Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as award number NIHR133547. The protocol was agreed in April 2021. The draft manuscript began editorial review in October 2022 and was accepted for publication in April 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Asgharzadeh et al. This work was produced by Asgharzadeh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Asgharzadeh et al.
Chapter 1 Background
Description of health problem
Type 1 diabetes mellitus (T1DM) was formerly known as insulin-dependent diabetes. It is the result of an autoimmune process that leads to the destruction of the insulin-producing beta cells in the pancreas. The cause of this autoimmune process is not known.
Aetiology, pathology and prognosis
Insulin is essential for survival. Diabetes is characterised by high blood glucose levels, known as hyperglycaemia. Injected insulin lowers blood glucose. It can cause abnormally low glucose, known as hypoglycaemia. The aim of insulin treatment is to keep plasma glucose as close to normal as possible and so prevent the development of the long-term complications of diabetes due to hyperglycaemia.
Treatment also aims to reduce the increased risk of cardiovascular disease (CVD) seen in diabetes. Deficiency of insulin can lead to diabetic ketoacidosis (DKA), which can be fatal.
Epidemiology
Type 1 diabetes usually comes in late childhood or early adolescence but can develop at any age. T1DM accounts for 5–10% of diabetes cases. The prevalence of T1DM is higher in adults than in children; the highest prevalence is observed in adults aged ≥ 30 years. 1,2 There are about 250,000 people with T1DM in the UK.
Impact of health problem
Hypoglycaemia
Hypoglycaemia can be mild, moderate or severe.
People with diabetes are rightly scared of hypoglycaemia, and this fear may lead to them allowing blood glucose to run higher than is desirable, which can increase the risk of long-term complications. Episodes of hypoglycaemia are usually called ‘hypos’.
The American Diabetes Association3 defines hypoglycaemia as follows:
-
Severe hypoglycaemia: an event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. These episodes may be associated with sufficient neuroglycopaenia to induce seizure or coma.
-
Documented symptomatic hypoglycaemia: an event during which typical symptoms of hypoglycaemia are accompanied by a measured plasma glucose concentration of (3.9 mmol/l).
-
Asymptomatic hypoglycaemia: an event not accompanied by typical symptoms of hypoglycaemia but with a measured plasma glucose concentration of 70 mg/dl (3.9 mmol/l).
Non-severe hypoglycaemia can be mild or moderate. Mild hypoglycaemia may present with symptoms, such as sweating, shaking, hunger and nervousness. Some symptoms are due to the release of adrenaline. Mild hypoglycaemia is easily self-managed by taking rapidly absorbed carbohydrate.
Moderate hypoglycaemia can cause difficulty concentrating or speaking, confusion, weakness, vision changes and mood swings.
Mild and moderate hypos can usually be managed by people with diabetes themselves, but moderate hypos often lead to the interruption of activities.
Severe hypoglycaemia can lead to cognitive impairment, unconsciousness and convulsions and can be fatal. People having severe hypos need assistance and may need to attend an accident and emergency department or seek support from paramedics. They may require admission to hospital.
Hypoglycaemia can trigger an adrenergic response that acts as a warning that glucose should be consumed. Unfortunately, in some people, after repeated hypos, this warning may be lost. This is known as hypoglycaemic unawareness, and such people are at increased risk of severe hypoglycaemia and its effects. These individuals are covered by the recommendation in NICE diagnostics guidance (DG21)4 and technology appraisal guidance TA1515 on insulin pumps.
Nocturnal hypoglycaemia occurs during sleep and may not be detected. However, it may disturb sleep and wake the person up. It can have two adverse effects. One is rebound hyperglycaemia, the result of the body’s reactions to hypoglycaemia, such as releasing other hormones that increase blood glucose, meaning that nocturnal hypoglycaemia may result in unusually high blood glucose levels around breakfast time. The other consequence is that nocturnal hypoglycaemia may itself contribute to hypoglycaemic unawareness.
Past appraisals
In a technology appraisal (TA53) of long-acting insulin analogues (at that time only glargine),6 the NICE Appraisal Committee accepted that both hypoglycaemic episodes and the fear of such episodes recurring caused significant disutility. A utility decrement of 0.0052 per non-severe hypoglycaemic event (NSHE) was accepted. As regards fear of hypos, NICE’s guidance (TA53)6 states:
The Committee accepted that episodes of hypoglycaemia are potentially detrimental to an individual’s quality of life. This is partly the result of an individual’s objective fear of symptomatic hypoglycaemic attacks as indicated in the economic models reviewed in the Assessment Report. In addition, as reported by the experts who attended the appraisal meeting, individuals’ quality of life is affected by increased awareness and uncertainty of their daily blood glucose status and their recognition of the need to achieve a balance between the risk of hypoglycaemia and the benefits of longer-term glycaemic control. The Committee understood that improvement in this area of concern regarding the balance between hypoglycaemia and hyperglycaemia could have a significant effect on an individual’s quality of life.
Guidance on the Use of Long‑acting Insulin Analogues for the Treatment of Diabetes – Insulin Glargine. Available from www.nice.org.uk/guidance/ta53. All rights reserved. Subject to Notice of rights NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
© NICE (2022)
However, the guidance did not specify the amount of utility lost because of fear of hypos, and nor did the Technology Assessment Report7 because it was based on the industry submission from Aventis, which was classed as confidential. However, clearly the utility gain from reducing the fear of hypoglycaemia was enough to change a substantial cost per quality-adjusted life-year (QALY) to an affordable one. There is the probability that a reduction in the rate (events recorded/unit time) of severe hypoglycaemia events may reduce the fear of severe hypoglycaemia events, although the impact of this seems likely to be variable across patients. The quality-of-life impact arising from this would be over and above the direct quality-of-life impact of the severe hypoglycaemia events themselves.
In the type 2 guidelines developed in 2008, fear of severe hypos was estimated to reduce quality of life by 0.020. The assessment group (Waugh et al., Aberdeen8) considered the reasonableness of this:
This fear effect may only apply to a sub-group of patients, but as an illustration of the possible impact of this, the social tariffs derived by Dolan and colleagues9 suggest that a move from level 2 within the anxiety subscale of EQ-5D to level 1 would be associated with a 0.07 QoL gain. In a similar vein, the coefficients derived by Brazier and colleagues10 for the SF-6D questionnaire for the consistent model using standard gamble valuations suggest that a movement within the social dimension from health problems interfering moderately to not interfering would be associated with a 0.022 QoL improvement. Similarly, an improvement in the mental health subscale from feeling downhearted some of the time to little or none of the time would be associated with a 0.021 QoL improvement.
Waugh et al.
Studies of the disutility of hypoglycaemia
Brod et al. 11 carried out a survey to estimate the effect of non-severe hypos on work in terms of productivity, costs and self-management. The authors used telephone interviews and focus groups, supplemented by a literature review. Respondents were required to have had a NSHE in the previous month. A NSHE was defined as a hypo event not requiring assistance from anyone else, with or without blood glucose measurement, and with or without symptoms. The respondents were asked about duration, effect on work, and likely cause of the hypo, and whether the hypo occurred at work, at other times of the day, or during sleep. Seven hundred and thirteen respondents had T1DM, and half of this group had NSHEs at least once per week, with 27% having at least one per month. Twenty-two per cent had hypos only a few times per year.
About 95% of people identified hypos by symptoms, and about 60% of episodes were confirmed by a blood glucose test. The average duration of a NSHE was 33 minutes, but the effect on self-management lasted a week, with an extra six blood glucose tests, a reduction in insulin dose by an average of 6.5 units per day for 4 days in 25% of people, and an unplanned contact with a healthcare professional in 25%.
The effects on work included:
-
Leaving early or missing a full day in 18% of people. The average work time lost was 10 hours.
-
Missing meetings or being unable to finish a task in 24% of people.
Work time was lost not only because of NSHEs occurring at work but also because of those outside work, including nocturnal hypos. No breakdown by insulin regimen was reported, such as continuous subcutaneous insulin infusion (CSII) compared with multiple daily injection (MDI).
Leckie et al. 12 recruited 243 people with diabetes (216 people with type 2 diabetes mellitus (T2DM) and some with T2DM on insulin) who were in employment. The participants’ insulin regimens included mostly MDI, but 51 were taking twice-daily mixtures of soluble and Neutral Protamine Hagedorn. Over a 12-month follow-up, every month they recorded their hypo events, the severity of these and the effect on work. A total of 1955 NSHEs were reported, plus 238 severe hypos (some involving unconsciousness and seizures, and a few resulting in soft-tissue injuries). However, 66% of patients had no severe hypos. Most (62%) of the severe episodes occurred at home and 52% occurred during sleep, but 15% occurred at work. Fifty-five per cent of the NSHEs occurred at home and 30% occurred at work. It should be noted that the mean HbA1c in most patients was > 9%, except for patients who had more than two severe hypos over the year, in whom it was 8.4%, still far above the target.
Frier et al. 13 carried out a survey of 466 people with T1DM about the frequency of non-severe hypoglycaemia and found that people with T1DM had an average of 2.4 episodes per week (median 2 episodes per week), with around one-quarter of these being nocturnal. The after-effects include fatigue and reduced alertness, and they persisted longer after nocturnal NSHEs (10 hours) than after daytime episodes (5 hours). Among those in employment, 20% of NSHEs led to a loss of work time. Most did not contact their healthcare professionals. Self-testing of blood glucose increased in the week after the episode, with an average of four extra tests. The survey showed that NSHEs are troublesome for patients and have effects lasting at least into the following day. The commonest after-effects were tiredness, reduced alertness and feeling emotionally down.
Choudhary et al. 14 reported that the use of pumps with a low glucose suspend (LGS) facility meant that 66% of NSHEs lasted < 10 minutes and only 12% lasted up to 2 hours. Nocturnal hypos were greatly reduced.
About 30% of people with T1DM have an impaired awareness of hypos,15 and these people are three to six times more likely to have severe hypos. The Gold scale rates awareness on a scale of 1–7, where 7 means a complete absence of symptoms of hypoglycaemia. Structured education, such as Dose Adjustment for Normal Eating (DAFNE), restores awareness in about half of people with impaired awareness. Better control of hypoglycaemia avoidance can also restore awareness. A trial by Little et al. 16 (the HypoCOMPass trial) showed that better control for 24 weeks improved the Gold score by 1 point and reduced the fear of hypo level from 58 to 45 (higher scores indicate greater fear, with the maximum being 132) without adversely affecting HbA1c.
Evans et al. 17 used the time trade-off (TTO) method to estimate the disutility of hypos on the health-related quality of life (HRQoL) scale (0–1, where 1 is perfect health and 0 is death). They interviewed 551 people with T1DM and 8286 people with no diabetes. They note that hypos can affect HRQoL in two ways: first through the direct effects of the episodes, and second through fear of future hypos, which can lead to precautions, such as taking an insufficient insulin dose (increasing the risk of complications), restricting physical activity, and overeating. In addition, repeated hypos can lead to hypoglycaemic unawareness, which increases the risk of future hypos. The authors estimated that daytime NSHEs reduce HRQoL in a range of 0.032 for one event per month to 0.071 for three episodes per week. Nocturnal NSHEs reduce it by slightly more. Severe events, even only once or twice per year, reduce HRQoL by about 0.08.
The general public’s valuation of disutility per event per year ranged from 0.004 for non-severe daytime hypos to 0.06 per severe event. People with T1DM had slightly lower estimates of the disutility of severe events, at 0.047.
Using data from this study, Lauridson et al. 18 reported that the disutility of NSHEs may diminish if there are repeated events.
The study by Harris et al. 19 reports the Canadian results from this study.
Levy et al. 20 elicited utility values for non-severe hypoglycaemia from 51 Canadians (but only half had T1DM) and control participants with no diabetes. The disutility from a single NSHE was 0.0033. Levy et al. 20 argue that a minimum significant utility loss is 0.03, which would be reached by people having 10 NSHEs per year.
Adler et al. 21 found that severe, frequent and nocturnal hypoglycaemia reduced quality of life, ranging from 0.84 in people with diabetes who had the least severe state (non-severe, daytime only, only once per year, not causing any worry) to 0.40 (severe frequent hypoglycaemia day and night, causing anxiety).
Currie et al. 22 surveyed 1305 UK patients with T1DM and T2DM using both the Hypoglycaemia Fear Survey (HFS) and the EuroQol-5 Dimensions (EQ-5D). Each severe hypoglycaemic event (SHE) avoided was associated with a change of 5.9 on the HFS. Given a further estimate that each unit change on the HFS was associated with an EQ-5D quality-of-life change of 0.008, this led to an estimated benefit from reduced fear of SHEs of 0.047 per annual event avoided. This was coupled with a direct utility loss associated with a SHE in T1DM of 0.00118 to yield an overall patient benefit of 0.05 per unit reduction in annual SHEs. Currie et al. 22 also reported direct disutilities in T1DM of 0.0036 per NSHE.
Conclusions on hypoglycaemia
Hypoglycaemia remains a major problem in T1DM and has not improved over recent decades. This may be because the increased emphasis on improving glycaemic management, through more intensive insulin treatment, has offset other advances in treatment; tightly managed diabetes can make it more likely that hypoglycaemia might occur. The frequency and severity of hypos can be reduced by structured education and using CSII (insulin pumps), but hypos remain a problem that leads to economic disutilities. For individual events, disutilities and costs are much greater for severe hypos, but the much larger number of NSHEs lead to significant impacts on quality of life.
Current service provision
Management of disease
In people without T1DM, the pancreas produces a little insulin throughout the day but peaks of insulin release after meals. The release after meals is very fast and enables the body to handle and store nutrients. The pancreas releases insulin into the portal vein that goes into the liver, its main site of action.
Treatment with insulin is aimed at replicating the function of the pancreas. Insulin is injected under the skin (subcutaneously). Modern insulin regimens have two components: short-acting insulin to cover mealtimes, and long-acting insulin to cover the rest of the day, usually given twice per day. The long-acting form is called basal, and the combination is often referred to as ‘basal-bolus’ insulin, or as MDI – with three injections of short-acting insulins and two of long-acting insulins (glargine or detemir). However, subcutaneous insulin injections cannot achieve as rapid an effect as pancreatic insulin, and because of the slower onset of action and more prolonged effects, hyperglycaemia is common shortly after meals, often followed by later hypoglycaemia.
Control within target of plasma glucose by intensified insulin therapy requires more than just insulin injections. It also requires regular monitoring of blood glucose by finger-pricking and measurement using a portable meter, or by using a continuous blood glucose measurement device, and then adjusting the insulin dose to take account of calorie intake from food and energy expenditure on exercise. People with diabetes usually manage their own diabetes, supported by structured education packages, such as DAFNE.
The aim of treatment is to manage hyperglycaemia and avoid hypoglycaemia. Glycaemic management is assessed using glycated haemoglobin (HbA1c), which gives an average measure over 2–3 months. The NICE target for T1DM is 48 mmol/mol (formerly 6.5%), but few people with T1DM achieve this. With the spread of continuous glucose measurement devices, ‘time in range’ is increasingly used as another measure of glycaemic management.
The alternative to MDI is CSII using an insulin pump. CSII was approved by NICE with restrictions. 5
NICE guidance: Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus [TA151].
Continuous subcutaneous insulin infusion (or ‘insulin pump’) therapy is recommended as a treatment option for adults and children aged ≥ 12 years with T1DM provided that:
-
attempts to achieve target haemoglobin A1c (HbA1c) levels with MDI result in the person experiencing disabling hypoglycaemia. For the purpose of this guidance, disabling hypoglycaemia is defined as the repeated and unpredictable occurrence of hypoglycaemia that results in persistent anxiety about recurrence and is associated with a significant adverse effect on quality of life
or
-
HbA1c levels have remained high [i.e. at 8.5% (69 mmol/mol) or above] on MDI therapy (including, if appropriate, the use of long-acting insulin analogues) despite a high level of care.
CSII therapy is recommended as a treatment option for children aged < 12 years with T1DM provided that:
-
MDI therapy is considered to be impractical or inappropriate, and
-
children on insulin pumps would be expected to undergo a trial of MDI therapy between the ages of 12 and 18 years.
The guidance on the use of the VeoTM pump also had restrictions. 4
NICE guidance: Integrated sensor-augmented pump therapy systems for managing blood glucose levels in type 1 diabetes [the MiniMedTM Paradigm Veo system and the Vibe and G4 PLATINUMTM continuous glucose monitoring (CGM) system] (DG21)
-
The MiniMed Paradigm Veo system is recommended as an option for managing blood glucose levels in people with T1DM only if they have episodes of disabling hypoglycaemia despite optimal management with CSII.
-
The MiniMed Paradigm Veo system should be used under the supervision of a trained multidisciplinary team who are experienced in CSII and CGM for managing T1DM only if the person or their carer agrees to use the sensors for at least 70% of the time; understands how to use it and is physically able to use the system; and agrees to use the system while having a structured education programme on diet and lifestyle, and counselling.
-
People who start to use the MiniMed Paradigm Veo system should continue to use it only if they have a decrease in the number of hypoglycaemic episodes that is sustained. Appropriate targets for such improvements should be set.
The guidance did not comment on reduction of severity of hypos.
In people with no diabetes, hypoglycaemia is rare, because if the blood glucose drops, a counter-regulatory mechanism kicks in, including the release of glucagon (which raises blood glucose) and adrenaline and the cessation of insulin release. In people receiving MDI, there are pools of long-acting and short-acting insulin under the skin (subcutaneous) that, unlike pancreatic insulin, cannot be switched off. People receiving CSII have only a little short-acting insulin, so stopping the pump gives a quick response. (There can be a hazard here, in that should a pump fail, the patient soon will have no insulin and be at risk of hyperglycaemia and DKA.)
Interventions to reduce hypoglycaemia
One intervention to reduce the risk of hypoglycaemia is structured education, such as the DAFNE programme. Structured education is recommended in NICE guideline NG17. 2 The assessment report for the original appraisal of patient education in diabetes has been published in the NIHR Health Technology Assessment (HTA) monograph series. 23
Iqbal and Heller24 have provided a more recent review of the role of structured education and hypoglycaemia. They note that until recently, severe hypoglycaemia had not become less frequent over the last 20 years despite advances in treatment. They conclude that structured education can reduce the incidence of severe hypoglycaemia by about 50%, and that there is some evidence, albeit from an observational study with no control group, that the DAFNE-Hypoglycaemia Awareness Restoration Training (DAFNE-HART) programme can reduce hypoglycaemia even in patients with hypoglycaemia unawareness.
Continuous glucose monitoring
There are various forms of CGM. The term ‘continuous’ is slightly misleading: glucose levels are measured every few minutes. The device measures the level of glucose under the skin (‘interstitial glucose’), which reflects the level in the blood, but with a slight delay.
There are three elements in CGM:
-
a sensor that sits just underneath the skin and measures glucose levels
-
a transmitter that is attached to the sensor and sends the results to a display device
-
a display device that shows the glucose level.
The person with diabetes checks the CGM data and adjusts insulin dose, calorie intake or activity levels to maintain blood glucose levels.
So, the traditional ‘loop’ involves CGM, the patient using the data, and insulin dosage.
Autosuspend pumps
The mechanism here is that the CGM–patient–pump loop is augmented by direct communication between CGM device and the pump. If blood glucose is falling too low, the CGM device communicates with the pump and switches off the insulin infusions for, say, 2 hours. This is particularly useful in nocturnal hypoglycaemia when the patient is asleep.
Closed-loop systems
This term refers to systems with three components: the CGM, a microprocessor with algorithms and a pump. In effect, the microprocessor replaces the person. The microprocessor (in effect a small computer) receives data from the CGM and adjusts the infusion rate from the pump.
Devices such as the Veo only control the pump when hypoglycaemia is occurring. They may switch off the insulin infusion when blood glucose falls too low, or if it is heading in that direction.
Closed-loop systems can also control insulin infusion if blood glucose is too high. The most advanced system is the iLet from BetaBionics, which is a dual pump that infuses insulin if blood glucose is too high and glucagon if it is too low.
Relevant national guidelines, including National Service Frameworks
The National Institute of Health and Care Excellence (NICE) guideline covers care and treatment for adults (aged ≥ 18 years) with T1DM, including advice on diagnosis, education and support, blood glucose management, cardiovascular risk, and identifying and managing long-term complications. 2 Evidence reviews by NICE evaluated the most effective method of glucose monitoring to improve glycaemic management in adults with T1DM. Overall, 17 studies were included in the clinical effectiveness analysis to examine real-time continuous glucose monitoring (rtCGM) versus intermittently scanned continuous glucose monitoring (isCGM), rtCGM versus standard self-monitoring of blood glucose (SMBG), and isCGM versus SMBG. Two UK studies among 14 primary studies that contained cost–utility analyses were included in this evidence review. The results show time in range (TIR) to be a better measure than HbA1c as it captures variation and can be more directly linked to risk of complications. There was a clinically meaningful positive effect on TIR for rtCGM versus both isCGM and SMBG, as well as for isCGM versus SMBG, on the pre-set minimally important difference of a 5% change. 25 The authors clarified that the service user should consult with a member of the diabetes care team who has expertise in the use of CGM. This guideline reported both published UK cost-effectiveness studies (one on rtCGM and one on isCGM) and found these technologies to be cost-effective compared with intermittent capillary blood glucose monitoring. Based on the results of economic modelling (using clinical data from the randomised controlled trials (RCTs) included in the clinical review), isCGM glucose monitoring was clearly cost-effective for the overall population of people with T1DM, and this finding was robust to all the sensitivity analyses undertaken. 25
The Scottish Health Technologies Group (SHTG) review examined the cost-effectiveness of using closed-loop systems and the artificial pancreas for the management of T1DM compared with current diabetes management options, and considered clinical effectiveness, safety and patient aspects. 26
The evidence reviewed on clinical effectiveness consisted of small crossover RCTs that tested the use of closed-loop systems over relatively short periods of time in people with well-managed diabetes who had had the condition for several years and often had experience with using insulin pumps. The results of a network meta-analysis (NMA) and three pairwise meta-analyses show significant improvements in mean percentage TIR for people with T1DM using a closed-loop system compared with other insulin-based therapies. The pairwise meta-analyses also reported statistically significant reductions in mean percentage time spent in hyperglycaemia and hypoglycaemia. High heterogeneity was present in all meta-analyses, for all outcomes. This is potentially a result of the small study size, multiple different closed-loops systems in the intervention group, and the use of a variety of insulin therapy methods in the control groups. It should be noted that some of the secondary evidence reviewed might have been based on technologies that have since been superseded by newer models because of the rapidly changing nature of these systems.
In addition, adverse events were rarely reported in either the closed-loop system or the control groups.
The SHTG economic model showed that closed-loop systems were associated with the highest costs and QALYs in a Scottish adult population with T1DM, except in the comparison with CGM + CSII. The base-case results showed that the technology is cost-effective compared with CGM + CSII, but not cost-effective in comparison with flash or CGM combined with MDI in people with well-controlled T1DM. There are some uncertainties because of a lack of published studies underpinning the assumptions in the model.
Description of technology under assessment
Summary of intervention
The intervention of interest is a class of automated insulin delivery systems called HCL systems, which have three components: a CGM, a microprocessor with control algorithms and a pump. The microprocessor receives data from the CGM and adjusts the infusion rate from the pump to help keep glucose levels in a healthy range. These systems are aimed at reducing user or caregiver input in insulin dosing, and some only require users to deliver meal boluses by entering the estimated amount of carbohydrates in meals at the time they are eaten. Carbohydrate counting is essential for diabetes management and necessitates matching insulin doses to food choices. Some people find carbohydrate counting challenging because they do not have the skills, tend to eat out (which can be difficult to estimate) or have unhealthy eating habits.
Several HCL systems are available in the UK. Some of these systems have received regulatory approval for a fixed combination of CGM, control algorithm and insulin pump. However, some systems involve combining interoperable devices. The following systems are representative of the intervention of interest and have been identified by NICE as currently available in the UK.
Advanced hybrid closed loop
Hybrid closed-loop (HCL) systems use control algorithms to automate basal insulin delivery based on glucose sensor values in order to increase the time that a patient spends in the target range and thus reduce the frequency and duration of hypoglycaemia. The user of the HCL system is required to enter their carbohydrate intake before each meal, so that the appropriate mealtime insulin bolus can be delivered by the system.
Advanced HCL (AHCL) systems have additional features that include automated correction of bolus insulin delivered up to every 5 minutes when glucose levels are elevated. These systems may also enable greater personalisation of insulin delivery and monitoring and can include meal detection modules that allow the system to deliver more aggressive auto-correction boluses. 27
A number of HCL models, systems and apps are presented in Appendix 1.
Identification of important subgroups
The NICE scope (March 2022) includes people with T1DM (of any age), and the following subgroups if evidence permits:
-
Women with T1DM who are pregnant and those planning pregnancy (not including gestational diabetes). Note that in this assessment this subpopulation does not need to fulfil the criterion of prior use of at least one technology.
-
Children with T1DM.
-
If possible, evidence should be analysed based on the following age groups:
-
≤ 5 years
-
6–11 years
-
12–19 years.
-
-
People with extreme fear of hypoglycaemia.
-
People with diabetes-related complications that are at risk of deterioration.
Current usage in the National Health Service
The management of T1DM involves lifestyle adjustments, monitoring of blood glucose levels, and insulin replacement therapy, with the aim of recreating normal fluctuations in circulating insulin concentrations. Blood glucose levels are monitored to determine the type and amount of insulin needed to regulate blood glucose levels and reduce the risk of complications.
National Institute for Health and Care Excellence guidelines recommend that adults and pregnant women with T1DM be empowered to self-monitor their blood glucose, supported by structured education packages (e.g. DAFNE) on how to measure glucose levels and interpret the results. 2 NICE also recommends that children and young people (CYP) with T1DM and their families or carers be offered a continuing programme of education from diagnosis. Several systems of monitoring glucose levels and delivering insulin are available in clinical practice. The system recommended for an individual is based on their age, whether they are pregnant, their glycaemic control and their personal preferences.
Blood glucose monitoring
Capillary blood glucose monitoring
Blood glucose concentrations in diabetes can vary considerably from day to day and over a 24‑hour period. Routine blood glucose testing is typically done using capillary blood glucose monitoring. Capillary blood glucose monitoring involves pricking a part of the body (usually the finger) with a lancet device to obtain a small blood sample at certain times of the day. The drop of blood is then applied to a test strip, which is inserted into a blood glucose meter for an automated determination of the glucose concentration in the blood sample at the time of the test. Blood glucose measurements are taken after several hours of fasting, usually in the morning before breakfast, and before and after each meal to measure the change in glucose concentration.
Real-time continuous blood glucose measurement
Real-time continuous blood glucose measurement is an alternative to routine finger-prick blood glucose monitoring for people (including pregnant women) aged ≥ 2 years who have diabetes, have MDI of insulin or use insulin pumps, and are self-managing their diabetes. This involves measuring interstitial fluid glucose levels throughout the day and night.
A rtCGM system comprises three parts:
-
a sensor that sits just underneath the skin and measures glucose levels
-
a transmitter that is attached to the sensor and sends glucose levels to a display device
-
a display device that shows the glucose level – a separate handheld device (known as a ‘standalone’ CGM) or a pump (known as an ‘integrated system’).
With most rtCGM systems, calibration by checking the finger-prick blood glucose level is needed once or twice per day. rtCGM systems monitor glucose levels regularly (approximately every 5 minutes), and alerts can be set for high, low or rate of change.
Flash/intermittently scanned glucose monitoring
Flash glucose monitoring (FGM) systems comprise a reader and a sensor applied to the skin to measure interstitial fluid glucose levels. It provides a reading or trends only when the sensor is scanned.
Glycated haemoglobin
Longer-term control is measured using HbA1c levels, which reflect the average blood glucose levels over 2–3 months. HbA1c is correlated to CGM results over the preceding 8–12 weeks. 28 NICE guidelines on diabetes (T1DM and T2DM) in CYP, in adults, and in pregnancy recommend that people with T1DM aim for a target HbA1c level of ≤ 6.5% (48 mmol/mol) to minimise the risk of long-term complications from diabetes. Control above target glucose levels may trigger a discussion about different options for insulin administration.
Insulin regimens
Multiple daily injections
Insulin is injected subcutaneously. Modern insulin regimens have two components: short-acting insulin to cover mealtimes, and long-acting insulin to cover the rest of the day, which is usually given twice per day. The long-acting form is called basal, and the combination is often referred to as ‘basal-bolus’ insulin, or as MDI, with three injections of short-acting insulins and one or two of long-acting insulin. However, subcutaneous insulin injections cannot achieve as rapid an effect as pancreatic insulin, and because of the slower onset of action and more prolonged effect, hyperglycaemia is common shortly after meals, often followed by hypoglycaemia later.
Continuous subcutaneous insulin infusion
The alternative to MDI is CSII using an insulin pump. It makes use of an external pump that delivers insulin continuously from a refillable storage reservoir by means of a subcutaneously placed cannula. CSII was approved by NICE as a treatment option for adults and children aged ≥ 12 years with T1DM, provided that:
-
attempts to achieve target HbA1c levels with MDI result in the person experiencing disabling hypoglycaemia. For this guidance, disabling hypoglycaemia is defined as the repeated and unpredictable occurrence of hypoglycaemia that results in persistent anxiety about recurrence and is associated with a significant adverse effect on quality of life or
-
HbA1c levels have remained high [i.e. at ≥ 8.5% (69 mmol/mol)] on MDI therapy (including, if appropriate, the use of long-acting insulin analogues) despite a high level of care.
CSII therapy is recommended as a treatment option for children aged < 12 years with T1DM provided that:
-
MDI therapy is considered to be impractical or inappropriate, and
-
children on insulin pumps would be expected to undergo a trial of MDI therapy between the ages of 12 and 18 years.
For pregnant women with T1DM, NICE recommends that CSII be offered to those who are using MDI and do not achieve blood glucose management without significant disabling hypoglycaemia.
Integrated sensor-augmented pump therapy systems
Integrated sensor-augmented pump (SAP) therapy systems combine rtCGM with CSII. The systems are designed to measure interstitial glucose levels (every few minutes) and allow immediate real‑time adjustment of insulin therapy. The systems may produce alerts if the glucose levels become too high or too low. NICE’s DG21 on integrated SAP therapy systems for managing blood glucose levels in T1DM recommends the MiniMed Paradigm Veo system as an option for managing blood glucose levels in people with T1DM only if they have episodes of disabling hypoglycaemia despite optimal management with CSII. 4 As with other pumps, the user can programme one or more basal rate settings for different times of the day/night. A built-in bolus calculator works out how much insulin is needed for a meal following the input of carbohydrates consumed. The advanced feature of SAP is that the rtCGM–patient–pump loop is augmented by direct communication between the rtCGM device and the pump. If blood glucose is falling too low, the rtCGM device communicates with the pump and automatically switches off (suspends) the insulin infusions. Depending on the device, either the user must restart insulin delivery or the pump resumes insulin delivery after 2 hours.
Low glucose suspend/predictive low glucose suspend
Sensor-augmented pump systems can operate in standard (manual) and advanced (automatic) modes. In the manual open loop mode, the continuous glucose monitor and glucose pump do not communicate with each other, and insulin doses are programmed by the user, who makes manual adjustments.
In advanced, automatic mode, the CGM device and pump can communicate with each other automatically, based on real-time glucose data, to adjust the insulin basal rate and suspend the insulin infusion without the input of the wearer in order to prevent potential hypoglycaemia. Glucose suspension can be a simple ‘low glucose suspend’ function, in which insulin infusion is suspended when the glucose monitoring system detects that glucose levels have fallen below a specific hypoglycaemia threshold. In this case, insulin is suspended for a period of time and may resume when the system determines that glucose levels have returned to within the target range or when the glucose suspension is overridden by the patient.
Predictive low glucose suspend (PLGS) is a more advanced use of technology in which prediction algorithms are used that essentially forecast future hypoglycaemia (e.g. within the next half-hour) and pre-emptively suspend insulin delivery before hypoglycaemia develops. PLGS systems will then automatically resume insulin infusions if the user overrides the suspension, or if glucose levels begin to rise or rise above a specific threshold. 29,30
Chapter 2 Definition of the decision problem
Decision problem
Interventions
The interventions of interest are HCL systems, a class of automated insulin delivery system that have three components: a CGM, a microprocessor with control algorithms and a pump.
Several HCL systems are available in the UK, such as MiniMed 670G and MiniMed 780G. The systems are representative of the intervention of interest and have been identified by NICE as currently available in the UK.
Population including subgroups
The population and subgroups are per NICE scope (published March 2022).
| Populations | People who have T1DM and who are having difficulty managing their condition despite prior use of at least one of the following technologies: continuous subcutaneous insulin infusion, real-time continuous glucose monitoring, flash glucose monitoring.a,b |
| If evidence permits, the following T1DM subpopulations will be included: | |
|
Relevant comparators
| Comparator |
|
| Where evidence permits, scenarios assessing the following comparators will be presented for women with T1DM who are pregnant/planning pregnancy: | |
|
Outcomes
Intermediate measures
-
Time in target range (percentage of time a person spends with blood glucose level in target range of 3.9–10 mmol/l).
-
Time below and above target range.
-
Change in HbA1c.
-
Rate of glycaemic variability.
-
Fear of hypoglycaemia.
-
Rate of SHE (events recorded/unit time).
-
Rate of severe hyperglycaemic events (events recorded/unit time).
-
Episodes of DKA (events recorded/unit time).
-
Rate of ambulance call-outs (events recorded/unit time).
-
Rate of hospital outpatient visits (events recorded/unit time).
-
Measures of weight gain.
Clinical outcomes
-
Retinopathy.
-
Neuropathy.
-
Cognitive impairment.
-
End-stage renal disease (ESRD).
-
CVD.
-
Mortality.
Additional clinical outcomes in women who are pregnant/have recently given birth
-
Premature birth.
-
Miscarriage related to fetal abnormality.
-
Increased proportion of babies delivered by caesarean section.
-
Macrosomia (excessive birthweight).
-
Respiratory distress syndrome in the newborn.
Device-related outcomes
-
Adverse events related to the use of devices.
Patient-reported outcomes
-
HRQoL.
-
Psychological well-being.
-
Impact on patient (time spent managing the condition, time spent off work or school, ability to participate in daily life, time spent at clinics, impact on sleep).
-
Anxiety about experiencing hypoglycaemia.
-
Acceptability of testing and method of insulin administration.
Carer-reported outcomes
Impact on carer (fear of hypoglycaemia, time spent managing the condition, time spent off work, ability to participate in daily life, time spent at clinics, impact on sleep).
Overall aims and objectives of assessment
The overall objectives of this project are to examine the clinical effectiveness and cost-effectiveness of HCL systems for managing glucose levels in people who have T1DM. The key questions for this review are provided below.
Key question 1
What is the clinical effectiveness of HCL systems for managing glucose in people who have T1DM and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
Subquestions
-
What is the clinical effectiveness of HCL systems for managing glucose in pregnant women who have T1DM?
-
What is the clinical effectiveness of HCL systems for managing glucose in children who have T1DM and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
-
What is the clinical effectiveness of HCL systems for managing glucose in people who have T1DM, an extreme fear of hypoglycaemia, and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
-
What is the clinical effectiveness of HCL systems for managing glucose in people who have T1DM, have diabetes-related comorbidities that are at risk of deterioration, and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
Key question 2
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
Subquestions
-
What is the cost-effectiveness of HCL systems for managing glucose in pregnant women who have T1DM?
-
What is the cost-effectiveness of HCL systems for managing glucose in children who have T1DM and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
-
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM, have an extreme fear of hypoglycaemia, and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
-
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM, have diabetes-related comorbidities that are at risk of deterioration, and are having difficulty managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGM?
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
The systematic review methods followed the principles outlined in the Cochrane Handbook for Diagnostic Test Accuracy Reviews31 and the NICE Diagnostics Assessment Programme Manual. 32 A PRISMA Checklist for systematic review reporting is provided in Report Supplemenatry Material 1
Methods for reviewing effectiveness
Identification of studies
Search strategy
The search strategy comprised the following main elements:
-
searching of electronic bibliographic databases and other online sources
-
contacting experts in the field
-
scrutiny of references of included studies, relevant systematic reviews, and the most recent NICE guidance on systems that combine CGM and CSII. 4
A comprehensive search was developed iteratively and undertaken in a range of relevant bibliographic databases and other sources, following the recommendations in chapter 4 of Cochrane Handbook for Systematic Reviews of Interventions. 33 Search terms were related to type 1 diabetes (including a separate set of terms relating to pregnant women and women planning pregnancy) and technologies to manage blood glucose levels. Search strings applied in the previous technology assessment on integrated SAP therapy systems (DG21)34 were used as the basis for developing selected lines relating to T1DM, insulin pumps, SAP and MDI, and other systematic reviews informed the lines relating to pregnancy. 35–37 The main MEDLINE search strategies were independently peer reviewed by a second information specialist.
Date limits were used to identify records added to databases since the searches for DG21 (run in 2014). 34 Searches were conducted in March and April 2021, and updated in April 2022, in the following resources: MEDLINE ALL (via Ovid), EMBASE (via Ovid), Science Citation Index and Conference Proceedings (via Web of Science), Cochrane Database of Systematic Reviews (via Wiley), CENTRAL (via Wiley), ClinicalTrials.gov; the HTA database [via Centre for Reviews and Dissemination (CRD)], the international HTA database (INAH); and the NIHR Journals Library. Searches were also conducted of the following websites:
-
US Food and Drug Administration (FDA).
-
Medicines and Healthcare products Regulatory Agency (MHRA).
-
Agency for Healthcare Research and Quality (AHRQ).
-
Canadian Agency for Drugs and Technologies in Health (CADTH).
-
Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU)
The search was developed in MEDLINE (via Ovid) and adapted as appropriate for other resources. Full search strategies are provided in Appendix 2.
Records were exported to EndNote X9, where duplicates were systematically identified and removed. Where available, alerts were set up so that the team were aware of any new, relevant publications added to databases after the original search date.
Inclusion and exclusion criteria
Studies that satisfied the inclusion/exclusion criteria reported in Appendix 3 were included.
Research papers were included in which it could not be established if all study participants had difficulty managing their condition (defined by HbA1c, fasting plasma glucose, non-fasting plasma glucose, or TIR as above), if the group mean met this criterion.
Papers that fulfilled the following criteria were excluded:
-
non-human studies, letters, editorials and communications qualitative studies
-
studies conducted outside routine clinical care settings, for example, inpatient research facilities, diabetes summer camps
-
studies where > 10% of the sample did not meet the inclusion criteria (e.g. > 10% were inpatients)
-
studies without extractable numerical data
-
studies that provided insufficient information for assessment of methodological quality/risk of bias
-
articles not available in the English language
-
studies evaluating individual components and not complete HCL systems
-
studies of DIY (do-it-yourself) closed-loop systems, which are not approved by regulatory bodies38
-
studies evaluating automated insulin delivery systems that only suspend insulin delivery when glucose levels are low/are predicted to get low.
Review strategy
Prioritisation strategy for full-text assessment
We applied a two-step approach for identifying and assessing relevant evidence. We applied stricter criteria at the point of data extraction/risk of bias than at title and abstract assessment to prioritise and select the best available evidence. 39–41 The elements used to prioritise evidence (study design, study length, sample size) were chosen in collaboration with NICE and diabetes clinicians as those that would provide the most applicable evidence.
Step 1
The studies were scoped in EndNote before deciding which studies qualified for full-text assessment (step 2). Records were coded in terms of study design and study duration. RCTs were prioritised over controlled trials. Non-randomised controlled trials/comparative effectiveness studies were prioritised over non-comparative studies. Longer-term studies (≥ 6 months) were prioritised over shorter-term studies.
Step 2
Studies identified in step 1 went through the standard systematic reviewing approach of full-text assessment. We followed the predefined PICO (see Chapter 2) to assess the eligibility of studies.
Prioritisation strategy for data extraction and risk of bias
Given the limited time and resources available, deprioritised studies, that is the large number of observational studies that otherwise met the inclusion criteria for this review, were narratively reported and listed. RCTs were prioritised for data extraction and quality assessment. 41
Data extraction strategy
We extracted the following study characteristics (informed by the scope): details on study design (parallel, factorial or crossover) and methodology, participant characteristics, intervention characteristics, comparator characteristics, outcomes, outcome measures and additional notes (such as funding).
Two reviewers extracted data independently using a piloted data extraction form. Disagreements was resolved through consensus, with the inclusion of a third reviewer if required.
Critical appraisal strategy
The risk of bias of randomised trials was assessed using the revised Cochrane risk-of-bias tool for randomised trials. 42 Risk of bias in controlled trials, non-randomised trials and cohort studies was assessed using the Cochrane Risk Of Bias In Non-randomised Studies of Interventions tool. 43 Risk of bias for case–control studies and controlled before-and-after studies was assessed using Effective Practice and Organisation of Care risk of bias tool. 44 Two reviewers assessed risks of bias. Disagreements were resolved through consensus, with the inclusion of a third reviewer if required.
Methods of data analysis/synthesis
We synthesised the RCT evidence statistically. The NMA was conducted using a frequentist approach and a random-effects model.
Subgroup analyses were undertaken where possible for the different combinations of interventions study participants had previously used to manage their blood glucose (i.e. flash glucose monitor and multiple daily insulin injections, flash glucose monitor and CSII, rtCGM and multiple daily insulin injections, rtCGM and CSII, self-blood glucose monitoring and CSII).
Pairwise and network meta-analysis
The analysis compared HCL systems and relevant comparators for managing blood glucose levels in T1DM. The primary effectiveness outcome was HbA1c. Other clinically relevant outcomes include the ‘time in target range’, which gives the percentage of time that a person spends with blood glucose level in target range of 70–180 mg/dl, and adverse events (e.g. severe hypoglycaemia, DKA).
Decisions about information to include in the NMA were informed by relevance to the decision problem and sufficient similarity across studies (e.g. patient characteristics and study design) to reduce the risk of violating underlying assumptions of transitivity/coherence when pooling direct and indirect evidence across studies. We used an iterative process45 to define the extent of the treatment network and to identify studies for inclusion. This involved first defining an initial core set of interventions that met the criteria set out in the projects’ scope and included trials of such interventions in T1DM populations.
Publication bias was assessed visually using a comparison-adjusted funnel plot, where publication bias is present if the funnel plot is asymmetrical. Egger’s test was also used, where publication bias is considered to exist if the p-value is < 0.05.
Transitivity was assessed by looking at the distributions of potential effect modifiers across all studies included in the systematic review.
To check for consistency of each network, net splitting can be performed, which splits the estimates in the network into direct and indirect estimates. Statistically significant inconsistency is present between the direct and indirect estimates if the p-value of the difference between effect estimates (ES) is < 0.05. However, owing to the small number of studies and treatments in each network, net splitting was not feasible. Loop consistency was also not tested as there were no closed loops in the networks for any of the outcomes.
Treatments were ranked using P-score, which measures the certainty that one treatment is better than another treatment, averaged over all competing treatments.
Statistical analyses were performed using RStudio version 4.1.0 (Posit Software, Boston MA, USA).
Dealing with missing data
We conducted the review according to the registered protocol.
Chapter 4 Patient and public involvement and engagement
At the start of the project, we followed the collaborative consultation approach that was considered most suitable for this review. One service user participated as a consultant. The consultant provided feedback on the scope, review and writing of the plain language summary, answered technical and user queries about T1DM technologies and provided feedback on our interpretation of the condition/technology. A public user was a member of the NICE committee. The public representative accessed our report, attended meetings, presented their views at the committee meetings and raised discussion points on the report findings.
Chapter 5 Equality, diversity and inclusion
We set out to explore the clinical effectiveness and cost-effectiveness of HCL systems for managing glucose levels in people who have T1DM. We examined the evidence in different age groups (children and adults) and in pregnant women. The evidence did not permit an examination of the effectiveness of HCL systems by patient ethnicity as these data were not clearly reported across studies.
Results
Number of studies identified
The literature search provided 12,890 records potentially related to the area of interest; 7292 records remained after duplicates were removed. After the abstract screening, 1364 records were identified for full-paper screening. A further 1326 articles were excluded at the full-text stage mainly as a result of incorrect intervention/comparators, study design, incorrect population, abstract/poster presentation only or further duplication identified. Fourteen records (12 RCTs)27,46–58 and nine observational studies27,59–64 are presented for this systematic review of clinical effectiveness. Three papers drew on the same study participants. External submissions, including NHS England evidence and company submissions, are also presented in this report.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is shown in Figure 1.
FIGURE 1.
The PRISMA flow diagram (14 RCTs were included; however, two studies were treated as single-arm studies).
Number and type of studies included
Randomised controlled trials
Twelve RCTs (one53 with two relevant intervention arms)46–56,58 were identified that yielded data of potential relevance to the decision problem assessing HCL against a comparator. RCTs in which HCL treatment was received for ≥ 4 weeks (range 4–26 weeks) were included if the comparator was relevant to the decision problem (comparators were classified as CSII + CGM and LGS/PLGS).
Most of these studies reported results for outcomes relevant to monitoring glycaemic management.
These data were assembled using CGM technology that accumulates a large number of data, and they assessed change in % TIR over a specified period of observation (start/baseline to final). Most studies reported change in HbA1c% level (final minus start/baseline) values. The RCTs thus provided quantitative data potentially amenable to NMA. Two publications27,57 were derived from the Fuzzy Logic Automated Insulin Regulation (FLAIR) study and presented data comparing HCL with AHCL; as HCL has been viewed here as a generic intervention, the FLAIR study can be considered more similar to a single-arm study (with two subgroups) than to an RCT and is considered in Table 2.
These RCTs were heterogeneous in multiple respects, including trial design (parallel-group or crossover design with washout phase between different treatments), participants’ age, number of participants and other demographics including run-in times, duration of observation periods, and numbers and types of previous treatments. Studies screened relatively small numbers of patients. The number of participants randomised ranged from 16 to 135, and studies were included whose authors classified recruiting participants variously as very young children, children, adolescents, young adults and older adults.
Table 1 summarises the main characteristics of patients recruited in RCTs with treatment lasting 4–26 months. Most studies were conducted in children or young adults. For young children it would likely be difficult to clearly establish whether they were having difficulty in managing glycaemia prior to recruitment. Only McAuley et al. 50 and Boughton et al. 47 looked at HCL use in elderly patients (aged > 60 years); in the control arm, for practical reasons and because of their familiarity with the method, the participants continued with their previous method of glycaemic management, which presumably was long-established (i.e. they were not ‘re-trained’ in a new non-HCL method). In treatment arms, participants were trained in the use of devices before performance was assessed. Both these studies in elderly people enrolled relatively few patients.
| Study | Change in HbA1c% | % time > 10 mM | % time 3.9–10 mM | % time < 3.9 mM | % time < 3.5 mM | % time < 3.3 mM | % time < 3.0 mM | % time < 2.8 mM | Hypo events | Ketotic events |
|---|---|---|---|---|---|---|---|---|---|---|
| Ware et al. 202255 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Population (N = 74): diagnosed ≥ 0.5 years previous; pump ≥ 3 months; HbA1c < 11% no previous HCL. Very young children aged 1–7 years | ||||||||||
| von dem Berge et al. 202254 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Population (N = 38): pump ≥ 3 months; total insulin > 8 U/day; HbA1c 7.4% (± 0.9); no severe hypo in last 3 months. Pre-school and school children aged 2–14 years | ||||||||||
| Thabit et al. 201553 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Population (N = 25): diagnosed ≥ 0.5 years previous; aged ≥ 6 years; pump ≥ 3 months; HbA1c < 10%. Children/adolescents aged 6–18 years Population (N = 33): diagnosed ≥ 0.5 years previous; aged ≥ 18 years; pump ≥ 0.5 year; HbA1c 7.5–10% |
||||||||||
| Ware et al. 202256 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Population (N = 135): diagnosed ≥ 1 year previous; pump ≥ 3 months; HbA1c 7.5–10%. Children/adolescents aged 6–18 years | ||||||||||
| Tauschmann et al. 201852 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Population (N = 86): diagnosed ≥ 1 year previous; aged ≥ 6 to 20 years; pump ≥ 3 months; HbA1c 7.5–10%; no CGM previous 3 months. Children and young adults aged 22 years (13–26 years) | ||||||||||
| Benhamou et al. 201965 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Population (N = 63): diagnosed ≥ 2 years previous; aged ≥ 18 years; ≤ 50 U per day; HbA1c ≤ 10%. Adults aged 48.2 years (± 13.4 years) | ||||||||||
| Boughton and Hovorka 201947 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Population (N = 37): diagnosed ≥ 2 years previous; aged ≥ 18 years; ≤ 50 U per day; HbA1c ≤ 10%. Adults aged 48.2 years (± 13.4 years) | ||||||||||
| McAuley et al. 202250 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Population (N = 30): diagnosed ≥ 10 years; age ≥ 60 years; using insulin pump; HbA1c ≤ 10.5%; no dementia. Elderly people aged 67 years (± 5 years) | ||||||||||
| Collyns et al. 202148 and Wheeler et al. 202258 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Population (N = 60): diagnosed ≥ 1 years; age 7–80 years; pump ≥ 6 months; daily insulin minimum 8 units; HbA1c < 10%; no pregnancy. Children aged 7–13 years, n = 19; adolescents aged 14–21 years, n = 14; adults aged 22–80 years, n = 26 | ||||||||||
| Kariyawasam et al. 202249 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Population (N = 22): diagnosed ≥ 1 year; age 6–12 years; pump ≥ 3 months; HbA1c ≤ 9.0%; hospital 3 days then 6 weeks post-hospital phase. Young people aged 6–12 years | ||||||||||
| Stewart et al. 201851 | ✓ | ✓ | a | ✓ | ||||||
| Population (N = 16): women (singleton pregnancy); diagnosed ≥ 1 year prior to pregnancy; aged 18–45 years; HbA1c (8% (± 1.1); excluded if insulin dose ≥ 1.5 U per kg | ||||||||||
The major outcomes reported in the RCTs related to monitoring glycaemic management. These included change in HbA1c% and % time within, above or below a defined blood glucose level (mmol/l), including % time within range indicating satisfactory control (3.9–10 mmol/l, % time in a hyperglycaemic range (> 10 mmol/l), and % time in a hypoglycaemic range, variously < 3.9, < 3.5, < 3.3, < 3.0 and < 2.8 mmol/l depending on the study. Low rates (events recorded/unit time) of severe hypoglycaemia and of ketotic episodes were also reported; it may be that the small number of participants and relatively short treatment periods mean that accurate estimates of the rates of these events are difficult to obtain. The outcomes reported in RCTs are summarised in Tables 2 and 3.
| Study | Design | Comment |
|---|---|---|
| NHS pilot study adults; HCL | Analysis of an audit of routinely available data (from 31 diabetes centres) of a cohort of NHS adult patients with elevated HbA1c who received HCL intervention starting between August and December 2021. Data collection to May 2022 | Outcome values reported for start of HCL and at end of study |
| Forlenza et al.64 2022; HCL | A prospective study. Selection of patients for enrolment unclear. Enrolled patients were screened for inclusion according to prespecified criteria, and those satisfying criteria entered a run phase followed by 3-month study phase. Methods were referenced to an earlier study | Results represent a subset of children able to use the HCL system |
| Beato-Vibora et al.60 2021; ‘group 4’ HCL (MM670G) | Cross-sectional study of HCL (MiniMed 670G System with Guardian Sensor 3) recipients followed (at a hospital) for about 13 months (other treatment modalities were also reported) | |
| Bassi et al.59 2022; two AHCLs (A, MM780G; B, Control-IQ) | Retrospective comparison of two AHCLs (Medtronic 780G and Control-IQ) after propensity matching of patients who upgraded to an AHCL system for 1 month; HbA1c was not reported | Propensity matching selects patient subgroups and may render study population less ‘real world’ |
| Beato-Vibora et al.61 2021; AHCL MM780G | Prospective study of patients transferred to an AHCL system (c780G AHCL) from previously used modality. Method of patient selection unspecified | |
| Breton and Kovatchev62 2021; AHCLAHCL slim X2 pump with Control-IQ | A retrospective analysis of a sample (n = 7801) of a self-selected group of patients who used AHCL slim X2 pump with Control-IQ. Subjects self-selected by uploading data to ‘Tandem’s t:connect web application as of February 11, 2021’; 9451 patients met inclusion criteria; 83% had T1DM; results for 7801 T1DM subjects were presented | Non-self-selectors not represented. Ratio of self-selectors to non-self-selectors not reported |
| Carlson et al.63 2022; AHCL MM | Prospective ‘safety’ study of (a) adolescent and (b) adult patients using an AHCL (MiniMed closed-loop system) for 45 days. Industry-sponsored study | Restrictive inclusion and exclusion criteria |
| Bergenstal et al.27 2021; HCL MM 670G; AHCL as but with updated software. Crossover study | Because in the present report HCL and AHCL are both considered HCL interventions, Bergenstal et al.26 has been classified as an ‘observational’ single-arm study (single population) observed prospectively for two examples of the HCL intervention. There was no washout period at time of switch between the two HCL interventions; the first HCL intervention used was determined randomly | |
| NHS pilot study CYP; HCL | Analysis of an audit of routinely available data (from eight paediatric centres) of a cohort of young NHS patients with elevated HbA1c who received HCL intervention starting between August and December 2021. Data collection to May 2022 | Outcome values reported for start of HCL and at end of study |
| Study | Population at recruitment | Age description | N |
|---|---|---|---|
| NHS pilot study adults; HCL (report provided to EAG by NICE, 17 June 2022) | NHS services adults with T1DM managed with an insulin pump and flash glucose monitor with an HbA1c ≥ 8.5%; aged > 18 years | Adult; median 40 years (IQR 28–50 years) | 640 (63 lost to follow-up) |
| Forlenza et al.64 2022; HCL | Diagnosed ≥ 0.25 years; pump ≥ 3 months; HbA1c < 10%; total insulin ≥ 8 U per day; no severe hypo in last 3 months | Children; 2 to < 7 years | 46 |
| Beato-Vibora et al.60 2021; ‘group 4’ HCL (MM670G) | T1DM for 29 years (± 9.4 years); pregnant women excluded. Cross-sectional study | Adult; 38 years (± 11 years) | 43 |
| Bassi et al.59 2022; two AHCLs (A = MM780G; B = Control-IQ) | Diagnosed ≥ 1 years; previous CSII or MDI; use of CGM: ≥ 1 month before and after starting the AHCL. Dropouts from AHCL before 1 month of use were excluded | 24.4 years (± 15.7 years) | A 51; B 39 |
| Beato-Vibora et al.61 2021; AHCL MM780G | HbA1c% 7.23 (± 0.86); pregnant women excluded | Adult 43 years (± 12 years) | 52 |
| Breton and Kovatchev62 2021; AHCLAHCL slim X2 pump with Control-IQ | Users of AHCL; patients in US ‘Tandem’s Customer Relations Management database’ | Range 6–91 years | 7801 |
| Carlson et al.63 2022; AHCL MM | Diagnosed ≥ 2 years; T1DM for at least 2 years. Minimum daily insulin ≥ 8 U; HbA1c% < 10; willingness to use device. Excluded if history of severe hypos, diabetic ketosis | Adolescents and adults. 38.3 years (± 17.6 years) | 157 |
| Bergenstal et al.27 2021; HCL MM 670G; AHCL as but with updated software. Crossover study | Diagnosed ≥ 1 year; aged 14–29 years; HbA1c 7.0–11.0%; excluded if ≥ 1 severe hypo | 14–29 years | 112 |
| NHS pilot study CYP; HCL (report provided to EAG by NICE, 17 June 2022) | Children or young people aged 1 to < 19 years; TIDM for ≥ 1 year; minimum of 2 prior HbA1c measures | 6.6 years (± 3.7 years), range 2–18.9 years | 251 |
Outcome results reported in the RCTs are summarised in Appendix 4, Table 25. Glycaemic management outcomes by study arm were reported in various ways, as mean [standard deviation (SD)] or median [interquartile range (IQR)] values; often start/baseline values for each arm were not reported or were unclear so that change from baseline was sometimes unreported and only end-of-treatment values were provided. Trials reported a difference and its range between arms, whether this was represented as a median or a mean for a particular outcome. These reported values were available for NMA. Where necessary some outcome results have been calculated from numerical data in the relevant published reports; these, together with most other data reported, were often strongly rounded to only a few significant figures. Appendix 4, Table 25, summarises the data extracted from the included RCTs.
Because many different outcomes were reported by authors, Appendix 4, Table 25 is organised by columns specifying outcomes in the following order: HbA1c%; % time > 10 mmol/l; % TIR (3.9–10 mmol/l); % time < 3.9 mmol/l; % time < 3.5 mmol/l; % time < 3.0 mmol/l; % time < 2.8 mmol/l; non-severe hypo events; severe hypo events; DKA events.
Each study outcome value within these columns is represented by trial arm as (1) start of study, (2) end of study and (3) end of study minus start of study difference. The start/baseline value was not necessarily the same in both arms of a trial.
The last row for each study represents the value reported for the difference between arms and is labelled NET effect.
Authors reported values sometimes as mean (SD), sometimes as median or median (IQR), and sometimes as mean [95% confidence interval (CI)]. Values that are medians are asterisked.
Individual study details are itemised as n = number of participants; age of participants in years; interventions HCL, CSII + CGM OR LGS/PLGS; Tx = treatment period in weeks; study design, parallel-arm or crossover study.
Data input for meta-analyses
Outcome results reported in the RCTs are summarised in Appendix 4, Table 25. For reader information, some summary values (e.g. change from start to end in a trial arm) that were unreported in published reports have been estimated from numerical data elsewhere in the trial reports.
The results from standard random-effects meta-analyses are presented as forest plots. The effect size is the mean difference (MD) between trial arms in the mean change from start to end of study (MD is sometimes called weighted MD).
Published data available for extraction and further use in meta-analysis presented several difficulties. Most data reported were strongly rounded so that outcomes (end of study minus start of study) by study arm, and also net effect (MD) of intervention versus comparator, were usually reported to only two significant figures. These estimates were sometimes reported as a mean (SD, or 95% CI) or as a median (IQR). This was not consistent for a particular outcome across different studies; for example, the hyperglycaemic outcome ‘time in range at glucose concentration > 10 mmol/l’ was reported in some studies as median (IQR) and in others as mean (SD). The measure chosen by authors appeared to depend on the skewness of the data distribution. Sometimes start/baseline or end-of-study values for each arm were not reported or were unclear, and change from baseline in each arm was sometimes unreported.
Trials reported the difference between arms (difference between intervention and comparator in change from start to end of study) sometimes as mean (SD) difference and sometimes as median (IQR) differences for a particular outcome. As meta-analysis of medians is problematic, where reported values were deemed useful, median (IQR) values were converted to means and variance values using the Vasserstats website (http://vassarstats.net/median_range.html). The difference between arms reported in some RCTs was developed using complex models and therefore did not necessarily correspond to separate data reported for each arm. In these circumstances the model-adjusted outcome was used for meta-analysis.
These characteristics of the available data represent limitations to the accuracy of meta-analytic results.
Glycated haemoglobin per cent
Figure 2 shows the reported change (start to end of study) in HbA1c% for each arm over the treatment period. The standard meta-analysis shows the MD between treatment arms. A negative ES is presented in Appendix 5, Figure 19, comparing HCL with comparator, infers superior management of glycaemia with HCL.
FIGURE 2.
Change (end minus start as mean ± SD or median) by arm in HbA1c% over treatment period in RCTs.
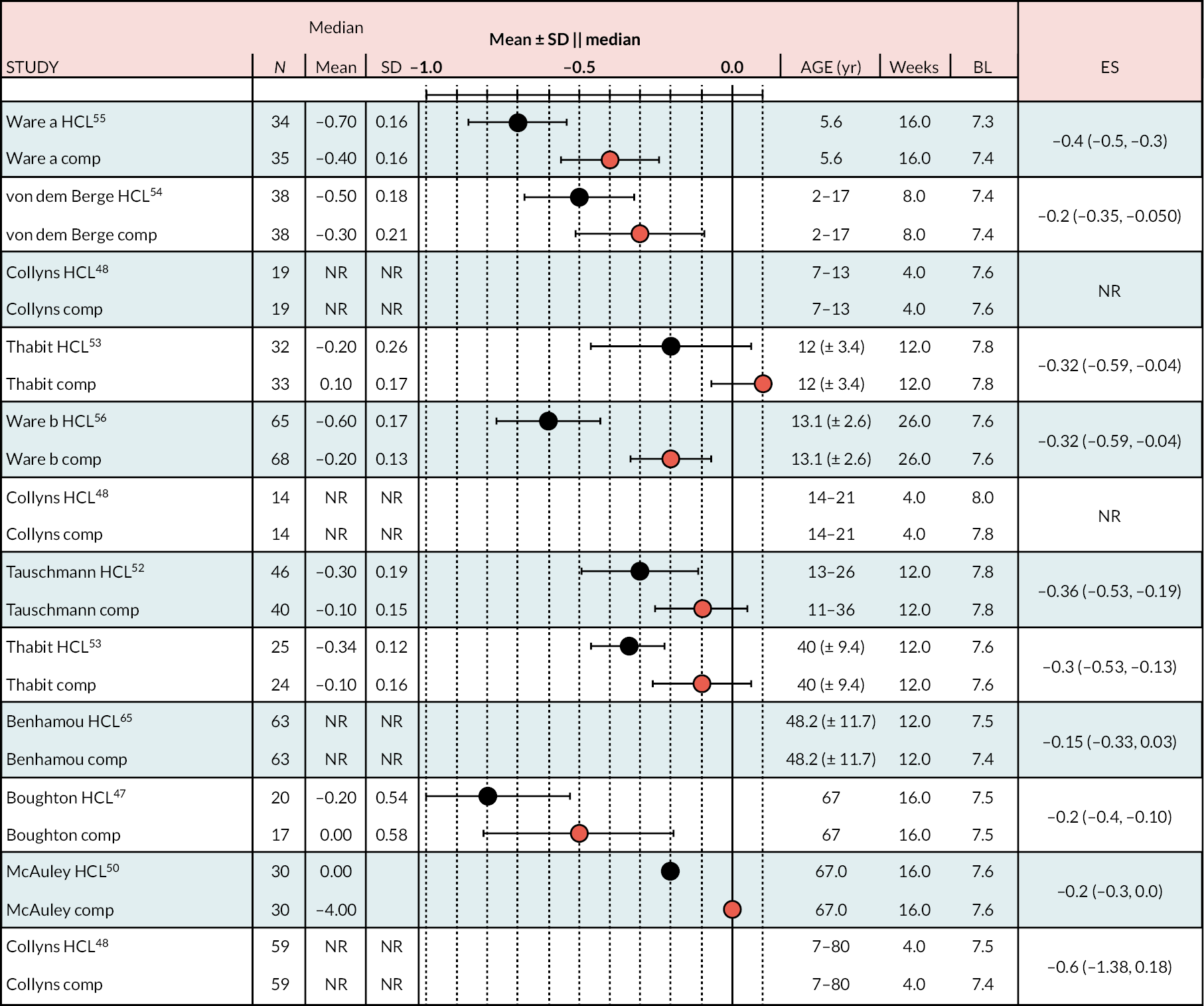
The range of mean baseline HbA1c% in the RCTs was narrow: 7.4–8.3. In all studies the reduction in HbA1c% (end minus start of study) was greater in the HCL arm than in the comparator arm (either CSII + CGM or LGS/PLGS). The change in HbA1c% over the treatment period is modest in the HCL arm (range −0.2 to −0.8). Net effect sizes (MD between treatments, HCL vs. comparator) are modest, ranging from approximately −0.15 to approximately −0.4. Relative to the NHS ‘real world’ adult pilot study, in RCTs the start/baseline HbA1c% is lower (NHS baseline = 9.4 HbA1c%) and the MD is smaller (NHSES = −1.6). In the NHS pilot study (described in Methods for assessing cost-effectiveness evidence) treatment with HCL brings the mean HbA1c% to 7.9, approaching a level comparable with the upper range values seen in RCTs after HCL use. Not included in the forest plot is the FLAIR study27 comparing two AHCL versus HCL with baseline HbA1c% = 7.9. Change from baseline was similar to that in the RCTs: −0.5 (± 0.10) with HCL and −0.3 (± 0.09) with AHCL.
