Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/27/01. The contractual start date was in July 2016. The draft report began editorial review in September 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Costa et al. This work was produced by Costa et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Costa et al.
Chapter 1 Introduction
Background
Fractures of the distal radius are extremely common injuries. In the developed world, 6% of women will have sustained such a fracture by the age of 80 years and 9% will have done so by the age of 90 years. 1 As the population continues to age, these figures are likely to increase. There is a bimodal distribution in terms of age. Younger patients frequently sustain complicated, high-energy injuries involving the wrist joint; however, fractures of the distal radius are also common in older patients, who are more likely to sustain low-energy fractures related to osteoporosis. 2 The optimal management of fractures of the distal radius in adults remains controversial. This study was designed to address both groups of patients, as the key management decisions pertain to all patients with a fracture of the distal radius.
In general, if the bone fragments are undisplaced, that is the bone fragments remain in anatomical alignment, fractures of the distal radius are treated non-operatively. However, if the bone fragments have moved out of their normal alignment, the treating clinician will usually recommend a ‘manipulation’ of the bone fragments to restore the normal anatomy. Manipulating a fracture is painful and, therefore, is carried out using local, regional or general anaesthetic.
Following the manipulation, the bone fragments can fall back out of normal alignment. Therefore, the treating clinician will apply support to the bone fragments while they heal.
This trial compared two techniques for holding the position of the bone fragments following a manipulation of a dorsally displaced fracture of the distal radius: a moulded cast and surgical fixation with Kirschner wires (K-wires).
Relevance of project
Handoll and Madhok3 summarised the results of a series of Cochrane reviews of randomised controlled trials (RCTs) of the treatment of fractures of the distal radius and ‘exposed the serious deficiency in the available evidence’. Furthermore, they were able to identify key areas for future research, including ‘when and what type of surgery is indicated’. 3 In 2014, we published the results of the Distal Radius Acute Fracture Fixation Trial (DRAFFT) [National Institute for Health Research (NIHR) Health Technology Assessment 08/116/974]. 5 In that study, we randomly assigned 461 adult patients undergoing surgery for a dorsally displaced fracture of the distal radius to either a K-wire fixation group or a locking plate fixation group. Contrary to the existing literature, and against the increasing use of plate fixation, the DRAFFT study found that, if a closed reduction of the fracture is possible, then there is no difference in outcomes between the use of K-wires and locking plates for patients with fractures of the distal radius. K-wire fixation is less expensive and quicker to perform than locking plate fixation. This evidence led to a change in clinical practice in the UK6 and changes to national guidelines on the management of this injury. 7
However, there remained unanswered questions, specifically ‘Is there any need to perform surgical fixation of the fracture following a closed reduction of the distal radius, or is a simple cast as effective as the insertion of metalwork?’.
In 2020, Karantana et al. 8 published the results of a Cochrane review of percutaneous wire fixation compared with plater cast use for adult patients with a dorsally displaced fracture of the distal radius. This review included a total of 11 randomised or quasi-randomised trials of manipulation and plaster cast compared with manipulation and K-wire fixation, involving 917, mostly older, participants. Unfortunately, the authors concluded that the quality of the existing evidence was very low. All of the trials included in the review were found to be at a high risk of bias, and allocation concealment was considered adequate in only one trial. Furthermore, all trials reported outcomes incompletely, to the extent that the authors were unable to draw a conclusion with regard to the effect of the interventions on patient-reported function. 8 They did, however, find that the risk of re-displacement of the fracture in participants treated with manipulation and plaster cast was, on average, 12%, (range 3–75%). A review of the published literature8 found no other trials addressing this question since the date of this Cochrane review search (in June 2019).
We conducted a RCT of manipulation and surgical fixation with K-wires compared with manipulation and moulded casting in the treatment of adult patients with a dorsally displaced fracture of the distal radius.
Objectives
The aim of this trial was to improve wrist function and reduce pain by determining the best treatment strategy for adult patients undergoing manipulation for a dorsally displaced fracture of the distal radius.
The primary objective was to quantify and draw inferences on observed differences in the Patient-Rated Wrist Evaluation (PRWE),9 a validated assessment of wrist function and pain, between manipulation and surgical fixation with K-wires and manipulation followed by a moulded cast in the first year post randomisation.
The secondary objectives were to:
-
quantify and draw inferences on differences in the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) [a validated assessment of health-related quality of life (HRQoL)], between surgical fixation with K-wires and moulded casting over the first year post randomisation
-
determine the complication rate, including the need for further surgery, between surgical fixation with K-wires and moulded casting during the first year post randomisation
-
investigate, using appropriate statistical and economic analysis methods, the health-care resource use and comparative cost-effectiveness of surgical fixation with K-wires and moulded casting in the first year post randomisation.
Chapter 2 Methods
The final protocol for this trial has been published and some of the content has been reproduced in this monograph. 10 Copyright © Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
See Appendix 3, Table 24, for a summary of the changes implemented with each protocol version followed during the conduct of the trial.
Trial design
The project was designed as a two-phase study. An internal pilot was to be conducted to confirm the expected rate of recruitment in a large-scale multicentre RCT. The main RCT was planned for a minimum of 24 trauma centres.
Internal pilot summary
The pilot took place at five centres over a period of 6 months. The aim of this initial phase was to determine the number of eligible and recruited patients in a small number of representative recruitment centres over 6 months.
Recruitment during the pilot phase was successful and, therefore, the project continued to the main trial without pause. Participants from the internal pilot were included in the final analysis.
Main randomised controlled trial summary
All adult patients presenting at the recruitment centres with an acute dorsally displaced fracture of the distal radius suitable for manipulation were potentially eligible to take part in the trial. The broad eligibility criteria ensured that the results of the study can be readily generalised to the wider patient population.
Prior to the manipulation, a member of the local research team, for example the research associate, collected baseline demographic data, radiographs and functional data using the PRWE and collected HRQoL data using the EQ-5D-5L. Participants were asked to complete a HRQoL questionnaire to ascertain both their current (injured) and typical (pre-injury) status.
A computer-generated randomisation sequence, stratified by recruitment centre, intra-articular extension of the fracture and age of the participant (aged < 50 or ≥ 50 years), was prepared, with the allocation provided through a secure, centralised, web-based randomisation service by the Oxford Clinical Trials Research Unit. Each participant was randomly allocated to either ‘manipulation and surgical fixation with K-wires’ (the K-wire group) or ‘manipulation and moulded casting’ (the cast group). Both of these treatments are widely used in the NHS, and all of the surgeons were familiar with both techniques. Post treatment, each participant was issued with standardised written rehabilitation instructions.
A member of the local research team performed a clinical assessment and made a record of any early complications at 6 weeks. Routine radiographs were also collected at the 6-week post-randomisation review. Functional outcome data were collected by the central trial team using the PRWE and EQ-5D-5L questionnaires at 3, 6 and 12 months post randomisation. These questionnaires were administered centrally. The participants were also asked to fill out a health-care resource use questionnaire and provide details of any late complications or interventions related to their injury.
Participants
Patients were screened in the emergency department or trauma unit of participating trial recruitment centres. Screening logs were kept at each recruitment centre to determine the number of patients assessed for eligibility and reasons for any exclusion. In addition, the number of eligible and recruited patients and the number of patients who declined consent/withdrew were recorded.
Inclusion criteria
Patients were eligible for Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT 2) if:
-
they had sustained a dorsally displaced fracture of the distal radius, which was defined as a fracture within 3 cm of the radiocarpal joint
-
they were over the age of 16 years and able to give informed consent
-
the treating consultant surgeon believed that they would benefit from manipulation of the fracture.
Exclusion criteria
Patients were excluded from participation in DRAFFT 2 if:
-
the injury was more than 2 weeks old
-
the fracture extended > 3 cm from the radiocarpal joint
-
the fracture was open with a Gustilo grading > 1°
-
the articular surface of the fracture (specifically the radiocarpal joint) could not be reduced by indirect techniques (in a minority of fractures, the joint surface is so badly disrupted that the surgeon has to open up the fracture to restore the anatomy)
-
there was evidence, such as cognitive impairment, that the patient would be unable to adhere to trial procedures or complete questionnaires.
Patients who sustained injuries to areas of the body other than the wrist that might have affected the primary outcome measure were still included in the analysis. Information with regard to additional injuries was recorded at baseline, and analysis was adjusted when necessary.
Consent
Recruitment took place in 36 NHS trusts in the UK that treat patients with distal radius fractures. Eligible patients were identified by the clinical team. A member of the local research team presented the eligible patient with the participant information sheet and provided them with a verbal explanation of the trial procedures. The patients were then given the opportunity to discuss any issues related to the trial with the local research and clinical team, as well as members of their family and friends. In general, patients who are admitted with a fracture of the distal radius have their treatment on the following day(s), so there was sufficient time for the patients to consider taking part in the trial.
Decline consent and withdrawals
Patients were able to decline to take part in the trial without prejudice. Participants could withdraw from the trial at any point. A decision to decline consent or withdraw did not affect the standard of care the patient received. Once withdrawn, the patient was advised to discuss their further care plan with their surgeon. On withdrawal of the patient, any data collected up until the time of withdrawal were retained by the research team and included in the final analysis.
Randomisation
Those patients who consented to take part in the trial had their treatment allocated using a secure, centralised, online randomisation service. The randomisation occurred after the manipulation of the fracture, when the surgeon had determined that the fracture could be adequately reduced without the need to open the fracture.
Although the great majority of these injuries were managed on planned trauma operating lists during normal working hours, the randomisation service was open 24 hours each day to facilitate the inclusion of all potentially eligible patients. Randomisation was on a 1 : 1 basis, stratified by recruitment centre, intra-articular extension of the fracture and age of the patient (< 50 or ≥ 50 years). The sequence was generated by the trial statistician based at the Centre for Statistics in Medicine in Oxford.
Stratification by recruitment centre helped to ensure that any clustering effect related to the recruitment centre itself was equally distributed in the trial treatments. Although it was possible that the surgeons at one recruitment centre were more experienced in one or other treatment than those at another centre, all of the recruiting hospitals, and indeed all hospitals throughout the NHS, use both techniques as part of their normal practice so staff and surgeons were already familiar with both forms of treatment. Although this may not eliminate any individual surgeon-specific effect at any one recruitment centre,11 the manipulation of a fracture of the distal radius is a common procedure, so at each recruitment centre many surgeons were involved in the management of this group of patients (between 10 and 30 surgeons, including both consultants and trainees). Therefore, it was anticipated that each surgeon would operate on only a small number of patients enrolled in the trial, thus greatly reducing the risk of surgeon-specific influences affecting the outcome.
Stratification on the basis of intra-articular extension of the fracture addressed a major potential confounder, as disruption of this articular surface of the distal radiocarpal joint may predispose a patient to secondary osteoarthritis of the wrist. 12
Evidence suggests that other associated features of fractures of the distal radius that commonly appear in the classification systems, such as involvement of the ulnar styloid process, do not actually affect the functional outcome of the injury. 13 Therefore, no other fracture variables were included in the stratification of the randomisation.
Stratification on the basis of age was used in an attempt to discriminate between younger patients with normal bone quality sustaining high-energy fractures and older patients with low-energy (fragility) fractures related to osteoporosis. Empirically, both of these groups of patients could benefit, or not, from surgical fixation. However, stratification by age could also help to identify any effect related to the quality of the patients’ bone. The use of dual-energy X-ray absorptiometry is widely regarded as the gold standard for the assessment of bone density. However, this investigation was not routinely available at all recruitment centres. Therefore, we used age as a surrogate for bone density. In a large study in Norway involving 7600 participants,14 it was demonstrated that forearm bone mineral density remains stable up until the age of 50 years. In males, bone mineral density decreases steadily after the age of 50 years, whereas in females there is an initial rapid decline between the ages of 50 and 65 years, with a further decline thereafter. 14 A study by Court-Brown et al. 15 assessed > 1000 patients with a fracture of the distal radius. The study found a clear bimodal distribution according to the age of the patient. The crossover of the two peaks of incidence was around 50 years of age. These studies provide strong evidence that patients aged > 50 years are increasingly vulnerable to fragility fractures of the distal radius. Furthermore, the study by Court-Brown et al. 15 demonstrated that in the UK approximately 60% of patients sustaining a fracture of the distal radius are aged > 50 years, whereas 40% are aged < 50 years. Therefore, we chose age under or over 50 years as the stratification criterion for this trial.
Blinding
Participants could not be blinded to their treatment allocation because the wires had to be removed, usually in the outpatient clinic at the 6-week follow-up appointment. Nor could the treating surgeons be blind to the surgical/non-surgical treatment that they were providing; however, the treating clinical team did not take part in the postoperative assessment of the patients. The central administrative team was not blind to the treatment allocation; however, the outcome data were provided independently by the participants via questionnaires.
Trial treatments
All participants underwent a closed (i.e. without making any incisions in the skin) manipulation of the fracture. The manipulation was carried out in an operating theatre under either regional or general anaesthetic. The choice of anaesthetic was left to the discretion of the treating surgeon/anaesthetist, as per their normal practice. An X-ray image intensifier machine allowed the surgeon to judge that an adequate closed reduction had been achieved during the manipulation. The decision to accept or not the reduction was left to the discretion of the treating surgeon, as per their normal practice. Only after this decision was made was the participant randomised to one treatment or the other. This trial compared two techniques for holding the position of the bone fragments following the manipulation of a dorsally displaced fracture of the distal radius.
Moulded cast
This technique involves the application of a cast that is shaped (moulded) over the skin to hold the bone fragments in position. This technique is simple and quick to perform, there is little risk of complications and the materials used are cheap. However, the cast is not applied directly to the bone fragments and, therefore, it is possible for the bone fragments to re-displace under the cast, particularly when the swelling starts to settle a few days after the surgery. The principles of applying a moulded cast are inherent in the technique, but in this pragmatic trial the type of casting material, the extent of the cast and the details of the moulding technique were left to the discretion of the treating surgeon, as per their usual technique. Relevant information about the initial technique used and any subsequent interventions, such as cast replacement or removal, was recorded.
Surgical fixation with wires
After the skin has been covered in antiseptic, sharp wires are passed through the skin over the back of the wrist and directly into the bone to hold the bone fragments in the correct position. The principles of K-wire fixation are also inherent in the technique, although there are several different options for the positioning of wires. The size and number of wires, the insertion technique and the configuration of wires were left to the discretion of the surgeon, as per their normal practice. A neutralising cast is applied at the end of the procedure, as per standard surgical practice, but this does not need to be specifically moulded as the wires themselves hold the bone in position. Relevant information on the initial technique used and any subsequent interventions, such as metalwork removal and cast replacement, was recorded.
Rehabilitation
All patients randomised, regardless of group, received the same standardised, written physiotherapy advice detailing the exercises that they needed to perform for rehabilitation following their injury. The written information was developed and tested by experienced physiotherapists/hand therapists as part of the original DRAFFT trial. 4 All patients in both groups were advised to move their shoulder, elbow and finger joints fully within the limits of their comfort. Participants were asked during the 3-, 6- and 12-month follow-ups to say if they had undergone any other investigations/interventions, including any hand therapy.
Participant care pathway
Participants were clinically reviewed at around 6 weeks, as per routine practice after this type of injury. The decision on whether or not to record details of additional rehabilitation and follow-up appointments was left to the discretion of the treating clinicians, as per their normal practice.
Primary outcome
The primary outcome measure for this study was the PRWE at 12 months post randomisation. The PRWE is a 15-item questionnaire designed specifically for the assessment of distal radial fractures and wrist injuries and comprises range of questions in two (equally weighted) sections concerning the patient’s experience of pain and function. All questions are scored on an 11-point, ordered, categorical scale ranging from ‘no pain’ or ‘no difficulty’ (0) to ‘worst ever pain’ or ‘unable to do’ (10). Five questions relate to a patient’s experience of pain and 10 questions relate to function and disability; scores for the 10 function items are summed and divided by 2 and added to the total score for the five pain items to give a score out of 100 (best score = 0 and worst score = 100). The PRWE was administered at baseline (at which point wrist function both pre and immediately post injury was assessed) and then again 3, 6 and 12 months post randomisation. Table 1 shows the collection times for all of the trial outcomes. The PRWE is the most sensitive outcome measure available for patients sustaining this specific injury. 16
| Outcome measure | Time point | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre injurya | Post injuryb | Pre reduction | Post reduction | 6 weeks | 3 months | 6 months | 12 months | |
| Wrist function and pain (PRWE) | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| HRQoL (EQ-5D-5L) | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Radiographs | ✗ | ✗ | ✗ | |||||
| Resource use | ✗ | ✗ | ✗ | |||||
| Complications (including further treatment) | ✗ | ✗ | ✗ | |||||
Secondary outcomes
The secondary outcome measures in this trial were as follows.
Patient-Rated Wrist Evaluation score and general health-related quality of life
The PRWE score at 3 and 6 months was one secondary outcome measure, as was the area under the PRWE curve using data from all time points over the first 12 months post randomisation.
Health-related quality of life at 3, 6 and 12 months was measured using the EuroQol-5 Dimensions (EQ-5D) at 3, 6 and 12 months, and area under the curve (AUC) over the first 12 months post randomisation using all time points. The EQ-5D-5L is a validated, generalised, HRQoL questionnaire consisting of two parts. The first part, the EQ-5D-5L index, includes five domains related to daily activities, each domain being rated on a five-level scale,17 with answers converted into multiattribute utility score. 18 This conversion was carried out, in accordance with the recommendation of the UK National Institute for Health and Care Excellence (NICE), at the time of finalising the statistical health economics analysis plan (SHEAP). 19 The second part, the EuroQol visual analogue scale (EQ-VAS), is measure of health on a scale of 0 to 100, from the worst (0) to best health imaginable (100).
The EQ-5D-5L index ranges from –0.285 to 1, with the EQ-5D-5L crosswalk ranging from –0.594 to 1, with higher scores indicating better quality of life (QoL) and 0 being equivalent to death. EQ-VAS scores range from 0 to 100, with higher scores indicating better QoL.
Complications over the 12 months post randomisation
Further secondary outcome measures were the complications recorded included infection; damage to nerves, tendons or blood vessels; and further surgical interventions for loss of reduction/malunion after the index procedure.
Imaging outcomes
An assessment of the quality of the reduction, and the risk of subsequent loss of reduction, was made using the criteria set out by Costa et al. 4 Standard posterior–anterior and lateral radiographs were taken at baseline (pre and post reduction using the routine intraoperative fluoroscopic images from the operating theatre) and at the routine follow-up appointment 6 weeks post randomisation. These radiographs are those used for the investigation of patients with a suspected fracture of the distal radius and for the follow-up of such patients following any intervention, so there was no need to request any additional or special investigations.
Health-care resource use
Health-care resource use was monitored for the economic analysis. Unit cost data were obtained from the latest available national databases, such as the NHS Supply Chain Catalogue,20 NHS Reference Cost,21 NHS Electronic Drug Tariff,22 British National Formulary (BNF)23 and Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2019. 24 The unit cost was estimated using local sources if the unit cost could not be obtained from the national databases. The costs incurred following discharge, including NHS costs and patients’ out-of-pocket expenses, were recorded in a short questionnaire that was completed by participants at 3, 6 and 12 months post randomisation. Patient self-reported information on service use has been shown to be accurate in terms of the intensity of use of different services. 25
Data management: questionnaire completion
Patient-reported outcome data for the 3-, 6- and 12-month outcome points were collected through a questionnaire sent from the trial central office. Those participants who had provided their mobile telephone number and/or e-mail address to the research team received a text message and/or e-mail prior to the first post-out to advise them that they should expect the questionnaire. Reminder text messages and/or e-mails were sent to participants who did not return the questionnaire in a prespecified time frame. Participants who failed to respond to the questionnaire after two attempts were contacted by telephone to minimise the loss of follow-up information.
Participants were offered the option of receiving a £10 gift voucher with their 12-month questionnaire to compensate them for their time.
Adverse event management
Adverse events were listed on the appropriate case report form (CRF) for routine return to the ‘DRAFFT 2’ central office. Serious adverse events (SAEs) were entered onto the SAE reporting form and reported to the central study team. However, some adverse events that were foreseeable as part of the proposed treatment were not reported on a SAE reporting form and were instead recorded on a complication form. These events included complications of anaesthesia or surgery (e.g. wound infection or damage to nerves, tendons or blood vessels), complex regional pain syndrome (CRPS), deep-vein thrombosis (DVT)/pulmonary embolism (PE) and further planned surgery for the removal of symptomatic metalwork or for the loss of fracture position/malunion. All participants experiencing SAEs were followed up as per protocol until the end of the trial.
Statistical analysis
Sample size
The DRAFFT trial,4 which included the same patient population of patients with a fracture of the distal radius, estimated that the standard deviation (SD) of the PRWE score at 12 months post randomisation was 16 points. However, other studies of patients with a fracture of the distal radius have reported SDs for PRWE score in the range of 16–23 points. 26 Therefore, we chose a conservative estimate for the SD of 18 points. The DRAFFT results also showed an approximate normal distribution of PRWE scores at 12 months post randomisation. 5 A 6-point mean difference between groups equates to a standardised effect size of 0.33 for an assumed SD of 18 points. MacDermid et al. 16 found that the PRWE is sensitive enough to detect subtle but clinically relevant changes in wrist function of this order of magnitude in patients sustaining a fracture of the distal radius. A mean difference in PRWE scores of 6 points is slightly higher than the difference in scores that would result if all the participants in one group reported a response to any of the PRWE’s constituent questions (e.g. one point less difficulty in turning a doorknob) just one point better than the other group (each point in response contributes 5 points to the overall score). We believed the target difference (i.e. 6 points) would be important to patients on both an individual and a population level and could lead to a change in clinical practice in the UK. The total number of participants required to obtain a statistical power of 90% at the two-sided 5% significance level to detect a 6-point difference between groups for the primary outcome measure was 382, that is 191 participants were required in each treatment group. Making a conservative allowance of 20% for patients ‘lost to follow-up’, we planned to recruit a minimum of 476 patients.
Analysis plan
Statistical significance and multiple testing
There is no multiple testing as only a single primary outcome was considered. Therefore, the significance levels used is two-sided 5%, and 95% confidence intervals (CIs) are reported.
Definition of analysis populations
Populations for analysis were defined as follows.
Intention to treat
In the intention-to treat (ITT) analysis, all available randomised participants were analysed in the groups to which they were randomly allocated. All participants with observed data at any time point were included in the analysis.
Per protocol
The per-protocol (PP) analysis included only eligible participants who received the treatment to which they were randomised and for whom data on the primary outcome were available at 12 months post randomisation. Participants who crossed over, had other types of surgery (e.g. plate fixation), did not have surgery or were randomised in error were not included in this population.
Withdrawal from treatment and/or follow-up
Some data were missing because of participant voluntary withdrawal, lack of completion of individual data items or general loss to follow-up. When possible, the reasons for missing data were ascertained and are reported.
Withdrawals/losses to follow-up together with reasons were reported by treatment, and any deaths (and their causes) were reported separately.
Baseline comparability of randomised groups
Baseline characteristics are reported by treatment group, including the stratification/minimisation factors, when applicable, and important prognostic, demographic and clinical covariates.
Numbers (with percentages) for binary and categorical variables and means (with SDs) or medians (with lower and upper quartiles) for continuous variables are presented; no tests of statistical significance or CIs for differences between randomised groups on any baseline variable were performed.
Treatment compliance
Compliance with treatment is reported by treatment group. Non-compliance was defined as failure, for any reason, to perform the original treatment to which a participant was randomised, for example if there was a need to perform an open reduction of the fracture after fracture reduction was lost after randomisation.
Reliability
To ensure consistency, validation checks were carried out on the data, including checking for duplicate records, range checking of the variables’ values and validating potential outliers by comparing with CRFs and referring back to recruitment centres if necessary. Calculations and processes performed by a computer program were checked by hand calculations (when possible) for a minimum of 5% of the available data or 20 patients randomly sampled. This included checking that data had been imported correctly.
For each variable, missing value codes were checked for consistency and the proportion of missing values per variable was presented. Patterns of missing data were explored.
The reliability of the PRWE was checked through the methods described above with particular focus on the number of missing items that made up each subscale.
Analysis of primary outcome
The primary outcome measure, the role of the moulded cast compared with K-wire fixation after manipulation of the PRWE score at 12 months post randomisation, was modelled using a mixed-effects model. This model accounted for recruitment centre (random effect), baseline (post injury) values, values at other time points, articular extension of the fracture (intra- or extra-articular) and age of the patient (≥ 50 or < 50 years) (fixed effects). Treatment by time point interactions were also included in the model to allow time-specific treatment effects to be calculated. This is a different approach from that outlined in the DRAFFT 2 protocol10 (linear regression with cluster robust option to control for recruitment centre), but it is considered in keeping with the principle outlined there (i.e. allowing for adjustment for clustering by recruitment centre as well as the other stratification variables as fixed effects). Baseline was included as a response, rather than adjusted for as a continuous covariate, to obtain time point-specific estimates to be used in the AUC analysis (see below for details on the analysis of the secondary AUC outcomes). Model fit and whether or not the model assumptions were met were assessed graphically.
Analysis of secondary outcomes
The EQ-5D-5L outcomes [index and visual analogue scale (VAS)] were measured at 3, 6 and 12 months post randomisation. A mixed-effects model, as used in the primary outcome, was used to assess this outcome, with recruitment centre as a random effect and baseline values, values at previous time points, articular extension of the fracture (intra- or extra-articular) and age of the patient (≥ 50 or < 50 years) as fixed effects. Treatment group by time point interactions were included in the model to allow time-specific treatment effects to be calculated. Comparisons were performed on an ITT basis and the results were presented as comparative summary statistics (i.e. adjusted mean difference) with 95% CIs. The distribution of the five domains was also reported separately in tabular and graphical form (see Table 22 and Figures 17–21) by treatment.
The secondary outcome of complications, including those needing further surgery at 1 year post randomisation, was analysed as follows. The total number of further surgeries, including those in the cast group who received any surgery, was analysed by calculating the odds ratio and 95% CI using logistic regression. Other generic, non-surgical, complications [i.e. CRPS and DVT and PE (pooled)] were analysed using the same method. Other individual complications were summarised by treatment group, but not analysed, and reported in tabular form (see Table 12). As the total number of further surgeries is not a binary variable but a count, a Poisson regression adjusting for randomised treatment and randomisation variables was used for that analysis.
An AUC analysis was performed on PRWE and EQ-5D scores (both the EQ-5D-5L index and EQ-VAS). The estimates obtained in the mixed model for each time point used in the primary analysis were used to calculate the AUC. Using the estimates from the mixed model rather than raw, unadjusted estimates results in less biased estimates of the AUC when data are missing. 27 The PRWE AUC estimates were computed from the mixed model used for the primary analysis, and the standard errors (SEs) for these estimates were calculated using the delta method. The EQ-5D AUC results were produced in the same manner.
Missing data
The missing items required for definition of outcomes were investigated. Missing data were reported and summarised by treatment. An ITT analysis strategy to assess assumptions about departures from randomisation policies and handling of missing data was performed to examine the robustness of the primary analysis results. For each missing data point, the median PRWE score in the treatment group into which that patient was randomised was imputed and this ‘complete’ data set was analysed using the mixed-effects model used in the primary analysis. This analysis was repeated on a population that had the 60th quantile imputed for one group’s missing values and on the 40th quantile for the other, then again using the 70th and 30th quantiles. The process was repeated but the treatment groups were swapped. In total, five such sensitivity analyses on PRWE scores were performed. The adjusted mean differences and 95% CIs for these analyses were plotted.
The instructions to users of the PRWE suggest a mechanism to deal with missing data: if an item is missing, then this can be replaced with the mean score of the subscale. However, no limit to the number of items that can be replaced is specified; for example, it is theoretically possible, if a value is available for only one item is on a subscale, to substitute that value for all missing values. Therefore, we explored how missing values on the PRWE subscales affected the outcome by calculating the PRWE scores obtained when two, three or four items per subscale were completed and comparing the results with the score obtained on the basis of a single item.
Sensitivity analysis
Sensitivity analysis of the primary outcome and secondary PRWE outcomes was carried out on a PP basis to examine robustness of the conclusions to different assumptions about departures from randomised policies.
An analysis was performed using a three-level model with participant within surgeon within recruitment centre to examine the potential surgeon (random) effects. This model incorporated terms that allowed for possible heterogeneity in patient’s responses because there were a number of recruiting surgeons as well as recruitment centres. In addition, the fixed effects of the treatment groups, patient age and intra-articular extension also allowed for possible heterogeneity in responses.
Prespecified subgroup analysis
Subgroup analyses of the effect of the two clinical stratifying variables (age < 50 or ≥ 50 years and intra- or extra-articular extension) on the primary outcome were performed. These were undertaken for each of the stratifying variables using an extended model to formally test the interaction between each stratifying variable and the treatment factor; appropriate 95% CIs were reported for the interaction effects, in addition to p-values. These analyses were considered exploratory and results should be interpreted with due caution, and are reported as such in line with recommendations for subgroup analysis made elsewhere. 28 The subgroup results for the primary outcome are presented in a forest plot (see Figure 9).
Health economic analysis plan
A within-trial economic evaluation was conducted from the recommended NHS and Personal Social Services (PSS) perspective in the base-case (primary) analysis. 29
Measurement of health-care resource use
Resource use in the first 6 weeks was collected on CRFs that were completed by the research team, whereas resource use during follow-up was collected through trial questionnaires given to participants at 3, 6 and 12 months post randomisation. Recall period was baseline to 3 months, 3 months to 6 months and 6 months to 12 months post randomisation. The questionnaire captured resource use information related to distal radius fracture, such as the number and duration of admissions to inpatient wards [classified as orthopaedics (wrist/arm), orthopaedics (any bones) and rehabilitation unit], number of diagnostic tests, use of outpatient services (classified as orthopaedics, physiotherapy, pathology and radiology), and use of community-based health and social care services. Participants also recorded direct medical costs (e.g. medications), direct non-medical costs (e.g. aids and adaptations, help with housework/child care and travel) and indirect costs (i.e. lost productivity) attributable to the participant’s injury.
Free-text responses were reclassified to the appropriate cost category, and were removed if deemed unrelated/irrelevant to the trial by clinical experts (e.g. cardiology) or were analysed collectively as ‘other’ in the descriptive analysis.
Valuation of costs
The intraoperative consumable costs for both cast and K-wire fixation were obtained from the latest NHS Supply Chain catalogue. 30 (see Appendix 2, Table 21). The duration of surgery was recorded and included in the analysis as a unit cost per minute of theatre time. According to an operating theatre benchmarking study in 2013,31 the cost of each theatre hour that included staff cost was, on average, £561. The unit cost of the initial distal radius surgery fracture fixation was assessed using the NHS Reference Costs21 and the Healthcare Resource Group32 code (HT45Z) for ‘Minor Hand Procedures for Trauma’ was assigned to both trial groups. Day-case costs or overnight admission costs were obtained from the latest NHS Reference Costs. 21 If a participant reported a length of stay longer than the trim point specified in the NHS Reference Costs, then the inpatient excess day cost from the NHS Reference Costs was used.
Direct medical costs, such as the inpatient care, outpatient care and community care, were sourced from the latest available NHS Reference Costs21 and PSSRU Unit Costs of Health and Social Care 2019. 24 The unit cost of medication related to the distal radius fracture (e.g. analgesia) was sourced using the latest available BNF33 or the NHS Electronic Drug Tariff. 22 The cost of medication taken over the study period was computed using the cost per dose for each product and the mean quantity taken per day for the reported number of days. All medications were assumed to be in generic tablet form unless stated otherwise.
The unit cost of direct non-medical items, such as help with housework/child care and travel incurred by participant’s carers, was obtained directly from the questionnaire. The unit costs of aids and adaptations were obtained from the latest NHS Supply Chain Catalogue. The cost of lost productivity was computed using the human capital approach from the Office for National Statistics,34 in which the daily median wage was multiplied by the number of days that the participant had to take off work because of their injury.
The cost of health-care resource use per participant was computed by multiplying the reported frequency of health-care resource utilisation by the unit cost of each resource item and adding the direct non-medical cost, which was obtained directly from the questionnaire. The base currency of all costs was 2018/19 Great British pounds (GBP), and the unit costs were adjusted to 2018/19 prices using the NHS Cost Inflation Index, which supersedes the Hospital and Community Health Services Index.
Valuation of health outcomes
Responses to the EQ-5D-5L were converted to EuroQol-5 Dimensions, three-level version (EQ-5D-3L), utility scores using the crosswalk algorithm18 recommended by NICE35 and valued using the UK EQ-5D-3L time trade-off tariff. 36 Quality-adjusted life-years (QALYs) were calculated as the AUC of utility scores from baseline, 3-, 6- and 12-month data using the trapezoidal rule. 37 Estimates of mean QALYs were then adjusted using regression methods with baseline EQ-5D as the independent variable. Utilities were set to zero at the studied time point if patient had died just before that time point (e.g. if a patient died between 3 months and 6 months, then the EQ-5D score of that patient at 6 months would be zero). 38
Data analysis
Resource use items were summarised by trial allocation group and follow-up period and differences between groups were analysed using t-tests for continuous variables and Pearson chi-squared (χ2) tests for categorical variables. The means and SEs for values of each cost category were estimated by treatment allocation and follow-up period. Differences in mean costs were assessed using t-tests. The bootstrap 95% CI was computed based on 10,000 replications. No discounting of costs and QALYs was applied because the time horizon was 1 year. A two-sided significance level of 0.05 was used throughout.
Cost-effectiveness analysis
The cost-effectiveness of K-wires compared with a cast was assessed from the UK NHS and PSS perspective in the base-case analysis. 39 The base-case analysis adopted an ITT (‘as-randomised’ with imputation of missing data using multiple imputation) perspective, and an incremental cost-effectiveness ratio (ICER) was calculated as the difference in mean costs divided by the difference in mean QALYs between the treatments.
The NICE40 cost-effectiveness threshold of £20,000 to £30,000 per additional QALY was used to determine the cost-effectiveness of K-wires compared with a cast after the manipulation of a fracture of the distal radius. An additional £15,000 per QALY cost-effectiveness threshold was also included to reflect recent trends in health-care decision-making. 41 The treatment was considered to be cost-effective if it had an ICER below the specified cost-effectiveness threshold. The net monetary benefit (NMB) of K-wires compared with a cast was computed across different cost-effectiveness thresholds. A positive incremental NMB would indicate that K-wires are cost-effective, compared with a cast, at the given cost-effectiveness threshold.
Non-parametric bootstrapping was also performed to assess the uncertainty on the ICERs. The probability that K-wires are cost-effective compared with a cast was plotted at varying levels of cost-effectiveness thresholds for an additional QALY and presented in cost-effectiveness acceptability curves. The cost-effectiveness plane was drawn based on bootstrap estimates of the differences in cost and effectiveness for the K-wire group compared with the cast group and the ellipses represented 50%, 75% and 95% confidence regions around the ICER for the K-wire group compared with the cast group. The ellipses were computed based on the methods described by Nixon et al. 42
Missing data
Under the missing at random (MAR) assumption, multiple imputation analysis was used to impute missing costs and QALYs. This was carried out using chained regression equations predicting missing values from the observed covariates (observed responses of participant) and creating sets of multiple data sets containing possible values for missing observations. 43 Pooled estimates were then computed using Rubin’s rules44 to obtain an overall mean estimate of the costs or QALYs.
