Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/106/01. The contractual start date was in September 2013. The draft report began editorial review in November 2020 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Constable et al. This work was produced by Constable et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Constable et al.
Chapter 1 Introduction
Parts of this chapter are reproduced from Constable et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Male synthetic sling versus Artificial urinary Sphincter Trial: Evaluation by Randomised controlled trial
In 2013, the UK Government’s National Institute for Health and Care Research Health Technology Assessment programme funded MASTER (the Male synthetic sling versus Artificial urinary Sphincter Trial: Evaluation by Randomised controlled trial); this report describes the research.
MASTER was a multicentre, non-inferiority randomised controlled trial (RCT), with a non-randomised cohort (NRC) that investigated the clinical effectiveness (including safety) and cost-effectiveness of male synthetic sling surgery compared with that of the established artificial urinary sphincter (AUS) surgery in men with urodynamic stress incontinence (USI) after prostate surgery (for cancer or benign disease). MASTER also included an embedded qualitative component to explore the participant-perceived importance of outcomes, as well as exploration of participant and surgeon perspectives.
Background
Stress urinary incontinence (SUI) is common in men after prostate surgery [radical prostatectomy for cancer or transurethral resection of prostate (TURP) for benign disease] and can be difficult to improve. Urinary incontinence (UI) has a major impact on quality of life resulting from a profound loss of self-esteem and restrictions on work, social interaction and personal relationships, including sex life.
In two parallel RCTs,2,3 75% of men who were incontinent 6 weeks after prostate surgery still had some leakage 12 months after surgery, despite receiving physiotherapy. Pelvic floor muscle treatment and drug treatments can be suboptimal, with some men continuing to cope only with the aid of containment products. Other treatments, such as injectables and inflatable balloons, have been reviewed, but there is insufficient evidence to support their use. 1,4
For some men, surgery for persistent bothersome SUI remains the only option for active management. AUS is the recommended surgical procedure for men still affected by troublesome SUI > 12 months after prostate surgery. 5,6 Despite this, a large proportion of men (around 8% after radical prostatectomy and 2% after TURP) continue to have severe disabling incontinence following AUS implantation, which greatly impacts their quality of life, and many of these men (27% and 6%, respectively) remain reliant on containment measures, such as pads [unpublished data from 4–6-years’ follow-up of Men After Prostate Surgery (MAPS) trial responders (Charis Glazener, University of Aberdeen, 2019, personal communication)].
More recently, the male synthetic sling (male sling), which treats SUI by elevating the urethra, has been developed. In the last decade, a variety of slings have been offered to a minority of men seeking surgical treatment for post prostatectomy incontinence (PPI) from the NHS, dependent on surgeon enthusiasm and local arrangements. Male slings, of many varieties, have been reported in case series over the last 10 years and have been increasingly used for the treatment of PPI; however, thorough evaluation and high-quality RCT evidence are lacking. 5–7 National Institute for Health and Care Excellence (NICE) guidance5 also states that the male sling should be used only as part of a well-developed RCT.
Additional background information is given in the previously published protocol. 1
Scale of the problem
The number of men undergoing radical prostatectomy for localised prostate cancer is increasing [it increased from 5600 in 2011 (when MASTER was being developed), to 7704 in 2017 and 9413 in 2018]. 8,9 This trend is likely to continue as the population ages and as localised prostate cancer case finding using prostate-specific antigen testing increases, potentially leading to more men subsequently requiring surgery for prostate cancer treatment-related UI. As an indication, if 50 more people required an AUS each year, this would cost the NHS an additional £450,000 per annum.
Benign enlarged prostate occurs in around 30% (1.8 million) of men in the UK aged > 60 years and is a major cause of lower urinary tract symptoms (LUTSs), with around 25,000 TURP procedures carried out annually in England alone. 4,10
The prevalence of SUI in men after radical prostatectomy is unclear, but is estimated to be between 5% and 57%, depending on definition, timing of assessment after surgery and population characteristics. The prevalence of SUI in men after TURP is clearer, and ranges from 0% to 8%. 6
The rate of recovery of continence plateaus at around 12 months after surgery. The MAPS trial found that 40% of men with incontinence 6 weeks after radical prostatectomy continued to have persistent incontinence at 12 months, and this incontinence was severe in 20% of men (requiring the use of pads or other containment measure). Levels of continence did not improve in the next 12 months, up to 24 months after surgery. 2,3
Rationale for MASTER
An AUS is invasive, and voiding involves manual manipulation of a pump located in the scrotum, which requires a sufficient degree of manual dexterity. In addition, the device is relatively expensive, its implantation requires specialist surgical skill and, in time, revision surgery may be necessary. For all these reasons, other surgical methods have been tested, but a Cochrane review in 20144 found no adequately powered RCTs to guide practice, and there have been no new RCTs since. 5,11
Although treatment with the male sling is thought to be less invasive, is more acceptable to some men and appears to be less expensive, harms, further treatment, revision surgery and longer-term effects need to be considered to determine full comparative effectiveness.
To the best of our knowledge, there are no published high-quality RCTs comparing the male sling with the AUS. A Cochrane systematic review4 concluded that there was not enough evidence to guide practice for men or surgeons considering surgery for USI after prostate surgery. The authors of the review found only one small, poor-quality RCT that compared implantation of an AUS and treatment with injectable bulking agent. 11 In this RCT, the men treated with AUS were more likely to be cured (18/20, 82%) than those who had the injectable treatment (11/23, 46%) [odds ratio (OR) 5.67, 95% confidence interval (CI) 1.28 to 25.10]. The review highlighted the need for adequately powered comparative RCTs to guide practice. 4
Current NICE guidance (CG97), last updated in 2015 (accessed August 2019), remains unchanged, recommending AUS for persistent SUI with further guidance and evaluation needed on male slings. 5 Current NHS guidance also states that the male synthetic sling should be used only in RCTs against the AUS. AUS continues to be the preferred intervention, but there is increasing opinion that the male sling is an acceptable alternative for men with mild to moderate PPI. 6,7
Both clinicians and patients lack the evidence required to make an informed choice between the AUS and the male sling, and NHS policy-makers lack information on cost-effectiveness to plan service provision. Owing to these uncertainties, MASTER was developed to compare the male sling with the AUS in men who have SUI after prostate surgery (for cancer or benign disease).
MASTER will provide the required robust evidence to allow informed treatment and health-care provision decisions for patients, clinicians and health-care policy-makers, in terms of the clinical effectiveness, cost-effectiveness and adverse events associated with the male sling and AUS.
Surgical procedures
When MASTER was designed, and to date, the design and function of the AUS appeared suitable, and, despite attempts to improve on the existing device, there are still no signs of significant innovations that would have needed to be considered prior to or during this trial. However, sling technology was, and is, less mature; we anticipated that during the trial recruitment period there would be a greater choice of implants from different manufacturers. For that reason, we did not specify which brand of male sling should be used. However, at the time of trial design, almost all slings being implanted were passive, that is non-adjustable, in type. Although there seem to have been no significant developments in the passive slings used in MASTER, there are developments in adjustable slings, although the European Association of Urology (EAU) guidelines 202063 reiterate that there are no data to suggest an advantage of the adjustable over the simpler passive slings. There was some information on adjustable slings, but there was inadequate evidence of efficacy and some evidence that the adjustable slings required more frequent additional surgery and were associated with more complications than passive slings. Hence, we stipulated that the male slings should be of the non-adjustable, suburethral, transobturator type only.
Male sling
The male sling (i.e. the non-absorbable polypropylene mesh implant) is placed under the urethra to elevate it and is held in place by passing it through the obturator foramen of the pelvis bilaterally. It has a passive mode of action. The aim of the sling is to stop the loss of urine on exertion and the operation is effective immediately.
Artificial urinary sphincter
The AUS consists of an inflatable cuff that is placed around the urethra, a pressure-regulating balloon that keeps the cuff inflated and a pump that is placed in the scrotum, which the man squeezes to enable voiding. The aim is to close the urethra so that the patient is dry, except when he wants to void. Once implanted, the device is deactivated in the open position for 4–6 weeks to allow postoperative swelling to subside. The device is then activated in clinic to ensure that the man can use the device correctly.
Questions addressed by MASTER
The aim of this study was to determine whether or not the male sling is non-inferior to implantation of the AUS for men with SUI after prostate surgery (for cancer or benign disease), judged primarily on clinical effectiveness, but also considering the relative harms and cost-effectiveness. To determine whether the male sling or AUS is cost-effective for the NHS in the UK, the interventions were compared in terms of continence in men after prostate surgery; the relative harms of the interventions; the costs to the patients and to the NHS, including the need for repeat surgery in both groups; and overall patient satisfaction.
Principal objectives
The primary objective was to compare (1) participant-reported continence 12 months after randomisation [a composite outcome derived from responses indicating any loss of urine to either of the two questions ‘How often do you leak urine?’ and ‘How much urine do you leak?’ from the validated International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF)] and (2) the corresponding cost-effectiveness of a policy of primary implantation of the male sling compared with AUS, measured as the incremental cost per quality-adjusted life-year (QALY), 24 months after randomisation.
Secondary objectives
Secondary objectives compared (1) adverse events (AEs); (2) the costs of the benefits and harms; (3) the need for further treatments; (4) the differential effects on other outcomes, such as quality of life and general health; and (5) participant satisfaction with the male sling and the AUS surgeries up to 24 months after randomisation.
Qualitative component
MASTER included a comprehensive embedded qualitative component to establish the patient-perceived importance of outcomes and to explore participant and surgeon experiences of surgery and acceptable inferiority margins, and to determine reasons for failure that resulted in crossover to alternative surgery.
Chapter 2 Methods and practical arrangements
Parts of this chapter are reproduced from Constable et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
MASTER was a multicentre, non-inferiority RCT that included a NRC and compared the clinical effectiveness and cost-effectiveness of male synthetic sling and AUS surgeries in men with PPI.
Participating centres were asked to consent and randomise (RCT) -or to consent (NRC) participants as close to surgery as was practical to minimise participant drop-out. This resulted in some delays from the date of consent to the date of randomisation/date of surgery. A significant health economic component was also included as part of MASTER to establish costs, cost-effectiveness and long-term modelling. The economic methods and evaluation are described in full in Chapter 5.
An embedded qualitative component was also included as part of MASTER to establish the importance of the main outcomes to patients, explore patient and surgeons’ experiences, and explore reasons for reoperation. The qualitative component methods and findings are discussed in full in Chapter 6.
Further details of the study design, methodology and management [eligibility, consent, treatment allocation, sample size calculation and serious adverse event (SAE) reporting] described herein are also in the published protocol1 and are represented in Figure 1.
FIGURE 1.
MASTER flow diagram. a, Men in the NRC are followed up by questionnaire and electronic follow-up but not clinical review at 1 year. This figure is reproduced from Constable et al. 1 This article is distributed under the terms of Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided appropriate credit is given to the original authors(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies, unless otherwise stated. The figure contains minor additions and formatting changes to the original figure.
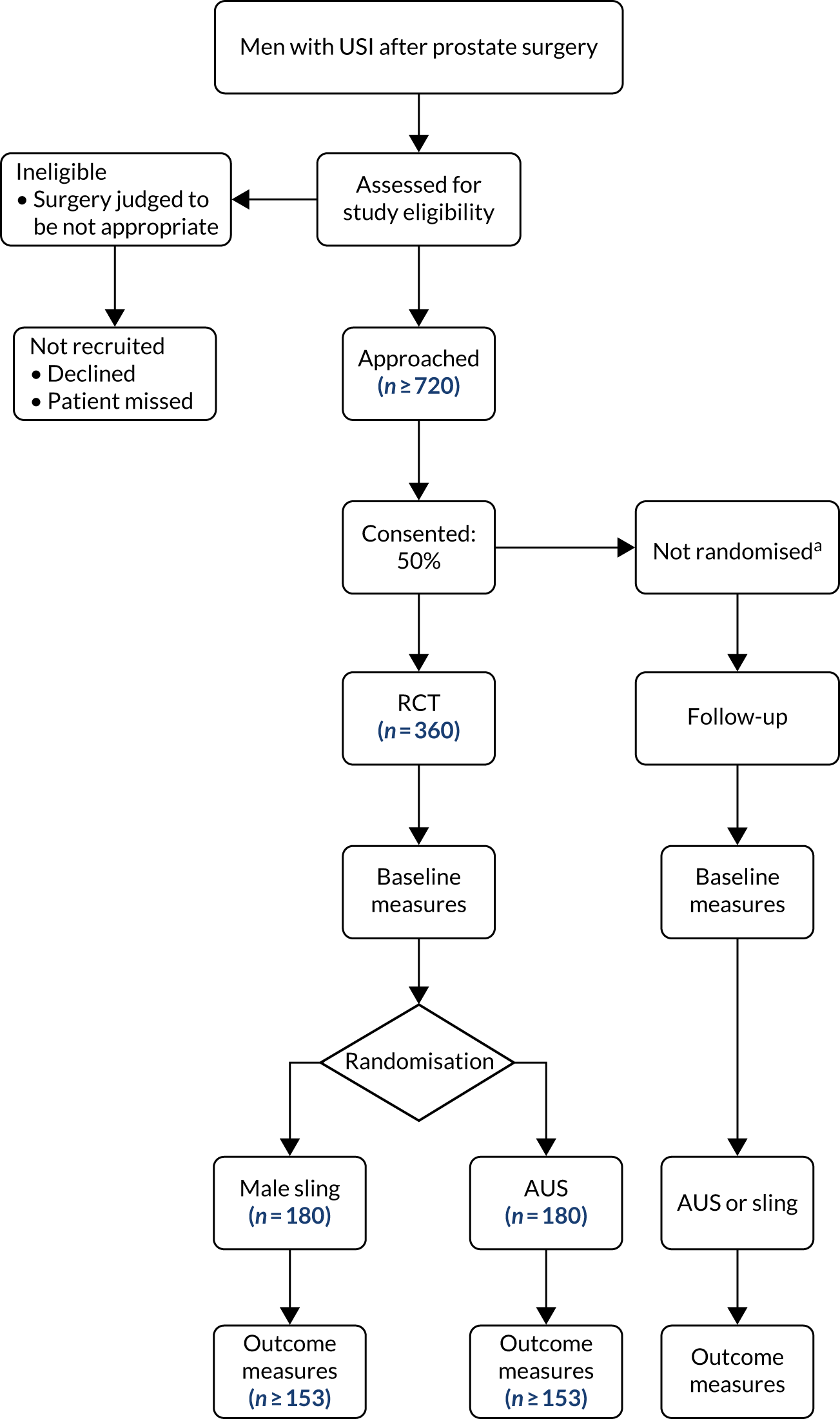
Study population
Men with USI after prostate surgery (radical prostatectomy or TURP) whose incontinence was considered to require surgery were eligible for MASTER.
Men were excluded if they had previously undergone sling or AUS surgery, had unresolved bladder neck contracture or urethral stricture after their prostate surgery, had insufficient manual dexterity to operate the AUS, or were unable to give informed consent or complete trial documentation. In addition, after the closure of the NRC, men who did not want to be randomised were excluded.
Treatment allocation
Eligible men consenting to the RCT were randomised to receive either male sling or AUS surgery in a 1 : 1 allocation ratio, using the randomisation application at the trial office at the Centre for Healthcare Randomised Trials (CHaRT). The randomisation application was available as both an interactive voice-response telephone system and an internet-based application, using a minimisation algorithm based on type of previous prostate surgery (radical prostatectomy or TURP) and radiotherapy or not, in addition to the patients’ prostate surgery and centre.
Men eligible and consenting to the NRC chose, in discussion with their surgeon, to receive either male sling or AUS surgery.
Data collection during follow-up
Participant-reported outcomes were assessed using self-completed questionnaires at baseline (before surgery), 6 months after surgery, and 12 and 24 months after randomisation. This meant that no 6-month questionnaires were issued if a participant did not have surgery, but the 12- and 24-month questionnaires were issued to all. A self-completed 3-day bladder diary was also collected at these time points. Up to two reminders were sent to participants by post or telephone.
Men in the RCT were also reviewed in clinic 12 months after surgery, which included a 24-hour pad leakage test.
Study outcome measures
The clinical primary outcome of MASTER was participant self-report of continence following implantation of the male sling compared with AUS 12 months after randomisation.
The economic primary outcome was the cost-effectiveness of the male sling compared with that of AUS measured as the incremental cost per QALY 24 months after randomisation (methods described in Chapter 5).
The primary outcome measure was the man’s report of continence 12 months after randomisation, derived from the responses to questions 3 and 4 in the ICIQ-UI SF questionnaire. 12 The ICIQ-UI SF has been used in research and clinical practice across the world. It is a short and simple questionnaire that gives a comprehensive summary of the symptoms of incontinence and has been used to facilitate patient–clinician discussions.
Question 3 asked about the frequency of urinary leakage and question 4 asked about the volume of urinary leakage. Question 3 is scored from 0 to 5 (0 = never, 5 = all the time) and question 4 is scored 0, 2, 4 or 6 (0 = none, 6 = a large amount). To be classed as continent, men had to answer ‘never’ to question 3 and ‘none’ to question 4. A response to only one of the questions of either ‘never’ or ‘none’ was also taken to indicate continence. Men who provided any other pair of responses were classed as incontinent. The Data Monitoring Committee (DMC) recommended (on 17 October 2017) that we also report a less strict definition of absolute continence to include ‘once a week or less often’ and ‘a small amount’ as being compatible with being ‘adequately dry’ owing to a lower than anticipated success rate of both interventions.
Secondary outcomes
The secondary outcomes were used to further assess continence and its impact on men’s quality of life. These were included as part of the postal participant questionnaires issued at baseline, 6 months after surgery, and 12 and 24 months after randomisation. The secondary outcomes were:
-
ICIQ-UI SF score. The ICIQ-UI SF score comprises the two questions from which the primary outcome was derived and a question on the overall impact of UI on everyday life. This question is scored from 0 to 10 (0 = not at all, 10 = a great deal) and the other two questions are scored from 0 to 5 and 0 to 6. The score was obtained by summing these three questions to give a score out of 21, where a higher score indicates more severe incontinence.
-
International Consultation on Incontinence Questionnaire-Urinary Incontinence – Male Lower Urinary Tract Symptoms (ICIQ-UI MLUTS) voiding and incontinence scores. 13 The ICIQ-MLUTS module has 11 questions that are used to produce the scores, all of which are answered on a scale of 0 to 4 (0 = never, 4 = all of the time). The scores were obtained by summing the relevant questions. The voiding score is based on the answers to five questions and ranges from 0 to 20. The incontinence score is based on the answers to six questions and ranges from 0 to 24. In both cases, a higher score indicates a poorer outcome. The ICIQ-MLUTS has a comprehensive approach to assessing all LUTS. It was developed by patients for patients. It has been tested and proven reliable in numerous research and clinical settings, and is easy to understand and complete.
-
International Consultation on Incontinence Questionnaire-Male Sexual Matters Associated with Lower Urinary Tract Symptoms (ICIQ-MLUTSsex) module. 14 The ICIQ-MLUTSsex uses four questions to evaluate male sexual matters associated with LUTS. All four questions are scored from 0 to 3, with 0 indicating no problem and 3 indicating the highest level of problem. The ICIQ-MLUTSsex is obtained by summing the responses to the four questions. It provides a robust measure of the impact of sexual matters.
-
Short Form questionnaire-12 items (SF-12), version 2. 15 The SF-12 version 2 is a multipurpose short-form generic measure of health status. It measures eight health domains and provides physical component and mental component scores. The survey has 12 questions, with one or two questions for each health domain. The questions have between three and five possible responses. The total score for each component is obtained by summing the questions that relate to the component. Higher scores indicate better outcomes and, to achieve this, reverse scoring of questions 1, 8, 9 and 10 was implemented. The SF-12 version 2 is practical, reliable and valid and has been widely used in monitoring population health, comparing and analysing condition burden and predicting medical expenses.
-
EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 16 The EQ-5D-3L is a standardised generic instrument for use as a self-completed measurement of health. It provides a simple descriptive profile and a single value that can be used in the clinical and economic evaluation of health care, and population health surveys. It has five items on mobility, self-care, pain, usual activities and psychological status, with three possible answers for each item (1 = no problem, 2 = moderate problem and 3 = severe problem). The responses for each item/domain are converted to quality-of-life estimates using an algorithm to produce EQ-5D-3L index scores for each participant. The five scores are summed to produce a variable on the scale –0.594 to 1, where negative scores represent health states worse than death, 0 represents the state of worst health and 1 represents full health.
-
Continence at 6 and 24 months. This variable was formed in the same way as the primary outcome but uses the data at 6 and 24 months, respectively.
-
Pad weight at 12 months. Participants were asked to wear a pad for 24 hours, which was then weighed to determine the urine leakage (weight of urine leaked was determined by subtracting the weight of the dry pad from the pad weight after 24 hours). Where the participant told us that they were dry and did not need to wear pads, this was accepted and they were not required to do the pad test at 12 months.
-
Urine leakage at 12 months after randomisation compared with that before surgery. Participants were asked how their urine leakage compared with that before their MASTER surgery. Participants answered on the seven-point Patient Global Impression of Improvement (PGI-I) scale: very much better, much better, a little better, no change, a little worse, much worse or very much worse.
-
Satisfaction with surgery results at 12 months. Participants were given five possible responses to choose from: completely satisfied, fairly satisfied, fairly dissatisfied, very dissatisfied or not sure.
-
Whether or not the participant would recommend the surgery at 12 months. Participants answered ‘yes’ or ‘no’ to the question of whether or not they would recommend their surgery to a friend.
-
Time until participant was able to return to work, assessed at 12 months. Participants were asked how many months it took until they were able to return to their normal daily activities.
-
Other secondary outcomes included surgical complications and AEs, operating time, length of hospital stay, number of re-admissions to hospital and time to receiving further surgery.
Choice of validated outcome measures
Outcome measures were chosen to reflect current international standards of reporting to ensure that findings would be relevant to patients, clinicians and policy-makers. Outcomes were measured at baseline to provide values for later statistical adjustments. The primary measure of continence was the man’s subjective report derived from two questions on the ICIQ-UI SF questionnaire, developed and validated in a variety of populations for both research and clinical practice.
Blinding
Baseline data were reported by men before randomisation using self-completed questionnaires. Blinding in theatre was not possible, and participants could not be blinded to surgery received owing to the nature of the devices. Outcome assessment was largely by participant-completed questionnaires. Outcome assessors for the 12-month clinic appointment were blinded to randomisation where possible and were asked to record if they knew which operation was received prior to undertaking the outcome assessment.
Important changes to the methods after trial commencement
Closure of the non-randomised cohort
Recruitment to the NRC closed in October 2015, after 100 participants had consented, because it was adversely affecting recruitment to the RCT. 1 Those men already consented to the NRC remained in the study and were followed up as per protocol.
Extension to recruitment
Slower than anticipated recruitment meant that a 9-month recruitment extension was required to achieve the full sample size.
Safety reporting
Adverse events were either notified to the study office by the local research team or reported by the men in their follow-up questionnaires. If a SAE was suspected, it was verified by the local research team, where possible. Unrelated AEs were not recorded. The published protocol1 provides more details on relatedness, potentially expected AEs and reporting.
Sample size
Evidence suggested that 20% of men would still be incontinent 12 months after receiving an AUS, whereas 35% of men would still be incontinent after receiving a male sling. 1 The sample size calculation was undertaken by simulation. Assuming that there was no difference between the arms of the trial, 310 participants would give 90% power to show that male slings were non-inferior to AUS by a margin of ≤ 15%. To allow for 15% loss to follow-up, the sample size was increased to 360.
Statistical analysis of outcomes
Predefined statistical analyses were included in the published protocol. 1 Statistical analyses were based on all randomised men, regardless of whether or not they complied with their randomised surgery. The comparisons were between those who were randomised to receive a male sling and those who were randomised to receive an AUS.
The primary outcome was analysed using a generalised linear model, clustering by centre and with adjustment for previous radiotherapy and the 24-hour pad test weight at baseline as fixed effects. Statistical significance was at 5%, with a corresponding CI, equivalent to a one-sided test for non-inferiority at 2.5%. An intention-to-treat (ITT) analysis was performed with all of the participants remaining in their randomised group. ORs are presented for the primary outcome and the less strict definition to aid interpretation.
A subgroup analysis of the primary outcome on baseline urine leakage (≤ 250 g vs. > 250 g) was also performed. It was also planned to perform a subgroup analysis on previous prostate surgery (radical prostatectomy vs. TURP); however, given that 93% of randomised participants underwent radical prostatectomy, the groups were too unevenly balanced to make this worthwhile. It is also for this reason that the analysis does not adjust for type of prostate surgery. The subgroup analysis was at the stricter, two-sided, 1% level.
Secondary outcomes with multiple categories (e.g. satisfaction categories) were analysed using ordered logistic regression clustered by centre and with adjustment for previous radiotherapy and baseline pad weight. Secondary outcomes that are continuous outcomes and measured repeatedly (e.g. EQ-5D-3L, ICIQ-UI SF, ICIQ-MLUTS incontinence and voiding scores) were analysed using a mixed-effects repeated-measures model, with random effects for centre and participant and fixed effects for treatment, time point and the respective outcome at baseline, as well as for the baseline pad test weight and previous radiotherapy.
The primary outcome was tested in a non-inferiority framework with a non-inferiority margin of 15%, thought to be an acceptable lower effectiveness, in return for reduced AEs and easier operation of the sling. The null hypothesis was that the male sling was inferior to the AUS by at least 15% and a p-value of < 0.025 would indicate that the null hypothesis could be rejected, suggesting the male sling was non-inferior to the sphincter.
Secondary outcomes that measure quality of life, such as EQ-5D-3L and SF-12 version 2, were tested under a superiority framework. Continence-specific secondary outcomes, such as ICIQ-UI SF and ICIQ-MLUTS incontinence, voiding and sexual functioning scores, were also tested under a superiority framework.
Non-responder analysis
Descriptive data comparing the baseline characteristics of participants who did and did not respond at 12 months are displayed and the t-test (continuous outcomes) and chi-squared test (categorical outcomes) were used to estimate the statistical significance of the differences between responders and non-responders.
Missing data
Where an outcome was to be analysed using a model that adjusted for the baseline outcome, any individuals for whom this was missing at baseline had their outcome imputed by the centre mean or, in the case of the baseline 24-hour pad test, the overall mean.
Missing outcome data were imputed using a multiple imputation model (using chained equations) with randomised intervention, number of times leaked, quantity leaked, previous radiotherapy, previous prostate surgery and age used as covariates. Pattern-mixture models made the following assumptions: all missing data being continent; all missing being incontinent; all missing slings were continent and all missing AUSs were incontinent; and, finally, all missing slings were incontinent and all missing AUSs were continent.
Patient and public involvement
Pre-funding application and design of the research
Prior to the initial funding application, we had the benefit of a patient advisor and an expert panel of service users who were extensively involved in all stages of the initial application, including the outline stage, to ensure that the trial addressed matters of most concern to men with incontinence. Members of this panel were interviewed on their leakage problems, expectations from surgical treatment and willingness to participate in the planned research. We also benefited from their review and approval of the proposed trial documentation [including the draft application, proposed questionnaires and instructions for the 24-hour urine leakage (pad) test]. Feedback from the expert panel helped to refine the study to understand what outcomes were important.
We also had a patient advisor grant holder who advised throughout. His thoughts were:
It is helpful to the trial for the consumer rep[resentative] to have some experience of the problems being investigated. Thank you for asking me to join the PMG [Project Management Group]. As someone with the problem of leakage and an AUS fitted for many years, I was able to help with the wording and arrangements of the questionnaires given to potential participants. Being a member of the PMG I was able from time to time to ask for clarification of the wording of some of the professional statements. Receiving the paperwork and documents of the TSC [Trial Steering Committee] is very important to know how the trial is proceeding and the problems occurring. It is important to know that the trial progress and problems are circulated to other parties to gain information or avoid duplication. It has been nice to observe and know that all professionals involved in this trial have ‘gone about’ the investigations in a keen and interested manner. From my eyes the trial has been worthwhile and has found other unknown problems.
Embedded qualitative component
MASTER incorporated a significant qualitative component, which was embedded at each stage of the study, to include patient and public involvement in the following:
-
establishment of patient-perceived importance of different outcomes
-
exploration of patients’ perspectives on acceptable inferiority margins
-
patients’ experiences of procedures
-
reasons for failure resulting in crossover to alternative surgery
-
surgeons’ experiences of procedures.
The full methods, findings and evaluation of the qualitative component are reported in Chapter 6.
Oversight of the study
One of the independent members of the TSC was a patient representative. The TSC met throughout the study and reviewed all study documentation, including participant-facing documents and questionnaires that were sent to participants.
Report writing, academic paper preparation and dissemination
The patient and public involvement partner on the TSC has been actively involved in discussions of the trial results with the TSC and has been supportive of the study in report preparation.
Chapter 3 Description of the study population, baseline characteristics and treatment received
This chapter describes the baseline characteristics of and treatment received by the MASTER study population.
Throughout this chapter, the RCT data are separated from those of the NRC. Data tables for the RCT are presented in this chapter and Appendix 1, and the NRC data are presented in Appendix 2.
Recruitment to the study
Participants were recruited from 28 centres in the UK (Table 1). A total of 27 centres were recruited to the RCT, whereas one centre was recruited to the NRC only. Thirteen centres were recruited to both the RCT and the NRC. In total, 480 participants were recruited to MASTER: 190 consented and were randomised to receive a male sling, 190 consented and were randomised to receive an AUS, and 100 consented to the NRC. Participants were recruited to MASTER from 1 January 2014 to 31 December 2017 (Figure 2).
| Centre | Number (%) of participants | |||
|---|---|---|---|---|
| RCT | NRC | Total | ||
| Male sling group | AUS group | |||
| Southmead Hospital, Bristol | 24 (12.6) | 25 (13.2) | 11 (11.0) | 60 (12.5) |
| University College London Hospital, London | 18 (9.5) | 19 (10.0) | 15 (15.0) | 52 (10.8) |
| James Cook University Hospital, Middlesbrough | 12 (6.3) | 14 (7.4) | 11 (11.0) | 37 (7.7) |
| Freeman Hospital, Newcastle | 17 (8.9) | 16 (8.4) | 0 | 33 (6.9) |
| Guy’s Hospital, London | 11 (5.8) | 12 (6.3) | 8 (8.0) | 31 (6.5) |
| St Richard’s Hospital, Chichester | 6 (3.2) | 6 (3.2) | 15 (15.0) | 27 (5.6) |
| Addenbrookes Hospital, Cambridge | 6 (3.2) | 6 (3.2) | 13 (13.0) | 25 (5.2) |
| Queen Elizabeth Hospital, Birmingham | 6 (3.2) | 6 (3.2) | 13 (13.0) | 25 (5.2) |
| Western General Hospital, Edinburgh | 12 (6.3) | 12 (6.3) | 0 | 24 (5.0) |
| Royal Hallamshire Hospital, Sheffield | 9 (4.7) | 10 (5.3) | 2 (2.0) | 21 (4.4) |
| Stepping Hill Hospital, Stockport | 9 (4.7) | 10 (5.3) | 1 (1.0) | 20 (4.2) |
| St George’s Healthcare NHS Trust, London | 9 (4.7) | 9 (4.7) | 0 | 18 (3.8) |
| East Sussex Healthcare NHS Trust, Eastbourne | 8 (4.2) | 7 (3.7) | 0 | 15 (3.1) |
| University Hospital, Southampton | 6 (3.2) | 6 (3.2) | 1 (1.0) | 13 (2.7) |
| St James’s University Hospital, Leeds | 5 (2.6) | 4 (2.1) | 3 (3.0) | 12 (2.5) |
| Imperial College Hospital, London | 5 (2.6) | 5 (2.6) | 0 | 10 (2.1) |
| Royal Salford, Salford | 5 (2.6) | 5 (2.6) | 0 | 10 (2.1) |
| Pinderfields General Hospital, Wakefield | 5 (2.6) | 4 (2.1) | 0 | 9 (1.9) |
| Leicester General Hospital, Leicester | 1 (0.5) | 2 (1.1) | 4 (4.0) | 7 (1.5) |
| Wrexham Maelor Hospital, Wrexham | 3 (1.6) | 3 (1.6) | 0 | 6 (1.3) |
| Lister Hospital, Stevenage | 2 (1.1) | 3 (1.6) | 0 | 5 (1.0) |
| Southern General Hospital, Glasgow | 2 (1.1) | 1 (0.5) | 1 (1.0) | 4 (0.8) |
| Bradford Royal Infirmary, Bradford | 2 (1.1) | 2 (1.1) | 0 | 4 (0.8) |
| Aintree University Hospital, Liverpool | 2 (1.1) | 2 (1.1) | 0 | 4 (0.8) |
| Royal Gwent Hospital, Newport | 2 (1.1) | 1 (0.5) | 0 | 3 (0.6) |
| City Hospital, Nottingham | 0 | 0 | 2 (2.0) | 2 (0.4) |
| Salisbury NHS Foundation Trust, Salisbury | 2 (1.1) | 0 | 0 | 2 (0.4) |
| Bedford Hospital, Bedford | 1 (0.5) | 0 | 0 | 1 (0.2) |
| Total | 190 | 190 | 100 | 480 |
FIGURE 2.
Recruitment graph.
