Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/13/02. The contractual start date was in April 2018. The draft manuscript began editorial review in October 2022 and was accepted for publication in February 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Santer et al. This work was produced by Santer et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Santer et al.
Chapter 1 Introduction
Some text in this chapter has been reproduced with permission from Renz et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Acne vulgaris (hereon acne) is one of the most common inflammatory dermatoses seen globally. 2 Acne typically starts in adolescence with 15–20% of people affected showing moderate or severe acne, often persisting to adulthood. 3 Acne induced scarring occurs in approximately 20% of people with acne and negative social and psychological impact can be substantial. 4,5 The prevalence of acne in adult women is considerable6–10 and results in substantial health service use. 11
National Institute for Health and Care Excellence (NICE) guidance recommends topical combination preparations containing retinoids, benzoyl peroxide or antibiotics as first line treatment for mild/moderate acne or, for moderate/severe acne, a combination treatment alone or together with oral lymecycline or doxycycline. 12 The NICE guideline recommends that treatment regimens that include an antibiotic (topical or oral) should not be continued for more than 6 months unless exceptional circumstances apply, although other guidelines limit the use of oral antibiotics to 3 months. 13–15
In the UK, oral isotretinoin can be used under the supervision of a dermatologist for indications including severe acne, such as acne at risk of scarring that has not responded to other recommended treatments. Oral isotretinoin is a highly effective treatment for acne but may be contraindicated or deemed unacceptable by some due to concerns about potential serious adverse effects and teratogenicity, which requires robust pregnancy prevention management. In addition, referrals from primary to secondary care for oral isotretinoin often involve long waits, a situation worsened by the impact on health services of the coronavirus pandemic.
A third of people who consult with acne are prescribed prolonged courses of oral antibiotics16 and acne has been shown to account for the majority of antibiotic exposure amongst young people. 17 This may be because people with acne find topical treatments difficult to use, or experience side effects such as stinging or redness (although these can be mitigated with appropriate advice). 16 Sebum is integral to acne pathogenesis, yet antibiotics have no effect on sebum production18 and are therefore less effective than is sometimes estimated by clinicians19 and patients. 20 Rising rates of antibiotic resistance mean that non-antibiotic alternatives are urgently needed. 21,22
Spironolactone, a potassium-sparing diuretic, is widely used in the UK for indications including hypertension23 and has been prescribed off licence for women with acne for many years due to its anti-androgenic properties. US and European Guidelines suggest a role for spironolactone in the management of female acne. 13,14 Spironolactone is more widely used in the USA, where database studies have shown that women who have taken spironolactone for acne were found to receive almost three fewer months of oral antibiotics than those who were not. 24 However, despite expert opinion suggesting spironolactone has a role in acne management, systematic reviews have highlighted a paucity of high-quality evidence from randomised controlled trials (RCTs). 25,26
This trial aimed to answer whether spironolactone improves acne-related quality of life (QoL) in adult women with persistent facial acne compared to placebo. As we wished to evaluate spironolactone as it would be used in a real-life context in the clinical pathway, we chose a pragmatic trial design, which included concomitant use of topical treatments in both groups.
Chapter 2 Methods
Trial design
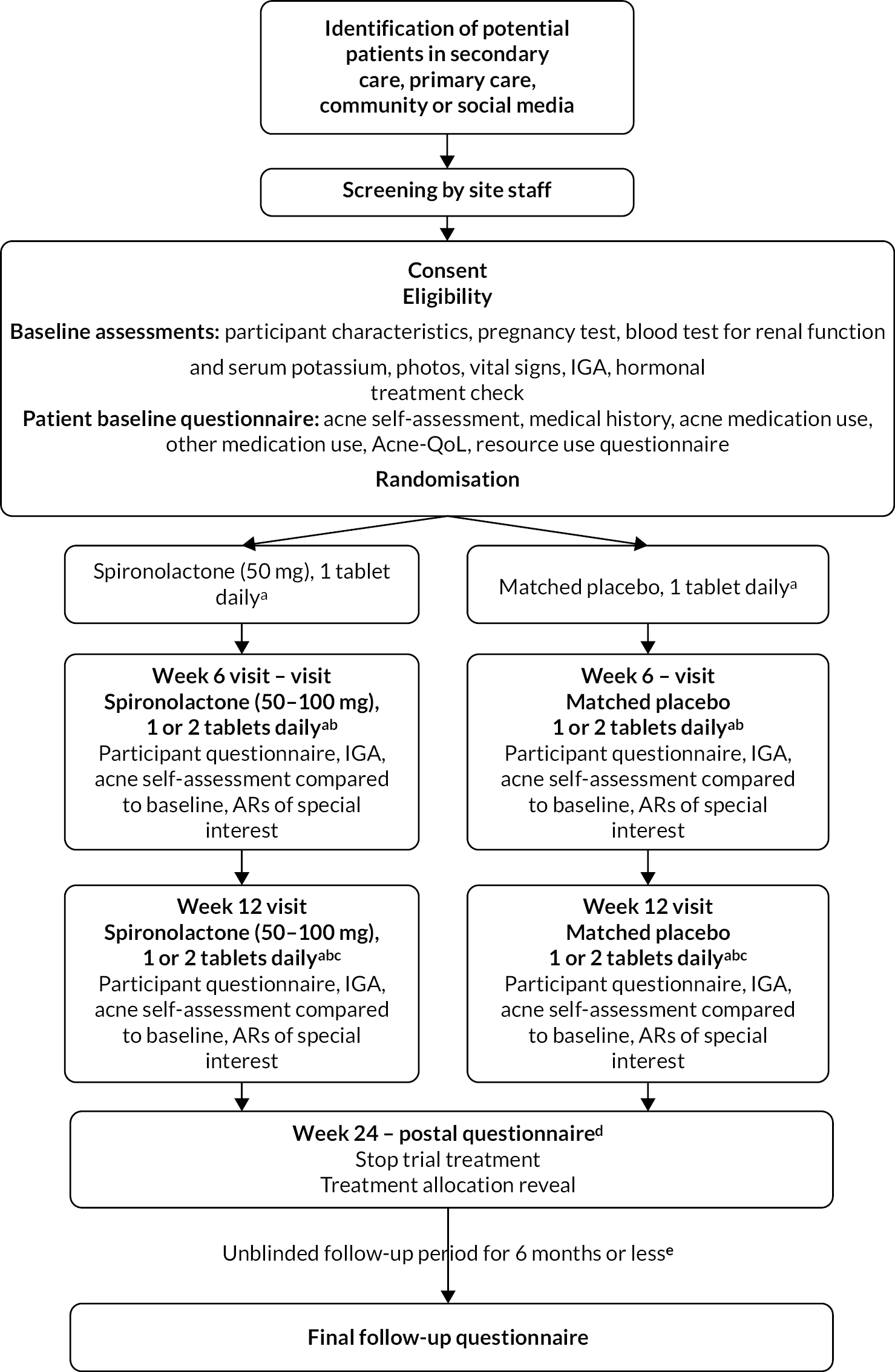
The spironolactone for adult female acne (SAFA) trial was a pragmatic, multicentre, double-blind, superiority RCT with two (1 : 1) parallel treatment groups – spironolactone plus standard topical therapy compared to placebo plus standard topical therapy – to investigate the clinical effectiveness (see Chapter 3) and cost-effectiveness (see Chapter 4) of spironolactone for persistent facial acne. A pragmatic trial design was chosen in order to test the intervention in a population as similar as possible to the context in which the intervention would be used clinically. 27,28 We therefore chose a participant-reported outcome measure, allowed concomitant use of topical treatments and pursued follow-up for as long a duration as possible. Because we wished to include clinically important outcomes to inform decision-making by health professionals and patients, we also looked systematically for adverse effects. The trial protocol paper was published in a peer-reviewed journal1 and is available in full (see www.fundingawards.nihr.ac.uk/award/16/13/02). The study design is represented in Figure 1.
FIGURE 1.
Trial flow diagram. AR, adverse reaction; IGA, Investigator’s Global Assessment. a, Allow use of topical therapy (creams/lotions/gels). b, Escalate dose if study tablet is tolerated, otherwise maintain on 1 tablet. c, Option to add antibiotic taken by mouth/change topical therapy if response to study tablet is inadequate. d, Participants in either arm may seek to use spironolactone or other acne treatments after this time. e, All participants are followed up until the last participant has reached week 24 after baseline.
Ethical approval for the trial was given by Wales Research Ethics Committee (REC) 3 in January 2019 (18/WA/0420). The trial was registered prospectively ISRCTN (ISRCTN12892056) and EudraCT (2018-003630-33).
Changes to protocol
Ethical approval for the trial was given by Wales Research Ethics Committee (REC) 3 in January 2019 (reference number: 18/WA/0420). The trial is registered on ISRCTN (ISRCTN12892056) and EudraCT (2018-003630-33). A table of changes made to the protocol is given in Appendix 1 (see Table 41). These can be summarised as:
-
clarification of exclusion criteria
-
changes to social media recruitment strategy
-
changes to recruitment material to attempt to improve response rates to mail-out (including development of brief summary sheet in addition to full participant information sheet, signposting to video)
-
adaptations to allow retention of research participants during COVID through flexible trial procedures, clinical trials unit (CTU) taking over some roles from recruiting sites and subsequent changes to reopen to recruitment following COVID
-
downward revision of target sample size to reflect the correlation of the Acne-Specific Quality of Life (Acne-QoL) subscale at 12 weeks with baseline
-
addition of qualitative substudy [not funded by Health Technology Assessment (HTA) and not reported further here].
Participants’ eligibility criteria
Participants were invited through primary care, secondary care and advertising through community and social media, and trial procedures were carried out in secondary care (further details below).
Eligibility criteria reflected safety (i.e. contraindications to spironolactone treatment, including intention to become pregnant), likely appropriateness of use of spironolactone to reflect real life context (e.g. acne sufficient to warrant oral treatment, not previously used spironolactone) and recent changes to therapy that may have impacted on acne (e.g. starting or stopping hormonal treatment within past 3 months).
Inclusion criteria
-
Women aged 18 years or over.
-
Facial acne vulgaris, symptoms present since at least 6 months.
-
Acne of sufficient severity to warrant treatment with oral antibiotics, as judged by the trial clinician; women with an Investigator’s Global Assessment (IGA) ≥ 2 were eligible to participate in the trial.
-
Women of childbearing potential at risk of pregnancy must be willing to use their usual hormonal or barrier method of contraception for the first 6 months of the trial [while taking the trial investigational medicinal product (IMP)] and for at least 4 weeks (approximately one menstrual cycle) afterwards.
-
Willing to be randomised to either treatment.
-
Willing and able to give informed consent.
-
Sufficient English to carry out primary care Acne-QoL.
Exclusion criteria
-
Acne grades 0–1 using IGA (i.e. clear or almost clear).
-
Has ever taken spironolactone.
-
Oral antibiotic treatment (lasting longer than 1 week) for acne within the past month.
-
Oral isotretinoin treatment within the past 6 months.
-
Started, stopped or changed long-term (lasting more than 2 weeks) hormonal contraception, co-cyprindiol or other hormonal treatment within the past 3 months.
-
Planning to start, stop or change long-term (lasting more than 2 weeks) hormonal contraception, co-cyprindiol or other hormonal treatment within the next 3 months.
-
Pregnant/breastfeeding.
-
Intending to become pregnant in the next 6 months.
-
Contraindicated to spironolactone:
-
currently taking potassium-sparing diuretic, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker or digoxin
-
hereditary problems of galactose intolerance, lactase deficiency or glucose–galactose malabsorption (as the spironolactone and placebo tablets contain lactose)
-
androgen-secreting adrenal or ovarian tumour
-
Cushing syndrome
-
congenital adrenal hyperplasia
-
estimated glomerular filtration rate below 60 ml/minute/1.73 m2
-
serum potassium level above the upper limit of reference range for the laboratory processing the sample.
-
Participant identification
Potential participants were identified through primary and secondary care, community advertising and social media advertising. All trial documentation such as invitation letters and patient information sheets directed potential participants to the trial specific website (see www.fundingawards.nihr.ac.uk/award/16/13/02) which provided information about the trial, eligibility questions, as well as contact details. CTU trial staff triaged interested potential patients to the closest open recruiting trial centre.
Primary care
Targeted mailings
General practitioner (GP) practices local to the recruiting trial sites were identified as participant identification centres (PICs). PICs searched patient lists for potential participants and sent the invitation packs using a secure online mailing service.
Opportunistic recruitment
General practitioners local to recruiting trial sites were provided with business cards containing broad eligibility criteria and a QR code linking to the trial-specific website. GPs were asked to give these cards to patients during consultations if the GPs felt that the patient may potentially be eligible.
Secondary care
Trial staff at recruitment sites screened referral letters and opportunistically invited patients in outpatient clinics. Where there was an available database and patients had given permission to be contacted about research, potential patients were contacted by the local site trial team by e-mail or mail-out.
Community advertising
The trial was advertised through media local to recruiting trial sites and advertising in appropriate institutions, such as posters in universities, pharmacies and hospitals, directing potential patients to the trial-specific website.
Social media advertising
Digital marketing company
An external digital marketing company conducted a social media advertising campaign (on Facebook/Instagram) which displayed advertisements to people who had shown an interest in acne or relevant organisations linked with the condition and who met the profile demographic (gender, age and location). The adverts were shown to people in the area of three initial trial centres. People interested in the trial were able to click on a link to the trial-specific website. If an individual no longer wanted to see the trial advert, they had the option of clicking a link that closed the advert and was not shown again. Adverts managed by the external digital marketing company were run over 3 non-consecutive months. The advertising campaign was stopped due to a pause in recruitment related to COVID-19 in March 2020.
In-house by Southampton clinical trial units
Social media advertising campaigns on Facebook and Instagram (co-ordinated in Facebook Ads Manager) were run in-house and co-ordinated by Southampton clinical trial units (CTU) from June 2020 to August 2021 (non-consecutive). Adverts were displayed to people who had shown an interest in acne or relevant organisations linked with the condition and who fitted the profile demographic (gender, age and geographical location). People interested in the trial could then click on a link to the trial-specific website. If an individual no longer wanted to see the trial advert, they could click a link that ensured it was not shown again.
Baseline and follow-up data collection
Baseline and follow-up appointments were carried out by secondary care dermatology centres to ensure standardisation of the clinical assessments, as Investigator Global Assessment of acne is not generally assessed in primary care and was an important secondary outcome. See Figure 1 for illustration of participant flow through the trial and Table 1 for schedule of observations.
| Outcome measure | 6 weeks | 12 weeks (primary end point) | 24 weeks (end of treatment) | Follow-up (52 weeks or sooner)a |
|---|---|---|---|---|
| Primary outcome measure | ||||
| Acne-QoL symptom subscale score | X | |||
| Secondary outcome measures | ||||
| Acne-QoL symptom subscale score | X | X | X | |
| Acne-QoL other subscalesb | X | X | X | X |
| Acne-QoL total score | X | X | X | X |
| Participant self-assessed overall improvementc | X | X | X | X |
| Investigator’s Global Assessmentd | X | X | ||
| Participant’s Global Assessmente | X | X | X | X |
| Participant satisfaction with trial treatmentf | X | |||
| Health-related quality of life using EQ-5D-5Lg | X | X | X | X |
| Resource use/costs incurred | X | X | X | X |
| Cost-effectivenessh | X | |||
Baseline assessment
After initial telephone contact from site staff to carry out initial eligibility check, potential participants were invited to attend a baseline appointment (face-to-face) at their local trial site. Baseline appointment included:
-
discussion of participant information sheet and written informed consent
-
clinician assessed the eligibility
-
pregnancy test
-
blood test for renal function and potassium level
-
height, weight, waist circumference, polycystic ovary syndrome (PCOS) characteristics, blood pressure
-
acne medication and oral medication use
-
Investigator’s Global Assessment (IGA) to assess acne severity
-
the site team also took photos on an instant camera or the participant took selfies of their acne in order to compare progress at the follow-up visits
-
questionnaires as outlined in schedule of observations (see Table 1).
Participants were offered a £20 voucher at baseline appointment to thank them for their time and a £10 voucher at subsequent appointments (weeks 6 and 12).
Follow-up
As shown in Figure 1, initial trial design included follow-up based on week-6 and week-12 visits which were initially intended to be held face-to-face in secondary care clinics, in order to discuss dose adjustment (see Interventions below), carry out IGA and reiterate contraceptive counselling. However, following redesign of trial delivery during the COVID-19 pandemic, sites were given the option of holding follow-up visits either remotely or face-to-face (if local trust guidance permitted). Additionally, (1) participants had the option to send digital photos of their acne for the clinician to assess during the consultation and grade the participant’s acne using IGA and (2) site teams had the option to post the trial medication directly to the participants. These modifications ensured that participants who had been recruited to the trial were not lost to follow-up and also allowed for flexibility during subsequent lockdowns.
Week 6 and 12 follow-up visits (remote or face-to-face)
Participants attended their week-6 and week-12 visit either remotely or face-to-face. If attending remotely, participants were asked to send photos (‘selfies’) of their face for the site team to score the IGA, providing the usual data protection measures of secure storage of the photos and prompt deletion (once no longer needed) were applied.
At week 6, the site team assessed treatment response and whether the participant was experiencing side effects from the study medication before escalating the dose to two tablets daily. If the dose hadn’t been increased to two tablets daily at week 6, treatment response was assessed again at week 12 and increased to two tablets daily. The participant’s GP was informed of the dose increase (if applicable).
At weeks 6 and 12, the participant completed a participant questionnaire on acne medication use, adverse reactions (ARs), self-assessment of acne improvement compared to baseline, resource use in the period between baseline and week 6, Acne-Quality of Life Questionnaire (Acne-QoL), EuroQol-5 Dimensions, five-level version (EQ-5D-5L) and NHS resource use in the period between baseline and week 6 and between week 6 and week 12.
The investigator assessed the participant’s acne using the IGA and recorded the number of remaining tablets in the IMP bottle prescribed at week 6 or week 12 respectively.
Week 24 (postal questionnaire)
The participant completed the week-24 questionnaire containing the Acne-QoL, EQ-5D-5L, self-assessment of acne improvement compared to baseline, number of tablets left from week 12 IMP bottle(s), acne medication use, ARs, serious adverse events (SAEs), satisfaction with the trial treatment and NHS resource use in the period between week 12 and week 24.
Unblinded follow-up period (questionnaire)
Participants were unblinded at week 24 (if returning the week 24 questionnaire) or at week 28 (if the week 24 questionnaire was not returned) and then entered an unblinded follow-up period. Originally, all participants were to be followed up for 52 weeks and would be sent the final follow-up questionnaire 52 weeks after baseline. However, in order to deliver the trial on time, the unblinded follow-up period was truncated so that, once the last participant received the week 24 questionnaire, all remaining participants who had not already received the final follow-up questionnaire were sent it at this point.
Interventions
Trial participants received one tablet per day (50 mg spironolactone or matched placebo) for the first 6 weeks of the trial. At or any time after the week-6 visit, the dose was escalated to two tablets daily (total 100 mg spironolactone or matched placebo) by the trial clinician, providing the participant was tolerating any side effects. All participants were instructed to take their total dose once per day in the morning to avoid diuresis later in the day.
All participants were instructed that they could continue to use their usual topical treatments throughout the trial, but adherence to topicals was not actively promoted. Participants were asked not to change their topical treatments or hormonal treatments between baseline and week 12.
Outcome measures
Primary outcome
The primary outcome was comparison of mean Acne-QoL symptom subscale score between groups at 12 weeks, adjusted for baseline variables. Of the many different participant-reported outcome measures available, the Acne-QoL is the most extensively validated. 29,30 The Acne-QoL contains 19 questions with 7 response categories, each referring to the past week, organised into 4 domains (self-perception, role-social, role-emotional, acne symptoms).
Effectiveness of acne treatments is usually judged clinically at 8–12 weeks so primary outcome at 12 weeks was chosen. A survey carried out prior to the trial (reported in the published protocol paper1) suggested that people with persistent acne may not be willing to stay on a trial treatment of unknown benefit for longer than 12 weeks, which is why this time point was chosen, with blinded treatment continuing to 24 weeks to assess medium term outcomes and continuing to follow-up participants beyond this to investigate longer-term outcomes.
No changes were made to trial outcomes after the trial commenced.
Secondary outcomes
-
Acne-QoL symptom subscale score at 6 weeks and at end of treatment (24 weeks). 29,30
-
Acne-QoL other subscales (self-perception, role-emotional and role-social), and total score, at 6, 12 and 24 weeks.
-
Participant self-assessed overall improvement at 6, 12 and 24 weeks recorded on a six-point Likert scale with photographs taken at the baseline visit to aid recall, as was carried out in the previous HTA-funded trial on acne. 31
-
FDA IGA of acne at 6 and 12 weeks (5-point scale: 0 – Clear; 1 – Almost clear; 2 – Mild; 3 – Moderate; 4 – Severe). 32
-
PGA at 6, 12 and 24 weeks (5-point scale same as IGA but written in plain language).
-
Participant satisfaction with trial treatment (asked prior to revealing treatment allocation at 24 weeks).
-
Health-related quality of life using EQ-5D-5L at 6, 12 and 24 weeks.
-
Cost at 6, 12 and 24 weeks (participant report) and cost-effectiveness over 24 weeks.
Other outcomes
-
Acne-QoL symptom subscale score at up to 52 weeks.
-
Acne-QoL other subscales (self-perception, role-emotional and role-social), and total score, at up to 52 weeks.
-
Participant self-assessed overall improvement at up to 52 weeks recorded on a six-point Likert scale.
-
Participant’s Global Assessment at up to 52 weeks.
-
ARs of special interest.
-
Use of other oral acne treatment (e.g. antibiotics, isotretinoin) (participant report).
-
Health-related quality of life using EQ-5D-5L up to 52 weeks.
-
Cost up to 52 weeks (participant report).
-
Participant experiences during the trial (qualitative interviews).
Sample size
Original sample size calculation
Based on comparison of mean Acne-QoL scores between groups at 12 weeks, power 90%, alpha 0.05 and seeking a difference between groups of two points on the symptom subscale [standard deviation (SD) 5.8, effect size 0.35], we calculated that 346 participants would be needed. Allowing for 20% loss to follow-up gave a total target of 434 participants (217 per group). 30,33
Revised sample size
No assumption was made initially regarding anticipated correlation between baseline and 12 weeks Acne-QoL subscale (r = 0). Following preliminary data, an adjustment was made on the basis of correlation between baseline and 12-week Acne-QoL subscale (r = 0.293). A deflation factor of 1-ρ2,34 allowing a reduction in the required sample size (including allowing for 20% loss to follow-up) to 398 participants (199 per group). This revision was approved in consultation with the Trial Steering Committee (TSC), Data Monitoring Committee, REC and the Funder.
Randomisation, concealment and blinding
Participants were randomised in a 1 : 1 ratio to either spironolactone plus standard topical therapy or matched placebo plus standard topical therapy, using an independent web-based system (TENELEA) using varying blocks of size two and four, stratified by recruitment centre and by baseline severity (IGA ˂ 3 vs. IGA 3 or 4).
Participants, local site staff and investigators were blind to treatment allocation until the end of the treatment phase (week 24). At this point participants and local site staff were unblinded. The research team remained blinded to treatment allocation until all analyses were complete and checked; however the statisticians were unblinded throughout the course of the trial.
Participants were asked prior to unblinding to guess which treatment they thought they had received, although it is noted that this may measure an individual’s prior belief in interventions’ effectiveness rather than a successful guess of treatment allocation.
Withdrawal, unblindings and protocol deviations
Participants were free to withdraw from the treatment or trial at any time without providing a reason:
-
participants could stop treatment but remain in follow-up (level 1 withdrawal)
-
participants could stop treatment and withdraw from follow-up (level 2 withdrawal)
-
participants could stop treatment and withdraw from follow-up and their data would not be used within the analysis (level 3 withdrawal).
Emergency unblinding was available in case of adverse events (AEs) where treatment allocation may affect the required participant care. If unblinding was performed, site staff informed Southampton Clinical Trial Unit (SCTU) trial team with details (date, time, reason for unblinding, name of staff requesting the code break and name of person breaking the code) and unblinding reports were filed in the participant medical records at site.
Serious adverse events were reviewed in a blinded manner.
If a participant became pregnant while taking part in the trial, the local site team was required to inform the SCTU as soon as they became aware of the pregnancy. The pregnancy itself was not a SAE; however the outcome of the pregnancy may be a SAE. When becoming pregnant, the participant was asked to stop taking the trial medication and was withdrawn from the trial (level 2 withdrawal).
If an informed consent for pregnancy follow-up was completed, the SCTU Quality and Regulatory team would enquire about the outcome of the pregnancy with the site staff.
If no informed consent for pregnancy follow-up was received, no further follow-up of the pregnancy outcome was possible.
There were a total of 206 protocol deviations (Table 2). Most of these related to the visit schedule, where appointments were late or cancelled, primarily due to the pandemic, meaning no IGA was recorded. The 31 deviations related to study procedure primarily related to the timing of tests at baseline or the timing of receiving IMP. The 12 deviations related to patient identifiable data involved the use of the safa@soton.ac.uk when not appropriate. The 15 deviations related to consent procedures related primarily to sites using an older version of the consent form.
| Reason for deviation | Number |
|---|---|
| Consent procedures | 15 |
| Case report form completion | 6 |
| Delegation log | 1 |
| Standard operating procedure adherence | 4 |
| Inclusion/exclusion | 5 |
| Lab assessments/procedures | 3 |
| Study procedures | 31 |
| SAE reporting | 3 |
| Registration/randomisation/unblinding | 5 |
| Study drug management | 13 |
| Visit schedule | 104 |
| Patient identifiable data | 12 |
| Other | 4 |
Statistical methods
A statistical analysis plan was written and reviewed by the TSC and Data Monitoring and Ethics Committee (DMEC) prior to the trial database being locked.
The complete cases population consisted of all participants who had been randomised to a treatment arm, regardless of compliance, and had complete data for the outcome and time point being analysed. The level of missing data is reported for all outcomes, unless otherwise stated. The frequency and pattern of missing data were examined, and a sensitivity analysis was carried out using a chained-equations multiple imputation model, including all outcomes and covariates used in the final analysis.
For the primary analyses, descriptive statistics were used to characterise recruited participants and assess baseline comparability between groups. For the primary outcome, a linear regression model was used to analyse Acne-QoL symptom subscale at week 12, adjusting for baseline variables (including stratification factors, baseline Acne-QoL symptom subscale score, topical treatment use, hormonal treatment use, age and PCOS status). A 95% confidence interval (CI) for the least squares mean difference between groups in Acne-QoL symptom subscale at week 12 was calculated. The same analysis methods were used to summarise Acne-QoL symptom subscale at other time points (weeks 6, 24 and up to week 52 after baseline) and for the other Acne-QoL subscales (self-perception, role-emotional and role-social) and total score.
Investigator’s Global Assessment and PGA at 6, 12 and 24 were dichotomised as success or failure as recommended by the US Food and Drug Administration32 (with success for IGA and Participant’s Global Assessment defined as clear or almost clear (grade 0 or 1) and at least a two-grade improvement from baseline; this represents a clinically meaningful outcome). The dichotomised outcomes were summarised by frequencies and percentages and compared by group using logistic regression, adjusting for stratification factors [recruitment centre and baseline acne severity (IGA < 3 vs. IGA ≥ 3)], baseline Acne-QoL symptom subscale score, topical treatment use (yes/no), hormonal treatment use (yes/no), age and PCOS status (as below).
