Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR127569. The contractual start date was in December 2019. The draft manuscript began editorial review in December 2022 and was accepted for publication in February 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Black et al. This work was produced by Black et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Black et al.
Chapter 1 Introduction
Background
Induction of labour (IOL) is the most common obstetric intervention offered to women when risks of continuing the pregnancy are thought to outweigh risks of birth. Rates of IOL in the UK were above 40% in the 1970s, but halved over the next decade, before increasing again from the late 1990s. 1 Current IOL rates mean that 33% of pregnant women in England have their labour induced, compared with 21% 10 years ago. 2 Elective IOL at term, when compared with expectant management of pregnancy, reduces caesarean birth and maternal hypertensive disease, as well as being associated with a reduction in perinatal mortality and maternal complications. 3–5 It thus seems likely that demand for IOL will continue. Maternity services are struggling to accommodate increasing rates of IOL. 6 Although IOL (compared with expectant management) reduces overall hospital stay, it increases the amount of time spent on antenatal wards and on labour wards, with a major impact on maternity resources and staffing, and on women’s experience of labour. 3,7–9
Cervical ripening is a key component of IOL, whereby application of a drug or mechanical method over several hours, causes softening, shortening and opening of the cervix in preparation for labour. 10 Cervical ripening may itself initiate labour but is often followed by artificial rupture of membranes (ARM) with or without intravenous infusion of oxytocin (both inpatient procedures). National Institute for Health and Care Excellence (NICE) guidance recommends that all women having IOL have prior cervical ripening, unless there is a contraindication. 11
Traditionally, all cases of cervical ripening have been performed in hospital, to allow monitoring of maternal and fetal well-being and recognition of complications such as uterine hyperstimulation (frequent/sustained contractions that increase the risk of hypoxic birth injury; incidence 2–3%). 12 More recently, however, a rising number of maternity units give women the option of home cervical ripening. Home cervical ripening means that women attend hospital for initial assessment and administration of cervical ripening agent before returning home (to their own home or that of a friend/relative/birth partner) for a period of time (usually 24 hours), before reassessment in hospital. This arrangement has the potential to reduce women’s overall hospital stay during IOL, thus reducing costs to health service providers. However, the safety and acceptability of home cervical ripening has not been fully evaluated. Any NHS cost savings could potentially be offset by increased costs of any additional morbidity resulting from home cervical ripening; costs to parents may be increased and acceptability of home cervical ripening is not fully understood. Health services need to balance the full resource impact of IOL with the need to provide safe and acceptable care.
In the CHOICE study, we addressed the question ‘Is it safe, effective, cost-effective and acceptable to women to carry out home cervical ripening during induction of labour (IOL)?’ We also performed descriptive analyses of the process and outcomes of IOL. These analyses will provide information to help women and their caregivers make informed decisions around when and how to have IOL.
Rationale and justification for study
As the rate of IOL is increasing, home cervical ripening may provide opportunities to reduce the burden on the NHS. However, there are evidence gaps in whether home cervical ripening is safe, acceptable to women, reduces hospital stay and is cost-effective. NICE identified the need to assess the safety, efficacy and clinical and cost-effectiveness of outpatient and inpatient IOL in the UK setting, considering women’s views. 11 Maternity service users have identified IOL as an important research topic and women have reported specific negative experiences such as increased pain and anxiety and lack of support, which may be alleviated by home cervical ripening. 9,13
Home cervical ripening has the potential to reduce separation of women from their families and increase choice regarding the timing and setting for labour and delivery. Existing evidence suggest that home cervical ripening is feasible and adverse outcomes appear to be rare, but trials have been underpowered to confirm safety. 14 Importantly, studies have not confirmed anticipated reductions in length of hospital stay or cost-effectiveness. 4,15 However, no studies have investigated the acceptability to women or their families, or whether choice is increased in a UK setting, apart from a small feasibility study conducted by some of the investigators in qCHOICE. 9 This study will provide much needed evidence on women’s and partners experiences of home cervical ripening, IOL and costs from the service user perspectives.
Despite the lack of evidence on home cervical ripening, the practice is becoming increasingly common in UK practice. In preparation for this study (August 2018), we obtained information on IOL policies from 128/167 (77%) obstetric units in Scotland and England and found that 54% (69 of 128) of units offered, or would shortly start to offer, home cervical ripening. This was a large and rapid increase – a 2014 survey found only 17% of UK maternity units offered home cervical ripening. 16
There is variation in the population of women offered home cervical ripening between hospitals. However, most units only offer home cervical ripening to women with ‘low risk’ pregnancies (i.e. women with uncomplicated pregnancies). Most units that offered home cervical ripening in 2018 (> 90%) used topical prostaglandin applied intravaginally as a slow-release pessary of 10 mg dinoprostone, which stays in place for 24 hours. This was in line with NICE guidance, which recommended prostaglandin as the primary method of IOL for all women. 11 Balloon catheters, which involve inserting either the balloon from a foley catheter or a specially designed cervical ripening catheter into the cervix and inflating it with saline to mechanically open the cervix, have also been shown to be effective. 11 Compared with prostaglandin, balloon catheters have a lower incidence of uterine hyperstimulation (2% vs. 3%) and operative delivery indicated by fetal heart rate abnormalities (12% vs. 18%). 12 However, they may be less acceptable to women. 17 Whereas in 2014 no units offered home cervical ripening with balloon catheters, our survey suggested that at least six UK units currently, or soon would, offer balloon catheters as the primary method of home cervical ripening. 16 Other methods of cervical ripening are not currently used at home. Oral misoprostol has high rates of uterine hyperstimulation and is not used outside hospitals in the UK. 18 Osmotic dilators (an alternative mechanical method) are under evaluation in hospitals (SOLVE trial; ISRCTN20131893) but have not yet been shown to be effective or established in UK practice.
The aim of the overall CHOICE study was to assess the safety, clinical effectiveness, cost-effectiveness and acceptability of home cervical ripening. This was to be achieved by performing a prospective multicentre observational cohort study, using real-world data obtained from hospital electronic health records (CHOICE prospective observational cohort study) linked with a process evaluation using a questionnaire-based survey and nested case studies (qCHOICE) with economic evaluation.
Chapter 2 Observational cohort study
Observational cohort study objectives/research questions
This study addressed the research question:
Is home cervical ripening:
-
as safe as in-hospital cervical ripening in terms of neonatal unit (NNU) admission (primary outcome) and other secondary outcomes of maternal and neonatal morbidity?
-
effective in reducing the amount of time women spend in hospital during the IOL process?
-
cost-effective from the NHS perspective?
Preplanned primary comparison
The planned primary comparison was home dinoprostone (a prostaglandin) cervical ripening (exposure) versus in-hospital dinoprostone cervical ripening (comparison) from 39 weeks of gestation. A secondary exploratory comparison was planned to be undertaken to explore home cervical ripening with balloon catheter (exposure) versus home cervical ripening with dinoprostone (comparator).
Additional supplementary research questions to be applied to the cohort study, should numbers suffice, were:
-
How do IOL rates, methods and outcomes vary across the UK?
-
What are the outcomes of IOL in different subgroups of women (e.g. women with multiple pregnancy, women with IOL after a previous caesarean section) and at each week of gestation (37, 38, 39, 40 and 41+ weeks)?
-
Can we predict which women will have caesarean section after IOL?
However, these supplementary questions are not addressed in this report.
Revised primary comparison
Following preplanned pilot analysis at 156 site months of data collection, the primary comparison was changed to ensure feasibility and to reflect current practice. The revised primary comparison became ‘Home balloon cervical ripening (exposure) versus in-hospital prostaglandin cervical ripening (comparator) from 37 weeks gestation’.
Throughout the report, these main two comparator groups are referred to as ‘home balloon’ and ‘hospital prostaglandin’, respectively.
Observational cohort study methods
Study design as planned at study outset
We planned to perform a prospective multicentre observational cohort study with an internal pilot phase. 19 We set out to obtain data from electronic health records from at least 14 maternity units offering only in-hospital cervical ripening and 12 offering dinoprostone home cervical ripening. The expected sample size was 8533 women with singleton pregnancies undergoing IOL at 39 + 0 weeks’ gestation or more. To achieve this and to contextualise our findings, we planned to collect data relating to a cohort of approximately 41,000 women undergoing IOL after 37 weeks. We planned to use mixed-effects logistic regression for the non-inferiority comparison of NNU admission and propensity score matched adjustment to control for treatment indication bias.
A secondary exploratory comparison was planned if sufficient cases allowed: home cervical ripening with balloon catheter (exposure) versus home cervical ripening with dinoprostone (comparator), to explore whether there are any indications of different safety profiles of these two methods of home cervical ripening.
Changes to study design
Results of the planned interim analysis after 156 site months of data led to a change in study design and expected sample size. Three key changes were made:
-
Data would be obtained from a total of 26 NHS maternity units, of which 18 offered only in-hospital cervical ripening (predominantly with prostaglandin) and 8 offered home cervical ripening using balloon catheters, meaning that around 25,000 women would be in the initial data extract from which the analysis sample would be drawn.
-
Instead of including a population of women from 39 weeks’ gestation onwards, women were included if they underwent IOL at 37 completed weeks of gestation onwards.
-
Instead of comparing home cervical ripening using dinoprostone with in-hospital cervical ripening using dinoprostone, home cervical ripening with balloon would be compared with in-hospital cervical ripening using any prostaglandin.
-
Planned analysis would use multivariable logistic regression without propensity score matching for the primary outcome. All other outcomes would be reported using descriptive statistics.
Justification of change in study design
It was recognised following the pilot analysis that it was not feasible to answer the original research question relating to safety of home cervical ripening using dinoprostone, as dinoprostone was so infrequently used in home settings. If the number of home cervical ripening cases using dinoprostone had continued to accrue at the same rate, it was calculated that it would take over 10 years to reach the planned sample size. Instead, it was recognised that as balloon cervical ripening had become the dominant method in home settings, a pragmatic research question would ask what the difference in safety (and cost) is between home cervical ripening using balloon and in-hospital cervical ripening using any prostaglandin.
It was also recognised that home cervical ripening using balloon was being used for multiple indications from 37 weeks’ gestation and was not limited to post-dates IOL. Thus, it was considered relevant to include women from 37 completed weeks of gestation in the primary analysis.
To complete the study within the funded period, it was decided to cease data collection on 30 June 2022, 1 month earlier than originally planned. This decision was made to maximise time available for data analysis within funded study resources and to ensure that the maximum descriptive analyses could be performed to provide details of the context in which the study was set. This was considered particularly relevant given the perceived practice changes that had occurred during the study period, including those changes in setting related to the COVID-19 pandemic, increased IOL rates, wide variation in indications for IOL, substantial delays during IOL and earlier gestation at onset of IOL.
Participants
Data on all women having IOL at 37 + 0 weeks’ gestation or more were extracted. Women who opted out of data provision were excluded from the data set by the BadgerNet (System C, Stratford-upon-Avon, UK) maternity system analysts before data were extracted, so the number of such women is not known to the research team.
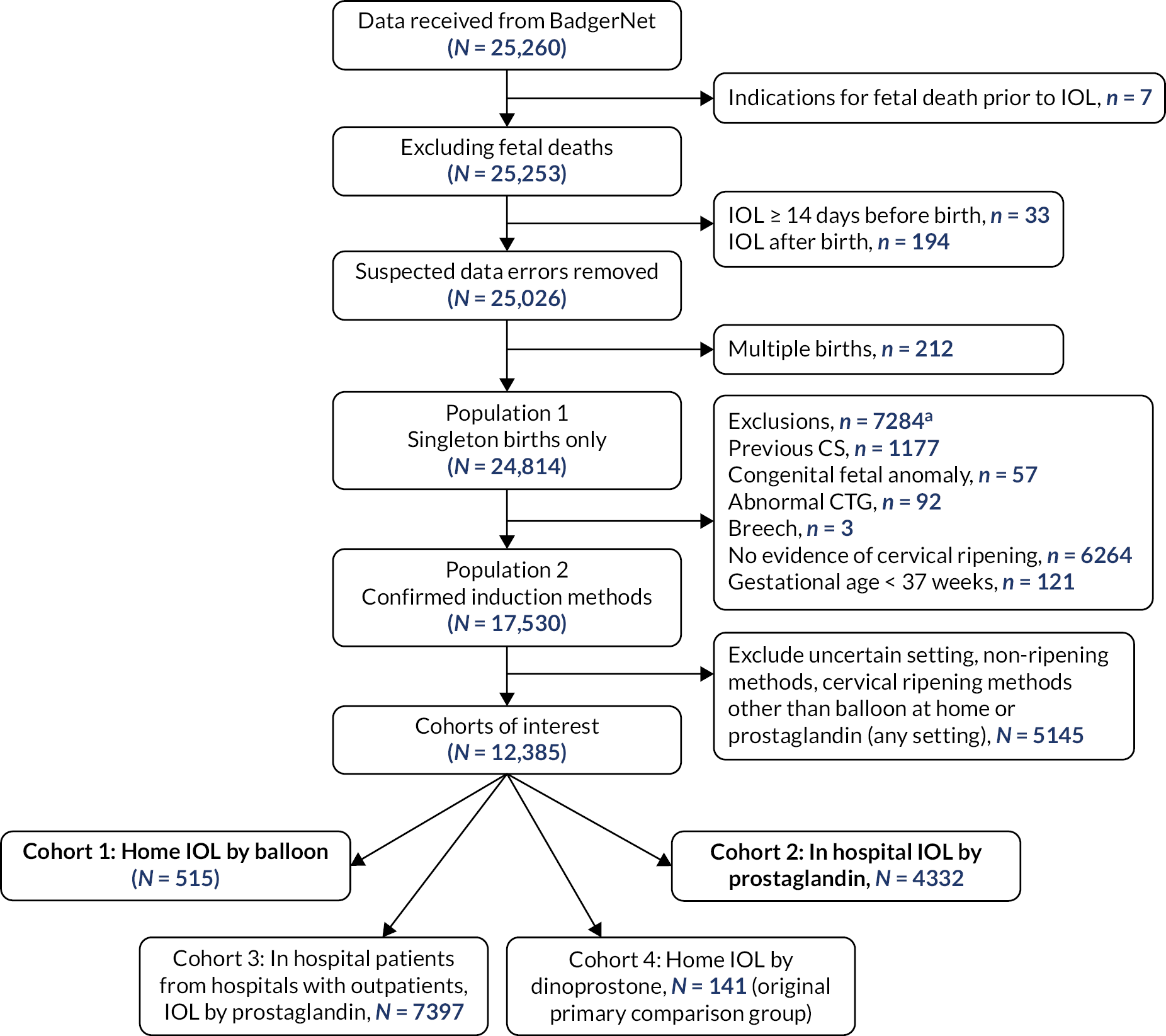
More stringent inclusion and exclusion criteria were applied at the analysis stage. These are summarised in the flowchart in Figure 1. In the primary analysis, a cohort of women was created with broadly comparable level of risk in pregnancy (i.e. those without key risk factors for adverse maternal or perinatal outcomes defined below) in whom there was no contraindication to home cervical ripening, who had singleton pregnancies with IOL at 37 weeks’ gestation or more. This group included women having IOL for post dates, because of maternal or clinician preference, for maternal age, for discomfort or social indications, for hypertension, diabetes, fetal concerns (oligohydramnios, reduced liquor volume, macrosomia, intrauterine growth restriction, static growth, polyhydramnios), fetal movement concerns, obstetric cholestasis, antepartum haemorrhage, previous obstetric history, prelabour rupture of membranes (ROM) documented as primary or other indication for IOL (prolonged ROM, spontaneous ROM and suspected spontaneous ROM) and other maternal reasons, such as raised body mass index (BMI), in vitro fertilisation pregnancy, musculoskeletal pain, thrombophilia. Exclusion criteria consisted of previous caesarean section, antepartum stillbirth (before cervical ripening initiated), congenital fetal anomaly, abnormal cardiotocograph (CTG)/Doppler, breech presentation.
FIGURE 1.
Identification of CHOICE observational cohort study populations and analysis cohorts by setting and method of cervical ripening. a, Some participants may have more than one reason for exclusion. CS, caesarean section.
Participants were identified from data recorded in specified fields in BadgerNet electronic maternity records. We used data fields indicating IOL, estimated due date and date of IOL to identify women having IOL at 37 weeks of gestation or more.
Women were made aware of the CHOICE study through posters in participating sites, business cards, information leaflets, online adverts on hospital/maternity websites and relevant social media sites, and information in maternal electronic maternity records.
Women were able to opt out of data provision by notifying their clinician or midwife or e-mailing the study research midwife at the local site, following which their opt-out status was recorded on their electronic record. This became a conditional field, where there must be no opt-out selected for the data to be extracted for the study. There was no restriction on co-enrolment in other studies.
Study settings
The study was performed in 26 UK obstetric units, 8 of which offered cervical ripening both in-hospital (mix of methods) and at home (predominantly balloon method) and 18 of which offered exclusively in-hospital cervical ripening (predominantly prostaglandin method). All included sites used the BadgerNet maternity record system.
The maternity units included were selected to represent the diverse range of maternity service settings in the UK, and included urban tertiary referral units, mid-sized urban district general hospitals and small, more isolated, rural units.
Participating trusts/boards/units included:
-
NHS Borders
-
NHS Fife
-
NHS Grampian
-
NHS Highland
-
NHS Lanarkshire
-
NHS Tayside
-
Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity)
-
Birmingham Women’s and Children’s NHS Foundation Trust
-
Darlington Memorial Hospital
-
University Hospital of North Durham (Maternity)
-
Dorset County Hospital
-
Epsom Hospital (Maternity)
-
St Helier Hospital (Maternity)
-
Hereford County Hospital (Maternity)
-
Princess Royal University Hospital
-
King’s College Hospital (Maternity)
-
Chorley and South Ribble Hospital
-
Royal Preston Hospital
-
Cumberland Infirmary (Maternity)
-
West Cumberland Hospital (Maternity)
-
Queen Elizabeth Hospital, Gateshead (Maternity)
-
Warwick Hospital
-
Queen Elizabeth Hospital Kings Lynn (Maternity)
-
New Cross Hospital, Wolverhampton (Maternity)
-
Walsall Manor Hospital
-
Worcestershire Royal Maternity Hospital
No data were collected from each site for the first 14 days after their ‘go live’ date to ensure that women had the opportunity to see and read study materials such as posters and study cards, and to opt-out of data collection if preferred.
Clinical and sociodemographic characteristics
The following demographic and clinical characteristics were described across cohorts: number of women undergoing IOL by study site; age of women in years; BMI of women; class III obesity at booking (≥ BMI 40 kg/m2); marker of deprivation [index of multiple deprivation (IMD)]; parity (number of parity 0, 1, 2, 3 +); gestation at IOL (completed weeks) plus number at each completed week; birth weight (grams) and indication for IOL.
Indications for IOL (as defined by study sites) were categorised as follows: post dates; hypertension (pregnancy-induced hypertension or pre-eclampsia), antepartum haemorrhage; diabetes; obstetric cholestasis; obstetric history; maternal age; maternal request (including social indications); other maternal reasons (including raised BMI, in vitro fertilisation; musculoskeletal pain, thrombophilia); fetal concern (excessive growth, polyhydramnios, reduced liquor volume, small for gestational age, static growth, suboptimal growth, abnormal dopplers); previous precipitate labour; ROM (preterm, prolonged, suspected, confirmed); reduced fetal movements (isolated or recurrent episodes).
Exposures
The exposure group consisted of women who, at the start of the cervical ripening process, planned to have home cervical ripening and who underwent this procedure using a balloon catheter as the first method of IOL. This group is referred to as the ‘home balloon’ group in the report.
The comparator group included women who planned to have in-hospital cervical ripening from maternity units not offering home cervical ripening, and who underwent this using prostaglandin methods. This design minimised potential bias arising from the fact that, in maternity units which offer both home and in-hospital cervical ripening, the risk of complications in the babies of women having home cervical ripening (lower-risk pregnancies) is inherently different from that of babies of women having in-hospital cervical ripening (higher-risk pregnancies).
A further two cohorts were identified (in-hospital cervical ripening using prostaglandin where the hospital also offers home cervical ripening, and at home cervical ripening using dinoprostone – the original exposure group), to provide context to the primary outcome, although the latter group was not used in any of the secondary outcome analyses. Figure 1 outlines the derivation of each cohort.
Outcomes
Primary outcome
The primary outcome was admission to a NNU/special care baby unit for 48 hours or longer, initiated within 48 hours of birth. NNU admission is a marker of neonatal morbidity and is the leading core outcome defined for studies of IOL. 19 Any increase in NNU admission of term babies is undesirable due to the separation of mother and baby. However, NNU admission rates are highly variable between maternity units and are likely to depend on local policies and culture. 20 For this reason, we used a primary outcome that represents more severe neonatal morbidity (admission to a NNU within 48 hours of birth for 48 hours or longer), which is less likely to be influenced by site-specific factors than NNU admissions for shorter durations.
Secondary outcomes
The core outcomes set for IOL includes many outcomes for inclusion in studies of IOL. 13 These outcomes were prespecified as exploratory secondary outcomes in the published study protocol. Further mother and baby outcomes were suggested by our lay consultation as important to include. Not all these outcomes were included in the updated statistical analysis plan following pilot analysis, as many outcomes were recognised to be poorly recorded or not recorded at all within the pilot data extract. The outcomes considered for reporting (where enough data exist) are outlined below.
Maternal outcomes
Mode of birth (unassisted vaginal, caesarean, forceps/ventouse-assisted); postpartum haemorrhage 1000 ml or more; maternal pyrexia 38 °C or more after starting cervical ripening (exploratory outcome); obstetric anal sphincter injury; retained placenta.
Offspring outcomes
Apgar score at 1, 5 and 10 minutes; NNU admission (any); duration of NNU stay; duration of NICU stay; neonatal seizures; meconium-stained liquor, mechanical ventilation; intracranial haemorrhage; serious morbidity (at least one of neonatal seizures, intracranial haemorrhage, stillbirth, neonatal death); stillbirth after admission/first attendance for IOL (excluding deaths from congenital anomalies); early neonatal death up to 7 days after birth (day 0–6; excluding deaths from congenital anomalies); hypoxic-ischaemic encephalopathy.
Effectiveness outcomes
Birth in obstetric unit; birth in alongside midwifery unit (if available at that site); methods used to start (first method) IOL (e.g. type of prostaglandin, ARM); number of IOL methods used by type; number of women undergoing cervical ripening as part of IOL at any point (balloon, prostaglandin or cervical dilator); number of women undergoing each type of cervical ripening, first versus subsequent; number of women in whom more than one cervical ripening agent was used; number of women receiving oxytocin during labour.
Labour progress
Cervical dilation at commencement of IOL; time (duration in minutes before birth) cervix fully dilated; cervical dilation at time of caesarean section; length of time between IOL starting and transfer to labour ward (minutes); length of time between IOL starting and birth (minutes); duration of antenatal hospital stay for cervical ripening (in-hospital) (minutes); duration of discharge during cervical ripening (at-home; minutes); duration of antenatal hospital stay for cervical ripening (at home; minutes); duration of labour ward admission until birth (minutes); duration of postnatal hospital stay (mother; minutes); total hospital stay (minutes); oxytocin use.
Reporting of small numbers
To preserve anonymity of participants, it was agreed that where fewer than five outcomes were reported in a group then findings for that outcome would not be reported by group.
Changes to outcomes
The primary outcome remained the same, although following the pilot analysis, it was agreed that it was unlikely that there would be sufficient power to achieve our primary objective of assessing safety of home versus in-hospital cervical ripening. To compare outcomes across groups, a simplified analysis with two non-matched cohorts replaced the original analysis plan, which had involved a propensity-score matching process.
Sample size
The original sample size required 1920 home IOL with dinoprostone to be matched with 1920 in-hospital IOL with prostaglandin from hospitals not offering home IOL. However, at the time of the pilot study, only 150 women had been given prostaglandin for IOL at home, with even fewer having received dinoprostone. Our stop–go criteria at this point required us to have achieved at least 400. With the changes in practice necessitated by the COVID-19 pandemic, 1920 was not going to be achievable in a practicable time frame. The requirement to match was dropped to increase the power from the in-hospital cohort.
Overall, the lower numbers of women in the home setting than expected meant that the study was deemed to be underpowered to assess the primary outcome in any other way than exploratory. The primary end point was presented with a 95% confidence interval (CI), but should only be considered as hypothesis generating rather than hypothesis testing.
Data sources
Data were collected directly from electronic maternity and neonatal records for participants who had babies cared for by neonatal staff. These data are recorded by clinical staff (midwives, doctors and neonatal nurses) while providing antenatal, intrapartum and postpartum care. Existing data fields were used. Data were assumed to reflect the understanding of the clinical staff who entered the data. Diagnoses were assumed to reflect national guidance.
Unless women opted out of secondary data use, deidentified data were transferred from BadgerNet participating sites to a secure University of Edinburgh server for analysis.
No personal data were collected. Potentially identifiable data, such as the date and time of birth, date of events such as commencing cervical ripening and hospital discharge, were converted into gestation at birth (weeks + days); and antenatal and postnatal events into ‘t – x’ and ‘t + x’ minutes, respectively.
Data from a total of 40 tables in the BadgerNet system were extracted. These included details of the following:
-
neonatal care admission
-
critical incident affecting baby
-
birth details of baby
-
discharge details of baby
-
baby examination details
-
baby feeding details
-
operative birth details
-
internal transfer of baby
-
medications administered to baby
-
microbiological tests performed in relation to baby
-
baby patient index (demographic and clinical details)
-
risk assessment relating to baby
-
transfer of baby’s care
-
maternal admission
-
maternal analgesia
-
maternal antenatal assessment
-
maternal care plan
-
maternal communication details
-
maternal critical incident
-
maternal discharge details
-
maternal perineal tears or trauma
-
maternal health history
-
maternal induction of labour details
-
maternal induction of labour booking details
-
maternal internal transfer
-
maternal labour and birth details
-
maternal lifestyle
-
maternal death
-
maternal observations
-
maternal partogram
-
maternal patient index
-
maternal pre-operative checklist
-
maternal previous pregnancy
-
maternal risk assessment
-
maternal ROM
-
maternal sepsis pathway
-
maternal transfer of care
-
maternal transfusion
-
maternal vaginal examination
-
summary of neonatal care.
Algorithm to determine setting of cervical ripening
Women in the study data set were categorised as having either had home cervical ripening, in-hospital cervical ripening or unclear setting of cervical ripening. The categorisation took place using the following variables: mother_induction (first induction), mother_admission (admissions after IOL), mother_patientindex, mother_discharge (excluding discharge before IOL), mother_communication (phone call between ‘patient’ and other), mother_previouspregnancy and baby_deliverydetails.
The main analysis population was identified following the exclusion of cases listed below:
-
Remove if fetal death is reason for IOL.
-
Remove if IOL time < 14 days before birth (likely coding error).
-
Remove if IOL time is after birth (likely coding error).
-
Remove multiple births (population 1 in flowchart).
-
Remove if reason for IOL is:
-
congenital fetal abnormality
-
abnormal CTG
-
breech.
-
-
Remove if any previous pregnancy involved caesarean section.
-
Remove if not confirmed IOL.
-
Remove if gestational age at IOL < 37 weeks (population 2 in flowchart).
The algorithm used to determine induction of labour setting (‘IOL type’) was as follows (where 'y' = yes and 'n' = no):
-
If the hospital does not offer home cervical ripening, then IOLtype = ‘In hospital only’.
-
If the hospital is a mixed hospital (allows both at home and in-hospital cervical ripening) AND dischargedhome = ‘y’, then IOLtype = ‘home’.
-
If the hospital is a mixed hospital (allows both at home and in-hospital cervical ripening) AND dischargedhome = ‘n’, then IOLtype = ‘inhospital mixed’.
-
For those still undefined at this point:
-
If baby born outside hospital, then IOLtype = ‘home’.
-
If the hospital allows home IOL AND there is no admission time, then IOLtype = ‘inhospital mixed’ (data sets only include admissions after IOL, so mother must already be in hospital if there is no admission time after IOL has commenced).
-
If place of birth in hospital that allows home IOL AND there is no discharge note AND first admission > 120 hours, then IOLtype = ‘uncertain’.
-
If place of birth in hospital that allows home IOL AND there is no discharge note AND first or second admission is 6–120 hours after birth, then IOLtype = ‘home’.
-
If place of birth in hospital that allows home IOL AND there is no discharge note AND only one admission AND admission ≤ 6 hours after birth, then IOLtype = ‘uncertain’.
-
If place of birth in hospital that allows home IOL AND there is no discharge note and time from IOL to discharge > 24 hours, then IOLtype = ‘inhospital mixed’.
-
If place of birth in hospital that allows home IOL AND IOL time < discharge time AND discharge ≤ 24 hours after birth, then IOLtype = ‘inhospital mixed’.
-
If place of birth in hospital that allows home IOL AND IOL time < discharge time AND discharge ≤ 24 hours before birth, then IOLtype = ‘home’.
-
If place of birth in hospital that allows home IOL AND first admission < 6 hours after birth, then IOLtype = ‘home’.
-
If place of birth in hospital that allows home IOL AND first discharge is after birth, then IOLtype = ‘inhospital mixed’.
-
If there is a record of a phone call between patient and other during period between IOL and birth, then IOLtype = ‘home’.
-
Definition of cohorts
-
If IOLtype = ‘home’ and induction method = ‘cervical balloon’, then cohort = 1.
-
If IOLtype = ‘In hospital only’ and induction method = ‘prostaglandins’, then cohort = 2.
-
If IOLtype = ‘inhospital mixed’ and induction method = ‘prostaglandins’, then cohort = 3.
-
If IOLtype = ‘home’ and prostaglandin type = ‘propess’, then cohort = 4.
Interim analyses and stopping guidelines
As proposed, we carried out a pilot phase analysis to determine the parameters of the primary outcome and feasibility of obtaining the planned sample size for the original preplanned analysis at 156 site months, using criteria as shown in Table 1. This was based on an evaluable comparison group of 1920 women in each arm, so acted as an inherent check on home cervical ripening eligibility and uptake rates. We assessed variation of the primary outcome at the pilot stage, along with that of other measures of neonatal morbidity included as secondary outcomes (e.g. any NNU admission). We retained the preplanned parameters of NNU admission used in the primary outcome after analysis of pilot data. We redefined the comparison groups following assessment of the pilot analysis data, which demonstrated much lower uptake of home cervical ripening using dinoprostone than expected, higher uptake of home cervical ripening using balloon, a much wider range of indications of IOL in home cervical ripening groups and earlier gestation at IOL in both settings. The decision to redefine the comparison groups was made in consultation between the expert project management group, the study steering committee and the funder.
| Criteria | Stop | Change | Go |
|---|---|---|---|
| N matched women in each arm | < 400 (< 4 SD of target) | 400–549 (2–4 SD of target) | 550–650 (2 SD of target) |
| ICC for NNU admission | > 0.0125 | > 0.01 but ≤ 0.0125 | ≤ 0.01 |
| ACTION | Stop study – unfeasible to assess safety outcomes | Consult with funder for extension to data collection period | Continue study as proposed |
Statistical methods
All analyses were fully specified in an updated comprehensive statistical analysis plan (version 2.0) and agreed by the steering committee. Analyses were carried out in accordance with relevant guidance, including Strengthening the Reporting of Observational Studies in Epidemiology. 15
The original design, which informed the sample size calculation, was a non-inferiority design, chosen with a non-inferiority margin of 4% (deemed as likely to be an important difference on consultation with women and clinicians) for the primary outcome of NNU admission within 48 hours of birth for 48 hours or more.
The non-inferiority margin was established based on a combination of what is acceptable to women and partners and what costs are acceptable to healthcare providers. However, following the pilot phase and the expected inability to reach the required sample size, the principal analysis as defined originally, was deemed unlikely to be sufficiently powered, and the primary analysis was changed to an estimation problem, rather than a hypothesis testing problem.
For the principal analysis of the primary outcome, we used mixed-effects logistic regression for the comparison of NNU admission within 48 hours of birth for 48 hours or longer (yes/no). Within the mixed model, site has been included as a random effect and all other factors are fixed effects. A random intercept for each site was included, rather than a common intercept to allow for baseline differences, with the assumption of no correlation between sites.
Potential confounding variables were identified from the pre-planned list including:
-
Gestational age at commencement of IOL (in weeks).
-
Maternal age at delivery (in years).
-
BMI at booking (or earliest record) in kg/m2.
-
Maternal medical conditions such as diabetes (pre-existing or gestational).
-
Hypertensive disorder (proteinuria, hypertension, pre-eclamptic toxaemia, pregnancy-induced hypertension, pre-eclampsia).
-
Other maternal risk factors (obstetric cholestasis, thrombophilia, in vitro fertilisation, past obstetric history).
-
Fetal concerns (fetal growth or liquor concerns, reduced fetal movement, other fetal reason).
-
ROM.
-
Smoking.
-
Deprivation.
-
Parity.
Descriptive statistics were used to describe all secondary outcomes. We described the duration of hospital stay during IOL, time spent at home, total hospital stay and time to birth using medians and interquartile ranges, while categorical outcomes such as mode of birth were described using proportions and percentages.
As outlined in the exposures section, data were reported for cohort 1 (women who had balloon cervical ripening at home), cohort 2 (women who had in-hospital cervical ripening using prostaglandin) and cohort 3 (women who had in-hospital cervical ripening using prostaglandin in a hospital that also offers home cervical ripening). Data from the larger cohort of women having IOL from 37 + 0 weeks’ gestation (population 2) and those relating specifically to in-hospital cervical ripening in units also offering home cervical ripening (cohort 3) were used to contextualise the findings on the background of unit practices and populations undergoing IOL. This was due to the considerable inter-unit variation in both the rates of IOL and the risk profile of women giving birth that needed to be considered. This helped to demonstrate the generalisability of the findings.
Missing data
We expected that up to 10% of women would have missing data on the primary outcome, eligibility criteria, setting of cervical ripening and/or have only part of the baseline data (age, comorbidities and any relevant identified hospital-level factors). Due to a lack of opportunity to put bespoke fields in place to address missing data as planned, only complete case analyses were conducted. Some of these missing fields made it difficult to identify whether women had gone home for IOL or were in hospital, and as such could not be included in the analysis data set. It is possible that, with better ability to determine which fields must be completed, we could have increased the sample size, as approximately 36% of the singleton births had insufficient detail. However, given the relative proportion of home IOL to the final complete cohort, it is still unlikely that we would have achieved sufficient power.
Additional analyses
The primary outcome was adjusted for potential confounders (as described above), but no further adjusted or subgroup analyses were performed.
Observational cohort study results
Recruitment context
Unexpectedly, the CHOICE study took place in an NHS under pressure to manage high IOL rates (range 31–49% of all births in study sites) during the COVID-19 pandemic, with long delays during the IOL process. Home cervical ripening using balloon was performed in women for a wide array of indications (low to moderate-risk groups) and from 37 weeks’ gestation onwards.
COVID-19 pandemic impact
The observational cohort study was due to commence site recruitment in spring of 2020. All sites were due to go live simultaneously. As a result of the COVID-19 pandemic, the CHOICE study was halted from all recruitment for 10 months in total. Following the restart of research, every research and development department had differing timelines for processing of CHOICE permissions for their site, with several unable to progress these for several months. This led to much less recruitment than planned and a much later pilot analysis than planned. The pilot analysis was however carried out at the same number of site months as originally intended (156 site months). Following completion of the pilot analysis (May 2022) only one further month of data extraction was planned, thus ending data extraction on 30 June 2022. This decision was taken to ensure that the study would be completed on time, within budget and with maximal use of available data (estimated at end of data collection period to include around 25,000 cases of IOL and around 500 having cervical ripening at home using balloon methods).
The data collection period extended from 3 February 2021 to 30 June 2022, 14 days after the first ‘go live’ date as shown in Table 2.
| Site | Live date | Months live |
|---|---|---|
| NHS Borders | 23 August 2021 | 10 |
| NHS Fife | 19 August 2021 | 10 |
| NHS Grampian | 18 October 2021 | 8 |
| NHS Highland | 13 September 2021 | 9 |
| NHS Lanarkshire | 5 July 2021 | 11 |
| NHS Tayside | 19 July 2021 | 11 |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity) | 02 August 2021 | 10 |
| Birmingham Women’s and Children’s NHS Foundation Trust | 8 March 2021 | 15 |
| Darlington Memorial Hospital | 1 April 2022 | 2 |
| University Hospital of North Durham (Maternity) | 1 April 2022 | 2 |
| Dorset County Hospital | 12 August 2021 | 10 |
| Epsom Hospital (Maternity) | 5 May 2021 | 13 |
| St Helier Hospital (Maternity) | 5 May 2021 | 13 |
| Hereford County Hospital (Maternity) | 4 May 2021 | 13 |
| Princess Royal University Hospital | 22 March 2022 | 3 |
| King’s College Hospital (Maternity) | 22 March 2022 | 3 |
| Chorley and South Ribble Hospital | 6 September 2021 | 9 |
| Royal Preston Hospital | 6 September 2021 | 9 |
| Cumberland Infirmary (Maternity) | 26 July 2021 | 11 |
| West Cumberland Hospital (Maternity) | 26 July 2021 | 11 |
| Queen Elizabeth Hospital, Gateshead (Maternity) | 20 January 2021 | 17 |
| Warwick Hospital | 15 September 2021 | 9 |
| Queen Elizabeth Hospital Kings Lynn (Maternity) | 10 September 2021 | 9 |
| New Cross Hospital, Wolverhampton (Maternity) | 15 July 2021 | 11 |
| Walsall Manor Hospital | 20 April 2022 | 2 |
| Worcestershire Royal Maternity Hospital | 7 June 2021 | 12 |
Participant flow
Participants were identified from the data transferred from BadgerNet as illustrated in Figure 1. Data for a total of 25,260 women were extracted.
Losses and exclusions
As outlined in the flowchart, seven women were excluded due to fetal death occurring before IOL started. A total of 212 women with multiple pregnancies were excluded. Additional exclusions (7284 in total) were those women where home cervical ripening would be contraindicated (previous caesarean birth, congenital fetal anomaly, abnormal CTG, breech fetal presentation, gestational age < 37 completed weeks) and those where there was no documented evidence that any cervical ripening had taken place. Further exclusions involved women where there was assumed to be a higher level of clinical risk than those who had cervical ripening at home since these women had in hospital cervical ripening in hospitals that also offered home cervical ripening (n = 7397, cohort 3). The final exclusions from the primary analysis were those women who had cervical ripening using dinoprostone at home (n = 141, cohort 4) given that these were no longer included in the primary comparison.