Mean imputation was used for missing baseline covariates whereas multiple imputation for QALYs was performed at the QALY level and costs were imputed at the total cost level (e.g. total outpatient cost) in each follow-up time point using Amelia II in R (The R Foundation for Statistical Computing, Vienna, Austria). This multiple imputation package has been shown to outperform other such packages [e.g. NORM (S-PLUS – TIBCO Spotfire, Somerville, USA or R), MICE (S-PLUS, R and Stata – StataCorp LP, College Station, TX, USA) and SPSS MI (SPSS Inc., Chicago, IL, USA)]. 45 Independent variables included in the imputation model consisted of baseline covariates, such as age, sex, articular extension of fracture, PRWE score, whether or not the participant had CRPS, pre-injury EQ-5D index and VAS scores, and post-injury EQ-5D VAS score. This imputation was run 40 times in accordance with the ‘rule of thumb’ that suggests that the number of imputations should be similar to the percentage of incomplete cases. 46
Subgroup and sensitivity analyses
Similarly to the statistical analysis, the economic evaluation was repeated for subgroups defined by the two clinical stratifying variables (age and articular extension of fracture). One-way sensitivity analyses, such as extending the study perspective to societal perspective by including costs of lost productivity and complete-case analysis to assess the impact of missing data on the ICERs, were conducted.
Ethics approval and monitoring
Ethics committee approval
The National Research Ethics Committee approved this study on 6 October 2016 (16/SC/0462).
Trial Management Group
The day-to-day trial management was the responsibility of the trial manager, based at Oxford Trauma/Oxford Clinical Trials Research Unit of the University of Oxford and supported by administrative staff. The Trial Management Group (TMG) met monthly to assess overall trial progress. It was also the responsibility of the trial manager to train local research staff at each of the trial recruitment centres. The trial’s statistical, health economics and programming team were closely involved in setting up data capture systems, design of databases and clinical reporting forms.
Trial Steering Committee
A Trial Steering Committee was appointed by the National Institute for Health Research (NIHR) and was responsible for overseeing, monitoring and supervising trial progress on behalf of the funder. The Trial Steering Committee consisted of three independent experts, a lay member and the chief investigator who met once per year during the recruitment period. Membership is listed in the Acknowledgements.
Data Safety and Monitoring Committee
The study Data Safety and Monitoring Committee (DSMC) adopted a DAta MOnitoring Committees: Lessons, Ethics, Statistics (DAMOCLES) charter, which defines its terms of reference and operation in relation to oversight of the trial. The DSMC was tasked with monitoring ethics, safety and data integrity. The trial statistician provided data and analyses requested by the DSMC so that the DSMC could review accruing data and summaries of the data presented by treatment group and assess the screening algorithm against the eligibility criteria. It also considered emerging evidence from other related trials or research and reviewed related SAEs that had been reported. The DSMC met twice during the recruitment period. Membership of the DSMC is listed in the Acknowledgements.
Patient and public involvement
Alongside the DRAFFT trial,4 a series of formal qualitative interviews were performed with patients suffering from fractures of the distal radius. The views and priorities of patients taking part in the DRAFFT trial4 were used to inform the trial treatments and processes in this study.
To ensure ongoing patient and public involvement, a patient/carer representative was actively involved in general trial management. In addition, a further independent patient representative was a member of the Trial Steering Committee.
Chapter 3 Results
Screening and randomisation
Patient screening for potential study participants was open from January 2017 to March 2019. A total of 2636 patients were screened, of whom 1441 were not eligible because they met one of the exclusion criteria. A further 495 were not included because of the clinician’s treatment preference, and 196 declined to participate in the study (Table 2 shows the reasons for declining consent and Table 3 shows the clinicians’ stated treatment preference). The Consolidated Standards of Reporting Trials (CONSORT) flow diagram shows the reasons for ineligibility (Figure 1).
| Reasons | Number of participants |
|---|---|
| Patient does not want to take part in research | 143 |
| Patient does not want to complete questionnaires | 9 |
| No reason given | 32 |
| Total | 184 |
| Treatment preference | Number of participants |
|---|---|
| Conservative | 31 |
| Surgical fixation with K-wires | 120 |
| Surgical fixation with plate | 183 |
| Other | 7 |
| Unknown | 154 |
| Total | 495 |
FIGURE 1.
The CONSORT flow diagram.
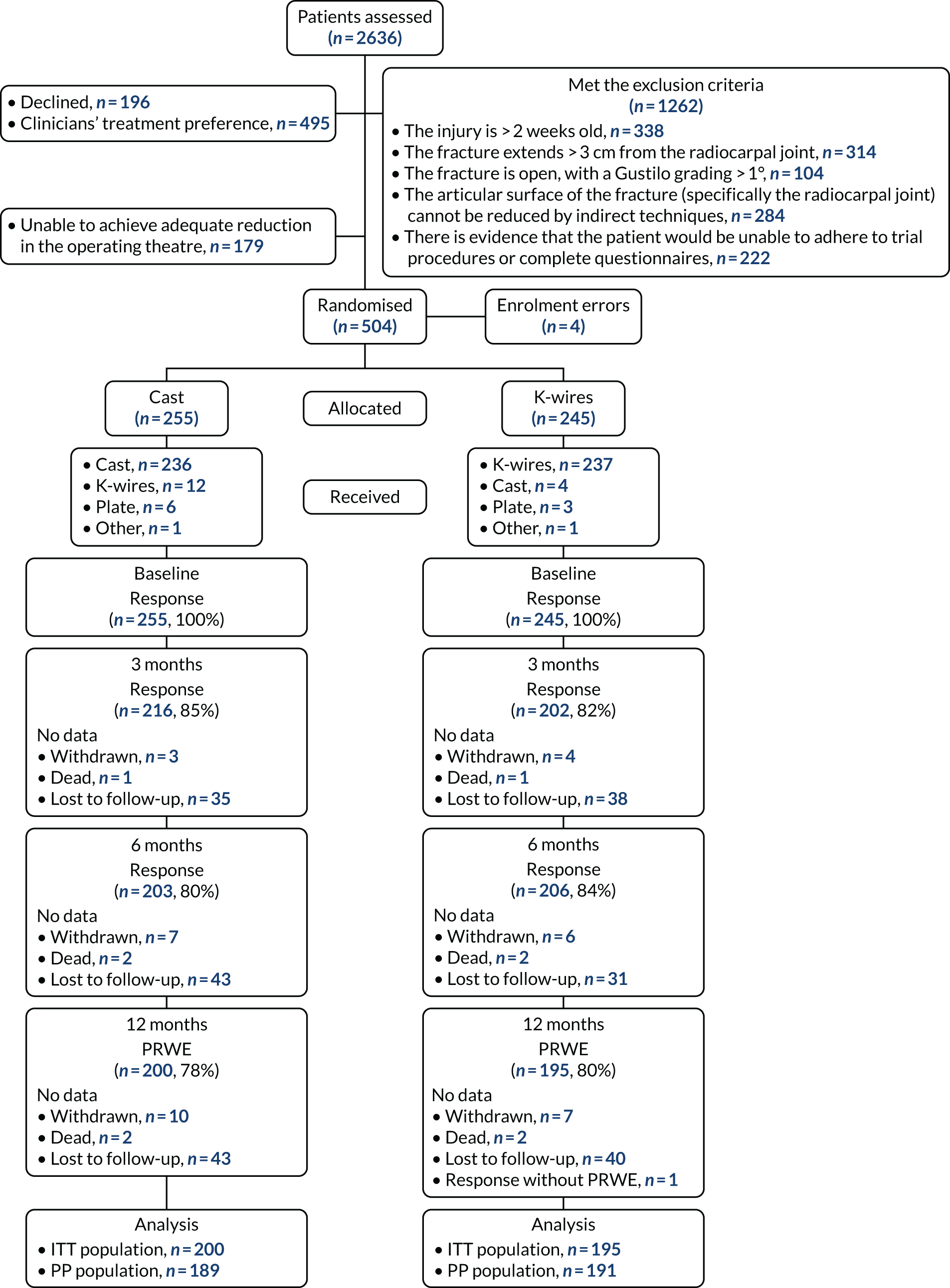
A total of 179 patients consented to enter the study but were not randomised because the articular surface of their fracture could not be reduced by indirect techniques during manipulation under anaesthetic in the operating theatre.
A total of 504 patients were entered into the randomisation system, but four were entered in error (without consent). Therefore, this trial randomised a total of 500 participants, of whom 255 were allocated to the cast group and 245 were allocated to the K-wire group.
Recruitment
The first randomisation took place on 31 January 2017 and the final randomisation was on 28 March 2019, and the final 12-month follow-up was received on 18 May 2020.
Baseline characteristics
Baseline participant characteristics
The randomisation was stratified by recruitment centre, articular extension (extra- or intra-articular) and age (< 50 or ≥ 50 years). Table 4 shows the allocation of the participants to the treatment groups for each stratification variable. Pinderfields Hospital and Addenbrooke’s Hospital were open to recruitment but did not randomise any participants and so were omitted from this table.
| Stratification variable | Treatment group, n (%) | |
|---|---|---|
| Cast (N = 255) | K-wire (N = 245) | |
| Recruitment centre | ||
| Basingstoke and North Hampshire Hospital | 5 (2.0) | 8 (3.3) |
| Blackpool Victoria Hospital | 2 (0.8) | 3 (1.2) |
| Cumberland Infirmary | 4 (1.6) | 5 (2.0) |
| Eastbourne District General Hospital | 10 (3.9) | 8 (3.3) |
| Ipswich Hospital | 1 (0.4) | 2 (0.8) |
| James Cook University Hospital | 16 (6.3) | 15 (6.1) |
| John Radcliffe Hospital | 12 (4.7) | 13 (5.3) |
| Leicester General Hospital | 10 (3.9) | 9 (3.7) |
| Luton & Dunstable Hospital | 7 (2.7) | 8 (3.3) |
| Manchester Royal Infirmary | 5 (2.0) | 5 (2.0) |
| Musgrove Park Hospital | 2 (0.8) | 3 (1.2) |
| North Tyneside General Hospital | 5 (2.0) | 6 (2.4) |
| Northampton General Hospital | 12 (4.7) | 8 (3.3) |
| Peterborough City Hospital | 6 (2.4) | 6 (2.4) |
| Poole Hospital | 4 (1.6) | 7 (2.9) |
| Royal Berkshire Hospital | 4 (1.6) | 5 (2.0) |
| Royal Blackburn Hospital | 9 (3.5) | 10 (4.1) |
| Royal Cornwall Hospital | 7 (2.7) | 7 (2.9) |
| Royal Derby Hospital | 7 (2.7) | 5 (2.0) |
| Royal Hampshire County Hospital | 1 (0.4) | 2 (0.8) |
| Royal Stoke University Hospital | 10 (3.9) | 7 (2.9) |
| Royal Victoria Infirmary | 13 (5.1) | 14 (5.7) |
| Salisbury District Hospital | 12 (4.7) | 9 (3.7) |
| Sandwell General Hospital | 7 (2.7) | 10 (4.1) |
| Southampton General Hospital | 6 (2.4) | 3 (1.2) |
| Southmead Hospital | 19 (7.5) | 21 (8.6) |
| St. George’s University of London | 3 (1.2) | 2 (0.8) |
| Tunbridge Wells Hospital | 5 (2.0) | 3 (1.2) |
| University Hospital (Coventry) | 21 (8.2) | 21 (8.6) |
| University Hospital Llandough | 10 (3.9) | 7 (2.9) |
| University Hospital of North Tees | 5 (2.0) | 3 (1.2) |
| Whiston Hospital | 3 (1.2) | 2 (0.8) |
| Wrightington Hospital | 3 (1.2) | 3 (1.2) |
| Wythenshawe Hospital | 9 (3.5) | 5 (2.0) |
| Age (years) | ||
| < 50 | 62 (24.3) | 51 (20.8) |
| ≥ 50 | 193 (75.7) | 194 (79.2) |
| Articular extension of the fracture | ||
| Extra-articular extension | 184 (72.2) | 176 (71.8) |
| Intra-articular extension | 71 (27.8) | 69 (28.2) |
| Total | 255 (51.0) | 245 (49.0) |
Table 5 shows the baseline continuous variables as means and SDs by treatment group. The mean age in the cast group was 59.6 (SD 17.2) years and in the K-wire group was 60.7 (SD 16.0) years. Baseline pre- and post-injury patient-reported outcomes (i.e. PRWE, EQ-5D-5L index and EQ-VAS scores) are also reported in Table 5 and were similar across the treatment groups. Table 6 shows the categorical baseline variables (presented as number of participants with percentages) of sex, treated wrist and grade of surgeon performing the procedure. Two-hundred and twelve (83.1%) participants in the cast group and 205 (83.7%) participants in the K-wire group were female.
| Variable | Treatment group | |||
|---|---|---|---|---|
| Cast (N = 255) | K-wire (N = 245) | |||
| n | Mean (SD) | n | Mean (SD) | |
| Age (years) | 255 | 59.6 (17.2) | 245 | 60.7 (16.0) |
| Baseline pre injury (retrospective) | ||||
| PRWE score (points) | 254 | 3.1 (10.3) | 245 | 4.5 (14.0) |
| EQ-5D-5L index score | 254 | 0.9 (0.2) | 244 | 0.9 (0.1) |
| EQ-VAS score, 0–100 | 254 | 84.6 (15.3) | 243 | 86.4 (13.9) |
| Baseline post Injury | ||||
| PRWE score (points) | 253 | 84.3 (13.3) | 243 | 81.9 (14.5) |
| EQ-5D-5L index score | 252 | 0.3 (0.3) | 241 | 0.4 (0.3) |
| EQ-VAS score, 0–100 | 253 | 63.9 (22.8) | 242 | 64.2 (23.1) |
| Variable | Treatment group, n (%) | |
|---|---|---|
| Cast (N = 255) | K-wire (N = 245) | |
| Sex | ||
| Female | 212 (83.1) | 205 (83.7) |
| Male | 43 (16.9) | 40 (16.3) |
| Treated wrist | ||
| Left | 147 (57.6) | 146 (59.6) |
| Right | 108 (42.4) | 98 (40.0) |
| Not documented | 0 (0.0) | 1 (0.4) |
| Surgeon’s grade | ||
| Consultant | 139 (54.5) | 104 (42.4) |
| Other | 11 (4.3) | 21 (8.6) |
| Specialist trainee | 73 (28.6) | 91 (37.1) |
| Staff grade/associate/specialist | 32 (12.5) | 28 (11.4) |
| Not documented | 0 (0.0) | 1 (0.4) |
Treatment information
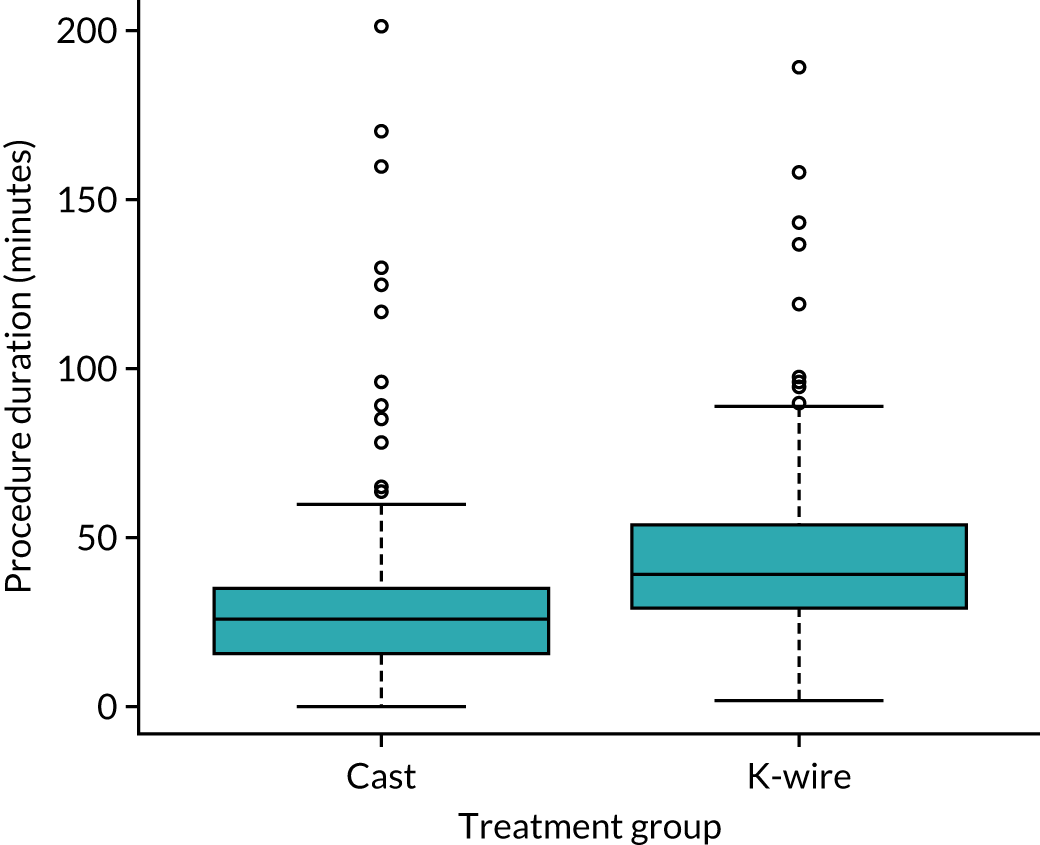
Information on treatments is presented in Figure 2 and Table 7. Figure 2 is a box plot of procedure duration for each group and shows that fixation with K-wire took longer than moulded casting. Table 7 shows other details about the treatments performed. The majority of treatments (87.1% and 82.4% in the cast and K-wire group, respectively) were performed by experienced surgeons who had performed over 20 procedures for both cast and K-wire interventions. A total of 361 surgeons performed the treatments as part of the trial. The median number of treatments per surgeon was 1, with a range of 1–11.
FIGURE 2.
Box plot of procedure duration time by treatment group.
| Treatment details | Treatment group, n (%) | |
|---|---|---|
| Cast (N = 255) | K-wire (N = 245) | |
| Perioperative antibiotic cover used | ||
| Yes | 48 (19.0) | 181 (74.8) |
| No | 204 (81.0) | 61 (25.2) |
| Number of prior patients with a displaced distal radius fracture treated by surgeon | ||
| 0 | 1 (0.4) | 5 (2.0) |
| < 5 | 4 (1.6) | 13 (5.3) |
| 5–10 | 11 (4.3) | 7 (2.9) |
| 11–20 | 17 (6.7) | 18 (7.4) |
| > 20 | 222 (87.1) | 201 (82.4) |
| K-wire information | ||
| Number of wires used | ||
| 1 | 0 (0.0) | 5 (2.0) |
| 2 | 4 (1.6) | 154 (63.1) |
| 3 | 7 (2.7) | 74 (30.3) |
| > 3 | 1 (0.4) | 3 (1.2) |
| N/A | 243 (95.3) | 8 (3.3) |
| Wire size (mm) | ||
| 1.1 | 0 (0.0) | 1 (0.4) |
| 1.6 | 9 (3.5) | 172 (72.6) |
| Other | 3 (1.2) | 56 (23.6) |
| N/A | 243 (95.3) | 8 (3.4) |
| Pinning technique used | ||
| Interfragmentary | 6 (2.4) | 91 (38.2) |
| Kapanji | 4 (1.6) | 93 (39.1) |
| Mixed technique | 2 (0.8) | 46 (19.3) |
| N/A | 243 (95.3) | 8 (3.4) |
| Cast informationa | ||
| Thumb included | ||
| Yes | 59 (23.2) | |
| No | 176 (69.3) | |
| N/A | 19 (7.5) | |
| Material used | ||
| Fibreglass | 13 (5.1) | |
| Plaster of Paris | 222 (87.1) | |
| Other | 1 (0.4) | |
| N/A | 19 (7.5) | |
| Coverage | ||
| Backslab | 19 (7.5) | |
| Full cast | 214 (84.9) | |
| N/A | 19 (7.5) | |
Treatment compliance
There was > 92% compliance for both treatments with 236 (92.5%) of those randomly allocated a moulded cast receiving one and 237 (96.7%) receiving their allocated K-wire (Table 8). More participants randomised to the cast group crossed over to receive K-wire than participants randomised to the K-wire group who crossed over to receive a cast. A small number from both groups (the cast group, n = 4; the K-wire group, n = 3) received a plate. The ‘other’ in the K-wire group received an external fixation (after a fasciotomy for compartment syndrome and decompression of carpal tunnel) and the ‘other’ treatment received for the participant allocated to the cast treatment could not be confirmed.
| Treatment received | Treatment group allocated, n (%) | Total, n (%) | |
|---|---|---|---|
| Cast | K-wire | ||
| Cast | 236 (92.5) | 4 (1.6) | 240 (48.0) |
| K-wire | 12 (4.7) | 237 (96.7) | 249 (49.8) |
| Plate | 6 (2.4) | 3 (1.2) | 9 (1.8) |
| Other | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Total | 255 (51.0) | 245 (49.0) | 500 (100.0) |
Deaths and withdrawals
Overall, there were 17 withdrawals (cast group, n = 10; K-wire group, n = 7) and four deaths (cast group, n = 2; K-wire group, n = 2). In most cases, the reason for withdrawals was not wanting to complete questionnaires (nine in total: cast group, n = 6; K-wire group, n = 3) or participant decision (four in total: cast group, n = 2; K-wire group, n = 2). Seventeen participants withdrew from the study; however, five of them agreed for medical routine data to still be collected for the trial by the local research team. There were four deaths, two of unknown cause, one was due to stroke and the other due to suspected graft-versus-host disease.
Primary outcome
Analysis of primary outcome: Patient-Rated Wrist Evaluation score at 12 months post randomisation
The PRWE score was assessed at baseline (pre and post injury), and at 3, 6 and 12 months post injury. The mean PRWE score and SD for each treatment group at each time point (excluding pre-injury baseline as this did not contribute to the analysis model) are provided in Table 9 and were based on all available data at each time point. The adjusted mean differences in PRWE score were obtained from a linear mixed-effects regression model adjusting for the stratification factors age and articular extension and observations within participants within recruitment centres as random effects. Baseline (post injury) was included as a response, rather than adjusted for as continuous covariate fixed effect, to obtain time point-specific estimates for baseline to be used in the AUC analysis. The number of observations for each treatment group is the number of participants who supplied PRWE data at that time point. The analysis was performed on the prespecified ITT population defined in Chapter 2, Definition of analysis populations. The adjusted analysis was prespecified as the primary trial results.
| Time point | Treatment group | Mean difference | p-value | ||||
|---|---|---|---|---|---|---|---|
| Cast | K-wire | ||||||
| n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusted (95% CI) | ||
| Baseline (post injury) | 253 | 84.3 (13.30) | 243 | 81.91 (14.52) | –2.39 | ||
| 3 months | 213 | 42.08 (23.85) | 201 | 41.56 (24.77) | –0.51 | –0.45 (–4.37 to 3.47) | 0.82 |
| 6 months | 202 | 28.35 (23.35) | 206 | 27.56 (22.33) | –0.79 | –0.32 (–4.26 to 3.62) | 0.87 |
| 12 months (primary outcome) | 200 | 21.16 (23.09) | 195 | 20.69 (22.33) | –0.47 | –0.34 (–4.33 to 3.66) | 0.87 |
| AUC over 12 months | 38.19a | 37.60a | –0.60 (–4.41 to 3.21) | 0.88 | |||
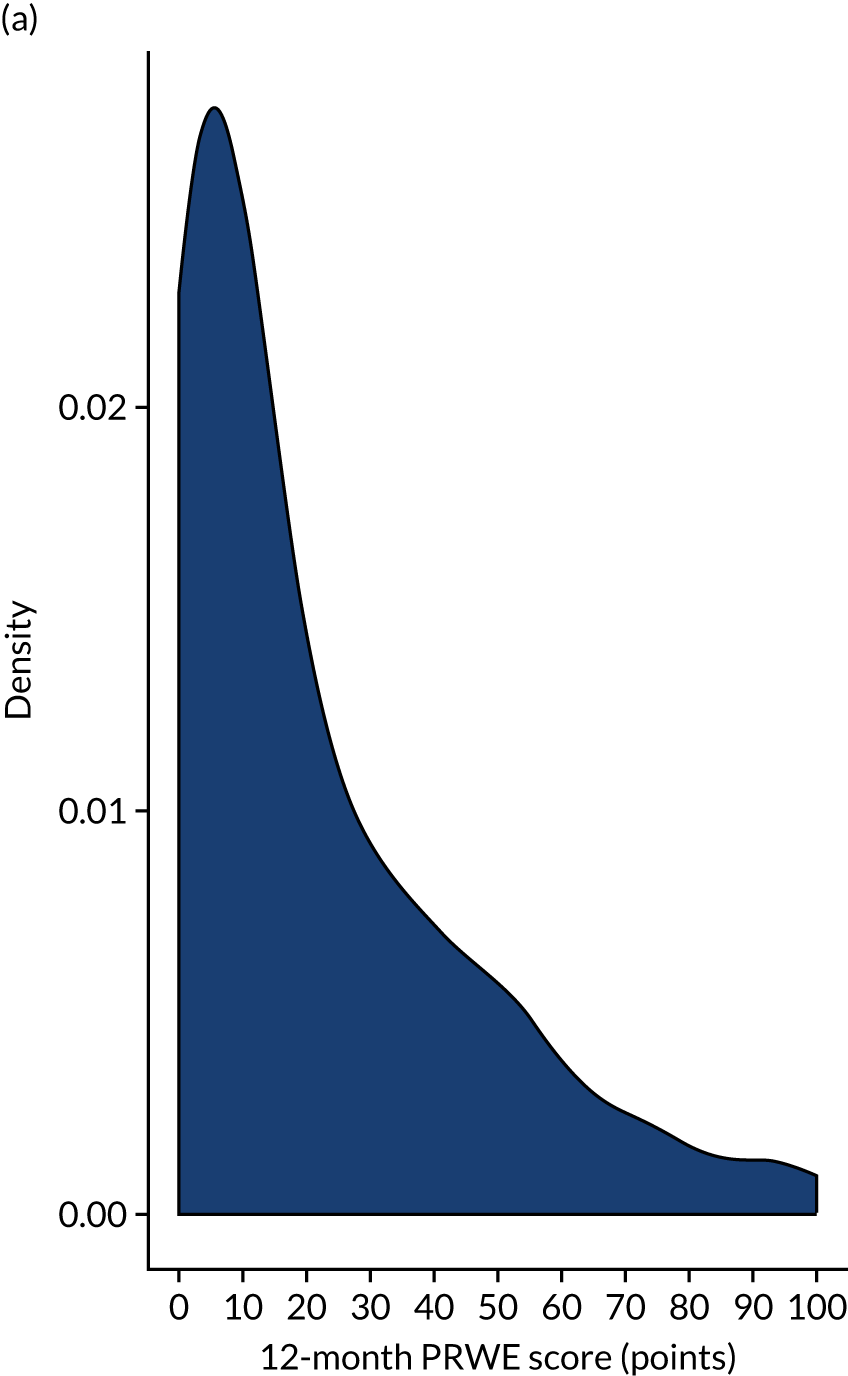
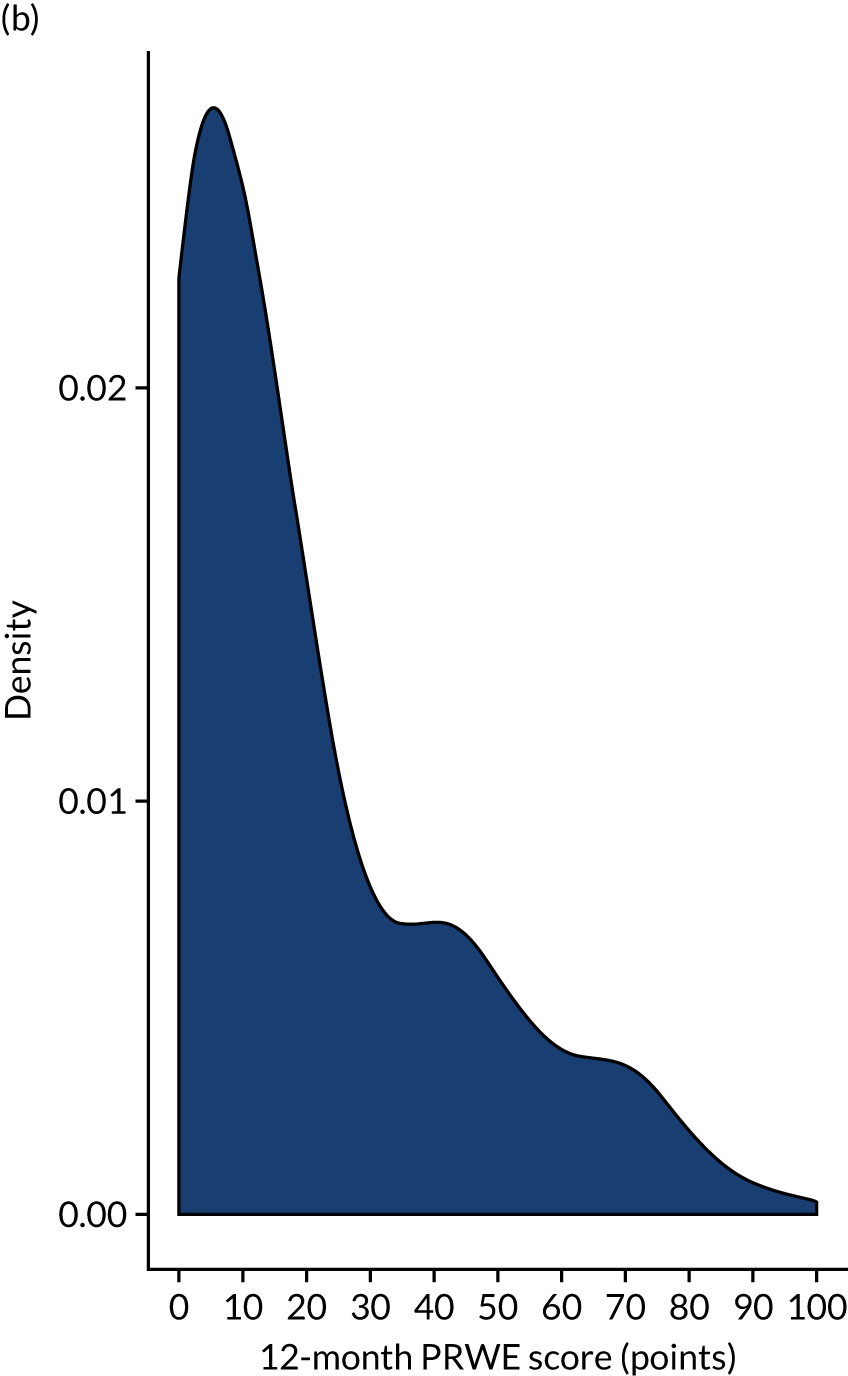
The adjusted results in Table 9 show no significant treatment effect at any of the time points or in the AUC analysis. The primary outcome (i.e. PRWE score at 1 year) shows a between-group difference of –0.34 (95% CI –4.33 to 3.66; p = 0.87). Figure 3 shows the means and SDs at each time point used in the analysis model (baseline post injury and 3, 6 and 12 months post randomisation). Figure 4 shows the density plot of the 12-month PRWE score for both the cast and the K-wire groups.
FIGURE 3.
Change in PRWE score by treatment group over time.
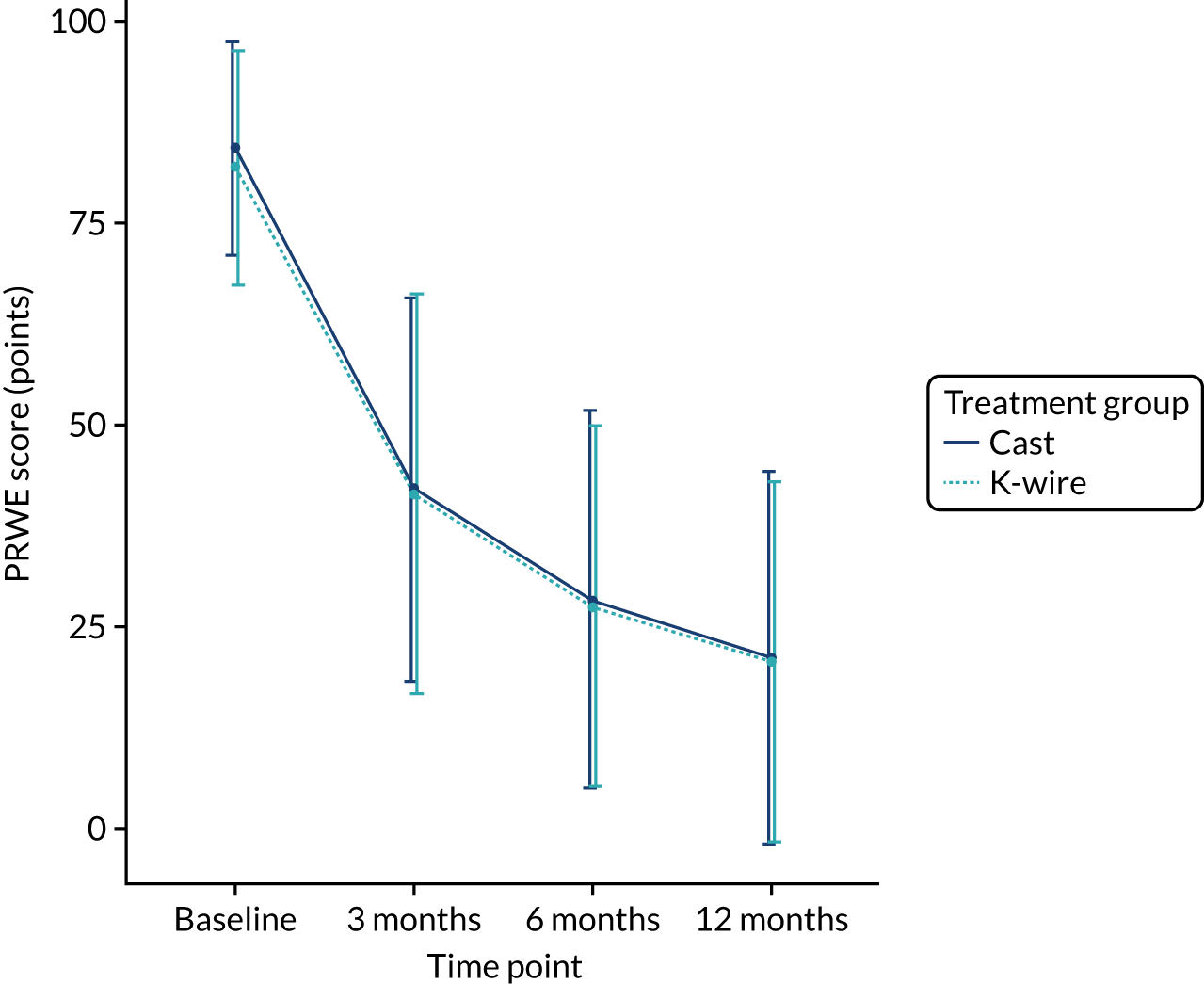
FIGURE 4.
Histograms of PRWE score at 12 months post randomisation by treatment group. (a) Cast group and (b) K-wire group.
Graphical model checks were performed as part of the analysis and indicated no issues with the model fit as well as no evidence of the underlying assumptions not being met. These checks are available on the project webpage (www.journalslibrary.nihr.ac.uk/programmes/hta/152701/#/; accessed October 2021) .