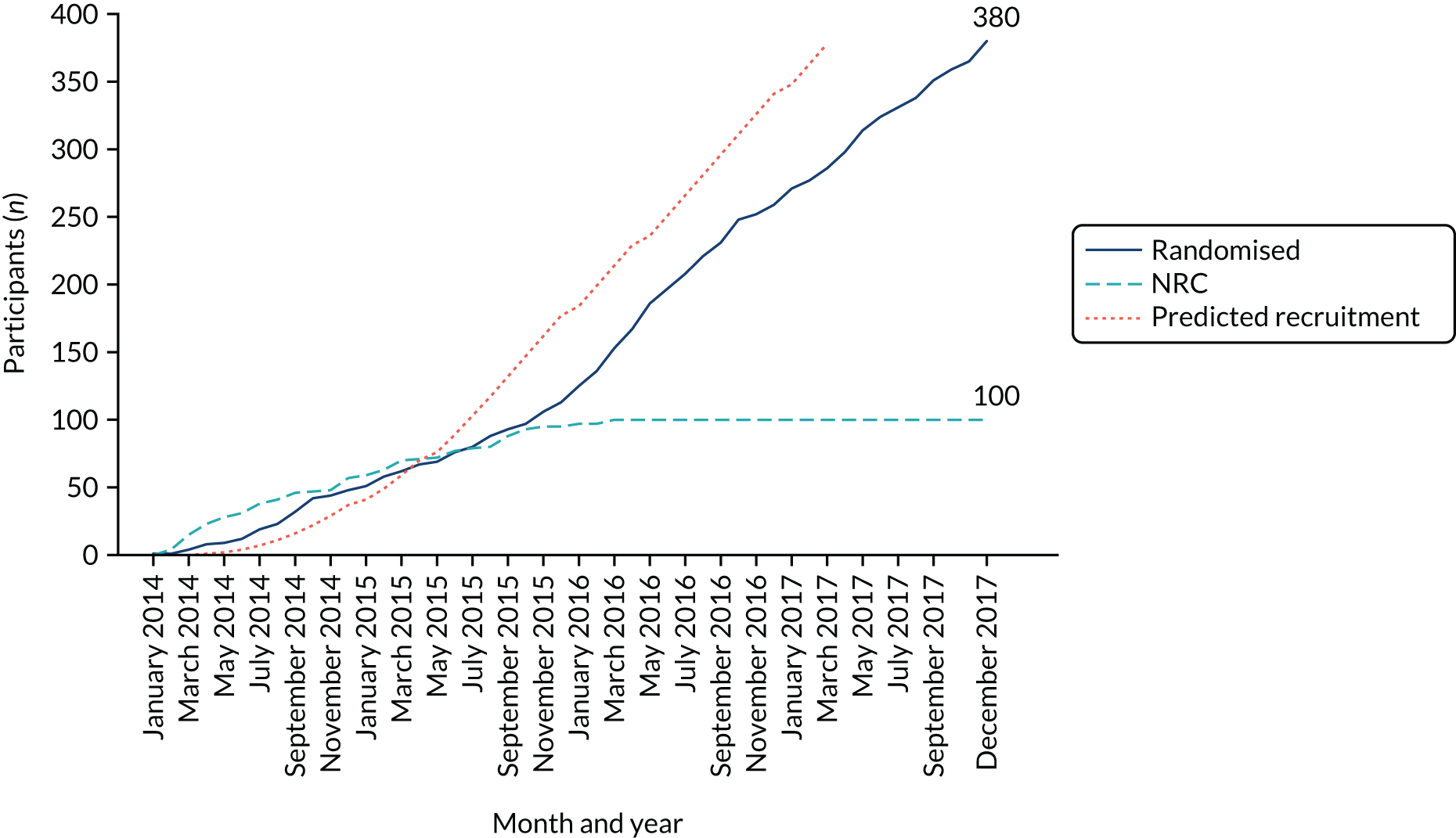
The centres were set up in a phased approach and did not all recruit for the full duration of the trial; therefore, the number of recruiting months (i.e. the time between the first and the last participant recruited in a centre) ranged from 1 to 47 months. Participants were recruited into the NRC until 27 October 2015, after which recruitment was stopped to the NRC because it was adversely affecting recruitment to the RCT. The database was closed to follow-up on 9 March 2020.
Study conduct
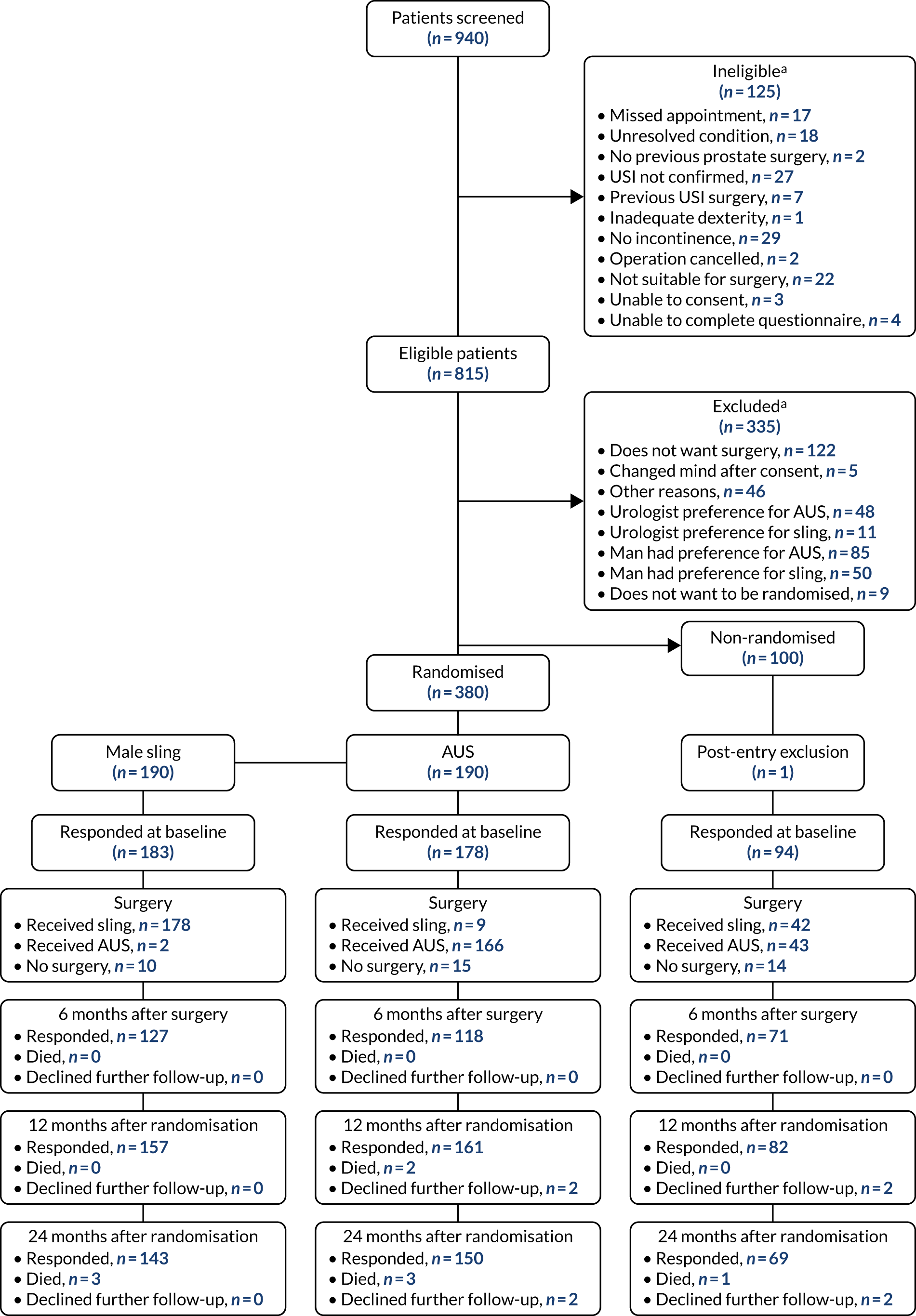
The progress of participants through the stages of the trial to follow-up is shown on the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 3). In total, 940 potential participants were considered for entry to MASTER. Of these, 125 (13.3%) individuals failed to meet one of the eligibility criteria. Of the 815 individuals who were eligible, 335 (41.1%) were excluded; the majority of individuals were excluded because the patient did not want surgery, or the patient or the urologist preferred the sling or the AUS. One participant was excluded from the NRC after entering the trial because they were not found to have any incontinence. The time from randomisation to the intervention and the time from randomisation to each of the follow-up points was similar between the two randomised groups (Table 2) and the NRC (see Appendix 2, Table 32).
FIGURE 3.
The CONSORT flow diagram of men recruited to MASTER. a, Patients can be ineligible and excluded for more than one reason.
| Time to events | Randomised group | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Days from randomisation to 6-month follow-up | ||
| Mean (SD); n | 308 (126); 125 | 318 (150); 116 |
| Median (IQR) | 275 (237–323) | 269 (224–365) |
| Minimum, maximum | 189, 1184 | 183, 1236 |
| Days from randomisation to 12-month follow-upa | ||
| Mean (SD); n | 370 (38); 152 | 362 (45); 146 |
| Median (IQR) | 360 (345–379) | 351 (342–364) |
| Minimum, maximum | 332, 552 | 323, 757 |
| Days from randomisation to 24-month follow-upb | ||
| Mean (SD); n | 760 (43); 132 | 748 (23); 138 |
| Median (IQR) | 747 (737–762) | 742 (735–755) |
| Minimum, maximum | 703, 1053 | 700, 853 |
| Days from surgery to 6-month follow-up | ||
| Mean (SD); n | 211 (31); 125 | 204 (32); 116 |
| Median (IQR) | 202 (189–218) | 195 (189–210) |
| Minimum, maximum | 177, 348 | 46, 355 |
| Days from surgery to 12-month follow-up | ||
| Mean (SD); n | 266 (123); 150 | 248 (121); 142 |
| Median (IQR) | 291 (210–338) | 267 (197–331) |
| Minimum, maximum | –630, 530 | –549, 573 |
| Days from surgery to 24-month follow-up | ||
| Mean (SD); n | 653 (125); 130 | 623 (130); 136 |
| Median (IQR) | 671 (611–718) | 652 (579–712) |
| Minimum, maximum | –257, 914 | –122, 774b |
| Days from randomisation to surgery | ||
| Mean (SD); n | 102 (110); 180 | 122 (129); 175 |
| Median (IQR) | 77 (36–133) | 92 (32–166) |
| Minimum, maximum | 1, 996 | 0, 939 |
The follow-up rates at 12 and 24 months for each randomised group were > 75% for the participants in the RCT. Although the follow-up rate is slightly higher for the AUS group, there was no substantive difference between the two randomised groups. Seven participants are known to have died up to the 2-year follow-up. These deaths were not related to participation in the trial.
Participant and sociodemographic factors
There was no difference in the age of the participants between the two randomised groups or the NRC (Table 3) (see Appendix 2, Table 33, for the NRC). The average age of the participants was between 67 and 68 years. All men had received a previous prostate operation and > 90% were not leaking urine prior to their prostate operation. Of those men in the RCT, 94% had their original prostate surgery for prostate cancer. Nearly all men received a radical prostatectomy and a smaller number of men underwent other procedures, such as channel TURP for obstructing prostate cancer, transurethral prostatectomy for benign prostatic obstruction or retropubic prostatectomy for benign prostatic obstruction. Approximately 50% of men had received physiotherapy for SUI and around 20% of men had received radiotherapy for prostatic disease. At least 90% of men had used pads or protection since their prostatic surgery because of leaking urine.
| Participant and sociodemographics | Randomised group | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Age (years), mean (SD) | 67 (6) | 68 (6) |
| Previous prostate surgery, n (%) | ||
| Surgery for prostate cancer | 178 (93.7) | 180 (94.7) |
| Surgery for benign prostate obstruction | 8 (4.2) | 9 (4.7) |
| Surgery for both | 4 (2.1) | 1 (0.5) |
| Type of previous surgery, n (%) | ||
| Radical prostatectomy | 183 (96.3) | 182 (95.8) |
| Channel TURP for obstructing prostate cancer | 2 (1.1) | 4 (2.1) |
| Transurethral prostatectomy for benign prostatic obstruction | 13 (6.8) | 7 (3.7) |
| Retropubic prostatectomy for benign prostatic obstruction | 1 (0.5) | 00 |
| Received radiotherapy for prostatic disease, n (%) | ||
| Yes | 38 (20.0) | 39 (20.5) |
| No | 152 (80.0) | 151 (79.5) |
| Leaking urine before first prostate operation, n (%) | ||
| Yes | 6 (3.2) | 10 (5.3) |
| No | 177 (93.2) | 174 (91.6) |
| Missing | 7 (3.7) | 6 (3.2) |
| Previous treatment for urinary/bladder problems, n (%) | ||
| Injectable treatment for SUI | 9 (4.7) | 8 (4.2) |
| Physiotherapy for SUI | 95 (50.0) | 83 (43.7) |
| Drug treatment with duloxetine for SUI | 23 (12.1) | 21 (11.1) |
| Drug treatment for other urinary/bladder problem | 40 (21.1) | 35 (18.4) |
| Any neurological disease | 2 (1.1) | 7 (3.7) |
| Do you wear pads or protection because of leaking urine?, n (%) | ||
| Yes | 175 (92.1) | 171 (90.0) |
| No | 5 (2.6) | 4 (2.1) |
| Missing | 10 (5.3) | 15 (7.9) |
| 24-hour pad test result (g) | ||
| Mean (SD); n | 384 (414); 159 | 433 (518); 159 |
| Median (IQR) | 256 (89–545) | 267 (130–554) |
| Pads used on an average day, mean (SD); n | 3.4 (1.8); 180 | 3.7 (2.2); 173 |
Clinical assessment at baseline
Prior to their MASTER surgery, men completed a 24-hour pad test and the pad weight increase caused by urinary leakage was recorded (see Table 3; see Appendix 2, Table 33, for the NRC). There were some extreme values in each group, leading to means being much higher than the medians. The mean number of pads used was similar in the two RCT groups and was slightly larger in the NRC (see Table 3; see Appendix 2, Table 33, for the NRC). In the NRC, the time to surgery was reported from the consent date rather than the randomisation date; therefore, in the two randomised groups, the median times from randomisation to surgery were 77 days (sling group) and 92 days (AUS group), whereas, in the NRC, the median time from consent to surgery was 95 days.
The responses to the two questions making up the primary outcome on the ICIQ-UI SF and the individual ICIQ-MLUTS questions for the RCT are shown in Table 4 (see Appendix 2, Table 34, for the responses for the NRC). The summary scores were similar across the RCT and the NRC. The questions that form the primary outcome show that > 90% of men leaked at least once per day when they consented to MASTER and more than one-third classed their leakage as ‘a large amount’. The ICIQ-UI SF score was similar in the RCT and the NRC. Incontinence and voiding scores were also similar in the RCT and NRC, with the low voiding scores indicating that incontinence problems were more severe than voiding problems. The sexual functioning score was high in the RCT and the NRC, which indicates that incontinence had a considerable effect on sexual functioning.
| Health status | Randomised group | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| How often do you leak urine? n (%) | ||
| Once per week or less | 0 | 0 |
| Two or three times per week | 3 (1.6) | 1 (0.5) |
| About once per day | 3 (1.6) | 2 (1.1) |
| Several times per day | 115 (60.5) | 104 (54.7) |
| All of the time | 54 (28.4) | 59 (31.1) |
| Question missing | 8 (4.2) | 12 (6.3) |
| Missing | 7 (3.7) | 12 (6.3) |
| How much urine do you usually leak? n (%) | ||
| A small amount | 38 (20.0) | 29 (15.3) |
| A moderate amount | 76 (40.0) | 84 (44.2) |
| A large amount | 68 (35.8) | 61 (32.1) |
| Question missing | 1 (0.5) | 4 (2.1) |
| Missing | 7 (3.7) | 12 (6.3) |
| Score for effect on everyday life, mean (SD); n | 7.5 (2.3); 178 | 7.8 (2.1); 176 |
| Number of times pass urine during the daytime, mean (SD); n | 7.4 (3.8); 172 | 8.4 (8.6); 155 |
| Number of times get up at night to pass urine, mean (SD); n | 1.8 (1.4); 179 | 1.8 (1.5); 175 |
| ICIQ-UI SF score, mean (SD); n | 16.1 (3.4); 172 | 16.4 (3.2); 166 |
| ICIQ-MLUTS incontinence score, mean (SD); n | 12.2 (3.9); 176 | 12.1 (4.2); 168 |
| ICIQ-MLUTS voiding score, mean (SD); n | 3.9 (3.5); 178 | 3.6 (3.4); 171 |
| ICIQ-MLUTSsex, mean (SD); n | 8.1 (1.3); 137 | 8.0 (1.5); 131 |
| SF-12 mental score, mean (SD); n | 47.5 (10.7); 169 | 49.7 (11.3); 168 |
| SF-12 physical score, mean (SD); n | 48.3 (9.2); 169 | 47.5 (9.7); 168 |
| EQ-5D-3L, mean (SD); n | 0.823 (0.221); 177 | 0.814 (0.241); 172 |
The urinary symptoms for men in the RCT, taken as responses from the ICIQ-UI SF, are shown in Appendix 1, Table 30 (see Appendix 2, Table 35, for responses for the NRC). These are similar between the randomised groups.
The SF-12 mental and physical scores were also similar. In all three groups, the mean for both scores was slightly less than 50, which is the median of the scale, suggesting that participants rated their mental and physical health slightly below the average for the general population when they entered the trial.
In the RCT, 180 of the 190 men randomised to the male sling group and 175 of the 190 randomised to the AUS group received surgery (Table 5). This means that 93.4% of the RCT population underwent surgery, with 178 and 166 men, respectively, receiving their allocated intervention (90.5% of the RCT men). Of the 25 men in the RCT who did not receive surgery, reasons were recorded for 22 (male sling group, n = 8; AUS group, n = 14): two died before receiving surgery (both AUS); 11 no longer wanted surgery (male sling group, n = 3; AUS group, n = 8); six had a change in their medical condition and either no longer required surgery or surgery was no longer appropriate (male sling group, n = 3; AUS group, n = 3); one man in the sling group did not want to self-catheterise; and two men (male sling group, n = 1; AUS group, n = 1) had current care responsibilities that made surgery impracticable.
| Surgery details | Randomised group | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Received surgery, n/N (%) | 180/190 (94.7) | 175/190 (92.1) |
| Received sling, n/N (%) | 178/180 (98.9) | 9/175 (5.1) |
| Received sphincter, n/N (%) | 2/180 (1.1) | 166/175 (94.9) |
| Device replacement/removal (within 24 months), n/N (%) | 20/180 (11.1) | 4/175 (2.3) |
| Sling replaced by sphincter (n) | 18 | 00 |
| Sling replaced with a new sling (n) | 2 | 00 |
| Sphincter replaced with a new sphincter (n) | 0 | 3 |
| Time from randomisation to surgery (days) | ||
| Mean (SD); n | 102 (110); 180 | 122 (129); 175 |
| Median (IQR) | 77 (36–133) | 92 (32–166) |
| Length of surgery (minutes) | ||
| Mean (SD); n | 101 (34); 175 | 128 (37); 168 |
| Median (IQR) | 98 (75–121) | 120 (102–150) |
| Length of hospital stay (days) | ||
| Mean (SD); n | 2 (2); 180 | 2 (2); 175 |
| Median (IQR) | 1 (1–2) | 1 (1–2) |
| Time to re-admission (days) | ||
| Mean (SD); n | 331 (155); 20 | 429 (332); 4 |
| Median (IQR) | 331 (203–392) | 376 (161–698) |
Two (1.1%) men were randomised to receive a male sling but received an AUS, one at his own choice and the other because his surgery was delayed and at a subsequent review it was decided that an AUS was more appropriate. Nine men randomised to receive an AUS received a male sling: seven because of participant preference and the other two because of clinician preference.
In the NRC, 85 men (male sling, n = 42/46, 91.3%; AUS, n = 43/46, 93.5%) received surgery (see Appendix 2, Table 36).
The length of hospital stay was similar in both of the randomised groups and in the NRC. As expected, surgery for men receiving an AUS took approximately 20 minutes longer than the surgery for men receiving a male sling.
The proportion of men who were re-admitted for further surgery was larger in the male sling group than in the AUS group (see Table 5). In the majority of cases, revision surgery in the male sling group was required because incontinence had not improved. Twenty men (11%) in this group were re-admitted to have their sling removed; 18 had their sling replaced with an AUS and two had a new sling inserted. Four men (2%) randomised to receive an AUS were re-admitted for further surgery. Three received a replacement AUS and one had his AUS removed but not replaced. In the NRC, there were eight re-admissions: six men had their male sling replaced by an AUS and two men had their AUS replaced with another AUS (see Appendix 2, Table 36). A small number of device failures occurred in both randomised groups and in the NRC. The length of time to re-admission was right skewed, but the median time in both randomised groups and the NRC was between 10 and 13 months.
Adverse events and deaths
Data on the AEs that occurred during the surgical procedures were also collected (Table 6) (see Appendix 2, Table 37, for the NRC). The majority of these were events that required additional oral pain relief (pain relief is standard therapy after these procedures). More men in the male sling group than in the AUS group required postoperative catheterisation and catheterisation for > 24 hours. Otherwise, the rates of AEs were low and similar between the groups.
| Adverse event | Randomised group, n (%) | |
|---|---|---|
| Male sling (N = 180) | AUS (N = 175) | |
| Total number of SAEs | 8 | 15 |
| Total number of AEs | 226 | 192 |
| Participants with any AEs | 152 (84.4) | 149 (85.1) |
| Number of AEs per participant | ||
| 0 | 28 (15.6) | 26 (14.9) |
| 1 | 101 (56.1) | 12 (68.6) |
| 2 | 35 (19.4) | 19 (10.9) |
| 3 | 11 (6.1) | 6 (3.4) |
| ≥ 4 | 5 (2.8) | 4 (2.3) |
| Postoperative catheter required | 28 (15.6) | 8 (4.6) |
| Catheter required for > 24 hours | 20 (11.1) | 6 (3.4) |
| Urinary tract infection | 0 | 2 (1.1) |
| Pyrexia | 1 (0.6) | 3 (1.7) |
| Wound infection | 3 (1.7) | 1 (0.6) |
| Sepsis, septicaemia or abscess | 1 (0.6) | 0 |
| Retention requiring surgery | 1 (0.6) | 0 |
| Bowel obstruction | 0 | 1 (0.6) |
| Constipation | 1 (0.6) | 3 (1.7) |
| New urinary tract symptoms | 2 (1.1) | 0 |
| Tape or sling complications | 1 (0.6) | 0 |
| Device exposure/extrusion requiring no treatment | 0 | 1 (0.6) |
| Acute or chronic pain | 1 (0.6) | 1 (0.6) |
| Oral pain relief given | 140 (77.8) | 139 (79.4) |
| Parenteral pain relief given | 13 (7.2) | 13 (7.4) |
| Antibiotic treatment for postoperative infection | 6 (3.3) | 10 (5.7) |
| Other AEs | 4 (2.2) | 2 (1.1) |
There were a small number of SAEs (see Table 6; see Appendix 2, Table 37, for the NRC), experienced by eight men in the male sling group (recatheterisation requiring or prolonging hospital stay, n = 3; mesh erosion n = 3; infection urosepsis, n = 1; developed coffee ground vomit, n = 1) and 13 men in the AUS group (recatheterisation requiring or prolonging hospital stay, n = 3; infection, n = 3; erosion of device, n = 2; haematoma, n = 1; bruising and inflammation, n = 1; urinary retention/voiding difficulties, n = 1; pain, n = 1; transient hypotension, n = 1; thrombosis, n = 1; exacerbation of asthma; n = 1; one man had three SAEs). Six men in the NRC had a SAE: five in the male sling group (anaphylaxis, n = 1; haematuria, n = 1; recatheterisation requiring or prolonging hospital stay, n = 2; and wound infection, n = 1) and one in the AUS group (dysuria, n = 1).
There were six deaths in the RCT (three in each of the randomised groups) and one death in the NRC, none of which was related to their MASTER surgery. Six of the deaths were a result of cancer and one was due to pneumonia.
Chapter 4 Clinical results
This chapter describes the comparison of the male synthetic sling with the AUS at 6 months after surgery, and at 12 and 24 months after randomisation.
Analysis populations
Throughout this chapter, the 380 participants in the RCT (randomised to receive either a male sling or an AUS) are separated from the 99 participants in the NRC, whose surgery was decided by a combination of surgeon and participant preference. All of the statistical analysis is a comparison between the two groups in the RCT only. No statistical analysis was performed on those in the NRC, but summary statistics for the NRC are presented in Appendix 2.
Operative details
The surgical details for the RCT are described in Chapter 3 and, for the NRC, in Appendix 2. In the RCT, 355 (male sling, n = 180; AUS, n = 175) of the 380 randomised men received surgery and 85 (male sling, n = 42; AUS, n = 43) of the 99 non-randomised men received surgery. Adherence to the randomised intervention was high, with nearly 97% of the men who received surgery receiving their randomised procedure. The surgery time in minutes for the men randomised to the sling group was statistically significantly shorter than that for men randomised to the AUS group (–24.67 minutes, 95% CI –33.62 to–15.72 minutes; p < 0.001) (see Table 5).
Primary outcome
The primary outcome was the proportion of men continent at 12 months post randomisation (male sling, n = 20, 13%; AUS, n = 25, 15.8%), with continence defined as a combined response of ‘never’ and ‘none’ to the questions ‘how often do you leak urine?’ and ‘how much urine do you leak?’ in the ICIQ-UI SF participant-reported questionnaire. On the advice of the DMC, a less strict version of this was included as a secondary outcome, for which ‘less than once a week’ and ‘a small amount’ were added to the definitions of success and are, therefore, included in the continence proportion. Using the less strict definition, 52 (33.8%) men in the male sling group and 55 (34.8%) men in the AUS group were continent 12 months after randomisation (Table 7 for the RCT; see Appendix 2, Table 38, for the NRC). Table 7 also includes the data for both the 6-month and the 24-month time points.
| Analysis of continence | Randomised group, n/N (%) | Difference (male sling – AUS)a (95% CI); p-value | OR (95% CI) | |
|---|---|---|---|---|
| Male sling | AUS | |||
| ITT | ||||
| Sample size | 190 | 190 | ||
| Primary outcome | ||||
| Continent (12 months) | 20/154 (13.0) | 25/158 (15.8) | –3.4 (–11.7 to 4.8); 0.003 | 0.75 (0.36 to 1.54) |
| Continent: less strict definition (12 months) | 52/154 (33.8) | 55/158 (34.8) | –1.9 (–10.4 to 6.6); 0.001 | 0.92 (0.62 to 1.35) |
| Other time points | ||||
| Continent (6 months) | 19/121 (15.7) | 24/117 (20.5) | –5.9 (–13.8 to 2.1); 0.012 | 0.67 (0.38 to 1.16) |
| Continent: less strict definition (6 months) | 45/121 (37.2) | 48/117 (41.0) | –5.1 (–14.9 to 4.7); 0.024 | 0.80 (0.53 to 1.23) |
| Continent (24 months) | 21/140 (15.0) | 22/148 (14.9) | –0.6 (–9.2 to 8.0); 0.001 | 0.95 (0.48 to 1.90) |
| Continent: less strict definition (24 months) | 44/140 (31.4) | 51/148 (34.5) | –4.0 (–16.7 to 8.8); 0.045 | 0.83 (0.46 to 1.51) |
| Per protocol | ||||
| Sample size | 178 | 166 | ||
| Primary outcome | ||||
| Continent (12 months) | 20/151 (13.2) | 22/146 (15.1) | –0.025 (–0.107 to 0.056); 0.001 | 0.81 (0.40 to 1.64) |
| Continent: less strict definition (12 months) | 52/151 (34.4) | 50/146 (34.2) | –0.010 (–0.094 to 0.075); 0.001 | 0.96 (0.65 to 1.40) |
| Other time points | ||||
| Continent (6 months) | 19/119 (16.0) | 20/112 (17.9) | –0.029 (–0.119 to 0.060); 0.004 | 0.81 (0.43 to 1.54) |
| Continent: less strict definition (6 months) | 44/119 (37.0) | 44/112 (39.3) | –0.037 (–0.133 to 0.059); 0.010 | 0.85 (0.56 to 1.29) |
| Continent (24 months) | 21/136 (15.4) | 19/139 (13.7) | 0.011 (–0.072 to 0.094); < 0.001 | 1.09 (0.56 to 2.14) |
| Continent: less strict definition (24 months) | 44/136 (32.4) | 47/139 (33.8) | –0.024 (–0.159 to 0.110); 0.033 | 0.89 (0.48 to 1.66) |
Under both definitions, most men reported that they were not continent at all three time points. The ITT-estimated absolute risk difference was –0.034 (95% CI –0.117 to 0.048; non-inferiority p = 0.003), indicating a lower success rate in those randomised to receive a male sling, but with a CI that excludes the predefined non-inferiority margin of –15%. This shows that the sling was non-inferior to the AUS.
At 6 months, there was a larger difference between the male sling and the AUS, but, at 24 months, there was almost no difference when the original definition of continence was used (see Table 7). The less strict definition showed a smaller difference at 6 and 12 months than for the original definition, but a larger difference was seen at 24 months when this alternative definition was used (see Table 7).
The per-protocol estimates at 6, 12 and 24 months were similar to those of the ITT analysis (see Table 7).
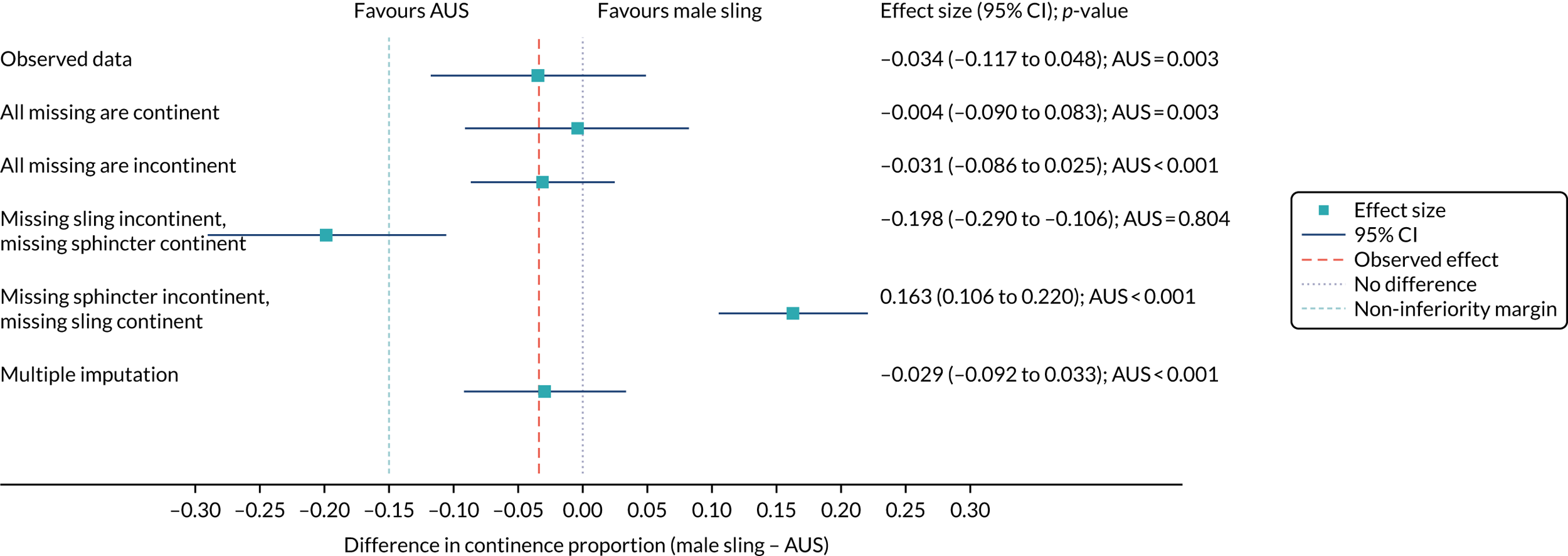
Sensitivity analysis
Figure 4 shows the sensitivity analysis of the primary outcome. Multiple imputation and pattern-mixture modelling were used. Estimates were consistent in all but the most extreme circumstances, which are unlikely to reflect the missing data.
FIGURE 4.
Forest plot showing the sensitivity analysis of the primary outcomes on missing data.
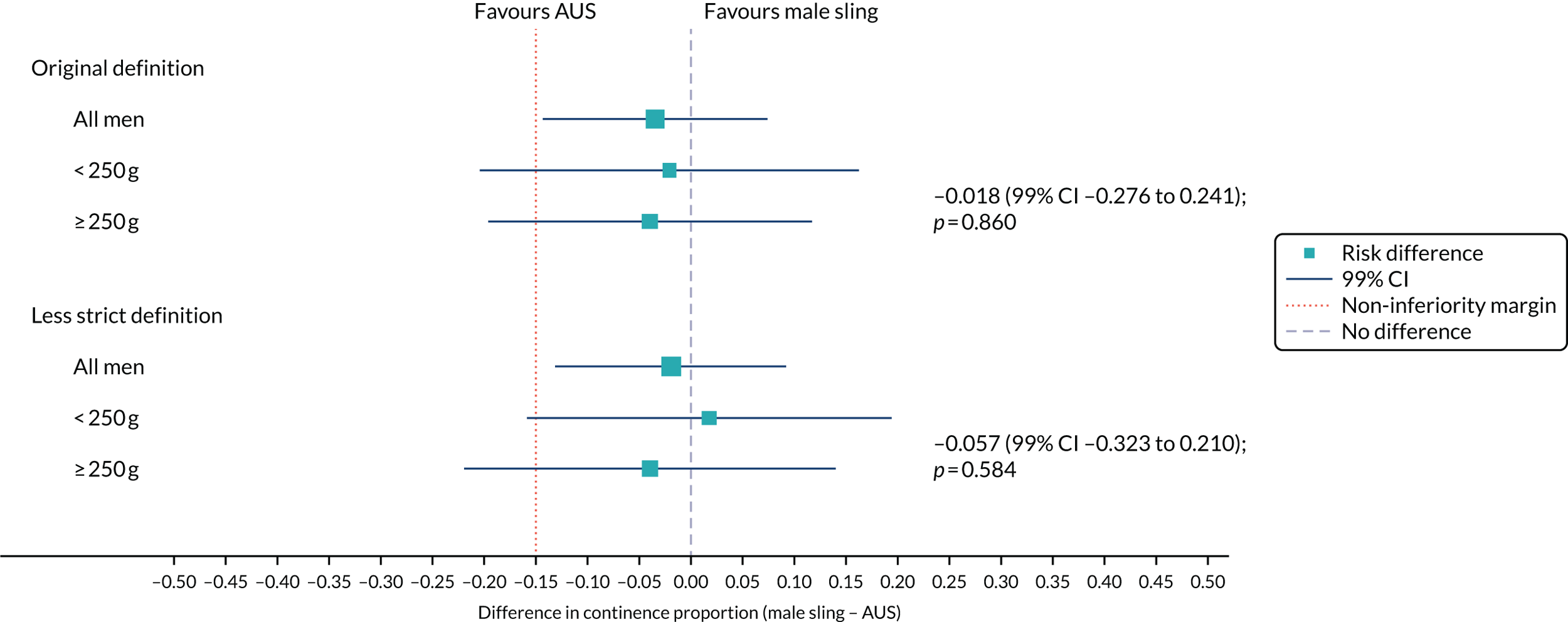
Subgroup analysis by baseline pad weight
The men’s report of continence at 12 months was available for 312 men in the RCT: 134 men with a pad weight at baseline of ≤ 250 g and 178 men with a pad weight at baseline of > 250 g.