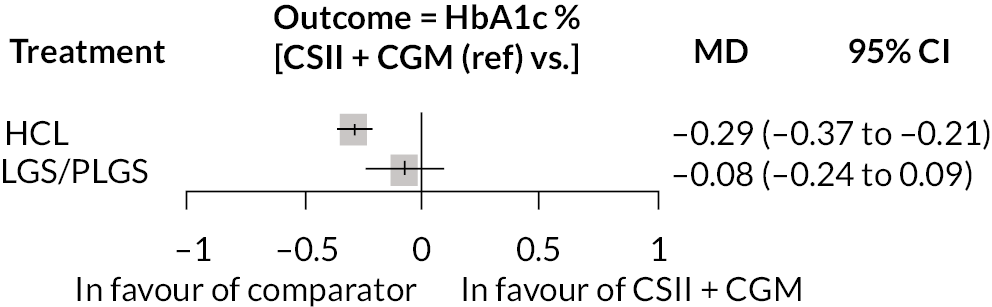
Ten estimates from nine studies (Figure 3) were included in this NMA because estimates from the Thabit study arms were split into adult and children estimates. The reference treatment class was CSII + CGM, where estimates > 0 favoured CSII + CGM. The forest plot of the NMA is presented in Figure 3. Compared with CSII + CGM, treatment with HCL decreased HbA1c% by 0.28 (95% CI −0.34 to −0.21). There was no statistically significant difference between CSII + GCM and LGS/PLGS.
FIGURE 3.
Results of the NMA of the outcome change in HbA1c% during observation period.
Per cent time within range (between 3.9 and 10.0 mmol/l)
In all the RCTs, the increase in % TIR was greater in the HCL arm than in the comparator arm (Figure 4), in all cases reaching statistical significance (p < 0.05). The lowest mean start/baseline value % TIR was 40%; in all other RCTs it was > 50%. In the NHS pilot study (described in Methods for assessing cost-effectiveness evidence), the start/baseline value was 34.2%, allowing considerable scope for improvement with HCL treatment, which was 28.5% (unadjusted; 95% CI 31.4 to 13.5). The change from start/baseline in the HCL arm of RCTs recruiting adults of similar age to those in the adult NHS pilot study ranged from ≈ 10% to ≈ 15%, approximately half of that in the pilot. The improvement in % TIR appears to be greater the smaller the start or baseline level.
FIGURE 4.
Change (end minus start as mean ± SD or median) by arm % TIR (3.9–10.0 mmol/l) over treatment period.
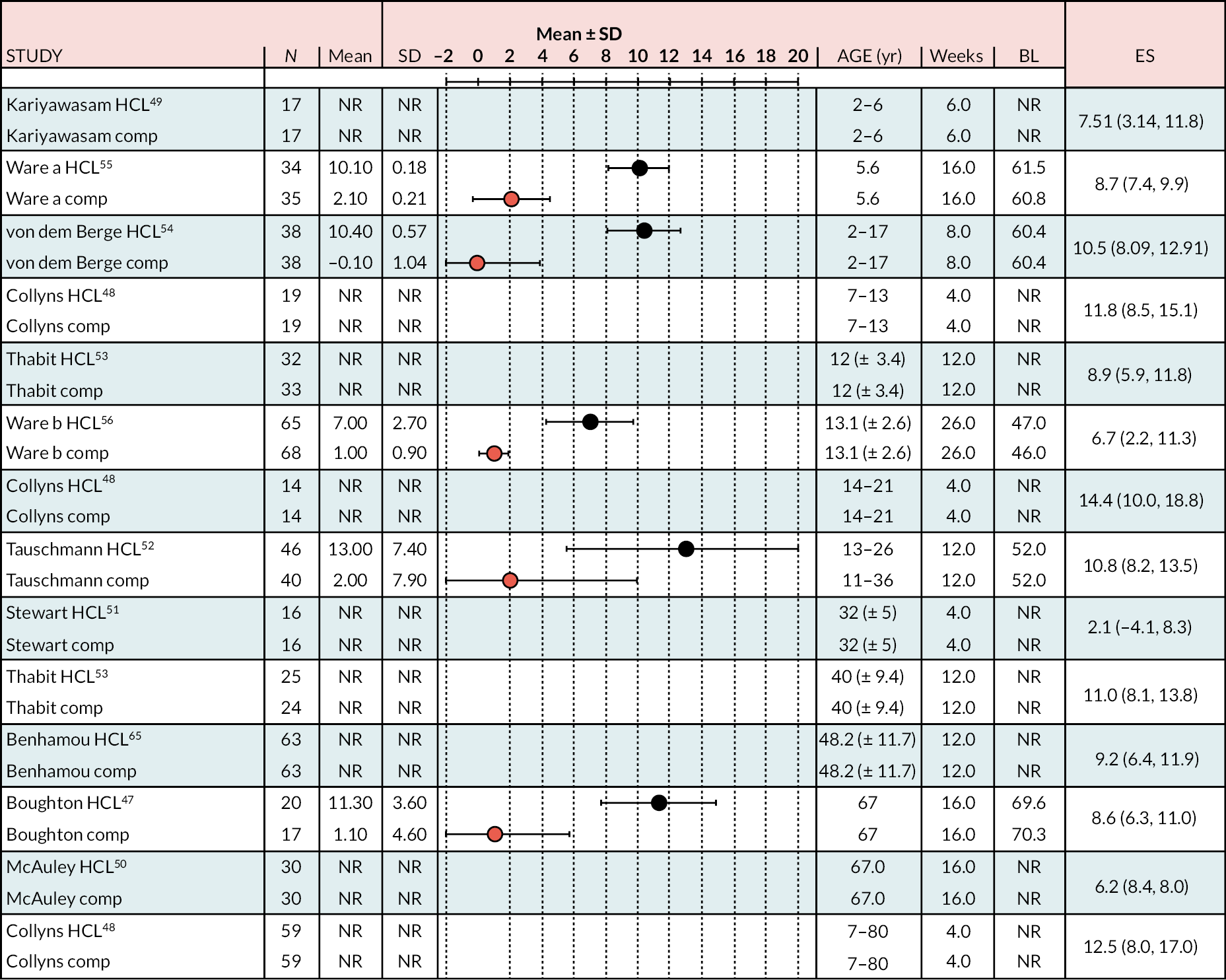
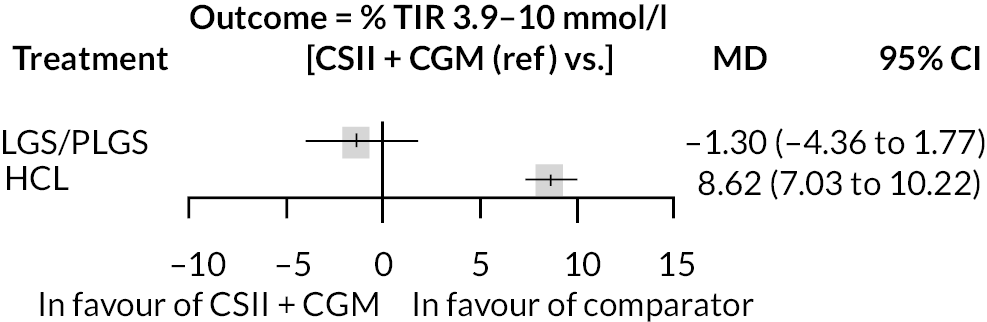
The meta-analysis pooled estimate for the comparison of HCL with CSII + CGM was considerably influenced by the inclusion of the Stewart et al. 51 study (see Appendix 6, Figure 20). This study included only pregnant women, and treatment duration was shorter than in other studies. The exclusion of Stewart et al. resulted in greater pooled estimate for HCL versus CSII + CGM (MD 9.1). There were 13 estimates from 12 studies that were included in this NMA, as estimates from Thabit were split into adult and children estimates. The reference treatment class was CSII + CGM, where estimates < 0 favoured CSII + CGM. The forest plot of the NMA is presented in Figure 5. Compared with the CSII + CGM treatment classification, HCL significantly increased % TIR (between 3.9 and 10.0 mmol/l), with a MD of 8.6 (95% CI 7.03 to 10.22). There was no statistically significant difference between CSII + GCM and LGS/PLGS.
FIGURE 5.
Results of the NMA of the outcome time in target range (% between 3.9 and 10.0 mmol/l).
Per cent time above range (> 10.0 mmol/l)
Figure 6 shows the change from start/baseline in % time in hyperglycaemic range (> 10.0 mmol/l) for each trial arm. Ware et al. 55 and Boughton et al. 47 reported baseline and follow-up % TIR as median (IQR) without specifying the IQR for the change from baseline; calculating the IQR was problematical and not attempted. The studies by Benhamou et al. 65 and Thabit et al. 53 only reported net ES (difference between arms).
FIGURE 6.
Change (end minus start as mean ± SD or median) by arm in % time in hyperglycaemia.
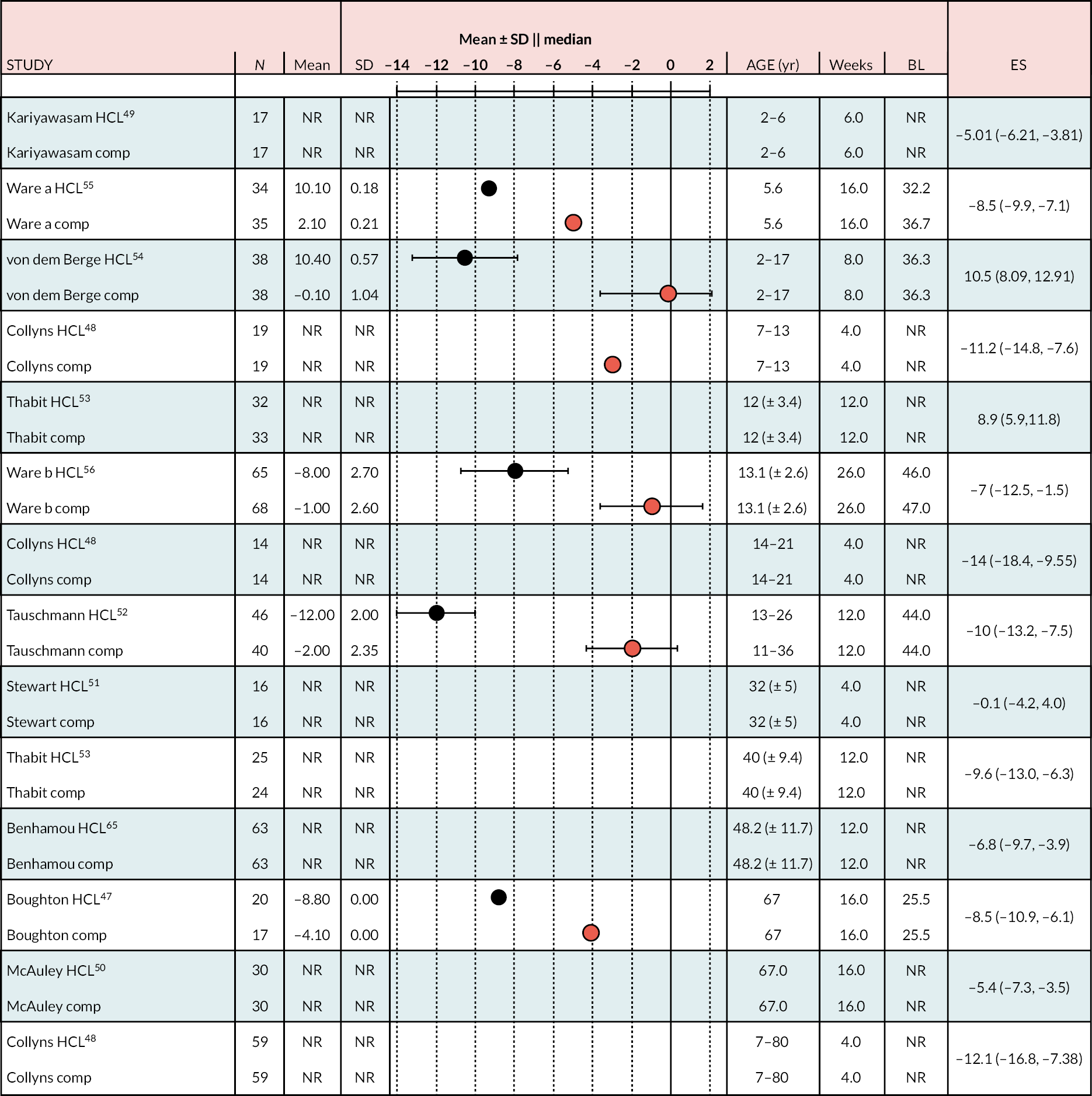
In all studies comparing HCL with CSII + CGM other than the small study in pregnant women by Stewart et al.,51 HCL reduced % time in hyperglycaemic range to a greater extent than in the CSII + CGM arm, and the MD between arms (net effect size; Appendix 7, Figure 21) was statistically significant (p < 0.05). The Stewart et al. study was influential for the comparison of HCL with CSII + CGM; when Stewart et al. was excluded from the meta-analysis the MD increased from −7.2 to −7.88 and heterogeneity reduced. The NHS pilot study (described in Methods for assessing cost-effectiveness evidence) reported an unadjusted reduction in % time in the hyperglycaemic range of ≥ 14 mmol/l (rather than 10 mmol/l) of 22.2%. There was marked heterogeneity in the studies comparing HCL with LGS/PLGS, although the pooled estimate was statistically significant.
There were the same 13 estimates from 12 studies in this NMA as for the outcome % TIR between 3.9 and 10.0 mmol/l. The reference treatment class was CSII + CGM, where estimates > 0 favoured CSII + CGM. Figure 7 presents the NMA results.
FIGURE 7.
Results of the NMA for % time above range 10.0 mmol/l.
Compared with CSII + CGM, HCL significantly decreased TIR (% above 10.0 mmol/l), with a MD of −7.2 (−8.89 to −5.51). There was no statistically significant difference between CSII + GCM and LGS/PLGSs.
Per cent time below range (< 3.9 mmol/l)
Figure 8 summarises % time in hypoglycaemic range of < 3.9 mmol/l (end study minus start) by study arm. The meta-analysis is presented in Appendix 8, Figure 22.
FIGURE 8.
Per cent time below range (< 3.9 mmol/l): change by arm (end minus start as mean ± SD or median) in % time below 3.9 mmol/l over treatment period in RCT.
Because of skewed data, authors mostly reported these results as medians with IQRs, with only a few studies reporting mean (SD). Estimation of the difference between interventions (ES) was also reported as median (IQR) in some studies and as mean (SD) in others, making estimation of the MD between HCL and a comparator problematic.
The published data indicate that at start of study the % time in hypoglycaemia was very low and that this generally became slightly smaller by the end of the study.
Thabit et al. and Benhamou et al. did not report before and after values; Thabit et al. presented ES as a ratio of medians, and Benhamou et al.’s ES was reported as −2.4 (95% CI −3.0 to −1.7). The NHS pilot study (described later in the report) did not report this outcome. In both arms the mean or median % TIR was small (≤ 6%), and the ES (difference between arms) was also small, not reaching statistical significance. Percentage of time in hypoglycaemic range of < 3.0 mmol/l study results were mostly reported as median with IQR; only a few studies reported mean (SD). The mean or median % TIR was < 1.5% in both arms, and the ES values (HCL vs. comparator) reported were very small. This outcome was reported in the NHS pilot study (described in Methods for assessing cost-effectiveness evidence). The % times in range were reported as baseline 0.36% and follow-up 0.34%, providing a difference for HCL of −0.02 (95% CI −0.01 to 0.2). A few studies reported alternative hypoglycaemic ranges (Table 4) with similar results.
| Variable | Audit in adults | National Diabetes Audita |
|---|---|---|
| Age (years) | 40b | 43.4 |
| Diabetes duration (years) | 21 | 24.9 |
| Gender (% male) | 32.8b | 42 |
| Ethnicity (%) | ||
| White | 83.9 | 87.2 |
| Asian | 2.6 | 2.1 |
| Black | 1.2 | 0.9 |
| Mixed | 1.8 | 0.8 |
| Other | 0.7 | 1.0 |
| Unknown | 7.7 | 8.1 |
| HbA1c (mmol/l) | 78.8 | 63.5 |
| HbA1c (%) | 9.4 | 8.0 |
Seven estimates from seven studies were included in this NMA. The reference treatment class was CSII + CGM, where estimates > 0 favoured CSII + CGM. The forest plot of the NMA is presented in Figure 9.
FIGURE 9.
Results of the NMA of the outcome % time below range (3.9 mmol/l).
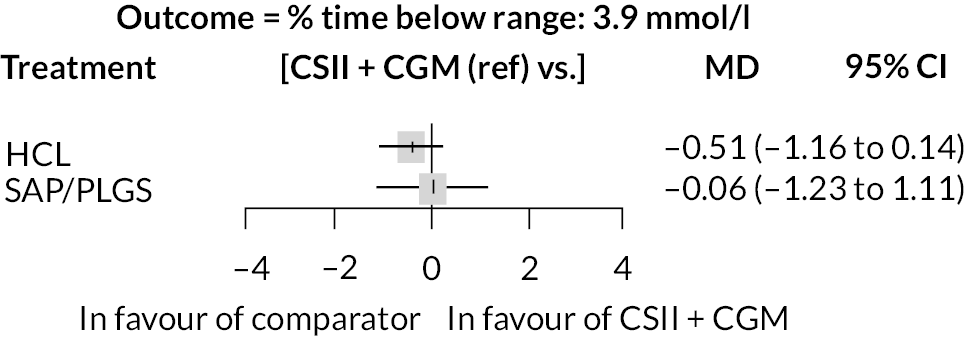
Despite a MD of < 0 for HCL compared with CSII + CGM, as the 95% CI crossed 0, there was no statistically significant difference between HCL and CSII + CGM, and similarly no statistically significant difference between CSII + CGM and LGS/PLGS.
Observational studies (studies or study arms with no intervention other than hybrid closed-loop or advanced hybrid closed-loop)
The study designs of the included single-arm observational studies are summarised in Tables 2 and 3. The main characteristics of the nine observational studies are also summarised in Tables 2 and 3. They provide outcome values indicating glycaemic management in T1DM patients using HCL or AHCL) systems. Two are NHS pilot studies, which are described in reports provided to the Evidence Assessment Group (EAG) (NICE, 17 June 2022), and seven are reported in published articles. 27,59–64 Properties of RCTs not included in the NMA but used for comparing HCL recipients in observational studies are presented in Appendix 9, Table 26.
Most observational studies employed similar inclusion criteria to those used in the RCTs. The NHS adult pilot (described in section 6.1.1) and NHS CYP pilot (described in section 6.1.2) studies were less narrow in recruitment than these and included adult participants who had poorer glycaemic management in terms of HbA1c% and hyperglycaemia (% time above 10 mmol/l (reported separately for ranges 1–14 mmol/l and > 10 mmol/l) at baseline than the other observational studies.
The number of participants across these NHS studies was greater than seen across the RCTs. The adult pilot study accumulated > 200 person-years of HCL observation (more than twice that in RCTs) and the CYP pilot more than approximately 100 person-years (the CYP pilot report was not clear about the numbers of participants with missing data).
Outcome results reported in observational studies are available upon request and are presented graphically in forest plots in which the change from baseline/start to end of study is compared with that seen in the HCL arms of the RCTs.
Figure 10 shows the change from baseline in HbA1c% (end of intervention minus start of intervention) experienced by HCL recipients (i.e. no intervention other than HCL and/or AHCL) reported in identified RCTs and in single-arm observational studies. The range of change is narrow across RCTs and most single-arm trials. The improvement in HbA1c% level (1.6%) was much greater in the NHS pilot adult study and the baseline level (9.4 %) was considerably above those in all other studies so that there was a greater scope for improvement. In the NHS pilot with CYP, the baseline HbA1c was lower (≈7.8%) and the benefit more modest (−0.64%) than in the adult study.
FIGURE 10.
Change in HbA1c (%) from baseline in study participants receiving HCL or AHCL intervention.
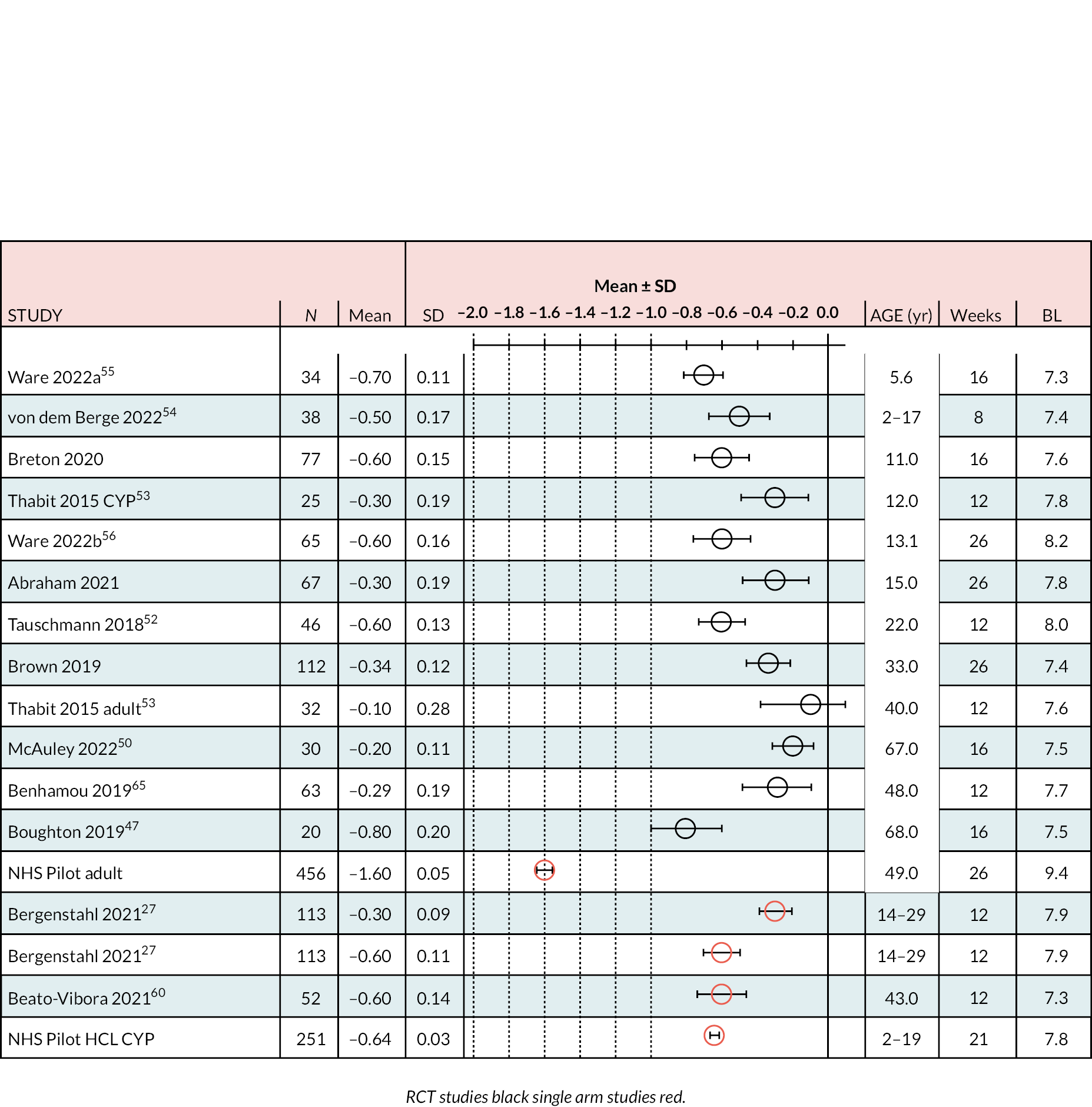
As the effect size (−1.6) and baseline value (9.4) are greater for the NHS adult pilot study than for other studies, it may be reasonable to assume that this may derive from greater scope for improvement (reduction in HbA1c%) at high baseline HbA1c%. We therefore assumed a linear regression (effect size vs. baseline) that minimised the difference between observed and regression-predicted effect sizes across all 17 studies. The results for each study were assessed (available upon request) and suggest that for the NHS adult study the difference between observed and regression-predicted effect size (0.290) is of comparable magnitude to that across the remaining 16 studies. The mean of the difference between observed and predicted values for 17 studies was 0.204 (range 0.002–0.392) and the difference for the NHS adult study was close to the mean (0.290 vs. 0.204) and smaller than that for some studies and larger than that for others.
The major difference between the NHS adult pilot study and other studies was baseline HbA1c% value. The adult pilot population may be described as adults ‘having difficulty managing their condition’. We compared the NHS adult study with other studies in terms of further baseline characteristics to see if there were further differences. The average age in the adult NHS study is 49 years, so we compared the NHS study with adult studies in participants of comparable age. The available data do not indicate any striking differences between studies.
We conducted a forest plot for % TIR (between 3.9 and 10 mmol/l); figures are available upon request. At baseline in most studies the TIR was > 50%. In the NHS pilot adult study, the baseline TIR was 34.2%; this likely reflects the broad inclusion of patients and indicates, along with HbA1c% baseline, that these patients have control above target glucose levels prior to receiving HCL intervention. Similarly in the NHS CYP pilot, baseline control was above target levels (48.7%). In the NHS adult pilot benefit from HCL was larger than in the other studies; the mean value at end of follow-up was 62.7% TIR; this compares fairly closely with values in other observational studies of 63.8% (Forlenza)64, 71% (Beato-Vibora cross-sectional study)60, 80% (Beato-Vibora prospective study)61 and 63% and 67% (Bergenstahl)27 (HCL and AHCL respectively). Similarly in the CYP pilot, the end-of-study TIR was near that in other studies, at 63%.
We examined forest plots of the change from baseline in the % time in the hyperglycaemic range of > 10 mmol/l (figures available upon request). All studies reported an improvement from baseline; improvement ranged from 3.0% to 14% reduction in % time in hyperglycaemic range. The NHS pilot study did not report this outcome but did report unadjusted (uncorrected) % TIR > 14 mmol/l. At baseline the % time > 14 mmol/l was 37.4%, and a further 26.6% of time was in the range between 10 and 14 mmol/l, indicating that at baseline the NHS pilot study patients had a large % of time in hyperglycaemic state (≈64% of time). Transfer to HCL resulted in large reduction of 22.6% time above the 14 mmol/l range. The benefit of HCL in the range 10–14 mmol/l was more modest (a reduction in % TIR of 4%); thus, these results suggest that HCL improved hypoglycaemia considerably in the upper range but that a substantial proportion remained slightly above the 10 mmol/l cut-off point.
The change in % time in hypoglycaemic ranges (< 3.9 mmol/l and < 3.0 mmol/l) was reported in most observational studies. We examined the mean (95% CI) change from baseline in % time < 3.9 mmol/l (figures available upon request); CIs were wide. The % time < 3.9 mmol/l both at baseline (range 2.1% in the NHS pilot adult study to 3.4%) and after the HCL intervention were small, so that the resulting mean improvement was approximately −1% or less, with CIs mostly crossing the null. The NHS pilot adult study reported a change of −0.5% and an associated p-value of < 0.001. The CYP pilot study also reported a statistically significant improvement. Only in one other study (Carlson et al.,63 adult patients) was the change statistically significant at a p-value of < 0.05.
Several single-arm studies reported other outcomes indicative of hypoglycaemic status, most commonly % TIR < 3.0 mmol/l. Changes from baseline were < 1% and with one exception did not reach statistical significance. The large survey study by Breton et al. (T1DM n = 7801) reported medians of 0.01 (IQR 0.00–0.35) before HCL and 0.02 (IQR 0.00–0.400) after, with a resulting p-value of < 0.001. These authors considered this small worsening in hypoglycaemia during HCL likely to be clinically meaningless.
Summary of observational studies
Observational studies were defined as studies that reported end of intervention minus start of intervention results for outcomes of interest (HbA1c%, % time in range 3.9–10 mmol/l, % time > 10 mmol/l) whether such data came from the HCL or AHCL arm of RCTs (irrespective of the comparator investigated) or from single-arm studies, such as the NHS pilot studies. Multiple designs, as summarised in Tables 2 and 3, were therefore included.
The outcome estimates reported from observational studies were quantitatively broadly in line with those from the RCTs. Measures of glycaemic performance, such as HbA1c%, % TIR (3.9–10 mmol/l), and % time above range > 10 mmol/l all improved on transfer to HCL (or to an AHCL) without any strong evidence that hypoglycaemia became more of a problem; however, changes in hypoglycaemia were mostly underpowered in these studies. In the largest studies (the NHS pilot audit study in adults and the very large survey study by Breton et al.), there was no persuasive indication of deterioration in hypoglycaemic states.
The NHS pilot adult audit study differed somewhat from most other studies in that it included a broader spectrum of patients. That these patients had a poor record of glycaemic management at baseline was indicated by high HbA1c% and low % TIR (3.9–10 mmol/l) measures; at baseline the proportion of hyperglycaemic participants was high, as indicated by the % time > 10 mmol/l. Transfer to HCL resulted in larger improvements than observed in other studies, likely partly due to the poorer starting status that would allow for greater scope for improvement. In the NHS pilot study, the post-HCL levels of measures of glycaemic management approached those seen for HCL groups in other studies (both RCT and single-arm studies). The NHS pilot studies in adults and in CYP may have enrolled patients atypical of the generality of the UK T1DM population; however, it is unlikely that all UK T1DM patients need to transfer to better control systems because many may be achieving control within target with their current practice. It appears likely that by recruiting patients with control above target glucose levels, the outcomes reported in the pilot studies may reflect the sort of improvements in glycaemic management that may be close to a group that would require access to better systems. The discontinuation rate in the use of HCL (temporary or permanent) in the adult pilot study was about 10%; there was no distinction made between permanent and temporary. Whether discontinuation would increase with time is unknown, but from a CE perspective permanent discontinuation represents a wastage of device(s). Discontinuations were reported in some RCTs; in most RCTs the observation time on treatment was too short and the numbers of participants too small to provide a meaningful idea of discontinuation rates.
Assumptions
Publication bias was visually assessed using a funnel plot and statistically assessed using Egger’s test for each of the outcomes. All four funnel plots were symmetric, suggesting a lack of publication bias, as well as the p-values of Egger’s test, all of which were p > 0.05. Consistency and inconsistency were measured using node-splitting, which compares the direct and indirect estimates of the network. Loop-consistency was not measured as the networks for each outcome had no closed loops. Node-splitting concluded that there were no issues with consistency in the models.
Subgroup and sensitivity analyses
The results of the subgroup and sensitivity analyses (as specified in the protocol) are presented in Table 7.
A subgroup analysis was performed where studies were categorised based on the mean or median age of participants at baseline. Those with a mean or median age < 18 years were classified as ‘children and young adults’, and studies with participants with a mean age ≥ 18 years were classed as ‘adults’.
The following sensitivity analyses were performed:
•The Stewart et al. 51 study, which was in pregnant women only, was removed from the analysis.
•The Benhamou et al. 65 study was removed from the analysis as it was identified as a potential outlier for the outcome ‘% time in range 3.9–10.0 mmol/l’, as the difference in arms was around 31 but larger than in the remaining studies.
•Compared with the overall results, there were no statistically significant changes to the results when removing pregnant participants (excluding Stewart et al. 51) or when removing the outlying study. 65
When splitting the study estimates into adults (≥ 18 years) and those aged < 18 years. There were no statistically significant subgroups when compared with the overall NMA results. When comparing the subgroups separately, for the outcome % TIR between 3.9 and 10 mmol/l, HCL was significantly statistically better than CSII + CGM (MD 7.74, 95% CI 6.87 to 8.62) in the under-18s, but not statistically significant in the ≥ 18 years group.
Additional outcomes
Adverse events
Studies did not consistently report additional outcomes. In the Benhamou et al. 46 trial, the authors observed one severe hypoglycaemia and one ketoacidosis occurring in two different patients during the extension phase. The ketoacidosis occurred while the patient was under closed loop and presented with an acute infection of the ear, whereas the severe hypoglycaemia occurred while the patient had temporarily switched to open loop treatment. In this study several device malfunctions were reported, including 21 events related to the pump (in 7 patients), 6 events related to the sensor (in 4 patients) and 4 events related to the handset (in 3 patients).
In the Ware et al. 56 study, seven severe hypoglycaemia events were reported in total (closed-loop group, n = 4; comparator group, n = 3), two DKA events (both in the closed-loop group) and two non-treatment-related serious adverse events (broken ankle in the control group and hospital admission for gastroenteritis in the closed-loop group) occurred after randomisation. There were 23 reportable hyperglycaemia events (closed-loop group, n = 11; control group, n = 12), which did not meet the criteria for DKA. A total of 155 adverse events were reported (closed-loop group, n = 67; control group, n = 88).
Tauschmann et al.’s52 study reported one DKA presenting in the closed-loop group due to infusion set failure that was not related to the closed-loop therapy. There were two instances of severe hypoglycaemia in both groups.
Thabit et al. 53 reported safety outcomes. In this study, one episode of severe hypoglycaemia occurred in adult participants during the intervention period when the closed-loop system was not in use because of loss of connectivity (low battery) and the participant was receiving insulin at the rate supplied by the study insulin pump. In the study involving children and adolescents, one adolescent participant had two severe hypoglycaemic episodes (seizures) during the intervention period; these episodes required third-party assistance but did not result in hospital admission. During the two episodes, the closed-loop system was not in use (the participant was using SAP therapy).
Seven adverse events were reported for 7 (6%) out of 112 participants while using the 670G system and 6 events for 6 (5%) out of 112 participants while using the AHCL system (see Table 4). Severe hypoglycaemia occurred in one participant while they were using the AHCL system and no instances occurred in patients using the 670G system. No cases of DKA were reported. Six cases of hyperglycaemia were reported, which were in relation to infusion-set obstruction, and four cases were observed in the comparator group of adults. In children and adolescents, this was reported for two cases in the intervention group only. 27
The FLAIR study reported two severe hypoglycaemia events in the HCL group. Two hyperglycaemia events were related to insulin pump issues (without DKA) in the HCL group. Pump issues were not specified.
The Boughton et al. study reported two events of severe hypoglycaemia in the SAP group. Four participants reported some adverse events in the HCL group and seven participants reported some in the SAP group.
The Kariyawasam et al. 49 study reported a mean number of hypoglycaemic (< 3.9 mmol/l) episodes of 25.51 [5.42 standard error (SE)] in the closed-loop group and 48.19 (5.39 SE) in the open-loop group. Von dem Berge et al.’s54 study reported the median of hypoglycaemic events (< 54 mg/dl), four in the intervention group and three in the comparison group.
Collyns et al.’s48 study reported five device-related adverse events in each study arm.
Stewart et al. 51 reported eight hypoglycaemic events in the HCL group and 12.5 in the comparator (CGM + CSII) group.
Ware et al. 55 reported one serious adverse event of severe hypoglycaemia that occurred during the closed-loop period.
Overall, the majority of the studies reported a small number of events in both trial groups. From the data presented above, there was no clear difference between the HCL and comparator groups. Studies included a small sample and were heterogeneous, which limits a quantitative synthesis.
Patient-reported outcomes and perspectives
Tauschmann et al. 52 used the (confidential information has been removed).
The FLAIR study66 reported mean scores on the glucose monitoring satisfaction survey of 2.76 points (SD 0.52 points) at screening, 2.65 points (SD 0.63 points) at the end of the period using the HCL system, and 2.80 points (SD 0.55 points) at the end of the period using the AHCL (p = 0.0030) comparing HCL with AHCL. The only two satisfaction subscales that changed and showed superiority of AHCL were emotional burden and behavioural burden.
Benhamou et al.’s46 study reported improved levels of satisfaction using the Diabetes Treatment Satisfaction Questionnaire score. The satisfaction improved significantly, with a Diabetes Treatment Satisfaction Questionnaire total score of 50.0 (Q1–Q3 48.5–53.5) at baseline in open loop, 65.0 (Q1–Q3 57–66.5) after the initial close loop period, and 60.0 (Q1–Q3 58.5–63) at the end of the extension period.
McAuley et al. 50 recorded Hypoglycemia Fear Survey score. The median score was 7.5 (IQR 4–10) and 7.5 (IQR 5–10) for HCL and SAP therapy, respectively. The difference between the two groups was not significant.
Wheeler et al.’s58 study compared technology satisfaction and sleep quality between AHCL and SAP + PLGM (predictive low-glucose management). Overall treatment satisfaction was significantly higher for the AHCL group than for those treated with SAP + PLGM. There was no significant difference in anticipated worry of hypoglycaemia. The results showed that no changes in the well-being index and hypoglycaemia fear/confidence.
Several studies that used various tools and different survey approaches for technology satisfaction. Only one study (Benhamou et al.), comparing an open-loop with a closed-loop system, found that user satisfaction had increased significantly. Other studies did not observe any significant changes.
Quantity and quality of research available
Of the 12 RCTs included in the analysis, seven were rated overall as having some concerns about risk of bias, and two were rated overall as having a high risk of bias. 48,54 Table 8 provides a visual summary of each domain. Risk of bias was noted for each domain as follows.
High risk of bias was most common in relation to domain 2 (deviations from intended interventions). In this domain, 4 out of 12 RCTs were deemed to be at low risk of bias;47,50–52 6 out of 12 had some concerns about risk of bias (Bergenstal,27 Thabit,53 Ware55, Kariyawasam,49 von dem Berge,54 Collyns48), and 2 out of 12 RCTs were deemed to be at high risk of bias in this domain (Benhamou,46 Weinzimer57).
In domain 1 (randomisation process), there were some concerns about risk of bias in 6 out of 12 RCTs (Benhamou,46 Bergenstal, Thabit,53 Weinzimer,57 Kariyawasam,49 von dem Berge,54 Collyns48), because there was no information available to answer the signalling questions for the domain (Benhamou,46 Thabit,53 Weinzimer,57 von dem Berge54); a lack of information on the randomisation process (Benhamou,46 Thabit,53 Weinzimer,57 von dem Berge,54 Collyns48); issues with allocation concealment (Benhamou,46 Tauschmann,52 Thabit,53 Ware, Weinzimer,57 Boughton,47 von dem Berge,54 Collyns48); or differences in the characteristics of participant groups at baseline (Bergenstal). The RCT by Collyns et al. 48 was deemed to be high risk of bias in relation to the randomisation process. The domains with the lowest risk of bias were in relation to missing outcome data (domain 3) and outcomes measurement (domain 4), with all 12 RCTs considered to be at low risk of bias for both of these.
In domain 5 (selection of the reported results), all but three RCTs were considered to be at low risk of bias. Those that had some concerns about risk of bias were the studies by Benhamou et al.,46 Boughton and Hovorka47 and von dem Berge. 54
External submissions
We received external submissions through NICE that included NHS England evidence and company submissions.
National Health Service England evidence
National Health Service (NHS) England submitted two observational audit studies, the first conducted in adults and the second in CYP. The pilot studies were non-randomised studies with no control group and a before-and-after study design. The before-and-after design limits the scientific value of the evidence as there is a greater risk of bias due to lack of randomisation, lack of a true control, and selection bias.
Additionally, the findings of the two pilots are interim and potentially do not reflect the full results.
National Health Service England hybrid closed-loop pilot in adults with type 1 diabetes
The study included adults with T1DM (n = 570 with complete follow-up data) from 31 diabetes centres across England that started HCL therapy. Inclusion criteria were use of an insulin pump and FGM and a HbA1c of ≥ 69 mmol/mol. Routinely collected, anonymised data were submitted to a secure online tool. Outcomes included in the analysis were those with both baseline and follow-up data available. The primary outcome was HbA1c; other outcomes related to the scope included diabetes distress scores and event rates (events recorded/unit time) (hospital admission, paramedic callouts and severe hypoglycaemia).
Participants had high HbA1c (≥ 9.4%; 78.9 mmol/mol). Participants in the pilot study had worse glycaemic management than those in the National Diabetes Audit (see Table 4). 67 The National Diabetes Audit shows that 16% of people with T1DM have an HbA1c > 86 mmol/mol or 10%. 67 This indicates that the pilot study participants are in the 20% of the population with the worst management.
Mean HbA1c% declined from baseline to 5-month follow-up (mean change −1.5, 95% CI −1.4 to −1.6; p < 0.0001). Time below target range ≤ 3.9 mmol/l showed some reductions, with a mean change of −0.5, 95% CI 0.2 to 0.7 for 3–3.8 mmol/l % times in range, and a mean change of −0.02, 95% CI −0.1 to 0.2. There are several points that require consideration:
-
Diabetes distress score measures improved; however, EQ-5D data measures were not collected. Therefore, utility measures are challenging to quantify.
-
The level and amount of patient education is not clearly defined. It is unclear if patients received structured education that may have improved glucose measures.
-
Patients enrolled in the study were on CSII therapy, which is one of NICE’s criteria for switching to HCL. However, the length of pump therapy was not clear. NICE recommends the suspension of pump therapy when glycaemic improvements are not achieved.
-
Cost data were not provided.
National Health Service England closed-loop study in children and young people
This study recruited 251 CYP (< 19 years old) who had had T1DM for at least 1 year and had two HbA1c measures before starting HCL. Participants were recruited from eight centres across England.
Participants with other medical conditions that have an impact on glucose measures and/or participants in other device evaluation trials were excluded. Outcomes (HbA1c, TIR, hypoglycaemia frequency) were assessed at baseline and at 3- and 6-month follow-up. The HFS was completed by participants aged ≥ 12 years and by parents of participants aged < 12 years.
At 6-month follow-up, HbA1c (mmol/l) was 7 mmol/l (95% CI 5.8 to 8.2 mmol/l; p < 0.001). The improvement observed (0.6%) was slightly above the clinically meaningful change (0.5%). This was accompanied by improvements in TIR (MD −14.3, 95% CI −15.9 to −12.4; p < 0.001), and hypoglycaemia (MD −1.2, 95% CI 0.82 to 1.74; p < 0.001). There are several points that require consideration:
-
Pre-HCL treatments (such as pump and CGM) were not clearly described.
-
Extent of severe hypoglycaemia that may affect the HFS was not described.
-
Parental/carer EQ-5D data were not collected.
-
The level and amount of patient education was not clearly defined.
-
Cost data were not provided.
Medtronic submission clinical effectiveness
The Medtronic submission compared the (advanced) HCL systems with rtCGM with CSII (non-integrated). The company described a number of studies, and edited extracts of its report are included in Appendix 10.
Medtronic submission clinical effectiveness: Evidence Assessment Group critique
Carlson et al.’s study63 was undertaken in a US context. The results of the extended study phase have not been published except in an abstract.
Da Silva et al.’s study reported data based on an ongoing trial of the MiniMed™ 780G AHCL system (Medtronic, Watford, UK), and it is the first report of outcomes. 68 There is a lack of demographic data, such as users’ duration of diabetes and previous therapies. The results are limited by the follow-up duration of the cohort, with a mean of 54 ± 32 days. There is some concern about reliability. The usability can only be inferred from the high percentage of time spent in AHCL and the small number of AHCL exits.
Medtronic suggests consistent effectiveness of the MiniMed 780G system in current users (over 20,000 in June 2022), reporting improvements in performance, safety and usability compared with MiniMed 670G, reducing the burden for people living with T1DM. It seems that these results are based on the same source as those of the ongoing trial. The source and history of participants is not clear.
Vigersky et al. 69 reported safety and effectiveness outcomes following the transition of participants to the MiniMed 780G system with the GuardianTM 4 sensor (Medtronic, Watford, UK) (NCT03959423). The results relate to a US population. It is not clear whether the authors used the Guardian 4 System (Guardian 4 sensor + Guardian 4 transmitter) or just the Guardian 4 sensor. The data are based on a poster presentation, and no more data about the patients were available.
The main issue with Arrieta et al. 70 is that it is not clear whether patients with T1DM had received different previous treatments. The only treatment information available is the percentage of MiniMed 780G system users, for two different age groups of people. Outcomes were analysed for three cohorts of users: cohort 1 (post AHCL), cohort 2 (longitudinal) and cohort 3 (pre vs. post AHCL). This study is related to several different countries’ populations and the results show differences with adults with T1DM in NHS England.
Choudhary et al. 71 is a retrospective analysis of CareLink™ (Medtronic, Northridge, CA, USA) data from people with T1DM in the UK and was conducted to determine the real-world effectiveness of sensor-integrated pump therapy with the MiniMed Paradigm Veo or MiniMed 640G systems. Comparisons of SAP with LGS, SAP with PLGM, and LGS with LGM were undertaken. There is no HCL arm in this study. The initial analysis was based on treatment groups of different sizes and durations of treatment. The reasons for using SAP therapy without any suspension mode activated, and for switching to LGS, were not available. The analysis was purely descriptive, and no formal statistical comparison has been carried out.
The FLAIR study,27 a randomised crossover trial conducted between June 3 and 22 August 2019, recruited 113 adolescents and young adults with T1DM. It was undertaken in the UK. The study period was only 3 months; thus, it is not possible to determine the sustainability of observed benefit over a longer period of time.
Collyn’s et al.’s study48 demonstrated a significant improvement in TIR, with no increase in hypoglycaemia for AHCL compared with SAP 1 PLGM during 4 weeks. The short study period limits the impact sustainability assessment. The age range of included participants is wide, and no stratified data have been reported based on the age group.
Petrovski et al.’s study72 assessed the use of a 10-day structured initiation protocol for the MiniMed 670G HCL system in individuals with T1DM on MDI therapy. It was a single-centre study with a small sample size for investigating the clinical outcomes of using HCL for patients on MDI with SMBG, with or without RT-CGM or isCGM, with no prior pump experience.
Farabi et al.’s study73 was a systematic evaluation of the relationship between routine, unstructured physical activity, and glucose variations across wake and sleep periods for multiple days in young adults with T1DM in their natural home/work environment. This study is limited by the lack of a control group. The study did not have any exclusion criteria based on patients’ history. There are also factors that can affect glucose levels, such as structured physical exercise, which have not been considered in this study.
Dexcom submission clinical effectiveness
Dexcom compares HCL with SAP. This is based on the results of one systematic review and NMA74 and eight RCTs. 55,56,75–80 The review was based on 52 RCTs, comprising 3975 participants, for T1DM. Comparators were SAP (rtCGM + CSII) and intermittently scanned glucose monitoring with CSII (FGM + CSII). The results of the NMA indicated that, in terms of HbA1c reduction, there is no significant difference between CGM + CSII, with a MD of −0.36 (95% CI −0.90 to 0.19). When simultaneously considering HbA1c and severe hypoglycaemia, integrated systems as well as MDI + CGM appeared to provide the highest composite ranking in cluster analysis of surface under the cumulative ranking curve values. Despite finding the most favourable results for HCL, it should be noted that the study authors recommended, ‘If only one technology is desired or practical, then CGM appears most favourable from composite ranking of A1c, hypoglycaemia, and QoL’. 74
All of the eligible trials included SAP as the main comparator; no studies compared HCL with FGM + CSII. The company described a number of studies, and edited extracts of its report are included in Appendix 10.
Dexcom submission clinical effectiveness: Evidence Assessment Group critique
The EAG has some concerns about the results of the existing NMA. 74 Performance bias is challenging to assess because of the impracticability of blinding participants and clinicians to the devices being compared. Inconsistent reporting of TIR outcome made it impossible to meta-analyse this outcome.
The EAG has not managed to source the result reported in the submission from the iDCL trial because in this study multiple daily insulin injections were used by 35 (21%) patients. 77 The authors reported more unscheduled contacts in the closed-loop group, which was attributed to the use of an investigational device, and the insulin pumps used by the control group did not have a feature for suspending insulin for predicted hypoglycaemia, which might have an effect on the amount of continuous glucose monitor-measured hypoglycaemia.
Kanapka et al.’s study was similar to the iDCL study, with 21% of patients in the closed-loop group and 17% in the control group having used MDI. 80 The amount of hypoglycaemia at baseline was unrepresentatively low in both treatment groups, which, in addition to the fact that most of the patients in the control group used a pump with a PLGS feature, limited the ability of the trial to assess the effect of the closed-loop system on hypoglycaemia. On the other hand, it is not possible to assess the sustainability of the treatment effect over a longer period because the trial period was only 4 months.
The EAG has some concerns about the participants’ characteristics. The participants came from a more advantaged socioeconomic background and had more experience with diabetes technology, which may have a better effect on glycaemic management.
The EAG has some concerns about the monitoring method used because the researchers used remote monitoring that might have improved the glycaemia compared with real-world control. In addition, they reported an error in the software. The small sample size and the different context of the UK cause some concerns regarding generalisability. 78 There are some concerns about Forlenza et al.’s study79 because in that study it was possible to achieve better management than could be seen in the real world. This occurred because a high degree of physician oversight was provided to both groups through continuous remote monitoring by a paediatric endocrinologist. This may have biased both the experimental and control groups, thereby limiting generalisability. There is risk of selection bias because subjects had enrolment HbA1c values of < 7.5% on average in both groups, which may further limit generalisability.
There are some concerns about the generalisability of Ware et al.’s study on closed-loop control in very young children with T1DM. 55 Highly motivated participants in closed-loop studies, and the crossover design, may limit the generalisability of these findings, because growth and development are rapid in very young children and might have affected the trial results. Furthermore, additional exclusion criteria that were unrelated to diabetes applied to participants at sites in Germany, which potentially affected the reported treatment effect.
There are also concerns about the generalisability of Boughton et al.’s study76 results because the authors enrolled participants who may not be fully representative of the general population of older adults with T1DM owing to the requirement for insulin pump therapy and the low baseline HbA1c. There was little ethnic diversity in the study population. The study participants had a relatively high level of educational attainment and might have had a higher level of technological proficiency than an age-matched population, which might limit the generalisability of the results.
CamDiab submission clinical effectiveness
CamDiab presented 10 studies as clinical effectiveness evidence. The company described a number of studies, and edited extracts of its report are included in Appendix 10.
CamDiab submission clinical effectiveness: Evidence Assessment Group critique
With regard to Boughton et al.’s study,76 there are some concerns about the generalisability of the results to the wider population of older adults with T1DM because there was little ethnic diversity in the study population. In the supplementary material, it is mentioned that the study participants had a relatively high level of educational attainment and might have had a higher level of technological proficiency than an age-matched population, which might limit the generalisability of the results.
For Bally et al.’s study,81 there may be some concerns around the duration (for 4 weeks, in the order assigned at randomisation, with a 2- to 4-week washout period in between). This might have been insufficient to assess long-term compliance. Some exclusion criteria, such as participants with hypoglycaemia unawareness, have restricted assessment of the closed-loop system to those who might benefit greatly. The heterogeneity of sensor use in the control period might have confounded the reported glycaemic outcomes.
Leelarathna et al.’s82 study results are based on a small sample size and a relatively short study duration. In this study, the system used was an early generation closed-loop system (which was not a commercially available product). Some failures were observed using closed loop during the home phase because of the unavailability of CGM data, a non-operational laptop, and an unreliable Bluetooth communication between pump and the computer. All of these limitations could have affected the results.
Stewart et al.’s51 study included pregnant participants who had had intensive insulin treatment (either MDI or CSII), with equal numbers of pump and MDI users. There are some concerns about the study duration (the short 4-week duration may have been insufficient for optimal closed-loop training, particularly for device-naive participants and those with less advanced self-management skills). It was the prototype version of the closed-loop system, which had frequent errors, and reduced the time that closed loop was operational.
One of Tauschmann et al.’s83 studies included a small sample size, and the need to carry multiple devices during the closed-loop intervention, in addition to the study duration, caused concerns about the findings. Another study by Tauschmann et al. 84 caused the same concerns, and also mentioned that the intervention was a prototype version of a closed-loop system and there was some restrictions in using this system during strenuous exercise.
The main concern about Tauschmann et al. 52 was the number of devices comprising a HCL system, which increased the risk of device and connectivity problems. This issue resulted in more frequent non-protocol contacts to address technical issues. Another concern is the systematic exclusion of participants with HbA1c outside the range of 7.5–10.0% and other groups, such as those with an impaired awareness of hypoglycaemia or a history of recurrent severe hypoglycaemia.
Ware et al. 56 (Cambridge HCL algorithm in children and adolescents with T1DM) used two different glucose sensors in the two closed-loop hardware configurations; although the two have been shown to be similarly accurate in the hypoglycaemic range (glucose < 3.9 mmol/l), the glucose sensors’ accuracy needs to be considered when interpreting the results. A prespecified analysis has been carried out to compare the entire closed-loop group with the control group, rather than each closed-loop system separately; the findings should be interpreted with caution.
The EAG’s main concern about the other Ware et al. 55 study (closed-loop control in very young children with T1DM) is the generalisability of data. Insulin-pump use was a prerequisite for trial participation and sensor use at enrolment was higher than average. A HbA1c level of < 11.0% (97 mmol per mole) was required for trial participation, which potentially limited enrolment. In addition, children from ethnic minorities were under-represented. Investigators were free to adjust insulin therapy according to clinical judgement before randomisation, which may have affected baseline characteristics. Research participants in closed-loop studies tend to be highly motivated, which may also limit generalisability. A crossover design was used, but because growth and development are rapid in very young children, this may have affected trial results. Additional exclusion criteria that were unrelated to diabetes applied to participants at sites in Germany, which potentially affected the reported treatment effect.
Tandem submission clinical effectiveness
Tandem presented three recent pieces as clinical effectiveness evidence in its submission. It described a number of studies, and edited extracts from its report are included in Appendix 10.
Tandem submission clinical effectiveness: Evidence Assessment Group critique
Assessing the quality of study and results based on Singh et al.’s poster is not possible because there are not enough data about history of patients or a description of the intervention and comparator. 85
In Forlenza et al.’s,86 500 users were affected by T2DM, whereas most patients had T1DM. In this cohort study 806 users transitioned from MDI therapy to Control-IQ therapy. There is reliance on a glucose management indicator (GMI) as a surrogate for biological HbA1c data because of a lack of follow-up on these data. There is concern about the generalisability of the results because of the need for device data to be uploaded by users. Those device users who did not upload their data would not be represented. The analyses were performed using a reporting dashboard of real-world data, which is a limitation because predetermined analyses existing within the dashboard tools were used.
(Confidential information has been removed.)
Assessment of effectiveness
Summary of information
The clinical evidence identified 12 RCTs that compared HCL with CSII + CGM or SAP therapy.
Studies were heterogeneous in terms of population, age groups, gender, RCT design (parallel or crossover), numbers of participants and variable adjustment methods for determining MD between intervention and comparators. Studies did not consistently describe comparators. Precision of reported values for outcomes was limited because of rounding to only two significant figures. Individual patient meta-analysis would be the preferred approach to assessing the relative effectiveness of different interventions; however, obtaining requisite IPD was infeasible within the resources available.
Overall, the HCL arm of RCTs achieved improvement in HbA1c%, % TIR (3.9–10 mmol/l), and hyperglycaemic levels. Comparator arms also often showed improvements, but these were usually smaller than that observed in the HCL arm. Irrespective of the type of intervention used in the comparator arms, these outcomes were statistically superior in the HCL arm compared with the control arm. Available evidence from the RCTs suggests that these gains in glycaemic management reported for HCL were not accompanied by a greater risk of hypoglycaemia; however, the power to detect small event sizes was limited because of the small size of study groups and the relatively short treatment duration.
The outcome estimates reported for observational studies were quantitatively broadly in line with those from the RCTs. Measures of glycaemic performance such as HbA1c%, % TIR, and % time above range all improved on transfer to HCL (or to AHCL) without any strong evidence that hypoglycaemia became more of a problem. However, changes in hypoglycaemia were mostly underpowered in these studies; in the largest studies (the NHS pilot and the survey study by Breton et al.), there was no persuasive indication of a deterioration in hypoglycaemic states.
The inclusion of RCTs was based on the presence of a relevant comparator arm, the inclusion of at least 90% HCL recipients in the intervention arm, and the reporting of outcome measures applicable to NMA. The aim of the RCTs was generally to demonstrate improvement in glycaemic management with the use of HCL. The study by Stewart et al. of pregnant women included only 16 participants followed for 4 weeks; the population, study design and outcomes in this study were clearly different from those in other studies, so transitivity of the NMA including Stewart is threatened.
In all there were relatively few RCT studies, they were of small size encompassing a total of ~450 HCL recipients followed for between 4 and 26 weeks accumulating approximately 110 person-years of observation. Inclusion criteria applied for the studies were relatively narrow and most participants had reasonably good glycaemic management at entry, as indicated in most of those studies reporting baseline TIR (3.9–10 mmol/l) at > 50% (range 47–62%), and baseline HbA1c at between 7% and 8%. There was considerable heterogeneity across studies regarding the age of participants, some studies presented results stratified by age groups. The relevance of the RCT populations and outcome measure results for the decision problem may be debatable and is not easy to judge.
The quality of RCT studies assessed according to Cochrane criteria (see Table 8) was associated with some concern.
In the HCL arm of RCTs the intervention achieved a statistically significant improvement in HbA1c%, in TIR between 3.9 and 10 mmol/l, and in hyperglycaemic levels. Control arms also showed improvement, but this was smaller than that seen with HCL. Irrespective of the type of intervention used in the control arms, these outcomes were statistically superior in the HCL arm compared with the control arm. Available evidence from the RCTs suggests that these gains in glycaemic management reported for HCL were not accompanied by a greater risk of hypoglycaemia; however, the power to detect small event sizes was limited because of small size of study groups and relatively short treatment duration.
The NHS adult pilot study differed somewhat from most other studies in that it included a broader spectrum of patients. These patients had a poor record of glycaemic management at baseline as indicated by high HbA1c% and low % TIR (3.9–10 mmol/l) measures; at baseline, the proportion of hyperglycaemic participants was high as indicated by the % time > 10 mmol/l. Transfer to HCL resulted in larger improvements than observed in other studies, likely partly due to the poorer starting status. In the NHS pilot study, the resulting levels of measures of glycaemic management after HCL intervention approached those seen for HCL groups in other studies (both RCTs and single-arm studies). The discontinuation rate in the use of HCL (temporary or permanent) in the pilot study was about 10%; whether this would increase with time is unknown, but from a clinical evidence perspective this represents a wastage of device(s).
Discussion
The evidence on closed-loop systems has been informed by short-duration RCT studies, with small number of participants and some uncertainty about the methodological quality of included studies. Closed-loop systems have been previously reviewed and have shown effectiveness in treating patients with T1DM. 87 In this review, the HCL arm of RCTs achieved improvement in HbA1c%, TIR (3.9–10 mmol/l), and hyperglycaemic levels. Comparator arms also often showed improvements, but these were smaller than that observed in the HCL arm. Irrespective of the type of intervention used in the comparator arms, these outcomes were usually statistically superior in the HCL arm compared with the comparator arm. In the NHS pilot study, the post-HCL levels of measures of glycaemic management approached those seen for HCL groups in other published studies (both RCTs and single-arm studies). The 2022 SHTG26 found significant improvements in mean percentage TIR for people with T1DM using a closed-loop system compared with other insulin-based therapies. We found similar trends to those in the SHGT work. However, it should be noted that the scope of the SHGT group differs from the scope of this work. Our NMA synthesis demonstrated a significant decrease in % TIR > 10.0 mmol/l, increase in % TIR (between 3.9 and 10.0 mmol/l), and a decrease in HbA1c% showing superiority of HCL to other treatments.
Evidence suggest that such technologies have the potential to improve the lives of people with T1DM and their families. People seem to report a better quality of life, improved diabetes burden and quality of sleep, and less anxiety with technologies. 88 The study by Wheeler showed no significant improvements in the anticipated worry about hypoglycaemia in children, parents and adults. Studies included in this review used various tools to assess technology satisfaction. Only one study (Benhamou et al.), which compared an open-loop and a closed-loop system, found that user satisfaction had increased. In the other studies, the difference between the HCL group and comparator was not statistically significant. RCTs included in this review reported a small number of adverse events in both treatment groups. Although some reports of hypoglycaemia were identified in the included studies, we did not identify any clear trends or differences between HCL and the comparator. It is worth noting that the studies included in this review are of short duration. The REPOSE study assessed the relative effectiveness of CSII therapy in comparison with MDI over 24 months. Adverse events (such as DKA) were higher at the initiation of therapy and reduced over time. Therefore, it is important to assess long-term adverse events to allow for an adjustment period in people with T1DM.
Chapter 6 Systematic review of existing cost-effectiveness evidence
Methods for assessing cost-effectiveness evidence: key questions
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM and are having difficulty managing their condition despite prior use of CSII and self-monitoring of blood glucose or glucose monitoring (rtCGM or FGM) and MDI?