Data on study population
Following initial exclusions, the total number of women who were coded as having had an IOL at each site is shown in Table 3 (population 1 as per Figure 1). Following exclusion of those in whom the method of IOL could not be established, the numbers of women with confirmed method of IOL at each site are shown in Table 3 (population 2). The IOL rate at each site (calculated as a proportion of all births at each site during the study period) is shown in Table 3 where denominator data were provided by sites.
| Hospital or NHS trust | All in data set (population 1), N (%) | Confirmed inductions with or without cervical ripening (population 2), N (%) | IOL rate during CHOICE study period (% of all registerable births)a |
|---|---|---|---|
| NHS Borders | 260 (1.0) | 170 (1.0) | 31.7 |
| NHS Fife | 1199 (4.8) | 756 (4.3) | 39.0 |
| NHS Grampian | 1190 (4.8) | 759 (4.3) | 33.0 |
| NHS Highland | 639 (2.6) | 462 (2.6) | 41.0 |
| NHS Lanarkshire | 1825 (7.4) | 1050 (6.0) | 33.5 |
| NHS Tayside | 1200 (4.8) | 753 (4.3) | Unknown |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity) | 1373 (5.5) | 852 (4.9) | 39.0 |
| Birmingham Women’s and Children’s NHS Foundation Trust | 4063 (16.4) | 2664 (15.2) | 34.6 |
| Darlington Memorial Hospital | 156 (0.6) | 143 (0.8) | 46.3 |
| University Hospital of North Durham (Maternity) | 209 (0.8) | 164 (0.9) | 46.3 |
| Dorset County Hospital | 463 (1.9) | 387 (2.2) | 35.7 |
| Epsom Hospital (Maternity) | 896 (3.6) | 653 (3.7) | 33.3 |
| St Helier Hospital (Maternity) | 890 (3.6) | 793 (4.5) | 33.3 |
| Hereford County Hospital (Maternity) | 821 (3.3) | 585 (3.3) | 38.2 |
| Princess Royal University Hospital | 299 (1.2) | 142 (0.8) | Unknown |
| King’s College Hospital (Maternity) | 272 (1.1) | 160 (0.9) | 30.6 |
| Royal Preston Hospital | 1223 (4.9) | 1070 (6.1) | 40.7 |
| Cumberland Infirmary (Maternity) | 586 (2.4) | 435 (2.5) | 36.5 |
| West Cumberland Hospital (Maternity) | 355 (1.4) | 191 (1.1) | 36.5 |
| Queen Elizabeth Hospital, Gateshead (Maternity) | 1258 (5.1) | 1009 (5.8) | 49.4 |
| Warwick Hospital | 851 (3.4) | 608 (3.5) | 34.8 |
| Queen Elizabeth Hospital, Kings Lynn (Maternity) | 667 (2.7) | 558 (3.2) | 40.1 |
| New Cross Hospital, Wolverhampton (Maternity) | 1776 (7.2) | 1380 (7.9) | 37.8 |
| Walsall Manor Hospital | 283 (1.1) | 224 (1.3) | Unknown |
| Worcestershire Royal Maternity Hospital | 2057 (8.3) | 1561 (8.9) | 41.1 |
| Total | 24,814 (100) | 17,530 (100) |
Following further exclusions (see Figure 1), the final comparison groups for the main analysis comprised 515 women who underwent home cervical ripening using balloon methods (cohort 1), 4332 women who underwent in-hospital cervical ripening using prostaglandin methods (cohort 2) and 7397 women in the additional comparison group (providing background context) who underwent in-hospital cervical ripening with prostaglandin at a site that also offered home cervical ripening (cohort 3) as shown in Table 4.
| IOL type | Mixed hospital, at home balloon cervical ripening (cohort 1), N (%) | In hospital only, prostaglandin cervical ripening (cohort 2), N (%) | Mixed hospital, in-hospital prostaglandin cervical ripening (cohort 3), N (%) |
|---|---|---|---|
| Total | 515 (100) | 4332 (100) | 7397 (100) |
Not all units offered cervical ripening using balloon method. The number of women undergoing balloon cervical ripening at any point during IOL by site is reported in Table 5.
| Hospital or NHS trust | Women having balloon cervical ripening at any point among all confirmed inductions (population 2), n/N (%) |
|---|---|
| NHS Borders | 93/170 (54.7) |
| NHS Fife | 12/756 (1.6) |
| NHS Grampian | 401/759 (52.8) |
| NHS Lanarkshire | 16/1050 (1.5) |
| NHS Tayside | 235/753 (31.2) |
| Ashford and St Peter’s Hospital NHS Foundation Trust (Maternity) | 59/852 (6.9) |
| Darlington Memorial Hospital | 28/143 (19.6) |
| Queen Elizabeth Hospital, Kings Lynn (Maternity) | 27/558 (4.8) |
| University Hospital of North Durham (Maternity) | 74/164 (45.1) |
| Dorset County Hospital | 20/387 (5.2) |
| Epsom Hospital (Maternity) | 108/653 (16.5) |
| St Helier Hospital (Maternity) | 174/793 (21.9) |
| Worcestershire Royal Maternity Hospital | 6/1561 (0.4) |
| Cumberland Infirmary (Maternity) | 6/435 (1.4) |
| West Cumberland Hospital (Maternity) | 8/191 (4.2) |
| Total | 1267/9225 (13.7) |
Cervical ripening using cervical dilator was performed at two sites as shown in Table 6.
| Hospital or NHS trust | Women having cervical ripening using cervical dilator at any point among all confirmed inductions (population 2), n/N (%) |
|---|---|
| Queen Elizabeth Hospital, Kings Lynn (Maternity) | 58/1009 (5.8) |
| Warwick Hospital | 12/608 (2.0) |
All sites used prostaglandin as a method of cervical ripening in some cases, as is shown in Table 6.
Of those in the exposed group (‘home balloon’) around 1 in 20 (24/515, 4.7%) received prostaglandin for cervical ripening after their balloon method was used.
The total number of women undergoing confirmed IOL (population 2) by parity and gestation are provided in Report Supplementary Material 1, Tables 1 and 2, respectively. The number of women undergoing IOL by setting (whether home or in-hospital, regardless of method) is provided in Report Supplementary Material 1, Table 3. Further breakdown of first method used in IOL (including but not exclusive to cervical ripening) by site is provided in Report Supplementary Material 1, Table 4. Median number of IOL attempts made by site, parity and gestation are provided in Report Supplementary Material 1, Tables 5–7. Median number of different methods used in IOL are provided in Report Supplementary Material 1, Tables 8–10. Number of women having balloon method for cervical ripening at any point by site, parity and gestation are provided in Report Supplementary Material 1, Table 11–13. Number of women having prostaglandin method for cervical ripening at any point by site, parity and gestation are provided in Report Supplementary Material 1, Tables 14–16. Number of women having non-ripening IOL methods at any point by site, parity and gestation are provided in Report Supplementary Material 1, Tables 17–19. Number of women having any cervical ripening method at any point by site, parity and gestation are provided in Report Supplementary Material 1, Tables 20–22. Subsequent method used in IOL after balloon or prostaglandin by site, parity, gestation and setting are provided in Report Supplementary Material 1, Tables 23–40. The proportion of women using more than one method of cervical ripening by site, parity and gestation are provided in Report Supplementary Material 1, Tables 41–43.
In all groups, the median cervical dilatation at onset of IOL was 1 cm, with an interquartile range of 0–1. A full breakdown of cervical dilatation at onset of IOL is provided in Report Supplementary Material 1, Tables 44–46.
Cohort characteristics
Characteristics of women included in the exposed group (‘home balloon’), in the comparison group (‘hospital prostaglandin’) and in the background context group (women who had in-hospital cervical ripening using prostaglandin in hospitals that also offered home cervical ripening – ‘hospital prostaglandin – mixed hospital’) are presented in Table 7. This demonstrated similarities across the groups with respect to maternal age, median BMI and the range of gestations at which cervical ripening was performed. Further breakdown of maternal age by site, parity, gestation and setting are provided in Report Supplementary Material 1, Tables 47–50, respectively. Further breakdown of maternal BMI by site, parity and gestation are provided in Report Supplementary Material 1, Tables 51–53, respectively.
| Exposed: home balloon, N = 515 | Comparison: hospital prostaglandin, N = 4332 | Background context group: hospital prostaglandin, mixed hospital, N = 7397 | |
|---|---|---|---|
| Parity N (%) | |||
| 0 | 339 (65.8) | 2465 (56.9) | 3973 (53.7) |
| 1 | 118 (22.9) | 1131 (26.1) | 1936 (26.2) |
| 2 | 40 (7.8) | 463 (10.7) | 906 (12.2) |
| 3 + | 18 (3.5) | 273 (6.3) | 582 (7.9) |
| Gestation N (%) | |||
| 37 weeks | 50 (9.7) | 584 (13.5) | 1056 (14.3) |
| 38 weeks | 85 (16.5) | 894 (20.6) | 1547 (20.9) |
| 39 weeks | 163 (31.7) | 1267 (29.2) | 2330 (31.5) |
| 40 weeks | 81 (15.7) | 826 (19.1) | 1448 (19.6) |
| 41 weeks | 129 (25) | 753 (17.4) | 980 (13.2) |
| 42 weeks | 7 (1.4) | 8 (0.2) | 36 (0.5) |
| Maternal age (years) | Median 30 (IQR 26–34) | Median 29 (IQR 25–33) | Median 30 (IQR 26–34) |
| BMI (kg/m2) | Median 26.8 (IQR 23.4–31.6) | Median 27.4 (IQR 23.6–32.2) | Median 27.0 (IQR 23.5–32.1) |
| Class III obesity n/N (%) | 24/487 (4.9) | 259/4013 (6.5) | 430/6962 (6.2) |
| Birthweight | Median 3540 g (IQR 3200–3830) | Median 3414 g (IQR 3080–3720) | Median 3380 g (IQR 3050–3710) |
| IMD N (%) | |||
| 1 | 58 (11.3) | 1239 (28.6) | 2272 (30.7) |
| 2 | 95 (18.5) | 770 (17.8) | 1709 (23.1) |
| 3 | 96 (18.7) | 920 (21.3) | 1352 (18.3) |
| 4 | 153 (29.8) | 824 (19.0) | 1206 (16.3) |
| 5 | 112 (21.8) | 573 (13.2) | 852 (11.5) |
The proportion of women in the home balloon group who were in their first pregnancy was 65.8%, while in the hospital prostaglandin group 56.9% were in their first pregnancy.
Class III obesity (BMI > 40 kg/m2) was present in 4.9% of women in the home balloon group and 6.5% in the hospital prostaglandin group. Further breakdown of class III obesity by site, parity, gestation and setting are provided in Report Supplementary Material 1, Tables 52–55.
The proportion of women in the home balloon group who were 41 weeks’ gestation at onset of IOL was 25%, while in the hospital prostaglandin group it was 17.4%. Respective proportions at 42 weeks’ gestation were 1.4% and 0.2%. Further breakdown of gestation by site, parity and setting are provided in Report Supplementary Material 1, Tables 56–58.
Median birth weight in the home balloon group was 3540 g compared with 3414 g in the hospital prostaglandin group. Further breakdown of birth weight by site, parity, gestation and setting are provided in Report Supplementary Material 1, Tables 59–62.
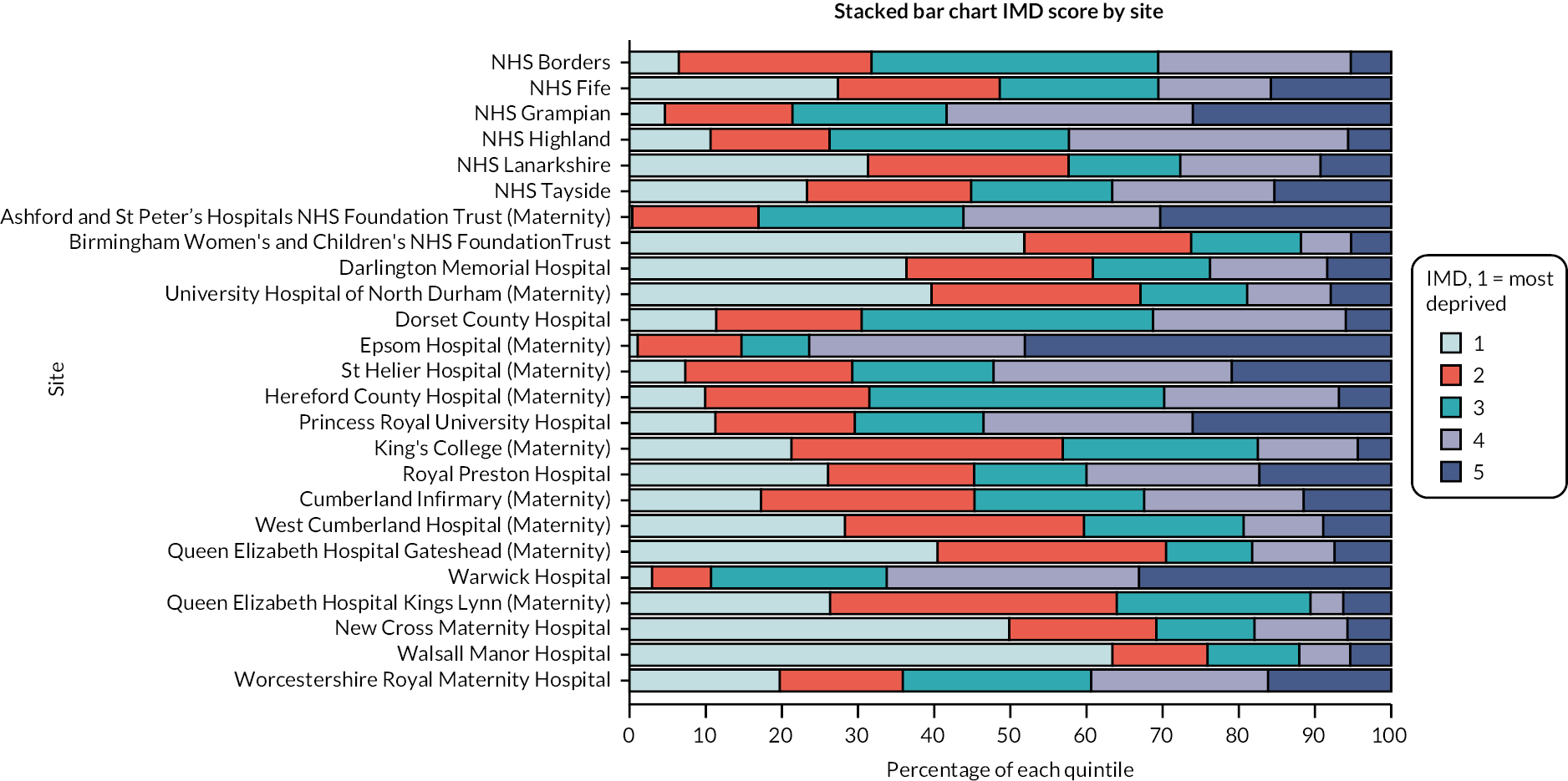
Index of multiple deprivation was 11.3% in the home balloon group being in the most deprived category compared to 28.6% in the hospital prostaglandin group. The wider group of women in the ‘hospital prostaglandin – mixed hospital’ group had 30.7% of women in the most deprived category.
Index of multiple deprivation by site is indicated in Figure 2. Further breakdown of IMD by parity, gestation and setting are provided in Report Supplementary Material 1, Tables 63–74.
FIGURE 2.
CHOICE cohort study participant IMD by study site (all IOL cases with confirmed method). Chorley and South Ribble Hospital is not included in the figures as fewer than five women from the unit were included in the study population.
Indications for induction of labour
Recorded indications for IOL appeared similar across the two groups except for the expected higher proportion of ‘post dates’ indication (27.2% vs. 15.8%) reflecting the higher proportion of women beyond 41 weeks gestation in the home balloon group, as shown in Table 8. More than one indication was recorded in some cases.
| Exposed: home balloon,a n/N (%) |
Unexposed: hospital prostaglandin, n/N (%) | Background context group: hospital prostaglandin – mixed hospital,a n/N (%) | |
|---|---|---|---|
| Post-dates | 150/515 (27.2) | 686/4332 (15.8) | 833/7397 (11.3) |
| Hypertensive disorder | 33/515 (6.4) | 361/4332 (8.3) | 583/7397 (7.9) |
| Diabetes | 59/515 (11.5) | 664/4332 (15.3) | 1050/7397 (14.2) |
| Obstetric cholestasis | 8/515 (1.6) | 103/4332 (2.4) | 191/7397 (2.6) |
| Antepartum haemorrhage | – | 11/4332 (0.3) | 50/7397 (0.7) |
| Maternal age | 14/515 (2.7) | 99/4332 (2.3) | 262/7397 (3.5) |
| Fetal concernsb | 166/515 (32.2) | 1491/4332 (34.4) | 2510/7397 (33.9) |
| Precipitate labour | – | 9/4332 (0.2) | – |
| Reduced fetal movement | 88/515 (17.1) | 895/4332 (20.7) | 1448/7397 (19.6) |
| ROM | – | 167/4332 (3.9) | 629/7397 (8.5) |
| Maternal request | 19/515 (3.7) | 70/4332 (1.62) | 200/7397 (2.7) |
| Other maternal reasons | 86/515 (16.7) | 441/4332 (10.2) | 868/7397 (11.7) |
Approximately one in three inductions (32.2% and 34.4%) were carried out due to fetal concerns in both home balloon and hospital prostaglandin groups. Fetal concerns included oligohydramnios, macrosomia, intrauterine growth, growth liquor concerns, polyhydramnios, reduced liquor volume, small gestational age, static growth, suboptimal growth, abnormal dopplers or other fetal reason.
A total of 17.1% of IOL in the home balloon group had the indication of reduced fetal movement, while 20.7% in the hospital prostaglandin group had this indication.
Diabetes was the indication for IOL in 11.5% of the home balloon group and 15.3% of the hospital prostaglandin group.
Hypertensive disorders were the indication for IOL in 6.4% of the home balloon groups and 8.3% of the hospital prostaglandin group.
Maternal age was the indication for IOL in 2.7% of the home balloon group and 2.3% of the hospital prostaglandin group.
Obstetric cholestasis was the indication for IOL in 1.6% of the home balloon group and 2.4% of the hospital prostaglandin group.
In total 3.7% of IOL were carried out due to maternal request in the home balloon group compared to 1.6% in the hospital prostaglandin group.
Further breakdown of indications for IOL by site, parity and gestation are provided in Report Supplementary Material 1, Tables 75–110.
Outcomes and estimation
Primary outcome
The total number of babies with a NNU stay within 48 hours of birth for 48 hours or more was divided by the population of babies without a NNU stay within 48 hours of birth for 48 hours of more, in the home balloon and hospital prostaglandin groups to calculate odds. The odds of the primary outcome in the home balloon group (16/499) was compared with the odds in the hospital prostaglandin group (206/4126) in the form of a ratio, as shown in Table 9. However, due to the influence of practice at various sites, this odds ratio was adjusted for site.
| Home balloon, n/N (%) | Hospital prostaglandin, n/N (%) | Bivariate odds ratio (95% CI),a N = 4847 | Multivariable odds ratio (95% CI),b N = 4366 | |
|---|---|---|---|---|
| NNU admission for at least 48 hours within 48 hours of birth | 16/515 (3.1) | 206/4332 (4.8) | 0.75 (0.35 to 1.64) | 0.81 (0.36 to 1.81) |
While adjusting for nothing other than site, the odds ratio of the primary outcome for home balloon to hospital prostaglandin is 0.75. This means that the chances of the primary outcome (NNU admission for 48 hours or more within 48 hours of birth) in the home balloon group are lower than in the hospital prostaglandin group, by 25%. Even with the adjustment for other potentially confounding factors, this remains similar (at 19% lower in home balloon group than hospital prostaglandin group). However, as expected, the 95% CI is quite large, meaning that the true odds ratio of home balloon to hospital prostaglandin groups could theoretically be between about one-third (0.36 or 74% lower odds) to almost twice as much (1.81 or 81% higher odds).
The effect of potential confounding factors was explored by including them in a multivariable model. The results are shown in Table 10. Most potential confounding factors had adjusted odds ratios of close to one, with confidence intervals crossing unity, indicating little influence on the primary outcome. However, women who had given birth to one or two previous babies had lower odds of the primary outcome than women with no previous births. Women in the third quintile of deprivation (IMD 3) had lower odds of the primary outcome than women in the most deprived quintile.
| n/N (%) | Multivariable odds ratio (95% CI), N = 4366 | |
|---|---|---|
| Gestation (weeks – referent 37 weeks) | 0.86 (0.75 to 0.99) | |
| Parity | ||
| 0 | 135/2549 (5.3) | Reference |
| 1 | 31/1107 (2.8) | 0.46 (0.31 to 0.70) |
| 2 | 8/458 (1.8) | 0.25 (0.12 to 0.53) |
| 3 + | 9/252 (3.6) | 0.50 (0.24 to 1.04) |
| Maternal age (year) | 1.01 (0.98 to 1.04) | |
| Booking BMI (n = 4500) (kg/m2) | 1.02 (0.99 to 1.04) | |
| Hypertension | ||
| None | 163/3831 (4.3) | Reference |
| Hypertension | 20/535 (3.7) | 0.71 (0.43 to 1.18) |
| Diabetes | ||
| None | 143/3616 (4.0) | Reference |
| Diabetes | 40/750 (5.3) | 1.17 (0.77 to 1.78) |
| Other maternal risk factor a | ||
| None | 176/4151 (4.2) | Reference |
| Maternal risk factora | 7/215 (3.3) | 0.66 (0.30 to 1.48) |
| Fetal risks b | ||
| None | 131/2875 (4.6) | Reference |
| Fetal riskb | 52/1471 (3.5) | 0.72 (0.47 to 1.09) |
| Reduced fetal movement | ||
| None | 144/3484 (4.1) | Reference |
| Reduced fetal movement | 39/882 (4.4) | 0.98 (0.66 to 1.46) |
| Other fetal concerns c | ||
| None | 169/3907 (4.3) | Reference |
| Other fetal concernsc | 14/459 (3.1) | 0.86 (0.46 to 1.61) |
| Prelabour ruptured membranes | ||
| None | 171/4219 (4.1) | Reference |
| Prelabour ruptured membranes | 12/147 (8.2) | 1.54 (0.80 to 2.97) |
| Smoking status at booking | ||
| Non-smoker | 162/3855 (4.2) | Reference |
| Smoker | 21/511 (4.1) | 1.18 (0.72 to 1.93) |
| IMD | ||
| 1 (most deprived) | 56/1138 (4.9) | Reference |
| 2 | 33/782 (4.2) | 0.90 (0.57 to 1.41) |
| 3 | 24/911 (2.6) | 0.54 (0.32 to 0.90) |
| 4 | 37/903 (4.1) | 0.83 (0.52 to 1.33) |
| 5 (least deprived) | 33/632 (5.2) | 1.11 (0.68 to 1.84) |
Further breakdown of the primary outcome by gestation are provided in Report Supplementary Material 1, Tables 111 and 112.
Of the 7397 women in the hospital prostaglandin – mixed hospital group, 556 (7.52%) experienced the primary outcome.
Of the 141 women who had home cervical ripening with dinoprostone (Propess®, FerringPharmaceuticals, West Drayton, UK) prostaglandin (cohort 4 in the flowchart in Figure 1, and the original exposure before pilot analysis), 10 experienced the primary outcome, a proportion of 7.09%.
Secondary outcomes
Secondary maternal outcomes compared by exposure group are reported in Table 11. Postpartum haemorrhage of 1000 ml or more occurred in similar proportions in all groups at 11–12%. Third- or fourth-degree perineal tears affected around 1 in 50 women in each group. Maternal pyrexia was reported in 1.75% of home balloon cases and 1.4% hospital prostaglandin cases.
| Home balloon, N = 515 (%) | Hospital prostaglandin, N = 4332 (%) | Background context group: hospital prostaglandin – mixed hospital, N = 7397 (%) | |
|---|---|---|---|
| Postpartum haemorrhage ≥ 1000 ml | 65 (12.6) | 482 (11.1) | 866 (11.7) |
| Third- or fourth-degree perineal tear | 11 (2.1) | 86 (2.0) | 141 (1.9) |
| Maternal pyrexia in labour | 9/515 (1.8) | 59/4332 (1.4) | 188/7397 (2.5) |
Further breakdown of postpartum haemorrhage over 1000 ml by site and setting are provided in Report Supplementary Material 1, Tables 113 and 114. Further breakdown of maternal pyrexia and retained placenta by setting are provided in Report Supplementary Material 1, Tables 115 and 116, respectively.
Neonatal care admission affected around 8% of babies in both home balloon and hospital prostaglandin groups. In the hospital prostaglandin – mixed hospital group (cohort 3 in the flow diagram in Figure 1), the proportion of babies admitted to neonatal care was 14%, as shown in Table 12. Median Apgar scores were the same across the three groups. Further breakdown of Apgar scores by setting are provided in Report Supplementary Material 1, Tables 117–121. Further breakdown of NNU admission by setting is provided in Report Supplementary Material 1, Table 122.
| At home balloon | Hospital prostaglandin | Background context group: hospital prostaglandin – mixed hospital | |
|---|---|---|---|
| Neonatal care admission (any) n/N (%) | 40/515 (7.8) | 371/4332 (8.6) | 1049/7397 (14.2) |
| Apgar score at 5 minutes | Median 9 (IQR 9–9), N = 511 | Median 9 (IQR 9–10), N = 4323 | Median 9 (IQR 9–10), N = 4331 |
| Apgar score at 10 minutes | Median 9 (IQR 9–9), N = 44 | Median 9 (IQR 9–10), N = 558 | Median 9 (IQR 9–10), N = 1296 |
Describing induction of labour practice
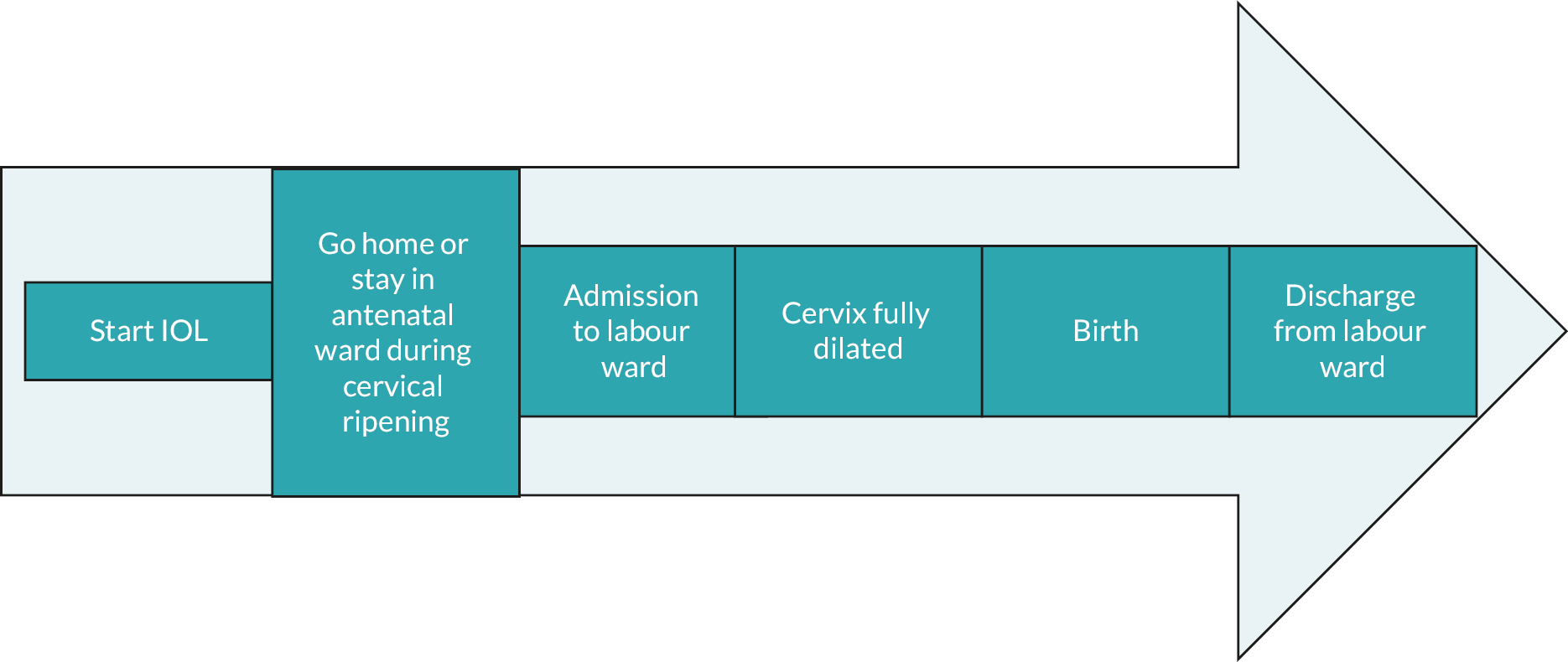
As shown in Figure 3, each induced labour follows a pattern of phases. The duration of each distinct phase (based upon location and labour events) is shown in Table 13. This shows that the home balloon group had an average duration of labour from IOL onset to birth of 2574 minutes (IQR 1777–3978 minutes) while the hospital prostaglandin group had a duration of 1906 minutes (IQR 1042–3514 minutes). These durations are broken down as follows: the home balloon group took 1875 minutes (IQR 1181–3080 minutes) to reach labour ward care while the hospital prostaglandin group took 1490 minutes (IQR 745–2915 minutes). The home balloon group took 624 minutes (IQR 383–950 minutes) to get from labour ward entry to birth, while the hospital prostaglandin group took 376 minutes (IQR 144–691 minutes). The average duration of the second stage of labour (from full dilatation of the cervix to birth) was 66 minutes (IQR 16–150 minutes) in the home balloon group and 29 minutes (IQR 9–96 minutes) in the hospital prostaglandin group. Women in the home balloon group spent on average 1043 minutes at home during cervical ripening.
FIGURE 3.
Simple timeline of potential events during IOL.
| Phase of labour | Home balloon, median minutes (IQR) | Hospital prostaglandin, median minutes (IQR) | Background context group: hospital prostaglandin – mixed hospital, median minutes (IQR) | |
|---|---|---|---|---|
| IOL onset to entry to labour warda | 1875 (1181–3080), N = 487 | 1490 (745–2915), N = 3565 | 1215 (650–2390), N = 5997 | |
| IOL onset to entry to labour warda by parity | Parity 0 | 1960 (1335–3130), N = 323 | 1618 (830–3060), N = 2038 | 1341 (709–2650), N = 3317 |
| Parity 1 | 1804.0 (1050–2955), N = 110 | 1337 (645–2733), N = 921 | 1052 (572–1945), N = 1522 | |
| Parity 2 | 1815.5 (1007–2998), N = 36 | 1211 (630–2625), N = 378 | 1115 (6510–2260), N = 698 | |
| Parity 3 + | 1887 (1141–3021), N = 18 | 1273 (667–2825), N = 228 | 1154 (586–2228), N = 460 | |
| IOL onset to entry to labour warda by gestation at induction onset | 37 weeks | 1995 (1288–3807), N = 47 | 1805 (859–3415), N = 461 | 1481 (705–2712), N = 838 |
| 38 weeks | 1875 (1050–3344), N = 83 | 1753 (881–3445), N = 712 | 1373 (677–2500), N = 1235 | |
| 39 weeks | 2130 (1280–3030), N = 157 | 1548 (810–3075), N = 1051 | 1336 (695–2740), N = 1866 | |
| 40 weeks | 1920 (1102–3575), N = 77 | 1258 (692–2619), N = 698 | 1025 (593–1995), N = 1205 | |
| 41 weeks | 1643 (1169–2808), N = 116 | 1115 (640–2300), N = 637 | 990 (577–1758), N = 826 | |
| 42 + weeks | 1800 (1365–3312), N = 7 | 1893 (750–2850), N = 6 | 785 (575–1551), N = 27 | |
| IOL onset to cervix fully dilated | 2285 (1638–3691), N = 324 | 1657 (910–3045), N = 2941 | 1411 (830–2497), N = 4910 | |
| IOL onset to cervix fully dilated by parity | Parity 0 | 2463 (1805–3860), N = 186 | 1858 (1070–3245), N = 1513 | 1660 (1005–2835), N = 2355 |
| Parity 1 | 2103 (1360–3300), N = 94 | 1423 (760–2724), N = 876 | 1166 (712–2118), N = 1490 | |
| Parity 2 | 2062 (1365–2919), N = 30 | 1395 (710–2760), N = 350 | 1190 (720–2230), N = 655 | |
| Parity 3 + | 2208 (1735–3384), N = 14 | 1387 (755–3010), N = 202 | 1197 (715–2205), N = 410 | |
| IOL onset to cervix fully dilated by gestation at induction onset | 37 weeks | 2340 (1740–4507), N = 35 | 1916 (950–3490), N = 394 | 1667 (890–2895), N = 701 |
| 38 weeks | 2310 (1435–3789), N = 59 | 1873 (1050–3638), N = 616 | 1555 (895–2647), N = 1021 | |
| 39 weeks | 2519 (1804–3644), N = 104 | 1773 (995–3196), N = 878 | 1465 (850–2615), N = 1591 | |
| 40 weeks | 2275 (1615–3653), N = 52 | 1491 (803–2535), N = 557 | 1238 (750–2165), N = 962 | |
| 41 weeks | 2025 (1495–3300), N = 69 | 1240 (785–2323), N = 493 | 1240 (750–2040), N = 618 | |
| 42 + weeks | 1635.0 (1435–4302), N = 5 | < 5 | 1138 (690–1700), N = 17 | |
| Entry to labour ward to birtha | 624 (383–950), N = 487 | 376 (144–691), N = 3565 | 412 (168–723), N = 5997 | |
| Entry to labour ward to birtha by parity | Parity 0 | 741 (488–1033), N = 323 | 544 (258, 833), N = 2038 | 568 (299–858), N = 3317 |
| Parity 1 | 434 (281–687), N = 110 | 223 (85–444), N = 921 | 238 (85–479), N = 1522 | |
| Parity 2 | 459 (330–677), N = 36 | 210 (73–438), N = 378 | 238 (90–486), N = 698 | |
| Parity 3 + | 344 (235–567), N = 18 | 260 (85–477), N = 228 | 257 (102–481), N = 460 | |
| Full dilation of cervix to birth | 66 (16–150), N = 324 | 29 (9–96), N = 2941 | 29 (10–105), N = 4910 | |
| Full dilation of cervix to birth by parity | Parity 0 | 116 (59–181), N = 186 | 76 (30–154), N = 1513 | 85 (31–171), N = 2355 |
| Parity 1 | 20 (7–82), N = 94 | 12 (5–29), N = 876 | 14 (6–38), N = 1490 | |
| Parity 2 | 12 (6–19), N = 30 | 8 (4–20), N = 350 | 9 (4–24), N = 655 | |
| Parity 3 + | 8 (5–17), N = 14 | 6 (3–18), N = 202 | 8 (3–19), N = 410 | |
| Full dilation of cervix to birth by gestation at induction onset | 37 weeks | 19 (7–118), N = 35 | 20 (7–58), N = 394 | 18 (7–67), N = 701 |
| 38 weeks | 27 (10–106), N = 59 | 25 (7–90), N = 616 | 26 (8–89), N = 1021 | |
| 39 weeks | 82 (17–159), N = 104 | 30 (10–99), N = 878 | 30 (9–110), N = 1591 | |
| 40 weeks | 71 (22–143), N = 52 | 31 (10–97), N = 557 | 36 (11–119), N = 962 | |
| 41 weeks | 94 (20–162), N = 69 | 42 (14–119), N = 493 | 52 (14–140), N = 618 | |
| 42 + weeks | 87 (60–165), N = 5 | – | 29 (10–105), N = 4910 | |
| Induction start to birth | 2574 (1777–3978), N = 515 | 1906 (1042–3514) N = 4332 | 1649 (934–2935), N = 7397 | |
| Induction start to birth | Parity 0 | 2791 (1920–4215), N = 339 | 2210 (1284–3928), N = 2465 | 2009 (1213–3374), N = 3973 |
| Parity 1 | 2315 (1426–3387), N = 118 | 1485 (807–2881), N = 1131 | 1228 (724–2247), N = 1936 | |
| Parity 2 | 2165 (1352–3017), N = 40 | 1461 (766–2859), N = 463 | 1285 (753–2419), N = 906 | |
| Parity 3 + | 2231 (1744–3386), N = 18 | 1514 (772–3188), N = 273 | 1295 (727–2426), N = 582 | |
| Induction start to birth | 37 weeks | 2623 (1857–4693), N = 50 | 2111 (1059–4099), N = 584 | 1842 (979–3173), N = 1056 |
| 38 weeks | 2519 (1676–4019), N = 85 | 2173 (1147–4161), N = 894 | 1813 (990–3141), N = 1547 | |
| 39 weeks | 2780 (1993–4007), N = 163 | 1992 (1096–3747), N = 126 | 1695 (935–3197), N = 2330 | |
| 40 weeks | 2427 (1704–4072), N = 81 | 1702 (945–3051), N = 826 | 1483 (880–2622), N = 1448 | |
| 41 weeks | 2309 (1541–3589), N = 129 | 1607 (912–2809), N = 753 | 1495 (868–2435), N = 980 | |
| 42 + weeks | 1977 (1632–4467), N = 7 | 2624 (954–3560), N = 8 | 1271 (826–2164), N = 36 | |
| Discharge during balloon cervical ripening at home | 1043.5 (642.00–1683.0), N = 110 | N/A | N/A | |
| Last admission to birth during balloon cervical ripening at home | 802 (510–1184), N = 493 | N/A | N/A | |
| Birth to discharge from labour ward care | 2075 (1356–2959), N = 514 | 1691 (1015–2554), N = 4225 | 2026 (1401–3071), N = 7350 |
Similar findings were observed when broken down by parity. For first births, the average total time from IOL onset to entry to labour ward was 1960 minutes (IQR 1335–3130 minutes) in the home balloon group and 1617 minutes (IQR 830–3060 minutes) in the hospital prostaglandin group. From labour ward entry to birth duration was 741 minutes (IQR 488–1033 minutes) in the home balloon group and 544 minutes in the hospital prostaglandin group.
Further breakdown of duration of each phase of labour by site, parity, gestation and setting is provided in Report Supplementary Material 1, Tables 123–150.
Breakdown of type of birth by exposure and comparison groups is provided in Table 14.
| Type of birth | Home balloon, N = 515 (%) | Hospital prostaglandin, N = 4332 (%) | Background context group: hospital prostaglandin – mixed hospital, N = 7397 (%) |
|---|---|---|---|
| Unassisted vaginal birth | 259 (50.3) | 2509 (57.9) | 4151 (56.1) |
| Caesarean birth | 175 (34.0) | 1174 (27.1) | 1995 (27.0) |
| Forceps | 74 (14.4) | 395 (9.1) | 808 (10.9) |
| Ventouse | 7 (1.4) | 254 (5.9) | 436 (5.9) |
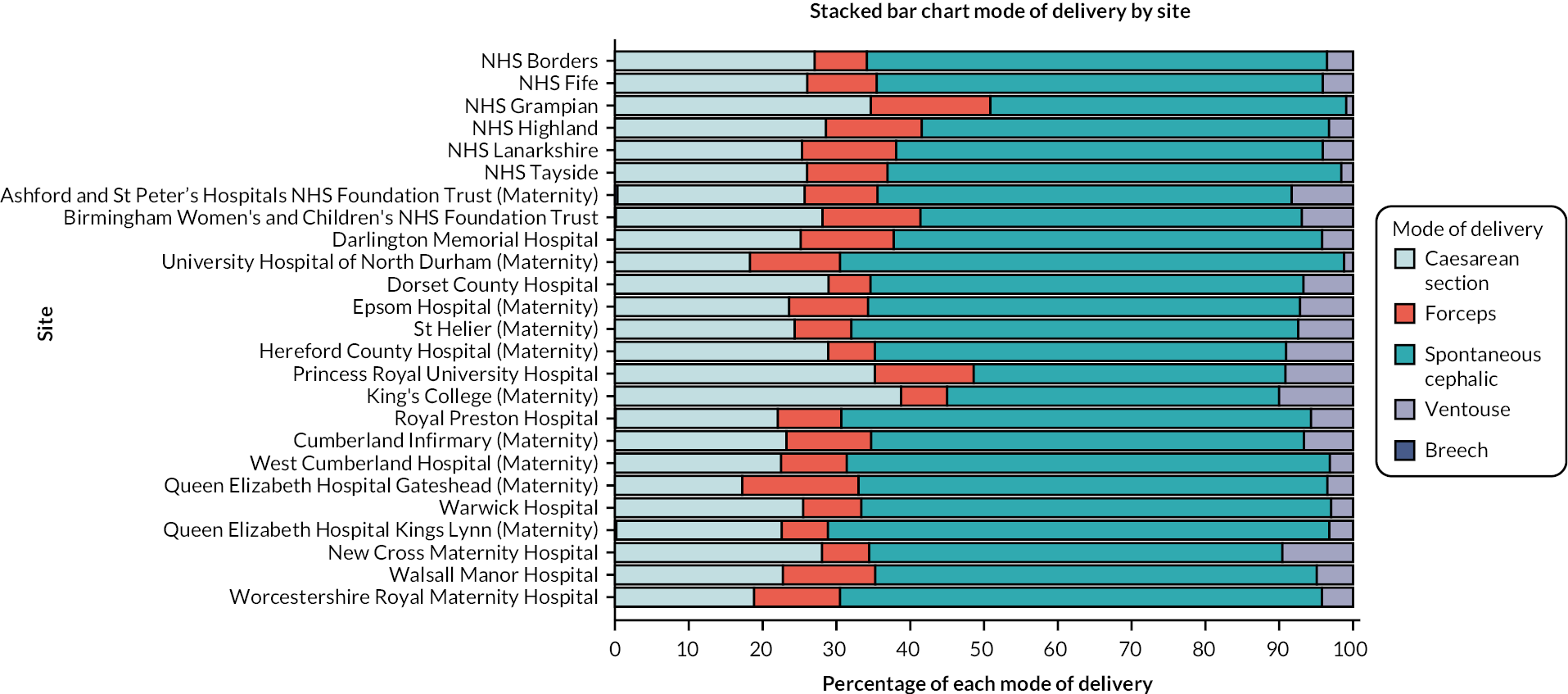
Type of birth in all IOL with known method (population 2) by site is shown in Figure 4 and type of birth by cohort and site is shown in Table 15. The breakdown of birth type by parity and gestation are shown in Report Supplementary Material 1, Tables 151–159.
FIGURE 4.