When the original continence definition was used, in both subgroups, the proportion of men incontinent was larger for those who received the male sling than for those who received the AUS (Table 8). When the less strict definition was used, the proportion of men incontinent in the > 250 g subgroup was larger for those who received the male sling than for those who received the AUS. In the subgroup for which the baseline pad weight was ≤ 250 g, the AUS group had a larger proportion of men who were incontinent than the male sling group.
| Definition | Randomised group, n/N (%) | Effect size (male sling – AUS)a (99% CI); p-value | |
|---|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | ||
| Original definition | |||
| All men | 20/154 (13.0) | 25/158 (15.8) | –0.034 (–0.143 to 0.074); 0.003 |
| ≤ 250 g pad weight | 10/68 (14.7) | 11/66 (16.7) | –0.021 (–0.203 to 0.162); 0.034 |
| > 250 g pad weight | 10/86 (11.6) | 14/92 (15.2) | –0.040 (–0.196 to 0.117); 0.034 |
| Less strict definition | |||
| All men | 52/154 (33.8) | 55/158 (34.8) | –0.019 (–0.131 to 0.092); 0.001 |
| ≤ 250 g pad weight | 26/68 (38.2) | 24/66 (36.4) | 0.018 (–0.159 to 0.194); 0.007 |
| > 250 g pad weight | 26/86 (30.2) | 31/92 (33.7) | –0.039 (–0.219 to 0.140); 0.056 |
The forest plot (Figure 5) displays this subgroup information visually and the size of the interaction effect. There is no evidence that baseline pad weight moderates the overall treatment effect.
FIGURE 5.
Forest plot showing the continence rate using the original and the less strict definitions based on baseline pad weight.
Questionnaire response over time
The 6-month questionnaire was sent post surgery and the 12- and 24-month questionnaires were sent post randomisation. Where there was a delay in surgery and the 6-month and 12-month questionnaires were due at the same time, the 12-month questionnaire was prioritised. This explains the slightly lower response rates at 6 months (which were shown in Chapter 3) (Table 9).
| Follow-up time point | Randomised group, n (%) | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| 6 months | 127 (66.8) | 118 (62.1) |
| 12 months | 157 (82.6) | 161 (84.7) |
| 24 months | 143 (75.3) | 150 (78.9) |
Secondary outcomes
Pad use reduced from baseline, but there was no difference between the two randomised groups in the proportion of men still using pads, and there was no indication of pad use reducing during follow-up. Daily pad use was consistently slightly higher in the male sling group than in the AUS group at all three time points (Table 10) (see Appendix 2, Table 39, for the NRC).
| Follow-up time point | Randomised group | Effect size (male sling – AUS)a (95% CI); p-value | |
|---|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | ||
| Wears pads or other protection,b n/N (%) | |||
| Baseline | 175/190 (92.1) | 171/190 (90.0) | |
| 6 months | 79/120 (65.8) | 68/112 (60.7) | 7.6 (–4.4 to 19.6); 0.212 |
| 12 months | 102/148 (68.9) | 102/150 (68.0) | 2.1 (–1.1 to 15.2); 0.750 |
| 24 months | 83/130 (63.8) | 96/142 (67.6) | –2.2 (–16.5 to 12.2); 0.768 |
| Pads used in an average day,c mean (SD); n | |||
| Baseline | 3.4 (1.8); 180 | 3.7 (2.2); 173 | |
| 6 months | 1.6 (2.0); 119 | 1.1 (1.5); 112 | 1.62 (1.16 to 2.25); 0.004 |
| 12 months | 1.6 (2.1); 146 | 1.3 (1.7); 150 | 1.25 (0.99 to 1.58); 0.062 |
| 24 months | 1.5 (1.8); 127 | 1.2 (1.2); 142 | 1.30 (0.92 to 1.85); 0.137 |
| Score for effect on everyday life, mean (SD); n | |||
| Baseline | 7.5 (2.3); 178 | 7.8 (2.1); 176 | |
| 6 months | 3.3 (3.4); 123 | 2.0 (2.4); 116 | 1.38 (0.68 to 2.09); < 0.001 |
| 12 months | 3.5 (3.4); 155 | 2.7 (2.9); 157 | 0.82 (0.16 to 1.47); 0.014 |
| 24 months | 3.2 (3.1); 141 | 2.6 (2.9); 147 | 0.76 (0.09 to 1.43); 0.026 |
| ICIQ-UI SF score, mean (SD); n | |||
| Baseline | 16.1 (3.4); 172 | 16.4 (3.2); 166 | |
| 6 months | 8.1 (6.0); 116 | 6.1 (4.4); 113 | 2.04 (0.75 to 3.33); 0.002 |
| 12 months | 8.7 (6.1); 151 | 7.5 (5.3); 153 | 1.30 (0.11 to 2.49); 0.032 |
| 24 months | 7.9 (5.5); 131 | 7.1 (5.0); 139 | 1.42 (0.18 to 2.65); 0.024 |
| ICIQ-MLUTS: incontinence, mean (SD); n | |||
| Baseline | 12.2 (3.9); 176 | 12.1 (4.2); 168 | |
| 6 months | 6.7 (5.0); 114 | 5.2 (3.9); 111 | 1.50 (0.47 to 2.53); 0.004 |
| 12 months | 7.3 (4.8); 146 | 5.7 (4.4); 146 | 1.66 (0.71 to 2.61); 0.001 |
| 24 months | 6.5 (4.2); 128 | 5.6 (3.9); 141 | 1.05 (0.08 to 2.03); 0.034 |
| ICIQ-MLUTS: voiding, mean (SD); n | |||
| Baseline | 3.9 (3.5); 178 | 3.6 (3.4); 171 | |
| 6 months | 4.6 (3.9); 115 | 3.0 (3.1); 108 | 1.25 (0.52 to 1.97); 0.001 |
| 12 months | 4.4 (4.0); 144 | 2.9 (2.9); 146 | 1.33 (0.66 to 2.00); < 0.001 |
| 24 months | 4.3 (3.7); 130 | 3.3 (3.3); 139 | 0.69 (0.00 to 1.37); 0.049 |
| ICIQ-MLUTSsex, mean (SD); n | |||
| Baseline | 8.1 (1.3); 137 | 8.0 (1.5); 131 | |
| 6 months | 7.6 (1.6); 82 | 7.6 (2.0); 77 | 0.22 (–0.20 to 0.64); 0.308 |
| 12 months | 7.6 (1.6); 104 | 7.6 (1.8); 117 | 0.08 (–0.30 to 0.46); 0.676 |
| 24 months | 7.7 (1.7); 89 | 7.5 (1.7); 97 | 0.19 (–0.21 to 0.59); 0.352 |
| EQ-5D-3L, mean (SD); n | |||
| Baseline | 0.823 (0.221); 177 | 0.814 (0.241); 172 | |
| 6 months | 0.822 (0.240); 125 | 0.827 (0.266); 112 | –0.023 (–0.070 to 0.025); 0.348 |
| 12 months | 0.809 (0.260); 151 | 0.813 (0.274); 158 | –0.019 (–0.062 to 0.025); 0.396 |
| 24 months | 0.820 (0.239); 136 | 0.828 (0.260); 147 | –0.014 (–0.059 to 0.030); 0.534 |
| SF-12: physical score, mean (SD); n | |||
| Baseline | 48.3 (9.2); 169 | 47.5 (9.7); 168 | |
| 6 months | 48.4 (10.0); 116 | 49.0 (10.4); 102 | –1.57 (–3.53 to 0.39); 0.116 |
| 12 months | 48.3 (10.3); 142 | 48.7 (10.3); 146 | –0.83 (–2.61 to 0.96); 0.364 |
| 24 months | 48.0 (10.1); 126 | 48.7 (10.8); 139 | –1.18 (–3.02 to 0.65); 0.207 |
| SF-12: mental score, mean (SD); n | |||
| Baseline | 47.5 (10.7); 169 | 49.7 (11.3); 168 | |
| 6 months | 49.8 (10.8); 116 | 49.8 (10.9); 102 | 0.19 (–1.90 to 2.28); 0.857 |
| 12 months | 48.8 (10.9); 142 | 51.3 (10.5); 146 | –1.09 (–2.98 to 0.81); 0.262 |
| 24 months | 49.4 (11.2); 126 | 51.2 (10.4); 139 | 0.04 (–1.91 to 1.99); 0.968 |
The effect of incontinence on everyday life was worse in men randomised to receive the male sling, and this difference is significant at all three time points (see Table 10). The ICIQ-UI SF score, which combines frequency, volume and effect of incontinence into a single outcome, is at the highest (and, therefore, worst) at 12 months. The difference between the two randomised groups was significant at all three time points, with a poorer outcome seen in the male sling group.
Urinary symptoms derived from the individual responses to the ICIQ-UI SF questionnaire are shown in Appendix 1, Table 31, for the RCT and Appendix 2, Table 41, for the NRC. There was a consistent pattern of men who received the male sling having worse outcomes on these questions than those who received the AUS.
Voiding scores and incontinence scores were worse in the male sling group than in the AUS group (see Table 10) and, although there was improvement from baseline for the incontinence score, the voiding score did not change over time. There was no difference between the groups in the sexual functioning score and there is only a small improvement from baseline across the groups.
The EQ-5D-3L score at baseline showed that men rated their quality of life as good and, although this dropped slightly at 12 months, it increased again at 24 months (see Table 10). The SF-12 physical and mental scores remain very similar to the baseline values at all three follow-up points. There were no statistically significant differences in the generic quality-of-life outcomes between the two randomised groups.
Some additional outcomes were collected at 12 months only (Table 11; see Appendix 2, Table 40, for the NRC). Men were asked to compare their urinary leakage with that before their surgery using the seven-point PGI-I scale. Of the men randomised to receive the male sling, 75 (40%) reported that they were very much better compared with 99 (52%) men in the AUS group. The volume of urinary leakage at 12 months was reported to be worse than baseline by 12 men in the male sling group and five men in the AUS group. The OR showed a difference between the groups, with the men in the sling group worse off than those in the AUS group (0.39, 95% CI 0.24 to 0.62; p < 0.001).
| Outcome | Randomised group | OR (95% CI); p-valuea | |
|---|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | ||
| Urine leakage compared with that before surgery, n (%) | |||
| Very much better | 75 (39.5) | 99 (52.1) | 0.39 (0.24 to 0.62); < 0.001 |
| Much better | 25 (13.2) | 21 (11.1) | |
| A little better | 19 (10.0) | 9 (4.7) | |
| No change | 11 (5.8) | 5 (2.6) | |
| A little worse | 5 (2.6) | 1 (0.5) | |
| Much worse | 5 (2.6) | 2 (1.1) | |
| Very much worse | 2 (1.1) | 2 (1.1) | |
| Missing | 48 (25.3) | 51 (26.8) | |
| Satisfaction with surgery results, n (%) | |||
| Completely satisfied | 58 (30.5) | 79 (41.6) | 0.44 (0.28 to 0.69); < 0.001 |
| Fairly satisfied | 46 (24.2) | 46 (24.2) | |
| Fairly dissatisfied | 17 (8.9) | 8 (4.2) | |
| Very dissatisfied | 19 (10.0) | 3 (1.6) | |
| Not sure | 4 (2.1) | 2 (1.1) | |
| Missing | 46 (24.2) | 52 (27.4) | |
| Recommend surgery to a friend, n (%) | |||
| Yes | 108 (78.8) | 123 (95.3) | 0.18 (0.07 to 0.48); 0.001 |
| No | 29 (21.2) | 6 (4.7) | |
| Missing | 53 (27.9) | 61 (32.1) | |
| 24-hour pad weight at 12 months (g) | Adjusted mean difference (95% CI); p-value | ||
| Mean (SD); n | 30 (85); 50 | 74 (452); 44 | –50 (–228 to 128); 0.561 |
| Median (IQR) | 0.1 (0.0–7.4) | 0.1 (0.0–6.2) | |
| Minimum, maximum | 0, 403 | 0, 3000 | |
| Time until return to normal activities (months) | |||
| Mean (SD); n | 2.6 (1.9); 131 | 2.2 (1.7); 124 | 0.39 (–0.09 to 0.88); 0.105 |
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | |
Men randomised to receive a male sling were less likely than those in the AUS group to be satisfied with the results of their surgery (OR 0.44, 95% CI 0.28 to 0.69; p < 0.001; see Table 11) and were also less likely to recommend their surgery to a friend (n = 108, 79%, in male sling group vs. n = 123, 95%, in AUS group; OR 0.18, 95% CI 0.07 to 0.48; p = 0.001).
Complications reported over the 24-month follow-up period
Complications reported by the men within their follow-up questionnaires are shown in Table 12. The higher frequency of catheter problems in the male sling group than the AUS group is also captured here. Device problems, which we described and explained more fully in Chapter 3 (device replacement/removal), are also shown here. For the other complications that are reported here, the rates are similar between the groups. Care needs to be taken when interpreting these because a device problem can be ‘need to pump several times’ or ‘small leakage’, whereas pain can be ‘soreness’ or an indication of pain that has now subsided.
| Complication | Randomised group, n (%) | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Participants with complications | 110 (57.9) | 95(50.0) |
| Number of complications per participant | ||
| 1 | 53 (27.9) | 48 (25.3) |
| 2 | 29 (15.3) | 24 (12.6) |
| 3 | 17 (8.9) | 10 (5.3) |
| 4 | 7 (3.7) | 8 (4.2) |
| ≥ 5 | 4 (2.1) | 5 (2.6) |
| Bowel obstruction | 3 (1.6) | 4 (2.1) |
| Constipation | 34 (17.9) | 27 (14.2) |
| New bladder or urinary symptoms | 25 (13.2) | 6 (3.2) |
| Urinary tract infection | 23 (12.1) | 20 (10.5) |
| Other infection | 10 (5.3) | 2 (1.1) |
| Device problems | 23 (12.1) | 37 (19.5) |
| Sexual problems | 34 (17.9) | 37 (19.5) |
| Wound breakdown | 3 (1.6) | 7 (3.7) |
| Pain at site of surgery | 56 (29.5) | 44 (23.2) |
Chapter 5 Economic analysis
Methods
Summary
The principal research question being addressed in the economic analysis was the cost-effectiveness of a policy of primary implantation of the male synthetic sling compared with AUS, measured by incremental cost per QALY at 24 months post randomisation. The within-trial economic evaluation analysis follows the ITT principle and is based on data collected alongside the RCT and the health economics plan. A Markov cohort decision analysis model was developed to consider a longer time horizon to provide additional information for policy-makers. The base-case analysis assessed the costs and cost-effectiveness of the interventions compared with the perspective of the NHS in accordance with the NICE recommendations. 17 Sensitivity analyses exploring a broader perspective of cost were also conducted. Data were collected on resource use for the delivery of the intervention; primary and secondary NHS resource use over 24 months’ follow-up, including referral for additional specialist management; and broader societal resource use, which included personal costs to the participants for containment products, private medical costs and lost productivity costs, mainly lost income, over the 24 months. All costs were expressed in Great British pounds and were valued in 2017–18 prices. Costs and benefits experienced after the first year were discounted at the recommended 3.5% per annum. 17 The sensitivity analysis explored the impact of varying key assumptions on the base-case estimates of cost-effectiveness in accordance with NICE recommendations. The methods for within-trial and modelling analyses are described here.
Within-trial economic evaluation
Measuring resource use
Intervention resource use was recorded prospectively for every participant in the study (Table 13). Intervention resource was collected using an intraoperative (theatre) case report form (CRF). This included the grade and type of staff who administered the intervention, the operating urologist (and whether or not they were supervised), the anaesthetist and nurses. The operation time was calculated from the time of entry into the anaesthetic room to the time of leaving the theatre. The type of anaesthesia administered was identified as general, spinal or local. Information that was collected included the device that participants received, either AUS or sling; the use of prophylactic antibiotic; pre-treatment and post-treatment catheterisation; the use of either oral or parenteral pain relief; the use of postoperative antibiotic treatment; and the length of stay.
| Resource | Unit cost (£) | Notes and source of information |
|---|---|---|
| Intervention device | ||
| Slings | 2281 | Average price paid for device over the study period (personal communication with three sitesa) |
| AUS | 4519 | Average price paid for device over the study period (personal communication with three sitesa) |
| Consultant surgeon | 109 | Cost per hour (PSSRU)18 |
| Specialty registrar | 47 | Cost per hour (PSSRU)18 |
| Consultant anaesthetist | 109 | Cost per hour (PSSRU)18 |
| Theatre nurse band 6 | 45 | Average cost per hour (PSSRU)18 |
| Theatre nurse band 5 | 34 | Average cost per hour (PSSRU)18 |
| Theatre nurse band 4 | 9 | Average cost per hour (PSSRU)18 |
| General anaesthesia | 22 | Anaesthesia consumables cost per average case (BNF);19 details in Appendix 3, Table 42 |
| Spinal anaesthesia | 2 | Anaesthesia consumables cost per average case (BNF);19 details in Appendix 3, Table 42 |
| Local anaesthesia | 2 | Anaesthesia consumables cost per average case (BNF);19 details in Appendix 3, Table 42 |
| Antibiotics | 1 | Prophylactic antibiotic Gentamicin (BNF)19 |
| Catheter | 2 | Foley (male) short/medium term (single use) |
| Inpatient stay | 413 | Weighted average of LB50 and LB21: excess elective inpatient stay (cost per day) (NHS Reference Costs)20 |
| Theatre services | 740 | ISD National Statistics Theatre costs per hour excluding staff and drugs (ISD)21 |
| Secondary care costs | ||
| Sling surgery | 4374 | Based on average cost of slings in the study |
| 5529 | Used for the sensitivity analysis. Weighted average of LB21 (with CC score of 0–1 and 2 +). Elective inpatient: major open, prostate or bladder neck procedures (male) (NHS Reference Costs)20 | |
| AUS surgery | 6612 | Based on average cost of AUS in the study |
| 8465 | LB50Z Elective inpatient implantation of artificial urinary sphincter (male and female) (NHS Reference Costs)20 | |
| Antibiotics | 2 | Weekly average cost of several antibiotics to treat wound infection details in appendix (BNF)19 |
| Incontinence tablets | 4 | Weekly average cost of several incontinence medications details in appendix (BNF)19 |
| Outpatient visit | 110 | Service code 101 total outpatient attendance: average of urology department outpatient appointment (NHS Reference Costs)20 |
| Sheath | 43 | Calculated average cost per week (NHS Electronic Drug Tariff 201922) (see Appendix 3, Table 44) |
| Permanent catheter | 5 | Calculated average cost per week (NHS Electronic Drug Tariff 201922) (see Appendix 3, Table 43) |
| Removal of AUS | 5529 | Weighted average of LB21 (with a CC score of 0–1 and 2 +); elective inpatient: major open, prostate or bladder neck procedures (male) (NHS Reference Costs)20 |
| Replacement of AUS parts | 2658 | Weighted average of LB20 (with a CC score of 0 to 7 +); elective inpatient. infection or mechanical problems related to genito-urinary prostheses, implants or grafts, with interventions (NHS Reference Costs)20 |
| Partial removal of tape | 2479 | Weighted average of MA03 (with CC score of 0–1 and 2 +); elective inpatient: major open lower genital tract procedures (NHS Reference Costs)20 |
| Ultrasound scan | 54 | Imaging outpatient RD40Z diagnostic imaging: ultrasound scan with duration of < 20 minutes, without contrast |
| Computerised tomography scan | 90 | Imaging outpatient RD20A diagnostic imaging: computerised tomography scan of one area, without contrast (NHS Reference Costs)20 |
| Urodynamics | 140 | Urology LB42A outpatient procedures: dynamic studies of urinary tract, 19 years and over (NHS Reference Costs)20 |
| Flexible cystoscopy | 151 | Urology LB72A outpatient procedures: diagnostic flexible cystoscopy, 19 years and over (NHS Reference Costs)20 |
| Uroflowmetry | 151 | Urology LB42A outpatient procedures: dynamic studies of urinary tract, 19 years and over (NHS Reference Costs)20 |
| Doctor | 39 | Based on surgery consultation lasting 9.22 minutes (PSSRU18) |
| Nurse | 12 | Based on surgery consultation (15.5 minutes) (PSSRU18) |
| Physiotherapist | 12 | Based on surgery consultation (15.5 minutes) (PSSRU18) |
| Participants’ personal expenses | ||
| Paid work | 575 | Office for National Statistics 2019.23 Based on weekly median gross pay for male employee jobs |
| Pads | 0.31 | Average cost per pad |
| Bed/chair covers | 0.25 | Average cost per chair or bed protector |
| Over-the-counter medications | Several | As reported by participants |
| Prescriptions | 9 | Cost of NHS prescription in England24 |
Further resource use data were collected using participant-reported questionnaires 6 months after surgery and at 12 and 24 months post randomisation. The questionnaires captured details of contacts with primary or community health-care and social care services, prescribed medications, the use of external sheaths or permanent catheters, details of inpatient and day case admissions, and outpatient attendances for reasons related to the surgery or leaking urine. Details of further hospitalisations and treatments for leaking urine and diagnostic tests were collected through a hospital admission CRF that was completed by the recruiting site. The costs of additional sling and AUS surgery related to urinary leakage were estimated using the cost estimates from the study because there is no specific Healthcare Resource Group (HRG) for the use of slings in men. The costs of additional surgery, such as removal and replacement of AUS and excision of slings, were estimated using national tariffs.
The total costs from the health services perspective were calculated by summing all intervention treatment and follow-up costs related to the incontinence symptoms for each participant. Participants were dropped from the complete-case analysis if one of the component costs was missing because of a non-returned questionnaire. In addition, the questionnaires captured the direct non-medical costs incurred by participants, as well as the number of days off work, to inform the gross loss of earnings attributable to the participant’s health state or contacts with care providers.
Valuing resource use
Resource use was valued by attaching unit costs derived from published sources. These included the British National Formulary,19 the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 201818 and the Department of Health and Social Care’s NHS Reference Costs 2017–18. 20 Data on the price of the devices were obtained from the clinical centres . The cost of the AUS consumables consisted of the cost of the control pump, pressure-regulating balloon, cuff with Inhibizone and control system accessory kit. Health services costs were summed across categories to generate a total cost to the NHS of the intervention and the follow-up care for each trial participant. Table 13 gives a summary of the unit costs values and data sources for resource use categories.
Participant perspective costs
Participant perspective costs included out-of-pocket expenditure on incontinence containment products, such as pads and over-the-counter medications, and visits to private care providers to treat incontinence symptoms. The cost of time off work (for those in paid employment) was also included. For time off work, the appropriate national average price of 1 week of work was used (see Table 13). Data for personal incurred costs (i.e. private medical costs, over-the-counter medications and other expenses) are sourced directly from the participant questionnaires. A wider cost perspective was obtained by combining the total NHS and participant perspective costs, and is considered as a sensitivity analysis.
Estimation of quality of life
A generic instrument, the EQ-5D-3L, was used to measure quality of life. Trial participants completed the EQ-5D-3L at baseline, 6 months after their operation, and at 12 and 24 months post randomisation. Responses to the EQ-5D-3L questionnaire were translated into utility values using the UK general population tariffs, based on the time trade-off method. 25 Quality-of-life data were also collected using the SF-12 questionnaire for comparison. SF-12 data were collected at baseline and at 6, 12 and 24 months. These data were converted into a Short Form questionnaire-6 Dimensions (SF-6D) utility index using a published algorithm. 26 Respondents who died during the trial were assigned a utility of 0 at each time point following death. QALYs were derived using an area-under-the-curve approach, assuming a linear extrapolation between utility measurement time points.
Data analysis
The within-trial economic analysis was conducted following the ITT principle. Components of costs and QALYs were described with the appropriate descriptive statistics when relevant: mean and standard deviation (SD) for continuous and count outcomes, and numbers and percentages for dichotomous and categorical outcomes (e.g. numbers reporting problems on EQ-5D-3L). Analyses were conducted using Stata, version 14.1® (StataCorp LP, College Station, TX, USA). To investigate the potential for skewed cost data (i.e. a small proportion of participants incurring very high costs), we used generalised linear models (GLMs), testing alternative model specifications for appropriate fit to the data. Cost data were analysed using GLMs, with adjustment for minimisation covariates and baseline utility score. 27 The models included a cluster effect for centre. GLMs allow correction for the potential for skewed cost data (i.e. a small proportion of participants incurring very high costs) by allowing specification of an appropriate distributional family and link function to best fit the data. Different distributional families offer alternative specifications to reflect the relationship between the mean and the variance of the estimates under consideration. 28,29 The most appropriate distributional family for each analysis was determined using a modified Park test. Appropriate link functions were identified using several tests (i.e. Pearson correlation test, Pregibon link test and a modified Hosmer and Lemeshow test). The decision on the appropriate link function to use was based on which function performed best on the most of the three tests, as confirmed by examination of the respective p-values of the tests. The process followed Glick et al. 28 and was performed in Stata 14 using the user-written ‘glmdiag’ command. The incremental treatment effect on QALYs and costs was represented by the coefficient on treatment in the respective analysis models. Marginal effects are estimated on the cost and QALY scale, and CIs were calculated using the delta method. The cost data were analysed using a gamma family with a log-link. A standard linear regression (ordinary least squares) model was applied to the analysis of incremental QALY gains.
Missing data
Missing data are a frequent problem in a cost-effectiveness analysis (CEA) within a RCT. Missing costs and health utility data were imputed at each time point using fully conditional multiple imputation by chain equations, implemented through the multiple imputation by chained equations (MICE) package (run within Stata, version 14.0; https://fmwww.bc.edu/RePEc/bocode/m; accessed 5 September 2020) under the missing at random assumption. The appropriateness of the missing at random assumption was assessed by (1) investigating the missing data patterns (monotonic vs. non-monotonic) and (2) comparing the attributes of participants with and participants without missing costs and health-related quality-of-life data at each follow-up time point. Regression models were used to generate multiple imputed Q70 data sets, in which missing values were predicted drawing on predictive covariates based on randomised treatment, type of prostate surgery (radical or TURP), whether or not they have had radiotherapy in addition to surgery, age and centre. Costs and EQ-5D-3L utility scores at each time point contributed as both predictors and imputed variables. Imputations were generated using predictive mean matching drawn from the five k-nearest-neighbours (knn = 5); predictive mean matching preserves distribution of the data and is more robust to violations of the normality assumption. The multiple imputation was run 20 times, generating 20 complete data sets that reflected the distributions of and correlations between variables.
Incremental cost per quality-adjusted life-year gained
Imputed data were analysed using the appropriately specified GLMs to estimate the costs and QALYs in each treatment group over the 24 months. Joint distributions of costs and outcomes from the original data set were generated through non-parametric bootstrapping and changes in costs and QALYs were calculated for each sample. A total of 1000 bootstrap samples were drawn and means for both incremental costs and incremental QALYs (with associated 95% CIs) were calculated. Measures of variance for these costs and QALYs were derived using bootstrapping. From the results of the bootstrapping, cost-effectiveness acceptability curves (CEACs) were created. CEACs were used to represent whether or not the various interventions are cost-effective at various threshold values for society’s willingness to pay for an additional QALY. CEACs present results when the analysis follows a net benefit approach. This approach utilises a straightforward rearrangement of the cost-effectiveness decision rule used when calculating incremental cost-effectiveness ratios (ICERs; see Incremental cost-effectiveness analysis) to create the net monetary benefit. The ICER provides a ratio of cost per additional QALY gained. The net monetary benefit (NMB) of the interventions in question is equal to:
where λ represents a decision-maker’s willingness to pay for incontinence avoided or a QALY. If the above expression holds true, the intervention is considered cost-effective. Given that society’s willingness to pay is unknown, the NMB was calculated for a number of possible λ values, including the usual £20,000–30,000 for QALY values, which is a threshold often adopted by policy-makers in the NHS. 17
Sensitivity and subgroup analysis
Sensitivity analyses were performed to gauge the impact of varying key assumptions and/or parameter values in the base-case analysis. Sensitivity analyses were performed in relation to the sources used for unit costs. The base-case analysis utilised cost estimation based on the resource utilisation. The first sensitivity analysis was conducted by varying the cost of the AUS device because the price list for the slings was constant across all centres. Sensitivity analysis was also conducted using the published HRG NHS reference costs for both groups. 20 As previously stated, there is no specified HRG code for male slings; therefore, the slings were considered as major open and prostrate or bladder neck procedures. Sensitivity analyses were also conducted using the utility data derived from the SF-12. The analysis also explored the effect of not discounting the year 2 data and increasing the discount rate to 6% on costs and QALYs. Given that the base-case analysis used multiple imputation of missing data, sensitivity analyses explored the effect of conducting analyses using complete cost and QALY pairs. Analysis was completed on the complete-case data, using the regression models specified in the base case.
Subgroup analysis
Subgroup analyses were undertaken based on the:
-
type of prostate surgery that the men had – radical prostatectomy or transurethral resection of prostrate
-
volume of urine leaked per 24 hours at baseline – > 250 ml or ≤ 250 ml.