Participants’ comparison with baseline photo at weeks 6, 12 and 24 weeks was presented by frequencies and percentages and compared by group using logistic regression with success defined as slight improvement/moderate improvement/excellent improvement/completely cleared and failure defined as no improvement/worse. Participants’ satisfaction with trial treatment was also presented with frequencies and percentages and compared by group using logistic regression.
Polycystic ovary syndrome status was defined as patients who stated they had a diagnosis or those who had suspected PCOS. Suspected PCOS was derived based on the Rotterdam criteria,35 where suspected PCOS is defined as having two or more of: oligo/anovulation (missed/infrequent periods), hyperandrogenism (evidence of excess facial and body hair or female pattern baldness) or polycystic ovaries on ultrasound. Since ultrasound was not performed in this study, participants needed to have both of the other criteria to qualify as having suspected PCOS.
Exploratory subgroup analyses were conducted to investigate how the treatment effect differs by whether participants have symptoms consistent with PCOS as recorded at the baseline visit, by age (below 25 years and 25 years and over),36 by higher and lower IGA scores at baseline (as per stratification), by the use of hormonal co-treatments (yes/no) and by the use of topical co-treatments (yes/no). Descriptive statistics were also used to observe the differences in mean Acne-QoL score pre- and post-COVID pandemic and amongst different ethnicities.
A complier-average causal effect (CACE) analysis was undertaken in order to compare ‘compliant’ participants (i.e. those who reported that they took their study medication) in the intervention group with those in the control group who would have complied with the intervention, given the opportunity to do so. Compliance was defined as taking at least 80% of the prescribed medication over the 12- to 24-week period. To explore the sensitivity of this analysis to the definition of ‘compliance’, we also completed two further sensitivity analyses defining compliance as taking 100% of the trial medication and 50% of the trial medication. Compliance was presented by frequencies and percentages and compared by groups with a single-equation instrumental-variables regression model adjusting for baseline variables.
Adverse reactions of special interest and SAEs were summarised by group with frequencies and percentages and compared with Pearson’s χ² tests.
The same analysis methods were applied to the outcomes collected at up to 52 weeks; however, the interpretation of these results was assessed with caution as participants were no longer blind to treatment use and could have started a different acne treatment. All analyses were carried out using Stata and/or SAS. No interim analysis was planned or conducted.
Health economics
For health economics methodologies, please see Chapter 4.
Trial oversight
The day-to-day management of the trial was co-ordinated through SCTU and through regular meetings with the Trial Management Group (TMG). The conduct of the trial was overseen by a TSC and a DMEC.
Chapter 3 Trial results
Trial population
A total of 1267 women were assessed for eligibility; 413 were consented and randomised from 10 hospitals in England and Wales. Recruitment period ran from 5 June 2019 to 31 August 2022 with a pause in recruitment from 23 March to 11 June 2020 due to the COVID-19 pandemic. All sites open by end of 2020 were affected by COVID-19, mostly due to staff redeployment and the pause of research at trusts, with each site closed for an average of 6 months with a range from 3 to 10 months (Table 3). Last participant’s final follow-up questionnaire was returned in March 2022 with data cleaning complete in April 2022.
| Site | Opened | Closed | Restart | Time closed total (months) |
|---|---|---|---|---|
| Queen Elizabeth Hospital (Birmingham) | 13 January 2020 | 23 March 2020 11 January 2021 |
16 October 2020 20 April 2021 |
7 |
| Bristol Royal Infirmary | 24 June 2019 | 23 March 2020 | 15 January 2021 | 3 |
| Epsom Hospital | 22 October 2019 | 23 March 2020 | 13 May 2021 | 10 |
| Harrogate District Hospital | 22 May 2019 | 23 March 2020 | 6 July 2020 | 4 |
| Poole General Hospital | 24 July 2019 | 23 March 2020 | 9 September 2020 | 6 |
| St Mary’s General Hospital (Portsmouth) | 17 June 2019 | 23 March 2020 | 21 July 2020 | 4 |
| Queen’s Medical Centre (Nottingham) | 19 October 2020 | 9 November 2020 | 10 May 2021 | 6 |
| University Hospital of Wales (Cardiff) | 28 October 2020 | 12 November 2020 | 5 May 2021 | 6 |
| Singleton Hospital (Swansea) | 13 January 2021 | N/A | N/A | N/A |
| St Mary’s Hospital (HQ) (London) | 21 May 2021 | N/A | N/A | N/A |
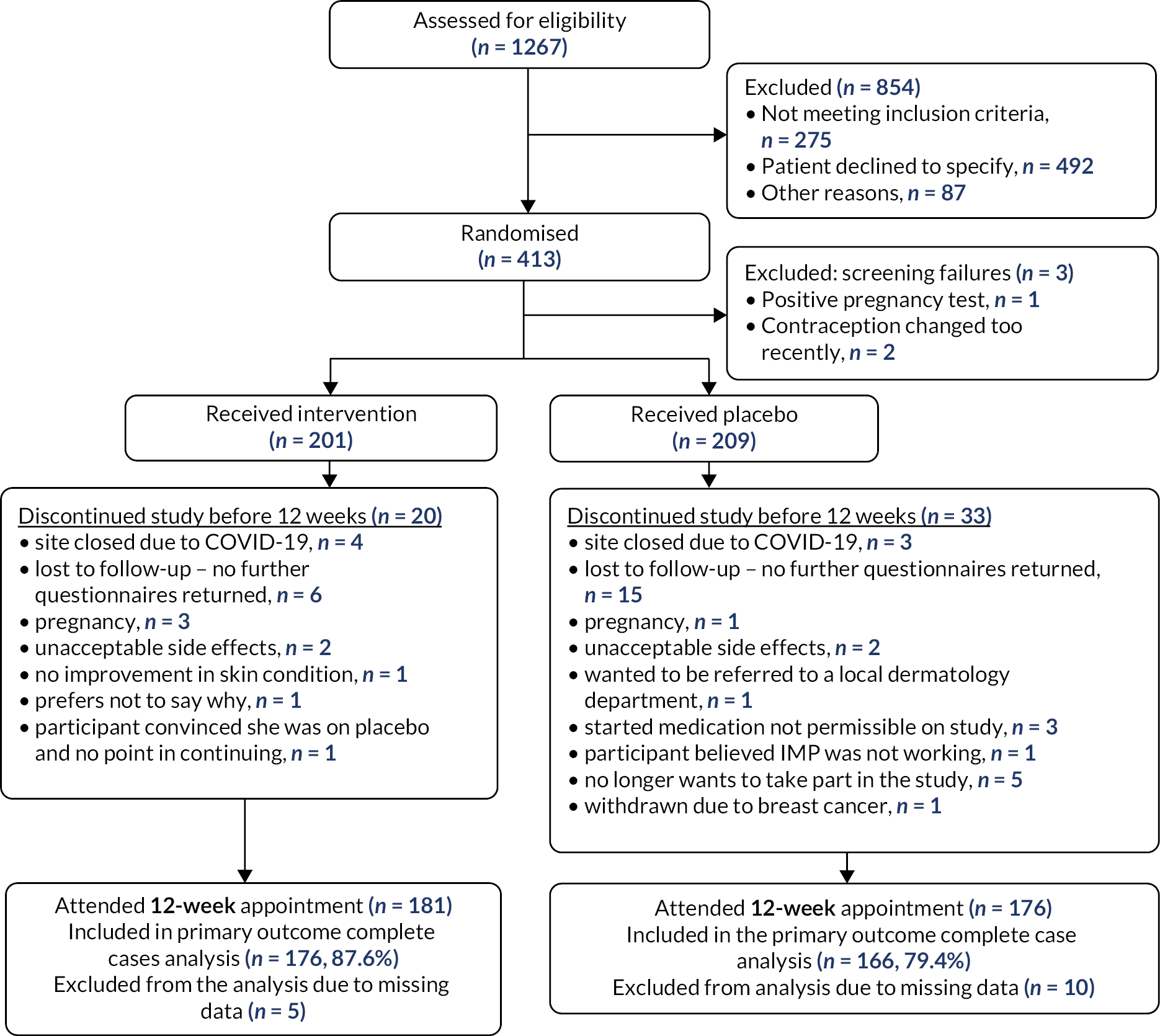
There were three post-randomisation screen failures (two where it was found after randomisation that contraception had been changed recently and the third where the pregnancy test was incorrectly done after randomisation) leading to 201 women being assigned the intervention and 209 being assigned placebo. Site closures due to COVID-19 resulted in the loss to follow-up of seven participants (Figure 2). Loss to follow-up was one of the main reasons for participant discontinuation in the trial, with 6 discontinuing for this reason before 12 weeks in the intervention group and 15 in the control group. There were seven pregnancies in total, with three in the intervention group and four in the control group. The primary outcome data was provided for 176/201 (87.6%) of participants in the intervention group and 166/209 (79.4%) in the control group.
FIGURE 2.
Consort diagram.
Social media advertising accounted for 47.6% (195/410) of the participant recruitment, 19.8% (81/410) through secondary care, 15.6% (64/410) through primary care, 6.6% (27/410) through community advertising, 6.6% (27/410) word of mouth and 3.9% (16/410) through the participants finding the trial via online search.
Baseline characteristics
Information on stratification factors (recruiting centre and baseline acne severity judged by IGA) can be seen in Table 4. The percentage of women who had an IGA score of more than or equal to three, denoting moderate or severe acne, was 53.7%.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Centre – n (%) b | |||
| Bristol Royal Infirmary | 34 (16.9%) | 36 (17.2%) | 70 (17.1%) |
| Epsom Hospital | 7 (3.5%) | 8 (3.8%) | 15 (3.7%) |
| Harrogate District Hospital | 43 (21.4%) | 46 (22.0%) | 89 (21.7%) |
| Nottingham University Hospitals NHS Trust – Queen’s Medical Centre Campus | 14 (7.0%) | 14 (6.7%) | 28 (6.8%) |
| Poole General Hospital | 26 (12.9%) | 26 (12.4%) | 52 (12.7%) |
| Queen Elizabeth Hospital (Birmingham) | 23 (11.4%) | 25 (12.0%) | 48 (11.7%) |
| Singleton Hospital (Swansea) | 3 (1.5%) | 4 (1.9%) | 7 (1.7%) |
| St Mary’s General Hospital (Portsmouth) | 42 (20.9%) | 42 (20.1%) | 84 (20.5%) |
| St Mary’s Hospital (HQ) (London) | 6 (3.0%) | 5 (2.4%) | 11 (2.7%) |
| University Hospital of Wales (Cardiff) | 3 (1.5%) | 3 (1.4%) | 6 (1.5%) |
| Missing – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Baseline severity – n (%) b | |||
| IGA 2 or less | 92 (45.8%) | 98 (46.9%) | 190 (46.2%) |
| IGA 3 or more | 109 (54.2%) | 111 (53.1%) | 220 (53.7%) |
| Missing – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Baseline demographics and source of recruitment are described in Table 5. The average age of participants was 29 years old and the average body mass index was 26. The majority of women were white and almost 50% were recruited through social media advertising.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Age (years) at baseline b | |||
| Mean | 29.6 | 28.7 | 29.2 |
| SD | 7.4 | 7.0 | 7.2 |
| Range | 18–59 | 18–51 | 18–59 |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Ethnicity | |||
| White | 158 (84.0%) | 170 (84.6%) | 328 (84.3%) |
| Asian or Asian British | 5 (2.7%) | 4 (2.0%) | 9 (2.3%) |
| Black, African, Black British or Caribbean | 4 (2.1%) | 2 (1.0%) | 6 (1.5%) |
| Mixed or multiple ethnic groups | 6 (3.2%) | 3 (1.5%) | 9 (2.3%) |
| Other ethnic group | 1 (0.5%) | 3 (1.5%) | 4 (1.0%) |
| Prefer not to say | 14 (7.4%) | 19 (9.5%) | 33 (8.5%) |
| Missing from eCRF – n (%)c | 13 (6.5%) | 8 (3.8%) | 21 (5.1%) |
| Body mass index b | |||
| Mean | 25.7 | 26.5 | 26.1 |
| SD | 5.3 | 5.9 | 5.6 |
| Range | 17.7–50.1 | 16.6–50.1 | 16.6–50.1 |
| Missing from eCRF – n (%)c | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
| Waist circumference (cm) b | |||
| Mean | 80.3 | 81.7 | 81.0 |
| SD | 12.2 | 13.2 | 12.7 |
| Range | 60.0–122.0 | 60.0–127.0 | 60.0–127.0 |
| Missing from eCRF – n (%)c | 1 (0.5%) | 1 (0.5%) | 2 (0.5%) |
| Where did the participant hear about the trial? – n (%) b | |||
| Community advertising | 12 (6.0%) | 15 (7.2%) | 27 (6.6%) |
| GP | 35 (17.4%) | 29 (13.9%) | 64 (15.6%) |
| Participant’s online search | 6 (3.0%) | 10 (4.8%) | 16 (3.9%) |
| Secondary care | 38 (18.9%) | 43 (20.6%) | 81 (19.8%) |
| Social media advertising | 98 (48.8%) | 97 (46.4%) | 195 (47.6%) |
| Word of mouth | 12 (6.0%) | 15 (7.2%) | 27 (6.6%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
The acne assessment information recorded at baseline can be seen in Table 6. It was observed that 1% of women described their acne as almost clear, 21% described their acne as mild severity, 53% described their acne as moderate severity and 25% as severe; 0.5% of women did not answer. This is in contrast with the clinicians’ views (IGA), who described 46% of women as having mild severity, 41% as moderate severity and 13% as severe. Hormonal contraception was being used by 58% of women, with 71% using the progesterone-only pill or other progesterone-only contraception.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| How would you describe the acne on your face at the moment? – n (%) b | |||
| Clear | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Almost clear | 3 (1.5%) | 1 (0.5%) | 4 (1.0%) |
| Mild severity | 37 (18.4%) | 49 (23.4%) | 86 (21.0%) |
| Moderate severity | 115 (57.2%) | 101 (48.3%) | 216 (52.7%) |
| Severe | 44 (21.9%) | 58 (27.8%) | 102 (24.9%) |
| Not answered | 2 (1.0%) | 0 (0.0%) | 2 (0.5%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Using the IGA scale for acne, how would you describe the participant’s facial acne – n (%)b | |||
| Clear | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Almost clear | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Mild severity | 92 (45.8%) | 98 (46.9%) | 190 (46.3%) |
| Moderate severity | 84 (41.8%) | 82 (39.2%) | 166 (40.5%) |
| Severe | 25 (12.4%) | 29 (13.9%) | 54 (13.2%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| If completed, how was IGA carried out? n – (%)b | |||
| Face-to-face | 198 (98.5%) | 208 (99.5%) | 406 (99.0%) |
| Photo | 3 (1.5%) | 0 (0.0%) | 3 (0.7%) |
| Video consultation | 0 (0.0%) | 1 (0.5%) | 1 (0.2%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Medical history information is presented in Table 7. More than half of the women in the trial had acne for over 5 years. The average age acne started was 16 years old and 19% of women either reported having a diagnosis of PCOS or, based on the information they provided to us, they met the Rotterdam criteria and therefore had suspected PCOS (see Methods section).
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| How long have you had your current episode of acne? – n (%) b | |||
| Less than 6 months | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 6 months to 2 years | 48 (23.9%) | 56 (26.8%) | 104 (25.3%) |
| 2–5 years | 44 (21.9%) | 49 (23.4%) | 93 (22.7%) |
| Over 5 years | 109 (54.2%) | 104 (49.8%) | 213 (52.0%) |
| Not answered | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Age acne started (years) b | |||
| Mean | 16.1 | 16.7 | 16.4 |
| SD | 5.4 | 5.8 | 5.6 |
| Range | 9.0–46.0 | 8.0–40.0 | 8.0–46.0 |
| Missing from eCRF – n (%)c | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
| PCOS diagnosis or suspected PCOS – n (%)b,d | |||
| Yese | 30 (15.4%) | 47 (23.3%) | 77 (19.4%) |
| No | 165 (84.6%) | 155 (76.7%) | 320 (80.6%) |
| Missing from eCRF – n (%)c,f | 6 (3.0%) | 7 (3.4%) | 13 (3.2%) |
The details of acne medication use are described in Table 8. There was a high proportion of women (85%) who said they had used or are currently using topical treatments. Benzoyl peroxide and combination were currently being used by 13% of women. Approximately 30% of women were taking topical treatments once a day. The full table of acne medication use at baseline can be seen in the Appendix 2 (see Table 42).
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Have you used, or are you currently using, topical treatments (creams/lotions/gels) for your acne? – n (%) b | |||
| Yes | 169 (84.9%) | 171 (82.2%) | 340 (83.5%) |
| No | 30 (15.1%) | 37 (17.8%) | 67 (16.5%) |
| Missing from eCRF – n (%)c | 2 (1.0%) | 1 (0.5%) | 3 (0.7%) |
| If ʻYesʼ d | |||
| Benzoyl peroxide | 22/165 (13.3%) | 30/168 (17.9%) | 52/333 (15.6%) |
| Azelaic acid | 10/165 (6.1%) | 15/167 (9.0%) | 25/332 (7.5%) |
| Topical adapalene | 22/167 (13.2%) | 21/167 (12.6%) | 43/334 (12.9%) |
| Nicotinamide | 9/165 (5.5%) | 3/167 (1.8%) | 12/332 (3.6%) |
| Antibiotic | 8/167 (4.8%) | 6/167 (3.6%) | 14/334 (4.2%) |
| Combination | 28/167 (16.8%) | 21/168 (12.5%) | 49/335 (14.6%) |
| Other | 20/137 (14.6%) | 25/148 (16.9%) | 45/285 (15.8%) |
| Not sure | 7/134 (5.2%) | 7/147 (4.8%) | 14/281 (5.0%) |
| If topical treatments have been prescribed, how often are they used? b | |||
| Not at all | 17 (8.5%) | 32 (15.3%) | 49 (12.0%) |
| Less than once a day | 16 (8.0%) | 19 (9.1%) | 35 (8.6%) |
| Once a day | 62 (31.0%) | 58 (27.8%) | 120 (29.3%) |
| Twice a day | 25 (12.5%) | 20 (9.6%) | 45 (11.0%) |
| More than twice a day | 1 (0.5%) | 2 (1.0%) | 3 (0.7%) |
| Not been prescribed topical treatments | 64 (32.0%) | 64 (30.6%) | 128 (31.3%) |
| Not answered | 15 (7.4%) | 14 (6.7%) | 29 (7.1%) |
| Missing from eCRF – n (%)c | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
| Is the participant currently using any hormonal treatment? – n (%) b | |||
| Yes | 81 (40.1%) | 91 (43.5%) | 172 (42.0%) |
| No | 120 (59.7%) | 118 (56.5%) | 238 (58.1%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| If ʻYesʼ, please state which hormonal treatment e | |||
| Combined oral contraceptionf | 27 (33.3%) | 22 (24.2%) | 49 (28.5%) |
| Progesterone-only pill or other progesterone-only contraceptiong | 54 (66.7%) | 69 (75.8%) | 123 (71.5%) |
| Missing from eCRF – n (%)c | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Table 9 presents the baseline values for all outcome measures. The mean Acne-QoL acne symptom subscale score at baseline was 13.0 (SD 4.7). The mean score of the other subscale scores including role-social, role-emotional and self-perception were 12.4 (SD 6.7), 10.7 (SD 6.6) and 8.9 (SD 6.5), respectively. The Acne-QoL total score at baseline was 45.0 (SD 21.1).
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL acne symptom subscale score at baseline | |||
| N | 201 | 209 | 410 |
| Mean (SD) | 13.2 (4.9) | 12.9 (4.5) | 13.0 (4.7) |
| Missing from eCRF – n (%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Acne-QoL role-social subscale score at baseline | |||
| N | 201 | 209 | 410 |
| Mean (SD) | 12.4 (6.6) | 12.5 (6.8) | 12.4 (6.7) |
| Missing from eCRF – n (%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Acne-QoL role-emotional subscale score at baseline | |||
| N | 201 | 209 | 410 |
| Mean (SD) | 10.8 (6.7) | 10.6 (6.4) | 10.7 (6.6) |
| Missing from eCRF – n (%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Acne-QoL self-perception subscale score at baseline | |||
| N | 201 | 209 | 410 |
| Mean (SD) | 9.2 (6.6) | 8.6 (6.4) | 8.9 (6.5) |
| Missing from eCRF – n (%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Acne-QoL total score at baseline | |||
| N | 201 | 209 | 410 |
| Mean (SD) | 45.6 (20.9) | 44.6 (21.2) | 45.0 (21.1) |
| Missing from eCRF – n (%)a | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Treatment information
Participants initially received one tablet per day (50 mg spironolactone or matched placebo) then at or any time after the 6-week visit, the dose was escalated to two tablets daily if the treatment was tolerated. Table 10 shows that the majority of women (98.1%) were advised to increase to two tablets per day at their 6-week visit. The percentage of women on two tablets per day between 6 and 12 weeks was 96% and between 12 and 24 weeks was 90%, with similar percentages for both groups.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| No. of participants with 6-week treatment information available – n (%)b | 188 (93.5%) | 193 (92.3%) | 381 (92.9%) |
| No. of participants who were advised to increase the dose to two tablets per day at 6-week visitc | 182 (98.9%) | 182 (97.3%) | 364 (98.1%) |
| Missing from eCRF – n (%)d | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| No. of participants with 12-week treatment information available – n (%)b | 181 (90.1%) | 177 (84.7%) | 358 (87.3%) |
| No. of participants whose dose per day between 6-week and 12-week visit wasc | |||
| One tablet | 4 (2.2%) | 4 (2.3%) | 8 (2.2%) |
| Two tablets | 174 (96.1%) | 169 (95.5%) | 343 (95.8%) |
| Missing from eCRF – n (%)d | 3 (1.7%) | 4 (2.3%) | 7 (2.0%) |
| No. of participants with a 24-week treatment information eCRF available – n (%)b | 168 (83.6%) | 146 (69.9%) | 314 (76.4%) |
| No. of participants whose dose per day between 12-week and 24-week visit was c | |||
| One tablet | 7 (4.2%) | 3 (2.1%) | 10 (3.2%) |
| Two tablets | 150 (89.3%) | 132 (90.4%) | 282 (89.8%) |
| Not answered | 9 (5.4%) | 11 (7.5%) | 20 (6.4%) |
| Missing from eCRF – n (%)d | 2 (1.2%) | 0 (0.0%) | 2 (0.6%) |
Primary outcome
Figure 3 displays the mean Acne-QoL symptom subscale over time for each treatment group. The acne symptoms of women improve over time for both groups, with higher scores seen in the spironolactone group, indicating greater improvement. After 12 weeks, improvement continues in the spironolactone group; however the placebo group flattens out. The largest difference between the groups can be seen at 24 weeks. It is important to note that at 24 weeks treatment ended; at this point participants could obtain any treatment that they wanted which could explain the smaller difference in scores at 52 weeks.
FIGURE 3.
Mean Acne-QoL symptom subscale score by time point for each treatment group.
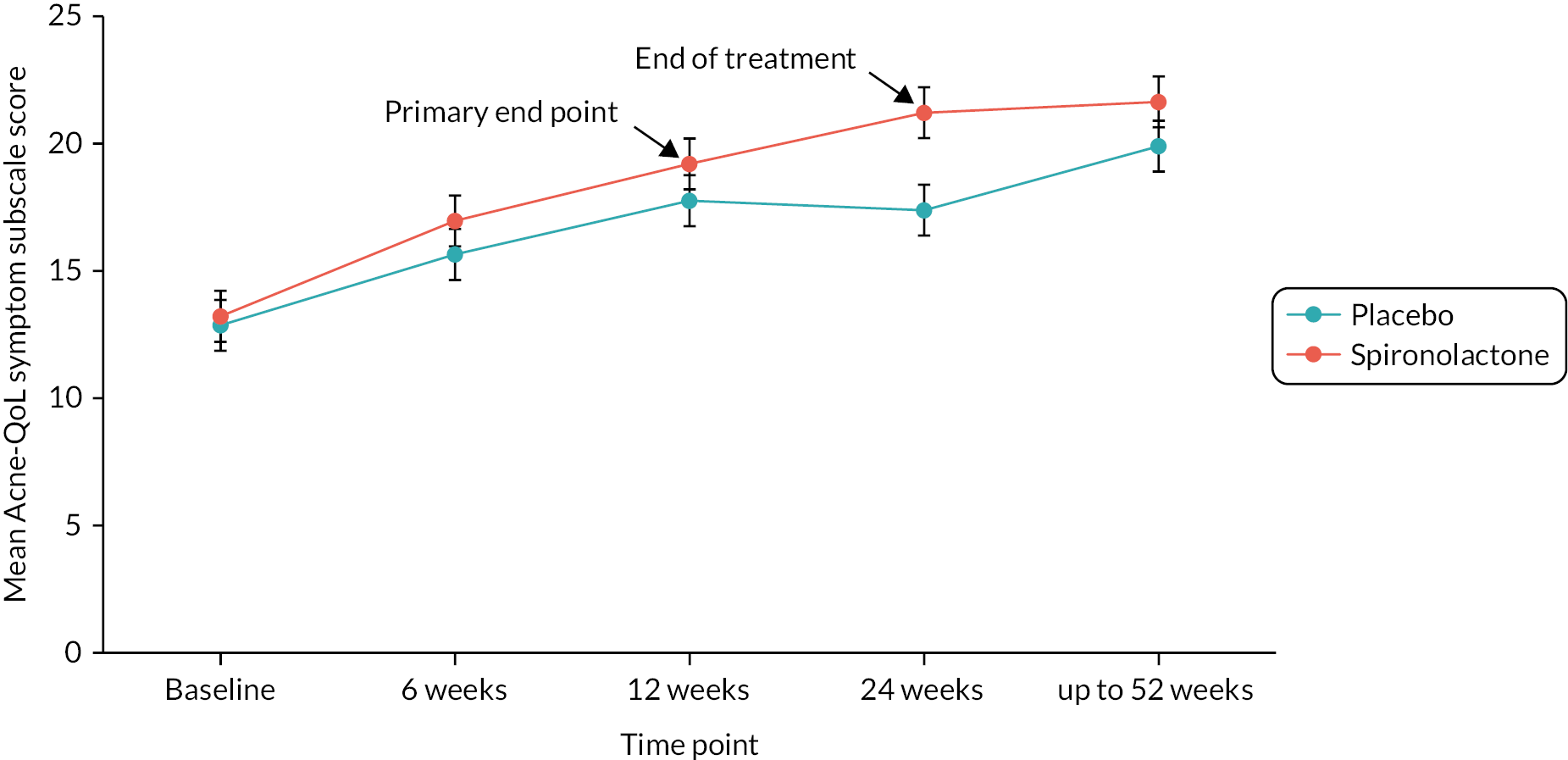
Table 11 presents the primary end-point information. The mean Acne-QoL symptom subscale score at 12 weeks was 19.2 (SD 6.1) in the spironolactone group and 17.8 (SD 5.6) in the placebo group. In the unadjusted model, the mean difference was 1.48 with 95% CI 0.30 to 2.67. The primary analysis adjusted for stratification factors (site and baseline IGA), baseline Acne-QoL symptom subscale score, topical treatment use, hormonal treatment use, age and PCOS status, resulting in mean difference 1.27 (95% CI 0.07 to 2.46). This represents a statistically significant improvement in the spironolactone group. The sensitivity analysis on multiply imputed data gave similar results and inferences (see Table 11).