CHOICE cohort type of birth following confirmed IOL by study site.
| Study site | Birth type | Home balloon, N = 515 (%) | Hospital prostaglandin, N = 4332 (%) | Background context group: hospital prostaglandin – mixed hospital, N = 7397 (%) |
|---|---|---|---|---|
| NHS Borders | Unassisted vaginal | 19 (57.6) | – | 17 (56.7) |
| Forceps assisted | < 5 | – | < 5 | |
| Ventouse assisted | < 5 | – | < 5 | |
| Caesarean | 10 (30.3) | – | 11 (36.7) | |
| NHS Fife | Unassisted vaginal | – | 372 (59.0) | – |
| Forceps assisted | – | 58 (9.2) | – | |
| Ventouse assisted | – | 25 (4.0) | – | |
| Caesarean | – | 176 (27.9) | – | |
| NHS Grampian | Unassisted vaginal | 111 (47.2) | – | 25 (24.0) |
| Forceps assisted | 43 (18.3) | – | 13 (12.5) | |
| Ventouse assisted | < 5 | – | < 5 | |
| Caesarean | 79 (33.6) | – | 63 (60.6) | |
| NHS Highland | Unassisted vaginal | – | – | 167 (50.0) |
| Forceps assisted | – | – | 48 (14.4) | |
| Ventouse assisted | – | – | 10 (3.0) | |
| Caesarean | – | – | 109 (32.6) | |
| NHS Lanarkshire | Unassisted vaginal | < 5 | – | 443 (55.8) |
| Forceps assisted | < 5 | – | 103 (13.0) | |
| Ventouse assisted | < 5 | – | 32 (4.0) | |
| Caesarean | < 5 | – | 216 (27.2) | |
| NHS Tayside | Unassisted vaginal | 89 (49.2) | – | 41 (50.6) |
| Forceps assisted | 21 (11.6) | – | 9 (11.1) | |
| Ventouse assisted | < 5 | – | < 5 | |
| Caesarean | 69 (38.1) | – | 30 (37.0) | |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity) | Unassisted vaginal | – | – | 339 (54.9) |
| Forceps assisted | – | – | 59 (9.5) | |
| Ventouse assisted | – | – | 54 (8.7) | |
| Caesarean | – | – | 164 (26.5) | |
| Birmingham Women’s and Children’s NHS Foundation Trust | Unassisted vaginal | – | – | 1190 (52.1) |
| Forceps assisted | – | – | 289 (12.6) | |
| Ventouse assisted | – | – | 158 (6.9) | |
| Caesarean | – | – | 646 (28.3) | |
| Darlington Memorial Hospital | Unassisted vaginal | 6 (54.5) | – | 26 (41.9) |
| Forceps assisted | < 5 | – | 10 (16.1) | |
| Ventouse assisted | < 5 | – | < 5 | |
| Caesarean | < 5 | – | 22 (35.5) | |
| University Hospital of North Durham (Maternity) | Unassisted vaginal | 28 (65.1) | – | < 5 |
| Forceps assisted | 5 (11.6) | – | < 5 | |
| Ventouse assisted | < 5 | – | < 5 | |
| Caesarean | 10 (23.3) | – | < 5 | |
| Dorset County Hospital | Unassisted vaginal | – | 144 (53.3) | – |
| Forceps assisted | – | 16 (5.9) | – | |
| Ventouse assisted | – | 17 (6.3) | – | |
| Caesarean | – | 93 (34.4) | – | |
| Epsom Hospital (Maternity) | Unassisted vaginal | < 5 | – | 103 (62.4) |
| Forceps assisted | < 5 | – | 10 (6.1) | |
| Ventouse assisted | < 5 | – | 13 (7.9) | |
| Caesarean | < 5 | – | 39 (23.6) | |
| St Helier Hospital (Maternity) | Unassisted vaginal | < 5 | – | 208 (67.3) |
| Forceps assisted | < 5 | – | 15 (4.9) | |
| Ventouse assisted | < 5 | – | 18 (5.8) | |
| Caesarean | < 5 | – | 68 (22.0) | |
| Hereford County Hospital (Maternity) | Unassisted vaginal | – | – | 268 (54.1) |
| Forceps assisted | – | – | 30 (6.1) | |
| Ventouse assisted | – | – | 46 (9.3) | |
| Caesarean | – | – | 151 (30.5) | |
| Princess Royal University Hospital | Unassisted vaginal | – | 59 (41.8) | – |
| Forceps assisted | – | 19 (13.5) | – | |
| Ventouse assisted | – | 13 (9.2) | – | |
| Caesarean | – | 50 (35.5) | – | |
| King’s College Hospital (Maternity) | Unassisted vaginal | – | – | 51 (44.0) |
| Forceps assisted | – | – | 8 (6.9) | |
| Ventouse assisted | – | – | 9 (7.8) | |
| Caesarean | – | – | 48 (41.4) | |
| Royal Preston Hospital | Unassisted vaginal | – | – | 554 (64.2) |
| Forceps assisted | – | – | 70 (8.1) | |
| Ventouse assisted | – | – | 54 (6.3) | |
| Caesarean | – | – | 184 (21.3) | |
| Cumberland Infirmary (Maternity) | Unassisted vaginal | – | 165 (56.7) | – |
| Forceps assisted | 34 (11.7) | |||
| Ventouse assisted | 15 (5.2) | |||
| Caesarean | 77 (26.5) | |||
| West Cumberland Hospital (Maternity) | Unassisted vaginal | – | 40 (60.6) | – |
| Forceps assisted | 7 (10.6) | |||
| Ventouse assisted | < 5 | |||
| Caesarean | 17 (25.8) | |||
| Queen Elizabeth Hospital, Gateshead (Maternity) | Unassisted vaginal | – | – | 473 (63.7) |
| Forceps assisted | 115 (15.5) | |||
| Ventouse assisted | 22 (3.0) | |||
| Caesarean | 133 (17.9) | |||
| Warwick Hospital | Unassisted vaginal | – | 281 (63.1) | – |
| Forceps assisted | 27 (6.1) | |||
| Ventouse assisted | 13 (2.9) | |||
| Caesarean | 124 (27.9) | |||
| Queen Elizabeth Hospital, Kings Lynn (Maternity) | Unassisted vaginal | – | – | 242 (61.7) |
| Forceps assisted | 27 (6.9) | |||
| Ventouse assisted | 12 (3.1) | |||
| Caesarean | 110 (28.1) | |||
| New Cross Hospital, Wolverhampton (Maternity) | Unassisted vaginal | – | 667 (54.9) | – |
| Forceps assisted | 78 (6.4) | |||
| Ventouse assisted | 118 (9.7) | |||
| Caesarean | 352 (29.0) | |||
| Walsall Manor Hospital | Unassisted vaginal | – | 103 (56.0) | – |
| Forceps assisted | 24 (13.0) | |||
| Ventouse assisted | 9 (4.9) | |||
| Caesarean | 48 (26.1) | |||
| Worcestershire Royal Maternity Hospital | Unassisted vaginal | – | 678 (62.3) | – |
| Forceps assisted | 132 (12.1) | |||
| Ventouse assisted | 42 (3.9) | |||
| Caesarean | 237 (21.8) |
Unassisted vaginal birth occurred in 50.3% of the home balloon group and 57.1% of the hospital prostaglandin group. Birth assisted by forceps occurred in 14% of the home balloon group and 9.1% of the hospital prostaglandin group. Ventouse birth took place in 1.4% of the home balloon group and 5.9% in hospital prostaglandin group. Caesarean birth occurred in 34% of women in the home balloon group and 27.1% in the hospital prostaglandin group.
Breakdown of vaginal birth by setting is provided in Report Supplementary Material 1, Table 160.
Total breakdown of type of birth by parity following IOL (population 2) is shown in Table 16. Proportions vary substantially by parity. One in three (36%) women giving birth for the first time had an unassisted birth while 83% giving birth for a second time had this birth type and 86% giving birth for the third and fourth time had this birth type. The proportion of forceps-assisted births was 17% for first births compared to 4, 2 and 1.5% for second, third and fourth births, respectively.
| Parity | Unassisted vaginal birth (%) | Forceps assisted (%) | Ventouse assisted (%) | Caesarean (%) |
|---|---|---|---|---|
| 0 | 3378 (36.0) | 1620 (17.2) | 736 (7.8) | 3660 (39.0) |
| 1 | 3864 (83.0) | 192 (4.1) | 158 (3.4) | 439 (9.4) |
| 2 | 1816 (86.5) | 43 (2.0) | 46 (2.2) | 194 (9.2) |
| 3 + | 1187 (86.1) | 21 (1.5) | 28 (2.0) | 140 (10.2) |
Breakdown of type of birth by parity and setting of IOL is shown in Table 17. Forceps were used in 18.9% of primiparous women after home balloon and 14.4% after hospital prostaglandin. In women with one previous birth, respective percentages were 6.8% and 2.6%. Caesarean birth was performed in 45% of primiparous women after home balloon and 39.1% after hospital prostaglandin. In women with one previous birth, caesarean took place in 12.7% after home balloon and 10.5% after hospital prostaglandin.
| Parity | Birth type | Home balloon, N = 515 (%) | Hospital prostaglandin, N = 4332 (%) | Background context group: hospital prostaglandin – mixed hospital, N = 7397 (%) |
|---|---|---|---|---|
| Parity 0 | Unassisted vaginal | 115 (33.9) | 947 (38.4) | 1336 (33.6) |
| Forceps assisted | 64 (18.9) | 356 (14.4) | 681 (17.1) | |
| Ventouse assisted | 6 (1.8) | 197 (8.0) | 328 (8.3) | |
| Caesarean | 154 (45.4) | 965 (39.1) | 1627 (41) | |
| Parity 1 | Unassisted vaginal | 94 (79.7) | 946 (83.6) | 1558 (80.5) |
| Forceps assisted | 8 (6.8) | 29 (2.6) | 89 (4.6) | |
| Ventouse assisted | < 5 | 37 (3.3) | 73 (3.8) | |
| Caesarean | 15 (12.7) | 119 (10.5) | 212 (11) | |
| Parity 2 | Unassisted vaginal | 34 (85.0) | 394 (85.1) | 762 (84.1) |
| Forceps assisted | < 5 | 6 (1.3) | 27 (3.0) | |
| Ventouse assisted | < 5 | 10 (2.2) | 22 (2.4) | |
| Caesarean | < 5 | 53 (11.4) | 95 (10.5) | |
| Parity 3 + | Unassisted vaginal | 16 (88.9) | 222 (81.3) | 495 (85.1) |
| Forceps assisted | < 5 | < 5 | 11 (1.9) | |
| Ventouse assisted | < 5 | 10 (3.7) | 13 (2.2) | |
| Caesarean | < 5 | 37 (13.6) | 61 (10.5) |
Postpartum haemorrhage over 1000 ml was reported by site and setting of cervical ripening, as shown in Table 18. Proportions ranged from 11% to 14% at the two sites with large enough numbers to report a proportion for the home balloon group and from 9% to 14% in the hospital prostaglandin group. The background context – ‘hospital prostaglandin – mixed hospital’ group had wider ranges at 9–23%.
| Hospital or NHS Trust | Confirmed inductions (population 2), n/N (%) | Exposed: at home balloon, n/N (%) | Unexposed: in hospital prostaglandin, n/N (%) | Background context group: in hospital prostaglandin in mixed hospital, n/N (%) |
|---|---|---|---|---|
| NHS Fife | 106/756 (14.0) | 93/631 (14.7) | ||
| NHS Grampian | 71/759 (9.4) | 26/235 (11.1) | 10/104 (9.6) | |
| NHS Highland | 55/462 (11.9) | 43/334 (12.9) | ||
| NHS Tayside | 75/753 (10.0) | 27/181 (14.9) | 11/81 (13.6) | |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity) | 130/852 (15.3) | 93/618 (15.1) | ||
| Birmingham Women’s and Children’s NHS Foundation Trust | 305/2664 (11.5) | 264/2286 (11.6) | ||
| Dorset County Hospital | 59/387 (15.3) | 40/270 (14.8) | ||
| Epsom Hospital (Maternity) | 85/653 (13.0) | < 5 | 17/165 (10.3) | |
| St Helier Hospital (Maternity) | 84/793 (10.6) | < 5 | 33/309 (10.7) | |
| Hereford County Hospital (Maternity) | 60/585 (10.3) | 48/495 (9.7) | ||
| Princess Royal University Hospital | 22/142 (15.5) | 22/141 (15.6) | ||
| King’s College Hospital (Maternity) | 20/160 (12.5) | 13/116 (11.2) | ||
| Royal Preston Hospital | 114/1070 (10.7) | 95/863 (11.0) | ||
| Cumberland Infirmary (Maternity) | 49/435 (11.3) | 28/291 (9.6) | ||
| West Cumberland Hospital (Maternity) | 30/191 (15.7) | 9/66 (13.6) |
Retained placenta requiring manual removal was reported in < 0.2% of cases in all settings and < 5 in the home balloon group, hence only the total for the whole IOL population is shown in Table 19.
| Confirmed inductions (population 2) n/N (%) | |
|---|---|
| Total | 15/17,530 (0.1) |
Pyrexia in labour was reported in 5% of cases of IOL, 7% in the home balloon group, 4% in the hospital prostaglandin group and 6% of women in the background context hospital prostaglandin mixed-hospital group, as shown in Table 20.
| Confirmed inductions (population 2), n/N (%) | Home balloon, n/N (%) | Hospital prostaglandin, n/N (%) | Background context group: hospital prostaglandin – mixed hospital, n/N (%) | |
|---|---|---|---|---|
| Total | 934/17,515 (5.3) | 39/515 (7.6) | 198/4330 (4.6) | 451/7395 (6.1) |
Percentage of pyrexia after induction of labour by setting
Place of birth is reported for all IOL cases (≥ 38 °C from observations) and by setting of cervical ripening, as shown in Table 21.
| Confirmed inductions (population 2), n/N (%) | Home balloon, n/N (%) | Hospital prostaglandin, n/N (%) | Background context group: hospital prostaglandin – mixed hospital, n/N (%) | |
|---|---|---|---|---|
| Hospital shared care | 14,819 (84.5) | 482 (93.6) | 4250 (98.1) | 5466 (73.9) |
| Midwife-led unit | 200 (1.1) | 8 (1.6) | 25 (0.6) | 102 (1.4) |
| Consultant-led unit | 2499 (14.3) | 20 (3.9) | 57 (1.3) | 1827 (24.7) |
Duration of first NNU admission was reported for all IOL cases and by setting of cervical ripening as shown in Table 22. These data show that median duration in the home balloon group was 2429 minutes (IQR 1121–6506 minutes) and in the hospital prostaglandin group was 3355 minutes (IQR 2220–6665 minutes). Further breakdown of duration of first and total NNU admission by setting of cervical ripening is provided in Report Supplementary Material 1, Tables 161–163.
| Confirmed inductions (population 2) median (IQR) | Home balloon median (IQR) | Hospital prostaglandin median (IQR) | Background context group: hospital prostaglandin – mixed hospital median (IQR) | |
|---|---|---|---|---|
| Duration of first NNU admission Median in minutes (IQR) |
3068 (2140–6341), N = 2110 | 2430 (1121–6506), N = 40 | 3355 (2220–6665), N = 369 | 3118 (2194–6391), N = 1048 |
| Total time spent in NNU Median in minutes (IQR) |
3321 (2268–6555), N = 2110 | 2833 (1302–7103), N = 40 | 3649 (2387–6776), N = 369 | 3350 (2310–6555), N = 1048 |
Exploratory neonatal outcomes
In population 2, the number of events of several neonatal outcomes were fewer than five in at least one of the individual cohorts; thus, these totals are provided for the whole of population 2 only with no further breakdown by setting of cervical ripening, as shown in Table 23.
| Outcomes | n events (N = 17,530) |
|---|---|
| Neonatal seizures | 25 |
| Mechanical ventilation | 61 |
| Neonatal death | 10 |
The total number of events in the composite serious neonatal morbidity (neonatal seizures, intracranial haemorrhage, stillbirth or neonatal death) was 23 in population 2, but with < 5 in one group, no further breakdown is reported.
For the following three outcomes, less than five events (including zero) were recorded in population 2, so are not reported here: intracranial haemorrhage, infant death, meconium-stained liquor, stillbirth after admission, hypoxic–ischaemic encephalopathy.
For a breakdown of neonatal care provision, recording of transitional care or postnatal ward care provided by neonatology service was not clearly recorded so is not reported. The same issue arose for treatment for neonatal sepsis and treatment in a NNU for infection.
Analysis of IOL after previous caesarean birth and IOL in multiple pregnancies were not performed at this stage as numbers were limited. The prediction of which women will have caesarean birth after IOL will be performed as part of a PhD project starting in 2023.
Observational cohort study discussion
The CHOICE observational cohort study used a large electronic data set to prospectively report on IOL practice across 26 UK maternity units. It explored the safety of cervical ripening at home using balloon methods, and while findings do not indicate safety concerns compared with hospital prostaglandin cervical ripening, the sample size was underpowered to provide a precise estimate. The study sample is likely to be largely representative of the wider UK population given the diverse set of study sites included.
Despite a push from policy-makers towards reducing hospital attendances and admissions as part of pandemic mitigation measures, the CHOICE study has demonstrated that intentions to move cervical ripening to home settings did not consistently translate into practice. In addition, the use of dinoprostone cervical ripening at home appears infrequent, with balloon cervical ripening being the predominant method used at home where home cervical ripening is offered at all. This contrasts with recommended practice at the time of protocol development, where only home cervical ripening with dinoprostone was endorsed. As such, the CHOICE study adapted to compare outcomes of home cervical ripening using balloon with in-hospital cervical ripening using prostaglandin. This pragmatic approach meant that the two predominating strategies to IOL setting in the UK were compared head to head. It does however mean that both method and setting of cervical ripening differed between the exposed and the comparator group. Thus, any potential differences identified between the two groups could not be attributed to either method or setting, but instead to a combination of both.
The primary safety outcome of admission to a NNU within 48 hours of birth for 48 hours or more occurred at a similar rate across the home balloon and hospital prostaglandin cervical ripening groups after adjustment for potential confounders. While this may suggest that the home balloon option is not inferior from a safety perspective, the wide confidence intervals reflect the smaller than anticipated sample size, so no such conclusions can be drawn regarding safety until larger studies are conducted. The smaller than anticipated number of women having home cervical ripening meant that only descriptive statistics were performed to explore secondary outcomes; thus larger studies are also required to identify precise differences in odds of these outcomes.
Of interest to health services and pathway development was the time taken from IOL to birth, and time spent in hospital across the home balloon and hospital prostaglandin groups. The home balloon cervical ripening group spent on average 1000 minutes at home during cervical ripening, while the hospital prostaglandin group spent around 1800 minutes in antenatal wards before moving to labour ward. However, interpretation of any apparent differences in the duration of each stage of labour by setting is limited by potential variation in practice across units, especially as most home balloon cases arose from just two units (NHS Grampian and NHS Tayside). It is possible that the duration of the initial stages of labour are influenced more by local practice than the setting per se, as prioritisation of women for transfer to labour ward may differ in each unit and management of first and second stage of labour may also vary. The role of differing prioritisation practices in increased duration of induced labour is supported by the findings from the background group of women having in-hospital cervical ripening in hospitals that also offer home cervical ripening. Women in this group have an overall duration from IOL onset to birth that was 257 minutes less than those having in-hospital prostaglandin cervical ripening in hospitals that only offered this option. Given that the former group are inherently higher risk (as they did not have home cervical ripening in a unit that also offered this option), the findings suggest that their risk status may affect prioritisation practices (and thus shortens their duration of IOL when managing flow of women through labour ward). Given that women who went home for balloon cervical ripening spend over 1000 minutes at home during the process, there is potential for substantial differences in both experiences and costs of this approach when compared with in-hospital cervical ripening.
Findings indicated that home balloon cervical ripening was carried out at 37 completed weeks of gestation in 47 cases in the cohort. While numbers are small, these suggest that use of home balloon cervical ripening may be as effective at allowing labour to establish at early term gestations as at term or post-dates.
The proportion of women having caesarean birth in the home balloon group (34%) is in keeping with the proportion of the group being in their first pregnancy and the proportion being beyond 41 weeks of pregnancy, as both are substantial risk factors for caesarean birth. Previous research would suggest that neither being at home nor having a balloon for cervical ripening increase caesarean birth rate, with the best available evidence on balloon versus prostaglandin cervical ripening to date being published in November 2022. 21 This individual participant data meta-analysis of almost 4000 women in randomised controlled trials showed no significant difference in caesarean birth rate between the two methods. The study did not, however, consider setting and randomised trial evidence of the impact of setting of cervical ripening on caesarean birth rate is lacking.
Use of routinely collected data and the inherent incompleteness of these led to several key variables not being available for analysis in the CHOICE study for some potential participants, for example, method used for cervical ripening. This led to a large proportion of women not being included in any of the analyses as either the method or the setting for IOL could not be confirmed; thus a complete case analysis was conducted. No imputation or sensitivity analyses were performed to explore the potential impact of missing data as it was not clear which data were missing and which simply did not apply to those individuals. This limits the ability to interpret the findings and how likely these are to represent the truth.
Lack of power raised risk of type 2 error in the primary outcome and limited the reporting of secondary outcomes to descriptive analyses only. This limited the ability to interpret the findings as its unclear whether apparent differences in proportions across study groups (e.g. caesarean birth) are fully explained by differences in baseline characteristics of each group (e.g. differences in parity, gestation at onset of IOL). Similarly, less than one-quarter of participants in the home balloon cervical ripening group had a re-admission time recorded after going home during cervical ripening such that duration of time spent at home is based upon only 110 women. Incomplete recording of information for specific outcomes (where no consistent variable is used for recording the outcome) appears to have been an issue in the CHOICE study, with the example of retained placenta where only 0.1% cases were affected, yet the published prevalence of this condition is 1–3%. 22 This is not an issue for variables, such as maternal age and mode of birth, where recording is complete. Data on maternal intensive care admission, umbilical cord prolapse or birth outside of hospital could not be identified (i.e. data were not consistently recorded using a single variable); thus these were not reported. Furthermore, several exploratory outcomes could not be reported due to less than five events in any cohort, including intracranial haemorrhage, infant death and meconium-stained liquor. Lack of available data on a breakdown of care type when under neonatal care meant that it was not possible to assess differences in provision of special, high dependency, intensive, transitional or postnatal ward care.
Lack of oxytocin data meant that it was not possible to compare dose or duration of oxytocin during labour across study groups. It is unclear whether such data were not recorded at all or whether they were not recorded in the fields that it had been anticipated to be recorded in.
Future research should seek to confirm the descriptive findings of the CHOICE study using high-quality routinely collected data that allow for adjusted analyses to account for potential confounding. The safety of home cervical ripening for women should be considered, along with the impact of the apparently longer duration of labour on experiences and outcomes. Future studies would benefit from detailed oxytocin usage data to allow exploration of the reason for longer second stage of labour in the home balloon cervical ripening group compared to in-hospital prostaglandin cervical ripening.
Overall, the CHOICE observational cohort study findings suggest that home cervical ripening using balloon at term in low- and moderate-risk pregnancies is a slow but potentially safe procedure for women in both first and subsequent pregnancies, but larger and more complete data sets are required to confirm these findings.
Chapter 3 qCHOICE process evaluation
qCHOICE objectives
The purpose of the process evaluation was:
-
to assess whether home cervical ripening is acceptable to women, their families and healthcare professionals and cost effective from the perspective of women and their partners (reported in Chapter 4)
-
to describe the contextual influences on the implementation and practices in relation to cervical ripening protocols, and outcomes of cervical ripening, in different settings (e.g. different size units, rural and urban settings).
Specific qCHOICE research questions:
-
In what ways does the service context influence cervical ripening approaches in hospital or out of hospital settings?
-
What is the acceptability of home or hospital and different methods of cervical ripening to women (and their birth partners) and implications for their experience of IOL and care?
-
What is the acceptability of home cervical ripening from the perspective of healthcare professionals?
-
What are the cost implications from the service user perspective?
-
What information and outcomes are important for pregnant women and their partners?
-
What are the psychological correlates of cervical ripening setting?
-
What potential factors mediate women’s experiences, for example, rurality, distance from hospital, information provision, professional support?
-
What are the service barriers and enablers of adoption of home cervical ripening?
-
Are there any unintended consequences associated with home cervical ripening, for individuals, families and or services involved?
qCHOICE process evaluation methods
Study design
The process evaluation comprised a questionnaire-based survey and case studies nested within the CHOICE observational cohort study. Case studies involved semistructured interviews with women and birth partners, and interviews and focus group discussions with professionals and key stakeholders.
An additional survey of all UK maternity units was undertaken to determine impact of the COVID-19 pandemic on IOL policy and practice.
Changes to study design
The process evaluation was undertaken during the COVID-19 pandemic, and this affected the study process in several ways.
Site visits and face-to-face contact with NHS staff and study participants were not possible for most of the study. All work was carried out remotely, with meetings, interviews and focus groups taking place online using video conferencing [Microsoft Teams (Microsoft Teams Microsoft Corporation, Redmond, WA, USA) and Zoom (Zoom Video Communications, San Bruno, CA, USA)]. The research team was unable to develop visual models to support the discussion and analysis of the pathway and network maps using Visio, as it was not accessible via Microsoft Teams and Zoom. It was not possible to complete planned observation of IOL consultation appointments or the associated short follow-up interviews, as access to NHS sites was restricted. A survey of all maternity units to understand changes to practice and policy related to the pandemic was added to the process evaluation.
Small numbers of women having home cervical ripening meant that psychological outcomes could not be compared between settings.
In addition, several changes to the recruitment strategy for the postnatal survey were made over the course of the process evaluation in response to initial low response rates when recruiting solely via the BadgerNet electronic maternity notes system. Detailed information about changes to the recruitment strategy is given in the section Data collection later.
Participants
-
Postnatal questionnaire-based survey.
Anyone who had undergone an IOL at 37 weeks or more at the 26 CHOICE study sites was initially eligible to participate. Those who suffered pregnancy or neonatal loss were excluded.
-
Case studies.
Participants in the case study interviews and focus groups were all drawn from the five case study sites and included:
-
A purposive sample of healthcare professionals and stakeholders, including: clinical directors, heads of midwifery, maternity commissioners, representatives from Maternity Voices Partnerships, obstetricians, midwives and other healthcare professionals involved in IOL policy development and/or clinical practice.
-
A purposive sample of women who had completed the questionnaire-based survey, given birth in one of the case study sites and had given consent for further contact on the survey.
-
A purposive sample of birth partners of the women who took part in the interviews. Birth partner was defined by the woman and included co-parents, friends and other relatives who had supported them during their IOL.
Study settings
-
Online postnatal questionnaire-based survey available initially in all CHOICE study sites.
-
Case study sites.
Five NHS trusts and health boards in England and Scotland were selected for variation in service context and configuration, setting and method of cervical ripening.
-
COVID impact survey.
All NHS trusts and boards in the UK that offer maternity services as identified through the NHS service directories for the four UK countries were invited to take part (n = 157).
Outcomes
The planned outcomes were to describe women, birth partner and stakeholder experiences of IOL and cervical ripening. Specifically, the intention was to identify barriers and enablers to offering home cervical ripening, and aspects of positive and negative experiences in relation to IOL and cervical ripening. Use of questions based on the Labour Agentry Scale were planned as a primary outcome when comparing home and hospital cervical ripening. 23
Changes to outcomes
The small number of people who were offered home cervical ripening and who returned home (13% of survey respondents) led to a change in available outcomes.
For the questionnaire-based survey, it was not possible to report inferential statistics comparing home and hospital cervical ripening, including on the primary outcome of labour agentry, and descriptive statistics were undertaken instead.
As women and birth partner interview participants were recruited through the survey, the number of participants who had direct experience of returning home during cervical ripening was lower than anticipated.
The COVID impact survey was added to the process evaluation and provided important additional data to inform context.
Sample size
Postnatal questionnaire-based survey
The original purpose of the postnatal survey was to compare women who had home cervical ripening with those who stayed in hospital in the five case study sites, with sense of control (Labour Agentry) as the primary comparative measure. The sample size required to compare the experiences of women who had home and hospital cervical ripening was estimated to be 89 per group (178 in total) for a probability of type 1 error set at 0.05 for a two-tailed comparison and an 80% power.
Based on the facility in BadgerNet to administer the survey via push notifications in women’s handheld electronic maternity notes, a decision was taken to open the survey to all 26 CHOICE sites and increase the target sample accordingly. However, this strategy proved ineffective in practice since it became apparent that few women accessed their electronic maternity notes in the postnatal period. In addition, realisation that there were very small numbers of home cervical ripening necessitated a change in analysis plan and a shift in target sample size. A pragmatic sample size of 300 respondents was deemed practical, achievable, and useful for the revised purpose of describing the experience of women who undergo cervical ripening at home and in hospital.
Case studies
A sample of five case studies was decided on, with selection designed to balance depth with breadth of information and analysis. The choice of case study sites formed a substudy of the CHOICE sample of sites, to provide diversity and balance of service types based on geography, service configuration and approaches to provision of IOL.
The target sample sizes, shown in Table 24, for the interviews and focus groups were also pragmatic and based on an estimation of numbers needed in a purposive sample to achieve information power. 24
| Method | Participant group | Number |
|---|---|---|
| Interviews | Healthcare professionals and stakeholders (10 per site) | 50 |
| Women (10–15 per site) | 50–75 | |
| Birth partners (5–7 per site) | 25–35 | |
| Focus groups | Healthcare professionals (3 per site with 6–8 participants) | 90–120 |
| Total | 215–280 | |
COVID impact survey
The target sample for the survey was representation of all UK NHS trusts and boards that offer maternity services (n = 156). It was anticipated that response rate may be low and having this ambitious target was deemed the best way to achieve a response rate that would enable adequate representation to assess the impact of the pandemic on factors that inform understanding of the context of the CHOICE study and qCHOICE process evaluation.
Data collection
The following methods were used to gather data.
Postnatal questionnaire-based survey
An online survey of postnatal women who had undergone IOL at 37 or more weeks of pregnancy. The questionnaire [Project Documents via National Institute for Health and Care Research (NIHR) Journal project web page: qCH-PD6] was designed to explore women’s views and experiences of key elements of the IOL process including cervical ripening at home and in hospital. It comprised fixed response questions and free-text options about key issues. Questions designed to assess satisfaction, labour agentry, and mental well-being were based on previously tested surveys. 23,25,26
A modified and abridged version of the IOL satisfaction questionnaire was used. 27 This focused on women’s experiences of aspects of IOL including information, anxiety and physical and emotional discomfort. Five positively and negatively worded statements about experiences of process of cervical ripening; 10 statements about the time from cervical ripening to labour room admission; 13 about the IOL process overall; 5 about time from admission to birth. Responses were a five-point Likert scale 'strongly agree' to 'strongly disagree', reported using N (%) agreement to create three categories: merging 'strongly agree’ with ‘agree’, and ‘strongly disagree’ with ‘disagree’.
A series of 10 questions from the Labour Agentry Scale (short; 23) were used to measure sense of control during childbirth. Responses were a six-point Likert-type scale and analysis was reported as percentage agreement across three categories: agree, neutral and disagree.
Demographic questions and questions about information and decision-making were based on questions in the Scottish National Maternity Survey altered to focus on IOL. 26
The questionnaire was primarily online, hosted by Online Surveys (www.onlinesurveys.ac.uk). Eligible participants could also request a paper version of the survey or to complete the survey verbally over the telephone. Use of an interpreter or translator could also be requested.
Recruitment
Women were initially invited to complete the questionnaire through their BadgerNet electronic maternity notes app in use at all CHOICE study sites. A push notification was automatically sent to eligible participants via the smartphone application (app) at the time of IOL booking. This notification directed them to view an electronic study leaflet within the BadgerNet maternity notes app. A second notification was sent automatically at point of transfer from maternity services, around 10 days postnatal. This notification contained a link to the online survey site.
However, recruitment via the BadgerNet maternity notes app was low and it was not possible to know whether push notifications were seen by women. This problem was addressed by implementation of further strategies to increase recruitment, in the five case study sites, as per the original plan for the survey.
Invitation by midwives was put in place at the five case study sites. The research midwife working at the maternity unit identified eligible women and provided them with a study card or sent them a letter with the URL and a QR code link to the online survey site.
Potential participants were also invited to take part via focused social media: (1) a paid-for Facebook (Facebook Inc., Menlo Park, CA, USA) advertising campaign targeted eligible participants in areas local to the five case study sites; (2) the survey information and link were shared on the Facebook pages of relevant organisations and groups local to case study sites. These included pages of the five case study sites maternity services and Maternity Voices Partnerships.
Information and consent for participation
The online survey landing page directed potential participants to the participant information sheet, consent form and contact details for researchers. It was not possible to access the survey without completing these steps.
Case studies
Interviews and focus groups were conducted with healthcare professionals and stakeholders from the five case study sites. The aim was to explore the IOL process locally at each site, acceptability of home cervical ripening, facilitators and barriers to home cervical ripening adoption and any unintended consequences of home cervical ripening. Topic guides were used for interviews and focus groups topic and covered implementation of local cervical ripening guidelines in practice, experiences of providing IOL care more generally, views on acceptability of home cervical ripening from a service perspective, and facilitators and barriers to offering at home cervical ripening safely (Project Documents via NIHR Journal project web page: qCH-PD9, qCH-PD10).
Interviews were conducted with women and birth partners from the five case study sites. The aim was to explore the acceptability of home cervical ripening, information and outcomes, factors mediating experiences of home cervical ripening and any unintended consequences of home cervical ripening. Topic guides were used for interview and covered experiences of receiving IOL care more generally and views on acceptability of home cervical ripening from a service user perspective (Project Documents via NIHR Journal project web page: qCH-PD7, qCH-PD8).
Recruitment
Due to COVID-19 restrictions, all recruitment, interviews and focus groups were conducted remotely by authors CY and MH, together with three MSc students based at City, University of London. All participants were sent a participant information sheet and consent form via e-mail at least 24 hours before the interview or focus group (Project Documents via NIHR Journal project web page: qCH-PD1, qCH-PD4). They were given an option of returning the consent form electronically or providing verbal consent before the data collection (Project Documents via NIHR Journal project web page: qCH-PD5).
Interviews and focus groups with healthcare professionals and stakeholders
Recruitment was conducted remotely through local principal investigators, who approached clinical directors, heads of midwifery, service managers, midwives, obstetricians, other NHS staff, such as pharmacists, and key stakeholders, such as members of local Maternity Voice Partnerships, to take part in either an interview or a focus group.
Interviews with women and birth partners
While completing the postnatal survey, women were given the option to provide their contact details and give permission for a member of the research team to contact them regarding a possible interview.
Consent to contact birth partners for interviews was discussed at the end of interviews with women, who provided their contact information to the research team. Birth partners were then contacted by the research team.
COVID impact survey
A questionnaire-based survey (Project Documents via NIHR Journal project web page: qCH-PD11) of healthcare professionals at all UK NHS Trusts and Boards to determine whether aspects of practice and policy around IOL had altered in response to the pandemic.
The research team carefully considered the process of IOL, with a focus on cervical ripening and key elements of that process formed the basis of a questionnaire. The questionnaire comprised fixed response questions and free-text options requesting additional detail, allowing respondents to provide written comments about key issues. The survey also included questions to determine perceived changes in women’s response to IOL in the context of COVID-19.
The questionnaire was available via Online Surveys (www.onlinesurveys.ac.uk), with a Microsoft Word (Microsoft Corporation, Redmond, WA, USA) version available should respondents have difficulties accessing the online survey site.
Recruitment
Senior midwifery and obstetric staff at all NHS trusts and boards in Scotland, Wales, England and Northern Ireland were contacted by e-mail through established networks including the Royal College of Midwives heads of midwifery network, professional contacts of CHOICE study team; local clinical research networks and the British Intrapartum Care Society.
Potential participants were provided with information about the survey and its purpose, a link to the online survey and a Microsoft Word version of the questionnaire. If the staff member who had been contacted initially was unable to complete the survey or did not feel that they had enough knowledge of IOL policy and practice to do so accurately, they were asked to pass the information to another colleague within the same trust or board who they felt could do this. Reminders were sent at 2 and 4 weeks after initial contact.
Analysis
Postnatal questionnaire-based survey
Quantitative data: survey data were exported to SPSS® (IBM, Armonk, NY, USA) software and descriptive statistics produced. Overall, 309 eligible responses were included in the analysis. Just 36 respondents (13%) returned home during cervical ripening and, as a result, it was not possible to report statistically significant differences in the primary outcome of sense of control (Labour Agentry) or by psychosocial outcome of postnatal psychological well-being score [Warwick–Edinburgh Mental Wellbeing Scales (WEMWBS)] between women with home cervical ripening and women with in-hospital ripening.
Qualitative data: free-text responses were deidentified and analysis support software NVivo (QSR International, Warrington, UK) was used to aid thematic content analysis of the data.
Case studies
All interviews and focus groups were video- or audio-recorded and then transcribed in full before being imported to a bespoke NVivo 12 (Lumivero, Denver, CO, USA) database to support data management and analysis. Documentary sources, particularly local IOL guidelines, were added to the NVivo project file as PDF files. An abductive approach was employed for the thematic analysis. 28,29
COVID impact survey
Survey data were imported to IBM SPSS software and descriptive statistics produced. Simple content and thematic analysis of free-text responses was also undertaken.
qCHOICE process evaluation results
Data collected
Postnatal questionnaire-based survey
The data collection period was extended after initial poor uptake, with the survey remaining open between February 2021 and April 2022; 309 eligible responses were received.
Detailed tables containing survey results are included in Appendix 1, Tables 43–109 and Appendix 2, Tables 110–117.
Case studies
Staff: interviews (n = 48) and four focus groups (n = 28) were conducted with midwives, obstetricians, other staff and stakeholders, between November 2020 and December 2021 with a total of 76 participants.
Service users: interviews with women and birth partners (n = 60) were conducted between April 2021 and May 2022.
The number of participants who took part in interviews and focus groups are shown in Table 25.
| Method | Participant group | Numbers |
|---|---|---|
| Interviews (N = 108) | Midwives | 29 |
| Obstetricians | 14 | |
| Other NHS staff | 2 | |
| Stakeholders | 3 | |
| Women | 43 | |
| Birth partners | 17 | |
| Focus groups (N = 4) | Midwives | 8 |
| Obstetricians | 20 | |
| Total | 136 |
COVID-19 impact survey
The COVID-19 impact survey was open between June and November 2020; 92 eligible responses were received, a response rate of 59%. Table 26 shows the professional role of the survey respondents.
| Work role of person completing the survey | Numbers |
|---|---|
| Senior specialist midwife or midwifery manager | 31 |
| Consultant obstetrician | 25 |
| Head of midwifery | 24 |
| Consultant midwife | 16 |
| Research midwife | 8 |
| Clinical director | 4 |
| Clinical midwife | 3 |
| Obstetric trainee (specialty trainee year 7) | 1 |
| Total | 112a |
Losses and exclusions
In the postnatal questionnaire-based survey, a total of 320 responses were received. Nine responses were excluded as respondents had not had an IOL and a further two because their IOL happened prior to the CHOICE study commencing. A total of 309 responses were included in analysis.
Findings
Postnatal questionnaire-based survey
Summary findings are presented below.
Respondents had given birth in Scotland, England and Wales at 19 CHOICE study sites (NHS boards and trusts) and a further six NHS areas. Summary statistics for the women who responded are given in Table 27. Appendix 1, Figure 9 provides a visual summary of the distribution of gestation at which respondents had IOL.
| First baby? N = 309 | Yes: 206 (67%) | No: 103 (33%) | ||||
|---|---|---|---|---|---|---|
| Maternal age (years) N = 309 |
Min: 19 | Max: 52 | Median: 31 | |||
| Baby’s birthweight (grams) N = 297 (12 missing) |
Min: 1790 | Max: 6600 | Median: 3500 | |||
| Ethnicity N (%) N = 307 (2 missing) |
White: 291 (95) | Asian/Asian British: 8 (3) | Black: 4 (1) | Mixed/multiple ethnicity: 4 (1) | ||
| Social deprivation index N (%) N = 306 (3 missing) |
1 (most deprived) 61 (20) |
2 57 (19) |
3 60 (20) |
4 73 (24) |
5 (least deprived) 55 (18) |
|
| Gestation at IOL (weeks) N = 309 |
Min: 37 | Max: 42 | Median: 39 | |||
| Reason for IOL N (%) N = 309 |
Medical (e.g. raised blood pressure) 146 (47) |
Length of pregnancy 70 (23) |
Size of baby (large or small) 37 (12) |
SROM 20 (7) |
RFM 19 (6) |
Other 14 (5) |
| Method of IOL N (%) N = 309 |
Prostaglandin gel/pessary 202 (65) |
Balloon catheter 43 (14) |
Non-cervical ripening methods: membrane sweep, amniotomy, intravenous oxytocic 38 (12) |
Prostaglandin gel/pessary and balloon catheter 12 (4) |
Osmotic dilator (e.g. Dilapan-S) 9 (3) |
Don’t know 5 (2) |
| Cervical ripening? N (%) N = 304 (5 missing) |
Yes: 266 (86) | No: 38 (12) | ||||
| Home cervical ripening N (%) N = 266 |
Offered option to return home 39 (15) |
Returned home 36 (14) |
Case studies
Appendix 1, Table 45 provides an overview of the case study sites, including their cervical ripening pathways, as reported in local guidelines and by participants during data collection. All five case study sites initially provided home cervical ripening (two using balloon catheter); however, their local guidelines changed due to COVID-19 restriction, with one site increasing this option and another one site suspending it completely for a majority of the study period. The local guidelines for IOL including methods used for cervical ripening, both at home and in hospital, and eligibility criteria for those offered the option to go home varied between sites.
Appendix 1, Tables 46–109 provide the full results from the postnatal survey items.
Appendix 2, Tables 110–117 provide the full qualitative (free-text) responses to the postnatal survey.
Appendix 1, Figures 9–13 provide visual illustrations of specific survey item responses.