Results
Table 14 details the level of missing data by treatment group and time point. The missing data were monotonic as individuals may miss one follow-up period and return in the next one. There were more missing data at 6 months than at 12 and 24 months.
| Variable | Randomised group: missing values, n (%) | Total missing, n (%) | |
|---|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | ||
| Resource use | |||
| For the intervention | 28 (15) | 25 (13) | 53 (14) |
| 6 months | 64 (34) | 74 (39) | 138 (37) |
| 12 months | 33 (17) | 30 (16) | 63 (17) |
| 24 months | 50 (26) | 42 (22) | 92 (24) |
| Total | 105 (55) | 103 (54) | 208 (55) |
| EQ-5D-3L score | |||
| Baseline | 13 (7) | 18 (9) | 31 (8) |
| 6 months | 65 (34) | 77 (41) | 142 (37) |
| 12 months | 39 (21) | 30 (16) | 69 (18) |
| 24 months | 52 (27) | 40 (21) | 92 (24) |
| QALY EQ-5D-3L | 97 (51) | 98 (52) | 195 (1) |
| SF-6D score | |||
| Baseline | 24 (13) | 31 (16) | 55 (14) |
| 6 months | 76 (40) | 92 (48) | 168 (44) |
| 12 months | 50 (26) | 46 (24) | 96 (25) |
| 24 months | 65 (34) | 56 (29) | 121 (32) |
| QALY SF-6D | 117 (62) | 121 (64) | 238 (63) |
Resource use
Table 15 details the resource use associated with the intervention and follow-up care in slings and AUS groups.
| Resource | Randomised group | |
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Had intervention, n (%) | ||
| Sling (N = 180) | 178 (99) | 9 (5) |
| AUS (N = 175) | 2 (1) | 166 (95) |
| Surgeon, n (%) | ||
| None | 10 (5) | 15 (8) |
| Surgeon | 158 (83) | 150 (79) |
| Supervised | 22 (12) | 25 (13) |
| Anaesthetist, n (%) | ||
| None | 9 (5) | 15 (8) |
| Surgeon | 170 (90) | 161 (85) |
| Supervised | 10 (5) | 13 (7) |
| Type of anaesthesia, n (%) | ||
| None | 6 (3) | 14 (7) |
| General | 161 (85) | 159 (84) |
| General and local | 11 (6) | 9 (5) |
| General and spinal | 1 (1) | 3 (2) |
| Local | 1 (1) | 0 (0) |
| Spinal | 6 (3) | 2 (1) |
| Missing | 4 (2) | 3 (2) |
| Prophylactic antibiotic, n (%) | ||
| No | 10 (5) | 18 (10) |
| Yes | 173 (91) | 164 (86) |
| Missing | 7 (4) | 8 (4) |
| Pre-operation catheter, n (%) | ||
| No | 11 (6) | 20 (10) |
| Yes | 175 (92) | 169 (89) |
| Missing | 4 (2) | 2 (1) |
| Postoperative pain relief, n (%) | ||
| None | 38 (20) | 38 (19) |
| Oral pain relief | 128 (67) | 127 (67) |
| Parental pain relief | 13 (7) | 13 (7) |
| Missing | 11 (6) | 13 (7) |
| Theatre, n (%) | ||
| No | 9 (5) | 18 (9) |
| Yes | 180 (95) | 172 (91) |
| Missing | 1 (0) | |
| Postoperative catheter, n (%) | ||
| No | 153 (80) | 178 (94) |
| Yes | 30 (16) | 10 (5) |
| Missing | 7 (4) | 2 (1) |
| Permanent catheter, n (%) | ||
| 6 months | ||
| No | 125 (66) | 114 (60) |
| Yes | 2 (1) | 3 (2) |
| Missing | 63 (33) | 73 (38) |
| Sheath | ||
| No | 120 (63) | 115 (61) |
| Yes | 7 (4) | 2 (2) |
| Missing | 63 (33) | 73 (38) |
| Medications | ||
| No | 108 (57) | 109 (57) |
| Yes | 19 (10) | 9 (5) |
| Missing | 63 (33) | 72 (38) |
| 12 months | ||
| No | 154 (81) | 160 (84) |
| Yes | 3 (2) | 1 (1) |
| Missing | 33 (17) | 29 (15) |
| Sheath | ||
| No | 148 (78) | 156 (82) |
| Yes | 9 (5) | 5 (3) |
| Missing | 33 (17) | 29 (15) |
| Medications | ||
| No | 136 (72) | 145 (76) |
| Yes | 21 (11) | 15 (8) |
| Missing | 33 (17) | 30 (16) |
| 24 months | ||
| No | 142 (75) | 142 (75) |
| Yes | 0 (0) | 7 (5) |
| Missing | 48 (25) | 41 (22) |
| Sheath | ||
| No | 139 (73) | 147 (77) |
| Yes | 3 (2) | 2 (1) |
| Missing | 48 (25) | 41 (22) |
| Medications | ||
| No | 119 (63) | 142 (75) |
| Yes | 22 (11) | 7 (4) |
| Missing | 49 (26) | 41 (21) |
| Hospital resources, n; mean (SD) | ||
| Operation time (minutes) | 184; 96 (39) | 183; 117 (49) |
| Inpatient stay during operation (days) | 189; 1.29 (1.20) | 190; 1.39 (1.89) |
| 6 months | ||
| Practice doctor | 127; 0.74 (1.85) | 118; 0.70 (1.54) |
| Practice nurse | 127; 0.38 (1.00) | 117; 0.52 (1.51) |
| Hospital doctor | 127; 0.59 (0.99) | 118; 0.49 (1.20) |
| Hospital nurse | 126; 0.20 (0.63) | 118; 0.28 (0.98) |
| Hospital physiotherapist | 127; 0.03 (0.28) | 118; 0.12 (0.67) |
| 12 months | ||
| Practice doctor | 157; 0.95 (2.70) | 161; 0.75 (2.17) |
| Practice nurse | 157; 0.53 (1.88) | 161; 0.40 (1.16) |
| Hospital doctor | 157; 0.49 (0.96) | 161; 0.35 (0.83) |
| Hospital nurse | 157; 0.19 (0.90) | 161; 0.12 (0.46) |
| Hospital physiotherapist | 157; 0.01 (0.08) | 161; 0.00 (0.00) |
| 24 months | ||
| Practice doctor | 141; 0.43 (1.41) | 149; 0.44 (1.65) |
| Practice nurse | 142; 0.53 (1.90) | 149; 0.35 (1.33) |
| Hospital doctor | 142; 0.44 (1.00) | 149; 0.33 (1.17) |
| Hospital nurse | 142; 0.13 (0.62) | 149; 0.17 (1.12) |
| Hospital physiotherapist | 142; 0.06 (0.68) | 149; 0.00 (0.00) |
| Medication | 141; 0.43 (1.41) | 149; 0.44 (1.65) |
Intervention
Among those who received the intervention, 178 (98%) participants in the sling group received the treatment that they were allocated to and two (1%) received AUS. In the AUS group, 166 (95%) received their allocated treatment and nine (5%) received slings. The intervention time for the male sling group was 96 minutes (SD 39 minutes), compared with 117 minutes (SD 50 minutes) for the AUS group. The rest of the resource use for the initial surgery was similar across both groups.
Follow-up care
The number of further operations is reported in Table 5 (see Chapter 3). The remainder of the follow-up resource utilisation, the use of catheters, sheaths, visits to primary and secondary health-care providers and use of medications, was similar across the groups and there was no statistically significant difference between the two groups (see Table 15).
Intervention and follow-up costs
The differences in costs reflect the differences reported in resource use. Details of costs are reported in Table 16. The cost of the intervention was, on average, lower for the male sling group than for the AUS group. Most of the differences in costs for the intervention components were statistically significantly lower for the male sling group than the AUS group, apart from the theatre, medications and inpatient stay. The main driver of the intervention cost savings for the male sling group was the difference in device costs. Overall, the total cost of the initial intervention was significantly lower in the male sling group than in the AUS group (–£2477, 95% CI –£2935 to –£2017). These findings are based on the complete-case data. The follow-up costs at 6, 12 and 24 months were not significantly different between the groups. However, over the 24-month follow-up, the total NHS perspective costs were lower for the male sling group than for the AUS group (–£2866, 95% CI –£3723 to –£2008).
| Resource | Randomised group, n; mean (£) (SD) | Incremental costs (£),a mean (95% CI) | |
|---|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | ||
| Surgeon | 184; 184 (79) | 183; 226 (99) | –50 (–68 to –33) |
| Anaesthetist | 184; 180 (77) | 183; 221 (99) | –50 (–68 to –32) |
| Nurses | 184; 174 (71) | 183; 206 (93) | –40 (–60 to –20) |
| Anaesthesia | 190; 20 (6) | 190; 20 (7) | 0 (–5 to 2) |
| Prophylactic antibiotic | 183; 1 (0) | 182; 1 (0) | 0 (0) |
| Device (sling/AUS) | 189; 2185 (565) | 190; 4056 (1282) | –1980 (–2159 to –1801) |
| Device activation (AUS) | 189; 1 (11) | 190; 94 (39) | –99 (–104 to –93) |
| Pre-operation catheter | 186; 2 (1) | 189; 2 (1) | 0 (0) |
| Pain relief | 179; 0 (0) | 177; 0 (0) | 0 (0) |
| Theatre | 184; 1189 (486) | 183; 1444 (616) | –306 (–425 to 187) |
| Postoperative catheter | 183; 0 (1) | 188; 0 (0) | 0 |
| Medication | 181; 8 (37) | 186; 3 (24) | 5 (–2 to 11) |
| Inpatient stay | 189; 532 (494) | 190; 578 (778) | –47 (–205 to 111) |
| Total operation cost | 162; 4374 (1295) | 165; 6753 (2395) | –2659 (–3259 to –2059) |
| 6 months | |||
| Permanent catheter | 127; 1 (8) | 117; 2 (10) | 0 (–3 to –2) |
| External sheath | 127; 28 (117) | 117; 9 (66) | 19 (–11 to 49) |
| Practice doctor | 127; 29 (72) | 118; 28 (60) | 4 (–14 to 24) |
| Practice nurse | 127; 5 (12) | 117; 6 (18) | 0 (–4 to 3) |
| Hospital doctor | 127; 65 (110) | 118; 55 (132) | 17 (–15 to 50) |
| Hospital nurse | 126; 2 (8) | 118; 3 (12) | 0 (–3 to 2) |
| Hospital physiotherapist | 127; 0 (3) | 118; 1 (8) | 0 (–2 to 1) |
| Medication | 126; 7 (17) | 118; 4 (13) | 4 (–0.3 to 7) |
| Total 6-month cost | 126; 138 (213) | 116; 108 (234) | 23 (–32 to 117) |
| 12 months | |||
| Permanent catheter | 157; 2 (18) | 161; 1 (10) | 1 (–2 to 5) |
| External sheath | 157; 15 (60) | 161; 8 (44) | 5 (–7 to 17) |
| Practice doctor | 157; 37 (105) | 161; 29 (85) | 5 (–20 to –30) |
| Practice nurse | 157; 6 (22) | 161; 5 (14) | 1 (–2 to 5) |
| Hospital doctor | 157; 54 (105) | 161; 38 (91) | 15 (–7 to 38) |
| Hospital nurse | 157; 2 (11) | 161; 1 (5) | 0 (–2 to 3) |
| Hospital physiotherapist | 157; 0 (1) | 161; 0 (0) | 0 (0) |
| Medication | 157; 13 (32) | 160; 9 (28) | 4 (–3 to 12) |
| Further surgery | 159; 703 (2007) | 155; 256 (1297) | 548 (82 to 1013) |
| Total 6- to 12-month cost | 155; 326 (1067) | 154; 281 (1438) | 27 (–195 to 251) |
| Total 12-month follow-up costs | 95; 5421 (2115) | 95; 7802 (1813) | –2242 (–2751 to –1732) |
| 12 to 24 months | |||
| Permanent catheter | 142; 0 (0) | 149; 13 (57) | –11 (–20 to 2) |
| External sheath | 142; 11 (71) | 149; 6 (57) | 4 (–12 to 19) |
| Practice doctor | 141; 16 (53) | 149; 17 (62) | –2 (–17 to 13) |
| Practice nurse | 142; 6 (22) | 149; 4 (15) | 2 (–4 to 8) |
| Hospital doctor | 142; 47 (106) | 149; 35 (124) | 10 (–14 to 34) |
| Hospital nurse | 142; 1 (7) | 149; 2 (13) | 0 (–3 to 2) |
| Hospital physiotherapist | 142; 1 (7) | 149; 0 (0) | 0 (0 to 2) |
| Medication | 141; 28 (66) | 149; 9 (39) | 20 (7 to 32) |
| Further surgery | 163; 190 (1021) | 157; 186 (1377) | –3 (–200 to 194) |
| Total 12- to 24-month costb | 139; 326 (1133) | 144; 289 (1441) | 18 (–246 to 282) |
| Total 24-month follow-up costs | 85; 5674 (2862) | 86; 8578 (4463) | –2866 (–3723 to –2008) |
Private health-care costs
A further analysis incorporated participants’ costs into the analysis over the 24-month period. Table 17 reports mean costs from a wider economic perspective. Like the base-case analysis, there were a lot of missing data and the reported summaries represent all available data. The main drivers of the private costs were the income lost, measured in the number of days off work, and the cost of pads. Overall, the participants in both groups had similar resource use over the follow-up period, apart from the use of pads and visits to the nurse, which were statistically significantly higher in the male sling group than in the AUS group.
| Resource | Randomised group, n; mean (£) (SD) | Incremental costs (£),a mean (95% CI) | |
|---|---|---|---|
| Male sling | AUS | ||
| Pads | 69; 291 (241) | 80; 221 (182) | 72 (3 to 141) |
| Chair/bed sheets | 87; 35 (135) | 95; 29 (93) | 3 (–33 to 38) |
| Private doctor | 88; 37 (74) | 92; 21 (55) | 18 (–1 to 37) |
| Private nurse | 81; 10 (38) | 90; 1 (7) | 9 (0 to 19) |
| Private physiotherapist | 115; 0 (0) | 112; 5 (58) | 0 |
| Medication | 62; 2 (10) | 69; 11 (43) | –11 (–24 to 2) |
| Other private care | 59; 0 (3) | 63; 159 (1260) | –124 (–399 to 150) |
| Income lost | 77; 401 (1235) | 86; 665 (2312) | –119 (–640 to 402) |
| Total private cost | 32; 924 (1643) | 42; 967 (2917) | 256 (–442 to 954) |
Quality-of-life measures
Table 18 provides descriptive data of the mean utility scores and QALYs generated by combining utilities with the duration of follow-up. The differences are based on ordinary least squares models, with adjustment for minimisation covariates and baseline EQ-5D-3L score. The utility scores derived using the EQ-5D-3L indicate that the quality-of-life score at 6 months was the same as at baseline for the sling group and higher for the AUS group, but declined at 12 months for both groups and improved for both groups at 24 months. There was no statistical difference in the 24-month QALY scores between the two groups. The utility scores derived using the SF-6D were higher at 6 months, but reduced at 12 and 24 months for the sling group and reduced at only 24 months for the AUS group. There was no statistically significant difference in the 24-month QALY scores based on the complete data. The visual analogue scale scores were highest at 6 months and lower at 12 and 24 months in both groups.
| Score | Randomised group, n; mean (SD) | Incremental QALYs,a mean (95% CI) | |
|---|---|---|---|
| Male sling | AUS | ||
| EQ-5D-3L | |||
| Baseline | 177; 0.823 (0.22) | 172; 0.814 (0.24) | |
| 6 months | 125; 0.822 (0.24) | 112; 0.827 (0.27) | –0.025 (–0.07 to 0.02) |
| 12 months | 151; 0.809 (0.26) | 160; 0.802 (0.29) | –0.014 (–0.07 to 0.04) |
| 24 months | 136; 0.820 (0.23) | 147; 0.828 (0.26) | 0.002 (–0.05 to 0.06) |
| QALYs | 92; 1.680 (0.38) | 92; 1.641 (0.46) | –0.016 (–0.08 to 0.05) |
| EQ-VAS | |||
| Baseline | 178; 72.88 (21.09) | 174; 71.91 (22.98) | |
| 6 months | 125; 74.54 (21.45) | 117; 77.60 (17.20) | –4.04 (–7.20 to –0.81) |
| 12 months | 157; 74.49 (21.58) | 157; 77.56 (17.12) | –3.86 (–7.33 to –0.39) |
| 24 monthsb | 141; 74.61 (19.06) | 145; 77.03 (18.25) | –3.40 (–6.60 to –0.19) |
| SF-6D | |||
| Baseline | 166; 0.729 (0.14) | 159; 0.743 (0.15) | |
| 6 months | 114; 0.760 (0.14) | 97; 0.773 (0.14) | –0.244 (–0.055 to 0.006) |
| 12 months | 140; 0.744 (0.15) | 142; 0.773 (0.15) | –0.023 (–0.059 to 0.013) |
| 24 monthsb | 123; 0.725 (0.15) | 134; 0.746 (0.15) | –0.003 (–0.033 to 0.026) |
| QALYs | 72; 1.503 (0.25) | 68; 1.550 (0.23) | –0.053 (–0.127 to 0.021) |
The proportion of men who reported any health problems on the EQ-5D-3L domains is illustrated in Figures 6–9. The descriptive data are based on all available data reported at all of the time points at which data were collected. They show the percentage of men who reported a problem on each of the domains of quality of life (i.e. a ‘2’ or ‘3’).
FIGURE 6.
Percentage of men experiencing any problems, by randomised group (baseline).
FIGURE 7.
Percentage of men experiencing any problems, by randomised group (6 months).
FIGURE 8.
Percentage of men experiencing any problems, by randomised group (12 months).
FIGURE 9.
Percentage of men experiencing any problems, by randomised group (24 months).
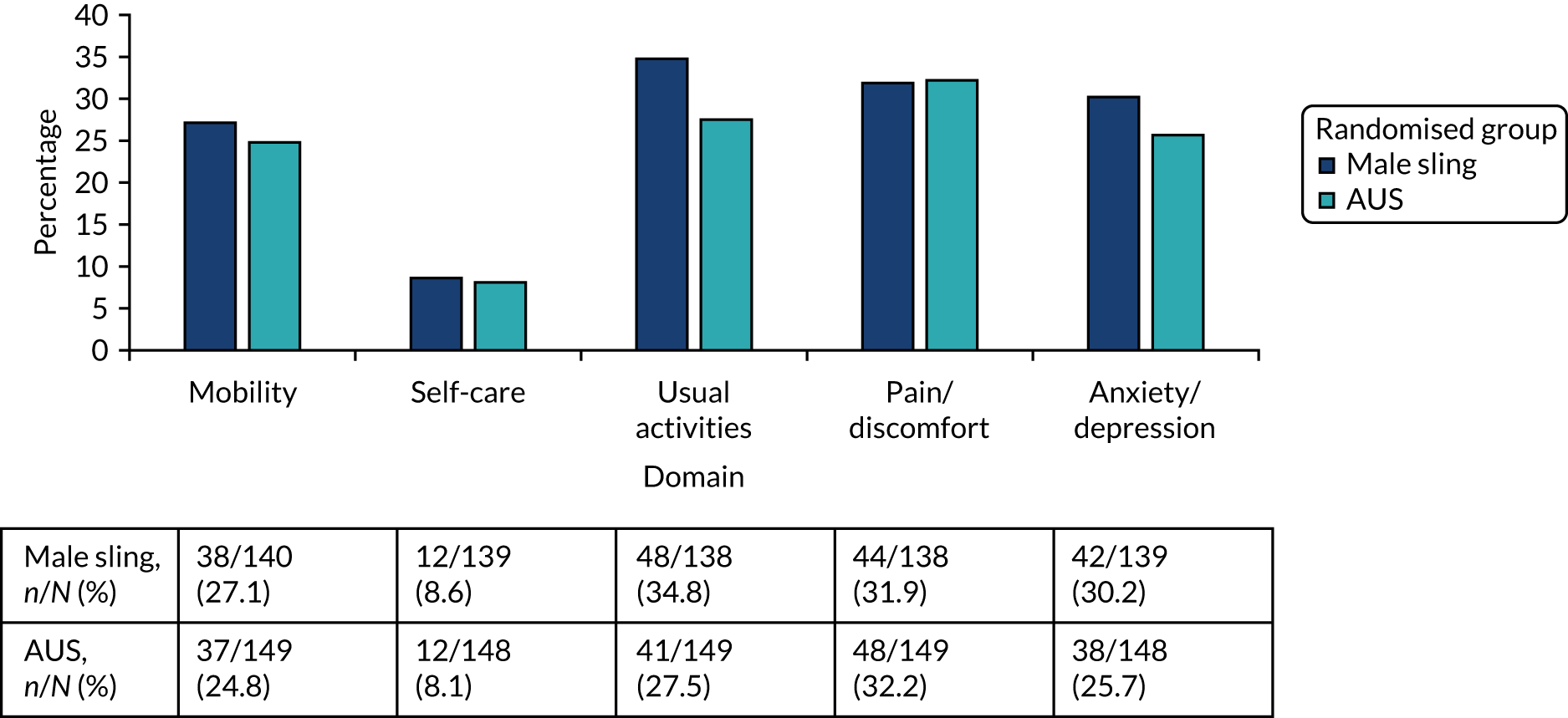
The domain with the lowest percentage of men with problems was self-care. More men seemed to experience more problems with all of the domains at 12 and 24 months than at 6 months.
The male sling group reported statistically significantly lower (worse) EuroQol-Visual Analogue Scale (EQ-VAS) scores than the AUS group over the different time points. The EQ-VAS scores ranged from 73 to 77. They also reported lower SF-6D utility scores at all of the different time points and lower SF-6D QALYs (–3.40, 95% CI –6.60 to –0.19) than the AUS group. The figures of EQ-VAS and SF-6D scores are presented in Appendix 3, Figures 16 and 17.
Incremental cost-effectiveness analysis
Results of the within-trial analysis are reported as ICERs, calculated as the difference in costs divided by the differences in QALYs (male slings vs. AUS) over the 24-month follow-up. Although the perspective of the primary analysis is the NHS, we have also included the costs to the individuals for containment products, namely pads, chair and mattress protectors and medications, as well as visits to private health-care providers as a sensitivity analysis. All sensitivity analyses apart from the complete-case analysis were conducted using multiple imputation methods.
Base-case analysis
The base-case results in Table 19 show significantly lower costs for the male sling group (£2497, 95% CI –£3167 to –£1875) and not significantly fewer QALYs (–0.006, 95% CI –0.06 to 0.054). This low uncertainty in the costs is illustrated in Figure 10, with the cost values lying below the horizontal axis. There is some uncertainty in the QALYs because some of the values are found on the right side of the vertical axis. The CEAC shown in Figure 11 illustrates that there is a high probability that male slings are the most cost-effective intervention. The probability that slings will be considered cost-effective is 99% at the £30,000 willingness-to-pay threshold for a QALY.
| Cost (£) | Δ Cost (£) | Effect | Δ Effect | ICER (male slings vs. AUS) (Δ cost/Δ effect) (£) | Probability cost-effective at alternative threshold values for WTP for a QALY gain (%) | ||
|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | ||||||
| Base-case analysis | |||||||
| Male sling | 5513 | 1.613 | 1 | 0.99 | |||
| AUS | 8010 | –2497 | 1.619 | –0.006 | 425,870 | 0 | 0.01 |
| Complete-case analysisa | |||||||
| Male sling | 5480 | 1.659 | 1 | 0.97 | |||
| AUS | 8209 | –2745 | 1.667 | –0.008 | 339,780 | 0 | 0.03 |
| SF-6D results | |||||||
| Male sling | 5513 | 1.464 | 1 | 0.99 | |||
| AUS | 8010 | –2497 | 1.484 | –0.019 | 128,043 | 0 | 0.01 |
| HRG costs | |||||||
| Male sling | 5385 | 1.606 | 1 | 1 | |||
| AUS | 8793 | –3407 | 1.621 | –0.015 | 222,986 | 0 | 0 |
| Societal costs | |||||||
| Male sling | 6221 | 1.609 | 0.99 | 0.96 | |||
| AUS | 8448 | –2226 | 1.624 | –0.015 | 152,439 | 0.01 | 0.04 |
| Lowest AUS cost | |||||||
| Male sling | 5538 | 1.621 | 0.96 | 0.84 | |||
| AUS | 7052 | –1513 | 1.636 | –0.015 | 100,871 | 0.04 | 0.16 |
| Undiscounted 2-year data | |||||||
| Male sling | 5553 | 1.621 | 1 | 0.97 | |||
| AUS | 7913 | –2360 | 1.636 | –0.015 | 154,965 | 0 | 0.03 |
| Six per cent discount | |||||||
| Male sling | 5530 | 1.597 | 1 | 0.98 | |||
| AUS | 7890 | –2360 | 1.612 | –0.015 | 160,178 | 0 | 0.02 |
| Subgroup analysis for those who had TURP | |||||||
| Male sling | 7441 | 1.630 | Dominant | 0.84 | 0.78 | ||
| AUS | 8364 | –924 | 1.627 | 0.003 | 0.16 | 0.22 | |
| Volume of urine ≤ 250 ml | |||||||
| Male sling | 5447 | 1.599 | 0.38 | 0.36 | |||
| AUS | 5607 | –161 | 1.621 | –0.021 | 7315 | 0.62 | 0.64 |
| Volume of urine > 250 ml | |||||||
| Male sling | 7853 | 1.616 | 0.45 | 0.43 | |||
| AUS | 7869 | –16 | 1.627 | –0.011 | –1462 | 0.55 | 0.57 |
FIGURE 10.
Scatterplot of incremental costs and QALYs for slings compared with AUS using EQ-5D-3L quality-of-life score.
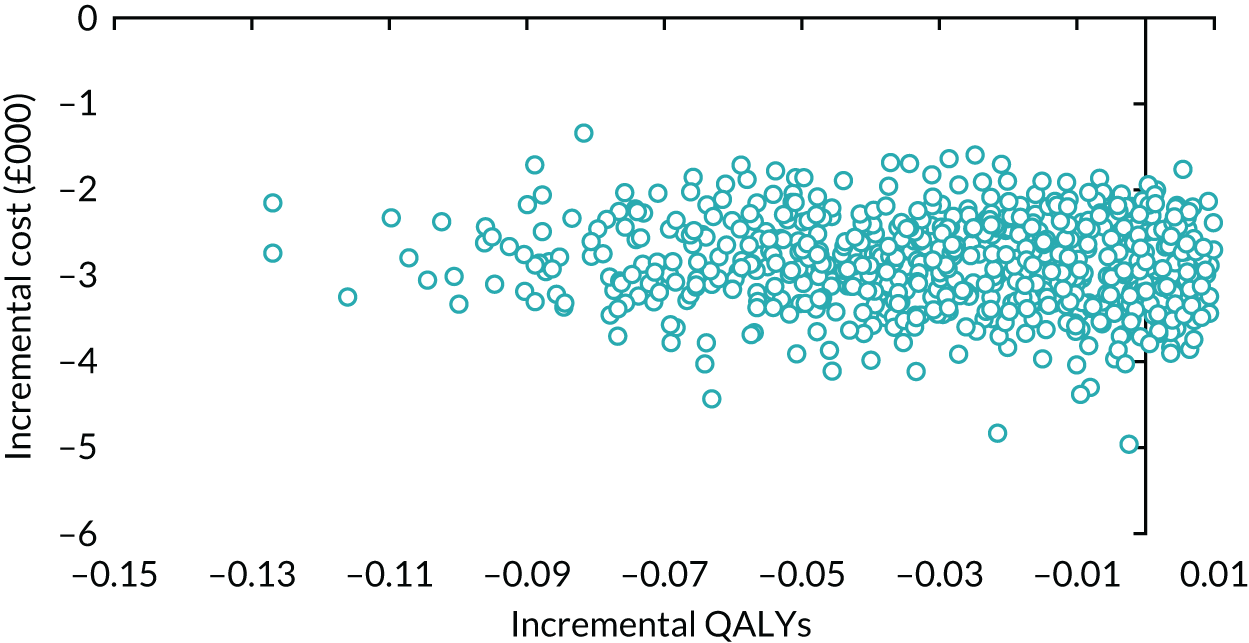
FIGURE 11.
Cost-effectiveness acceptability curves: male sling vs. AUS using EQ-5D-3L quality-of-life scores.
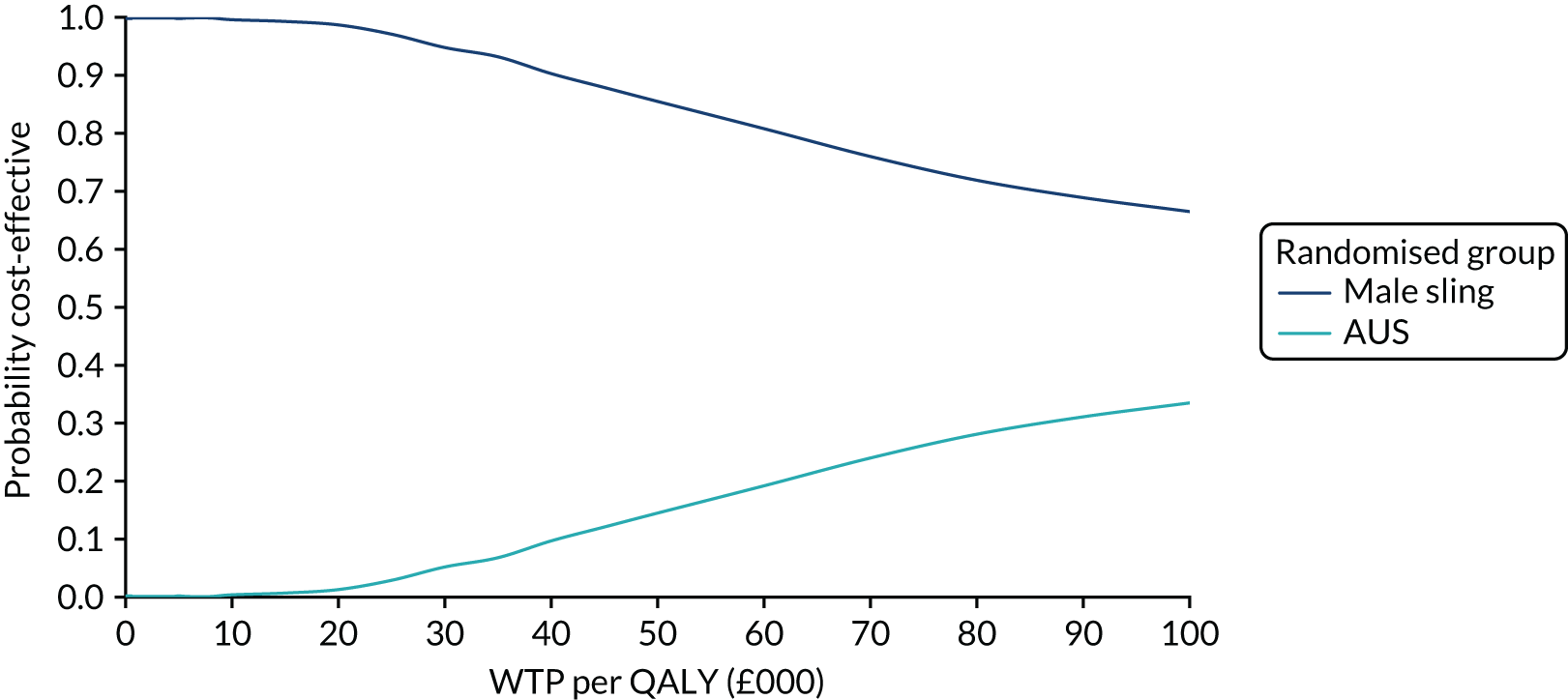
Sensitivity analysis
None of the findings is sensitive to the type of cost data used. The magnitude and direction of the cost and QALY difference remained the same and the probability that slings were cost-effective at the £30,000 willingness-to-pay threshold for an additional QALY ranged between 90% and 100%. The results of the subgroup analysis of men who had TURP indicate that slings were dominant because they cost less and were more effective than AUS. The results for the volume of urine leaked per 24 hours at baseline (> 250 ml vs. ≤ 250 ml) both had lower non-significant cost differences between the two groups, the ICERs were lower and the probability that slings would be considered to be cost-effective reduced to 36% and 43% at the £30,000 willingness to pay for an additional QALY. The scatterplot of incremental costs and QALYs and CEACs of the sensitivity analyses are reported in Appendix 3.
Discussion
Summary of the findings
For the base-case analysis, male sling group costs were lower (£2497) than those for AUS. This cost difference was mainly driven by the substantially more expensive AUS kits and the average time spent in theatre for the operation for the AUS group. Although the total costs at the various follow-up times were higher for the male sling group than for the AUS group, they were not statistically significant and did not have much of an impact on the overall total cost. On average, the male slings provided a lower QALY benefit (0.006) than AUS; however, this was not statistically significant. The very high cost difference and low QALY difference led to a high ICER of £425,870. Given that, on average, slings cost less and have fewer QALYs than AUS, the ICER indicates cost savings that will be accrued for loss of QALYs. According to Dowie et al. ,30 the case for adopting an intervention that costs less and has fewer QALYs becomes progressively stronger as the saving from the loss of a QALY increases.
Several deterministic sensitivity analyses were performed to address the areas of uncertainty in data collection. All of these analyses apart from the complete-case analysis were conducted using the imputed data set. The findings were not sensitive to the type of data used and were similar to the base-case analysis. It is reassuring that the results and conclusions of the imputed and the complete-case data did not differ. The probability of slings being cost-effective at a threshold of £20,000–30,000 in both data sets was > 95%. However, the subgroup analysis for men who had TURP showed that the slings were dominant because they had lower costs and higher QALYs than AUS. Data for the men who had a radical prostatectomy were too few to undertake any meaningful analysis. The results for the volume of urine leaked per 24 hours at baseline, > 250 ml compared with ≤ 250 ml, both reported lower ICERs than the baseline analysis.
As mentioned in Missing data, missing data are always a problem for the CEA. The results for those with complete data reported in Table 19 were derived from less than half of the participants (male sling group, n = 78; AUS, n = 75) because some participants’ data were missing at certain time points. Therefore, the base-case analysis was based on imputed data.
The estimates of incremental costs were sensitive to the method used to derive the intervention costs. Despite the fact that there is no HRG for the male slings, Office of Population Censuses and Surveys (OPCS) codes were used to map the costs to HRGs and the cost difference increased from £2497 to £3432 and the probability that slings would be considered cost-effective increased to 100%. When the lowest AUS price was used to estimate the costs of the intervention, the cost difference reduced to £1470 and the probability that slings would be considered cost-effective at the £30,000 willingness-to-pay threshold for an additional QALY reduced to 90%.
Health economics modelling analysis
Little is known about the long-term outcomes of slings in men because follow-up of men is modest but growing. A de novo economic model that considered a longer time horizon was developed to provide additional information for policy-makers.
Methods
Model type
A long-term Markov cohort decision analysis model was developed in TreeAge Pro™ 2018 software (TreeAge Pro 2018, R1.0. TreeAge Software, Williamstown, MA; www.treeage.com) to represent the treatment pathway for men with SUI. The model was developed to represent progression through the pathway of care. A cohort of 1000 men, with an average age of 67 years (trial baseline population), who were suffering from USI requiring surgical intervention enter the model and receive either a sling or an AUS and follow a treatment pathway based on risk of requiring further surgery. A maximum of three surgeries was modelled.
The structure of the model was developed in collaboration with clinicians and trial collaborators. The model allows the cohort to have further surgery for USI in the following sequence: (1) AUS/slings for the sling group and AUS only for the AUS group. The treatment pathway described in Figure 12 is identical for both model groups, with the only difference being that a proportion of the sling group could receive a sling. The base-case analysis model was run for a maximum of 20 years to take into account the life expectancy of a 67-year-old male in the UK. 31 The cycle length was 1 year and a half cycle correction was applied to the model. Cost and QALYs experienced after the first year were discounted at the recommended 3.5%. 17
FIGURE 12.
Model structure.
