Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 17/140/02. The contractual start date was in April 2019. The draft manuscript began editorial review in May 2023 and was accepted for publication in January 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Mihaylova et al. This work was produced by Mihaylova et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mihaylova et al.
Chapter 1 Introduction
Background and definition of the clinical problem
Statins are a class of lipid-lowering medications that have been reliably shown in large randomised controlled trials to reduce the risk of heart attacks, strokes and vascular mortality in different categories of patients. Since the 1990s, the Cholesterol Treatment Trialists’ Collaboration (CTTC) has co-ordinated a prospective meta-analysis of all large statin trials. 1 This work has demonstrated that statins produce similar proportional reductions of about one-quarter in cardiovascular disease (CVD) risk per 1-mmol/l reduction in low-density lipoprotein cholesterol (LDL-C) in a wide range of people [e.g. men and women; older and younger people; people with and without previous CVD; people with high and low CVD risk; people with diabetes or non-dialysis-dependent chronic kidney disease (CKD)], and that further reductions in LDL-C with more intensive statin therapy produce further reductions in CVD risk. 2–8
There is some controversy, however, over the size of statin therapy’s net benefits for people at low CVD risk, and for elderly people, and there are concerns about the safety of statins. Many of the safety concerns originate from non-randomised studies9,10 and may be unreliable. 11 Nevertheless, in randomised trials, standard statin dose regimens have been associated with a proportional increase of about 10% in incident diabetes,12 and more intensive statin regimens with about twice this risk. 13 Such adverse effects should be considered in evaluating the net effects of statins particularly in people at low CVD risk.
Cost-effectiveness analyses help decision-makers obtain better value for money by targeting healthcare resources at interventions and population groups where the net health gain is greatest in relation to the net cost. However, questions remain as to how such analyses should be performed, which leads decision-makers to question their reliability. Decision-analytic models, typically developed using summary data from multiple sources, and (summary) treatment effects from randomised clinical trials, are commonly used but are likely to be of limited reliability in particular categories of people defined by multiple characteristics (e.g. by disease risk, age, sex, comorbidities).
Rationale for the study
Statins are now cheap14 and the direct costs to the NHS are less of a concern, but the efforts required to initiate and support people on treatment should not be understated. In England and Wales, the National Institute for Health and Care Excellence (NICE) recommends treatment with high-intensity statin therapy for all individuals with a history of previous CVD or diabetes and, since 2014, treatment with medium-intensity statins for those without such a history who have a 10-year CVD risk ≥ 10%. 15,16 Furthermore, this guidance also states that statin treatment should not be ruled out if the estimated 10-year CVD risk was ˂ 10% if the patient had an informed preference for taking statin or their risk might be underestimated, opening the possibility for wider statin use but stopping short of concrete guidance. The economic evaluations of statin therapy for CVD prevention in these guidelines followed the conventional approach of decision- analytic modelling using published summary data. An evaluative cost-effectiveness framework based on summary data, however, does not allow for reliable assessment of disease risks and treatment effects over time or in categories of patients. Furthermore, in the absence of an assessment of model validity, the reliability of the results is unclear.
The NICE guidance affects a large section of the population (about 37% of 30- to 84-year-olds17). Unreliable cost-effectiveness results could lead to the consequence that people who do not derive worthwhile health benefit from the statin treatment are recommended for the treatment, while people who could derive worthwhile benefit from the statin treatment are not recommended for the treatment or are recommended for a suboptimal intensity of statin treatment. The uncertainty in evidence can also affect the level of implementation of guideline recommendations with ample evidence indicating suboptimal statin use among people recommended for treatment18–20 with both individual patient and prescriber factors likely contributing.
Consequently, in 2014 a research recommendation was made in NICE CG181 for the development of a cost-effectiveness analysis of statins informed by the individual participant data (IPD) of randomised clinical trials. 16 This recommendation was taken forward in this project. The following priority research areas were identified: (1) use of IPD from randomised clinical trials of statins to develop more detailed and reliable (e.g. based on time-to-event analysis) cost-effectiveness analyses and (2) use of such analyses to produce detailed results for the effectiveness and cost-effectiveness of statin therapies in categories of patients by CVD risk and other patient characteristics (e.g. age, sex, comorbidities). These analyses are timely in view of the increasing availability of statins (i.e. all widely used statins are now available generically in the UK); the growing evidence for the effectiveness of new treatments [e.g. ezetimibe, PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors]; and the developments in UK CVD risk scoring (e.g. QRISK®3 10-year CVD risk score21).
Cost-effectiveness studies embedded within individual statin trials have demonstrated an ability to evaluate the cost-effectiveness of statin regimens in categories of patients. 22–26 By combining IPD-based multivariable time-to-event disease risk equations with estimates of the relative effects of statins on disease risks into interlinked disease models, such frameworks have evaluated statins’ cost-effectiveness reliably in particular categories of participants in the trials (e.g. by CVD risk, age and gender). 23,25 The CTTC database is a unique resource for the development of further, more detailed analyses to guide statin recommendations for individual patients. It includes IPD from large statin trials among nearly 180,000 participants with well-documented baseline characteristics and first occurrence of major vascular, cancer and mortality events during the studies’ follow-up periods. However, trials in the database recruited their participants from different countries during the period 1980s to 2010 and, therefore, participants’ disease risks may potentially not generalise well to the contemporary UK population.
In this project we aimed to strengthen the evidence about the cost-effectiveness of statins using the CTTC IPD together with large contemporary UK population cohorts. The cost-effectiveness analysis of statin regimens developed using these data would account for the timing of disease events and the beneficial and adverse effects of statin therapies and allow for more reliable estimates of the net effects of statin therapies of different intensity on quality-adjusted life expectancy and healthcare costs in categories of patients.
The research question addressed in this project was ‘What is the cost-effectiveness of different statin therapies in different categories of people?’. We assessed the effects of standard statin therapy, defined as a therapy that achieves about 35–45% LDL-C reduction (e.g. atorvastatin 20 mg/day, rosuvastatin 5–10 mg/day or simvastatin 40–80 mg/day), and of higher-intensity statin therapy achieving ≥ 45% reduction (e.g. atorvastatin 40–80 mg/day, rosuvastatin 20–40 mg/day) (Table 1).
Changes to methods
The project was developed following a prospectively developed study protocol. Protocol versions 1 (April 2019) and 2 (November 2022) are available at https://fundingawards.nihr.ac.uk/award/17/140/02. 28 Three changes were made from version 1 to version 2 of the study protocol. First, the decision not to model recurrent CVD events of the same type was taken because such events were not available in the CTTC database29 at the onset of the project and the subsequently developed microsimulation model was judged to be of sufficient depth and to have an excellent performance in key participant categories. Second, we did not use the Heart Protection Study long-term follow-up data30 in the microsimulation model validation as the model validation in the UK Biobank (UKB) cohort31 indicated a need to calibrate the model in a contemporary population cohort such as UKB. Third, the decision was taken to use the UKB resurvey data to derive a cohort of people aged ≥ 70 years to guide the development of the assessment of value of statin therapy in older people.
Subsequent to protocol version 2, there have been only limited changes. First, the Whitehall II study data contributed more substantively to the development of the statin cost-effectiveness study in older people. In particular, we used the Whitehall II study32 data from Phase 9 onwards (2007–9 survey with 10–12 years’ follow-up thereafter) to validate the performance of the microsimulation model both in people 40–70 years and in people ≥ 70 years old. Second, to increase the size of the older people cohort, we used the UKB and Whitehall II studies’ data for participants ≥ 70 years old together, instead of UKB data alone, to project and present results for cost-effectiveness of statin therapy in older people. Finally, we decided against reporting results for cost-effectiveness of statin therapy by lifetime CVD risk (QRISKlifetime33), as statin therapy was already shown to be highly effective and cost-effective across all categories of people aged ≥ 40 years.
Organisation of the report
This report is compiled using a number of chapters presenting different components of the project.
Chapter 2 outlines the initial model development using the IPD of large statin trials in the CTTC database to estimate key CVD and nonvascular disease risk equations.
Chapter 3 describes the CVD microsimulation policy model calibration, further development, and validation in the UKB database and in the Whitehall II study.
Chapter 4 presents the hospital and primary care cost regression models, estimated using the UKB cohort and linked hospital admissions and primary care data, allowing an assessment of the healthcare costs associated with individual patient characteristics and history of vascular and nonvascular disease events.
Chapter 5 reports the quality-of-life (QoL) regression model, developed using IPD from the Health Survey for England (HSE) in 2006, 2011 and 2017, allowing an assessment of health-related quality of life (HRQoL) associated with individual patient characteristics and history of vascular and nonvascular disease events.
Chapter 6 reports our findings for the cost-effectiveness of statin therapy in categories of people 40–70 years old using the CVD microsimulation policy model.
Chapter 7 reports further validation of the CVD policy model among people aged ≥ 70 years in the UKB and Whitehall II cohorts and our findings for the cost-effectiveness of statin therapy among people aged ≥ 70 years in the UK.
Chapter 8 describes the web-based interface to the CVD microsimulation policy model to facilitate external use of the model.
Chapter 9 presents a general discussion and Chapter 10 summarises the key findings of this project.
Chapter 2 Development and internal validation of a cardiovascular disease microsimulation model using individual participant data from large randomised controlled trials of statin therapy
Aims and objectives
This chapter describes the development and internal validation of a model framework that predicts key CVD and nonvascular events among individuals without and with previous CVD using the IPD from 16 randomised statin versus control trials in the CTTC database.
Risk equations were developed for four vascular events, myocardial infarction (MI), stroke, coronary revascularisation (CRV) and vascular death (VD), and two nonvascular events, incident cancer and nonvascular death (NVD). These equations were developed separately for participants with and participants without previous CVD.
The risk equations formed the basis of a CVD microsimulation model to predict the risk of each of these events for each individual over time. Calibration plots were used to assess model performance in categories of individuals.
Methods
Data
There were 22 statin versus control trials in the CTTC that recruited over the years 1988–2000, with a median follow-up of 4.8 years. 6 Trials that recruited exclusively patients with CKD or chronic heart failure (4D, AURORA, CORONA and GISSI-HF) were excluded from the analysis. 6,29 Furthermore, two further trials, LIPS and PostCABG, were excluded because they did not record non-fatal stroke events. Following these exclusions, 68,018 participants without previous CVD at entry from 11 trials and 49,878 participants with previous CVD at entry from 16 trials contributed to the estimation of the risks of first post-randomisation occurrences of the aforementioned six disease events. Ten of the trials included both participants with and participants without previous CVD. Collectively, the trials recruited across Europe, North and South America, Australia, Israel, New Zealand and South Africa. Further details of the individual trials can be found elsewhere. 29
Handling of missing data
No ethnicity data were available for participants in the LIPID trial, and all 9014 participants were classified as ethnicity ‘not recorded’ and included in an ‘other’ category in analysis. Missing data for disease history at baseline, including treated hypertension, were assumed to indicate no such history. For 292 of participants with missing LDL-C levels, total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides were available and used to estimate their LDL-C level using the Friedewald formula. 34 The extent of further missing data was as follows: age (49 participants), smoking status (101 participants), body mass index (BMI) (530 participants), LDL-C (1551 participants), HDL-C (394 participants), creatinine (1331 participants), systolic blood pressure (BP) (364 participants) and diastolic BP (373 participants). All missing values for BMI were replaced with mean BMI values in categories by study, sex, age group and prior diabetes. All other missing values were replaced by respective mean values in categories by study, treatment allocation, sex, age group and treated hypertension.
Risk equations
Cox proportional hazard (PH) regression models were used to fit the hazard equations for each event. Trials were assumed to each have their own hazard but with the assumption of proportionality between trials over time, and trial indicators were included as binary covariates in the models to account for differences in hazards between trials. Time was measured in years from randomisation into the trial. Allocation to treatment was also included in risk equations (except the incident cancer and NVD equations) to account for effect of study treatments. Participants’ age and sex were retained in all risk equations.
Participant characteristics and interactions indicated in previous studies to be associated with disease risks of interest were considered in developing the modelling. The following baseline covariates were considered for inclusion in all risk equations: ethnicity, smoking status, BMI, LDL-C, HDL-C, creatinine, systolic BP, diastolic BP, treated hypertension and history of diabetes, cancer and CVD. Participant age was fitted as a time-varying covariate. Histories of previous non-fatal events during study follow-up (other than the event subject to modelling) were included as time-varying covariates when modelling each event of interest. In view of the large data set and to ensure robust risk models, variables were selected for inclusion at the 1% significance level using backward variable selection and checked for stability using forward selection with backward steps. 35 Forward variable selection with backward steps at the 1% significance level was then used to select the following six interactions for inclusion: sex and diabetes, age and LDL-C, age and BP, age and smoker, age and diabetes, and age and sex. These interactions were considered for inclusion in all models except those for CRV.
The PH assumption was checked prior to modelling using log–log plots and cumulative hazard plots and post modelling using PH assumption tests and plots of the scaled Schoenfeld residuals.
Microsimulation model
The risk equations were then used in a microsimulation model to predict the annual incidence (risk) of each event for each participant. The model was run 300 times for each participant over 5 years. For each participant, for each year in the model, the point estimate of the annual incidence of each event was the average number of occurrences across the 300 simulations. The cumulative sum of these incidences was then calculated to obtain the predicted cumulative incidence over time.
Internal model validation
The performance of the predictions from the microsimulation model was checked using calibration plots of observed versus predicted cumulative incidence for each event.
Results
At baseline, participants without and participants with previous CVD contributing to the risk models’ estimation had a mean age of 62 and 63 years, respectively. Most of them were male (65% and 78%), of white ethnicity (72% and 78%) and non-smokers (80% and 81%), and nearly half of them (42% and 48%) were overweight (BMI of 25–30 kg/m2). Among participants with previous CVD at baseline, 83% had a history of only MI (50%), other coronary heart disease (CHD) (19%), peripheral arterial disease (PAD) (6.4%) or stroke (7.8%), and 17% had a history of two or more of these conditions (Table 2).
| Characteristic | Without previous CVD historya,b | With previous CVDa,b |
|---|---|---|
| Number of participants | 68,018 | 49,878 |
| Age, years | 62.3 (9.2) | 62.7 (9.1) |
| Sex, male (%) | 43,972 (65%) | 39,085 (78%) |
| Ethnicity | ||
| Whiteb | 49,170 (72%) | 38,901 (78%) |
| Black | 6110 (9.0%) | 1226 (2.5%) |
| Otherc | 12,738 (19%) | 9751 (20%) |
| Current smoker | 13,873 (20%) | 9554 (19%) |
| BMI (kg/m2) | ||
| < 18.5 | 670 (0.99%) | 251 (0.50%) |
| 18.5–25 | 19,963 (29%) | 15,023 (30%) |
| 25–30 | 28,598 (42%) | 23,711 (48%) |
| 30–35 | 13,532 (20%) | 8487 (17%) |
| 35–40 | 3708 (5.5%) | 1806 (3.6%) |
| ≥ 40 | 1547 (2.3%) | 600 (1.2%) |
| LDL-C (mmol/l) | 3.5 (0.89) | 3.8 (0.85) |
| HDL-C (mmol/l) | 1.3 (0.38) | 1.1 (0.31) |
| Creatinine (μmol/l) | 91 (24) | 98 (23) |
| Systolic BP (mmHg) | 142 (20) | 139 (22) |
| Diastolic BP (mmHg) | 83 (11) | 81 (11) |
| On hypertension treatment | 35,478 (52%) | 25,472 (51%) |
| Prior diabetes | 15,131 (22%) | 7949 (16%) |
| Prior cancer | 32 (0.05%) | 25 (0.05%) |
| Previous CVD | ||
| MI only | 24,866 (50%) | |
| PAD only | 3186 (6.4%) | |
| Stroke only | 3875 (7.8%) | |
| Other CHD onlyd | 9631 (19%) | |
| Two or more of the above | 8320 (17%) | |
The number of participants experiencing disease end points during follow-up are reported in Table 3.
| Without previous CVD | With previous CVD | |
|---|---|---|
| Number of participants | 68,018 | 49,878 |
| MI | 1758 | 4134 |
| Stroke | 1133 | 2160 |
| CRV | 1424 | 4958 |
| Incident cancer | 3085 | 3450 |
| VD | 1118 | 3751 |
| NVD | 1767 | 2242 |
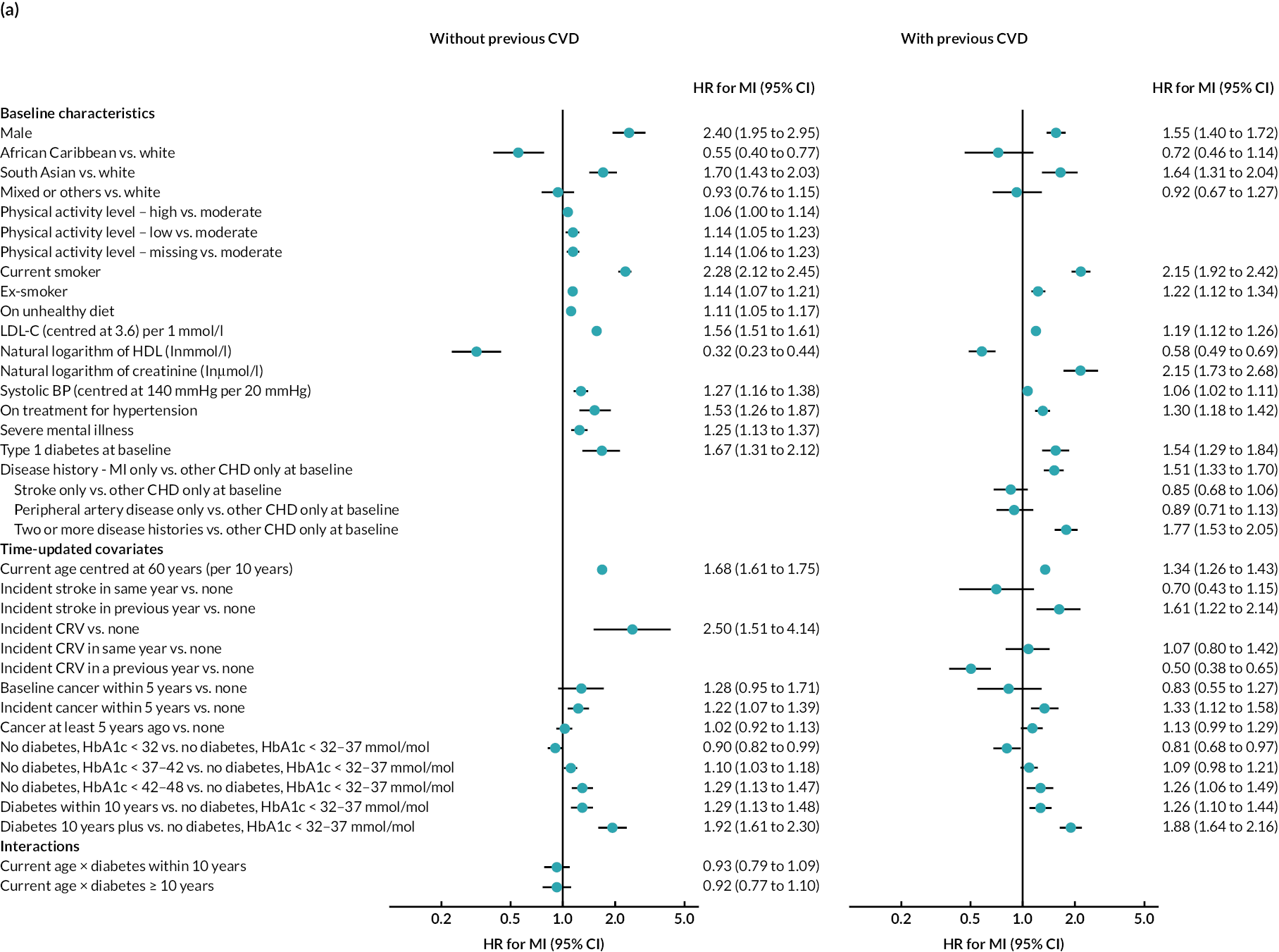
Log–log plots and cumulative hazard plots created prior to the Cox hazard regression modelling of events did not show any major violations of the PH assumption (not shown). Figure 1a–f shows the Cox hazard risk equations for each of the events in turn. With the exception of incident cancer, separate risk equations were fitted for participants without and participants with previous CVD. Contributions of risk factors to the hazard of incident cancer were similar in participants without and participants with previous CVD, and hence a single risk equation was fitted for all participants regardless of CVD history at baseline (see Figure 1f). The tests and plots of Schoenfeld residuals after fitting each of the Cox hazard regressions did not show any major violations of the PH assumption (not shown).
FIGURE 1.
Cox hazard equations estimated using IPD from large randomised controlled trials. (a) MI; (b) stroke; (c) CRV; (d) VD; (e) NVD; and (f) incident cancer. Participants with a history of cancer at baseline were excluded. CI. confidence interval; HR, hazard ratio.
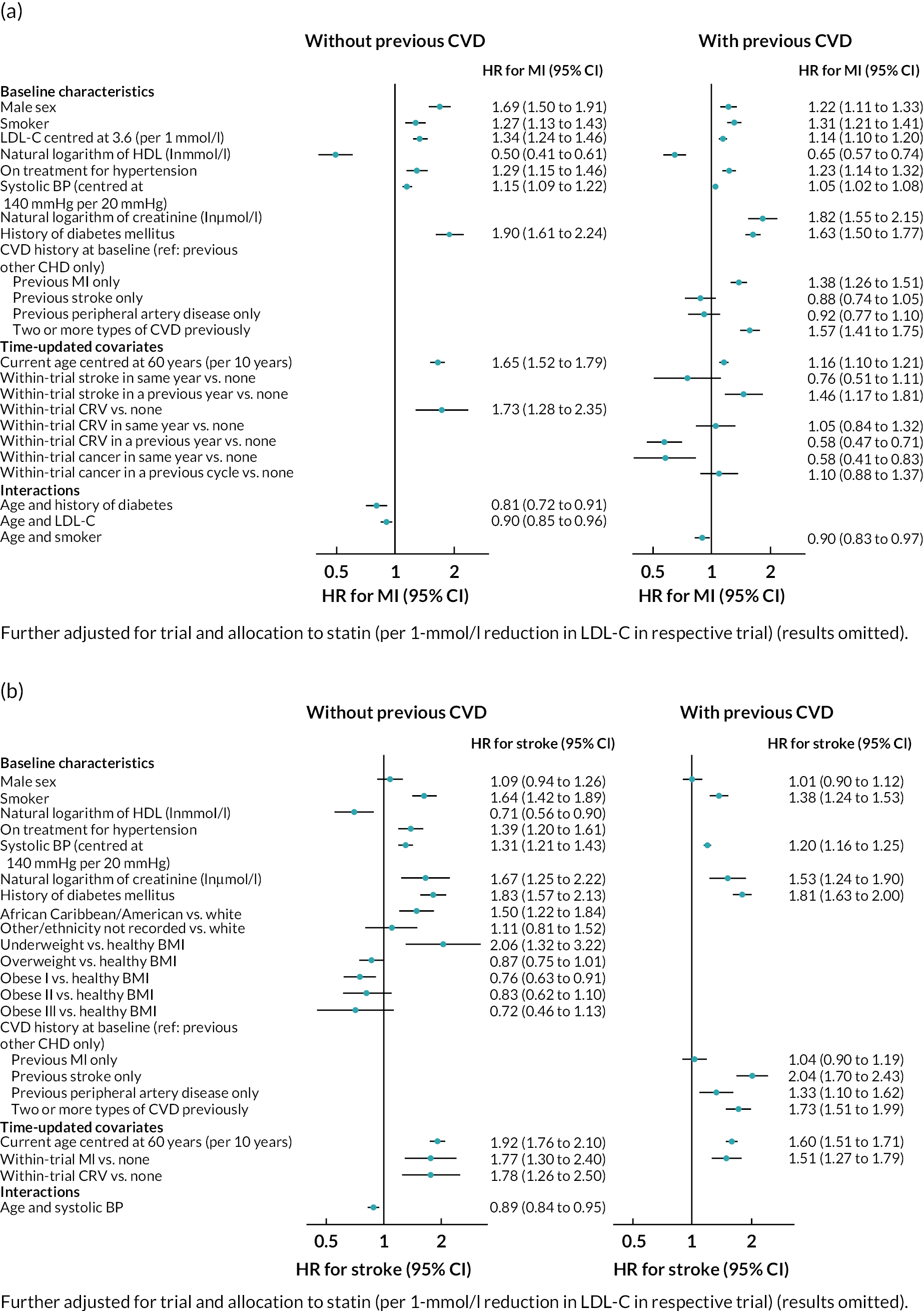
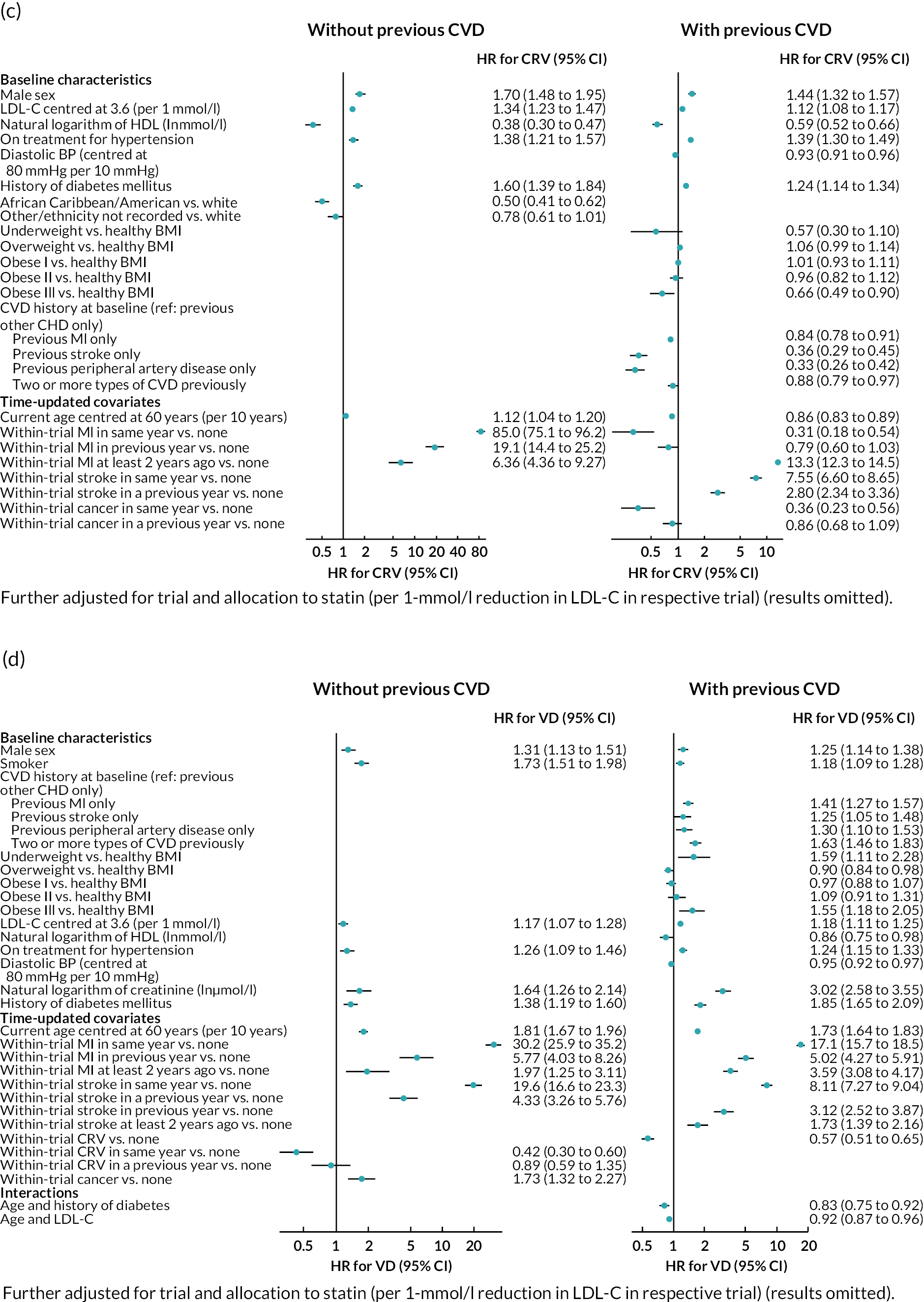
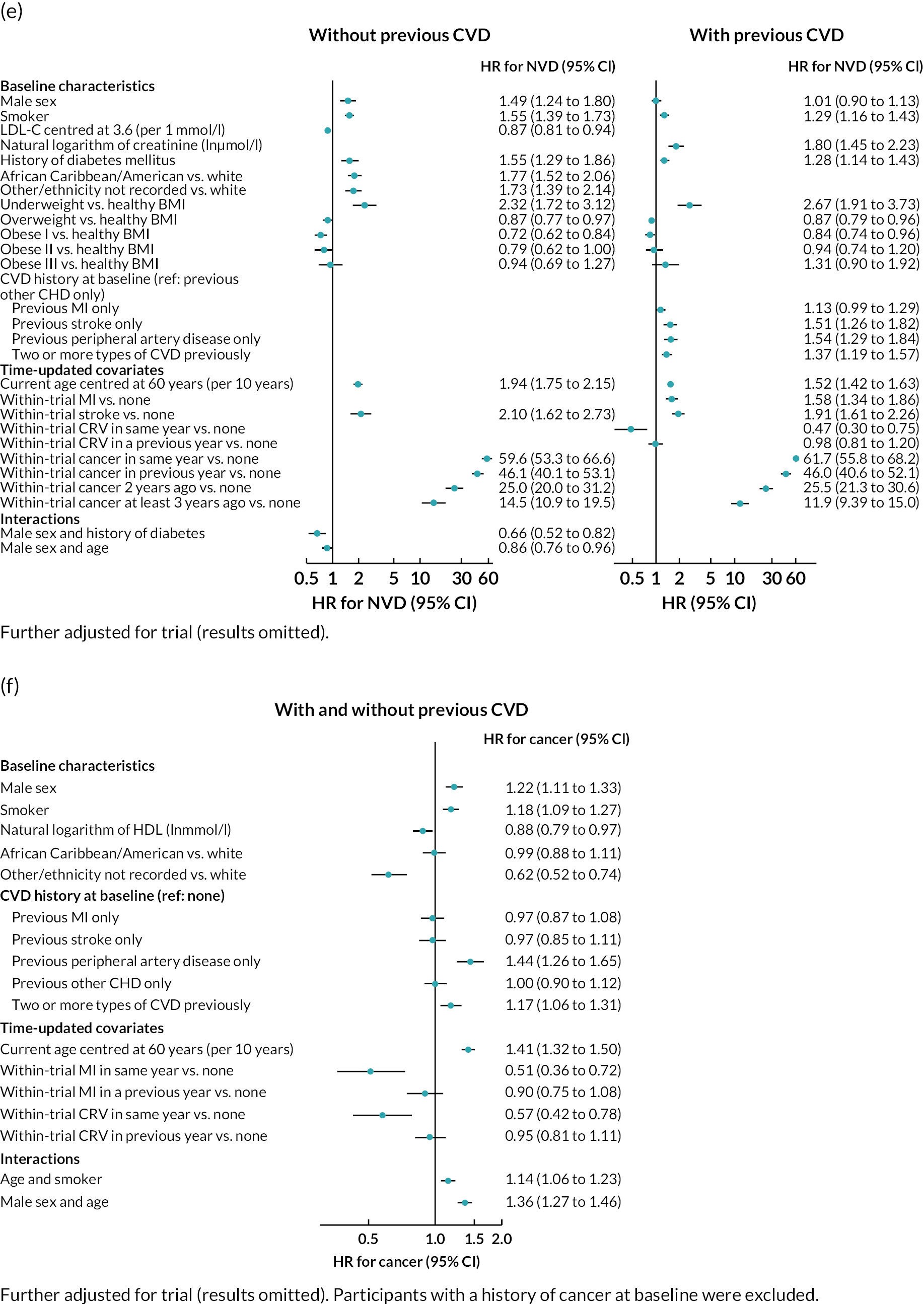
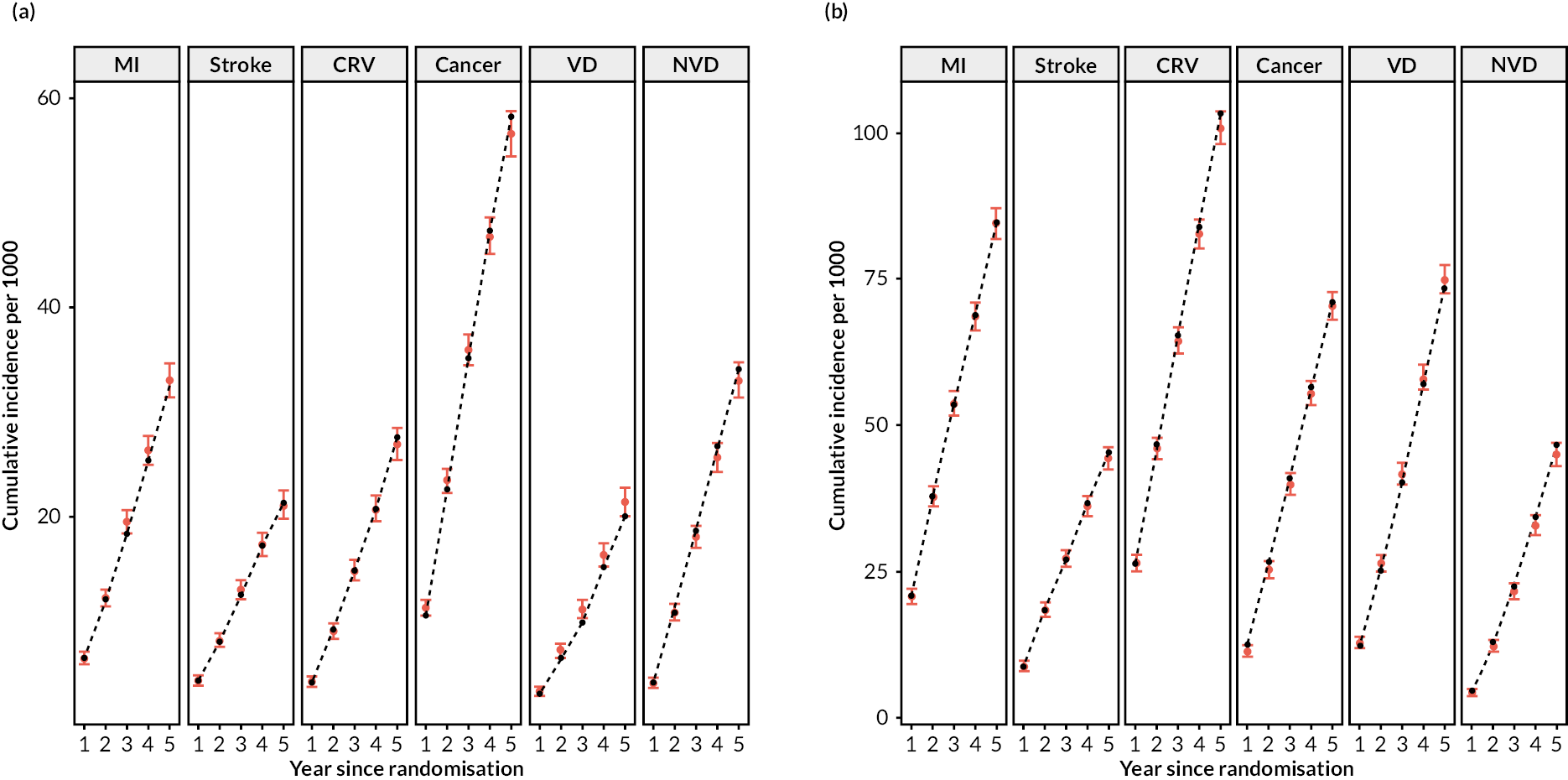
Figure 2 shows a schematic of the microsimulation model used to predict the annual incidence (risk) of each event for each participant. The model performed well in internal validation (Figure 3).
FIGURE 2.
Cardiovascular disease microsimulation model schematic.
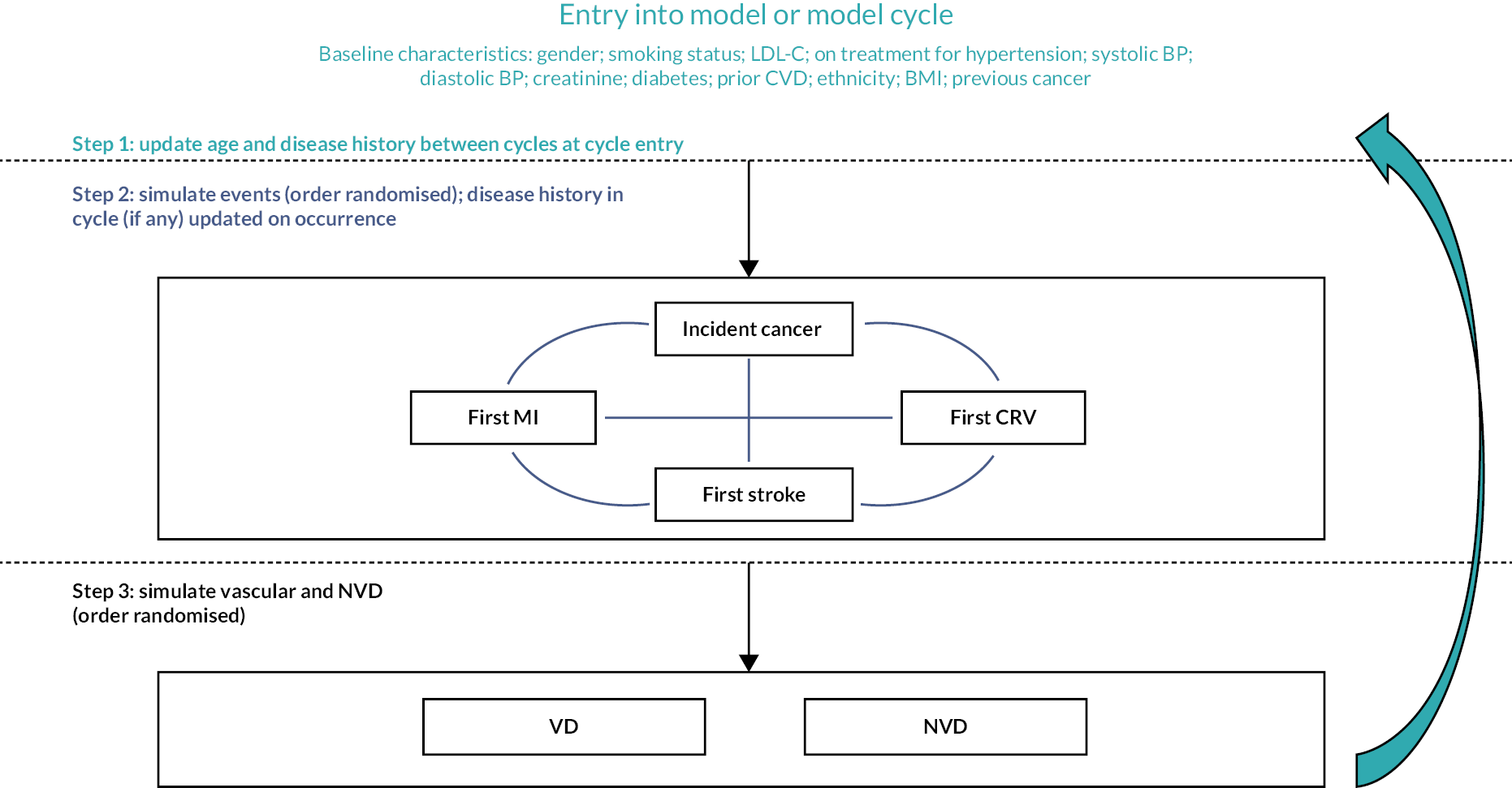
FIGURE 3.
Internal validation of the CVD model in the CTTC database. (a) Without previous CVD; and (b) with previous CVD. Red denotes observed with 95% confidence interval; black denotes prediction from model.
Summary
This chapter presented the development and internal validation of a model framework that predicts risks of key vascular and nonvascular events using the IPD from 16 randomised statin versus control trials in the CTTC. This initial model is further developed in a large contemporary UK cohort, the UKB, in Chapter 3.
Chapter 3 Calibration and further development of the cardiovascular disease microsimulation model
Aims and objectives
In this chapter, we discuss the calibration, further development and validation of the CVD microsimulation model in the UKB cohort and the Whitehall II study. Although CTTC trial data included well-adjudicated CVD end points, the model needs to represent well the contemporary UK CVD and mortality risks. Therefore, our aim was to use the large and contemporary UKB cohort to check the initial model performance and generate a reliable CVD microsimulation model capable of predicting CVD risks and mortality in different categories of the current UK population based on sociodemographic and clinical risk factors.
Methods
UK Biobank cohort
Between 2006 and 2010, the UKB cohort study recruited over 500,000 individuals aged 40–70 years from across the UK. 31 Study participants were followed using routine electronic healthcare databases to identify incidences of deaths, cancers and hospital admissions. Additionally, approximately 40% of the participants had their primary care records linked to the study. The UKB database contains comprehensive information on the sociodemographic, behavioural, physical and clinical characteristics of the participants at recruitment, as well as their disease histories both at recruitment and during follow-up. All UKB participants were included in the present study, with the exception of a small number of individuals with end-stage kidney disease at baseline. Appendix 1 describes the processing of UKB data to support CVD model development and QRISK3 calculation21 (see Appendix 1, Table 22).
Cardiovascular disease model development in UK Biobank
We utilised the follow-up data from the UKB up to 31 March 2017 to validate, calibrate and further develop the event risk equations for MI, stroke, CRV, incident cancer, VD and NVD. About 9500 participants experienced MI, 5000 experienced strokes and 3400 died from CVD causes, indicating sufficient sample sizes for the development of risk equations. During the external validation of the initial microsimulation model in the UKB database, we found that all risk equations required calibration.
The general approach to calibration and further development of the risk equations involved four steps. First, we calibrated the intercept and shape parameters of all trial data-based initial risk equations using the calculated linear predictor, which was calculated using initial risk equation coefficients and UKB participant characteristics. Second, we re-estimated the coefficients of covariates with different definitions in UKB (e.g. smoking and ethnicity categories and diabetes and cancer during follow-up) and related interaction terms by releasing them from the linear predictor. Third, further individual participant covariates of interest, such as physical activity, socioeconomic deprivation, diet quality, history of severe mental illness and type 1 diabetes, which were not available in CTTC data, were included in the risk equations. Finally, we fitted an incident diabetes risk equation with glycated haemoglobin (HbA1c) at entry as a further covariate using data from UKB participants with linked primary care records. The incident diabetes risk equation was estimated across both participants with and participants without previous CVD as there was no external evidence of difference in associations with risk factors, the PH assumptions were met and there were no substantive differences in estimated associations in the two populations. The incident diabetes equation was integrated as an additional end point in the microsimulation model.
Cox regression models were used for backward and forward stepwise covariate selection, with an inclusion threshold of 1% level of statistical significance. PH assumption of covariates in each model was tested using Schoenfeld residuals. Parametric PH models were then estimated for each end point, using three distributions (exponential, Weibull and Gompertz), and their Akaike information criterion (AIC) and Bayesian information criterion (BIC) were compared to identify the distributions best fitting each model end point. Lifetime simulations were used for the end points of death, incident cancer and incident diabetes to assess whether the model predicted lifetime risks were consistent with other UK population data. To account for parameter uncertainty, we generated 1000 sets of coefficients using bootstrapping (with replacement) of the respective UKB data and re-estimating the final risk equations. 36
Executing model predictions
We executed 1000 first-order simulations for each individual and used diagnostics to determine the sufficient number of microsimulations across study results. A simulation stops when the individual is predicted to die or reaches 110 years of age. We summarised risks of model events, survival and quality-adjusted life-years (QALYs) across the first-order simulations to minimise the Monte Carlo uncertainty in the model projection for each participant.
We employed the CVD microsimulation model to perform lifelong projections for all UKB participants. Individuals with cancer and/or diabetes history at entry were not at risk of incident cancer or diabetes. The lifetime risks of model events, remaining life expectancy and QALYs for each participant were derived by adding up the results across annual cycles.
We also ran 500 and 1000 simulations for probabilistic sensitivity analysis in participant categories without and with prior CVD, respectively, and checked that the 95% confidence intervals (CIs) for cost-effectiveness measures were reliably estimated.
