Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/57/32. The contractual start date was in September 2016. The draft report began editorial review in July 2021 and was accepted for publication in January 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Thornhill et al. This work was produced by Thornhill et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Thornhill et al.
Chapter 1 Background
Parts of this chapter have been adapted with permission from the study protocol. 1
Infective endocarditis
Infective endocarditis (IE) is a life-threatening infection of the endocardial lining of the heart, particularly the heart valves, with a high morbidity rate and ≈ 30% first-year mortality. 2 The infection leads to the formation of infected heart valve vegetations that may lead to obstruction of normal blood flow through the heart, destruction or perforation of valve leaflets, and damage to the chordae tendineae that can cause severe valvular regurgitation. These in turn may lead to heart failure. In addition, there may be perivalvular abscess formation, and fragments of infected vegetations may break off and embolise to distant capillary beds, causing strokes, retinal haemorrhages, brain abscesses and other distant site infections, vasculitis and characteristic nail bed splinter haemorrhages. 2
Currently, there are ≈ 3000 IE cases and ≈ 900 IE-related deaths annually in England, and the number has nearly doubled in the last 10 years. 3,4 All patients require hospitalisation and intensive long-term treatment with antibiotics. 2 A large proportion of patients require surgery to replace damaged heart valves and the long-term morbidity rate is high. 2 The resultant cost to individuals, families, society and the NHS is extremely high. 5
Risk of infective endocarditis
Although IE is relatively rare, affecting only 3–10 per 100,000 people per year,3,4,6,7 patients at increased risk of IE are comparatively common and increasing in number. A large number of individuals with predisposing cardiac conditions are at increased risk of IE. 7 Guideline committees around the world have generally stratified individuals into those at high, moderate and low risk of contracting IE, or developing complications from IE. 8–11 High-risk individuals include (1) those with a history of IE; (2) those with prosthetic heart valves, including transcatheter, bioprosthetic or homograft valves; (3) those for whom prosthetic material was used for valve repair; (4) those with any type of cyanotic congenital heart disease; and (5) those with any type of congenital heart disease repaired with prosthetic material, who are at high risk for 6 months following the repair or for life if there is a residual shunt of valvular regurgitation. Those at moderate risk include those with valvular stenosis or regurgitation, a bicuspid aortic valve or hypertrophic cardiomyopathy.
The role of oral bacteria
Infective endocarditis can result from bloodstream infection caused by a spectrum of bacterial and fungal organisms entering the circulation. However, certain organisms seem to have a higher propensity than others for causing IE. The possibility that some cases of IE might be linked to invasive dental procedures (IDPs) was first suggested by Lewis and Grant in 192312 and supported in 1935 by Okell and Elliott,13 who demonstrated that 61% of individuals develop a transient bacteraemia with oral viridans group streptococci (OVGS) following a dental extraction and that OVGS could be isolated from the heart valve vegetations of 40–45% of individuals with IE.
Use of antibiotic prophylaxis before invasive dental procedures to prevent infective endocarditis
Following the development of antibiotics, Glaser et al. 14 and Hirsch et al. 15 demonstrated that penicillin given prophylactically could reduce the bacteraemia caused by dental extractions, and this paved the way for the American Heart Association (AHA) to issue the first guidelines on the use of antibiotic prophylaxis (AP) to prevent IE in 1955. 16
Antibiotic prophylaxis was soon adopted globally as a standard of care for preventing IE in those at increased risk of IE who were undergoing IDP. However, to the best of our knowledge, there has never been a trial of AP to define its efficacy in IE prevention. 17 Furthermore, multiple studies have shown that low-level bacteraemia occurs frequently following daily activities such as tooth brushing, flossing and mastication, particularly in those with poor oral hygiene. 18 Some have argued that the risk of developing IE posed by these daily activities far exceeds any risk associated with IDP,19 and thus the case for giving AP to cover IDPs is flawed. 19 This, along with concerns about the risk of adverse drug reactions5,20 and the development of antibiotic resistance,21 has led to reductions in the number of individuals targeted for AP.
In 2007, the AHA recommended restricting AP to those at high risk of IE and its complications who were undergoing IDP. 11 The European Society of Cardiology (ESC) published similar guidance in 2009. 9 In the UK, however, where the important role of daily activities in causing OVGS IE was most strongly argued,19 the National Institute for Health and Care Excellence (NICE) concluded in 2008 that ‘the evidence does not show a causal relationship between having an interventional procedure and the development of IE’ and that ‘it is biologically implausible that a dental procedure would lead to a greater risk of IE than regular tooth brushing’ and recommended the complete cessation of AP (© NICE 2008 Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. Available from www.nice.org.uk/guidance/ng64. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.). 22,23
Nonetheless, concerns remain that IDPs could pose a risk for those at increased risk of IE and most guideline committees (e.g. AHA, ESC, Japanese Cardiac Society) continue to recommend AP for those at highest risk who are undergoing IDP. 9,11,24 In 2016, even NICE softened its position and no longer precludes the use of AP for those at increased risk of IE undergoing IDPs. 25,26
Lack of evidence to support the use of antibiotic prophylaxis to prevent infective endocarditis
Despite the wide adoption of AP before IDPs to prevent IE in many parts of the world, to the best of our knowledge, there has never been a randomised controlled trial (RCT) of AP’s efficacy. The evidence base for the use of AP is mainly limited to case–control studies, in which reduction in bacteraemia was used as a surrogate measure for reduction in IE, and the evidence base for the use of AP is therefore limited and heterogeneous. Many of the studies are of poor methodological quality. 17
There are several reasons why a RCT of AP has not been performed to date and is unlikely to be performed in the foreseeable future. 27 AP is not a treatment, but rather a prevention strategy, and IE is comparatively rare. This means that hundreds of individuals at risk of IE would need to receive AP to prevent one case of IE. Data from a study published in The Lancet suggested that the number of individuals that would need to receive AP to prevent one case of IE was 277 [95% confidence interval (CI) 156 to 1217 individuals]. 6 The need to randomise patients to placebo or active prophylaxis would double the size of the study.
Furthermore, AP has to be administered immediately before performing an IDP for it to be effective. This means that the decision to provide AP has to be made by dentists. However, each dental practice sees only a small number of individuals at risk of IE. Therefore, large numbers of dental practices across the country would need to be involved in recruiting and randomising patients to AP or placebo. All of this means that the size, cost and complexity of a RCT to evaluate AP would be substantial.
Several attempts have been made to fund such a RCT, including by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme and the US National Institutes of Health. Ultimately, the high cost of such studies has prevented these and other agencies from funding such a trial. A further barrier to a RCT, particularly in countries in which AP is the current standard of care, are ethics and medicolegal concerns about withholding AP from individuals at high risk of IE who would be randomised to placebo prophylaxis. This is because of concern that individuals randomised to placebo prophylaxis could develop IE and die. So far, this concern has prevented attempts to perform a RCT of AP in countries outside the UK. 27 Collectively, these factors explain why a RCT has not taken place to date and may never be conducted. 27
The aim of the study
Before a RCT would be meaningful, we need to be certain that there is an association between IDPs and IE; that is the purpose of the Invasive Dentistry–Endocarditis Association (IDEA) study. Indeed, such a study could render a RCT unnecessary. Without an association between IDPs and IE, there is no rationale for the use of AP. The IDEA study links national data concerning courses of dental treatment and hospital admissions for IE to investigate if there is a temporal link or association between IDPs and the subsequent development of IE.
The advantage of performing this study in England is that, between March 2008 and March 2016, NICE recommended against AP and there was a high level of compliance with this recommendation. 3,6,28 Any association between IDP and IE should therefore be fully exposed.
Chapter 2 Overview of prespecified study methodology
Introduction
The aim of this study was to investigate if there is a temporal association between IDPs and the development of IE.
Antibiotic prophylaxis makes sense only if there is a temporal association between IDPs and IE. Yet, to the best of our knowledge, few studies have specifically looked for the existence of such an association and the results of these investigations have been contradictory.
In 1995, Lacassin et al. 29 performed a small case–control study in France in which they compared the occurrence of IDPs in the 3 months prior to hospital admission in 171 patients with IE with a control population. They found a significantly elevated risk of IE in those who had undergone an IDP and estimated that AP could reduce the risk of IE by 5–10%. However, they acknowledged that their study was underpowered and the case–control design was criticised regarding the risk of selection bias and confounding due to potential differences in IE risk factors between cases and controls.
In 1998, Strom et al. 30 performed a similar case–control study in the Philadelphia area comparing the incidence rate of IDPs in the 3 months prior to hospital admission in 273 IE cases and control subjects. They found no evidence for an association between IDP and IE. However, the authors acknowledged that the study was underpowered to identify an association and was at risk of selection bias and confounding due to differences in the risk of IE in cases and control subjects.
In 2017, Tubiana et al. 31 examined the relationship between IE and IDPs in the 3 months prior to IE hospital admission in a cohort study of 138,876 high-risk individuals with prosthetic heart valves, of whom 267 developed IE associated with oral streptococci. They reported no significant association between IDP and the development of IE, but acknowledged a lack of power to demonstrate an association in their cohort study.
In an attempt to avoid the issues of selection bias and risk factor confounding, Porat Ben-Amy et al. 32 performed a case-crossover design study to address the same problem. They identified 170 patients with IE and used a patient questionnaire to identify any dental visits over the previous 2 years. The frequency of dental visits in the 3 months immediately before IE diagnosis was compared with the frequency in earlier 3-month periods. Again, the study suffered from a small sample size and recall bias caused by patients’ difficulty recalling the timing and nature of dental procedures performed over the preceding 2 years.
More recently, Chen et al. 33 performed a larger case-crossover design study using a Taiwanese longitudinal health insurance database. They identified 739 patients with IE and compared the incidence rate of IDPs in the 3 months immediately prior to IE diagnosis (cases) with earlier 3-month periods (matched control periods). They did not find a significant difference in the incidence rate of IDPs between cases and controls. However, they acknowledged the small number of IE cases in their study and the likelihood that they had insufficient statistical power to detect an association between IE and IDPs.
In 2017, after this study had already started, Tubiana et al. ,31 alongside the cohort study described above, reported the results of a further case-crossover analysis of 648 prosthetic valve patients who developed oral streptococcal IE. They found a higher frequency of IDP in the 3-month case period before IE hospital admission than in the control periods (5.1% vs. 3.2%, respectively; odds ratio 1.66, 95% CI 1.05 to 2.63; p = 0.03). 31
Apart from the Tubiana et al. case-crossover study,31 all of these studies were underpowered. 29,30,32,33 Even more importantly, they were all (including the study by Tubiana et al. 31) performed in populations in which the standard of care was to prescribe AP prior to IDP. At the time of the Lacassin et al. 29 and Strom et al. 30 studies, patients at moderate and high risk of IE would have been provided with AP, and in the case of the Porat Ben-Amy et al. 32, Chen et al. 33 and Tubiana et al. 31 studies it was recommended that those at high risk were provided with AP. Clearly, if AP has efficacy for preventing IE, any association between IDPs and IE would be underestimated in a situation where patients at increased risk of IE were being provided with AP.
The purpose of the IDEA study was to perform a much larger case-crossover study in a population in which the standard of care was not to provide AP. This is why we chose a study period from April 2009 to March 2016. Although the NICE guidelines recommending cessation of AP came into effect in March 2008 (and AP prescribing fell quickly after their introduction),6 by waiting an extra year before collecting data we could ensure that any carryover effect of AP prescribing was minimised. This should have optimised the chances of identifying any association between IDPs and IE that might exist and provide much more reliable data on this important issue. Because the UK was the only country in the world where AP was not recommended during this period, it was the only place where a study investigating the association between IDPs and IE could be performed without the effect of AP concealing any association.
The case-crossover design eliminates selection bias and confounding for risk of IE because each case acts as its own control. 34,35 Furthermore, by linking national dental and Hospital Episode Statistics (HES) data, we did not have to rely on patient recall to determine the timing and nature of any dental procedures that were performed.
Because the case-crossover methodology addresses problems of selection bias and confounding, and provides greater statistical power for addressing cause and effect issues of this type, we prespecified a case-crossover methodology for our primary analysis. This case-crossover methodology was first proposed by Maclure34 for studying the effect of transient events in triggering a subsequent acute outcome while eliminating control selection bias and confounding by constant within-subject characteristics. In case-crossover studies, each individual acts as their own control. The study examines individuals with a specific outcome – in this case, those who develop IE – and looks at their exposure to events that might precipitate that outcome – in this case, IDPs.
Primary aim of the study
Our primary aim was to perform a case-crossover design study34 to quantify the number of IDPs in the 3 months immediately preceding a hospital admission for IE (the case or exposure period) and to compare this with the number of IDPs in the preceding 12 months (i.e. months 4–15; the matched control periods) to determine if there is an association between IDPs and IE.
We chose a 3-month case/exposure period because previous research has shown that ≈ 90% of IE cases associated with an identifiable potential cause result in hospital admission and a definitive diagnosis of IE within 3 months. 36 A 3-month period is widely accepted within research studies for defining IE cases associated with a causal invasive procedure. 29–33 The preceding 12-month period was selected as the control period.
Possible outcomes
If there is a link between IDPs and IE, we would expect courses of dental treatment involving an IDP to occur with significantly higher frequency in the 3-month case/exposure period immediately preceding hospital admission for IE than in the matched control period.
Alternatively, if there is no link between IDP and IE, we would expect there to be no significant difference in the number of IDPs in the 3-month case/exposure period immediately preceding hospital admission for IE and the number of IDPs in the control period.
Data sources
All hospital admissions in England are recorded in the NHS Digital HES database. 37 We submitted a request to NHS Digital to search the HES database using International Classification of Diseases, Tenth Revision (ICD-10), codes to identify all IE hospital admissions between 1 April 2010 and 31 March 2016 and create two data sets:
-
Data set 1 – patient-identifying information (i.e. NHS number, name, date of birth, sex, address) for those admitted with IE. This list of identifiers, along with a unique HES study identifier generated randomly for each patient by NHS Digital, was transferred directly to the NHS Business Services Authority (NHSBSA).
-
Data set 2 – for all IE admissions identified, NHS Digital produced a full set of clinical, diagnostic and procedural HES data for the period 1 January 2000 to 31 March 2016. Data set 2 included the same unique study identifiers used in data set 1, but no other patient-identifying information. Data set 2 was transferred to the study team.
The NHSBSA maintains dental records for all patients receiving NHS dental treatment in England. Using the personal identifiers received in database 1, we requested that they extract the dental records of each of these individuals for the period 1 April 2009 to 31 March 2016, thereby providing the dental records of each individual for the year preceding their hospital admission for IE. The NHSBSA was requested to remove all patient identifiers (except the unique HES study identifier) from these data to create data set 3, which was transferred to the study team. Thus, the study team received and were able to link the medical (data set 2) and dental (data set 3) records of each individual using the common HES study identifier (common to all three data sets) without receiving patient-identifying details.
The study period from 1 April 2009 to 31 March 2016 was chosen for several reasons. First, in March 2008, the NICE guidelines recommending that dentists should cease to provide AP to patients for preventing IE came into effect and, by March 2009, AP prescribing had decreased by 76%. 3,22 Therefore, if IDPs are a risk factor for IE, the risk will have been maximised since then.
Second, from April 2008, dentists working in the English NHS were required to record if a patient had received a dental extraction, scale and polish or endodontic treatment (i.e. an IDP) as part of their course of dental treatment using NHS form FP17. Patient-identifying data, other types of dental treatment (i.e. non-invasive), and the start and end dates of the course of treatment are also recorded. Dentists must complete a FP17 form for each patient they treat and send it to the NHSBSA, where data are recorded to receive payment. Compliance is therefore high.
By waiting to commence data collection for 1 year after the introduction of the NICE guidelines and inclusion of dental procedure recording on the FP17 form, we minimised any carryover effect of AP prescribing or changeover effect on the recording of dental data.
Approvals
Because this study uses national data and necessitated the transfer of individually identifying information between NHS Digital and the NHSBSA, we were required to obtain both national Research Ethics Committee approval (reference 17/SC/0371) and Confidentiality Advisory Group (CAG) approval (reference 17/CAG/0076) under Regulation 5 of the Health Service (Control of Patient Information) Regulations 200238 to process patient-identifiable information without consent. We also had to obtain approval from NHS Digital Data Access Request Service (DARS) and the Independent Group Advising on the Release of Data (IGARD).
Identifying invasive dental procedures
Since April 2008, dentists working in the English NHS must record if a patient has had a dental extraction, dental scaling or endodontic treatment as part of any course of dental treatment. These procedures were considered IDPs for the purposes of this study. 11,39 We also identified ‘non-invasive’ courses of dental treatment, which included courses of treatment restricted to a simple dental examination, with or without radiography, and excluded any operative (including restorative) treatment. Courses of treatment that did not include IDPs or non-invasive dental procedures, as defined above, were labelled indeterminate courses of dental treatment. These included courses of dental treatment that involved restorative dental treatments, but no IDP.
Identifying infective endocarditis admissions and infective endocarditis risk stratification
An IE admission was defined as a hospital provider spell, that is ‘the total continuous stay of a patient on premises controlled by a Health Care Provider’, in line with the NHS Business definition (Information from NHS Digital, licensed under the current version of the Open Government Licence; www.nationalarchives.gov.uk/doc/open-government-licence/version/3/). 40 Each spell may comprise a number of consultant episodes, each with a primary ICD-10 discharge diagnosis code and up to 20 secondary or supplementary codes. An IE admission was defined as any spell where an ICD-10 I33.0 primary diagnosis code was used for any consultant episode. Patients discharged alive with a hospital stay of < 3 days in length (admission date – discharge date), re-admission within 30 days or elective admission [i.e. admission method recorded as waiting list (category 11), booked (category 12) or planned (category 13)] were excluded from analysis. We have labelled this definition of IE cases as the ‘narrow’ definition of IE or ‘IE narrow’. A recent analysis of English hospital admissions for IE showed that the use of this narrow definition of IE cases had the highest specificity (0.97) and positive predictive value (PPV; 0.88) for identifying Duke criteria-positive IE cases of any ICD-10 diagnostic codes, but low sensitivity (0.41). 41
To increase sensitivity for identifying IE cases, we also performed the analysis using the same criteria and any hospital admission with a primary or secondary ICD-10 discharge diagnosis code (i.e. I33.0, I33.9, I39.0, I39.1, I39.2, I39.3, I39.4 or I39.8), or a primary discharge diagnosis code of I38.X. This definition, labelled the ‘broad’ definition of IE or ‘IE broad’, has been shown to have higher sensitivity (0.65), but lower specificity (0.83, PPV 0.80). 41
To stratify individuals into those at high, moderate, or low/unknown risk of developing IE, the database was searched back to 1 January 2000 to identify any ICD-10 diagnosis or Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4 (OPCS-4), procedure codes occurring before an IE admission that would have placed an individual at high or moderate risk of IE, as defined by the 2007 AHA guidelines (see Appendix 1, Tables 24 and 25). 11,42 After an individual had an IE hospital admission, they were considered at high risk for further episodes of IE. New IE episodes were distinguished from re-admissions by accepting only IE admissions that were > 6 months apart as new episodes. 7,43 Individuals with a congenital heart condition that was completely repaired with prosthetic material or a device were considered to be at high risk of developing IE for 6 months after the procedure, and were then considered at low risk of developing IE, in line with AHA guidelines. 11 Individuals not identified as being at moderate or high risk were considered to be at low/unknown risk of IE.
Sample size and power calculations
Use of publicly available annualised data from NHS Digital allowed us to estimate that there were 10,593 IE diagnoses in England between 1 April 2009 and 31 March 2015. It also confirmed that the incidence rate of IE was increasing year on year. Between 1 April 2010 and 31 March 2016, we therefore expected the number of IE cases to be at least 10,593, and this is the figure we used in our power calculations. Publicly available NHSBSA dental services data for the same period showed that 56% of the population were regular NHS dental attenders (not accounting for any of those only attending an NHS dentist in an emergency). 44
Preliminary analysis of the dental data showed that there were 176.4M courses of dental treatment between 1 April 2009 and 31 March 2015. Of these, ≈ 90.6M included an IDP and ≈ 85.8M did not include an IDP. IDPs included ≈ 13.2M extractions, ≈ 78.6M scale and polishes and ≈ 3.6M endodontic treatments. The numbers of IDPs and the number of each type of IDP are therefore likely to be larger for the period from 1 April 2009 to 31 March 2016.
A case-crossover design study34 is a type of self-controlled case-series. 45–47 Sample sizes for self-controlled case series are given by Muscoda46 and available from the HyLown Consulting webpage. 48
The cases in our study were those patients with IE admissions who underwent an IDP in the 15 months before admission: the ‘observation period’. The ‘risk period’ was the last 3 months of the observation period (i.e. the 3 months just before the admission) and the ‘control period’ was the previous 12 months. Our data spanned the period between April 2009 and March 2016. In the 6 years from April 2010 to March 2016, we expected to identify 10,593 patients with IE admissions in HES data for England whose exposure to IDPs was observed in the 7 years of NHS dental service data from April 2009 to March 2016. We know that 56% of the population regularly use NHS dentists, and that at least half of all courses of dental treatment include an IDP. If we assume that regular NHS dental patients are seen once every 2 years, then we would expect that each patient would have an IDP at least every 4 years. Bearing in mind that IDPs include common procedures, such as a scale and polish, as well as less common procedures, such as extractions, this estimate was probably conservative.
Assuming that there was no association between IDPs and IE, we estimated that our sample size would consist of 10,593 × 0.56 × 0.25 = 1483 patients with admissions for IE who underwent an IDP in the previous 12 months. This gave 80% power to detect a relative incidence rate of 1.18 (i.e. + 18%) in the 3-month ‘risk period’ compared with control periods. If regular NHS dental patients have an IDP once every 2 years, then we should have 2966 cases and 80% power to detect a relative incidence rate of 1.12 (i.e. + 12%). This should give us the statistical power to detect any association between IDPs and IE that is likely to be of clinical significance, and it will greatly exceed the power of any previous study.
Statistical analysis
The planned case–cohort analysis used a longitudinal Poisson regression in which the outcome was the number of IDPs. Specifically, the primary planned analysis was to divide time into four intervals, each of 3 months, with the number of IDPs in the 3 months immediately prior to IE compared with that in months 3–6, 6–9 and 9–12. Hypothesis tests, using Wald tests, were performed to compare case and control periods. A larger number of IDPs in the case period than in the control periods would suggest that there is an association between IE and IDP events.
A second planned analysis was to consider the relative incidence rate of IDPs to non-IDPs during the case and control periods.
Alternative case and control periods were also considered, including:
-
4-monthly – months 0–4 (case) compared with months 4–8 and 8–12 (controls)
-
quarterly, but removing month 1 – months 2–4 (case) compared with months 5–7, 8–10 and 11–13 (controls).
In all cases, the model was a longitudinal Poisson regression using an exchangeable correlation matrix and robust standard errors. The Poisson model was tested for overdispersion by fitting a population-averaged negative binomial model with an overdispersion parameter proportional to the mean. The overall comparison of case and control periods was the averaged coefficient across the three control periods and all IE cases; however, period-specific incidence rates are also reported.
Reporting guidance
As this was an observational study, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement reporting guidance checklist for cohort studies49 was used to guide our reporting of this study.
Causes and consequences of delays in receipt of data
The project start date was 1 September 2016, and the original project end date was 30 November 2018. Within this, we had allowed 14 months to obtain all the regulatory permissions and receive the data needed for the study from NHS Digital and the NHSBSA (i.e. we should have received the data by 30 September 2017). However, we did not receive both data sets until 21 May 2020, some 32 months late. There were many delays in the regulatory and contractual process that led to this.
First, at the outset of the study, the Health Research Authority (HRA) informed us that we did not require NHS research ethics approval for the study, but would require CAG approval. As the University of Sheffield requires us to obtain University ethics approval for any studies not covered by NHS ethics approval, we sought and obtained this before submitting a CAG application. The CAG, however, rejected our application and indicated that it would not consider it without prior NHS ethics approval. The CAG would not accept the University of Sheffield ethics approval. It rejected the HRA advice on this. We therefore sought NHS ethics approval through the National Research Ethics Service (NRES). However, NRES insisted that this was not necessary for our study and that it was the CAG’s responsibility to provide any approval. It took some time to resolve this conflict of opinion and advice between HRA, NRES and CAG. Eventually, NRES undertook the ethics review, and once it had issued a favourable opinion we were allowed to submit the CAG application. Owing to these delays, we finally received CAG approval on 27 September 2017.
Second, once we had CAG approval, we submitted our application to the DARS at NHS Digital to obtain Section 263 approval under the Health and Social Care Act50 for the use of confidential patient information. Having already obtained both research ethics and CAG approval, we expected a relatively rapid turnaround. However, owing to the delays in obtaining these, we were caught by the introduction of a new regulatory step in the NHS Digital CAG approval process, with the additional need to obtain approval from the newly established IGARD. This changed the information we were required to submit. Nonetheless, we submitted our application to the new 13-stage DARS/IGARD process on 27 October 2017.
In January 2018, DARS/IGARD informed us that NHS Digital had reservations about providing the NHSBSA with a list of patients diagnosed with IE, as this would mean that NHSBSA staff would know that everyone on that list had had IE. To avoid this, it was suggested that the data sent to the NHSBSA should contain details of patients admitted for other conditions as well. As we thought it would be interesting to investigate any association between IDP and other acute medical conditions [e.g. myocardial infarction (MI), stroke and pulmonary embolism (PE)] as well, we suggested adding patients diagnosed with these conditions to the data set extracted by NHS Digital. Thus, NHSBSA staff would not know which of these conditions any individual might have experienced. This, however, resulted in a much larger data set, which in turn caused concern within the NHSBSA about its ability to manage a much larger data set with its existing resource and staff. We were eventually informed that we had received DARS/IGARD approval on the 23 February 2018.
Third, the data access charge estimates from NHS Digital and the NHSBSA on which our grant application was costed produced a combined cost of just under the £20,000 requested. However, because of the delays and the DARS/IGARD/NHS Digital requirement that we include non-IE data, both NHS Digital and the NHSBSA very substantially increased their data access charges: NHS Digital increased from £9560 to £25,800 and the NHSBSA increased from under £10,000 to £21,081.45 (i.e. £46,881.45 total). This was beyond the grant’s ability to fund. In discussion with NIHR, it was decided that we should seek a 4-month extension to the study (new end date 31 March 2019) and a budget increase to cover the increase in data access charges. The contract variation request was submitted to NIHR on 22 March 2018 and approved on 1 August 2018.
Fourth, during this period, NHS Digital and the NHSBSA also changed their views several times about the nature of the data-sharing agreement (DSA) arrangement they wanted to cover this study. NHS Digital’s opinion changed from wanting just an agreement between it and the University of Sheffield, to a three-way agreement involving NHS Digital, the NHSBSA and the University of Sheffield, and then to a series of two-way agreements. In late March 2018, NHS Digital finally informed us that it would enter into only a two-way agreement between NHS Digital and the University of Sheffield, and it would not permit the NHSBSA to be a signatory to the agreement (despite the NHSBSA being named in the agreement as a data processor). We therefore had to seek legal advice about how to proceed, as the NHSBSA was one of the named data processors and insisted on being a signatory to any agreement. We were advised to set up two separate DSAs – one between NHS Digital and the University of Sheffield, and another between the University of Sheffield and the NHSBSA. This was finally agreed by all parties in April 2018.
Fifth, simultaneous negotiations about the large increase in data access charges ultimately resulted in a reduction in the NHS Digital charge to £10,200. However, the NHSBSA informed us of a possible further increase in the data access charges. The reason it gave was that the government now required it to outsource all of its data activities to the private company Capita plc (London, UK), and Capita plc would want to perform its own costing, as the NHSBSA was no longer in a position to perform the work. The involvement of Capita plc was new information for us and meant that the DSAs and DARS approvals were no longer valid. NHS Digital required both the NHSBSA and Capita plc to be named as data processors on the DSAs, and we had to obtain separate details and evidence of the data security arrangements of Capita plc as well as the NHSBSA. It took time to identify the right people within Capita plc to provide the necessary details and several problems arose. These problems were due to NHS Digital/DARS’s dissatisfaction with the information provided by Capita plc and Capita plc’s unfamiliarity with the requirements of NHS Digital. The delays were exacerbated by these organisations’ failure to communicate directly with each other and the study team had to act as an intermediary in these processes and communications.
On 10 August 2018, we were finally able to provide NHS Digital/DARS with all of the information it required to approve the study and sign the DSAs. On 14 September 2018, NHS Digital/DARS informed us that it was now satisfied with all of the information and that the DSAs between NHS Digital and the University of Sheffield, and between the University of Sheffield and Capita plc were ready for signing. After much chasing, we received an e-mail from NHS Digital on 12 October 2018 informing us that it had had a change of mind and decided to send the project back to IGARD for review. By 23 November 2018, we had still not heard the outcome of that review or received either data set, and it was evident that it would be impossible to complete the project before the extension’s end date of 31 March 2019. In further discussions with NIHR, it was decided to stop all further work and expenditure (apart from data access charges) on the project on 30 November 2018 and effectively freeze the project until we could be certain of receiving both data sets.
Sixth, around the same time, the NHSBSA informed us that Capita plc’s contract to undertake all of the NHSBSA’s data-processing activities was coming to an end, with all data-processing activity being brought back in house. However, because the NHSBSA no longer had the staff and resources to undertake this work and because of the challenges associated with repatriating the work to the NHSBSA, it was unlikely that the NHSBSA would be able to deal with our data request for at least 1 year.
Seventh, in early 2019, we finally received IGARD approval again and the DSAs were finally signed off on 21 March 2019. In June 2019, NHS Digital finally provided the NHSBSA with data set 1 and the study team with data set 2. Unfortunately, the NHSBSA Dental Information Services Team in Eastbourne was still not in a position to extract the dental data we needed, despite now having all the information they needed from NHS Digital. After much further negotiation, the Eastbourne NHSBSA team enlisted the support of the NHSBSA Data Analytics Learning Laboratory team in Newcastle to carry out the work. This necessitated further adjustments to the DSA’s data security details, but, on 30 October 2019, we finally received a guarantee from the NHSBSA’s Chief Insight (Data) Officer that the NHSBSA would provide the dental data before 1 April 2020.
Eighth, we informed NIHR of this and it was agreed that we should request a further contract variation to allow the project to restart on 1 April 2020, with an end date of 31 March 2021. This contract variation was submitted on 25 October 2019. On 5 February 2020, NIHR approved the contract variation, with a financial reconciliation that would allow us to restart on 1 April 2020 and fund our work until 31 March 2021. However, although providing funding until 31 March only, NIHR extended the study end date by a further 3 months until the 30 June 2021. We finally received the dental data from the NHSBSA on 21 May 2020.
Ninth, with the release of the dental data to the study team, the NHSBSA informed us that it was unable to supply all of the data we had requested (i.e. data from 1 April 2009 until 31 March 2015). It informed us that this was because they have a policy of destroying data that are ≥ 10 years old. NHSBSA was, therefore, able to supply us with data only from 1 April 2010 until 31 March 2015, that is 60 months of data rather than the 72 months of data we had requested and had used to calculate the sample size and power of the data. Had the data been made available to us as planned by 30 September 2017 (or even up to 18 months after that), we could have still received the whole data set.
Last, on 16 March 2020, the government announced the UK COVID-19 lockdown. This necessitated a rapid change to home working that slowed the NHSBSA’s final delivery of the dental data. We finally received the data on 21 May 2020. The need for home working also had some effect on the study team’s ability to work with and analyse the data, which were stored on University of Sheffield computers, but had to be handled remotely due to home working. It also meant that the study team members could not work as closely and seamlessly together as normal. However, we adapted very effectively to home working.
These events were important because they caused enormous delays and disruptions to the study and led to a substantial (≈ 20%) loss of data, as noted above. Once we had received the data, we identified further problems that are described in the following chapters.
Chapter 3 Data checking, data linkage and preliminary analysis
We finally received the hospital admissions data from NHS Digital in June 2019 and received the dental data from the NHSBSA on 21 May 2020. Each data set was checked for completeness, integrity, fidelity to specification and duplicates. It was also cleaned and manipulated to comply with the methodological requirements. The two data sets were then linked using the common, random, individual-specific study identifier.
Data on infective endocarditis hospital admissions
The imported IE hospital admissions data from NHS Digital were manipulated to comply with the IE case definition. As described in Identifying infective endocarditis admissions and infective endocarditis risk stratification, we used two different definitions of an IE hospital admission:
-
a narrow definition – any consultant episode for which an ICD-10 I33.0 primary discharge diagnosis code was used
-
a broad definition – any consultant episode for which a primary or secondary ICD-10 discharge diagnosis code of I33.0, I33.9, I39.0, I39.1, I39.2, I39.3, I39.4 or I39.8, or a primary discharge diagnosis code of I38.X was used.
As a patient may be under the care of more than one consultant and even be transferred between hospitals during a single spell of admission for IE, we combined such episodes into a single continuous hospital admission spell or ‘index’ admission (i.e. we removed non-index admissions data).
New IE cases result in urgent or emergency hospital admission. However, some patients with an existing IE diagnosis are admitted to hospital for evaluation, or may be re-admitted for further evaluation or treatment. As these do not represent a new IE diagnosis, we excluded the following from analysis: all elective admissions (i.e. where admission method was recorded as waiting list, booked or planned), any hospital admission with a duration of < 3 days and any re-admission within 6 months. For the IE admission data counts at each stage of these manipulations, see Appendix 1, Table 26.
Because new IE episodes were distinguished from re-admissions by accepting only IE admissions > 6 months apart as new episodes,7,43 we analysed the data to determine how many patients had more than one IE admission and how many of those were > 6, > 12 or > 18 months apart. For data using the narrow and broad definitions for an IE admission, see Appendix 1, Table 27.
Data on courses of dental treatment
The data on courses of dental treatment received from the NHSBSA were checked, cleaned and characterised (Table 1).
| Characteristic | Value |
|---|---|
| Dental treatment start date | |
| Number | 3,751,621 |
| Earliest | 1 April 2010a |
| Latest | 17 March 2016 |
| Dental treatment end date | |
| Number | 3,745,728 |
| Earliest | 1 April 2010 |
| Latest | 17 March 2016 |
| Dental treatment duration (days) | |
| Number | 3,745,728 |
| Mean (standard deviation) | 10.8 (30.6) |
| Median (minimum, maximum) | 0 (0, 1848) |
| Match descriptionb | |
| Number | 3,751,621 |
| Legacy index does not appear in patient dimension, n (%) | 1,427,382 (38.0) |
| Legacy index matches multiple NHS numbers, n (%) | 140,076 (3.7) |
| Legacy index has unique 1 : 1 NHS number match, n (%) | 2,168,752 (57.8) |
| Legacy index matches a unique NHS number, but other LIDs also match the same NHS number, n (%) | 15,411 (0.4) |
| Dental procedure type | |
| Number | 3,751,621 |
| IDP, n (%) | 1,819,776 (48.5) |
| Non-invasive procedure, n (%) | 956,634 (25.5) |
| Indeterminate procedure, n (%) | 975,211 (26.0) |
| IDP type | |
| Number | 1,819,775 |
| Endodontic treatment, n (%) | 39,490 (2.2) |
| Extractions, n (%) | 286,360 (15.7) |
| Scale and polish, n (%) | 1,384,950 (76.1) |
| Mixed, n (%) | 108,975 (6.0) |
| Antibiotics usec | |
| Number | 3,751,621 |
| No, n (%) | 3,682,646 (98.2) |
| Yes, n (%) | 68,975 (1.8) |
During this process, NHSBSA dental data were linked to NHS Digital data on IE admissions using the person-specific study identifier generated by NHS Digital. Dental data with no associated IE admission data were removed and any courses of dental treatment that overlapped were combined into a single course of dental treatment. The counts of dental treatment courses at each stage of data cleaning and manipulation are provided in Appendix 1, Table 28.