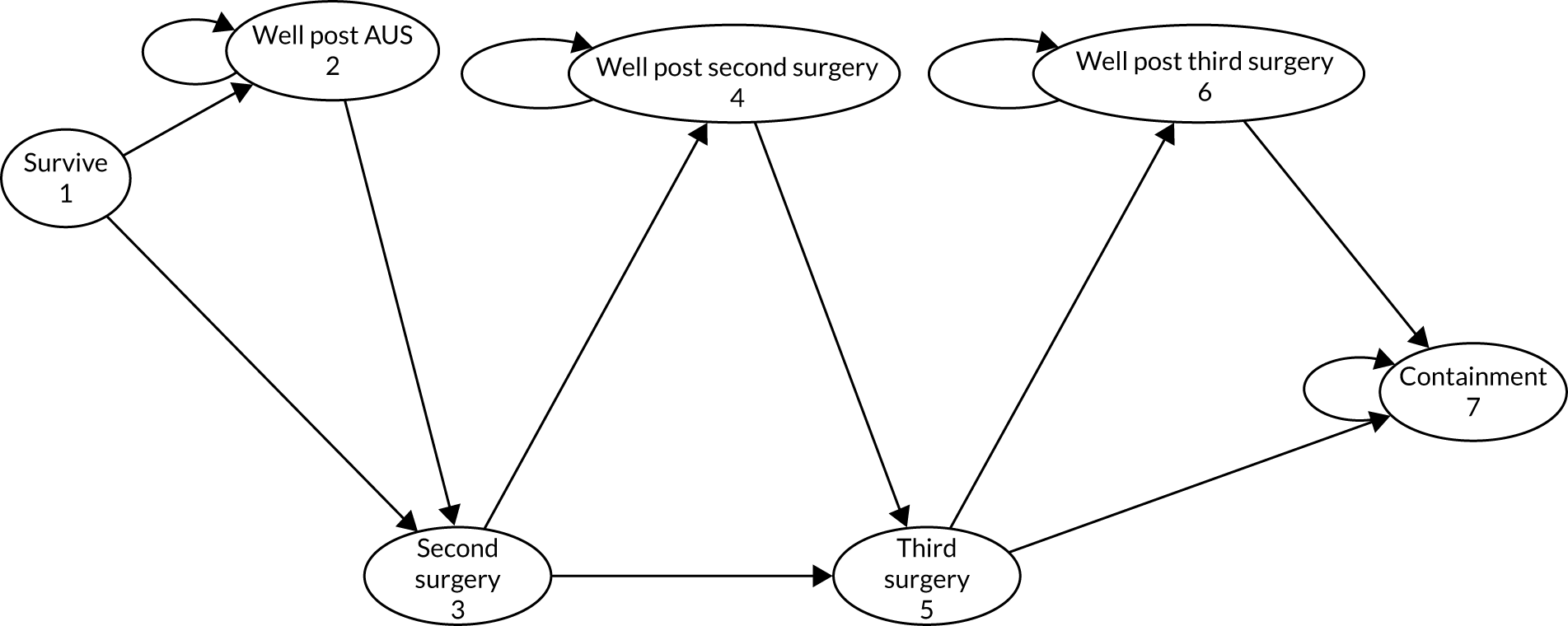
After each surgical procedure, the cohort can enter into one of four mutually exclusive states:
-
Well post surgery. This reflects the proportion of the cohort in any given cycle who do not require any further surgery for USI or complications, or who die naturally. They may, however, still experience some USI symptoms or other complications that do not require hospitalisation. Once entering this state, the cohort remains there over successive model cycles until they experience failure or death.
-
Complications. A proportion of the cohort can enter the complications state in the model following surgery, reflecting that men may experience complication risks in the short term following SUI surgery. The complications state reflects major complications that require hospitalisation or further surgery, such as failure of the device. The duration of the complications state is 3 months, reflecting that the complications will be identified early and resolved quickly to enable the patient to return to the well state. As the cohort progresses through successive surgeries for failures, each complication state relates to the most recent surgical procedure. For example, it is assumed that complications following second surgery relate to the second surgery.
-
Failure. Progression of the cohort along the sequence of surgeries is triggered by failure, as described in Figure 12, that is the need for repeat surgery is considered an indication of failure.
-
Containment. The proportion of the cohort having progressed through all surgical options described and still experiencing symptoms indicative of treatment failure is assumed to be managed conservatively and not to receive further surgical intervention. Conservative management includes the use of containment products.
-
Dead. The modelled cohort may die at any point in the model, following general population all-cause mortality rates. No additional surgical mortality rate is included because no one died during the surgical procedures.
Identification of model parameters
The parameter estimates for probability of failure and complications, and costs and utilities were derived mainly from the trial data. Data were sought from published studies to inform treatment failure and complication rates, but no data were identified that could be used to inform the model parameters, particularly the sling group. The model requires four main sets of parameters: (1) transition probabilities between health states, (2) treatment effect of the interventions, (3) quality-of-life data and (4) health-care costs. These data were based on the trial data and follow guidelines for good practice. 32
Treatment failures
Treatment failures varied by procedure and were based on the number of participants that had further surgery for USI symptoms and received either a sling or an AUS. Data were available for each year of the trial follow-up period (Table 20). There were 16 failures in the sling group in the first year and four in the second year. There was one failure in the AUS group in the first year and two failures in the second year. Uncertainty surrounding the treatment failure probabilities was incorporated into the model by using a beta distribution. The lack of data meant that the trial data were not extrapolated using survival models. The failure rate for the follow-up years was assumed to be that experienced in the second year.
| Variable | Point estimate | Comments |
|---|---|---|
| AUS fail: year 1 | 0.006 | Based on 1/175 participants who had surgery owing to the return of USI symptoms in the first year |
| AUS fail: year 2 | 0.011 | Based on 2/175 participants who had surgery owing to the return of USI symptoms in the second year |
| Sling fail: year 1 | 0.089 | Based on 16/180 participants who had surgery owing to the return of USI symptoms in the first year |
| Sling fail: year 2 | 0.022 | Based on 4/180 participants who had surgery owing to the return of USI symptoms in the second year |
| AUS complications: year 1 | 0.034 | Based on 6/175 participants who had surgery owing to complications in the first year |
| AUS complications: year 2 | 0.011 | Based on 2/175 participants who had surgery owing to complications in the second year |
| Sling complications: year 1 | 0.011 | Based on 2/180 participants who had surgery owing to complications in the first year |
| Sling complications: year 2 | 0.006 | Based on 1/180 participants who had surgery owing to complications in the second year |
Complications
The process for incorporating complications into the model was identical to that described for failures requiring further surgery. For the purposes of the transition probabilities, complications are defined here as any complication that required admission to hospital or further surgery. There were two complications that needed hospitalisation in the first year and two in the second year in the sling group (see Table 20). There were six complications that needed hospitalisation in the first year and the two in the second year in the AUS group. Uncertainty surrounding the treatment complication probabilities was incorporated into the model by using a beta distribution. The complication rate for the long-term follow-up was assumed to be that of the second year.
Mortality parameters
As men move through the model, the chance that they might die is based on the annual rates of age-specific all-cause mortality for men (Office for National Statistics interim life tables). 33 The cohort can die from any other model health state. It was assumed that there was no excess mortality for men related to their cancer procedure over and above general population all-cause mortality.
Resource use and costs
The costs of slings, AUSs and other interventions are based on the component costing approach used for the within-trial CEA. Costs for the remaining health states (i.e. failures, complications and well) are obtained from the trial data. Definitions of failures and complications are as described in the previous section for obtaining state transition probabilities. Men who were not categorised as having complications or failures were categorised as being in the ‘well’ health state. This does not mean that these men did not have USI symptoms and, therefore, the cost of the ‘well’ state implies that a proportion of the cohort have costs of consultations with health professionals and treatments based on the complete-case data reported in the trial. No additional costs were included to take into account the shelf life of the devices. Ordinary least square regression models were used to determine the impact of health state on costs. The health state dummy variables were interacted with treatment group to obtain specific health state costs where data were available to generate the mean and SD data. The base-case analysis uses treatment-specific health state costs and the sensitivity analysis considers state-specific costs that are averaged across all men experiencing a health state, regardless of randomised group in the studies. Costs obtained from the trial data reflect the annual cycle of the model. Exceptions to this include full costs applied for complication state at the point of entry to capture the full resource impact of high-cost surgical/hospitalisation procedures. The costs applied to failure after the third surgery were based on the average annual cost of pads.
All cost parameters are defined in the model as statistical distributions, assumed to follow a gamma distribution. Table 21 reports the mean, standard error (SD of the sampling distribution) and the alpha and beta parameters used to define the gamma distributions.
| Variable | Point estimate (£) | Robust standard errors (£) | Parameter | |
|---|---|---|---|---|
| Alpha | Beta | |||
| Initial sling surgery | 4631 | 61 | 5763 | 1.245 |
| Initial AUS surgery | 7422 | 93 | 6521 | 0.878 |
| Sling failure | 6903 | 295 | 1134 | 0.164 |
| AUS failure | 7562 | 295 | 168 | 0.09 |
| Sling complication | 5345 | 2923 | 3.34 | 6.26 |
| AUS complication | 7685 | 4495 | 2.92 | 3.80 |
| Sling well: year 1 | 198 | 29 | 45 | 0.23 |
| AUS well: year 1 | 182 | 28 | 40 | 0.22 |
| Sling well: year 2 | 249 | 58 | 18 | 0.07 |
| AUS well: year 2 | 89 | 56 | 3 | 0.03 |
Utilities
Health state utilities were derived for the health states: failure, complications and well. The utility measure reflects the utility of that state specific to each man in the trial. Sensitivity analysis explores the impact of using health state utilities that are not treatment specific. All utility data are included in the model as statistical distributions. The utilities accrued in each health state reflect the annual cycle of the model. Uncertainty in utility parameters is characterised and incorporated by sampling from beta distributions for the utility of each modelled health state. Alpha and beta parameters are calculated using the method of the moments approach and the parameters of the distribution are presented in Table 22.
| Utility state | Health state utility value | Standard error | Parameter | |
|---|---|---|---|---|
| Alpha | Beta | |||
| Sling: baseline | 0.823 | 0.017 | 414 | 89 |
| AUS: baseline | 0.814 | 0.018 | 380 | 87 |
| Sling failure: year 1 | 0.696 | 0.020 | 376 | 160 |
| AUS failure: year 1 | 0.673 | 0.042 | 83 | 41 |
| Sling failure: year 2 | 0.696 | 0.020 | 376 | 160 |
| AUS failure: year 2 | 0.673 | 0.042 | 83 | 41 |
| Sling complications: year 1 | 0.541 | 0.043 | 72 | 61 |
| AUS complications: year 1 | 0.366 | 0.031 | 88 | 152 |
| Sling complications: year 2 | 0.405 | 0.047 | 44 | 64 |
| AUS complications: year 2 | 0.529 | 0.027 | 180 | 161 |
| Sling well: year 1 | 0.852 | 0.021 | 241 | 42 |
| AUS well: year 1 | 0.843 | 0.021 | 251 | 47 |
| Sling well: year 2 | 0.823 | 0.022 | 244 | 52 |
| AUS well: year 2 | 0.839 | 0.021 | 256 | 49 |
Cost-effectiveness analysis
The model analysis was conducted using second-order Monte Carlo (probabilistic or second order) simulation with 1000 iterations. Each iteration resampled a value from each input parameter based on the defined distributions. The model was probabilistic, with 1000 expected values of costs and QALYs generated for each treatment strategy from the sampling distributions. Cost and QALY values were combined into a single measure of efficiency and reported as incremental costs per QALY gained, commonly referred to as the ICER. ICERs can have either negative or positive signs. ICERs with negative signs are easy to interpret. They suggest that the new intervention is either dominant or is dominated. Interventions that generate cost savings and higher QALYs than a comparator offer an even stronger case for cost-effectiveness. On the other hand, interventions that are more costly and generate fewer QALYs than a comparator are said to be dominated and are not considered a cost-effective use of resources. Positive ICERs that are more effective and more costly are assessed based on the maximum acceptable willingness-to-pay threshold. Interventions reporting an ICER of £20,000 to £30,000 per QALY gained are generally considered cost-effective. Conversely, interventions that cost more and generate fewer QALYs than a comparator are said to be dominated and are not considered a cost-effective use of resources. Sometimes positive ICERs are also derived when an intervention costs less but is less effective than the comparator. In this case, low values indicate a low probability of cost-effectiveness.
Cost-effectiveness acceptability curves were generated by assessing the probabilistic net monetary benefit for each treatment strategy. The probability that each strategy is cost-effective at alternative threshold values of willingness to pay for a QALY gain was calculated using the CEACs. Similarly, scatterplots of incremental costs and incremental QALYs were obtained for the male sling compared with the AUS and report the results on the cost-effectiveness plane. Both CEACs and the scatterplots illustrate the uncertainty surrounding the optimal treatment strategy caused by the combined statistical variability in the model’s parameter estimates.
Deterministic sensitivity analysis
Although the CEACs illustrate sampling uncertainty, they do not address underlying uncertainty driven by choice of data, structural assumptions, methodological choices or heterogeneity across subgroups of the modelled cohorts. Several deterministic sensitivity analyses were conducted to address the main areas of uncertainty in the model and to test the effect of the assumptions on the cost-effectiveness conclusions. All deterministic analyses are subjected to the same sampling uncertainty as described for the base case, ensuring that all presented ICERs are fully probabilistic apart from the one-way sensitivity ICER. Analyses were conducted to explore uncertainty surrounding the choice of model utilities and costs.
The base-case analysis uses treatment-specific utilities and costs for each health state in the model. These data are advantageous in that they preserve the impact of treatment on the costs and quality of life in any given health state. However, for rare events, such as the complications in our data, they were derived from trial data with a follow-up period that registered many events. Sensitivity analysis explores the impact of using utilities and costs that were defined for each modelled health state, but not split according to randomised treatment in the trials. This increases the sample for estimating costs and utilities but removes any assumption about treatment-specific effects on these parameters for the model.
Sensitivity analysis was also undertaken to determine the impact of methodological uncertainty on the results. Long-term data on the time to failure and complications are lacking and, therefore, estimates have been developed for the model based on the probability of events. The impact of any modelling inaccuracies on cost-effectiveness is likely to be magnified over longer-term time horizons. Sensitivity analyses, therefore, shortened the time horizon to 5 and 10 years.
Sensitivity analysis was also conducted to determine the impact of methodological uncertainty surrounding the appropriate discount rate to apply to future costs and QALYs. Sensitivity analyses vary the discount rate between 0% and 6% in line with NICE best practice guidelines. 17
Cost-effectiveness results
The base-case analysis over a 20-year time horizon indicates that the sling group had lower costs (£1511, 95% CI –£4597 to £5577) but also fewer QALYs (0.133, 95% CI –0.782. to 0.488) than the AUS group. The ICER is £11,385, which means that for each QALY lost there is a cost saving of £11,385. These are reported in Table 23. The scatterplot of cost-effectiveness is reported in Figure 13. The base-case CEAC illustrating uncertainty is reported in Figure 14. The results indicate that the probability that slings would be considered cost-effective at the £30,000 willingness to pay for a QALY threshold is 42%.
| Strategy | Cost (£) | Δ Cost (£) | QALYs | Δ QALYs | ICER | Probability cost-effective at £30,000 |
|---|---|---|---|---|---|---|
| Base-case | ||||||
| Sling | 8103 | 9.548 | 0.42 | |||
| AUS | 9615 | –1512 | 9.681 | –0.133 | 11,385 | 0.58 |
| Sensitivity analysis (using non-treatment group-specific costs and QALYs) | ||||||
| Sling | 7784 | 9.673 | 0.82 | |||
| AUS | 9435 | –1652 | 9.641 | 0.031 | Dominateda | 0.18 |
| 0% discount rate | ||||||
| Sling | 9030 | 12.409 | 0.38 | |||
| AUS | 10,215 | –1185 | 12.587 | –0.178 | 6641 | 0.62 |
| 6% discount rate | ||||||
| Sling | 7639 | 8.120 | 0.44 | |||
| AUS | 9316 | –1676 | 8.230 | –0.110 | 15,232 | 0.56 |
| 10-year follow-up | ||||||
| Sling | 7119 | 6.485 | 0.48 | |||
| AUS | 8972 | –1853 | 6.568 | –0.083 | 22,198 | 0.52 |
| 5-year follow-up | ||||||
| Sling | 6229 | 3.703 | 0.63 | |||
| AUS | 8389 | –2160 | 3.744 | –0.041 | 52,986 | 0.37 |
| One-way sensitivity analysis on the probability of sling failure: 0.054 | ||||||
| Sling | 9520 | 9.496 | NA | |||
| AUS | 9636 | –116 | 9.681 | –0.186 | 626 | NA |
| One-way sensitivity analysis on the probability of sling failure: 0.06 | ||||||
| AUS | 9636 | 9.681 | NA | |||
| Sling | 9953 | 317 | 9.483 | –0.199 | Dominateda | NA |
| One-way sensitivity analysis on the probability of sling complications: 0.15 | ||||||
| Sling | 9021 | 9.500 | NA | |||
| AUS | 9636 | –615 | 9.681 | 0.181 | 3398 | NA |
| One-way sensitivity analysis on the probability of sling complications: 0.26 | ||||||
| AUS | 9636 | 9.681 | NA | |||
| Sling | 10,056 | –420 | 9.464 | –0.217 | Dominateda | NA |
FIGURE 13.
Scatterplot of incremental costs and QALYs: male sling vs. AUS base-case model analysis.
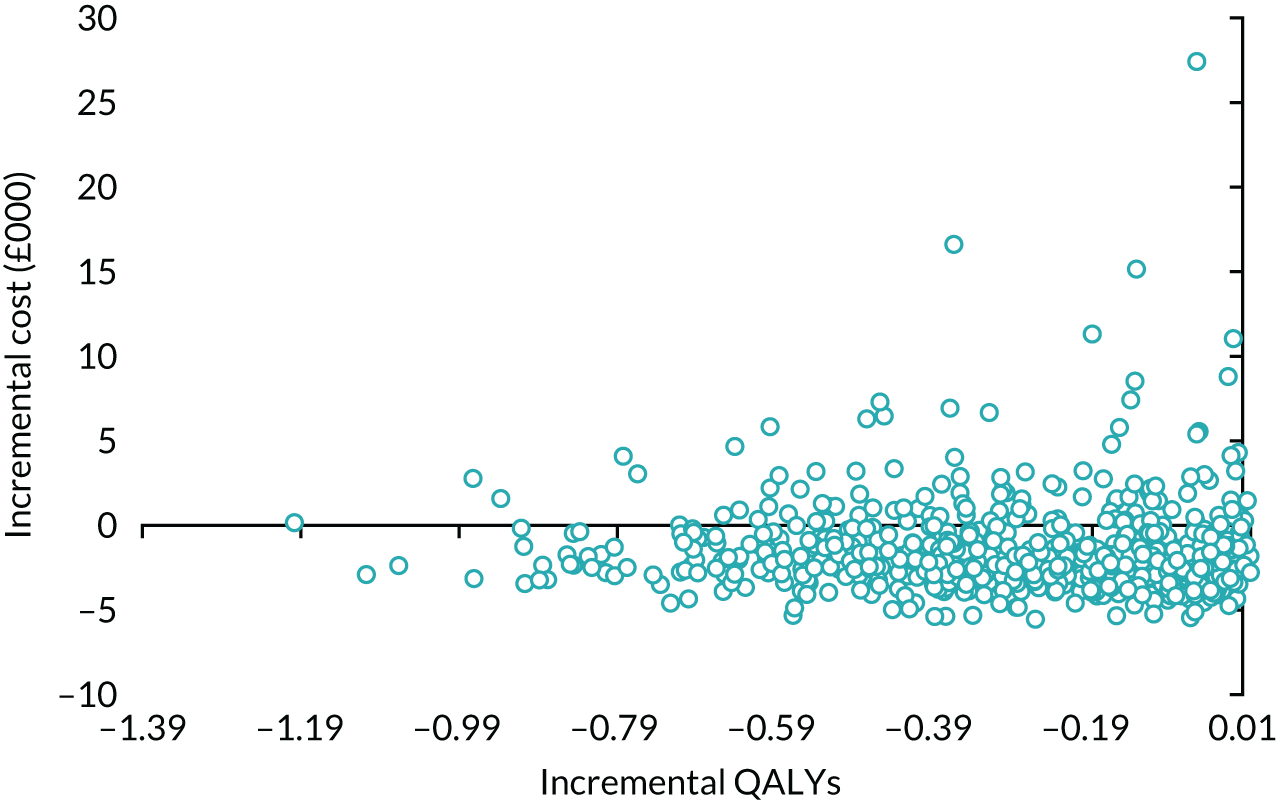
FIGURE 14.
Cost-effectiveness acceptability curves: male sling vs. AUS base-case model analysis. WTP, willingness to pay.
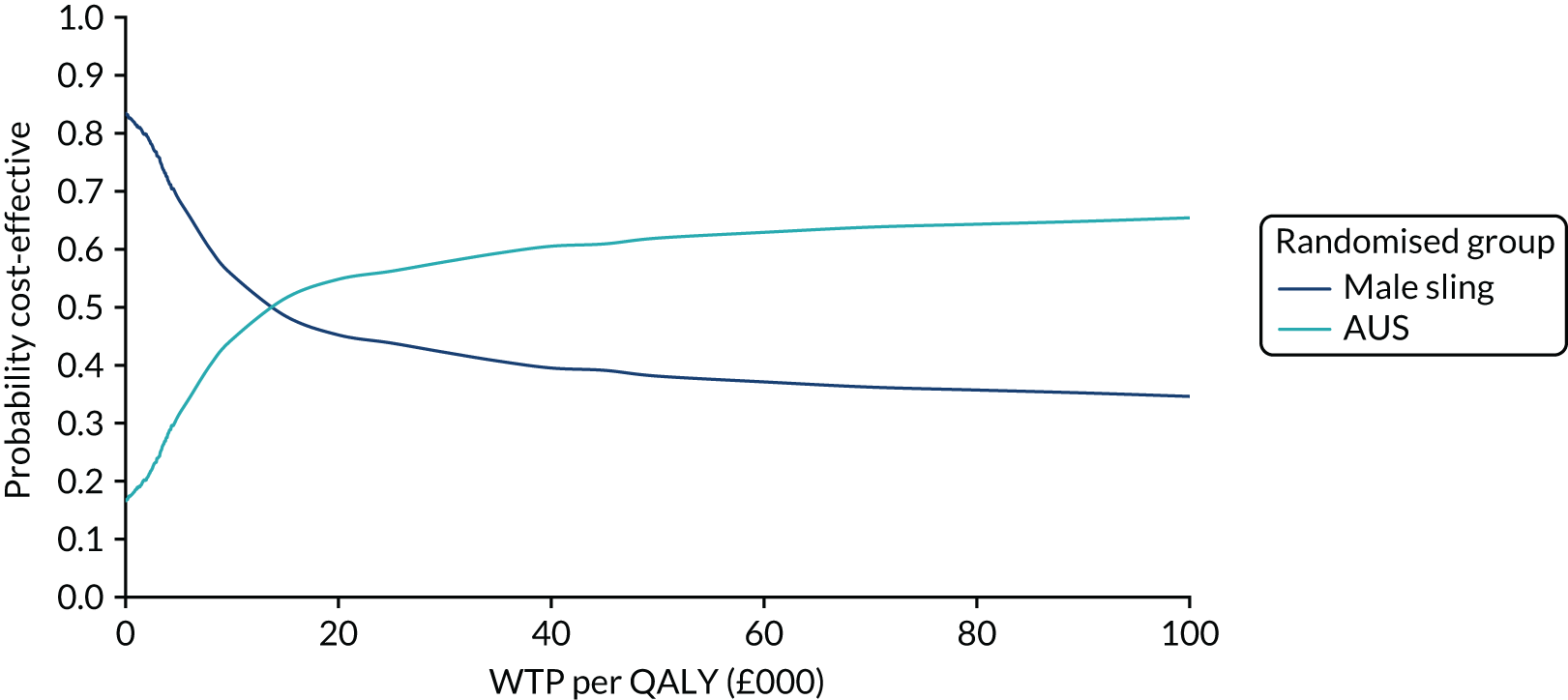
Sensitivity analysis
The results from all sensitivity analyses are reported in Table 23. On average, the cost difference in most of the analyses follows the same direction as the base-case analysis, although the magnitude in the differences reduces in most of the analyses. However, the results of the analyses that relate to non-treatment-specific costs and QALYs indicate that slings cost less and are more effective than AUS and, therefore, slings dominate. The results are sensitive to the probability of failure and complications as the costs of slings increase and QALYs reduce and the AUS dominates.
Interpretation of the model results
The model illustrates the long-term trade-offs between the choice of a male sling and the choice of an AUS and is populated with the data from the trial on failure and complications needing further treatment. The results are mainly driven by the higher QALYs experienced by those who enter the well state in the AUS group. The long-term results are similar to the within-trial analysis in that slings cost less but are still less effective than AUS. However, the magnitude of the ICER reduces over time, which means that the cost savings that are gained from the reduction of QALYs reduce. This also leads to a reduction in the probability that slings would be considered cost-effective at the £30,000 willingness-to-pay threshold, as illustrated in the results.
One of the main weaknesses of the model is the lack of long-term failure and complication data for the use of slings in men. The existing evidence tends to report success based on the number of pads used rather than the number of further surgeries. According to Leon et al. ,34 the major postoperative complication of the AUS in the long term is the need for explantation of the device because of erosion or infection. Studies suggest that the AUS has an average lifetime of 10 years,35,36 but there is no evidence of the average lifetime of male slings; therefore, this was not included in the model. Therefore, these results need to be interpreted with caution because the 2-year data are immature and there is a chance that all the important trade-offs, particularly complications, have not been observed in both groups. The ICER is sensitive to the long-term failure and complication values. There is currently too much uncertainty around the failure and complication rates and further follow-up is needed to reduce the uncertainty.
These results must be interpreted with caution, with the understanding that they would apply only in a situation in which relative differences in the effectiveness of the male sling compared with the AUS do not change over time. It is not clear at this stage if this assumption is valid and long-term follow-up is needed to inform this.
Chapter 6 Qualitative report
Introduction to the MASTER qualitative programme
The use of qualitative research methods to help to strengthen the findings of RCTs is increasingly employed. These methods allow for a thorough understanding of the views and experiences of the patients who are participating in the RCT. 37 These patient experiences may be missed if only quantitative measures are used, hence the importance of qualitative research. 38 To fully understand the experience of both patients and clinicians within MASTER, an embedded comprehensive programme of qualitative studies was included (Figure 15). The qualitative programme included five studies, the principal aims of which were:
-
study 1 – to establish the importance of the main outcomes to patients undergoing treatment for PPI (during study set-up)
-
study 2 – to explore experiences of PPI, surgical outcomes and perceptions regarding randomisation
-
study 3 – to explore the participant’s experience of the two procedures
-
study 4 – to explore the experiences of those participants’ requiring further surgery
-
study 5 – to explore the experience of surgeons who performed both procedures.
The findings of these studies are summarised in this report. They are arranged to reflect the sequence in which the studies were performed and are summarised in Figure 15.
FIGURE 15.
Sequence of studies and principal subject areas of the five qualitative studies.
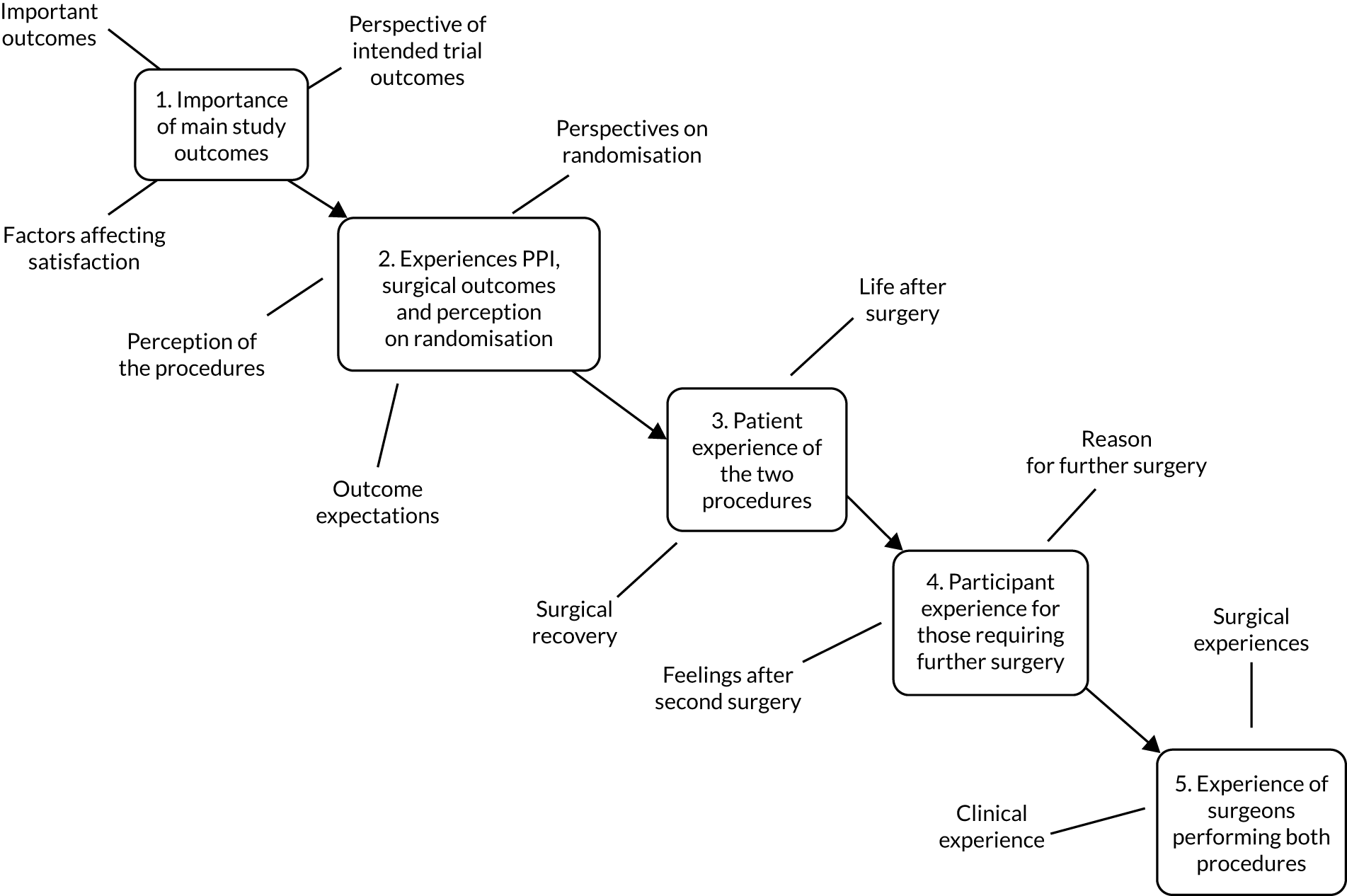
Methodology common to all studies
Recruitment and sampling
Participants for the qualitative substudies were identified through the main trial, using purposive sampling. 39 They received a qualitative participant information sheet and were asked to provide written informed consent. Arrangements to conduct the qualitative activities were made via telephone at the participants’ convenience.
The interviews were conducted either face to face or over the telephone, depending on geographical circumstances. Interview schedules were prepared to reflect the differing areas of investigation and were amended as required throughout to ensure responsiveness to the emerging findings. Participants were recruited until data saturation was reached and there were no new emerging themes. 40 All interviews were audio-recorded and transcribed verbatim, following which all data transcripts were coded and analysed using a thematic analysis. 41 Qualitative data management software NVivo 10 (QSR International, Warrington, UK) was used to facilitate data analysis.
Qualitative study participants
Participant details are provided in Table 24 and demonstrate comparable groups at each stage of the investigation in terms of age distribution.
| Group | Number of participants | Age range (years) |
|---|---|---|
| Focus group | 4 | 63–71 |
| Pre surgery | 25 | 51–76 |
| Post surgery | 15 | 58–74 |
| Further surgery | 10 | 58–73 |
| Surgeons | 20 | Unknown |
Study 1: patient focus group to establish the importance of the main study outcomes
Aims
Focus groups are used to allow unstructured discussions on particular topics with a group of potential participants to help to guide the study. 42 The areas for exploration for this focus group were:
-
important outcomes post surgery for the men
-
factors affecting men’s expectations following surgery and satisfaction post operation
-
men’s general comments and suggestions for amendments to the self-report questionnaires for inclusion as study outcomes.
Methods
Initially, patients who had not yet been invited to take part in the trial, but were otherwise eligible, were invited to take part in a focus group to ensure that the study was clear in its direction from the men’s perspectives. Twelve patients on the waiting list for surgery at Southmead Hospital (Bristol, UK) were invited and sent a participant information sheet and consent form; four men consented to take part.
Results
The men were aged 63–71 years. All were white, two were retired, one was semiretired and one was in full-time employment. They had PPI and were able to provide insights pertinent to the trial intention (Table 25).
| Theme | Subtheme |
|---|---|
| Desired outcomes from surgery | Pad use and leakage |
| Relationships, self-esteem and confidence | |
| Embarrassment | |
| Factors affecting satisfaction with treatment | Perceived promises |
| Perspectives of intended trial outcome measures | Suggestions |
Desired outcomes from surgery
There was a consensus between the men that the inconvenience associated with pad use was a central factor in their decision to seek treatment. Indeed, when asked to report on what the ideal outcome from surgery would be, the immediate answer from one participant was ‘not having the pads’. This was met with general agreement from the group. One of the men admitted to using up to 13 pads per day, before preferring to use a Conveen sheath® (Coloplast Ltd, Peterborough, UK), although this was also not an ideal solution:
I’m finding that the Conveen . . . sometimes make me sore.
P2
However, for most of the participants, the general inconvenience of pad or Conveen was not a barrier to leading a full and active life:
Not at all. I’m still walking, I’m still working, I still do a lot of things. I’ve never changed my lifestyle at all.
P1
Some of the men described how they were able to manage their symptoms to minimise the impact on their daily lives. Others described how leakage was a major inconvenience:
I manage it during the daytime – where is the nearest loo? The whole day was ‘where am I going to be’?
P3
Driving is a nightmare. It’s OK when you know that you’re going because when you’re sat in the car it’s OK, but as soon as I stand . . .
P4
Although the cessation of pad use was agreed to be the ideal outcome for their forthcoming surgery, this did not extend to the complete control of leakage for all of the participants. One man deemed it an acceptable outcome to his surgery if he was able to control his leakage during the day:
Yes. If that could be strengthened a little bit longer into the afternoon. If that was like it all day, I wouldn’t bother at all.