Secondary outcomes
EuroQol-5 Dimensions, five-level version, index
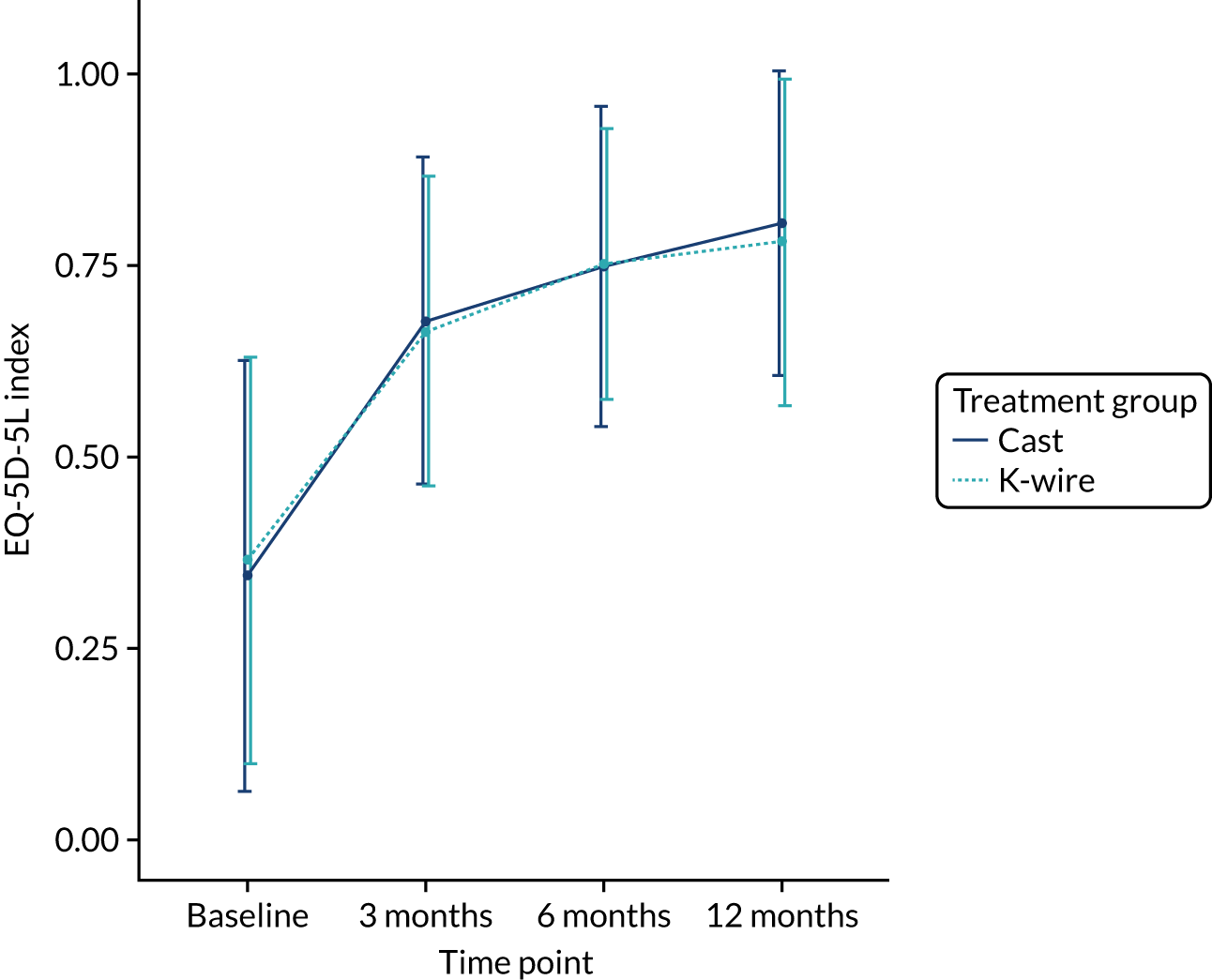
The secondary outcomes (i.e. EQ-5D-5L index at 3, 6 and 12 months post randomisation, as well as the EQ-5D-5L index AUC) are presented in Table 10. A mixed-effects model, as used in the primary outcome, was used to assess these outcomes. The means and SDs are presented, as well as adjusted mean differences, 95% CIs and p-values derived from the model. A graphical summary of the EQ-5D-5L index means and SDs for each time point by treatment group is shown in Figure 5. The EQ-5D-5L index showed improvement over time from injury until 12 months post randomisation, but there was no significant treatment effect at any time point or AUC analysis with a 12-month adjusted mean difference of –0.03 (95% CI –0.07 to 0.02). The distribution of the five domains is presented in Appendix 2, Figures 17–21.
| Time point | Treatment group | Mean difference | p-value | ||||
|---|---|---|---|---|---|---|---|
| Cast | K-wire | ||||||
| n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusted (95% CI) | ||
| Baseline (post injury) | 252 | 0.35 (0.28) | 241 | 0.37 (0.26) | 0.02 | ||
| 3 months | 217 | 0.68 (0.21) | 203 | 0.67 (0.20) | –0.01 | –0.02 (–0.06 to 0.02) | 0.37 |
| 6 months | 204 | 0.75 (0.21) | 207 | 0.75 (0.18) | 0.00 | 0.00 (–0.04 to 0.04) | 0.96 |
| 12 months | 199 | 0.81 (0.20) | 197 | 0.78 (0.21) | –0.02 | –0.03 (–0.07 to 0.02) | 0.26 |
| AUC over 12 months | 0.69a | 0.68a | –0.01 (–0.05 to 0.03) | 0.82 | |||
FIGURE 5.
Change in EQ-5D-5L index by treatment group over time.
EuroQol-5 Dimensions visual analogue scale
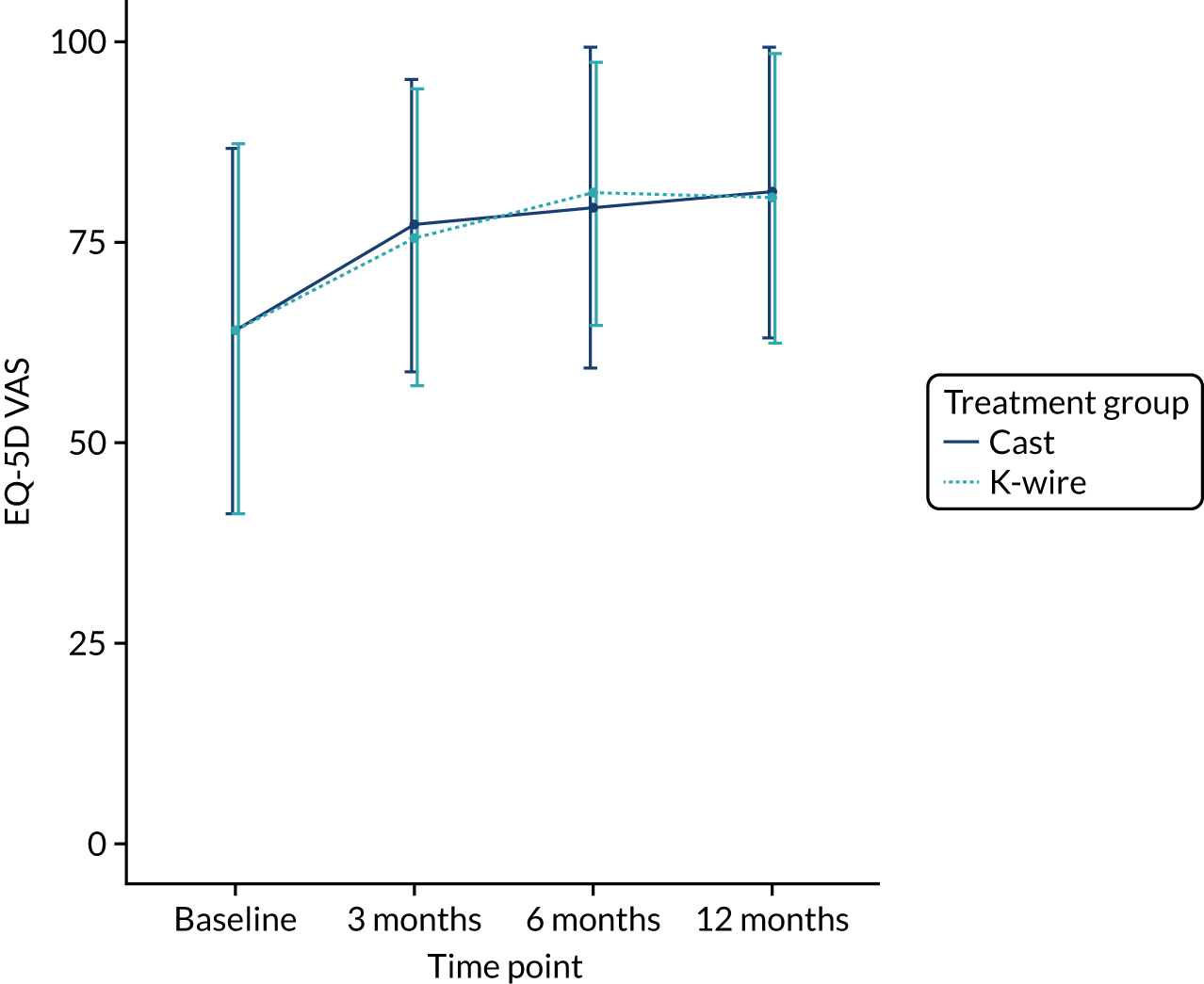
The secondary outcome EQ-VAS, a QoL score running from 0 to 100 with higher values indicating better QoL, at 3, 6 and 12 months post randomisation, and the EQ-VAS AUC scores are presented in Table 11. A linear mixed-effects model, as used in the primary outcome, was used to assess these outcomes. The means and SDs are given along with adjusted mean differences, 95% CIs and p-values derived from the model. A plot of the EQ-5D VAS means and SDs on all available data for each time point by treatment group is given in Figure 6. The EQ-5D VAS showed improvement over time but there was no significant treatment effect at any time point or AUC analysis with a 12-month adjusted mean difference of –0.51 (95% CI –4.30 to 3.29; p = 0.79). This result is supported by Figure 6, indicating no raw mean difference at any time.
| Time point | Treatment group | Mean difference | p-value | ||||
|---|---|---|---|---|---|---|---|
| Cast | K-wire | ||||||
| n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusted (95% CI) | ||
| Baseline (post injury) | 253 | 63.87 (22.76) | 242 | 64.19 (23.14) | 0.32 | ||
| 3 months | 216 | 77.08 (18.25) | 201 | 75.55 (18.51) | –1.53 | –1.73 (–5.44 to 1.98) | 0.36 |
| 6 months | 202 | 79.29 (20.02) | 204 | 81.09 (16.37) | 1.80 | 1.87 (–1.88 to 5.61) | 0.33 |
| 12 months | 199 | 81.11 (18.06) | 195 | 80.42 (18.00) | –0.69 | –0.51 (–4.30 to 3.29) | 0.79 |
| AUC over 12 months | 76.81a | 76.98a | 0.16 (–3.59 to 3.92) | 0.97 | |||
FIGURE 6.
Change in EQ-5D VAS by treatment group over time.
Complications
Complications, including the need for further surgery, CRPS and DVT/PE were analysed by calculating the odds ratio and 95% CI using logistic regression. The total number of further surgeries was analysed using a Poisson regression adjusting for randomised treatment and randomisation variables as that is a count variable, not binary. Other individual complications were summarised by treatment group, but not analysed, and reported in Table 12. The logistic regression and Poisson models adjusted for randomised treatment and the variables used in the stratification process (age group, extension of the fracture, and recruitment centre).
| Complication | Treatment group, n (%) | |
|---|---|---|
| Cast | K-wire | |
| Cast-specific complications (6 weeks) | ||
| Cast discomfort | 65 (25.5) | 32 (13.0) |
| Cast changed | 72 (28.2) | 105 (42.9) |
| Pressure sores | 11 (4.3) | 3 (1.2) |
| K-wire-specific complications (6 weeks) | ||
| Neurological injury | 0 (0.00) | 7 (2.9) |
| Vascular injury | 0 (0.00) | 0 (0.00) |
| Tendon injury | 0 (0.00) | 4 (1.6) |
| Redness around wound | 0 (0.00) | 20 (8.2) |
| Leaking from pin site | 0 (0.00) | 16 (6.5) |
| If leaking, purulent drainage | 0 (0.00) | 4 (1.6) |
| If leaking, confirmation of infection | 0 (0.00) | 4 (1.6) |
At 6 weeks post randomisation, there was evidence that K-wire fixation reduced the need for further surgery because of loss of fracture reduction (Table 13). There were 33 occurrences of loss of fracture reduction in the cast group, compared with one occurrence in the K-wire group (odds ratio 0.02, 95% CI 0.001 to 0.10). The majority of the participants who had further surgery because of a loss of reduction were those whose original fracture was extra-articular (70%) and were aged > 50 years (76%). There were only two additional surgeries in the first 6 weeks post randomisation that were not due to loss of reduction.
| Details of further surgery | Treatment group, n (%) | Odds ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Cast | K-wire | |||
| Reasons for further surgery | ||||
| Loss of reduction | 33 (12.94) | 1 (0.41) | 0.02 (0.001 to 0.10) | < 0.001 |
| Other reason for surgery | 1 (0.39) | 1 (0.41) | ||
| Fracture type in those with loss of reduction | ||||
| Extra-articular | 22 (8.63) | 1 (0.41) | ||
| Intra-articular | 11 (4.31) | 0 (0.00) | ||
| Age (years) of those with loss of reduction | ||||
| < 50 | 9 (3.53) | 0 (0.00) | ||
| ≥ 50 | 24 (9.41) | 1 (0.41) | ||
| Treatment received in further surgery | ||||
| Cast | 1 (0.39) | 0 (0.00) | ||
| K-wire | 11 (4.31) | 1 (0.41) | ||
| Plate | 22 (8.63) | 1 (0.41) | ||
During the 6-week to 12-month follow-up period there were six additional surgeries in the cast group, compared with three in the K-wire group. None of these surgeries was due to loss of reduction. The reasons for the additional surgeries included a second fall, carpal tunnel, tendon transfer and stiffness. The number of other generic complications during the first year post randomisation was similar in both treatment groups (cast group, n = 28; K-wire group, n = 22). These complications included osteoarthritis in the thumb, cellulitis around the pin site, stiffness, swelling, loss of radial height, numbness, sensitivity, a scapholunate injury and pain. No statistically significant result was found when testing for associations between the treatment groups and these complications (Table 14).
| Details of further surgery | Treatment group, n (%) | Odds ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Cast group | K-wire group | |||
| Total number of further surgeries | 40 (15.69) | 5 (2.04) | 0.13 (0.04 to 0.23) | < 0.001 |
| Number of participants who had further surgery | 37 (14.51) | 5 (2.04) | 0.08 (0.02 to 0.20) | < 0.001 |
| Time point of further surgery | ||||
| 6 weeks | 34 (13.33) | 2 (0.82) | ||
| 3 months | 1 (0.39) | 1 (0.41) | ||
| 6 months | 1 (0.39) | 2 (0.82) | ||
| 12 months | 4 (1.57) | 0 (0.00) | ||
| Generic complications | ||||
| CRPS | 5 (1.96) | 7 (2.86) | 1.45 (0.43 to 5.28) | 0.55 |
| Other complications | 28 (10.98) | 22 (8.98) | 0.74 (0.39 to 1.39) | 0.35 |
The PRWE score at 12 months post randomisation among those in the cast group who had further surgery because of loss of reduction [mean 20.79 points (SD 20.95 points)] was very similar to that in the cast treatment group as a whole [mean 21.16 points (SD 23.09 points)] (Table 15).
| Time point | Total cast group | Loss of reduction in the cast group | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline (post injury) | 253 | 84.3 (13.30) | 33 | 86.52 (9.51) |
| 3 months | 213 | 42.08 (23.85) | 32 | 45.46 (22.46) |
| 6 months | 202 | 28.35 (23.35) | 30 | 29.17 (22.61) |
| 12 months | 200 | 21.16 (23.09) | 28 | 20.79 (20.95) |
Missing data
The primary analysis was performed using a complete-case analysis, which assumed the data were MAR (i.e. the unobserved data were from the same conditional distribution as the observed data.) This seemed to be a plausible assumption for this trial as the number of patients lost to follow-up was low and there were roughly equal numbers of missing data across both treatment groups. However, two sensitivity analyses on missing data were performed.
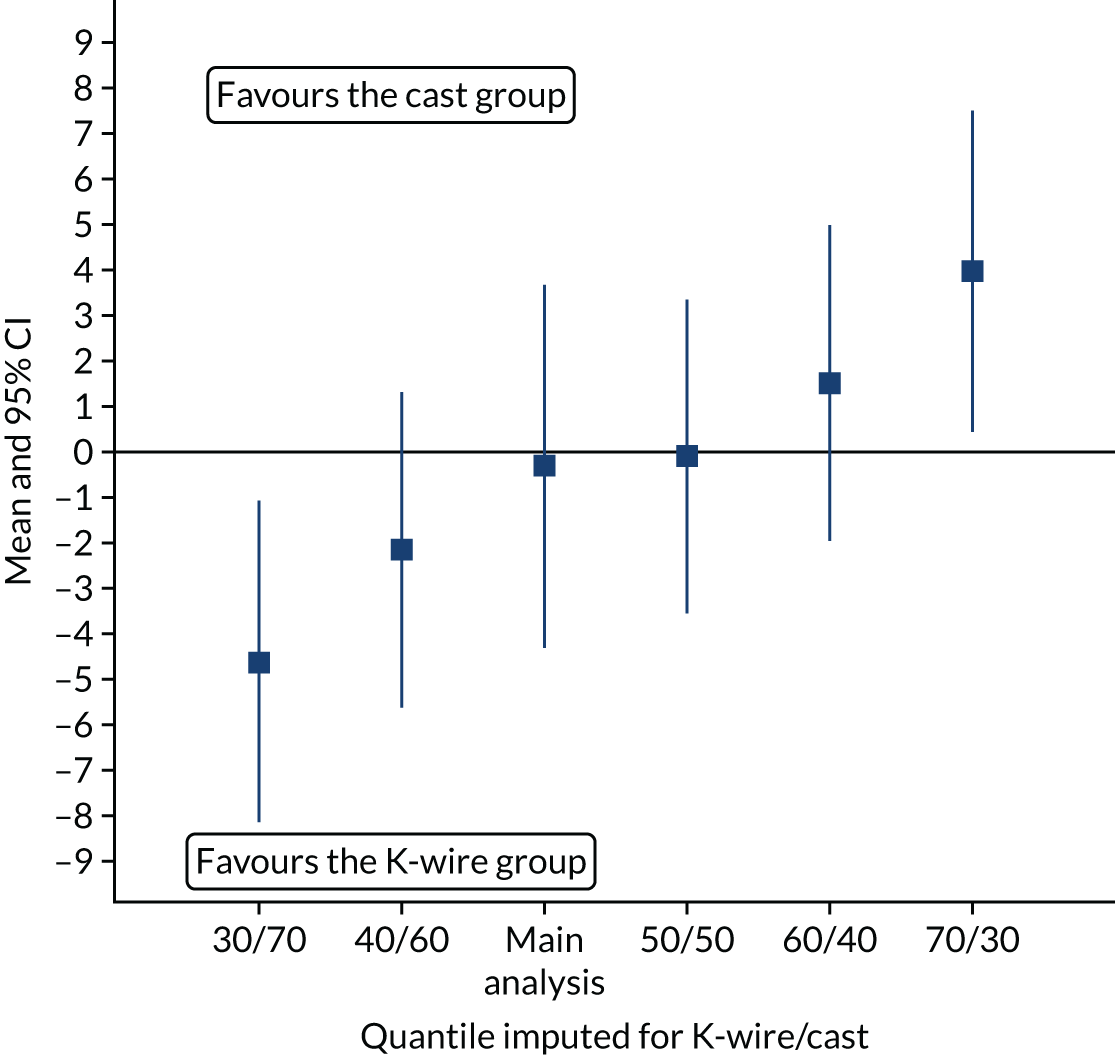
The first sensitivity analysis used imputation of quantiles. For each missing data point, the median value of the treatment group was imputed and analysed using the same mixed model as in the primary analysis. The analysis was repeated on a population that had the 60th quantile imputed for one group’s missing values and the 40th quantile for the other, then again using the 70th and 30th quantiles. The process was repeated but the treatment groups were swapped. In total, five sensitivity analyses were performed and the results were displayed graphically (see Figure 7).
Figure 7 shows how the estimates of the adjusted mean difference changed depending on the values imputed. This sensitivity analysis showed that there would need to be a large violation of the MAR assumption in the main analysis to render the results invalid and such a departure seems unlikely.
FIGURE 7.
The 12-month PRWE scores (points) for imputed quantiles.
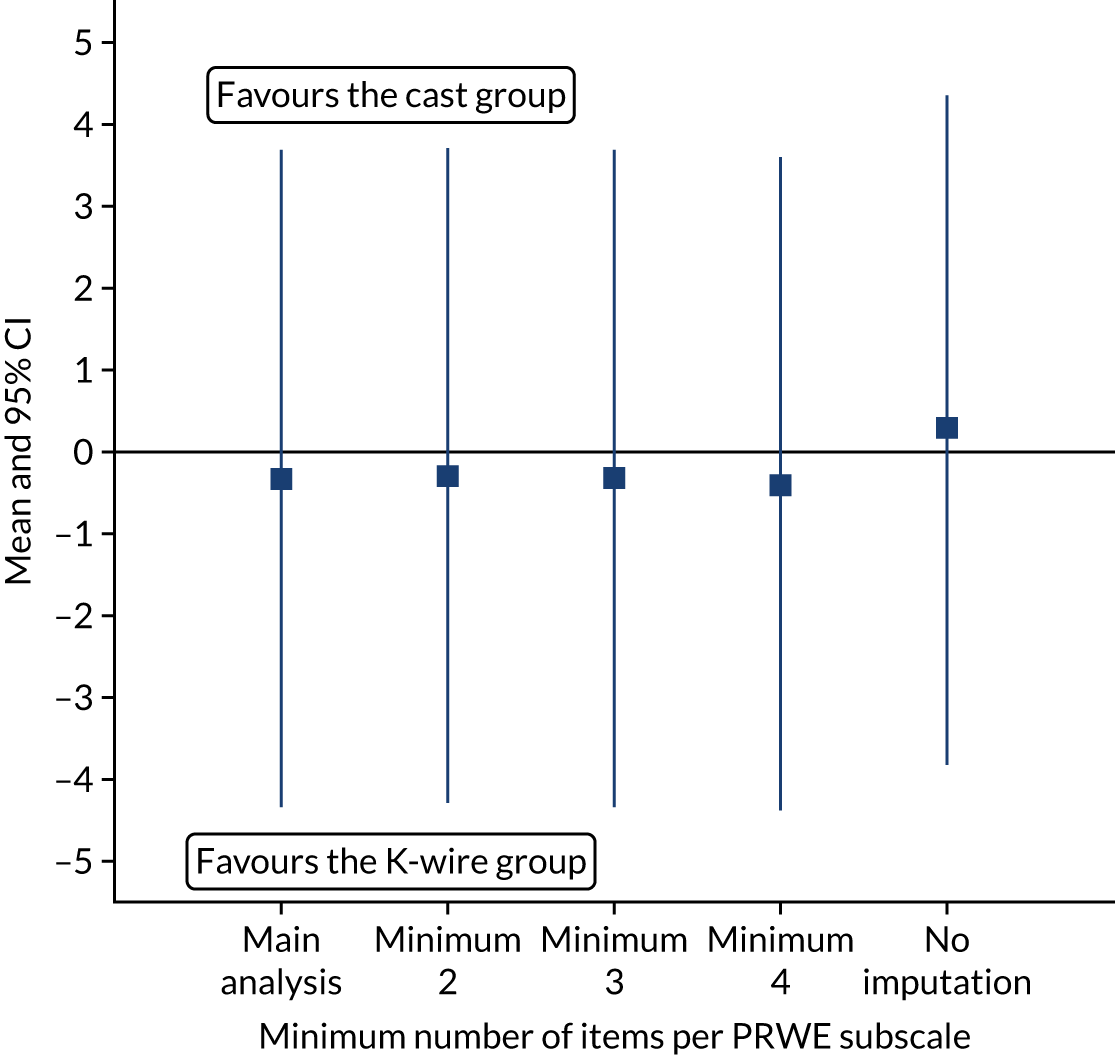
In the primary analysis it was assumed that one item present per subscale was not defined as missing; in the second sensitivity analysis, the effect of requiring at least two, three, four and all items present in a subscale to not be called missing; was examined. Figure 8 showed that there was no difference in the estimated treatment effect at 12 months post randomisation for different ways of handling missing data in the PRWE subscales.
FIGURE 8.
The 12-month PRWE scores (points) for different minimum number of items required in the PRWE subscales.
Sensitivity analysis
Two sensitivity analyses were specified in the SHEAP:19 a PP analysis on PRWE outcomes to examine different assumptions about departures from randomised policies and a three-level model with participant within surgeon within recruitment centre to examine potential surgeon (random) effects. This model incorporated terms that allowed for possible heterogeneity in patients’ responses as a result of there being multiple recruiting surgeons as well as recruitment centres.
Per protocol
This sensitivity analysis used the same mixed model as in the primary analysis, adjusting for person within recruitment centre as random effects and age and articular extension as fixed effects. Baseline and the other time points were included, with treatment by time point interactions also included to allow time-specific treatment effects to be calculated. The results are presented in Table 16. These results support the result from the main analysis that there was no treatment effect at any of the time points on the PRWE (12-month PRWE adjusted mean difference of –0.80 points, 95% CI –5.11 to 3.52 points; p = 0.72).
| Time point | Treatment group | Mean score difference (points) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Cast | K-wire | ||||||
| n | Mean score (SD) (points) | n | Mean score (SD) (points) | Unadjusted | Adjusted (95% CI) | ||
| Baseline (post injury) | 188 | 83.94 (13.62) | 189 | 81.51 (13.67) | –2.43 | ||
| 3 months | 179 | 42.11 (23.78) | 176 | 40.23 (24.75) | –1.88 | –2.12 (–6.51 to 2.27) | 0.34 |
| 6 months | 178 | 28.52 (23.16) | 186 | 27.53 (22.51) | –0.99 | –1.03 (–5.39 to 3.32) | 0.64 |
| 12 months | 189 | 21.73 (23.38) | 191 | 20.85 (22.42) | –0.88 | –0.80 (–5.11 to 3.52) | 0.72 |
| AUC over 12 months | 38.07a | 36.67a | –1.39 (–6.12 to 3.34) | 0.77 | |||
Multilevel analysis
An analysis using a three-level model with participant within surgeon within recruitment centre to examine potential surgeon (random) effects was fitted, and the results are shown in the Table 17. This model incorporated terms that allowed for possible heterogeneity in patients’ responses as a result of there being a number of recruiting surgeons as well as recruitment centres, in addition to the fixed effects of the treatment groups, patient age, articular extension and baseline PRWE score. This was different from the primary analysis model, which did not incorporate baseline PRWE score as a covariate as was needed for AUC analysis. As there was no AUC analysis from this model, baseline is included as a covariate, but interaction terms for other time points (3 and 6 months) were retained.
| Time point | Treatment group | Mean score difference (points) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Cast | K-wire | ||||||
| n | Mean score (SD) (points) | n | Mean score (SD) (points) | Unadjusted | Adjusted (95% CI) | ||
| Baseline (post injury) | 253 | 84.3 (13.30) | 243 | 81.91 (14.52) | –2.39 | ||
| 3 months | 213 | 42.08 (23.85) | 201 | 41.56 (24.77) | –0.51 | 0.55 (–3.70 to 4.79) | 0.80 |
| 6 months | 202 | 28.35 (23.35) | 206 | 27.56 (22.33) | –0.79 | 0.85 (–3.41 to 5.12) | 0.70 |
| 12 months | 200 | 21.16 (23.09) | 195 | 20.69 (22.33) | –0.47 | 0.65 (–3.70 to 4.99) | 0.77 |
Table 17 shows that the direction of the treatment effect on the 12-month PRWE score was the opposite of that in the main analysis (i.e. of opposite sign) and suggests that there is an adjusted mean difference of 0.65 points favouring cast treatment (95% CI –3.70 to 4.99 points). There was still no statistically significant result for any time point, which reinforced the overall interpretation of the clinical trial results.
Subgroup analysis
There were two predefined subgroups analyses for DRAFFT 2 based on the two clinical stratifying variables (i.e. age and articular extension). These were performed using an extended model to formally test the interaction between each stratifying variable and the treatment factor. The interaction test results are reported in Figure 9 as adjusted mean differences and 95% CIs. Two models were fitted: one fitting an interaction term with age and one fitting an interaction term with articular extension. A linear mixed model adjusting for baseline PRWE score, age and articular extension as fixed effects and participant within recruitment centre was used as a base model to perform these analyses; time point (3 and 6 months) interactions with treatment effect were fitted. It is important to note that subgroup analyses are exploratory and hypothesis generating, so results from Figure 9 should be interpreted with caution. No significant difference between any of the predefined subgroups in treatment effect on the 12-month PRWE score was found.
FIGURE 9.
Forest plot of predefined clinical subgroups’ 12-month PRWE score.
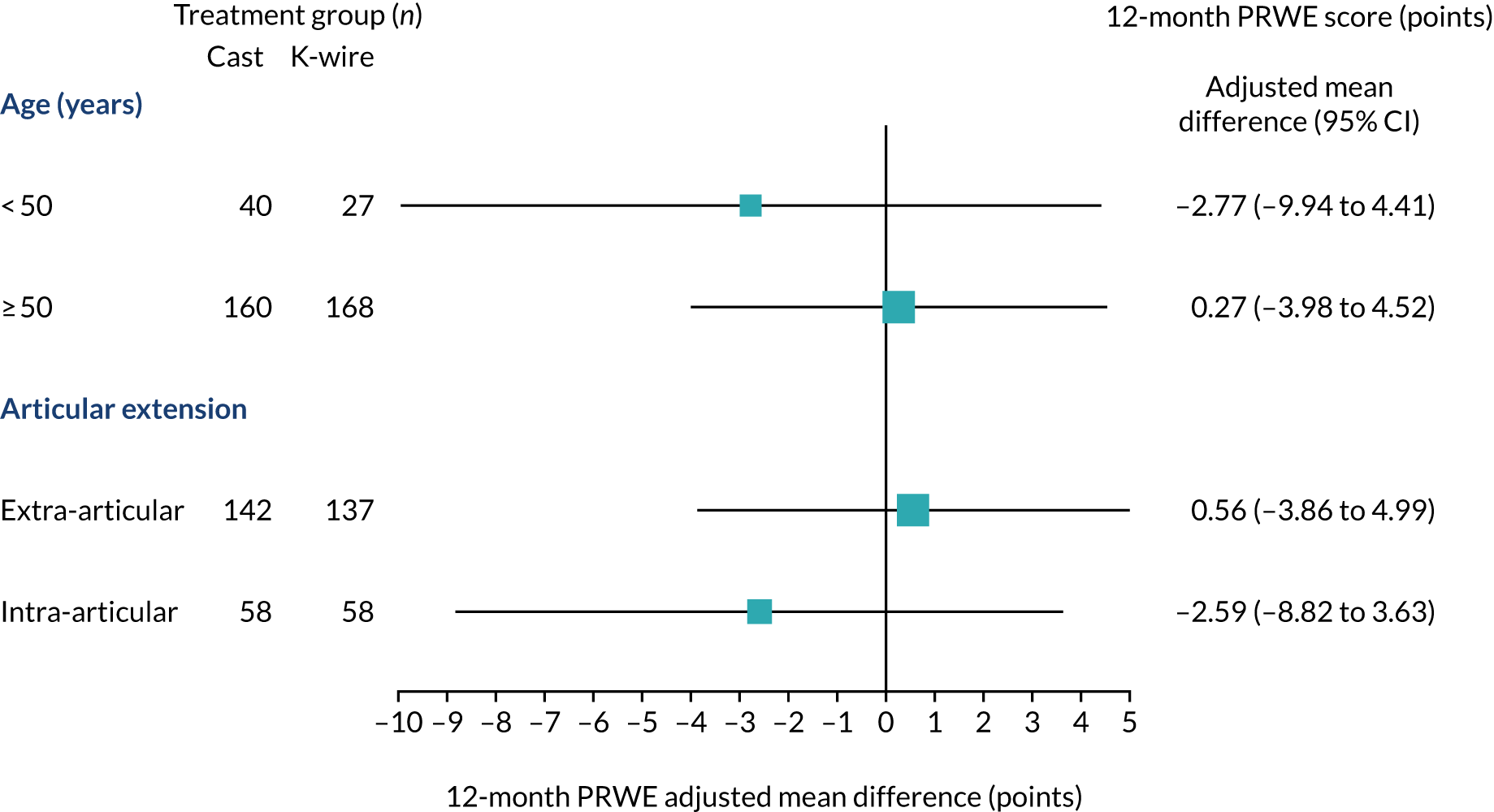
Adverse events
Most adverse events were reported as complications in Chapter 2, Secondary outcomes.
There were 10 other SAEs (cast group, n = 5; K-wire group, n = 5); all were classified as ‘unexpected’ and all but one were classified as ‘not related’. The other SAE, which was classified as ‘unlikely to be related’, was a patient readmitted with discitis and primary hyperparathyroidism. Other SAEs included a fall leading to hip fracture, chest pain, removal of a cataract and admission to a psychiatric hospital.
Chapter 4 Health economic evaluation
Completion rate
A complete set of EQ-5D and health-care resource use values (from the NHS and PSS perspective) from baseline to 12 months post randomisation was collected from 290 out of 500 participants (58.0%). Completion rates of all the health-care resource items by treatment group for each time point are shown in Table 18 and the response rate of each domain of the EQ-5D-5L is presented in Appendix 2, Table 22. Our data are non-monotonic; that is, participants who did not complete the 3-month questionnaire could have completed the 6- or 12-month questionnaire.
| Health-care resource use type | Treatment group, n (%) | |
|---|---|---|
| Cast (N = 255) | K-wire (N = 245) | |
| Data available on health-care resource use only | 147 (57.6) | 151 (61.6) |
| Data available on health-care resource use and quality of life | 143 (56.1) | 147 (60.0) |
| 3 months | 216 (84.7) | 202 (82.4) |
| Subsequent inpatient care | 214 (83.9) | 202 (82.4) |
| Outpatient care | 214 (83.9) | 202 (82.4) |
| Community care | 215 (84.3) | 202 (82.4) |
| Medications | 216 (84.7) | 201 (82.0) |
| PSS | 216 (84.7) | 202 (82.4) |
| Aids and adaptations | 216 (84.7) | 200 (81.6) |
| Time off work | 136 (53.3) | 126 (51.4) |
| Additional costsa | 213 (83.5) | 202 (82.4) |
| 6 months | 203 (79.6) | 206 (84.1) |
| Subsequent inpatient care | 203 (79.6) | 206 (84.1) |
| Outpatient care | 203 (79.6) | 206 (84.1) |
| Community care | 202 (79.2) | 206 (84.1) |
| Medications | 198 (77.6) | 203 (82.9) |
| PSS | 203 (79.6) | 205 (83.7) |
| Aids and adaptations | 202 (79.2) | 205 (83.7) |
| Time off work | 203 (79.6) | 203 (82.9) |
| Additional costsa | 202 (79.2) | 205 (83.7) |
| 12 months | 200 (78.4) | 196 (80.0) |
| Subsequent inpatient care | 199 (78.0) | 195 (79.6) |
| Outpatient care | 199 (78.0) | 196 (80.0) |
| Community care | 199 (78.0) | 195 (79.6) |
| Medications | 199 (78.0) | 195 (79.6) |
| PSS | 199 (78.0) | 196 (80.0) |
| Aids and adaptations | 199 (78.0) | 195 (79.6) |
| Time off work | 197 (77.3) | 196 (80.0) |
| Additional costsa | 197 (77.3) | 195 (79.6) |
Health-care resource use
The observed health-care resource use recorded by participants is presented Appendix 2, Table 23, by treatment group per follow-up time point. There were no significant differences in health-care resource utilisation rates between the treatment groups.