Further model validation in UK Biobank and Whitehall II studies
Cumulative end-point incidences predicted by the UKB-calibrated CVD microsimulation model were compared with the observed cumulative end-point incidences across UKB participants by previous CVD, sex and age at entry over the 9 years of UKB data used in the model calibration (until 31 March 2017; internal validation) and over further 3 years of UKB follow-up data not used in model estimation (until 29 February 2020; temporally external validation). The UKB-calibrated model was further externally validated in the Whitehall II cohort32 using the Phase 9 data of participants as baseline and comparing the model-predicted with the observed cumulative incidences of end points over the next 10 years of follow-up. Appendix 2 describes the Whitehall II data used in CVD model validation, including participants’ baseline characteristics (see Appendix 2, Table 23) and the number of events they experienced during follow-up (see Appendix 2, Table 24).
Results
The analytical data from the UKB study included 444,576 participants without previous CVD and 57,278 with previous CVD at entry. The average follow-up time was 8.1 years for participants without previous CVD and 7.9 years for those with previous CVD, resulting in 3,588,967 and 451,368 person-years of follow-up, respectively. At entry, the mean age of participants without previous CVD was 56 [standard deviation (SD) 8] years, with 56% being female, 95% being of white ethnicity, 5% having diabetes and 16% receiving hypertension treatment. The mean age of participants with previous CVD at entry was 60 (SD 7) years, and 41% were female, 95% were of white ethnicity, 14% had diabetes and 46% were receiving hypertension treatment (Table 4).
| Without CVD history | With CVD history | |
|---|---|---|
| N | 444,576 | 57,278 |
| Age, years | 56 (8.1) | 60.4 (7.0) |
| Sex, male (%) | 194,996 (44%) | 33,734 (59%) |
| Ethnicity | ||
| White | 420,409 (95%) | 54,488 (95%) |
| Black | 7268 (1.6%) | 770 (1.3%) |
| South Asian | 6946 (1.6%) | 1053 (1.8%) |
| Othera | 9953 (2.2%) | 967 (1.7%) |
| Smoking status | ||
| Non-smoker | 250,261 (56%) | 25,137 (44%) |
| Ex-smoker | 148,312 (33%) | 25,211 (44%) |
| Current smoker | 46,003 (10%) | 6930 (12%) |
| BMI (kg/m2) | ||
| < 18.5 | 2370 (0.53%) | 256 (0.45%) |
| 18.5–25 | 149,300 (34%) | 13,415 (23%) |
| 25–30 | 189,650 (43%) | 24,241 (42%) |
| 30–35 | 74,714 (17%) | 13,222 (23%) |
| 35–40 | 20,662 (4.6%) | 4328 (7.6%) |
| ≥ 40 | 7880 (1.8%) | 1816 (3.2%) |
| LDL-C (mmol/l) | 3.6 (0.82) | 3.1 (0.87) |
| HDL-C (mmol/l) | 1.5 (0.37) | 1.3 (0.36) |
| HbA1c (mmol/mol) | 35.8 (6.2) | 38.6 (8.7) |
| Creatinine (μmol/l) | 71 (15) | 77 (19) |
| Systolic BP (mmHg) | 138 (19) | 139 (19) |
| Diastolic BP (mmHg) | 82 (10) | 81 (10) |
| On hypertension treatment | 71,930 (16%) | 26,184 (46%) |
| Prior diabetes (any) | 21,567 (4.9%) | 8171 (14%) |
| Prior type 1 diabetes | 2741 (0.62%) | 1479 (2.6%) |
| Prior cancer | 32,713 (7.4%) | 5861 (10%) |
| Previous CVD | ||
| MI only | 2071 (3.6%) | |
| PAD only | 6806 (12%) | |
| Stroke only | 5137 (9.0%) | |
| Other CHD onlyb | 28,973 (51%) | |
| Two or more | 14,291 (25%) | |
| Townsend deprivation score quintile | ||
| 1 (least deprived) | 166,141 (37%) | 18,960 (33%) |
| 2 | 89,397 (20%) | 10,957 (19%) |
| 3 | 72,626 (16%) | 9034 (16%) |
| 4 | 64,448 (14%) | 9178 (16%) |
| 5 | 51,964 (12%) | 9149 (16%) |
| Physical activity level | ||
| High | 145,206 (33%) | 16,780 (29%) |
| Moderate | 146,156 (33%) | 17,679 (31%) |
| Low | 65,932 (15%) | 10,105 (18%) |
| Missing | 87,282 (20%) | 12,714 (22%) |
| History of severe mental illness | 36,087 (8%) | 6324 (11%) |
| Unhealthy diet (including uncertain) | 158,569 (36%) | 21,705 (38%) |
The numbers of events over follow-up that informed the estimation and calibration of model risk equations are summarised in Table 5.
| Without CVD history | With CVD history | |
|---|---|---|
| Number of participants | 444,576 | 57,278 |
| MI | 5427 | 2507 |
| Stroke | 4806 | 2132 |
| CRV | 6860 | 3451 |
| Incident cancer | 29,682 | 5221 |
| Incident diabetes | 9014 | 2772 |
| VD | 2142 | 1708 |
| NVD | 11,004 | 3170 |
Risk equations
The external validation of the initial microsimulation model (see Chapter 2) in the UKB cohort showed that the predicted cumulative incidence rates of CVD events were significantly higher than the observed incidence rates, indicating a need for model calibration to enhance the accuracy of predictions in this UK cohort.
The final parametric PH models, following the four steps of calibration and further development (see Appendix 3, Tables 25–27), are presented in Table 6 (type of parametric model and shape parameters with 95% CI) and Figure 4 [hazard ratio (HR) (95% CI)].
| Without CVD history | With CVD history | |
|---|---|---|
| MI | Weibull, shape 1.04 (1.01 to 1.07) | Exponential |
| Stroke | Weibull, shape 1.12 (1.09 to 1.16) | Exponential |
| CRV | Weibull, shape 1.13 (1.11 to 1.16) | Gompertz, shape –0.04 (–0.06 to –0.03) |
| Incident cancer | Gompertz, shape –0.01 (–0.01 to –0.00) | |
| Incident diabetes | Weibull, shape 1.44 (1.40 to 1.48) | |
| VD | Gompertz, shape 0.05 (0.03 to 0.07) | Gompertz, shape 0.07 (0.05 to 0.09) |
| NVD | Gompertz, shape 0.06 (0.05 to 0.06) | Gompertz, shape 0.05 (0.04 to 0.07) |
FIGURE 4.
Hazard ratios (95% CI) of risk factors in final risk equations. (a) MI; (b) stroke; (c) CRV; (d) incident diabetes; (e) incident cancer; (f) VD; and (g) NVD.
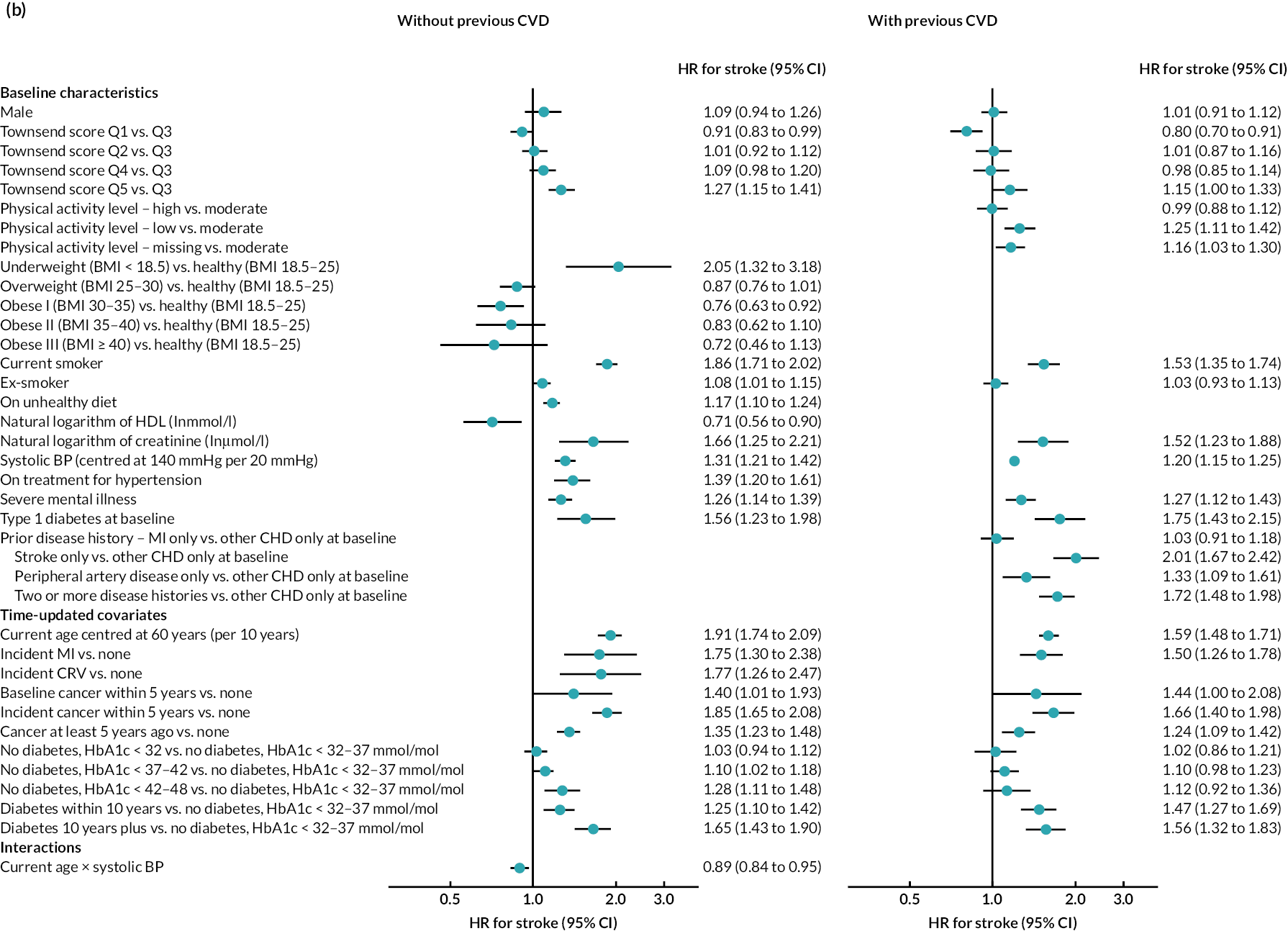
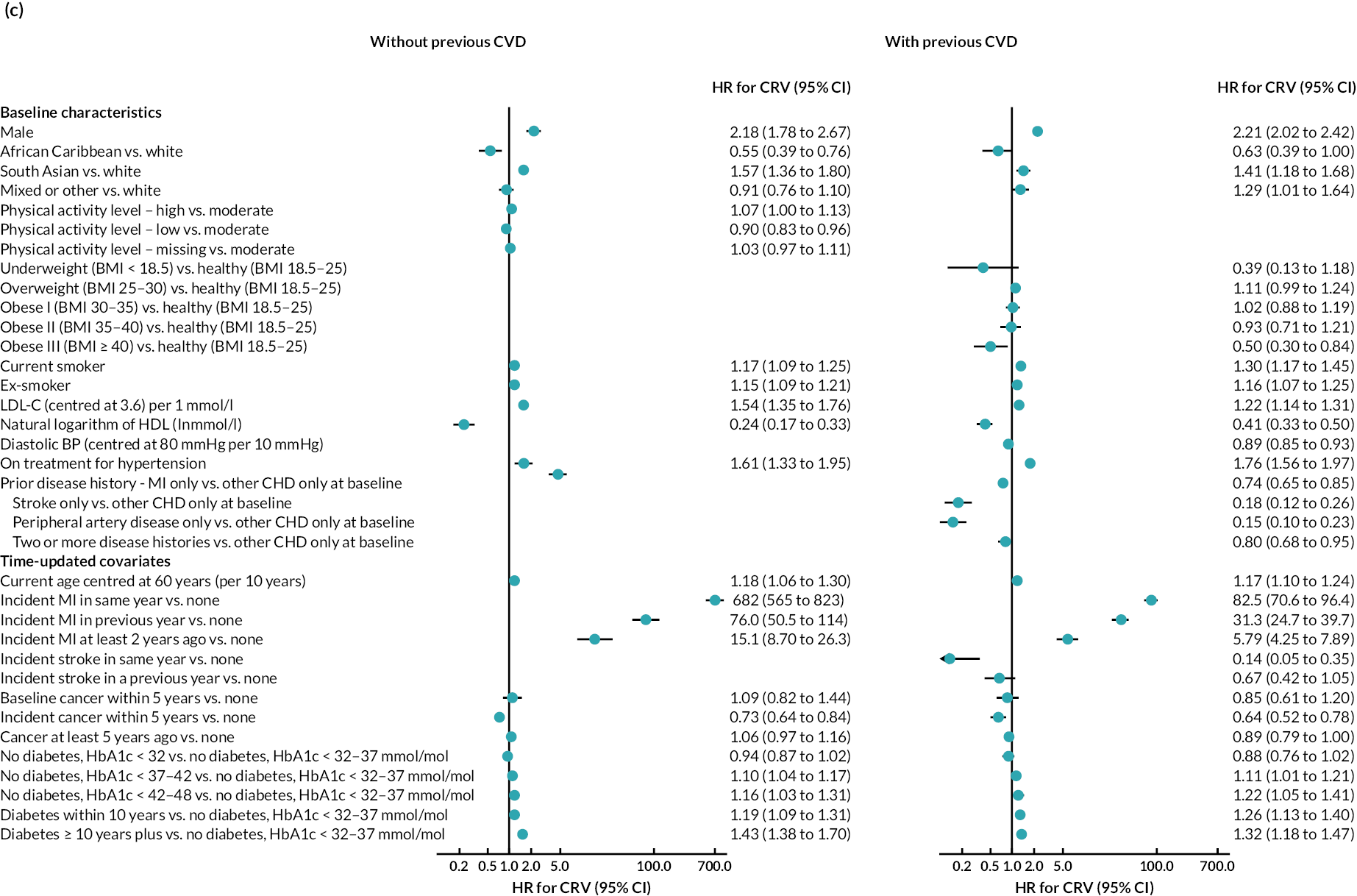
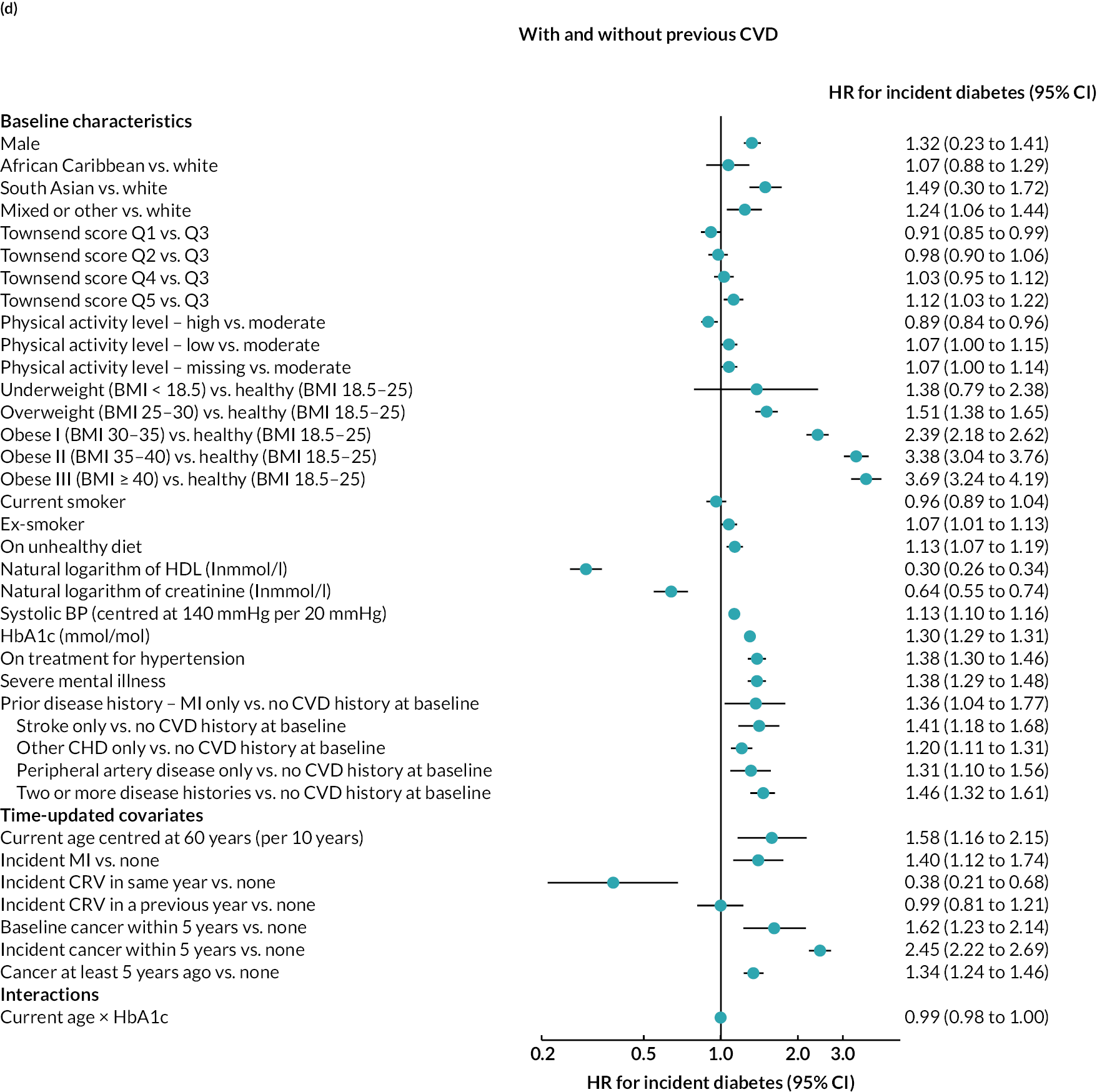
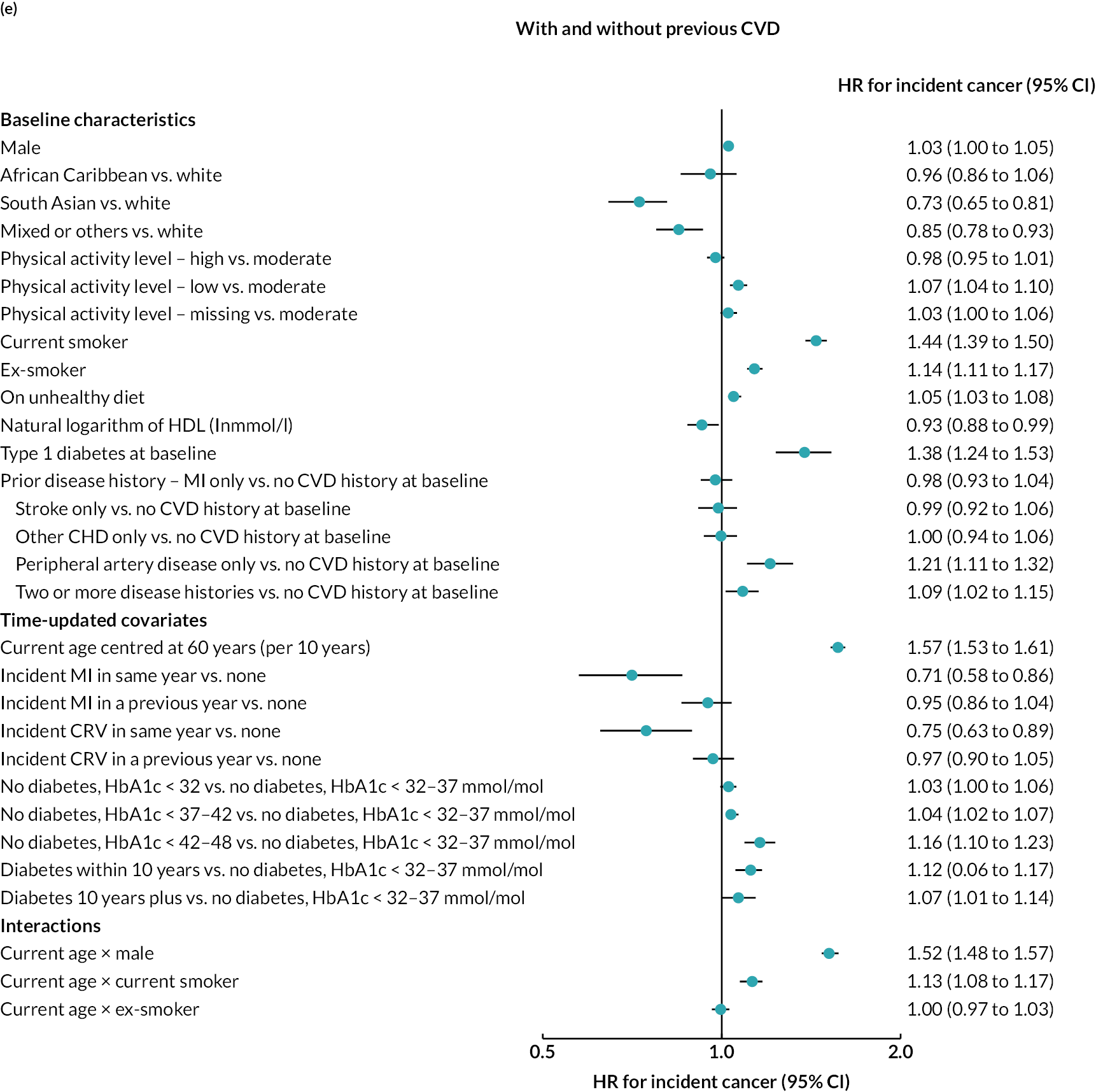
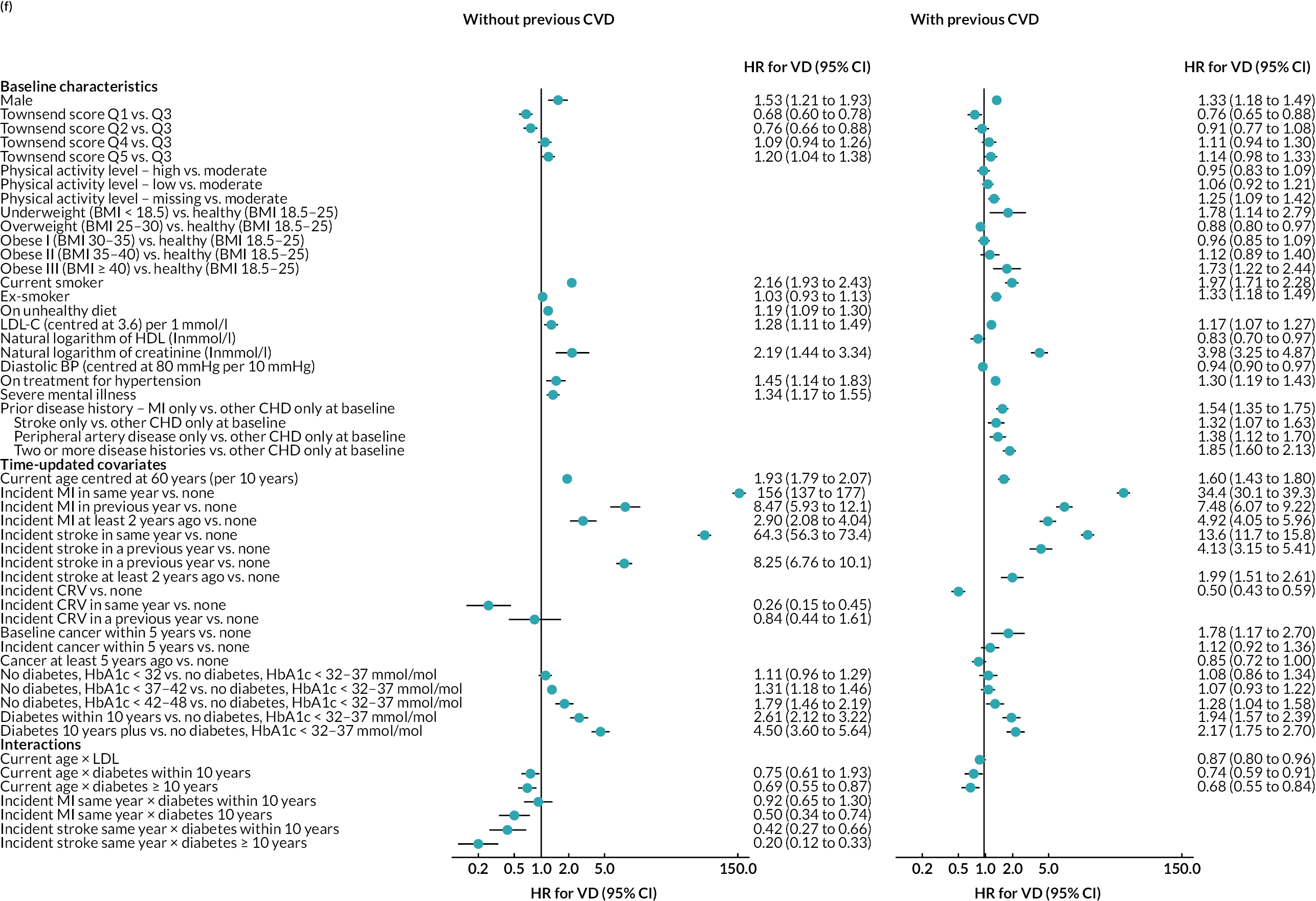
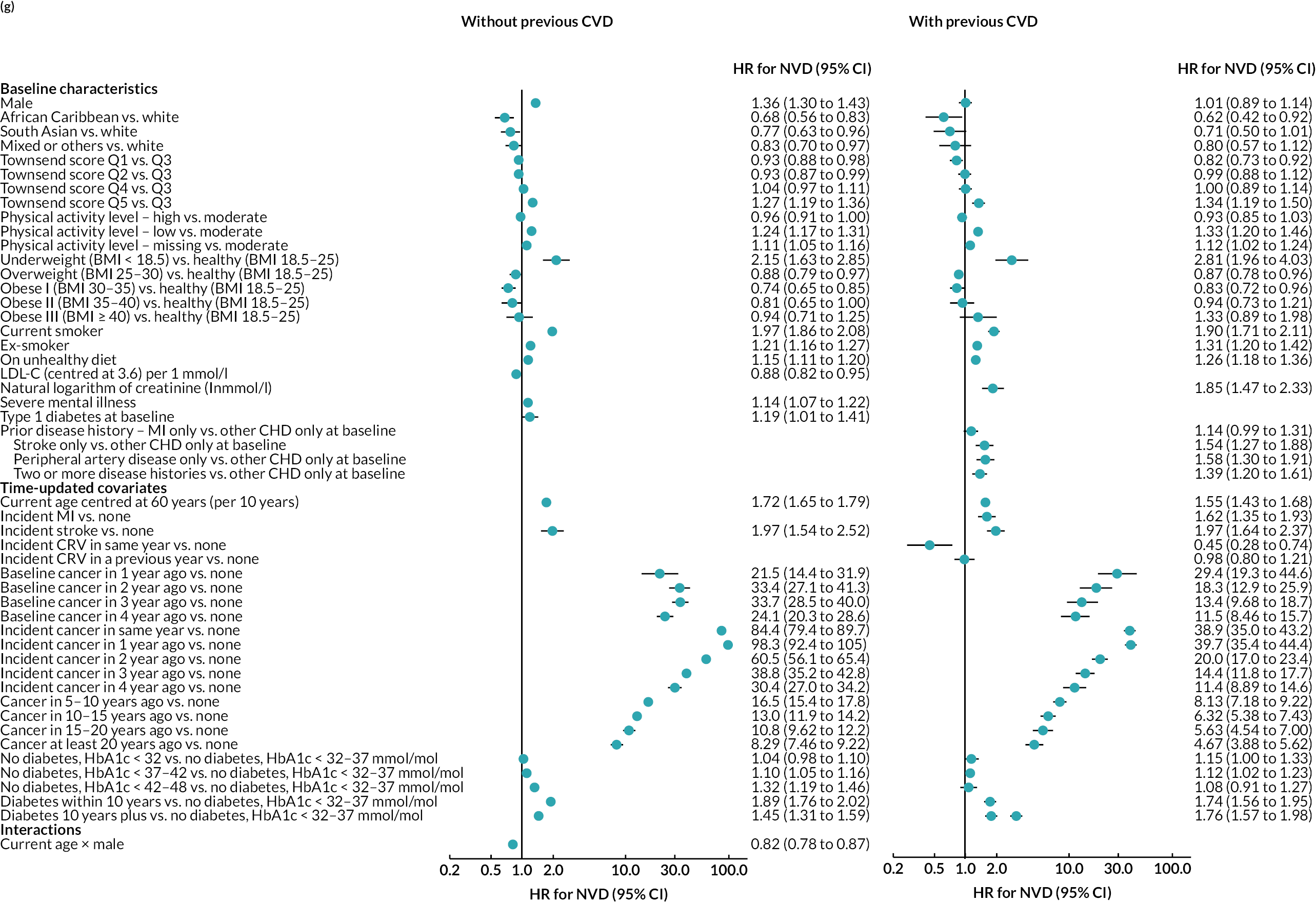
Age, male sex, smoking and treated hypertension were strongly associated with higher risks of CVD events. Higher socioeconomic deprivation was associated with higher risks of stroke, incident diabetes, VD and NVD. An unhealthy diet was associated with higher risks of MI, stroke and VD among people without CVD history. Lower physical activity was associated with higher risk of MI in people without CVD history; higher risk of stroke in people with CVD history; and higher risks of cancer, diabetes and NVD regardless of CVD history (see Figure 4).
MI, especially recent MI, was strongly associated with higher risk of CRV, and, separately, higher risk of stroke. MI and stroke were associated with higher risks of subsequent VD, with the greatest risks in the year of event. CRV was associated with lower risk of subsequent VD. Longer time since diabetes diagnosis and higher HbA1c level, in those without diabetes, were associated with higher risks of all CVD events. The patterns were similar in people without and people with CVD history, although magnitudes differed (see Figure 4). See Appendix 3 for the detailed risk equations (see Appendix 3, Table 28).
The diagnostics for the number of first-order simulations (see Appendix 4) indicated that 500 first-order simulations achieved stable estimates across the participant categories of interest (see Appendix 4, Figure 14). The diagnostics for the number of simulations for the probabilistic sensitivity analyses indicated that 500 and 1000 simulations in people without and people with previous CVD, respectively, were sufficient to achieve stable estimates of the 95% CIs (see Appendix 4, Figure 15).
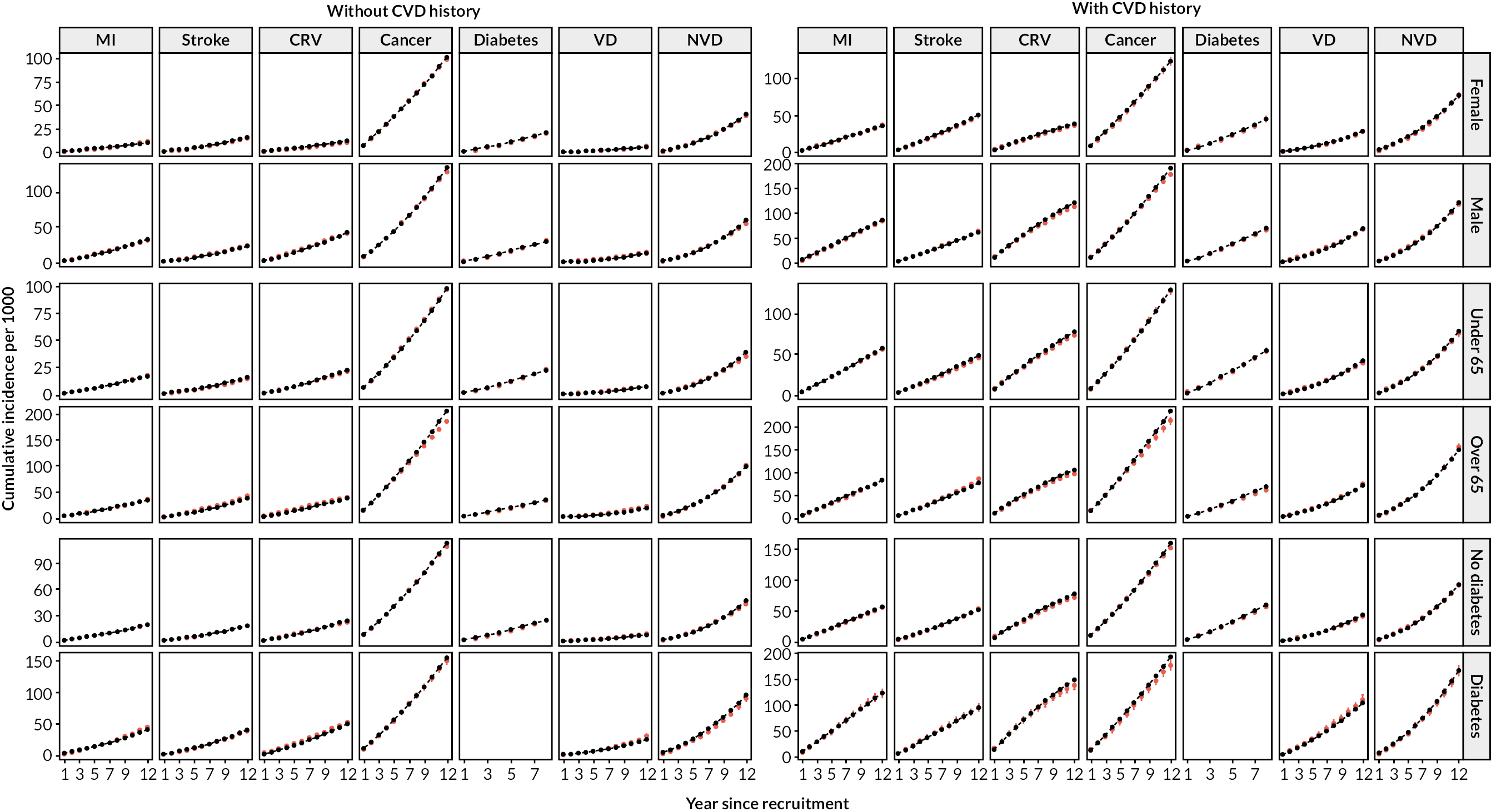
After calibration, the model’s predicted cumulative incidence rates for all end points across follow-up years showed good agreement with the observed rates in participant categories by previous CVD, age, sex and prior diabetes in the UKB (Figures 5a and 6). The UKB-calibrated model also demonstrated good overall performance in the external validation in the Whitehall II cohort, with only a slight overestimation of stroke risk among participants without previous CVD (see Figure 5b).
FIGURE 5.
Validation of the CVD model in UKB and Whitehall II cohorts. (a) Validation in UKB; and (b) validation in Whitehall II Phase 9. Red, observed; black, model. Validation covers 12 years in UKB (8 years for incidence diabetes due to stopping follow-up earlier) and 10 years in Whitehall II (7 years for incidence cancer due to stopping follow-up earlier).
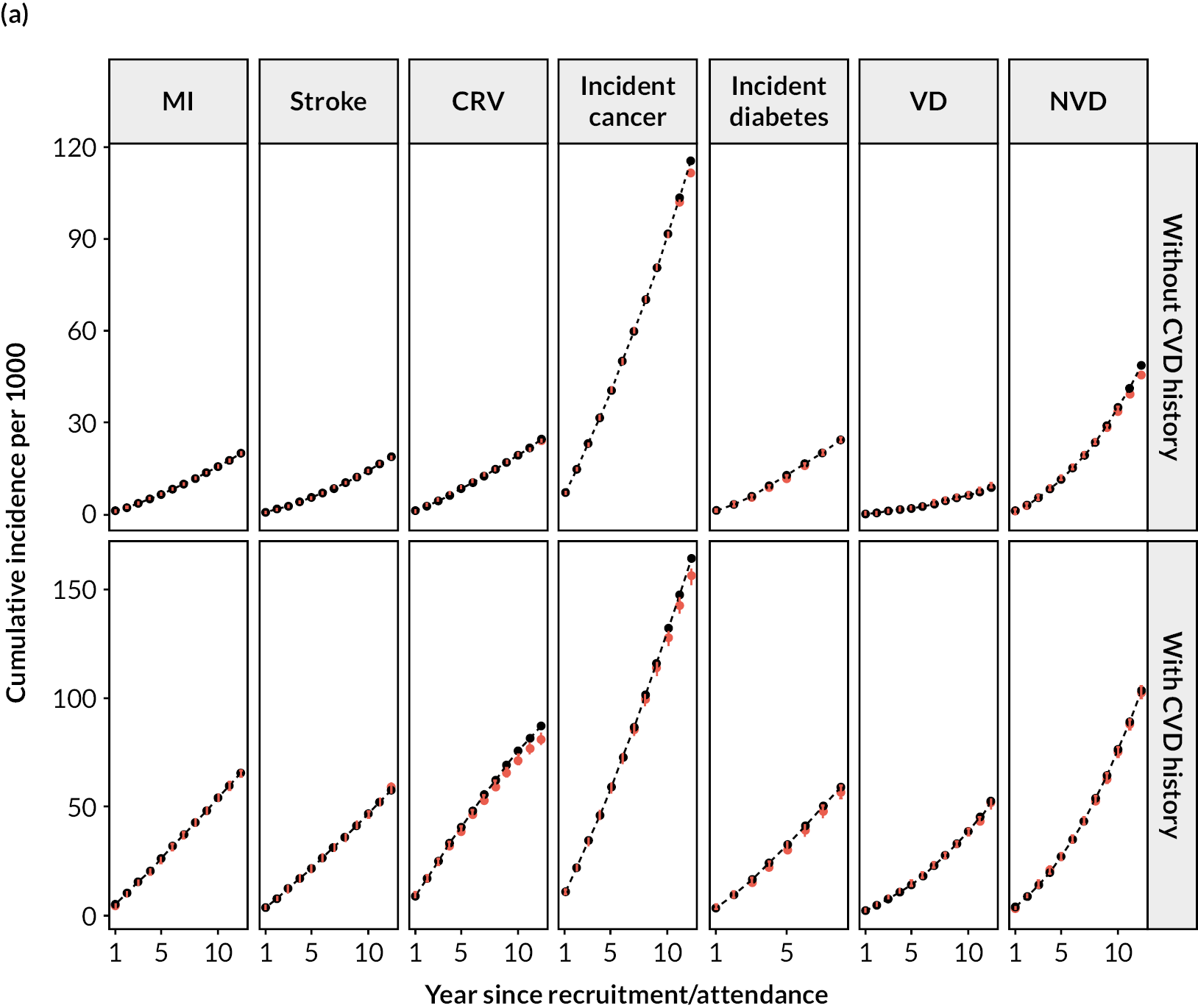
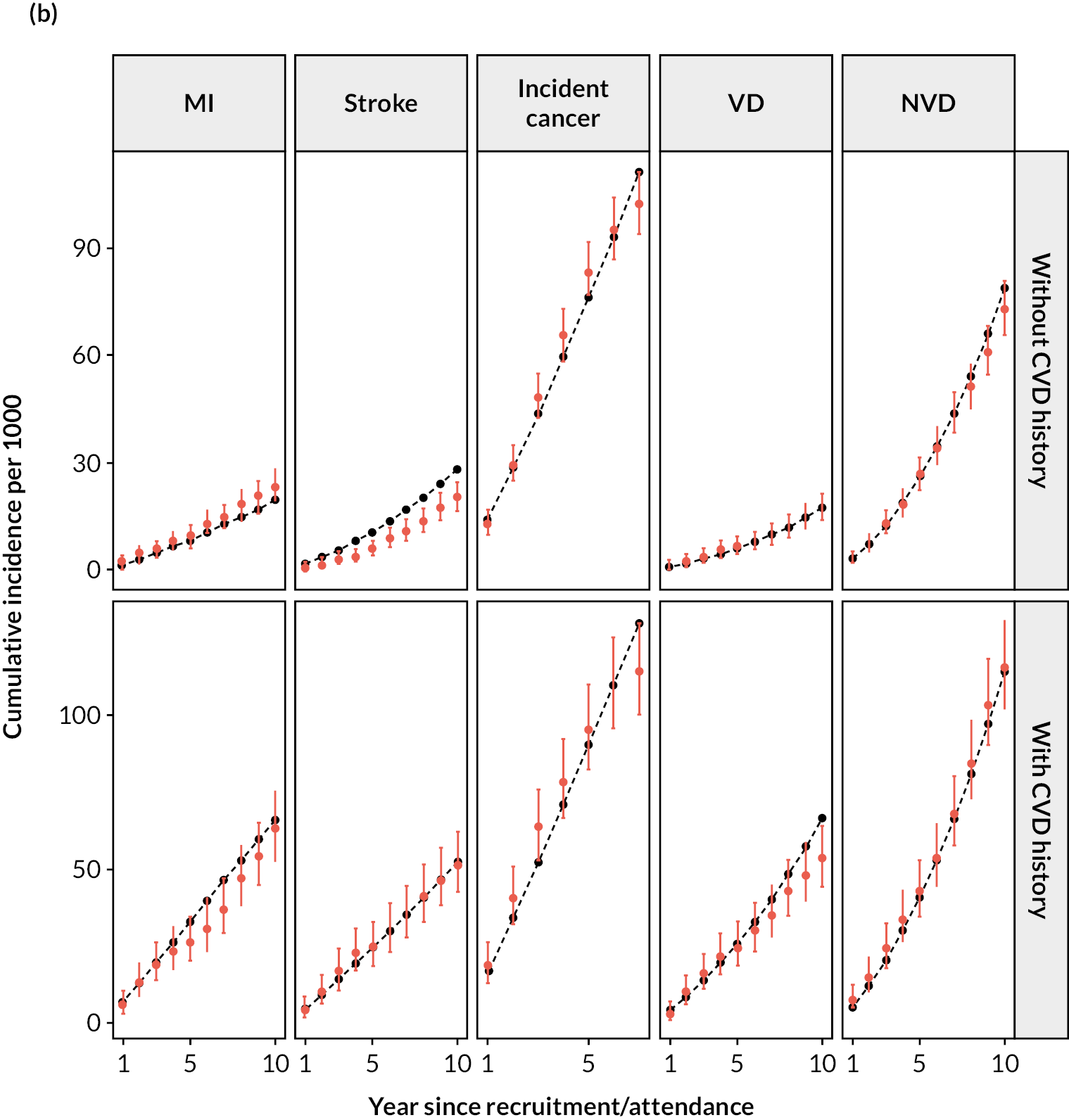
FIGURE 6.
Validation of the CVD model in UKB cohort, by sex, age and prior diabetes at entry. Red, observed; black, model. Validation includes 3 extra years that were not used to calibrate the model. Follow-up of incident diabetes partly relies on primary care records, which ended earlier than other data types in the UKB. Incident diabetes is not predicted for individuals with diabetes history.
Model prediction in UK Biobank
We used the calibrated model to execute individual projections for all UKB participants. For individuals in the same age and sex category, shorter life expectancy and fewer QALYs were predicted for those with CVD history or higher 10-year CVD risk. Men had shorter life expectancy but more QALYs as a proportion of their life expectancy than women, reflecting higher QoL in men all else equal. Taking participants aged 50–59 years as an example, the projected remaining life-years ranged between 24.2 (95% CI 23.1 to 25.5) and 34.6 (33.5 to 35.8) years for men [15.7 (15.1 to 16.5) to 29.4 (28.5 to 30.3) QALYs] and between 28.0 (27.7 to 30.1) and 37.2 (36.0 to 38.5) years for women [16.4 (16.0 to 17.4) to 29.0 (28.0 to 29.8) QALYs], depending on their CVD history or 10-year CVD risk (see the summary of model predictions and their parameter uncertainty presented in Appendix 5, Table 29).
Summary
The CVD microsimulation policy model, which was calibrated in the UKB cohort, demonstrated good performance across different categories of UKB participants and Whitehall II participants. The model can be used to project individuals’ lifetime risks of cardiovascular morbidity, incident diabetes, incident cancer, and vascular and nonvascular mortality, and long-term effects of strategies to reduce CVD risks.
Report Supplementary Material 1 includes the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) Checklist for the Prediction Model Development and Validation.
Chapter 4 Modelling the primary and hospital care costs associated with cardiovascular disease events
Aims and objectives
Our aim was to generate cost evidence for a patient-level microsimulation model that would be used in cost-effectiveness analyses of interventions to reduce CVD risk. This evidence had to be specific to patient characteristics and their experiences of CVD events. The objective was to estimate annual healthcare costs associated with patient characteristics and events, as used in the model, from the perspective of the UK NHS.
To achieve this, we developed separate cost regression models to predict the annual primary and hospital care costs based on the baseline characteristics of the participants and their time-updated experiences of key disease events. In particular, these cost models take into account the duration since adverse events, which can significantly impact healthcare costs.