Please refer to the section Sample size later for detailed findings from the case studies.
COVID impact survey
Responses were received from 92 of the 156 NHS trusts and boards offering maternity services across the UK, as shown in Table 28.
| Country (N) | Trust and board responses received, N (%) | Total responses, N (%) |
|---|---|---|
| England (130) | 71 (55) | 71 (77) |
| Scotland (14) | 14 (100) | 14 (15) |
| Wales (7) | 4 (57) | 4 (4) |
| Northern Ireland (5) | 3 (60) | 3 (3) |
| UK Total (156) | 92 (59) | 92 (100) |
Those who took part responded to questions about changes to IOL practice. While many stated that there was no change to professionals undertaking cervical ripening or approaches to post-dates IOL, there were indications of change regarding method of cervical ripening and criteria for home cervical, as shown in Table 29.
| Area of practice | Yes, N (%) | No change, N (%) |
|---|---|---|
| Has there been a change in method used for cervical ripening? | 21 (23) | 69 (75) |
| Has there been a change in professional undertaking cervical ripening? | 6 (7) | 85 (92) |
| Has there been a change in approach for post-dates IOL? | 5 (6) | 86 (93) |
| Has there been a change in criteria for offering home cervical ripening? | 26 (28) | 60 (65) |
A little more than half (56%) of the respondents also indicated there was no change to the number of women returning during cervical ripening, and 26% of respondents stated that this number increased in response to the pandemic, as shown in Table 30.
| More women discharged home after cervical ripening, N (%) | Fewer women discharged home after cervical ripening, N (%) | No change, N (%) | Missing, N (%) |
|---|---|---|---|
| 26 (28) | 5 (5) | 56 (61) | 7 () |
Findings by qCHOICE study objectives
-
In what ways does the service context influence cervical ripening approaches in hospital or out of hospital settings?
Women described variation in policy and practice across all sites. It was common to hear of care being planned around service capacity rather than in line with guidance/policy. At times, this proved detrimental.
Had balloon induction 8am Monday. Balloon out 8am Tuesday and was 2–3 cm. However, was sent home as there were not enough midwives to induce me further … was taken back in on Thursday 4pm. 7am Friday taken to the delivery room [for ARM] … at that point was then back to 1 cm.
Survey participant 89753808
There were significant delays to the IOL process. Of those who remained in hospital and had cervical ripening (n = 230), the range was 0–260 hours, with a mean stay prior to transfer to labour suite of 31.5 hours.
Staff were pushed to the brink, which is why I was in hospital for 11 days before my waters were broken.
Survey participant 89021852
Some women reported unsafe physically and emotionally unsafe situations caused by delays.
I was told I couldn’t have [an epidural] until I moved to labour ward but I couldn’t move to labour ward as it was full. I was only moved when I was pushing.
Survey participant 88910706
The case studies revealed variation in policy and practice across all sites (Table 31). Increased IOL rates and staffing shortages were the primary contextual influences shaping cervical ripening approaches to in-hospital and at home.
| Hospital or NHS Trust | Number of women having prostaglandin cervical ripening at any point among all confirmed inductions (population 2), n/N (%) |
|---|---|
| NHS Borders | 40/170 (23.5) |
| NHS Fife | 637/756 (84.3) |
| NHS Grampian | 122/759 (16.1) |
| NHS Highland | 341/462 (73.8) |
| NHS Lanarkshire | 813/1050 (77.4) |
| NHS Tayside | 97/753 (12.9) |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (Maternity) | 705/852 (82.8) |
| Birmingham Women’s and Children’s NHS Foundation Trust | 2355/2664 (88.4) |
| Darlington Memorial Hospital | 65/143 (45.5) |
| University Hospital of North Durham (Maternity) | 5/164 (3.1) |
| Dorset County Hospital | 274/387 (70.8) |
| Epsom Hospital (Maternity) | 183/653 (28.0) |
| St Helier Hospital (Maternity) | 420/793 (53.0) |
| Hereford County Maternity | 507/585 (86.7) |
| Princess Royal University Hospital | 141/142 (99.3) |
| King’s College Hospital (Maternity) | 125/160 (78.1) |
| Royal Preston Hospital | 929/1070 (86.8) |
| Cumberland Infirmary (Maternity) | 293/435 (67.4) |
| West Cumberland Hospital (Maternity) | 69/191 (36.1) |
| Total | 8121/12,189 (66.6) |
[I]t’s the induction of labour numbers … they’re just so high now. You know, that is one of our biggest pressures. We look at it every month on the dashboard and it just seems to be going … I mean God, I think at the end of last year, we hit a 58% induction rate. It was just chronic and the pressure on the service …
Site 3 midwife interview 063
[W]e date, we incident report every single delay we have, we put them through and you see the trends and when you have more staff, you see it go down and it’s just so hard.
Site 1 midwife interview 055
COVID-19 influenced approaches to cervical ripening, but little clarity or consistency rationality around decisions regarding changes in practice. One case study site increased home cervical ripening, while another completely suspended it.
[W]hen we got to March and COVID arrived and we didn’t want people in the hospital and women didn’t want to be in the hospital, that was the environment for change was there.
Site 4 midwife interview 075
Home cervical ripening was offered at just over half (54%) of trusts and boards represented in the COVID-19 impact survey responses. Criteria for offering home IOL varied considerably; often, this severely limited eligibility and meant that very few people were offered the option to return home.
Respondents at most maternity units reported that the COVID-19 pandemic did not impact IOL and cervical ripening practice: 75% no change in method used for cervical ripening; 93% no change in approach for ‘post-dates’ IOL; and 65% no change in criteria for offering home cervical ripening. The pandemic had only a small impact on the numbers of women being offered home cervical ripening: 61% of services reported no change; 28% reported more and 5% reported fewer women offered the choice to return home.
-
2. What is the acceptability of home or hospital and different methods of cervical ripening to women (and their birth partners) and implication for their experience of IOL and care?
Attitudes towards home cervical ripening varied among those who completed the postnatal survey. While attitudes were more positive among those who took part in interviews, especially towards the idea of home cervical ripening, experiences of those who actually went home were mixed. Positive experiences were most often associated with being in the comfort of one’s own environment.
I think for both of us, rather than be in hospital, we just got on with our normal day. I think that was definitely better for both of us.
Site 3 birth partner 027
Some, however, felt home to be risky and preferred to stay in hospital. Safety appeared to be the biggest concern for those who said they would not want to return home.
[C]oncerns about safety of induction and distance from hospital to home meant staying at home for induction wasn’t an option I would consider.
Survey participant 78644478
I wanted to be in the safety of the midwives, doctors, nurses and all the care team there … if I had questions, I know that the midwives were there immediately to hand.
Site 3 service user 082
[T]he care you’d getting in hospital, they’ve done it a million times, so I just feel a bit better when they’re on hand.
Site 5 birth partner 108
Crucially, women and birth partners wanted the choice to go home or stay in hospital. Lack of choice about place of cervical ripening was evident in the survey comments and in the case study data.
I was told I could have balloon induction and go home at consultant appointment, then when I attended hospital was told this wasn’t actually something I could have, and I would need the gel induction and would need to stay in – this made me feel disappointed and anxious.
Survey participant 88158381
[T]he nurse, said to us, I’m looking at probably five days in hospital. I wasn’t happy about that. I would rather have been at home with my husband. But no, I was told that there was no way that I would be able to go home.
Site 1 service user 028
I would have preferred to be monitored in hospital, but that wasn’t an option. So it was just, you know, I would be at home with it.
Site 4 service user 062
Women who had home cervical ripening were more likely to choose that option again (64%) and recommend it to others (61%), than those who stayed in hospital (55%, 54%).
There were mixed experiences of different cervical ripening methods. Level of discomfort and ability to cope with discomfort varied between different cervical ripening methods.
It wasn’t that pleasant experience for me at all. I was in a lot of pain having it put in, which I did say to them at the time. After the 24 hours when they checked they said I actually had their reaction to it. So I was very, very sore and swollen.
Site 4 service user 030 – pessary
When I had it done, it was as bad as I thought it was going to be. I mean, it wasn’t horrendous, but it wasn’t as breezy as they kind of made out to be. And it was certainly worse than getting the pessary.
Site 4 service user 071 – balloon
Women who had a balloon catheter inserted reported more discomfort than those who had a pessary or osmotic dilator, although ability to cope with discomfort was similar across all cervical ripening methods.
Attentive care and access to pain relief were important to women and birth partners.
I felt it was ok. I just relaxed, the midwives that were dealing with me were just so friendly and they really made you feel comfortable. So, I didn’t find it too unpleasant.
Site 5 service user 076
-
3. What is the acceptability of home cervical ripening from the perspective of clinicians and health professionals?
Attitudes towards home cervical ripening were generally positive.
Our understanding and perception is that it will be a lot easier to manage the stress and the anxiety associated with that delay if somebody is at home and in their own environment and relaxed rather than stuck in a hospital.
Site 2 midwife interview 109
Many saw home cervical ripening as a potential solution to workload and capacity issues.
We do have at the staffing issues, I’m sure quite a lot of hospitals do. I mean, I’m down to one ward at the moment, normally have two so if these women can stay at home safely, then it’s much better for us than have them just sitting here looking at us thinking, is it me next?
Site 4 midwife interview 090
It frees up hospital resources as well. So they’re not just having to stay in a hospital room by themselves in the hospital just waiting for things to happen.
Site 5 health professional interview 064
However, while there were no negative attitudes to home cervical ripening expressed, some did not perceive it to be the straightforward solution that others expected as there were unintended and unmonitored consequences for workload, such as additional scheduling work and management of re-admissions.
[I]t is acknowledged as a risk because with the staffing levels as they are at the moment, we can only get a woman to a certain point in that induction process, if we haven’t got a midwife available to do that and give them the one-to-one care on Labour Ward … it’s not uncommon to see delays of 24 hours plus with women just sitting in a ward waiting for that to happen, which I’m aware of the fact that with our staffing levels it may not completely remove that risk by doing an outpatient induction.
Site 2 midwife interview 109
There were concerns about appropriate method for home cervical ripening; many healthcare professionals perceived mechanical methods to be the safest for home cervical ripening.
Home induction with Propess makes me a little bit twitched as to what, if anything, goes wrong and they haven’t realised, and they’re not being monitored, are they?
Site 1 midwife interview 020
-
4. What are the cost implications from the service user perspective?
See health economics analysis in Chapter 4.
-
5. What information and outcomes are important for pregnant women and their partners?
Having information concerning cervical ripening setting, methods, length of time, and pain and having choice about cervical ripening were important to women and their birth partners.
I think they were very, very good with the literature that was provided and explaining the options when we came in for the induction. We spoke to both the midwife and a consultant who actually inserted the rod. They explained everything and how it would go.
Site 3 service user 003
[P]robably have anxiety just because it was overdue, but nowhere near as bad because they were keeping us informed of what the options are, what they’re going to do and what might work, and if not, then what would happen.
Site 4 birth partner 084
Half of survey respondents (50%) did not feel that they had enough information to fully understand what to expect during IOL; 53% reported having easy access to information about what to do; 52% of those who remained in hospital and 56% of those who returned home during cervical ripening had access to information about different types of IOL. More women who remained in hospital (66%) than who went home for cervical ripening (47%) felt that they were given clear information about IOL. However, women often reported in free-text comments that they lacked information about what to expect during cervical ripening.
I don’t think I was given enough information … I wasn’t told that this [painful CR] was normal.
Survey participant 88910706
This was also reflected in the interviews with women and birth partners, who reported receiving limited information on IOL more generally, including risks and benefits.
In terms of like the actual process of what was going to happen, she did go over it vaguely, but it was very much like ‘when you go in for your induction, they’ll explain everything once you get there’.
Site 4 service user 031
There is no risks or benefits discussed, it was pretty much just ‘this is what we would do in this circumstance. It’ll probably be really quick for you because of X, Y and Z’. And it wasn’t.
Site 4 service user 074
Over half of the women (57%) felt that they either had no choice or no alternative option when deciding whether to have an IOL. Women in the survey and interviews reported feeling ‘pushed’ into the choice or perceived it as a one-way choice where IOL was the only option for a safe labour and birth.
It was never something I had a choice in and I was told if I didn’t get induced there was a high chance of my baby being stillborn because I was almost 42 weeks so this scared me.
Survey participant 89942117
There was no kind of discussion as to what I wanted or what I was comfortable with. It was more ‘this is what’s going to happen’.
Site 3 service user 080
Some felt it was not possible to make a truly informed choice about IOL despite their own push for this.
I was induced because of my age. Whilst it was made clear that the decision was my choice, I also felt a lot of pressure from health professionals to be induced. I read up on the subject to inform my decision and asked a series of questions but felt a strong push to be induced quickly and before my due date which I was not comfortable with. It made my last couple of weeks of pregnancy quite stressful.
Survey participant 74256136
Many women stated they were not given a choice of method.
It was gel. Yes, I didn’t have a choice. They never gave me a choice, but I knew that my friend did the pessary.
Site 5 service user 016
-
6. What are the psychological correlates of cervical ripening setting?
It was not possible to report correlates of home and hospital cervical ripening due to the small numbers of respondents who returned home; however, other data informed the understanding of psychological impact and aspects of cervical ripening. The short WEMWBS was used to measure psychological well-being. The mean score was 24.5, above the population average of 23.2; 102 women (33%) scored below the population average; and 2 women had a score of just 7.
Forty-one women who left free-text comments described their experience of IOL as difficult or traumatic and/or having caused significant long-term negative impact on their physical and/or mental well-being.
It was all so horrendous I will never have another child. It gives me anxiety thinking about it all. Before this experience I did want more than one child.
Survey participant 89649436
Participants in the case studies reflected on their experiences psychologically, and some reported difficult or even traumatic experiences related to the providing and receiving cervical ripening care both at home and in-hospital.
I think I was just worried that I was going to be in absolutely horrific pain for hours on end and it was going to be the worst thing ever. That’s still how I remember it, but I think probably I’ve blurred my memory a little.
Site 5 service user 033
I would never have an induction again. I wouldn’t do it. If I was overdue like I was before to the point where it would need an induction, I would opt straight for a C-section … [I]f I was to get pregnant again, I would probably need counselling before I gave birth because it was such a horrific experience that I know I would need support around it.
Site 2 service user 093
I found it traumatic witnessing it because being completely helpless in a scenario, just watching somebody suffer for that length of time, trying your best to try and do anything to try and help them out and alleviate that pain in any way you can.
Site 1 birth partner 040
This also applied to maternity professionals, some of whom reported moral distress in relation to the process.
I feel like we had quite a few recently where it’s just not worked and it just feels a little bit like you’re playing into assault in some ways and playing into trauma and being a part of something that you know could well lead to trauma, but not really knowing how to get off the train because that’s what you do in a hospital.
Site 2 midwife interview 041
-
7. What potential factors mediate women’s experience, for example, rurality, distance from hospital, information provision, professional support?
There were several factors that mediated women’s experience: healthcare professional support, birth partner’s presence, information provision and having choice; privacy and having their own space; delays. Factors prevalent in poor experience were: lack of support; separation from birth partner; lack of privacy; inadequate pain relief; delays impacting progression of IOL process.
For women who completed the survey, the most important factor in a positive experience was support from kind, caring staff and feeling safe.
My midwife was incredibly supportive throughout labour and birth. We felt safe and well cared for.
Survey participant 83384325
In the interviews, most women and their birth partners spoke positively of the care they received. They highly valued healthcare professionals’ support throughout their IOL care, and this mediated whether an experience was positive or not, especially during CR.
Staff were amazing and I think I was on the induction ward for two days and every midwife I came into contact with was just a lovely. So, it was really nice that the staff are just so good with the patients.
Site 5 service user 076
Birth partner’s presence was one of the most important mediating factors for women.
I’m quite an anxious person anyway. It’s just having that partner to hold your hand and support you through and reassure you, tell you that things are going to be OK and that sort of thing. But I think the main thing for me was being that second voice.
Site 2 service user 096
Birth partners were an important source of support for women during their cervical ripening, but they often could not be present as much of the time as wanted and sometimes missed the birth. This was a source of anxiety for women and denied them important support during cervical ripening.
He [birth partner] had nowhere suitable to wait as we live 3 hours away from the hospital and this made him anxious as he wasn’t there to support me. Also made me anxious as I knew he was waiting for news and worrying while he was not with me.
Survey participant 87927535
Those who had their own space appeared to have more positive experiences, whether this was their space at home or a space in a separate room in hospital.
I went for the outpatient one. It was kind of positioned to me that that was the better option because you get to go home and be in your own house … It just seemed more relaxed and organised to me … those two combined were really big things for me: going home and passing in my own time.
Site 3 servicer user 023
I think the fact that I had a private room made it much better.
Site 5 service user 016
The physical environment of IOL suite/pre labour ward was often inappropriate and inadequate. Many women who stayed in ‘bays’ in the antenatal ward during cervical ripening spoke about the lack of privacy and how this sometimes negatively impacted their experience.
I was labouring behind a curtain, no privacy, others all around me … it was really hard to focus and stay calm and relax with no privacy of my own, no pain relief and no food.
Survey participant 82998991
[Redacted] was trying to sleep, and it’s difficult to sleep when people are constantly coming in and out of the room. You know, we’ve only got a curtain between you and next door, and people are in pain, screams. You can hear family on the phone, you know, there’s always some noise in the background.
Site 5 birth partner 097
Receiving information and being offered genuine choices about IOL contributed to positive experiences. Many women, however, reported not having genuine choices during IOL, as previously highlighted.
I really struggled with the pregnancy, and I’ve struggled since, but I think it’s just the lack of information … If I could have just sat down, had a conversation with somebody and asked what I needed to ask, I think it would have been different.
Site 2 service user 111
They [the staff] were open to questions. I know that they’re very busy, but I never felt that they were rushed with me. If I had anything to ask, they answered.
Site 4 service user 030
Delays in the IOL care, especially moving from having cervical ripening to having ARM in the labour ward, shaped women’s experiences. This was experienced by women receiving care in hospital and by those who went home.
I suppose the only major worry I had was how long it was taking them to get me into the hospital and getting bumped every day because I was a healthy, pregnant woman is awfully anxiety inducing.
Site 4 service user 019
-
8. What are the service barriers and enablers of adoption of home cervical ripening?
Staff shortages was one of the key barriers identified during the case studies. Delays during IOL were frequently reported, primarily connected to unit capacity, staffing and workload.
There will be delays unfortunately. I think just to manage it as best we can, but then the unpredictability of our workload hugely impacts on the ability to offer induction.
Site 4 midwife interview 067
Others touched on the complexity of implementing a new cervical ripening approach into practice.
[W]e just culturally have used inpatient induction for so long … I think the process is new and…I think new things scare people a bit.
Site 5 obstetrician interview 110
Enablers included cross-boundary collaboration, cross-trust/board knowledge sharing, consistent training and professional confidence.
It wasn’t a particular easy transition to out of hospital induction … If we offer it as a real option and a safe option with robust support and guidelines around about it, we can do this safely … [obstetrician co-lead on labour ward] was just so confident. She’s happy to look at the ones in the book that I look at am not sure why they’re coming in for an inpatient and say 'No she can go home' and it gives the confidence to the staff … But it was the coming together of the team that was really key to getting up [at home cervical ripening] and running.
Site 4 midwife interview 075
Response to the pandemic impacted offer of home cervical ripening in variable ways, acting as a facilitator at some sites but a barrier in others. Making home cervical ripening the default service also influenced the number of women who are offered and then go home for cervical ripening. However, interviews with women and birth partners revealed that home cervical ripening was not as acceptable when it was default as it would be if it were a genuine choice.
-
9. Are there any unintended consequences associated with home cervical ripening, for individuals, families and or services involved?
For service users, lack of choice was a key issue associated with home cervical ripening and IOL more generally. Choice about place of cervical ripening is individual, and some women who returned home felt they did not have a choice to stay in hospital, others who stayed would have liked the option to return home.
I would like to have been given the choice to go home or stay at hospital. I didn’t know there was an option without losing my place in the queue for the labour ward.
Survey participant 80858153
I was not offered the choice to stay in hospital after my balloon was inserted. This would have made all the difference with a toddler at home.
Survey participant 89706212
For staff, potential workload increases were an unintended consequence; however, much of this workload is hidden. Home cervical ripening implementation highlighted that there is potentially more of this hidden work associated with this approach as there is additional administrative work and stress associated with prioritisation, discharge and re-admission procedures for women going home and returning to hospital and in telephone communication with women undergoing cervical ripening at home.
It’s obviously hugely disheartening for the people who are waiting around, but I think it also just creates really undue levels of stress on the clinicians who are trying to manage those lists and trying to keep on top of who’s coming through.
Site 1 midwife interview 021
All women have had delays across the board and it’s been quite difficult for medical professionals and midwife leads to try to prioritise long lists of women that are waiting. It’s quite difficult because in maternity care in general, there is an issue with burnout and keeping staff and I think the pandemic has made this worse.
Site 4 obstetrician interview 084
If the woman on top of the list, we ring her, and she doesn’t answer the phone at 1 o’clock in the morning, and you think 'OK, what if she rings me back at 3 o’clock and I’ve brought the next one in and she’s delayed again?'. But it’s a process – it’s a system, and we have to follow it. If I’ve got capacity right now, she needs to come in. I will ring her three times if I have to, and I’ll document that, and I’ll move on to the next one. If the next woman doesn’t pick up, then I’ll move on to the next one. I’ll keep going until I’ve got them all in.
Site 1 midwife, focus group 031
Finally, there were potential safety issues regarding IOL, whether cervical ripening was in hospital or at home, given the staff shortages and delays. During data collection, several of the accounts recorded by the research team triggered a formal reporting to the trial steering committee about safety concerns and communication to site 1 about them.
qCHOICE discussion
Home cervical ripening appears acceptable to some women and partners but does not necessarily lead to positive care experiences. Women still seek support from staff (e.g. to manage pain) and require reassurance during long delays in the IOL process. Some women described anxieties about whether what they were experiencing was normal or indication of a problem. While the lower restrictions on birth partner support were appreciated, some partners were anxious that they had been left in the position of care provider, leading to potential for distress and trauma for the partner. Participants described staffing shortages as being a key reason for delays during IOL, which in turn led to dissatisfaction and substantial anxiety about the unborn baby, especially when IOL was commenced due to concerns about fetal well-being. Staff perceptions of home cervical ripening were positive in relation to it offering women more comfort and distraction from the ‘wait’ often encountered during IOL. Staff anxiety was evident when discussing potential risks of cervical ripening at home using prostaglandin due to the potential for unmonitored complications developing. Staff described additional workload and stress associated with organisation of care for women undergoing IOL in different locations.
Ultimately women made clear that they wanted genuine choice about whether to undergo cervical ripening at home, but equally the findings demonstrate an unmet need for balanced and useful information about IOL itself to inform a decision to embark upon IOL in the first place. In many cases, women perceived there to be no choice at all about starting IOL, either because no alternative was offered or because they felt ‘pushed’ into IOL, and in others there was no discussion about the risks and benefits of IOL prior to it taking place. Both women and staff acknowledged the major impact that IOL experiences can have on long-term psychological well-being.
A strength of the process evaluation is that the survey respondents represented a diverse range of backgrounds, with women from the most socially deprived settings being represented in similar proportions to their population prevalence. Similarly, a broad range of indications for IOL were represented in the survey respondents, providing a rich array of experiences around decisions for embarking upon IOL and experiences of the process itself. In addition to women, the views of their partners and health professionals were sought, providing multiple perspectives of the process of cervical ripening both at home and in-hospital. Limitations included that only 1 in 20 respondents were from non-white ethnic groups, so the needs of minority groups may not have been sufficiently identified in this work. In addition, despite the study objective to assess acceptability of home versus in-hospital cervical ripening, many qualitative interview findings were more focused on the overall experience of IOL (especially delays during IOL beyond the cervical ripening stage) rather than specifically the setting of cervical ripening. This led to some limitations in drawing conclusions that were specific to setting of cervical ripening. However, findings do suggest that in the context of genuine choice for women and adequate staffing to support the logistics of caring for all women on an IOL pathway (at home and in hospital), home cervical ripening will be acceptable to many women.
Chapter 4 Health economic analysis
Objectives
Aligning with the revised observational cohort primary comparison, the revised economic analysis aims were to: determine the cost-effectiveness of at-home cervical ripening with balloon catheter versus in-hospital cervical ripening with prostaglandin for pregnant women having IOL, from (1) NHS and Personal Social Services (PSS) perspective and (2) the patients’ perspective.
The health economic analysis addressed the specific research questions:
-
Is home cervical ripening using balloon cost-effective to the NHS compared with in-hospital cervical ripening using prostaglandin?
-
Is home cervical ripening using balloon cost-effective from the patient and family perspective compared with in-hospital cervical ripening using prostaglandin?
-
How do the different IOL methods impact on maternal time in hospital, time in antenatal ward, time in labour ward, time in postnatal wards?
-
What are the patient perspective costs and ‘spill over’ costs, for example, time away from family, cost of paid and unpaid child care?
-
Do the data from the CHOICE observational cohort and qCHOICE study show any hidden cost of services due to, for example: (1) delays in IOL; (2) midwifery time to support mother having delayed IOL, etc?
Methods
Study design as planned at outset
At study outset, we planned to undertake two economic evaluations alongside the CHOICE study: (1) to determine the cost-effectiveness of home cervical ripening with prostaglandin (exposure) versus in-hospital cervical ripening with prostaglandin (comparator) for pregnant women having IOL and (2) to determine the cost-effectiveness of home cervical ripening with balloon catheter (exposure) versus home cervical ripening with prostaglandin (comparator) for pregnant women having IOL. All costs were to be reported in UK pounds sterling (£) adjusted for inflation for the price of the year 2021.
The planned method was to undertake a within-study economic evaluation conducted alongside the CHOICE prospective cohort study utilising routine clinical electronic maternity (from BadgerNet) and neonatal data from the National Neonatal Research Database. The methods for analysis were in accordance with good practice guidelines for economic analyses based on observational data, using propensity score matching adjustment to control for treatment indication bias, which also aligned with the original statistics analysis plan. Extrapolation to the longer term via modelling was to be considered if the cohort data suggested a difference between arms in terms of the primary outcome – NNU admissions within 48 hours – or there were short- and medium-term complications for the mother or child, which could be important to capture over a longer time horizon.
A secondary analysis from the patient perspective was planned, using evidence on patient related costs and spill-over effects from the qCHOICE survey. As resource-use data from the patient perspective would not be available from the BadgerNet and NHS routine data sets, tailored questions related to economic resource use were embedded in the process evaluation survey questionnaire (qCHOICE).
A health economic analysis plan (HEAP) was developed in consultation with the CHOICE study statisticians and was reviewed regularly by the study management group. The HEAP was planned to be submitted for publication to a peer reviewed journal; however, following the internal pilot phase and revised analysis plans, publication plans were put on hold and, instead, a revised HEAP was developed alongside the revised main study analysis plan.
Changes to study design
In line with the key changes that were made to the CHOICE observational cohort study design and expected sample size, the HEAP was also revised and approved following the internal pilot. There are some maternity units in the UK who offer at-home IOL as default, and therefore the original question regarding cost-effectiveness of this approach remains valid, albeit amended to reflect current practice and the revised CHOICE primary study question (home cervical ripening using balloon vs. hospital cervical ripening using prostaglandin). Although the CHOICE study data set was underpowered to answer the primary efficacy question, a decision-analytic modelling approach (as opposed to an economic evaluation based solely on the observational cohort data set) has been used to estimate potential cost-effectiveness of at-home versus in-hospital cervical ripening, using as much of the CHOICE data as possible, and supplementing this with any additional evidence from the wider literature and clinical expert advice where needed. There were two main changes from the original protocol: (1) the analysis now focuses on the comparison of at-home cervical ripening with balloon catheter versus in-hospital cervical ripening with prostaglandin; (2) use of a decision tree model-based analysis (as opposed to individual patient data-based analysis), albeit using as many data from CHOICE as possible.
The revised key economic objectives are detailed in Chapter 4.
Health economic analysis overview
The economic evaluation explored the cost-effectiveness of home cervical ripening with balloon catheter (exposure) versus hospital cervical ripening with prostaglandin (comparator) for pregnant women having IOL. Cost-effectiveness analysis is a form of economic evaluation in which both the costs and effects of two or more health interventions are compared, and the results report the incremental difference between the alternatives under consideration as an incremental cost-effectiveness ratio (ICER). 30 The analysis was undertaken from the perspective of the UK NHS and PSS for price year 2021. Secondary analysis was undertaken from the patient perspective to account for IOL-related resource use incurred by women and their families. This analysis is informative for the home cervical ripening setting. The time horizon of the analysis was from the time of initial application of cervical ripening method up to postnatal discharge or 28 days post birth, to capture any cost and morbidity events incurred in the neonatal period. We used a short time horizon in line with the timeline and primary outcome of this study. Recent evaluations on IOL in other contexts have also used short-time horizon (from time of IOL to hospital discharge) for assessing cost-effectiveness of IOL at outpatient department using balloon catheter in Australia. 31 Costs and outcomes will not be discounted as the time horizon is restricted to < 1 year.
Data sources
Data used in the economic analysis are primarily from the CHOICE study sites consisting of 26 obstetric units across the UK (18 offered only in-hospital cervical ripening -predominantly with prostaglandin, and 8 offered home cervical ripening using balloon catheters). The maternity data were recorded by clinical staff (midwives, doctors and neonatal nurses) during the course of antenatal, intrapartum and postpartum care in the BadgerNet maternity system. Neonatal data were also obtained from BadgerNet. All data obtained for economic analysis were anonymised and accessed from the Edinburgh Clinical Trials Unit servers managed by the University of Edinburgh.
A rapid systematic review of economic evaluations of cervical ripening at home or in hospital was undertaken in August 2022 to explore: (1) a wider evidence base on the effectiveness and possible complications of home-based/outpatient cervical ripening and (2) cost-effectiveness studies and existing decision models of home/outpatient versus hospital IOL. 32 A detailed description of this systematic review is included in Appendix 3, with Appendix 3, Tables 119 and 120 containing the search terms used for the effectiveness and cost-effectiveness reviews, respectively. The papers from this review were used to inform, develop and potentially parameterise the CHOICE study economic model, as well as to aid reflection and comparison of results. We added an overview of the cost-effectiveness studies identified in the rapid systematic review in Appendix 3. A total of nine studies were included, conducted in various settings, such as Europe, USA, India and Australia. Most studies (seven of nine) used a short time frame, focusing on events from randomisation/admission/IOL to discharge. Two studies extended the follow-up period up to 4 weeks post delivery. Decision tree or decision-analytic modelling approaches were used in only two studies, while the remaining studies employed non-parametric bootstrap or logistic regression methods on study data sets. Three studies used NNU admission avoided as the primary outcome measure, and two studies estimated quality-adjusted life-years (QALYs) for the mothers (not babies). Other outcomes assessed in the selected studies included postpartum length of stay, asphyxia, postpartum haemorrhage and caesarean section rates. Notably, six of eight studies identified the use of Foley or Cook balloon in outpatient settings as a cost-saving strategy compared with inpatient settings. The other study did not use balloons. Further details of the rapid systematic review search strategy, databases and Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram results can be found in Appendix 3, Figures 14 and 15.
Decision model
The cost-effectiveness analysis was undertaken using a decision tree model to synthesise the parameter inputs of relevance for the economic analysis. Decision trees are economic models that illustrate alternative decision options and their possible consequences. The structure of the model was based upon the pathway that patients will follow in IOL in the UK setting, but is also similar to previously published decision models in this setting and patient group. 33 As per the CHOICE observational data set, the patient population for the economic model is women who had singleton pregnancies with IOL at 37 weeks’ gestation or more with broadly comparable levels of risk, such as those without key risk factors for adverse maternal or perinatal outcomes, and who had pregnancies in which there was no contraindication to home cervical ripening. Figure 5 illustrates the decision tree pathway. In the model, women either can receive balloon catheter cervical ripening and are then sent home for their cervical ripening stage of IOL (exposure arm) or can receive prostaglandin and are admitted to hospital into an antenatal ward. Women in the ‘home cervical ripening’ arm, can be admitted to antenatal ward at a later time, and may also call the maternity unit from home for advice and reassurance during their IOL period. The decision tree is split by type of delivery (vaginal, vaginal with instrumental, and caesarean section), as this impacts on the costs, duration of time in labour ward and possible complications. The primary outcome is NNU admission avoided, rather than NNUadmission, as this enables the outcome to represent a health benefit for easier interpretation of economic outcomes, rather than based on a detrimental effect of NNU admissions.
FIGURE 5.
Decision tree pathway displaying potential processes and outcomes of IOL using either balloon or prostaglandin cervical ripening.
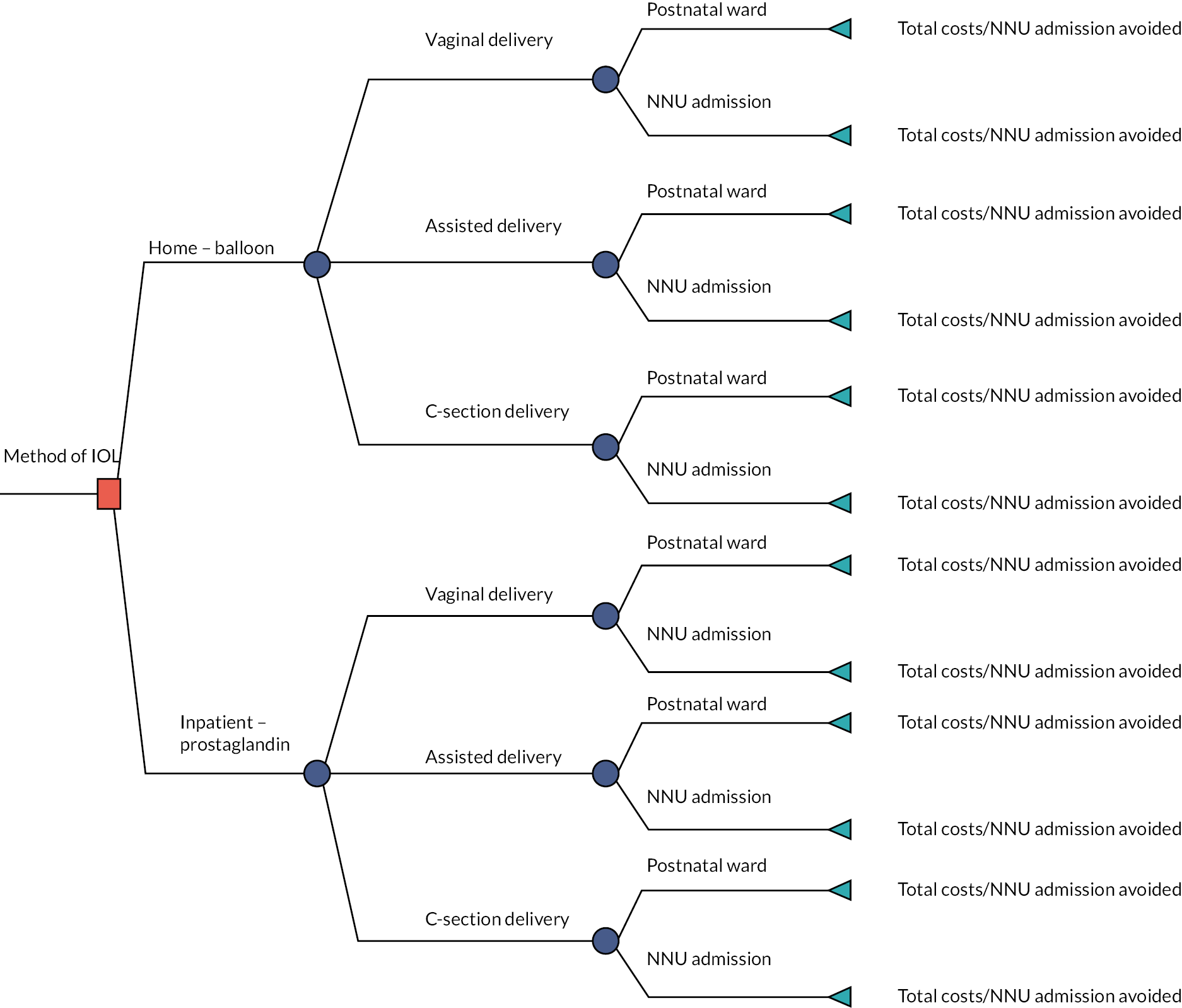
Model parameters/data
Effectiveness data and outcomes
Measuring health state utility value was not possible directly from the CHOICE study data since this is an observational study and neither quality of life nor utility data are collected routinely on NHS and maternity data sets. Previous studies of a similar nature in the UK have considered elapsed time intervals from hospital to delivery. 34 Merollini et al. 33 measured utility values via the EuroQol-5 Dimensions; however, this was for mothers, not babies/newborns, between time of IOL to hospital discharge. The study showed no significant difference in utility index (p-value = 0.642) for mothers between intervention (balloon catheter in outpatient) and comparison (prostaglandin in inpatient) groups. Based on the rapid review we conducted, we were unable to identify any relevant studies that demonstrated significant differences in the utility index for mothers. Additionally, we found that there was no available utility index specifically for term newborns.
Neonatal unit admission is a marker of neonatal morbidity and the number one core outcome defined for studies of IOL. 35 In line with the primary outcome of the cohort study, we used incremental cost per NNU admission within 48 hours avoided as the outcome to consider as the within-study morbidity. Additional outcomes were explored in terms of: (1) incremental cost per inpatient hour prevented in the interval between hospital admission to birth and (2) incremental cost per hour from IOL to birth. This enabled comparison with previous studies of a similar nature which have considered elapsed time interval from hospital to birth the economic outcome of interest. 34 Additionally, it is important to consider both the costs and short-term morbidity outcomes that could be impacted through changing the setting and type of cervical ripening, so impact of maternal complications and neonatal morbidity were explored, and if relevant, incorporated into the analysis.
Further, qCHOICE showed no evidence of difference in quality of life and anxiety between arms using the WEMWBS, although there were low numbers (n = 36) of responses for assessing regarding significance of a difference. Therefore, this factor was not incorporated into the economic analysis. The qualitative data were rich and showed that the factors that impacted on mothers’ stress levels were not related to the setting (home vs. in hospital) but more to the overall induction process.
Resource use
The sample patient pathway in Figure 6 illustrates time points that are relevant to resource use identification and outcome measurement from when pregnant women visit the hospital for their induction/cervical ripening method up to 28 days post birth in this study. It was formed following the NHS’s pregnancy and baby guide and NHS foundation trust resources. 35
FIGURE 6.
Sample patient pathway illustrating time points of relevance to resource use from onset of IOL.
