are left to each site. We hope the information is useful to you.
Important patient-specific information prior to the scan
When the patient attends the CT department, they should have their height and weight measured to enable their body mass index (BMI) to be calculated. BMI should be included on the report. The patient’s heart rate and rhythm should be recorded before and after beta-blockade. There are important details of the patient’s past medical history, current health and current medications that must be documented before the scan is performed.
The specific questions relating to contrast administration focus on history of allergy, recent contrast administration, use of metformin and renal function.
The questions relating to beta-blockers focus on specific contraindications. It is important to determine if the patient is asthmatic because beta-blockers should be administered with caution in asthmatic patients and should not be used in patients with severe asthma. Beta-blockers should not be administered in patients taking verapamil or in patients with severe aortic stenosis or second- or third-degree heart block.
Glyceryl trinitrate is usually well tolerated, although it should not be used in patients taking sildenafil (Viagra) or other such phosphodiesterase inhibitors because of the risk of profound hypotension.
Drug administration in preparation for computerised tomography coronary angiography
Heart rate control
Heart rate reduction to < 65 b.p.m. should be achieved in all patients in whom it is safe and practicable to do so to ensure absence of motion artefacts on derived images. The development of high-specification CT scanners (e.g. dual source, > 64-slice scanners) means that the likelihood of diagnostic image quality is higher than for 64-slice CT when heart rate control is not achieved, but image quality is improved even on these high-specification CT scanners when the heart rate is < 65 b.p.m.
Beta-blockers
Beta-blockers should be used as the first-line drugs for lowering heart rate prior to CTCA. They can also have the beneficial effect of reducing ectopic activity and heart rate variability. Beta-blockers should not be administered to patients already taking verapamil owing to the risk of ventricular standstill and cardiac arrest. Metoprolol is the most commonly used beta-blocker in this context. It can be administered intravenously or orally.
Intravenous dosing in computerised tomography coronary angiography
Metoprolol can be administered intravenously with the patient on the scanner table. This method is now first line in many UK centres and has the advantage of heart rate control being achieved quickly. A typical dose regime is as follows: initial dose of 5 mg given intravenously over 1 minute followed by a saline flush, with further administration of the same dose every 2–3 minutes until the heart rate is < 65 b.p.m. The maximum recommended intravenous dose of metoprolol quoted in the British National Formulary is 15 mg, although doses up to 20 mg, 25 mg and 30 mg have been quoted in the literature. Some UK centres titrate up to 50 mg without reported adverse events, although there are currently no published data to support this.
Treatment of symptomatic bradycardia/hypotension
Patients who suffer symptomatic bradycardia and/or hypotension should be treated with atropine and/or glucagon, as detailed in Appendix 5. Intravenous fluids may be used for patients with symptomatic hypotension in the absence of bradycardia.
Coronary artery vasodilation with glyceryl trinitrate
One or two puffs of sublingual glyceryl trinitrate (GTN) (400–800 μg) are frequently administered just prior to the scan. Although there may be some secondary reflex tachycardia, this does not seem to be a problem in practice. Headache is the most common side effect (≈ 10%), and patients should be specifically warned about this and possible dizziness prior to administration. GTN should be used with caution in patients with a systolic blood pressure of < 90 mmHg. Concomitant use with phosphodiesterase inhibitors, such as sildenafil, can result in significant hypotension.
Record keeping of drug administration
It is crucial to keep an accurate record of the drugs administered during CTCA within the report.
Iodine-based contrast safety
Contrast medium administration should be performed in accordance with the Royal College of Radiologists guidance on ‘Standards for Intravascular Contrast Agent Administration to Adult Patients’ (www.rcr.ac.uk/docs/radiology/pdf/BFCR(10)4_Stand_contrast.pdf).
Computerised tomography scanner technical requirements
General considerations
Minimum requirements:
-
A maintenance, servicing and repair programme should be in place for all scanners within a department.
-
A QA programme should be in place, supervised by appropriately trained radiographers and medical physicists.
Scanner capabilities
Minimum requirements:
-
ECG gating with prospective and retrospective gating capability is required.
-
Dose modulation should be available for retrospectively gated scanning to reduce the radiation dose to the patient.
-
Pre-scan contrast timing assessment, either by automated or by visual bolus tracking or with assessment of a test bolus, must be possible.
Hardware specification
Minimum requirements:
-
A 64-slice detector (or above) multidetector CT is required. The non-diagnostic rate of scanners with fewer detector rows is 10% greater than those with 64-slice detectors.
-
Detector width should be ≤ 0.625 mm.
-
Gantry rotation time should be < 350 milliseconds.
-
z-axis coverage of at least 20 mm and preferably 32 mm is required to ensure a realistic scan time (and therefore breath hold) and minimise misalignment artefact.
-
Temporal resolution should be < 175 milliseconds for a single sector.
Scanning modes
Electrocardiographic gating is essential for coronary artery imaging. To minimise vessel motion, points of relative cardiac rest are selected for image acquisition, usually at mid-diastole or at end-systole for slightly higher or variable heart rates. Previously, electrocardiographic gating has been either retrospective (helical) or prospective (axial), but the advent of wide detector arrays and dual-source scanners has blurred these traditional boundaries.
Retrospectively gated image acquisition occurs with the X-ray tube continuously on, over a number of cardiac cycles, before data at the required points of the R–R interval are retrospectively referenced to the patient’s ECG. The potentially high radiation dose with this method can be minimised with dose modulation; this is where the radiation dose is reduced significantly in parts of the cardiac cycle at which there is likely to be significant cardiac motion, such as during systole, and increased to diagnostic levels during mid to late diastole.
Prospectively gated image acquisition takes place at only the required phase of the cardiac cycle, which is anticipated by the scanner based on the patient’s ECG. This ‘step and shoot’ acquisition generates axial slices during sequential cycles, which are then combined to create the final volume. Scanners with wide detector arrays (up to 16 cm in the z axis) have the potential to image the entire heart prospectively in a single heart beat; this enables scanning of patients with arrhythmias (including atrial fibrillation) with no misregistration artefacts.
Dual-source scanners are also capable of prospective helical acquisition, using both X-ray sources to allow a high-pitch acquisition. Current systems are limited in this approach because a heart rate of < 60 b.p.m. is required to provide a sufficiently long period of diastasis. In addition, the second X-ray source is generally less powerful and has a smaller field of view.
Prospective gating
Minimum technical requirements:
-
Prospective gating, which permits a considerable reduction in radiation dose without reduction in image quality, should be used routinely unless there is a clear indication otherwise.
-
Where heart rate exceeds the recommended threshold for standard prospective gating, additional tube-on time may be utilised, a technique known as ‘padding’. This allows a greater proportion of the cardiac cycle to be imaged and, although this means a higher radiation dose than with standard prospective gating, this should be used in preference to retrospective gating where possible.
Retrospective gating
-
Retrospective gating should be reserved for use as a problem-solving tool, for example in patients with very high heart rates, or rarely where full-cycle acquisition is required, such as functional or heart valve assessment. This is less sensitive to alterations in rhythm than prospective gating. 6
-
Except where there is significant heart rate variability (such as atrial fibrillation), dose modulation techniques should be adopted, using the narrowest selectable window of diagnostic tube current. 7
-
The pitch should not be less than 0.2 (defined as table travel per rotation divided by the collimation of the X-ray beam).
-
Adaptive technologies should be utilised to permit the deletion of data from premature ventricular beats, the insertion of undetected R peaks, and the shifting of R peaks to adjust for arrhythmia, during retrospective gating. 7
Novel gating options
-
Prospective helical scanning may be utilised with dual-source scanners where patient heart rate is ≤ 60 b.p.m. and patients meet the limitations of the field of view.
Image quality and radiation dose optimisation
The objective is to obtain diagnostic-quality images while delivering the lowest reasonably achievable radiation dose to the patient. Image quality and radiation dose are intrinsically linked.
Setting the image quality
Temporal resolution is optimised by using a low tube rotation time. Dual-source scanners have intrinsically better temporal resolution than single-source scanners without any impact on patient dose. Multiphase reconstructions incur a dose penalty over single cardiac phase reconstructions because they require projection data to be acquired over a longer part of the cardiac cycle. Multisegment reconstructions incur a dose penalty over single-segment reconstructions because they require projection data to be acquired using retrospective ECG gating, whereas single-segment reconstructions can be generated from data using prospectively gated acquisitions, which are generally more dose efficient.
Spatial resolution is determined by the reconstruction kernel and the slice thickness. Both in turn affect image noise and, therefore, radiation dose.
Contrast resolution is determined by the iodine concentration in the coronary arteries and the tube voltage setting. The sensitivity to iodine is higher at lower tube voltages, so 80 kVp or 100 kVp is advantageous for coronary CTCA. If the tube voltage is reduced without changing the mAs value, the dose to the patient will drop but image noise will increase. Lower-energy X-ray beams may fail to penetrate through the anatomy of medium and large patients, leading to streak artefacts. The optimum choice of tube voltage is, therefore, a compromise dependent on the size of the patient.
Setting scan protocol parameters to optimise the radiation dose
-
Where possible, prospective electrocardiographic gating should be used.
-
In exceptional cases in which retrospective electrocardiographic gating is required, dose modulation or mA pulsing techniques should be used to keep the radiation dose as low as possible.
-
The scan range and field of view should be tailored for each patient. If possible, the scan range must be set inferior to the shoulders to avoid the prescribed mAs being set for the width of the shoulders rather than the thorax. The greater the detector coverage, the harder it is to tailor the scan range to the patient’s anatomy. In retrospective ECG-gated CTCA, the irradiated length may be up to 6 cm longer than the imaged length as a result of helical over-ranging.
-
Scan protocols should be size specific. This is achievable by activating tube current modulation with patient size or setting mAs in accordance with the patient’s weight or BMI.
-
The tube voltage should be reduced to 100 kVp for small and medium patients: iodine contrast agent is more conspicuous at lower tube voltages. The mAs value can be increased modestly to compensate for the increase in image noise. (The prescribed CTDIvol should still be lower at 100 kVp than at 120 kVp.)
-
Iterative reconstruction should be used if built into the CTCA protocol.
-
The CTDIvol and DLP predicted for the scan should be used to determine the effect of dose-reduction measures before the scan takes place.
Auditing patient doses
The DLP is the standard radiation dose measure that is used in CTCA. The DLPs recorded should be included in the report.
The use of iodinated contract medium in computerised tomography coronary angiography
To obtain optimal diagnostic accuracy in CTCA, it is essential that coronary artery contrast enhancement is homogenous and constant throughout the entire scan range. In addition, the contrast enhancement should be sufficiently intense to allow visualisation of small vessels but not so intense that it causes beam-hardening artefact. The contrast medium infusion protocol for CTCA, therefore, needs to be specifically planned for each patient.
Major factors that determine the intensity and homogeneity of contrast enhancement in the coronary arteries are BMI, cardiac output, the iodine dose and the rate at which contrast medium is injected. In a given patient, the iodine density in the image can be increased by increasing the iodine concentration, the rate of injection, or both. The ideal iodine delivery rate is between 1 g/second and 2 g/second depending on the kVp used.
Although greater intracoronary attenuation during coronary CTA leads to higher diagnostic accuracy in evaluating stenosis, very high degrees of enhancement may mean that the density of contrast overlaps that of coronary artery calcium, which can obscure calcified plaques.
Contrast infusion rates typically used for CTCA are higher than for general CT (up to 7.0 ml/s). The ideal situation is for maximal contrast enhancement in the ascending aorta and the left ventricle and little or no contrast in the right side of the heart. A saline chaser is usually used after the contrast medium injection through a dual-head power injector; this can decrease streak artefacts in the superior vena cava and maximise the effect of the contrast dose by flushing contrast out of the arm veins. The saline infusion is started immediately following the contrast medium infusion and is infused at the same rate.
The length of time that this opacification is required will depend on scan coverage and the time taken to scan (which will be scanner specific), so it is vital that the contrast protocol is tailored to the clinical question. For example, imaging a patient who has had previous coronary artery bypass graft surgery means that a 25% increase in contrast volume will typically be required because the scan will need to start from the level of the internal mammary artery origin.
Intravascular contrast attenuation can be optimised by decreasing the tube voltage (to 100 kVp or 80 kVp), which increases the opacification of the blood vessels because of an increase in the X-ray absorption of iodine at lower photon energies. This will also decrease radiation dose if the tube current is kept constant. It is possible to produce equivalent image quality and contrast opacification with equivalent radiation dose compared with higher kVp techniques using lower doses of IV contrast medium. This reduction in contrast dose reduces the risk of nephrotoxicity and can allow substantial cost savings. Because lower tube voltages produce less photons (for a given mA), low-kVp scanning is not possible in very obese patients.
Contrast protocols
Providing precise recommendations on contrast regimens that cover all clinical scenarios and CT scanner types is very difficult. However, a typical example of injection flow rates for CTCA using a 64-slice CT system for coronary artery imaging is given in Table 31 (assuming a contrast concentration of 350 mgI/ml). Contrast volumes are typically lower in CT scanners that are capable of single heart beat acquisition (either wide-area detector or high-pitch dual-tube scanners) (see Table 31).
| kVp | Flow rate (ml/s) | Flow volume (ml) | Saline (ml) |
|---|---|---|---|
| 80 | 3.5 | 60 | 25 |
| 100 | 5.0 | 75 | 35 |
| 120 | 6.5 | 95 | 50 |
| 140 | 7.0 | 100 | 60 |
Template for reporting computerised tomography coronary angiography: the RAPID-CTCA trial
Patient history/clinical question
Patient characteristics:
-
heart rhythm
-
initial heart rate, amount of beta-blockade administered and acquisition heart rate and rhythm during scan
-
patient’s BMI and scan compliance (e.g. achieved breath hold).