Methods
Data
This study was conducted using data from the UKB, the cohort used in the calibration of the microsimulation model. All UKB participants with established linkage to primary care (38.5%) or hospital inpatient care (100%) records were included, with the exception of a small number of participants with end-stage renal disease for whom the linked hospital data did not include dialysis information. The analyses focused on the primary and hospital inpatient care costs. Primary care services were costed by identifying categories of consultations, monitoring tests and prescription medications and costing them based on the Unit Costs of Health and Social Care,37–39 the national reference costs,40 and NHS prescription cost analysis,41 respectively. Hospital inpatient care services were costed by identifying the hospital episodes, grouping the episodes into Healthcare Resource Groups42 and costing them using the NHS England reference costs. 40,43 Costs were analysed in annual cycle from entry into the UKB and were generated by summing the costs incurred by each participant during each year of follow-up in the study. All costs were inflated to year 2020 using the NHS cost inflation index. 37 The conventional participant risk factors included in the disease risk models were also included in the cost models. We assessed the impact of the following four CVD events, namely MI, stroke, CRV and VD, and three nonvascular events, namely diabetes, cancer and NVD, on annual healthcare costs. Follow-up data from participants’ entry into the UKB until 31 March 2016 contributed to these analyses.
Statistical methods
The following participant characteristics at entry into UKB were considered in the cost models: sex, ethnicity, smoking status, physical activity, diet quality, BMI, LDL-C, HDL-C, serum creatinine, systolic and diastolic BP, treated hypertension, and histories of diabetes, severe mental illness and CVD. The models also included annually updated participant characteristics such as current age and time since previous CVD events or incident diabetes or cancer. The study used generalised linear regression models to model annual primary care costs and two-part models to model annual hospital care costs, with the first part modelling the probability of incurring any costs and the second part modelling the costs conditional on incurring any. The study considered six different generalised linear models (GLMs) using three distributions (Gaussian, Poisson and gamma) and two link functions (identity and natural log), and the best-fitting models were chosen based on specification tests, predictive performance and parsimony. Finally, the study used cluster robust standard errors (SEs) to account for the lack of independence between annual periods for the same participant and performed stepwise bidirectional covariate selection at the 1% level of statistical significance. Analyses were performed using R version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
All 501,807 participants of the UKB contributed to the hospital cost analyses, while 192,983 (38.5%) contributed to the primary care cost analyses over 3,798,324 and 1,255,741 annual follow-up periods, respectively. Participants with previous CVD were older (mean age of 60 and 56 years, respectively), more likely to be men (57–59% and 44%, respectively), from more deprived socioeconomic backgrounds, and more likely to be smokers, be less physically active, have an unhealthy diet, have a higher BMI and have disease histories such as treated hypertension, diabetes, cancer and severe mental illness (Table 7).
| Primary care costs analysis | Hospital care costs analysis | |||
|---|---|---|---|---|
| Without previous CVD (N = 168,205) | With previous CVD (N = 24,778) | Without previous CVD (N = 444,536) | With previous CVD (N = 57,271) | |
| Age (years) | 56.1 (8.0) | 60.3 (7.1) | 56.0 (8.1) | 60.4 (7.0) |
| Male | 73,573 (43.7) | 14,084 (56.8) | 194,979 (43.9) | 33,729 (58.9) |
| Ethnicity | ||||
| White | 159,517 (94.8) | 23,570 (95.1) | 417,964 (94) | 54,121 (94.5) |
| Black | 1851 (1.1) | 222 (0.9) | 7266 (1.6) | 770 (1.3) |
| South Asian | 2905 (1.7) | 495 (2) | 6983 (1.6) | 1058 (1.8) |
| Othera | 3170 (1.9) | 355 (1.4) | 9912 (2.2) | 962 (1.7) |
| Missing | 762 (0.5) | 136 (0.5) | 2411 (0.5) | 360 (0.6) |
| Townsend socioeconomic deprivation quintile | ||||
| 1 (least deprived) | 63,113 (37.5) | 8248 (33.3) | 166,039 (37.4) | 18,951 (33.1) |
| 2 | 34,644 (20.6) | 4880 (19.7) | 89,211 (20.1) | 10,939 (19.1) |
| 3 | 27,656 (16.4) | 3977 (16.1) | 72,492 (16.3) | 9019 (15.7) |
| 4 | 24,526 (14.6) | 3982 (16.1) | 64,358 (14.5) | 9155 (16.0) |
| 5 | 18,029 (10.7) | 3657 (14.8) | 51,883 (11.7) | 9138 (16.0) |
| Missing | 237 (0.1) | 34 (0.1) | 553 (0.1) | 69 (0.1) |
| Smoking | ||||
| Never | 94,763 (56.3) | 11,069 (44.7) | 248,296 (55.9) | 24,880 (43.4) |
| Ex-smoker | 55,607 (33.1) | 10,654 (43) | 147,781 (33.2) | 24,998 (43.6) |
| Current smoker | 17,016 (10.1) | 2874 (11.6) | 45,979 (10.3) | 6927 (12.1) |
| Missing | 819 (0.5) | 181 (0.7) | 2480 (0.6) | 466 (0.8) |
| Physical activity level | ||||
| Low | 24,777 (14.7) | 4265 (17.2) | 65,921 (14.8) | 10,104 (17.6) |
| Moderate | 55,199 (32.8) | 7676 (31) | 146,146 (32.9) | 17,677 (30.9) |
| High | 55,680 (33.1) | 7468 (30.1) | 145,192 (32.7) | 16,776 (29.3) |
| Missing | 32,549 (19.4) | 5369 (21.7) | 87,277 (19.6) | 12,714 (22.2) |
| Diet quality | ||||
| Healthy | 108,313 (64.4) | 15,553 (62.8) | 285,989 (64.3) | 35,570 (62.1) |
| Unhealthy | 56,945 (33.9) | 8643 (34.9) | 149,077 (33.5) | 20,166 (35.2) |
| Missing | 2947 (1.8) | 582 (2.3) | 9470 (2.1) | 1535 (2.7) |
| BMI (kg/m2) | ||||
| < 18.5 | 840 (0.5) | 128 (0.5) | 2364 (0.5) | 253 (0.4) |
| ≥ 18.5, < 25 | 55,226 (32.8) | 6035 (24.4) | 148,846 (33.5) | 13,352 (23.3) |
| ≥ 25, < 30 | 71,515 (42.5) | 10,344 (41.7) | 187,957 (42.3) | 23,874 (41.7) |
| ≥ 30, < 35 | 28,779 (17.1) | 5485 (22.1) | 74,396 (16.7) | 13,037 (22.8) |
| ≥ 35, < 40 | 7949 (4.7) | 1802 (7.3) | 20,645 (4.6) | 4312 (7.5) |
| ≥ 40 | 2990 (1.8) | 733 (3) | 7871 (1.8) | 1813 (3.2) |
| Missing | 906 (0.5) | 251 (1) | 2457 (0.6) | 630 (1.1) |
| LDL-C (mmol/l) | 3.6 (0.9) | 3.1 (0.9) | 3.6 (0.8) | 3.1 (0.9) |
| HDL-C (mmol/l) | 1.5 (0.4) | 1.3 (0.4) | 1.5 (0.4) | 1.3 (0.4) |
| Creatinine (μmol/l) | 71.5 (15.1) | 76.5 (19.5) | 71.5 (15.1) | 77.0 (19.8) |
| Systolic BP (mmHg) | 138.2 (18.7) | 139.1 (19.0) | 137.8 (18.6) | 138.9 (18.9) |
| Diastolic BP (mmHg) | 82.6 (10.1) | 81.0 (10.4) | 82.4 (10.1) | 80.9 (10.5) |
| On antihypertensive treatment | 27,240 (16.2) | 10,900 (44) | 71,925 (16.2) | 26,181 (45.7) |
| Prior diabetes | ||||
| Type 1 | 926 (0.6) | 558 (2.3) | 2487 (0.6) | 1378 (2.4) |
| Type 2 | 7134 (4.2) | 2694 (10.9) | 19,075 (4.3) | 6792 (11.9) |
| Prior cancer | 12,221 (7.3) | 2420 (9.8) | 32,712 (7.4) | 5859 (10.2) |
| Severe mental illness history | 17,549 (10.4) | 3374 (13.6) | 36,082 (8.1) | 6323 (11) |
| Previous CVD | ||||
| No | 168,205 (100) | 0 (0) | 444,536 (100) | 0 (0) |
| MI only | 776 (3.1) | 2070 (3.6) | ||
| Stroke only | 1991 (8) | 5137 (9) | ||
| PAD only | 3473 (14) | 6805 (11.9) | ||
| Other CHD onlyb | 12,642 (51) | 28,969 (50.6) | ||
| Two or more | 5896 (23.8) | 14,290 (25) | ||
During the 7.1-year mean follow-up period, a small percentage of participants experienced the key CVD events, with higher event risks among people with previous CVD (Table 8). Additionally, some participants without previous diabetes or cancer at recruitment were diagnosed with these conditions during the follow-up period. The duration of follow-up was similar between the primary and hospital care cost analyses, and between participants with and participants without previous CVD. On average, participants had several primary care consultations, diagnostic and monitoring tests, and prescription medications per year, with primary care costs totalling £409 per year and the hospital inpatient cost totalling £583 per year. People with previous CVD had higher rates and costs of primary and hospital care than those without (see Table 8).
| Primary care costs analysis | Hospital care costs analysis | |||
|---|---|---|---|---|
| Without previous CVD (N = 168,205) | With previous CVD (N = 24,778) | Without previous CVD (N = 444,536) | With previous CVD (N = 57,271) | |
| Duration of follow-up (years) | 7.1 (0.9) | 7.0 (1.2) | 7.1 (1.0) | 7.0 (1.3) |
| Number of participants with events during follow-up | ||||
| MI | 1841 (1.1) | 871 (3.5) | 4651 (1.0) | 2210 (3.9) |
| Stroke | 1730 (1.0) | 812 (3.3) | 4106 (0.9) | 1872 (3.3) |
| CRV | 2197 (1.3) | 1205 (4.9) | 5877 (1.3) | 3094 (5.4) |
| Incident diabetesa | 3425 (2.1) | 1077 (5.0) | 7395 (1.7) | 2301 (4.7) |
| Incident cancera | 9057 (5.8) | 1797 (8.0) | 25,376 (6.2) | 4498 (8.7) |
| VD | 521 (0.3) | 457 (1.8) | 1781 (0.4) | 1412 (2.5) |
| NVD | 2777 (1.7) | 869 (3.5) | 9067 (2.0) | 2632 (4.6) |
| Healthcare use during follow-up | ||||
| Total number of person-years | 1,096,034 | 159,707 | 3,371,754 | 426,570 |
| Person-years with primary/hospital care costs | 1,015,858 (92.7) | 156,379 (97.9) | 534,287 (15.8) | 121,167 (28.4) |
| Number of primary care consultations/hospital inpatient episodes per person-year (95% CI) | 5.10 (5.08 to 5.11) | 8.31 (8.24 to 8.38) | 0.34 (0.33 to 0.34) | 0.71 (0.70 to 0.72) |
| Number of diagnostic and monitoring tests per person-year (95% CI) | 2.98 (2.96 to 2.99) | 5.84 (5.77 to 5.91) | ||
| Number of prescription medications per person-year (95% CI) | 17.9 (17.8 to 18.0) | 48.3 (47.6 to 49.0) | ||
| Annual cost (£) (95% CI) | ||||
| Total primary/hospital inpatient care costs | 360 (356 to 363) | 746 (730 to 762) | 514 (510 to 518) | 1131 (1114 to 1149) |
| Primary care consultations | 148 (147 to 148) | 241 (239 to 243) | ||
| Diagnostic and monitoring tests (primary care) | 28 (28 to 28) | 49 (48 to 49) | ||
| Prescription medications (primary care) | 183 (180 to 187) | 456 (441 to 471) | ||
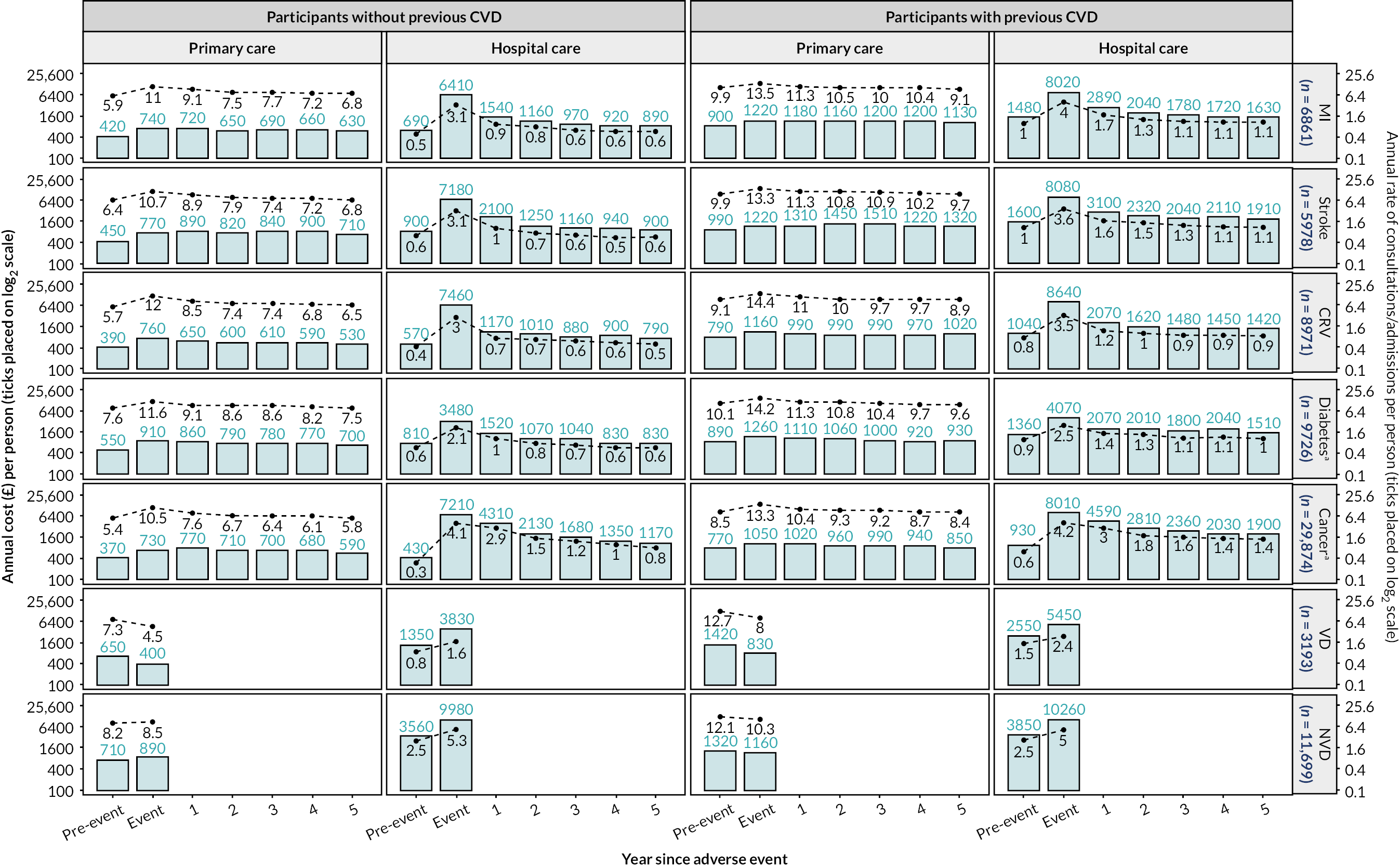
Annual primary care consultation rates and annual hospital admission rates peaked in the year of events such as MI, stroke, CRV, diabetes or cancer, and then decreased over the subsequent years. The annual costs had a similar pattern across event types, except that annual primary care costs continued to increase after the year of stroke. Participants with previous CVD had higher annual primary care consultation rates and hospital admission rates, and higher annual primary care and hospital care costs (Figure 7).
FIGURE 7.
Annual rates and costs of healthcare use by time since adverse event. a, Incident cases only. Mean annual costs are summarised for UKB participants experiencing the corresponding events. Annual periods are defined from date of participant’s entry into UKB. ‘Event’ years correspond to the annual periods with respective event; ‘pre-event’ years correspond to annual periods prior to annual periods with respective event. Thereafter data are presented for each subsequent annual period up to 5 years following the annual periods with events. Total numbers of corresponding first events in the study period are presented on the right-hand side. Reproduced with permission from Zhou et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
After statistical model selection, primary care costs were modelled using one-part GLMs with Poisson distribution and identity link function, while hospital care costs were modelled using two-part models with logistic regression model (part 1) and GLM with Poisson distribution and identity link function (part 2). The annual primary and hospital care costs were £262 and £244, respectively, for the reference individual in models for people without previous CVD. The reference annual primary and hospital care costs were £302 and £376, respectively, in models for people with previous CVD with the same reference characteristics except having a history of MI at baseline. Participant characteristics associated with higher primary care cost included older age, female sex, south Asian ethnicity, higher socioeconomic deprivation, smoking, low level of physical activities, unhealthy BMI, and morbidity, including treated hypertension, severe mental illness or prior diabetes or CVD (Table 9). Similar characteristics, except south Asian ethnicity, were also associated with higher hospital care costs (Table 10).
| Covariate | Participants without previous CVD, mean (SE)a | Participants with previous CVD, mean (SE)a |
|---|---|---|
| Intercept | 262 (4) | 302 (22) |
| Baseline characteristics | ||
| Male (ref: female) | –51 (3) | –50 (13) |
| Ethnicity (ref: white) | ||
| Black | –3 (11) | 61 (127) |
| South Asian | 57 (9) | 24 (36) |
| Othersb | –3 (2) | –107 (24) |
| Townsend socioeconomic deprivation quintile (ref: quintile 3) | ||
| 1 (least deprived) | –5 (1) | 6 (17) |
| 2 | 4 (2) | 25 (17) |
| 4 | 6 (3) | 49 (21) |
| 5 | 50 (6) | 132 (34) |
| Smoking (ref: never) | ||
| Ex-smoker | 22 (2) | c |
| Current smoker | 50 (5) | c |
| Physical activity level (ref: moderate) | ||
| Low | 30 (5) | 117 (21) |
| High | –2 (2) | –8 (10) |
| Missing | 32 (4) | 96 (21) |
| BMI (kg/m2) (ref: ≥ 18.5, < 25) | ||
| < 18.5 | 36 (15) | 182 (107) |
| ≥ 25, < 30 | 3 (2) | 8 (11) |
| ≥ 30, < 35 | 31 (4) | 58 (16) |
| ≥ 35, < 40 | 91 (9) | 194 (46) |
| ≥ 40 | 132 (13) | 329 (44) |
| LDL-C (centred at 3.6; per 1 mmol/l) | –6 (2) | c |
| Natural logarithm of HDL-C (lnmmol/l) | –32 (6) | c |
| Systolic BP (centred at 140 mmHg; per 20 mmHg) | c | –20 (6) |
| On antihypertensive treatment (ref: no) | 127 (5) | 93 (14) |
| Severe mental illness history (ref: no) | 152 (6) | 280 (31) |
| Prior type 1 diabetes (ref: no) | 643 (45) | 731 (78) |
| Previous CVD (ref: MI only) | ||
| PAD only | NA | 66 (19) |
| Stroke only | NA | 124 (37) |
| Other CHD onlyd | NA | 84 (17) |
| Two or more | NA | 221 (22) |
| Time-varying characteristics | ||
| Current age (centred at 60 years; per 10 years) | 57 (2) | 62 (7) |
| Incident MI (ref: no) | 194 (24) | 231 (54) |
| Incident stroke (ref: no) | 362 (56) | 428 (82) |
| Incident CRV (ref: no) | ||
| Same year | 391 (40) | 233 (33) |
| ≥ 1 year ago | 69 (15) | 10 (26) |
| Diabetes (ref: no) | ||
| < 10 years ago | 360 (11) | 343 (24) |
| ≥ 10 years ago | 560 (19) | 568 (39) |
| Cancer (ref: no) | ||
| < 5 years | 357 (11) | 236 (25) |
| ≥ 5, < 10 years | 159 (13) | 123 (28) |
| ≥ 10 years ago | 77 (6) | e |
| VD (ref: no) | –95 (30) | –16 (91) |
| NVD (ref: no) | 389 (37) | 198 (65) |
| Interactions | ||
| Any incident MI and same year CRV (ref: no) | –226 (47) | c |
| Any incident MI and same year VD (ref: no) | c | –500 (109) |
| Any incident stroke and same year VD (ref: no) | –277 (86) | –439 (133) |
| < 5 years cancer and same year NVD (ref: no) | –245 (47) | c |
| Covariate | Participants without previous CVD | Participant with previous CVD | ||
|---|---|---|---|---|
| Part 1: likelihood of incurring cost, OR (95% CI)a | Part 2: cost (£), if any incurred, mean(SE)a | Part 1: likelihood of incurring cost, OR (95% CI)a | Part 2: cost (£), if any incurred, mean(SE)a | |
| Intercept | 0.13 (0.13 to 0.13) | 2102 (23) | 0.19 (0.18 to 0.21) | 2326 (119) |
| Baseline characteristics | ||||
| Male (ref: female) | 0.92 (0.91 to 0.93) | –65 (14) | 0.87 (0.84 to 0.89) | –125 (54) |
| Ethnicity (ref: white) | ||||
| Black | 1.04 (1 to 1.08) | –117 (68) | 1.06 (0.97 to 1.16) | –412 (128) |
| South Asian | 1.14 (1.1 to 1.18) | –168 (48) | 1.21 (1.12 to 1.31) | –426 (102) |
| Othersb | 1.03 (1 to 1.06) | –165 (49) | 1.09 (1.01 to 1.19) | –246 (145) |
| Townsend socioeconomic deprivation quintile (ref: quintile 3) | ||||
| 1 (least deprived) | 0.95 (0.94 to 0.96) | –81 (20) | 0.91 (0.89 to 0.94) | c |
| 2 | 0.99 (0.98 to 1.01) | –55 (22) | 0.95 (0.92 to 0.99) | c |
| 4 | 1.07 (1.05 to 1.08) | 24 (27) | 1.06 (1.02 to 1.1) | c |
| 5 | 1.17 (1.15 to 1.19) | 94 (27) | 1.15 (1.11 to 1.19) | c |
| Smoking (ref: never) | ||||
| Ex-smoker | 1.11 (1.1 to 1.12) | 40 (15) | 1.06 (1.04 to 1.09) | 8 (45) |
| Current smoker | 1.2 (1.18 to 1.22) | 183 (24) | 1.13 (1.09 to 1.17) | 276 (85) |
| Physical activity level (ref: moderate) | ||||
| Low | 1.1 (1.09 to 1.12) | 110 (23) | 1.25 (1.21 to 1.29) | 415 (74) |
| High | 1.07 (1.05 to 1.08) | –12 (16) | 1.04 (1.02 to 1.07) | –13 (46) |
| Missing | 1.14 (1.13 to 1.16) | 72 (20) | 1.17 (1.13 to 1.2) | 156 (51) |
| Unhealthy diet (ref: healthy diet) | 1.06 (1.05 to 1.07) | c | 1.06 (1.04 to 1.09) | c |
| BMI (kg/m2) (ref: ≥ 18.5, < 25) | ||||
| < 18.5 | 1.13 (1.06 to 1.2) | 298 (169) | 1.43 (1.23 to 1.66) | 1007 (796) |
| ≥ 25, < 30 | 1.12 (1.11 to 1.13) | 68 (16) | 1.04 (1.01 to 1.07) | 16 (50) |
| ≥ 30, < 35 | 1.24 (1.23 to 1.26) | 239 (20) | 1.14 (1.1 to 1.17) | 177 (58) |
| ≥ 35, < 40 | 1.36 (1.33 to 1.39) | 451 (35) | 1.21 (1.16 to 1.27) | 381 (87) |
| ≥ 40 | 1.51 (1.46 to 1.56) | 649 (66) | 1.34 (1.26 to 1.42) | 840 (178) |
| LDL-C (centred at 3.6 mmol/l; per 1 mmol/l) | 0.97 (0.96 to 0.97) | –36 (8) | c | c |
| Natural logarithm of HDL-C (lnmmol/l) | 0.86 (0.84 to 0.88) | c | c | c |
| Natural logarithm of creatinine centred at 4.4; per 0.2 lnμmol/l | 0.98 (0.98 to 0.99) | c | 1.02 (1.01 to 1.04) | 107 (24) |
| Systolic BP (centred at 140 mmHg; per 20 mmHg) | 0.93 (0.93 to 0.94) | c | 0.95 (0.94 to 0.96) | c |
| Diastolic BP (centred at 80 mmHg; per 10 mmHg) | 1.02 (1.01 to 1.02) | c | c | c |
| On antihypertensive treatment (ref: no) | 1.14 (1.13 to 1.16) | 141 (20) | 1.11 (1.09 to 1.14) | c |
| Severe mental illness history (ref: no) | 1.43 (1.41 to 1.45) | 193 (25) | 1.39 (1.34 to 1.43) | 227 (66) |
| Prior type 1 diabetes (ref: no) | 1.83 (1.74 to 1.93) | 702 (119) | 1.69 (1.58 to 1.82) | 792 (148) |
| Previous CVD (ref: MI only) | ||||
| PAD only | NA | NA | 1.19 (1.12 to 1.27) | 498 (122) |
| Stroke only | NA | NA | 1.11 (1.04 to 1.19) | 113 (118) |
| Other CHD onlyd | NA | NA | 1.27 (1.2 to 1.34) | 105 (106) |
| Two or more | NA | NA | 1.43 (1.35 to 1.52) | 381 (114) |
| Time-varying characteristics | ||||
| Current age (centred at 60 years; per 10 years) | 1.38 (1.37 to 1.39) | 173 (9) | 1.25 (1.23 to 1.27) | 121 (31) |
| Incident MI (ref: no) | ||||
| Same year | 47.09 (38.69 to 57.32) | 3054 (167) | 47.33 (33.93 to 66.02) | 3965 (241) |
| 1 year ago | 1.76 (1.59 to 1.95) | 670 (153) | 1.71 (1.52 to 1.92) | 1011 (303) |
| 2 years ago | 1.44 (1.29 to 1.61) | 304 (117) | 1.28 (1.17 to 1.41) | 696 (171) |
| ≥ 3 years ago | 1.35 (1.22 to 1.49) | e | e | e |
| Incident stroke (ref: no) | ||||
| Same year | 47.08 (41.91 to 52.9) | 4485 (142) | 46.65 (36.98 to 58.85) | 4591 (208) |
| 1 year ago | 2.58 (2.39 to 2.8) | 2192 (296) | 2.19 (1.96 to 2.46) | 1561 (260) |
| 2 years ago | 1.78 (1.62 to 1.95) | 833 (137) | 1.52 (1.38 to 1.67) | e |
| ≥ 3 years ago | 1.49 (1.37 to 1.61) | e | e | e |
| Incident CRV (ref: no) | ||||
| Same year | f | 5186 (114) | f | 5117 (146) |
| 1 year ago | 1.66 (1.53 to 1.81) | 137 (82) | 1.54 (1.41 to 1.68) | 599 (192) |
| 2 years ago | 1.51 (1.37 to 1.65) | e | 1.32 (1.23 to 1.41) | 4 (110) |
| ≥ 3 years ago | 1.32 (1.22 to 1.43) | e | e | e |
| Diabetes (ref: no) | ||||
| < 10 years ago | 1.36 (1.33 to 1.39) | 274 (35) | 1.36 (1.32 to 1.41) | 408 (80) |
| ≥ 10 years ago | 1.2 (1.16 to 1.23) | 158 (51) | 1.22 (1.17 to 1.28) | e |
| Cancer (ref: no) | ||||
| Same year | 40.92 (39.33 to 42.58) | 5380 (56) | 24.59 (22.17 to 27.27) | 5160 (150) |
| 1 year ago | 6.04 (5.87 to 6.21) | 4620 (86) | 3.81 (3.55 to 4.08) | 3475 (181) |
| 2 years ago | 3.03 (2.94 to 3.12) | 2332 (88) | 2.39 (2.23 to 2.56) | 1863 (172) |
| 3 years ago | 2.46 (2.38 to 2.54) | 1899 (89) | 2.09 (1.95 to 2.25) | 1601 (176) |
| 4 years ago | 2.23 (2.16 to 2.31) | 1502 (86) | 1.92 (1.79 to 2.07) | 947 (75) |
| ≥ 5 years ago | 1.69 (1.66 to 1.72) | 1159 (38) | 1.6 (1.54 to 1.65) | e |
| VD (ref: no) | 2.32 (2.03 to 2.64) | 4318 (491) | 2.38 (2.07 to 2.74) | 4749 (420) |
| NVD (ref: no) | 11.4 (10.69 to 12.16) | 6792 (145) | 9.1 (7.97 to 10.38) | 6412 (260) |
| Event interactions | ||||
| Same year MI and same year CRV (ref: no) | f | –3848 (227) | f | –3358 (364) |
| Same year MI and same year VD (ref: no) | 0.03 (0.02 to 0.04) | –4694 (670) | 0.02 (0.01 to 0.03) | –4874 (722) |
| Same year stroke and same year VD (ref: no) | 0.22 (0.16 to 0.32) | –4171 (685) | 0.09 (0.06 to 0.14) | –4308 (691) |
| Same year cancer and same year NVD (ref: no) | 0.35 (0.26 to 0.47) | –1725 (291) | 0.37 (0.21 to 0.66) | –1529 (617) |
Summary of findings
Our research offers valuable contemporary insights into the long-term primary and hospital care costs associated with CVD events, providing models to predict healthcare costs at the individual level. The cost models are intended to be used in cost-effectiveness assessments of therapies to reduce CVD risk, using CVD decision models.
The cost models can also be used to derive the marginal effects of CVD events on primary and hospital care cost. Both the cost models and the derived marginal effects can inform economic and policy assessments of the value of health interventions aimed at reducing CVD risk and burden, as well as cost analyses. Beyond the impact of conditions such as CVD, diabetes and cancer, our research identified key individual factors that play a significant role in primary and hospital care costs. These factors include smoking, obesity and low physical activity, and addressing them could help reduce the demand and costs associated with healthcare services.
Chapter 5 Modelling health-related quality of life associated with cardiovascular disease events
Aims and objectives
Cardiovascular disease can have a significant impact on an individual’s HRQoL. 45 To estimate this impact, we developed a regression model to predict an individual’s HRQoL based on their age, sex, socioeconomic status, BMI, smoking status, and history of CVD and other diseases. Our goal was to integrate this QoL model into the CVD microsimulation model to provide annual QoL prediction for individuals in the model.
Methods
Health Survey for England
The QoL model was developed using data from the HSE in 2006, 2011 and 2017. The HSE is an annual cross-sectional survey designed to monitor the national health. In addition to core questions, the HSE includes yearly questionnaires on different topics. In 2006, 2011 and 2017 the survey included both the EuroQoL-5 Dimensions (EQ-5D) questionnaire and the detailed CVD questionnaire. The EQ-5D questionnaire measures participants’ generic HRQoL and the CVD questionnaire queries their first and latest experiences of different CVD events.
Missing data
We aligned the specification of participants’ baseline characteristics and disease event histories with those in the CVD microsimulation model to ensure compatibility. The dependent variable, the ED-5D QoL utility index, was derived from the five scores of the five-dimension EQ-5D questionnaire, which measures mobility, self-care, usual activities, pain and anxiety. The EQ-5D-3L questionnaire was administered in HSE 2006 and 2011, with QoL utility values ranging from − 0.594 for the worst health state to 1 for full health, where 0 represents a health state equivalent to death and higher values indicate better QoL. 46 In HSE 2017, the EQ-5D-5L questionnaire was used with a similar QoL utility range after mapping the 5L value set to the 3L values. 47 The independent variables included age, sex, ethnicity, socioeconomic deprivation [indicated by the Index of Multiple Deprivation (IMD)], BMI, current smoking status, treated hypertension, mental illness, cancer, diabetes, MI in last 12 months, stroke in last 12 months, CRV in last 12 months, any angina, MI that happened more than 1 year ago, stroke that happened more than 1 year ago, and CRV that happened more than 1 year ago.
Apart from the EQ-5D (10% missing) and BMI (16.5% missing), only small proportions of missing values were observed for other participant characteristics. Based on the assumption of missing at random, multiple imputation was used to impute missing data while estimating the QoL model. The R package ‘mice’ was used, which imputes multivariate missing data based on fully conditional specification, where each incomplete variable is imputed by a separate model. 48 All covariates included in the QoL model were included in the imputation models. The dependent variable, the EQ-5D utility index, was continuous and skewed, with lower and upper bounds. An ordered logit model has been shown more appropriate for imputing missing dimensions of the EQ-5D questionnaire data,49 and was thus used in the present study. For other missing variables, logistic regression was used for binary variables, multinomial logit for ethnicity, and ordered logit for BMI category. Twenty imputations and 100 iterations for each imputation were performed. Following imputation, the EQ-5D utility index was calculated.
Quality-of-life regression model
An ordinary linear regression is a popular choice for modelling EQ-5D utility, as it allows the estimation of direct QoL decrements associated with individual characteristics and disease events, and there is no convincing evidence that other estimators are clearly superior. A linear regression model was estimated for QoL utility following multiple imputation of missing data. Owing to the non-linear effect of age on EQ-5D utility, age splines with a knot at 70 years were used. In a sensitivity analysis, the QoL model coefficients remained similar following the exclusion of participants aged < 40 years. Further checks did not indicate important interactions between recent MI and CRV events or between CVD histories and recent events.
Results
There were 24,231 participants included in the QoL model estimation (Table 11). The average EQ-5D utility across study population was 0.83 (SD 0.24). Participants’ mean age was 54 years; 55% were women; 91% were of white ethnicity; 20% were current smokers; and 68% were overweight or obese. Among the study participants, 19% had treated hypertension, 7.6% had diabetes, 5.2% had long-term mental illness and 2.4% had a history of cancer. Additionally, 2.3% were diagnosed with angina; 1.3% with MI more than 1 year ago without other CVDs; 1.7% with stroke more than 1 year ago without other CVDs; 2% with more than 1 CVDs. 1.9% had received CRV more than 1 year ago. MI, stroke and CRV events that had occurred in the last 12 months were rare, accounting for 0.4%, 0.5% and 0.2% of participants, respectively.
| Characteristic | n (%)/mean (SD) (N = 24,231 participants) |
|---|---|
| EQ-5D utility | 0.83 (0.24) |
| Missing | 2436 (10%) |
| Age, years | 53.9 (15.2) |
| Male | 10,822 (44.7%) |
| Ethnicity | |
| White | 21,907 (90.7%) |
| Black | 584 (2.4%) |
| South Asian | 1102 (4.6%) |
| Others | 550 (2.3%) |
| Missing | 88 (0.4%) |
| Deprivation | |
| IMD 1 | 5214 (21.5%) |
| IMD 2 | 5390 (22.2%) |
| IMD 3 | 5033 (20.8%) |
| IMD 4 | 4544 (18.8%) |
| IMD 5 (most deprived) | 4050 (16.7%) |
| BMI category (kg/m2) | |
| < 18.5 | 166 (0.8%) |
| 18.5–25 | 6222 (30.8%) |
| 25–30 | 8046 (39.8%) |
| 30–35 | 3869 (19.1%) |
| 35–40 | 1351 (6.7%) |
| ≥ 40 | 573 (2.8%) |
| Missing | 4004 (16.5%) |
| Smoking status | |
| Current smoker | 4929 (20.4%) |
| Ex-smoker | 9283 (38.4%) |
| Missing | 65 (0.3%) |
| Treatment of hypertension | 4695 (19.4%) |
| Missing | 24 (0.1%) |
| Mental illness | 1254 (5.2%) |
| Missing | 8 (0.0%) |
| Cancer | 588 (2.4%) |
| Missing | 8 (0.0%) |
| Diabetes history | |
| Diabetes ≤ 10 years | 1204 (5.0%) |
| Diabetes > 10 years | 617 (2.6%) |
| Missing | 54 (0.2%) |
| CVD history | |
| Angina only (ever) | 564 (2.3%) |
| MI only ≥ 1 year | 318 (1.3%) |
| Stroke only ≥ 1 year | 421 (1.7%) |
| Two or more conditions | 483 (2.0%) |
| Missing | 21 (0.1%) |
| CRV ≥ 1 year ago | 448 (1.9%) |
| Missing | 15 (0.1%) |
| MI < 12 months ago | 85 (0.4%) |
| Missing | 4 (0.0%) |
| Stroke < 12 months ago | 114 (0.5%) |
| Missing | 4 (0.0%) |
| CRV < 12 months ago | 50 (0.2%) |
| Missing | 5 (0.0%) |
Table 12 presents the results of the QoL regression model. Previous experiences of CVD events were associated with lower QoL. MI was associated with 0.10 (95% CI 0.03 to 0.16) lower QoL in the year of the event and 0.07 (0.04 to 0.10) lower QoL in the following years. Stroke was associated with 0.09 (0.04 to 0.13) lower QoL in the year of the event and 0.13 (0.11 to 0.16) lower QoL in subsequent years. On the other hand, CRV was associated with higher QoL of 0.04 (0.02 to 0.07) in years following the procedure. Diabetes was associated with 0.04 (0.03 to 0.06) lower QoL in the first 10 years from diagnosis and 0.08 (0.06 to 0.10) lower QoL in the following years. Cancer affecting daily activities was associated with 0.13 (0.11 to 0.14) lower QoL. Mental illness was associated with 0.26 (0.24 to 0.27) lower QoL.
| Coefficient (95% CI) | |
|---|---|
| Intercept | 0.879 (0.869 to 0.889) |
| Age spline 1 (< 70 years)a | –0.028 (–0.031 to –0.025) |
| Age spline 2 (≥ 70 years)a | –0.057 (–0.067 to –0.048) |
| Male | 0.034 (0.028 to 0.040) |
| Ethnicity (ref: white) | |
| Black | –0.002 (–0.022 to 0.018) |
| South Asian | –0.026 (–0.041 to –0.011) |
| Others | –0.016 (–0.036 to 0.004) |
| Deprivation quintiles (ref: IMD 3) | |
| IMD 1 | 0.026 (0.017 to 0.035) |
| IMD 2 | 0.010 (0.001 to 0.019) |
| IMD 4 | –0.019 (–0.029 to –0.010) |
| IMD 5 | –0.055 (–0.065 to –0.045) |
| BMI categories (ref: 18.5–25 kg/m2) | |
| < 18.5 | –0.031 (–0.066 to 0.004) |
| 25–30 | –0.014 (–0.021 to –0.006) |
| 30–35 | –0.039 (–0.049 to –0.03) |
| 35–40 | –0.086 (–0.101 to –0.071) |
| ≥ 40 | –0.128 (–0.152 to –0.105) |
| Current smoker | –0.057 (–0.066 to –0.049) |
| Ex-smoker | –0.021 (–0.028 to –0.014) |
| Treatment of hypertension | –0.026 (–0.034 to –0.017) |
| Mental illness | –0.267 (–0.281 to –0.253) |
| Cancer | –0.128 (–0.147 to –0.109) |
| Diabetes ≤ 10 years | –0.043 (–0.059 to –0.028) |
| Diabetes > 10 years | –0.085 (–0.105 to –0.065) |
| CVD history | |
| Angina only (ever) | –0.118 (–0.14 to –0.096) |
| MI only ≥ 1 year | –0.071 (–0.100 to –0.042) |
| Stroke only ≥ 1 year | –0.129 (–0.155 to –0.104) |
| Two or more conditions | –0.187 (–0.212 to –0.162) |
| CRV ≥ 1 year ago | 0.041 (0.014 to 0.067) |
| MI < 12 months ago | –0.101 (–0.164 to –0.038) |
| Stroke < 12 months ago | –0.087 (–0.136 to –0.039) |
| CRV < 12 months ago | 0.025 (–0.051 to 0.102) |
Summary of findings
We developed a QoL model using data from HSE 2006, 2011 and 2017, after aligning the definitions of covariates and model specification with characteristics in the CVD microsimulation model. This allowed the QoL model to be integrated into the CVD microsimulation model and used to estimate QALYs during model simulation.
It is worth noting that although the estimate of the cancer-related utility decrement was 0.13, the incident cancer’s contribution in the CVD microsimulation model was revised to 0.03. The main reason is that the HSE only recorded cancer affecting daily life, whereas the incident cancer in the CVD microsimulation model included any cancer except non-melanoma skin cancer. For example, in the 2011 HSE questionnaire, the related question was ‘do you have any long-standing illness, disability or infirmity? By long-standing I mean anything that has troubled you over a period of time, or that is likely to affect you over a period of time?’. Further cross-tabulating the reported cancer with the follow-up question, ‘does this illness or disability/do any of these illnesses or disabilities limit your activities in any way’, more than 60% of participants reporting a cancer history reported limited activities due to the illness in all the 2006, 2011 and 2017 surveys of HSE. However, the percentages of limited activities associated with cancer reported in other studies ranged between 10% and 50%. 50–52 This indicated that the estimate based on HSE data is likely to overstate the impact of any previous cancer on QoL, and a cancer-related utility decrement of 0.03, informed by the literature,53–59 was used, instead.
Chapter 6 Cost-effectiveness of statin therapies for people 40–70 years old in the UK
Aims and objectives
Our aim was to assess the net health effects and cost-effectiveness of lifetime statin therapy of different intensity in the contemporary UK population from the perspective of the UK NHS.
To achieve this, we used the CVD microsimulation policy model with integrated healthcare costs and QoL models to project CVD disease progression, QoL-adjusted life expectancy, and healthcare costs with and without statin treatment.
Methods
The cardiovascular disease microsimulation policy model
The CVD microsimulation policy model (see Chapter 3) with integrated primary care and hospital inpatient care cost models (see Chapter 4) and QoL model (see Chapter 5) informed the assessment of the cost-effectiveness of statin therapy. The model was used to project event risks, survival, primary and hospital care costs and QoL and to summarise life years, QALYs and healthcare costs over individuals’ remaining lifetimes (i.e. until death or 110 years of age) without and with statin treatment, and to assess the cost-effectiveness of statin therapies in categories of individuals.
Health-related quality of life
We estimated the HRQoL associated with participant characteristics, disease histories and events by employing a linear regression model using data from the HSE conducted in 2006, 2011 and 2017 (see Chapter 5), which measured participants’ QoL using the EQ-5D questionnaire. We valued participants’ QoL using UK valuations of QoL utility. 46,47 The estimated QoL model was then integrated into the CVD model to forecast individuals’ QoL in each year in the model.
Effects and costs of statin therapy
Table 13 presents the effects and costs of statin therapy used in the analyses. We evaluated the effects of standard statin therapy (achieving 35–45% reduction in LDL-C) and higher-intensity statin therapy (achieving ≥ 45% LDL-C reduction) (see Table 1) compared with no statin treatment. The absolute reduction in LDL-C was calculated based on the proportional reduction achieved by the statin regimen and the individual’s pre-treatment LDL-C level. Reductions in cardiovascular event risks with statin regimens were calculated based on the absolute reduction in LDL-C with the particular statin regimen and the relative reduction of cardiovascular event risks per 1 mmol/l LDL-C reduction with statin therapy, reported by the CTTC IPD meta-analysis of randomised trials of statin therapy. 5 The analyses took into account the excess rates of new-onset diabetes,12,13 myopathy and rhabdomyolysis60 reported with statin therapy (see Table 13).
| Item | Value | Source |
|---|---|---|
| Effects of statin therapy on cardiovascular events per 1-mmol/l reduction in LDL-C, RR (95% CI) | CTTC IPD meta-analysis5 | |
|
0.76 (0.73 to 0.79) | |
|
0.84 (0.80 to 0.89) | |
|
0.75 (0.73 to 0.78) | |
|
0.88 (0.85 to 0.91) | |
| Adverse effects of statin therapy on: | ||
| Incident diabetes, OR (95% CI) | ||
| With standard statin therapy compared with no statin treatment | 1.09 (1.02 to 1.17) | Meta-analyses of randomised controlled trials12 |
| With higher-intensity statin therapy compared with standard statin therapy | 1.12 (1.04 to 1.22) | Meta-analyses of randomised controlled trials13 |
| Myopathy | ||
| Excess per 100,000 person-years on statin therapy (95% CI) | 11 (4 to 27) | Overview of cohort studies60 |
| Occurrence of myopathy is associated with reduction in QoL over 30 days’ recovery period. Statin treatment is stopped | 0.017 QALY reduction in year | Modelling study61 |
| Rhabdomyolysis | ||
| Excess per 100,000 person-years on statin therapy (95% CI) | 3.4 (1.6 to 6.5) | Overview of cohort studies60 |
| Case fatality | 10% | Overview of cohort studies60 |
| Reduction in QoL | 50% over 7.5 days hospital admission and by 20% for further 30 days recovery | Modelling study61 |
| LDL-C reductions with statin therapy: | ||
| With standard statin therapy (e.g. atorvastatin 20 mg/day, rosuvastatin 5–10 mg/day or simvastatin 40–80 mg/day) | 37–43%; 43% used in base-case | Meta-analysis of randomised controlled trials27 |
| With higher-intensity statin therapy (e.g. atorvastatin 40–80 mg/day, rosuvastatin 20–80 mg/day) | 48–58%; 55% used in base-case | Meta-analysis of randomised controlled trials27 |
| Statin therapy costs (£) | ||
| Standard statin therapy (e.g. atorvastatin 20 mg/day, rosuvastatin 5-10 mg/day or simvastatin 40–80 mg/day) | £14.09–19.57 per year; £14.35 used in base-case | NHS drug tariff, December 202114 |
| Higher-intensity statin therapy (e.g. atorvastatin 40–80 mg/day, rosuvastatin 20–40 mg/day) | £15.91–27.91 per year; £21.91 used in base-case | NHS drug tariff, December 202114 |
| Statin initiation and monitoring healthcare costs (£) | ||
| • In year of initiation (a doctor and a nurse consultations; tests of blood lipids, HbA1c, thyroid function) | £54.65 | Unit Costs of Health and Social Care;37 NHS reference costs62 |
| • In subsequent years: a nurse consultation and a blood lipids test (for people with previous CVD) | £12.05 | Unit Costs of Health and Social Care;37 NHS reference costs62 |
Cost of statin treatment included the cost of generic statin medication14 and the costs of consultations37 and blood lipid tests62 for the initiation and monitoring of statin prescribing in the NHS (see Table 13).