P1
Embarrassment caused by leakage was a recurring theme from each of the participants, ranging from brief annoyance to an overwhelming impact on their lives:
For me it’s very embarrassing. I lead a very active life.
P1
I personally . . . from an embarrassment point. At very odd times I have leaked.
P2
The biggest embarrassment is if you have a partner . . . When you are in bed and it happens to me, and you end up peeing on her bum.
P2
Really bad. I haven’t a lot of confidence. I hate it, I really hate it.
P4
Embarrassment extended to the impact on relationships, self-esteem and confidence as important factors. In particular, this was mentioned as having a major effect on their sex lives, including on sexual confidence, which was also compounded by the side effects of the prostate surgery.
There was some recognition that sex was perhaps less frequent than when they were younger. Nevertheless, it was still stressed by several of the men as being a very important part of their lives and loss of full sexual function was recognised as a threat to the masculine identity:
I mean because there is the sex life business. You become insecure. That’s number one.
P4
Then I had a lot of trouble with erections. But then I was bent a bit. You can have an operation for that as well, but I didn’t think I wanted to go for that . . . I’m married, our sex life is not what it was when we were young but you know.
P4
But at the same time, you’re a man. Like for her, if you lose your breasts you don’t feel a full woman any more, you don’t feel a full man any more . . . If you’re married it’s probably fine but in my case I’m separated and if things start to come together you think ‘oh no, my God’, it’s an awful thing to have to explain . . .
P3
Factors affecting satisfaction with treatment
The expected outcome of the men’s treatment was highly influenced by the predicted prognoses of the medical professionals. After prostate surgery, when the side effects did not improve in line with their expectation of the outcome of treatment set by the medical staff, this had a large effect on their level of satisfaction:
They told me I would be dry within 3 months. Didn’t happen.
P1
Yes, I’d been told. You’d be dry within 3 months.
P2
Other factors that contributed to the level of satisfaction of treatment included the level of confidence in their surgeon and other staff. This was influenced by their personal perception of competence, but also by familiarity:
The guys here at [name of hospital] know me, they know all about it. The guys in [name of hospital] didn’t know about it.
P1
I had a lady come in one morning at half seven to do my dressing. She had gloves on and she moved the bed because she said there wasn’t room for the trolley. Then she proceeded to clean me up with the same pair of gloves.
P2
The length of stay in hospital after surgery, length of operation, scarring, risk of infection and general fear of surgery were mentioned in the course of the discussion as being important factors to the men. However, this was not universal:
I have had a lot of operations in my life . . . I’ve never been afraid of surgery – it’s always been good.
P4
Perspectives of the intended trial outcome measures
Participants in the focus group were also asked for their views on the appropriateness of the intended outcome measures. The questionnaires that they were asked to comment on were the International Consultation on Incontinence Questionnaire Lower Urinary Tract Symptoms Quality of Life (ICIQ-LUTSqol) module43 to assess the impact on quality of life; the ICIQ-MLUTS,44 which incorporates evaluation of urinary storage, and voiding and incontinence symptoms; and the ICIQ-MLUTSsex45 to assess male sexual matters associated with their LUTSs and impact on quality of life.
The focus group confirmed the importance of leakage and sexual matters as outcomes, which are well covered by the baseline questionnaire and the key outcomes that matter to individuals with PPI. The baseline questionnaire also covered the medically related outcomes well, for example the volume of leakage, urgency, nocturia and depression. However, it was not picking up on the impact of symptoms and issues, such as inconvenience to daily life, embarrassment, distress, frustration, effect on relationships with family and friends or sexual confidence. It was suggested that the inclusion of the ‘how much does this bother you?’ items from the ICIQ-MLUTS and ICIQ-MLUTSsex would provide an evaluation of the impact of these issues on patients. This was intended to achieve an evaluation of the differential effects of the operations on other outcomes, such as quality of life and general health.
Although inclusion of a condition-specific quality-of-life questionnaire, such as the ICIQ-LUTSqol, was considered advantageous, it was considered more appropriate for a later-stage study in the evaluation of these surgeries. The SF-1215 was included, recognising that it may lack sensitivity for the specific issues highlighted by this focus group but would enable the capture of high-level quality-of-life data.
Discussion
The men who took part in the focus group discussed their satisfaction with surgery as being dry, using fewer or no pads, being able to regain their social life and having improved sexual matters. It was felt by some that the use of pads was an inconvenience and that it had an impact on the activities that they felt able to do. This confirmed the importance of the areas covered by the baseline questionnaires. It also highlighted that complete cure of incontinence was not the only outcome to secure satisfaction with treatment. These findings were important to underpin the study and ensure measurement of the most appropriate indicators of outcome.
Study 2: explore experiences of post-prostatectomy incontinence, perceptions regarding randomisation and surgical outcomes
Aims
Initially, this study explored the rationale behind participants’ decisions to be randomised or not randomised to suggest possible recommendations for the improvement of recruitment strategies. Many trials struggle to recruit enough participants, threatening their overall validity. Any subjective view of the characteristics of each surgical procedure may have implications for participants’ eventual choice. The following provides an insight into how participants perceived the male sling and AUS prior to surgery. Participants’ expectations for the outcome of their surgery were also explored, as satisfaction with a procedure is known to be closely linked with met expectations. 46,47 This had the intention of informing the criterion of non-inferiority for the two surgeries.
Methods
The second subgroup were recruited between February and August 2014. Purposeful selection of participants was undertaken to represent both the randomised cohort and the NRC. All interviews were conducted before the scheduled surgery and within approximately 1 month of initial recruitment to MASTER. The foci of investigation with this group were:
-
What are participants’ reasons for choosing to be randomised or to decline randomisation?
-
How do participants perceive each of the two procedures?
-
What are participants’ expectations for the outcomes of their surgery?
Results
Twenty-five men, aged between 51 and 76 years, from seven centres, were recruited to participate in this subgroup [10 consented to randomisation and 15 consented to the NRC (seven men in the NRC had the male sling and eight had the AUS)]. The main purpose of this substudy was to explore motivations surrounding trial participation to extend learning regarding trial conduct. Experiences of symptoms following initial prostatectomy surgery were also explored in this baseline group to examine the breadth of symptoms across the cohort (Table 26).
| Theme | Subtheme |
|---|---|
| Perspectives on randomisation | Current lifestyle, symptoms and situations |
| ‘Doing your bit for society’ | |
| Equipoise issues | |
| ‘Leave it to the experts’ | |
| Randomisation issues | |
| Having a preference | |
| Centre inconsistency | |
| Perception of the procedures | Perceptions of the AUS |
| Perceptions of the sling | |
| Outcome expectations | Expectation of success |
| Incontinence | |
| Continence aids | |
| Lifestyle and quality of life | |
| Mental health | |
| Sex life |
Perspectives on randomisation
Seven main themes were identified as prominent when considering the main reasons for men deciding whether or not to be randomised. These were (1) current lifestyle, symptoms and situation; (2) ‘doing your bit for society’; (3) ‘equipoise issues’; (4) ‘leave it to the experts’; (5) randomisation issues; (6) having a strong preference; and (7) centre inconsistencies.
Current lifestyle, symptoms and situation
All of the men in the NRC felt that they had made their decision in agreement with their surgeon based on their current symptoms and situation. The men had generally been living with incontinence for several years prior to being considered for surgery, so were looking for a reliable and ‘final solution’. A primary concern for participants was to receive the surgery that had the least chance of requiring future corrective surgical procedures. For this reason, high success rates and a low chance of mechanical failure were deemed important. Some men viewed the sling as the ‘simpler’ and superior option, with greater longevity, owing to there being fewer components. However, the success rate was perceived as being lower because it was a ‘new’ procedure. The sling was generally viewed as less suitable by those with particularly severe symptoms. Current lifestyle was also important. One participant felt that the sling would not be suitable owing to his active occupation. Another participant, a keen cyclist, was doubtful that the sling would be effective. In all cases, there was considerable trust that the surgeon would advise on what was best for them.
Well, I trust his judgment. And as I say, that was based on symptoms that I exhibited last July. Things have got a lot worse since then, so I want the best solution that’s on offer.
12003
The AUS, as the ‘tried and tested’ procedure, was judged as having a higher success rate by some participants, but with a higher risk of mechanical failure. The AUS was often expected to last between 5 and 10 years before corrective surgery or replacement parts may be required. Many were told that, in the event of sling failure, they could subsequently have the AUS. For this reason, age also had a bearing on whether or not participants saw reliability and longevity as having particular relevance:
I think the decision at the end of the day He [the surgeon] said at the end of the day, it is going to be your final decision. I mean I have kind of the idea of having the sling first and then if that didn’t work, then I would go for the AUS. But I’m not getting any younger. And I don’t really want too many more operations. And I was thinking to myself well, is it better to have more success, with one operation . . . I felt I’d suffered enough over the last 3 and a half years.
13010
‘Doing your bit for society’
When asked what their most important reasons were for participating in the trial, one of the most common answers was for altruistic reasons. The randomised men attributed a high value to the idea of ‘helping others’, when considering their incentives. Connected to this was their willingness to contribute to ongoing research and the advancement of medical knowledge. For these reasons, a slight preference for one of the procedures did not dissuade some participants from opting to be randomised. Some idea of a personal disadvantage to taking part may have been suggested, but this was deemed insufficient for refusal of randomisation:
Well if I didn’t go on the trial, then I could choose which one I wanted, but if I don’t go on the trial then it doesn’t help you or anybody else does it?
15003
For the non-randomised participants, as with the randomised participants, it was clear that some of the men valued their inclusion as an altruistic contribution to science. However, for these men, follow-up in the interest of science was seen as secondary to their primary concern, which was to receive their procedure of choice:
I don’t mind doing like information stuff and that but I wanted to have the sling.
11002
Equipoise issues
The majority of the participants had weighed up the relative merits of the two procedures in their minds during their decision-making process. For some, this resulted in a favoured procedure, so randomisation was not an option, but for others the decision was less clear cut. One medically informed participant held a position of clear equipoise so concluded that randomisation was his best course of action:
So because it is actually quite a difficult decision because we don’t know the answers, in a way randomisation helps make that decision for me. Quite constructive.
12001
For other men who chose to be randomised, their position was more ambivalent. The outcome of the surgery was likely to be an improvement on their current symptoms, so they saw no reason to make a decision either way. They chose to be randomised because the outcome was perceived to be the same:
I don’t mind. I’d be happy with either really as long as they do the job you know.
13004
Clinical equipoise communicated by the surgeon was highly influential. Prior to consultation, some of the randomised men held an initial preference for one or other of the surgeries but were swayed by their surgeon that they were ‘an ideal candidate for the MASTER study’. However, the non-randomised men were advised in some cases that one of the procedures was ‘the best option’ for them. The sling was recommended for some younger participants, the rationale being its longevity, the less invasive operation and, if necessary, the option of having the AUS at a later date:
I was put on to her by my urology team, they were looking at the AUS and they never even mentioned the sling, but when I saw [name of surgeon], she said probably the sling would be a better option for me because of my age.
20001
The AUS was recommended by some of the consenting surgeons as suitable for more active participants and those with a higher severity of symptoms because it had a higher success rate:
Following my morning here with [name of surgeon] and a conversation with him, he thought that for my symptoms the sling wouldn’t do the job.
12003
‘Leave it to the experts’
An influential factor on decisions in both randomised and non-randomised participants was the view that the health professionals ‘know best’. Participants mentioned certain centres that they believed to have a particular body of expertise. This contributed to their confidence in receiving the treatment, which was thought to be best for them. It appeared that the surgeon had the most capacity to be influential:
I’ll think well, that’s what they’ve gone with, that’s fair enough, you know best, you guys, you’re the doctors, you’re the experts.
16009
For a few randomised participants, complete confidence in the surgeon allowed them to actively avoid having an opinion on the two procedures. This distanced them from the decision-making process, with the explanation that ‘too much information is dangerous sometimes’. One participant acknowledged that this would also maximise his chances of being satisfied with the procedure:
I don’t, well I tried not to form any opinion because I’m not an expert . . . I mean if I were to have the sling and the sling was ineffective, I would be very frustrated and wish that I’d had the other, so I’m just leaving that up to the surgeon.
15006
Randomisation issues
Most participants saw randomisation as ‘a lottery’ that decided the surgery that they were to have. All randomised participants were confident with the knowledge that they would not know which procedure they would receive until the day of the operation. However, for several participants, the scientific purpose of randomisation was not understood. For example, one participant thought it was primarily intended to produce an even number of participants for each procedure. Others who agreed to be randomised had a more abstract sense of community spirit, rather than specifically understanding or considering the main aims of the trial:
I don’t have any opinions on that [the process of being randomised]. If there is some benefit to the team by being randomised I’m very happy.
15006
The non-randomised participants, in particular, saw randomisation as when ‘someone else’ chose the procedure, and not necessarily the one which was best for them. A general misconception was that either the surgeon or the ‘computer’ decides. The uncertainty associated with randomisation was uncomfortable, particularly if they had a favoured procedure. It was also important for some participants that they had overall responsibility and control over the final decision:
I wasn’t sort of as keen on the random factor where somebody else chose my outcome. [I prefer] being in command of my own sort of decisions.
16007
Despite this, many of the current non-randomised participants expressed no issue with the concept of being randomised per se. They acknowledged that if they did not have a preference then randomisation would have been an option for them.
Having a preference
For all of the non-randomised participants, a strong preference for one procedure was the main reason for their choice not to be randomised. Although there was usually at least one factor that resulted in them favouring one procedure, for some participants the formation of this preference may have preceded their first consultation with a surgeon. In particular, for several men, the influence of success stories featured on the Channel 4 programme Embarrassing Bodies48 was an important influence on initial treatment preferences preceding consultation. Other preferences stemmed from personal research online, the hospital or information provided by other health professionals. However, sometimes, despite the participant initially having a preference, the preferred treatment procedure was not the procedure undertaken. The communication and discussion with trial recruiters (mainly the surgeon) were sufficiently influential, in many cases, for the participants to change their minds from their initial preference:
I’ve done lots of research obviously online and I saw the sling as my preferred option . . . But following my morning here with [name of surgeon] and a conversation with him, he thought that for my symptoms the sling wouldn’t do the job.
12003
In some men who had formed a very strong preference prior to consultation, there was a reluctance to consider the alternative procedure. They were ignorant of the details of the other surgery and were reluctant to discuss their treatment views as, in their minds, the decision was already made:
It was my decision and he [the surgeon] recognised I was rather determined about it.
18003
I’ve always been in favour of the sphincter anyway, so it probably to me wouldn’t be a fair comparison to talk too much about the sling because I haven’t really considered it to be honest.
13001
Many of the participants had a strong preference for or an aversion to characteristics of the two procedures. For this reason, these men were extremely opposed to the prospect of being randomised to either surgery. The AUS was perhaps easier than the sling to visualise for some participants because they saw it as being more ‘mechanical’, giving a greater sense of security in operational terms:
With the AUS, I know it’s more intricate but you seem to have more control . . .
13010
The sling was generally favoured by those participants who saw it as a less-invasive procedure. Several of the men were attracted to the sling because it would allow them to go to the toilet normally, as they were very uncomfortable with the idea of having to press a button to urinate. The AUS was seen as having too many ‘foreign objects’ in the body, and several expressed concerns about the consequences of a mechanical failure:
I’d like to be dry, but I had worries about what it would do to me mentally. Having to press a button when I need to urinate and in the back of my mind thinking does this malfunction at any time and what do I do if it does?
13007
Centre inconsistencies
There were some inconsistencies between centres over whether the sling was offered independently or the sling was offered as part of MASTER only. This may have had implications for some of the choices made. Two of the men who were offered the sling independently of MASTER owing to a slight preference were not averse to having the AUS:
If it didn’t work and was just the same, or a little bit better, I’ve no problem going for the other thing you know. Anything to get rid of this business.
11003
Several of the randomised participants had a preference for the sling, but it was not available to them outside the trial at their particular centre. One might expect this to be an incentive to take part in the trial to have the chance of receiving the sling. However, this was not directly reported to be the case by any of the current participants. All randomised participants were happy to receive either of the procedures:
If I had a choice then I would pick to have the sling done but I don’t have a choice then no it doesn’t make any difference.
15003
At a centre that does not officially offer the sling outside the trial, one randomised participant described how his surgeon favoured the sling because he had previously received radiotherapy. Nevertheless, the participant was happy to have either procedure:
I believe it’s supposed to be randomised, but [name of surgeon] is pushing for a sling rather than a sphincter.
12007
Perceptions of the procedures
Every participant was asked for their opinion on each of the two procedures and had a good or reasonable grasp of the main differences between the surgeries. For this reason, the following sections present the views of the participants from all interviews, both randomised and non-randomised.
Perceptions of the artificial urinary sphincter
When questioned about how the AUS worked, there was a range of answers. It was described as a ‘tap you are able to squeeze off’ with a ‘button’ or ‘switch’ in the scrotum, which is used to control the flow of urine. The most common perception of the AUS was that it had a high success rate. For this reason, it was seen as very reliable owing to the confidence associated with it being ‘tried and tested’:
From what I’ve read, the percentage is, the higher percentage of success, with the artificial sphincter . . . because that’s has been running for 20 or 30 years or something.
12004
Many of the points raised by participants about the AUS were concerned with its use and operation. The more mechanical nature of the AUS was highlighted by some as being a potential issue, as there was some concern over a risk of malfunction owing to the number of components. Some participants identified possible risks with having to receive replacement parts at a later date or having more immediate emergency problems:
My concern is what if it goes wrong and the cuff is closed around the urethra and your bladder is filling and you can’t release the cuff?
16009
There were conflicting expectations from different participants for the longevity or lifespan of the AUS. Some saw the AUS as a more ‘permanent’ solution, but others anticipated that it would work for between 5 and 10 years before the possibility of requiring further surgery. Although the AUS was sometimes seen as a temporary solution, this was a concern for only some of the men. Others were quite happy if it worked for this length of time:
I mean 5 years down the line something might go amiss and they might have another look at it like but 5 years that would be great for me at the moment.
13001
One advocate of the AUS talked about the switch in the scrotum being desirable because it allowed a mechanical ‘control’ of continence. However, other participants thought that it might be possible to press the button in the scrotum inadvertently or that it would be uncomfortable. There were also concerns associated with visibility owing to the external part of the AUS and how inconvenient it might be to have to use a cubicle rather than a urinal in future:
I don’t know whether if it’s easy enough if you still want to use the urinal just to stand there and fiddle around or whether it would mean really that if you want to pass urine you’ve got to find a cubicle and half undress to do it.
12001
However, despite mentioning some of these potential issues, for participants who were prepared to have the AUS, their primary concern was that their incontinence was cured. As covered by the previous section, other factors were generally stated as being more important when deciding on their treatment options:
The only slight thing that does bother me is this pressing button business like you know if that’s going to be alright, with the little balloon inside. It does concern me slightly but then again I think when I’ve read how long you’ve been doing it, the success rate [is encouraging].
13004
Perceptions of the sling
The participants found the functionality of the sling more difficult to describe than the AUS, but it was seen as a ‘support’ that ‘holds the water pipe in place’. The most common perception of the sling was that it was referred to as the ‘simpler option’. This was because of it being comparatively less complex mechanically and having fewer components. The advantage to this was that there were ‘fewer things to go wrong’, with the unobtrusiveness of the sling being attractive to several of the participants:
You don’t really know it’s there, you just carry on life as normal.
12001
The surgical procedure for this was also seen as less complex and less invasive, with the possibility of having the operation and being discharged the same day. There was an expectation of being able to carry on with normal life within a few weeks of the operation, with no need for activation. The recovery period was expected to be shorter than that for the AUS:
Well this is such a simple device that the surgery I understand takes about an hour . . . I understand the recovery is rapid, for want of a better word.
18003
The sling was also expected to last longer without the need for replacement parts and was seen as the ‘final solution’ by these participants. For one relatively young participant, this was seen as advantageous because there would be less chance of further surgery in the future. However, several acknowledged this with a caveat, such as ‘if it works’, as the success rate of the sling was generally perceived to be lower than that of the AUS at around 60%. The AUS was seen as a back-up option or, alternatively, it might be possible for the sling to be adjusted at a later date in the event of sling failure. Some participants had a lower expectation of a full reduction in leakage, but, nevertheless, any improvement was welcome:
The sling is presumably just helping one to control leakage, and so therefore there is some doubt as to how effective it will be. If it’s 50% effective it would be a big improvement for me, that’s what I thought.
16004
There was some doubt that the sling would be effective for those with an active lifestyle. In addition, one participant mentioned that he worried about urine retention and pain after the operation. More generally, the sling was perceived as a ‘new procedure’ that was yet to be tried and tested:
I fancied the sling a bit more but then again, apparently from what I’ve read it’s not been too much tried and tested over the years so slight quandary either way really.
13004
Outcome expectations
The main hopes for post-surgery outcomes were explored, with participants’ responses largely aligned with the participant reports from the initial focus group. Primary expectations were for an improvement in incontinence and for a reduction or complete cessation of pad use or other continence aids. However, when questioned further, there were many other expectations and hopes for positive changes to their lives. The following section presents the views of the participants from all interviews, randomised and non-randomised.
Expectations of success
Most participants expressed their hope for a ‘complete cure’, but tempered their expectations with what they viewed as a realistically achievable outcome. Only a small minority had strong expectations that they would be completely dry after the operation. Any potential improvement was generally seen as a successful result. Participants were also clear that there was a chance that the procedure may not be effective at alleviating their symptoms. Several drew on previous experiences of their prostatectomy surgery and the consequences of this:
Well, really I suppose I’m looking for it to be 100%, but you know I’m a realist as well and . . . I mean when I read all about the actual operation for the prostatectomy I was convinced I wouldn’t be one of the 1 in 50 or so where it doesn’t work out but I was unfortunately.
11003
Well, that’s the goal but I realise when you read all the particulars that there is no guarantee. But they term as a successful operation if they can stop it altogether or you get a 50% reduction in your leakage problems, which is 50% better than it was before.
13002
I’m not expecting 100% prevention of leakage from the sling.
16004
Oh, simply stated, to restore continence and eradicate the need to wear pads . . . On the other hand, if I am wearing a pad, well, there again I have been warned of the consequences.
18003
Incontinence
The primary and obvious desired outcome for the men was the reduction or complete cure of their incontinence. All of the men consistently mentioned that they would like to be dry or at least have an improvement in their symptoms. Many saw leakage as their only major health issue:
If I can restore continence, then I’ll consider myself to be as fit as a fiddle.
18003
Several of the men talked about the feeling of urgency as a major outcome that they hoped would improve. Many spoke of the inconvenience that this caused them, including planning where toilets are, having to rush to toilets unexpectedly or planning journeys around toilet stops. Others also mentioned the inconvenience of the frequency that they needed to go to the toilet and how they hoped that this would change. Stress incontinence symptoms, such as leakage when active or when laughing, sneezing or coughing, was also mentioned by several participants. The effect on their lives, inconvenience and associated embarrassment were all mentioned as outcomes that the men hoped would change for the better:
To do everyday things without dashing off to a loo or considering where the nearest one is, or ending up with embarrassing situations where I have leakage.
16007
I’m hoping I can get up out of a chair or sneeze, laugh, cough, whatever, without having to think about changing my pad afterwards.
15004
Continence aids
The reduction or cessation of the use of pads, a Conveen or Dribblestop® (Rennich Industries Ltd, Calgary, AB, Canada) was consistently mentioned as a major outcome that was expected post surgery. The use of pads was described as extremely inconvenient when going anywhere, travelling or attending social occasions. They wanted to be liberated from the necessity of carrying bags of pads around and having to plan ahead around the location of suitable changing areas:
I have, in a way, to prepare my days if I am going anywhere, to have the right kind of pads and to be, remember to take a change of pads where I’m going and then there are difficulties often at finding a place where I can change the pads conveniently.
15006
Continence aids were also very uncomfortable. Two of the men were especially keen not to have to wear pads at night, as the discomfort while sleeping was particularly acute. There was also the associated embarrassment of wearing pads, the worry that pads were visible through clothing and the worry of whether or not there was any odour:
So it is quite annoying. I’m constantly aware of the problem, you’re always aware of having a wet pad and whether or not it’s obvious to anybody else.
15006
The financial burden of having to buy pads was also mentioned by several men:
I’m only down to one a day now, one maybe two if I have a really strenuous day, but it’s mainly one a day. But I mean over the 3 and a half years it’s cost me thousands.
20001
Lifestyle and quality of life
When asked how they expect that their lives might change after the operation, the men mentioned many aspects of their lives that they felt had been restricted or made impossible by their incontinence. There was a real sense of optimism about the future as they looked forward to their lives improving or at least returning to ‘normal’:
Well, just to be able to try and lead a normal life . . . like I used to be, basically.
13001
Many were hoping for a more active lifestyle and being able to participate in more sporting activities. These included being able to go for a swim; walking; going for a bike ride; playing football, golf and skittles; and spending more time in the garden. They felt that the constant threat of leaking when under any physical stress inhibited their ability to partake in these activities:
I can’t do anything, I’m 69 this year and, reasonably physically fit. I can’t run, I can’t, I could run but I can’t do these things, I can’t play any sport of any kind.
15006
Others talked about the effect that their incontinence had on their social lives. They felt that incontinence was curtailing their ability to attend social gatherings and events or spend time with their extended families. They felt that this had a severe impact on their quality of life:
Going out and just general socialising with people . . . It just puts a general dampener on things like. Just lifestyle I would feel a lot better.
13004
Several men described how their sleep was disturbed by the necessity of getting up to go to the toilet up to six times in one night. It was very important that this would change:
Maybe I could sleep through the night better, to get up four or five times every night. It’s not easy.
12005
Many of the men were retired, but a few of the men spoke of the effect that their symptoms had on their working lives. One man was not able to work in his managerial position as a result of his symptoms. He was hopeful that, if his incontinence was cured, he would be able to return to work. Another spoke of the problems associated with being a lorry driver with his symptoms and saw the operation as a way of returning to full-time work. Going back to work was seen as part of the expectation of returning to a normal, symptom-free lifestyle:
Yeah, basically just to get me life back as well in a sense.
16003
Mental health
A number of men described the profound effect that they felt that their symptoms had on their general well-being and mental health. Two of the men spoke of symptoms of depression and disturbed sleep owing to general anxiety surrounding their incontinence. During social occasions with other people, these men spoke of the anxiety and embarrassment associated with leaking and the effect that this had on their confidence:
I’m standing around talking to people, I don’t necessarily leak a large amount but I can see it coming down and that’s disturbing psychologically when I’m with other people. I feel dirty really, if you know what I mean.
16004
There was an expectation that their mental health would improve after the operation if they felt that they were able to return to a ‘normal’ life:
I must say I have lost a bit of confidence, but I’ll soon get that back. When I start going out normally.
13007
One man spoke of the challenge that he felt that his symptoms were to his masculinity because of the necessity of having to use a cubicle instead of a urinal. In his mind, the restoration of continence would allow him to feel better about himself as a man:
So, if it’s a success, I will, in my own feelings, I’ll be a whole man again.
13002
Sex life
There were varying expectations concerning any improvements to sex lives after their surgery. The men described difficulties achieving erections, or impotence following their original prostatectomy. The resulting effect on their sex lives was more of a problem for some men than for others. For some, an active sex life was still a high priority, so there were considerable hopes for improvements. This included the control of urine when aroused, the improvement of erections and the general effect on sexual confidence:
Hopefully I might get a sex life back. At the moment it’s non-existent.
13005
Again, it boosts confidence, it boosts morale.
12007
For others, an improvement in sex life was not seen as a particularly important outcome. They were conscious that the surgery was unlikely to help with impotence:
Well, I hope very much it will improve the incontinence. I doubt very much it will do anything for the erections and the pain.
12001
For some of the older men, other improvements to their lifestyle were seen as a higher priority:
I think when you get to a little bit older in life, you realise it’s not the be all and end all of everything, so, you know, it’s not something I’m doing this for.
15004
Discussion
Perspectives on randomisation
As covered earlier, a patient’s perception of the two surgeries may be shaped by a variety of influences. Information from the media, personal research online and word of mouth can affect opinion. Most importantly, it is the information supplied during consultation with the surgeon that appears to have the greatest influence. It is important to understand the effect that this information has on the resultant subjective opinion of each patient, as it may have implications for their eventual choice of procedure.
Within MASTER, both operations were highly likely to be beneficial, which allowed some men to be fairly indifferent about their treatment choice. Those who chose randomisation were secure in the knowledge that either treatment was likely to have a positive outcome, allowing underlying altruistic motivations to be realised with relatively little risk. Non-randomised participants were able to fulfil any altruistic tendencies by participating in all follow-up research, yet also have their preferred procedure. This would suggest that having a trial arm that follows up non-randomised participants is not an optimal strategy for RCT recruitment rates.
Participants can find the reasons for randomisation confusing and the scientific rationale is often not understood. A common reason for declining trial entry is ‘dislike of the idea of randomisation’. 49 This was true for those who felt that randomisation would deny them any control over the final decision. However, several of the non-randomised men indicated their willingness to be randomised, had they not developed a preference.
Perspectives of the procedure
Some men were concerned about the longevity of the AUS and any necessity for corrections or future replacement parts at a later date. The recommendation is that patients should be advised clearly of the revision and malfunction rates for the AUS,50 which is particularly important given that patient satisfaction is highly related to whether or not expectations are met. 46,47 It is also important that patients have the opportunity to discuss any concerns regarding the function or usability of the AUS during consultation.
For many men, it was more difficult to verbalise ‘how the sling works’. Conceptually, it was more difficult to understand owing to the necessity for visualising the physiology of the urethra. However, most understood and could describe the basic concept of its functionality. Nevertheless, this highlights the importance of any supportive information on the two procedures to clarify any potential confusion.
For many, the sling was seen as having better longevity because the surgery was less invasive, with a shorter recovery period, and replacement parts were not required. The overall satisfaction of patients with these expectations is likely to be related to whether or not these high expectations are realistic and, thus, if patients can make fully informed decisions; the limited evidence for relative success rates of the procedures should be clear.
There were various uncertainties surrounding the relative suitability of the sling for those with an active lifestyle and these should be addressed.
Outcome expectations
There were a large number and complex set of expected outcomes mentioned by the participants. The importance of an improvement in continence and reduction in the use of continence aids was confirmed as the primary outcomes for all men. In addition, with the expected reduction in their symptoms, there were high expectations of improvements to their quality of life. This included hopes for an improved social life, a more active lifestyle (e.g. gardening, swimming and cycling), reduction in nocturia and going back to work.
The effect of their incontinence on their confidence, mental health and general well-being were mentioned by several men, and UI is known to be associated with higher rates of depression. 51 Improvements in the level of continence was perceived to have a complementary positive effect on well-being and mental health, thereby underpinning a broader expectation following surgery.
Study 3: explore the participants’ experiences of the two surgical procedures
Aims
The focus of investigation with this group was to explore the experiences of participants who had undergone both trial procedures. This investigation explored two distinct areas:
-
surgical recovery – how participants felt during their recovery period, how long they spent in hospital, the symptoms that they experienced, the immediate perspective for those who had the sling and for those who had an AUS fitted, and their experience of the AUS being activated
-
life after surgery – what differences surgery had made to their life, how successful their surgery had been and the symptoms they experienced post surgery.
Methods
The third subgroup was recruited 6–24 months after their surgical procedure to explore the post-surgical experience of men undergoing a sling or an AUS within MASTER. They were interviewed once and the interviews were conducted between January 2015 and November 2018.
Results
Having extensively investigated the men’s perspectives pre operation, we compared the experiences after surgery for both procedures and evaluated the differences in experiences between the two groups (Table 27). Fifteen men were recruited to this subgroup, aged 58–74 years: seven men who received an AUS and eight men who received a sling.
| Theme | Subtheme |
|---|---|
| Surgical recovery | Inpatient stay |
| Immediate perspective of sling | |
| Activating the AUS | |
| Sore and swollen | |
| Life after surgery | Adapting to the AUS |
| Level of continence | |
| Life changing | |
| Perception of success |
Surgical recovery
Inpatient stay
Owing to the length of time that had elapsed between the procedure and the interview, participants were unable to recall the exact length of the hospital stay; however, a number speculated that it was within 24 hours for either procedure. One man who had undergone an AUS, however, described a delayed release from hospital as a result of an allergy to the anaesthetic. Overall, both procedures resulted in a short stay in hospital of 1–2 nights for those whose procedure had gone as planned:
I was in for about 3 days, I was in a bit longer than I should have been because I didn’t take kindly to the general anaesthetic and I was being violently sick for a while.
12027 AUS
Well the day I arrived there and I think it was 2 days and then I was allowed out.
18066 AUS
I was overnight and out. I walked out without any issues, it was quite surprising.
18064 sling
A number of undesirable experiences that occurred following both procedures were discussed, ranging from longer surgery to pain, retention and swelling:
. . . when the surgeon did the operation he said the tissue in the muscles and that area was really toughened and hardened with the radiotherapy so he found it difficult to operate, it was difficult to cut and carry out the operation.
15010 sling
What happened was I had developed a massive haematoma into the scrotum and basically ended up being in hospital for 3 weeks.
32031 AUS
I had bladder retention; I couldn’t go to the toilet so they did take it out but they had to replace it to let me go to the toilet. They said it was due to postoperative swelling.
11022 sling
There were positive comments from many men about the staff and their overall hospital experience during their stay:
The staff were very good; they look after you.
11022 sling
They [the staff] were marvellous, even with a sense of humour.
12025 AUS
All the people [staff] have been brilliant.
12013 AUS
Longer hospital stays and some bumps along the road were not something that featured in the discussion about expectations, and have relevance for improved pre-operative counselling.