Health-care costs
The mean costs of the key resource inputs associated with the trial are summarised in Table 19. The mean unadjusted costs (SE) by treatment groups and follow-up time points, in 2018/19 prices (GBP) (available case). There were no significant differences in the mean cost between treatment groups except for the following: the mean initial treatment cost was higher in the K-wire group [£1520.86 (SE £16.52)] than in the cast group [£1363.69 (SE £15.76); t-test, p < 0.001], but the mean cost of treatment of complications between baseline and 12 months post randomisation was lower in the K-wire group [£11.59 (SE £6.28)] than in the cast group [£105.15 (SE £18.10); t-test, p < 0.001]. In terms of medications from baseline to 12 months post randomisation, those randomised to the cast group incurred a higher mean cost [£2.75 (SE £0.75)] than those in the K-wire group [£0.98 (SE £0.25); t-test, p = 0.027]. The mean additional cost, which refers to additional (private) cost items incurred by participants and their next of kin (e.g. travel expenditure, child care and help with housework), between baseline and 3 months post randomisation was higher in the K-wire group [£42.09 (SE £10.49)] than in the cast group [£19.03 (SE £3.58); t-test, p = 0.039].
| Health-care resource use type | Treatment group, mean unadjusted costs (SE) (£) | Mean difference (£) | Bootstrapped 95% CI (£) | p-valuea | |
|---|---|---|---|---|---|
| Cast | K-wire | ||||
| Baseline to 12 months | |||||
| Initial treatment costb | 1363.69 (15.76) | 1520.86 (16.52) | 157.18 | 111.78 to 202.96 | < 0.001 |
| Treatment of complicationsc | 105.15 (18.10) | 11.59 (6.28) | –93.56 | –131.73 to –57.63 | < 0.001 |
| Subsequent inpatient care | 24.08 (10.80) | 5.58 (5.58) | –18.50 | –42.92 to 3.99 | 0.129 |
| Outpatient care | 272.79 (27.72) | 267.92 (24.11) | –4.87 | –77.47 to 66.40 | 0.895 |
| Community care | 38.56 (6.49) | 43.13 (6.49) | 4.58 | –13.68 to 22.49 | 0.618 |
| Medications | 2.75 (0.75) | 0.98 (0.25) | –1.76 | –3.43 to –0.37 | 0.027 |
| PSS | 20.51 (15.89) | 12.16 (10.79) | –8.36 | –49.04 to 26.6 | 0.664 |
| Aids and adaptations | 8.28 (1.06) | 7.92 (1.65) | –0.36 | –4.05 to 3.79 | 0.854 |
| Total cost, NHS and PSS | 1835.40 (46.96) | 1870.15 (38.04) | 34.75 | –82.51 to 153.21 | 0.566 |
| Medications, out of pocket | 0.78 (0.15) | 0.77 (0.19) | –0.01 | –0.46 to 0.50 | 0.982 |
| Time off work | 807.70 (158.89) | 943.89 (197.48) | 136.19 | –358.39 to 635.40 | 0.591 |
| Additional costs | 22.65 (4.31) | 43.96 (10.25) | 21.30 | 1.25 to 44.50 | 0.056 |
| Total cost, societal | 2666.52 (169.03) | 2858.77 (211.42) | 192.24 | –333.73 to 729.72 | 0.478 |
| Breakdown: baseline to discharge | |||||
| Initial treatment costb | 1363.69 (15.76) | 1520.86 (16.52) | 157.18 | 111.78 to 202.96 | < 0.001 |
| Treatment of complicationsc | 105.15 (18.10) | 11.59 (6.28) | –93.56 | –131.73 to –57.63 | < 0.001 |
| Total cost, NHS and PSS | 1468.43 (24.18) | 1532.45 (17.73) | 64.02 | 4.50 to 123.22 | 0.033 |
| Breakdown: discharge to 3 months | |||||
| Subsequent inpatient care | 11.45 (8.13) | 0.00 (0.00) | –11.45 | –29.26 to 0.00 | 0.160 |
| Outpatient care | 155.63 (14.10) | 162.38 (14.45) | 6.74 | –32.00 to 46.28 | 0.739 |
| Community care | 25.36 (5.38) | 23.62 (4.01) | –1.74 | –15.57 to 11.00 | 0.796 |
| Medications | 1.89 (0.70) | 0.86 (0.24) | –1.04 | –2.65 to 0.23 | 0.166 |
| PSS | 24.19 (18.75) | 14.80 (13.15) | –9.39 | –57.91 to 32.99 | 0.682 |
| Aids and adaptations | 5.26 (0.69) | 5.46 (1.61) | 0.20 | –2.67 to 4.09 | 0.909 |
| Total cost, NHS and PSS | 187.86 (22.77) | 170.64 (18.19) | –17.22 | –76.39 to 38.88 | 0.555 |
| Medications, out of pocket | 0.77 (0.16) | 0.77 (0.17) | 0.00 | –0.46 to 0.45 | 0.999 |
| Time off work | 1060.71 (210.84) | 1104.92 (214.37) | 44.21 | –547.71 to 627.81 | 0.883 |
| Additional costs | 19.03 (3.58) | 42.09 (10.49) | 23.06 | 3.94 to 46.95 | 0.039 |
| Total cost, societal | 770.17 (120.86) | 765.34 (119.28) | –4.83 | –342.56 to 326.69 | 0.977 |
| Breakdown: 3–6 months | |||||
| Subsequent inpatient care | 0.00 (0.00) | 6.61 (6.61) | 6.61 | 0.00 to 19.83 | 0.318 |
| Outpatient care | 102.73 (14.24) | 100.70 (13.41) | –2.03 | –40.23 to 36.35 | 0.918 |
| Community care | 15.42 (4.23) | 17.45 (3.72) | 2.03 | –9.33 to 12.57 | 0.718 |
| Medications | 0.42 (0.16) | 0.18 (0.06) | –0.24 | –0.61 to 0.07 | 0.165 |
| PSS | 0.02 (0.02) | 0.00 (0.00) | –0.02 | –0.07 to 0.00 | 0.318 |
| Aids and adaptations | 2.67 (0.75) | 2.12 (0.70) | –0.54 | –2.55 to 1.50 | 0.598 |
| Total cost, NHS and PSS | 97.13 (13.13) | 107.19 (14.90) | 10.06 | –27.44 to 50.11 | 0.613 |
| Medications, out of pocket | 0.30 (0.13) | 0.26 (0.17) | –0.04 | –0.42 to 0.41 | 0.866 |
| Time off work | 1236.75 (428.96) | 2046.50 (671.53) | 809.75 | –652.09 to 2376.41 | 0.316 |
| Additional costs | 5.59 (2.75) | 5.60 (2.17) | 0.01 | –7.22 to 6.35 | 0.998 |
| Total cost, societal | 218.15 (47.94) | 312.51 (79.44) | 94.36 | –79.97 to 290.85 | 0.310 |
| Breakdown: 6–12 months | |||||
| Subsequent inpatient care | 18.31 (10.66) | 0.00 (0.00) | –18.31 | –41.31 to 0.00 | 0.087 |
| Outpatient care | 74.83 (18.18) | 59.77 (9.75) | –15.07 | –57.27 to 23.13 | 0.466 |
| Community care | 7.70 (2.21) | 12.35 (4.87) | 4.65 | –4.85 to 15.86 | 0.385 |
| Medications | 1.16 (0.59) | 0.21 (0.12) | –0.95 | –2.28 to 0.04 | 0.114 |
| PSS | 0.00 (0.00) | 0.02 (0.02) | 0.02 | 0.00 to 0.05 | 0.319 |
| Aids and adaptations | 2.13 (0.67) | 2.06 (0.79) | –0.08 | –2.05 to 2.07 | 0.942 |
| Total cost, NHS and PSS | 81.98 (18.37) | 59.87 (10.16) | –22.11 | –64.66 to 17.02 | 0.293 |
| Medications, out of pocket | 0.00 (0.00) | 0.07 (0.06) | 0.06 | –0.01 to 0.19 | 0.318 |
| Time off work | 2134.93 (1228.59) | 3471.31 (1228.91) | 1336.37 | –2066.78 to 4526.59 | 0.449 |
| Additional costs | 2.83 (1.64) | 5.44 (2.96) | 2.61 | –3.27 to 10.02 | 0.442 |
| Total cost, societal | 209.78 (80.14) | 248.47 (83.23) | 38.69 | –193.97 to 261.49 | 0.738 |
Health utilities
The summary statistics of the EQ-5D utility scores for all observed cases across all time points by treatment groups are presented in Tables 10 and 11. There was no evidence of a difference in the EQ-5D utility between treatment groups at any time point and the mean QALY gain in the cast group [0.706 (SE 0.013)] was also not statistically significant from that in the K-wire group [0.707 (SE 0.011); t-test, p = 0.955].
Cost-effectiveness results
The incremental cost-effectiveness analysis results for the K-wire group compared with the cast group in the base-case analysis and in each of the sensitivity and subgroup analyses are shown in Table 20. The probability that manipulation with K-wire fixation is cost-effective at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY gained is also presented. The NMB, cost-effectiveness acceptability curve and cost-effectiveness plane with the confidence ellipse for the base-case analysis and in each of the sensitivity and subgroup analyses are presented in Figures 10–16.
| Analysis | Incremental cost (£) (bootstrapped 95% CI) | Incremental QALYs (bootstrapped 95% CI) | ICER (£/QALY) | Probability K-wire is cost-effective at specified cost-effectiveness threshold | ||
|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||
| Base-case | ||||||
| NHS and PSS perspective: imputed costs and QALYs, covariate adjusted | 29.65 (–94.85 to 154.15) | –0.007 (–0.03 to 0.016) | –4296.24 (dominated; north-west quadrant) | 0.24 | 0.24 | 0.25 |
| Sensitivity | ||||||
|
1. Societal perspective: imputed costs and QALYs, covariate adjusted |
399.44 (–147.73 to 946.6) | –0.007 (–0.03 to 0.016) | –57,870.32 (dominated; north-west quadrant) | 0.06 | 0.07 | 0.09 |
|
2. NHS and PSS perspective: complete-case costs and QALYs, covariate adjusted |
59.57 (–91.96 to 211.09) | –0.006 (–0.037 to 0.026) | –10,674.63 (dominated; north-west quadrant) | 0.28 | 0.30 | 0.32 |
| Subgroup | ||||||
|
1. NHS and PSS perspective: imputed costs and QALYs, covariate adjusted |
||||||
|
i.< 50 years |
49.02 (–222.92 to 320.97) | –0.012 (–0.055 to 0.031) | –4106.06 (dominated; north-west quadrant) | 0.26 | 0.26 | 0.27 |
|
ii.≥ 50 years |
21.58 (–119.61 to 162.78) | –0.005 (–0.032 to 0.022) | –4188.54 (dominated; north-west quadrant) | 0.32 | 0.33 | 0.34 |
|
2. NHS and PSS perspective: imputed costs and QALYs, covariate adjusted |
||||||
|
i. Extra-articular extension |
94.85 (–41.14 to 230.84) | –0.007 (–0.034 to 0.019) | –12,896.62 (dominated; north-west quadrant) | 0.17 | 0.19 | 0.22 |
|
ii. Intra-articular extension |
–148.54 (–427.29 to 130.22) | –0.007 (–0.055 to 0.042) | 22,801.42 (south-west quadrant) | 0.55 | 0.51 | 0.48 |
FIGURE 10.
Base-case analysis of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
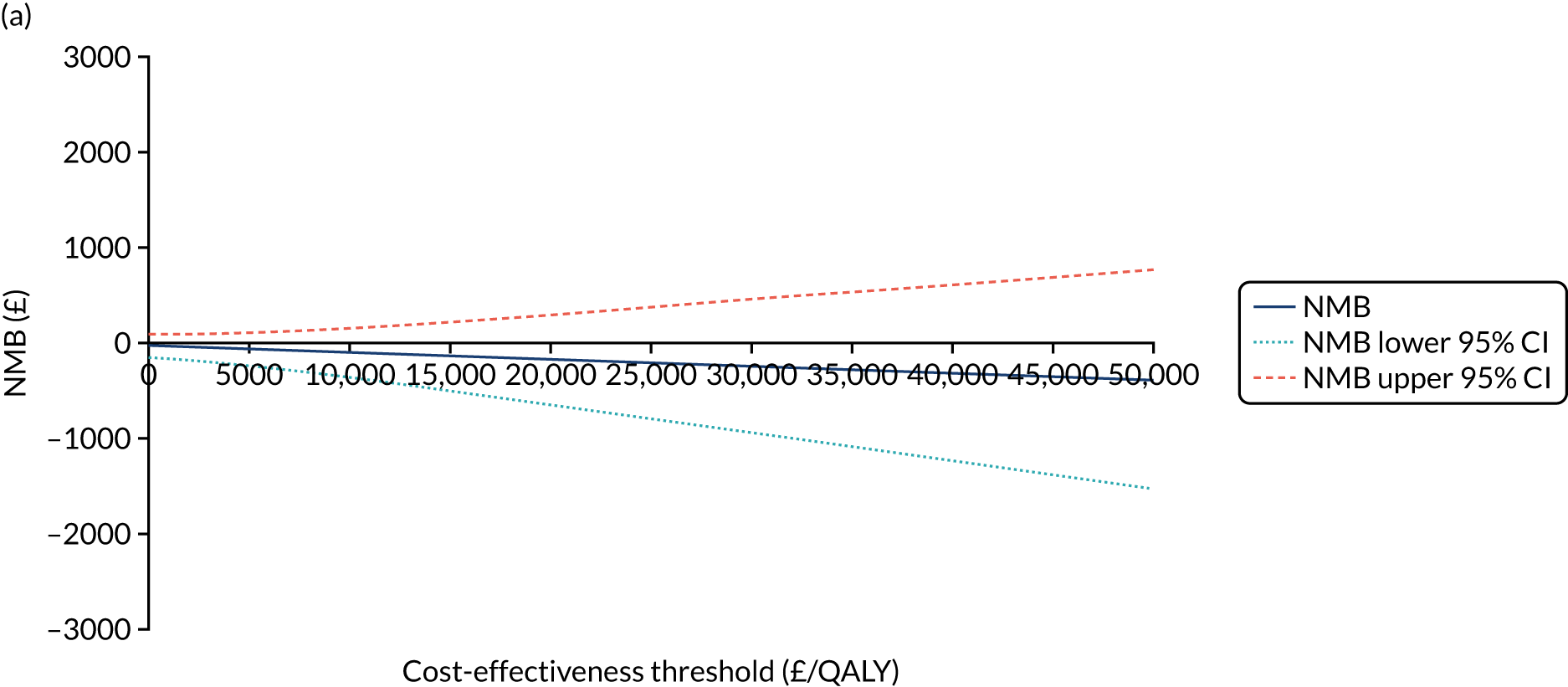
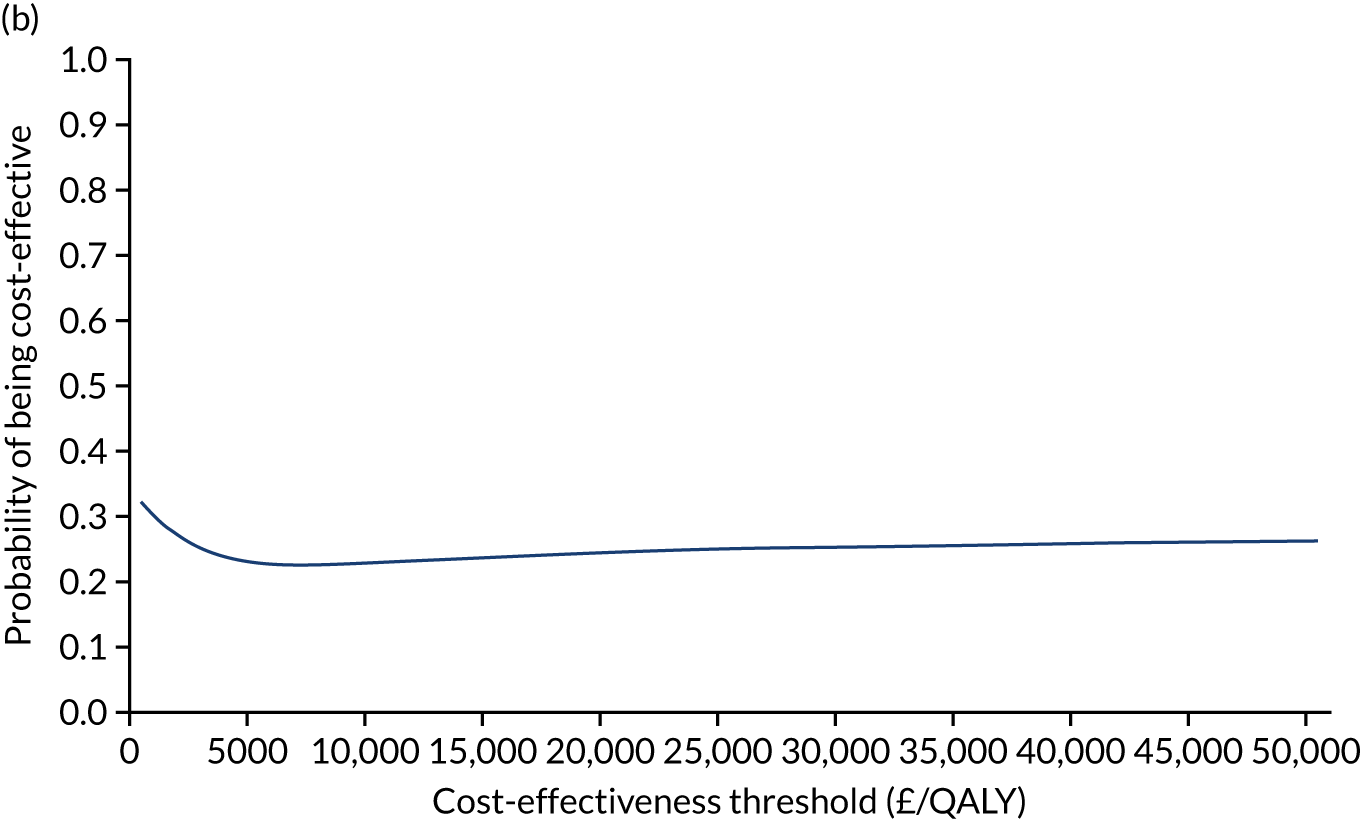
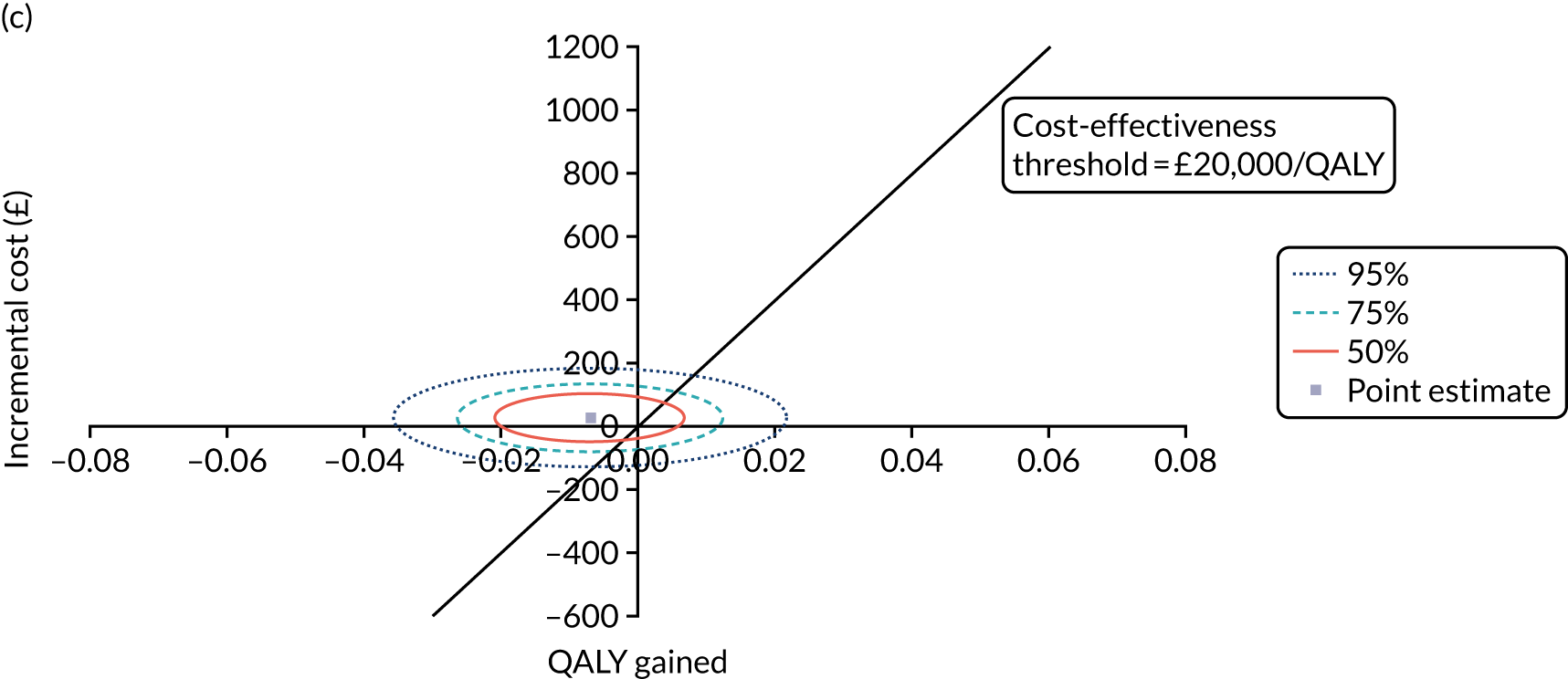
FIGURE 11.
Sensitivity analysis (societal perspective) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
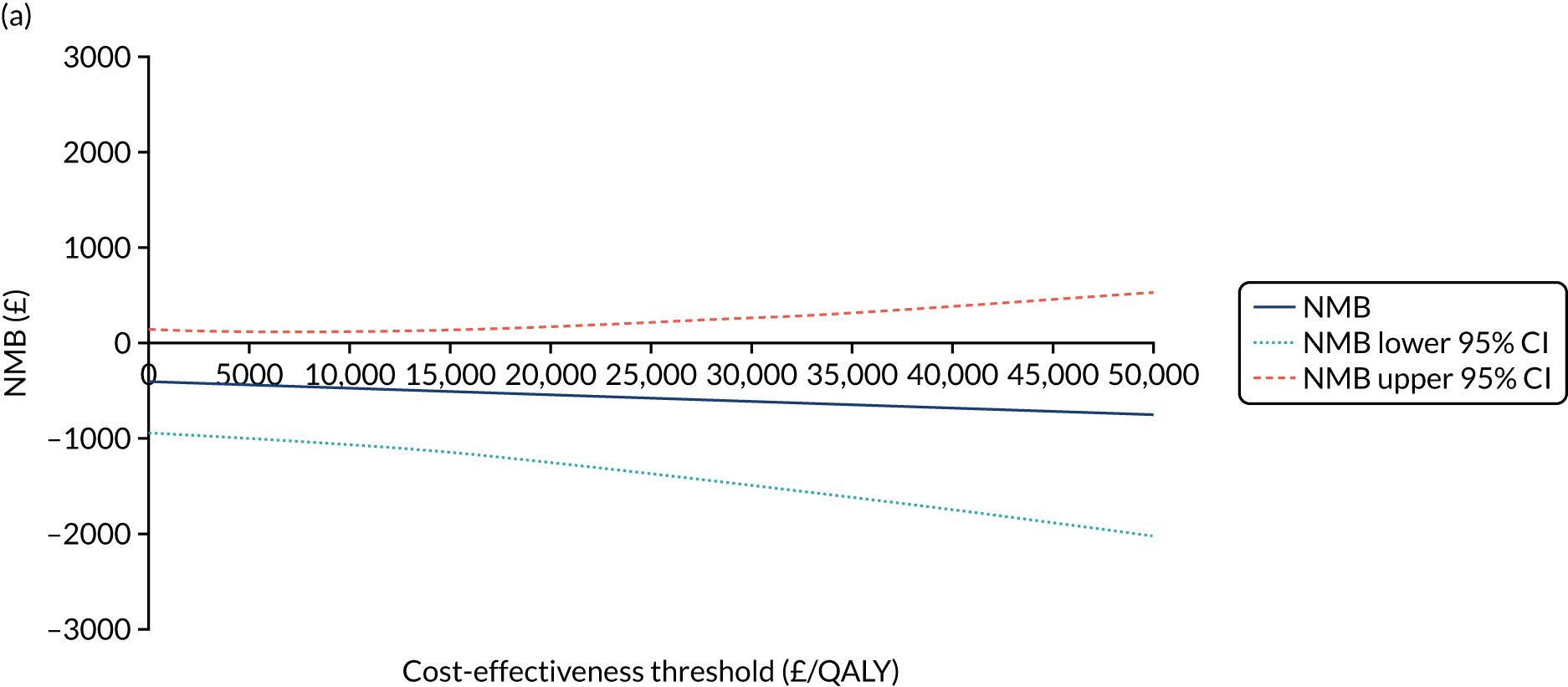
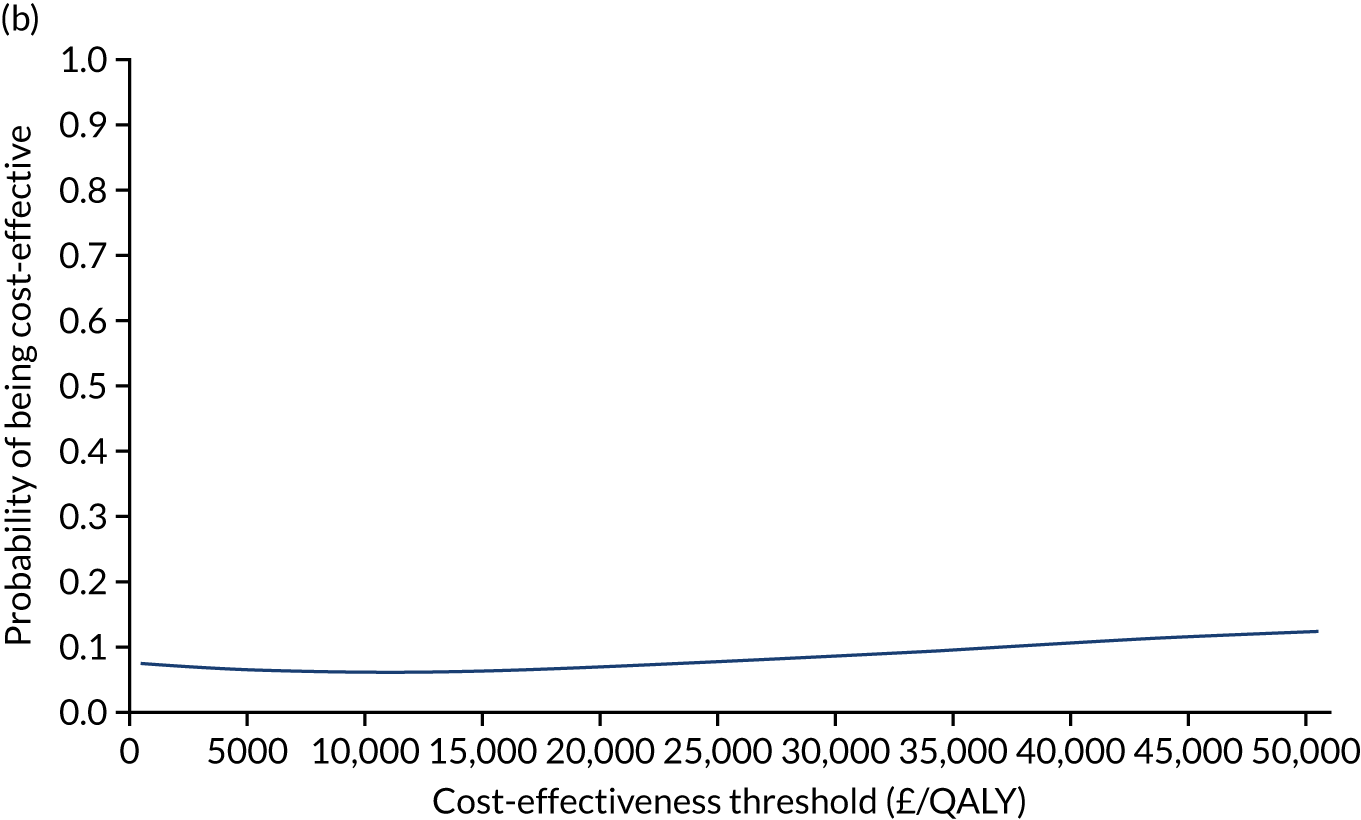
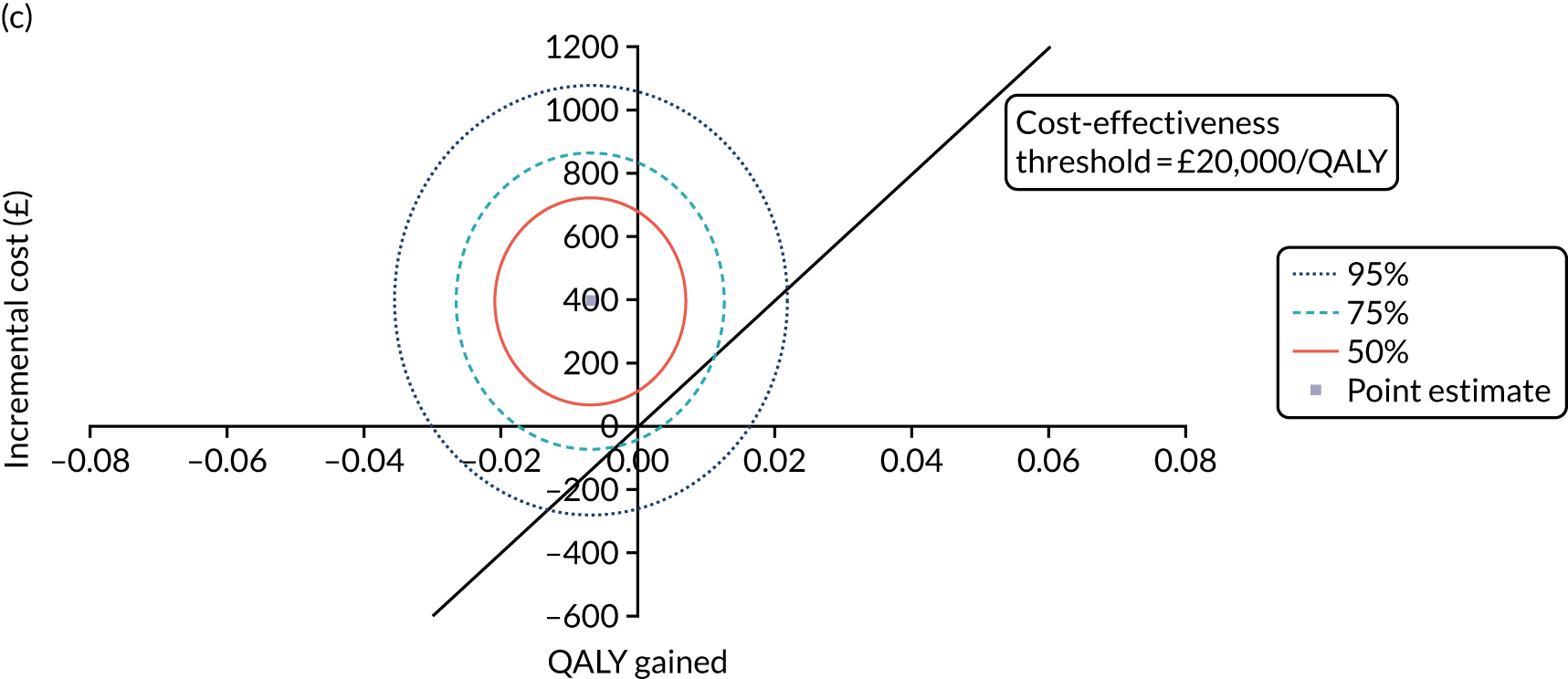
FIGURE 12.
