Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/192/89. The contractual start date was in April 2016. The draft report began editorial review in July 2020 and was accepted for publication in July 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Harris et al. This work was produced by Harris et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – Journals Library, and the DOI of the publication must be cited.
2023 Harris et al.
Chapter 1 Introduction
Background and rationale of the ADAPTT study
Dual antiplatelet therapy (DAPT), a combination of aspirin and clopidogrel, prasugrel or ticagrelor, is recommended for secondary prevention of ischaemic events (i.e. heart attack and stroke) among people with coronary artery disease. Guidelines recommend that patients are treated with DAPT for 6–12 months following myocardial infarction (MI) and coronary interventions [percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG)],1–4 and support the use of the more potent antiplatelet inhibitors ticagrelor and prasugrel. 3 Antiplatelet agents reduce the risk of ischaemic events by preventing the formation of clots in atherosclerotic coronary arteries and within stents (following PCI) or grafts (following CABG), but increase the risk of bleeding. 5 Randomised controlled trials (RCTs) have shown that adding clopidogrel to aspirin leads to a 1% excess risk of major bleeding (necessitating admission to hospital) compared with aspirin alone. 6,7 Prasugrel and ticagrelor reduce the risk of ischaemic events further, but also further increase the risk of bleeding. 8 Some patients [e.g. those with existing atrial fibrillation (AF), or those who develop AF after PCI, CABG or acute coronary syndrome (ACS)] are prescribed an anticoagulant (e.g. warfarin, dabigatran, rivaroxaban, apixaban) in addition to DAPT [known as triple therapy (TT)], which further increases the risk of bleeding.
‘Real-world’ bleeding events that do not require any intervention are likely to be much more frequent than those reported in RCTs, which exclude patients at high risk of bleeding and mainly report only on major bleeding. Bleeding events that do not result in hospitalisation are largely managed in primary care and may have a significant clinical and economic impact. 9 Minor and nuisance bleeding (nose and gum bleeds, bruising and prolonged bleeding from cuts) may also reduce adherence to DAPT, thereby reducing the benefit of DAPT among non-adherent patients,10 who are at increased risk of a secondary ischaemic coronary episode. 11 Only three studies have reported the incidence and consequences of nuisance bleeding after DAPT;12–14 these suggest that nuisance bleeding is common (affecting 29–38% of patients) and affects adherence (11% of patients in one study discontinued clopidogrel13).
The economic impact of bleeding events, particularly minor bleeding events, associated with DAPT is poorly characterised, as is their impact on health-related quality of life (HRQoL). 9 This is not surprising given that health economic analyses often lack detailed data on adverse effects of interventions, despite consensus that such effects should be considered. 15,16 To ensure that appropriate decisions are made about which DAPT regimens to use in clinical practice, the health and resource use consequences of minor and major bleeding events should be incorporated into assessments of cost-effectiveness. For DAPT, this entails accounting for uncertainty in the absolute risk of bleeding; the impact of different bleeding events on HRQoL and treatment adherence, and subsequent risk of secondary ischaemic events; and the cost implications of managing these bleeding events.
In the ADAPTT study, we used Hospital Episode Statistics (HES) and the Clinical Practice Research Datalink (CPRD) databases to conduct three non-randomised studies of interventions to estimate the incidence of all bleeding events occurring among patients prescribed different DAPT or TT regimens after undergoing coronary interventions (i.e. PCI and CABG) and in conservatively managed ACS patients. We used the framework recommended by the Cochrane Bias Methods Group and the Cochrane Non-Randomised Studies for Interventions Methods Group for establishing appropriate patient populations, interventions and follow-up to emulate the following three hypothetical RCTs (hereafter referred to as the target trials):17
-
for patients who have undergone PCI, estimate the effect on bleeding events of assignment to DAPT with aspirin and clopidogrel (AC) (reference), compared with assignment to DAPT with aspirin and prasugrel (AP) or DAPT with aspirin and ticagrelor (AT)
-
for patients who have undergone CABG, estimate the effect on bleeding events of assignment to aspirin (reference), compared with assignment to DAPT with AC
-
for patients who are conservatively managed after presenting with ACS, estimate the effect on bleeding events of assignment to aspirin (reference), compared with assignment to DAPT with AC.
The Cochrane Bias Methods Group and the Cochrane Non-Randomized Studies for Interventions Methods Group17 also recommended that confounders should be specified a priori, using clinician expertise and literature review, although no method of doing this was specified. In the context of ADAPTT, we carried out a literature review, surveys and semistructured interviews with clinicians to identify confounders and relevant co-interventions (medications that a patient might receive with or after starting the antiplatlet regimen, which may both be related to the antiplatelet regimen and be prognostic for bleeding) (see Chapter 2). The confounders identified were taken forward for the analyses of the NRSIs emulating the three target trials (see Chapter 3).We also estimated rates of minor and major bleeding in patients receiving TT (see Chapter 3).We also specified relevant co-interventions, that is medications that a patient might receive with or after starting the antiplatelet regimen, which may both be related to antiplatelet regimen and be prognostic for bleeding. The confounders identified were taken forward for the analyses of the non-randomised studies of interventions emulating the three target trials (see Chapter 2). We also estimated rates of minor and major bleeding among patients receiving TT (see Chapter 3).We conducted a qualitative study exploring patient perspectives on adherence and nuisance bleeding when on DAPT (see Chapter 4). We also conducted a health economic analysis, including a literature review to estimate the deterioration in utility [i.e. quality-adjusted life-years (QALYs)] of patients who have minor or major bleeding events, and a health elicitation study (see Chapter 5). Finally, patient and public involvement was extensive and was used to guide the ADAPTT study (see Chapter 6). Patient and public involvement identified the need for the qualitative study with patients and informed the decision-making process with regard to assembling the target trials from the data sets.
Research objectives
The following objectives were defined in the application for funding:
-
Estimate the rates of major and minor bleeding events with different DAPT (and TT) exposures in each target trial (PCI, CABG, ACS but no procedure).
-
Estimate hazard ratios (HRs) for bleeding for different antiplatelet regimens: for the PCI cohort, we will compare AC with AP or AT; for the CABG and ACS no-procedure cohorts, we will compare aspirin with AC.
-
Review the literature to estimate the deterioration in utility (i.e. QALYs) of patients who have minor or major bleeding events.
-
Revise/extend existing economic models of the cost-effectiveness of different DAPT regimens to include estimates of the incidence of minor and major bleeding events and associated impacts on utility in the general population.
-
Estimate the resources required and associated costs incurred of treating major and minor events of the alternative DAPT (TT) exposures in the three specified patient populations.
-
Understand patients’ perspectives of DAPT, and the factors that influence responses to nuisance bleeding, focusing on adherence and information-seeking.
This last objective was identified through the patient and public involvement work after the start of the ADAPTT study. The patient and public involvement group discussed their own experiences of nuisance bleeding symptoms, prompting the research team to identify this as a topic warranting further investigation.
Changes to the ADAPTT study since the start of the study
We made the following additions/changes to the study that were not specified in the original application for funding:
-
We included a study to identify confounders systematically, as recommended by the Cochrane Bias Methods Group and the Cochrane Non-Randomized Studies for Interventions Methods Group. 17 This involved a systematic review; semistructured interviews with cardiologists, cardiac surgeons and general practitioners (GPs); and a survey to assess the extent to which the confounders identified by the first two methods were considered by different medical practitioners when making prescribing decisions (see Chapter 2).
-
For the PCI target trial, we excluded patients with stable angina undergoing PCI (elective PCI) because > 90% of these patients were prescribed DAPT with AC (see Chapter 3).
-
For the emergency PCI target trial, we conducted two analyses, one including the entire ACS population (for the comparison of DAPT with ticagrelor vs. DAPT with clopidogrel) and another restricted to the ST-elevation myocardial infarction (STEMI) population (for the comparison of DAPT with prasugrel vs. DAPT with clopidogrel), as only STEMI patients were prescribed DAPT with AP (see Chapter 3).
-
We did not attempt to estimate HRs for DAPT compared with TT because the number of patients who could be assigned to TT was too small to justify meaningful comparative analyses (see Chapter 3).
-
We conducted a qualitative study with patients to explore patients’ perspectives on adherence and nuisance bleeding (see Chapter 4).
-
We did not revise existing cost-effectiveness models or attempt to build a new model because our estimates for bleeding were at risk of bias and confounding (see Chapter 5).
Chapter 2 Confounders study
This chapter describes the systematic identification of confounders using systematic review and qualitative interviews with clinicians, including a survey of clinicians to describe DAPT prescribing practice in the UK and to assess the extent to which the confounders identified by the first two methods were considered by different medical practitioners when making prescribing decisions.
Systematic review
Methods
Study eligibility criteria
We reasoned that the number of confounders was likely to be limited and that most would be repeated across multiple studies and study designs. We, therefore, took a pragmatic approach and restricted the study designs to RCTs and cohort studies, which we believed would be the most likely to yield confounders. We included all RCTs and cohort studies (prospective or retrospective) that compared different DAPT interventions (or DAPT and anticoagulants) in our populations, regardless of intervention duration, and any prognostic studies that investigated the relationship between DAPT and bleeding.
Search methods for the identification of studies
The search strategy is shown in Appendix 1. Search terms included the population (e.g. ACS, PCI and CABG), the intervention (e.g. DAPT, TT and P2Y12 inhibitor) and a filter for study design (RCT and cohort study). We searched the following electronic databases: MEDLINE (via Ovid), 1950 to 24 August 2016; The Cochrane Library (issue 7, 2016); and EMBASE (via Ovid), 1970 to 24 August 2016.
Study selection
One review author (MP) triaged the titles and abstracts identified by the search and obtained the full text of studies identified as relevant to the review. Because of the large number of relevant studies identified, we included only the studies for which full text was available to download electronically (no attempt was made to obtain the full text of studies without online access or unpublished studies). We considered studies published in the English language only.
Quality assessment
We did not perform a risk-of-bias assessment because the output of the review is descriptive (i.e. a list of confounders and co-interventions) and there are no established criteria for assessing the validity of the methods used by primary researchers to consider potential confounders and co-interventions. It would, therefore, be inappropriate to apply a risk-of-bias tool for studies estimating a treatment effect.
Data extraction and checking
Data on potential confounders and co-interventions were extracted by two researchers (MP and KM) independently. Variables extracted included study characteristics, population characteristics (reported in the tables of baseline characteristics), study design (RCT or cohort study), interventions considered, factors adjusted for in the statistical analyses and factors reported to predict risk of bleeding in our populations. We anticipated that potential confounders would be identified from multiple studies and that the list of potential confounders would reach an asymptote, so it would not be necessary to extract data from all studies identified. We, therefore, used ‘saturation’ as a criterion for discontinuing data extraction, defined as review of the full text of 10 consecutive studies without identifying an additional confounder/co-intervention. Given the large number of studies identified, we initially selected a random sample of 70 studies for data extraction. All identified potential confounders were grouped into demographic factors, medical history, comorbidity, presentation risk factors, biomarkers, procedural risk factors and other factors (for those that did not fit into these categories).
Results
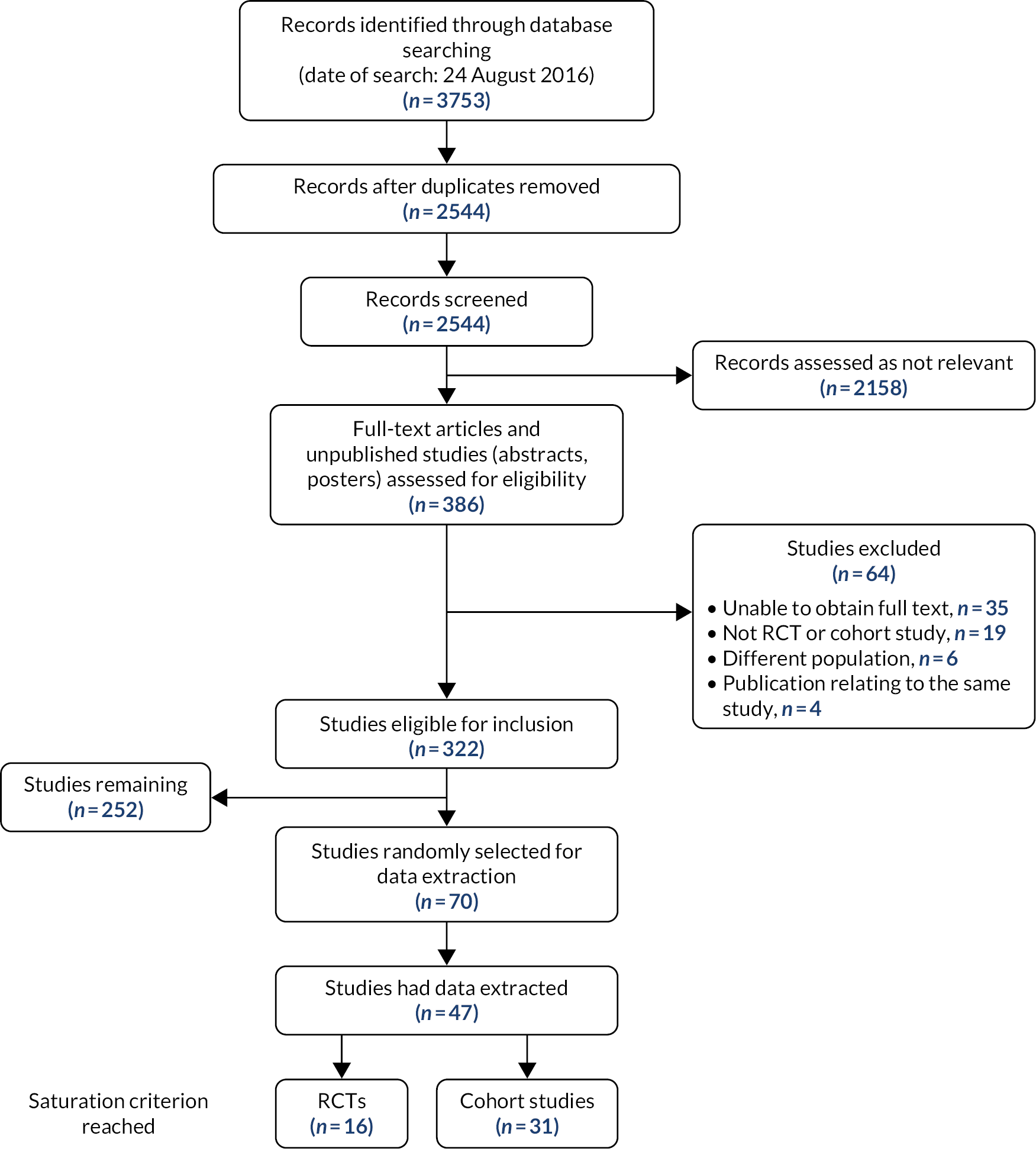
We screened 2544 records, identified 322 studies eligible for inclusion and selected a random sample of 70 for initial data extraction. The saturation criterion (no further new factors identified in 10 consecutive studies) was reached after data extraction from 47 studies (16 RCTs and 31 cohort studies) (Figure 1). We identified 59 potential confounders (seven demography, five medical history, 16 comorbidities, six presentation risk, four risk scores, seven biochemical markers and 14 procedural risk), as shown in Table 1.
| Confounders (N = 59) | |||||||
|---|---|---|---|---|---|---|---|
| Demography (n = 7) | Medical history (n = 5) | Comorbidity (n = 16) | Presentation risk (n = 6) | Ischaemic/bleeding risk scores (n = 4) | Biomarkers (n = 7) | Procedural risk (n = 14) | Co-interventions (N = 10) |
|
|
|
|
|
|
|
|
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Qualitative study with clinicians
Methods
Recruitment and sampling
Cardiologists, cardiac surgeons and GPs based in one of four UK regions [Bristol (University Hospitals Bristol NHS Foundation Trust), Gloucestershire (Gloucestershire Hospitals NHS Foundation Trust), Oxford (Oxford Health NHS Foundation Trust) and Cardiff (Cardiff and Vale University Health Board)] were invited to take part in individual, face-to-face or telephone semistructured interviews. These clinicians are responsible for initiating DAPT or continuing to prescribe DAPT in the light of patients’ experiences and symptoms, in tertiary, secondary or primary care settings. Potential participants were identified by clinicians who were part of the ADAPTT study team, using purposive sampling. The participants selected regularly prescribed DAPT, and practised over a wide geographical area, ensuring that a variety of different practice settings were included. The aim was to recruit six participants from each of the three clinician groups. This number was considered adequate for identifying the range of factors involved in shaping DAPT prescribing decisions. 18,19 Potential participants who expressed an interest in the study when approached by study team members were contacted by the qualitative researcher via e-mail and were provided with a participant information sheet. A suitable date for the interview was arranged if the clinician was still able to participate within the study period.
Data collection
Face-to-face or telephone interviews were conducted between June and October 2017. Face-to-face interviews took place at the Bristol Royal Infirmary and the Bristol Heart Institute. Before each interview, participants signed a consent form or, in the case of telephone interviews, participants had a choice of providing oral informed consent or signing and returning a digital copy. All interviews were audio-recorded.
A clinical vignette-based topic guide was used to guide discussions and elicit clinician prescribing judgements and the range of prescribing decisions when considering prescribing DAPT and/or DAPT and an anticoagulant (TT). 20,21 Four vignettes presenting different clinical scenarios were generated for cardiologist and cardiac surgeon interviews, and three for GP interviews (see Appendix 2). Participants were asked to comment on (1) the clinical decisions that would need to be made for each case vignette; (2) whether they would prescribe DAPT, or DAPT and an anticoagulant, or change the regimen presented; (3) their choice of pharmacotherapeutic agents; and (4) the factors that would influence their decisions (see Appendix 4). Participants were also asked to comment on their use of guidelines and evidence for each case vignette. They were also asked about their links to pharmaceutical companies to ascertain possible conflicts of interest when making prescribing decisions. Vignettes were designed by the ADAPTT study chief investigator and co-investigators, which included a consultant cardiologist; the cardiac surgeon topic guide was piloted with one cardiac surgeon to test the overall structure and relevance of scenarios and questions.
Data analysis
Interview audio-recordings were transcribed by a professional transcription service. All transcripts were checked for accuracy against the original audio-recordings and anonymised. Transcripts were imported into NVivo 11 data management software (QSR International,Warrington, UK) to aid data coding and management. Initially, data were analysed as three separate data sets, using a framework approach. 22 A framework approach was considered to be the most appropriate method for guiding data analysis because:
-
it sits well within pragmatic applied health research in which qualitative methods are used to address a real-life issue, rather than generate theory
-
it allows for analysing data by case (i.e. individual participant or clinician group) and by code using a matrix output, to explore differences or similarities between cases in judgements and views on DAPT.
Initial codes were created representing the clinical vignettes, and the topics of interest under each one: stated prescribing intention, factors considered and sources of information. Transcript data were indexed based on these codes and participants’ responses to each question were deductively mapped to these codes. Open coding (i.e. inductive coding) was then used to extract the individual factors reported by each participant and capture items of interest to the research question emerging from participants’ narratives. Following the coding of the first three transcripts, an analytical framework was developed. When analysis of the three data sets was complete, in-depth coding of the totality of the transcripts as one large data set was carried out to identify a detailed list of factors reported by participants to influence their decision to prescribe, or not prescribe, antiplatelet and anticoagulant medication. Descriptive labels capturing the factors, and clinical or non-clinical indicators linked to these factors (e.g. indicators linked to a patient’s risk profile), were then categorised under higher-order codes to capture broader descriptive categories. Framework matrices were created in NVivo 11 to address specific research questions (e.g. when and why clinicians prescribe DAPT) and to allow for comparisons to be made between and within the three clinician groups in their responses on codes of interest. The analytical framework and findings were presented to the ADAPTT study team at different stages during the analysis to obtain clinical input on the relevance, significance and authenticity of the findings, and to explore clinical concerns arising from these data to guide subsequent analysis and interpretation of data. Findings were also presented to the patient and public involvement group for comments (see Chapter 6).
Results
Eight cardiac surgeons, six cardiologists and eight GPs were initially approached. Six interviews with cardiac surgeons, six with cardiologists and five with GPs were organised. The remainder of the clinicians either did not reply to the researcher’s e-mails or declined to participate, citing lack of time as the reason. Five interviews were conducted face to face and the rest were conducted over the telephone. Interviews lasted between 26 and 45 minutes.
Differences in prescribing decisions between clinician groups
Differences emerged in the prescribing practices between GPs and secondary care specialists. Cardiologists and cardiac surgeons would make independent decisions about whether a patient required DAPT or DAPT and anticoagulant. GPs, on the other hand, were mostly involved in medication regimen management, and would not be independently prescribing or changing specialist medication and regimens without first consulting secondary care specialists:
The only treatment that I would start in primary care is aspirin, so if a change is required, and obviously apixaban for an AF or something like that, so therefore if there was a problem I would probably go back to the specialist rather than change it myself.
GP010
[Bleeding] would be a difficult situation and would almost certainly be left to the specialist in the hospital to agonise over.
GP012
I’m questioning here kind of where the aspirin and ticagrelor’s come from [...] I would [...] probably ring the cardiology on call on the day.
GP010
I would probably again phone the cardiologist to say do I need to keep this patient on aspirin as well and simply give them some gastric prophylaxes and cross my fingers or can I safely have them just on warfarin?
GP012
I wouldn’t [change the prescription] routinely unless the patient was unhappy, we won’t change drugs that have been issued by the hospital. We will stick with what the hospital said.
GP013
General practitioners differed from the other two groups in that they would consider the DAPT regimen in relation to a patient’s other medication and medical history:
When I see a summary printout from the hospital, if I have seen that these drugs are incompatible or there’s a problem with them, then yes, I would go back to the [hospital].
GP010
One always has to consider [patient ischaemic risk and medication history] and look at the list of medication; for example if we see [a patient on DAPT] with a painful ankle and we’re thinking about using an anti-inflammatory, for example, you know, we’d perhaps be a bit more reluctant if we can see that they’re on dual antiplatelet therapy as well.
GP013
The prescribing decisions of each clinician group are reported Table 2. In summary, ticagrelor was the most common choice of cardiologists when prescribing DAPT, whereas clopidogrel was the one routinely used by cardiac surgeons. Clopidogrel was the agent of choice of the majority of participants when it came to prescribing TT, and for patients who were assessed to be at high risk of bleeding. Cardiologists were more likely than cardiac surgeons to prescribe DAPT in all four scenarios, whereas cardiac surgeons were more likely to discontinue DAPT because of bleeding risks if a patient was also on anticoagulant medication, or if the prescription of an anticoagulant agent was being considered.
| Scenario | ||||
|---|---|---|---|---|
| Group | Patient, elderly and diabetic, develops unstable angina; initiate DAPT | Patient on long-term anticoagulation undergoing PCI for new-onset angina; initiate DAPT | Patient on DAPT following STEMI; develops AF; initiate anticoagulant | Patient on DAPT with ticagrelor following PCI; presents with nosebleeds and bruising |
| Cardiologists (n = 6) | All participants would initiate DAPT | Five participants would prescribe TT | All would prescribe TT | All would change to clopidogrel |
| Cardiac surgeons (n = 8) | Five participants would initiate DAPT |
|
|
|
| GPs (n = 8) |
|
|
|
|
Table 3 presents the factors that informed clinician prescribing decisions within factor categories, with examples. A detailed report of the factors, along with their constituent indicators, is presented in Appendix 4.
| Patient-related factors | Clinician-related factors | Drug characteristics | Local contexts |
|---|---|---|---|
| Patient risk profile: | Clinical guidelines and evidence-based medicine | Potency of drug | Commissioning and organisation budget policy |
|
|
|
|
| Previous and planned revascularisation procedures: | Professional opinion and experience | Licensing | Local culture of prescribing |
|
|
||
| Factors specific to the pharmacotherapeutic regimen of the patient: | Local prescribing protocols and decision support tools | ||
|
|||
| Multidisciplinary team-working | |||
|
|||
| Patient views and preferences |
Patient-related factors
Patient bleeding and ischaemic risk profile
The starting point for all clinician groups and all participants was an assessment of ischaemic and bleeding risk. Factors relating to a patient’s clinical presentation and risk profile were the most frequently raised by all participants (a comprehensive list of the indicators emerging from the analysis is presented in Appendix 4). The following excerpt is illustrative of the judgements described by participants:
You are making a balanced judgement between the benefit of anticoagulation or antiplatelet therapy in terms of its ischaemia reduction, versus the risk which is bleeding, [...] so you’re looking at the presentation and judging whether it’s a high ischaemic risk presentation such as STEMI or a low ischaemic risk presentation such as stable angina and then the risk of bleeding, which would be low in healthy, young diabetic men, and would be very high in elderly, low body weight, hypertensive females with renal failure, so you’re trying to make a judgment taking all those factors.
Previous and planned revascularisation procedures
The patients’ previous and planned revascularisation procedures influenced prescribing decisions, including the duration of treatment. The most frequently mentioned factors to guide decisions were the stenting procedure and stent attributes. Interviewees considered time since stents were inserted, the types and number of stents inserted, and the success of the revascularisation procedure:
If let’s say [it is] 3 months from the time of the stent, we are a lot safer. For certain stents, even after 6 weeks, you are a lot safer.
Cardiac surgeon 007
The period of time really would depend on the actual procedure performed, how complex it is, how many stents he’s put in and how worried you are about the stent failing over the next few months.
Cardiologist 014
If the patient has had a successful revascularisation, the questions are what should they be restarted on.
Cardiac surgeon 015
I would [consider prescribing a second antiplatelet]. Only on the basis that, presumably, [the patient] is going for revascularisation [...] in preparation for a stenting procedure.
Cardiologist 002
Patient reactions to the pharmacotherapeutic regimen
When deciding to initiate a pharmacotherapeutic agent, or make changes to an existing regimen, interviewees considered factors related to a patient’s medication history, for example presenting with side effects, resistance or allergic reactions to agents:
This is someone who’s already on anticoagulation, so I’d look and see if they were on warfarin already and, [if] they’d had good control, I would carry on with the warfarin. If they were on a NOAC [non-vitamin K oral anticoagulant], I probably would put them onto a reduced dose of the NOAC.
Cardiologist 009
If a patient is having intolerable side effects [...] such as breathing difficulties, which I know is a potential side effect with ticagrelor [then would swap to clopidogrel].
GP 018
We test the patients if they are previously on clopidogrel and they are proven to have high resistance to clopidogrel, then I will swap them to aspirin and ticagrelor.
Cardiac surgeon 007
Risk of non-adherence
Interviewees considered individual patient characteristics that might compromise adherence to the pharmacotherapeutic regimen. They raised concerns for non-adherence more often when explaining their judgements of prescribing anticoagulant agents:
The problem with warfarin is it doesn’t have a fixed dose, so patients need to undergo blood tests [...] you have to consider which patient you have so sometimes you have very old patients, they live alone, it’s difficult for them to have blood tests. Maybe they’ve got very difficult veins to access to do the blood test and in this case it would be much easier to give another anticoagulant drug.
Cardiac surgeon 004
I always [...] ask [patients] ‘are you good at taking tablets? Do you struggle? Do you sometimes miss tablets?’ [...] if they’re telling me that they miss their evening tablets, I’m not going to give them a BD [bis in die (twice a day)] medication, try and give them once a day.
Cardiologist 003
Patient views and preferences
In some instances, interviewees would consider patient preferences in their prescribing decision-making. For example, patient preferences would be taken into account when choosing between anticoagulant agents because of the impact of different regimens on a patient’s lifestyle, and the regimen’s dosage requirements. Some interviewees also reported that they would consider patient views when balancing risk of ischaemia with risk of bleeding, and ways to manage nuisance bleeding:
Warfarin is very much a lifestyle-changing medication and you do need to have that discussion with them and they do need to be on board with attending warfarin clinics and for their prescribing of warfarin, along with modification of their lifestyle, in order to be safe when taking warfarin.
Cardiac surgeon 008
You have to have a discussion with the patient to say ‘I think this is due to the medication we’re on. You’re on it because you’ve got a high risk of recurrent myocardial infarction. How bad are these nosebleeds? What are the consequences of the nosebleeds?’ and then you have to consider the position as to what to do depending on what the patient tells you really.
GP 006
You chat to the patient, you know you talk to them about the risk of a stroke versus the risk of a bleed and they have to help you to make a decision.
Cardiologist 003
Clinician-related factors
Guided by clinical guidelines and evidence-based medicine
Clinical guidelines and evidence-based medicine did not inform decisions for some case vignettes. Several factors influenced the use of guidelines, including awareness of the guidelines and research evidence for specific case vignettes. Several interviewees, in particular cardiac surgeons, commented on the lack of research evidence to inform decisions in some scenarios. Multiple, and sometimes conflicting, sources of evidence could also present challenges:
Clopidogrel would be another [choice] and, again, we wouldn’t use that because I think NICE [National Institute for Health and Care Excellence] guidance suggests ticagrelor as the best treatment option in this scenario.
GP 006
As far as I know, there are no randomised clinical trials that demonstrate clearly that, after elective CABG, dual antiplatelet is better.
Cardiac surgeon 004
You need to have enough evidence, clinical evidence, of the use of the medication [to inform the decision of whether or not to prescribe it].
Cardiac surgeon 007
And the trouble is, you’ve got so much data [...] the more data we get, the more confused we seem to be.
Cardiologist 001
The quality and credibility of guidelines and available evidence and their relevance to complex patient cases also influenced attitudes towards the use of clinical guidelines and evidence-based medicine:
The guidelines and the studies are done on patients maybe up to 70 or 75 years of age. They don’t help you when you have someone who’s 90, there isn’t any data for that and there isn’t data for the patients who are complex, like the ones in hospital, because in the study [they are] not the ones who have dementia, falls, emphysema, all the other problems that you have to try and consider.
Cardiologist 003
What the guidelines and the trials are desperately trying to do, and I’m not sure it’s actually possible, is they’re trying to make all patients the same and give you a simple answer. I personally have always felt that’s a gross oversimplification because all patients are different and all scenarios are different.
Cardiologist 001
[Clinical guidelines] are useful. As a reference point, no doubt about it, and some of them are [...] a general one-size-fits-all approach.
GP010
I read them, I know them, but I don’t always follow them. [...] guidelines are written by committees that may or may not have vested interests about what they’re writing the guidelines about and they may have an inadequate evidence base on which to do it.
Cardiologist 005
The problem, particularly in surgery, is then a lot of those guidelines are based on weak evidence, not on significant sizeable randomised studies.
Cardiac surgeon 015
Other considerations were their clarity and level of complexity:
[...] NICE particularly [...] they’re not necessarily good at helping you weigh two treatments against each other, they’re just saying ‘This is an appropriate treatment to give which you should consider and offer where appropriate’.
Cardiologist 009
[Local guidelines] change a lot and still some people maybe ignore little bits and pieces, but they change and they’re becoming really complicated because of the scenarios [...] [there are] so many boxes you have to follow to go down to tell you what to do.
Cardiologist 003
I think we need risk calculators to predict for specific situations what the best strategy for antiplatelet therapy is. Otherwise it does take quite some time to try to ascertain from the guidelines what should we do with specific cases.
Cardiac surgeon 008
I think sometimes they’re very long and they are difficult to get through.
GP010
Professional opinion
Individual professional opinion was an important determinant of prescribing behaviours when prescribing guidelines and evidence were not thought of as relevant or useful. Clinicians would tend to prescribe agents that they were familiar with and had used in the past:
I think what influences prescribing, certainly in my experience, and I think in lots of surgeons’ experience, is their own practice. [...] Since the guidelines are not very clear or not supported by very strong evidence, often you find individual surgeons will have their own opinion.
Cardiac surgeon 015
There are no clear indications taken by the guidelines [to support DAPT] so, really, if you have, let’s say, 30 years’ good experience with aspirin, maybe you would prefer to continue giving aspirin.
Cardiac surgeon 004
From experience, what you’ve used in other patients in the past [would help decide what agent to prescribe].
GP006
That really comes down to one of comfort and what you’ve been used to and what you use a lot of [...] since sort of late in my training a few years ago I’ve just been using more of apixaban and that’s what you get comfortable with. I’m happy using apixaban because I’ve been prescribing it quite a lot, I’m used to the potential side effects.
Cardiologist 014
Drug characteristics
When choosing between agents, agent potency was an important consideration for managing the risk of bleeding for a patient:
The reason I’m choosing apixaban is it’s the anticoagulant which probably has [...] the lowest bleeding risk, so because you’re also giving a patient two other drugs which cause bleeding, I would go for the drug which has the lowest bleeding risk.
Cardiologist 001
In this case, there really only is the clopidogrel that we would use because we would not want to combine a very potent antiplatelet agent such as ticagrelor or prasugrel with anticoagulation.
Cardiologist 014
Ticagrelor is a very powerful antiplatelet agent, so [...] I would stop the aspirin and see whether, with ticagrelor only, the nose bleeding and the bruising reduced. If it [...] I would stop the ticagrelor and put the patient back on aspirin and clopidogrel.
Cardiac surgeon 015
The licensing of agents for specific clinical scenarios would also influence prescribing:
For any valvular disease, like if you have AF and you have had a mitral valve repair, mitral valve replacement, or aortic valve replacement, you cannot currently use NOACs or apixaban because they are not licensed for it at the moment.
Cardiac surgeon 011
Local contexts
Commissioning and organisation budget policy
Prescribing behaviours were influenced by the local budgets, commissioning decisions and prescribing protocols:
At the moment in the unit, we only [prescribe] clopidogrel, we’re not using ticagrelor.
Cardiac surgeon 015
If you asked me, I would use NOAC or apixaban, not warfarin; however, at the moment, after cardiac surgery, NOACs are not licensed to be used, number one. Number two [...] it’s more expensive, it’s not allowed to be used, I cannot use it.
Cardiac surgeon 011
Clopidogrel is much more [often prescribed] locally and I actually don’t know why. I think it’s an expense issue. I think clopidogrel has been around longer and is now much cheaper.
GP012
When you’re prescribing, there is a software that actually tells you, first of all, it can link you to the guidance and the CCG [Clinical Commissioning Group].
GP006
Guided by local prescribing culture
Prescribing culture would also influence prescribing behaviours, meaning that clinicians would tend to prescribe agents routinely prescribed within the unit. How familiar other clinicians and staff members who were involved in a patient’s care were with specific agents was thought to be important for patient safety and team-working:
Because there’s no clear statement about cardiac surgery for the moment, we stick to clopidogrel, which is a bit more known by clinicians, and known by GPs as well.
Cardiac surgeon 004
Familiarity of the medication in the unit and people who treat the patient, so if you get some that nobody is familiar, they don’t know what to do with it, how rapid is the response and if it is possible to be reversed, especially for cardiac surgery because you may have to take the patient back for bleeding or something.
Cardiac surgeon 007
Interviewees based in one hospital setting reported the importance of standardising prescribing practices in secondary care settings through local prescribing protocols to promote patient safety, improve multidisciplinary team (MDT) communication and support junior doctors and other members of staff in their roles:
In the past, there were 10 surgeons, you had 11 different [prescribing] policies. Now it’s not the case because you want to run things in a simple way and not as confusing as it was in the past, not least for the nurses and the juniors, so generally most units will have protocols which have been agreed by everybody. So I think it makes life easier for everybody concerned.
Cardiac surgeon 015
We have what we call trust protocols or trust guidelines [...] it’s easier; it’s much quicker and easier to read and to understand [than individual guidelines and research evidence].
Cardiac surgeon 004
There’s multiple different antiplatelet regimes and, to some extent, there’s only certain evidence base for them and it’s very confusing for juniors to have a lot of different approaches [...] so reducing variants is sort of one of the tenets of safe care in hospital. [...] So the EC [European Community] guideline approach would say ticagrelor. I think the clinical scenario says ticagrelor and the hospital protocol says ticagrelor, so I’d go ticagrelor.
Cardiologist 005
Interviewees respected colleagues’ clinical decision-making autonomy and were reluctant to change medication prescribed by other clinicians:
I would carry on with aspirin and clopidogrel because I am worried about the stent that might block and the cardiologists are going to be pretty upset if they find that the clopidogrel has been stopped.
Cardiac surgeon 011
If the clinician in the hospital had recommended ticagrelor, I would continue it because I’d be concerned if there was some specific reason why they chose that one.
GP012
Guided by multidisciplinary team opinion
A decision on which regime was the most appropriate would be guided by members of the MDT if the individual clinician felt that they lacked the expertise to make an informed decision. Most interviewees stated that their decisions would be informed by cardiology expert opinion, whereas a minority referred to other members of the MDT:
I would expect my interventional colleagues to be saying, you know, ‘We’ve reviewed the data, we’ve reviewed the international guidelines and then this is how we think they should be interpreted in our settings’.
Cardiologist 009
Haematology can be useful [...] regarding anticoagulation, and pharmacies, pharmacy are very good at being able to guide us regarding evidence.
Cardiac surgeon 008
We try and work as a team, so I might, if I’m in any discomfort about making this decision, I might discuss it with my GP colleagues as well as the patient’s consultant cardiologist. Or actually the prescribing adviser at the CCG [Clinical Commissioning Group] can often be very helpful in producing guidelines and protocols if there are any.
GP018
Conflicts of interest and pharmaceutical company influence
Two interviewees reported being involved in research funded by pharmaceutical companies, and two stated that they made an effort to maintain independence because of their academic roles. Some GPs reported their surgery’s policy to block access of pharmaceutical company representatives to individual doctors. None of the interviewees reported being directly influenced by pharmaceutical companies in what they prescribed, even in the cases of participants reporting direct involvement with pharmaceutical companies through their research activities:
I have to be very careful not to have too many links with pharma in that particular area, otherwise I can’t be involved in that particular kind of, you know [...] reviewing of the evidence and providing the guidelines.
Cardiologist 009
I’ve never held any consultancy with any company. I’ve always refused because I wanted to maintain my independence.
Cardiac surgeon 015
I have a good relationship with companies in terms of looking at data and them funding some of my research occasionally, but I certainly wouldn’t let that affect my prescribing.
Cardiologist 001
I don’t see any drug rep[resentative]s at all. I don’t know if anyone in our practice does. I try not to engage with them, personally.
GP013
Most interviewees described the role of pharmaceutical companies in continuing professional education and dissemination of clinical trial findings. Some believed that this involvement had the potential to indirectly influence prescribing behaviours through the relationships created:
We do a journal club where [...] we also have a rep from one of the pharma companies who is providing the lunch, often telling us about an [research] update [...] Their education support sometimes is [a] double-edged sword that, although it’s supposed to be neutral of a product [...] they’ve gone for subtle forms of influencing [...] It’s making you associate their product with some good feeling so that when you have a choice that’s equal, you think ‘actually here I’m going to use that drug because I feel more confident about it’.
Cardiologist 009
If somebody takes you to a big meeting and you have a great time, then the next time you have to prescribe a drug which is produced by that particular company, willingly or unwillingly, you will be more disposed isn’t it, it’s human nature.
Cardiac surgeon 015
Some interviewees believed that pharmaceutical companies had some influence in the content of guideline recommendations through funding the clinical trials that provided the evidence for the recommendations, promoting individuals who supported their products within key committees and lobbying decision-makers:
By definition, they have a role because if you look at major trials they have done, especially with the new drugs [...]. [Guidelines] are not as much independent, but they are by definition influenced one way or another by the companies and the drug-producing manufacturer.
Cardiac surgeon 007
I think that the pharma companies are trying to target it at a bigger level so they’ve got key opinion leaders that you associate with particular brands [...] They’ve tried to push those people forward so [...] they’re not necessarily directly promoting their drug, but are finding ways to make that person have influence by linking them up with other leaders in the research world or in national or international societies.
Cardiologist 009
They [pharmaceutical companies] produce the drugs and they pay for the trials, so they’re obviously massively important [...] [named pharmaceutical company] basically lobbied government saying, ‘our drug isn’t being prescribed’ and ‘the guidelines say it should and why not?’ so and they started to get very political.
Cardiologist 001
Survey of clinicians
Methods
Two online surveys (one for cardiologists and one for cardiac surgeons) were developed by the study team, including a methodologist with expertise in survey design, a consultant cardiologist and a consultant surgeon. The surveys were designed to do the following:
-
Describe DAPT prescribing practice among various patient subgroups [based on age, type of event (e.g. ACS vs. non-ACS), concomitant anticoagulant use for cardiology patients and type of surgery, anticoagulant use for cardiac surgery patients].
-
Identify the five most important factors that influence the choice of DAPT prescription in each of six separate domains (e.g. demography, comorbidity and procedure related-characteristics) for cardiology patients and two domains (age and comorbidity only) for cardiac surgery patients. The factors included in the survey were those identified from the systematic review (see Table 1) and additional factors identified from the clinician interviews.
-
Identify whether or not the chosen factors influenced prescribing decisions because they increased risk of ischaemia, risk of bleeding or risk of both ischaemia and bleeding.
The factors and their respective domains were those identified from the systematic review and clinician interviews. The surveys were uploaded to SurveyMonkey® (Palo Alto, CA, USA) and the online surveys were piloted among a small group of cardiologists and cardiac surgeons to ensure ease of use and to test face and content validity. An invitation including a link to the survey was disseminated by e-mail via the Society for Cardiothoracic Surgery (cardiac surgeons) and the British Cardiovascular Intervention Society (cardiologists) to all individual fellows and members of the societies. The data analysis tools in SurveyMonkey and Microsoft Excel® (Microsoft Corporation, Redmond,WA, USA) were used to calculate descriptive statistics.
Characteristics of survey respondents (cardiologists and cardiac surgeons)
There were 101 cardiologists and 36 cardiac surgeons who initiated the survey. Of these, 22 cardiologists (22%) and five cardiac surgeons (14%) consented to participate (selected the ‘agree’ electronic consent button) but did not complete any survey questions, so they were removed, leaving a total of 79 cardiologist and 31 cardiac surgeon respondents (Table 4). Of the cardiologists, almost two-thirds of respondents were consultant grade and were evenly distributed across years of practice categories, whereas, for cardiac surgeons, the majority of respondents (90%) were consultants and over half of them had practised for > 15 years. Respondents represented all regions of the UK, but the regions most represented were London, the south west and the north west. Just under two-thirds of cardiologist respondents prescribed DAPT daily, and the majority of the remainder prescribed DAPT two or three times per week. The most common guidelines used by both clinician groups were NICE1 and European Society of Cardiology2 guidelines, although just under half of cardiac surgeon respondents (42%) reported using none of the guidelines. Most cardiologists reported that local protocols for DAPT prescribing were available, whereas two-thirds of cardiac surgeons reported that they had no local protocols for antiplatelet prescribing.
| Demographic details | Respondents, n (%) | |
|---|---|---|
| Cardiologists (N = 79) | Cardiac surgeons (N = 31) | |
| Grade | ||
| Consultant | 50 (63) | 28 (90) |
| Fellow/specialist registrar | 25 (32) | 2 (6) |
| Associate specialist/staff grade | 4 (5) | 1 (3) |
| Subspecialty (consultants only) | ||
| Interventional cardiology | 45 (90) | – |
| Heart failure | 4 (8) | – |
| Cardiac imaging | 1 (2) | – |
| Years of practice (consultants only) | N = 28 | |
| < 5 | 12 (24) | 4 (14) |
| 5–10 | 14 (28) | 5 (18) |
| 11–15 | 11 (22) | 4 (14) |
| > 15 | 13 (26) | 15 (54) |
| Location | ||
| North West | 6 (8) | 6 (19) |
| North East | 1 (1) | 1 (3) |
| Yorkshire and the Humber | 4 (5) | 1 (3) |
| East Midlands | 5 (6) | 1 (3) |
| West Midlands | 6 (8) | 2 (6) |
| Eastern England | 6 (8) | 1 (3) |
| London | 16 (20) | 5 (16) |
| South East Coastal | 4 (5) | 1 (3) |
| South Central | 6 (8) | 3 (10) |
| South West | 11 (14) | 4 (13) |
| Scotland | 10 (13) | 4 (13) |
| Wales | 2 (3) | 1 (3) |
| Northern Ireland | 2 (3) | 1 (3) |
| How often DAPT is prescribed | ||
| Daily | 48 (61) | – |
| Two or three times per week | 25 (32) | – |
| Less than once per week | 6 (8) | – |
| Guidelines used for DAPT prescribinga | ||
| NICE | 37 (47) | 12 (39) |
| European Society of Cardiology | 64 (81) | 16 (52) |
| American College of Cardiology/American Heart Association | 8 (10) | 9 (29) |
| None of the above | 6 (8) | 13 (42) |
| Are local protocols for DAPT prescribing available? | ||
| Yes | 63 (80) | 12 (39) |
| No | 16 (20) | 19 (61) |
Survey results: cardiologists
Dual antiplatelet therapy prescribing practice for ACS and stable angina patients is shown in Table 5. The default prescribing regimen for ACS STEMI patients was AT (more than two-thirds of respondents, with most prescribing for 12 months). Relatively few STEMI patients were prescribed AP (12% and 5% in those aged ≤ 75 years and > 75 years, respectively).
| Patients, split by age (years), n/N (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAPT regimen and duration of treatment | ACS STEMI (PCI) | ACS NSTEMI (PCI) | ACS unstable angina (PCI) | Stable angina (PCI) | ACS (conservatively managed) | |||||||
| ≤ 75 | > 75 | ≤ 75 | > 75 | ≤ 75 | > 75 | ≤ 75 | > 75 | ≤ 75 | > 75 | |||
| AC | 8/68 (12) | 14/64 (22) | 18/68 (22) | 26/64 (41) | 29/67 (43) | 34/64 (53) | 59/67 (88) | 57/63 (90) | 40/71 (56) | 46/67 (69) | ||
| 1 month | 0 | 1/14 (7) | 0 | 0 | 1/29 (3) | 0 | 1/59 (2) | 1/57 (2) | 0 | 0 | ||
| 3 months | 0 | 0 | 0 | 1/26 (4) | 1/29 (3) | 1/34 (3) | 2/59 (3) | 1/57 (2) | 2/46 (5) | 1/46 (2) | ||
| 6 months | 2/8 (25) | 3/14 (21) | 2/18 (11) | 3/26 (12) | 1/29 (3) | 3/34 (9) | 20/59 (34) | 24/57 (42) | 3/46 (8) | 7/46 (15) | ||
| 12 months | 5/8 (63) | 9/14 (64) | 15/18 (83) | 20/26 (77) | 24/29 (83) | 28/34 (82) | 35/59 (59) | 30/57 (53) | 34/46 (85) | 37/46 (80) | ||
| > 12 months | 1/8 (13) | 1/14 (7) | 1/15 (7) | 2/26 (8) | 2/29 (7) | 2/34 (6) | 1/59 (2) | 1/57 (2) | 1/46 (3) | 1/46 (2) | ||
| AP | 8/68 (12) | 3/64 (5) | 4/68 (6) | 2/64 (3) | 2/67 (3) | 1/64 (2) | 1/67 (1) | 1/63 (2) | 2/71 (3) | 1/67 (1) | ||
| 1 month | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1/1 (100) | 0 | 0 | ||
| 3 months | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 6 months | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 12 months | 8/8 (100) | 3/3 (100) | 4/4 (100) | 2/2 (100) | 2/2 (100) | 1/1 (100) | 1/1 (100) | 0 | 2/2 (100) | 1/1 (100) | ||
| > 12 months | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AT | 52/68 (76) | 47/64 (73) | 46/68 (59) | 36/64 (56) | 36/67 (54) | 29/64 (45) | 7/67 (10) | 5/63 (8) | 29/71 (41) | 20/67 (30) | ||
| 1 month | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 months | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2/29 (7) | 2/20 (10) | ||
| 6 months | 5/52 (10) | 3/47 (6) | 5/46 (11) | 3/36 (8) | 3/36 (8) | 2/29 (7) | 5/7 (71) | 3/5 (60) | 4/29 (14) | 3/20 (15) | ||
| 12 months | 47/52 (90) | 42/47 (89) | 40/46 (87) | 32/36 (89) | 32/36 (89) | 27/29 (93) | 2/7 (29) | 2/5 (40) | 27/29 (79) | 15/20 (75) | ||
| > 12 months | 0 | 2/47 (4) | 1/46 (2) | 1/36 (3) | 1/36 (3) | 0 | 0 | 0 | 0 | 0 | ||
Among patients who had a non-ST elevation myocardial infarction (NSTEMI), just over half of all respondents prescribed AT for both younger and older patients, whereas, for conservatively managed ACS patients, 41% (for patients aged ≤ 75 years) and 30% (for patients aged > 75 years) of respondents prescribed AT. The use of AP was infrequent, except for STEMI patients aged ≤ 75 years, for whom just over 12% of respondents prescribed this regimen.
Across all ACS groups, approximately 10% of respondents prescribed DAPT for 6 months only (all regimens and age groups), although there was variation in the duration of DAPT treatment in the ACS conservatively managed patient group, with DAPT prescribing ranging from 3 months to > 12 months. Among patients with stable angina undergoing PCI, the default DAPT regimen was AC (for ≈ 90% of patients across both age groups), with variation in duration of treatment ranging from 1 month to > 12 months. Fewer than 10% of stable angina patients were prescribed AT.
Antiplatelet prescribing practice for ACS patients who also need anticoagulants is shown in Table 6. The majority of respondents (63–70%) prescribe TT with AC to ACS patients undergoing PCI (STEMI, NSTEMI and unstable angina patients), with the duration of TT ranging from 1 to 6 months, although 1 month was most frequent. About one-third of respondents prescribe antiplatelet monotherapy, with the majority (80%) prescribing it for 12 months. Most respondents (84%) reported stopping aspirin when stepping down from TT to dual therapy; only 16% reported stopping the P2Y12 inhibitor.
| Patients, n/N (%) | ||||
|---|---|---|---|---|
| Antiplatelet regimen and duration of treatment | ACS STEMI (PCI) | ACS NSTEMI (PCI) | ACS unstable angina (PCI) | ACS conservatively managed |
| Monotherapy | 16/60 (27) | 20/65 (31) | 20/65 (31) | 36/58 (62) |
| 1 month | 1/16 (6) | 2/20 (10) | 1/20 (5) | 3/36 (8) |
| 3 months | 1/16 (6) | 1/20 (5) | 1/20 (5) | 2/36 (6) |
| 6 months | 0 | 0 | 1/20 (5) | 6/36 (17) |
| 12 months | 13/16 (81) | 16/20 (80) | 16/20 (80) | 24/36 (67) |
| > 12 months | 1/16 (6) | 1/20 (5) | 1/20 (5) | 1/36 (3) |
| AC | 42/60 (70) | 41/65 (63) | 41/65 (63) | 20/58 (34) |
| 1 month | 21/42 (50) | 21/41 (51) | 23/41 (56) | 12/20 (60) |
| 3 months | 16/42 (38) | 15/41 (37) | 13/41 (32) | 5/20 (25) |
| 6 months | 5/42 (12) | 5/41 (12) | 5/41 (12) | 2/20 (10) |
| 12 months | 0 | 0 | 0 | 1/20 (5) |
| > 12 months | 0 | 0 | 0 | 0 |
| AP | 0 | 0 | 0 | 0 |
| 1 month | 0 | 0 | 0 | 0 |
| 3 months | 0 | 0 | 0 | 0 |
| 6 months | 0 | 0 | 0 | 0 |
| 12 months | 0 | 0 | 0 | 0 |
| > 12 months | 0 | 0 | 0 | 0 |
| AT | 7/60 (12) | 4/65 (6) | 4/65 (6) | 3/58 (5) |
| 1 month | 5/7 (71) | 3/4 (75) | 3/4 (75) | 2/3 (67) |
| 3 months | 1/7 (14) | 1/4 (25) | 0 | 1/3 (33) |
| 6 months | 1/7 (14) | 0 | 1/4 (25) | 0 |
| 12 months | 0 | 0 | 0 | 0 |
| > 12 months | 0 | 0 | 0 | 0 |
For patients with conservatively treated ACS, antiplatelet monotherapy was most commonly prescribed (62% of respondents), followed by TT with AC (34% of respondents), mostly prescribed for 1–3 months. None of these patients was prescribed AP.
The patient factors that cardiologists take into account when prescribing DAPT are shown in Figure 2a. Patient age, use of concomitant anticoagulation, and whether or not a patient had a current, or had experienced a previous, bleed were considered important by 73%, 72% and 70% of respondents, respectively, followed by disease complexity (40%), anaemia (37%) and renal impairment (33%). About one-quarter considered peptic ulcer diseases, body mass index (BMI), diabetes and ACS risk score as important when prescribing DAPT for their patients.
FIGURE 2.
Patient, procedure- and presentation-related factors and blood test results that cardiologists consider important when prescribing DAPT to their patients. (a) Patient factors; (b) presentation-related factors; (c) blood test results; and (d) procedure-related factors. BMS, bare-metal stent; BVS, bioabsorbable vascular scaffold; CKD, chronic kidney disease; CRUSADE, Can rapid Risk stratification of Unstable angina patients suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guideline; DES, drug-eluting stent; ECG, electrocardiogram; GFR, glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; HAS-BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile international normalised ratio, elderly, drug/alcohol usage; HbA1c, glycated haemoglobin; IABP, intra-aortic balloon pump; ISR, in-stent restenosis; LV, left ventricular; ST, stent thrombosis; SYNTAX, SYNergy between PCI with TAXus and cardiac surgery; TIMI, thrombolysis in myocardial infarction.
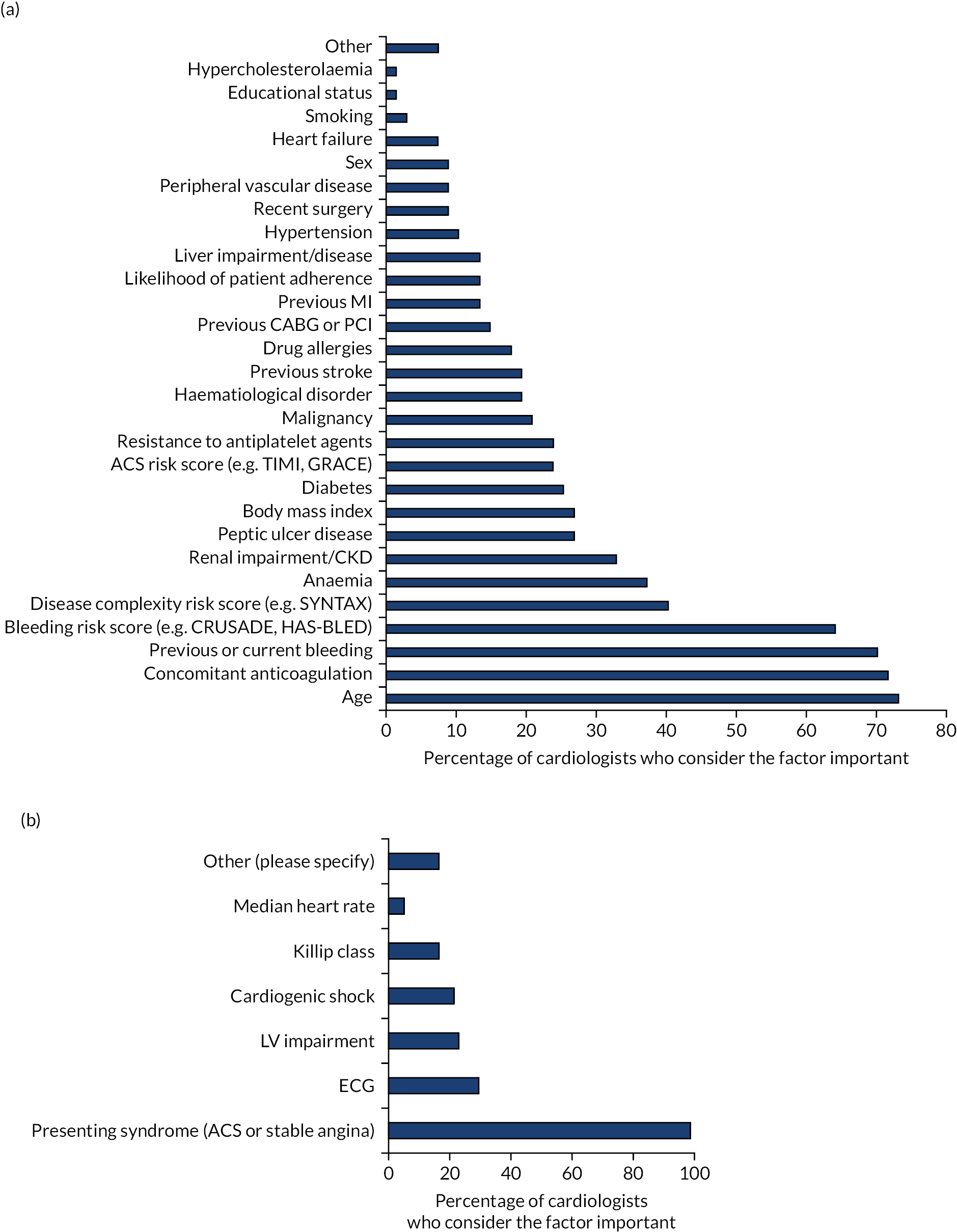
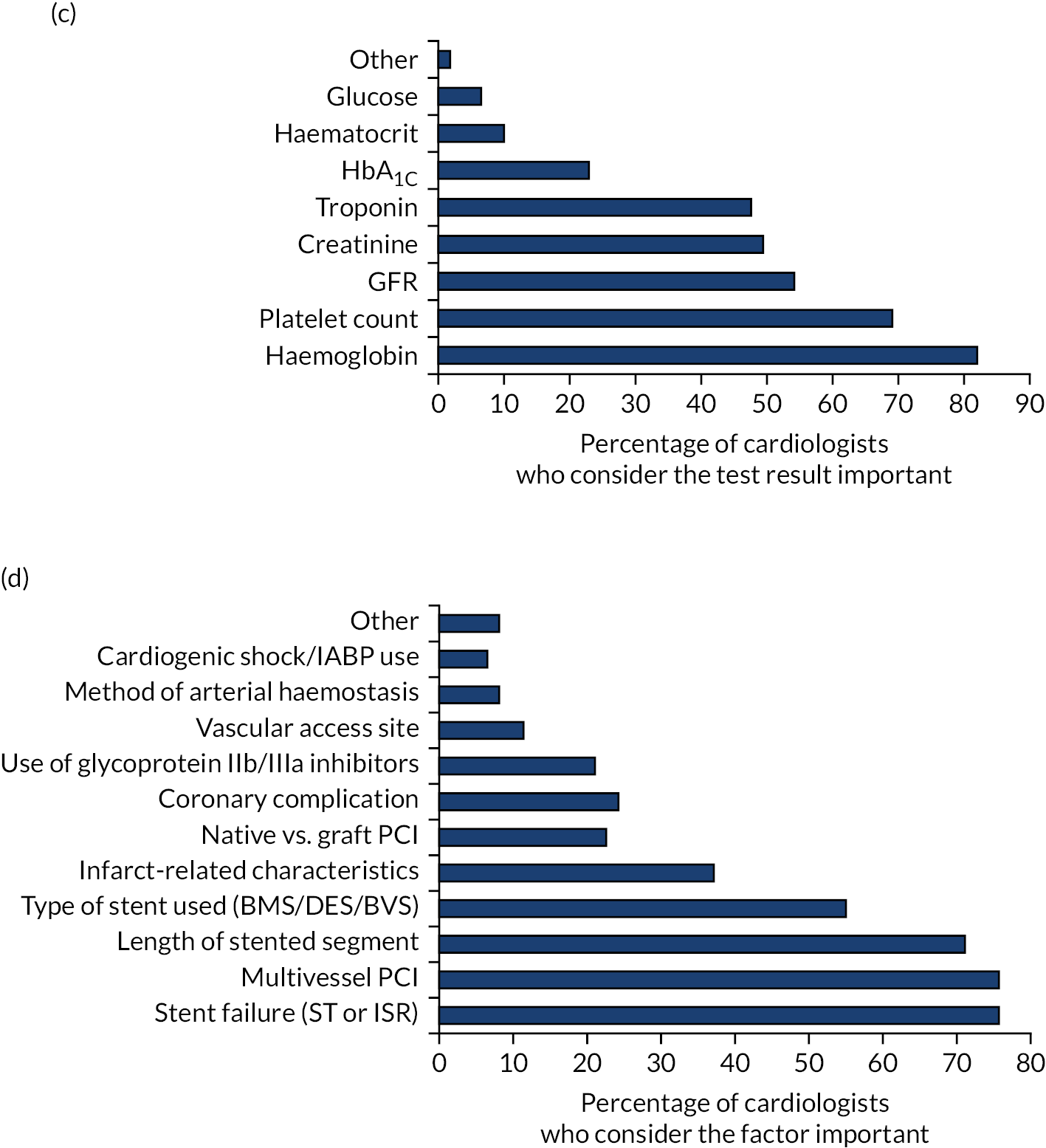
Presentation-related factors and blood test results that cardiologists consider when they prescribe DAPT are shown in Figure 2b and c, respectively. Presenting syndrome (ACS or stable angina) was the single most important presentation-related factor taken into consideration when prescribing DAPT (98% of all respondents). In terms of blood test results, haemoglobin (82%), platelet count (69%), glomerular filtration rate (GFR) (54%), creatinine (49%) and troponin (47.5%) were considered to be important.
Procedure-related factors that influence DAPT prescription are shown in Figure 2d. More than 70% of respondents thought that stent failure (76%), multivessel PCI (76%) and length of stented segment (71%) were important factors that influenced their DAPT prescription; 55% thought that type of stent used was important when prescribing DAPT. Just over one-third (37%) considered infarct-related characteristics, and about one-quarter considered coronary complications or whether a native or graft vessel had been stented.
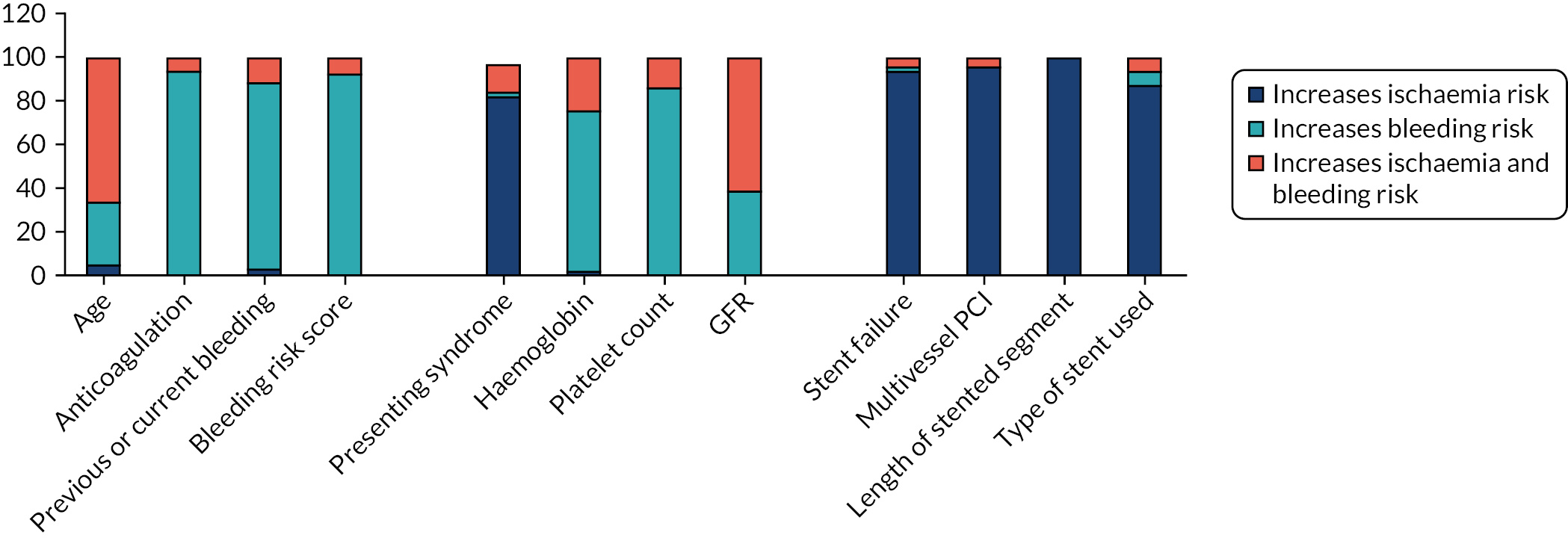
Respondents were asked to indicate whether or not the factors that influence their decision-making process when prescribing DAPT did so because of their association with ischaemia risk, bleeding risk, or both ischaemia and bleeding risk. Figure 3 shows the contribution of each risk (ischaemia, bleeding or both) to the decision-making process for the most commonly considered factors (i.e. those regarded as important by ≥ 50% of respondents). Figure 2 indicates that cardiologists consider bleeding and ischaemia risks equally when prescribing DAPT. Five of the 12 factors (presenting syndrome, ACS or stable angina, and those related to the PCI procedure) were chosenmainly on the basis that they increase ischaemia risk, 5 of the 12 (concomitant anticoagulation, previous or current bleeding, bleeding risk score, haemoglobin level and platelet count) were chosen mainly on the basis that they increase bleeding risk, and 2 of the 12 (age and GFR) were chosen mainly on the basis that they increase both bleeding and ischaemia risks.
FIGURE 3.
The contribution of each risk (ischaemia, bleeding or both) to the cardiologist decision-making process for the most commonly considered patient, presentation- and procedure-related factors (i.e. those regarded as important by ≥ 50% of respondents).
Survey results: cardiac surgeons
Table 7 shows antiplatelet prescribing for the various patient subgroups. Antiplatelets were commonly prescribed for the following patient subgroups: CABG and recent ACS (81%), CABG with poor vessel/conduit quality (71%) and CABG and previous stent (61%). Relatively few respondents prescribed DAPT as a substitute for vitamin K antagonist prophylaxis for CABG and tissue valve surgery (13%) and post-operative AF (3%).
| Respondents, n (%) | |||||
|---|---|---|---|---|---|
| Patient subgroup | Low-dose aspirin (75–150 mg) | High-dose aspirin (150–300 mg) | Low-dose AC (75 mg once per day) | Low-dose AT (90 mg twice per day) | Low-dose AT (60 mg twice per day) |
| CABG plus recent ACS | 7 (24) | 6 (21) | 15 (52) | 18 (62) | 3 (10) |
| CABG with poor vessel/conduit quality | 0 | 0 | 3 (10) | 4 (14) | 1 (3) |
| Off-pump CABG | 10 (35) | 18 (62) | 8 (28) | 6 (17) | 17 (58) |
| CABG plus tissue valve (as substitute for VKA prophylaxis) | 11 (38) | 4 (14) | 3 (10) | 2 (7) | 6 (21) |
| CABG plus previous stent | 1 (3) | 1 (3) | 0 | 0 | 2 (7) |
| Post-operative AF (as substitute for VKA prophylaxis) | 20 (69) | 2 (7) | 3 (10) | 2 (7) | 2 (7) |
For the CABG with recent ACS, CABG with poor vessel/conduit quality and CABG with previous stent patient subgroups, DAPT was the preferred treatment (76%, 79% and 86%, respectively). DAPT with clopidogrel was most frequently prescribed for the last two patient subgroups, although, for the CABG and recent ACS patient subgroup, respondents were equally likely to prescribe DAPT with ticagrelor. Low-dose aspirin was preferred for patients undergoing off-pump CABG (62% of respondents), patients undergoing CABG and valve surgery (76%) and patients with post-operative AF (76%). Just under two-thirds of respondents (18/29; 62%) never prescribe DAPT after surgery for patients who require thromboprophylaxis with warfarin or a NOAC, whereas the remainder (38%) prescribe DAPT only in very selected patients requiring warfarin or a NOAC.
The patient factors that cardiac surgeons take into account when prescribing DAPT are shown in Figure 4. Previous or current bleeding and previous CABG or PCI were considered important by 59% and 66% of respondents, respectively, followed by age, concomitant anticoagulation and bleeding risk score (48%). About one-third considered previous stroke and peptic ulcer disease, one-quarter considered disease complexity and one-fifth considered resistance to antiplatelet agents to be important.
FIGURE 4.
Patient factors that cardiac surgeons consider important when prescribing antiplatelet agents to their patients. CKD, chronic kidney disease; CRUSADE, Can rapid Risk stratification of Unstable angina patients suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guideline; GRACE, Global Registry of Acute Coronary Events; HAS-BLED, hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, labile international normalised ratio, elderly, drug/alcohol use; SYNTAX, SYNergy between PCI with TAXus and cardiac surgery; TIMI, thrombolysis in myocardial infarction.
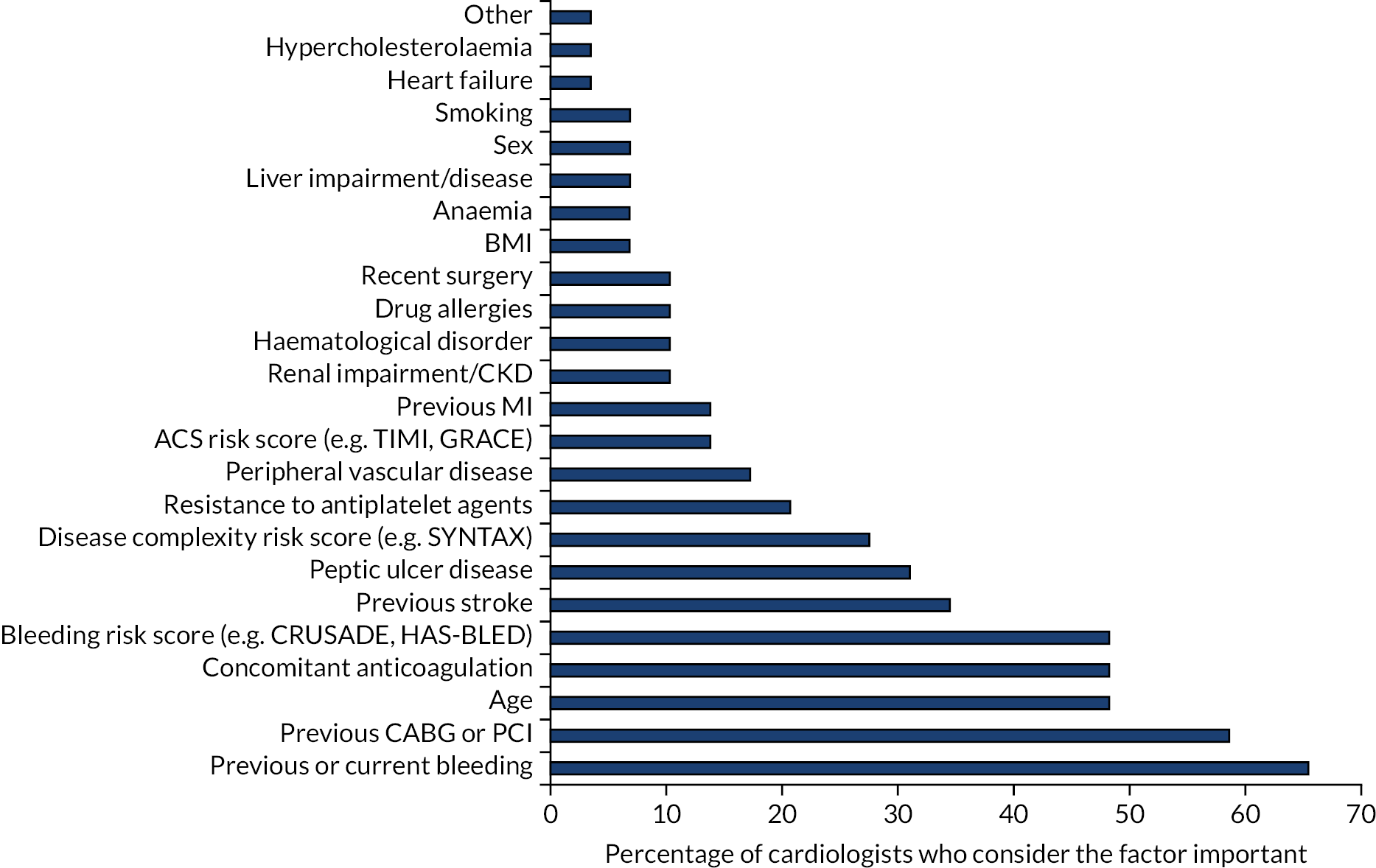
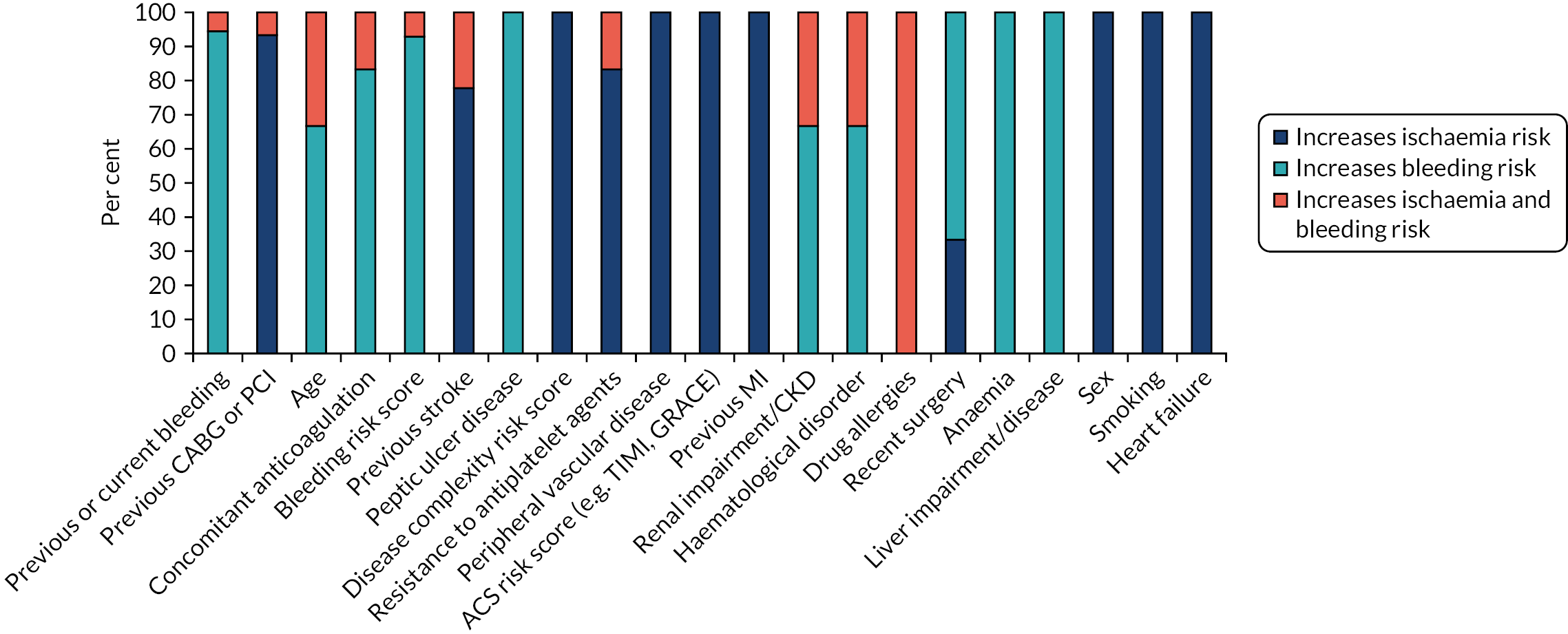
Respondents were asked to indicate whether or not the factors that influence their decision-making process when prescribing DAPT did so because of their association with ischaemia risk, bleeding risk, or both ischaemia and bleeding risk. Figure 5 shows the contribution of each risk (ischaemia, bleeding or both) to the decision-making process for all patient factors. Both bleeding and ischaemia risks were considered equally in the decision-making process, but concern about bleeding risk featured more prominently in the factors that > 48% of surgeons considered to be important when prescribing (previous or current bleeding, age, concomitant anticoagulation and bleeding risk score).