Other questions are as follows:
-
What is the cost-effectiveness of HCL systems for managing glucose in pregnant women who have T1DM?
-
What is the cost-effectiveness of HCL systems for managing glucose in children who have T1DM and are having difficulty managing their condition despite prior use of CSII and self-monitoring of blood glucose or glucose monitoring (rtCGM or FGM) and MDI?
-
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM, have an extreme fear of hypoglycaemia, and are having difficulty managing their condition despite prior use of CSII and self-monitoring of blood glucose or glucose monitoring (rtCGM or FGM) and MDI?
-
What is the cost-effectiveness of HCL systems for managing glucose in people who have T1DM and diabetes-related comorbidities that are at risk of deterioration, and are having difficulty managing their condition despite prior use of CSII and self-monitoring of blood glucose or glucose monitoring (rtCGM or FGM) and MDI?
Systematic review of existing cost-effectiveness evidence
As per the protocol, a systematic review of the existing cost-effectiveness evidence surrounding HCL was commenced using the following methods.
Study identification
A comprehensive search of the literature for published economic evaluations was performed in a range of relevant bibliographic databases in April 2021 and updated in April 2022. The database searches were developed using search strings applied in the previous technology assessment on integrated SAP therapy systems (DG21)34 as the basis for selected lines relating to T1DM, insulin pumps, sensor augmented pumps and MDI, and other systematic reviews for lines relating to pregnancy. 35–37 The search was informed by the strategy developed for the clinical effectiveness review [see per cent time within range (between 3.9 and 10.0 mmol/l)] and established economic terms based on the CRD NHS EED (NHS Economic Evaluation Database) filter. 89 A date limit in 2014 was applied for each database, based on the search dates for DG21. 34 The search was limited to the English language to reflect the inclusion criteria. Full details of the search strategies are provided in Appendix 2.
The following databases were searched, from 2014: MEDLINE ALL (via Ovid); EMBASE (via Ovid); EconLit (via EBSCOhost); HTA database (via CRD); the international HTA database (INAHTA); EconPapers (via RePEc); AHRQ website; CADTH website; SBU website; Cost-Effectiveness Analysis (CEA) Registry; and School of Health and Related Research Health Utilities Database (ScHARRHUD).
The reference lists of included studies and results of the clinical effectiveness search were also checked.
Records were exported to EndNote X9, where duplicates were systematically identified and removed.
An additional, scoping search for hypoglycaemia and HRQoL in MEDLINE ALL (via Ovid) was conducted from 1 January 2020 to 10 June 2022 for studies on hypoglycaemia and quality of life in people with diabetes. The search was limited to 2020 onwards because searches for a recent economic report for NG1790 were undertaken in May 2020. 91 The targeted search included terms for hypoglycaemia and HRQoL and used a recognised search filter (sensitivity maximising health utilities search filter from Arber et al. 92). The full search strategy is provided in Appendix 2.
Additionally, the Hypo-RESOLVE website was checked. 93
Potentially relevant literature identified during the systematic review of economic evaluations and sent by topic experts was also examined for relevance.
One hundred and twenty-seven records were retrieved and sifted by the health economists.
Inclusion and exclusion of relevant studies
Studies that satisfied the following criteria were included in the review.
Population
People who have T1DM and are having difficulty managing their condition despite prior use of CSII and self-monitoring of blood glucose or glucose monitoring (rtCGM or FGM).
The following T1DM subpopulations were included:
-
pregnant women and those planning pregnancies (excluding gestational diabetes)
-
children (aged ≤ 5 years, 6–11 years, 12–19 years)
-
people with extreme fear of hypoglycaemia
-
people with diabetes-related complications that are at risk of deterioration.
For this review, difficulty refers to not maintaining HbA1c levels of ≤ 6.5% (48 mmol/mol), not maintaining at least 70% TIR of 3.9–10 mmol/l, or repeated hypoglycaemia that causes anxiety about recurrence and is associated with a significant adverse effect on quality of life.
Pregnant women and those planning pregnancies were not required to have previously used CSII and glucose monitoring (self-monitoring, or rtCGM/FGM) with MDI.
Intervention
Hybrid closed-loop systems.
Comparators
-
rtCGM with CSII (non-integrated).
-
Intermittently scanned (flash) glucose monitoring with CSII.
For women with T1DM who are pregnant/planning pregnancy, the following comparators were also included:
-
real-time continuous glucose monitoring with multiple daily insulin injections
-
intermittently scanned (flash) glucose monitoring with multiple daily insulin injections
-
self-blood glucose monitoring with CSII.
Outcome measures
-
Cost and cost-effectiveness outcomes [costs for each treatment technology, direct medical care costs, incremental cost-effectiveness ratios (ICERs), for example, cost per QALY gained].
Study design
-
Studies comprising an economic evaluation (cost analysis, cost–consequences analysis, cost-effectiveness analysis, cost–utility analysis and cost–benefit analysis), and any model-based economic evaluation involving a direct comparison between HCL and non-integrated CGM and CSII therapy in T1DM.
Other inclusion criteria
-
Full-text reports published in the English language.
-
Abstracts (only if they were companion publications to full text included studies or contained extractable numerical data).
Papers that fulfilled the following criteria were excluded: studies evaluating automated insulin-delivery systems that suspend insulin delivery only when glucose levels are low/are predicted to get low; non-human studies; letters, editorials and communications; and articles not available in the English language.
Methods
The searches were developed and run by our information specialists (Anna Brown and Rachel Court). Sifting was undertaken by two reviewers. Mary Jordan led the review sifting the abstracts and titles of all identified studies, while Felix Achana and Lena Al-Khudairy acted jointly as second reviewer. Results between first and second reviewer were then compared and anomalies resolved through discussion or, where this was not possible, by recourse to the full team of reviewers. Full texts of the result of the first sift were obtained and screened using the same process.
Data extraction and quality assessment
As per the protocol, it was intended that information would be extracted by one reviewer (MJ) using a pre-piloted data extraction form for full economic evaluation studies, and that the reporting quality of studies included in the systematic review would be assessed against the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist94 and the Philips’ checklist,95 respectively. Where search results rendered this process unnecessary, quality appraisal was undertaken narratively, guided by the criteria detailed in these checklists. 94,95
Data synthesis
A narrative synthesis of findings and assessment of study quality is presented, and recommendations for future economic models are discussed.
Results
The literature search identified 745 records through electronic database searches and other sources. After removing duplicates, 516 records were screened for inclusion. On the basis of title and abstract, 497 records were excluded. The remaining 19 records were included for full-text screening. A further 13 articles were excluded at the full-text stage mainly because of incorrect intervention/comparator,96–100 incorrect study design,101 being abstract/poster presentation only102–104 or identification of further duplication. 105–107
The literature search (Figure 11) identified six studies that were included in the review. 26,108–112
FIGURE 11.
Search strategy flow diagram.
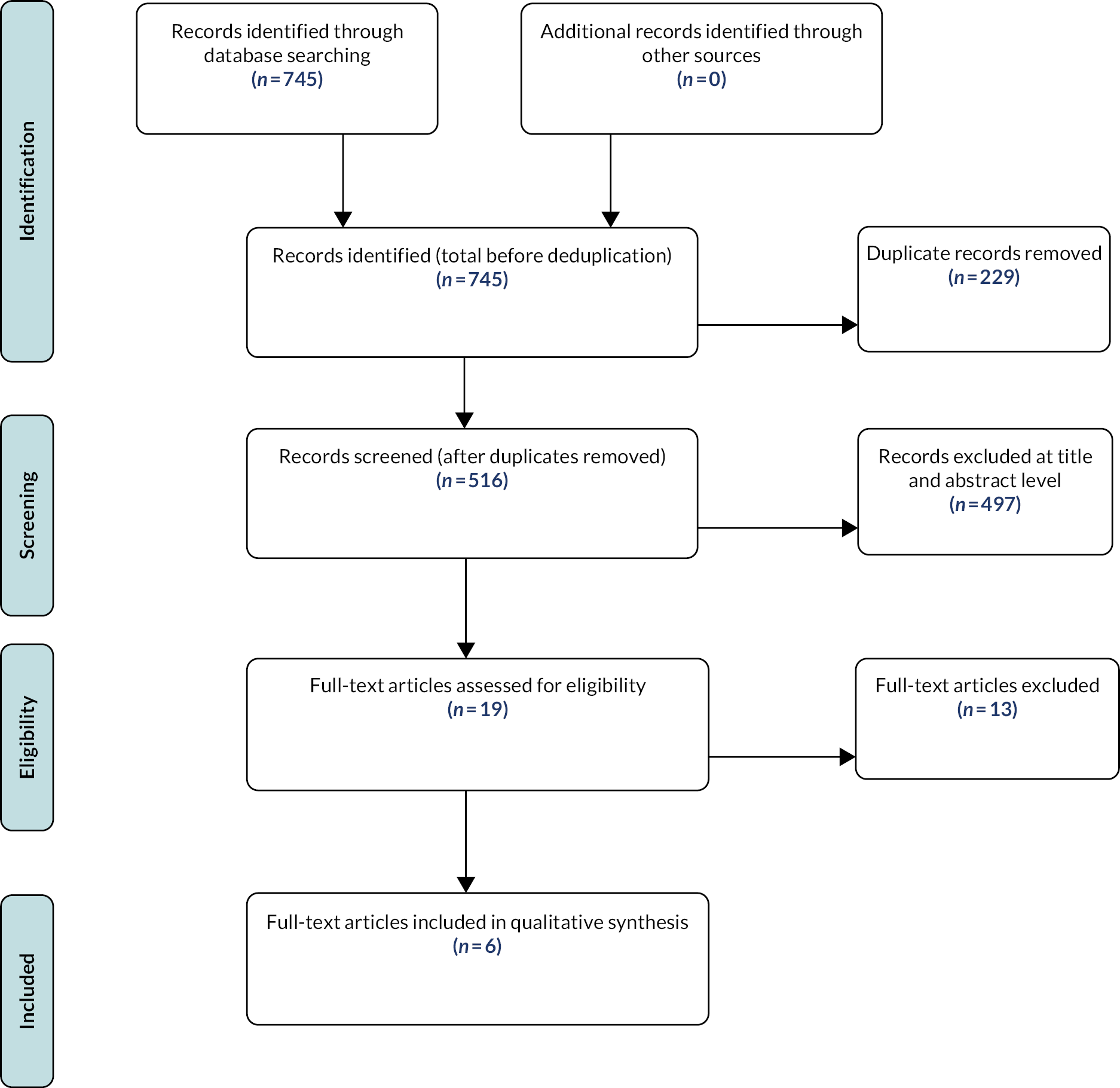
Summary of the economic analyses undertaken
In this section, we summarise the economic analyses undertaken and discuss the approach taken and relevance to assessing HCL compared with CGM/FGM and CSII in adults with T1DM.
The first four studies use the iQVIA CORE Diabetes Model (CDM) to conduct their economic evaluations, whereas the study in the SHTG report26 used the Sheffield type 1 diabetes model. Both the iQVIA CDM and the Sheffield type 1 diabetes model are validated models in which Monte Carlo methods are employed to estimate the cost-effectiveness of diabetes-related technologies including HCL systems. The study presented in the CADTH report108 is a budget impact analysis and was conducted using a customised Microsoft Excel® tool (Microsoft Corporation, Redmond, WA, USA).
Jendle et al.109
Jendle et al. 109 used the CDM to assess the cost-effectiveness of the MiniMed 670G HCL system compared with CSII in people in Sweden who have T1DM.
Baseline cohort characteristics, and both treatment effect on HbA1c and rate (events recorded/unit time) of SHEs for the HCL system, were taken from a single-arm before-and-after clinical study. 113,114 Other clinical inputs were either assumed or derived from the literature, and costs were obtained from a variety of published sources.
All costs included in the model were reported in 2018 Swedish krona (SEK). The analysis was conducted from a Swedish societal perspective, over a lifetime horizon, with future clinical and economic costs discounted at a rate of 3% per annum. A human capital approach to costing lost productivity was used. The results were presented in terms of an ICER expressed as cost per QALY gained. The authors undertook scenario analyses around the costs of HCL, costs of comparator, rate of SHEs, impact of fear of hypoglycaemia and cost-effectiveness in patients with worse management (HbA1c ≥ 7.5%).
The base-case deterministic results showed that the MiniMed 670G HCL system when compared with CSII had an ICER of SEK 164,236 (1 SEK = £0.082) per QALY gained. This resulted from an increase of 1.90 QALYs but higher overall costs despite a lower cumulative incidence of diabetes-related complications and reduced productivity losses.
The results of the scenario analyses showed that the ICER was most sensitive to assumptions relating to the impact of fear of hypoglycaemia on quality of life, treatment comparator costs and reductions in SHE rates.
Although the study added to the literature on the cost-effectiveness of HCL systems by conducting a cost-effectiveness analysis of the MiniMed 670G system in Sweden, the authors acknowledged and discussed the limitations associated with the analysis.
Roze et al.111
Roze et al. 111 used the CDM to assess the cost-effectiveness of the MiniMed 670G HCL system compared with CSII in people in the UK who have T1DM.
Baseline cohort characteristics, and both treatment effect on HbA1c and rate of SHEs for the HCL system, were taken from a single-arm before-and-after clinical study. 113,114 Other clinical inputs were either assumed or derived from the literature, and costs were obtained from a variety of published sources.
All costs included in the model were reported in 2018 Great British pounds (GBP). The analysis was conducted from a UK healthcare system perspective, over a lifetime horizon, with future clinical and economic costs discounted at a rate of 3.5% per annum. Results were presented in terms of an ICER expressed as cost per QALY gained.
Base-case deterministic results showed that use of the MiniMed 670G HCL system led to an increase of 1.73 QALYs compared with CSII, with higher total lifetime direct costs of £35,425. This resulted in an ICER of £20,421 per QALY gained.
Sensitivity analyses showed sensitivity of the ICER to assumptions surrounding glycaemic management and quality-of-life benefits associated with reduction in fear of hypoglycaemia.
The authors ultimately concluded that in the UK, over patient lifetimes, using the MiniMed 670G HCL system is likely to be cost-effective relative to the continued use of CSII in people with T1DM, particularly those with fear of hypoglycaemia and control above target glucose levels at baseline. The main contribution to knowledge was that, unlike the previous analysis of the MiniMed 670G in Sweden109 that considered a societal perspective, Roze et al. 111 adopted a UK healthcare system perspective.
Serne et al.112
Serne et al. 112 used the CDM to determine the cost-effectiveness of the MiniMed 670G HCL system compared with isCGM with MDI or CSII in people with T1DM. The study extended the evidence base on the cost-effectiveness of the MiniMed 670G HCL system by conducting a study in the Netherlands.
The baseline cohort characteristics and treatment effect data for isCGM with MDI/CSII were taken from a prospective observational real-world cohort study (FUTURE) in Belgium. 115 Treatment effect for the HCL cohort was sourced from a retrospective analysis of patients transitioning from SAP to the MiniMed 670G in the USA. 116
A societal perspective was taken for the analysis, over a lifetime time horizon, with future costs specific to the Netherlands discounted at 4% and clinical outcomes at 1.5% per annum. All direct and indirect costs included were reported in 2020 euros, with a human capital approach taken to calculate the cost of lost productivity.
Using the MiniMed 670G HCL system increased mean QALYs by 2.231 compared with isCGM in the deterministic base case. Total mean lifetime costs were also higher in the HCL cohort, at EUR 13,683, resulting in an ICER of EUR 6133 per QALY gained.
Sensitivity analyses highlighted that ICER results were sensitive to assumptions around SHE rates and the quality-of-life benefit associated with reduced fear of hypoglycaemia.
Some discussion of the limitations of the data sources for this economic analysis was provided by the authors. They concluded that using the MiniMed 670G system is likely to be cost-effective relative to isCGM + MDI or CSII for adults based in the Netherlands who have long-standing T1DM.
Jendle et al.110
Jendle et al. 110 use the CDM (version 9.0) to evaluate the long-term cost-effectiveness of the MiniMed 780G AHCL system against isCGM + MDI CSII in people in Sweden who have T1DM.
Baseline characteristics and treatment effect data for the isCGM with MDI/CSII cohort were taken from the FUTURE clinical trial in Belgium,115 with an assumed treatment effect applied for the HCL cohort based on Collyns et al. 48
The cost-effectiveness analysis was conducted from a societal perspective projected over patients’ lifetimes with results presented in SEK, although no cost year was explicitly stated. Future clinical and cost benefits were discounted at 3.0% per annum, and the results were presented in terms of an ICER expressed as cost per QALY gained.
Using the MiniMed 780G system was associated with an improvement of 1.95 QALYs compared with isCGM + MDI or CSII. Clinical benefits accrued from reduced incidence and delayed time to onset of diabetes-related complications. The total costs were estimated to be SEK 727,408, producing an ICER of SEK 373,700 per QALY gained.
Jendle et al. 110 contributed to the literature by showing that the MiniMed 780G system is expected to be cost-effective compared with isCGM + MDI or CSII for the treatment of T1DM in Sweden, at a willingness-to-pay threshold of SEK 500,000 per QALY gained.
Scottish Health Technologies Group (2022)26
The study in the 2022 SHTG report used the Sheffield type 1 diabetes model to examine the clinical effectiveness and cost-effectiveness of closed-loop systems and the artificial pancreas for the management of T1DM. In particular, the study compared closed-loop systems with five comparator interventions: SMBG + MDI, CGM + MDI, isCGM + MDI, CSII + MDI and CSII + CGM.
The baseline characteristics and treatment effects for the simulation cohort were obtained from a 2017 Scottish T1DM cohort study and a NMA of the published literature. The cohort study was a nationally representative sample of individuals living with T1DM in Scotland.
The analysis adopted a healthcare payer perspective, with patients’ lifetimes as the time horizon. The indirect costs associated with lost work productivity due to diabetes morbidity were not included, and all the other costs were expressed in GBP. The costs and utilities were discounted at 3.5% per annum following the NICE methods of technology appraisal guidance.
The base-case results showed that the ICERs of closed-loop systems compared with SMBG + MDI, CGM + MDI and isCGM + MDI were £44,920, £58,996 and £79,664 per QALY gained, respectively. In all these pairwise comparisons, closed-loop systems had higher costs and QALYs than all of the comparators. It was, however, also noted that closed-loop systems had lower costs and higher QALYs than CSII + MDI and were thus cost-effective in this group. The deterministic sensitivity analyses showed that the findings were sensitive to changes in the assumed effects on hypoglycaemia and the per-event disutility value associated with NSHE, whereas the results of the probability sensitivity analysis were very similar to the base-case results.
The main limitation of the study was that it relied on an algorithm to convert improvements in percentage TIR to measures of reduction in HbA1c, which potentially resulted in inaccurate estimates. Nevertheless, the fact that the study used a nationally representative simulation cohort for Scotland meant that the findings were generalisable to the population, unlike the results of the other identified economic studies that used baseline data for different countries. Furthermore, unlike the previous analyses in the literature that considered either the MiniMed 670G or the MiniMed 780G compared with isCGM + CSII or CSII alone, the study provided a more comprehensive analysis of closed-loop systems in general compared with multiple configurations of the comparator technologies.
Canadian Agency for Drugs and Technology in Health108
The study in the 2021 CADTH report had three objectives. First, it extended the evidence base by using a budget impact analysis to estimate the financial impact of introducing HCL systems for individuals with T1DM. Second, it assessed the perspectives, experiences and expectations of individuals living with T1DM as well those of as their carers. Third, it assessed the ethical aspects associated with the use of HCL systems.
The analysis was conducted from the perspective of the Canadian publicly funded healthcare system with a time horizon of 3 years. The base-case results of the budget impact analysis showed that an additional CA$823 million would be needed to reimburse HCL systems for the eligible population. Specifically, an additional CA$131 million would be needed in year 1, an additional CA$271 million in year 2 and an additional CA$421 million in year 3. The scenario analyses showed that the results were sensitive to changes in the population of eligible individuals. In particular, increasing the HCL coverage levels to 100% translated to an increase of CA$916 million needed to finance the provision of HCL systems. The results were also sensitive to changes in the price of CGM and the uptake of HCL systems among the users of MDI.
The main limitation of the analysis was that the epidemiological measures used to inform the budget impact analysis, that is the prevalence of T1DM, the annual incidence of T1DM and the population growth rate, were proxy measures derived from the literature and may thus not have been accurate. These measures were obtained from a 2014 report, but the cost estimates for the base case were for 2020. The study also made several assumptions about the coverage levels of insulin-pump use, glucometers, CGM and SMBG test strips, which had an impact on the accuracy of the results.
Characteristics of retained studies
The characteristics of the six retained studies are summarised following CHEERS. Five of these studies were economic evaluations of HCL systems, whereas one was a budget impact analysis that aimed at estimating the financial impact of reimbursing HCL systems for individuals with T1DM. The economic evaluation studies compared the cost-effectiveness of HCL systems with various diabetes management technologies, such as isCGM + MDI, CSII and SMBG. Four studies used the iQVIA CDM to conduct their analyses,109–112 while the study in the SHTG report26 used the Sheffield type 1 diabetes model. Of the six studies, two were conducted in Sweden109,110 and one each was conducted in the UK,111 the Netherlands,112 Scotland26 and Canada. 108
The studies modelled their outcomes over patients’ lifetimes and reported their outcomes as cost per QALY gained, except from Roze et al. 111 and the study in the CADTH report that considered a healthcare payer perspective. 108 All the studies discounted their costs and outcomes in line with their national guidelines. An interesting point to note, however, is that there was substantial heterogeneity in the choice of baseline cohort data as well as the data for the treatment effects. For instance, Serne et al. 112 used different data sources for both the treatment effects and the simulation cohort. Moreover, the data were not for the Netherlands. Similarly, the studies by Roze et al. 111 and Jendle et al. 110 used a baseline simulation cohort comprising individuals from the USA, yet the studies were aimed at informing long-term cost-effectiveness for the UK and Swedish populations, respectively. Jendle et al.,109 despite being conducted in Sweden, used simulation cohort data sourced from a Belgian study. Only the study in the SHTG report26 used baseline data for its population of interest.
To characterise uncertainty in the base-case results, in all of the included studies the authors performed several one-way sensitivity/scenario analyses. The studies that employed the iQVIA CDM and the study in the SHTG report that used the Sheffield type 1 diabetes model further conducted probabilistic sensitivity analyses and presented the results in the form of cost-effectiveness acceptability curves (CEACs). An interesting point to note is that the base-case results were found to be very sensitive to the severe hypoglycaemic rates (events recorded/unit time) (SHE) and changes in the assumptions relating to the quality-of-life benefit associated with reduced fear of hypoglycaemia in four out of the five cost-effectiveness studies. 26,110–112 Furthermore, the CEACs showed that HCL systems are expected to be cost-effective compared with the comparator technologies at various hypothetical willingness-to-pay thresholds.
Quality assessment of the modelling methods and economic analyses
Structure
The budget impact analysis in the CADTH report108 was conducted using a customised Microsoft Excel tool and used several epidemiological measures obtained from the literature, such as the prevalence of T1DM, incidence rates and population growth rates, to estimate the market size and coverage levels of HCL systems in Canada. Financial projections were then made using these measures by adjusting the base-year HCL costs over a 3-year time horizon.
The structure of the models used in the cost-effectiveness studies was judged to be of good quality. The studies clearly stated their decision problem/research question, the viewpoint of their analyses and their modelling objectives, which were coherent with the decision problem. Both the iQVIA CDM and the Sheffield type 1 diabetes model are validated models for evaluating diabetes technologies. The studies that used the iQVIA CDM described the model as one with a complex semi-Markov model structure with interdependent sub-models, so more thorough, easier access to its reported features would be of benefit to the intended audience. None of the studies clearly showed the illustrative model structure, which depicted the clinical pathway for T1DM, although references were given to previous publications that outline this. The model is capable of capturing both long- and short-term clinical complications and costs associated with T1DM and has been extensively validated for use in this condition since its inception. 117,118
The Sheffield type 1 diabetes model is discussed more extensively in the SHTG report,26 unlike the iQVIA CDM studies that merely provide brief descriptions. The model also has a Markov model structure with several sub-models. The first Markov model predicts mortality in each cycle and is characterised by two states, that is alive or dead. If a particular individual is alive, then that individual can develop microvascular complications or CVD and can experience severe or NSHE. A five-state model for nephropathy (i.e. no nephropathy, microalbuminuria, macroalbuminuria, ESRD and death from ESRD), a three-state neuropathy model (no neuropathy, neuropathy and amputation) and a five-state model for retinopathy (i.e. no retinopathy, background retinopathy, proliferative retinopathy, macular oedema and blindness) is used to capture the progression of microvascular complications. A key difference between the STHG study that used the Sheffield type 1 diabetes model and the studies that used the iQVIA CDM is that the SHTG study used a published algorithm to model CVD and convert improvements in TIR to reductions in HbA1c, which was deemed to be a more relevant outcome measure. The algorithm assumed the form of a multivariable model where the 5-year risk of CVD depended on several individual characteristics, including duration of diabetes, age, systolic blood pressure, HbA1c levels, previous CVD, presence of macroalbuminuria and cholesterol levels.
Data
All the studies required data to undertake the economic analyses. For the cost-effectiveness studies to be conducted, both clinical and cost information as well as baseline characteristics for the simulation cohorts had to be input into the analytic models prior to the simulation process. The cost-effectiveness analyses also required data on the disutilities associated with diabetes-related complications as well as data on the utility benefits from the reduction in the fear of hypoglycaemia, which were largely obtained from the published literature. The budget impact analysis in the CADTH report108 used national statistics to inform the key epidemiological measures (i.e. the prevalence of T1DM, the annual incidence of T1DM and the population growth rate) and cost data required to estimate the market size and the amount of money needed to reimburse HCL systems.
In two studies, Serne et al. 112 and Jendle et al.,110 the authors obtained their baseline data and data for the treatment effect of their comparators from a prospective cohort study conducted in Belgium115 but used different data sources for their intervention treatment effects. The study by Serne et al. 112 obtained the treatment effect for the intervention from a retrospective US-based study of patients transitioning from SAP to the MiniMed 670G HCL system,116 whereas the study by Jendle et al. 110 obtained the intervention treatment effect from a randomised crossover trial conducted in New Zealand that comprised T1DM patients using the MiniMed 780G HCL system. 48 It is, however, not clear how the treatment effect was elicited as this is not explicitly stated in the text. Furthermore, the New Zealand study reported the treatment effects of the MiniMed 780G system on TIR. Yet TIR was not one of the outcomes of interest in Jendle et al. 110
Roze et al. 111 and Jendle et al. 109 obtained their baseline data from a study similar to the one used by Serne et al. 112 for the intervention treatment effect,113,114 but Roze et al. 111 used a NMA of the literature to obtain the treatment effects, whereas Jendle et al. 109 sourced the treatment effects from the simulation cohort. Like Roze et al.,111 the study in the SHTG report conducted a NMA of the published literature to get estimates of the treatment effects, but, unlike Roze et al.,111 the baseline characteristics were sourced from a 2017 Scottish T1DM cohort study.
The relevant cost inputs were obtained from the published literature, and they reflected the perspective of each study as reported. Where suitable resource use data were not available, for example, for treatment mix of the comparator, limitations were acknowledged and the authors justified the assumption of using a more conservative approach to costing. An important point to note is that the methods of identifying the relevant information sources were not clearly stated, although justifications for the chosen data sources were made and appropriate references were provided. It was not clear if a quality appraisal of the studies serving as data sources was undertaken, and, to the best of our knowledge, the studies did not undertake systematic reviews to identify the studies reporting key inputs. With respect to the risk equations underlying clinical progression within the validated models (i.e. the iQVIA CDM and the Sheffield type 1 diabetes model), the sources and choice of source where multiple options were available were not provided or justified. Therefore, the appropriateness of these sources for use with the specific decision problem cannot be assessed.
Uncertainty
The budget impact analysis presented in the CADTH report108 included scenario analyses where universal HCL coverage was assumed. All five cost-effectiveness studies also conducted several deterministic analyses by varying key input parameters to reflect lower and upper limits, or by making changes to input parameters if multiple sources of information were available to assess the impact on the base-case ICER and/or to determine the key drivers of the economic model. It was unclear in some analyses whether the sensitivity analyses were exhaustive as no tornado plots were reported. However, results were presented for all sensitivity and scenario analyses.
Four out of the five cost-effectiveness studies, that is Serne et al.,112 Roze et al.,111 SHTG26 and Jendle et al.,109 noted a substantial negative relationship between reducing the utility benefit for the HCL users due to an expected relatively lower fear of hypoglycaemia than among the users of the comparator technologies and the incremental QALY gain. To the best of our knowledge, however, ‘best-case’ and ‘worst-case’ analyses were not undertaken. It appears that probabilistic sensitivity analyses were performed as CEACs were presented showing the probabilities at which the HCL systems under investigation were likely to be cost-effective at various willingness-to-pay thresholds. This was, however, not explicitly stated in the texts.
Assumptions
The studies made several assumptions depending on the type of economic analysis being undertaken. There was significant overlap between studies about the assumptions made, likely to be due to the homogeneity of the economic analyses. For instance, the budget impact analysis in the CADTH report assumed particular figures for the epidemiological measures needed to estimate the market size and financial impact of reimbursing HCL systems. The study also assumed that the reimbursement would be limited to the eligible population but explored this assumption in a scenario analysis by varying the population coverage levels.
All of the cost-effectiveness analyses except that from the study in the SHTG report26 assumed that their findings were generalisable to their target populations despite using baseline data for other countries. The studies also used short-term simulation data to make long-term projections over patients’ lifetimes. The study in the SHTG report used an algorithm to convert improvements in TIR to reductions in HbA1c and assumed that the converted measures compared favourably with their actual estimates. To show that HCL systems were more cost-effective than their comparator technologies, the majority of the cost-effectiveness analyses assumed a utility benefit to HCL users due to the expected greater reduction in diabetes-related complications for this group than for those using the other technologies.
Discussion
The systematic review identified six studies containing economic analyses of HCL systems. Of the six studies, five were cost-effectiveness analyses comparing HCL systems with various diabetes management technologies, whereas one was a budget impact analysis that estimated the financial impact of reimbursing HCL systems over a 3-year time horizon. Two studies were conducted in Sweden109,110 and one study each was conducted in the UK,111 the Netherlands,112 Scotland26 and Canada. 108 These studies were assessed using the CHEERS and Philips checklists where applicable.
According to the assessment, four studies were identified as cost-effectiveness analyses in their titles: Jendle et al.,110 Serne et al.,112 Roze et al. 111 and Jendle et al. 109 The other two studies, the study in the SHTG report26 and the one in the CADTH report,108 did not have the phrase ‘cost-effectiveness analysis’ or other similar terminology in their titles that would have identified them as economic evaluations; however, on further scrutiny of the studies, we noted that the SHTG report contained a cost-effectiveness analysis in addition to a systematic review and NMA, while the CADTH report contained a budget impact analysis in addition to a review of the perspectives of HCL users and their carers as well as the ethical considerations of using HCL systems.
All of the studies except for the one in the SHTG report26 had structured abstracts containing information on the background, methods, study perspective, results and conclusions. Although the study in the SHTG report did not contain an abstract, it had several sections providing the relevant information that would normally be found in an abstract. The overall objective of Jendle et al. 110 was to evaluate the long-term cost-effectiveness of the MiniMed 780G HCL system (i.e. AHCL system) compared with isCGM + MDI or CSII. The study in the SHTG report examined the clinical effectiveness and cost-effectiveness of closed-loop systems and the artificial pancreas for the management of T1DM compared with current diabetes management options. Serne et al.,112 Roze et al. 111 and Jendle et al. 109 assessed the cost-effectiveness of the MiniMed 670G HCL system compared with CSII but differed in the way the comparator intervention was configured. Serne et al. 112 considered the users of isCGM + MDI or CSII, whereas Roze et al. 111 and Jendle et al. 109 considered only CSII users.
All of the cost-effectiveness studies noted that HCL systems were cost-effective over the lifetime compared with their comparator interventions. This inference was, however, subjective as the studies chose arbitrary willingness-to-pay thresholds. For instance, despite both Jendle et al. 110 and Jendle et al. 109 being conducted in Sweden, Jendle et al. 109 found that the MiniMed 670G HCL system was associated with an ICER of SEK 164,236 per QALY gained and was thus cost-effective at a threshold of SEK 300,000 per QALY gained. Jendle et al.,110 on the other hand, showed that the MiniMed 780G HCL system was associated with an ICER of SEK 373,700 per QALY gained and was cost-effective at a willingness-to-pay threshold of SEK 500,000 per QALY gained. If a threshold of SEK 300,000 per QALY gained had been used instead, then the MiniMed 780G HCL system would not have been cost-effective. The results in Serne et al. 112 showed that the MiniMed 670G HCL system had an ICER of EUR 6133 per QALY gained compared with the comparator technology and was thus cost-effective at willingness-to-pay thresholds of EUR 20,000, EUR 50,000 and EUR 80,000 per QALY gained. Roze et al. 111 noted that the MiniMed 670G HCL systems had an ICER of GBP 20,421 per QALY gained, which was below GBP 30,000 per QALY gained. The study in the SHTG report26 noted that closed-loop systems were not cost-effective compared with CGM + MDI, SMBG + MDI and CGM + MDI as their ICERs were GBP 58,996, GBP 44,920 and GBP 79,604 per QALY gained, respectively, and all were above a threshold of GBP 30,000 per QALY gained. If the study had considered a willingness-to-pay threshold of GBP 80,000 per QALY gained, then closed-loop systems would not have been found to be cost-effective in all these pairwise comparisons. This therefore indicates that economic evaluations should be undertaken with better justification for the chosen willingness-to-pay thresholds.
While the iQVIA CDM and the Sheffield type 1 diabetes model are both suitable for conducting economic analyses of diabetes management technologies, allowing for both deterministic and probabilistic sensitivity analyses to be undertaken; the four studies that used the iQVIA CDM109–112 are limited in the sense that the model considers only life expectancy, quality-adjusted life expectancy, cumulative incidence and time to onset of long-term complications as the outcomes of interest. These outcome measures are, however, sufficient for eliciting the population health gains (or health losses by extension) associated with the various diabetes management technologies.
The iQVIA CDM uses time, time in state and diabetes-dependent probabilities to simulate the progression of diabetes and diabetes-related complications with both diabetes and non-diabetes mortality accounted for. The model allows for both clinical and cost data to be input directly into the model or for the default parameters to be used instead. The studies identified in this review used the literature to obtain this information. The clinical data include baseline characteristics, such as age, sex, duration of diabetes, total daily insulin dose and HbA1c levels as well as data on the disutilities associated with diabetes-related complications. The cost data include the cost of insulin pumps and accessories, for example, infusion sets and reservoirs, sensors, transmitters, serters, batteries and self-monitored plasma glucose testing, the direct costs of diabetes-related complications and the indirect costs if a societal perspective is adopted. The Sheffield type 1 diabetes model used by the study in the SHTG report26 is also limited in the sense that it relies on published data from outside the UK to define the risk of long-term complications. Furthermore, this risk largely depends on HbA1c, ignoring the effects of the other risk factors, and could thus introduce bias into the results when interventions are evaluated that affect other factors besides HbA1c. 119 Given that our objective is to provide evidence to NICE on the cost-effectiveness of HCL systems in general and our scope is not limited to the interventions that only affect HbA1c, we find the iQVIA CDM to be more appealing than the Sheffield type 1 diabetes model.
A major limitation of most of the cost-effectiveness studies is that their findings might not be generalisable. This is because the studies did not use baseline characteristics and treatment effects data for their target populations. The studies relied on studies conducted in the USA for the treatment effects of the MiniMed 670G HCL system, a prospective cohort study conducted in Belgium for the simulation data and treatment effects of isCGM + MDI or CSII as well as a randomised crossover trial in New Zealand for the treatment effect of the MiniMed 780G HCL system, despite some controversy around the elicitation of the treatment effect. Only the SHTG study used data for its study setting. The assumption made in these studies was that the simulation cohorts, despite being for the USA, Belgium and New Zealand, were representative of the Netherlands, Sweden and the UK, which is a rather strong assumption. Furthermore, the chosen data sources had varying study designs with different identification assumptions, which potentially affected the validity of the results. To extend these studies, therefore, cost-effectiveness analyses with appropriate simulation cohorts are needed. Our study does this by using real-world data for the UK to serve as the simulation cohort. We also extend the SHTG study that used the Sheffield type 1 diabetes model to simulate Scottish data by using the iQVIA CDM, which obviates some of the limitations of the Sheffield type 1 diabetes model.
Chapter 7 Companies’ submissions of cost-effectiveness evidence
Medtronic submission economics
The Medtronic submission used the iQVIA CDM, henceforth referred to as the iQVIA CDM and described in more detail in Figure 12, to compare the AHCL 780G MiniMed pump with the CSII using the 640G MiniMed pump. Two comparisons were made with CSII + CGM, the first with rtCGM using the Guardian sensor and transmitter and the second with isCGM using the Freestyle Libre sensor.
FIGURE 12.
iQVIA CDM structure. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CHF, congestive heart failure; MI, myocardial infarction; mort, mortality; PVD, peripheral vascular disease; spec mort, specific mortality (i.e. condition-related deaths).
HCL was associated with an HbA1c reduction of 0.8% and both CSII + rtCGM and CSII + isCGM were associated with no change. Thereafter a common annual worsening of the iQVIA default of 0.045% was applied.
The change in HbA1c was derived from the Collyns et al. 48 Medtronic-funded open-label RCT two-sequence crossover study of HCL compared with SAP + PLGM. Collyns et al. 48 used the HCL 670G MiniMed pump, revising the operational mode to implement SAP + PLGM. Collyns et al. 48 reported a mean baseline of 9.3 mmol/l, with this improving to 8.5 mmol/l in the AHCL arm and worsening slightly to 9.5 mmol/l in the PLGS arm, equivalent to approximately 7.5% HbA1c at baseline and 7.0% HbA1c for AHCL and 7.6% HbA1c for PLGS.
No difference in NSHE was assumed, although it can be noted that time < 3.9 mmol/l improved from a baseline of 3.1%–2.1% for HCL.
Both HCL and CSII + rtCGM were assumed to have no SHEs. For the comparison with CSII + isCGM, annual rates of SHEs not requiring medical assistance and requiring medical assistance of 0.65 and 0.25 were stated as being sourced from Östenson et al. 120
Patient population characteristics at baseline were taken from Collyns et al.,48 with a mean age of 23 years, a duration of diabetes of 13 years, a baseline HbA1c of 7.6% and 42% male.
Total annual technology costs were £5420 for A/HCL 780G, £5342 for CSII + rtCGM and £3516 for CSII + isCGM. Other costs were largely sourced from NG17.
For the comparison of 780G with CSII + rtCGM, the company estimated totals of 13.89 QALYs and 13.67 QALYs, respectively, yielding a net gain of 0.21 QALYs. Total costs of £253,583 and £259,400 were estimated, yielding a net cost saving of £5816 and hence dominance for HCL 780G over CSII + rtCGM. A scenario analysis using the net HbA1c gain of 0.3% from the Isganaitis study roughly halved the gain to 0.12 QALYs but net savings of £4765 persisted, so HCL 780G remained dominant over CSII + rtCGM.
For the comparison of HCL 780G with CSII + isCGM, the company estimated totals of 13.89 QALYs and 13.19 QALYs, respectively, yielding a net gain of 0.69 QALYs. Total costs of £253,583 and £240,526 were estimated, suggesting a net cost of £13,057 and an ICER of £18,672 per QALY. The scenario analysis using the net HbA1c gain of 0.3% from the Isganaitis study slightly reduced the estimated gain to 0.61 QALYs and net costs increased to £14,758, resulting in an ICER of £23,873 per QALY.
The EAG makes the following observations:
-
The results of Collyns et al. 48 are for AHCL compared with PLGS rather than for HCL compared with CSII + CGM.
-
Östenson et al.,120 the reference for SHE rates for CSII + CGM, does not specify that patients with T1DM were on CSII + isCGM. The only treatment information available is the types of insulin that were received, with 8% receiving only long-acting insulin, 65% receiving both short- and long-acting insulin and 27% receiving other types of insulin. There is no obvious reason why the SHE rates are specific to CSII + isCGM and do not include other regimens, such as MDI.
-
The EAG is unable to source the annual SHE rates not requiring medical assistance and requiring medical assistance of 0.65 and 0.25 from Östenson et al. 120 who reported a mean annual SHE rate of 0.7 among those with T1DM.
-
It appears that the iQVIA CDM default quality-of-life values were used throughout. These relate to T2DM patients with a quality-of-life value of 0.752 when having no complications, rather than the 0.839 for T1DM patients. Additional survival might have been undervalued.
-
The sensors and transmitters for the Guardian system within the costing of the 780G system and CSII + rtCGM were costed at the anticipated April 2023 list price rather than the current list price.
-
Both CSII + rtCGM and CSII + isCGM were costed as using the Medtronic 640G pump. There may be a range of other pumps that can be used within both CSII + rtCGM and CSII + isCGM, the costs of which may differ from that of the Medtronic 640G.
-
The sensors and transmitters for a CSII + rtCGM assumed the Guardian system. There may be a range of other sensors and transmitters that can be used, the costs of which may differ.
Dexcom submission economics
(Confidential information has been removed.)
Tandem submission economics
The Tandem submission referenced the Dexcom submission economics and provides no additional cost-effectiveness estimates.
CamDiab submission economics
(Confidential information has been removed.)
CamDiab Dan05 study economics
(Confidential information has been removed.)
CamDiab KidsAP02 study economics
(Confidential information has been removed.)
Summary of companies’ economic modelling
The inputs and outputs of the companies’ economic modelling are summarised in Tables 5 and 6.
| Medtronic | DexCom/Tandem | CamDiab Dan05 | CamDiab KidsAP02 | |
|---|---|---|---|---|
| Baseline characteristic | ||||
| Mean age | 23.5 (7.0) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Male % | 42% | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Duration of diabetes | 13 (10.2) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| HbA1c | 7.6% (0.9) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Costs of hypoglycaemic events | ||||
| NSHE | £0 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE non-medical | £489 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE medical | £2358 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Disutilities hypoglycaemic events | ||||
| NSHE daytime | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| NSHE night-time | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE non-medical | −0.0137 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE medical | −0.0578 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE any daytime | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE any night-time | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Company | Medtronic | DexCom/Tandem | CamDiab Dan05 | CamDiab KidsAP02 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model | iQVIA CDM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||
| Regime | HCL | CSII + rtCGM | CSII + isCGM | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed a | Confidential information has been removed b | Confidential information has been removed | Confidential information has been removed |
| Pump | 780G | 640G | NR | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Clinical effects | |||||||||
| HbA1c | −0.8% | 0.0% | 0.0% | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| NSHE | – | – | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE non-medical | 0 | 0 | 0.65 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE medical | 0 | 0 | 0.25 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SHE total | 0 | 0 | 0.90 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| QoL direct effect | – | – | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Annual cost | Confidential information has been removed | Confidential information has been removed | £3516 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removedd | Confidential information has been removed | Confidential information has been removed |
| Results | |||||||||
| Life-year undiscounted | 42.79 | 41.67 | 41.67 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Life-year discounted | 20.57 | 20.34 | 20.34 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| QALYs | 13.89 | 13.67 | 13.19 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Net vs. comp. | 0.21 | 0.70 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Costs | £253,583 | £259,400 | £240,526 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Net vs. comp. | −£5816 | £13,057 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| ICER vs. comp. | Dominant | £18,672 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
Independent economic assessment
Methods
Patient population
The key baseline characteristics are drawn from the 2019 to 2020 National Diabetes Audit subgroup of those on pump therapy. For the scenario analyses using the adult NHS pilot data, the baseline characteristics are taken from the pilot (reported earlier in NHS evidence).
Other baseline characteristics needed as inputs to the iQVIA CDM are taken from NG17, these largely being derived from the Repose trial of pumps compared with MDI as reported in Heller et al. 121 It can be noted that these characteristics relate to a slightly worse-managed group of patients, their baseline HbA1c being 9.1% at baseline. Patients were excluded if they had used a pump in the last 3 years, and among those randomised to pump therapy a 0.85% improvement was observed, which brings the similarity of the audit subgroup into line with that of the National Diabetes Audit pump subgroup. Unfortunately, as in the HCL trials the Repose trial did not report changes in other baseline characteristics that might have been affected by pump adoption, such as systolic blood pressure.
Treatment options to be evaluated
The cost-effectiveness analysis considers the three comparators in the EAG NMA:
-
CSII + CGM non-integrated
-
LGS/PLGS
-
HCL.
CSII + CGM is not separately evaluated as CSII + rtCGM and CSII + isCGM. Based on feedback from the Diabetes Technology Network, the balance is assumed to be 10% CSII + rtCGM and 90% CSII + isCGM for adult patients (paediatric patients may have a higher rtCGM proportion of around 25%, in part due to higher Omnipod use), although this may underestimate CSII + isCGM use. The EAG scenario analysis that applies the NHS adult pilot data CSII + CGM applies 100% CSII + isCGM because prior use of CSII + isCGM is reported as a requirement.
Framework: methods of synthesis
Glycated haemoglobin effects
The EAG base case applies the results of the NMA. The EAG also presents scenarios restricting the NMA evidence base [HCL −0.28% (0.033%), PLGS −0.06% (0.079%)] to adult trials [HCL −0.24% (0.043%), PLGS −0.01% (0.115%)] and applying the mean change of the NHS adult pilot [HCL −1.50% (0.051%)].
The base case assumes that the HbA1c effect endures for the model time horizon of 50 years. Scenarios of durations of 5 years, 10 years and 20 years are presented.
Non-severe hypoglycaemic event and severe hypoglycaemic event rates
Non-severe hypoglycaemic event rates were not reported in the trials. As reviewed in more detail below, where they were reported they were typically based on proxies, such as the number of periods of ≥ 20 minutes spent < 3.0 mmol/l. The EAG presents a brief review of the literature on NSHE and SHE rates before presenting scenario analyses that estimate NSHE and SHE rates based on estimates in the literature coupled with the EAG NMA results for time below range.
The SHTG report estimated NSHEs from Donnelly et al.,122 a randomly drawn sample of 267 T1DM and T2DM insulin-treated patients in Tayside during 2001. These patients were asked to record their hypoglycaemic events for 1 month. Among the T1DM patients (n = 94), who had a mean age of 41 years, a mean duration of diabetes 10 years, were 49% male and had a mean HbA1c of 8.5%, the numbers of NSHEs and SHEs were 327 and 9, respectively, suggesting per-patient average annual rates of 42 for NSHEs and 1.15 for SHEs. The SHTG assumed that these rates apply to MDI + SMBG as is reasonable given the 2001 data and that patients were advised to check their blood glucose 2–4 times daily with a portable glucose meter. The SHTG coupled these with reductions of 50% for HCL from McAuley et al.,123 35% for MDI + rtCGM from Beck et al.,124 25% for MDI + isCGM from Bolinder et al. 125 and an assumption of 30%, the mid-point of the MDI + rtCGM and MDI + isCGM values, for CSII + CGM. This implies annual NSHE rates of 21 for HCL and 29 for CSII + CGM.
It should be noted in passing that the 1.15 annual average for SHEs of Donnelly et al. 122 is an order of magnitude greater than the 0.115 annual rate for SHEs requiring NHS resource use that Leese et al. 126 estimated across all T1DM patients in Tayside (n = 977), who had an average age of 33 years, an average diabetes duration of 17 years, were 57% male and had a mean 7.92% HbA1c. These estimates if taken together suggest that only 10% of SHEs require NHS attention, which is somewhat less than the EAG base case of 37.9% as summarised in Evidence Assessment Group base case.
McAuley et al.,123 sponsored by JDRF Australia, compared HCL using the Medtronic 670G with MDI + SMBG or CSII + SMBG over 6 months among 120 T1DM patients, who had a mean age of 44 years, a mean diabetes duration of 24 years, were 47% male and had a mean of 7.4% HbA1c. In the HCL group (n = 61) there were eight SHEs, of which four were attributed to the study device, while in the control group (n = 59) there were seven SHEs. These correspond to annual SHE rates of 0.26 and 0.24, respectively, a ratio of 111%, but when only including SHEs attributable to HCL annual SHE rates of 0.13 and 0.24, respectively, a ratio of 55%. Unfortunately, McAuley et al. 123 do not specify how SHEs were attributed to device or other causes. Turning to the time below range, both HCL and control showed improvements over the course of the trial. The net effects favoured HCL, with the percentage time below range improving by 2.0%, 0.8%, 0.6% and 0.4% for 3.9 mmol/l, 3.3 mmol/l, 3.0 mmol/l and 2.8 mmol/l, respectively. Applying these net changes to the end of trial control arm time below ranges of 3.8%, 1.4% 0.9% and 0.6%, the ratios of time below range (while a percentage of e.g. 0.9% may at first sight seem small it corresponds with an hourly 1.5 per week) that result are 47%, 43%, 33% and 33%. These ratios may be subject to quite considerable rounding error but show some alignment with the 55% SHE ratio that excludes SHEs not attributable to HCL. However, it must be acknowledged that this in turn begs the question of how to handle SHEs not attributable to HCL in the HCL arm for any comparison with the control arm.
In a similar vein, the RCTs of HCLs that reported SHEs and ratios of time below range are presented below. Few papers reported NSHEs, and those that did used proxies:
-
Kariyawasam et al. 127 used the number of events < 3.9 mmol/l.
-
Brown et al. 77 and Breton et al. 75 used the median numbers of events of at least 15 minutes ≤ 3.0 mmol/l.
-
Abraham et al. 128 used the median numbers of events of at least 20 minutes ≤ 3.0 mmol/l.
The median weekly NSHE rates at the end of trial reported by Abraham et al. 128 of 2.1 for control and 1.1 for HCL are notably different from the numbers of moderate hypoglycaemia events reported in the supplementary appendix of 7 and 13, respectively. The former imply annual event rates of 57 for HCL and 109 for control, while the latter imply annual event rates of 0.21 and 0.38. However, the ratios of these events are similar, at 53% and 55%, which are also quite similar to the ratios of the time below range, as reported in Table 7.
| Lead author | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraham | Brown | McAuley | Ware | Boughton | Breton | Ware | Benhamou | Tauschmann | Thabit | Thabit | Kariyawasam | |
| Published | 2021 | 2019 | 2020 | 2022 | 2019 | 2022 | 2022 | 2019 | 2018 | 2015a | 2015b | 2021 |
| Study weeks | 26 | 26 | 26 | 26 | 16 | 16 | 16 | 12 | 12 | 12 | 12 | 6 |
| Comparator | Mixed | CSII* | Mixed | CSII* | CSII* | CSII* | CSII* | CSII* | CSII* | CSII* | CSII* | CSII* |
| Age (years) | 15 | 33 | 44 | 13 | 68 | 11 | 5.6 | 48 | 22 | 40 | 12 | 8.2 |
| Duration of diabetes (years) | 7.7 | 17 | 24 | 6.5 | 38 | 5.2 | 2.6 | 28 | 12 | 21 | 4.7 | 5.5 |
| Male (%) | 44 | 50 | 46 | 43 | 57 | 50 | 58 | 38 | 49 | 55 | 56 | 47 |
| HbA1c base (%) | 7.75 | 7.40 | 7.80 | 8.25 | 7.45 | 7.7 | 7.35 | 7.60 | 7.90 | 7.60 | 7.80 | 7.25 |
| NSHEs annual | ||||||||||||
| Comparator | 109.2 | 26.0 | NR | NR | NR | 31.2 | NR | NR | NR | NR | NR | 24.5 |
| HCL | 57.2 | 20.8 | NR | NR | NR | 20.8 | NR | NR | NR | NR | NR | 13.0 |
| Ratio | 52% | 80% | – | – | – | 67% | – | – | – | – | – | 53% |
| SHEs annualised | ||||||||||||
| Comparator | 0.00 | 0.00 | 0.24 | 0.00 | 0.38 | 0.00 | 0.00 | 0.19 | 0.20 | 0.00 | 0.00 | 0.00 |
| HCL | 0.00 | 0.00 | 0.26 | 0.06 | 0.00 | 0.00 | 0.04 | 0.32 | 0.17 | 0.13 | 0.35 | 0.00 |
| Ratio | 100% | 100% | 111% | – | 0% | 100% | – | 167% | 86% | – | – | 100% |
| Excl. non attr. | – | – | 0.13 | – | – | – | – | – | – | – | – | – |
| Ratio | – | – | 55% | – | – | – | – | – | – | – | – | – |
| Time ratios (%) | ||||||||||||
| ≤ 3.9 mmol/l | 54 | 61 | 47 | 110 | 94 | 78 | 102 | 44 | 79 | 81 | 83 | 50 |
| ≤ 3.5 mmol/l | NR | NR | NR | NR | 100 | NR | 102 | NR | 84 | NR | NR | NR |
| ≤ 3.3 mmol/l | 44 | NR | 43 | NR | NR | NR | NR | 35 | NR | NR | NR | NR |
| ≤ 3.0 mmol/l | 50 | 97 | 33 | NR | 100 | 77 | 102 | NR | NR | NR | NR | 56 |
| ≤ 2.8 mmol/l | 50 | NR | 33 | NR | NR | NR | NR | 29 | 118 | 45 | 47 | NR |
For individual studies, the reductions in time below range tend to be similar across the thresholds, although Brown et al. 77 and Thabit et al. 53 do not follow this pattern.
Among the papers that report NSHEs there is a reasonable if imperfect correspondence between the reduction in NSHEs and the reduction in time below range. There is a degree of circularity in this due to the definition of NSHEs not being symptomatic events, but the number of times patients fell below a mmol/l threshold for at least a given amount of time.
Rates of SHEs are low but vary between the papers even for just their HCL arms. There is no obvious pattern between comparator and HCL, or with the time below range ratios.
Turning to rates of NSHEs within the two main quality-of-life studies reviewed in more detail in Health valuation, Gordon et al. 129 and Currie et al.,22 NSHEs were defined symptomatically, with Gordon et al. 129 relying on trial data and Currie et al. 22 relying on postal questionnaire 3-month recall data with a 31% response rate. Gordon et al. 129 did not report NSHE rates. Currie et al. 22 reported an annualised symptomatic NSHE rate for the T1DM subset of 37.6, which, given that the surveys were in 2000 and 2006, probably related mainly to MDI. This needs to be read in conjunction with the reported annual SHE rate of 1.47 and the 31% response rate. But the 37.6 annual NSHE rate corresponds quite closely to the 42 annual NSHE rate reported in Donnelly et al. 122 from which the SHTG inferred annual NSHE rates of 21 for HCL and 29 for CSII + CGM. This in turn corresponds quite closely with the common 20.8 annual NSHE rate for HCL reported in Brown et al. and Breton et al.
Because there was no direct RCT evidence of the effects of HCL on NSHEs, the EAG does not include NSHE effects in its base case. Given the range of reported SHE rates, the EAG also does not include SHE effects in its base case.
For NSHEs, the EAG presents a scenario analysis that couples the 20.8 annual NSHE rate for HCL of Brown et al. and Breton et al. with the EAG NMA time < 3.0 mmol/l net ES, the weighted mean of the end of trials’ time < 3.0 mmol/l for the CSII + CGM and the assumption that the number of NSHEs is proportionate to the time < 3.0 mmol/l. Scenarios of annual NSHE rates of 57.2 and 13.0 for HCL are presented.
For SHEs, the EAG adopts the same approach in exploratory scenarios that assumes SHE rates are proportionate to time < 3.0 mmol/l. Note that this is not saying that the threshold for SHEs is 3.0 mmol/l, only that the best measure of whatever is the appropriate threshold for SHEs is likely to be itself proportionate to time < 3.0 mmol/l. Coupling this with the annual SHE rate for HCL of 0.26 as reported in McAuley et al., chosen because it was a 26-week study and a reasonable mid-point, results in the estimates in Table 8. (The annual SHE rate is reasonably similar to the 0.20 annual SHE rate for CSII + CGM that was applied in the DG21 assessment of sensor augmented pump therapy for T1DM patients. The mean annual SHEs of 0.1855 for rtCGM and 0.1358 for isCGM of NG17 suggest an annual rate of around 0.14. The second year annual SHE rate of 0.30 for those on pumps in the Repose trial is also reasonably aligned with this, bearing in mind that CGM was not a requirement.)
| Time < 3.0 mmol/l | NSHEs | SHEs | |||
|---|---|---|---|---|---|
| NMA net | Absolute | Ratio | |||
| HCL | −0.14% | 0.46% | 100% | 20.8 | 0.26 |
| PLGS | −0.16% | 0.44% | 96% | 19.9 | 0.25 |
| CSII | Reference | 0.60% | 130% | 25.9 | 0.32 |
The annual SHE rates correspond reasonably closely to the NHS adult pilot annual rates of 0.21 at baseline and 0.34 at 6 months.
Treatment pathways and modelling
Treatment pathway
The treatment pathway assumes that patients remain on a single treatment option throughout: CSII + CGM, PLGS or HCL.
Modelling of glycated haemoglobin effects: iQVIA CORE Diabetes Model summary
In line with DG21 and NG17, the EAG uses the iQVIA CDM (see Figure 12) to model the micro- and macro-vascular complications of diabetes and patients’ overall survival. This decision is in part due to its availability to the EAG at the start of the DAR process but is mainly due to precedents, with NG172 noting:
The previously published IQVIA CDM (CDM) version 9.5, which has been validated against clinical and epidemiological data, was used for the analysis. This was decided on due to the need for a model accounting for the long-term complications of diabetes within a lifetime time horizon as agreed upon by the Guideline Committee. Given the complexity of modelling type 1 diabetes and the timeline constraints associated with this clinical guideline development, the committee agreed this was a more robust approach than attempting to develop a new model framework from scratch.
Type 1 Diabetes in Adults: Diagnosis and Management. Available from www.nice.org.uk/guidance/ng17. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
© NICE (2015)
There is also the benefit of direct comparability with most of the industry submissions’ economic modelling, but it should be borne in mind that the SHTG modelling used the Sheffield model.
In brief, as shown in the model diagram above, the iQVIA CDM predicts the progress of patients with T1DM over their lifetime, modelling the incidences of the 11 macro- and micro-vascular complications, the likelihoods of which are affected by T1DM. The default and recommended settings are to sample 1000 patients from the patient characteristics group and run each of these patients through the model 1000 times.
The iQVIA team has advised the EAG that for modelling a T1DM cohort only the non-specific mortality approach should be use as per the diagram above, and not the combined approach of the T2DM United Kingdom Prospective Diabetes Study (UKPDS) 62 and UKPDS 82 studies. Given the event-specific mortality, to estimate the non-specific mortality by age, ‘Other Mort’ in the diagram, the EAG adjusts UK life table data to remove deaths due to the International Statistical Classification of Diseases and Related Health Problems, Tenth Edition (ICD-10), codes for CVD, cerebrovascular disease and renal failure. The iQVIA modelling team has indicated that removing deaths attributable to the ICD-10 codes for hypertension may also be reasonable, and the EAG presents this in a scenario analysis. The iQVIA CDM team indicates that for T1DM this approach requires that the non-combined modelling of mortality be selected.
Modelling of glycated haemoglobin effects: iQVIA CORE Diabetes Model validation work
Both Palmer et al. 117 and McEwan et al. 118 presented model validation work for previous versions of what was then the IMS CDM. McEwan et al. is the more recent paper; it probably used a more recent version of the CDM and with the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study (Table 9) has a large number of patients and a long follow-up and is consequently preferred by the EAG. However, only Palmer et al. reported validation work around overall survival, and the EAG turns to this at the end of the review.
| Study | Event | Trial observed | CDM version 8.5 modelled | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Control | Net | Treatment | Control | Net | ||
| DCCT; N = 1441; 5.0–6.5 years’ follow-up | Retinopathy | 23 | 91 | −68 | 18 | 91 | −73 |
| Neuropathy | 7 | 28 | −21 | 8 | 30 | −22 | |
| Microalb. | 55 | 103 | −48 | 72 | 105 | −33 | |
| Albuminuria | 9 | 9 | 0 | 6 | 10 | −4 | |
| DCCT/EDIC; N = 1226; 17–30 years’ follow-up | Cardiovascular events | 25 | 38 | −13 | 38 | 43 | −5 |
| Retinopathy | 153 | 356 | −203 | 200 | 211 | −11 | |
| Neuropathy | 66 | 178 | −112 | 101 | 83 | 18 | |
| CVD | 66 | 100 | −34 | 115 | 118 | −3 | |
| ESRD | 7 | 14 | −7 | 26 | 23 | 3 | |
McEwan et al. modelled the internal validity of what was then the CDM version 8.5 in predicting events for the DCCT cohort with follow-up of 5.0–6.5 years and the EDIC cohort with follow-up of 17–30 years.
Validation is reasonable for the DCCT study, suggesting that the CDM is relatively good at modelling events over a medium time horizon, but, given the lifetime modelling of most cost-effectiveness analyses, the validation for the DCCT/EDIC study is the more relevant. McEwan et al. reported the relative risks of events for the CDM compared with the trial, but for cost-effectiveness modelling the differences in the absolute numbers of events are the more relevant metric. It is not reported why McEwan et al. group cardiovascular events given the CDM model structure, but it may have been that trial reporting necessitated this.
The control arm of the DCCT/EDIC is now obsolete. Concentrating on the DCCT/EDIC intensive treatment arm, the iQVIA CDM overestimated all events for the treatment arm, this being most serious for ESRD, for which the model estimate was 26 compared with the observed 7, more than triple the observed at 371%. But cardiovascular events, retinopathy, neuropathy and CVD were also overestimated, the modelled incidences being 152%, 131%, 153% and 174%, respectively, of those observed in the trial. The EAG presents a scenario analysis that reduces these costs proportionately to their overestimation as reported in McEwan et al. This mainly affects the costs of eye and renal complications because of their high annual costs. This scenario does not address the effects of any possible overestimation of eye and renal complications on quality of life and overall survival.
It can be noted that Palmer et al. also examined the observed versus the modelled incidences of ESRD over time and found a very good correspondence with data from 1075 US T1DM patients recruited prior to the age of 18 years, a 25-year cumulative incidence of 9.1% observed compared with 8.9% modelled. It is unclear whether this model validation was internal, using a study used to construct the CDM, or external, trying to model the outcomes of a study not used in the construction of the CDM.