Sensitivity analysis (complete-case analysis) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
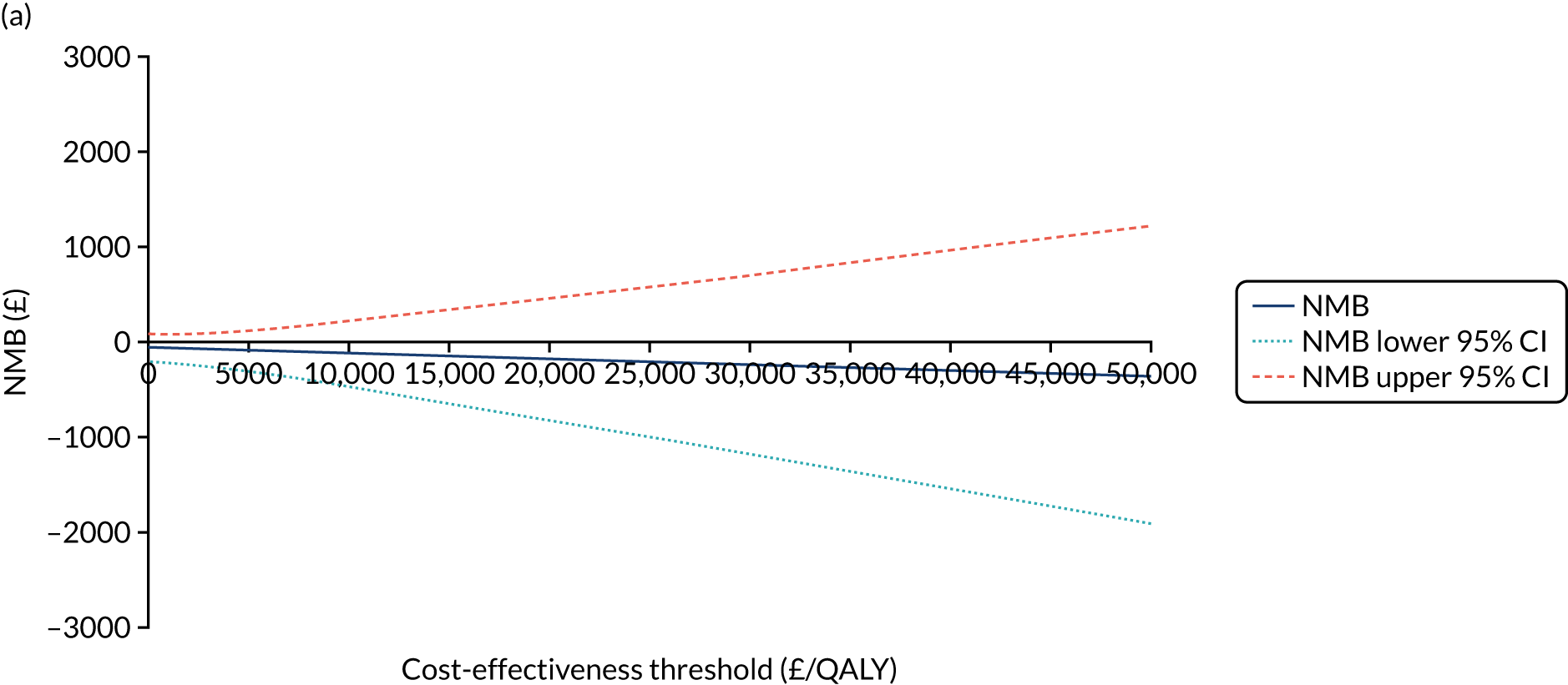
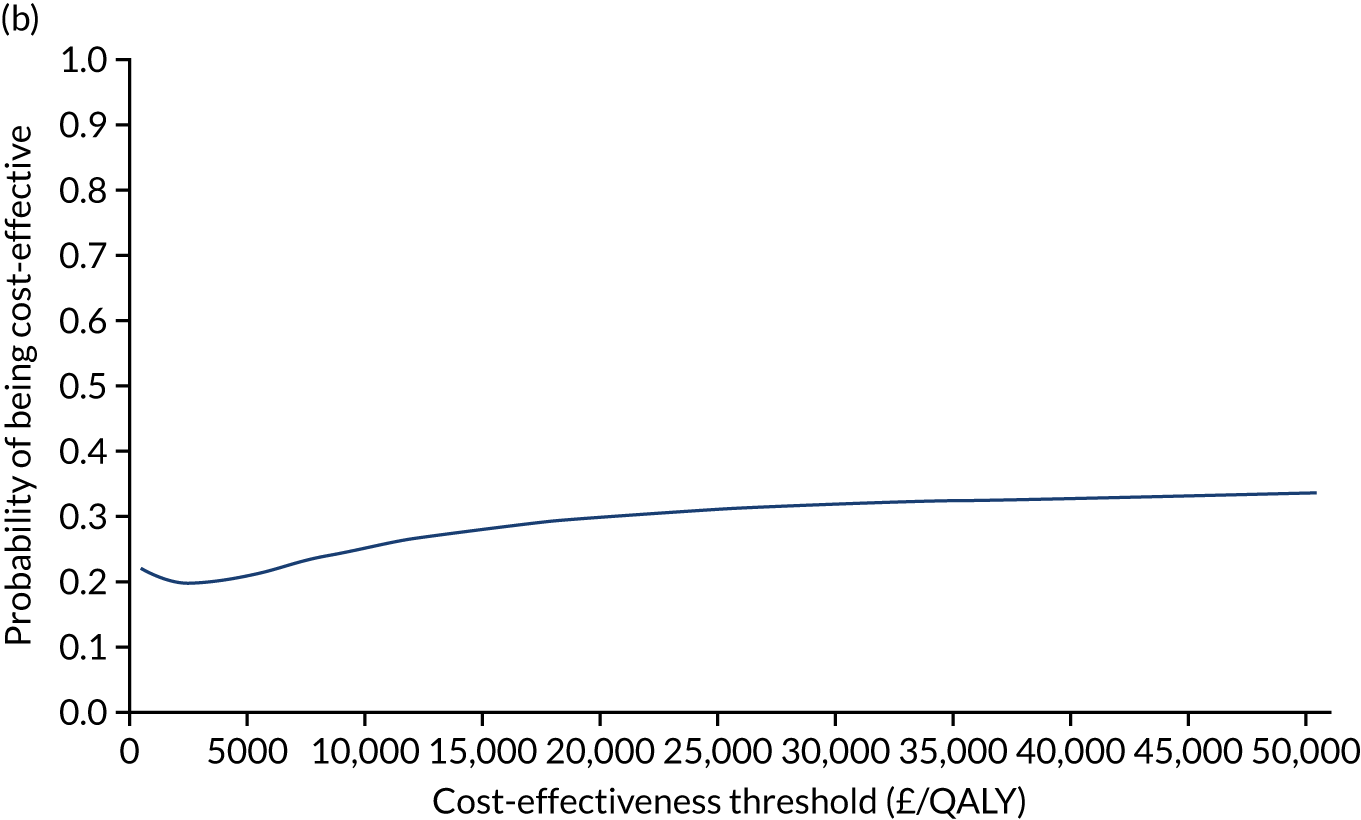
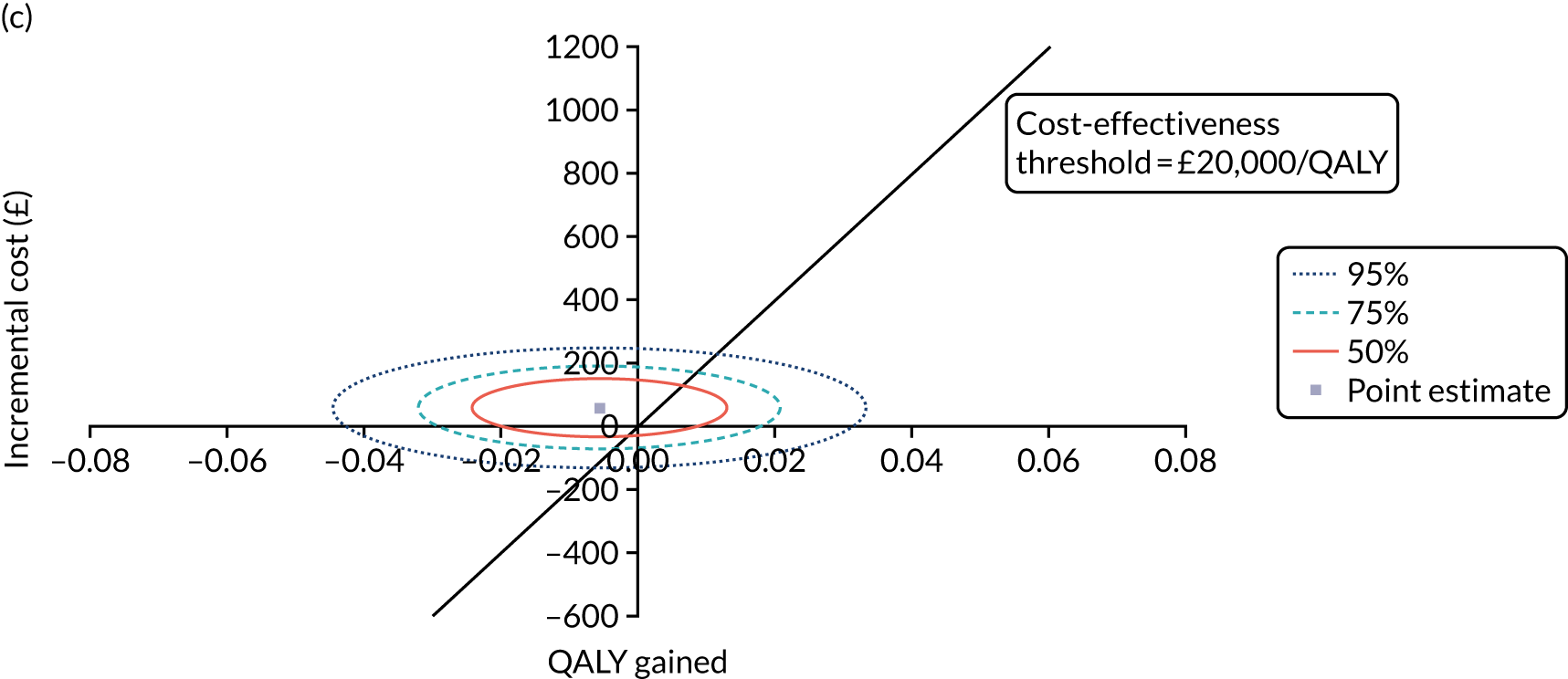
FIGURE 13.
Subgroup analysis (aged < 50 years) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
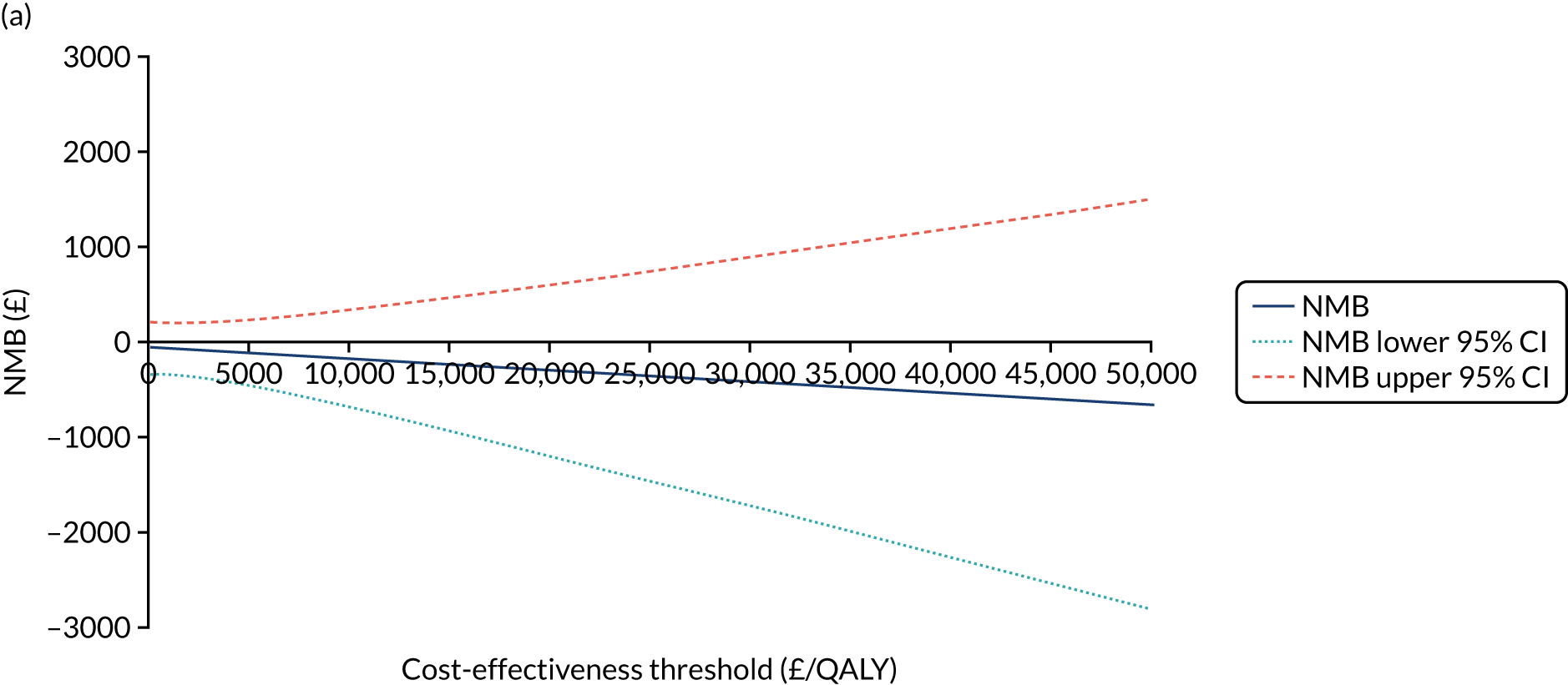
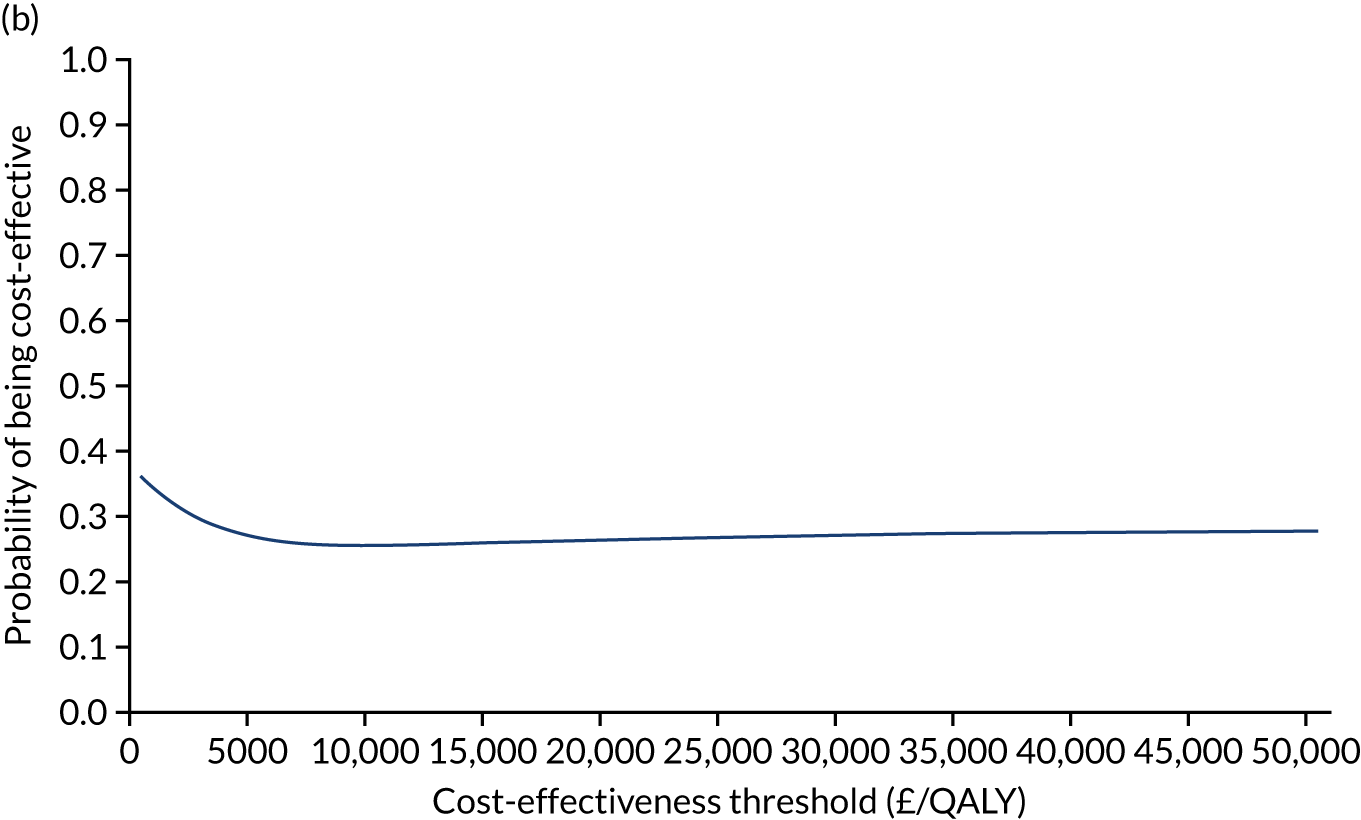
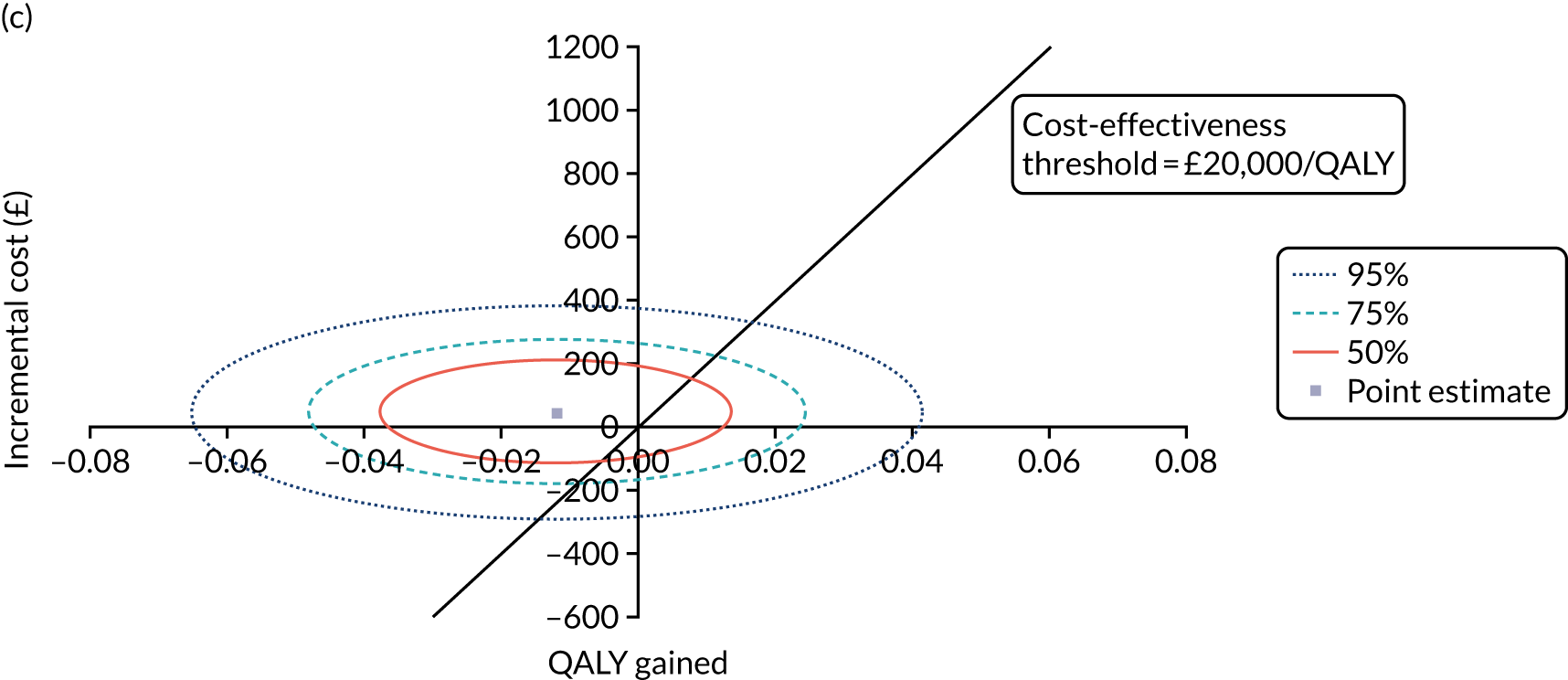
FIGURE 14.
Subgroup analysis (aged ≥ 50 years) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
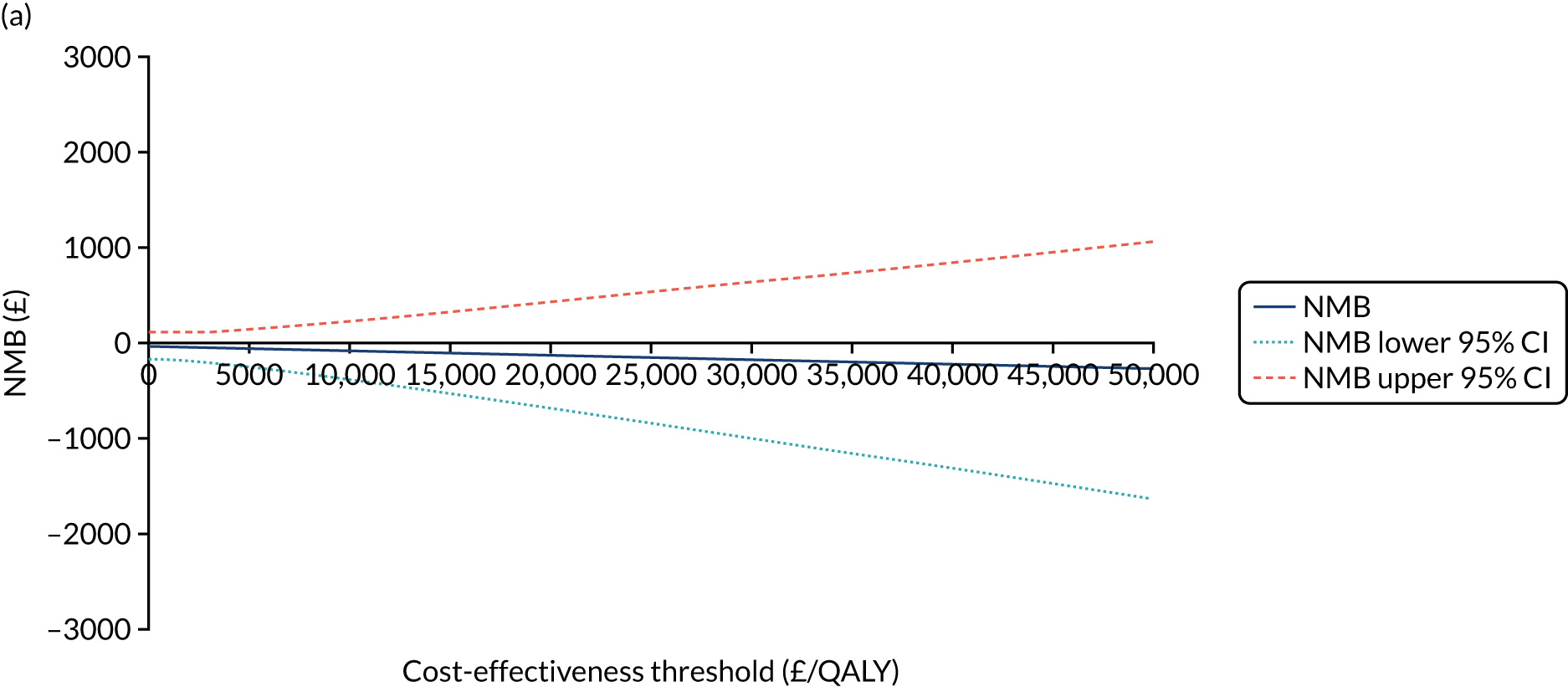
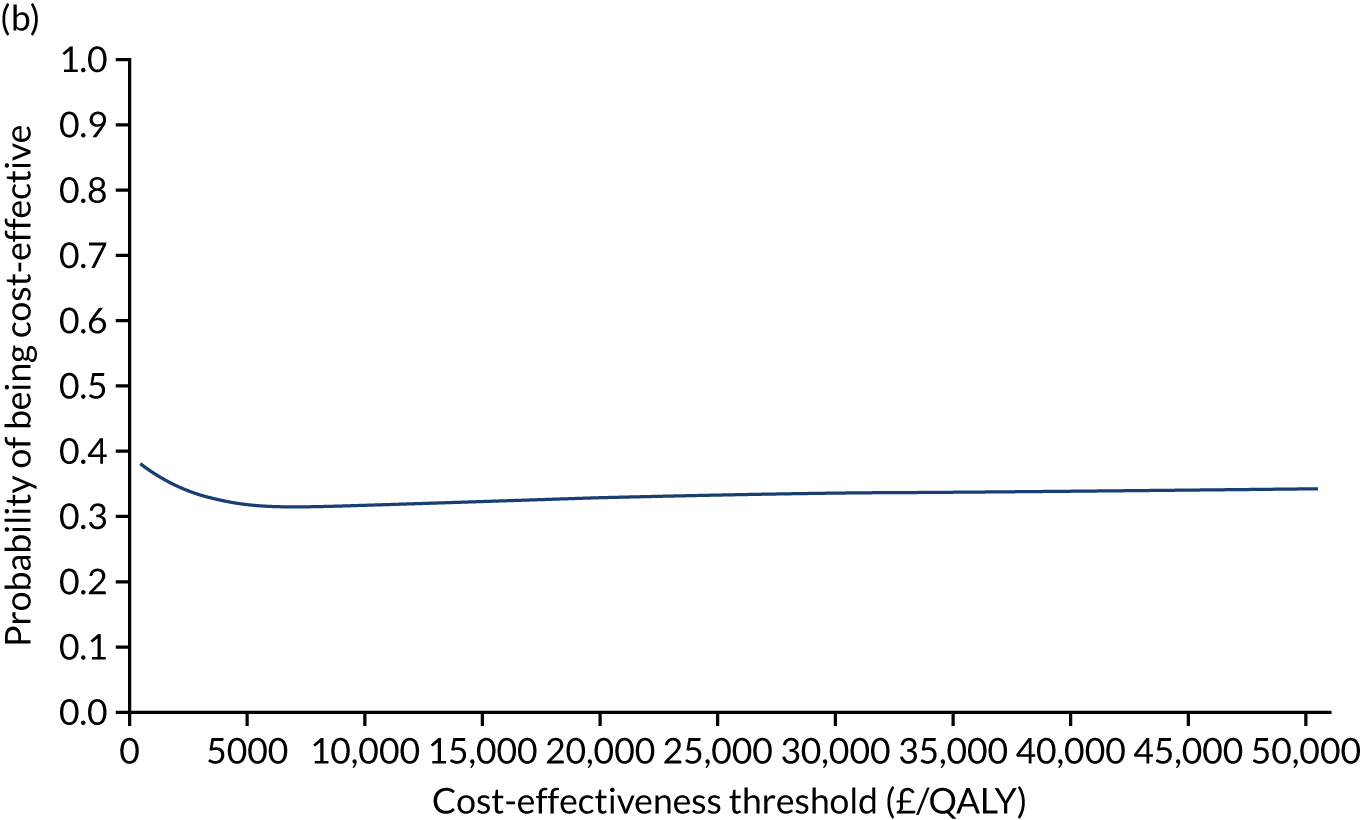
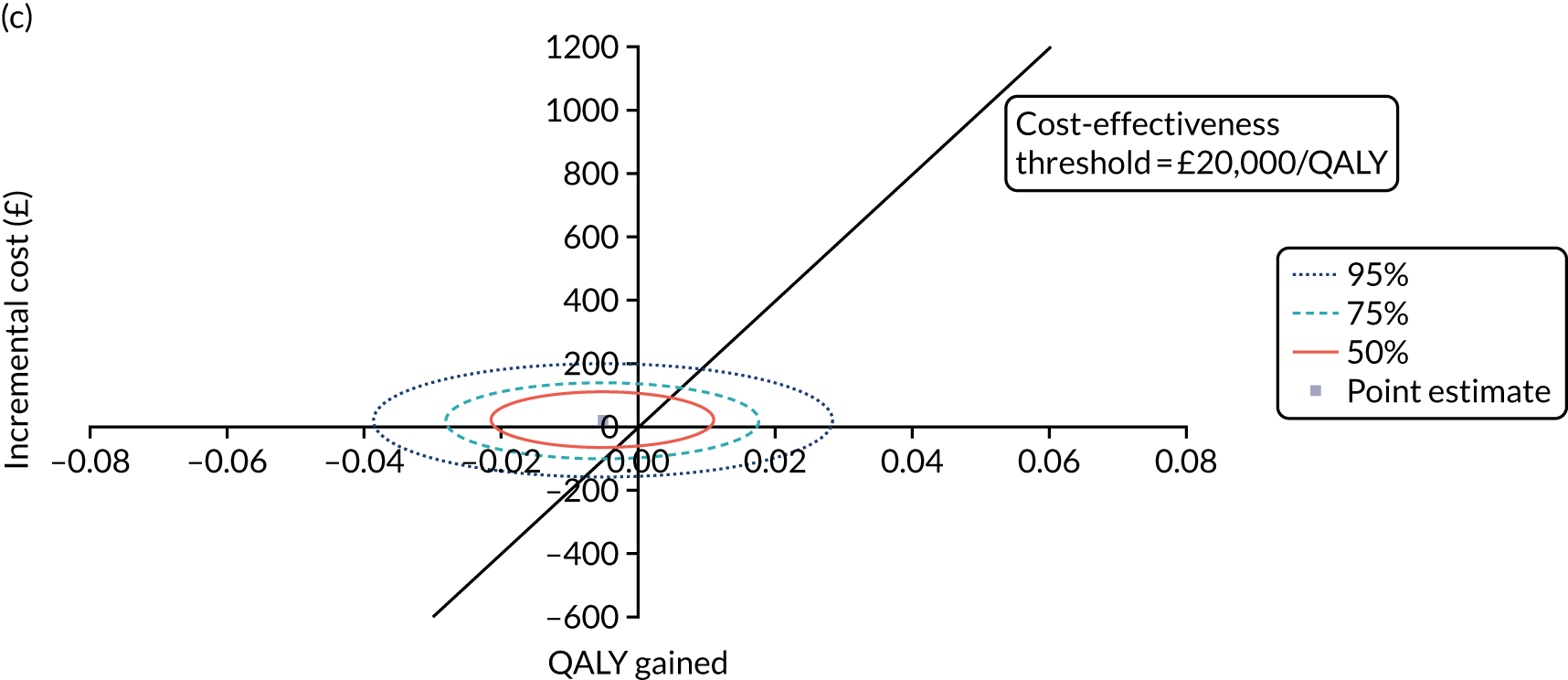
FIGURE 15.
Subgroup analysis (extra-articular extension) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
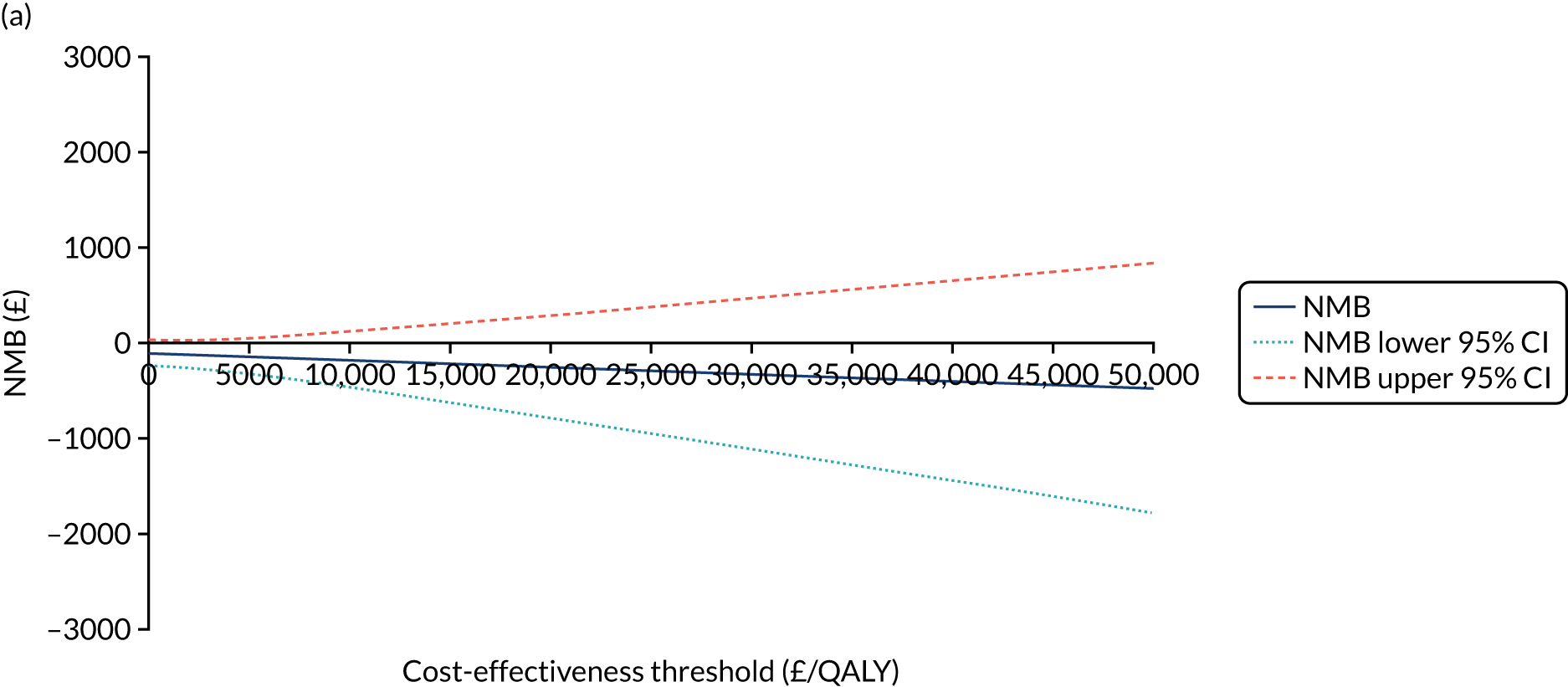
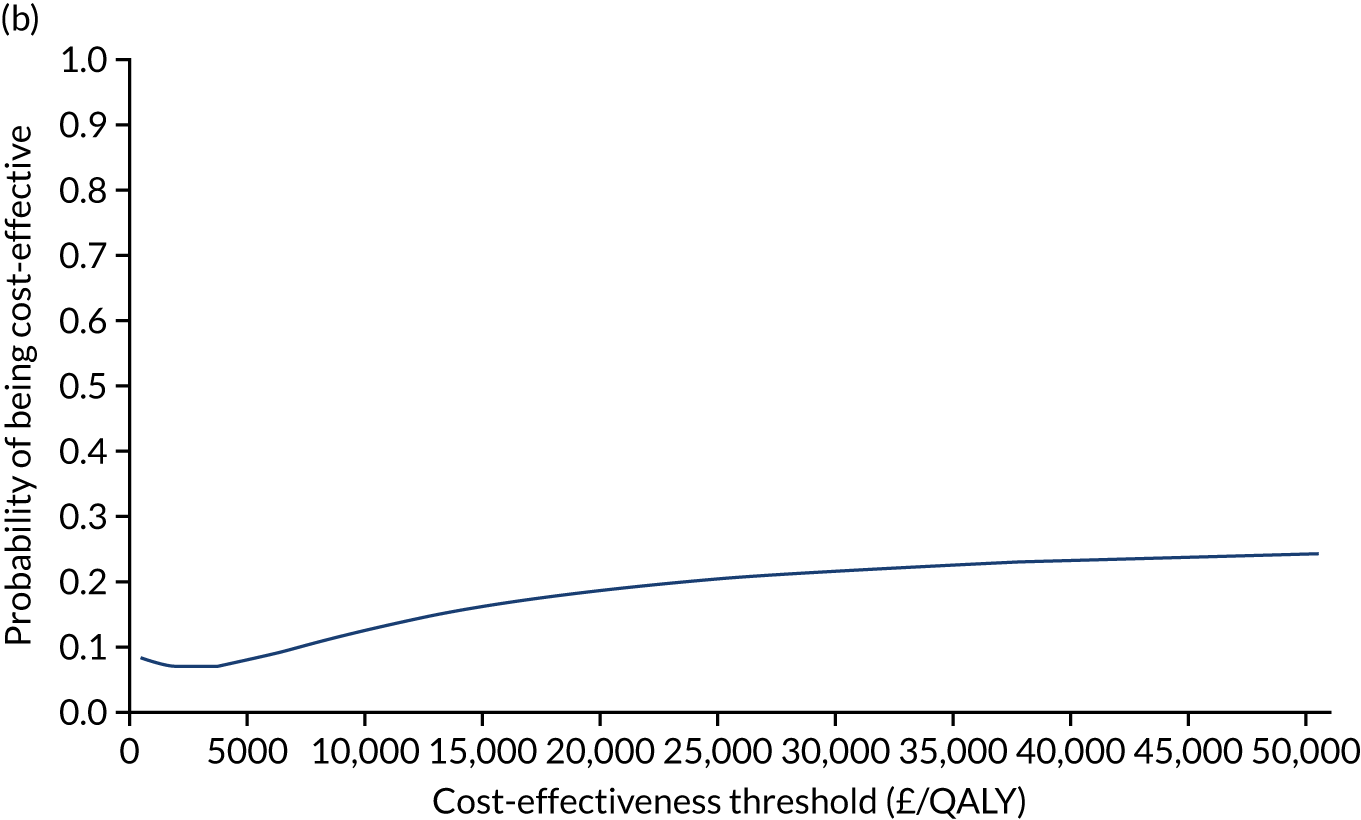
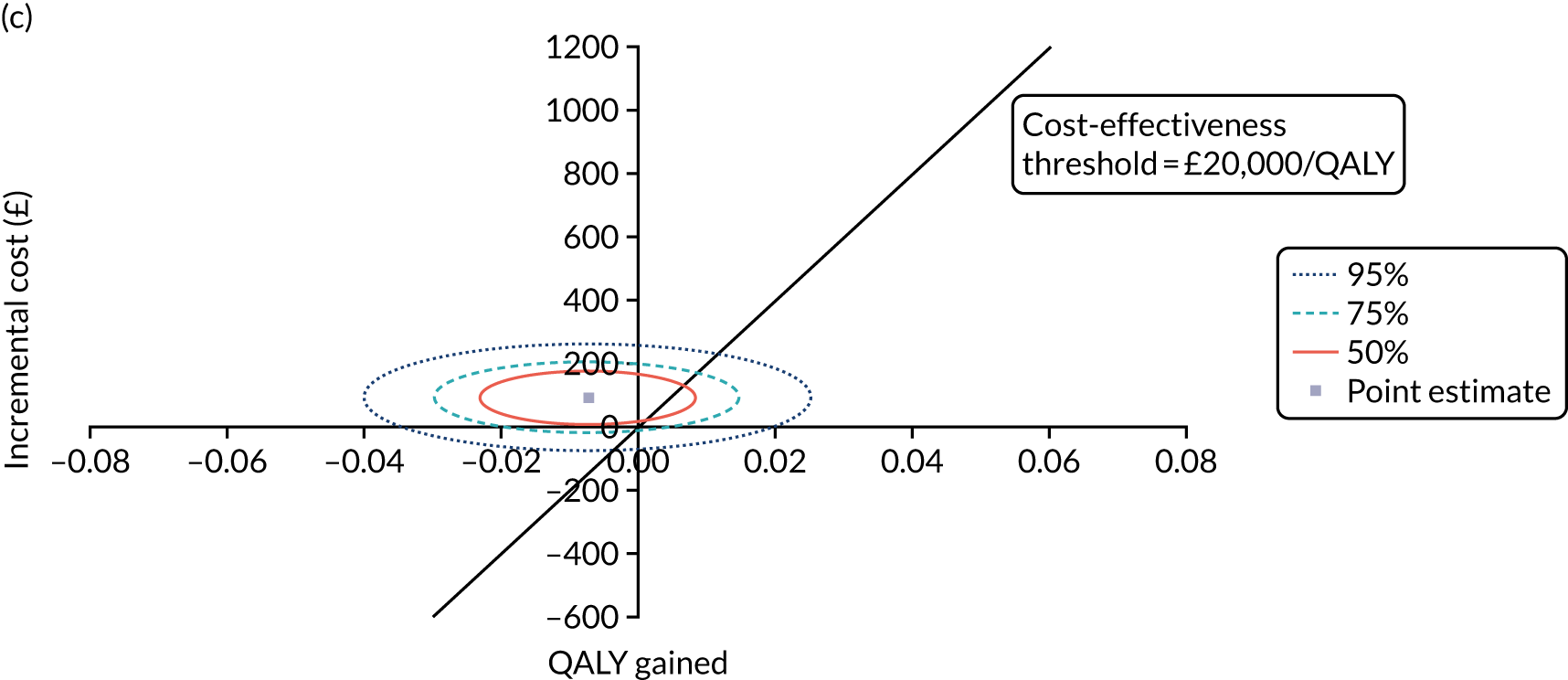
FIGURE 16.