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL symptom subscale score at 12-weeks | |||
| N | 176 | 166 | 342 |
| Mean (SD) | 19.2 (6.1) | 17.8 (5.6) | 18.5 (5.9) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted – only including baseline Acne-QoL score) | 1.48 (0.30 to 2.67) | REF | N/A |
| Complete cases (adjusted)b | 1.27 (0.07 to 2.46) | REF | N/A |
| Missing data imputed (adjusted – 100 imputations) | 1.26 (0.04 to 2.48) | REF | N/A |
Secondary and tertiary outcomes
Details of Acne-QoL information at 12 weeks are displayed in Table 12. There was a statistically significant improvement in mean total Acne-QoL score and all Acne-QoL subscales at 12 weeks in the spironolactone group compared to placebo.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL role-social subscale score at 12 weeks | |||
| N | 176 | 165 | 341 |
| Mean (SD) | 18.7 (6.1) | 17.0 (6.7) | 17.8 (6.4) |
| Mean difference Acne-QoL role-social subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.88 (0.64 to 3.13) | REF | N/A |
| Complete cases (adjusted) | 1.99 (0.70 to 3.28) | REF | N/A |
| Acne-QoL role-emotional subscale score at 12 weeks | |||
| N | 175 | 166 | 341 |
| Mean (SD) | 20.2 (7.8) | 17.5 (8.1) | 18.9 (8.1) |
| Mean difference Acne-QoL role-emotional subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 2.66 (1.08 to 4.25) | REF | N/A |
| Complete cases (adjusted) | 2.75 (1.11 to 4.39) | REF | N/A |
| Acne-QoL self-perception subscale score at 12 weeks | |||
| N | 175 | 166 | 341 |
| Mean (SD) | 19.2 (8.5) | 16.9 (8.2) | 18.1 (8.4) |
| Mean difference Acne-QoL self-perception subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 2.21 (0.55 to 3.86) | REF | N/A |
| Complete cases (adjusted) | 2.10 (0.39 to 3.82) | REF | N/A |
| Acne-QoL total score at 12 weeks | |||
| N | 176 | 166 | 342 |
| Mean (SD) | 77.0 (26.5) | 69.0 (26.2) | 73.2 (26.6) |
| Mean difference Acne-QoL total score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 8.18 (2.98 to 13.38) | REF | N/A |
| Complete cases (adjusted) | 8.03 (2.68 to 13.37) | REF | N/A |
Details of Acne-QoL information at 24 weeks is displayed in Table 13. The mean Acne-QoL symptom subscale score was 21.2 (SD 5.9) in the spironolactone group and 17.4 (SD 5.8) in the placebo group. The mean difference between the groups in the adjusted analysis was 3.45 with 95% CI 2.16 to 4.75, which represents a statistically significant greater improvement in the spironolactone group. The results for all the other Acne-QoL subscales and for total Acne-QoL score at 24 weeks were also statistically significant in favour of the spironolactone group.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL symptom subscale score at 24 weeks | |||
| N | 163 | 136 | 299 |
| Mean (SD) | 21.2 (5.9) | 17.4 (5.8) | 19.5 (6.1) |
| Mean difference Acne-QoL symptom subscale score at 24 weeks (95 CI%) | |||
| Complete cases (unadjusted) | 3.77 (2.50 to 5.03) | REF | N/A |
| Complete cases (adjusted) | 3.45 (2.16 to 4.75) | REF | N/A |
| Acne-QoL role-social subscale score at 24 weeks | |||
| N | 163 | 136 | 299 |
| Mean (SD) | 19.6 (5.6) | 16.7 (6.8) | 18.3 (6.3) |
| Mean difference Acne-QoL role-social subscale score at 24 weeks (95% CI) | |||
| Complete cases (unadjusted) | 3.03 (1.78 to 4.28) | REF | N/A |
| Complete cases (adjusted) | 3.06 (1.77 to 4.35) | REF | N/A |
| Acne-QoL role-emotional subscale score at 24 weeks | |||
| N | 164 | 137 | 301 |
| Mean (SD) | 21.1 (7.3) | 16.5 (8.2) | 19.0 (8.0) |
| Mean difference Acne-QoL role-emotional subscale score at 24 weeks (95% CI) | |||
| Complete cases (unadjusted) | 4.50 (2.81 to 6.18) | REF | N/A |
| Complete cases (adjusted) | 4.35 (2.64 to 6.05) | REF | N/A |
| Acne-QoL self-perception subscale score at 24 weeks | |||
| N | 164 | 137 | 301 |
| Mean (SD) | 21.3 (7.7) | 16.3 (8.7) | 19.1 (8.5) |
| Mean difference Acne-QoL self-perception subscale score at 24 weeks (95% CI) | |||
| Complete cases (unadjusted) | 4.98 (3.23 to 6.74) | REF | N/A |
| Complete cases (adjusted) | 4.77 (2.97 to 6.56) | REF | N/A |
| Acne-QoL total score at 24 weeks | |||
| N | 164 | 137 | 301 |
| Mean (SD) | 83.0 (25.0) | 66.7 (27.5) | 75.6 (27.4) |
| Mean difference Acne-QoL total score at 24 weeks (95% CI) | |||
| Complete cases (unadjusted) | 16.25 (10.66 to 21.84) | REF | N/A |
| Complete cases (adjusted) | 15.73 (10.04 to 21.42) | REF | N/A |
Table 14 describes Acne-QoL information at 6 and (up to) 52 weeks, although these time points are less informative as 6 weeks would clinically be judged to be too early to expect to see a treatment difference from spironolactone for acne and after 24 weeks participants were unblinded and able to seek any treatment that they wished, including spironolactone in the control group.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL symptom subscale score at 6 weeks | |||
| N | 176 | 179 | 355 |
| Mean (SD) | 17.0 (5.7) | 15.6 (5.7) | 16.3 (5.7) |
| Acne-QoL symptom subscale score at 52 weeks | |||
| N | 95 | 81 | 176 |
| Mean (SD) | 21.7 (6.3) | 20.0 (5.7) | 20.9 (6.1) |
| Acne-QoL role-social subscale score at 6 weeks | |||
| N | 176 | 179 | 355 |
| Mean (SD) | 16.2 (6.5) | 15.2 (6.8) | 15.7 (6.6) |
| Acne-QoL role-social subscale score at 52 weeks | |||
| N | 95 | 81 | 176 |
| Mean (SD) | 20.1 (5.7) | 17.8 (6.4) | 19.1 (6.1) |
| Acne-QoL role-emotional subscale score at 6 weeks | |||
| N | 178 | 183 | 361 |
| Mean (SD) | 15.7 (7.8) | 14.4 (7.6) | 15.0 (7.7) |
| Acne-QoL role-emotional subscale score at 52 weeks | |||
| N | 96 | 82 | 178 |
| Mean (SD) | 22.4 (7.7) | 18.6 (8.7) | 20.7 (8.4) |
| Acne-QoL self-perception subscale score at 6 weeks | |||
| N | 178 | 183 | 361 |
| Mean (SD) | 15.2 (8.2) | 13.4 (8.0) | 14.3 (8.2) |
| Acne-QoL self-perception subscale score at 52 weeks | |||
| N | 96 | 82 | 178 |
| Mean (SD) | 22.6 (7.9) | 18.8 (8.7) | 20.8 (8.5) |
| Acne-QoL total score at 6 weeks | |||
| N | 179 | 184 | 363 |
| Mean (SD) | 63.3 (26.1) | 57.7 (26.0) | 60.4 (26.2) |
| Acne-QoL total score at 52 weeks | |||
| N | 96 | 82 | 178 |
| Mean (SD) | 86.3 (26.1) | 74.7 (28.0) | 81.0 (27.5) |
Information on participant’s and investigator’s global assessment (PGA and IGA, respectively) at 12 and 24 weeks can be seen in Table 15. PGA and IGA were dichotomised as success or failure with success defined as clear or almost clear (grade 0 or 1) and at least a two-grade improvement from baseline, in line with FDA guidance. The adjusted odd ratio for PGA score at 12 weeks was not statistically significant; however the PGA at 24 weeks showed a statistically meaningful result with adjusted odds ratio 3.76 and 95% CI 1.95 to 7.28. This implies that the odds of success on the PGA was higher in the spironolactone group. The adjusted odds ratio for IGA at 12 weeks was also statistically significant with odds ratio 5.18 and 95% CI 2.18 to 12.2. Therefore, the odds of success on the IGA were significantly higher in the spironolactone group.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| PGA score at 12 weeks | |||
| N | 176 | 166 | 342 |
| Success | 36 (20.5%) | 20 (12.1%) | 56 (16.4%) |
| Failure | 140 (79.6%) | 146 (88.0%) | 286 (83.6%) |
| Complete cases (unadjusted) – odds ratio (95% CI) | 1.91 (1.05 to 3.45) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI) | 1.69 (0.89 to 3.19) | REF | N/A |
| PGA score at 24 weeks | |||
| N | 164 | 136 | 300 |
| Success | 53 (32.3%) | 15 (11.0%) | 68 (22.7%) |
| Failure | 111 (67.8%) | 121 (89.0%) | 232 (77.3%) |
| Complete cases (unadjusted) – odds ratio (95% CI) | 3.93 (2.09 to 7.37) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI) | 3.76 (1.95 to 7.28) | REF | N/A |
| If completed, how was IGA carried out? – n (%)3 | |||
| Face-to-face | 97 (57.7%) | 95 (59.8%) | 192 (58.7%) |
| Photo | 55 (32.7%) | 47 (29.6%) | 102 (31.2%) |
| Video consultation | 16 (9.5%) | 17 (10.7%) | 33 (10.1%) |
| Missing from eCRF – n (%)4 | 0 (0.0%) | 1 (0.6%) | 1 (0.3%) |
| IGA score at 12 weeks | |||
| N | 168 | 160 | 328 |
| Success | 31 (18.5%) | 9 (5.6%) | 40 (12.2%) |
| Failure | 137 (81.6%) | 151 (94.4%) | 288 (87.8%) |
| Complete cases (unadjusted) – odds ratio (95% CI) | 3.78 (1.73 to 8.27) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI) | 5.18 (2.18 to 12.28) | REF | N/A |
Table 16 presents participants’ self-assessed overall improvement and participants’ satisfaction with trial treatment. Participant self-assessed overall improvement was measured by asking a single question ‘Using the photograph taken at your first visit if you have it, how do you think your acne today compares to your acne then?’ on a six-point Likert scale from 1 ‘Worse’ to 6 ‘Completely Cleared’. Participants in the spironolactone group were more likely than those in the placebo group to report overall acne improvement, compared with baseline photo, at both 12 weeks {72.2% vs. 67.9% [adjusted OR 1.16 (95% CI 0.70 to 1.91)]} and at 24 weeks {81.9% vs. 63.3% [adjusted OR 2.72 (95% CI 1.50 to 4.93)]}. Post hoc analyses showed that this equated to number needed to treat (NNT) of 23 at 12 weeks and NNT of 5 at 24 weeks. Participants were asked about their satisfaction with the trial treatment through a single question ‘Do you think the tablets you received in this trial have helped your skin?’. This was measured on a scale of 0 (‘not at all’) to 5 (‘a lot’) with higher scores indicating increased satisfaction with treatment. There was a significantly higher proportion of people who were satisfied (scored 3 or more) with the trial treatment compared with placebo, 70.6% versus 43.1%. The adjusted odds ratio was statistically significant with odds ratio 3.12 (95% CI 1.80 to 5.41). Therefore, the odds of participant satisfaction were significantly higher in the spironolactone group.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Participant’s self-assessed overall improvement at 12 weeks a | |||
| N | 169 | 159 | 328 |
| 1–2 | 47 (27.8%) | 51 (32.1%) | 98 (29.9%) |
| 3–6 | 122 (72.2%) | 108 (67.9%) | 230 (70.1%) |
| Mean (SD) | 3.4 (1.3) | 3.1 (1.2) | 3.2 (1.2) |
| Complete cases (unadjusted) – odds ratio (95% CI)b | 1.23 (0.76 to 1.96) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI)b | 1.16 (0.70 to 1.91) | REF | N/A |
| Participant’s self-assessed overall improvement at 24 weeks a | |||
| N | 160 | 128 | 288 |
| 1–2 | 29 (18.1%) | 47 (36.7%) | 76 (26.4%) |
| 3–6 | 131 (81.9%) | 81 (63.3%) | 212 (73.6%) |
| Mean (SD) | 4.0 (1.3) | 3.1 (1.3) | 3.6 (1.4) |
| Complete cases (unadjusted) – odds ratio (95% CI)b | 2.62 (1.53 to 4.50) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI)b | 2.72 (1.50 to 4.93) | REF | N/A |
| Participant’s satisfaction with trial treatment at 24 weeks c | |||
| 0–2 | 42 (29.4%) | 70 (56.9%) | 112 (42.1%) |
| 3–5 | 101 (70.6%) | 53 (43.1%) | 154 (57.9%) |
| Not sure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not answered | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Complete cases (unadjusted) – odds ratio (95% CI) | 3.18 (1.91 to 5.27) | REF | N/A |
| Complete cases (adjusted) – odds ratio (95% CI) | 3.12 (1.80 to 5.41) | REF | N/A |
Table 17 details the information on other oral acne treatment used at 24 and 52 weeks. At the time of the final follow-up questionnaire, 35% of women in the intervention group and 28% in the control group reported that they were taking spironolactone. This shows that, following unblinding at 24 weeks, a substantial proportion of women in the intervention group had continued this medication and a substantial proportion in the control group had commenced it after unblinding. Although numbers are small, it is notable that fewer women in the intervention group than the control group reported that they were taking oral antibiotics or isotretinoin at the time of the final follow-up questionnaire.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| At 24 weeks | |||
| Are you using topical treatment? | |||
| N | 168 | 146 | 314 |
| Yes | 112 (66.7%) | 99 (67.8%) | 211 (67.2%) |
| No | 52 (31.0%) | 37 (25.3%) | 89 (38.3%) |
| Not answered | 4 (3.4%) | 10 (6.7%) | 14 (4.5%) |
| Are you using oral antibiotic? | |||
| N | 168 | 146 | 314 |
| Yes | 4 (2.4%) | 6 (4.1%) | 10 (3.2%) |
| Not answered | 164 (97.6%) | 140 (95.9%) | 304 (96.8%) |
| Are you using combined oral contraceptive? | |||
| N | 168 | 146 | 314 |
| Yes | 26 (15.5%) | 16 (11.0%) | 42 (13.4%) |
| Not answered | 142 (84.5%) | 130 (89.0%) | 272 (86.6%) |
| Are you using oral isotretinoin? | |||
| N | 168 | 146 | 314 |
| Yes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not answered | 168 (100.0%) | 146 (100.0%) | 314 (100.0%) |
| Are you using progesterone-only contraception? | |||
| N | 168 | 146 | 314 |
| Yes | 47 (28.0%) | 48 (32.9%) | 95 (30.3%) |
| Not answered | 121 (72.0%) | 98 (67.1%) | 219 (69.8%) |
| At 52 weeks | |||
| Are you using topical treatment? | |||
| N | 103 | 89 | 192 |
| Yes | 88 (85.4%) | 79 (88.8%) | 167 (87.0%) |
| No | 5 (4.8%) | 2 (2.2%) | 7 (3.6%) |
| Not answered | 10 (9.7%) | 8 (9.0%) | 18 (9.4%) |
| Are you using oral antibiotic? | |||
| N | 103 | 89 | 192 |
| Yes | 6 (5.8%) | 12 (13.5%) | 18 (9.4%) |
| Not answered | 97 (94.2%) | 77 (86.5%) | 174 (90.6%) |
| Are you using spironolactone? | |||
| N | 103 | 89 | 192 |
| Yes | 36 (35.0%) | 25 (28.1%) | 61 (31.8%) |
| Not answered | 67 (65.0%) | 64 (71.9%) | 131 (68.2%) |
| Are you using combined oral contraceptive? | |||
| N | 103 | 89 | 192 |
| Yes | 13 (12.6%) | 8 (9.0%) | 21 (10.9%) |
| Not answered | 90 (87.4%) | 81 (91.0%) | 171 (89.1%) |
| Are you using oral isotretinoin? | |||
| N | 103 | 89 | 192 |
| Yes | 2 (1.9%) | 6 (6.7%) | 8 (4.2%) |
| Not answered | 101 (98.1%) | 83 (93.3%) | 184 (95.8%) |
| Are you using progesterone-only contraception? | |||
| N | 103 | 89 | 192 |
| Yes | 33 (32.0%) | 32 (36.0%) | 65 (33.9%) |
| Not answered | 70 (68.0%) | 57 (64.0%) | 127 (66.1%) |
Additional analyses
Subgroup analysis
Table 18 describes the subgroup analysis that was performed to compare results in certain pre-specified populations. Interaction terms were included in the model; however age (categorised as below 25 years and 25 years and over)36 and treatment were the only significant interaction terms. The coefficient and 95% CI for this interaction was: 4.2 (1.3 to 7.1). The difference in Acne-QoL symptom subscale score in the 25 years and above population was 2.42 (95% CI 1.00 to 3.84), which was statistically significant and reached the desired two-point mean difference between groups in favour of spironolactone. The population of those below 25 years was considerably smaller (44 participants) and therefore it is difficult to draw definitive conclusions, although the difference of –0.87 (95% CI –3.67 to 1.92), which does not favour the spironolactone group, suggests that spironolactone may be less effective in younger women.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Including only participants with PCOS or suspected PCOS | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 26 | 35 | 61 |
| Mean (SD) | 17.4 (6.0) | 17.3 (5.2) | 17.3 (5.5) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 0.60 (–2.14 to 3.34) | REF | N/A |
| Complete cases (adjusted) | 1.37 (–1.40 to 4.14) | REF | N/A |
| Including only participants without PCOS or suspected PCOS | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 144 | 124 | 268 |
| Mean (SD) | 19.4 (6.2) | 17.7 (5.8) | 18.6 (6.0) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.74 (0.36 to 3.12) | REF | N/A |
| Complete cases (adjusted) | 1.46 (0.12 to 2.80) | REF | N/A |
| Including only participants 25 years and above | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 132 | 120 | 252 |
| Mean (SD) | 20.0 (6.0) | 17.4 (5.6) | 18.7 (5.9) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 2.63 (1.27 to 3.98) | REF | N/A |
| Complete cases (adjusted) | 2.42 (1.00 to 3.84) | REF | N/A |
| Including only participants below 25 years | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 44 | 46 | 90 |
| Mean (SD) | 16.9 (5.8) | 18.7 (5.6) | 17.8 (5.8) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | –1.79 (–4.13 to 0.55) | REF | N/A |
| Complete cases (adjusted) | –0.87 (–3.67 to 1.92) | REF | N/A |
| Including only participants with a BMI ≤ 25 | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 99 | 82 | 181 |
| Mean (SD) | 19.8 (5.7) | 17.7 (5.2) | 18.9 (5.5) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 2.17 (0.60 to 3.75) | REF | N/A |
| Complete cases (adjusted) | 1.89 (0.32 to 3.46) | REF | N/A |
| Including only participants with a BMI > 25 | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 77 | 84 | 161 |
| Mean (SD) | 18.4 (6.6) | 17.8 (6.0) | 18.1 (6.3) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 0.61 (–1.19 to 2.40) | REF | N/A |
| Complete cases (adjusted) | 0.34 (–1.56 to 2.24) | REF | N/A |
| Including only participants with baseline IGA < 3 | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 79 | 80 | 159 |
| Mean (SD) | 20.4 (5.8) | 18.2 (5.8) | 19.3 (5.9) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.90 (0.20, 3.61) | REF | N/A |
| Complete cases (adjusted) | 1.63 (–0.10 to 3.35) | REF | N/A |
| Including only participants with baseline IGA ≥ 3 | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 97 | 86 | 183 |
| Mean (SD) | 18.3 (6.2) | 17.4 (5.4) | 17.8 (5.8) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.06 (–0.60 to 2.73) | REF | N/A |
| Complete cases (adjusted) | 1.35 (–0.33 to 3.03) | REF | N/A |
| Including only participants not taking hormonal treatment | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 102 | 90 | 192 |
| Mean | 18.8 (6.1) | 17.5 (5.7) | 18.2 (5.9) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.49 (–0.12 to 3.11) | REF | N/A |
| Complete cases (adjusted) | 1.79 (0.12 to 3.46) | REF | N/A |
| Including only participants taking the combined oral contraceptive a | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 27 | 18 | 45 |
| Mean (SD) | 21.1 (6.7) | 19.2 (6.1) | 20.4 (6.5) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 2.13 (–1.89 to 6.14) | REF | N/A |
| Complete cases (adjusted) | 2.70 (–1.77 to 7.17) | REF | N/A |
| Including only participants taking progesterone-only contraception | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 47 | 58 | 105 |
| Mean (SD) | 18.9 (5.8) | 17.7 (5.3) | 18.2 (5.5) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 0.58 (–1.33 to 2.50) | REF | N/A |
| Complete cases (adjusted) | 1.03 (–0.83 to 2.90) | REF | N/A |
| Including only participants who reported they were not taking topical treatment at baseline | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 24 | 27 | 51 |
| Mean (SD) | 18.8 (5.8) | 17.2 (6.7) | 17.9 (6.3) |
| Mean difference Acne-QoL symptom subscale score at 12 weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.96 (–1.19 to 5.10) | REF | N/A |
| Complete cases (adjusted) | 1.91 (–1.82 to 5.65) | REF | N/A |
| Including only participants who reported they were taking a topical treatment at baseline | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 150 | 138 | 288 |
| Mean (SD) | 19.2 (6.1) | 17.9 (5.4) | 18.6 (5.8) |
| Mean difference Acne-QoL symptom subscale score at 12-weeks (95% CI) | |||
| Complete cases (unadjusted) | 1.27 (–0.17 to 2.56) | REF | N/A |
| Complete cases (adjusted) | 1.22 (–0.09 to 2.53) | REF | N/A |
Summary statistics are presented in Table 19 for the primary outcome pre and post COVID as well as for ethnicity. The mean Acne-QoL symptom subscale score at 12 weeks pre and post COVID was similar for both groups. Those who identified as non-white had on average a better score in the placebo group; however this population was small (n = 22), therefore it is difficult to draw meaningful conclusions.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Pre COVID | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 35 | 32 | 67 |
| Mean (SD) | 20.1 (6.3) | 17.7 (4.7) | 19.0 (5.7) |
| Post COVID | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 141 | 134 | 275 |
| Mean (SD) | 19.0 (6.1) | 17.8 (5.8) | 18.4 (6.0) |
| Ethnicity – white | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 143 | 149 | 292 |
| Mean (SD) | 19.6 (6.1) | 17.4 (5.6) | 18.5 (5.9) |
| Ethnicity – non-white | |||
| Acne-QoL symptom subscale score at 12 weeks | |||
| N | 15 | 7 | 22 |
| Mean (SD) | 16.1 (5.6) | 19.9 (5.3) | 17.3 (5.7) |
Complier-average causal effect analysis
The results of the CACE analysis performed on self-reported treatment adherence at 24 weeks can be seen in Table 20. ‘Compliance’ was defined as participants reporting that they had taken either 50%, 80% or 100% of the prescribed medication over the 12- to 24-week period. Compliance was only considered over the 12- to 24-week period due to the collection of these data before this time point being poorly reported in part due to the COVID-19 pandemic, as pill count at 6 and 12 weeks was initially planned but often not possible. When considering 80% as the threshold for compliance, we observed 74% of women complied. The adjusted mean difference between groups was 5.13 (95% CI 3.17 to 7.08), suggesting a greater treatment effect amongst women who took 80% or more of the prescribed medication. For all thresholds of compliance (50%, 80% and 100%), the proportion of participants achieving ‘compliance’ were similar in both spironolactone and placebo groups, suggesting that spironolactone was well-tolerated.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Acne-QoL symptom subscale score at 24 weeks | |||
| CACE analysis – 50% compliance | |||
| Was the participant compliant? | |||
| Yes | 144 (98.0%) | 115 (98.3%) | 259 (98.1%) |
| No | 3 (2.0%) | 2 (1.7%) | 5 (1.9%) |
| Mean difference Acne-QoL symptom subscale score at 24 weeks (95% CI) | |||
| Complete cases (unadjusted) | 4.33 (2.82 to 5.84) | REF | N/A |
| Complete cases (adjusted) | 3.89 (2.48 to 5.30) | REF | N/A |
| Acne-QoL symptom subscale score at 24 weeks | |||
| CACE analysis – 80% compliance | |||
| Was the participant compliant? | |||
| Yes | 110 (74.8%) | 85 (72.7%) | 195 (73.9%) |
| No | 37 (25.2%) | 32 (27.4%) | 69 (26.1%) |
| Mean difference Acne-QoL symptom subscale score at 24 weeks | |||
| Complete cases (unadjusted) | 5.67 (3.60 to 7.74) | REF | N/A |
| Complete cases (adjusted) | 5.13 (3.17 to 7.08) | REF | N/A |
| Acne-QoL symptom subscale score at 24 weeks | |||
| CACE analysis – 100% compliance | |||
| Was the participant compliant? | |||
| Yes | 33 (22.4%) | 29 (24.8%) | 62 (23.5%) |
| No | 114 (77.6%) | 88 (75.2%) | 202 (76.5%) |
| Mean difference Acne-QoL symptom subscale score at 24 weeks | |||
| Complete cases (unadjusted) | 18.89 (9.64 to 28.14) | REF | N/A |
| Complete cases (adjusted) | 17.13 (8.34 to 25.93) | REF | N/A |
Adverse reactions
Adverse reactions of special interest were included in the participant questionnaire and can be seen in Tables 21–23. Table 21 shows that menstrual irregularities are reported by approximately a quarter of women at all time points, with similar rates in spironolactone and placebo groups. Table 22 shows that menstrual irregularities are also similar across both groups when comparing across all time points. Table 23 shows that most ARs were experienced at similar rates in the spironolactone group compared to the placebo group. Pearson’s χ² test showed that the only statistically significant difference was that headaches were more commonly reported amongst women on spironolactone.