It is particularly important to model ESRD correctly within the CDM because of its large effect on quality of life, a disutility of 0.164 for haemodialysis and 0.204 for peritoneal dialysis compared with a patient who has no complications, and its very high ongoing annual cost of £34,613 for haemodialysis and £31,139 for peritoneal dialysis. The effects of the modelled ESRD on QALYs, costs and the ICER bear particular scrutiny.
Unfortunately, McEwan et al. did not report the corresponding survival percentages. Any modelled differences in overall survival may drive the ICER to a somewhat greater extent than the modelled differences in vascular events and albuminuria. This somewhat limits the usefulness of the validation exercise for assessing the reasonableness of using the CDM for economic assessments. This may also be the reason why the incidence of ESRD is modelled as higher in the treatment arm than in the control arm, the reverse of that observed. Time spent with ESRD would have been a better comparison, but data for this comparison might not have been available for the trial.
Turning back to Palmer et al., they reported the observed overall proportion surviving compared with that modelled for a cohort of 142 US T1DM patients in the Joslin clinic (Table 10), all of whom were recruited before they were 21 years old.
| Observed (%) | Modelled (%) | |
|---|---|---|
| At 4 years | 99 | 99 |
| At 10 years | 97 | 95 |
| At 15 years | 96 | 87 |
| At 20 years | 88 | 79 |
| At 25 years | 81 | 70 |
Again, the observed values and the CDM modelled values were reasonably aligned in the medium term but diverged somewhat in the longer term. This may provide an argument for exploring the effect that shorter time horizons have on the ICER, and if modelling children or adolescents keeping a weather eye on the considerably longer time horizons that have to be modelled to effect a lifetime time horizon.
The Mount Hood challenges invite diabetes modellers to test their models against long-term follow-up data in competition with other modellers. The EAG has identified the first, fourth, fifth, eighth and ninth challenges as being published in peer-reviewed journals, but of these only the fourth, held in 2004, reported validation data on model performance for T1DM patients.
The Mount Hood 4 Modelling Group130 reported the results for two models that attempted to replicate the DCCT for the primary prevention cohort at 9 years, CORE (Table 11) and Archimedes (a third model, EAGLE, attempted to reproduce results for the secondary prevention cohort). Only the micro-vascular complications that could be compared with published DCCT data were presented, results for the Archimedes model being very similar to those of the CORE model.
| Arm | DCCT | CORE | ||||
|---|---|---|---|---|---|---|
| Control (%) | Intense (%) | Net (%) | Control (%) | Intense (%) | Net (%) | |
| Microalbuminuria | 27.3 | 16.0 | −11.3 | 27.7 | 14.9 | −12.8 |
| Background retinopathy | 52.2 | 14.3 | −37.9 | 39.4 | 14.4 | −25.0 |
| Peripheral neuropathy | 63.2 | 27.7 | −35.5 | 64.0 | 25.0 | −39.0 |
The CORE model estimated 9-year cumulative incidences for the intensive care arm quite well, but the estimates for the control arm were more variable. This caused the net estimates of microalbuminuria to be closely aligned, peripheral neuropathy to be reasonably aligned and background retinopathy to be poorly aligned with those of the DCCT. Within the above it should be borne in mind that the control arm of the DCCT is obsolete and that only the intensive treatment arm has any relevance today.
The above may appear critical of the validity of the iQVIA CDM as longer time horizons are modelled. It is almost inevitable that uncertainty around modelled outputs will increase as the time horizon extends and that observed values will diverge to some extent from that modelled. Although the validation work suggests a less than perfect correspondence between the model and real life, the availability of the validation work is a strength. Much of the economic modelling presented to NICE within other workstreams such as Single Technology Appraisals relies on short-term trials extrapolated to lifetime horizons for which no parallel validation work is possible. It should also be borne in mind that the iQVIA CDM continues to evolve.
The ability of the iQVIA CDM to reliably simulate a T1DM paediatric population is an open question, being affected by both the longer duration required for a lifetime horizon and the degree to which the risk equations of the model relate to a paediatric population. A key source of T1DM model inputs appears to be the DCCT/EDIC trial, which recruited patients between 13 and 39 years, with a mean baseline age of 27 years and a SD of 7.1 years. If normally distributed this would imply that of the 1441 recruited at baseline around 24 (2%) would have been up to 12 years, 40 (3%) between 13 and 15 years and 80 (6%) between 16 and 18 years: a total of 144 (10%) being up to 18 years of age at baseline. At close of the DCCT, the mean age had increased to 33 years while at EDIC 18 years’ follow-up it had risen to 52 years, meaning that the great majority of the DCCT/EDIC data will relate to an adult population. An alternative to the EDIC CVD model in the iQVIA CDM is the Pittsburgh CVD model, which was based on the Epidemiology of Diabetes Complications Study (EDC) that recruited 658 subjects with childhood onset of diabetes before the age of 17 years and has followed them up for 22 years. If modelling a younger population this suggests at a minimum exploring the effect of the Pittsburgh CVD model. The EAG remains uncomfortable simulating a paediatric population using the iQVIA CDM but presents a scenario of this.
Modelling of glycated haemoglobin effects: glycated haemoglobin progression
The iQVIA CDM default for HbA1c progression is an annual 0.045% worsening. This is drawn from the DCCT/EDIC trial as reported in Nathan et al. 131 The DCCT trial compared intensive therapy with conventional therapy among 1441 patients with T1DM. A primary prevention cohort with a duration of diabetes of 1–5 years had to have no history of hypertension, CVD, neuropathy requiring treatment or retinopathy. A secondary intervention cohort could have a duration of diabetes of 1–15 years and had to have at least one microaneurysm on one eye. Intensive therapy included MDI with a minimum of three daily injections or CSII with patient-specific HbA1c goals. Conventional therapy was standard of care in the 1980s, typically one or two daily injections and SMBG or urine testing, with the only HbA1c goal being the avoidance of values over 13.5%. EDIC provided long-term follow-up to the DCCT. After the DCCT and before enrolment in EDIC, all in the conventional therapy arm were offered training in intensive therapy. The DCCT was a controlled trial and the EDIC was observational.
Tabulated data suggest that at the end of the DCCT for the intensive therapy arm the median HbA1c was 7.2%. The EAG reproduced figures from Nathan et al. and extracted the values.
The reasons for downturn at the end of intensive therapy are unclear, the graphed value appearing to be below the reported 7.2% for the end of the DCCT phase. Values prior to this also appear slightly higher than 7.2%.
The EAG estimates that in the intensive therapy arm the median HbA1c at 6 months was 6.88% while at 9 years it was 7.48%, which suggests an annual worsening of 0.07%. Applying the stated end of DCCT value of 7.2% suggests an annual worsening of 0.04%, which is reasonably aligned with the 0.045% default of the iQVIA CDM, but this ignores the long-term EDIC follow-up.
The EAG estimates that among those initially on intensive therapy who continued it during EDIC, the median HbA1c was 7.64% at EDIC baseline and 7.71% at 18 years, which suggests little to no annual worsening during EDIC. Nathan et al. tabulate an end of EDIC value of 8.0%, which over the course of EDIC might suggest an annual worsening of 0.02% in the intensive care arm.
Combining the tabulated 8.0% end of EDIC value with the EAG estimates of a 6-month DCCT of 6.88% suggests an annual worsening over the 26.5 years (ignoring the intervening training period) of 0.042%, which is aligned with the iQVIA CDM value of 0.045%.
It should be noted that both the DCCT and the EDIC are relatively old and of questionable relevance to the current appraisal. The DCCT control arm is obsolete. There was a slight upwards trend among the intensive care arm during the DCCT, but this may have reflected ‘trial fatigue’, or the incidence of hypos, or in the early years concern about retinopathy and ‘glycaemic re-entry’. Follow-up in the DCCT intensive care arm was intensive, with frequent visits. This intensity of follow-up was not carried through to EDIC, which could account for any general worsening during EDIC rather than this being due to any underlying disease progression. It can also be noted that when the DCCT control group moved to EDIC and transferred to the intensified insulin regime they saw an initial fall in their HbA1c but no general upwards trend thereafter.
Turning to the UK National Diabetes Audit 2019–20, the median HbA1c by age among those with T1DM is shown below.
While this does not follow individual patients over time, there is no obvious worsening of the median HbA1c with age. HbA1c appears to become better controlled in early adulthood. This is mirrored in Acharya et al.,132 who in a cross-sectional study of 255 young Scottish participants with T1DM found that those in the youngest age group had statistically significantly higher mean HbA1c than those in the eldest age group, with means of 9.9% for those aged 15–18 years, 9.4% for those aged 18–22 years and 8.8% for those aged 22–25 years. Turning back to the National Audit data, HbA1c remains reasonably constant throughout middle age, possibly showing a slight further improvement above the age of 60 years, although this might be the result of survivor bias, with it not rising above the values of middle age until patients are in their eighties.
In the light of the above, for the base case the EAG will assume no annual worsening of HbA1c over time as would be expected in a disease where beta cell capacity is mostly lost by diagnosis. A scenario analyses of an annual worsening of 0.045% will be presented, in part to aid comparison with other modelling efforts.
Modelling of other clinical effects: non-severe hypoglycaemic events and severe hypoglycaemic events
There is some lack of clarity around the iQVIA CDM implementation of the quality-of-life decrements for NSHEs, as reviewed in greater detail in Health valuation. Coupled with a wish to simplify the implementation of scenario analyses, the EAG uses the iQVIA CDM to model the effects of HbA1c on survival and the micro- and macro-vascular complications of diabetes. The iQVIA CDM overall survival curve for each comparator is then coupled with comparator specific treatment costs and in scenario analyses with the comparator-specific NSHE rate and SHE rate. With the addition of the events’ unit costs and disutilities, this enables technologies’ other effects to be incorporated into the cost-effectiveness analysis.
Note that this assumes that there are no deaths from SHEs, in common with iQVIA CDM defaults and the NG17 model inputs.
Perspective, discount rates and time horizon
As per the NICE methods guide, the perspective for costs is the NHS and Personal Social Services, the perspective for benefits is that of the patient, and costs and benefits are discounted at 3.5%.
The base case assumes a 50-year time horizon, which is effectively a lifetime horizon for all but an insignificant proportion of patients.
Given the uncertainty around the iQVIA CDM outputs for longer time horizons as reviewed in Treatment pathways and modelling above, time horizons of 8, 12 and 24 years will also be explored. Multiples of 4 years correspond to pumps’ lifespans.
Health valuation
Quality of life without complications and disutilities of micro- and macro-vascular complications
The 0.839 values for quality of life without complications for patients with T1DM, based on Peasgood et al.,133 and the disutilities of micro- and macro-vascular complications (Table 12) are taken from the default values of the iQVIA CDM (the iQVIA CMD team stated that the default utilities for complications relate to T2DM patients and that to derive utilities for T1DM patients the T2DM disutilities should be calculated and applied to the T1DM quality-of-life value for no complications). This is in line with NG17.
| Complication | Disutility |
|---|---|
| MI event | −0.055 |
| MI subsequent | −0.055 |
| Angina | −0.090 |
| CHF | −0.108 |
| Stroke event | −0.164 |
| Stroke subsequent | −0.164 |
| PVD | −0.061 |
| Gross proteinuria | −0.048 |
| Haemodialysis | −0.164 |
| Peritoneal dialysis | −0.204 |
| Renal transplant | −0.023 |
| Background diabetic retinopathy | −0.040 |
| Background diabetic retinopathy wrongly treated | −0.040 |
| Proliferative diabetic retinopathy | −0.070 |
| Proliferative diabetic retinopathy lasered | −0.070 |
| Macular oedema | −0.040 |
| Severe vision loss | −0.074 |
| Cataract | −0.016 |
| Neuropathy | −0.084 |
| Ulcer | −0.170 |
| Amputation | −0.280 |
| Post amputation | −0.280 |
Disutilities of hypoglycaemia events
Given previous reviews of the effects of hypoglycaemia on quality of life, the EAG largely relies on NG17 coupled with the systematic reviews of Chatwin et al.,134 Coolen et al.,135 Jensen et al. 136 and Matlock et al. 137 to extract and review papers that may report values compatible with the NICE reference case. The EAG augments this with a systematic literature search from 2020 to find papers that may have been published after previous reviews’ date cut-offs.
The EAG first summarises the papers underlying the iQVIA defaults, appending the review of Gordon et al. 129 to this due to the similarity of their method to that of Currie et al. 22 It then turns to other papers in the literature, these mostly being more recent publications.
If a constant disutility per NSHE is applied, the iQVIA CDM default is 0.00335 per event as drawn from the poorly reported US data of Foos and McEwan. 138 But the preference appears to be for non-linear models and diminishing marginal disutilities, in which case the iQVIA CDM defaults for the effect of NSHEs on quality of life are to choose either the analyses of Lauridsen et al.,18 based on the TTO data of Evans et al.,139 or the analyses of Currie et al. 22
The study by Foos and McEwan138 is only available in abstract with minimal information, other than it being a US-based survey that collected 6-month data about mild, moderate, severe and very severe hypoglycaemia events. No information about how quality of life was calculated or measured is provided, but this coupled with mean event rates within the categories resulted in annual disutility scores of −0.0011, −0.0062, −0.0148 and −0.0586 for mild, moderate, severe and very severe hypoglycaemia events, the weighted average for mild and moderate events of −0.00340 being essentially the same as the −0.00335 iQVIA CDM default if a linear disutility is selected.
Evans et al.,139 sponsored by Novo Nordisk, undertook an internet-based TTO exercise among three samples from the general population, patients with T1DM and patients with T2DM from an existing panel in Canada, the USA, Germany, Sweden and the UK. Evans et al. did not state how many of those in the existing general population panel chose not to start the questionnaire, but of the 11,196 who did, 90% completed it, among whom a further 17% were excluded, leaving 8286, or 82%.
The central estimates suggested that respondents were willing to sacrifice 3.8% of their future survival to go from one quarterly daytime NSHE to none, and to sacrifice 4.1% to go from one quarterly nocturnal NSHE, that is sacrifices of around 2 weeks’ survival per year. Similarly, to go from no to one annual SHE respondents were willing to sacrifice around 10% of future survival, around 5 weeks per year. The decrements for going from some to no events seem quite high and may not be reasonable. If so, this also carries through to the functions of Lauridsen et al. 18
Evans et al. report mean decrements per event among the T1DM subgroup of 0.004 for a daytime NSHE, 0.008 for a nocturnal NSHE, 0.047 for a daytime SHE and 0.051 for a nocturnal SHE, the values for severe events being slightly less than those reported for the general population of 0.057 and 0.062. (Evans et al. imply that their TTO study does not take discounting into account. Given T1DM respondents’ mean age of 39 years they might reasonably expect to live for at least another 30 years. Time preferences among respondents of the NICE reference case discount rate of 3.5% would reduce, for example, the disutility for one annual SHE from 0.082 to 0.049, a 40% reduction. But it can be noted that Dolan et al. 9 in a study of 39 members of the general public estimated individual discount rates scattered around 0%, and it appears standard in TTO to not estimate individuals’ time preferences alongside their quality-of-life estimates.) The EAG assumes that these are disutilities per annual event and includes the step going from no to some NSHEs.
Lauridsen et al.,18 sponsored by Novo Nordisk, used the TTO values for NSHEs of Evans et al. 139 to estimate the quality-of-life impact of NSHEs, recognising the apparent diminishing marginal disutilities as graphed in Figure 13. The non-linearity appears to be mainly driven by the step going from no to some NSHEs. A two-stage estimation procedure that modelled this step separately from subsequent increases in the NSHE rate might result in a smaller and more linear effect for the subsequent increases after the initial step.
FIGURE 13.
Non-severe hypoglycaemic event disutilities for the iQVIA CDM defaults and Gordon et al. 129
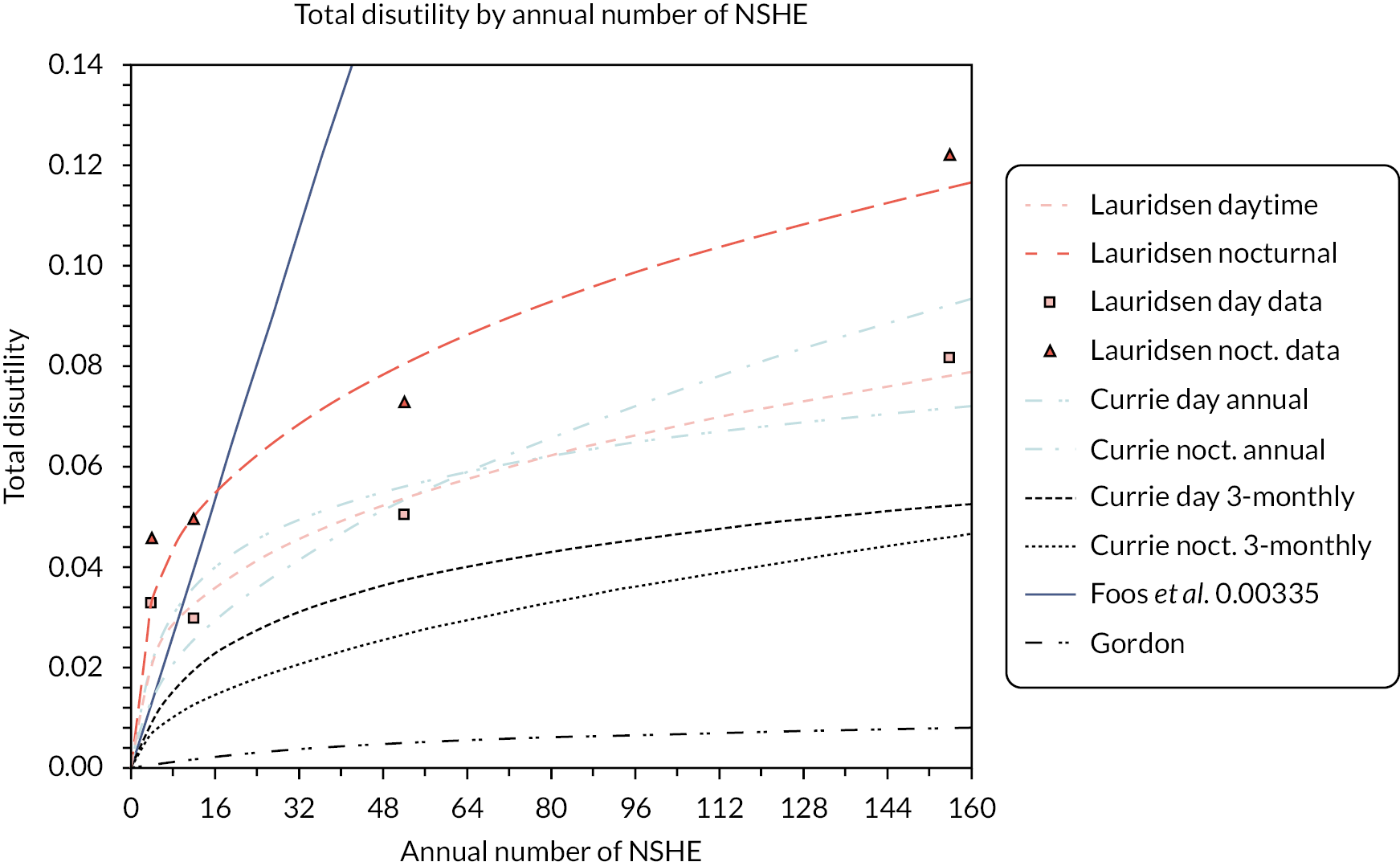
Currie et al.,22 sponsored by Novo Nordisk, used the results of postal questionnaires mailed to UK patients, with an average age 63 years, identified as having either T1DM (34%) or T2DM (66%), in two surveys of 1500 and 3200 people, respectively, with some overlap between the surveys. The overall response rate across the two surveys was 31%, which is quite low and may reflect self-selection bias; those responding might have tended to have been those whose NSHEs and SHEs had a greater impact on their quality of life.
The authors collected data on patient characteristics, comorbidities, the number of NSHEs and the presence of SHEs during a 3-month recall period, the HFS version 1 worry subscale (HFS1-ws) and the EQ-5D. For patients who responded to both surveys their second response was chosen. The effect of this choice was not explored, but it can be noted that the mean HFS score for the first survey of 6.76 was somewhat lower than the 9.39 of the second survey.
Reported rates of SHEs among those experiencing them, 10.3% of T1DM patients, 8.3% of T2DM patients in insulin and 1.8% of T2DM patients taking oral antidiabetic drugs, were quite high (Table 3 is poorly labelled but states the total number of patients, the proportion of patients experiencing SHEs and an annualised SHE rate. For it to be possible for the annualised rate to apply only to those experiencing a SHE during the 3-month recall period the minimum possible annualised rate would be four. Table 4 gives annualised rates of 1.47, 1.86 and 0.14. The EAG concludes that these annualised rates must be across the entire patient number and not the subgroup who experienced SHEs): annualised rates of 14.3, 22.3 and 7.6, respectively, yielding an overall sample mean of 14.9 among those experiencing SHEs. This contrasts with annual rates from the UK hypoglycaemia study group among those experiencing SHEs of 5.1 and 6.9 for T1DM patients of < 5 years and > 15 years duration, and 1.5, 1.4 and 2.8 for T2DM patients on oral antidiabetic drugs, insulin for < 2 years and insulin for > 5 years.
Among the 84.7%, 78.0% and 49.5% of patients reporting symptomatic NSHEs, the corresponding annual rates are 44.4, 31.2, and 48.7, with an average of 45.5. Nocturnal NSHEs were reported by fewer patients, 30.1%, 25.6% and 4.2%, respectively, these patients reporting annual event rates of 21.3, 17.7 and 30.6, yielding an overall average of 21.7. While only a relatively small proportion of patients reported SHEs, their average number of SHEs may be a concern, particularly when interpreting their estimated effect on the HFS1-ws as a result of this being the presence or absence of SHEs rather than the number of SHEs.
In a two-stage analysis, the HFS1-ws was modelled as a function of age, insulin use, the logarithm of the number of NSHEs and the presence or absence of SHEs. Two separate HFS1-ws regressions were undertaken, one for symptomatic NSHEs and one for nocturnal NSHEs. Unfortunately, Currie et al. 22 were not explicit about the time period that should be used when calculating the number of NSHEs, but it can be noted that the presence or absence of SHEs can only have been calculated based on the 3-month recall period of the questionnaires. [The EAG contacted Currie as the corresponding author about this but did not receive a reply. It appears that the iQVIA CDM may input an annual rate of NSHEs to the HFS1-ws function(s) of Currie et al. when calculating their effect. The EAG contacted the iQVIA about this but did not receive a reply. Partly because of the uncertainty about its implementation in the iQVIA CDM, the EAG estimates the effects of NSHEs separately from the modelling that uses the iQVIA CDM through application of the modelled overall survival curve to event rates, disutilities and costs. The EAG adopts a parallel approach for estimating the treatment costs and the costs and quality of life effects of NSHEs and SHEs.] The EQ-5D was modelled as a function of the HFS1-ws, age, body mass index and the presence or absence of a range of comorbidities.
Currie et al. 22 report disutilities for symptomatic and nocturnal NSHEs of 0.0142 (1.42%) and 0.0084 (0.84%), implicitly suggesting that these are additive. Given the regression analyses and probability of positive covariance between symptomatic and nocturnal NSHEs, the EAG thinks that only one of the HFS1-ws regressions should be applied, this also avoiding double-counting the effects of SHEs. The stated disutility values also apply only when patients are moving from experiencing no NSHEs to experiencing a small number of NSHEs. The functions are non-linear and have a quite rapidly declining marginal disutility for NSHEs.
The more recent paper by Gordon et al.,129 sponsored by AstraZeneca, very closely mirrors the analysis of Currie et al.,22 both being co-authored by McEwan. As with Currie et al.,22 Gordon et al. used the EQ-5D and did not specify that the UK social tariff was used, although this seems likely.
Gordon et al. 129 were explicit about the time period that should be used when calculating the NSHE event rate and the presence or absence of SHE events within their functions: a common 4-week period for both. In the light of the common co-authorship and similarity of analyses of Gordon et al. and Currie et al.,22 the EAG thinks that the most reasonable assumption about the time period that should be used when calculating the NSHE event rate and the presence or absence of SHE events for the functions of Currie et al. 22 is a common 3-month period in line with the recall period of the questionnaires. (Currie et al. 22 noted that the more numerous second questionnaire recall period was 3 months. The EAG assumes that this also applies to the first questionnaire.)
Turning to other papers in the literature, Yfantopoulos et al. 140 recruited 938 adult subjects with T2DM who were receiving insulin and had an average age of 67 years, these being split into an estimation sample of 489 and a validation sample of 449. EQ-5D data were valued using the UK social tariff. Within a multivariate analysis the presence of severe hypoglycaemia was estimated to reduce the EQ-5D by a disutility of −0.050, this being statistically significant. Unfortunately, the period over which SHEs were recorded is not reported.
Zhang et al. 141 analysed the records of 7081 Chinese patients with T2DM receiving oral agents who had an average age of 60 years. EQ-5D data were collected and valued using a Chinese tariff. Unfortunately, the paper does not report the data period or recall period for the hypoglycaemia event rates. An ordinary least squares regression that controlled for various patient characteristics and comorbidities estimated that an ‘additional’ NSHE relative to none had a disutility of −0.007 while SHEs has a disutility of −0.008, both being statistically significant. The similarity of disutilities for NSHEs and SHEs suggests that they relate to the presence or absence of events, rather than a disutility per event.
Nauck et al.,142 sponsored by Novo Nordisk, analysed the LEADER cardiovascular outcomes trial among patients with T2DM who had a high risk of CVD and were randomised to liraglutide (n = 4668) or placebo (n = 4672). The trial followed patients for 3.5–5.0 years and collected the EQ-5D at baseline, 12 months, 24 months and study completion, which was valued using the UK social tariff. A linear mixed repeated measurements model estimated that severe hypoglycaemia had a disutility of −0.029 but that this did not quite reach statistical significance, with a p-value of 0.073, due to the small number of events. The text does not specify whether this related to any severe hypoglycaemia events during follow-up or was, for example, an annualised event rate, but it appears to be the former.
Levy et al.,20 sponsored by Novo Nordisk, elicited quality-of-life values using the TTO for quarterly, monthly and weekly NSHEs from 51 Canadians with diabetes, and from 79 and 75 members of the Canadian and UK general populations. For those with diabetes, the central TTO values reported for annualised NSHE rates of 0, 4, 12 and 52 were 0.92, 0.91, 0.87 and 0.75, which suggest a more linear relationship than the TTO values of Evans et al. An ordinary least squares regression estimated that the number of NSHEs had a coefficient of −0.0033 while with a Flogit analysis the coefficient was −0.0247, both of which were statistically significant. They conclude that a NSHE is associated with a −0.0033 disutility for those with diabetes compared with an estimate of −0.0032 for the general public, these estimates being aligned with the −0.00335 that the iQVIA CDM estimates from Foos and McEwan.
Briggs et al.,143 sponsored by BMS, analysed the 2-year data from the SAVOR-TIMI 53 trial of saxagliptin against placebo among 16,488 patients with T2DM. Patients were followed for 2 years, with the EQ-5D being collected alongside event rates and valued using the UK social tariff. This was focused on the impact of cardiovascular events but also included a dichotomous variable for whether the patient had a history of on-trial hypoglycaemic events, which the EAG assumes were SHEs. This estimated a decrement of −0.027 with a p-value of 0.157, this being similar to the −0.029 estimate of Nauck et al.
Pratipanawatr et al.,144 sponsored by MSD, analysed EQ-5D data valued using the UK social tariff from a Thai cross-sectional study of sulfonylurea compared with sulfonylurea with metformin among 659 patients with T2DM. Data on hypoglycaemia events were collected using 6-month recall data with patients being classified by their most severe hypoglycaemia event – none, mild, moderate and severe – with 202 (31%) patients having experienced some hypoglycaemia during the preceding 6 months. A multivariate regression that controlled for age, sex, vascular complication, treatment, weight, medication adherence, worry about hypoglycaemia, worry about weight gain and overall satisfaction found that the presence of hypoglycaemia during the preceding 6 months was statistically significantly associated with reduction in quality of life: a worst experienced hypoglycaemia event of mild, moderate or severe reduced quality of life by 0.156, 0.096 or 0.198, respectively.
Peasgood et al. 133 analysed data from 2469 UK patients with T1DM taking part in a DAFNE course who were followed up for 2 years. Quality-of-life data were collected using the EQ-5D, the SF-36 and the EQ-5D VAS. They imply that the EQ-5D was valued using the UK social tariff with a baseline average of 0.839 among a patient group with an average age of 39 years and a duration of diabetes of 16 years. Questionnaires were administered at baseline, 1 year and 2 years, with follow-up rates of 58% and 24%, respectively, with the mean EQ-5D remaining reasonably constant at 0.851 and 0.840, respectively.
Peasgood et al. report the distribution of the number of SHEs during the preceding year (Table 13).
| Baseline (%) | Year 1 (%) | Year 2 (%) | |
|---|---|---|---|
| 0 | 78.4 | 89.9 | 90.5 |
| 1 | 9.4 | 5.0 | 5.4 |
| 2 | 4.4 | 2.0 | 1.8 |
| 3 | 2.2 | 1.0 | 1.0 |
| 4 | 1.4 | 0.7 | 0.8 |
| 5 + | 4.2 | 1.4 | 0.6 |
| Distribution of the annual number of SHEs among those experiencing | |||
| 1 | 43.5 | 49.5 | 56.5 |
| 2 | 20.4 | 19.8 | 18.7 |
| 3 | 10.2 | 9.9 | 10.4 |
| 4 | 6.5 | 6.9 | 8.3 |
| 5 + | 19.4 | 13.9 | 6.3 |
Although an underestimate, if those experiencing five or more SHEs are assumed to have experienced five SHEs, the above suggests annual event rates per patient of 0.51, 0.22 and 0.18 for baseline, year 1 and year 2. It can also be noted that in year 1 and year 2 the proportion reporting SHEs is reasonably similar to the 10.3% 3-monthly proportion reported in Currie et al. 22
Around half of those experiencing SHEs only experienced one during the preceding year. The vast majority, over 80% at all time points, experienced at most four per year. If it is assumed that those experiencing five or more experienced only five SHEs, among those having had a SHE during the preceding year these correspond to annual rates of 2.38, 2.16 and 1.90 at baseline, year 1 and year 2, respectively. These contrast with the EAG inferred annual rate among the T1DM patients who experienced a SHE of 14.3 in Currie et al. 22
Peasgood et al. undertook linear modelling of the EQ-5D that controlled for a large number of the complications of diabetes. This estimated a −0.0020 fixed-effects coefficient and a −0.0022 random-effects coefficient for the number of SHEs in the preceding year, although only the random-effects coefficient was statistically significant. There may be the possibility of confounding variables or multicollinearity with HbA1c having a statistically significant negative coefficient and the Hospital Anxiety and Depression Scale depression score also having a statistically significant coefficient. These might artificially reduce the estimated effect of SHEs on quality of life.
For the disutility of NSHEs, Gordon et al. and Currie et al. 22 are the papers that provide estimates that conform most closely to the NICE reference case. The key differences between Gordon et al. and Currie et al. 22 are:
-
Gordon et al. was specific to T1DM patients receiving insulin, while Currie et al.’s22 study had a majority of T2DM patients.
-
Gordon et al. used data from the RCT of dapagliflozin against placebo within which the trial data definitions, interpretation and collection seem likely to have been more stringently defined and consistently applied than within the postal recall questionnaires of Currie et al. 22
-
The response rate of Gordon et al. was high, at around 80% of the baseline population, and more relevantly at around 90% of those remaining in the trial at the 52-week data analysis point, compared with only 31% for Currie et al. 22
This leads the EAG to prefer the estimates of Gordon et al. over those of Currie et al. 22 The EAG provides a scenario analyse of the estimates of Currie et al. 22 assuming that the NSHE rate should be 3-monthly and that the 69% non-responders had the same preferences as the 31% responders.
For the disutility of SHEs, most papers provide estimates for the presence of SHEs rather than the disutility per annual SHE. If annual SHE rates are of the order reported in Currie et al. 22 then this is problematic. But if annual SHE rates are more in line with those reported in Peasgood et al. this may be less problematic. Subsequent to DAFNE, over half of those reporting SHEs had only one SHE during the preceding year. In this situation any treatment effects on SHE event rates are more likely to determine their presence or absence, that is going from one to no or from no to one SHE.
The EAG adopts the estimates of Gordon et al. for SHE disutilities and applies these to the SHE event rate. For relatively rare events such as SHEs, the short DEPICT-2 4-week window of Gordon et al. may be a concern. The EAG supplies a scenario analysis that applies the coefficient of Nauck et al.
Hypoglycaemia events and carer disutilities
Parents are affected by their children having hypoglycaemia events and are fearful of these events occurring. Friends and relatives caring for people with T1DM may be similarly affected. The EAG has not identified any research that quantifies these disutilities.
A reasonable upper limit for the effect on carers might be to assume that they have the same disutility as the patient with T1DM for whom they are caring.
The EAG will provide a scenario analysis that simply doubles the disutilities associated with hypoglycaemia events, that is that relates to the subset of patients being cared for and that assumes carers experience the same disutility as the patient.
Costs
Training costs
The Diabetes Technology Network has provided estimates of the number of outpatient visits and the nursing time required to move from MDI + CGM to CSII + CGM and from MDI + CGM to HCL. There is no difference between these estimates; that is, going onto a pump using CSII + CGM involves much the same visits and staff time as going onto a pump using HCL. As a consequence, the EAG base case ignores training costs.
This does not cover the situation of moving from CSII + CGM to HCL, with most patients moving from isCGM to rtCGM and with some further training required for changing to HCL pump use. The Diabetes Technology Network indicates that pre-fitment, fitment and additional post-fitment visits would total three consultant-led outpatient visits, three nurse-led outpatient visits, and three nurse follow-up calls or e-mails plus an additional nurse hour for a fitment visit. Costing these at £208 and £144 of the Diabetic Medicine WF01A NHS 2020–1 NHS Schedule of Costs and £51 per hour for band 5 nursing time spent on patient activities from the 2021 Personal Social Services Research Unit Unit Costs of Health and Social Care, with an assumption of an average 10 minutes per telephone call or e-mail, results in an additional cost of £1132.
Treatment costs
To cost the technologies the EAG uses current list prices supplied by the NHS Supply Chain. Although the costs of HCL pumps and consumables differ slightly between systems, the total 4-year costs are similar, with the exception of one system that is around an annual average of £500 more than the unweighted average. This also applies to the LGS/PLGS systems. The EAG applies the unweighted averages for year 1 and years 2, 3 and 4 and provides a scenario analysis that increases these by £500 for both HCL and LGS/PLGS.
In response to EAG clarification questions, Dexcom provided data suggesting that the average G6 sensor duration was slightly less than the maximum 10 days, with around 87% lasting for 10 days and a mean duration of 9.5 days or 95% of maximum duration. (Confidential information has been removed.) This is reasonably aligned with the 95% mean of Dexcom. The EAG inflates the cost of all CGM sensors by 5% to account for this.
The EAG assumes that only 10% of Dexcom users require a dedicated receiver due to the near ubiquity of smartphones.
The EAG adds an additional annual average insulin cost of £315 to all regimes, based on a daily average of 50IU.
It should also be noted that because the clinical effectiveness estimates for CSII + CGM are not differentiated by CGM type, CSII + CGM is treated as a pooled comparator of 90% CSII + isCGM and 10% CSII + rtCGM. Costs for CSII + isCGM are somewhat lower than those for CSII + rtCGM: annual averages of £5620 and £4024, respectively.
Companies have indicated that prices will change for the next financial year, and some products have confidential volume discounts. The EAG addresses these aspects in the confidential patient access scheme appendix submitted to NICE.
Ongoing visits and the costs of micro- and macro-vascular complications
It is assumed that without complications that, once established on treatment, the average patient is seen in outpatient clinic once per quarter. This is costed at the NHS reference cost for a consultant-led, non-admitted, face-to-face follow-up appointment for diabetic medicine. This cost is reasonably different for 2019–20, at £154, compared with 2020–1, at £208. The proportion of follow-up visits that were not face to face also differed, at 9.6% compared with 49.6%. It seems reasonable to assume that the 2020–1 costs were in part driven by the COVID-19 pandemic, during which only the more serious cases would have been seen in clinic. For this reason the EAG will apply the 2019–20 price of £154, uprated by the NHS pay and prices index by 3.08% to £160 in 2020–1 prices, resulting in an annual routine outpatient cost of £640.
The costs of other routine management for, for example, angiotensin-converting enzyme inhibitors and the proportion in receipt of these and the costs of micro- and macro-vascular complications are taken from NG17, inflated to 2019–20 prices. All patients are assumed to receive screening. Table 14 provides the costs of ongoing management and micro- and macro-vascular complications.
| Complication | Cost (£) | In receipt | |
|---|---|---|---|
| Primary prevention (%) | Secondary prevention (%) | ||
| Statins | 28.42 | 47 | 84 |
| Aspirin | 16.96 | 59 | 88 |
| ACE-I/ARB | 23.71 | 21 | 76 |
| Stopping ACE-I/ARB due to AEs | 40.72 | ||
| Microalbuminuria screening | 4.41 | ||
| Gross proteinuria screening | 4.41 | ||
| Eye screening | 56.44 | ||
| MI first year | 4231 | ||
| MI subsequent years | 894 | ||
| Angina first year | 7265 | ||
| Angina subsequent years | 327 | ||
| CHF first year | 4077 | ||
| CHF subsequent years | 2945 | ||
| Stroke first year | 4728 | ||
| Stroke subsequent years | 175 | ||
| Stroke death within 30 days | 1332 | ||
| PVD first year | 1380 | ||
| PVD subsequent years | 600 | ||
| Haemodialysis first year | 34,855 | ||
| Peritoneal dialysis | 31,357 | ||
| Renal transplant (first year) | 21,810 | ||
| Renal transplant (second year) | 8649 | ||
| Laser treatment | 151 | ||
| Cataract operation | 962 | ||
| Following cataract operation | 211 | ||
| Blindness first year | 7858 | ||
| Blindness subsequent years | 7592 | ||
| Neuropathy first year | 39 | ||
| Neuropathy subsequent years | 39 | ||
| Active ulcer | 3654 | ||
| Amputation event | 8761 | ||
| Post amputation | 26,653 | ||
Non-severe hypoglycaemic event costs
It is assumed that there are no costs to the NHS or Personal Social Services from NSHEs.
Severe hypoglycaemic event costs
A number of previous NICE assessments have applied the resource use estimates of Leese et al. 126 to estimate the cost per SHE that requires medical attention. Leese et al. identified 244 hypoglycaemia events requiring medical attention in Tayside during the year from June 1997, the balance between these being roughly equally split between T1DM and T2DM (even rates of 11% for T1DM and 1.7% for T2DM patients were balanced out by the larger number of T2DM patients). These were estimated to cost £141,120 when uprated from 2002 to 2021 prices, equivalent to an average of £578 per event requiring outside medical assistance.
NG17 used Heller et al. 121 to cost severe SHEs separately for those with T1DM, those with T2DM on insulin and those with T2DM on oral antidiabetic drugs. They analysed 15 trials, the mean ages being around 42 years for T1DM, 58 years for T2DM on insulin and 57 years for T2DM on oral antidiabetic drugs. The trials yielded 536 severe glycaemia events for analysis, the proportion of T1DM patients with severe hypoglycaemia being around 11% for the two 26-week trials, and 12% and 15% for the two 52-week trials. The majority of events (78%, n = 420) occurred among the T1DM patients. The use of medical services for T1DM patients was slightly lower, at 37.9% of events, than the 47.4% of T2DM patients but given that most SHEs were among T1DM patients this was little different from the overall average of 39.9%. Across all events 29.3% required an ambulance or emergency room team, 11.9% led to hospital or emergency room assistance and 6.7% required hospital admission for at least 24 hours, these averages being only slightly different for T1DM patients at 31.0%, 9.5% and 5.0%, respectively.
NG17 also cited Hammer et al. 2009, sponsored by Novo Nordisk, who used resource use questionnaire data from 201 UK T1DM and T2DM patients, all of whom were using insulin and had experienced at least one SHE in the last year. The mean direct costs per SHE, inflated to 2021 prices using the HCHS to 2015 and the NHSCII thereafter, were estimated as £36 for those not requiring external medical assistance, these costs being mostly due to follow-up contacts, £327 for those requiring medical treatment in the community and £1113 for those requiring hospital treatment. The weighted average of these was £374 which is aligned with the £370 of NG17.
Applying the weights of Heller et al. for T1DM patients results in a lower cost of £260, which is the weighted mean of £36 for those with no outside medical assistance and £628 for those requiring outside medical assistance. It is uncertain how accurately subsequent follow-up contacts and visits can be ascribed exclusively to preceding SHEs given that these patients will be receiving ongoing care. Excluding these costs and using the T1DM weights of Heller et al. for T1DM patients results in a lower average cost of £206, which is the weighted mean of £1.83 for those with no outside medical assistance and £542 for those requiring outside medical assistance. The cost of between £542 and £628 for events requiring outside medical assistance is quite well aligned with the £578 cost of Leese et al., although it should be borne in mind that the latter is a roughly equal mix between events among T1DM patients and T2DM patients.
In the light of the above, for its base case the EAG will apply a cost of £1.83 for SHEs not requiring outside medical attention and of £542 for those requiring medical attention, with it being assumed that 37.9% of SHEs require medical attention. A scenario analysis that applies £36 for SHEs not requiring outside medical attention and of £628 for those requiring medical attention will be supplied. A scenario that costs all SHEs at the 2021 updated £381 of NG17 will also be supplied, somewhat higher than the base-case average of £207 despite the same sources being cited.
Evidence Assessment Group cost-effectiveness modelling results
Evidence Assessment Group base case
The base-case modelling provides the following disaggregate estimates presented in Table 15.
| CSII | PLGS | HCL | |||
|---|---|---|---|---|---|
| Value | Net vs. CSII | Value | Net vs. CSII | ||
| Life-Years undiscounted | 32.499 | 32.685 | 0.186 | 32.957 | 0.458 |
| QALYs | |||||
| iQVIA CDM modelled | 14.232 | 14.291 | 0.059 | 14.392 | 0.160 |
| NSHEs | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| SHEs | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Total QALYs | 14.232 | 14.291 | 0.059 | 14.392 | 0.160 |
| Costs | |||||
| Treatment | £86,564 | £105,258 | £18,694 | £117,749 | £31,185 |
| Routine outpatient | £12,182 | £12,222 | £40 | £12,279 | £97 |
| SHEs | £0 | £0 | £0 | £0 | £0 |
| Other management | £1700 | £1708 | £8 | £1721 | £21 |
| CVD | £4691 | £4649 | −£42 | £4531 | −£160 |
| Renal | £10,365 | £10,367 | £3 | £9943 | −£421 |
| Ulcer/amp./neuropathy | £889 | £898 | £9 | £880 | −£9 |
| Eye | £18,270 | £17,604 | −£666 | £16,185 | −£2085 |
| Total costs | £134,661 | £152,706 | £18,045 | £163,289 | £28,628 |
Undiscounted survival is estimated to increase by 0.458 years using HCL compared with CSII + CGM. But in part due to discounting, which reduces the net survival gain to 0.149, the patient gain is only 0.160 QALYs. The net treatment cost of £31,185 is partly offset by renal savings of £421 and eye savings of £3085, resulting in a net cost of £28,628. This results in the cost-effectiveness estimates presented in Table 16.
| CSII | PLGS | HCL | |
|---|---|---|---|
| Life-Years undiscounted | 32.499 | 32.685 | 32.957 |
| Total QALYs | 14.232 | 14.291 | 14.392 |
| Total costs | £134,661 | £152,706 | £163,289 |
| ICER vs. CSII | – | £305,852 | £178,925 |
The results suggest that PLGS is extendedly dominated by HCL, but that HCL has a poor cost-effectiveness estimate of £179,000 per QALY.
The iQVIA CDM does not permit periodic capital costs to be modelled, so for the deterministic modelling the EAG uses the modelled overall survival curves to estimate treatment costs. This approach cannot be adapted to the probabilistic modelling, so the EAG approximates these costs within the iQVIA CDM by applying the 4-yearly annual average costs for CSII + CGM and HCL respectively, the iQVIA CDM only permitting pairwise comparisons. This results in a central cost-effectiveness estimate of £186,000 per QALY for HCL compared with CSII + CGM, which is similar to the deterministic estimate, and probabilities of HCL being cost-effective at thresholds of £20,000, £30,000, £50,000 and £100,000 per QALY of 21%, 31%, 39% and 47%, respectively (Figure 14).
FIGURE 14.
Base-case CEAC.
Evidence Assessment Group scenario analyses
The EAG presents the following scenario analyses:
-
SA01: revising the NMA to65 (a) be restricted to only adult studies and (b) exclude Banhamou et al. 65
-
SA02: application of the NHS adult pilot – (a) patients’ baseline characteristics and (b) patients’ baseline characteristics and HbA1c change of −1.5% for HCL with an assumption of no change for CSII + CGM and (c) SA02b with the costs of complications reduced by their possible overestimation as identified in McEwan et al. 117
-
SA03: time horizons of 8, 12 and 24 years.
-
SA04: durations of HbA1c effect of 5, 10 and 20 years.
-
SA05: inclusion of NSHEs, based on an HCL annual rate of (a) 20.8, (b) 57.2 and (c) 13.0 with comparator rates based on the ratio of time < 3 mmol/l, valued using Gordon et al. 129
-
SA06: inclusion of NSHEs as per SA05a and SHEs, valued using Gordon et al. 129
-
SA07: inclusion of NSHEs as per SA05a valued using Currie et al. 22 and SHEs valued using (a) Currie et al. 22 and (b) Nauck et al. 142
-
SA08: SA06 with SHEs costed at (a) £36 for no medical attention and £628 for medical attention and (b) £381 for all SHEs.
-
SA09: SA06 with a doubling of the NSHE and SHE quality-of-life effects to reflect possible carer effects.
-
SA10: CSII is (a) 85% isCGM and 15% rtCGM and (b) 95% isCGM and 5% rtCGM.
-
SA11: HCL and PLGS average annual cost being £500 higher.
-
SA12: additional £1132 training cost for transferring from CSII + CGM to either PLGS or HCL. (The EAG did not ask the Diabetes Technology Network about transferring from CSII + CGM to PLGS. But as the main issue identified in transferring to HCL was the move from isCGM to rtCGM, the EAG assumes that the same costs will be incurred transferring to PLGS.)
-
SA13: revising non-specific mortality to (a) all-cause mortality and (b) non-specific mortality that also excludes all deaths associated with hypertension.
-
SA14: annual 0.045% HbA1c worsening.
Within these results PLGS is extendedly dominated throughout, and for reasons of space the EAG does not consider it further. EAG scenario analyses are presented in Appendix 12, Table 27.
Chapter 8 Discussion
Summary of key results
The aim of the RCTs was generally to demonstrate improvement of glycaemic management with use of HCL. We identified one study, by Stewart et al., of pregnant women, which included only 16 participants followed for 4 weeks; the population, study design and outcomes in this study were clearly different from those in other studies so that transitivity in NMA that include Stewart et al. is threatened. This was addressed by conducting a sensitivity analysis (see Subgroup and sensitivity analyses).
There were relatively few studies, and they were small, encompassing a total of ≈ 450 HCL recipients followed for between 4 and 26 weeks, accumulating approximately 110 person-years of observation. Inclusion criteria applied to the studies were relatively narrow and most participants had reasonably good glycaemic management at entry, as indicated in most of those studies reporting baseline TIR (3.9–10 mmol/l) at > 50% (range 47–62%) and baseline HbA1c at between 7% and 8%. There was considerable heterogeneity across studies regarding the age of participants, and some studies presented results stratified by age groups. The relevance of the RCT populations and outcome measure results to the decision problem is debatable and not easy to judge. The quality of studies assessed according to Cochrane criteria was associated with either low risk of bias or some concern.
In the HCL arm of RCTs, the interventions achieved a statistically significant improvement in HbA1c% that decreased MD (0.28, 95% CI −0.34 to −0.21), significantly increased % TIR (between 3.9 and 10.0 mmol/l) (MD 8.6, 95% CI 7.03 to 10.22), and in hyperglycaemic levels significantly decreased TIR (% > 10.0 mmol/l) (MD −7.2, 95% CI −8.89 to −5.51). Control arms also showed improvement, but this was smaller than that seen with HCL. Irrespective of the type of intervention used in the control arms, these outcomes were statistically superior in the HCL arm versus the control arm. Available evidence from the RCTs suggests that these gains in glycaemic management reported for HCL were not accompanied by a greater risk of hypoglycaemia; however, the power to detect small event sizes was limited because of small study groups and relatively short treatment duration. Adverse events were reported in some studies and were mainly low. Patient-reported outcomes were assessed using various methods and did not result in clear trends.
The estimated cost-effectiveness of PLGS compared with CSII + CGM is consistently worse than that of HCL compared with CSII + CGM, for both the base case and the scenario analyses. PLGS is extendedly dominated by HCL and the EAG does not consider it further.
Given the NMA estimated effect on HbA1c of −0.29% for HCL compared with CSII + CGM, the cost-effectiveness of HCL is poor. Net treatment costs are estimated to be £31,185; cost offsets from fewer complications and in particular −£2085 from reduced eye complications, probably mostly severe visual loss, and −£421 from reduced renal complications, probably mostly ESRD, reduce the net total cost to £28,628. The net undiscounted survival gain is 0.458 years, this contributing to a patient gain of 0.160 QALYs. This results in a base-case deterministic cost-effectiveness estimate of £179,000 per QALY, a probabilistic central estimate of £186 per QALY and probabilities of HCL being cost-effective at £20,000 per QALY and £30,000 per QALY thresholds of 21% and 31%, respectively.
The NHS adult pilot baseline patient characteristics result in a reasonable improvement to £126,000 per QALY. Assuming that the pilot’s 1.5% improvement in HbA1c is the net effect for HCL over CSII + CGM results in net treatment costs of £35,912. Cost offsets from reduced eye complications of −£16,442 and from reduced renal complications of −£6731 help reduce the net total cost to £12,447. The net undiscounted survival gain increases to 3.1 years, contributing to the increased patient gain of 1.004 QALYs. The resulting cost-effectiveness estimate of £12,398 per QALY is an order of magnitude better than the EAG base case. The EAG review of the published model validation work highlights that incidences of renal and eye complications may be overestimated. Adjusting the costs of these roughly doubles the NHS pilot scenario cost-effectiveness estimate to £21,583 per QALY. Note that this does not take into account any possible effects on quality of life or life expectancy.
The EAG review of the published model validation work also highlights that the modelling of longer-term effects is more uncertain. Time horizons of 8, 12 and 24 years worsen the cost-effectiveness estimate to £910,000, £664,000 and £328,000 per QALY, respectively.
The duration of the HbA1c effect is also uncertain. Limiting this to 5, 10 and 20 years while retaining a time horizon of 60 years worsens the cost-effectiveness estimate to £657,000, £425,000 and £247,000 per QALY, respectively.
The EAG base case does not include the effects of symptomatic or severe hypoglycaemia events due to the high uncertainty around annual event rates and the lack of direct evidence that HCL has an effect on these. Incorporating non-severe symptomatic hypoglycaemia event rates, inferred from an annual rate of 20.8 for HCL with an annual rate of 27.1 for CSII + CGM based on the ratio of times < 3.0 mmol/l, improves the cost-effectiveness estimate to £169,000 per QALY. Annual rates of 57.1 and 13.0 for HCL result in cost-effectiveness estimates of £166,000 and £170,000 per QALY. Including severe hypoglycaemia events improves the cost-effectiveness to £163,000 per QALY.
If both non-severe and severe hypoglycaemia events are included and are valued using the same source as NG17, the cost-effectiveness improves £121,000 per QALY, while if severe events are valued using another reasonable source within the literature the cost-effectiveness improves further to £109,000.
Doubling the quality-of-life effect of hypoglycaemia events to reflect possible carer effects improves the cost-effectiveness estimate from £169,000 to £151,000 per QALY. Increasing the costs of severe hypoglycaemia events has relatively little effect on the cost-effectiveness estimate.
As the clinical effectiveness estimates for CSII + CGM are not differentiated by CGM type, CSII + CGM is treated as a pooled comparator of 90% CSII + isCGM and 10% CSII + rtCGM. Costs for CSII + rtCGM are somewhat higher than those for CSII + isCGM: annual averages of £5620 and £4024, respectively. Because of the non-differentiation of clinical effect by CGM type, differentiating by only treatment costs would cause CSII + rtCGM to be extendedly dominated. Reducing the proportion of CSII + CGM that is isCGM from 90% to 85% improves the cost-effectiveness to £169,000 per QALY, while increasing it to 95% worsens it to £188,000 per QALY. Additional annual HCL costs of £500, as may apply to some HCL systems, worsen the cost-effectiveness to £239,000 per QALY, while training costs for crossover from CSII + CGM to HCL of £1132 worsen it to £186,000 per QALY.
The EAG non-specific mortality estimates may be too low if there are competing risks. All-cause mortality is too high but it forms an upper bound. Its application results in a cost-effectiveness estimate of £200,000 per QALY. There may be an argument for removing deaths associated with hypertension from the non-specific mortality. This improves the cost-effectiveness estimate to £167,000 per QALY.
If T1DM is associated with an annual worsening of 0.045% in HbA1c, then this improves the cost-effectiveness estimate by a reasonable amount, to £153,000 per QALY.
The key model inputs are:
-
the net effect on HbA1c
-
the duration of the net effect on HbA1c
-
the model time horizon
-
treatment costs.
Other important model inputs are:
-
hypoglycaemia event rates
-
what source is used to value the disutilities of hypoglycaemia event rates
-
what non-specific mortality is applied
-
whether HbA1c worsens annually among T1DM patients and if so by how much.
The key modelling uncertainties are around:
-
overall survival gains
-
severe visual loss and its effects on survival, quality of life and costs
-
ESRD and its effects on survival, quality of life and costs.
Generalisability of results
The modelled cost-effectiveness of HCL is driven by the change in HbA1c and how long that change persists, the latter depending on modelling assumptions and the baseline patient age. The larger is the HbA1c effect, and the longer it persists, the greater is the difference in the modelled proportions having serious visual loss and ESRD. Assuming an annual worsening of HbA1c compounds this effect. If it is assumed that the HbA1c effect persists for the patient lifetime, the baseline age determines the duration of the HbA1c effect. The EAG base case applies the national diabetes audit mean age of those on pumps, sampling this using the SD.
Exploratory modelling of a paediatric population very broadly mirrors the adult results, but the EAG has reservations about the reliability the iQVIA CDM for modelling a paediatric population. It also raises questions about durations of effects and how the transition from childhood to adulthood may affect these.
The EAG has not considered the cost-effectiveness of HCL for pregnant women due to the lack of evidence. It notes the relationship between HbA1c and birth defects. If HCL reduces HbA1c in pregnant women to the same extent as in the adult population the short-term additional costs of HCL will have some immediate cost offsets from reduced birth defects, with the potential for additional benefits to the child at no additional cost. It also seems likely that the baseline age of pregnant women is below the national diabetes audit mean age, which is likely to further improve cost-effectiveness. If after giving birth women remain on HCL into the long term, the cost-effectiveness estimate of HCL will trend towards that of the adult female T1DM population of the same age but remain superior to it.
Strengths and limitations of analysis
The clinical analysis prioritised randomised controlled evidence that provides superior evidence to that from other study designs. The clinical evidence also provided additional observational evidence to compare with the NHS audit studies. The analysis was conducted following Cochrane Handbook for Systematic Reviews of Interventions. Forest plots and NMA results were presented. Transitivity of the network is threatened because the RCTs were heterogeneous in multiple respects including trial design (parallel-group or crossover design with washout phase between different treatments), participants’ age, number of participants and other demographics, including run-in times, duration of observation periods, and number and types of previous treatments. Studies screened relatively small numbers of patients. The number of participants randomised ranged from < 20 to 135. However, sensitivity and subgroup analysis were performed and provided some reassurance in our findings. The quality of observational studies is generally poor. Nevertheless, the outcome estimates reported for observational studies were quantitatively broadly in line with those from the RCTs. Half of the included studies included UK centres and therefore represent some relevance to UK settings. There was very limited evidence on pregnancy, and the effectiveness of HCL in pregnant women remains unclear.
A strength and a weakness of the analysis is the availability of published iQVIA CDM validation data against long-term observational studies. The validation data relate at least in part to earlier model iterations of the iQVIA CDM than that used by the EAG. The strength is its availability, it often being absent from other NICE assessments. But it highlights some uncertainty about the reliability of the modelling of the incidence of retinopathy, in one validation exercise this having been overestimated by around 30% for the intervention arm of the EDIC trial, and of the incidence of ESRD, this having been overestimated by around 250% for the intervention arm of the EDIC trial. Modelling of survival appears reasonable in the medium term, but the longer-term modelling of survival is subject to more uncertainty.
The net HbA1c effect, its duration and the resulting costs offsets from reduced eye and renal complications determine whether HCL is likely to be estimated to be cost-effective at conventional thresholds. The trials were of relatively short duration, which argues for consideration of shorter effect durations than the maintenance of effect for the patient lifetime as assumed in the base case.
There is an argument for reducing the eye and renal cost offsets proportionately to their possible overestimation within the iQVIA CDM. Uncertainty around the modelled overall survival argues for consideration of shorter time horizons.
The uncertainty around the modelled long-term survival coupled with uncertainty about how much of the clinical data underlying model construction was drawn from a paediatric population causes the EAG to view paediatric modelling using the iQVIA CDM with some caution.
A weakness of the analysis is the lack of data on the effect of HCL on symptomatic and severe hypoglycaemia events. The EAG has inferred these from the ratio of time < 3.0 mmol/l for HCL compared with that of the other comparators, coupled with event rates for HCL. There is considerable uncertainty around these, and the EAG only presents the possible effects of hypoglycaemic events within scenario analyses. It should also be noted that the EAG preferred quality-of-life function for hypoglycaemia events differs from that of NG17 and suggests a somewhat smaller effect.
Conclusions
Randomised controlled trials of HCL interventions in comparison with CSII + CGM or sensor augmented pump therapy achieved a statistically significant improvement in HbA1c%, in TIR between 3.9 and 10 mmol/l, and in hyperglycaemic levels. The outcome estimates reported for observational studies were quantitatively broadly in line with those for the RCTs. Measures of glycaemic performance, such as HbA1c%, % TIR (3.9–10 mmol/l), and % time above range > 10 mmol/l all improved on transfer to HCL.
Well-designed RCTs are needed to explore the effectiveness of HCL systems in larger samples of people, with longer follow-ups, and in pregnant women. Trials that include a wider variety of participants, for example, people with control above target glucose levels, or who live in remote or rural areas, would be helpful. Trials that collect data to support economic modelling of HCL systems, such as on quality of life and adverse events, would be very beneficial. Studies are required to clearly describe comparators and should ideally use real-time GM + CSII or FGM + CSII as the control, as these are the most relevant comparators. There is a lack of evidence on the long-term effect of the HCL system and especially on clinical outcomes, such as CVD. Carer outcomes and patient-reported outcomes are not systematically captured or reported.
Chapter 9 Consultation and additional requests from the National Institute for Health and Care Excellence
Regression analyses
The EAG identified eight studies that reported change in HbA1c for an HCL recipient adult population. The evidence comprised six RCTs27,49,50,52,53,60 and two single-arm studies (Beato–Vibora et al. 61 and the NHS adult pilot study).
In addition, NICE drew our attention to an abstract of the Steno trial145 (appraisal consultation document). Steno was an RCT study reporting a change of 1% from a baseline of 8.3%. NICE requested a regression analysis of effect size versus baseline and taking into consideration the NHS pilot study. The included studies were predominantly conducted in adult populations; however, the age distribution varied considerable between studies, and the age range of the additional abstract population was not disclosed. It should be emphasised that the number of studies is relatively small and that the studies are heterogeneous in design, duration, and age range of patients.
Unweighted regression analyses
Briefly, we performed unweighted regression on the included studies and a range of sensitivity analyses (Figure 15). We were unable to weight the Steno trial because of missing data, and therefore the weighted regressions do not include this study.
FIGURE 15.
Results of unweighted regression. (a) All nine studies; (b) only RCTs (n = 7); (c) RCTs: Boughton et al. and abstract plus NHS pilot; and (d) RCTs minus abstract (n = 6).
The regression slope for all nine studies and for the seven RCTs were similar. The EAG would like to highlight that (1) it was not possible to estimate a variance around the Steno abstract, and (2) two RCTs, Thabit et al. and McAuley et al., yielded almost identical results, differing only in the uncertainty around effect size; these regressions appear reasonably consistent with the result from the NHS pilot study. A much flatter regression was obtained after the exclusion of data from the Steno abstract from the RCT regression. The RCT by Boughton et al. included a greater proportion of elderly patients than the other RCTs. The exclusion of Boughton et al. from the regression of RCTs resulted in a regression slope somewhat steeper than that of all RCTs (or all studies) but consistent with that of the NHS pilot study that exhibited a larger effect size at a higher baseline value. Sensitivity analyses yielded similar regression slopes to the analysis of all nine studies.
-
Sensitivity analysis 1: including RCTs + NHS and excluding Boughton et al.
-
Sensitivity analysis 2: including RCTs + NHS and excluding Boughton et al. and the Steno abstract.
Weighted regression analyses
The weighted regression relates to the inverse of the SE of the effect size.
The analyses are presented in Figure 16, where the vertical axis is the effect size.
FIGURE 16.
Results of weighted regression.
The inclusion of all studies (including the NHS pilot) resulted in a regression line that aligns with the NHS pilot study. The weighted regression of RCT studies indicated a poor alignment with the NHS pilot study and with the Steno abstract (orange data point). The EAG notes the large effect size of Boughton et al., and this may be an outlier.