Using the two different definitions for an IE admission (narrow and broad), we then determined the number of these admissions for which we had 12 months of linked data on preceding dental courses of treatment. We quantified this in two ways: (1) starting from the day of IE hospital admission (months 0–12) and (2) skipping the first month before hospital admission (months 1–13). The counts for these are shown in Appendix 1, Table 29.
Infective endocarditis patient characteristics using the narrow definition of infective endocarditis
Using the narrow definition of IE, we analysed the age, sex and previous IE risk status (high, moderate or low/unknown risk) of each IE admission patient. We also identified the specific heart condition that categorised an individual as high risk or moderate risk for IE, and if there was any IE causal organism data for that individual. We quantified this information for all IE admissions in the data set and for those IE admissions for which we had linked dental data (Table 2; see also Appendix 2, Figures 19 and 21).
| Characteristic | IE narrow admission | |
|---|---|---|
| All | With linked dental data | |
| Age of the patient (years) | ||
| Number | 11,570 | 3163 |
| Mean (standard deviation) | 61.2 (19.8) | 61.6 (18.7) |
| Median (minimum, maximum) | 65 (0, 101) | 66 (1, 96) |
| Sex | ||
| Number | 11,575 | 3160 |
| Male, n (%) | 7900 (68.3) | 2135 (67.5) |
| Female, n (%) | 3675 (31.7) | 1030 (32.5) |
| Level of IE risk before IE admission | ||
| Number | 11,575 | 3160 |
| High risk, n (%) | 3285 (28.4) | 1050 (33.2) |
| Moderate risk, n (%) | 1875 (16.2) | 520 (16.4) |
| Low/unknown risk, n (%) | 6415 (55.4) | 1590 (50.2) |
| Reason for high-risk stratification, n (%)a | ||
| Previous IE | 530 (16.1) | 155 (14.8) |
| Previous IE: I38X | 180 (5.5) | 35 (3.3) |
| Replacement heart valve | 2090 (63.6) | 700 (66.7) |
| Repaired heart valve | 280 (8.5) | 105 (10.0) |
| Cyanotic congenital heart disease | 155 (4.7) | 35 (3.3) |
| Repaired congenital heart disease | – | – |
| Palliative shunt or conduit | 35 (1.1) | 20 (1.9) |
| Prosthetic heart/ventricular assist device | – | – |
| Reason for moderate-risk stratification, n (%)a | ||
| Previous rheumatic fever | 580 (30.9) | 155 (29.8) |
| Non-rheumatic valve | 1190 (63.5) | 340 (65.4) |
| Congenital valve anomalies | 55 (2.9) | 20 (3.9) |
| Hypertrophic cardiomyopathy | 50 (2.7) | 10 (1.9) |
| Causal organism reported | ||
| Number | 11,575 | 3160 |
| No, n (%) | 3175 (27.4) | 870 (27.5) |
| Yes, n (%) | 8400 (72.6) | 2290 (72.4) |
| Type of causal organism | ||
| Number | 8400 | 2290 |
| Oral streptococci, n (%) | 2620 (31.2) | 755 (33.0) |
| Other streptococci, n (%) | 610 (7.3) | 175 (7.6) |
| Staphylococci, n (%) | 2630 (31.3) | 680 (29.7) |
| Other causal organism, n (%) | 1395 (16.6) | 375 (16.4) |
| Mixed, n (%) | 1145 (13.6) | 305 (13.3) |
We also extracted the following data for each IE admission: whether the admission was an elective or non-elective admission, the start and end date of the admission episode, the length of hospital stay, and whether the patient was discharged from hospital alive or dead (providing the inpatient IE mortality rate). Table 3 provides these data for all IE admissions recorded using the narrow definition of IE and those narrow-definition IE admissions that had linked dental data. For the duration of hospital admission for all narrow-definition IE admissions, see Appendix 2, Figure 21, and for the duration of hospital admission for all narrow-definition IE admissions with linked dental data, see Appendix 2, Figure 22.
| Characteristics | IE narrow admission | |
|---|---|---|
| All | With linked dental data | |
| Admission method | ||
| Number | 11,575 | 3165 |
| Non-elective, n (%) | 11,250 (97.2) | 3165 (100.0) |
| Elective, n (%) | 325 (2.8) | 0 (0.0) |
| Admission date | ||
| Number | 11,574 | 3163 |
| Earliest | 1 April 2010a | 1 April 2011a |
| Latest | 28 March 2016 | 26 March 2016 |
| Discharge date | ||
| Number | 11,297 | 3134 |
| Earliest | 2 April 2010 | 6 April 2011 |
| Latest | 31 March 2016 | 31 March 2016 |
| Hospital length of stay | ||
| Number | 11,297 | 3134 |
| Mean (standard deviation) (days) | 31.7 (24.5) | 32.0 (21.7) |
| Median (minimum, maximum) (days) | 29 (0, 315) | 30 (1, 208) |
| Discharged alive? | ||
| Number | 11,285 | 3130 |
| No, n (%) | 1720 (15.2) | 410 (13.1) |
| Yes, n (%) | 9560 (84.7) | 2720 (86.9) |
Infective endocarditis patient characteristics using the broad definition of infective endocarditis
Using the broad definition of IE, we analysed the age, sex and previous IE risk status (high, moderate or low/unknown risk) of each IE admission patient. We also identified the specific heart condition that categorised an individual as at high or moderate risk for IE, and if there were any IE causal organism data for that individual. We quantified this information for all IE admissions in the data set and for those IE admissions for which we had linked dental data (Table 4).
| Characteristic | IE broad admission | |
|---|---|---|
| All | With linked dental data | |
| Age of the patient (years) | ||
| Number | 17,732 | 4293 |
| Mean (standard deviation) | 60.9 (21.0) | 62.3 (18.6) |
| Median (minimum, maximum) | 66 (0, 103) | 66 (1, 103) |
| Sex | ||
| Number | 17,740 | 4490 |
| Male, n (%) | 11,665 (65.8) | 2830 (65.9) |
| Female, n (%) | 6075 (34.2) | 1460 (34.0) |
| Level of IE risk before IE admission | ||
| Number | 17,740 | 4295 |
| High risk, n (%) | 4075 (23.0) | 1250 (29.1) |
| Moderate risk, n (%) | 3065 (17.3) | 745 (17.3) |
| Low/unknown risk, n (%) | 10,600 (59.8) | 2300 (53.6) |
| Reason for high-risk stratification, n (%)a | ||
| Previous IE | 405 (9.9) | 100 (8.0) |
| Previous IE: I38X | 115 (2.8) | 20 (1.6) |
| Replacement heart valve | 2860 (70.2) | 920 (73.6) |
| Repaired heart valve | 405 (9.9) | 135 (10.8) |
| Cyanotic congenital heart disease | 220 (5.4) | 50 (4.0) |
| Repaired congenital heart disease | – | – |
| Palliative shunt or conduit | 50 (1.2) | 20 (1.6) |
| Prosthetic heart/ventricular assist device | 10 (0.3) | – |
| Reason for moderate-risk stratification, n (%)a | ||
| Previous rheumatic fever | 1000 (32.6) | 235 (31.5) |
| Non-rheumatic valve | 1915 (62.5) | 465 (62.4) |
| Congenital valve anomalies | 75 (2.5) | 25 (3.4) |
| Hypertrophic cardiomyopathy | 75 (2.5) | 20 (2.7) |
| Causal organism reported | ||
| Number | 17,740 | 4295 |
| No, n (%) | 6730 (37.9) | 1385 (32.2) |
| Yes, n (%) | 11,010 (62.1) | 2910 (67.8) |
| Type of causal organism | ||
| Number | 11,010 | 2910 |
| Oral streptococci, n (%) | 3020 (27.4) | 850 (29.2) |
| Other streptococci, n (%) | 780 (7.1) | 215 (7.4) |
| Staphylococci, n (%) | 3540 (32.2) | 895 (30.8) |
| Other causal organism, n (%) | 2130 (19.3) | 545 (18.7) |
| Mixed, n (%) | 1540 (14.0) | 405 (13.9) |
We also extracted the admission data for each IE admission recorded using the broad definition of IE and those broad-definition IE admissions that had linked dental data (Table 5).
| Characteristic | IE broad admissions | |
|---|---|---|
| All | With linked dental data | |
| Admission method | ||
| Number | 17,740 | 4295 |
| Non-elective, n (%) | 16,680 (94.0) | 4295 (100.0) |
| Elective, n (%) | 1060 (6.0) | 0 (0.0) |
| Admission date | ||
| Number | 17,741 | 4293 |
| Earliest | 1 April 2010a | 1 April 2011a |
| Latest | 31 March 2016 | 30 March 2016 |
| Discharge date | ||
| Number | 17,259 | 4255 |
| Earliest | 1 April 2010 | 6 April 2011 |
| Latest | 31 March 2016 | 31 March 2016 |
| Hospital length of stay | ||
| Number | 17,259 | 4255 |
| Mean (standard deviation) (days) | 29.6 (29.1) | 31.7 (24.2) |
| Median (minimum, maximum) (days) | 24 (0, 1,027) | 28 (0, 229) |
| Discharged alive? | ||
| Number | 17,235 | 4250 |
| No, n (%) | 2820 (16.4) | 655 (15.4) |
| Yes, n (%) | 14,420 (83.7) | 3595 (84.6) |
Dental data matched to infective endocarditis admissions
Having cleaned the data sets, we were able to look in more detail at all of the dental data provided by the NHSBSA and compare these with the dental treatment data in the 12 months immediately prior to admission for those individuals admitted to hospital with an IE diagnosis. We did this for individuals admitted to hospital using both the narrow and broad definitions of IE (see Data on infective endocarditis hospital admissions). The numbers of courses of dental treatment for each definition were compared (Table 6). We plotted the distribution of courses of dental treatment of different length for the entire dental data set (see Appendix 2, Figure 23), and for those courses of dental treatment matched to patients who developed IE (see Appendix 2, Figure 24).
| Characteristics | Courses of dental treatment | ||
|---|---|---|---|
| All | Courses matched to narrow definition of IE | Courses matched to broad definition of IE | |
| Dental treatment start date | |||
| Number | 3,751,621 | 5426 | 7338 |
| Earliest | 1 April 2010 | 7 April 2010 | 7 April 2010 |
| Latest | 17 March 2016 | 2 March 2016 | 7 March 2016 |
| Dental treatment end date | |||
| Number | 3,751,621 | 5426 | 7338 |
| Earliest | 1 April 2010 | 23 April 2010 | 12 April 2010 |
| Latest | 17 March 2016 | 4 March 2016 | 7 March 2016 |
| Dental treatment duration | |||
| Number | 3,751,621 | 5426 | 7338 |
| Mean (standard deviation) (days) | 10.8 (30.5) | 12.0 (34.9) | 11.9 (33.9) |
| Median (minimum, maximum) (days) | 0 (0, 1848) | 0 (0, 725) | 0 (0, 725) |
| Match descriptiona | |||
| Number | 3,751,620 | 5425 | 7340 |
| Legacy index does not appear in patient dimension, n (%) | 1,427,380 (38.0) | 2545 (46.9) | 3695 (50.3) |
| Legacy index has multiple matching NHS numbers, n (%) | 140,075 (3.7) | 160 (2.9) | 190 (2.6) |
| Legacy index has unique 1 : 1 NHS number match, n (%) | 2,168,750 (57.8) | 2710 (50.0) | 3445 (46.9) |
| Legacy index matches a unique NHS number, but other LIDs also match the same NHS number, n (%) | 15,410 (0.4) | 10 (0.2) | 10 (0.1) |
| Procedure type | |||
| Number | 3,751,620 | 5425 | 7340 |
| Invasive procedure, n (%) | 1,819,775 (48.5) | 2585 (47.6) | 3455 (47.1) |
| Non-invasive procedure, n (%) | 956,635 (25.5) | 1460 (26.9) | 2000 (27.2) |
| Indeterminate procedure, n (%) | 975,210 (26.0) | 1380 (25.4) | 1885 (25.7) |
| Invasive procedure type | |||
| Number | 1,819,775 | 2585 | 3455 |
| Endodontic treatment, n (%) | 39,490 (2.2) | 65 (2.5) | 75 (2.2) |
| Extractions, n (%) | 286,360 (15.7) | 445 (17.2) | 615 (17.8) |
| Scale and polish, n (%) | 1,384,950 (76.1) | 1885 (72.9) | 2520 (72.9) |
| Mixed, n (%) | 108,980 (6.0) | 185 (7.2) | 245 (7.1) |
| Antibiotics useb | |||
| Number | 3,751,620 | 5425 | 7340 |
| No, n (%) | 3,682,645 (98.2) | 5325 (98.2) | 7205 (98.2) |
| Yes, n (%) | 68,975 (1.8) | 105 (1.9) | 135 (1.8) |
Timing of dental procedures in the 12 months before infective endocarditis hospital admission
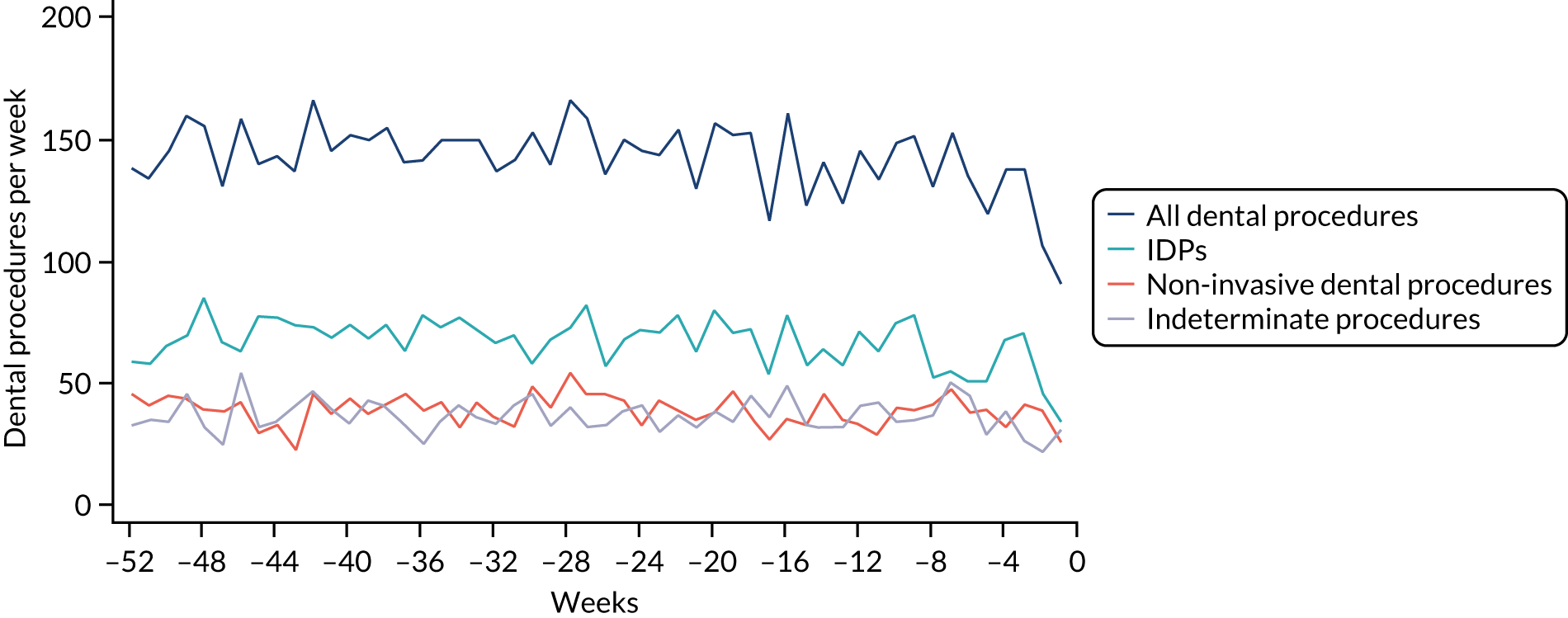
Because a case-crossover analysis compares the number of events (IDPs) in the case period immediately before the outcome (IE) with the number in earlier control periods, the duration and timing of the case and control periods is crucial. Although we had predefined a 3-month case period, this was based on the choices made in other studies, rather than on evidence. We therefore plotted the weekly numbers of all dental procedures, IDPs, non-invasive dental procedures and indeterminate procedures over the 52 weeks immediately preceding each IE hospital admission for which we had linked dental data. We repeated this analysis using the narrow (Figure 1) and broad (Figure 2) definitions for IE.
FIGURE 1.
Weekly number of dental procedures over the 52 weeks preceding IE hospital admission using the narrow definition of IE. The x-axis shows the number of weeks before an IE hospital admission (using the narrow definition of IE), with the time of admission being week 0 and using the start date for each course of dental treatment.
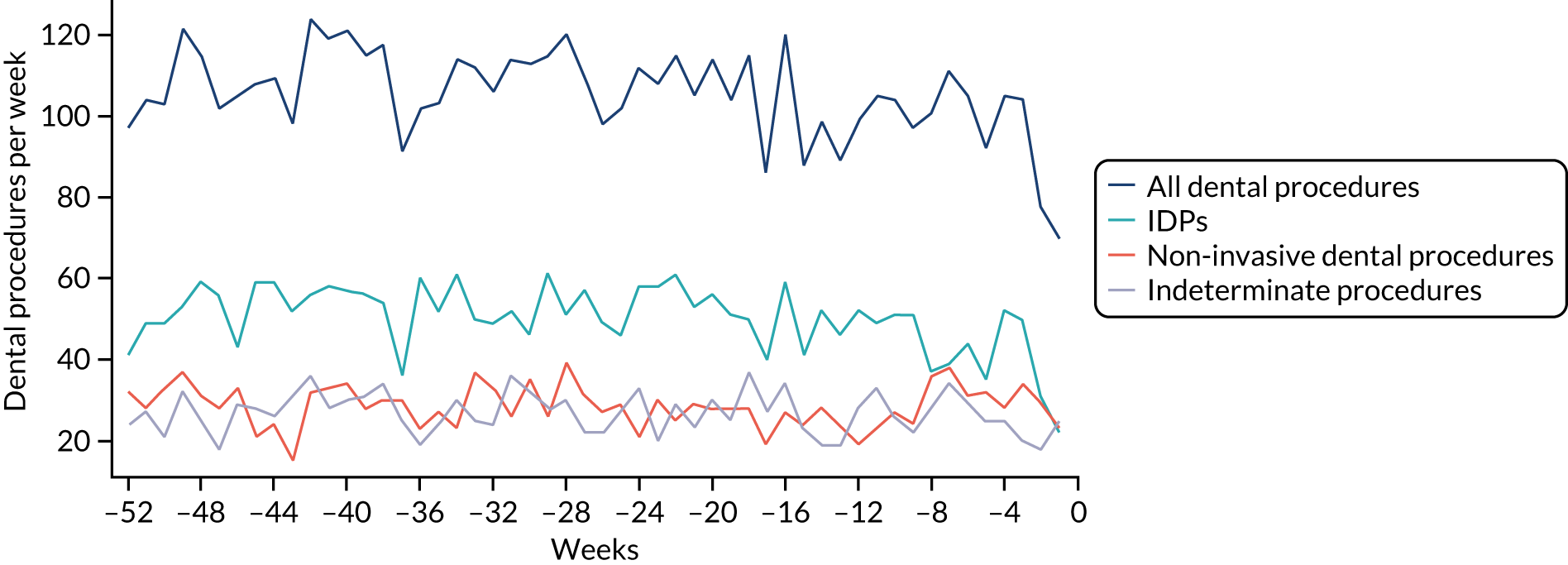
FIGURE 2.
Weekly number of dental procedures over the 52 weeks preceding IE hospital admissions using the broad definition of IE. The x-axis shows the number of weeks before an IE hospital admission (using the broad definition of IE), with the time of admission being week 0 and using the start date for each course of dental treatment.
Concerns raised by the timing of dental procedures in the 12 months before infective endocarditis admission
The results shown in Figures 1 and 2 were a surprise for two main reasons. The first was that we had anticipated that, if there were a link between IDP and IE, this would occur in the 3 months (12 weeks) immediately preceding any admission to hospital for IE. This 3-month window was derived from the period used in other IE studies,29–33 and we adopted this to define the case period in our planned (prespecified) case-crossover analysis comparing the incidence rate of IE in the case period (months 0–3) with that in the earlier control period (months 4–15). However, the time course studies did not support this. They showed that any change in IE incidence rate was largely confined to the 4 weeks immediately before admission. It is notable that although several studies have adopted 3 months as the exposure period between a triggering event (e.g. an IDP) and the onset of IE, none of these studies had actually plotted a time course to confirm this relationship. Indeed, the only study to specifically investigate the time between exposure and onset of IE (the incubation period) found that this period was as short as 7 days in 62% of cases, that 92% of IE cases developed within 4 weeks of an exposure and that only 8% of exposures occurred ≥ 4 weeks before IE diagnosis. 36 Our time course data are, therefore, much more consistent with a 4-week exposure/case period than the 12-week case period we predefined for our analysis.
The other surprise was that our time course data showed a decrease in the number of all types of dental procedures in the 4 weeks preceding IE admission. If there was a link between IDPs and IE, we would have expected an increase in the number of IDPs in the 4 weeks before IE admission, but this was not the case. Alternatively, if there was no association between IDPs and IE, we would expect no change in the number of IDPs in the 4 weeks preceding IE admission, but this was also not the case. The decrease in all types of dental procedures was unexpected and raised concerns that this might be caused by some other issue affecting the data on dental treatment in the weeks before admission to hospital with IE.
We therefore undertook further investigations to examine the possible reasons for this observation.
Chapter 4 Investigation of the reasons for the decrease in the number of dental procedures in the 4 weeks before infective endocarditis admission
Initial considerations
The time course data prompted a reconsideration of the ideas underlying our study and further discussion with our clinical colleagues.
One consideration was the fact that the main association between IDPs and IE (if it exists) should be for those at the highest risk of IE. As the proportion of the entire population that is at high risk of IE is very small, we might be losing any signal of an association within the larger population. There is also some debate about which IDPs are real risk factors for IE. The procedure around which there is most consensus is dental extractions; in countries where AP is recommended for IE, it is universally recommended for individuals at high risk of IE who are undergoing dental extractions.
We therefore stratified individuals in the data set according to their IE risk status (high, moderate or low/unknown) and looked at the number of dental extractions in the 12 months before an IE hospital admission (see Appendix 2, Figure 25).
A decrease in the number of non-extraction dental visits was still evident in the data in the 4 weeks before IE admission, and this was as true for those at low/unknown risk of IE as for those at high or moderate risk of IE. This decrease was also evident for dental extractions in those at low/unknown risk of IE, but there was little evidence of any change in the general trend for those at moderate or high risk of IE (although the numbers were very small).
The decrease in the number of all types of dental visits (both extraction and non-extraction), particularly in those at low/unknown risk of IE, suggested some inherent reduction in the number of dental visits or a loss of data in the 4 weeks before hospital admission for IE. There was no reduction (or a much smaller reduction) in the number of dental extractions for those at high risk of IE compared with those at low/unknown risk of IE in the 4 weeks before IE admission. This suggests that those at high risk of IE who are undergoing dental extractions may be more likely to be admitted to hospital with an IE diagnosis in the subsequent 4 weeks than those at low/unknown risk of IE.
Infective endocarditis diagnosis is not simple, and patients who develop IE often become quite ill before a diagnosis is made. Symptoms may mimic the symptoms of flu, making diagnosis difficult, and may include a high temperature, night sweats, shortness of breath on exertion, tiredness, muscle and joint pains, and weight loss. 51 We speculated that one explanation for the unexplained decrease in all types of dental visits in the 4 weeks before IE admission might be because patients were incubating IE and becoming too ill to visit the dentist, cancelling appointments or failing to attend. However, it was also possible that something else could be causing a loss of data on dental treatments in the days before a hospital admission.
There was no direct way we could test if patients were too unwell to visit the dentist, but we realised that we might be able to address this indirectly by looking at the number of dental treatments in the weeks prior to other types of hospital admission. Within our data set, we had the data to perform this analysis for acute hospital admissions for MI, PE and stroke, and we had the research ethics approval to support this. Unlike hospital admission for IE, hospital admission for the majority of MI, PE and stroke patients occurs without warning or prior illness that might prevent dental attendance. Hence, if illness in the lead-up to hospital admission with IE was the cause of the decrease in dental attendance in the 4 weeks prior to their hospital admission, we would not expect to see a similar decrease in the numbers admitted to hospital with MI, PE or stroke. We therefore directly compared the monthly number of extraction and non-extraction dental visits in the 12 months before hospital admission for individuals admitted to hospital with IE (using both the narrow and broad definitions of IE), MI, PE and stroke (see Appendix 2, Figure 26).
The time course data showed a decrease in the number of dental visits (both extraction and non-extraction) in the 4 weeks before hospital admission for IE (both narrow and broad definition), MI, PE or stroke. In other words, the decrease in the number of dental visits was common to all types of acute hospital admission, not specific to IE admissions. This strongly suggested that there was a universal reduction in the number of dental visits or a loss of data occurring in the 4 weeks before emergency hospital admissions for serious illnesses with a significant mortality.
To explore possible reasons for this, we conducted a series of discussions with the NHSBSA and general dental practitioners.
For each course of NHS dental treatment, dentists complete a FP17 form. This is sent electronically to the dental division of the NHSBSA, based in Eastbourne, where it is analysed. The main purpose of the form is to ensure that dentists are fulfilling their dental services contract with the NHS, but it is also used to account for the patient charges that dentists are obliged to collect from patients, and the payment of dentists.
Patient charges and payments to dentists are based on bands of treatment (i.e. bands 1–3) or special treatment categories (e.g. prescription only and denture repairs). These are detailed in Part 5 of the form. Treatment bands do not provide much detail of the treatment provided and only cover broad cost categories of treatment. For this reason, in April 2008, the form was changed to collect some basic information on the types of dental treatment provided. This information is collected in Part 5a of the form, the ‘Clinical Data Set’, which is intended to provide the NHS and researchers with more information on the types of treatment provided by dentists.
Collection of these data became a mandatory requirement for all dentists from 1 April 2008, but some leeway was permitted in the first 12 months after its introduction. It is for this reason that we were only able to collect data for this study from April 2009. For the purposes of the study, we considered the following items in the ‘Clinical Data Set’ to be IDPs: item 1, ‘Scale and polish’; item 5, ‘Endodontic treatment’; and item 7, ‘Extractions’ (for more details, see Identifying invasive dental procedures). Part 2 of the form collects the patient details and Part 3 provides the course of treatment start and end dates; this information was also important to the study.
Results of the investigation conducted into the reasons for the decrease in the recorded number of dental treatments in the weeks preceding infective endocarditis hospital admission
We engaged in a series of discussions with staff at the NHSBSA Dental Data Processing Centre at Eastbourne to investigate if there could have been any data loss. These discussions eventually revealed that although we had asked for the details of all courses of dental treatment during the period 1 April 2009 through 31 March 2015, these had not in fact been provided. There were two main reasons for this.
The first reason was that, because of all the regulatory, administrative and organisational delays in providing us with the data, the NHSBSA did not actually perform the data extraction until April 2020 (instead of by 31 October 2017, as originally planned). NHSBSA also informed us that it had introduced a policy of destroying all data that was ≥ 10 years old. Because of this, it had supplied us with data only from 1 April 2010 until 31 March 2015 (i.e. 60 months’ data rather than the 72 months’ data we had requested – a 17% reduction in the size of the data set).
The second was that the NHSBSA also informed us that it had not provided us with any data for courses of dental treatment that had no Part 5a Clinical Data Set content. We were surprised to hear that any FP17 could be returned and processed that did not contain Part 5a information, as dentists have been required to complete Part 5a since April 2008. However, further questioning revealed that the NHSBSA do not enforce this requirement for incomplete courses of treatment, although it does enforce it for all complete courses of dental treatment.
Dentists are required to record the date when a patient is accepted for a course of dental treatment (even if only for an oral examination with no actual treatment) in Part 3 of the FP17 form. Because many courses of treatment are a single visit (e.g. a patient has a dental examination but no treatment), there is a tick box for when a course of treatment starts and finishes on the same day. If a dentist ticks this box or provides an end date for the course of treatment, they must also complete Part 5, which provides details of the band of dental treatment provided and is used to manage the business transactions involved (i.e. patient charges and payment due to the dentist). When this is complete, the dentist must also record clinical details in Part 5a.
However, Part 3 of the form also contains three tick boxes for incomplete courses of treatment (i.e. courses of treatment for which there is a start date but no end date). To deal with the business aspects of an incomplete course of treatment, the dentist is required to tick one of three boxes in Part 3 to denote what band of dental treatment they provided and any patient payment collected. However, the NHSBSA do not require dentists to complete Part 5a of the form (where the clinical details are recorded), as long as one of these three boxes is ticked. Furthermore, the NHSBSA does not perform follow-up to ensure that these details are provided in such situations, whereas it does perform follow-up if an end of treatment date was entered or the ‘Completion same day as acceptance’ box was ticked. Because such courses of dental treatment contain no clinical details of the treatment provided, the NHSBSA removed these from the provided data set. This meant that we had no data for any incomplete courses of treatment.
We investigated the possibility of the NHSBSA providing us with these data. However, we identified this problem only in November 2020, by which time any re-run of the data by the NHSBSA would have resulted in a further loss of 7 months of data owing to its policy of destroying data that are ≥ 10 years old. Moreover, although we would receive the start date for these incomplete courses of treatment, we would not receive any clinical details of the treatment provided and would hence be unable to determine whether or not the treatment was invasive – the essential item of information for our study.
We also talked to a number of dentists working in general dental practice to obtain a better understanding of how they handled incomplete courses of treatment. It would appear that, although some dentists record the clinical details for incomplete courses of treatment, many do not, as it is time-consuming and an unnecessary requirement for payment. Furthermore, some dental practice software used in completing such forms does not allow the inclusion of treatment details for an incomplete course of treatment. The information from dental practitioners therefore confirmed the information from the NHSBSA and suggested inconsistencies in the way incomplete courses of treatment are recorded.
We also asked dentists to tell us about the types of situation that most often result in an incomplete course of treatment. These included patients abandoning a course of treatment, moving out of the area, being admitted to hospital as an emergency with a serious life-threatening disease or dying. Emergency admission to hospital, death and serious illness were considered not uncommon reasons for a patient to fail to complete a course of treatment.
This observation is important, as IE hospital admissions are nearly all unexpected emergencies. Around 20% of patients admitted to hospital with IE die during the initial admission, and around 30% die within 1 year of the admission. Owing to their serious nature and the frequent need for long-term intravenous antibiotics and cardiac surgical intervention, hospital stays for IE are among the longest of any condition.
Furthermore, survivors often have serious debilitating and long-term morbidity. This suggests that, if a patient is admitted to hospital with IE soon after initiating a course of dental treatment, there is a significant possibility that the course of treatment will not be completed, and that the clinical treatment data will not be provided to the NHSBSA. In addition, many patients admitted with IE, particularly those requiring valve intervention, will be evaluated and treated by the maxillofacial surgery team while in hospital before valve surgery, providing another reason for any course of treatment initiated by the patient’s primary care dentist to be abandoned and incomplete.
In any of these circumstances, the record of the treatment being started will be lost from the data provided to us by the NHSBSA. Furthermore, the fact that data loss is most likely in the period immediately before IE admission explains (at least in part) the decrease in the number of all types of dental procedures in the 4 weeks before emergency hospital admission that we saw with other serious acute medical conditions associated with a significant mortality rate and long-term morbidity (i.e. MI, PE and stroke). Unfortunately, this data loss, which was focused on the weeks before high-mortality-rate emergency hospital admissions, directly and adversely impacted our case-crossover analysis, which relies on comparing the number of IDPs in the weeks immediately before IE admission (when the data loss is focused) with that in earlier periods (when data loss would be much less likely to occur).
In conclusion, we have two major reasons for data loss: (1) loss of all types of data from earlier years owing to the NHSBSA’s policy of destroying data that are ≥ 10 years old, and (2) loss of clinical treatment data for incomplete courses of dental treatment. Although the former significantly reduces the sample size and, therefore, the statistical power of the study, it does not affect the comparison of case and control periods in our case-crossover analysis. Unfortunately, however, the loss of data from incomplete courses of treatment is disproportionally focused on case periods, rather than control periods. This is therefore likely to significantly bias the outcome of any case-crossover analysis.
Chapter 5 The search for alternative methods of analysis
Initial considerations
The loss of data in the critical few weeks before IE hospital admission seriously undermined our ability to investigate any link between IDPs and IE using the study’s case-crossover methodology design. Our investigations determined that this data loss was, at least in part, due to the NHSBSA not requiring dentists to provide clinical details for incomplete courses of dental treatment.
We therefore investigated ways of using the data to answer the original question that did not depend on the incomplete course of treatment data. Possibilities included using:
-
data for single-visit courses of dental treatment
-
courses of dental treatment defined by the end date.
Single-visit courses of dental treatment
Single-visit courses of treatment are courses of dental treatment that start and finish on the same day (i.e. the start and finish dates are the same). Restricting the data to single-visit treatments would ensure that all courses of treatment recorded were complete before IE hospital admission and, therefore, could not be affected by any data lost as a result of incomplete courses of treatment. However, using single-visit treatment could introduce a new bias if the mix of invasive and non-invasive dental treatments was significantly different for single-visit courses of treatment and longer courses of treatment.
The entire dental data set consisted of 3,751,621 records, of which 2,629,502 (70.1%) were single session (i.e. the start date and end date were identical). Of those, 6623 (0.25%) related to individuals requiring IE hospital admission within 13 months of that date – using the broad definition of an IE hospital admission.
These single-visit courses of treatment were divided into those that included an IDP, an indeterminate procedure or a non-invasive procedure. They were tabulated according to the number of months (30-day periods) before the IE admission (Table 7; see also Appendix 2, Figure 27).
| Months before IE admission | All procedures (n) | IDPs (n) | Indeterminate procedures (n) | Non-invasive procedures (n) | ||||
|---|---|---|---|---|---|---|---|---|
| All courses | Single visit | All courses | Single visit | All courses | Single visit | All courses | Single visit | |
| 13 | 801 | 570 | 377 | 250 | 213 | 140 | 211 | 180 |
| 12 | 729 | 498 | 331 | 206 | 176 | 106 | 222 | 186 |
| 11 | 747 | 519 | 361 | 219 | 205 | 148 | 181 | 152 |
| 10 | 751 | 518 | 349 | 217 | 190 | 136 | 212 | 165 |
| 9 | 728 | 510 | 345 | 212 | 175 | 125 | 208 | 173 |
| 8 | 742 | 540 | 352 | 243 | 203 | 144 | 187 | 153 |
| 7 | 771 | 540 | 352 | 209 | 187 | 135 | 232 | 196 |
| 6 | 728 | 505 | 326 | 201 | 207 | 142 | 195 | 162 |
| 5 | 725 | 523 | 347 | 231 | 175 | 125 | 203 | 167 |
| 4 | 704 | 481 | 321 | 190 | 193 | 135 | 190 | 156 |
| 3 | 672 | 483 | 313 | 203 | 180 | 137 | 179 | 143 |
| 2 | 802 | 514 | 381 | 180 | 201 | 153 | 220 | 181 |
| 1 | 564 | 422 | 242 | 177 | 141 | 101 | 181 | 144 |
| Total | 9464 | 6623 | 4397 | 2738 | 2446 | 1727 | 2621 | 2158 |
| Single-visit courses as a percentage of the total | 70.0 | 62.3 | 70.6 | 82.3 | ||||
The time sequence plots (see Appendix 2, Figure 27) show that single-visit data largely abrogate the decrease in the number of IDPs seen in the month before IE admission for all courses of dental treatment. This suggests that using the single-visit data helps to remove the effect of incomplete courses of treatment. However, this effect is not seen for indeterminate procedures or non-invasive dental procedures.