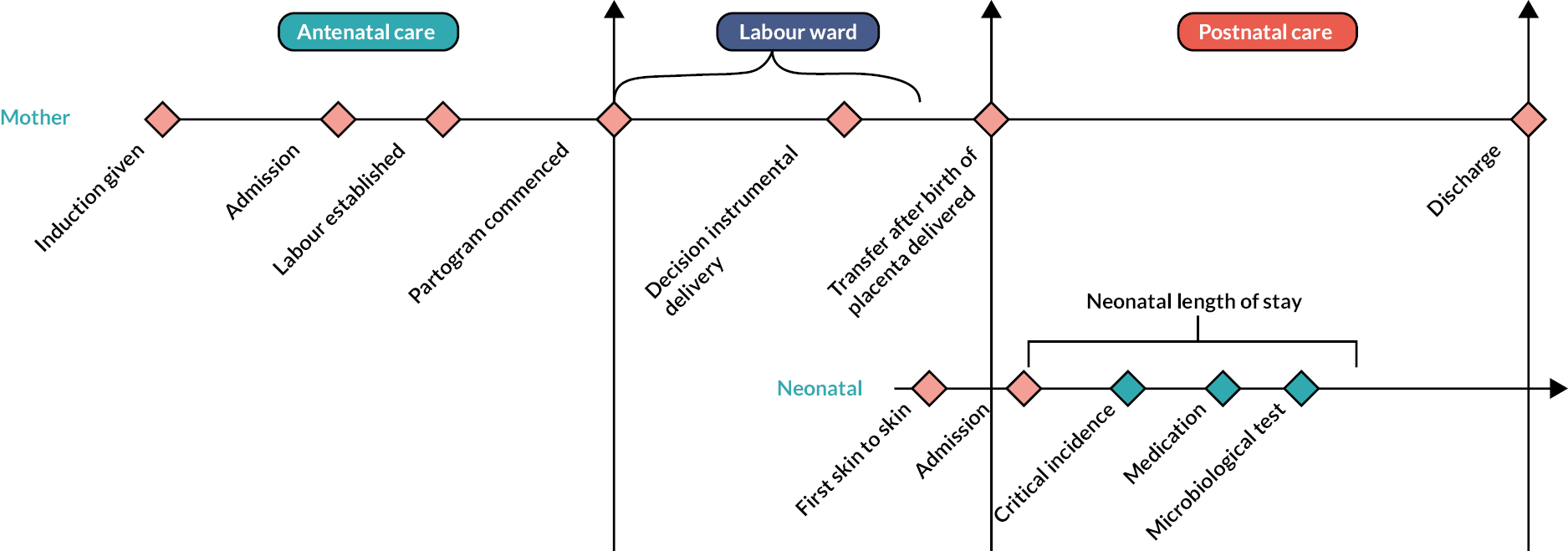
All resource use was measured according to the relevant units, such as length of stay, number of medications, type of delivery, as detailed in the full list of parameters in Tables 33 and 34.
| Resource use items | Unit cost (in £; year 2021) | HRG | Sources |
|---|---|---|---|
| Cervical ripening methods | |||
| Prostin E2 vaginal gel (1.5 mg on average) | 13.4 | n/a | BNF36 |
| Propess 10 mg dinprostone with vaginal delivery system | 165 | BNF36 | |
| Foley balloon catheter | 5.87 | Alfirevic et al. 201646 (inflation-adjusted using HCHS pay and prices inflators from PSSRU 2021)37 | |
| Cook’s double-balloon catheter | 70.3 | Alfirevic et al. 201646 (inflation-adjusted using HCHS pay and prices inflators from PSSRU 2021)37 | |
| Antenatal ward | |||
| Admission to antenatal ward (cost per episode) | 1785.0 | n/a | (1) 2020–1 National Tariff Payment System: non-mandatory prices47 and (2) NHS Maternity Statistics, England – 2020–12 |
| Midwife (cost per hour) | 41.0 | n/a | PSSRU, 202137 |
| Delivery types (delivery phase only) | |||
| Vaginal deliverya (cost per episode) | 2176.0 | NZ30A | (1) 2020–1 National Tariff Payment System: non-mandatory prices47 and (2) NHS Reference Costs Year 2015–638 |
| Assisted deliverya (cost per episode) | 2903.0 | NZ40A | (1) 2020–1 National Tariff Payment System: non-mandatory prices47 and (2) NHS Reference Costs Year 2015–638 |
| Caesarean sectiona (cost per episode) | 5299.0 | NZ51B | (1) 2020–1 National Tariff Payment System: non-mandatory prices47 and (2) NHS Reference Costs Year 2015–638 |
| Postnatal period | |||
| Maternal stay in postnatal ward (cost per episode) | 319.0 | (1) 2020–1 National Tariff Payment System: non-mandatory prices47 and (2) NHS Maternity Statistics, England – 2020–12 | |
| Neonatal care | |||
| NNU admission (cost per episode) | 935.7 | XA02Z, XA03Z, XA04Z, XA05Z | (1) NHS Reference Costs Year 2020–147 and (2) NDAU, 201653 |
| Average income and nursery cost | |||
| Average income (cost per hour) | 19.1 | Office for National Statistics48 | |
| Nursery charge (cost per hour) | 5.78 | Statista 202249 | |
| Intervention | Parameter description | Transitional probabilities | Cost parameters (£) | |||
|---|---|---|---|---|---|---|
| Point estimate | Distribution | Point estimate | Standard error | Probabilistic distribution | ||
| Type of balloon catheter | ||||||
| Home | Foley balloon | 0.50a | Beta | 5.6 | 1.12 | Normal |
| Cook balloon | 0.50a | Beta | 70.3 | 14.06 | Normal | |
| Types of deliveries | ||||||
| Home | Vaginal delivery after home IOL | 0.50b (259) | Dirichlet | 1828.6 | 104.2 | Normal |
| Assisted delivery after home IOL | 0.16b (81) | Dirichlet | 1830.0 | 138.2 | Normal | |
| C-section after home IOL | 0.34b (175) | Dirichlet | 3029.4 | 196.4 | Normal | |
| Hospital | Vaginal delivery after hospital IOL | 0.58b (2509) | Dirichlet | 3193.6 | 74.1 | Normal |
| Assisted delivery after hospital IOL | 0.15b (649) | Dirichlet | 3765.0 | 127.4 | Normal | |
| C-section after hospital IOL | 0.27b (1174) | Dirichlet | 4021.9 | 108.4 | Normal | |
| NNU admission | ||||||
| Home | NNU admission after home IOL and vaginal delivery | 0.05b (13) | Beta | 60.6 | 23.4 | Normal |
| Postnatal ward with mother after home IOL and vaginal delivery | 0.95b (246) | 1-NNU admission | 0.0 | |||
| NNU admission after home IOL and assisted delivery | 0.07b (6) | Beta | 44.2 | 21.3 | Normal | |
| Postnatal ward with mother after home IOL and assisted delivery | 0.93b (75) | 1-NNU admission | 0.0 | |||
| NNU admission after home IOL and C-section | 0.07b (13) | Beta | 59.8 | 21.4 | Normal | |
| Postnatal ward with mother after home IOL and C-section | 0.93b (162) | 1-NNU admission | 0.0 | |||
| Hospital | NNU admission after hospital IOL and vaginal delivery | 0.05b (119) | Beta | 40.6 | 4.8 | Normal |
| Stays at postnatal ward with the mother after hospital IOL and vaginal delivery | 0.95b (2390) | 1-Above | 0.0 | |||
| NNU admission after hospital IOL and assisted delivery | 0.11b (73) | Beta | 94.1 | 12.5 | Normal | |
| Stays at postnatal ward with the mother after hospital IOL and assisted delivery | 0.89b (576) | 1-Above | 0.0 | |||
| NNU admission after hospital IOL and caesarean section | 0.12b (142) | Beta | 117.3 | 12.4 | Normal | |
| Stays at postnatal ward with the mother after hospital IOL and caesarean section | 0.88b (1032) | 1-Above | 0.0 | |||
| Type of delivery | Resource-use variable | Home cervical ripening | Hospital cervical ripening | Incrementala | ||||
|---|---|---|---|---|---|---|---|---|
| N | Minutes | SE | N | Minutes | SE | |||
| Vaginal delivery | ||||||||
| Mother length of stay | ||||||||
| Antenatal ward | 248 | 380 | 50 | 2159 | 2046 | 45 | −1666 | |
| Labour ward | 248 | 1049 | 66 | 2156 | 775 | 65 | 273 | |
| Postnatal ward | 259 | 3283 | 476 | 2443 | 3190 | 174 | 93 | |
| Total | 248 | 4818 | 501 | 2097 | 6148 | 208 | −1330 | |
| Time length induction given to birth | 259 | 2811 | 112 | 2509 | 2249 | 44 | 562 | |
| Neonatal length of stay in NNU | 19 | 5091 | 1649 | 133 | 4720 | 387 | 370 | |
| Assisted delivery | ||||||||
| Mother length of stay | ||||||||
| Antenatal ward | 81 | 465 | 87 | 598 | 2306 | 93 | −1841 | |
| Labour ward | 81 | 1414 | 120 | 597 | 1096 | 96 | 318 | |
| Postnatal ward | 81 | 3384 | 408 | 632 | 5386 | 458 | −2002 | |
| Total | 81 | 5263 | 400 | 580 | 8727 | 479 | −3464 | |
| Time length induction given to birth | 81 | 3327 | 213 | 649 | 2846 | 91 | 481 | |
| Neonatal length of stay in NNU | 6 | 3675 | 1105 | 78 | 4820 | 385 | −1145 | |
| C-section | ||||||||
| Mother length of stay | ||||||||
| Antenatal ward | 158 | 633 | 108 | 803 | 2727 | 85 | −2094 | |
| Labour ward | 158 | 1682 | 141 | 803 | 1086 | 35 | 597 | |
| Postnatal ward | 174 | 4204 | 448 | 1150 | 6342 | 369 | −2138 | |
| Total | 158 | 6636 | 507 | 785 | 9395 | 388 | −2759 | |
| Time length induction given to birth | 175 | 3437 | 151 | 1174 | 3444 | 73 | −7 | |
| Neonatal length of stay in NNU | 15 | 4295 | 1147 | 158 | 5369 | 404 | −1074 | |
| Total | ||||||||
| Mother length of stay | ||||||||
| Antenatal ward | 487 | 476 | 46 | 3560 | 2243 | 37 | −1767 | |
| Labour ward | 487 | 1315 | 61 | 3556 | 899 | 43 | 416 | |
| Postnatal ward | 514 | 3611 | 291 | 4225 | 4376 | 159 | −766 | |
| Total | 487 | 5482 | 312 | 3462 | 7317 | 175 | −1835 | |
| Time length induction given to birth | 515 | 3105 | 84 | 4332 | 2662 | 36 | 442 | |
| Neonatal length of stay in NNU | 40 | 4580 | 896 | 369 | 5019 | 237 | −439 | |
There are five main resource categories of relevance: cervical ripening method, maternal time in hospital [antenatal ward, labour ward (including type of delivery), postnatal ward], time spent by midwives on maternal phone calls, complications and costs to parents (patient perspective). Cost of NNU admissions are included. The base case total cost (CT) is a function of the cost of cervical ripening method (Ccr), time in hospital (Cthosp), telephone calls (Ccalls) and complication costs (Ccomp). In the sensitivity analysis, the cost to parents is also included (Cparents). Equation 1 illustrates the main components of total cost.
Unit cost
Unit cost information was combined with the resource use data collected. Valuing the resource use using the unit costs provided an estimate of the total cost for each resource. These estimates were aggregated to estimate total patient costs within each arm and the mean cost per patient per arm. The difference in average costs (and significance) between the two study arms was estimated. All unit costs were collected in UK pounds sterling (£) adjusted for inflation using the NHS Cost Inflation Index for price year 2021. Cost information was derived from routine sources, such as the British National Formulary,36 Personal Social Services Resource Unit37 and NHS Reference Costs. 38 Some unit costs were obtained from published literature or from clinical expert opinion (i.e. CHOICE study team), if not available from these sources. For the scenario analysis adopting a patient perspective, the human capital approach was used and average UK salary to reflect cost of child care. 39 Resource use data were combined with unit costs to calculate the costs for each group. Table 32 outlines the unit costs for all resources identified for the economic model and their sources.
Model parameters
Table 33 details the parameter inputs for the model and corresponding standard errors and distributional forms used in the probabilistic analysis. We estimated all transitional probabilities from the CHOICE study data set (BadgerNet data), with the exception of use of Foley and Cook balloons, which was an assumption based on clinical expert advice. These estimated probabilities and cost parameters were used in the decision tree model to calculate the ICER.
In terms of cervical ripening method, the type of balloon catheter used varies throughout the UK (typically Foley balloon or Cook balloon), yet there is a considerable difference in cost between the two types. The CHOICE data set did not detail the type of balloon catheter used for each patient and therefore it was assumed a 50% split between the two types based on clinical experts’ advice. The type of prostaglandin administered for the ‘in-hospital’ group was detailed in the CHOICE data set and therefore the economic model used the proportions from the CHOICE data set for the economic model. The CHOICE data set distinguished between time in hospital in the various wards, from admission to discharge, enabling a detailed breakdown of cost of time in antenatal ward, labour ward (by delivery type) and postnatal ward until discharge or 28 days post birth, whichever came first. It is assumed that patients in the ‘hospital’ arm, are admitted upon receiving prostaglandin, whereas in the ‘home’ arm patients are not admitted until they return to hospital to give birth. Therefore, we hypothesise a longer period of time in antenatal ward in the ‘hospital’ arm than in the ‘home’ arm. However, it may be that the patients in the home arm may experience a longer delivery duration given the nature of the different cervical ripening methods, so it is important to capture the duration in each ‘ward/stage’ and differences between arms as best possible from the data set, as well as representing the overall time in hospital. Cost of telephone calls includes both maternal and partners’ phone calls to the ward/unit as they both take up midwife time, and therefore there can be a cost of phone calls to both arms (not just the home arm).
In the patient perspective sensitivity analyses, costs were incorporated based on resource use estimates from the qCHOICE study data comprising paid and unpaid child care, transport to hospital, phone calls to the hospital and partner’s time at hospital during the antenatal and labour ward period.
Stata® version 17 (StataCorp LP, College Station, TX, USA) was used to carry out analysis of the CHOICE data sets and to combine resource use estimates with unit cost information.
Presentation of results and uncertainty
Incremental costs (ΔC) and effects (ΔE) were used to estimate the ICER (where ICER = ΔC/ΔE). Cost-effectiveness is typically expressed as an ICER, that is the incremental cost per NNU admission avoided; however, given the original study hypothesis of non-inferiority within a margin of 4% increase in NNU admissions (i.e. no difference between arms in terms of NNU admissions, and if any minimal difference is observed it would be acceptable up to a 4% increase in NNU admissions), it is likely that there will be no or minimal difference in effects between the home cervical ripening and hospital cervical ripening groups. The traditional ICER presents results as a ratio representing the relation between difference in costs and difference in effects, and in the case of a non-inferiority effectiveness measure (and depending on the cost outcome) could result in a ‘dominated’ or ‘dominant’ ICER in relation to the comparator.
A 1000-iteration Monte Carlo simulation was undertaken for probabilistic sensitivity analysis (PSA) to represent uncertainty in each of the model parameter inputs and to illustrate the impact of this on the overall cost and effect results. The stability of the 1000-iteration Monte Carlo simulation was tested and was found to be within reasonable bounds; 95% uncertainty intervals were presented with the mean difference in cost and effect estimates. 40 To visualise this uncertainty, the 1000 iterations from the PSA were plotted on a cost-effectiveness plane, with the non-inferiority margin for effects presented. Non-inferiority of the effect outcome could be illustrated by a narrow spread of cost–effect pairs very close to zero (and within the bounds of the non-inferiority margin) on the horizontal axis on a cost-effectiveness plane.
Subgroup and sensitivity analysis
As per the main study analyses, the economic analysis plan intended to explore potential subgroup analyses of: (1) nulliparous and parous women and (2) indication for IOL, however, due to little difference between groups and insufficient numbers to allow meaningful analyses, we did not conduct these in the final analyses.
Additional sensitivity analysis was undertaken to explore alternative IOL methods, taking into account possibility of variations in the utilisation rates based on clinical experts’ advice. The base case analysis assumed a 50% split between the Foley and Cook balloons. Four scenarios were generated, as follows: (1) Foley balloon (100%), Cook balloon (0%), (2) Foley balloon (0%), Cook balloon (100%), (3) Foley balloon (75%), Cook balloon (25%), (4) Foley balloon (25%), Cook balloon (75%). We conducted a separate sensitivity analysis considering societal perspective cost (i.e. including both NHS and patient’s costs).
Health economic analysis results
Resource use
Table 34 presents the mean resource quantities per arm.
Table 34 presents the resource use in each group (based on the CHOICE study data set). The length of stay of the women who received balloon catheter at home was significantly lower than those who received prostaglandin ‘in hospital’ (mean 5482 vs. 7317 minutes; p < 0.01). We estimated the average length of stay in antenatal, labour and postnatal wards. Mothers who had home cervical ripening spent significantly less time in the antenatal ward and more time in the labour ward compared with mothers who had hospital cervical ripening. There was no significant difference in the length of postnatal stay between the two groups, which could indicate no or minimal difference in maternal complications that required a longer stay in hospital. For vaginal and assisted deliveries, mothers’ length of stay was significantly higher in the antenatal ward when they received the in-hospital cervical ripening method. Mothers who had caesarean delivery after home cervical ripening stayed significantly less time in each ward except labour ward than who had caesarean delivery after having hospital cervical ripening. The admission to and length of stay of newborns in the NNU was less in home arm compared with the hospital arm, irrespective of delivery types and in total.
Table 35 presents the proportion of mothers or partners who communicated with their midwife during the antenatal period. Higher proportions of mothers/partners in the home cervical ripening arm (75.4%) communicated with the midwife during the period than in the hospital cervical ripening arm (2.4%). Similarly, the number of calls and average duration of calls were higher for mothers who had home cervical ripening compared to mothers who had cervical ripening at the hospital.
| Phone call | Home cervical ripening | Hospital cervical ripening | ||||
|---|---|---|---|---|---|---|
| N = 515 | Mean or % | SE | N = 4332 | Mean or % | SE | |
| Mother or partner communicated (%) | 388 | 75.34 | 1.90 | 102 | 2.35 | 0.23 |
| Number of phone calls | 388 | 2.05 | 0.07 | 102 | 1.60 | 0.15 |
| Call duration (minutes) | 388 | 5.98 | 0.37 | 102 | 3.65 | 0.65 |
Table 36 presents the mean cost (£) per service by delivery types and model groups. Overall, the home cervical ripening using a balloon catheter method reduced the average total cost by £1423 compared with prostaglandin at hospital (£2237 vs. £3660). The antenatal and postnatal ward stay cost was lower for home cervical ripening than hospital ripening. However, the costs of midwifery staff dealing with phone calls and maternal duration in labour ward were higher for the home arm than hospital arm. For both cervical ripening approaches, the delivery cost was the highest for caesarean section followed by assisted and vaginal delivery, respectively. The NNU stay cost was highest for newborns in the ‘in hospital’ group than newborns in the ‘home’ group for all types of deliveries except vaginal delivery. Overall, the cost of NNU admissions was greatest in the ‘in hospital’ group.
| Cost items | Home IOL | Hospital IOL | ||||
|---|---|---|---|---|---|---|
| N | Mean | SE | N | Mean | SE | |
| Vaginal delivery | ||||||
| Prostaglandin | – | – | – | 2453 | 85 | 1.45 |
| Foley/Cook balloon catheter | 259 | 38.06 | – | – | – | – |
| Phone call | 198 | 4.42 | 0.37 | 57 | 2.77 | 0.63 |
| Antenatal ward cost | 248 | 479.13 | 62.50 | 2159 | 2581.64 | 57.30 |
| Labour ward cost | 248 | 839.06 | 52.54 | 2156 | 620.26 | 51.72 |
| Postnatal ward cost | 259 | 464.28 | 67.28 | 2443 | 451.07 | 24.63 |
| NNU cost | 19 | 826.72 | 267.73 | 133 | 766.56 | 62.88 |
| Total | 259 | 1828.56 | 108.20 | 2509 | 3317.38 | 74.23 |
| Assisted delivery | ||||||
| Prostaglandin | – | – | – | 628 | 94.01 | 2.89 |
| Foley balloon catheter | 81 | 38.06 | – | – | – | – |
| Phone call | 68 | 3.61 | 0.64 | 14 | 3.56 | 1.26 |
| Antenatal ward cost | 81 | 586.88 | 110.32 | 598 | 2909.71 | 116.72 |
| Labour ward cost | 74 | 743.63 | 56.03 | 370 | 603.85 | 17.07 |
| Postnatal ward cost | 81 | 478.47 | 57.67 | 632 | 761.58 | 64.70 |
| NNU cost | 6 | 596.77 | 179.45 | 78 | 782.73 | 62.60 |
| Total | 81 | 1830.01 | 134.71 | 649 | 3952.06 | 128.46 |
| Caesarean section | ||||||
| Prostaglandin | – | – | – | 1144 | 92.37 | 2.14 |
| Foley/Cook balloon catheter | 175 | 38.06 | – | – | – | – |
| Phone call | 122 | 3.81 | 0.41 | 31 | 1.50 | 0.65 |
| Antenatal ward cost | 158 | 798.96 | 136.42 | 803 | 3440.59 | 107.36 |
| Labour ward cost | 158 | 1790.40 | 150.39 | 803 | 1155.21 | 37.72 |
| Postnatal ward cost | 174 | 594.43 | 63.40 | 1150 | 896.75 | 52.14 |
| NNU cost | 15 | 697.45 | 186.24 | 158 | 871.85 | 65.58 |
| Total | 175 | 2997.16 | 202.10 | 1174 | 4229.27 | 109.66 |
| All types of deliveries | ||||||
| Prostaglandin | – | – | – | 4225 | 88.26 | 1.11 |
| Foley/Cook balloon catheter | 515 | 38.06 | – | – | – | – |
| Phone call | 388 | 4.09 | 0.25 | 102 | 2.49 | 0.44 |
| Antenatal ward cost | 487 | 600.82 | 57.76 | 3560 | 2830.50 | 47.03 |
| Labour ward cost | 480 | 1137.49 | 60.73 | 3329 | 747.48 | 34.99 |
| Postnatal ward cost | 514 | 510.57 | 41.17 | 4225 | 618.83 | 22.52 |
| NNU cost | 40 | 743.75 | 145.57 | 369 | 815.06 | 38.43 |
| Total | 515 | 2236.83 | 93.42 | 4332 | 3659.59 | 56.03 |
Effectiveness outcome
The base case economic analysis uses NNU admission (within 48 hours of birth) avoided, transforming the study primary outcome of NNU admission (a detrimental outcome, which the original cohort study aimed to show non-inferiority within a 4% margin) into a health benefit scale. Table 37 illustrates that home cervical ripening compared with hospital cervical ripening reduced NNU admission by 1.5% (p = 0.224), which is statistically insignificant, yet well below the non-inferiority margin of a 4% increase.
| Outcome | IOL | Total observation | Number of deliveries/admissions | Proportion (%) | 95% CI | Incremental (%) | |
|---|---|---|---|---|---|---|---|
| Lower (%) | Upper (%) | ||||||
| Types of delivery | |||||||
| Vaginal delivery | Hospital IOL | 4332 | 2509 | 58 | 56 | 59 | 7.6 |
| Home IOL | 515 | 259 | 50 | 46 | 55 | ||
| Assisted delivery | Hospital IOL | 4332 | 649 | 15 | 14 | 16 | −0.7 |
| Home IOL | 515 | 81 | 16 | 13 | 19 | ||
| Caesarean section | Hospital IOL | 4332 | 1174 | 27 | 26 | 28 | −6.9 |
| Home IOL | 515 | 175 | 34 | 30 | 38 | ||
| NNU admission (admitted within 48 hours) | Hospital IOL | 4332 | 334 | 7.7 | 6.9 | 8.5 | 1.5 |
| Home IOL | 515 | 32 | 6.2 | 4.1 | 8.3 | ||
| Long stay in NNU (stayed > 48 hours) | Hospital IOL | 4332 | 218 | 5.0 | 4.4 | 5.7 | 1.7 |
| Home IOL | 515 | 17 | 3.3 | 1.8 | 4.8 | ||
| Admitted early and stayed long (admitted within 48 hours and stayed > 48 hours) | Hospital IOL | 4332 | 200 | 4.6 | 4.0 | 5.2 | 1.7 |
| Home IOL | 515 | 15 | 2.9 | 1.5 | 4.4 | ||
Base case economic analysis results
Table 38 presents the results of the economic analysis, showing the deterministic results and the probabilistic results, which account for the underlying uncertainty in the model inputs and how this impacts on cost-effectiveness outcomes.
| Intervention | Mean cost (£): without NNU cost | Mean effect: NNU admission avoided in the last 48 hours (%) | Cost difference (£) | Effect difference | ICER (£) |
|---|---|---|---|---|---|
| Home IOL | 2527 | 93.8 | −1009 | 0.015 | Home dominates |
| Hospital IOL | 3536 | 92.3 | |||
| Probabilistic analysis results a | |||||
| Home IOL | 2524 | 97.6 | −993 | 0.005 | Home dominates |
| Hospital IOL | 3517 | 97.1 | (−1198 to −783) | (−0.005 to 0.013) | |
The economic analysis found that home cervical ripening with balloon led to a cost savings of £993 (−£1198, −£783) per woman, with no difference in NNU admissions avoided (mean 0.005, 95% CI −0.05 to 0.013), and is therefore considered to be the dominant strategy compared with in-hospital cervical ripening with prostaglandin.
Cost-effectiveness plane
The outputs from the probabilistic analysis (indicated in the uncertainty intervals) can be illustrated on a cost-effectiveness plane. Figure 7 illustrates the distribution of 1000 incremental cost and effect estimates from the PSA – this represents the uncertainty surrounding the expected incremental costs and incremental effect in terms of NNU admissions avoided. The figure shows that there is little uncertainty regarding the extent and existence of the expected cost saving (shown in the vertical plane – the distribution of outcomes are all below zero), but considerable uncertainty regarding the existence and extent of any difference in terms of NNU admission avoided (horizontal plane). The original study non-inferiority margin of a 4% increase in NNU admissions has also been included in this figure, illustrating that overall the mean point estimate shows an improvement in terms of NNU admissions avoided with the ‘home’ arm, and that the range of uncertainty is well within (below) the predefined non-inferiority margin of a 4% increase in NNU admissions.
FIGURE 7.
CHOICE economic analysis cost-effectiveness plane.
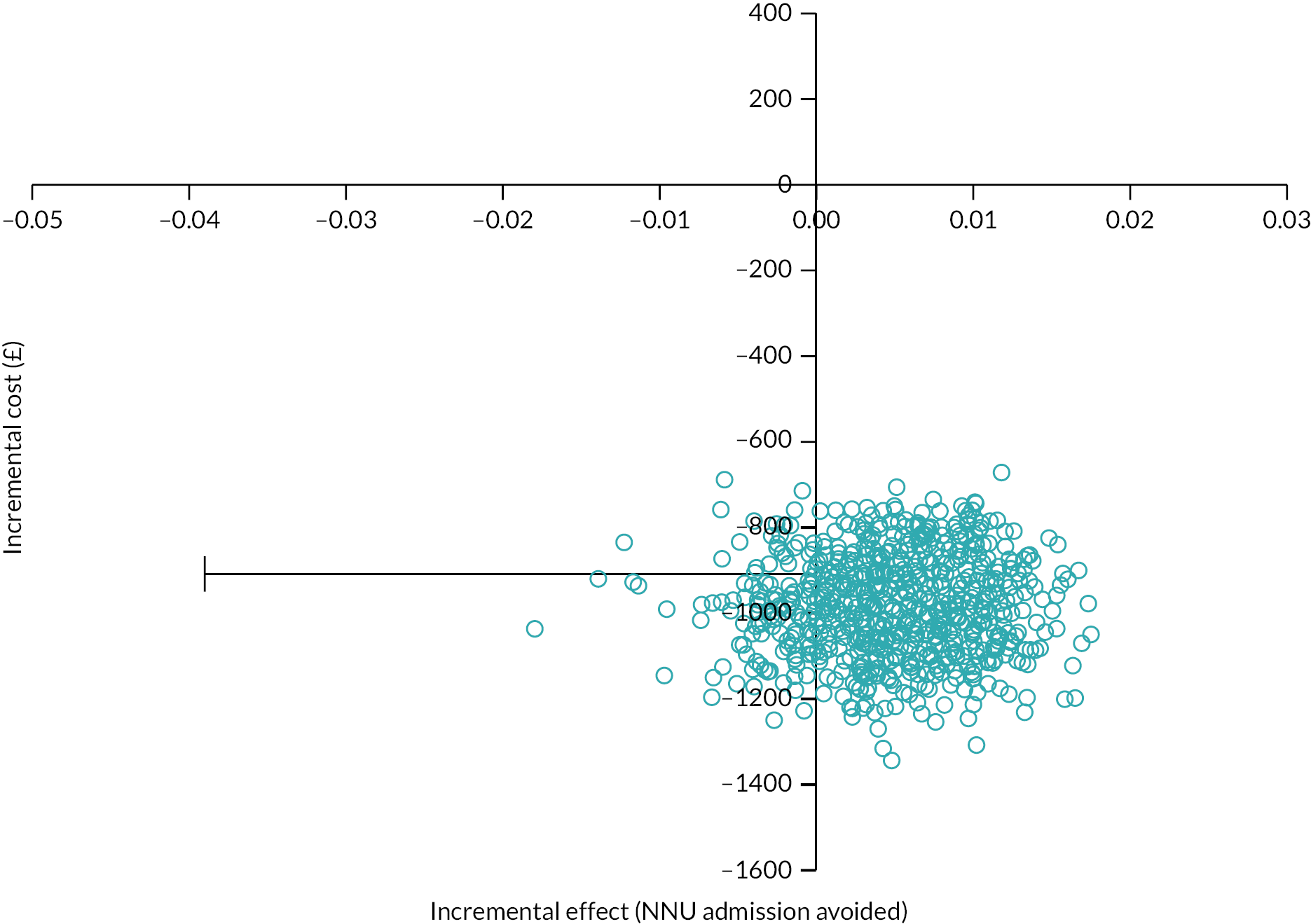
The cost-effectiveness acceptability curve in Figure 8 illustrates that at willingness-to-pay thresholds above £3000, there is an 82% probability that home cervical ripening is the optimal/cost-effective option. Across a broad range of threshold, the home cervical ripening method is the optimal strategy.
FIGURE 8.
CHOICE economic analysis cost-effectiveness acceptability curve.
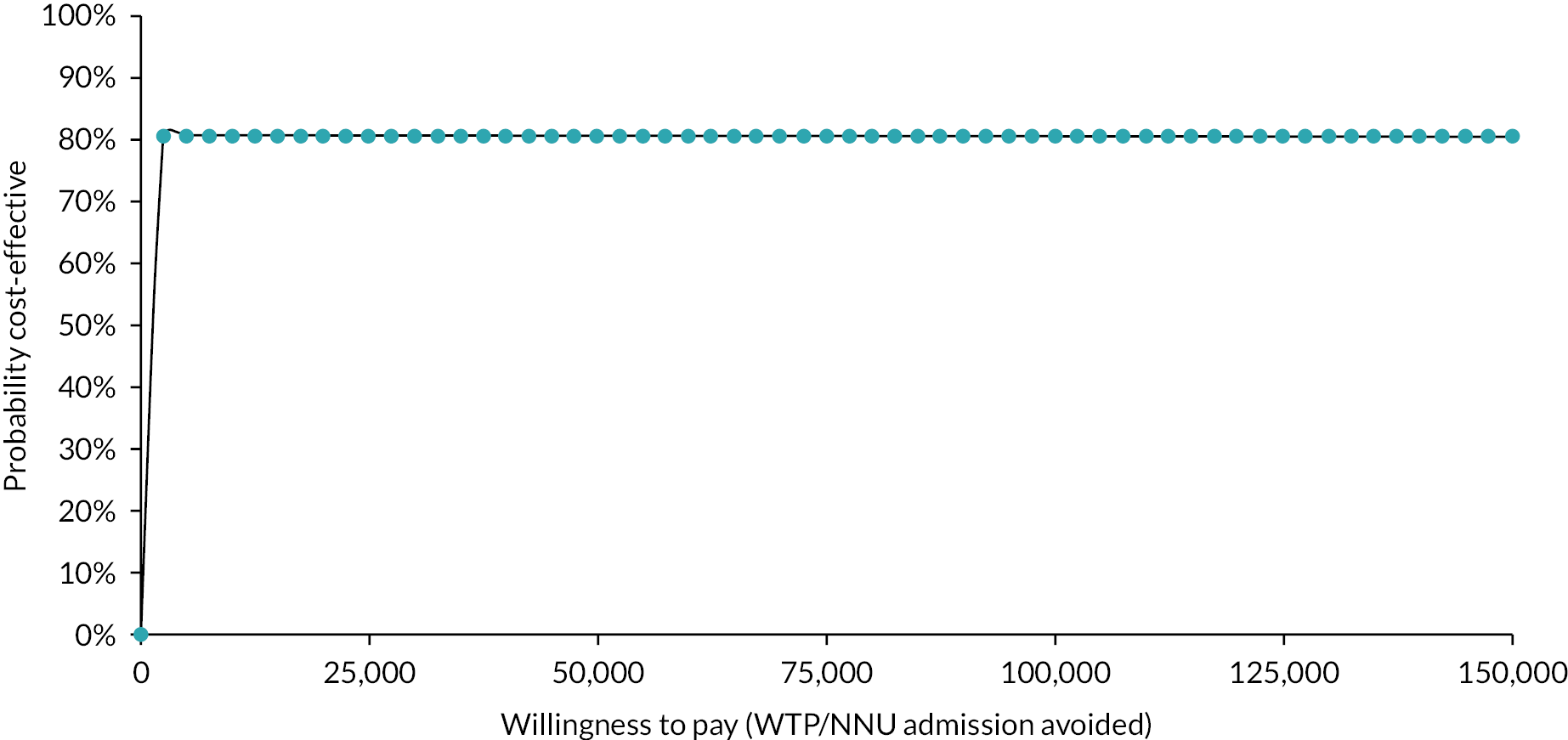
Alternative outcomes: incremental cost per time in hospital
Reporting cost-effectiveness in terms of: (1) incremental cost per time in hospital and (2) incremental cost per time from IOL to birth can be important for consideration of alternative outcomes (beyond the CHOICE primary outcome) and also for comparison with other similar studies. Table 34 reports the resource use summary results from the CHOICE study, detailing both total time in hospital for each arm, and time from IOL to birth. In terms of time in hospital, the home cervical ripening arm has a total of 5482 minutes (3.8 days), compared with 7317 minutes (5 days) in the in-hospital cervical ripening arm, a reduction of 1895 minutes. In terms of an ICER, the home cervical ripening arm is the dominant strategy, both cost saving and reducing overall time in hospital.
When the outcome of interest is time from IOL to birth (regardless of where that time is spent), Table 34 shows that the home cervical ripening arm has the longer duration [3105 minutes (51.7 hours) compared with 2662 minutes (44.4 hours)]. This translates to an ICER of £137 per additional hour from IOL to birth. While this is not a large difference in time duration between arms, it does show that the at-home balloon catheter cervical ripening option is longer overall, which could impact on patients’ perspectives and preferences for method of cervical ripening. The longer duration may be due to the cervical ripening method of balloon catheter as opposed to prostaglandin, where IOL time is longer.
Sensitivity analyses – use of Foley and Cook balloons
Table 39 reports the sensitivity analysis of alternative IOL methods considering changes in utilisation rate from our assumption 50% split between the Foley and Cook balloons based on clinical experts’ advice. The increased use of the Foley balloon led to a lower cost estimate for the home IOL arm, as this balloon is less expensive compared with the Cook balloon. In all four different scenarios, home cervical ripening remained a cost-effective strategy. We considered two balloons equally efficacious in this analysis.
| Scenarios | Description (use of type of balloon) (%) | Home IOL cost (£) | Hospital IOL (£) | ΔC | ΔE | ICER |
|---|---|---|---|---|---|---|
| Base case | Foley balloon (50), Cook balloon (50) | 2548 | −961 | Home IOL dominates | ||
| Scenario 1 | Foley balloon (100), Cook balloon (0) | 2516 | −993 | Home IOL dominates | ||
| Scenario 2 | Foley balloon (0), Cook balloon (100) | 2581 | 3510 | −929 | 0.015 | Home IOL dominates |
| Scenario 3 | Foley balloon (75), Cook balloon (25) | 2532 | −977 | Home IOL dominates | ||
| Scenario 4 | Foley balloon (25), Cook balloon (75) | 2565 | −945 | Home IOL dominates |
Sensitivity analyses – patient perspective costs
Table 40 shows the resource items of relevance from the patient perspective. While this is a small sample (the qCHOICE study surveyed a subsample of the larger CHOICE cohort study), this is indicative data of the resource use from the patient perspective and the point estimates (along with standard errors) can be useful inputs to the economic model. The data indicate that 74.1% and 97.2% of partner or caregivers stayed with the mother who had hospital and home cervical ripening, respectively. The percentage of mothers who relied on paid or unpaid child-care services was higher among mothers who had cervical ripening in hospital (30.4%) compared with at home (17.1%). Among the mothers who had home cervical ripening, 63.9% called the midwife for consultation. Around 47% of the mothers in the ‘home’ arm travelled to hospital and came back to home when they were waiting for initiation of labour at home. There was little difference between average transport costs, which overall were higher among mothers who had hospital cervical ripening (£23.1) than mothers who had home cervical ripening (£18.74).
| Cost items/questions | Home IOL | Hospital IOL | ||||
|---|---|---|---|---|---|---|
| N | Yes | % or Mean (SD) | N | Yes | % or Mean (SD) | |
| Anyone stayed with mother | ||||||
| Partner or other caregiver stayed with mother before admission (hospital only) | – | – | – | 228 | 169 | 74.1% |
| Partner or other caregiver stayed with mother at home before move back to hospital | 36 | 35 | 97.2% | – | – | – |
| Paid or unpaid child care | ||||||
| Used paid or unpaid child care | 35 | 6 | 17.1% | 181 | 55 | 30.4% |
| Used paid child care | 35 | 1 | 2.9% | 181 | 7 | 3.9% |
| Average hour paid | – | – | – | 181 | 7 | 10.86 (6.67) |
| Average hour unpaid | – | – | – | 181 | 50 | 50.46 (61.43) |
| Phone call and return to hospital | ||||||
| Phone call to midwife while stay at home | 36 | 23 | 63.9% | – | – | – |
| Average number of times called | 36 | 23 | 1.91 (0.24) | –- | – | – |
| Returned to hospital but go home again without being admitted | 36 | 17 | 47.2% | – | – | – |
| Returned to hospital once | 36 | 13 | 36% | – | – | – |
| Returned to hospital two/three times | 36 | 4 | 11% | – | – | – |
| Transport cost | ||||||
| Average transport cost (£) | 36 | 35 | 18.74 (14.4) | 230 | 223 | 23.13 (37.93) |
Table 41 shows the cost from the patients’ perspectives in the home and hospital study arms. The total patient perspective cost was higher in the hospital arm (£954) compared with the home arm (£665); this is predominantly driven by the opportunity cost of partners’/other caregivers’ time supporting the mother either at home or in hospital ‘away from other activities’, There was little reported difference in terms of child-care cost (both paid and unpaid) and transportation costs between arms.
| Cost items | Home IOL | Hospital IOL | ||||
|---|---|---|---|---|---|---|
| N | Mean cost (£) | SE | N | Mean cost (£) | SE | |
| Anyone (partner) stayed with mothera | 35 | 570 | – | 169 | 1000 | – |
| Paid or unpaid child care | 4 | 821 | – | 55 | 821 | – |
| Phone call cost | 23 | 2 | – | – | – | – |
| Transport cost (direct cost) | 36 | 18 | 2 | 230 | 22 | 2 |
| Total cost | 36 | 665 | 39 | 230 | 954 | 35 |
Table 42 reports the economic analysis outcomes, including the patient perspective costs in addition to the NHS costs; that is, the ‘societal’ perspective. The addition of patient perspective costs makes both arms more expensive, yet, overall, home cervical ripening remains the optimal strategy, saving costs to NHS and patients while showing little difference in terms of NNU admissions avoided.
| Intervention | Mean cost (£): without NNU cost | Mean effect: NNU admission avoided in the last 48 hours (%) | Cost difference (£) | Effect difference | ICER (£) |
|---|---|---|---|---|---|
| Base case (NHS costs) probabilistic results | |||||
| Home IOL | 2524 | 97.6 | −993 | 0.005 | (Home dominates) |
| Hospital IOL | 3517 | 97.1 | (−1198, −783) | (−0.005, 0.013) | |
| Societal perspective (NHS and patient costs) probabilistic results | |||||
| Home IOL | 3184 | 97.6 | −1289 | 0.005 | (Home dominates) |
| Hospital IOL | 4473 | 97.1 | (−1541, −1059) | (−0.005, 0.013) | |
Discussion
The economic analysis found home cervical ripening to be cost saving in comparison with in-hospital cervical ripening with no detrimental effect on NNU admissions. Indeed, the effect point estimate indicates an improvement in terms of NNU admissions avoided (although this was not statistically significant as per non-inferiority study design), and variation within the confidence and uncertainty interval was well below the bounds of the original study non-inferiority margin of a 4% increase in NNU admissions. 41 The probabilistic analysis indicates that at willingness-to-pay thresholds above £3000, there is an 82% probability that home cervical ripening is the optimal/cost-effective option. Typically, a willingness-to-pay threshold, such as the NICE recommended threshold of £20,000/QALY is used to interpret such results, however, as this cost-effectiveness analysis uses NNU admissions avoided, the cost/QALY threshold is likely too high and, arguably, would not be applicable in this study. 41 A suitable willingness-to-pay value per NNU admission avoided is difficult to determine, yet anecdotally a £3000 could be acceptable. Other studies of a similar nature report a notional willingness-to-pay threshold of £100 for each inpatient hour averted,34 and that probability that cervical ripening with isosorbide mononitrate is cost-effective was estimated at 0.67. This increases to 0.77 at a notional willingness-to-pay threshold of £1000. If a net monetary benefit approach had been applied (using a willingness-to-pay threshold of £30,000), the incremental net benefit in favour of the home cervical ripening group was £133 per person. Likewise, a cost minimisation analysis would have shown the home cervical ripening arm to be the cost-saving option.
The cost savings are driven primarily by reduced time in antenatal ward for the home cervical ripening group, with an average of 476 minutes (7.9 hours) compared with 2243 minutes (37.4 hours). The analysis also showed that this method of cervical ripening is likely to lead to a longer time in labour ward (although the difference was not substantial between groups), and there was little difference in antenatal ward time. The resource use and cost data also indicate some of the ‘hidden’ or displaced costs of the home cervical ripening option, with an increased number and duration of phone calls from women and their partners to the hospital compared with those in the in-hospital group. This cost reflects, yet does not fully capture, some of the additional administrative burden that home cervical ripening could place on midwives and the maternity unit. Cost savings in terms of reduced antenatal ward time and freed up bed space (which are captured in our analysis) could potentially be offset somewhat by additional ‘hidden’ administrative burden. The base case analysis indicates some of the administrative or hidden costs that home IOL could incur, through longer duration in labour ward associated with home IOL via balloon catheter, and the increased number of and duration of phone calls to maternity unit, which will be answered by a midwife; however, there are other potential hidden costs that the study did not capture. The qCHOICE study case studies explored this further in qualitative interviews and found potential workload implications and stress inducing factors for staff, which were not accounted for in the economic analysis.