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Experienced irregular menstrual bleeding at baseline a | |||
| Yes | 44 (22.2%) | 58 (28.0%) | 102 (25.2%) |
| No | 119 (60.1%) | 107 (51.7%) | 226 (55.8%) |
| Don’t have periods | 35 (17.7%) | 42 (20.3%) | 77 (19.0%) |
| Missing from eCRF – n (%)b | 3 (1.5%) | 2 (1.0%) | 5 (1.2%) |
| Experienced irregular menstrual bleeding at 6 weeks a | |||
| Yes | 45 (25.3%) | 46 (25.3%) | 91 (25.3%) |
| No | 100 (56.2%) | 102 (56.0%) | 202 (56.1%) |
| Don’t have periods | 33 (18.5%) | 34 (18.7%) | 67 (18.6%) |
| Missing from eCRF – n (%)b | 9 (4.8%) | 9 (4.7%) | 18 (4.8%) |
| Experienced irregular menstrual bleeding at 12 weeks a | |||
| Yes | 38 (22.0%) | 46 (27.7%) | 84 (24.8%) |
| No | 98 (56.7%) | 79 (47.6%) | 177 (52.2%) |
| Don’t have periods | 37 (21.4%) | 41 (24.7%) | 78 (23.0%) |
| Missing from eCRF – n (%)b | 7 (3.9%) | 9 (5.1%) | 16 (4.5%) |
| Experienced irregular menstrual bleeding at 24 weeks a | |||
| Yes | 42 (25.9%) | 37 (27.2%) | 79 (26.5%) |
| No | 82 (50.6%) | 64 (47.1%) | 146 (49.0%) |
| Don’t have periods | 38 (23.5%) | 35 (25.7%) | 73 (24.5%) |
| Missing from eCRF – n (%)b | 6 (3.6%) | 10 (6.9%) | 16 (5.1%) |
| Experienced irregular menstrual bleeding at 52 weeks a | |||
| Yes | 20 (21.1%) | 21 (25.9%) | 41 (23.3%) |
| No | 46 (48.4%) | 39 (48.2%) | 85 (48.3%) |
| Don’t have periods | 29 (30.5%) | 21 (25.9%) | 50 (28.4%) |
| Missing from eCRF – n (%)b | 8 (7.8%) | 8 (9.0%) | 16 (8.3%) |
| Characteristic | Spironolactone (n = 201) (%) | Placebo (n = 209) (%) | Total (n = 410) (%) |
|---|---|---|---|
| Experienced irregular menstrual bleeding at some point in the 6–24-week period a | |||
| Yes | 73 (39.0) | 72 (37.7) | 145 (38.4) |
| No/don’t have periodsb | 114 (61.0) | 119 (62.3) | 233 (61.6) |
| Missing from eCRF – n (%)c | 14 (7.0) | 18 (8.6) | 32 (7.8) |
| Characteristic | Spironolactone (n = 201) (%) | Placebo (n = 209) (%) | Total (n = 410) (%) | p-value from Pearson’s χ² test |
|---|---|---|---|---|
| Number of participants who experienced at least one AR – n (%)a | 128 (63.7) | 107 (51.2) | 235 (57.3) | 0.01 |
| Summary of ARs – n (%) a | ||||
| Abdominal pain | 9 (4.5) | 10 (4.8) | 19 (4.6) | 0.88 |
| Breast enlargement | 31 (15.4) | 25 (12.0) | 56 (13.7) | 0.31 |
| Diarrhoea | 7 (3.5) | 11 (5.3) | 18 (4.4) | 0.38 |
| Dizziness/vertigo/lightheadedness | 38 (18.9) | 26 (12.4) | 64 (15.6) | 0.07 |
| Drowsiness/sleepiness | 14 (7.0) | 18 (8.6) | 32 (7.8) | 0.53 |
| Fatigue/tiredness | 23 (11.4) | 29 (13.9) | 52 (12.7) | 0.46 |
| Headache | 41 (20.4) | 25 (12.0) | 66 (16.1) | 0.02 |
| Indigestion/heartburn/dyspepsia | 23 (11.4) | 17 (8.1) | 40 (9.8) | 0.26 |
| Nausea/feeling sick | 21 (10.5) | 16 (7.7) | 37 (9.0) | 0.32 |
| Polyuria (passing much more urine than usual) | 62 (30.9) | 52 (25.9) | 114 (27.8) | 0.18 |
| Reduced libido | 15 (7.5) | 11 (5.3) | 26 (6.3) | 0.36 |
| Tenderness of the breasts | 40 (19.9) | 37 (17.7) | 77 (18.8) | 0.57 |
| Tingling | 6 (3.0) | 10 (4.8) | 16 (3.9) | 0.35 |
| Vomiting/being sick | 4 (2.0) | 1 (0.5) | 5 (1.2) | 0.16 |
| Weight gain | 13 (6.5) | 17 (8.1) | 30 (7.3) | 0.52 |
| Other | 34 (16.9) | 22 (10.5) | 56 (13.7) | 0.06 |
Table 24 details the overall ARs by common terminology criteria for adverse events (CTCAE) grade v5. Approximately 23% of people experienced a mild AE and 25% experienced a moderate AE. Less than 10% of women experienced a severe AE.
| Characteristic | Spironolactone (n = 201) (%) | Placebo (n = 209) (%) | Total (n = 410) (%) |
|---|---|---|---|
| Adverse reactions – n (%) a , b | |||
| Mild | 52 (25.9) | 44 (21.1) | 96 (23.4) |
| Moderate | 55 (27.4) | 48 (23.0) | 103 (25.1) |
| Severe | 18 (9.0) | 13 (6.2) | 31 (7.6) |
| Life-threatening | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not answered | 3 (1.5) | 2 (1.0) | 5 (1.2) |
| Severe (Grade 3 or above) AR – n (%)a | 18 (9.0) | 13 (6.2) | 31 (7.6) |
Safety
Reported serious adverse events/serious adverse reactions and suspected unexpected serious adverse reactions
Table 25 details the SAEs reported. There were nine SAEs reported in total which were split evenly across the groups. There was one grade 2, six grade 3s, one grade 4 and one grade 5. None of the SAEs were related to treatment.
| PI assess-ment | Groupa | System Organ Class | Date SAE reported | CR assessmentb | Date treatment last received | Date of onset of SAE | CTCAE gradec | Seriousd | Actione | Causalityf | Status when resolvedg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SAE | Spironolactone | Infections and infestations | 20 September 2021 | SAE | Unknown | Unknown | 3 | 3 | Unknown | 4 | Unknown |
| SAE | Spironolactone | Injury, poisoning and procedural complications |
26 August 2021 | SAE | 12 July 2021 | 12 July 2021 | 3 | 3 | 0 | 5 | 1 |
| SAE | Spironolactone | Respiratory, thoracic and mediastinal disorders |
2 September 2021 | SAE | 22 April 2021 | 23 April 2021 | 3 | 3 | 0 | 5 | 1 |
| SAE | Spironolactone | Surgical and medical procedures | 2 October 2020 | SAE | 17 July 2020 | 1 October 2020 | 2 | 3 | 0 | 4 | 1 |
| SAE | Placebo | Gastrointestinal disorders | 1 September 2021 | SAE | 26 August 2021 | 31 August 2021 | 3 | 3 | 0 | 5 | 2 |
| SAE | Placebo | Neoplasms benign, malignant and unspecified (incl cysts and polyps) |
27 October 2021 | SAE | 21 September 021 | 21 September 2021 | 3 | 6 | 4 | 5 | Ongoing |
| SAE | Placebo | Nervous system disorders | 3 January 2020 | SAE | 11 December 2019 | 11 December 2019 | 3 | 2 | 0 | 4 | 1 |
| SAE | Placebo | Psychiatric disorders | 21 April 2021 | SAE | 27 March 2021 | 27 March 2021 | 4 | 2 | 0 | 5 | 1 |
| SAE | Placebo | Respiratory, thoracic and mediastinal disorders |
23 September 2021 | SAE | 29 March 2021 | 25 May 2021 | 5 | 1 | 0 | 5 | 5 = Fatal |
Pregnancies
Seven participants became pregnant while in the trial. The pregnancies were recorded at different sites and different contraception methods were used. Contraception was discussed with each participant at their baseline visit. Pregnant participants were withdrawn from the trial as soon as site staff became aware of the pregnancy (level 2 withdrawal). Pregnant participants were approached for consent for pregnancy follow-up. Three participants did not consent to the outcome of the pregnancy being followed up. Four participants consented to pregnancy outcome follow-up. All pregnancies were reported to Medicines and Healthcare products Regulatory Agency (MHRA) as part of the Development Safety Update Reports over the course of the trial following recommendation from the DMEC group, although the pregnancies did not meet the requirements to trigger an urgent safety measure.
Chapter 4 Economic evaluation
Introduction
The primary objective of this within-trial economic evaluation was to estimate, via a cost–utility analysis, the cost-effectiveness of spironolactone used with routine topical treatment compared to no active treatment (placebo) in addition to routine topical treatment for moderate to severe persistent adult female acne over 24 weeks, using individual level data collected within the SAFA trial. Literature searches did not find any previously published economic evidence for this question.
The secondary objective was to undertake a cost-effectiveness (CEA) analysis using the disease-specific Acne-QoL to estimate incremental cost per unit of change on the Acne-QoL symptom subscale over 24 weeks.
In addition, we set out to measure health-related quality of life using the EQ-5D-5L at week 6, 12, 24 and up to week 52 in order to compare quality-adjusted life-years (QALYs) between groups at week 24 and up to week 52. We also compared cost at week 24 and up to 52, based on resource use data collected at the individual participant level at week 6, 12, 24 and up to week 52, including use of other oral treatments for acne during follow-up.
Methods
Overview of economic analysis
The base (or reference) case within-trial economic analysis uses individual participant level data collected over 24 weeks from the SAFA trial to compare the cost-effectiveness of spironolactone used alongside routine topical treatment compared to no active treatment (placebo) alongside routine topical treatment for moderate to severe persistent adult female acne. This comparison was chosen because it reflected the clinical question being addressed and data being collected within the SAFA trial. There are potential questions with alternative comparisons that may be of more interest to clinicians. For instance, in practice women with persistent acne are likely to be given an active treatment on top of routine topical treatment. These women would most likely receive an oral antibiotic (lymecycline or doxycycline) as per NICE guidance12 suggesting a systemic antibiotic alongside topicals for more moderate to severe disease. We explore this question, which is more reflective of clinical practice, in sensitivity analysis but there are limitations to such an analysis given the data we have available.
The base case analysis undertakes a cost–utility analysis from an NHS and Personal Social Services (PSS) perspective, where outcome is defined in terms of incremental difference in the mean number of QALYs. A secondary analysis is undertaken using a cost-effectiveness analysis approach. This was chosen as the secondary analysis due to uncertainties about what an incremental cost per point change on the Acne-QoL symptom subscale means or how much a decision maker would value this.
Published guidelines for the economic evaluation of healthcare interventions were followed as appropriate. 37,38 NICE published updated guidance after analysis for this trial had started. 39–41 While much of the guidance remains unchanged, the preferred mapping function to be used in reference case analyses for the EQ-5D-5L was updated from that published by van Hout et al. 42 to that of Alava et al. 43,44 We have adhered to the earlier guidelines as the analysis started prior to the change in guidelines.
A copy of the health economic analysis plan that was written and reviewed prior to the trial database being locked can be found in the project documents.
Resource use
In keeping with the trial being conducted in the UK, where the NHS provides publicly funded health care, the analysis takes a health service perspective which is also in line with the NICE reference case. 45 Resource use was collected at baseline (for the 6 weeks prior to the baseline visit), week 6, week 12 and week 24 via case report forms and participant questionnaires. PSS resource use was not captured explicitly, as it was anticipated that these types of services would be unlikely to be incurred as a result of acne. Costs incurred by the participant and/or a family member or friend were also collected, informed by input with the patient and public involvement (PPI) members of the study team. This included personal out-of-pocket costs (including complementary therapists, non-prescribed medication, travel costs to healthcare appointments, parking costs at healthcare appointments, cosmetic and skin care products, and other costs), impact on employment (productivity costs) for the woman or a family member/friend, and support services outside of official services.
Valuing costs
The cost of the intervention was estimated using data collected within the SAFA trial and costed using published unit costs for spironolactone in the prescription cost analysis (PCA). 46 In the base case analysis, the intervention is costed as per the schedule shown in Table 26, where the intervention is delivered in primary care. The delivery of the intervention in a primary care setting (i.e. GP practice) reflects how the intervention would be delivered in clinical practice. In the trial, the medication was either posted directly to participants’ homes or participants collected their medication at secondary care follow-up visits (if face-to-face). In clinical practice, patients would collect their medication via repeat prescription from their GP surgery or pharmacy. As a result, postage costs were not included in the economic evaluation.
| Cost item | Unit cost (£) | Unit | Source, assumptions |
|---|---|---|---|
| Intervention | |||
| Spironolactone with dose escalation | 49.37 | 1 × 50 mg tablet daily for 6 weeks followed by 2 × 50 mg tablet daily for 18 weeks. PCA 2021. | |
| GP visit related to intervention | 33.00 | Cost per visit. Number of anticipated visits varies depending on patient age. PSSRU Unit Costs 2020–1. Chapter 10.3a, p. 110, per surgery consultation lasting 9.22 minutes. Including direct care staff costs, without qualification costs. | |
| Serum pregnancy test | 2.61 | National Schedule of NHS Costs 2020. Assumes a single blood test taken during GP visit and analysed by pathology as a single haematology sample. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Blood test for renal function (eGFR) and potassium level (K serum) | 5.22 | National Schedule of NHS Costs 2020. Assumes a single blood test taken during GP visit and analysed by pathology as two tests on a single haematology sample. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Mean cost per quantity (£) | |||
| Medication costs | |||
| Topical preparations for acne | 0.96 | gram/ml | Prescription Cost Analysis England 2021. Mean across all medications in each medication type. Weighted averages taken where listed > 1x. Assumed generic version unless brand name given. Where details (e.g. strength) not given, assumed most commonly prescribed version. |
| Other topical preparation | 0.03 | gram/ml | |
| Oral contraceptives | 0.08 | tablet | |
| Oral antibiotics | 0.22 | capsule/tablet | |
| Anti-depressants | 0.20 | capsule/tablet | |
| Analgesics | 0.04 | capsule/tablet | |
| PCOS/diabetes medication | 0.03 | tablet | |
| Other medications | 0.40 | various | |
| Doxycycline/lymecycline weighted average | 0.25 | capsule | Prescription Cost Analysis England 2021. Weighted average for estimating oral antibiotic control for SA3 (see Table 11). Assumes 1 × 100 mg (doxycycline)/408 mg (lymecycline) per day for 24 weeks in SA3 analysis. |
| Community-based HCP contacts | |||
| GP visit unrelated to intervention | 33.00 | PSSRU Unit Costs 2020–1. Chapter 10.3a, p. 110, per surgery consultation lasting 9.22 minutes. Including direct care staff costs, without qualification costs. | |
| Practice nurse | 14.13 | PSSRU Unit Costs 2020–1 and PSSRU Unit Costs 14/15. Chapter 10, p. 109. Primary Care Practice Nurse. Including direct care staff costs, without qualification costs. PSSRU 2015, p. 174 direct to indirect time: 1 : 0.30. Duration of contact 15.5 minutes. | |
| NHS walk-in centre | 71.99 | National Schedule of NHS Costs 2020. Assumed weighted average of all community health services. Weighted average for all outpatient appointments £137 inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Cost 2021, p. 145). | |
| Community dermatology service | 121.01 | National Schedule of NHS Costs 2020. Weighted average for dermatology: consultant led and non-consultant led. | |
| Healthcare assistant | 14.44 | PSSRU Unit Costs 2020–1 and UKHCA Commissioning Survey 2012. PSSRU 2021 spreadsheet ‘community-based’ social care professionals – home care working (note that the cost per hour differs from that in the publication, p. 126, but think the one quoted in the publication is the error). PSSRU 2021, section 11.5, p. 126.1 : 0.25 Direct to indirect time on face-to-face contact (includes travel). Assumes day-time week price. Assumes 30-minute visit duration based on majority of visits (63%) being 16–30 minutes (primary ref: UKHCA Commissioning Survey 2012. ‘Care is not a commodity’). | |
| Pharmacist | 6.99 | PSSRU Unit Costs 2020–1 and PSNC Pharmacy Advice Audit 2021. PSSRU 2015 ratio of direct to indirect time on patient related activities: 1 : 0.43. PSNC Pharmacy advice audit: 5.48 minutes – mean length of time a pharmacist spent with the patient, p. 18. | |
| Physiotherapist | 66.82 | National Schedule of NHS Costs 2020. Physiotherapist, adult, one to one. | |
| Dietitian | 82.46 | National Schedule of NHS Costs 2020. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Other (community) | 33.00 | Used most common visit: GP visit. PSSRU Unit Costs 2020–1. Chapter 10.3a, p. 110, per surgery consultation lasting 9.22 minutes. Including direct care staff costs, without qualification costs. | |
| Hospital outpatient contacts | |||
| Dermatologist | 128.25 | National Schedule of NHS Costs 2019–20. Consultant-led outpatient attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Dermatology nurse | 100.71 | National Schedule of NHS Costs 2019–20. Non-consultant led outpatient attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Ear, nose and throat (ENT) | 116.11 | National Schedule of NHS Costs 2019–20. Consultant-led outpatient attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Interventional radiology | 137.64 | National Schedule of NHS Costs 2019–20. Total attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Trauma and orthopaedics | 125.67 | National Schedule of NHS Costs 2019–20. Total attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Respiratory medicine | 161.07 | National Schedule of NHS Costs 2019–20. Total attendance. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Other (outpatient) | 137.10 | National Schedule of NHS Costs 2019–20. Total attendance for all outpatient types. Inflated to 2021 prices as per NHSCII Pay and Prices (PSSRU Unit Costs 2021, p. 145). | |
| Hospital admission | |||
| Accident and Emergency | 182.28 | National Schedule of NHS Costs 2019–20. Index/Accident and Emergency. | |
| Personal out-of-pocket expenses | |||
| Lost work time | 18.01 | Office for National Statistics Annual Survey of Hours and Earnings time series of selected estimates. Mean hourly earnings, excluding overtime (£), 2021 – provisional. | |
Resource use relevant to the NHS perspective was valued using UK unit costs (Great British pounds) for the most current price year available at the start of the analysis (2021). Unit costs were identified from published sources and are clearly reported in the results section.
A mean cost (SD) per participant per intervention group was estimated for 24 weeks.
Outcomes
The primary economic outcome measure was incremental cost per QALYs over 24 weeks. The EQ-5D-5L is a generic preference-based measure of health-related quality of life instrument with five dimensions (mobility, pain/discomfort, usual activities, self-care, anxiety/depression) with five levels ranging from ‘no problems’ through to ‘unable to’ or ‘extreme problems’. 47,48 Responses were converted to utility scores using the EQ-5D-5L Crosswalk UK preference weights, as this was in line with recommendations at the point analysis started, where utility ranges from –0.594 to 1. 42 Utility values were used to estimate QALYs over 24 weeks, using both linear interpolation and area under the curve analysis with and without baseline adjustment. 48
Secondary analysis reports a cost-effectiveness analysis (CEA) where the incremental cost per unit change on the Acne-QoL symptom subscale score is estimated (i.e. summing items 15–19, see the Health Economics Analysis Plan for details). The Acne-QoL symptom subscale consists of five questions, each with answers on a scale of 0–6 (‘extensive, a whole lot, a lot, a moderate amount, some, very few, none’ for items 15–17 and ‘extremely, very much, quite a bit, a good bit, somewhat, a little bit, and not at all’ for items 18–19) with total scores ranging from 0 to 30 [Acne-Specific Quality of Life Questionnaire (Acne-QoL) Manual and Interpretation Guide]. 49 We undertook a cost-effectiveness analysis despite it not being clear what decision makers would be willing to pay for a unit change on the Acne-QoL symptom subscale because Klassen et al. 50 found that clinical measures were more responsive to change than the generic measures (shown by larger effect sizes) and concluded that it is desirable to combine generic preference-based measures with the use of disease-specific measures for acne.
Economic evaluation at 24 weeks
A full economic evaluation was only planned if spironolactone was found to be clinically effective. The economic base case analysis incorporates all randomised participants with complete cost and outcome data available. Given that the time horizon was 24 weeks, costs and benefits are not discounted. 45 The main base case analysis was a cost–utility analysis to estimate the incremental cost per QALY to enable comparison with the cost-effectiveness of other interventions. A cost-effectiveness threshold (ʎ) of £30,000 (£20,000) per QALY is used in line with NICE guidance. 45
Mean (SD) resource use and mean (SD) cost per participant is estimated per randomised group. The incremental cost (95% CI) between groups is estimated adjusted for randomisation stratification variables [centre, baseline severity (IGA < 3 vs. 3 or more)], baseline variables as in the primary analysis [including baseline Acne-QoL symptom subscale score and use of topical treatments (Y/N)] and baseline outcome variable (baseline EQ-5D) and unadjusted. Given that baseline characteristics and baseline outcome measurements are often predictive of total costs and QALYs, it is usual to give more weight to adjusted analyses than unadjusted analyses. 48,51 Mean (SD) utility and mean (SD) QALYs per participant per randomised group are presented along with the incremental QALYs (95% CI) between groups adjusted and unadjusted. The base case cost–utility analysis and secondary cost-effectiveness analysis are undertaken using a regression-based approach, seemingly unrelated regression equations [sureg command was used in Stata 17 (StataCorp LP, College Station, TX)], which allows covariate adjustment without requiring covariates for cost and effectiveness be the same. 51
To determine the level of sampling uncertainty surrounding the mean incremental cost-effectiveness ratios (ICERs), non-parametric bootstrapping was conducted to generate 10,000 estimates of incremental costs and benefits. From this, cost-effectiveness acceptability curves (CEACs) were generated to show the probability that the intervention is cost-effective at different values of willingness to pay. Stata MP version 17 was used to conduct the analysis.
A single subgroup analysis was undertaken for age (categorised as below 25 years and 25 years and over) as the clinical analysis found age to be a significant interaction term (see Subgroup analysis).
Sensitivity analysis
The following sensitivity analyses were undertaken to explore key uncertainties around important parameters in the economic evaluation:
Sensitivity analysis 1 (SA1): The base case economic evaluation over 24 weeks did not impute missing data, instead a complete case analysis (CCA) was undertaken. To evaluate the impact of missing data on the cost-effectiveness estimates, multiple imputation was employed, assuming that the data was missing at random (MAR). The mi impute chained [pmm, knn(10)] command was used in Stata 17, which imputes missing data using chained equations (MICE) and predictive mean matching (to ensure only plausible values are imputed). The model included the outcome measure (cost or utility), baseline value of the outcome (cost or utility), randomisation group, and all covariates {randomisation stratification variables [centre, baseline severity (IGA < 3 vs. 3 or more)] and baseline variables [including baseline Acne-QoL symptom subscale score, use of topical treatments (Y/N)]} included in the analysis model. To handle the missing cost and outcome data to assess the impact on the conclusions reached,52 a further sensitivity analysis (SA1a) was undertaken to test the assumption of MAR. A common concern in economic evaluations, particularly if data are missing to different degrees in different treatments groups, is that the probability of being missing may depend on the underlying unobserved values, for example the chances of completing a QoL questionnaire may depend on the patients (unobserved) QoL status: the lower their QoL, the less likely they are to complete a questionnaire. This is referred to as ‘missing not at random’ (MNAR). 53 Therefore, following review of the patterns of missingness, to test for the impact of possible MNAR on the results, we use a pattern-mixture model. 53 This model involves modifying the multiply imputed data to reflect possible departures from the MAR assumption, by multiplying the imputed values by a plausible value, and reanalysing the results. We tested the following scenarios:
-
missing EQ-5D data were 5% lower than imputed in both groups of the study
-
missing EQ-5D data were 10% lower than imputed in both groups of the study
-
missing cost data were 5% higher than that imputed in both groups of the study
-
missing cost data were 10% higher than that imputed in both groups of the study
-
missing EQ-5D data were 5% higher and cost data 5% lower than imputed in both groups of the study
-
missing EQ-5D data were 10% higher and cost data 10% lower than imputed in both groups of the study.
Sensitivity analysis 2 (SA2): The cost–utility analysis was repeated, but the intervention was costed as per the SAFA trial protocol (i.e. intervention was accessed via secondary care) in order to provide a range on the possible cost-effectiveness estimate from the base case analysis (i.e. where intervention accessed via primary care). This was a more conservative analysis as secondary care delivery of the intervention is more costly.
Sensitivity analysis 3 (SA3): The cost–utility analysis was repeated assuming the NICE guidance12 on acne was followed, meaning all women in the placebo group were assumed to have taken oral antibiotics (lymecycline or doxycycline, one tablet daily for 24 weeks), in addition to topical treatment. As this patient population had persistent acne of sufficient severity to warrant treatment with oral antibiotics, this is a reasonable assumption. In this analysis we assumed that the health-related quality of life (measured using the EQ-5D-5L) remained unchanged from that which was collected in the SAFA trial. In reality we did not know what the health-related quality of life of these women would be. However, assuming that these women took oral antibiotics (and the antibiotics were effective), the incremental QALYs would be less than that observed in the SAFA trial for women in the placebo group. A threshold analysis was performed to ascertain what level of QALYs would switch the intervention between cost-effective and cost-ineffective.
Sensitivity analysis 4 (SA4): The cost–utility analysis was repeated taking a wider perspective by including the participants’ and family/friends’ out-of-pocket costs, productivity costs and non-official services costs.
Sensitivity analysis 5 (SA5): We performed a descriptive analysis of data collected after participants were unblinded to treatment (at week 24), from week 25 and up to week 52. The mean costs (SD) and mean (SD) QALYs by randomisation group are reported together with incremental costs and QALYs for up to week 52. Due to the number of missing data up to week 52, costs and QALYs were not combined.
This economic evaluation is reported in line with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidance. 41 We did not, however, examine the distributional effects, that is how costs and outcome were distributed across different individuals, as this item was not on the previous version of the CHEERS checklist at the time the HEAP was written.
Results
The clinical trial results, including details on sample size and participant characteristics, are reported in Chapter 3. Of the 410 women recruited to the trial, 201 were randomly assigned to spironolactone alongside routine topical treatment and 209 allocated to no active treatment (placebo) alongside routine topical treatment at the start of the trial. Sixty women either withdrew, were lost to follow-up, or did not attend the 24-week visit, 19 in the spironolactone group and 41 in the placebo group. Three hundred and one women completed the questionnaire at week 24 (164 in the spironolactone group and 137 in the placebo group). Of these 301 women, 126 women in the spironolactone group and 109 women in the placebo group had complete cost and outcome data. Data from these 235 women were analysed in the base case unadjusted complete case cost–utility analysis over 24 weeks.