Immediate perspective of the male sling
Following surgery, participants were eager to have immediate results. These were anticipated to be much sooner for those who had received a sling than for those who had undergone an AUS. A range of accounts were provided; however, only one out of the eight men who underwent the sling procedure described an immediate improvement at the point of discharge:
. . . after the first few days I was fine anyway, I could get about and I wasn’t particularly delicate. I mean it’s an operation at the end of the day, you’ve got to be careful with what you do but there weren’t any major restrictions on doing anything.
33025 sling
No there were no issues, I had my operation and then a follow-up visit to the hospital fairly quickly after that.
34007 sling
I woke up and I did feel a little bit sore but that was it but the relief of my problem was almost, it was immediate you know I mean I had to pass urine before I was allowed to leave the hospital obviously and it’s transformed my life.
20005 sling
Contrasting accounts were put forward by participants who described immediate feelings that the surgery had been ineffective or a sense of waiting for improvement as postoperative symptoms settled:
It was good surgery, unfortunately it didn’t work. More or less straight away, it was just immediately you know . . . it was a slight improvement.
27022 sling
I think it was 3 or 4 weeks just to settle down and there was no effect whatsoever.
15010 sling
Variable experiences with postoperative catheterisation were also reported:
She took the catheter out and said go and sit in the other room which was a public room. I didn’t have any pads and I ended up with wet trousers because I didn’t have anywhere to go to change. I was not entirely impressed by that, I was quite upset by that.
15033 sling
I was as right as rain for 2 days, I mean I was up in about 6 hours and walking up and down the corridor and I thought this is great I thought you know the whole thing seemed to be fine you know. There’s no squirts or anything like that going on and then 2 days later I suddenly seized up and then I had to go into [hospital name] at 4 o’clock in the morning to get the catheter fitted. I had a catheter in for 2 weeks.
11026 sling
Activating the artificial urinary sphincter
Where men with a sling had been immediately able to describe how they felt about the success of their surgery, those who had an AUS reported their need to wait prior to activating the device. During this period, men recalled continuous pad use and leakage. This was not deemed particularly problematic because the men had become accustomed to this; however, some anxiety was present regarding the anticipated outcome:
It was no different really to what it was for the previous what 2 years? You know you had to wear a pad because you were leaking.
12024 AUS
I was more apprehensive of that than the actual operation because you’re thinking ‘what is going to happen? How am I going to manage?’.
12025 AUS
Well the surgery afterwards, the way I was feeling after I was questioning whether it was worth it, because you feel, the first couple of weeks because the sphincter’s not activated, you’re sore, weak and slightly worse and I think ‘I hope this is going to be worth it’ because you don’t know how the sphincters going to work, is it going to be 100% effective, 60% effective or 30% effective.
18066 AUS
Participants also described their initial daily awareness of having an internal device; however, it soon became a regular feature of their life:
I know it was a foreign body inside you for the first what, 6 weeks you knew it was there.
12024 AUS
. . . no no you’re not aware of it [AUS] at all.
12027 AUS
I know it sounds funny but even nowadays sometimes you know you think ‘oh no I haven’t hit the pump’, but I mean I can still feel obviously the pump and I’ve still got like the lump where they put presumably the reservoir.
12025 AUS
They talked about their ability to adapt to passing urine using the AUS. These discussions revealed that men had found them fairly easy to use, although some did describe experiences, such as accidental AUS activation, when sitting a certain way:
You get used to it very quickly . . . apart from squeezing the bulb in the scrotum it was just like going to the toilet normally, there was no pain.
12027 AUS
It doesn’t happen very often but occasionally when you’re swinging your legs in and out of the car you sit on the edge of the seat it can if you’re in the wrong position trigger the bulb.
12027 AUS
. . . sometimes if I sit on something and say that might dislodge the cuff and then I might have slight leak . . . it’s only when I’m sitting.
12024 AUS
The AUS was largely believed to have been successful immediately following activation, with one respondent noting that improvements were evident within 24 hours. One man suffered from an infection as a result of the AUS, but detailed his satisfaction at having a device that had worked, albeit temporarily:
As soon as it was activated it proved that you know I wasn’t constantly wetting myself, I could just switch it on and off you know whenever I wanted to.
12027 AUS
It worked perfectly, I felt really comfortable, no problem whatsoever.
18022 AUS
For the short period before it was infected I was you know quite, I was dry and leak free.
12066 AUS
Sore and swollen
Participants who underwent the procedures provided detailed descriptions of their physical experiences, including swelling and a range of sensations suggestive of pain. The swelling eventually subsided after 1 month for those undergoing an AUS and some weeks longer for those who received a male sling. Some participants also described how they felt more comfortable than they had expected after having the sling fitted:
When they did the prostatectomy she said ‘you’ll be a bit swollen,’ she said ‘you’ll be like if you can imagine an aubergine that’s what you’ll be like,’ well this was more like a melon with this job it was horrendous.
12066 AUS
I was quite sore but not you know it wasn’t unbearable. You know these big purple gelato plums that you get in the supermarket? Well that’s what my bits and pieces felt like and looked like, they were blue.
15033 sling
The wounds were kind of sore.
18066 AUS
I was really surprised at how little discomfort I had.
18064 sling
For a few participants, the swelling had resulted in discomfort that made activating the AUS somewhat difficult. Participants felt that this had contributed to a prolonged recovery, as they noted that passing urine and using the device could be fairly painful:
The surgeon said what you must do is keep pulling it down a couple of times a day. Now that was difficult because, it’s an extremely painful area, it was very swollen and you were searching for this thing which threw you a little bit because they suggested it could disappear up into your body if you didn’t pull it down.
12027 AUS
Until a lot of the swelling went down it was a bit uncomfortable. I think it was tissue more than anything, I couldn’t even feel the cuff around the urethra.
12024 AUS
Testicular pain was also explicitly described by those who had undergone a sling surgery, and one man noted that this continued to the point of the interview. Another participant, however, highlighted that this had improved within 1 week. Participants also discussed the specific pain that they had been subjected to as a result of their wound:
My testicles hurt like hell . . . my testicles aren’t anywhere near as sore as they were for about 2 or 3 months after the operation.
11026 sling
The scar in between the legs was quite sore and the testicles were quite sore for a week or so but you know again they get better.
15033 sling
Wound complications were described, along with the associated pain and practical hygiene issues. Some felt that they had received very limited information about this aspect, with one man suggesting that he received information about care for his wound only after actively pursuing this:
The wound between the legs seemed to take a long time to heal up; it carried on bleeding and weeping for some time.
12027 AUS
Terrible because it was all swollen and there was three wounds.
12066 AUS
You were sent home with a dressing on in the various places and nobody said you’ve got to change the dressing, you can wash yourself. I asked the nurses and they didn’t know either whether I should change the dressing so they gave me some dressings and said ‘well here you are’.
12027 AUS
Life after surgery
The following sections examine the differences after surgery between the sling and the AUS, looking at how the men adapted to their life after surgery and the impact that the results of surgery had on their life, for both procedures.
Adaptation
Men who received the sling were not required to adapt to new procedures in the same way as those who received the AUS. Largely, men described a fairly easy transition to passing urine when activating their AUS, although they mentioned that it required some getting used to, as it was possible to forget:
The nurse actually switches it on, activates it for you and shows you where to find it and at first um the first few days or so it seemed difficult to find.
12027 AUS
The lady [nurse] was very, very good and now it’s, it seems natural, I know it’s not but . . . I’ve got to go and you’re going and you think nothing’s ‘oh I’ve got to switch on’.
12025 AUS
You need to have two hands obviously, one’s got to hold the hose pipe and the other one’s got to squeeze the thing but you could get into a pickle with it, I see what she meant by you need somebody else to know how to operate it in case you can’t and I thought ‘why would that be,’ but when it happens see you’ve only got to bust your hand perhaps or strain a wrist or whatever you’re going to be stuck aren’t you, you can’t do it.
12066 AUS
Further to this, a key theme that emerged centred on the difficulties that these men encountered when using public toilets. More time was needed to pass urine using an AUS and the men said that they preferred to activate and use their AUS within a cubicle for the sake of privacy. This also appeared to show that participants were self-conscious when doing this within a public setting:
I try to avoid urinals if I can, if I can just go into a toilet and close the door and then you know I just got to take my trousers down but that’s not a problem, but after using urinals you know I think this is something that’s a bit more personal and that’s why I try to avoid urinals.
12024 AUS
Only slight downside is like all men I can’t go to a public urinal I’ve got to look for a cubicle because you’ve got to sort of half undress yourself to get at it, you can’t just unzip your fly and do it that way, it doesn’t work like that.
12027 AUS
Level of continence
The majority of the men who underwent the AUS procedure were continent, and this was also temporarily experienced by one participant who had suffered from AUS erosion. For this participant, the eventual removal of the AUS had resulted in a painful experience of urinary retention, severe infection and a return to pre-operative incontinence and bleeding. This was, however, viewed as an isolated incident and was not perceived by the participant as indicative of the effectiveness of every AUS. This participant repeatedly speculated that there had been an issue with his particular device, resulting in it becoming septic. Nonetheless, the majority of AUS participants were extremely satisfied with their procedure and the ability to finally have some control over their bladder:
I accepted the fact that probably it would never go completely but the fact it’s working 95% now is a blessing so even if, heaven forbid, it packed up next week or I had to revert at least I would have had a whole year without any worries or such.
12025 AUS
The men commonly provided figures to show the extent of their improvement post operation; those who had undergone the AUS repeatedly asserted that, although their continence had not been achieved completely, their continence was now much better than it had been pre operation. Some LUTS were described by a number of men, including stress incontinence, which was mentioned by two men who needed to use pads as a preventative measure to ensure that their clothing would not become wet. This was, however, considered to be an acceptable outcome because the number of pads and volume of leakage had reduced significantly:
I was hoping to be fully continent again and basically afterwards I am . . . The fact that you can’t control yourself in any shape or form before, now I’ve got virtually full control, 90% control.
12027 AUS
To be somewhat drier than I was you know, and I certainly am not 100% but compared with what it was, a lot drier and I mean I didn’t let it sort of affect me day to day like although it was very difficult. But now oh I feel like yeah it’s marvellous like a weight’s been lifted.
17006 AUS
Residual leaking when sitting was reported by a man who had received an AUS, although the rarity of this occurrence meant that the participant did not view this as particularly problematic. Moreover, he had speculated that this was largely because of the position in which he sat. He further noted that he had continued to wear pads, but the number of pads had reduced:
I could sit on something and say that it might dislodge the cuff that’s going under the urethra and then I might have a slight leak and I know then I think I must shift. It’s only when I’m sitting not when I’m standing, walking around its 100%.
12024 AUS
I play golf totally dry, go on long walks now with my wife, carry the shopping home sometimes you know with a little walk, carry the shopping home where you really exercise the abdominal muscles, excellent.
18066 AUS
I’m happy with the performance of the sphincter.
18066 AUS
Those who had been randomised to the sling procedure, comparing their improvements with their pre-operative symptoms, felt a sense of relief because, for many, the symptoms were no longer debilitating:
So to go from being totally wet to being not incontinent it was great, you know I was very, very happy and I was happy with the outcome.
34007 sling
Well I mean 40 ml is not hugely leaking is it, really in the great scheme of things, nobody wants to be, it didn’t go down to zero though which was a disappointment. Not totally successful but a success in as much as it reduced it quite a lot.
33025 sling
So in the first couple of weeks I actually felt like He-Man [™ Mattel, Inc., Los Angeles, CA, USA], I was absolutely bone dry, I thought this was superb. Even now when I do heavy physical stuff it’s a pad a day.
18064 sling
For one man, an incident of severe leakage following a sling procedure was considered to be a cause for concern; however, following this, his ability to pass urine had immediately returned to what he felt was ‘normal’, with the exception of a tendency to stop and start:
It’s literally like it was before I had the original operation you know no problems whatsoever, I can feel when I need to go . . . I mean when I go for a pee I do have to sort of stop and start again just to make sure that I’ve emptied my bladder out sort of thing.
15033 sling
Stress incontinence was also reported by a man who had initially presented with severe incontinence that required a Conveen. Although he now wore a pad to protect himself from leakage when he expected to drink large volumes of liquid, he repeatedly asserted that his outcome had been more than satisfactory, despite the fact that he had recently developed stress incontinence:
I don’t wear a pad generally, only if I’m going out for a long period of time or if I’m going to a social event where we’re going to be drinking I might wear one then and it’s only just in the last 6 months that I’ve stress incontinence occasionally . . . I mean the improvement is huge I would recommend it to anybody.
20005 sling
However, not all of the men in the sling group considered the presence of any level of LUTS to be acceptable. Two men emphasised that, for them, the sling had been completely ineffective and their incontinence had continued with minimal improvements. One man stressed his dissatisfaction with the procedure because of his inability to retain quantities of urine, some dribbling and a weak stream; however, he accepted that his incontinence had significantly improved:
It’s infinitely better than what I had before you know where I just used to walk around peeing you know . . . I can only retain about 300 ml of urine then I get the urge and by the time I reach 400 ml I’ve got to go you know my whole brain tells me ‘go for a pee go for a pee go for a pee’ . . . I can’t pee standing up properly, if I do about a minute or so afterwards I suddenly have a big squirt.
11027 sling
As far as I am concerned, the sling hasn’t worked at all, it’s made a little bit of improvement but very little. I’m still leaking up to maybe 700 ml a day which is a lot of leaked liquid.
27022 sling
Just total incontinence, it’s continued to this day.
15010 sling
Life changing
For all men who reported continence following their surgery, their specific procedure and its subsequent effects were thought to have had a substantial impact on their lives. To illustrate this, the men presented comparisons of their life with incontinence with their life at the point of interview and discussed a wide range of changes. The ability to control when and where they would pass urine was, understandably, the most important change, but they also felt that this had enabled them to do things that previously their level of incontinence had prevented:
Very pleased about it, I’m glad that I had it done.
34007 sling
It’s the answer to all your prayers if you’ve had no control and all of a sudden, pardon the pun, but the thing is the control’s in your hands.
17006 AUS
But it’s so much better than I had for 2 years. I mean I can’t tell you, I can go in the sea, I can wear swimming costumes you know I can do anything.
15033 sling
It doesn’t cause me problems and I can walk a lot um I mean, was it last week, you know we’ve done 6-mile walks, 2-mile walks across the cliffs at Bude.
12025 AUS
I think it’s had a big impact in as much as before the operation I was looking at, you know, it’s bad to say it but you know when you were young and you see all these old men and all that type of thing and I didn’t want to end up as one of them and I feel as though I’m not going to do that now.
18066 AUS
Men also stressed that the presence of LUTSs may be considered a negative outcome for some, but emphasised that, in comparison with their experience of severe incontinence, this was the preferred option. This highlighted the individuality of the man’s perspectives because it recognised that not all men would be satisfied with this outcome:
Compared with as I was before, this is a godsend. If I hadn’t experienced all that before and just come from being dry to having these leaks, I think it would be bad but now I’ve experienced what I had before.
17006 AUS
The men also appeared to draw on the concept of masculinity during these discussions, which emphasised the psychological impact that their previous symptoms, and surgery, had had on their mental well-being and identity. They suggested that a lack of bladder control had been humiliating and in regaining control they were able to rebuild the self-esteem that they had lost. For one man who had been previously catheterised, the ability to simply pass urine standing had made a tremendous difference to his psychological frame of mind. It was further stressed that the positive improvements meant that they were able to fully engage with their daily lives rather than remain preoccupied with their incontinence and the need for the toilet:
I should say it’s given me back 90–95% of my dignity really.
20005 sling
It’s very much a mental thing but it’s sort of demeaning almost for a bloke and it’s just so nice, it’s such a relief to be able to stand there.
15033 sling
You don’t have to plan thinking ‘oh now where is there a toilet to?’. You just take every day as it comes now.
12024 AUS
Before was horrible, the after is great. It is like being a different person.
12025 AUS
However, not all men were able to share such positive experiences and, for some, the lack of improvement had been particularly disappointing. This was largely because of their expectation that such improvement would have ultimately enabled them to do things that they regarded as normal. In such instances, some had become almost resigned to the fact that the improvements and changes that they had hoped for were not attainable. The resulting frustration meant that pursuing any further surgery was out of the question:
I hoped to have control back and a life back really so that I could go out and about and do normal things which I haven’t been able to do, it’s very disappointing I’ve got to accept I think how it is.
12066 AUS
Perceptions of success
The men who had reported improvements to their incontinence and quality of life viewed their procedure as successful. Some men drew on their previous views regarding the success rate of an AUS and, in doing so, perpetuated the view that the AUS was a superior procedure to the sling:
I have read different reports that call it five-star deluxe and I agree with them.
12024 AUS
It was a lot of pain and discomfort to go through, but the end result was worth it.
12027 AUS
However, for those men who reported an effective sling, a shifting perspective towards the procedure was presented. It was highlighted that the procedure had exceeded expectations, given that some men had felt that the sling would not be as effective as an AUS in terms of treating their incontinence. Thus, having a less invasive surgical option and being able to control their ability to pass urine after severe incontinence were sources of satisfaction:
I couldn’t see how a sling would help me control it as much as it did but it’s just helped me control it completely, so it’s so much better I can’t tell you, it’s such a relief.
15033 sling
For one participant, however, the LUTSs that he experienced after his sling procedure were a source of great disappointment and anger. He repeatedly asserted that he had felt that an AUS would have been the appropriate option for his symptoms as the effect of the sling was less than satisfactory. This participant felt that the trial, and the NHS, had failed him. This view was partly shaped by previous surgical procedures and errors in treating other unrelated health problems. This account emphasised how previous experiences could have a bearing on the present procedure:
The bottom line is that I’m in better condition now than I was previous to it but it isn’t a 100% and for me now it appears to me as though I had the wrong operation, I’d be better off with a sphincter.
11027 sling
Another man had to have the AUS removed and saw this as a retrograde step; he then felt reluctant to have another operation:
I ended up having to have the artificial urinary sphincter removed and all the haematoma taken out and since then, basically I’m back to one pad a day.
32031 AUS
I am reluctant to get back to having a further operation unless I can be more or less guaranteed it’s going to work.
32031 AUS
Furthermore, questions remained regarding the longevity of their particular procedure. It was widely believed that the AUS would work for up to 10 years, which was a matter of concern for older men who had discussed the possible problems that an invasive procedure could have in the future:
The only thing that I have in the back of my mind all the time is that they give this sphincter a 10-year life span, not necessarily 10 years but that is the figure that’s been bandied about. My thoughts will be I’d be 80 then and such an operation would be to redo it in anyway would be probably a little more hazardous.
17006 AUS
If I’d have been told then that if it comes back and you need radiotherapy it’s going to be destroyed and it might have to be removed or whatever I might then have said alright let’s not bother I’ll just carry on with the Conveens, I wouldn’t go through the operation in that case. It was something I never gave a thought to.
12027 AUS
Discussion
Surgical recovery
Despite some recall difficulties, most men reported that hospital stays ‘appeared’ relatively short. Few men who received the sling experienced an immediate improvement post surgery, although there was some report of slight improvement. By comparison, those who had an AUS fitted, although they had to wait 4–6 weeks after surgery for it to be activated, found it to be largely successful, with many experiencing positive results immediately after the AUS had been activated. Discomfort post surgery was experienced by all; however, there were varying degrees of discomfort, with some finding it better than expected and others experiencing swelling for a few weeks after. This resulted in some pain when trying to use their AUS once it was activated. Further information regarding wound care was highlighted as an unmet need for some men. This has potential implications for improving the patient experience. Realistic explanation of the recovery period and timelines for improvement are key to managing men’s expectations in the post operative period. Most men interviewed highlighted that any reported issues largely resolved over time.
Life after surgery
For both procedures, most men agreed that their quality of life had improved and, in line with reported expectations pre operation, complete continence was not necessarily required for a successful outcome. Inevitably, experiences varied from being straightforward and without incident to having complications along the way. It was recognised that there were variable circumstances for these experiences that were not necessarily related to the procedures.
The impact of success following surgery was farther reaching than symptom experience, with many men feeling that they were able to return to a sense of normality that reflected their life prior to their original prostate surgery. The associated emotional boost described by some provided a new lease of life that led to endorsements for both procedures. For some men, however, surgery was unsuccessful and the requirement for further intervention is discussed below.
Study 4: explore participants’ experiences for those requiring further surgery
Aims
In the previous substudies, we reported that surgery was regarded as successful by some men, but some men did not achieve the outcome that they were hoping for. During the study period, some men underwent further surgery and it was important to explore the reasons for this and the eventual outcomes.
Methods
The final subgroup were recruited from the main trial participants who had undergone an initial surgery for their PPI but required further surgery. The qualitative study team were informed of those requiring subsequent surgery and were all invited to participate to give an account of their opinions and the variability of reasons for further surgical input. The focus of enquiry with this subgroup was:
-
reasons for further surgery – why patients needed a second operation, what their symptoms were after their first operation and how they felt about this
-
feelings after second surgery – the results of the second surgery, their perception of how successful it had been and the impact that it had on their lives.
Results
Ten men aged 58–73 years who consented to participate in this substudy were interviewed. Three men had received an AUS as their first surgery and the other seven had received the sling; nine men went on to have an AUS fitted as their second surgery and one who had received an AUS was waiting to hear from the consultant about reoperation at the time of interview (Table 28).
| Theme | Subtheme |
|---|---|
| Reasons for reoperation | Failure after surgery |
| Feelings about procedure failure | |
| Feelings about second surgery | |
| Feelings after second surgery | Satisfaction |
| Impact on life |
Reasons for reoperation
The definitions of success following surgery have been explored during this study; it is important to look closely at the factors driving the requirement for further surgery to understand the potential threshold for lack of success.
Failure after surgery
Some of the men experienced immediate failure of their surgery, with no improvement at all:
Nothing happened with the sling it just didn’t work at all . . . it just made no made no difference really.
20021 sling
There was no improvement . . . I was just back to where I started.
11022 sling
After about a week, when I was leaking like I was, [I realised] this isn’t working.
24023 sling
Others found that their procedure was partially successful, but over just a few months they experienced a decline to the point that they were back to where they had been prior to surgery:
But I still leaked a bit, after the operation [and then] it got worse. I was peeing like a tap, like an open tap.
24023 sling
It got worse you know to the point that I wouldn’t say I was house bound but I was almost not waiting to go out because I knew what would be happening . . . I was significantly worse after the operation than I was before.
20009 sling
It’s suddenly gone [and] started leaking again . . . I think it was, it was over a period of time I think, it was getting worse and worse.
12017 AUS
. . . it was fine and then all of a sudden the unit broke down again and I was back and it was as if somebody had clicked the switch overnight.
13016 AUS
Feelings about procedure failure
When they realised that their procedure had not worked, many men were frustrated, disappointed and resigned to the fact that they might never regain continence or that they would require further surgery and have to go through the pain and recovery again:
Yes, it is a bit frustrating yeah because I’m thinking you know what are they going to do to me next? You know, they try this and try that you know, as I say I don’t mind in a way really because I just want something if they could do something for me.
12017 AUS
I was resigned to the fact that I didn’t feel that there would be any improvement.
11022 sling
. . . you know very disappointed because I mean I know that I’m facing surgery again which is obviously not something that you want and clearly now I know what the pain is going to be like so you’re looking at another 6–8 weeks of awful pain.
16055 sling
One participant described how having the AUS fitted lifted his depression but then, with the failure of the device, his depression returned instantly. Another participant, who was depressed after the failure of his AUS, felt that nothing was being undertaken to solve the problem. He was no further forward, having to use pads daily because he was still incontinent. It was evident not only that there was an impact on physical health, but also that there are intrinsic links with mental health that emerge as a result of being incontinent, with feeling the necessity to carry on with ‘normal’ day-to-day life. Men reported that quality of life was greatly affected by this:
My depression I’d say lifted, I was happy, I was more back to my normal self how I used to be with people and it was fine and then all of a sudden the unit broke down again . . . it was really depressing, it drags you down day after day after day.
13016 AUS
I’m depressed at the moment because after 5 years of wearing pads I’ve been to the hospital so many times and thinking I’m back to square one again no ones’ going to do anything about it you know.
12017 AUS
Feelings about a second surgery
After discussing their options for the next step and being offered a second procedure, feelings were mixed among the men. Some men were hopeful that they would gain control over their incontinence having been given a second opportunity, whereas others, having had a sling that failed, were considering an AUS, seeing it as a last chance. One man even felt that he was at a point at which he would do anything to regain control over his incontinence:
. . . obviously when you’ve got a second option to look forward to it gives you hope . . . I’ve got a second chance.
11022 sling
I have high hopes [for the second surgery].
26005 sling
The last chance sort of situation isn’t it really? . . . It’s something that you know you need to do so you don’t have a choice.
16055 sling
I’d do anything basically to cure this problem.
26005 sling
Feelings after a second surgery
An exploration of the men’s feelings and experiences was undertaken after their second surgery to reflect the anticipation of the results, having already experienced the disappointment of a failed procedure. As with the first surgery, all of the men had hoped for an improvement in symptoms and a reduction, if not eradication, in the use of pads.
Satisfaction
Once the second surgery was complete and the AUS had been fitted, most men were satisfied with the result, reporting a significant improvement, if not complete dryness:
Instant dryness.
12046 sling
As it stands at the moment, I’m probably looking at a 90–95% you know sort of better.
20009 sling
I’m quite pleased in as much that I don’t have to worry about having a dry pad with me all the time.
20021 sling
Impact on life
The impact on their life was also important, and many men described feeling ‘normal’ and being able to do all of the things they had not been able to do previously:
It was just an elated feeling knowing now that I’ve got you know sort of most of my previous lifestyle back.
20009 sling
Back to normal.
12046 sling
Well yes I’ve got my life back.
24023 sling
Despite the men’s satisfaction with the final outcome after their second procedure, many found the wait excessive between having the first procedure, realising that it had failed, deciding to have another procedure and actually having the second procedure. They felt that it would have been better if they could have been booked in sooner for their second operation, having already waited initially for the first operation:
It would have been nice if they could have said ‘Oh it’s not working let’s book you in for the next session we can do this’.
24023 sling
Yeah I’m satisfied with what’s been done but it’s just taken so long.
26005 sling
Discussion
Reasons for reoperation
The 10 men who were interviewed were all offered a second procedure to try and help to control and manage their incontinence. Many felt frustrated and disappointed after discovering that their first procedure had not worked, whether this was immediate or showed a decline over a period of time. This affected men both mentally and physically, with some feeling depressed and others feeling that they were back to where they started with their incontinence in terms of the impact on their social lives. The opportunity for a second surgery brought about mixed feelings: some felt hopeful and that they had been given a second chance, whereas others felt that they had no other option or were resigned to the fact that they were unlikely to ever regain continence.
Feelings after a second surgery
Once they had their second surgery, many men reported satisfaction with the outcome. Many men felt that they had got their lives back. Most of the men felt that the wait between the decision to have a second surgery and the procedure being carried out was too long and that there should be some recognition of the previous experience that they had been through. The majority of the men interviewed who went on to have a second surgery had initially had a sling fitted and all received an AUS for their second surgery. This served to reinforce the preconceptions about differences between the two procedures for some of the men, with the AUS being perceived as the superior and more definitive solution.
Study 5: explore the experience of surgeons who perform both procedures
Aims
As discussed earlier, the surgeons counselling men for these procedures are influential in the decision-making process and are key to the discussions that underpin surgical options. It was, therefore, imperative that we explored the perspectives of these surgeons regarding the clinical experience of conducting these procedures, as well as their viewpoints on the relative merits or limitations of each.
Methods
Surgeons performing both procedures within MASTER were invited to participate in interviews. The experience of performing these operations and the surgical considerations involved are crucial to understanding how the findings might be taken forward by clinicians. A total of 20 surgeons were recruited. Areas for exploration included:
-
surgical experiences – the perspective of the surgeons of each procedure, the differences and similarities between each, complications and duration, and if surgeons have a preference and any reasoning behind this
-
clinical experiences – looking at the suitability of patients for each surgery and those patients who have previously undergone radiotherapy, the perspective of the surgeons on their patients’ preferences and meeting their expectations, complications that can occur and the surgeons’ own perspective on the procedures (Table 29).
Results
| Theme | Subtheme |
|---|---|
| Surgical experiences | Invasiveness of procedures |
| Procedure preference | |
| Procedure complications | |
| Procedure length | |
| Clinical experiences | Patients’ preference |
| Complications | |
| Meeting patients’ expectations | |
| Patient suitability and surgeon perception of procedures | |
| Patient suitability after radiotherapy | |
| Own perspective |
Surgical experiences
Invasiveness of procedures
Most surgeons considered the AUS to be more invasive than the sling, which may have an impact on its consideration as a surgical option:
I’d say the artificial sphincter is more invasive in terms of you’ve got two incisions and also you’re placing something in the scrotum which is uncomfortable for the patient.
13
There is an additional incision when you do the sphincter clearly because of the groin incision to place the pump and the reservoir.
4
Well I think that the sphincter’s certainly more invasive, because you know as well as doing two incisions you’ve also got to create space in the scrotum; so you know there’s often a bit more bruising, well a lot more bruising after a sphincter . . . definitely more invasive but I’m not sure that’s a very important concept per se.
15
I mean because of the needs or the way I perceive the need for the second incision with sphincter, they are a little bit sorer and it is more invasive, yes.
16
Procedure preference
Surgeons tended to prefer the AUS; this was because they had more experience of the procedure. Although the AUS was considered a more challenging procedure than the sling, the sling was a relatively new procedure for many surgeons, so they were not as comfortable with it. It was noted that the sling was often reported to be a slightly simpler operation:
The sphincter is a completely different dissection, you have to go round the urethra and then there’s a separate abdominal incision and actually I feel happier doing a sphincter than I do a sling because with the sphincter you know exactly where everything’s going at every point.
01
I actually have done more sphincters than male slings and probably found male slings a little bit, I wouldn’t say less but I’m less comfortable with it. I actually have done many more artificial sphincters than male slings so I think in terms of technically for me that’s my preference.
11
I’ve been a consultant now for 16 years and I’ve been doing sphincters all that time and I’ve done slings I think for about the last 3 years.
18
My experience of sphincters is greater really.
15
Some surgeons talked about how there was one part of the sling procedure that they found quite tricky: they were not always able to see where they were guiding an instrument. This was one of the reasons that they preferred the AUS over the sling:
The bit I don’t like about the sling is the sort of kind of blind, albeit guided, passage in a very short metallic instrument around the pelvis so that’s maybe you know something I don’t like too much.
12
With the sling procedure you have this moment where you’re passing the trocar through the obturator foramen and you’re never quite sure exactly what you’re going through.
01
The actual sling procedure putting the trocars in you know for me is not the most comfortable.
10
Procedure complications
Surgeons discussed complications with both procedures. Reported complications with the AUS included infection, difficulty placing the AUS and revision surgery for the AUS. However, the complications were perceived to be in only a few cases:
Obviously the complications are related to infection or erosion.
08 on AUS
I’ve only had one sphincter that I’ve not been able to place intraoperatively because of a urethral compromise and that was not part of the MASTER trial, the guy had had radiotherapy and everything was stuck down so and that was fine because all I did was then place a sort of fat pack behind the urethra and then come back and put it in about 3 months later and everything was fine.
01
I’ve had a couple of persistent two or three, three patients I think with persistent leakage that have required additional cuff placement which has been successful.
05 on AUS revision
The artificial sphincter, the only real issue is having the re-dos which is not part of the MASTER study you know so revision surgery is more, technically more difficult.
14
By comparison, the complications encountered with the male sling were generally risk of bleeding, the difficulty of manoeuvring anatomy if the patient has a small pelvis and the possibility of causing damage to the urethra during the operation:
With the sling you’ve got to cut further back off the central tendon so there is a risk, there’s more a risk of bleeding with putting in the sling.
02
I do occasionally encounter a small amount of bleeding when I place the trocar for the sling and that’s always slightly worrying because you’ve got no direct pressure point to put it on.
01
The male sling, if the pelvis is very small and deep then I’ve had a couple of occasions where it’s been very difficult to get the trocars around the obturator canal underneath the pubic bones to be honest so that’s been quite difficult. I’ve had one patient who I’ve not managed to do that on and had to abandon.
14
Procedure length
Surgeons noted that the sling procedure was shorter than that for the AUS, reporting a varying difference in the time that it took for each procedure; however, all surgeons reported that the sling took less time because it was a simpler procedure that required fewer incisions:
If it’s a simple straightforward artificial sphincter I can do that in an hour and a half, if it’s a male sling it’s probably just over an hour. From a patient point of view there’s no substantive difference.
14
The advanced male sling is certainly quicker, probably takes around 30 minutes. The artificial sphincter takes about an hour and a half, 2 hours.
13
It’s a lot shorter once you’ve done that it only takes about 30 minutes to do whereas the sphincter’s sort of 45 minutes skin to skin.
01
It takes probably about 30 minutes to do a male sling, 30, 35, maybe 40, but it takes about an hour and five to do a sphincter so there’s also a few more steps in putting in a sphincter and a bit more dissection.
15
And the sling is much more straightforward, so 30/45 minutes.
16
Although the AUS was seen as a more invasive procedure, it was also favoured by most surgeons. This was mainly owing to most surgeons being more familiar with AUS surgery and, therefore, they felt more comfortable with it. Both procedures had their own complications. The time for each procedure varied among the surgeons, with all reporting that the sling was a shorter procedure because it was more ‘straightforward’, with most reporting a time of around 30 minutes. However, there was a variety of times reported for completing an AUS, with no clear consensus and reports ranging from 45 minutes to 2 hours.
Clinical experiences
The following sections explore the clinical experiences of surgeons with both the sling and the AUS, and their views of their patients’ preferences, expectations and suitability.
Patients’ preference
It was felt that, in general, patients had a preconception of what would be most suitable for them or an overall preference before seeing their surgeon, which influenced consultations. Prior to seeing the urologist, patients have often read about the procedures, looked them up on the internet or spoken to their general practitioner (GP):
Patients often come in with a preconception of what’s right for them.