Overall, single visits account for 70.0% of all courses of treatment associated with IE admission in the subsequent 13 months (Table 7). However, single visits account for a smaller proportion of all courses of treatment that include an IDP (62.3%) and a larger proportion of those that include a non-invasive procedure (82.3%). This is probably because single visits are more likely to include a simple dental examination in which no further treatment needs are identified. In contrast, invasive treatments are more likely to require multiple visits.
To examine this further, we looked at IDPs in isolation and divided these procedures into (1) all IDPs, (2) endodontic treatments, (3) dental extractions and (4) scale and polishes. We compared these data for all courses of dental treatment and single-visit courses of treatment over the 13 months (30-day periods) before IE admission (Table 8; see also Appendix 2, Figure 28).
| Months before IE admission | All IDPs (n) | Endodontic treatments (n) | Extractions (n) | Scale and polishes (n) | ||||
|---|---|---|---|---|---|---|---|---|
| All courses | Single visit | All courses | Single visit | All courses | Single visit | All courses | Single visit | |
| 13 | 377 | 250 | 17 | 3 | 81 | 35 | 311 | 216 |
| 12 | 331 | 206 | 12 | 0 | 79 | 32 | 261 | 176 |
| 11 | 361 | 219 | 15 | 2 | 80 | 30 | 289 | 191 |
| 10 | 349 | 217 | 9 | 0 | 92 | 39 | 267 | 183 |
| 9 | 345 | 212 | 10 | 0 | 73 | 20 | 285 | 193 |
| 8 | 352 | 243 | 11 | 0 | 66 | 30 | 290 | 216 |
| 7 | 352 | 209 | 13 | 0 | 88 | 36 | 277 | 176 |
| 6 | 326 | 201 | 13 | 3 | 80 | 24 | 269 | 180 |
| 5 | 347 | 231 | 11 | 3 | 69 | 31 | 285 | 198 |
| 4 | 321 | 190 | 11 | 2 | 72 | 25 | 257 | 164 |
| 3 | 313 | 203 | 7 | 1 | 74 | 31 | 246 | 173 |
| 2 | 381 | 180 | 12 | 0 | 57 | 28 | 221 | 153 |
| 1 | 242 | 177 | 3 | 1 | 44 | 27 | 202 | 150 |
| Total | 4397 | 2738 | 144 | 15 | 955 | 388 | 3460 | 2369 |
| Single-visit courses as a percentage of the total | 62.3 | 10.4 | 40.6 | 68.5 | ||||
As described above, the proportion of all courses of dental treatment that were single visit and included an IDP was 62.3%. However, this was not evenly distributed between the types of IDP studied (Table 8). Most notably, only 10.4% of endodontic treatments and 40.6% of extractions were single visit. In contrast, 68.5% of scale and polish courses of treatment were single visit. Confining the data to single visits therefore results in an over-representation of scale and polish procedures and an under-representation of extractions and (even more so) endodontic procedures, which often take several visits for completion.
On the other hand, examining the time course data (see Appendix 2, Figure 28) shows that use of single-visit data reduces the substantial decrease in all courses of dental treatment seen in the few weeks before IE admission. This is most notable for dental extractions, less so for scale and polish procedures and there are too few data to draw any conclusions about the effect on endodontic procedures.
Courses of dental treatment defined by the end date
Our data analysis relied on the start date to define the date of a course of dental treatment. However, if we used the end date of a course of treatment instead, we would retain the data on single-visit and multivisit courses, but the data would not be affected by missing data on courses of treatment that were incomplete only because they had been scheduled to finish after the date when the patient was admitted to hospital for IE as an emergency. Although these courses of treatment are incomplete, using the end date to define the date of treatment means that, even if they had been completed, they would have been excluded from the data, as their end date did not fall within the admissible time frame (i.e. up to 16 months before admission for IE).
We therefore compared the number of each type of dental procedures over the 13 months before IE admission using either the start date or end date of a course of treatment to define the timing of the course of treatment (Table 9; see also Appendix 2, Figure 29). The numbers of the different procedures did not differ markedly using these two methods.
| Months before IE admission | All procedures (n) | Invasive procedures (n) | Indeterminate procedures (n) | Non-invasive procedures (n) | ||||
|---|---|---|---|---|---|---|---|---|
| Start date | End date | Start date | End date | Start date | End date | Start date | End date | |
| 13 | 801 | 795 | 377 | 391 | 213 | 196 | 211 | 208 |
| 12 | 729 | 748 | 331 | 342 | 176 | 185 | 222 | 221 |
| 11 | 747 | 732 | 361 | 349 | 205 | 200 | 181 | 183 |
| 10 | 751 | 744 | 349 | 348 | 190 | 203 | 212 | 193 |
| 9 | 728 | 745 | 345 | 353 | 175 | 181 | 208 | 211 |
| 8 | 742 | 730 | 352 | 349 | 203 | 191 | 187 | 190 |
| 7 | 771 | 780 | 352 | 351 | 187 | 191 | 232 | 238 |
| 6 | 728 | 718 | 326 | 332 | 207 | 193 | 195 | 193 |
| 5 | 725 | 741 | 347 | 362 | 175 | 179 | 203 | 200 |
| 4 | 704 | 684 | 321 | 309 | 193 | 195 | 190 | 180 |
| 3 | 672 | 696 | 313 | 330 | 180 | 188 | 179 | 178 |
| 2 | 802 | 701 | 381 | 292 | 201 | 196 | 220 | 213 |
| 1 | 564 | 628 | 242 | 282 | 141 | 157 | 181 | 189 |
| Total | 9464 | 9442 | 4397 | 4390 | 2446 | 2455 | 2621 | 2597 |
The time plots indicated a smaller decrease in the number of dental procedures of all types in the few weeks before IE admission when using the end date rather than the start date to define course of treatment (see Appendix 2, Figure 29). However, using the end date did not eliminate the decrease completely.
A reduction in the decrease in the number of each type of IDP was also seen when using the end date rather than the start date to define courses of treatment (Table 10; see also Appendix 2, Figure 30); however, the decrease was not completely abrogated.
| Months before IE admission | All IDPs (n) | Type of IDP (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Endodontic procedure | Dental extraction | Scale and polish | ||||||
| Start date | End date | Start date | End date | Start date | End date | Start date | End date | |
| 13 | 377 | 410 | 17 | 13 | 81 | 75 | 311 | 322 |
| 12 | 331 | 371 | 12 | 15 | 79 | 86 | 261 | 270 |
| 11 | 361 | 376 | 15 | 17 | 80 | 81 | 289 | 278 |
| 10 | 349 | 374 | 9 | 13 | 92 | 78 | 267 | 283 |
| 9 | 345 | 369 | 10 | 9 | 73 | 75 | 285 | 285 |
| 8 | 352 | 372 | 11 | 12 | 66 | 69 | 290 | 291 |
| 7 | 352 | 375 | 13 | 10 | 88 | 91 | 277 | 274 |
| 6 | 326 | 356 | 13 | 14 | 80 | 72 | 269 | 270 |
| 5 | 347 | 391 | 11 | 13 | 69 | 87 | 285 | 291 |
| 4 | 321 | 327 | 11 | 9 | 72 | 70 | 257 | 248 |
| 3 | 313 | 356 | 7 | 12 | 74 | 83 | 246 | 261 |
| 2 | 381 | 305 | 12 | 9 | 57 | 66 | 221 | 230 |
| 1 | 242 | 295 | 3 | 12 | 44 | 63 | 202 | 220 |
| Total | 4397 | 4677 | 144 | 158 | 955 | 996 | 3460 | 3523 |
It is likely that this is because we are not correcting for the loss of incomplete course of treatment data, but shifting the timing of longer courses of treatment further away from the date of IE admission, with little effect on shorter courses of treatment and no effect on single-visit courses of treatment.
Conclusions
Using single-visit data is the most effective way of mitigating the data loss caused by incomplete courses of dental treatment in the period just before high-mortality emergency hospital admissions. However, it causes a significant further loss of data, and this loss is greater for IDPs than for non-invasive dental procedures. Furthermore, the extent of the data loss differs according to the specific type of IDP: it is highest for endodontic procedures, significant for extractions and lowest for scale and polishes.
In contrast, using the end date to define courses of dental treatment has less of a mitigating effect on the loss of data due to incomplete courses of dental treatment, but results in minimal further loss of data and a reduction in the selective loss of IDPs. However, it does not avoid the selective loss of IDPs completely. Yet, on balance, it would appear preferable to use the end date to define courses of dental treatment and we will adopt this for any subsequent analysis. Nonetheless, use of the end date to define courses of dental treatment does not alter the critical loss of dental procedural data in the few weeks immediately before any acute hospital admission for a serious disease. The fact that this data loss is focused on the case period (and not the control period) of any case-crossover analysis fundamentally undermines and biases any such analysis.
Chapter 6 Final analysis using the end date for courses of dental treatment and narrow or broad definition of infective endocarditis
Initial considerations
Owing to the issues identified earlier and the work performed in Chapter 5, we reanalysed the data, using the end date to define the timing of dental procedures and courses of treatment relative to hospital admissions for IE. We repeated this analysis using both the narrow and broad definitions of IE.
A recent study showed that the narrow definition of IE had the highest specificity and PPV for identifying Duke criteria-positive IE cases (specificity 0.97, PPV 0.88), but low sensitivity (0.41). 41 To increase the sensitivity and the number of IE cases identified, we also used a broad definition of IE that included all hospital admissions with a primary or secondary ICD-10 discharge diagnosis code of I33.0, I33.9, I39.0, I39.1, I39.2, I39.3, I39.4 or I39.8, or a primary discharge diagnosis code of I38.X. This increased the sensitivity to 0.65, but reduced the specificity to 0.83 and the PPV to 0.80. 41 Nonetheless, the number of IE cases identified using the broad definition was larger. A total of 11,574 IE admissions were identified using the narrow definition, compared with 17,741 using the broad definition (Table 11).
| Category of admission | IE risk level (n) | |||
|---|---|---|---|---|
| High | Moderate | Low/unknown | All | |
| Using the narrow definition of IE | ||||
| All IE admissions meeting narrow definition of IE | 3283 | 1875 | 6416 | 11,574 |
| Narrow-definition IE admissions linked to at least one dental treatment record | ||||
| Using start or end date | 1177 | 591 | 1743 | 3511 |
| Using start date | 1162 | 579 | 1713 | 3454 |
| Using end date | 1166 | 579 | 1726 | 3471 |
| Dental treatment records linked to at least one narrow-definition IE admission | ||||
| Using start or end date | N/A | N/A | N/A | 6440 |
| Using start date | N/A | N/A | N/A | 6268 |
| Using end date | N/A | N/A | N/A | 6322 |
| With same start and end date | N/A | N/A | N/A | 4393 |
| Total linkages between a dental treatment record and a narrow-definition IE admission | ||||
| Using start date | 2158 | 1035 | 3095 | 6288 |
| Using end date | 2165 | 1051 | 3125 | 6341 |
| With same start and end date | 1542 | 721 | 2144 | 4407 |
| Using the broad definition of IE | ||||
| All admissions meeting broad definition of IE | 4074 | 3065 | 10,602 | 17,741 |
| Broad-definition IE admissions linked to at least one dental treatment record | ||||
| Using start or end date | 1486 | 969 | 2747 | 5202 |
| Using start date | 1464 | 950 | 2704 | 5118 |
| Using end date | 1471 | 951 | 2717 | 5139 |
| Dental treatment records linked to at least one broad-definition IE admission | ||||
| Using start or end date | N/A | N/A | N/A | 9578 |
| Using start date | N/A | N/A | N/A | 9309 |
| Using end date | N/A | N/A | N/A | 9385 |
| With same start and end date | N/A | N/A | N/A | 6583 |
| Total linkages between a dental treatment record and a broad-definition IE admission | ||||
| Using start date | 2714 | 1749 | 4901 | 9364 |
| Using end date | 2728 | 1772 | 4942 | 9442 |
| With same start and end date | 1931 | 1239 | 3453 | 6623 |
Summary data on infective endocarditis admissions and linked dental treatment records using the narrow and broad definitions of infective endocarditis
Table 11 summarises all of the IE admissions meeting the narrow definition of IE and those occurring in individuals at high, moderate, or low/unknown risk of IE. It also shows the number of dental treatment records linked to at least one narrow definition IE admission using the start or end date to identify courses of dental treatment occurring in the 13 months before IE admission. It also summarises the same data using the broad definition of IE. These data explicitly count admissions, dental treatment records and linkages (rather than patients), because a single admission is often linked to more than one course of dental treatment and a single dental treatment record may, very occasionally, be linked to more than one admission.
All of the other tables in this chapter deal with dental treatment records as unique linkages using the end date to define the date of the dental treatment.
Table 12 shows the number and nature of dental treatment records in the 13 months prior to all IE admissions, and IE admissions in those at high, moderate, or low/unknown risk of developing IE. Table 13 shows the number of extractions, scale and polishes, and endodontic procedures in the 13 months prior to IE admission in each patient risk category. These data are shown using either the narrow or broad definition of IE.
| Risk category | Type of dental procedure (n) | ||
|---|---|---|---|
| IDP | Indeterminate | Non-invasive | |
| Using the narrow definition of IE | |||
| High | 951 | 562 | 652 |
| Moderate | 506 | 285 | 260 |
| Low/unknown | 1541 | 777 | 807 |
| All | 2998 | 1624 | 1719 |
| Using the broad definition of IE | |||
| High | 1201 | 724 | 803 |
| Moderate | 822 | 495 | 455 |
| Low/unknown | 2367 | 1236 | 1339 |
| All | 4390 | 2455 | 2597 |
| Risk category | Procedure (n) | Total (n) | ||
|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | ||
| Using the narrow definition of IE | ||||
| High | 216 | 1002 | 53 | 1271 |
| Moderate | 190 | 663 | 32 | 885 |
| Low/unknown | 590 | 1858 | 73 | 2521 |
| Total | 996 | 3523 | 158 | 4677 |
| Using the broad definition of IE | ||||
| High | 216 | 1002 | 53 | 1271 |
| Moderate | 190 | 663 | 32 | 885 |
| Low/unknown | 590 | 1858 | 73 | 2521 |
| Total | 996 | 3523 | 158 | 4677 |
Number of dental procedures over the 13 months before infective endocarditis admission using the narrow and broad definitions of infective endocarditis
We quantified the number of all dental procedures and those of different levels of invasiveness over the 13 months before IE admission for all individuals using the narrow and broad definitions of IE (Table 14), and plotted these in Figure 3 (narrow definition) and Figure 4 (broad definition). The same quantification was also undertaken for those patients at high risk of IE [Table 15, Figure 5 (narrow definition) and Figure 6 (broad definition)], moderate risk of IE [Table 16, Figure 7 (narrow definition) and Figure 8 (broad definition)] or low/unknown risk of IE [Table 17, Figure 9 (narrow definition) and Figure 10 (broad definition)].
| Months before IE admission | Type of dental procedure (n) | All (n) | ||
|---|---|---|---|---|
| IDP | Indeterminate | Non-invasive | ||
| Using the narrow definition of IE | ||||
| 13 | 248 | 136 | 142 | 526 |
| 12 | 229 | 120 | 151 | 500 |
| 11 | 252 | 124 | 119 | 495 |
| 10 | 249 | 146 | 127 | 522 |
| 9 | 234 | 122 | 132 | 488 |
| 8 | 240 | 132 | 136 | 508 |
| 7 | 251 | 124 | 154 | 529 |
| 6 | 241 | 119 | 117 | 477 |
| 5 | 247 | 126 | 130 | 503 |
| 4 | 214 | 130 | 115 | 459 |
| 3 | 226 | 120 | 109 | 455 |
| 2 | 185 | 124 | 157 | 466 |
| 1 | 182 | 101 | 130 | 413 |
| Total | 2998 | 1624 | 1719 | 6341 |
| Using the broad definition of IE | ||||
| 13 | 391 | 196 | 208 | 795 |
| 12 | 342 | 185 | 221 | 748 |
| 11 | 349 | 200 | 183 | 732 |
| 10 | 348 | 203 | 193 | 744 |
| 9 | 353 | 181 | 211 | 745 |
| 8 | 349 | 191 | 190 | 730 |
| 7 | 351 | 191 | 238 | 780 |
| 6 | 332 | 193 | 193 | 718 |
| 5 | 362 | 179 | 200 | 741 |
| 4 | 309 | 195 | 180 | 684 |
| 3 | 330 | 188 | 178 | 696 |
| 2 | 292 | 196 | 213 | 701 |
| 1 | 282 | 157 | 189 | 628 |
| Total | 4390 | 2455 | 2597 | 9442 |
FIGURE 3.
Number of each type of dental procedure over the 13 months before IE admission for patients at all levels of risk using the narrow definition of IE.
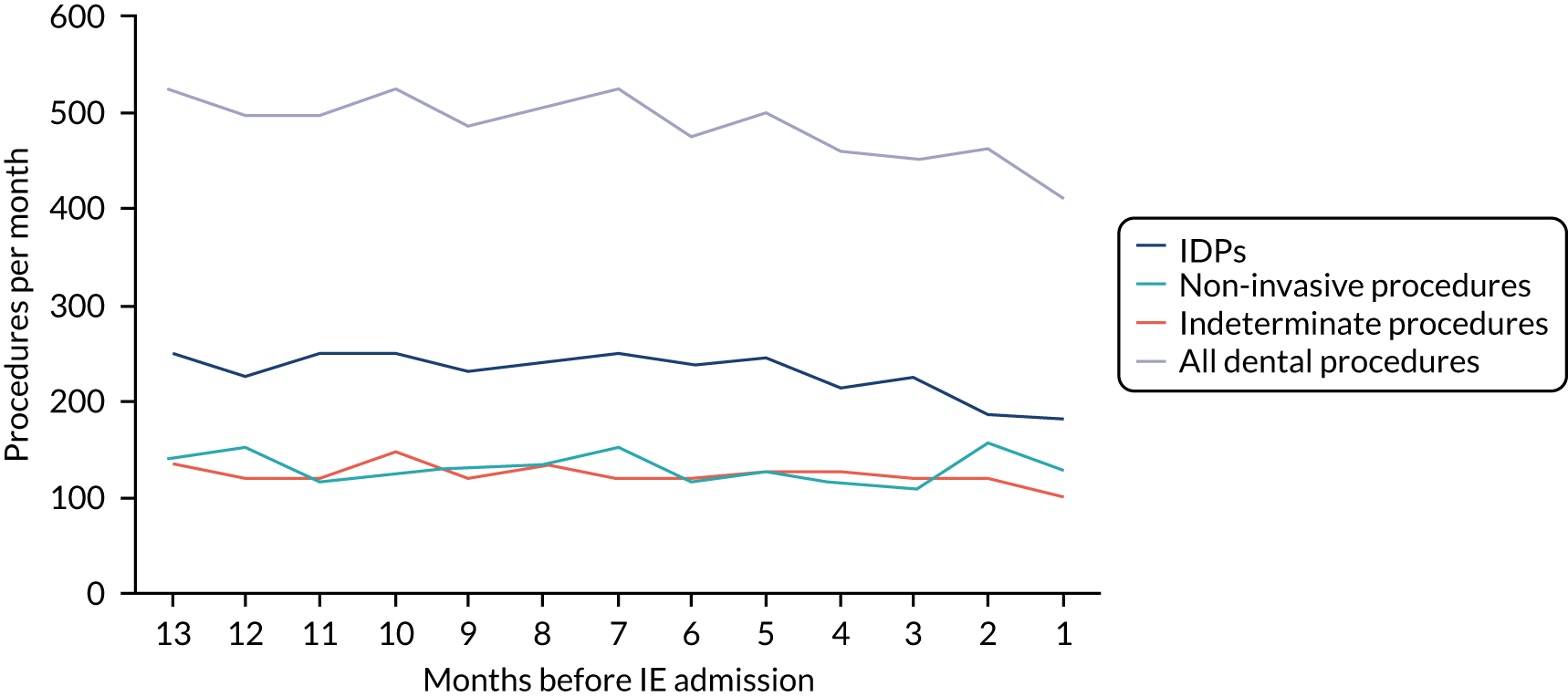
FIGURE 4.
Number of each type of dental procedure over the 13 months before IE admission for patients at all levels of risk using the broad definition of IE.
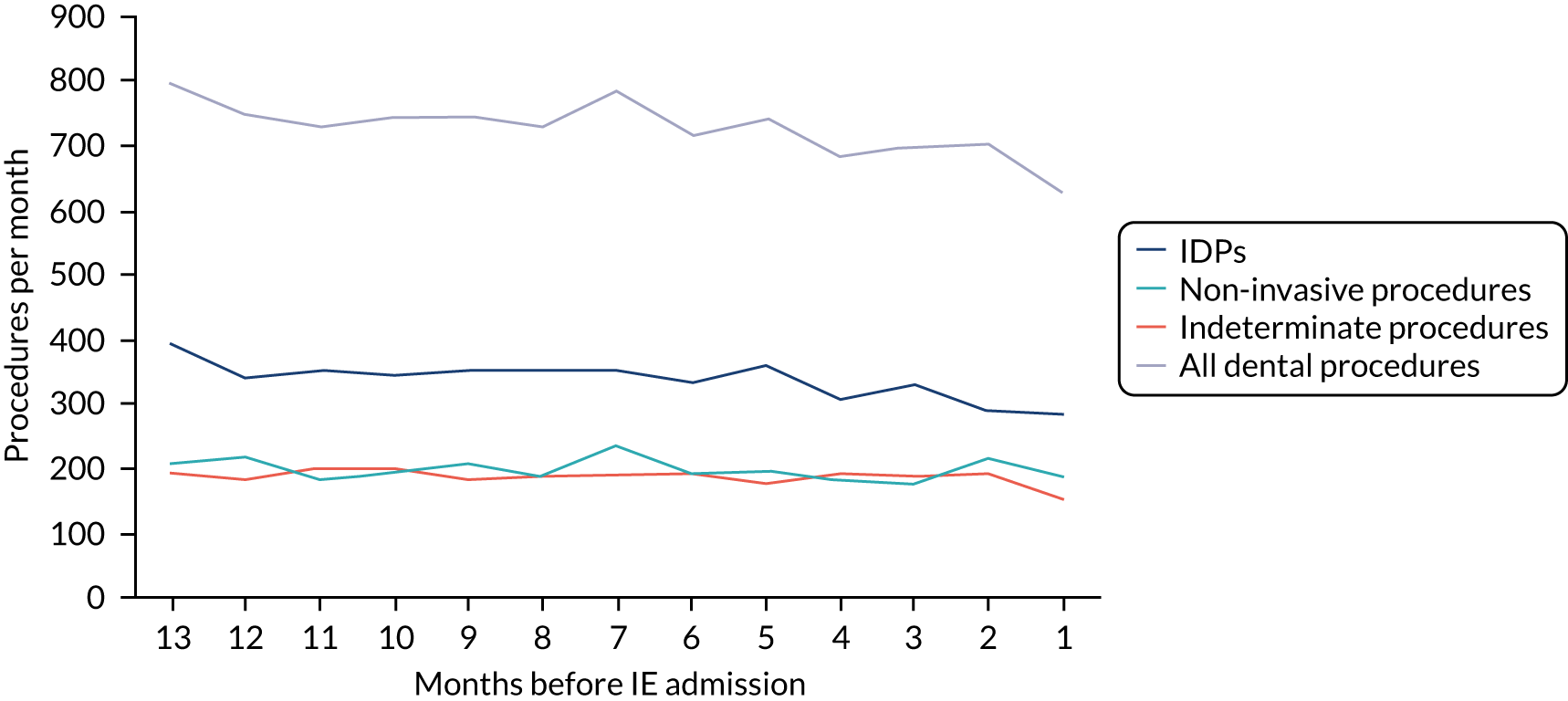
| Months before IE admission | Type of dental procedure (n) | All (n) | ||
|---|---|---|---|---|
| IDP | Indeterminate | Non-invasive | ||
| Using the narrow definition of IE | ||||
| 13 | 72 | 52 | 56 | 180 |
| 12 | 85 | 39 | 59 | 183 |
| 11 | 66 | 41 | 39 | 146 |
| 10 | 82 | 51 | 42 | 175 |
| 9 | 73 | 46 | 64 | 183 |
| 8 | 78 | 44 | 47 | 169 |
| 7 | 87 | 44 | 54 | 185 |
| 6 | 82 | 33 | 50 | 165 |
| 5 | 83 | 49 | 46 | 178 |
| 4 | 60 | 42 | 42 | 144 |
| 3 | 80 | 41 | 41 | 162 |
| 2 | 50 | 40 | 62 | 152 |
| 1 | 53 | 40 | 50 | 143 |
| Total | 951 | 562 | 652 | 2165 |
| Using the broad definition of IE | ||||
| 13 | 100 | 62 | 64 | 226 |
| 12 | 105 | 40 | 69 | 214 |
| 11 | 83 | 59 | 50 | 192 |
| 10 | 95 | 56 | 57 | 208 |
| 9 | 105 | 55 | 75 | 235 |
| 8 | 94 | 55 | 52 | 201 |
| 7 | 103 | 58 | 64 | 225 |
| 6 | 98 | 46 | 62 | 206 |
| 5 | 105 | 59 | 62 | 226 |
| 4 | 82 | 60 | 54 | 196 |
| 3 | 92 | 59 | 56 | 207 |
| 2 | 71 | 58 | 72 | 201 |
| 1 | 68 | 57 | 66 | 191 |
| Total | 1201 | 724 | 803 | 2728 |
FIGURE 5.
Number of each type of dental procedure over the 13 months before IE admission for patients at high risk of IE using the narrow definition of IE.
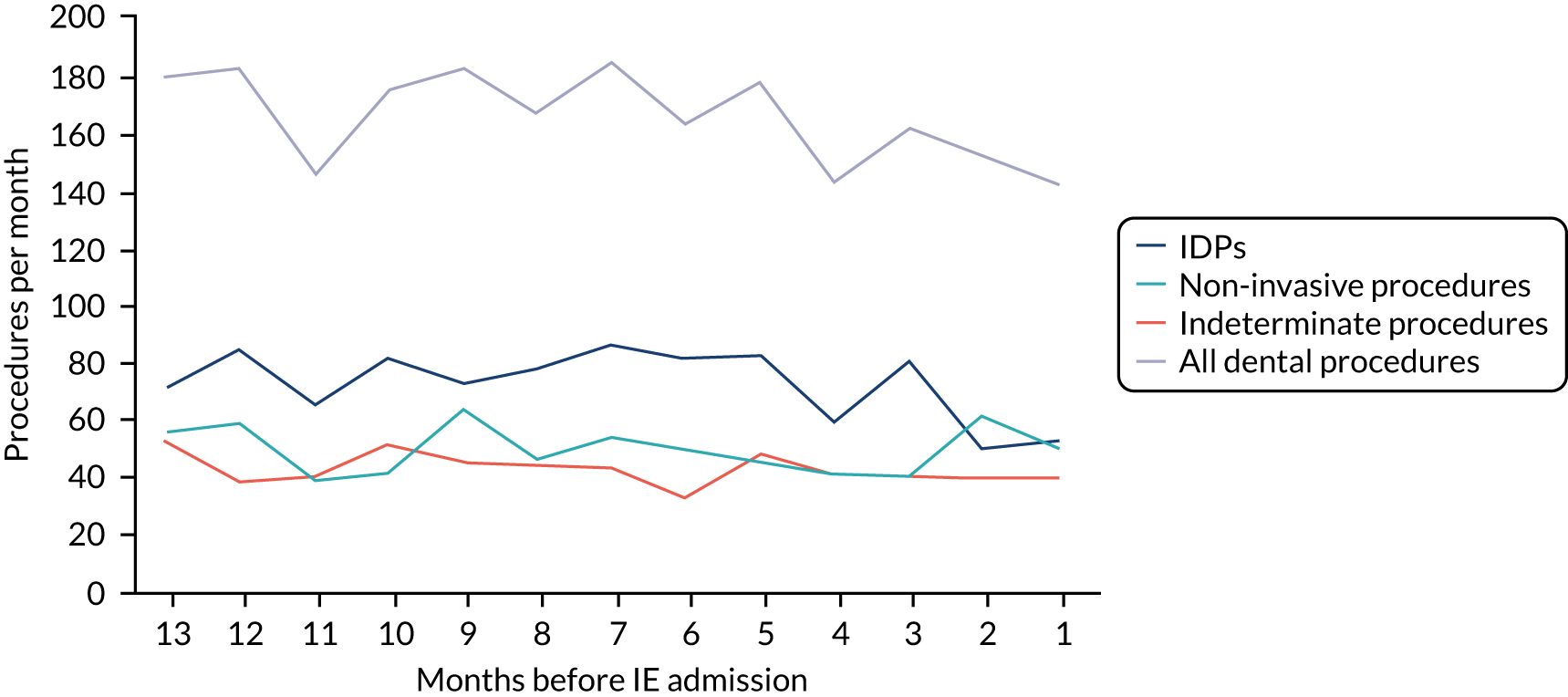
FIGURE 6.
Number of each type of dental procedure over the 13 months before IE admission for patients at high risk of IE using the broad definition of IE.
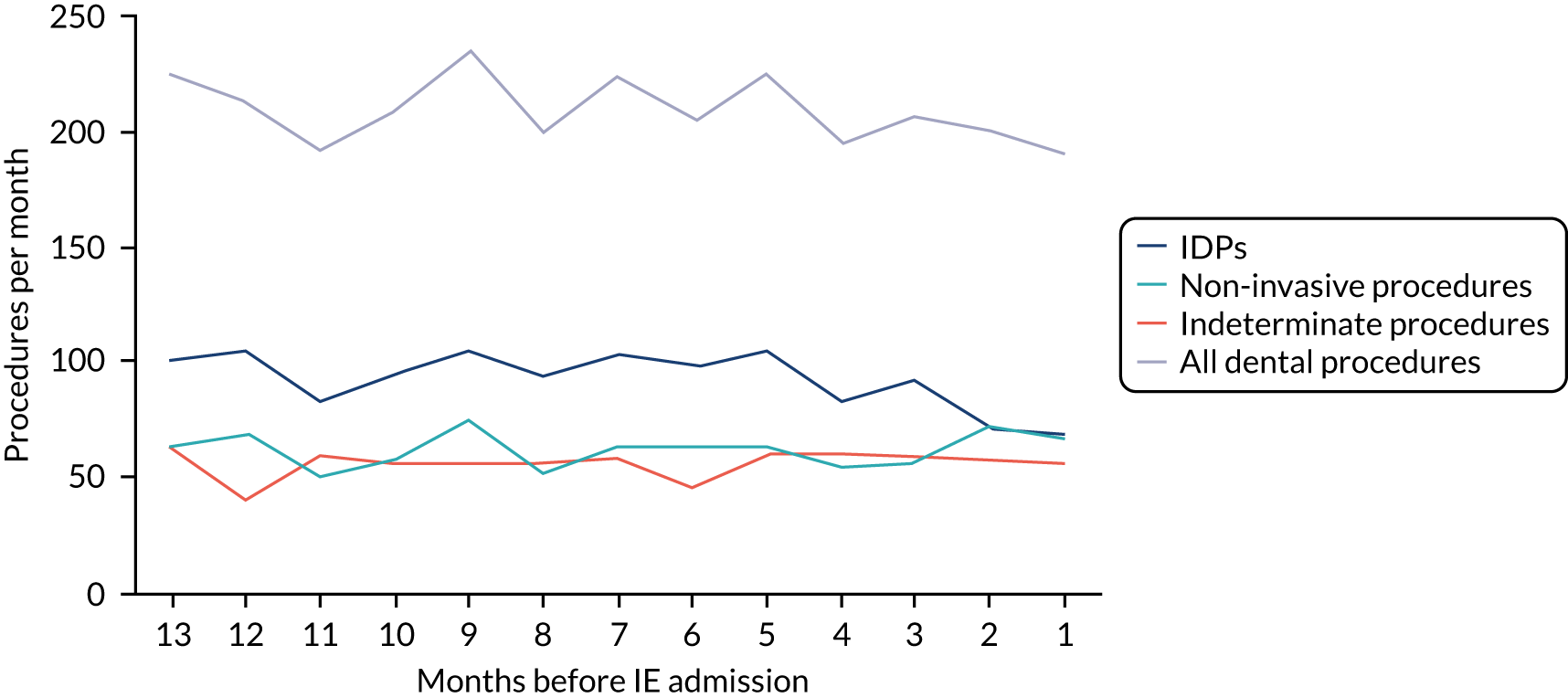
| Months before IE admission | Type of dental procedure (n) | All (n) | ||
|---|---|---|---|---|
| IDP | Indeterminate | Non-invasive | ||
| Using the narrow definition of IE | ||||
| 13 | 50 | 21 | 18 | 89 |
| 12 | 36 | 19 | 25 | 80 |
| 11 | 41 | 19 | 20 | 80 |
| 10 | 37 | 23 | 23 | 83 |
| 9 | 48 | 27 | 25 | 100 |
| 8 | 39 | 27 | 25 | 91 |
| 7 | 31 | 20 | 18 | 69 |
| 6 | 32 | 23 | 12 | 67 |
| 5 | 44 | 23 | 22 | 89 |
| 4 | 38 | 21 | 13 | 72 |
| 3 | 32 | 24 | 15 | 71 |
| 2 | 37 | 25 | 19 | 81 |
| 1 | 41 | 13 | 25 | 79 |
| Total | 506 | 285 | 260 | 1051 |
| Using the broad definition of IE | ||||
| 13 | 80 | 41 | 35 | 156 |
| 12 | 58 | 38 | 45 | 141 |
| 11 | 70 | 33 | 36 | 139 |
| 10 | 65 | 36 | 37 | 138 |
| 9 | 69 | 44 | 42 | 155 |
| 8 | 60 | 37 | 39 | 136 |
| 7 | 48 | 37 | 35 | 120 |
| 6 | 51 | 41 | 31 | 123 |
| 5 | 76 | 33 | 35 | 144 |
| 4 | 63 | 40 | 26 | 129 |
| 3 | 61 | 45 | 26 | 132 |
| 2 | 61 | 43 | 30 | 134 |
| 1 | 60 | 27 | 38 | 125 |
| Total | 822 | 495 | 455 | 1772 |
FIGURE 7.
Number of each type of dental procedure over the 13 months before IE admission for patients at moderate risk of IE using the narrow definition of IE.
FIGURE 8.
Number of each type of dental procedure over the 13 months before IE admission for patients at moderate risk of IE using the broad definition of IE.
| Months before IE admission | Type of dental procedure (n) | All (n) | ||
|---|---|---|---|---|
| IDP | Indeterminate | Non-invasive | ||
| Using the narrow definition of IE | ||||
| 13 | 126 | 63 | 68 | 257 |
| 12 | 108 | 62 | 67 | 237 |
| 11 | 145 | 64 | 60 | 269 |
| 10 | 130 | 72 | 62 | 264 |
| 9 | 113 | 49 | 43 | 205 |
| 8 | 123 | 61 | 64 | 248 |
| 7 | 133 | 60 | 82 | 275 |
| 6 | 127 | 63 | 55 | 245 |
| 5 | 120 | 54 | 62 | 236 |
| 4 | 116 | 67 | 60 | 243 |
| 3 | 114 | 55 | 53 | 222 |
| 2 | 98 | 59 | 76 | 233 |
| 1 | 88 | 48 | 55 | 191 |
| Total | 1541 | 777 | 807 | 3125 |
| Using the broad definition of IE | ||||
| 13 | 211 | 93 | 109 | 413 |
| 12 | 179 | 107 | 107 | 393 |
| 11 | 196 | 108 | 97 | 401 |
| 10 | 188 | 111 | 99 | 398 |
| 9 | 179 | 82 | 94 | 355 |
| 8 | 195 | 99 | 99 | 393 |
| 7 | 200 | 96 | 139 | 435 |
| 6 | 183 | 106 | 100 | 389 |
| 5 | 181 | 87 | 103 | 371 |
| 4 | 164 | 95 | 100 | 359 |
| 3 | 177 | 84 | 96 | 357 |
| 2 | 160 | 95 | 111 | 366 |
| 1 | 154 | 73 | 85 | 312 |
| Total | 2367 | 1236 | 1339 | 4942 |
FIGURE 9.