FIGURE 5.
The contribution of each risk (ischaemia, bleeding or both) to the cardiac surgeon decision-making process for the most commonly considered factors (i.e. those regarded as important by ≥50% of respondents). CKD, chronic kidney disease; GRACE, Global Registry of Acute Coronary Events; TIMI, thrombolysis in myocardial infarction.
Discussion
We identified 70 factors and 10 co-interventions by systematic review, clinician interview and clinician survey (Table 8). Of the 70 factors identified, 59 (84%) were identified by systematic review, 25 (36%) were identified by clinician interview and 46 (66%) were confirmed by clinician survey. Only 25 (36%) were identified by all three methods. The clinician interviews identified an additional 10 factors (14%) not identified by the systematic review (four were confirmed by clinician survey), including antiplatelet cost considerations, local/international prescribing guidelines, adherence issues among patients, clinician professional opinion and resistance to antiplatelet agents.
| Factors identified | Source | Confounder, cause of exposure, cause of outcome, none | Direction of effect for risk of bleeding |
|---|---|---|---|
| Demography (n = 7 ) | |||
| Older age | SR, I, CS | Confounder | Increases risk |
| Female sex | SR, CS | Confounder | Increases risk |
| Decreasing BMI | SR, I, CS | Confounder | Increases risk |
| South Asian ethnicity | SR | Confounder | Increases risk |
| Smoker | SR, CS | Cause of exposure | – |
| Lower educational level | SR | None | – |
| Family history of IHD | SR | None | – |
| Medical history (n = 5 ) | |||
| Previous MI | SR, I, CS | Cause of exposure | – |
| Previous CABG or PCI | SR, I, CS | Cause of exposure | – |
| Previous bleeding | SR, I, CS | Confounder | Increases risk |
| Dyspnoea | SR, I | None | – |
| Recent surgery | SR, CS | Confounder | Increases risk |
| Comorbidity (n = 16 ) | |||
| IHD | SR, I, CS | Cause of exposure | – |
| Diabetes | SR, I, CS | Confounder | – |
| Hypertension | SR, I, CS | Confounder | Increases risk |
| Hypercholesterolaemia | SR | Cause of exposure | – |
| Peripheral vascular disease | SR, I, CS | Cause of exposure | – |
| Stroke or TIA | SR, I, CS | Confounder | Increases risk |
| Heart failure | SR, CS | Confounder | Increases risk |
| Peptic ulcer disease | SR, CS | Confounder | Increases risk |
| Chronic kidney disease | SR, I, CS | Confounder | Increases risk |
| Cancer | SR, CS | Confounder | Increases risk |
| Haematological disorder | SR, CS | Confounder | Increases risk |
| AF/thrombosis/valve disease requiring warfarin or NOAC | SR, I, CS | Confounder | Increases risk |
| Anaemia | SR, I, CS | Confounder | Increases risk |
| Lung disease (e.g. COPD and asthma) | SR | None | – |
| Liver disease (e.g. cirrhosis) | SR, I, CS | Confounder | Increases risk |
| Gout | SR | None | – |
| Presentation risk (n = 6) a | |||
| ACS risk scores | SR, I, CS | Confounder | Increases risk |
| LV impairment | SR, I, CS | Confounder | Increases risk |
| Cardiogenic shock | SR, CS | Confounder | Increases risk |
| Killip class | SR, CS | Confounder | Increases risk |
| ECG | SR, I | Cause of exposure | – |
| Median heart rate | SR | None | – |
| Ischaemic/bleeding risk scores (n = 4) a | |||
| SYNTAX | SR, I, CS | Cause of exposure | – |
| CRUSADE | SR, I, CS | Confounder | Increases risk |
| HAS-BLED | SR, I, CS | Confounder | Increases risk |
| CHA2DS2-VASc | SR, I | None | – |
| Biochemical markers (proxies of disease) ( n = 7) a | |||
| Troponin (ACS) | SR, I, CS | Confounder | Increases risk |
| Glucose or HbA1c (diabetes) | SR, CS | Confounder | Increases risk |
| Creatinine or GFR (kidney disease) | SR, CS | Confounder | Increases risk |
| Haemoglobin or haematocrit (anaemia) | SR, CS | Confounder | Increases risk |
| Platelet count | SR, I, CS | Confounder | Increases risk |
| CRP or ESR (inflammation) | SR, CS | Cause of outcome | – |
| Leucocytes (infection, malignancy) | SR | None | – |
| Procedural risk (PCI) (n = 14)a | |||
| IABP use | SR, CS | Confounder | Increases risk |
| Total ischaemic time | SR | None | – |
| Clopidogrel loading dose | SR | Cause of outcome | Increases risk |
| Glycoprotein IIb/IIIa inhibitor use | SR | Confounder | Increases risk |
| Radial access site | SR | Cause of outcome | Decreases risk |
| Method of arterial haemostasis | SR, CS | Cause of exposure | – |
| Type of stent used (BMS vs. DES) | SR, I | None | – |
| Length of stented segment | SR, I, CS | Cause of exposure | – |
| Stent failure | SR, I, CS | Cause of exposure | – |
| TIMI flow pre/post procedure | SR | None | – |
| Multivessel PCI | SR, I, CS | Cause of exposure | – |
| Native vs. graft PCI | SR, CS | Cause of exposure | – |
| Infarct-related characteristics (no reflow/reduced TIMI flow/MVO) | SR, CS | Cause of exposure | – |
| Coronary complication (perforation, dissection) | SR, CS | Confounder | Increases risk |
| Other (n = 11) a | |||
| Drug potency | I | Confounder | – |
| Drug allergies | I, CS | Cause of exposure | – |
| Resistance to antiplatelet agents | I, CS | Confounder | Decreases risk |
| Adherence to clinical guidelines | I, CS | None | – |
| Commissioning and organisation budget policy | I | Cause of exposure | – |
| Local DAPT prescribing culture | I | Cause of exposure | – |
| MDT opinion | I | Cause of exposure | – |
| Adherence-related factors | I, CS | Confounder | Decreases risk |
| Patient views and preferences | I | Cause of exposure | – |
| Individual clinician professional opinion | I | Cause of exposure | – |
| Conflicts of interest and pharmaceutical company influence | I | Cause of exposure | – |
| Co-interventions (n = 10) | |||
| Statin | SR | None | – |
| Beta-blocker | SR | None | – |
| ACE-I | SR | None | – |
| Calcium channel blocker | SR | None | – |
| Diuretic | SR | None | – |
| RAS | SR | None | – |
| NSAIDs | SR | Confounder | Increases risk |
| Steroids | SR | Confounder | Increases risk |
| Co-intervention | SR | None | – |
| Statin | SR | Confounder | Increases risk |
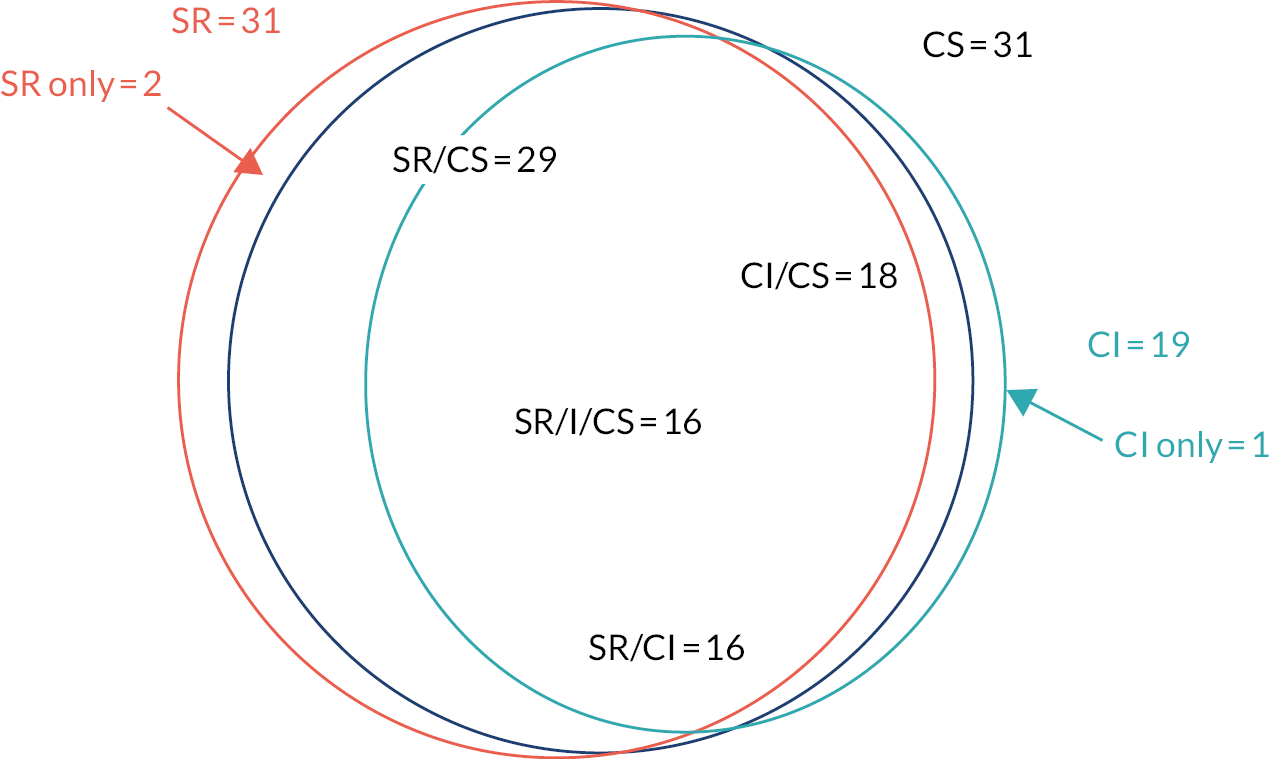
Only 34 out of 70 (49%) of the factors identified were classified as true confounders (factors that influence both DAPT prescribing and risk of bleeding). The decision regarding classification of potential confounders as true confounders was based on survey results and clinician expertise in the research team. The overlap between systematic review, clinician interview and clinician survey is shown in Figure 6. Of the 34 true confounders, no data were available to characterise 17 (50%).We had data to identify all demographic (n = 4), medical history (n = 2) and comorbidity (n = 11) confounders, but not all presentation risk (n = 4), risk score (n = 2), biochemical marker (n = 5) procedural risk (n = 3) and other factor (n = 3) confounders. We also identified 10 co-interventions from the systematic review. Of these, only three (judged to influence both what antiplatelet regimens a patient might receive and bleeding risk) were classified as true confounders.
FIGURE 6.
Overlap of 34 true confounders between those identified by SR, CI and CS. CS, clinician survey; I, interview; SR, systematic review.
We did not attempt to classify the factors we identified as potential confounders into confounding domains, that is domains that can be characterised by measuring one or more of a range of the identified variables. Such an approach is logical and could reduce the number of covariates used for statistical adjustment, given that many of these will be highly correlated. For example, bleeding risk could, in theory, be identified from several factors: previous bleed; increasing age; presence of anaemia; biomarkers such as haemoglobin, haematocrit and platelet count, etc. However, we were not certain if individual variables that might be grouped within a domain such as bleeding risk would generate an equal amount of bias nor if all are equally valid and reliable measures of the bleeding risk confounding domain. Classification into domains would require further input from cardiologists and cardiac surgeons, which was beyond the scope of this project. Nevertheless, we chose not to include biochemical markers as confounders in the statistical models, given that they are proxies of diseases captured through comorbidities.
The process of identifying confounders was systematic. There was good agreement between the three methods used (i.e. systematic review, clinician interviews and clinician surveys). The clinician interviews identified hard-to-measure factors not identified in the systematic review, such as clinician concerns regarding patient adherence; patient preferences; cost; the influence of local protocols and guidelines on prescribing practice; and patient drug allergies or resistance to medication. Some of these factors may influence eligibility criteria in RCTs and lead to the exclusion of certain patients (e.g. those deemed not likely to comply with medication regimen, or those with drug allergies or resistance to antiplatelets).
The inclusion of clinician interviews and surveys alongside the systematic review identified the main factors that influence bleeding risk and confirmed that similar risk factors influence both ischaemic and bleeding risk. Reliance on the literature only may be misleading; for example, in our review, most of the studies used for data extraction had ischaemic end points as their primary outcome [e.g. major adverse cardiovascular events (MACEs)]. It is, therefore, plausible that some of the variables reported in the descriptive tables or adjusted for in the statistical analyses of these studies influenced ischaemic outcomes, but not bleeding. This highlights the importance of using multiple sources of information for identifying confounders. The research team included broad expertise (clinical, epidemiological, qualitative methods, survey design), which contributed to the cohesiveness of the study and its findings.
We did not attempt to select factors on the basis of causality/understanding of underlying mechanisms or consideration of clinician behaviour, mainly because we do not have full knowledge of the structure of the causal diagram that relates all covariates to each other and to the DAPT prescription and risk of bleeding. Therefore, we cannot be certain that the covariates we selected as true confounders would be sufficient to control for confounding bias. 23
There is currently no guidance on how to extract data on confounders using literature review, given the variety of study designs potentially eligible for inclusion (e.g. RCTs, prospective/retrospective cohort studies/registries, some descriptive and some comparative, prognostic/risk prediction studies). Non-randomised studies in particular have different designs, different and inconsistent methods of reporting, and often do not justify their rationale for statistical adjustment. 24 Given these issues and the lack of guidance, we took a broad approach to data extraction and included every factor considered by the authors of these studies as a potential confounder for our study.
All of the studies from which we extracted data included only cardiology populations. There are few RCTs testing antiplatelet regimens among cardiac surgery populations; none of these was included in our randomly generated list for data extraction. Cardiac surgery patients have the same underlying disease and, therefore, should have the same risk factors for bleeding. However, although the factors influencing the decision-making process identified by the clinician interviews were similar between cardiac surgeons and cardiologists, our surveys highlighted some differences between the two clinician groups in the decision-making process for antiplatelet prescribing. Moreover, procedural risk factors are different for PCI (cardiology) and CABG (cardiac surgery), although we had no data on any of these factors, so they represent unmeasured confounding.
Chapter 3 The ADAPTT study
Methods
The protocol for the ADAPTT study has been published. 25
Data sources
The CPRD is a database of primary care electronic health record data (available online via CPRD GOLD) from participating general practices, covering 7% of the UK population. 26 Patients included in CPRD are largely representative of the UK population in terms of age, sex, ethnicity and BMI. HES cover all hospital admissions for all English patients whose treatment is funded by the NHS, whether treated by the NHS or by independent providers. 27 Seventy-five per cent of English general practices included in CPRD are linked to HES data. 26 We obtained data from 1 April 2009 to 31 July 2017; this period covers the introduction of the newer antiplatelet agents prasugrel and ticagrelor. The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (protocol number 16_126R).
Study populations
We initially specified three target trials for (1) patients undergoing CABG, (2) patients hospitalised and conservatively managed for ACS and (3) patients undergoing PCI. Eligibility and exclusion criteria for the three target trials are listed in Table 9. However, for the purpose of the statistical analysis, patients undergoing PCI were separated into emergency PCI and stable PCI, as these represent different populations: patients undergoing emergency PCI have ACS, which is associated with poorer short-term prognosis than PCI for stable coronary artery disease. Some analyses in the emergency PCI population were restricted to the STEMI population only.
| PICO component | Target trial | Issues in emulating the target trial using observational data |
|---|---|---|
| Eligibility criteria | Target trial 1 (CABG) Consecutive patients (aged ≥ 18 years) undergoing CABG (urgent and elective). Exclusions: DAPT or anticoagulant use in the previous 3 months; other concomitant cardiac surgery (e.g. valve surgery); major bleed necessitating hospitalisation in previous 1 year; renal failure necessitating dialysis; intolerance/allergy to aspirin, clopidogrel, prasugrel or ticagrelor |
CPRD–HES linked data set contains information that allows us to identify all eligible patients for the three target trials. The study period is April 2009 to July 2017. All eligible patients will have sufficient data (1 year) preceding their index event to apply the exclusion criteria and characterise the population (e.g. comorbidities) and sufficient follow-up data (1 year) to identify outcomes. It is not possible to capture intolerance/allergy to aspirin, clopidogrel, prasugrel or ticagrelor |
| Target trial 2 (conservatively managed ACS) | ||
| Consecutive patients (aged ≥ 18 years) hospitalised for ACS: MI with or without ST-elevation or unstable angina. Exclusions: PCI or CABG performed at time of ACS diagnosis; major bleed necessitating hospitalisation in previous 12 months; renal failure necessitating dialysis; intolerance/allergy to aspirin, clopidogrel, prasugrel or ticagrelor | ||
| Target trial 3 (PCI) | ||
| Consecutive patients (aged ≥ 18 years) undergoing PCI (emergency or elective). Exclusions: DAPT or anticoagulant use in the previous 3 months; major bleed necessitating hospitalisation in previous 12 months; renal failure necessitating dialysis; intolerance/allergy to aspirin, clopidogrel, prasugrel or ticagrelor | ||
| Interventions | Target trial 1 (CABG) Clopidogrel (75 mg) in addition to aspirin (at a dose of 75 mg daily, in line with current guidelines) or aspirin only (any dose, reflecting variation in usual care) | Relevant interventions can be identified as CPRD has information on all medications (including doses) prescribed in primary care |
| Target trial 2 (conservatively managed ACS) | ||
| As for target trial 1 | ||
| Target trial 3 (PCI) | ||
| Clopidogrel (75 mg daily) or prasugrel (5 mg or 10 mg daily) or ticagrelor (90 mg twice daily). All patients will receive aspirin (at a dose of 75 mg daily, in line with current guidelines) | ||
| Assignment to interventions | Participants are assigned to DAPT interventions in hospital | Participants enter the study at index procedure date for PCI and CABG, and episode start date for ACS, and will be assigned to DAPT interventions using first prescription in CPRD (within 2 months of hospitalisation) as a proxy for what they were prescribed in hospital (there are no medications data in HES). This assignment will exclude a proportion of eligible patients (those who died or experienced a major bleed that caused them to stop DAPT, or patients who have no prescription for DAPT within the 2-month window); we will identify and describe the characteristics of these excluded patients |
| In sensitivity analyses, we will address the robustness of results to different assumptions about the intervention group among those patients for whom the DAPT medication is unknown or a major bleed occurs prior to the first DAPT medication, by using multiple imputation models for handling missing data. Prior known information regarding the likely prescription based on patient characteristics or general policies will be incorporated in these analyses | ||
| Follow-up | Starts at assignment to intervention and ends at first bleed or 1 year from assignment (whichever comes first) | Starts at time of hospitalisation for PCI, CABG or ACS and ends at first bleed or 12 months from hospitalisation (whichever comes first) |
| Primary outcome | Any bleed within 12 months of the start of DAPT (DAPT is prescribed at hospitalisation for PCI, CABG or ACS) | Any bleed within 12 months of hospitalisation for PCI, CABG or ACS |
| Analysis | Intention to treat | According to first prescription for DAPT in CPRD |
Patients were included if they had a PCI, CABG or ACS diagnosis (index event) recorded in HES during the study period (1 April 2010 to 31 January 2017) and had at least 1 year of linked CPRD–HES data before the date of their index event. They must also have been prescribed one of the treatment regimens being compared in the target trial corresponding to their index event. One year’s data preceding eligibility for the target trial is adequate to apply most of the exclusion criteria and determine comorbidities and medication history; such information would be collected at baseline in a randomised trial. The following Office of Population Censuses and Surveys (OPCS) procedure codes (PCI and CABG) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), codes (ACS no procedure) were used to identify patients: PCI: K49, K50 and K75; CABG: K40, K41, K42, K43, K44, K45 and K46; ACS without a procedure: I20.0, I21, I22, I24.9 with no OPCS code for PCI or CABG in the same hospital admission; PCI STEMI: K49, K50 and K75 and I21.0-I21.3 or I22.0–I22.8 as primary or secondary diagnosis.
Interventions
The interventions of interest for the three target trials are shown in Table 9. Guidelines recommend low-dose aspirin (75–100 mg per day) plus either clopidogrel (75 mg per day), prasugrel (5 mg or 10 mg per day) or ticagrelor (90 mg twice per day) for PCI and conservatively managed ACS patients. For PCI patients, the interventions of interest were AC, AP and AT. In conservatively managed ACS patients, clopidogrel is the most commonly prescribed second antiplatelet agent (in addition to aspirin), and a large proportion of patients are prescribed aspirin only; therefore, the interventions of interest are aspirin only (any dose) and AC. There is variation in aspirin prescription for CABG patients (75–300 mg per day). Surgeons may also prescribe an additional antiplatelet agent, most commonly clopidogrel. Therefore, the comparisons of interest for CABG patients are aspirin only (any dose, reflecting variations in usual care in different hospitals) and AC (75 mg per day).We specified these comparisons based on preliminary feasibility counts from the CPRD, which showed that few CABG and conservatively managed ACS patients are prescribed AP or AT. Product codes for the antiplatelets are detailed in Appendix 5.
In the target trials, the interventions would be assigned during the hospital stay, as soon as patients were eligible for antiplatelet therapy. Our observational data set does not have information on medication given to patients at discharge because HES does not include medications data. Therefore, the first time at which we have information on the antiplatelet regimen to which patients were assigned in hospital is when they receive their first primary care prescription(s) for aspirin or DAPT, recorded in the CPRD.We used the first prescription in the CPRD as a proxy for the medications that patients started in hospital. This is justified because the qualitative study with clinicians (see Chapter 2) supported the assumption that GPs are unlikely to change the prescriptions that were started in hospital.
We classified patients according to the first prescription recorded in the CPRD in the first 2 months after hospitalisation for PCI, CABG or ACS. This 2-month window was based on variability in the amount of DAPT medication provided to patients in hospital following their PCI, CABG or ACS treatment, and hence variability in the time when they first requested a repeat prescription from their general practice. A preliminary investigation showed that > 75% of eligible patients had a prescription for one or more antiplatelet agents during this time period. Patients were assigned to intervention groups as follows: (1) if a patient had a prescription only for aspirin during the 2-month window after hospital discharge, they were assigned to an aspirin-only intervention; (2) if a patient also received a prescription for clopidogrel, prasugrel or ticagrelor, they were assigned to AC, AP or AT; and (3) if there was a prescription for more than one additional antiplatelet agent in the 2-month window, the patient was assigned to an intervention based on the agent prescribed first.
For example, if a patient had an aspirin prescription and a prescription for clopidogrel before a prescription for ticagrelor, the patient was assigned to the AC intervention. Patients with no prescriptions in the CPRD for aspirin or AC, AP or AT within the 2-month window were excluded from the main analysis. Patients who experienced a major bleed or a MACE prior to the first antiplatelet prescription(s) occurring in the CPRD within 2 months of the index event were also excluded from the analysis because we could not assume that the antiplatelet prescription observed in the CPRD would be the same as that assigned in hospital at the time of the index event. Both these groups of patients (those with no antiplatelet prescriptions and those who experienced an event) were included in a sensitivity analysis in which assignment to DAPT was performed using multiple imputation for missing data based on propensity scores; see Sensitivity analysis 1: multiple imputation for unknown intervention group.
Outcomes
The primary outcome was any bleeding event. For each patient, we identified all bleeding events in HES and the CPRD during follow-up (365 days after the index event). We originally planned to classify bleeding events according to the Bleeding Academic Research Consortium (BARC) bleeding scale;28 however, the data sets did not contain all of the information required to allow BARC classification. We have specified a comprehensive list of bleeding codes in the CPRD and HES (see Appendix 6). These were categorised according to anatomical site for descriptive purposes. Secondary outcomes were as follows: any major bleeding event, any minor bleeding event, all-cause mortality, cardiovascular mortality, mortality from bleeding (these would be identified from linked Office for National Statistics data), MI, stroke, additional coronary intervention and MACEs (defined as any of MI, stroke, cardiovascular mortality or additional coronary intervention). Major bleeding events were defined as any HES bleed, and minor bleeding events were defined as any CPRD bleed without any HES bleed being recorded within 28 days (i.e. ± 14 days of the CPRD bleeding event). This was to ensure that the record in the CPRD is not a duplicate count of a HES bleeding event, as the CPRD records GP-reported bleeds and may also record bleeds leading to hospital admission.
Follow-up
The start of follow-up (the index event) was the date of the index hospital procedure (PCI and CABG groups) or the start date of the hospital episode that contained the ACS diagnosis (ACS group). Patients were followed up for 365 days after the index event.
Confounding and co-interventions
Confounders (variables that predict both risk of bleeding and intervention group) were specified a priori29,30 (see Chapter 2). Read, ICD-10 and product code lists were prepared to identify all confounders, either from published sources31 or created by the study team [methodologists familiar with Read (CPRD) and ICD-10 (HES) coding systems and clinicians]. Code lists for the confounders are shown in Appendix 7.
Sample size
The estimated rates of any bleeding with the different therapies are 5% for aspirin, 9% for AC and 12% for AP and AT. 6,7,32,33 We used preliminary feasibility counts provided by the CPRD to identify numbers of patients eligible for each target trial: (1) PCI: AC (reference: 6738 patients) versus AP (842 patients) or AT (770 patients), (2) CABG: aspirin (reference: 2556 patients) versus AC (595 patients) and (3) conservatively managed ACS: aspirin (reference: 8148 patients) versus AC (3082 patients). These estimates gave expected numbers of bleeding events of at least 700 for PCI, 180 for CABG and 680 for ACS, assuming ratios of 8 : 1 (AC : AP or AC : AT) for PCI, 4 : 1 (aspirin : AC) for CABG and 2.5 : 1 (aspirin : AC) for ACS. The HRs detectable with 90% and 80% power at 5% statistical significance, assuming the group ratios given previously, are shown in Table 10. The correlation of the DAPT with other covariates adjusted for was unknown and we assessed the impact of a range of correlations (0, 0.3 and 0.5).
| Ratio of presence: absence of covariate | Squared correlation with other covariates | HR detectable | |
|---|---|---|---|
| 90% power | 80% power | ||
| PCI | |||
| 8 : 1 | 0 (i.e. unadjusted) | 1.48 | 1.41 |
| 0.3 | 1.60 | 1.50 | |
| 0.5 | 1.74 | 1.62 | |
| CABG | |||
| 4 : 1 | 0 (i.e. unadjusted) | 1.83 | 1.69 |
| 0.3 | 2.06 | 1.87 | |
| 0.5 | 2.35 | 2.10 | |
| Conservatively managed ACS | |||
| 2.5 : 1 | 0 (i.e. unadjusted) | 1.32 | 1.27 |
| 0.3 | 1.39 | 1.33 | |
| 0.5 | 1.48 | 1.40 | |
Statistical analyses
We examined temporal changes in DAPT prescribing and bleeding for PCI, CABG and ACS populations between 2010 and 2017. Descriptive statistics were used to summarise the characteristics of the different intervention groups and standardised mean differences (SMDs) were used to compare them. We estimated the rates of any bleeding (number of events/person-time) with 95% confidence intervals (CIs) for each group. We separated major (leading to hospital admission, i.e. HES inpatient data) and minor (CPRD) bleeding because adverse events of each type have different health and resource use consequences. We censored all bleeds at the GP transfer-out date or last collection date, thereby ignoring any bleeds in the HES data set recorded after this period.
Data-cleaning and dealing with missing data
The most recent record of smoking status and BMI were used; data-cleaning rules suggested by Atkinson et al. 34 were used for smoking and rules suggested by Bhaskaran et al. 35 were used for BMI. All data, such as prescription dates, recorded after the date of death were set to missing. For binary variables, we took no record of a code in the data sets to mean absence of event. We examined all non-binary variables for missing data. Smoking and BMI were missing 4% and 8% of values, respectively, for the emergency PCI group; 1% and 5%, respectively, for the CABG group; and 4% and 7%, respectively, for the ACS group. These missing data were replaced with age- and sex-adjusted averages.
Comparative analysis
Analyses estimated the effects of assigned intervention (analogous to an intention-to-treat analysis of a randomised trial) for the antiplatelet regimens corresponding to the first prescription of aspirin or DAPT in the CPRD (see Interventions). For each target trial, we calculated propensity scores for the comparative antiplatelet regimens using a backward stepwise logistic regression with significance level for removal from the model set at 0.25. For the emergency PCI target trial (2012–17), we report only results pertaining to AT versus AC, as AP is almost exclusively prescribed for STEMI patients. The AC versus AP versus AT analysis was performed in the STEMI population only, using a multinomial logistic regression to accommodate the three interventions (AC, AP and AT).
All confounders identified as possibly being related to the bleeding outcome were included in these stepwise models. Criteria for excluding tails of propensity score distributions were decided by reviewing the bleeding events between interventions, based on cut-off points of the propensity score at fifth, 25th, 50th, 75th and 95th percentiles. We excluded subjects in the extreme tails of the propensity score distribution (lower fifth percentile of propensity scores) only for CABG analyses when few patients or deaths in either intervention were observed. There was good overlap of covariate distributions for emergency PCI, STEMI PCI and conservatively managed ACS. All subsequent analyses were based on data with these tails excluded (CABG analyses only). This made it more likely that analyses were restricted to patients eligible to receive either intervention. Kaplan–Meier curves were generated after adjusting by the inverse probability of treatment weights using the propensity scores,36 where the weights were defined as 1/propensity score for the treatment received.
We used Cox regression models to estimate crude and adjusted HRs with 95% CIs for the time to first bleeding event, comparing intervention groups for each target trial. Participants free from a bleeding event were censored at 12 months after the index event. For each target trial, we adjusted for all potential confounders identified in Chapter 2 and the propensity score. 37,38 All continuous variables (calendar year, age, BMI and propensity scores) were included in models as cubic splines with knots set at the 25th and 75th percentiles. Visual assessments of these splines were undertaken to check that these were appropriate. Confounders were included using a backward stepwise approach with significance level for removal from the model set at 0.25, and additionally adjusted for propensity scores. The intervention group was included in all models. We could not formally compare interventions among the stable PCI patients because there was no variability in treatment: > 93% were prescribed AC.
For all secondary end points, we used survival models to estimate adjusted HRs with 95% CIs for time to first event, as detailed previously. For mortality outcomes, we adjusted for a smaller list of confounders (year, age, sex, BMI, ethnic group, smoking, Charlson Comorbidity Index score and propensity scores). 39 The Charlson Comorbidity Index score was calculated separately using Read codes and ICD-10 codes for the year prior to the index event. The Read codes were extracted from Khan et al. 40 and the ICD-10 codes were extracted from Maringe et al. 41 For both the Read codes and the ICD-10 codes, each diagnosis was considered only once and the Charlson Comorbidity Index score was calculated by adding the scores associated with these diagnoses. The final Charlson Comorbidity Index score was taken as the higher value calculated via Read codes and ICD-10 codes for each patient. In the very few instances [20 (0.2%) emergency PCI patients and three (0.1%) ACS patients] for which there were no GP data or HES data in the preceding year, the Charlson Comorbidity Index score was set to zero.
Sensitivity analyses
Sensitivity analysis 1: multiple imputation for unknown intervention group
An intervention could not be derived from prescription data for some eligible patients (i.e. those who died before receiving their first prescription, had a major bleed or further ACS event that may have caused them to change antiplatelet regimen, or had no aspirin/DAPT prescription recorded in the CPRD within the 2-month window). Instead, an intervention was assigned, using multiple imputation methods, based on the propensity scores calculated from the main analysis populations. Twenty randomly generated values from a uniform distribution were generated and each of these was used to assign 20 imputed interventions. If the propensity score was smaller than the uniform generated value, then the patient was placed in the reference intervention group; if it was greater, they were placed in the more potent intervention group (AP or AT). The estimated bleeding risks by intervention were then pooled across the 20 data sets using Rubin’s rules. 42 This approach was modified for the emergency PCI and STEMI PCI populations by including propensity scores for all three interventions.
Sensitivity analysis 2: exclusion of patients who changed medication before first observed bleeding event
This sensitivity analysis aimed to address the possibility that some minor bleeding events were not documented in the CPRD, but nevertheless prompt medication changes. We specified a priori that this sensitivity analysis would be undertaken only if > 10% of people changed medication before their first observed bleeding event. We investigated the proportions of people who changed medication before their first recorded bleed in the CPRD and HES; these were 3% for the emergency PCI, 3% for the STEMI PCI, 4% for the conservatively managed ACS and 3% for CABG populations. Therefore, this sensitivity analysis was not performed.
Sensitivity analysis 3: restricted to patients at low risk of bleeding
This sensitivity analysis excluded patients at high risk of bleeding;43 we hypothesised that restricting to a subpopulation of patients at low risk of bleeding would result in the lowest risk of residual confounding. Excluded patients were those with stage 4/stage 5 chronic kidney disease, anaemia, a clotting disorder, cancer (excluding non-melanoma skin cancer), liver cirrhosis with portal hypertension, stroke or recent surgery within the preceding 30 days.
Sensitivity analysis 4: repeating primary outcome analysis without censoring of any Clinical Practice Research Datalink or Hospital Episode Statistics bleed at transfer out or last collection date
This sensitivity analysis assessed whether or not censoring at first bleeding event or death, rather than at the GP transfer-out date or last collection date, had any impact on the results. We, therefore, included all HES bleeds that occurred after a patient had transferred out of a general practice or that occurred after the last collection date for that general practice.
Sensitivity analysis 5: instrumental variable analysis
This sensitivity analysis was proposed as a method of controlling for confounding by indication. We tested the feasibility of prescribing preference of the treating physician as the potential instrument in the instrumental variable analysis. 44,45 The prescribing preference of the treating physician was derived from the first prescription written in primary care, as GPs typically represcribe the treatment prescribed in hospital. The treatment assigned to the previous patient eligible for inclusion in the same target trial and seen by the same physician was identified by the method described above; when there was more than one patient with a relevant procedure or diagnosis on any given day, the previous patient was selected at random.
Subgroup analyses
For each subgroup analysis, the main primary outcome analysis (adjusted by propensity scores and all selected confounders) was repeated including an interaction term for the subgroup. The following subgroups were investigated: ACS versus non-ACS (CABG population), defined by the presence or absence of a diagnosis of ACS during the same continuous inpatient spells as the CABG procedure; diabetes versus non-diabetes, defined by the presence or absence of a diagnosis of diabetes in the year prior to the index procedure/event; chronic kidney disease versus non-chronic kidney disease, defined by the presence or absence of a diagnosis of kidney disease in the year prior to the index procedure/event; concurrent prescription for proton pump inhibitors (PPIs), defined by the presence or absence of a prescription for PPIs in the window between discharge after the index event and 2 months (61 days) later. The codes used to identify subgroups were the same as in the confounder coding process.
Treatment switches and adherence
Treatment switch/discontinuation was defined as starting a second antiplatelet or stopping aspirin in the aspirin group or stopping clopidogrel or aspirin in the AC group. Starting a second antiplatelet was defined as a patient receiving at least one prescription for a second antiplatelet during follow-up. Stopping clopidogrel or aspirin was defined as a gap between repeat prescriptions of > 1.5 times the number of days’ supply of the last prescription.
Adherence was defined using the medication possession ratio (MPR). 46 The MPR was calculated as the total number of days of available medication (quantity of drug prescribed divided by the daily dose) divided by 12 (12 months of follow-up). For the AC group, the overall MPR was calculated as the average MPR of AC. Non-adherence was defined as a MPR of < 80%. 47
Statistical analyses were undertaken using Stata® 15.1 (StataCorp LP, College Station, TX, USA).
Coronary artery bypass grafting
Trends in antiplatelet prescribing and bleeding over time
Figure 7 shows the trends in prescription of antiplatelet therapy between 2010 and 2017. Prescriptions with aspirin monotherapy decreased (from 70.9% in 2010 to 52.2% in 2017), whereas prescriptions of DAPT with clopidogrel increased (from 13.2% in 2010 to 29.0% in 2017). Prescriptions of P2Y12 monotherapy were stable over time (average 3.5%) and a proportion of patients (average 12.9%) received no antiplatelet therapy. Rates of bleeding did not change markedly over time; on average, the rates of major and minor bleeds were 18.0 and 38.3 events, respectively, per 1000 person-years.
FIGURE 7.
Proportion of CABG patients being prescribed different antiplatelet regimes by year and rates of major (HES), minor (CPRD) and total bleeding events by year among CABG patients. (a) Different antiplatelet regimes; and (b) bleeding events.
The total number of bleeds (major and minor bleeds combined) appeared to increase slightly over time, consistent with the increase in the proportion of patients receiving AC.
Figure 8 shows how the CABG target trial was constructed from the available data. There were 5335 CABG patients in the linked CPRD–HES data set, and 2783 (52%) of them were eligible for inclusion in the target trial (Table 11). Of these, 482 (17%) were excluded because they had no antiplatelet prescription data in the first 2 months after hospital discharge, leaving 2301 (83%) included in the primary analysis.
| Characteristics | Aspirin (N = 1702) | AC (N = 599) | SMD | Unknowna (N = 482) | Overall (N = 2783) | SMDb |
|---|---|---|---|---|---|---|
| Demography | ||||||
| Year of event, n (%) | ||||||
| 2010/11 | 392 (23) | 73 (12) | 0.35 | 101 (21) | 566 (20) | 0.09 |
| 2011/12 | 350 (21) | 99 (17) | 92 (19) | 541 (19) | ||
| 2012/13 | 305 (18) | 124 (21) | 103 (21) | 532 (19) | ||
| 2013/14 | 255 (15) | 105 (18) | 74 (15) | 434 (16) | ||
| 2014/15 | 211 (12) | 93 (16) | 60 (12) | 364 (13) | ||
| 2015/16 | 117 (7) | 65 (11) | 34 (7) | 216 (8) | ||
| 2016/17 | 72 (4) | 40 (7) | 18 (4) | 130 (5) | ||
| Age (years), mean (SD) | 67.6 (9.3) | 66.3 (9.8) | 0.13 | 67.0 (11.3) | 67.2 (9.8) | 0.02 |
| Sex, n (%) | ||||||
| Male | 1375 (81) | 508 (85) | 0.11 | 387 (80) | 2270 (82) | 0.04 |
| Female | 327 (19) | 91 (15) | 95 (20) | 513 (18) | ||
| BMIc (kg/m2), mean (SD) | 28.7 (4.6) | 28.3 (4.4) | 0.08 | 28.9 (4.8) | 28.7 (4.6) | 0.06 |
| Ethnic group, n (%) | ||||||
| White | 1599 (94) | 524 (87) | 0.22 | 444 (92) | 2567 (92) | 0.01 |
| Other than white | 103 (6) | 75 (13) | 38 (8) | 216 (8) | ||
| Smoking category,d n (%) | ||||||
| Ex-smoker | 747 (44) | 245 (42) | 0.07 | 202 (43) | 1194 (44) | 0.02 |
| Non-smoker | 694 (41) | 245 (42) | 197 (42) | 1136 (41) | ||
| Smoker | 242 (14) | 96 (16) | 74 (16) | 412 (15) | ||
| Medical history , n (%) | ||||||
| History of MI (ever) | 646 (38) | 308 (51) | 0.27 | 177 (37) | 1131 (41) | 0.10 |
| History of CABG/PCI (ever) | 147 (9) | 58 (10) | 0.04 | 58 (12) | 263 (9) | 0.10 |
| Bleeding | 49 (3) | 17 (3) | 0.002 | 13 (3) | 79 (3) | 0.01 |
| Previous surgery | 64 (4) | 23 (4) | 0.004 | 31 (6) | 118 (4) | 0.12 |
| Comorbidity, n (%) | ||||||
| History of IHD (ever) | 1686 (99) | 596 (99) | 0.05 | 463 (96) | 2745 (99) | 0.21 |
| Diabetes | 486 (29) | 183 (31) | 0.04 | 130 (27) | 799 (29) | 0.05 |
| Hypertension | 1110 (65) | 419 (70) | 0.10 | 311 (65) | 1840 (66) | 0.04 |
| Hypercholesterolaemia | 806 (47) | 302 (50) | 0.06 | 204 (42) | 1312 (47) | 0.12 |
| Peripheral vascular disease | 171 (10) | 52 (9) | 0.05 | 58 (12) | 281 (10) | 0.08 |
| Stroke | 8 (1) | 8 (1) | 0.09 | 7 (1) | 23 (1) | 0.07 |
| Heart failure | 197 (12) | 71 (12) | 0.01 | 74 (15) | 342 (12) | 0.11 |
| Peptic ulcer disease | 5 (0.3) | < 5 | 0.01 | < 5 | 8 (0.3) | 0.02 |
| Haemodialysis or renal disease | 104 (6) | 43 (7) | 0.04 | 27 (6) | 174 (6) | 0.03 |
| Cancer | 96 (6) | 24 (4) | 0.08 | 36 (7) | 156 (6) | 0.09 |
| Clotting disorder | 16 (1) | – | 0.14 | < 5 | ** | 0.04 |
| Anaemia | 70 (4) | 32 (5) | 0.06 | 18 (4) | 120 (4) | 0.04 |
| Liver cirrhosis | < 5 | < 5 | 0.05 | – | < 5 | 0.06 |
| Co-interventions , n (%) | ||||||
| NSAIDs | 292 (17) | 117 (20) | 0.06 | 93 (19) | 502 (18) | 0.04 |
| Steroids | 115 (7) | 30 (5) | 0.07 | 45 (9) | 190 (7) | 0.11 |
| PPIs | 729 (43) | 227 (38) | 0.10 | 201 (42) | 1157 (42) | 0.003 |
| Anticoagulants | 14 (1) | < 5 | 0.06 | 8 (2) | ** | 0.09 |
FIGURE 8.
Flow diagram describing the construction of the CABG target trial. C, clopidogrel; P, prasugrel; SA, sensitivity analysis; T, ticagrelor.
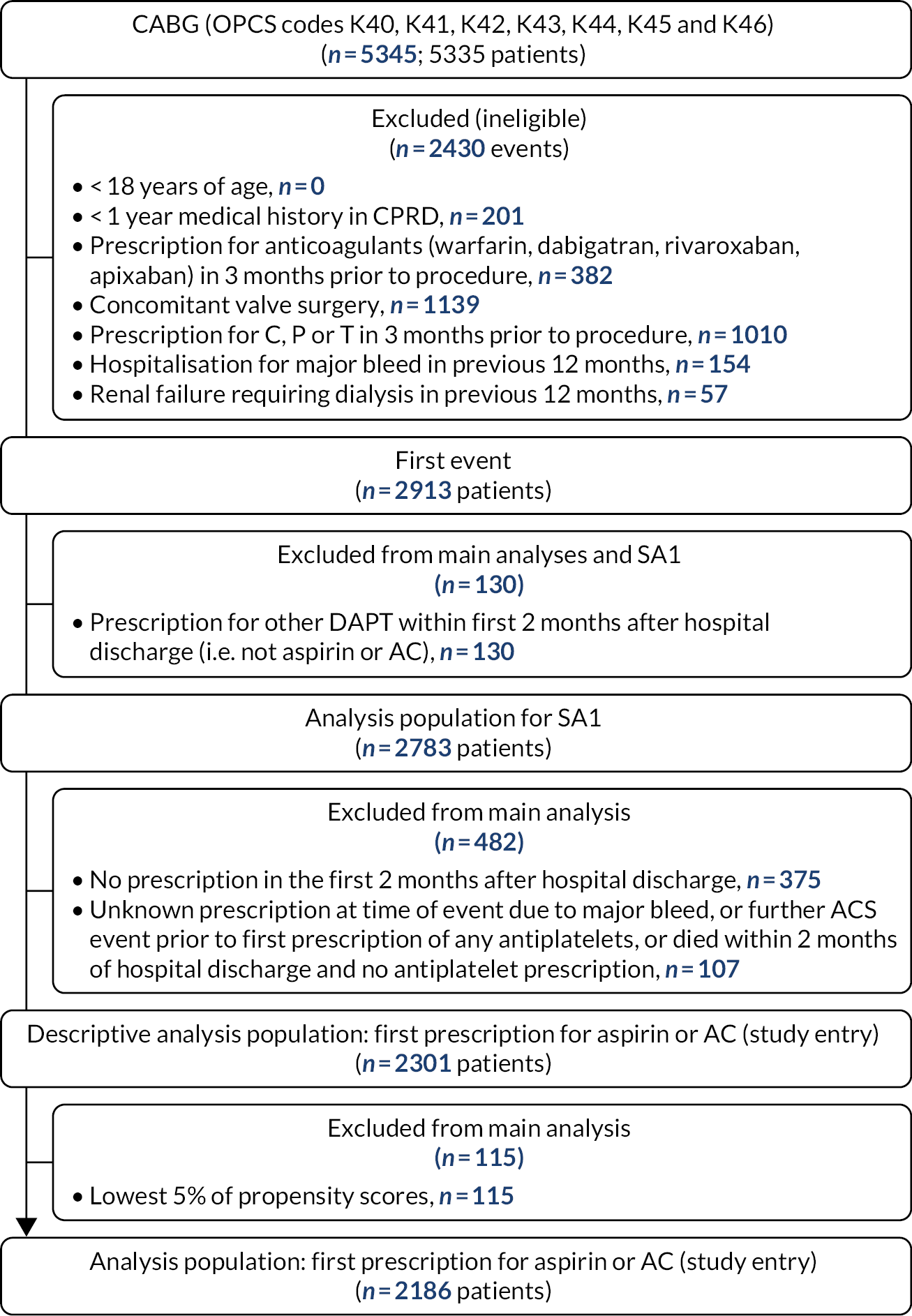
Baseline characteristics of participants included in and participants excluded from the target trial
The baseline characteristics of participants in the CABG target trial are shown in Table 11. We used the SMD to express the size of the difference between groups relative to the variability observed for each patient characteristic. A SMD of 0.10 is the threshold used to denote a meaningful imbalance in the covariates between groups. 48,49 The number of eligible CABG patients decreased every year between 2010 and 2017 because of the decline in the number of practices in the CPRD GOLD over time (Nafiu Ismail, Keele University, 2019, personal communication). 50 For aspirin versus AC, there was fairly good balance in the covariates between groups. Relatively few covariates had SMDs of > 0.10: age (0.13, lower in AC), sex (0.11, more women received AC), ethnic group (0.22, higher proportion received AC among those other than white), history of MI (0.27, higher proportion in the AC group) and clotting disorder (0.14, higher proportion in the aspirin group). There was no difference of in length of hospital stay between the aspirin and the AC groups [median 6, interquartile range (IQR) 5–9, and median 6, IQR 5–8, respectively].
We also used SMDs to identify potential differences between the group of patients for whom the intervention was unknown (n = 482) and the group for whom an intervention could be assigned (aspirin or AC, n = 2301). The unknown intervention group had lower proportions of patients with a history of ischaemic heart disease (IHD) (96% vs. 99%) and hypercholesterolaemia (42% vs. 48%), and higher proportions of patients with heart failure (15% vs. 12%), patients who had had previous surgery (6% vs. 4%) and patients taking steroids (9% vs. 6%) (all SMDs > 0.10). Of the 482 patients without an intervention, 107 (22%) either died or had a major bleed or ACS event before their first prescription and 375 (78%) had no prescription of an antiplatelet agent in the first 2 months after their index event.
The characteristics of patients who died or had a major bleed or ACS event, compared with those who had no antiplatelet prescription within 2 months of discharge, are shown in Table 12. The group of patients who experienced an event included older patients (aged 72 years vs. 66 years, SMD 0.6); more women (23% vs. 19%, SMD 0.12); more ex-smokers (49% vs. 41%, SMD 0.16); and more patients with a history of MI (50% vs. 33%, SMD 0.34), hypertension (70% vs. 63%, SMD 0.15), peripheral vascular disease (17% vs. 11%, SMD 0.18), stroke (3% vs. 1%, SMD 0.13), heart failure (26% vs. 12%, SMD 0.36), renal disease (11% vs. 4%, SMD 0.27), cancer (12% vs. 6%, SMD 0.21) and anaemia (7% vs. 3%, SMD 0.17); and fewer patients with hypercholesterolaemia (37% vs. 44%, SMD 0.13). Furthermore, more patients who experienced an event were taking steroids (15% vs. 8%, SMD 0.23).
| Characteristics | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 107) | No prescription in the CPRD within 2 months of discharge (N = 375) | SMD | Total known (N = 2301) |
|---|---|---|---|---|
| Demography | ||||
| Year of event, n (%) | ||||
| 2010/11 | 24 (22) | 77 (21) | 0.19 | 465 (20) |
| 2011/12 | 18 (17) | 74 (20) | 449 (20) | |
| 2012/13 | 21 (20) | 82 (22) | 429 (19) | |
| 2013/14 | 21 (20) | 53 (14) | 360 (16) | |
| 2014/15 | 14 (13) | 46 (12) | 304 (13) | |
| 2015/16 | 6 (6) | 28 (7) | 182 (8) | |
| 2016/17 | < 5 | 15 (4) | 112 (5) | |
| Age (years), mean (SD) | 71.9 (9.1) | 65.7 (11.4) | 0.60 | 67.2 (9.4) |
| Sex, n (%) | ||||
| Male | 82 (77) | 305 (81) | 0.12 | 1883 (82) |
| Female | 25 (23) | 70 (19) | 418 (18) | |
| BMIa (kg/m2), mean (SD) | 28.8 (4.6) | 28.9 (4.8) | 0.03 | 28.6 (4.6) |
| Ethnic group, n (%) | ||||
| White | 97 (91) | 347 (93) | 0.07 | 2123 (92) |
| Other than white | 10 (9) | 28 (7) | 178 (8) | |
| Smoking category,b n (%) | ||||
| Ex-smoker | 51 (49) | 151 (41) | 0.16 | 992 (44) |
| Non-smoker | 38 (36) | 159 (43) | 939 (41) | |
| Smoker | 16 (15) | 58 (16) | 338 (15) | |
| Medical history, n (%) | ||||
| History of MI (ever) | 53 (50) | 124 (33) | 0.34 | 954 (41) |
| History of CABG/PCI (ever) | 11 (10) | 47 (13) | 0.07 | 205 (9) |
| Bleeding | < 5 | 12 (3) | 0.16 | 66 (3) |
| Previous surgery | 9 (8) | 22 (6) | 0.10 | 87 (4) |
| Comorbidity, n (%) | ||||
| History of IHD (ever) | 103 (96) | 360 (96) | 0.01 | 2282 (99) |
| Diabetes | 31 (29) | 99 (26) | 0.06 | 669 (29) |
| Hypertension | 75 (70) | 236 (63) | 0.15 | 1529 (66) |
| Hypercholesterolaemia | 40 (37) | 164 (44) | 0.13 | 1108 (48) |
| Peripheral vascular disease | 18 (17) | 40 (11) | 0.18 | 223 (10) |
| Stroke | < 5 | < 5 | 0.13 | 16 (1) |
| Heart failure | 28 (26) | 46 (12) | 0.36 | 268 (12) |
| Peptic ulcer disease | 0 | < 5 | 0.07 | 7 (0.3) |
| Haemodialysis or renal disease | 12 (11) | 15 (4) | 0.27 | 147 (6) |
| Cancer | 13 (12) | 23 (6) | 0.21 | 120 (5) |
| Clotting disorder | 0 | < 5 | 0.10 | 16 (1) |
| Anaemia | 7 (7) | 11 (3) | 0.17 | 102 (4) |
| Liver cirrhosis | 0 | – | – | < 5 |
| Valve disease | 17 (16) | 32 (9) | 0.23 | 231 (10) |
| Co-interventions , n (%) | ||||
| NSAIDs | 20 (19) | 73 (19) | 0.02 | 409 (18) |
| Steroids | 16 (15) | 29 (8) | 0.23 | 145 (6) |
| PPIs | 43 (40) | 158 (42) | 0.04 | 956 (42) |
| Anticoagulants | < 5 | 7 (2) | 0.08 | 16 (1) |
Bleeding events among participants included in and those excluded from the target trial
Of the 2186 patients included in the target trial, 111 (5%) experienced at least one bleeding event: 69/1596 (4%) in aspirin and 42/590 (7%) in the AC group. With regards to major and minor bleeding events, 38/2186 (2%) patients experienced a major bleed and 79/2186 (4%) experienced a minor bleed. The proportion of patients experiencing a major and minor bleeding event in aspirin were 20/1596 (1%) and 53/1596 (3%), respectively, while in the AC group the proportion of patients experiencing a major and minor bleeding event were 18/590 (3%) and 26/590 (4%), respectively.
Figure 9 shows the Kaplan–Meier curves of cumulative bleeding in AC versus aspirin groups [any bleed, major (HES reported) and minor (CPRD reported)], and Table 13 shows the follow-up time and the number of bleeding events for major and minor bleeding. The cumulative incidence of any bleeding was higher with AC than with aspirin (see Figure 9a). The curves diverged early (approximately 1 month) after the index event. This was also reflected in the Kaplan–Meier curve for minor bleeding events (see Figure 9c), whereas, for major bleeding events, the curves diverged after approximately 3 months (see Figure 9b). The crude incidence rate of major bleeds in the AC group was more than double that of the aspirin group (30.9 vs. 12.6 events per 1000 person-years, respectively). The crude incidence rate of minor bleeds was also higher in the AC group than in the aspirin group (45.3 vs. 33.8 events per 1000 person-years, respectively) (see Table 13). Of the 111 (5%) patients who experienced bleeding events, the majority (n = 88, 79%) experienced a single bleed and 23 (21%) experienced more than one bleed. Over half of all bleeds were gastrointestinal in origin; just over one-quarter were ear, nose and throat; and just over 10% were skin and soft-tissue bleeds (Table 14). Bleed sites did not differ markedly between the aspirin and the AC groups, with the exception of ear, nose and throat bleeds, which were more prevalent in the AC group.
| Aspirin | AC | |||||
|---|---|---|---|---|---|---|
| Bleeding events | Number of patients with at least one bleed | Person-years | Rate per 1000 person-years (95% CI) | Number of patients with at least one bleed | Person-years | Rate per 1000 person-years (95% CI) |
| Major (HES) | 20 | 1584 | 12.6 (8.1 to 19.6) | 18 | 582 | 30.9 (19.5 to 49.1) |
| Minor (CPRD) | 53 | 1564 | 33.8 (25.9 to 44.4) | 26 | 574 | 45.3 (30.9 to 66.6) |
| All (CPRD and HES) | 69 | 1440 | 47.9 (37.9 to 60.7) | 42 | 517 | 81.2 (60.0 to 109.9) |
| Bleeds recorded (HES or CPRD), n (%) | |||
|---|---|---|---|
| Site | Aspirin (N = 1596) | AC (N = 590) | Total (N = 2186) |
| Ear, nose or throat | 9 (11) | 14 (26) | 23 (17) |
| Gastrointestinal | 49 (57) | 28 (53) | 77 (55) |
| Genitourinary | < 5 | 0 | < 5 |
| Intracranial | < 5 | 0 | < 5 |
| Ocular | 6 (7) | < 5 | ** |
| Skin or soft tissue | 10 (12) | 5 (9) | 15 (11) |
| Other anatomical site | < 5 | 0 | < 5 |
| Unspecified anatomical site | 5 (6) | 2 (4) | 7 (5) |
| Total | 86 | 53 | 139 |
FIGURE 9.
Kaplan–Meier curves displaying cumulative bleeding according to intervention group. (a) Any bleeding; (b) major bleeding; and (c) minor bleeding. Plots are weighted according to the inverse probability of treatment received, and so compare outcomes if all eligible patients received aspirin or AC.
Patients who could not be assigned an intervention either because they experienced a bleeding or ischaemic event or died before their first prescription, or because they had no prescription in CPRD within 2 months of discharge (17% of the eligible population) had a slightly higher bleeding rate than the patients included in the target trial (7% vs. 5%) (see Table 15). The bleeding rate was markedly higher in those who experienced an ischaemic or bleeding event or died than in those who had no prescription in CPRD within 2 months of discharge (15% vs. 5%).
| Bleeding events, n/N (%) | Bleeding events, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Number of patients (n) | Aspirin | AC | HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in CPRDa | No prescription in CPRD within 2 months of discharge | Overall, n/N (%) |
| Primary outcome | |||||||
| Crude | 2186 | 69/1596 (4) | 42/590 (7) | 1.69 (1.15 to 2.48) | 16/107 (15) | 17/375 (5) | 33/482 (7) |
| Adjusted | 1.72 (1.15 to 2.57)b,c,d | ||||||
| SA1: multiple imputation for unknown intervention group | 2638 | Not possible to calculate from imputed data | 1.53 (1.02 to 2.29)b,c | Multiple imputation for unknown intervention group | |||
| SA3: restricted to patients at low risk of bleeding | 1980 | 58/1453 (4) | 38/527 (7) | 1.94 (1.26 to 2.98)b,e | – | – | |
| SA4: primary adjusted analysis without censoring of any CPRD or HES bleed at transfer-out or last collection date | 2186 | 71/1596 (4) | 43/590 (7) | 1.74 (1.17 to 2.59)b,f | – | – | |
| Major bleeding (HES reported) | 2186 | 20/1596 (1) | 18/590 (3) | 2.89 (1.48 to 5.64)b,g | 12/107 (11) | 10/375 (3) | 22/482 (5) |
| Minor bleeding (CPRD reported) | 2186 | 53/1596 (3) | 26/590 (4) | 1.22 (0.74 to 1.99)b,h | < 5 | 8/375 (2) | ** |
Analyses for the primary outcome (bleeding)
The primary analysis excluded patients for whom we could not assign an intervention (n = 482) and those in the lowest fifth percentile of propensity score (n = 115). The patients who could not be assigned an intervention had a higher rate of any bleeding and major bleeding than those included in the target trial (any bleeding: 7% vs. 5%, respectively; major bleeding: 5% vs. 4%, respectively) (Table 15). The crude HR indicated an increase in the hazard of bleeding in the AC group, compared with the aspirin group (1.69, 95% CI 1.15 to 2.48) (see Table 15). The HR was similar after adjustment for propensity scores and confounders (1.72, 95% CI 1.15 to 2.57). When separated by major (HES-reported) and minor (CPRD-reported) bleeding, there was an increased hazard of major bleeding (adjusted HR 2.89, 95% CI 1.48 to 5.64), but not of minor bleeding (adjusted HR 1.22, 95% CI 0.74 to 1.99), in the AC group, compared with the aspirin group.
Sensitivity analyses
The sensitivity analyses were conducted only for the primary outcome (any bleeding event). The inclusion of the 482 patients with unknown intervention using multiple imputation to assign patients to an intervention group (sensitivity analysis 1) did not materially alter the adjusted HR (1.53, 95% 1.02 to 2.29) (see Table 15). The exclusion of patients deemed to have a high risk of bleeding (sensitivity analysis 3) slightly increased the HR to 1.94 (95% CI 1.26 to 2.98). Repeating the analysis without censoring of any HES bleed at transfer-out or last collection date in the CPRD (therefore including HES bleeds that may have occurred after data collection in the CPRD had stopped) (sensitivity analysis 4) did not change the HR (1.74, 95% CI 1.17 to 2.59).
We did not conduct sensitivity analysis 2 (exclusion of patients who changed medication before first bleeding event) because very few patients (< 5/120) changed medication before their first bleeding event (so this did not meet our prespecified threshold of > 10% of the population).We also did not conduct sensitivity analysis 5. The proposed instrument was a consultant’s antiplatelet prescription at the time of the index event for their previous CABG patient (who is in the linked HES–CPRD data set and is eligible for the target trial). There was evidence of an association between previous prescription and current prescription [odds ratio (OR) 4.37, 95% CI 3.51 to 5.44; p < 0.001], but there was little evidence of an association between previous prescription and bleeding (OR 1.08, 95% CI 0.69 to 1.69; p = 0.74). Moreover, further investigation of consultant episodes in HES data in relation to actual procedures carried out by individual consultants in Bristol also revealed that the consultant who carries out the surgery (and is likely to prescribe antiplatelet medication) for an individual patient is not necessarily the consultant named on the finished episode for that patient. Therefore, the instrumental variable analysis was not explored any further.
Subgroup analyses
There was no evidence of any subgroup effects for people with diabetes compared with people without diabetes (p = 0.62, interaction test), people with chronic kidney disease compared with people without chronic kidney disease (p = 0.48) or concurrent prescription for PPIs compared with no concurrent prescription for PPIs (p = 0.36).
Bleeding events among patients eligible for the target trial but not included in the primary analysis
Table 15 shows the number of bleeds among patients who were not included in the analysis. Patients who experienced an event or died before their first prescription in the CPRD had a higher rate of major bleeding than those included in the target trial (15% vs. 5%, respectively). Those with no prescription in the CPRD within 2 months of discharge had the same bleeding rate as those included in the target trial (5% vs. 5%).
Mortality and ischaemic events among participants included in and those excluded from the target trial
Figure 10 shows the Kaplan–Meier curves for the secondary outcomes of all-cause and cardiovascular mortality, mortality from bleeding, MI, stroke, additional coronary intervention and the composite outcome of MACE. The event rates for all secondary outcomes were higher among patients excluded from the target trial (15% of the eligible CABG population) (Table 16). However, this event rate was driven entirely by the 107 patients (3% of the eligible population) who died or experienced another ACS event, rather than those with no prescription in the CPRD within the 2-month time window (the latter had an incidence of all secondary outcomes similar to that of patients included in the target trial).
FIGURE 10.
Kaplan–Meier curves displaying cumulative all-cause and cardiovascular mortality, mortality from bleeding and ischaemic events (MI, stroke, additional PCI) and cumulative MACEs, according to intervention group. (a) All-cause mortality; (b) cardiovascular mortality; (c) mortality from bleeding; (d) MI; (e) stroke; (f) additional PCI; and (g) MACE.
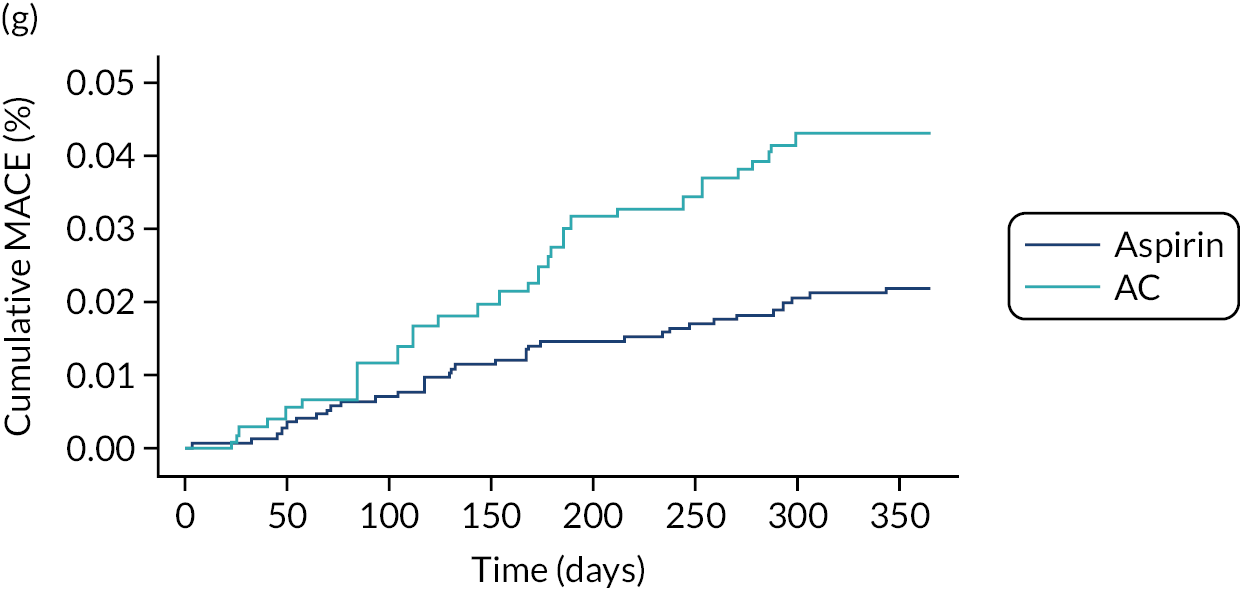
| Secondary outcomes | Included in target trial, n (%) | Not included in target trial, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Aspirin (N = 1596) | AC (N = 590) | Overall (N = 2186) | Adjusted HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in CPRDa (N = 107) | No prescription in CPRD within 2 months of discharge (N = 375) | Overall (N = 482) | |
| All-cause mortality | 21 (1) | 11 (2) | 32 (1) | 1.34 (0.63 to 2.85)b | 70 (65) | 4 (1) | 74 (15) |
| Cardiovascular mortality | 15 (1) | 5 (1) | 20 (1) | 0.82 (0.29 to 2.34)c | 58 (54) | 2 (1) | 60 (12) |
| Mortality from bleeding | < 5 | 0 | < 5 | – | < 5 | < 5 | < 5 |
| MI | 12 (1) | 13 (2) | 25 (1) | 2.51 (1.13 to 5.58)d | < 5 | < 5 | 7 (1) |
| Stroke | < 5 | 0 | < 5 | – | – | – | |
| Additional coronary intervention | 14 (1) | 12 (2) | 26 (1) | 2.73 (1.22 to 6.12)e | < 5 | < 5 | 5 (1) |
| MACE | 34 (2) | 27 (5) | 61 (3) | 2.06 (1.23 to 3.46)f | 70 (65) | < 5 | ** |
Analyses for the secondary outcomes (mortality and ischaemic events)
With regard to ischaemic outcomes, AC increased the hazards of MI (HR 2.45, 95% CI 1.10 to 5.45), additional coronary intervention (HR 2.48, 95% CI 1.13 to 5.46) and MACEs (HR 1.95, 95% CI 1.17 to 3.26). AC did not increase the hazard of mortality or of cardiovascular mortality (see Table 16).
Treatment switches and adherence
Treatment switches in the aspirin and AC groups by type of switch and whether the switch occurred before or after bleeding or an ischaemic event are shown in Table 17.
| Bleed occurred, n/N (%) | Ischaemic eventb occurred, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Type of switch, n/N (%) | Median (IQR) time to switch (months) | Before switcha | After switch | Before switch (within 2 months) | Before switch | After switch | No ischaemic or bleeding events, n/N (%) |
| Aspirin | Discontinued aspirin, 281/1702 (17) | 8.1 (5.7–10.3) | 11/281 (4) | 6/281 (2) | < 5 | < 5 | 1/281 (0.3) | 261/281 (93) |
| Initiated second antiplatelet, 75/1702 (4) | 5.9 (3.7–8.7) | < 5 | < 5 | 8/75 (11) | 9/75 (12) | 0/75 (0) | 61/75 (81) | |
| AC | Discontinued aspirin, 85/599 (14) | 8.4 (6.5–10.6) | 8/85 (9) | < 5 | 0/85 (0) | < 5 | 0/85 (0) | 72/85 (85) |
| Discontinued clopidogrel, 41/599 (14) | 7.2 (5.7–10.2) | 0/41 (0) | < 5 | 0/41 (0) | < 5 | 0/41 (0) | 40/41 (98) | |
| Discontinued AC, 22/599 (4) | 6.4 (5.7–8.8) | 0/22 (0) | < 5 | 0/22 (0) | < 5 | 0/22 (0) | 21/22 (95) | |
| Initiated a different P2Y12 inhibitor, < 5 | 6.7 (5.7–8.2) | 0/3 (0) | 0/3 (0) | < 5 | < 5 | 0/3 (0) | < 5 | |
In the 12 months after the index event, 341 out of 1702 (20%) patients in the aspirin group were identified as ‘switchers’. There were 356 treatment switches; 281 (79%) were aspirin discontinuations, and 75 (21%) initiated a different P2Y12 inhibitor. The median time to switching was > 6 months in the two groups of switchers. On average, patients who initiated a second antiplatelet received 5.9 prescriptions of the second antiplatelet (6 months’ supply). The median time to switching was between 6 and 8 months in all groups of switchers.
Among patients assigned AC, 106 out of 599 (18%) were identified as switchers. There were 151 treatment switches; 85 (56%) were aspirin discontinuations, 41 (27%) were clopidogrel discontinuations, 22 (15%) were aspirin and clopidogrel discontinuations and < 5 were initiations of a different P2Y12 inhibitor. The median time to switching was ≥ 6 months among all those who switched.
Across both groups (aspirin and AC), 42 switchers had a bleed or ischaemic events, 29 (69%) in aspirin and 13 (31%) in AC. Most of these events occurred before the switch.
In the aspirin group, the majority of switchers (> 80%) did not experience a bleeding or ischaemic event; 6% experienced a bleeding event (most before the switch and distributed equally between the two groups of switchers) and 4% experienced an ischaemic event, with the majority of these in the group of switchers who initiated a second antiplatelet agent before the switch.
In the AC group, just 2% of switchers experienced a bleed, with most of these occurring before the switch and in the group of switchers who discontinued aspirin; 1% experienced an ischaemic event, with most of these also occurring before the switch and also in the group that discontinued aspirin.
Adherence, defined as a MPR of ≥ 0.8, was 70% in the aspirin group and 54% in the AC group.
Discussion
This is the first study using routinely collected data to examine the incidence of bleeding among patients undergoing CABG in the 12 months after their procedure. Only 5% of the study population experienced any bleeding event; this is the same as the incidence for any bleeding reported in a 2018 meta-analysis (including five RCTs and eight observational studies),51 but lower than the incidence of major bleeding (7%) from eight observational studies reported in another meta-analysis. 52 These discrepancies probably arise because of different criteria for reporting bleeding events being used in individual studies and different methods of data collection.
The data suggest that there is underascertainment of minor bleeding (including ‘nuisance’ bleeding) in the CPRD. The incidence of minor bleeding was 4% across the CABG population, which is much lower than the incidence of nuisance bleeding (29–38%) reported in previous studies of patients on antiplatelet medication (in which patients were interviewed about bleeding events). 12-14 This suggests that the vast majority of nuisance bleeding does not prompt patients to go to their GP.
In our population, DAPT with AC, compared with aspirin monotherapy, was associated with an increased hazard of any bleeding (HR 1.72, 95% CI 1.15 to 2.57) and major bleeding (HR 2.89, 95% CI 1.48 to 5.64), but not minor bleeding (HR 1.22, 95% CI 0.74 to 1.99). Recent meta-analyses52,53 suggest that DAPT with AC does not increase the risk of major bleeding, compared with aspirin monotherapy. Agarwal et al. 52 pooled data from five RCTs and seven non-randomised studies [RCTs with, pooled, 16 events/446 participants in the AC group and eight events/445 participants in the aspirin group, relative risk (RR) 1.82, 95% CI 0.78 to 4.25; cohort studies with, pooled, 281 events/4398 participants in the DAPT group and 294 events/4327 participants in the aspirin group, RR 1.10, 95% CI 0.94 to 1.29]. The size and direction of the point estimate for the RCT meta-analysis was similar to our analysis, but the CIs were wide, reflecting the small sample size and low frequency of events.
Solo et al. 53 conducted a network meta-analysis comprising 3745 patients and investigating different antithrombotic regimens (DAPT with clopidogrel and ticagrelor, antiplatelet monotherapy, vitamin K antagonists and rivaroxaban). It showed no increase in major bleeding with DAPT with AC versus aspirin monotherapy (RR 0.85, 95% CI 0.30 to 2.37), although the CIs were wide (reflecting the small sample sizes and low frequency of events of the included trials). None of the published meta-analyses evaluated minor bleeding.
The differences in effect size between our study and the meta-analyses are likely to be a result of differences in design, populations and methods of data collection. We also excluded a group of patients eligible for inclusion (15% of the eligible population) who could not be assigned an intervention because they died, had a major bleed or ACS event or had no prescription in the CPRD in the specified time window for assigning the intervention. This may have introduced selection bias into our study, particularly as these patients had a higher rate of bleeding than the included population.