Study population
UK Biobank individual participants’ characteristics at entry into the study informed the analyses of statin cost-effectiveness in people 40–70 years old. We report results in categories of participants by history of CVD, sex, age, untreated LDL-C level, and, for those without previous CVD at entry, by their estimated 10-year CVD risk, calculated using the QRISK3 risk score. 21
Cost-effectiveness analyses
Base-case analysis
The cost-effectiveness of lifetime statin therapy was evaluated from the perspective of the UK NHS. The following assumptions were made: (1) the reduction in LDL-C levels with specific statin regimen corresponded to the average proportional reduction achieved with that regimen and the pre-treatment LDL-C level, (2) the relative effects of statin therapy on event risks were similar in categories of participants and remained unchanged over the duration of therapy, (3) disease events did not differ in severity irrespective of treatment regimen and (4) statin therapy did not affect the risks of cancer or other nonvascular events63 (except the risk of incident diabetes, myopathy and rhabdomyolysis as the adverse events), nor cause any discomfort or disutility beyond the CVD events and adverse events previously specified.
For each individual, we ran 500 microsimulations to address the first-order uncertainty. We reported the gained life-years and QALYs, additional statin and healthcare costs, and incremental costs per QALY for standard and higher-intensity statin therapy. The analysis followed the NICE manual for health technology evaluations with future life-years, QALYs and costs discounted at 3.5% per year. 64
Assessment of parameter uncertainty
We summarised parameter uncertainty using 500 and 1000 sets of parameter values, including uncertainty in statin effects on event risks, risk equations, QoL and healthcare costs, for participants without and participants with previous CVD history, respectively. Values for treatment effects were sampled from log-normal distributions based on the relative risk reductions with statin therapy (see Table 13). Bootstrapping was used to derive the values for (1) the parameters of the events risk equations and of the healthcare cost equations from the UKB study population and (2) the parameters of the QoL equation from the HSE participants’ data. Cost-effectiveness acceptability curves and probability of cost-effectiveness at willingness-to-pay thresholds of £20,000 and £10,000 per QALY were reported.
Sensitivity and scenario analyses
We varied key parameters in sensitivity analyses. First, we assumed the relative risk reductions of cardiovascular events with statin therapy to increase annually, as suggested by Mendelian randomisation studies,65 with a further 1.5% proportional reduction per 1 mmol/l reduction in LDL-C each year following the first 5 years of treatment. In another scenario analysis, we assumed the base-case relative risk reduction to decline by 10% annually from year 6 onwards. Second, we applied smaller relative risk reductions in cardiovascular events per 1 mmol/l LDL-C after 75 years of age, informed from data only among people aged > 75 years in the IPD meta-analysis. 5 Third, we ran scenario analyses with small detrimental or beneficial statin effect on incident cancer, informed by the 95% CI limits reported in an IPD meta-analysis of randomised statin trials. 63 Fourth, we implemented a scenario analysis that assessed statin cost-effectiveness under real-world compliance with statin therapy over time, derived from routine UK data,66 with statin effects and costs discontinued with therapy discontinuation. Fifth, we included analyses with hypothetical extra disutility associated with statin treatment equal to 0.001 or 0.002 each year. 67 In further sensitivity analyses, we reduced the effects of cardiovascular events or diabetes adverse effects on QoL by 50% and used discount rates of 1.5% instead of 3.5% per year. We also presented results of a scenario analysis in which only healthcare costs for CVD and incident diabetes were included and a sensitivity analysis with higher costs of statin therapy.
Further details of the methods of cost-effectiveness analyses are available in Appendix 6, including further details of the sensitivity and scenario analyses (see Appendix 6, Tables 30 and 31).
Results
Categories of people 40–70 years old
Table 14 presents the number of UKB participants in categories. Participants who were identified to have a lower risk of CVD were predominantly women, younger, and with lower LDL-C levels. There were no men aged 60–70 years with a 10-year CVD risk of ˂ 5%. A small number of men and women aged 40–49 years with a 10-year CVD risk ˃ 15% were grouped together with those with a 10-year CVD risk of 10–15% in the corresponding categories by LDL-C for the presentation of results.
| Sex, age (years) | Without CVD, by 10-year CVD risk (QRISK3, %) | With CVD | ||||
|---|---|---|---|---|---|---|
| < 5 | 5–10 | 10–15 | 15–20 | ≥ 20 | ||
| Pre-treatment LDL-C (mmol/l): < 3.4 | ||||||
| Men | ||||||
| 40–49 | 13,497 | 4037 | 711 | 239a | 228a | 939 |
| 50–59 | 2281 | 9055 | 4759 | 1847 | 1622 | 2263 |
| 60–70 | 0 | 1535 | 6073 | 6513 | 9113 | 6218 |
| Women | ||||||
| 40–49 | 32,831 | 986 | 146a | 60a | 119a | 1430 |
| 50–59 | 18,843 | 6707 | 1025 | 308a | 366a | 1868 |
| 60–70 | 1522 | 9822 | 5742 | 1994 | 1589 | 2956 |
| Pre-treatment LDL-C (mmol/l): ≥ 3.4, < 4.1 | ||||||
| Men | ||||||
| 40–49 | 10,436 | 5629 | 1099 | 332a | 289a | 853 |
| 50–59 | 1255 | 9445 | 7190 | 3079 | 2408 | 2317 |
| 60–70 | 0 | 908 | 5919 | 8003 | 14,028 | 6607 |
| Women | ||||||
| 40–49 | 17,285 | 1214 | 178a | 59a | 86a | 827 |
| 50–59 | 17,471 | 11,228 | 1980 | 540 | 454a | 2109 |
| 60–70 | 1304 | 14,829 | 11,580 | 4693 | 3039 | 4298 |
| Pre-treatment LDL-C (mmol/l): ≥ 4.1 | ||||||
| Men | ||||||
| 40–49 | 5623 | 5310 | 1605 | 645 | 660 | 1006 |
| 50–59 | 423a | 5790 | 6616 | 4044 | 5005 | 3581 |
| 60–70 | 0 | 260 | 2891 | 5634 | 18,960 | 9950 |
| Women | ||||||
| 40–49 | 6943 | 1247 | 295a | 128a | 214a | 557 |
| 50–59 | 10,022 | 12,220 | 3574 | 1280 | 1300 | 2645 |
| 60–70 | 568 | 12,079 | 14,068 | 8507 | 9135 | 6854 |
Base-case cost-effectiveness of statin therapy in categories of 40–70 years old
In participant categories defined by age, sex, previous CVD and 10-year CVD risk, standard statin therapy (20 mg of atorvastatin daily) was projected to increase individual QALYs (undiscounted) by 0.20–1.09, and higher-intensity statin therapy (80 mg of atorvastatin daily) by a further 0.03–0.20 QALYs (see Figure 8). Across population categories, standard statin therapy compared with no statin therapy had an incremental cost per QALY ranging from £280 to £8530, with higher-intensity statin therapy realising additional QALYs compared with standard statin therapy at an incremental cost per QALY ranging from £2610 to £47,640 (see Figure 9 and Appendix 7, Table 32). At a £20,000-per-QALY threshold, it was certain that either standard or higher-intensity statin therapy was cost-effective (see Appendix 7, Table 32). Higher-intensity statin therapy had the higher probability of being cost-effective in most participant categories and standard statin therapy was most likely to be cost-effective in categories of younger participants with lower LDL-C and/or lower CVD risk levels (see Figure 10). The probability that either standard or higher-intensity statin therapy was cost-effective remained above 80% at the £10,000-per-QALY threshold across all participant categories, with higher-intensity statin therapy having higher probability of being cost-effective at higher CVD risk and/or higher LDL-C levels (see Figure 10 and Appendix 7, Table 32).
FIGURE 8.
Undiscounted QALYs gained with lifetime statin therapy in categories by sex, age, CVD risk and pre-treatment LDL-C level. NA, not applicable (no UKB participants in this category).
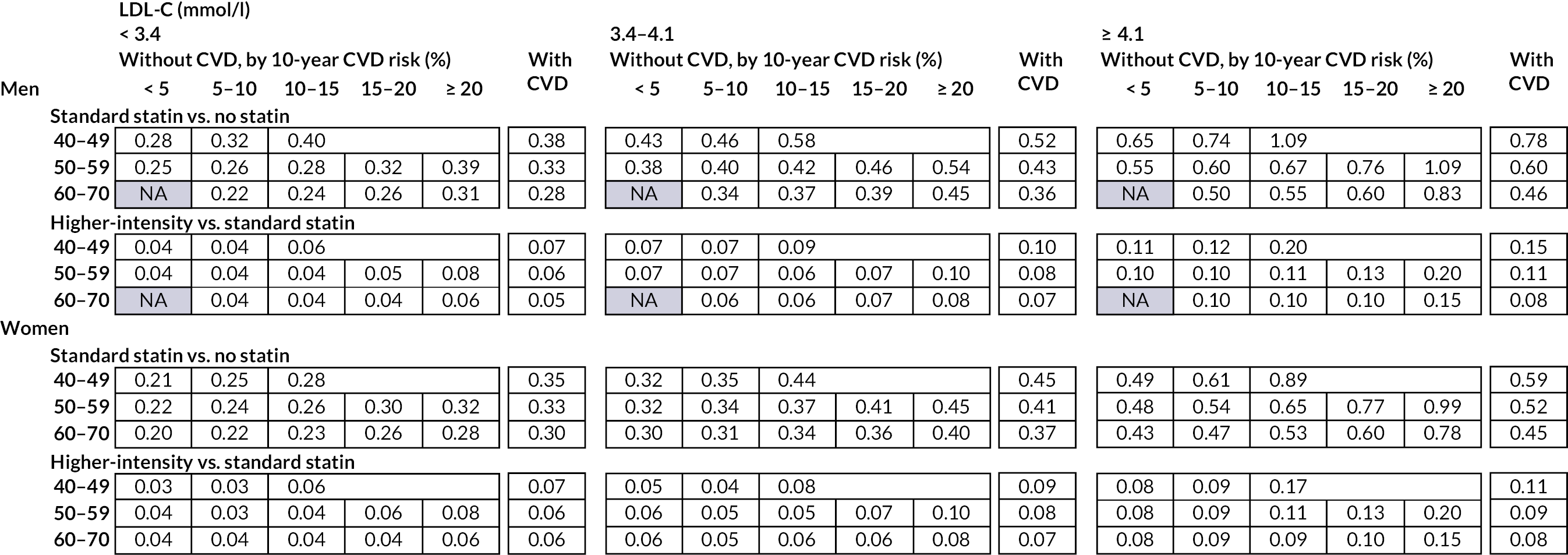
FIGURE 9.
Incremental cost per QALY gained (£/QALY gained) with lifetime statin therapy in categories by sex, age, CVD risk and pre-treatment LDL-C level. ICER, incremental cost-effectiveness ratio with costs and QALYs discounted at 3.5% per year; NA, not applicable (no UKB participants in this category).
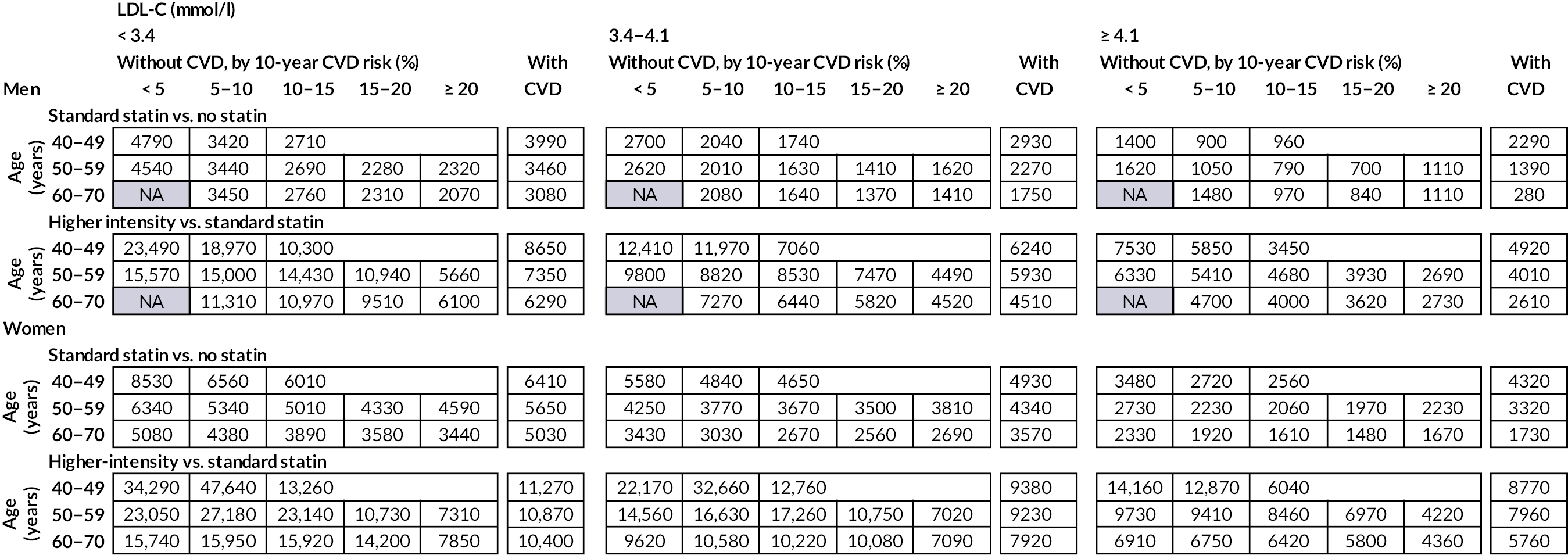
FIGURE 10.
Probability of cost-effectiveness of lifetime statin therapy in categories by sex, age, CVD risk and pre-treatment LDL-C level. (a) At £20,000-per-QALY-gained threshold for cost-effectiveness and (b) at £10,000-per-QALY-gained threshold for cost-effectiveness. NA, not applicable (no UKB participants in this category)
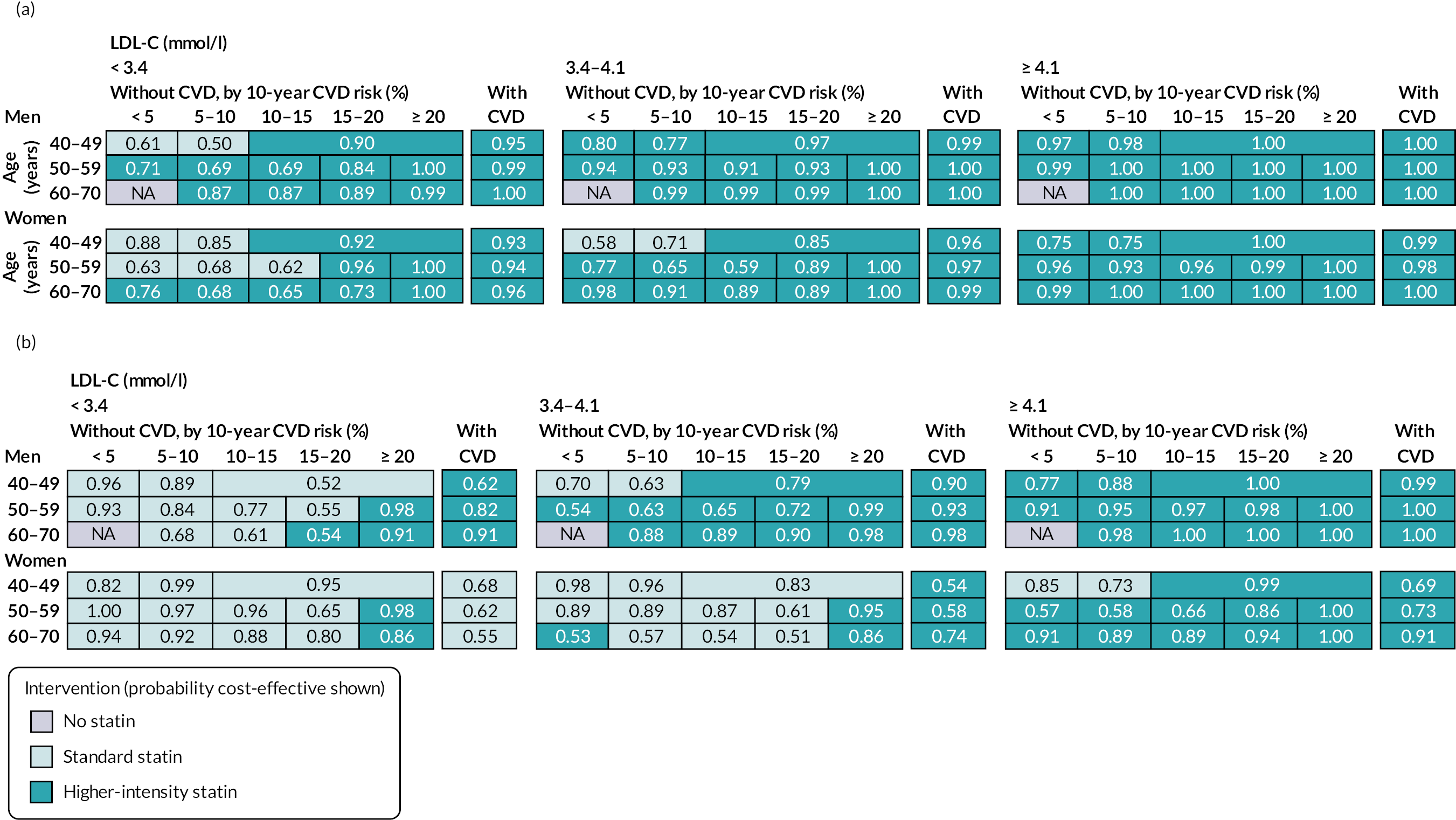
Sensitivity analyses of statin cost-effectiveness in categories of 40–70 years old
Sensitivity analyses found robust cost-effectiveness results across most categories of participants, except for the participants who were younger and had low 10-year CVD risk (Table 15). For these participants, the cost-effectiveness results were moderately impacted by the following hypothetical assumptions: (1) declining treatment effect after 5 years on statin treatment; (2) hypothetical increase in cancer incidence risk with statin treatment; (3) increased cost of statin medication and (4) extra QoL disutility with statin treatment ≥ 0.002 per year.
| People without CVD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex, CVD risk | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men, high | Men, moderate | Women, high | Women, moderate | Men, high | Men, moderate | Women, high | Women, moderate | |
| Age (years) | 60–70 | 40–49 | 60–70 | 40–49 | 60–70 | 40–49 | 60–70 | 40–49 |
| 10-year CVD risk (%) | 15–20 | 5–10 | 15–20 | 5–10 | 15–20 | 5–10 | 15–20 | 5–10 |
| LDL-C (mmol/l) | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 |
| People with previous CVD | ||||||||
| Sex, CVD risk | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men, very high | Men, high | Women, very high | Women, high | Men, very high | Men, high | Women, very high | Women, high | |
| Age (years) | 60–70 | 40–49 | 60–70 | 40–49 | 60–70 | 40–49 | 60–70 | 40–49 |
| LDL-C (mmol/l) | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 | ≥ 4.1 | < 3.4 |
| Base-case (£/QALY gained) | 840 | 3420 | 1480 | 6560 | 3620 | 18,970 | 5800 | 47,640 |
| Relative reduction in CVD events with statin therapy increasing annually (£) | 1300 | 2360 | 1640 | 4150 | 2750 | 7800 | 4320 | 12,370 |
| Relative reduction in CVD events with statin therapy decreasing annually (£) | 1290 | 20,040 | 2800 | 42,850 | 12,480 | –23,820a | 24,940 | –22,690a |
| Relative reduction in CVD events with statin therapy reduced in elderly (£) | 1120 | 3790 | 1990 | 7410 | 5170 | 28,220 | 8370 | 135,170 |
| RR in incident cancer with statin therapy of 0.96 (£) | 390 | 2120 | 1090 | 4230 | 3590 | 18,360 | 5750 | 47,000 |
| RR in incident cancer with statin therapy of 1.05 (£) | 1790 | 9720 | 2230 | 18,340 | 3660 | 19,680 | 5850 | 49,190 |
| Risk of NVD increased by 20% (£) | 650 | 3420 | 1400 | 6700 | 3550 | 20,560 | 5840 | 57,920 |
| Compliance with statin therapy as in routine care (£) | 1260 | 4520 | 1920 | 7480 | 4800 | 33,030 | 7500 | 101,540 |
| With QoL disutility of daily statin pill of 0.001/year (£) | 910 | 4390 | 1610 | 9440 | 3630 | 19,010 | 5810 | 47,830 |
| With QoL disutility of daily statin pill of 0.002/year (£) | 980 | 6130 | 1770 | 16,790 | 3630 | 19,060 | 5820 | 48,020 |
| QoL disutilities of CVD events reduced by 50% (£) | 870 | 3550 | 1540 | 6990 | 3790 | 20,690 | 6150 | 59,350 |
| QoL disutilities of CVD events increased by 50% (£) | 820 | 3300 | 1430 | 6190 | 3470 | 17,520 | 5490 | 39,790 |
| QoL disutilities of diabetes reduced by 50% (£) | 830 | 3280 | 1450 | 6130 | 3360 | 13,760 | 5160 | 25,390 |
| Discount rates for costs and outcomes at 1.5% per annum (£) | 1420 | 3140 | 1910 | 5700 | 3710 | 13,500 | 5420 | 28,210 |
| Include only healthcare costs for CVD and incident diabetes (£) | (–400) | 2120 | 190 | 4490 | 2260 | 17,040 | 4280 | 42,940 |
| Cost of statin × 1.5 (£) | 1360 | 5010 | 2060 | 8750 | 5300 | 26,240 | 7680 | 63,090 |
| Cost of statin × 2 (£) | 1890 | 6590 | 2640 | 10,930 | 6980 | 33,510 | 9570 | 78,530 |
| Cost of statin × 5 (£) | 5020 | 16,130 | 6120 | 24,040 | 17,050 | 77,140 | 20,900 | 171,210 |
| Base-case (£) | 280 | 3990 | 1730 | 6410 | 2610 | 8650 | 5760 | 11,270 |
| Relative reduction in CVD events with statin therapy increasing annually (£) | 1400 | 3420 | 2140 | 4140 | 2630 | 5470 | 4330 | 6090 |
| Relative reduction in CVD events with statin therapy decreasing annually (£) | (–200) | 11,080 | 2410 | 22,320 | 4000 | –93,080a | 15,120 | –823,410a |
| Relative reduction in CVD events with statin therapy reduced in elderly (£) | 790 | 4020 | 2080 | 6980 | 3790 | 9710 | 7440 | 13,710 |
| RR in incident cancer with statin therapy of 0.96 (£) | 270 | 3270 | 1580 | 5000 | 2640 | 8610 | 5760 | 11,170 |
| RR in incident cancer with statin therapy of 1.05 (£) | 290 | 5260 | 2000 | 9320 | 2570 | 8730 | 5770 | 11,430 |
| Risk of NVD increased by 20% (£) | (–100) | 3950 | 1530 | 6540 | 2170 | 8840 | 5570 | 11,790 |
| Compliance with statin therapy as in routine care (£) | 900 | 4980 | 2450 | 7710 | 4240 | 10,830 | 8250 | 14,500 |
| With QoL disutility of daily statin pill of 0.001/year (£) | 300 | 4640 | 1900 | 7950 | 2610 | 8670 | 5780 | 11,290 |
| With QoL disutility of daily statin pill of 0.002/year (£) | 320 | 5540 | 2090 | 10,470 | 2620 | 8690 | 5790 | 11,310 |
| QoL disutilities of CVD events reduced by 50% (£) | 270 | 4010 | 1770 | 6720 | 2550 | 8760 | 6040 | 12,050 |
| QoL disutilities of CVD events increased by 50% (£) | 290 | 3970 | 1700 | 6130 | 2670 | 8550 | 5520 | 10,580 |
| QoL disutilities of diabetes reduced by 50% (£) | 270 | 3850 | 1670 | 6250 | 2340 | 7450 | 4950 | 9940 |
| Discount rates for costs and outcomes at 1.5% per annum (£) | 1480 | 4080 | 2680 | 5640 | 3800 | 7990 | 6410 | 9380 |
| Include only healthcare costs for CVD and incident diabetes (£) | (–2940) | 1550 | (–1700) | 3950 | (–1190) | 5610 | 1530 | 8420 |
| Cost of statin × 1.5 (£) | 710 | 4990 | 2350 | 7800 | 3900 | 11,660 | 7790 | 15,320 |
| Cost of statin × 2 (£) | 1140 | 6000 | 2960 | 9190 | 5190 | 14,670 | 9810 | 19,380 |
| Cost of statin × 5 (£) | 3730 | 12,020 | 6660 | 17,530 | 12,950 | 32,720 | 21,950 | 43,720 |
Summary of findings
This study evaluated the cost-effectiveness of statin therapy in categories of men and women aged 40–70 years in the UK, based on their CVD risk and pre-treatment LDL-C levels. We report that lifetime standard statin therapy increased QoL adjusted survival in all categories studied and, at current UK cost of generic statin therapy, was highly cost-effective. Higher-intensity statin therapy was also found to be cost-effective in many categories with higher CVD risk or higher pre-treatment LDL-C levels. Sensitivity analyses confirmed the robustness of the results. The study highlights the need to improve statin uptake among eligible people and consider widening statin eligibility to optimise the benefits from statin therapy in the population.
Report Supplementary Material 1 includes the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Checklist.
Chapter 7 Cost-effectiveness of statin therapies for older people in the UK
Aims and objectives
The use of statin therapy in older people has been more limited than in other patient categories68,69 despite increasing CVD risks with increasing age and the growing number and share of older people. In particular, there is less definitive evidence of statin benefit among older people without previous CVD,5 and guidelines often fall short of making specific recommendations on the use of statins for primary CVD prevention in older people and instead suggest patient–physician discussion about individual patient circumstances. 15,70,71
We set out to assess the lifetime net health effect and cost-effectiveness of statin treatment in older people in the present-day UK using the UK CVD microsimulation model, together with the effects of statin in older people reported by an IPD meta-analysis of large statin trials. 5
Methods
Study population
We assessed the lifetime effectiveness and cost-effectiveness of statin therapy in categories of UK adults ≥ 70 years old in the UKB31 and the Whitehall II32 studies by sex, previous CVD and pre-treatment LDL-C levels. All UKB participants ≥ 70 years old at recruitment into the study or at any subsequent study resurvey were included in the present study from their earliest eligible attendance. All Whitehall II participants ≥ 70 years old at Phase 9 of the study were included in the present study.
Cardiovascular disease microsimulation model validation in older people
The development and assessment of the CVD microsimulation policy model have been reported in Chapters 2 and 3 of this report and the healthcare costs and QoL models in Chapters 4 and 5, respectively. Here, the model performance was further assessed among participants ≥ 70 years old in UKB and Whitehall II studies using their linked electronic death records, hospital admissions, primary care records (UKB only) and cancer registrations to identify MI, strokes, CRV (UKB only), incident diabetes (UKB only) and incident cancers and deaths during follow-up.
Cost-effectiveness of statin therapy in older people
The effects and costs of standard and higher-intensity statin therapy are described in Table 13 and Chapter 6.
In the base-case analysis of cost-effectiveness of statin therapy in older people, we assumed that similar rate ratio (RR) reductions in cardiovascular events per 1-mmol/l LDL-C reduction were achieved independently of age at statin initiation as reported in the IPD meta-analysis. 5 In a key scenario analysis, we applied RR reductions in cardiovascular end points per 1 mmol/l LDL-C reduction with statin therapy from age 75 years onwards, informed from data only among people > 75 years of age in that meta-analysis. Finally, to further explore the limited randomised evidence in older people without previous CVD, we report a further scenario analysis employing the effect of statin therapy on the risk of major vascular event per 1-mmol/l LDL-C reduction, as reported in this category of trial participants by the CTTC (RR 0.92, 99% CI 0.73 to 1.16)5 for the risks of MI and stroke of statin-treated individuals in the model. In this scenario analysis, we also assumed that statin treatment did not affect risks of CRV and VD.
Our assessment of parameter uncertainty and the sensitivity analyses for statin cost-effectiveness in older people followed the approach described in Chapter 6 and Appendix 6, Table 30, for people 40–70 years old. Further scenario analyses with doubled risk of NVD, with general QoL reduced by 0.1 and with both assumptions together, were executed to assess sensitivity to further reduced potential in older people to benefit from preventive cardiovascular treatment.
Results
The baseline characteristics of participants ≥ 70 years old identified from both UKB and Whitehall II studies are presented in Table 16. There were 15,019 (52% men; mean age 72.5 years) participants without previous CVD and 5103 (66% men; mean age 72.9 years) with previous CVD. The derived untreated mean LDL-C levels were 4.2 mmol/l (SD 0.78 mmol/l) and 4.3 mmol/l (SD 0.98 mmol/l) among participants without and participants with previous CVD, respectively.
| Participants without previous CVD | Participants with previous CVD | |
|---|---|---|
| Number of participants | 15,019 | 5103 |
| Age, years | 72.5 (2.5) | 72.9 (2.7) |
| Male sex | 7838 (52%) | 3389 (66%) |
| Ethnicity | ||
| White | 14,686 (98%) | 4916 (96%) |
| Black | 55 (0%) | 13 (0%) |
| South Asian | 166 (1%) | 134 (3%) |
| Othera | 112 (1%) | 40 (1%) |
| Townsend socioeconomic deprivation quintile | ||
| 1 (least deprived) | 6370 (42%) | 1926 (38%) |
| 2 | 3066 (20%) | 1005 (20%) |
| 3 | 2879 (19%) | 1123 (22%) |
| 4 | 1774 (12%) | 693 (14%) |
| 5 | 930 (6%) | 356 (7%) |
| Smoking status | ||
| Never | 8523 (57%) | 2486 (49%) |
| Ex-smoker | 6034 (40%) | 2444 (48%) |
| Current smoker | 462 (3%) | 173 (3%) |
| Physical activity level | ||
| High | 5257 (35%) | 1694 (33%) |
| Moderate | 5486 (37%) | 1934 (38%) |
| Low | 1806 (12%) | 688 (13%) |
| Missing | 2470 (16%) | 787 (15%) |
| Unhealthy diet (including uncertain) | 4363 (29%) | 1765 (35%) |
| BMI (kg/m2) | 27 (4.1) | 27 (4.3) |
| < 18.5 | 99 (1%) | 24 (0%) |
| 18.5–25 | 5642 (38%) | 1478 (29%) |
| 25–30 | 6674 (44%) | 2380 (47%) |
| 30–35 | 2084 (14%) | 941 (18%) |
| 35–40 | 422 (3%) | 222 (4%) |
| ≥ 40 | 98 (1%) | 58 (1%) |
| LDL-C (mmol/l) | 3.7 (0.65) | 3.2 (0.74) |
| HDL-C (mmol/l) | 1.7 (0.31) | 1.6 (0.32) |
| On statin treatment | 4289 (29%) | 2979 (58%) |
| Derived untreated LDL-C (mmol/l)b | 4.2 (0.78) | 4.3 (0.98) |
| Creatinine (μmol/l) | 78 (13) | 84 (19) |
| Systolic BP (mmHg) | 146 (18) | 142 (19) |
| Diastolic BP (mmHg) | 79 (10) | 77 (11) |
| Treated hypertension | 4076 (27%) | 2631 (52%) |
| Prior diabetes | 1154 (8%) | 782 (15%) |
| Prior cancer | 2040 (14%) | 774 (15%) |
| Severe mental illness | 1206 (8%) | 452 (9%) |
| Previous CVD history | ||
| MI only | 103 (2%) | |
| PAD only | 380 (7%) | |
| Other CHDc only | 2910 (57%) | |
| Stroke only | 343 (7%) | |
| Two or more of MI, PAD, other CHD or stroke | 1367 (27%) | |
In model validation, the cumulative event rates predicted by the CVD microsimulation model, using the baseline characteristics of participants ≥ 70 years in UKB and Whitehall II cohorts, corresponded well to observed event rates (Figure 11).
FIGURE 11.
Cardiovascular disease microsimulation model validation among UKB and Whitehall II participants aged ≥ 70 years. In the Whitehall II study, no linked data for CRV and diabetes were available and, therefore, CRV and diabetes were excluded from model validation.
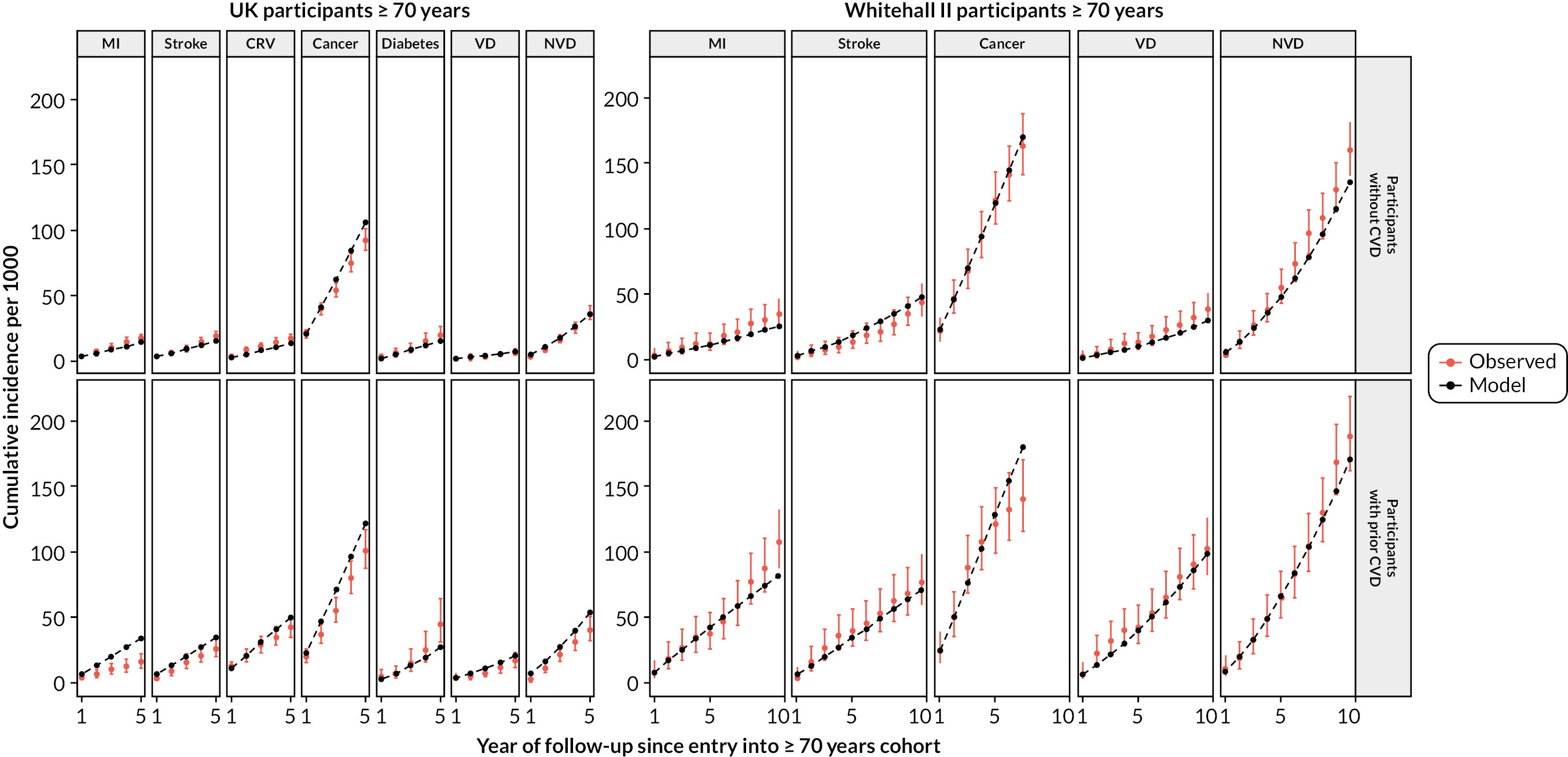
In participant categories by sex, previous CVD and pre-treatment LDL-C level, in the base-case analysis standard statin therapy was projected to increase individual QALYs (undiscounted) by 0.24–0.70, and higher-intensity statin therapy by a further 0.04–0.13 QALYs (Table 17). Across these categories, standard statin therapy compared with no statin had an incremental cost per QALY gained ranging from £120 to £3500 and higher-intensity compared with standard statin therapy ranging from £2210 to £11,780 (Table 18, and see Appendix 8, Table 33). In our key scenario analysis, the cost-effectiveness was not materially affected if relative risk reductions of cardiovascular events were equal to those reported only in the subgroup of participants > 75 years old, although the gains in QALYs were somewhat smaller (0.15–0.48 with standard statin and 0.02–0.09 with higher-intensity statin across participant categories) (see Tables 17 and 18). In the further scenario analysis using statin effects among older people without previous CVD for risks of MI and stroke and no statin effects on risks of CRV and VD, a smaller benefit was projected with lifetime statin therapy, although standard statin therapy remained cost-effective in participant categories by sex and level of pre-treatment LDL-C (see Tables 17 and 18).
| LDL-C (mmol/l) | Base-case analysis | Key scenario analysis | Further scenario analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without CVD | With CVD | Without CVD | With CVD | Without CVD | |||||||
| < 3.4 | 3.4–4.1 | ≥ 4.1 | < 3.4 | 3.4–4.1 | ≥ 4.1 | < 3.4 | 3.4–4.1 | ≥ 4.1 | |||
| Standard statin vs. no statin | |||||||||||
| Men | 0.25 | 0.41 | 0.70 | 0.33 | 0.17 | 0.28 | 0.48 | 0.26 | 0.07 | 0.12 | 0.20 |
| Women | 0.24 | 0.34 | 0.51 | 0.34 | 0.15 | 0.22 | 0.34 | 0.24 | 0.06 | 0.10 | 0.14 |
| Higher-intensity vs. standard statin | |||||||||||
| Men | 0.05 | 0.08 | 0.13 | 0.06 | 0.03 | 0.05 | 0.09 | 0.05 | 0.00 | 0.02 | 0.03 |
| Women | 0.04 | 0.07 | 0.10 | 0.06 | 0.02 | 0.04 | 0.06 | 0.04 | 0.00 | 0.01 | 0.02 |
| LDL-C (mmol/l) | Base-case analysis | Key scenario analysis | Further scenario analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Without CVD | With CVD | Without CVD | With CVD | Without CVD | |||||||
| < 3.4 | 3.4–4.1 | ≥ 4.1 | < 3.4 | 3.4–4.1 | ≥ 4.1 | < 3.4 | 3.4–4.1 | ≥ 4.1 | |||
| Standard statin vs. no statin | |||||||||||
| Men | 2940 | 1760 | 1650 | 120 | 4120 | 2600 | 2150 | 2310 | 7870 | 4510 | 3060 |
| Women | 3500 | 2280 | 1780 | 1720 | 5050 | 3280 | 2590 | 3080 | 10,420 | 6140 | 4300 |
| Higher-intensity vs. standard statin | |||||||||||
| Men | 7430 | 4180 | 3180 | 2210 | 11,450 | 6360 | 4610 | 5220 | 89,550 | 18,350 | 9620 |
| Women | 11,780 | 5860 | 4950 | 5080 | 20,770 | 8940 | 7190 | 8360 | 2,088,810 | 26,040 | 19,540 |
The analyses of parameter uncertainty indicated that, in the base-case analysis, either standard or higher-intensity statin therapy was certain to be cost-effective at willingness-to-pay thresholds as low as £5000 per QALY (Figure 12, and see Appendix 8, Table 33). At a willingness-to-pay threshold of £20,000 per QALY, higher-intensity statin therapy had the highest probability of being cost-effective across all categories of men and women ≥ 70 years old in the base-case cost-effectiveness analysis (see Figure 12). At a cost-effectiveness threshold of £5000 per QALY, however, standard statin therapy had the highest probability of being cost-effective among women with pre-treatment LDL-C lower than 4.1 mmol/l and men with pre-treatment LDL-C lower than 3.4 mmol/l (see Figure 12). In the key scenario analysis, although at the £20,000-per-QALY threshold higher-intensity statin therapy retained the highest probability of being cost-effective, at £5000 per QALY standard statin therapy had the highest probability of cost-effectiveness in most categories of older men and women (see Figure 12). In the further scenario analysis among older people without previous CVD, at the £20,000-per-QALY threshold, higher-intensity statin therapy had the highest probability of being cost-effective for men and women with LDL-C ≥ 3.4 mmol/l but standard statin therapy was most probably cost-effective at lower LDL-C level (see Figure 12). In this scenario, at the £5000-per-QALY threshold, standard statin therapy was cost-effective only for older men with LDL-C ≥ 3.4 mmol/l and older women with LDL-C ≥ 4.1 mmol/l. The analyses of parameter uncertainty indicated that in the key and further scenario analyses standard or higher-intensity statin therapy remained more likely to be cost-effective than no statin treatment across all patient categories at a willingness-to-pay threshold of £20,000 per QALY, but the level of uncertainty was larger (see Figure 12 and Appendix 8, Table 34).
FIGURE 12.
Probability that lifetime statin therapy is cost-effective in older people. (a) Men; and (b) women.