Subgroup analysis (intra-articular extension) of K-wire vs. cast. (a) NMB; (b) cost-effectiveness acceptability curve; and (c) confidence ellipse on the cost-effectiveness plane.
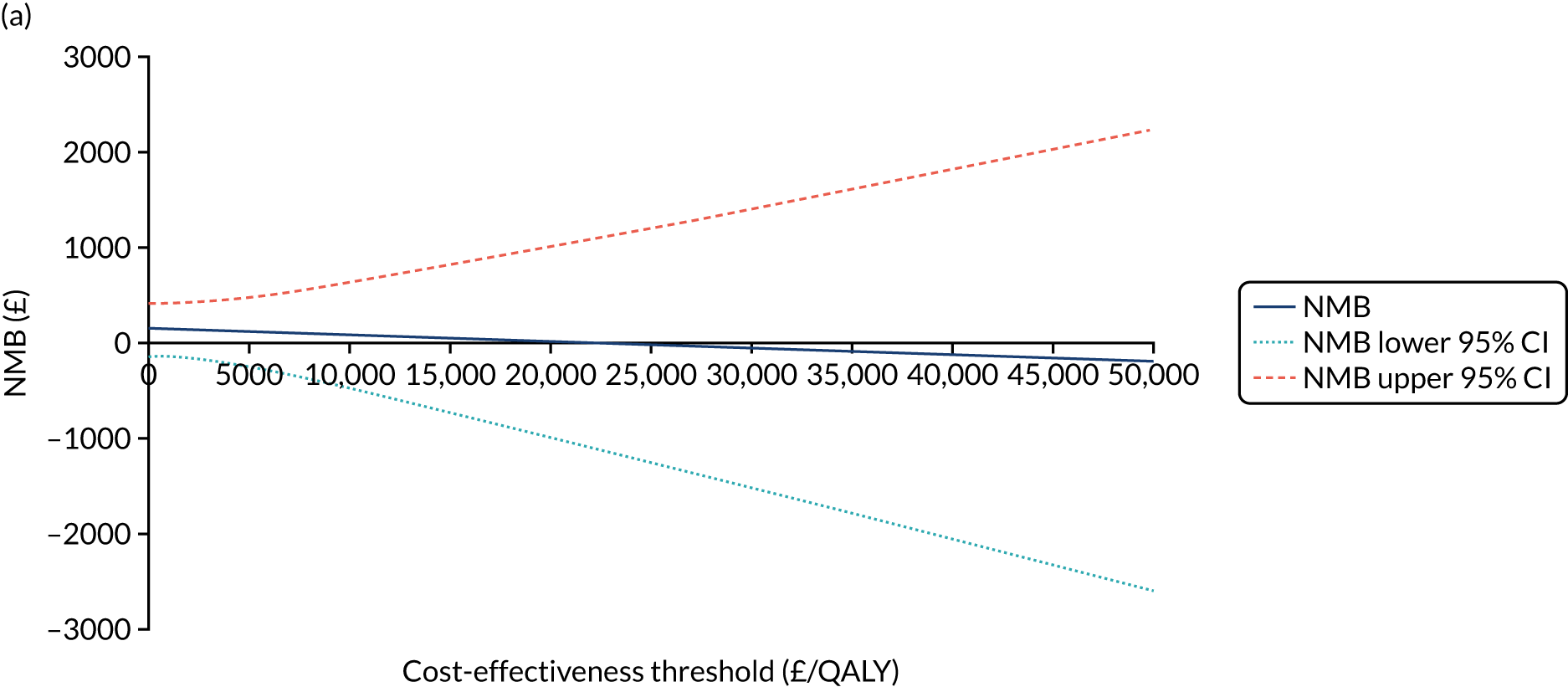
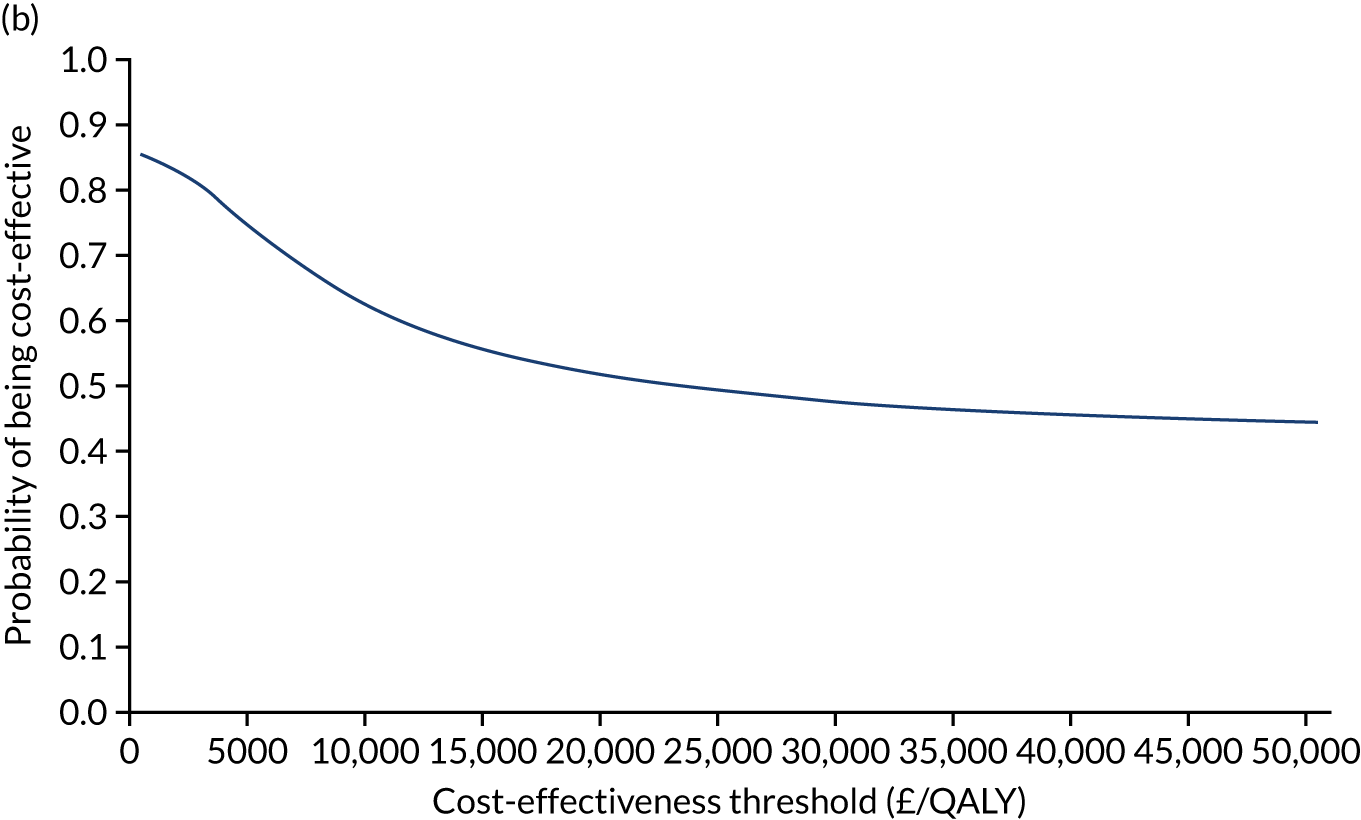
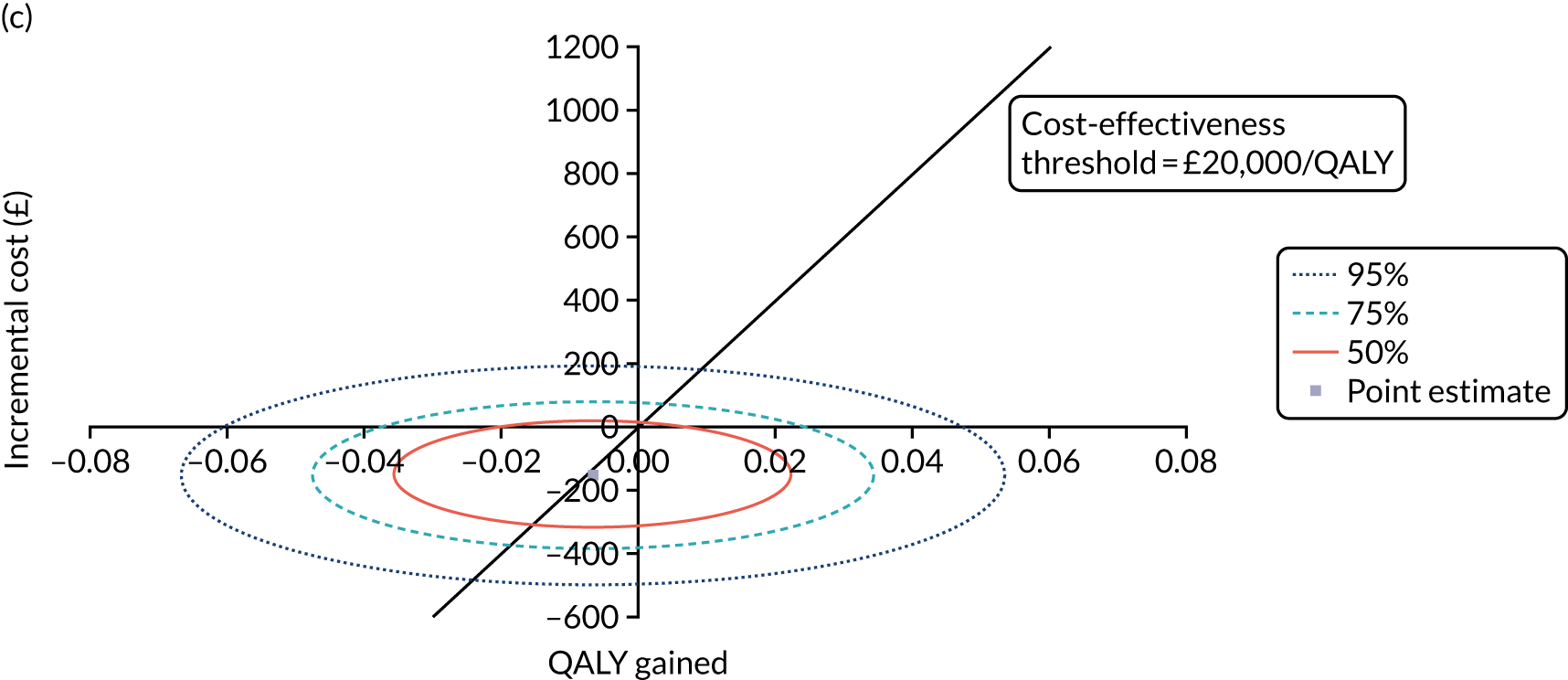
The base-case analysis showed that manipulation with K-wire fixation was dominated [higher cost (£29.65, 95% CI –£94.85 to £154.15)] and almost as effective (–0.007, 95% CI –0.03 to 0.016) when compared with the cast group from the NHS and PSS perspective. In addition, the probability of manipulation with K-wire fixation being cost-effective was < 0.5 at the assumed cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY, whereas the NMB was negative. Therefore, the base-case analysis indicated that manipulation with K-wire fixation is unlikely to be cost-effective relative to the use of a cast among the studied population.
Both sensitivity analyses (societal perspective and complete-case) showed similar results that supported the base-case finding that manipulation with K-wire fixation was dominated. The probability of manipulation with K-wire fixation being cost-effective relative to the use of a cast among the studied population was < 0.5 in both sensitivity analyses, and the NMB was negative.
The subgroup analyses showed similar results to the base-case finding in that manipulation with K-wire fixation was found to be unlikely to be cost-effective relative to manipulation followed by a cast in the studied population (probability < 0.5 and negative NMB) using the prespecified cost-effectiveness thresholds. In the intra-articular extension group, the ICER was £22,801 per QALY (a point estimate), which suggests that manipulation with K-wire fixation would not be cost-effective relative to the use of a cast at a cost-effectiveness threshold of £15,000 per QALY or £20,000 per QALY. In the intra-articular extension group, the ICER was £22,801 per QALY (a point estimate), which suggests that manipulation with K-wire fixation would likely not be cost-effective (probability of being cost-effective at 0.55) relative to the use of a cast at a cost-effectiveness threshold of £15,000 or £20,000 per QALY.
Chapter 5 Discussion
Recruitment
Between January 2017 and March 2019, a total of 2636 patients with a dorsally displaced fracture of the distal radius were screened in the recruitment centres. A total of 1441 patients met one or more of the exclusion criteria. Somewhat surprisingly, 338 of these patients were excluded because the injury was > 2 weeks old. Although some patients with an injury to the distal radius do try to manage this themselves without visiting the hospital, this is not very common in the case of a displaced fracture as, in such cases, there is often a visible deformity. It seems more likely that this exclusion criterion was met by patients whose fracture was initially managed non-operatively, but in whom fracture displacement or re-displacement subsequently occurred or was managed > 2 weeks after the index injury. It is common practice in many NHS hospitals for patients to undergo manipulation of a displaced distal radius fracture in the emergency department or a minor injuries unit and to then be followed up with repeat radiographs in the orthopaedic fracture clinic. Re-displacement can then occur in the second or third week. This in itself does raise questions about the optimal initial management of these injuries (see Chapter 6, Future research recommendations). A total of 104 patients were excluded because their fracture was open with a Gustilo grading > 1°. As the outcome in such cases is usually dictated more by the associated soft-tissue injury than by the underlying fracture, it is appropriate that these patients were excluded. A total of 222 patients were excluded because they were deemed to be unable to adhere to the trial procedures or complete questionnaires. This proportion is similar to that found in the first DRAFFT trial,4 and is in keeping with the fact that distal radius fractures are common in patients with cognitive impairment. A total of 284 patients were excluded during screening because the treating surgeon felt that the fracture would require open reduction and hence could not be treated with either of the two trial treatments. Again, this is in keeping with the fact that a proportion of patients with intra-articular fractures have an obvious step or gap in the radiocarpal joint surface that cannot be reduced by a closed manipulation of the fracture.
The great majority of patients who met the eligibility criteria and who were approached for their informed consent were happy to take part in the trial, but 196 patients declined, most commonly because they ‘did not want to take part in research’.
In addition to the 196 patients who declined to consent, 495 potentially eligible patients were not approached for consent into the trial because the treating clinician had a strong preference for a particular treatment. In 151 cases, the surgeon preferred one of the two treatments under investigation (cast group, n = 31; K-wire group, n = 120). Although the exclusion of these patients does affect the external validity of the trial, this is a relatively small number compared with the 500 patients who were included in the trial. The remaining patients (n = 344) were not offered the opportunity to enter the trial because the consulting surgeon had a preferred ‘other’ treatment. In 154 cases, this other treatment was not specified in the screening data. For the other 190 patients, some surgeons recommended external fixation, but the most commonly recommended treatment was plate fixation.
There is likely to have been some overlap in this group with the exclusion criterion ‘the articular surface of the fracture cannot be reduced by indirect means’, but we do not have radiographical data to confirm this. However, despite the results of the DRAFFT trial (which found no evidence that plate fixation was superior to K-wire fixation if a dorsally displaced fracture of the distal radius could be reduced without opening the fracture),4 and the subsequent national guidelines,47 some surgeons still routinely offer plate fixation to all patients for whom they are recommending surgical fixation for a distal radius fracture. 48 Excluding this group of patients may also have affected the generalisability of the trial but, again, this is a relatively small number compared with the 500 participants who were offered the chance to take part and consented to do so.
In the DRAFFT trial,4 patients were randomised to their treatment group before their surgery. This was in keeping with common practice at the time but did lead to some potentially eligible patients being excluded preoperatively if the surgeon was not confident that a closed reduction of the fracture could be achieved during surgery. Therefore, to prevent unnecessary exclusions, and facilitated by the increased availability of real-time online randomisation via operating theatre computers, DRAFFT 2 was designed such that randomisation was planned to take place after a closed reduction of the fracture had been achieved in the operating theatre, but before any fixation was performed. Patients who gave their informed consent before the reduction was attempted were advised that, if their surgeon felt that the manipulation of the fracture was unsatisfactory and that the fracture could not be reduced without opening the skin, they would not be randomised into the trial but the surgeon would proceed with open reduction and fixation, as per routine clinical practice. A total of 179 patients who consented to enter the trial were subsequently excluded before randomisation because the surgeon was unable to achieve or maintain a closed reduction intraoperatively. This is lower than the proportion of patients in the DRAFFT trial4 who were excluded preoperatively because the surgeon was uncertain that a closed reduction of the fracture could be achieved (40% of potentially eligible patients in the DRAFFT trial4 were excluded on this criterion), indicating that the use of real-time randomisation following the manipulation of the fracture did help to improve the external validity of the trial. This is worthy of note in the design of future studies.
Participants and treatment groups
Five hundred participants were randomised into the trial. The larger centres screened more eligible patients, but recruitment of participants was distributed across all of the recruitment centres apart from two that did not recruit any participants. The recruitment centres represent the range of major trauma centres, trauma units and local emergency hospitals that routinely treat patients with distal radius fracture in the NHS. Therefore, we can be confident that the participants were representative of the UK population with distal radius fractures.
A total of 255 participants were allocated to the cast group and 245 were allocated to the K-wire group. We anticipated some crossover of participants immediately after randomisation but, in fact, only 27 participants did not receive their allocated treatment. Twelve participants in the cast group crossed over to the K-wire group and four participants in the K-wire group crossed over to the cast group. The remaining participants underwent plate fixation (n = 10) or other surgery (n = 2). As part of the statistical analysis plan, we included a secondary PP analysis of the PRWE outcomes to supplement the primary ITT analysis, but, given the relatively small number of participants who did not receive their allocated treatment, we were reassured that non-compliance was unlikely to affect the integrity of the trial findings.
The baseline characteristics of the two groups were well balanced in terms of both participant demographics and pre-injury patient-reported wrist function and HRQoL. Fracture characteristics were also well balanced, with 72% of participants in both treatment groups having extra-articular fractures. This distribution was expected, as intra-articular fractures are those more likely to require open reduction and hence be excluded from the trial as per the eligibility criteria.
In both groups, the treatments were most commonly performed by an orthopaedic consultant (48%) or a specialist trainee under supervision (33%), which reflects routine clinical practice in the UK. As with all surgical interventions, the experience of the surgeon may affect the outcome of the surgery beyond the choice of treatment. Therefore, we were pleased to note that there was reasonable balance of senior surgeons (consultants or staff and associate specialists) across the groups. If anything, the proportion of procedures performed by senior surgeons was higher in the cast group (67%) than in the K-wire group (54%), which is interesting given that the K-wire fixation treatment may be considered the more ‘technically demanding’ of the two. However, both treatments are used routinely throughout the NHS. and the surgeons in both groups were very experienced in performing both treatments: over 80% of the 361 surgeons involved in the trial reported that they had performed > 20 procedures of each type before the trial started. In the group undergoing surgical fixation with K-wires, two or three 1.6-mm wires was the most common option, with surgeons using an equal mix of interfragmentary and Kapanji insertion techniques, which reflects routine clinical practice in the UK. In the cast group, the great majority of participants were treated with a plaster of Paris cast moulded around the full circumference of the wrist and forearm in the manner of a ‘close-contact’ cast. This again reflects routine practice, as plaster of Paris can be moulded to maintain fracture reduction more easily than the alternative cast material, fibreglass.
As anticipated, the number of patients who withdrew or were lost to follow-up increased slightly during the 1 year following randomisation. In the sample size calculation, we estimated the loss to follow-up to be 20% and, indeed, follow-up was 79% in the cast group and 80% in the K-wire group at the 12 months post randomisation point. In total, 499 out of 500 participants provided data to inform the primary analysis (as data from earlier time points were used in this analysis). As the trial also exceeded the minimum recruitment target of 460 participants (500 participants being randomised), we can be confident that the trial fulfilled the assumptions made in the sample size calculation.
Results
Primary outcome
This trial found no evidence that manipulation and surgical fixation with K-wires was superior to manipulation followed by a moulded cast in the management of acute dorsally displaced distal radius fractures. The CIs excluded our target difference of 6 points. There was no evidence of a difference between the treatments at 3 or 6 months, or in the AUC for the PRWE score over the 12 months post randomisation. There was no evidence of a difference in the secondary PP analysis of the PRWE score or in the pre-planned sensitivity analysis to examine surgeon within recruitment centre effects. Nor was there any evidence of a difference in the pre-planned subgroup analyses for patients above and below 50 years of age and for patients with extra-articular compared with intra-articular fractures.
These data provide strong evidence that, if a closed reduction of the fracture can be achieved, surgical fixation with K-wires does not provide superior wrist function to moulded casting for patients with a dorsally displaced fracture of the distal radius.
Secondary outcomes
The trial also found no evidence that manipulation and surgical fixation with K-wires was superior to manipulation and a moulded cast in the management of acute dorsally displaced distal radius fractures in terms of HRQoL. There was no evidence of a difference between the treatments at 3 or 6 months, or in the AUC for the EQ-5D index score in the 12 months post randomisation. Nor was there any evidence of a difference in the EQ-5D VAS scores at any time point. These data provide corroborating evidence to support the analysis of the primary outcome measure.
In terms of complications, the overall number of complications was small in both treatment groups. In the 12 months post randomisation, one DVT was reported in each group and there were five diagnoses of CRPS in the cast group and seven in the K-wire group. These findings are in keeping with the low rate of complications reported in the literature and the complication profile reported in the DRAFFT trial. 4
However, there was a statistically significant and clinically important difference in the number of further interventions performed for loss of fracture reduction in the 6 weeks following the index manipulation of the fracture: 33 (13%) participants in the cast group compared with one participant (< 1%) in the K-wire group required a further intervention. This finding is not unexpected, as the purpose of surgical fixation is to secure the reduction of the fracture by holding the bones in position using metalwork, in this case by passing metal wires across the fracture to hold the bone fragments in their anatomical position while they heal. By contrast, a moulded cast provides only indirect support to the bones following their manipulation. As soft-tissue swelling settles after the injury, the cast becomes looser and the reduction of the bone fragments may be lost. These data provide important information for clinicians advising patients about their treatment options following this common injury. If treated in a moulded cast, 13% of patients (one in eight) will require a further intervention for loss of fracture reduction in the first 6 weeks. This is in keeping with a recent Cochrane review, which found a 12% ‘re-displacement’ rate among patients treated with a plaster cast after manipulation. 8
To investigate the outcome of those patients who required a further intervention for loss of fracture reduction compared with those in the cast group as a whole, we performed an unplanned secondary analysis of the PRWE outcomes in these participants. The mean PRWE score in those participants requiring further intervention following their cast treatment (n = 33) was 21 (SD 21) points at 12 months post randomisation, which is the same as in the cast group as a whole [mean 2 (SD 23 points)]. Although these data are reassuring, in that participants did not seem to suffer longer-term deficits in wrist function despite requiring a further intervention to treat their fracture, this review was unplanned and should be interpreted with caution.
Health economic evaluation
The economic evaluation in this trial indicates that manipulation and K-wire fixation is dominated by manipulation followed by a moulded cast (i.e. K-wire fixation is more costly but no more effective). Therefore, K-wire fixation is unlikely to be cost-effective among adult patients who have sustained an acute dorsally displaced fracture of the distal radius.
This finding was consistent across the pre-planned sensitivity analyses and subgroup analyses except in the intra-articular extension subgroup, in which the probability that K-wire would be cost-effective relative to a cast was slightly above 0.5 when a cost-effectiveness threshold of £15,000 per QALY or £20,000 per QALY was applied. This appears to be driven by the difference in the mean cost of treatment of complications, complications being more common in the subgroup with intra-articular extension. The mean cost of treatment of complications in the cast group [£147.03 (SE £47.62)] was higher than that in the K-wire group [£2.95 (SE £0.50)] in the intra-articular extension subgroup analysis.
The initial treatment cost was higher in the K-wire group than in the cast group because of the cost of the K-wires, the cost of the theatre consumables and the longer time in the operating theatre. However, the cost of treatment of complications was higher in the cast group. This was because those in the cast group reported more further surgeries than those in the K-wire group between baseline and 6 weeks after discharge, specifically the loss of fracture reduction leading to further surgical intervention.
The mean cost of medications from baseline to 12 months post randomisation was higher in the cast group than in the K-wire group, and this was attributable to two participants taking risedronate (one for 30 days and one for 42 days), which has a higher unit cost than other medications. It is unlikely that the use of risedronate is associated with the choice of treatment in the trial, but both participants happened to be randomised to the cast group. If a t-test was performed without these two observations, the mean cost of the medication in the cast group would drop from £2.75 (SE £0.75) to £1.94 (SE £0.48) and there would not be a statistically significant difference in the mean cost of medications between the K-wire and cast groups (t-test, p = 0.074). Likewise, additional (non-NHS) costs were statistically significantly different between the K-wire and cast groups because one participant incurred an exceptionally high travel and help with housework cost between discharge and 3 months. If a t-test was performed without this single observation, there would not be a statistically significant difference in the mean additional costs between the K-wire and cast groups.
Of note, the analysis presented here differs from the published SHEAP19 in two ways. First, it differs in the measurement of the cost of the initial treatment. As the duration of the operation was significantly different between the two groups [31.2 minutes (SE 1.7 minutes) in the cast group compared with 44.2 minutes (SE 1.7 minutes) in the K-wire group; t-test, p < 0.001], we used the cost associated with the time that each patient spent in the operating theatre, instead of using only the cost of surgical staff, to obtain more comprehensive estimate of the overall cost of the treatment. It has been shown that staff costs account for only 65% of operating theatre time. 31 To avoid double-counting the cost of the surgeon’s time, the cost of surgical staff was not computed. Of note, the Healthcare Resource Group code includes a standard cost for operating theatre time, thus overestimating the cost of surgery, but, because this code is assigned to patients undergoing both treatments, this would not lead to any difference between groups. Second, we added a cost-effectiveness threshold of £15,000 per QALY in our analysis to reflect current trends in health-care decision-making.
Limitations
Recruiting patients to clinical trials in the context of urgent surgery is difficult. A concern before this trial started was that patients and/or surgeons would not be willing to take part. In fact, only 196 patients declined to take part in the study. A bigger issue was equipoise in the surgical community. A total of 495 potentially eligible patients were not offered the chance to take part in the trial because the treating surgeon had a clinical preference for a particular treatment; 120 patients were offered manipulation and K-wire fixation, 31 manipulation and cast and 344 other treatments or unspecified. Although these numbers are low in relation to the total of 2636 patients who were screened as part of the study, they do have an impact on the external validity of the trial.
A further anticipated limitation was crossover of participants from the allocated treatment. However, only 27 of the 500 participants did not receive their allocated treatment. The secondary PP analysis, that is the analysis that included only those participants who received their allocated treatment, confirmed the results of the primary ITT analysis, finding no evidence of a difference between the two treatment groups.
There was also some loss to follow-up in the trial, but this was in keeping with the loss to follow-up of 20% used in the sample size calculation, which indicated that a minimum of 460 participants should be recruited. As the trial recruited 500 participants, we can be confident that DRAFFT 2 fulfilled the assumptions made in the sample size calculation.
The final major limitation of the trial was that neither the treating clinicians nor the participants could be blind to the treatments. This is, of course, inevitable in the case of surgical interventions, as the wires are clearly visible to the patient and surgeon when removed. Assessment bias was minimised by the fact that the surgeons providing the interventions played no part in the assessment of the participants’ outcomes.
Chapter 6 Conclusions
Surgical fixation with K-wires was not found to be superior to moulded casting in terms of wrist function following manipulation of an acute dorsally displaced fracture of the distal radius. If a closed reduction of the fracture can be achieved, clinicians may consider the application of a moulded cast as a safe and cost-effective alternative to surgical fixation.
Future research recommendations
With regard to fractures of the distal radius, further health technology assessments are recommended to investigate the following:
-
What is the optimal technique for immobilisation of a dorsally displaced distal radius fracture that does not require manipulation to achieve reduction?
-
What are the optimal techniques for immobilisation of a dorsally displaced distal radius fracture following the first manipulation of the fracture?
-
What is the optimal technique for providing pain relief to patients who require a reduction of a dorsally displaced distal radius fracture?
-
What is the optimal regime for rehabilitation of the patient after immobilisation of their wrist for a fracture of the distal radius?
Furthermore, further research to explore patient treatment preferences could inform all future research in this area.
Acknowledgements
Trial team
Trial management team
Professor Matthew Costa, chief investigator; Mr Alexander Ooms and Dr William Sones, trial statisticians; Dr Jonathan Cook, senior trial statistician; Dr Juul Achten, senior research fellow; Dr Robin Lerner, Dr Marta Campolier and Dr Katy Mironov, trial managers; Mrs Kylea Draper, trial co-ordinator; Mr Damian Haywood and Mrs Amrita Athwal, senior trial managers; Dr May Ee Png, Dr Melina Dritsaki and Dr Helen Dakin, trial health economists; and Mrs Alwin McGibbon, public member.
Trial admin
Dr Shakil Patel and Miss Bojana Selinšek, trial administrative co-ordinators; and Miss Alice Brealy, Miss Kinzah Abbasi, Mr Hugo Strachwitz, Miss Catherine Thompsett and Ms Francis Taylor, data clerks.
Trial applicants
Professor Matthew Costa, chief investigator.
Co-applicants: Dr Juul Achten, Dr Nicholas Parsons, Professor Alastair Gray, Mrs Helen Hedley, Professor Sarah Lamb, Mrs Alwin McGibbon, Dr Melina Dritsaki and Professor Joseph Dias.
Trial Steering Committee
Mr Daniel Perry, Consultant Paediatric Orthopaedic Surgeon (chairperson); Mr Michael Whitehouse, Consultant Trauma and Orthopaedic Surgeon (chairperson); Mr Grey Giddins, Consultant Orthopaedic and Hand Surgeon (independent member); and Mrs Penelope Harris (lay independent member).
Data Monitoring Committee
Professor Lee Shepstone, Professor of Medical Statistics (chairperson); Professor Simon Donell, Consultant Orthopaedic Surgeon (independent member); and Dr Jean Craig, Research Methodologist (independent member).
Statisticians
Mr Alexander Ooms, Dr William Sones and Dr Jonathan Cook.
Health economists
Dr May Ee Png, Dr Melina Dritsaki and Dr Helen Dakin.
Programming team
Ms Lucy Eldridge, Mr Malcolm Hart, Mr Patrick Julier and Dr Simon Shayler.
Radiographical complications team
Mr Matt Cehic and Dr Caroline Gaymer.
Research associates
Emma Stoddard, Ella Riedel, James Glen, Jazmine McCooey, Heather Jarvis, Mat Williams, Toni De Freitas, Janet Sinclair, Liz Still, Glaxy Gray, Ian Venables, Alice Panes, David Triggs, Bradley Tallon, Monah Fareh, Anna Mcskeane, Nicci Kelsall, Zoe Saunders, Stephen Duberley, Maria Ciaccio, Alison Whitcher, Esther Zebracki, Eliza Foster, Steven Barnfield, Katherine Coates, Josephine Morley, Paula Brock, Angie Dempster, Elizabeth Corbishley, Deborah Bunn, Helen Howlett, Helen Shirley, Debbie Wilson, Lucy Dudgeon, Sue Brown, Kate Smith, Andrea Jones, Kathryn Lewis, Maria Mestre, Zoe Warnock, Martin Austin, Sangeetha Prasath, Carolyn Colvin, Charlotte Humphrey, Sarah Buckley, Thelma Darian, Elizabeth Denis, Susan O’Sullivan, Emma Craig, Emily Nash, Rosie Murdoch, Toby Nisbett, Gabbie Young, Jessica Summers, Eve Fletcher, Debbie Walters, Sarah Diment, Benjamin Wilson, Julie Cronin, Rachel Cowey Julie Colley, Ashley Turner, Liam Preece, Maria Baggott, Maria Letts, Elizabeth Cruddas, Abby Newdick, Tereze Cepelkova, Cherrelle DeSouza, Alexandra Bak, Michael Dunn, Isabel Carballo Horton, Damian Dunn, Charlotte Boustead, Joanna Teuke, Tracey Lee, Tracy Brear, Holly Maguire, Ida Ponce, Joanne Hiden, Nicola Collins, Clare Harrop, Lindsay Cunningham, Georgina Williamson, Richard Mccormick, Joanne Bradley-Potts, Leila Dehghani, Cathleen Chabo, Helen Sankey, Karen Smith, Jenny Baron, Stuart Watson, Donna Simpson, Nick Aitken and Heidi McColm.
Other acknowledgements
The DRAFFT 2 trial was supported by the NIHR Oxford Biomedical Research Centre.
List of recruitment centres
Addenbrooke’s Hospital – Cambridge University Hospitals NHS Foundation Trust.
Basingstoke and North Hampshire Hospital – Hampshire Hospitals NHS Foundation Trust.
Blackpool Victoria Hospital – Blackpool Teaching Hospitals NHS Foundation Trust.
Cumberland Infirmary – North Cumbria University Hospitals NHS Trust.
Eastbourne District General Hospital – East Sussex Healthcare NHS Trust.
Ipswich Hospital – East Suffolk and North Essex NHS Foundation Trust.
James Cook University Hospital – South Tees NHS Hospital Trust.
John Radcliffe Hospital – Oxford University Hospitals NHS Foundation Trust.
Leicester General Hospital – University Hospitals of Leicester NHS Trust.
Luton & Dunstable Hospital – Luton & Dunstable University Hospital NHS Foundation Trust.
Manchester Royal Infirmary – Manchester University NHS Foundation Trust.
Musgrove Park Hospital – Somerset NHS Foundation Trust.
North Tyneside General Hospital – Northumbria Healthcare NHS Foundation Trust.
Northampton General Hospital – Northampton General Hospital NHS Trust.
Peterborough City Hospital – North West Anglia NHS Foundation Trust.
Pinderfields Hospital – Mid Yorkshire Hospitals NHS Trust.
Poole Hospital – Poole Hospital NHS Foundation Trust.
Royal Berkshire Hospital – Royal Berkshire NHS Foundation Trust.
Royal Blackburn Hospital – East Lancashire Hospitals NHS Trust.
Royal Cornwall Hospital – Royal Cornwall Hospitals NHS Trust.
Royal Derby Hospital – University Hospitals of Derby and Burton NHS Trust.
Royal Hampshire County Hospital – Hampshire Hospitals NHS Foundation Trust.
Royal Stoke University Hospital – University Hospitals of North Midlands NHS Trust.
Royal Victoria Infirmary – Newcastle upon Tyne NHS Trust.