Number of each type of dental procedure over the 13 months before IE admission for patients at low/unknown risk of IE using the narrow definition of IE.
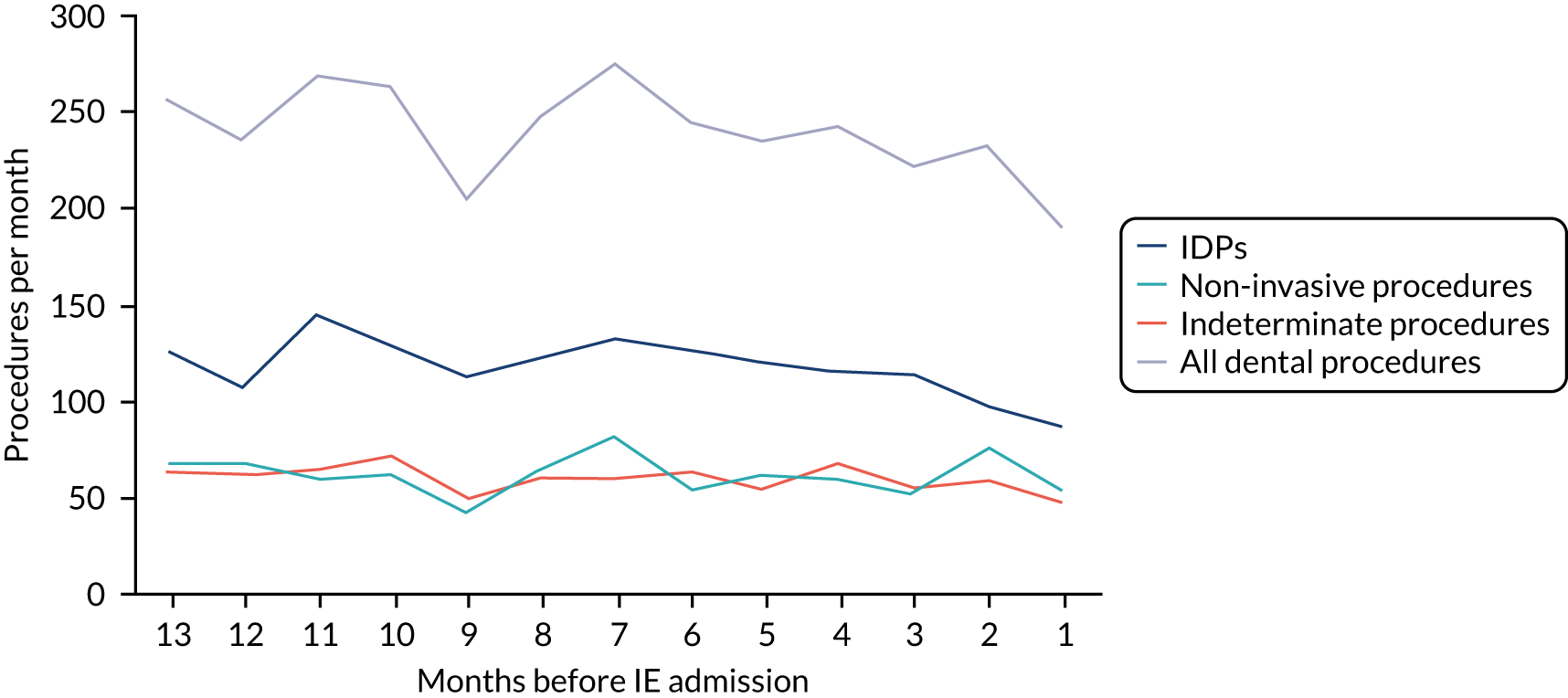
FIGURE 10.
Number of each type of dental procedure over the 13 months before IE admission for patients at low/unknown risk of IE using the broad definition of IE.
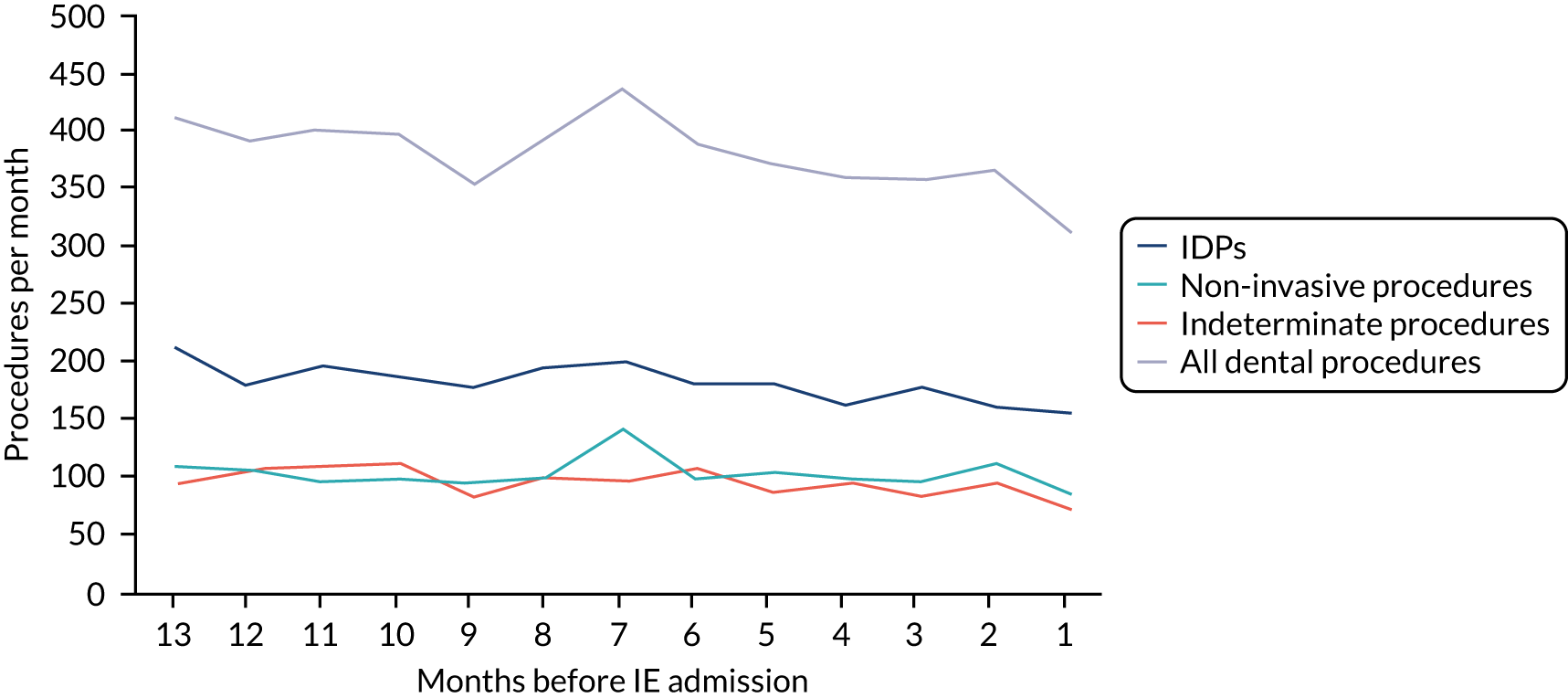
As would be expected, the data plots for all individuals (Figures 3 and 4) had larger numbers when the broad definition was used rather than the narrow definition of IE. However, both exhibited a decrease in the overall number of dental procedures in the month before IE admission, demonstrating the impact of the loss of the data from incomplete courses of treatment in the weeks before any acute hospital admission.
Even when analysis was confined to those at high risk of IE, we still observed a small decrease in the overall number of dental procedures in the month before IE hospital admission (Figures 5 and 6). The distribution of this fall between the types of dental procedure (IDP, indeterminate and non-invasive) was difficult to ascertain, reflecting the overall fluctuation of the data over time. However, there appeared to be more of a decrease in the IDP data than the non-invasive data. Any such difference could be the result of the disproportionately greater loss of IDP data resulting from the loss of data from incomplete courses of treatment in the few weeks before IE admission.
Number of each type of invasive dental procedure over the 13 months before infective endocarditis admission using the narrow and broad definitions of infective endocarditis
As any relationship between dentistry and IE is likely to relate to IDPs, we looked more specifically at the numbers of the three types of IDP for which we were able to obtain data (i.e. extractions, endodontic procedures and scale and polishes). Again, we quantified the monthly numbers of these procedures over the 13 months prior to hospital admission for all individuals who were admitted to hospital with an IE diagnosis using either the narrow or broad definition of IE.
We collected these data for individuals at all levels of IE risk (Table 18) and plotted the data (Figures 11 and 12), including the data for all IDPs and non-invasive dental procedures for comparison. This was then repeated for those at high (Table 19, Figures 13 and 14), moderate (Table 20, Figures 15 and 16) and low/unknown risk of IE (Table 21, Figures 17 and 18).
| Months before IE admission | Type of IDP (n) | All IDPs (n) | Non-invasive procedures (n) | ||
|---|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | |||
| Using the narrow definition of IE | |||||
| 13 | 44 | 203 | 6 | 253 | 142 |
| 12 | 55 | 181 | 12 | 248 | 151 |
| 11 | 55 | 207 | 11 | 273 | 119 |
| 10 | 53 | 202 | 11 | 266 | 127 |
| 9 | 57 | 184 | 7 | 248 | 132 |
| 8 | 47 | 200 | 9 | 256 | 136 |
| 7 | 71 | 191 | 8 | 270 | 154 |
| 6 | 53 | 194 | 11 | 258 | 117 |
| 5 | 57 | 199 | 11 | 267 | 130 |
| 4 | 48 | 173 | 6 | 227 | 115 |
| 3 | 61 | 175 | 9 | 245 | 109 |
| 2 | 36 | 150 | 5 | 191 | 157 |
| 1 | 49 | 135 | 8 | 192 | 130 |
| Total | 686 | 2394 | 114 | 3194 | 1719 |
| Using the broad definition of IE | |||||
| 13 | 75 | 322 | 13 | 410 | 208 |
| 12 | 86 | 270 | 15 | 371 | 221 |
| 11 | 81 | 278 | 17 | 376 | 183 |
| 10 | 78 | 283 | 13 | 374 | 193 |
| 9 | 75 | 285 | 9 | 369 | 211 |
| 8 | 69 | 291 | 12 | 372 | 190 |
| 7 | 91 | 274 | 10 | 375 | 238 |
| 6 | 72 | 270 | 14 | 356 | 193 |
| 5 | 87 | 291 | 13 | 391 | 200 |
| 4 | 70 | 248 | 9 | 327 | 180 |
| 3 | 83 | 261 | 12 | 356 | 178 |
| 2 | 66 | 230 | 9 | 305 | 213 |
| 1 | 63 | 220 | 12 | 295 | 189 |
| Total | 996 | 3523 | 158 | 4677 | 2597 |
FIGURE 11.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for all patients using the narrow definition of IE.
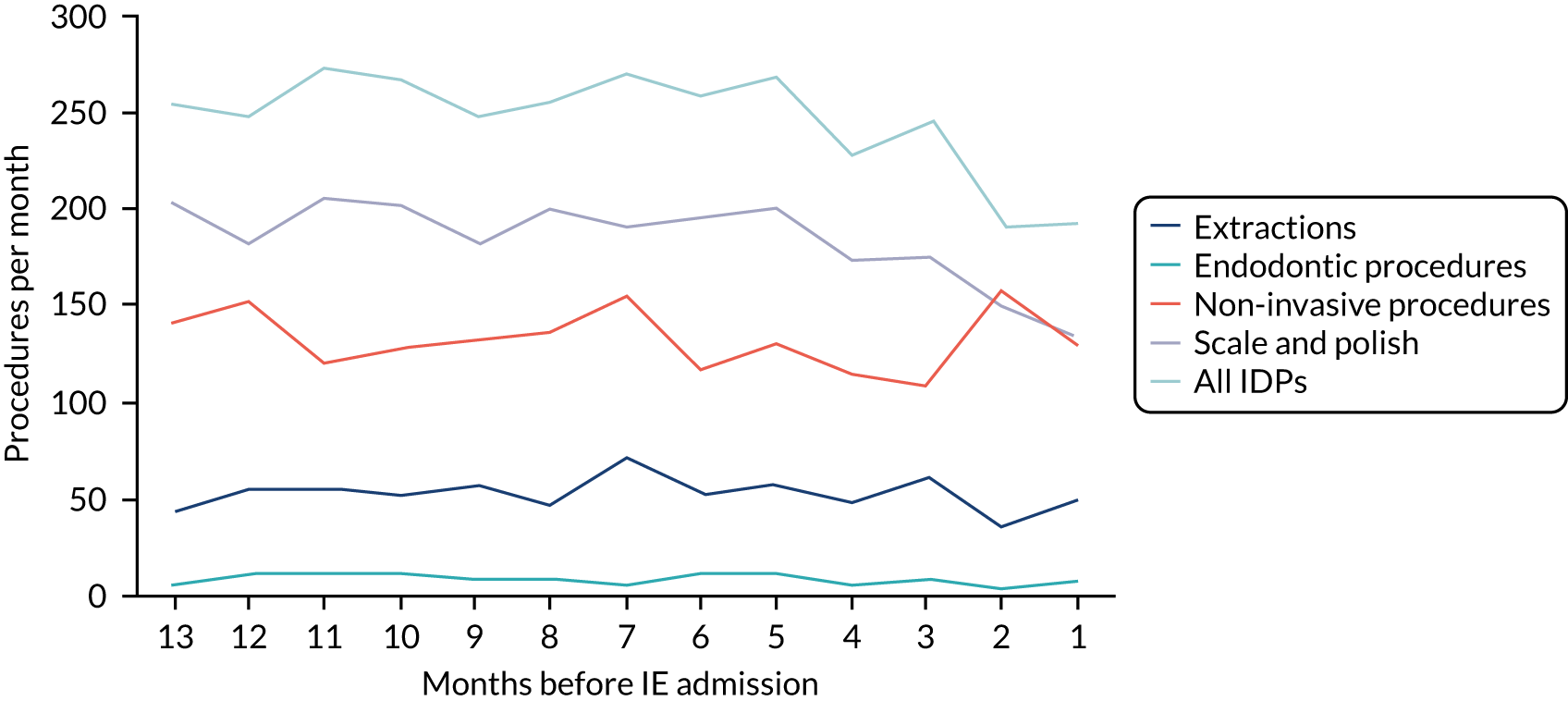
FIGURE 12.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for all patients using the broad definition of IE.
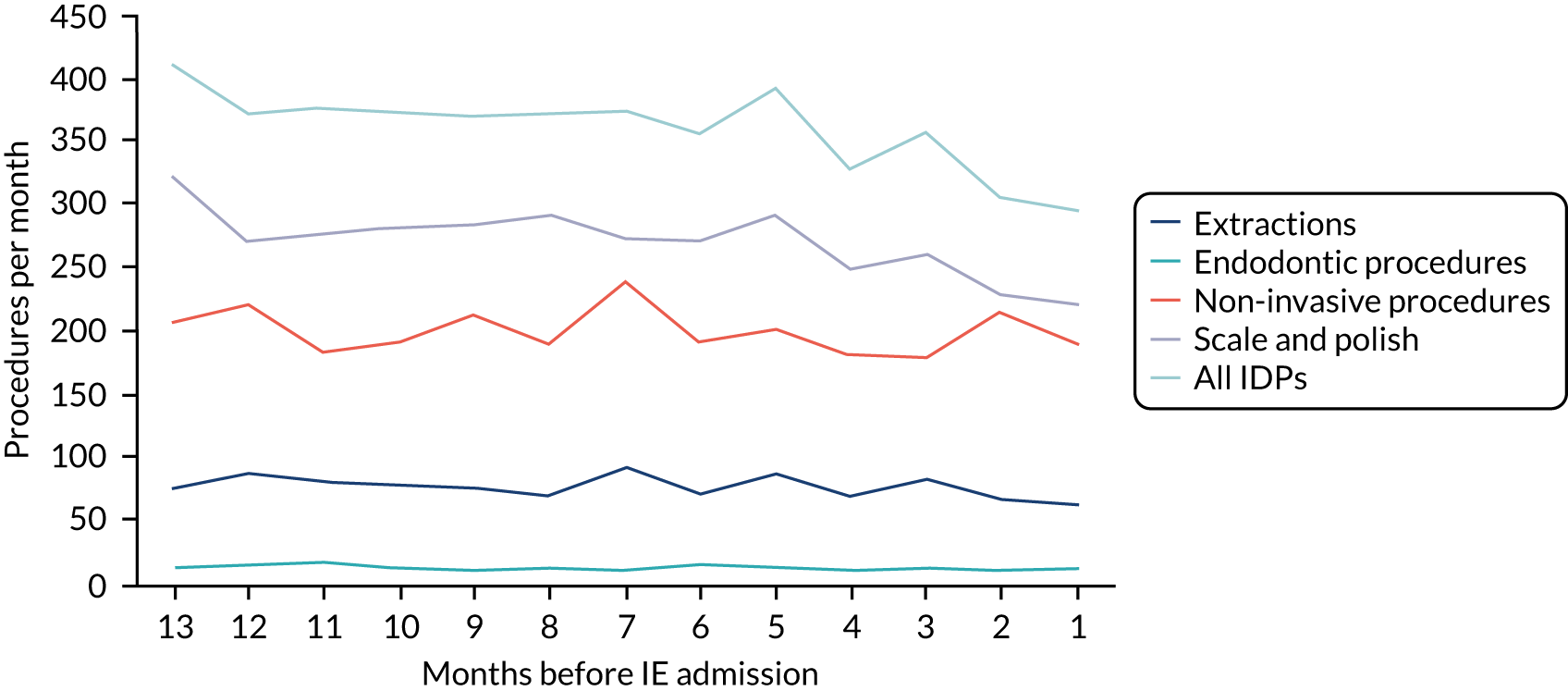
| Months before IE admission | Type of IDP (n) | All IDPs (n) | Non-invasive procedures (n) | ||
|---|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | |||
| Using the narrow definition of IE | |||||
| 13 | 10 | 60 | 2 | 72 | 56 |
| 12 | 19 | 66 | 6 | 91 | 59 |
| 11 | 12 | 58 | 1 | 71 | 39 |
| 10 | 16 | 67 | 5 | 88 | 42 |
| 9 | 13 | 65 | 1 | 79 | 64 |
| 8 | 13 | 65 | 3 | 81 | 47 |
| 7 | 21 | 70 | 5 | 96 | 54 |
| 6 | 14 | 68 | 4 | 86 | 50 |
| 5 | 10 | 72 | 5 | 87 | 46 |
| 4 | 11 | 48 | 2 | 61 | 42 |
| 3 | 19 | 63 | 4 | 86 | 41 |
| 2 | 6 | 41 | 3 | 50 | 62 |
| 1 | 14 | 38 | 3 | 55 | 50 |
| Total | 178 | 781 | 44 | 1003 | 652 |
| Using the broad definition of IE | |||||
| 13 | 15 | 85 | 3 | 103 | 64 |
| 12 | 21 | 85 | 5 | 111 | 69 |
| 11 | 17 | 69 | 4 | 90 | 50 |
| 10 | 18 | 79 | 5 | 102 | 57 |
| 9 | 17 | 94 | 1 | 112 | 75 |
| 8 | 15 | 80 | 4 | 99 | 52 |
| 7 | 25 | 83 | 5 | 113 | 64 |
| 6 | 15 | 81 | 5 | 101 | 62 |
| 5 | 18 | 90 | 4 | 112 | 62 |
| 4 | 15 | 67 | 3 | 85 | 54 |
| 3 | 18 | 76 | 6 | 100 | 56 |
| 2 | 10 | 58 | 5 | 73 | 72 |
| 1 | 12 | 55 | 3 | 70 | 66 |
| Total | 216 | 1002 | 53 | 1271 | 803 |
FIGURE 13.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for high-risk patients using the narrow definition of IE.
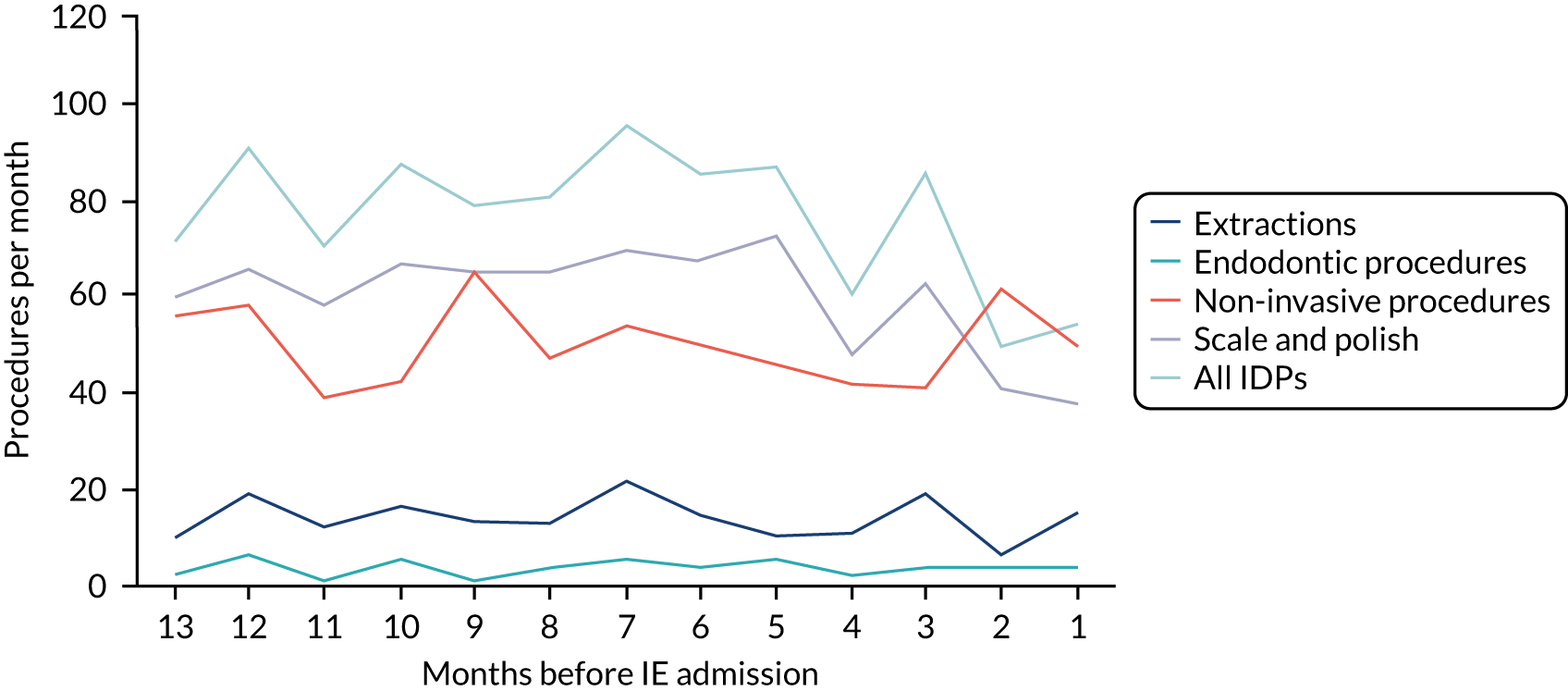
FIGURE 14.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for high-risk patients using the broad definition of IE.
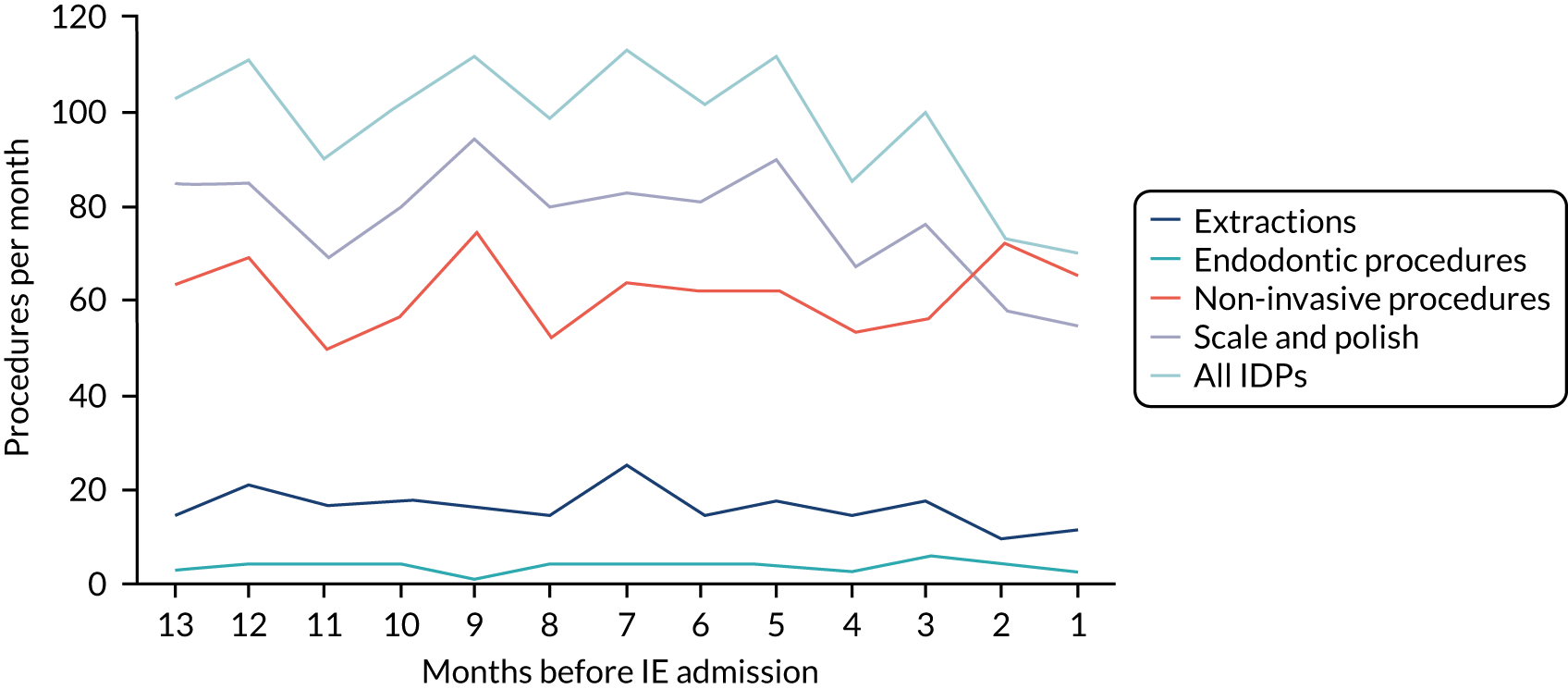
| Months before IE admission | Type of IDP (n) | All IDPs (n) | Non-invasive procedures (n) | ||
|---|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | |||
| Using the narrow definition of IE | |||||
| 13 | 13 | 37 | 1 | 51 | 18 |
| 12 | 7 | 31 | 2 | 40 | 25 |
| 11 | 8 | 36 | 2 | 46 | 20 |
| 10 | 10 | 29 | 0 | 39 | 23 |
| 9 | 16 | 35 | 1 | 52 | 25 |
| 8 | 6 | 34 | 1 | 41 | 25 |
| 7 | 10 | 23 | 0 | 33 | 18 |
| 6 | 5 | 27 | 3 | 35 | 12 |
| 5 | 11 | 35 | 2 | 48 | 22 |
| 4 | 9 | 32 | 1 | 42 | 13 |
| 3 | 10 | 26 | 2 | 38 | 15 |
| 2 | 8 | 29 | 1 | 38 | 19 |
| 1 | 11 | 31 | 0 | 42 | 25 |
| Total | 124 | 405 | 16 | 545 | 260 |
| Using the broad definition of IE | |||||
| 13 | 21 | 61 | 4 | 86 | 35 |
| 12 | 11 | 48 | 4 | 63 | 45 |
| 11 | 17 | 57 | 3 | 77 | 36 |
| 10 | 14 | 54 | 0 | 68 | 37 |
| 9 | 20 | 51 | 3 | 74 | 42 |
| 8 | 8 | 52 | 2 | 62 | 39 |
| 7 | 12 | 37 | 1 | 50 | 35 |
| 6 | 11 | 42 | 4 | 57 | 31 |
| 5 | 20 | 59 | 4 | 83 | 35 |
| 4 | 12 | 55 | 3 | 70 | 26 |
| 3 | 16 | 51 | 3 | 70 | 26 |
| 2 | 12 | 50 | 0 | 62 | 30 |
| 1 | 16 | 46 | 1 | 63 | 38 |
| Total | 190 | 663 | 32 | 885 | 455 |
FIGURE 15.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for moderate-risk patients using the narrow definition of IE.
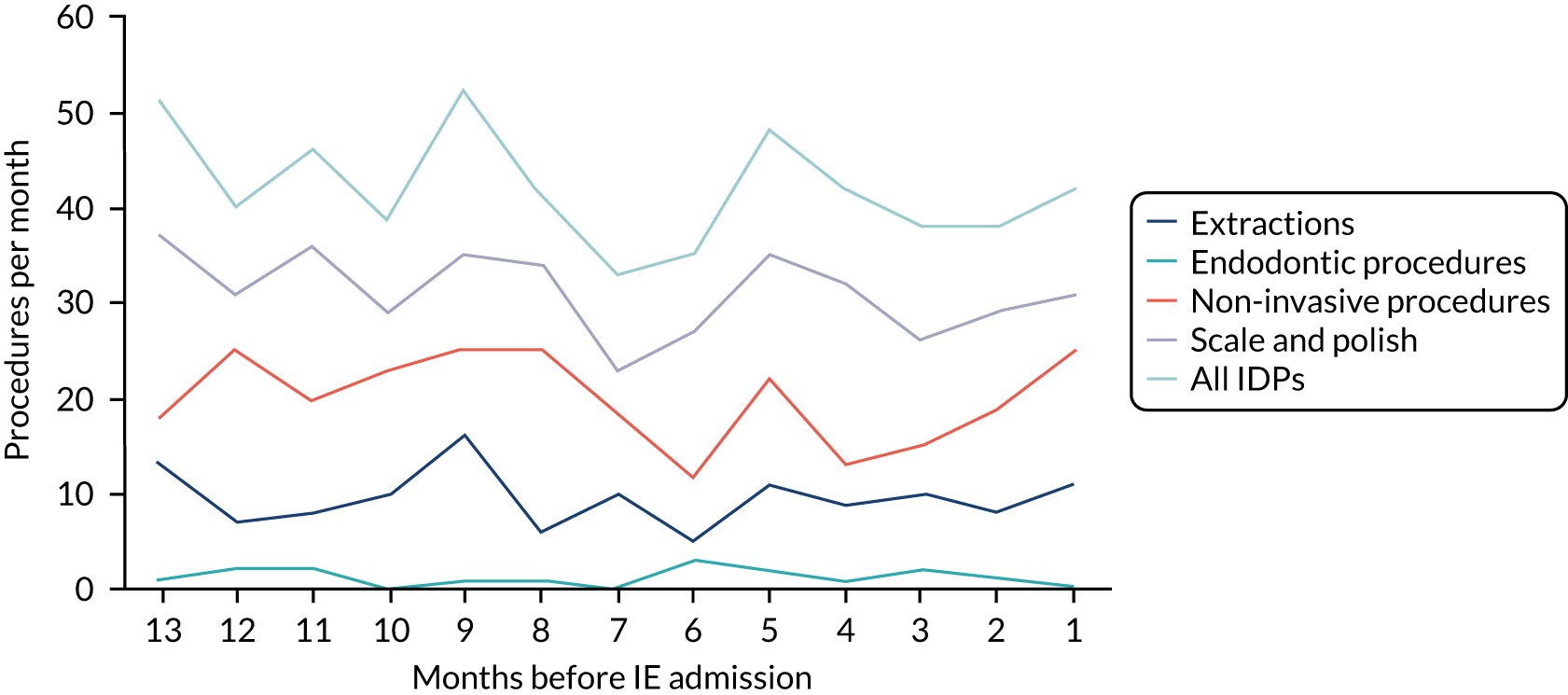
FIGURE 16.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for moderate-risk patients using the broad definition of IE.