Scan acquisition parameters
-
type of scanner (e.g. 64-slice company name and model)
-
scanning mode (e.g. retrospective), including degree of dose modulation (as percentage of cardiac cycle) and padding (in milliseconds) if relevant
-
kVP and mA used
-
total DLP.
Imaging findings: coronary
-
The results from calcium scoring – if performed – describing the distribution (localised or diffuse) and total Agatson score.
-
The quality of the CTCA data (excellent, diagnostic, moderate, non-diagnostic).
-
Describe the coronary anatomy, including dominance and presence of coronary artery anomalies.
-
Each coronary artery is separately commented on with regard to the presence and type of plaques (i.e. non-calcified, mixed or calcified).
-
Semiquantitative reporting of the degree of stenosis using the terms mild (< 50% luminal narrowing), moderate (50–75%), severe (≥ 75%) and occlusion (100%) for each coronary artery.
-
When bypass grafts are present, the type, origin and site of anastomosis, as well as the degree of stenosis, should be included.
Imaging findings: cardiac
This section is dedicated to information about other non-coronary cardiac findings, such as the morphology of the valves, aortic root, size of the pulmonary trunk and arteries, the ventricular and atrial septum, as well as the presence of myocardial disease (e.g. areas of infarction, scars or aneurysm formation).
Imaging findings: non-cardiac
Extra cardiac findings in the mediastinum, lung and chest wall, including the analysis of the images from the upper abdomen.
Conclusions
Specify conclusions and recommendations.
Further guidance
For further advice please contact:
Professor Carl Roobottom
E-mail address:
Telephone number:
Dr Graham McKillop
E-mail address:
Telephone number:
This reporting guideline closely follows guidance from The Royal College of Physicians. 72
Appendix 2 RAPID-CTCA scan report form
Appendix 3 Computerised tomography coronary angiography quality assurance working practice document form
Background
Computerised tomography coronary angiography results will usually be reported in the RAPID-CTCA trial by a trained radiologist or cardiologist at recruiting centres as soon as possible, ideally within 2 hours, and communicated immediately to the treating clinician. These reports will be written according to the Society of Cardiovascular CT guidelines; to confirm that the scanning process is consistent across the ≈ 37 UK centres participating in the trial, a proportion of these scans will be subject to dual QA reporting.
The purpose of this is to ensure that (1) reporting at recruiting centres is of suitable quality to ensure patient safety and (2) the technical quality of the CTCA scans is of suitable quality to enable accurate interpretation.
Timelines and process for quality assurance reporting
This process applies to the first 10 scans from each centre. The sites should send the scans for QA reporting as soon as possible (ideally within 7 days of the scan being completed). The full reporting process should be completed within 28 days of the scan being delivered.
Transfer process: CD/DVD
Posting the scan on a CD/DVD is the preferred method of transfer. Scans must be anonymised (using the participant number as the only identifier) and copied to a disk by the research team at the site before being sent to Plymouth (for which the address is shown below) for QA reporting.
Please ensure that the scan file (not just the disk) is labelled with the participant number so that it can be easily identified once it has been uploaded for review. To guarantee that there are no technical issues reading the disks, scans should be saved from PACs (picture archiving and communication) or the scanner as a DICOM (digital imaging and communications in medicine) file (not a picture/JPEG file) with no ‘viewer’/’reader’ software attached to the image.
The disks should not be encrypted as this can cause issues accessing and using the files, even if a password is provided. To help aid the review process, please e-mail , copying in rapid.ctca@ed.ac.uk when any scans have been transferred to Plymouth to ensure prompt identification and review. This e-mail should include the participant number and the date that the scan was carried out (note that this does not apply to the Plymouth site). The database QA section should also be updated entering the date that the scan(s) were sent (see Appendix 2) for instructions.
Scans to be posted to: Professor Carl Roobottom, FAO: Kym Luke, . Plymouth only: EMERGE Office. FAO: Rachel O’Brien, .
Transfer process: image exchange portal
After a trial period, it has been decided that the IEP method of transfer cannot be accommodated. Contact rapid.ctca@ed.ac.uk if you cannot send the disks by post.
Review process
Two trained radiologists/cardiologists (the QA reporters) will dual report each scan, and will be blinded to the original recruiting centre and clinical radiology/cardiology report. QA reporting will be completed on the same reporting form provided to the clinician reporting the scan (see Appendix 2). Once these data have been entered and saved onto the database, the QA reporters will be able to access the primary CT scan from the site to compare the reports.
If any discrepancies or concerns are identified by the QA reporters between the clinical and the research report, this will be detailed on CRF 08 ‘Quality Assurance Check of CTCA Scans’ (see Appendix 1) and further actions recommended. Where immediate clinical concerns are raised, the QA reporters should contact the chief investigator or trials office using the numbers provided on the CRF.
Where (non-urgent) feedback to the site is required, the trial manager will download and e-mail a copy of CRF 08 to the principal investigator and radiologist at the site, copying in Alasdair Gray (chief investigator) who will review and provide any further feedback. A decision as to whether or not any further action is required will be made by the Trial Management Group. Any feedback given by the site as part of this process will be recorded in the trial office section of CRF 08 on the eCRF.
The outcomes of this QA reporting process will be compiled for the review of the TSC and the Data Monitoring Committee.
Appendix 4 Quality assurance reporting form
Appendix 5 RAPID-CTCA treatment guideline
| CTCA result | Troponin level result | Trial treatment recommendation |
|---|---|---|
| Obstructive disease: stenosis ≥ 70% | Positive or negative |
|
| Moderate non-obstructive disease: stenosis 50–69% | Positive |
|
| Moderate non-obstructive disease: stenosis 50–69% | Negative |
|
| Mild non-obstructive disease: stenosis < 50% | Positive |
|
| Mild non-obstructive disease: stenosis < 50% | Negative |
|
| Normal (no evidence of coronary artery disease) |
|
Appendix 6 Summary of protocol changes during the trial duration
| Version No | Date | Changes made | Date implemented |
|---|---|---|---|
| 7 | 24 February 2020 | Updates to primary and secondary end points and outcomes. Addition of CTCA scan research repository. Updates to statistics and health economics sections to better reflect SAP and HEAP | 19 June 2020 |
| 6 | 19 July 2018 | Changes to sample size and power calculation to reflect extension request | 19 September 2018 |
| 5 | 17 October 2016 | Update to allow 24 hours, rather than 18, to randomise participants. CTCA delivery section reworded to clarify the deviation/violation reporting process when high radiation doses are identified. QA reporting: update to ‘experts independent to the trial site’. Updated to section 6 and 9.2.1 to include ‘alternative heart rate limiting medication (instead of beta-blocker)’ | 25 November 2016 |
| 4 | 23 May 2016 | ≈ 35 sites rather than 30. Primary/secondary end points clarified to better reflect proposed statistical analysis. Clarification: now deviation if participants randomised to have a CTCA do not receive the scan within 72 hours. Clarification to ambulatory scan process. CTCA radiation dose protocol deviations now recorded/reported in DLP not mSV. Update of management guidelines to include functional testing as a suggested treatment | 29 June 2016 |
| 3 | 13 November 2015 | Update to primary and secondary end points for planned statistical analysis of the study. New secondary end point ‘Clinician certainty of presenting diagnosis after CTCA’ inclusion/exclusion updates: troponin level rise now included definition: increase in high-sensitivity troponin level meeting European Society of Cardiology criteria for ‘rule-in’ or MI; invasive coronary angiography only exclusion if on day of trial eligibility assessment; beta-blocker intolerance now only exclusion if no alternative heart rate limiting agent available. Exclusions: atrial fibrillation now only exclusion if mean heart rate is anticipated to be greater than 75 b.p.m. after beta-blockade. Update to clarify the timeline in which the scan can be completed | 15 January 2016 |
| 2 | 9 December 2014 | Secondary end points clarified. Screening process clarified | At study start |
| 1 | 27 October 2014 | N/A: first draft | Never used by sites – study started on version 2 |
Appendix 7 Supplementary tables
| Site | Troponin level assay | Change in assay during trial | |||
|---|---|---|---|---|---|
| Type of assay | 99th centile (ng/l) | Date assay changed | Type of assay | 99th centile (ng/l) | |
| Royal Infirmary of Edinburgh | Hs Tn I | Male: 34 | |||
| Female: 16 | |||||
| Northern General Hospital, Sheffield | Hs Tn T | 14 | |||
| Derriford Hospital, Plymouth | Hs Tn T | 13 | 6 November 2016 | Hs Tn I | Male: 34 |
| Female: 16 | |||||
| Torbay Hospital, Torquay | Hs Tn T | 14 | |||
| Victoria Hospital, Kirkcaldy | Hs Tn T | 13 | |||
| Russells Hall Hospital, Dudley | Hs Tn T | Male: 15 | |||
| Female: 10 | |||||
| Royal Berkshire Hospital, Reading | Hs Tn T | 14 | |||
| Bradford Royal Infirmary, Bradford | Hs Tn I | 50 | |||
| Royal Bournemouth Hospital, Bournemouth | Hs Tn T | 14 | |||
| Jersey General Hospital, St Helier | Hs Tn I | 33 | |||
| Borders General Hospital, Melrose | C Tn I | 50 | |||
| Royal Victoria Hospital, Newcastle | Hs Tn T | Male: 14 | |||
| Female: 9 | |||||
| University Hospital Lewisham, London | C Tn I | 100 | 20 March 2017 | Hs Tn T | 13 |
| Glasgow Royal Infirmary, Glasgow | Hs Tn I | 33 | |||
| Milton Keynes University Hospital, Milton Keynes | C Tn I | 10 | |||
| Royal Stoke University Hospital, Stoke-on-Trent | Hs Tn I | 39 | |||
| Sandwell General Hospital, West Bromwich | Hs Tn T | 14 | |||
| St Thomas’ Hospital, London | Hs Tn T | 13 | |||
| Rotherham Hospital, Rotherham | C Tn I | 39 | |||
| Leeds General Infirmary, Leeds | Hs Tn I | 49 | |||
| Queen Elizabeth Hospital, Birmingham | Hs Tn T | 14 | |||
| East Surrey Hospital, Redhill | Hs Tn T | 14 | |||
| Southampton General Hospital, Southampton | Hs Tn I | 39 | |||
| Wythenshawe Hospital, Manchester | Hs Tn I | Male: 33 | |||
| Female: 15 | |||||
| Luton and Dunstable University Hospital, Luton | Hs Tn T | 14 | |||
| Royal London Hospital, London: laboratory assay | Hs Tn T | 14 | |||
| Royal London Hospital, London: point-of-care assay | Tn I | 23 | |||
| Whipps Cross University Hospital, London | C Tn I | 39 | |||
| Worcestershire Royal Hospital, Worcester | Hs Tn T | 20 | |||
| Ulster Hospital, Belfast | Hs Tn T | 14 | |||
| University Hospital of North Tees, Stockton-on-Tees | Hs Tn T | 39 | |||
| Ninewells Hospital, Dundee | C Tn I | 44 | |||
| Queen Alexandra Hospital, Portsmouth | Hs Tn I | 39 | |||
| Wrexham Maelor Hospital, Wrexham | C Tn I | 40 | |||
| Basildon University Hospital, Basildon | Hs Tn T | 14 | |||
| New Cross Hospital, Wolverhampton | Hs Tn I | Male: 34 | |||
| Female: 15 | |||||
| Raigmore Hospital, Inverness | C Tn I | 40 | 3 June 2017 | Hs Tn I | Male: 33 |
| Female: 15 | |||||
| Queen Elizabeth University Hospital, Glasgow | Hs Tn I | Male: 33 | |||
| Female: 15 | |||||
| Site | Trial arm, n (%) | Overall (N = 1748), n (%) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Southampton General Hospital, Southampton | 98 (11.2) | 95 (10.9) | 193 (11.0) |
| Derriford Hospital, Plymouth | 84 (9.6) | 84 (9.6) | 168 (9.6) |
| Royal Infirmary of Edinburgh | 75 (8.6) | 75 (8.6) | 150 (8.6) |
| Royal Berkshire Hospital, Reading | 62 (7.1) | 60 (6.9) | 122 (7.0) |
| Milton Keynes University Hospital | 59 (6.7) | 60 (6.9) | 119 (6.8) |
| Torbay Hospital, Torquay | 46 (5.2) | 46 (5.3) | 92 (5.3) |
| Russells Hall Hospital, Dudley | 35 (4.0) | 35 (4.0) | 70 (4.0) |
| University Hospital Lewisham, London | 35 (4.0) | 34 (3.9) | 69 (3.9) |