The observational study analysis and economic analysis of the CHOICE study data set found no difference in terms of maternal complications or neonatal morbidity, and therefore these were not incorporated into the economic model, nor a longer-term model extrapolation undertaken. Duration of postnatal stay is another indicator of maternal complications, and the CHOICE data set and economic analysis found little difference between arms, again indicating no detrimental impact of the home cervical ripening method.
While the home cervical ripening option was found to be the optimal strategy in our base case analysis, when the outcome of interest is time from IOL to birth (regardless of where that time is spent), the home cervical ripening group had the longer duration (51.7 hours compared with 44.4 hours). While this is not a large difference in time duration between arms, it does show that the at home balloon catheter cervical ripening option is longer overall, which could impact on patients’ perspectives and preferences for method of cervical ripening. The longer duration may be due to the nature of the cervical ripening method of balloon catheter as opposed to prostaglandin.
We identified several studies on IOL have been undertaken in the UK setting, yet there remains a lack of established economic evidence comparing cervical ripening methods in both in-hospital and home settings. Despite this, home cervical ripening is frequently cited in reviews as favourable due to its cost saving nature. 42,43 Alfirevic et al. 44 conducted a systematic review and meta-analysis to assess the safety and cost-effectiveness of various IOL methods (including both mechanical and complementary methods). The authors found Foley and Cook balloon catheters were both dominated (more expensive and less effective) by an oral misoprostol solution. The authors found that titrated misoprostol solution had the greatest probability of being the cost-effective option; however, given the increased rates of uterine hyperstimulation of misoprostol compared with mechanical methods, it may not be appropriate for home cervical ripening. The findings are not directly comparable to this study since the authors did not find studies with outpatient settings in their review. Eddama et al. 34 conducted a cost-effectiveness analysis of outpatient (at home) cervical ripening with isosorbide mononitrate. The study found a fewer number of hours’ stay in antenatal and labour wards than women allocated to the placebo group. The incremental cost per hour prevented from hospital admission to delivery was £7.53. Petrou et al. 45 estimated the cost-effectiveness of prostaglandin vaginal gel for the IOL from the perspective of the UK NHS. The study found that mothers experienced a significantly reduced interval between IOL and delivery (average 1711 minutes for prostaglandin E2 gel vs. 2765 minutes for prostaglandin E2 tablets; p = 0.03) and concluded that prostaglandin E2 gel is probably more cost-effective than prostaglandin E2 tablets for the IOL.
A study similar to this analysis was conducted by Merollini et al. in Australia settings. 33 The authors assessed the cost-effectiveness of IOL with outpatient balloon catheter versus inpatient prostaglandin vaginal gel or tape using a decision tree model. The authors used QALYs gained as the outcome and found that outpatient balloon cervical ripening was probably cost saving compared with inpatient IOL with prostaglandin. Similar to our findings, the study estimated reduced hours of antenatal ward stay (12.5 reduced hours) in outpatient cervical ripening compared to the prostaglandin in inpatients. However, inpatient stay was higher at the birth suite/labour ward and postnatal ward in the balloon outpatient strategy compared with the prostaglandin in inpatient. Another study in South Australia conducted by compared inpatient with outpatient care for cervical ripening using prostaglandin E2 among women with healthy, low-risk prolonged pregnancies. 15 Women randomised to outpatient care had an overall cost saving of A$319 (approximately £518) (53) per woman (95% CI A$104 to A$742) compared with women randomised to usual care. Merollini et al. 33 recommended that future studies should compare safety, cost-effectiveness and acceptability of alternative at outpatient cervical ripening using mechanical methods.
The economic analysis used the rich CHOICE study data set but adopted a decision modelling approach as more appropriate given power and sample issues with the cohort data set. This approach enabled the synthesis of the study data with other evidence identified in a systematic literature review, particularly regarding parameters of high uncertainty. PSA was undertaken to account for parameter uncertainty. The analysis time horizon was limited to 28 days post birth to align with the CHOICE cohort study. As per protocol, maternal and neonatal complications were explored, and as there was no indication of medium- or long-term morbidity or mortality implications, we did not extrapolate beyond the study time horizon. One limitation of this health economic analysis is the absence of a utility-based outcome, such as QALYs, which is recommended by NICE. This limitation hinders the ability to compare the cost-effectiveness of the intervention with other healthcare resource allocations.
This economic analysis enabled exploration of the potential cost saving from home cervical ripening and its relation to factors that may offset it, such as increased costs of any additional morbidity, and greater demand on midwifery time on phone calls with women at home. The economic analysis has shown that home cervical ripening is likely to be cost saving, with no detrimental impact on NNU admissions or maternal complications and, in terms of the economic outcomes, it would be the optimal approach. However, additionally, the incorporation of costs to women and their families (via questions embedded in the process evaluation survey) was a particularly novel and important aspect of the CHOICE economic analysis, allowing sensitivity analyses to adopt a broader patient perspective. Including costs of such ‘spill over’ effects for patients and their families due to additional time supporting/waiting in hospital (e.g. time away from family), showed an increased time burden for the in-hospital group, but also highlighted that the home cervical ripening arm often had multiple trips to and from the hospital prior to admission. These findings, alongside the base case results, are of particular relevance and importance for policy implications decisions.
Overall, the economic analyses from both the NHS and patient perspectives have shown that home cervical ripening using balloon is likely to be cost saving, with no detrimental impact on NNU admissions or length of stay in postnatal wards. However, the implications for a service change strategy need to be thought through thoroughly and considered in tandem with the qCHOICE findings. Unless women feel supported to choose between home and in-hospital settings, and they have sufficient information to allow realistic expectations of the IOL process, women will not have positive birth experiences.
Chapter 5 Discussion and conclusions
Main findings
This study has assessed the safety, effectiveness and cost-effectiveness of home cervical ripening compared with in-hospital cervical ripening in a UK NHS setting. Conducted in the context of the COVID-19 pandemic, lower home cervical ripening rates than anticipated and maternity units overwhelmed by demand for IOL with long delays throughout the process, the study suggests that home cervical ripening may be safe and is likely to be cost saving. However, the acceptability of home cervical ripening is hugely dependent on context and relational aspects of care, and thus is not guaranteed. Sample size in the CHOICE cohort was smaller than anticipated due to fewer mothers undergoing home cervical ripening and later recruitment of sites during the pandemic, thus the study is underpowered and firm conclusions around safety cannot be drawn.
The CHOICE cohort study shows no clear signal of safety concerns during home cervical ripening with balloon compared with in-hospital cervical ripening with prostaglandin, but substantial uncertainty remains surrounding safety outcomes due to an underpowered sample size. The findings show that woman on the home balloon cervical ripening pathway take around 10 hours longer from start of cervical ripening until birth. The majority of this time is spent at home, which partly explains why, on average, IOL with home cervical ripening using balloon costs around £933 less to the NHS and £289 less to women in the health economic analyses. These differences were seen despite the consideration of less obvious costs such as telephone communication between women and midwifery staff while awaiting re-admission for labour ward care. Together, these findings suggest that home cervical ripening with balloon dominates as a cheaper approach to IOL with no clear signal of safety concerns, but more evidence on safety outcomes is essential from a larger study sample to allow adequate assessment. The detailed process evaluation shows that current practice does not adequately equip women with the information they need to make informed choices or develop realistic expectations about IOL, nor does it ensure that women feel physically and emotionally safe during IOL, whether at home or in-hospital. There is some evidence that home cervical ripening is acceptable to women, but no suggestion that it is necessarily more acceptable than in-hospital care. Acceptability appears to be dependent upon how supported and safe women feel throughout the process of IOL.
Strengths
The CHOICE study has provided a unique multilayered insight into contemporary IOL practice in the UK that will inform multiple aspects of clinical practice going forward. These include the provision of evidence-based information on the process of balloon cervical ripening at home that can be shared with women and staff, and informing NHS trusts and boards of the potential cost savings involved in offering home cervical ripening with balloon as an alternative to the default approach of in-hospital cervical ripening with prostaglandin. The relatively early gestation (10% of home cervical ripening occurred at 37 weeks of gestation), the wide array of indications (only one in four home cervical ripening cases were for post dates) and the importance of how care is delivered to women undergoing home cervical ripening may each influence clinical guideline development going forward.
Limitations
Multiple elements of the CHOICE study were not conducted as planned and described in the study protocol. This largely related to the impact of the COVID-19 pandemic on site recruitment and IOL practice (setting) such that fewer women in the cohort and health economic analysis sample underwent home cervical ripening than anticipated. An attempt to continue the study to reach the original sample size was judged to be futile. Instead, the primary comparison was changed to ensure that the most common approach to home cervical ripening (balloon method) was compared with the most common approach to in-hospital cervical ripening (prostaglandin). However, the disadvantage was that both method and setting then differed between comparison groups, not just setting, making any conclusions about safety of setting alone impossible to reach. The smaller sample size than expected meant that the original sophisticated analysis plan could not be carried out. It is not possible to know whether the absence of evidence of any difference in safety outcome between home balloon cervical ripening and in-hospital prostaglandin cervical ripening would have been rejected in a larger sample size.
The cohort study analysis took the approach of including women where all relevant variables were recorded or were assumed not to have been relevant to the woman (e.g. no cervical ripening method recorded so assumed not to have had cervical ripening, no admission of baby to the NNU was assumed to mean that the baby was not admitted to the NNU). This ‘complete case analysis’ was performed as it was not possible to establish whether a variable was really ‘missing’ or simply did not apply to that individual woman/baby. Alternative options such as imputing a variable (inserting a value for the missing variable, estimating what that value should be) could have been considered if it had been clear which variables were ‘missing’. Similarly, if it had been known which values were missing, a sensitivity analysis could have been performed to look at the extremes of possible findings if all missing values were positive (i.e. the event did occur) or all missing values were negative (i.e. the event did not occur). As a result of the approach taken, it is possible that women who underwent cervical ripening but did not have full details recorded were inadvertently excluded from analyses, and that women who were included may have been inappropriately recorded as not having experienced an outcome that they did experience due to it not being recorded. The implications of this for the study are unknown, but could include higher incidences of certain outcomes than reported, or that a larger sample of women could have been included had the missing data been included. This issue does not apply to those variables where recording is virtually complete, for example, maternal age, postcode (used to assess deprivation status), mode of birth, blood loss.
Ultimately, the safety of home cervical ripening would ideally be assessed in a randomised trial to avoid the potential for confounding and reduce missing data, but the rare nature of key safety outcomes makes such a trial unfeasible due to the required sample size being excessive.
Generalisability
The findings of the CHOICE study are expected to be relevant across the UK and also in settings with similar government-funded maternity service provision and IOL policies and procedures.
Interpretation
The strengths of the overall CHOICE study lie in its pragmatic approach to quantifying and exploring experiences of home cervical ripening with balloon simultaneously, addressing both what is happening and why compared with in-hospital cervical ripening. It has also addressed financial implications of home cervical ripening using balloon methods in the context of a resource-constrained setting. The qualitative study findings have a major focus on experiences and unintended consequences of home cervical ripening, but also of offering IOL in the first place, especially on grounds of safety. It became clear that women viewed a recommendation for IOL to mean it was unsafe to remain pregnant and thus this raised alarm when IOL processes were delayed.
The study makes an important contribution to understanding of current IOL practice and experiences, and a range of implications of home cervical ripening using balloon. A further observation from across the CHOICE study is that service limitations (rather than policies or guidelines) influenced practice such that even if new evidence led to a change in guidance, there is no guarantee that this would lead to a change in clinical practice where high IOL rates and lack of resources to support these remain a major issue. The safety implications of the current situation were highlighted in accounts of labour being uncontrolled and unmonitored where no space on labour ward was available while labour established. This also raised issues around emotional safety of IOL in the current context given the lack of analgesia options and dedicated support out with the labour ward setting.
Future research should explore how IOL rates and staff levels combined are linked to safety outcomes, as there may be a point at which volume of work leads to unmet healthcare needs resulting in greater harm than benefit from IOL.
Implications for practice
While the CHOICE study did not identify any signals of safety concerns following home cervical ripening, the lower than anticipated sample size within the CHOICE cohort means an absence of conclusive evidence on neonatal and maternal outcomes persists.
Some very positive experiences of IOL were described when supportive factors were in place. Factors that mediated women’s experience of cervical ripening during IOL included support from maternity staff and birth partners’ presence, while factors prevalent in poor experience included lack of support, separation from birth partner, lack of privacy, inadequate pain relief, delays impacting progression of IOL process, inadequate information provision and choice and lack of privacy. These issues should each be considered in detail before units embark upon a change in IOL pathway.
Transitioning to home cervical ripening required a change of mindset, cross-professional working, cross-trust/board working, consistent training and professional confidence, for example, obstetric leadership in deciding who is safe to go home. Making home cervical ripening the default service also influenced the number of women who are offered and then go home for cervical ripening. This approach should be considered and implemented only after extensive consultation with service users, as interviews with women and birth partners revealed that home cervical ripening was not as acceptable when it was default as it would be if it were a genuine choice.
Summary of research recommendations
To better understand the potential differences in outcomes between home and in-hospital cervical ripening, future research should ensure that study cohorts are large enough to facilitate adjustment for potential confounders, and that findings are reported in a manner that facilitates meta-analysis. Data on uncommon but important safety outcomes, such as cord prolapse and intrapartum stillbirth, are important to fully understand the potential implications of home cervical ripening. Adequately powered future studies may thus benefit from focusing on maternity units who conduct a large proportion of cervical ripening in the home setting, with a similar number of units offering only in-hospital cervical ripening.
Given the prominent issue of delays during the IOL process, future research should explore how such delays impact upon experiences and outcomes of the IOL process. This includes how IOL rates and staffing levels may interact to impact upon safety outcomes.
The amount of oxytocin used following cervical ripening should be compared across mechanical and pharmacological methods and across home and hospital settings. This would ensure a better understanding of why home balloon cervical ripening was linked to longer second stage of labour than in-hospital cervical ripening.
Studies should consider how to ensure that informed choices regarding IOL and IOL setting are made. This could involve training of staff to engage in supported decision-making, development and evaluation of information resources or decision aids for women, and implementation research to assess what aspects of IOL services are most strongly linked to positive IOL experiences.
Equality, diversity and inclusion statement
The CHOICE study took initial steps during study set-up to optimise the participation of relevant people, including translating key patient-facing materials into seven languages and offering interpreter options for the qCHOICE survey. Images and pictures used in patient-facing materials and public engagement were reviewed by the parent advisory group (PAG) and patient and public involvement (PPI) co-applicants to ensure that they were inclusive and accessible.
Participation in qCHOICE was not as inclusive as it could have been, despite a number of strategies employed to improve recruitment, especially for the postnatal questionnaire-based survey. There are key recommendations below that could improve inclusivity in research participation, which future research designs should take into consideration.
In December 2021, the CHOICE research team organised two meetings with the PAG and PPI co-applicants to discuss widening participation in the study. Because certain demographic groups tend to participate more often and, thus, are more likely to be represented in research than others, it was important to understand what steps could be taken to ensure this research reflected the communities in which it was being conducted.
Though the research team took steps to increase accessibility for relevant people, the meetings with the PAG and PPI co-applicants highlighted that the research team could be doing more. There were three key recommendations:
-
increased compensation for service user participants
-
long-term engagement by research teams with the communities with which they work
-
employment of lay researchers with strong ties to local communities.
The PAG and PPI co-applicants recommended that compensation for surveys and interviews could be increased, as many of those who are underrepresented in research are more likely to have income- and time-related barriers to taking part. Demographic data from the UK show correlations between socioeconomic status, ethnicity and higher education status, meaning that any widening participation efforts must recognise this intersection.
They also emphasised that long-term engagement with local communities fosters trust between researchers and participants, and is particularly pertinent when working with groups who experience discrimination within health care and those who have experienced trauma during their care.
Finally, our PAG members raised the important point that academic teams may not the best people to be conducting research within communities, in which case the employment of lay researchers or organisations with strong ties locally would be beneficial for widening participation.
While the timeline for CHOICE did not allow for the enactment of all these key recommendations, they should be considered in future research design and data collection planning.
Patient and public involvement
The aim of PPI in the study was to provide public and patient involvement during every stage of the research from pretrial funding to report writing and dissemination.
From the start, two PPI co-applicants were involved in the study throughout the bid stage.
During the post-funding preparatory work, the PPI co-applicants were included in communications (e-mail, online meetings and face-to-face meetings) for development of the study protocol.
During the study, a PAG was established in July 2020, which included the two PPI co-applicants and two service users. A PPI framework was developed following the NIHR’s framework (INVOLVE) for public involvement in research. It outlined the values, roles, payment and recognition for PPI.
The PPI co-applicants on the team are involved in the production of the final report to the NIHR and publications of findings in peer-reviewed journals.
The PAG met several times through the study and provided feedback on patient-facing materials and ad-hoc consultation on specific aspects of the study, including:
-
CHOICE information leaflet
-
qCHOICE participant information sheets
-
qCHOICE study leaflets and poster
-
CHOICE BadgerNet PUSH notifications
-
qCHOICE questionnaire-based survey
-
qCHOICE social media strategy and Facebook ad campaign.
All documentation and media were amended in light of their comments, including changing pictures used in the Facebook advertising campaign to illustrations, reflecting one member’s experience that the women she works with respond better to these media types. The PAG was also consulted on widening participation in the study, the results of which are reported in the equality, diversity and inclusion statement.
The PPI positively influenced the development and progress of the study, in particular qCHOICE, and improved the quality and accessibility of the patient-facing study materials.
The PAG membership was small, and the study would have benefited from a larger group that was more representative and inclusive. More consultation with the PAG during data collection may have been beneficial, particularly during qCHOICE when survey recruitment was low.
The CHOICE team’s consultation regarding widening participation was a strong point during the study PPI, as it has produced key recommendations (outlined in the equality, diversity and inclusion statement) for researchers and future projects.
Conclusions
This study has assessed the safety, effectiveness and cost-effectiveness of home cervical ripening compared with in-hospital cervical ripening in a UK NHS setting. The study was conducted in the context of the COVID-19 pandemic, lower home cervical ripening rates than anticipated, and maternity units overwhelmed by demand for IOL with long delays throughout the process. The study suggests that home cervical ripening using balloon methods may be safe, but with substantial uncertainty around neonatal and maternal outcomes due to sample size limitations. Home cervical ripening appears to be cost saving. However, the acceptability of home cervical ripening is hugely dependent on context and relational aspects of care, and thus is not guaranteed.
Practice changes to be considered in light of the findings of the CHOICE study are the cost and potential experiential benefits of offering home cervical ripening using balloon as an option in UK maternity units, while taking into account the key features of care that are linked to positive IOL experiences.
Future research should include large study populations for further study of safety outcomes of home cervical ripening, including meta-analyses. Studies should also focus on improving informed choice for women relating to accepting IOL and determining setting of cervical ripening.
Chapter 6 Other information
Study registration
The CHOICE protocol is published in BMJ Open: Cervical ripening at home or in-hospital – prospective cohort study and process evaluation (CHOICE) study: a protocol. 19
The CHOICE study is registered on the ISRCTN database, with registration number: ISRCTN32652461.
Protocol
The most recent version of the CHOICE study protocol (V6.0) will be available on the NIHR website.
Funding sources
Funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme. This was a commissioned call. The funder was not involved in conducting the research.
Additional information
Contributions of authors
Mairead Black (https://orcid.org/0000-0002-6841-8601) (Senior Clinical Lecturer in Obstetrics) contributed to study design, co-ordinated the observational cohort study analysis, interpreted the findings, prepared the observational cohort study and overall discussion chapters and had oversight of the writing of this report.
Cassandra Yuill (https://orcid.org/0000-0002-3918-5917) (Research Fellow, Maternal and Child Health) collected and analysed data, interpreted the qCHOICE findings and wrote sections of the qCHOICE chapter in this report.
Mairi Harkness (https://orcid.org/0000-0002-1007-7648) (Research Fellow, Maternal and Child Health) collected and analysed data, interpreted the qCHOICE findings and wrote sections of the qCHOICE chapter in this report.
Sayem Ahmed (https://orcid.org/0000-0001-9499-1500) (Research Associate, Health Economics) conducted the health economics analysis and interpreted the findings and contributed to the writing of the health economics analysis chapter in this report.
Linda Williams (https://orcid.org/0000-0002-6317-1718) (Research Fellow, Statistics) conducted the analysis of the observational cohort data and edited this report.
Kathleen A Boyd (https://orcid.org/0000-0002-9764-0113) (Reader in Health Economics and Health Technology Assessment) designed and supervised the health economic analysis, interpreted the findings, wrote the health economic analysis chapter and edited this report.
Maggie Reid (https://orcid.org/0000-0002-5458-2902) (Midwife and Clinical Operations Lead, Clevermed Ltd) contributed to study design and conduct, and approved the content of this report.
Amar Bhide (https://orcid.org/0000-0003-2393-7501) (Consultant and Reader in Obstetrics and Fetal Medicine) contributed to study design and conduct, and approved the content of this report.
Neelam Heera (Founder of Cysters) contributed to study design and approved the content of this report.
Jane Huddleston (Public representative) contributed to study design and approved the content of this report.
Neena Modi (https://orcid.org/0000-0002-2093-0681) (Professor of Neonatal Medicine) contributed to study design and approved the content of this report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Chair of Medical Statistics and Trial Methodology) contributed to study design and approved the content of this report.
Dharmintra Pasupathy (https://orcid.org/0000-0002-6722-1006) (Reproductive and Perinatal Health) contributed to study design and approved the content of this report.
Julia Sanders (https://orcid.org/0000-0001-5712-9989) (Professor of Clinical Midwifery) contributed to study design and interpretation of findings, including drafting of key study messages, and approved the content of this report.
Gordon C S Smith (https://orcid.org/0000-0003-2124-0997) (Professor and Head of Department, Obstetrics and Gynaecology) contributed to study design and interpretation of findings, and approved the content of this report.
Rosemary Townsend (https://orcid.org/0000-0002-3438-7069) (Senior Research Fellow in Obstetrics) contributed to co-ordination of the observational cohort study analysis, interpretation of findings, including drafting of key study messages, wrote sections and approved the content of this report.
Helen Cheyne (https://orcid.org/0000-0001-5738-8390) (Professor of Maternal and Child Health) designed the qCHOICE study, supervised conduct of qCHOICE research, interpreted qCHOICE and the wider CHOICE findings, wrote sections and approved the content of this report.
Christine McCourt (https://orcid.org/0000-0003-4765-5795) (Professor of Maternal and Child Health) designed the qCHOICE study, supervised conduct of qCHOICE research, interpreted qCHOICE and the wider CHOICE findings, wrote sections and approved the content of this report.
Sarah Stock (https://orcid.org/0000-0003-4308-856X) (Professor of Maternal and Fetal Health) conceived the idea for the CHOICE study, designed and led the conduct of the study, including interpreting all findings, and edited this report.
Posthumous acknowledgement
Professor Fiona Denison (Professor of Translational Obstetrics) contributed to study design and set-up.
Study management
The Edinburgh Clinical Trials Unit supported the conduct of the CHOICE study through its study co-ordination service.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
The study data generated are not suitable for sharing beyond that contained within the report. Further information can be obtained from the corresponding author.
Ethics statement
The CHOICE study was approved by the York and Humber-Sheffield Ethics Committee on 17 June 2020, reference number 20/YH/0145.
Information governance statement
The University of Edinburgh is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection legislation, The University of Edinburgh is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: dpo@ed.ac.uk.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/LPYT7894.
Primary conflicts of interest: Mairead Black has received funding from the NIHR as Chief Investigator of the Plan-A study 2022–5. She has received research funding from NIHR HTA (NIHR204294, NIHR204156, OBSUK NIHR152057). Kathleen A. Boyd has funding from NIHR HTA for health economics research (NIHR131352, NIHR 12/211/54, NIHR 15/55/54). Maggie Reid had a contract with Clevermed Limited to provide midwifery expertise. Amar Bhide had an NIHR grant for the TRUFFLE-II study. He has received fundings from the NIHR HTA (NIHR 204647, NIHR 21/582 and NIHR Innovate UK Project No: 10053908). He received royalties for the book High Risk Pregnancy and Delivery: A South Asian Perspective (Elsevier, India). He has support for attending meetings/trave from Nordic Federation of Obstetrics and Gynaecology. He is in a leadership or fiduciary role at Acta Obstetricia Gynaecologia Scandinavia. Receipt of equipment from St George’s charity. Neelam Heera is founder and chair of trustees at Cysters and receives payment for her time for patient and public involvement in related research projects. Neena Modi receives NIHR funding to her institution (NIHR150958, NIHR153935, NIHR203323, HS&DR Project: 15/70/104, PR-PRU-1217-21202). John Norrie receives research funding to the University of Edinburgh from NIHR HTA programmes (NIHR127569, NIHR133092, NIHR151601, NIHR155294, NIHR132594 (19/162/02), NIHR152877, NIHR133776, NIHR129801, C-10333879 NIHR131352, NIHR131118, 15/130/95, 2019-002479-33, NIHR155342, NIHR155477, 16/93/01, NIHR131855, 17/147/47, 17/30/02, 15/150/05, 17/22/02 125193). He is Chair of MRC/NIHR Efficacy and Mechanism Evaluation Board, 2019 to present. He was on the HTA Commissioning Sub-Board (EOI) 2016–17, NIHR CTU Standing Advisory Committee 2018–23, NIHR HTA & EME Editorial Board 2015–19, Pre-Exposure Prophylaxis Impact Review Panel 2017, EME – Funding Committee Members 2019–22, HTA General Committee 2016–19, HTA Post-Funding Committee teleconference 2016–19 and HTA Funding Committee Policy Group 2016–19. Dharmintra Pasupathy was the obstetric lead for the National Maternity Perinatal Audit UK 2016–20. Julia Sanders received funded time as part of the NIHR grant that funded the study. She has held grants in the past 36 months from the NIHR, Scottish Government and the Burdett Trust for Nursing (NIHRDH-16/149/01, NIHR127325). She is a member of study steering committees for various NIHR funded studies, a well-being of women funding panel member, NIHR PCAF funding panel member and NPEU, Oxford University and trials advisory group member. She also receives funding for self-employed midwifery witness work. Gordon C S Smith receives financial support for consumables for research including screening for complications of pregnancy near term. He is also principal investigator and co-principal Investigator on NIHR-funded research projects (71276070, HTA 17.148.07, HER00660, NIHRDH-HTA/15/105/01). Rosemary Townsend has a Chief Scientist Office early postdoctoral fellowship. Helen Cheyne has research grant funding from NIHR (NIHR130619, HS&DR 17/105/16, NIHR133727, NIHR133727, NIHR130619, 17/105/16). She had evaluation of peer support project from Aberlour and MAHP Research unit core funding from the Scottish Government Chief Scientist office. She has participated on an NIHR and University of Aberdeen project advisory board. Sarah Stock has received research funding from NIHR HTA (NIHR131352, NIHR HTA 17/22/02 and 16/151/01), Wellcome Trust, MRC, CSO Scotland and Tommy’s charity. She was a member of an HTA funding committee 2016–20, received consultancy fees from Natera and honoraria from Hologic. She has HTA DMC and TSC membership. Linda Williams and Jane Huddleston have no interests to declare.
Publications
Stock SJ, Bhide A, Richardson H, Black M, Yuill C, Harkness M, et al. Cervical ripening at home or in-hospital: prospective cohort study and process evaluation (CHOICE) study: a protocol. BMJ Open 2021;11:e050452. https://doi.org/10.1136/bmjopen-2021-050452
Harkness M, Yuill C, Cheyne H, Stock SJ, McCourt C. Induction of labour during the COVID-19 pandemic: a national survey of impact on practice in the UK. BMC Pregnancy Childbirth 2021;21:310. https://doi.org/10.1186/s12884-021-03781-x
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Public Health Scotland . Births in Scottish Hospitals; Maternity and Births 2018 19 2020. https://publichealthscotland.scot/our-areas-of-work/early-years-and-young-people/maternity-and-births (accessed 5 April 2024).
- NHS Digital Community and Mental Health Team . NHS Maternity Statistics, England: 2020–21 2021. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics/2020-21# (accessed 5 April 2024).
- Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network . Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 2018;379:513-23.
- Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ 2012;344.
- Knight HE, Cromwell DA, Gurol-Urganci I, Harron K, van der Meulen JH, Smith GCS. Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: an English national cohort study. PLOS Med 2017;14.
- Royal College of Midwives . The Gathering Storm: England’s Midwifery Workforce Challenges 2017.
- Shetty A, Burt R, Rice P, Templeton A. Women’s perceptions, expectations and satisfaction with induced labour – a questionnaire-based study. Eur J Obstet Gynecol Reprod Biol 2005;123:56-61.
- Henderson J, Redshaw M. Women’s experience of induction of labor: a mixed methods study. Acta Obstet Gynecol Scand 2013;92:1159-67.
- Coates R, Cupples G, Scamell A, McCourt C. Women’s experiences of induction of labour: qualitative systematic review and thematic synthesis. Midwifery 2019;69:17-28.
- Norman JE, Stock S. Intracervical foley catheter for induction of labour. Lancet 2011;378:2054-5.
- National Institute for Health and Care Excellence . Induction of Labour: Clinical Guideline 2008.
- Jozwiak M, Oude Rengerink K, Benthem M, van Beek E, Dijksterhuis MGK, de Graaf IM, et al. PROBAAT Study Group . Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial. Lancet 2011;378:2095-103.
- Cheyne H, McCourt C, Semple K. Mother knows best: developing a consumer led, evidence informed, research agenda for maternity care. Midwifery 2013;29:705-12.
- Vogel JP, Osoti AO, Kelly AJ, Livio S, Norman JE, Alfirevic Z. Pharmacological and mechanical interventions for labour induction in outpatient settings. Cochrane Database Syst Rev 2017;9.
- Adelson PL, Wedlock GR, Wilkinson CS, Howard K, Bryce RL, Turnbull DA. A cost analysis of inpatient compared with outpatient prostaglandin E2 cervical priming for induction of labour: results from the OPRA trial. Aust Health Rev 2013;37:467-73.
- Sharp AN, Stock SJ, Alfirevic Z. Outpatient induction of labour in the UK: a survey of practice. Eur J Obstet Gynecol Reprod Biol 2016;204:21-3.
- Levine LD, Downes KL, Parry S, Elovitz MA, Sammel MD, Srinivas SK. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol 2018;218:254.e1-7.
- Wing DA, Brown R, Plante LA, Miller H, Rugarn O, Powers BL. Misoprostol vaginal insert and time to vaginal delivery: a randomized controlled trial. Obstet Gynecol 2013;122:201-9.
- Stock SJ, Bhide A, Richardson H, Black M, Yuill C, Harkness M, et al. Cervical ripening at home or in-hospital – prospective cohort study and process evaluation (CHOICE) study: a protocol. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2021-050452.
- NHS England . The Maternity Transformation Report: Reducing Admission of Full Term BABIES to Neonatal Units n.d. www.england.nhs.uk/mat-transformation/reducing-admission-of-full-term-babies-to-neonatal-units (accessed May 2023).
- Jones MN, Palmer KR, Pathirana MM, Cecatti JG, Filho OBM, Marions L, et al. Balloon catheters versus vaginal prostaglandins for labour induction (CPI Collaborative): an individual participant data meta-analysis of randomised controlled trials. Lancet 2022;400:1681-92.
- Perlman NC, Carusi DA. Retained placenta after vaginal delivery: risk factors and management. Int J Womens Health 2019;11:527-34.
- Hodnett ED, Simmons-Tropea DA. The labour agentry scale: psychometric properties of an instrument measuring control during childbirth. Res Nurs Health 1987;10:301-10.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick–Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5.
- Scottish Government . Maternity Care Survey 2018: National Results 2019. www.gov.scot/publications/maternity-care-survey-2018-national-results/documents (accessed 5 April 2024).
- Henry A, Madan A, Reid R, Tracy SK, Austin K, Welsh A, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth 2013;13:25-2393.
- Timmermans S, Tavory I. Theory construction in qualitative research from grounded theory to abductive analysis. Sociol Theory 2012;30:167-86.
- Meyer S, Lunnay B. The application of abductive and retroductive inference for the design and analysis of theory-driven sociological research. Sociol Res 2013;18:86-9.
- Robinson R. Cost-effectiveness analyses. BMJ 1993;307:793-5.
- Merollini K, Beckmann M. Induction of labor using balloon catheter as an outpatient versus prostaglandin as an inpatient: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol 2021;260:124-30.
- Garritty C, Gartlehner G, Nussbaumer-Streit B, King V, Hamel C, Kamel C, et al. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 2020;130:13-22.
- Merollini KMD, Beckmann M. Induction of labor using balloon catheter as an outpatient versus prostaglandin as an inpatient: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol 2021;260:124-30.
- Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, et al. The cost-effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. Br J Obstet Gynaecol 2009;116:1196-203.
- Dos Santos F, Drymiotou S, Antequera Martin A, Mol BW, Gale C, Devane D, et al. Development of a core outcome set for trials on induction of labour: an international multistakeholder Delphi study. Br J Obstet Gynaecol 2018;125:1673-80.
- National Institute for Health and Care Excellence . British National Formulary 2021. https://bnf.nice.org.uk (accessed 5 April 2024).
- Jones K, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: Personal Social Services Research Unit, University of Kent; 2021.
- Department of Health . NHS Reference Costs: Financial Year 2015–2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 5 April 2024).
- van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis 2010;69:i89-91.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual. NICE Process and Methods PMG20 2024. www.nice.org.uk/process/pmg20/chapter/introduction (accessed 22 May 2024).
- Dong S, Khan M, Hashimi F, Chamy C, D’Souza R. Inpatient versus outpatient induction of labour: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2020;20. https://doi.org/10.1186/s12884-020-03060-1.
- Kelly AJ, Alfirevic Z, Ghosh A. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD007372.pub3.
- Alfirevic Z, Keenev E, Dowswell T, Welton N, Medley N, Dias S, et al. Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2016;20:1-584.
- Petrou S, Taher S, Abangma G, Eddama O, Bennett P. Cost-effectiveness analysis of prostaglandin E2 gel for the induction of labour at term. Br J Obstet Gynaecol 2011;118:726-34.
- Alfirevic Z, Keeney EDT, Dowswell T, Welton NJ, Medley N, Dias S, et al. Methods to induce labour: a systematic review, network meta-analysis and cost-effectiveness analysis. Br J Obstet Gynaecol 2016;123:1462-70.
- NHS England . 2020/21/National/Tariff/Payment/System 2020. www.england.nhs.uk/pay-syst/national-tariff/national-tariff-payment-system (accessed 5 April 2024).
- Office for National Statistics . Census 2021. Earnings and Working Hours 2021. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours (accessed 5 April 2024).
- Statista . Average Cost of Full-Time (50 Hours a Week) Childcare at Nurseries in Great Britain in 2022 2022.
- Gupta JK, Maher A, Stubbs C, Brocklehurst P, Daniels JP, Hardy P. Synthetic Osmotic Cervical Dilator for Induction of Labor in Comparison to Dinoprostone Vaginal insErt (SOLVE) Collaborative Group . A randomized trial of synthetic osmotic cervical dilator for induction of labor vs dinoprostone vaginal insert. Am J Obstet Gynecol 2022;4. https://doi.org/10.1016/j.ajogmf.2022.100628.
- Abel GA, Barclay ME, Payne RA. Adjusted indices of multiple deprivation to enable comparisons within and between constituent countries of the UK including an illustration using mortality rates. BMJ Open 2016;6. https://bmjopen.bmj.com/content/6/11/e012750.full.
- Fat LN, Scholes S, Boniface S, Mindell J, Stewart-Brown S. Evaluating and establishing national norms for mental wellbeing using the short Warwick–Edinburgh Mental Well-being Scale (SWEMWBS): findings from the Health Survey for England. Qual Life Res 2017;26:1129-44. https://doi.org/10.1007/s11136-016-1454-8.
- Ougham K, Modi N. The NDAU Report 2016. London: Neonatal Data Analysis Unit, Imperial College London; 2017.
Appendix 1 qCHOICE additional data tables: postnatal survey findings
| Agree (%) | Neutral (%) | Disagree (%) | |
|---|---|---|---|
| I felt a lot of discomfort | |||
| Balloon catheter | 31 (72) | 2 (5) | 10 (23) |
| Pessary | 97 (48) | 7 (3) | 97 (48) |
| Balloon and pessary | 9 (75) | 0 | 3 (25) |
| Osmotic dilatora | 4 (50) | 0 | 4 (50) |
| I was able to cope with the discomfort | |||
| Balloon catheter | 34 (79) | 0 | 9 (21) |
| Pessary | 167 (83) | 5 (2) | 29 (14) |
| Balloon and pessary | 8 (67) | 1 (8) | 3 (35) |
| Osmotic dilatora | 7 (87) | 0 | 1 (13) |
| Minimum score | Maximum score | Mean score (population average, women = 23.2) | |
|---|---|---|---|
| All respondents, N = 309 (0 missing) | 7 | 35 | 24.5 |
| Cervical ripening only, N = 266 | 7 | 35 | 24.5 |
| Hospital cervical ripening, N = 230 | 7 | 35 | 24.5 |
| Home cervical ripening, N = 36 | 14.75 | 35 | 24.2 |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | |
|---|---|---|---|---|---|
| Type of unit | OU and AMU | Two OU and two AMUs | OU | OU and two FMUs | OU and AMU |
| Geographical location | England (Mid) | England (South) | England (North) | Scotland (East) | Scotland (West) |
| Area type | Urban inner city | Suburban | Mixed urban and rural | Mixed urban and rural | Mixed urban and rural |
| IOL rate (%) | 35–45 | 45 | 55–60 | 33 | 34 |
| IOL information first given | 35–37 weeks | 34–36 weeks | 38 and 40 weeks | 38–39 weeks | 38–39 weeks |
| Membrane sweeps offered | 40 weeks | 38 weeks | 1–2 per week with > 48 hours in between | 40 weeks | 40 weeks |
| Gestation IOL offered (low risk/prolonged pregnancy) | 41–42 weeks (41+3 suggested) | 41–42 weeks | 41–42 weeks | Offer at 40+7; IOL booked at 40+12 |
Offer at 40+7; IOL booked at 40+12 |
| Who offers IOL | Midwives (low risk, PROM at term) and obstetricians (high risk) | Midwives and obstetricians (high risk) | Midwives (low risk) and obstetricians (high risk) | Midwives (low risk) and obstetricians (high risk) | Midwives (low risk, shared high risk) and obstetricians (high risk) |
| Offer home cervical ripening | Yes | No (suspended) | Yes | Yes | Yes |
| Who does CR? | Midwives | Midwives (prostaglandin) Obstetricians (balloon catheter) |
Midwives (prostaglandin, low risk) | Midwives | Midwives (prostaglandin, balloon catheter) Obstetricians (balloon catheter) |
| Home cervical ripening eligibility | Low-risk post-dates pregnancy | N/A | Low-risk post-dates pregnancy | All unless significant concern about pregnancy | Low-risk post-dates pregnancy |
| Cervical ripening methods offered | Prostaglandin Dilapan-S during SOLVE Trial50 | Prostaglandin (parous) Balloon catheter (nulliparous) |
Prostaglandin Dilapan-S | Prostaglandin Balloon catheter |
Prostaglandin Balloon catheter Foley catheter |
| Methods for home cervical ripening | Prostaglandin | N/A | Dilapan-S |
Balloon catheter | Balloon catheter |
| Protocol for those who defer or decline cervical ripening | Individualised plan made by Consultant Obstetrician. Offer of twice weekly monitoring and membrane sweeps | Consultant obstetrician appointment and management plan. Offer of twice weekly monitoring | Offer of at least twice weekly monitoring | Counselling with a senior clinician. Offer of at least twice weekly monitoring | Documentation and risk discussion with senior obstetrician. Twice weekly monitoring |
| Where does cervical ripening take place (if not home) | 8-bed IOL suite or 4-bed bay on antenatal ward | Unit 1: antenatal ward dedicated bay Unit 2: room allocated for cervical ripening on antenatal ward. |
Single ensuite rooms on labour suite | Antenatal ward (maternity assessment area) | Antenatal ward dedicated bay and single rooms |
| Women’s journey during hospital cervical ripening | Admitted to IOL suite for assessment and start of CR. Transfer to labour ward when able to ARM, in labour or clinical reason | Admitted to antenatal ward for assessment and start of CR. Transfer to labour ward when able to ARM, in labour or clinical reason. If balloon was used and not in labour, then Prostaglandin for 6 hours | Admitted to IOL suite for assessment and start of CR. Transfer to labour ward when able to ARM, in labour or clinical reason | Admitted to antenatal ward for assessment and start of CR. Transfer to labour ward when able to ARM, in labour or clinical reason. After 24 hours (if not in labour), reassessed and receive Prostin | Admitted to antenatal ward for assessment and start of CR. Transfer to labour ward when able to ARM, in labour or clinical reason. If using balloon, ARM must be performed within 2 hours of removal |
| Women’s journey during home cervical ripening | Attend IOL suite for assessment, monitoring and start of CR. Returnhome for up to 24 hours | – | Attend IOL suite for assessment, monitoring and start of CR. Return home, with plan to return at an agreed time | Attend antenatal ward for assessment, monitoring and start of CR. Return home for up to 24 hours. Then progress to ARM, return home to await ARM appointment or receive prostaglandin and stay in hospital | Attend antenatal ward for assessment, monitoring and start of CR. Return home for up to 24 hours. If using Cook balloon, ARM must be performed within 2 hours of removal |
Postnatal survey: descriptive statistics
There were 320 responses, of which 309 were eligible. Nine respondents had not had IOL and two stated that their IOL was prior to the start of the study.