Resource use and costs
Unit costs, together with their source and any assumptions made, are presented in Table 26. Mean baseline resource use per participant for each randomised group is summarised in Table 27 using the available case data. The level of resource use in each group was very similar prior to randomisation.
| Resource | Spironolactone (N = 201) | Placebo (N = 209) | Mean difference | ||
|---|---|---|---|---|---|
| Mean (n) | SD | Mean (n) | SD | (95% CI) | |
| Community-based HCP contacts | |||||
| GP (number of visits) | 0.19 (200) | 0.452 | 0.139 (209) | 0.422 | 0.051 (–0.034 to 0.136) |
| Practice nurse (number of visits) | 0.025 (200) | 0.157 | 0.038 (209) | 0.237 | –0.013 (–0.053 to 0.026) |
| NHS walk-in centre (number of visits) | 0.005 (200) | 0.071 | 0 (209) | 0 | 0.005 (–0.005 to 0.015) |
| Community dermatology service (number of visits) | 0.04 (200) | 0.221 | 0.048 (209) | 0.044 | –0.008 (–0.052 to 0.037) |
| Healthcare assistant (number of visits) | 0.01 (200) | 0.141 | 0 (209) | 0 | 0.01 (–0.009 to 0.029) |
| Other (number of visits) | 0 | 0 | 0 | 0 | 0 |
| Total community-based HCP visits | 0.27 (200) | 0.616 | 0.225 (209) | 0.590 | 0.045 (–0.072 to 0.162) |
| Hospital contacts | |||||
| Dermatologist outpatient (number of visits) | 0.075 (199) | 0.300 | 0.083 (205) | 0.381 | –0.008 (–0.075 to 0.060) |
| Dermatology nurse outpatient (number of visits) | 0.010 (199) | 0.142 | 0.005 (205) | 0.070 | 0.005 (–0.017 to 0.027) |
| Other outpatient (number of visits) | 0.015 (199) | 0.158 | 0.005 (205) | 0.069 | 0.010 (–0.014 to 0.034) |
| A&E admission (number of visits) | 0.015 (194) | 0.160 | 0 (204) | 0 | 0.015 (–0.007 to 0.038) |
| Total hospital contacts | 0.119 (193) | 0.446 | 0.095 (200) | 0.396 | 0.024 (–0.059 to 0.108) |
| Prescription medications | |||||
| Topical acne preparations – quantity (number) | 7.065 (201) | 23.035 | 5.510 (206) | 17.966 | 1.555 (–2.465 to 5.575) |
| Other topical preparations – quantity (number) | 2.736 (201) | 35.425 | 0.243 (206) | 3.484 | 2.493 (–2.382 to 7.370) |
| Oral contraceptives – quantity (number) | 0 (201) | 0 | 0.102 (206) | 1.463 | –0.102 (–0.305 to 0.101) |
| Oral antibiotics – quantity (number) | 0.488 (201) | 3.536 | 1.272 (206) | 7.329 | –0.784 (–1.910 to 0.342) |
| Anti-depressants – quantity (number) | 0.697 (201) | 5.192 | 0.388 (206) | 3.210 | 0.308 (–0.531 to 1.147) |
| Analgesics – quantity (number) | 0.149 (201) | 2.116 | 0 (206) | 0 | 0.149 (–0.141 to 0.439) |
| PCOS/diabetes drugs – quantity (number) | 0 (201) | 0 | 0.272 (206) | 3.902 | –0.272 (–0.813 to 0.269) |
| Other drugs – quantity (number) | 0.577 (201) | 8.182 | 0.117 (206) | 1.672 | 0.461 (–0.684 to 1.605) |
| All medications – quantity (number) | 11.711 (201) | 46.065 | 7.903 (206) | 21.570 | 3.809 (–3.174 to 10.791) |
| Personal out-of-pocket expenses | |||||
| Complementary therapist (number of visits) | 0.154 (188) | 0.703 | 0.087 (196) | 0.461 | 0.068 (–0.051 to 0.186) |
| Cosmetic skincare products (number) | 1.574 (188) | 2.159 | 1.622 (196) | 2.103 | –0.048 (–0.048 to 0.380) |
| Non-NHS-prescribed medication (number) | 0.202 (188) | 0.848 | 0.148 (196) | 0.539 | 0.054 (–0.088 to 0.196) |
| Parking costs at healthcare appointments (number) | 0.053 (188) | 0.322 | 0.046 (196) | 0.369 | 0.007 (–0.062 to 0.077) |
| Travel costs to healthcare appointments (number) | 0.037 (188) | 0.190 | 0.031 (196) | 0.200 | 0.007 (–0.033 to 0.046) |
| Other out-of-pocket expense (number) | 0.005 (188) | 0.073 | 0.005 (196) | 0.071 | 0.000 (–0.014 to 0.015) |
| Total out-of-pocket items | 2.027 (188) | 2.735 | 1.939 (196) | 2.438 | 0.088 (–0.432 to 0.607) |
| Productivity losses | |||||
| Lost patient work time (number reporting) | 0.020 (197) | 0.141 | 0.034 (205) | 0.182 | –0.014 (–0.046 to 0.018) |
| Lost carer work time (number reporting) | 0.015 (194) | 0.124 | 0.030 (203) | 0.170 | –0.014 (–0.044 to 0.015) |
Intervention resource use and costs
If implemented in clinical practice, spironolactone would most likely be used alongside a topical treatment and would be obtained by the patient through their GP (i.e. primary care). Figure 4 shows a flow chart of resource use for the intervention group (spironolactone) as if it was being delivered in primary care. The majority of women (182/184, 99%) increased to two tablets of spironolactone at week 6. In the 24-week period where spironolactone was used and data were available, the mean number of doses per participant was 293 (SD 9.47). The standard of care approach (expected resource use if implemented widely in primary care) used in the base case economic evaluation, gave rise to a mean cost of £122.87 (SD £13.04) per participant (including medication, GP visits and blood tests) (refer Table 28 for breakdown).
FIGURE 4.
Intervention resource use as per proposed standard of care (base case). a, Assumes all patients escalated to two 50 mg tablets spironolactone at 6-week visit. Based on the data from the trial, this was the case for 182/184 (99%) of available patients in the spironolactone group at 6 weeks. b, As those with relevant comorbidities or on treatments with increased risk were not included in the trial, it is not possible to estimate the proportion of such patients that might receive spironolactone in reality and that would need blood test monitoring.
| Resource | Spironolactone (N = 201) | Placebo (N = 209) | Mean difference | ||
|---|---|---|---|---|---|
| Mean (n) | SD | Mean (n) | SD | (95% CI) | |
| Intervention | |||||
| Spironolactone (number) | 294 (201) | 0 | 0 (209) | 0 | – |
| GP visits related to intervention (number of visits)a | 2.060 (201) | 0.341 | 0 (209) | 0 | – |
| Blood tests – renal function (eGFR) and potassium level (number) | 1.060 (201) | 0.341 | 0 (209) | 0 | – |
| Community-based HCP contacts | |||||
| GP (number of visits) | 0.1 (150) | 0.380 | 0.081 (124) | 0.351 | 0.019 (–0.068 to 0.107) |
| Practice nurse (number of visits) | 0.013 (150) | 0.115 | 0.008 (124) | 0.090 | 0.005 (–0.001 to 0.023) |
| NHS walk-in centre (number of visits) | 0.013 (150) | 0.163 | 0 (124) | 0 | 0.013 (–0.016 to 0.042) |
| Community dermatology service (number of visits) | 0.013 (150) | 0.115 | 0.008 (124) | 0.090 | 0.005 (–0.020 to 0.030) |
| Healthcare assistant (number of visits) | 0 (150) | 0 | 0 (124) | 0 | 0 |
| Other (number of visits) | 0.007 (150) | 0.082 | 0 (124) | 0 | 0.007 (–0.008 to 0.021) |
| Total community-based HCP visits | 0.147 (150) | 0.510 | 0.097 (124) | 0.431 | 0.050 (–0.064 to 0.164) |
| Hospital contacts | |||||
| Dermatologist outpatient (number of visits) | 0.02 (150) | 0.140 | 0.024 (127) | 0.152 | –0.004 (–0.038 to 0.031) |
| Dermatology nurse outpatient (number of visits) | 0.02 (150) | 0.140 | 0.039 (127) | 0.232 | –0.019 (–0.064 to 0.025) |
| Other outpatient (number of visits) | 0.013 (150) | 0.163 | 0 (127) | 0 | 0.013 (–0.015 to 0.042) |
| A&E admission (number of visits) | 0 (134) | 0 | 0 (116) | 0 | 0 |
| Total hospital contacts | 0.061 (132) | 0.296 | 0.052 (115) | 0.260 | 0.008 (–0.062 to 0.079) |
| Prescription medications | |||||
| Topical acne preparations – quantity (number) | 9.898 (147) | 25.398 | 9.879 (124) | 37.092 | 0.019 (–7.493 to 7.531) |
| Other topical preparations – quantity (number) | 0 (147) | 0 | 0 (124) | 0 | 0 |
| Oral contraceptives – quantity (number) | 0 (147) | 0 | 0.677 (124) | 7.543 | –0.677 (–1.902 to 0.547) |
| Oral antibiotics – quantity (number) | 1.143 (147) | 10.300 | 2.532 (124) | 13.653 | –1.389 (–4.258 to 1.479) |
| Anti-depressants – quantity (number) | 0.381 (147) | 4.619 | 1.355 (124) | 9.348 | –0.974 (–2.697 to 0.749) |
| Analgesics – quantity (number) | 0 (147) | 0 | 1.935 (124) | 21.553 | –1.935 (–5.434 to 1.563) |
| PCOS/diabetes drugs – quantity (number) | 0 (147) | 0 | 1.581 (124) | 17.601 | –1.581 (–4.438 to 1.277) |
| Other drugs – quantity (number) | 0 (147) | 0 | 5.403 (124) | 60.168 | –5.403 (–15.170 to 4.364) |
| All medications – quantity (number) | 11.422 (147) | 29.653 | 23.363 (124) | 96.804 | –11.941 (–28.507 to 4.625) |
| Personal out-of-pocket expenses | |||||
| Complementary therapist (number of visits) | 0.122 (131) | 0.608 | 0.230 (113) | 1.044 | –0.108 (–0.320 to 0.104) |
| Cosmetic skincare products (number) | 3.267 (131) | 5.657 | 3.903 (113) | 6.049 | –0.635 (–2.113 to 0.842) |
| Non-NHS-prescribed medication (number) | 0.115 (131) | 0.506 | 0.292 (113) | 1.200 | –0.178 (–0.404 to 0.049) |
| Parking costs at healthcare appointments (number) | 0.031 (131) | 0.173 | 0 (113) | 0 | 0.031 (–0.001 to 0.063) |
| Travel costs to healthcare appointments (number) | 0.031 (131) | 0.213 | 0.009 (113) | 0.094 | 0.020 (–0.021 to 0.064) |
| Other out-of-pocket expenses (number) | 0.023 (131) | 0.150 | 0.053 (113) | 0.397 | 0.037 (–0.104 to 0.044) |
| Total out-of-pocket items | 3.588 (131) | 5.955 | 4.487 (113) | 6.666 | –0.899 (–2.491 to 0.693) |
| Productivity losses | |||||
| Lost patient work time (number reporting) | 0.000 (186) | 0.000 | 0.021 (191) | 0.144 | –0.021 (–0.042 to –0.000) |
| Lost carer work time (number reporting) | 0.005 (185) | 0.074 | 0.021 (190) | 0.144 | –0.016 (–0.039 to 0.008) |
Other health resource use and costs
Mean resource use for the spironolactone and placebo groups are shown in Table 28. Table 29 reports the disaggregated mean discounted costs per participant for both groups using available case data. When intervention use was combined with other health resource use, the mean difference per participant was £126.35 (95% CI, £112.88 to £139.82) for women receiving spironolactone compared to women receiving placebo in the base case (see Table 29). The difference in total costs between both groups largely reflects the cost of the intervention; other NHS costs were not significantly different between groups (£3.98 higher per participant, on average, in the intervention group, 95% CI, –£9.30 to £17.26).
| Resource | Spironolactone (N = 201) | Placebo (N = 209) | Mean difference | ||
|---|---|---|---|---|---|
| Mean (n) | SD | Mean (n) | SD | (95% CI) | |
| Intervention (standard of care) | |||||
| Spironolactone | 49.36 (201) | 0 | 0 (209) | 0 | 49.36 (49.36 to 49.36) |
| GP visits related to intervention | 67.97 (201) | 11.26 | 0 (209) | 0 | 67.97 (66.44 to 69.50) |
| Blood tests – renal function (eGFR) and potassium level | 5.53 (201) | 1.78 | 0 (209) | 0 | 5.53 (5.29 to 5.78) |
| All intervention costs | 122.87 (201) | 13.04 | 0 (209) | 0 | 122.87 (121.09 to 124.64) |
| Community-based HCP contacts | |||||
| GP | 3.30 (150) | 12.54 | 2.66 (124) | 11.60 | 0.64 (–2.26 to 3.53) |
| Practice nurse | 0.19 (150) | 1.63 | 0.11 (124) | 1.27 | 0.07 (–0.28 to 0.43) |
| NHS walk-in centre | 0.96 (150) | 11.76 | 0 (124) | 0 | 0.96 (–1.12 to 3.04) |
| Community dermatology service | 1.61 (150) | 13.92 | 0.98 (124) | 10.87 | 0.64 (–2.38 to 3.66) |
| Healthcare assistant | 0 (150) | 0 | 0 (124) | 0 | 0 |
| Other | 0.22 (150) | 2.69 | 0 (124) | 0 | 0.22 (–0.26 to 0.70) |
| All community-based HCP costs | 6.28 (150) | 24.83 | 3.75 (124) | 16.46 | 2.53 (–2.60 to 7.66) |
| Hospital contacts | |||||
| Dermatologist outpatient | 2.57 (150) | 18.02 | 3.03 (127) | 19.55 | –0.46 (–4.91 to 3.98) |
| Dermatology nurse outpatient | 2.01 (150) | 14.15 | 3.96 (127) | 23.40 | –1.95 (–6.45 to 2.55) |
| Other outpatient | 1.83 (150) | 22.39 | 0 (127) | 0 | 1.83 (–2.08 to 5.74) |
| A&E admission | 0 (134) | 0 | 0(116) | 0 | 0 |
| All hospital contact costs | 7.28 (132) | 36.42 | 5.73 (115) | 28.09 | 1.55 (–6.70 to 9.79) |
| Prescription medications | |||||
| Topical acne preparations | 4.01 (147) | 10.94 | 4.63 (124) | 17.75 | –0.62 (–4.09 to 2.85) |
| Other topical preparations | 0 (147) | 0 | 0 (124) | 0 | 0 |
| Oral contraceptives | 0 (147) | 0 | 0.04 (124) | 0.45 | –0.04 (–0.11 to 0.03) |
| Oral antibiotics | 0.29 (147) | 2.70 | 0.63 (124) | 3.50 | –0.34 (–1.08 to 0.40) |
| Anti-depressants | 0.08 (147) | 0.91 | 0.20 (124) | 1.60 | –0.13 (–0.43 to 0.18) |
| Analgesics | 0 (147) | 0 | 0.07 (124) | 0.82 | –0.07 (–0.21 to 0.06) |
| PCOS/diabetes drugs | 0 (147) | 0 | 0.05 (124) | 0.57 | –0.05 (–0.14 to 0.04) |
| Other drugs | 0 (147) | 0 | 0.28 (124) | 3.17 | –0.28 (–0.80 to 0.23) |
| All medication costs | 4.37 (147) | 11.77 | 5.91 (124) | 18.93 | –1.54 (–5.25 to 2.17) |
| Total costs (NHS perspective) | 141.99 (128) | 57.90 | 15.64 (110) | 45.62 | 126.35 (112.88 to 139.82) |
| Total costs (NHS perspective), excluding intervention | 19.61 (128) | 56.65 | 15.64 (110) | 45.62 | 3.98 (–9.30 to 17.26) |
| Personal out-of-pocket expenses | |||||
| Complementary therapist | 9.96 (139) | 57.54 | 17.37 (120) | 106.11 | –7.41 (–27.93 to 13.11) |
| Cosmetic skincare products | 56.77 (139) | 82.90 | 60.05 (120) | 78.00 | –3.28 (–23.07 to 16.52) |
| Non-NHS-prescribed medication | 1.84 (139) | 7.97 | 4.23 (120) | 18.13 | –2.39 (–5.74 to 0.96) |
| Parking costs at healthcare appointments | 0.08 (139) | 0.50 | 0 (120) | 0 | 0.08 (–0.01 to 0.17) |
| Travel costs to healthcare appointments | 0.23 (139) | 1.84 | 0.48 (120) | 5.20 | –0.25 (–1.18 to 0.68) |
| Other out-of-pocket expenses | 0.53 (139) | 4.38 | 0.44 (120) | 3.41 | 0.08 (–0.89 to 1.06) |
| All personal out-of-pocket costs | 69.41 (139) | 113.05 | 82.57 (120) | 148.60 | –13.15 (–45.23 to 18.92) |
| Productivity losses | |||||
| Lost patient and carer productivity | 27.87 (177) | 354.76 | 15.95 (179) | 183.54 | 11.93 (–46.86 to 70.71) |
| Total costs (wider perspective) | 252.67 (113) | 490.19 | 93.53 (100) | 144.02 | 159.14 (58.86 to 259.41) |
Participant out-of-pocket and productivity costs
Personal out-of-pocket expenses costs were £69.41 (SD £113.05) per participant for the spironolactone group compared to £82.57 (SD 148.60) for the placebo group, giving a mean difference of –£13.15 (95% CI –£45.23 to £18.92) per participant. Out of the six categories of personal costs explicitly asked about, cosmetics skincare products formed the largest cost category, with a mean cost of £56.77 (SD 82.90) per participant in the intervention group and £60.05 (SD 78.00) per participant in the control group, followed by complementary therapy, with a mean cost of £9.96 (SD 57.54) per participant in the intervention group and £17.37 (SD 106.11) per participant in the control group. The five main categories included under out-of-pocket expenses, as informed by input with the study team’s PPI members, captured nearly all out-of-pocket costs; the ‘other’ category included to capture anything that may have been missed was rarely used.
Four participants reported using other support services, including an online acne support forum or social media groups for PCOS/Acne, but did not report any costs for using these.
In terms of productivity costs, response rates to these questions varied between 98% at baseline, 70% at week 24 and < 7% at up to week 52. Very few women, in paid employment, reported an impact on their employment as a result of their acne. The number of women reporting an impact on their paid employment was in single figures. It was not always possible to attach a cost on the basis of the information provided.
In terms of whether the participants had a family member or friend who had taken time off paid work to accompany them to healthcare appointments related to their acne, six women reported this at baseline, three at 6 weeks, four at 12 weeks, one at 24 weeks and none at 52 weeks.
Outcomes
Table 30 shows outcomes for both groups unadjusted for the available case data. Over 24 weeks, complete responses to the EQ-5D-5L were received from 99.8% of participants at baseline, 86.6% at week 6, 82.9% at week 12 and 72.9% at week 24. The mean (SD) QALYs in the spironolactone group were 0.4169 (0.0580) per participant compared to 0.4036 (0.0790) per participant in the placebo group, giving an incremental difference of 0.0133 (95% CI –0.0024 to 0.0289) QALYs. Table 31 shows that 91 (46%) of women in the spironolactone group and 89 (43%) women in the placebo group started the trial in perfect health (indicated by answering 11111 in EQ-5D-5L). Thus, there was no potential for the EQ-5D-5L to detect any improvement in the health-related quality of life as a result of the intervention for these 91 women. The proportion of participants in the perfect health state increased throughout the trial, suggesting the intervention had a positive impact on the health-related quality of life for participants who entered the trial in any health state lower than perfect health. One participant in the placebo group entered the trial in the worst self-reported health state (indicated by answering 55555 on the EQ-5D-5L). None of the participants in the spironolactone group entered the trial in the worst self-reported health state. Table 32 shows the number and percentage of women reporting each level on each dimension of the EQ-5D-5L at baseline, week 6, week 12, week 24 and up to week 52. The impact of acne on health-related quality of life is picked up primarily on the pain and anxiety dimensions of the EQ-5D-5L.
| Spironolactone (n = 201) | Placebo (n = 209) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Baseline EQ-5D-5L | 0.8866 | 0.1477 (200) | 0.8601 | 0.1999 (209) | 0.0265 (–0.0078 to 0.0608) |
| 6 weeks EQ-5D-5L | 0.8939 | 0.1354 (176) | 0.8625 | 0.1679 (179) | 0.0314 (–0.0005 to 0.0633) |
| 12 weeks EQ-5D-5L | 0.9043 | 0.1383 (174) | 0.8772 | 0.1768 (166) | 0.0271 (–0.0067 to 0.0608) |
| 24 weeks EQ-5D-5L | 0.9090 | 0.1530 (163) | 0.8901 | 0.1797 (136) | 0.0190 (–0.0189 to 0.0568) |
| QALYs at 24 weeks | 0.4169 | 0.0580 (162) | 0.4036 | 0.0790 (136) | 0.0133a (–0.0024 to 0.0289) |
| 52 weeks EQ-5D-5L | 0.9208 | 0.1516 (92) | 0.8291 | 0.2664 (79) | 0.0918 (0.0274 to 0.1561) |
| QALYs at 52 weeks | 0.9158 | 0.1364 (88) | 0.8515 | 0.2154 (74) | 0.0644 (0.0093 to 0.1194) |
| EQ-5D-5La | Spironolactone (n = 201) | Standard care (n = 209) | Total | |||
|---|---|---|---|---|---|---|
| 11111 | 55555 | 11111 | 55555 | 11111 | 55555 | |
| Baseline | 91 (46%) (n = 200) | 0 (0%) | 89 (43%) (n = 209) | 1 (0.48%) | 180 (44%) | 1 (<1%) |
| 6 weeks | 85 (48%) (n = 176) | 0 (0%) | 71 (40%) (n = 179) | 0 (0%) | 156 (44%) | 0 (0%) |
| 12 weeks | 93 (53%) (n = 174) | 0 (0%) | 79 (48%) (n = 166) | 0 (0%) | 172 (51%) | 0 (0%) |
| 24 weeks | 95 (58%) (n = 163) | 0 (0%) | 70 (51%) (n = 136) | 0 (0%) | 165 (55%) | 0 (0%) |
| 52 weeks | 59 (64%) (n = 92) | 0 (0%) | 32 (41%) (n = 79) | 0 (0%) | 91 (53%) | 0 (0%) |
| EQ-5D-5L domains | Spironolactone (n = 201) | Standard care (n = 209) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Care | Activities | Pain | Anxiety | Mobility | Care | Activities | Pain | Anxiety | |
| Baseline | ||||||||||
| 1 | 185 (93) | 197 (98) | 184 (92) | 159 (79) | 101 (50) | 194 (93) | 200 (96) | 189 (90) | 155 (74) | 101 (48) |
| 2 | 11 (6) | 4 (2) | 13 (6) | 28 (14) | 63 (31) | 11 (5) | 8 (4) | 11 (5) | 34 (16) | 61 (29) |
| 3 | 3 (1) | 0 (0) | 3 (1) | 8 (4) | 30 (15) | 3 (1) | 0 (0) | 5 (2) | 13 (6) | 36 (17) |
| 4 | 1 (0.5) | 0 (0) | 1 (0.5) | 6 (3) | 6 (3) | 0 (0) | 0 (0) | 1 (0.5) | 5 (2) | 8 (4) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 3 (1) | 2 (1) | 3 (1) |
| 6 weeks | ||||||||||
| 1 | 167 (95) | 173 (98) | 166 (94) | 137 (77) | 97 (55) | 167 (93) | 170 (95) | 163 (91) | 134 (75) | 83 (46) |
| 2 | 5 (3) | 3 (2) | 6 (3) | 29 (16) | 49 (28) | 8 (4) | 6 (3) | 8 (4) | 28 (16) | 55 (30) |
| 3 | 4 (2) | 0 (0) | 5 (3) | 6 (3) | 27 (15) | 1 (1) | 2 (1) | 6 (3) | 14 (8) | 29 (16) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 5 (3) | 4 (2) | 3 (2) | 1 (1) | 2 (1) | 2 (1) | 11 (6) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| 12 weeks | ||||||||||
| 1 | 167 (95) | 174 (99) | 161 (91) | 146 (83) | 104 (59) | 153 (92) | 161 (97) | 149 (90) | 125 (75) | 91 (55) |
| 2 | 6 (3) | 1 (1) | 11 (6) | 20 (11) | 39 (22) | 5 (3) | 3 (2) | 11 (6) | 25 (15) | 42 (25) |
| 3 | 3 (2) | 0 (0) | 4 (2) | 6 (3) | 27 (15) | 6 (3) | 1 (1) | 5 (3) | 12 (7) | 27 (16) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 4 (2) | 4 (2) | 2 (1) | 1 (1) | 1 (1) | 2 (1) | 4 (2) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 2 (1) |
| 24 weeks | ||||||||||
| 1 | 156 (96) | 159 (98) | 153 (94) | 137 (84) | 100 (61) | 125 (92) | 131 (96) | 127 (93) | 112 (82) | 79 (58) |
| 2 | 5 (3) | 4 (2) | 6 (4) | 20 (12) | 39 (24) | 5 (4) | 2 (1) | 5 (4) | 15 (11) | 28 (21) |
| 3 | 2 (1) | 0 (0) | 4 (2) | 4 (2) | 18 (11) | 5 (4) | 3 (2) | 1 (1) | 6 (4) | 25(18) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 5 (3) | 1 (1) | 0 (0) | 3 (2) | 1 (1) | 2 (1) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 2 (1) |
| 52 weeks | ||||||||||
| 1 | 89 (96) | 92 (99) | 88 (95) | 78 (85) | 61 (66) | 70 (89) | 73 (92) | 66 (84) | 55 (70) | 36 (46) |
| 2 | 2 (2) | 1(1) | 4 (4) | 8 (9) | 20 (22) | 2 (3) | 4 (5) | 6 (8) | 14 (18) | 24 (30) |
| 3 | 2 (2) | 0 (0) | 1 (1) | 4 (4) | 10 (11) | 5 (6) | 1 (1) | 2 (3) | 6 (8) | 15 (19) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 1 (1) | 2 (3) | 1 (1) | 4 (5) | 0 (0) | 2 (3) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (1) | 4 (5) | 2 (3) |
The mean score of the Acne-QoL symptom subscale at 24 weeks was 21.215 (SD 5.859) in the spironolactone group compared to 17.389 (SD 5.802) in the placebo group (Table 33). Thus, the incremental difference in score was 3.852 (95% CI 2.492 to 5.158) (27% missing data at week 24), refer to Table 34.
| Spironolactone (n = 201) | Placebo (n = 209) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Mean | SD (n) | Mean | SD (n) | ||
| Baseline symptom Acne-QoL | 13.219 | 4.943 (201) | 12.866 | 4.546 (209) | 0.353 (–0.569 to 1.274) |
| 6 weeks symptom Acne-QoL | 16.967 | 5.715 (176) | 15.648 | 5.691 (179) | 1.319 (0.129 to 2.510) |
| 12 weeks symptom Acne-QoL | 19.205 | 6.118 (176) | 17.759 | 5.580 (166) | 1.446 (0.198 to 2.693) |
| 24 weeks symptom Acne-QoL | 21.215 | 5.859 (163) | 17.389 | 5.802 (136) | 3.825 (2.492 to 5.158) |
| Symptom Acne-QoL change at 24 weeks compared to baseline | 8.147 | 6.118 (163) | 4.463 | 6.337 (136) | 3.684 (2.262 to 5.105) |
| 52 weeks symptom Acne-QoL | 21.634 | 6.257 (95) | 19.963 | 5.697 (81) | 1.671 (–0.122 to 3.464) |
| Symptom Acne-QoL change at 52 weeks compared to baseline | 8.613 | 7.154 (95) | 6.951 | 6.500 (81) | 1.663 (–0.385 to 3.710) |
| Analysis (N s, N p) | Incremental cost (95% CI) | QALY change (95% CI) | ICER (£) | CEAC at £20,000 thresholda (%) | CEAC at £30,000 thresholda (%) |
|---|---|---|---|---|---|
| Base case, CCA, adjusted (118,101) | 125.36 (111.13 to 139.58) | 0.0019 (–0.0096 to 0.0133) | 67,191 | 23 | 35 |
| Base case, CCA, unadjusted (126,109) | 125.53 (112.15 to 138.91) | 0.0036 (–0.0117 to 0.0189) | 34,770 | 37 | 47 |
| SA2: per protocol, CCA, adjusted (118,101) | 265.67 (250.52 to 280.82) | 0.0019 (–0.0096 to 0.0133) | 141,955 | 3 | 12 |
| SA3a: oral antibiotic control, CCA, adjusted (118,101) | 17.11 (2.88 to 31.33) | 0.0019b (–0.096 to 0.0133) | 9169 | 57 | 59 |
| SA3b: oral antibiotic control, multiple imputation (MI), adjusted (201,209) | 11.53 (–0.26 to 23.32) | 0.004 (–0.004 to 0.013) | £2683 | 81 | 82 |
| SA4a: wider perspective, CCA, adjusted (97,85) | 102.07 (64.21 to 139.92) | –0.0027 (–0.0139 to 0.0085) | Dominated | – | – |
| SA4b: wider perspective, MI, adjusted (201,209) | 133.25 (72.52 to 193.93) | 0.0044 (–0.004 to 0.013) | 30,249 | 31 | 50 |
| Subgroup analysis: < 25 years, CCA, adjusted: (28,29) | 108.23 (89.09 to 127.37) | 0.0004 (–0.0141 to 0.0150) | 263,871 | 25 | 33 |
| Subgroup analysis: ≥ 25 years, CCA, adjusted: (90,72) | 133.06 (114.97 to 151.16) | 0.0067 (–0.0079 to 0.0213) | 19,994 | 50 | 62 |
Base case cost–utility analysis
Table 34 presents the results of the cost–utility analysis in terms of incremental costs and QALYs, together with an estimate of the ICER and separately the CEAC (see Table 34). In the CCA, the incremental cost for the spironolactone group (n = 118) compared to the no active intervention (placebo) group (n = 101) was £125.36 (95% CI, £111.13 to £139.58) [unadjusted this was £125.53 (95% CI £112.15 to £138.91)]. The adjusted incremental QALYs for the spironolactone group compared with the no active intervention (placebo) group was 0.0019 (95% –0.0096 to 0.0133) (unadjusted was 0.0036, 95% CI –0.0117 to 0.0189). The ICER was £67,191 (unadjusted £34,770) per QALY, which given a threshold value of £30,000 (£20,000),45 means the intervention appears cost-ineffective in both the adjusted and unadjusted analyses at baseline without taking account of missing data. At a willingness to pay of £30,000 per QALY there was a 35% (unadjusted 47%) chance of spironolactone being cost-effective in this population for women with persistent moderate to severe acne.