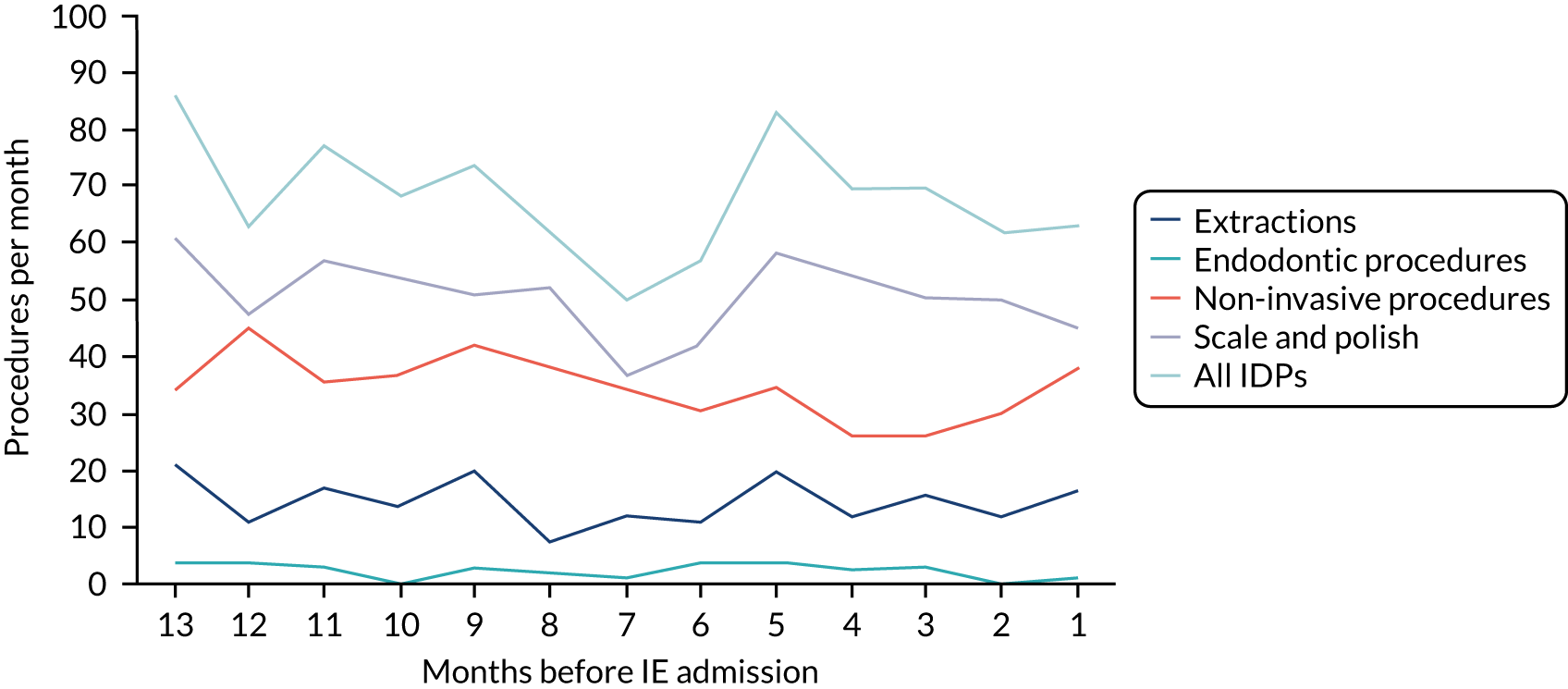
| Months before IE admission | Type of IDP (n) | All IDPs (n) | Non-invasive procedures (n) | ||
|---|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | |||
| Using the narrow definition of IE | |||||
| 13 | 21 | 106 | 3 | 130 | 68 |
| 12 | 29 | 84 | 4 | 117 | 67 |
| 11 | 35 | 113 | 8 | 156 | 60 |
| 10 | 27 | 106 | 6 | 139 | 62 |
| 9 | 28 | 84 | 5 | 117 | 43 |
| 8 | 28 | 101 | 5 | 134 | 64 |
| 7 | 40 | 98 | 3 | 141 | 82 |
| 6 | 34 | 99 | 4 | 137 | 55 |
| 5 | 36 | 92 | 4 | 132 | 62 |
| 4 | 28 | 93 | 3 | 124 | 60 |
| 3 | 32 | 86 | 3 | 121 | 53 |
| 2 | 22 | 80 | 1 | 103 | 76 |
| 1 | 24 | 66 | 5 | 95 | 55 |
| Total | 384 | 1208 | 54 | 1646 | 807 |
| Using the broad definition of IE | |||||
| 13 | 39 | 176 | 6 | 221 | 109 |
| 12 | 54 | 137 | 6 | 197 | 107 |
| 11 | 47 | 152 | 10 | 209 | 97 |
| 10 | 46 | 150 | 8 | 204 | 99 |
| 9 | 38 | 140 | 5 | 183 | 94 |
| 8 | 46 | 159 | 6 | 211 | 99 |
| 7 | 54 | 154 | 4 | 212 | 139 |
| 6 | 46 | 147 | 5 | 198 | 100 |
| 5 | 49 | 142 | 5 | 196 | 103 |
| 4 | 43 | 126 | 3 | 172 | 100 |
| 3 | 49 | 134 | 3 | 186 | 96 |
| 2 | 44 | 122 | 4 | 170 | 111 |
| 1 | 35 | 119 | 8 | 162 | 85 |
| Total | 590 | 1858 | 73 | 2521 | 1339 |
FIGURE 17.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for low/unknown-risk patients using the narrow definition of IE.
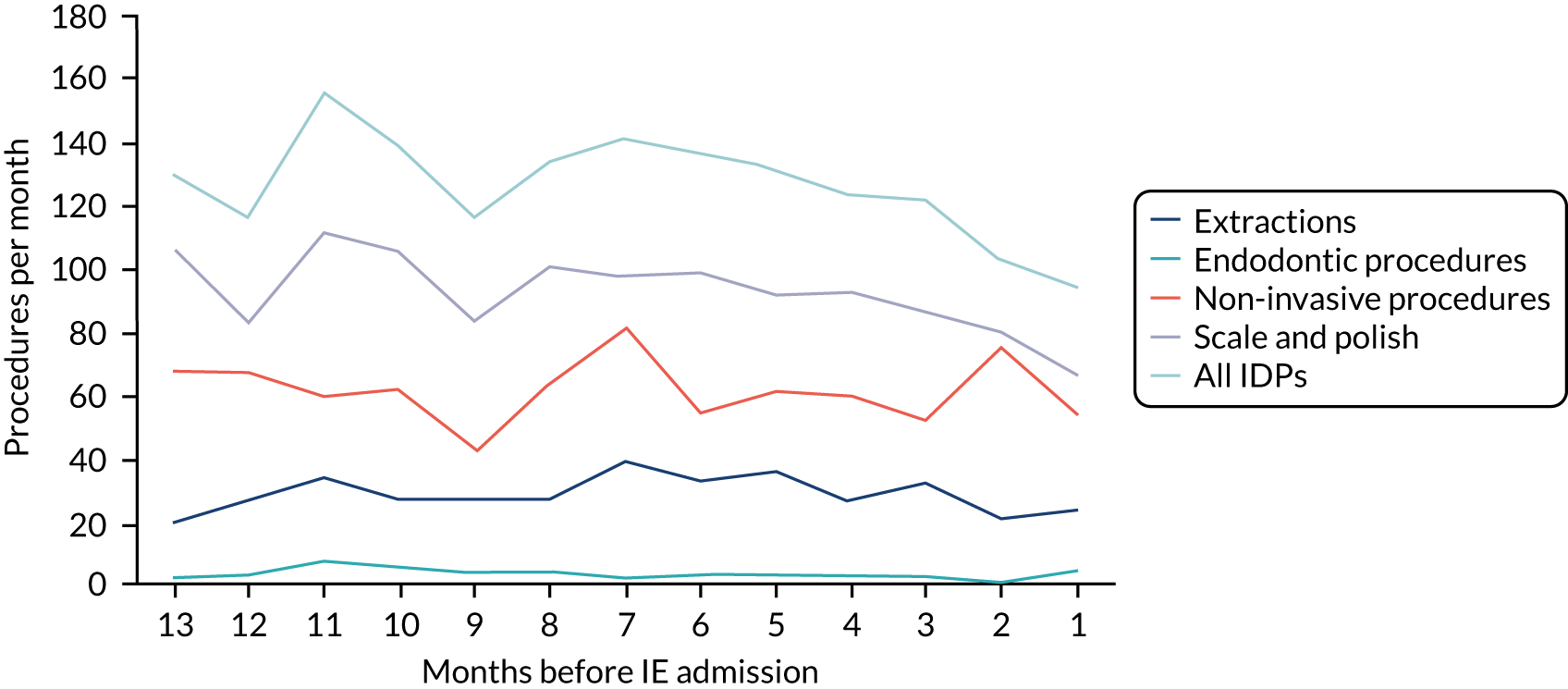
FIGURE 18.
Number of extractions, scale and polishes, and endodontic procedures over the 13 months before IE admission for low/unknown-risk patients using the broad definition of IE.
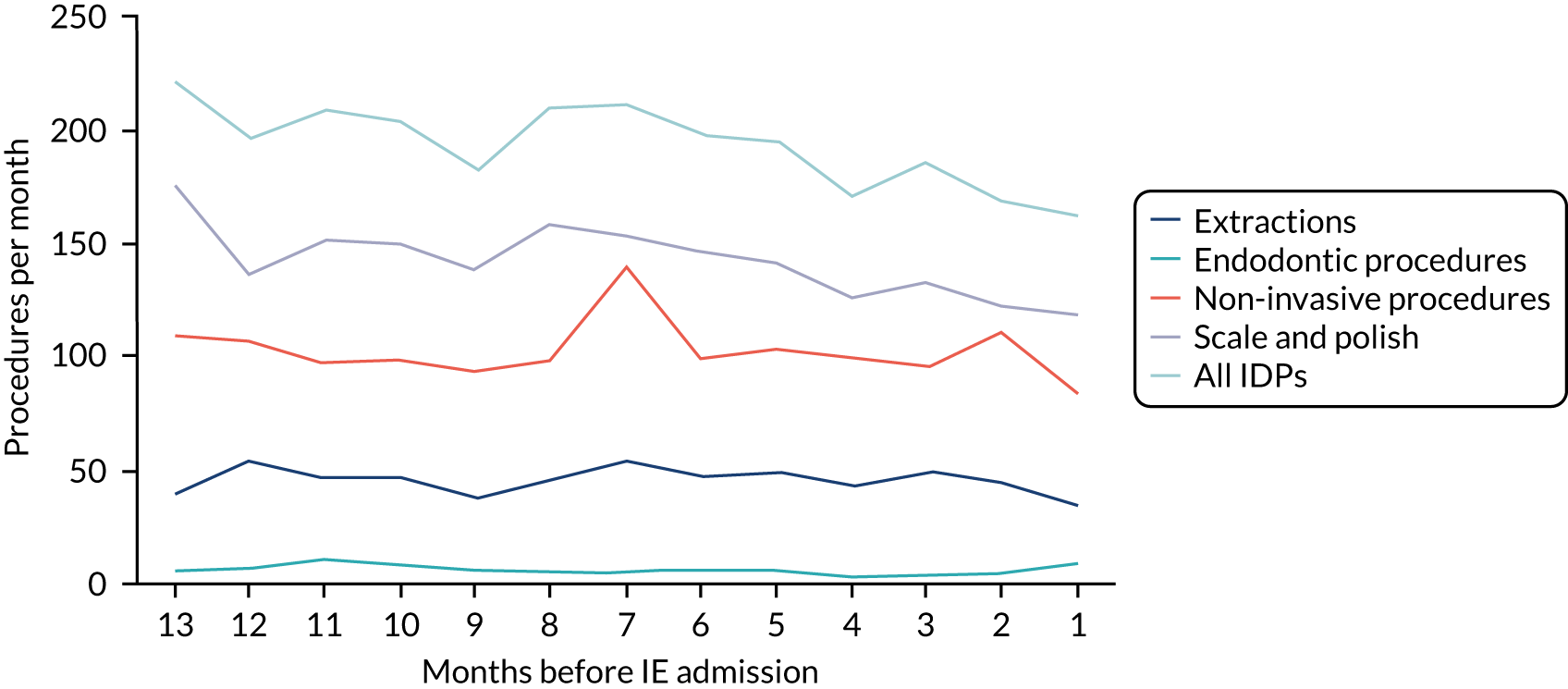
Any relationship between dental procedures and IE should be seen most strongly in individuals who are at high risk of and develop IE (Table 19, Figures 13 and 14). It is notable that there was no apparent change in the number of extractions or endodontic procedures (outside the extent of fluctuation in the data over the full 13 months) in the period prior to IE admission. Table 22 (using the narrow definition of IE) and Table 23 (using the broad definition of IE) show the incidence rate ratios (IRRs) for the quarterly control periods compared with those of the 3 months immediately preceding IE.
Using the narrow definition of IE (Table 22), the incidence rate of extractions was similar in months 4–12 compared with the 3 months prior to IE admission (months 3–12 vs. 0–3: IRR 1.18, 95% CI 0.77 to 1.62; p = 0.23) and endodontic dentistry was uncommon across all periods (IRR 1.00, 95% CI 0.46 to 2.16; p = 1.00). By contrast, there was evidence of a decrease in the incidence rate of scale and polishes in the months prior to IE admission (months 3–12 vs. 0–3: IRR 1.48, 95% CI 1.23 to 1.78; p < 0.001). As scale and polishes account for the majority of IDPs, this pattern was reflected in the data for all recorded invasive procedures (months 3–12 vs. 0–3: IRR 1.34, 95% CI 1.13 to 1.58; p = 0.004).
| Months before IE admission | Type of IDP, IRR (95% CI) | All IDPs, IRR (95% CI) | Non-invasive procedures, IRR (95% CI) | ||
|---|---|---|---|---|---|
| Extractions | Scale and polish | Endodontic | |||
| Case | |||||
| 0–3 | – | – | – | – | – |
| Control | |||||
| 3–6 | 0.85 (0.52 to 1.39) | 1.47 (1.17 to 1.85) | 1.00 (0.40 to 2.52) | 1.30 (1.05 to 1.60) | 0.97 (0.75 to 1.25) |
| 6–9 | 1.29 (0.84 to 1.99) | 1.52 (1.24 to 1.86) | 1.00 (0.40 to 2.52) | 1.37 (1.14 to 1.64) | 1.13 (0.91 to 1.41) |
| 9–12 | 1.26 (0.82 to 1.96) | 1.45 (1.17 to 1.80) | 1.00 (0.40 to 2.52) | 1.34 (1.10 to 1.64) | 1.00 (0.78 to 1.27) |
| Combined | 1.18 (0.77 to 1.62) | 1.48 (1.23 to 1.78) | 1.00 (0.46 to 2.16) | 1.34 (1.13 to 1.58) | 1.03 (0.85 to 1.26) |
| Months before IE admission | Type of IDP, IRR (95% CI) | All IDPs, IRR (95% CI) | Non-invasive procedures, IRR (95% CI) | ||
|---|---|---|---|---|---|
| Extraction | Scale and polish | Endodontic | |||
| Case | |||||
| 0–3 | – | – | – | – | – |
| Control | |||||
| 3–6 | 1.12 (0.72 to 1.74) | 1.45 (1.17 to 1.79) | 0.75 (0.32 to 1.78) | 1.33 (1.10 to 1.62) | 0.99 (0.79 to 1.25) |
| 6–9 | 1.38 (0.90 to 2.11) | 1.53 (1.28 to 1.84) | 0.83 (0.36 to 1.93) | 1.42 (1.20 to 1.67) | 1.06 (0.86 to 1.30) |
| 9–12 | 1.47 (0.96 to 2.25) | 1.38 (1.12 to 1.68) | 0.83 (0.37 to 1.86) | 1.32 (1.10 to 1.59) | 0.95 (0.76 to 1.19) |
| Combined | 1.32 (0.92 to 1.89) | 1.45 (1.22 to 1.72) | 0.81 (0.41 to 1.56) | 1.36 (1.16 to 1.59) | 1.00 (0.84 to 1.20) |
The non-invasive dental procedure data were also plotted for comparison. Although there was a suggestion of an increase in non-invasive dental procedures in the 2 months before IE admission, this was within the extent of the data fluctuation over the full 13 months, with no evidence of any significant change in incidence rate (months 3–12 vs. 0–3: IRR 1.03, 95% CI 0.85 to 1.26; p = 0.54).
Conclusion
Although the narrow definition of IE identified ≈ 35% fewer IE cases than the broad definition, it did so with higher specificity and, therefore, there was a greater likelihood that these cases really were modified Duke criteria-positive IE cases. Plotting the number of dental procedures over the 13 months before IE admission did not reveal significantly different patterns of dental procedural numbers over the few weeks before IE admission between these two different definitions, (although the number of IE cases was 35% higher using the broad definition). Accordingly, we have focused on data using the narrow definition of IE.
The data plots for dental procedural numbers in all those admitted to hospital with IE (using the narrow definition) appeared to show a decrease in the number of all dental procedures in the 1–3 months before IE hospital admission. This decrease seemed to be larger for IDP than for non-invasive or indeterminate procedures. However, this could reflect the fact that the loss of data from incomplete courses of treatment had a disproportionately larger effect on IDPs than non-invasive procedures. The same general pattern was also seen in individuals at high risk of IE who went on to be admitted to hospital with IE (i.e. those in whom any association between IDPs and subsequent IE would be expected to be strongest).
When we assessed the number of each type of IDP (i.e. scale and polishes, extractions and endodontic procedures) over the 13 months before IE hospital admission, we identified different patterns. For both all individuals and those at high risk of IE, there was no clear pattern of a decrease in the number of extractions or endodontic procedures in the month (or 2 months) before IE admission. Any change was within the fluctuation in number for that type of procedure over the 13 months studied. Given the disproportionate loss of IDP data in the few weeks before IE admission caused by the loss of data from incomplete courses of treatment, this could mean that there was an increase in the number of extractions and endodontic treatments in the weeks before IE admission. Such an increase would have suggested an association between these procedures and the subsequent development of IE. However, we have no evidence of that and are therefore unable to draw any definite conclusions.
In contrast, nearly all of the decrease in the number of IDPs identified by the plots in the 1–2 months immediately before IE admission appeared to be accounted for by scale and polishes. There appeared to be a significant decrease in the number of scale and polishes, which was in contrast to the observed pattern for extractions and endodontic procedures. Again, this may be an aberration caused by the incomplete course of treatment data loss or could suggest that any association between IDP and subsequent IE relates to dental extractions or endodontic treatment, rather than scale and polishes. Unfortunately, the uncertainties caused by the loss of incomplete course of treatment data make it impossible to draw any firm conclusions.
Owing to the uncertainties caused by the loss of incomplete course of treatment data, we felt unable to further analyse or interpret the data, or present any implications concerning the association between IDP and IE.
Chapter 7 Discussion
Background
Infective endocarditis is a life-threatening infection of the endocardial lining of the heart, particularly the heart valves, with a high morbidity rate and a first-year mortality rate of ≈ 30%. 2
Although IE is relatively rare, affecting only 3–10 per 100,000 people per year,3,4,6,7 patients at increased risk of IE are comparatively common and increasing in number. A large number of individuals with predisposing cardiac conditions are at increased risk of IE. 7 Guideline committees around the world have generally stratified these individuals into those at high risk of IE and those at moderate risk of IE. The rest of the population is considered to be at low risk. 8–11
Infective endocarditis can result from bacteraemia caused by a broad spectrum of bacterial and fungal organisms entering the circulation. However, the possibility that some cases of IE might be caused by oral bacteria entering the circulation during IDPs was first suggested by Lewis and Grant in 1923,12 and was supported in 1935 by Okell and Elliott, who demonstrated that 61% of individuals develop a transient bacteraemia with OVGS following a dental extraction and that OVGS could be isolated from the heart valve vegetations of 40–45% of individuals with IE. 13 Although the proportion of IE cases caused by OVGS has probably decreased since then, OVGS still compete with staphylococci as the most frequent cause of IE and are likely to account for 35–45% of cases.
A putative link between IDPs and IE led the AHA to issue the first guidelines on the use of AP to prevent IE in 1955,16 and AP became the worldwide standard of care for preventing IE in those at increased risk of IE. However, to the best of our knowledge, there has never been a trial of AP to define its efficacy in IE prevention. 17 Furthermore, multiple studies have shown that low-level bacteraemia occurs frequently following daily activities such as tooth brushing, flossing and mastication, particularly in those with poor oral hygiene. 18 It has been argued that the risk of developing IE posed by these daily activities far exceeds any risk associated with IDP,19 and thus the case for giving AP to cover IDPs is flawed. 19 This, along with concerns about the risk of adverse reactions5,20 and the development of antibiotic resistance,21 has led to reductions in the individuals targeted for AP.
In 2007, the AHA recommended restricting AP to those at high risk of IE and its complications who were undergoing an IDP. 11 The ESC published similar guidance in 2009. 9 In the UK, however, where the importance of daily activities as the cause for OVGS IE was most strongly argued,19 NICE concluded in 2008 that ‘the evidence does not show a causal relationship between having an interventional procedure and the development of IE’ and that ‘it is biologically implausible that a dental procedure would lead to a greater risk of IE than regular tooth brushing’, and recommended the complete cessation of AP (© NICE 2008 Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. Available from www.nice.org.uk/guidance/ng64. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.). 22,23
Nonetheless, concerns remain that IDPs could pose a risk for those at increased risk of IE and most guideline committees (e.g. AHA, ESC, Japanese Cardiac Society) continue to recommend AP for those at highest risk undergoing an IDP. 9,11,24 In 2016, even NICE changed its position and no longer precludes the use of AP for those at increased risk of IE undergoing an IDP. 25,26
Despite the ongoing controversy over the value of AP to prevent IE, to the best of our knowledge, there has never been a RCT of AP’s efficacy. The evidence base for the use of AP is limited to a small number of case–control studies, in which reduction in bacteraemia has been used as a surrogate measure for reduction in IE. The evidence base for the use of AP is therefore limited and heterogeneous, and many of the studies are of poor methodological quality. 17 Furthermore, for reasons discussed in Chapter 1, it is unlikely that a RCT will be performed in the foreseeable future, and before such a RCT is deemed worthwhile, we need to be certain that there is an association between IDP and IE. Indeed, such a study could render a RCT unnecessary.
The aim of this study
The aim of the IDEA study, therefore, was to investigate if there is a temporal link (or association) between IDP and subsequent occurrence of IE by linking English national data on hospital admissions for IE with data on courses of dental treatment.
The advantage of performing this study in England between April 2009 and March 2016 was that NICE recommended against all use of AP to prevent IE in the UK during this period. Furthermore, studies have shown high compliance with the recommendation during this period. 3,6,28 As a consequence, any association between IDPs and IE should have been fully exposed.
To study this association, we chose a case-crossover methodology, as it is specifically designed for studying the effect of transient events (e.g. an IDP) in triggering a subsequent outcome (e.g. IE). The advantage of the case-crossover methodology is that it removes many of the issues of confounding and selection bias that affect cohort and case–control studies, as each patient acts as their own control. 34,35 In addition, by linking national dental and HES data, we would not have to rely on patient recall to determine the timing and nature of any dental procedures that were performed. The case-crossover methodology was first proposed by Maclure34 and provides greater statistical power for addressing this type of cause and effect issue because it addresses the problems of selection bias and confounding. Furthermore, the study can be performed by selecting individuals who develop the disease of interest (in this case, IE), without the need to select a matched control population.
Outcome
Unfortunately, although the data we received on IE hospital admissions from NHS Digital were to specification, the dental data we received from the NHSBSA were not. This fundamentally undermined our ability to perform the study as planned and generate reliable results using the case-crossover methodology. There were two main shortcomings of the NHSBSA data.
The first was that the delays in the regulatory process and supply of data by the NHSBSA, along with the introduction of a policy to destroy all records that were ≥ 10 years old, meant that we only received 60 months of data (rather than the 72 months we had requested and had used to calculate the sample size and power of the study; see Chapter 2 for details).
The second was that when we analysed the data, we identified a decrease in the number of all types of dental procedures in the few weeks immediately preceding emergency hospital admission with a number of acute medical conditions, including IE, MI, stroke and PE. These conditions, like IE, are associated with high in-hospital and first-year mortality rates. After further investigation, it became apparent that the reason for this was that dentists are not required to provide the NHSBSA with any details of the treatment they had provided if a course of dental treatment was not completed (as often occurs if treatment is interrupted by an emergency hospital admission, death or long-term debilitating illness). The result was a critical loss of data on any dental procedures performed in the few weeks immediately preceding emergency hospital admission (but not at other times).
The data loss caused by the first consideration reduced the overall sample size and thus the statistical power of the study. The data loss caused by the second consideration critically affected the case period of the case-crossover methodology, but not the control period. Hence, our ability to detect any increase in the number of IDPs in the case period of the case-crossover study compared with the control period (as would be expected if there were an association between IDPs and IE) was fundamentally undermined.
We experimented with using the end date of courses of dental treatment instead of the start date as a way of excluding data on incomplete courses of treatment from analysis. We also limited our analysis to courses of dental treatment that started and finished on the same day as another method of excluding the effect from incomplete courses of treatment. However, neither approach resolved the fact that we had lost critical data on dental procedures being performed in the few weeks before emergency admission to hospital with IE. The resulting decrease in the number of dental procedures in the weeks before IE admission could give the impression that dental procedures are protective against IE. Although they might not be linked to IE hospital admission, it is difficult and counterintuitive to explain why they might protect against IE hospital admission. Furthermore, the decrease in the number of dental procedures in the weeks before IE admission affected both IDPs and non-invasive dental procedures.
We also considered comparing the decrease in the number of IDPs with the decrease in the number of non-invasive dental procedures in the weeks before IE admission. In other words, we considered performing a comparative case-crossover analysis of IDPs and non-invasive dental procedures.
However, this was also flawed because the data loss caused by incomplete courses of treatment was not equal for all types of dental procedures. The most common non-invasive dental procedures are a simple dental examination and radiography. As these are diagnostic procedures, they are usually the first procedures a dentist performs before deciding on the need for any other treatment.
By contrast, a dentist is unlikely to perform an extraction without performing an initial dental examination and radiography. Usually, therefore, the examination and radiography will be performed at the first dental visit, and the patient booked to return once the need for an extraction is identified (unless the extraction is particularly urgent and the dentist has time during the first visit). Other IDPs, such as endodontic treatments, are longer and usually performed over more than one visit, whereas a scale and polish is often performed at the conclusion of a course of treatment. This means that the loss of incomplete course of treatment data is likely to have a disproportionately greater effect on IDPs than non-invasive dental procedures, as, by definition, a course that is incomplete must include more than one visit to the dentist.
This was confirmed when we studied single-visit courses of dental treatment. Restricting the analysis to single-visit courses of treatment resulted not only in a significant further loss of data, but also in a loss of data greater for IPDs than for non-IDPs. Furthermore, the extent of the data loss differed according to the type of IDP (greatest for endodontic procedures, followed by extractions, and lowest for scale and polishes).
Using the end date to identify courses of dental treatment resulted in minimal further loss of data and appeared to slightly mitigate the effects of the loss of data on incomplete courses of treatment by shifting the timing of procedures relative to IE admission. Hence, we used the end date to perform our final analyses. Nonetheless, this approach did not alter the critical loss of dental procedure data in the few weeks immediately before any acute hospital admission. Because this data loss is focused on the case period rather than the control period of any case-crossover analysis, it fundamentally undermines (and introduces bias to) any case-crossover analysis. Furthermore, because this data loss disproportionately affects IDPs more than non-invasive dental procedures, it introduces further bias and undermines any comparison of the two entities in the few weeks before IE admission.
As a consequence, although we have plotted the time course of the number of different dental procedures over the 13 months before IE hospital admission using both narrow and broad definitions of IE (see Chapter 5), we think that it is difficult to draw any firm conclusions about the relationship between each type of dental procedure and IE owing to the unreliability of the data in the critical weeks before admission, caused by the loss of the incomplete course of treatment data. Given this, we feel that it would be inappropriate to submit these observations to formal statistical analysis that might invest them with greater credibility than is justified.
Impact of the results on previous knowledge
Unfortunately, for the reasons described above, the results of this study did not fulfil our aim of determining if there is a causal temporal association between IDPs and subsequent IE. Apart from providing some new descriptive data on the epidemiology of IE in England, we were unable to contribute to the existing knowledge base on IE in the way we had intended or hoped to do.
Implications of the results for clinical practice/policy
Regrettably, because of the unreliability of the dental data associated with IE admissions, we were unable to draw any conclusions about the relationship between IDPs and subsequent IE or the value of AP in preventing IE following IDPs that could be used to inform either clinical practice or the current NICE guidelines. 10
Recommendations for data collection/future research
Data collection
The first cause of data loss described above was the delay in the regulatory process and supply of data by the NHSBSA, along with its policy to destroy all records that were ≥ 10 years old. Had the regulatory process and supply of data after approval not been delayed (as detailed in Chapter 2), we would have received the full 72 months of data we requested, despite the NHSBSA 10-year data destruction rule. Alternatively, we would have received the full 72 months of data we needed from the NHSBSA, despite the regulatory and other delays, if it did not have a policy of destroying data that are ≥ 10 years old. Given that supporting research is now part of the stated objectives of the NHSBSA, it is difficult to understand why the 10-year destruction rule was adopted when the other major supplier of NHS research data (NHS Digital) does not destroy data after 10 years.
A more streamlined system of research governance and approval for Department of Health and Social Care-funded research using NHS data could dramatically reduce the impediments that currently delay research that is important for informing NHS clinical practice. This is particularly the case for large-scale epidemiological studies, such as the IDEA study, that do not require patient-identifying information as part of the study. This study was subject to the differing and conflicting advice and opinions of the NHS Research Ethics Service, CAG, DARS and IGARD. Despite the overarching responsibility of the HRA, these organisations failed to co-ordinate or work together to facilitate the approval process. Indeed, they often held opposing views on the same subject, disregarded the advice of the HRA and replicated each other’s functions. In our experience, even interventional clinical research on patients is not subject to the same level of overburdensome regulatory approval in the UK, nor is this the case for similar data-driven research in North America or Europe.
Once regulatory approval was obtained, considerable further delay resulted from the need to obtain a DSA to access the data. Although it may make operational sense to split NHS data between two NHS organisations (NHS Digital and the NHSBSA), it is hard to understand why these organisations cannot work together or share data for research purposes when it is within both their remits to support research activity. Yet, we had to not only act as an intermediary for the two organisations, but also establish separate DSAs with both organisations to allow NHS Digital to send data to the NHSBSA. In an ideal world, these NHS organisations would communicate directly with each other, share data and work to common standards – particularly in the support of research. Moreover, it is hard to understand why one government-funded organisation, NIHR, is required to pay substantial data access charges to two other government-funded organisations (NHS Digital and the NHSBSA) to access data that aims to improve government-funded, NHS clinical care.
The second cause of data loss described above is different in nature and relates to the unexpected loss of all dental procedure data for incomplete courses of dental treatment (as explained more fully in Chapter 4). Unfortunately, because one of the most common reasons for an incomplete course of dental treatment is an emergency admission to hospital, the data loss occurs with higher frequency in the case period than in the control periods of a case-crossover study, rendering this analysis unreliable. This unexpected data loss means we could not reliably interpret the data from this study on the association between IDP and admission to hospital with IE.
The data loss occurred only in association with incomplete courses of dental treatment, as the NHSBSA does not require dentists to provide the details of what dental treatment they provided up to the point when the course of treatment was discontinued. Dentists can simply tick a box indicating the band of treatment provided (Part 3 of the FP17 form) and that the course of treatment was not completed. In all other situations, dentists are required to complete Part 5a (Clinical Data Set) of the FP17 form, which records the dental procedures performed. It is hard to see why dentists are not required to provide this information for incomplete courses of dental treatment as well. Had this been the case, we would have been able to complete the study as planned.
The loss of clinical data on incomplete courses of treatment occurs because dentists are only required to record the start and end date of a course of treatment (or only the start date when the course of treatment is incomplete). This issue would be resolved if dentists recorded the precise date when each dental procedure was performed (rather than just the start and end date of a course of treatment). This was the case before the capitation system of payment to dentists was introduced in the UK and still is the case in many countries, including the USA. If the precise date on which each dental procedure was performed was recorded then, even if a course of treatment was interrupted by an emergency hospital admission, we would still know when any procedures prior to the course of treatment being abandoned were performed. Thus, we would not lose data on dental procedures performed just before an emergency hospital admission.
In addition, in doing so we would know more precisely when a procedure occurred in relationship to another event (e.g. hospital admission). With longer courses of dental treatment, the precise timing of a dental procedure is not known, as only the start and end date of a course of treatment are recorded. Although our data on the duration of courses of treatment (see Appendix 2, Figures 23 and 24) show that most courses of treatment were single visits, there was a long tail to the curve, confirming that some courses of treatment are weeks or even months long. In such cases, the start and end date only give us a window in which a procedure could have occurred; with long courses of dental treatment that window could be large, making the timing of procedures in relation to another event very imprecise.
Research
Although we were unable to answer questions about the relationship between IDPs and IE in England because the NHSBSA does not collect data on incomplete courses of dental treatment in association with emergency IE hospital admissions, this data loss will not necessarily affect dental data collected in other countries. We will therefore explore the possibility of performing this study elsewhere, where such data loss is not an issue.
The loss of dental data was confined to incomplete courses of dental treatment occurring immediately before unexpected emergency hospital admission for conditions that frequently result in death (e.g. IE, MI, stroke and PE). The loss was not seen with less urgent or less fatal hospital admission types. Presumably, this is because those affected have more opportunity to advise their dentist of their hospital admission and make arrangements to continue their treatment after discharge, and are more likely to survive their hospital admission and complete their dental treatment. Hence, the issues we identified are unlikely to afflict research into any association between IDPs and other less urgent or less fatal reasons for hospital admission.
Although we were unable to use a case-crossover study to investigate any association between IDPs and IE, this was entirely due to a problem with the dental procedure data collected from general dental practitioners in the community. Data on other types of medical or surgical procedure (including dental) performed in a hospital setting were not afflicted by this problem as they were collected, along with the IE admissions data, as part of the HES data set collected by NHS Digital for all inpatient (and outpatient) admissions. This means that, although we could not study the association between IDPs (performed in general dental practice) and IE, we can study the association between invasive medical and surgical procedures and subsequent IE using the case-crossover methodology. We can even study the relationship between IDPs and subsequent IE for the comparatively small number of dental procedures that are performed during a hospital inpatient or outpatient admission, rather than in general dental practice. Indeed, we have obtained funding from the British Heart Foundation (London, UK) to investigate the relationship between specific medical and surgical procedures occurring in the hospital setting (including hospital-based dental procedures) and subsequent IE. This research is ongoing.
Conclusion
Unfortunately, the missing data on incomplete courses of treatment concerning what dental procedures were performed by dentists in the weeks before patients were admitted to hospital with IE prevented definite conclusions being drawn about the relationship between IDPs and the subsequent risk of developing IE. Future studies using data systems in which such data are not missing may still be able to answer this question.
Acknowledgements
Patient and public involvement in this research
During the design and funding phase of the study, it was taken to two patient and public involvement (PPI) groups organised by the University of Sheffield and Sheffield Teaching Hospitals NHS Foundation Trust for evaluation and comment: (1) the Cardiovascular Research Patient Panel and (2) the Dental PPI Group. There were a total of 11 individuals in these groups.
Prior to meeting with them, both groups were sent a lay summary of the study. They were asked to evaluate how easy the summary was to read and their perceptions of the study’s value to the NHS; all 11 gave the top score for both. After receiving the responses, the study’s principal investigator presented an overview of the study and answered questions. They were asked about any ethical concerns – particularly relating to the linkage of individual data on two national data bases (patients were happy with our proposed arrangements for maintaining the anonymity of these data). Their feedback was used to improve the grant proposal and both groups were directly involved in editing the lay summary used in the grant submission and regulatory approvals.
Two PPI members were appointed to the Project Advisory Group and participated in Project Advisory Group meetings.
Contributions of authors
Martin H Thornhill (https://orcid.org/0000-0003-0681-4083) (Professor of Translational Research in Dentistry) contributed to the study design, study management, data interpretation and first draft of the final report.
Annabel Crum (https://orcid.org/0000-0001-9310-8536) (Data Specialist) contributed to the data specification, safeguarding, management and analysis.
Saleema Rex (https://orcid.org/0000-0002-0318-6125) (Statistician) contributed to the development of the statistical analysis plan and statistical analysis of the data.
Richard Campbell (https://orcid.org/0000-0002-0975-9270) (Data Specialist) contributed to the data management, data analysis and editing of the final report.
Tony Stone (https://orcid.org/0000-0002-0167-3800) (Senior Data Manager) contributed to the data management, data safeguarding and oversight of the data analysis.
Mike Bradburn (https://orcid.org/0000-0002-3783-9761) (Senior Statistician) contributed to the development of the statistical analysis plan and statistical analysis of the data.
Veronica Fibisan (https://orcid.org/0000-0001-9082-8255) (Study Manager) contributed to the writing, editing and reviewing of the final report.
Mark J Dayer (https://orcid.org/0000-0002-0976-9743) (Consultant Cardiologist) contributed to the original study design, data interpretation and cardiological aspects of the study.
Bernard D Prendergast (https://orcid.org/0000-0002-6031-2124) (Consultant Cardiologist) contributed to the original study design, data interpretation and cardiological aspects of the study.
Peter B Lockhart (https://orcid.org/0000-0003-3293-3430) (Research Professor in Oral Medicine and Dental Research) contributed to the original study design, data interpretation and dental aspects of the study.
Larry M Baddour (https://orcid.org/0000-0002-4473-7077) (Professor of Medicine, Infectious Diseases) contributed to the original study design, data interpretation and infectious disease aspects of the study.
Jon Nicholl (https://orcid.org/0000-0001-5436-1264) (Professor of Health Services Research) contributed to the study design, study management, data/statistical analysis, data interpretation and drafting of the final report.
All authors contributed to, edited or reviewed the final report.
Publication
Cahill TJ, Dayer M, Prendergast B, Thornhill M. Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ 2017;358:j3942.