Regression analyses: baseline glycated haemoglobin per cent versus net change in glycated haemoglobin in hybrid closed-loop randomised controlled trials
Seven RCTs with sufficient reported data were included: five compared HCL with CSII + rtCGM (Benhamou,65 Boughton,47 Tauschmann,52 Ware A55 and Ware B56), and two compared HCL with CSII + CGM [Thabit (adults) and Thabit (children)]. The effect size was defined as the change in HbA1c% in the HCL arm minus that in the comparator arm (net change in HbA1c). The change in HbA1c% was calculated from the HbA1c% at the start of the intervention minus that at the end of the study or treatment period (for crossover trials). All studies reported greater reduction in HbA1c% in the HCL arm than in the comparator arm (net change negative) Table 17. Where baseline HbA1c% differed between arms, a pooled estimate was calculated weighted by the number of participants per arm. The Benhamou et al. RCT had a crossover design, and baseline HbA1c% for each treatment group necessitated an estimation from available but incomplete reported data, resulting in two different estimates. Studies reported precision for each arm to only a single decimal place; consequently, the calculation of the difference between arms had little precision. Linear weighted regression analyses were conducted using the ‘metareg’ command in Stata. Three weightings were explored: (1) according to SD effect size, (2) according to SE effect size and (3) according to sample size. It should be noted that relatively few studies were available and that baseline HbA1c% contributes to both ordinate and abscissa axes of regressions.
| Baseline HbA1c | Change | Change SD | n | Change SE | Study |
|---|---|---|---|---|---|
| 7.69a | −0.15b | 0.107 | 126 | 0.009532 | Benhamou et al. |
| 7.45 | −0.3 | 0.095 | 37 | 0.015618 | Boughton et al. |
| 7.9 | −0.5 | 0.027 | 86 | 0.002911 | Tauschmann et al. |
| 7.35 | −0.3 | 0.039 | 69 | 0.004695 | Ware A |
| 8.25 | −0.4 | 0.026 | 133 | 0.002254 | Ware B |
| 7.6 | −0.3 | 0.056 | 65 | 0.006946 | Thabit et al. (adult) |
| 7.8 | −0.3 | 0.063 | 49 | 0.009 | Thabit et al. (children) |
Regression using all seven studies, and sensitivity analyses omitting specified studies
Data and regression parameters are available upon request for (1) analysis with Benhamou et al. baseline HbA1c at 7.69%, and (2) analysis with Benhamou et al. baseline HbA1c at 7.26%. Few studies were available for this analysis, and those that were available were heterogeneous (e.g. with regard to study design and age distribution of participants). Effect size precision was poor because the values reported by authors were usually rounded to a single decimal place. Baseline HbA1c% in Benhamou et al. 65 was incompletely recorded and necessitated estimation from textual data and published supplementary material; of the two estimates, the 7.69% (based on supplementary data) reasonably aligned with the pooled baseline for all studies and, therefore, on balance, this value is preferred. Because of a paucity of studies and alignment with NMA, the EAG included Benhamou et al. in the analysis. As a consequence of data deficiencies, the CIs around regression parameters were wide, with p-values for one or both parameters exceeding 0.05; an exception was when baseline for Benhamou et al. was set at 7.26% and the study by Tauschmann et al. was omitted. The omission of Tauschmann et al., one of the five studies of HCL versus CSII + rtCGM, did not appear to justify the economic analysis. The regression selected for use in economic analysis was based on using Benhamou et al. baseline at 7.69%, weighting by effect size SD and omitting the Thabit studies. The omission or inclusion of the Thabit studies and the use of (A) or (B) weighting had minimal influence on regression lines.
Hybrid closed loop performance in pregnancy
The EAG identified four studies describing HCL use in pregnancy. The studies included (a) a crossover RCT by Stewart et al. 51 with 16 patients; (b) the AiDAPT trial,146 (confidential information has been removed) that was submitted by the company and (c) an RCT identified as CRISTAL, which at the time of writing is still recruiting patients. 147 These studies were different from the nine studies in relation to gender, age and patient characteristics and therefore were excluded from the regression. HbA1c values were not consistently reported across the studies. For instance, the AiDAPT trial reported mean HbA1c% at baseline (7.6%) but not at endline. The AiDAPT trial reported the percentage of patients who satisfied NICE targets of < 6.5% for HbA1c and therefore these data could not be synthesised.
Stewart et al. reported HbA1c values to a single decimal place. Baseline HbA1c was 6.6% (SD 2.8%), which dropped to 6.4% (SD 2.7%) at endline. The EAG estimated that the change from baseline followed the metan command in Stata by employing the number of participants, effect size, and SD of effect size. We plotted the change in HbA1c of the HCL group in comparison with the regression line of all nine studies discussed earlier. Baseline HbA1c% was lower in Stewart et al. than in other studies, which indicates minimal improvement. The SD for baseline change was relatively large, with only 16 participants in the trial.
Indirect comparison: published data on intermittently scanned continuous glucose monitoring compared with real-time continuous glucose monitoring
Results
The EAG followed a pragmatic approach (following discussions with the NICE technical team) and included studies from NG17 that involved rtCGM in comparison with isCGM. Three additional studies were included to the original NMA that was reported in the EAG report. The EAG did not have access to the full-text publication of the abstract submitted by the company. 145 This abstract was not included in the main indirect comparison (the EAG evaluated the abstract and the results remained similar to those of the main analysis). Briefly, two studies (CORRIDA148 and I 1HART CGM149) reported five outcomes that included HbA1c%, TIR (% between 3.9 and 10 mmol/l), time above range (% above 10 mmol/l), and time below range (% below 3.9 and 3.0 mmol/l). One study (ALERTT1150) reported three outcomes: HbA1c%, TIR (% between 3.9 and 10 mmol/l), and time < 3.0 mmol/l. Studies were heterogeneous in terms of population, age groups, gender, RCT design (parallel crossover), numbers of participants and variable adjustment methods for determining MD between intervention and comparators. Additionally, rtCGM versus isCGM involved participants receiving MDI and/or pump therapy. Studies did not consistently describe comparators. Crossover studies did not provide data at different crossover time points (results are presented in Table 18).
| Reference CSII + CGM | Results, MD (95% CI) | ||||
|---|---|---|---|---|---|
| HbA1c% | TIR (% between 3.9 and 10 mmol/l) | Time above range (% > 10 mmol/l) | Time below range (% < 3.9 mmol/l) | Time below range (% < 3.0 mmol/l) | |
| HCL | −0.26 (−0.41 to −0.10) | 8.38 (6.26 to 10.50) | −7.83 (−11.18 to −4.49) | −0.47 (−3.15 to 2.21) | −0.03 (−0.20 to 0.14) |
| rtCGM | 0.02 (−0.15 to 0.19) | −0.22 (−2.75 to 2.30) | −0.57 (−4.39 to 3.24) | 0.36 (−2.61 to 3.34) | −0.03 (−0.26 to 0.19) |
| isCGM | 0.38 (0.15 to 0.62) | −6.27 (−10.24 to −2.31) | 5.12 (−0.70 to 10.95) | −3.91 (−8.02 to 0.20) | 0.29 (−0.05 to 0.64) |
| SAP/PLGS | 0.34 (−0.46 to 1.15) | −4.12 (−21.13 to 12.90) | 4.27 (−2.90 to 11.43) | −0.07 (−4.63 to 4.49) | 0.07 (−1.33 to 1.47) |
Change in glycated haemoglobin level (%)
Thirteen estimates from 12 studies informed this outcome. HCL demonstrated superiority, and this was statistically significant. The isCGM group did not perform as well as other groups, and this was statistically significant.
Time in target range (% between 3.9 and 10 mmol/l)
There were 15 estimates from 14 studies included in this network. HCL was the only treatment that demonstrated significant superiority to the reference treatment (CSII + CGM).
Time below target range (% below 3.9 and 3.0 mmol/l)
Nine estimates were included in the analysis for both the time < 3.9 mmol/l (9 studies) and the time < 3.0 mmol/l (10 studies) outcomes. For both outcomes, there were no statistically significant differences between the reference treatment of CSII + CGM and the other treatment groups.
Time above target range (% above 10 mmol/l)
There were 14 estimates from 13 studies that were included in this analysis. HCL demonstrated superiority, and that was statistically significant. The other treatment groups did not show a significant difference from the reference group (CSII + CGM).
The costs of stroke
The previous EAG report drew its costs from NG17, uprating these for inflation to yield a cost of a stroke of £4728 in the first year and £175 in subsequent years. NG17 notes that these costs were based on work completed for the NICE CVD risk guidance CG181. CG181 is reported as calculating first 6-month and 1-year post-event costs using information from the NHS drug tariff, NHS reference costs, Personal Social Services Research Unit Unit Costs of Health and Social Care and the British National Formulary. The EAG has not been able to source the relevant costs in the CG181 publicly available documents.
Insulet highlights a paper in the literature by Xu et al. 151 that estimated the costs of stroke patients using data from the medical records of 84,184 English, Welsh and Northern Irish NHS patients with a diagnosis of stroke between April 2015 and March 2016, as included in the Sentinel Stroke National Audit Programme. An individual patient simulation model was constructed that estimated first year and subsequent year healthcare costs, when uprated by 9.3% for inflation, of £14,702 and £1233, respectively, and social care costs of £9811 and £5219, respectively, yielding an average total 5-year cost of £46,039.
The total 5-year health and social care costs increased markedly with age. For ischaemic stroke these were reasonably constant at around £20,000 for those between 25 years and 60 years of age, increasing thereafter. For intracerebral haemorrhage these increase from around £20,000 for those of 25 years of age to around £32,000–£35,000 for those between 40 and 60 years of age, increasing thereafter. Around 60% of patients appear to have been classified as having ischaemic stroke.
A possible problem with the analysis is that not all the estimated costs might relate solely to stroke. Within the healthcare cost elements the authors tried to control for this by only including ambulance, magnetic resonance imaging and computed tomography scans, thrombolysis, acute stroke unit care, rehabilitation stroke unit care, general medical ward care, community rehabilitation, general practitioner (GP) visits, secondary prevention and ESD therapists. The balance between these costs is not stated, and general medical ward care is of particular concern. The social care cost elements may be more subject to this criticism. It is also unclear whether care home costs took into account self-funding.
Given the baseline mean age of 43 years in the modelling for the current assessment and of 40 years in the NHS adult pilot, the total 5-year healthcare costs estimated by Xu et al. 151 appear to be around 40–45% of their overall mean estimate for ischaemic stroke and around 70% of their overall mean cost for intracerebral haemorrhage. Unfortunately, it is not possible to further disaggregate these percentages when applying them to healthcare costs and social care costs. Applying them uncritically suggests healthcare costs of £7680 in the first year and £644 in subsequent years, social care costs of £5125 in the first year and £2726 in subsequent years and total costs of £12,805 in the first year and £3370 in subsequent years.
However, it should also be noted that beyond 60 years of age the estimated costs increase. Some if not much of the time modelled as being spent with stroke in the modelling for the current assessment will occur later in life and above the age of 60 years.
A possible additional source for the first year and subsequent costs of stroke are the UKPDS estimates for type 2 diabetes patients, as presented in UKPDS. The main benefit of this is that it controls for the costs associated with the other complications of diabetes and can be used to calculate the additional costs of stroke compared with having no complications, albeit in a T2DM population. Inpatient costs are estimated separately from non-inpatient costs, the latter covering elements such as GP visits using questionnaire data. A drawback is that it does not present any estimates of the social care costs. For stroke, uprating by 15.7% for inflation, the additional annual healthcare costs in excess of having no complications for a man of 40 years are £5610 for a non-fatal stroke, £625 for a history of stroke and £3517 for a fatal stroke. For a woman aged 40 years the costs are £6011, £673 and £3727, respectively. Increasing the patient age to 60 years increases these costs to £7989, £1030 and £4044 for a man and to £8360, £1115 and £4198 for a woman.
These costs are higher than those drawn from NG17. They are reasonably aligned with the healthcare costs for 40-year-olds estimated by the EAG from Xu et al.,151 although the estimates of Xu et al. 151 increase more rapidly with age. The EAG will revise its base-case costs of stroke estimates to the UKPDS healthcare costs for a 40-year-old female, providing scenarios of:
-
the UKPDS healthcare costs for a 60-year-old female
-
adding 30% (based on the proportion self-funding their residential care, as estimated by Meades and Hyde,152 when estimating the costs of blindness) of the social care costs of £5125 for the first year and £2726 in subsequent years
-
applying the original EAG report costs to illustrate the effect of this change.
Baseline glycated haemoglobin and net change in glycated haemoglobin
As reviewed in more detail in Regression analyses: baseline glycated haemoglobin per cent versus net change in haemoglobin A1c or glycated haemoglobin in hybrid closed-loop randomised controlled trials, comments were received and opinions expressed at the last NICE Appraisal Committee meeting that a worse baseline HbA1c is typically associated with a greater capacity to benefit. The EAG’s preferred regression of the net effect HbA1c by baseline HbA1c for HCL over CSII + rtCGM is, as per the NMA, to weight studies by their standard errors and to include Benhamou et al. but with the additional required assumption of a baseline 7.69% for Benhamou. The NMA results suggest little difference in net effect between the HCL versus CSII + rtCGM studies and the Thabit studies of HCL against CSII + CGM, with the latter actually suggesting a smaller effect size. It may be reasonable to include the Thabit studies in the regression of net effect against baseline, in effect assuming Thabit to be HCL against CSII + rtCGM. This has little effect on the regressions’ central estimates, as outlined in Figure 17.
FIGURE 17.
Net change in HbA1c by baseline HbA1c: HCL vs. CSII + rtCGM.
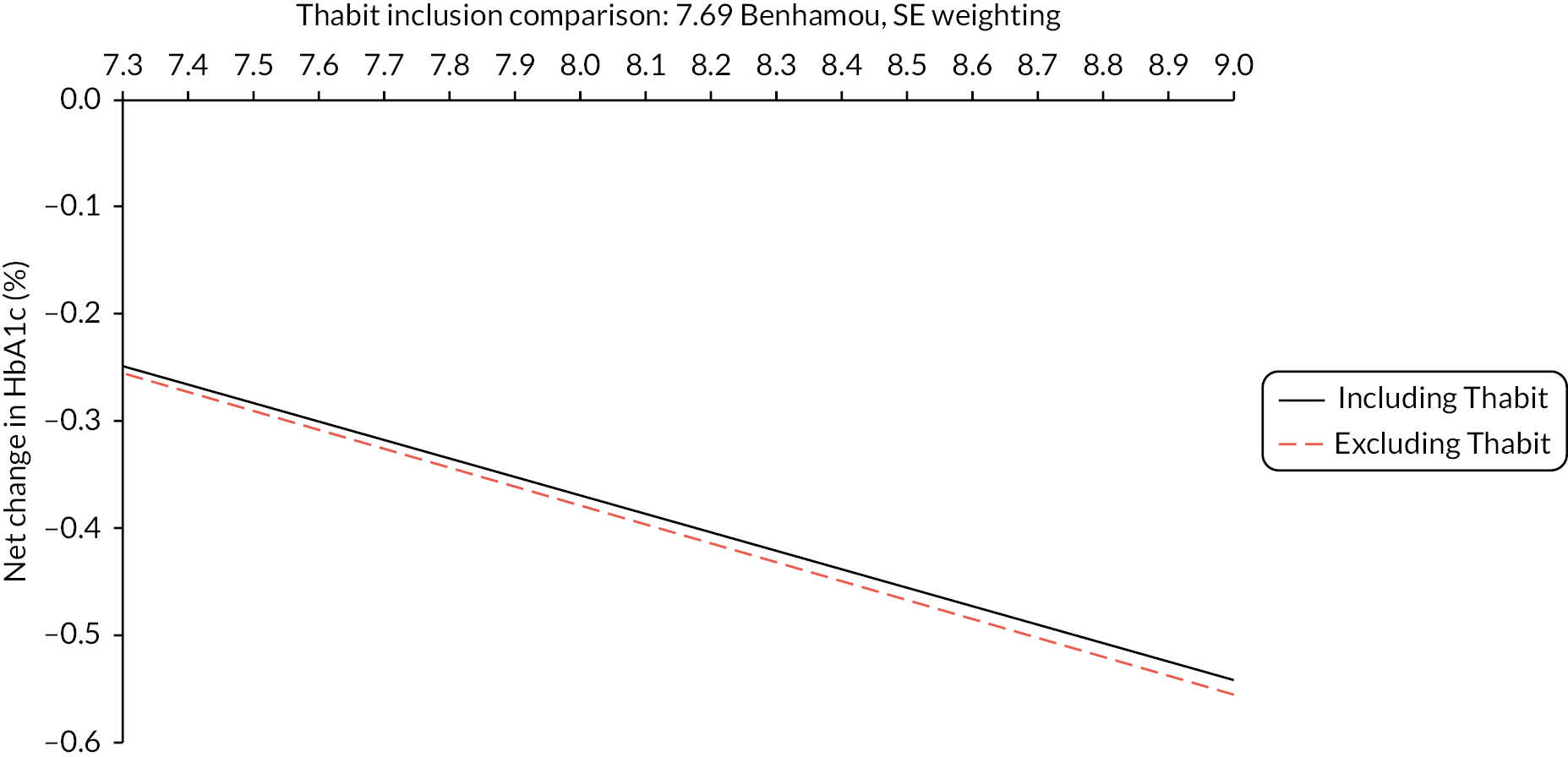
For the overall weighted mean baseline of around 7.8%, the NMA estimates a net effect for HCL over CSII + rtCGM of −0.28%, while the above regressions estimate a net effect size of around −0.34%. Although imperfect, given the centrality of the NMA to the clinical ES and the uncertainty surrounding the regressions the EAG will apply the slope parameter of the regression that excludes the Thabit studies to the central NMA estimate at the mean baseline of 7.8% to arrive at the following estimated net effect sizes for HCL against CSII + rtCGM. When coupled with the net effect from the NMA for rtCGM against rtCGM of −0.36%, this results in the following net effects. The above scenario analyses do not apply any adjustment for the effect of baseline HbA1c on the net effect of rtCGM against isCGM. The EAG views this regression as highly uncertain and unreliable, also bearing in mind that much of the comparison was between MDI + rtCGM and MDI + isCGM rather than between CSII + rtCGM and CSII + isCGM. However, for completeness a similar exercise can be performed. Noting the weighted mean baseline of 7.5% across the relevant studies, the regression estimates a net effect of −0.30% compared with the NMA estimate of 0.36%. As a consequence, the EAG will similarly apply the regression slope to the NMA estimate at a baseline of 7.5% to arrive at the following net effects by baseline HbA1c. When coupled with the net effects for HCL against CSII + rtCGM, this results in the following net effects for HCL against CSII + isCGM (Table 19).
| Exploration of HbA1c net effect by baseline HbA1c: HCL vs. CSII + rtCGM | |||||||||
| Baseline HbA1c (%) | 7.4 | 7.6 | 7.8 | 8.0 | 8.2 | 8.4 | 8.6 | 8.8 | 9.0 |
| Net HbA1c (%) | −0.21 | −0.24 | −0.28 | −0.32 | −0.35 | −0.39 | −0.42 | −0.46 | −0.49 |
| Using single regression: modelled effect sizes by baseline HbA1c | |||||||||
| Baseline HbA1c (%) | 7.4 | 7.6 | 7.8 | 8.0 | 8.2 | 8.4 | 8.6 | 8.8 | 9.0 |
| CSII + isCGM (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CSII + rtCGM (%) | −0.36 | −0.36 | −0.36 | −0.36 | −0.36 | −0.36 | −0.36 | −0.36 | −0.36 |
| HCL (%) | −0.57 | −0.60 | −0.64 | −0.68 | −0.71 | −0.75 | −0.78 | −0.82 | −0.85 |
| Speculation on HbA1c net effect by baseline HbA1c: rtCGM vs. isCGM | |||||||||
| Baseline HbA1c (%) | 7.4 | 7.6 | 7.8 | 8.0 | 8.2 | 8.4 | 8.6 | 8.8 | 9.0 |
| Net HbA1c (%) | −0.35 | −0.37 | −0.38 | −0.40 | −0.42 | −0.43 | −0.45 | −0.46 | −0.48 |
| Using both regression: modelled effect sizes by baseline HbA1c | |||||||||
| Baseline HbA1c (%) | 7.4 | 7.6 | 7.8 | 8.0 | 8.2 | 8.4 | 8.6 | 8.8 | 9.0 |
| CSII + isCGM (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CSII + rtCGM (%) | −0.35 | −0.37 | −0.38 | −0.40 | −0.42 | −0.43 | −0.45 | −0.46 | −0.48 |
| HCL (%) | −0.56 | −0.61 | −0.66 | −0.71 | −0.77 | −0.82 | −0.87 | −0.92 | −0.97 |
Estimating rates of severe hypoglycaemic events and non-severe hypoglycaemic events
The earlier EAG work estimated rates of SHEs and NSHEs based on what it felt were reasonable baseline rates for HCL, coupled with an assumption that the rates of SHEs were proportionate to time < 3.0 mmol/l and the rates of NSHEs were proportionate to time < 3.9 mmol/l. When coupled with mean baseline values in the HCL studies of 0.64 for time < 3.0 mmol/l and 4.56 for time < 3.9 mmol/l, the NMA provides the following estimates: for time < 3.9 mmol/l – HCL of 3.37, CSII + rtCGM of 4.56, CSII + isCGM of 0.29; for time < 3.0 mmol/l – HCL of 0.64, CSII + rtCGM of 0.64, CSII + isCGM of 0.29. The value for CSII + isCGM for TBR < 3.9 mmol/l is very much less than that of CSII + rtCGM. It should be noted that the isCGM versus rtCGM studies that this is based on had somewhat higher absolute TBR baseline values than the HCL studies. NG17 estimated a ratio between isCGM and rtCGM of 94% for NSHEs and 73% for SHEs. When exploring the rates of NSHEs the EAG will typically apply the 94% ratio from NG17 but will provide a scenario analysis that applies the full set of NMA estimates. These ratios are applied to the HCL rates of 20.8 for NSHEs and 0.26 for SHEs, as presented in Table 20, with the EAG also providing scenarios for NSHEs of rates of 57.2 and 13.0.
| NSHEs | SHEs | ||
|---|---|---|---|
| NMA and NG17 | NMA alone | NMA alone | |
| HCL | 20.80 | 20.80 | 0.26 |
| CSII + rtCGM | 25.43 | 25.43 | 0.26 |
| CSII + isCGM | 24.03 | 1.60 | 0.39 |
It should be noted that for rtCGM NG17 estimated annual rates of 0.19 for SHEs and 20.5 for NSHEs. A full account of the NSHE and SHE rates assumed for HCL is provided in the EAG’s earlier work.
Valuing severe hypoglycaemic events
The EAG retains its preference for Gordon et al. 129 in valuing NSHEs for the reasons outlined in its earlier work. This also outlined that Gordon et al. 129 observed very few severe hypoglycaemic episodes and suggested that Nauck et al. 142 provided an estimate that was reasonably representative of the alternatives in the literature. The EAG exploration of hypoglycaemic events will use Gordon et al. 129 to value non-severe hypoglycaemia and Nauck et al. 142 to value severe hypoglycaemia. Scenarios using only Gordon et al. 129 and the historically more commonly applied Currie et al. 22 will be presented.
Costing non-severe hypoglycaemic events
In common with a number of other NICE assessments, including NG17, the EAG previously assumed that NSHEs result in no costs to the NHS or Personal Social Services. NG17 highlighted Geelhoed et al.,153 who surveyed 1631 European T1DM patients and defined NSHEs as hypoglycaemia symptoms that occurred during the previous 7 days or episodes of blood glucose < 3.1 mmol/l that patients could manage without third-party assistance. Data were also collected on healthcare resource use following a NSHE. The mean weekly frequency of 1.8 NSHEs, or an annual rate of 91, was somewhat higher than those of NG17 and the EAG exploratory analyses. Following a NSHE, 2.3% of T1DM patients contacted a healthcare professional and SMBG increased by 12%, with 13.6% also reducing their insulin dose. If a GP appointment is assumed, if the increase in monitoring applies only for the week after a NSHE and if the reduced insulin dose is ignored, these estimates might suggest an additional cost of £2.15 following a NSHE, or, given the average weekly rate of 1.8 for modelling purposes, around £1.20 per event.
In contrast to this, Brod et al. 11 surveyed 193 and 192 UK patients with T1DM and T2DM respectively, who had had at least one NSHE during the previous month. Among the T1DM patients, 47% experienced NSHEs between daily and weekly, 28% experienced them several times to once per month and the remainder experienced them less frequently than this. Arbitrarily assuming 10, 3 and 0.25 per month, respectively, suggests a roughly similar mean frequency to the 1.8 per week in Geelhoed et al. 153 Across all UK patients, 25.7% ‘contacted a healthcare professional after last NSHE’, this not being limited to primary care. This is an order of magnitude greater than Geelhoed et al. 153 estimated, part of which may be due to the longer recall period in Brod et al.
Assuming that the contacts are per month with a NSHE rather than per event suggests costs that are roughly three times those of Geelhoed et al.,153 or around £3.60. Orozco-Beltrán et al. 154 surveyed 294 Spanish patients with T1DM who experienced an average of 1.7 NSHEs per week. The authors reported that NSHEs were associated with an additional SMBG cost of £1.20 per event, while 8% of daytime and 12% of nocturnal NSHEs during the study period led to a healthcare contact. The balance between daytime and nocturnal events is only provided across T1DM and T2DM patients but suggests an overall contact rate of 8.9%. Assuming these are GP visits suggests a total cost per event of £4.93. The EAG will present a scenario that costs NSHEs at £5 per event. This is unlikely to have any material effect on the results. The common weekly rate of around 1.8 NSHEs in the three papers may suggest an additional scenario of an annual 90 NSHEs, but it should be borne in mind that these rates were among patients responding to questionnaires about NSHEs and so may not constitute a representative sample.
Costs of the technologies
The costings used in the previous EAG report incorrectly applied the costs of the Freestyle Libre 3 to CSII + isCGM. These should have applied the costs of the Freestyle Libre 2 and taken into account the costs of Dexcom One sensor use. The previous EAG costings also slightly inflated the costs of HCL systems to take into account that some sensors do not last their full lifespan, for example because they are accidentally knocked, using survival curve data supplied by the companies. All the companies have since indicated that users can telephone for a free replacement sensor should this be required. As a consequence, the EAG removes this element from the costing. The costs for HCL and CSII + rtCGM have been provided by the NHS supply chain. These do not include any volume discounts or any proposed future discounts and are based on the costs current to the NHS supply chain system. It is assumed that only 10% of Dexcom 6 users require a receiver. The EAG has estimated costs for CSII + isCGM by using the CSII + rtCGM costs and substituting the NHS drug tariff isCGM sensor and transmitter costs for the rtCGM costs. Professor Partha Kar has provided market share estimates for most systems. The EAG takes the mid-point of these estimates; for example, the MiniMed 780G market share estimates of 60–65% result in a 62.5% estimate. For systems without a market share estimate the residual market share is split equally between them, indicated by a superscript ‘a’ in Tables 21 and 22. Given the uncertainty around market shares in addition to the base-case weighted average costs the EAG supplied a full set of scenario analyses that apply the lowest cost system in each subtype. Note that for CSII + rtCGM this retains the balance of 3% Freestyle Libre 3 and 97% Dexcom 6, and similarly for CSII + isCGM this retains the balance of 80% Freestyle Libre 2 and 20% Dexcom One. These lowest system costing analyses worsen the HCL versus pooled CSII + CGM base-case ICER by 10% and the scenario analyses ICERs by between 10% and 12%. This results in the system costs listed in Table 21.
| Year 1 | Years 2–4 | 4-year | Share(%) | |
|---|---|---|---|---|
| HCL system costs | ||||
| Ypsomed | £8171 | £5706 | £25,289 | 10a |
| Advanced therapeutics | £7650 | £4975 | £22,575 | 10a |
| Air Liquide | £7785 | £4980 | £22,724 | 18 |
| Medtronic | £8051 | £4768 | £22,355 | 63 |
| HCL weighted average | £7976 | £4920 | £22,735 | – |
| HCL cheapest | £8051 | £4768 | £22,355 | – |
| CSII + rtCGM system costs | ||||
| CSII + rtCGM: Freestyle Libre 3 | 3 | |||
| Ypsomed | £5952 | £3488 | £16,415 | 33a |
| Medtronic | £5829 | £2944 | £14,660 | 33 |
| Insulet | £4115 | £4115 | £16,459 | 33a |
| CSII + rtCGM: Dexcom 6 | 97 | |||
| Ypsomed | £7371 | £4906 | £22,089 | 33a |
| Medtronic | £7248 | £4362 | £20,334 | 33 |
| Insulet | £5533 | £5533 | £22,133 | 33a |
| CSII + rtCGM weighted average | £6675 | £4891 | £21,348 | – |
| CSII + rtCGM cheapest | £7205 | £4319 | £20,163 | – |
| CSII + isCGM system costs | ||||
| CSII + isCGM: Freestyle Libre 2 | 80 | |||
| Ypsomed | £5607 | £3142 | £15,033 | 33a |
| Medtronic | £5484 | £2598 | £13,278 | 33 |
| Insulet | £3769 | £3769 | £15,077 | 33a |
| CSII + rtCGM: Dexcom One | 20 | |||
| Ypsomed | £5597 | £3132 | £14,993 | 33a |
| Medtronic | £5474 | £2588 | £13,238 | 33 |
| Insulet | £3759 | £3759 | £15,037 | 33a |
| CSII + isCGM weighted average | £4951 | £3168 | £14,454 | – |
| CSII + isCGM cheapest | £5482 | £2596 | £13,270 | – |
Due to different ES for CSII + rtCGM and CSII + isCGM these are modelled separately. Results being pooled assuming that CSII + CGM is 90% CSII + isCGM and 10% CSII + rtCGM leads to the following base-case treatment costs. Note that the year 1 and years 2–4 costs are applied in the model, with the annual average presented here to ease comparison (see Table 22).
| Year 1 | Years 2–4 | 4-year | Annual | |
|---|---|---|---|---|
| Base-case treatment costs: weighted average | ||||
| HCL | £7976 | £4920 | £22,735 | £5684 |
| CSII + rtCGM | £6675 | £4891 | £21,348 | £5337 |
| CSII + isCGM | £4951 | £3168 | £14,454 | £3614 |
| CSII pooled (90% CSII + isCGM) | £5124 | £3340 | £15,144 | £3786 |
| Treatment cost scenario: lowest cost system | ||||
| HCL | £8051 | £4768 | £22,355 | £5589 |
| CSII + rtCGM | £7205 | £4319 | £20,163 | £5041 |
| CSII + isCGM | £5482 | £2596 | £13,270 | £3317 |
| CSII pooled (90% CSII + isCGM) | £5654 | £2768 | £13,959 | £3490 |
Because the Freestyle Libre 3 is relatively new to the market and also rather cheaper than the Dexcom 6, if the Freestyle Libre 3 becomes more prevalent the cost of CSII + rtCGM will fall somewhat. However, as it is assumed that 90% of CSII + CGM is CSII + isCGM even if all CSII + rtCGM used the Freestyle Libre 3 the pooled annual average cost of CSII + CGM would fall by around only £140. The EAG does not explore this further. An additional £830 is added to these annual costs to account for insulin, lancets and test strips, these estimates being taken from the Medtronic submission. Routine outpatient costs add a further annual £640.
Analyses
In addition to its base case the EAG conducted the following scenarios:
-
SA01: applying the regression results for net effect for HCL over CSII + rtCGM by baseline HbA1c.
-
SA02: applying the regression results for both net effect for HCL over CSII + rtCGM by baseline HbA1c and net effect for rtCGM over isCGM by baseline HbA1c.
-
SA03: applying the various costs of stroke as outlined above.
-
SA04: assuming an annual 0.045% worsening in HbA1c.
-
SA05: applying the NMA HbA1c results that exclude Benhamou et al.
-
SA06: adjusting the costs of complications to account for their possible overestimation within the iQVIA CDM, as described in greater detail in the original EAG report.
-
SA07: estimating NSHEs using annual rates of (a) 20.8, (b) 57.2, (c) 13.0 and (d) 90.0 for HCL as outlined above.
-
SA08: estimating HSEs using annual rates of 20.8 for NSHEs and 0.64 for SHEs for HCL as outlined above.
-
SA09: SA08 and valuing HEs using (a) Currie et al. 22 and (b) Gordon et al. 129
-
SA10: SA08 and assuming SHE costs of (a) £36 for non-medical and £628 for medical and (b) £381 on average.
-
SA11: SA08 with NSHE £5 cost per event.
-
SA12: SA08 with double the HE quality of life effect to account for possible carer effects.
-
SA13: estimating NSHE for CSII + isCGM using the NMA TBR < 3.9 mmol/l estimates.
Results: base case – weighted average costing of technologies
The revised base-case estimates the following in Tables 23 and 24.
| CSII + | HCL | ||||
|---|---|---|---|---|---|
| isCGM | rtCGM | HCL | vs. isCGM | vs. rtCGM | |
| Life-years undiscounted | 32.499 | 32.962 | 33.471 | 0.972 | 0.509 |
| QALYs | |||||
| CDM modelled | 14.232 | 14.400 | 14.581 | 0.349 | 0.181 |
| NSHEs | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| SHEs | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Total QALYs | 14.232 | 14.400 | 14.581 | 0.349 | 0.181 |
| Costs | |||||
| Treatment | £85,540 | £119,353 | £127,707 | £42,168 | £8355 |
| Routine OP | £12,182 | £12,279 | £12,393 | £211 | £114 |
| HEs | £0 | £0 | £0 | £0 | £0 |
| Other management | £1700 | £1722 | £1742 | £43 | £21 |
| CVD | £4878 | £4663 | £4479 | −£399 | −£184 |
| Renal | £10,365 | £9774 | £9284 | −£1080 | −£490 |
| Neuropathy/amp. | £889 | £877 | £816 | −£72 | −£61 |
| Eye | £18,270 | £15,745 | £14,262 | −£4008 | −£1483 |
| Total costs | £133,824 | £164,412 | £170,685 | £36,861 | £6273 |
| CSII + isCGM | CSII + rtCGM | HCL | |
|---|---|---|---|
| Life-years undiscounted | 32.499 | 32.962 | 33.471 |
| Total QALYs | 14.232 | 14.400 | 14.581 |
| Total costs | £133,824 | £164,412 | £170,685 |
| ICER: fully incremental | Reference | Extendedly dominated | £105,620 |
| ICER: pooled CSII + CGM | Reference | £101,753 | |
Results: scenario analyses – weighted average costing of technologies
In the fully incremental analyses, including SA01 (applying the regression results for net effect for HCL over CSII + rtCGM by baseline HbA1c) and SA02 (applying the regression results for both net effect for HCL over CSII + rtCGM by baseline HbA1c and net effect for rtCGM over isCGM by baseline HbA1c), CSII + rtCGM is extendedly dominated throughout.
The results are presented in Appendix 11, Table 27.
From the pooled analyses that apply the regression of the net effect of HCL over CSII + rtCGM, SA01, and that apply this and the regression of the net effect of isCGM over rtCGM, SA02, the following results (Figure 18).
FIGURE 18.
Scenario analyses: net effects by baseline HbA1c.
Note that for a baseline of 8.0% SA01 estimates an ICER of £101,146 per QALY while SA02 estimates an ICER of £99,544 per QALY. These are slightly lower than the base-case ICER of £101,753 per QALY as it is assumed that the regression is centred around the trial baseline HbA1c values, which are < 8.0%.
Results: comparison with NG17
NG17 estimated an annual cost of rtCGM of £2000 based on the September 2020 ceiling cost permitted for pregnant women in the NHS and NHS Improvement funding document and an annual isCGM cost of £910 based on 26 Freestyle Libre 2 sensors costing £35 each.
This yielded an annual net cost for rtCGM compared with isCGM of £1090. This compares with the current assessment’s annual net cost for CSII + rtCGM compared with CSII + isCGM of £1723, or roughly 60% higher. NG17 modelled a total net cost for rtCGM compared with isCGM of £14,512 with net QALYs of 0.123 and an implied ICER of £118,000 per QALY. The most comparable EAG analysis is SA09, which estimates a total net cost for CSII + rtCGM compared with CSII + isCGM of £30,084, roughly double that of NG17. Net gains are also greater at 0.236 QALYs, yielding an ICER of £127,000 per QALY, which is reasonably aligned with that of NG17. The implied NG17 ICER of £118,000 per QALY for rtCGM compared with isCGM is presumably the reason why, although both rtCGM and isCGM were approved in NG17, the recommendation was that ‘when choosing a CGM device … if multiple devices meet their needs and preferences, offer the device with the lowest cost’. This and/or patient preferences may explain the current preponderance of isCGM, and why if HCL is recommended it will mainly displace isCGM.
Results: validity of pooling continuous subcutaneous insulin infusion + intermittently scanned continuous glucose monitoring and continuous subcutaneous insulin infusion + real-time continuous glucose monitoring
The EAG has presented fully incremental results in line with the NICE methods guide. It then pools the modelled results for CSII + isCGM and CSII + rtCGM, with their total costs and total QALYs being weighted 90% and 10%, respectively. This glosses over that the baseline for CSII + sCGM is 8.00% but the baseline for CSII + rtCGM includes the net effect and so is 8.00% – 0.36% = 7.64%. If this is felt to be the true picture it is unproblematic, but there may be some concerns about this.
An alternative is to model both CSII + isCGM patients and CSII + rtCGM patients having a baseline of 8.00% with the net effect of HCL compared with CSII + isCGM being −0.64% and the net effect of HCL compared with CSII + rtCGM being −0.28%. Adopting this approach and pooling 90 : 10 results in a total net cost of £33,717, a net 0.330 QALY gain and an ICER of £102,050 per QALY, which is very similar to the £101,753 per QALY in the base case. This approach can be criticised due to the common baseline HbA1c suggesting that the CSII + isCGM and CSII + rtCGM patient populations are different, and so may beg more questions than it answers.
Questions for committee
Economic questions:
-
Is a 90% share for CSII + isCGM the most reasonable estimate?
-
How reasonable are the market share costing assumptions and what weight should be given to the scenario that assumes the cheapest system will be mainly used?
-
Should one or both the regressions of net change by baseline HbA1c be applied?
-
Should estimates of SHE be included, and, if so, what are the most reasonable estimates, how should they be valued in terms of the quality of life of patients and possibly carers, and what cost should be applied?
-
Should estimates of NSHE be included, and, if so, what are the most reasonable estimates, how should they be valued in terms of the quality of life of patients and possibly carers, and what cost should be applied?
Weighted average costings of incremental and pooled analyses (HCL vs. CSII + CGM) are presented in Appendix 12, Table 28. Incremental and pooled analyses (HCL vs. CSII + CGM) by the costly system are presented in Appendix 12, Table 29.
Additional information
Contributions of authors
Asra Asgharzadeh (https://orcid.org/0000-0002-1068-8537): senior clinical effectiveness (screening for clinical evidence, assessment of study inclusion, data abstraction, write-up of clinical results).
Mubarak Patel (https://orcid.org/0000-0001-7573-1447): statistical analysis (network meta-analysis, write-up of results).
Martin Connock (https://orcid.org/0000-0002-5353-9988): statistical analysis (clinical study analysis, write-up of results).
Sara Damery (https://orcid.org/0000-0003-3681-8608): clinical effectiveness (screening for clinical evidence, assessment of study inclusion, data abstraction, risk of bias write-up).
Iman Ghosh (https://orcid.org/0000-0002-7073-7468): clinical effectiveness (quality appraisal).
Mary Jordan (https://orcid.org/0000-0002-0497-8634): systematic review of cost-effectiveness.
Karoline Freeman (https://orcid.org/0000-0002-9963-2918): clinical effectiveness (screening for clinical evidence, assessment of study inclusion, data abstraction).
Anna Brown (https://orcid.org/0000-0002-4541-6232): information specialist (designing searches, locating records).
Rachel Court (https://orcid.org/0000-0002-4567-2586): senior information specialist (reviewing and updating searches, locating records, references).
Sharin Baldwin (https://orcid.org/0000-0002-2374-5844): clinical effectiveness (screening for clinical evidence, assessment of study inclusion).
Fatai Ogunlayi (https://orcid.org/0000-0002-4707-3448): clinical effectiveness (screening for clinical evidence, assessment of study inclusion).
Chris Stinton (https://orcid.org/0000-0001-9054-1940): clinical effectiveness (screening for clinical evidence, assessment of study inclusion).
Ewen Cummins: senior cost-effectiveness (cost-effectiveness lead and production).
Lena Al-Khudairy (https://orcid.org/0000-0003-0638-583X): clinical effectiveness lead.
Acknowledgements
Professor Norman Waugh, Warwick University, provided clinical and topic support. Professor Aileen Clark assessed the quality of the work. Dr Nitin Gholap, Consultant in Endocrinology and Acute Medicine – University Hospital Coventry and Warwickshire, provided clinical support. Leigh Carr, NHS Supply Chain, provided the costings of pumps and their consumables. Ben McGough, Digital Lead, NHS Diabetes Programme Team, liaised with the Diabetes Technical Network. Dr Fiona Green, Consultant in Diabetes and Endocrinology, provided additional expert opinion. Dr Mark Lamotte and the iQVIA team provided advice on inputs to and the use of the iQVIA Core Diabetes Model. Mrs Pearl Pawson reviewed the report.
Data-sharing statement
All data requests should be submitted to the corresponding author or Warwick Evidence for consideration.
Ethics statement
This study is a secondary analysis of published evidence and therefore ethics was not required.
Information governance statement
This study did not handle any personal information.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/JYPL3536.
Primary conflicts of interest: Karoline Freeman received a grant for NIHR Evidence Synthesis Programme NIHR135325, Rachel Court received a grant for NIHR Evidence Synthesis Programme NIHR135326, and Chris Stinton received a grant for NIHR Evidence Synthesis Programme NIHR135326.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. The Management of Type 1 Diabetes in Adults . A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44:2589-625. https://doi.org/10.2337/dci21-0043.
- National Institute for Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management [NG17] 2015. www.nice.org.uk/guidance/ng17 (accessed 19 February 2021).
- Defining and Reporting Hypoglycemia in Diabetes . A report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245-9. https://doi.org/10.2337/diacare.28.5.1245.
- National Institute for Health and Care Excellence . Integrated Sensor-Augmented Pump Therapy Systems for Managing Blood Glucose Levels in Type 1 Diabetes (the MiniMed Paradigm Veo System and the Vibe and G4 PLATINUM CGM System) 2016. www.nice.org.uk/guidance/dg21 (accessed 18 February 2021).
- National Institute for Health and Care Excellence . Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus 2008. www.nice.org.uk/guidance/ta151 (accessed 19 February 2021).
- National Institute for Health and Care Excellence . Guidance on the Use of Long‑acting Insulin Analogues for the Treatment of Diabetes – Insulin Glargine 2002.
- Warren E, Weatherley-Jones E, Chilcott J, Beverley C. Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8450.
- Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14360.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. University of York; 1995.
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 health survey. J Clin Epidemiol 1998;51:1115-28. https://doi.org/10.1016/S0895-4356(98)00103-6.
- Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health 2011;14:665-71. https://doi.org/10.1016/j.jval.2011.02.001.
- Leckie AM, Graham MK, Grant JB, Ritchie PJ, Frier BM. Frequency, severity, and morbidity of hypoglycemia occurring in the workplace in people with insulin-treated diabetes. Diabetes Care 2005;28:1333-8. https://doi.org/10.2337/diacare.28.6.1333.
- Frier BM, Jensen MM, Chubb BD. Hypoglycaemia in adults with insulin-treated diabetes in the UK: self-reported frequency and effects. Diabet Med 2016;33:1125-32. https://doi.org/10.1111/dme.12878.
- Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023-5. https://doi.org/10.2337/dc10-2411.
- Choudhary P. Insulin pump therapy with automated insulin suspension: toward freedom from nocturnal hypoglycemia. JAMA 2013;310:1235-6. https://doi.org/10.1001/jama.2013.278576.
- Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114-22. https://doi.org/10.2337/dc14-0030.
- Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther 2020;11:83-95. https://doi.org/10.1007/s13300-019-00720-0.
- Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res 2014;23:2645-50. https://doi.org/10.1007/s11136-014-0712-x.
- Harris S, Mamdani M, Galbo-Jørgensen CB, Bøgelund M, Gundgaard J, Groleau D. The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes 2014;38:45-52. https://doi.org/10.1016/j.jcjd.2013.09.001.
- Levy AR, Christensen TLU, Johnson JA. Utility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-73.
- Adler A, Jofre-Bonet M, Wilkinson G, Martin A, Mcguire A. Quantifying the loss of health-related quality of life from hypoglycemia. 2220-PO. Diabetes 2014;63. https://doi.org/10.2337/db14-2207-2292.
- Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34. https://doi.org/10.1185/030079906X115757.
- Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N. The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7220.
- Iqbal A, Heller SR. The role of structured education in the management of hypoglycaemia. Diabetologia 2018;61:751-60. https://doi.org/10.1007/s00125-017-4334-z.
- National Institute for Health and Care Excellence (NICE) NG17 Guideline Development Team . Type 1 Diabetes in Adults: Diagnosis and Management [B] Evidence Reviews for Continuous Glucose Monitoring in Adults With Type 1 Diabetes NICE Guideline NG17 Evidence Reviews Underpinning Recommendations 1.6.10 to 1.6.18 and Recommendations for Research in the NICE Guideline 2022. www.nice.org.uk/guidance/ng17/evidence/b-continuous-glucose-monitoring-in-adults-with-type-1-diabetes-pdf-11013435182 (accessed 20 September 2022).
- Harbour J, Dimitrova M, Colthart I, Stewart J, Herbert P. Closed Loop Systems and the Artificial Pancreas for the Management of Type 1 Diabetes [SHTG Recommendation]. Glasgow/Edinburgh: Healthcare Improvement Scotland, Scottish Health Technologies Group (SHTG); 2022.
- Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208-19. https://doi.org/10.1016/S0140-6736(20)32514-9.
- Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007;50:2239-44. https://doi.org/10.1007/s00125-007-0803-0.
- Lunati ME, Morpurgo PS, Rossi A, Gandolfi A, Cogliati I, Bolla AM, et al. Hybrid close-loop systems versus predictive low-glucose suspend and sensor-augmented pump therapy in patients with type 1 diabetes: a single-center cohort study. Front Endocrinol 2022;13. https://doi.org/10.3389/fendo.2022.816599.
- Messer LH, Berget C, Forlenza GP. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther 2019;21:462-9. https://doi.org/10.1089/dia.2019.0105.
- Cochrane Collaboration . Handbook for Diagnostic Test Accuracy Reviews 2021. https://methods.cochrane.org/sdt/handbook-dta-reviews (accessed 18 February 2021).
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 21 February 2021).
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane; 2021.
- Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20170.
- Tessier V. Périnatalité: Perinatality: Rappel favorisé sur la précision. Canadian Health Libraries Association – Association des bibliothèques de la santé du Canada; 2017.
- Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi M, Athanasiou A, Martin-Hirsch PP, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2015;2016. https://doi.org/10.1002/14651858.CD008478.pub2.
- Cochrane Pregnancy and Childbirth . Cochrane Pregnancy and Childbirth’s Trials Register: Detailed Search Methods Used to Maintain and Update the Specialised Register 2018. https://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/public/uploads/cochrane_pregnancy_and_childbirth_search_methods_2018_1.docx (accessed 29 June 2022).
- Royal College of Nursing . Do It Yourself (DIY) Closed Loop for People Living With Type 1 Diabetes: Position Statement 2020. www.diabetes.org.uk/about-us/about-the-charity/our-strategy/position-statements/do-it-yourself-closed-loop (accessed 25 March 2021).
- National Institute for Health and Care Excellence . 6.1 Identifying and Selecting Relevant Evidence. Developing NICE Guidelines: The Manual 2014. www.nice.org.uk/process/pmg20/ (accessed 22 March 2021).
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual 2014. www.nice.org.uk/process/pmg20/ (accessed 22 March 2021).
- Wade R, Sharif-Hurst S, Harden M, Walton M, Claxton L, Hodgson R, et al. Methods for selecting the best evidence to inform a NICE technology appraisal on selective internal radiation therapies for hepatocellular carcinoma. Syst Rev 2020;9. https://doi.org/10.1186/s13643-020-01447-x.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Cochrane Effective Practice and Organisation of Care (EPOC) . EPOC Resources for Review Authors 2021. https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 24 March 2021).
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane; 2020.
- Benhamou PY, Lablanche S, Vambergue A, Pou S, Madrolle S, Romero-Ugalde H, et al. The beneficial effects of closed-loop insulin delivery in patients with highly unstable type 1 diabetes eligible for islet transplantation are maintained over 6 months: an extension study of the DBLHU-WP10 trial. Diabetes Obes Metab 2022;24:956-61. https://doi.org/10.1111/dom.14654.
- Boughton CK, Hovorka R. Advances in artificial pancreas systems. Sci Transl Med 2019;11. https://doi.org/10.1126/scitranslmed.aaw4949.
- Collyns OJ, Meier RA, Betts ZL, Chan DSH, Frampton C, Frewen CM, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care 2021;44:969-75. https://doi.org/10.2337/dc20-2250.
- Kariyawasam D, Morin C, Casteels K, Le Tallec C, Sfez A, Godot C, et al. Hybrid closed-loop insulin delivery versus sensor-augmented pump therapy in children aged 6–12 years: a randomised, controlled, cross-over, non-inferiority trial. Lancet Digit Health 2022;4:e158-68. https://doi.org/10.1016/S2589-7500(21)00271-5.
- McAuley SA, Trawley S, Vogrin S, Ward GM, Fourlanos S, Grills CA, et al. Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care 2022;45:381-90. https://doi.org/10.2337/dc21-1667.
- Stewart ZA, Wilinska ME, Hartnell S, O’Neil LK, Rayman G, Scott EM, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care 2018;41:1391-9. https://doi.org/10.2337/dc17-2534.
- Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321-9. https://doi.org/10.1016/S0140-6736(18)31947-0.
- Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129-40. https://doi.org/10.1056/NEJMoa1509351.
- von dem Berge T, Remus K, Biester S, Reschke F, Klusmeier B, Adolph K, et al. In-home use of a hybrid closed loop achieves time-in-range targets in preschoolers and school children: results from a randomized, controlled, crossover trial. Diabetes Obes Metab 2022;24:1319-27. https://doi.org/10.1111/dom.14706.
- Ware J, Allen JM, Boughton CK, Wilinska ME, Hartnell S, Thankamony A, et al. KidsAP Consortium . Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386:209-19. https://doi.org/10.1056/NEJMoa2111673.
- Ware J, Boughton CK, Allen JM, Wilinska ME, Tauschmann M, Denvir L, et al. DAN05 Consortium . Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health 2022;4:e245-55. https://doi.org/10.1016/S2589-7500(22)00020-6.
- Weinzimer S, Bailey R, Bergenstal RM, Nimri R, Beck RW, Schatz D, et al. A Comparison of post-prandial glucose control in the Medtronic advanced hybrid closed-loop system vs 670G. Diabetes Technol Ther 2022;1. https://doi.org/10.1089/dia.2021.0568.
- Wheeler BJ, Collyns OJ, Meier RA, Betts ZL, Frampton C, Frewen CM, et al. Improved technology satisfaction and sleep quality with Medtronic MiniMed R advanced hybrid closed-loop delivery compared to predictive low glucose suspend in people with type 1 diabetes in a randomized crossover trial. Acta Diabetol 2022;59:31-7. https://doi.org/10.1007/s00592-021-01789-5.
- Bassi M, Teliti M, Lezzi M, Iosca A, Strati MF, Carmisciano L, et al. A comparison of two hybrid closed-loop systems in Italian children and adults with type 1 diabetes. Front Endocrinol 2021;12. https://doi.org/10.3389/fendo.2021.802419.
- Beato-Vibora PI, Gallego-Gamero F, Ambrojo-Lopez A. Real-world outcomes with different technology modalities in type 1 diabetes. Nutr Metab Cardiovasc Dis 2021;31:1845-50. https://doi.org/10.1016/j.numecd.2021.02.028.
- Beato-Vibora PI, Gallego-Gamero F, Ambrojo-Lopez A, Gil-Poch E, Martin-Romo I, Arroyo-Diez FJ. Amelioration of user experiences and glycaemic outcomes with an advanced hybrid closed loop system in a real-world clinical setting. Diabetes Res Clin Pract 2021;178. https://doi.org/10.1016/j.diabres.2021.108986.
- Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601-8. https://doi.org/10.1089/dia.2021.0097.
- Carlson AL, Sherr JL, Shulman DI, Garg SK, Pop-Busui R, Bode BW, et al. Safety and glycemic outcomes during the MiniMed™ advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178-89. https://doi.org/10.1089/dia.2021.0319.
- Forlenza GP, Ekhlaspour L, DiMeglio LA, Fox LA, Rodriguez H, Shulman DI, et al. Glycemic outcomes of children 2–6 years of age with type 1 diabetes during the pediatric MiniMedTM 670G system trial. Pediatr Diabetes 2022;23:324-9. https://doi.org/10.1111/pedi.13312.
- Benhamou PY, Franc S, Reznik Y, Thivolet C, Schaepelynck P, Renard E, et al. DIABELOOP WP7 Trial Investigators . Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health 2019;1:e17-25. https://doi.org/10.1016/s2589-7500(19)30003-2.
- Hood KK, Laffel LM, Danne T, Nimri R, Weinzimer SA, Sibayan J, et al. Lived Experience of advanced hybrid closed-loop versus hybrid closed-loop: patient-reported outcomes and perspectives. Diabetes Technol Ther 2021;23:857-61. https://doi.org/10.1089/dia.2021.0153.
- NHS Digital . National Diabetes Audit, 2019–20, Type 1 Diabetes 2021. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/national-diabetes-audit-2019-20-type-1-diabetes (accessed 6 September 2022).
- Da Silva JD, Lepore G, Battelino T, Arrieta A, Castañeda J, Grossman B, et al. Real-world performance of the MiniMed™ 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther 2022;24:113-9. https://doi.org/10.1089/dia.2021.0203.
- Vigersky RA, Bode BW, Brazg RL, Buckingham BA, Carlson AL, Kaiserman KB, et al. Glycemic control using recommended settings in youth and adults with type 1 diabetes (T1D) – Minimed 780G system with the calibration-free guardian 4 sensor results. Diabetes 2022;71. https://doi.org/10.2337/db22-100-LB.
- Arrieta A, Battelino T, Scaramuzza AE, Da Silva J, Castañeda J, Cordero TL, et al. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: evidence from 12 870 real-world users. Diabetes Obes Metab 2022;24:1370-9. https://doi.org/10.1111/dom.14714.
- Choudhary P, de Portu S, Arrieta A, Castañeda J, Campbell FM. Use of sensor-integrated pump therapy to reduce hypoglycaemia in people with type 1 diabetes: a real-world study in the UK. Diabet Med 2019;36:1100-8. https://doi.org/10.1111/dme.14043.
- Petrovski G, Al Khalaf F, Campbell J, Fisher H, Umer F, Hussain K. 10-day structured initiation protocol from multiple daily injection to hybrid closed-loop system in children and adolescents with type 1 diabetes. Acta Diabetol 2020;57:681-7. https://doi.org/10.1007/s00592-019-01472-w.
- Farabi SS, Carley DW, Quinn L. Glucose variations and activity are strongly coupled in sleep and wake in young adults with type 1 diabetes. Biol Res Nurs 2017;19:249-57. https://doi.org/10.1177/1099800416685177.
- Pease A, Lo C, Earnest A, Kiriakova V, Liew D, Zoungas S. The efficacy of technology in type 1 diabetes: a systematic review, network meta-analysis, and narrative synthesis. Diabetes Technol Ther 2020;22:411-21. https://doi.org/10.1089/dia.2019.0417.
- Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836-45. https://doi.org/10.1056/NEJMoa2004736.
- Boughton CK, Hartnell S, Thabit H, Mubita WM, Draxlbauer K, Poettler T, et al. Hybrid closed-loop glucose control compared with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longev 2022;3:e135-42. https://doi.org/10.1016/s2666-7568(22)00005-8.
- Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707-17. https://doi.org/10.1056/NEJMoa1907863.
- Ekhlaspour L, Forlenza GP, Chernavvsky D, Maahs DM, Wadwa RP, Deboer MD, et al. Closed loop control in adolescents and children during winter sports: use of the Tandem Control-IQ AP system. Pediatr Diabetes 2019;20:759-68. https://doi.org/10.1111/pedi.12867.
- Forlenza GP, Ekhlaspour L, Breton M, Maahs DM, Wadwa RP, DeBoer M, et al. Successful at-home use of the Tandem Control-IQ artificial pancreas system in young children during a randomized controlled trial. Diabetes Technol Ther 2019;21:159-69. https://doi.org/10.1089/dia.2019.0011.
- Kanapka LG, Wadwa RP, Breton MD, Ruedy KJ, Ekhlaspour L, Forlenza GP, et al. iDCL Trial Research Group . Extended use of the Control-IQ closed-loop control system in children with type 1 diabetes. Diabetes Care 2021;44:473-8. https://doi.org/10.2337/dc20-1729.
- Bally L, Thabit H, Kojzar H, Mader JK, Qerimi-Hyseni J, Hartnell S, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261-70. https://doi.org/10.1016/s2213-8587(17)30001-3.
- Leelarathna L, Dellweg S, Mader JK, Allen JM, Benesch C, Doll W, et al. AP@home Consortium . Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care 2014;37:1931-7. https://doi.org/10.2337/dc13-2911.
- Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: a 3-week, free-living, randomized crossover trial. Diabetes Care 2016;39:2019-25. https://doi.org/10.2337/dc16-1094.
- Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168-74. https://doi.org/10.2337/ dc15-2078.
- Singh H, Mueller L, Alencar G, Habif S, Pinsker J. Real-World Evidence for Long-Term Improvements in Glycemic Control Using Control-IQ Technology in a Large Cohort With Type 1 Diabetes from the United States. n.d. www.xcdsystem.com/adc/program/VY6jrda/index.cfm?pgid=2619&sid=21183&abid=116199 (accessed 16 September 2022).
- Forlenza GP, Carlson AL, Galindo RJ, Kruger DF, Levy CJ, McGill JB, et al. Real-World evidence supporting Tandem Control-IQ hybrid closed-loop success in the Medicare and Medicaid type 1 and type 2 diabetes populations. Diabetes Technol Ther 2022;24:814-23. https://doi.org/10.1089/dia.2022.0206.
- Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361. https://doi.org/10.1136/bmj.k1310.
- Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia 2021;64:1007-15. https://doi.org/10.1007/s00125-021-05391-w.
- Centre for Reviews and Dissemination . NHS EED MEDLINE Using OvidSP [Search Filter] n.d. www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 2 April 2022).
- National Institute for Health and Care Excellence . Type 1 Diabetes in Adults: Diagnosis and Management: Insulin Therapy 2021. www.nice.org.uk/guidance/ng17/update/ng17-update-1/documents/economic-report (accessed 29 June 2022).
- National Institute for Health and Care Excellence . Diabetes Type 1: Insulin Therapies: Health Economics Search Strategies 2021. www.nice.org.uk/guidance/ng17/update/ng17-update-1/documents/search-strategies (accessed 29 June 2022).
- Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville JM. Performance of Ovid MEDLINE search filters to identify health state utility studies. Int J Technol Assess Health Care 2017;33:472-80. https://doi.org/10.1017/s0266462317000897.
- Hypo-RESOLVE . Publications 2022. https://hypo-resolve.eu/publications (accessed 29 June 2022).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. https://doi.org/10.1007/s10198-013-0471-6.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Herman WH, Braffett BH, Kuo S, Lee JM, Brandle M, Jacobson AM, et al. The 30-year cost-effectiveness of alternative strategies to achieve excellent glycemic control in type 1 diabetes: an economic simulation informed by the results of the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC). J Diabetes Complications 2018;32:934-9. https://doi.org/10.1016/j.jdiacomp.2018.06.005.
- Haahtela T. Real option approach for comparing lifetime costs of alternative diabetes type I treatment methods. Fuzzy Econ Rev 2016;21:71-9. https://doi.org/10.25102/fer.2016.02.04.
- Pease A, Zomer E, Liew D, Earnest A, Soldatos G, Ademi Z, et al. Cost-effectiveness analysis of a hybrid closed-loop system versus multiple daily injections and capillary glucose testing for adults with type 1 diabetes. Diabetes Technol Ther 2020;22:812-21. https://doi.org/10.1089/dia.2020.0064.
- Lambadiari V, Ozdemir Saltik AZ, de Portu S, Buompensiere MI, Kountouri A, Korakas E, et al. Cost-effectiveness analysis of an advanced hybrid closed loop insulin delivery system in people with type 1 diabetes in Greece. Diabetes Technol Ther 2021;28. https://doi.org/10.1089/dia.2021.0443.
- Vallarino CR, Wong-Jacobson SH, Benneyworth BD, Meadows ES. Costs and outcomes comparison of diabetes technology usage among people with type 1 or 2 diabetes using rapid-acting insulin. J Diabetes Sci Technol 2021;17:439-48. https://doi.org/10.1177/19322968211052081.
- Pease A, Zomer E, Liew D, Lo C, Earnest A, Zoungas S. Cost-effectiveness of health technologies in adults with type 1 diabetes: a systematic review and narrative synthesis. Syst Rev 2020;9. https://doi.org/10.1186/s13643-020-01373-y.
- Cohen O, Ledgaard Holm A, Buompensiere MI, Jendle J. Cost-effectiveness analysis of the MiniMed 780g system versus multiple daily injections with intermittently scanned continuous glucose monitoring in individuals with type 1 diabetes in Sweden. Diabetes Technol Ther 2021;23. https://doi.org/10.1089/dia.2021.2525.abstracts.
- Cohen O, Saltik AZO, Buompensiere MI, Walter E. Cost‐effectiveness analysis of the MiniMedTM 780g system versus multiple daily injections with intermittently scanned continuous glucose monitoring in individuals with type 1 diabetes in Austria. Diabetes Technol Ther 2021;23:A13-4. https://doi.org/10.1089/dia.2021.2525.abstracts.
- Rai P, Zhang Y, Stover A, Iloabuchi C, Chiumente M, Kamal KM. Insulin Delivery Systems for Type 1 Diabetes Mellitus: A Comparison using a Decision Analysis Modeling Approach n.d. www.researchgate.net/publication/326181840_Insulin_Delivery_Systems_for_Type_1_Diabetes_Mellitus_-_A_Comparison_Using_a_Decision_Analysis_Modeling_Approach.
- Jendle J, de Portu S, Roze S. Cost Effectiveness Analysis of MiniMed 670G System versus Continuous Subcutaneous Insulin Infusion, in Individuals with Type 1 Diabetes n.d.