| University Hospital of North Tees, Stockton-on-Tees | 34 (3.9) | 33 (3.8) | 67 (3.8) |
| Royal Victoria Infirmary, Newcastle | 29 (3.3) | 31 (3.6) | 60 (3.4) |
| Queen Elizabeth University Hospital, Glasgow | 29 (3.3) | 28 (3.2) | 57 (3.3) |
| Sandwell General Hospital, West Bromwich | 26 (3.0) | 27 (3.1) | 53 (3.0) |
| Royal London Hospital, London | 24 (2.7) | 24 (2.8) | 48 (2.7) |
| Ulster Hospital, Belfast | 21 (2.4) | 22 (2.5) | 43 (2.5) |
| Leeds General Infirmary, Leeds | 21 (2.4) | 18 (2.1) | 39 (2.2) |
| Jersey General Hospital, St Helier | 19 (2.2) | 20 (2.3) | 39 (2.2) |
| St Thomas’ Hospital, London | 19 (2.2) | 20 (2.3) | 39 (2.2) |
| Wythenshawe Hospital, Manchester | 18 (2.1) | 19 (2.2) | 37 (2.1) |
| Royal Bournemouth Hospital, Bournemouth | 16 (1.8) | 16 (1.8) | 32 (1.8) |
| New Cross Hospital, Wolverhampton | 16 (1.8) | 14 (1.6) | 30 (1.7) |
| Royal Stoke University Hospital, Stoke-on-Trent | 14 (1.6) | 14 (1.6) | 28 (1.6) |
| Basildon University Hospital, Basildon | 10 (1.1) | 11 (1.3) | 21 (1.2) |
| Victoria Hospital, Kirkcaldy | 10 (1.1) | 10 (1.1) | 20 (1.1) |
| Borders General Hospital, Melrose | 10 (1.1) | 9 (1.0) | 19 (1.1) |
| Northern General Hospital, Sheffield | 10 (1.1) | 8 (0.9) | 18 (1.0) |
| Queen Alexandra Hospital, Portsmouth | 8 (0.9) | 9 (1.0) | 17 (1.0) |
| Queen Elizabeth Hospital, Birmingham | 8 (0.9) | 7 (0.8) | 15 (0.9) |
| Rotherham Hospital, Rotherham | 7 (0.8) | 6 (0.7) | 13 (0.7) |
| Whipps Cross University Hospital, London | 7 (0.8) | 6 (0.7) | 13 (0.7) |
| Bradford Royal Infirmary, Bradford | 6 (0.7) | 6 (0.7) | 12 (0.7) |
| Raigmore Hospital, Inverness | 5 (0.6) | 6 (0.7) | 11 (0.6) |
| East Surrey Hospital, Redhill | 5 (0.6) | 5 (0.6) | 10 (0.6) |
| Luton and Dunstable University Hospital, Luton | 3 (0.3) | 4 (0.5) | 7 (0.4) |
| Ninewells Hospital, Dundee | 3 (0.3) | 4 (0.5) | 7 (0.4) |
| Glasgow Royal Infirmary, Glasgow | 3 (0.3) | 2 (0.2) | 5 (0.3) |
| Wrexham Maelor Hospital, Wrexham | 1 (0.1) | 2 (0.2) | 3 (0.2) |
| Worcestershire Royal Hospital, Worcester | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Characteristic | Trial arm, n (%) | Overall (N = 1748) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Type of pain | |||
| Burning | 62 (7.1) | 65 (7.5) | 127 (7.3) |
| Stabbing | 40 (4.6) | 37 (4.3) | 77 (4.4) |
| Sharp | 98 (11.2) | 97 (11.1) | 195 (11.2) |
| Aching | 95 (10.9) | 86 (9.9) | 181 (10.4) |
| Dull | 99 (11.3) | 101 (11.6) | 200 (11.5) |
| Gripping | 44 (5.0) | 42 (4.8) | 86 (4.9) |
| Crushing | 119 (13.6) | 89 (10.2) | 208 (11.9) |
| Heavy | 107 (12.2) | 117 (13.4) | 224 (12.8) |
| Tight | 158 (18.1) | 172 (19.8) | 330 (18.9) |
| Other | 37 (4.2) | 47 (5.4) | 84 (4.8) |
| No pain | 16 (1.8) | 17 (2.0) | 33 (1.9) |
| Total | 875 | 870 | 1745 |
| Missing | 2 | 1 | 3 |
| Pattern of pain | |||
| Continuous | 525 (60.1) | 526 (60.4) | 1051 (60.3) |
| Intermittent | 313 (35.9) | 314 (36.1) | 627 (36.0) |
| Discrete | 20 (2.3) | 13 (1.5) | 33 (1.9) |
| No pain | 15 (1.7) | 18 (2.1) | 33 (1.9) |
| Total | 873 | 871 | 1744 |
| Missing | 4 | 0 | 4 |
| Location of pain | |||
| Central | 616 (70.6) | 624 (71.6) | 1240 (71.1) |
| Left | 157 (18.0) | 158 (18.1) | 315 (18.1) |
| Right | 10 (1.1) | 16 (1.8) | 26 (1.5) |
| Epigastrium | 26 (3.0) | 8 (0.9) | 34 (1.9) |
| Neck | 10 (1.1) | 10 (1.1) | 20 (1.1) |
| Jaw | 6 (0.7) | 3 (0.3) | 9 (0.5) |
| Multiple | 24 (2.7) | 29 (3.3) | 53 (3.0) |
| None | 24 (2.7) | 23 (2.6) | 47 (2.7) |
| Total | 873 | 871 | 1744 |
| Missing | 4 | 0 | 4 |
| Any radiation of pain | |||
| Yes | 540 (68.7) | 580 (72.1) | 1120 (70.4) |
| No | 246 (31.3) | 224 (27.9) | 470 (29.6) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to shoulders | |||
| Yes | 168 (21.4) | 162 (20.1) | 330 (20.8) |
| No | 618 (78.6) | 642 (79.9) | 1260 (79.2) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to left arm | |||
| Yes | 307 (39.1) | 344 (42.8) | 651 (40.9) |
| No | 479 (60.9) | 460 (57.2) | 939 (59.1) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to right arm | |||
| Yes | 109 (13.9) | 121 (15.0) | 230 (14.5) |
| No | 677 (86.1) | 683 (85.0) | 1360 (85.5) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to neck | |||
| Yes | 133 (16.9) | 156 (19.4) | 289 (18.2) |
| No | 653 (83.1) | 648 (80.6) | 1301 (81.8) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to jaw | |||
| Yes | 97 (12.3) | 102 (12.7) | 199 (12.5) |
| No | 689 (87.7) | 702 (87.3) | 1391 (87.5) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Radiation to back | |||
| Yes | 115 (14.6) | 139 (17.3) | 254 (16.0) |
| No | 671 (85.4) | 665 (82.7) | 1336 (84.0) |
| Total | 786 | 804 | 1590 |
| Missing | 91 | 67 | 158 |
| Any aggravating factors | |||
| Yes | 379 (49.4) | 376 (49.5) | 755 (49.4) |
| No | 388 (50.6) | 384 (50.5) | 772 (50.6) |
| Total | 767 | 760 | 1527 |
| Missing | 110 | 111 | 221 |
| Exertion as aggravating factor | |||
| Yes | 261 (34.0) | 266 (35.0) | 527 (34.5) |
| No | 506 (66.0) | 494 (65.0) | 1000 (65.5) |
| Total | 767 | 760 | 1527 |
| Missing | 110 | 111 | 221 |
| Position as aggravating factor | |||
| Yes | 68 (8.9) | 71 (9.3) | 139 (9.1) |
| No | 699 (91.1) | 689 (90.7) | 1388 (90.9) |
| Total | 767 | 760 | 1527 |
| Missing | 110 | 111 | 221 |
| Eating as aggravating factor | |||
| Yes | 15 (2.0) | 25 (3.3) | 40 (2.6) |
| No | 752 (98.0) | 735 (96.7) | 1487 (97.4) |
| Total | 767 | 760 | 1527 |
| Missing | 110 | 111 | 221 |
| Other aggravating factor(s) | |||
| Yes | 59 (7.7) | 57 (7.5) | 116 (7.6) |
| No | 708 (92.3) | 703 (92.5) | 1411 (92.4) |
| Total | 767 | 760 | 1527 |
| Missing | 110 | 111 | 221 |
| Any relieving factors | |||
| Yes | 573 (71.2) | 584 (72.3) | 1157 (71.7) |
| No | 232 (28.8) | 224 (27.7) | 456 (28.3) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| GTN as relieving factor | |||
| Yes | 282 (35.0) | 295 (36.5) | 577 (35.8) |
| No | 523 (65.0) | 513 (63.5) | 1036 (64.2) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| Antacid as relieving factor | |||
| Yes | 18 (2.2) | 16 (2.0) | 34 (2.1) |
| No | 787 (97.8) | 792 (98.0) | 1579 (97.9) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| Analgesia as relieving factor | |||
| Yes | 139 (17.3) | 149 (18.4) | 288 (17.9) |
| No | 666 (82.7) | 659 (81.6) | 1325 (82.1) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| Rest as relieving factor | |||
| Yes | 196 (24.3) | 200 (24.8) | 396 (24.6) |
| No | 609 (75.7) | 608 (75.2) | 1217 (75.4) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| Other relieving factor(s) | |||
| Yes | 73 (9.1) | 68 (8.4) | 141 (8.7) |
| No | 732 (90.9) | 740 (91.6) | 1472 (91.3) |
| Total | 805 | 808 | 1613 |
| Missing | 72 | 63 | 135 |
| Characteristic | Trial arm, n (%) | Overall (N = 1748) | |
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Mode of presentation | |||
| 999 | 473 (53.9) | 484 (55.6) | 957 (54.7) |
| NHS 24 | 12 (1.4) | 13 (1.5) | 25 (1.4) |
| General practitioner | 97 (11.1) | 91 (10.4) | 188 (10.8) |
| Transfer | 3 (0.3) | 7 (0.8) | 10 (0.6) |
| Self | 262 (29.9) | 259 (29.7) | 521 (29.8) |
| Other | 30 (3.4) | 17 (2.0) | 47 (2.7) |
| Total | 877 | 871 | 1748 |
| Initial area of assessment | |||
| ED | 789 (90.0) | 768 (88.2) | 1557 (89.1) |
| Observation unit | 4 (0.5) | 5 (0.6) | 9 (0.5) |
| Acute medical unit | 53 (6.0) | 53 (6.1) | 106 (6.1) |
| Cardiology | 19 (2.2) | 22 (2.5) | 41 (2.3) |
| Other | 12 (1.4) | 23 (2.6) | 35 (2.0) |
| Total | 877 | 871 | 1748 |
| Trial arm, n (%) | Overall (N = 1748) | ||
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Initial cardiac troponin elevated | |||
| Yes | 492 (56.1) | 512 (58.8) | 1004 (57.4) |
| No | 385 (43.9) | 359 (41.2) | 744 (42.6) |
| Total | 877 | 871 | 1748 |
| Initial ECG result | |||
| Normal | 328 (37.4) | 356 (40.9) | 684 (39.1) |
| T wave inversion | 220 (25.1) | 205 (23.5) | 425 (24.3) |
| ST depression | 87 (9.9) | 69 (7.9) | 156 (8.9) |
| ST elevation | 37 (4.2) | 47 (5.4) | 84 (4.8) |
| Bundle branch block | 63 (7.2) | 57 (6.5) | 120 (6.9) |
| Other abnormality | 142 (16.2) | 137 (15.7) | 279 (16.0) |
| Total | 877 | 871 | 1748 |
| Initial ECG rhythm | |||
| Sinus | 811 (93.1) | 800 (92.4) | 1611 (92.7) |
| Atrial fibrillation | 26 (3.0) | 20 (2.3) | 46 (2.6) |
| Other | 34 (3.9) | 46 (5.3) | 80 (4.6) |
| Total | 871 | 866 | 1737 |
| Missing | 6 | 5 | 11 |
| Final cardiac troponin level elevated | |||
| Yes | 521 (59.4) | 540 (62.0) | 1061 (60.7) |
| No | 356 (40.6) | 331 (38.0) | 687 (39.3) |
| Total | 877 | 871 | 1748 |
| Final ECG result | |||
| Normal | 364 (41.5) | 388 (44.5) | 752 (43.0) |
| T wave inversion | 224 (25.5) | 236 (27.1) | 460 (26.3) |
| ST depression | 61 (7.0) | 30 (3.4) | 91 (5.2) |
| ST elevation | 36 (4.1) | 45 (5.2) | 81 (4.6) |
| Bundle branch block | 59 (6.7) | 51 (5.9) | 110 (6.3) |
| Other abnormality | 133 (15.2) | 121 (13.9) | 254 (14.5) |
| Total | 877 | 871 | 1748 |
| Final ECG rhythm | |||
| Sinus | 809 (92.8) | 806 (93.1) | 1615 (92.9) |
| Atrial fibrillation | 19 (2.2) | 13 (1.5) | 32 (1.8) |
| Other | 44 (5.0) | 47 (5.4) | 91 (5.2) |
| Total | 872 | 866 | 1738 |
| Missing | 5 | 5 | 10 |
| Trial arm, n (%) | Overall (N = 1748) | ||
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Treating clinician impression at time of randomisation | |||
| Acute coronary syndrome, n (%) | |||
| Highly suspicious | 319 (36.4) | 323 (37.1) | 642 (36.7) |
| Moderately suspicious | 399 (45.5) | 393 (45.1) | 792 (45.3) |
| Slightly or not suspicious | 159 (18.1) | 155 (17.8) | 314 (18.0) |
| Total | 877 | 871 | 1748 |
| Clinical impression, n (%) | |||
| NSTEMI | 334 (38.1) | 351 (40.3) | 685 (39.2) |
| Chest pain: no clear diagnosis | 261 (29.8) | 259 (29.7) | 520 (29.7) |
| Unstable angina | 162 (18.5) | 151 (17.3) | 313 (17.9) |
| Stable angina | 54 (6.2) | 51 (5.9) | 105 (6.0) |
| Gastrointestinal pain | 18 (2.1) | 4 (0.5) | 22 (1.3) |
| Musculoskeletal pain | 7 (0.8) | 12 (1.4) | 19 (1.1) |
| Anxiety | 5 (0.6) | 6 (0.7) | 11 (0.6) |
| Oesophageal spasm | 2 (0.2) | 1 (0.1) | 3 (0.2) |
| Aortic dissection | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Pulmonary embolism | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Other | 33 (3.8) | 34 (3.9) | 67 (3.8) |
| Total | 877 | 871 | 1748 |
| Certainty they felt with their diagnosisa | |||
| Mean (SD); n | 7.1 (1.8); 877 | 7.1 (1.9); 871 | 7.1 (1.8); 1748 |
| Median (Q1, Q3) | 7 (6, 8) | 7 (6, 8) | 7 (6, 8) |
| Minimum, Maximum | 0, 10 | 1, 10 | 0, 10 |
| Trial arm, n (%) | Overall (N = 1748) | ||
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Home | 195 (22.3) | 181 (20.8) | 376 (21.5) |
| Cardiology: admit | 246 (28.1) | 226 (25.9) | 472 (27.0) |
| Medicine: admit | 195 (22.3) | 213 (24.5) | 408 (23.4) |
| Critical care | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Observation unit | 19 (2.2) | 17 (2.0) | 36 (2.1) |
| CCU | 179 (20.4) | 177 (20.3) | 356 (20.4) |
| Transfer to another hospital | 26 (3.0) | 44 (5.1) | 70 (4.0) |
| Other | 15 (1.7) | 12 (1.4) | 27 (1.5) |
| Total | 876 | 871 | 1747 |
| Missing | 1 | 0 | 1 |
| Study site | Number of patients | Trial arm, n/N (%) | Adjusted HRa (95% CI) | |
|---|---|---|---|---|
| CTCA plus standard care | Standard care alone | |||
| Southampton General Hospital | 193 | 8/98 (8.2) | 5/95 (5.3) | 1.45 (0.47 to 4.44) |
| Derriford Hospital, Plymouth | 168 | 5/84 (6.0) | 7/84 (8.3) | 0.76 (0.24 to 2.39) |
| Royal Infirmary of Edinburgh | 150 | 8/75 (10.7) | 9/75 (12.0) | 0.96 (0.37 to 2.50) |
| Royal Berkshire Hospital, Reading | 122 | 1/62 (1.6) | 3/60 (5.0) | 0.28 (0.03 to 2.72) |
| Milton Keynes University Hospital | 119 | 4/59 (6.8) | 4/60 (6.7) | 0.93 (0.23 to 3.74) |
| Trial arm, n (%) | Overall (N = 1748), n (%) | ||
|---|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | ||
| Patient died before questionnaire due | 4 (0.5) | 3 (0.3) | 7 (0.4) |
| Follow-up discontinued before questionnaire due | 1 (0.1) | 2 (0.2) | 3 (0.2) |
| Non-responder | 178 (20.3) | 238 (27.3) | 416 (23.8) |
| Respondera | 694 (79.1) | 628 (72.1) | 1322 (75.6) |
| Investigation | Trial arm, n (%) | |
|---|---|---|
| CTCA plus standard care (N = 877) | Standard care alone (N = 871) | |
| Upper gastrointestinal endoscopy | 34 (3.9) | 27 (3.1) |
| Other gastrointestinal investigations | 24 (2.7) | 28 (3.2) |
| Abdominal ultrasound | 48 (5.5) | 36 (4.1) |
| CT chest | 22 (2.5) | 20 (2.3) |
| CT aorta/CT aortogram | 6 (0.7) | 4 (0.5) |
| CT pulmonary angiogram | 19 (2.2) | 15 (1.7) |
| V/Q scan | 2 (0.2) | 4 (0.5) |
| Other respiratory investigations | 12 (1.4) | 7 (0.8) |
| Other relevant investigations | 5 (0.6) | 5 (0.6) |
Appendix 8 Supplementary figures
FIGURE 18.