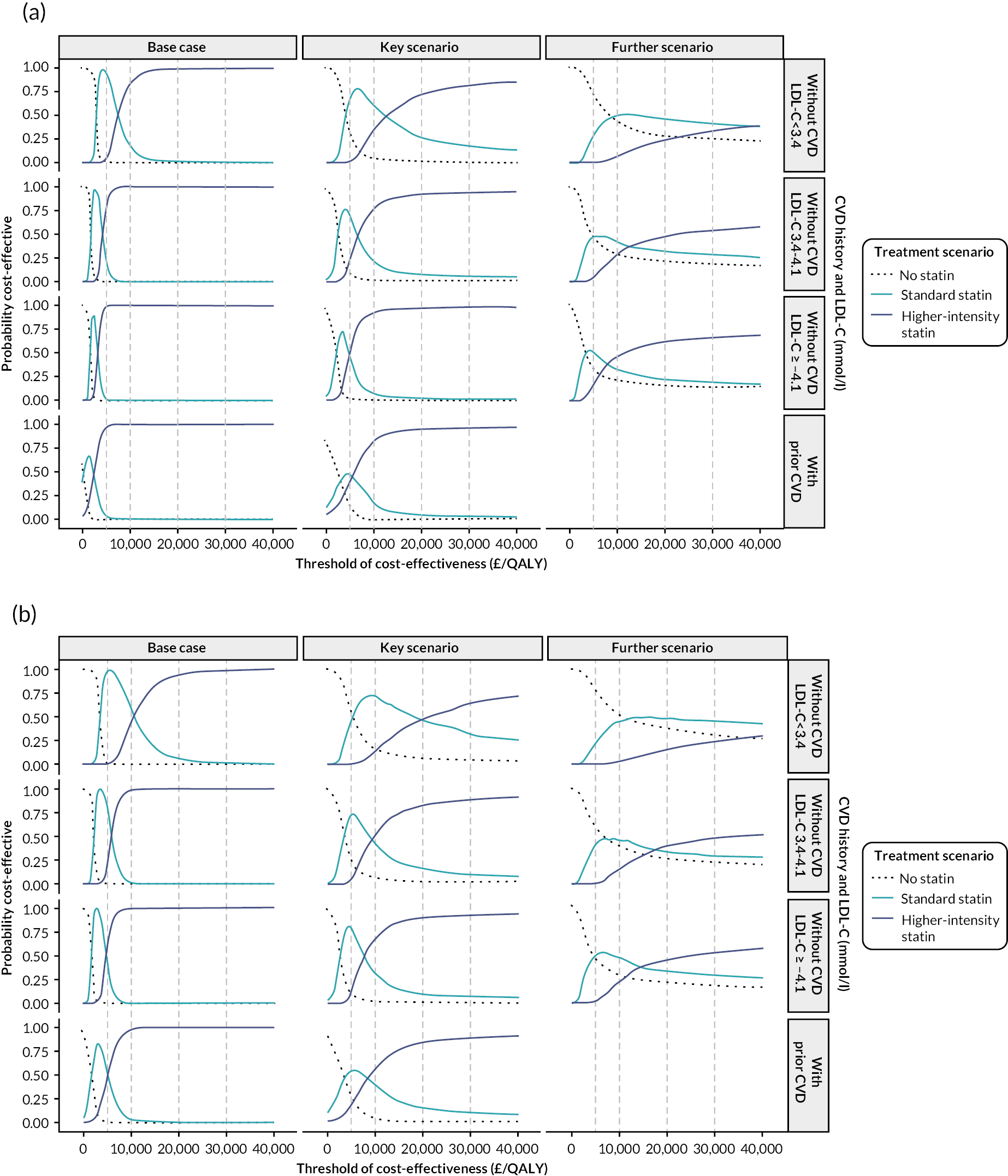
The cost-effectiveness results remained robust in a wide range of sensitivity analyses for the base-case scenario analysis (Table 19) with higher sensitivity noted for higher-intensity statin at a price five times higher. The results remained robust in the sensitivity analyses for the key scenario analysis, including with further annual decline in RR reductions of cardiovascular events with statin therapy, although higher sensitivity was noted for cost-effectiveness of higher-intensity statin in older women and in older men with further declining risk reductions and lower pre-treatment LDL-C level at a price five times higher (Table 20). The results were not materially different in scenario analyses with doubled risk of NVD, with general QoL reduced by 0.1 and with both (results not shown).
| People without previous CVD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men | Men | Women | Women | Men | Men | Women | Women | |
| LDL-C (mmol/l) | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 |
| People with previous CVD | ||||||||
| Sex | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men | Women | Men | Women | |||||
| Base-case | 1650 | 1760 | 1780 | 2280 | 3180 | 4180 | 4950 | 5860 |
| RR in incident cancer with statin therapy of 0.96 | 1220 | 1050 | 1320 | 1530 | 3160 | 4140 | 4920 | 5820 |
| RR in incident cancer with statin therapy of 1.05 | 2380 | 3460 | 2640 | 4190 | 3200 | 4210 | 5010 | 5900 |
| Compliance with statin therapy as in routine care | 2000 | 2130 | 2190 | 2740 | 4090 | 5320 | 6060 | 7400 |
| With QoL disutility of daily statin pill of 0.001/year | 1730 | 1900 | 1920 | 2570 | 3190 | 4180 | 4960 | 5870 |
| With QoL disutility of daily statin pill of 0.002/year | 1800 | 2060 | 2090 | 2950 | 3190 | 4190 | 4970 | 5880 |
| With QoL disutility of daily statin pill of 0.005/year | 2080 | 2770 | 2840 | 5290 | 3210 | 4210 | 4990 | 5910 |
| Discount rates for costs and outcomes at 1.5% per annum | 2240 | 2200 | 2080 | 2350 | 3640 | 4340 | 4830 | 5410 |
| Include only healthcare costs for CVD and incident diabetes | 30 | 230 | 470 | 1010 | 1440 | 2510 | 3470 | 4490 |
| Cost of statin × 1.5 | 1950 | 2280 | 2320 | 3090 | 4040 | 5660 | 6500 | 8090 |
| Cost of statin × 2 | 2240 | 2800 | 2850 | 3910 | 4890 | 7150 | 8060 | 10,320 |
| Cost of statin × 5 | 4000 | 5930 | 6050 | 8820 | 10,020 | 16,060 | 17,380 | 23,680 |
| Base-case | 120 | 1720 | 2210 | 5080 | ||||
| RR in incident cancer with statin therapy of 0.96 | 100 | 1500 | 2250 | 5050 | ||||
| RR in incident cancer with statin therapy of 1.05 | 170 | 2110 | 2190 | 5080 | ||||
| Compliance with statin therapy as in routine care | 940 | 2720 | 3560 | 6990 | ||||
| With QoL disutility of daily statin pill of 0.001/year | 120 | 1900 | 2220 | 5100 | ||||
| With QoL disutility of daily statin pill of 0.002/year | 140 | 2120 | 2220 | 5110 | ||||
| With QoL disutility of daily statin pill of 0.005/year | 180 | 3230 | 2240 | 5140 | ||||
| Discount rates for costs and outcomes at 1.5% per annum | 1240 | 2490 | 3330 | 5660 | ||||
| Include only healthcare costs for CVD and incident diabetes | (–2910) | (–1410) | (–1220) | 1370 | ||||
| Cost of statin × 1.5 | 620 | 2390 | 3610 | 7080 | ||||
| Cost of statin × 2 | 1120 | 3060 | 5010 | 9070 | ||||
| Cost of statin × 5 | 4110 | 7070 | 13,400 | 21,040 | ||||
| People without previous CVD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex, CVD risk | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men | Men | Women | Women | Men | Men | Women | Women | |
| People with previous CVD | ||||||||
| Sex | Standard statin vs. no statin therapy | Higher-intensity vs. standard statin therapy | ||||||
| Men | Women | Men | Women | |||||
| LDL-C (mmol/l) | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 | ≥ 4.1 | 3.4–4.1 |
| Key scenario | 2150 | 2600 | 2590 | 3280 | 4610 | 6360 | 7190 | 8940 |
| Relative reduction in CVD events with statin therapy decreasing annually by 10% each year from year 6 onwards | 2230 | 3760 | 4580 | 7260 | 7520 | 14,560 | 20,720 | 31,280 |
| RR in incident cancer with statin therapy of 0.96 | 1490 | 1420 | 1790 | 1970 | 4570 | 6290 | 7130 | 8880 |
| RR in incident cancer with statin therapy of 1.05 | 3440 | 6510 | 4410 | 8710 | 4650 | 6430 | 7260 | 8990 |
| Compliance with statin therapy as in routine care | 2580 | 3090 | 3140 | 3930 | 5760 | 7990 | 9160 | 11,640 |
| With QoL disutility of daily statin pill of 0.001/year | 2290 | 2910 | 2900 | 3970 | 4620 | 6370 | 7200 | 8950 |
| With QoL disutility of daily statin pill of 0.002/year | 2440 | 3290 | 3310 | 5000 | 4630 | 6380 | 7210 | 8970 |
| With QoL disutility of daily statin pill of 0.005/year | 3030 | 5450 | 5670 | 23,230 | 4650 | 6400 | 7250 | 9010 |
| Discount rates for costs and outcomes at 1.5% per annum | 2640 | 2890 | 2730 | 3130 | 4930 | 6270 | 6760 | 7960 |
| Include only healthcare costs for CVD and incident diabetes | 510 | 1010 | 1210 | 1980 | 2740 | 4540 | 5590 | 7490 |
| Cost of statin × 1.5 | 2570 | 3350 | 3370 | 4520 | 5780 | 8510 | 9490 | 12,410 |
| Cost of statin × 2 | 2980 | 4100 | 4150 | 5750 | 6950 | 10,660 | 11,800 | 15,880 |
| Cost of statin × 5 | 5480 | 8600 | 8830 | 13,140 | 13,980 | 23,550 | 25,640 | 36,720 |
| Key scenario | 2310 | 3080 | 5220 | 8360 | ||||
| Relative reduction in CVD events with statin therapy decreasing annually by 10% each year from year 6 onwards | 2720 | 5180 | 8410 | 22,620 | ||||
| RR in incident cancer with statin therapy of 0.96 | 1850 | 2540 | 5210 | 8310 | ||||
| RR in incident cancer with statin therapy of 1.05 | 3380 | 4210 | 5240 | 8380 | ||||
| Compliance with statin therapy as in routine care | 3250 | 4480 | 7470 | 11,730 | ||||
| With QoL disutility of daily statin pill of 0.001/year | 2530 | 3540 | 5240 | 8380 | ||||
| With QoL disutility of daily statin pill of 0.002/year | 2790 | 4160 | 5250 | 8400 | ||||
| With QoL disutility of daily statin pill of 0.005/year | 4070 | 8760 | 5280 | 8460 | ||||
| Discount rates for costs and outcomes at 1.5% per annum | 3110 | 3590 | 6030 | 8580 | ||||
| Include only healthcare costs for CVD and incident diabetes | (–910) | (–90) | 1460 | 4310 | ||||
| Cost of statin × 1.5 | 2930 | 4010 | 7050 | 11,470 | ||||
| Cost of statin × 2 | 3550 | 4940 | 8870 | 14,580 | ||||
| Cost of statin × 5 | 7270 | 10,530 | 19,810 | 33,230 | ||||
Summary of findings
This assessment of the long-term effectiveness and cost-effectiveness of statin therapy in people aged ≥ 70 years in the UK used a validated CVD microsimulation model and the totality of randomised evidence of effects of statin treatment effects in older people. It concluded that lifetime statin treatment was likely to increase QoL adjusted survival in older men and women, irrespective of their CVD history or level of LDL-C and, at the current UK cost of generic statins, was likely to be cost-effective for all patient categories studied. Higher-intensity statin therapy was the strategy likely to bring the highest health benefit cost-effectively, although standard statin regimens would also achieve most of the benefit and were preferred in some scenarios and sensitivity analyses. These cost-effectiveness results remained robust in sensitivity analyses, including those with somewhat smaller CVD risk reductions with statin therapy in older individuals. It is noted that reductions of major CVD events with statin therapy have not been independently established among older people without prior CVD in randomised clinical trials and, therefore, the conclusions for cost-effectiveness may be less certain in this category.
Report Supplementary Material 1 includes the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Checklist.
Chapter 8 Web-based user interface of the cardiovascular disease policy model
Aims and objectives
The CVD microsimulation policy model was developed using R. To facilitate model use by patients, physicians, researchers and health policy-makers, we developed a user-friendly web-based interface together with a user guide. The interface allows users to modify model inputs and adapt the model for their needs. Patients can use this model to better understand their risk of CVDs and the projected consequences with or without treatments; physicians can use this model to facilitate their clinical decisions; and researchers and policy-makers can use this model to assess different CVD policies.
Interface development
The web-based user interface was developed using the R ShinyApps interface. 72 The interface includes the three sections, ‘introduction’, ‘model parameters’ and ‘run analyses’, which are described in the following sections.
Introduction
The ‘introduction’ section is designed to facilitate the use of the interface. It includes the following panels:
-
Model overview – describes the model and cites the references so that users can understand the functionality of the model and where to find further information of the model.
-
Glossary – explains the terminology used in the interface.
-
User guide and files – includes the user guide and examples of model inputs formatting to facilitate the use of model.
Model parameters
This section is designed to help users input the necessary model inputs. It includes prefilled default values.
To use the CVD microsimulation policy model, profiles are prepared for patients whose outcomes are simulated, treatment scenarios with relevant treatment effects are specified, and the model is run using the integrated model risk equations over a certain time horizon. Therefore, the ‘model parameters’ section includes the following panels:
-
patient characteristics – to enter the profiles of a single patient or a group of patients
-
treatment profiles – to enter treatment effects and costs
-
cost inflation index – to allow users to modify the cost year (default healthcare costs in UK 2021 Great British pounds) by specifying an inflation index using 2021 as the reference year.
Model execution
The section ‘run analyses’ is designed to execute model analyses and report results. After the inputs are filled in the ‘model parameters’ section, the user should use this section and click buttons to run the analyses. This section includes the following panels:
-
Base-case analysis – to run the base-case analysis, make predictions for patients under each treatment scenario and summarise the incremental analysis across treatment scenarios.
-
Probabilistic analysis – provides an option for the user to specify the uncertainty ranges of some inputs to facilitate the analysis of parameter uncertainty.
Figure 13 presents the first look of the ShinyApps. Details of the models can be accessed by visiting and using the ShinyApps from this website (https://livedataoxford.shinyapps.io/shiny_ctt_ukb_model/).
FIGURE 13.
Screenshot of the ShinyApps interface showing the sections and the ‘model overview’ panel.
Model application
The CVD microsimulation model interface and user guide are available at https://livedataoxford.shinyapps.io/shiny_ctt_ukb_model/. The model interface enables users to project outcomes for particular patient profiles. To illustrate its use, the model predicted a 39.3% lifetime cumulative incidence of a vascular event (MI, stroke, CRV or VD), 29.3 years’ further lifespan, and 24.5 QALYs over the lifetime of a healthy 60-year-old man [of white ethnicity, in quintile 3 of socioeconomic deprivation, non-smoker, with moderate physical activities, a healthy diet and normal BMI level (18.5–25 kg/m2), a LDL-C of 3.6 mmol/l, a HDL-C of 1 mmol/l, creatinine of 82 μmol/l, BP of 140/80 mmHg, HbA1c of 32 mmol/mol, not on antihypertensive treatment, and without histories of severe mental illness, cancer or diabetes].
Summary
The CVD microsimulation policy model is a CVD decision-analytic cost-effectiveness model built in R. Its ShinyApps interface facilitates the use of the model by a broader set of users interested in assessing the long-term effectiveness and cost-effectiveness of CVD treatments and policies.
Chapter 9 Discussion
Statement of principal contributions
Cardiovascular disease microsimulation policy model
A new UK CVD microsimulation policy model has been developed to predict an individual’s lifetime risks of CVD and death. In addition to sociodemographic and clinical characteristics, the model incorporates socioeconomic status and modifiable lifestyle factors (physical activity, diet quality and BMI) and tracks medical history and comorbidities. It has shown good accuracy across various categories of the UK population. Moreover, the model includes individualised predictions of HRQoL associated with individuals’ characteristics and adverse events. A significant decrease in QoL during the year of acute events, with partial recovery of QoL in subsequent years, except for stroke and longer diabetes duration, is reported. The study also quantified the temporal impacts of cardiovascular events on UK primary and hospital care costs, revealing substantial excess primary and hospital care costs during the years with events compared with the years prior to event. However, although the excess hospital care costs decreased substantially in the years following the event, the excess primary care costs remained relatively stable. The model’s web interface includes all the features mentioned, providing lifetime individual-level projections that can help inform economic assessments of the value of health interventions to reduce CVD risk. 73 The model could be particularly useful for a cost-effectiveness assessment of CVD prevention treatments such as lipid- or BP-management therapies.
Statin cost-effectiveness findings
We conducted an assessment of the cost-effectiveness of statin therapy for men and women aged ≥ 40 years in the UK, taking into account the contemporary CVD incidence and mortality and the potential trade-offs between statin benefits and risks. We considered sex, age, previous CVD status or estimated CVD risk, and pre-treatment LDL-C level, the key patient characteristics by which to present our findings. Our findings show that lifetime statin therapy increased QoL-adjusted survival and was highly cost-effective in all patient categories studied. Higher-intensity statin therapy was also cost-effective in many categories of patients at higher CVD risk or higher LDL-C levels. These results remained robust in sensitivity analyses and at lower cost-per-QALY-gained cost-effectiveness thresholds.
There is greater uncertainty about the value of statin treatment for people ≥ 70 years old. A recent IPD meta-analysis noted trends across age categories towards smaller proportional reductions in major coronary events and VDs per 1.0-mmol/l reduction in LDL-C with statin therapy. 5 Data were particularly limited among participants > 75 years old without previous CVD, for whom there was no direct evidence for statistically significant CVD risk reductions with statin therapy. In this study we included three scenarios for size of statin effects in the cost-effectiveness assessment among older people. First, we used the overall relative risk reductions in cardiovascular events per 1-mmol/l LDL-C reduction with statin therapy. Second, we used the relative risk reductions in cardiovascular events only among participants aged > 75 years at time of randomisation in trials. 5 Finally, we used even smaller relative reduction in cardiovascular events per 1-mmol/l LDL-C reduction with statin therapy among older people without previous CVD. 5 Despite smaller net health benefits in the later scenarios, statin therapy remained cost-effective, with standard statin therapy preferred in the latest scenario, although the level of uncertainty was substantially higher. Our findings contradict the observation in an earlier study of cost-effectiveness of statin therapy for the primary prevention of CVD in people ≥ 75 years old,74 in which the authors reported that even a small hypothetical increase in a geriatric-specific adverse effect (i.e. reducing QoL by 0.003–0.004) would offset the cardiovascular benefit of statin therapy. In this study, we explicitly integrated the known small excesses of myopathy, rhabdomyolysis and increased treatment intensity-dependent hazard of incident diabetes with statin treatment. In addition, the results remained robust to further hypothetical statin-associated reductions in QoL up to 0.005 per year, as well as to lower effectiveness of statin treatment in older people, suggesting that the uncertainty in the value of statin therapy for older people may be more limited than previously suggested.
Our research suggests that the entire UK population aged ≥ 40 years would benefit from statin therapy cost-effectively, including the 56% of those aged 40–70 years (or 14 million individuals), many of whom have raised LDL-C levels, who are not currently recommended for statin therapy. 75 Patients at only moderately raised CVD risk who have higher LDL-C levels are likely to derive benefits comparable to or exceeding those in patient categories currently treated. Our results could be particularly useful in discussions between patients and doctors as part of shared decision-making. More efforts are needed to ensure clear communication of the evidence to doctors, individual patients and populations groups, and the efficient integration of statin treatment into the health care of a wider range of population categories. While initiating statin treatment is cost-effective also in the presence of treatment discontinuation, long-term persistence with statin therapy is crucial to maximise benefits.
Our findings present a dilemma for policy-makers faced with constrained resources. For example, it is uncertain whether UK primary care has the resources to expand services at the same level of per-person cost of initiating and monitoring statin treatment. Nonetheless, our findings are robust to some increases in costs. For example, at current costs, the incremental cost per QALY gained with statin treatment is much lower than the threshold of £20,000–30,000 per QALY gained used for new treatments. 64
Strengths of the assessment
The key strengths of the study include the use of high-quality evidence and robust methodology. The CVD microsimulation policy model was derived using large and rich IPD with substantial duration of follow-up, enabling us to reliably model the disease risks, QoL-adjusted survival and healthcare costs of individuals over time using their characteristics at entry. The model integrated the underlying risk of incident diabetes, which enabled us to project the excess diabetes risk with statin therapy and its consequences, as well as the risk of incident cancer, the largest competing risk affecting HRQoL and survival of individuals, in parallel with any benefits from CVD risk reductions. The ability to report results for distinct categories of actual person profiles and fully assess parameter uncertainty is a further strength.
This cost-effectiveness study of statin therapy has key further advantages over previous studies. Effects of statin therapies were informed by an individual participant meta-analysis of all large statin trials. It improves on the CVD decision-analytic modelling informing the current NICE guideline of statin treatment to reduce CVD risk15 by using recent IPD and integrating all known adverse effects of statin therapy. Recently, Kohli-Lynch et al. 76 reported population-level cost-effectiveness results in Scotland, using recent population data, at different thresholds of CVD risk, CVD risk and LDL level, and age. The present study instead reports detailed results in distinct UK population categories and for both standard and higher-intensity statin regimens, directly informing decision-makers’ trade-offs at different cost-effectiveness thresholds and presenting compelling evidence that, at currently stated thresholds, initiating statin therapy could be considered good value for money for all UK adults ≥ 40 years old. This study improves on the 10-year results reported by Greving et al. 77 by extending the time horizon to a person’s lifetime and reaches different conclusions about the cost-effectiveness of statin therapy. In general, the results over a limited time horizon, despite avoiding the assumptions needed for extrapolation, result in a downwards bias of the estimated benefits and cost-effectiveness of preventive treatments that reduce disease risks and should be discouraged. Recent US cost-effectiveness analyses of statin therapy, although based on older data, also reported evidence for broader statin cost-effectiveness in adults, largely irrespective of level of CVD risk, but sensitive to level of possible ‘disutility’ of taking a daily statin pill. 61,78,79 Our analysis suggests that this sensitivity is likely to be relevant only among people at lower CVD risk or with lower LDL-C levels.
Limitations of the assessment
The study has several limitations.
First, while we explicitly modelled the effects of evolving cancer and diabetes risks, further morbidities likely to accumulate in older age, together with the need for co-medication, were beyond the scope of the current project. While the increasing hazard of NVD captures some of these effects, and the scenario analyses (e.g. higher cancer risk with statin therapy, further QoL disutilities) provide assurance that these are unlikely to materially affect statins’ value, further work to assess the implications of multimorbidity and polypharmacy will be helpful.
Second, the risk equations used in our framework were derived and validated in population cohorts, such as UKB and Whitehall II, which may limit the generalisability of our findings due to the potential presence of a healthier volunteer effect. The under-representation of ethnic minority groups in these cohorts is a further limitation. However, the individual equations include a broad range of characteristics to account for the effects of lifestyle, socioeconomic and clinical factors, enabling us to extend findings to populations with varying distributions of these characteristics. Nevertheless, some selection bias may persist, and scenario analyses are employed to further check for the impact of this on study findings.
Third, in the analytical framework we modelled the first occurrences only of the disease events of interest. While subsequent disease events from same type were not explicitly modelled, the use of the same population data to model risks of further events, in particular vascular and NVD risks, would have also accounted for the impacts of subsequent events (as both risk factors and first occurrences of events contribute to subsequent risks of events as well as deaths).
Fourth, a significant limitation of our cost-effectiveness results is the currently limited direct evidence for the effects of statins in older people without previous CVD and the absence of randomised data on the long-term effects of statin therapy. Nonetheless, we found that our results were not highly sensitive to hypothesised lower efficacy in sensitivity analyses. While ongoing large, randomised trials in older primary prevention populations will improve the evidence, our findings’ general robustness to variations in key parameters suggests that delaying statin treatment for millions of older people while awaiting new evidence is not justified.
Fifth, our analytical framework was developed and assessments were performed for individuals aged ≥ 40 years, so we are unable to provide information on the effects of initiating statin treatment on younger individuals.
Finally, developing a new CVD model using contemporary population data was motivated by the need to reflect current disease risks in view of decreasing cardiovascular mortality and incidence over recent decades. While the findings in this report are likely to describe well the current disease risks, further changes in disease risks will necessitate an update of the model framework. The approach taken in the present study could be helpful in calibrating the reported risk equations with new population data.
Equality, diversity and inclusion
Participant representation
Reflect on the important population characteristics for your study. What active steps were taken to optimise participation of relevant people?
This study aimed to provide an effectiveness and cost-effectiveness assessment of statin treatment in people ≥ 40 years old in the general UK population. With this focus, we used the data from participants (≥ 100,000) in the trials included in the CTTC to inform the treatments’ effect as well as an initial estimation of disease risks and disease progression, and we used the data from participants (aged 40–70, ≥ 500,000) in the UKB to further inform the disease progression and healthcare costs, and used data from the HSE (nationally representative) to inform the QoL assessment. As we do not exclude specific populations from the analysis, all types of people were included in our research. As the UKB participants could be healthier than the general UK population, we also checked our projections using follow-up data on participants in the Whitehall II study, a cohort of British civil servants. We present results across categories by age, sex, CVD history or CVD risk and LDL-C level that are more relevant to the corresponding categories in the general UK population.
Was the participant population inclusive? If not, an explanation should be provided as to what prevented this or any improvements that could be made for future study designs
We believe that the participant population is reasonably inclusive and can answer the question relevant to general UK population. However, we relied on available IPD from UK cohorts. We acknowledge an under-representation of minority ethnic groups in UKB. Future studies could consider including further population data to better represent the UK general population, such as the UK Clinical Practice Research Datalink databases, despite there being more limited data on participant characteristics in these data sets.
If applicable, provide comments on how gaps in prior evidence/data were identified and how were these addressed during the project. Did this research uncover any gaps in knowledge/data during the project; how could this be addressed in future research?
As noted in the 2014 NICE guideline ‘Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease’,80 the prior economic evidence is limited in its methodological ability to predict the impact of statin treatment on an individual’s disease risks over a lifetime. This prior evidence is also limited in its ability to project QoL of and costs for individuals with different characteristics. We addressed these issues by using the IPD from large statin trials to allow a reliable estimation of disease progression using adjudicated outcomes in the trials; using a large population cohort to calibrate the disease models and inform the healthcare costs; and using national population surveys to inform QoL assessment. Future research should focus on estimating an even more detailed set of outcomes and using a broader population with longer follow-up data to support the modelling.
How have you made sure text and pictures, used as part of participant and public engagement, were inclusive and accessible and not discouraging to under-represented groups?
Our public representatives’ members on the project management and oversight groups helped us identify the key concern of the general public and helped us develop project outreach materials that were clear and conveyed well the key message from the project to the general public. In particular, we presented results across diverse patient categories and focused on clarity in our presentation, which involved simplifying text and figures.
Reflections on your research team and wider involvement
Does your research team include those from groups who are generally under-represented in your field of study? If not, please state why this is the case.
The research team was reasonably broad and included good representation of areas of expertise, gender and national identities. Unfortunately, the team did not include representation of key minority ethnic groups directly and instead we relied on discussing our findings more broadly, including with the Oxford Population Health (OxPop) Public Advisory Panel to improve our coverage of broader and more diverse views and opinions.
Was there a range of experience and expertise across the research team? How were development opportunities provided, particularly for more junior members of the team?
Our research team included people with ranges of experiences and expertise, from a postdoctoral researcher to senior postdoctoral researchers, associate professors and professors. They are from different backgrounds, including health economists, epidemiologists, statisticians, a pharmacologist, clinicians, and patient and public representatives. More junior researchers were well supervised, benefited from advice from more experienced project members, and had a range of training opportunities in specialist and general research skills and development.
Were participants and members of the public involved representative of the disease and geographic areas? What role/s did they provide and how did they benefit the project?
Three patient and public representatives were included in the study, and we also presented our work to a broader public panel consisting of 13 individuals who represent the target population and geographic areas affected by the disease. Their input was invaluable in improving the dissemination of the study results and helping them understand the scientific work behind the messages sent to the public. They were also the first group to learn about the results from our project, which provided them with the opportunity to benefit from the project.
Patient and public involvement and engagement
We reported the patient and participant involvement following the structure of the GRIPP2 Short Form (see Report Supplementary Material 1). 81
Aim
We aimed to include patient and public involvement representation throughout the project to make the project more relevant to the patient group and the wider public and to facilitate the dissemination of project results.
Methods
We involved two members of the public from the start of this project. The first member (PPI-A1) was expected to be involved in all nine meetings of the project management group to discuss the progress of the project every 3 or 4 months until the end of the project. The second member (PPI-B) was expected to be involved in all the three meetings of the project oversight group to discuss the project’s progress and emerging results yearly until the end of the project. In the last year of the project, we included a further PPI member, PPI-A2, to replace PPI-A1 in the management group, as PPI-A1 stepped down due to ill health. The PPI members had some direct experience with the statin intervention as well as some indirect experience with CVD in relatives.
During the project, we held further separate meetings with our PPI members to discuss the study methodology, model outline and, later, the emerging results and solicit their feedback on whether (1) we included all important patient characteristics and outcomes; (2) we used language that is easily understood by the patients and the public; and (3) the presentation of the emerging results was relevant to the patients and the public. With their feedback, we modified the model and further developed the dissemination slides of the results of the cost-effectiveness study presented to the OxPop Public Advisory Panel, which included 13 patient and public representatives with diverse ages, ethnicities and backgrounds, for further feedback. At these meetings the final versions of the slides were formed for further dissemination of the results to the general public.
Results
During the project management group meetings, PPI-A1 expressed concerns about the muscle side effect of statin. During the Project Oversight Group meetings and in separate PPI meetings, PPI-B noted that the models omitted some important individual factors, including lifestyle (e.g. physical activity and diet quality) and mental health status. These outcomes and factors were subsequently captured in the model development and in the cost-effectiveness analysis of statin therapy. PPI members also appreciated model validation and that the model was able to predict rates of disease events corresponding to observed rates in external data.
The separate meetings with our PPI representatives helped us to shape the slides for the presentation to the OxPop Public Advisory Panel. We first presented our emerging results from a scientific researcher’s perspective to PPI-B. With feedback from PPI-B and PPI-A2, we reduced the number of slides from 56 to 13, restricting the content to what would be relevant to and understandable by the patient group and the general public. We modified our research messages into plain language, such as mentioning that the LDL-C reduced by statin was the ‘bad’ cholesterol, while HDL-C was the ‘good’ cholesterol; referring to level of health with respect to QALYs (e.g. 1 QALY = 1 year in good health); and using value-for-money when referring to cost-effectiveness.
Patient and public involvement member A2 helped to make sure that we addressed all key questions and considered how to enhance the project dissemination, including (1) what did we say we would do in the protocol for this project?; (2) can we learn or use the ideas from similar projects?; (3) what are the key messages from the project?; (4) who are the target audiences?; and (5) who are our partners and allies?
With these questions in mind, we refined the slides for the presentation to the OxPop Public Advisory Panel to showcase the health benefits and value-for-money of statins in the present-day UK. The slides focused on the main results that are expected to be of interest to a general audience.
The panel understood well the results indicating the benefits of statins and provided further helpful comments to improve the presentation of results to the general public, such as a suggestion to present statins’ benefits in different time frames as well as lifetime.
Discussion and reflections/critical perspective
Our PPI members provided important contributions to the study design and made an invaluable contribution to disseminating the research results to the general public using language that can be easily understood and to restrict the results to only those relevant to the general public.
Dissemination of the results of the study
We aimed to disseminate our findings to audiences with different backgrounds, including academia, research community, trainees, policy-makers, people from the industry, patients and the general public.
Table 21 summarises the undertaken project dissemination activities.
| Date, format | Audience | Event | Title | Content |
|---|---|---|---|---|
| 13 November 2020, presentation and discussion | Academic and professional | Biomedical Research Centre Oxford, University of Oxford | Cardiovascular disease models to inform policy considerations | Methodology: CVD microsimulation policy model |
| 25–26 March 2021, presentation | Academic, research, students, trainees | Nuffield Department of Population Health 2021 Annual Symposium | Estimating risk equations to support a CVD policy model | Methodology: risk equations |
| 29 August 2021, presentation | Academic, research, trainees, policy, industry | ESC Congress 2021 The Digital Experience | A model of lifetime health outcomes in CVD based on clinical trials and large cohort data | Methodology and predictions: calibrated CVD microsimulation policy model |
| 30 November 2021–3 December 2021, presentation | Policy, industry, academic, research, trainees | Virtual ISPOR Europe 2021 | Calibrating CVD policy model using large cohort data | Methodology: model calibration |
| 30 November 2021–3 December 2021, presentation | Policy, industry, academic, research, trainees | Virtual ISPOR Europe 2021 | Excess annual hospital costs due to cardiovascular events in a contemporary UK population to inform health technology assessments | Methodology: hospital costs model |
| 26–29 August 2022 | Academic, research, trainees, policy, industry | ESC Congress 2022 | Excess primary and secondary care costs for CVD events Benefit loss with early stopping of statin Cost-effectiveness of statins in patient categories |
Main results |
| November 2022 | Patients, public | Wolfson Institute of Population Health, QMUL | Discuss emerging results from the project | Emerging main results |
| November 2022 | Patients, public | OxPop Public Advisory Panel, Oxford | Discuss emerging results from the project | Emerging results |
| December 2022 | Policy-makers | The NICE Guideline development group ‘Cardiovascular disease: risk assessment and reduction, including lipid modification’ | Shared draft outputs from the project | Emerging results |
Chapter 10 Conclusion
Implications for service provision
Using a UK CVD policy model, developed using large contemporary UK population data, and the current best evidence for the beneficial and adverse effects of statin therapy, this study has shown that lifetime statin therapy is highly cost-effective across all UK adults ≥ 40 years old, suggesting that both improvements in statin uptake and widening of statin eligibility need to be considered in addition to monitoring the impact on statin uptake and adherence across population strata including hard-to-reach groups.
The CVD microsimulation model, and healthcare costs and QoL models, developed in this project are freely available to researchers, practitioners and policy-makers and could inform further strategies for individualised CVD management.
Suggested research needs
The following areas for further research were identified during the project development:
-
The value of statin treatment for people at moderate CVD risk would decrease if there were further, as yet unknown, adverse effects of statin therapy. The ongoing work of the CTTC has the potential to importantly strengthen the evidence for the efficacy and safety of statin therapy.
-
There is no conclusive direct evidence of cardiovascular risk reductions with statin therapy among participants > 75 years old without previous CVD. Two large, ongoing randomised trials in older people, scheduled to complete in 2026, will add to the evidence. 82,83
-
It has been also hypothesised that many people < 40 years of age are also likely to benefit; however, data on the effects of statins in younger people, beyond the category of familial hypercholesterolemia, are limited.
-
To provide a more definitive value assessment of statin treatment in younger and older categories of the population, future decision-analytic models would require high-quality cohort data in younger adults and people in their 80s and 90s, categories not typically well represented in research.
-
In view of some evidence of a potentially more limited impact of key cardiovascular events, including MI, on QoL, high-quality contemporary studies of the temporal impact of cardiovascular and other disease events on HRQoL are also required to inform future economic analyses.
6.Further research into the implications of multimorbidity and polypharmacy for prioritising effective preventive interventions in older people is likely to be informative.
Additional information
CRediT contribution statement
Borislava Mihaylova (https://orcid.org/0000-0002-0951-1304) (Professor of Health Economics): Conceptualization, Methodology, Software, Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Runguo Wu (https://orcid.org/0000-0002-6523-5754) (Senior Health Economist): Methodology, Software, Validation, Formal analysis, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization.
Junwen Zhou (https://orcid.org/0000-0002-6812-0944) (Senior Researcher in Health Economics): Methodology, Software, Validation, Formal analysis, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization.
Claire Williams (https://orcid.org/0000-0002-3005-1541) (Senior Researcher in Health Economics): Methodology, Validation, Formal analysis, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization.
Iryna Schlackow (https://orcid.org/0000-0002-4154-1431) (Senior Researcher in Health Economics): Methodology, Software, Formal analysis, Data Curation, Writing - Review & Editing, Funding acquisition.
Jonathan Emberson (https://orcid.org/0000-0001-7792-9422) (Professor of Medical Statistics and Epidemiology): Methodology, Investigation, Resources, Writing - Review & Editing, Funding acquisition.
Christina Reith (https://orcid.org/0000-0001-7448-9847) (Senior Clinical Research Fellow): Investigation, Resources, Writing - Review & Editing, Funding acquisition.
Anthony Keech (https://orcid.org/0000-0002-9426-9136) (Professor of Medicine, Cardiology and Epidemiology): Methodology, Investigation, Resources, Writing - Review & Editing.
John Robson (https://orcid.org/0000-0001-6889-0415) (Clinical Reader in Primary Care): Methodology, Investigation, Writing - Review & Editing, Supervision.
Richard Parnell (Public Representative): Investigation, Writing - Review & Editing, Visualization.
Jane Armitage (https://orcid.org/0000-0001-8691-9226) (Professor of Clinical Trials and Epidemiology): Methodology, Investigation, Resources, Writing - Review & Editing, Funding acquisition.
Alastair Gray (https://orcid.org/0000-0003-0239-7278) (Professor of Health Economics): Methodology, Investigation, Writing - Review & Editing, Funding acquisition.
John Simes (Professor of Clinical Epidemiology): Methodology, Investigation, Resources, Writing - Review & Editing.
Colin Baigent (https://orcid.org/0000-0003-4856-7420) (Professor of Epidemiology): Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Supervision, Funding acquisition.
Acknowledgements
Members of the project oversight group: Colin Baigent, Alison Gater, Borislava Mihaylova, Stephen Morris, Paul Roderick (chairperson), Natalie Rowland, Peter Sever and Liam Smeeth.
We thank the external members of our project oversight group for their guidance and support throughout the project development. Particular thanks are due to Alison Gater, a representative of the general public on the committee, for her helpful insights throughout the project and her contribution to project dissemination. We also thank Kenneth Wilkinson, a member of the project management group representing the general public from April 2019 to May 2022, and further members of the public with whom we discussed the project and emerging results, including members of the OxPop Public Advisory Panel, participating in the meeting held on 26 November 2022 in Oxford, UK.
This research has been conducted using data from Cholesterol Treatment Trialists’ Collaboration www.cttcollaboration.org/, UK Biobank Resource under Application Number 56757 www.ukbiobank.ac.uk, and Whitehall II study www.ucl.ac.uk/epidemiology-health-care/research/epidemiology-and-public-health/research/whitehall-ii. We thank all the participants, staff and other contributors to these resources. Cholesterol Treatment Trialists’ Collaborators.
CTT secretariat: J Armitage, C Baigent, E Barnes, L Blackwell, R Collins, K Davies, J Emberson, J Fulcher, H Halls, WG Herrington, L Holland, A Keech, A Kirby, B Mihaylova, R O’Connell, D Preiss, C Reith, J Simes and K Wilson.
CTT Collaborating trialists: A to Z trial (phase Z) M Blazing, E Braunwald, J de Lemos, S Murphy; T Pedersen, M Pfeffer, H White and S Wiviott; AFCAPS/TEXCAPS (AirForce/Texas Coronary Atherosclerosis Prevention Study) M Clearfield, JR Downs, A Gotto and S Weis; ALERT (Assessment of Lescol in Renal Transplantation) B Fellström, H Holdaas (deceased), A Jardine and TR Pedersen; ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) D Gordon, B Davis; C Furberg, R Grimm, S Pressel, J Probstfield, M Rahman and L Simpson; ALLIANCE (Aggressive Lipid-Lowering Initiation Abates New Cardiac Events) M Koren; ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) B Dahlöf, A Gupta, N Poulter, P Sever and H Wedel; ASPEN (Atorvastatin Study for the Prevention of Coronary Heart Disease Endpoints in Non-Insulin Dependent Diabetes Mellitus) RH Knopp (deceased); AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events) S Cobbe, B Fellström, H Holdaas (deceased), A Jardine, R Schmieder and F Zannad; CARDS (Collaborative Atorvastatin Diabetes Study) DJ Betteridge (deceased), HM Colhoun, PN Durrington, J Fuller (deceased), GA Hitman and A Neil; CARE (Cholesterol And Recurrent Events Study) E Braunwald, B Davis, CM Hawkins, L Moyé, M Pfeffer and F Sacks; CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Kjekshus, H Wedel and J Wikstrand; 4D (Die Deutsche Diabetes Dialyse Studie) C Wanner and V Krane; GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico) Heart Failure and Prevention trials: MG Franzosi, R Latini, D Lucci, A Maggioni;, R Marchioli, EB Nicolis, L Tavazzi and G Tognoni; HOPE-3 J Bosch, E Lonn and S Yusuf; HPS (Heart Protection Study) J Armitage, L Bowman, R Collins, A Keech, M Landray, S Parish, R Peto and P Sleight (deceased); IDEAL (Incremental Decrease in Endpoints through Aggressive Lipid-lowering) JJP Kastelein and TR Pedersen; JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) R Glynn, A Gotto, JJP Kastelein, W Koenig, J Macfadyen and PM Ridker; LIPID (Long-term Intervention with Pravastatin in Ischaemic Disease) A Keech, S MacMahon, I Marschner, A Tonkin, J Shaw, J Simes and H White; LIPS (Lescol Intervention Prevention Study) PW Serruys; Post-CABG (Post-Coronary Artery Bypass Graft Study) G Knatterud (deceased); PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) GJ Blauw, S Cobbe, I Ford, M Murphy, GJ Blauw, P Macfarlane, C Packard, N Sattar, J Shepherd (deceased) and S Trompet; PROVE-IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) E Braunwald, CC Cannon and S Murphy; SEARCH (Study of Effectiveness of Additional Reductions in Cholesterol and Homocysteine) R Collins, J Armitage, L Bowman, R Bulbulia, R Haynes, S Parish, R Peto and P Sleight (deceased); SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) P Amarenco and K Welch; 4S (Scandinavian Simvastatin Survival Study) J Kjekshus, T Pedersen and L Wilhelmsen; TNT (Treating to New Targets) P Barter, A Gotto, J La Rosa, JJP Kastelein and J Shepherd (deceased); WOSCOPS (West of Scotland Coronary Prevention Study) S Cobbe, I Ford, S Kean, P Macfarlane, C Packard, M Roberston, N Sattar, J Shepherd (deceased) and R Young. Other CTT Members: H Arashi, R Clarke, M Flather, S Goto, U Goldbourt, J Hopewell, K Hovingh, G Kitas, C Newman, M Sabatine, GG Schwartz, L Smeeth, J Tobert, J Varigos and J Yamamguchi.
Further support from the British Heart Foundation, the UK Medical Research Council, the National Institute for Health Research Barts Biomedical Research Centre (NIHR203330) and NHMRC – Australia is acknowledged.
Data-sharing statement
Requests for individual patient data from trials contributing into the Cholesterol Treatment Trialists’ Collaboration should be made directly to the data custodians of each trial (see Cholesterol Treatment Trialists’ data policy on www.cttcollaboration.org/). Other data underlining this work may be obtained from third parties (UK Biobank www.ukbiobank.ac.uk/; Whitehall II study www.ucl.ac.uk/epidemiology-health-care/research/epidemiology-and-public-health/research/whitehall-ii; Health Survey for England https://beta.ukdataservice.ac.uk/datacatalogue/series/series?id=2000021) and are not publicly available. Researchers can apply to use the UK Biobank resource, Whitehall II study data, and UK Data Service data. For all other queries, please contact the corresponding author.
Ethics statement
Ethics committee approval was not required for this secondary research study.
Information governance statement
University of Oxford and Queen Mary University of London are committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Oxford is the Data Controller in relation to use of UK Biobank and Cholesterol Treatment Trialists’ Collaboration data in the study, and you can find out more about how we handle personal data, including how to exercise your individual rights, and the contact details for our Data Protection Officer here: https://compliance.admin.ox.ac.uk/data-protection-policy. Under the Data Protection legislation, Queen Mary University of London is the Data Controller in relation to use of UK Biobank and Whitehall II data in the study, and you can find out more about how we handle personal data, including how to exercise your individual rights, and the contact details for our Data Protection Officer here: https://www.qmul.ac.uk/privacy/media/arcs/policyzone/Data-Protection-Policy-v03.1.pdf.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/KDAP7034.
Primary conflicts of interest: Borislava Mihaylova reports grants from the National Institute for Health and Care Research (NIHR) and Barts Charity (London, UK) during the conduct of the study and membership of the following committees: NIHR Programme Grants for Applied Research (PGfAR) Subpanel A (2016–20), Value in Health Advisory Board (2019–present), the European Society of Cardiology (ESC) Clinical Practice Guidelines committee (2022–present) and the Task Force for the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. Christina Reith reports grants from the NIHR, British Heart Foundation, Medical Research Council (MRC) and National Health and Medical Research Council (NHMRC)-Australia during the conduct of the study and membership of the Clinical Data Interchange Standards Consortium (CDISC) Board. Anthony Keech reports grants from the NHMRC-Australia, Amgen (Thousand Oaks, CA, USA), Mylan (Canonsburg, PA, USA) and Abbott Laboratories (Chicago, IL, USA) during the conduct of the study; consulting fees from AstraZeneca (Cambridge, UK); and speaker fees from Sanofi (Paris, France) and Pfizer (Sandwich, UK). John Robson reports grants from Barts Charity and NIHR during the conduct of the study. Richard Parnell reports membership of the following committees: EME Prioritisation Group 1 June 2015–1 June 2016, EME Strategy Advisory Committee 1 November 2019–31 December 2020, and EME – Funding Committee Members 1 June 2016–1 June 2020. Jane Armitage reports grants from the NIHR, British Heart Foundation, MRC and NHMRC-Australia and Novartis (Basel, Switzerland) during the conduct of the study and membership of the of BHF Clinical Studies Committee. Alastair Gray reports grants from the NIHR, British Heart Foundation and MRC during the conduct of the study. John Simes reports grants from Bayer (Leverkusen, Germany), Roche (Basel, Switzerland), Bristol Myers Squibb (Uxbridge, UK), AstraZeneca, MSD (London, UK), Pfizer, NHMRC-Australia and consulting fee paid to his institution from Detsamma Investments Pty Ltd as FivepHusion (NSW, Australia) during the conduct of the study. Colin Baigent reports grants from the NIHR, MRC, Boehringer Ingelheim (Ingelheim am Rhein, Germany) and Health Data Research UK (London, UK) during the conduct of the study and membership of the European Society of Cardiology’s Committee on Practice Guidelines (2018–present).