Salisbury District Hospital – Salisbury NHS Foundation Trust.
Sandwell General Hospital – Sandwell and West Birmingham Hospitals NHS Trust.
Southampton General Hospital – University Hospital Southampton NHS Foundation Trust.
Southmead Hospital – North Bristol NHS Trust.
St. George’s University of London – St George’s University Hospitals NHS Foundation Trust.
Tunbridge Wells Hospital – Maidstone and Tunbridge Wells NHS Trust.
University Hospital (Coventry) – University Hospitals Coventry and Warwickshire NHS Trust.
University Hospital Llandough – Cardiff and Vale University Health Board.
University Hospital of North Tees – North Tees and Hartlepool NHS Foundation Trust.
Whiston Hospital – St Helens and Knowsley Teaching Hospitals NHS Trust.
Wrightington Hospital – Wrightington, Wigan and Leigh NHS Foundation Trust.
Wythenshawe Hospital – Manchester University NHS Foundation Trust.
Contributions of authors
Matthew L Costa (https://orcid.org/0000-0003-3644-1388) (Chief Investigator) was involved in the study conception and design, developed the protocol, had clinical responsibility, and was involved in writing and reviewing the report.
Juul Achten (https://orcid.org/0000-0002-8857-5743) was involved in the study conception and design, developed the protocol, was a member of the TMG, and was involved in writing and reviewing the report.
Alexander Ooms (https://orcid.org/0000-0003-1877-8323) was a member of TMG, was responsible for the statistical analysis of the trial, and was involved in writing and reviewing the report.
May Ee Png (https://orcid.org/0000-0001-5876-9363) was a member of the TMG, was responsible for the health economics analysis, and was involved in writing and reviewing the report.
Jonathan Cook (https://orcid.org/0000-0002-4156-6989) developed the protocol, was a member of TMG, was responsible for the statistical analysis of the trial, and was involved in writing and reviewing the report.
Melina Dritsaki (https://orcid.org/0000-0002-1673-3036) developed the protocol, was a member of TMG and was involved in reviewing the health economic analyses and the report.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) was involved in the study conception and design, developed the protocol, was a member of the TMG and was involved in reviewing the report.
Robin Lerner (https://orcid.org/0000-0002-8372-8181) was involved in study management, was a member of the TMG and was involved in reviewing the report.
Kylea Draper (https://orcid.org/0000-0001-6900-6134) was involved in study management, was a member of the TMG and was involved in writing the report.
Marta Campolier (https://orcid.org/0000-0002-7168-2534) was involved in study management, was a member of the TMG, and was involved in writing and reviewing the report.
Helen Dakin (https://orcid.org/0000-0003-3255-748X) was involved in reviewing the health economic analyses and the report.
Alwin McGibbon (https://orcid.org/0000-0002-5116-9063) developed the protocol, was a member of TMG and was involved in reviewing the report.
Nicholas Parsons (https://orcid.org/0000-0001-9975-888X) was involved in study conception and design, developed the protocol, was a member of the TMG and was involved in reviewing the report.
Helen Hedley (https://orcid.org/0000-0001-5324-9019) was involved in study conception and design, developed the protocol, was a member of the TMG and was involved in reviewing the report.
Joseph Dias (https://orcid.org/0000-0001-5360-4543) was involved in study conception and design, developed the protocol, was a member of the TMG, and was involved in writing and reviewing the report.
Publication
Costa ML, Achten J, Ooms A, Png ME, Cook JA, Lamb SE, et al. Surgical fixation with K-wires versus casting in adults with fracture of distal radius: DRAFFT2 multicentre randomised clinical trial. BMJ 2022;376:e068041.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol 1999;52:243-9. https://doi.org/10.1016/S0895-4356(98)00167-X.
- Chen NC, Jupiter JB. Management of distal radial fractures. J Bone Joint Surg Am 2007;89:2051-62. https://doi.org/10.2106/00004623-200709000-00025.
- Handoll HH, Madhok R. From evidence to best practice in the management of fractures of the distal radius in adults: working towards a research agenda. BMC Musculoskelet Disord 2003;4. https://doi.org/10.1186/1471-2474-4-27.
- Costa ML, Achten J, Plant C, Parsons NR, Rangan A, Tubeuf S, et al. UK DRAFFT: a randomised controlled trial of percutaneous fixation with Kirschner wires versus volar locking-plate fixation in the treatment of adult patients with a dorsally displaced fracture of the distal radius. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19170.
- Costa ML, Achten J, Parsons NR, Rangan A, Griffin D, Tubeuf S, et al. DRAFFT Study Group . Percutaneous fixation with Kirschner wires versus volar locking plate fixation in adults with dorsally displaced fracture of distal radius: randomised controlled trial. BMJ 2014;349. https://doi.org/10.1136/bmj.g4807.
- Costa ML, Jameson SS, Reed MR. Do large pragmatic randomised trials change clinical practice?. Bone Joint J 2016;98–B:410-3. https://doi.org/10.1302/0301-620X.98B3.36730.
- National Institute for Health and Care Excellence . Improving Health and Social Care Through Evidence-Based Guidance 2020. https://www.nice.org.uk/guidance/ng38/resources/fractures-noncomplex-assessment-and-management-pdf-1837399081669 (accessed 24 March 2020).
- Karantana A, Handoll HH, Sabouni A. Percutaneous pinning for treating distal radial fractures in adults. Cochrane Database Syst Rev 2020;2. https://doi.org/10.1002/14651858.CD006080.pub3.
- MacDermid JC, Turgeon T, Richards RS, Beadle M, Roth JH. Patient rating of wrist pain and disability: a reliable and valid measurement tool. J Orthop Trauma 1998;12:577-86. https://doi.org/10.1097/00005131-199811000-00009.
- Achten J, Sones W, Dias J, Hedley H, Cook JA, Dritsaki M, et al. Surgical fixation with K-wires versus plaster casting in the treatment of dorsally displaced distal radius fractures: protocol for Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT 2). BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028474.
- Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5120.
- Downing ND, Karantana A. A revolution in the management of fractures of the distal radius?. J Bone Jt Surg – Ser B n.d.:1271-5. https://doi.org/10.1302/0301-620X.90B10.21293.
- Zenke Y, Sakai A, Oshige T, Moritani S, Nakamura T. The effect of an associated ulnar styloid fracture on the outcome after fixation of a fracture of the distal radius. J Bone Joint Surg Br 2009;91:102-7. https://doi.org/10.1302/0301-620X.91B1.21026.
- Berntsen GK, Fønnebø V, Tollan A, Søgaard AJ, Magnus JH. Forearm bone mineral density by age in 7,620 men and women: the Tromsø study, a population-based study. Am J Epidemiol 2001;153:465-73. https://doi.org/10.1093/aje/153.5.465.
- Court-Brown CM, Rimmer S, Prakash U, McQueen MM. The epidemiology of open long bone fractures. Injury 1998;29:529-34. https://doi.org/10.1016/S0020-1383(98)00125-9.
- MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the short form-36, disability of the arm, shoulder, and hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am 2000;25:330-40. https://doi.org/10.1053/jhsu.2000.jhsu25a0330.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Ooms AG, Png ME, Cook JA, Dritsaki M, Dakin HA, Costa ML. Statistical and health economic analysis plan for a randomized controlled trial of surgical fixation with K-wires versus plaster casting in the treatment of dorsally displaced distal radius fractures: DRAFFT2. Bone Jt Open 2020;1:245-52. https://doi.org/10.1302/2633-1462.16.BJO-2020-0044.R1.
- NHS Business Services Authority . NHS Supply Chain Catalogue 2020. https://my.supplychain.nhs.uk/catalogue (accessed 24 March 2020).
- NHS . National Cost Collection for the NHS 2020. https://improvement.nhs.uk/resources/national-cost-collection/ (accessed 24 March 2020).
- NHS . NHS Electronic Drug Tariff 2020. www.drugtariff.nhsbsa.nhs.uk/#/00770298-DC/DC00770293/Home (accessed 9 January 2020).
- National Institute for Health and Care Excellence . British National Formulary 2020. https://bnf.nice.org.uk/ (accessed 24 March 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Brink M, Van den Hout W, Stiggelbout A, Kievit J, Van de Velde C. Self-Reports of Health Care Utilization: Can a Questionnaire Replace a Diary n.d.
- MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord 2003;4. https://doi.org/10.1186/1471-2474-4-24.
- Bell ML, King MT, Fairclough DL. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data. SAGE Open 2014;4. https://doi.org/10.1177/2158244014534858.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5330.
- National Institute for Health and Care Excellence (NICE) . The Social Care Guidance Manual 2016.
- NHS Business Services Authority . NHS Supply Chain Catalogue 2018 19 2018. https://my.supplychain.nhs.uk/catalogue (accessed 14 September 2021).
- Foundation Trust Network . Operating Theatres – Maximising a Valuable Resource 2013. https://nhsproviders.org/media/1128/operating-theatres-final.pdf (accessed 24 October 2019).
- NHS Digital . Recent Archived Costing Material 2020. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/reference-costs-groupers (accessed 2 September 2021).
- Joint Formulary Committee . British National Formulary (online) 2019. www.medicinescomplete.com (accessed 1 July 2019).
- Office for National Statistics . Employee Earnings in the UK: 2019 2019. www.ons.gov.uk/releases/employeeearningsintheuk2019 (accessed 24 March 2020).
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 14 September 2021).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Parsons N, Griffin XL, Achten J, Chesser TJ, Lamb SE, Costa ML. Modelling and estimation of health-related quality of life after hip fracture: a re-analysis of data from a prospective cohort study. Bone Joint Res 2018;7:1-5. https://doi.org/10.1302/2046-3758.71.BJR-2017-0199.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- National Institute for Health and Care Excellence . Judging Whether Public Health Interventions Offer Value for Money 2013. www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities (accessed 1 July 2018).
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Nixon R, Wonderling D, Grieve R. How to estimate cost-effectiveness acceptability curves, confidence ellipses and incremental net benefits alongside randomised controlled trials. Health Econ 2010;19:316-33. https://doi.org/10.1002/hec.1477.
- Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Ltd; 1987.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Blankers M, Koeter MW, Schippers GM. Missing data approaches in eHealth research: simulation study and a tutorial for nonmathematically inclined researchers. J Med Internet Res 2010;12. https://doi.org/10.2196/jmir.1448.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- National Institute for Health and Care Excellence . Improving Health and Social Care Through Evidence-Based Guidance 2020. www.nice.org.uk/ (accessed 27 July 2020).
- Fullilove S, Gozzard C. Dorsally displaced fractures of the distal radius. Bone Jt J 2016:298-300. https://doi.org/10.1302/0301-620X.98B3.36771.
- NHS . Archived NHS Reference Costs 2020. https://improvement.nhs.uk/resources/reference-costs/ (accessed 24 March 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- The Physiotherapy Centre . Prices 2020. www.thephysiocentre.co.uk/how-much/ (accessed 24 March 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- University of East Anglia Medical Services Occupational Health Department . Price List 2020. www.umsoccupationalhealth.co.uk/page1.aspx?p=17&t=7 (accessed 24 January 2020).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Merton Voluntary Service Council . Group Details – Meals on Wheels (LBM) 2020. www.mvsc.co.uk/civicrm/profile/view?reset=1&id=94191&gid=1/ (accessed 24 March 2020).
- North Yorkshire County Council . Paying For Care At Home 2020. www.northyorks.gov.uk/paying-care-home (accessed 24 March 2020).
- Murgia M. NHS to trial artificial intelligence app in place of 111 helpline. Financial Times n.d.
Appendix 1 The DRAFFT 2 Collaborators
Aamer Ullah, University Hospitals of Leicester NHS Trust.
Aaron Ng, Mid Yorkshire Hospitals NHS Trust.
Adam Watts, Wrightington, Wigan & Leigh NHS Foundation Trust.
Amr Elseehy, Central Manchester University Hospitals NHS Foundation Trust.
Andrew McAndrew, Royal Berkshire NHS Foundation Trust.
Andrew McKee, North West Anglia NHS Foundation Trust.
Antony Bateman, University Hospitals of Derby and Burton NHS Trust.
Barnaby Sheridan, Somerset NHS Foundation Trust.
Caroline Hing, St George’s University Hospitals NHS Foundation Trust.
Charalambos P Charalambous, Blackpool Teaching Hospitals NHS Foundation Trust.
Chris Peach, Manchester University NHS Foundation Trust.
Christopher Roberts, East Suffolk and North Essex NHS Foundation Trust.
David Gidden, Northampton General Hospital NHS Trust.
David Mackay, North Cumbria University Hospitals NHS Trust.
David Warwick, University Hospital Southampton NHS Foundation Trust.
Iain Macleod, Hampshire Hospitals NHS Foundation Trust.
Jaime Candal-Couto, Northumbria Healthcare NHS Foundation Trust.
John Williams, Newcastle upon Tyne NHS Trust.
Jonathan Young, University Hospitals Coventry and Warwickshire NHS Trust.
Kanthan Theivendran, Sandwell and West Birmingham Hospitals NHS Trust.
Kevin Smith, University Hospitals of North Midlands NHS Trust.
Khitish Mohanty, Cardiff & Vale University Health Board.
Makaram Srinivasan, East Lancashire Hospitals NHS Trust.
Mark Farrar, Poole Hospital NHS Foundation Trust.
Michael Butler, Royal Cornwall Hospitals NHS Trust.
Mohamed Hamadto, University Hospitals of North Midlands NHS Trust.
Nigel Rossiter, Hampshire Hospitals NHS Foundation Trust.
Oliver Keast-Butler, East Sussex Healthcare NHS Trust.
Phillip Johnston, Cambridge University Hospitals NHS Foundation Trust.
Praveen Sarda, Central Manchester University Hospitals NHS Foundation Trust.
Pulimamidi Sanjay, Bedfordshire Hospitals NHS Foundation Trust.
Rajesh Nanda, North Tees and Hartlepool NHS Foundation Trust.
Richard Benson, Maidstone & Tunbridge Wells NHS Trust.
Sridharrao Sampalli, Salisbury NHS Foundation Trust.
Stephen Lipscombe, St Helens and Knowsley Teaching Hospitals NHS Trust.
Steve Gwilym, Oxford University Hospitals NHS Foundation Trust.
Timothy Chesser, University Hospitals Bristol and Weston NHS Foundation Trust.
William Eardley, South Tees Hospitals NHS Foundation Trust.
Amit Sharma, Bedfordshire Hospitals NHS Foundation Trust.
Andreas Fontalis, St George’s University Hospitals NHS Foundation Trust.
Arshad Iqbal, Poole Hospital NHS Foundation Trust.
Asanka Wijendra, Oxford University Hospitals NHS Foundation Trust.
Catherine Gibson, Hampshire Hospitals NHS Foundation Trust.
Craig Zhao, East Sussex Healthcare NHS Trust.
Damir Rasidovic, University Hospitals Coventry and Warwickshire NHS Trust.
Dilip Pillai, University Hospitals of Derby and Burton NHS Trust.
Harry Beale, Hampshire Hospitals NHS Foundation Trust.
Hytham Afifi, Northampton General Hospital NHS Trust.
James Duncan, Royal Berkshire NHS Foundation Trust.
James Kennedy, Manchester University NHS Foundation Trust.
John Baha Tadros, East Sussex Healthcare NHS Trust.
Jonathan Crosby, North West Anglia NHS Foundation Trust.
Jonathan Hobby, University Hospitals of Derby and Burton NHS Trust.
Ken Wong, Ipswich Hospital NHS Trust.
Khaled Aneiba, North Tees and Hartlepool NHS Foundation Trust.
Laura Beddard, Poole Hospital NHS Foundation Trust.
Louise Nordin, Hampshire Hospitals NHS Foundation Trust.
Luke Hughes, Manchester University NHS Foundation Trust.
Lydia Jenner, North Tees and Hartlepool NHS Foundation Trust.
Marie-Clare Killen, Newcastle upon Tyne NHS Trust.
Mark Williamson, Maidstone & Tunbridge Wells NHS Trust.
Matt Jones, Royal Cornwall Hospitals NHS Trust.
Matt Weston, University Hospitals Coventry and Warwickshire NHS Trust.
Mohammad Iqbal, Bedfordshire Hospitals NHS Foundation Trust.
Olivia Payton, University Hospitals Coventry and Warwickshire NHS Trust.
Pradeep Kankanalu, University Hospitals of North Midlands NHS Trust.
Rebecca Emily Beamish, Hampshire Hospitals NHS Foundation Trust.
Robert Jennings, University Hospital Southampton NHS Foundation Trust.
Ryan Higgin, Salisbury NHS Foundation Trust.
Sally Kerr, Hampshire Hospitals NHS Foundation Trust.
Simon J Parker, Royal Berkshire NHS Foundation Trust.
Steph Buchan, Salisbury NHS Foundation Trust.
Steve Milner, University Hospitals of Derby and Burton NHS Trust.
Sukhdeep Gill, Hampshire Hospitals NHS Foundation Trust.
Thomas Corbett, St George’s University Hospitals NHS Foundation Trust.
Tofi Oni, East Sussex Healthcare NHS Trust.
Venkat Vardhan, University Hospitals of North Midlands NHS Trust.
Warran Wignadasan, Poole Hospital NHS Foundation Trust.
Wystan Chevannes, North West Anglia NHS Foundation Trust.
Appendix 2 Health economics supplementary data
| Resource item | Unit type | Unit cost (£) | Source |
|---|---|---|---|
| DRAFFT 2 cost | |||
| Fixation surgery | |||
| K-wires | Each | 2.96 | NHS Supply Chain Catalogue 20 |
| Reinforced gown XL | Each | 1.76 | NHS Supply Chain Catalogue 20 |
| Surgical visor masks | Each | 0.48 | NHS Supply Chain Catalogue 20 |
| Theatre masks | Each | 0.09 | NHS Supply Chain Catalogue 20 |
| Biogel gloves | Each | 1.90 | NHS Supply Chain Catalogue 20 |
| Image intensifier cover | Each | 4.26 | NHS Supply Chain Catalogue 20 |
| Laceration pack | Each | 13.55a | DRAFFT4 |
| Sterilisation of drill | Each | 10.23a | DRAFFT4 |
| Sterile dressing gauze | Each | 0.24 | NHS Supply Chain Catalogue 20 |
| Full cast | Each | 3.21 | NHS Supply Chain Catalogue 20 |
| Non-sterile gloves | Each | 0.58 | NHS Supply Chain Catalogue 20 |
| Surface wipes | Each | 0.05 | NHS Supply Chain Catalogue 20 |
| Black bag | Each | 0.03 | NHS Supply Chain Catalogue 20 |
| Yellow bag | Each | 0.09 | NHS Supply Chain Catalogue 20 |
| Polythene bag for extras | Each | 0.02 | NHS Supply Chain Catalogue 20 |
| Incontinence pad | Each | 0.11 | NHS Supply Chain Catalogue 20 |
| Hospital stay | |||
| Intensive care unit | Per day | 834.00 | NHS Reference Costs 2018/1921 |
| Acute trauma ward | Per day | 353.99a | NHS Reference Costs 2017/1849 |
| Rehabilitation ward | Per day | 443.00 | NHS Reference Costs 2018/1921 |
| Treatment after index surgery | |||
| Ulna shortening | LOS of 6 days | 5662.79 | NHS Reference Costs 2018/1921 |
| Revision to plate and screw/removal of metalwork/washout of joint | LOS of 6 days | 4144.66 | NHS Reference Costs 2018/1921 |
| Debridement | LOS of 2 days | 1209.00 | NHS Reference Costs 2018/1921 |
| Injection | LOS of 1 day | 190.00 | NHS Reference Costs 2018/1921 |
| Carpal tunnel decompression/surgery to tendon/manipulation/wire fixation | LOS of 4 days | 5471.73 | NHS Reference Costs 2018/1921 |
| Inpatient care | |||
| Orthopaedics: wrist/arm | LOS of 2 days | 1043.00 | NHS Reference Costs 2018/1921 |
| Orthopaedics: other bones | LOS of 4 days | 5776.00 | NHS Reference Costs 2018/1921 |
| Orthopaedics: other bones | Per excess bed-day | 373.43a | NHS Reference Costs 2017/1849 |
| Other non-surgery | Per day | 353.99a | NHS Reference Costs 2017/1849 |
| Day case | LOS of 1 day | 1094.00 | NHS Reference Costs 2018/1921 |
| Rehabilitation ward | Per day | 443.00 | NHS Reference Costs 2018/1921 |
| Outpatient care | |||
| Orthopaedics/fracture clinic | Per visit | 120.00 | NHS Reference Costs 2018/1921 |
| Physiotherapist (NHS) | Per hour | 38.88a | Unit Costs of Health and Social Care 2015 50 |
| Physiotherapist (private) | Per hour | 75.00 | The Physiotherapy Centre 202051 |
| Pathology (blood tests) | Per test | 2.79 | NHS Reference Costs 2018/1921 |
| Radiology | Per test | 30.59 | NHS Reference Costs 2018/1921 |
| Emergency department: wrist | Per visit | 168.00 | NHS Reference Costs 2018/1921 |
| Emergency department: others | Per visit | 168.00 | NHS Reference Costs 2018/1921 |
| Others: | |||
| Bone health assessment | Per visit | 71.00 | NHS Reference Costs 2018/1921 |
| Falls clinic | Per visit | 83.21a | Unit Costs of Health and Social Care 2018 52 |
| Injection | Per visit | 204.00 | NHS Reference Costs 2018/1921 |
| Neurophysiologist | Per visit | 425.00 | NHS Reference Costs 2018/1921 |
| Occupational health advisor | Per hour | 90.00 | UMS Occupational Health Department 202053 |
| Occupational therapist | Per visit | 83.00 | NHS Reference Costs 2018/1921 |
| Psychologist | Per visit | 183.00 | NHS Reference Costs 2018/1921 |
| Rheumatologist | Per visit | 147.00 | NHS Reference Costs 2018/1921 |
| Primary and community care | |||
| General practitioner (surgery visit) | Per minute | 4.30 | Unit Costs of Health and Social Care 2019 24 |
| General practitioner (home visit) | Per minute | 5.32a | Unit Costs of Health and Social Care 2010 54 |
| General practitioner (telephone contact) | 7.1 minutes | 27.62a | Unit Costs of Health and Social Care 2015 50 |
| Practice nurse (surgery visit) | Per hour | 42.00 | Unit Costs of Health and Social Care 2019 24 |
| Practice nurse (home visit) | Per hour | 45.02a | Unit Costs of Health and Social Care 2010 54 |
| Practice nurse (telephone contact) | Per hour | 36.83a | Unit Costs of Health and Social Care 2010 54 |
| District nurse (surgery visit) | Per hour | 51.16a | Unit Costs of Health and Social Care 2015 50 |
| District nurse (home visit) | Per hour | 93.10a | Unit Costs of Health and Social Care 2010 54 |
| Physiotherapist (surgery visit) | Per hour | 36.83a | Unit Costs of Health and Social Care 2014 55 |
| Physiotherapist (home visit) | Per hour | 36.83a | Unit Costs of Health and Social Care 2014 55 |
| Occupational therapist (surgery visit) | Per hour | 48.00 | Unit Costs of Health and Social Care 2019 24 |
| Occupational therapist (home visit) | Per visit | 47.06a | Unit Costs of Health and Social Care 2010 54 |
| Other | |||
| Radiology | Per hour | 37.00 | Unit Costs of Health and Social Care 2019 24 |
| Aids and adaptations | |||
| Wrist support (e.g. brace/splint) | Each | 4.54 | NHS Supply Chain Catalogue 20 |
| Grab rail | Each | 48.00 | Unit Costs of Health and Social Care 2019 24 |
| Dressing aids | Each | 6.78 | NHS Supply Chain Catalogue 20 |
| Long-handled aids (e.g. shoe horn, reacher) | Each | 4.25 | NHS Supply Chain Catalogue 20 |
| Bathing aids | Each | 3.92 | NHS Supply Chain Catalogue 20 |
| Kitchen aids (e.g. jar/tin openers) | Each | 5.86 | NHS Supply Chain Catalogue 20 |
| Other | |||
| Computer aids (e.g. ergonomic keyboard) | Each | 8.18 | NHS Supply Chain Catalogue 20 |
| Exercise aids (e.g. exercise band, putty) | Each | 30.08 | NHS Supply Chain Catalogue 20 |
| PSS | |||
| Frozen Meals on Wheels | Per meal | 3.17 | Meals on Wheels 202056 |
| Hot Meals on Wheels | Per meal | 6.75a | Unit Costs of Health and Social Care 2014 55 |
| Laundry services | Per load | 4.90 | North Yorkshire County Council 202057 |
| Calls to NHS 111 | Per call | 14.32a | Financial Times 201758 |
| Social worker | Per hour | 51.00 | Unit Costs of Health and Social Care 2019 24 |
| Care worker/help at home | Per hour | 28.00 | Unit Costs of Health and Social Care 2019 24 |
| Lost productivity | |||
| Median wage | Per week | 585.00 | Office for National Statistics34 |
| Medications | |||
| Analgesic | |||
| Algesal cream 50 g | Each | 1.49 | NHS Electronic Drug Tariff 22 |
| Aspirin 75 mg | Pack of 28 | 1.47 | BNF23 |
| Co-codamol 8 mg/500 mg | Pack of 100 | 3.17 | NHS Electronic Drug Tariff 22 |
| Co-codamol 30 mg/500 mg | Pack of 100 | 4.60 | NHS Electronic Drug Tariff 22 |
| Codeine 15 mg | Pack of 28 | 1.04 | NHS Electronic Drug Tariff 22 |
| Codeine 30 mg | Pack of 28 | 1.21 | NHS Electronic Drug Tariff 22 |
| Codeine 60 mg | Pack of 28 | 1.79 | NHS Electronic Drug Tariff 22 |
| Co-dydramol 10 mg/500 mg | Pack of 30 | 0.95 | NHS Electronic Drug Tariff 22 |
| Diclofenac 50 mg | Pack of 28 | 7.41 | NHS Electronic Drug Tariff 22 |
| Dihydrocodeine 30 mg | Pack of 28 | 1.19 | NHS Electronic Drug Tariff 22 |
| Gabapentin 100 mg | Pack of 100 | 1.98 | NHS Electronic Drug Tariff 22 |
| Gabapentin 300 mg | Pack of 100 | 3.04 | NHS Electronic Drug Tariff 22 |
| Gabapentin 600 mg | Pack of 100 | 7.93 | NHS Electronic Drug Tariff 22 |
| Lidocaine 5% | Pack of 30 | 72.40 | NHS Electronic Drug Tariff 22 |
| Ibuprofen 200 mg | Pack of 24 | 1.09 | NHS Electronic Drug Tariff 22 |
| Ibuprofen 400 mg | Pack of 24 | 1.05 | NHS Electronic Drug Tariff 22 |
| Ibuprofen 600 mg | Pack of 84 | 4.08 | NHS Electronic Drug Tariff 22 |
| Morphine 5 mg | Pack of 60 | 3.29 | NHS Electronic Drug Tariff 22 |
| Morphine 10 mg | Pack of 60 | 5.20 | NHS Electronic Drug Tariff 22 |
| Morphine 15 mg | Pack of 60 | 9.10 | NHS Electronic Drug Tariff 22 |
| Morphine 20 mg | Pack of 56 | 10.61 | NHS Electronic Drug Tariff 22 |
| Morphine 30 mg | Pack of 60 | 12.47 | NHS Electronic Drug Tariff 22 |
| Morphine 60 mg | Pack of 60 | 24.32 | NHS Electronic Drug Tariff 22 |
| Naproxen 250 mg | Pack of 28 | 3.29 | NHS Electronic Drug Tariff 22 |
| Naproxen 500 mg | Pack of 28 | 3.02 | NHS Electronic Drug Tariff 22 |
| Nefopam 30 mg | Pack of 90 | 5.66 | NHS Electronic Drug Tariff 22 |
| Oxycodone 5 mg | Pack of 28 | 12.52 | NHS Electronic Drug Tariff 22 |
| Oxycodone 10 mg | Pack of 56 | 25.04 | NHS Electronic Drug Tariff 22 |
| Oxycodone 15 mg | Pack of 56 | 38.12 | NHS Electronic Drug Tariff 22 |
| Paracetamol 500 mg | Pack of 100 | 2.19 | NHS Electronic Drug Tariff 22 |
| Solpadeine 12.8 mg/500 mg | Pack of 30 | 4.91 | NHS Electronic Drug Tariff 22 |
| Tramadol 50 mg | Pack of 60 | 4.60 | NHS Electronic Drug Tariff 22 |
| Tramadol 100 mg | Pack of 60 | 14.47 | NHS Electronic Drug Tariff 22 |
| Tramadol 150 mg | Pack of 60 | 21.71 | NHS Electronic Drug Tariff 22 |
| Flucloxacillin 250 mg | Pack of 28 | 1.67 | NHS Electronic Drug Tariff 22 |
| Flucloxacillin 500 mg | Pack of 28 | 2.68 | NHS Electronic Drug Tariff 22 |
| Pregabalin 75 mg | Pack of 56 | 2.47 | NHS Electronic Drug Tariff 22 |
| Pregabalin 150 mg | Pack of 56 | 3.09 | NHS Electronic Drug Tariff 22 |
| Pregabalin 300 mg | Pack of 56 | 5.28 | NHS Electronic Drug Tariff 22 |
| Clopidogrel 75 mg | Pack of 28 | 1.49 | NHS Electronic Drug Tariff 22 |
| Heparinoid 0.30% | Each | 3.99 | NHS Electronic Drug Tariff 22 |
| Other bone protection medication | |||
| Alendronate 10 mg | Pack of 28 | 2.63 | NHS Electronic Drug Tariff 22 |
| Bisphosphonate | |||
| Risedronate 35 mg | Pack of 4 | 11.34 | NHS Electronic Drug Tariff 22 |
| Supplement and vitamin | |||
| Adcal 1.5 g | Pack of 100 | 8.70 | NHS Electronic Drug Tariff 22 |
| Calcichew 800 units/2.5 g | Pack of 30 | 6.75 | NHS Electronic Drug Tariff 22 |
| Calcium and colecalciferol 400 units/1.5 g | Pack of 60 | 2.95 | NHS Electronic Drug Tariff 22 |
| Vitamin D 800 units/1.25 g | Pack of 30 | 4.21 | NHS Electronic Drug Tariff 22 |
| Vitamin D 20,000 units | Pack of 30 | 29.00 | NHS Electronic Drug Tariff 22 |
| Vitamin D 50,000 units | Pack of 3 | 4.95 | NHS Electronic Drug Tariff 22 |
| Domain | Levels | Time point | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre injury | Post injury | 3 months | 6 months | 12 months | |||||||
| Cast (n = 255) | K-wire (n = 245) | Cast (n = 255) | K-wire (n = 245) | Cast (n = 216) | K-wire (n = 202) | Cast (n = 203) | K-wire (n = 206) | Cast (n = 200) | K-wire (n = 196) | ||
| Mobility | 1 | 229 (89.8) | 219 (89.4) | 199 (78.0) | 185 (75.5) | 181 (83.8) | 167 (82.7) | 172 (84.7) | 168 (81.6) | 168 (84.0) | 161 (82.1) |
| 2 | 11 (4.3) | 12 (4.9) | 22 (8.6) | 30 (12.2) | 12 (5.6) | 18 (8.9) | 13 (6.4) | 21 (10.2) | 14 (7.0) | 18 (9.2) | |
| 3 | 9 (3.5) | 9 (3.7) | 20 (7.8) | 17 (6.9) | 16 (7.4) | 13 (6.4) | 9 (4.4) | 15 (7.3) | 13 (6.5) | 11 (5.6) | |
| 4 | 5 (2.0) | 4 (1.6) | 7 (2.7) | 2 (0.8) | 7 (3.2) | 4 (2.0) | 8 (3.9) | 1 (0.5) | 3 (1.5) | 6 (3.1) | |
| 5 | 0 (0.0) | 0 (0.0) | 5 (2.0) | 9 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Self-care | 1 | 241 (94.5) | 241 (98.4) | 7 (2.7) | 11 (4.5) | 108 (50.0) | 96 (47.5) | 135 (66.5) | 141 (68.4) | 160 (80.0) | 150 (76.5) |
| 2 | 8 (3.1) | 2 (0.8) | 59 (23.1) | 59 (24.1) | 75 (34.7) | 70 (34.7) | 52 (25.6) | 51 (24.8) | 25 (12.5) | 32 (16.3) | |
| 3 | 3 (1.2) | 2 (0.8) | 92 (36.1) | 101 (41.2) | 30 (13.9) | 27 (13.4) | 13 (6.4) | 12 (5.8) | 14 (7.0) | 11 (5.6) | |
| 4 | 1 (0.4) | 0 (0.0) | 58 (22.7) | 40 (16.3) | 2 (0.9) | 8 (4.0) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 2 (1.0) | |
| 5 | 1 (0.4) | 0 (0.0) | 37 (14.5) | 31 (12.7) | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Usual activities | 1 | 231 (90.6) | 233 (95.1) | 5 (2.0) | 7 (2.9) | 52 (24.1) | 42 (20.8) | 92 (45.3) | 88 (42.7) | 111 (55.5) | 101 (51.5) |
| 2 | 13 (5.1) | 5 (2.0) | 24 (9.4) | 18 (7.3) | 87 (40.3) | 76 (37.6) | 75 (36.9) | 80 (38.8) | 64 (32.0) | 61 (31.1) | |
| 3 | 5 (2.0) | 5 (2.0) | 70 (27.5) | 74 (30.2) | 63 (29.2) | 66 (32.7) | 31 (15.3) | 32 (15.5) | 22 (11.0) | 27 (13.8) | |
| 4 | 3 (1.2) | 2 (0.8) | 47 (18.4) | 53 (21.6) | 6 (2.8) | 12 (5.9) | 3 (1.5) | 5 (2.4) | 2 (1.0) | 7 (3.6) | |
| 5 | 2 (0.8) | 0 (0.0) | 107 (42.0) | 91 (37.1) | 8 (3.7) | 6 (3.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Pain | 1 | 200 (78.4) | 194 (79.2) | 5 (2.0) | 5 (2.0) | 28 (13.0) | 15 (7.4) | 44 (21.7) | 42 (20.4) | 88 (44.0) | 71 (36.2) |
| 2 | 32 (12.5) | 34 (13.9) | 45 (17.6) | 44 (18.0) | 98 (45.4) | 103 (51.0) | 109 (53.7) | 115 (55.8) | 70 (35.0) | 90 (45.9) | |
| 3 | 18 (7.1) | 15 (6.1) | 107 (42.0) | 111 (45.3) | 81 (37.5) | 64 (31.7) | 40 (19.7) | 43 (20.9) | 32 (16.0) | 24 (12.2) | |
| 4 | 4 (1.6) | 2 (0.8) | 64 (25.1) | 59 (24.1) | 7 (3.2) | 18 (8.9) | 7 (3.4) | 4 (1.9) | 8 (4.0) | 10 (5.1) | |
| 5 | 0 (0.0) | 0 (0.0) | 31 (12.2) | 24 (9.8) | 2 (0.9) | 2 (1.0) | 2 (1.0) | 1 (0.5) | 1 (0.5) | 1 (0.5) | |
| Anxiety/depression | 1 | 198 (77.6) | 193 (78.8) | 131 (51.4) | 122 (49.8) | 127 (58.8) | 130 (64.4) | 140 (69.0) | 147 (71.4) | 144 (72.0) | 141 (71.9) |
| 2 | 28 (11.0) | 31 (12.7) | 55 (21.6) | 70 (28.6) | 54 (25.0) | 42 (20.8) | 36 (17.7) | 38 (18.4) | 31 (15.5) | 34 (17.3) | |
| 3 | 21 (8.2) | 17 (6.9) | 39 (15.3) | 34 (13.9) | 22 (10.2) | 25 (12.4) | 21 (10.3) | 14 (6.8) | 19 (9.5) | 14 (7.1) | |
| 4 | 6 (2.4) | 4 (1.6) | 21 (8.2) | 5 (2.0) | 8 (3.7) | 4 (2.0) | 2 (1.0) | 5 (2.4) | 4 (2.0) | 5 (2.6) | |
| 5 | 1 (0.4) | 0 (0.0) | 6 (2.4) | 11 (4.5) | 5 (2.3) | 1 (0.5) | 3 (1.5) | 1 (0.5) | 0 (0.0) | 2 (1.0) | |
FIGURE 17.