Cumulative probability of CHD death or non-fatal MI.
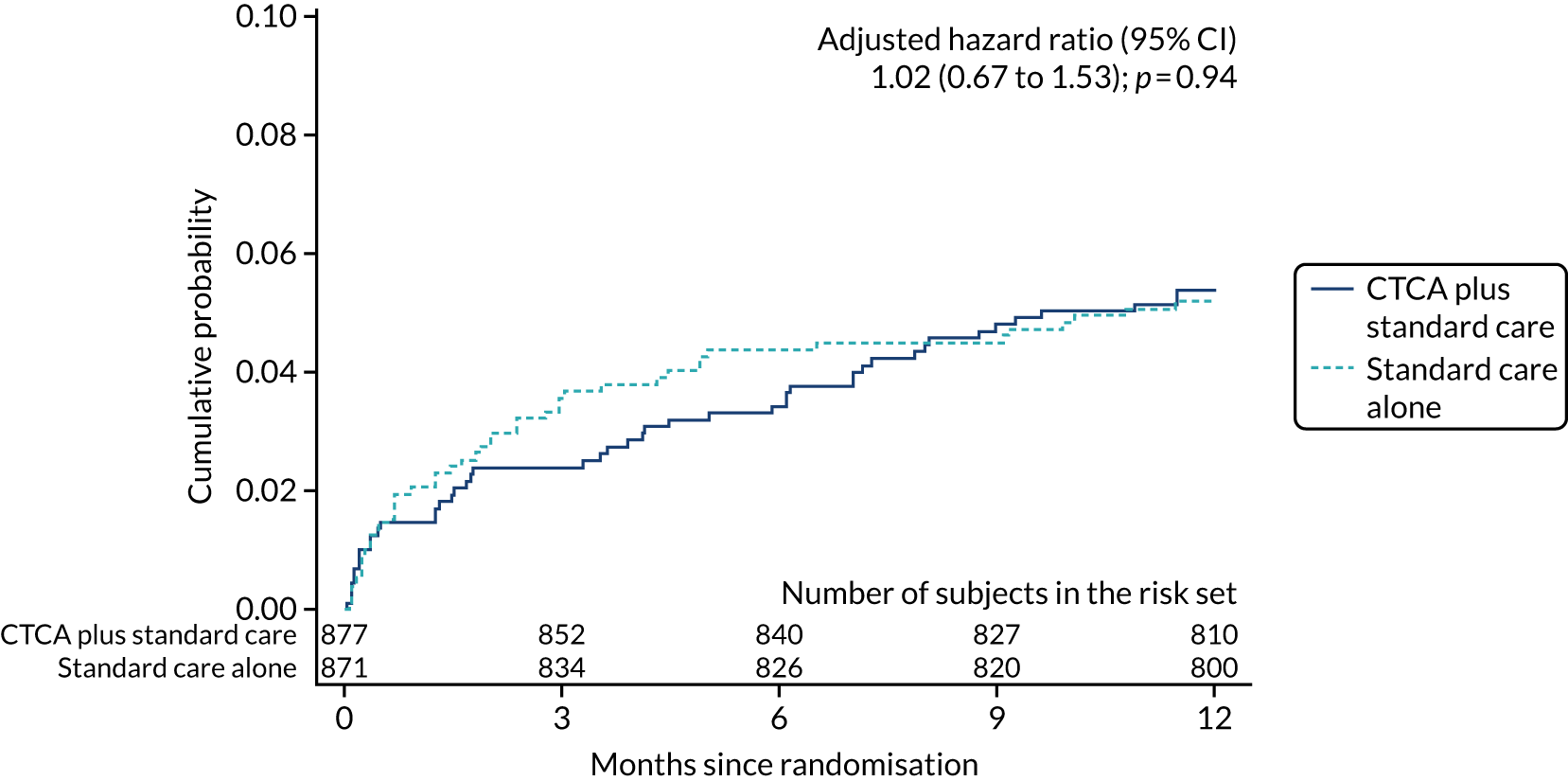
FIGURE 19.
Cumulative probability of cardiovascular disease death or non-fatal MI.
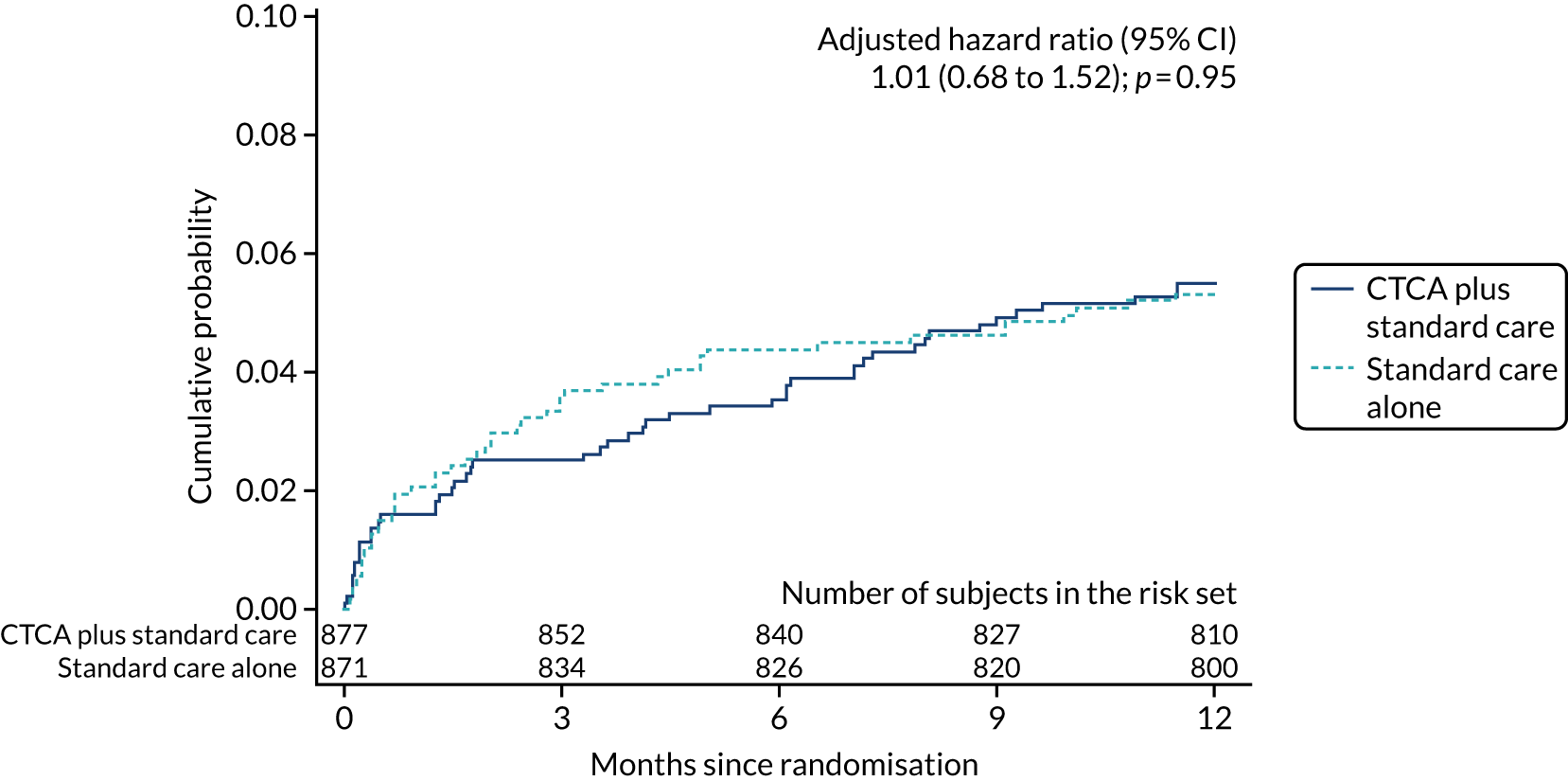
FIGURE 20.
Cumulative probability of non-fatal MI.
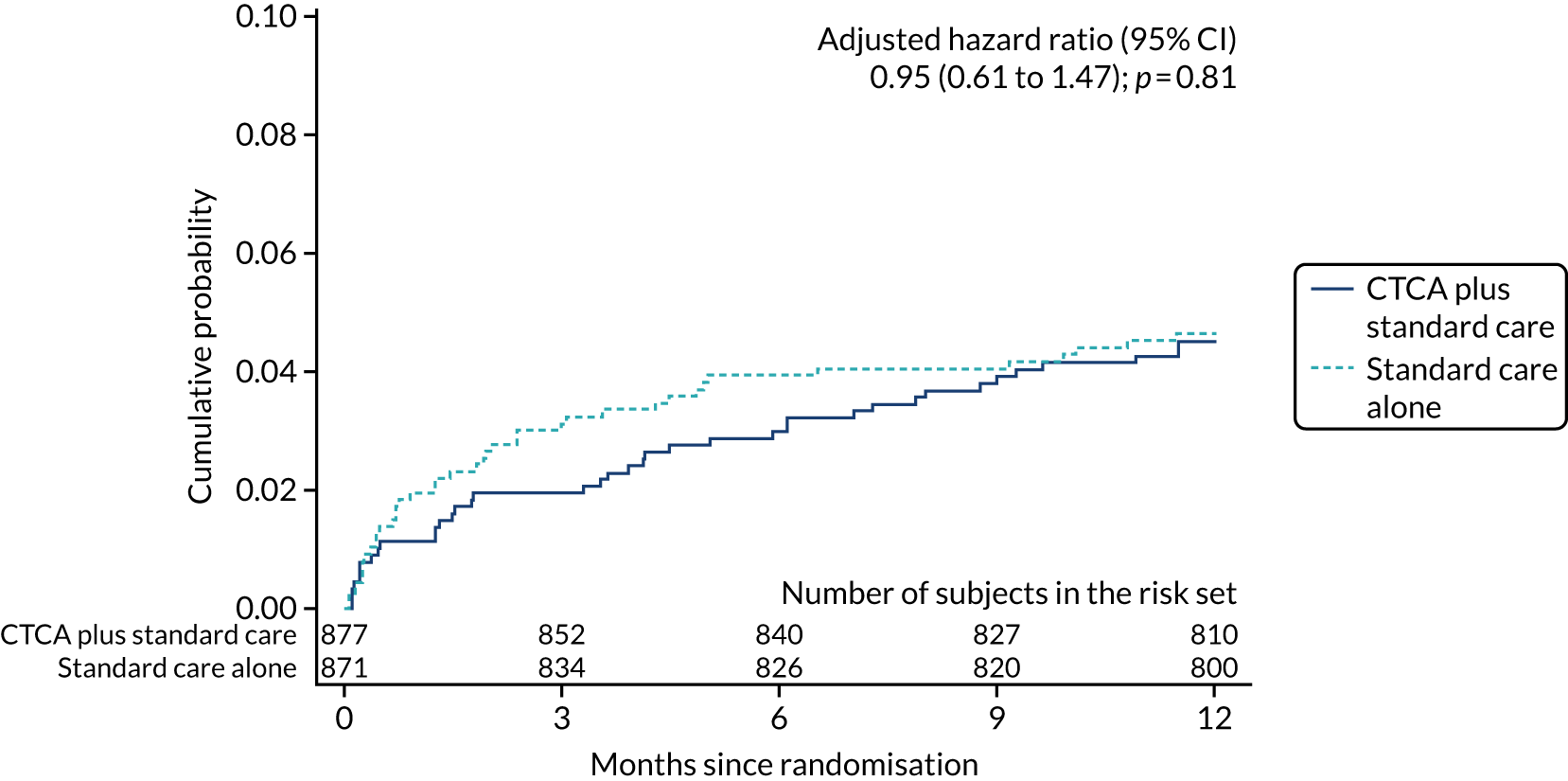
FIGURE 21.