Our results were robust to the different assumptions tested in the sensitivity analyses (multiple imputation for unknown intervention groups, exclusion of patients with high risk of bleeding and no censoring for bleeding events after the last collection date in the CPRD). HRs were comparable across all analyses. A limitation of the multiple imputation is that it was based on patient characteristics for the cohort of patients with known intervention. However, the subgroup of patients who could not be assigned an intervention (482 patients, 15% of the eligible population) had a higher risk of bleeding, and were, therefore, different from the population with known intervention.
Surprisingly, our study also showed an increased hazard of MI, additional coronary interventions and MACE (composite of MI, coronary reinterventions and death) in the AC group, compared with the aspirin group. The meta-analysis by Agarwal et al. 52 found a decreased risk of all-cause mortality (RR 0.67, 95% CI 0.48 to 0.94) and MACEs (composite of MI, stroke and death, RR 0.84, 95% CI 0.71 to 0.99) in the AC group, compared with the aspirin group, with the effect size similar across RCTs and observational studies. Other meta-analyses, for example Verma et al. ,54 showed a similar effect of DAPT on mortality (RR 0.68, 95% CI 0.43 to 1.08) and MACEs (0.86, 95% CI 0.73 to 1.03) among patients after CABG. The network meta-analysis by Solo et al. 53 showed a similar effect size for AC compared with aspirin for all-cause mortality (RR 0.70, 95% CI 0.11 to 4.50) and MI (RR 0.71, 95% CI 0.26 to 1.96).
The unexpected increase in MACEs that we observed in our study suggests that CABG patients who were prescribed DAPT were higher-risk patients. The baseline covariates show that the AC population was younger, had a higher proportion of individuals who were other than white and a higher rate of previous MI. Although these covariates were adjusted for in the statistical analysis, they reflect a population with presumed increasing complexity of disease who will be more likely to experience secondary events. Furthermore, we did not have data on a substantial proportion of potential confounders, in particular procedure-related characteristics. Our results are, therefore, likely to reflect a certain degree of confounding by indication.
We excluded 15% of the eligible population from the primary analysis because they could not be assigned an intervention. Excluding patients who experienced a major bleed or MACE prior to the first (if any) antiplatelet prescription(s) occurring in CPRD within 2 months of the surgery was necessary because we could not reliably assume that the observed treatment would be the same as the assigned intervention at baseline for those patients. However, this may have induced selection bias, as we excluded a high-risk population. For example, a true higher risk of MACE in the aspirin group may be masked or even reversed by excluding susceptible patients (high-risk patients who experienced a major bleeding event or MACE prior their first prescription in primary care) from the risk set. It is well established that depletion of susceptible populations from an analysis population, for example by including prevalent users, could make harmful interventions appear protective. 55–57 This is an issue that was difficult to overcome with the data that were available, as reliably imputing the assigned intervention at baseline is difficult given that the excluded population is likely to be a quite distinct population from the included population. Nevertheless, selection bias is unlikely to be solely responsible for the observed effect, as the Kaplan–Meier curves for ischaemic outcomes (see Figure 10) diverge for the entire follow-up period; the inclusion of the population with early events would have influenced the curves only in the first few months.
It is also important to note that there is considerable uncertainty about the benefits of adding a P2Y12 inhibitor to aspirin monotherapy after CABG. There are no large, pragmatic, multicentre RCTs of DAPT versus aspirin monotherapy in CABG populations, and most of the RCTs included in the meta-analyses summarised above52–54 were at high risk of bias and had saphenous vein graft failure as a primary outcome. Saphenous vein graft failure is not a clinical end point and does not correlate well with hard clinical end points (mortality, MI and repeat intervention). 58
Our analysis was intention to treat, so patients were analysed according to intervention groups assigned at baseline, regardless of adherence or switches in antiplatelet treatment. Generally, studies report that between 45% and 65% of patients adhere to medications prescribed for secondary prevention, with few differences between drug classes. 59–61 Several studies have highlighted potential adherence issues among patients taking antiplatelet therapy/anticoagulants59–61 and studies conducted in real-world settings suggest that non-adherence to DAPT is a common problem, affecting up to 48% of patients. 59 This study reflects this, showing that non-adherence in the DAPT group was 46%, which was much higher than in the aspirin group (30%). Furthermore, over one-quarter of all patients in the DAPT group were identified as having switched from DAPT, meaning that they stopped aspirin, clopidogrel or both. Just under half of all MIs in the AC group (n = 6/13) occurred among the switchers. It is possible that, in this study, non-adherence in a high-risk population prescribed DAPT contributed to the high incidence of MACEs that we observed.
Conservatively managed acute coronary syndrome
Trends in antiplatelet prescribing and rates of bleeding over time
Figure 11 describes how the target trial population was assembled from the available data sets. Figure 12 shows the trends in antiplatelet prescriptions and rates of bleeding between 2010 and 2017 in the target trial population. There was a slight decrease in both aspirin and DAPT (AC) prescriptions over time (from 30.2% in 2010 to 23.5% in 2017 for aspirin and from 42.9% in 2010 to 32.7% in 2017 for AC). There was also a slight increase in the number of patients being prescribed no antiplatelet therapy or being prescribed some other regimen (e.g. one or more P2Y12 inhibitors). Both major and minor bleeding rates decreased, from 50.0 and 80.0 events, respectively, per 1000 person-years in 2010 to 13.7 and 51.3 events, respectively, per 1000 person-years in 2017.
FIGURE 11.
Flow diagram describing the construction of the conservatively managed ACS target trial. C, clopidogrel; P, prasugrel; SA, sensitivity analysis; T, ticagrelor.
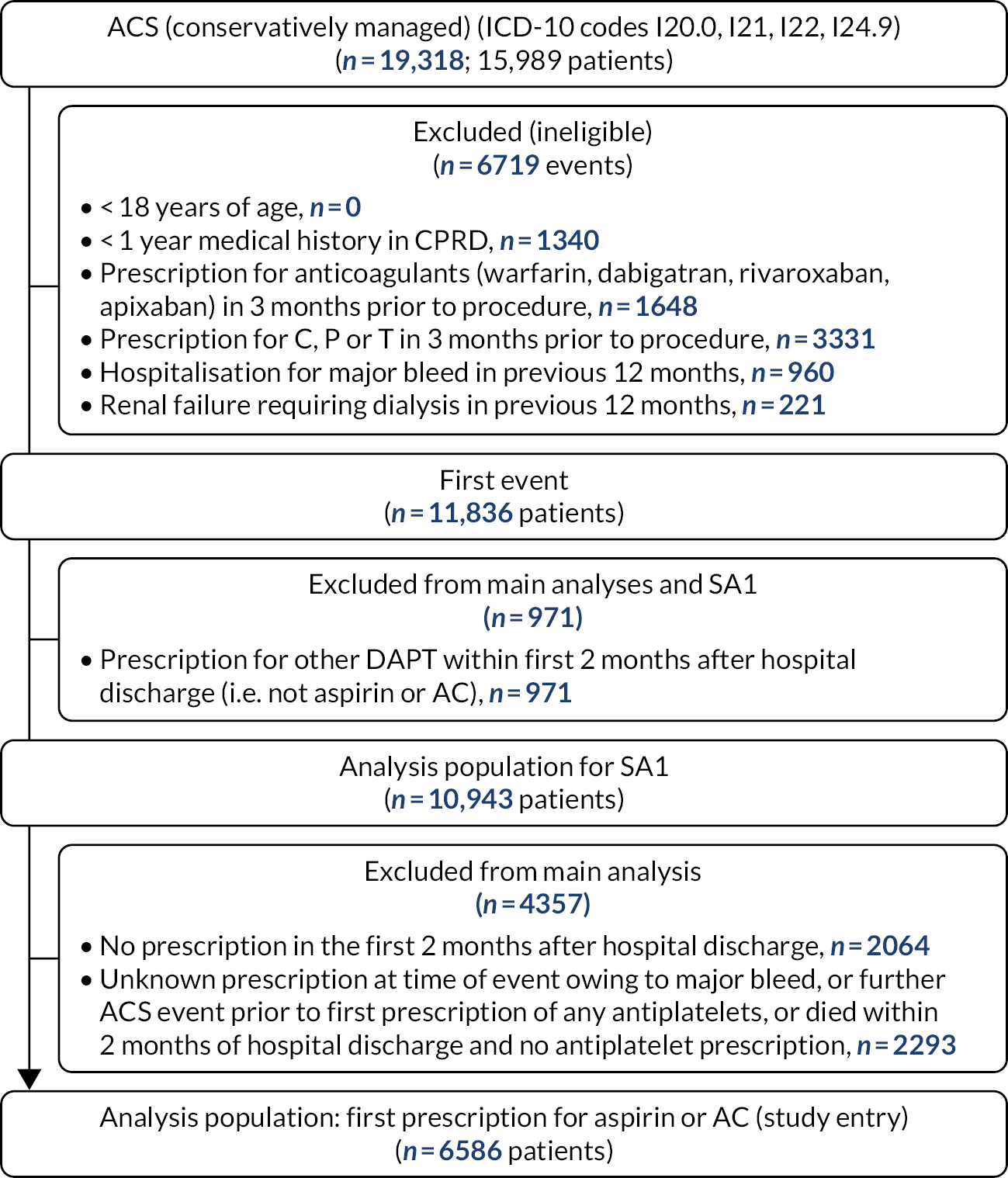
FIGURE 12.
Proportion of conservatively managed ACS patients being prescribed different antiplatelet regimes by year and rates of major (HES), minor (CPRD) and total bleeding events by year among conservatively managed ACS patients. (a) Different antiplatelet regimes; and (b) bleeding events.
Baseline characteristics of participants included in and those excluded from the target trial
Table 18 shows the baseline characteristics of patients included in and those excluded from the primary analysis. Of the 15,989 patients with linked CPRD–HES data, 10,943 (68%) were eligible and included in the target trial; 4357 of these patients (40%) were excluded because they could not be assigned to an intervention group. The number of eligible patients decreased every year between 2010 and 2017 (as a result of the decline over time in the number of practices in the CPRD GOLD). 50 The covariates with an imbalance between the aspirin and the AC groups were smoking (14% were smokers in the aspirin group and 18% were smokers in the AC group, SMD 0.11), history of MI (39% in the aspirin group vs. 57% in the AC group, SMD 0.38), history of CABG/PCI (21% in the aspirin group vs. 14% in the AC group, SMD 0.20), hypercholesterolaemia (17% in the aspirin group vs. 11% in the AC group, SMD 0.17) and use of PPIs (51% in the aspirin group vs. 43% in the AC group, SMD 0.15). For patients with unknown intervention (excluded from the primary analysis), compared with patients with known intervention (included in the primary analysis), the covariates with an imbalance were history of MI (50% in the included population and 35% in the excluded population, SMD 0.32), history of CABG/PCI (17% in the included population vs. 8% in the excluded population, SMD 0.26) and history of IHD (77% in the included population vs. 62% in the excluded population, SMD 0.31). There was a difference of 2 days in length of hospital stay between the aspirin and the AC groups [median 3 (IQR 1–7) days and median 5 (IQR 3–9) days, respectively].
| Characteristics | Aspirin (N = 2609) | AC (N = 3977) | SMD | Unknowna (N = 4357) | SMDb | Overall (N = 10,943) |
|---|---|---|---|---|---|---|
| Demography | ||||||
| Year of event, n (%) | ||||||
| 2010/11 | 690 (26) | 980 (25) | 0.07 | 915 (23) | 0.17 | 2665 (24) |
| 2011/12 | 562 (22) | 837 (21) | 987 (23) | 2657 (24) | ||
| 2012/13 | 453 (17) | 782 (20) | 787 (18) | 2186 (20) | ||
| 2013/14 | 377 (14) | 595 (15) | 787 (18) | 2022 (18) | ||
| 2014/15 | 258 (10) | 380 (10) | 638 (15) | 1610 (15) | ||
| 2015/16 | 176 (7) | 274 (7) | 550 (13) | 1188 (11) | ||
| 2016/17 | 93 (4) | 129 (3) | 392 (9) | 842 (8) | ||
| Age (years), mean (SD) | 73.2 (13.5) | 74.1 (13.7) | 0.07 | 73.7 (15.7) | 0.004 | 73.7 (14.5) |
| Sex, n (%) | ||||||
| Male | 1401 (54) | 2194 (55) | 0.03 | 2311 (53) | 0.03 | 5906 (54) |
| Female | 1208 (46) | 1783 (45) | 2046 (47) | 5037 (46) | ||
| BMIc (kg/m2), mean (SD) | 28.2 (5.8) | 27.6 (5.7) | 0.10 | 27.3 (5.9) | 0.09 | 27.6 (5.8) |
| Ethnic group, n (%) | ||||||
| White | 2411 (92) | 3733 (94) | 0.06 | 4029 (92) | 0.03 | 10,173 (93) |
| Other than white | 198 (8) | 244 (6) | 328 (8) | 770 (7) | ||
| Smoking category,d n (%) | ||||||
| Ex-smoker | 983 (39) | 1460 (38) | 0.11 | 1574 (37) | 0.03 | 4017 (38) |
| Non-smoker | 1196 (47) | 1680 (44) | 1927 (46) | 4803 (45) | ||
| Smoker | 352 (14) | 672 (18) | 716 (17) | 1740 (16) | ||
| Medical history , n (%) | ||||||
| History of MI (ever) | 1014 (39) | 2286 (57) | 0.38 | 1504 (35) | 0.32 | 4804 (44) |
| History of CABG/PCI (ever) | 549 (21) | 544 (14) | 0.20 | 359 (8) | 0.26 | 1452 (13) |
| Bleeding | 89 (3) | 141 (4) | 0.01 | 180 (4) | 0.03 | 410 (4) |
| Previous surgery | 136 (5) | 164 (4) | 0.05 | 202 (5) | 0.004 | 502 (5) |
| Comorbidity , n (%) | ||||||
| History of IHD (ever) | 2032 (78) | 3011 (76) | 0.05 | 2718 (62) | 0.31 | 7761 (71) |
| Diabetes | 668 (26) | 1013 (25) | 0.003 | 1008 (23) | 0.06 | 2689 (25) |
| Hypertension | 1223 (47) | 1670 (42) | 0.10 | 1743 (40) | 0.08 | 4636 (42) |
| Hypercholesterolaemia | 450 (17) | 452 (11) | 0.17 | 485 (11) | 0.08 | 1387 (13) |
| Peripheral vascular disease | 126 (5) | 186 (5) | 0.01 | 247 (6) | 0.04 | 559 (5) |
| Stroke | 39 (1) | 64 (2) | 0.01 | 80 (2) | 0.02 | 183 (2) |
| Heart failure | 289 (11) | 436 (11) | 0.004 | 519 (12) | 0.03 | 1244 (11) |
| Peptic ulcer disease | 10 (0.4) | 18 (1) | 0.01 | 26 (1) | 0.02 | 54 (1) |
| Haemodialysis or renal disease | 227 (9) | 377 (9) | 0.03 | 432 (10) | 0.03 | 1036 (9) |
| Cancer | 205 (8) | 239 (6) | 0.07 | 396 (9) | 0.09 | 840 (8) |
| Clotting disorder | 8 (0.3) | 10 (0.3) | 0.01 | 28 (1) | 0.05 | 46 (0.4) |
| Anaemia | 204 (8) | 274 (7) | 0.04 | 366 (8) | 0.04 | 844 (8) |
| Liver cirrhosis | < 5 | < 5 | 0.02 | 7 (0.2) | 0.01 | 14 (0.1) |
| Co-interventions , n (%) | ||||||
| NSAIDs | 495 (19) | 725 (18) | 0.02 | 698 (16) | 0.07 | 1918 (18) |
| Steroids | 361 (14) | 564 (14) | 0.01 | 596 (14) | 0.01 | 1521 (14) |
| PPIs | 1324 (51) | 1725 (43) | 0.15 | 1810 (42) | 0.10 | 4859 (44) |
| Anticoagulants | 60 (2) | 51 (1) | 0.08 | 117 (3) | 0.07 | 228 (2) |
Of the 4357 patients who could not be assigned to an intervention group, 2293 (53%) either died or had a major bleed or ACS event before their first prescription, and 2064 (47%) had no prescription for an antiplatelet agent in the 2 months after their index event. The characteristics of patients who died or had a major bleed or ACS event compared with those who had no antiplatelet prescription within 2 months of discharge are shown in Table 19. There were large differences in age (79 vs. 67 years, respectively, SMD 0.83) and history of MI (42% vs. 26%, respectively, SMD 0.34). There were also differences in BMI (26.6 vs. 28.1 kg/m2, respectively, SMD 0.25) and in the proportion of patients with heart failure (15% vs. 8%, respectively, SMD 0.22), renal disease (13% vs. 7%, respectively, SMD 0.20), cancer (12% vs. 6%, respectively, SMD 0.18) and diabetes (26% vs. 20%, respectively, SMD 0.16). There were also differences in ethnic group (6% other than white vs. 9% other than white, respectively, SMD 0.13), the proportion of current smokers (16% vs. 18%, respectively, SMD 0.13) and the proportion of patients with hypercholesterolaemia (9% vs. 13%, respectively, SMD 0.13) and anaemia (10% vs. 7%, respectively, SMD 0.11).
| Characteristic | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 2293) | No prescription in the CPRD within 2 months of discharge (N = 2064) | SMD | Total known (N = 6586) |
|---|---|---|---|---|
| Demography | ||||
| Year of event, n (%) | ||||
| 2010/11 | 512 (22) | 475 (23) | 0.07 | 1670 (25) |
| 2011/12 | 400 (17) | 387 (19) | 1399 (21) | |
| 2012/13 | 436 (19) | 351 (17) | 1235 (19) | |
| 2013/14 | 333 (15) | 305 (15) | 972 (15) | |
| 2014/15 | 283 (12) | 267 (13) | 638 (10) | |
| 2015/16 | 216 (9) | 176 (9) | 450 (7) | |
| 2016/17 | 113 (5) | 103 (5) | 222 (3) | |
| Age (years), mean (SD) | 79.4 (12.4) | 67.3 (16.5) | 0.83 | 73.7 (13.6) |
| Sex, n (%) | ||||
| Male | 1232 (54) | 1079 (52) | 0.03 | 3595 (55) |
| Female | 1061 (46) | 985 (48) | 2991 (45) | |
| BMIa (kg/m2), mean (SD) | 26.6 (5.6) | 28.1 (6.1) | 0.25 | 27.8 (5.7) |
| Ethnic group; n (%) | ||||
| White | 2158 (94) | 1871 (91) | 0.13 | 6144 (93) |
| Other than white | 135 (6) | 193 (9) | 442 (7) | |
| Smoking category,b n (%) | ||||
| Ex-smoker | 900 (40) | 674 (34) | 0.13 | 2443 (39) |
| Non-smoker | 983 (44) | 944 (48) | 2876 (45) | |
| Smoker | 356 (16) | 360 (18) | 1024 (16) | |
| Medical history , n (%) | ||||
| History of MI (ever) | 966 (42) | 538 (26) | 0.34 | 3300 (50) |
| History of CABG/PCI (ever) | 177 (8) | 182 (9) | 0.04 | 1093 (17) |
| Bleeding | 102 (4) | 78 (4) | 0.03 | 230 (3) |
| Previous surgery | 102 (4) | 100 (5) | 0.02 | 300 (5) |
| Comorbidity , n (%) | ||||
| History of IHD (ever) | 1423 (62) | 1295 (63) | 0.01 | 5043 (77) |
| Diabetes | 605 (26) | 403 (20) | 0.16 | 1681 (26) |
| Hypertension | 965 (42) | 778 (38) | 0.09 | 2893 (44) |
| Hypercholesterolaemia | 211 (9) | 274 (13) | 0.13 | 902 (14) |
| Peripheral vascular disease | 150 (7) | 97 (5) | 0.08 | 312 (5) |
| Stroke | 49 (2) | 31 (2) | 0.05 | 103 (2) |
| Heart failure | 350 (15) | 169 (8) | 0.22 | 725 (11) |
| Peptic ulcer disease | 13 (1) | 13 (1) | 0.01 | 28 (0.4) |
| Haemodialysis or renal disease | 293 (13) | 139 (7) | 0.20 | 604 (9) |
| Cancer | 264 (12) | 132 (6) | 0.18 | 444 (7) |
| Clotting disorder | 18 (1) | 10 (0.4) | 0.04 | 18 (0.3) |
| Anaemia | 226 (10) | 140 (7) | 0.11 | 478 (7) |
| Liver cirrhosis | < 5 | < 5 | 0.02 | 7 (0.1) |
| Valve disease | 105 (5) | 68 (3) | 0.07 | 277 (4) |
| Co-interventions , n (%) | ||||
| NSAIDs | 328 (14) | 370 (18) | 0.10 | 1220 (19) |
| Steroids | 352 (15) | 244 (12) | 0.10 | 925 (14) |
| PPIs | 994 (43) | 816 (40) | 0.08 | 3049 (46) |
| Anticoagulants | 58 (3) | 59 (3) | 0.02 | 111 (2) |
Bleeding events among participants included in and those excluded from the target trial
Of the 6586 patients included in the target trial, 688 (10%) experienced at least one bleeding event: 216 out of 2609 (8%) in the aspirin group and 472 out of 3977 (12%) in the AC group. With regard to major and minor bleeding events, 290 out of 6586 (4%) patients experienced a major bleed and 463 out of 6586 (7%) experienced a minor bleed. The proportions of patients experiencing a major and a minor bleeding event in the aspirin group were 96 out of 2609 (4%) and 141 out of 2609 (5%), respectively, whereas, in the AC group, the proportions of patients experiencing a major and a minor bleeding event were 194 out of 3977 (5%) and 322 out of 3977 (8%), respectively.
Figure 13 shows the Kaplan–Meier curves of cumulative bleeding (any bleed, major bleed and minor bleed) in the AC compared with the aspirin groups. The cumulative incidence of any bleeding increased steadily over the 12 months, but was higher in the AC group than in the aspirin group. The survival curves crossed after approximately 35 days, with a lower incidence of bleeding in the AC group before this point. This was reflected in the survival curves for both major and minor bleeding.
FIGURE 13.
Kaplan–Meier curves displaying cumulative bleeding according to intervention group. (a) Any bleeding; (b) major bleeding; and (c) minor bleeding. Plots are weighted according to the inverse probability of treatment received, and so compare outcomes if all eligible patients received aspirin or AC.
The crude incidence rates of major and minor bleeds were 24% higher (38 vs. 50 events per 1000 person-years) and 49% higher (56 vs. 84 events per 1000 person-years), respectively, in the AC group than in the aspirin group (Table 20). Of those who experienced a bleeding event within 12 months, the majority (489/688; 71%) of patients experienced only one bleeding event; 132 out of 688 (19%) experienced two bleeding events, and the remainder (67/688; 10%) experienced three or more bleeds. Over 40% of bleeds were gastrointestinal in origin; skin or soft-tissue bleeds and ear, nose and throat bleeds each accounted for just under one-fifth of bleeds (Table 21). More participants in the aspirin group than in the AC group had gastrointestinal bleeds (54% vs. 39%, respectively), but slightly fewer had skin or soft-tissue bleeds (15% vs. 21%, respectively) and ear, nose and throat bleeds (10% vs. 22%, respectively).
| Aspirin | AC | |||||
|---|---|---|---|---|---|---|
| Bleeds | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) |
| Major bleeds (HES) | 96 | 2550 | 37.6 (30.8 to 46.0) | 194 | 3855 | 50.3 (43.7 to 57.9) |
| Minor bleeds (CPRD) | 141 | 2512 | 56.1 (47.6 to 66.2) | 317 | 3769 | 84.1 (75.4 to 93.9) |
| All bleeds (CPRD and HES) | 216 | 2131 | 101.4 (88.7 to 115.8) | 467 | 3182 | 146.7 (134.0 to 160.7) |
| Bleeds recorded (HES or CPRD), n (%) | |||
|---|---|---|---|
| Bleed site | Aspirin (N = 2609) | AC (N = 3977) | Total (N = 6586) |
| Ear, nose or throat | 29 (10) | 155 (22) | 184 (18) |
| Gastrointestinal | 162 (54) | 274 (39) | 436 (43) |
| Genitourinary | 19 (6) | 25 (4) | 44 (4) |
| Intracranial | 10 (3) | 37 (5) | 47 (5) |
| Ocular | 14 (5) | 27 (4) | 41 (4) |
| Skin or soft tissue | 44 (15) | 151 (21) | 195 (19) |
| Other anatomical site | 9 (3) | 19 (3) | 28 (3) |
| Unspecified anatomical site | 14 (5) | 20 (3) | 34 (3) |
| Total | 301 | 708 | 1009 |
Patients who could not be assigned an intervention because they experienced a bleed or ischaemic event or died before their first prescription, or because they had no prescription in the CPRD within 2 months of discharge (40% of the eligible population), had a lower bleeding rate than the patients included in the target trial (7% vs. 10%, respectively) (Table 22). The bleeding rate was slightly higher among those who had no prescription in the CPRD within 2 months of discharge than among those who experienced an event or died (9% vs. 6%, respectively).
| Bleeding events | Bleeding events, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Number of patients (n) | Aspirin, n/N (%) | AC, n/N (%) | HR (95% CI) | Unknown prescriptiona | No prescription in CPRD within 2 months of discharge | Overall, n/N (%) |
| Primary outcome | |||||||
| Crude | 6586 | 216/2609 (8) | 472/3977 (12) | 1.44 (1.23 to 1.70) | 132/2293 (6) | 178/2064 (9) | 310/4357 (7) |
| Adjusted | 1.43 (1.21 to 1.69)b,c | ||||||
| SA1: multiple imputation for unknown intervention group | 10,744 | 1.28 (1.09 to 1.51)b,c | Multiple imputation for unknown intervention group | ||||
| SA3: restricted to patients at low risk of bleeding | 5687 | 174/2236 (8) | 412/3451 (12) | 1.56 (1.30 to 1.88)b,d | – | – | |
| SA4: primary adjusted analysis without censoring of any CPRD or HES bleed at transfer-out or last collection date | 6586 | 223/2609 (9) | 488/3977 (12) | 1.38 (1.18 to 1.62)b,e | – | – | |
| Major bleeding (HES reported) | 6586 | 96/2609 (4) | 194/3977 (5) | 1.34 (1.04 to 1.72)b,f | 90/2293 (4 | 107/2064 (5) | 197/4357 (4) |
| Minor bleeding (CPRD reported) | 6586 | 141/2609 (5) | 322/3977 (8) | 1.51 (1.23 to 1.86)b,g | 55/2293 (2) | 103/2064 (5) | 158/4357 (4) |
Analyses for the primary outcome (bleeding)
The primary analysis excluded patients to whom we could not assign an intervention (n = 4357, 40% of the eligible population). The crude and adjusted HRs indicated an increase of about 40% in the hazard of bleeding in the AC group compared with the aspirin group (HR 1.44, 95% CI 1.23 to 1.70, and HR 1.43, 95% CI 1.21 to 1.69, respectively) (see Table 22). When split into major and minor bleeding, the hazard of major bleeding increased by 34% (HR 1.34, 95% CI 1.04 to 1.72), and the hazard of minor bleeding increased by 51% (HR 1.51, 95% CI 1.23 to 1.86) in the AC group compared with the aspirin group.
Sensitivity analyses
The HRs were slightly attenuated in sensitivity analysis 1 (multiple imputation for patients with unknown intervention; HR 1.28, 95% CI 1.09 to 1.51), but did not change substantially for sensitivity analyses 3 or 4 (see Table 22).We did not conduct sensitivity analysis 2 (exclusion of patients who changed medication before first bleeding event) because very few patients (29/688, 4%) were identified as having changed medication before their first bleeding event (this did not meet the prespecified threshold of > 10% of the population).
We did not conduct the instrumental variable analysis (sensitivity analysis 5). There was evidence of an association between previous prescription and current prescription (OR 1.31, 95% CI 1.15 to 1.49; p < 0.001), but there was no evidence of an association between previous prescription and bleeding (OR 0.91, 95% CI 0.74 to 1.13; p = 0.41). Furthermore, we were not confident that the treating consultant was the same as the prescribing cardiologist; therefore, the instrumental variable analysis was not explored any further.
Subgroup analyses
There was no evidence of any subgroup effects for people with diabetes compared with people without diabetes (p = 0.33, interaction test), or for people with chronic kidney disease compared with people without chronic kidney disease (p = 0.52). There was a weak interaction (p = 0.05) between a concurrent prescription for PPIs (OR 1.25, 95% CI 1.00 to 1.55) and no concurrent prescription for PPIs (OR 1.73, 95% CI 1.33 to 2.24), meaning that the increase in bleeding in the AC group was smaller among patients with a concurrent prescription for a PPI than among those without.
Mortality and ischaemic events among participants included in and those excluded from the target trial
Figure 14 shows the Kaplan–Meier curves for the secondary outcomes of all-cause and cardiovascular mortality, mortality from bleeding, MI, stroke, additional coronary intervention and the composite outcome of MACE.
FIGURE 14.
Kaplan–Meier curves displaying cumulative all-cause and cardiovascular mortality, mortality from bleeding and ischaemic events, according to intervention group. (a) All-cause mortality; (b) cardiovascular mortality; (c) mortality from bleeding; (d) MI; (e) stroke; (f) additional PCI; and (g) MACE.
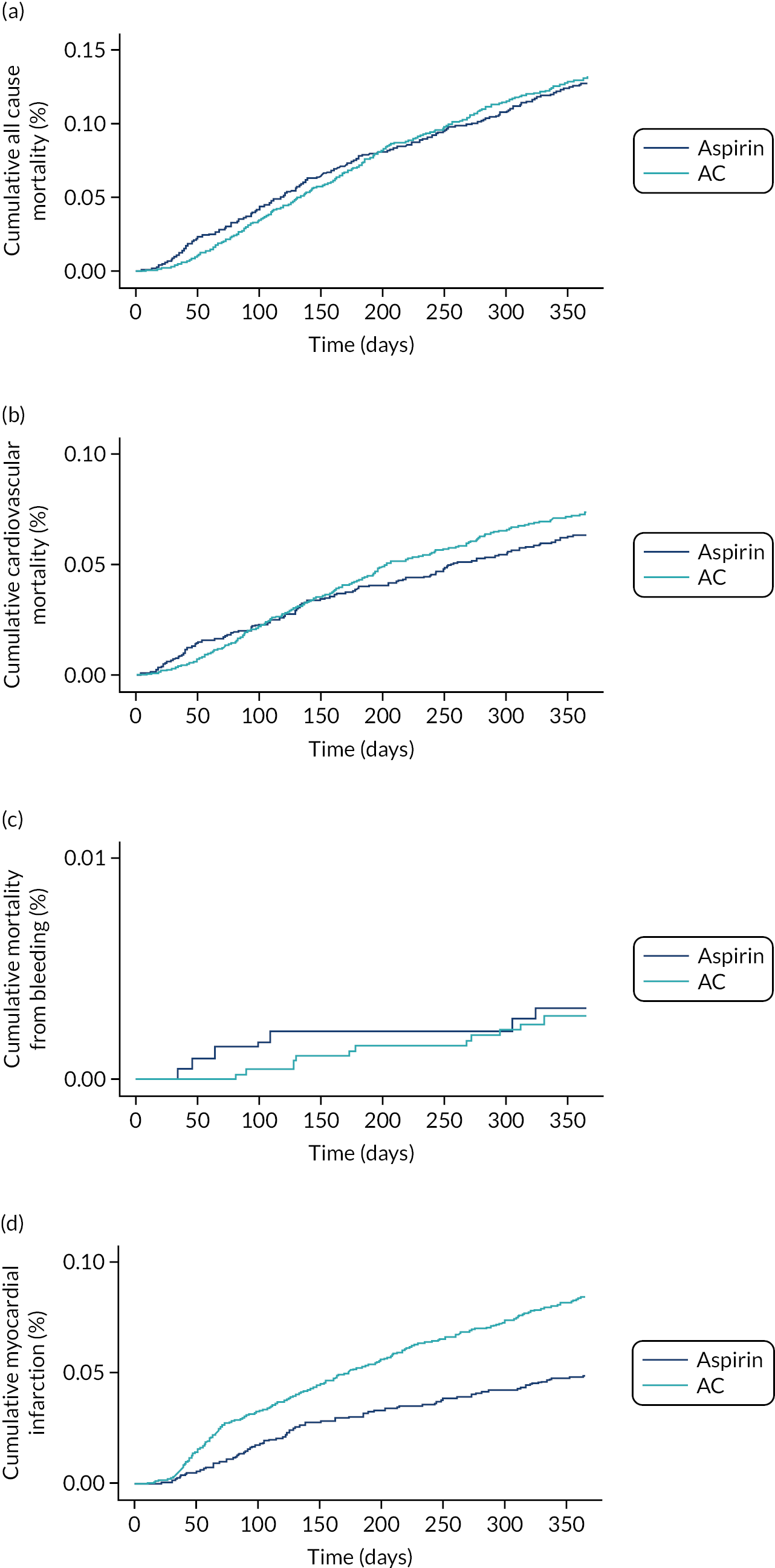
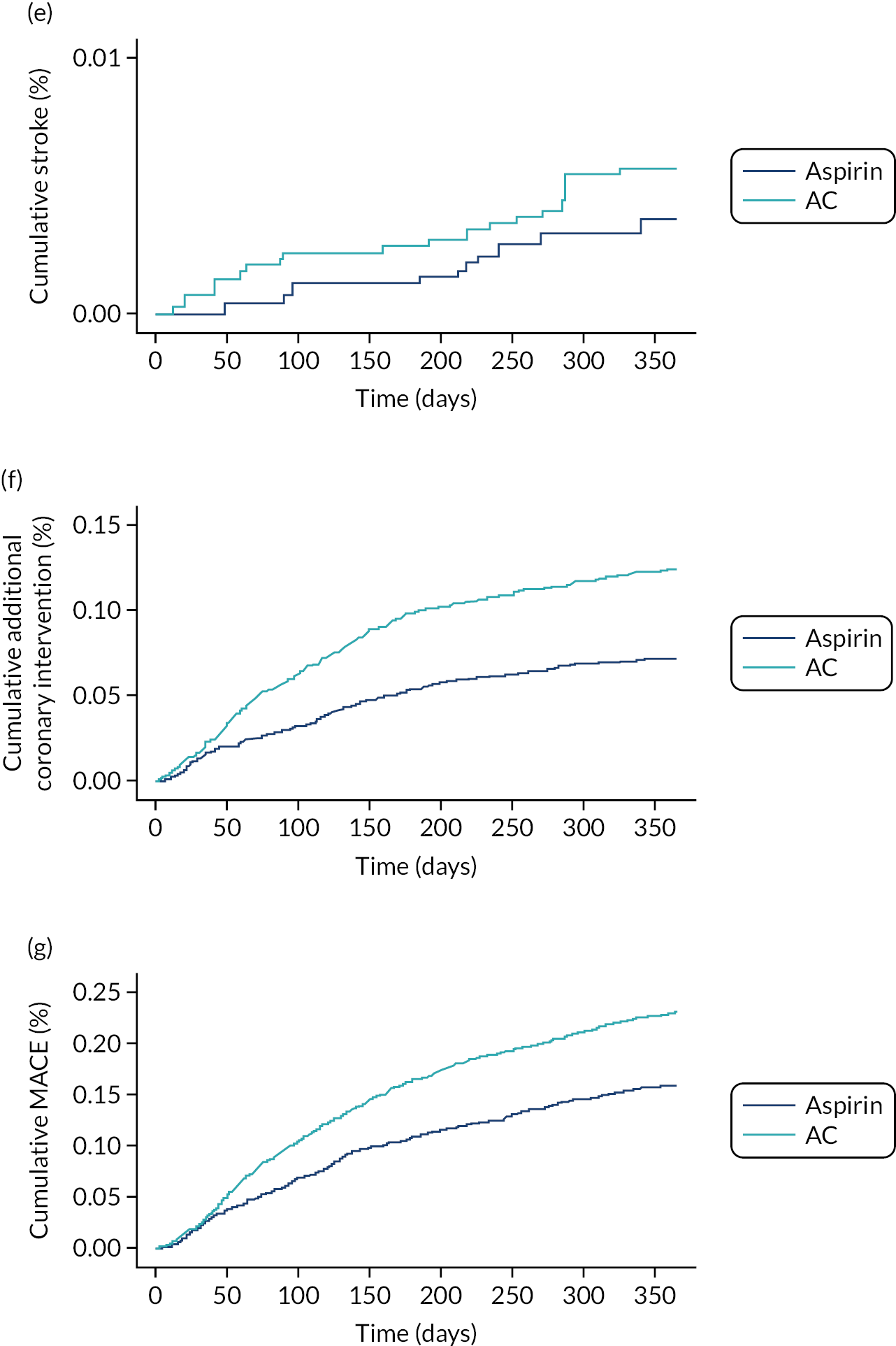
There were large differences in mortality and ischaemic events between patients included in the target trial and patients who were eligible for inclusion but were not included (40% of all eligible patients) (Table 23), although these were driven entirely by the group of patients excluded because they had a bleeding or ischaemic event prior to their first prescription in the CPRD.
| Included in target trial | Not included in target trial, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Secondary outcomes | Aspirin (N = 2609), n (%) | AC (N = 3977), n (%) | Overall (N = 6586), n (%) | Adjusted HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in CPRDa (N = 2293) | No prescription in CPRD within 2 months of discharge (N = 2064) | Overall (N = 4357) |
| All-cause mortality | 299 (11) | 534 (13) | 833 (13) | 1.03 (0.89 to 1.19)b | 1640 (72) | 197 (10) | 1837 (42) |
| Cardiovascular mortality | 140 (5) | 298 (7) | 438 (7) | 1.20 (0.98 to 1.08)c | 1215 (53) | 79 (4) | 1294 (30) |
| Mortality from bleeding | 7 (0.3) | 11 (0.3) | 18 (0.3) | 0.82 (0.31 to 2.16)d | 38 (2) | 7 (0.3) | 45 (1) |
| MI | 106 (4) | 328 (8) | 434 (7) | 1.87 (1.49 to 2.34)e | 113 (5) | 84 (4) | 197 (5) |
| Stroke | 10 (0.4) | 17 (0.4) | 27 (0.4) | 1.44 (0.63 to 3.28)f | 7 (0.3) | 14 (1) | 21 (0.5) |
| Additional coronary intervention | 176 (7) | 461 (12) | 637 (10) | 1.86 (1.55 to 2.24)g | 200 (9) | 94 (5) | 294 (7) |
| MACE | 374 (14) | 903 (23) | 1277 (19) | 1.57 (1.38 to 1.78)h | 1460 (64) | 224 (11) | 1684 (39) |
Analyses for the secondary outcomes (mortality and ischaemic events)
There was no association between antiplatelet prescription (AC vs. aspirin) and all-cause mortality, cardiovascular mortality, mortality from bleeding or stroke (see Table 23).
Treatment switches and adherence
Treatment switches are shown in Table 24. In the 12 months after the index event, 608 out of 2609 (23%) patients in the aspirin group were identified as ‘switchers’. There were 657 treatment switches; 431 (66%) initiated a second antiplatelet and 226 (34%) stopped aspirin. The median time to switching was 5 months among those who initiated a second antiplatelet and 8 months among those who stopped aspirin. On average, patients who initiated a second antiplatelet received 6.5 prescriptions of the second antiplatelet (6 months’ supply).
| Bleed occurred, n/N (%) | Ischaemic eventb occurred, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Type of switch, n/N (%) | Median (IQR) time to switch (months) | Before switcha | After switch | Before switch (within 2 months) | Before switch | After switch | No ischaemic or bleeding events, n/N (%) |
| Aspirin | Discontinued aspirin, 431/2609 (17) | 7.6 (5.7–9.9) | 18/431 (4) | 11/431 (3) | 4/431 (1) | 14/431 (3) | 7/431 (2) | 381/431 (88) |
| Initiated second antiplatelet, 226/2609 (9) | 4.8 (3.3–6.9) | 17/226 (8) | 16/226 (7) | 36/226 (16) | 42/226 (19) | 10/226 (4) | 146/226 (65) | |
| AC | Discontinued aspirin, 531/3977 (13) | 7.8 (5.5–10.3) | 59/531 (11) | 19/531 (4) | 11/531 (2) | 53/531 (10) | 17/531 (3) | 394/531 (74) |
| Discontinued clopidogrel, 269/3977 (7) | 7.7 (5.6–10.2) | 31/269 (12) | 6/269 (2) | 11/269 (4) | 37/269 (14) | 7/269 (3) | 194/269 (72) | |
| Discontinued AC, 156/3977 (4) | 6.4 (4.7–8.7) | 17/156 (11) | 10/156 (6) | 7/156 (4) | 20/156 (13) | 9/156 (6) | 106/156 (68) | |
| Initiated a different P2Y12 inhibitor, 30/3977 (1) | 5.0 (2.2–7.9) | < 5 | < 5 | 15/30 (50) | 16/30 (53) | 0/30 | 12/30 (40) | |
Among patients assigned AC, 668 out of 3977 (24%) were identified as switchers in the 12 months after the index event. There were 986 treatment switches; of these, 531 (54%) were aspirin discontinuations; 269 (27%) were clopidogrel discontinuations, 156 (16%) were aspirin and clopidogrel discontinuations and 30 (3%) were initiations of a different P2Y12 inhibitor. The median time to switching was between 5 and 8 months in all groups of switchers.
Across the groups, 283 switchers had a bleed or ischaemic event, 108 (38%) in aspirin and 175 (62%) in AC. Most of these events occurred before the switch. In the aspirin group, 12% of those who discontinued aspirin had a bleeding or ischaemic event, but 35% who initiated a second antiplatelet had a bleeding (33 patients) or ischaemic event (55 patients). The numbers of bleeding events before and after switching were similar in both groups of switchers, although the number of ischaemic events was highest before the switch among those who initiated a second antiplatelet, suggesting that the ischaemic event triggered the switch.
In the AC group, the proportion of patients experiencing bleeding and ischaemic events was highest among the switchers who initiated a different P2Y12 inhibitor (60%, 16 ischaemic events, all before the switch, and five bleeding events), but was also relatively high among those who discontinued both aspirin and clopidogrel (32%, 27 bleeds and 29 ischaemic events); it was lowest among the switchers who discontinued aspirin only (26%, 78 bleeds and 70 ischaemic events). Across all groups of switchers, a higher proportion experienced both bleeding and ischaemic events before, rather than after, the switch.
Adherence, defined as a MPR of ≥ 0.8, was 56% in the aspirin group and 60% in the AC group.
Discussion
In the conservatively managed ACS target trial, including 6586 patients, the overall rate of bleeding was 10%, whereas the rates of major and minor bleeding were 4% and 7%, respectively. The rates of major bleeding with aspirin and DAPT with clopidogrel that we observed in our population (4% for aspirin and 5% with DAPT) were slightly higher than, but comparable to, those reported in the conservatively managed ACS population (n = 7985) in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) RCT: 3% for aspirin and 4% for DAPT with AC. 62,63
The main finding in our conservatively managed ACS target trial was that, compared with aspirin, DAPT with clopidogrel increased the risk of any bleed by 43%, and the risk of major and minor bleeding by 34% and 51%, respectively. None of the sensitivity analyses markedly attenuated these HRs for bleeding. There was no evidence of subgroup effects, although a concurrent prescription for PPIs attenuated the effect of DAPT on any bleeding events. In the CURE trial, DAPT with clopidogrel also increased the risk of major bleeding by 38% (RR 1.38, 95% CI 1.13 to 1.67), which was comparable to the increase in risk we observed in our population.
Similar to the finding in the CABG target trial, DAPT with clopidogrel increased the hazards of MI, additional coronary intervention and MACEs by 88%, 86% and 58%, respectively, compared with aspirin. This is in contrast to the finding in the CURE RCT, which showed that DAPT with clopidogrel was associated with a 20% reduction in the combined end point of cardiovascular mortality, non-fatal MI and stroke (9% vs. 11%, RR 0.80, 95% CI 0.69 to 0.92). Other RCTs in ACS populations show a 20% decrease in the risk of secondary ischaemic events with DAPT with clopidogrel, regardless of revascularisation status. 64,65
Potential reasons for the discrepancy between our findings and those of the CURE trial are confounding, selection bias and treatment switches/adherence. Most baseline characteristics were reasonably balanced between the aspirin and the DAPT groups, and the risk factors for ischaemia that showed an imbalance between the aspirin and DAPT groups (smoking, hypercholesterolaemia, previous MI and revascularisation) were not uniformly in one direction, that is not always higher in the AC group (the proportion of smokers and of those with a history of MI was greater in the DAPT group, whereas the proportion of those who had a previous revascularisation and hypercholesterolaemia was higher in the aspirin group). These factors were all adjusted for in the analysis. Furthermore, unmeasured confounding is likely to be less of an issue in the conservatively managed ACS target trial than in the CABG or PCI target trials because there are no definitive clinical guidelines to guide antiplatelet selection and no procedure-specific characteristics to influence DAPT prescribing. Nevertheless, we cannot rule out that patients perceived to be at high risk of secondary ischaemic events were more likely to be prescribed DAPT, and indeed those in the AC group had a longer median length of stay (by 2 days) than those in the aspirin group, suggesting that the former had more significant ACS events.
Selection bias may also be an explanation for the observed results, as selection for the target trial is likely to be associated with both the assignment to intervention and the outcome. We excluded 40% (4357/10,943) of the eligible population because they experienced a major bleed or ischaemic event before their first prescription or because they had no prescription within 2 months of hospital discharge. The former (just over half of the excluded population, 2293/4357) were much older, with a higher incidence of previous MI, heart failure, renal disease and cancer, and so were likely to be at higher risk of secondary ischaemic events. An older, more frail population is more likely to be prescribed aspirin. It is possible that a true higher risk of MACE in the aspirin group may be masked by excluding this susceptible population from the risk set. Although we imputed intervention status at baseline in a sensitivity analysis, this was driven by the characteristics of patients included in the target trial, and, therefore, may not truly reflect the actual prescription at baseline. It is debatable the extent to which imputation for such a large number of missing data is effective. The extent to which selection bias is responsible for the observed results is not clear. It is worth noting that the survival curves for MACE (see Figure 14) continue to diverge until the end of follow-up, suggesting a true higher risk of MACE in the included population, rather than an effect driven by the exclusion of eligible participants with an early event, which would have affected the shape of the survival curves early during follow-up, but not later on.
Non-adherence was 40% in the DAPT group and 44% in the aspirin-only group. These DAPT adherence rates reflect those reported by other studies in real-world populations59–61 and mirror those in the CABG target trial. Over one-quarter of patients in the conservatively managed ACS target trial switched prescription (either stopped aspirin or initiated a second antiplatelet agent in the aspirin group, or stopped aspirin or clopidogrel or both aspirin and clopidogrel in the DAPT group). The proportion of patients experiencing bleeding and ischaemic events was highest among the switchers who discontinued both aspirin and clopidogrel (32%). It is possible that non-adherence in the higher-risk population prescribed DAPT contributed to the high incidence of MACEs that we observed.
Emergency percutaneous coronary intervention
Trends in antiplatelet prescribing and rates of bleeding over time
Figure 15 shows how the target trial population was assembled. Figure 16 show the trends in antiplatelet prescriptions and rates of bleeding between 2010 and 2017. There was a large decrease in AC (from 80.4% in 2010 to 30.9% in 2017) and a large increase in AT (from 0% and 0.4% in 2010/11, to 12.4% in 2012 and 54.4% in 2017) over time. There was a small increase in AP between 2010 and 2013, followed by a consistent decrease thereafter. Other prescriptions (P2Y12 inhibitor monotherapy, aspirin only and other, e.g. more than one P2Y12 inhibitor) remained steady, but were below 7%. Major bleeding rates were similar over time, at 29.6 and 26.7 events per 1000 person-years in 2010 and 2017, respectively. Minor bleeding rates increased slightly, from 75.1 to 89.5 events per 1000 person-years, in 2010 and 2017, respectively.
FIGURE 15.
Flow diagram describing the construction of the emergency PCI target trial. C, clopidogrel; P, prasugrel; SA, sensitivity analysis; T, ticagrelor.
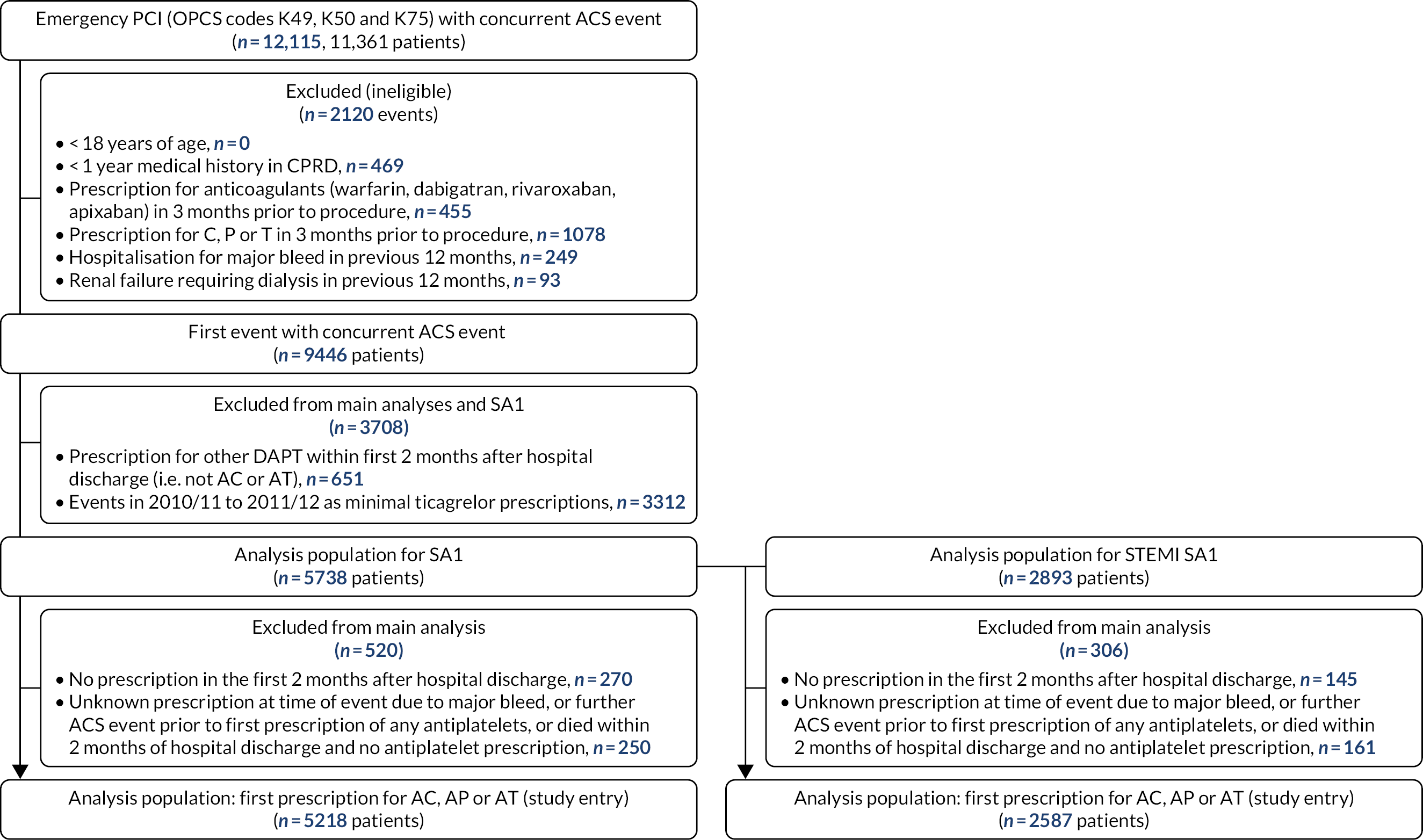
FIGURE 16.
Proportion of emergency PCI patients being prescribed different antiplatelet regimes by year and rates of major (HES), minor (CPRD) and total bleeding events by year among emergency PCI patients. (a) Different antiplatelet regimes; and (b) bleeding events.
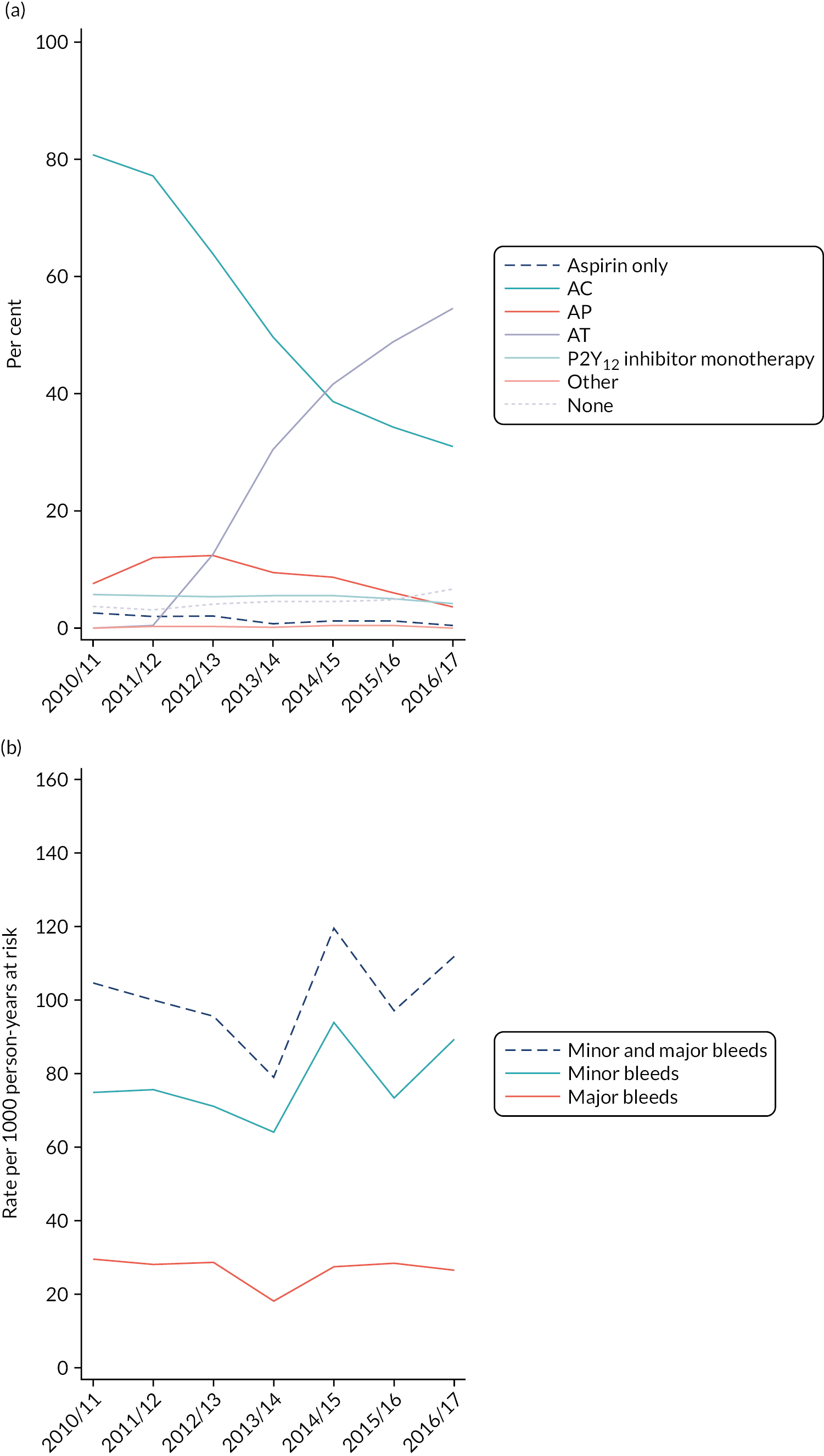
Baseline characteristics of participants included in and those excluded from the target trial
Table 25 shows the baseline characteristics of participants included in and those excluded from the target trial. Of the 11,361 patients with linked CPRD–HES data, 5738 (51%) were eligible and included, and 520 (9%) were excluded because they could not be assigned to an intervention group. The covariates with an imbalance between the AC and the AT groups were age (mean age of 66.1 years in the AC group vs. 62.5 years in the AT group, SMD 0.3), smoking (27% were smokers in the AC group vs. 34% in the AT group, SMD 0.15), history of IHD (90% in the AC group vs. 83% in the AT group, SMD 0.19), hypertension (43% in the AC group vs. 35% in the AT group, SMD 0.17), hypercholesterolaemia (22% in the AC group vs. 16% in the AT group, SMD 0.16), peripheral vascular disease (5% in the AC group vs. 3% in the AT group, SMD 0.11) and renal disease (5% in the AC group vs. 3% in the AT group, SMD 0.11). There was no difference in median length of hospital stay between the AC and the AT groups [median 2 (IQR 1–3) days for both groups].
| Characteristic | AC (N = 2769) | AP (N = 529) | AT (N = 1920) | SMD (AC vs. AT)a | Unknownb (N = 520) | SMDc | Overall (N = 5738) |
|---|---|---|---|---|---|---|---|
| Demography | |||||||
| Year of event, n (%) | |||||||
| 2012/13 | 1090 (39) | 212 (40) | 213 (11) | 0.78 | 136 (26) | 0.08 | 1651 (29) |
| 2013/14 | 710 (26) | 134 (25) | 437 (23) | 124 (24) | 1405 (24) | ||
| 2014/15 | 493 (18) | 110 (21) | 532 (28) | 118 (23) | 1253 (22) | ||
| 2015/16 | 302 (11) | 53 (10) | 431 (22) | 84 (16) | 870 (15) | ||
| 2016/17 | 174 (6) | 20 (4) | 307 (16) | 58 (11) | 559 (10) | ||
| Age (years), mean (SD) | 66.1 (12.4) | 58.8 (10.4) | 62.5 (11.9) | 0.30 | 67.5 (14.1) | 0.26 | 64.4 (12.5) |
| Sex, n (%) | |||||||
| Male | 2007 (72) | 425 (80) | 1411 (73) | 0.02 | 374 (72) | 0.04 | 4217 (73) |
| Female | 762 (28) | 104 (20) | 509 (27) | 146 (28) | 1521 (27) | ||
| BMId (kg/m2), mean (SD) | 28.3 (5.1) | 28.2 (4.9) | 28.4 (5.3) | 0.01 | 27.4 (5.2) | 0.18 | 28.3 (5.2) |
| Ethnic group, n (%) | |||||||
| White | 2520 (91) | 484 (91) | 1755 (91) | 0.01 | 470 (90) | 0.03 | 5229 (91) |
| Other than White | 249 (9) | 45 (9) | 165 (9) | 50 (10) | 509 (9) | ||
| Smoking category,e n (%) | |||||||
| Ex-smoker | 891 (33) | 136 (27) | 552 (30) | 0.15 | 164 (33) | 0.08 | 1743 (32) |
| Non-smoker | 1047 (39) | 144 (28) | 652 (36) | 162 (33) | 2005 (36) | ||
| Smoker | 730 (27) | 227 (45) | 627 (34) | 166 (34) | 1750 (32) | ||
| Medical history , n (%) | |||||||
| History of MI (ever) | 2153 (78) | 381 (72) | 1463 (76) | 0.03 | 316 (61) | 0.35 | 4313 (75) |
| History of CABG/PCI (ever) | 899 (32) | 115 (22) | 573 (30) | 0.03 | 162 (31) | 0.02 | 1749 (30) |
| Bleeding | 50 (2) | 13 (2) | 42 (2) | 0.03 | 10 (2) | 0.01 | 115 (2) |
| Previous surgery | 126 (5) | 15 (3) | 52 (3) | 0.10 | 26 (5) | 0.06 | 219 (4) |
| Comorbidity , n (%) | |||||||
| History of IHD (ever) | 2489 (90) | 410 (78) | 1595 (83) | 0.19 | 352 (68) | 0.43 | 4846 (84) |
| Diabetes | 568 (21) | 72 (14) | 356 (19) | 0.05 | 92 (18) | 0.04 | 1088 (19) |
| Hypertension | 1192 (43) | 124 (23) | 665 (35) | 0.17 | 186 (36) | 0.05 | 2167 (38) |
| Hypercholesterolaemia | 597 (22) | 56 (11) | 306 (16) | 0.15 | 69 (13) | 0.14 | 1028 (18) |
| Peripheral vascular disease | 141 (5) | 16 (3) | 57 (3) | 0.11 | 25 (5) | 0.03 | 239 (4) |
| Stroke | 12 (0.4) | < 5 | 5 (0.3) | 0.04 | < 5 | 0.06 | ** |
| Heart failure | 193 (7) | 35 (7) | 115 (6) | 0.03 | 58 (11) | 0.16 | 401 (7) |
| Peptic ulcer disease | 10 (0.4) | 0 | < 5 | 0.03 | < 5 | 0.02 | 16 (0.3) |
| Haemodialysis or renal disease | 139 (5) | 9 (2) | 57 (3) | 0.11 | 31 (6) | 0.09 | 236 (4) |
| Cancer | 134 (5) | 14 (3) | 57 (3) | 0.10 | 39 (8) | 0.15 | 244 (4) |
| Clotting disorder | 5 (0.2) | 0 | < 5 | 0.001 | < 5 | 0.01 | 10 (0.2) |
| Anaemia | 80 (3) | 6 (1) | 26 (1) | 0.11 | 19 (4) | 0.09 | 131 (2) |
| Liver cirrhosis | 0 | 0 | < 5 | 0 | < 5 | 0.08 | < 5 |
| Co-interventions , n (%) | |||||||
| NSAIDs | 552 (20) | 87 (16) | 351 (18) | 0.04 | 74 (14) | 0.13 | 1064 (19) |
| Steroids | 256 (9) | 33 (6) | 161 (8) | 0.03 | 59 (11) | 0.09 | 509 (9) |
| PPIs | 994 (36) | 159 (30) | 618 (32) | 0.08 | 158 (30) | 0.08 | 1929 (34) |
| Anticoagulants | 17 (1) | < 5 | 6 (0.3) | 0.04 | 8 (2) | 0.10 | ** |
For patients excluded from the primary analysis, compared with patients included, the covariates with an imbalance were age (mean 64.0 years in the included population vs. 67.5 years in the excluded population, SMD 0.26), BMI (mean 28.4 kg/m2 in the included population vs. 27.4 kg/m2 in the excluded population, SMD 0.18), history of MI (77% in the included population vs. 61% in the excluded population, SMD 0.35), history of IHD (86% in the included population vs. 68% in the excluded population, SMD 0.43), hypercholesterolaemia (18% in the included population vs. 13% in the excluded population, SMD 0.14), heart failure (7% in the included population vs. 11% in the excluded population, SMD 0.16), cancer (4% in the included population vs. 8% in the excluded population, SMD 0.15) and prescription for non-steroidal anti-inflammatory drugs (NSAIDs) (19% in the included population vs. 14% in the excluded population, SMD 0.13).
Of the 520 patients without intervention, 250 (48%) died or had a major bleed or ACS event before their first prescription, and 270 (52%) had no prescription for an antiplatelet agent in the 2 months after their index event. The characteristics of patients who died or had a major bleed or ACS event, compared with those who had no antiplatelet prescription within 2 months of discharge, are shown in Table 26. The former were older (72 years vs. 63 years, respectively, SMD 0.70); had a greater proportion of women (33% vs. 23%, respectively, SMD 0.22); had fewer smokers (28% vs. 39%, respectively, SMD 0.25); had greater proportions of patients with a history of CABG/PCI (37% vs. 26%, respectively, SMD 0.25), previous surgery (7% vs. 3%, respectively, SMD 0.16), diabetes (24% vs. 12%, respectively, SMD 0.30), hypertension (43% vs. 29%, respectively, SMD 0.30), peripheral vascular disease (6% vs. 4%, respectively, SMD 0.11), heart failure (14% vs. 9%, respectively, SMD 0.15), renal disease (99% vs. 3%, respectively, SMD 0.26) and liver cirrhosis (1% vs. 0%, respectively, SMD 0.13); and a smaller proportion of patients with previous bleeding (7% vs. 3%, respectively, SMD 0.16). Patients who died or had an event also had more prescriptions of steroids, PPIs and anticoagulants (all SMDs > 0.1).
| Characteristic | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 250) | No prescription in the CPRD within 2 months of discharge (N = 270) | SMD | Total known (N = 5218) |
|---|---|---|---|---|
| Demography | ||||
| Year of event, n (%) | ||||
| 2012/13 | 67 (27) | 69 (26) | 0.18 | 1515 (29) |
| 2013/14 | 60 (24) | 64 (24) | 1281 (25) | |
| 2014/15 | 61 (24) | 57 (21) | 1135 (22) | |
| 2015/16 | 41 (16) | 43 (16) | 786 (15) | |
| 2016/17 | 21 (8) | 37 (14) | 501 (10) | |
| Age (years), mean, (SD) | 72.3 (12.9) | 62.9 (13.6) | 0.70 | 64.0 (12.3) |
| Sex, n (%) | ||||
| Male | 167 (67) | 207 (77) | 0.22 | 3843 (74) |
| Female | 83 (33) | 63 (23) | 1375 (26) | |
| BMIa (kg/m2), mean (SD) | 27.6 (5.3) | 27.3 (5.2) | 0.04 | 28.4 (5.1) |
| Ethnic group, n (%) | ||||
| White | 226 (90) | 244 (90) | 0.001 | 4759 (91) |
| Other than white | 24 (10) | 26 (10) | 459 (9) | |
| Smoking category,b n (%) | ||||
| Ex-smoker | 92 (38) | 72 (28) | 0.25 | 1579 (32) |
| Non-smoker | 79 (33) | 83 (33) | 1843 (37) | |
| Smoker | 68 (28) | 98 (39) | 1584 (32) | |
| Medical history , n (%) | ||||
| History of MI (ever) | 149 (60) | 167 (62) | 0.05 | 3997 (77) |
| History of CABG/PCI (ever) | 93 (37) | 69 (26) | 0.25 | 1587 (30) |
| Bleeding | < 5 | 8 (3) | 0.16 | 105 (2) |
| Previous surgery | 17 (7) | 9 (3) | 0.16 | 193 (4) |
| Comorbidity , n (%) | ||||
| History of IHD (ever) | 165 (66) | 187 (69) | 0.07 | 4494 (86) |
| Diabetes | 59 (24) | 33 (12) | 0.30 | 996 (19) |
| Hypertension | 108 (43) | 78 (29) | 0.30 | 1981 (38) |
| Hypercholesterolaemia | 34 (14) | 35 (13) | 0.02 | 959 (18) |
| Peripheral vascular disease | 15 (6) | 10 (4) | 0.11 | 214 (4) |
| Stroke | < 5 | < 5 | 0.09 | 18 (0.3) |
| Heart failure | 34 (14) | 24 (9) | 0.15 | 343 (7) |
| Peptic ulcer disease | < 5 | 0 (0) | 0.13 | 14 (0.3) |
| Haemodialysis or renal disease | 23 (9) | 8 (3) | 0.26 | 205 (4) |
| Cancer | 22 (9) | 17 (6) | 0.09 | 205 (4) |
| Clotting disorder | < 5 | 0 (0) | 0.09 | 9 (0.2) |
| Anaemia | 11 (4) | 8 (3) | 0.08 | 112 (2) |
| Liver cirrhosis | < 5 | 0 (0) | 0.13 | 1 (0.02) |
| Valve disease | 14 (6) | 13 (5) | 0.04 | 178 (3) |
| Co-interventions , n (%) | ||||
| NSAIDs | 40 (16) | 34 (13) | 0.10 | 990 (19) |
| Steroids | 34 (14) | 25 (9) | 0.14 | 450 (9) |
| PPIs | 93 (37) | 65 (24) | 0.29 | 1771 (34) |
| Anticoagulants | 7 (3) | < 5 | 0.20 | 27 (1) |
Bleeding events among participants included in and those excluded from the target trial
In the emergency PCI target trial, comprising all patients with ACS undergoing emergency PCI (3845 STEMI, 3082 NSTEMI and 4186 unstable angina patients), we compared AC with AT. AP was prescribed to ACS STEMI patients only; therefore, the comparison of AC with AP was restricted to the STEMI population. Of the 4689 patients prescribed AC or AT, 416 (9%) experienced at least one bleeding event: 209 out of 2769 (8%) patients prescribed AC and 207 out of 1920 (11%) patients prescribed AT. With regard to major and minor bleeding events, 117 out of 4689 (3%) patients experienced a major bleed and 332 out of 4689 (7%) experienced a minor bleed. The proportion of patients experiencing a major or a minor bleeding event was 63 out of 2769 (2%) and 161 out of 2769 (6%), respectively, in the AC group and 54 out of 1920 (3%) and 171 out of 1920 (9%), respectively, in the AT group.
Figure 17 shows the Kaplan–Meier curves of cumulative bleeding events [any, major (HES) and minor (CPRD)] in the AC and AT groups. The cumulative incidence of any bleeding increased steadily over the 12 months, but was higher in the AT group than in the AC group. The survival curves crossed twice. Major bleeds were initially more frequent in the AT group, until approximately 50 days, then were more frequent in the AC group, until approximately 200 days (6.5 months), and thereafter were more frequent in the AT group. Minor bleeds were consistently more frequent in the AT group than in the AC group.
FIGURE 17.
Kaplan–Meier curves displaying cumulative bleeding according to intervention group. (a) Any bleeding; (b) major bleeding; and (c) minor bleeding. Plots are weighted according to the inverse probability of treatment received, and so compare outcomes if all eligible patients received AC or AT.