Study sites (Q8 what hospital did you give birth in?)
Of the responses, 50% came from women who had an IOL at one of the five qCHOICE sites, rising to 61% at the seven sites focused on for recruitment; 34 responses (11%) came from women who had had an IOL at NHS trusts and boards that were not participating in the CHOICE study.
| NHS trust/board | Frequency (N = 309) n (%) |
|---|---|
| Gateshead Health NHS Foundation Trust | 26 (8.4) |
| Epsom and St Helier NHS Trust | 23 (7.4) |
| Birmingham Women’s and Children’s NHS Foundation Trust | 17 (5.5) |
| NHS Lanarkshire | 38 (12.3) |
| NHS Tayside | 55 (17.8) |
| Worcestershire Acute Hospitals NHS Trust | 30 (9.7) |
| NHS Grampian | 7 (2.3) |
| Mid Yorkshire Teaching NHS Trust | 8 (2.6) |
| Ashford and St Peter’s Hospitals NHS Foundation Trust | 8 (2.6) |
| NHS Fife | 11 (3.6) |
| Lancashire Teaching Hospitals NHS Foundation Trust | 7 (2.3) |
| South Warwickshire University NHS Foundation Trust | 10 (3.2) |
| North Cumbria Integrated Care NHS Foundation Trust | 6 (1.9) |
| Royal Wolverhampton NHS Trust | 11 (3.6) |
| NHS Greater Glasgow and Clyde | 10 (3.2) |
| Other | 42 (13.5) |
| NHS trust/board | Frequency (N = 36) n (%) |
|---|---|
| Gateshead Healthy NHS Foundation Trust | 3 (8.3) |
| NHS Lanarkshire | 2 (5.6) |
| NHS Tayside | 22 (61.1) |
| Ashford and St Peter’s Hospitals NHS Foundation Trust | 3 (8.3) |
| Other | 6 (16.6) |
Summary statistics
| Response | All (N = 309) n (%) | Home cervical ripening (N = 36) n (%) | Hospital cervical ripening (N = 230) n (%) |
|---|---|---|---|
| Yes | 206 (67) | 27 (75) | 157 (68) |
| No | 103 (33) | 9 (25) | 73 (32) |
| Cervical ripening | Minimum (years) | Maximum (years) | Median (years) |
|---|---|---|---|
| All (N = 309) | 19 | 52 | 31 |
| Home (N = 36) | 19 | 40 | 29 |
| Hospital (N = 230) | 19 | 52 | 31 |
| Cervical ripening | Minimum (weeks) | Maximum (weeks) | Median (weeks) |
|---|---|---|---|
| All (N = 309) | 37 | 42 | 39 |
| Home (N = 36) | 38 | 42 | 40 |
| Hospital (N = 230) | 37 | 42 | 39 |
FIGURE 9.
Gestation at time of IOL in survey respondents. SD, standard deviation.
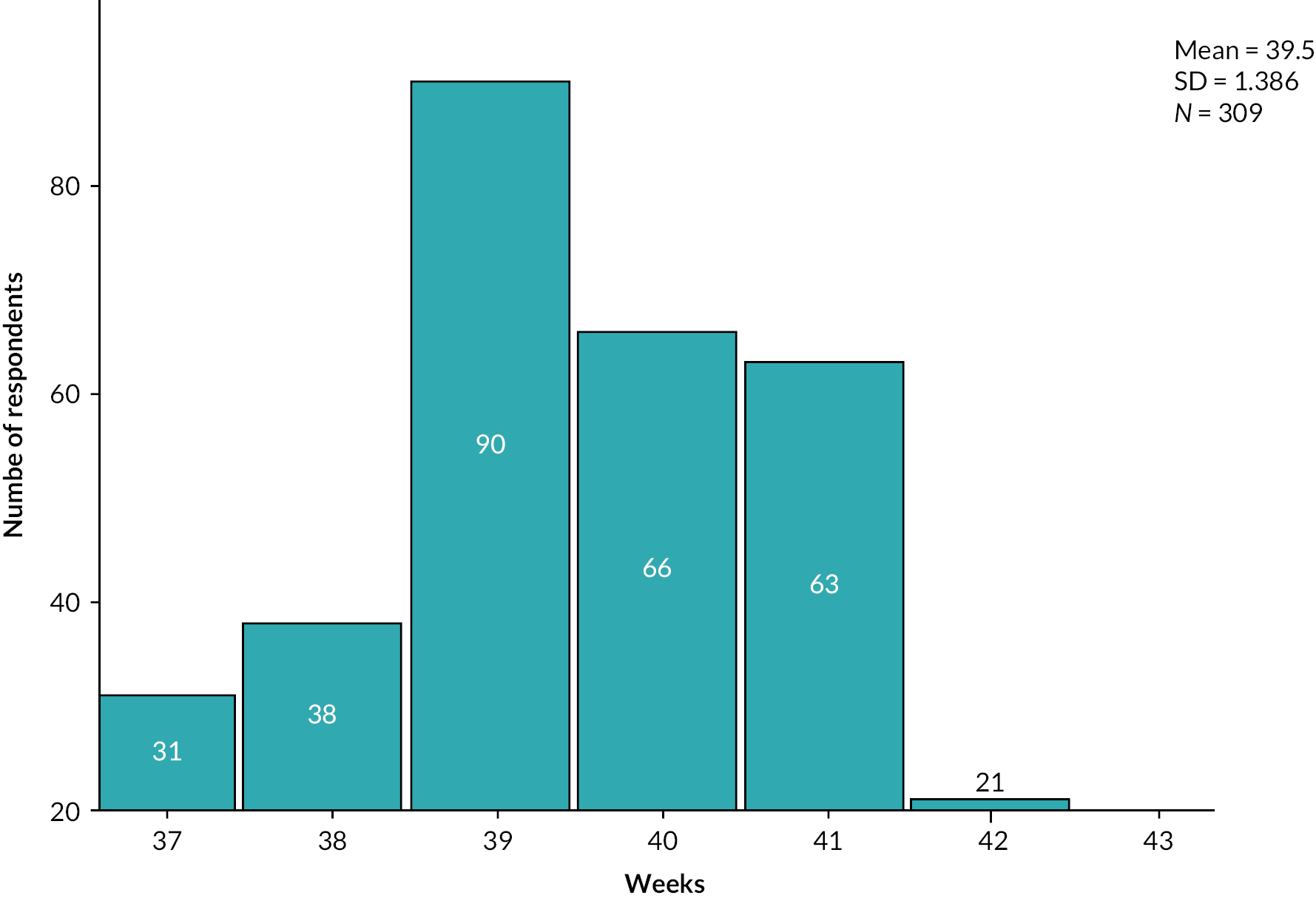
| Ethnicity | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Totals (N) | 307 (2 missing) | 36 | 228 (2 missing) |
| White | 291 (95) | 35 (97) | 215 (94) |
| Asian/Asian British | 8 (3) | 1 (3) | 6 (3) |
| Black | 4 (1) | 0 | 4 (2) |
| Mixed/multiple ethnicity | 4 (1) | 0 | 3 (1) |
| IMD quintile:1 = most deprived, 5 = least deprived | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Hospital n (%) | Home n (%) | |
| 1 | 61 (20) | 51 (22) | 4 (11) |
| 2 | 57 (19) | 41 (18) | 11 (30) |
| 3 | 60 (19) | 44 (19) | 8 (22) |
| 4 | 73 (24) | 51 (22) | 7 (19) |
| 5 | 55 (18) | 40 (18) | 6 (17) |
| Total responses | 306, 3 missing | 227, 3 missing | 36, 0 missing |
Other sample characteristics
| Cervical ripening | Minimum (g) | Maximum (g) | Median (g) |
|---|---|---|---|
| All (N = 297, 12 missing) | 1790 | 6600 | 3500 |
| Home (N = 35, 1 missing) | 2771 | 4900 | 3640 |
| Hospital (N = 221, 9 missing) | 1790 | 6600 | 3440 |
| Reason | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Medical reason (e.g. high blood pressure) | 146 (47) | 13 (36) | 117 (51) |
| Length of pregnancy | 70 (23) | 15 (42) | 44 (20) |
| Size of baby: large or small | 37 (12) | 4 (11) | 30 (13) |
| Spontaneous rupture of membranes | 20 (7) | 0 | 12 (5) |
| Reduced fetal movements | 19 (6) | 1 (3) | 14 (6) |
| Other | 14 (5) | 3 (8) | 12 (5) |
| Total responses (N) | 309 | 36 | 230 |
| Place | All n (%) | Home cervical ripening n (%) | Hospital cervical ripening n (%) |
|---|---|---|---|
| Hospital delivery suite | 160 (52) | 13 (36) | 132 (57) |
| Hospital-based MWU/birth centre | 124 (40) | 18 (50) | 86 (37) |
| Freestanding MWU/birth centre | 14 (4) | 3 (8) | 6 (3) |
| At home | 5 (2) | 0 | 4 (2) |
| Hadn’t decided yet | 6 (2) | 2 (6) | 2 (1) |
| Total responses (N) | 309 | 36 | 230 |
| Response | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Yes | 148 (48) | 19 (53) | 102 (44) |
| No | 153 (59) | 16 (44) | 121 (53) |
| I’m not sure | 8 (3) | 1 (3) | 7 (3) |
| Total responses (N) | 309 | 36 | 230 |
Subsequent question If yes, how? Simple content analysis on free-text responses given in content and thematic analysis document.
| Response | Cervical ripening | ||
|---|---|---|---|
| All N (%) | Home n (%) | Hospital n (%) | |
| Yes, I felt it was fully my decision | 122 (39) | 14 (39) | 87 (38) |
| Yes, but I felt there was no other option | 117 (38) | 11 (31) | 92 (40) |
| Not really, as I didn’t have enough information | 10 (3) | 1 (3) | 8 (3) |
| No, I didn’t feel I was given a choice | 60 (19) | 10 (28) | 43 (19) |
| Total responses (N) | 309 | 36 | 230 |
| Response | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Yes, I felt I fully understood | 205 (66) | 21 (58) | 149 (65) |
| Partly | 70 (23) | 8 (22) | 57 (25) |
| Not really | 19 (6) | 5 (14) | 12 (5) |
| I’m not sure | 2 (0.6) | 0 | 2 (1) |
| No | 13 (4) | 2 (6) | 10 (4) |
| Total responses (N) | 309 | 36 | 230 |
| Response | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Yes, I felt I fully understood | 155 (50) | 15 (42) | 113 (49) |
| Partly | 90 (29) | 10 (28) | 66 (29) |
| Not really | 38 (12) | 8 (22) | 29 (13) |
| I’m not sure | 0 | 0 | 0 |
| No | 26 (8) | 3 (8) | 22 (10) |
| Total responses (N) | 309 | 36 | 230 |
| Method of induction | Cervical ripening | ||
|---|---|---|---|
| All n (%), N = 309 | Home n (%), N = 36 | Hospital n (%), N = 230 | |
| Gel pessaries | 202 (65) | 7 (19) | 195 (85) |
| Catheter | 43 (14) | 24 (67) | 19 (8) |
| Non-cervical ripening methodsa | 38 (12) | 0 | 0 |
| Pessary and catheter | 12 (4) | 3 (8) | 9 (4) |
| Dilapan | 9 (3) | 2 (6) | 7 (3) |
| Don’t know | 5 (2) | 0 | 0 |
| Total responses (N) | 309 | 36 | 230 |
| Response (all cervical ripening; N = 264, 2 missing)a | Strongly agree/agree n (%) | Unsure n (%) | Disagree/strongly disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 141 (53) | 9 (3) | 114 (43) |
| I was about able to cope with the discomfort | 216 (82) | 6 (2) | 42 (16) |
| I felt tense during the insertion | 116 (44) | 24 (9) | 124 (47) |
| I felt anxious the induction wouldn’t work | 149 (57) | 34 (13) | 81 (31) |
| Response (home cervical ripening; N = 36)a | Strongly agree/agree n (%) | Unsure n (%) | Disagree/strongly disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 30 (83) | 1 (3) | 5 (14) |
| I was about able to cope with the discomfort | 28 (78) | 1 (3) | 7 (19) |
| I felt tense during the insertion | 22 (61) | 4 (11) | 10 (28) |
| I felt anxious the induction wouldn’t work | 22 (61) | 5 (14) | 9 (25) |
| Response (hospital cervical ripening; N = 228, 2 missing)a | Strongly agree/agree n (%) | Unsure n (%) | Disagree/strongly disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 112 (49) | 8 (3) | 109 (48) |
| I was about able to cope with the discomfort | 188 (82) | 5 (2) | 35 (15) |
| I felt tense during the insertion | 94 (41) | 20 (9) | 114 (50) |
| I felt anxious the induction wouldn’t work | 127 (56) | 29 (13) | 26 (11) |
| Responsea | Cervical ripening | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Yes | 239 (91) | 29 (81) | 210 (91) |
| No | 25 (9) | 7 (19) | 18 (8) |
| Total responses (N) | 264, 2 missing | 36 | 228, 2 missing |
| Cervical ripeninga | Minimum (minutes) | Maximum (minutes) | Median (minutes) |
|---|---|---|---|
| All (N = 237, 29 missing) | 1 | 4230 | 30 |
| Home (N = 29, 7 missing) | 1 | 4230 | 30 |
| Hospital (N = 208, 22 missing) | 7 | 2880 | 30 |
| Responsea | Cervical ripeningb | ||
|---|---|---|---|
| All n (%) | Home n (%) | Hospital n (%) | |
| Yes | 39 (15) | 32 (89) | 7 (3) |
| No | 227 (85) | 4 (11) | 223 (97) |
| Total responses (N) | 266 | 36 | 230 |
| Response | Respondents (all; N = 309, 0 missing) n (%) |
|---|---|
| Yes | 41 (13) |
| No | 21 (7) |
| Wasn’t offered; I added/coded this | 247 |
| Response | Respondents (all who had cervical ripening; N = 266, 0 missing)a n (%) |
|---|---|
| Yes | 36 (14) |
| No | 17 (6) |
| Wasn’t offered; I added/coded this | 213 (80) |
| Response | All cervical ripening (N = 266, 0 missing)a | |||
|---|---|---|---|---|
| Went home | Didn’t go home | Not offered | Total | |
| Yes | 32 | 7 | 0 | 39 |
| No | 4 | 10 | 213 | 227 |
| Total | 36 | 266 | ||
| Response | Respondents (N = 7)a |
|---|---|
| I didn’t want to go home | 6 |
| I initially wanted to go home but changed my mind | 0 |
| I was recommended to stay after monitoring | 1 |
Those who remained in hospital and had cervical ripening (N = 230)
| Respondents (N = 227, 3 missing) | Minimum (hours) | Maximum (hours/days) | Median (hours) |
|---|---|---|---|
| In hospital | 0 | 260/10 | 22 |
FIGURE 10.
Time spent in hospital before moving to labour ward (hours). SD, standard deviation.
FIGURE 11.
Time spent in hospital before moving to labour ward (days). SD, standard deviation.
| Response | Respondents (N = 228, 2 missing) n (%) |
|---|---|
| No | 59 (26) |
| Birth partner | 159 (70) |
| Othera | 10 (4) |
| Response | Respondents (N = 181, 49 missing)a n (%) |
|---|---|
| Yes | 55 (30) |
| No | 126 (70) |
| Response | Respondents (N = 55) n (%) |
|---|---|
| Paid | 4 (7) |
| Unpaid | 48 (87) |
| Paid and unpaid | 3 (5) |
| Response | Minimum (hours) | Maximum (hours) | Median (hours) |
|---|---|---|---|
| Paid (N = 7) | 3 | 22 | 10 |
| Unpaid (N = 50) | 1 | 361 | 38 |
| Response | Respondents (N = 229, 1 missing) n (%) |
|---|---|
| Car | 212 (93%) |
| Taxi | 10 (4%) |
| Walked | 5 (2%) |
| Ambulance | 1 |
| Car and taxi | 1 |
| Public transport | 0 |
| Response (N = 223) | Minimum (£) | Maximum (£) | Median (£) |
|---|---|---|---|
| Cost | 0 | 300 | 10 |
| Response | Respondents (N = 229, 1 missing) n (%) |
|---|---|
| Not in paid employment | 13 (6) |
| Full-time employed | 180 (79) |
| Part-time employed | 6 (3) |
| Self-employed | 28 (12) |
| Other | 2 (1) |
| Response (N = 227, 3 missing) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 143 (63) | 7 (3) | 77 (34) |
| I was able to cope with the discomfort | 155 (68) | 13 (6) | 59 (26) |
| I felt anxious about being in hospital | 115 (51) | 16 (7) | 96 (42) |
| I was able to relax on the antenatal ward | 101 (44) | 23 (10) | 103 (45) |
| I was able to rest on the antenatal ward | 103 (45) | 15 (7) | 109 (48) |
| I had good family support in hospital | 151 (66) | 16 (7) | 60 (26) |
| I had easy access to information from the staff | 127 (56) | 24 (11) | 76 (33) |
| I was worried the induction might not be safe | 47 (21) | 29 (13) | 151 (66) |
| I would have preferred to go home | 97 (43) | 27 (12) | 103 (45) |
| I felt embarrassed by the catheter/gel | 21 (9) | 10 (4) | 196 (86) |
| Response (N = 227, 3 missing) | Agree n (%) | Unsure and disagree n (%) |
|---|---|---|
| I felt a lot of discomfort | 143 (63) | 84 (37) |
| I was able to cope with the discomfort | 155 (68) | 72 (32) |
| I felt anxious about being in hospital | 115 (51) | 112 (49) |
| I was able to relax on the antenatal ward | 101 (44) | 126 (56) |
| I was able to rest on the antenatal ward | 103 (45) | 124 (55) |
| I had good family support in hospital | 151 (67) | 76 (33) |
| I had easy access to information from the staff | 127 (56) | 100 (44) |
| I was worried the induction might not be safe | 47 (21) | 180 (79) |
| I would have preferred to go home | 97 (43) | 130 (57) |
| I felt embarrassed by the catheter/gel | 21 (9) | 206 (91) |
| Response (N = 230, 0 missing) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt anxious about being induced | 168 (73) | 15 (6) | 47 (20) |
| I felt in control | 62 (27) | 50 (22) | 118 (51) |
| I understood what was happening | 174 (76) | 25 (11) | 31 (13) |
| I felt relaxed | 62 (27) | 38 (16) | 130 (56) |
| Everything made sense | 137 (60) | 32 (14) | 61 (26) |
| I was given clear information | 151 (66) | 28 (12) | 51 (22) |
| I felt comfortable with my choice about my care | 145 (63) | 31 (13) | 54 (23) |
| I had access to information about the types of induction available | 119 (52) | 27 (12) | 84 (36) |
| I had easy access to information about what to do | 122 (53) | 37 (16) | 71 (31) |
| I found the induction process uncomfortable | 144 (63) | 20 (9) | 66 (29) |
| I was worried about when my labour would begin | 176 (76) | 13 (6) | 41 (18) |
| I would choose staying in hospital again | 126 (55) | 35 (15) | 69 (30) |
| I would recommend staying in hospital during induction to other women | 125 (54) | 36 (16) | 69 (30) |
| Response (N = 230, 0 missing) | Agree n (%) | Unsure and disagree n (%) |
|---|---|---|
| I felt anxious about being induced | 168 (73) | 62 (27) |
| I felt in control | 62 (27) | 168 (73) |
| I understood what was happening | 174 (76) | 56 (24) |
| I felt relaxed | 62 (27) | 168 (73) |
| Everything made sense | 137 (60) | 93 (40) |
| I was given clear information | 151 (66) | 79 (34) |
| I felt comfortable with my choice about my care | 145 (63) | 85 (37) |
| I had access to information about the types of induction available | 119 (52) | 111 (48) |
| I had easy access to information about what to do | 122 (53) | 108 (47) |
| I found the induction process uncomfortable | 144 (63) | 86 (37) |
| I was worried about when my labour would begin | 176 (76) | 54 (23) |
| I would choose staying in hospital again | 126 (55) | 104 (45) |
| I would recommend staying in hospital during induction to other women | 125 (54) | 105 (46) |
Those who had home induction of labour and cervical ripening (N = 36)
Of 309 women, 46 (15%) answered yes when asked if they were offered the opportunity to go home for cervical ripening; 41 (13%) stated that they did return home and 36 (12%) stated that they had returned home and also had some form of cervical ripening.
| Response (N = 35, 1 missing) | Minimum (hours) | Maximum (hours) | Median (hours) |
|---|---|---|---|
| Time at home | 3 | 168 | 24 |
FIGURE 12.
Time spent at home during cervical ripening. SD, standard deviation.
| Response (N = 36, 0 missing) | Respondents n (%) |
|---|---|
| Yes | 23 (64) |
| No | 13 (36) |
| Response (N = 36, 0 missing) | Minimum (n) | Maximum (n) | Median (n) |
|---|---|---|---|
| Phone calls | 0 | 5 | 1 |
| Response (N = 36, 0 missing) | Respondents n (%) |
|---|---|
| Yes | 17 (47) |
| No | 23 (56) |
| Response (N = 36, 0 missing) | Minimum (n) | Maximum (n) | Median (n) |
|---|---|---|---|
| Returns to hospital | 0 | 3 | 0 |
| Response (N = 36, 0 missing) | Respondents n (%) |
|---|---|
| Yes | 35 (97) |
| No | 1 (3) |
| Response (N = 36, 6 missing) | Respondents (N = 30), n |
|---|---|
| Yes | 4 |
| No | 26 |
| Response | Minimum (hours) | Maximum (hours) | Median (hours) |
|---|---|---|---|
| Paid (N = 1) | 7 | 7 | 7 |
| Unpaid (N = 3) | 12 | 48 | 24 |
| Response (N = 36, 0 missing) | Respondents n (%) |
|---|---|
| Car | 33 (92) |
| Taxi | 2 (6) |
| Ambulance | 1 (3) |
| Response (N = 35, 1 missing) | Minimum (£) | Maximum (£) | Median (£) |
|---|---|---|---|
| Cost of travel | 0 | 60 | 15 |
| Response (N = 36, 0 missing) | Respondents n (%) |
|---|---|
| Not in paid employment | 1 (3) |
| Full time employed | 31 (86) |
| Part time employed | 1 (3) |
| Self-employed | 4 (8) |
| Response (N = 36, 0 missing) | Agree n (%) | Unsure and disagree n (%) |
|---|---|---|
| I felt a lot of discomfort | 28 (78) | 8 (22) |
| I was able to cope with the discomfort | 29 (81) | 7 (19) |
| I felt anxious about going home | 14 (39) | 22 (61) |
| While at home I felt anxious about being at home not hospital | 13 (36) | 23 (64) |
| I was able to relax at home | 20 (56) | 16 (44) |
| I was able to rest at home | 24 (67) | 12 (33) |
| I had good family support at home | 35 (97) | 1 (3) |
| I had easy access to information from the staff | 23 (64) | 13 (36) |
| I was worried it might not be safe to be at home | 11 (31) | 25 (69) |
| I would have preferred to stay at the hospital | 12 (33) | 24 (67) |
| I felt embarrassed by the catheter/gel | 3 (8) | 33 (92) |
| Response (N = 36, 0 missing) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt anxious about being induced | 31 (86) | 1 (3) | 4 (11) |
| I felt in control | 9 (25) | 4 (11) | 23 (64) |
| I understood what was happening | 24 (67) | 2 (5) | 10 (28) |
| I felt relaxed | 8 (22) | 9 (25) | 19 (53) |
| Everything made sense | 18 (50) | 3 (8) | 15 (42) |
| I was given clear information | 17 (47) | 4 (11) | 15 (42) |
| I felt comfortable with my choice about my care | 20 (56) | 7 (19) | 9 (25) |
| I had access to information about the types of induction available | 20 (56) | 2 (6) | 14 (39) |
| I had easy access to information about what to do | 19 (53) | 6 (17) | 11 (31) |
| I found the induction process uncomfortable | 32 (89) | 1 (3) | 3 (8) |
| I was worried about when my labour would begin | 26 (72) | 5 (14) | 5 (14) |
| I would choose going home again | 23 (64) | 6 (17) | 7 (19) |
| I would recommend going home during induction to other women | 22 (61) | 8 (22) | 6 (17) |
| Response (N = 36) (0 missing) | Agree n (%) | Unsure and disagree n (%) |
|---|---|---|
| I felt anxious about being induced | 31 (86) | 5 (14) |
| I felt in control | 9 (25) | 27 (75) |
| I understood what was happening | 24 (67) | 12 (33) |
| I felt relaxed | 8 (22) | 28 (78) |
| Everything made sense | 18 (50) | 18 (50) |
| I was given clear information | 17 (47) | 19 (33) |
| I felt comfortable with my choice about my care | 20 (56) | 16 (44) |
| I had access to information about the types of induction available | 20 (56) | 16 (44) |
| I had easy access to information about what to do | 19 (53) | 17 (47) |
| I found the induction process uncomfortable | 32 (89) | 4 (11) |
| I was worried about when my labour would begin | 26 (72) | 10 (28) |
| I would choose going home again | 23 (64) | 13 (36) |
| I would recommend going home during induction to other women | 22 (61) | 14 (39) |
All respondents (N = 309)
| Response (all; N = 309) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 250 (81) | 11 (4) | 48 (15) |
| I was about able to cope with the discomfort | 174 (56) | 24 (8) | 111 (36) |
| I felt tense and anxious | 185 (60) | 34 (11) | 90 (29) |
| I felt anxious the induction wouldn’t work | 126 (41) | 37 (12) | 146 (47) |
| I felt that my labour had started | 213 (69) | 37 (12) | 59 (19) |
| Response (home cervical ripening; N = 36) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 32 (89) | 0 | 4 (11) |
| I was about able to cope with the discomfort | 21 (58) | 4 (11) | 11 (30) |
| I felt tense and anxious | 26 (72) | 1 (3) | 9 (25) |
| I felt anxious the induction wouldn’t work | 20 (51) | 4 (11) | 12 (33) |
| I felt that my labour had started | 19 (53) | 5 (14) | 12 (33) |
| Response (hospital cervical ripening; N = 230) | Agree n (%) | Unsure n (%) | Disagree n (%) |
|---|---|---|---|
| I felt a lot of discomfort | 188 (82) | 7 (3) | 35 (15) |
| I was about able to cope with the discomfort | 124 (54) | 16 (7) | 90 (39) |
| I felt tense and anxious | 134 (58) | 28 (12) | 68 (30) |
| I felt anxious the induction wouldn’t work | 95 (41) | 26 (11) | 109 (47) |
| I felt that my labour had started | 162 (70) | 26 (11) | 42 (18) |
Q42 Labour Agentry Scale: 10 questions
| Respondents | Minimum score (n) | Maximum score (n) | Median score (n) |
|---|---|---|---|
| All (N = 309) | 10 | 60 | 40 |
| Home cervical ripening (N = 36) | 13 | 59 | 39 |
| Hospital cervical ripening (N = 230) | 10 | 60 | 39 |
| Response (all; N = 309) | Agree (3 categories) n (%) | Neutral n (%) | Disagree (2 categories) n (%) |
|---|---|---|---|
| I felt tense | 188 (61) | 33 (11) | 88 (28) |
| I felt important | 215 (69) | 30 (10) | 64 (21) |
| I felt confident | 149 (48) | 65 (21) | 95 (31) |
| I felt in control | 140 (45) | 44 (14) | 125 (40) |
| I felt fearful | 152 (49) | 52 (17) | 105 (34) |
| I felt relaxed | 95 (31) | 68 (22) | 146 (47) |
| I felt good about my behaviour | 243 (79) | 27 (9) | 39 (13) |
| I felt helpless (powerless) | 122 (39) | 39 (13) | 148 (48) |
| I felt like a failure | 96 (31) | 21 (7) | 192 (62) |
| I felt I was with people who care about me | 249 (81) | 22 (7) | 38 (12) |
| Response | Agree n (%) | ||
|---|---|---|---|
| All respondents (N = 309) | In-hospital cervical ripening (N = 230) | Home cervical ripening (N = 36) | |
| I felt tense | 188 (61) | 143 (62) | 23 (64) |
| I felt important | 215 (69) | 149 (65) | 28 (78) |
| I felt confident | 149 (48) | 102 (44) | 19 (53) |
| I felt in control | 140 (45) | 98 (43) | 18 (50) |
| I felt fearful | 152 (49) | 113 (49) | 19 (53) |
| I felt relaxed | 95 (31) | 68 (30) | 10 (28) |
| I felt good about my behaviour | 243 (79) | 183 (80) | 27 (75) |
| I felt helpless (powerless) | 122 (39) | 92 (40) | 15 (42) |
| I felt like a failure | 96 (31) | 71 (31) | 12 (33) |
| I felt I was with people who care about me | 249 (81) | 179 (78) | 31 (86) |
| Response (N = 309, 0 missing) | Minimum (n) | Maximum (n) | Median (n) |
|---|---|---|---|
| Nights | 1 | 11 | 2 |
| Response (N = 308, 1 missing) | Respondents n (%) |
|---|---|
| Yes | 27 (9) |
| No | 281 (91) |
| Response (N = 26, 1 missing) | Minimum (n) | Maximum (n) | Median (n) |
|---|---|---|---|
| Nights | 1 | 10 | 1.5 |
| Response (N = 293, 16 missing) | Minimum (n) | Maximuma (n) | Median (n) |
|---|---|---|---|
| Weeks | 1 | 20 | 4 |
Q46 Short Warwick–Edinburgh Mental Wellbeing Scales score (transformed score)
Short WEMWBS: population average is 23.2 for women (23.7 for men). 52
| Respondents | Minimum score | Maximum score | Median score |
|---|---|---|---|
| All respondents (N = 309, 0 missing) | 7 | 35 | 24.11 |
| Cervical ripening only (N = 266) | 7 | 35 | 24.11 |
| Hospital cervical ripening (N = 230) | 7 | 35 | 24.11 |
| Home cervical ripening (N = 36) | 14.75 | 35 | 24.11 |
FIGURE 13.
Short WEMWBS score distribution across respondents. SD, standard deviation.
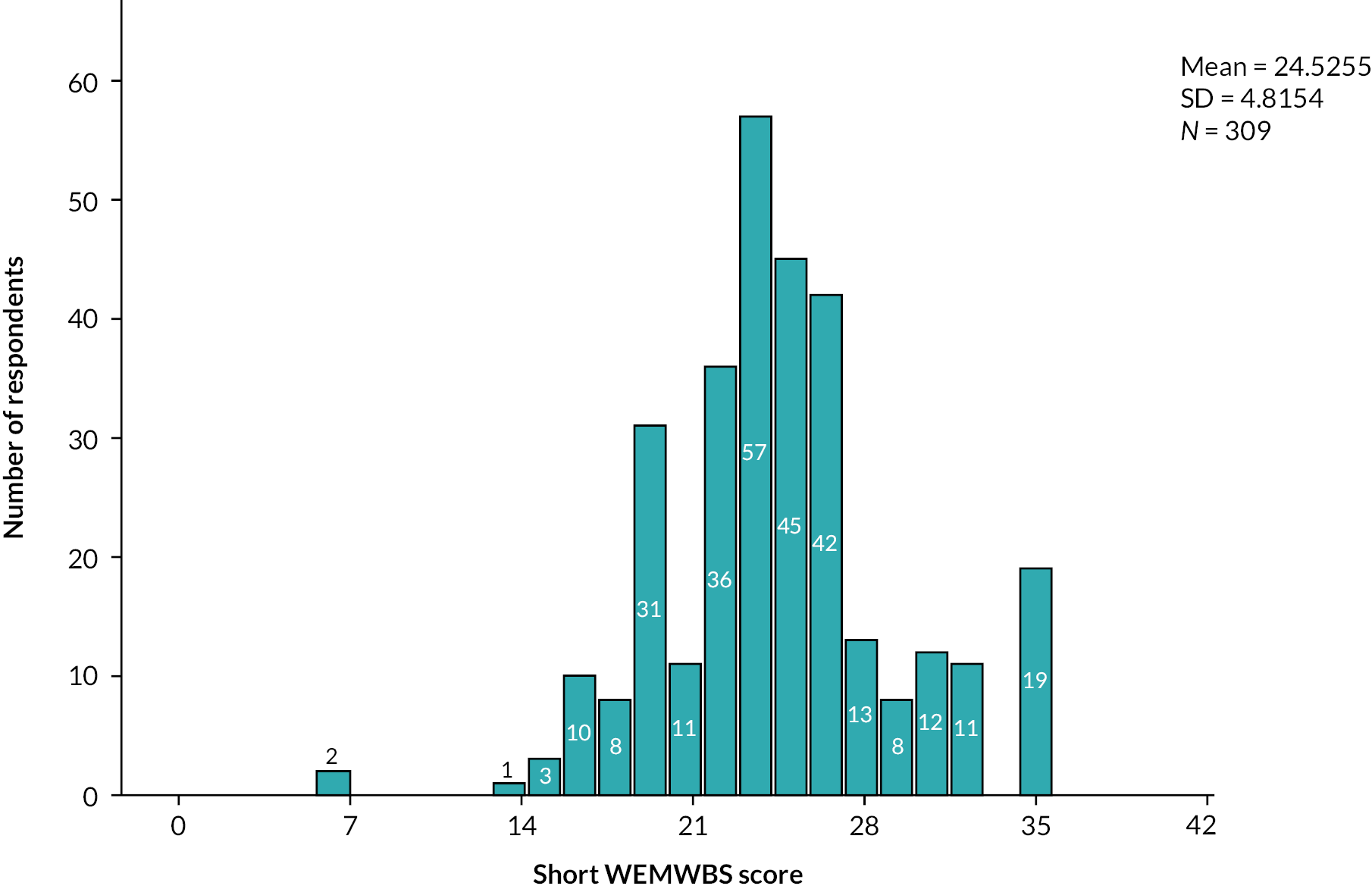
| Response | All (N = 309) n (%) | Home cervical ripening (N = 36) n (%) | Hospital cervical ripening (N = 230) n (%) |
|---|---|---|---|
| Yes | 96 (31) | 10 (28) | 73 (32) |
| No | 213 (69) | 26 (72) | 157 (68) |
If yes, please provide details.
Simple content analysis on free-text responses in content and thematic analysis document.
All respondents
| Response | All (N = 308, 1 missing) n (%) | Home cervical ripening (N = 36) n (%) | Hospital cervical ripening (N = 229, 1 missing) n (%) |
|---|---|---|---|
| Yes | 10 (3) | 2 (6) | 6 (3) |
| No | 61 (20) | 22 (61) | 33 (14) |
| Not applicable | 237 (77) | 12 (33) | 190 (83) |
If yes, please provide details.
Simple content analysis in content and thematic analysis document.
Q49 Please add any other comments about how your experience of induction or feelings about it were affected, if at all, by the COVID-19 pandemic.
Free-text responses; content and thematic analysis being completed separately.
Q50 Thinking back about your induction and birth experiences, is there anything else you would like to tell us?
Free-text responses; content and thematic analysis being completed separately.
Appendix 2 qCHOICE detailed qualitative findings
Survey free text content analysis.
Q14: Did induction of labour lead to any change in your birthplace plans?
Yes: 148 (48%) No: 153 (49%) I’m not sure: 8 (3%).
If yes, please explain the reason.
| Changes stated | Respondents who stated this (n)a |
|---|---|
| Unable to use water during labour or birth | 46 |
| Unable to attend birth centre/midwifery led unit | 43 |
| Unwanted interventions: monitoring, epidural, syntocinon drip, caesarean section etc. | 29 |
| Hospital labour ward only option | 25 |
| Birth in different town | 8 |
| Had planned for early labour at home | 7 |
| Unable to have planned homebirth | 6 |
| Movement restricted during labour | 4 |
Q47: There have been many changes in maternity services due to COVID-19. Did your feelings about induction of labour change at all due to the COVID-19 pandemic?
Yes: 96 (31%) No: 213 (69%)
Q47a: If yes, please provide details.
| Features described | Respondents who stated those features (n)a |
|---|---|
| Birth partner restrictions | 50 |
| Visiting restrictions | 17 |
| Risk of COVID infection while in hospital | 15 |
| Fear of being alone or unsupported | 12 |
| Impact on staffing leading to delays or reduced care | 9 |
| PPE/mask wearing | 4 |
| Change to place of birth | 2 |
| Food options restricted | 2 |
| Movement restricted | 1 |
| No choice on method (balloon only) | 1 |
| Increased IOL rate | 1 |
Q48 If you were offered at home cervical ripening, did your feelings about the choice to go home/stay in hospital after the catheter or gel insertion change at all due to COVID-19 pandemic?
| Respondents | Feelings changed, n (%) | ||
|---|---|---|---|
| Yes | No | Not applicable | |
| All (N = 309) | 10 (3) | 61 (20) | 237 (77) |
| Returned home and had cervical ripening only (N = 36) | 2 (6) | 22 (61) | 12 (33) |
Q48a: If yes, please provide details.
| Changes described | Respondents (n)a |
|---|---|
| Preferred home | 2 |
| Would be better supported at home if birth partner restrictions in place | 2 |
| Would have had better care at home as unsupported in hospital | 2 |
| Wish had stayed in hospital as painful and unsupported at home | 1 |
| Prefer hospital | 1 |
Q49: Please add any other comments about how your experience of induction or feelings about it were affected, if at all, by the COVID-19 pandemic.
Many answers included comments not related to COVID-19. Those are included in wider thematic analysis but not in the simple content analysis of this question.
| Response | Respondents (n) |
|---|---|
| Partner excluded or anxious about that possibility | 44 |
| Staff shortages leading to delays or negative impact on care | 20 |
| Family separation and restricted visiting | 15 |
| Positive experience (without other context) | 11 |
| Changed place of labour and/or birth | 7 |
| Restricted movement while in hospital | 4 |
| Worried about catching COVID-19 in hospital | 3 |
| Worried about changes to restrictions | 3 |
| COVID-19 testing | 1 |
| Personal protective equipment and mask wearing | 1 |
| Effect on communication and interaction with staff | 1 |
Thematic analysis
Content and thematic analysis was performed on free-text responses left throughout the survey. Most of this text was left in response to Q50: Thinking about your induction and birth experiences, is there anything else you would like to tell us?
Overall experience: positive, negative, mixed
The text was read and, where possible, categorised according to whether the women’s description of their experience was positive, negative or mixed overall. It was possible to categorise 190 survey responses in this way.
| Categorisation of experience overall | Women (n) |
|---|---|
| Positive | 35 |
| Negative | 133 |
| Mixed | 27 |
The analysis also identified themes within the women’s descriptions that were linked to their negative or positive experiences. Some women’s responses covered more than one of these themes.
| Factors mentioned in relation to a negative experience of IOL | Women who mentioned this (n) |
|---|---|
| Partner being excluded for part or all of their induction experience, and/or worrying that this might happen | 60 |
| Lack of support during cervical ripening (pre admission to labour ward) | 44 |
| Delays at all stages of the process but most notably waiting for transfer to labour ward for ARM or when in labour | 41 |
| IOL associated with increased intervention and/or poor outcomes | 36 |
| Poor, or lack of, communication | 35 |
| Decision-making about IOL, not being aware of the risks until afterwards, pressure to have an IOL or unwanted IOL | 29 |
| Concerns about poor clinical care and the safety of themselves and their baby | 28 |
| Staffing levels | 28 |
| Family separation, especially other young children | 20 |
| Physical environment of IOL/cervical ripening area (lack of privacy, sleep, food) | 13 |
| Long hospital stay | 10 |
| Factors mentioned in relation to a positive experience of IOL | Women who mentioned this (n) |
|---|---|
| Staff | 39 |
| Good clinical care and feeling safe | 15 |
| IOL as a generally positive experience | 8 |
| Quick IOL | 8 |
| Information and communication | 6 |
Of the women who had a positive or a mixed experience, 14 described the way that staff made them feel that contributed to this experience. The words they used to describe how they were made to feel were: safe, relaxed, comfortable, at ease, supported, in control, powerful, important.
Thematic analysis: negative experiences
Anxiety about partners being excluded from some or all of the time that women were in hospital, particularly during the period of their induction before they moved to the labour ward, was a significant theme throughout the survey. This anxiety was often exacerbated by poor staffing levels and fear that they may spend the early part of their induction completely alone. Several women described feeling very worried about this during the last weeks of pregnancy. An understanding that IOL would mean increased length of time spent in hospital increased that anxiety.