- Serne E, Roze S, Buompensiere MI, De Valk H. Cost-effectiveness analysis of the MiniMed 670g system versus multiple daily injections with intermittently scanned continuous glucose monitoring in individuals with type1 diabetes in the Netherlands. Diabetes Technol Ther 2021;23:A15-A6. https://doi.org/10.1089/dia.2021.2525.abstracts.
- Roze S, Buompensiere I, Ozdemir Saltik Z, Cohen O. Cost effectiveness analysis of MiniMedTM 670g system versus continuous subcutaneous insulin infusion, in individuals with type1 diabetes in the United Kingdom. Diabetes Technol Ther 2020;22. https://doi.org/10.1089/dia.2020.2525.abstracts.
- Young C, Hill S, Kim J, Cornelissen T, Herington E, Smith A, et al. Hybrid closed-loop insulin delivery systems for people with type 1 diabetes. Can J Health Technol 2021;1. https://doi.org/10.51731/cjht.2021.50.
- Jendle J, Pohlmann J, de Portu S, Smith-Palmer J, Roze S. Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther 2019;21:110-8. https://doi.org/10.1089/dia.2018.0328.
- Jendle J, Buompensiere MI, Holm AL, de Portu S, Malkin SJP, Cohen O. The cost-effectiveness of an advanced hybrid closed-loop system in people with type 1 diabetes: a health economic analysis in Sweden. Diabetes Ther 2021;12:2977-91. https://doi.org/10.1007/s13300-021-01157-0.
- Roze S, Buompensiere MI, Ozdemir Z, de Portu S, Cohen O. Cost-effectiveness of a novel hybrid closed-loop system compared with continuous subcutaneous insulin infusion in people with type 1 diabetes in the UK. J Med Econ 2021;24:883-90. https://doi.org/10.1080/13696998.2021.1939706.
- Serne EH, Roze S, Buompensiere MI, Valentine WJ, De Portu S, de Valk HW. Cost-effectiveness of hybrid closed loop insulin pumps versus multiple daily injections plus intermittently scanned glucose monitoring in people with type 1 diabetes in the Netherlands. Adv Ther 2022;39:1844-56. https://doi.org/10.1007/s12325-022-02058-9.
- Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407-8. https://doi.org/10.1001/jama.2016.11708.
- Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155-63. https://doi.org/10.1089/dia.2016.0421.
- Charleer S, De Block C, Van Huffel L, Broos B, Fieuws S, Nobels F, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care 2020;43:389-97. https://doi.org/10.2337/dc19-1610.
- Akturk HK, Giordano D, Champakanath A, Brackett S, Garg S, Snell-Bergeon J. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab 2020;22:583-9. https://doi.org/10.1111/dom.13933.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20:S27-40. https://doi.org/10.1185/030079904X2006.
- McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health 2014;17:714-24. https://doi.org/10.1016/j.jval.2014.07.007.
- Thokala P, Kruger J, Brennan A, Basarir H, Duenas A, Pandor A, et al. The Sheffield Type 1 Diabetes Policy Model. HEDS Discussion Paper 13/05. Sheffield: School of Health and Related Research; 2013.
- Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med 2014;31:92-101. https://doi.org/10.1111/dme.12261.
- Heller SR, Frier BM, Hersløv ML, Gundgaard J, Gough SCL. Severe hypoglycaemia in adults with insulin-treated diabetes: impact on healthcare resources. Diabet Med 2016;33:471-7. https://doi.org/10.1111/dme.12844.
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, et al. DARTS/MEMO Collaboration . Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med 2005;22:749-55. https://doi.org/10.1111/j.1464-5491.2005.01501.x.
- McAuley SA, Lee MH, Paldus B, Vogrin S, de Bock MI, Abraham MB, et al. Australian JDRF Closed-Loop Research Group . Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care 2020;43:3024-33. https://doi.org/10.2337/dc20-1447.
- Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371-8. https://doi.org/10.1001/jama.2016.19975.
- Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254-63. https://doi.org/10.1016/s0140-6736(16)31535-5.
- Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, et al. DARTS/MEMO Collaboration . Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003;26:1176-80. https://doi.org/10.2337/diacare.26.4.1176.
- Kariyawasam D, Morin C, Casteels K, Le Tallec C, Godot C, Sfez A, et al. Diabeloop DBL4K hybrid closed-loop system improves time-in-range without increasing time-in-hypoglycemia in children aged 6-12 years. Diabetes 2021;70. https://doi.org/10.2337/db21-98-LB.
- Abraham MB, de Bock M, Smith GJ, Dart J, Fairchild JM, King BR, et al. Australian Juvenile Diabetes Research Fund Closed-Loop Research group . Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr 2021;175:1227-35. https://doi.org/10.1001/jamapediatrics.2021.3965.
- Gordon J, Beresford-Hulme L, Bennett H, Tank A, Edmonds C, McEwan P. Relationship between hypoglycaemia, body mass index and quality of life among patients with type 1 diabetes: observations from the DEPICT clinical trial programme. Diabetes Obes Metab 2020;22:857-65. https://doi.org/10.1111/dom.13972.
- Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46. https://doi.org/10.2337/dc07-9919.
- Nathan DM. DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9-16. https://doi.org/10.2337/dc13-2112.
- Acharya SH, Philip S, Viswanath AK, Boroujerdi M, Waugh NR, Pearson DWM. Glycaemic control and body mass index in late-adolescents and young adults with type 1 diabetes mellitus: a population-based study. Diabet Med 2008;25:360-4. https://doi.org/10.1111/j.1464-5491.2007.02372.x.
- Peasgood T, Brennan A, Mansell P, Elliott J, Basarir H, Kruger J. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Making 2016;36:1020-33. https://doi.org/10.1177/0272989x16658660.
- Chatwin H, Broadley M, Speight J, Cantrell A, Sutton A, Heller S, et al. Hypo-RESOLVE Consortium . The impact of hypoglycaemia on quality of life outcomes among adults with type 1 diabetes: a systematic review. Diabetes Res Clin Pract 2021;174. https://doi.org/10.1016/j.diabres.2021.108752.
- Coolen M, Broadley M, Hendrieckx C, Chatwin H, Clowes M, Heller S, et al. Hypo-RESOLVE Consortium . The impact of hypoglycemia on quality of life and related outcomes in children and adolescents with type 1 diabetes: a systematic review. PLOS ONE 2021;16. https://doi.org/10.1371/journal.pone.0260896.
- Jensen MV, Broadley M, Speight J, Scope A, Preston L, Heller S, et al. Hypo-RESOLVE Consortium . The impact of hypoglycaemia on the quality of life of family members of adults with type 1 or type 2 diabetes: a qualitative systematic review. Diabet Med 2021;38. https://doi.org/10.1111/dme.14666.
- Matlock KA, Broadley M, Hendrieckx C, Clowes M, Sutton A, Heller SR, et al. Hypo-RESOLVE consortium . Changes in quality of life following hypoglycaemia in adults with type 2 diabetes: a systematic review of longitudinal studies. Diabet Med 2022;39. https://doi.org/10.1111/dme.14706.
- Foos V, McEwan P. Conversion of hypoglycemia utility decrements from categorical units reflecting event history into event specific disutility scores applicable to diabetes decision models. Paper presented at 23rd Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research, ISPOR 2018. Baltimore, MD United States. Value Health 2018;21.
- Evans M, Khunti K, Mamdani M, Galbo-Jørgensen CB, Gundgaard J, Bøgelund M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-90.
- Yfantopoulos J, Chantzaras A. Health-related quality of life and health utilities in insulin-treated type 2 diabetes: the impact of related comorbidities/complications. Eur J Health Econ 2020;21:729-43. https://doi.org/10.1007/s10198-020-01167-y.
- Zhang Y, Wu J, Chen Y, Shi L. EQ-5D-3L Decrements by diabetes complications and comorbidities in China. Diabetes Ther 2020;11:939-50. https://doi.org/10.1007/s13300-020-00788-z.
- Nauck MA, Buse JB, Mann JFE, Pocock S, Bosch-Traberg H, Frimer-Larsen H, et al. LEADER Publication Committee for the LEADER Trial Investigators . Health-related quality of life in people with type 2 diabetes participating in the LEADER trial. Diabetes Obes Metab 2019;21:525-32. https://doi.org/10.1111/dom.13547.
- Briggs AH, Bhatt DL, Scirica BM, Raz I, Johnston KM, Szabo SM, et al. Health-related quality-of-life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the Saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus (SAVOR)-TIMI 53 trial. Diabetes Res Clin Pract 2017;130:24-33. https://doi.org/10.1016/j.diabres.2016.12.019.
- Pratipanawatr T, Satirapoj B, Ongphiphadhanakul B, Suwanwalaikorn S, Nitiyanant W. Impact of hypoglycemia on health-related quality of life among type 2 diabetes: a cross-sectional study in Thailand. J Diabetes Res 2019;2019. https://doi.org/10.1155/2019/5903820.
- Christensen B, Ranjan AG, Rytter K, McCarthy OM, Schmidt S, Nørgaard K. Automated insulin delivery use in adults with type 1 diabetes (T1D) treated with insulin pump and continuous glucose monitoring (CGM) but not meeting glycemic targets: a randomized controlled trial [published online ahead of print 11 April 2024]. J Diabetes Sci Technol 2024.
- Lee TTM, Collett C, Bergford S, Hartnell S, Scott EM, Lindsay RS, et al. AiDAPT Collaborative Group . Automated insulin delivery in women with pregnancy complicated by type 1 diabetes. N Engl J Med 2023;389:1566-78. https://doi.org/10.1056/NEJMoa230391.
- Beunen K, Van Wilder N, Ballaux D, Vanhaverbeke G, Taes Y, Aers XP, et al. Closed-loop insulin delivery in pregnant women with type 1 diabetes (CRISTAL): a multicentre randomized controlled trial–study protocol. BMC Pregnancy Childbirth 2023;23.
- Hásková A, Radovnická L, Petruželková L, Parkin CG, Grunberger G, Horová E, et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care 2020;43:2744-50.
- Reddy M, Jugnee N, Anantharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Technol Ther 2018;20:751-7.
- Visser MM, Charleer S, Fieuws S, De Block C, Hilbrands R, Van Huffel L, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet 2021;397:2275-83.
- Xu XM, Vestesson E, Paley L, Desikan A, Wonderling D, Hoffman A, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J 2018;3:82-91. https://doi.org/10.1177/2396987317746516.
- Meands C, Hyde C. What is the cost of blindness?. Br J Ophthalmol 2003;87:1201-4.
- Geelhoed-Duijvestijn PH, Pedersen-Bjergaard U, Weitgasser R, Lahtela J, Jensen MM, Östenson CG. Effects of patient-reported non-severe hypoglycemia on healthcare resource use, work-time loss, and wellbeing in insulin-treated patients with diabetes in seven European countries. J Med Econ 2013;16:1453-61. https://doi.org/10.3111/13696998.2013.852098.
- Orozco-Beltrán D, Mezquita-Raya P, Ramírez de Arellano A, Galán M. Self-reported frequency and impact of hypoglycemic events in Spain. Diabetes Ther 2014;5:155-68. https://doi.org/10.1007/s13300-014-0057-z.
- Slover R, Pihoker C, Shulman D, Kaiserman K, Liljenquist D, Sherr J, et al. Safety and glycemic control during the Medtronic advanced hybrid closed-loop (Ahcl) pivotal trial in youth aged 7–17 years with type 1 diabetes (T1d). Diabetes Technol Ther 2022;24. https://doi.org/10.1177/2164957X221096590.
- Breton MD, Cherñavvsky DR, Forlenza GP, DeBoer MD, Robic J, Wadwa RP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial Pancreas Ski study. Diabetes Care 2017;40:1644-50. https://doi.org/10.2337/dc17-0883.
- Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275-80. https://doi.org/10.2337/dc18-1581.
Appendix 1 Hybrid closed-loop models, systems and apps
MiniMed 670G
MiniMed 670G (Medtronic) is a CE-marked HCL system that uses a control algorithm called SmartGuard. SmartGuard technology has a manual mode and an auto-mode. In manual mode, the 670G works just like other SAP systems. In auto-mode function, blood glucose data measured by the CGM (Guardian sensor) is sent wirelessly to the insulin pump (670G) to enable adjustment of basal insulin every 5 minutes to maintain sensor glucose levels near a target glucose of 120 mg/dl (6.7 mmol/l). The system requires some user interaction to administer mealtime bolus doses. The 670G is not licensed for use in children under 7 years old. The device is also not to be used in people who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
MiniMed 780G
MiniMed 780G (Medtronic) is a CE-marked HCL system that was launched in 2020. It has a more advanced algorithm than that used in the 670G system and has Bluetooth connectivity. The system includes different glucose targets according to users’ needs. In addition to the target glucose of 120 mg/dl (6.7 mmol/l), users can select to achieve a tighter glucose target of 5.5–6.1 mmol/l. In contrast to its predecessor system, the 780G has an ‘autocorrection feature’ that delivers correction boluses automatically when sustained hyperglycaemia is detected. This requires minimal user or carer interaction. The CGM (Guardian sensor) is connected to the MiniMed mobile app via Bluetooth, which optionally automatically uploads data to the CareLink connect system to notify carers or for clinician review. The 780G is not licensed for use in children under 7 years or in people who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Control IQTM
Control-IQ (Tandem Diabetes Care) is a CE-marked system that combines t:slimX2TM insulin pump and Control-IQ technology. This system can be interlinked with a compatible CGM to form a HCL system that suspends insulin delivery in response to predicted hypoglycaemia or gives a correction bolus in response to predicted hyperglycaemia. Control-IQ has six settings, including optional settings for sleep and exercise, to adjust basal insulin delivery depending on user need. Mealtime bolus doses are administered manually. Data from Control-IQ can be uploaded on the Diasend or Tidepool data clouds for clinician review. Control-IQ is not licensed for use in children under 6 years or in people who require less than a total daily insulin dose of 10 units per day or who weigh < 55 pounds, as those are the required minimum values the system needs to operate safely.
CamAPS FXTM
CamAPS FX (CamDiab) is a CE-marked Android app developed at the University of Cambridge. The app can be interlinked with a compatible CGM (DexcomTM G6) and insulin pump (DanaTM RS or Dana-I) to form a HCL system. CamAPS FX can operate on an auto-mode ‘off’ whereby basal insulin delivery is pre-programmed by the user or on an auto-mode ‘on’ whereby insulin delivery is directed by the app. In auto-mode ‘on’, a bolus dose calculator embedded in the app allows the user to initiate the delivery of mealtime insulin dose. If the auto-mode ‘on’ feature is prevented from coming on, an auto-mode ‘attempting’ feature is initiated to revert insulin delivery to pre-programmed basal rates. Data from CamAPS FX can be uploaded to the Diasend data cloud for clinician review. CamAPS FX is licensed for use in people aged ≥ 1 year and in pregnancy; however, other age restrictions may apply depending on the chosen CGM and insulin pump.
Appendix 2 Literature search strategies
Record of searches: clinical effectiveness
Overview
| Database/website | Date searched (date updated) | Number of records + update number of records = total |
|---|---|---|
| MEDLINE ALL (via Ovid) | 31 March 2021 (11 April 2022) | 1914 + 789 = 2703 |
| EMBASE (via Ovid) | 31 March 2021 (11 April 2022) | 4267 + 1210 = 5477 |
| Science Citation Index & Conference Proceedings - Science (Web of Science) | 31 March 2021 (12 April 2022) | 2190 + 514 = 2704 |
| Cochrane Library (Wiley) | 31 March 2021 (12 April 2022) | 1327 [all CENTRAL, 0 CDSR] + 159 [all CENTRAL, 0 CDSR] = 1486 |
| ClinicalTrials.gov | 12 April 2021 (12 April 2022) | 392 + 57 = 449 |
| HTA database (CRD) | 7 April 2021 | 16a |
| International HTA database (INAHTA) | 7 April 2021 (6 April 2022) | 22 + 10 = 32 |
| NIHR Journals Library | 12 April 2021 (12 April 2022) | 5 + 1 = 6 |
| AHRQ website | 12 April 2021 (6 April 2022) | 1 + 0 + 1 |
| CADTH website | 12 April 2021 (7 April 2022) | 14 + 2 = 16 |
| SBU website | 12 April 2021 (7 April 2022) | 0 + 0 = 0 |
Note: The WHO International Clinical Trials Registry Platform (ICTRP) was not searched because it was unavailable between 12 April 2021 and 22 April 2021.
Total results: 10,148 + 2742 from update = 12,890
Total after 4211 duplicates removed + 1005 duplicates within update results + 382 duplicates with original results removed = 7292
Also searched for background information about HCL technologies:
| Website | Date searched | Number of records |
|---|---|---|
| FDA devices databases | 21 April 2021 | 12 |
| MHRA (via www.gov.uk) | 22 April 2021 | 7 |
Search strategies:
Note: see below each database strategy for details of update searches.
MEDLINE (via Ovid)
Date searched: 31 March 2021
Database: Ovid MEDLINE(R) ALL 1946 to 30 March 2021
Search strategy
-
Diabetes Mellitus, Type 1/ (77,349)
-
Diabetic Ketoacidosis/ (6613)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ab,kf,ti. (56,549)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ab,kf,ti. (28,252)
-
((insulin$ adj2 depend$) or insulindepend$).ab,kf,ti. (33,812)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ab,kf,ti. (23,572)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis or dka).ab,kf,ti. (11,574)
-
Hyperglycemia/ (28,751)
-
Hypoglycemia/ (27,924)
-
(hyperglyc?em$ or hypoglyc?em$).ab,kf,ti. (116,536)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ab,kf,ti. (151,415)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [population: T1DM] (365,002)
-
Pancreas, Artificial/ (816)
-
closed loop.ab,kf,ti. (10,516)
-
(artificial adj2 (pancreas or beta cell$)).ab,kf,ti. (1729)
-
(bionic adj2 pancreas).ab,kf,ti. (25)
-
(Automat$ adj2 (insulin deliver$ or insulin dosing or glucose control$ or glyc?emic control$)).ab,kf,ti. (285)
-
((minimed or medtronic) and (670G or 780G)).ab,kf,ti. (57)
-
(tslim or t slim or control iq or camAPS or camdiab or dexcom G6 or dexcom G7 or smartguard or smart guard or diabeloop or dblg1 or ilet or beta bionics or (omnipod and horizon) or (mylife and loop) or (tidepool and loop) or bigfoot or anydana loop).ab,kf,ti. (175)
-
13 or 14 or 15 or 16 or 17 or 18 or 19 [Intervention: closed loop systems] (12,163)
-
(sensor? adj3 (augment$ or integrat$ or pump? or insulin)).ab,kf,ti. (7798)
-
SAPT.ab,kf,ti. (533)
-
predictive low glucose.ab,kf,ti. (95)
-
basal iq.ab,kf,ti. (9)
-
((minimed or medtronic) and 640G).ab,kf,ti. (33)
-
(paradigm$ adj3 (veo or pump$)).ab,kf,ti. (57)
-
(veo adj3 pump$).ab,kf,ti. (9)
-
(g4 adj3 platinum).ab,kf,ti. (58)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ab,kf,ti. (14)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 [sensor augmented pumps] (8467)
-
Insulin Infusion Systems/ (5477)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ab,kf,ti. (14,806)
-
(pump$ adj2 (therap$ or treatment$)).ab,kf,ti. (3223)
-
((subcutaneous adj2 insulin$) or CSII).ab,kf,ti. (3863)
-
(minimed or dana diabecare or dana R or dana RS or kaleido or omnipod or medtrum or touchcare or ypsopump or cellnovo).ab,kf,ti. (376)
-
(medtronic adj3 (pump$ or system$ or deliver$)).ab,kf,ti. (719)
-
(tandem adj3 (pump$ or system$ or deliver$)).ab,kf,ti. (925)
-
((accu-chek or accuchek) adj3 (pump$ or system$ or deliver$ or combo or insight or solo)).ab,kf,ti. (34)
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 [insulin pumps/CSII] (20,952)
-
((continu$ or flash or intermittent$ or sensor or sensors or real time) adj4 glucose adj4 (monitor$ or measurement$)).ab,kf,ti. (5859)
-
(glucose adj (sensor$ or sensing)).ab,kf,ti. (4186)
-
(CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS).ab,kf,ti. (4526)
-
(dexcom or freestyle or libre or enlite or (guardian and (medtronic or sensor)) or eversense or glucomen day).ab,kf,ti. (2410)
-
40 or 41 or 42 or 43 [continuous or flash glucose monitors] (13,031)
-
(2014082* or 2014083* or 201409* or 201410* or 201411* or 201412* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).dt,ez,da. [added to database since search for previous DAR in 2014] (8,960,844)
-
12 and 20 and 45 [T1DM and closed loop + date limit] (1134)
-
12 and 30 and 45 [T1DM and SAPT + date limit] (498)
-
12 and 39 and 44 and 45 [T1DM and pumps and GMs + date limit] (1090)
-
46 or 47 or 48 (1951)
-
limit 49 to english language (1903)
-
exp Pregnancy/ (912,957)
-
exp Pregnancy Complications/ (435,723)
-
Perinatal Care/ or Preconception Care/ or Prenatal Care/ (35,143)
-
exp Cesarean Section/ (46,694)
-
Pregnant Women/ (9180)
-
(pregnan$ or ante natal$ or antenatal$ or pre natal$ or prenatal$ or (expectant$ adj2 mother$) or “mother? to be” or matern$ or conception$ or preconception$ or “trying to conceive” or prepregnan$ or periconception$ or giving birth or childbirth$ or labo?r or newborn$ or new born$ or neonat$ or neo nat$ or baby or babies).ab,kf,ti. (1,208,728)
-
(miscarr$ or abort$ or cesarean or caesarean or c section$ or (prematur$ and (birth$ or rupture$ or infant$)) or preterm or pre term or prematurity or prom or macrosomia$ or birth weight$ or birthweight$ or eclamp$ or preeclamp$ or stillbirth$ or still birth$ or stillborn$ or still born$).ab,kf,ti. (352,238)
-
(perinatal or peri natal or fetal or foetal or intrauterine or intra uterine).ab,kf,ti. (364,876)
-
apgar.ab,kf,ti. (12,586)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 [pregnancy, planning pregnancy, pregnancy complications; broad] (1,735,176)
-
exp Insulin/ and Injections, Subcutaneous/ (2455)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (1309)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (563)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (10,207)
-
MDI.ti,ab,kf. (3832)
-
(injection adj3 therapy).ti,ab,kf. (4196)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,kf. (1376)
-
(short acting adj3 insulin).ti,ab,kf. (576)
-
(rapid acting adj3 insulin).ti,ab,kf. (799)
-
or/61-69 [insulin injections] (21,919)
-
Blood Glucose Self-Monitoring/ (7126)
-
Blood Glucose/ (167,907)
-
(blood glucos$ or blood sugar$).ab,kf,ti. (87,354)
-
72 or 73 (210,595)
-
(self monitor$ or test$ strip$ or finger prick$ or fingerprick$ or finger stick$ or fingerstick$ or lancet? or meter?).ab,kf,ti. (43,222)
-
(capillary adj4 (test$ or measur$)).ab,kf,ti. (5082)
-
75 or 76 (47,993)
-
74 and 77 (5789)
-
SMBG.ab,kf,ti. (1195)
-
glucometer$.ab,kf,ti. (1146)
-
71 or 78 or 79 or 80 [self monitoring of blood glucose] (11,381)
-
44 and 70 [continuous or flash GMs AND MDI] (488)
-
81 and 39 [SMBG AND CSII] (1709)
-
82 or 83 (2022)
-
12 and 60 and 84 and 45 [T1DM and pregnancy and any of the comparator groups specific to this population + date limit] (55)
-
limit 85 to english language (54)
-
50 or 86 (1914)
Update
Date searched: 11 April 2022
Re-ran above search with search line 45 altered to:
45 (“20210331” or 202104* or 202105* or 202106* or 202107* or 202108* or 202109* or 202110* or 202111* or 202112* or 2022*).dt,ez,da. [added to database since original MTA search in March 2021]
Total:
87 50 or 86 (789)
Search strings used in the previous technology assessment on integrated sensor-augmented pump therapy systems were used as the basis for developing selected lines relating to type 1 diabetes, insulin pumps, sensor augmented pumps and multiple daily injections:
Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20(17). https://doi.org/10.3310/hta20170
The following were used as sources of search terms for pregnancy and related concepts:
Tessier V. Périnatalité: Perinatality: Rappel favorisé sur la précision. Canadian Health Libraries Association – Association des bibliothèques de la santé du Canada; 2017. URL: https://extranet.santecom.qc.ca/wiki/!biblio3s/doku.php?id=concepts:perinatalite (accessed 26 April 2021).
Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi M, Athanasiou A, Martin‐Hirsch PPL, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2015;9:CD008478. https://doi.org/10.1002/14651858.CD008478.pub2
Cochrane Pregnancy and Childbirth’s Trials Register: Detailed Search Methods Used to Maintain and Update the Specialised Register. 2018. URL: https://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/public/uploads/cochrane_pregnancy_and_childbirth_search_methods_2018_1.docx (accessed 26 April 2021).
EMBASE (via Ovid)
Date searched: 31 March 2021
Database: EMBASE 1974 to 30 March 2021
Search strategy
-
insulin dependent diabetes mellitus/ (120,636)
-
diabetic ketoacidosis/ (13,211)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ab,kw,ti. (89,362)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ab,kw,ti. (39,641)
-
((insulin$ adj2 depend$) or insulindepend$).ab,kw,ti. (42,438)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ab,kw,ti. (41,350)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis or dka).ab,kw,ti. (17,665)
-
hypoglycemia/ or insulin hypoglycemia/ or nocturnal hypoglycemia/ or hyperglycemia/ (169,981)
-
(hyperglyc?em$ or hypoglyc?em$).ab,kw,ti. (171,413)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ab,kw,ti. (219,463)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 [population: T1DM] (552,812)
-
exp artificial pancreas/ (2518)
-
“glucose monitoring/insulin pump system”/ (19)
-
closed loop.ab,kw,ti. (13,542)
-
(artificial adj2 (pancreas or beta cell$)).ab,kw,ti. (2728)
-
(bionic adj2 pancreas).ab,kw,ti. (84)
-
(automat$ adj2 (insulin deliver$ or insulin dosing or glucose control$ or glyc?emic control$)).ab,kw,ti. (501)
-
((minimed or medtronic) and (670G or 780G)).ab,dm,dv,kw,ti. (204)
-
(tslim or t slim or control iq or camAPS or camdiab or dexcom G6 or dexcom G7 or smartguard or smart guard or diabeloop or dblg1 or ilet or beta bionics or (omnipod and horizon) or (mylife and loop) or (tidepool and loop) or bigfoot or anydana loop).ab,dm,dv,kw,ti. (452)
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 [Intervention: closed loop systems] (16,556)
-
(sensor? adj3 (augment$ or integrat$ or pump? or insulin)).ab,kw,ti. (9751)
-
SAPT.ab,kw,ti. (498)
-
predictive low glucose.ab,kw,ti. (216)
-
basal iq.ab,dm,dv,kw,ti. (35)
-
((minimed or medtronic) and 640G).ab,dm,dv,kw,ti. (162)
-
(paradigm$ adj3 (veo or pump$)).ab,dm,dv,kw,ti. (251)
-
(veo adj3 pump$).ab,dm,dv,kw,ti. (63)
-
(g4 adj3 platinum).ab,dm,dv,kw,ti. (215)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ab,dm,dv,kw,ti. (56)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 [sensor augmented pumps] (10,819)
-
insulin infusion/ (8355)
-
insulin pump/ or implantable insulin pump/ (7934)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ab,kw,ti. (23,686)
-
(pump$ adj2 (therap$ or treatment$)).ab,kw,ti. (6128)
-
((subcutaneous adj2 insulin$) or CSII).ab,kw,ti. (7275)
-
(minimed or dana diabecare or dana R or dana RS or kaleido or omnipod or medtrum or touchcare or ypsopump or cellnovo).ab,dm,dv,kw,ti. (1653)
-
(medtronic adj3 (pump$ or system$ or deliver$)).ab,dm,dv,kw,ti. (3028)
-
(tandem adj3 (pump$ or system$ or deliver$)).ab,dm,dv,kw,ti. (1170)
-
((accu-chek or accuchek) adj3 (pump$ or system$ or deliver$ or combo or insight or solo)).ab,dm,dv,kw,ti. (174)
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 [insulin pumps/CSII] (36,787)
-
((continu$ or flash or intermittent$ or sensor or sensors or real time) adj4 glucose adj4 (monitor$ or measurement$)).ab,kw,ti. (10,566)
-
(glucose adj (sensor$ or sensing)).ab,kw,ti. (5539)
-
(CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS).ab,kw,ti. (8864)
-
(dexcom or freestyle or libre or enlite or (guardian and (medtronic or sensor)) or eversense or glucomen day).ab,dm,dv,kw,ti. (4605)
-
41 or 42 or 43 or 44 [continuous or flash glucose monitors] (20,571)
-
11 and 20 [T1DM and closed loop] (4001)
-
11 and 30 [T1DM and SAPT] (1703)
-
11 and 40 and 45 [T1DM and pumps and GMs] (4215)
-
46 or 47 or 48 (7448)
-
limit 49 to dc=20140825-20210331 (4300)
-
limit 50 to english language (4177)
-
exp pregnancy/ (688,558)
-
exp pregnancy disorder/ (555,248)
-
exp cesarean section/ (101,840)
-
pregnant woman/ (87,032)
-
pregnancy outcome/ (63,986)
-
perinatal care/ or prepregnancy care/ or prenatal care/ (57,151)
-
(pregnan$ or ante natal$ or antenatal$ or pre natal$ or prenatal$ or (expectant$ adj2 mother$) or “mother? to be” or matern$ or conception$ or preconception$ or “trying to conceive” or prepregnan$ or periconception$ or giving birth or childbirth$ or labo?r or newborn$ or new born$ or neonat$ or neo nat$ or baby or babies).ab,kw,ti. (1,447,977)
-
(miscarr$ or abort$ or cesarean or caesarean or c section$ or (prematur$ and (birth$ or rupture$ or infant$)) or preterm or pre term or prematurity or prom or macrosomia$ or birth weight$ or birthweight$ or eclamp$ or preeclamp$ or stillbirth$ or still birth$ or stillborn$ or still born$).ab,kw,ti. (455,281)
-
(perinatal or peri natal or fetal or foetal or intrauterine or intra uterine).ab,kw,ti. (465,863)
-
apgar.ab,kw,ti. (19,929)
-
52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 [pregnancy, planning pregnancy, pregnancy complications; broad] (1,956,753)
-
blood glucose monitoring/ (28,256)
-
glucose blood level/ (263,683)
-
(blood glucos$ or blood sugar$).ab,kw,ti. (130,425)
-
64 or 65 (300,041)
-
self monitoring/ (8173)
-
(self monitor$ or test$ strip$ or finger prick$ or fingerprick$ or finger stick$ or fingerstick$ or lancet? or meter?).ab,kw,ti. (67,932)
-
(capillary adj4 (test$ or measur$)).ab,kw,ti. (6773)
-
67 or 68 or 69 (76,712)
-
66 and 70 (9965)
-
SMBG.ab,kw,ti. (2497)
-
glucometer$.ab,kw,ti. (2300)
-
63 or 71 or 72 or 73 [self monitoring of blood glucose] (35,552)
-
insulin/ and exp injection/ (5679)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (2612)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (783)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (15,088)
-
MDI.ab,kw,ti. (6716)
-
(injection adj3 therapy).ab,kw,ti. (6291)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ab,kw,ti. (2369)
-
(short acting adj3 insulin).ab,kw,ti. (969)
-
(rapid acting adj3 insulin).ab,kw,ti. (1412)
-
75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 [insulin injections/ MDI] (34,854)
-
45 and 84 [continuous or flash GMs AND MDI] (1390)
-
74 and 40 [SMBG AND CSII] (5410)
-
85 or 86 (6238)
-
11 and 62 and 87 [T1DM and pregnancy and any comparator group specific to the pregnancy population] (443)
-
limit 88 to dc=20140825-20210331 (240)
-
limit 89 to english language (233)
-
51 or 90 (4267)
Update
Date searched: 11 April 2022
Re-ran above search with search lines 50 and 89 altered to:
50 limit 49 to dc=20210331-20220411
89 limit 88 to dc=20210331-20220411
Total:
91 51 or 90 (1210)
Science Citation Index – Expanded & Conference Proceedings Citation Index - Science (via Web of Science)
Date searched: 31 March 2021
| #69 | 2190 | #68 OR #43 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
|---|---|---|
| # 68 | 43 | (#66 AND #48 AND #8) AND
LANGUAGE: (English) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 67 | 47 | #66 AND #48 AND #8 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 66 | 605 | #65 OR #64 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 65 | 248 | #55 AND #33 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 64 | 400 | #63 AND #38 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 63 | 6208 | #62 OR #61 OR #60 OR #59 OR #58 OR #57 OR #56 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 62 | 1189 | TS=(insulin* NEAR/0 inject*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 61 | 338 | TS=(“rapid acting” NEAR/3 insulin) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 60 | 137 | TS=(“short acting” NEAR/3 insulin) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 59 | 1994 | TS=(injection NEAR/3 therapy) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 58 | 2420 | TS=MDI Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 57 | 109 | TS=(“multiple dose” NEAR/3 (inject* OR insulin* OR regime* OR routine*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 56 | 737 | TS=(“multiple daily” NEAR/3 (inject* OR insulin* OR regime* OR routine*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 55 | 2407 | #54 OR #53 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 54 | 1088 | TS=(SMBG OR glucometer*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 53 | 1823 | #52 AND #49 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 52 | 57,400 | #51 OR #50 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 51 | 2658 | TS=(capillary NEAR/4 (test* OR measur*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 50 | 54,859 | TS=(“self monitor*” OR “test* strip*” OR “finger prick*” OR fingerprick* OR “finger stick*” OR fingerstick* OR lancet* OR meter*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 49 | 32,964 | TS=(“blood glucos*” OR “blood sugar*”) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 48 | 450,041 | #47 OR #46 OR #45 OR #44 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 47 | 3630 | TS=apgar Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 46 | 103,621 | TS=(perinatal OR “peri natal” OR fetal OR foetal OR intrauterine OR “intra uterine”) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 45 | 124,549 | TS=(miscarr* OR abort* OR cesarean OR caesarean OR “c section*” OR (prematur* AND (birth* OR rupture* OR infant*) ) OR preterm OR “pre term” OR prematurity OR prom OR macrosomia* OR “birth weight*” OR birthweight* OR eclamp* OR preeclamp* OR stillbirth* OR “still birth*” OR stillborn* OR “still born*”) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 44 | 379,961 | TS=(pregnan* OR “ante natal*” OR antenatal* OR “pre natal*” OR prenatal* OR (expectant* NEAR/2 mother*) OR “mother* to be” OR matern* OR conception* OR preconception* OR “trying to conceive” OR prepregnan* OR periconception* OR “giving birth” OR childbirth* OR labo*r OR newborn* OR “new born*” OR neonat* OR “neo nat*” OR baby OR babies) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 43 | 2175 | (#41 OR #40 OR #39) AND
LANGUAGE: (English) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 42 | 2255 | #41 OR #40 OR #39 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 41 | 983 | #38 AND #33 AND #8 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 40 | 593 | #25 AND #8 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 39 | 1445 | #15 AND #8 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 38 | 14,694 | #37 OR #36 OR #35 OR #34 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 37 | 1701 | TS=(dexcom OR freestyle OR libre OR enlite OR (guardian AND (medtronic OR sensor) ) OR eversense OR “glucomen day”) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 36 | 7203 | TS=(CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 35 | 4043 | TS=(glucose NEAR/0 (sensor* OR sensing) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 34 | 4292 | TS=((continu* OR flash OR intermittent* OR sensor OR sensors or “real time”) NEAR/4 glucose NEAR/4 (monitor* OR measurement*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 33 | 9131 | #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 32 | 26 | TS=((accu-chek OR accuchek) NEAR/3 (pump* OR system* OR deliver* OR combo OR insight OR solo) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 31 | 1121 | TS=(tandem NEAR/3 (pump* OR system* OR deliver*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 30 | 310 | TS=(medtronic NEAR/3 (pump* OR system* OR deliver*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 29 | 232 | TS=(minimed OR “dana diabecare” OR “dana R” OR “dana RS” OR kaleido OR omnipod OR medtrum OR touchcare OR ypsopump OR cellnovo) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 28 | 1748 | TS=((subcutaneous NEAR/2 insulin*) OR CSII) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 27 | 2715 | TS=(pump* NEAR/2 (therap* OR treatment*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 26 | 5555 | TS=(insulin* NEAR/3 (pump* OR infus* OR deliver* OR catheter*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 25 | 14,388 | #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 24 | 12 | TS=((animas OR vibe) NEAR/3 (pump* OR infus* OR system*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 23 | 53 | TS=(g4 NEAR/3 platinum) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 22 | 7 | TS=(veo NEAR/3 pump*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 21 | 40 | TS=(paradigm* NEAR/3 (veo OR pump*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 20 | 45 | TS=((minimed OR medtronic) AND 640G) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 19 | 12 | TS=“basal iq” Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 18 | 115 | TS=“predictive low glucose” Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 17 | 440 | TS=SAPT Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 16 | 13,776 | TS=(sensor$ NEAR/3 (augment* OR integrat* OR pump$ OR insulin) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 15 | 42,226 | #14 OR #13 OR #12 OR #11 OR #10 OR #9 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 14 | 177 | TS=(tslim OR “t slim” OR “control iq” OR camAPS OR camdiab OR “dexcom G6” OR “dexcom G7” OR smartguard OR “smart guard” OR diabeloop OR dblg1 OR ilet OR “beta bionics” OR (omnipod AND horizon) OR (mylife AND loop) OR (tidepool AND loop) OR bigfoot OR “anydana loop”) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 13 | 88 | TS=((minimed OR medtronic) AND (670G OR 780G) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 12 | 258 | TS=(automat* NEAR/2 (“insulin deliver*” OR “insulin dosing” OR “glucose control*” OR “glyc$emic control*”) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 11 | 124 | TS=(bionic NEAR/2 pancreas) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 10 | 1299 | TS=(artificial NEAR/2 (pancreas OR “beta cell*”) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 9 | 41,216 | TS=“closed loop” Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 8 | 146,413 | #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 7 | 78,894 | TS=((high OR higher OR low OR lower OR increas* OR decreas* OR deficien* OR sufficien* OR insufficien* OR reduce* OR reduction* OR fluctuat* OR fallen OR falling OR threshold OR safe) NEAR/3 (glucose* OR sugar* OR hba1c OR “hb a1” OR hba1 OR a1c OR h$emoglob* OR glycoh$emoglob*) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 6 | 47,313 | TS=(hyperglyc$em* OR hypoglyc$em*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 5 | 4801 | TS=(ketoacidosis OR acidoketosis OR “keto acidosis” OR ketoacidemia OR ketosis OR dka) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 4 | 11,210 | TS=(dm1 OR “dm 1” OR dmt1 OR “dm t1” OR t1dm OR “t1 dm” OR t1d OR iddm) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 3 | 3716 | TS=((insulin* NEAR/2 depend*) OR insulindepend*) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 2 | 11,031 | TS=(diabet* NEAR/3 (britt* OR juvenil* OR pediatric OR paediatric OR early OR keto* OR labil* OR acidos* OR autoimmun* OR “auto immun*” OR “sudden onset”) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
| # 1 | 27,913 | TS=(diabet* NEAR/3 (“typ* 1” OR “typ* i” OR type1 OR typei OR “typ* one”) ) Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2021 |
Update
Date searched: 12 April 2022
Original search above was not fully saved in Web of Science because it is over 40 lines, so strategy was re-entered using fewer lines (one line for each concept), combined as above and run with Timespan altered to:
Timespan: 2021-03-31 to 2022-04-12 (Index Date)
Total: 514
The Ovid MEDLINE search strategy was translated for use in Web of Science with the aid of the Polyglot Search Translator:
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. https://doi.org/10.5195/jmla.2020.834
Cochrane Database of Systematic Reviews (CDSR) & Cochrane Central Register of Controlled Trials (CENTRAL) (via Wiley Cochrane Library)
Date searched: 31 March 2021
Search interface: www.cochranelibrary.com/advanced-search/search-manager
| #1 | [mh ^“Diabetes Mellitus, Type 1”] | 5614 |
|---|---|---|
| #2 | [mh ^“Diabetic Ketoacidosis”] | 139 |
| #3 | (diabet* NEAR/3 ((typ* NEXT 1) OR (typ* NEXT i) OR type1 OR typei OR (typ* NEXT one))):ti,ab,kw | 10,200 |
| #4 | (diabet* NEAR/3 (britt* OR juvenil* OR pediatric OR paediatric OR early OR keto* OR labil* OR acidos* OR autoimmun* OR (auto NEXT immun*) OR “sudden onset”)):ti,ab,kw | 3429 |
| #5 | ((insulin* NEAR/2 depend*) OR insulindepend*):ti,ab,kw | 22,663 |
| #6 | (dm1 OR (dm NEXT 1) OR dmt1 OR (dm NEXT t1) OR t1dm OR “t1 dm” OR t1d OR iddm):ti,ab,kw | 3481 |
| #7 | (ketoacidosis OR acidoketosis OR “keto acidosis” OR ketoacidemia OR ketosis OR dka):ti,ab,kw | 1174 |
| #8 | [mh ^Hyperglycemia] | 1952 |
| #9 | [mh ^Hypoglycemia] | 2258 |
| #10 | (hyperglyc?em* OR hypoglyc?em*):ti,ab,kw | 24,948 |
| #11 | ((high OR higher OR low OR lower OR increase* OR decreas* OR deficien* OR sufficien* OR insufficien* OR reduce* OR reduction* OR fluctuat* OR fallen OR falling OR threshold OR safe) NEAR/3 (glucose* OR sugar* OR hba1c OR (hb NEXT a1) OR hba1 OR a1c OR h?emoglob* OR glycoh?emoglob*)):ti,ab,kw | 23,784 |
| #12 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 | 59,772 |
| #13 | [mh ^“Pancreas, Artificial”] | 73 |
| #14 | “closed loop”:ti,ab,kw | 1264 |
| #15 | (artificial NEAR/2 (pancreas OR (beta NEXT cell*))):ti,ab,kw | 365 |
| #16 | (bionic NEAR/2 pancreas):ti,ab,kw | 47 |
| #17 | (automat* NEAR/2 ((insulin NEXT deliver*) OR “insulin dosing” OR (glucose NEXT control*) OR (glyc?emic NEXT control*))):ti,ab,kw | 117 |
| #18 | ((minimed OR medtronic) AND (670G OR 780G)):ti,ab,kw | 32 |
| #19 | (tslim OR “t slim” OR “control iq” OR camAPS OR camdiab OR “dexcom G6” OR “dexcom G7” OR smartguard OR “smart guard” OR diabeloop OR dblg1 OR ilet OR “beta bionics” OR (omnipod AND horizon) OR (mylife AND loop) OR (tidepool AND loop) OR bigfoot OR “anydana loop”):ti,ab,kw | 152 |
| #20 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 | 1564 |
| #21 | (sensor? NEAR/3 (augment* OR integrat* OR pump? OR insulin)):ti,ab,kw | 838 |
| #22 | SAPT:ti,ab,kw | 48 |
| #23 | “predictive low glucose”:ti,ab,kw | 63 |
| #24 | “basal iq”:ti,ab,kw | 11 |
| #25 | ((minimed OR medtronic) AND 640G):ti,ab,kw | 30 |
| #26 | (paradigm* NEAR/3 (veo OR pump*)):ti,ab,kw | 42 |
| #27 | (veo NEAR/3 pump*):ti,ab,kw | 24 |
| #28 | (g4 NEAR/3 platinum):ti,ab,kw | 39 |
| #29 | ((animas OR vibe) NEAR/3 (pump* OR infus* OR system*)):ti,ab,kw | 17 |
| #30 | #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 | 984 |
| #31 | [mh ^“Insulin Infusion Systems”] | 669 |
| #32 | (insulin* NEAR/3 (pump* OR infus* OR deliver* OR catheter*)):ti,ab,kw | 4129 |
| #33 | (pump* NEAR/2 (therap* OR treatment*)):ti,ab,kw | 1666 |
| #34 | ((subcutaneous NEAR/2 insulin*) OR CSII):ti,ab,kw | 1528 |
| #35 | (minimed OR “dana diabecare” OR “dana R” OR “dana RS” OR kaleido OR omnipod OR medtrum OR touchcare OR ypsopump OR cellnovo):ti,ab,kw | 203 |
| #36 | (medtronic NEAR/3 (pump* OR system* OR deliver*)):ti,ab,kw | 214 |
| #37 | (tandem NEAR/3 (pump* OR system* OR deliver*)):ti,ab,kw | 57 |
| #38 | ((accu-chek OR accuchek) NEAR/3 (pump* OR system* OR deliver* OR combo OR insight OR solo)):ti,ab,kw | 17 |
| #39 | #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 | 5680 |
| #40 | ((continu$ or flash or intermittent$ or sensor or sensors or real time) NEAR/4 glucose NEAR/4 (monitor* OR measurement*)):ti,ab,kw | 625 |
| #41 | (glucose NEXT (sensor? OR sensing)):ti,ab,kw | 348 |
| #42 | (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS):ti,ab,kw | 2033 |
| #43 | (dexcom OR freestyle OR libre OR enlite OR (guardian AND (medtronic OR sensor)) OR eversense OR “glucomen day”):ti,ab,kw | 1563 |
| #44 | #40 OR #41 OR #42 OR #43 | 3621 |
| #45 | #12 AND #20 | 861 |
| #46 | #12 AND #30 | 556 |
| #47 | #12 AND #39 AND #44 | 853 |
| #48 | #45 OR #46 OR #47 | 1520 |
| #49 | #45 OR #46 OR #47 with Limits: Cochrane Library publication date from Sep 2014 to Apr 2021 |
1319 |
| #50 | [mh Pregnancy] | 22,393 |
| #51 | [mh “Pregnancy Complications”] | 12,074 |
| #52 | [mh ^“Perinatal Care”] OR [mh ^”Preconception Care”] OR [mh ^”Prenatal Care”] | 1792 |
| #53 | [mh “Cesarean Section”] | 3153 |
| #54 | [mh ^“Pregnant Women”] | 297 |
| #55 | (pregnan* OR (ante NEXT natal*) OR antenatal* OR (pre NEXT natal*) OR prenatal* OR (expectant* NEAR/2 mother*) OR (mother? NEAR/2 “to be”) OR matern* OR conception* OR preconception* OR “trying to conceive” OR prepregnan* OR periconception* OR “giving birth” OR childbirth* OR labo?r OR newborn* OR (new NEXT born*) OR neonat* OR (neo NEXT nat*) OR baby OR babies):ti,ab,kw | 107,835 |
| #56 | (miscarr* OR abort* OR cesarean OR caesarean OR (c NEXT section*) OR (prematur* AND (birth* OR rupture* OR infant*)) OR preterm OR “pre term” OR prematurity OR prom OR macrosomia* OR (birth NEXT weight*) OR birthweight* OR eclamp* OR preeclamp* OR stillbirth* OR (still NEXT birth*) OR stillborn* OR (still NEXT born*)):ti,ab,kw | 46,780 |
| #57 | (perinatal OR “peri natal” OR fetal OR foetal OR intrauterine OR “intra uterine”):ti,ab,kw | 21,877 |
| #58 | apgar:ti,ab,kw | 4463 |
| #59 | #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 | 122,190 |
| #60 | [mh Insulin] AND [mh ^“Injections, Subcutaneous”] | 454 |
| #61 | (“multiple daily” NEAR/3 (inject* OR insulin* OR regime* OR routine*)):ti,ab,kw | 714 |
| #62 | (“multiple dose” NEAR/3 (inject* OR insulin* OR regime* OR routine*)):ti,ab,kw | 249 |
| #63 | (multiple NEAR/3 (inject* OR insulin* OR regime* OR routine*)):ti,ab,kw | 2186 |
| #64 | MDI:ti,ab,kw | 2986 |
| #65 | (injection NEAR/3 therapy):ti,ab,kw | 2610 |
| #66 | ((basal* AND bolus) NEAR/3 (injection* OR regime* OR routine* OR system*)):ti,ab,kw | 3745 |
| #67 | (“short acting” NEAR/3 insulin):ti,ab,kw | 363 |
| #68 | (“rapid acting” NEAR/3 insulin):ti,ab,kw | 417 |
| #69 | #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 | 11,689 |
| #70 | [mh ^“Blood Glucose Self-Monitoring”] | 805 |
| #71 | [mh ^“Blood Glucose”] | 16,258 |
| #72 | ((blood NEXT glucose*) OR (blood NEXT sugar*)):ti,ab,kw | 34,151 |
| #73 | #71 OR #72 | 34,151 |
| #74 | ((self NEXT monitor*) OR (test* NEXT strip*) OR (finger NEXT prick*) OR fingerprick* OR (finger NEXT stick*) OR fingerstick* OR lancet? OR meter?):ti,ab,kw | 14,651 |
| #75 | (capillary NEAR/4 (test* OR measur*)):ti,ab,kw | 600 |
| #76 | #74 OR #75 | 15,159 |
| #77 | #73 AND #76 | 2965 |
| #78 | SMBG:ti,ab,kw | 797 |
| #79 | glucometer*:ti,ab,kw | 401 |
| #80 | #70 OR #77 OR #78 OR #79 | 3438 |
| #81 | #44 AND #69 | 400 |
| #82 | #39 AND #80 | 513 |
| #83 | #81 OR #82 | 822 |
| #84 | #12 AND #59 AND #83 | 52 |
| #85 | #12 AND #59 AND #83 with Limits: Cochrane Library publication date from Sep 2014 to Apr 2021 |
44 |
| #86 | #49 OR #85 | 1327 |
| #87 | #49 OR #85 with Limits: Cochrane Library publication date from Sep 2014 to Apr 2021, in Cochrane Reviews and Cochrane Protocols |
0 |
| #88 | #49 OR #85 with Limits: Cochrane Library publication date from Sep 2014 to Apr 2021, in Trials |
1327 |
Update
Date searched: 12 April 2022
Re-ran above search with limit for search lines 49, 85, 87 and 88 altered to:
Cochrane Library publication date from April 2021 to April 2022
Results:
| #87 | #49 OR #85 with Limits: Cochrane Library publication date from Apr 2021 to Apr 2022, in Cochrane Reviews and Cochrane Protocols |
0 |
|---|---|---|
| #88 | #49 OR #85 with Limits: Cochrane Library publication date from Apr 2021 to Apr 2022, in Trials |
159 |
The Ovid MEDLINE search strategy was translated for use in the Cochrane Library with the aid of the Polyglot Search Translator:
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. https://doi.org/10.5195/jmla.2020.834
ClinicalTrials.gov
Date searched: 12 April 2021
Search interface: ‘Advanced search’ https://clinicaltrials.gov/ct2/search/advanced
| Original search | Results | Update | Results |
|---|---|---|---|
| “closed loop” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 190 | “closed loop” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 29 |
| “artificial pancreas” OR “artificial endocrine pancreas” OR “bionic pancreas” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 158 | “artificial pancreas” OR “artificial endocrine pancreas” OR “bionic pancreas” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 15 |
| “minimed 670G” OR “minimed 780G” OR “control iq” OR camaps OR camdiab OR “dexcom G6” OR “dexcom G7” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 83 | “minimed 670G” OR “minimed 780G” OR “control iq” OR camaps OR camdiab OR “dexcom G6” OR “dexcom G7” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 30 |
| “sensor augmented” OR SAPT OR “predictive low glucose” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 79 | “sensor augmented” OR SAPT OR “predictive low glucose” [other terms] | (diabetes AND “type 1”) OR hypoglycemia OR hyperglycemia [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 1 |
| insulin AND infusion AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 95 | insulin AND infusion AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 11 |
| insulin AND infusion AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 107 | insulin AND infusion AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 11 |
| (“insulin pump” OR “insulin pumps” OR “subcutaneous insulin”) AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 197 | (“insulin pump” OR “insulin pumps” OR “subcutaneous insulin”) AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 27 |
| (“insulin pump” OR “insulin pumps” OR “subcutaneous insulin”) AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 210 | (“insulin pump” OR “insulin pumps” OR “subcutaneous insulin”) AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 27 |
| CSII AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 39 | CSII AND (“glucose monitor” OR “glucose monitors” OR “glucose monitoring”) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 6 |
| CSII AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 42 | CSII AND (CGM OR CGMs OR FGM OR FGMs OR iCGM OR iCGMs OR rtCGM OR rtCGMS) [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 5 |
| (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND injection AND “self monitoring” [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 6 | (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND injection AND “self monitoring” [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 0 |
| (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND injection AND SMBG [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 4 | (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND injection AND SMBG [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 1 |
| (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND MDI AND SMBG [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 5 | (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND MDI AND SMBG [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 0 |
| (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND MDI AND “self monitoring” [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 01/01/2014 to 04/12/2021 | 5 | (pregnancy OR pregnant OR conception OR preconception OR childbirth OR fetus) AND MDI AND “self monitoring” [other terms] | diabetes AND “type 1” [condition or disease] | First posted from 04/12/2021 to 04/12/2022 | 0 |
| Total | 1220 | 163 | |
| Total after duplicate removal (using EndNote) | 392 | 57 |
Update
Date searched: 12 April 2022. For update search and numbers, see right-hand columns in original strategy table above. 57 new.
Health Technology Assessment database (via CRD website)
Date searched: 7 April 2021
Search interface: www.crd.york.ac.uk/CRDWeb/
| ((closed loop) OR (artificial NEAR2 pancreas) OR (bionic NEAR2 pancreas)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 2 |
| ((minimed or control iq or camAPS or camdiab or dexcom)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| ((sensor augmented) OR (SAPT)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| ((automat* NEAR2 (insulin OR glucose OR glycemic OR glycaemic))) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 0 |
| ((insulin NEAR2 (pump* OR infus*)) OR (subcutaneous NEAR2 insulin*) OR (CSII)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 10 |
| ((continu* or flash or intermittent* or sensor or sensors or real time) AND (glucose) AND (monitor* or measurement*)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 6 |
| ((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 3 |
| ((diabet* or insulin*) AND (pregn*) AND (injection* or MDI or self monitoring or SMBG)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| Total unique records | 16 |
No new records so update search not needed.
International HTA database (via INAHTA website)
Date searched: 7 April 2021
Search interface: Advanced search builder https://database.inahta.org/search/advanced
| (closed loop) FROM 2014 TO 2021 | 0 |
| (artificial pancreas) FROM 2014 TO 2021 | 2 |
| (bionic pancreas) FROM 2014 TO 2021 | 0 |
| (minimed OR “control iq” OR camAPS OR camdiab OR dexcom) FROM 2014 TO 2021 | 2 |
| (“Pancreas, Artificial”[mh]) FROM 2014 TO 2021 | 2 |
| (“sensor augmented”) FROM 2014 TO 2021 | 1 |
| (SAPT) FROM 2014 TO 2021 | 0 |
| (“Insulin Infusion Systems”[mh]) FROM 2014 TO 2021 | 7 |
| (insulin AND (pump* OR infusion* OR subcutaneous)) FROM 2014 TO 2021 | 8 |
| (CSII) FROM 2014 TO 2021 | 2 |
| ((continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*)) FROM 2014 TO 2021 | 15 |
| ((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) FROM 2014 TO 2021 | 7 |
| ((diabet* or insulin*) AND pregn* AND (injection* or MDI or “self monitoring” or SMBG)) FROM 2014 TO 2021 | 4 |
| Total | 50 |
| Total after duplicate removal (using EndNote) | 22 |
Update
Date searched: 6 April 2022
Re-ran search above search in one line with end date altered to 2022:
(((diabet* or insulin*) AND pregn* AND (injection* or MDI or “self monitoring” or SMBG)) FROM 2014 TO 2022) OR (((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) FROM 2014 TO 2022) OR (((continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*)) FROM 2014 TO 2022) OR ((CSII) FROM 2014 TO 2022) OR ((insulin AND (pump* OR infusion* OR subcutaneous)) FROM 2014 TO 2022) OR ((“Insulin Infusion Systems”[mh]) FROM 2014 TO 2022) OR ((SAPT) FROM 2014 TO 2022) OR ((“sensor augmented”) FROM 2014 TO 2022) OR ((“Pancreas, Artificial”[mh]) FROM 2014 TO 2022) OR ((minimed OR “control iq” OR camAPS OR camdiab OR dexcom) FROM 2014 TO 2022) OR ((bionic pancreas) FROM 2014 TO 2022) OR ((artificial pancreas) FROM 2014 TO 2022) OR ((closed loop) FROM 2014 TO 2022)
Total: 32
Notes: after checking several lines from the original search above and finding some of the new records were for HTAs published before 2021, it was decided that all 32 should be exported and deduplicated with the previous results in EndNote.
Total after deduplication in EndNote: 10
NIHR Journals Library
Date searched: 12 April 2021
Search interface: basic search www.journalslibrary.nihr.ac.uk/#/
| Search terms | Total results | Total at update | Number of new (not in previous results or sets), possibly relevant results |
|---|---|---|---|
| “closed loop” | 3 | 3 | 0 |
| “closed-loop” | 2 | 3 | 1 |
| “artificial pancreas” | 2 | 1 | 0 |
| “bionic pancreas” | 0 | 0 | 0 |
| Minimed | 5 | 5 | 0 |
| “Control IQ” | 0 | 0 | 0 |
| “Control-IQ” | 0 | 0 | 0 |
| camAPS | 0 | 1 | 0 |
| Camdiab | 0 | 0 | 0 |
| dexcom | 0 | 1 | 0 |
| “automated insulin delivery” | 0 | 0 | 0 |
| Total unique results, added since 2014 | 5 | 1 |
Update
Date searched: 12 April 2022. For numbers see right-hand column in original strategy table above. 1 new, 1 potentially relevant.
Agency for Healthcare Research and Quality website
Date searched: 12 April 2021
Search publications: www.ahrq.gov/research/publications/search.html
| Search terms | Total results | Comments | Total at update 04/22 | Comments at update 04/22 |
|---|---|---|---|---|
| closed loop | 0 | 0 | ||
| artificial pancreas | 0 | 0 | ||
| diabetes | 6 | 0 relevant | 6 (0 new) | |
| insulin | 0 | 0 |
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 0 new.
Search Evidence-Based Reports: www.ahrq.gov/research/findings/evidence-based-reports/search.html
| Search terms/method | Total results | Comments | Total at update 04/22 | Comments at update 04/22 |
|---|---|---|---|---|
| closed loop | 0 | 0 | ||
| artificial pancreas | 1 | 0 relevant; about pancreatic adenocarcinoma | 1 (0 new) | |
| Browsed Topic: Endocrine conditions | 25 reports, of which 10 published 2014–present | 0 relevant | 26 reports, of which 11 published 2014–present (1 new) | 0 relevant |
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 1 new, 0 relevant.
Full Research Reports: www.ahrq.gov/research/findings/final-reports/index.html
Checked 10 reports listed; none relevant.
Update. Checked again 6 April 2022. 0 new reports listed.
Technology Assessment Program: www.ahrq.gov/research/findings/ta/index.html Checked all reports and projects listed; none relevant.
Update. Checked again 6 April 2022. 0 new published reports listed. 1 new revised report listed, but not relevant.
Technology Assessment Archive (up to 2016): https://archive.ahrq.gov/clinic/techarch.htm
Used ctrl + F to search web page for:
-
diabet
-
closed
-
pancreas
-
insulin
-
glucose
-
- nothing relevant found
AHRQ Research Studies: www.ahrq.gov/research/findings/studies/index.html
| Search term | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| Closed loop | 4 | 0 relevant (all about closed loop communi-cation systems; not diabetes) | 5 (1 new) | 0 relevant (all about closed-loop communi-cation systems; not diabetes) |
| Artificial pancreas | 0 | 0 | ||
| Bionic pancreas | 0 | 0 | ||
| insulin delivery | 3 | 0 relevant | 0 | |
| minimed | 0 | 0 | ||
| control iq | 0 | 527 (technical changes to search likely) | See new search in row below | |
| control iq AND diabetes | – | – | 58 | Checked 2021 and 2022. None relevant |
| camAPS | 0 | 0 | ||
| camdiab | 0 | 0 | ||
| dexcom | 0 | 0 | ||
| insulin pump | 0 | 0 | ||
| insulin pumps | 0 | 0 | ||
| insulin infusion | 1 | 0 relevant | 1 (0 new) | |
| insulin infusions | 0 | 0 | ||
| CSII | 0 | 0 | ||
| glucose monitoring | 3 | 0 relevant (2 × type 2 diabetes, 1 about behaviour change) | 6 (3 new) | 0 relevant |
| glucose monitors | 0 | 0 | ||
| glucose monitor | 1 | 1 possibly relevant | 1 (0 new) | |
| flash | 0 | 0 | ||
| insulin AND injections | 0 | 0 | ||
| daily injections | 0 | 0 | ||
| blood glucose | 13 | 0 relevant; either type 2 diabetes, or not about self-monitoring | 15 (2 new) | 0 relevant |
| smbg | 0 | 0 | ||
| Total possibly relevant studies: | 1 | 0 | ||
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 6 new, 0 relevant.
Canadian Agency for Drugs and Technologies in Health website
Date searched: 12 April 2021
Search box on homepage www.cadth.ca/
Limit results by ‘Result Type: Reports; Projects in Progress’.
Sort by newest to oldest (to enable easy exclusion of pre-2014 records)
| Search terms | Total results | Number of new (not in previous sets), possibly relevant results | Total at update April 2022 | Number of new (not in previous results or sets), possibly relevant results |
|---|---|---|---|---|
| “closed loop” | 34 | 5 | 19 | 1 |
| artificial pancreas | 22 | 2 | 9 | 0 |
| bionic pancreas | 5 | 0 | 2 | 0 |
| automated insulin delivery | 18 | 0 | 10 | 0 |
| minimed | 16 | 1 | 5 | 0 |
| “control IQ” | 2 | 0 | 1 | 0 |
| camAPS | 0 | 0 | 0 | 0 |
| camdiab | 0 | 0 | 0 | 0 |
| Dexcom | 10 | 1 | 2 | 0 |
| “insulin pump” | 41 | 1 | 12 | 0 |
| “insulin infusion” | 51 | 0 | 5 | 0 |
| CSII | 23 | 0 | 3 | 0 |
| “glucose monitor” | 25 | 0 | 10 | 0 |
| “glucose monitoring” | 80 | 4 | 29 | 1 |
| “insulin injections” | 41 | 0 | 3 | 0 |
| “daily injections” | 43 | 0 | 8 | 0 |
| “self monitoring” AND glucose | 124 | 0 | 0 | 0 |
| SMBG | 31 | 0 | 5 | 0 |
| Total unique, possibly relevant results | 14 | 2 | ||
Update
Date searched: 7 April 2022. For numbers see right-hand column in original strategy table above. 2 new, 2 potentially relevant.