The CEACs (Figure 5) of the adjusted and unadjusted base case analysis show that the probability of spironolactone being cost-effective only approaches 50% as the threshold value approaches £120,000 (adjusted), demonstrating a high degree of uncertainty associated with the decision under these conditions.
FIGURE 5.
Cost-effectiveness acceptability curve, CCA, adjusted and unadjusted QALYs.
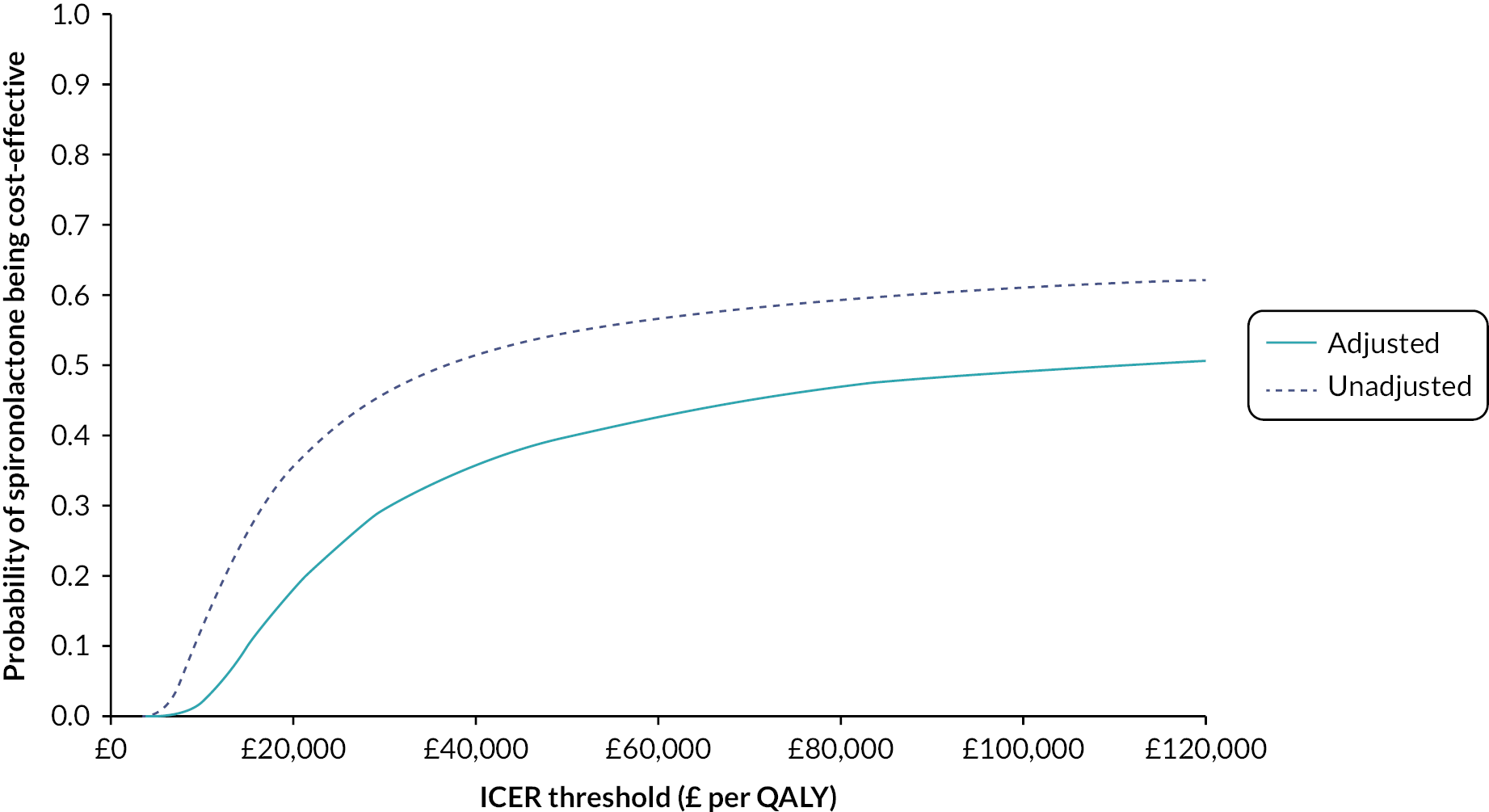
Secondary cost-effectiveness analysis
Refer to Table 35 for a summary of results. The adjusted incremental difference in cost per point change on the Acne-QoL symptom subscale for the spironolactone group (n = 119) compared to the non-active treatment (placebo) group (n = 102) was £38.21 (unadjusted £35.91) based on a CCA. The adjusted mean point change on the Acne-QoL symptom subscale was higher in the spironolactone group suggesting their acne-related quality of life improved more than for those in the placebo group. The spironolactone group was more expensive and more effective than the placebo group. It is impossible to say whether the ICER reported is deemed cost-effective or not as there is uncertainty about how much a decision maker would value a point change on the Acne-QoL symptom subscale.
| Analysis (N s, N p) | Incremental cost (95% CI) | Acne-QoL symptom scale score change (95% CI) | Incremental cost per unit change (£) |
|---|---|---|---|
| Secondary analysis, CCA, adjusted: (119, 102) | 126.57 (112.35 to 140.78) | 3.31 (1.90 to 4.72) | 38.21 |
| Secondary analysis, CCA, unadjusted (127, 110) | 126.52 (113.00 to 140.04) | 3.52 (1.94 to 5.11) | 35.91 |
Pre-specified subgroup analysis
The mean costs and QALYs over 24 weeks for the subgroup analysis by age are shown in Table 36. The ICER was £263,871 per QALY for women under 25 years compared to £19,994 for women over 25 years of age. This result suggests that spironolactone is likely to be cost-effective for women aged over 25 years. While this finding is in line with what might have been anticipated looking at the results found in the clinical pre-specified subgroup analysis on age, which suggested spironolactone is more effective for women aged 25 years or over in terms of the mean difference in Acne-QoL symptom subscale score, it ought to be interpreted with caution given the smaller sample sizes necessitated by splitting the data set into subgroups. Further research to understand if and why there may be a difference in (cost-)effectiveness of spironolactone by age is warranted before drawing any conclusions.
| Spironolactone (N = 201) | Placebo (N = 209) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Mean (n) | SD | Mean (n) | SD | ||
| Subgroup < 25 years | |||||
| Total costs at 24 weeks | 127.38 (31) | 22.89 | 20.70 | 44.52 | 106.67 (88.55 to 124.80) |
| QALYs at 24 weeks | 0.4231 (31) | 0.0364 | 0.4178 (29) | 0.0598 | 0.0053 (–0.0201 to 0.0307) |
| Subgroup ≥ 25 years | |||||
| Total costs at 24 weeks | 145.86 (95) | 64.42 | 14.00 (80) | 46.40 | 131.86 (114.82 to 148.91) |
| QALYs at 24 weeks | 0.4130 (95) | 0.0630 | 0.4097 (80) | 0.0637 | 0.0033 (–0.0157 to 0.0223) |
Sensitivity analyses
Sensitivity analyses around the key uncertainties in the week-24 evaluation are detailed below with some aspects proving influential to the conclusions reached.
Missing data analysis (SA1)
Using multiple imputation, under the assumption of MAR, to evaluate the impact of missing data on the cost–utility results, it can be seen that the ICER is less than in the complete case, adjusted analysis (£27,879 compared with £67,191 per QALY in the adjusted base case). The probability of spironolactone being cost-effective at £30,000 per QALY was estimated as 53%.
Table 37 reports the proportion of missing values (%) for key variables – it can be seen that there was differential rates of attrition for cost and outcome variables, with greater missing data in the placebo group, compared to the spironolactone group, at 12- and 24-week follow-up for costs and outcome variables. There also seems to be a pattern of increasing missingness with time, suggesting some patients are being lost to follow-up.
| Variable | Spironolactone | Placebo | Total |
|---|---|---|---|
| Baseline variables | |||
| Treatment allocation | 0 | 0 | 0 |
| Centre | 0 | 0 | 0 |
| Baseline severity (IGA) | 0 | 0 | 0 |
| Acne-QoL symptom subscale score at baseline | 0 | 0 | 0 |
| Use of topical treatments (y/n) | 1.00 | 0.48 | 0.73 |
| EQ-5D at baseline | 0.50 | 0.00 | 0.24 |
| Costs at baseline | 4.48 | 5.74 | 5.12 |
| Cost variables a | |||
| Costs at 6 weeks | 17.91 | 18.18 | 18.05 |
| Costs at 12 weeks | 14.43 | 23.44 | 19.02 |
| Costs at 24 weeks | 23.88 | 38.76 | 31.46 |
| Costs at 52 weeks | 92.54 | 94.26 | 93.41 |
| Outcome variables for health-related quality of life | |||
| EQ-5D at 6 weeks | 12.44 | 14.35 | 13.41 |
| EQ-5D at 12 weeks | 13.43 | 20.57 | 17.07 |
| EQ-5D at 24 weeks | 20.40 | 33.49 | 27.07 |
| EQ-5D at 52 weeks | 54.73 | 61.72 | 58.29 |
| Outcome variables for acne-related quality of life | |||
| Acne-QoL at 6 weeks | 12.44 | 14.35 | 13.41 |
| Acne-QoL at 12 weeks | 12.44 | 20.57 | 16.59 |
| Acne-QoL at 24 weeks | 18.91 | 34.93 | 27.07 |
| Acne-QoL at 52 weeks | 52.74 | 61.24 | 57.07 |
| Outcomes for cost–utility and cost-effectiveness analyses a | |||
| Total costs (treatment period) | 36.32 | 47.38 | 41.95 |
| Total QALYS (treatment period) | 20.90 | 33.49 | 27.32 |
| Change Acne-QoL symptoms (treatment period) | 18.91 | 34.93 | 27.07 |
Logistic regression was used to explore any association between total costs over the treatment period, QALY score at 24 weeks, and Acne-QoL subscale score change at 24 weeks with randomisation group, randomisation stratification variables [site and baseline severity (IGA < 3 vs. ≥ 3)], baseline variables (Acne-QoL symptom subscale score and use of topical treatments) and value of baseline outcome (EQ-5D).
The fact that some baseline variables, including treatment allocation, predict missingness confirms that data are not MCAR and support the methods for SA1 under a MAR assumption as more plausible (Table 38). However, MNAR is a possibility given that missingness is associated with treatment allocation: patients with worse outcomes may be dropping out at higher rates in the control arm if placebo plus usual treatment is not effective. As it is not possible to ascertain the extent to which this may have occurred, this supports the need for SA1a to explore the impact MNAR may have on outcomes.
| Odds ratio in logistic regression for missing data (95% CI) | |||
|---|---|---|---|
| Missing data on costs | Missing data on QALYs | Missing data on Acne-QoL symptom subscale change | |
| Treatment allocation | 0.67 (0.44–1.02) | 0.43 (0.26–0.69)a | 0.43 (0.26–0.69)a |
| Age | 0.98 (0.95–1.01) | 0.96 (0.93–1.00)b | 0.96 (0.93–1.00) |
| Baseline severity (IGA < 3 vs. ≥ 3) | 0.83 (0.53–1.30) | 0.97 (0.59–1.61) | 1.00 (0.60–1.66) |
| Use of topical treatments (yes/no) | 0.51 (0.29–0.91)b | 0.55 (0.29–1.02) | 0.54 (0.29–1.01) |
| Baseline EQ-5D | 0.61 (0.18–2.03) | 0.65 (0.18–2.41) | 0.69 (0.18–2.57) |
| Baseline Acne-QoL symptom subscale score | 1.01 (0.96–1.01) | 1.02 (0.97–1.07) | 1.02 (0.97–1.07) |
The results of SA1a are presented in Table 39 and demonstrate that changing costs to assume that patients with missing data may have had higher resource use, did not have a large impact on results. Changing QoL data to assume that patients with missing data had lower QoL scores, did have an impact on results, reducing the ICER and increasing the probability that spironolactone is cost-effective compared with placebo. Indeed, assuming a 10% reduction in QoL scores pushed the ICER below the £20,000 threshold.
| Scenario description | Incremental cost (95% CI) | QALY change (95% CI) | ICER (£) | CEAC at £20,000 threshold (%) | CEAC at £30,000 threshold (%) |
|---|---|---|---|---|---|
| MAR: SA1 | 119.78 (107.99 to 131.57) | 0.0043 (–0.0041 to 0.0127) | 27,879 | 35 | 53 |
| MNAR: –5% QoL | 119.78 (107.99 to 131.57) | 0.0057 (–0.0027 to 0.0141) | 21,053 | 47 | 65 |
| MNAR: –10% QoL | 119.78 (107.99 to 131.57) | 0.0071 (–0.0013 to 0.0155) | 16,912 | 60 | 76 |
| MNAR: +5% cost | 119.71 (107.74 to 131.67) | 0.0043 (–0.0041 to 0.0127) | 27,860 | 35 | 53 |
| MNAR: +10% cost | 119.63 (107.48 to 131.78) | 0.0043 (–0.0041 to 0.0127) | 27,842 | 35 | 53 |
| MNAR: –5% QoL and + 5% cost | 119.70 (107.73 to 131.67) | 0.006 (–0.003 to 0.014) | 21,038 | 47 | 65 |
| MNAR: –10% QoL and + 10% cost | 119.62 (107.47 to 131.78) | 0.007 (–0.001 to 0.016) | 16,889 | 60 | 76 |
Intervention costed as per protocol with delivery of spironolactone in secondary care (SA2)
In a sensitivity analysis, costing the intervention as it was delivered in the trial (i.e. in secondary care, see Figure 6 for detail), the mean cost of the spironolactone intervention (medication, dermatology nurse visits, blood tests and pregnancy test) was £264.94 (SD £36.14). The ICER was estimated as £141,955 per QALY and a probability of 12% of spironolactone being cost-effective at a £30,000 threshold. The increased ICER reflects the cost of delivering the intervention through regular appointments with a dermatology nurse (trial protocol), rather than in primary care, as would be the expected standard of care if delivered in practice.
FIGURE 6.
Intervention resource use as per trial protocol (i.e. accessed via secondary care) (SA2).
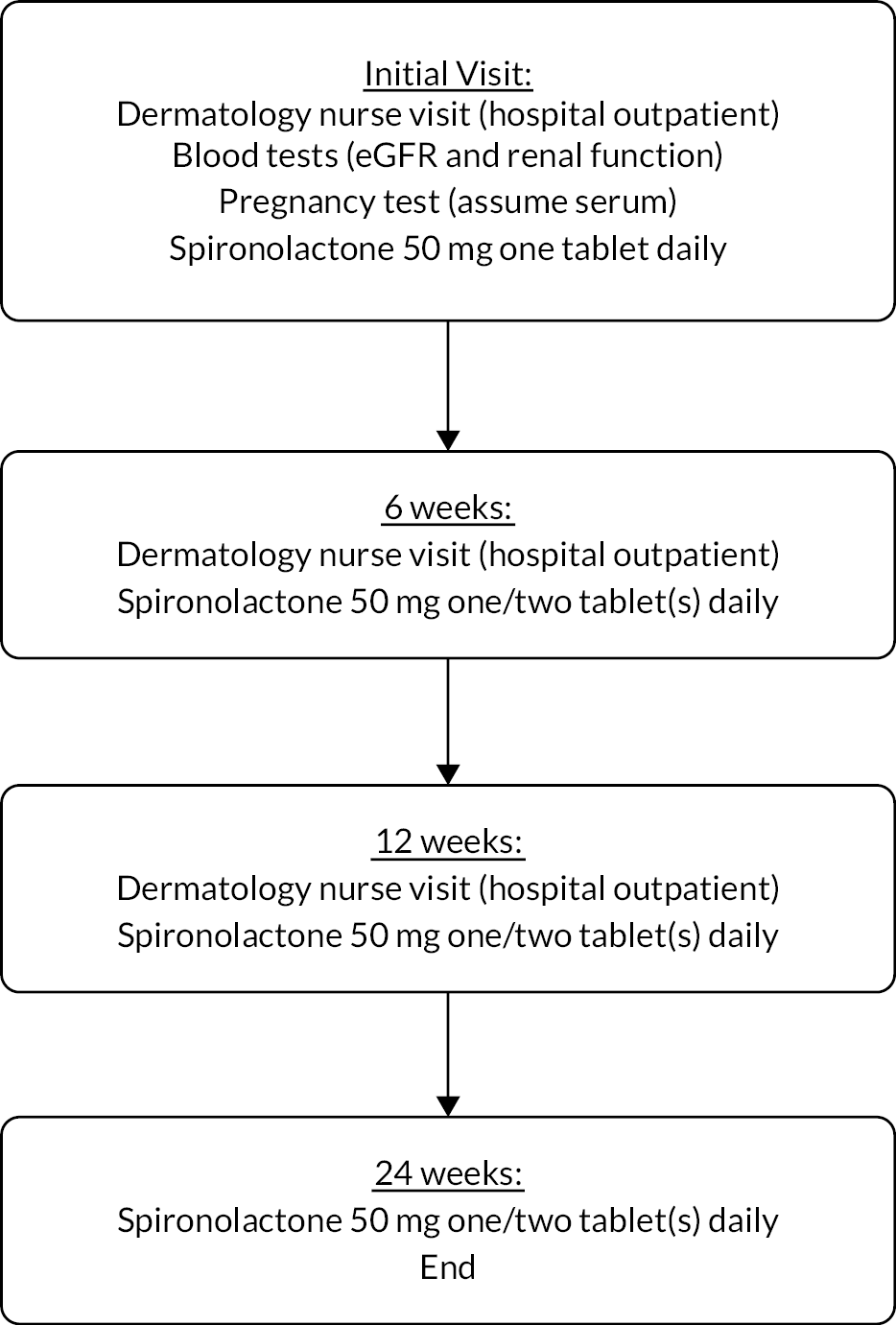
Cost-effectiveness of spironolactone compared to treatment with oral antibiotics in line with NICE acne guidance (National Institute for Health and Care Excellence, 2021) (SA3a: complete case and SA3b: multiple imputation)
There were 13 participants who reported taking an oral antibiotic (lymecycline/oxytetracycline/doxycycline) between baseline and week 24, 9 participants in the placebo group and 4 participants in the spironolactone group. In a sensitivity analysis undertaking a CCA, assuming all women in the placebo group took an oral antibiotic for the 24-week treatment period in line with NICE guidance,12 an ICER of £9169 per QALY was estimated with a probability of 59% of spironolactone being cost-effective at a £30,000 per QALY threshold. Repeating this but using multiple imputation to deal with missing data, the ICER was £2683 per QALY with a probability of 82% that spironolactone would be cost-effective at a £30,000 threshold (see Table 34). This sensitivity analysis assumed that the incremental QALYs observed in the SAFA trial would still be valid, if oral antibiotics are effective, we would expect the incremental QALYs to be less than that observed in the trial. A threshold analysis was performed which found that incremental QALYs would have to decrease to 0.00057 in order to switch the ICER from cost-effective to cost-ineffective at a £30,000 threshold.
Wider case (SA4a: complete case and SA4b: multiple imputation)
Sensitivity analysis was undertaken to take into account costs outside the NHS, including personal out-of-pocket expenses and lost patient and carer productivity as a result of participant’s acne. This wider perspective CCA only had complete cost and QALY data for 97 (48%) women in the spironolactone group and 85 (40%) of women in the control group. The CCA found spironolactone to be dominated by the control group, that is the spironolactone group had higher costs and lower QALYs than those in the control group. This is likely to reflect the small numbers in this analysis. Due to the level of missing data when combining responses from multiple questionnaires, multiple imputation was also undertaken in this sensitivity analysis. Handling the missing data in this way resulted in an estimated ICER of £30,249 per QALY with a probability of 50% of spironolactone being cost-effective at a £30,000 threshold (see Table 34). Changes to reported productivity were found to be minimal, with only nine such changes to paid employment reported for participants themselves and six reported for a family member or friend taking time off work to accompany them to appointments for their acne.
Costs and outcomes over 52 weeks
As described in the methods, data were also collected beyond the treatment period (24 weeks) for up to 52 weeks. Response rates were, however, very low at this time point, with 58% of participants missing EQ-5D data and 93% missing resource use data (see Table 37). As such, it is difficult to draw conclusions from these results, but as presented in Table 29, incremental QALYs over 52 weeks was 0.0644 (95% CI 0.0093 to 0.1194) and incremental cost (NHS perspective) (Table 40) over the same period was £95.44 (95% CI 8.29 to 182.70).
| Resource | Spironolactone (N = 201) | Placebo (N = 209) | Mean difference | ||
|---|---|---|---|---|---|
| Mean (n) | SD | Mean (n) | SD | (95% CI) | |
| All community-based HCP costs | 19.64 (16) | 33.25 | 33.24 (13) | 42.00 | –13.60 (–42.25 to 15.05) |
| Total hospital contacts costs | 17.10 (15) | 45.13 | 9.87 (13) | 35.57 | 7.23 (–24.70 to 39.17) |
| All medication costs | 4.81 (16) | 11.23 | 9.66 (13) | 19.41 | –4.85 (–16.65 to 6.96) |
| Total costs (NHS perspective), 25–52 weeks | 39.89 (15) | 67.47 | 54.41 (12) | 79.00 | –14.52 (–72.57 to 43.52) |
| Total costs (NHS perspective), 0–52 weeks | 179.21 (13) | 76.99 | 83.76 (10) | 123.54 | 95.44 (8.29 to 182.60) |
Health economic discussion
Main findings
Our economic evaluation provides a range of estimates for the cost-effectiveness of spironolactone compared to no active treatment (both groups could use routine topical treatment). In the base case analysis, where the comparator is that used in the trial (no active treatment) and the delivery of the intervention is costed as delivered via primary care, we found no evidence of cost-effectiveness for spironolactone in the adjusted analysis. However, this is not particularly meaningful as women are unlikely to be given no treatment in clinical practice, so a more appropriate comparator would be another oral treatment, such as oral antibiotics.
In adjusted analysis using multiple imputation (MI), spironolactone was estimated to be cost-effective at the £30,000 per QALY threshold. This divergence in conclusion between the complete case and MI analysis demonstrates the impact of missing data (41.95% missing for costs and 27.32% for QALYs at 24 weeks). The cost-effectiveness of spironolactone is potentially understated by the decision to use the CCA as the base case. Furthermore, exploration of the MAR assumption for this MI analysis, given the greater proportions of missingness in the placebo arm, suggests that this MI analysis may under-estimate the cost-effectiveness of spironolactone. The large difference in the estimated ICER for the adjusted and unadjusted complete case analyses reflects the non-significant differential mean utility scores between study groups at baseline.
The base case within trial economic evaluation is limited to the question and data explored in the trial; for this study the comparator was not that which would be considered to represent current practice. 12 Rather, given the dearth of evidence of benefit for spironolactone for acne vulgaris in adult women,25,54 the comparator was a placebo with routine topical treatment in order to show any benefit. To test the impact of this on the findings of the economic study, we present a sensitivity analysis where the placebo group was assumed to take oral antibiotics in addition to topical treatment in line with current NICE guidelines for moderate acne. 12 In this analysis we assumed that antibiotics plus routine topical treatment would convey no more benefit (as measured by the EQ-5D-5L) than placebo plus routine topical treatment. We believe this to be reasonable given that the available evidence from a systematic review demonstrates little to no benefit of combined oral antibiotics with topical treatments compared to topical treatment combinations alone. 55 Furthermore, placebos are known to convey some effectiveness through the placebo effect. 56,57 This analysis found that spironolactone is highly cost-effective with an ICER of £9169 per QALY in a CCA and £2683 per QALY using multiple imputation. Though there are limitations of this analysis, notably we cannot be certain as to the impact on utility of taking an oral antibiotic on top of topical treatment, as this was not the comparator within the trial. To explore this, we undertook a threshold analysis to estimate how much incremental QALYs would have to change in order for the conclusion to move from cost-effective to cost-ineffective. We found that as long as the incremental difference in QALYs was more than 0.00057 QALYs when the willingness-to-pay threshold is £30,000 per QALY in the CCA, then spironolactone would remain cost-effective compared to oral antibiotic use. We await further evidence to determine whether this is a likely scenario.