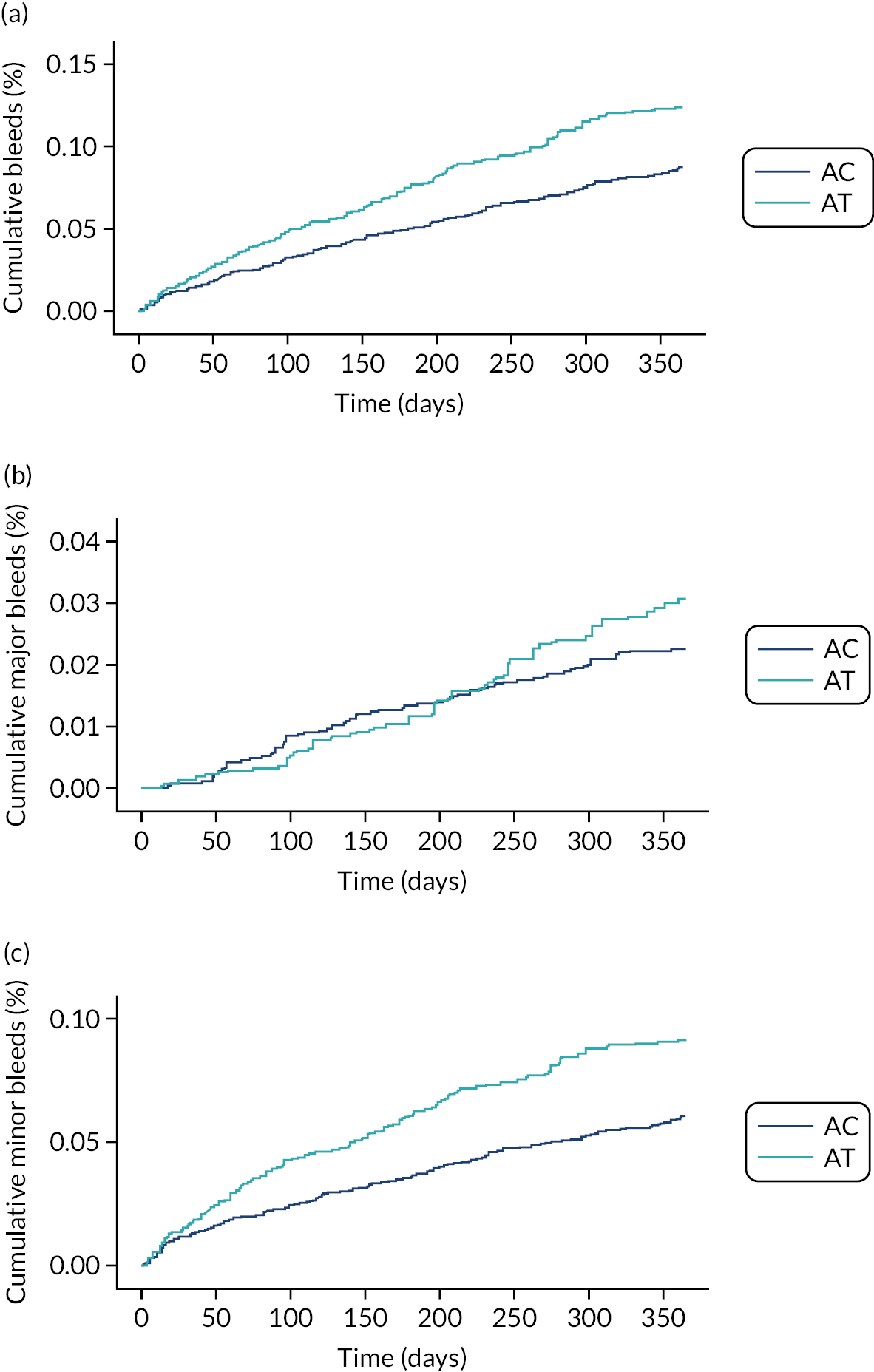
In the AT group, compared with the AC group, the crude incidence rates of major and of minor bleeds were 24% higher (29 vs. 23 events per 1000 person-years) and 56% higher (94 vs. 60 events per 1000 person-years), respectively (Table 27). Of those who experienced a bleeding event within 12 months, the majority of patients experienced only one bleeding event (310/416, 75%), 78 out of 416 (19%) experienced two bleeding events, and the remainder (28/416, 7%) experienced three or more bleeds. Bleeds by site are shown in Table 28; there were no major differences between intervention groups.
| AC | AT | |||||
|---|---|---|---|---|---|---|
| Bleeds | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) |
| Major (HES) | 63 | 2731 | 23.1 (18.0 to 29.5) | 54 | 1888 | 28.6 (21.9 to 37.3) |
| Minor (CPRD) | 161 | 2671 | 60.3 (51.7 to 70.3) | 170 | 1807 | 94.1 (81.0 to 109.4) |
| All (CPRD and HES) | 209 | 2344 | 89.2 (77.9 to 102.1) | 206 | 1543 | 133.5 (116.5 to 153.1) |
| Bleeds recorded (HES or CPRD), n (%) | |||
|---|---|---|---|
| Bleed site | AC (N = 2769) | AT (N = 1920) | Total (N = 4689) |
| Ear, nose or throat | 47 (17) | 65 (22) | 112 (19) |
| Gastrointestinal | 117 (42) | 108 (36) | 225 (39) |
| Genitourinary | 8 (3) | 6 (2) | 14 (2) |
| Intracranial | 8 (3) | < 5 | ** |
| Ocular | 11 (4) | 9 (3) | 20 (3) |
| Skin or soft tissue | 76 (27) | 100 (30) | 176 (30) |
| Other anatomical site | 5 (2) | < 5 | ** |
| Unspecified anatomical site | 6 (2) | 7 (2) | 13 (2) |
| Total | 278 | 301 | 579 |
Patients who could not be assigned an intervention because they experienced a bleed or ischaemic event or died before their first prescription, or because they had no prescription in the CPRD within 2 months of discharge (9% of the eligible population), had a lower bleeding rate than the patients included in the target trial (3% vs. 9%) (see Table 29). The bleeding rate was slightly higher among those who had no prescription in the CPRD within 2 months of discharge than among those who experienced an event or died (3% vs. 2%).
| Bleeding events, n/N (%) | Bleeding events, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Patients (n) | AC | AT | HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in the CPRDa | No prescription in the CPRD within 2 months of discharge | Overall, n/N (%) |
| Primary outcome | |||||||
| Crude | 4689 | 209/2769 (8) | 207/1920 (11) | 1.48 (1.22 to 1.80) | 5/250 (2) | 9/270 (3) | 14/520 (3) |
| Adjusted | 1.47 (1.19 to 1.82)b,c | ||||||
| SA1: multiple imputation for unknown intervention group | 5209 | 1.45 (1.28 to 2.39)b,c | |||||
| SA3: restricted to patients at low risk of bleeding | 4409 | 196/2565 (8) | 194/1844 (11) | 1.44 (1.16 to 1.80)b,d | |||
| SA4: primary adjusted analysis without censoring of any CPRD or HES bleed at transfer-out or last collection date | 4689 | 215/2769 (8) | 212/1920 (11) | 1.46 (1.18 to 1.80)b,e | |||
| Major bleeding (HES reported) | 4689 | 63/2769 (2) | 54/1920 (3) | 1.33 (0.89 to 1.99)b,f | 6/250 (2) | 11/270 (4) | 17/520 (3) |
| Minor bleeding (CPRD reported) | 4689 | 161/2769 (6) | 171/1920 (9) | 1.60 (1.26 to 2.03)b,g | < 5 | < 5 | < 5 |
Analyses for the primary outcome (bleeding)
The primary analysis excluded patients for whom we could not assign an intervention (n = 4689). The crude and adjusted HRs indicated an increase of about 50% in the hazard of bleeding in the AT group compared with the AC group (HR 1.48, 95% CI 1.22 to 1.80, and HR 1.47, 95% CI 1.19 to 1.82, respectively) (Table 29). When split into major and minor bleeding, there was a 33% increased hazard of major bleeding (HR 1.33, 95% CI 0.89 to 1.99) and a 60% increased hazard of minor bleeding (HR 1.60, 95% CI 1.26 to 2.03) in the AT group compared with the AC group.
Sensitivity analyses
The HRs did not change substantially for sensitivity analysis 1 (multiple imputation for 5209 patients with unknown intervention, HR 1.45, 95% CI 1.28 to 2.39) or for sensitivity analyses 3 or 4 (Table 30). We did not conduct sensitivity analysis 2 (exclusion of patients who changed medication before first bleeding event) because very few patients (14/475, 3%) changed medication before their first bleeding event (so this did not meet our prespecified threshold of > 10% of the population).We also did not conduct sensitivity analysis 5, the instrumental variable analysis. There was evidence of an association between previous prescription and current prescription (OR 10.61, 95% CI 9.12 to 12.34; p < 0.001), but no evidence of an association between previous prescription and bleeding (OR 1.18, 95% CI 0.95 to 1.46; p = 0.25). The instrumental variable analysis was not explored any further.
| Included in target trial | Not included in target trial,a n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Secondary outcomes | AC (N = 2769), n (%) | AT (N = 1920), n (%) | Overall (N = 4689), n (%) | Adjusted HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 250) | No prescription in the CPRD within 2 months of discharge (N = 270) | Overall (N = 520) |
| All-cause mortality | 70 (3) | 34 (2) | 104 (2) | 0.94 (0.60 to 1.47)b | 219 (88) | 11 (4) | 230 (44) |
| Cardiovascular mortality | 32 (1) | 16 (1) | 48 (1) | 0.92 (0.48 to 1.78)c | 195 (78) | 6 (2) | 201 (39) |
| Mortality from bleeding | 6 (0.2) | < 5 | ** | 0.33 (0.04 to 2.86)d | 7 (3) | 0 | 7 (1) |
| MI | 85 (3) | 48 (3) | 133 (3) | 0.91 (0.61 to 1.34)e | < 5 | 9 (3) | ** |
| Stroke | 5 (0.2) | < 5 | ** | 1.56 (0.40 to 6.03)f | < 5 | < 5 | < 5 |
| Additional coronary intervention | 272 (10) | 203 (11) | 475 (10) | 1.03 (0.85 to 1.26)g | 8 (3) | 29 (11) | 37 (7) |
| MACE | 337 (12) | 249 (13) | 586 (12) | 1.06 (0.89 to 1.27)h | 203 (81) | 40 (15) | 243 (47) |
Subgroup analyses
There was no evidence of any subgroup effects for people with diabetes compared with people without diabetes (p = 0.13, interaction test), for people with chronic kidney disease compared with people without chronic kidney disease (p = 0.22) or for a concurrent prescription for PPIs compared with no concurrent prescription for PPIs (p = 0.98).
Mortality and ischaemic events among participants included in and those excluded from the target trial
Figure 18 shows the Kaplan–Meier curves for the secondary outcomes of all-cause and cardiovascular mortality, mortality from bleeding, MI, stroke, additional coronary intervention and the composite outcome of MACE. There were large differences in mortality and ischaemic events between patients included in the target trial and patients who were eligible for inclusion but were not included (9% of all eligible patients) (see Table 30).
FIGURE 18.
Kaplan–Meier curves displaying cumulative all-cause and cardiovascular mortality, mortality from bleeding and ischaemic events, according to intervention group. (a) All-cause mortality; (b) cardiovascular mortality; (c) mortality from bleeding; (d) MI; (e) stroke; (f) additional PCI; and (g) MACE.
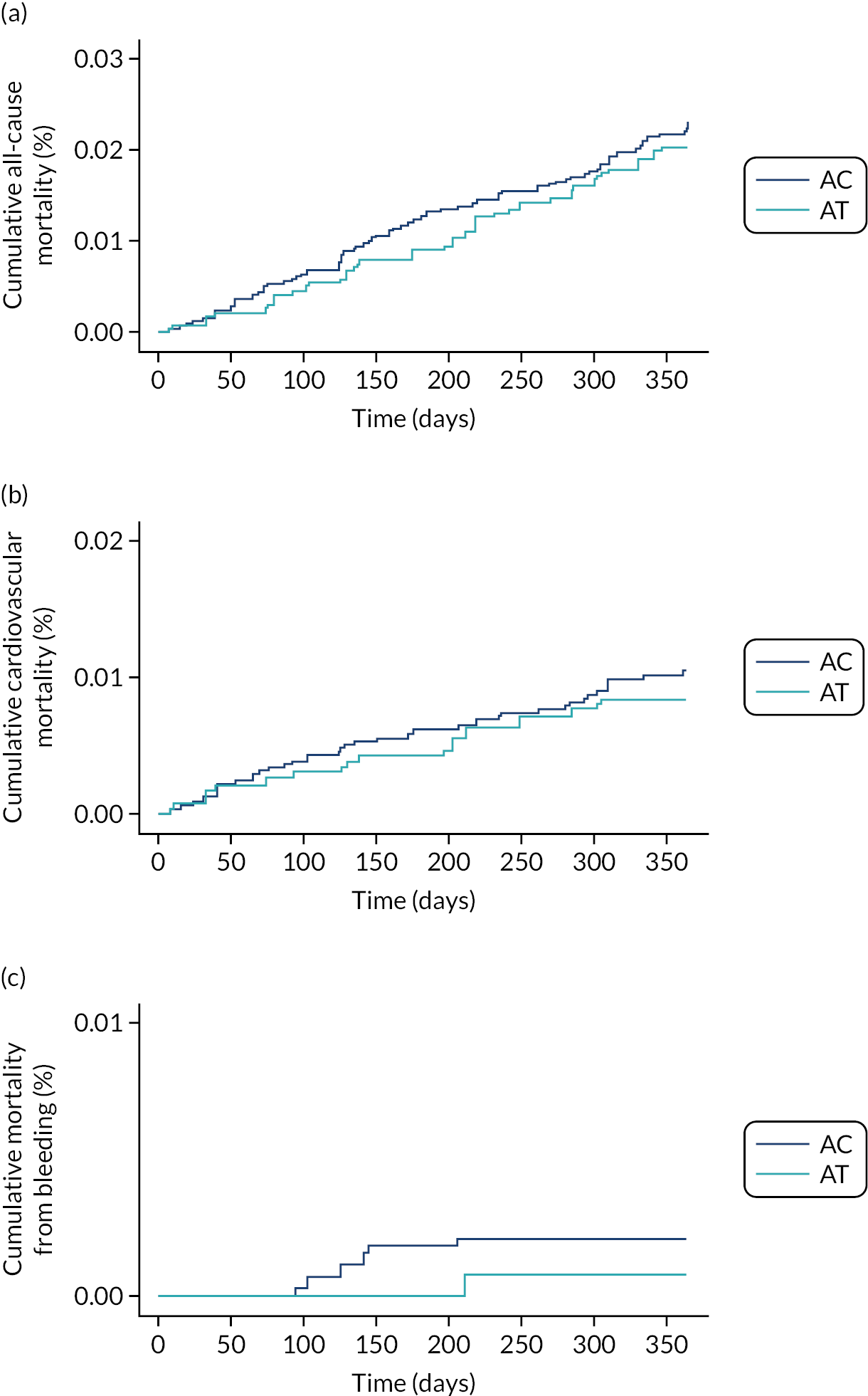
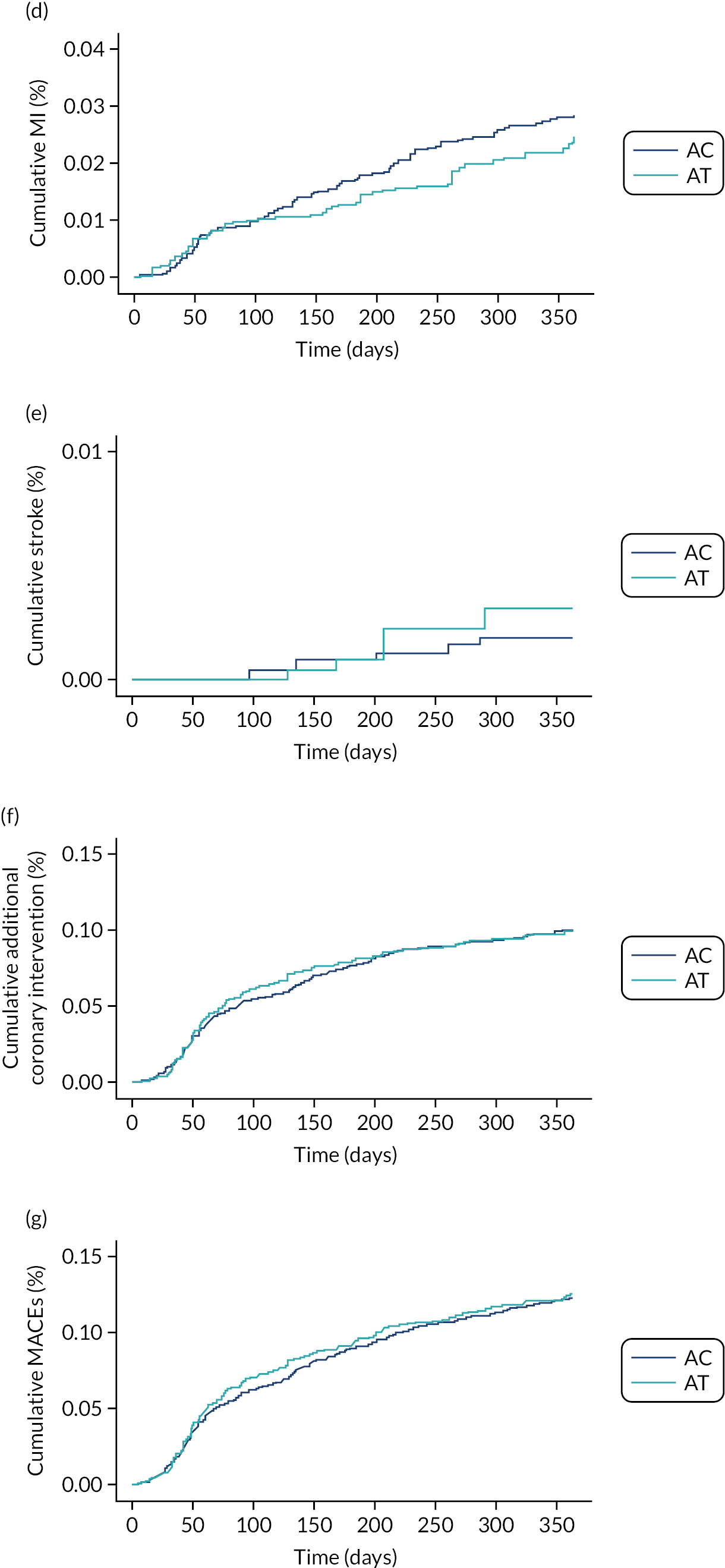
Analyses for the secondary outcomes (mortality and ischaemic events)
There was no association between antiplatelet prescription (AT vs. AC) and any of the secondary outcomes (see Table 30).
Treatment switches and adherence
Table 31 shows treatment switches in the emergency PCI population by intervention group (AC and AT), and by type of switch and whether the switch occurred before or after a bleeding or ischaemic event.
| Bleed occurred, n/N (%) | Ischaemic eventb occurred, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Type of switch, n/N (%) | Median (IQR) time to switch (months) | Before switcha | After switch | Before switch (within 2 months) | Before switch | After switch | No ischaemic or bleeding events, n/N (%) |
| AC | Discontinued aspirin, 300/2769 (11) | 8.0 (5.6–10.9) | 19/300 (6) | 8/300 (3) | 5/300 (2) | 19/300 (6) | 6/300 (2) | 251/300 (84) |
| Discontinued clopidogrel, 124/2769 (4) | 8.0 (5.9–10.2) | 8/124 (6) | < 5 | < 5 | 12/124 (10) | < 5 | 102/124 (82) | |
| Discontinued AC, 84/2769 (3) | 7.9 (5.5–9.9) | 5/84 (6) | < 5 | < 5 | 8/84 (10) | < 5 | 66/84 (79) | |
| Initiated a different P2Y12 inhibitor, 52/2769 (2) | 2.0 (1.0–3.8) | < 5 | < 5 | 11/52 (21) | 11/52 (21) | < 5 | 34/52 (65) | |
| AT | Discontinued aspirin, 210/1920 (11) | 8.0 (6.0–10.3) | 22/210 (10) | < 5 | < 5 | 8/210 (4) | < 5 | 177/210 (84) |
| Discontinued ticagrelor, 154/1920 (8) | 8.1 (6.3–10.3) | 12/154 (8) | < 5 | < 5 | 8/154 (5) | < 5 | 129/154 (84) | |
| Discontinued AT, 85/1920 (4) | 7.6 (6.1–9.7) | 7/85 (8) | < 5 | < 5 | 5/85 (6) | < 5 | 69/85 (81) | |
| Initiated a different P2Y12 inhibitor, 151/1920 (8) | 3.3 (1.9–6.0) | 11/151 (7) | 7/151 (5) | < 5 | < 5 | < 5 | 128/151 (85) | |
In the 12 months after the index event, 379 out of 2769 (14%) patients in the AC group were identified as ‘switchers’. There were 560 treatment switches; 300 of these (54%) were aspirin discontinuations, 124 (22%) were clopidogrel discontinuations, 84 (15%) were aspirin and clopidogrel discontinuations and 52 (9%) were initiations of a different P2Y12 inhibitor.
Among patients assigned AT, 404 out of 1920 (21%) were identified as switchers. There were 454 treatment switches; of these, 200 (44%) were aspirin discontinuations, 154 (34%) were ticagrelor discontinuations, 85 (19%) were aspirin and ticagrelor discontinuations and 15 (3%) were initiations of a different P2Y12 inhibitor.
The median time to switch was around 8 months in all groups of switchers. Across AC and AT, 125 switchers had a bleed or ischaemic events, 65 (52%) in AC and 60 (48%) in AT. Most of these events occurred before the switch.
In all intervention groups, the number of ischaemic events was larger among those who switched, compared with event rates in the population overall. Adherence, defined as a MPR of ≥ 0.8, was 71% in the AC group, 69% in the AP group and 68% in the AT group.
Emergency percutaneous coronary intervention restricted to ST-elevation myocardial infarction patients
Trends in antiplatelet prescribing and rates of bleeding over time
The target trial population is shown in Figure 15. Trends in antiplatelet prescriptions and rates of bleeding between 2010 and 2017 are shown in Figure 19. There was a large decrease in DAPT prescriptions with AC (from 74.5% in 2010 to 16.7% in 2017) and a large increase in DAPT prescriptions with AT (from 0% and 0.6% in 2010/11, to 14.6% in 2012 and 67.0% in 2017) over time. Prescriptions of DAPT with AP increased from 13.3% in 2010 to 21.3% in 2011, remained at this level until 2012 and then decreased thereafter to reach 5.9% in 2017. Prescriptions of aspirin and P2Y12 inhibitor monotherapy remained steady, and a small proportion of patients (about 5% over 2010–17) received no antiplatelet prescription at all. Despite the large increase in AT prescriptions over time, major bleeding rates increased only marginally, from 28.4 events per 1000 person-years to 36.4 events per 1000 person-years in 2017. Minor bleeding rates increased over time, from 73.8 to 113.8 events per 1000 person-years in 2010 and 2017, respectively.
FIGURE 19.
Proportion of emergency PCI STEMI patients being prescribed different antiplatelet regimes by year and rates of major (HES), minor (CPRD) and total bleeding events by year among emergency PCI STEMI patients. (a) Different antiplatelet regimes; and (b) bleeding events.
Baseline characteristics of participants included in and those excluded from the target trial
Table 32 shows the baseline characteristics of patients included in and those excluded from the primary analysis. Of the 5738 patients with linked CPRD–HES data and eligible for the emergency PCI analysis, 2893 (50%) were STEMI patients. Of these patients, 306 (11%) were excluded because they could not be assigned to an intervention group.
| Characteristics | AC (N = 1023) | AP (N = 406) | AT (N = 1158) | SMD (AC vs. AP)a | SMD (AC vs. AT)a | Unknownb (N = 306) | Overall (N = 2893) | SMDc |
|---|---|---|---|---|---|---|---|---|
| Demography | ||||||||
| Year of event, n (%) | ||||||||
| 2012/13 | 425 (42) | 170 (42) | 118 (10) | 0.12 | 0.91 | 84 (27) | 797 (28) | 0.06 |
| 2013/14 | 288 (28) | 98 (24) | 243 (21) | 70 (23) | 699 (24) | |||
| 2014/15 | 163 (16) | 78 (19) | 323 (28) | 65 (21) | 629 (22) | |||
| 2015/16 | 99 (10) | 43 (11) | 281 (24) | 56 (18) | 479 (17) | |||
| 2016/17 | 48 (5) | 17 (4) | 193 (17) | 31 (10) | 289 (10) | |||
| Age (years), mean (SD) | 65.8 (12.7) | 58.8 (10.0) | 62.0 (12.0) | 0.61 | 0.31 | 67.1 (14.6) | 63.4 (12.6) | 0.31 |
| Sex, n (%) | ||||||||
| Male | 736 (72) | 331 (82) | 856 (74) | 0.23 | 0.04 | 213 (70) | 2136 (74) | 0.11 |
| Female | 287 (28) | 75 (18) | 302 (26) | 93 (30) | 757 (26) | |||
| BMId (kg/m2), mean (SD) | 27.8 (4.8) | 28.1 (4.6) | 28.0 (5.1) | 0.07 | 0.04 | 27.3 (4.9) | 27.9 (4.9) | 0.14 |
| Ethnic group, n (%) | ||||||||
| White | 944 (92) | 372 (92) | 1066 (92) | 0.02 | 0.01 | 281 (92) | 2663 (92) | 0.01 |
| Other than white | 79 (8) | 34 (8) | 92 (8) | 25 (8) | 230 (8) | |||
| Smoking category,e n (%) | ||||||||
| Ex-smoker | 295 (30) | 105 (27) | 322 (29) | 0.27 | 0.11 | 91 (32) | 813 (30) | 0.07 |
| Non-smoker | 359 (37) | 107 (28) | 363 (33) | 88 (31) | 917 (33) | |||
| Smoker | 318 (33) | 177 (46) | 414 (38) | 108 (38) | 1017 (37) | |||
| Medical history, n (%) | ||||||||
| History of MI (ever) | 797 (78) | 290 (71) | 854 (74) | 0.14 | 0.09 | 163 (53) | 2104 (73) | 0.47 |
| History of CABG/PCI (ever) | 243 (24) | 71 (17) | 279 (24) | 0.10 | 0.05 | 87 (28) | 680 (24) | 0.13 |
| Bleeding | 24 (2) | 11 (3) | 28 (2) | 0.02 | 0.01 | 6 (2) | 69 (2) | 0.03 |
| Previous surgery | 34 (3) | 10 (2) | 31 (3) | 0.05 | 0.04 | 12 (4) | 87 (3) | 0.06 |
| Comorbidity , n (%) | ||||||||
| History of IHD (ever) | 842 (82) | 305 (75) | 905 (78) | 0.17 | 0.10 | 174 (57) | 2226 (77) | 0.50 |
| Diabetes | 167 (16) | 47 (12) | 183 (16) | 0.14 | 0.01 | 41 (13) | 438 (15) | 0.06 |
| Hypertension | 306 (30) | 84 (21) | 315 (27) | 0.21 | 0.05 | 91 (30) | 796 (28) | 0.06 |
| Hypercholesterolaemia | 126 (12) | 29 (7) | 108 (9) | 0.16 | 0.10 | 26 (8) | 289 (10) | 0.06 |
| Peripheral vascular disease | 36 (4) | 10 (2) | 26 (2) | 0.06 | 0.08 | 15 (5) | 87 (3) | 0.11 |
| Stroke | < 5 | < 5 | < 5 | 0.05 | 0.05 | < 5 | 13 (0.4) | 0.03 |
| Heart failure | 61 (6) | 23 (6) | 71 (6) | 0.004 | 0.02 | 28 (9) | 183 (6) | 0.12 |
| Peptic ulcer disease | < 5 | 0 (0) | < 5 | 0.04 | 0.02 | 0 (0) | < 5 | 0.05 |
| Haemodialysis or renal disease | 29 (3) | 5 (1) | 21 (2) | 0.12 | 0.08 | 14 (5) | 69 (2) | 0.14 |
| Cancer | 34 (3) | 11 (3) | 35 (3) | 0.04 | 0.02 | 18 (6) | 98 (3) | 0.14 |
| Clotting disorder | < 5 | 0 | < 5 | 0.04 | 0.05 | 0 (0) | 5 (0.2) | 0.06 |
| Anaemia | 10 (1) | < 5 | 11 (1) | 0.001 | 0.003 | 6 (2) | ** | 0.08 |
| Liver cirrhosis | 0 (0) | 0 (0) | 0 (0) | – | – | < 5 | < 5 | 0.08 |
| Co-interventions , n (%) | ||||||||
| NSAIDs | 185 (18) | 65 (16) | 200 (17) | 0.06 | 0.02 | 34 (11) | 484 (17) | 0.18 |
| Steroids | 87 (9) | 25 (6) | 97 (8) | 0.09 | 0.01 | 31 (10) | 240 (8) | 0.07 |
| PPIs | 318 (31) | 114 (28) | 353 (30) | 0.07 | 0.01 | 83 (27) | 868 (30) | 0.07 |
| Anticoagulants | < 5 | < 5 | < 5 | 0.05 | 0.01 | 6 (2) | 17 (1) | 0.14 |
Compared with patients in the AC group, the population in the AP group was younger; had a higher proportion of men; had more smokers; was less likely to have had a history of MI and IHD; and was also less likely to have diabetes, hypertension, hypercholesterolaemia and renal disease (all SMDs > 0.10). Patients in the AT group were also younger, with more smokers than in the AC group, but were otherwise balanced with regard to all other baseline characteristics. There was no difference in median length of stay between the AT, the AP and the AC groups [3 (IQR 2–4) days, 3 (IQR 2–3) days and 3 (IQR 2–3) days, respectively].
Of the 306 patients without intervention, 161 (53%) either died or had a major bleed or ACS event before their first prescription, and 145 (47%) had no prescription for an antiplatelet agent in the 2 months after their index event. Compared with the 2587 out of 2893 (89%) patients with known intervention (included in the primary analysis), the population with unknown intervention (excluded from the primary analysis) was older; had a higher proportion of women; had more smokers/ex-smokers; and had a higher proportion of patients with a history of CABG/PCI, previous surgery, a history of IHD, hypertension, heart failure, renal disease, cancer and liver cirrhosis (see Table 32). Fewer patients with unknown intervention were taking NSAIDs, but more of them were taking anticoagulants.
Compared with the population who had no prescription in the CPRD within 2 months of discharge, the population who had a bleed or ischaemic event was older; had a higher proportion of women; had fewer smokers; had a higher proportion of patients with a history of CABG/PCI, previous surgery, history of IHD, diabetes, hypertension, heart failure, renal disease and cancer; and had a higher proportion of patients using NSAIDs, steroids, PPIs and anticoagulants (Table 33).
| Characteristics | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 161) | No prescription in the CPRD within 2 months of discharge (N = 145) | SMD | Total known (N = 2587) |
|---|---|---|---|---|
| Demography | ||||
| Year of event, n (%) | ||||
| 2012/13 | 44 (27) | 40 (28) | 0.18 | 713 (28) |
| 2013/14 | 35 (22) | 35 (24) | 629 (24) | |
| 2014/15 | 39 (24) | 26 (18) | 564 (22) | |
| 2015/16 | 29 (18) | 27 (19) | 423 (16) | |
| 2016/17 | 14 (9) | 17 (12) | 258 (10) | |
| Age (years), mean (SD) | 72.5 (13.7) | 61.2 (13.3) | 0.83 | 63.0 (12.3) |
| Sex, n (%) | ||||
| Male | 97 (60) | 116 (80) | 0.44 | 1923 (74) |
| Female | 64 (40) | 29 (20) | 664 (26) | |
| BMIa (kg/m2), mean (SD) | 27.2 (4.9) | 27.3 (4.9) | 0.03 | 28.0 (4.9) |
| Ethnic group, n (%) | ||||
| White | 149 (93) | 132 (91) | 0.06 | 2382 (92) |
| Other than white | 12 (7) | 13 (9) | 205 (8) | |
| Smoking category,b n (%) | ||||
| Ex-smoker | 54 (36) | 37 (27) | 0.33 | 722 (29) |
| Non-smoker | 52 (34) | 36 (27) | 829 (34) | |
| Smoker | 46 (30) | 62 (46) | 909 (37) | |
| Medical history , n (%) | ||||
| History of MI (ever) | 88 (55) | 75 (52) | 0.06 | 1941 (75) |
| History of CABG/PCI (ever) | 58 (36) | 29 (20) | 0.36 | 593 (23) |
| Bleeding | 0 (0) | 6 (4) | 0.29 | 63 (2) |
| Previous surgery | 10 (6) | < 5 | 0.25 | 75 (3) |
| Comorbidity , n (%) | ||||
| History of IHD (ever) | 97 (60) | 77 (53) | 0.14 | 2052 (79) |
| Diabetes | 32 (20) | 9 (6) | 0.41 | 397 (15) |
| Hypertension | 69 (43) | 22 (15) | 0.64 | 705 (27) |
| Hypercholesterolaemia | 13 (8) | 13 (9) | 0.03 | 263 (10) |
| Peripheral vascular disease | 7 (4) | 8 (6) | 0.05 | 72 (3) |
| Stroke | < 5 | < 5 | 0.01 | 11 (0.4) |
| Heart failure | 20 (12) | 8 (6) | 0.24 | 155 (6) |
| Peptic ulcer disease | 0 (0) | 0 (0) | – | < 5 |
| Haemodialysis or renal disease | 12 (7) | < 5 | 0.30 | 55 (2) |
| Cancer | 14 (9) | < 5 | 0.26 | 80 (3) |
| Clotting disorder | 0 (0) | 0 (0) | – | 5 (0.2) |
| Anaemia | < 5 | < 5 | 0.01 | 25 (1) |
| Liver cirrhosis | < 5 | 0 (0) | 0.11 | 0 (0) |
| Co-interventions , n (%) | ||||
| NSAIDs | 21 (13) | 13 (9) | 0.13 | 450 (17) |
| Steroids | 20 (12) | 11 (8) | 0.16 | 209 (8) |
| PPIs | 54 (34) | 29 (20) | 0.31 | 785 (30) |
| Anticoagulants | 6 (4) | 0 (0) | 0.28 | 11 (0.4) |
Bleeding events among participants included in and those excluded from the target trial
Of the 2587 STEMI patients, 260 (10%) experienced at least one bleeding event: 80 out of 1023 (8%) in the AC group, 46 out of 406 (11%) in the AP group and 134 out of 1158 (12%) in the AT group. With regard to major and minor bleeding events, 70 out of 2587 (3%) patients experienced a major bleed and 208 out of 2587 (8%) experienced a minor bleed. The proportions of patients experiencing a major and a minor bleeding event were 22 out of 1023 (2%) and 62 out of 1023 (6%), respectively, in the AC group; 9 out of 406 (2%) and 39 out of 406 (10%), respectively, in the AP group; and 39 out of 1158 (3%) and 107 out of 1158 (9%), respectively, in the AT group.
Figure 20 shows the Kaplan–Meier curves of cumulative bleeding (any bleed) in the AC versus the AP versus the AT groups. The cumulative incidence of any bleeding increased steadily over the 12 months, but was higher in the AP and AT groups than in the AC group. The number of major bleeds was larger in the AT group and the numbers of minor bleeds were larger in the AP and AT groups than in the AC group. The crude incidence rates of major and minor bleeds were 3% higher (23 vs. 22 events per 1000 person-years) and 62% higher (102 vs. 63 events per 1000 person-years) in the AP group than in the AC group, and were 58% higher (34 vs. 22 events per 1000 person-years) and 54% higher (97 vs. 63 events per 1000 person-years) in the AT group than in the AC group (Table 34). Of those who experienced a bleeding event within 12 months, the majority of patients experienced only one bleeding event (187/260, 72%); 57 out of 260 (22%) experienced two bleeding events; and the remainder (16/260, 6%) experienced three or more bleeds. Bleeds by site are shown in Table 35; there were slightly larger numbers of ear, nose and throat bleeds in the AP group than in the AC or AT groups, and a larger number of gastrointestinal bleeds in the AC group than in the AP or AT groups.
| AC | AP | AT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bleeds | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) | Number of bleeds | Person-years | Rate per 1000 person-years (95% CI) |
| Major (HES) | 22 | 1008 | 21.8 (14.4 to 33.2) | 9 | 400 | 22.5 (11.7 to 43.2) | 39 | 1133 | 34.4 (25.1 to 47.1) |
| Minor (CPRD) | 62 | 982 | 63.2 (49.2 to 81.0) | 39 | 381 | 102.3 (74.8 to 140.1) | 106 | 1089 | 97.4 (80.5 to 117.8) |
| All (CPRD and HES) | 80 | 862 | 92.8 (74.6 to 115.6) | 46 | 334 | 137.8 (103.2 to 183.9) | 133 | 928 | 143.4 (121.0 to 170.0) |
| Bleeds recorded (HES or CPRD), n (%) | ||||
|---|---|---|---|---|
| Bleed site | AC (N = 1023) | AP (N = 406) | AT (N = 1158) | Total (N = 2587) |
| Ear, nose or throat | 16 (16) | 18 (28) | 44 (22) | 78 (21) |
| Gastrointestinal | 44 (44) | 20 (31) | 82 (41) | 146 (40) |
| Genitourinary | < 5 | 0 | < 5 | 8 (2) |
| Intracranial | < 5 | 5 (8) | < 5 | 10 (3) |
| Ocular | 6 (6) | < 5 | 6 (3) | ** |
| Skin or soft tissue | 22 (22) | 16 (25) | 55 (28) | 93 (26) |
| Other anatomical site | < 5 | 0 | < 5 | 6 (2) |
| Unspecified anatomical site | < 5 | < 5 | < 5 | 8 (2) |
| Total (N) | 101 | 64 | 199 | 364 |
FIGURE 20.
Kaplan–Meier curves displaying cumulative bleeding according to intervention group. (a) Any bleeding; (b) major bleeding; and (c) minor bleeding. Plots are weighted according to the inverse probability of treatment received, and so compare outcomes if all eligible patients received AC, AP or AT.
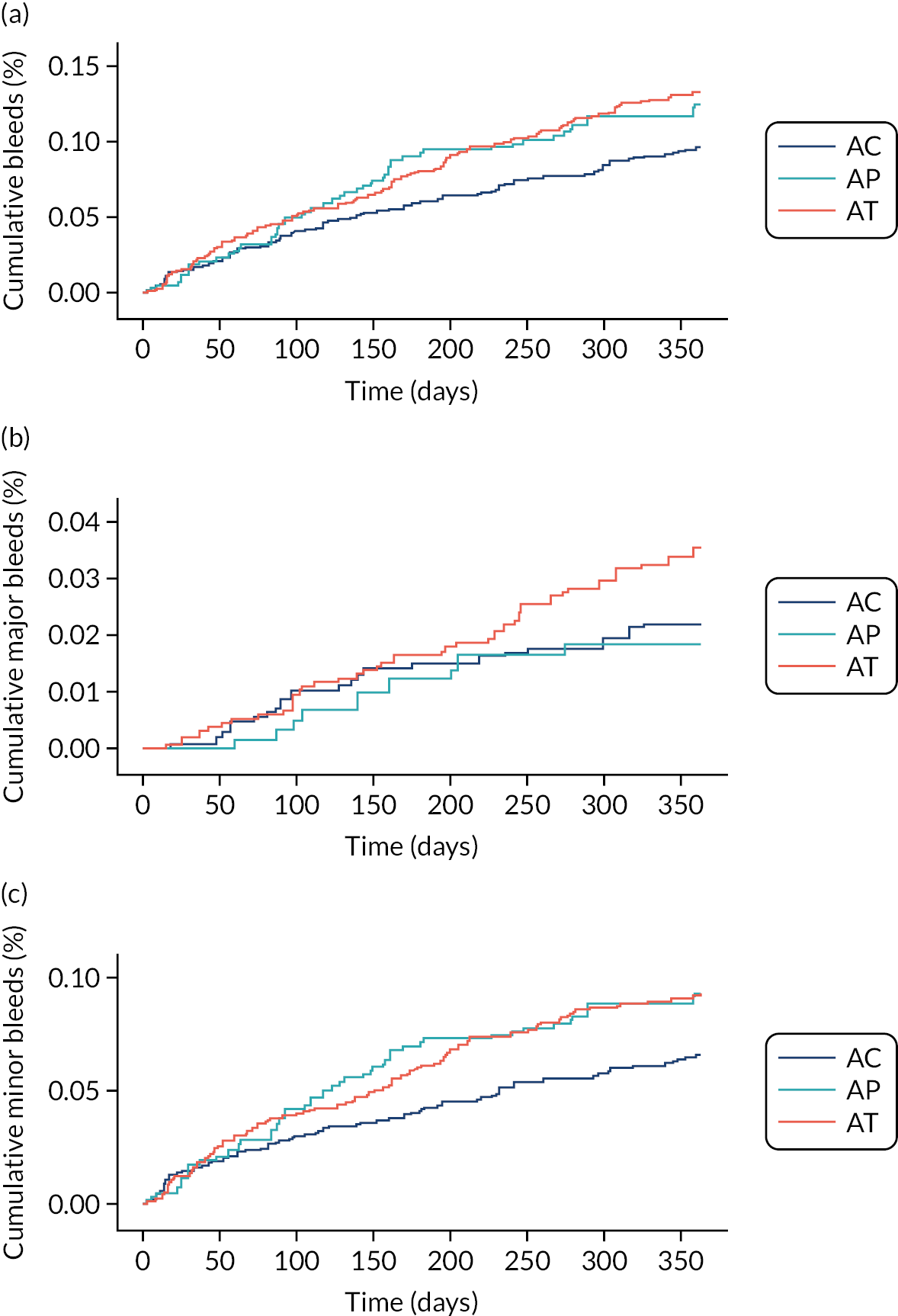
Patients who could not be assigned an intervention because they experienced a bleed or ischaemic event or died before their first prescription or because they had no prescription in the CPRD within 2 months of discharge (11% of the eligible population) had a lower bleeding rate than the patients included in the target trial (3% vs. 10%, respectively) (Table 36). The bleeding rate was slightly higher among those who had no prescription in the CPRD within 2 months of discharge than among those who experienced an event or died (4% vs. 2%, respectively).
| Bleeding events, n/N (%) | Bleeding events, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Patients included in target trial (n) | AP | AP | AT | HR (95% CI) | Ischaemic/major bleeding event or death before first prescription in the CPRDa | No prescription in the CPRD within 2 months of discharge | Overall |
| Primary outcome | ||||||||
| Crude | 2587 | 80/1023 (8) | 46/406 (11) | 134/1158 (12) | AP: 1.48 (1.02 to 2.12) | < 5 | 6/145 (4) | ** |
| Adjusted | AT: 1.53 (1.16 to 2.01) | |||||||
| AP: 1.77 (1.21 to 2.59)b,c | ||||||||
| AT: 1.50 (1.10 to 2.05)b,c | ||||||||
| SA1: multiple imputation for unknown intervention group | 2893 | AP: 1.77 (1.21 to 2.58)b,c | ||||||
| AT: 1.47 (1.08 to 2.00)b,c | ||||||||
| SA3: restricted to patients at low risk of bleeding | 2488 | 78/982 (8) | 45/394 (11) | 125/1112 (11) | AP: 1.76 (1.20 to 2.58)b,d | |||
| AT: 1.46 (1.07 to 2.00)b,d | ||||||||
| SA4: primary adjusted analysis without censoring of any CPRD or HES bleed at transfer-out or last collection date | 2587 | 81/1023 (8) | 47/406 (12) | 137/1158 (12) | AP: 1.81 (1.24 to 2.63)b,e | |||
| AT: 1.53 (1.12 to 2.07)b,e | ||||||||
| Major bleeding (HES reported) | 2587 | 22/1023 (2) | 9/406 (2) | 39/1158 (3) | AP: 1.33 (0.59 to 3.01)b,f | < 5 | 5/145 (3) | ** |
| AT: 1.86 (1.05 to 3.32)b,f | ||||||||
| Minor bleeding (CPRD reported) | 2587 | 62/1023 (6) | 39/406 (10) | 107/1158 (9) | AP: 1.86 (1.23 to 2.82)b,g | 0 | < 5 | < 5 |
| AT: 1.49 (1.06 to 2.09)b,g | ||||||||
Analyses for the primary outcome (bleeding)
The primary analysis excluded patients for whom we could not assign an intervention (306/2993, 11%). The crude and adjusted HRs indicated an increase in the hazard of bleeding in the AP group compared with the AC group (HR 1.48, 95% CI 1.02 to 2.12, and HR 1.77, 95% CI 1.21 to 2.59, respectively). The crude and adjusted HRs also indicated an increase in the hazard of bleeding in the AT group compared with the AC group (HR 1.53, 95% CI 1.16 to 2.01) and HR 1.50, 95% CI 1.10 to 2.05, respectively) (see Table 36). When split by major and minor bleeding, there was an increased hazard of major bleeding for both the AP versus the AC groups (HR 1.33, 95% CI 0.59 to 3.01) and the AT versus the AC groups (HR 1.86, 95% CI 1.05 to 3.32), although the estimate for AP versus AC is quite imprecise. The hazards for minor bleeding increased for the AP group, compared with the AC group (HR 1.86, 95% CI 1.23 to 2.82), and for the AT group, compared with the AC group (HR 1.49, 95% CI 1.06 to 2.09).
Sensitivity analyses
The HRs did not change substantially for sensitivity analysis 1 (multiple imputation for 2893 patients with unknown intervention) (HR 1.77, 95% CI 1.21 to 2.58, for AP vs. AC, and HR 1.47, 95% CI 1.08 to 2.00, for AT vs. AC) or for sensitivity analyses 3 or 4 (see Table 36).
We did not conduct sensitivity analysis 2 (exclusion of patients who changed medication before first bleeding event) because very few patients (7/260, 3%) changed medication before their first bleeding event (so this did not meet our prespecified threshold of > 10% of the population).We also did not conduct sensitivity analysis 5, the instrumental variable analysis. Although there was evidence of an association between previous prescription and current prescription (OR 5.71, 95% CI 4.31 to 7.57, for AP vs. AC, and OR 17.78, 95% CI 13.87 to 22.80, for AT vs. AC; p < 0.001), there was less evidence of an association between previous prescription and bleeding (OR 1.20, 95% CI 0.82 to 1.77, for AP vs. AC, and OR 1.24, 95% CI 0.92 to 1.67, for AT vs. AC; p = 0.34).
Subgroup analyses
There was no evidence of any subgroup effects for people with diabetes versus people without diabetes (p = 0.44, interaction test), for people with chronic kidney disease versus people without chronic kidney disease (p = 0.11) or for a concurrent prescription for PPIs versus no concurrent prescription for PPIs (p = 0.77).
Mortality and ischaemic events among participants included in and those excluded from the target trial
Figure 21 shows the Kaplan–Meier curves for the secondary outcomes of all-cause and cardiovascular mortality, mortality from bleeding, MI, stroke, additional coronary intervention and the composite outcome of MACE. Patients not included in the target trial (11% of all eligible patients) had higher event rates than those included, although these were driven largely by the group of patients excluded because they had a bleeding or ischaemic event prior to first prescription in the CPRD (Table 37). The 145 patients excluded because they had no prescription in the CPRD within 2 months of discharge had event rates comparable to those of the included population.
| Included in target trial | Not included in target trial,a n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Secondary outcomes | AC (N = 1023), n (%) | AP (N = 406), n (%) | AT (N = 1158), n (%) | Overall (N = 2587), n (%) | Adjusted HR (for AP/AT vs. AC) (95% CI) | Ischaemic/major bleeding event or death before first prescription in the CPRD (N = 161) | No prescription in the CPRD within 2 months of discharge (N = 145) | Overall (N = 306) |
| All-cause mortality | 31 (3) | 7 (2) | 22 (2) | 60 (2) | AP: 1.32 (0.54 to 3.20)b | 141 (88) | < 5 | ** |
| AT: 0.96 (0.52 to 1.75)b | ||||||||
| Cardiovascular mortality | 15 (1) | < 5 | 8 (1) | ** | AP: 1.42 (0.43 to 4.68)c | 127 (79) | < 5 | ** |
| AT: 0.61 (0.24 to 1.54)c | ||||||||
| Mortality from bleeding | < 5 | < 5 | 0 | 6 (0.2) | AP: 2.48 (0.32 to 19.21)d | < 5 | 0 | < 5 |
| AT: – | ||||||||
| MI | 27 (3) | 12 (3) | 33 (3) | 72 (3) | AP: 1.20 (0.60 to 2.42)e | < 5 | < 5 | 5 (2) |
| AT: 1.22 (0.69 to 2.14)e | ||||||||
| Stroke | < 5 | < 5 | < 5 | 5 (0.2) | AP: 0.57 (0.03 to 11.14)f | < 5 | – | < 5 |
| AT: 1.25 (0.13 to 12.21)f | ||||||||
| Additional coronary intervention | 109 (11) | 52 (13) | 132 (11) | 293 (11) | AP: 1.14 (0.82 to 1.60)g | 6 (4) | 20 (14) | 26 (8) |
| AT: 1.16 (0.88 to 1.54)g | ||||||||
| MACE | 132 (13) | 57 (14) | 161 (14) | 350 (14) | AP: 1.10 (0.80 to 1.51)h | 133 (83) | 23 (16) | 156 (51) |
| AT: 1.21 (0.94 to 1.56)h | ||||||||
FIGURE 21.
Kaplan–Meier curves displaying cumulative all-cause and cardiovascular mortality, mortality from bleeding and ischaemic events, according to intervention group. (a) All-cause mortality; (b) cardiovascular mortality; (c) mortality from bleeding; (d) MI; (e) stroke; (f) additional PCI; and (g) MACE.
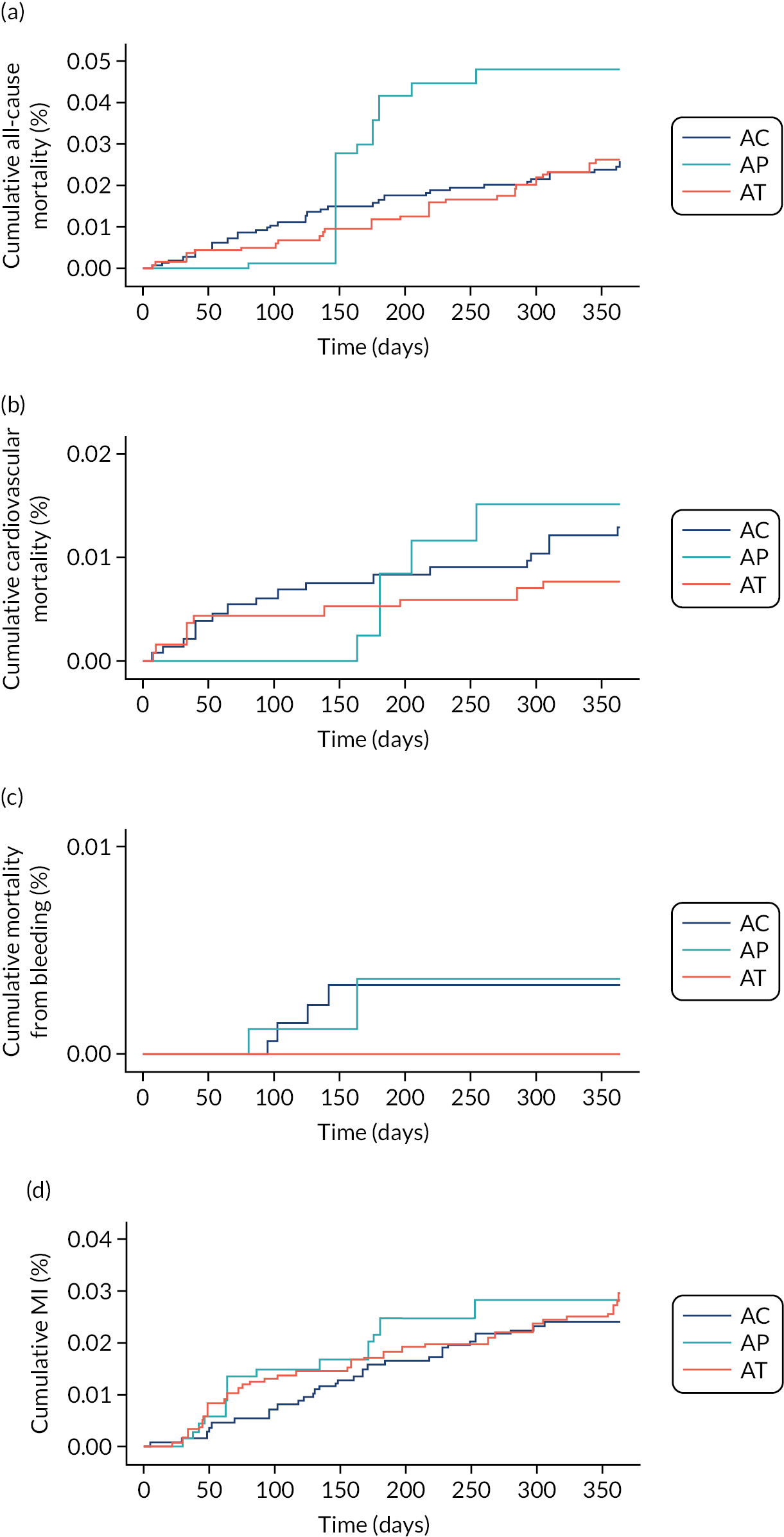
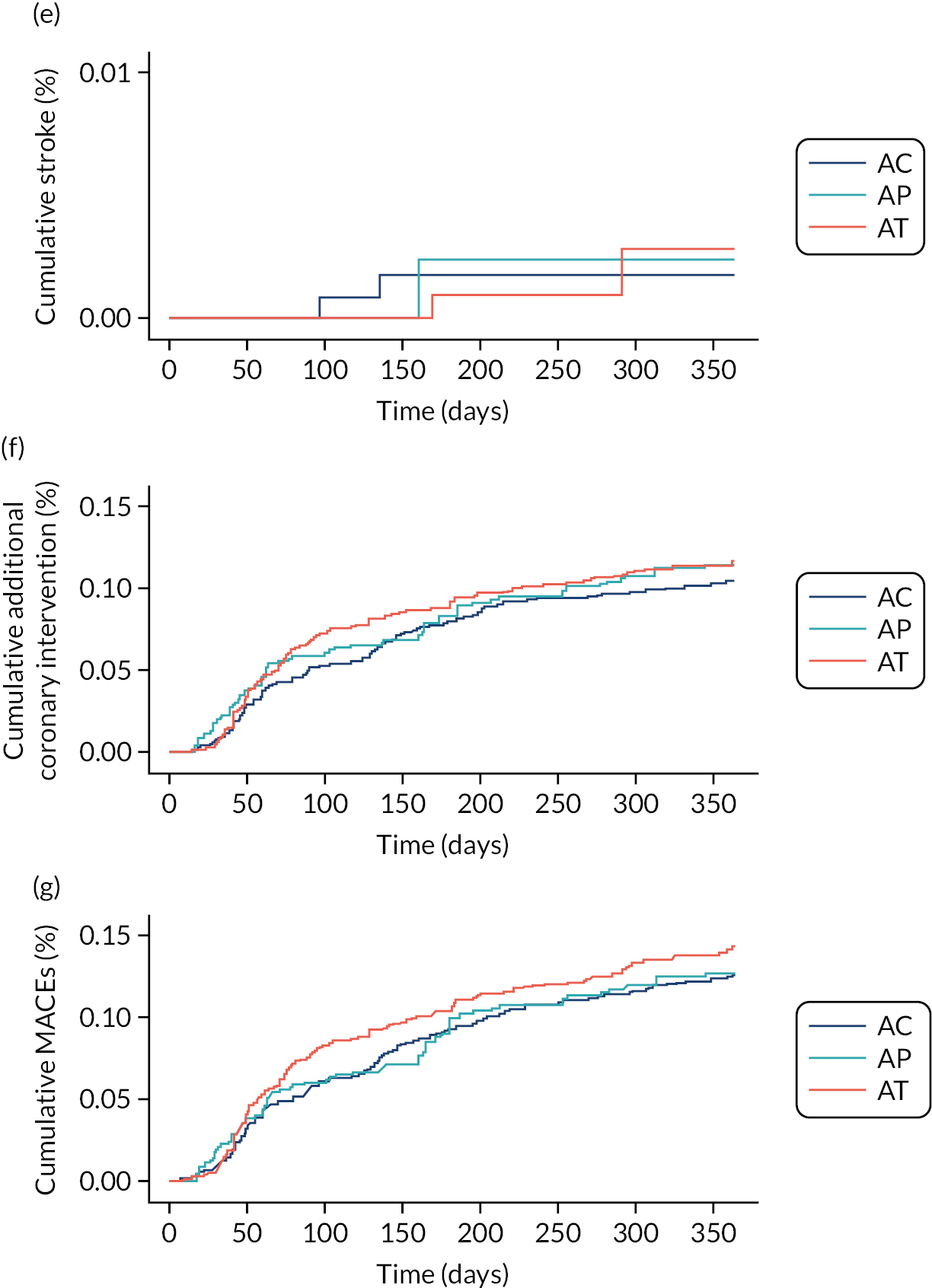
Analyses for the secondary outcomes (mortality and ischaemic events)
There was no association between antiplatelet prescription (AP vs. AC and AT vs. AC) and any outcome (see Table 37).
Treatment switches and adherence
In the AC group, 141 out of 1023 (14%) patients were identified as ‘switchers’. There were 205 treatment switches (Table 38). Of these, 114 (56%) were aspirin discontinuations, 43 (21%) were clopidogrel discontinuations, 30 (15%) were aspirin and clopidogrel discontinuations and 18 (9%) were initiations of a different P2Y12 inhibitor. The median time to switch was between 7 and 8 months, although those who initiated a different P2Y12 inhibitor switched at a median time of 1 month.
| Bleed occurred, n/N (%) | Ischaemic eventb occurred, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Type of switch, n/N (%) | Median (IQR) time to switch (months) | Before switcha | After switch | Before switch (within 2 months) | Before switch | After switch | No ischaemic or bleeding events, n/N (%) |
| AC | Discontinued aspirin, 114/1023 (11%) | 7.9 (5.6–11.2) | 11/114 (10) | 0/114 | < 5 | < 5 | < 5 | 96/114 (84) |
| Discontinued clopidogrel, 43/1023 (4%) | 7.9 (6.4–9.5) | 7/43 (16) | 0/43 | < 5 | 6/43 (14) | 0/43 | 32/43 (74) | |
| Discontinued AC, 30/1023 (3%) | 7.2 (5.1–9.1) | 5/30 (17) | 0/30 | < 5 | < 5 | < 5 | 22/30 (73) | |
| Initiated a different P2Y12 inhibitor, 18/1023 (2%) | 1.2 (0.8–3.1) | < 5 | < 5 | < 5 | < 5 | < 5 | 13/18 (72) | |
| AP | Discontinued aspirin, 38/406 (9%) | 8.7 (6.4–10.9) | < 5 | 0/38 | < 5 | < 5 | < 5 | 32/38 (84) |
| Discontinued prasugrel, 16/406 (4%) | 9.9 (7.9–11.6) | < 5 | 0/16 | < 5 | < 5 | 0/16 | 12/16 (75) | |
| Discontinued AP, 14/406 (3%) | 8.8 (6.3–11.3) | < 5 | 0/14 | < 5 | < 5 | < 5 | 11/14 (80) | |
| Initiated a different P2Y12 inhibitor, 22/406 (5%) | 2.9 (1.5–4.6) | < 5 | 0/22 | 0/22 | 0/22 | 0/22 | 21/22 (95) | |
| AT | Discontinued aspirin, 128/1158 (11%) | 7.7 (5.9–9.9) | 16/128 (13) | < 5 | < 5 | 5/128 (4) | < 5 | 103/128 (80) |
| Discontinued ticagrelor, 92/1158 (8%) | 7.8 (6.0–9.6) | 7/92 (8) | < 5 | < 5 | 5/92 (5) | < 5 | 74/92 (80) | |
| Discontinued AT, 50/1158 (4%) | 7.2 (6.1–8.7) | 5/50 (10) | < 5 | < 5 | < 5 | < 5 | 38/50 (76) | |
| Initiated a different P2Y12 inhibitor, 84/1158 (7%) | 3.3 (1.7–6.8) | 8/84 (10) | 5/84 (6) | 0/84 | < 5 | < 5 | 68/84 (76) | |
In the AP group, 60 out of 406 (15%) were identified as switchers. There were 90 treatment switches in total; of these, 38 (42%) were aspirin discontinuations, 16 (18%) were prasugrel discontinuations, 14 (16%) were aspirin and prasugrel discontinuations and 22 (24%) were initiations of a different P2Y12 inhibitor. The median time to switching was between 9 and 10 months, but in those who initiated a different P2Y12 inhibitor the median time to switch was 3 months.
Among patients assigned AT, 242 out of 1158 (21%) were identified as switchers. There were 354 treatment switches, 128 (36%) aspirin discontinuations, 92 (26%) ticagrelor discontinuations, 50 (14%) aspirin and ticagrelor discontinuations and 84 (24%) initiations of a different P2Y12 inhibitor. The median time to switching was between 7 and 8 months, except for those who initiated a different P2Y12 inhibitor, in whom the median time to switch was 3 months.
Across all groups, 76 switchers had a bleeding or ischaemic event, 24 (32%) in AC, 8 (10.5%) in AP and 44 (58%) in AT. Most of these events occurred before the switch.
In all intervention groups, the number of ischaemic events was larger among those who switched, compared with event rates in the population overall. Adherence, defined as a MPR of ≥ 0.8, was 71% in the AC group, 69% in the AP group and 68% in the AT group.
Discussion (emergency percutaneous coronary intervention and ST-elevation myocardial infarction percutaneous coronary intervention)
We conducted two analyses in the emergency PCI population: one for a comparison of DAPT with ticagrelor versus DAPT with clopidogrel, including the entire ACS population (STEMI, NSTEMI and unstable angina), and another restricted to the STEMI population only, to allow a comparison of DAPT with prasugrel, as well as ticagrelor, versus DAPT with clopidogrel.
The emergency PCI population included 5738 patients, half of whom were patients with STEMI and half of whom were patients with NSTEMI or unstable angina. The overall incidence of bleeding in the population was 9%, and the incidence of major and minor bleeding was 2% and 7%, respectively. The incidence of bleeding in the STEMI-only population was similar. The incidence of bleeding reported in RCTs and observational studies is about 11% overall (major bleeds, 6%; minor bleeds, 4.5%);66,67 the discrepancies between major and minor bleeding rates in this study and those from other studies are largely because of different definitions of major and minor bleeding.
This study showed a 47% increased risk of overall bleeding (HR 1.47, 95% CI 1.19 to 1.82), and a 33% increased risk of major (HR 1.33, 95% CI 0.89 to 1.99) and minor (HR 1.33, 95% CI 0.89 to 1.99) bleeding with DAPT with ticagrelor, compared with DAPT with clopidogrel. These results were similar when restricted to the STEMI population. These results reflect the results from two recent meta-analyses comparing DAPT with ticagrelor versus DAPT with clopidogrel. Guan et al. 67 included 16 studies (11 RCTs and five observational studies) with 25,632 ACS patients, > 90% of whom had been revascularised by PCI. Ticagrelor increased the risk of both minor (OR 1.57, 95% CI 1.30 to 1.89) and major (OR 1.52, 95% CI 1.01 to 2.29) bleeding. Fan et al. 66 included 11 studies, six RCTs [20,992 participants, including the Platelet Inhibition and Patient Outcomes (PLATO) RCT33] and five observational studies (7992 participants), which showed an increased risk of major (OR 1.36, 95% CI 1.02 to 1.82) and minor (OR 1.43, 95% CI 1.25 to 1.63) bleeding.
We found that bleeding events were similar between patients receiving prasugrel and patients receiving ticagrelor in the STEMI population (11% vs. 12%, respectively). This confirms the finding from a recent head-to-head comparison of DAPT with prasugrel or ticagrelor among 4018 participants with ACS undergoing PCI. 68 In this RCT, major bleeding (BARC types 3–5) was observed in 5% of patients receiving ticagrelor and in 5% of patients receiving prasugrel (HR 1.12, 95% CI 0.83 to 1.51), whereas minor bleeding (BARC types 1 or 2) was observed in 14% and 15% of patients in the ticagrelor and prasugrel groups, respectively (HR 0.90, 95% CI 0.76 to 1.06).
Ticagrelor is the preferred P2Y12 inhibitor as part of DAPT for patients with ACS undergoing PCI, largely based on the results of the PLATO RCT,33 which randomised 18,624 patients with ACS. The PLATO RCT showed reduced odds of MACEs with ticagrelor, compared with clopidogrel, in the ACS population undergoing PCI (OR 0.83, 95% CI 0.76 to 0.92). In our study, DAPT with ticagrelor did not reduce the risk of death or MACE in the PCI population with ACS [HR 0.94 (95% CI 0.60 to 1.47) and HR 1.06 (95% CI 0.89 to 1.27), respectively] or in the PCI population with STEMI [HR 0.96 (95% CI 0.52 to 1.75) and HR 1.21 (95% CI 0.94 to 1.51), respectively], compared with DAPT with clopidogrel. Our results reflect the 2019 meta-analysis of 11 clinical trials by Fan et al. ,66 which included six RCTs (20,992 participants, including the PLATO RCT) and five observational studies (7992 participants) and showed no significant difference between DAPT with ticagrelor and DAPT with clopidogrel with regard to MACEs (OR 0.83, 95% CI 0.66 to 1.03). Interestingly, although the meta-analysis66 showed a reduced risk of death from any cause (OR 0.81, 95% CI 0.72 to 0.91) and of cardiovascular death (OR 0.76, 95% CI 0.65 to 0.89), this was driven entirely by data from RCTs (largely the PLATO RCT). Similarly, the meta-analysis by Guan et al. 67 (11 RCTs and five observational studies) did not show significant differences in all-cause mortality (OR 0.83, 95% CI 0.67 to 1.03), MI (OR 0.77, 95% CI 0.57 to 1.03), stroke (OR 0.85, 95% CI 0.57 to 1.26) or MACEs (OR 0.64, 95% CI 0.41 to 1.01), despite including > 25,000 patients in the analysis.
In our study, among STEMI patients undergoing PCI, there was no association between DAPT with prasugrel versus DAPT with clopidogrel and death (HR 1.32, 95% CI 0.54 to 3.20) or MACE (HR 1.10, 95% CI 0.80 to 1.51). Meta-analyses69,70 that have compared DAPT with prasugrel versus DAPT with clopidogrel generally report lower mortality and smaller numbers of MACEs among those receiving prasugrel. For example, in a meta-analysis including two RCTs and one observational study, including > 5000 patients with ACS (mostly STEMI), rates of all-cause mortality (OR 0.49, 95% CI 0.28 to 0.85), MI (OR 0.68, 95% CI 0.57 to 0.81), stroke (OR 0.55, 95% CI 0.34 to 0.89) and MACEs (OR 0.59, 95% CI 0.42 to 0.82) were significantly lower with prasugrel. 69 However, this meta-analysis included trials with different lengths of follow-up (1 month, 1–5 years), which was not taken into account in the analyses.
Similar to the target trials for CABG and conservatively managed ACS, the results of the emergency PCI target trial may be affected by residual confounding and selection bias. Patients assigned to DAPT with clopidogrel were older and had more comorbidities than patients assigned DAPT with ticagrelor.
Although these factors were adjusted for in the analyses, there remains the possibility that the two groups still had different underlying risks of bleeding and ischaemia. Furthermore, we had no data on half of all identified confounders (see Chapter 2), for example PCI procedural characteristics or severity of underlying disease (angiographic features), so these factors could not be adjusted for in the analysis. We had no strong evidence that care pathways and PCI outcomes in the population changed between 2012 and 2017; patients from earlier years (when they were more likely to be prescribed DAPT with clopidogrel because ticagrelor was not widely available) were not markedly different from those included from later years (when patients were more likely to be prescribed DAPT with ticagrelor).
Of the emergency PCI population eligible for the target trial, 9% could not be assigned an intervention. The excluded population comprised two distinct groups: one group was of people who died or experienced a major bleed or ischaemic event (4% of the eligible population), which would have changed their DAPT prescription assigned in hospital, and the other group had no prescription in the CPRD within 2 months of discharge (5% of the eligible population). These two subgroups differed from each other and from the included population. Both groups had higher rates of bleeding and ischaemic events than the included population. Therefore, it is possible that their exclusion because they could not be assigned an intervention may have biased results for both bleeding and ischaemic outcomes.
Non-adherence was 33% in the DAPT with ticagrelor group, 31% in the DAPT with prasugrel (STEMI-only) group and 28% in the DAPT with clopidogrel group. These non-adherence rates are slightly lower than those we observed for the CABG and conservatively managed ACS target trials (≥ 40%), possibly reflecting the fact that cardiologists are able to stress the importance of adherence to their patients more effectively in the emergency PCI setting, owing to availability of evidence-based clinical guidelines. 10 However, the rates of non-adherence are lower than those reported in RCTs; for example, the rate of non-adherence in the PLATO trial was only 17%. 33 Up to one-fifth of patients in the DAPT with clopidogrel, DAPT with prasugrel (STEMI only) and DAPT with ticagrelor groups were identified as switchers (stopped aspirin or the P2Y12 inhibitor or both aspirin and P2Y12 inhibitor). In all intervention groups (and for both emergency PCI and STEMI PCI populations), the rate of ischaemic events was higher among those who switched than for the target trial populations overall. However, it is unlikely that non-adherence/switching influenced the findings with regard to bleeding or ischaemic outcomes, given that rates of non-adherence/switching were similar between DAPT groups.
Triple therapy
About 5–10% of patients with ACS have an indication for anticoagulants, mainly for AF, mechanical heart valves, but less commonly for concurrent left ventricular thrombus and thromboembolic disorders. Triple antithrombotic therapy (or TT) increases the risk of bleeding twofold to threefold compared with DAPT. 3
Methods
Two distinct TT (anticoagulant plus aspirin plus additional antiplatelet) populations of interest were decided a priori: TT patients who had received a prescription for anticoagulants in the 6 months prior to their first PCI, CABG or ACS event, and TT patients who were anticoagulant-naive. Patients who were aged < 18 years; had < 1 year of medical history prior to the event; had a prescription for clopidogrel, prasugrel or ticagrelor in the 3 months prior to the event; and who had a PCI, CABG or ACS or bleeding event prior to the first prescription were excluded from each population. The study populations are described in Figure 22. Product codes for the anticoagulants are detailed in Appendix 5. Analyses of these populations was descriptive; duration of TT was described, along with rates of bleed per 1000 person-years with 95% CIs, and numbers of bleeds by site. Outcomes including MACE and mortality were assessed as for the target trials. These outcomes were additionally presented by different types of TT, with SMDs calculated as before for TTwith warfarin versus TTwith NOAC.
FIGURE 22.
The construction of the TT populations. a, CIPS that include both PCI and CABG are considered CABG; CIPS that include both ACS and PCI are considered PCI; CIPS that include both ACS and CABG are considered CABG; b, warfarin, dabigatran, rivaroxaban and apixaban; c, patients may be excluded for more than one reason; d, bleeding event captured within hospital admission or GP appointment. C, clopidogrel; CIPS, continuous inpatient stays; P, prasugrel; T, ticagrelor. Note that prescriptions before are those within 6 months prior to index event; anticoagulants after are those with prescriptions within 2 months of discharge after the index event.
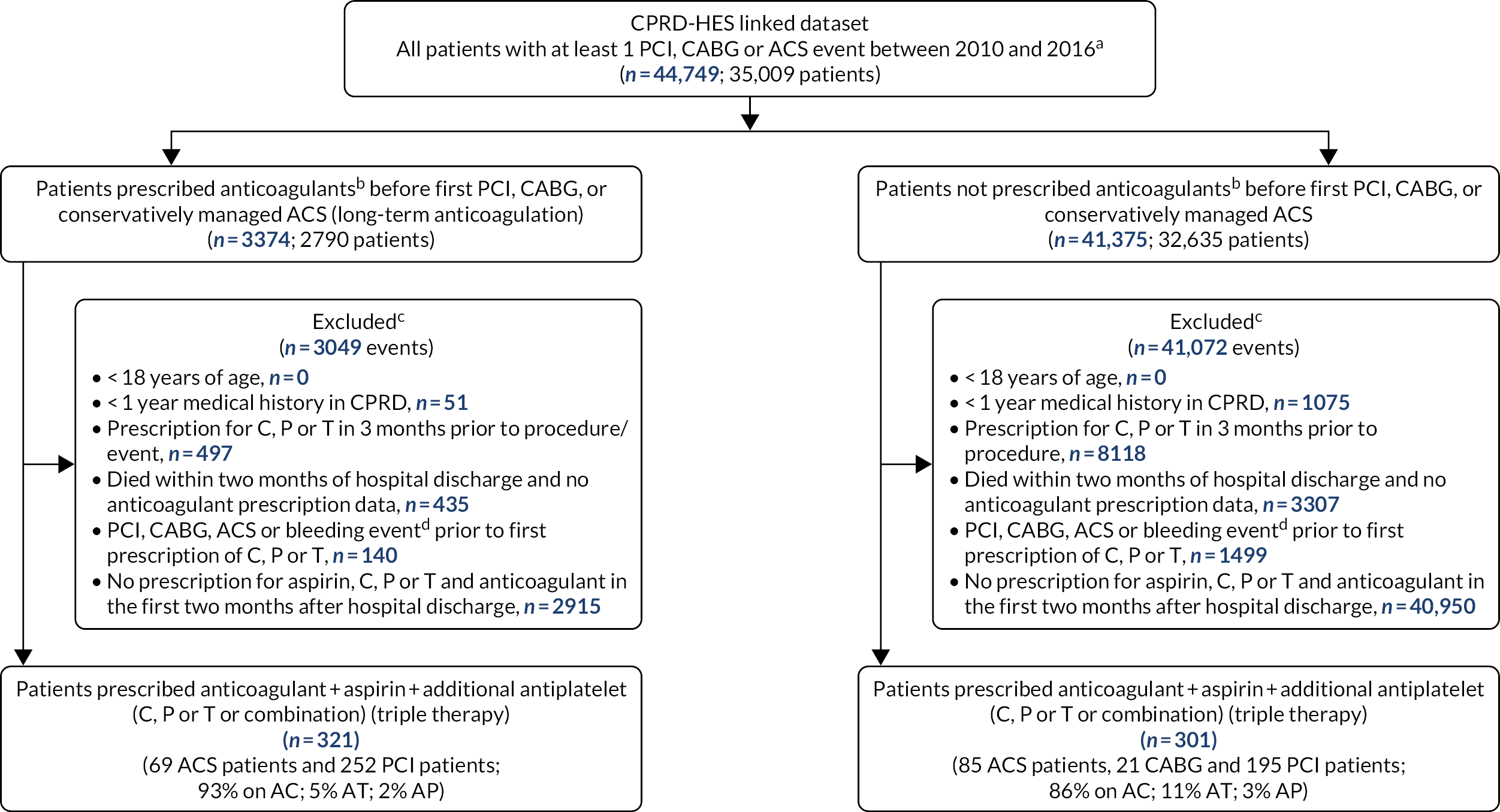
Results
Table 39 shows the participants in the three target trials who were on TT categorised into those receiving long-term anticoagulation and those who were prescribed an anticoagulant after the index event. All patients receiving long-term anticoagulation underwent PCI or were medically treated.
| Characteristic | Patients on long-term anticoagulation (N = 321) | Patients prescribed anticoagulants after first CABG, PCI or ACS event, but not before (N = 301) | SMD | All (N = 622) |
|---|---|---|---|---|
| Demography | ||||
| Cohort, n (%) | ||||
| ACS | 69 (21) | 85 (28) | 0.44 | 154 (25) |
| CABG | 0 (0) | 21 (7) | 21 (3) | |
| PCI | 252 (79) | 195 (65) | 447 (72) | |
| DAPT group, n (%) | ||||
| AC | 299 (93) | 259 (86) | 0.24 | 558 (90) |
| AP | 7 (2) | 10 (3) | 17 (3) | |
| AT | 15 (5) | 32 (11) | 47 (8) | |
| Year of event, n (%) | ||||
| 2010/11 | 36 (11) | 38 (13) | 0.16 | 74 (12) |
| 2011/12 | 49 (15) | 35 (12) | 84 (14) | |
| 2012/13 | 51 (16) | 61 (20) | 112 (18) | |
| 2013/14 | 53 (17) | 44 (15) | 97 (16) | |
| 2014/15 | 57 (18) | 52 (17) | 109 (18) | |
| 2015/16 | 45 (14) | 41 (14) | 86 (14) | |
| 2016/17 | 30 (9) | 30 (10) | 60 (10) | |
| Age (years), mean (SD) | 73.8 (10.0) | 69.6 (12.6) | 0.37 | 71.8 (11.5) |
| Sex, n (%) | ||||
| Male | 235 (73) | 233 (77) | 0.10 | 468 (75) |
| Female | 86 (27) | 68 (23) | 154 (25) | |
| BMI (kg/m2), mean (SD)a | 28.9 (4.5) | 29.3 (5.7) | 0.06 | 29.1 (5.1) |
| Ethnic group, n (%) | ||||
| White | 311 (97) | 284 (94) | 0.12 | 595 (96) |
| Non white | 10 (3) | 17 (6) | 27 (4) | |
| Duration of TT (months), median (IQR) | 3.8 (2.0–7.7) | 3.3 (2.1–6.3) | 0.08 | 3.5 (2.0–6.6) |
| Primary outcomes | ||||
| Any bleed, n (%) | 57 (18) | 54 (18) | 0.01 | 111 (18) |
| Rate per 1000 person-years (95% CI) | 220.1 (169.8 to 285.4) | 211.4 (161.5 to 276.7) | 215.8 (179.1 to 260.2) | |
| Major bleed (HES), n (%) | 23 (7) | 21 (7) | 0.01 | 44 (7) |
| Rate per 1000 person-years (95% CI) | 75.4 (50.1 to 113.4) | 73.4 (47.9 to 112.6) | 74.4 (55.4 to 100.0) | |
| Minor bleed (CPRD), n (%) | 38 (12) | 37 (12) | 0.01 | 75 (12) |
| Rate per 1000 person-years (95% CI) | 130.1 (94.7 to 178.8) | 131.5 (94.9 to 182.3) | 130.8 (104.1 to 164.3) | |
| Total number of bleeds, n (%) | 85 | 85 | 170 | |
| Ear, nose or throat bleed | 21 (25) | 33 (36) | – | 54 (31) |
| Gastrointestinal bleed | 19 (22) | 34 (37) | – | 53 (30) |
| Genitourinary bleed | < 5 | < 5 | – | < 5 |
| Intracranial bleed | 7 (8) | 6 (7) | – | 13 (7) |
| Ocular bleed | 12 (14) | 5 (5) | – | 17 (10) |
| Skin or soft-tissue bleed | 18 (21) | 12 (13) | – | 30 (17) |
| Other anatomical site bleed | < 5 | < 5 | – | < 5 |
| Unspecified anatomical site bleed | 5 (6) | 0 (0) | – | 5 (3) |
| Secondary outcomes, n (%) | ||||
| All-cause mortality | 22 (7) | 21 (7) | 0.01 | 43 (7) |
| Cardiovascular mortality | 10 (3) | 13 (4) | 0.06 | 23 (4) |
| Mortality from bleeding | 0 | 5 (2) | 0.18 | 5 (1) |
| MI | 18 (6) | 12 (4) | 0.08 | 30 (5) |
| Stroke | < 5 | < 5 | 0.04 | 7 (1) |
| Additional coronary intervention | 37 (12) | 30 (10) | 0.05 | 67 (11) |
| MACE | 56 (17) | 48 (16) | 0.04 | 104 (17) |
The majority of patients (93%) initiating an anticoagulant after their index event were PCI or medically treated ACS patients. Over 85% of all patients in both groups had DAPT with clopidogrel as part of their TT. The group on long-term anticoagulation were older (74 years vs. 70 years) and had a lower proportion of individuals who were other than white (3% vs. 6%) than the group prescribed an anticoagulant after the index event. There were no major differences in the incidence of bleeding events or in the total number of bleeds between the groups. The incidence and rate of bleeding were similar between groups (18% and just over 210 per 1000 person-years). However, compared with patients on long-term anticoagulation, more patients who initiated an anticoagulant after the index event had ear, nose or throat bleeds (25% vs. 36%, respectively) and gastrointestinal bleeds (22% vs. 37%, respectively), but fewer had ocular bleeds (14% vs. 5%, respectively) and skin or soft-tissue bleeds (21% vs. 13%, respectively). The duration of TT was between 3 and 4 months for both groups. Mortality and MACE rates were similar between groups.