Data-sharing statement
The original data, from which the aggregated data shown in this report are derived, is the subject of DSAs between the University of Sheffield, NHS Digital and the NHSBSA. These agreements restrict the sharing of data and require its destruction after study completion. We are, therefore, unable to share the original data. However, the NHS Digital and NHSBSA data we used may be obtained by application to these organisations after appropriate regulatory approval. All data requests should be submitted to the corresponding author for consideration.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Thornhill M, Nicholl J, Prendergast B, Dayer M, Lockhart P. Protocol (Version 1.0): NIHR–HTA Funded Study: 15 57 32. The Invasive Dentistry–Endocarditis Association Study – The IDEA Study 2016. www.fundingawards.nihr.ac.uk/award/15/57/32 (accessed 4 April 2022).
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2015;387:882-93. https://doi.org/10.1016/S0140-6736(15)00067-7.
- Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011;342. https://doi.org/10.1136/bmj.d2392.
- Thornhill MH, Dayer MJ, Nicholl J, Prendergast BD, Lockhart PB, Baddour LM. An alarming rise in incidence of infective endocarditis in England since 2009: why?. Lancet 2020;395:1325-7. https://doi.org/10.1016/S0140-6736(20)30530-4.
- Thornhill MH, Dayer MJ, Prendergast B, Baddour LM, Jones S, Lockhart PB. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother 2015;70:2382-8. https://doi.org/10.1093/jac/dkv115.
- Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219-28. https://doi.org/10.1016/S0140-6736(14)62007-9.
- Thornhill MH, Jones S, Prendergast B, Baddour LM, Chambers JB, Lockhart PB, et al. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. Eur Heart J 2018;39:586-95. https://doi.org/10.1093/eurheartj/ehx655.
- Cahill TJ, Dayer M, Prendergast B, Thornhill M. Do patients at risk of infective endocarditis need antibiotics before dental procedures?. BMJ 2017;358. https://doi.org/10.1136/bmj.j3942.
- Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. https://doi.org/10.1093/eurheartj/ehp285.
- National Institute for Health and Care Excellence (NICE) . Prophylaxis Against Infective Endocarditis Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures 2008. www.nice.org.uk/guidance/cg64/ (accessed 19 June 2015).
- Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736-54. https://doi.org/10.1161/CIRCULATIONAHA.106.183095.
- Lewis T, Grant R. Observations relating to subacute infective endocarditis. Heart 1923;10:21-77.
- Okell CC, Elliott SD. Bacteraemia and oral sepsis: with special reference to the aetiology of subacute endocarditis. Lancet 1935;226:869-72. https://doi.org/10.1016/S0140-6736(00)47788-3.
- Glaser RJ, Dankner A, Mathes SB, Harfoed CG. Effect of penicillin on the transient bacteremia following dental extraction. Am J Med 1947;3. https://doi.org/10.1016/0002-9343(47)90116-2.
- Hirsch HL, Vivino JJ, Merril A, Dowling HF. Effect of prophylactically administered penicillin on incidence of bacteremia following extraction of teeth. Arch Intern Med 1948;81:868-78. https://doi.org/10.1001/archinte.1948.00220240077005.
- American Heart Association . Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1960;21:151-5. https://doi.org/10.1161/01.CIR.21.1.151.
- Cahill TJ, Harrison JL, Jewell P, Onakpoya I, Chambers JB, Dayer M, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart 2017;103:937-44. https://doi.org/10.1136/heartjnl-2015-309102.
- Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc 2009;140:1238-44. https://doi.org/10.14219/jada.archive.2009.0046.
- Roberts GJ. Dentists are innocent! ‘Everyday’ bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr Cardiol 1999;20:317-25. https://doi.org/10.1007/s002469900477.
- Thornhill MH, Dayer MJ, Durkin MJ, Lockhart PB, Baddour LM. Risk of adverse reactions to oral antibiotics prescribed by dentists. J Dent Res 2019;98:1081-7. https://doi.org/10.1177/0022034519863645.
- Lesho EP, Laguio-Vila M. The slow-motion catastrophe of antimicrobial resistance and practical interventions for all prescribers. Mayo Clin Proc 2019;94:1040-7. https://doi.org/10.1016/j.mayocp.2018.11.005.
- National Institute for Health and Care Excellence (NICE) . Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. 2008. www.nice.org.uk/guidance/cg64 (accessed March 2008).
- Watkin RW, Roberts GJ, Elliott TS. BSAC Working Party on Infective Endocarditis . New guidance from NICE regarding antibiotic prophylaxis for infective endocarditis – response by the BSAC working party. J Antimicrob Chemother 2008;62:1477-8. https://doi.org/10.1093/jac/dkn404.
- Nakatani S, Ohara T, Ashihara K, Izumi C, Iwanaga S, Eishi K, et al. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J 2019;83:1767-809. https://doi.org/10.1253/circj.CJ-19-0549.
- National Institute for Health and Care Excellence (NICE) . Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures – Recommendations 2008. www.nice.org.uk/guidance/cg64/chapter/Recommendations (accessed 28 November 2016).
- Thornhill MH, Dayer M, Lockhart PB, McGurk M, Shanson D, Prendergast B, et al. A change in the NICE guidelines on antibiotic prophylaxis. Br Dent J 2016;221:112-14. https://doi.org/10.1038/sj.bdj.2016.554.
- Thornhill MH, Lockhart PB, Prendergast B, Chambers JB, Shanson D. NICE and antibiotic prophylaxis to prevent endocarditis. Br Dent J 2015;218:619-21. https://doi.org/10.1038/sj.bdj.2015.496.
- Rawlins MD. National Institute for Clinical Excellence: NICE works. J R Soc Med 2015;108:211-19. https://doi.org/10.1177/0141076815587658.
- Lacassin F, Hoen B, Leport C, Selton-Suty C, Delahaye F, Goulet V, et al. Procedures associated with infective endocarditis in adults. A case control study. Eur Heart J 1995;16:1968-74. https://doi.org/10.1093/oxfordjournals.eurheartj.a060855.
- Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med 1998;129:761-9. https://doi.org/10.7326/0003-4819-129-10-199811150-00002.
- Tubiana S, Blotiére PO, Hoen B, Lesclous P, Millot S, Rudant J, et al. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: nationwide population based cohort and a case crossover study. BMJ 2017;358. https://doi.org/10.1136/bmj.j3776.
- Porat Ben-Amy D, Littner M, Siegman-Igra Y. Are dental procedures an important risk factor for infective endocarditis? A case-crossover study. Eur J Clin Microbiol Infect Dis 2009;28:269-73. https://doi.org/10.1007/s10096-008-0622-3.
- Chen PC, Tung YC, Wu PW, Wu LS, Lin YS, Chang CJ, et al. Dental procedures and the risk of infective endocarditis. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000001826.
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133:144-53. https://doi.org/10.1093/oxfordjournals.aje.a115853.
- Maclure M, Mittleman MA. Should we use a case-crossover design?. Annu Rev Public Health 2000;21:193-221. https://doi.org/10.1146/annurev.publhealth.21.1.193.
- Starkebaum M, Durack D, Beeson P. The ‘incubation period’ of subacute bacterial endocarditis. Yale J Biol Med 1977;50:49-58.
- NHS Digital . Hospital Episode Statistics (HES) n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 24 April 2022).
- Great Britain . The Health Service (Control of Patient Information) Regulations 2002 2002.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. https://doi.org/10.1093/eurheartj/ehv319.
- NHS Data Model and Dictionary Service . NHS Data Model and Dictionary n.d. www.datadictionary.nhs.uk (accessed 9 July 2020).
- Fawcett N, Young B, Peto L, Quan TP, Gillott R, Wu J, et al. ‘Caveat emptor’: the cautionary tale of endocarditis and the potential pitfalls of clinical coding data – an electronic health records study. BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1390-x.
- Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. Circulation 1997;96:358-66. https://doi.org/10.1161/01.cir.96.1.358.
- Chu VH, Sexton DJ, Cabell CH, Reller LB, Pappas PA, Singh RK, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis 2005;41:406-9. https://doi.org/10.1086/431590.
- NHS Digital . NHS Dental Statistics for England – 2020–2021 Annual Report n.d. www.gov.uk/government/statistics/nhs-dental-statistics-for-england-2020-21-annual-report (accessed 30 November 2021).
- Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics 1995;51:228-35. https://doi.org/10.2307/2533328.
- Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med 2006;25:2618-31. https://doi.org/10.1002/sim.2477.
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768-97. https://doi.org/10.1002/sim.2302.
- HyLown Consulting LLC. Calculate Sample Size Needed to Test Relative Incidence in Self Controlled Case Series Studies: SCCS, Alt-2 n.d. http://powerandsamplesize.com/Calculators/Test-Relative-Incidence-in-Self-Controlled-Case-Series-Studies/SCCS-Alt-2 (accessed 24 April 2022).
- STROBE . STROBE Checklists n.d. www.strobe-statement.org/checklists/ (accessed 28 April 2022).
- Great Britain . Health and Social Care Act 2012 2012.
- Thornhill MH, Dayer M, Lockhart PB, McGurk M, Shanson D, Prendergast B, et al. Guidelines on prophylaxis to prevent infective endocarditis. Br Dent J 2016;220:51-6. https://doi.org/10.1038/sj.bdj.2016.49.
- World Health Organization . International Classification of Diseases 11th Revision n.d. https://icd.who.int/en (accessed 5 April 2022).
- NHS Digital . ICD-10 n.d. https://hscic.kahootz.com/t_c_home/view?objectID=14232080 (accessed 18 May 2022).
- NHS Digital . OPCS-4 n.d. https://hscic.kahootz.com/t_c_home/view?objectID=14270896 (accessed 18 May 2022).
Appendix 1 Supplementary tables
| Cardiac condition | ICD-10a diagnosis codes and OPCS-4b procedure codes for identifying those at high risk of IEc |
|---|---|
| Previous IE | ICD-10 diagnosis codes:
|
| Prosthetic replacement of heart valve | OPCS-4 procedure codes:
|
| Valve repair using prosthetic material | OPCS-4 procedure codes:
|
| Prosthetic heart or ventricular assist device | OPCS-4 procedure codes:
|
| Congenital heart condition for which a palliative shunt or conduit has been used | OPCS-4 procedure codes:
|
| Unrepaired cyanotic congenital heart conditiond | ICD-10 diagnosis codes:
|
| Completely repaired congenital heart condition defect with prosthetic material or device, whether placed by surgery or catheter intervention, during first 6 months after the procedure onlye | OPCS-4 procedure codes:
|
| Cardiac condition | ICD-10a diagnosis codes for identifying those at moderate risk of IEb |
|---|---|
| Previous rheumatic fever | ICD-10 diagnosis codes:
|
| Non-rheumatic valve disease | ICD-10 diagnosis codes:
|
| Hypertrophic cardiomyopathy | ICD-10 diagnosis codes:
|
| Congenital valve anomalies | ICD-10 diagnosis codes:
|
| IE data manipulations | Count (n) | Comments |
|---|---|---|
| Split IE records | ||
| IE narrow | 16,845 | IE consultant episodes identified by the narrow definition of IE |
| IE broad | 26,356 | IE consultant episodes identified by the broad definition of IE |
| Removed IE non-index data | ||
| IE narrow | 11,574 | 5271 non-index admissions removed |
| IE broad | 17,741 | 8615 non-index admissions removed |
| Removed all elective IE admissions | ||
| IE narrow | 10,436 | 1138 elective admissions removed |
| IE broad | 16,345 | 2458 elective admissions removed |
| Removed IE admissions with hospital stay of < 3 days (if discharged alive) | ||
| IE narrow | 8034 | 2402 hospital admissions of < 3 days removed |
| IE broad | 11,298 | 5047 hospital admissions of < 3 days removed |
| Number of distinct IE admissions per patient | Period between distinct IE admissions, n (%) | ||||
|---|---|---|---|---|---|
| < 6 months | 6–12 months | 13–18 months | > 18 months | Overall | |
| Using the narrow definition of IE | |||||
| 1 | 8388 (74.34) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 8388 (74.34) |
| 2 | 1751 (15.52) | 50 (0.44) | 30 (0.27) | 66 (0.58) | 1897 (16.81) |
| 3 | 587 (5.20) | 23 (0.20) | 13 (0.12) | 43 (0.38) | 666 (5.90) |
| 4 | 104 (0.92) | 19 (0.17) | 11 (0.10) | 21 (0.19) | 155 (1.37) |
| 5 | 29 (0.26) | 1 (0.01) | 6 (0.05) | 6 (0.05) | 42 (0.37) |
| > 5 | 104 (0.92) | 5 (0.04) | 6 (0.05) | 20 (0.18) | 135 (1.20) |
| Total | 10,963 (97.16) | 98 (0.87) | 66 (0.58) | 156 (1.38) | 11,283 (100.00) |
| Using the broad definition of IE | |||||
| 1 | 12,567 (73.29) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 12,567 (73.29) |
| 2 | 2522 (14.71) | 116 (0.68) | 60 (0.35) | 104 (0.61) | 2802 (16.34) |
| 3 | 962 (5.61) | 69 (0.40) | 28 (0.16) | 76 (0.44) | 1135 (6.62) |
| 4 | 209 (1.22) | 36 (0.21) | 13 (0.08) | 55 (0.32) | 313 (1.83) |
| 5 | 57 (0.33) | 10 (0.06) | 13 (0.08) | 20 (0.12) | 100 (0.58) |
| > 5 | 144 (0.84) | 21 (0.12) | 15 (0.09) | 49 (0.29) | 229 (1.34) |
| Total | 16,461 (96.00) | 252 (1.47) | 129 (0.75) | 304 (1.77) | 17,146 (100.00) |
| Dental data manipulations | Count (n) | Comments |
|---|---|---|
| Imported dental treatment records (courses of treatment) | 3,751,621 | Dental data file received from the NHSBSA |
| Number with missing treatment acceptance (start) date | 0 | |
| Number with missing treatment end date | 7 | |
| Number of invalid treatment end dates removed | 5886 | |
| Number of unique dental treatment records | 3,750,484 | Determined using HES identifier, acceptance and treatment end date |
| Duplicate records | 3206 | Determined using HES identifier, acceptance and treatment end date |
| Number of dental records taken forward | 3,748,881 | |
| Dental data matched with any associated IE admissions data | 51,181 | Dental data without any associated IE admissions data removed |
| Combining overlapping courses of dental treatment | 50,785 |
| Data description | IE admissions (n) | Patient count (n) |
|---|---|---|
| IE (narrow definition) linked to 0–12 months dental data | 3163 | 3115 |
| IE (broad definition) linked to 0–12 months dental data | 4293 | 4217 |
| IE (narrow definition) linked to 1–13 months dental data (first month skipped) | 3135 | 3094 |
| IE (broad definition) linked to 1–13 months dental data (first month skipped) | 4252 | 4187 |
Appendix 2 Supplementary figures
FIGURE 19.
Age distribution of all IE cases using the narrow definition of IE.
FIGURE 20.
Age distribution of IE cases with linked dental data using the narrow definition of IE.
FIGURE 21.
Length of hospital stay for all IE cases using the narrow definition of IE. Length of stay restricted to 100 days maximum.
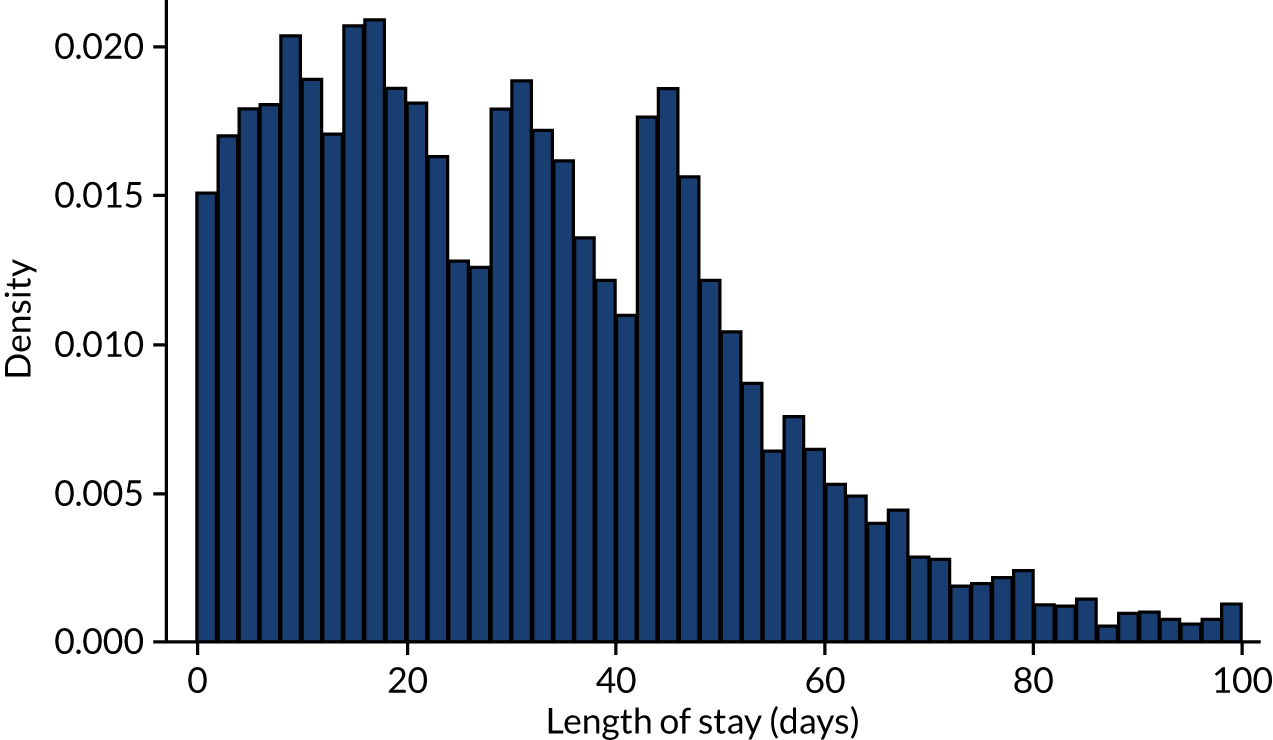
FIGURE 22.
Length of hospital stay for all IE cases with linked dental data using the narrow definition of IE. Length of stay restricted to 100 days maximum.
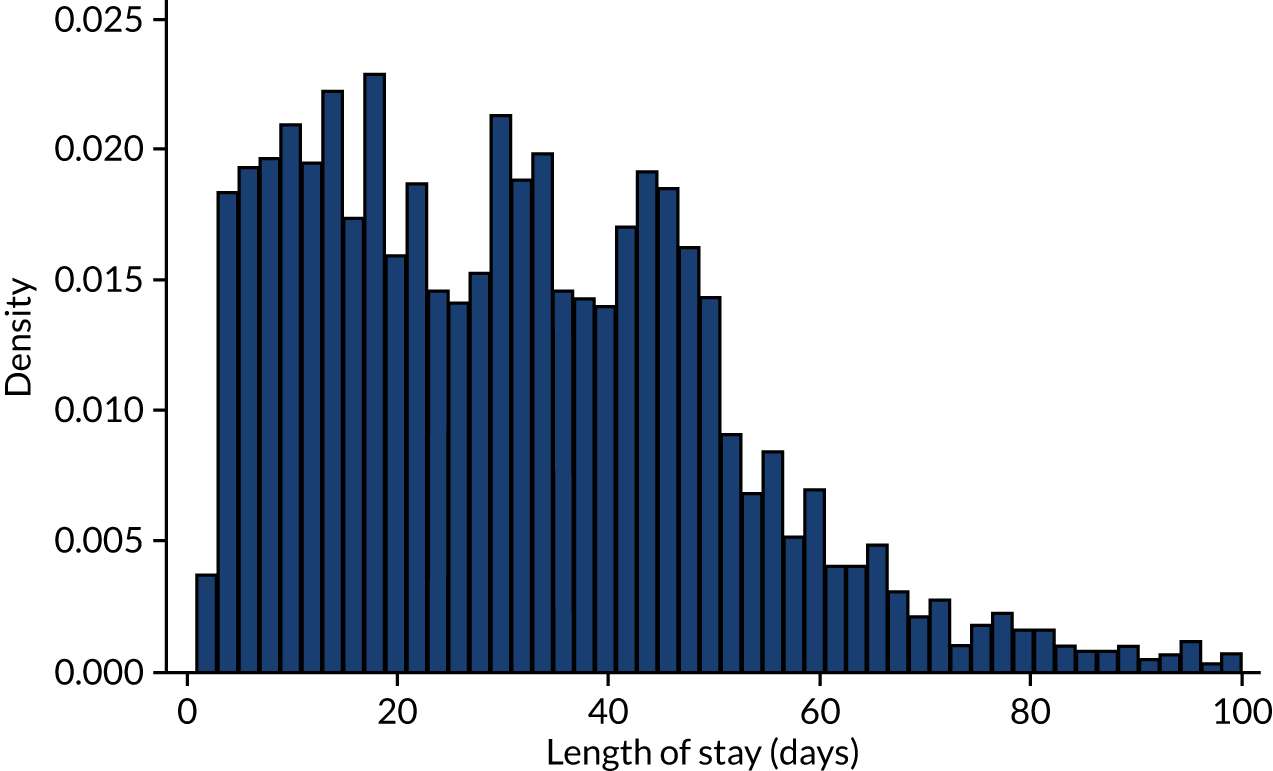
FIGURE 23.
Duration of courses of dental treatment (all) in days. Length of course restricted to 100 days maximum.
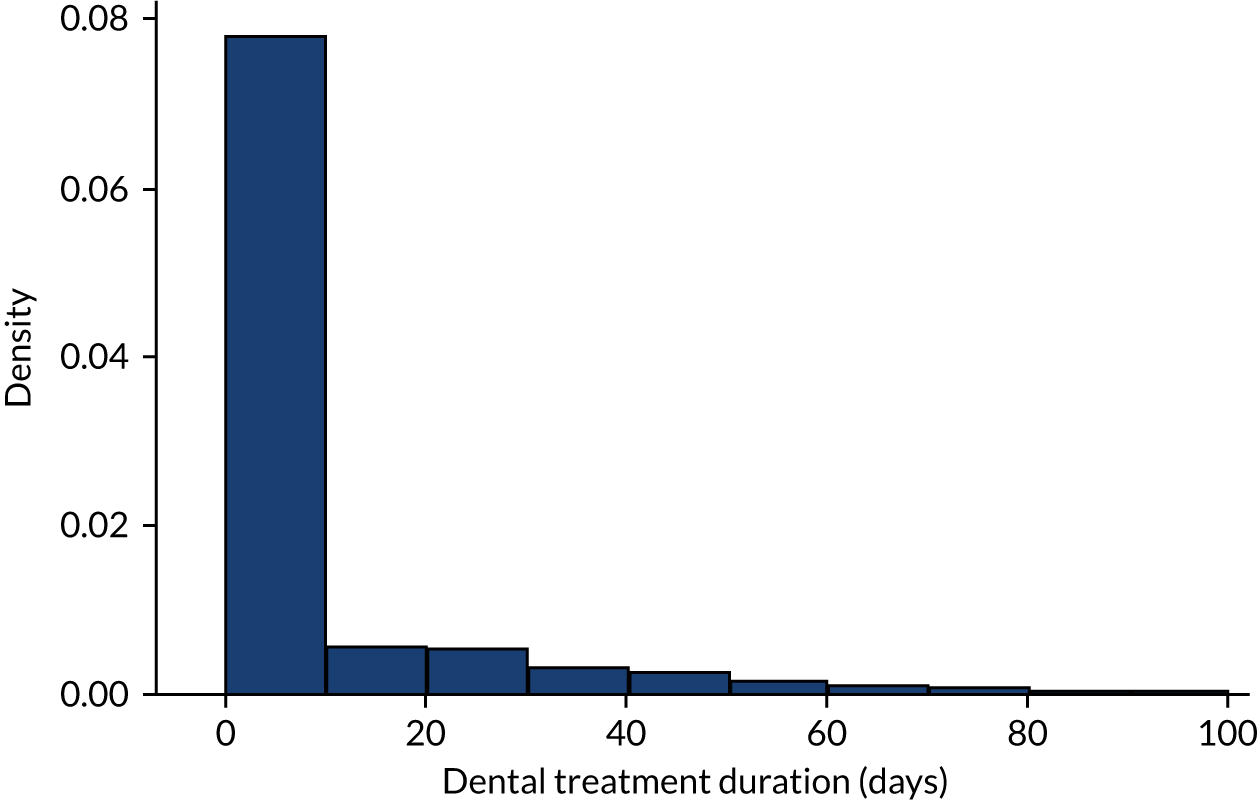
FIGURE 24.
Duration of courses of dental treatment matched to IE admissions in days. Length of course restricted to 100 days maximum.
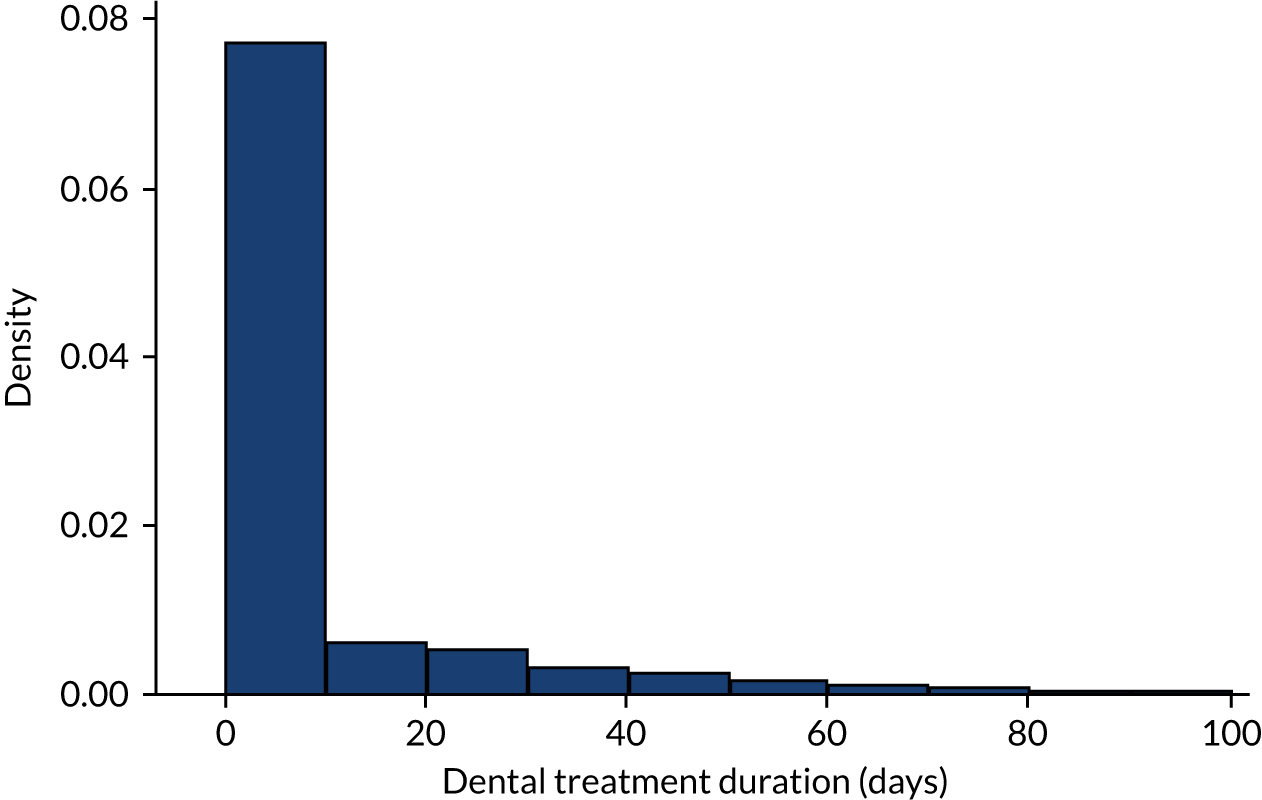
FIGURE 25.
Monthly number of extraction or non-extraction dental visits in those at high, moderate, or low/unknown risk of IE in the 12 months before IE admission. (a) Non-extraction; and (b) extraction. The x-axis shows the number of months before an IE hospital admission, with the time of admission being week 0 and using the start date for each course of dental treatment.
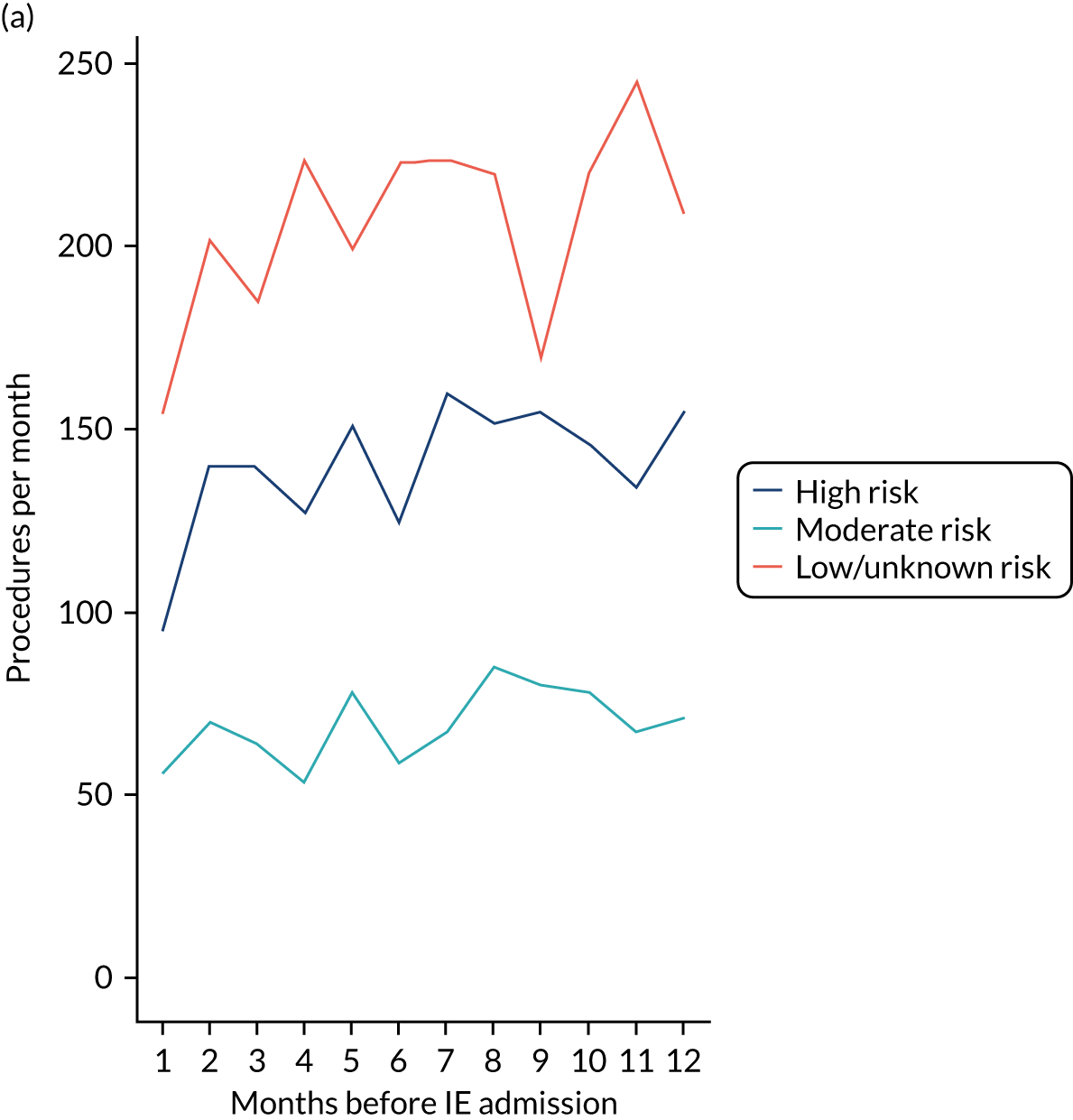
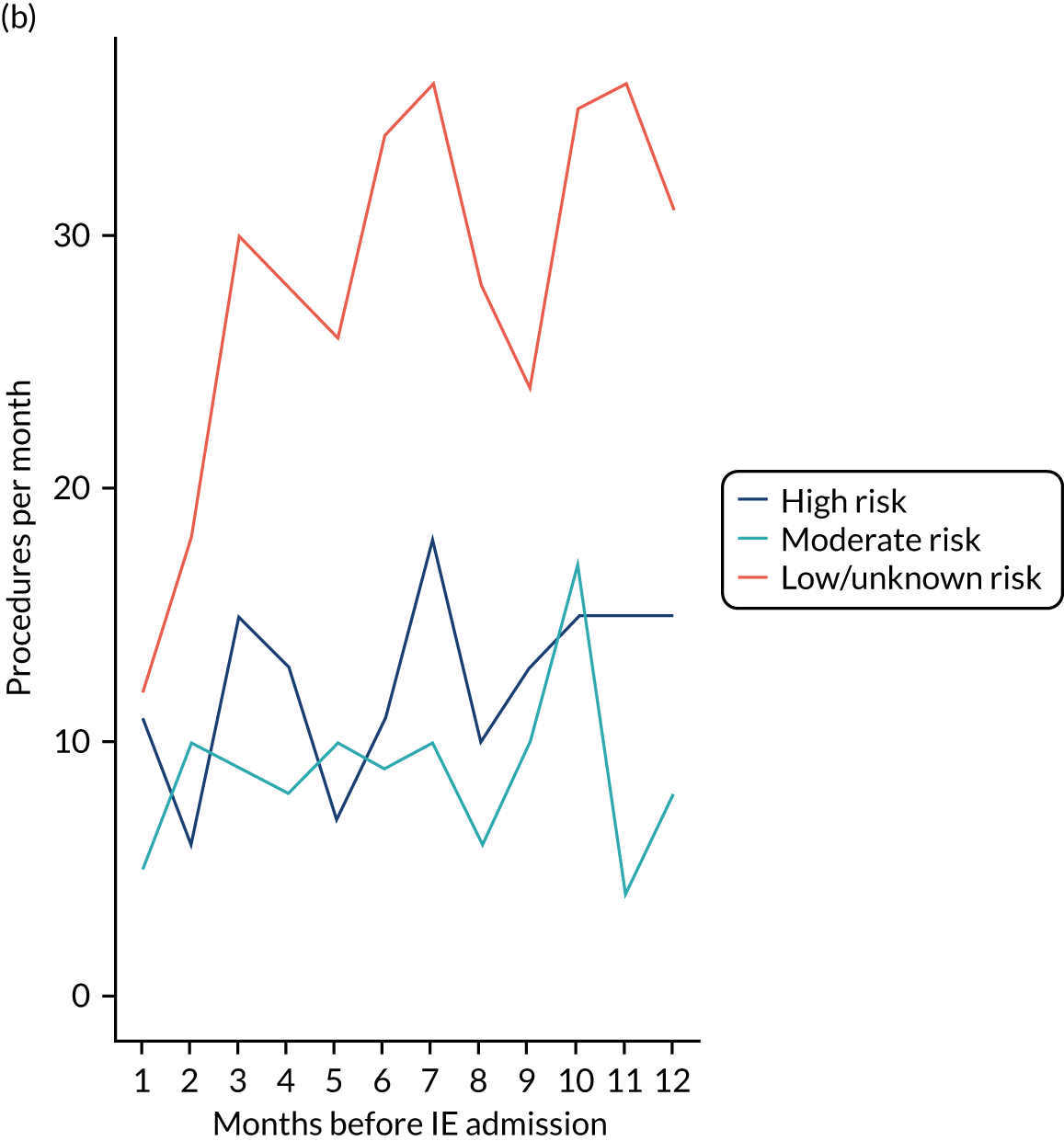
FIGURE 26.