Change in EQ-5D-5L mobility domain by treatment group over time.
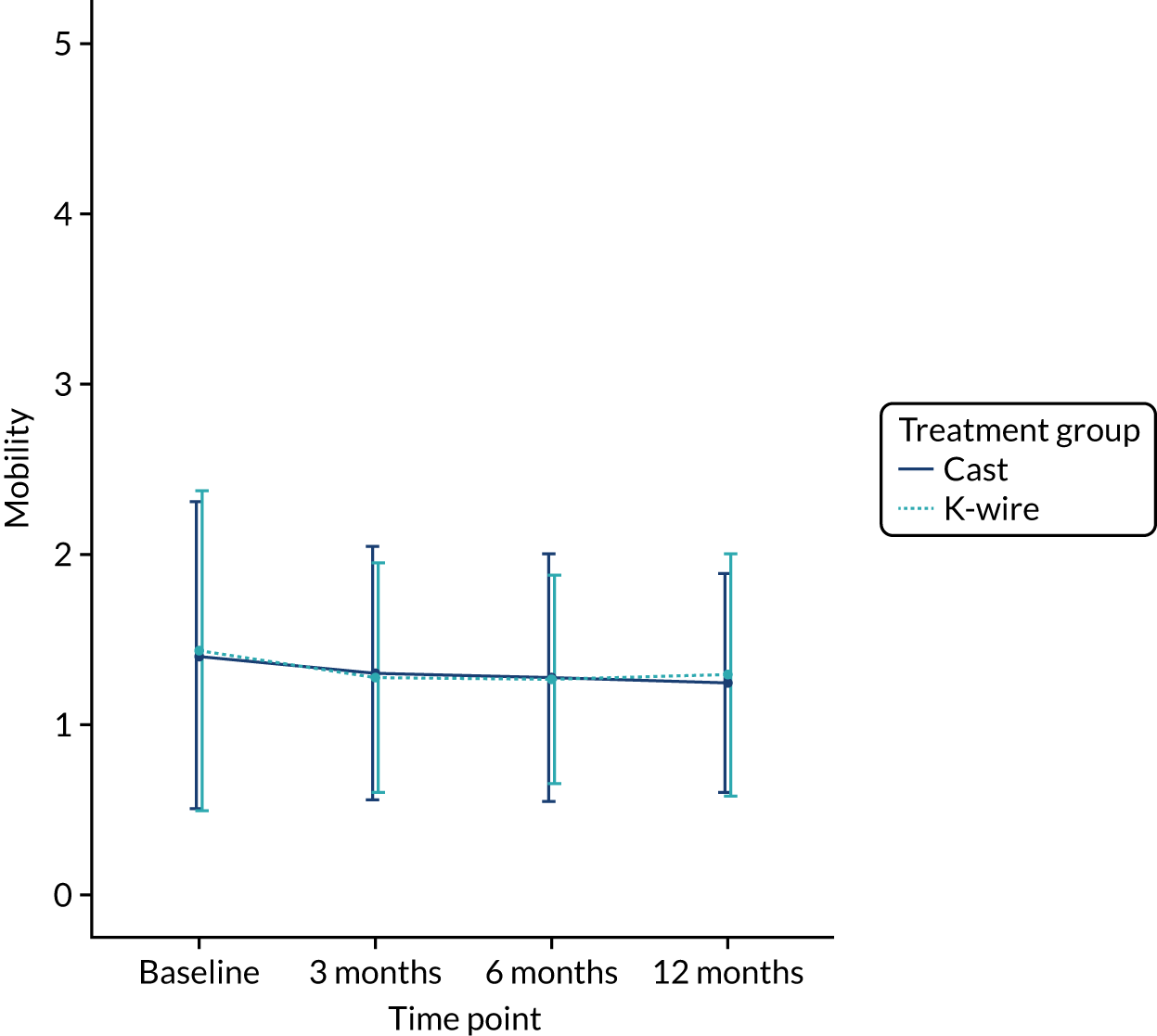
FIGURE 18.
Change in EQ-5D-5L self-care domain by treatment group over time.
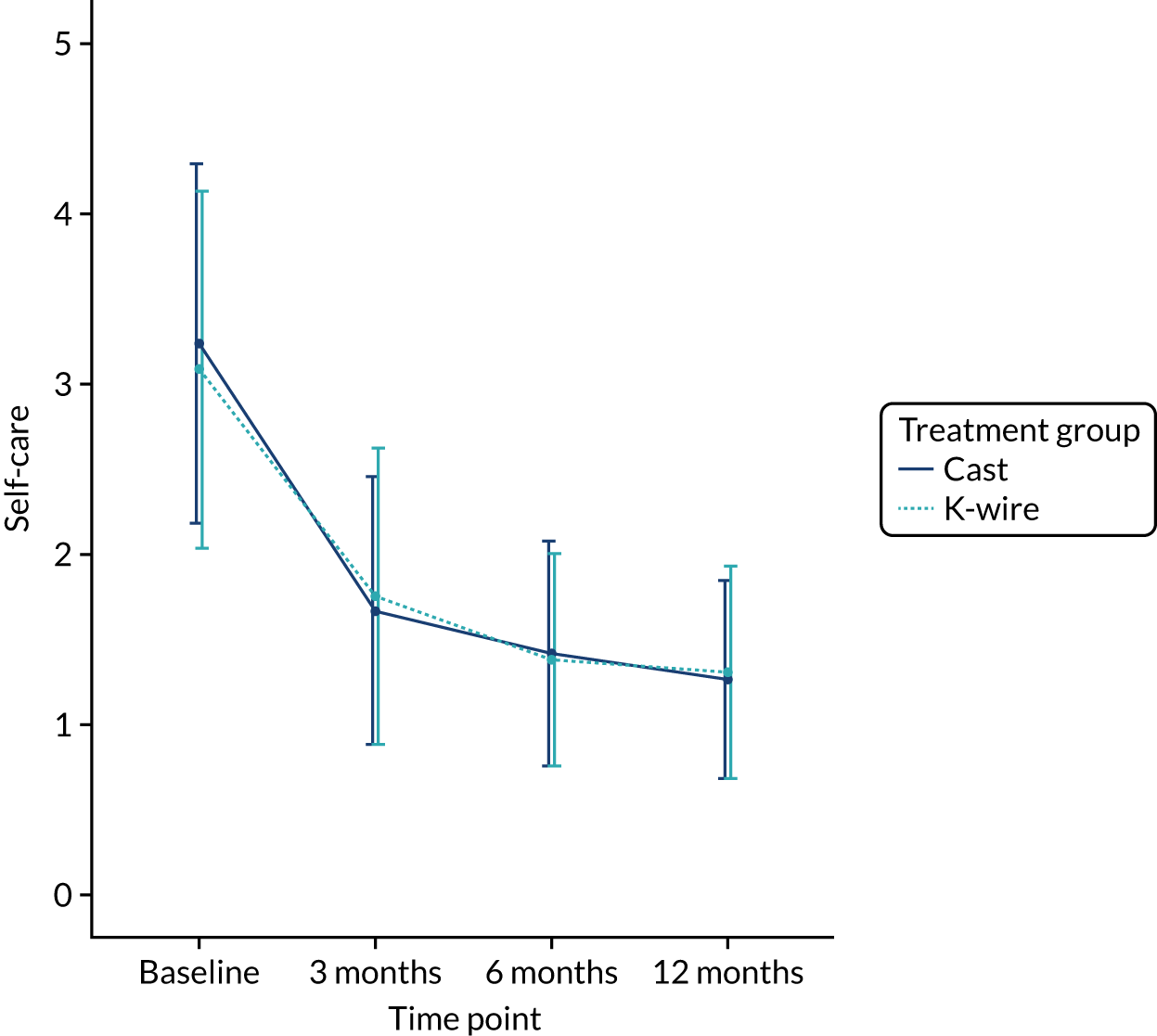
FIGURE 19.
Change in EQ-5D-5L usual activities domain by treatment group over time.
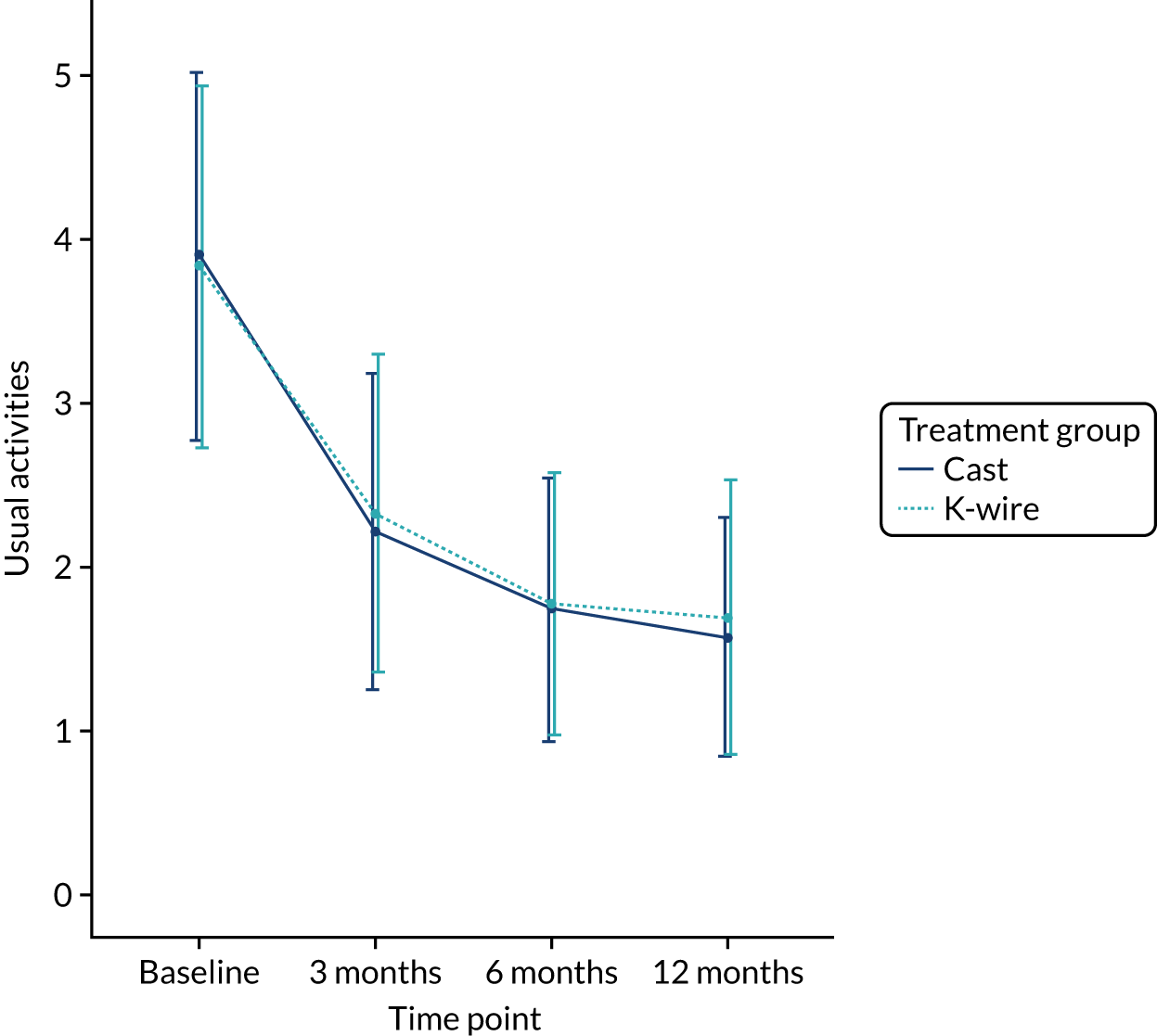
FIGURE 20.
Change in EQ-5D-5L pain domain by treatment group over time.
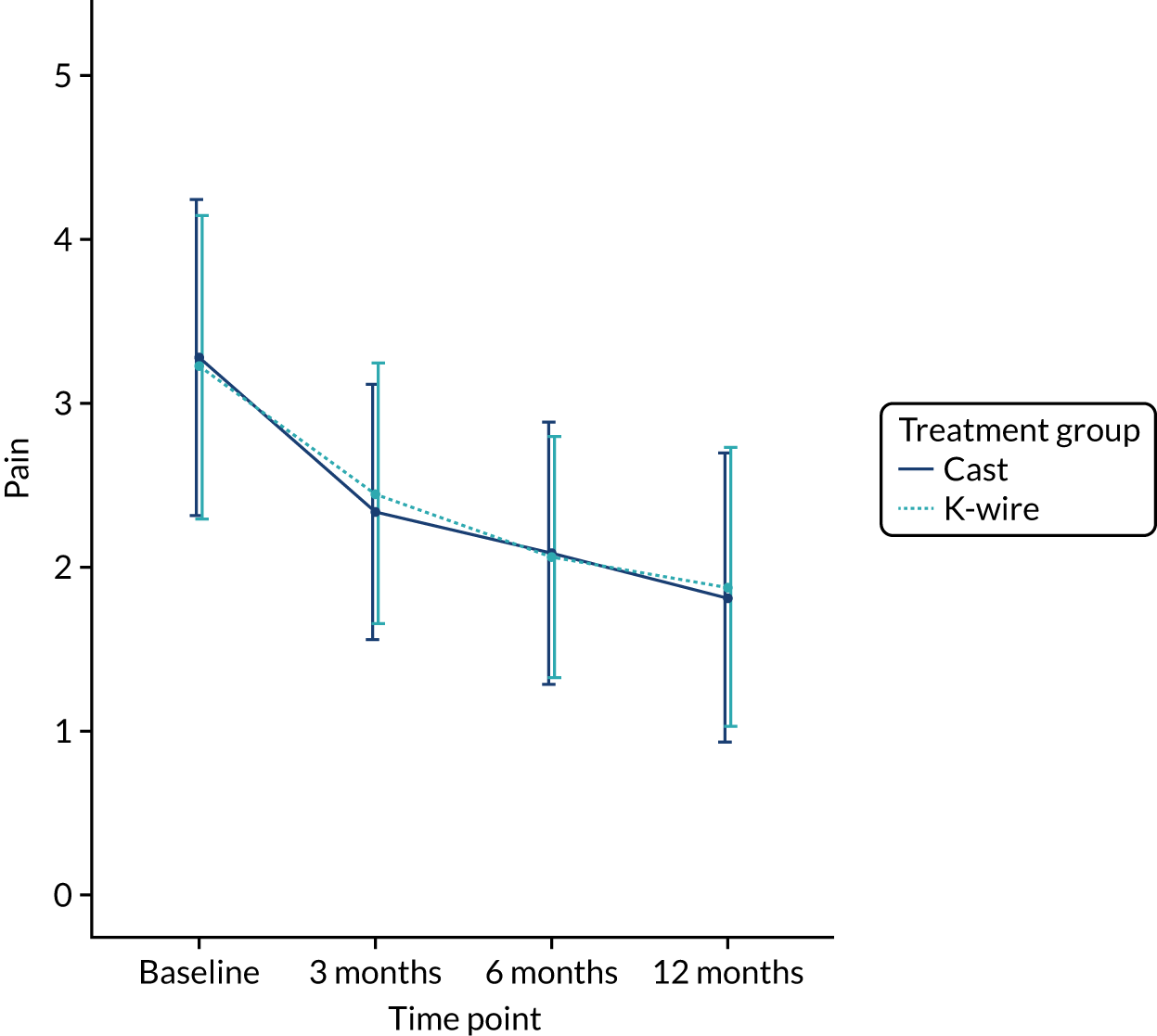
FIGURE 21.
Change in EQ-5D-5L anxiety/depression domain by treatment group over time.
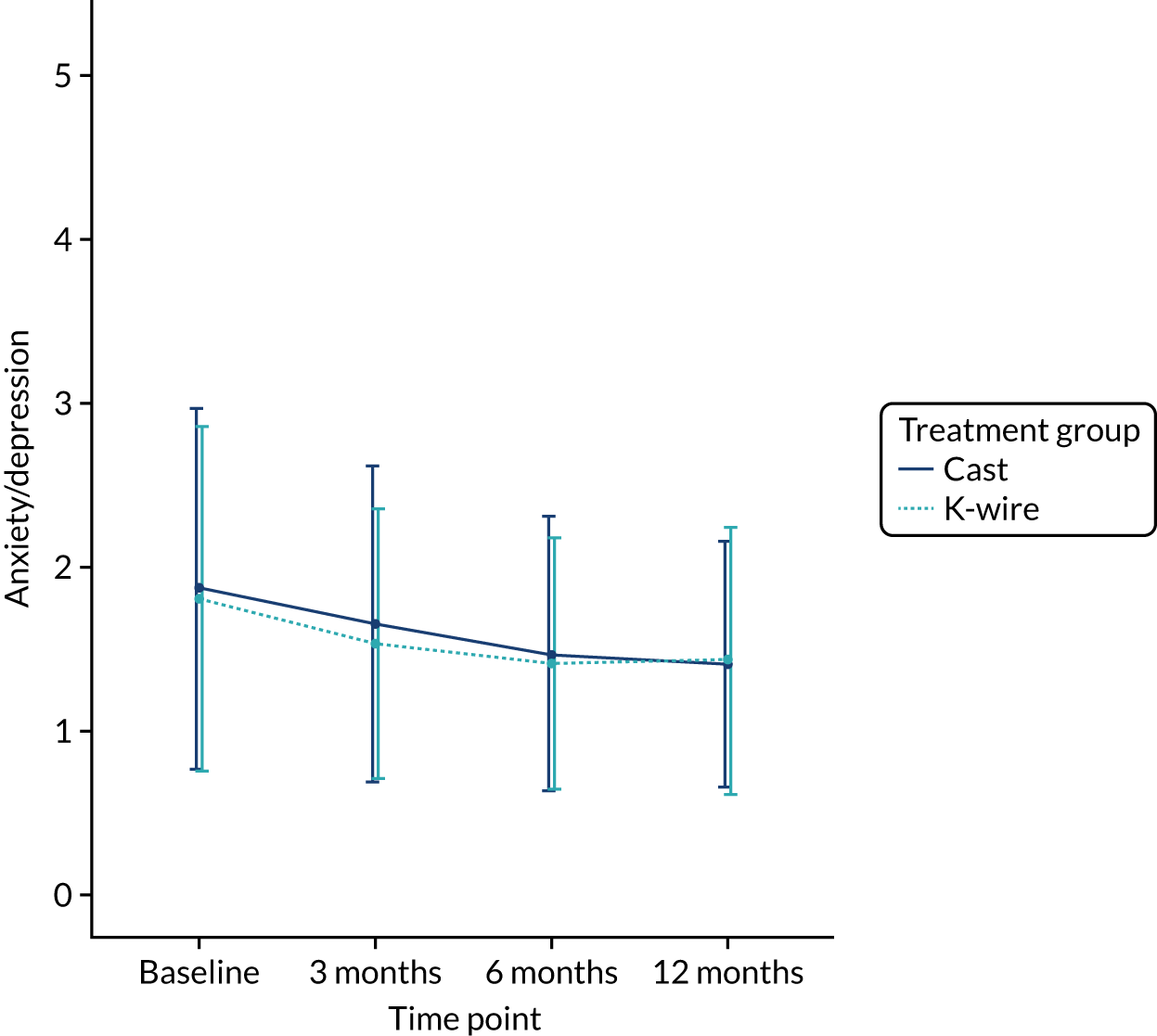
| Health-care resource type | Treatment group | Bootstrapped 95% CI | p-value | |
|---|---|---|---|---|
| Cast | K-wire | |||
| Discharge to 3 months | ||||
| Subsequent inpatient care, mean number of days (SE) | 0.005 (0.005) | 0 (0) | –0.014 to 0 | 0.318 |
| Outpatient care, mean number of visits (SE) | ||||
| Orthopaedics/fracture | 0.678 (0.091) | 0.752 (0.096) | –0.184 to 0.333 | 0.571 |
| Physiotherapist: NHS | 0.028 (0.015) | 0.079 (0.024) | –0.002 to 0.106 | 0.067 |
| Physiotherapist: private | 0.140 (0.038) | 0.153 (0.036) | –0.090 to 0.116 | 0.800 |
| Pathology: blood tests | 1.336 (0.127) | 1.248 (0.124) | –0.444 to 0.251 | 0.617 |
| Radiology | 0.140 (0.062) | 0.233 (0.073) | –0.098 to 0.282 | 0.335 |
| Emergency department: wrist | 0.009 (0.007) | 0.005 (0.005) | –0.019 to 0.010 | 0.594 |
| Emergency department: others | 0.028 (0.016) | 0.005 (0.005) | –0.061 to 0.005 | 0.172 |
| Others | 0.014 (0.008) | 0.005 (0.005) | –0.028 to 0.010 | 0.336 |
| Community care, mean duration in minutesa (SE) | ||||
| General practitioner: surgery visit | 1.540 (0.345) | 2.839 (0.617) | –0.046 to 2.763 | 0.067 |
| General practitioner: home visit | 0 (0) | 0 (0) | – | – |
| General practitioner: telephone contact | 0.376 (0.206) | 0.542 (0.255) | –0.452 to 0.839 | 0.612 |
| Practice nurse: surgery visit | 0.558 (0.441) | 0.995 (0.514) | –0.888 to 1.801 | 0.519 |
| Practice nurse: home visit | 0.419 (0.419) | 0 (0) | –1.256 to 0 | 0.318 |
| Practice nurse: telephone contact | 0.047 (0.047) | 0.050 (0.050) | –0.14 to 0.149 | 0.965 |
| District nurse: surgery visit | 4.000 (3.908) | 0 (0) | –11.907 to 0 | 0.307 |
| District nurse: home visit | 0 (0) | 0 (0) | – | – |
| Physiotherapist: surgery visit | 11.611 (2.962) | 10.149 (3.207) | –9.842 to 7.120 | 0.738 |
| Physiotherapist: home visit | 1.612 (1.121) | 2.040 (1.278) | –2.876 to 3.881 | 0.802 |
| Occupational therapist: surgery visit | 4.116 (3.055) | 0.746 (0.498) | –10.388 to 0.925 | 0.277 |
| Occupational therapist: home visit | 0.977 (0.977) | 0.199 (0.199) | –2.930 to 0.398 | 0.436 |
| Others | 0 (0) | 0 (0) | – | – |
| Medications, proportion of participantsb | ||||
| Analgesic | 0.398 (–) | 0.396 (–) | – | 0.904 |
| Antibiotic | 0 (–) | 0 (–) | – | – |
| Bisphosphonate | 0.009 (–) | 0 (–) | – | 0.169 |
| Other bone protection medication | 0.014 (–) | 0.040 (–) | – | 0.094 |
| Supplement and vitamin | 0.046 (–) | 0.050 (–) | – | 0.873 |
| Aids and adaptations, mean number (SE) | ||||
| Wrist support (e.g. brace/splint) | 0.414 (0.045) | 0.420 (0.038) | –0.11 to 0.119 | 0.919 |
| Grab rail | 0.023 (0.012) | 0.020 (0.012) | –0.037 to 0.031 | 0.855 |
| Dressing aids | 0.079 (0.058) | 0.030 (0.030) | –0.19 to 0.06 | 0.453 |
| Long-handled aids (e.g. shoe horn, reacher) | 0.023 (0.010) | 0.025 (0.011) | –0.027 to 0.031 | 0.902 |
| Bathing aids | 0.074 (0.021) | 0.055 (0.016) | –0.073 to 0.032 | 0.468 |
| Kitchen aids (e.g. jar/tin openers) | 0.042 (0.018) | 0.040 (0.016) | –0.049 to 0.042 | 0.944 |
| Others | 0.014 (0.008) | 0.010 (0.007) | –0.023 to 0.016 | 0.704 |
| PSS, mean number (SE) | ||||
| Frozen Meals on Wheels | 0 (0) | 0 (0) | – | – |
| Hot Meals on Wheels | 0.005 (0.005) | 0 (0) | –0.014 to 0 | 0.318 |
| Laundry services | 0 (0) | 0 (0) | – | – |
| Calls to NHS 111 | 0 (0) | 0 (0) | – | – |
| PSS | ||||
| Social worker, mean duration in minutesa (SE) | 0 (0) | 0 (0) | – | – |
| Care worker/help at home, mean duration in minutesa (SE) | 53.958 (40.927) | 31.650 (28.113) | –129.008 to 70.087 | 0.653 |
| Time off work, mean number of days (SE) | 47.704 (4.824) | 48.644 (5.199) | –12.908 to 14.430 | 0.895 |
| Additional costs, proportion of participantsb | 0.241 (–) | 0.243 (–) | – | 0.971 |
| 3 months to 6 months | ||||
| Subsequent inpatient care, mean number of days (SE) | 0 (0) | 0 (0) | – | – |
| Outpatient care, mean number of visits (SE) | ||||
| Orthopaedics/fracture | 0.187 (0.058) | 0.180 (0.043) | –0.155 to 0.129 | 0.916 |
| Physiotherapist: NHS | 0.034 (0.019) | 0.049 (0.021) | –0.044 to 0.073 | 0.624 |
| Physiotherapist: private | 0.153 (0.029) | 0.112 (0.027) | –0.119 to 0.037 | 0.299 |
| Pathology: blood tests | 1.128 (0.172) | 1.277 (0.204) | –0.363 to 0.684 | 0.578 |
| Radiology | 0.182 (0.063) | 0.214 (0.111) | –0.190 to 0.313 | 0.806 |
| Emergency department: wrist | 0.020 (0.016) | 0.005 (0.005) | –0.049 to 0.010 | 0.363 |
| Emergency department: others | 0.025 (0.013) | 0.034 (0.014) | –0.030 to 0.048 | 0.630 |
| Others | 0.059 (0.026) | 0.029 (0.014) | –0.094 to 0.019 | 0.302 |
| Community care, mean duration in minutesa (SE) | ||||
| General practitioner: surgery visit | 1.154 (0.362) | 2.488 (0.626) | –0.029 to 2.825 | 0.066 |
| General practitioner: home visit | 0 (0) | 0 (0) | – | – |
| General practitioner: telephone contact | 0.040 (0.031) | 0.340 (0.196) | –0.025 to 0.736 | 0.133 |
| Practice nurse: surgery visit | 0.025 (0.025) | 0 (0) | –0.074 to 0 | 0.319 |
| Practice nurse: home visit | 0 (0) | 0 (0) | – | – |
| Practice nurse: telephone contact | 0.020 (0.020) | 0 (0) | –0.059 to 0 | 0.319 |
| District nurse: surgery visit | 0.000 (0.000) | 0.388 (0.388) | 0.000 to 1.165 | 0.318 |
| District nurse: home visit | 0 (0) | 0 (0) | – | – |
| Physiotherapist: surgery visit | 8.375 (2.765) | 7.146 (2.460) | –8.677 to 5.873 | 0.740 |
| Physiotherapist: home visit | 2.970 (2.970) | 1.165 (0.899) | –8.717 to 2.621 | 0.561 |
| Occupational therapist: surgery visit | 3.582 (3.582) | 0 (0) | –10.746 to 0 | 0.319 |
| Occupational therapist: home visit | 0 (0) | 0 (0) | – | – |
| Others | 0.074 (0.074) | 0 (0) | –0.223 to 0 | 0.319 |
| Medications, proportion of participantsb | ||||
| Analgesic | 0.123 (–) | 0.146 (–) | – | 0.964 |
| Antibiotic | 0 (–) | 0 (–) | – | – |
| Bisphosphonate | 0.005 (–) | 0 (–) | – | 0.271 |
| Other bone protection medication | 0.015 (–) | 0.015 (–) | – | 0.820 |
| Supplement and vitamin | 0.034 (–) | 0.029 (–) | – | 0.506 |
| Aids and adaptations, mean number (SE) | ||||
| Wrist support: (e.g. brace/splint) | 0.104 (0.034) | 0.117 (0.028) | –0.076 to 0.097 | 0.766 |
| Grab rail | 0.020 (0.012) | 0.005 (0.005) | –0.045 to 0.005 | 0.253 |
| Dressing aids | 0.030 (0.030) | 0.020 (0.012) | –0.084 to 0.039 | 0.750 |
| Long-handled aids (e.g. shoe horn, reacher) | 0 (0) | 0.015 (0.008) | 0 to 0.034 | 0.083 |
| Bathing aids | 0.010 (0.010) | 0.029 (0.015) | –0.015 to 0.058 | 0.289 |
| Kitchen aids (e.g. jar/tin openers) | 0.015 (0.011) | 0.034 (0.014) | –0.015 to 0.054 | 0.293 |
| Others | 0.030 (0.014) | 0.010 (0.007) | –0.055 to 0.010 | 0.196 |
| PSS, mean number (SE) | ||||
| Frozen Meals on Wheels | 0 (0) | 0 (0) | – | – |
| Hot Meals on Wheels | 0 (0) | 0 (0) | – | – |
| Laundry services | 0.005 (0.005) | 0 (0) | –0.015 to 0 | 0.319 |
| Calls to NHS 111 | 0 (0) | 0 (0) | – | – |
| PSS | ||||
| Social worker, mean duration in minutesa (SE) | 0 (0) | 0 (0) | – | – |
| Care worker/help at home, mean duration in minutesa (SE) | 0 (0) | 0 (0) | – | – |
| Time off work, mean number of days (SE) | 21.864 (7.242) | 26.652 (6.916) | –14.629 to 23.288 | 0.635 |
| Additional costs, proportion of participantsb | 0.064 (–) | 0.092 (–) | – | 0.288 |
| 6 months to 12 months | ||||
| Subsequent inpatient care, mean number of days (SE) | 0 (0) | 0 (0) | – | – |
| Outpatient care, mean number of visits (SE) | ||||
| Orthopaedics/fracture | 0.176 (0.047) | 0.163 (0.044) | –0.139 to 0.119 | 0.845 |
| Physiotherapist: NHS | 0.060 (0.023) | 0.097 (0.029) | –0.035 to 0.113 | 0.329 |
| Physiotherapist: private | 0.116 (0.035) | 0.122 (0.034) | –0.089 to 0.103 | 0.889 |
| Pathology: blood tests | 0.437 (0.132) | 0.474 (0.146) | –0.332 to 0.427 | 0.850 |
| Radiology | 0.176 (0.070) | 0.071 (0.039) | –0.271 to 0.041 | 0.192 |
| Emergency department: wrist | 0.055 (0.036) | 0.010 (0.007) | –0.126 to 0.015 | 0.227 |
| Emergency department: others | 0.055 (0.037) | 0.036 (0.013) | –0.110 to 0.041 | 0.621 |
| Others | 0 (0) | 0.026 (0.014) | 0.005 to 0.057 | 0.059 |
| Community care, mean duration in minutesa (SE) | ||||
| General practitioner: surgery visit | 1.396 (0.676) | 1.042 (0.443) | –2.116 to 1.112 | 0.661 |
| General practitioner: home visit | 0.503 (0.362) | 0.000 (0.000) | –1.307 to 0.000 | 0.166 |
| General practitioner: telephone contact | 0 (0) | 0 (0) | – | – |
| Practice nurse: surgery visit | 0 (0) | 4.639 (4.639) | 0 to 13.918 | 0.319 |
| Practice nurse: home visit | 0 (0) | 0 (0) | – | – |
| Practice nurse: telephone contact | 0.025 (0.025) | 0 (0) | –0.075 to 0 | 0.319 |
| District nurse: surgery visit | 0 (0) | 0 (0) | – | – |
| District nurse: home visit | 0 (0) | 0 (0) | – | – |
| Physiotherapist: surgery visit | 4.924 (2.353) | 9.359 (5.515) | –5.311 to 17.652 | 0.460 |
| Physiotherapist: home visit | 0 (0) | 0 (0) | – | – |
| Occupational therapist: surgery visit | 0.303 (0.303) | 0 (0) | –0.909 to 0 | 0.319 |
| Occupational therapist: home visit | 0 (0) | 0.256 (0.184) | 0 to 0.667 | 0.166 |
| Others | 0 (0) | 0 (0) | – | – |
| Medications, proportion of participantsb | ||||
| Analgesic | 0.080 (–) | 0.082 (–) | – | 0.768 |
| Antibiotic | 0 (–) | 0 (–) | – | – |
| Bisphosphonate | 0.005 (–) | 0.000 (–) | – | 0.323 |
| Other bone protection medication | 0.020 (–) | 0.015 (–) | – | 0.731 |
| Supplement and vitamin | 0.025 (–) | 0.015 (–) | – | 0.477 |
| Aids and adaptations, mean number (SE) | ||||
| Wrist support (e.g. brace/splint) | 0.101 (0.033) | 0.092 (0.027) | –0.094 to 0.073 | 0.848 |
| Grab rail | 0.015 (0.011) | 0.010 (0.010) | –0.035 to 0.026 | 0.751 |
| Dressing aids | 0.010 (0.010) | 0.005 (0.005) | –0.030 to 0.015 | 0.665 |
| Long-handled aids (e.g. shoe horn, reacher) | 0.015 (0.011) | 0.021 (0.010) | –0.025 to 0.031 | 0.720 |
| Bathing aids | 0.015 (0.011) | 0.010 (0.007) | –0.035 to 0.021 | 0.718 |
| Kitchen aids (e.g. jar/tin openers) | 0.030 (0.014) | 0.056 (0.028) | –0.030 to 0.093 | 0.407 |
| Others | 0.015 (0.015) | 0 (0) | –0.045 to 0 | 0.319 |
| PSS, mean number (SE) | ||||
| Frozen Meals on Wheels | 0.000 (0.000) | 0.005 (0.005) | 0.000 to 0.015 | 0.319 |
| Hot Meals on Wheels | 0 (0) | 0 (0) | – | – |
| Laundry services | 0 (0) | 0 (0) | – | – |
| Calls to NHS 111 | 0 (0) | 0 (0) | – | – |
| PSS | ||||
| Social worker, mean duration in minutesa (SE) | 0 (0) | 0 (0) | – | – |
| Care worker/help at home, mean duration in minutesa (SE) | 0 (0) | 0 (0) | – | – |
| Time off work, mean number of days (SE) | 45.385 (20.833) | 63.636 (21.170) | –38.196 to 73.924 | 0.545 |
| Additional costs, proportion of participantsb | 0.030 – | 0.046 – | – | 0.418 |
Appendix 3 Changes to protocol
All protocol versions can be found on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/152701/#/documentation (accessed 8 September 2020). Table 24 shows the summary of changes implemented with each protocol version.
| Version and date | Summary of changes |
|---|---|
| 1.0: 9 August 2016 | None. This was the first version approved by IRAS and given to recruiting centres |
| 2.0: 25 June 2019 | Inclusion of e-mail/SMS messages, extension of the trial duration and update on committee members |
| 3.0: 27 September 2019 | Inclusion of intention to send patient incentive gift vouchers |
List of abbreviations
- AUC
- area under the curve
- BNF
- British National Formulary
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRPS
- complex regional pain syndrome
- DRAFFT
- Distal Radius Acute Fracture Fixation Trial
- DRAFFT 2
- Distal Radius Acute Fracture Fixation Trial 2
- DSMC
- Data Safety and Monitoring Committee
- DVT
- deep-vein thrombosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-VAS
- EuroQol visual analogue scale
- GBP
- Great British pounds
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- K-wire
- Kirschner wire
- MAR
- missing at random
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PE
- pulmonary embolism
- PP
- per protocol
- PRWE
- Patient-Rated Wrist Evaluation
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SHEAP
- statistical health economics analysis plan
- TMG
- Trial Management Group
- VAS
- visual analogue scale