Table 40 shows the frequency and rate of bleeding, total number of bleeds and bleeds by site according to type of TT. The median duration of TT was 1 month less for TT with warfarin than for TT with a NOAC. Patients on TT with warfarin had slightly more minor bleeds that patients on TT with NOAC (13% vs. 9%), a larger number of total bleeds (138 vs. 27), but less mortality from bleeding (0.4% vs. 2%). The site of bleeding differed between those on TT with warfarin and those on TT with NOAC; the former had more ear, nose and throat bleeds (34% vs. 18%); ocular bleeds (12% vs. 3%); and skin or soft-tissue bleeds (19% vs. 10%), but fewer gastrointestinal bleeds (25% vs. 41%) and intracranial bleeds (4% vs. 21%).
| TT with warfarin | TT with NOACa | TT with LMWH | TT with apixaban | TT with dabigatran | TT with rivaroxaban | ||
|---|---|---|---|---|---|---|---|
| (N = 472), n (%) | (N = 90), n (%) | SMDb | (N = 60), n (%) | (N = 31), n (%) | (N = 9), n (%) | (N = 47) | |
| Duration of TT (months), median (IQR) | 3.7 (2.2–7.1) | 3.6 (1.8–6.2) | 0.28 | 2.4 (1.3–4.2) | 2.6 (1.8–5.9) | 4.6 (2.5–8.0) | 3.8 (1.9–6.2) |
| Any bleed, n (%) | 87 (18) | 16 (18) | 0.06 | 8 (13) | 8 (26) | < 5 | 6 (13) |
| Rate per 1000 person-years (95% CI) | 219.9 (178.0 to 271.6) | 202.5 (135.8 to 302.2 | 159.4 (79.7 to 318.7) | 360.7 (180.4 to 721.2) | 133.2 (18.8 to 945.6) | 161.7 (72.6 to 359.9) | |
| Major bleed (HES), n (%) | 33 (7) | 9 (10) | 0.01 | < 5 | < 5 | < 5 | < 5 |
| Rate per 1000 person-years (95% CI) | 73.3 (52.1 to 103.1) | 77.9 (43.1 to 140.6) | 34.1 (8.5 to 136.3) | 148.2 (55.6 to 394.8) | 122.6 (17.3 to 870.7) | 66.7 (21.5 to 206.9) | |
| Minor bleed (CPRD), n (%) | 61 (13) | 8 (9) | 0.11 | 6 (10) | < 5 | 0 (0) | < 5 |
| Rate per 1000 person-years (95% CI) | 140.1 (108.8 to 180.4) | 101.9 (60.3 to 172.0) | 108.3 (48.7 to 241.2) | 151.0 (56.7 to 402.4) | – | 67.7 (21.8 to 210.0) | |
| Total number of bleeds, n (%) | 138 | 27 | – | 12 | 13 | < 5 | 10 |
| Ear, nose or throat bleed | 47 (34) | < 5 | – | < 5 | 0 (0) | 0 (0) | < 5 |
| Gastrointestinal bleed | 37 (27) | 11 (41) | – | 5 (42) | 5 (39) | < 5 | < 5 |
| Genitourinary bleed | < 5 | 0 (0) | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intracranial bleed | 5 (4) | 6 (22) | – | < 5 | 5 (39) | 0 (0) | 0 (0) |
| Ocular bleed | 16 (12) | < 5 | – | 0 (0) | 0 (0) | 0 (0) | < 5 |
| Skin or soft-tissue bleed | 26 (19) | < 5 | – | < 5 | < 5 | 0 (0) | 0 (0) |
| Other anatomical site bleed | < 5 | < 5 | – | 0 (0) | 0 (0) | 0 (0) | < 5 |
| Unspecified anatomical site bleed | < 5 | < 5 | – | 0 | < 5 | 0 (0) | < 5 |
| All-cause mortality | 33 (7) | 6 (7) | 0.01 | < 5 | < 5 | 0 (0) | < 5 |
| Cardiovascular mortality | 17 (4) | < 5 | 0.02 | < 5 | < 5 | 0 (0) | 0 (0) |
| Mortality from bleeding | < 5 | < 5 | 0.14 | < 5 | < 5 | 0 (0) | 0 (0) |
| MI | 24 (5) | < 5 | 0.03 | < 5 | 0 (0) | 0 (0) | < 5 |
| Stroke | < 5 | < 5 | 0.26 | < 5 | < 5 | 0 (0) | < 5 |
| Additional coronary intervention | 47 (10) | 11 (12) | 0.07 | 9 (15) | < 5 | < 5 | 8 (17) |
| MACE | 75 (16) | 17 (19) | 0.08 | 12 (20) | < 5 | < 5 | 11 (23) |
Discussion
The incidence of any bleeding among patients on TT was double that in the target trial populations taking antiplatelets only (TT 18%, compared with 8% and 12% among conservatively managed ACS patients taking aspirin monotherapy and DAPT with clopidogrel, respectively; and compared with 8% and 11% among emergency PCI patients taking DAPT with clopidogrel and DAPT with ticagrelor, respectively). The rates of mortality and MACEs at 1 year were higher among patients who were prescribed TT than among emergency PCI patients prescribed DAPT only (mortality: 7% vs. 2%, respectively; MACEs: 17% vs. 12%, respectively), but lower than among the conservatively managed ACS patients receiving aspirin or DAPT (mortality: 7% vs. 13%, respectively; MACEs: 17% vs. 19%, respectively).
There are several systematic reviews comparing DAPT with TT among patients undergoing PCI. 71–76 All included between 7000 and > 20,000 patients. All show, unequivocally, an increased risk of bleeding with TT (by about 1.5 times), and, although TT decreases the risk of stent thrombosis, it does not appear to decrease risks of death and ischaemic end points at 1 year.
We observed no major differences between TT with warfarin and TT with NOACs in the incidences of bleeding, mortality or MACEs, although slightly more patients experienced a stroke in the NOAC group. We could not perform comparative analyses between the different TT groups because the numbers of patients in each group were too small. A 2019 network meta-analysis77 including > 10,000 patients from four RCTs did not show a reduced risk of major bleeding (OR 0.70, 95% CI 0.38 to 1.23), or MACE (OR 1.02, 95% CI 0.71 to 1.47) for TT with NOAC, compared with TT with warfarin [the four RCTs were as follows: What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST); an open-label, randomised, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin k antagonist treatment strategy in subjects with AF who undergo PCI (PIONEER AF-PCI); a randomised evaluation of dual antithrombotic therapy with dabigatran vs. TT with warfarin in patients with non-valvular AF undergoing PCI (RE-DUAL PCI); and an open-label, 2 × 2 factorial, randomised controlled, clinical trial to evaluate the safety of apixaban vs. vitamin k antagonist and aspirin vs. aspirin placebo in patients with AF and ACS or PCI (AUGUSTUS)]. We could not draw conclusions about the risks and benefits of different NOACs as part of TT, as the numbers of patients in each group were < 50.
Chapter 4 Qualitative study with patients
The extent of under-recording of bleeding events in primary care is unknown. One issue is under-recording of bleeding events by GPs,78 and another issue is the extent to which patients under-report bleeding to their GP, particularly the nuisance bleeding likely to be experienced while taking DAPT or anticoagulants. 79 Nuisance bleeding is reported by up to 38% of patients initiating DAPT14, yet the rate of minor (CPRD-reported) bleeding events in the ADAPTT study across all populations was only 4–7%. Nuisance bleeding may not result in patients seeking medical care or hospitalisations, and such events are believed not to need active intervention. 28 At the same time, there is concern that nuisance bleeding may influence adherence to DAPT80 and limit patient quality of life,14 and that it can result in premature discontinuation of DAPT. 81 This qualitative study was conducted to improve our understanding of patients’ experiences with nuisance bleeding and the factors that prompt them to seek help and/or medication changes (illness behaviours) while on DAPT.
Methods
Study design
We conducted qualitative focus groups with two groups of patients who had undergone PCI or CABG:
-
group 1: antiplatelet therapy for 0–3 months (start of DAPT therapy)
-
group 2: antiplatelet therapy for 9–12 months (coming to the end of DAPT therapy).
Focus groups were used because of their distinct ability to identify the range of views and experiences of patients through group interaction. 82 Two focus groups for each of the two treatment duration groups were organised to allow for any differences in experiences, perceptions or needs that might be present between patients at different stages in their therapy to emerge through the narratives.
Recruitment and sampling
The focus groups were conducted during June and July 2017. Participants were patients who had been treated at the Bristol Heart Institute, identified from hospital wards pre discharge and hospital theatre/catheter laboratory lists and approached by research nurses and consultant cardiologists during follow-up and post-surgery clinics, cardiac rehabilitation sessions and day clinics.
The target sample size was 10 participants per focus group (two groups with patients at the start of DAPT therapy and two groups with patients at the end of DAPT therapy; 40 participants in total), aiming to meet the recruitment needs of the patient elicitation exercise performed as part of the health economics analysis (see Chapter 5), while maintaining a sample size appropriate for focus groups. 82 The patient elicitation exercise and focus groups were independent in terms of their aims and methodologies, but were conducted on the same day because of logistical considerations. Approximately 1 week after the initial contact, patients who expressed interest in participating were contacted again by members of the ADAPTT study team to confirm attendance. The voluntary nature of participation in the focus groups was made clear to all individuals and informed consent was obtained. The study was approved by the South West – Cornwall and Plymouth Research Ethics Committee (reference number 17/SW/0092).
Data collection
All discussions were audio-recorded. A topic guide was used that covered the attribution of symptoms to DAPT, the range of thresholds for seeking further information and help, the range of thresholds for requesting a change in medication, and issues related to adherence and quality of life. Generally, sampling of participants who share attributes of interest and focusing group discussion on a limited number of topics will require fewer focus groups to meet the aims of a study and achieve saturation. 82 For our purposes, four focus groups were considered adequate to address the aims of the study.
Data analysis
Focus group audio-recordings were transcribed by a professional transcription service. All transcripts were checked for accuracy against the original audio-recordings and anonymised. Transcripts were imported into NVivo 11 data management software to aid data coding and management. Data were analysed as one data set using a framework approach. 22 Following familiarisation with the transcripts, initial codes were created representing the topics guiding the discussion: information and knowledge about DAPT, issues related to adherence, issues related to bleeding and the role of family members in adherence. These topics were informed by the study objectives. Transcript data were indexed based on these codes. In iterative rounds of analysis, further codes were inductively created within these initial categories to reflect the issues spontaneously raised by participants during the discussion as related to intentions to stop taking medication, accessing care and/or information on DAPT. 83 Following the coding of the first two transcripts, an analytical framework was developed. Framework matrices were created in NVivo 11 to identify differences and similarities within and across themes and focus groups/time frames for antiplatelet therapy. One researcher led the analysis, with the coding frame being developed in collaboration with the co-investigators. The team met regularly to discuss the coding framework and themes, and any implications for ongoing data collection. Findings were presented to the patient and public involvement group for further comments and feedback to enhance trustworthiness, credibility and rigour. The patient and public involvement group confirmed the relevance of the findings to the group’s experiences.
Results
Figure 23 shows the flow diagram of participants through the study. In total, 150 individuals were identified as being eligible for inclusion and were approached by telephone; 68 were invited to participate in the study. Of these, 37 agreed to participate and received a participant information leaflet, but only 21 patients attended their assigned focus groups. Focus group discussions lasted for between 60 and 90 minutes.
FIGURE 23.
Participant flow diagram. PIL, participant information leaflet.
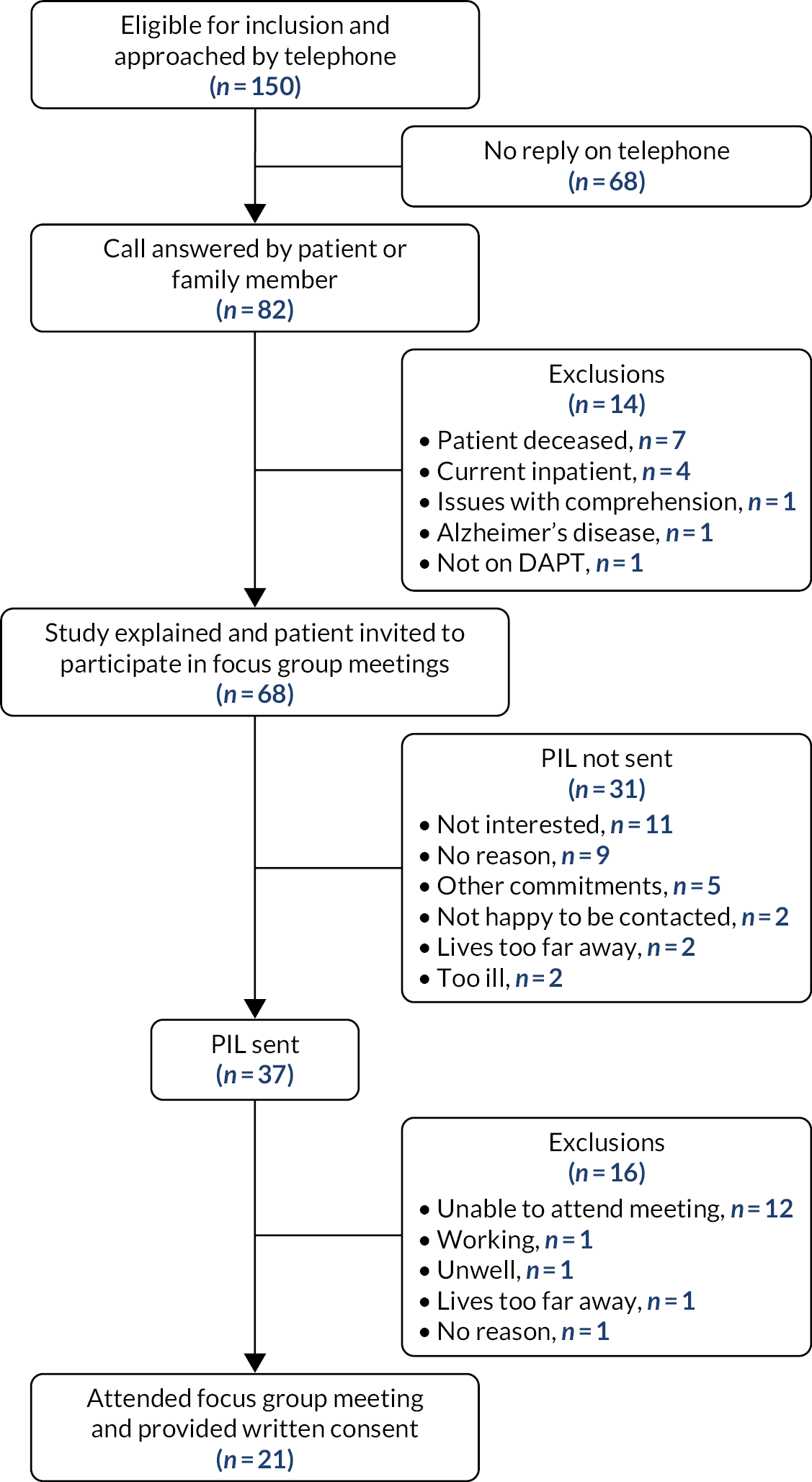
Table 41 reports on the demographic characteristics of the 21 patients participating in the study and the 47 patients who were approached but declined participation. Only one of the nine women approached (11%) accepted participation, compared with 34% of the men approached. All individuals who participated were white. Fewer patients who had CABG (25%) than had PCI (34%) accepted participation. Of the 21 participants, 14 had undergone PCI, six had CABG and one had not received a revascularisation intervention (pharmacotherapy only). Nine participants had been taking DAPT for ≤ 3 months and 12 had been taking it for 9–12 months. The average age of participants was 66 years. The spouse of a male participant in one of the early DAPT groups participated in the discussion, but was not counted as a patient participant and not included in the participant demographic information.
| Participants | |||
|---|---|---|---|
| Characteristics | Patient information leaflet sent and attended focus groups (N = 21) | Approached but declined participation (N = 47) | Total (N = 68) |
| Sex, n (%) | |||
| Female | 1 (5) | 8 (17) | 9 (13) |
| Male | 20 (95) | 39 (83) | 59 (87) |
| Age (years), mean (SD) | 66.3 (11.3) | 64.5 (9.4) | 65 (10) |
| Ethnic group, n (%) | |||
| White | 21 (100) | 37 (79) | 58 (95) |
| Asian | 0 (0) | 1 (2) | 1 (1) |
| Not recorded | 0 (0) | 9 (19) | 9 (13) |
| Procedure, n (%) | |||
| CABG | 6 (29) | 18 (39)a | 24 (36)b |
| PCI | 14 (67) | 27 (59)a | 41 (61)b |
| PCI and CABG | 0 (0) | 1 (2)a | 1 (1)b |
| Medical management | 1 (5) | 0 (0) | 1 (1) |
| Antiplatelet regimen, n (%) | |||
| AC | 14 (67) | 27 (59)a | 41 (61)b |
| AP | 1 (5) | 2 (4)a | 3 (4)b |
| AT | 6 (29) | 16 (35)a | 22 (33)b |
| Clopidogrel only | 0 (0) | 1 (2)a | 1 (1)b |
| Duration of DAPT (months), n (%) | |||
| ≤ 6 | 9 (43) | 18 (42) | 27 (42) |
| > 6 | 12 (57) | 25 (58) | 37 (58) |
| Duration (months) of DAPT for ≤ 6 months, median (IQR) | 1.3 (1.0–2.9) | 2.0 (1.9–3.0) | 2.0 (1.0–3.0) |
| Duration (months) of DAPT for > 6 months, median (IQR) | 11.8 (10.6–12.7) | 12.0 (12.0–12.0) | 12.0 (12.0–12.0) |
| Reported previously experiencing a minor bleed while on DAPT, n (%) | 10 (48) | – | – |
Five themes capturing the enablers of and barriers to adherence and triggers of information- and care-seeking were identified (Table 42). The two treatment duration groups did not differ in their attitudes towards nuisance bleeding, DAPT or perceptions of care. Differences in experiences between the two groups are reported where relevant.
| Themes | Subthemes |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Theme 1: patient medication counselling during hospital stay
Being offered patient medication counselling and quality of the interaction
Participants in both groups recounted being given information about their medication before leaving hospital. For many, this was the only instance of medication counselling received, mostly delivered by nurses when dispensing medication. Only a small number of participants recounted being counselled about their medication by health-care professionals after leaving hospital. Often, participants challenged the quality of the medication counselling received while in hospital, on both the type and the quantity of information given.
Focus group 1, 9–12 months
204 male (M): I can’t remember my consultant telling me any of the side effects that may well happen to be quite honest with you [...].
M: I had a special meeting with one of the nurses beforehand and it was all written out, what they are going to tell you, and he went through it and said this is a possibility with these chances [...].
205M: It was the same with me, I went in and had the stents done and when I came back out [...] you just relied on the nurse that was looking after you at the time. She brought the discharge forms and all the rest of it and tablets, medication to take [...].
201M: I picked the hospital in my case, nothing from the GP particularly [...] and the guy that looks after me is fantastic and he just, he gives me lots of information about things.
202M: It’s the same with the GP, not very good, it was the nurse when I had the stents put in that told me most of the information and then when you go back and see whoever you see afterwards, they are very good [...].
206M: [I was given] loads of information. Explanations on each pill and an indication of how long they should be taken, certainly in my case.
207M: The only time I basically knew how long I had to take mine for was because it was on the pillbox, taking until 5 June and consequently they have now put me on this other medication [...] and then I suppose I will get told what to do, what medication to take out of that [...].
205M: When I was discharged as a day case, I was told about the possible risk of bleeding while I was on the aspirin and ticagrelor.
Focus group 2, 0–3 months
102M: Oh yeah, the surgeon told me all about it, what they were for.
101M: Nobody told me anything.
101_wife: Nothing. No, nothing at all.
103M: If you asked, they would say, ‘Oh that’s so and so, that’s so and so.’. Oh right, OK then, that was it.
Focus group 4, 0–3 months
The single female participant pointed out the sex bias inherent in cardiovascular disease and revascularisation information given to patients, and how it misses out the specific needs of women patients:
The information in the leaflet by [doctor], it was directed at men and I understand because it’s themajority of men that seem to have the heart problems. However women are being ignored [...] so yes, from a woman’s point of view there needs to be a lot more information for us, it’s not just about the boobs, it’s about the emotional side. [...] Also with the bleeding with women that are still having periods, that needs to be discussed as well.
109 female (F)
Barriers to participation
Discussions suggested that, in most cases, participants had only a limited understanding of their treatment following the initial medication counselling. Participants in both groups reported barriers to engaging with health-care professionals during their time in hospital. These barriers related to the timing of counselling, the setting in which the discussion took place (i.e. busy hospital wards) and the communication style adopted by the clinician. In many cases, participants recounted being approached shortly after their revascularisation intervention or diagnosis, and right before they left hospital, when their physical and emotional state hindered participation in the communication process. At this time, medication was not a priority.
Focus group 3, 9–12 months
220M: [...] at the end of the operation or the procedure, you’re left there recuperating, waiting to go home, be collected, and a nurse comes along with a bag of medication.
215M: The trouble is, when you’re, when you’re given the medication, you’re ill. [...] You’ve just had a bloody heart attack.
[Agreement from group.]
216M: And you’ve got a lot more things on your mind [...] than worrying about that [the side effects of the medication].
Focus group 4, 0–3 months
109F: [The surgeon] came round after my surgery but I was completely out of it. The alarms were going because there was an incident going on [...] he said ‘you’re OK, everything was successful [...] do you have any questions?’.Well I was, I had so much morphine [laughs] my husband [said] ‘Do you have any questions?’ and I was, yeah but I couldn’t focus mentally, come out with it [...].
108M: [...] The nurses basically when they, when I left hospital, just sort of went through the drugs and said ‘That’s for that, that’s for that, that’s for that’ and I was by that time climbing the walls because I wanted to get out, so I didn’t take a lot of that on board.
Opportunities for patient medication counselling after leaving hospital
Following discharge from hospital, most participants recounted limited opportunities for receiving medication counselling from specialists, with the exception of participants referred to a cardiac rehabilitation clinic post CABG. One participant referred to the pharmacist as a source of information on statins.
Focus group 1, 9–12 months
204M: And just going on to the rehab[ilitation], [...] and part of the handout booklet did go into the drugs with us and just go ‘what are you on?’, definitely what they were there for, and so in general terms [...] within the rehab time and the literature they issued, that was quite good [...]
206M: Talking of the statins, I went to see the pharmacist [...] he was so helpful, he explained about every statin virtually on the market and what it did and what it didn’t.
Several participants were dissatisfied with the opportunities available to receive information after discharge into the community, and with the quality of the communication during secondary care follow-up appointments. Factors influencing the opportunities for receiving information, and the quality of the information received, included uncertainty about their care pathway, lack of continuity of care, emphasis on clinical procedures, and fragmented communication pathways between different care providers. These are the issues discussed in the following excerpt by participants in one of the two focus groups that consisted of people on DAPT for 0–3 months.
Focus group 4, 0–3 months
108M: [...] but I do think there is a lack of communication to the individual post op[eration], I’ve never seen my surgeon [who did the operation], I’ve never met him, I had to ask when I went back exactly what they had done and where they’ve done it. So that would be [the] downside from my experience.
107M: But you [108M] said you had nothing from the hospital when you left. They didn’t do another appointment. No paperwork, because I was in the [hospital] and shortly after I got home, I had a letter through to say there was a date to go and see my surgeon.
108M: Yes well I had that but I didn’t see my surgeon [during the follow-up appointment the surgeon] talked at 90 miles an hour, dah dah dah dah dah dah blah blah blah. ‘Any questions?’. [...] And so I did have some questions and to answer them he had to go back through the notes [...] which was a big disappointment.
109F: I’m the same as [108M], I haven’t seen my surgeon either [...]
105M: So when will you next be tested to see what the medication is doing to you?
106M: Don’t know. I suppose it’s up to me to make an appointment.
105M: That’s the problem with it, isn’t it, waiting for you to have a symptom to go back and say so and so.
For the majority of participants, the medication package insert was the main source of information. Only a very small minority accessed information online. Some participants found written information difficult to understand.
Focus group 1, 9–12 months
[...] I did experience chest pain subsequently, not like the angina that I had before, and from the literature with the medication and looking on the internet, I discovered that it was a possible side effect of ticagrelor, but I wasn’t warned about that.
205M
Focus group 4, 0–3 months
Interviewer: Were you given any information what side effects to expect, what to look out for?
109F: Only what I read in the box [...].
M: Only what we get in the box.
110M: No, 16 or 17 pages to read because each medication has its own list of side effects [...].
109F: The leaflets did [...] give me enough information, you know. Like we all said, they very much merged into one because it was like ‘OK, that one’s saying the same as that one with the side effects’ so they were all pretty – pretty similar.
107M: But they say everything, they say – you can suffer from anything by reading one of those leaflets [...].
109F: It was just the numbers were slightly different, you know this is from one to 100 or this is one, two or three out of 1000 people. That was just the slight difference to it.
110M: No, but I mean there’s so many [...] you know, instructions and what you shouldn’t do and what you should do and what could happen.
M: It could happen by the time you’ve read one.
[Laughter.]
110M: And at the end of it, you don’t know what the medication is for anyway.
Theme 2: perceptions of care and medication counselling after leaving hospital
The interface between secondary and primary care
Contact with GPs was needed for instigating repeat prescriptions of medication initiated in the hospital, but participants were not always clear on what the care pathway was after leaving hospital, nor the responsibilities of the different professionals involved in their care.
Focus group 4, 0–3 months
106M: I was under the understanding that the hospital sent a letter to the doctors to say ‘He’s on these medication for a year, from now’ but it never appeared on my repeat prescription. So then I [...] phoned the surgery and said ‘Can you just put it on the repeat prescription?’. ‘No, you’ve got to see the doctor’ [...].
108M: It’s all a bit sort of vague but I’m pretty sure that they told me I need to go see my GP after a month to review my medication and that he would have a copy of the letter that they gave me on my medication, which a bit like yourself, I took that letter with me [...] he just took the letter off me and read through it and said ‘Oh right yeah you’re on blah blah blah’.
Participants would access a GP in case of medication concerns, primarily because they ultimately wanted to see a specialist and GPs were believed to be their gateway to secondary care. Participants described several barriers to accessing specialist care, including the long waiting time between secondary care follow-up appointments and long waiting lists for specialist appointments.
Focus group 4, 0–3 months
Yes [the first port of call would be the GP], it has to be to then be referred on in case you get something more.
109F
Focus group 1, 9–12 months
206M: Well you have got to go to the GP first and then whatever. I mean at the moment, hospital is sort of, every 9 months I get a note saying ‘[name] you are on this, you are going to see them and in the meantime if anything happens, you can’t phone up them because you have got your appointment’, so you go to the GP.
207M: I know the letter I got from my consultant, in the bottom paragraph, said if you experience any problems ring the secretary on this and we will gladly see you back into our outpatient clinic, so rather than go to my own GP, who only knew part of the problem, I could go back to the consultant who knew all the problem and then get it sorted [...] but how long that waiting list would be to get in to see him is a different matter.
The role of primary care in overseeing medication management
The majority of participants expressed scepticism when discussing the role of primary care in medication management. Overall, participants believed that GPs lacked the knowledge to oversee their medication and did not trust GPs’ ability to give informed advice. The following quotation is illustrative of the opinions expressed by the majority of participants when discussing their perceptions of GPs’ knowledge of their conditions and medication.
Focus group 4, 0–3 months
I don’t think local GPs in most cases, or certainly in my experience, I don’t think they have enough understanding of the cocktails of drugs I was taking and to be able to say ‘That’s what we need to check’. [...] when I go back to my local GP there’s no discussion that takes place, that’s the letter from my consultant, that’s what I need and you know there’s no – I don’t even ask whether I should change anything because they just don’t know.
105M
Some participants discussed instances where their GP’s advice contradicted the information received from specialists, while many perceived their GP’s advice to be unreliable. In the following excerpt, participants discuss their experiences with seeing their GP for reviewing their medication. In these encounters, participants expected GPs to revise their prescriptions based on guidance given by secondary care specialists and information included in medication package inserts but the advice received did not meet these expectations compromising their trust in the ability of GPs to oversee their care.
Focus group 1, 9–12 months
204M: [...] I mean there were issues when [medication management is] outsourced to the GP because the GPs acknowledged the letter [from the hospital reporting on the patient’s medication regime], but then suggested ‘well you could just stay on the clopidogrel because it might help strokes’.
202M: GPs are useless.
204M: And it’s sort of an issue of ‘well that’s not what the heart experts are saying’ [...] so you are getting one decision from the experts from the hospital which, you know, is quite conclusive in a way and there may be tiny differences but you know where you are going, but once it goes outsource to the GP, I think that’s when it gets a bit blurred from the patient side [...].
202M: I had the same thing with my GP to review [my medication] [...], he said ‘well it’s up to you’ [to decide whether to stop or continue with the medication]. I said ‘well it’s not up to me’ and he said ‘well you can take it if you want to’. So I was really upset about that and I had recently been back to the surgeon and he has reviewed it all properly and he said you can stop that, we will put that down to 2.5 instead of 5 and away you go and you think ‘well what’s the point in going to the GP for?’, because they don’t say anything.
In addition to the knowledge and expertise of GPs, several participants raised the issue of continuity when accessing GPs, with many sharing their experiences of seeing a different GP during their appointments, as many surgeries assign patients to available appointment slots, which might not necessarily be with their named GP. These experiences influenced participants’ decisions of whether or not and when to access primary care.
Focus group 4, 0–3 months
108M: I mean GP is a title isn’t it, how often do you see the same one?
107M: Well you see, you’re seeing too many GPs, you know, one you get used to and then anybody’s got access to him, I mean you haven’t got a GP as such. You go in and end up seeing one of maybe seven or eight, you know; otherwise you’ve got to wait perhaps a month to see that particular one you want and you don’t want to wait a month. There’s too many people.
109F: I haven’t had that experience, I’ve consistently seen the same doctor all the way through. My surgery have been quite proactive in making sure that I do see that same GP that I saw at the beginning [...].
108M: I think if you get contact with a GP and you have confidence in that GP then that’s great [...] I would need to be very concerned about something to go back to my GP, fortunately I’m literally 150 yards away from my practice and, I have to be desperate to see [doctor], I would want to see the guy that I’ve got the trust in [...].
M: But that can be up to 3 weeks can’t it?
Theme 3: making sense of treatment and symptoms
Experiences and perceptions of nuisance bleeding
Experiences of nuisance bleeding varied from bruising to bleeding that had a more compromising effect on quality of life. Of the participants who shared their experiences with the group, just under half at the early stages of treatment (44%) and half coming to the end of treatment reported experiencing nuisance bleeding. Those who had already come to the end of their treatment and were now on only one antiplatelet reported subsiding of symptoms.
Focus group 3, 9–12 months
220M: The only other thing is [...] I had slight problems with haemorrhoids [prior to DAPT]. [...] With taking the medication, both medications, the aspirin and the other, there was bleeding. So it did increase the bleeding there. I was quite concerned about it. But now that I’ve been off the tablet [...] it’s all improved. So hopefully my bruises will go down [...]
215M: And then they just bunged me on all this medication, very much like [220M], just loads of bruises, very much like you cut yourself shaving; it bled for hours. And then I had a defibrillator fitted, when I had the defibrillator fitted they took me off the aspirin and since I’ve been off the aspirin, I still take the other, whatever it’s called, I don’t get bruising anymore.
Most participants felt that they had control over their symptoms and shared ways of dealing with nuisance bleeding; for the majority, nuisance bleeding was not a cause of major concern.
Focus group 4, 0–3 months
108M: I have cut myself a couple of times and noticed that it’s a bit runny and hold a tissue over it and it stops [...].
106M: I’m not shaving very much and gone back to the electric because when you do cut yourself, obviously you bleed a little bit, [...] but it just takes that bit longer to stop it and it’s a bit awkward.
Most participants were aware of the link between antiplatelet medication and bleeding because of the timing of the symptoms, because they were told by health professionals, through interactions with other patients or through reading information leaflets. Being aware of the bleeding risk involved in DAPT medication decreased levels of anxiety when experiencing symptoms.
Focus group 4, 0–3 months
106M: When I was given the medication at the hospital and they gave me a months’ worth [of medication], the nurse did explain what it was and what would happen, so it wasn’t a surprise that I was bleeding more. I understand what was causing it, so that was fine [...].
105M: I’ve had this [DAPT] since, over the last 12 years, so 12 years ago when I had a stent I was put on aspirin and something else. Can’t remember what it was at the time but that was for a 12-month period and basically it was to stop the body rejecting the stent or trying to cover it. Because obviously if you’ve got something in that tube and it’s something that gets furred up its going to block it even more solidly so that was what that was about [...].
109F: [I would link bleeding to DAPT] because when I was talking or somebody was talking again in my rehab group they’d brought up the thing of bruising and I thought ‘Oh yeah that’s been happening to me’ and I hadn’t put the two together.
Focus group 2, 0–3 months
101M: [this patient had not yet seen their GP after leaving hospital, a requirement for being prescribed medication initiated in hospital] [...] I don’t know [whether I would attribute bleeding to the antiplatelet medication]. I’m a very trusting person.
102M: I would yes, I would put it down to the [anti]platelets straight away [...].
101M: I never knew before, in fact, that any drug could cause damage.
Weighing the costs and benefits of dual antiplatelet therapy before acting on symptoms
The majority of participants believed that the benefits of DAPT outweighed any potential risks or impact of side effects experienced so far. The antithrombotic qualities of the drugs, along with the fact that they were taken for only a short period of time, made them more appealing than other medication, whereas they perceived the implications of not taking them as life-threatening. Some participants compared nuisance bleeding with other more serious side effects, which, in some cases, led to discontinuation of treatment, to emphasise that they regarded nuisance bleeding as being less severe than other side effects. Some participants also emphasised that they would not discontinue DAPTwithout the advice of a clinician. Nuisance bleeding would trigger accessing emergency services if it was persistent and unmanageable.
Focus group 1, 9–12 months
204M: [...] If you were bleeding, I would think.
206M: I am certainly not stopping the ticagrelor because I am only going to get it for 12 months and I have got me money’s worth [laughs]. Obviously at the moment, I feel that’s a more important drug, whereas the statins is something else.
204M: I stopped [statins] for a while and after [experiencing other symptoms] I started taking it [...] but on the blood ones [...], [side effects] wasn’t a consideration to stop [...]. It’s they’re doing a purpose to keep [the blood] thin so I think even if there is bruising or slight bleeding, if it became serious that could be an issue [...], but if it’s just a bruise, that goes with the territory really.
205M: None of the side effects were severe enough to make me consider stopping them, if they had been I wouldn’t stop them without reference to a GP. [...] Exactly the same thing, minor things, but not if you can’t stop. If it was more serious I think I would be going down 999 [...] because it depends how much it is, if it’s quite a lot, rather than try and go to a walk-in, depends how far away you are and GP, well you won’t see a nurse for 5 days either, so you need more response [...].
202M: [...] with my gums and that, if it bled and bled and bled for 2 or 3 days, then I probably would phone a doctor and it always stops after an hour or so, I don’t see a problem really. Same with the nose bleeds, they only last for 10 minutes, average, and then they stop.
Focus group 3, 9–12 months
220M: I think, I think we all accepted bruising and that as just a side effect that you’ll accept [...].
216M: For me, there’s no option [not taking the medication], because if it’s going to thin your blood, which is what I want to do to keep the stent working and not clogging up, then you’ve got to accept some disadvantages. So I’m all for carrying on taking the tablets.
The importance placed on adherence was heightened by participants’ family experiences of heart disease.
Focus group 1, 9–12 months
My brothers had stents and my father had triple heart bypass and died of a massive heart attack afterward [...] but I do wonder if [...] taking this sort of drugs for longer would actually keep you going. Every morning I wake up, to me it’s a bonus [...] so if I could take something for longer that you could sort of guarantee, it might make you a little bit better.
201M
Focus group 2, 0–3 months
My sister [...] had never had any heart conditions but had been on statins because [...] of our family history and she stopped taking the statins against doctors’ advice because they were causing her leg problems and she ended up having a triple bypass so, you know, they advised her to carry on taking them and it would have probably have prevented that.
105M
Some participants in one focus group pointed out that their perceptions of and reactions to nuisance bleeding might be different had they experienced more severe symptoms, giving as examples those described in medication insert leaflets, and the scenarios presented in the patient elicitation exercise carried out prior to the focus groups.
Focus group 4, 0–3 months
106M: No [bleeding is not a concern].
108M: I think if any of us have experienced any of the things that you’ve written in there [refers to the questionnaire scenario], then I think you’d get a different answer. It’s very difficult, you know, alright we probably all read that, you know, excessive nose bleed or bleeding from behind or wherever, never experienced it.
Taking multiple medications can hinder making sense of and receiving care for symptoms
Taking multiple medications presented challenges when trying to make sense of symptoms and acting on these symptoms.
Focus group 4, 0–3 months
Well I’ve never had any bleeding apart from a little spot here which refuses to go away, whether that’s associated with it I don’t know, but as far as the antiplatelet medication is concerned when you take a cocktail of your medication you don’t know which one’s doing what, so you can’t really answer that fully.
110M
Participants thought polypharmacy made conversations with clinicians about their medication concerns more challenging. It was not always clear which agent had caused the symptoms causing concern, and clinicians were thought to focus on agents or symptoms falling under their own expertise, while at the same time being unwilling to make changes to medication prescribed by other specialists.
Focus group 4, 0–3 months
If you [ask around the room] about all of our different medications. I’m diabetic as well [...] so I’m probably on a different cocktail, and therefore the side effects could be apportioned to all sorts of different things, so it is a case of sucking and seeing it and if you’re not feeling that great on it, going back and discussing it, which isn’t easy [...] because, you know, from the consultant’s point of view he’s tackling the cholesterol so that’s his war is on that and if anything else as a side effect is ‘Oh well you have to put up with that to get this beaten’ [...].
105M
Focus group 1, 9–12 months
I’ve had aches, I have had aches and I’ve referred to my GP and he said, considering it’s so close since the op, he doesn’t want to mess with them. [...] And that was the problem, having a multitude of medications, you don’t know which one is causing it, and the answer by the GP was just give you another one [to deal with the side effects].
109F
Perceptions of patient involvement in medication management
Several participants from both groups discussed their views on patient involvement in care and medication management. Some participants believed that adherence to and being engaged in their treatment was necessary because ‘it’s our responsibility as a patient’ (focus group 4, 0–3 months, 109F). Medication self-management was believed to be made pertinent by gaps in patient counselling and the challenges of accessing medication advice that they considered to be trustworthy. Several participants emphasised the importance of taking control of their treatment themselves to ensure adherence and address uncertainty resulting from conflicting advice. For others, being informed could guide appropriate help-seeking and inform their discussions with clinicians.
Focus group 1, 9–12 months
204M: [...] I don’t think patients are necessarily highlighted with [how long they need to take medication for], if they don’t look themselves. I think [you] have to read what you are taking and see in perhaps 6 months ‘am I taking it for too long?’, etc.
207M: But isn’t it the same old story, sort of given a pill and within that pillbox there is a load of literature, how many people read it when they get round to the side effects maybe or what have you, I mean they take the pill for so long, go and get a repeat prescription. I will be honest with you, I very rarely read the literature inside the pillbox [...].
205M: [...] I do read all the literature in tablets now, because I am on 23 tablets a day so I am worried that how do they know that one isn’t reacting on another one down the line [...].
Focus group 3, 9–12 months
Well, the reason I disagree [with not reading the medication information leaflets] is because [you cannot answer the question] ‘what the hell’s going wrong with me?’. You read the leaflets and you think, ‘Oh’, if you get this happening or that happening then you can speak to your doctor about it or – but do not stop taking these tablets. [...] And I think it’s easier when you know what’s causing the problem than when you’ve got the problem it’s not being explained to you really. So that’s why I read the leaflet, but that’s myself.
216M
Theme 4: experiences of everyday adherence to treatment regimen
Barriers to adherence
Polypharmacy and regimen complexity made it difficult for participants to take medication as advised. When taking a multitude of medications, the physical attributes of tablets also became important enablers of, or barriers to, adherence.
Focus group 1, 9–12 months
I take 23 [tablets] a day and take 17 in the morning and six at night, but some of the ones you have to take an hour before food, some you have to take an hour after food, but I just haven’t got enough time in the day to do that, so you tend to take the whole lot.
201M
Focus group 2, 0–3 months
The trouble you find is that, say you’ve got eight tablets in a pot, or something like that, and two of them are very, very small. They could be on the tablecloth perhaps.
101M
Focus group 3, 9–12 months
215 M: And I was just saying earlier, one of the things that really gets me is they keep changing the bloody colour and the shape of them [...]
M: That’s annoying, isn’t it?
215M: Yes it is, but if you’re elderly and get a bit confused.
M: Yes, yes.
215M: I mean, I have to read them and see what they are.
M: I agree with you, that is annoying.
M: Yes.
215M: If that was my mother, my mother would have been in a hell of a state with it. I think it’s just stupid.
Adherence-promoting strategies
Participants discussed strategies that they used to help them take their medication every day. Sticking to a routine, using medication dispensers and automatic prescription renewal and delivery schemes were some strategies raised.
Focus group 4, 0–3 months
107M: It is [easy] for me [to remember to take my medication] because I got a routine and I stick to that routine and it doesn’t change. You know, if I’ve got a tablet missing I’m straight up the chemist and say ‘Look, you know, can you get this for me’.
105M: Yeah well you’ve gone – you can set it up on a website now, Pharmacy2U [Leeds, UK] and they’ll just post it out to you.
M: That’s right, yeah.
106M: So I used the patient access apps at home; if I don’t need just tick it or send it off a few days later, go down collect it from the doctor, the chemist then in the doctors’ surgery. Just go in and it’s there waiting, which I could have delivered if I wanted to, but I don’t feel I should do that personally.
Focus group 3, 9–12 months
220M: I don’t know about you, because you’ve got to take, well, it’s five a day now, I got one of those tablet dispensers.
M: Yes, yes.
220M: And make it up for a week and that is the best way to do it.
Focus group 1, 9–12 months
204M: Yes it wasn’t a problem for me because it was only once a day and once you have had a routine and when to take it [...] it wasn’t a problem at all really.
205M: No problem at all.
M: I had no problems.
M: Dead easy.
Theme 5: support from family network
During discussions, the central role of partners in the participants’ care and recovery was often mentioned. Partners were reported to be active members in the discussions during consultations, or searching for information afterwards, when patients themselves might not have. Family members also supported participants in taking their medication.
Focus group 4, 0–3 months
[...] Apart from ‘you may bleed a bit more’ there was nothing else said about any other side effects, but my wife, um, is very nosey and she googles everything so – so we learnt quite a lot from – from that side of it.
106M
Focus group 1, 9–12 months
My daughter sorted me out with those big pill things, Monday, Tuesday, Wednesday, filled it all up and said get on with it. You just fall into it, easy.
201M
Focus group 2, 0–3 months
101_wife: Our daughter [...] said to me, ‘Mum, make sure dad does this’. And she’s done a list and put them all in these little [...]
101M: As they do.
101_wife: Just in case I do overprescribe them [laughs].
Discussion
Participants’ perceptions of, and reactions to, nuisance bleeding were shaped by their understanding and knowledge of why they were taking DAPT and the risks involved in taking, as well as not taking, the medication, and by their understanding of symptoms, including making sense of their experiences, and whether or not these were thought to significantly compromise their health and quality of life. Participants described being given information about their medication when this was dispensed prior to their discharge from hospital, but few reported this encounter to result in adequate knowledge of their treatment. Several factors influenced the outcome of medication counselling, including the timing of counselling, and whether or not a participant’s physical and psychological state at the time enabled engagement with the information provided. Following discharge into the community, however, participants had few opportunities to access medication counselling. Other than scheduled specialist outpatient appointments, participants reported few opportunities to see a specialist if they had medication concerns. Most contact with health professionals would be through primary care; however, most perceived primary care as lacking the expertise and capacity to successfully address participants’ concerns and symptoms in a timely and appropriate manner. Taking control of one’s care through medication self-management and access to informal support networks was also found to act as an adherence enabler.
These qualitative findings reflect similar findings from a USA-based study reporting on patients’ motivation to continue with their DAPT medication despite the risk of nuisance bleeding,84 reflecting current understandings of nuisance bleeding as not resulting in seeking care. 28 The importance of medication knowledge and patient participation in their care for promoting adherence is highlighted by these findings, whereby participants reported being less concerned about their symptoms when they were aware of the cause,84 especially when polypharmacy increased uncertainty about the cause of symptoms. 85 Several other studies have also emphasised the role of patient counselling and health literacy in adherence and continuation with antiplatelet therapies. 80,86,87 Findings also highlight the need for care pathways that span the secondary–primary care continuum to ensure access points to medication counselling after a patient leaves hospital. Physical and psychological barriers might make it difficult for patients to participate in counselling when in hospital,87 and medication concerns often emerge after a patient is discharged into the community. Informal care networks are also a facilitator of adherence and play an important role in medication self-management. 88
These findings highlight the role of health literacy (e.g. knowledge, confidence and ability to access information; quality of patient–provider communication; trust in the primary care physician; and care expectations, as well as care pathways) in influencing the way that individuals act on their concerns and symptoms. Not taking action on nuisance bleeding experiences might be the result not only of perceived low severity of symptoms, but also of being able to make sense of these symptoms and feeling confident and able to access the health-care system for support.
Chapter 5 Health economics
Parts of this chapter have been reproduced from Doble et al. 8 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) International license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Health-related quality-of-life impact of minor and major bleeding events during dual antiplatelet therapy: a systematic literature review and patient preference elicitation study
A lack of reliable estimates on the HRQoL impacts of bleeds could lead to inappropriate decisions about which DAPT regimens to use in clinical practice. It is not clear to what extent primary research has determined the impact of bleeding events on HRQoL or what evidence has been used to populate existing decision-analytic models assessing DAPT. Furthermore, NICE in the UK requires the use of the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), a generic health-status questionnaire,89 when assessing the HRQoL impacts of interventions. 90 Therefore, it is important to identify whether or not health-state utility decrements for bleeding events (hereafter referred to as ‘utility decrements’) derived from the EQ-5D-3L are available for use in cost-effectiveness analyses. The EQ-5D-3L has been shown to be a valid, reliable and responsive instrument to measure HRQoL in patients with ACS,91,92 and is a suitable questionnaire to use to derive such utility decrements. However, it is unclear if the recently developed EuroQol-5 Dimensions, five-level version (EQ-5D-5L), with improved sensitivity and reduced ceiling effects,93 would also be a suitable instrument to estimate the impact of bleeding on HRQoL. Therefore, our study first aimed to review the evidence regarding utility decrements of bleeding events among patients receiving DAPT after coronary interventions. Second, we sought to derive robust UK utility decrements for use in future cost-effectiveness analyses of DAPT, through a patient elicitation exercise using vignettes and both the EQ-5D-3L and the EQ-5D-5L.
Methods
Literature review and quality assessment
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement94 was used as a guideline for the design of the review, with adaptations made for the focus on utility decrements.
Eligibility criteria
Studies published in English that reported utility decrements associated with bleeds among adults taking DAPT were considered. Included studies could be primary research that prospectively collected HRQoL information from which utility decrements could be estimated or decision-analytic models of DAPT that incorporated utility decrements [derived directly from time trade-off/standard gamble/expert elicitation methods or indirectly using a HRQoL questionnaire such as the EuroQol-5 Dimensions (EQ-5D)]. Specific populations that were considered included patients receiving DAPT who had previously had a PCI or CABG, and ACS patients receiving medication only. Studies assessing antiplatelet monotherapy in these populations were excluded. Studies reporting HRQoL information from which utility decrements could not be derived (e.g. condition-specific, non-preference-based HRQoL questionnaires) were excluded.
Information sources, search and data collection
Two databases (Ovid MEDLINE and PubMed) were searched from inception to 23 July 2018 (see Appendix 8). Search terms were developed for three categories: coronary interventions, DAPT nomenclature and HRQoL terminology. In addition, a hand-search of references from included articles was conducted. One author (BD) screened the titles and abstracts of all of the citations identified from the search strategies, reviewed the full-text articles identified after screening and extracted the data from the included studies.
Data items and synthesis of results
The synthesis of the literature search results was stratified by study type (primary research or decision-analytic model). Data were extracted on the following: study design, patient population, DAPT regime, categorisation of bleeding, HRQoL instrument, and the valuation approach used to estimate health-state utility values and utility decrements for minor, major and other bleeds reported. It is quite common for utility decrements to be reported in decision-analytic modelling studies with no more than a citation provided and no additional details as to how the decrements were derived. In such cases, the cited references were also reviewed to extract information on the derivation methods. The quality and relevance of the utility decrements identified in each included study were assessed using the checklist outlined by Ara et al. 95 Note that, as part of the checklist, the utility decrements were assessed for their adherence to reimbursement agency requirements specifically using the NICE reference case. 90
Patient elicitation exercise using vignettes and the EuroQol-5 Dimensions
Study design, recruitment and participants
The elicitation exercise was a standalone study conducted alongside the qualitative study involving the two groups of participants described in Chapter 4. Two focus groups were organised for each of the two treatment-duration groups: group 1 – antiplatelet therapy for 0–3 months (start of DAPT therapy) – and group 2 – antiplatelet therapy for 9–12 months (coming to the end of DAPT therapy).
Data collection
Participants were randomly allocated a colour-coded study booklet (see Appendix 10) containing a patient demographics questionnaire and one of four sequences of six EQ-5D questionnaires and associated vignettes (see Appendix 9). The sequence of the EQ-5D questionnaires and vignettes was varied to avoid ordering effects in participants’ responses. To allocate study booklets, a randomisation scheme was used with block sizes of two, four and six, stratified by duration of DAPT exposure (≤6 or > 6 months).
Participants first completed the demographics and baseline EQ-5D-3L and EQ-5D-5L questionnaires as they pertained to their health on that day. Given that the EQ-5D-3L is the NICE-recommended instrument for assessing the HRQoL impacts of interventions, its inclusion allowed our derived decrements to constitute potential evidence for future cost-effectiveness analyses conducted in the UK. Inclusion of the EQ-5D-5L allowed us to compare themagnitude of utility decrements derived from different EQ-5D questionnaires. Participants then completed the EQ-5D-3L and EQ-5D-5L modified questionnaires in relation to two vignettes describing minor (vignette A) and major (vignette B) bleeds (see Appendix 10). Modified versions of the EQ-5D questionnaires were approved by the EuroQoL Research Foundation on 21 June 2017 and were used to improve the clarity of the elicitation exercise (e.g. questionnaires completed in relation to vignettes rather than the respondent’s ‘own’ health) and to minimise the burden on participants (e.g. removal of the visual analogue scale). Vignettes were used because there are few opportunities to administer HRQoL questionnaires to patients experiencing bleeds. Patients may not seek medical care for minor bleeds, precluding researchers from interacting with patients at the time of event, and major bleeds often represent medical emergencies that incapacitate patients.
The vignettes were developed based on the BARC definitions,28 which provided standardised nomenclature to differentiate the descriptions of minor (i.e. a bleed that does not result in patients seeking medical care) and major (i.e. a bleed that does result in patients seeking medical care) bleeds. Both vignettes were also reviewed for face validity and updated based on feedback received from two clinicians (a GP and a cardiologist). For each vignette, participants completed both the EQ-5D-3L and the EQ-5D-5L. All participants completed each of the questionnaires individually and did not discuss their answers with other participants. At the bottom of each EQ-5D questionnaire, a supplementary question asked how long participants expected their HRQoL to be affected by the bleed described in the vignette. We expected that this information would be poorly quantified in the literature, yet this information is essential to estimate appropriate utility decrements (i.e. it is required to standardise the loss in HRQoL estimated from the EQ-5D for a specific time period). Therefore, we sought to directly quantify values by asking study participants. It should also be noted that many of the participants (10/21, 48%) reported previously experiencing a minor bleed while on DAPT during the focus group interviews, and research has shown that most patients who have received, or are currently receiving, DAPT are cognisant of the range of bleeding risks associated with DAPT. 84 It is, therefore, likely that all participants would have actively considered the risk of bleeding separately from the elicitation exercise, thus making them suitable surrogates to comment on the impact of bleeding on HRQoL.
Missing data and extreme values
As the elicitation exercise was conducted in small groups with oversight from at least one study co-ordinator, missing data were anticipated to be minimal. Owing to the open-ended nature of the supplementary questions, there was the potential for participants to report extreme values relative to other participants (the limits for defining an extreme value were differences of > 6 months and of 1 year from the next closest reported value for minor and major bleeds, respectively) or nonsensical values (e.g. HRQoL time impact greater for minor bleeds than for major bleeds). In such scenarios, we planned to consider reported values as missing and substitute mean values.
Data analysis
Responses to the EQ-5D questionnaires were used to estimate mean utility decrements for both minor and major bleeds. Responses were converted to health-state utility values using the UK EQ-5D-3L tariff,96 the UK EQ-5D-5L tariff97 and the UK EQ-5D-5L crosswalk to UK EQ-5D-3L value set. 98 The last one uses a mapping function to convert EQ-5D-5L responses to health-state utility values from the EQ-5D-3L tariff. Utility decrements were then derived using linear regression as the primary analysis. EQ-5D-3L or EQ-5D-5L utility values associated with either vignette A or vignette B were the dependent variables adjusted for baseline EQ-5D utility value, age, sex, coronary intervention received (PCI, CABG or ACS with medical management) and number of days since commencing DAPT therapy. Control groups were created by duplicating baseline utility values and assuming that these values represented hypothetical participants not experiencing a bleed. The regression coefficient for the variable indicating the presence/absence of a bleed represented the mean utility decrement if the effects on HRQoL were to persist for 1 year. Using responses from the supplementary questions, the regression coefficients of the bleeding event identifier variables were multiplied by the mean number of days the event was predicted to affect HRQoL and the product was divided by 365 days.
An alternative approach to estimating utility decrements was used in a sensitivity analysis to test the robustness of the decrements derived from the primary analysis. By subtracting the utility values for vignette A or B from a value of 1 (perfect health), a utility decrement for a bleed if the effects on HRQoL were to persist for 1 year for each participant was estimated. Adjustments were made by multiplying these values by the mean number of days that the event was predicted to affect HRQoL (derived from the supplementary questions) and dividing the product by 365 days. The mean decrements for the two bleed types were then determined. Note that the calculation approach used in the sensitivity analysis will exaggerate the utility decrement for any patient not otherwise describing their health as perfect and was used to identify maximum plausible values for the minor and major bleeding utility decrements.
Utility decrements from the primary analysis for each EQ-5D questionnaire were compared with each other, as well as with decrements from the sensitivity analysis and estimates from the literature review. As it is likely that existing utility decrements identified in the literature review might have been derived for use in cost-effectiveness analyses from the US perspective, responses to the EQ-5D-3L and EQ-5D-5L were also converted to health-state utility values using the US EQ-5D-3L tariff99 and the US EQ-5D-5L crosswalk to US EQ-5D-3L value set. 98 The primary and sensitivity analyses were repeated and the were results compared with utility decrements identified in the literature review.
Results
Literature review
We identified a total of 459 citations. After removing duplicates (n = 86), 373 unique titles and abstracts were screened. Of these, 330 were excluded and 43 were reviewed in full text. Twelve studies were judged eligible and included in the review (Figure 24).
FIGURE 24.
Flow diagram for selection of studies.
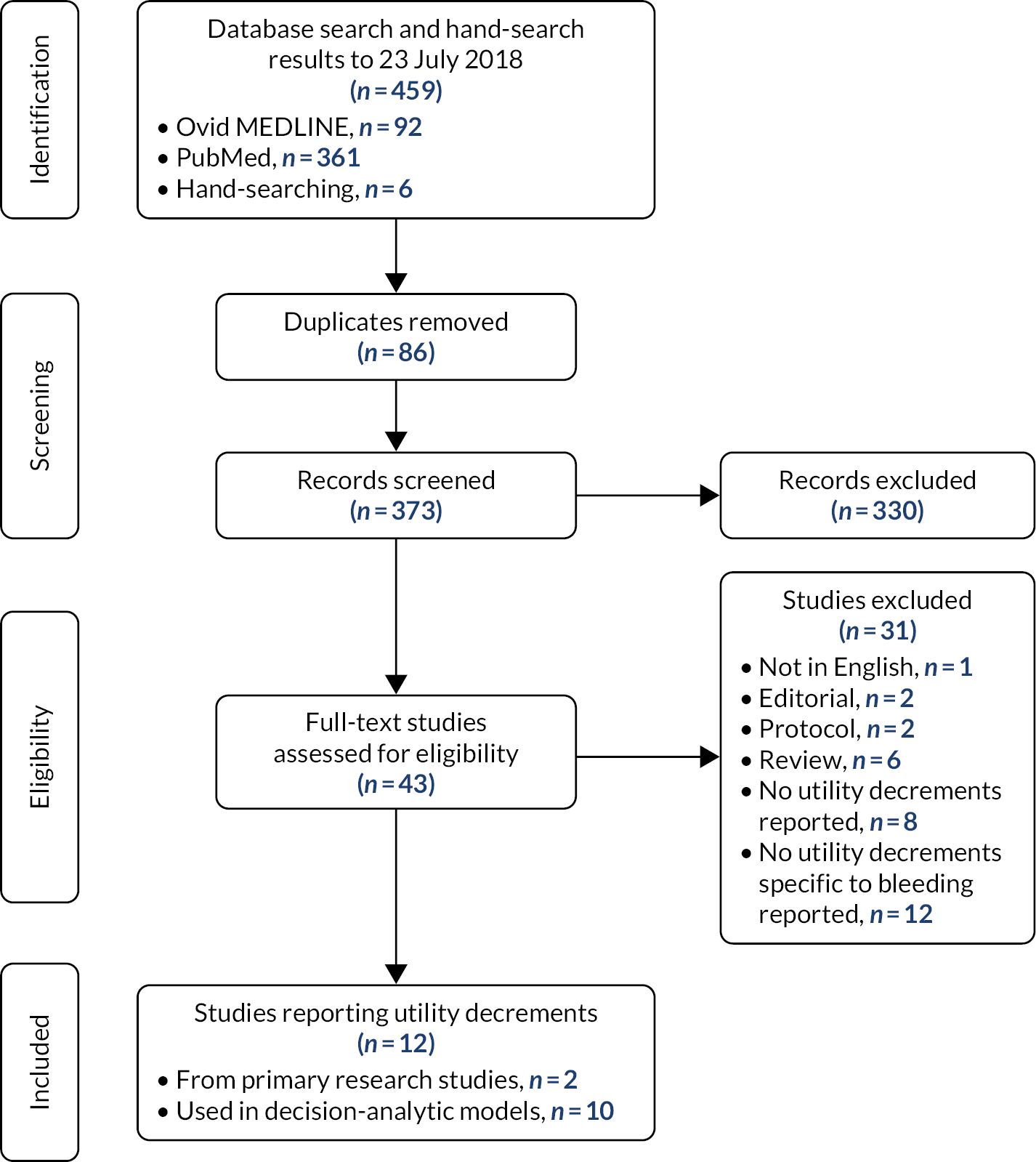
The 12 eligible studies comprised two primary research studies14,100 (Table 43) and 10 decision-analytic modelling studies101–110 (Table 44). Utility decrements from the primary research studies, derived using differences in baseline and 6-month follow-up responses from the EQ-5D-3L, ranged from –0.0257 (95% CI –0.0365 to –0.0148) for minor bleeds to –0.0445 (95% CI –0.073 to –0.016) for major bleeds (see Table 43). Utility decrements from decision-analytic models ranged from –0.002 to –0.02 for minor bleeds and from –0.007 to –0.05 for major bleeds. Utility decrements were also reported for general bleeding terms such as ‘gastrointestinal bleeds’, ranging from –0.005 to –0.016, and decrements of –0.01, –0.02, –0.03, –0.13 and –0.25 were reported for ‘CABG-related’, ‘bleeding in general’, ‘extracranial’, ‘serious’ and ‘non-fatal bleeds’, respectively (see Table 44). A summary of the sources of utility decrements reported in the decision-analytic models is provided in Appendix 11.
| Study; country | Study design | Patient population | Antiplatelet regime | Definition and categorisation of bleeding | Instrument used to measure QoL | Valuation method | Utility decrements for | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Any bleed | Minor bleeds | Major bleeds | ||||||||
| Amin et al.;14 USA | Prospective, multicentre cohort study (TRIUMPH) | 3560 AMI patients who had been hospitalised | DAPT post AMI (85% and 13% of patients who had a nuisance bleed at any time point received thienopyridine and warfarin, respectively, at discharge) | Nuisance bleeding (BARC type 1a), defined as the occurrence of any of the four bruising/bleeding eventsb that did not lead to hospitalisation, blood transfusion or change of medications by a physician | EQ-5D VAS at baseline and at 1, 6 and 12 months | VAS | NR | BARC type 1: –2.81 (95% CI 1.09 to 5.64) for VAS at 1 month | NR | |
| Amin et al.;100 USA | Prospective, observational, longitudinal, multicentre registry (TRANSLATE-ACS) | 9290c AMI patients treated with PCI | DAPT post PCI (clopidogrel, 68%; prasugrel, 29%; ticagrelor, 2%) | Any bleeding or severe bruising event that was patient reported, associated with an antiplatelet medication change or independently adjudicated rehospitalisation as a result of bleeding based on medical record review; BARCa | EQ-5D-3L questionnaire to calculate index score and VAS at baseline and 6 months | D1 valuation model99 for index score and direct valuation using VAS | Bleeding associated with a change of –0.033 (95% CI –0.041 to –0.026) in the index score and of −2.5 (95% CI –3.3 to –1.8) in the VAS | BARC type 1 vs. none: –0.0257 (95% CI –0.0365 to –0.0148) for the index score; –2.04 (95% CI –3.15 to –0.093) for the VAS | BARC types 2–4 vs. none: –0.0381 (95% CI –0.047 to –0.0293) for the index score; –2.79 (95% CI –3.70 to –1.88) for the VAS | BARC types 3–4 vs. none: –0.0445 (95% CI –0.073 to –0.016) for the index score; –7.10 (95% CI –10.04 to –4.16) for the VAS |
| Hypothetical patient population modelled | Antiplatelet regime | Definition and categorisation of bleeding | Instrument and population used to measure QoL | Valuation method | Utility decrements for minor bleeds | Utility decrements for major bleeds | Utility decrements for other bleeds | ||
|---|---|---|---|---|---|---|---|---|---|
| Greenhalgh et al.101 | Four subgroups:
|
DAPT: prasugrel plus low-dose aspirin compared with clopidogrel plus low-dose aspirin | MS model definition for bleed does not exclude CABG-related bleeds; non-fatal bleeds not treated as ongoing health states within model [such events incur only temporary reduction (14 days) in HRQoL] | MS: EQ-5D-3L; UK population norms | MS: time trade-off techniques | NR | MS: 25% decrement to UK population norms (free of disease) for 14 days; equal to a disutility of -0.007 | NR | |
| Garg et al.102 | ACS patients who had PCI (i.e. a DES) | DAPT: clopidogrel plus low-dose aspirin; durations of 12 and 30 months | Major and minor bleeds based on TIMI bleeding risk score (disutility applied during the year in which event occurred) | NR; see Appendix 12 for more details | NR: see Appendix 12 for more details | −0.002 | −0.025 | NR | |
| Kazi et al.103 | ACS patients who had PCI | Five strategies:
|
Minor haemorrhage and CABG-related bleeding based on TIMI bleeding risk score and extracranial haemorrhage based on TIMI score | NR; see Appendix 12 for more details | NR; see Appendix 12 for more details | 0.2 for 2 days (−0.004) | NR | Extracranial: 0.2 for 14 days (−0.0308) | CABG-related bleed: 0.5 for 7 days (−0.01) |
| Liew et al.104 | ACS patients (trial data used included patients scheduled to undergo medical or invasive management, e.g. PCI or CABG) | DAPT: ticagrelor plus aspirin compared with clopidogrel plus aspirin | No clinical definitions reported [disutilities applied during the cycle (1-year cycle length) in which the event occurred] | EQ-5D-3L | NR | −0.02 | −0.05 | NR | |
| Gupta et al.105 | CAS patients who had PCI, receiving either a DES or a BMS | DAPT: clopidogrel plus aspirin | Gl bleeding | Based on the average duration of hospitalisation | NA | NR | NR | Gl haemorrhage: toll of 6 days (–0.016) | |
| Schleinitz and Heidenreich106 | High-risk ACS patients: unstable angina and electrocardiographic changes or non-Q-wave Ml | DAPT: clopidogrel plus aspirin compared with aspirin alone | Gl bleeding | Assumption | NA | NR | NR | Gl bleeding: –0.005 | |
| Latour-Pérez et al.107 | NSTEMI ACS patients who had a hospital admission | DAPT: clopidogrel plus aspirin compared with aspirin alone | Gl bleeding [disutility counted only in the cycle (1-month cycle length) in which it occurred] | NR; see Appendix 12 for more details | NR; see Appendix 12 for more details | NR | NR | Serious haemorrhage disutility –0.13; Gl bleeding referred to in methods section, but no associated disutility value reported | |
| Jiang and You108 | ACS patients who had PCI | DAPT: three strategies –
|
Non-fatal bleeding | NR; see Appendix 12 for more details | NR; see Appendix 12 for more details | NR | NR | Non-fatal bleeding: −0.250 | |
| Wang et al.109 | 60-year-old Chinese (north Asian) ACS patients who underwent PCI | DAPT: three strategies –
|
Bleeding | NR; see Appendix 12 for more details | NR; see Appendix 12 for more details | NR | NR | Bleeding: −0.02 | |
| Jiang and You110 | 60-year-old ACS patients undergoing PCI | DAPT: four strategies –
|
Non-fatal bleeding | NR; see Appendix 12 for more details | NR; see Appendix 12 for more details | NR | NR | Non-fatal bleeding: −0.250 |
The results of our quality and relevance assessment are based on the information provided in the text of the included studies and in associated references, and are provided in Appendix 12. Overall, the utility decrements for bleeding events in the included studies were derived mainly from studies with limited relevance to the population of interest and lacked comprehensive reporting to accurately assess their risk of bias. Only half of the studies provided adequate details concerning the measurement and valuation of the reported utility decrements and none of the included studies was completely aligned with reimbursement agency requirements in the UK.
Patient elicitation exercise using vignettes and the EuroQol-5 Dimensions
The characteristics of participants are shown in Table 41. DAPT exposure times were ≤ 6 and > 6 months for nine and 12 of the participants, respectively. Ten out of 21 participants (48%) reported experiencing a minor bleed while on DAPT (ascertained in the discussions that occurred during the qualitative interviews). Baseline EQ-5D health-state utility values were as follows: EQ-5D-3L UK tariff, 0.760 (95% CI 0.159 to 1); EQ-5D-3L US tariff, 0.816 (95% CI 0.446 to 1); EQ-5D-5L UK tariff, 0.824 (95% CI 0.197 to 1); EQ-5D-5L UK crosswalk, 0.760 (95% CI 0.221 to 1); and EQ-5D-5L US crosswalk, 0.817 (95% CI 0.440 to 1).
All but one participant (20/21) completed the demographics questionnaire fully (i.e. no missing data); the remaining participant did not report the number of months over which they had taken DAPT. The two baseline EQ-5D questionnaires were completed fully. Complete data were obtained for the EQ-5D-3L for vignettes A and B, one participant did not complete the EQ-5D-5L for either vignette A or vignette B, and one participant responded only to the pain and anxiety/depression domains for the EQ-5D-5L for vignette A. In addition, five participants did not respond to the supplementary question (i.e. duration of decrement in HRQoL) for both vignette A and vignette B with the EQ-5D-3L; missing values were imputed with mean values of 7.60 and 45.38 days, respectively. Five and four participants did not respond to this question for vignettes A and B, respectively, with the EQ-5D-5L; missing values were imputed with mean values of 10.93 and 48.75 days, respectively.
One participant reported extreme values of 10 years for vignette A and 4 years for vignette B for the EQ-5D-5L (next closest values were 3 and 10 months, respectively), which is perhaps counterintuitive given that vignette A represents a less severe health state (minor bleed) than vignette B (major bleed). The same participant also reported an extreme value of 1 year for vignette A (next closest value was 3 months) and no response for vignette B for the EQ-5D-3L. These three extreme values were set to missing and imputed with the respective mean values.
Utility decrements for both minor and major bleeding events derived using linear regression (primary analysis) and the alternative approach (sensitivity analysis) are presented in Table 45. For the primary analysis, the utility decrements estimated using the two EQ-5D questionnaires and different valuation methods are relatively similar (range –0.000848 to –0.00250 for minor bleeds and –0.0187 to –0.0297 for major bleeds). The EQ-5D-3L UK tariff resulted in the largest utility decrement for both minor and major bleeds (–0.00250 and –0.0297, respectively). Applying the US tariff to the EQ-5D-3L resulted in slightly smaller decrements (–0.00180 and –0.0203). The EQ-5D-5L UK tariff resulted in the smallest utility decrement for minor bleeds (–0.000848) and a smaller utility decrement for major bleeds than the respective values for the EQ-5D-3L UK tariff (0.0222 vs. 0.0297, respectively). Utility decrements derived from crosswalk values were smaller than the values estimated from the EQ-5D-3L using both the UK and the US tariffs for both major and minor bleeds (see Table 45). Complete regression results are provided in Appendix 13.
| Primary analysis, mean (SD) | Sensitivity analysis, mean (SD) | |||
|---|---|---|---|---|
| Instrument | Minor bleeda | Major bleedb | Minor bleedc | Major bleedd |
| EQ-5D-3L UK tariff (n = 21) | –0.00250 (0.00265) | –0.0297 (0.0478) | –0.00828 (0.0155) | –0.0621 (0.103) |
| EQ-5D-3L US tariff (n = 21) | –0.00180 (0.00190) | –0.0203 (0.0328) | –0.00584 (0.0102) | –0.0441 (0.0705) |
| EQ-5D-5L to EQ-5D-3L UK value set (n = 19; n = 20)e | –0.00140 (0.00280) | –0.0258 (0.0421) | –0.00661 (0.00911) | –0.0552 (0.0830) |
| EQ-5D-5L to EQ-5D-3L US value set (n = 19; n = 20)e | –0.00137 (0.00275) | –0.0187 (0.0305) | –0.00566 (0.00880) | –0.0405 (0.0597) |
| EQ-5D-5L UK tariff (n = 19; n = 20)e | –0.000848 (0.00170) | –0.0222 (0.0362) | –0.00453 (0.00614) | –0.0465 (0.0700) |
Using the alternative estimation approach resulted in utility decrements that were larger than the values estimated in the primary analysis (range 0.00453 to 0.00828 for minor bleeds and 0.0405 to 0.0621 for major bleeds) (see Table 45). The relative magnitude of the utility decrements followed the same pattern as observed in the primary analysis. For both minor and major bleeds, the largest differences between the utility decrements estimated in the primary and sensitivity analyses were for the EQ-5D-3L UK tariff (differences of 0.00578 and 0.0324 for minor and major bleeds, respectively).