Strengths and limitations
The strength of this economic evaluation is that it is able to provide reliable estimates of cost-effectiveness based on individual participant level data collected at little marginal cost alongside a RCT. This is, however, also a potential limitation in that within trial health, economic evaluations are constrained by the question and data collected in the RCT. This is particularly so for placebo-controlled trials. The base case set out to answer the question of whether spironolactone is cost-effective compared to no active treatment (both groups could use routine topical medications) to align with the clinical question funded. In line with recommendations, the costs related to the placebo tablets were excluded as these were a research cost. However, it should be acknowledged that placebos may themselves result in some effectiveness through a placebo effect that could potentially lower cost and that were such an effect present it would not be possible to observe the size of this effect given the study design or account for it in the economic analysis. In practice this analysis may also not be the most informative for clinicians who would be unlikely to send women away with no active treatment if they consulted with acne persisting beyond 6 months. We therefore performed a sensitivity analysis assuming all women in the placebo group received an oral antibiotic for 24 weeks. Limitations of this analysis were acknowledged above, and a further potential limitation is that this approach assumes that the downstream resources used, if receiving oral antibiotics, will be the same as in the placebo arm. In reality, patients may use fewer resources if their acne is well-managed and/or use more resources in order to manage AEs. However, despite these limitations and while the results should be interpreted with caution, the analysis serves to provide a lower range estimate for the cost-effectiveness of spironolactone that better reflects accepted standard of care based upon current NICE guidelines. 12 Other studies of spironolactone are underway in France and the USA,58,59 which compare spironolactone directly to oral antibiotic treatment, and therefore the economic results of these studies will add to the evidence base in this regard in due course. It should also be acknowledged that this study was not able, given the time frame, to capture the potential costs and benefits of changes to antimicrobial resistance that may result from less prescribing of antibiotics for acne if the use of spironolactone resulted in such. Since acne is one of the most common dermatological conditions and since the prescription of antibiotics is one of the most common systemic treatments prescribed for acne worldwide, the impact of increased use of spironolactone on antimicrobial resistance is likely to be significant. Further research based on this sensitivity analysis comparing spironolactone to oral antibiotics could be undertaken to conduct a model-based cost–utility analysis using the results from the aforementioned ongoing trials comparing spironolactone to doxycycline, when their results are published. 58,59
At the design stage there was discussion about whether the EQ-5D-5L was an appropriate instrument to use in a study of acne. The EQ-5D was included to enable a cost–utility analysis to be performed. Such an analysis enables cost-effectiveness to be compared across a range of health conditions and interventions and is most useful to decision makers who have to prioritise health care. The limited published evidence available at that time supported the use of the EQ-5D for acne, although this evidence was based on the EQ-5D-3L, whereas the 5L version increases the potential for the instrument to pick up smaller changes in health-related quality of life. Yang et al. 60 systematically searched for evidence on the reliability, validity and responsiveness of three generic preference-based measures [EQ-5D, short form questionnaire-6 dimensions (SF-6D) and Health Utilities Index (HUI)] in skin conditions. They only found evidence, albeit a limited amount, to support the use of the EQ-5D in skin diseases; they found no studies looking at measurement properties for the SF-6D or HUI in skin disease. For acne there was one paper by Klassen et al. 50 who found that problems on the EQ-5D-3L domains were substantially higher in the acne sample than in an age-truncated population sample (aged 20–39 years) particularly on the pain/discomfort (42.1% in the acne sample vs. 17.7% in an age-truncated population sample) and anxiety/depression domains (52.8% vs. 12.5% respectively). It also found the EQ-5D-3L to be responsive to change, with moderate effect sizes at 4 and 12 months (–0.44 and –0.53 respectively). The authors argued that the use of the EQ-5D would ensure the benefits of acne treatments could be compared to those of other treatments (Klassen et al.,50 p. 232).
In this study, which uses the EQ-5D-5L as opposed to the 3L version, the conclusion reached about cost-effectiveness was sensitive to the estimates of QALYs generated using it. This may reflect a number of factors. Firstly, at baseline there was a non-significant difference in utility between study groups with higher mean utility in the intervention group. This meant that the complete case adjusted analysis, which takes into account baseline utility, reached a different conclusion to the unadjusted CCA, not taking account of baseline variables. Secondly, there were differential rates of missing data on the EQ-5D-5L between study groups, with lower completion rates in the control group (65%) than the intervention group (81%) at 24 weeks. When multiple imputation in an adjusted analysis is used, the incremental CCA, unlike the complete case analysis, fell below the £30,000 NICE threshold, suggesting that spironolactone alongside topical treatment is borderline cost-effective compared to no active treatment (placebo) alongside topical treatment. Finally, at baseline, 46% in the intervention group and 43% in the control group were in perfect health (the health state 11111) according to the EQ-5D-5L. For these participants, the EQ-5D-5L had no potential to measure any change in health-related quality of life from the treatments. It is unclear to what extent this differs from the proportion we would expect to be in the perfect health state in the general population as there do not appear to be published population norms for the EQ-5D-5L in the UK (Kind et al., 1998 and EuroQOL website). 61–63 Comparison can be made to other studies of different conditions, for instance in a recent study of the skin condition vitiligo, 55% of participants were in the perfect health state on the EQ-5D-5L at baseline suggesting that acne may have more impact on health-related quality of life than vitiligo as captured on the EQ-5D-5L. Like Klassen et al.,50 we find that women with moderate to severe acne report most problems on the pain/discomfort and anxiety/depression dimensions of the EQ-5D. Further research using the EQ-5D data generated in this study alongside that elicited in other studies of acne would be useful in order to inform future studies about the validity and responsiveness of using the instrument for acne, while acknowledging that this may vary depending on the severity of acne amongst the study group.
The health economics analysis plan specified that a CCA would be undertaken in the base case; this was to be consistent with the approach undertaken in the statistical analysis for the clinical primary outcome. However, such an approach is less reliable where there is missing economic data and where this missingness is at differential rates between study groups at follow-up (attrition bias). With the benefit of hindsight, primary concern ought to have been around the level of missing economic data, which is known to often be greater than that for clinical outcomes. However, both complete case and multiple imputation analyses are reported as planned so that the impact of missing data on the results can be clearly seen. Taking account of missing data in the analysis improves the incremental cost per QALY estimated and thus the cost-effectiveness of spironolactone.
Finally, given the timing of the study, with recruitment and follow-up occurring during the COVID-19 pandemic, it is probable that patient resource utilisation was affected by this. For example, patients may have had fewer opportunities to access healthcare professionals, which could have reduced the downstream resource use, thus potentially reducing the incremental cost in favour of no active treatment. This could have resulted in an under-estimation of the cost-effectiveness of spironolactone and the generalisability of these results should be considered in this context.
Chapter 5 Discussion
Summary of findings
The SAFA trial is the largest randomised trial to date evaluating the effectiveness of spironolactone in the treatment of acne. Women taking spironolactone reported greater improvements than those on placebo in 12-week Acne-QoL symptom subscale score [mean difference between groups 1.48 (95% CI 0.30 to 2.670)], with more substantial differences at 24 weeks [mean difference between groups 3.77 (95% CI 2.50 to 5.03)]. All secondary outcomes favoured spironolactone over placebo, with greater differences between groups seen at 24 weeks than 12 weeks in favour of spironolactone on all measured outcomes.
Participants in the spironolactone group were more likely than those in the placebo group to report overall acne improvement, compared with baseline photo. At 12 weeks this difference was not statistically significant [72.2% vs. 67.9% (OR 1.16 (95% CI 0.70 to 1.91)] but at 24 weeks it was [81.9% vs. 63.3% (OR 2.72 (95% CI 1.50 to 4.93)]. This equates to a NNT of 23 at 12 weeks and a NNT of 5 at 24 weeks in order for participants to report that their acne had improved. Self-assessed improvement in acne, PGA of acne and IGA of acne also improved to a greater extent on spironolactone: the IGA was judged successful at week 12 for 19% of participants in the spironolactone group and 6% in the placebo group [OR 5.18 (95% CI 2.18 to 12.28)]. IGA was not assessed at 24 weeks. Participants also rated that they were satisfied that the treatment had improved their skin more in the spironolactone group [70.6% vs. 43.1% (NNT4)].
There was higher loss to follow-up for the health economic data than anticipated, which makes the CCA (which does not find evidence of cost-effectiveness for spironolactone) more difficult to interpret. In contrast, the analysis using the imputed data allowing for attrition bias suggested that spironolactone was likely to be cost-effective at accepted NICE thresholds. Comparison with oral antibiotics, which would be a common comparator in routine practice, also suggested that spironolactone is likely to be cost-effective.
Adverse reactions to study medications were commonly reported in both groups, with the only statistically significant difference in reporting between groups being for headaches [41/201 (20.4%) in spironolactone group and 25/209 (12.0%) in placebo group (p = 0.02)]. Trial design included commencing all participants on 50 mg spironolactone or matched placebo one tablet daily and increasing this to two tablets daily at 6 weeks if tolerated. Over 95% of participants in both groups tolerated the treatment and increased their dosage. Treatment adherence was similar in both groups, further supporting the suggestion that spironolactone was well-tolerated on this dosing regimen.
Strengths and limitations
The pragmatic design of SAFA was developed to inform real-world decision-making for women with acne and this influenced a number of features of trial design:
-
Broad eligibility and recruitment strategies involved primary care, secondary care, and community and social media advertising, ensuring that participants were broadly reflective of women who could be offered spironolactone in routine care.
-
Participants in both groups continued to use their usual prescribed topical treatments (creams, gels, lotions), if desired, in order to reflect the place of oral treatments as part of the treatment regimen in the acne care pathway.
-
A PROM was chosen as the primary outcome (see further discussion of primary outcome below).
The consensus process regarding PROMs in acne is still ongoing and, at the time of designing the trial, there were fewer validated measures available. We chose the Acne-QoL symptoms subscale as primary outcome as this was the PROM with the best validity data at the time. While an exploration of the minimum clinically important difference for the Acne-QoL had been published, no strong conclusions were drawn and further research was suggested. 33 We based our sample size calculation on seeking to detect a difference between groups of two points at week 12 and, though all of the results were statistically significant, the week-12 symptoms subscale score (primary outcome) point estimate of 1.27 does not exceed the two-point target although the 95% confidence interval of 0.07 to 2.46 does include this value. At week 24 the difference of 3.45 clearly exceeds this target (95% CI 2.16 to 4.75). Furthermore, the strength of evidence from the secondary outcomes, including IGA at 12 weeks, PGA, participants’ self-assessed improvement and satisfaction with treatment, all point towards spironolactone as an effective treatment for women with acne.
Adaptations during the COVID-19 pandemic were crucial to completing the trial, particularly for the many participants already recruited, yet led to limitations, including that remote follow-up visits (via phone or video call) limited collection of investigator-assessed acne severity (IGA), sometimes led to delays in participants obtaining treatment and led to reduced availability of data on treatment adherence. It was originally planned that the treatment adherence analysis would have been based on pill return following the initial 12-week period, in line with the primary outcome measure. However, as many participants were not seen face-to-face at 12 weeks, the analysis was based on self-report over the 12- to 24-week period, as a useful secondary analysis and the best estimate of treatment adherence available. Limitations related to pandemic may also have contributed to the follow-up rate of 83.4% at the primary time point of 12 weeks (87.6% in spironolactone group 79.4% in control group).
Interpretation of results in context of existing evidence
Patient-reported outcome measures in acne
Due to the pragmatic trial design, we wished to include a PROM for acne as the primary outcome as this is in line with what people with acne say is important to them, that is overall appearance, rather than counting the number of spots. 64 Furthermore, previous trials have concluded that lesion counts are time-consuming, show wide inter-assessor variation and give little additional information to global assessments.
However, although the Acne Core Outcomes Research Network65 has developed consensus around ‘what to measure’ in acne trials there is, as yet, no strong consensus on which specific measurement tools to use. At the time of designing the trial, there were few validated measures available, and we chose the Acne-QoL symptoms subscale as primary outcome on the basis of a mixed methods evaluation of existing scales. 66 More recent evidence suggests that, while evidence on measurement properties is lacking for all acne PROMs, newer measures (COMPAQ and Acne-Q) may have greater content validity. 67
Ongoing randomised controlled trials of spironolactone for acne
There are currently two further RCTs of spironolactone recruiting: spironolactone (100 mg daily) versus doxycycline (100 mg daily) in a non-inferiority trial in the USA59 and spironolactone (150 mg daily) versus doxycycline (100 mg daily) superiority trial in France. 58 It is hoped that the outcomes of these may provide opportunities for individual patient meta-analysis and greater power to examine effects within subgroups, particularly age, body mass index (BMI) and ethnicity.
Spironolactone dose
In the SAFA trial, women commenced on 50 mg daily of spironolactone, increased to 100 mg daily at or after 6 weeks if tolerated. We found that over 95% of women did increase their dose to 100 mg. It could be speculated that our finding of greater effect at 24 weeks than 12 weeks might not have been observed if participants had commenced on 100 mg, and it may be that starting at the higher dose and then down-titrating only if side effects develop may lead to more rapid improvement in acne symptoms. This treatment regimen was chosen on the basis of a survey of clinicians carried out at the design stage and reported in our protocol paper. 1
The ongoing US and French trials both chose a higher starting dose of spironolactone (100 mg and 150 mg respectively) and it will be interesting to compare their data on tolerability, adherence and adverse effects in these trials. With more data it may also be possible to explore whether people with PCOS or higher BMI benefit more from higher doses, as there is literature to suggest that this may be the case. 25
Side effects of spironolactone in this patient group
The most common side effect found in observational studies was menstrual irregularities (15–25%), although this was thought to be less common at lower doses (100 mg/day or less). 25 It is notable that reports of menstrual irregularities were very similar across both groups (39% in the spironolactone group and 38% in the placebo group combined across all time points). Menstrual irregularities have been reported as a very frequent side effect amongst women taking spironolactone at higher doses (particularly 200 mg daily),25 but it is reassuring that, in comparison with placebo, the proportion experiencing this side effect does not appear to be high amongst women taking 100 mg daily spironolactone.
Other side effects, also dose-related, reported in previous studies include breast tenderness, dizziness, nausea, headache, polyuria and fatigue. 25 It is a useful addition to the literature to be able to report the frequency of these adverse effects in a placebo-controlled trial. Whereas SAFA participants in both groups commonly reported several of these symptoms, in general, differences between groups were not substantial and the only statistically significant difference was headaches, experienced by 20% in the spironolactone group and 12% in the placebo group. Clearly the analyses of differences in reporting are not powered to detect small differences and, for instance, the difference in dizziness/vertigo/lightheadedness between groups suggests this may be more common for spironolactone than placebo, whereas there are some instances, such as fatigue, that were actually slightly more common in the placebo group.
Pregnancies
Spironolactone is cautioned against in pregnancy, due to a theoretical risk of feminising the male fetus in the third trimester, although this has not been observed in humans. 68 Spironolactone is likely to be less teratogenic than oral tetracyclines, commonly used for acne in young women, where there is concern about dental discolouration and possible effect on fetal bone growth in the second or third trimester,68 so it seems appropriate that in usual practice, spironolactone would be treated the same as these agents.
The SAFA trial took a pragmatic approach to pregnancies, again in terms of seeking to reflect real-life practice. SAFA participants were advised to use contraception at the baseline visit and at subsequent visits, but pregnancy tests were only carried out at baseline and not at every visit. It was felt that a baseline test would be necessary in the context of a RCT, although this is unlikely to be usual practice. Seven pregnancies were observed in the trial, which would suggest the importance of regular counselling about contraception in this age group.
Renal function monitoring
There is increasing consensus that, while baseline check of renal function and potassium levels is likely to be advisable prior to commencing spironolactone, ongoing monitoring is unlikely to be necessary for most young women. 13 The largest study on this topic to date found that 13 (0.7%) of 1802 women commencing spironolactone were found to have raised potassium, but that subsequent testing showed this to be either erroneous or transient or quickly self-resolved. 69 A study using routinely collected US data found that, of 618 women starting spironolactone, 145 had no blood tests either at baseline or for ongoing monitoring and concluded that raised potassium rarely occurs in otherwise healthy women aged 45 years or less. 70
While UK guidelines make no mention of the use of spironolactone for acne, US guidelines13 advise that:
Serum potassium testing is therefore not required, but should be considered in older patients and in patients who are also taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and digoxin.
Economic evaluation conclusions
The results of the economic evaluation suggest that cost-effectiveness depends upon the assumptions made and methods used. While the base case adjusted analysis would not conclude that spironolactone is cost-effective compared to no active treatment other than topical treatments, sensitivity analyses, taking into account missing data and comparing to an antibiotic, showed spironolactone to be cost-effective. Furthermore, for reasons given in Chapter 4, it is likely that this study under-estimates the economic benefits of spironolactone. Our results add an important contribution to the evidence available to inform any future decisions in this clinical area and should be considered along with the economic results of future studies in this area.
Equality, diversity and inclusivity
Gaps in prior evidence
Prior to carrying out this research we had previously carried out systematic reviews, both of the use of spironolactone for acne25,26 and on the views and experiences of people with acne. 71 The systematic review of spironolactone for acne showed that, unfortunately, spironolactone is not a suitable treatment for men due to related gynaecomastia (breast swelling).
The systematic review of the views and experiences of people with acne showed that under-represented groups include younger people, people from ethnic minority backgrounds and men. Unfortunately, men cannot use spironolactone, as above, so were not included in this trial. The HTA call sought a proposal on persistent acne, and we therefore chose to limit the trial to women aged 18 or over, which is also clinically where more consensus for the use of spironolactone previously converged. Regarding ethnicity, we aimed to address this by including sites covering geographical areas with high proportions of populations from ethnic minority backgrounds.
The INCLUDE Ethnicity Framework72 will be helpful in designing future trials and seeking to maximise inclusion and applicability to underserved groups, although was not available at the start of designing this trial. It is unfortunate that ethnicity was not included initially in study documents although during the progress of the trial, efforts from the trial staff and site staff to collect this data for participants who had already been recruited mean that it has been possible to report this, though with some missing data.
Members of research team
The research team was made up predominantly of women, including the two Co-Chief Investigators. There was no great ethnic diversity within the research team, although at least five members were of European background and did not have English as their first language.
Both the TSC and Data Monitoring Committee were chaired by women, and the DMEC chair was of SE Asian background.
Participant representation
Of 356 participants where ethnicity data were available, 92.1% were white and 7.9% were from non-white background. The reason for the predominantly white trial participant population is unknown but may have related to the ability of participants to travel to recruiting centres during the COVID-19 pandemic. The three recruiting centres with the highest number of participants (accounting for 59.3% of recruited participants) were located in Portsmouth, Bristol and Harrogate.
Public involvement
Public contributors were relatively representative of the target population, as both the two public contributors on the TMG and the public contributor on the TSC had had acne for many years and had been frustrated by treatments on offer. They represented differing geographic areas (NE, SE, SW) but no non-white ethnicity.
Public involvement
How public contributors were involved prior to the randomised controlled trial
A James Lind Alliance Priority Setting Partnership, funded by the National Institute for Health and Care Research (NIHR), identified the need to establish the best way to manage acne in women who may or may not have underlying hormonal abnormalities. 25
We gained feedback on key questions relating to research design from a virtual acne-specific patient panel, convened through ‘People in Research’ (www.peopleinresearch.org), as well as a patient survey carried out with the support of the UK Dermatology Clinical Trials Network (UK DCTN). Findings suggested that participants would find it difficult to abstain from using topical treatments and that asking participants to take a placebo for 1 year would also be a barrier to recruitment. This strongly influenced design decisions around use of topical treatments in the trial and choice of primary outcome at 12 weeks with unblinding at 24 weeks.
Two public contributors (IS and KaT) with experience of acne were co-applicants on the grant and influenced design decision, for instance highlighting that the originally planned upper age limit of 50 years was arbitrary, which was then abandoned, and contributing to the choice of primary and secondary outcomes.
How public contributors were involved in delivering the randomised controlled trial
Two public contributors (IS and KaT) with experience of acne attended all TMG meetings to ensure that decisions around trial design were informed by their perspective, trial procedures were feasible for participants and trial materials were readable and included all the relevant information that participants would want. Public contributors influenced the trial design and delivery, for instance by advocating the use of social media advertising to improve recruitment, designing and later redesigning recruitment materials. Due to the COVID pandemic, face-to-face meetings were limited. Indeed, the ‘results reveal’ meeting, planned as face-to-face in London, became a largely hybrid meeting due to so many attendees having COVID at that time.
Although there are not specific charities to liaise with in the field of acne, public contributors will also be involved in disseminating the trial and have suggested routes to dissemination.
Guidance for reporting involvement of patients and the public 2 short form
| Section and topic | Item 73 |
|---|---|
| 1: Aim | The aim of public involvement was to ensure that at all stages of design, execution, interpretation and dissemination public contributors were involved in decision-making and sharing their experience. |
| 2: Methods | Two core public contributors with extensive experience of acne were members of the TMG, contributing to all decision-making and reviewing all public-facing documents, including study website. A third public contributor with extensive experience of acne as well as professional skills in marketing contributed to study website design and social media recruitment strategy. Prior to starting recruitment, a survey was sent to public contributors involved with the Centre of Evidence Based Dermatology, in order to ascertain broader views on key design decisions (reported in protocol paper)1 Extensive public involvement activities were carried out in the early stages of recruitment in order to revise invitation materials with the aim of boosting the response rate. These involved a Southampton-based ‘Young Adults’ PPI Group’, some of whom had experience of acne but all of whom were in the target age range. |
| 3: Trial results | Through ensuring that all trial procedures were feasible and acceptable, public involvement was necessary to the successful delivery of this trial. |
| 4: Discussion and conclusions | Outcomes – Comment on the extent to which PPI influenced the trial overall. Describe positive and negative effects. |
| 5: Reflections/critical perspective | Involvement of public contributors in TMG meetings may have added to time taken in some meetings, but this is not viewed as a negative as it is recognised that multiple views strengthen the research and feeding these in take time. It is possible that a more time-efficient and cost-efficient model may be to have had some separate meetings of core staff with public contributors, rather than attendance of public contributors at all TMGs, but we chose to continue attendance at all TMGs in order to ensure that public contributors remain a core part of the team, and because it may not always be apparent from the agenda when the public perspective is going to be crucial. |
Chapter 6 Conclusions
Implications for health care
This trial has demonstrated that oral spironolactone provides a safe alternative to systemic antibiotics for adult women with persistent facial acne judged to be of sufficient severity to warrant treatment with oral antibiotics.
Although spironolactone has been used widely in the community to treat hypertension and some related disorders, it is not currently licensed for the management of acne and consequently GPs may be reticent to prescribe it off licence for this indication. The results from this trial not only demonstrate efficacy but also safety of spironolactone in the populations of adult females with acne many of whom are maintained on long-term antibiotic therapies. Adopting a combined approach using oral spironolactone and topical agents has the potential to reduce the long-term prescribing of oral antibiotics and therefore reduce the likelihood of emerging bacterial resistance, a well-recognised global concern. It is likely that treatment courses of spironolactone over 3 months are of greater benefit than shorter treatment duration. The economic evaluation shows that oral spironolactone is likely to be a cost-effective alternative to oral antibiotics, when used alongside topical treatments for persistent acne.
Research recommendations
Clinical questions
-
What is the comparative effectiveness of oral antibiotics compared with spironolactone for the treatment of acne?
-
What is the optimal dosing of spironolactone in acne, particularly for women of higher BMI?
-
Which women with acne are most likely to benefit from spironolactone, for instance which age groups, ethnicities and acne types?
Mechanistic questions
-
Further research into the mechanism of action of spironolactone for acne could inform which patient groups and clinical subtypes of acne may respond better and which treatments could most usefully be co-prescribed with spironolactone, or help develop new treatments.
Health economics research recommendations
-
Further research based on this sensitivity analysis comparing spironolactone to oral antibiotics could be undertaken to conduct a model-based cost–utility analysis using the results from the aforementioned ongoing trials comparing spironolactone to doxycycline, when their results are published.
-
Further research using the EQ-5D data generated in this study alongside that elicited in other studies of acne would be useful in order to inform future studies about the validity and responsiveness of using the instrument for acne, while acknowledging that this may vary depending on the severity of acne amongst the study group.
Additional information
Contributions of authors
Miriam Santer (https://orcid.org/0000-0001-7264-5260) (Professor of Primary Care Research) contributed to the development of the protocol, oversaw day-to-day management of the study and drafted the paper.
Megan Lawrence (https://orcid.org/0000-0003-4776-0616) (Senior Medical Statistician) contributed to the development of the protocol, the management of the study and the drafting of the paper, and carried out statistical analyses.
Sarah Pyne (https://orcid.org/0000-0003-0093-9125) (Senior Research Associate in Health Economics) contributed to the development of the protocol, the management of the study and the drafting of the paper, and carried out health economic analyses.
Susanne Renz (https://orcid.org/0000-0003-0241-7240) (Trial Manager) contributed to the development of the protocol, led day-to-day management of the study and drafted the paper.
Beth L Stuart (https://orcid.org/0000-0001-5432-7437) (Professor of Medical Statistics and Clinical Trials) contributed to the development of the protocol and the drafting of the paper, oversaw day-to-day management of the study and carried out statistical analyses.
Tracey Sach (https://orcid.org/0000-0002-8098-9220) (Professor of Health Economics) contributed to the development of the protocol and the management of the study, carried out health economic analyses and drafted the paper.
Matthew Ridd (https://orcid.org/0000-0002-7954-8823) (Professor of Primary Health Care) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Kim S Thomas (https://orcid.org/0000-0001-7785-7465) (Professor of Applied Dermatology Research) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Jacqueline Nuttall (https://orcid.org/0000-0002-5826-2594) (Head of Trial Management, Non-Cancer) contributed to the development of the protocol and the drafting of the paper, and oversaw day-to-day management of the study.
Natalia Permyakova (https://orcid.org/0000-0003-3549-3215) (Medical Statistician) contributed to the development of the protocol, the management of the study and the drafting of the paper, and carried out statistical analyses.
Zina Eminton (Senior Trial Manager) contributed to the development of the protocol and the drafting of the paper, and oversaw day-to-day management of the study.
Nick Francis (https://orcid.org/0000-0001-8939-7312) (Professor of Primary Care Research) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Paul Little (https://orcid.org/0000-0003-3664-1873) (Professor of Primary Care Research) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Ingrid Muller (https://orcid.org/0000-0001-9341-6133) (Professor of Health Psychology) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Irene Soulsby (https://orcid.org/0000-0002-2291-831X) (Public contributor) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Karen Thomas (https://orcid.org/0000-0001-8991-3380) (Public contributor) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Gareth Griffiths (https://orcid.org/0000-0002-9579-8021) (Director, Southampton Clinical Trials Unit) contributed to the development of the protocol, the management of the study and the drafting of the paper.
Alison M Layton (https://orcid.org/0000-0003-0473-3319) (Professor of Dermatology) contributed to the development of the protocol, the management of the study and the drafting of the paper.