Note: assume website has been restructured or search interface/system changed since original search. Searched for words without quotation marks in ‘Contains all the words’ and terms in quotation marks in ‘Advanced Search’. Sorted by last updated and checked records for 2021 and 2022.
Swedish Agency for Health Technology Assessment and Assessment of Social Services website
Date searched: 12 April 2021
Search box on home page: www.sbu.se/en/
| Search terms/method | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| closed loop | 0 | 0 | ||
| artificial pancreas | 1 | not relevant; ‘dialysis for acute hepatic failure’ | 1 (0 new) | |
| bionic pancreas | 0 | 0 | ||
| diabetes > Filter on subject and publication type > Publication year From 2014 to 2021 | 30 | 0 relevant | 5 new | 0 relevant |
| insulin > Filter on subject and publication type > Publication year From 2014 to 2021 | 5 | 0 relevant | 1 new | 0 relevant |
| Total possibly relevant studies, published since 2014 | 0 | 0 | ||
Update
Date searched: 7 April 2022. For numbers see right-hand column in original strategy table above. 0 relevant.
US Food & Drug Administration Premarket Notification, Premarket Approval & De novo databases (via FDA website)
Date searched: 21 April 2021
Search interfaces:
-
devices@FDA (searches PMN-510(k) Premarket Notification and PMA-Premarket Approval databases) www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm
-
De novo database, ‘device name’ field www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/denovo.cfm
| Search terms | devices@FDA results | De novo database results | Documents downloaded (judged to contain potentially useful/relevant information not already identified in previous sets) |
|---|---|---|---|
| dexcom | 13 | 2 | 3 decision summaries, 1 classification order |
| control-IQ | 4 | 1 | 2 decision summaries, 1 classification order |
| control iq | Same results as control-IQ | 0 | |
| t:slim | 0 | 1 | 1 decision summary, 1 classification order |
| t slim | 3 | 1 | 0 |
| tslim | 1 | 0 | 0 |
| camaps | 0 | 0 | 0 |
| camdiab | 0 | 0 | 0 |
| minimed 670G | 7 | 0 | 2 summaries of safety and effectiveness data |
| minimed 780G | 0 | 0 | 0 |
| minimed | – | 0 | 0 |
| smartguard | 8 | 0 | 0 |
| smart guard | 2 | 0 | 0 |
| ilet | 0 | 0 | 0 |
| beta bionics | 0 | 0 (also tried ‘Requester name’ field) | 0 |
| closed loop | 13 | – | 1 summary of safety and effectiveness data |
| artificial pancreas | 1 | – | 0 |
| bionic pancreas | 0 | – | 0 |
Medicines and Healthcare products Regulatory Agency (via gov.uk website)
Date searched: 22 April 2021
Search interface: www.gov.uk/
Filters selected:
About (Topic): Health and social care and Medicines, medical devices
Updated after:1 January 2014
| Search term | Results | Documents downloaded (judged to contain potentially useful/relevant information not already identified in previous sets) |
|---|---|---|
| dexcom | 6 | 2 Field Safety Notices, 1 gov.uk web page |
| “control-iq” | 0 | 0 |
| “control iq” | 0 | 0 |
| “t:slim” | 2 | 1 Field Safety Notice, 1 gov.uk web page |
| “t slim” | 1 | 0 |
| tslim | 0 | 0 |
| camaps | 0 | 0 |
| camdiab | 0 | 0 |
| “minimed 670G” | 2 | 2 Field Safety Notices |
| minimed 780G | 1 | 0 |
| smartguard | 0 | 0 |
| “smart guard” | 0 | 0 |
| ilet | 0 | 0 |
| “beta bionics” | 0 | 0 |
| “closed loop” | 3 | 0 |
| “artificial pancreas” | 0 | 0 |
| “bionic pancreas” | 0 | 0 |
Record of searches: cost-effectiveness
Overview:
| Database/website | Date searched (date updated) | Number of records + update = total |
|---|---|---|
| MEDLINE ALL (via Ovid) | 7 April 2021 (5 April 2022) | 162 + 56 = 218 |
| EMBASE (via Ovid) | 7 April 2021 (5 April 2022) | 312 + 91 = 403 |
| EconLit (via EBSCOhost) | 7 April 2021 (5 April 2022) | 7 + 1 = 8 |
| HTA database (via CRD) | 7 April 2021a | 16 |
| International HTA database (INAHTA) | 7 April 2021 (6 April 2022) | 22 + 10 = 32 |
| EconPapers (RePEc) | 7 April 2021 (6 April 2022) | 16 + 6 = 22 |
| AHRQ website | 12 April 2021 (6 April 2022) | 1 + 0 = 1 |
| CADTH website | 12 April 2021 (7 April 2022) | 14 + 2 = 16 |
| SBU website | 12 April 2021 (7 April 2022) | 0 + 0 = 0 |
| CEA registry | 14 April 2021 (7 April 2022) | 27 + 2 = 29 |
| ScHARRHUD | 14 April 2021a | 0 |
Total results: 577 + 168 from update = 745
Total after 158 duplicates + 43 duplicates within update results + 28 duplicates with original results removed = 516
Additional targeted searches were made for other parameters later (see end).
Search strategies:
Note: see below each database strategy for details of update searches.
MEDLINE (via Ovid)
Date searched: 7 April 2021
Database: Ovid MEDLINE(R) ALL 1946 to 6 April 2021
Search strategy
-
Diabetes Mellitus, Type 1/ (77,411)
-
Diabetic Ketoacidosis/ (6618)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ab,kf,ti. (56,642)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ab,kf,ti. (28,281)
-
((insulin$ adj2 depend$) or insulindepend$).ab,kf,ti. (33,825)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ab,kf,ti. (23,617)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis or dka).ab,kf,ti. (11,593)
-
Hyperglycemia/ (28,779)
-
Hypoglycemia/ (27,948)
-
(hyperglyc?em$ or hypoglyc?em$).ab,kf,ti. (116,710)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ab,kf,ti. (151,670)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 [population: T1DM] (365,496)
-
Pancreas, Artificial/ (816)
-
closed loop.ab,kf,ti. (10,542)
-
(artificial adj2 (pancreas or beta cell$)).ab,kf,ti. (1730)
-
(bionic adj2 pancreas).ab,kf,ti. (25)
-
(Automat$ adj2 (insulin deliver$ or insulin dosing or glucose control$ or glyc?emic control$)).ab,kf,ti. (287)
-
((minimed or medtronic) and (670G or 780G)).ab,kf,ti. (58)
-
(tslim or t slim or control iq or camAPS or camdiab or dexcom G6 or dexcom G7 or smartguard or smart guard or diabeloop or dblg1 or ilet or beta bionics or (omnipod and horizon) or (mylife and loop) or (tidepool and loop) or bigfoot or anydana loop).ab,kf,ti. (176)
-
13 or 14 or 15 or 16 or 17 or 18 or 19 [Intervention: closed loop systems] (12,190)
-
(sensor? adj3 (augment$ or integrat$ or pump? or insulin)).ab,kf,ti. (7831)
-
SAPT.ab,kf,ti. (536)
-
predictive low glucose.ab,kf,ti. (97)
-
basal iq.ab,kf,ti. (9)
-
((minimed or medtronic) and 640G).ab,kf,ti. (33)
-
(paradigm$ adj3 (veo or pump$)).ab,kf,ti. (58)
-
(veo adj3 pump$).ab,kf,ti. (9)
-
(g4 adj3 platinum).ab,kf,ti. (58)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ab,kf,ti. (14)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 [sensor augmented pumps] (8503)
-
Insulin Infusion Systems/ (5481)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ab,kf,ti. (14,832)
-
(pump$ adj2 (therap$ or treatment$)).ab,kf,ti. (3232)
-
((subcutaneous adj2 insulin$) or CSII).ab,kf,ti. (3868)
-
(minimed or dana diabecare or dana R or dana RS or kaleido or omnipod or medtrum or touchcare or ypsopump or cellnovo).ab,kf,ti. (380)
-
(medtronic adj3 (pump$ or system$ or deliver$)).ab,kf,ti. (720)
-
(tandem adj3 (pump$ or system$ or deliver$)).ab,kf,ti. (926)
-
((accu-chek or accuchek) adj3 (pump$ or system$ or deliver$ or combo or insight or solo)).ab,kf,ti. (34)
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 [insulin pumps/CSII] (20,986)
-
((continu$ or flash or intermittent$ or sensor or sensors or real time) adj4 glucose adj4 (monitor$ or measurement$)).ab,kf,ti. (5882)
-
(glucose adj (sensor$ or sensing)).ab,kf,ti. (4191)
-
(CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS).ab,kf,ti. (4544)
-
(dexcom or freestyle or libre or enlite or (guardian and (medtronic or sensor)) or eversense or glucomen day).ab,kf,ti. (2422)
-
40 or 41 or 42 or 43 [continuous or flash glucose monitors] (13,072)
-
(2014082* or 2014083* or 201409* or 201410* or 201411* or 201412* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).dt,ez,da. [added to database since search for previous DAR in 2014] (8,999,414)
-
12 and 20 and 45 [T1DM and closed loop + date limit] (1143)
-
12 and 30 and 45 [T1DM and SAPT + date limit] (505)
-
12 and 39 and 44 and 45 [T1DM and pumps and GMs + date limit] (1100)
-
46 or 47 or 48 (1967)
-
limit 49 to english language (1919)
-
exp Pregnancy/ (913,489)
-
exp Pregnancy Complications/ (435,971)
-
Perinatal Care/ or Preconception Care/ or Prenatal Care/ (35,179)
-
exp Cesarean Section/ (46,725)
-
Pregnant Women/ (9210)
-
(pregnan$ or ante natal$ or antenatal$ or pre natal$ or prenatal$ or (expectant$ adj2 mother$) or “mother? to be” or matern$ or conception$ or preconception$ or “trying to conceive” or prepregnan$ or periconception$ or giving birth or childbirth$ or labo?r or newborn$ or new born$ or neonat$ or neo nat$ or baby or babies).ab,kf,ti. (1,210,177)
-
(miscarr$ or abort$ or cesarean or caesarean or c section$ or (prematur$ and (birth$ or rupture$ or infant$)) or preterm or pre term or prematurity or prom or macrosomia$ or birth weight$ or birthweight$ or eclamp$ or preeclamp$ or stillbirth$ or still birth$ or stillborn$ or still born$).ab,kf,ti. (352,725)
-
(perinatal or peri natal or fetal or foetal or intrauterine or intra uterine).ab,kf,ti. (365,250)
-
apgar.ab,kf,ti. (12,609)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 [pregnancy, planning pregnancy, pregnancy complications; broad] (1,736,892)
-
exp Insulin/ and Injections, Subcutaneous/ (2457)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (1309)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (564)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ti,ab,kf. (10,216)
-
MDI.ti,ab,kf. (3837)
-
(injection adj3 therapy).ti,ab,kf. (4204)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ti,ab,kf. (1376)
-
(short acting adj3 insulin).ti,ab,kf. (576)
-
(rapid acting adj3 insulin).ti,ab,kf. (799)
-
or/61-69 [insulin injections] (21,941)
-
Blood Glucose Self-Monitoring/ (7144)
-
Blood Glucose/ (168,038)
-
(blood glucos$ or blood sugar$).ab,kf,ti. (87,483)
-
72 or 73 (210,806)
-
(self monitor$ or test$ strip$ or finger prick$ or fingerprick$ or finger stick$ or fingerstick$ or lancet? or meter?).ab,kf,ti. (43,311)
-
(capillary adj4 (test$ or measur$)).ab,kf,ti. (5095)
-
75 or 76 (48,093)
-
74 and 77 (5795)
-
SMBG.ab,kf,ti. (1197)
-
glucometer$.ab,kf,ti. (1147)
-
71 or 78 or 79 or 80 [self monitoring of blood glucose] (11,403)
-
44 and 70 [continuous or flash GMs AND MDI] (488)
-
81 and 39 [SMBG AND CSII] (1715)
-
82 or 83 (2028)
-
12 and 60 and 84 and 45 [T1DM and pregnancy and any of the comparator groups specific to this population + date limit] (56)
-
limit 85 to english language (55)
-
50 or 86 (1930)
-
Economics/ (27,310)
-
exp “costs and cost analysis”/ (243,824)
-
Economics, Dental/ (1915)
-
exp economics, hospital/ (25,035)
-
Economics, Medical/ (9127)
-
Economics, Nursing/ (4002)
-
Economics, Pharmaceutical/ (2977)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (852,480)
-
(expenditure$ not energy).ti,ab. (31,555)
-
value for money.ti,ab. (1740)
-
budget$.ti,ab. (30,786)
-
88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 (1,007,726)
-
((energy or oxygen) adj cost).ti,ab. (4248)
-
(metabolic adj cost).ti,ab. (1480)
-
((energy or oxygen) adj expenditure).ti,ab. (26,059)
-
100 or 101 or 102 (30,788)
-
99 not 103 (1,000,667)
-
letter.pt. (1,129,857)
-
editorial.pt. (563,250)
-
historical article.pt. (362,940)
-
105 or 106 or 107 (2,035,927)
-
104 not 108 (963,183)
-
exp animals/ not humans/ (4,809,908)
-
109 not 110 [economic studies filter] (901,889)
-
87 and 111 (162)
Update
Date searched: 5 April 2022
Re-ran above search with search line 45 altered to:
45 (202104* or 202105* or 202106* or 202107* or 202108* or 202109* or 202110* or 202111* or 202112* or 2022*).dt,ez,da. [added to database since original search for this MTA]
Total: 112 87 and 111 (56)
The economics terms (lines 88–111) are based on the CRD NHS EED filter:
Centre for Reviews and Dissemination. Search Strategies: NHS EED MEDLINE Using OvidSP. York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedmedline (accessed 27 April 2021).
Search strings used in the previous technology assessment on integrated SAP therapy systems were used as the basis for developing selected lines relating to type 1 diabetes, insulin pumps, sensor-augmented pumps and MDI:
Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess 2016;20(17). https://doi.org/10.3310/hta20170
The following were used as sources of search terms for pregnancy and related concepts:
Tessier V. Périnatalité: Perinatality: Rappel favorisé sur la précision. Canadian Health Libraries Association - Association des bibliothèques de la santé du Canada; 2017. URL: https://extranet.santecom.qc.ca/wiki/!biblio3s/doku.php?id=concepts:perinatalite (accessed 26 April 2021).
Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi M, Athanasiou A, Martin‐Hirsch PPL, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2015;9:CD008478. https://doi.org/10.1002/14651858.CD008478.pub2
Cochrane Pregnancy and Childbirth’s Trials Register. Detailed Search Methods Used to Maintain and Update the Specialised Register. 2018. URL: https://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/public/uploads/cochrane_pregnancy_and_childbirth_search_methods_2018_1.docx (accessed 26 April 2021).
EMBASE (via Ovid)
Date searched: 7 April 2021
Database: EMBASE 1974 to 6 April 2021
Search strategy
-
insulin dependent diabetes mellitus/ (120,816)
-
diabetic ketoacidosis/ (13,238)
-
(diabet$ adj3 (typ$ 1 or typ$ i or type1 or typei or typ$ one)).ab,kw,ti. (89,502)
-
(diabet$ adj3 (britt$ or juvenil$ or pediatric or paediatric or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset)).ab,kw,ti. (39,710)
-
((insulin$ adj2 depend$) or insulindepend$).ab,kw,ti. (42,510)
-
(dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ab,kw,ti. (41,428)
-
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis or dka).ab,kw,ti. (17,695)
-
hypoglycemia/ or insulin hypoglycemia/ or nocturnal hypoglycemia/ or hyperglycemia/ (170,292)
-
(hyperglyc?em$ or hypoglyc?em$).ab,kw,ti. (171,683)
-
((high or higher or low or lower or increas$ or decreas$ or deficien$ or sufficien$ or insufficien$ or reduce$ or reduction$ or fluctuat$ or fallen or falling or threshold or safe) adj3 (glucose$ or sugar$ or hba1c or hb a1 or hba1 or a1c or h?emoglob$ or glycoh?emoglob$)).ab,kw,ti. (219,849)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 [population: T1DM] (553,786)
-
exp artificial pancreas/ (2523)
-
“glucose monitoring/insulin pump system”/ (22)
-
closed loop.ab,kw,ti. (13,576)
-
(artificial adj2 (pancreas or beta cell$)).ab,kw,ti. (2733)
-
(bionic adj2 pancreas).ab,kw,ti. (84)
-
(automat$ adj2 (insulin deliver$ or insulin dosing or glucose control$ or glyc?emic control$)).ab,kw,ti. (501)
-
((minimed or medtronic) and (670G or 780G)).ab,dm,dv,kw,ti. (204)
-
(tslim or t slim or control iq or camAPS or camdiab or dexcom G6 or dexcom G7 or smartguard or smart guard or diabeloop or dblg1 or ilet or beta bionics or (omnipod and horizon) or (mylife and loop) or (tidepool and loop) or bigfoot or anydana loop).ab,dm,dv,kw,ti. (452)
-
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 [Intervention: closed loop systems] (16,596)
-
(sensor? adj3 (augment$ or integrat$ or pump? or insulin)).ab,kw,ti. (9770)
-
SAPT.ab,kw,ti. (499)
-
predictive low glucose.ab,kw,ti. (216)
-
basal iq.ab,dm,dv,kw,ti. (35)
-
((minimed or medtronic) and 640G).ab,dm,dv,kw,ti. (162)
-
(paradigm$ adj3 (veo or pump$)).ab,dm,dv,kw,ti. (251)
-
(veo adj3 pump$).ab,dm,dv,kw,ti. (63)
-
(g4 adj3 platinum).ab,dm,dv,kw,ti. (215)
-
((animas or vibe) adj3 (pump$ or infus$ or system$)).ab,dm,dv,kw,ti. (56)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 [sensor augmented pumps] (10,839)
-
insulin infusion/ (8362)
-
insulin pump/ or implantable insulin pump/ (7947)
-
(insulin$ adj3 (pump$ or infus$ or deliver$ or catheter$)).ab,kw,ti. (23,717)
-
(pump$ adj2 (therap$ or treatment$)).ab,kw,ti. (6135)
-
((subcutaneous adj2 insulin$) or CSII).ab,kw,ti. (7277)
-
(minimed or dana diabecare or dana R or dana RS or kaleido or omnipod or medtrum or touchcare or ypsopump or cellnovo).ab,dm,dv,kw,ti. (1656)
-
(medtronic adj3 (pump$ or system$ or deliver$)).ab,dm,dv,kw,ti. (3033)
-
(tandem adj3 (pump$ or system$ or deliver$)).ab,dm,dv,kw,ti. (1171)
-
((accu-chek or accuchek) adj3 (pump$ or system$ or deliver$ or combo or insight or solo)).ab,dm,dv,kw,ti. (174)
-
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 [insulin pumps/CSII] (36,842)
-
((continu$ or flash or intermittent$ or sensor or sensors or real time) adj4 glucose adj4 (monitor$ or measurement$)).ab,kw,ti. (10,589)
-
(glucose adj (sensor$ or sensing)).ab,kw,ti. (5548)
-
(CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS).ab,kw,ti. (8880)
-
(dexcom or freestyle or libre or enlite or (guardian and (medtronic or sensor)) or eversense or glucomen day).ab,dm,dv,kw,ti. (4614)
-
41 or 42 or 43 or 44 [continuous or flash glucose monitors] (20,610)
-
11 and 20 [T1DM and closed loop] (4008)
-
11 and 30 [T1DM and SAPT] (1705)
-
11 and 40 and 45 [T1DM and pumps and GMs] (4222)
-
46 or 47 or 48 (7461)
-
limit 49 to dc=20140825-20210331 (4304)
-
limit 50 to english language (4181)
-
exp pregnancy/ (689,502)
-
exp pregnancy disorder/ (556,137)
-
exp cesarean section/ (102,040)
-
pregnant woman/ (87,246)
-
pregnancy outcome/ (64,095)
-
perinatal care/ or prepregnancy care/ or prenatal care/ (57,272)
-
(pregnan$ or ante natal$ or antenatal$ or pre natal$ or prenatal$ or (expectant$ adj2 mother$) or “mother? to be” or matern$ or conception$ or preconception$ or “trying to conceive” or prepregnan$ or periconception$ or giving birth or childbirth$ or labo?r or newborn$ or new born$ or neonat$ or neo nat$ or baby or babies).ab,kw,ti. (1,450,554)
-
(miscarr$ or abort$ or cesarean or caesarean or c section$ or (prematur$ and (birth$ or rupture$ or infant$)) or preterm or pre term or prematurity or prom or macrosomia$ or birth weight$ or birthweight$ or eclamp$ or preeclamp$ or stillbirth$ or still birth$ or stillborn$ or still born$).ab,kw,ti. (456,116)
-
(perinatal or peri natal or fetal or foetal or intrauterine or intra uterine).ab,kw,ti. (466,666)
-
apgar.ab,kw,ti. (19,962)
-
52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 [pregnancy, planning pregnancy, pregnancy complications; broad] (1,960,053)
-
blood glucose monitoring/ (28,324)
-
glucose blood level/ (264,217)
-
(blood glucos$ or blood sugar$).ab,kw,ti. (130,659)
-
64 or 65 (300,664)
-
self monitoring/ (8184)
-
(self monitor$ or test$ strip$ or finger prick$ or fingerprick$ or finger stick$ or fingerstick$ or lancet? or meter?).ab,kw,ti. (68,060)
-
(capillary adj4 (test$ or measur$)).ab,kw,ti. (6781)
-
67 or 68 or 69 (76,851)
-
66 and 70 (9977)
-
SMBG.ab,kw,ti. (2499)
-
glucometer$.ab,kw,ti. (2303)
-
63 or 71 or 72 or 73 [self monitoring of blood glucose] (35,625)
-
insulin/ and exp injection/ (5682)
-
(multiple daily adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (2615)
-
(multiple dose adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (783)
-
(multiple adj3 (inject$ or insulin$ or regime$ or routine$)).ab,kw,ti. (15,107)
-
MDI.ab,kw,ti. (6724)
-
(injection adj3 therapy).ab,kw,ti. (6301)
-
((basal$ and bolus) adj3 (injection$ or regime$ or routine$ or system$)).ab,kw,ti. (2372)
-
(short acting adj3 insulin).ab,kw,ti. (969)
-
(rapid acting adj3 insulin).ab,kw,ti. (1412)
-
75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 [insulin injections/ MDI] (34,894)
-
45 and 84 [continuous or flash GMs AND MDI] (1390)
-
74 and 40 [SMBG AND CSII] (5427)
-
85 or 86 (6255)
-
11 and 62 and 87 [T1DM and pregnancy and any comparator group specific to the pregnancy population] (446)
-
limit 88 to dc=20140825-20210331 (242)
-
limit 89 to english language (235)
-
51 or 90 (4272)
-
Health Economics/ (33,568)
-
exp Economic Evaluation/ (318,503)
-
exp Health Care Cost/ (302,491)
-
pharmacoeconomics/ (7520)
-
92 or 93 or 94 or 95 (558,862)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (1,149,601)
-
(expenditure$ not energy).ti,ab. (43,069)
-
(value adj2 money).ti,ab. (2579)
-
budget$.ti,ab. (40,898)
-
97 or 98 or 99 or 100 (1,188,152)
-
96 or 101 (1,417,777)
-
letter.pt. (1,175,320)
-
editorial.pt. (692,507)
-
note.pt. (850,530)
-
103 or 104 or 105 (2,718,357)
-
102 not 106 (1,310,667)
-
(metabolic adj cost).ti,ab. (1614)
-
((energy or oxygen) adj cost).ti,ab. (4538)
-
((energy or oxygen) adj expenditure).ti,ab. (33,372)
-
108 or 109 or 110 (38,389)
-
107 not 111 [economic studies filter] (1,302,843)
-
91 and 112 (312)
Update
Date searched: 5 April 2022
Re-ran above search with search lines 50 and 89 altered to:
50 limit 49 to dc=20210405-20220405
89 limit 88 to dc=20210405-20220405
Total: 113 91 and 112 (91)
The economics terms (lines 92–112) are based on the CRD NHS EED filter:
Centre for Reviews and Dissemination. Search Strategies: NHS EED Embase Using OvidSP. York: Centre for Reviews and Dissemination; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp#nhseedembase (accessed 27 April 2021).
EconLit with Full Text (via EBSCOhost)
Date searched: 7 April 2021
Search screen: Advanced Search
| # | Query | Limiters/expanders | Results |
|---|---|---|---|
| S27 | S4 AND S26 | Limiters – Published Date: 20140101-20210431 Search modes – Boolean/Phrase |
7 |
| S26 | S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | Search modes – Boolean/Phrase | 11,027 |
| S25 | TI (minimed or medtronic or tslim or “t slim” or “control iq” or “basal iq” or camAPS or camdiab or dexcom or smartguard or “smart guard” or diabeloop or dblg1 or ilet or “beta bionics” or omnipod or mylife or tidepool or bigfoot or anydana or paradigm* or veo or platinum or animas or vibe or dana or kaleido or medtrum or touchcare or ypsopump or cellnovo or tandem or “accu chek” or accuchek or freestyle or libre or enlite or (guardian and sensor) or eversense or glucomen) OR AB (minimed or medtronic or tslim or “t slim” or “control iq” or “basal iq” or camAPS or camdiab or dexcom or smartguard or “smart guard” or diabeloop or dblg1 or ilet or “beta bionics” or omnipod or mylife or tidepool or bigfoot or anydana or paradigm* or veo or platinum or animas or vibe or dana or kaleido or medtrum or touchcare or ypsopump or cellnovo or tandem or “accu chek” or accuchek or freestyle or libre or enlite or (guardian and sensor) or eversense or glucomen) | Search modes – Boolean/Phrase | 10,312 |
| S24 | TI (SMBG or glucometer*) OR AB (SMBG or glucometer*) | Search modes – Boolean/Phrase | 1 |
| S23 | TI ((“blood glucos*” or “blood sugar*”) AND (“self monitor*” or “test* strip*” or “finger prick*” or fingerprick* or “finger stick*”or fingerstick* or lancet* or meter* or (capillary N4 (test* or measur*)))) OR AB ((“blood glucos*” or “blood sugar*”) AND (“self monitor*” or “test* strip*” or “finger prick*” or fingerprick* or “finger stick*”or fingerstick* or lancet* or meter* or (capillary N4 (test* or measur*)))) | Search modes – Boolean/Phrase | 4 |
| S22 | TI ((“short acting” or “rapid acting”) N3 insulin*) OR AB ((“short acting” or “rapid acting”) N3 insulin*) | Search modes – Boolean/Phrase | 1 |
| S21 | TI ((basal* and bolus) N3 (injection* or regime* or routine* or system*)) OR AB ((basal* and bolus) N3 (injection* or regime* or routine* or system*)) | Search modes – Boolean/Phrase | 0 |
| S20 | TI injection N3 therapy OR AB injection N3 therapy | Search modes – Boolean/Phrase | 1 |
| S19 | TI MDI OR AB MDI | Search modes – Boolean/Phrase | 21 |
| S18 | TI (multiple N4 (inject* or insulin* or regime* or routine*)) OR AB (multiple N4 (inject* or insulin* or regime* or routine*)) | Search modes – Boolean/Phrase | 275 |
| S17 | TI (insulin* N3 (inject* or therapy*)) OR AB (insulin* N3 (inject* or therapy*)) | Search modes – Boolean/Phrase | 9 |
| S16 | TI (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS) OR AB (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS) | Search modes – Boolean/Phrase | 45 |
| S15 | TI (“glucose sensor*” or “glucose sensing”) OR AB (“glucose sensor*” or “glucose sensing”) | Search modes – Boolean/Phrase | 0 |
| S14 | TI ((continu* or flash or intermittent* or sensor or sensors or “real time”) N4 glucose N4 (monitor* or measurement*)) OR AB ((continu* or flash or intermittent* or sensor or sensors or “real time”) N4 glucose N4 (monitor* or measurement*)) | Search modes – Boolean/Phrase | 1 |
| S13 | TI ((subcutaneous N2 insulin*) or CSII) OR AB ((subcutaneous N2 insulin*) or CSII) | Search modes – Boolean/Phrase | 2 |
| S12 | TI ((pump* N2 (therap* or treatment*)) OR AB ((pump* N2 (therap* or treatment*)) | Search modes – Boolean/Phrase | 2 |
| S11 | TI ((insulin* N3 (pump* or infus* or deliver* or catheter*)) OR AB ((insulin* N3 (pump* or infus* or deliver* or catheter*)) | Search modes – Boolean/Phrase | 2 |
| S10 | TI (SAPT or “predictive low glucose”) OR AB (SAPT or “predictive low glucose”) | Search modes – Boolean/Phrase | 0 |
| S9 | TI (sensor* N3 (augment* or integrat* or pump* or insulin)) OR AB (sensor* N3 (augment* or integrat* or pump* or insulin)) | Search modes – Boolean/Phrase | 12 |
| S8 | TI (automat* N2 (“insulin deliver*” or “insulin dosing” or “glucose control*” or “glyc#emic control*”)) OR AB (automat* N2 (“insulin deliver*” or “insulin dosing” or “glucose control*” or “glyc#emic control*”)) | Search modes – Boolean/Phrase | 0 |
| S7 | TI bionic N2 pancreas OR AB bionic N2 pancreas | Search modes – Boolean/Phrase | 0 |
| S6 | TI (artificial N2 (pancreas or “beta cell*”)) OR AB (artificial N2 (pancreas or “beta cell*”)) | Search modes – Boolean/Phrase | 0 |
| S5 | TI “closed loop” OR AB “closed loop” | Search modes – Boolean/Phrase | 354 |
| S4 | S1 OR S2 OR S3 | Search modes – Boolean/Phrase | 688 |
| S3 | TI (hyperglyc#em* OR hypoglyc#em*) OR AB (hyperglyc#em* OR hypoglyc#em*) | Search modes – Boolean/Phrase | 19 |
| S2 | TI (ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis or dka) OR AB (ketoacidosis or acidoketosis or “keto acidosis” or ketoacidemia or ketosis or dka) | Search modes – Boolean/Phrase | 0 |
| S1 | TI (diabet* or insulin* or insulindepend* or dm1 or dmt1 or t1dm or t1d or iddm or “dm 1” or “dm t1” or “t1 dm”) OR AB (diabet* or insulin* or insulindepend* or dm1 or dmt1 or t1dm or t1d or iddm or “dm 1” or “dm t1” or “t1 dm”) | Search modes – Boolean/Phrase | 683 |
Update
Date searched: 6 April 2022
Re-ran above search with line 27 changed to: Published Date: 20210101-20220431
Total: 1
Health Technology Assessment database (via CRD website)
Date searched: 7 April 2021
Search interface: www.crd.york.ac.uk/CRDWeb/
| ((closed loop) OR (artificial NEAR2 pancreas) OR (bionic NEAR2 pancreas)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 2 |
| ((minimed or control iq or camAPS or camdiab or dexcom)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| ((sensor augmented) OR (SAPT)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| ((automat* NEAR2 (insulin OR glucose OR glycemic OR glycaemic))) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 0 |
| ((insulin NEAR2 (pump* OR infus*)) OR (subcutaneous NEAR2 insulin*) OR (CSII)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 10 |
| ((continu* or flash or intermittent* or sensor or sensors or real time) AND (glucose) AND (monitor* or measurement*)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 6 |
| ((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 3 |
| ((diabet* or insulin*) AND (pregn*) AND (injection* or MDI or self monitoring or SMBG)) and (Project record:ZDT OR Full publication record:ZDT) IN HTA FROM 2014 TO 2021 | 1 |
| Total unique records | 16 |
No new records so update search not needed.
International Health Technology Assessment database (via INAHTA website)
Date searched: 7 April 2021
Search interface: advanced search builder https://database.inahta.org/search/advanced
| (closed loop) FROM 2014 TO 2021 | 0 |
| (artificial pancreas) FROM 2014 TO 2021 | 2 |
| (bionic pancreas) FROM 2014 TO 2021 | 0 |
| (minimed OR “control iq” OR camAPS OR camdiab OR dexcom) FROM 2014 TO 2021 | 2 |
| (“Pancreas, Artificial”[mh]) FROM 2014 TO 2021 | 2 |
| (“sensor augmented”) FROM 2014 TO 2021 | 1 |
| (SAPT) FROM 2014 TO 2021 | 0 |
| (“Insulin Infusion Systems”[mh]) FROM 2014 TO 2021 | 7 |
| (insulin AND (pump* OR infusion* OR subcutaneous)) FROM 2014 TO 2021 | 8 |
| (CSII) FROM 2014 TO 2021 | 2 |
| ((continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*)) FROM 2014 TO 2021 | 15 |
| ((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) FROM 2014 TO 2021 | 7 |
| ((diabet* or insulin*) AND pregn* AND (injection* or MDI or “self monitoring” or SMBG)) FROM 2014 TO 2021 | 4 |
| Total | 50 |
| Total after duplicate removal (using EndNote) | 22 |
Update
Date searched: 6 April 2022
Re-ran search above search in one line with end date altered to 2022:
(((diabet* or insulin*) AND pregn* AND (injection* or MDI or “self monitoring” or SMBG)) FROM 2014 TO 2022) OR (((diabet* or insulin*) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS)) FROM 2014 TO 2022) OR (((continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*)) FROM 2014 TO 2022) OR ((CSII) FROM 2014 TO 2022) OR ((insulin AND (pump* OR infusion* OR subcutaneous)) FROM 2014 TO 2022) OR ((“Insulin Infusion Systems”[mh]) FROM 2014 TO 2022) OR ((SAPT) FROM 2014 TO 2022) OR ((“sensor augmented”) FROM 2014 TO 2022) OR ((“Pancreas, Artificial”[mh]) FROM 2014 TO 2022) OR ((minimed OR “control iq” OR camAPS OR camdiab OR dexcom) FROM 2014 TO 2022) OR ((bionic pancreas) FROM 2014 TO 2022) OR ((artificial pancreas) FROM 2014 TO 2022) OR ((closed loop) FROM 2014 TO 2022)
Total: 32
Notes: after checking several lines from the original search above and finding some of the new records were for HTAs published before 2021, it was decided that all 32 should be exported and de-duplicated with the previous results in EndNote.
Total after de-duplication in EndNote: 10
EconPapers (via Research Papers in Economics)
Date searched: 7 April 2021
Search interface: advanced search https://econpapers.repec.org/scripts/search.pf
Filters selected: Working Papers, Journal Articles, Books & Chapters.
Sort by date modified (to enable easy exclusion of pre-2014 records)
| Search terms (entered in ‘Free text search’) | Update | |
|---|---|---|
| (diabet* OR insulin* OR hyperglyc* OR hypoglyc* OR dm1 OR dmt1 OR t1dm OR t1d OR iddm OR “dm 1” OR “dm t1” OR “t1 dm”) AND (“closed loop” OR “artificial pancreas” OR “artificial endocrine pancreas” OR “bionic pancreas”) | 13 | 5 |
| (diabet* OR insulin* OR hyperglyc* OR hypoglyc* OR dm1 OR dmt1 OR t1dm OR t1d OR iddm OR “dm 1” OR “dm t1” OR “t1 dm”) AND (minimed OR “control iq” OR camAPS OR camdiab OR excom) | 0 | 0 |
| (diabet* OR insulin* OR hyperglyc* OR hypoglyc* OR dm1 OR dmt1 OR t1dm OR t1d OR iddm OR “dm 1” OR “dm t1” OR “t1 dm”) AND (“sensor augmented” OR SAPT) | 0 | 0 |
| insulin AND (pump* OR infusion* OR subcutaneous) AND (continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*) | 3 | 2 |
| insulin AND (pump* OR infusion* OR subcutaneous) AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS) | 2 | 1 |
| CSII AND (continu* OR flash OR intermittent* OR sensor OR sensors OR “real time”) AND (glucose) AND (monitor* or measurement*) | 2 | 1 |
| CSII AND (CGM or CGMs or FGM or FGMs or iCGM or iCGMs or rtCGM or rtCGMS) | 1 | 0 |
| (diabet* OR insulin* OR hyperglyc* OR hypoglyc* OR dm1 OR dmt1 OR t1dm OR t1d OR iddm OR “dm 1” OR “dm t1” OR “t1 dm”) AND pregn* AND (injection* OR MDI OR “self-monitoring” OR SMBG) | 2 | 0 |
| Total | 23 | 9 |
| Total after duplicate removal (using EndNote) | 16 | 6 |
Update
Date searched: 6 April 2022
Re-ran search above searches with box ticked for added to EconPapers in the last 1 year (new or updated items, selected Modified last 1 year and Date is Creation/revision of Metadata). For numbers see right-hand column in original strategy table above.
Agency for Healthcare Research and Quality website
Date searched: 12 April 2021
Search publications: www.ahrq.gov/research/publications/search.html
| Search terms | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| closed loop | 0 | – | 0 | – |
| artificial pancreas | 0 | – | 0 | – |
| diabetes | 6 | 0 relevant | 6 (0 new) | – |
| insulin | 0 | 0 | – |
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 0 new.
Search Evidence-Based Reports: www.ahrq.gov/research/findings/evidence-based-reports/search.html
| Search terms/method | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| closed loop | 0 | 0 | – | |
| artificial pancreas | 1 | 0 relevant; about pancreatic adenocarcinoma | 1 (0 new) | – |
| Browsed Topic: Endocrine conditions | 25 reports, of which 10 published 2014–present | 0 relevant | 26 reports, of which 11 published 2014–present (1 new) | 0 relevant |
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 1 new, 0 relevant.
Full Research Reports: www.ahrq.gov/research/findings/final-reports/index.html
Checked 10 reports listed; none relevant.
Update. Checked again 6 April 2022. 0 new reports listed.
Technology Assessment Program: www.ahrq.gov/research/findings/ta/index.html
Checked all reports and projects listed; none relevant.
Update. Checked again 6 April 2022. 0 new published reports listed. 1 new revised report listed, but not relevant.
Technology Assessment Archive (up to 2016): https://archive.ahrq.gov/clinic/techarch.htm
Used ctrl + F to search web page for:
-
diabet
-
closed
-
pancreas
-
insulin
-
glucose
-
- nothing relevant found
AHRQ Research Studies: www.ahrq.gov/research/findings/studies/index.html
| Search term | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| Closed loop | 4 | 0 relevant (all about closed-loop communication systems; not diabetes) | 5 (1 new) | 0 relevant (all about closed-loop communication systems; not diabetes) |
| Artificial pancreas | 0 | – | 0 | – |
| Bionic pancreas | 0 | – | 0 | – |
| insulin delivery | 3 | 0 relevant | 0 | – |
| minimed | 0 | – | 0 | – |
| control iq | 0 | – | 527 (technical changes to search likely) | See new search in row below |
| control iq AND diabetes | – | – | 58 | Checked 2021 and 2022. None relevant |
| camAPS | 0 | – | 0 | – |
| camdiab | 0 | – | 0 | – |
| dexcom | 0 | – | 0 | – |
| insulin pump | 0 | – | 0 | – |
| insulin pumps | 0 | – | 0 | – |
| insulin infusion | 1 | 0 relevant | 1 (0 new) | – |
| insulin infusions | 0 | – | 0 | – |
| CSII | 0 | – | 0 | – |
| glucose monitoring | 3 | 0 relevant (2 x type 2 diabetes, 1 about behaviour change) | 6 (3 new) | 0 relevant |
| glucose monitors | 0 | – | 0 | – |
| glucose monitor | 1 | 1 possibly relevant | 1 (0 new) | – |
| flash | 0 | – | 0 | – |
| insulin AND injections | 0 | – | 0 | – |
| daily injections | 0 | – | 0 | – |
| blood glucose | 13 | 0 relevant; either type 2 diabetes, or not about self-monitoring | 15 (2 new) | 0 relevant |
| smbg | 0 | – | 0 | – |
| Total possibly relevant studies: | 1 | – | 0 | |
Update
Date searched: 6 April 2022. For numbers see right-hand column in original strategy table above. 6 new, 0 relevant.
Canadian Agency for Drugs and Technologies in Health website
Date searched: 12 April 2021
Search box on homepage www.cadth.ca/
Limit results by ‘Result Type: Reports; Projects in Progress’.
Sort by newest to oldest (to enable easy exclusion of pre-2014 records)
| Search terms | Total results | Number of new (not in previous sets), possibly relevant results | Total at update April 2022 | Number of new (not in previous results or sets), possibly relevant results |
|---|---|---|---|---|
| “closed loop” | 34 | 5 | 19 | 1 |
| artificial pancreas | 22 | 2 | 9 | 0 |
| bionic pancreas | 5 | 0 | 2 | 0 |
| automated insulin delivery | 18 | 0 | 10 | 0 |
| minimed | 16 | 1 | 5 | 0 |
| “control IQ” | 2 | 0 | 1 | 0 |
| camAPS | 0 | 0 | 0 | 0 |
| camdiab | 0 | 0 | 0 | 0 |
| Dexcom | 10 | 1 | 2 | 0 |
| “insulin pump” | 41 | 1 | 12 | 0 |
| “insulin infusion” | 51 | 0 | 5 | 0 |
| CSII | 23 | 0 | 3 | 0 |
| “glucose monitor” | 25 | 0 | 10 | 0 |
| “glucose monitoring” | 80 | 4 | 29 | 1 |
| “insulin injections” | 41 | 0 | 3 | 0 |
| “daily injections” | 43 | 0 | 8 | 0 |
| “self monitoring” AND glucose | 124 | 0 | 0 | 0 |
| SMBG | 31 | 0 | 5 | 0 |
| Total unique, possibly relevant results | 14 | 2 | ||
Update
Date searched: 7 April 2022. For numbers see right-hand column in original strategy table above. 2 new, 2 potentially relevant.
Note: assume website has been restructured or search interface/system changed since original search. Searched for words without quotation marks in ‘Contains all the words’ and terms in quotation marks in ‘Advanced Search’. Sorted by last updated and checked records for 2021 and 2022.
Swedish Agency for Health Technology Assessment and Assessment of Social Services website
Date searched: 12 April 2021
Search box on home page: www.sbu.se/en/
| Search terms/method | Total results | Comments | Total at update April 2022 | Comments at update April 2022 |
|---|---|---|---|---|
| closed loop | 0 | – | 0 | – |
| artificial pancreas | 1 | Not relevant; ‘dialysis for acute hepatic failure’ | 1 (0 new) | – |
| bionic pancreas | 0 | – | 0 | – |
| diabetes > Filter on subject and publication type > Publication year From 2014 to 2021 | 30 | 0 relevant | 5 new | 0 relevant |
| insulin > Filter on subject and publication type > Publication year From 2014 to 2021 | 5 | 0 relevant | 1 new | 0 relevant |
| Total possibly relevant studies, published since 2014 | 0 | – | 0 | |
Update
Date searched: 7 April 2022. For numbers see right-hand column in original strategy table above. 0 relevant.
Cost-Effectiveness Analysis Registry (via Tufts Medical Center)
Date searched: 14 April 2021
Search interface: Basic search, Search for: Methods http://healtheconomicsdev.tuftsmedicalcenter.org/cear2/search/search.aspx
| Search terms | Total results | Results published since 2014 | Number of new (not in previous sets), possibly relevant results | Results added since 2021 | Number of new (not in previous CEA search or sets), possibly relevant results |
|---|---|---|---|---|---|
| closed loop | 0 | 0 | 0 | 0 | – |
| artificial pancreas | 0 | 0 | 0 | 0 | – |
| bionic pancreas | 0 | 0 | 0 | 0 | – |
| insulin delivery | 4 | 4 | 4 | 0 | – |
| minimed | 2 | 2 | 1 | 0 | – |
| control IQ | 0 | 0 | 0 | 0 | – |
| camAPS | 0 | 0 | 0 | 0 | – |
| camdiab | 0 | 0 | 0 | 0 | – |
| dexcom | 1 | 1 | 1 | 1 | 1 |
| insulin pump | 10 | 9 | 7 | 0 | – |
| insulin pumps | 3 | 2 | 0 | 0 | – |
| insulin infusion | 20 | 15 | 5 | 0 | – |
| insulin infusions | 0 | 0 | 0 | 0 | – |
| CSII | 19 | 14 | 0 | 0 | – |
| glucose monitoring | 16 | 14 | 6 | 2 | 0 |
| glucose monitors | 0 | 0 | 0 | 0 | – |
| glucose monitor | 16 | 14 | 0 | 2 | 0 |
| flash | 6 | 2 | 0 | 0 | – |
| insulin injections | 5 | 5 | 0 | 1 | 1 |
| daily injections | 17 | 11 | 1 | 1 | 0 |
| blood glucose | 47 | 22 | 2 | 3 | 0 |
| smbg | 17 | 10 | 0 | 1 | 0 |
| Total unique, possibly relevant results: | 27 | 2 | |||
Update
Date searched: 7 April 2022. For numbers see right-hand column in original strategy table above. Two potentially relevant, but duplicates of those found in MEDLINE in original search.
ScHARRHUD
Date searched: 14 April 2021
Search interface: www.scharrhud.org/index.php?recordsN1&m=search (closed July 2024)
| closed loop OR artificial pancreas OR bionic pancreas AND 2014 > 2021:YR | 0 |
| (minimed OR control iq OR camAPS OR camdiab OR dexcom) AND 2014 > 2021:YR | 0 |
| sensor augmented OR sapt AND 2014 > 2021:YR | 0 |
| automated insulin OR insulin delivery AND 2014 > 2021:YR | 0 |
| insulin pump* OR insulin infusion* OR CSII AND 2014 > 2021:YR | 1 (not relevant; type 2 diabetes) |
| glucose monitor* AND 2014 > 2021:YR | 0 |
| flash AND 2014 > 2021:YR | 0 |
| insulin inject* AND 2014 > 2021:YR | 0 |
| insulin injections AND 2014 > 2021:YR | 0 |
| daily injections AND 2014 > 2021:YR | 0 |
| MDI AND 2014 > 2021:YR | 0 |
| blood glucose AND 2014 > 2021:YR | 0 |
| smbg AND 2014 > 2021:YR | 0 |
Update
Note (07/04/22): Searching * in any field limited to 2021 to 2022 in Date in ScHARRHUD retrieved 0 results. Searching * in any field limited to 2020 to 2022 in Date in ScHARRHUD retrieved 302 results so no new records have been added since 2020. Therefore, the searches were not re-run.
Additional targeted searches for individual parameters
Hypoglycaemia and Quality of Life
Date: 10 June 2022
Ovid MEDLINE(R) ALL 1946 to 9 June 2022
-
hypoglycemia/ or insulin coma/29,970
-
(hypoglycemi* or hypoglycaemi*).ti,ab,kf.63,398
-
1 or 270,791
-
Quality-Adjusted Life Years/14,835
-
(quality adjusted or adjusted life year$).tw,kf.20,920
-
(qaly$ or qald$ or qale$ or qtime$).tw,kf.13,223
-
(illness state$1 or health state$1).tw,kf.7688
-
(hui or hui1 or hui2 or hui3).tw,kf.1807
-
(multiattribute$ or multi attribute$).tw,kf.1133
-
(utility adj3 (score$1 or valu$ or health$ or cost$ or measur$ or disease$ or mean or gain or gains or index$)).tw,kf.18,324
-
utilities.tw,kf.8545
-
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eur qol5d or eur?qul or eur?qul5d or euro$ quality of life or european qol).tw,kf.15,107
-
(euro$ adj3 (d or 5d or 5 dimension$ or 5dimension$ or 5 domain$ or 5domain$)).tw,kf.5797
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).tw,kf.25,017
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw,kf.2184
-
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw,kf.14,297
-
quality of life/ and ec.fs.10,868
-
quality of life/ and (health adj3 status).tw,kf.10,904
-
(quality of life or qol).tw,kf. and Cost-Benefit Analysis/7271
-
((qol or hrqol or quality of life).ti,kf. or *quality of life/) and ((qol or hrqol$ or quality of life) adj2 (increas$ or decrease$ or improv$ or declin$ or reduc$ or high$ or low$ or effect or effects or worse or score or scores or change$1 or impact$1 or impacted or deteriorat$)).ab.47,789
-
Cost-Benefit Analysis/ and (cost-effectiveness ratio$ and (perspective$ or life expectanc$)).tw,kf.4707
-
*quality of life/ and (quality of life or qol).ti.61,866
-
quality of life/ and ((quality of life or qol) adj3 (improv$ or chang$)).tw,kf.36,382
-
quality of life/ and health-related quality of life.tw,kf.40,638
-
models,economic/11,001
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25202,159
-
3 and 26907
-
limit 27 to yr=“2020 -Current”177
-
(hypoglycemi* or hypoglycaemi*).ti.21,153
-
1 or 2936,314
-
26 and 30358
-
limit 31 to yr=“2020 -Current”55[Hypos and QoL 2020 onwards hypo terms in title or MeSH indexing]
-
28 not 32122[Hypos and QoL 2020 onwards hypo terms only in abstract or keywords]
Total: 177 exported in two batches [55 (line 32) and 122 (line 33)]
Website searches
Date: 10 June 2022
Checked:
https://hypo-resolve.eu/publications
Quantitative papers sent by team members and noted in original sifting for economic evaluations.
Appendix 3 Inclusion and exclusion criteria
| Populations | People who have T1DM who are having difficultya,b managing their condition despite prior use of at least one of the following technologies: CSII, rtCGM, FGMa,b
If evidence permits the following T1DM subpopulations will be included: |
|
|
| Target condition | T1DM |
| Intervention | HCL systems |
| Comparator |
|
| Outcomes | Intermediate measures |
|
|
| Clinical outcomes | |
|
|
| Additional clinical outcomes in women who are pregnant/have recently given birth | |
|
|
| Device-related outcomes | |
|
|
| Patient-reported outcomes | |
|
|
| Carer-reported outcomes | |
|
|
| Study design | HCL systems studies |
|
|
| All comparator studies | |
|
|
| Healthcare setting | Self-use supervised by primary or secondary care |
| Publication type | Peer-reviewed papers Abstracts and manufacturer data will be included only if they provide numerical data and sufficient detail on methodology to enable assessment of study quality/risk of bias. Furthermore, only data on outcomes that have not been reported in peer-reviewed full-text papers will be extracted and reported |
| Language | English |
Appendix 4 Summary of main outcome measures reported in randomised controlled trials
| HbA1c%, mean SD,*median IQR | % TIR > 10 mmol/l, mean SD,*median IQR | % TIR 3.9–10.0 mmol/l, mean SD *median IQR | % TIR < 3.9 mmol/l (70 mg/dl), mean SD,*median IQR | % TIR < 3.5 mmol/l (63 mg/dl), mean SD,*median IQR | % TIR < 3.3 mmol/l (60 mg/dl), mean SD,*median IQR | % TIR < 3.0 mmol/l (54 mg/dl), mean SD, *median IQR | % TIR < 2.8 mmol/l (50 mg/dl), mean SD,*median IQR | N hypo non-severe mean SD*,**median IQR | N hypo severe, mean SD* | N DKA, event, *mean SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tauschmann et al. 201852 HCL vs. CSII + CGM; 22 years, 21 years; n = 86; Tx 12 weeks; parallel-arm study; age HCL 22* CSII + CGM 21* Lancet 2021;392:1321–9 | |||||||||||
| Inter base | 8.0 (0.6) | 44 (11) | 52 (10) | *3.5 (2.0, 5.4) | *1.8 (0.8, 3.2) | NR | NR | * 0.4 (0.1,1.0) | |||
| Inter end | 7.4 (0.6) | 32 (8) | 65(8) | * 2.6 (1.9, 3.6) | * 1.4 (0.9, 1.9) | NR | NR | * 0.3 (0.2, 0.6) | |||
| Diff calc | −0.6 (0.125) | −12 (2.0) | 13 | *−0.9 | *−0.4 | NR | NR | * 0.1 | NR | 2 | 1 |
| Comp base | 7.8 (0.6) | 44 (11) | 52 (9) | *3.3 (1.2, 5.5) | *1.9 (0.6, 3.30) | NR | NR | * 0.5 (0.1, 1.0) | |||
| Comp end | 7.7 (0.5) | 42 (10) | 54 (9) | * 3.9 (1.7, 5.3) | * 2.0 (0.9, 3.0) | NR | NR | * 05.(0.2, 0.9) | NR | 2 | 0 |
| Diff calc | −0.1 (0.123) | −2 (2.35) | 2 | *0.6 | * 0.1 | NR | NR | * 0.0 | |||
| Rep. net effect (95% CI) | −0.36 (−0.53 to −0.19) | −10 (−13.2 to −7.5) | 10.8 (8.2 to 13.5) | *−0.83 (−1.4 to −0.16) | *−0.33 (−0.81 to 0.04) | NR | NR | *−0.09 (−0.24 to 0.1) | 0 | +1 | |
| Ware et al. 2022:54 HCL vs. CSII + CGM; aged 5.6 years (1.61 years) very young children; crossover study; n = 74; Tx 16 weeks. N Engl J Med 2022;386:209–19 | |||||||||||
| Inter base | 7.3 (0.7) | *32.2 (24.0, 42.7) | 61.5 (9.5) | *4.5 (2.4, 6.7) | NR | NR | *0.8 (0.2, 1.8) | NR | NR | ||
| Inter end | 6.6 (0.6) | *22.9 (19.3, 27.3) | 71.6 (5.9) | *4.9 (3.3, 6.7) | *2.6 (1.8, 3.7) | NR | *1.0 (0.6, 1.4) | NR | NR | ||
| Diff calc | −0.7 (0.16) | *−9.3 | 10.1 | *0.3 | NR | *0.2 | NR | NR | 1 | 0 | |
| Comp base | 7.4 (0.6) | *36.7 (21.6, 41.8) | 60.8 (10.9)_ | *3.9 (2.0, 7.4) | NR | *0.6 (0.3, 1.4) | NR | NR | |||
| Comp end | 7.0 (0.7) | *31.7 (23.4, 40.1) | 62.9 (9.0) | *4.5 (2.9, 7.3) | *2.4 (1.4, 4.2) | NR | *0.9 (0.4, 1.6) | NR | NR | ||
| Diff calc | −0.4 (0.16) | *−5.0 | 2.1 | *0.6 | NR | *0.3 | NR | NR | 0 | 0 | |
| Net effect (95% CI) | −0.4 (−0.5 to −0.3) | *−8.5 (−9.9 to −7.1) | 8.7 (7.4 to 9.9) | *0.1 (−0.4 to 0.5), NS | *0.04 (−0.3 to 0.3), NS | NR | *0.02 (−0.1 to 0.1), NS | NR | NR | 1 | 0 |
| Ware et al. 2022:56 HCL vs. CSII + CGM; children/adolescents: parallel-arm study; age 13.1 years (2.6) and 12.8 (2.9) years; n = 135; Tx 6 months | |||||||||||
| Inter base | 8.2 (0.7) | 46 (15) | 47 (12) | *6.1(2.7, 9.5) | NR | NR | NR | NR | NR | NR | NR |
| Inter end | 7.6 (1.1) | 38 (20) | 54 (17) | *6.1 (3.0, 12.1) | NR | NR | NR | NR | NR | NR | NR |
| Diff calc | −0.6 (0.17) | −8 (3.1) | 7 | * 0 | NR | NR | NR | NR | 11 | 2 | 2 |
| Comp base | 8.3 (0.7) | 47 (16) | 46 (13) | *4.9 (0.32, 9.4), | NR | NR | NR | NR | NR | NR | NR |
| Comp end | 8.1 (0.8) | 46 (15) | 47 (12) | *5.4 (2.0, 12.0) | NR | NR | NR | NR | NR | NR | NR |
| Diff calc | −0.2 (0.13) | −1 (2.6) | 1 | * 0.5 | NR | NR | NR | NR | 12 | 0 | 0 |
| Net effect (95% CI) | −0.32 (−0.59 to −0.04) | −7.0 (−12.5 to −1.5) | 6.7 (2.2 to 11.3) | *−0.53 (−1.78 to 2.83) | NR | NR | NR | NR | 1 | 2 | 2 |
| Benhamou et al. 2019:65 HCL vs. CSII + CGM; adults aged 48.2 (11.7) years; n = 63; Tx 12 weeks. Crossover trial. Lancet Digit Health 2019;1:e17–25 | |||||||||||
| HCL | −0.29 (0.6) | 29.5 (10.2) | 68.5 (9.4) | 2 (2.40) | NR | 0.8 (0.8) | NR | 0.2 (0.8) | NR | 5 | 0 |
| Comparator | −0.14 (0.6) | 36.3 (10.20) | 59.4 (10.20) | 4.3 (2.40) | NR | 2 (1.6) | NR | 0.7 (0.8) | NR | 3 | 0 |
| Net effect (95% CI) | −0.15 (−0.33 to 0.03) | −6.8 (−9.7 to −3.9) | 9.2 (6.4 to 11.9) | −2.4 (−3.0 to −1.7) | NR | −1.3 (−1.6 to −0.9) | NR | −0.5 (−0.33 to 0.03) | NR | 2 | 0 |
| Thabit et al. 2015 children/adolescents:53 HCL vs. CSII + CGM; aged 12 (3.4) years; n = 25; crossover study; Tx 12 weeks. N Engl J Med 2015;373:2129–40 | |||||||||||
| Inter base | 7.8 (0.7) | NR | NR | NR | NR | NR | NR | 2 | |||
| Inter end | 7.6 (1.1) | NR | NR | NR | NR | NR | NR | 0 | |||
| Diff calc | −0.2 | 36.0 (12.5) | 61.2 (11.9) | *2.9 (1.4, 4.5) | NR | NR | NR | *0.2 (0.1, 0.4) | NR | 2; 1 pnt HCL off | 2 |
| Comp base | 7.8 (0.6) | NR | NR | NR | NR | NR | NR | ||||
| Comp end | 7.9 (10.6) | NR | NR | NR | NR | NR | *0.4 (0.2, 0.7) | NR | |||
| Diff calc | 0 | 44.5 (12.7) | 51.6 (11.8) | *3.0 (1.8, 6.1) | NR | NR | NR | NR | |||
| Net effect (95% CI) | −0.3 (−0.6 to 0.1) | −7.7 (−11.0 to −4.4) | 8.9 (5.9 to 11.8) | a083 (0.62 to 1.1); p=0.18 | NR | NR | NR | ¥0.47 (0.22 to 1.1); p = 0.05 | NR | ||
| Thabit et al. 2015 adults:53 HCL vs. CSII + CGM; aged 40 (9.4) years; n = 33; crossover study; Tx 12 weeks. N Engl J Med 2015;373:2129–40 | |||||||||||
| Inter base | 7.6 (0.9) | NR | NR | NR | NR | NR | NR | ||||
| Inter end | 7.3 (0.8) | NR | NR | NR | NR | NR | NR | ||||
| Diff calc | −0.3 (0.21) | 29.2 (11.4) | 67.(10.60) | *2.9 (1.4, 4.5) | NR | NR | NR | *0.3 (0.1, 0.7) | NR | 1 | 1 |
| Comp base | 7.6 (0.8) | NR | NR | NR | NR | NR | NR | ||||
| Comp end | 7.6 (1.1) | NR | NR | NR | NR | NR | *0.4 (0.1, 0.9) | NR | 0 | 1 | |
| Diff calc | 0 (0.24) | 38.9 (16.6) | 56.8 (14.2) | *3.0 (1.8, 6.1) | NR | NR | NR | NR | |||
| Net effect (95% CI) | −0.3 (−0.5 to −0.1) | −9.6 (−13.0 to −6.3) | 11.0 (8.1 to 13.8) | a0.81 (0..68 to 0.96); p = 0.02 | NR | NR | NR | ¥0.45 (0.31 to 0.56) p < 0.001 |
NR | 1 | 0 |
| McAuley et al. 2022:50 intervention: HCL vs. LGS/PLGS; elderly adults aged 67 years (5); n = 30; crossover study; Tx 4 months | |||||||||||
| Inter base | 7.5 (6) | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Inter end | *7.3 (7.1, 7.5) | 23.6 (6.6) | 75.2 (6.3) | *1.21 (0.6, 1.68) | NR | *0.37 (0.12, 0.49) | *0.13 (0.03, 0.24) | NR | NR | 3 | 0 |
| DIFF | NR | NR | NR | NR | NR | NR | -NR | NR | NR | ||
| Comp base | 7.5 (6) | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Comp end | *7.5 (7.1, 7.9) | 29.0 (9.8) | 69.0 (9.1) | *1.69 (1.0, 2.54) | NR | *0.41 (0.2, 0.78) | *0.16 (0.10, 0.38) | NR | NR | 2 | 1 |
| Diff | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Net effect (95% CI) | −0.2 (−0.3 to 0.0) | −5.4 (−7.3 to −3.5) | 6.2 (4.4 to 8.0) | *−0.47 (−1.05 to −0.25) | NR | *−0.19 (−0.36 to −0.06) | *−0.11 (−0.16 to −0.05) | NR | NR | + 1 | −1 |
| In the 12 months pre trial there were n = 5 single severe hypo events and n = 4 patients with ≥ 2 severe hypo events. A minimum of 13 severe hypo events in 30 person-years ≈0.43/person-year. HCL rate was 0.3/person-year and SAP rate was diff 0.2/person-year | |||||||||||
| Boughton et al.47 HCL (CamAPS FX, CamDiabTM, Cambridge, UK) vs. CSII+CGM; crossover study; aged 68 years (63, 70) vs. 67 years (62, 70); n = 20 vs. n = 17; Tx 16 weeks. Sci Transl Med 2019;11 | |||||||||||
| Inter base | 7.5 (1.0) | *25.5 (15.1, 41.9) | 69.6 (14.1) | *1.8 (0.8, 3.2) | NR | NR | *0.1 (0.0, 0.4) | NR | NR | NR | |
| Inter end | 6.7 (0.7) | *16.7 (11.4, 23.9) | 79.9 (7.9) | *1.7 (1.3, 2.4) | *0.7 (0.5, 1.1) | NR | *0.2 (0.1, 0.3) | NR | NR | NR | |
| Diff | −0.8 (0.27) | *−8.8 | 10.3 | *−0.1 | NR | NR | NR | NR | NR | 0 | NR |
| Comp base | 7.4 (0.9) | *25.5 (15.9, 39.8) | 70.3 (13.7) | *1.6 (0.4, 2.7) | NR | NR | *0.1 (0.0, 0.4) | NR | NR | NR | |
| Comp end | 6.9 (0.9) | *21.4 (16.9, 36.50) | 71.4 (13.2) | *1.7 (0.9, 2.7) | *0.7 (0.4, 1.2) | NR | *0.2 (0.1, 0.3) | NR | NR | NR | |
| Diff | −0.5 (0.31) | *−4.1 | 1.1 | *0.1 | NR | NR | NR | NR | NR | 2 | NR |
| Net effect (95% CI) | −0.2 (−0.4 to −0.10) | *−8.5 (−10.9 to −6.1) | 8.6 (6.3 to 11.0) | *−0.1 (−0.3 to 0.2) | *0.0 (−0.2 to 0.1) | NR | *0.0 (−0.1 to 0.1) | NR | NR | −2 (17.6/100 person-years) | NR |
| Von dem Berge et al. 202254 HCL vs. LGS/PLGS; n = 38: aged 2–6 years (n = 18) and 14–17 years (n = 20); Tx 8 weeks. Crossover trial; Diabetes Obes Metab 2022;1–9 | |||||||||||
| Inter base | 7.4 (0.9) | 36.3 (14.5) | 60.4 (12.3) | NR | NR | NR | 0.8 (0.9) | 0 | 0 | ||
| Inter end | 6.9 (0.5) | 25.8 (8.1) | 70.8 (7.2) | NR | NR | NR | 0.8 (0.7) | 0 | 0 | ||
| Diff calc | −0.5 (0.17) | −10.5 (2.7) | 10.4 | NR | NR | NR | 0 | < 3.9 mM**16 (13.5, 19.0); < 3 mM**4 (3.4, 5.9) | NR | ||
| Comp base | 7.4 (0.9) | 36.3 (14.5) | 60.4 (12.3) | NR | NR | NR | 0.8 (0.9) | 0 | 0 | ||
| Comp end | 7.1 (0.6) | 36.5 (15.2) | 60.3 (13.9) | NR | NR | NR | 0.6 (0.50) | 0 | 0 | ||
| Diff calc | −0.3 (0.18) | −0.2 (3.41) | −0.1 | NR | NR | NR | −0.2 | < 3.9 mM**18 (13.7, 20.6);< 3 mM**3 (2.6, 4.6) | NR | ||
| Net effect 95% CI | p < 0.0002 | p < 0.0001 | p < 0.0001 | NR | NR | NR | NS | NS; NS | 0 | NR | |
| Kariyawasam et al. 2022:49 HCL vs. CSII + CGM; n = 20 (n = 17 for 6-week home phase); aged 2–6 years; crossover study; Tx 6 weeks. Lancet Digit Health; crossover RCT | |||||||||||
| Inter base | 7.6 (0.52) | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | |
| Inter end | NR | 31.1 (7.7) | 66.19 (6.5) | 2.62 (2.39) | NR | NR | 0.57 (0.77) | NR | 0 | 0 | |
| Diff calc | NR | NR | NR | NR | NR | NR | NR | NR | *13 (11.6)/person-year | NR | |
| Comp base | 7.4 (0.95) | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | |
| Comp end | NR | 36.11 (7.7) | 58.68 (6.5) | 5.24 (2.39) | NR | NR | 1.01 (0.77) | NR | 0 | 0 | |
| Diff calc | NR | NR | 7.51 | NR | NR | NR | NR | NR | *24.57 (12)/person-year | NR | |
| Net effect 95% CI (calc); reported p | NR | −5 (−10.2 to 0.18); p < 0.015 | 7.51 (3.14 to 11.8); p < 0.001 | −2.62 (−4.22 to −1.01); p < 0.0001 | NR | NR | −0.44 (−0.96 to −0.08); p < 0.003 | NR | −11.57 (−19.5 to −3.6); p < 0.0001 | 0 | 0 |
| Collyns et al.48 HCL vs. LGS/PLGS; n = 60; aged 23.5 years (7–65 years); Tx 4 weeks with 2–4 weeks’ run-in; crossover trial; all three age groups. ALL 59 (completed) | |||||||||||
| Inter base | 7.6 (0.9) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Inter end | NR | 27.5 (8.1) | 70.4 (8.1) | 2.1 (1.4) | NR | NR | 0.5 (0.5) | NR | 0 | 0 | 0 |
| Diff calc | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Comp base | 7.6 (0.9) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Comp end | NR | 39.6 (12.1) | 57.9 (11.7) | 2.5 (1.6) | NR | NR | 0.5 (0.5) | NR | 0 | 0 | 1 |
| Diff calc | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Net effect 95% CI (rep); reported p | −0.6 (−1.38 to 0.18) | −12.1 (9.0); p < 0.001 | 12.5 (8.5); p < 0.001 | −0.4 (1.3); p < 0.0318 | NR | NR | −0.1(0.4); p < 0.025 | NR | 0 | 0 | −1 |
| Collyns et al.48 HCL vs. LGS/PLGS; n = 19; aged 7–13 years; Tx 4 weeks with 2 weeks’ run-in; crossover RCT; children | |||||||||||
| Net effect 95% CI (rep); reported p | NR | −11.2 (8.0); p < 0.001 | 11.8 (7.4); p < 0.001 | −0.7 (1.8); p < 0.1216 | NR | NR | −0.2 (0.5); p < 0.067 | NR | NR | NR | NR |
| Collyns et al.48 HCL vs. LGS/PLGS; n = 14; aged 14–21 years; Tx 4 weeks with 2 weeks’ run-in; crossover RCT; adolescents | |||||||||||
| Net effect 95% CI (rep); reported p | NR | −14.0 (8.5); p < 0.001 | 14.4 (8.4); p < 0.001 | −0.74 (1.1); p < 0.1804 | NR | NR | −0.1 (0.3); p < 0.2441 | NR | NR | NR | NR |
| Collyns et al.48 HCL vs. LGS/PLGS; n = 26; aged 22–80 years; Tx 4 weeks with 2 weeks’ run-in; crossover RCT; adults | |||||||||||
| Net effect 95% CI (reported p) | NR | −11.8 (10); p < 0.001 | 11.9 (9.5); p < 0.001 | −0.1 (0.9); p < 0.5184 | NR | NR | −0.0 (0.2); p < 0.5462 | NR | NR | NR | NR |
| HbA1c% | % TIR > 10 mmol/l | % TIR > 7.8 mmol/l | % TIR 3.5–7.8 mmol/l | % TIR < 3.5 mmol/l | % TIR < 2.8 mmol/l | Hypo events median (range), unclear if IQR | N severe hypo | DKA event | |||
| Stewart et al. 201851 HCL vs. CSII + CGM; n = 16; aged 32.8 years (SD 5 years); Tx 4 weeks; crossover RCT; adult pregnant women; study reported TIRs that were in most cases atypical of other studies | |||||||||||
| Inter end | 6.6% | 14.6 | 36.1 | 62.3 | 1.6 | 0.2 | 8 (1 to 17) | 0 | NR | ||
| Comp end | 6.4% | 14.8 | 36.6 | 60.1 | 2.7 | 0.5 | 12.5 (1 to 53) | 0 | NR | ||
| Net effect 95% CI (rep) p | p 0.15 | −0.1 (−4.2 to 4.0); p 0.94 | −0.6 (−7.4 to 6.30); p 0.86 | 2.1 (−4.1 to 8.3); p 0.47 | −1.1 (−0.2 to −2.1); p 0.02 | −0.2 (−0.0 to −0.5); p 0.03 | p 0.04 | NR | |||
| No statistically significant improvement in glycaemic management over 4 weeks except for less time in hypoglycaemic range possible reflected in fewer hypo (non-severe) events | |||||||||||
Appendix 5 Glycated haemoglobin per cent additional figure
FIGURE 19.