An ordering by magnitude of the derived and existing utility decrements for minor and major bleeds is presented in Table 46. For minor bleeds, the utility decrements ranged from –0.000848 to –0.0257, whereas, for major bleeds, the utility decrements ranged from –0.005 to –0.250.
| Source | Utility decrement |
|---|---|
| Minor bleeds | |
| EQ-5D-5L UK tariff – PA | –0.000848 |
| EQ-5D-5L to EQ-5D-3L US value set – PA | –0.00137 |
| EQ-5D-5L to EQ-5D-3L UK value set – PA | –0.00140 |
| EQ-5D-3L US tariff – PA | –0.00180 |
| Garg et al.102 | –0.002 |
| EQ-5D-3L UK tariff – PA | –0.00250 |
| Kazi et al.103 | –0.004 |
| EQ-5D-5L UK tariff – SA | –0.00453 |
| EQ-5D-5L to EQ-5D-3L US value set – SA | –0.00566 |
| EQ-5D-3L US tariff – SA | –0.00584 |
| EQ-5D-5L to EQ-5D-3L UK value set – SA | –0.00661 |
| EQ-5D-3L UK tariff – SA | –0.00828 |
| Liew et al.104 | –0.02 |
| Amin et al.100 | –0.0257 (BARC type 1) |
| Major bleeds | |
| Schleinitz and Heidenreich106 | –0.005 (GI bleeding) |
| Greenhalgh et al.101 | –0.007 |
| Kazi et al.103 | –0.01 (CABG-related) |
| Gupta et al.105 | –0.016 (GI haemorrhage) |
| EQ-5D-5L to EQ-5D-3L US value set – PA | –0.0187 |
| Wang et al.109 | –0.02 (bleeding in general) |
| EQ-5D-3L US tariff – PA | –0.0203 |
| EQ-5D-5L UK tariff – PA | –0.0222 |
| Garg et al.102 | –0.025 |
| EQ-5D-5L to EQ-5D-3L UK value set – PA | –0.0258 |
| EQ-5D-3L UK tariff – PA | –0.0297 |
| Kazi et al.103 | –0.0308 (extra-cranial) |
| Amin et al.100 | –0.0381 (BARC type 2–4) |
| EQ-5D-5L to EQ-5D-3L US value set – SA | –0.0405 |
| EQ-5D-3L US tariff – SA | –0.0441 |
| Amin et al.100 | –0.0445 (BARC type 3–4) |
| EQ-5D-5L UK tariff – SA | –0.0465 |
| Liew et al.104 | –0.05 |
| EQ-5D-5L to EQ-5D-3L UK value set – SA | –0.0552 |
| EQ-5D-3L UK tariff – SA | –0.0621 |
| Latour-Pérez et al.107 | –0.13 (serious haemorrhage) |
| Jiang and You108 | –0.250 (non-fatal bleeding) |
| Jiang and You110 | –0.250 (non-fatal bleeding) |
Discussion
The evidence of utility decrements for bleeds among patients receiving DAPT after coronary interventions is limited. Data sources used to estimate utility decrements lack relevance to the population of interest and have been inadequately reported, precluding an accurate assessment of their susceptibility to bias. Adequate details of measurement and valuation are provided for only half of the studies and no study completely aligned with reimbursement agency requirements in the UK, according to the NICE reference case. The highest-quality evidence was reported by Amin et al. ,100 but this study used a US population, applying the EQ-5D-3L US tariff (which limits generalisability to other jurisdictions). The decrements were also based on differences in HRQoL estimated over 6 months, which is an overestimation of the length of time a bleed would affect HRQoL, compared with responses from the supplementary questions in our study (8–11 days and 45–49 days for minor and major bleeds, respectively). On the other hand,
some major bleeds are likely to have a much more prolonged effect on HRQoL, such as stroke. Our primary research study attempted to elicit the length of time that a bleed would affect HRQoL from patients who either had experienced a minor bleed or were highly likely to have actively considered the risk of bleeding outside the elicitation exercise, whereas existing studies have based this length of time on clinical assumptions or used the time difference between study follow-up points.
Utility decrements derived from the patient elicitation exercise were consistent with some of the existing estimates (see Table 46). The utility decrement for minor bleeds estimated from the EQ-5D-3L UK tariff in the primary analysis of our study (–0.00250) is similar to decrements reported by Garg et al. 102 and Kazi et al. 103 (–0.002 and -0.004, respectively), which were both based on an unclear synthesis of values reported from the consensus of three internists111 and a direct elicitation using standard gamble methods. 112 In contrast, there is a large difference between the decrements estimated from the EQ-5D-3L US tariff in the primary and sensitivity analyses for our study (–0.00180 and –0.00584, respectively) and the decrement reported by Amin et al. ,100 who also used the EQ-5D-3L US tariff (–0.0257). In comparison to EQ-5D-3L US tariff utility decrements for other conditions,113 the utility decrement for minor bleeding reported by Amin et al. 100 seems large. Similar decrements are reported for mononeuritis of the upper limb (–0.0244), chronic ulcer of the skin (–0.0272) and migraine (–0.0297). These conditions would seem to be associated with greater HRQoL affects than minor bleeds that, by the BARC definition, do not cause patients to seek treatment. In contrast, the utility decrements for minor bleeds derived in our study are comparable to decrements reported for chronic sinusitis (–0.0022) and other dental disorders (–0.003), which are likely to have an effect on HRQoL that is similar to that of minor bleeds.
The utility decrements for major bleeds estimated from the EQ-5D-3L and EQ-5D-5L using the UK tariffs in the primary analysis of our study (–0.0297 and –0.0222, respectively) are similar to decrements reported by Garg et al. 102 and Kazi et al. 103 (–0.025 and –0.0381, respectively). Decrements estimated from the EQ-5D-3L US tariff in the primary and sensitivity analyses for our study (–0.0203 and –0.0441, respectively) are similar to the decrements reported by Amin et al. 100 for BARC types 2–4 and types 3–4 bleeds (–0.0381 and –0.0445, respectively).
From our elicitation exercise, it is apparent that utility decrements estimated from the EQ-5D-3L are consistently larger than decrements estimated from the EQ-5D-5L. The differences in decrements were larger when EQ-5D-3L values were compared with EQ-5D-5L values directly (differences of 0.00165 and 0.0075 for minor and major bleeds, respectively), with small differences observed when EQ-5D-3L values were compared with values obtained using the EQ-5D-5L to EQ-5D-3L crosswalk value set (differences of 0.0011 and 0.0039, respectively). This is not surprising, as the EQ-5D-5L has been shown to shift mean utility values closer to 1 (full health), compressing them into a smaller range than the EQ-5D-3L does. 114 This difference can potentially cause improvements in HRQoL to be valued less when using the EQ-5D-5L, compared with the EQ-5D-3L. However, the impact of using utility decrements derived from the different versions of the EQ-5D questionnaires on the cost-effectiveness of DAPT has yet to be elucidated and will be a valuable line of future research.
Our study has several limitations. First, our derived utility decrements are based on responses to the EQ-5D associated with vignettes describing minor and major bleeds and responses from participants estimating the length of time that a bleed would impact their HRQoL. Participants completing the elicitation exercise may not have directly experienced a major bleed, but most had previously experienced a minor bleed while on DAPT. All participants were, however, recruited to the study because of their current or past experience taking DAPT, and research has shown that most patients on DAPT are aware of the range of bleeding risks associated with DAPT. 84 Therefore, it is likely that all participants would have been informed of the risk of bleeds while on DAPT by their treating physician, thus making them suitable surrogates. Furthermore, there are a number of existing studies that have successfully employed the vignette approach to elicit utility values/decrements using participant samples with no first-hand experience or knowledge of the health states they were being asked to value. 115–117 These existing studies have justified the vignette approach, as existing evidence was of poor quality and of little relevance (which we also showed in our review) and direct measurement in affected patients would be difficult (which is also the case for major bleeds, as patients are incapacitated at the time of the event, and minor bleeds, as patients do not interact with the health-care system at the time of the event).
Second, our study population was small (n = 21) and homogeneous, potentially limiting generalisability. Furthermore, 16 of the 37 participants who agreed to participate in the study did not attend their assigned group session. The reasons for non-attendance are not clear, but it could be due to reduced HRQoL, employment status or a greater travel distance to the study location. These potential differences may bias our results, but the direction of such bias is unclear. That being said, our sample is broadly comparable in demographic and treatment characteristics to those individuals who were invited to participate, but did not attend, as well as to a whole-of-England PCI registry that reports demographics of 74% male and 90% white ethnicity. 118 In addition, given the questionable quality and relevance to the UK context of the existing evidence identified in our review (some decrements were derived from expert elicitation of only three medical internists or a single clinician),102,103,107,109 we believe that our larger sample and applied methods represent an improvement over approaches used previously.
Third, the elicitation exercise required cognitive processing that may have been difficult for some participants owing to advanced age (some participants were aged > 80 years and noticeably fatigued/lost concentration during the 20-minute exercise; this was in addition to a 1-hour group discussion). A few participants commented that it was difficult to imagine that they were the individual described in the vignettes. However, as the groups were small, the study co-ordinators ensured that all of the participants understood the exercise and completed all of the questionnaires to the best of their ability.
Fourth, some of the participants reported difficulty in assessing the impact of a major bleed (i.e. a bleed that results in patients seeking medical care) on HRQoL, given the range of different examples presented in the vignette (e.g. persistent nose bleed, blood in your bowel movement, vomiting blood or bleeding in your eye). As we were interested in estimating an average utility decrement for a major bleeding event, in general, it was not possible to limit the vignette description to a specific type of bleed. Furthermore, the vignette for major bleeds was developed using the BARC definitions, which encompass several concepts of seriousness when classifying bleeds considered ‘major’. 28 For the few participants expressing difficulty, guidance from the supervising researcher was provided, indicating that the participant should try to account for all potential impacts of the bleeds described in the vignette in their responses. It is, however, possible that participants limited their responses to the impact of only one of the example bleeds described, but it is not clear if participants would have selected the ‘less’ or more ‘severe’ example bleed in their responses.
Despite the limitations, the patient elicitation exercise provides a clear approach to estimating utility decrements for adverse events that may otherwise be difficult to obtain. For minor bleeds, alternative approaches, such as expert elicitation, might be less reliable because clinicians have limited ability to observe the HRQoL impacts of such events, as, by definition, minor bleeds do not cause patients to seek medical care. 28 The elicitation exercise also has added advantages over direct elicitation approaches (e.g. time trade-off119 or standard gamble120) in that it both captures the patients’ understandings of the HRQoL impacts and allows for the use of general population preferences in estimating utility values, as recommended by many reimbursement agencies, such as NICE. 90
Our study has also raised the question of whether or not the EQ-5D is a suitable instrument to capture HRQoL impacts of adverse events. This was reflected in our study by the confusion experienced by many participants when trying to understand why certain questions of the EQ-5D were relevant to the health state described in the vignettes. For example, one participant asked ‘Why would my ability to walk be affected by a nose bleed?’. It seemed that participants were expecting questions to be directly related to the event described in the vignettes, such as those likely to be included in a preference-based, condition-specific measure of HRQoL. It may, therefore, be of interest to explore such HRQoL questionnaires when using the patient elicitation vignette approach.
Comparative effect of different combinations of antiplatelet therapy on total health-care costs: inverse probability-weighted analyses of three population-based cohorts
Several studies have compared economic outcomes associated with different antiplatelet regimens among patients undergoing PCI. 101,121–123 One modelling study from the UK estimated the cost-effectiveness of clopidogrel and aspirin versus aspirin monotherapy among patients with non-ST segment elevation ACS. 6 However, estimates are lacking for the UK of the cost-effectiveness of using ticagrelor instead of prasugrel for DAPT with patients who undergo PCI or the comparative cost-effectiveness of clopidogrel and aspirin versus aspirin monotherapy for patients who undergo CABG or have conservatively managed ACS. To inform such models, we evaluated the total health-care costs associated with different antiplatelet therapy regimens using real-world data.
Methods
Data on health-care use were derived from the same data sets we used in the ADAPTT study: the CPRD GOLD database and linked HES data. 26,124 Patients included in the CPRD are largely representative of the UK population. 26 We included data from 1 April 2009 to 31 July 2017, a period covering the introduction of the newer antiplatelet drugs prasugrel and ticagrelor.
Study populations
Study populations were defined in line with the statistical analysis evaluating the effect of different antiplatelet therapies on clinical outcomes (see Chapter 3). The flow diagrams of participant selection are shown in Figures 8, 11 and 15.
Interventions
The first prescription in the CPRD within 2 months after the index hospitalisation for PCI, CABG or ACS was used as a proxy for the antiplatelet therapy that the patient started in hospital. Chapter 3 describes how patients were assigned to their intervention groups.
Resource use and associated costs
We compared total health-care costs associated with different antiplatelet treatment regimens among three different populations: patients undergoing CABG, conservatively managed ACS patients and patients with ACS undergoing PCI (emergency PCI). To avoid attributing costs directly associated with the index event to the antiplatelet regimen, we defined the start of follow-up as the day after the end of the finished consultation episode in which the hospital procedure (CABG or PCI) or first ACS diagnosis occurred. The total health-care costs associated with the different treatment regimens were measured at 1, 2 and 3 years after the start of follow-up.
Primary care health-care use was based on consultations captured by the CPRD.We included conventional, out-of-hours and telephone consultations, as well as home visits. Costs associated with these different types of consultations were based on the Unit Costs of Health and Social Care 2018. 125
Data on secondary care health-care use were based on data from HES. HES data contain details of all hospital NHS patient care episodes, private patients treated in NHS hospitals and care delivered to NHS patients by independent treatment centres. For the current analysis, we used HES data sets on admitted patient care,124 outpatient care,126 accident and emergency (A&E) care, and adult critical care.
For each HES data set, we derived associated Healthcare Resource Groups (HRGs) using the HRG4 + 2017/18 Reference Costs Grouper software. 127 For admitted patient care, HRGs were created at the finished consultation level. The 2017/18 national schedule of reference costs was used to attach costs to the different forms of resource use. 128 All costs were discounted at an annual rate of 3.5%.
Statistical analysis
Because the decision to prescribe one antiplatelet regimen and not another is likely, at least partly, to be driven by patient characteristics, we used inverse probability of treatment weighting to adjust for measured potential confounders. We considered the same confounders for inclusion in the model to create the weights as we did for the main analysis (see Chapter 3), but added total health-care costs in the year before the index date as an additional potential confounder. Total health-care costs in the year prior the index date can be considered as a proxy of the general health status of the patient and is likely to be a strong prognostic factor of future health-care costs. These prior health-care costs were split into five categories for the conservatively managed ACS and emergency PCI populations (< £400, £400–1699, £1700–3749, £3750–7799 and ≥ £7800) and five categories for the CABG population (< £9800, £9800–12,599, £12,600–14,199, £14,200–16,999 and ≥ £17,000). Other continuous variables were modelled using restricted cubic splines, with the number of knots determined by the Akaike information criterion.
For the CABG and conservatively managed ACS populations, the weights were constructed using logistic regression models for the probability of being initiated on DAPT with AP versus aspirin monotherapy. For the emergency PCI population, we restricted the analysis period to 2012–17 because, during the first 2 years of the study (2010–11), virtually no patients received ticagrelor prescriptions. For this 2012–17 emergency PCI population, we used multinomial logistic regression to estimate the probabilities of being treated with DAPT with AC versus AP versus AT. Confounders were included in the final models using a backward stepwise approach, with significance level for removal from the model set at 0.25. For variables that had a strong association with the outcome in a multivariable model (p < 0.01), we took a more liberal threshold of 0.5 for removal from the model. Only results restricted to AC versus AT were reported for the emergency PCI population, because AP is virtually exclusively prescribed for STEMI patients. A comparison of AC versus AP versus AT was performed in the subgroup of STEMI patients.
Subsequently, a weight was assigned to individuals based on the inverse of the model-predicted probability of being in the group for the treatment actually received. To prevent problems that can arise with very large weights when simply taking the inverse of the model-predicted probabilities,129,130 we estimated stabilised weights using a (multinomial) logistic regression with an intercept only (in case of no effect modification) or with the relevant main term(s) in the presence of effect modification by one of the variables considered a priori as potential effect modifiers: diabetes, chronic kidney disease and concurrent use of PPIs. These potential effect modifiers were prespecified in the published protocol. 25 When (stabilised) weights are estimated in this way, one can estimate the average treatment effect, that is the mean costs between patients assigned to one treatment regimen and patients assigned to the other treatment regimen in the case of two treatment options. When estimating the average treatment effect, one can also estimate, for example, what would happen if all patients with ACS undergoing PCI had received AC compared with what would have happened if all of them were initially prescribed AT.
Some patients were administratively censored because of the end of the study period, they were registered at a practice that stopped contributing data to the CPRD or they left a registered practice. Although such censoring events can often be considered non-informative when analysing clinical outcomes such as MI, they are typically informative when focusing on health-care costs because of the great variation between patients in cost accumulation over time. 131 To overcome this, we estimated the inverse probability of censoring weights. As the number of censored individuals was relatively small, we could include only a few covariates in the logistic regression model used for estimating the probability of censoring for each patient. For all populations we included the following covariates in this model: antiplatelet treatment regimen, age (restricted cubic spline with four degrees of freedom) and sex. Censoring weights were assigned to individuals based on the inverse of one minus the model-predicted probability of being censored for uncensored patients and a weight of zero for censored patients. Final weights for the analysis were subsequently estimated by multiplying the stabilised inverse probability of treatment weights by the inverse probability of censoring weights.
Total health-care costs at years 1, 2 and 3 of follow-up were estimated by fitting weighted generalised linear models (GLMs) with gamma distribution and log-link. A GLM with gamma distribution can handle positive values only. For the ACS population, a very small number of uncensored patients (< 0.3%) had no health-care costs recorded in the year after the index date. Therefore, there are insufficient data to inform a two-part model. 21 Instead we added a small increment (10−6) to patients with zero health-care costs to be able to fit a GLM with gamma distribution.
The models were fitted with an indicator of the antiplatelet regimens. In the presence of effect modification, an interaction with the relevant effect modifier, including the main term for the potential effect modifier, were also included. These weighted regression models were then used to predict what the mean total health-care costs would be under the different antiplatelet treatment regimens. For example, we predicted what the mean total health-care costs would be if all patients in the CABG cohort received AC versus aspirin monotherapy.
Smoking and BMI values were missing for 4% and 8%, respectively, of the emergency PCI population; for 2% and 7%, respectively, of the CABG population; and for 6% and 12%, respectively, of the ACS population. These missing values were replaced with age- and sex-specific modes for smoking, and age- and sex-specific averages for BMI.We estimated 95% CIs by performing 1000 bootstrap samples, with a single imputation for smoking and BMI nested in each bootstrap sample. All analyses were performed in R (packages: sqldf; dplyr; tidyr; doParallel; snow; splines, ggplot2, nnet) (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the CABG, the conservatively managed ACS and the emergency PCI (including STEMI only) populations are shown in Chapter 3 (see Tables 11, 18, 25 and 32). Table 47 shows, per patient population, total health-care costs incurred during the year before the index date.
| Total health-care costs (£) in | Antiplatelet regimen, n (%) | |||||
|---|---|---|---|---|---|---|
| Patient population | the year before the index date | Aspirin | AC | AP | AT | p-value |
| CABG | < 9800 | 320 (19) | 147 (24) | – | – | < 0.001 |
| 9801–12,599 | 363 (21) | 87 (14) | – | – | ||
| 12,600–14,199 | 359 (21) | 116 (19) | – | – | ||
| 14,200–16,999 | 352 (20) | 121 (20) | – | – | ||
| ≥ 17,000 | 331 (19) | 139 (23) | – | – | ||
| ACS | < 400 | 538 (20) | 1100 (26) | – | – | < 0.001 |
| 400–1699 | 846 (31) | 1382 (33) | – | – | ||
| 1700–3749 | 546 (20) | 735 (17) | – | – | ||
| 3750–7799 | 393 (15) | 513 (12) | – | – | ||
| ≥ 7800 | 384 (14) | 492 (12) | – | – | ||
| Emergency PCI | < 400 | – | 584 (19) | 300 (52) | 679 (35) | < 0.001 |
| 400–1699 | – | 359 (12) | 125 (22) | 369 (19) | ||
| 1700–3749 | – | 681 (22) | 74 (13) | 319 (16) | ||
| 3750–7799 | – | 1047 (34) | 53 (9) | 442 (23) | ||
| ≥ 7800 | – | 437 (14) | 27 (5) | 151 (8) | ||
The mean total health-care costs in the year prior to the index date were much higher for CABG patients (£13,601) than for ACS patients (£3528) or emergency PCI patients (£3625). Although there were some differences in the distribution of prior health-care costs between the different antiplatelet regimens within the CABG cohort, the mean costs were very similar (£13,623 for aspirin monotherapy and £13,537 for AC). Within the conservatively managed ACS group, patients receiving AC had lower mean total health-care costs in the year prior to the index date than patients receiving aspirin monotherapy (£3317 vs. £3857). Within the emergency PCI group, patients initiated on AC had higher mean total health-care costs prior to the index date (£4492) than patients initiated on AP (£1660) or AT (£2829), suggesting that sicker patients with more morbidity were assigned to AC.
Total health-care costs associated with different antiplatelet regimens
Across all patient groups, total health-care costs were larger in the first year after the index date than in the subsequent years (Tables 48 and 49). Although health-care costs in the year before the index date were particularly high among patients with CABG (see Table 47), cumulative health-care costs after the index date were substantially higher among patients with ACS who were initially conservatively managed with treatment medication alone. It should, however, be noted that our analyses were set up to compare different antiplatelet treatment regimens, and not for comparisons between ACS patients initiated on antiplatelet therapy only and ACS patient undergoing PCI and initiated on antiplatelet therapy.
| Mean (95% CI) health-care costs (£) under different antiplatelet regimens | |||
|---|---|---|---|
| Population and year | Aspirin | AC | AC vs. aspirin |
| CABG | |||
| Year 1 | 4130 (3762 to 4526) | 4224 (3711 to 4779) | 94 (–555 to 763) |
| Year 2 | 6464 (5965 to 6993) | 6701 (5841 to 7619) | 236 (–831 to 1223) |
| Year 3 | 8294 (7668 to 8947) | 8181 (7185 to 9317) | 113 (–1318 to 1102) |
| Conservatively managed ACS | |||
| Year 1 | 7761 (7963 to 8982) | 8371 (8061 to 8707) | 610 (–626 to 1516) |
| Year 2 | 11,151 (10,205 to 12,536) | 12,269 (11,871 to 12,756) | 1118 (–226 to 2206) |
| Year 3 | 14,155 (13,058 to 15,597) | 15,380 (14,867 to 15,931) | 1225 (–426 to 2423) |
| Population and year | Total health-care costs (£) under different antiplatelet regimens, mean (95% CI) | |||||
|---|---|---|---|---|---|---|
| AC | AP | AT | AP vs. AC | AT vs. AC | AT vs. AP | |
| 2012–17, no concurrent PPI | ||||||
| Year 1 | 3505 (3167 to 3855) | – | 3577 (3090 to 4098) | – | 72 (–532 to 762) | – |
| Year 2 | 5686 (5136 to 6248) | – | 5530 (4892 to 6239) | – | –156 (–974 to 754) | – |
| Year 3 | 7074 (6445 to 7758) | – | 7804 (6778 to 8943) | – | 730 (–453 to 2029) | – |
| 2012–17, concurrent PPI | ||||||
| Year 1 | 4364 (4048 to 4711) | – | 5509 (4676 to 6527) | – | 1145 (269 to 2195) | – |
| Year 2 | 7488 (6958 to 8076) | – | 8435 (7269 to 9856) | – | 947 (–364 to 2419) | – |
| Year 3 | 9878 (9151 to 10,698) | – | 12,090 (9699 to 15,165) | – | 2212 (–382 to 5182) | – |
| 2012–17, STEMI, no concurrent PPI | ||||||
| Year 1 | 3336 (2788 to 3932) | 3425 (2546 to 4579) | 3727 (3196 to 4298) | 90 (–1001 to 1286) | 391 (–451 to 1166) | 302 (–953 to 1344) |
| Year 2 | 5560 (4716 to 6597) | 5114 (3811 to 6502) | 6253 (5198 to 7359) | –445 (–2100 to 1105) | 694 (–708 to 2044) | 1139 (–668 to 2793) |
| Year 3 | 6809 (5824 to 7940) | 6630 (4820 to 8677) | 8469 (6670 to 10,378) | –179 (–2316 to 1953) | 1660 (–474 to 3899) | 1840 (–869 to 4627) |
| 2012–17, STEMI, concurrent PPI | ||||||
| Year 1 | 4529 (3993 to 5077) | 8720 (3504 to 16307) | 5820 (4779 to 7138) | 4190 (–1149 to 12,142) | 1291 (79 to 2739) | –2899 (–10,842 to 2622) |
| Year 2 | 7199 (6425 to 8065) | 11,265 (4922 to 19,065) | 8765 (7223 to 10,628) | 4066 (–2309 to 11,986) | 1566 (–159 to 3663) | –2500 (–10,644 to 4247) |
| Year 3 | 9293 (8231 to 10,426) | 14,376 (6524 to 23,584) | 12,019 (9426 to 15,509) | 5083 (–3339 to 14,558) | 2725 (–109 to 6337) | –2357 (–11,802 to 6459) |
We predicted the total health-care costs if all patients were initiated on one of the antiplatelet treatment regimens of interest (aspirin and AC for CABG and conservatively managed ACS patients; AC, AP and AT for emergency PCI patients). For the CABG patient population, cumulative health-care costs were comparable if all patients were initiated on aspirin monotherapy, compared with all patients being initiated on AC (see Table 48). For example, the discounted mean health-care costs at 1 year were predicted to be £4130 (95% CI £3762 to £4526) if all CABG patients were initiated on aspirin monotherapy, compared with £4224 (95% CI £3711 to £4779) if all CABG patients were initiated on AC.
Among patients with conservatively managed ACS, predicted cumulative health-care costs were estimated to be slightly higher if all patients were treated with AC than if they were all treated with aspirin monotherapy. The mean cumulative difference between the two regimens was estimated to be £610 (95% CI –£626 to £1516) at year 1, increasing to £1225 (95% CI –£426 to £2423) at year 3. However, there was still substantial overlap between the CIs of the predicted mean health-care costs for all years (see Table 48).
Interactions between the antiplatelet regimen and the concurrent PPI prescriptions were found among the emergency PCI population. Therefore, we tabulated cumulative health-care costs separately for patients with and for patients without concurrent PPI prescriptions (see Table 49). Cumulative healthcare costs were higher among those receiving concurrent PPI prescriptions, potentially reflecting frailty and higher risk of (gastrointestinal) bleeding among those receiving these prescriptions. Differences between antiplatelet regimens were larger among patients with concurrent PPI prescriptions than among those not receiving concurrent PPI prescriptions (see Table 49). For example, although there was hardly any difference in predicted mean health-care costs at 1 year among those not receiving concurrent PPI therapy if all patients received AT, compared with AC (£72, 95% CI –£532 to £762), patients on concurrent PPIs were predicted to have higher mean health-care costs if they were receiving AT, compared with AC (£1145, 95% CI £269 to £2195).
Discussion
This study estimated mean cumulative health-care costs, including costs incurred by primary care consultations, A&E visits, outpatient visits and intensive care unit stays, under different antiplatelet regimens across three populations (patients undergoing CABG or emergency PCI, or those with ACS who are conservatively managed). Mean cumulative health-care costs were much lower the year after a CABG procedure than the year before. However, we did not find strong evidence for a difference in mean cumulative health-care costs with initiation of aspirin monotherapy versus DAPT with AC.
For the conservatively managed ACS population, mean cumulative health-care costs were substantially higher the year after the index event than the year before. This is in line with expectations, because in the absence of effective revascularisation, health-care costs are expected to rise after a first ACS event. Average cumulative health-care costs were estimated to be slightly higher in this population of patients if all patients were treated with DAPT with clopidogrel than with aspirin monotherapy, although there was considerable overlap between CIs.
Among emergency PCI patients, estimated cumulative health-care costs were comparable under the different antiplatelet regimens among patients not receiving concurrent PPI prescriptions. This may be partly because of clinicians deciding that a PPI co-prescription is not necessary among patients who have a relatively low risk of gastrointestinal bleeding, meaning that such patients may have fewer underlying health problems than patients for whom clinicians decide to co-prescribe a PPI. Among STEMI patients receiving concurrent PPI prescriptions, AP treatment initiation was associated with higher costs than AC or AT.
We analysed the data according to the intention-to-treat principle and did not record actual prescriptions of antiplatelet therapy and adherence to this therapy. In March 2018, the cost of 12 months of low-dose aspirin (75 mg per day) treatment ranged between £6.76 and £8.84, the cost of 1 year of clopidogrel treatment was £15.08, the cost of 1 year of ticagrelor treatment was £655.20 and the cost of 1 year of prasugrel treatment was £570.72 (these costs decreased to £85.06 for 10-mg tablets and £263.64 for 5-mg tablets in March 2020). 132–134 Therefore, the impact of also accounting for antiplatelet therapy costs would have only a small impact for most comparisons, except the comparison between DAPT with clopidogrel and treatment with one of the more potent antiplatelet agents (prasugrel or ticagrelor).
A previous study compared the cost-effectiveness of DAPT with prasugrel versus DAPT with clopidogrel among patients with ACS (including both STEMI and NSTEMI patients) and planned PCI in the USA, based on the results of the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38), which included data from eight countries, including the UK. 121 Over a median follow-up of 14.7 months, average rehospitalisation costs, excluding study drug costs, were US$517 (95% CI US$25 to US$1040) lower per patient for DAPT with prasugrel (£459, 95% CI £22 to £924 in 2018 Great British pounds). In approximately 80% of bootstrap replicates, prasugrel was a dominant strategy, and results were similar for STEMI and NSTEMI patients. TRITON-TIMI 38 was also used to inform a cost-effectiveness modelling study for the UK in which 1-year health-care costs, excluding drug costs, were estimated to be £274 lower among patients with diabetes treated with prasugrel than among those with diabetes treated with clopidogrel. 101
The cost-effectiveness of treating ACS patients with DAPT with ticagrelor for 12 months instead of DAPT with clopidogrel in the Swedish setting was evaluated using data from the PLATO randomised trial. 135 This study found comparable total health-care costs, including the cost of study drugs, between the two treatment strategies (€96, 95% CI –€360 to €553 in 2010 Euros). However, excluding drug costs, health-care costs were €402 lower in the ticagrelor arm, mainly owing to savings in the number of bed-days. Results were similar for ACS patients with intent for conservative management using medication only, with a difference of €79 (95% CI –€775 to €933, ticagrelor vs. clopidogrel). A 2015 non-randomised study from the USA also found no significant difference in total health-care costs between ACS patients who underwent PCI initiated on ticagrelor and ACS patients who underwent PCI initiated on prasugrel after 1 year of follow-up (US$5456 vs. $4844; p = 0.37). 122 A 2017 study of patients with ACS receiving drug-eluting stents found that the overall costs per patient were higher in a cohort of patients receiving prasugrel than among patients receiving clopidogrel (€1163, 95% CI €1062 to €1170 in 2014 Euros) with no difference in QALYs (–0.027, 95% CI –0.064 to 0.011). 136
In contrast to most of these previous studies, we included costs incurred by primary care consultations, A&E visits, outpatient visits and intensive care unit stays, as well as costs for hospital admissions. We focused on total health-care costs from a UK NHS perspective, the most relevant cost outcome. Furthermore, acute events may be poorly coded in primary care,137 which could result in significant underestimation of total costs when restricting to consultations with a code for minor bleed. Even major bleeding events necessitating, or occurring during, hospital admission may be missed in more than one-third of the cases using diagnostic codes alone. 138
The current study is limited by several biases we identified in the target trials and by small sample sizes, potentially resulting in confounded estimates and wide CIs. For example, TRITON-TIMI 38 included 13,608 ACS patients scheduled for PCI,32 whereas we included 5647 ACS patients undergoing PCI, of whom only 579 patients received DAPT with prasugrel. Although we adjusted for the confounders identified systematically by literature review, clinician interviews and surveys (see Chapter 2), adjusted for health-care costs accrued in the year before the index date, and adjusted for informative censoring using inverse probability weighting, we cannot exclude the possibility that our results are affected by unmeasured confounding. The exclusion of patients with a MACE before first prescription in the CPRD or no prescription in the CPRD within 2 months of discharge is also likely to have resulted in selection bias. Along the same lines, concurrent PPI use may actually be a collider, being an effect of the outcome and the antiplatelet regimen started at the index date, meaning that conditioning on this factor may have increased, instead of resolving, any bias. 139 Given these limitations, a formal cost-effectiveness evaluation was deemed not to be appropriate.
Chapter 6 Summary of the main findings and future research recommendations
Underascertainment of minor/nuisance bleeding
In the populations in this study, the incidences of any bleeding and of minor bleeding were between 5% and 10% and between 4% and 7%, respectively. These are almost certainly affected by underascertainment of nuisance bleeding, which has been reported to be as high as 38% in previous studies of patients on antiplatelet medication in which patients were interviewed about bleeding events. 12–14 Half of all patients in our qualitative study and in the patient and public involvement group reported experiencing a nuisance bleed while taking DAPT, but none of them reported their bleed to a health-care provider. Although the qualitative study was small and does not constitute definitive evidence, it certainly suggests that the main factor responsible for the low rates of bleeding observed in the CPRD is under-reporting by patients, rather than GPs failing to submit all data to the CPRD. Future studies will require prospective data collection on nuisance bleeding, given that this is under-reported, and given the impact it has on quality of life.
More potent antiplatelet therapy was associated with an increase in the hazard of bleeding
Compared with aspirin monotherapy, DAPT was associated with an increase in the hazard of any bleeding among CABG patients (by about 1.7 times) and conservatively managed ACS patients (by about 1.4 times). Similarly, compared with less potent DAPT with clopidogrel, more potent DAPT with ticagrelor or prasugrel (STEMI only) increased the hazard of bleeding by about 1.5 and 1.8 times, respectively. All of these comparisons excluded a decreased hazard (i.e. the lower 95% CI for the HR was > 1). Evidence from recent meta-analyses of RCTs and non-randomised studies is not conclusive. Meta-analyses in the CABG population show an increase in bleeding, but the CIs around the point estimates are wide and do not exclude a decreased risk. 52,53 Some meta-analyses in ACS populations show a significant increased risk of bleeding with more potent DAPT,66,67 but a 2020 large network meta-analysis conducted as part of a NICE evidence review, including > 20,000 ACS participants with/without revascularisation, showed no clinically important difference in bleeding between DAPT with clopidogrel and DAPT with ticagrelor at 1 year, or between DAPT with clopidogrel and DAPT with prasugrel. 140
More potent antiplatelet therapy was not associated with a decreased risk of major adverse cardiovascular events
In the ADAPTT study, we did not observe the expected decrease in MACEs with DAPT versus aspirin monotherapy or with more potent DAPT versus less potent DAPT. Indeed, we observed the opposite effect: an increase in MACEs in the CABG (twofold increase) and conservatively managed ACS (1.6-times increase) populations. Large meta-analyses (8000 to > 25,000 participants)66,67 investigating ischaemic outcomes in CABG or ACS populations (with and without revascularisation) are not all conclusive, with some not excluding an increased risk with DAPT versus aspirin and with more potent DAPT versus less potent DAPT. 52–54,66 However, in all of these meta-analyses, the direction of effect suggests a protective effect of more potent antiplatelet therapy.
The ADAPTT study analyses are at risk of bias
In the ADAPTT study, we identified several factors that may have influenced the results: biases owing to imperfect emulation of the defined target trials and differential switching from treatment assigned at baseline, and non-adherence between intervention groups (Table 50).
| Target trial | |||
|---|---|---|---|
| CABG | ACS (conservatively managed) | ACS (PCI treated) | |
| Eligible population in HES–CPRD, n (%) | 2783 (100) | 10,943 (100) | 5738 (100) |
| Potential for selection bias | |||
| Could not assign intervention at baseline; therefore, excluded from analysis, n (%) | 482 (17) | 4357 (40) | 520 (9) |
| Differences in event rates (bleeding and MACEs) between participants included/excluded |
Yes | Yes | Yes |
|
|
|
|
| Differences in median length of hospital stay between those included/excluded |
|
|
|
| Selection of participants based on exposure and disease status | Yes (some excluded participants were older with more comorbidities and had higher rates of bleeding and ischaemic events) | Yes (fewer excluded participants had previous MI or CABG/PCI or history of IHD, and had higher rates of bleeding and ischaemic events) | Yes (some excluded participants were older with more comorbidities and had higher rates of bleeding and ischaemic events) |
| Potential for confounding | |||
| Confounders for which no data available | Yes (procedure characteristics and severity of disease) | Yes (severity of disease) | Yes (procedure characteristics, presentation risk factors, severity of disease) |
| Differences in baseline characteristics between intervention groups |
Aspirin vs. AC | Aspirin vs. AC | AC vs. AT |
|
|
|
|
| Differences in median length of hospital stay between intervention groups (proxy for health/illness) | No (6 days for the aspirin and AC groups) | Yes (3 days in the aspirin group vs. 5 days in the AC group), suggesting a sicker population in the AC group | No (2 days in the AC and AT groups) |
| Differences in health-care costs in the year prior to event (proxy for health/illness) | No (£13,623 in the aspirin group and £13,537 in the AC group) | Yes (£3317 in the aspirin groups vs. £3857 in the AC group), suggesting a sicker population in the AC group | Yes (£4492 in the AC group and £2829 in the AT group) |
| Non-adherence and treatment switches | |||
| Differences in non-adherence between groups | Yes, non-adherence higher in the AC group (aspirin, 30%; AC, 46%) | Yes, non-adherence slightly higher in the aspirin group (aspirin, 44%; AC, 40%) | Yes, non-adherence higher in the AT group (AC, 28%; AT, 33%) |
| Differences in switching from treatment assigned at baseline between groups | Similar proportion of ‘switchers’ in the aspirin (20%) and AC (18%) groups | Similar proportion of ‘switchers’ in the aspirin (23%) and AC (24%) groups | Yes, fewer ‘switchers’ in the AC group (14%) than in the AT group (21%) |
| Event rates, HR (95% CI) (AC vs. aspirin for CABG and conservatively managed ACS; AT vs. AC for PCI-treated ACS) | |||
| Bleeding (any) | 1.72 (1.15 to 2.57) | 1.43 (1.21 to 1.69) | 1.47 (1.19 to 1.82) |
| MACE | 2.06 (1.23 to 3.46) | 1.57 (1.38 to 1.78) | 1.06 (0.89 to 1.27) |
| All-cause mortality | 1.34 (0.63 to 2.85) | 1.03 (0.89 to 1.19) | 0.94 (0.60 to 1.47) |
Selection bias
We excluded a subgroup of the eligible population because they could not be assigned to an intervention. We had no data on hospital prescribing of antiplatelet therapy; therefore, we assumed that the first prescription recorded in primary care within 2 months of the index date was the same as the regimen started at the time of the index event in hospital. We could not, therefore, assign an intervention to those who died before a first prescription could be observed in the CPRD. Some patients had no prescription data within the 2-month time window that we specified. We also excluded patients experiencing a major bleed or MACE necessitating hospitalisation before first prescription in the CPRD because DAPT prescriptions are changed after such events. Collectively, these situations resulted in 17%, 40% and 9% of the eligible CABG, conservatively managed ACS and PCI-treated ACS populations, respectively, being excluded from the analysis. For the conservatively managed ACS target trial, the proportion of excluded patients was substantial.
Across all three target trial populations, the excluded patients comprised two distinct groups (roughly 50 : 50): one older and with more comorbidities, who experienced an early major bleed or MACE, and the second younger, with a higher proportion of smokers but with fewer comorbidities than the included population. The two excluded groups are likely to have different underlying risks of bleeding and ischaemia, and are, therefore, likely to be prescribed different antiplatelet regimens. The distribution of these two groups of excluded patients (and their even rates) between our intervention groups is unknown. If this distribution is uneven, which is likely given that cardiologists prescribe less potent antiplatelet therapy to older and more frail patients and more potent antiplatelet therapy for secondary prevention among younger, less comorbid patients (see interviews with clinicians and survey results in Chapter 2), then it is possible for their exclusion to influence the results. Several studies have shown that including/excluding certain populations from an analysis data set, for example including prevalent rather than incident users of medications, could make protective interventions appear harmful and vice versa. 55–57 Although we imputed the original assigned intervention to allow inclusion of all eligible participants in a sensitivity analysis, it is questionable whether or not imputations based on a population that differed in general baseline characteristics from the excluded population and did not experience a major event early after the start of follow-up could be used reliably to impute unobserved treatment assignment. The assignment to intervention is, therefore, probably not missing at random.
The exclusion of the eligible population with an early event would be expected to influence the curves at the beginning of follow-up, but most Kaplan–Meier curves (see Figures 9, 10, 13, 14, 17, 18, 20 and 21) for most outcomes continued to diverge until the end of follow-up, indicating that the included populations (with late events) in the different intervention groups really had a different underlying risk. However, roughly half of the excluded population in all target trials had no early event and their contribution to the event rate (if included) and influence on the Kaplan–Meier curves are unknown.
Confounding
We identified confounders systematically using literature review, clinician interviews and surveys, so that we could adjust for confounders in the analyses and attempt to emulate random assignment. The different sources we used to identify confounders highlighted that, for both cardiologists and cardiac surgeons, balancing ischaemic risk with bleeding risk is the primary guiding criterion when prescribing antiplatelet drugs. Although we suspected a priori that this might be true, independent confirmation from different sources (interviews and surveys) decreased our motivation to attempt to conduct an instrumental variable analysis. The instrumental variable analysis was also not feasible for other reasons (see Chapter 3).
Although intervention groups were reasonably balanced with regard to baseline characteristics and these were adjusted for in the analysis, no data were available for half of the confounders identified, such as procedure-related characteristics and complexity of disease (see Chapter 2). These are important factors that clinicians consider when prescribing antiplatelet therapy. Furthermore, in the conservatively managed ACS and PCI-treated ACS target trials, we had other indicators of differences in baseline risk. In the former target trial, participants in the DAPT with clopidogrel intervention group had higher health-care costs and a longer hospital stay than those in the aspirin monotherapy group, suggesting that those prescribed DAPT were a sicker, higher-risk group. By contrast, in the PCI-treated ACS target trial, those assigned more potent DAPT with ticagrelor had lower health-care costs than those assigned less potent DAPT with clopidogrel (see Table 50). Similarly, the finding in the PCI-treated ACS target trial that patients concurrently treated with PPIs have higher mean costs is likely to be partly a reflection of high-risk patients being co-prescribed PPIs. The possibility of residual confounding (e.g. measurement error in measured confounders) cannot be ruled out.
Given all the limitations highlighted so far (underascertainment of minor bleeding, potential selection bias and confounding), we did not conduct a formal cost-effectiveness evaluation because it would not have been appropriate.
Non-adherence to antiplatelet interventions assigned at baseline was high
As in a RCT, we conducted an intention-to-treat analysis. However, non-adherence to the treatment assigned at baseline was up to 46% across the target trials, in particular in the CABG and conservatively treated ACS populations (see Table 50). Non-adherence rates are very similar to those reported in studies in which adherence rates were assessed prospectively through questionnaires,59–61 which are higher than those observed in RCTs. The PLATO RCT, for example, reported a non-adherence rate of 17% with AT,33 whereas the non-adherence rate in the AT group of our PCI-treated ACS target trial was 33%. Non-adherence may have influenced our findings; for example, the high non-adherence rate in the DAPT with clopidogrel intervention group in the CABG population (see Table 50) may have increased MACE rates among those assigned to this regimen. It is worth noting that prescription data in the CPRD are regarded as a valid reflection of prescriptions issued in primary care. 141
Patient and public involvement
Patient and public involvement in research is defined as research actively carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. 142 In clinical trials (whether observational studies or RCTs), the main tasks perceived to be under patient and public involvement remit are clearly defined, such as reviewing participant-facing materials and data collection methods, exploring the burden being placed on research participants and ethics issues. However, existing guidelines do not provide clear advice on how to involve patients in observational studies using routinely collected data, which do not involve recruiting patients or collecting data. Most of these studies report no patient involvement in setting the research question or the outcome measures, design or implementation of the study,143–145 although a few have used patient groups to identify relevant research topics and meaningful outcomes within the routinely collected data sets and to review results. 146,147
How the ADAPTT study patient and public involvement group was established
Twenty-five patient and public involvement members were recruited from a pool of patients who had received treatment for a heart attack at the Bristol Heart Institute in 2016. Patients were approached by research nurses and consultant cardiologists during follow-up and post-surgery clinics and given information in the form of a leaflet explaining the ADAPTT study, the role of patient and public involvement and what potential members were expected to do. Interested patients contacted the patient and public involvement facilitator, who provided further details and invited them to attend the first patient and public involvement meeting.
How patient and public involvement steered the ADAPTT study
Patient and public involvement members covered a broad range of ages (55–80 years) and social classes and represented patients from all of the ADAPTT study target trials. After the first meeting, a further three meetings were organised between October 2016 and June 2019. Table 51 provides a summary of the meetings and their outcomes, and how they informed the ADAPTT study.
| Meeting | Meeting date and number of attendees | Meeting objectives | Summary of discussions | Outcome | |
|---|---|---|---|---|---|
| 1 | September 2016; 25 patient and public involvement members |
|
|
|
|
|
|
||||
|
|
||||
|
|||||
| 2 | November 2017; 12 patient and public involvement members |
|
|
The research team:
|
|
|
|
|
|||
|
|||||
|
|||||
| 3 | February 2018; 10 patient and public involvement members |
|
Patient and public involvement members reported similar experiences to patients in the qualitative studies. They highlighted the following:
|
|
|
|
|||||
|
|
||||
| 4 | June 2019; six patient and public involvement members |
|
Patient and public involvement members reported that:
|
The research team and the patient and public involvement group agreed to conduct a patient and public involvement process evaluation |
In summary, patient and public involvement was successfully implemented in the ADAPTT study, which was designed to answer a research question solely on the basis of routinely collected data. Patient and public involvement informed the decision-making process with regard to assembling the target trials from the data sets, for example when deciding on a time window for antiplatelet prescriptions in the CPRD for assignment to interventions, because we did not fully understand the patient pathway with regard to repeat prescriptions following discharge from hospital.
Patient and public involvement also provided context to the findings of the ADAPTT study and addressed several uncertainties for which we found little UK-relevant research, for example (1) whether or not and how patients on DAPT report nuisance bleeding to health-care providers, (2) whether or not nuisance bleeding among DAPT users affects adherence (given that DAPT use is time-limited) and (3) whether or not our data set reflects real-world bleeding of patients on DAPT in the UK.
Patient and public involvement members highlighted important issues affecting patients with respect to antiplatelet prescribing after a heart attack (which was the catalyst for the qualitative study with patients): (1) poor information provision in hospital with regard to side effects of DAPT and what to do about these; (2) nuisance bleeding is common, affecting > 50% of people taking antiplatelet drugs; (3) but it may not impact strongly on adherence because the use of DAPT is time limited; and (4) there was no shared decision-making with regard to DAPT prescribing, but patients felt no need to be involved in the decision process so soon after an acute event. Patient and public involvement members suggested ways of improving the information provision with regard to medications prescribed at hospital discharge. Interestingly, patient and public involvement findings mirrored those of the qualitative study with patients, increasing confidence in the findings of the qualitative study. However, patients participating in patient and public involvement and in the qualitative study reported no issues with adherence, which does not reflect the ADAPTT study data, in which non-adherence was high. This highlights that patients who participate in patient and public involvement may not be representative of the group of patients they represent.
Patient and public involvement process evaluation using the ‘cube’ framework
Patient and public involvement is becoming increasingly accepted as a means to ensure the relevance and acceptability of health research;148 as patient and public involvement becomes more ingrained in health research, ‘robust measurement of the impact of involvement is needed’. 149 We, therefore, evaluated the patient and public involvement that we conducted throughout the ADAPTT study using a cube evaluation150 workshop with our public contributors.
Method
The cube is a ‘four-dimensional theoretical framework that describes the fundamental elements for successful knowledge exchange, and which could be used for mapping and analysing the quality of the interactions that take place within knowledge spaces’ (Figure 25). 151
FIGURE 25.
The cube framework.
We chose to use the cube framework for our evaluation because of its theoretical grounding and interactive nature. The cube creates an immediate visual representation of a participant’s views, which makes a real-time discussion of the findings possible.
All of the public contributors recruited to the ADAPTT study patient and public involvement group were invited to attend the cube workshop. We explained that the meeting would be a time for them to talk about their experiences of being involved in the study, which would help the research team to develop and improve the patient and public involvement work going forward. Six members of the patient and public involvement group were able to attend. The group was facilitated by the patient and public involvement lead, Andy Gibson. It was intentional that the researchers involved in the ADAPTT study did not facilitate this workshop to ensure that the patient and public involvement group members had the freedom to be honest in their responses.
The group was asked to reflect on its involvement in the ADAPTT study; as guided by the cube, this was undertaken with particular focus on the four different elements, which were as follows:
-
whether they had a strong or weak voice in the study
-
the ways in which they could be involved (few or many)
-
their impact on changes in the study (little/a lot)
-
organisation concerns versus their own concerns.
The group members were given Post-It® Notes (3M, Saint Paul, MN, USA) and asked to place these onto the corresponding wall chart, the idea being that they would put themselves (via the Post-It Note) onto a sliding scale to reflect their positions. The group members were also encouraged to write comments on the Post-It Notes to give context to their visual answers. Collectively this process enabled a visual representation of the group’s experiences, and a narrative was produced that stimulated subsequent discussion.
Findings
The group members largely shared the perspectives of the researchers regarding their impact within the patient and public involvement group; however, disparities were evident in the perceived impact of the group’s work on the study itself:
-
patient and public involvement members had a strong voice within the group
-
their collective voice had less impact within the study
-
the group’s opinions were listened to and questions answered
-
the group’s ideas were discussed in detail and taken seriously
-
the group had some impact on changes in the study, but this was minimal
-
there were not enough options for involvement
-
organisation concerns were dominant
-
there was not enough communication from the study team to the patient and public involvement group (including to the group members who had not been able to attend meetings)
-
the group members were unsure of their overall impact.
Strengths and limitations of the study
The main strength of the ADAPTT study is that we quantified bleeding rates across different populations prescribed antiplatelet drugs (with or without an anticoagulant) in a UK-relevant population. Another strength is that we identified confounders systematically using different sources; the clinician interviews and surveys also gave us important insight into how clinicians prescribe antiplatelet drugs in the real world. A further strength is the use of target trial emulation; there is growing evidence that observational studies explicitly emulating existing RCTs can result in similar effect estimates to those of the RCT they are emulating,152,153 avoiding the different direction of effect that can result from less well-designed observational studies. 55 Finally, we also assessed the patient perspectives on DAPT and factors that influence adherence and health-seeking behaviours, and estimated utility decrements in a relevant UK patient population, based on standardised definitions of minor and major bleeding events, using a validated HRQoL instrument for the patient population of interest and valued using general population tariffs.
The main limitation is that we did not conduct a cost-effectiveness analysis because the target trial emulations were not perfect and may have produced biased estimates. Causal inference based on observational data requires high-quality data on exposures, confounders and outcomes, but our data sets had inherent limitations. Given the poor coding of acute events in some large primary care databases, mediocre sensitivity of diagnostic codes for detecting major bleeds in hospital databases137,138 and lack of information on medication use in databases, such as the HES, routine electronic health-care records may (depending on the question of interest) not always be the right source of data.
Identifying confounders in the way that we did is resource intensive. Resources are an important consideration when deciding whether or not to use the methods we adopted, given that the main output is a judgement about the risk of bias from unmeasured confounding. It is unclear how the risk of unmeasured confounding affects the interpretation of a target trial conducted using observational data. However, the confounders we identified could be used in future observational studies in the same populations, for example studies planning prospective data collection and studies assembling retrospective data sets. It also provides reliable information as to the variables we would need to collect to allow us to perform a formal quantitative bias analysis. 154
Implications for decision-makers
Despite the potential for bias, the results from this study using routinely collected data suggest that clinicians should exercise caution when prescribing more potent antiplatelet therapy to their patients, given that the increased risk of bleeding we observed was not offset by a reduced risk of ischaemic events. Several recent large meta-analyses52–54,66,67,69,70 of RCTs have also failed to show a conclusive benefit of more potent antiplatelet therapy on cardiovascular events, highlighting that the DAPT landscape is complex and that data from non-trial populations (representing the ‘real world’) should be carefully considered by decision-makers alongside RCT evidence when making recommendations about DAPT.
Future research recommendations
Future research could explore the feasibility of using other UK data sets of routinely collected data, less susceptible to bias, to estimate the benefit and harm of antiplatelet interventions. For example, it may be feasible to conduct the ADAPTT study emulations of two of the three target trials (conservatively managed ACS and PCI-treated ACS) using the UK National Institute for Cardiovascular Outcomes Research (NICOR) Myocardial Ischaemia National Audit Project and PCIs audit. 155 These data sets contain information on initial assignment to medication and confounding factors not available in our data sets, namely disease complexity and periprocedural information. Although the NICOR data sets are not currently linked with either primary care data or hospital episode data, in principle such linkages should be possible (and are being carried out at the local level in parts of the UK, e.g. Bristol) and should be explored in the future.
Randomised controlled trials of DAPT with bleeding as the primary outcome are unlikely to be conducted in the future. There is, therefore, still a need for prospective observational studies with high-quality data on outcomes and health-care costs, important potential confounders identified in Chapter 2, and, importantly, data on prescriptions in hospital. If high-quality observational data become available, they should be incorporated, together with estimates of the impact on quality of life, into a cost–utility analysis to assess which antiplatelet regimen is the preferred option in which patient population. We recommend that our utility decrements are used in future cost-effectiveness analyses of DAPT in a UK setting, particularly for minor bleeding events, when existing evidence is limited. In addition, rather than using a range of alternative sources in cost-effectiveness models, some of which may be unreliable, we recommend that future research focus on quantifying the value of information from reducing the uncertainty of our estimated utility decrements. This research would demonstrate whether or not conducting a larger, more robust, study to collect additional information on the HRQoL impact of minor and major bleeds for patients taking DAPT would be an efficient use of resources.
The qualitative study with patients highlighted that medication knowledge and understanding, and confidence in dealing with symptoms, facilitate positive attitudes towards adherence to DAPT, but that currently there are limited opportunities for patients to access relevant, timely and appropriate DAPT medication counselling. Additional qualitative research is needed to develop an intervention to support service users taking DAPT, which should explore (1) what informational and practical support service users think they need to make more informed decisions about their health and medications; (2) how it should be conveyed, for example written information, face-to-face counselling, through peer support and/or group rehabilitation, via digital resources; (3) when is it best to convey this information and support along their recovery and care journey (e.g. while in hospital), shortly after going home, in the community; and (4) by whom this information should be conveyed, for example cardiologists/cardiac surgeons, GPs, cardiac nurses, pharmacists. There is evidence that such interventions improve medication adherence in other populations,156 and, given the high rate of non-adherence to DAPT, this should be explored further.
Interest and controversy about the value to decision-makers of estimates of effectiveness based on observational studies have increased in equal measure in recent years. The principle of designing an observational study to emulate a RCT by first defining a target trial appears to be a robust approach, highlighting where the emulation succeeds or fails (as in the ADAPTT study). Nevertheless, further research is required to validate instances in which an emulation is considered to have been successful. Although there are examples of retrospective validation (typically, reanalysis of observational data using the emulation approach, when previous published effect estimates from RCTs and observational studies are known to differ153), there has been no prospective validation of target trials (i.e. using observational data to emulate ongoing RCTs before their data are analysed and the results are known). This may require collaboration between triallists and epidemiologists, and, potentially, require setting up the target trial alongside the real trial. Such research has the potential to improve future observational studies and give more confidence when decisions need to be made on the basis of observational estimates (if the emulation is successful) when RCTs are not possible.
The Cochrane Non-Randomized Studies for Interventions Methods Group recommended specifying confounders a priori some years ago. 29 However, no guidance was provided on how to identify potential confounding factors:
There is no established method for identifying a pre-specified set of important confounders. Listing potential confounding factors should certainly be done ‘independently’ and, one might argue, ‘systematically’. The list should not be generated solely on the basis of factors considered in primary studies included in the review (at least, not without some form of independent validation), since the number of potential confounders is likely to increase over time (hence, older studies may be out of date) and researchers themselves may simply choose to measure confounders considered in previous studies (hence, such a list could be selective). (Researchers investigating aetiological associations often do not explain their choice of confounding factors [Pocock SJ, Collier TJ, Dandreo KJ, de Stavioa BL, Goldman MB, Kalish LA, et al. Issues in the reporting of epidemiological studies: a survey of recent practice. BMJ 2004;329:883.].) Rather, the list should be based on evidence (although undertaking a systematic review to identify all potential prognostic factors is extreme) and expert opinion from members of the review team and advisors.
Reeves et al. 29 Reproduced with permission from The Cochrane Handbook.
This recommendation was endorsed in the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool for assessing risk of bias in non-randomised studies of interventions,17,157 but the revised Cochrane Handbook chapter158 provided no more information about how to identify confounding domains.
In the ADAPTT study, we used literature review and clinician expertise to identify confounding domains. We found it difficult to extract data on confounders from published studies, given the variety of study designs potentially eligible for inclusion (e.g. RCTs; prospective/retrospective cohort studies/registries, some descriptive and some comparative; prognostic/risk prediction studies) and the lack of standardised reporting in many of these study designs. Future research to develop guidance for identifying confounders and how confounders should be organised into confounding domains is urgently needed to facilitate consistent implementation of the ROBINS-I tool.
Funders need to consider how to identify emulations of RCTs that will be successful. Although the investment in an emulation will be much less than in a definitive pragmatic RCT, the investment might be considered to have been wasted if the emulation is unsuccessful and conclusions to inform patient care cannot be drawn. Triallists are often required to demonstrate that their trial is feasible through predefined progression criteria agreed between the triallists and the funder. Feasibility of the target trial should be determined in the same way prior to conducting a full analysis, centred around an assessment of the likely bias arising in the context of the available data sets, and should include stop/go criteria for progression to a full analysis. Stop/go criteria should address:
-
availability of the proposed data (and sample size) for the emulation
-
availability of data for assigning participants to the defined intervention in the target trial and validity of the method used for assignment
-
little or no selection of the cohort for analysis after the defined point of entry into the target trial
-
little or no missing follow-up time (potentially giving rise to immortal time bias) after the defined point of entry into the target trial
-
identification of confounding domains and the availability of data to characterise them
-
few or no missing data for group assignment and outcome
-
validity of the outcome measurement data.
In the ADAPTT study, it can be argued that we met only two of these seven criteria (numbers 1 and 4). The funder of the ADAPTT study (the Health Technology Assessment programme) highlighted two main concerns prior to the decision to fund the study: to what extent confounding by indication will influence the results of the study and whether or not data from CPRD would capture the true incidence of minor bleeds. However, in the absence of clear stop/go criteria, it was difficult for us and the funder to undertake a true assessment of feasibility and halt the study.
Acknowledgements
We would like to thank Dr Andy Gibson, associate professor in patient and public involvement at the University of the West of England, Bristol, for helping to facilitate the patient and public involvement process evaluation and Tarita Murray-Thomas, CPRD researcher, for extracting the ADAPTT study data sets from CPRD Gold. We would like to thank the patient and public involvement contributors for their continued dedication to and support of the ADAPTT study.
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme. The British Heart Foundation and the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol funded some staff time (MP, JH, BR and TJ). This study is based on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency and data from NHS Digital. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript for submission.
Contributions of authors
Dr Jessica Harris (https://orcid.org/0000-0002-4605-7710) (Senior Lecturer) created the ADAPTT study analysis data sets (linked HES–CPRD data), conducted data cleaning and performed all the statistical analyses. She had full access to all the data in the study and takes responsibility for the integrity of the analysis data sets and the accuracy of the results.
Dr Koen B Pouwels (https://orcid.org/0000-0001-7097-8950) (Senior Researcher) conducted the analyses of the comparative effect of different antiplatelet regimens on total health-care costs. He wrote Chapter 5 of the study, advised with regard to interpretation of the main ADAPTT study target trial results and revised parts of the manuscript for intellectual content.
Dr Thomas Johnson (https://orcid.org/0000-0003-4638-601X) (Associate Professor) contributed to study conceptualisation and provided clinical cardiology expertise. He wrote the clinical vignettes for the qualitative study with clinicians and helped to design the clinician surveys. He advised with regard to interpretation of the main ADAPTT study target trial results.
Professor Jonathan Sterne (https://orcid.org/0000-0001-8496-6053) (Professor of Medical Statistics and Epidemiology) contributed to the design and steered the statistical analysis of the ADAPTT study target trials. He also helped to interpret the results and revised the manuscript for intellectual content.
Dr Christalla Pithara (https://orcid.org/0000-0003-2958-5201) (Senior Research Associate) helped to design and conduct the qualitative studies with clinicians and patients. She also wrote Chapter 4.
Dr Kalaivani Mahadevan (https://orcid.org/0000-0002-9414-6945) (Consultant Interventional Cardiologist) performed the systematic review for identification of confounders. She extracted the data from the studies and grouped the confounders into relevant clinical subgroups. She also provided clinical cardiology expertise.
Professor Barney Reeves (https://orcid.org/0000-0002-5101-9487) (Professorial Research Fellow in Health Services Research) contributed to study conceptualisation, helped to prepare the application for funding, contributed to the interpretation of the findings and revised the manuscript for intellectual content.
Mr Umberto Benedetto (https://orcid.org/0000-0002-7074-7949) (Professor of Cardiac Surgery) provided cardiac surgery expertise and helped to conduct the survey with cardiac surgeons. He also provided input into the interpretation of the findings with regard to the CABG target trial.
Professor Yoon Loke (https://orcid.org/0000-0001-9109-2307) (Professor of Medicine and Pharmacology) provided CPRD and pharmacoepidemiology expertise throughout the study.
Professor Daniel Lasserson (https://orcid.org/0000-0001-8274-5580) (Professor of Ambulatory Care) provided general practice and CPRD expertise throughout the study.
Dr Brett Doble (https://orcid.org/0000-0002-4948-8831) (Assistant Professor) performed the systematic literature review of the HRQoL impact of major and minor bleeding events during DAPT and designed and conducted the patient preference elicitation study. He also wrote Chapter 5.
Dr Noreen Hopewell-Kelly (https://orcid.org/0000-0002-0699-0178) (Research Fellow) conducted and facilitated patient and public involvement in the ADAPTT study. She also conducted the patient and public involvement process evaluation and wrote this section of the report.
Dr Sabi Redwood (https://orcid.org/0000-0002-2159-1482) (Associate Professor) designed the qualitative studies with clinicians and patients and helped with the interpretation of the results of these studies and with writing Chapter 4.
Professor Sarah Wordsworth (https://orcid.org/0000-0002-2361-3040) (Professor of Health Economics) contributed to the design and interpretation of the health economics studies.
Professor Andrew Mumford (https://orcid.org/0000-0002-5523-511X) (Professor of Haematology) contributed to study conceptualisation and provided clinical haematology expertise.
Professor Chris Rogers (https://orcid.org/0000-0002-9624-2615) (Professor of Medical Statistics) provided advice with regard to some of the statistical analyses in the ADAPTT study target trials.
Dr Maria Pufulete (https://orcid.org/0000-0002-1775-1949) (Senior Research Fellow) wrote the application for funding, designed and conducted the confounders study, interpreted the results of the ADAPTT study target trials and wrote the manuscript.
Publications
Doble B, Pufulete M, Harris JM, Johnson T, Lasserson D, Reeves BC, Wordsworth S. Health-related quality of life impact of minor and major bleeding events during dual antiplatelet therapy: a systematic literature review and patient preference elicitation study. Health Qual Life Outcomes 2018;16:191.
Pufulete M, Harris J, Sterne JAC, Johnson TW, Lasserson D, Mumford A, et al. Comprehensive ascertainment of bleeding in patients prescribed different combinations of dual antiplatelet therapy (DAPT) and triple therapy (TT) in the UK: study protocol for three population-based cohort studies emulating ‘target trials’ (the ADAPTT Study). BMJ Open 2019;9:e029388.
Pithara C, Pufulete M, Johnson TW, Redwood S. Patient perspectives of nuisance bleeding and adherence to dual antiplatelet therapy: a qualitative study. Open Heart 2020;7:e001405.
Data-sharing statement
This retrospective observational study used electronic medical records from the CPRD and HES. The data-sharing agreements do not permit further sharing of the data, although data can be obtained directly from the CPRD and NHS Digital. The statistical code used to produce the results can be requested from jessica.harris @bristol.ac.uk. Any queries with regard to the data or the results of the analyses should be submitted to the corresponding author in the first instance.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
Appendix 1 Confounders: study literature searches
| Database | Set 1. Population + intervention + studies (RCTs/cohort – DAPT/TT) (n) | Set 2. Population + intervention + outcome (bleeding) (n) | Set 3. Population + outcome + risk (risk of bleeding after a coronary intervention) (n) |
|---|---|---|---|
| CENTRAL (database of controlled studies: RCTs, CCTs, ITS, CBA) | 775 | Included in set 1 (CENTRAL database) | 720 |
| MEDLINE | 1822 | 558 (deduplicated against set 1 MEDLINE) | 5001 |
| EMBASE | 1156 | 520 (deduplicated against set 1 EMBASE) | 1582 |
| Total | 3753 | 1078 | 7303 |
| After deduplication | 2544 | 849 | 6273 |
| Set 3 (Population + outcome + risk) | Search-within-a-search 1: (score or scores or model or models or tool or tools or algorithm* or prognosis or predict or prediction or cohort):ti,ab (n = 1843) |
| Search-within-a-search 2: (risk near3 (score* or factor or factors or model or models or prediction or stratification or category or bleed*)):ti,ab (n = 3300) (prior to deduplication) |
1. Ovid MEDLINE
Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Date range searched: 1946 to present.
Date searched: 24 August 2016.
-
Acute Coronary Syndrome/
-
(acute coronary adj3 syndrome*).ti,ab,kf.
-
ACS.ti,ab,kf.
-
heart attack*1.ti,ab,kf.
-
exp Myocardial Infarction/
-
myocardial infarct*.ti,ab,kf.
-
(MI or AMI).ti,ab,kf.
-
(stemi or non-stemi or nstemi).ti,ab,kf.
-
exp Angina, Unstable/
-
(angina adj3 unstable).ti,ab,kf.
-
exp Percutaneous Coronary Intervention/
-
(percutaneous coronary adj3 intervention).ti,ab,kf.
-
(PCI or PPCI or PCI-S).ti,ab,kf.
-
exp Angioplasty/
-
angioplasty.ti,ab,kf.
-
exp Stents/
-
stent*1.ti,ab,kf.
-
exp Coronary Artery Bypass/
-
CABG.ti,ab,kf.
-
coronary artery bypass.ti,ab,kf.
-
or/1-20 [Population]
-
(dual antiplatelet adj (therapy or treatment)).ti,ab,kf.
-
(DAPT or DAT).ti,ab,kf.
-
or/22-23
-
Aspirin/
-
(aspirin or acetylsalicylic acid or ASA).ti,ab,kf,rn,nm.
-
or/25-26
-
(clopidrogel or prasugrel or ticagrelor or plavix or efient or brilinta).ti,ab,kf,rn,nm,sh.
-
PURINERGIC P2Y RECEPTOR ANTAGONISTS/
-
(P2Y12 adj2 (antagonist* or inhibitor*)).ti,ab,kf,rn,nm.
-
or/28-30
-
24 or (27 and 31)
-
exp Anticoagulants/
-
(anticoagul* or antithrombo* or anti-coagul* or anti-thrombo* or OAC* or DOAC* or NOAC*).ti,ab, kf,rn,nm.
-
(coumarin* or coumadin* or warfarin or marevan or dicoumarol or dicoumarin or dicumarin or dicumarol or acenocoumarol or phenindione or aldocumar or dabigatram or pradaxa or BIBR1048 or Apixaban or Eliquis or BMS-562247-01 or Edoxaban or Lixiana or savaysa or DU-176b or betrixaban or PRT-054021 or PRT0504021 or rivaroxaban or xarelto or BAY-59739 or Erixaban or D0913).ti,ab,kf,rn,nm.
-
((Vitamin K or Factor Xa or Factor 10a or Factor IIa) adj2 (antagonist* or inhibitor*)).ti,ab,kw,rn,nm.
-
or/33-36
-
(triple therapy or triple antiplatelet therapy or triple antithrombotic therapy or triple antithrombotic combination therapy).ti,ab,kf.
-
(TAPT or TOAT).ti,ab,kf.
-
((24 or 31) and 37) or 38 or 39
-
32 or 40 [Intervention]
-
(bleed*1 or bleeding).ti,ab,kf.
-
Hemorrhage/
-
(hemorrhag* or haemorrhag*).ti,ab,kf.
-
or/42-44 [Outcome]
-
risk/ or risk assessment/ or risk factors/
-
risk stratification.ti,ab,kf.
-
(risk adj3 model*).ti,ab,kf.
-
risk factor*.ti,ab,kf.
-
or/46-49 [Risk]
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
(RCT or randomi*).ti,ab,kf.
-
placebo.ab.
-
(random* adj (assign* or allocat* or divide* or division)).ti,ab,kf.
-
trial.ti,ab.
-
groups.ab.
-
or/51-57
-
cohort studies/ or follow-up studies/ or longitudinal studies/ or prospective studies/ or retrospective studies/
-
longitudinal.ab.
-
(prospective or retrospective).ab.
-
(CCT or (control* adj (trial*1 or study or studies))).ti,ab,kf.
-
(Follow up adj2 (study or studies)).ti,ab,kf.
-
follow up assessment.ti,ab,kf.
-
(compar* and group*).ab.
-
cohort.ti,ab,kf.
-
(register or registry).ti,ab,kf.
-
or/59-67
-
58 or 68 [Study Design Filter]
-
21 and 41 and 69
-
21 and 41 and 45
-
21 and 41 and 50
-
21 and 41 and 45 and 50
-
21 and 45 and 50
2. The Cochrane Library, Issue 7, 2016
Date range searched: issue 1, 2003 to 24 August 2016.
Date searched: 24 August 2016.
-
MeSH descriptor: [Acute Coronary Syndrome] explode all trees
-
“acute coronary syndrome”:ti,ab,kw (Word variations have been searched)
-
ACS:ab (Word variations have been searched)
-
heart attack*:ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Myocardial Infarction] explode all trees
-
myocardial next infarct*:ti,ab,kw (Word variations have been searched)
-
MI or AMI:ab (Word variations have been searched)
-
(stemi or non-stemi or nstemi):ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Angina, Unstable] explode all trees
-
(angina near unstable):ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees
-
(percutaneous next coronary) and intervention:ti,ab,kw (Word variations have been searched)
-
PCI or PPCI or PCI-S:ab (Word variations have been searched)
-
MeSH descriptor: [Angioplasty] explode all trees
-
angioplasty:ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Stents] explode all trees
-
stent or stents or stenting:ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Coronary Artery Bypass] explode all trees
-
CABG:ab (Word variations have been searched)
-
“coronary artery bypass”:ti,ab,kw (Word variations have been searched)
-
(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20)
-
(dual next antiplatelet) and (therapy or treatment):ti,ab,kw (Word variations have been searched)
-
(DAPT or DAT):ti,ab,kw (Word variations have been searched)
-
#22 or #23
-
MeSH descriptor: [Aspirin] explode all trees
-
aspirin or “acetylsalicylic acid”:ti,ab,kw (Word variations have been searched)
-
ASA:ab (Word variations have been searched)
-
#25 or #26 or #27
-
(clopidrogel or prasugrel or ticagrelor or plavix or efient or brilinta):ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Purinergic P2Y Receptor Antagonists] explode all trees
-
(P2Y12 near (antagonist* or inhibitor*)):ti,ab,kw (Word variations have been searched)
-
#29 or #30 or #31
-
#24 or (#28 and #32)
-
MeSH descriptor: [Anticoagulants] explode all trees
-
(anticoagul* or antithrombo* or anti-coagul* or anti-thrombo* or OAC* or DOAC* or NOAC*):ti,ab,kw (Word variations have been searched)
-
(coumarin* or coumadin* or warfarin or marevan or dicoumarol or dicoumarin or dicumarin or dicumarol or acenocoumarol or phenindione or aldocumar):ti,ab,kw (Word variations have been searched)
-
dabigatram or pradaxa or BIBR1048 or Apixaban or Eliquis or BMS-562247-01 or Edoxaban or Lixiana or savaysa or DU-176b or betrixaban or PRT-054021 or PRT0504021 or rivaroxaban or xarelto or BAY-59739 or Erixaban or D0913:ti,ab,kw (Word variations have been searched)
-
“vitamin K” and (antagonist* or inhibitor*):ti,ab,kw (Word variations have been searched)
-
(“vitamin K” or “factor Xa” or “factor 10a” or “factor IIa”) and (antagonist* or inhibitor*):ti,ab,kw (Word variations have been searched)
-
#34 or #35 or #36 or #37 or #38 or #39
-
triple near therapy:ti,ab,kw (Word variations have been searched)
-
TAPT or TOAT:ab (Word variations have been searched)
-
((#24 or #32) and #40) or #41 or #42
-
#33 or #43
-
MeSH descriptor: [Hemorrhage] explode all trees
-
bleed*:ti,ab,kw (Word variations have been searched)
-
hemorrhag* or haemorrhag*:ti,ab,kw (Word variations have been searched)
-
#45 or #46 or #47
-
MeSH descriptor: [Risk] explode all trees
-
“risk stratification”:ti,ab,kw (Word variations have been searched)
-
risk near (factor* or model*):ti,ab,kw (Word variations have been searched)
-
#49 or #50 or #51
-
#21 and #44
-
#21 and #48 and #52
-
(#54 not #53)
3. Ovid EMBASE
Date range searched: 1974 to date.