Publications
Peer-reviewed publications
Zhou J, Wu R, Williams C, Emberson J, Reith C, Keech A, et al. Prediction models for individual-level healthcare costs associated with cardiovascular events in the UK. PharmacoEconomics 2023;41:547–59. https://doi.org/10.1007/s40273-022-01219-6
Wu R, Williams C, Zhou J, Schlackow I, Emberson J, Reith C, et al. Long-term cardiovascular risks and statin treatment impact on socioeconomic inequalities: a microsimulation model. Br J Gen Pract 2024;74:e189–98. https://doi.org/10.3399/BJGP.2023.0198
Zhou J, Williams C, Keng MJ, Wu R, Mihaylova B. Estimating costs associated with disease model states using generalized linear models: a tutorial. PharmacoEconomics 2023;74:e189–e198. https://doi.org/10.1007/s40273-023-01319-x
Mihaylova B, Wu R, Zhou J, Williams C, Emberson J, Schlackow I, et al. Lifetime effects and cost-effectiveness of standard and higher-intensity statin therapy across population categories in the UK: a microsimulation modelling study. Lancet Regional Health-Europe 2024;40:100887. https://doi.org/10.1016/j.lanepe.2024.100887
Mihaylova B, Wu R, Zhou J, Williams C, Schlackow I, Emberson J, et al. Lifetime effects and cost-effectiveness of statin therapy for older people in the United Kingdom: a modelling study. Heart 2024;110:1277–85. https://doi.org/10.1136/heartjnl-2024-324052
Published conference abstracts
Wu R, Williams C, Schlackow I, Zhou J, Emberson J, Reith C, et al. A model of lifetime health outcomes in cardiovascular disease based on clinical trials and large cohorts. Eur Heart J 2021;42:ehab724.3149. https://doi.org/10.1093/eurheartj/ehab724.3149
Mihaylova B, Wu R, Williams C, Zhou J, Schlackow I, Emberson J, et al. Cost-effectiveness of statin therapy in categories of patients in the UK. Eur Heart J 2022;43(Suppl. 2). https://doi.org/10.1093/eurheartj/ehac544.2841
Wu R, Williams C, Schlackow I, Zhou J, Emberson J, Reith C, et al. P70 calibrating cardiovascular disease policy model using large cohort data. Value Health 2022;25:S16. https://doi.org/10.1016/j.jval.2021.11.068. (www.sciencedirect.com/science/article/pii/S1098301521018635).
Wu R, Williams C, Zhou J, Schlackow I, Emberson J, Reith C, et al. Benefit accrual with cardiovascular disease prevention and effects of discontinuation: a modelling study. Eur Heart J 2022;43(Suppl. 2). https://doi.org/10.1093/eurheartj/ehac544.2850
Zhou J, Wu R, Williams C, Emberson J, Reith C, Keech A, et al. Impact of cardiovascular events on primary and hospital care costs: findings from UK Biobank study. Eur Heart J 2022;43(Suppl. 2). https://doi.org/10.1093/eurheartj/ehac544.2852
Zhou J, Wu R, Williams C, Mihaylova B. P55 excess annual hospital costs due to cardiovascular events in a contemporary UK population to inform health technology assessments. Value Health 2022;25:S12. https://doi.org/10.1016/j.jval.2021.11.053. (www.sciencedirect.com/science/article/pii/S1098301521018489).
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Protocol for a prospective collaborative overview of all current and planned randomized trials of cholesterol treatment regimens. Am J Cardiol 1995;75:1130-4. https://doi.org/10.1016/s0002-9149(99)80744-9.
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. https://doi.org/10.1016/s0140-6736(05)67394-1.
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. https://doi.org/10.1016/s0140-6736(10)61350-5.
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397-405. https://doi.org/10.1016/s0140-6736(14)61368-4.
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407-15. https://doi.org/10.1016/S0140-6736(18)31942-1.
- Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90. https://doi.org/10.1016/s0140-6736(12)60367-5.
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016;4:829-39. https://doi.org/10.1016/s2213-8587(16)30156-5.
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators . Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117-25. https://doi.org/10.1016/s0140-6736(08)60104-x.
- Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin?. BMJ 2013;347. https://doi.org/10.1136/bmj.f6123.
- Malhotra A. Saturated fat is not the major issue. BMJ 2013;347. https://doi.org/10.1136/bmj.f6340.
- Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532-61. https://doi.org/10.1016/s0140-6736(16)31357-5.
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735-42. https://doi.org/10.1016/s0140-6736(09)61965-6.
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556-64. https://doi.org/10.1001/jama.2011.860.
- NHS Prescription Services . Drug Tariff December 2021 2021. www.nhsbsa.nhs.uk/sites/default/files/2021-11/Drug%20Tariff%20December%202021.pdf (accessed 17 March 2022).
- National Institute for Health and Care Excellence . Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease 2014. www.nice.org.uk/guidance/ng238/evidence (accessed 26 September 2024).
- National Institute for Health and Care Excellence . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification: Clinical Guideline 2023. www.nice.org.uk/guidance/ng238 (accessed 16 December 2023).
- Ueda P, Lung TW, Clarke P, Danaei G. Application of the 2014 NICE cholesterol guidelines in the English population: a cross-sectional analysis. Br J Gen Pract 2017;67:e598-608. https://doi.org/10.3399/bjgp17X692141.
- Kotseva K, Wood D, De Bacquer D, De Backer G, Ryden L, Jennings C, et al. EUROASPIRE Investigators . EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636-48. https://doi.org/10.1177/2047487315569401.
- Matthews A, Herrett E, Gasparrini A, Van Staa T, Goldacre B, Smeeth L, et al. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016;353. https://doi.org/10.1136/bmj.i3283.
- Achelrod D, Gray A, Preiss D, Mihaylova B. Cholesterol- and blood-pressure-lowering drug use for secondary cardiovascular prevention in 2004–2013 Europe. Eur J Prev Cardiol 2016;24:426-36. https://doi.org/10.1177/2047487316676906.
- Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017;357. https://doi.org/10.1136/bmj.j2099.
- Heart Protection Study Collaborative Group . Statin cost-effectiveness in the United States for people at different vascular risk levels. Circ Cardiovasc Qual Outcomes 2009;2:65-72. https://doi.org/10.1161/CIRCOUTCOMES.108.808469.
- Heart Protection Study Collaborative Group . Lifetime cost effectiveness of simvastatin in a range of risk groups and age groups derived from a randomised trial of 20,536 people. BMJ 2006;333:1145-8. https://doi.org/10.1136/bmj.38993.731725.BE.
- Heart Protection Study Collaborative Group . Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals. Lancet 2005;365:1779-85. https://doi.org/10.1016/S0140-6736(05)63014-0.
- Schlackow I, Kent S, Herrington W, Emberson J, Haynes R, Reith C, et al. SHARP Collaborative Group . A policy model of cardiovascular disease in moderate-to-advanced chronic kidney disease. Heart 2017;103:1880-90. https://doi.org/10.1136/heartjnl-2016-310970.
- Mihaylova B, Schlackow I, Herrington W, Lozano-Kuhne J, Kent S, Emberson J, et al. SHARP Collaborative Group . Cost-effectiveness of simvastatin plus ezetimibe for cardiovascular prevention in CKD: results of the study of heart and renal protection (SHARP). Am J Kidney Dis 2016;67:576-84. https://doi.org/10.1053/j.ajkd.2015.09.020.
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7404.1423.
- National Institute for Health and Care Research . Cost-Effectiveness of Statin Therapies Evaluated Using Individual Participant Data from Large Randomised Clinical Trials n.d. https://fundingawards.nihr.ac.uk/award/17/140/02 (accessed 8 November 2023).
- Cholesterol Treatment Trialists’ (CTT) Collaboration . CTT Collaboration n.d. www.cttcollaboration.org/participating-trials (accessed 28 April 2023).
- Heart Protection Study Collaborative Group . Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 2011;378:2013-20. https://doi.org/10.1016/s0140-6736(11)61125-2.
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001779.
- Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251-6. https://doi.org/10.1093/ije/dyh372.
- Hippisley-Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using Qresearch database. BMJ 2010;341. https://doi.org/10.1136/bmj.c6624.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
- Smith G. Step away from stepwise. J Big Data 2018;5. https://doi.org/10.1186/s40537-018-0143-6.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: University of Kent, PSSRU; 2020.
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. National Institute for Health Research School for Primary Care Research . Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30. https://doi.org/10.1016/S0140-6736(16)00620-6.
- NHS Digital . GP Workload Survey 2006 07 2007. https://digital.nhs.uk/data-and-information/areas-of-interest/workforce/gp-workload-survey (accessed 30 September 2021).
- NHS Improvement . National Cost Collection Guidance 2019 2019. https://improvement.nhs.uk/documents/4883/National_cost_collections_19.pdf (accessed 31 January 2021).
- NHS Business Services Authority . Prescription Cost Analysis - England 2020–21 2021. www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-202021 (accessed 30 September 2021).
- NHS Digital . HRG4+ 2018/19/Reference/Costs/Grouper 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing---hrg4-2018-19-reference-costs-grouper (accessed 31 January 2021).
- NHS Improvement . Archived Reference Costs - 2017 2018 Reference Costs 2018. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 9 August 2024).
- Zhou J, Wu R, Williams C, Emberson J, Reith C, Keech A, et al. Prediction models for individual-level healthcare costs associated with cardiovascular events in the UK. PharmacoEconomics 2023;41:547-59. https://doi.org/10.1007/s40273-022-01219-6.
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321-9. https://doi.org/10.1056/NEJMoa1012848.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Hernandez Alava M, Pudney S, Wailoo A. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: results from a UK population study. PharmacoEconomics 2023;41:199-207. https://doi.org/10.1007/s40273-022-01218-7.
- van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Software 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Gomes M, Gutacker N, Bojke C, Street A. Addressing missing data in patient‐reported outcome measures (PROMS): implications for the use of PROMS for comparing provider performance. Health Econ 2016;25:515-28. https://doi.org/10.1002/hec.3173.
- Grov EK, Fosså SD, Dahl AA. Activity of daily living problems in older cancer survivors: a population‐based controlled study. Health Soc Care Community 2010;18:396-40. https://doi.org/10.1111/j.1365-2524.2010.00912.x.
- Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst 2009;101:1206-15. https://doi.org/10.1093/jnci/djp239.
- Sulicka J, Pac A, Puzianowska-Kuźnicka M, Zdrojewski T, Chudek J, Tobiasz-Adamczyk B, et al. Health status of older cancer survivors: results of the PolSenior study. J Cancer Surviv 2018;12:326-33. https://doi.org/10.1007/s11764-017-0672-6.
- Wu M, Brazier JE, Kearns B, Relton C, Smith C, Cooper CL. Examining the impact of 11 long-standing health conditions on health-related quality of life using the EQ-5D in a general population sample. Eur J Health Econ 2015;16:141-51. https://doi.org/10.1007/s10198-013-0559-z.
- Tsiplova K, Pullenayegum E, Cooke T, Xie F. EQ-5D-derived health utilities and minimally important differences for chronic health conditions: 2011 Commonwealth Fund Survey of Sicker Adults in Canada. Qual Life Res 2016;25:3009-16. https://doi.org/10.1007/s11136-016-1336-0.
- Borchert K, Jacob C, Wetzel N, Jänicke M, Eggers E, Sauer A, et al. Application study of the EQ-5D-5L in oncology: linking self-reported quality of life of patients with advanced or metastatic colorectal cancer to clinical data from a German tumor registry. Health Econ Rev 2020;10. https://doi.org/10.1186/s13561-020-00297-6.
- Keng MJ, Leal J, Bowman L, Armitage J, Mihaylova B. Decrements in health-related quality of life associated with adverse events in people with diabetes. Diabetes Obes Metab 2021;24:530-8. https://doi.org/10.1111/dom.14610.
- Geessink N, Schoon Y, van Goor H, Olde Rikkert M, Melis R. TOPICS-MDS Consortium . Frailty and quality of life among older people with and without a cancer diagnosis: findings from TOPICS-MDS. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0189648.
- Kroep S, Chuang LH, Cohen A, Gumbs P, van Hout B, Monreal M, et al. The impact of co-morbidity on the disease burden of VTE. J Thromb Thrombolysis 2018;46:507-15. https://doi.org/10.1016/j.jval.2017.08.1151.
- Krishnan A, Teixeira-Pinto A, Lim WH, Howard K, Chapman JR, Castells A, et al. Health-related quality of life in people across the spectrum of CKD. Kidney Int Rep 2020;5:2264-74. https://doi.org/10.1016/j.ekir.2020.09.028.
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol 2006;97:S52-60. https://doi.org/10.1016/j.amjcard.2005.12.010.
- Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation 2011;124:146-53. https://doi.org/10.1161/CIRCULATIONAHA.110.986349.
- NHS England . 2019/20/National/Cost/Collection/Data/Publication 2021. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 2 September 2022).
- Cholesterol Treatment Trialists’ (CTT) Collaboration . Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0029849.
- National Institute for Health and Care Excellence . NICE Health Technology Evaluations: The Manual (PMG36) 2022. www.nice.org.uk/process/pmg36 (accessed 19 March 2022).
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459-72. https://doi.org/10.1093/eurheartj/ehx144.
- Pate A, Elliott RA, Gkountouras G, Thompson A, Emsley R, van Staa T. The impact of statin discontinuation and restarting rates on the optimal time to initiate statins and on the number of cardiovascular events prevented. Pharmacoepidemiol Drug Saf 2020;29:644-52. https://doi.org/10.1002/pds.5023.
- Hutchins R, Viera AJ, Sheridan SL, Pignone MP. Quantifying the utility of taking pills for cardiovascular prevention. Circ Cardiovasc Qual Outcomes 2015;8:155-63. https://doi.org/10.1161/CIRCOUTCOMES.114.001240.
- Thalmann I, Preiss D, Schlackow I, Gray A, Mihaylova B. Population-wide cohort study of statin use for the secondary cardiovascular disease prevention in Scotland in 2009–2017. Heart 2023;109:388-95. https://doi.org/10.1136/heartjnl-2022-321452.
- Panozzo CA, Curtis LH, Marshall J, Fine L, Wells BL, Brown JS, et al. Incidence of statin use in older adults with and without cardiovascular disease and diabetes mellitus, January 2008–March 2018. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0223515.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. https://doi.org/10.1093/eurheartj/ehz455.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/AphA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019;139:e1082-143. https://doi.org/10.1161/CIR.0000000000000625.
- Chang W, Cheng J, Allaire J, Sievert C, Schloerke B, Xie Y, et al. Shiny: Web Application Framework for R (R Package Version 1.7.4.90022023) n.d. https://shiny.rstudio.com/ (accessed 30 April 2023).
- Kuntz K, Sainfort F, Butler M, Taylor B, Kulasingam S, Gregory S, et al. Decision and Simulation Modeling in Systematic Reviews 2013. www.ncbi.nlm.nih.gov/books/NBK127482/ (accessed 11 August 2022).
- Odden MC, Pletcher MJ, Coxson PG, Thekkethala D, Guzman D, Heller D, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533-41. https://doi.org/10.7326/M14-1430.
- Office for National Statistics . Population Estimates for the UK, England and Wales, Scotland and Northern Ireland: Mid-2020 2021. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020 (accessed 26 August 2022).
- Kohli-Lynch CN, Lewsey J, Boyd KA, French DD, Jordan N, Moran AE, et al. Beyond 10-year risk: a cost-effectiveness analysis of statins for the primary prevention of cardiovascular disease. Circulation 2022;145:1312-23. https://doi.org/10.1161/circulationaha.121.057631.
- Greving JP, Visseren FL, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ 2011;342. https://doi.org/10.1136/bmj.d1672.
- Heller DJ, Coxson PG, Penko J, Pletcher MJ, Goldman L, Odden MC, et al. Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation 2017;136:1087-98. https://doi.org/10.1161/CIRCULATIONAHA.117.027067.
- Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA 2015;314:142-50. https://doi.org/10.1001/jama.2015.6822.
- National Institute for Health and Care Excellence . Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease 2014. www.nice.org.uk/guidance/ng238/evidence/full-guideline-july-2014-pdf-13254225661 (accessed 6 February 2024).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- ClinicalTrials.gov . A Clinical Trial of Statin Therapy for Reducing Events in the Elderly (STAREE) n.d. https://clinicaltrials.gov/ct2/show/NCT02099123 (accessed 27 October 2022).
- ClinicalTrials.gov . Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults (PREVENTABLE) n.d. https://clinicaltrials.gov/show/NCT04262206 (accessed 27 October 2022).
- Li Y, Sperrin M, van Staa T. R package ‘QRISK3’: an unofficial research purposed implementation of ClinRisk’s QRISK3 algorithm into R. F1000Res 2020;8. https://doi.org/10.12688/f1000research.21679.3.
- UK Biobank . Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) 2005. https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/ipaq_analysis.pdf (accessed 24 August 2022).
- Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hypponen E, Kuzma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019;322:430-7. https://doi.org/10.1001/jama.2019.9879.
- Carter AR, Gill D, Davey Smith G, Taylor AE, Davies NM, Howe LD. Cross-sectional analysis of educational inequalities in primary prevention statin use in UK Biobank. Heart 2022;108:536-42. https://doi.org/10.1136/heartjnl-2021-319238.
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884-90. https://doi.org/10.1001/jama.290.14.1884.
Appendix 1 Processing the UK Biobank study data
Handling missing values in UK Biobank
Following the specification of participant characteristics, including those required for QRISK calculation, several characteristics, such as ethnicity, smoking status/cigarettes per day, BMI (including height, weight), total cholesterol, LDL-C, HDL-C, creatinine and BP measures, and Townsend score, had some missing values (see Table 22). Missing ethnicities were imputed as white, the majority category. Missing smoking statuses were imputed as the majority smoking status by sex, age category and education level. Missing cigarettes per day were imputed as the majority category, that is 10–20. Missing Townsend scores were imputed by regressing on IMD scores, years and sources (England, Wales or Scotland) if IMD scores were available, and imputed by looking up an average Townsend score according to the rounded Ordnance Survey co-ordinates if IMD scores were missing, or, finally, imputed by looking up an average Townsend score for the areas of the participant UKB assessment centres if both IMD scores and Ordnance Survey co-ordinates were missing. The remaining continuous variables with missing values were imputed using multiple imputation by chained equations with 20 imputations and 10 iterations for each using the package ‘mice’ in R, with weight, height, LDL-C, HDL-C, triglycerides, creatinine, systolic BP (two measures) and diastolic BP (two measures), and with age, sex, (imputed) ethnicity, (imputed) smoking status, baseline CVDs, treated hypertension, statin treatment status and diabetes added as auxiliary variables. After imputation, participants’ QRISK3 scores were calculated using an external R package. 84
| QRISK3 parameter | Variable generation from UKB for calculating QRISK3 | Missing |
|---|---|---|
| Sex | Original UKB variable | |
| Age | Must be 25–84 years. All UKB participants are eligible at entry into UKB | |
| Height (cm) | Original UKB variable | 3087 |
| Weight (kg) | Derived from BMI and weight | 2757 |
| Ethnicity |
|
2771 |
| Townsend score | Original UKB variable | 622 |
| Smoking status |
|
2946 |
| Diabetes type 1 | UKB algorithm, ICD10: E10 Insulin-dependent diabetes mellitus, E14 Unspecified diabetes mellitus (diagnosis age ≤ 20 years) | |
| Diabetes type 2 | UKB algorithm, ICD10: E11 Non-insulin-dependent diabetes mellitus; E12 Malnutrition-related diabetes mellitus; E13 Other specified diabetes mellitus; E14 Unspecified diabetes mellitus (diagnosis age > 20 years) | |
| Atrial fibrillation | UKB algorithm, ICD10: I48 Atrial fibrillation and flutter | |
| CKD (stage 3, 4 or 5) | ICD10: N18 Chronic renal failure | |
| Rheumatoid arthritis | UKB algorithm, ICD10: M05 Seropositive rheumatoid arthritis; M06 Other rheumatoid arthritis | |
| Migraine | UKB algorithm, ICD10: G43 Migraine | |
| Systemic lupus erythematosus | UKB algorithm, ICD10: M32 Systemic lupus erythematosus | |
| Severe mental illness (schizophrenia, bipolar disorder and moderate/severe depression) | UKB algorithm, ICD10: F20 Schizophrenia; F23 Acute and transient psychotic disorders; F31 Bipolar affective disorder; F32 Depressive episode; F33 Recurrent depressive disorder | |
| Erectile dysfunction or treatment | Combination of nurse interview medical conditions and medications data3 | |
| Angina or heart attack in a first-degree relative < 60 years | Including history of ‘heart disease’ of father, mother and siblings. There is no information about whether the disease happened before 60 years old. Therefore, there are two assumptions: (1) heart disease was angina or heart attack, and (2) all the diseases happened under 60 years old | |
| Total cholesterol/HDL | Total cholesterol/HDL | 72,530 |
| Systolic BP | Mean, if there are two successive measures | 1319 |
| SD of systolic BP | SD of two successive measures | 1826 |
| BP treatment | As defined in the main text | |
| Regular steroid tablets | Used nurse interview medications data86 | |
| Atypical antipsychotic medication | Used nurse interview medications data86 |
The physical activity level is indicated by the International Physical Activity Questionnaire (IPAQ) activity group, the UKB-derived variable based on Metabolic Equivalent Task (MET) scores and categorised following the IPAQ guidelines into three levels:85
-
Low: no activity or not enough to meet moderate or high level.
-
Moderate: 3 or more days of vigorous-intensity activity of at least 20 minutes/day, or
-
Five or more days of moderate-intensity activity and/or walking of at least 30 minutes/day, or
-
Five or more days of any combination of walking, moderate or vigorous-intensity activities achieving a minimum of at least 600 MET-minutes/week.
-
High: vigorous-intensity activity on at least 3 days and accumulating at least 1500 MET-minutes/week, or
-
Seven or more days of any combination of walking, moderate or vigorous-intensity activities achieving a minimum of at least 3000 MET-minutes/week.
-
About 20% of the IPAQ values at entry into UKB were missing; these are used in analyses as a separate category.
-
Daily diet is considered to be healthy if it meets at least four of the following seven components of food frequency,86 and otherwise unhealthy:
-
fruits: ≥ 3 servings/day
-
vegetables: ≥ 3 servings/day
-
fish: ≥ 2 servings/week
-
processed meats: ≤ 1 serving/week
-
unprocessed red meats: ≤ 1.5 servings/week
-
whole grains: ≥ 3 servings/day
-
refined grains: ≤ 1.5 servings/day.
The 11,018 (2%) uncertain cases due to missing data in some categories of food intake were combined with the unhealthy diet category, as an early model indicated similar associations.
Derivation of 10-year cardiovascular risk using QRISK3 for participants at entry into the UK Biobank
Further to participant characteristics previously defined or derived from UKB, the following assumptions were made. First, we assumed that all unspecified black ethnicity was Black Caribbean. Second, at the entry into UKB (2006–10), few histories of CKD were coded as CKD stages 3–5 and many were coded as unspecified CKD; the whole chronic renal failure category was used to mean ‘CKD stage 3–5’ for the calculation of QRISK3. Third, the categories of depressive episode and recurrent depressive disorder were used to mean ‘moderate/severe depression’ required for the calculation of QRISK3, because many were coded as unspecified depression in UKB. Fourth, we used history of heart disease of father, mother and sibling to substitute angina or heart attack in a first-degree relative < 60 required in QRISK3, as this is the most relevant information available in UKB. The code lists of medications for antihypertension, erectile dysfunction treatment, regular steroid tablets and atypical antipsychotic treatment were sourced from a published study using UKB data. 87 Finally, for participants who were on statin treatment at baseline, their estimated 10-year CVD risks were adjusted to the pre-treated level using their derived pre-treated LDL-C levels (the derivation method is described in the following section), the proportional LDL-C reduction of the statin regimen they were on at baseline and the treatment effect for this LDL-C reduction, indicated by the RR of major vascular events per 1-mmol/l-LDL-C reduction with statin therapy in CTTC meta-analysis. 5
Derivation of pre-treatment low-density lipoprotein cholesterol levels for statin-treated UK Biobank participants
We adjusted upwards the LDL-C levels of UKB participants who were on statin treatment at entry into UKB to derive ‘pre-treatment’ LDL-C levels using the potency of their statin regimen (see Table 1).
Statin dosage information was not collected at UKB baseline interview, but for participants with linked primary care prescription records both type of statin and dosage were available. However, more than half of the UKB participants did not have linked primary care data and, therefore, no linked primary care prescription records. Additionally, there were some discrepancies between reported statin use at UKB recruitment and available statin prescription records; in such cases a report of statin use in either source was accepted, and, if more than one source was available, the more intensive regimen was used.
The statin treatment reports with an exact drug type and dosage were directly associated with the proportional LDL-C reductions according to Table 1. The statin treatment reports with unknown dosage were assumed to derive the average proportional LDL-C reduction according to weighted frequency of statin regimens within the primary or secondary prevention populations with known statin regimens.
The pre-treatment LDL-C of statin-treated participants was calculated as:
Appendix 2 Description of Whitehall II study data
Whitehall II is a cohort study of 10,308 participants aged 35–55 years at recruitment from the British Civil Service in 1985, with periodic resurveys (called phases) of participants thereafter and derived further participant health outcomes using linked routine hospital records, cancer registrations and deaths data. The Whitehall II study data include Phases 1–9 and 11 at present. Owing to attrition/no response and deaths, the numbers of participants declined from 10,308 in Phase 1 (1985–8) to 6308 in Phase 11 (2012–4). During even-numbered phases, more limited baseline data were collected and no biomarkers were measured. After weighing the data size and the length of follow-up, we used all participants with Phase 9 data (2007–9) to validate the CTTC-derived and UKB-calibrated CVD model. Definitions and specifications are the same as data preparation in the UKB cohort data. The characteristics of Whitehall II Phase 9 participants are summarised in Table 23.
| Characteristic | Without prior CVD (N = 4874) | With prior CVD (N = 1887) |
|---|---|---|
| Mean (SD) or n (%) | ||
| Age at entry at Phase 9 (years) | 65.4 (5.8) | 67.5 (6.1) |
| Male | 3439 (71%) | 1320 (70%) |
| Female | 1435 (29%) | 567 (30%) |
| Ethnicity | ||
| White | 4554 (93%) | 1680 (89%) |
| South Asian | 320 (7%) | 207 (11%) |
| Smoking status | ||
| Non-smoker | 2601 (53%) | 919 (49%) |
| Ex-smoker | 1911 (39%) | 811 (43%) |
| Current smoker | 362 (7%) | 157 (8%) |
| BMI (kg/m2) | 27 (4.4) | 28 (4.7) |
| < 18.5 | 49 (1%) | 11 (1%) |
| 18.5–25 | 1778 (36%) | 523 (28%) |
| 25–30 | 2080 (43%) | 814 (43%) |
| 30–35 | 757 (16%) | 387 (21%) |
| 35–40 | 162 (3%) | 119 (6%) |
| ≥ 40 | 48 (1%) | 33 (2%) |
| LDL (mmol/l) | 3.1 (0.93) | 2.8 (0.93) |
| On statin | 1255 (26%) | |
| HDL (mmol/l) | 1.6 (0.44) | 1.6 (0.43) |
| Creatinine (μmol/l) | 84 (20) | 89 (30) |
| Systolic BP (mmHg) | 126 (16) | 126 (17) |
| Diastolic BP (mmHg) | 72 (10) | 70 (10) |
| Baseline diabetes | 667 (14%) | 402 (21%) |
| Type 1 diabetes | 3 (0%) | 2 (0%) |
| Hypertension treatment | 1404 (29%) | 1111 (59%) |
| Baseline cancer | 400 (8%) | 161 (9%) |
| CVD history | ||
| Other CHD only | 1242 (66%) | |
| MI only | 97 (5%) | |
| PAD only | 90 (5%) | |
| Stroke only | 57 (3%) | |
| Two or more | 401 (21%) | |
| Townsend score quintile | ||
| 1 (least deprived) | 0 (0%) | 0 (0%) |
| 2 | 358 (7%) | 112 (6%) |
| 3 | 3357 (69%) | 1265 (67%) |
| 4 | 1157 (24%) | 509 (27%) |
| 5 | 2 (0%) | 1 (0%) |
| Physical activity level | ||
| High | 1832 (38%) | 613 (32%) |
| Moderate | 2829 (58%) | 1138 (60%) |
| Low | 119 (2%) | 77 (4%) |
| Missing | 94 (2%) | 59 (3%) |
| Severe mental illness | 87 (2%) | 39 (2%) |
| Unhealthy diet (including uncertain) | 3051 (63%) | 1192 (63%) |
Electronic death records, NHS hospital records and cancer registry entries were linked for Whitehall II participants and used to identify events during follow-up. The data were made available until particular time points for different types of events. For incident CRVs and incident diabetes, only questionnaire data were available as a result of unavailable linked healthcare data, so we did not validate the two end points considering that the questionnaire data were unreliable. Among the participants in Phase 9, deaths have the longest follow-up periods, with an average of 11.7 years, followed by MI and stroke with an average of 9.9 years. The follow-up periods of cancers are shorter, with an average of 6.1 years. The numbers of follow-up events for the older cohort are summarised in Table 24.
| MI | Stroke | Incident cancer | VD | NVD | |
|---|---|---|---|---|---|
| Without CVD history | 122 | 110 | 459 | 125 | 530 |
| With CVD history | 128 | 104 | 198 | 135 | 318 |
| Total | 250 | 214 | 657 | 260 | 848 |
Appendix 3 Final, calibrated in UK Biobank disease risk equations
Choice of parametric survival model specifications for event risk equations
After the specifications of calibrated risk equations were finalised, three types of parametric PH models were fitted (exponential, Weibull and Gompertz) for each end point and their AIC and BIC were compared (Table 25).
| Event | Level of prevention | Distribution information | |||||
|---|---|---|---|---|---|---|---|
| Exponential | Weibull | Gompertz | |||||
| AIC | Shape | AIC | Shape | AIC | Shape | ||
| MI | Primary | 75,892 | NA | 75,885 | 1.039 | 75,887 | 0.013 |
| Secondary | 29,360 | NA | 29,357 | 1.022 | 29,359 | 0.015 | |
| Stroke | Primary | 69,860 | NA | 69,810 | 1.119 | 69,818 | 0.039 |
| Secondary | 26,133 | NA | 26,132 | 1.014 | 26,132 | 0.005 | |
| CRV | Primary | 57,057 | NA | 57,019 | 1.132 | 57,062 | 0.038 |
| Secondary | 31,009 | NA | 30,964 | 0.891 | 30,972 | –0.042 | |
| VD | Primary | 27,137 | NA | 27,116 | 1.139 | 27,116 | 0.049 |
| Secondary | 17,735 | NA | 17,713 | 1.155 | 17,706 | 0.067 | |
| NVD | Primary | 108,167 | NA | 107,929 | 1.232 | 108,011 | 0.061 |
| Secondary | 29,247 | NA | 29,183 | 1.197 | 29,200 | 0.057 | |
| Cancer | All | 328,901 | NA | 328,901 | 1.005 | 328,895 | –0.007 |
| Diabetes | All | 71,964 | NA | 71,237 | 1.450 | 71,142 | 0.161 |
First, we followed the AIC to initially identify best-fitting distributions (the smaller the AIC value, the better). If the AIC statistic of selected specification was within five units of the AIC of exponential distribution, then the exponential distribution was chosen initially.
Second, we listed the PH models with close AICs to be further checked. We plotted the predicted risks against observed risks across full duration of follow-up in UKB (note that we did not use the latest 3 years of UKB data in model estimation/calibration, which was to allow the use of these 3 years of data for temporal validation here). If the predictions did not correspond well to observed cumulative risks, we tried the alternative PH model specifications. Table 26 lists the PH model specifications compared by plotting the predicted against the observed risks and the choices made.
| Comparison of distributions using model simulated vs. observed data | Better performance | |
|---|---|---|
| MI secondary prevention | Exponential vs. Weibull | Exponential |
| CRV secondary prevention | Weibull vs. Gompertz | Gompertz |
| VD primary prevention | Weibull vs. Gompertz | Gompertz |
| NVD primary prevention | Weibull vs. Gompertz | Weibulla |
| NVD secondary prevention | Weibull vs. Gompertz | Weibulla |
| Incident cancer | Exponential vs. Weibull vs. Gompertz | All similarb |
| Incident diabetes | Weibull vs. Gompertz | Gompertzc |
Third, we simulated risks over lifetime with the selected distributions after the above two steps, and checked if the model predicted lifetime risks are plausible compared with population lifetable statistics and epidemiological studies’ data.
We found that the lifetime risk of incident diabetes estimated using Gompertz distribution was implausibly high, at about 50–80% across ages. Replacing Gompertz with Weibull distribution, the second-best AIC statistic, produced a predicted lifetime risk of incident diabetes of around 20%, which is consistent with NHANES (National Health and Nutrition Examination Survey) data for white ethnicity in the USA. 88 Weibull distribution was therefore chosen for the incident diabetes risk equation.
We also checked if the ratio of lifetime VD and NVD is plausible. We found a lifetime mortality cause prediction model based on 1999 and 2014 US Underlying Cause of Death data, which predict around 40% deaths from vascular causes for people aged 40–70 years (https://flowingdata.com/2016/01/19/how-you-will-die/). Our lifetime simulation results using Gompertz distribution for VD and Weibull distribution for NVD give the closest prediction in respect of the ratio.
Furthermore, we checked the life expectancy by age group predicted by the model using different combinations of distributions for VD and NVD against the Office for National Statistics life expectancy prediction and confirmed the choice of distributions for VD and NVD.
Additionally, incident cancer risk peaks at ages 85–89 years according to UK cancer statistics data, and the Gompertz distribution for incident cancer captured well the decreasing risk in later years.
The final choice of distributions (shapes) is listed in Table 27 and the full risk equations are reported in Table 28.