Monthly number of extraction or non-extraction dental visits in the 12 months before hospital admission for IE (broad and narrow definitions), MI, PE or stroke. (a) Broad definition of IE, non-extraction; (b) broad definition of IE, extraction; (c) narrow definition of IE, non-extraction; (d) narrow definition of IE, extraction; (e) MI, non-extraction; (f) MI, extraction; (g) PE, non-extraction; (h) PE, extraction; (i) stroke, non-extraction; and (j) stroke, extraction.
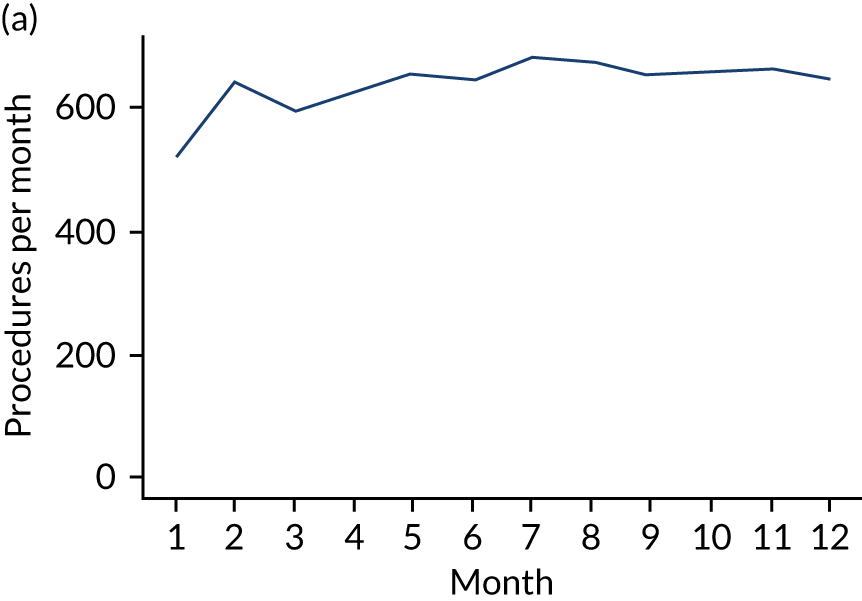
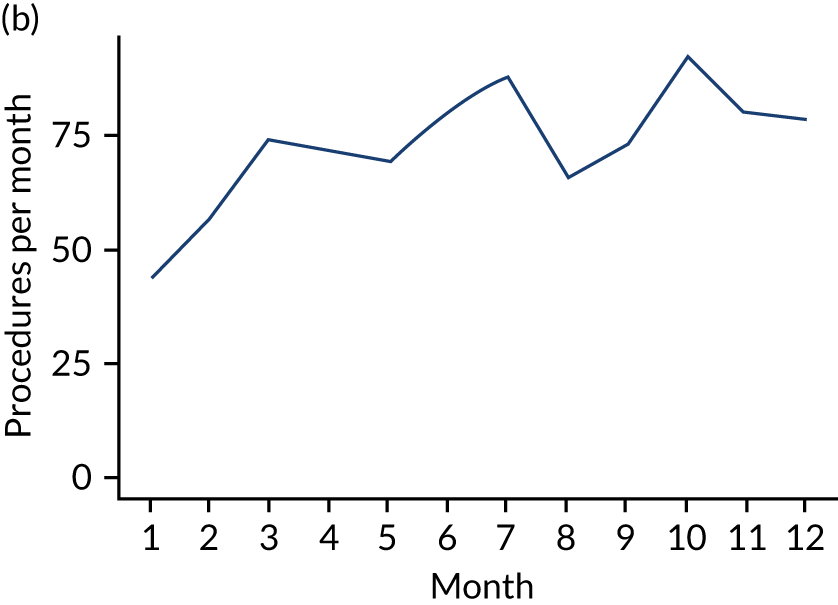
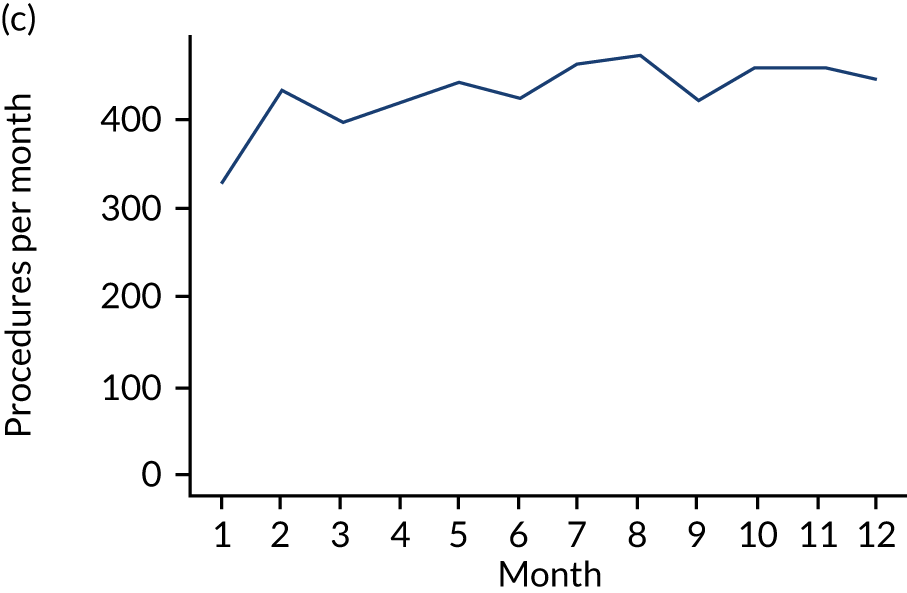
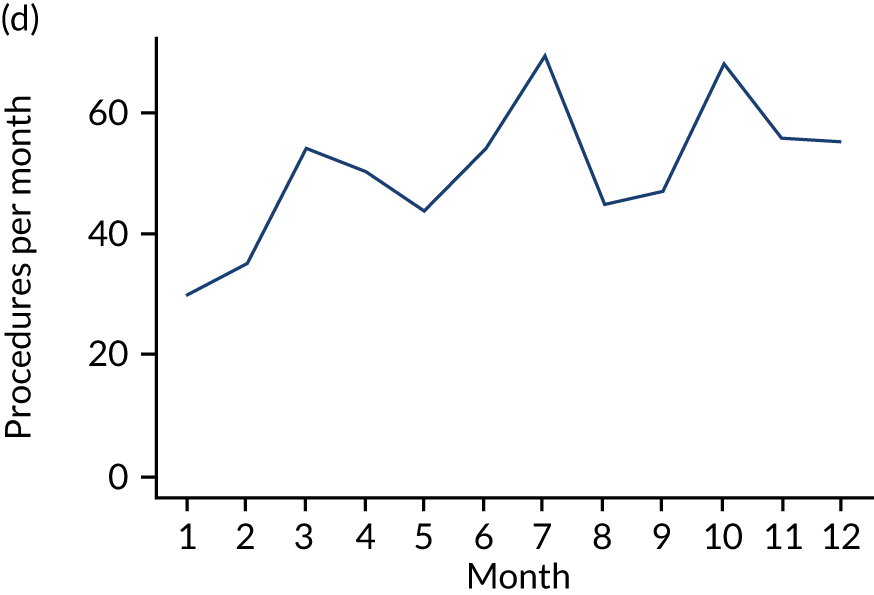
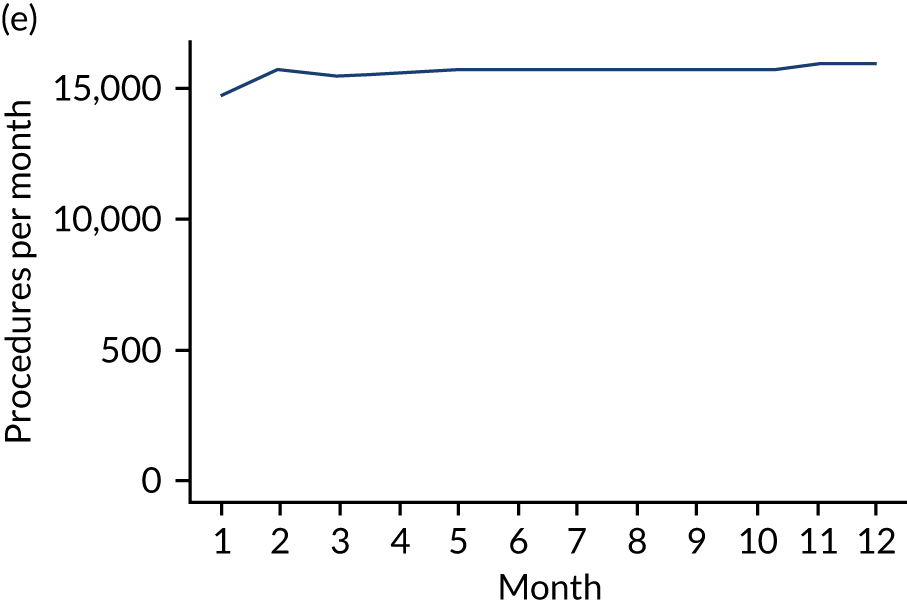
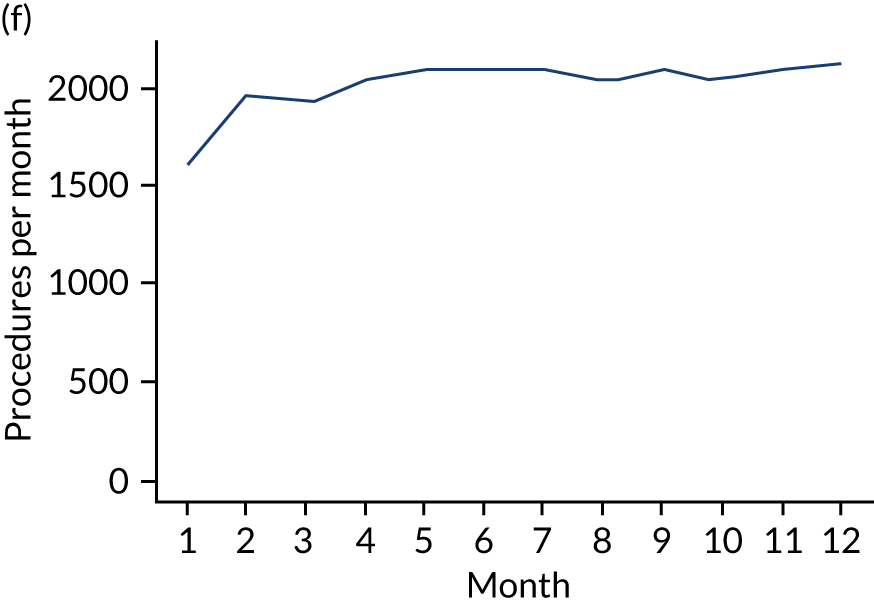
FIGURE 27.
All courses of dental treatment and single-visit courses of treatment by procedure type over the 13 months before IE admission. (a) All types of dental procedure; (b) IDPs; (c) indeterminate dental procedures; and (d) non-invasive dental procedures.
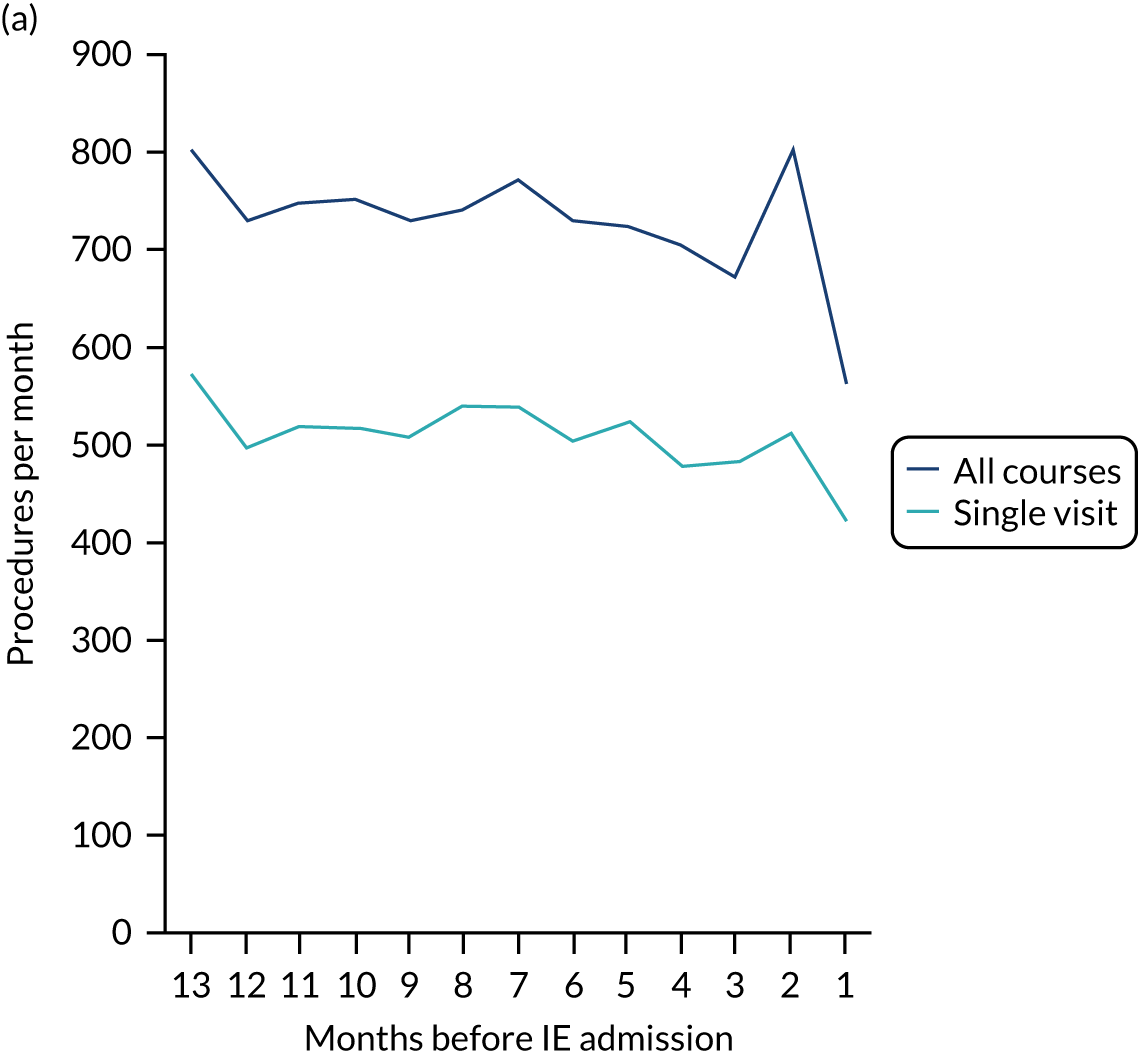
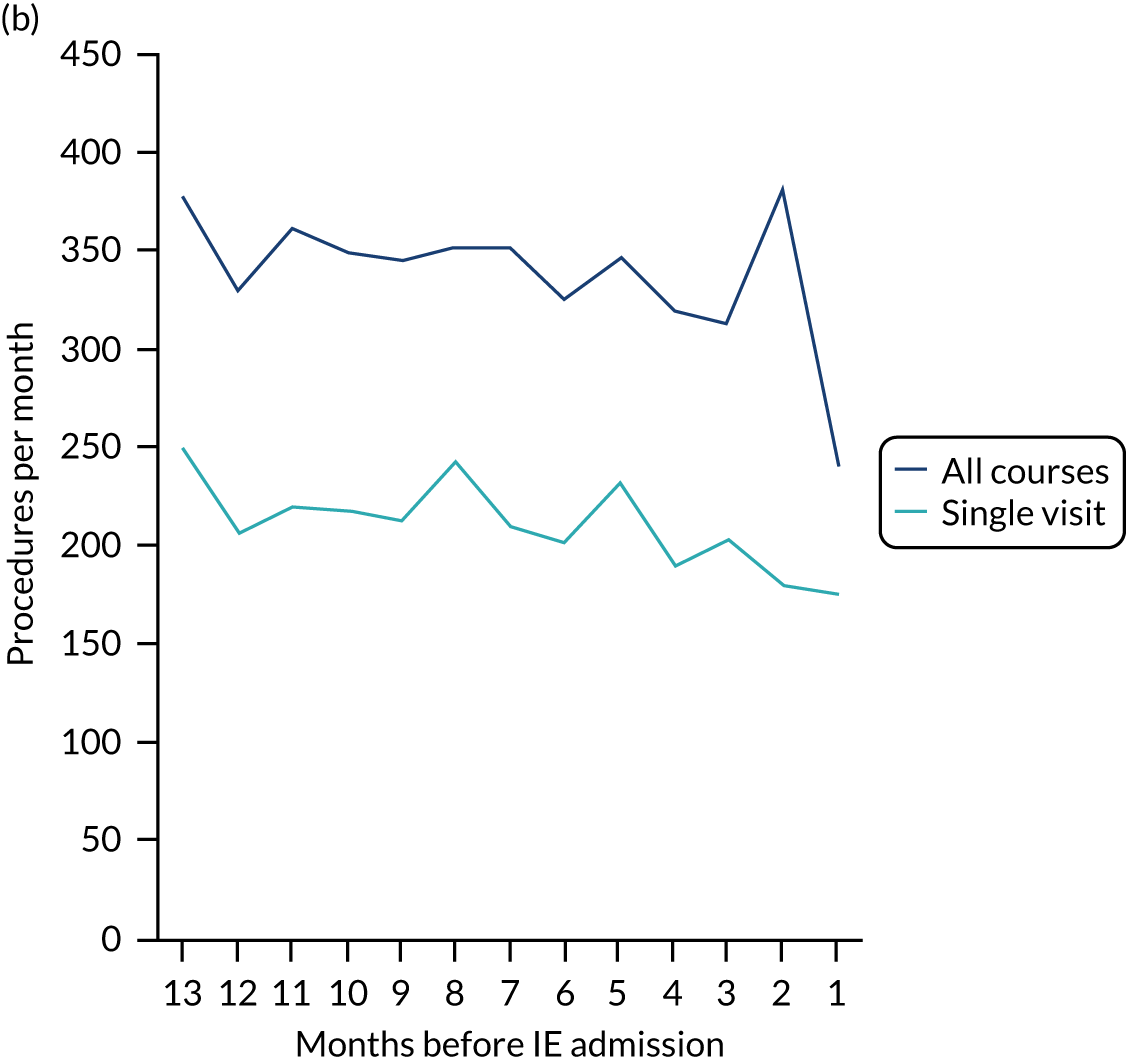
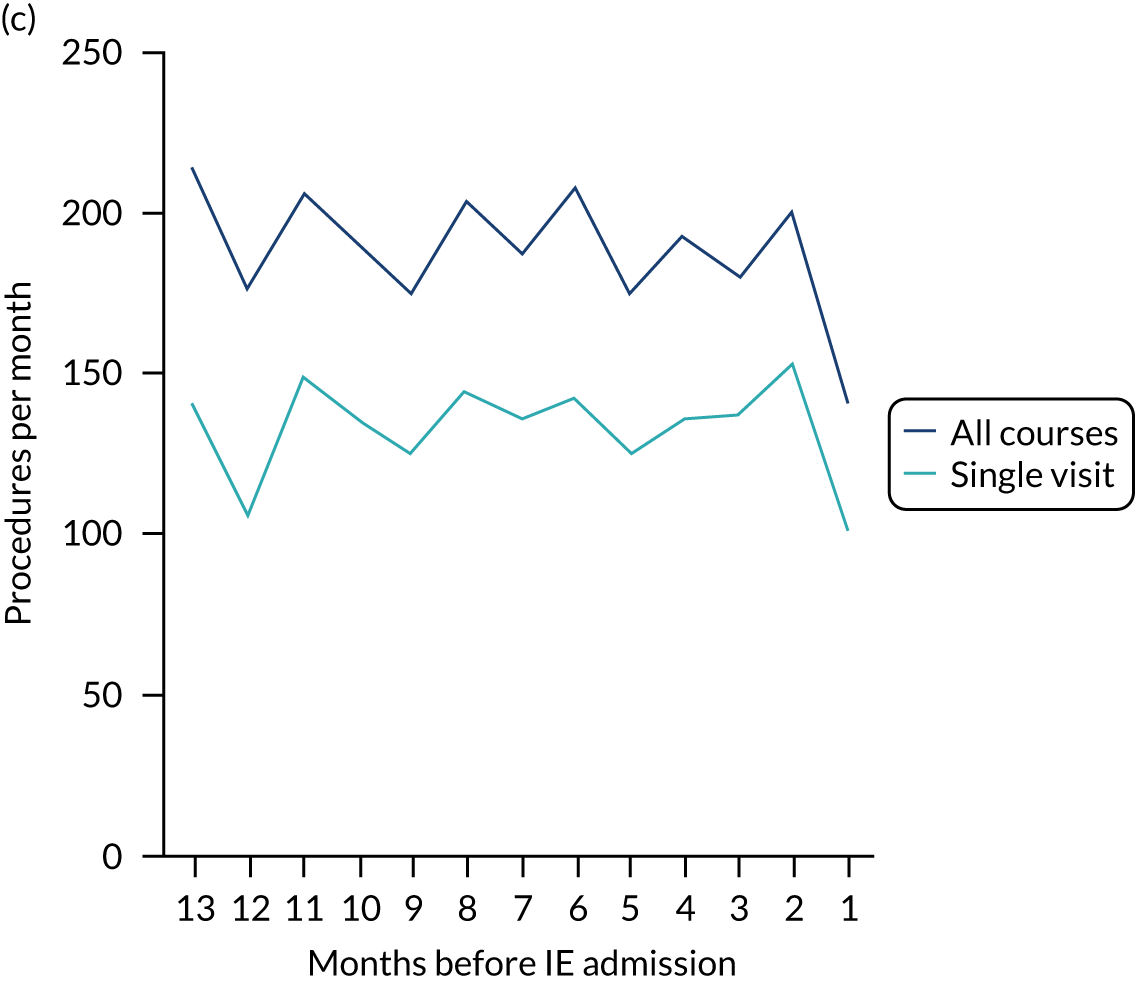
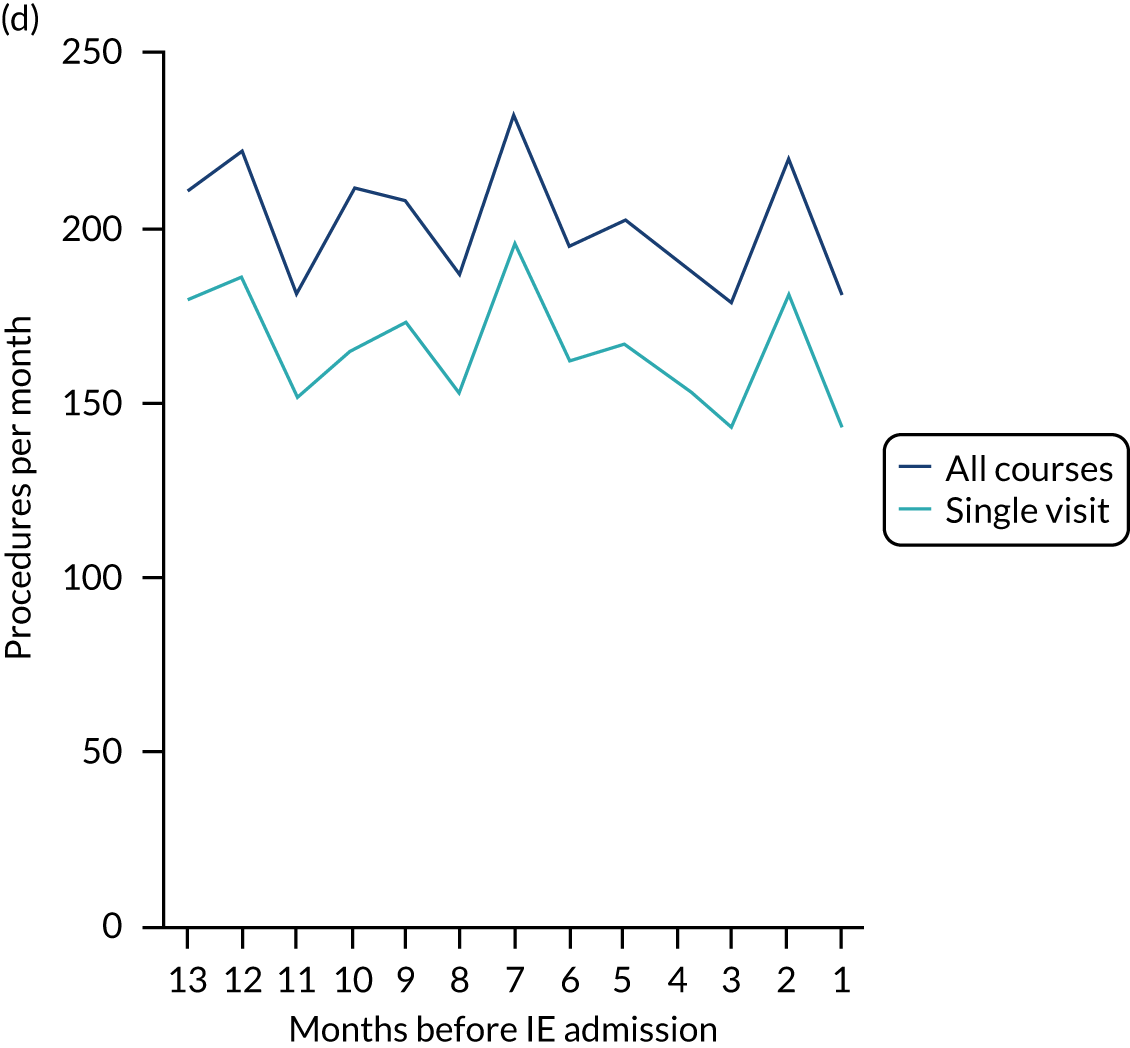
FIGURE 28.
All courses of IDPs and single-visit courses of IDPs by IDP type over the 13 months before IE admission. (a) All types of IDPs; (b) endodontic procedures; (c) dental extraction procedures; and (d) scale and polishes.
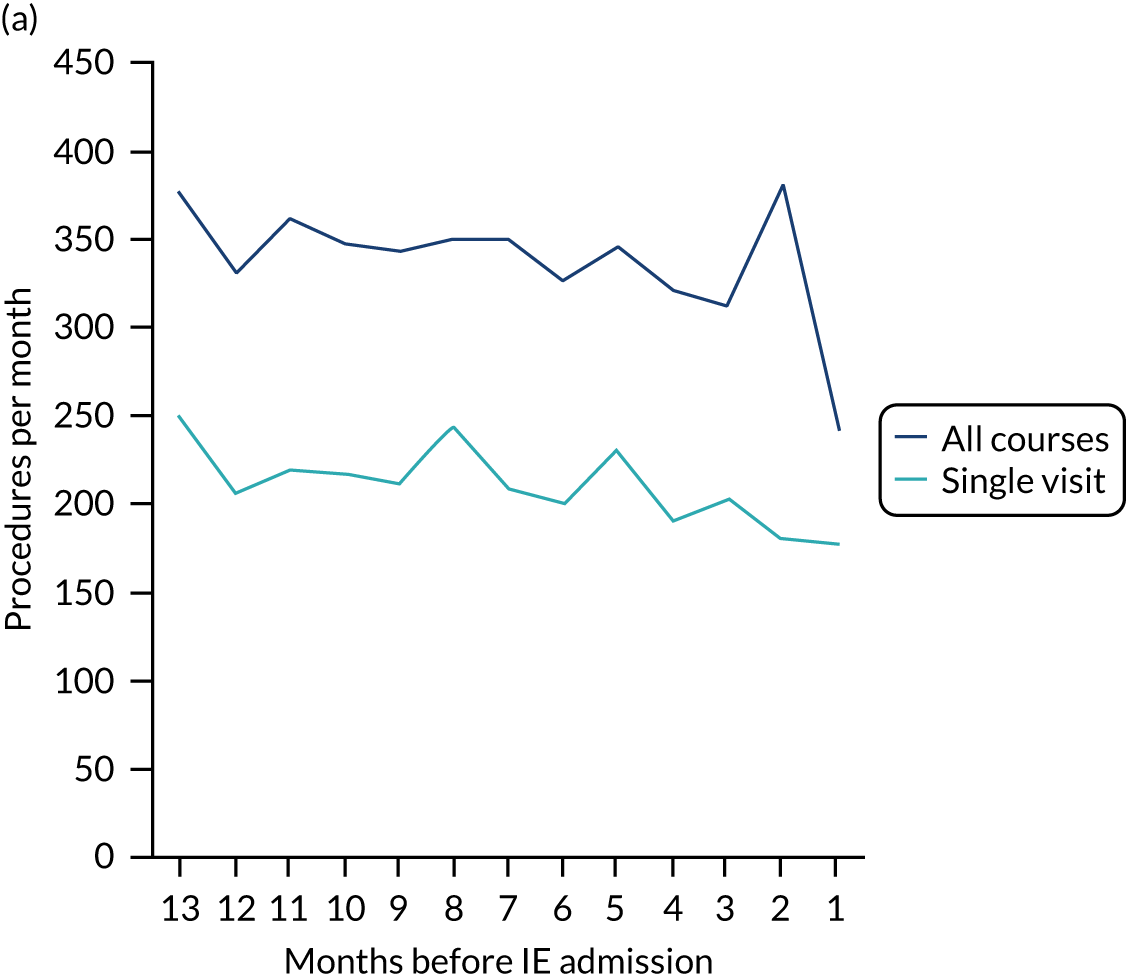
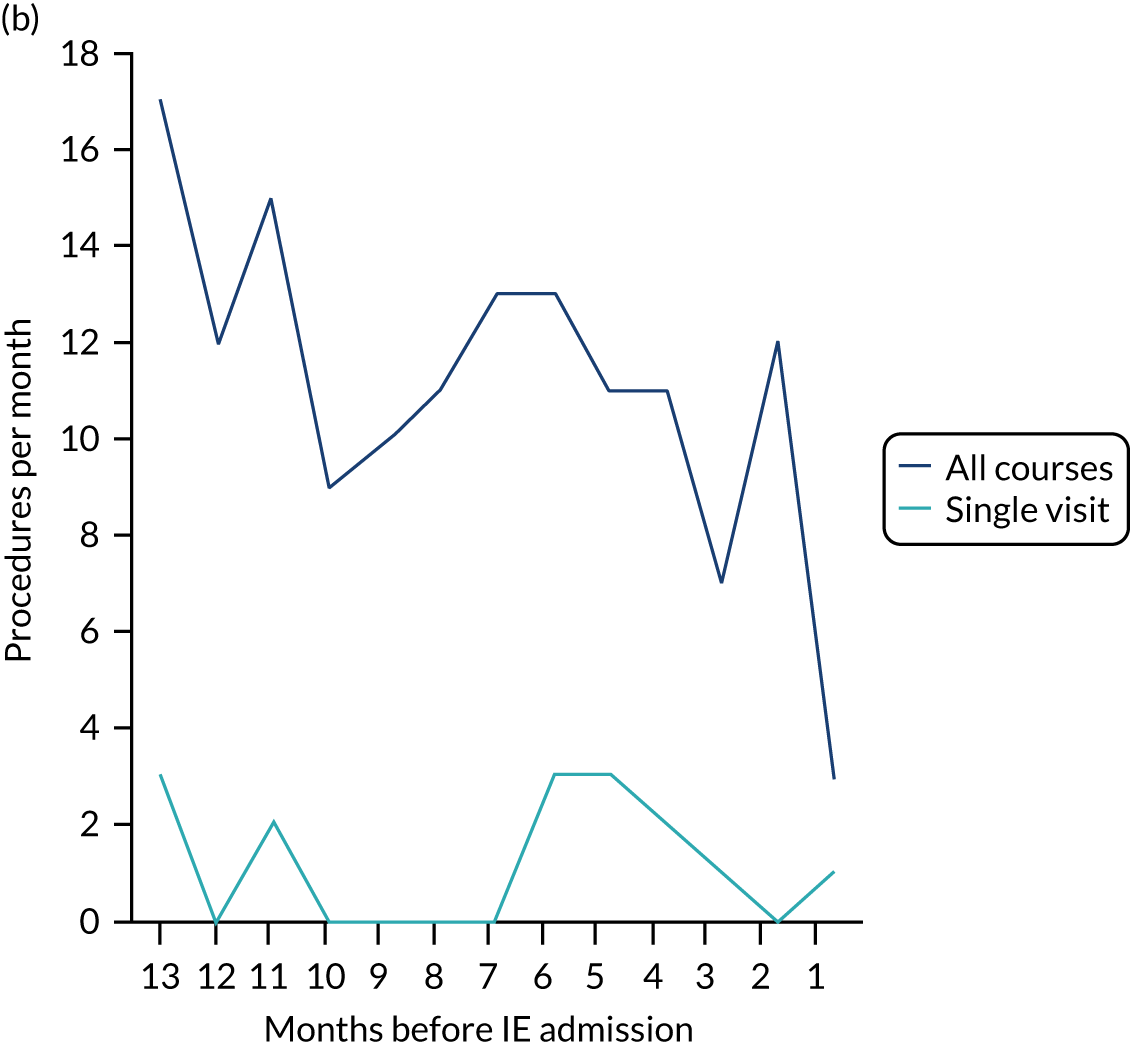
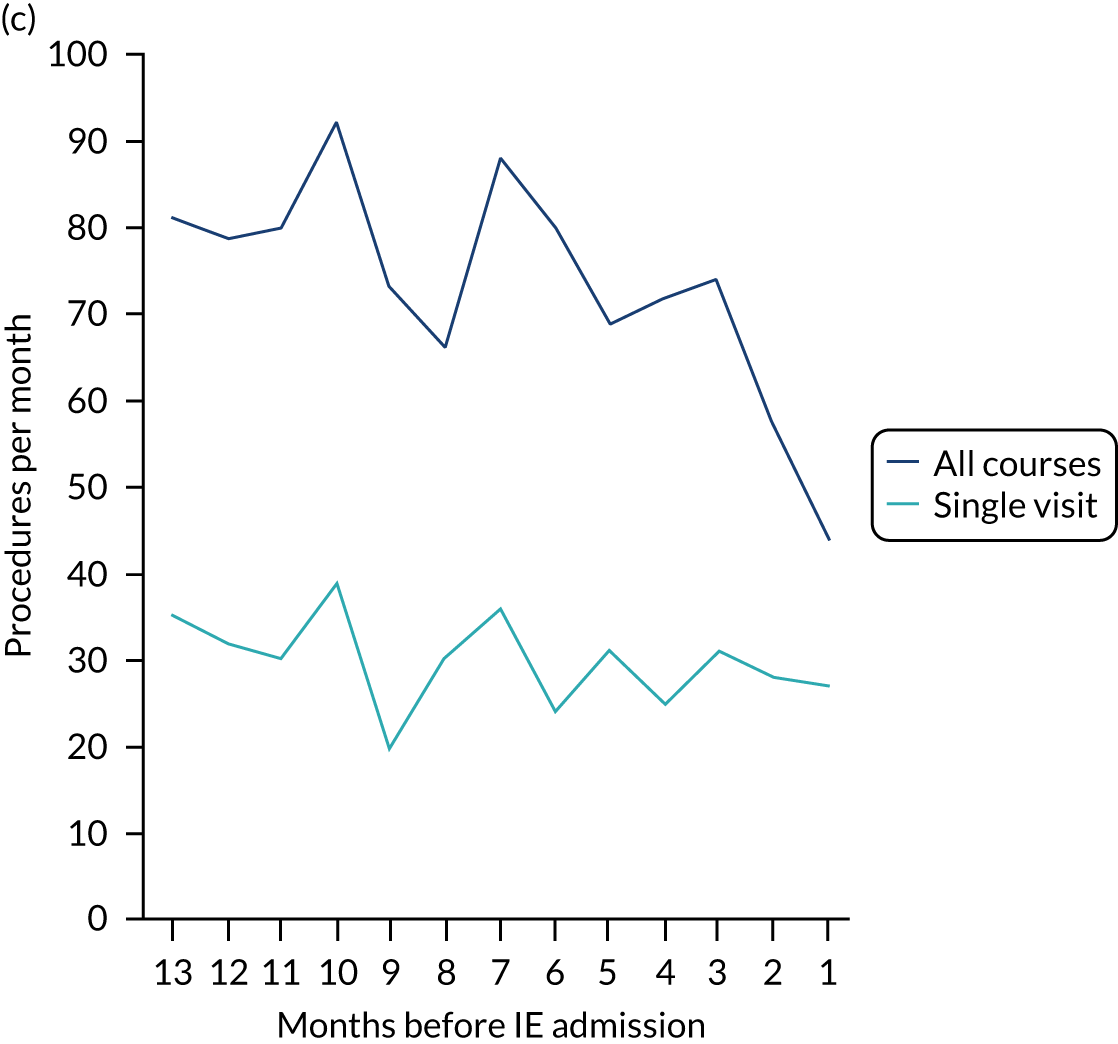
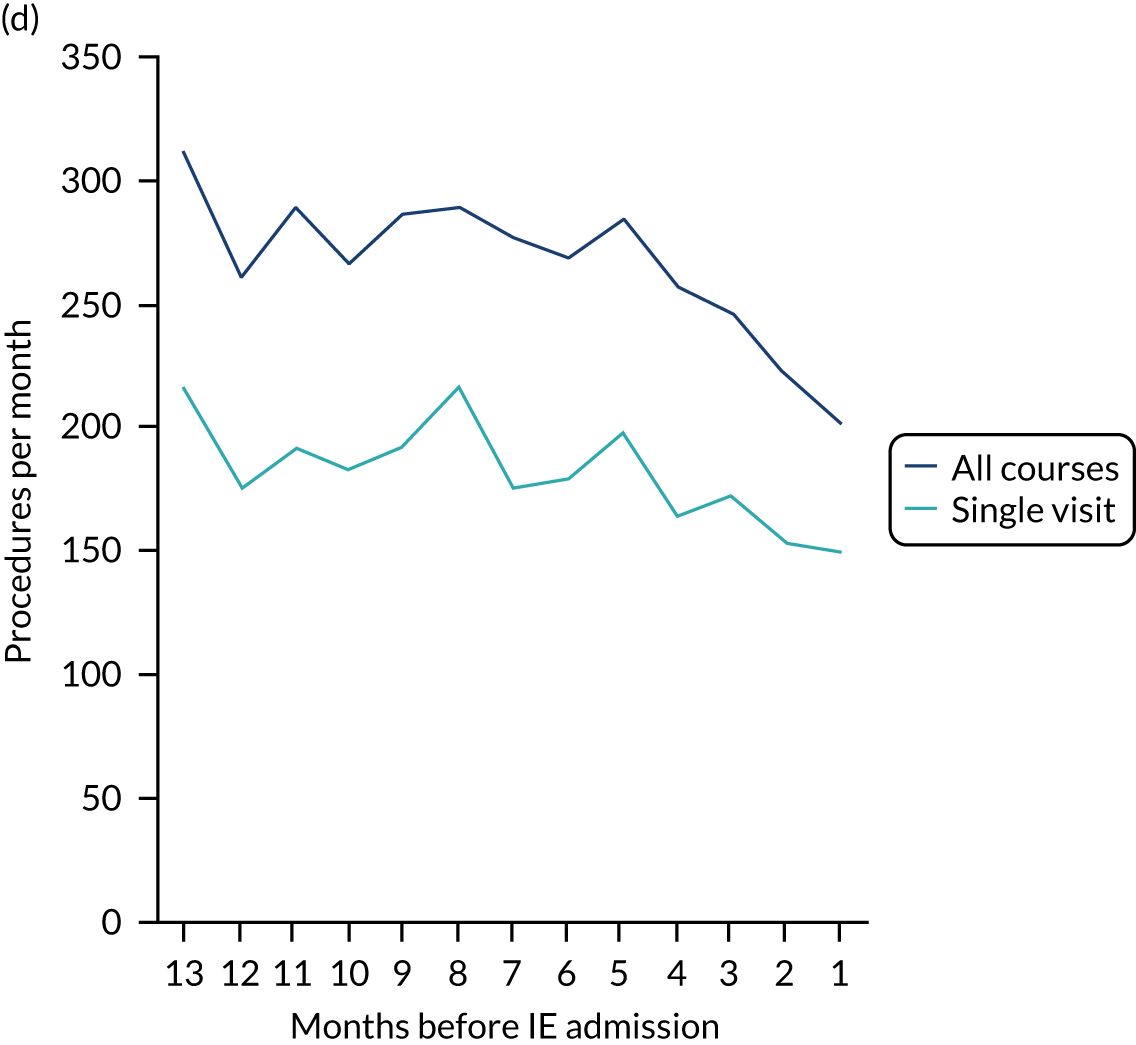
FIGURE 29.