Date searched: 24 August 2016.
-
exp acute coronary syndrome/
-
(acute coronary adj3 syndrome*).ti,ab,kw.
-
ACS.ti,ab,kw.
-
heart attack*1.ti,ab,kw.
-
exp heart infarction/
-
myocardial infarct*.ti,ab,kw.
-
(MI or AMI).ti,ab,kw.
-
(stemi or non-stemi or nstemi).ti,ab,kw.
-
exp unstable angina pectoris/
-
(angina adj3 unstable).ti,ab,kw.
-
exp percutaneous coronary intervention/
-
(percutaneous coronary adj3 intervention).ti,ab,kw.
-
(PCI or PPCI or PCI-S).ti,ab,kw.
-
exp angioplasty/
-
angioplasty.ti,ab,kw.
-
exp stent/
-
stent*1.ti,ab,kw.
-
coronary artery bypass graft/
-
CABG.ti,ab,kw.
-
coronary artery bypass.ti,ab,kw.
-
or/1-20
-
(dual antiplatelet adj (therapy or treatment)).ti,ab,kw.
-
(DAPT or DAT).ti,ab,kw.
-
or/22-23
-
acetylsalicylic acid/
-
(aspirin or acetylsalicylic acid or ASA).ti,ab,kw,rn,tn.
-
or/25-26
-
(clopidrogel or prasugrel or ticagrelor or plavix or efient or brilinta).ti,ab,kw,rn,tn,sh.
-
antithrombocytic agent/
-
(P2Y12 adj2 (antagonist* or inhibitor*)).ti,ab,kw,rn.
-
exp purinergic receptor blocking agent/
-
or/28-31
-
24 or (27 and 32)
-
exp anticoagulant agent/
-
(anticoagul* or antithrombo* or anti-coagul* or anti-thrombo* or OAC* or DOAC* or NOAC*).ti, ab,kw.
-
(coumarin* or coumadin* or warfarin or marevan or dicoumarol or dicoumarin or dicumarin or dicumarol or acenocoumarol or phenindione or aldocumar).ti,ab,kw,rn,tn.
-
(dabigatram or pradaxa or BIBR1048 or Apixaban or Eliquis or BMS-562247-01 or Edoxaban or Lixiana or savaysa or DU-176b or betrixaban or PRT-054021 or PRT0504021 or rivaroxaban or xarelto or BAY-59739 or Erixaban or D0913).ti,ab,kw,rn,tn.
-
(vitamin K adj2 (antagonist$ or inhibitor$)).ti,ab,kw,rn.
-
(factor Xa adj2 (antagonist$ or inhibitor$)).ti,ab,kw,rn.
-
(factor 10a adj2 (antagonist$ or inhibitor$)).ti,ab,kw,rn.
-
(factor IIa adj2 (antagonist$ or inhibitor$)).ti,ab,kw,rn.
-
(adjunct* or combin* or concurrent or cotherap* or co-therap* or dual or plus or triple).ti,ab,kw.
-
drug combination/
-
or/34-43
-
(triple therapy or triple antiplatelet therapy or triple antithrombotic therapy or triple antithrombotic combination therapy).ti,ab,kw.
-
(TAPT or TOAT).ti,ab,kw.
-
((24 or 32) and 44) or 45 or 46
-
33 or 47
-
(bleed*1 or bleeding).ti,ab,kw.
-
exp Bleeding/
-
(hemorrhag* or haemorrhag*).ti,ab,kw.
-
or/49-51
-
risk assessment/ or risk factor/ or patient risk/ or risk/ or high risk patient/
-
risk stratification.ti,ab,kw.
-
(risk adj3 model*).ti,ab,kw.
-
risk factor*.ti,ab,kw.
-
or/53-56
-
Randomized Controlled Trial/
-
Randomization/
-
(random* adj (assign* or allocat* or divide* or division)).ti,ab,kw.
-
(RCT or randomi*).ti,ab,kw.
-
trial.ti,ab.
-
placebo.ti,ab,kw.
-
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab,kw.
-
double blind procedure/
-
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti.
-
or/58-66
-
Controlled Clinical Trial/
-
(CCT or (controlled adj7 (study or design or trial))).ti,ab,kw.
-
cohort analysis/
-
cohort.ti,ab,kw.
-
longitudinal.ab.
-
(prospective or retrospective).ab.
-
follow up assessment.ti,ab,kw.
-
clinical trial/ or multicenter study/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
-
clinical study/ or exp longitudinal study/ or major clinical study/ or prospective study/ or retrospective study/
-
(Follow up adj2 study).ti,ab,kw.
-
register.ti,ab,kw.
-
or/68-78
-
67 or 79
-
Animal experiment/ not (human experiment/ or human/)
-
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
-
or/81-82
-
80 not 83
-
21 and 48 and 84
-
limit 85 to exclude medline journals
-
21 and 48 and 52
-
limit 87 to exclude medline journals
-
21 and 48 and 57
-
limit 89 to exclude medline journals
-
21 and 48 and 52 and 57
-
86 or 88 or 90 or 91
Appendix 2 Vignettes presenting four clinical scenarios
Interview case scenarios (cardiologists)
Scenario 1
On a post-take ward round you assess a 75-year-old type 1 diabetic patient describing crescendo symptoms of angina with T-wave inversion across the chest lead. Troponin measurements are within normal range. The patient is already on long-term aspirin treatment. You have elected to admit the patient for inpatient angiography.
Scenario 2
A patient with AF on long-term anticoagulation has been investigated for new-onset angina symptoms and is now awaiting PCI.
Scenario 3
You review a patient on the cardiac ward 2 days post STEMI [primary PCI to proximal left anterior descending (LAD)] with severe left ventricular impairment. The patient has developed AF and is currently prescribed aspirin and ticagrelor.
Scenario 4
A patient presents to your outpatient clinic 2 months following PCI (stenting) to their right coronary artery. They are taking aspirin and ticagrelor but have been struggling with frequent and heavy nosebleeds and have noticed significant bruising with minor trauma.
Interview case scenarios (cardiac surgeons)
Scenario 1
You have just undertaken successful complete revascularisation for an elective patient with stable angina and severe three-vessel coronary disease. Prior to surgery, the patient was taking 75 mg of aspirin once daily.
Scenario 2
A patient with AF on long-term anticoagulation has now undergone surgical revascularisation for severe three-vessel disease.
Scenario 3
On review of a patient, day 4 post CABG, they are found to have developed AF. Surgical revascularisation (CABG) had been undertaken following an acute presentation and initial stenting of a culprit lesion in the proximal right coronary artery. The severe nature of the proximal left coronary disease resulted in use of a left internal mammary artery (LIMA) graft to LAD and vein grafts to obtuse marginal (OM) 1 and OM2. The patient is on AC.
Scenario 4
A patient presents to your post-surgical clinic. Unfortunately, 2 weeks post surgery, the patient had presented to their local cardiac department with inferior ST elevation and required acute stenting of the native right coronary because of sub-acute failure of the vein graft. They are now taking aspirin and ticagrelor, but have been struggling with frequent and heavy nosebleeds and have noticed significant bruising with minor trauma.
Interview case scenarios (GPs)
Scenario 1
A patient attends your practice 2 weeks after discharge following a NSTEMI and PCI (stenting). They have been prescribed AT for 12 months.
Scenario 2
A patient presents to your practice with symptoms of palpitations 1 week after an acute myocardial infarction. The patient was prescribed AT on discharge from hospital. On examination the patient is found to be in AF.
Scenario 3
A patient presents to your practice 2 months after PCI (stenting) of their right coronary artery. They are taking AT, but have been struggling with frequent and heavy nosebleeds and have noticed significant bruising with minor trauma.
Appendix 3 Based on topic guides
Cardiac surgeon interviews: topic guide
Thank you for agreeing to take part in this interview to discuss dual antiplatelet therapy and anticoagulation in acute coronary syndrome.
The aim of this study is to understand the factors that influence clinicians’ decision-making when it comes to antiplatelet agents and anticoagulation pharmacotherapy in acute coronary syndrome; it is not an assessment of your individual knowledge or practice.
-
Before we begin, could you describe your role and responsibilities with regard to patients on dual antiplatelet therapy and anticoagulation in acute coronary syndrome? (Number of years in consultant/GP role.)
To help us understand in more detail how prescribing practices might vary, we are going to look at different case scenarios where dual antiplatelet therapy might be initiated or the pharmacotherapeutic regimen changed.
What we’d like is to hear how you would go about making decisions, and what you would consider when deciding (1) whether to prescribe a specific regimen and (2) which agent to prescribe in different situations.
Initiation of therapy
You have just undertaken successful complete revascularisation for an elective patient with stable angina and severe three-vessel coronary disease. Prior to surgery, the patient was taking 75 mg of aspirin once daily.
-
What is your standard practice for prevention of graft failure?
-
What would you be looking out for after the surgery?
-
What clinical decisions need to be made?
-
-
If you would prescribe a second antiplatelet agent, which one would you prescribe?
-
Is this the one you routinely prescribe?
-
(If participant prescribes all three): in the last 3 months, what proportions of your patients have received clopidogrel, ticagrelor and prasugrel?
-
What factors influence your decision-making? (Balancing ischaemic and bleeding risks: concomitant drugs, additional planned procedures, etc.)
-
Could you describe the factors that you would consider when deciding which (second) antiplatelet agent (if any) to prescribe?
-
What would lead you to select a particular additional antiplatelet agent?
-
Patient on anticoagulation and need for dual antiplatelet therapy addition
A patient with atrial fibrillation on long-term anticoagulation has now undergone surgical revascularisation for severe three-vessel disease.
-
In this scenario, what decisions need to be made?
-
Would you consider use of dual antiplatelet therapy (aspirin and clopidogrel) in addition to oral anticoagulant treatment? Reasons?
-
If no, then ask: are there any occasions when you have had to initiate DAPT in a patient with obligate need for an oral anticoagulant? (If so, then expand on clinical scenario.)
-
Which antiplatelet agents would you prescribe?
-
Would you continue the anticoagulant? Reasons?
-
Do you have a preferred anticoagulant? Reasons?
-
Patient on dual antiplatelet therapy developing need for anticoagulation
On review of a patient, day 4 post CABG, they are found to have developed AF. Surgical revascularisation (CABG) had been undertaken following an acute presentation and initial stenting of a culprit lesion in the proximal right coronary artery. The severe nature of the proximal left coronary disease resulted in the use of a LIMA graft to LAD and vein grafts to OM1 and OM2. The patient is on aspirin and clopidogrel.
-
In this scenario, what decisions would you make in relation to DAPT and anticoagulation?
-
How would you deal with this person’s ongoing thromboembolic risk relating to the new-onset AF?
-
-
Would you be happy initiating an oral anticoagulant? If so, what would determine the choice of agent that you would use? (If the respondent discusses only warfarin, then the interviewer should probe them about the use of the NOACs.)
-
Would you want to alter the antiplatelet regime that the patient is already prescribed? Reasons? Circumstances when you would want/not want to alter?
-
Are there any local/national/international guidelines specific to triple therapy that clinicians might use? Are these guidelines important when you consider your decision? Why are they important/ not important?
Patient on dual antiplatelet therapy presenting with bleeding
A patient presents to your post-surgical clinic. Unfortunately, 2 weeks post surgery, the patient had presented to their local cardiac department with inferior ST elevation and required acute stenting of the native right coronary owing to sub-acute failure of the vein graft. They are now taking aspirin and ticagrelor, but have been struggling with frequent and heavy nosebleeds and have noticed significant bruising with minor trauma.
-
In this scenario, what decisions would you make in relation to DAPT? Would you recommend any changes to the patient’s pharmacotherapy?
-
What influences your decision regarding therapy modification? (Are they balancing bleeding and ischaemic risk, i.e. location of stents, area at jeopardy?)
-
Are there any special considerations relating to how you convert from ticagrelor to another agent? What are these considerations?
-
Would you liaise with the cardiologist responsible when making these changes? What would determine whether or not you would contact the cardiologist responsible?
-
How long would you continue with dual antiplatelet therapy? What factors would you consider when deciding how long to continue DAPT therapy for?
-
You have/haven’t mentioned the use of guidelines and recommendations as a determinant of prescribing decisions. Could you tell me a bit about the presence or absence of guidelines when it comes to the above scenarios (local/national/international guidelines)? Are these important? Why?
-
Do you try to keep your practice in line with the evidence?
-
-
Do you think external factors, such as big pharma companies, play a role in prescribing decisions?
-
What is your experience with pharma companies? In what ways do you think pharma companies might be a factor influencing clinicians’ prescribing (e.g. through funding conferences and conference attendance, through distribution of free samples, interactions with pharma reps)?
-
[AstraZeneca plc (Cambridge, UK) for ticagrelor (Brilique®) and Daiichi Sankyo Company, Limited (Tokyo, Japan), for prasugrel (Efient®).]
-
-
Before we end this interview, is there anything you want to add about current practices and the key factors that influence prescribing among your colleagues?
Thank you.
Cardiologist interviews: topic guide
Thank you for agreeing to take part in this interview to discuss dual antiplatelet therapy and anticoagulation in acute coronary syndrome.
The aim of this study is to understand the factors that influence clinicians’ decision-making when it comes to antiplatelet agents and anticoagulation pharmacotherapy in acute coronary syndrome; it is not an assessment of your individual knowledge or practice.
Take verbal informed consent.
-
Before we begin, could you describe your role and responsibilities with regard to patients on dual antiplatelet therapy and anticoagulation in acute coronary syndrome? (Number of years in consultant/GP role.)
To help us understand in more detail how prescribing practices might vary, we are going to look at different case scenarios where dual antiplatelet therapy might be initiated or the pharmacotherapeutic regimen changed.
What we’d like is to hear how you would go about making decisions, and what you would consider when deciding (1) whether to prescribe a specific regimen and (2) which agent to prescribe in different situations.
Initiation of therapy
On a post-take ward round you assess a 75-year-old type 1 diabetic patient describing crescendo symptoms of angina with T-wave inversion across the chest lead. Troponin measurements are within normal range. The patient is already on long-term aspirin treatment. You have elected to admit the patient for inpatient angiography.
-
In this scenario, would you consider prescribing a second antiplatelet agent?
-
If you would prescribe a second antiplatelet agent, which one would you prescribe?
-
I understand that there are several choices when it comes to antiplatelet agents (clopidogrel, ticagrelor and prasugrel); is the agent you have chosen what the majority of your patients are being prescribed?
-
Approximately, what proportions of your patients have received each one during the last 3 months?
-
-
What factors would you consider when prescribing DAPT (Choosing to prescribe a second antiplatelet agent.) (Balancing ischaemic and bleeding risks: concomitant drugs, additional planned procedures etc.) (e.g. comorbidities, age, guidelines, other?) Which are the most important factors?
Patient on anticoagulation and need for dual antiplatelet therapy addition
A patient with atrial fibrillation on long-term anticoagulation has been investigated for new-onset angina symptoms and is now awaiting percutaneous coronary intervention.
-
In this scenario, what decisions might be made that are relevant to DAPT?
-
What are the factors you would consider when making prescribing decisions specific to DAPT?
-
Would you consider use of dual antiplatelet therapy (aspirin and clopidogrel) in addition to oral anticoagulant treatment (triple therapy)? Could you explain why?
-
If no, then ask: are there any occasions when you have had to initiate DAPT in a patient with obligate need for an oral anticoagulant? (If so, then expand on clinical scenario.)
-
-
Which antiplatelet agents would you prescribe?
-
Do you have a preferred anticoagulant? Could you share the reasons behind this?
-
Would you continue the anticoagulant?
-
Could you explain the reasons behind these decisions?
-
Patient on dual antiplatelet therapy developing need for anticoagulation
You review a patient on the cardiac ward 2 days post STEMI (primary PCI to proximal LAD) with severe left ventricular impairment. The patient has developed atrial fibrillation and is currently prescribed aspirin and ticagrelor.
-
In this scenario, what decisions would you make in relation to DAPT and anticoagulation?
-
How would you deal with this person’s ongoing thromboembolic risk relating to the new-onset AF?
-
Would you be happy initiating an oral anticoagulant? Why?
-
If so, what would determine the choice of agent that you would use? (If the respondent only discusses warfarin, then the interviewer should probe them about the use of the NOACs.)
-
Would you want to alter the antiplatelet regime that the patient is already prescribed?
-
-
Factors influencing your decision.
Patient on dual antiplatelet therapy presenting with bleeding
A patient presents to your outpatient clinic 2 months following percutaneous coronary intervention (stenting) to their right coronary artery. They are taking aspirin and ticagrelor but have been struggling with frequent and heavy nosebleeds and have noticed significant bruising with minor trauma.
-
In this scenario, what decisions would you make in relation to DAPT? Would you recommend any changes to the patient’s pharmacotherapy?
-
What would be the factors you would consider when making therapy modification decisions specific to DAPT? (Are they balancing bleeding and ischaemic risk, i.e. location of stents, area at jeopardy?)
-
Are there any special considerations relating to how you convert from ticagrelor to another agent? What are these considerations?
-
How long would you continue with dual antiplatelet therapy?
-
What factors would you consider when deciding how long to continue DAPT therapy for?
-
You have/haven’t mentioned the use of guidelines and recommendations as a determinant of prescribing decisions. Could you tell be a bit about the presence or absence of guidelines when it comes to the above scenarios (local/national/international guidelines)? Are these important? Why?
-
Do you try to keep your practice in line with the evidence?
-
-
Do you think external factors, such as big pharma companies, play a role in prescribing decisions?
-
What is your experience with pharma companies? In what ways do you think pharma companies might be a factor influencing clinicians’ prescribing (e.g. through funding conferences and conference attendance, through distribution of free samples, interactions with pharma reps)?
-
[AstraZeneca for ticagrelor (Brilique) and Daiichi Sankyo for prasugrel (Efient).]
-
-
Before we end this interview, is there anything you want to add about current practices and the key factors that influence prescribing among your colleagues?
Thank you.
General practitioner interviews: topic guide
Thank you for agreeing to take part in this interview to discuss dual antiplatelet therapy and anticoagulation in acute coronary syndrome.
The aim of this study is to understand the factors that influence clinicians’ decision-making when it comes to antiplatelet agents and anticoagulation pharmacotherapy in acute coronary syndrome; it is not an assessment of your individual knowledge or practice.
-
Before we begin, could you describe your role and responsibilities with regard to patients on dual antiplatelet therapy and anticoagulation in acute coronary syndrome? (Number of years in consultant/GP role.)
To help us understand in more detail how prescribing practices might vary, we are going to look at different case scenarios where dual antiplatelet therapy might be initiated or the pharmacotherapeutic regimen changed.
What we’d like is to hear how you would go about making decisions, and what you would consider when deciding (1) whether to prescribe a specific regimen and (2) which agent to prescribe in different situations.
Patient prescribed dual antiplatelet therapy in secondary care
A patient attends your practice 2 weeks after discharge following a non-ST elevation myocardial infarction and percutaneous coronary intervention (stenting). They have been prescribed aspirin and ticagrelor for 12 months.
-
Are there any circumstances under which you would decide to change this prescription? (Commissioning decisions/cost; practice protocols; balancing ischaemic and bleeding risks – concomitant drugs, additional planned procedures, etc.)
-
Do you have any concerns about this prescription? Would you change this prescription?
-
-
If you would consider changing, is there an antiplatelet agent you commonly prescribe?
-
Which antiplatelet drug do you most commonly prescribe (clopidogrel, prasugrel or ticagrelor)?
-
In the last 3 months what proportions of your patients received clopidogrel, ticagrelor and prasugrel?
Patient on dual antiplatelet therapy developing need for anticoagulation
A patient presents to your practice with symptoms of palpitations 1 week following an acute myocardial infarction. The patient was prescribed aspirin and ticagrelor on discharge from hospital. On examination the patient is found to be in AF.
-
What are your first thoughts on this scenario? How would you manage this person’s ongoing thromboembolic risk?
-
Would you be happy initiating an oral anticoagulant?
-
If so, what would determine the choice of agent that you would use? (If the respondent only discusses warfarin, then the interviewer should probe them about the use of the NOACs, e.g. dabigatran, rivaroxaban, apixaban, edoxaban.)
-
Would you want to alter the antiplatelet regime that the patient is already prescribed?
-
Under what circumstances would you want to alter the regime? Factors influencing the decision.
Patient on dual antiplatelet therapy presenting with bleeding
A patient presents to your practice 2 months following percutaneous coronary intervention (stenting) of their right coronary artery. They are taking aspirin and ticagrelor but have been struggling with frequent and heavy nosebleeds and noticed significant bruising with minor trauma.
-
What are your thoughts on this scenario? How would you deal with this patient?
-
Would you recommend any changes to the patient’s pharmacotherapy?
-
What would be the factors you would consider when making therapy modification decisions specific to DAPT? (Are they balancing bleeding and ischaemic risk, i.e. location of stents, area at jeopardy...)
-
Are there any special considerations relating to how you convert from ticagrelor to another antiplatelet agent?
-
Would you liaise with the cardiologist responsible when making these changes?
-
What would determine whether or not you would contact the cardiologist responsible?
-
How long would you continue dual antiplatelet therapy?
-
You have/haven’t mentioned the use of guidelines and recommendations as a determinant of prescribing decisions. Could you tell be a bit about the presence or absence of guidelines when it comes to above scenarios? (Local/national/international guidelines.) Are these important? Why?
-
Do you try to keep your practice in line with the evidence?
-
-
Do you think external factors such as big pharma companies play a role in prescribing decisions?
-
What is your experience with pharma companies? In what ways do you think pharma companies might be a factor influencing clinician’s prescribing (e.g. through funding conferences and conference attendance, through distribution of free samples, interactions with pharma reps)?
-
[AstraZeneca for ticagrelor (Brilique) and Daiichi Sankyo for Prasugrel (Efient).]
-
-
Before we end this interview, is there anything you want to add about current practices and the key factors that influence prescribing among your colleagues?
Thank you.
Appendix 4 Factors and constituent indicators from clinician interviews
Summary of factors influencing clinician decision-making (Cardiologist; Cardiac Surgeons; GPs)
| Category | Factors | Items in factors |
|---|---|---|
| 1. Patient factors |
|
|
|
||
|
||
|
||
|
|
|
|
||
|
||
|
|
|
| 2. Clinician factors |
|
|
|
|
|
|
||
|
||
|
||
|
||
|
||
| 3. Pharmacotherapeutic agents |
|
|
|
||
| 4. Organisational factors |
|
|
|
||
|
Appendix 5 Product codes for antiplatelet and anticoagulant prescriptions
| Product code | Product name |
|---|---|
| Aspirin | |
| 3 | Aspirin 75-mg dispersible tablets |
| 16 | Aspirin 75-mg tablets |
| 34 | Aspirin 75-mg gastro-resistant tablets |
| 111 | Aspirin 40-mg CAP |
| 216 | Aspirin 70-mg tablets |
| 254 | Aspirin 300-mg tablets |
| 377 | Aspirin 300-mg dispersible tablets |
| 383 | Aspirin 60-mg tablets |
| 393 | Disprin 300-mg dispersible tablets [Reckitt Benckiser Healthcare (UK) Ltd, Slough, UK] |
| 395 | Aspirin mixture |
| 434 | Aspirin 300-mg gastro-resistant tablets |
| 1137 | Nu-seals aspirin ec 300-mg gastro-resistant tablet (Eli Lilly and Company, Indianapolis, IN, USA) |
| 1486 | Aspirin 75-mg SUP |
| 2105 | Solprin 300-mg tablet [Reckitt Benckiser Healthcare (UK) Ltd] |
| 2607 | Paynocil tablet (Beecham Research Laboratories, Brentford, UK) |
| 2628 | Nu-Seals aspirin ec 75-mg gastro-resistant tablet (Eli Lilly and Company Ltd) |
| 2754 | Aspirin soluble 150-mg tablets |
| 2924 | Aspirin 150-mg tablets |
| 4271 | Aspirin soluble 200-mg tablets |
| 4523 | Aspirin 50-mg CAP |
| 6006 | Nu-Seals 75-mg gastro-resistant tablets (Alliance Pharmaceuticals Ltd, Chippenham, UK) |
| 6007 | Nu-Seals 300-mg gastro-resistant tablets (Alliance Pharmaceuticals Ltd) |
| 6696 | Micropirin 75-mg gastro-resistant tablets (Dexcel Pharma Ltd, Daventry, UK) |
| 7417 | Aspirin 40-mg tablets |
| 7462 | Aspirin 325-mg CAP |
| 7486 | Aspirin 37.5-mg tablets |
| 7516 | Aspirin 300-mg effervescent tablets, sugar-free |
| 7665 | Aspirin sr 300-mg tablets |
| 7915 | Aspirin sr 100-mg tablets |
| 7944 | Aspirin soluble 40-mg CAP |
| 8185 | Disprin CV 300-mg modified-release tablets [Reckitt Benckiser Healthcare (UK) Ltd] |
| 8186 | Aspirin 300-mg modified-release tablets |
| 8424 | Aspirin paed 81-mg tablets |
| 8645 | Aspirin 300-mg effervescent tablets |
| 8733 | Junior aspirin 37.5-mg tablets |
| 8734 | Aspirin disp 37.5-mg tablets |
| 8843 | Aspirin 325-mg tablets |
| 9027 | Aspirin disp 150-mg tablets |
| 9144 | Caprin 75-mg gastro-resistant tablets (Wockhardt UK Ltd, Wrexham, UK) |
| 9301 | Aspirin 100-mg modified-release tablets |
| 10305 | Aspirin 162.5-mg capsules |
| 10310 | Aspirin powder |
| 11941 | Aspirin sachets 30 mg |
| 11977 | Aspro® Clear maximum-strength tablets (Bayer plc, Reading, UK) |
| 12102 | Aspirin soluble 100-mg tablets |
| 13882 | Imazin XL tablets (Napp Pharmaceuticals Ltd, Cambridge, UK) |
| 15397 | Aspirin soluble 50-mg tablets |
| 15517 | Aspirin 100-mg sup |
| 17704 | Platet 100-mg effervescent tablet (Roche Products Ltd, Welwyn Garden City, UK) |
| 17920 | Disprin cv 100-mg modified-release tablet (Reckitt Benckiser Healthcare (UK) Ltd) |
| 18030 | Imazin XL Forte tablets (Napp Pharmaceuticals Ltd) |
| 18217 | Aspirin 300-mg orodispersible tablets, sugar-free |
| 18329 | Enprin 75-mg gastro-resistant tablets (Galpharm International Ltd, Braunton, UK) |
| 19189 | Micropirin 75-mg gastro-resistant tablet (Ratiopharm UK Ltd, London, UK) |
| 19577 | Nu-Seals aspirin |
| 19674 | Aspirin dispersible |
| 19797 | Nu-Seals aspirin |
| 19813 | Aspirin soluble |
| 20206 | Aspirin 50-mg sup |
| 20840 | Acetylsalicylic acid mix |
| 21380 | Aspirin 70-mg/isosorbide mononitrate 60-mg modified-release tablets |
| 21382 | Aspirin 150-mg/isosorbide mononitrate 60-mg modified-release tablets |
| 21921 | PostMI ec 300 mg gastro-resistant tablet (Ashbourne Pharmaceuticals Ltd, Northampton, UK) |
| 22107 | Aspirin disp 200-mg tablets |
| 22138 | Aspirin 324-mg modified-release tablets |
| 22232 | Disprin Direct 300-mg orodispersible tablets [Reckitt Benckiser Healthcare (UK) Ltd] |
| 22618 | Solprin 75-mg tablet [Reckitt Benckiser Healthcare (UK) Ltd] |
| 22864 | Aspirin paed mix |
| 23488 | Claradin 300-mg tablet (Nicholas Laboratories Ltd, Welwyn Garden City, UK) |
| 23495 | Aspirin |
| 23593 | PostMI 75-mg dispersible tablets (Ashbourne Pharmaceuticals Ltd) |
| 23878 | Nu-Seals cardio ec 75-mg gastro-resistant tablet (Genus Pharmaceuticals Ltd, Huddersfield, UK) |
| 23932 | Aspro Clear 300-mg effervescent tablets (Bayer plc) |
| 24025 | Caprin 300-mg gastro-resistant tablets (Pinewood Healthcare, Wrexham, UK) |
| 24960 | Aspirin 300 mg tablets (Vantage) |
| 25335 | PostMI 75-mg EC tablets (Ashbourne Pharmaceuticals Ltd) |
| 25718 | Angettes 75-mg tablets (Bristol-Myers Squibb Pharmaceuticals Ltd, Uxbridge, UK) |
| 27467 | Aspirin soluble 400-mg tablets |
| 28707 | Aspirin m/f 324-mg tablets |
| 29515 | Acetylsalicylic acid |
| 29759 | Aspro tablet (Roche Consumer Health) |
| 29848 | Aspirin 300-mg with glycine 150-mg chewable tablets |
| 30920 | Aspirin 300-mg dispersible tablet (M&A Pharmachem Ltd, Manchester, UK) |
| 31210 | Aspirin 300-mg tablet (Co-operative) |
| 31211 | Aspirin 75-mg dispersible tablet (AAH Pharmaceuticals Ltd, Coventry, UK) |
| 31858 | Caspac XL 162.5-mg capsule (Pharmacia Ltd, Sandwich, UK) |
| 31870 | Aspirin 320-mg tablets |
| 31938 | Aspirin 75-mg gastro-resistant tablets (Sandoz Ltd, Camberley, UK) |
| 31953 | Aspirin 75-mg dispersible tablets (IVAX Pharmaceuticals UK Ltd, Birmingham, UK) |
| 31954 | Aspirin 75-mg dispersible tablets (Teva UK Ltd, Castleford, UK) |
| 31956 | Aspirin 75-mg gastro-resistant tablets (Kent Pharmaceuticals Ltd, Ashford, UK) |
| 32036 | Aspirin 75-mg dispersible tablets (Actavis UK Ltd, Barnstaple, UK) |
| 32210 | Aspirin 300-mg dispersible tablets (Actavis UK Ltd) |
| 32992 | Aspirin 75-mg gastro-resistant tablets (Mylan, Hatfield, UK) |
| 33293 | Aspirin 75-mg gastro-resistant tablets (Sterwin Medicines) |
| 33320 | Aspirin 75-mg dispersible tablet (Sovereign Medical Ltd, Stansted, UK) |
| 33656 | Aspirin 75-mg dispersible tablets (AAH Pharmaceuticals Ltd) |
| 33662 | Aspirin 300-mg dispersible tablet (AAH Pharmaceuticals Ltd) |
| 33668 | Aspirin 300-mg dispersible tablet (Rusco Ltd, Kibworth, UK) |
| 33676 | Aspirin 75-mg dispersible tablets (Kent Pharmaceuticals Ltd) |
| 34309 | Aspirin 300-mg dispersible tablets (AAH Pharmaceuticals Ltd) |
| 34385 | Aspirin 75-mg soluble tablet (Co-operative) |
| 34386 | Aspirin 300-mg tablets (Actavis UK Ltd) |
| 34434 | Aspirin 75-mg dispersible tablets (Thornton & Ross Ltd, Huddersfield, UK) |
| 34485 | Aspirin 75-mg gastro-resistant tablets (IVAX Pharmaceuticals UK Ltd) |
| 34611 | Aspirin 75-mg gastro-resistant tablets (C P Pharmaceuticals Ltd, Wrexham, UK) |
| 34666 | Aspirin ec 300-mg gastro-resistant tablet (AAH Pharmaceuticals Ltd) |
| 34762 | Aspirin 300-mg gastro-resistant tablet (Galen Ltd, Craigavon, UK) |
| 34796 | Aspirin 75-mg gastro-resistant tablet (Galen Ltd) |
| 34797 | Aspirin 75-mg gastro-resistant tablets (Actavis UK Ltd) |
| 34942 | Aspirin 75-mg dispersible tablet (NuCare plc, Telford, UK) |
| 36543 | Aspirin 100-mg effervescent tablets |
| 37541 | Aspirin 227-mg medicated chewing gum |
| 39738 | Aspirin 162.5-mg modified-release capsules |
| 40144 | Aspirin 300-mg dispersible tablet (Thornton & Ross Ltd) |
| 40381 | Aspirin 75-mg soluble tablet (C P Pharmaceuticals Ltd) |
| 41512 | Aspirin 75-mg gastro-resistant tablets (Teva UK Ltd) |
| 41569 | Aspirin 300-mg tablets (AAH Pharmaceuticals Ltd) |
| 41594 | Aspirin 300-mg dispersible tablet (Teva UK Ltd) |
| 42061 | Aspirin 65 mg SUP |
| 43060 | Aspirin 300-mg soluble tablet (Celltech Pharma Europe Ltd, Slough, UK) |
| 43434 | Aspirin 300-mg gastro-resistant tablets (AAH Pharmaceuticals Ltd) |
| 43679 | Flamasacard® 162.5-mg modified-release capsule (Abbey Pharmaceuticals Ltd, Maidenhead, UK) |
| 43709 | Aspirin 75-mg gastro-resistant tablets (Almus Pharmaceuticals Ltd, Weybridge, UK) |
| 43806 | Aspirin 300-mg gastro-resistant tablets (Sandoz Ltd) |
| 44639 | Aspirin 300-mg dispersible tablet (NuCare plc) |
| 45643 | Aspirin 75-mg soluble tablet (Celltech Pharma Europe Ltd) |
| 45840 | Aspirin 300-mg dispersible tablet (Numark Management Ltd, Runcorn, UK) |
| 45851 | Aspirin 300-mg soluble tablet [Ranbaxy (UK) Ltd, Uxbridge, UK] |
| 47937 | Aspirin 75-mg dispersible tablets (Wockhardt UK Ltd) |
| 47992 | Aspirin 75-mg gastro-resistant tablets (AAH Pharmaceuticals Ltd) |
| 48000 | Aspirin 300-mg tablets (Sigma Pharmaceuticals plc, North Watford, UK) |
| 48021 | Aspirin 75-mg tablet (Hillcross Pharmaceuticals Ltd, Coventry, UK) |
| 48165 | Aspirin 300-mg tablets (Aspar Pharmaceuticals Ltd, St Albans, UK) |
| 48974 | Aspirin 75-mg tablets (Phoenix Healthcare Distribution Ltd, Runcorn, UK) |
| 49060 | Aspirin 75-mg dispersible tablets [Alliance Healthcare (Distribution) Ltd, Chessington, UK] |
| 49220 | Aspirin 300-mg tablets (Kent Pharmaceuticals Ltd) |
| 49685 | Aspirin 75-mg dispersible tablets (Sigma Pharmaceuticals plc) |
| 50555 | Aspirin 300-mg dispersible tablets (DE Pharmaceuticals, Prudhoe, UK) |
| 50926 | Aspirin 75-mg dispersible tablets (The Boots Company plc, Beeston, UK) |
| 50949 | Aspirin 75-mg tablets (AAH Pharmaceuticals Ltd) |
| 51561 | Aspirin 75-mg gastro-resistant tablets (Zanza Laboratories Ltd, Liverpool, UK) |
| 52044 | Aspirin 300-mg caplets (The Boots Company plc) |
| 52280 | Aspirin 300-mg tablet (Wockhardt UK Ltd) |
| 52618 | Aspirin 75-mg dispersible tablets (Bristol Laboratories Ltd, Berkhamsted, UK) |
| 52905 | Aspirin 300-mg tablets (Lloyds Pharmacy Ltd, Coventry, UK) |
| 53178 | Aspirin 75-mg gastro-resistant tablets (Wockhardt UK Ltd) |
| 53622 | Aspirin 300-mg tablet (M&A Pharmachem Ltd) |
| 53711 | Aspirin 300-mg tablet (NuCare plc) |
| 53791 | Aspirin 150-mg suppositories [Alliance Healthcare (Distribution) Ltd] |
| 53804 | Aspirin 300-mg gastro-resistant tablets [Alliance Healthcare (Distribution) Ltd] |
| 53816 | Aspirin 300-mg dispersible tablets [Alliance Healthcare (Distribution) Ltd] |
| 54284 | Aspirin 75-mg dispersible tablets (Almus Pharmaceuticals Ltd) |
| 54430 | Aspirin 75-mg tablets [Alliance Healthcare (Distribution) Ltd] |
| 54526 | Aspirin 300-mg tablets [Alliance Healthcare (Distribution) Ltd] |
| 54565 | Aspirin 75-mg dispersible tablets (Lloyds Pharmacy Ltd) |
| 54734 | Aspirin 300-mg tablets (Wockhardt UK Ltd) |
| 54997 | Aspirin 75-mg dispersible tablets (Dowelhurst Ltd, Leeds, UK) |
| 55230 | Aspirin 300-mg dispersible tablets (Kent Pharmaceuticals Ltd) |
| 55579 | Aspirin 300-mg tablets (Almus Pharmaceuticals Ltd) |
| 56007 | Aspirin 300-mg dispersible tablets (Sigma Pharmaceuticals Plc) |
| 56736 | Aspirin 300-mg tablets (Waymade Healthcare plc, Basildon, UK) |
| 56883 | Aspirin 75-mg tablets (Waymade Healthcare plc) |
| 56995 | Aspirin 75-mg dispersible tablets (Phoenix Healthcare Distribution Ltd) |
| 56996 | Aspirin 75-mg dispersible tablets (Waymade Healthcare Plc) |
| 57057 | Aspirin 75-mg dispersible tablets (Wockhardt UK Ltd) |
| 58331 | Aspirin 300-mg gastro-resistant tablets (Mylan) |
| 59021 | Aspirin 75-mg gastro-resistant tablets (Bristol Laboratories Ltd) |
| 59244 | Aspirin 100-mg capsules |
| 59253 | Aspirin 75-mg gastro-resistant tablets (Waymade Healthcare plc) |
| 59728 | Aspirin 75-mg tablets (Alissa Healthcare Research Ltd, Fareham, UK) |
| 59791 | Aspirin 75-mg dispersible tablets (Aspar Pharmaceuticals Ltd) |
| 60127 | Aspirin 75-mg tablets (DE Pharmaceuticals) |
| 60278 | Aspirin 300-mg tablets (DE Pharmaceuticals) |
| 60693 | Aspirin 15-mg/5-ml oral solution |
| 60694 | Aspirin 25-mg/5-ml oral solution |
| 60777 | Aspirin 75-mg gastro-resistant tablets (DE Pharmaceuticals) |
| 62334 | Aspirin 300-mg caplets (Wockhardt UK Ltd) |
| 62430 | Aspirin 300-mg suppositories (AAH Pharmaceuticals Ltd) |
| 63603 | Laboprin tablet (Laboratories For Applied Biology Ltd, South Ruislip, UK) |
| 64071 | Aspirin powder (J M Loveridge Ltd, Andover, UK) |
| 65027 | Bisoprolol 5-mg/aspirin 100-mg capsules |
| 66345 | Aspirin 75-mg dispersible tablets (DE Pharmaceuticals) |
| 66546 | Aspirin 75-mg dispersible tablets (Numark Ltd) |
| 66563 | Aspirin 75-mg gastro-resistant tablets (Phoenix Healthcare Distribution Ltd) |
| 66861 | Aspirin 75-mg effervescent tablets |
| 67124 | Bisoprolol 10-mg/aspirin 75-mg capsules |
| 67160 | Aspirin 300-mg dispersible tablets (Lloyds Pharmacy Ltd) |
| 67362 | Aspirin 300-mg suppositories [Alliance Healthcare (Distribution) Ltd] |
| 67521 | Aspirin 15-mg/5-ml oral suspension |
| 67754 | Aspirin 300-mg dispersible tablets (Almus Pharmaceuticals Ltd) |
| 67858 | Aspirin 25-mg capsules |
| 68051 | Aspirin 150-mg suppositories (Colorama Pharmaceuticals Ltd, London, UK) |
| 68752 | Aspirin 75-mg tablets (Sigma Pharmaceuticals Plc) |
| 70549 | Danamep® 75-mg dispersible tablets (Ecogen Europe Ltd, Leicester, UK) |
| 70841 | Aspirin 300-mg dispersible tablet (Family Health) |
| 71078 | Aspirin 300-mg dispersible tablets (Mawdsley-Brooks & Company Ltd, Salford, UK) |
| 71192 | Aspirin 75-mg tablets (Kent Pharmaceuticals Ltd) |
| Clopidogrel | |
| 489 | Clopidogrel 75-mg tablets |
| 836 | Plavix® 75-mg tablets (Sanofi SA, Paris, France) |
| 17816 | Plavix FC |
| 17817 | Clopidogrel FC |
| 38349 | Clopidogrel 300-mg tablets |
| 38998 | Plavix 300-mg tablets (Sanofi) |
| 40913 | Grepid® 75-mg tablets (Kent Pharmaceuticals Ltd) |
| 42750 | Clopidogrel 75-mg tablets (Actavis UK Ltd) |
| 45905 | Clopidogrel 1-mg/ml oral suspension |
| 46891 | Clopidogrel 75-mg/5-ml oral suspension |
| 52761 | Clopidogrel 75-mg tablets [Dr Reddy’s Laboratories (UK) Ltd, Beverley, UK] |
| 53751 | Clopidogrel 75-mg tablets (Phoenix Healthcare Distribution Ltd) |
| 54700 | Clopidogrel 75-mg tablets (A A H Pharmaceuticals Ltd) |
| 55161 | Clopidogrel 75-mg tablets (Wockhardt UK Ltd) |
| 56807 | Clopidogrel 75-mg tablets (Teva UK Ltd) |
| 57036 | Clopidogrel 75-mg tablets (Mylan) |
| 58347 | Clopidogrel 75-mg tablets (DE Pharmaceuticals) |
| 58448 | Clopidogrel 75-mg tablets (Aspire Pharma Ltd, Godalming, UK) |
| 59904 | Clopidogrel 75-mg/5-ml oral solution |
| 62855 | Clopidogrel 75-mg tablets [Alliance Healthcare (Distribution) Ltd] |
| 62978 | Clopidogrel 75-mg tablets (Sandoz Ltd) |
| 63450 | Clopidogrel 75-mg tablets (Almus Pharmaceuticals Ltd) |
| 65909 | Clopidogrel 75-mg tablets (Milpharm Ltd, South Ruislip, UK) |
| 67037 | Clopidogrel 75-mg tablets (Zentiva Group a.s., Prague, Czech Republic) |
| Prasugrel | |
| 39932 | Prasugrel 10-mg tablets |
| 40114 | Prasugrel 5-mg tablets |
| 40591 | Efient 5-mg tablets (Eli Lilly and Company Ltd) |
| 41229 | Efient 10-mg tablets (Eli Lilly and Company Ltd) |
| Ticagrelor | |
| 45576 | Ticagrelor 90-mg tablets |
| 47895 | Brilique 90-mg tablets (AstraZeneca UK Ltd) |
| 66973 | Ticagrelor 60-mg tablets |
| 68710 | Brilique 60-mg tablets (AstraZeneca UK Ltd) |
| 70606 | Ticagrelor 90-mg orodispersible tablets, sugar-free |
| Anticoagulants | |
| 45 | Warfarin 1-mg tablets |
| 61 | Warfarin 3-mg tablets |
| 833 | Warfarin 3-mg/5-ml oral solution |
| 1781 | Warfarin 5-mg tablets |
| 2675 | Fragmin® 10,000 IU/4-ml solution for injection ampoules (Pfizer Ltd, Sandwich, UK) |
| 2676 | Fragmin 5000 IU/0.2-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 2677 | Clexane® 100-mg/ml injection (Aventis Pharma, Reading, UK) |
| 3744 | Heparin 10-IU/ml flush solution |
| 3895 | Heparin sodium 1000-IU/ml injection |
| 4446 | Acenocoumarol 1-mg tablets |
| 4888 | Heplok 10-IU/ml oral solution (LEO Pharma A/S, Copenhagen, Denmark) |
| 4995 | Enoxaparin 100-mg/ml injection |
| 5305 | Sinthrome 1-mg tablets (Merus Labs Luxco II S.à.R.L., Luxembourg) |
| 5526 | Fragmin 2500 IU/0.2-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 5747 | Fragmin 25,000-IU/ml solution for injection (Pfizer Ltd) |
| 5998 | Fragmin 10,000-IU/ml solution for injection (Pfizer Ltd) |
| 6262 | Warfarin 500-μg tablets |
| 6478 | Enoxaparin sodium 20-mg/0.2-ml solution for injection pre-filled syringes |
| 6695 | Dalteparin sodium 2500 IU/0.2-ml solution for injection pre-filled syringes |
| 6822 | Elmiron 100-mg capsules (Teva UK Ltd) |
| 6860 | Fragmin 15,000 IU/0.6-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 7154 | Clexane Forte 120-mg/0.8-ml solution for injection pre-filled syringes (Sanofi) |
| 7199 | Enoxaparin sodium 40-mg/0.4-ml solution for injection pre-filled syringes |
| 7307 | Clexane 40-mg/0.4-ml solution for injection pre-filled syringes (Sanofi) |
| 7371 | Clexane 100-mg/1-ml solution for injection pre-filled syringes (Sanofi) |
| 8466 | Marevan™ 1-mg tablets (AMCo) |
| 8467 | Marevan 3-mg tablets (AMCo) |
| 8664 | Heparin sodium 5000-IU/ml injection |
| 9140 | Dalteparin sodium 10,000 IU/4-ml solution for injection ampoules |
| 9593 | Dalteparin 25,000-IU/ml injection solution |
| 9605 | Dalteparin sodium 5000 IU/0.2-ml solution for injection pre-filled syringes |
| 9610 | Tinzaparin 20,000-IU/ml injection |
| 9640 | Tinzaparin 10,000-IU/ml injection |
| 10002 | Dalteparin 10,000-IU/1-ml injection solution |
| 10004 | Clexane 80-mg/0.8-ml solution for injection pre-filled syringes (Sanofi) |
| 10044 | Dalteparin sodium 10,000 IU/0.4-ml solution for injection pre-filled syringes |
| 10072 | Fragmin 10,000 IU/0.4-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 10170 | Dalteparin sodium 15,000 IU/0.6-ml solution for injection pre-filled syringes |
| 10194 | Dalteparin sodium 12,500 IU/0.5-ml solution for injection pre-filled syringes |
| 10240 | Tinzaparin sodium 14,000 IU/0.7-ml solution for injection pre-filled syringes |
| 10532 | Minihep calcium 5000 IU/0.2-ml Injection (LEO Pharma) |
| 10533 | Calciparine® 25,000-IU/ml injection (Sanofi-Synthelabo Ltd, Reading, UK) |
| 10560 | Warfarin 10-mg tablets |
| 11372 | Heparin 100-IU/ml flush solution |
| 12681 | Heparin calcium 25,000-IU/ml injection |
| 12974 | Clexane 150-mg/ml injection (Aventis Pharma) |
| 13058 | Enoxaparin 150-mg/ml injection |
| 13097 | Clexane 20-mg/0.2-ml solution for injection pre-filled syringes (Sanofi) |
| 13210 | Enoxaparin sodium 80-mg/0.8-ml solution for injection pre-filled syringes |
| 13270 | Enoxaparin sodium 120-mg/0.8-ml solution for injection pre-filled syringes |
| 13348 | Marevan 5-mg tablets (AMCo) |
| 13501 | Dindevan® 50-mg tablet (Goldshield Pharmaceuticals Ltd, Croyden, UK) |
| 13502 | Dindevan 10-mg tablet (Goldshield Pharmaceuticals Ltd) |
| 13503 | Phenindione 50-mg tablets |
| 13504 | Phenindione 25-mg tablets |
| 13505 | Phenindione 10-mg tablets |
| 13568 | Heparin sodium 25,000 IU/ml subcutaneous injection |
| 13644 | Dindevan 25-mg tablet (Goldshield Pharmaceuticals Ltd) |
| 13663 | Innohep® 20,000-IU/ml injection (LEO Pharma) |
| 13716 | Heparin sodium 25,000-IU/ml Injection |
| 14099 | Clexane Forte 150-mg/ml injection (Aventis Pharma) |
| 14110 | Tinzaparin sodium 10,000 IU/0.5-ml solution for injection pre-filled syringes |
| 14138 | Enoxaparin sodium 60-mg/0.6-ml solution for injection pre-filled syringes |
| 14212 | Tinzaparin sodium 3500 IU/0.35-ml solution for injection pre-filled syringes |
| 14308 | Tinzaparin sodium 18,000 IU/0.9-ml solution for injection pre-filled syringes |
| 14341 | Clexane Forte 150-mg/1-ml solution for injection pre-filled syringes (Sanofi) |
| 14788 | Innohep 10,000 IU/0.5-ml solution for injection pre-filled syringes (LEO Pharma) |
| 14794 | Monoparin 1000-IU/ml injection (C P Pharmaceuticals Ltd) |
| 14851 | Tinzaparin sodium 4500 IU/0.45-ml solution for injection pre-filled syringes |
| 14891 | Dalteparin sodium 18,000 IU/0.72-ml solution for injection pre-filled syringes |
| 15006 | Sinthrome® 4-mg tablet (Alliance Pharmaceuticals Ltd) |
| 15293 | Heparin sodium 5000-IU/ml pre-filled injection |
| 15376 | Acenocoumarol 4-mg tablets |
| 15709 | Tinzaparin 3500-IU/0.3-ml sterile solution |
| 16061 | Innohep 3500 IU/0.35-ml solution for injection pre-filled syringes (LEO Pharma) |
| 16476 | Fragmin 18,000 IU/0.72-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 16530 | Fragmin 12,500 IU/0.5-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 17004 | Tinzaparin sodium 20,000 IU/2-ml solution for injection vials |
| 17007 | Tinzaparin sodium 2500 IU/0.25-ml solution for injection pre-filled syringes |
| 17049 | Innohep 18,000 IU/0.9-ml solution for injection pre-filled syringes (LEO Pharma) |
| 17484 | Innohep 10,000-IU/ml injection (LEO Pharma) |
| 17592 | Innohep 4500 IU/0.45-ml solution for injection pre-filled syringes (LEO Pharma) |
| 17664 | Clexane 60-mg/0.6-ml solution for injection pre-filled syringes (Sanofi) |
| 17791 | Innohep 5000-IU/5-ml sterile solution (LEO Pharma) |
| 17965 | Marevan 500-μg tablets (AMCo) |
| 18209 | Fragmin 7500 IU/0.3-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 18732 | Innohep 3500-IU/0.3-ml sterile solution (LEO Pharma) |
| 19280 | Innohep 14,000 IU/0.7-ml solution for injection pre-filled syringes (LEO Pharma) |
| 19337 | Multiparin 125,000 IU/5-ml solution for injection vials (Wockhardt UK Ltd) |
| 19486 | Dalteparin sodium 7500 IU/0.3-ml solution for injection pre-filled syringes |
| 19989 | Tinzaparin sodium 40,000 IU/2-ml solution for injection vials |
| 20010 | Uniparin calcium 25,000-IU/ml subcutaneous injection (C P Pharmaceuticals Ltd) |
| 20024 | Uniparin Forte 10,000-IU/0.4-ml subcutaneous injection (C P Pharmaceuticals Ltd) |
| 20028 | Multiparin 5000 IU/5-ml solution for injection vials (Wockhardt UK Ltd) |
| 20029 | Multiparin 25,000 IU/5-ml solution for injection vials (Wockhardt UK Ltd) |
| 20153 | Enoxaparin sodium 150-mg/1-ml solution for injection pre-filled syringes |
| 20154 | Enoxaparin sodium 100-mg/1-ml solution for injection pre-filled syringes |
| 20411 | Alphaparin 3000-IU/0.5-ml Injection (Grifols UK Ltd,Waterbeach, UK) |
| 20754 | Warfarin |
| 21233 | Innohep 20,000 IU/2-ml solution for injection vials (LEO Pharma) |
| 21316 | Innohep 40,000 IU/2-ml solution for injection vials (LEO Pharma) |
| 21365 | Uniparin 5000-IU/0.2-ml injection (C P Pharmaceuticals Ltd) |
| 21490 | Monoparin 5000-IU/ml injection (C P Pharmaceuticals Ltd) |
| 21518 | Monoparin 25,000-IU/ml injection (C P Pharmaceuticals Ltd) |
| 22428 | Dalteparin sodium 100,000 IU/4-ml solution for injection vials |
| 23078 | Warfarin 1-mg tablet (WB Pharmaceuticals Ltd, Leicester, UK) |
| 23570 | Fondaparinux sodium 7.5-mg/0.6-ml solution for injection pre-filled syringes |
| 23573 | Fondaparinux sodium 5-mg/0.4-ml solution for injection pre-filled syringes |
| 23579 | Fondaparinux sodium 2.5-mg/0.5-ml solution for injection pre-filled syringes |
| 24896 | Heparin low molecular weight 2500-IU/0.2-ml sterile solution |
| 25155 | Fragmin 100,000 IU/4-ml solution for injection vials (Pfizer Ltd) |
| 25195 | Heparin sodium 25,000-IU/ml pre-filled injection |
| 25287 | Unihep leo 1000 IU/ml injection (LEO Pharma) |
| 26146 | Heparin low molecular weight 10,000-IU/ml sterile solution |
| 27035 | Pump-hep 1000 IU/ml infusion (LEO Pharma) |
| 27139 | Pentosan polysulfate sodium 100-mg capsules |
| 27325 | Innohep 2500 IU/0.25-ml solution for injection pre-filled syringes (LEO Pharma) |
| 28506 | Heparin low molecular weight 3500-IU/0.3-ml sterile solution |
| 28593 | Heparin sodium 1000-IU/ml pre-filled injection |
| 29043 | Arixtra® 2.5-mg/0.5-ml solution for injection pre-filled syringes (Aspen Pharma Trading Ltd, London, UK) |
| 29207 | Innohep 5000-IU/0.5-ml sterile solution (LEO Pharma) |
| 29317 | Tinzaparin 5000-IU/0.5-ml sterile solution |
| 29318 | Heparin low molecular weight 2500-IU/ml sterile solution |
| 30108 | Heparin calcium 5000-IU/0.2-ml injection |
| 30202 | Warfarin wbp 1-mg tablet (Boehringer Ingelheim Ltd, Bracknell, UK) |
| 30203 | Warfarin wbp 3-mg tablet (Boehringer Ingelheim Ltd) |
| 30396 | Unihep leo 5000-IU/ml injection (LEO Pharma) |
| 31148 | Flolan 500-μg powder and solvent (pH 10.5) for solution for infusion vials (GlaxoSmithKline UK Ltd, Brentford, UK) |
| 31511 | Warfarin 3-mg tablet (WB Pharmaceuticals Ltd) |
| 31937 | Warfarin 5-mg tablets (Teva UK Ltd) |
| 32511 | Tinzaparin 5000-IU/5-ml sterile solution |
| 32645 | Heparin sodium 25,000-IU/ml injection |
| 33307 | Heparin sodium 5000 IU/1-ml solution for injection ampoules |
| 33558 | Monoparin calcium 5000 IU/0.2-ml solution for injection ampoules (Wockhardt UK Ltd) |
| 33711 | Warfarin 5-mg tablet (WB Pharmaceuticals Ltd) |
| 34019 | Warfarin 1-mg tablets (IVAX Pharmaceuticals UK Ltd) |
| 34086 | Warfarin 3-mg tablet (Celltech Pharma Europe Ltd) |
| 34087 | Warfarin 1-mg tablet (Celltech Pharma Europe Ltd) |
| 34088 | Warfarin 5-mg tablet (Celltech Pharma Europe Ltd) |
| 34095 | Warfarin wbp 5-mg tablet (Boehringer Ingelheim Ltd) |
| 34299 | Warfarin 1-mg tablets (Teva UK Ltd) |
| 34416 | Warfarin 1-mg tablets (Kent Pharmaceuticals Ltd) |
| 34417 | Warfarin 3-mg tablets (Teva UK Ltd) |
| 34418 | Warfarin 5-mg tablets (Mylan) |
| 34517 | Warfarin 1-mg tablets (Mylan) |
| 34526 | Warfarin 3-mg tablets (Mylan) |
| 34576 | Warfarin 1-mg tablet (Lagap) |
| 34691 | Warfarin 5-mg tablet (Regent Laboratories Ltd, London, UK) |
| 34758 | Warfarin 3-mg tablets (IVAX Pharmaceuticals UK Ltd) |
| 34864 | Warfarin 5-mg tablets (IVAX Pharmaceuticals UK Ltd) |
| 34918 | Warfarin 5-mg tablets (Actavis UK Ltd) |
| 35033 | Heparin sodium 5000 IU/5-ml solution for injection vials |
| 35941 | Heparin sodium 5000 IU/5-ml solution for injection ampoules |
| 36099 | Warfarin 1-mg/5-ml oral suspension |
| 36142 | Heparin sodium 25,000 IU/1-ml solution for injection ampoules |
| 36172 | Clexane 300-mg/3-ml solution for injection multidose vials (Sanofi) |
| 36196 | Heparin sodium 1000 IU/1-ml solution for injection ampoules |
| 36911 | Fragmin 10,000 IU/1-ml solution for injection ampoules (Pfizer Ltd) |
| 36989 | Fragmin 10,000 IU/1-ml solution for injection pre-filled syringes (Pfizer Ltd) |
| 37086 | Enoxaparin sodium 300-mg/3-ml solution for injection vials |
| 37131 | Heparin sodium 25,000 IU/5-ml solution for injection vials |
| 37613 | Heparin sodium 10,000 IU/10-ml solution for injection ampoules |
| 37616 | Heparin sodium 10 IU/ml solution |
| 37678 | Heparin sodium 5000 IU/0.2-ml solution for injection ampoules |
| 37704 | Minihep 25,000-IU/ml subcutaneous preparation (LEO Pharma) |
| 38041 | Warfarin sodium 5-mg/ml oral suspension |
| 38044 | Warfarin 5-mg/5-ml oral solution |
| 38327 | Arixtra 7.5-mg/0.6-ml solution for injection pre-filled syringes (Aspen Pharma Trading Ltd) |
| 38536 | Fondaparinux sodium 1.5-mg/0.3-ml solution for injection pre-filled syringes |
| 38839 | Arixtra 5-mg/0.4-ml solution for injection pre-filled syringes (Aspen Pharma Trading Ltd) |
| 39119 | Rivaroxaban 10-mg tablets |
| 39444 | Dabigatran etexilate 110-mg capsules |
| 39503 | Dabigatran etexilate 75-mg capsules |
| 39639 | Xarelto® 10-mg tablets (Bayer plc) |
| 39755 | Pradaxa® 110-mg capsules (Boehringer Ingelheim Ltd) |
| 39866 | Warfarin 1-mg tablets (Almus Pharmaceuticals Ltd) |
| 40143 | Warfarin 500-μg tablets (AAH Pharmaceuticals Ltd) |
| 40715 | Heparin 100-IU/ml oral solution (LEO Pharma) |
| 42106 | Unihep leo 25,000-IU/ml injection (LEO Pharma) |
| 42474 | Pradaxa 75-mg capsules (Boehringer Ingelheim Ltd) |
| 42853 | Heparin calcium 25,000-IU/ml injection |
| 43407 | Warfarin 3-mg tablets (AAH Pharmaceuticals Ltd) |
| 43408 | Warfarin 1-mg tablets (AAH Pharmaceuticals Ltd) |
| 43409 | Warfarin 5-mg tablets (AAH Pharmaceuticals Ltd) |
| 43655 | Warfarin sodium oral solution |
| 44238 | Heparin 50-IU/5-ml flush solution (Wockhardt UK Ltd) |
| 44491 | Heparin sodium 125,000 IU/5-ml solution for injection vials |
| 44866 | Warfarin sodium 1-mg/ml oral supension SF |
| 45597 | Lepirudin 50-mg powder for solution for injection vials |
| 45911 | Arixtra 1.5-mg/0.3-ml solution for injection pre-filled syringes (Aspen Pharma Trading Ltd) |
| 46632 | Dabigatran etexilate 150-mg capsules |
| 46678 | Pradaxa 150-mg capsules (Boehringer Ingelheim Ltd) |
| 46924 | Phenindione 10-mg tablets (AMCo) |
| 47207 | Rivaroxaban 20-mg tablets |
| 47353 | Rivaroxaban 15-mg tablets |
| 47397 | Heparin sodium 25,000 IU/5-ml solution for injection ampoules |
| 47566 | Apixaban 2.5-mg tablets |
| 47925 | Xarelto 20-mg tablets (Bayer plc) |
| 47944 | Warfarin 1-mg tablets (Actavis UK Ltd) |
| 48070 | Warfarin sodium tablets |
| 48134 | Xarelto 15-mg tablets (Bayer plc) |
| 48673 | Dalteparin sodium 10,000 IU/1-ml solution for injection ampoules |
| 48869 | Warfarin 1-mg/ml oral suspension, sugar-free |
| 48966 | Rivaroxaban 15-mg tablets |
| 49578 | Dalteparin sodium 10,000 IU/1-ml solution for injection pre-filled syringes |
| 50000 | Warfarin 1-mg/ml oral suspension, sugar-free (AAH Pharmaceuticals Ltd) |
| 50391 | Fragmin 18,000 IU/0.72-ml solution for injection pre-filled syringes (Waymade Healthcare plc) |
| 50994 | Heparin sodium 500 IU/500-ml infusion bags |
| 51006 | Clexane 80-mg/0.8-ml solution for injection pre-filled syringes (DE Pharmaceuticals) |
| 51350 | Fragmin 15,000 IU/0.6-ml solution for injection pre-filled syringes (Waymade Healthcare plc) |
| 51484 | Warfarin 1-mg tablets (Bristol Laboratories Ltd) |
| 51496 | Warfarin 1-mg tablets (Phoenix Healthcare Distribution Ltd) |
| 51509 | Warfarin 1-mg tablets [APC Pharmaceuticals & Chemicals (Europe) Ltd, Market Harborough, UK] |
| 51642 | Clexane 100-mg/1-ml solution for injection pre-filled syringes [Lexon (UK) Ltd, Redditch, UK] |
| 52004 | Fragmin 12,500 IU/0.5-ml solution for injection pre-filled syringes (Waymade Healthcare plc) |
| 52841 | Heparin calcium 5000 IU/0.2-ml solution for injection ampoules |
| 53350 | Heparin sodium 1000 IU/500-ml infusion bags |
| 53740 | Eliquis® 2.5-mg tablets (Bristol-Myers Squibb Pharmaceuticals Ltd) |
| 53745 | Warfarin 3-mg tablets (Bristol Laboratories Ltd) |
| 53752 | Warfarin 1-mg tablets [Alliance Healthcare (Distribution) Ltd] |
| 54066 | Apixaban 5-mg tablets |
| 54234 | Heparin sodium 1000 IU/500-ml infusion Viaflex bags (Baxter Healthcare Ltd, Northampton, UK) |
| 54451 | Rivaroxaban 20-mg tablets |
| 54892 | Warfarin 1-mg/ml oral suspension, sugar-free [Alliance Healthcare (Distribution) Ltd] |
| 54927 | Heparin sodium 2000 IU/1-l infusion bags |
| 54946 | Warfarin 3-mg tablets (Actavis UK Ltd) |
| 55096 | Fragmin 5000 IU/0.2-ml solution for injection pre-filled syringes (Waymade Healthcare plc) |
| 55316 | Warfarin 3-mg/5-ml oral suspension |
| 55490 | Heparin sodium 10,000-IU/ml injection |
| 55565 | Clexane 100-mg/1-ml solution for injection pre-filled syringes (DE Pharmaceuticals) |
| 55577 | Sinthrome 1-mg tablets [Lexon (UK) Ltd] |
| 55604 | Orgaran® 750 IU/0.6-ml solution for injection ampoules (Aspen Pharma Trading Ltd) |
| 56166 | Heparin sodium 100 IU/1-ml solution for injection ampoules |
| 56289 | Xarelto 20-mg tablets (Bayer plc) |
| 56314 | Warfarin 3-mg tablets (Kent Pharmaceuticals Ltd) |
| 56315 | Anticoagulant citrate–dextrose solution formula A infusion 500-ml bags |
| 56398 | Fragmin 5000 IU/0.2-ml solution for injection pre-filled syringes (Mawdsley-Brooks & Company Ltd) |
| 56640 | Xarelto 15-mg tablets (Bayer plc) |
| 57032 | Warfarin 1-mg/ml oral suspension, sugar-free (Rosemont Pharmaceuticals Ltd, Leeds, UK) |
| 58519 | Warfarin 1-mg tablets (DE Pharmaceuticals) |
| 58594 | Eliquis 5-mg tablets (Bristol-Myers Squibb Pharmaceuticals Ltd) |
| 58787 | Warfarin 5-mg tablets [Alliance Healthcare (Distribution) Ltd] |
| 58962 | Warfarin 3-mg tablets (DE Pharmaceuticals) |
| 59400 | Warfarin 500-μg tablets (Sigma Pharmaceuticals plc) |
| 59578 | Warfarin 3-mg tablets (Phoenix Healthcare Distribution Ltd) |
| 59761 | Heparin sodium 1000 IU/1-ml solution for injection ampoules (Wockhardt UK Ltd) |
| 60041 | Danaparoid sodium 750 IU/0.6-ml solution for injection ampoules |
| 60188 | Heparin sodium 5000 IU/1-• infusion bags |
| 60589 | Warfarin 500-μg tablets (Actavis UK Ltd) |
| 60949 | Warfarin 5-mg/5-ml oral suspension |
| 61949 | Fondaparinux sodium 10-mg/0.8-ml solution for injection pre-filled syringes |
| 62150 | Rivaroxaban 2.5-mg tablets |
| 62309 | Warfarin 500-μg tablets (Kent Pharmaceuticals Ltd) |
| 62310 | Warfarin 500-μg tablets (AMCo) |
| 62856 | Tinzaparin sodium 12,000 IU/0.6-ml solution for injection pre-filled syringes |
| 62902 | Tinzaparin sodium 16,000 IU/0.8-ml solution for injection pre-filled syringes |
| 62959 | Heparin calcium 5000 IU/0.2-ml solution for injection ampoules (AAH Pharmaceuticals Ltd) |
| 63071 | Warfarin 4-mg tablets |
| 63101 | Tinzaparin sodium 8000 IU/0.4-ml solution for injection pre-filled syringes |
| 63146 | Heparin sodium 20,000 IU/20-ml solution for injection ampoules |
| 63169 | Innohep 12,000 IU/0.6-ml solution for injection pre-filled syringes (LEO Pharma) |
| 63297 | Heparin sodium 5000 IU/5-ml solution for injection vials (LEO Pharma) |
| 63440 | Epoprostenol 500-μg powder and solvent (pH 10.5) for solution for infusion vials |
| 63571 | Innohep 16,000 IU/0.8-ml solution for injection pre-filled syringes (LEO Pharma) |
| 64133 | Heparin sodium 5000 IU/0.2-ml solution for injection ampoules (AAH Pharmaceuticals Ltd) |
| 64315 | Anticoagulant solution ACD-A 500-ml bags (Haemonetics® Ltd, Boston, MA, USA) |
| 64500 | Xarelto 2.5-mg tablets (Bayer plc) |
| 64559 | Heparin sodium 1000 IU/1-ml solution for injection ampoules (AAH Pharmaceuticals Ltd) |
| 64581 | Innohep 8000 IU/0.4-ml solution for injection pre-filled syringes (LEO Pharma) |
| 64678 | Edoxaban 60-mg tablets |
| 64969 | Clexane 20-mg/0.2-ml solution for injection pre-filled syringes (Sigma Pharmaceuticals plc) |
| 64998 | Epoprostenol 1.5-mg powder and solvent (pH 10.5) for solution for infusion vials |