| Event | Level of prevention | Distribution |
|---|---|---|
| MI | Primary | Weibull |
| Secondary | Exponential | |
| Stroke | Primary | Weibull |
| Secondary | Exponential | |
| CRV | Primary | Weibull |
| Secondary | Gompertz | |
| VD | Primary | Gompertz |
| Secondary | Gompertz | |
| NVD | Primary | Gompertz |
| Secondary | Gompertz | |
| Cancer | All | Gompertz |
| Diabetes | All | Weibull |
| MI and stroke | ||||
|---|---|---|---|---|
| MI, HR (95% CI) | Stroke, HR (95% CI) | |||
| Without CVD history, Weibull | With CVD history, exponential | Without CVD history, Weibull | With CVD history, exponential | |
| Coronary revascularisation incident cancer and incident diabetes | ||||
| Coronary revascularisation, HR (95% CI) | Cancer, HR (95% CI) | Diabetes | ||
| Without CVD history, Weibull | With CVD history, Gompertz | Without cancer history, Gompertz | Without diabetes history, Weibull | |
| VD and NVD | ||||
| VD, HR (95% CI) | NVD, HR (95% CI) | |||
| Baseline variable | ||||
| Male sex | 2.4 (1.95 to 2.95) | 1.55 (1.4 to 1.72) | 1.09 (0.94 to 1.26) | 1.01 (0.91 to 1.12) |
| Ethnicity (ref: white) | ||||
| Black ethnicity | 0.55 (0.4 to 0.77) | 0.72 (0.46 to 1.14) | ||
| South Asian ethnicity | 1.7 (1.43 to 2.03) | 1.64 (1.31 to 2.04) | ||
| Other ethnicity | 0.93 (0.76 to 1.15) | 0.92 (0.67 to 1.27) | ||
| Townsend deprivation score quintile (ref: third quintile) | ||||
| 1 | 0.91 (0.83 to 0.99) | 0.8 (0.7 to 0.91) | ||
| 2 | 1.01 (0.92 to 1.12) | 1.01 (0.87 to 1.16) | ||
| 4 | 1.09 (0.98 to 1.2) | 0.98 (0.85 to 1.14) | ||
| 5 | 1.27 (1.15 to 1.41) | 1.15 (1 to 1.33) | ||
| Smoking status (ref: non-smoker) | ||||
| Ex-smoker | 1.14 (1.07 to 1.21) | 1.22 (1.12 to 1.34) | 1.08 (1.01 to 1.15) | 1.03 (0.93 to 1.13) |
| Current smoker | 2.28 (2.12 to 2.45) | 2.15 (1.92 to 2.42) | 1.86 (1.71 to 2.02) | 1.53 (1.35 to 1.74) |
| Unhealthy diet | 1.11 (1.05 to 1.17) | 1.17 (1.1 to 1.24) | ||
| Physical activity (ref: moderate) | ||||
| Physical activity: low | 1.14 (1.05 to 1.23) | 1.25 (1.11 to 1.42) | ||
| Physical activity: high | 1.06 (1 to 1.14) | 0.99 (0.88 to 1.12) | ||
| Physical activity: missing | 1.14 (1.06 to 1.23) | 1.16 (1.03 to 1.3) | ||
| LDL-C (centred at 3.6 mmol/l) per 1 mmol/l | 1.56 (1.51 to 1.61) | 1.19 (1.12 to 1.26) | ||
| Natural logarithm of HDL-C (lnmmol/l) | 0.32 (0.23 to 0.44) | 0.58 (0.49 to 0.69) | 0.71 (0.56 to 0.9) | |
| On treatment for hypertension | 1.53 (1.26 to 1.87) | 1.3 (1.18 to 1.42) | 1.39 (1.2 to 1.61) | |
| Systolic BP (centred at 140 mmHg per 20 mmHg) | 1.27 (1.16 to 1.38) | 1.06 (1.02 to 1.11) | 1.31 (1.2 to 1.42) | 1.2 (1.15 to 1.25) |
| Natural logarithm of creatinine (lnµmol/l) | 2.15 (1.73 to 2.68) | 1.66 (1.25 to 2.21) | 1.52 (1.23 to 1.88) | |
| BMI category (kg/m2) (ref: 18.5–25 healthy) | ||||
| Underweight (< 18.5) | 2.05 (1.32 to 3.18) | |||
| Overweight (25–30) | 0.87 (0.76 to 1.01) | |||
| Obese I (30–35) | 0.76 (0.63 to 0.92) | |||
| Obese II (35–40) | 0.83 (0.62 to 1.1) | |||
| Obese III (≥ 40+) | 0.72 (0.46 to 1.13) | |||
| Severe mental illness | 1.25 (1.13 to 1.37) | 1.26 (1.14 to 1.39) | 1.27 (1.12 to 1.43) | |
| Type 1 diabetes at baseline | 1.67 (1.31 to 2.12) | 1.54 (1.29 to 1.84) | 1.56 (1.23 to 1.98) | 1.75 (1.43 to 2.15) |
| CVD history (ref: other CHD only) | ||||
| Previous MI only | 1.51 (1.33 to 1.7) | 1.03 (0.91 to 1.18) | ||
| History of a stroke only | 0.85 (0.68 to 1.06) | 2.01 (1.67 to 2.42) | ||
| History of peripheral artery disease only | 0.89 (0.71 to 1.13) | 1.33 (1.09 to 1.61) | ||
| Two or more disease histories | 1.77 (1.53 to 2.05) | 1.72 (1.48 to 1.98) | ||
| Time-updating variables | ||||
| Current age centred at 60 years (per 10 years) | 1.68 (1.61 to 1.75) | 1.34 (1.26 to 1.43) | 1.91 (1.74 to 2.09) | 1.59 (1.48 to 1.71) |
| Incident MI (ref: none) | ||||
| Any incident MI | 1.75 (1.3 to 2.38) | 1.5 (1.26 to 1.78) | ||
| Incident stroke (ref: none) | ||||
| Incident stroke in same year | 0.7 (0.43 to 1.15) | |||
| Incident stroke in a previous year | 1.61 (1.22 to 2.14) | |||
| Incident CRV (ref: none) | ||||
| Incident CRV in same year | 1.07 (0.8 to 1.42) | |||
| Incident CRV in a previous year | 0.5 (0.38 to 0.65) | |||
| Any incident CRV | 2.5 (1.51 to 4.14) | 1.77 (1.26 to 2.47) | ||
| Diabetes (ref: no diabetes, HbA1c 32–37 mmol/mol) | ||||
| No diabetes, HbA1c < 32 mmol/mol | 0.9 (0.82 to 0.99) | 0.81 (0.68 to 0.97) | 1.03 (0.94 to 1.12) | 1.02 (0.86 to 1.21) |
| No diabetes, HbA1c 37–42 mmol/mol | 1.1 (1.03 to 1.18) | 1.09 (0.98 to 1.21) | 1.1 (1.02 to 1.18) | 1.1 (0.98 to 1.23) |
| No diabetes, HbA1c 42–48 mmol/mol | 1.29 (1.13 to 1.47) | 1.26 (1.06 to 1.49) | 1.28 (1.11 to 1.48) | 1.12 (0.92 to 1.36) |
| Diabetes duration 0–10 years | 1.29 (1.13 to 1.48) | 1.26 (1.1 to 1.44) | 1.25 (1.1 to 1.42) | 1.47 (1.27 to 1.69) |
| Diabetes duration ≥ 10 years | 1.92 (1.61 to 2.3) | 1.88 (1.64 to 2.16) | 1.65 (1.43 to 1.9) | 1.56 (1.32 to 1.83) |
| Cancer (ref: none) | ||||
| Incident cancer 0–5 years ago | 1.22 (1.07 to 1.39) | 1.33 (1.12 to 1.58) | 1.85 (1.65 to 2.08) | 1.66 (1.4 to 1.98) |
| Baseline cancer 0–5 years ago | 1.28 (0.95 to 1.71) | 0.83 (0.55 to 1.27) | 1.4 (1.01 to 1.93) | 1.44 (1 to 2.08) |
| All cancer ≥ 5 years ago | 1.02 (0.92 to 1.13) | 1.13 (0.99 to 1.29) | 1.35 (1.23 to 1.48) | 1.24 (1.09 to 1.42) |
| Interactions | ||||
| Current age × systolic BP | 0.89 (0.84 to 0.95) | |||
| Current age × diabetes 0–10 years | 0.93 (0.79 to 1.09) | |||
| Current age × diabetes ≥ 10 years | 0.92 (0.77 to 1.1) | |||
| Shape a | 1.04 | NA | 1.12 | NA |
| Baseline variable | ||||
| Male sex | 2.18 (1.78 to 2.67) | 2.21 (2.02 to 2.42) | 1.03 (1 to 1.05) | 1.32 (1.23 to 1.41) |
| Ethnicity (ref: white) | ||||
| Black ethnicity | 0.55 (0.39 to 0.76) | 0.63 (0.39 to 1) | 0.96 (0.86 to 1.06) | 1.07 (0.88 to 1.29) |
| South Asian ethnicity | 1.57 (1.36 to 1.8) | 1.41 (1.18 to 1.68) | 0.73 (0.65 to 0.81) | 1.24 (1.06 to 1.44) |
| Other ethnicity | 0.91 (0.76 to 1.1) | 1.29 (1.01 to 1.64) | 0.85 (0.78 to 0.93) | 1.49 (1.3 to 1.72) |
| Townsend deprivation score quintile (ref: third quintile) | ||||
| 1 | 0.91 (0.85 to 0.99) | |||
| 2 | 0.98 (0.9 to 1.06) | |||
| 3 | 1.03 (0.95 to 1.12) | |||
| 5 | 1.12 (1.03 to 1.22) | |||
| Smoking status (ref: non-smoker) | ||||
| Ex-smoker | 1.15 (1.09 to 1.21) | 1.16 (1.07 to 1.25) | 1.14 (1.11 to 1.17) | 1.07 (1.01 to 1.13) |
| Current smoker | 1.17 (1.09 to 1.25) | 1.3 (1.17 to 1.45) | 1.44 (1.39 to 1.5) | 0.96 (0.89 to 1.04) |
| Unhealthy diet | 1.05 (1.03 to 1.08) | 1.13 (1.07 to 1.19) | ||
| Physical activity level (ref: moderate) | ||||
| Low | 0.9 (0.83 to 0.96) | 1.07 (1.04 to 1.1) | 1.07 (1 to 1.15) | |
| High | 1.07 (1 to 1.13) | 0.98 (0.95 to 1.01) | 0.89 (0.84 to 0.96) | |
| Missing | 1.03 (0.97 to 1.11) | 1.03 (1 to 1.06) | 1.07 (1 to 1.14) | |
| LDL-C (centred at 3.6 mmol/l) per 1 mmol/l | 1.54 (1.35 to 1.76) | 1.22 (1.14 to 1.31) | ||
| Natural logarithm of HDL-C (lnmmol/l) | 0.24 (0.17 to 0.33) | 0.41 (0.33 to 0.5) | 0.93 (0.88 to 0.99) | 0.3 (0.26 to 0.34) |
| On treatment for hypertension | 1.61 (1.33 to 1.95) | 1.76 (1.56 to 1.97) | 1.38 (1.3 to 1.46) | |
| Systolic BP (centred at 140 mmHg per 20 mmHg) | 1.13 (1.1 to 1.16) | |||
| Diastolic BP (centred at 80 mmHg per 10 mmHg) | 0.89 (0.85 to 0.93) | |||
| Natural logarithm of creatinine (lnµmol/l) | 0.64 (0.55 to 0.74) | |||
| HbA1c (mmol/mol) | 1.3 (1.29 to 1.31) | |||
| BMI categories (kg/m2) (ref: 18.5–25 kg/m2, healthy) | ||||
| Underweight (< 18.5 kg/m2) | 0.39 (0.13 to 1.18) | 1.38 (0.79 to 2.38) | ||
| Overweight (25–30 kg/m2) | 1.11 (0.99 to 1.24) | 1.51 (1.38 to 1.65) | ||
| Obese I (30–35 kg/m2) | 1.02 (0.88 to 1.19) | 2.39 (2.18 to 2.62) | ||
| Obese II (35–40 kg/m2) | 0.93 (0.71 to 1.21) | 3.38 (3.04 to 3.76) | ||
| Obese III (≥ 40 kg/m2) | 0.5 (0.3 to 0.84) | 3.69 (3.24 to 4.19) | ||
| Severe mental illness | 1.38 (1.29 to 1.48) | |||
| Type 1 diabetes at baseline | 1.38 (1.24 to 1.53) | |||
| CVD history (ref: other CHD only for the CRV equation/none for the cancer and diabetes equations) | ||||
| Underweight (< 18.5 kg/m2) | 0.39 (0.13 to 1.18) | 1.38 (0.79 to 2.38) | ||
| Overweight (25–30 kg/m2) | 1.11 (0.99 to 1.24) | 1.51 (1.38 to 1.65) | ||
| Obese I (30–35 kg/m2) | 1.02 (0.88 to 1.19) | 2.39 (2.18 to 2.62) | ||
| Obese II (35–40 kg/m2) | 0.93 (0.71 to 1.21) | 3.38 (3.04 to 3.76) | ||
| Obese III (≥ 40 kg/m2) | 0.5 (0.3 to 0.84) | 3.69 (3.24 to 4.19) | ||
| Severe mental illness | 1.38 (1.29 to 1.48) | |||
| Type 1 diabetes at baseline | 1.38 (1.24 to 1.53) | |||
| CVD history (ref: other CHD only for the CRV equation/none for the cancer and diabetes equations) | ||||
| Other CHD only | 1 (0.94 to 1.06) | 1.2 (1.11 to 1.31) | ||
| Previous MI only | 0.74 (0.65 to 0.85) | 0.98 (0.93 to 1.04) | 1.36 (1.04 to 1.77) | |
| History of a stroke only | 0.18 (0.12 to 0.26) | 0.99 (0.92 to 1.06) | 1.41 (1.18 to 1.68) | |
| History of peripheral artery disease only | 0.15 (0.1 to 0.23) | 1.21 (1.11 to 1.32) | 1.31 (1.1 to 1.56) | |
| Two or more disease histories | 0.8 (0.68 to 0.95) | 1.09 (1.02 to 1.15) | 1.46 (1.32 to 1.61) | |
| Time-updating variables | ||||
| Current age centred at 60 years (per 10 years) | 1.18 (1.06 to 1.3) | 1.17 (1.1 to 1.24) | 1.57 (1.53 to 1.61) | 1.58 (1.16 to 2.15) |
| Incident MI (ref: none) | ||||
| Incident MI in same year | 682.04 (565.35 to 822.8) | 82.48 (70.57 to 96.39) | 0.71 (0.58 to 0.86) | |
| Incident MI in previous year | 75.99 (50.47 to 114.41) | 31.31 (24.69 to 39.7) | ||
| Incident MI at least 2 years ago | 15.14 (8.7 to 26.34) | 5.79 (4.25 to 7.89) | ||
| Incident MI in a previous year | 0.95 (0.86 to 1.04) | |||
| Any incident MI | 1.4 (1.12 to 1.74) | |||
| Incident stroke (ref: none) | ||||
| Incident stroke in same year | 0.14 (0.05 to 0.35) | |||
| Incident stroke in a previous year | 0.67 (0.42 to 1.05) | |||
| Incident CRV (ref: none) | ||||
| Incident CRV in same year | 0.75 (0.63 to 0.89) | 0.38 (0.21 to 0.68) | ||
| Incident CRV in a previous year | 0.97 (0.9 to 1.05) | 0.99 (0.81 to 1.21) | ||
| Diabetes (ref: no diabetes, HbA1c 32–37 mmol/mol) | ||||
| No diabetes, HbA1c < 32 mmol/mol | 0.94 (0.87 to 1.02) | 0.88 (0.76 to 1.02) | 1.03 (1 to 1.06) | |
| No diabetes, HbA1c 37–42 mmol/mol | 1.1 (1.04 to 1.17) | 1.11 (1.01 to 1.21) | 1.04 (1.02 to 1.07) | |
| No diabetes, HbA1c 42–48 mmol/mol | 1.16 (1.03 to 1.31) | 1.22 (1.05 to 1.41) | 1.16 (1.1 to 1.23) | |
| Diabetes duration 0–10 years | 1.19 (1.09 to 1.31) | 1.26 (1.13 to 1.4) | 1.12 (1.06 to 1.17) | |
| Diabetes duration ≥ 10 years | 1.53 (1.38 to 1.7) | 1.32 (1.18 to 1.47) | 1.07 (1.01 to 1.14) | |
| Cancer (ref: none) | ||||
| Incident cancer 0–5 years ago | 0.73 (0.64 to 0.84) | 0.64 (0.52 to 0.78) | 2.45 (2.22 to 2.69) | |
| Baseline cancer 0–5 years ago | 1.09 (0.82 to 1.44) | 0.85 (0.61 to 1.2) | 1.62 (1.23 to 2.14) | |
| All cancer ≥ 5 years ago | 1.06 (0.97 to 1.16) | 0.89 (0.79 to 1) | 1.34 (1.24 to 1.46) | |
| Interactions | ||||
| Current age × male | 1.52 (1.48 to 1.57) | |||
| Current age × current smoker | 1.13 (1.08 to 1.17) | |||
| Current age × ex-smoker | 1 (0.97 to 1.03) | |||
| Current age × HbA1c | 0.99 (0.98 to 1) | |||
| Shape a | 1.13 | –0.04 | –0.01 | 1.44 |
| Without CVD history, Gompertz | With CVD history, Gompertz | Without CVD history, Gompertz | With CVD history, Gompertz | |
| Baseline variables | ||||
| Male sex | 1.53 (1.21 to 1.93) | 1.33 (1.18 to 1.49) | 1.36 (1.3 to 1.43) | 1.01 (0.89 to 1.14) |
| Ethnicity (ref: white) | ||||
| Black ethnicity | 0.68 (0.56 to 0.83) | 0.62 (0.42 to 0.92) | ||
| South Asian ethnicity | 0.77 (0.63 to 0.96) | 0.71 (0.5 to 1.01) | ||
| Other ethnicity | 0.83 (0.7 to 0.97) | 0.8 (0.57 to 1.12) | ||
| Townsend deprivation score quintile (ref = third) | ||||
| 1 | 0.68 (0.6 to 0.78) | 0.76 (0.65 to 0.88) | 0.93 (0.88 to 0.98) | 0.82 (0.73 to 0.92) |
| 2 | 0.76 (0.66 to 0.88) | 0.91 (0.77 to 1.08) | 0.93 (0.87 to 0.99) | 0.99 (0.88 to 1.12) |
| 4 | 1.09 (0.94 to 1.26) | 1.11 (0.94 to 1.3) | 1.04 (0.97 to 1.11) | 1 (0.89 to 1.14) |
| 5 | 1.2 (1.04 to 1.38) | 1.14 (0.98 to 1.33) | 1.27 (1.19 to 1.36) | 1.34 (1.19 to 1.5) |
| Smoking status (ref = non-smoker) | ||||
| Ex-smoker | 1.03 (0.93 to 1.13) | 1.33 (1.18 to 1.49) | 1.21 (1.16 to 1.27) | 1.31 (1.2 to 1.42) |
| Current smoker | 2.16 (1.93 to 2.43) | 1.97 (1.71 to 2.28) | 1.97 (1.86 to 2.08) | 1.9 (1.71 to 2.11) |
| Unhealthy diet | 1.19 (1.09 to 1.3) | 1.15 (1.11 to 1.2) | 1.26 (1.18 to 1.36) | |
| Physical activity level (ref: moderate) | ||||
| Low | 1.06 (0.92 to 1.21) | 1.24 (1.17 to 1.31) | 1.33 (1.2 to 1.46) | |
| High | 0.95 (0.83 to 1.09) | 0.96 (0.91 to 1) | 0.93 (0.85 to 1.03) | |
| Missing | 1.25 (1.09 to 1.42) | 1.11 (1.05 to 1.16) | 1.12 (1.02 to 1.24) | |
| LDL-C (centred at 3.6 mmol/l) per 1 mmol/l | 1.28 (1.11 to 1.49) | 1.17 (1.07 to 1.27) | 0.88 (0.82 to 0.95) | |
| Natural logarithm of HDL-C (lnmmol/l) | 0.83 (0.7 to 0.97) | |||
| On treatment for hypertension | 1.45 (1.14 to 1.83) | 1.3 (1.19 to 1.43) | ||
| Systolic BP (centred at 140 mmHg per 20 mmHg) | ||||
| Diastolic BP (centred at 80 mmHg per 10 mmHg) | 0.94 (0.9 to 0.97) | |||
| Natural logarithm of creatinine (lnµmol/l) | 2.19 (1.44 to 3.34) | 3.98 (3.25 to 4.87) | 1.85 (1.47 to 2.33) | |
| BMI categories (kg/m2) (ref: 18.5–25 healthy) | ||||
| Underweight (< 18.5) | 1.78 (1.14 to 2.79) | 2.15 (1.63 to 2.85) | 2.81 (1.96 to 4.03) | |
| Overweight (25–30) | 0.88 (0.8 to 0.97) | 0.88 (0.79 to 0.97) | 0.87 (0.78 to 0.96) | |
| Obese I (30–35) | 0.96 (0.85 to 1.09) | 0.74 (0.65 to 0.85) | 0.83 (0.72 to 0.96) | |
| Obese II (35–40) | 1.12 (0.89 to 1.4) | 0.81 (0.65 to 1) | 0.94 (0.73 to 1.21) | |
| Obese III (≥ 40) | 1.73 (1.22 to 2.44) | 0.94 (0.71 to 1.25) | 1.33 (0.89 to 1.98) | |
| Severe mental illness | 1.34 (1.17 to 1.55) | 1.14 (1.07 to 1.22) | ||
| Type 1 diabetes at baseline | 1.19 (1.01 to 1.41) | |||
| CVD history (ref: other CHD only) | ||||
| Previous MI only | 1.54 (1.35 to 1.75) | 1.14 (0.99 to 1.31) | ||
| History of a stroke only | 1.32 (1.07 to 1.63) | 1.54 (1.27 to 1.88) | ||
| History of peripheral artery disease only | 1.38 (1.12 to 1.7) | 1.58 (1.3 to 1.91) | ||
| Two or more disease histories | 1.85 (1.6 to 2.13) | 1.39 (1.2 to 1.61) | ||
| Time-updating variables | ||||
| Current age centred at 60 years (per 10 years) | 1.93 (1.79 to 2.07) | 1.6 (1.43 to 1.8) | 1.72 (1.65 to 1.79) | 1.55 (1.43 to 1.68) |
| Incident MI (ref: none) | ||||
| Any incident MI | 1.62 (1.35 to 1.93) | |||
| Incident MI in same year | 155.63 (136.92 to 176.91) | 34.37 (30.05 to 39.32) | ||
| Incident MI in previous year | 8.47 (5.93 to 12.1) | 7.48 (6.07 to 9.22) | ||
| Incident MI at least 2 years ago | 2.9 (2.08 to 4.04) | 4.92 (4.05 to 5.96) | ||
| Incident stroke (ref: none) | ||||
| Incident stroke in same year | 64.26 (56.28 to 73.37) | 13.6 (11.7 to 15.8) | ||
| Incident stroke in a previous year | 8.25 (6.76 to 10.07) | |||
| Incident stroke in previous year | 4.13 (3.15 to 5.41) | |||
| Incident stroke at least 2 years ago | 1.99 (1.51 to 2.61) | |||
| Any incident stroke | 1.97 (1.54 to 2.52) | 1.97 (1.64 to 2.37) | ||
| Incident CRV (ref: none) | ||||
| Incident CRV in same year | 0.26 (0.15 to 0.45) | 0.45 (0.28 to 0.74) | ||
| Incident CRV in a previous year | 0.84 (0.44 to 1.61) | 0.98 (0.8 to 1.21) | ||
| Any incident CRV | 0.5 (0.43 to 0.59) | |||
| Diabetes (ref: no diabetes, HbA1c 32–37) | ||||
| No diabetes, HbA1c < 32 mmol/mol | 1.11 (0.96 to 1.29) | 1.08 (0.86 to 1.34) | 1.04 (0.98 to 1.1) | 1.15 (1 to 1.33) |
| No diabetes, HbA1c 37–42 mmol/mol | 1.31 (1.18 to 1.46) | 1.07 (0.93 to 1.22) | 1.1 (1.05 to 1.16) | 1.12 (1.02 to 1.23) |
| No diabetes, HbA1c 42–48 mmol/mol | 1.79 (1.46 to 2.19) | 1.28 (1.04 to 1.58) | 1.32 (1.19 to 1.46) | 1.08 (0.91 to 1.27) |
| Diabetes duration 0–10 years | 2.61 (2.12 to 3.22) | 1.94 (1.57 to 2.39) | 1.89 (1.76 to 2.02) | 1.74 (1.56 to 1.95) |
| Diabetes duration ≥ 10 years | 4.5 (3.6 to 5.64) | 2.17 (1.75 to 2.7) | 1.45 (1.31 to 1.59) | 1.76 (1.57 to 1.98) |
| Cancer (ref: none) | ||||
| Incident cancer 0–5 years ago | 1.12 (0.92 to 1.36) | |||
| Baseline cancer 0–5 years ago | 1.78 (1.17 to 2.7) | |||
| All cancer ≥ 5 years ago | 0.85 (0.72 to 1) | |||
| Incident cancer in same year | 84.4 (79.39 to 89.73) | 38.89 (35.01 to 43.21) | ||
| Incident cancer 1 year ago | 98.29 (92.38 to 104.59) | 39.66 (35.41 to 44.43) | ||
| Incident cancer 2 years ago | 60.54 (56.08 to 65.36) | 19.97 (17 to 23.45) | ||
| Incident cancer 3 years ago | 38.8 (35.19 to 42.79) | 14.43 (11.8 to 17.66) | ||
| Incident cancer 4 years ago | 30.39 (26.97 to 34.24) | 11.41 (8.89 to 14.65) | ||
| Baseline cancer 1 year ago | 21.46 (14.45 to 31.89) | 29.36 (19.31 to 44.64) | ||
| Baseline cancer 2 years ago | 33.43 (27.07 to 41.29) | 18.33 (12.95 to 25.93) | ||
| Baseline cancer 3 years ago | 33.72 (28.47 to 39.95) | 13.44 (9.68 to 18.66) | ||
| Baseline cancer 4 year ago | 24.13 (20.34 to 28.62) | 11.52 (8.46 to 15.68) | ||
| All cancer 5–10 years ago | 16.52 (15.37 to 17.76) | 8.13 (7.18 to 9.22) | ||
| All cancer 10–15 years ago | 13.02 (11.91 to 14.23) | 6.32 (5.38 to 7.43) | ||
| All cancer 15–20 years ago | 10.82 (9.62 to 12.17) | 5.63 (4.54 to 7) | ||
| All cancer ≥ 20 years ago | 8.29 (7.46 to 9.22) | 4.67 (3.88 to 5.62) | ||
| Interactions | ||||
| Current age × LDL-C | 0.87 (0.8 to 0.96) | |||
| Current age × male | 0.82 (0.78 to 0.87) | |||
| Current age × diabetes 0–10 years | 0.75 (0.61 to 0.93) | 0.74 (0.59 to 0.91) | ||
| Current age × diabetes ≥ 10 years | 0.69 (0.55 to 0.87) | 0.68 (0.55 to 0.84) | ||
| MI same year × diabetes 0–10 years | 0.92 (0.65 to 1.3) | |||
| MI same year × diabetes ≥ 10 years | 0.5 (0.34 to 0.74) | |||
| Stroke same year × diabetes 0–10 years | 0.42 (0.27 to 0.66) | |||
| Stroke same year × diabetes ≥ 10 years | 0.2 (0.12 to 0.33) | |||
| Shape a | 0.05 | 0.07 | 0.06 | 0.05 |
Appendix 4 Diagnostics of convergence of results from model simulations
Number of first-order microsimulations
We executed up to 1500 first-order model microsimulations for UKB participants, by prior CVD, 10-year CVD risk, LDL-C and age, and averaged the simulation results, including QALYs gained, total incremental cost and incremental cost-effectiveness ratio (ICER), across different numbers of microsimulations (e.g. first 50, 100, 150, …, 1000) to check convergence, by comparing these averages with the averages across all 1000 or 1500 microsimulations. Figure 14 presents these comparisons for ICERs for standard statin compared with no statin therapy. The results indicate that the averages become more stable as the number of microsimulations increases. We decided to use 500 first-order microsimulations, which achieves good stability in all categories as well as allowing the execution of probabilistic sensitivity analyses in practicable time.
FIGURE 14.
Diagnostics for number of first-order microsimulations for the ICER of standard statin compared with no statin therapy in categories of UKB participants.
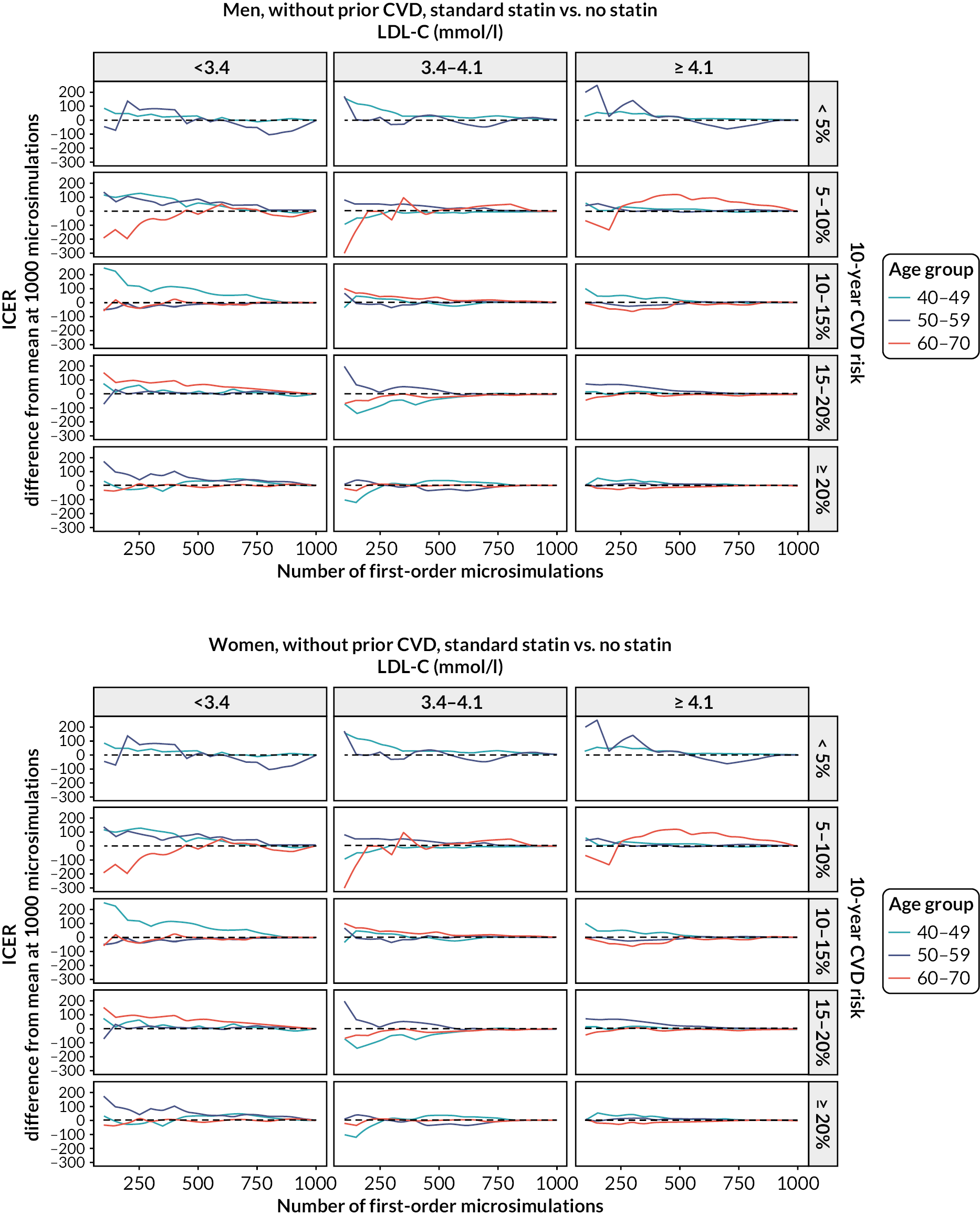
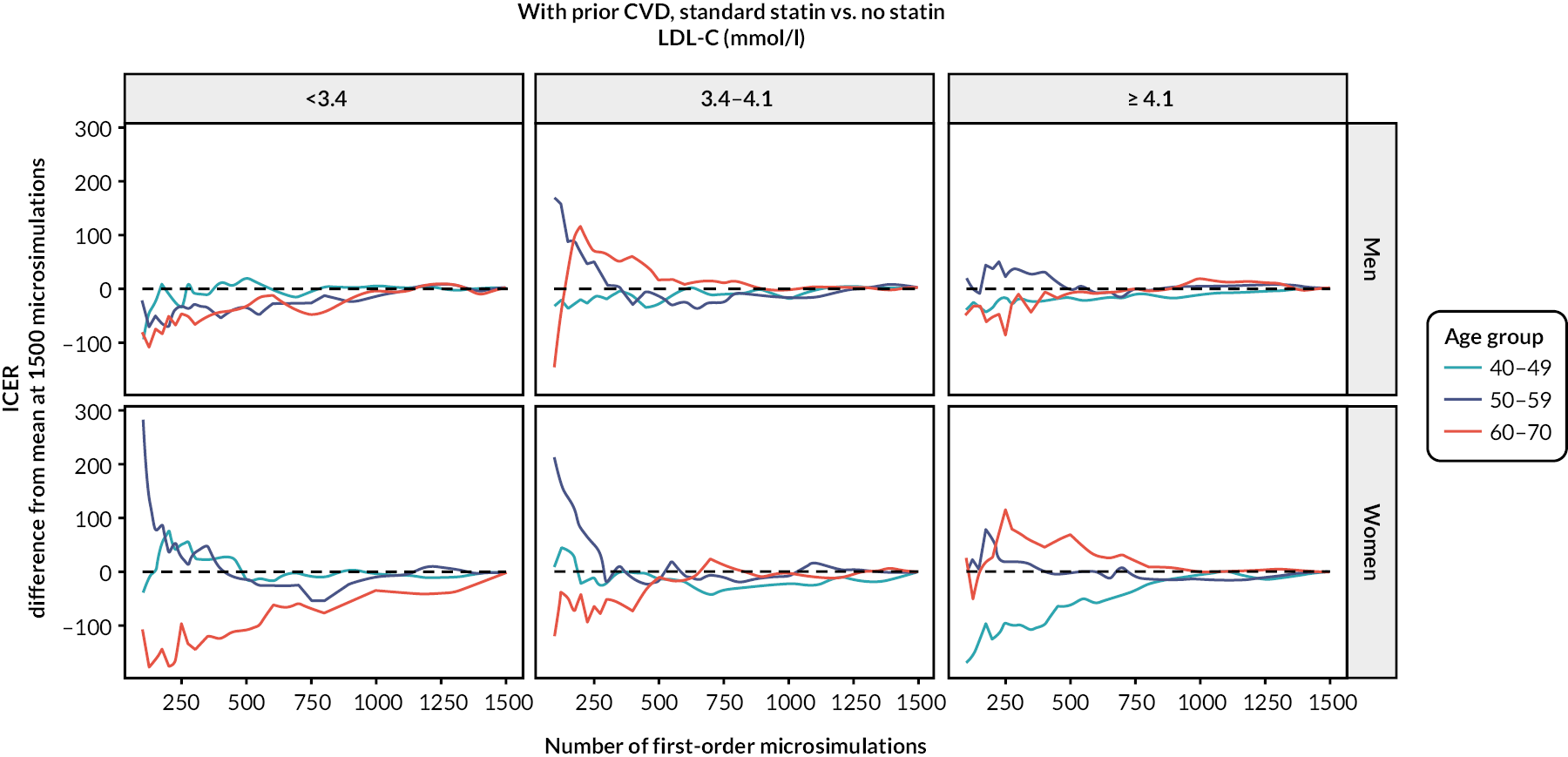
Number of simulations for probabilistic sensitivity analyses
We executed 500 and 1000 simulations for the probabilistic sensitivity analysis for UKB participants without and with prior CVD, respectively. To check if the numbers of simulations were sufficient, we examined the CIs at different numbers of simulations for QALYs gained, incremental cost, and ICER in categories of participants. Figure 15 presents this comparison for the ICERs of standard statin compared with no statin therapy. The results indicate that the Cis become stable quickly.
FIGURE 15.
Diagnostics for number of probabilistic sensitivity analyses simulations for the ICER of standard statin compared with no statin therapy, in categories of UKB participants.
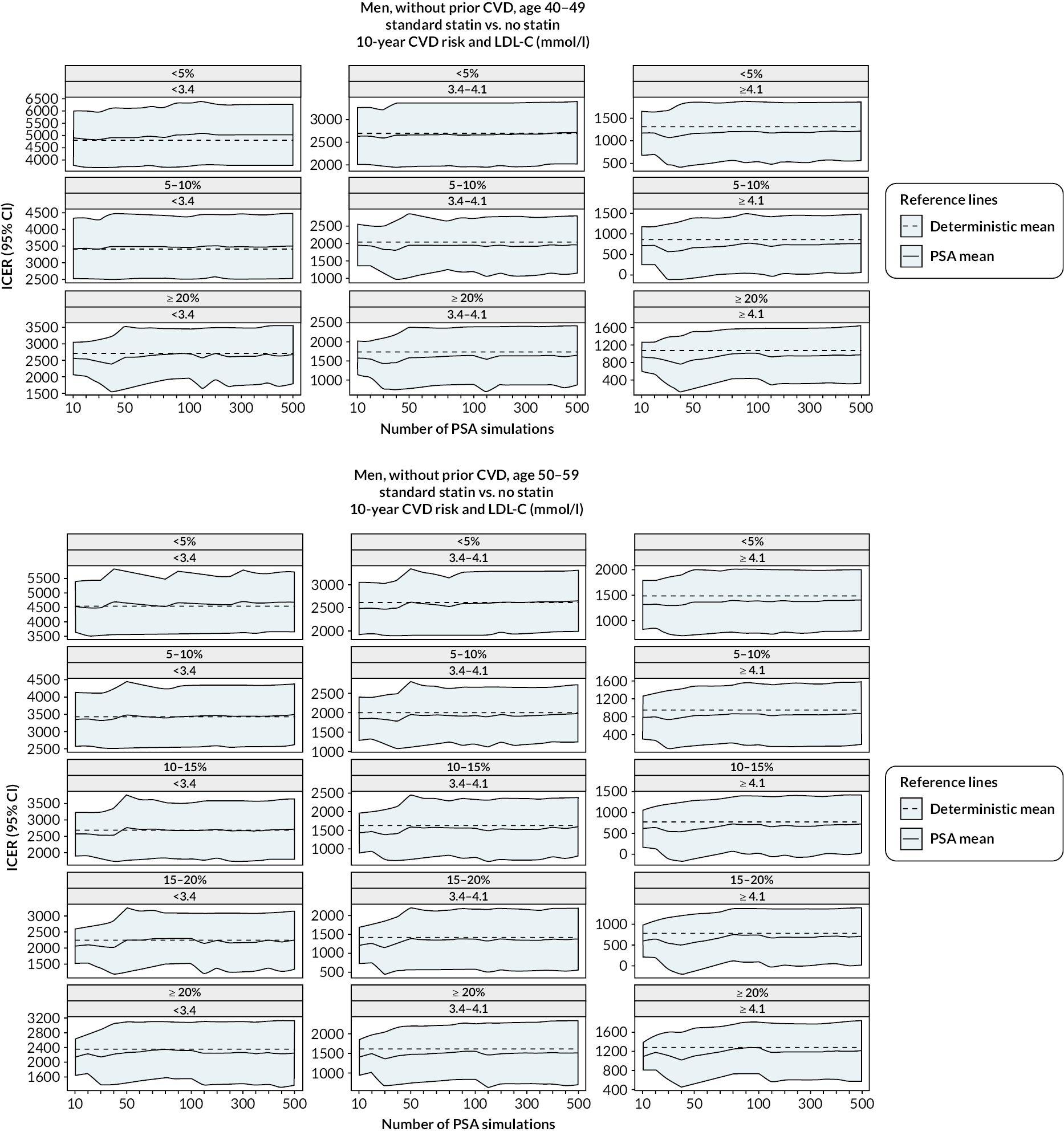
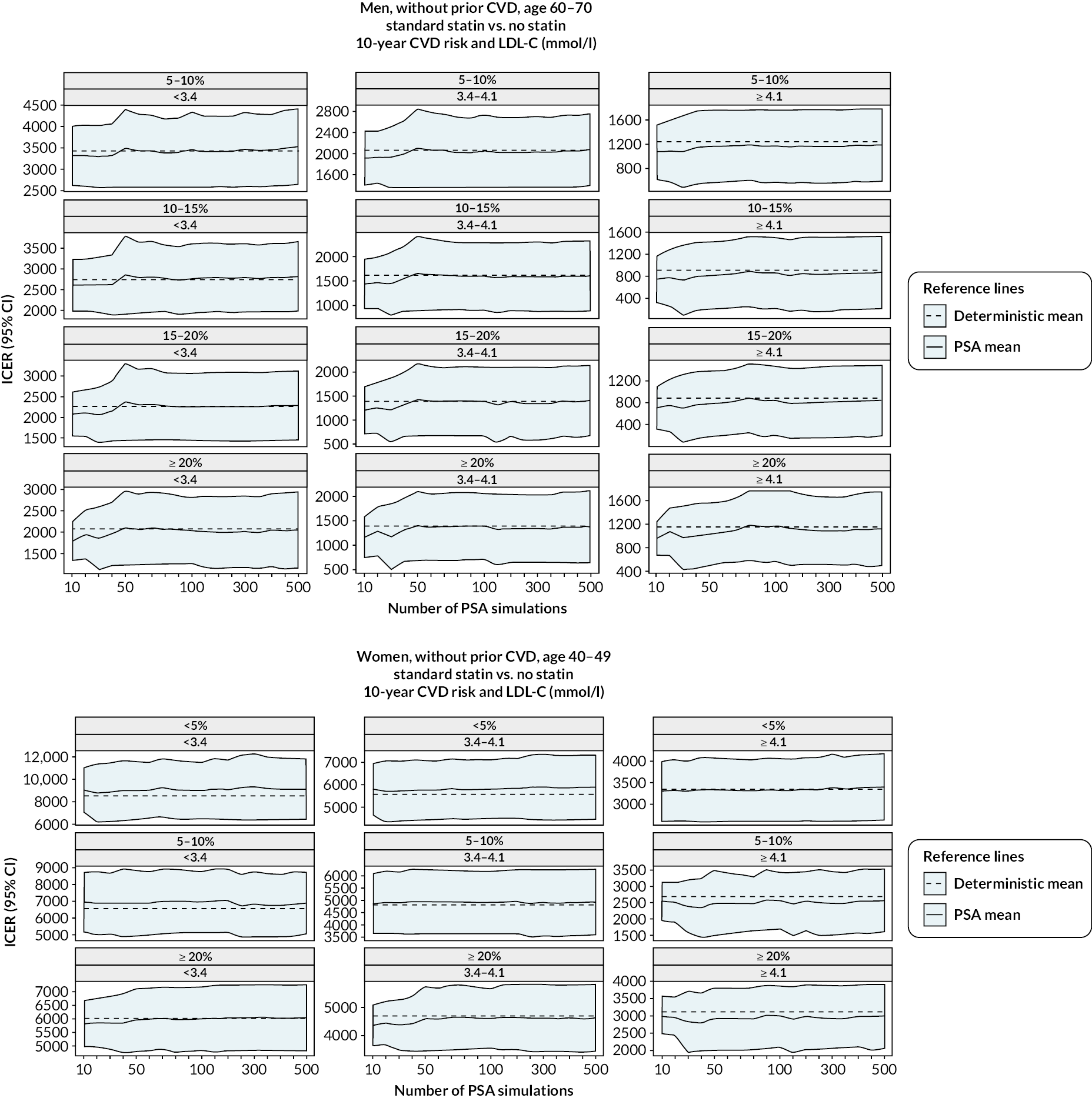
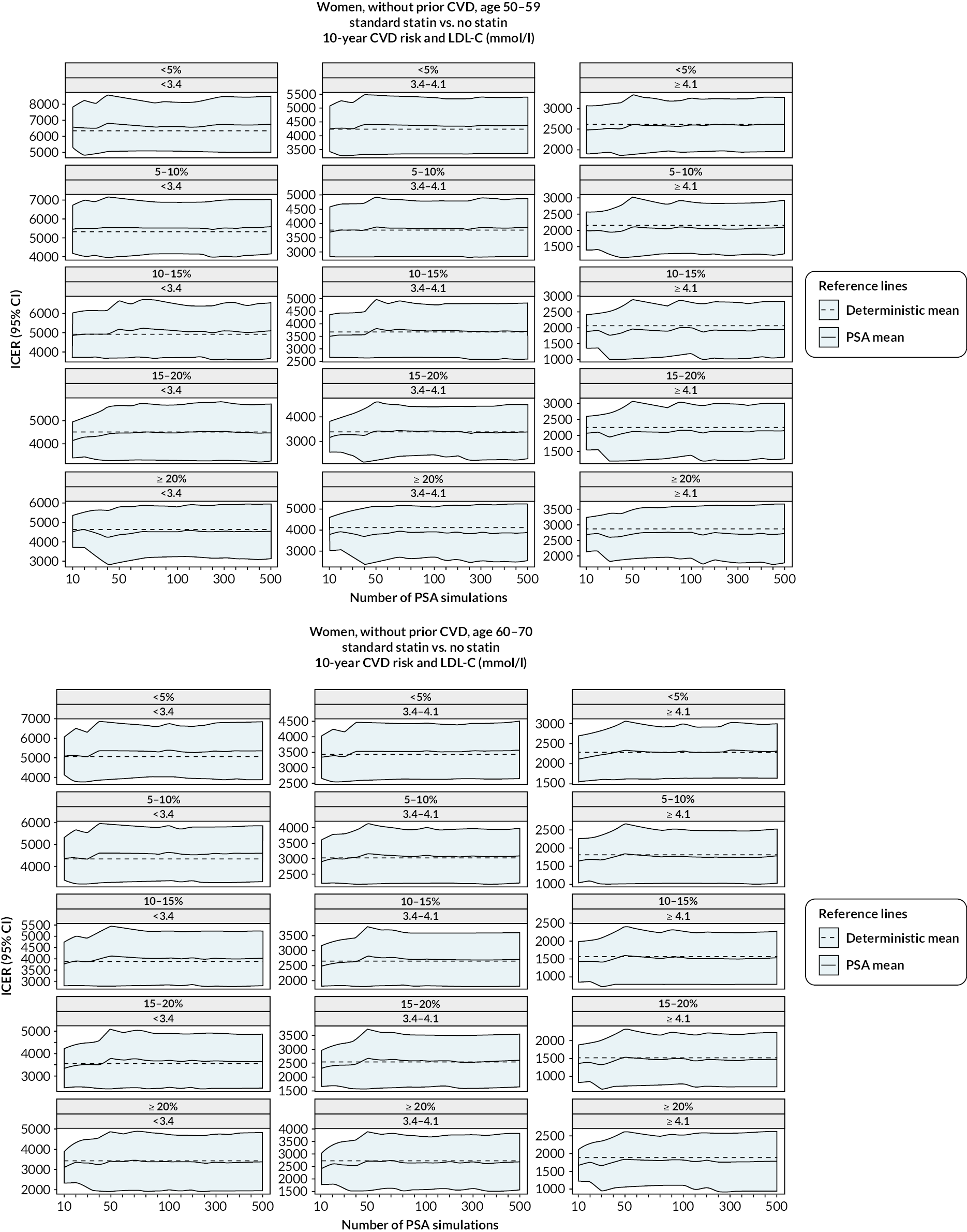
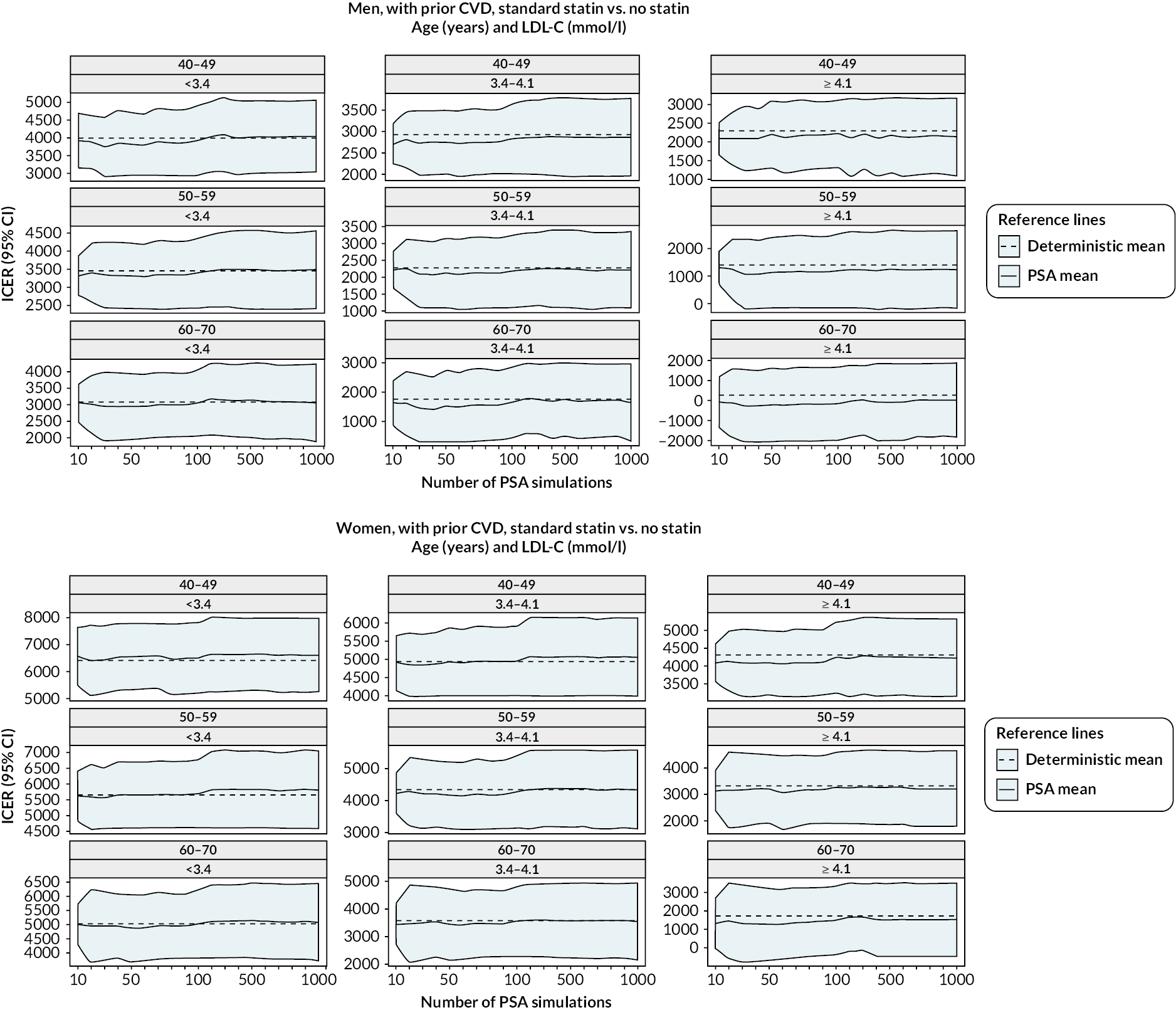
Appendix 5 Lifelong projections (without statin therapy) using the cardiovascular disease microsimulation model
| Men without CVD history | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at entry | 10-year CVD risk | Life-years | QALYs | MI | Stroke | CRV | Diabetesa | Cancera | VD | NVD |
| Number of individuals experiencing at least one event per 1000 individuals | ||||||||||
| Women without CVD history | ||||||||||
| Age at entry | 10-year CVD risk | Life years | QALYs | MI | Stroke | CRV | Diabetesa | Cancera | VD | NVD |
| Number of individuals experiencing at least one event per 1000 individuals | ||||||||||
| Men and women with CVD history | ||||||||||
| Sex | Age at entry | Life-years | QALYs | MI | Stroke | CRV | Diabetesa | Cancera | VD | NVD |
| Number of individuals experiencing at least one event per 1000 individuals | ||||||||||
| 40–49 | < 5% | 38.9 (37.3 to 40.2) | 33 (31.8 to 34) | 104 (91 to 116) | 129 (106 to 149) | 135 (121 to 147) | 130 (118 to 143) | 592 (518 to 654) | 260 (168 to 376) | 737 (623 to 826) |
| 5–10% | 35.4 (34 to 36.6) | 28.5 (27.5 to 29.4) | 167 (149 to 185) | 146 (122 to 167) | 206 (188 to 223) | 195 (179 to 209) | 581 (512 to 640) | 306 (218 to 411) | 692 (589 to 778) | |
| 10–15% | 32.6 (31.2 to 33.7) | 24.8 (23.9 to 25.5) | 235 (213 to 257) | 165 (138 to 189) | 278 (256 to 297) | 262 (243 to 278) | 543 (476 to 601) | 360 (275 to 459) | 639 (541 to 722) | |
| 15–20% | 30.7 (29.3 to 31.8) | 22.3 (21.4 to 23) | 287 (263 to 314) | 182 (151 to 209) | 330 (306 to 352) | 316 (295 to 334) | 505 (441 to 565) | 405 (318 to 502) | 594 (498 to 681) | |
| ≥ 20% | 28.5 (27.1 to 29.7) | 19.4 (18.4 to 20.2) | 356 (323 to 386) | 207 (171 to 241) | 399 (369 to 424) | 367 (344 to 388) | 447 (383 to 507) | 473 (380 to 563) | 527 (437 to 620) | |
| 50–59 | < 5% | 34.6 (33.3 to 35.7) | 29.4 (28.5 to 30.3) | 79 (69 to 88) | 137 (116 to 155) | 96 (87 to 104) | 96 (86 to 106) | 671 (611 to 718) | 219 (145 to 315) | 772 (682 to 842) |
| 5–10% | 32.1 (31 to 33.1) | 26.4 (25.6 to 27.2) | 124 (111 to 137) | 149 (128 to 168) | 150 (137 to 161) | 134 (122 to 146) | 677 (621 to 722) | 246 (175 to 336) | 746 (660 to 813) | |
| 10–15% | 29.8 (28.8 to 30.7) | 23.6 (22.9 to 24.3) | 176 (159 to 192) | 164 (142 to 183) | 207 (191 to 221) | 181 (167 to 195) | 670 (619 to 713) | 279 (210 to 364) | 715 (634 to 782) | |
| 15–20% | 28 (27 to 28.8) | 21.4 (20.7 to 21.9) | 227 (207 to 246) | 180 (156 to 201) | 261 (243 to 277) | 224 (208 to 239) | 649 (599 to 692) | 315 (250 to 396) | 681 (603 to 746) | |
| ≥ 20% | 25.1 (24.2 to 25.9) | 17.9 (17.3 to 18.5) | 306 (282 to 333) | 211 (184 to 237) | 341 (320 to 361) | 279 (261 to 295) | 596 (547 to 641) | 376 (304 to 451) | 622 (549 to 692) | |
| 60–70 | < 5% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 5–10% | 28.2 (27.3 to 29) | 23.2 (22.5 to 23.8) | 87 (78 to 97) | 158 (137 to 176) | 99 (91 to 108) | 92 (82 to 101) | 741 (700 to 775) | 204 (148 to 275) | 778 (711 to 828) | |
| 10–15% | 26.6 (25.8 to 27.4) | 21.3 (20.7 to 21.9) | 132 (119 to 145) | 169 (147 to 187) | 150 (138 to 162) | 124 (113 to 135) | 738 (700 to 770) | 233 (177 to 304) | 752 (685 to 803) | |
| 15–20% | 24.9 (24.2 to 25.6) | 19.4 (18.9 to 19.9) | 171 (156 to 186) | 182 (160 to 201) | 193 (179 to 207) | 154 (142 to 166) | 734 (697 to 765) | 259 (202 to 326) | 728 (665 to 781) | |
| ≥ 20% | 22.3 (21.6 to 22.9) | 16.5 (16 to 16.9) | 249 (228 to 270) | 210 (187 to 231) | 274 (256 to 292) | 199 (187 to 214) | 708 (672 to 738) | 308 (255 to 371) | 683 (623 to 735) | |
| 40–49 | < 5% | 42.1 (40.3 to 43.7) | 33 (31.8 to 34) | 42 (36 to 48) | 132 (108 to 153) | 46 (41 to 51) | 101 (90 to 111) | 400 (358 to 442) | 213 (119 to 343) | 766 (645 to 858) |
| 5–10% | 36.7 (35.1 to 38) | 24.6 (23.7 to 25.4) | 102 (89 to 116) | 161 (134 to 184) | 110 (97 to 122) | 245 (226 to 264) | 412 (374 to 452) | 293 (196 to 414) | 699 (583 to 793) | |
| 10–15% | 34.9 (33.2 to 36.2) | 21.4 (20.5 to 22.2) | 134 (117 to 155) | 183 (152 to 212) | 148 (130 to 164) | 313 (292 to 334) | 398 (358 to 438) | 336 (232 to 455) | 660 (545 to 760) | |
| 15–20% | 34.8 (32.9 to 36.4) | 21.1 (20 to 22) | 163 (140 to 192) | 196 (159 to 230) | 186 (164 to 208) | 372 (346 to 395) | 380 (339 to 422) | 361 (247 to 481) | 635 (517 to 745) | |
| ≥ 20% | 32.7 (30.9 to 34.3) | 19.3 (18.2 to 20.3) | 181 (153 to 210) | 215 (175 to 253) | 192 (168 to 216) | 485 (462 to 504) | 400 (356 to 444) | 364 (257 to 479) | 634 (521 to 741) | |
| 50–59 | < 5% | 37.2 (35.8 to 38.4) | 29 (28 to 29.8) | 49 (42 to 56) | 149 (128 to 168) | 49 (44 to 54) | 97 (87 to 107) | 422 (386 to 458) | 212 (130 to 319) | 729 (640 to 808) |
| 5–10% | 34.2 (32.9 to 35.3) | 24.9 (24 to 25.5) | 86 (75 to 97) | 166 (142 to 184) | 88 (79 to 97) | 167 (153 to 181) | 432 (398 to 467) | 257 (170 to 360) | 692 (609 to 770) | |
| 10–15% | 31.4 (30.2 to 32.4) | 20.8 (20.1 to 21.5) | 132 (117 to 148) | 189 (162 to 211) | 137 (123 to 152) | 249 (231 to 266) | 434 (401 to 466) | 306 (222 to 404) | 664 (581 to 741) | |
| 15–20% | 29.7 (28.4 to 30.7) | 18.4 (17.7 to 19) | 169 (148 to 196) | 212 (181 to 239) | 180 (162 to 202) | 306 (286 to 325) | 425 (392 to 458) | 344 (256 to 438) | 638 (551 to 718) | |
| ≥ 20% | 28 (26.6 to 29.1) | 16.4 (15.6 to 17) | 204 (175 to 240) | 243 (206 to 274) | 214 (190 to 243) | 353 (332 to 375) | 425 (389 to 461) | 359 (264 to 457) | 630 (539 to 717) | |
| 60–70 | < 5% | 33.4 (32.3 to 34.3) | 26.1 (25.3 to 26.8) | 40 (35 to 46) | 161 (141 to 179) | 37 (33 to 41) | 66 (59 to 75) | 427 (395 to 459) | 187 (119 to 273) | 702 (634 to 766) |
| 5–10% | 31.5 (30.5 to 32.4) | 23.6 (22.9 to 24.2) | 68 (60 to 78) | 170 (149 to 187) | 66 (59 to 72) | 112 (101 to 123) | 433 (403 to 462) | 220 (150 to 308) | 674 (608 to 735) | |
| 10–15% | 29.3 (28.3 to 30) | 21 (20.4 to 21.5) | 101 (89 to 114) | 184 (162 to 201) | 99 (90 to 108) | 155 (142 to 168) | 434 (406 to 462) | 253 (184 to 337) | 648 (584 to 705) | |
| 15–20% | 27.5 (26.6 to 28.2) | 18.8 (18.3 to 19.4) | 136 (122 to 152) | 200 (177 to 219) | 136 (124 to 149) | 197 (182 to 212) | 436 (409 to 463) | 286 (216 to 365) | 630 (569 to 688) | |
| ≥ 20% | 25.2 (24.2 to 25.9) | 16 (15.4 to 16.4) | 186 (162 to 215) | 234 (207 to 258) | 190 (171 to 213) | 240 (223 to 256) | 437 (410 to 463) | 323 (247 to 403) | 622 (558 to 681) | |
| Men | 40–49 | 30 (28.3 to 31.9) | 20.8 (19.7 to 22) | 183 (164 to 204) | 130 (114 to 149) | 178 (153 to 208) | 221 (201 to 241) | 398 (335 to 462) | 421 (293 to 552) | 579 (448 to 706) |
| 50–59 | 24.2 (23.1 to 25.5) | 15.7 (15.1 to 16.5) | 219 (202 to 240) | 156 (140 to 174) | 235 (212 to 262) | 242 (224 to 263) | 499 (446 to 548) | 366 (276 to 469) | 633 (530 to 723) | |
| 60–70 | 19.5 (18.7 to 20.4) | 12.2 (11.7 to 12.8) | 232 (214 to 254) | 175 (159 to 194) | 265 (247 to 288) | 233 (217 to 254) | 596 (556 to 633) | 317 (250 to 397) | 680 (600 to 746) | |
| Women | 40–49 | 34.9 (32.6 to 37.3) | 23.4 (22 to 24.7) | 87 (76 to 101) | 135 (116 to 160) | 49 (40 to 61) | 141 (126 to 158) | 336 (297 to 380) | 364 (217 to 517) | 634 (481 to 777) |
| 50–59 | 29.8 (28 to 31.6) | 18.2 (17.3 to 19.2) | 121 (106 to 139) | 175 (154 to 200) | 80 (68 to 95) | 194 (177 to 215) | 371 (339 to 407) | 324 (213 to 456) | 662 (530 to 773) | |
| 60–70 | 25.4 (24.1 to 26.7) | 14.7 (14 to 15.5) | 147 (128 to 170) | 210 (188 to 235) | 112 (98 to 131) | 215 (196 to 235) | 393 (366 to 421) | 294 (205 to 405) | 661 (556 to 748) | |
Appendix 6 Supplementary methods for the cost-effectiveness analyses
Integrating treatment effects of statin therapy in the cardiovascular disease microsimulation model
Statin treatment effects based on the RR per 1-mmol/l reduction in LDL-C, as reported by the CTTC meta-analysis5 informed effects of statin therapy on cardiovascular events and further meta-analyses informed effects on incident diabetes in the model. 12,13 In the base-case analysis, it was assumed that statins do not affect cancer incidence and NVD.