Husband could only visit at restricted times and had to be booked. Older child couldn’t visit at all. This made going to hospital much harder as I was alone most of the time.
89309337
It was a lonely experience, my husband was not allowed to come in until I was on active labor.
89426953
Being in hospital for days with only 2 hours’ visiting per day was extremely isolating and scary. Staff were ridiculously busy so care was not consistent.
89824481
I was in hospital being induced for 5 days and most of the time I was left alone with no check-up from any staff; was told at night time that they wouldn’t be able to do anything with me due to staff shortages, etc.
92395163
Many of the women believed or had been told by staff that restrictions on partner’s attendance was in place because of the pandemic. However, at many maternity units it is usual practice for partners only able to be present on antenatal and IOL suites during specified visiting hours.
The sense of being ‘allowed’ was present throughout women’s accounts.
My waters broke at around 1.30am, but I wasn’t allowed to call my husband until 4.30am, by which point I was almost 10 cm dilated.
87348075
I was lucky that my labour was during visiting hours on the induction ward, otherwise my partner would not have been allowed in until after I had started pushing.
88910706
Having an induction could also have impacted on me having my partner there. I was in active labour with very painful contractions for 7 hours before being moved to the labour ward when I was pushing. Luckily this was during the visiting hours of 7am and 7pm, but if it hadn’t been, my partner would not have been allowed in and may have missed the birth.
88910706
The birth experience is important to both parents, and birth partners are a vitally important source of support for most women, but women often described failure to acknowledge and accommodate this fact, and there were some instances of partners being treated poorly.
the induction also meant my husband actually missed our son being born because I progressed quickly in the end.
92222997
He [birth partner] had nowhere suitable to wait as we live 3 hours away from the hospital and this made him anxious as he wasn’t there to support me. Also made me anxious as I knew he was waiting for news and worrying while he was not with me.
87927535
The need to be with their partner during this important and difficult time led some women to change their behaviour.
I was very worried that if induction was prolonged and I had to be admitted my partner would not be allowed to visit/be with me till I was in established labour. It was why I laboured so long at home.
88963822
Women associated IOL and cervical ripening with additional time spent in hospital. As well as separation from their partner, this extra length of stay caused anxiety for some women who worried about exposure to COVID-19 infection, and others described being anxious about being separated from other members of their family, especially their other children.
I felt most upset about not being able to see my older son for the duration of my stay in hospital. With an induction, you never know how long you will be there, this was the biggest concern for me and had the biggest impact on my well-being during my stay.
92222997
Some stated that being able to go home during this time would have made a difference to them.
would have preferred to have been able to go home I have two children prior to the baby and had to spend a total of six nights away from them.
74204525
I was not offered the choice to stay in hospital after my balloon was inserted. This would have made all the difference with a toddler at home.
89706212
Length of stay during an IOL was a feature of many women’s experiences, and anxiety related to this was significantly exacerbated by delays. Women described delays at almost every stage of the IOL process; however, the most significant and impactful delay described by the women was the wait to be transferred from IOL suite to labour ward after cervical ripening had commenced, either to have an ARM or because they were in labour. This often caused anxiety and distress, and some women described threat to their physical well-being too.
My contractions started really quickly after induction and were coming every 2 minutes, but I was left in a room with no additional pain medication. I screamed for more drugs but was told by my midwife she needed to convince the labour ward that I was in labour as they didn’t want to give up a room for me.
77466659
You’re told you’ll be in and out of hospital but you’re not. I’d liken it to actual torture. My actual birth was beautiful but the 5 days I was stuck on a ward where I felt I was just left was the worst 5 days of my life.
84023732
I was really lucky that my husband could be with me for the majority of my very long hospital stay. The staff were pushed to the brink which is why I was in hospital for 11 days before my waters were broken. Pessaries were inserted on days 1 and 2 but with little or no effect. I can not fault the care from all the staff, especially during what turned out to be a quick but challenging birth. There is simply not enough staff available at the moment to run these units as they should be but those that are working are angels.
89021852
Being taken up to labour ward and then sent back as the room was needed by someone else just crushed me.
89666256
The staff were just so busy and clearly understaffed. I would sometimes be waiting hours for something to happen even if I was told it would be soon. I was put on long waiting lists which wasn’t pleasant. I was awoken in the middle of the night to be told I would be moved imminently and was not moved for hours (about 6). I was moved at least twice before I gave birth. Often I would be told the midwife would come at a certain time, but they would be late/only come if I called. I was also forgotten about including the checks after the second Propess® fell out.
89200550
The women often described the IOL process being interrupted by delays, impacting the progress of their labour and birth.
My catheter induction balloon fell out after 13 hours, it was presumed that this meant my cervix was open and I had a sweep the next day. A midwife gave me a sweep and said my cervix was nice and spongey and open. It wasn’t until a week later that I went into hospital to be induced into labour and my cervix was not open so when the midwife tried to break my waters it was excruciating, worse than the labour and she struggled to break them and eventually a doctor had to break them. I have never felt pain like it and was embarrassed at the screaming I was doing and I worried more about labour.
89503513
I feel my long-term health has been affected due to having to wait 3 full days between the pessary and oxytocin drip. My pre-eclampsia became a lot worse and I am still on a significant amount of medication.
90565214
Once the balloon was removed, I just had to phone the labour suite every night to see if there was room to take me in or not and for 2 days there wasn’t.
89668206
Induction took 4 days overall. Started with pessary and spent one night in hospital. Was then given catheter balloon and allowed to go home. Took 2 days to be taken back in due to ward being busy. Required waters broken and oxytocin drip before labour started.
90037129
I feel as if the hospital did not care about my whilst being in there was meant to get my waters broken and it got pushed back that many times I need up with a UTI [urinary tract infection] and hit a C-section in the end.
87646126
The balloon induction worked however due to lack of staff I did not have my waters broken as planned. Instead I was given Prostin® gel to continue ripening but delay labour. The Prostin gave me rapid and intense contractions about a minute apart. I was in agony for hours and afraid I was going to give birth in the toilet on the induction ward due to the pressure. As soon as my waters were broken I delivered very quickly (from 4 cm to 10 in just a few minutes) and with one push, Had my waters gone themselves I am sure I would have delivered in the toilet. Despite labouring for hours I was unable to access morphine or an epidural despite having a much more intense and painful labour than either of my previous non- induced labours. I wish when they decided that it was too busy to break my waters that I had been asked to come back the next day instead of accepting the Prostin gel and enduring the pain and fear that I experienced.
84619720
For some women, delay between decision being made for IOL and it actually happening, and delays during the process sat in opposition to their understanding of being induced because their baby was at risk.
My induction was delayed for a week because there were no midwives. I was told I needed to be induced early for safety reasons and ended up getting a c section on my daughters due date.
89503513
I think someone should have spoken to me and explained that I didn’t need to worry, as when you have been told for 3 months that your baby could be in danger if you reach 39 weeks and then have to go beyond that because they don’t have a bed for you, it’s a very scary time.
86991374
I was told for 2 weeks that I would be induced tomorrow, my blood pressure was so high I had to be admitted for observation I felt the doctors would tell me I needed to be induced asap then midwives would tell me there were no beds. I felt extremely stressed.
89475068
The physical environment and support on IOL suite were often criticised. Women described lack of privacy, lack of sleep, lack of food. Some noted that having a single room would have made a difference to their experience. It was another reason that women felt home IOL may have been preferrable.
I thought it was ridiculous that I was induced and then put on the ward with other ladies who were there just to be monitored over night or for what other reason. I was embarrassed to have to go through that pain with others hearing me during the night who were nowhere near full term. I laboured through the whole night trying to keep myself quiet to respect them but yet having to respect that I myself was going through horrific pain.
90570161
Not able to have my own room to labour in was the biggest downfall for me.
90570161
There is no reason why I couldn’t have been allowed home during the first part of the induction process and to make women labour behind a curtain for hours until there is a bed on delivery is unfair. I was labouring behind a curtain, no privacy, others all around me watching iPads with boyfriends there. It was really hard to focus and stay calm and relax with no privacy of my own, no pain relief and no food as I couldn’t get up to get lunch etc due to the contractions being so close together.
82998991
[W]as in labour for 9 hours. I had been in hospital for 72 hours and had not slept in nearly 3 days.
83683466
I spent 3 days crying in pain unable to eat or sleep in hospital.
89394152
There was an apparent deficit in the support that midwives were able to provide women on the IOL suite, before they moved to labour suite. This manifested in lack of appropriate pain relief, lack of support and concerns over lack of clinical care. These factors were exacerbated by the length of time some women spent in the prelabour ward area and delays in transferring them to labour ward.
I was in hospital being induced for 5 days and most of the time I was left alone with no check-up from any staff, was told at night time that they wouldn’t be able to do anything with me due to staff shortages, etc.
92395163
My contractions started really quickly after induction and were coming every 2 minutes, but I was left in a room with no additional pain medication. I screamed for more drugs but was told by my midwife she needed to convince the labour ward that I was in labour as they didn’t want to give up a room for me.
77466659
I think there was a lack of staff, which led to me being unable to access adequate pain relief as I was kept in the induction ward to labour until the changeover of staff due to no availability of staff in the labour ward.
84619720
because my Bishops score had been 2 at the start I don’t think anybody believed me when I said I wasn’t coping with pain and just kept offering me a shower and paracetamol.
85703546
I was only monitored at the start of the induction process and was not checked or examined until I was in significant distress due to the frequency and strength of my contractions, while the midwifery staff were attentive when I sought them out, they were not proactive in monitoring or assessing the progress of my induction. This resulted in a very rushed trip to delivery suite when I was finally assessed and baby being delivered 20 minutes after arrival on labour ward. I felt that I very much needed to be my own advocate on antenatal ward in order to be assessed.
86980502
The cervical ripening procedure and process was described by some women as extremely painful.
I also don’t feel I was made aware of how uncomfortable and painful repeated pessaries and breaking my waters would be.
83732110
I found the pessary extremely uncomfortable and had some swelling while it was inserted. Staff were attentive and provided lidocaine gel however subsequent examinations were painful.
87480189
Pessary was not inserted correctly so it irritated my insides to the point of making it sore and inflamed and swollen. Had to have gas and air to have it fitted again 2 days later. Made me feel so anxious about giving birth because of how painful the pessary and the checks on dilation were after the incorrect placing. It gave me doubts about labour that I hadn’t had previously.
89649436
The balloon was horrendous. I struggled to pee with it in and I was up all night in agony from it. It was also the source of a very serious infection I developed that so I had 4 days of IV [intravenous] antibiotics three times a day. The other issue was when it was removed I wore maternity pads because of the mucus plug. This made me unaware that my waters broke so my baby also developed an infection and had 3 days of IV antibiotics. I would never ever have this procedure done again.
89668206
screamed in pain as the pessary was inserted and the midwife did not address this, they were cold in approach.
90565214
Pain relief should have been offered for the balloon being fitted as the pain was horrendous.
92959966
The women also described pain not being taken seriously as they were not perceived by staff as being in established labour.
I wasn’t able to move from the bed because of the pain and felt spaced out due to pain for about 5 hours. I was not given any pain relief until late on even though I was begging for it.
87186151
Being induced was not a good experience for me. I was in a lot of pain before visiting hours ended and was pretty sure that labour had started. Despite this my birth partner was not allowed to stay. I was left to labour on my own on the ward with minimal pain relief overnight.
87348075
Poor communication, including not feeling listened to or believed, was an important factor for many of the women who had a negative experience of their induction. Women also described this impacting their clinical care.
[M]idwives should listen to the patients more, as they understand their body’s. My midwife told me I was not near 4 cm and I told her I can feel the head coming down and my waters broke in the toilet. I gave birth 10 minutes later.
83766849
When I told the midwives I needed to push I was told I was wrong and couldn’t be dilated enough. He was born three pushes later.
89666256
I felt like things happened to me rather than being part of any decisions.
88781722
I opted to wait for 48 hours, but then felt that choices were taken away from me, as I was told if I waited longer I wouldn’t be allowed the pessary and would have to go straight to the drip, which I wanted to avoid. I don’t think I was given enough information about what induction would be like. It was extremely painful and felt like one continuous contraction with no break in between. I wasn’t told whether this was normal.
88910706
When induction led to a quicker than usual labour, women described distress compounded by staff not listening to or believing them.
I felt I was not listened to fully. My birth was very quick (35 minutes for active labour). I kept communicating to the midwives that the induction was working quickly; however, they did not believe me as it is not normal for it to work so quickly.
89290205
Only had the Propess but progressed really quickly and was in active labour and 4 cm dilated within a few hours. Throughout this time I was told this pain would last for days and I was only in the early stages. Psychologically this destroyed me and the rest of the labour went downhill from there, I was terrified throughout.
90013746
Communication also featured in women’s reflection on their experience of deciding to have an IOL in the first place. Lack of information, or inaccurate information about what IOL actually involves, and feeling pressured or coerced into choosing IOL for safety reasons featured strongly.
It was never something I had a choice in and I was told if I didn’t get induced there was a high chance of my baby being stillborn because I was almost 42 weeks so this scared me.
89942117
I was induced because of my age. Whilst it was made clear that the decision was my choice, I also felt a lot of pressure from health professionals to be induced. I read up on the subject to inform my decision and asked a series of questions but felt a strong push to be induced quickly and before my due date which I was not comfortable with. It made my last couple of weeks of pregnancy quite stressful and that there was a ticking clock for Labour to start naturally.
74256136
The induction caused fetal distress and led me to have an emergency C-section. I was terrified. I had no birth partner present because of the hurry. I don’t feel I was made aware of the risks of induction and now feel incredibly anxious I caused my baby distress when I needn’t have.
89211828
It wasn’t fully explained to me just how long I would potentially be in hospital for, and how limited my movement would be once in labour due to being hooked up to both the monitor and the drip. I also don’t feel I was made aware of how uncomfortable and painful repeated pessaries and breaking my waters would be.
83732110
The clinical environment makes it harder to relax. This is what made me slightly resistant to the idea of induction, but I went through with it for the health and well being of my baby.
85728074
I did not feel I had a choice, and felt pressured to agree to the induction immediately without being able to speak to my husband as he was not allowed to attend the appointment.
89218266
It was the worst decision I could have made. The reason for the induction was totally false, I was told based on a growth scan my baby could die at any moment so needed to be born, but everything they claimed in the scan was wrong.
89218266
The consultant I spoke to used the word ‘stillborn’ about 20 times in a 5-minute conversation to try and convince me to have an induction. She also brought up my previous miscarriage as a means to coerce me into agreeing into an induction. It feels like everyone in maternity services are just trying to make sure that they aren’t liable for anything going wrong rather than practising true person centre care. I was not at the centre of my care I was made to fit into a system through fear and inexperience.
89561206
I was planning a home birth but instead was told my baby could die at any time so needed to come out.
89218266
Established labour away from labour ward, trauma and long-term negative effects
A significant number of women who had an IOL described being in established labour and/or second stage away from the labour ward, and some also wrote of trauma and long-term negative impact on their physical and/or mental well-being. Simple quantitative content analysis was performed on those responses.
A total of 28 women described being in established labour for a prolonged period and/or approaching second stage while on the IOL suite.
I was told I couldn’t have one {an epidural} until I moved to the labour ward but I couldn’t move to the labour ward as it was full. I was only moved when I was pushing. This meant I had a painful Ventouse delivery with only two paracetamol.
88910706
I was left to labour on my own on the ward with minimal pain relief overnight. My waters broke at around 1:30am but I wasn’t allowed to call my husband until 4:30am, by which point I was almost 10cm dilated.
87348075
Some 41 women described their experiences as traumatic and/or causing significant long-term negative impact on their physical and/or mental well-being.
My experience of induction was traumatic and something I’m trying to forget and move on from.
91696749
My partner and I feel like one of the most important experiences of our lives was stolen from us. The whole induction was deeply traumatic for us both and I still struggle to think about it today.
92802911
It was all so horrendous that I will never have another child. It gives me anxiety thinking about it all. Before this experience I did want more than one child.
89649436
None of the downsides/risks of induction were discussed with me; in my case it was probably the cause of a more painful labour, episiotomy, instrumental delivery, vagina wall tear and major haemorrhage. This impacted on my ability to breastfeed my baby and has left me with urinary incontinence.
88910706
Fourteen women described an experience that included both labouring away from a labour ward setting and suffering long-term negative effects from their IOL experience.
Ended up fully dilated in a multi-bedded ward thrown onto bed and wheeled to birthing ward. Staff did not examine me or take my concerns seriously. Left me traumatised with postnatal depression. A horrendous experience from start to finish. Nothing positive at all.
89675299
Thematic analysis: positive experiences
A total of 35 women described experiences that could be categorised as positive and 27 as mixed: responses that contained both positive and negative elements to their description of their experience.
When women described a positive experience of IOL, they most often mentioned the staff, or their care, as making a difference. Their responses tended to be generalised, with little specific information about what it was that made the experience good, although women expressed feeling ‘safe’ and ‘well cared for’.
The staff at [name] hospital where amazing and made the experience nice.
74048230
I had amazing care!
80251309
All the staff at [name] hospital were fantastic especially one midwife that stands out to me. She made the whole birthing experience relaxed and done anything she could to make me feel good.
81879255
My midwife [name] was incredibly supportive throughout labour and birth. We felt safe and cared for.
83384325
The induction was amazing thanks to the amazing midwives at [name] hospital.
85072501
The staff at [name] hospital were amazing, as was my midwife at [place], [midwife’s name]. We were supported at every step of our induction and birth journey.
89531292
I felt very safe in the hospital.
90841586
The staff at the labour ward in [maternity unit] were amazing. They made me and my partner feel safe and looked after. Nothing was an issue. Made my birthing experience really special.
90599187
I felt very safe and relaxed in the care of all staff at {name} hospital. I cannot speak more highly of them – amazing care.
90039516
For some, a quick and successful IOL was mentioned in relation to their positive experience.
Very positive and straight forward experience overall compared to my first baby. Waters were broken at 6.30am and baby was born at 9.35am with no other help needed (e.g. drip).
83143364
In the end, my labour went exactly as I wanted and my baby arrived safely within 4 hours.
87529588
However, women for whom the IOL was not successful or that led to intervention and/or surgical delivery also described a positive experience.
I had a good experience in [maternity unit]. Supportive staff, well informed and felt every decision was genuinely done for our well-being. I found the pessary extremely uncomfortable and had some swelling while it was inserted. Staff were attentive and provided lidocaine gel however subsequent examinations were painful. Induction failed and baby was delivered via planned C-section.
87480189
However, the birth experience overall was fairly positive, even with the pain relief and interventions that I eventually required (epidural and Ventouse).
88158381
Good communication and provision of information was a factor that women described as making a difference to their experience.
Felt supported and cared for. I was not talked down to.
88874756
We were left alone a lot in day. Short staffed. Only night sister explained clearly what was happening and felt like she cared.
90471336
I had a great experience. All of the midwives and doctors were excellent. They really put me at ease, explained everything that was happening, what I should expect next, etc.
90841586
I felt very informed by midwives and consultants throughout my pregnancy regarding induction of labour.
91510533
Having privacy of a single room with partner present was also mentioned.
Was in room on delivery suite throughout and birth partner was allowed to stay. Very good service.
84263761
I got my own room. This made a huge difference. If I was on a ward I would have had a much worse experience.
85203048
Home and hospital cervical ripening
There were a handful of comments about home and hospital cervical ripening, and the responses varied, although the comfort, convenience and support of their partner while at home was a draw. For some women, safety, the uncertainty of a quick labour, and being able to return to hospital in time to birth were of greater concern and meant that they chose to stay in hospital. Choice, lack of choice, and the information provided to enable choice about place of cervical ripening were raised as issues that impacted women’s experience.
COVID-19 did make me think about at home induction as I then knew my partner would be there throughout. But concerns about safety of induction and distance from hospital to home meant staying at home for induction wasn’t an option I would consider.
78644478
I had a lengthy induction with my first and was concerned about being in the hospital environment BUT I wouldn’t have gone home either.
85703546
While I appreciate being in hospital isn’t ideal or pleasant and at the time I would have liked to have gone home after being inserted, due to how quickly I went into labour I am pleased I didn’t have a choice and stayed overnight. I did have a comfort I was in the right place.
86994086
I was told I could have balloon induction and go home at consultant appointment, then when I attended hospital was told this wasn’t actually something I could have, and I would need the gel induction and would need to stay in – this made me feel disappointed and anxious.
88158381
I wouldn’t have chosen to go home if offered as I found just going into hospital stressful and preferred to stay to avoid repeating that.
89508785
My experience was overall good and birth was very quick I’m glad I stayed in hospital throughout as I would not have made it back before they were born.
92488159
I would have loved to go home during the first part of the induction. At one point I was in the antenatal ward for 24+ hours with zero intervention to wait for a fourth pessary (through no fault of the staff). I asked to go home on several occasions and I also asked many times for a C-section as I just wanted out of the ward. It definitely affected my mental health being in hospital, especially alone. Although I am forever grateful for the staff.
92995693
I was not allowed home once being induced so ended up having a week in hospital before baby was born.
89649436
I was not offered the choice to stay in hospital after my balloon was inserted. This would have made all the difference with a toddler at home.
89706212
I’d of liked my labour to start naturally so my family could be with me more of the time and my son could have stayed at home longer but was happy to be induced due to risk factors.
92134697
Three women described being sent home during cervical ripening to suit system (all at same maternity unit).
Had balloon induction 8am Monday. Balloon out 8am Tuesday and was 2–3 cm. However, was sent home as there were not enough midwives to induce me further and keep me in. I had to keep phoning them to find out what was going on as no one was contacting me about when I could go in. Was taken back in on Thursday 4pm … 7am Friday morning taken into delivery room to be finally put on the drip at that point was then back to 1 cm.
89753808
I was also told I wasn’t allowed to leave hospital once I was induced, but after three Propess not working, I was sent home because they had no room on the delivery suite for a week due to a long waiting list. As I was told I was extremely high risk due to diabetes and blood clots and that I wasn’t allowed to get to 39 weeks, I then became extremely worried as by the time i gave birth, I was only 3 days away from my due date.
86991374
Change in plans because of induction of labour
Most of this free text was provided in response to Q14: Did having an induction lead to any change in your birthplace plans?
Quantitative content analysis of the answers to this question is also reported.
Q14: Did IOL lead to any change in your birthplace plans?
Yes: 148 (48%); no: 153 (49%); I’m not sure: 8 (3%).
If yes, please explain the reason.
Some women gave more than one change to their plans (e.g. ‘Unable to attend midwife-led unit or have a waterbirth’).
In addition to change of place, some women described a change in the options available to them for pain relief, particularly no longer having the option of using water and the impact of restricted movement (due to increased monitoring and/or intravenous infusion and physical environment of IOL suite). A number of the women associated change in their plans with increased and unwanted intervention and poorer outcomes.
I was devastated I couldn’t go home for my birth of my first baby.
82988043
I was keen for another water birth but understood medical intervention may have been required.
82761125
Is a shame there us not a way of using a birthing pool when being induced, as this would’ve helped me to relax more. I also found that staff were quite surprised that I wanted to find ways of making the birth space less clinical (e.g. with soft lighting). The clinical environment makes it harder to relax. This is what made me slightly resistant to the idea of induction, but I went through with it for the health and well being of my baby.
85728074
I would have liked a water birth but was told it was no longer an option.
86991374
| Response | Respondents (n) |
| Unable to use water during labour or birth | 46 |
| Unable to attend birth centre/midwifery led unit | 43 |
| Unwanted interventions: monitoring, epidural, syntocinon drip, caesarean section etc. | 29 |
| Hospital labour ward only option | 25 |
| Birth in different town | 8 |
| Had planned for early labour at home | 7 |
| Unable to have planned homebirth | 6 |
| Movement restricted during labour | 4 |
I strongly did not want to be induced and have little intervention as possible but induction process led to lots of medical interventions and ultimately an emergency C-section. Which on reflection, resulted in a traumatic experience for me.
87395303
I would have much preferred to either be at home for the early stages or have my partner with me.
88278047
As I was placed on the drip, I was no longer able to move around to ease the pain or help progress labour. No longer able to use water to provide relief. It was suggested I had an epidural which I wound up taking. I would like to think this would have been avoided if I had been able to move around more.
89021852
I was planning a home birth but instead was told my baby could die at any time so needed to come out.
89218266
No waterbirth allowed, had to give birth lying on back and in labour suite.
89476394
I wanted to be active in labour and couldn’t.
89461132
Wanted to birth naturally at [town] maternity unit but was transferred to [city hospital] to have my waters broken which then lead to a caesarean section.
89491266
Had to have an epidural, which led to forceps delivery.
89497692
No pool, wasn’t able to be upright.
89498233
I was disappointed the balloon didn’t work which ultimately – after 3 days and three pessaries – meant I required the oxytocin drip to induce labour. This changed my birth preferences completely as I was physically and mentally drained and essentially chained to a bed for labour. I was unable to use natural methods or minimal pain relief due to the drip and therefore required an epidural. Pushing was extremely difficult and almost landed up as forceps delivery as I was laid on my back pushing uphill.
89531566
The whole experience of staying in hospital was extremely stressful. When I went to labour suite my birth plan was thrown out the window (for no medical reason) and it suddenly felt like a medics procedure I was discharged 16 hours after delivery with a baby who wasn’t feeding, an infection and in severe pain.
89536877
I planned to have a water birth with no drugs. I ended up on the bed on my back with an epidural.
89821844
Couldn’t have water birth and was tied to the bed because unit had no wireless monitoring devices working for babies heartbeat and contractions.
91696749
Appendix 3 Economic analysis rapid systematic review
A rapid systematic review of the (1) effectiveness and (2) cost-effectiveness of cervical ripening at home or in-hospital
Objectives
The objective of this review is to conduct a systematic literature review to explore:
-
Effectiveness and complications of home-based labour induction.
-
Cost-effectiveness studies and existing decision models of home versus hospital IOL.
This information will be used to develop and potentially provide parameters for the CHOICE study economic model.
Types of study to be included
Trial or effectiveness studies (e.g. randomised controlled trial, observational, cohort studies), cost analysis, economic evaluation, decision model, systematic review were included.
Interventions and exposures
Outpatient (home) cervical ripening.
Search process and databases
Five selected databases (MEDLINE, EMBASE, Web of Science, Cumulative Index to Nursing and Allied Health Literature and Cochrane Central Register of Controlled Trials).
Inclusion criteria
Effectiveness studies
-
No restriction by study type.
-
Home IOL.
-
Induction approach balloon/bulb (all other excluded).
-
Outcomes: NNU admission avoided, inpatient hour prevented.
-
Complications (e.g. pre-eclampsia).
Cost-effectiveness studies
All economic or cost-effectiveness studies comparing home versus hospital IOL.
Effectiveness review
| Topic | Search terms | |
|---|---|---|
| Populations | Pregnancy OR “pregnant women” OR pregnant OR Pregnan* | AND |
| Home/outpatient/Hospital/inpatient | (“out patient” OR outpatient OR “out ward” OR outward OR ambula* OR home) OR (hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) | AND |
| Labor induction or cervical ripening | (“labor induction” OR “Labor, Induced” OR (cervi* OR uter*) OR (rip* OR matur*) OR (“cervix ripening”) OR (“Cervical Ripening”)) | AND |
| Induction approach | (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”) | |
Search date: 5 August 2022
Search Terms for each database for review of safety and effectiveness studies:
MEDLINE
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”)
722 results (From 2000–22, language-English, Humans, Female)
EMBASE
((Pregnancy or “pregnant women” or pregnant or Pregnan*) and (“out patientor outpatient” or outward or ambula or home or hospital or hospitaliz* or hospitalis or admission or discharge or inpatient or “in patient” or “hospital-based” or “general ward*”) and (“labor induction” or “Labor Induced” or “cervix ripening” or “Cervical Ripening”) and (dinoproston* or PGE2 or “PG E 2” or PG or prostaglandin* or foley or balloon or bulb or “Foley balloon catheter”)).af.
limit to (human and english language and english and yr=“2000 -Current” and (article or article in press or “review”))
940 results
Web of Science Core Collection
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”)
23 results (705 duplicates with MEDLINE, year 2000–22, Language-English, article and review article)
Cumulative Index to Nursing and Allied Health Literature
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”)
262 results (Year 2000–22, English)
Cochrane Central Register of Controlled Trials
(Pregnancy OR pregnant OR Pregnan) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz or hospitalis OR admission OR discharge OR inpatient OR “in patient” OR “general ward”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston OR “PG” OR “prostaglandin” OR foley OR balloon OR “Foley balloon catheter”)
64 results (Year 2000–22, Intervention) Appendix 3.
FIGURE 14.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the review of safety and effectiveness studies.
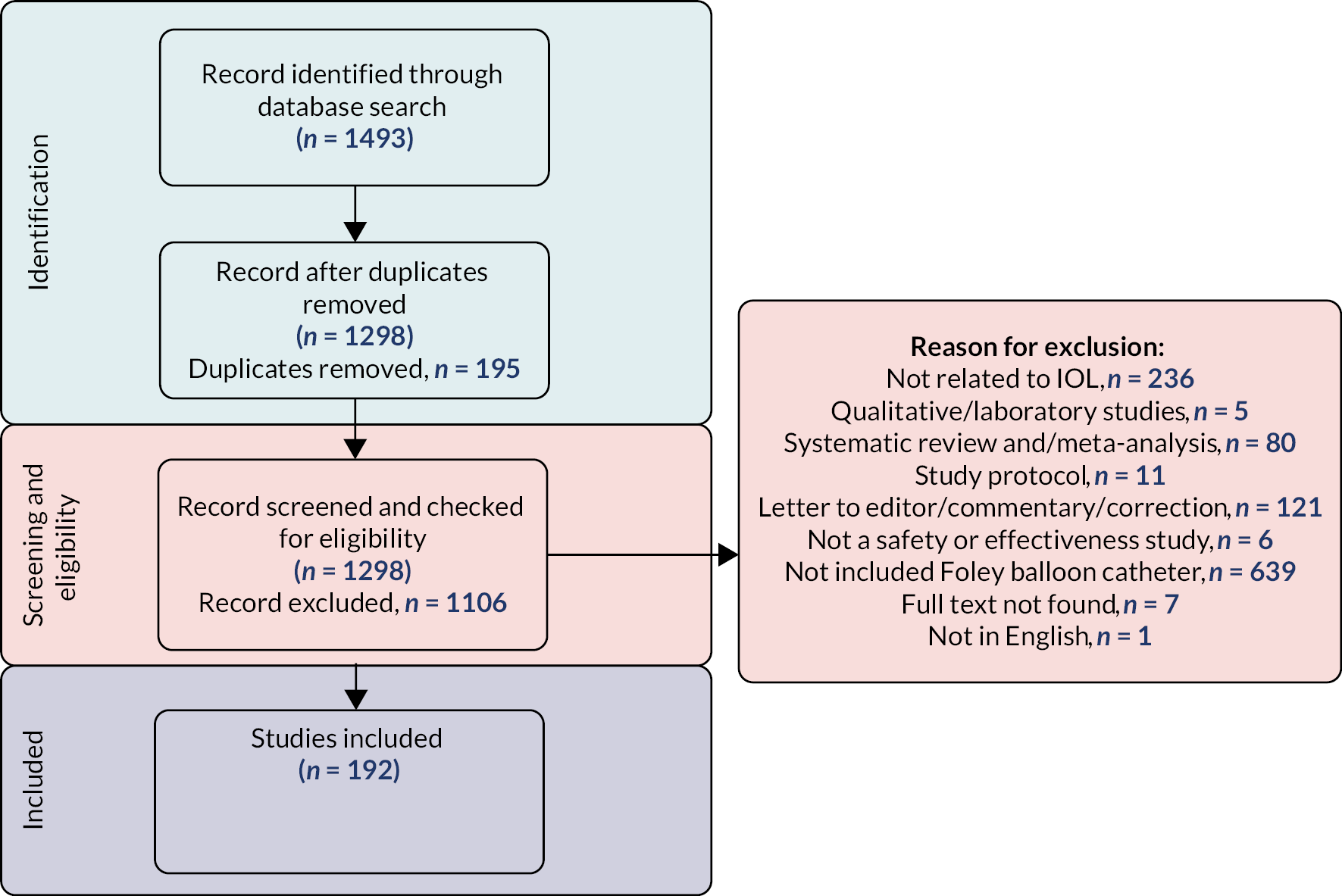
Cost-effectiveness review
| Topic | Search terms | |
|---|---|---|
| Populations | Pregnancy OR “pregnant women” OR pregnant OR Pregnan* | AND |
| Home/outpatient/Hospital/inpatient | (“out patient” OR outpatient OR “out ward” OR outward OR ambula* OR home) OR (hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) | AND |
| Labor induction or cervical ripening | (“labor induction” OR “Labor, Induced” OR (cervi* OR uter*) OR (rip* OR matur*) OR (“cervix ripening”) OR (“Cervical Ripening”)) | AND |
| Induction approach | (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2” OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”) | AND |
| Cost-effectiveness | (analyses, cost benefit or analyses, cost-benefit or analyses, cost-utility or analyses, marginal or analysis, cost benefit or analysis, cost-benefit or analysis, cost-effectiveness or analysis, cost-utility or analysis, marginal or “benefit and cost” or “benefits and costs” or cost benefit or cost benefit analyses or cost benefit analysis or cost benefit data or cost effectiveness or cost effectiveness analysis or cost utility analysis or “cost and benefit” or cost-benefit analyses or cost-benefit analysis or cost-benefit data or cost-effectiveness analysis or cost-utility analyses or cost-utility analysis or “costs and benefits” or data, cost-benefit or economic evaluation or economic evaluations or effectiveness, cost or evaluation, economic or evaluations, economic or marginal analyses or marginal analysis) | |
Search date: 7 August 2022
| Databases | Cost-effectiveness studies | |
|---|---|---|
| Hits (n) | After removing duplicates (using Endnote) (n)a | |
| MEDLINE | 27 | 27 |
| EMBASE | 37 | 26 |
| Web of Science | 33 | 1 |
| CINAHL | 13 | 8 |
| Cochrane | 9 | 7 |
| Total | 119 | 69 |
MEDLINE
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”) AND (“analyses, cost benefit” OR “analyses, cost-benefit” OR “analyses, cost-utility” OR “analyses, marginal” OR “analysis, cost benefit” OR “analysis, cost-benefit” OR “analysis, cost-effectiveness” OR analysis, cost-utility OR analysis, marginal OR “benefit and cost” OR “benefits and costs” OR “cost benefit” OR “cost benefit analyses” OR “cost benefit analysis” OR “cost benefit data” OR “cost effectiveness” OR “cost effectiveness analysis” OR “cost utility analysis” OR “cost and benefit” OR “cost-benefit analyses” OR “cost-benefit analysis” OR “cost-benefit data” OR “cost-effectiveness analysis” OR “cost-utility analyses” OR “cost-utility analysis” OR “costs and benefits” OR “data, cost-benefit” OR “economic evaluation” OR “economic evaluations” OR “effectiveness, cost” OR “evaluation, economic” OR “evaluations, economic” OR “marginal analyses” OR “marginal analysis”)
27 results (from 2000 to 2022, language: English, humans)
EMBASE
((Pregnancy or “pregnant women” or pregnant or Pregnan*) and (“out patient or outpatient” or outward or ambula or home or hospital or hospitaliz* or hospitalis or admission or discharge or inpatient or “in patient” or “hospital-based” or “general ward*”) and (“labor induction” or “Labor Induced” or “cervix ripening” or “Cervical Ripening”) and (dinoproston* or PGE2 or “PG E 2” or PG or prostaglandin* or foley or balloon or bulb or “Foley balloon catheter”) and (“analyses, cost benefit” or “analyses, cost-benefit” or “analyses, cost-utility” or “analyses, marginal” or “analysis, cost benefit” or “analysis, cost-benefit” or “analysis, cost-effectiveness” or analysis, cost-utility or analysis, marginal or “benefit and cost” or “benefits and costs” or “cost benefit” or “cost benefit analyses” or “cost benefit analysis” or “cost benefit data” or “cost effectiveness” or “cost effectiveness analysis” or “cost utility analysis” or “cost and benefit” or “cost-benefit analyses” or “cost-benefit analysis” or “cost-benefit data” or “cost-effectiveness analysis” or “cost-utility analyses” or “cost-utility analysis” or “costs and benefits” or “data, cost-benefit” or “economic evaluation” or “economic evaluations” or “effectiveness, cost” or “evaluation, economic” or “evaluations, economic” or “marginal analyses” or “marginal analysis”)).af.
37 (from 2000 to 2022, language: English, humans)
Web of Science Core Collection
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR albula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”) AND (“analyses, cost benefit” OR “analyses, cost-benefit” OR “analyses, cost-utility” OR “analyses, marginal” OR “analysis, cost benefit” OR “analysis, cost-benefit” OR “analysis, cost-effectiveness” OR analysis, cost-utility OR analysis, marginal OR “benefit and cost” OR “benefits and costs” OR “cost benefit” OR “cost benefit analyses” OR “cost benefit analysis” OR “cost benefit data” OR “cost effectiveness” OR “cost effectiveness analysis” OR “cost utility analysis” OR “cost and benefit” OR “cost-benefit analyses” OR “cost-benefit analysis” OR “cost-benefit data” OR “cost-effectiveness analysis” OR “cost-utility analyses” OR “cost-utility analysis” OR “costs and benefits” OR “data, cost-benefit” OR “economic evaluation” OR “economic evaluations” OR “effectiveness, cost” OR “evaluation, economic” OR “evaluations, economic” OR “marginal analyses” OR “marginal analysis”)
1 (MEDLINE duplicates excluded 32, year 2000–22, language: English, humans)
Cumulative Index to Nursing and Allied Health Literature
(Pregnancy OR “pregnant women” OR pregnant OR Pregnan*) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz* or hospitalis* OR admission* OR discharge* OR inpatient OR “in patient” OR “hospital-based” OR “general ward*”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston* OR PGE2 OR “PGE 2” OR “PG E 2”OR “PG” OR “prostaglandin*” OR foley OR balloon OR bulb OR “Foley balloon catheter”) AND (“analyses, cost benefit” OR “analyses, cost-benefit” OR “analyses, cost-utility” OR “analyses, marginal” OR “analysis, cost benefit” OR “analysis, cost-benefit” OR “analysis, cost-effectiveness” OR analysis, cost-utility OR analysis, marginal OR “benefit and cost” OR “benefits and costs” OR “cost benefit” OR “cost benefit analyses” OR “cost benefit analysis” OR “cost benefit data” OR “cost effectiveness” OR “cost effectiveness analysis” OR “cost utility analysis” OR “cost and benefit” OR “cost-benefit analyses” OR “cost-benefit analysis” OR “cost-benefit data” OR “cost-effectiveness analysis” OR “cost-utility analyses” OR “cost-utility analysis” OR “costs and benefits” OR “data, cost-benefit” OR “economic evaluation” OR “economic evaluations” OR “effectiveness, cost” OR “evaluation, economic” OR “evaluations, economic” OR “marginal analyses” OR “marginal analysis”)
13 results (year 2000–22, English)
Cochrane Central Register of Controlled Trials
(Pregnancy OR pregnant OR Pregnan) AND (“out patient” OR outpatient OR outward OR ambula OR home OR hospital OR hospitaliz or hospitalis OR admission OR discharge OR inpatient OR “in patient” OR “general ward”) AND (“labor induction” OR “Labor Induced” OR “cervix ripening” OR “Cervical Ripening”) AND (dinoproston OR “PG” OR “prostaglandin” OR foley OR balloon OR “Foley balloon catheter”) AND (“analyses, cost benefit” OR “analyses, cost-benefit” OR “analyses, cost-utility” OR “analyses, marginal” OR “analysis, cost benefit” OR “analysis, cost-benefit” OR “analysis, cost-effectiveness” OR analysis, cost-utility OR analysis, marginal OR “benefit and cost” OR “benefits and costs” OR “cost benefit” OR “cost benefit analyses” OR “cost benefit analysis” OR “cost benefit data” OR “cost effectiveness” OR “cost effectiveness analysis” OR “cost utility analysis” OR “cost and benefit” OR “cost-benefit analyses” OR “cost-benefit analysis” OR “cost-benefit data” OR “cost-effectiveness analysis” OR “cost-utility analyses” OR “cost-utility analysis” OR “costs and benefits” OR “data, cost-benefit” OR “economic evaluation” OR “economic evaluations” OR “effectiveness, cost” OR “evaluation, economic” OR “evaluations, economic” OR “marginal analyses” OR “marginal analysis”)
9 results (year 2000–22, intervention).
FIGURE 15.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the review of cost-effectiveness studies.
List of abbreviations
- ARM
- artificial rupture of membranes
- BMI
- body mass index
- CTG
- cardiotocograph
- HEAP
- health economic analysis plan
- ICER
- incremental cost-effectiveness ratio
- IMD
- index of multiple deprivation
- IOL
- induction of labour
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NNU
- neonatal unit
- PAG
- parent advisory group
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- ROM
- rupture of membranes
- WEMWBS
- Warwick–Edinburgh Mental Wellbeing Scales
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/LPYT7894).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