| 65247 | Edoxaban 30-mg tablets |
| 65285 | Warfarin 1-mg tablets (Crescent Pharma Ltd, Basingstoke, UK) |
| 65496 | Warfarin 500-μg tablets (Phoenix Healthcare Distribution Ltd) |
| 65538 | Elmiron® 100-mg capsules [imported (USA)] |
| 65746 | Warfarin 500-μg tablets (DE Pharmaceuticals) |
| 65850 | Lixiana® 60-mg tablets (Daiichi Sankyo UK Ltd, Uxbridge, UK) |
| 65876 | Edoxaban 15-mg tablets |
| 66286 | Warfarin 2.5-mg/5-ml oral solution |
| 66529 | Lixiana 30-mg tablets (Daiichi Sankyo UK Ltd) |
| 66570 | Warfarin 1-mg tablets (Waymade Healthcare plc) |
| 68591 | Warfarin 500-μg tablets [Alliance Healthcare (Distribution) Ltd] |
| 68667 | Warfarin 5-mg capsules |
| 68795 | Warfarin 1-mg capsules |
| 69128 | Warfarin 500-μg/5-ml oral solution |
| 69194 | Heparin low molecular weight 5000-IU/0.2-ml sterile solution |
| 70831 | Phenindione 50-mg tablets (AMCo) |
| 70866 | Inhixa 40-mg/0.4-ml solution for injection pre-filled syringes (Techdow Pharma England Ltd, Guildford, UK) |
| 71132 | Clexane 20-mg/0.2-ml solution for injection pre-filled syringes (DE Pharmaceuticals) |
| 71196 | Warfarin 1.5-mg/5-ml oral solution |
| 71274 | Inhixa 60-mg/0.6-ml solution for injection pre-filled syringes (Techdow Pharma England Ltd) |
| 71303 | Rivaroxaban 15-mg tablets and rivaroxaban 20-mg tablets |
| 71386 | Warfarin 1-mg/5-ml oral solution (special order) |
Appendix 6 Clinical Practice Research Datalink and Hospital Episode Statistics bleeding codes
| Medical code | Read code | Description | Type |
|---|---|---|---|
| 501 | R047.00 | [D] Epistaxis | ENT |
| 1557 | R047.11 | [D] Nosebleed | ENT |
| 2634 | 2D85.00 | O/E – blood in auditory canal | ENT |
| 4594 | 1C62.00 | Has nosebleeds – epistaxis | ENT |
| 5382 | 2D25.00 | O/E – epistaxis | ENT |
| 5785 | 1C6..11 | Epistaxis symptom | ENT |
| 5793 | 1C6..00 | Nosebleed symptom | ENT |
| 6958 | F586200 | Otorrhagia | ENT |
| 9395 | 1928.00 | Bleeding gums | ENT |
| 15540 | 1C6Z.00 | Nosebleed symptom NOS | ENT |
| 18281 | SP21300 | Primary post-tonsillectomy haemorrhage | ENT |
| 19221 | SP21400 | Secondary post-tonsillectomy haemorrhage | ENT |
| 26065 | F501G00 | Haemorrhagic otitis externa | ENT |
| 29281 | 2556 | O/E – bleeding gums | ENT |
| 38184 | 7404 | Surgical arrest of bleeding from internal nose | ENT |
| 38851 | R048.00 | [D] Throat haemorrhage | ENT |
| 42443 | 2D66.00 | O/E – blood from ear | ENT |
| 49563 | 2D65.00 | O/E – bloodstained ear discha | ENT |
| 51571 | 7405300 | Insertion of Brighton epistaxis balloon | ENT |
| 51717 | H5y0000 | Tracheostomy haemorrhage | ENT |
| 55166 | J017200 | Teeth staining due to pulpal bleeding | ENT |
| 62741 | 7404z00 | Surgical arrest of bleeding from internal nose NOS | ENT |
| 68624 | 7404y00 | Surgical arrest of bleeding from internal nose OS | ENT |
| 71829 | 2DE7.00 | O/E – throat haemorrhage | ENT |
| 621 | J573011 | Rectal bleeding | GI |
| 1642 | J68z.11 | GIB – Gastrointestinal bleeding | GI |
| 2044 | J510900 | Bleeding diverticulosis | GI |
| 2150 | J68z100 | Intestinal haemorrhage NOS | GI |
| 2814 | J12y100 | Unspec duodenal ulcer with haemorrhage | GI |
| 2832 | G848000 | Bleeding haemorrhoids NOS | GI |
| 3097 | J68..00 | Gastrointestinal haemorrhage | GI |
| 3600 | SE23111 | Perianal haematoma | GI |
| 3872 | J573.11 | Bleeding PR | GI |
| 4354 | J68z200 | Upper gastrointestinal haemorrhage | GI |
| 4636 | J68zz00 | Gastrointestinal tract haemorrhage NOS | GI |
| 6554 | J573012 | PRB – rectal bleeding | GI |
| 6574 | J573000 | Rectal haemorrhage | GI |
| 7096 | G844.11 | Perianal haematoma | GI |
| 9761 | G842000 | Internal bleeding haemorrhoids | GI |
| 11124 | J110111 | Bleeding acute gastric ulcer | GI |
| 11698 | 196C.00 | Painless rectal bleeding | GI |
| 11718 | 196B.00 | Painful rectal bleeding | GI |
| 12471 | J68z.00 | Gastrointestinal haemorrhage unspecified | GI |
| 15257 | G845000 | External bleeding haemorrhoids | GI |
| 15517 | J68z000 | Gastric haemorrhage NOS | GI |
| 16114 | J10y000 | Haemorrhage of oesophagus | GI |
| 18001 | J120100 | Acute duodenal ulcer with haemorrhage | GI |
| 18625 | J121111 | Bleeding chronic duodenal ulcer | GI |
| 19271 | J573.00 | Haemorrhage of rectum and anus | GI |
| 23813 | 7619100 | Gastrotomy and ligation of bleeding point of stomach | GI |
| 24989 | G850.00 | Oesophageal varices with bleeding | GI |
| 28366 | J12yy00 | Unspec duodenal ulcer; unspec haemorrhage and/or perforation | GI |
| 29492 | J150000 | Acute haemorrhagic gastritis | GI |
| 30054 | J110100 | Acute gastric ulcer with haemorrhage | GI |
| 32446 | J573100 | Anal haemorrhage | GI |
| 36583 | J111111 | Bleeding chronic gastric ulcer | GI |
| 44637 | J130100 | Acute peptic ulcer with haemorrhage | GI |
| 45304 | J130300 | Acute peptic ulcer with haemorrhage and perforation | GI |
| 45981 | 761D500 | Endoscopic injection haemostasis of duodenal ulcer | GI |
| 46479 | J573z00 | Haemorrhage of rectum and anus NOS | GI |
| 48730 | J120300 | Acute duodenal ulcer with haemorrhage and perforation | GI |
| 48951 | J121100 | Chronic duodenal ulcer with haemorrhage | GI |
| 53126 | J131100 | Chronic peptic ulcer with haemorrhage | GI |
| 57958 | J11y100 | Unspecified gastric ulcer with haemorrhage | GI |
| 60346 | J14y100 | Unspecified gastrojejunal ulcer with haemorrhage | GI |
| 63582 | J111100 | Chronic gastric ulcer with haemorrhage | GI |
| 63718 | 761D600 | Endoscopic injection haemostasis of gastric ulcer | GI |
| 70456 | J13y100 | Unspecified peptic ulcer with haemorrhage | GI |
| 62038 | 7609y11 | Tanner devascularisation for bleeding varices | GI |
| 71881 | J121300 | Chronic duodenal ulcer with haemorrhage and perforation | GI |
| 71897 | J111300 | Chronic gastric ulcer with haemorrhage and perforation | GI |
| 93436 | J12y300 | Unspecified duodenal ulcer with haemorrhage and perforation | GI |
| 94397 | J11yy00 | Unspec gastric ulcer; unspec haemorrhage and/or perforation | GI |
| 96622 | J13y300 | Unspecified peptic ulcer with haemorrhage and perforation | GI |
| 96628 | J140100 | Acute gastrojejunal ulcer with haemorrhage | GI |
| 96756 | G852000 | Oesophageal varices with bleeding in diseases EC | GI |
| 103474 | S73A100 | Perianal haematoma | GI |
| 3122 | 7736000 | Evacuation of perianal haematoma | GI |
| 71403 | J110300 | Acute gastric ulcer with haemorrhage and perforation | GI |
| 179 | K59z.11 | Breakthrough bleeding | GU |
| 183 | 158..12 | Vaginal bleeding | GU |
| 1039 | K59y300 | Intermenstrual bleeding | GU |
| 1583 | K5A1.00 | Postmenopausal bleeding | GU |
| 1941 | K597.00 | Postcoital bleeding | GU |
| 2283 | K596.00 | Metrorrhagia | GU |
| 2384 | K59yx11 | Dysfunctional uterine bleeding | GU |
| 3312 | K5C2.00 | Haematocolpos | GU |
| 3487 | K59y.11 | Metropathia haemorrhagica | GU |
| 3707 | 7D05200 | Evacuation of haematoma of vulva | GU |
| 5018 | K286v00 | Male genital haematoma NOS | GU |
| 5779 | K596.11 | Intermenstrual bleeding – irregular | GU |
| 5808 | K5E..00 | Other abnormal uterine and vaginal bleeding | GU |
| 6309 | K56y111 | Bleeding – vaginal NOS | GU |
| 6931 | 7D1C000 | Evacuation of haematoma from vagina | GU |
| 7733 | K19y411 | Urethral bleeding | GU |
| 9106 | 1584 | Heavy episode of vaginal bleeding | GU |
| 10118 | K19y400 | Bleeding from urethra | GU |
| 10425 | K59yx00 | Dysfunctional uterine haemorrhage NOS | GU |
| 11725 | K599.00 | Mid-cycle bleeding | GU |
| 12426 | K587.00 | Contact bleeding of cervix | GU |
| 15925 | K56y100 | Haemorrhage of vagina | GU |
| 16419 | K286w00 | Male genital haemorrhage NOS | GU |
| 16525 | K575.00 | Haematoma of vulva | GU |
| 21946 | K5E1.00 | Abnormal uterine bleeding, unspecified | GU |
| 23439 | SP03216 | Bleeding due to intrauterine contraceptive device | GU |
| 24349 | K286300 | Testicular haematoma – non-traumatic cause | GU |
| 25124 | K56y112 | BPV – vaginal bleeding | GU |
| 28242 | K5E2.00 | Abnormal vaginal bleeding, unspecified | GU |
| 29820 | SP03217 | Contraception IUCD causing bleeding | GU |
| 29903 | K59yy00 | Functional uterine haemorrhage NOS | GU |
| 31002 | K544.00 | Haematometra | GU |
| 31918 | K5E0.00 | Abnormal uterine bleeding unrelated to menstrual cycle | GU |
| 33676 | K5Ez.00 | Abnormal uterine and vaginal bleeding, unspecified | GU |
| 34757 | K566.00 | Vaginal haematoma | GU |
| 35767 | K55y300 | Haemorrhage of cervix | GU |
| 36070 | S760100 | Kidney haematoma without mention of open wound into cavity | GU |
| 36735 | K53y600 | Haematosalpinx | GU |
| 46997 | K59B.00 | Postmenopausal postcoital bleeding | GU |
| 47026 | K59A.00 | Premenopausal postcoital bleeding | GU |
| 48181 | K221100 | Prostatic haemorrhage | GU |
| 49111 | 66UI.00 | Hormone replacement therapy bleed pattern – abnormal | GU |
| 49162 | K286400 | Testicular haemorrhage | GU |
| 49487 | K537.00 | Haematoma of the broad ligament | GU |
| 50097 | K167.00 | Haemorrhage into bladder wall | GU |
| 52186 | K275200 | Corpus cavernosum haemorrhage | GU |
| 52215 | S761100 | Kidney haematoma with open wound into cavity | GU |
| 52896 | Kyu9D00 | [X] Other specified abnormal uterine and vaginal bleeding | GU |
| 62410 | 7E0F500 | Uterus operation haemostasis | GU |
| 71564 | 7B37400 | Open haemostasis of prostate | GU |
| 108636 | SP07R00 | Bleeding due to intrauterine contraceptive device | GU |
| 23601 | K221.00 | Prostatic congestion or haemorrhage | GU |
| 37882 | S760111 | Renal haematoma without mention of open wound into cavity | GU |
| 71783 | K221z00 | Prostatic congestion or haemorrhage NOS | GU |
| 48086 | K138100 | Renal artery haemorrhage | GU |
| 1786 | G60..00 | Subarachnoid haemorrhage | IC |
| 3535 | G61z.00 | Intracerebral haemorrhage NOS | IC |
| 4107 | 7032000 | Evacuation of extradural haematoma | IC |
| 4917 | 7017000 | Evacuation of subdural haematoma | IC |
| 5051 | G61..00 | Intracerebral haemorrhage | IC |
| 5682 | S62..00 | Cerebral haemorrhage following injury | IC |
| 6569 | S62..13 | Subdural haemorrhage following injury | IC |
| 7017 | 7004300 | Evacuation of intracerebral haematoma NEC | IC |
| 7862 | S629.00 | Traumatic subdural haematoma | IC |
| 8181 | S628.00 | Traumatic subdural haemorrhage | IC |
| 9696 | G604.00 | Subarachnoid haemorrhage from posterior communicating artery | IC |
| 13564 | G613.00 | Cerebellar haemorrhage | IC |
| 17734 | G622.00 | Subdural haematoma – non-traumatic | IC |
| 18411 | S62 A.00 | Traumatic extradural haematoma | IC |
| 19201 | G61X100 | Right-sided intracerebral haemorrhage, unspecified | IC |
| 19412 | G602.00 | Subarachnoid haemorrhage from middle cerebral artery | IC |
| 20284 | G62z.00 | Intracranial haemorrhage NOS | IC |
| 23580 | G60z.00 | Subarachnoid haemorrhage NOS | IC |
| 27661 | S62..11 | Extradural haemorrhage following injury | IC |
| 28077 | S62..14 | Traumatic cerebral haemorrhage | IC |
| 28314 | G61X000 | Left-sided intracerebral haemorrhage, unspecified | IC |
| 28807 | S62..12 | Subarachnoid haemorrhage following injury | IC |
| 28914 | 662o.00 | Haemorrhagic stroke monitoring | IC |
| 30045 | G616.00 | External capsule haemorrhage | IC |
| 30202 | G617.00 | Intracerebral haemorrhage, intraventricular | IC |
| 31060 | G61X.00 | Intracerebral haemorrhage in hemisphere, unspecified | IC |
| 31500 | 7004100 | Evacuation of haematoma from temporal lobe of brain | IC |
| 31595 | G610.00 | Cortical haemorrhage | IC |
| 31805 | G62..00 | Other and unspecified intracranial haemorrhage | IC |
| 35867 | S630.12 | Intracranial haematoma following injury | IC |
| 36178 | G620.00 | Extradural haemorrhage – non-traumatic | IC |
| 38304 | S620.00 | Closed traumatic subarachnoid haemorrhage | IC |
| 39274 | K138300 | Intrarenal haematoma | IC |
| 40338 | G611.00 | Internal capsule haemorrhage | IC |
| 41910 | G605.00 | Subarachnoid haemorrhage from basilar artery | IC |
| 42283 | S63z.00 | Other cerebral haemorrhage following injury NOS | IC |
| 42331 | G603.00 | Subarachnoid haemorrhage from anterior communicating artery | IC |
| 42581 | 25T0.00 | Bleeding stoma | IC |
| 43418 | S624.11 | Epidural haematoma following injury | IC |
| 43682 | 7004200 | Evacuation of haematoma from cerebellum | IC |
| 45421 | S624.00 | Closed traumatic extradural haemorrhage | IC |
| 45489 | ZA13600 | Drainage of subungual haematoma | IC |
| 45670 | K275100 | Corpus cavernosum haematoma | IC |
| 46152 | 7J01300 | Reopen cranium re-exploration op site arrest post op bleeding | IC |
| 46179 | 7008200 | Aspiration of haematoma of brain tissue | IC |
| 46316 | G612.00 | Basal nucleus haemorrhage | IC |
| 46545 | S62z.00 | Cerebral haemorrhage following injury NOS | IC |
| 51504 | S626.00 | Epidural haemorrhage | IC |
| 52968 | S63..00 | Other cerebral haemorrhage following injury | IC |
| 53810 | Gyu6200 | [X] Other intracerebral haemorrhage | IC |
| 53980 | S629000 | Traumatic subdural haematoma without open intracranial wound | IC |
| 56007 | G601.00 | Subarachnoid haemorrhage from carotid siphon and bifurcation | IC |
| 57315 | G618.00 | Intracerebral haemorrhage, multiple localised | IC |
| 58545 | S627.00 | Traumatic subarachnoid haemorrhage | IC |
| 60692 | G606.00 | Subarachnoid haemorrhage from vertebral artery | IC |
| 65745 | Gyu6100 | [X] Other subarachnoid haemorrhage | IC |
| 73471 | S625.00 | Open traumatic extradural haemorrhage | IC |
| 94351 | S623.00 | Open traumatic subdural haemorrhage | IC |
| 96630 | Gyu6F00 | [X] Intracerebral haemorrhage in hemisphere, unspecified | IC |
| 96717 | S621.00 | Open traumatic subarachnoid haemorrhage | IC |
| 4273 | G621.00 | Subdural haemorrhage – non-traumatic | IC |
| 6960 | G61..11 | Cerebrovascular accident due to intracerebral haemorrhage | IC |
| 17326 | G60X.00 | Subarachnoid haemorrhage from intracranial artery, unspec | IC |
| 37249 | K13y800 | Perirenal haematoma | IC |
| 37250 | K16y200 | Bladder haemorrhage | IC |
| 2883 | S622.00 | Closed traumatic subdural haemorrhage | IC |
| 7912 | G614.00 | Pontine haemorrhage | IC |
| 18604 | G61..12 | Stroke due to intracerebral haemorrhage | IC |
| 18912 | G623.00 | Subdural haemorrhage NOS | IC |
| 1819 | G8y0.00 | Haemorrhage NOS | NS |
| 3020 | 7M0G400 | Evacuation of haematoma NEC | NS |
| 4028 | SE4z.11 | Haematoma NOS | NS |
| 5422 | SK02.12 | Secondary and recurrent haemorrhage | NS |
| 8775 | SP21.11 | Haematoma – post operative | NS |
| 9571 | SP21100 | Post-operative haemorrhage | NS |
| 16848 | 7H02200 | Reopen chest, re-explore intra-abdominal operation site, surg arr post-operative bleed | NS |
| 17825 | SP21.12 | Haemorrhage – post operative | NS |
| 18677 | SK02.00 | Secondary and recurrent haemorrhage | NS |
| 20828 | 7M0U400 | Reexploration of organ and surgical arrest post-operative bleeding NOC | NS |
| 20857 | SP21.00 | Perioperative haemorrhage or haematoma | NS |
| 27956 | TA0..11 | Accidental haemorrhage during medical care | NS |
| 28144 | 7H22600 | Reopen abdo re-explore intra-abdominal operation site surg arr post-operative bleed | NS |
| 28652 | SP21000 | Intraoperative haemorrhage | NS |
| 31521 | SP21200 | Post-operative haematoma formation | NS |
| 37772 | 851..00 | Haemorrhage control by packing | NS |
| 45372 | 7G2H400 | Liposuction removal of haematoma | NS |
| 49374 | SK02.11 | Secondary and recurrent haemorrhage | NS |
| 53054 | D305.00 | Haemorrhagic disorder due to circulating anticoagulants | NS |
| 63620 | D305000 | Haemorrhagic disorder due to antithrombinaemia | NS |
| 64687 | D305100 | Haemorrhagic disorder due to hyperheparinaemia | NS |
| 87845 | 7L1L300 | Haemostasis of unspecified organ | NS |
| 94146 | Ryu7300 | [X] Haemorrhage, NEC | NS |
| 712 | F4C7100 | Subconjunctival haemorrhage | O |
| 1105 | F4C7200 | Conjunctival haemorrhage NOS | O |
| 1201 | F4K2800 | Vitreous haemorrhage | O |
| 2629 | F404500 | Intraocular haemorrhage | O |
| 3039 | F42y500 | Retinal haemorrhage NOS | O |
| 3822 | 2BB8.00 | O/E – vitreous haemorrhages | O |
| 8742 | 2BB5.00 | O/E – retinal haemorrhages | O |
| 10779 | F42y.11 | Haemorrhage – retinal | O |
| 12615 | SE1..11 | Bruise of eye | O |
| 15464 | F436000 | Unspecified choroidal haemorrhage | O |
| 28763 | F436100 | Expulsive choroidal haemorrhage | O |
| 28765 | F42y400 | Subretinal haemorrhage | O |
| 29702 | FyuH400 | [X] Vitreous haemorrhage in diseases EC | O |
| 33360 | F4G3200 | Exophthalmos due to orbital haemorrhage | O |
| 37550 | F436.00 | Choroidal haemorrhage and rupture | O |
| 38180 | F4H4100 | Optic nerve sheath haemorrhage | O |
| 46591 | SE11.12 | Bruise of periocular tissue | O |
| 46938 | F42y100 | Superficial retinal haemorrhage | O |
| 59812 | F436z00 | Choroidal haemorrhage or rupture NOS | O |
| 69892 | F424300 | Retinal pigment epithelium haemorrhagic detachment | O |
| 71197 | F437200 | Haemorrhagic choroidal detachment | O |
| 71253 | F42y300 | Deep retinal haemorrhage | O |
| 16510 | 22E9.00 | O/E – subconjunctival haemorrhage | O |
| 21799 | F4K7.00 | Retrobulbar haemorrhage | O |
| 62342 | G615.00 | Bulbar haemorrhage | O |
| 1155 | SE...11 | Haematoma with intact skin | SST |
| 1372 | 16B3.00 | Spontaneous bruising | SST |
| 2400 | SE10.00 | Black eye NOS | SST |
| 4702 | K286000 | Scrotal haematoma – non-traumatic cause | SST |
| 5130 | SE4..11 | Leg bruise | SST |
| 6070 | 16B..00 | Bruising symptom | SST |
| 6711 | R027.11 | [D] Spontaneous bruising | SST |
| 7144 | SE43.11 | Toenail bruise | SST |
| 7183 | R09z000 | [D] Umbilical bleeding | SST |
| 7472 | SE46.00 | Traumatic haematoma | SST |
| 8197 | SE2..11 | Bruise, trunk | SST |
| 8845 | SE3..11 | Arm bruise | SST |
| 9740 | SE0..12 | Bruise of head | SST |
| 10764 | SE42011 | Heel bruise | SST |
| 10984 | SE22300 | Haematoma of rectus sheath | SST |
| 12142 | SE0..11 | Bruise of face | SST |
| 12729 | SE30011 | Shoulder bruise | SST |
| 15444 | K31y000 | Breast haematoma – non-traumatic cause | SST |
| 16949 | F503100 | Haematoma of pinna | SST |
| 20946 | SE24211 | Bruise of scrotum | SST |
| 21161 | SE11.11 | Bruise of eyelids | SST |
| 21263 | SE05.11 | Bruise of ear | SST |
| 22176 | F4Ey000 | Haemorrhage of eyelid | SST |
| 22651 | G77z000 | Capillary haemorrhage | SST |
| 23695 | 16BZ.00 | Bruising symptom NOS | SST |
| 24324 | K286100 | Scrotal haemorrhage | SST |
| 27711 | 16B2.00 | Bruises easily | SST |
| 28511 | SE4z.12 | Intramuscular haematoma NOS | SST |
| 34284 | SE06.00 | Bruise of mandibular joint area | SST |
| 36873 | 7303000 | Drainage of haematoma of external ear | SST |
| 37853 | ZA13700 | Drainage of subungual haematoma with hot wire | SST |
| 39516 | ZA13800 | Drainage of subungual haematoma with drill | SST |
| 39775 | SE05.12 | Bruise of auricle | SST |
| 87841 | 7303200 | Drainage haematoma external ear control cavity c bolster suture | SST |
| 97046 | 7G31400 | Drainage of subungual haematoma | SST |
| 3170 | SE33011 | Subungual haematoma | SST |
| 4398 | SE45.11 | Haematoma of leg | SST |
| 6191 | 2I15.00 | O/E – bruising | SST |
| 24981 | 16B4.00 | Post-traumatic bruising | SST |
| 7285 | R063100 | [D] Pulmonary haemorrhage NOS | Other |
| 7290 | 7M0G000 | Aspiration of haematoma of organ NOC | Other |
| 8239 | R063000 | [D] Cough with haemorrhage | Other |
| 9759 | G718.00 | Leaking abdominal aortic aneurysm | Other |
| 15534 | G530.00 | Haemopericardium | Other |
| 27337 | J56y000 | Haemoperitoneum – non-traumatic | Other |
| 39108 | S750100 | Spleen haematoma without mention of open wound into cavity | Other |
| 39575 | C063000 | Thyroid haemorrhage | Other |
| 46267 | S740100 | Liver haematoma and contusion without open wound into cavity | Other |
| 55153 | C154200 | Adrenal haemorrhage | Other |
| 64982 | S751100 | Spleen haematoma with open wound into cavity | Other |
| 65976 | C12y100 | Haemorrhage of parathyroid | Other |
| 24126 | G360.00 | Haemopericardium/current comp following acute MI | Other |
| ICD-10 78 | Description | Type |
|---|---|---|
| I85.0 | Oesophageal varices with bleeding | GI |
| K25.0 | Gastric ulcer, acute with haemorrhage | GI |
| K25.2 | Gastric ulcer, acute with both haemorrhage and perforation | GI |
| K25.4 | Gastric ulcer, chronic or unspecified with haemorrhage | GI |
| K25.6 | Chronic or unspecified with both haemorrhage and perforation | GI |
| K26.0 | Duodenal ulcer, acute with haemorrhage | GI |
| K26.2 | Duodenal ulcer, acute with both haemorrhage and perforation | GI |
| K26.4 | Duodenal ulcer, chronic or unspecified with haemorrhage | GI |
| K26.6 | Chronic or unspecified with both haemorrhage and perforation | GI |
| K27.0 | Peptic ulcer, acute with haemorrhage | GI |
| K27.2 | Peptic ulcer, acute with both haemorrhage and perforation | GI |
| K27.4 | Peptic ulcer, chronic or unspecified with haemorrhage | GI |
| K27.6 | Chronic or unspecified with both haemorrhage and perforation | GI |
| K28.0 | Gastrojejunal ulcer, acute with haemorrhage | GI |
| K28.2 | Acute with both haemorrhage and perforation | GI |
| K28.4 | Gastrojejunal ulcer, chronic or unspecified with haemorrhage | GI |
| K28.6 | Chronic or unspecified with both haemorrhage and perforation | GI |
| K29.0 | Acute haemorrhagic gastritis | GI |
| K62.5 | Haemorrhage of anus and rectum | GI |
| K66.1 | Haemoperitoneum | GI |
| K92.0 | Haematemesis | GI |
| K92.1 | Melaena | GI |
| K92.2 | Gastrointestinal haemorrhage, unspecified | GI |
| I60 | Subarachnoid haemorrhage | IC |
| I60.0 | Subarachnoid haemorrhage from carotid siphon and bifurcation | IC |
| I60.1 | Subarachnoid haemorrhage from middle cerebral artery | IC |
| I60.2 | Subarachnoid haemorrhage from anterior communicating artery | IC |
| I60.3 | Subarachnoid haemorrhage from posterior communicating artery | IC |
| I60.4 | Subarachnoid haemorrhage from basilar artery | IC |
| I60.5 | Subarachnoid haemorrhage from vertebral artery | IC |
| I60.6 | Subarachnoid haemorrhage from other intracranial arteries | IC |
| I60.7 | Subarachnoid haemorrhage from intracranial artery, unspecified | IC |
| I60.8 | Other subarachnoid haemorrhage | IC |
| I60.9 | Subarachnoid haemorrhage, unspecified | IC |
| I61 | Intracerebral haemorrhage | IC |
| I61.0 | Intracerebral haemorrhage in hemisphere, subcortical | IC |
| I61.1 | Intracerebral haemorrhage in hemisphere, cortical | IC |
| I61.2 | Intracerebral haemorrhage in hemisphere, unspecified | IC |
| I61.3 | Intracerebral haemorrhage in brain stem | IC |
| I61.4 | Intracerebral haemorrhage in cerebellum | IC |
| I61.5 | Intracerebral haemorrhage, intraventricular | IC |
| I61.6 | Intracerebral haemorrhage, multiple localised | IC |
| I61.8 | Other intracerebral haemorrhage | IC |
| I61.9 | Intracerebral haemorrhage, unspecified | IC |
| I62 | Other non-traumatic intracranial haemorrhage | IC |
| I62.0 | Subdural haemorrhage (acute) (non-traumatic) | IC |
| I62.1 | Non-traumatic extradural haemorrhage | IC |
| I62.9 | Intracranial haemorrhage (non-traumatic), unspecified | IC |
| I69.0 | Sequelae of subarachnoid haemorrhage | IC |
| I69.1 | Sequelae of intracerebral haemorrhage | IC |
| I69.2 | Sequelae of other non-traumatic intracranial haemorrhage | IC |
| S06.4 | Epidural haemorrhage | IC |
| N93.8 | Other specified abnormal uterine and vaginal bleeding | GU |
| N93.9 | Abnormal uterine and vaginal bleeding, unspecified | GU |
| R04.0 | Epistaxis | ENT |
| R04.1 | Haemorrhage from throat | ENT |
| R04.2 | Haemoptysis | Other |
| R04.8 | Haemorrhage from other sites in respiratory passages | Other |
| R04.9 | Haemorrhage from respiratory passages, unspecified | Other |
| I23.0 | Haemopericardium as current comp following acute MI | Other |
Appendix 7 Code lists for confounders
| Confounders | CPRD source | HES source |
|---|---|---|
| Bleeding outcomes | ||
| Year of event | Date of PCI/CABG or date of start of first episode with record of ACS | |
| Age | Patient details | – |
| Sex | Patient details | – |
| BMI | Height and weight in clinical details | – |
| Ethnic group | – | Patient data |
| Smoking | Clinical details | – |
| Previous MI | Clinical details | Diagnoses by episodes |
| Previous CABG or PCI | – | Procedures by episodes |
| Previous bleeding | Clinical details | – |
| Previous surgery | – | Procedures by episodes |
| IHD | Clinical details | Diagnoses by episodes |
| Diabetes | Clinical details | Diagnoses by episodes |
| Hypertension | Clinical details | Diagnoses by episodes |
| Hypercholesterolaemia | Clinical details | Diagnoses by episodes |
| Peripheral vascular disease | Clinical details | Diagnoses by episodes |
| Stroke | Clinical details | Diagnoses by episodes |
| Heart failure | Clinical details | Diagnoses by episodes |
| Peptic ulcer disease | Clinical details | Diagnoses by episodes |
| Chronic kidney disease | Clinical details | Diagnoses by episodes |
| Cancer | Clinical details | Diagnoses by episodes |
| Haematological disorder | Clinical details | Diagnoses by episodes |
| Anaemia | Clinical details | Diagnoses by episodes |
| Liver disease | Clinical details | Diagnoses by episodes |
| Valve disease (CABG only) | – | Diagnoses by episodes |
| NSAIDs | Therapy details | – |
| Steroids | Therapy details | – |
| PPIs | Therapy details | – |
| Anticoagulants | Therapy details | – |
| MACE outcomes | ||
| Year of event | Date of PCI/CABG or date of start of first episode with record of ACS | |
| Age | Patient details | – |
| Sex | Patient details | – |
| BMI | Height and weight in clinical details | – |
| Ethnic group | – | Patient data |
| Smoking | Clinical details | – |
| Previous MI | Clinical details | Diagnoses by episodes |
| Previous CABG or PCI | – | Procedures by episodes |
| Previous bleeding | Clinical details | – |
| Previous surgery | – | Procedures by episodes |
| IHD | Clinical details | Diagnoses by episodes |
| Diabetes | Clinical details | Diagnoses by episodes |
| Hypertension | Clinical details | Diagnoses by episodes |
| Hypercholesterolaemia | Clinical details | Diagnoses by episodes |
| Peripheral vascular disease | Clinical details | Diagnoses by episodes |
| Stroke | Clinical details | Diagnoses by episodes |
| Heart failure | Clinical details | Diagnoses by episodes |
| Chronic kidney disease | Clinical details | Diagnoses by episodes |
| Cancer | Clinical details | Diagnoses by episodes |
| Haematological disorder | Clinical details | Diagnoses by episodes |
| Anaemia | Clinical details | Diagnoses by episodes |
| Liver disease | Clinical details | Diagnoses by episodes |
| Valve disease (CABG only) | – | Diagnoses by episodes |
| Mortality outcomes | ||
| Year of event | Date of PCI/CABG or date of start of first episode with record of ACS | |
| Age | Patient details | – |
| Sex | Patient details | – |
| BMI | Height and weight in clinical details | – |
| Ethnic group | – | Patient data |
| Smoking | Clinical details | – |
| Charlson Comorbidity Index | Clinical details | Diagnoses by episodes |
Appendix 8 Search strategy for health economics literature review
Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINER(R)
Date range searched: 1946 to present.
Date searched: 18 November 2016.
-
atrial fibrillation/ or heart arrest/ or myocardial ischaemia/ or *acute coronary syndrome/ or coronary disease/ or coronary artery disease/ or *coronary thrombosis/ or *myocardial infarction/ or *thromboembolism/ or *thrombosis/ or “*coronary artery disease”/
-
acute coronary syndrome.ab,hw,kf,kw,ot,sh,ti,tw.
-
myocardial infarction.ab,hw,kf,kw,ot,sh,ti,tw.
-
coronary artery disease.ab,hw,kf,kw,ot,sh,ti,tw.
-
coronary thrombosis.ab,hw,kf,kw,ot,sh,ti,tw.
-
1 or 2 or 3 or 4 or 5
-
heart bypass, right/ or *angioplasty, balloon, coronary/ or *atherectomy, coronary/ or *coronary artery bypass/ or *angioplasty/ or *angioplasty, balloon/ or *percutaneous coronary intervention/
-
coronary artery bypass grafting.ab,hw,kf,kw,ot,sh,ti,tw.
-
coronary stent.ab,hw,kf,kw,ot,sh,ti,tw.
-
percutaneous coronary intervention.ab,hw,kf,kw,ot,sh,ti,tw.
-
coronary interventions.ab,hw,kf,kw,ot,sh,ti,tw.
-
heart bypass surgery.ab,hw,kf,kw,ot,sh,ti,tw.
-
7 or 8 or 9 or 10 or 11 or 12
-
platelet aggregation inhibitors/ or aspirin/ or aspirin, dipyridamole drug combination/ or dipyridamole/ or prasugrel hydrochloride/ or exp ticlopidine/
-
antiplatelet therapy.ab,hw,kf,kw,ot,sh,ti,tw.
-
dual antiplatelet therapy.ab,hw,kf,kw,ot,sh,ti,tw.
-
aspirin.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
clopidogrel.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
prasugrel.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
ticagrelor.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
14 or 15 or 16 or 17 or 18 or 19 or 20
-
anticoagulants/ or *warfarin/ or *dabigatran/ or *factor xa inhibitors/ or *rivaroxaban/
-
anticoagulant therapy.ab,hw,kf,kw,ot,sh,ti,tw.
-
vitamin k antagonists.ab,hw,kf,kw,ot,sh,ti,tw.
-
triple therapy.ab,hw,kf,kw,ot,sh,ti,tw.
-
warfarin.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
dabigatran.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
rivaroxaban.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
apixaban.ab,hw,kf,kw,ot,sh,ti,nm,tw.
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
-
21 and 30
-
exp “quality of life”/ or *comparative effectiveness research/ or *health status indicators/ or *self report/ or exp patient outcome assessment/
-
quality of life.ab,hw,kf,kw,ot,sh,ti,tw.
-
health-related quality of life.ab,hw,kf,kw,ot,sh,ti,tw.
-
health state utility$.ab,hw,kf,kw,ot,sh,ti,tw.
-
multi-attribute utilit$.ab,hw,kf,kw,ot,sh,ti,tw.
-
preference-based measure.ab,hw,kf,kw,ot,sh,ti,tw.
-
quality-adjusted life-years.ab,hw,kf,kw,ot,sh,ti,tw.
-
EQ-5D.ab,hw,kf,kw,ot,sh,ti,tw.
-
SF-6D.ab,hw,kf,kw,ot,sh,ti,tw.
-
HUI-III.ab,hw,kf,kw,ot,sh,ti,tw.
-
AQoL.ab,hw,kf,kw,ot,sh,ti,tw.
-
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42
-
hemorrhage/ or *ecchymosis/ or *epistaxis/ or *exsanguination/ or *gastrointestinal hemorrhage/ or *gingival hemorrhage/ or *uterine hemorrhage/
-
$bleeding$.ab,hw,kf,kw,ot,sh,ti,tw.
-
44 or 45
-
6 and 13 and 21 and 43 – total hits: 89
Update 21 November 2016 to 14 August 2017 – total hits: 3.
Update 21 August 2017 to 23 July 2018 – total hits: 0.
Total hits: 92.
PubMed
Date searched: 28 November 2016.
Date searched: 1996 to 28 November 2016.
-
(((((((((heart arrest[MeSH Terms]) OR myocardial ischaemia[MeSH Terms]) OR acute coronary syndrome[MeSH Terms]) OR coronary artery disease[MeSH Terms]) OR coronary thrombosis [MeSH Terms]) OR myocardial infarction[MeSH Terms]) OR thromboembolism[MeSH Terms]) OR coronary artery disease[MeSH Terms]) OR atrial fibrillation[MeSH Terms]) OR coronary disease[MeSH Terms]
-
acute coronary syndrome[Title/Abstract]
-
myocardial infarction[Title/Abstract]
-
coronary artery disease[Title/Abstract]
-
coronary thrombosis[Title/Abstract]
-
1 or 2 or 3 or 4 or 5
-
(((((((heart bypass, right[MeSH Terms]) OR heart bypass, left[MeSH Terms]) OR angioplasty, balloon, coronary[MeSH Terms]) OR atherectomy, coronary[MeSH Terms]) OR coronary artery bypass[MeSH Terms]) OR angioplasty[MeSH Terms]) OR angioplasty, balloon[MeSH Terms]) OR angioplasty, transluminal, percutaneous coronary[MeSH Terms]
-
coronary artery bypass grafting[Title/Abstract]
-
coronary stent[Title/Abstract]
-
percutaneous coronary intervention[Title/Abstract]
-
coronary intervention[Title/Abstract]
-
heart bypass surgery[Title/Abstract]
-
7 or 8 or 9 or 10 or 11 or 12
-
((((((blood platelet aggregation inhibitors[MeSH Terms]) OR platelet aggregation inhibitors[MeSH Terms]) OR aspirin[MeSH Terms]) OR dipyridamole[MeSH Terms]) OR ticlopidine[MeSH Terms]) OR antiplatelet agents[MeSH Terms]) OR antiplatelet drugs[MeSH Terms]
-
antiplatelet[Title/Abstract]
-
dual antiplatelet therapy[Title/Abstract]
-
aspirin[Title/Abstract]
-
clopidogrel[Title/Abstract]
-
prasugrel[Title/Abstract]
-
ticagrelor[Title/Abstract]
-
14 or 15 or 16 or 17 or 18 or 19 or 20
-
((anticoagulant agents[MeSH Terms]) OR anticoagulant drugs[MeSH Terms]) OR warfarin[MeSH Terms]
-
anticoagulant therapy[Title/Abstract]
-
vitamin k antagonists[Title/Abstract]
-
triple therapy[Title/Abstract]
-
warfarin[Title/Abstract]
-
dabigatran[Title/Abstract]
-
rivaroxaban[Title/Abstract]
-
apixaban[Title/Abstract]
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
-
21 and 30
-
(((((quality of life[MeSH Terms]) OR comparative effectiveness research[MeSH Terms]) OR index, health status[MeSH Terms]) OR health status indicator[MeSH Terms]) OR assessment, patient outcome[MeSH Terms]) OR life year, quality adjusted[MeSH Terms]
-
quality of life[Title/Abstract]
-
health-related quality of life[Title/Abstract]
-
health state utilit*[Title/Abstract]
-
multi-attribute utilit*[Title/Abstract]
-
preference-based measure[Title/Abstract]
-
quality-adjusted life-year*[Title/Abstract]
-
EQ-5D*[Title/Abstract]
-
SF-6D[Title/Abstract]
-
HUI-III[Title/Abstract]
-
AQoL[Title/Abstract]
-
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42
-
(((((ecchymosis[MeSH Terms]) OR epistaxis[MeSH Terms]) OR exsanguination[MeSH Terms]) OR gastrointestinal hemorrhage[MeSH Terms]) OR gingival hemorrhage[MeSH Terms]) OR uterine hemorrhage[MeSH Terms]
-
*bleeding*[Title/Abstract]
-
44 or 45
-
6 and 13 and 21 and 43 – total hits: 321
Update 5 December 2016 to 14 August 2017 – total hits: 23.
Update 21 August 2017 to 23 July 2018 – total hits: 17.
Total hits: 361.
Appendix 9 Different sequences of the six EuroQol-5 Dimensions questionnaires for the patient elicitation exercise
| Order of the questionnaires | ||||||
|---|---|---|---|---|---|---|
| Sequence Number | First questionnaire | Second questionnaire | Third questionnaire | Fourth questionnaire | Fifth questionnaire | Sixth questionnaire |
| 1 | EQ-5D-3L baseline | EQ-5D-5L baseline | EQ-5D-3L vignette A | EQ-5D-3L vignette B | EQ-5D-5L vignette A | EQ-5D-5L vignette B |
| 2 | EQ-5D-5L baseline | EQ-5D-3L baseline | EQ-5D-5L vignette A | EQ-5D-5L vignette B | EQ-5D-3L vignette A | EQ-5D-3L vignette B |
| 3 | EQ-5D-3L baseline | EQ-5D-5L baseline | EQ-5D-3L vignette B | EQ-5D-3L vignette A | EQ-5D-5L vignette B | EQ-5D-5L vignette A |
| 4 | EQ-5D-5L baseline | EQ-5D-3L baseline | EQ-5D-5L vignette B | EQ-5D-5L vignette A | EQ-5D-3L vignette B | EQ-5D-3L vignette A |
Appendix 10 Example participant study booklet
The participant study booklet contains a demographics questionnaire followed by two baseline EQ-5D questionnaires (EQ-5D-3L and EQ-5D-5L) for assessing the participants’ own health. Some participants completed the EQ-5D-3L first and some completed the EQ-5D-5L first, depending on the colour-coded study booklet randomly allocated to them at the beginning of the study. These questionnaires were completed before the focus group interviews commenced. On the subsequent pages, four more EQ-5D questionnaires were provided, each associated with one of two vignettes describing an individual experiencing either a minor or a major bleeding event while on antiplatelet therapy. Each EQ-5D questionnaire was prefaced with instructions on how the elicitation exercise should be completed, followed by one of the two vignettes. Vignette A described an individual experiencing a minor bleed, whereas vignette B described an individual experiencing a major bleed. At the bottom of each EQ-5D questionnaire, there was a supplementary question that asked the participant how long they would expect their HRQoL to be affected by the bleeding event described in the respective vignette. Each participant completed both a EQ-5D-3L and a EQ-5D-5L questionnaire for each of the two vignettes. The order in which they were completed depended on the colour-coded study booklet randomly allocated to them at the beginning of the study, in that some participants completed the EQ-5D for vignette A first and others completed it for vignette B first. The four EQ-5D questionnaires associated with the two vignettes were completed after the completion of the focus group interviews. It should be noted that the EuroQol Research Foundation approved the use of the modified EQ-5D questionnaires on 21 June 2017 for the conduct of this study.
Reproduced with permission from Doble et al. 9 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The following questionnaires include minor additions and formatting changes to the original documents.
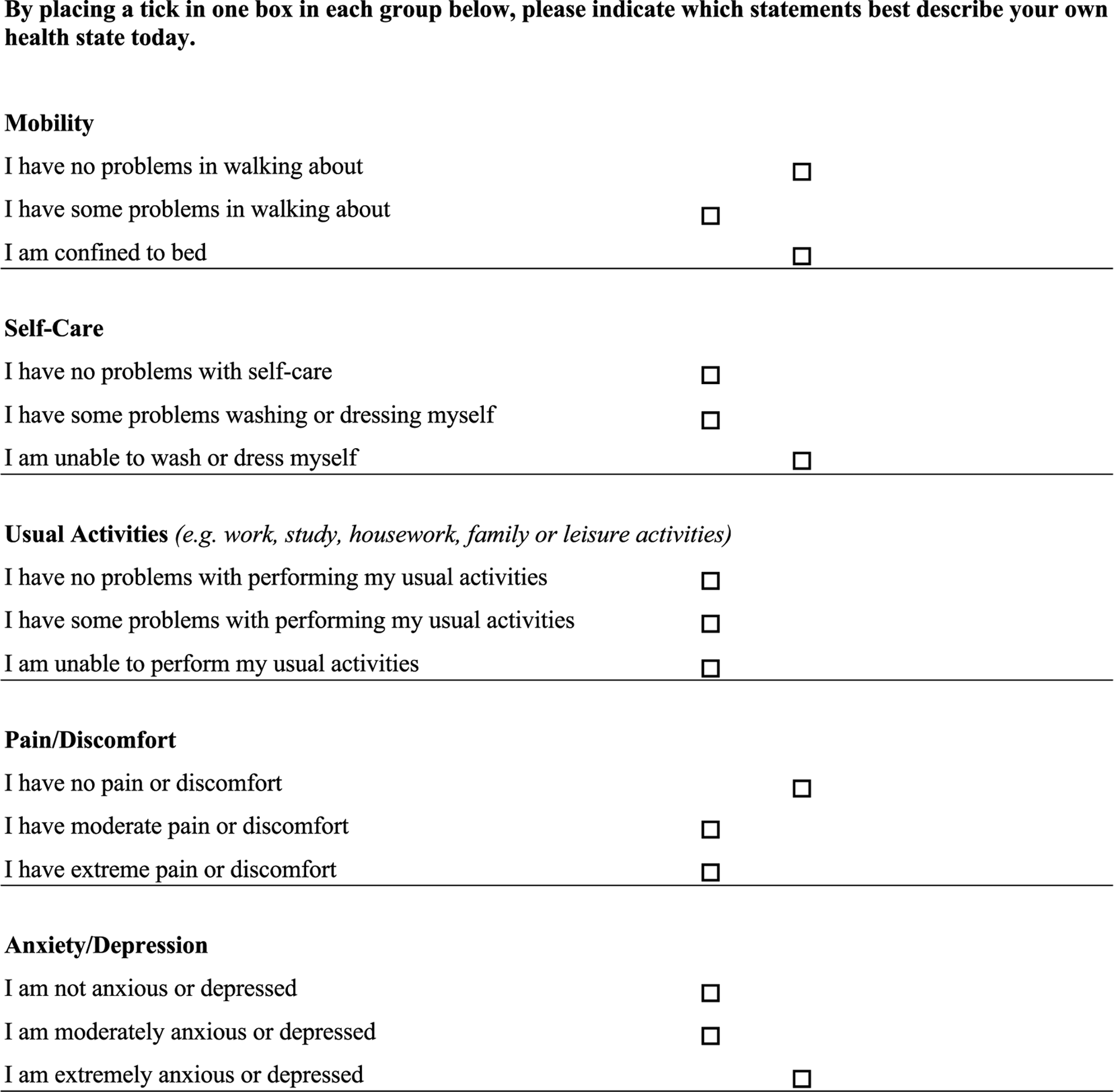
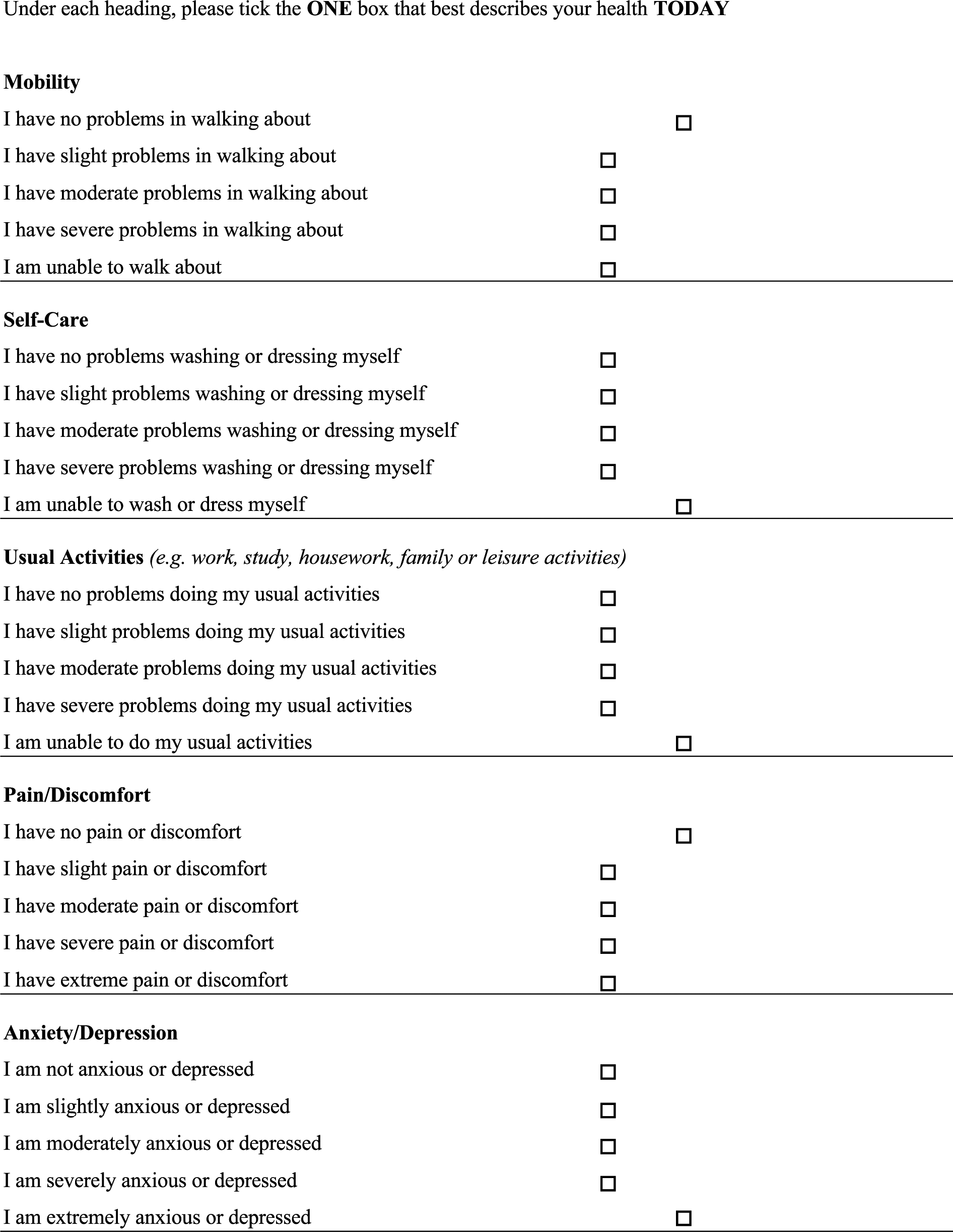

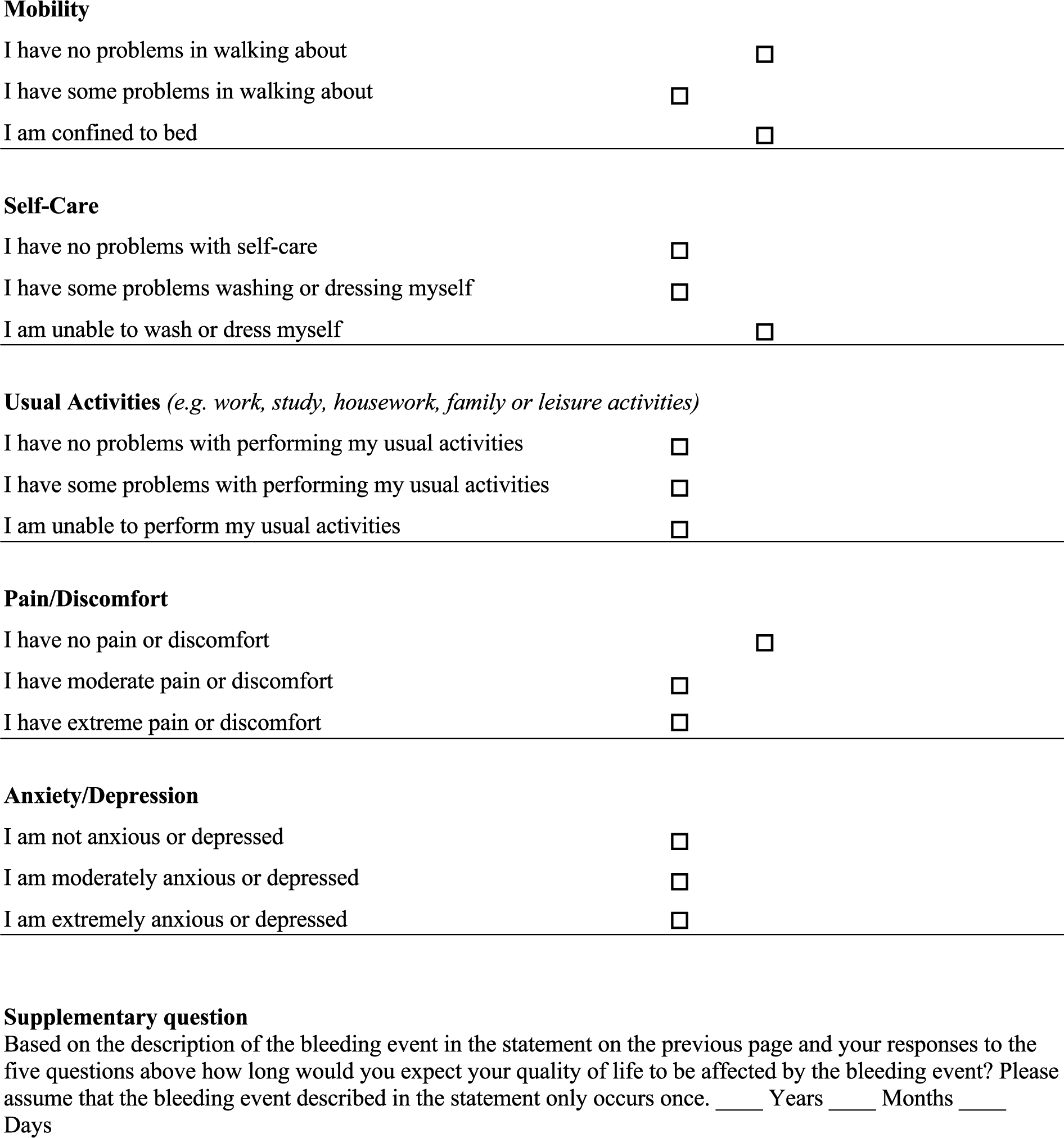

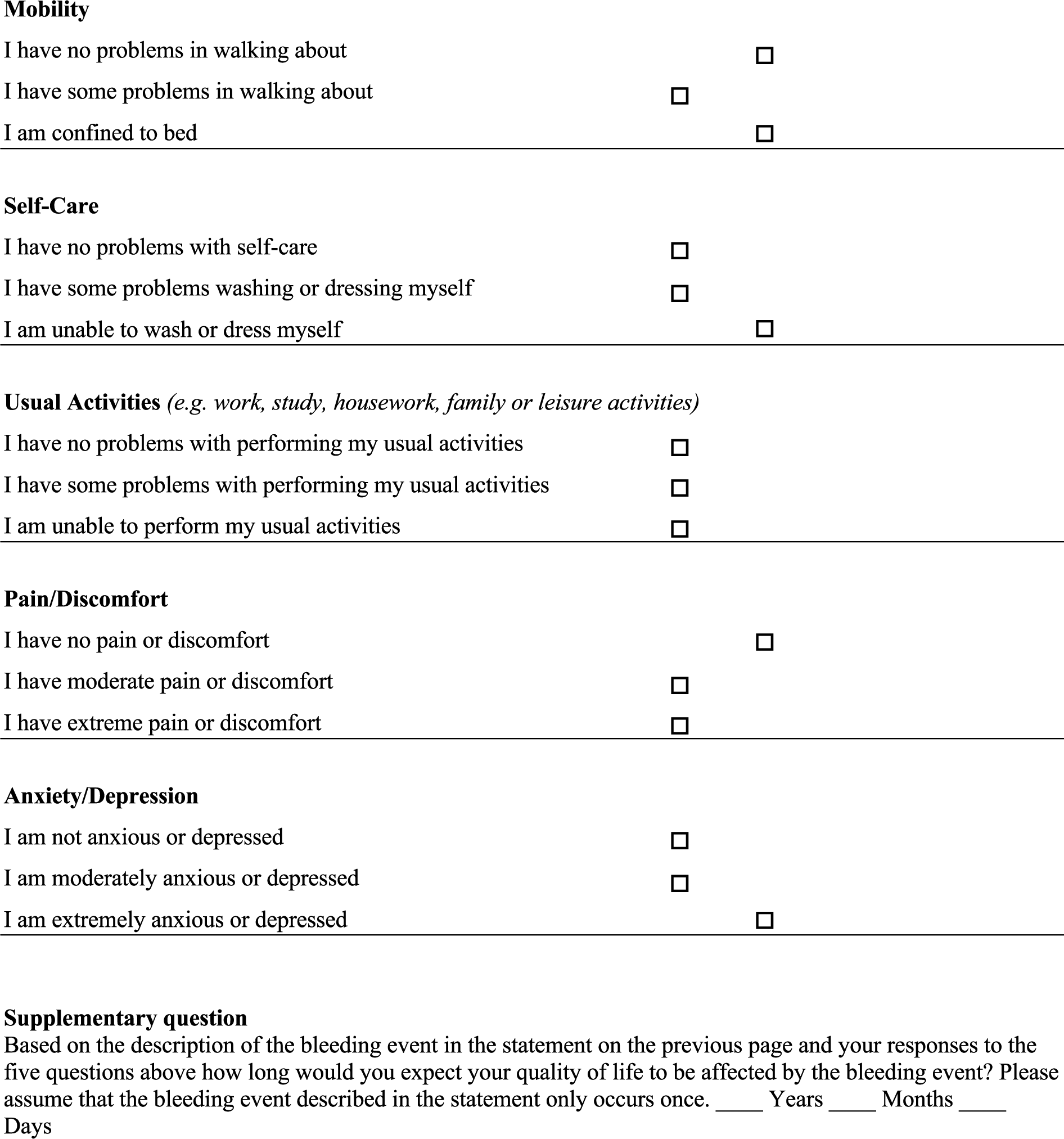


Appendix 11 Sources of utility decrements reported in the decision-analytic models
A summary of the sources of utility decrements reported in the decision-analytic model for DAPT is provided in Table 52. Only one study106 directly stated the source of/methods used to derive the reported decrements. Utility decrements were mainly derived based on assumptions101,105,107 or unpublished data from trial sponsors,104 or were listed as being obtained from a compendium of values;108,110 no utility decrements for bleeds were identified from these compendia. Three studies102,103,109 cited multiple references as the sources of the reported decrements and included one reference in common, namely a decision-analytic model that used a utility decrement of –0.03 for bleeds that result in short-term morbidity. 162 This decrement was derived from a consensus of three medical internists who designated a health-state utility value of 0.75 for 1-month or a utility decrement of –0.0208 for short-term morbidity bleeds in elderly patients with AF. 111 Other sources cited, identified after retrieving multiple references, used standard gamble methods to elicit utility values for major bleeds (0.841) from elderly patients with AF,112 an assumption of a utility value of 0.8 for 2 days or utility decrement of –0.00110 for a minor haemorrhage in patients with chronic AF164 or methods indiscernible based on an inaccessible report172 and utility values for bleeds not reported in the cited reference. 161
| Study | Source one and values reported | Source two and values reported |
|---|---|---|
| Greenhalgh et al.101 | Major bleed: | NA |
|
||
| Garg et al.102 | Minor bleed:
Extracranial major bleed:
|
|
| Kazi et al.103 | A number of references are listed under the general heading of bleeding, but no attempt has been made to assign specific reference to the different types of bleeding considered (minor, extracranial and CABG-related). In addition, no clear synthesis methods are described as to how the information from each of the references was used to obtain the final estimates
|
|
|
||
| Liew et al.104 | Minor and major bleeds: | NA |
|
||
| Gupta et al.105 | GI haemorrhage: | NA |
|
||
| Schleinitz and Heidenreich106 | GI bleed:
|
|
| Latour-Pérez et al.107 | Serious haemorrhage: | NA |
|
||
| Jiang and You108 | Non-fatal bleeding: | NA |
| Wang et al.109 | Major bleeding:
|
|
|
||
| Jiang and You110 | Non-fatal bleeding: | NA |
| Sullivan and Ghushchyan113 report utility decrements for a number of chronic conditions based on ICD-9 codes using the EQ-5D-3L in a US population; not clear where utility decrement for non-fatal bleeding was obtained as no such value is reported by Sullivan and Ghushchyan113 |
Appendix 12 Quality assessment and relevance of utility decrements from the included studies
The results of the quality and relevance assessment is provided in Table 53. Only three studies14,100,104 were judged to have patient characteristics very closely matched to our population of interest (i.e. post-coronary intervention on DAPT) and, therefore, were deemed to be of high relevance. The remaining studies used patients judged to be closely related (e.g. single-vessel disease treated with stenting or unstable angina on DAPT)105,106 or not to be closely related (e.g. general population, elderly AF or stroke patients and heart disease patients on anticoagulant therapy)101–103,107–110 and, therefore, were deemed to be of moderate and low relevance, respectively.
| Study | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category/Questions | Amin et al.14 | Amin et al.100 | Greenhalgh et al.101 | Garg et al.102 | Kazi et al.103 | Liew et al.104 | Gupta et al.105 | Schleinitz and Heidenreich106 | Latour-Pérez et al.107 | Jiang and You108 | Wang et al.109 | Jiang and You110 |
| Relevance to the decision problem | ||||||||||||
| How closely do the patient characteristics in the study match those described in the decision problem? | Very close; post-AMI on DAPT | Very close; post-PCI on DAPT | Not close; age-matched UK general population | Not close; elderly AF patients and US adults | Not close; elderly AF patients and US adults | Very close; post-ACS on DAPT | Close; single-vessel disease patients treated by stenting | Close; unstable angina or non-Q-wave MI on DAPT | Not close; heart disease patients on anticoagulant therapy | Not close; general population with self-reported medical diagnoses | Not close; elderly AF patients and stroke patients | Not close; general population with self-reported medical diagnoses |
| Does respondent selection and recruitment result in a population comparable to that being modelled? | Yes | Yes | Somewhat | No | No | Yes | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Unclear | NA; estimate based on expert consensus and unknown source | Unclear |
| Do the inclusion/exclusion criteria exclude any individuals? | Yes; if transferred from another facility > 25 hours after index event | Yes; those who died, missing baseline or 6-month data, incomplete or unvalidated hospital records | Yes; individuals in institutions, hostels, elderly homes or bed and breakfast accommodation | Yes; history of bleeds, falls and excessive alcohol intake | Yes; history of bleeds, falls and excessive alcohol intake | Yes; excluded if needed oral anticoagulation therapy or had risk of bradycardia | NA; estimate based on assumption | NA; estimates based on assumption | NA; estimate based on assumption | Not reported | NA; estimate based on expert consensus and unknown source |
Not reported |
| How closely do the inclusion criteria match people who would receive the intervention in routine practice? | Very close | Very close | Not close | Not close | Not close | Very close | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Unclear | NA; estimate based on expert consensus and unknown source | Unclear |
| Quality assessment: free from bias | ||||||||||||
| Is the precision of the estimate reflected in the variance around any estimate used in the model? | Yes | Yes | No | No | Yes; range noted for sensitivity analysis | No | No | Yes; range noted to create distribution | Yes; range noted for sensitivity analysis | Yes; range noted for sensitivity analysis | Yes; range noted for sensitivity analysis | Yes; range noted for sensitivity analysis |
| Are response rates reported and if so are the rates likely to be a threat to the validity of the estimated values? | No; potential threat to validity | No; potential threat to validity | Yes; 24% refused to take part; potential threat to validity | Yes; 23% of patients will not have AF diagnosis recorded in medical records; potential threat to validity; 11% refused participation | Yes; 23% of patients will not have AF diagnosis recorded in medical records; potential threat to validity; 11% refused participation | No; potential threat to validity | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | No; potential threat to validity | NA; estimate based on expert consensus and unknown source | No; potential threat to validity |
| How large is the loss to follow-up and are reasons given? | 16% with missing follow-up; no reasons provided | 16.5% with missing follow-up; no reasons provided | NA; no follow-up | NA; no follow-up | NA; no follow-up | Not reported | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Not reported | NA; estimate based on expert consensus and unknown source | Not reported |
| Are any losses to follow-up reported likely to threaten the validity of the estimates? | Potential threat to validity | Potential threat to validity | NA; no follow-up | NA; no follow-up | NA; no follow-up | Not reported | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Not reported | NA; estimate based on expert consensus and unknown source | Not reported |
| What are the levels of missing data and how are they dealt with? | Not reported | 25% missing data; excluded from study | Very small number of missing data points for each domain; unclear how they were handled | Not reported | Not reported | Not reported | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Not reported | NA; estimate based on expert consensus and unknown source | Not reported |
| Are there details on the causes of missing data? | No | Yes | No | Not reported | Not reported | Not reported | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Not reported | NA; estimate based on expert consensus and unknown source | Not reported |
| Could any missing data reported threaten the validity of the estimates? | Potential threat to validity | Potential threat to validity | Unlikely threat to validity | Potential threat to validity | Potential threat to validity | Potential threat to validity | NA; estimate based on assumption | NA; estimate based on assumption | NA; estimate based on assumption | Potential threat to validity | NA; estimate based on expert consensus and unknown source | Potential threat to validity |
| Utility values are measured and valued appropriately | ||||||||||||
| If valuation methods are used, are they used appropriately? | NA | NA | NA | Unclear; details not reported | Unclear; details not reported | NA | No; assumption based on average duration of hospitalisation | No; assumption no details | No; assumption based on clinical experience | NA | No; expert consensus | NA |
| Does the valuation method provide preference-based values anchored at 1 as equivalent to full health and 0 as equivalent to dead? | NA | NA | NA | Yes | Yes | NA | Unclear | Unclear | Unclear | NA | Unclear | NA |
| Are adequate details of the valuation method provided to allow judgement on appropriateness? | NA | NA | NA | No | No | NA | No | No | No | NA | No | NA |
| Are adequate details of the preference-based method provided? | Yes | Yes | Yes | NA | NA | Somewhat; no details of the tariff used with the EQ-5D | NA | NA | NA | Yes | NA | Yes |
| Was the generic preference-based measure delivered as intended? | Somewhat; used only EQ-5D VAS | Yes | Yes | NA | NA | Yes | NA | NA | NA | Yes | NA | Yes |
| Is the measure used for the group for which it was intended? | Yes | Yes | Yes | NA | NA | NA | Yes | NA | Yes | |||
| If a health state is valued using a vignette, can the appropriateness of the vignette be assessed? | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| In line with reimbursement agency requirements (i.e. align with the NICE reference case) | ||||||||||||
| Is the geographical area of recruitment relevant for the reimbursement agency? | USA; no | USA; no | UK; yes | UK and USA; somewhat | UK and USA; somewhat | USA; no | USA; no | USA; no | USA; no | USA; no | USA; no | USA; no |
| Does the measure used to collect utility values match the requirements of the decision problem and reimbursement agency? | Somewhat; used only EQ-5D VAS | Yes; EQ-5D-3L | Yes; EQ-5D-3L | No; combination of SG, Quality of Well-being Scale and TTO, and expert opinion | No; combination of SG, Quality of Well-being Scale and TTO, assumption and expert opinion | Yes; EQ-5D-3L | No; assumption | No; assumption | No; assumption | Yes; EQ-5D-3L | No; expert opinion and unknown source | Yes; EQ-5D-3L |
| Who completes the measure and does it satisfy the requirements of the decision problem and reimbursement agency? | Patients; yes | Patients; yes | General population; no | Patients/clinical assumption; somewhat | Patients/clinical assumption; somewhat | Patients; yes | Clinical assumption; no | Clinical assumption; no | Clinical assumption; no | General population/patients; somewhat | Clinical assumption; no | General population/patients; somewhat |
| Was mode of administration standardised across participants and in line with reimbursement agency requirements? | Yes | Unclear; via patient interview | Yes | Yes | Yes | Unclear; mode not specified | NA | NA | NA | Yes | NA | Yes |
| Who values the health states and does this satisfy the requirements of the reimbursement authority of interest? | Patients with VAS; no | General population; yes | General population; yes | Patients/clinical assumption; no | Patients/clinical assumption; no | Unclear; not reported | Clinical assumption; no | Clinical assumption; no | Clinical assumption; no | General population; yes | Clinical assumption; no | General population; yes |
| What techniques are used to value the health state and does this satisfy the requirements of the reimbursement authority of interest? | VAS; no | TTO, but US tariff; somewhat | TTO, UK tariff; yes | Combination of SG, TTO and expert consensus; somewhat | Combination of SG, TTO, assumption and expert consensus; somewhat | Unclear; not reported | Assumption based on the average duration of hospitalisation; no | Assumption with no details; no | Assumption based on clinical experience; no | TTO, but US tariff; somewhat | Combination of expert consensus and unknown source; somewhat | TTO, but US tariff; somewhat |
In terms of the quality/free-from-bias assessment, it was difficult to ascertain details concerning response rates, loss to follow-up and missing data for the majority of the studies. Even for studies that did report details for one or more of the characteristics,14,100–103 reasons for deficiencies or how they were accounted for were not reported. There were additional difficulties assessing the risk of bias for three of the studies,102,103,109 for which multiple sources were used in estimating the utility decrements and no details were provided concerning the synthesis methods used to combine the information. Three studies105–107 obtained utility decrements for bleeds based on assumptions, which made the questions concerning response rates, loss to follow-up and missing data not applicable. Overall, the identified studies were judged to be at high risk of bias, given the lack of detailed reporting.
Most studies using a generic preference-based instrument provided adequate details of the version and tariff used, delivered the instrument as intended and applied it to its intended population. 14,100,101,104,108,110 The remaining studies using valuation methods to elicit utility decrements (e.g. time-trade-off, standard gamble)102,103 or studies that based estimated utility decrements on assumptions/expert consensus105–107,109 provided very little detail to judge whether or not the approaches were appropriate.
Finally, none of the included studies was completely in line with the requirements for health-state utility values outlined in the NICE reference case. 90 The two studies that were the closest to the requirements were Greenhalgh et al. ,101 who used EQ-5D-3L utility values age-matched from the UK general population and applied an assumed utility decrement from these values for a bleeding event, and Amin et al. ,100 who used responses to the EQ-5D-3L from post-PCI patients receiving DAPT who experienced either minor or major bleeds, but used the US EQ-5D-3L tariff to derive utility decrements.
Appendix 13 Full regression results
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.120 | –0.252 to 0.0121 |
| Baseline health-state utility value | 0.776 | 0.473 to 1.0800 |
| Age | –0.00284 | –0.00936 to 0.00369 |
| Sex (male reference) | ||
| Female | 0.0327 | –0.306 to 0.372 |
| Intervention (PCI reference) | ||
| CABG | –0.0477 | –0.210 to 0.115 |
| Medical management | 0.0153 | –0.342 to 0.373 |
| Days since started DAPTa | 0.0000220 | –0.000455 to 0.000499 |
| Constant | 0.364 | –0.128 to 0.857 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.239 | –0.384 to –0.0933 |
| Baseline health-state utility value | 0.541 | 0.206 to 0.876 |
| Age | 0.00403 | –0.00316 to 0.0112 |
| Sex (male reference) | ||
| Female | –0.180 | –0.553 to 0.194 |
| Intervention (PCI reference) | ||
| CABG | –0.0741 | –0.253 to 0.105 |
| Medical management | –0.0848 | –0.478 to 0.309 |
| Days since started DAPTa | –0.000349 | –0.000875 to 0.000177 |
| Constant | 0.197 | –0.346 to 0.740 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.0863 | –0.175 to 0.00203 |
| Baseline health-state utility value | 0.762 | 0.456 to 1.0680 |
| Age | –0.00148 | –0.00583 to 0.00287 |
| Sex (male reference) | ||
| Female | 0.0489 | –0.178 to 0.276 |
| Intervention (PCI reference) | ||
| CABG | –0.0414 | –0.150 to 0.0675 |
| Medical management | 0.0132 | –0.225 to 0.000346 |
| Days since started DAPTa | 0.0000276 | –0.000291 to 0.000346 |
| Constant | 0.294 | –0.0853 to 0.674 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.164 | –0.260 to –0.0672 |
| Baseline health-state utility value | 0.536 | 0.202 to 0.869 |
| Age | 0.00308 | –0.00166 to 0.00782 |
| Sex (male reference) | ||
| Female | –0.102 | –0.349 to 0.146 |
| Intervention (PCI reference) | ||
| CABG | –0.0532 | –0.172 to 0.0655 |
| Medical management | –0.0461 | –0.306 to 0.214 |
| Days since started DAPTa | –0.000234 | –0.000581 to 0.000114 |
| Constant | 0.252 | –0.162 to 0.666 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.0514 | –0.129 to 0.0262 |
| Baseline health-state utility value | 0.760 | 0.570 to 0.950 |
| Age | –0.000527 | –0.00456 to 0.00350 |
| Sex (male reference) | ||
| Female | –0.0199 | –0.215 to 0.175 |
| Intervention (PCI reference) | ||
| CABG | –0.0168 | –0.115 to 0.0811 |
| Medical management | –0.205 | –0.408 to –0.00156 |
| Days since started DAPTa | –0.000109 | –0.000389 to 0.000171 |
| Constant | 0.259 | –0.0247 to 0.542 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.193 | –0.315 to –0.0713 |
| Baseline health-state utility value | 0.508 | 0.204 to 0.812 |
| Age | 0.00111 | –0.00509 to 0.00732 |
| Sex (male reference) | ||
| Female | 0.0217 | –0.288 to 0.331 |
| Intervention (PCI reference) | ||
| CABG | 0.000178 | –0.149 to 0.149 |
| Medical management | –0.178 | –0.503 to 0.147 |
| Days since started DAPTa | –0.000101 | –0.000546 to 0.000344 |
| Constant | 0.331 | –0.107 to 0.769 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.0505 | –0.102 to 0.00105 |
| Baseline health-state utility value | 0.758 | 0.578 to 0.938 |
| Age | –0.000313 | –0.00300 |
| Sex (male reference) | ||
| Female | 0.00758 | –0.122 to 0.137 |
| Intervention (PCI reference) | ||
| CABG | –0.0135 | –0.0786 to 0.0515 |
| Medical management | –0.115 | –0.249 to 0.0197 |
| Days since started DAPTa | –0.0000605 | –0.000246 to 0.000125 |
| Constant | 0.242 | 0.0313 to 0.452 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.140 | –0.222 to –0.0584 |
| Baseline health-state utility value | 0.501 | 0.211 to 0.791 |
| Age | 0.000839 | –0.00327 to 0.00495 |
| Sex (male reference) | ||
| Female | 0.0216 | –0.186 to 0.229 |
| Intervention (PCI reference) | ||
| CABG | –0.00152 | –0.102 to 0.0985 |
| Medical management | –0.113 | –0.329 to 0.104 |
| Days since started DAPTa | –0.0000486 | –0.000346 to 0.000248 |
| Constant | 0.368 | 0.0357 to 0.700 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.0312 | –0.0992 to 0.0369 |
| Baseline health-state utility value | 0.708 | 0.516 to 0.901 |
| Age | –0.0000328 | –0.00356 to 0.00349 |
| Sex (male reference) | ||
| Female | 0.0259 | –0.144 to 0.196 |
| Intervention (PCI reference) | ||
| CABG | –0.0364 | –0.124 to 0.0509 |
| Medical management | –0.125 | –0.304 to 0.0531 |
| Days since started DAPTa | –0.0000942 | –0.000336 to 0.000147 |
| Constant | 0.280 | 0.0234 to 0.536 |
| Variable | Coefficient | 95% CI |
|---|---|---|
| Bleeding event identifier | –0.166 | –0.278 to –0.0549 |
| Baseline health-state utility value | 0.459 | 0.139 to 0.779 |
| Age | 0.00130 | –0.00435 to 0.00694 |
| Sex (male reference) | ||
| Female | 0.0131 | –0.269 to 0.295 |
| Intervention (PCI reference) | ||
| CABG | 0.0128 | –0.126 to 0.151 |
| Medical management | –0.159 | –0.456 to 0.138 |
| Days since started DAPTa | –0.0000126 | –0.000413 to 0.000387 |
| Constant | 0.366 | –0.0489 to 0.780 |
List of abbreviations
- AC
- aspirin and clopidogrel
- ACS
- acute coronary syndrome
- A&E
- accident and emergency
- AF
- atrial fibrillation
- AP
- aspirin and prasugrel
- AT
- aspirin and ticagrelor
- BARC
- Bleeding Academic Research Consortium
- BMI
- body mass index
- CABG
- coronary artery bypass grafting
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CURE
- Clopidogrel in Unstable Angina to Prevent Recurrent Events
- DAPT
- dual antiplatelet therapy
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- F
- female
- GFR
- glomerular filtration rate
- GLM
- generalised linear model
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IHD
- ischaemic heart disease
- IQR
- interquartile range
- LAD
- left anterior descending
- LIMA
- left internal mammary artery
- M
- male
- MACE
- major adverse cardiovascular event
- MDT
- multidisciplinary team
- MI
- myocardial infarction
- MPR
- medication possession ratio
- NICE
- National Institute for Health and Care Excellence
- NICOR
- National Institute for Cardiovascular Outcomes Research
- NOAC
- non-vitamin K oral anticoagulant
- NSAID
- non-steroidal anti-inflammatory drug
- NSTEMI
- non-ST elevation myocardial infarction
- OM
- obtuse marginal
- OPCS
- Office of Population Censuses and Surveys
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- PLATO
- Platelet Inhibition and Patient Outcomes
- PPI
- proton pump inhibitor
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROBINS-I
- Risk Of Bias In Non-randomized Studies – of Interventions
- RR
- relative risk
- SMD
- standardised mean difference
- STEMI
- ST-elevation myocardial infarction
- TRITON-TIMI 38
- Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38
- TT
- triple therapy