Standard meta-analysis of MD between arms in change in HbA1c%: HCL vs. comparator.
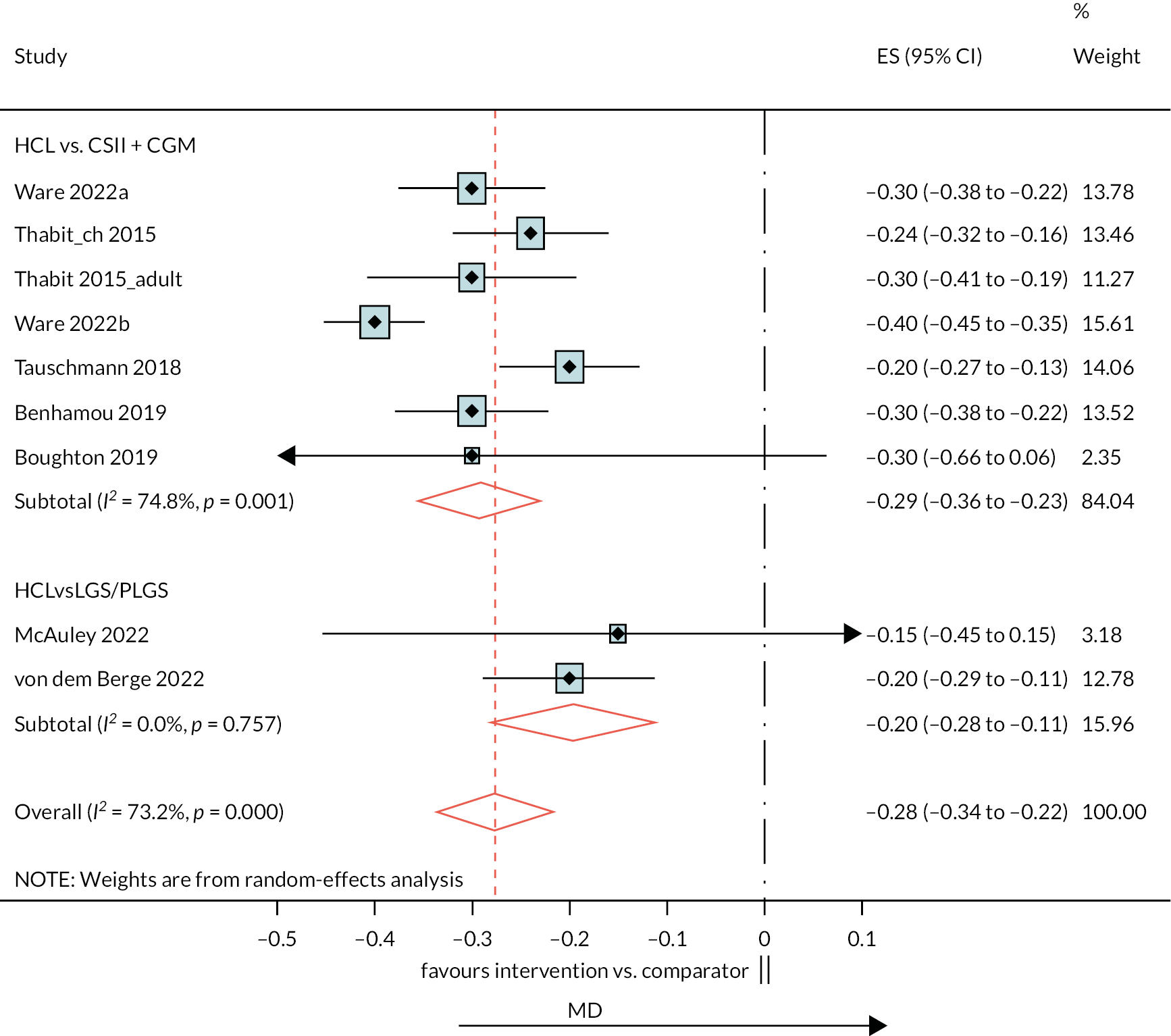
Appendix 6 Per cent time in range 3.9–10 mmol/l additional figure
FIGURE 20.
Standard meta-analysis of MD between arms in change in % TIR 3.9–10 mmol/l; HCL vs. comparator.
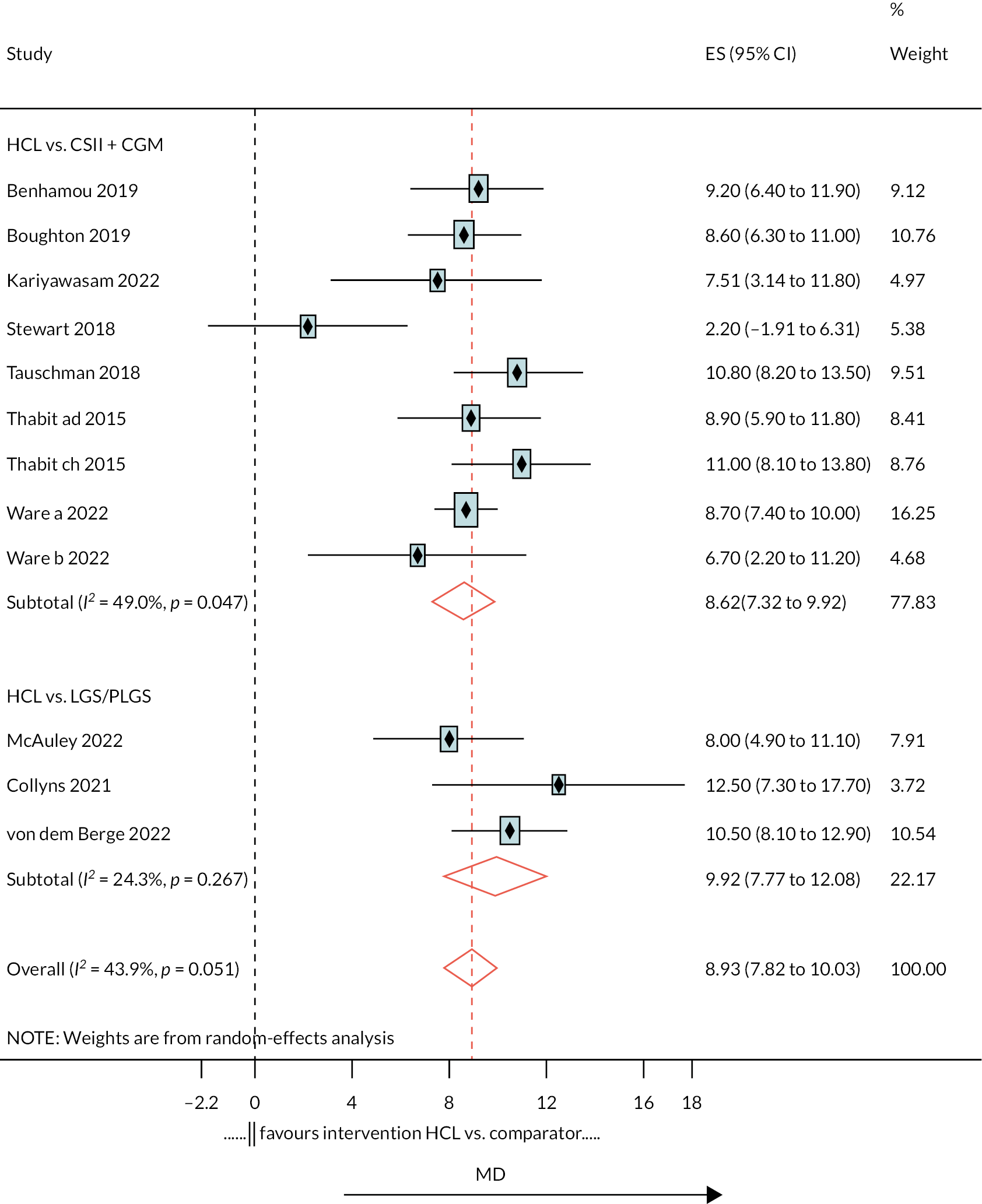
Appendix 7 Per cent of time above range > 10 mmol/l additional figure
FIGURE 21.
Standard meta-analysis of MD between arms in change in % TIR > 10 mmol/l.
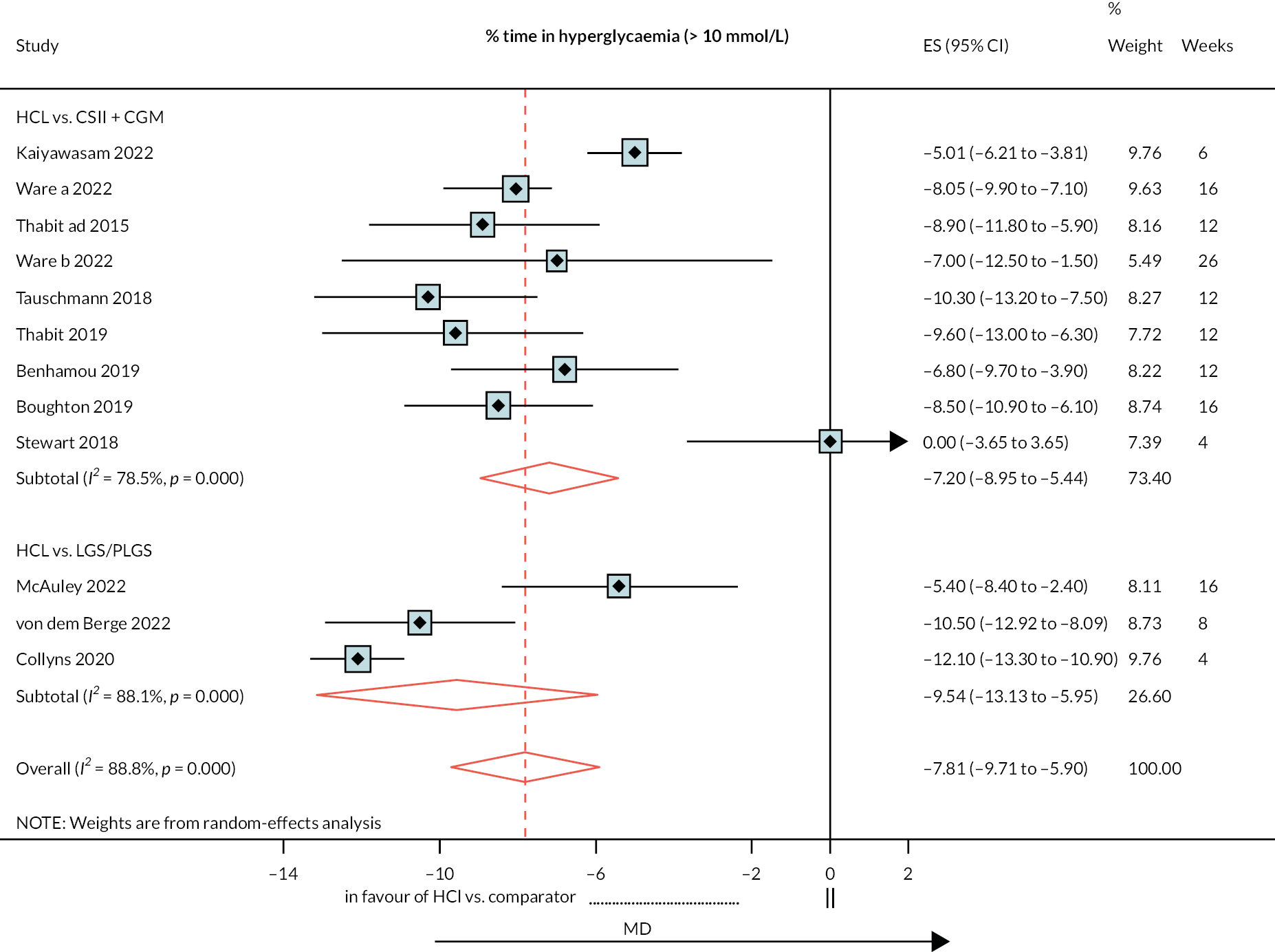
Appendix 8 Per cent time in range < 3.9 mmol/l additional figure
FIGURE 22.
Standard meta-analysis of MD between arms in change in % TIR < 3.9 mmol/l: HCL vs. comparator.
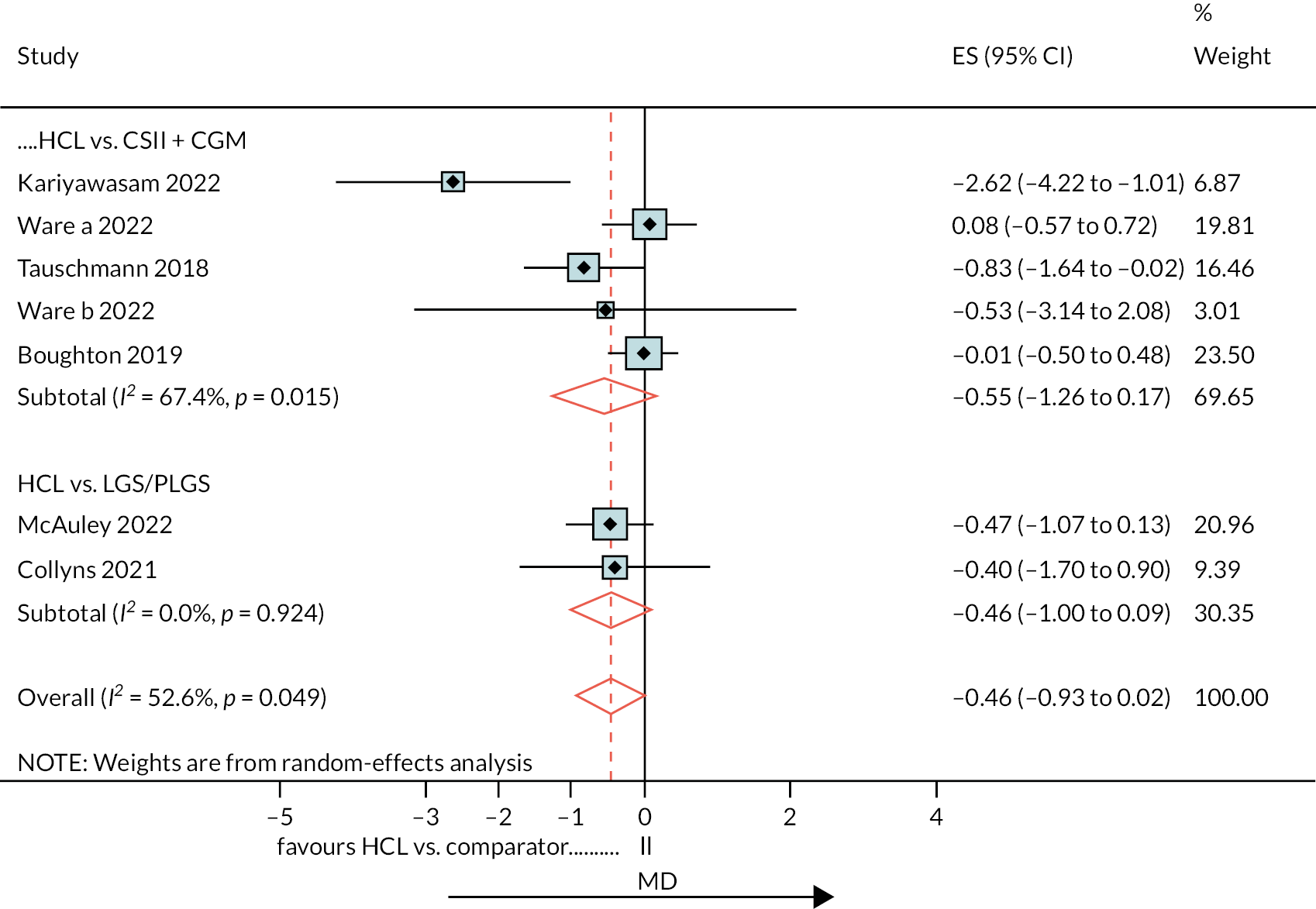
Appendix 9 Properties of randomised controlled trials not included in network meta-analysis but used for comparing hybrid closed-loop recipients in observational studies
| HbA1c%, mean SD | % TIR > 10 mmol/l, mean SD,*median IQR | % TIR 3.9–10.0 mmol/l, mean SD *median IQR | % TIR < 3.9 mmol/l (70 mg/dl), mean SD,*median IQR | % TIR < 3.5 mmol/l (63 mg/dl), mean SD,*median IQR | % TIR < 3.3 mmol/l (60 mg/dl), mean SD,*median IQR | % TIR < 3.0 mmol/l (54 mg/dl), mean SD,*median IQR | % TIR < 2.8 mmol/l (50 mg/dl), mean SD, *median IQR | N hypo non-severe *mean SD,**median IQR | N hypo severe *mean SD | DKA event *mean SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraham et al. 2021:128 HCL MiniMed 670G (Guardian 3 sensor, Guardian Link 3 transmitter) vs. CSII or 10% on multiple injections/day ± CGM vs. 5 years (3.1); n = 135; Tx 26 weeks | |||||||||||
| Inter base | 7.8 (1.0) | 41.8 (15.4) | 53.1 (13.0) | *2.9 (1.7, 6.4) | NR | *1.1 (0.6, 3.2) | *0.6 (0.2, 1.8) | 0.4 (0.1, 1.0) | NR | * 3 (3.0) | * 3(4.5) |
| Inter end | 7.5 (1.1) | 34.4 (13.0) | 62.5 (12.0) | *2.2 (1.7, 6.4) | NR | *0.8 (0.4, 2.0) | *0.4 (0.2, 1.8) | 0.3 (0.1, 0.5) | |||
| DIFF | −0.3 | −7.4 | 9.4 | *−0.7 | NR | *−0.3 | *−0.2 | −0.1 | 7 | ||
| Comp base | 7.7 (0.8) | 39.4 (14.5) | 54.6 (12.5) | *4.8 (2.6, 9.0) | NR | *2.2 (0.8, 4.60) | *1.3 (0.3, 2.8) | 0.7 (0.2, 1.7) | NR | * 3(4.4) | * 3(4.4) |
| Comp end | 7.6 | 37.9 (13.8) | 56.1 (12.2) | *4.1 (2.6, 8.7) | NR | *1.8 (0.7, 4.1) | *1.0 (0.4, 2.3) | 0.6 (0.2, 1.6) | |||
| DIFF | −0.1 | −1.5 | 1.5 | *−0.7 | NR | *−0.4 | *−0.3 | −0.1 | 13 | ||
| Rep. net effect (95% CI) | −0.3 (−0.5 to 0.0) | −4.3 (−8.8 to 0.2) | 6.7 (2.7 to 10.8) | *−1.9 (−2.5 to −1.3) | NR | *−1.0 (−1.2 to −0.50) | *−0.5 (−0.7 to −0.3) | −0.3 (−0.4 to −0.2) | - 6 | * 0 | * 0 |
| Breton et al. 2020:75 HCL vs. SAP; 11.3 years vs. −10.8 years; n = 78 vs. n = 23: Tx 16 weeks | |||||||||||
| Inter base N78 | 7.6 (1.0) | 45 (18) | 53 (17) | *1.2 (0.5, 2.4) | NR | NR | *0.1 (0.0, 0.4) | NR | NR | NR | NR |
| Inter end | 7.0 (0.8) | 31 (10) | 67 (10) | *1.6 (0.8, 2.4) | NR | NR | *0.2 (0.1, 0.4) | NR | NR | NR | NR |
| DIFF | −0.6 | −14 | 14 | 0.4 | NR | NR | 0.1 | NR | *0.5/week (0.1, 0.8) | 0 | 0 |
| Comp base N23 | 7.9 (0.9) | 47 (17) | 51 (16) | *1.0 (0.2, 2.1) | NR | NR | *0.1 (0.0, 0.3) | NR | NR | NR | NR |
| Comp end | 7.6 (0.9) | 43 (14) | 55 (13) | *1.8 (1.1, 3.0) | NR | NR | *0.3 (0.1. 0.6) | NR | NR | NR | NR |
| DIFF | −0.3 | −4 | 4 | 0.8 | NR | NR | 0.2 | NR | *0.6/week (0.1, 1.0) | 0 | 0 |
| Net effect (95% CI) | −0.4 (−0.9 to 0.1) | −10 (−14 to −6) | −10 (−14 to −6) | *−0.4 (−0.83 to −0.02) | NR | NR | *−0.07 (−0.19 to 0.02) | NR | P 0.16 | 0 | 0 |
| Brown et al. 2019:77 HCL vs. SAP; 33 years; n = 112 vs. n = 56; Tx 6 months | |||||||||||
| Inter base N112 | 7.40 (9.6) | 36 (19) | 61 (17) | 3.58 (3.39) | NR | NR | 0.90 (1.36) | NR | NR | NR | NR |
| Inter end | 7.06 (0.79) | 27 (12) | 71 (12) | 1.58 (1.15) | NR | NR | 0.29 (0.29) | NR | NR | NR | NR |
| DIFF | −0.34 | −SD | 10 | −2 | NR | NR | −0.61 | NR | *0.4/week (0.1, 0.9) | 0 | 1 (dev rel) |
| Comp base N56 | 7.4 (0.76) | 38 (15) | 59 (14) | 2.84 (2.54) | NR | NR | 0.56 (0.79) | NR | NR | NR | |
| Comp end | 7.39 (0.92) | 38 (15) | 59 (14) | 2.25 (1.46) | NR | NR | 0.35 (0.32) | NR | NR | NR | |
| DIFF | 0.01 | 0 | 0 | −0.59 | NR | NR | −0.21 | NR | *0.5/week (0.2,0.9) | 0 | 0 |
| Net effect (95% CI) | −0.3 (−0.53 to −0.13) | −10 (−13 to −8) | 11 (9 to 14) | −0.88 (−1.19 to −0.57) | NR | NR | −0.01 (−0.19 to −0.02) | NR | P 0.06 | 0 | 1 (dev rel) |
Appendix 10 Additional details on company submissions
Medtronic
-
Carlson et al.’s study63 assessed safety and change in glycaemia in adolescents and adults with T1DM during the Medtronic safety evaluation of the AHCL system. Both the run-in period and the study phase involved use of the AHCL study device that included the MiniMed 670G insulin pump (version 4.0 algorithm) with CGM system [the Guardian Sensor (version 3) glucose sensor and Guardian Link (version 3) transmitter]. This 3-month trial with a total 14,134 days of AHCL auto-basal and auto-correction use had no device-related SAEs and no serious or unanticipated device-related effects. There were no episodes of severe hypoglycaemia or DKA during the auto-basal and auto-correction-enabled study phase. Glycaemic-related outcomes of this study demonstrated reduced A1C and increased overall (24 hours per day) TIR in adolescents and adults using the AHCL system, when compared with a run-in period of SAP, PLGMs or automated basal insulin delivery use.
-
Da Silva et al.,68 in a report from 4120 users, analysed the safety and outcomes results of the MiniMed 780G system, which includes an AHCL algorithm that provides both automated basal and correction bolus insulin delivery in real-world settings. An improvement was reported over standard of care based on the ongoing trial (NCT03959423) that was confirmed by real-world evidence: 80% of the first 4120 AHCL users have reached glycaemic targets, that is TIR > 70% and a GMI < 7.0%.
-
Vigereski et al. 69 analysed safety and effectiveness outcomes of individuals using the MiniMed 780G system with the no-calibration Guardian 4 sensor during the first 3 months of use. Data are based on the published poster. There are inadequate data on participant history.
-
The FLAIR study27 compared the existing MiniMed 670G system with the new Medtronic AHCL system in adolescents and young adults with T1DM in a crossover trial at seven academic-based endocrinology practices (four in the USA, and one each in Germany, Israel and Slovenia). Both the MiniMed 670G and the AHCL system consisted of the same Medtronic 670G insulin pump and Guardian Sensor 3 continuous glucose monitor, with only the software differing between systems. The AHCL system was found to induce a greater reduction in hyperglycaemia during the day without an increase in hypoglycaemia than did the MiniMed 670G system. Time in the target glucose range increased from 57% to 67% with the AHCL system compared with 57% to 63% with the 670G system.
-
For the comparison between AHCL and SAP 1 PLGM in a two-sequence crossover study in New Zealand, 59 participants (35 female), with a mean age of 23.5 years, were recruited. AHCL improved % TIR 3.9–10.0 mmol/l (70–180 mg/dl) compared with SAP. There was one episode of mild DKA in the study, which occurred in the SAP 1 PLGM treatment period due to possible infusion set occlusion and a concurrent viral infection. There were no episodes of severe hypoglycaemia in the study. 48
-
Petrovsky et al.’s study72 described a structured initiation protocol of the MiniMed 670G HCL system in individuals with T1DM receiving MDI. This non-randomised single-centre study was conducted in Doha, Qatar, and enrolled individuals aged 7–18 years with T1DM > 1 year, receiving MDI with SMBG, with or without RT-CGM or isCGM, with no prior pump experience, and with an HbA1c level < 12.5%. An improvement in TIR was observed after 3 days in auto-mode; TIR continuously improved over time until it reached a plateau after 2 months. The authors reported that the improved clinical outcomes observed in the study were achieved in a safe manner, with no events of DKA or severe hypoglycaemia, and with no hospital admission, similar to the MiniMed 670G trials.
-
In an abstract, Slover et al. 155 evaluated whether the MiniMed 780G AHCL system may be effective in adult individuals with T1DM naive to CSII and CGM technologies. The report shows that people with T1DM naive to CSII and CGM technologies who switched directly to AHCL improved their glycaemic management, but there is no further information on participant history and intervention details.
Dexcom
The iDCL Trial Research Group conducted several feasibility and pilot studies of the Control-IQ system, and in 2019, Brown et al. 77 published the results of a 6-month randomised trial of this system. A multicentre RCT conducted across several centres in the USA evaluated a total of 168 patients who were randomly assigned in a 2 : 1 ratio to either the Control-IQ system (n = 112; HCL group) or the control group (n = 56; SAP therapy).
Breton et al. 75 conducted a 16-week RCT across four paediatric diabetes centres in the USA. A total of 101 patients were randomly assigned in a 3 : 1 ratio to either the Control-IQ system (n = 78; HCL group) or the control group (n = 23; SAP therapy). Patients in both groups attended follow-up visits at 2, 8 and 16 weeks.
Kanapka et al. 80 further evaluated the efficacy and safety of the Control-IQ system in the same cohort of children aged 6–13 years with a 12-week extension phase. A total of 100 patients who completed the 16-week RCT were entered into the extension phase and monitored for a further 12 weeks (a total of 28 weeks’ follow-up).
Ware et al. 56 recently published a study with the aim of assessing the efficacy and safety of the Cambridge HCL algorithm in children and adolescents with T1DM. This study was a parallel RCT conducted across seven UK and five US paediatric diabetes centres. A total of 133 patients were randomly assigned in a 1 : 1 ratio to either the CamAPS FX system (n = 65; HCL group) or the control group (n = 68; SAP therapy with or without glucose sensor). Patients in both groups attended follow-up visits at 13 and 26 weeks.
Some studies reported results of RCTs across different ski camps. Breton et al.’s156 study was a multisite, parallel RCT conducted across two ski camps (5-day ski camp; ≈5 hours skiing/day) in the USA. A total of 32 adolescents were randomised in a 1 : 1 ratio to either the UVA AP system (n = 16; HCL group) or the control group (n = 16); RM-SAP therapy. Ekhlaspour et al. 78 conducted the first superiority trial of the Control-IQ system in children and adolescents aged 6–18 years under real-world conditions. The study was a multisite, parallel RCT conducted across three ski camps (2-day ski-camp; ≈5 hours skiing/day) in the USA. A total of 48 participants were randomised in a 1 : 1 ratio to either the control-IQ system (n = 24; HCL group) or the control group (n = 24; RM-SAP therapy).
Forlenza et al. 79 conducted a 3-day home-use superiority trial in the 24 school children aged 6–12 years who participated in the 48-hour ski camp trial above. The study was a multisite, parallel RCT conducted during 3 days of home use at two clinical sites in the USA. A total of 24 school children were randomly assigned in a 1 : 1 ratio to either the Control-IQ system (n = 12; HCL group) or the control group (n = 12; SAP therapy).
Ware et al.,55 in a different study, aimed to evaluate the efficacy and safety of longer-term use of the Control-IQ system in young children using a larger sample size than in previously conducted trials. The study was a multicentre, crossover RCT conducted across diabetes centres in Europe over 16 weeks. A total of 74 children were first randomly assigned in a 1 : 1 ratio to either the Control-IQ system (n = 39; HCL group) or the control group (n = 35; SAP therapy). As the trial used a crossover design, participants received their assigned initial therapy for 16 weeks and then crossed over to the second trial therapy after a washout period of 1−4 weeks. Patients in both groups attended a follow-up visits every 4 weeks.
Boughton et al. 76 recently conducted one of the only multinational studies of HCL use specifically in older adults. The study adopted a multicentre, randomised, crossover (two-period) design across diabetes clinics at three UK centres and one Austrian centre. A total of 37 older adults were first randomly assigned in a 1 : 1 ratio to either the CamAPS FX system (n = 20; HCL group) or the control group (n = 17; SAP therapy). As the trial used a crossover design, participants received their assigned initial therapy for 16 weeks and then crossed over to the second trial therapy after a washout period of 4 weeks. Patients in both groups attended a follow-up visit every 4 weeks.
Overall, all studies except Breton et al. 75 reported a statistically significant between-group difference in HbA1c (%) reduction in favour of HCL compared with SAP systems, although statistical significance between systems was not reached in Breton et al. 75 In addition, all studies reported a statistically significant between-group difference in TIR (70–180 mg/dl) in favour of HCL compared with SAP systems.
The median number of hypoglycaemic events across trial periods was reported in two studies,75,77 although statistical significance was not reached between groups. The difference in the median number of hypoglycaemic events per week in the iDCL study77 was approaching statistical significance. 76
CAM-D
Boughton et al.’s study76 tested the hypothesis that using the Cambridge closed-loop algorithm in older adults with T1DM is safe and improves glucose management compared with SAP therapy. The study was a multicentre, multinational, crossover design contrasting 16 weeks of HCL insulin delivery with 16 weeks of sensor augmented pump therapy in 38 participants at three centres in the UK (Cambridge, Manchester and Birmingham) and one centre in Austria (Graz). The result shows that HCL algorithm is safe, and significantly improves glycaemic management compared with SAP therapy, without increasing hypoglycaemia in older adults with T1DM. The time spent in the target glucose range (3.9–10.0 mmol/l) with closed loop in this study population was high at 80%, and the 8.6%-point additional TIR compared with SAP therapy equates to an additional 2 hours each day in the target glucose range. Results show improvement in glycaemic management with closed loop without any increase in hypoglycaemia and in the context of a population with tight glycaemic management at baseline (baseline HbA1c 7.4%; 57 mmol/mol).
Bally et al.’s81 randomised crossover study recruited 31 adults (aged ≥ 18 years) attending diabetes clinics at Cambridge, UK, and Graz, Austria. Participants were randomly assigned to receive either day-and-night closed-loop insulin delivery followed by usual pump therapy with blinded CGM, or vice versa. The results of the study show that day-and-night HCL insulin delivery significantly improved overall glucose management while reducing hypoglycaemia progressively by 50–75% at lower glucose thresholds compared with usual insulin pump therapy. The findings of increased time spent in the glucose concentration target range, reduced hypoglycaemia and decreased glycaemic variability were similarly observed during night-time and daytime periods. These outcomes were achieved without change in total insulin delivery.
Leelarathna et al.’s study82 adopted a prospective multinational three-centre randomised crossover design with 17 adults with T1DM on insulin pump therapy over a 7-day home phase and a 1-day stay at a clinical research facility.
Stewart et al. 51 conducted a randomised two-period crossover study in pregnant women with T1DM to evaluate the safety, efficacy and longer-term feasibility of day-and-night closed-loop insulin delivery versus SAP therapy. Participants were randomly assigned to either 4 weeks of closed-loop (intervention) insulin delivery or 4 weeks of real-time CGM and CSII without the closed-loop system (SAP control) with a 1- to 2-week washout period before crossing to the alternate phase. No difference was found in the primary outcome of percentage of time in the target glucose range (63–140 mg/dl) during closed-loop and SAP therapy (62.3 vs. 60.1%; absolute difference 2.1%, 95% CI 24.1 to 8.3; p = 0.47). No episodes of severe hypoglycaemia occurred. The mean (SD) HbA1c was 6.6% (2.8%) [48.5 mmol/mol (7.5 mmol/mol)], 6.4% (2.7%) [46.3 mmol/mol (5.6 mmol/mol)] and 6.3% (2.7%) [45.9 mmol/mol (5.5 mmol/mol)] at baseline, end of closed-loop and end of SAP therapy, respectively.
Three studies by Tauschmann et al. reported results of a day-and-night closed-loop home trial in adolescents with T1DM under free-living conditions. 52,83 One study is a randomised two-period crossover design comparing automated closed-loop insulin delivery with SAP therapy over two 21-day periods in 12 subjects from paediatric diabetes clinics in the UK. 83 The results show that no serious adverse events or severe hypoglycaemic episodes were observed during either study period. The proportion of time that sensor glucose was in the target glucose range of 3.9–10.0 mmol/l (primary end point) was increased during closed-loop delivery compared with the control period (p = 0.001). The mean glucose level was significantly lower with closed-loop use (p = 0.001), as was the time spent above the target glucose range (p = 0.001).
The study extended findings from previous home trials in children and adolescents that were limited by a shorter intervention period. One of the previous trials was a prospective, single-centre, randomised crossover design contrasting automated closed-loop insulin delivery with sensor augmented pump therapy over 7 days. 84 Results show the proportion of time that the sensor glucose level was in the target glucose range of 3.9–10.0 mmol/l significantly increased during closed loop (p = 0.001). Closed-loop insulin delivery significantly reduced the mean glucose level (p = 0.028) and the time spent above target glucose level (p = 0.005) without increasing the time spent in hypoglycaemia. No serious adverse events or severe hypoglycaemic episodes were observed during either study period.
Tauschmann et al.’s study published in 2018 was a randomised, parallel design in multiple centres52 from the UK and the USA for comparing day-and-night HCL (closed-loop group) or SAP therapy (control group) during free-living over 12 weeks. The study reported a 10.8 percentage-point increase in time with glucose concentrations within the target glucose range across all age groups. This improvement resulted from a reduction in the time spent in hyperglycaemia without a change in total insulin delivery. The researchers observed a lower amount of bolus insulin and a higher amount of basal insulin in the closed-loop group than in the control group. Post randomisation, no severe hypoglycaemia occurred in either study group.
Ware et al. 55 evaluated the efficacy and safety of longer-term use of the Control-IQ system in young children in an open-label, multicentre, crossover RCT conducted across diabetes centres in Europe over 16 weeks. A total of 74 children were first randomly assigned in a 1 : 1 ratio to either the Control-IQ system (n = 39; HCL group) or the control group (n = 35; SAP therapy). As the trial used a crossover design, participants received their assigned initial therapy for 16 weeks and then crossed over to the second trial therapy after a washout period of 1−4 weeks. Patients in both groups attended a follow-up visit every 4 weeks. The primary outcome was the between treatment difference in the % TIR of 70−180 mg/dl.
In a separate study, Ware et al. 56 adopted an open-label, multicentre, multinational, one-period, randomised design comparing HCL insulin delivery with insulin pump therapy, with and without glucose sensor, over 6 months. Participants were recruited from diabetes outpatient clinics at seven UK and five US paediatric diabetes centres. One hundred and thirty-three eligible participants were randomly assigned to treatment (65 to the closed-loop group and 68 to the control group). The study reported a difference in efficacy between the two closed-loop system hardware configurations using the same algorithm, with an 11.5 mmol/mol (1.05%) reduction in HbA1c in the CamAPS FX cohort compared with the control, and no reduction in HbA1c in the FlorenceM cohort. No treatment effect in the cohort using the FlorenceM hardware was observed, contrasting with a treatment effect observed in the CamAPS FX cohort, which used more reliable components and a factory-calibrated glucose sensor.
Tandem
One is a poster that was presented at the Australian Diabetes Conference. 85 Of the two papers presented, one went through peer review and has been published online in Diabetes Technology and Therapeutics85 and the other is a version before peer review that has been submitted to the Diabetes Care journal.
Singh’s study (presented as a poster)85 reported an analysis of 71,686 people from the USA with T1DM who were on-boarded to Control-IQ technology between August 2020 and February 2022. The authors reported stratified data based on the prior therapy and age group. The results show that by using Control-IQ technology, GMI reflected clinically significant glycaemic improvement [7.1%, (95% CI 6.8–7.5), p < 0.001].
Glycaemic improvements were also demonstrated by prior therapy: prior MDI users at baseline = 8.2% (95% CI 7.2–9.5) to 7.2% (95% CI 6.9–7.6) at post, p < 0.001; prior pump users = 7.5% (95% CI 6.9–8.3) to 7.1% (95% CI 6.8–7.5), p < 0.001, and by age group: paediatrics at baseline = 8.2% (95% CI 7.3–9.3) to 7.5% (95% CI 7.1–7.9) at post, p < 0.001; adults = 7.7% (95% CI 7.0–8.8) to 7.1% (95% CI 6.8–7.5), p < 0.001; and older adults = 7.3% (95% CI 6.8–8.0) to 7.0% (95% CI 6.7–7.2), p < 0.001.
Forlenza et al.’s study86 includes 5575 patients in the USA who were covered by Medicare insurance (over the age of 65 years) or Medicaid insurance (disadvantaged youth) in a real-world retrospective analysis to assess glycaemic management outcomes with CIQ use among Medicare and Medicaid beneficiaries with any type of diabetes and those with T2DM with either type of insurance. Glycaemic outcomes were calculated for all participants who had at least 30 days of CGM data with ≥ 75% CGM availability before and after Control-IQ initiation. In this cohort 806 users who transitioned from MDI therapy to CIQ therapy had a higher baseline GMI at 7.9% and saw a significant decline in GMI to 7.1% (difference of −0.8%; p < 0.0001). [GMI = 3.31 + 0.02392 × (mean glucose in mg/dl). Average glucose is calculated over the entire time a customer used a Tandem pump in accordance with the guidelines above.]157 Across all age groups TIR also significantly increased without a significant change in level 1 or level 2 hypoglycaemia. The results show a significant reduction in GMI in the Medicare group of 0.3% and in the Medicaid group of 0.4%. There was also a significant improvement in TIR in the Medicare group of 10%, in the Medicaid group of 14%, and in the T2D subset of 8%.
(Confidential information has been removed.)
Appendix 11 Evidence Assessment Group scenario analyses’ incremental cost-effectiveness ratios: hybrid closed loop versus continuous subcutaneous insulin infusion + continuous glucose monitoring
| Δ Costs (£) | Δ QALYs | ICER (£) | |
|---|---|---|---|
| Base case | 28,628 | 0.160 | 179,000 |
| SA01a: Only adult studies | 28,734 | 0.141 | 204,000 |
| SA01b: Benhamou et al. excluded | 28,096 | 0.169 | 166,000 |
| SA02a: NHS adult pilot baseline characteristics | 25,775 | 0.205 | 126,000 |
| SA02b: NHS adult pilot characteristics and effect | 12,447 | 1.004 | 12,398 |
| SA02c: SA02b + reduced complication costs | 21,669 | 1.004 | 21,583 |
| SA03a: 8-year time horizon | 12,740 | 0.014 | 910,000 |
| SA03b: 12-year time horizon | 16,601 | 0.025 | 664,000 |
| SA03c: 24-year time horizon | 23,975 | 0.073 | 328,000 |
| SA04a: 5-year HbA1c effect | 29,571 | 0.045 | 657,000 |
| SA04b: 10-year HbA1c effect | 28,887 | 0.068 | 425,000 |
| SA04c: 20-year HbA1c effect | 28,369 | 0.115 | 247,000 |
| SA05a: NSHEs with HCL 20.8 annual | 28,628 | 0.170 | 169,000 |
| SA05b: NSHEs with HCL 57.2 annual | 28,628 | 0.173 | 166,000 |
| SA05c: NSHEs with HCL 13.0 annual | 28,628 | 0.168 | 170,000 |
| SA06: HEs: NSHEs and SHEs | 28,325 | 0.174 | 163,000 |
| SA07a: SA06 + HEs Currie values | 28,325 | 0.235 | 121,000 |
| SA07b: SA06 + HEs Currie and Nauck values | 28,325 | 0.260 | 109,000 |
| SA08a: SA06 + £36/£628 SHE cost | 28,246 | 0.174 | 162,000 |
| SA08b: SA06 + £381 SHE cost | 28,069 | 0.174 | 161,000 |
| SA09: SA06 + HEs double quality-of-life effect | 28,325 | 0.188 | 151,000 |
| SA10a: CSII 85% isCGM 15% rtCGM | 27,117 | 0.160 | 169,000 |
| SA10b: CSII 95% isCGM 5% rtCGM | 30,139 | 0.160 | 188,000 |
| SA11: HCL/PLGS annual cost £500 more | 38,244 | 0.160 | 239,000 |
| SA12: CSII to HCL training cost £1132 | 29,760 | 0.160 | 186,000 |
| SA13a: All-cause mortality | 27,846 | 0.139 | 200,000 |
| SA13b: Non-specific mortality excluding H.T. | 28,556 | 0.171 | 167,000 |
| SA14: Annual 0.045% HbA1c worsening | 27,694 | 0.181 | 153,000 |
Appendix 12 Incremental and pooled analysis (hybrid closed loop vs. continuous subcutaneous insulin infusion + continuous glucose monitoring)
| Description | CSII + isCGM | CSII + rtCGM | HCL | Net QALY | Net cost (£) | ICER | |
|---|---|---|---|---|---|---|---|
| BASE | Base case | Reference | Extendedly dominated | 105,620 | 0.332 | 33,802 | £101,753 |
| SA01A | HCL-rtCGM regression, baseline HbA1c 7.4% | Reference | Extendedly dominated | 160,700 | 0.228 | 36,109 | £158,444 |
| SA01B | HCL-rtCGM regression, baseline HbA1c 7.6% | Reference | Extendedly dominated | 133,382 | 0.273 | 35,410 | £129,896 |
| SA01C | HCL-rtCGM regression, baseline HbA1c 7.8% | Reference | Extendedly dominated | 120,924 | 0.292 | 34,249 | £117,410 |
| SA01D | HCL-rtCGM regression, baseline HbA1c 8% | Reference | Extendedly dominated | 105,053 | 0.331 | 33,500 | £101,146 |
| SA01E | HCL-rtCGM regression, baseline HbA1c 8.2% | Reference | Extendedly dominated | 87,375 | 0.386 | 32,522 | £84,231 |
| SA01F | HCL-rtCGM regression, baseline HbA1c 8.4% | Reference | Extendedly dominated | 81,780 | 0.398 | 31,496 | £79,037 |
| SA01G | HCL-rtCGM regression, baseline HbA1c 8.6% | Reference | Extendedly dominated | 75,545 | 0.415 | 30,049 | £72,478 |
| SA01H | HCL-rtCGM regression, baseline HbA1c 8.8% | Reference | Extendedly dominated | 64,255 | 0.467 | 28,591 | £61,222 |
| SA01I | HCL-rtCGM regression, baseline HbA1c 9% | Reference | Extendedly dominated | 52,980 | 0.541 | 27,207 | £50,243 |
| SA02A | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.4% | Reference | Extendedly dominated | 150,086 | 0.247 | 36,335 | £146,869 |
| SA02B | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.6% | Reference | Extendedly dominated | 129,407 | 0.280 | 35,392 | £126,265 |
| SA02C | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.8% | Reference | Extendedly dominated | 113,742 | 0.309 | 34,095 | £110,341 |
| SA02D | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8% | Reference | Extendedly dominated | 101,816 | 0.334 | 33,287 | £99,544 |
| SA02E | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.2% | Reference | Extendedly dominated | 84,500 | 0.394 | 32,331 | £82,058 |
| SA02F | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.4% | Reference | Extendedly dominated | 74,194 | 0.426 | 30,852 | £72,371 |
| SA02G | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.6% | Reference | Extendedly dominated | 67,379 | 0.460 | 29,827 | £64,883 |
| SA02H | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.8% | Reference | Extendedly dominated | 57,288 | 0.519 | 28,603 | £55,112 |
| SA02I | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 9% | Reference | Extendedly dominated | 47,109 | 0.590 | 26,696 | £45,277 |
| SA03A | Stroke costs 60-year-old | Reference | Extendedly dominated | 105,565 | 0.332 | 33,784 | £101,697 |
| SA03B | Stroke costs with social care costs | Reference | Extendedly dominated | 105,549 | 0.332 | 33,778 | £101,681 |
| SA03C | Stroke costs as per previous EAG base case | Reference | Extendedly dominated | 105,664 | 0.332 | 33,817 | £101,799 |
| SA04 | Annual 0.045% HbA1c worsening | Reference | Extendedly dominated | 99,958 | 0.328 | 31,946 | £97,396 |
| SA05 | Excluding Benhamou et al. | Reference | Extendedly dominated | 109,831 | 0.315 | 33,405 | £105,981 |
| SA06 | Adjusted complication costs | Reference | Extendedly dominated | 111,139 | 0.332 | 35,617 | £107,215 |
| SA07A | Include NSHEs with HCL 20.8 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 104,271 | 0.337 | 33,802 | £100,307 |
| SA07B | Include NSHEs with HCL 57.2 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 104,000 | 0.338 | 33,802 | £100,004 |
| SA07C | Include NSHEs with HCL 13.0 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 104,484 | 0.336 | 33,802 | £100,538 |
| SA07D | Include NSHEs with HCL 90.0 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 103,974 | 0.338 | 33,802 | £99,967 |
| SA08 | Include NSHEs as per SA07A and SHEs (base TBR < 3.0) | Reference | Extendedly dominated | 85,970 | 0.399 | 33,358 | £83,520 |
| SA09A | SA08 using Currie values for NSHE and SHE | Reference | Extendedly dominated | 90,516 | 0.384 | 33,358 | £86,907 |
| SA09B | SA08 using Gordon values for NSHE and SHE | Reference | Extendedly dominated | 101,022 | 0.343 | 33,358 | £97,310 |
| SA10A | SA08 with £36/£628 SHE cost | Reference | Extendedly dominated | 85,665 | 0.399 | 33,242 | £83,230 |
| SA10B | SA08 with £381 SHE cost | Reference | Extendedly dominated | 84,982 | 0.399 | 32,982 | £82,580 |
| SA11 | SA08 with NSHE cost £5 | Reference | Extendedly dominated | 85,324 | 0.399 | 33,069 | £82,797 |
| SA12 | SA08 with double HE quality-of-life effect | Reference | Extendedly dominated | 73,169 | 0.467 | 33,358 | £71,491 |
| SA13 | SA08 but NSHE all TBR < 3.9 estimates | Reference | Extendedly dominated | 99,126 | 0.349 | 33,358 | £95,615 |
| Description | CSII + isCGM | CSII + rtCGM | HCL (£) | Net QALY | Net cost (£) | ICER (£) | |
|---|---|---|---|---|---|---|---|
| BASE | Base case | Reference | Extendedly dominated | 115,473 | 0.332 | 37,246 | 112,118 |
| SA01A | HCL-rtCGM regression, baseline HbA1c 7.4% | Reference | Extendedly dominated | 175,019 | 0.228 | 39,622 | 173,858 |
| SA01B | HCL-rtCGM regression, baseline HbA1c 7.6% | Reference | Extendedly dominated | 145,456 | 0.273 | 38,903 | 142,711 |
| SA01C | HCL-rtCGM regression, baseline HbA1c 7.8% | Reference | Extendedly dominated | 132,139 | 0.292 | 37,718 | 129,305 |
| SA01D | HCL-rtCGM regression, baseline HbA1c 8% | Reference | Extendedly dominated | 114,936 | 0.331 | 36,943 | 111,544 |
| SA01E | HCL-rtCGM regression, baseline HbA1c 8.2% | Reference | Extendedly dominated | 95,753 | 0.386 | 35,937 | 93,078 |
| SA01F | HCL-rtCGM regression, baseline HbA1c 8.4% | Reference | Extendedly dominated | 89,822 | 0.398 | 34,888 | 87,549 |
| SA01G | HCL-rtCGM regression, baseline HbA1c 8.6% | Reference | Extendedly dominated | 83,266 | 0.415 | 33,422 | 80,612 |
| SA01H | HCL-rtCGM regression, baseline HbA1c 8.8% | Reference | Extendedly dominated | 71,068 | 0.467 | 31,929 | 68,370 |
| SA01I | HCL-rtCGM regression, baseline HbA1c 9% | Reference | Extendedly dominated | 58,800 | 0.541 | 30,502 | 56,329 |
| SA02A | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.4% | Reference | Extendedly dominated | 163,368 | 0.247 | 39,846 | 161,060 |
| SA02B | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.6% | Reference | Extendedly dominated | 141,113 | 0.280 | 38,885 | 138,727 |
| SA02C | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 7.8% | Reference | Extendedly dominated | 124,333 | 0.309 | 37,563 | 121,564 |
| SA02D | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8% | Reference | Extendedly dominated | 111,448 | 0.334 | 36,733 | 109,847 |
| SA02E | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.2% | Reference | Extendedly dominated | 92,657 | 0.394 | 35,747 | 90,729 |
| SA02F | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.4% | Reference | Extendedly dominated | 81,628 | 0.426 | 34,243 | 80,325 |
| SA02G | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.6% | Reference | Extendedly dominated | 74,325 | 0.460 | 33,195 | 72,210 |
| SA02H | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 8.8% | Reference | Extendedly dominated | 63,368 | 0.519 | 31,937 | 61,535 |
| SA02I | HCL-rtCGM and rtCGM-isCGM regressions, baseline HbA1c 9% | Reference | Extendedly dominated | 52,379 | 0.590 | 29,989 | 50,863 |
| SA03A | Stroke costs 60-year-old | Reference | Extendedly dominated | 115,418 | 0.332 | 37,227 | 112,062 |
| SA03B | Stroke costs with social care costs | Reference | Extendedly dominated | 115,402 | 0.332 | 37,222 | 112,046 |
| SA03C | Stroke costs as per previous EAG base case | Reference | Extendedly dominated | 115,517 | 0.332 | 37,261 | 112,163 |
| SA04 | Annual 0.045% HbA1c worsening | Reference | Extendedly dominated | 109,582 | 0.328 | 35,311 | 107,656 |
| SA05 | Excluding Benhamou et al. | Reference | Extendedly dominated | 120,196 | 0.315 | 36,851 | 116,912 |
| SA06 | Adjusted complication costs | Reference | Extendedly dominated | 120,993 | 0.332 | 39,060 | 117,580 |
| SA07A | Include NSHEs with HCL 20.8 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 113,998 | 0.337 | 37,246 | 110,525 |
| SA07B | Include NSHEs with HCL 57.2 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 113,702 | 0.338 | 37,246 | 110,191 |
| SA07C | Include NSHEs with HCL 13.0 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 114,232 | 0.336 | 37,246 | 110,779 |
| SA07D | Include NSHEs with HCL 90.0 annual (base TBR < 3.9 and NG17) | Reference | Extendedly dominated | 113,673 | 0.338 | 37,246 | 110,150 |
| SA08 | Include NSHEs as per SA07A and SHEs (base TBR < 3.0) | Reference | Extendedly dominated | 94,100 | 0.399 | 36,801 | 92,141 |
| SA09A | SA08 using Currie values for NSHE and SHE | Reference | Extendedly dominated | 99,075 | 0.384 | 36,801 | 95,877 |
| SA09B | SA08 using Gordon values for NSHE and SHE | Reference | Extendedly dominated | 110,575 | 0.343 | 36,801 | 107,355 |
| SA10A | SA08 with £36/£628 SHE cost | Reference | Extendedly dominated | 93,795 | 0.399 | 36,685 | 91,851 |
| SA10B | SA08 with £381 SHE cost | Reference | Extendedly dominated | 93,111 | 0.399 | 36,426 | 91,201 |
| SA11 | SA08 with NSHE cost £5 | Reference | Extendedly dominated | 93,453 | 0.399 | 36,512 | 91,418 |
| SA12 | SA08 with double HE quality-of-life effect | Reference | Extendedly dominated | 80,087 | 0.467 | 36,801 | 78,870 |
| SA13 | SA08 but NSHE all TBR < 3.9 estimates | Reference | Extendedly dominated | 108,499 | 0.349 | 36,801 | 105,485 |
List of abbreviations
- AHCL
- advanced hybrid closed loop
- AHRQ
- Agency for Healthcare Research and Quality
- CADTH
- Canadian Agency for Drugs and Technology in Health
- CDM
- CORE Diabetes Model
- CEAC
- cost-effectiveness acceptability curve
- CGM
- continuous glucose monitoring plus rtCGM
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CSII
- continuous subcutaneous insulin infusion
- CVD
- cardiovascular disease
- CYP
- children and young people
- DAFNE
- Dose Adjustment for Normal Eating
- DAFNE-HART
- DAFNE-Hypoglycaemia Awareness Restoration Training
- DCCT
- Diabetes Control and Complications Trial
- DKA
- diabetic ketoacidosis
- EAG
- Evidence Assessment Group
- EDIC
- Epidemiology of Diabetes Interventions and Complications
- EQ-5D
- EuroQol-5 Dimensions
- ES
- effect estimate
- ESRD
- end-stage renal disease
- FGM
- flash glucose monitoring
- FLAIR
- Fuzzy Logic Automated Insulin Regulation
- GMI
- glucose management indicator
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCL
- hybrid closed loop
- HFS
- Hypoglycaemia Fear Survey
- HFS1-ws
- Hypoglycaemia Fear Survey version 1 worry subscale
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- isCGM
- intermittently scanned continuous glucose monitoring
- LGS
- low glucose suspend
- MD
- mean difference
- MDI
- multiple daily injections
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NSHE
- non-severe hypoglycaemic event
- PLGM
- predictive low-glucose management
- PLGS
- predictive low glucose suspend
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- rtCGM
- real-time continuous glucose monitoring
- SAP
- sensor-augmented pump
- SEK
- Swedish krona
- SHE
- severe hypoglycaemic event
- SHTG
- Scottish Health Technologies Group
- SMBG
- standard self-monitoring of blood glucose
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- TIR
- time in range
- TTO
- time trade-off
- UKPDS
- United Kingdom Prospective Diabetes Study
Note
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/JYPL3536).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