Cumulative probability of CHD death.
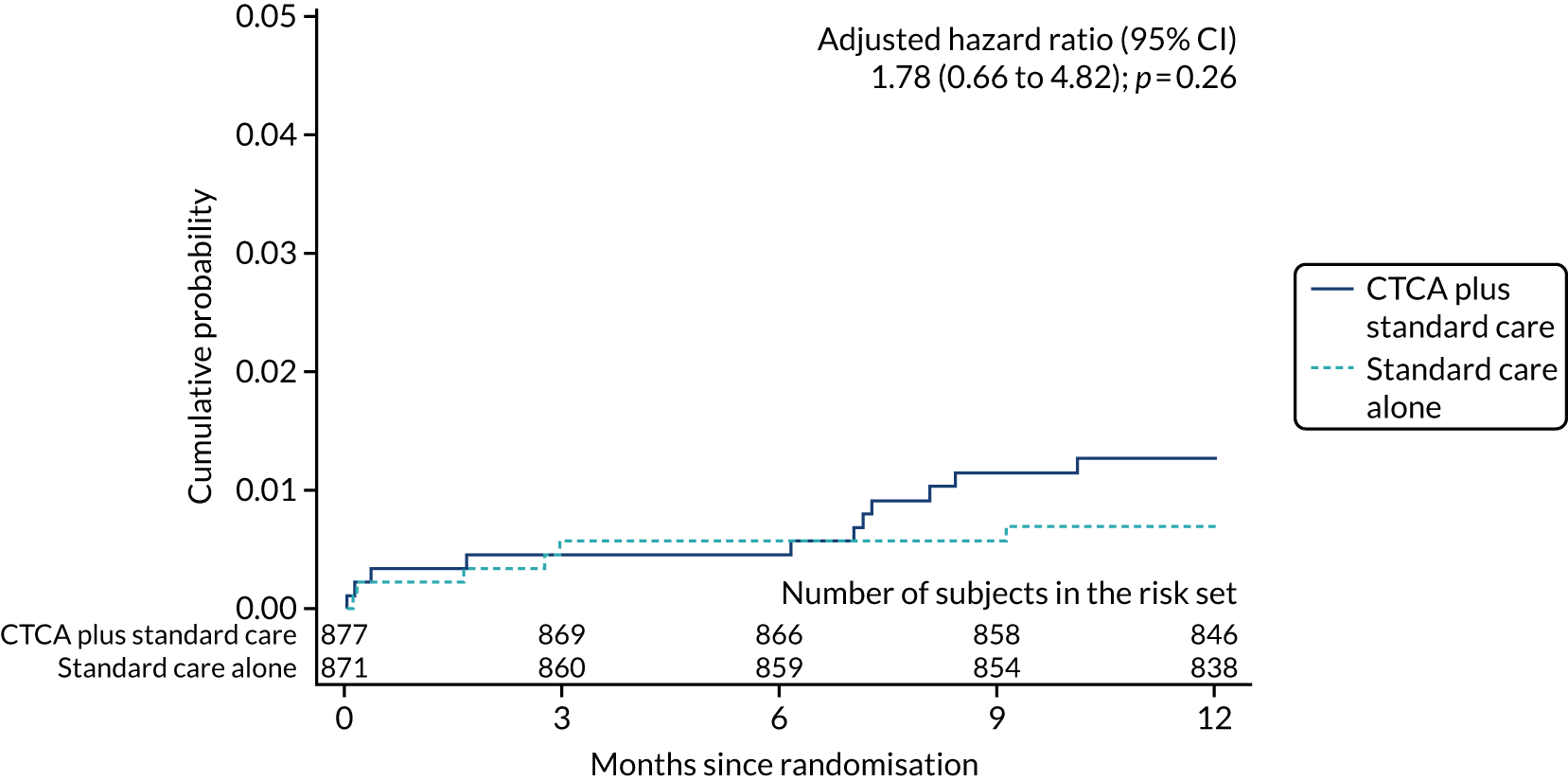
FIGURE 22.
Cumulative probability of cardiovascular disease death.
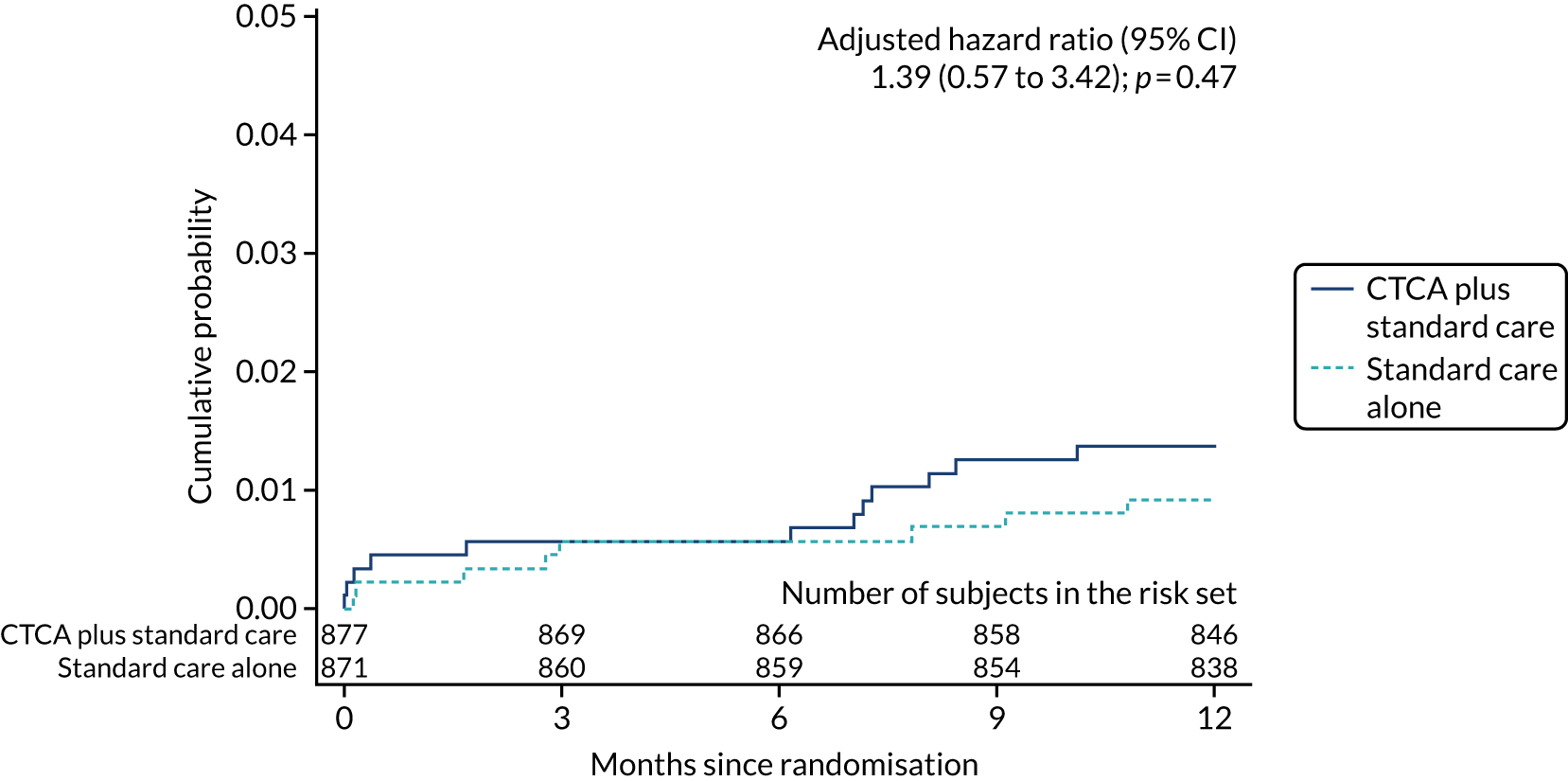
FIGURE 23.
Cumulative probability of all-cause death.
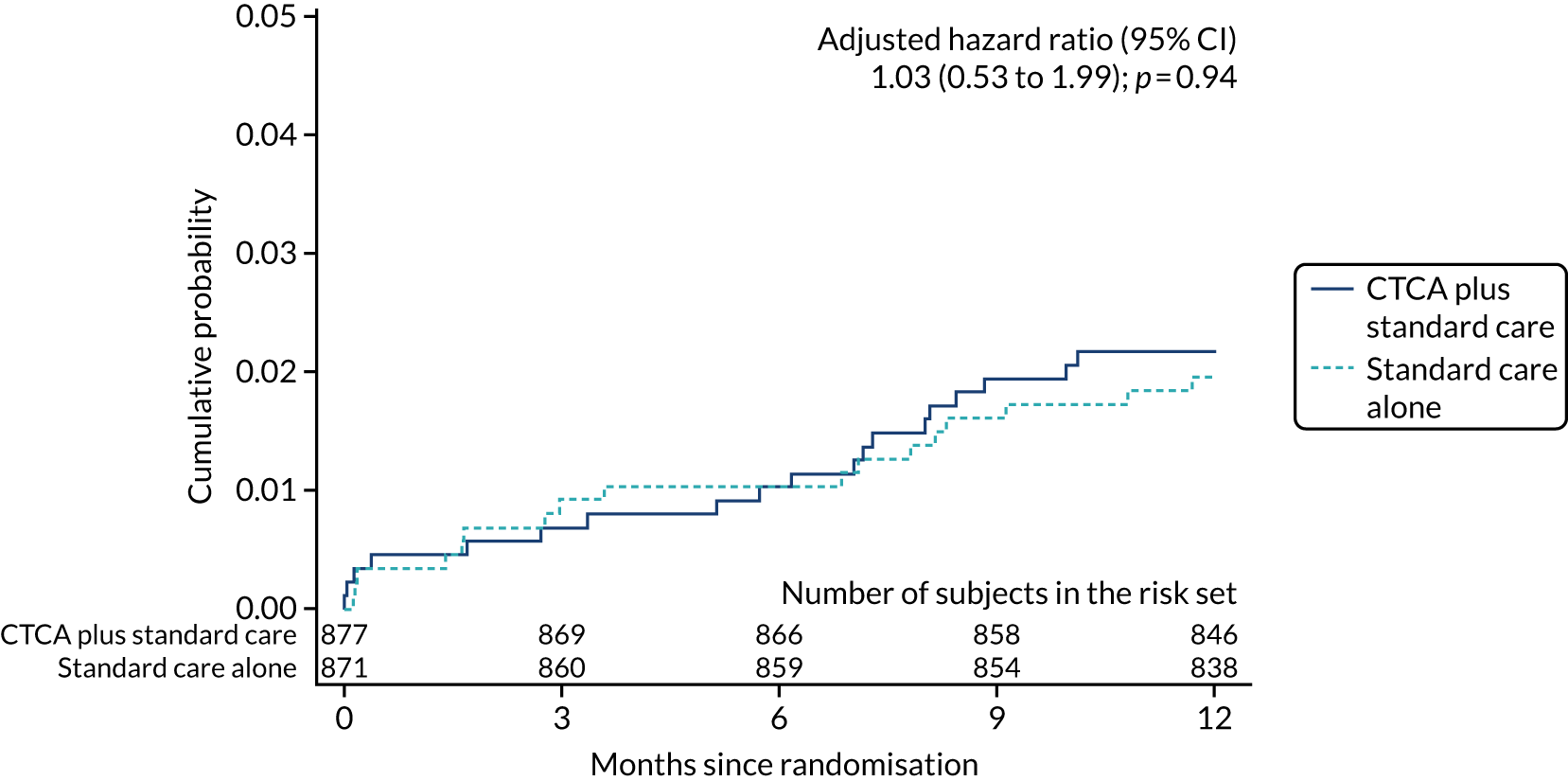
FIGURE 24.
Cumulative probability of CHD death or non-fatal MI (type 1 or 4b).