All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the following:
-
All trial participants
-
All sites participating in the trial:
Queen Elizabeth Hospital, Birmingham Hospitals NHS Foundation Trust
Solihull Hospital, Birmingham Hospitals NHS Foundation Trust
Bristol Royal Infirmary Dermatology Centre, University Hospitals Bristol NHS Foundation Trust
University Hospital of Wales, Cardiff and Vale University Health Board
Epsom General Hospital, Epsom and St Helier University Hospitals NHS Trust
Harrogate District Hospital, Harrogate and District NHS Foundation Trust
St Mary’s Hospital, Imperial College NHS Trust
Queen’s Medical Centre, Nottingham University Hospitals NHS Trust
Poole General Hospital, University Hospitals Dorset NHS Foundation Trust
St Mary’s General Hospital Dermatology Centre, Portsmouth Hospitals NHS Trust
Singleton Hospital, Swansea Bay University Health Board
-
TSC and DMEC members: Catherine Smith, Fiona Collier, William Henley, Sarah Heyworth, Shernaz Walton, Elaine Nicholls, Christian Mallen
-
The study was developed with support from the UK Dermatology Clinical Trials Network (UK DCTN). The UK DCTN is grateful to the British Association of Dermatologists and the University of Nottingham for financial support of the Network
-
Virtual patient panel for their ad
-
PICs for patient list searches for mailouts to identify potential eligible patients
-
Clinical Research Networks (CRNs) for their assistance in identifying potential PICs
-
Wessex CRN for providing the initial funding of a social media advertising campaign
-
Southampton CTU staff (past and present) who are not listed among the co-authors for their support of this trial
This project was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme (ref: 16/13/02) and supported by NIHR CTU support funding at Southampton CTU.
Data-sharing statement
Pseudonymised individual participant data (IPD) within the clinical study data set will be available for sharing via controlled access by authorised Southampton CTU staff (as delegated to Southampton CTU by the study sponsor). Data access can be requested via a Southampton CTU Data Release application form (available from www.southampton.ac.uk/ctu/about/index.page) after the trial is published. Please e-mail the completed form to the Southampton CTU Data Release Committee Co-ordinator at ctu@soton.ac.uk. Data access requests are reviewed against specific eligibility criteria by the Southampton CTU data custodian and key members of the study team, including a statistician and chief investigator or by an external Independent Review Panel. Decisions about requests are made promptly and usually no more than 3 months after receipt of request. Responses to all data requests, with a clear rationale for any refusals, will be sent promptly to the data requester. Any other queries should be addressed to the corresponding author for consideration.
Ethics statement
Ethical approval for the trial was given by Wales Research Ethics Committee 2 (Wales REC 3) in January 2019 (reference number: 18/WA/0420). This trial was conducted in accordance with the Declaration of Helsinki.
Regulatory approval was given by MHRA in January 2019.
University of Southampton sponsored this trial and approved the original protocol and subsequent amendments to the trial.
Information governance statement
The University of Southampton is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Southampton is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.southampton.ac.uk/legalservices/what-we-do/data-protection-and-foi.page.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/MYJT6804.
Primary conflicts of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/. We declare no support from any organisation other than the National Institute for Health and Care Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work. ICMJE declarations cover the following:
Miriam Santer: Subpanel member NIHR Programme Grants for Applied Research 2018–23. Academic PPIE lead and Board Member NIHR School for Primary Care Research 2022 onwards. RAPID and Efficient Eczema Trials (RAPID programme) – lead applicants Thomas and Roberts. NIHR PGfAR NIHR203279 funding to University of Southampton for 5% of my time. Trial of IGe tests for Eczema Relief (TIGER): randomised controlled trial of test-guided dietary advice for children with eczema, with internal pilot and nested economic and process evaluations – lead applicant Ridd. NIHR HTA NIHR133464 funding to University of Southampton for 10% of my time. Pragmatic, primary care, multicentre, randomised superiority trial of four emollients in children with eczema, with internal pilot and nested qualitative study (Best Emollients for Eczema – BEE) – lead applicant Ridd. NIHR HTA 15/130/07 completed August 2020 – funding to University of Southampton for 10% of my time. Cellulitis Optimal Antibiotic Treatment: COAT study – lead applicant Francis. NIHR HTA NIHR134867 funding to University of Southampton for 5% of my time. Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne – lead applicant Santer NIHR202852 – funding to University of Southampton for 20% of my time. Benefits and Harms of Reduced Dose Oral Isotretinoin in the Management of Acne Vulgaris – lead applicant Burden – NIHR151318 funding to University of Southampton for 2% of my time.
Beth L Stuart: Funding panel member NIHR HTA Programme November 2020–24.
Tracey Sach: Chair of the NIHR Research for Patient Benefit Regional Advisory Panel for the East of England. Member of the NIHR HTA Efficient Study Designs – 2, HTA Efficient Study Designs Board, HTA End of Life Care and Add-on Studies, HTA Primary Care Themed Call Board, and NIHR HTA Commissioning Board, Steering committee member of the UK Dermatology Clinical Trials Network. Cellulitis Optimal Antibiotic Treatment: COAT study – lead applicant Francis NIHR HTA NIHR134867 funding to University of East Anglia for 5% of my time. Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne – lead applicant Santer NIHR202852 – funding to University of East Anglia for 20% of my time. Benefits and Harms of Reduced Dose Oral Isotretinoin in the Management of Acne Vulgaris – lead applicant Burden – NIHR151318 funding to University of East Anglia for 5%–20% of my time. Proactive against reactive therapy for the prevention of lichen sclerosus exacerbation and progression of disease – a pragmatic, parallel group randomised controlled trial with embedded economic evaluation and process evaluation (PEARLS). Lead applicant Simpson – NIHR135121 funding to University of East Anglia for 5% to 20% of my time. Best systemic treatments for adults with atopic eczema over the long term (BEACON): a randomised, assessor-blind trial comparing ciclosporin, methotrexate and dupilumab. Lead applicant Smith – NIHR129926 funding to University of East Anglia for 5%–20% of my time.
Matthew Ridd: Co-Chair SAPC & NIHR SPCR skin/allergy research groups. Committee member NIHR In Practice Fellowship. RAPID and Efficient Eczema Trials (RAPID programme). NIHR PGfAR NIHR203279. Trial of IGe tests for Eczema Relief (TIGER): randomised controlled trial of test-guided dietary advice for children with eczema, with internal pilot and nested economic and process evaluations. NIHR HTA NIHR133464. Pragmatic, primary care, multicentre, randomised superiority trial of four emollients in children with eczema, with internal pilot and nested qualitative study (Best Emollients for Eczema – BEE). NIHR HTA 15/130/07 completed August 2020. Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in NIHR202852. Talking in Primary Care 2: Testing the effects of communication skills e-learning for practitioners on patients’ musculoskeletal pain and enablement. NIHR SPCR. ATLANTIS (Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment): a double-blind placebo-controlled trial, NIHR HTA. Eczema Care Online (ECO): Supporting self-care for eczema in the community, NIHR PGfAR. The association between the COVID-19 vaccine and skin conditions, NIHR SPCR. Improving the DIagnostic accuracy of referrals for Papilloedema (DiPP) from primary to secondary care: the development of clinical guidelines and educational material. NIHR SPCR/PDG. Rapid respiratory microbiological point-of-care-testing in primary care: a randomised controlled trial with internal pilot and qualitative and quantitative investigation of microbial, behavioural and antibiotic mechanisms (the RAPID-TEST RCT), NIHR EME NIHR131758. AmiTritypline for the prevention of post-HErpetic NeuralgiA (ATHENA): multicentre, individually randomised, pragmatic, placebo-controlled superiority trial with internal pilot, study within a trial and nested qualitative study, NIHR HTA. Barriers Enhancement for Eczema Prevention (BEEP): A randomised controlled trial to determine whether skin barrier enhancement with emollients can prevent eczema in high risk children. NIHR HTA. TRIUMPH: TReating Urinary symptoms in Men in Primary Healthcare using non-pharmaceutical and non-surgical interventions, NIHR HTA. Surgical interventions to treat severe pressure sores (SIPS), NIHR HTA.
Paul Little: Lead and Board Member NIHR School for Primary Care Research 2022 to present day. 2021–3 Platform Adaptive trial of NOvel antiviRals for eArly treatment of COVID-19 In the Community (PANORAMIC) (NB 132k is the Southampton resource – for my salary and Dr Lown).
Alison M Layton: Co-Clinical Lead in Dermatology for the regional NIHR comprehensive research network. Associate Medical Director for Research for local trust Harrogate and District NHS Foundation Trust. Clinical Lead for Skin Research Centre, Hull York Medical School, University of York. Member of UKDCTN prioritisation panel. Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne – lead co-applicant NIHR202852 – funding to University of York for time. Served as advisor, consultant and/or investigator for research (funded to institution) for Galderma, GSK, Origimm. Received honoraria for unrestricted educational events from Almirall, Beiersdorf, Galderma, La Roche-Posay, LEO Pharma, L’Oréal, Mylan, Origimm, Proctor & Gamble. Received remuneration to support travel to attend and give invited lectures at educational meetings from Almirall, Galderma, La Roche-Posay, L’Oreal, Leo, Origimm.
Ingrid Muller: RAPID and Efficient Eczema Trials (RAPID programme) – lead applicants Thomas and Roberts. NIHR PGfAR NIHR203279 funding to University of Southampton for 8% of my time. Trial of IGe tests for Eczema Relief (TIGER): randomised controlled trial of test-guided dietary advice for children with eczema, with internal pilot and nested economic and process evaluations – lead applicant Ridd. NIHR HTA NIHR133464 funding to University of Southampton for 8% of my time. Digital Sleep Support for Children with Attention-Deficit/Hyperactivity Disorder (The DISCA study) – lead applicants Hill and Cortese. NIHR PGfAR funding to University of Southampton for 10% of my time. Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne – lead applicant Santer NIHR202852 – funding to University of Southampton for 60% of my time.
Kim Thomas: Developing and testing an online intervention to support self-management, improve outcomes and reduce antibiotic use in acne – lead applicant Santer NIHR202852 – funding to University of Nottingham. Benefits and Harms of Reduced Dose Oral Isotretinoin in the Management of Acne Vulgaris – lead applicant Burden – NIHR151318 funding to University of Nottingham.
Jacqueline Nuttall: Cellulitis Optimal Antibiotic Treatment: COAT study – lead applicant Francis. NIHR HTA NIHR134867 funding to University of Southampton for 5% of my time. Multicentre trial of the clinical and cost-effectiveness of a novel urinary catheter design in reducing catheter-associated urinary tract infection compared with the traditional Foley design for adults requiring long-term catheterisation (CADET). Lead applicant C Murphy NIHR131172 – funding to University of Southampton for 5% of my time.
Publication
Renz S, Chinnery F, Stuart B, Day L, Muller I, Soulsby I, et al. Spironolactone for adult female acne (SAFA): protocol for a double-blind, placebo-controlled, phase III randomised trial of spironolactone as systemic therapy for acne in adult women. BMJ Open 2021;11:e053876. https://doi.org/10.1136/bmjopen-2021-053876
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Renz S, Chinnery F, Stuart B, Day L, Muller I, Soulsby I, et al. Spironolactone for adult female acne (SAFA): protocol for a double-blind, placebo-controlled, phase III randomised study of spironolactone as systemic therapy for acne in adult women. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2021-053876.
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527-34. https://doi.org/10.1038/jid.2013.446.
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris [Erratum appears in Lancet. 2012;379(9813):314]. Lancet 2012;379:361-72.
- Tan J, Kang S, Leyden J. Prevalence and risk factors of acne scarring among patients consulting dermatologists in the USA. J Drugs Dermatol 2017;16:97-102.
- Layton AM, Thiboutot D, Tan J. Reviewing the global burden of acne: how could we improve care to reduce the burden?. Br J Dermatol 2021;184:219-25. https://doi.org/10.1111/bjd.19477.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol 2014;27:3-8. https://doi.org/10.1159/000354887.
- Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol 2012;11:708-13.
- Perkins AC, Maglione J, Hillebrand GG, Miyamoto K, Kimball AB. Acne vulgaris in women: prevalence across the life span. J Women Health 2012;21:223-30. https://doi.org/10.1089/jwh.2010.2722.
- Tan JK, Tang J, Fung K, Gupta AK, Thomas DR, Sapra S, et al. Development and validation of a comprehensive acne severity scale. J Cutan Med Surg 2007;11:211-6. https://doi.org/10.2310/7750.2007.00037.
- Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther 2016;7:13-25. https://doi.org/10.2147/ahmt.S55832.
- Purdy S, Langston J, Tait L. Presentation and management of acne in primary care: a retrospective cohort study. Br J Gen Pract 2003;53:525-9.
- Xu J, Mavranezouli I, Kuznetsov L, Stephen Murphy M, Healy E. Management of acne vulgaris: summary of NICE guidance. BMJ 2021;374.
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol 2016;74:945.e-73.e33. https://doi.org/10.1016/j.jaad.2015.12.037.
- Thiboutot DM, Dréno B, Abanmi A, Alexis AF, Araviiskaia E, Barona Cabal MI, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 2018;78:S1-S23.e1.
- Le Cleach L, Lebrun‐Vignes B, Bachelot A, Beer F, Berger P, Brugère S, et al. Guidelines for the management of acne: recommendations from a French multidisciplinary group. Br J Dermatol 2017;177:908-13.
- Francis NA, Entwistle K, Santer M, Layton AM, Eady EA, Butler CC. The management of acne vulgaris in primary care: a cohort study of consulting and prescribing patterns using the Clinical Practice Research Datalink. Br J Dermatol 2017;176:107-15. https://doi.org/10.1111/bjd.15081.
- Lown M, McKeown S, Stuart B, Francis N, Santer M, Lewith G, et al. Prescribing of long-term antibiotics to adolescents in primary care: a retrospective cohort study. Br J Gen Pract 2021;71:e887-94. https://doi.org/10.3399/bjgp.2021.0332.
- Khondker L, Khan SI. Acne vulgaris related to androgens: a review. Mymensingh Med J 2014;23:181-5.
- Platt D, Muller I, Sufraz A, Little P, Santer M. GPs’ perspectives on acne management in primary care: a qualitative interview study. Br J Gen Pract 2021;71:e78-84. https://doi.org/10.3399/bjgp20X713873.
- Santer M, Chandler D, Lown M, Francis NA, Muller I. Views of oral antibiotics and advice seeking about acne: a qualitative study of online discussion forums. Br J Dermatol 2017;177:751-7.
- Del Rosso JQ, Webster GF, Rosen T, Thiboutot D, Leyden JJ, Gallo R, et al. Status Report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: Part 1: Antibiotic Prescribing Patterns, Sources of Antibiotic Exposure, Antibiotic Consumption and Emergence of Antibiotic Resistance, Impact of Alterations in Antibiotic Prescribing, and Clinical Sequelae of Antibiotic Use. J Clin Aesth Dermatol 2016;9:18-24.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis 2016;16:e23-33. https://doi.org/10.1016/s1473-3099(15)00527-7.
- British National Formulary . British Medical Association and the Royal Pharmaceutical Society n.d. www.bnf.org (accessed 23 April 2016).
- Park JH, Bienenfeld A, Orlow SJ, Nagler AR. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol 2018;19:449-55. https://doi.org/10.1007/s40257-018-0349-6.
- Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol 2017;18:169-91.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol 2020;21:303-5. https://doi.org/10.1007/s40257-020-00511-5.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic–explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Martin AR, Lookingbill DP, Botek A, Light J, Thiboutot D, Girman CJ. Health-related quality of life among patients with facial acne: assessment of a new acne-specific questionnaire. Clin Exp Dermatol 2001;26:380-5. https://doi.org/10.1046/j.1365-2230.2001.00839.x.
- McLeod LD, Fehnel SE, Brandman J, Symonds T. Evaluating minimal clinically important differences for the acne-specific quality of life questionnaire. PharmacoEconomics 2003;21:1069-79. https://doi.org/10.2165/00019053-200321150-00001.
- Ozolins M, Eady EA, Avery A, Cunliffe W, O’Neill C, Simpson N, et al. Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne. Health Technol Assess 2005;9:iii-212. https://doi.org/10.3310/hta9010.
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) . Guidance for Industry; Acne Vulgaris: Developing Drugs for Treatment (Draft Guidance) 2005:1-14. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071292.pdf.
- Fehnel SE, McLeod LD, Brandman J, Arbit DI, McLaughlin-Miley CJ, Coombs JH, et al. Responsiveness of the Acne-Specific Quality of Life Questionnaire (Acne-QoL) to treatment for acne vulgaris in placebo-controlled clinical trials. Qual Life Res 2002;11:809-16. https://doi.org/10.1023/a:1020880005846.
- Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol 2007;60:1234-8. https://doi.org/10.1016/j.jclinepi.2007.02.006.
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602-18.
- Jansen T, Janßen OE, Plewig G. Acne tarda [Acne in adults]. Hautarzt 2013;64:241-51. https://doi.org/10.1007/s00105-012-2458-0.
- Drummond MF, Sculpger MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 15 October 2016).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical trials. Oxford: Oxford University Press; 2014.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care 2022;25:3-9.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Alava MH, Wailoo A, Pudney S. Methods for Mapping Between the EQ 5D 5L and the 3L: NICE Decision Support Unit Report 2017. www.sheffield.ac.uk/nice-dsu/methods-development/mapping-eq-5d-5l-3l (accessed 19 July 2022).
- Alava MH, Pudney S, Wailoo A. The EQ-5D-5L value set for England: findings of a quality assurance program. Value Health 2020;23:642-48.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Health and Social Care Information Centre . Prescription Cost Analysis 2018. https://webarchive.nationalarchives.gov.uk/20180328135205/http://digital.nhs.uk/catalogue/PUB20200.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Girman C, Lookingbell D, Thiboutot D, Johnson J, Light J, Hartmaier S. Acne-Specific Quality of Life Questionnaire (Acne-QoL) Manual &Amp; Interpretation Guide 2003. Anzctr.org.au (accessed 30 June 2020).
- Klassen AF, Newton JN, Mallon E. Measuring quality of life in people referred for specialist care of acne: comparing generic and disease-specific measures. J Am Acad Dermatol 2000;43:229-33. https://doi.org/10.1067/mjd.2000.105507.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. PharmacoEconomics 2018;36:889-901.
- Tan J. Acne guidelines: pearls, pitfalls and questions. Br J Dermatol 2017;177:892-3.
- Mavranezouli I, Daly CH, Welton NJ, Deshpande S, Berg L, Bromham N, et al. A systematic review and network meta‐analysis of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Br J Dermatol 2022;187:639-49.
- Chiou WL. Low intrinsic drug activity and dominant vehicle (placebo) effect in the topical treatment of acne vulgaris. Int J Clin Pharmacol Ther 2012;50:434-7.
- van Laarhoven AI, van der Sman-Mauriks IM, Donders ART, Pronk MC, van de Kerkhof PCM, Evers AWM. Placebo effects on itch: a meta-analysis of clinical trials of patients with dermatological conditions. J Investig Dermatol 2015;135:1234-43.
- Poinas A, Lemoigne M, Le Naour S, Nguyen JM, Schirr-Bonnans S, Riche VP, et al. FASCE, the benefit of spironolactone for treating acne in women: study protocol for a randomized double-blind trial. Trials 2020;21:1-16.
- Barbieri Lab . Spironolactone Versus Doxycycline for Acne: A Comparative Non-Inferiority Evaluation (SD-ACNE) Research Study n.d. https://barbierilab.bwh.harvard.edu/clinical-trial-opportunities/ (accessed 7 September 2022).
- Yang Y, Brazier J, Longworth L. EQ-5D in skin conditions: an assessment of validity and responsiveness. Eur J Health Econ 2015;16:927-39.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41.
- EuroQOL . EQ-5D-Y User Guide V2.0 2020. https://euroqol.org/publications/user-guides/ (accessed 8 March 2021).
- EuroQol . EQ-5D 5L | Population Norms n.d. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/population-norms/ (accessed 10 September 2022).
- Layton AM, Whitehouse H, Eady EA, Cowdell F, Warburton KL, Fenton M. Prioritizing treatment outcomes: how people with acne vulgaris decide if their treatment is working. J Evid-Based Med 2017;10:163-70. https://doi.org/10.1111/jebm.12249.
- Layton AM, Eady EA, Thiboutot DM, Tan J. Acne Core Outcomes Research Network (ACORN) Outcomes Identification Group . Identifying what to measure in acne clinical trials: first steps towards development of a core outcome set. J Invest Dermatol 2017;137:1784-6. https://doi.org/10.1016/j.jid.2017.04.017.
- Hornsey S, Stuart B, Muller I, Layton AM, Morrison L, King J, et al. Patient-reported outcome measures for acne: a mixed-methods validation study (acne PROMs). BMJ Open 2021;11.
- Hopkins ZH, Thiboutot D, Homsi HA, Perez-Chada LM, Barbieri JS. Patient-reported outcome measures for health-related quality of life in patients with acne vulgaris: a systematic review of measure development and measurement properties. JAMA Dermatol 2022;158.
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol 2019;80:1147-8.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol 2015;151:941-4.
- Thiede RM, Rastogi S, Nardone B, Sadowsky LM, Rangel SM, West DP, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Women Dermatol 2019;5:155-7. https://doi.org/10.1016/j.ijwd.2019.04.024.
- Ip A, Muller I, Geraghty AW, Platt D, Little P, Santer M. Views and experiences of people with acne vulgaris and healthcare professionals about treatments: systematic review and thematic synthesis of qualitative research. BMJ Open 2021;11.
- Trial Forge . The INCLUDE Ethnicity Framework n.d. www.trialforge.org/trial-forge-centre/include/ (accessed 14 September 2022).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358.
Appendix 1 Amendments to protocol and trial documentation
| Amendment number | Date approved | Change |
|---|---|---|
| Substantial amendments | ||
| 01 | 12 April 2019 |
|
| 02 | 5 June 2019 | Unblinding letter to participants |
| 04 | 4 September 2019 | Addition of two additional sites |
| 05 | 3 December 2019 |
|
| 06 | 26 February 2020 | New trial documents: Pregnancy PIS and ICF |
| 07 | 26 February 2020 |
|
| 10 | 15 June 2020 |
|
| 12 | 1 July 2020 |
|
| 16 | 6 April 2021 |
|
| 17 | 2 August 2021 |
|
| Non-substantial amendments | ||
| 03 | 16 July 2019 | Correction of typographical error in protocol |
| 08 | 16 March 2020 | In response to COVID-19 pandemic, trial sites to run follow-up visits remotely and option to post/deliver IMP to participants |
| 09 | 1 April 2020 | Permission for Southampton CTU trial team to receive participant contact details in order to co-ordinate mail-out of follow-up questionnaires during COVID-19 |
| 11 | 12 June 2020 | Restart of recruitment |
| 13 | 10 July 2020 | Updated wording in PIS SAFA summary and patient invitation letter:_GP to reflect the option to deliver the follow-up assessments remotely |
| 14 | 20 November 2020 | Changes of principal investigator arrangements at one site |
| 15 | 16 December 2020 | Option for IMP at baseline to be posted/delivered directly to the participant |
| 18 | 12 October 2021 | Change of principal investigator at Imperial trial site |
Appendix 2 Acne medication use at baseline
| Characteristic | Spironolactone (n = 201) | Placebo (n = 209) | Total (n = 410) |
|---|---|---|---|
| Have you used, or are you currently using, topical treatments (creams/lotions/gels) for your acne? – n (%) b | |||
| Yes | 169 (84.9%) | 171 (82.2%) | 340 (83.5%) |
| No | 30 (15.1%) | 37 (17.8%) | 67 (16.5%) |
| Missing from eCRF – n (%)c | 2 (1.0%) | 1 (0.5%) | 3 (0.7%) |
| If Yes d | |||
| Benzoyl peroxide | |||
| Now | 17 (10.3%) | 27 (16.1%) | 44 (13.2%) |
| Now and in the past | 5 (3.0%) | 3 (1.8%) | 8 (2.4%) |
| In the past | 101 (61.2%) | 83 (49.4%) | 184 (55.3%) |
| Never | 18 (10.9%) | 24 (14.3%) | 42 (12.6%) |
| Not answered | 24 (14.5%) | 31 (18.5%) | 55 (16.5%) |
| Azelaic acid | |||
| Now | 10 (6.1%) | 15 (9.0%) | 25 (7.5%) |
| Now and in the past | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| In the past | 22 (13.3%) | 19 (11.4%) | 41 (12.4%) |
| Never | 94 (57.0%) | 83 (49.7%) | 177 (53.3%) |
| Not answered | 39 (23.6%) | 50 (29.9%) | 89 (26.8%) |
| Topical adapalene | |||
| Now | 19 (11.4%) | 19 (11.4%) | 38 (11.4%) |
| Now and in the past | 3 (1.8%) | 2 (1.2%) | 5 (1.5%) |
| In the past | 54 (32.3%) | 57 (34.1%) | 111 (33.2%) |
| Never | 59 (35.3%) | 53 (31.7%) | 112 (33.5%) |
| Not answered | 32 (19.2%) | 36 (21.6%) | 68 (20.4%) |
| Nicotinamide | |||
| Now | 9 (5.5%) | 2 (1.2%) | 11 (3.3%) |
| Now and in the past | 0 (0.0%) | 1 (0.6%) | 1 (0.3%) |
| In the past | 12 (7.3%) | 9 (5.4%) | 21 (6.3%) |
| Never | 100 (60.6%) | 100 (59.9%) | 200 (60.4%) |
| Not answered | 44 (26.7%) | 55 (32.9%) | 99 (29.8%) |
| Antibiotic | |||
| Now | 8 (4.8%) | 6 (3.6%) | 14 (4.2%) |
| Now and in the past | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| In the past | 92 (55.1%) | 85 (50.9%) | 177 (53.0%) |
| Never | 40 (24.0%) | 41 (24.6%) | 81 (24.3%) |
| Not answered | 27 (16.2%) | 35 (21.0%) | 62 (18.6%) |
| Combination | |||
| Now | 24 (14.4%) | 19 (11.3%) | 43 (12.8%) |
| Now and in the past | 4 (2.4%) | 2 (1.2%) | 6 (1.8%) |
| In the past | 62 (37.1%) | 62 (36.9%) | 124 (37.1%) |
| Never | 49 (29.3%) | 47 (28.0%) | 96 (28.7%) |
| Not answered | 28 (16.8%) | 38 (22.6%) | 66 (19.7%) |
| Other | |||
| Yes | 20 (14.6%) | 25 (16.9%) | 45 (15.8%) |
| If topical treatments have been prescribed, how often are they used? b | |||
| Not at all | 17 (8.5%) | 32 (15.3%) | 49 (12.0%) |
| Less than once a day | 16 (8.0%) | 19 (9.1%) | 35 (8.6%) |
| Once a day | 62 (31.0%) | 58 (27.8%) | 120 (29.3%) |
| Twice a day | 25 (12.5%) | 20 (9.6%) | 45 (11.0%) |
| More than twice a day | 1 (0.5%) | 2 (1.0%) | 3 (0.7%) |
| Not been prescribed topical treatments | 64 (32.0%) | 64 (30.6%) | 128 (31.3%) |
| Not answered | 15 (7.4%) | 14 (6.7%) | 29 (7.1%) |
| Missing from eCRF – n (%)c | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
List of abbreviations
- AE
- adverse event
- AR
- adverse reaction
- CCA
- complete case analysis
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CTCAE
- common terminology criteria for adverse events
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- IGA
- Investigator’s Global Assessment
- IMP
- investigational medicinal product
- MAR
- missing at random
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- NIHR
- National Institute for Health and Care Research
- PCA
- prescription cost analysis
- PCOS
- polycystic ovary syndrome
- PIC
- participant identification centre
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS
- Personal Social Services
- QALYs
- quality-adjusted life-years
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAFA
- spironolactone for adult female acne
- SCTU
- Southampton Clinical Trials Unit
- SF-6D
- Short Form questionnaire-6 Dimensions
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