The transition probabilities (tp) of events in the absence of statin treatment in the model in each cycle are calculated as:
where u is the length of the cycle (i.e. 1 year), H(t–u) and H(t) are the cumulative hazards at time t–u and t, respectively.
The treatment effects of statin (tx) are calculated as:
where RR is the rate ratio per 1-mmol/l LDL-C reduction with statin and ALR is the absolute LDL-C reduction with the statin therapy, which is product of pre-treatment LDL-C level and the proportional reduction in LDL-C with corresponding statin regimen.
The transition probabilities for events with statin treatment (tptx) in each cycle of the model is calculated as:
The excess rates on myopathy and rhabdomyolysis of statin treatment were applied as constant annual rate each year on statin treatment in the model.
| Scenario | Parameters |
|---|---|
| Relative risk reduction in CVD events with statin therapy increase/decrease annually | Increase: Further 1.5% relative risk reduction per 1-mmol/l reduction of LDL-C65 added each year from year 6 onwards Decrease: Relative risk reduction reduced by 10% each year from year 6 onwards Lifetime statin use and statin costs retained in simulations |
| RR in incident cancer events with statin therapy higher or lower | Using the 95% CI boundaries reported in an IPD meta-analysis of randomised statin trials63 |
| Risk of NVD higher | 20% higher than in base-case |
| Real-world compliance with statin therapy | Using observed statin discontinuation and restarting rates for the first discontinuation and first restarting,66 the derived probabilities of complying with statin therapy (see Table 31) were applied to each individual in the respective years in model simulation. Both statin effects and costs discontinued with no statin use |
| QoL disutilities of daily statin pill | 0.001 or 0.002 QALYs were deducted in each model year |
| QoL disutilities of CVD events | 50% or 150% of base-case decrements in QoL related to CVD events were applied |
| QoL disutilities of diabetes | Apply 50% of base-case decrement in QoL related to diabetes |
| Discount rates for costs and outcomes of 1.5% | Annual discount rates to 1.5% were used for costs and QALYs (instead of the 3.5% base-case rates) |
| Include healthcare costs only for CVD and incident diabetes | Healthcare costs associated with CVD and incident diabetes only included (i.e. unrelated healthcare costs were excluded) |
| Increased cost of statin therapy | The base-case costs of statin therapy increased 1.5, 2 or 5 times |
| Vary statin treatment effect on cancer incidence | RR of 0.96 or 1.05, respectively, applied for incident cancer with statin therapy based on 95% CI of the CTTC IPD meta-analysis reporting RR of 1.00 (95% CI 0.96 to 1.05)63 |
| Reduced statin treatment effects on cardiovascular events in the elderly | Smaller relative risk reductions per 1-mmol/l-LDL-C reduction with statin therapy on cardiovascular events were applied in people from 76 years of age onward in model simulation. The applied effects based CTTC IPD meta-analysis, reporting effects only among participants > 75 years of age were (per 1-mmol/l-LDL-C reduction):5 MI: Major coronary event RR 0.82 (99% CI 0.70 to 0.96) Stroke RR 0.89 (99% CI 0.71 to 1.10) CRV RR 1.02 (99% CI 0.75 to 1.40) VD RR 0.95 (99% CI 0.83 to 1.07) |
| Year | Cumulative probability (%)66 | On statin treatment (%) | ||
|---|---|---|---|---|
| Discontinuation, % | Restarting, % | On, % | Off, % | |
| 1 | 30 | 50 | 70 | 30 |
| 2 | 38 | 59 | 77 | 23 |
| 3 | 43 | 64 | 79 | 21 |
| 4 | 47 | 68 | 80 | 20 |
| 5 | 50 | 70 | 81 | 19 |
| 6 | 52 | 72 | 81 | 19 |
| 7 | 54 | 74 | 82 | 18 |
| 8 | 56 | 76 | 82 | 18 |
| 9 | 58 | 77 | 83 | 17 |
| 10 | 60 | 79 | 83 | 17 |
Appendix 7 Cost-effectiveness of statin therapies for people 40–70 years old in the UK: base-case results
| LDL-C | Sex | Statin therapy | Age | 10-year CVD risk/with CVD | QALYs gained, discounted | Total cost (£), discounted | ICER (£/QALY), £ | Probability cost-effective (at £20,000) | Probability cost-effective (at £10,000) | Probability cost-effective (at £5000) |
|---|---|---|---|---|---|---|---|---|---|---|
| < 3.4 | Men | Standard | 40–49 | < 5 | 0.07 | 331 | 4790 | 0.61 | 0.96 | 0.60 |
| < 3.4 | Men | Standard | 40–49 | (5, 10) | 0.09 | 310 | 3420 | 0.50 | 0.89 | 0.99 |
| < 3.4 | Men | Standard | 40–49 | (10, 15) | 0.13 | 361 | 2710 | 0.10 | 0.52 | 0.98 |
| < 3.4 | Men | Standard | 40–49 | With CVD | 0.14 | 539 | 3990 | 0.05 | 0.38 | 0.93 |
| < 3.4 | Men | Standard | 50–59 | < 5 | 0.07 | 322 | 4540 | 0.29 | 0.93 | 0.76 |
| < 3.4 | Men | Standard | 50–59 | (5, 10) | 0.08 | 285 | 3440 | 0.31 | 0.84 | 1.00 |
| < 3.4 | Men | Standard | 50–59 | (10, 15) | 0.10 | 265 | 2690 | 0.31 | 0.77 | 0.99 |
| < 3.4 | Men | Standard | 50–59 | (15, 20) | 0.12 | 273 | 2280 | 0.16 | 0.55 | 0.97 |
| < 3.4 | Men | Standard | 50–59 | ≥ 20 | 0.16 | 367 | 2320 | 0.00 | 0.02 | 0.72 |
| < 3.4 | Men | Standard | 50–59 | With CVD | 0.14 | 494 | 3460 | 0.01 | 0.18 | 0.91 |
| < 3.4 | Men | Standard | 60–70 | (5, 10) | 0.08 | 277 | 3450 | 0.13 | 0.68 | 1.00 |
| < 3.4 | Men | Standard | 60–70 | (10, 15) | 0.09 | 257 | 2760 | 0.13 | 0.61 | 0.99 |
| < 3.4 | men | Standard | 60–70 | (15, 20) | 0.11 | 249 | 2310 | 0.11 | 0.46 | 0.97 |
| < 3.4 | Men | Standard | 60–70 | ≥ 20 | 0.14 | 290 | 2070 | 0.01 | 0.09 | 0.76 |
| < 3.4 | Men | Standard | 60–70 | With CVD | 0.14 | 432 | 3080 | 0.00 | 0.09 | 0.81 |
| < 3.4 | Men | Higher intensity | 40–49 | < 5 | 0.01 | 191 | 23,490 | 0.39 | 0.04 | 0.00 |
| < 3.4 | Men | Higher intensity | 40–49 | (5, 10) | 0.01 | 199 | 18,970 | 0.50 | 0.11 | 0.00 |
| < 3.4 | Men | Higher intensity | 40–49 | ≥ 10 | 0.02 | 194 | 10,300 | 0.90 | 0.48 | 0.02 |
| < 3.4 | Men | Higher intensity | 40–49 | With CVD | 0.02 | 207 | 8650 | 0.95 | 0.62 | 0.03 |
| < 3.4 | Men | Higher intensity | 50–59 | < 5 | 0.01 | 176 | 15,570 | 0.71 | 0.07 | 0.00 |
| < 3.4 | Men | Higher intensity | 50–59 | (5, 10) | 0.01 | 169 | 15,000 | 0.69 | 0.16 | 0.00 |
| < 3.4 | Men | Higher intensity | 50–59 | (10, 15) | 0.01 | 169 | 14,430 | 0.69 | 0.23 | 0.01 |
| < 3.4 | Men | Higher intensity | 50–59 | (15, 20) | 0.02 | 171 | 10,940 | 0.84 | 0.45 | 0.03 |
| < 3.4 | Men | Higher intensity | 50–59 | ≥ 20 | 0.03 | 165 | 5660 | 1.00 | 0.98 | 0.28 |
| < 3.4 | Men | Higher intensity | 50–59 | With CVD | 0.03 | 191 | 7350 | 0.99 | 0.82 | 0.09 |
| < 3.4 | Men | Higher intensity | 60–70 | (5, 10) | 0.01 | 151 | 11,310 | 0.87 | 0.32 | 0.00 |
| < 3.4 | Men | Higher intensity | 60–70 | (10, 15) | 0.01 | 148 | 10,970 | 0.87 | 0.39 | 0.01 |
| < 3.4 | Men | Higher intensity | 60–70 | (15, 20) | 0.02 | 147 | 9510 | 0.89 | 0.54 | 0.03 |
| < 3.4 | Men | Higher intensity | 60–70 | ≥ 20 | 0.02 | 146 | 6100 | 0.99 | 0.91 | 0.24 |
| < 3.4 | Men | Higher intensity | 60–70 | With CVD | 0.03 | 164 | 6290 | 1.00 | 0.91 | 0.19 |
| < 3.4 | Women | Standard | 40–49 | < 5 | 0.05 | 393 | 8530 | 0.88 | 0.82 | 0.00 |
| < 3.4 | Women | Standard | 40–49 | (5, 10) | 0.07 | 441 | 6560 | 0.85 | 0.99 | 0.02 |
| < 3.4 | Women | Standard | 40–49 | ≥10 | 0.09 | 512 | 6010 | 0.08 | 0.95 | 0.04 |
| < 3.4 | Women | Standard | 40–49 | With CVD | 0.10 | 670 | 6410 | 0.07 | 0.68 | 0.01 |
| < 3.4 | Women | Standard | 50–59 | < 5 | 0.06 | 362 | 6340 | 0.63 | 1.00 | 0.03 |
| < 3.4 | Women | Standard | 50–59 | (5, 10) | 0.07 | 378 | 5340 | 0.68 | 0.97 | 0.32 |
| < 3.4 | Women | Standard | 50–59 | (10, 15) | 0.08 | 419 | 5010 | 0.62 | 0.96 | 0.49 |
| < 3.4 | Women | Standard | 50–59 | [15,20) | 0.10 | 445 | 4330 | 0.04 | 0.65 | 0.84 |
| < 3.4 | Women | Standard | 50–59 | ≥ 20 | 0.11 | 516 | 4590 | 0.00 | 0.02 | 0.71 |
| < 3.4 | Women | Standard | 50–59 | With CVD | 0.12 | 661 | 5650 | 0.06 | 0.62 | 0.13 |
| < 3.4 | Women | Standard | 60–70 | < 5 | 0.06 | 314 | 5080 | 0.24 | 0.94 | 0.42 |
| < 3.4 | Women | Standard | 60–70 | (5, 10) | 0.07 | 319 | 4380 | 0.32 | 0.92 | 0.78 |
| < 3.4 | Women | Standard | 60–70 | (10, 15) | 0.08 | 330 | 3890 | 0.35 | 0.88 | 0.94 |
| < 3.4 | Women | Standard | 60–70 | (15, 20) | 0.1 | 351 | 3580 | 0.27 | 0.80 | 0.98 |
| < 3.4 | Women | Standard | 60–70 | ≥ 20 | 0.12 | 398 | 3440 | 0.00 | 0.14 | 0.98 |
| < 3.4 | Women | Standard | 60–70 | With CVD | 0.12 | 628 | 5030 | 0.04 | 0.55 | 0.47 |
| < 3.4 | Women | Higher intensity | 40–49 | < 5 | 0.01 | 199 | 34,290 | 0.12 | 0.00 | 0.00 |
| < 3.4 | Women | Higher intensity | 40–49 | (5, 10) | 0.01 | 240 | 47,640 | 0.15 | 0.01 | 0.00 |
| < 3.4 | Women | Higher intensity | 40–49 | ≥ 10 | 0.02 | 221 | 13,260 | 0.92 | 0.05 | 0.00 |
| < 3.4 | Women | Higher intensity | 40–49 | With CVD | 0.02 | 214 | 11,270 | 0.93 | 0.32 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | < 5 | 0.01 | 187 | 23,050 | 0.37 | 0.00 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | (5, 10) | 0.01 | 206 | 27,180 | 0.32 | 0.03 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | (10, 15) | 0.01 | 224 | 23,140 | 0.38 | 0.04 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | (15, 20) | 0.02 | 208 | 10,730 | 0.96 | 0.35 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | ≥ 20 | 0.03 | 198 | 7310 | 1.00 | 0.98 | 0.00 |
| < 3.4 | Women | Higher intensity | 50–59 | With CVD | 0.02 | 227 | 10,870 | 0.94 | 0.38 | 0.00 |
| < 3.4 | Women | Higher intensity | 60–70 | < 5 | 0.01 | 162 | 15,740 | 0.76 | 0.06 | 0.00 |
| < 3.4 | Women | Higher intensity | 60–70 | (5, 10) | 0.01 | 174 | 15,950 | 0.68 | 0.08 | 0.00 |
| < 3.4 | Women | Higher intensity | 60–70 | (10, 15) | 0.01 | 187 | 15,920 | 0.65 | 0.12 | 0.00 |
| < 3.4 | Women | Higher intensity | 60–70 | (15, 20) | 0.01 | 197 | 14,200 | 0.73 | 0.20 | 0.00 |
| < 3.4 | Women | Higher intensity | 60–70 | ≥ 20 | 0.02 | 178 | 7850 | 1.00 | 0.86 | 0.01 |
| < 3.4 | Women | Higher intensity | 60–70 | With CVD | 0.02 | 230 | 10,400 | 0.96 | 0.45 | 0.01 |
| 3.4–4.1 | Men | Standard | 40–49 | < 5 | 0.11 | 287 | 2700 | 0.20 | 0.70 | 1.00 |
| 3.4–4.1 | Men | Standard | 40–49 | (5, 10) | 0.13 | 266 | 2040 | 0.23 | 0.63 | 0.98 |
| 3.4–4.1 | Men | Standard | 40–49 | ≥ 10 | 0.19 | 328 | 1740 | 0.03 | 0.21 | 0.85 |
| 3.4–4.1 | Men | Standard | 40–49 | With CVD | 0.19 | 556 | 2930 | 0.01 | 0.10 | 0.83 |
| 3.4–4.1 | Men | Standard | 50–59 | < 5 | 0.11 | 284 | 2620 | 0.06 | 0.46 | 1.00 |
| 3.4–4.1 | Men | Standard | 50–59 | (5, 10) | 0.12 | 249 | 2010 | 0.07 | 0.37 | 0.97 |
| 3.4–4.1 | Men | Standard | 50–59 | (10, 15) | 0.14 | 233 | 1630 | 0.09 | 0.35 | 0.92 |
| 3.4–4.1 | Men | Standard | 50–59 | (15, 20) | 0.17 | 237 | 1410 | 0.07 | 0.28 | 0.84 |
| 3.4–4.1 | Men | Standard | 50–59 | ≥ 20 | 0.22 | 355 | 1620 | 0.00 | 0.01 | 0.41 |
| 3.4–4.1 | Men | Standard | 50–59 | With CVD | 0.18 | 416 | 2270 | 0.01 | 0.07 | 0.69 |
| 3.4–4.1 | Men | Standard | 60–70 | (5, 10) | 0.12 | 252 | 2080 | 0.01 | 0.12 | 0.95 |
| 3.4–4.1 | Men | Standard | 60–70 | (10, 15) | 0.14 | 227 | 1640 | 0.01 | 0.11 | 0.82 |
| 3.4–4.1 | Men | Standard | 60–70 | (15, 20) | 0.16 | 212 | 1370 | 0.01 | 0.10 | 0.70 |
| 3.4–4.1 | Men | Standard | 60–70 | ≥ 20 | 0.20 | 278 | 1410 | 0.00 | 0.02 | 0.42 |
| 3.4–4.1 | Men | Standard | 60–70 | With CVD | 0.18 | 313 | 1750 | 0.00 | 0.02 | 0.41 |
| 3.4–4.1 | Men | Higher intensity | 40–49 | < 5 | 0.02 | 188 | 12,410 | 0.80 | 0.30 | 0.00 |
| 3.4–4.1 | Men | Higher intensity | 40–49 | (5, 10) | 0.02 | 193 | 11,970 | 0.77 | 0.37 | 0.02 |
| 3.4–4.1 | Men | Higher intensity | 40–49 | ≥ 10 | 0.03 | 197 | 7060 | 0.97 | 0.79 | 0.15 |
| 3.4–4.1 | Men | Higher intensity | 40–49 | With CVD | 0.04 | 224 | 6240 | 0.99 | 0.90 | 0.17 |
| 3.4–4.1 | Men | Higher intensity | 50–59 | < 5 | 0.02 | 172 | 9800 | 0.94 | 0.54 | 0.00 |
| 3.4–4.1 | Men | Higher intensity | 50–59 | (5, 10) | 0.02 | 168 | 8820 | 0.93 | 0.63 | 0.03 |
| 3.4–4.1 | men | Higher intensity | 50–59 | (10, 15) | 0.02 | 169 | 8530 | 0.91 | 0.65 | 0.08 |
| 3.4–4.1 | Men | Higher intensity | 50–59 | (15, 20) | 0.02 | 172 | 7470 | 0.93 | 0.72 | 0.16 |
| 3.4–4.1 | Men | Higher intensity | 50–59 | ≥ 20 | 0.04 | 179 | 4490 | 1.00 | 0.99 | 0.59 |
| 3.4–4.1 | Men | Higher intensity | 50–59 | With CVD | 0.03 | 191 | 5930 | 1.00 | 0.93 | 0.31 |
| 3.4–4.1 | Men | Higher intensity | 60–70 | (5, 10) | 0.02 | 150 | 7270 | 0.99 | 0.88 | 0.05 |
| 3.4–4.1 | Men | Higher intensity | 60–70 | (10, 15) | 0.02 | 146 | 6440 | 0.99 | 0.89 | 0.18 |
| 3.4–4.1 | Men | Higher intensity | 60–70 | (15, 20) | 0.02 | 144 | 5820 | 0.99 | 0.90 | 0.30 |
| 3.4–4.1 | Men | Higher intensity | 60–70 | ≥ 20 | 0.03 | 151 | 4520 | 1.00 | 0.98 | 0.58 |
| 3.4–4.1 | Men | Higher intensity | 60–70 | With CVD | 0.03 | 146 | 4510 | 1.00 | 0.98 | 0.59 |
| 3.4–4.1 | Women | Standard | 40–49 | < 5 | 0.07 | 398 | 5580 | 0.58 | 0.98 | 0.20 |
| 3.4–4.1 | Women | Standard | 40–49 | (5, 10) | 0.09 | 455 | 4840 | 0.71 | 0.96 | 0.57 |
| 3.4–4.1 | Women | Standard | 40–49 | ≥ 10 | 0.13 | 603 | 4650 | 0.15 | 0.83 | 0.68 |
| 3.4–4.1 | Women | Standard | 40–49 | With CVD | 0.14 | 668 | 4930 | 0.04 | 0.46 | 0.54 |
| 3.4–4.1 | Women | Standard | 50–59 | < 5 | 0.08 | 359 | 4250 | 0.23 | 0.89 | 0.90 |
| 3.4–4.1 | Women | Standard | 50–59 | (5, 10) | 0.10 | 377 | 3770 | 0.35 | 0.89 | 0.99 |
| 3.4–4.1 | Women | Standard | 50–59 | (10, 15) | 0.12 | 435 | 3670 | 0.41 | 0.87 | 0.99 |
| 3.4–4.1 | Women | Standard | 50–59 | (15, 20) | 0.14 | 485 | 3500 | 0.11 | 0.61 | 0.99 |
| 3.4–4.1 | Women | Standard | 50–59 | ≥ 20 | 0.16 | 618 | 3810 | 0.00 | 0.05 | 0.96 |
| 3.4–4.1 | Women | Standard | 50–59 | With CVD | 0.15 | 631 | 4340 | 0.04 | 0.43 | 0.84 |
| 3.4–4.1 | Women | Standard | 60–70 | < 5 | 0.09 | 314 | 3430 | 0.02 | 0.47 | 1.00 |
| 3.4–4.1 | Women | Standard | 60–70 | (5, 10) | 0.1 | 313 | 3030 | 0.09 | 0.57 | 1.00 |
| 3.4–4.1 | Women | Standard | 60–70 | (10, 15) | 0.12 | 320 | 2670 | 0.11 | 0.54 | 0.99 |
| 3.4–4.1 | Women | Standard | 60–70 | (15, 20) | 0.14 | 351 | 2560 | 0.11 | 0.51 | 0.98 |
| 3.4–4.1 | Women | Standard | 60–70 | ≥ 20 | 0.16 | 437 | 2690 | 0.00 | 0.14 | 0.93 |
| 3.4–4.1 | Women | Standard | 60–70 | With CVD | 0.15 | 546 | 3570 | 0.01 | 0.26 | 0.92 |
| 3.4–4.1 | Women | Higher intensity | 40–49 | < 5 | 0.01 | 213 | 22,170 | 0.42 | 0.02 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 40–49 | (5, 10) | 0.01 | 254 | 32,660 | 0.29 | 0.04 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 40–49 | ≥ 10 | 0.02 | 272 | 12,760 | 0.85 | 0.17 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 40–49 | With CVD | 0.02 | 231 | 9380 | 0.96 | 0.54 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | < 5 | 0.01 | 194 | 14,560 | 0.77 | 0.11 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | (5, 10) | 0.01 | 216 | 16,630 | 0.65 | 0.11 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | (10, 15) | 0.01 | 240 | 17,260 | 0.59 | 0.13 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | (15, 20) | 0.02 | 245 | 10,750 | 0.89 | 0.39 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | ≥ 20 | 0.03 | 228 | 7020 | 1.00 | 0.95 | 0.02 |
| 3.4–4.1 | Women | Higher intensity | 50–59 | With CVD | 0.03 | 233 | 9230 | 0.97 | 0.58 | 0.01 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | < 5 | 0.02 | 166 | 9620 | 0.98 | 0.53 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | (5, 10) | 0.02 | 178 | 10,580 | 0.91 | 0.43 | 0.00 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | (10, 15) | 0.02 | 191 | 10,220 | 0.89 | 0.46 | 0.01 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | (15, 20) | 0.02 | 205 | 10,080 | 0.89 | 0.49 | 0.02 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | ≥ 20 | 0.03 | 205 | 7090 | 1.00 | 0.86 | 0.07 |
| 3.4–4.1 | Women | Higher intensity | 60–70 | With CVD | 0.03 | 214 | 7920 | 0.99 | 0.74 | 0.06 |
| ≥ 4.1 | Men | Standard | 40–49 | < 5 | 0.16 | 228 | 1400 | 0.03 | 0.23 | 0.90 |
| ≥ 4.1 | Men | Standard | 40–49 | (5, 10) | 0.21 | 190 | 900 | 0.02 | 0.12 | 0.67 |
| ≥ 4.1 | Men | Standard | 40–49 | ≥ 10 | 0.37 | 353 | 960 | 0.00 | 0.00 | 0.10 |
| ≥ 4.1 | Men | Standard | 40–49 | With CVD | 0.31 | 717 | 2290 | 0.00 | 0.02 | 0.53 |
| ≥ 4.1 | Men | Standard | 50–59 | < 5 | 0.16 | 254 | 1620 | 0.01 | 0.09 | 0.86 |
| ≥ 4.1 | Men | Standard | 50–59 | (5, 10) | 0.19 | 196 | 1050 | 0.00 | 0.05 | 0.62 |
| ≥ 4.1 | Men | Standard | 50–59 | (10, 15) | 0.23 | 178 | 790 | 0.00 | 0.03 | 0.44 |
| ≥ 4.1 | Men | Standard | 50–59 | (15, 20) | 0.27 | 188 | 700 | 0.00 | 0.02 | 0.26 |
| ≥ 4.1 | Men | Standard | 50–59 | ≥ 20 | 0.44 | 491 | 1110 | 0.00 | 0.00 | 0.01 |
| ≥ 4.1 | Men | Standard | 50–59 | With CVD | 0.27 | 381 | 1390 | 0.00 | 0.00 | 0.26 |
| ≥ 4.1 | Men | Standard | 60–70 | (5, 10) | 0.18 | 265 | 1480 | 0.00 | 0.02 | 0.35 |
| ≥ 4.1 | Men | Standard | 60–70 | (10, 15) | 0.20 | 199 | 970 | 0.00 | 0.00 | 0.20 |
| ≥ 4.1 | Men | Standard | 60–70 | (15, 20) | 0.23 | 198 | 840 | 0.00 | 0.00 | 0.14 |
| ≥ 4.1 | Men | Standard | 60–70 | ≥ 20 | 0.37 | 409 | 1110 | 0.00 | 0.00 | 0.01 |
| ≥ 4.1 | Men | Standard | 60–70 | With CVD | 0.23 | 66 | 280 | 0.00 | 0.00 | 0.06 |
| ≥ 4.1 | Men | Higher intensity | 40–49 | < 5 | 0.02 | 184 | 7530 | 0.97 | 0.77 | 0.10 |
| ≥ 4.1 | Men | Higher intensity | 40–49 | (5, 10) | 0.03 | 184 | 5850 | 0.98 | 0.88 | 0.33 |
| ≥ 4.1 | Men | Higher intensity | 40–49 | ≥ 10 | 0.06 | 211 | 3450 | 1.00 | 1.00 | 0.90 |
| ≥ 4.1 | Men | Higher intensity | 40–49 | With CVD | 0.06 | 279 | 4920 | 1.00 | 0.99 | 0.47 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | < 5 | 0.03 | 170 | 6330 | 0.99 | 0.91 | 0.14 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | (5, 10) | 0.03 | 163 | 5410 | 1.00 | 0.95 | 0.38 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | (10, 15) | 0.04 | 164 | 4680 | 1.00 | 0.97 | 0.56 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | (15, 20) | 0.04 | 164 | 3930 | 1.00 | 0.98 | 0.74 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | ≥ 20 | 0.08 | 208 | 2690 | 1.00 | 1.00 | 0.99 |
| ≥ 4.1 | Men | Higher intensity | 50–59 | With CVD | 0.05 | 197 | 4010 | 1.00 | 1.00 | 0.74 |
| ≥ 4.1 | Men | Higher intensity | 60–70 | (5, 10) | 0.03 | 155 | 4700 | 1.00 | 0.98 | 0.65 |
| ≥ 4.1 | Men | Higher intensity | 60–70 | (10, 15) | 0.04 | 143 | 4000 | 1.00 | 1.00 | 0.80 |
| ≥ 4.1 | Men | Higher intensity | 60–70 | (15, 20) | 0.04 | 141 | 3620 | 1.00 | 1.00 | 0.86 |
| ≥ 4.1 | Men | Higher intensity | 60–70 | ≥ 20 | 0.06 | 175 | 2730 | 1.00 | 1.00 | 0.99 |
| ≥ 4.1 | Men | Higher intensity | 60–70 | With CVD | 0.04 | 110 | 2610 | 1.00 | 1.00 | 0.94 |
| ≥ 4.1 | Women | Standard | 40–49 | < 5 | 0.11 | 384 | 3480 | 0.25 | 0.85 | 1.00 |
| ≥ 4.1 | Women | Standard | 40–49 | (5, 10) | 0.16 | 445 | 2720 | 0.25 | 0.73 | 1.00 |
| ≥ 4.1 | Women | Standard | 40–49 | ≥ 10 | 0.28 | 716 | 2560 | 0.00 | 0.01 | 0.86 |
| ≥ 4.1 | Women | Standard | 40–49 | With CVD | 0.20 | 881 | 4320 | 0.01 | 0.31 | 0.90 |
| ≥ 4.1 | Women | Standard | 50–59 | < 5 | 0.13 | 346 | 2730 | 0.04 | 0.43 | 1.00 |
| ≥ 4.1 | Women | Standard | 50–59 | (5, 10) | 0.16 | 355 | 2230 | 0.07 | 0.42 | 0.98 |
| ≥ 4.1 | Women | Standard | 50–59 | (10, 15) | 0.20 | 420 | 2060 | 0.04 | 0.34 | 0.94 |
| ≥ 4.1 | Women | Standard | 50–59 | (15, 20) | 0.26 | 507 | 1970 | 0.01 | 0.14 | 0.88 |
| ≥ 4.1 | Women | Standard | 50–59 | ≥ 20 | 0.37 | 825 | 2230 | 0.00 | 0.00 | 0.13 |
| ≥ 4.1 | Women | Standard | 50–59 | With CVD | 0.20 | 659 | 3320 | 0.02 | 0.27 | 0.94 |
| ≥ 4.1 | Women | Standard | 60–70 | < 5 | 0.13 | 303 | 2330 | 0.01 | 0.09 | 0.97 |
| ≥ 4.1 | Women | Standard | 60–70 | (5, 10) | 0.16 | 297 | 1920 | 0.00 | 0.11 | 0.92 |
| ≥ 4.1 | Women | Standard | 60–70 | (10, 15) | 0.18 | 297 | 1610 | 0.00 | 0.11 | 0.83 |
| ≥ 4.1 | Women | Standard | 60–70 | (15, 20) | 0.22 | 330 | 1480 | 0.00 | 0.06 | 0.70 |
| ≥ 4.1 | Women | Standard | 60–70 | ≥ 20 | 0.31 | 525 | 1670 | 0.00 | 0.00 | 0.29 |
| ≥ 4.1 | Women | Standard | 60–70 | With CVD | 0.19 | 336 | 1730 | 0.01 | 0.09 | 0.67 |
| ≥ 4.1 | Women | Higher intensity | 40–49 | < 5 | 0.02 | 226 | 14,160 | 0.75 | 0.15 | 0.00 |
| ≥ 4.1 | Women | Higher intensity | 40–49 | (5, 10) | 0.02 | 267 | 12,870 | 0.75 | 0.27 | 0.00 |
| ≥ 4.1 | Women | Higher intensity | 40–49 | ≥ 10 | 0.05 | 291 | 6040 | 1.00 | 0.99 | 0.14 |
| ≥ 4.1 | Women | Higher intensity | 40–49 | With CVD | 0.04 | 309 | 8770 | 0.99 | 0.69 | 0.01 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | < 5 | 0.02 | 200 | 9730 | 0.96 | 0.57 | 0.00 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | (5, 10) | 0.02 | 222 | 9410 | 0.93 | 0.58 | 0.02 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | (10, 15) | 0.03 | 255 | 8460 | 0.96 | 0.66 | 0.06 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | (15, 20) | 0.04 | 277 | 6970 | 0.99 | 0.86 | 0.12 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | ≥ 20 | 0.07 | 293 | 4220 | 1.00 | 1.00 | 0.87 |
| ≥ 4.1 | Women | Higher intensity | 50–59 | With CVD | 0.03 | 260 | 7960 | 0.98 | 0.73 | 0.05 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | < 5 | 0.02 | 172 | 6910 | 0.99 | 0.91 | 0.03 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | (5, 10) | 0.03 | 182 | 6750 | 1.00 | 0.89 | 0.08 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | (10, 15) | 0.03 | 196 | 6420 | 1.00 | 0.89 | 0.17 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | (15, 20) | 0.04 | 211 | 5800 | 1.00 | 0.94 | 0.30 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | ≥ 20 | 0.06 | 240 | 4360 | 1.00 | 1.00 | 0.71 |
| ≥ 4.1 | Women | Higher intensity | 60–70 | With CVD | 0.03 | 182 | 5760 | 1.00 | 0.91 | 0.33 |
Appendix 8 Cost-effectiveness of statin therapies for older people in the UK
| Sex | Statin therapy | LDL-C | QALYs gained, discounted | Total cost (£), discounted, £ | ICER (£/QALY), £ | Probability cost-effective (at £20,000) | Probability cost-effective (at £10,000) | Probability cost-effective (at £5000) |
|---|---|---|---|---|---|---|---|---|
| Men | Standard | < 3.4 | 0.12 | 365 | 2940 | 0.01 | 0.18 | 0.95 |
| Men | Standard | 3.4–4.1 | 0.20 | 347 | 1760 | 0.00 | 0.00 | 0.20 |
| Men | Standard | ≥ 4.1 | 0.35 | 574 | 1650 | 0.00 | 0.00 | 0.01 |
| Men | Standard | With CVD | 0.18 | 21 | 120 | 0.00 | 0.00 | 0.03 |
| Women | Standard | < 3.4 | 0.10 | 355 | 3500 | 0.07 | 0.60 | 0.98 |
| Women | Standard | 3.4–4.1 | 0.14 | 328 | 2280 | 0.00 | 0.02 | 0.83 |
| Women | Standard | ≥ 4.1 | 0.22 | 394 | 1780 | 0.00 | 0.00 | 0.47 |
| Women | Standard | With CVD | 0.16 | 282 | 1720 | 0.00 | 0.03 | 0.54 |
| Men | Higher intensity | < 3.4 | 0.02 | 162 | 7430 | 0.99 | 0.82 | 0.06 |
| Men | Higher intensity | 3.4–4.1 | 0.04 | 154 | 4180 | 1.00 | 1.00 | 0.80 |
| Men | Higher intensity | ≥ 4.1 | 0.06 | 204 | 3180 | 1.00 | 1.00 | 0.99 |
| Men | Higher intensity | With CVD | 0.03 | 76 | 2210 | 1.00 | 1.00 | 0.97 |
| Women | Higher intensity | < 3.4 | 0.02 | 185 | 11,780 | 0.93 | 0.40 | 0.00 |
| Women | Higher intensity | 3.4–4.1 | 0.03 | 165 | 5860 | 1.00 | 0.98 | 0.17 |
| Women | Higher intensity | ≥ 4.1 | 0.04 | 201 | 4950 | 1.00 | 1.00 | 0.53 |
| Women | Higher intensity | With CVD | 0.03 | 149 | 5080 | 1.00 | 0.97 | 0.46 |
| Sex | Statin therapy | LDL-C | Key scenario analysis | Further scenario analysis for people without CVD at statin initiation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICER (£/QALY), £ | Probability cost-effective (at £20,000) | Probability cost-effective (at £10,000) | Probability cost-effective (at £5000) | ICER (£/QALY), £ | Probability cost-effective (at £20,000) | Probability cost-effective (at £10,000) | Probability cost-effective (at £5000) | |||
| Men | Standard | < 3.4 | 4120 | 0.27 | 0.60 | 0.68 | 7870 | 0.47 | 0.50 | 0.29 |
| Men | Standard | 3.4–4.1 | 2600 | 0.07 | 0.23 | 0.70 | 4510 | 0.32 | 0.43 | 0.48 |
| Men | Standard | ≥ 4.1 | 2150 | 0.02 | 0.07 | 0.48 | 3060 | 0.22 | 0.33 | 0.51 |
| Men | Standard | With CVD | 2310 | 0.05 | 0.17 | 0.46 | ||||
| Women | Standard | < 3.4 | 5050 | 0.46 | 0.71 | 0.47 | 10,420 | 0.48 | 0.45 | 0.22 |
| Women | Standard | 3.4–4.1 | 3280 | 0.16 | 0.42 | 0.72 | 6140 | 0.34 | 0.46 | 0.41 |
| Women | Standard | ≥ 4.1 | 2590 | 0.10 | 0.30 | 0.78 | 4300 | 0.33 | 0.48 | 0.51 |
| Women | Standard | With CVD | 3080 | 0.15 | 0.40 | 0.54 | ||||
| Men | Higher intensity | < 3.4 | 11,450 | 0.72 | 0.35 | 0.01 | 89,550 | 0.24 | 0.07 | 0.00 |
| Men | Higher intensity | 3.4–4.1 | 6360 | 0.92 | 0.77 | 0.23 | 18,350 | 0.47 | 0.28 | 0.04 |
| Men | Higher intensity | ≥ 4.1 | 4610 | 0.98 | 0.93 | 0.51 | 9620 | 0.62 | 0.46 | 0.16 |
| Men | Higher intensity | With CVD | 5220 | 0.95 | 0.83 | 0.40 | ||||
| Women | Higher intensity | < 3.4 | 20,770 | 0.48 | 0.14 | 0.00 | 2,088,810 | 0.16 | 0.03 | – |
| Women | Higher intensity | 3.4–4.1 | 8940 | 0.82 | 0.51 | 0.04 | 26,040 | 0.40 | 0.16 | 0.01 |
| Women | Higher intensity | ≥ 4.1 | 7190 | 0.89 | 0.68 | 0.13 | 19,540 | 0.45 | 0.23 | 0.02 |
| Women | Higher intensity | With CVD | 8360 | 0.84 | 0.56 | 0.19 | ||||
List of abbreviations
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- BMI
- body mass index
- BP
- blood pressure
- CHD
- coronary heart disease
- CKD
- chronic kidney disease
- CRV
- coronary revascularisation
- CTTC
- Cholesterol Treatment Trialists’ Collaboration
- CVD
- cardiovascular disease
- EQ-5D
- EuroQoL-5 Dimensions
- GLM
- generalised linear model
- HbA1c
- glycated haemoglobin
- HDL-C
- high-density lipoprotein cholesterol
- HRQoL
- health-related quality of life
- HSE
- Health Survey for England
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IPAQ
- International Physical Activity Questionnaire
- IPD
- individual participant data
- LDL-C
- low-density lipoprotein cholesterol
- MET
- Metabolic Equivalent Task
- MI
- myocardial infarction
- NA
- not applicable
- NICE
- National Institute for Health and Care Excellence
- NVD
- nonvascular death
- OxPop
- Oxford Population Health
- PAD
- peripheral arterial disease
- PH
- proportional hazard
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- UKB
- UK Biobank
- VD
- vascular death
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/KDAP7034).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