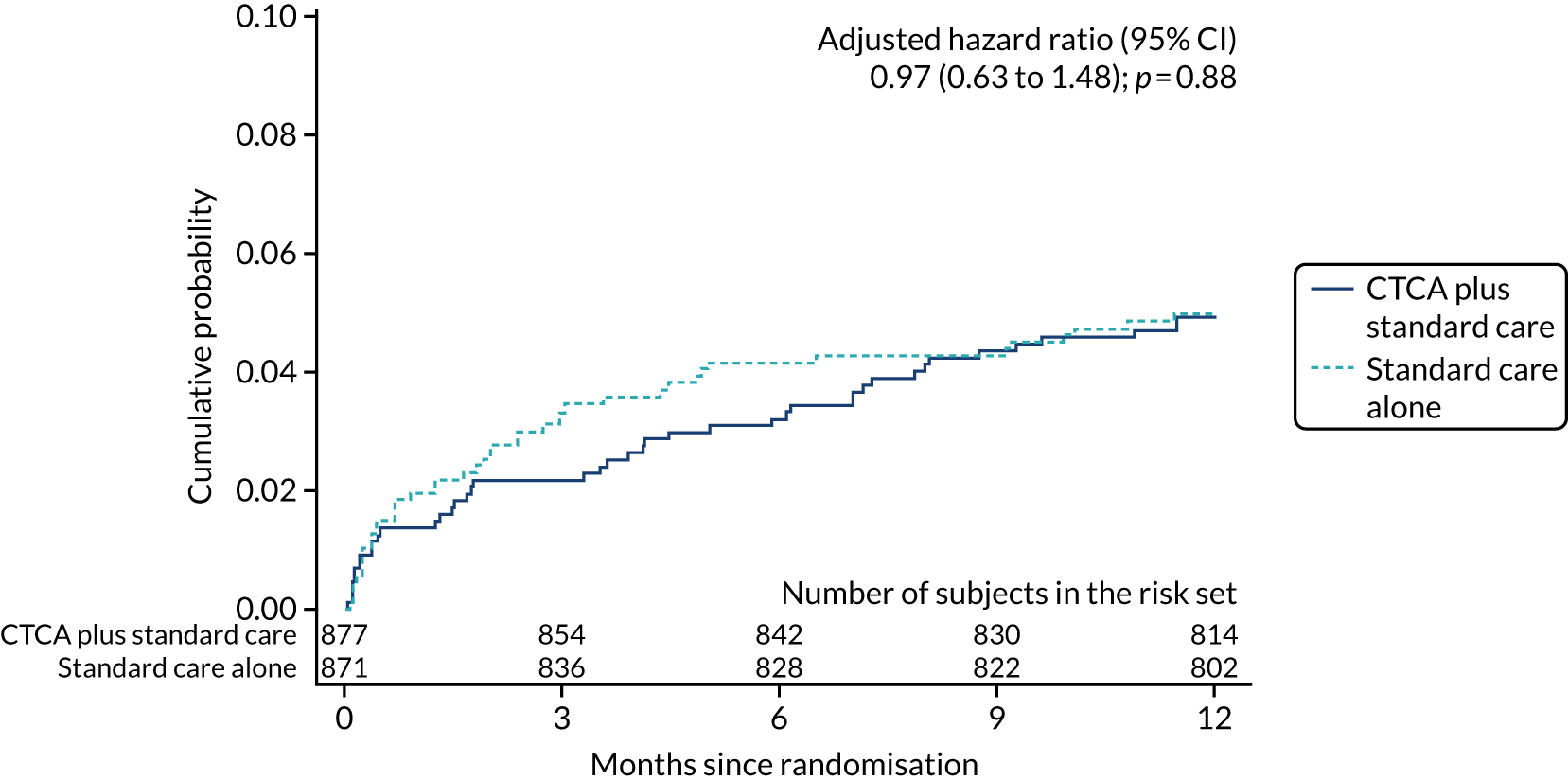
FIGURE 25.
Cumulative probability of non-fatal MI (type 1 or 4b).
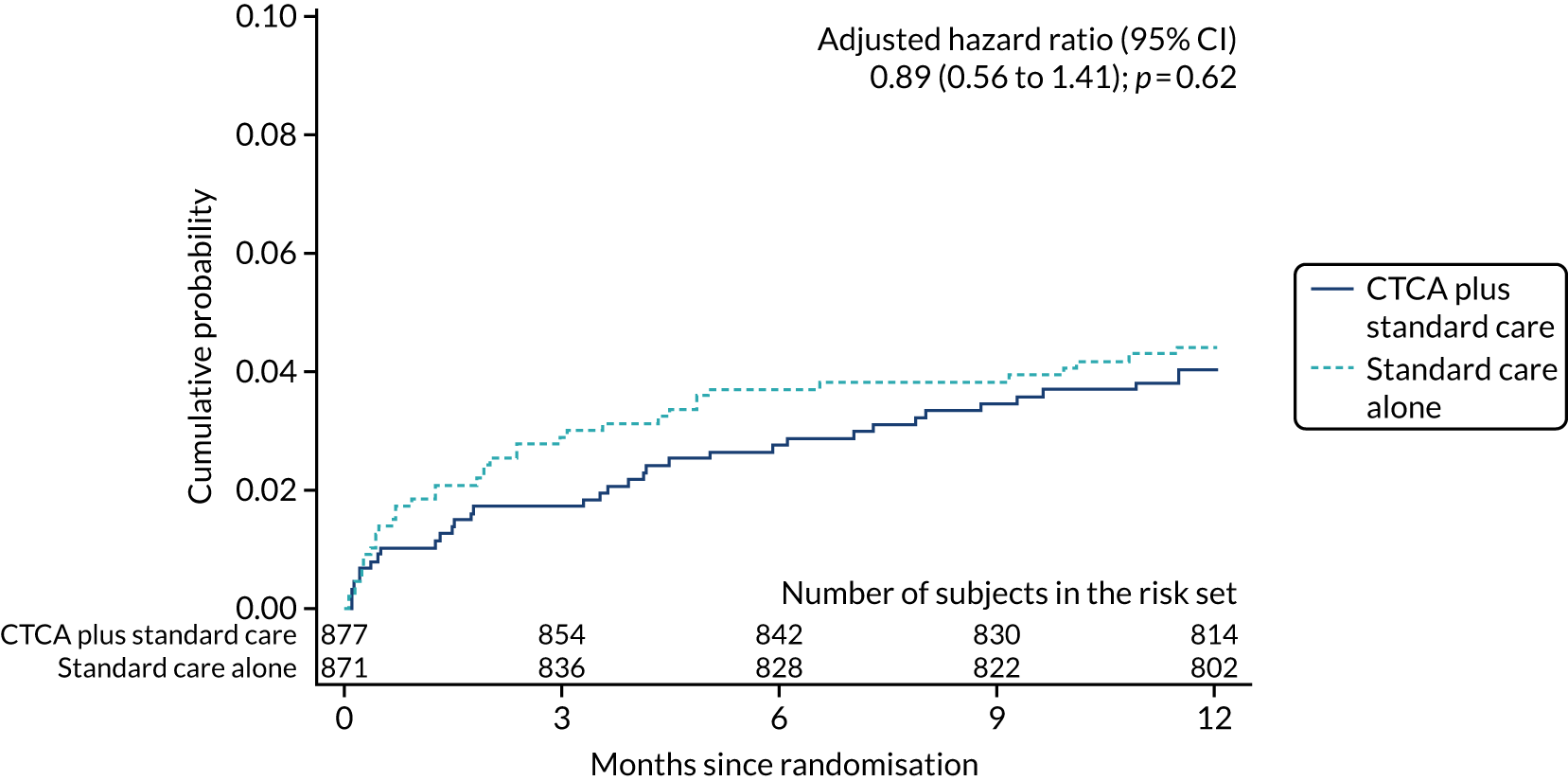
FIGURE 26.
Cumulative probability of non-cardiovascular disease death. The date of percutaneous coronary intervention was not known for one patient in the CTCA arm, and this patient was not included in estimates of cumulative probability.
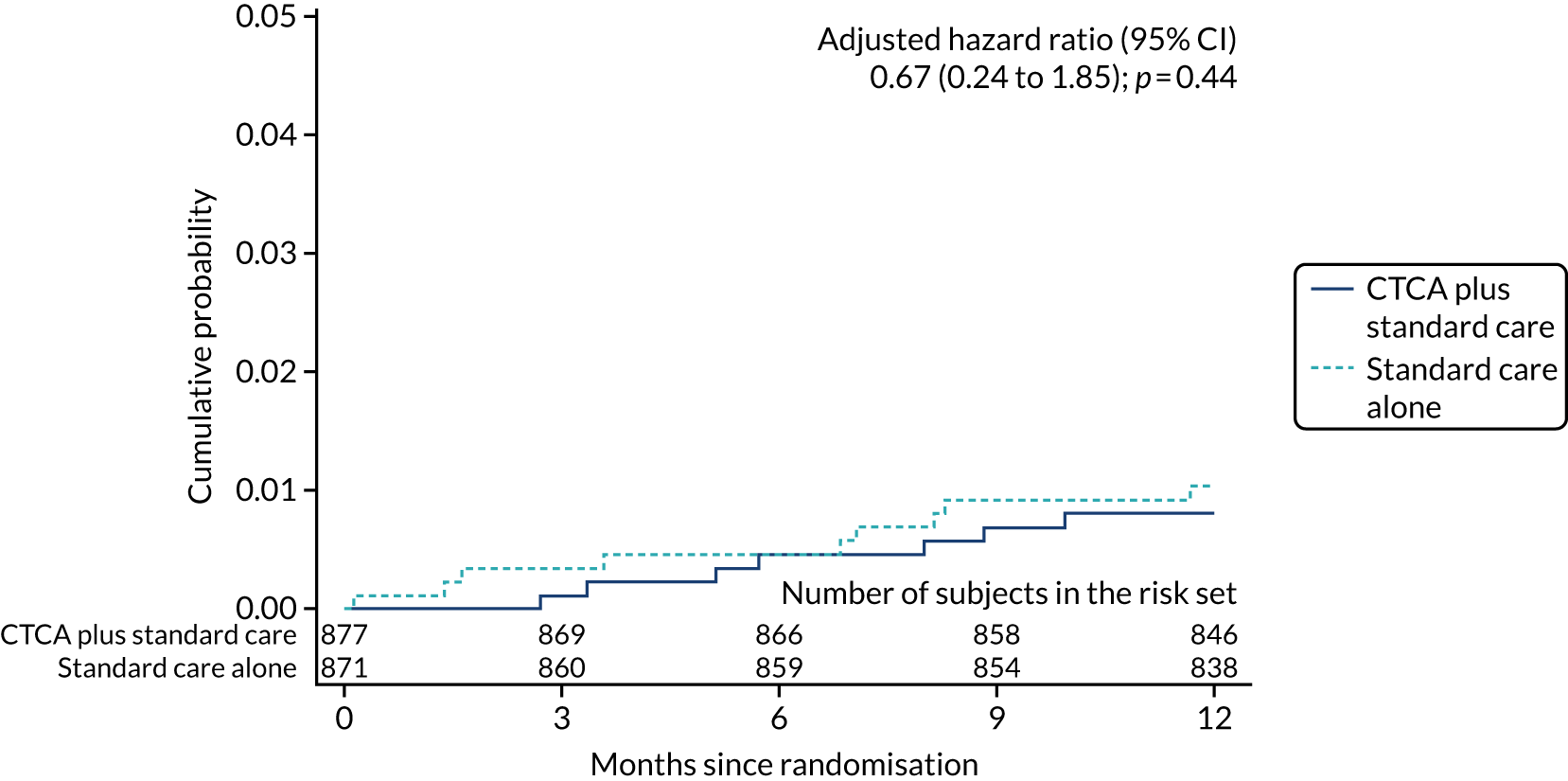
FIGURE 27.
Cumulative probability of percutaneous coronary intervention.
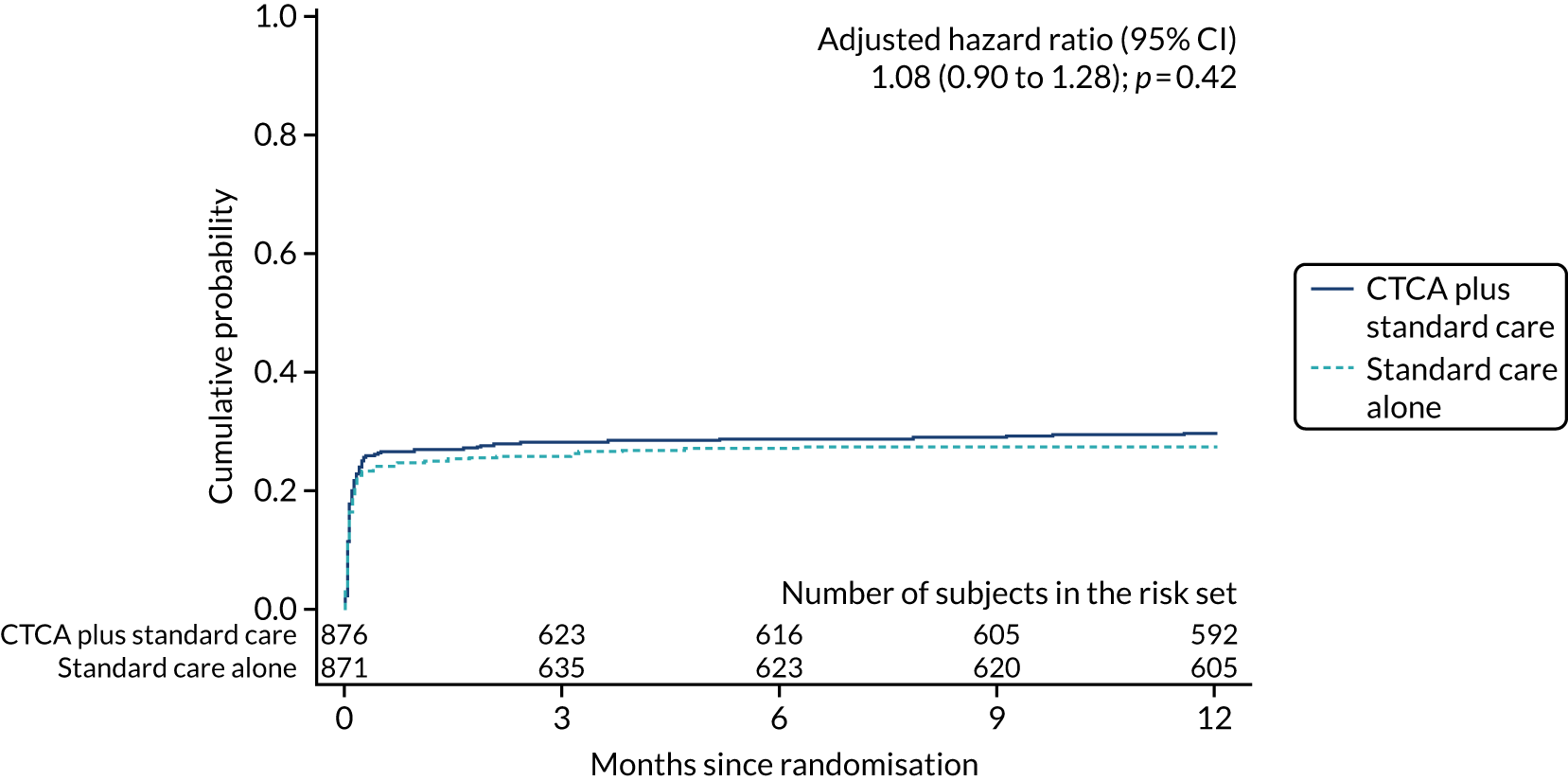
FIGURE 28.