The number of each type of dental procedure over the 13 months before IE admission using the start or end date to define the timing of the procedure. (a) All types of dental procedures; (b) IDPs; (c) indeterminate dental procedures; (d) non-invasive dental procedures.
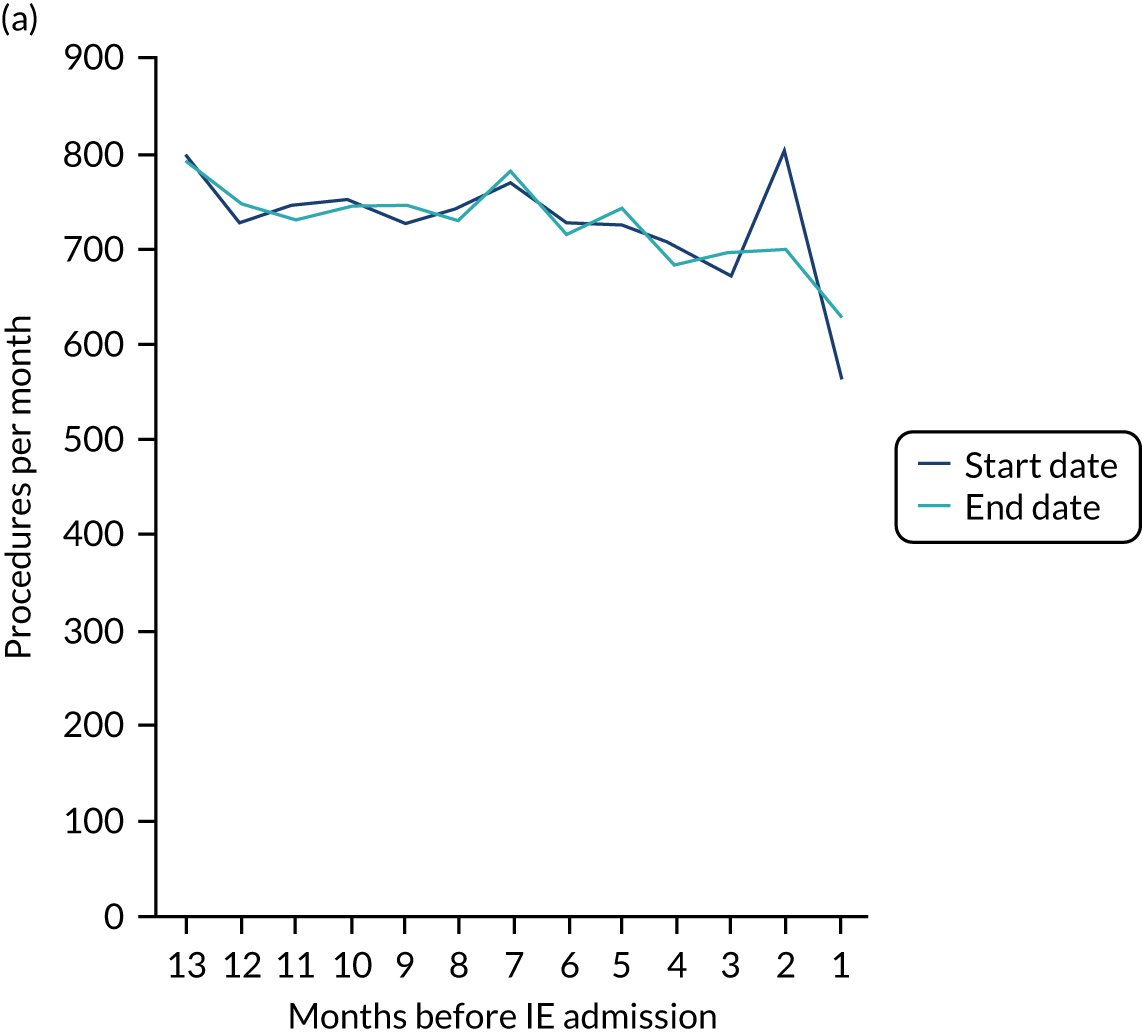
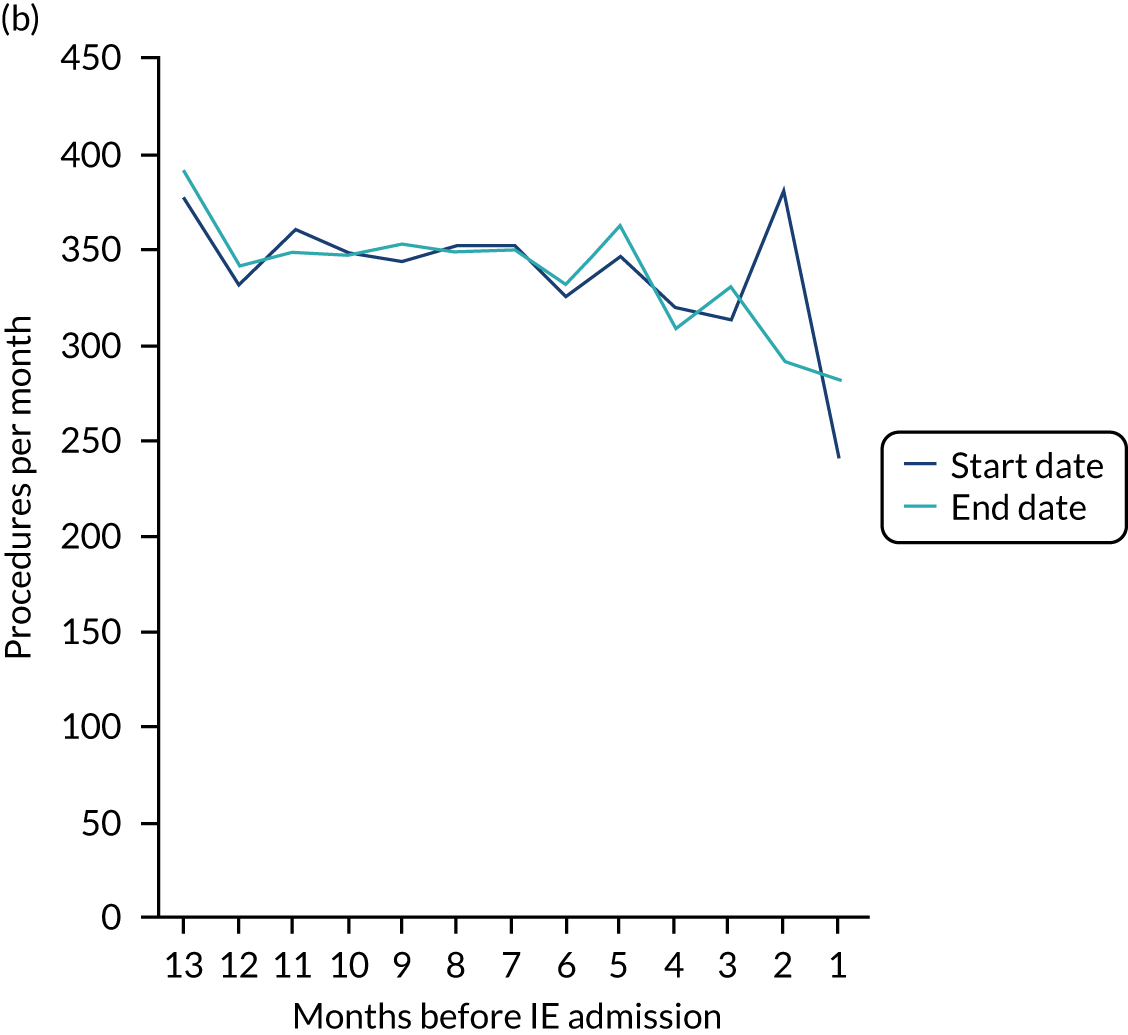
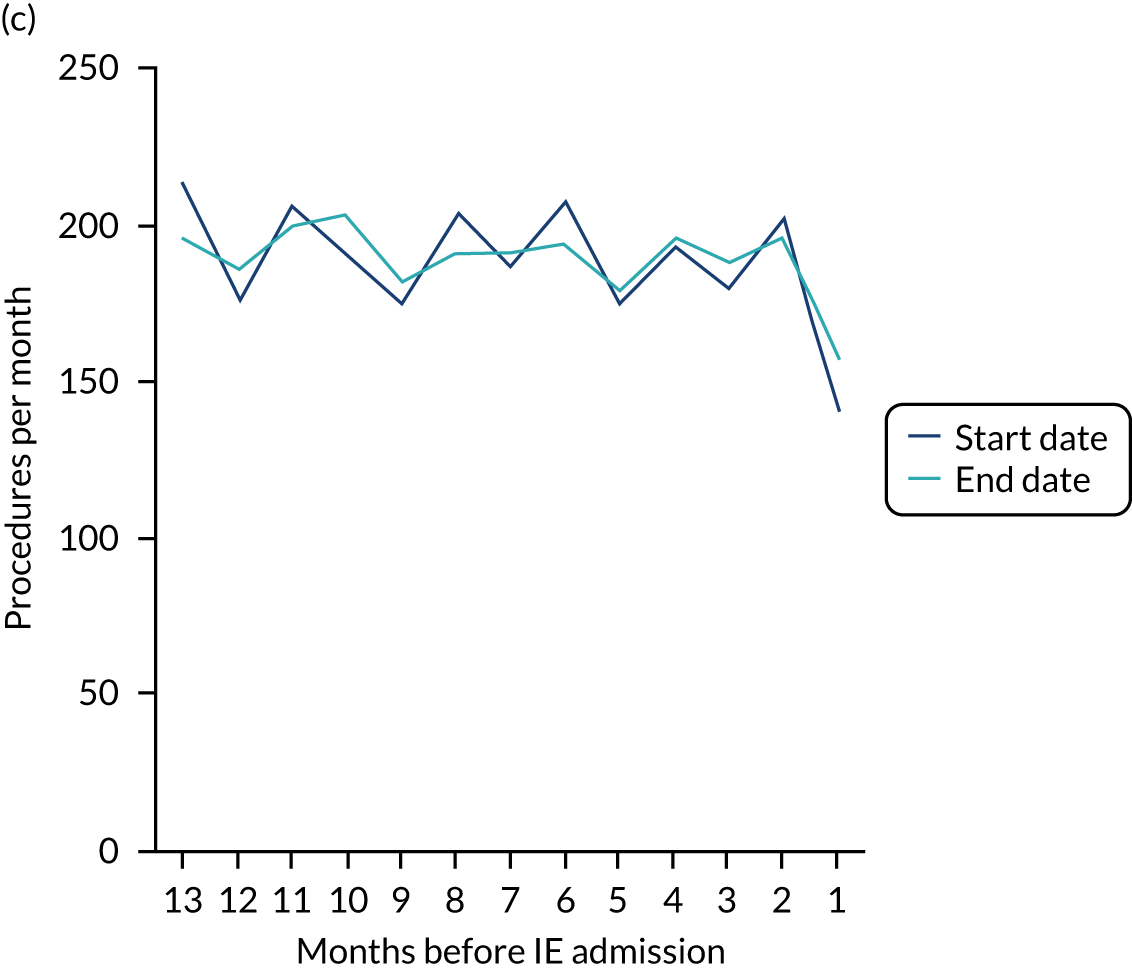
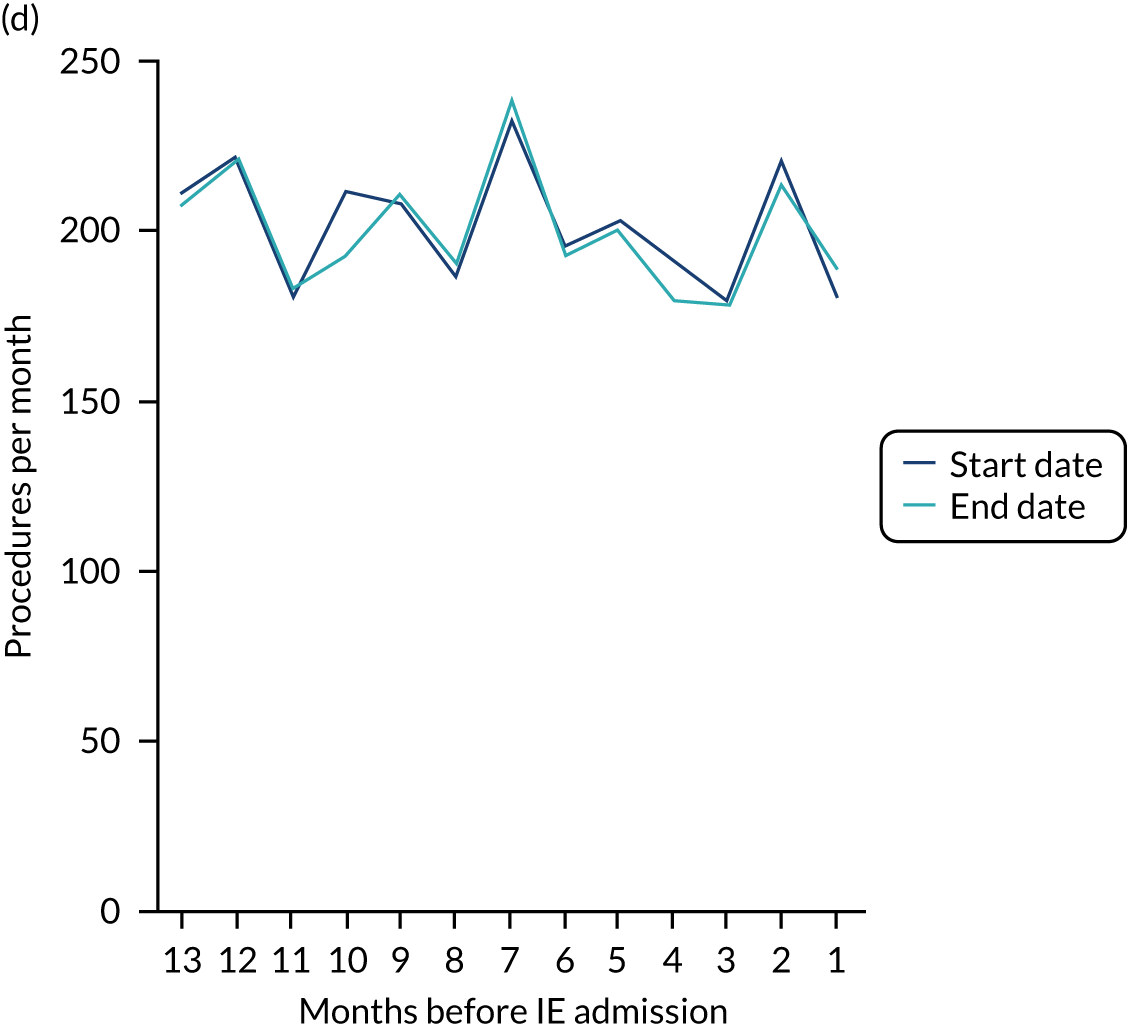
FIGURE 30.
The number of each type of IDP over the 13 months before IE admission using the start or end date to define the timing of the procedure. (a) All IDPs; (b) endodontic procedures; (c) dental extractions; and (d) scale and polishes.
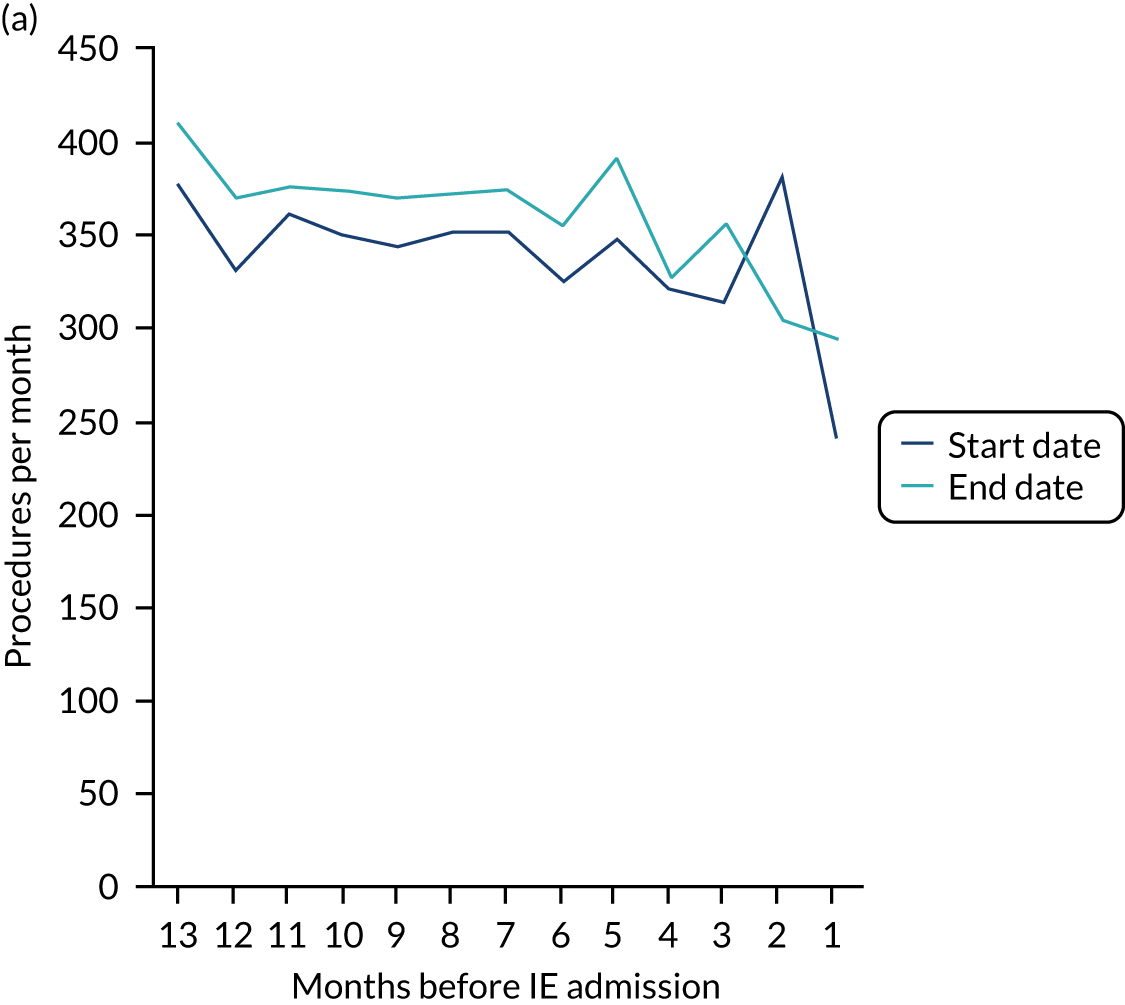
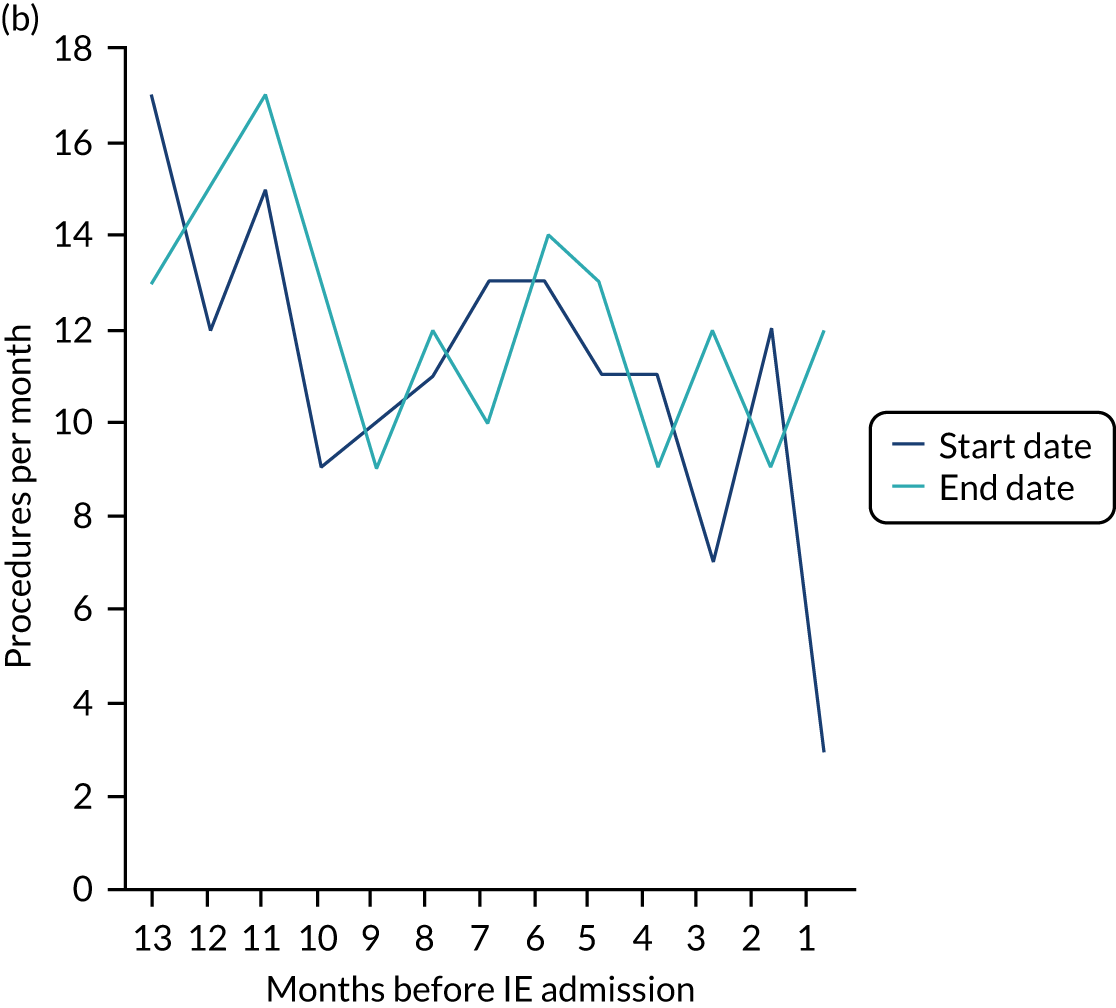
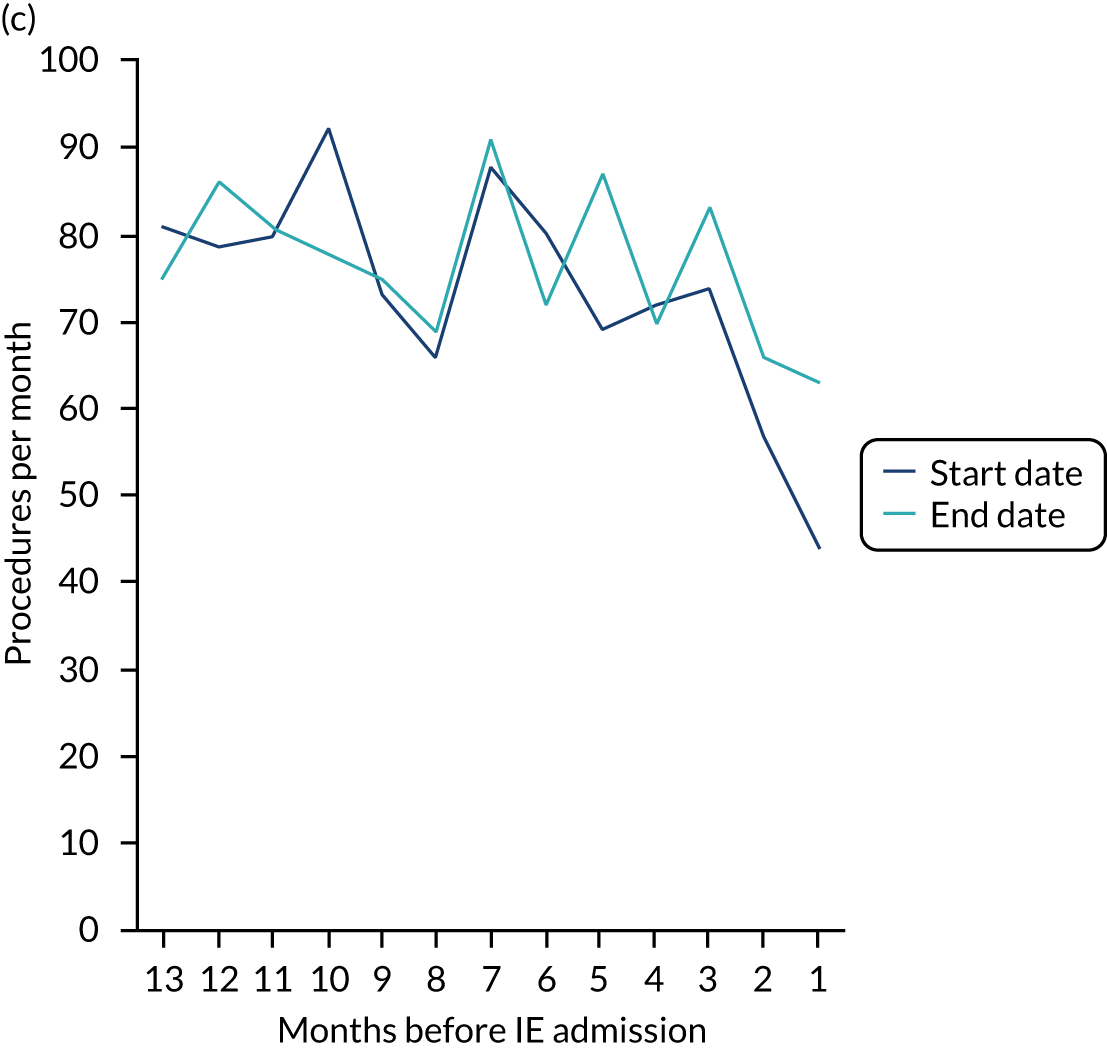
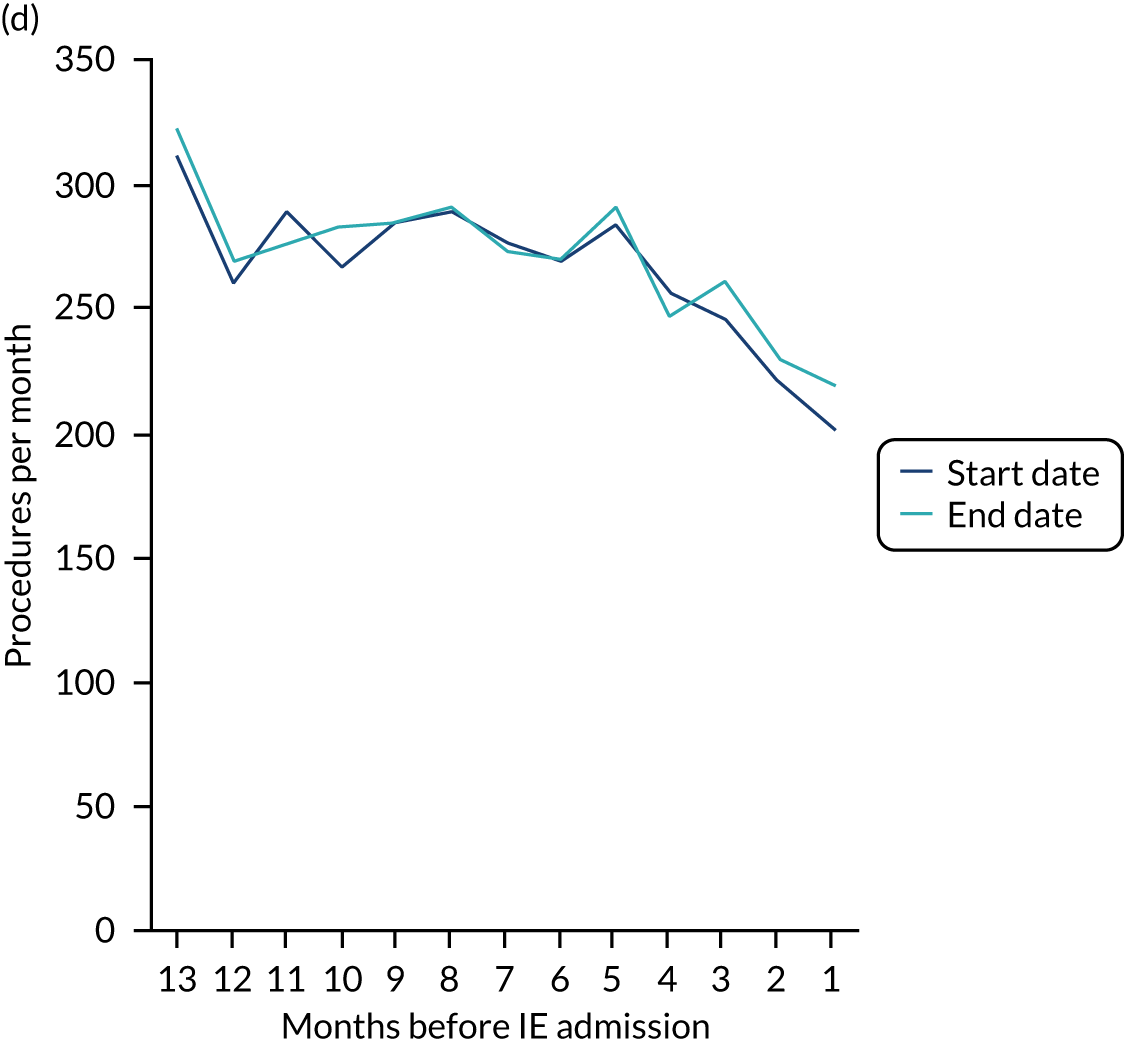
List of abbreviations
- AHA
- American Heart Association
- AP
- antibiotic prophylaxis
- CAG
- Confidentiality Advisory Group
- CI
- confidence interval
- DARS
- Data Access Request Service
- DSA
- data-sharing agreement
- ESC
- European Society of Cardiology
- HES
- Hospital Episode Statistics
- HRA
- Health Research Authority
- ICD-10
- International Classification of Diseases, Tenth Revision
- IDEA
- Invasive Dentistry–Endocarditis Association
- IDP
- invasive dental procedure
- IE
- infective endocarditis
- IGARD
- Independent Group Advising on the Release of Data
- IRR
- incidence rate ratio
- MI
- myocardial infarction
- NHSBSA
- NHS Business Services Authority
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NRES
- National Research Ethics Service
- OPCS-4
- Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4
- OVGS
- oral viridans group streptococci
- PE
- pulmonary embolism
- PPI
- patient and public involvement
- PPV
- positive predictive value
- RCT
- randomised controlled trial