Cumulative probability of coronary artery bypass graft surgery. The date of echocardiography was not known for one patient in the standard care alone arm and this patient is not included in estimates of cumulative probability.
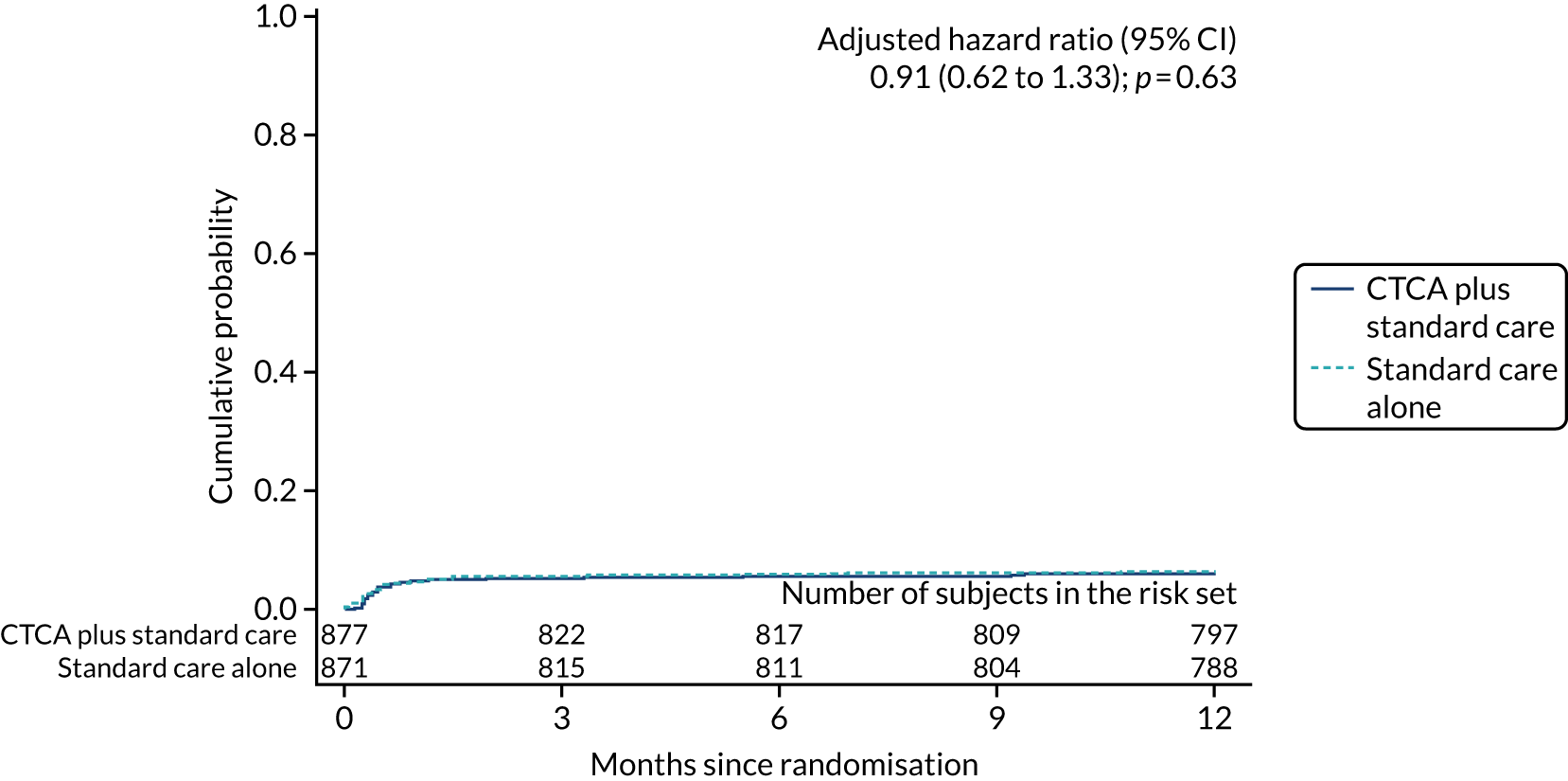
FIGURE 29.
Cumulative probability of echocardiogram.
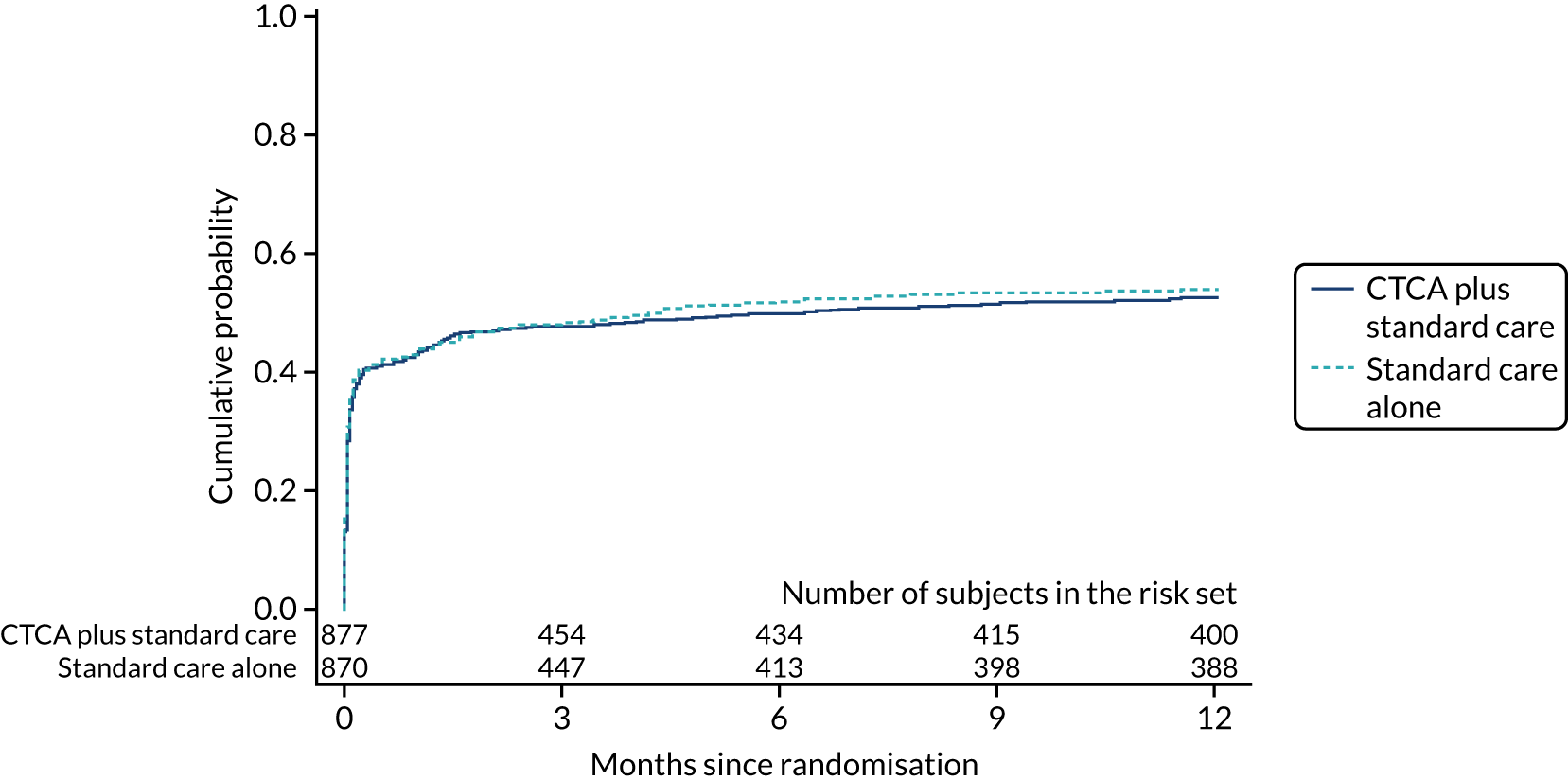
Appendix 9 Health economic analysis data dictionary
| Column name | Description |
|---|---|
| SubjectNo | Participant number |
| Randomisation_Date | Randomisation date |
| AllocatedTreatment | Allocated treatment arm (1 = CTCA + Standard Care; 2 = Standard Care alone) |
| Age | Age (years) |
| Gender | Gender (1 = Male; 2 = Female) |
| IndexStay_Days | Length of stay for index hospitalisation (calendar days) |
| FollowUp | Follow-up (1 = Follow-up completed to 12 months or death, if earlier; 2 = Follow-up discontinued prematurely) |
| LastContact_Date | Date of last contact with participant when follow-up discontinued prematurely |
| Death | Participant died within 12 months of randomisation (1 = Yes, 2 = No) |
| Death_Date | Date of death |
| Death_Index | Participant died during index hospitalisation (1 = Yes, 2 = No) |
| AdjudicatedMI1_4b | Non-fatal type 1 or type 4b MI within 12 months of randomisation (1 = Yes, 2 = No) |
| AdjudicatedMI1_4b_Date | Date of non-fatal type 1 or type 4b MI |
| AdjudicatedMI | Non-fatal MI within 12 months of randomisation (1 = Yes, 2 = No) |
| AdjudicatedMI_Date | Date of non-fatal MI |
| CTCA_Count | Number of CT coronary angiograms including those performed as trial intervention in CTCA + Standard Care arm |
| Angiography_Count | Number of invasive coronary angiograms |
| Percutaneous coronary intervention_Count | Number of percutaneous coronary interventions |
| Coronary artery bypass graft surgery_Count | Number of coronary artery bypass graft surgeries |
| Echo_Count | Number of echocardiograms |
| RadionuclideScan_Count | Number of radionuclide myocardial perfusion scans |
| TwentyFourHourTape_Count | Number of 24-hour tapes (ECG monitoring) |
| ExerciseTest_Count | Number of exercise ECG tests |
| Magnetic Resonance Angiography_Count | Number of magnetic resonance imaging angiograms |
| Stress Echo_Count | Number of stress echocardiograms |
| OtherECGMonitoring_Count | Number of other ECG monitoring |
| Cardiac magnetic resonance imaging_Count | Number of cardiac magnetic resonance imaging scans |
| Stress magnetic resonance imaging_Count | Number of stress magnetic resonance imaging perfusion scans |
| Column name | Description |
|---|---|
| ConMedID | Database medication record unique ID |
| SubjectNo | Participant number |
| ConMedCategoryID | Medication [1 = TNK/tPA; 2 = Buccal or sublingual nitrate; 3 = I.V. nitrate (including infusion); 4 = Aspirin; 5 = Clopidogrel; 6 = Fondaparinux; 7 = Unfractionated heparin; 8 = Novel anticoagulant (Dabigatran, Rivaroxaban, Apixaban); 9 = Tirofiban or equivalent IIbIIIa inhibitor (Abciximab, Eptifibatide); 10 = I.V. Morphine (or other I.V. opiate); 11 = Diuretic; 12 = Insulin; 13 = PPI; 14 = Beta blocker; 15 = Calcium channel blocker; 16 = Oral hypoglycaemic; 17 = Statin; 18 = Prasugrel; 19 = Ticagrelor; 20 = Warfarin; 21 = ACE inhibitor or ARB; 22 = Nicorandil; 23 = LMWH (Enoxaparin, Dalteparin); 24 = Oral nitrate; 25 = Bivalirudin; 26 = Ivabradine; 27 = Ranolazine] |
| PrescribedBeforeAdmission | Prescribed before admission? (1 = Yes; 2 = No) |
| StartDate | If prescribed before admission is no, Start date |
| StartTimeHH | If prescribed before admission is no, Hour component of start time |
| StartTimeMM | If prescribed before admission is no, Minute component of start time |
| EndDate | If stopped before discharge, Date stopped |
| EndTimeHH | If stopped before discharge, Hour component of time stopped |
| EndTimeMM | If stopped before discharge, Minute component of time stopped |
| PrescribedOnDischarge | Prescribed on discharge? (1 = Yes; 2 = No) |
| DoseAltered | If prescribed on both admission and discharge, Was the dose altered during this hospitalisation? (1 = Yes; 2 = No) |
| IncreasedDecreased | If dose altered is yes, Dose increased or decreased? (1 = Increased; 2 = Decreased) |
| case report formVersion | case report form version number |
| Column name | Description |
|---|---|
| SubjectNo | Participant number |
| TimepointID | Time point (1 = Pre-Randomisation; 6 = One Month Questionnaires; 7 = Six Month Questionnaires; 8 = Twelve Month Questionnaires) |
| Mobility | Mobility (1 = I have no problems in walking about; 2 = I have slight problems in walking about; 3 = I have moderate problems in walking about; 4 = I have severe problems in walking about; 5 = I am unable to walk about) |
| SelfCare | Self-Care (1 = I have no problems washing or dressing myself; 2 = I have slight problems washing or dressing myself; 3 = I have moderate problems washing or dressing myself; 4 = I have severe problems washing or dressing myself; 5 = I am unable to wash or dress myself) |
| UsualActvities | Usual activities (1 = I have no problems doing my usual activities; 2 = I have slight problems doing my usual activities; 3 = I have moderate problems doing my usual activities; 4 = I have severe problems doing my usual activities; 5 = I am unable to do my usual activities) |
| PainDiscomfort | Pain/Discomfort (1 = I have no pain or discomfort; 2 = I have slight pain or discomfort; 3 = I have moderate pain or discomfort; 4 = I have severe pain or discomfort; 5 = I have extreme pain or discomfort) |
| AnxietyDepression | Anxiety/Depression (1 = I am not anxious or depressed; 2 = I am slightly anxious or depressed; 3 = I am moderately anxious or depressed; 4 = I am severely anxious or depressed; 5 = I am extremely anxious or depressed) |
| HealthState | Your health today (scale from 0 to 100) |
| Column name | Description |
|---|---|
| SubjectNo | Participant number |
| TimepointID | Time point (6 = One Month Questionnaires; 7 = Six Month Questionnaires; 8 = Twelve Month Questionnaires) |
| Telephone | Number of times used telephone health advice (e.g. General Practitioner, NHS Direct) in the last month or last 3 months |
| General PractitionerSurgery | Number of times seen your General Practitioner at the surgery in the last month or last 3 months |
| General PractitionerHome | Number of times been visited at home by your General Practitioner in the last month or last 3 months |
| NurseHome | Number of times been visited at home by a nurse in the last month or last 3 months |
| SocialWorker | Number of times been visited by a social worker in the last month or last 3 months |
| AccidentAndEmegancy | Number of times visited an Accident and Emergency department since your hospital admission 1 month ago or in the last 3 months |
| AccidentAndEmegancyHospital | Which hospital did you go to for the A&E visit? (text) |
| OutpatientHospital | Number of times attended the hospital as an outpatient in the last month or last 3 months |
| OutpatientHospitalAppointment | Which hospital did you go to for this appointment? (text) |
| InpatientHospital | Have you spent any nights in hospital as an inpatient in the last month or last 3 months? (1 = Yes; 2 = No) |
| InpatientNightsInHospital | If Yes, number of nights you were in hospital for |
| InpatientHospitalAttend | Which hospital did you attend? (text) |
| Column name | Description |
|---|---|
| SubjectNo | Participant number |
| TimepointID | Time point (9 = Twelve Month case report form) |
| ED_AMUAttendancesACSCount | Number of ED (or similar alternative) attendances with suspected or proven acute coronary syndrome |
| ED_AMUAttendancesCount | Number of ED (or similar alternative) attendances (any reason other than acute coronary syndrome) |
| HospitalAttendanceCount | Number of elective attendances to hospital (any reason) |
| CardiologyOutpatientVisitCount | Number of cardiology outpatient visits |
| OutpatientVisitCount | Number of other outpatient visits (any reason) |
| DaysSpentOnCCU | Total days spent on Coronary Care Unit |
| DaysSpentOnICU | Total days spent on ICU |
| DaysSpentInHospital | Total days spent in hospital (any location, excluding CCU/ICU) |
Appendix 10 Health economic analysis unit costs
| Category | Description | Unit cost (£) | Source |
|---|---|---|---|
| IndexStay_Days | Length of stay for index hospitalisation (calendar days) | 402 | Assumed to be same as ward cost |
| MI1_4b | Non-fatal type 1 or type 4b MI | 2360 | NHS reference costs64 (weighted average of all MI procedures) |
| MI | Non-fatal MI | 2360 | NHS reference costs64 (weighted average of all MI procedures) |
| CTCA | CT coronary angiograms | 195 | NHS reference costs64 |
| Angiography | Invasive coronary angiograms | 1685 | Moss et al.73 |
| percutaneous coronary intervention | Percutaneous coronary interventions | 2930 | NHS reference costs64 (weighted average of all percutaneous coronary intervention procedures) |
| coronary artery bypass graft surgery | Coronary artery bypass graft surgeries | 11,760 | NHS reference costs64 (weighted average of all coronary artery bypass graft surgery procedures) |
| Echo | Echocardiograms | 120 | NHS reference costs64 (weighted average of simple and complex echocardiograms) |
| RadionuclideScan | Radionuclide myocardial perfusion scans | 306 | NHS reference costs64 (RN20Z for myocardial perfusion scan) |
| TwentyFourHourTape | 24-hour tapes (ECG monitoring) | 102 | NHS reference costs64 (stress echo) |
| ExerciseTest | Exercise ECG test | 219 | NHS reference costs64 for cardiopulmonary exercise testing |
| Magnetic Resonance Angiography | Magnetic resonance angiogram | 142 | NHS reference costs64 (weighted average of all magnetic resonance imaging scans) |
| StressEcho | Stress echocardiogram | 219 | NHS reference costs64 for cardiopulmonary exercise testing |
| OtherECGMonitoring | Other ECG monitoring | 102 | Assumed to be same as stress echo |
| Cardiac magnetic resonance imaging | Cardiac magnetic resonance imaging scan | 272 | NHS reference costs64 (weighted average of all cardiac magnetic resonance imaging scans) |
| Stress magnetic resonance imaging | Stress magnetic resonance imaging perfusion scans | 389 | NHS reference costs64 (myocardial perfusion scan, stress only) |
| ED_AMUAttendancesACS | ED (or similar alternative) attendances with suspected or proven acute coronary syndrome | 168 | NHS reference costs64 (code: 180) |
| ED_AMUAttendances | ED (or similar alternative) attendances (any reason other than acute coronary syndrome) | 168 | NHS reference costs64 (code: 180) |
| HospitalAttendance | Elective attendances to hospital (any reason) | 148 | NHS reference costs64 (code: OPROC) |
| CardiologyOutpatientVisit | Cardiology outpatient visits | 148 | NHS reference costs64 (code: OPROC) |
| OutpatientVisit | Outpatient visits (any reason) | 148 | NHS reference costs64 (code: OPROC) |
| DaysCCU | Cost per day in coronary care unit | 917 | De Nigris et al.74 |
| DaysICU | Cost per day in ICU | 1340 | Schroeder et al.75 |
| DaysHospital | Cost per day spent in hospital (any location, excluding CCU/ICU) | 402 | Driessen et al.76 |
| Telephone | Telephone health advice (e.g. general practitioner, NHS Direct) | 12 | PSSRU77 (£7.80 per nurse telephone consultation and £15.52 per general practitioner telephone consultation) |
| General PractitionerSurgery | General practitioner at the surgery | 32.12 | PSSRU 201977 (per consultation) |
| General PractitionerHome | General practitioner home visit | 133 | Schroeder et al.75 (assuming visit lasting 23.4 minutes) |
| NurseHome | Nurse home visit | 20 | PSSRU 201977 nurse cost of £51 per hour (average of bands 4–8b) |
| SocialWorker | Social worker visit | 20 | PSSRU 201977 (£45 per hour without qualifications and £51 per hour with qualifications) |
| Medication | Cost (£) | Size | Dosage | Cost per 5 days (£) | 1-year cost (£) |
|---|---|---|---|---|---|
| TNK/TPA | 602.70 | 1 | One off | 602.70 | 602.70 |
| Buccal or sublingual nitrate | 1.12 | 100 | One pack lasts for 1 year | 0.06 | 1.12 |
| I.V. nitrate (including infusion) | 2.31 | 1 | One-off infusion | 2.31 | 2.31 |
| Aspirin | 0.31 | 100 | One per day | 0.02 | 1.13 |
| Clopidogrel | 0.72 | 28 | One per day | 0.13 | 9.39 |
| Fondaparinux | 62.79 | 10 | 2.5 mg/5 mg daily up to 8 days | 31.40 | 62.79 |
| Unfractionated heparin | 11.37 | 10 | Only in hospital | 5.69 | 5.69 |
| Novel anticoagulants (dabigatran, rivaroxaban, apixaban) | 50.40 | 56 | Twice per day | 9.00 | 657.00 |
| Tirofiban or equivalent 2B3 A inhibitors (abciximab, eptifibatide) | 50.63 | 1 | Two infusions (IV for 12 hours) | 566.63 | 566.63 |
| IV morphine (or other IV opiate) | 2.67 | 1 | Once or twice in the hospital | 5.34 | 5.34 |
| Diuretic | 0.11 | 28 | Once per day | 0.02 | 1.43 |
| Proton pump inhibitor | 0.34 | 28 | Once per day | 0.06 | 4.43 |
| Beta-blocker | 0.10 | 28 | Once per day | 0.02 | 1.30 |
| Calcium channel blocker | 0.21 | 28 | Once per day | 0.04 | 2.74 |
| Oral hypoglycaemic | 2.88 | 84 | Twice per day | 0.34 | 25.03 |
| Statin | 0.20 | 28 | Once per day | 0.04 | 2.61 |
| Prasugrel | 47.56 | 28 | Once per day | 8.49 | 619.98 |
| Ticagrelor | 54.60 | 56 | Twice per day | 9.75 | 711.75 |
| Warfarin | 0.20 | 28 | Once per day | 0.04 | 2.61 |
| ACE inhibitor or ARB | 0.20 | 28 | Once per day | 0.04 | 2.61 |
| Nicorandil | 1.36 | 60 | Twice per day | 0.23 | 16.55 |
| LMWH (enoxaparin, dalteparin) | 54.23 | 10 | Twice per day, only in hospital | 54.23 | 54.23 |
| Oral nitrate | 0.67 | 56 | Once per day | 0.06 | 4.37 |
| Bivalirudin | 775.50 | 5 | Infusion for up to 72 hours, 2 vials | 826.20 | 826.20 |
| Ivabradine | 5.40 | 56 | Twice per day | 0.96 | 70.39 |
| Ranolazine | 48.98 | 60 | Twice per day | 8.16 | 148.98 |
Appendix 11 Scenario analysis estimating within-trial quality-adjusted life-years including all patients
All patients (877 randomised to early CTCA and 871 to standard care) completed the EQ-5D-5L questionnaire at baseline. The mean utility at baseline was 0.752 in the CTCA arm and 0.760 in the standard-care arm. However, there were 113 patients in the CTCA arm and 158 patients in the standard-care arm without any follow-up data (i.e. did not respond to any EQ-5D-5L questionnaires). Scenario analysis using all patients (i.e. including those with no follow-up data) is presented here.
The average baseline utility was 0.752 in the CTCA arm (n = 877) and 0.760 in the standard-care arm (n = 871), suggesting a slight imbalance in baseline values. In the CTCA arm, there were 185, 215 and 243 patients who did not respond to EQ-5D-5L questionnaires at 1, 6 and 12 months, respectively. Similarly, in the standard-care arm, there were 250, 275 and 301 patients who did not respond to EQ-5D-5L questionnaires at 1, 6 and 12 months, respectively. The responses on the individual items of the EQ-5D-5L questionnaire were used to estimate utility scores using the mapping algorithm by van Hout et al. 63 as recommended by NICE. The value set for the UK was used to estimate the utilities using the Excel crosswalk calculator. The patients who died during the trial were included with zero utility from the time of death, and the average of all of the patients was used to estimate the mean values of the utilities at different time points in both arms. These are presented in Table 49.
| Trial arm | Follow-up time point | |||
|---|---|---|---|---|
| Baseline | 1 month | 6 months | 12 months | |
| CTCA | 0.752 | 0.726 | 0.746 | 0.745 |
| Standard care | 0.760 | 0.724 | 0.757 | 0.746 |
Multiple imputation techniques were used to impute the utility values for patients with missing data at 1, 6 and 12 months. This multiple imputation was performed using the package MICE in R software. The missing utility values were estimated as average of values of data sets estimated using multiple imputations.
The QALYs were estimated using the trapezoidal rule for calculating the area under the curve. The utility values at baseline and 1, 6 and 12 months were multiplied with the corresponding time that they spent in these utilities. It was assumed that the patients stay in the same utility until the midpoint of the time difference to the next follow-up point. For example, utility in month 1 was assumed to last until 3.5 months (i.e. the midpoint of the follow-ups at months 1 and 6) and the utility at month 6 was used from month 3.5 to 9 months (i.e. the midpoint of the follow ups at months 6 and 12). Mean QALYs and CIs estimated using bootstrapping are presented in Table 50.
| Trial arm | Mean QALYs | 95% CIs |
|---|---|---|
| CTCA | 0.7317 | 0.7165 to 0.7470 |
| Standard care | 0.7369 | 0.7221 to 0.7518 |
List of abbreviations
- BMI
- body mass index
- b.p.m.
- beats per minute
- CCU
- coronary care unit
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- CT
- computerised tomography
- CTCA
- computerised tomography coronary angiography
- DLP
- dose-length product
- DMC
- Data Monitoring Committee
- ECG
- electrocardiogram
- ECTU
- Edinburgh Clinical Trials Unit
- ED
- emergency department
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GRACE
- Global Registry of Acute Coronary Events
- GTN
- glyceryl trinitrate
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IQR
- interquartile range
- MI
- myocardial infarction
- MICE
- mulitple imputation using chained equations
- MINOCA
- Myocardial Infarction with Non-Obstructive Coronary Artery disease
- NICE
- National Institute for Health and Care Excellence
- NSTEMI
- non-ST elevation myocardial infarction
- OR
- odds ratio
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- QA
- quality assurance
- QALY
- quality-adjusted life-year
- RAPID
- Rapid Assessment of Potential Ischaemic Heart Disease
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SMR
- standardised mortality ratio
- STEMI
- ST elevation myocardial infarction
- TSC
- Trial Steering Committee