15
Some of them do, almost all of them, have a preference.
18
They’ve often read about what’s available to them before they’ve come. You know these are informed, they’ve been on the internet, they’ve spoken to their GP.
19
By the time they’ve come to me, they’ve seen physios, oncology people, other urologists, registrars and friends, support groups and somebody along the way has influenced them one way or the other as to what their treatment option should be. It’s very rare that they come and they’re completely happy to have either.
20
Surgeons thought that many patients would prefer the sling because the thought of having to ‘operate’ the AUS might discourage many patients. A successful sling would mean one procedure for the patients and then they are almost back to normal, without having to do anything further. In the surgeons’ opinion, this made the sling more attractive to patients:
They don’t like the idea of manipulating a pump and then I go through the risk of erosion, infection and device failure, so they don’t like that side of it. So if it was up to the patient I’m sure they’d choose the sling every time.
01
Some men don’t like the idea of having to press something and yeah I like the idea that with the sling you’re almost back to normal if you like, that you don’t have to do anything.
13
I just think that a lot of men don’t like the idea of having to do something so I think there’s two reasons for patient preference. One is that men don’t want to have to fiddle with their scrotums before they have a pee, which will be the case of an artificial sphincter, and the other one is they have this slightly misguided, in my opinion, view that the suburethral sling is much less invasive.
12
I think they find that option attractive that they don’t have to do anything once the surgery has been performed. There’s no pushing, there’s no switch, there’s no pump.
09
Some men really think that the male sling is less invasive, it’s less involved because they don’t have to do anything to actually pass urine and they prefer that.
11
Some want the sling because it’s less bulky and it’s a new procedure so some patients want that.
07
Perceived preferences as a result of the sling being new and a keenness to have the latest procedure were tempered with concerns around newer procedures with fewer follow-up data available. There are data for the AUS on how it performs and how long it lasts, whereas these are unknown factors for the sling:
The artificial sphincter has been there for 30 years, we know the ins and outs of it, while in a sling it’s new. We don’t know how the surgical technique, how important is this, we don’t know in the long term, in 5–10 years’ time, how those people will behave.
06
I just wonder how efficacious the sling will really prove to be and how long it will last.
10
The sling it’s a much more of an unknown quantity in the long term, there’s been a lot of issues coming through on females with meshes, whether we will see anything similar in the future and that didn’t come through till 10, 15 years down the line.
02
Recently, the polypropylene mesh implant used in female incontinence and prolapse surgeries has been in the media, causing public concern about its use owing to some women suffering from long-term side effects. NICE guidelines have since been updated and the mesh implant is still advised for the treatment of male incontinence. 52 This media coverage of the female mesh implant53–55 was also a concern for some of the surgeons, who felt that the male sling may result in similar problems in the future. However, it has not been in use long enough for this to be evaluated.
Preference was exhibited by surgeons for specific procedures based on clinical effectiveness and suitability for their patient, dependent on patient history. Some considered the AUS to be more reliable, but it may not necessarily make the patient 100% dry. It was seen as a significant improvement, although the patient might experience a degree of stress incontinence, but generally be socially dry:
Artificial sphincter is more reliable in terms of my experience, again more reliable in terms of a dryness rate going to be 80% to 90%.
05
I always tell them that with a sphincter we don’t expect them to be 100% dry, we say we expect them to be socially dry and with a very significant improvement but it’s not uncommon for them to have a bit of leakage on coughing, heavy coughing or lifting.
02
The artificial sphincter to me is a much better although slightly more major operation with more satisfied patients.
08
Complications
With all surgical procedures, there are risks of complications, which will vary depending on a multitude of factors. With regard to those fitted with an AUS, cases of mechanical failure of the AUS itself were seen, as well as erosion of the device, which resulted in some surgeons experiencing a higher revision rate. There were also some anxieties about infection control, with the AUS having a higher risk of infection than the sling:
More mechanical failure problems and higher revision rate due to mechanical problems and complications.
05 on AUS
We counsel patients for the failure in a sphincter device [and] the risk of erosion.
04
Obviously you know great anxiety about infection control with the sphincter which you’re slightly less worried about with the male sling.
15
The main complications relating to sling insertion were considered to be retention, voiding function or, in some men, not making any difference at all to the level of incontinence:
With regard to the slings the complication that I see the most is retention, the inability to void after the procedure.
12
The main one with the male sling is voiding dysfunction so difficulty in voiding afterwards.
14
The biggest complication we’ve had is when the slings don’t work.
02
Some surgeons thought that the slings were fine when they worked well because it meant that the patients were dry and did not have to squeeze a device every time that they needed to empty their bladder:
When the slings work well they are fantastic, the men are dry and they’re not having to use a device.
02
Meeting patients’ expectations
The surgeons’ main aim was to restore some normality for their patients and, although many patients hoped to be completely dry, surgeons often prepared them for the chance that they may need to use a pad for safety or they may still suffer from some stress incontinence after the procedure. Many saw dryness as their patients’ main goal. This was not always achievable, but surgeons thought that, if they improved their patients’ continence, they would be able to return to normal activities; most patients were satisfied with this:
I think most of them want to be dry, most of them don’t mind if they have to wear one pad for security because I’ll always tell them they won’t necessarily be pad free and most of them have said ‘as long as it’s better than what I have now, I don’t mind wearing a pad’.
11
First of all they want dryness, now what you mean by dryness is also a very important perception because it’s very important whether we say you’re going to be absolutely dry or whether you’re going to be a lot better than you are but you may still have to use a safety pad or incontinence pad . . . I mean at the end of the day what you’re looking for is a patient to return to some sort of normality when we’re talking about quality of life.
14
It’s really all about continence for them.
12
Their expectations are to be completely continent . . . I mean you know I think men are often happy with you know not necessarily total continence which is a bit of a you know fake concept anyway but you know if the men are leaking quite large volumes which is uncomfortable. I think men don’t mind leaking a few drops here and there.
15
I think most patients want to get away from pads, and want to return to activities.
20
As clinicians we might be interested in the 24-hour leak . . . but that’s absolutely meaningless to a patient. What the patient is interested in, and I think these things all should be patient sensitive, are they happy? Are they satisfied? And is their quality of life better?
09
Patient suitability and surgeon perception of procedures
In certain situations, surgeons felt that some patients would not be suitable for an AUS, such as those who were mildly incontinent, because the procedure was seen as large scale and, therefore, possibly unnecessarily complex for milder symptoms. Some surgeons were anxious about placing an AUS in young patients owing to the AUS’s life span and the number of procedures that they might require over the rest of their lives. In addition, it was thought that, for those patients with hobbies such as cycling, the AUS would interfere in the way that they sat on the bike and could be activated accidentally:
Patients who are very mildly incontinent with good-quality sphincter, good urodynamics, no other complication factors and a low volume leaked then you could probably argue that an artificial sphincter’s overkill.
14
The other group of patients that we come across are those a very keen cyclists. I have quite a number of them. Clearly a sphincter would be a disadvantage.
04
I have anxieties about putting sphincters in very young patients because they will have to have a number of procedures over the years . . . if they’re in their 50s and 60s, which patients are now, I think it is a concern about putting a sphincter in them.
18
Many surgeons felt that those men with severe incontinence would not fare as well with a sling as with an AUS. Some surgeons reported that this was the case in the men they had seen who were severely incontinent; the results were not as satisfactory with the sling:
Patients who are completely incontinent, who have got no control, then a male sling is not appropriate.
14
This is just sort of anecdotal experience that the very wet men are not doing as well on the slings which is what all the research has been suggesting, so I mean do feel a little bit uncomfortable offering a sling to a very wet man.
02
I know one patient very well, leaks about 850 grams on average and he’s miserable, it’s just that he has absolutely no sphincter, no muscles to contract when we did the cystoscopy, so I just wonder what’s the chance of this guy getting dry by putting in a sling?
04
One surgeon estimated that 50% of slings failed, which resulted in patients going on to having the AUS fitted as a second procedure to see if that would improve their continence; concerns for younger patients were also voiced:
Or they’re young and they’re very active, well I have no idea how things are going to work in 10 years’ time, you’re only 60 you know or 50 you know it would be wrong of me to suggest you have a sling because in 10 years’ time you’ll be 60 and might be looking at having extra surgery that perhaps you didn’t need to have.
19
Patient suitability after radiotherapy
Many surgeons had concerns over the success of a sling in patients who had received radiotherapy owing to the previous damage caused. Some surgeons had a degree of success with this group of patients, but an equal number of men encountered problems after having a sling fitted:
Although two or three patients have done well with radiotherapy, I’ve had at least three or four patients who have had big problems after male sling after radiotherapy with worsening incontinence, no better incontinence and then difficulty with their sphincter afterwards with infection or erosion.
14
Unfortunately, no matter which way you look at it it’s not number one evidence but there are definitely worse outcomes with the sling in radiotherapy and for me that is a slight problem.
09
I think that I generally don’t offer the sling to people who’ve had radiotherapy although I’m aware that there was a study in the European Journal of Urology which didn’t find radiotherapy as one of the factors that predicted an adverse outcome or a worse outcome . . . I, like most people, still haven’t really got away from thinking that patients with radiotherapy probably aren’t going to respond well.
20
I have actually just put someone into the trial who’s recently had radiotherapy, but I’m quite uncomfortable with slings and radiotherapy.
16
I actually spoke to a couple of my colleagues who also recruit for the trial and asked them what their outcomes had been for men who had had male slings and had had radiotherapy and in terms of what they had said, and they’re all fairly experienced, they’d all said that it hadn’t been worse than those who’d had sphincters.
11
Because of the possible damage caused by radiotherapy, one surgeon felt that both the sling and the AUS would be less effective in patients who had undergone this treatment:
My personal view about post radiotherapy patients is that both sphincter and the sling will have less effectual outcome whatever you do. The post-radiotherapy patients will have a not as good outcome as the non-radiotherapy patients.
17
Personal perspective
Similar to patient preferences, surgeons held beliefs regarding both procedures that were often entrenched. Some displayed more evidence of shifting perceptions and some simply reflected the evidence base that there is no robust data to suggest if either procedure was superior:
I start every conversation by saying that whatever level of incontinence they’ve got, I start by saying NICE recommend the artificial urinary sphincter and that is a gold standard and it’s been around for 30 years and I just focus on the sphincter.
01
. . . it’s very hard to argue against the sphincter – because it really is a good procedure and we really do have the data for it you know.
02
We’ve been doing the male sling for some time and I think it’s now a widely used procedure outside of trials both in England and both in the United Kingdom and the rest of the world.
06
. . . if someone’s using two or three pads that used to be my sort of cut off whereas now I’m happier to put it in people who are wetter than that as part of the trial.
01
I do not believe that sphincter or sling is superior by any means at this stage.
17
Discussion
The surgeons who were interviewed largely described increased confidence and expertise with performing AUS surgery given the longstanding history of its use and their perceived surgical competence with the procedure. Some were more familiar with the sling and reported increased use in their centres over recent years, which fosters confidence. Consensus was evident that the length of surgery is shorter for the sling, but there were mixed views on the perceived ‘simplicity’ of the procedure.
Both procedures present considerations with regard to complications. The AUS has more components that cause concern around direct complications, such as infection and erosion, whereas problems with voiding dysfunction are perceived to be a bigger issue for those undergoing sling insertion. Concern regarding potential long-term complications of the sling were expressed, particularly given the lessons learned from female mesh procedures, which may have an impact on surgical decision-making. Patient characteristics also influence surgical recommendations, with age, lifestyle and previous medical procedures guiding preferences.
It was collectively recognised that patients are seeking a return to normality in their symptom management and a reduction in product use, and not necessarily complete resolution of incontinence symptoms. This supports earlier findings from the men’s perspectives and is appropriate for the likely outcomes. The surgeons who were interviewed described discussing realistic perspectives of outcomes with patients and that complete ‘dryness’ is less likely. It was recognised that patients present with their own preferences that are shaped by varying sources of information and influence the clinical exchange when deciding the appropriate surgical directions. The perceived simplicity of adapting to life following surgery was also recognised as an influencing factor, with an assumption that men prefer the straightforward option afforded by the sling compared with the learning required to use the AUS.
These insights have relevance with regard to influencing surgical choices and beyond the study when data can influence these preferences further. It is recognised that surgeons are a key influence in clinical decision-making for men with PPI, and these decisions will be informed by the findings from MASTER.
Main conclusions of the MASTER qualitative programme
The inclusion of the qualitative studies in MASTER afforded valuable insights into the experiences of men undergoing surgery for PPI. Exploring both the participants’ and the surgeons’ perspectives enabled the factors in determinants of success and the decisions that influence outcomes to be fully investigated and provided opportunities for informed conversations. This provides opportunities to influence current practice and will have particular relevance for the results of MASTER.
Expectations regarding outcomes
Of interest at the outset was that complete continence, although desired, was realistically not the expected goal; this was expressed by both the men and the surgeons. Achieving a satisfactory outcome was dependent on the improvement in continence rather than the complete resolution. They hoped that surgery would allow them to regain their social and sexual lives and to be in a position where they needed to use fewer or no pads or incontinence products, as these were restrictive in their lives. Being able to return to ‘normal’ was key. The resumption of activities and engagements that had ceased owing to leakage, the improvements in confidence and self-esteem, and the reduction in negative emotions were intrinsically linked and important outcomes for men with PPI.
Expression of preferences
Preferences among both men and surgeons emerged as a noticeable finding within the qualitative research and, in the absence of a robust evidence base, the sources on which to base these preferences were explored. Participants often had preferences based on complex considerations and varying sources of evidence: expectations for outcome, desire to minimise the invasiveness of surgery, concerns around operating a device, their personal and work life circumstances, requirements for heavy lifting, hobbies such as cycling, willingness to use a cubicle instead of a urinal, the internet and anecdotal evidence. Key to influencing men’s perspectives was the opinion of their surgeon. Respecting the experts’ opinion was a driving force behind treatment preferences, which highlights the importance of the surgeons’ perspectives being grounded in the evidence where available.
Surgeon preferences were two-fold: based on technical aspects of the two procedures and clinical perceptions of outcomes. There was a technical preference towards the AUS owing to most surgeons having greater experience in fitting this device than the newer sling. Technically difficult aspects of sling surgery were discussed, but there was recognition that it was a quicker procedure that has benefits in itself, with fewer components that can cause problems such as infection and erosion. The lack of long-term data for the sling was also a consideration, particularly in view of the emerging picture in the field of female mesh surgery. 56,57 Many also perceived that their patients had an inclination towards the sling because it required fewer mechanical parts. This was not reflected in the discussions with patients, in which we saw a split, with some being attracted to the sling because of its ease of use and others liking the control of the AUS and understanding its mechanical function better.
Surgeons’ preferences were also based on clinical judgement of their patients, with many reluctant to put a sling in a man with heavy incontinence, judging that it would be more suited to a man with milder incontinence symptoms. Previous history of radiotherapy was also a consideration, along with the type of activities that the man was likely to undertake, expressing that one procedure or the other was more appropriate for different circumstances. It was highlighted by the surgeons that without the findings from MASTER there is no evidence base on which to make these recommendations and the gravity of the decisions being made cannot be overlooked.
Methodological considerations
Methodologically, the existence of preferences had an influence on the study that required further consideration. Participants reported a desire to be altruistic and support research for the benefit of others, but there existed a spectrum of willingness to consent. For those who had no surgical preference and did not feel best placed to make a decision, the process of randomisation assisted the treatment pathway; however, in cases where an individual had a clear preference or a surgeon expressed a likelihood that one procedure would be more suited to them, this was prohibitive to the randomisation process. Provision of the NRC provided a vehicle to still contribute to the research findings but also receive their surgical preference; however, this was somewhat of a distraction to the main study aims, despite its original intentions. This has implications for future research and contributes to the growing body of evidence surrounding the mechanisms of trial conduct and the importance of clarity regarding central trial processes, such as randomisation. 58,59
Procedure comparisons
The participants described some soreness and discomfort post surgery for both procedures, with some experiencing more than expected. Most were satisfied with their eventual outcome, but stressed the importance of fully understanding the recovery period pre operation. This in-depth exploration provides unique insights to inform patient information and counselling for surgery to fully inform patients of the likely recovery trajectory. It is crucial to manage the expectations of men following surgery, and the experiences described above can be used to provide more accurate information for men undergoing these procedures in the future.
Participants who had a successful surgery, whether it was a sling or an AUS procedure, found the results life-changing, with a positive effect both physically and mentally. It had a significant impact on their quality of life. It gave the participants back their independence and, although some men still sustained some leakage, it was vastly improved from their earlier symptoms. Having experienced the previous increased levels of incontinence, most were satisfied even with a small volume of leakage as it did not control their lives the way that their symptoms had prior to surgery.
For those for whom the treatment failed, it was a significant disappointment and threatened their belief in ever recovering in some cases. There were frustrations in the length of time that it took for men to receive further surgery when they discovered that their original surgery was not successful. They perceived that they were ‘back to square one’ instead of progressing along a treatment pathway and felt that their subsequent procedure should be expedited. Despite this perceived loss of time, men were satisfied generally with their final outcome following further surgery. Among the 10 men who were interviewed who required further surgery, seven had previously had a sling procedure. The main trial findings provide robust data comparisons for the success of the two procedures, but it is important to raise the problem caused by sling failures here. Within the preference findings, it was often described that the sling was viewed as a possibly ‘simpler’ treatment prior to a potentially definitive AUS and this was borne out in these interviews. This narrative will require exploration in the light of the overall study findings to ensure that the place of both treatments in the surgical armoury is evidence based and accurately communicated to inform decision-making going forward.
Reflections for future practice
The unique insights gained through these in-depth interview studies have provided first-hand accounts of the experience of these two procedures, in the words of the men themselves. The knowledge derived from these qualitative studies provides key considerations for improving future practice when in discussion with men considering PPI surgical procedures and also for the methodology of future trials.
Both surgeons and participants were clear about the likely successes of surgery overall, with symptom alleviation rather than symptom resolution being the primary expectation. This is a strong foundation and identifies that robust counselling exists within the clinical trial situation. These findings should be helpful when used in routine clinical practice by providing an opportunity to improve further the counselling and management of expectations pre operation, as guided by the evidence included in this chapter. Clarity regarding recovery periods, symptom alterations and comparisons of the two procedures, in the words of the men themselves, will promote readiness for surgery and manage expectations following surgery, which is directly related to perceived satisfaction with outcomes. Ensuring that these findings are implemented into practice is key to mobilising the knowledge gained from this specific element of the MASTER study. Alternative sources of patient counselling to the traditional patient leaflet might be considered to take advantage of the valuable findings from this study and include the words of the men in counselling formats; for example, explainer videos. One study found that showing patients a video of the procedure that they were going to have led to great patient satisfaction than giving them a leaflet. 60 This is something that could be explored further because patients may find the video easier to understand and, therefore, have a better pre-surgery education.
Second, this study has highlighted the clear preconceptions that exist surrounding these procedures that, to date, have been reliant on experiences and opinions without the high-quality data that come from a robust study, such as MASTER. These findings exist for the benefit of both the men and their surgeons. Going forward, it is imperative that the narrative that surrounds decision-making in relation to these surgical procedures reflects the evidence-base to appropriately inform patients of the considerations that apply to their personal circumstances. Within the trial confines, it was easier and necessary to advocate a position of equipoise for the purposes of study fidelity and owing to the lack of sufficient research evidence to suggest otherwise. Beyond the trial, findings can be interpreted to improve the advice provided to men considering surgery for PPI, but it is recognised that these emerging findings will have to be assimilated within the context of some entrenched beliefs. Widespread dissemination among the surgeons providing these procedures is essential and particularly relevant for those who were not part of MASTER.
Last, we identified issues around trial methodology that provide learning for future studies. Inclusion of the NRC provided an ideal vehicle for the collection of data for men who were not prepared to be randomised, which offered additional findings for consideration. However, our findings supported other in-trial findings that suggested that the NRC detracted recruitment to the main randomised trial. The NRC was, therefore, closed to avoid this. The qualitative enquiry provided invaluable insights into this element of trial methodology to enable us to respond within the trial timeframe and achieve the outcomes of the main study. Interesting findings were also highlighted about how researchers discuss trials and how the language used can infer altered messaging to participants, which is vital for the informed consent process. This contributes to the growing body of evidence regarding the process of trial recruitment and reinforces existing findings regarding the importance of focusing on getting the consent stage as good as it can be to improve trial outcomes from the outset.
Chapter 7 Discussion and conclusions
MASTER was conceived because of the lack of high-quality research into the surgical treatments for men with incontinence after radical prostatectomy who had an inadequate response to conservative measures, including pelvic floor exercises. The AUS had been the standard treatment since the 1970s, but ‘less-invasive’ operations, such as a variety of male slings, were being increasingly used as an alternative. Although there was a lot of historical evidence that the AUS worked, because the slings were novel, their acceptance into surgical practice was based on poor information from low-quality case series, each with small numbers of men, often from only one centre. Although these new procedures appeared successful, there was no quality data comparing the male sling with the AUS. At our preparatory meeting for MASTER, the future PIs openly discussed the factors that prevented them from having equipoise, which meant that they would not feel comfortable to randomise most men into a high-quality RCT. At the time, few surgeons in the UK were implanting the male sling; indeed, NICE had reported that the sling should be used only in comparison with the AUS in high-quality research studies. We spent a long time going through what we knew, what we thought we knew and what we needed to know. We realised that many of our preferences and, therefore, the advice that we gave to men were based on poor-quality data. Many of the thoughts and beliefs of surgeons were well illustrated by the qualitative study in which their views were sought. Therefore, it was concluded that we needed to design a trial that would address the unknowns and provide data on the relative advantages and disadvantages of the AUS and the male sling. This then allowed us to ensure fully informed consent with the men who came asking for help with their PPI.
We wrote an outline protocol for a pragmatic trial that accepted all men who had failed conservative treatment and had ongoing troublesome SUI. We also minimised the exclusion criteria, unless there was clear evidence that including the men with any feature would either do them harm or not be expected to improve their symptoms. In the interests of generalisability, we included men who had developed UI after TURP for benign disease; however, there were only eight out of 190 in the sling group and nine out of 190 in the AUS group, and these numbers were too small for meaningful conclusions, although there was no glaring difference between the groups in outcomes. We then asked CHaRT if they were willing to be partners in the trial and to administer the study. Their expertise allowed a full protocol to be written and submitted successfully to the Health Technology Assessment programme.
Reviewing the existing literature revealed that there were considerable problems in several areas: there was a variety of definitions of ‘cure’ and improvement; emphasis was placed on a quantitative outcome measure, such as pad testing, rather that listening to what the men viewed as important; and there was poor trial design, with only a single, small, poor-quality RCT that compared the AUS with an injectable.
Hence, we decided to use a clear and unambiguous definition of ‘cure’ based on two ICIQ-UI SF questions as the primary outcome measure. As we suspected, MASTER showed a much greater prevalence of incontinence than the literature had reported. The 2018 AUA guidelines61 report a ‘cure’ rate for the male sling of 62%, but point out that the definition of cure varies between the 14 papers, as do the definitions of ‘improved’. A recent review,62 which included data from 780 men, gives an ‘overall dry rate’ of 58% for the AUS. However, in MASTER, only 15% of men in both groups said that they were dry after either the AUS or the sling. However, as stated, the previously reported case series are characterised by a range of definitions of ‘cure’ and little use of patient-reported outcome measures, which we believe is the reason for the much higher ‘cure’ rates.
There are hydrodynamic reasons why a low cure rate is not unexpected. The physiological continence sphincter mechanism is situated just above the level of the pelvic floor, allowing raised intra-abdominal pressure to enhance sphincteric pressure and preserve continence. However, both the AUS and the sling have to be placed under the pelvic floor so that the flutter valve effect of the normal anatomy is lost. Furthermore, although the AUS has a circumferential balloon mimicking a sphincter, the pressure that can be used has to be limited so that the viability of the urethra is preserved; therefore, when abdominal pressure rises above the AUS cuff pressure, leakage should be expected. Similarly, the male sling works by applying pressure in a unidirectional fashion as it elevates the urethra. Once again, tension has to be limited or else the urethra will be obstructed, preventing micturition and possibly causing erosion into the urethra.
Although the majority of men still had some degree of urinary leakage after implantation of an AUS or a sling, this degree of leakage was reduced. The qualitative component of MASTER has shown that men do not all expect to be dry and any reasonable improvement is greatly appreciated. The use of the satisfaction questionnaire, at 12 months, in MASTER bears this out, with a large majority of men being satisfied with both of the procedures used in the study and data from the ICIQ-UI SF and the ICIQ-MLUTS show that LUTSs, including incontinence, are much improved in most men, even when not dry. This has confirmed our clinical experience that men after surgery are often satisfied with their operation despite not being cured, as long as their leakage is significantly reduced. The comparisons between the two groups do not change significantly from 6 to 12 to 24 months.
As the first qualitative study indicated, the involvement of men in the planning stage proved invaluable and their endorsement of our plans to make extensive use of patient-reported outcome measures for both primary and secondary outcome measures were important. We did use some quantitative tests at baseline, including urodynamics to confirm that the man’s report of stress incontinence was verified during a urodynamic study. By doing this, we can say that the low cure rate was not because of us operating on men who did not have the condition that both the AUS and the sling are designed to cure or improve, namely stress incontinence. We also used the 24-hour pad test to give a baseline assessment of the severity of the man’s urine loss. One reason for doing this was to provide evidence as to whether or not one treatment was best for mild or severe leakage. Although both treatments worked, for leakage of > 250 ml per 24 hours, the AUS provided better results. Hence, if a man wanted to have a sling and not an AUS, he can be warned of this and told that we now have definitive data rather than just expert opinion on this matter. Similarly, the other secondary outcomes provide data that allow a more complete discussion with the man as part of the consent process. A desire for fully informed pre-operative counselling was a further key finding from the qualitative study, with men reporting a requirement for realistic expectations following surgery.
The health economic analyses were part of the planned 24-month follow-up and have been completed. These are important because they add cost-effectiveness data to longer-term clinical effectiveness data to guide overall decision-making on service design and provision. The health economic analysis was comprehensive and included, for example, use of medications and reoperations and other relevant admissions to hospital. In the base-case analysis the sling group costs were lower (–£2497, 95% CI –£3167 to –£1875) owing to the substantially more expensive AUS kits and the average time spent in theatre for the operation for the AUS group. However, the expectations that there would be shorter hospital stay and fewer complications were not realised and, therefore, had no cost advantage to either the AUS or the sling group. There were no differences in the follow-up costs. On average, the slings provided a lower QALY benefit (–0.006, 95% CI –0.06 to 0.054) than AUS; however, this was not statistically significant. The very high cost difference and low QALY difference led to a high ICER (£425,870). Several deterministic sensitivity analyses were performed to address the areas of uncertainty in data collection. All of these analyses were conducted using the imputed data set. The findings were sensitive to the type of data used and, although the sign of the difference did not change, the ICERs were lower than those reported in the base-case analysis. It is reassuring that the results and conclusions of imputed and complete-case data did not differ. The probability of the male sling being cost-effective at a threshold of £20,000–30,000 in both data sets was > 95%, although there is a lot of uncertainty in the data owing to the relatively few men with complications or requiring further surgery over the 24-month follow-up period. Longer-term analysis is crucial to determine if this holds true over the longer term.
The estimates of the incremental costs were sensitive to the approach taken to intervention costing. The base-case analysis estimated costs using the device costs, as reported by sites, and the operation details were collected using the CRFs, which included higher device costs and longer time in theatre for the AUS group. Estimates used from mapping the OPCS codes to HRGs using national reference cost-based tariffs indicated that, on average, sling procedure costs were less costly than AUS procedures.
Strengths and limitations
To the best of our knowledge, MASTER is the first RCT comparing the male sling with AUS surgery. The pragmatic nature of the trial and participation from 28 urological centres across the UK has generated robust, reliable and generalisable results.
MASTER provides an extensive data set that will be used to write a series of papers that will further add to our knowledge on PPI surgery. The assessment of baseline incontinence has been carried out in a comprehensive manner and will hopefully be regarded as a standard way of assessing men before surgery for PPI. Because of the quality of the published literature, it is an inevitable limitation of this study that we were unable to compare the severity of the MASTER men’s incontinence with men in previously published papers.
Although, to date, there seem to be no significant developments in the passive sling used in MASTER, there have been developments in adjustable slings; however, the EAU guidelines63 reiterate that there are no data to suggest an advantage of the adjustable over the simpler passive slings.
Future research
Given that device-related complications do not always occur in the first 24 months, longer-term data acquisition has been advised for all surgical devices, as highlighted recently by the Royal College of Surgeons of England;64 therefore, we will seek 5-year follow-up of our established MASTER trial cohort.
Although we do not feel that chronic pain is a major problem for men after PPI surgery, because of the controversies in women over mesh used for stress incontinence and pelvic organ prolapse surgery, we are planning an early, more detailed, review of pain in our participant group.
Conclusion
The majority of men reported that their continence improved from baseline, with the sling being non-inferior to AUS. The results of MASTER indicate that men can be told that both operations are successful in reducing leakage, satisfaction is high and improvement in quality of life, as a result of reduced leakage, is worthwhile. In terms of secondary outcomes, almost all favour the AUS over the sling. There were few SAEs, with slightly more participants affected in the AUS group than in the male sling group. The rate of reoperation in the first year was greater in the sling group, chiefly because it was ineffective in improving the men’s PPI and, hence, an AUS was implanted. Men should be told that only a minority will be cured, although most will have a significant worthwhile reduction in leakage and pad use. A full discussion on the benefits and possible problems of both devices is essential and men will then be able to decide which they want if they decide to go ahead with surgery. The results from MASTER will allow a more informed conversation between the man and his surgeon.
Acknowledgements
The authors wish to thank the men who participated in MASTER. We also thank Fiona Cherry, Lara Mitchell, Georgia Mannion-Krase, Denise Will, Rebecca Bruce, Margery Heath, Maria Ntessalen, Andrea Fraser, Dianne Dejean and Janice Cruden for their secretarial support and data management; Kirsty Shearer, Caroline Peet, Lynda Constable, Karen Campbell and Elerita Flammini for the trial management; Graeme MacLennan (CHaRT director from 2017), the programming team in CHaRT, led by Gladys McPherson (to 2016), Mark Forrest (2016-present) and members of the Project Management Group for their ongoing support and advice; Cynthia Fraser (Health Services Research Unit) for literature searches; Nikki Cotterill, Megan Pardoe, Alan Uren and Rafiyah Khan for the qualitative research carried out in MASTER; Marcus Jepson, Jenny Donovan and the Quintet team for exploring surgeon equipoise; and the independent members of the TSC and DMC, and the staff at the recruiting sites (listed below), who facilitated recruitment, treatment and follow-up of trial participants.
The independent members of the TSC who oversaw this study were Howard Kynaston (chairperson), Neville Goodman (consumer representative), Christopher Walker (patient representative), Suzanne Hagen and Tom McNicholas. The members of the DMC who oversaw this study were Jonathan Cook (chairperson), John Parry and Mark Speakman.
We would like to thank the collaborators of the MASTER trial: Cathryn Glazener, Marcus Drake, Anthony Mundy, Kirsty McCormack, Mary Kilonzo, Charles Boachie, Craig Ramsay, Nikki Cotterill, Robert Pickard, Alison McDonald, Gladys McPherson and John Norrie.
Members of the MASTER trial group responsible for recruitment in the clinical centres were as follows:
-
Bedford, Bedford Hospital NHS Trust– Liaqat Chowoo (principal investigator), Graham Smith, Charina Smith, Ann Britchford, Beena David and Retno Wulandari
-
Birmingham, Queen Elizabeth Hospital: Yz Almallah (principal investigator), Mohammed Belal, Yuko Smith, Alice Longe, Annette Nilsson, Carol Green, Daniella Lynch and Martin Joinson
-
Bradford, Bradford Royal Infirmary – John Bolton (principal investigator), Richard Benton, Linda Bamford, Helen Robertshaw, Hayley Inman, Anne Kay, Agapios Grentzis, Jane Sewell, Kelvin Stewart and Kay Cockroft
-
Bristol, Southmead Hospital – Hashim Hashim (principal investigator), Debbie Delgado, Constance Shiridzinomwa, Markus Drake, Helena Burden, Kate Warren, Julie Plant, Leigh Morrison, Katie McDonald, Carol Brain and Lyndsey Johnson
-
Cambridge, Addenbrookes Hospital – Nikesh Thiruchelvam (principal investigator), Kelly Leonard, Lisa Geoghegan and Suzanne Biers
-
Chichester, St Richard’s Hospital – Suzie Venn (principal investigator), Yolanda Baird, Isobel Amey, Alison Misselbrook, Sally Moore, Susanna Greenslade, Marian Flynn-Batham, Matthew Smith and Julie Wheatley
-
Eastbourne, Eastbourne District General Hospital – James Moore (principal investigator), Emma Edmunds, Anne Cowley, Penny Whithing, Jenni Law, Jackie Terry and Kelly Mintrim
-
Edinburgh, Western General Hospital – Amar Alhasso (principal investigator), Emma Whitecross, Shiobhan McCaskey, Charlotte Smith, Laurence Stewart, Emma Fleming, Rachel Campbell and Katherine Laurence
-
Glasgow, Southern General Hospital – Graeme Conn (principal investigator), Simon Morton and Alastair McKay
-
Guy’s and St Thomas’ NHS Foundation Trust, Guy’s Hospital – Arun Sahai (principal investigator), Nigel Butter, Jai Seth, Claire Taylor, Angel Garcia-Imhor, Sachin Malde, Matthew Hogben, Nicholas Favre Walker, Temitope Bankole, Naomi Hare and Samantha Broadhead
-
Imperial College Healthcare NHS Trust, Charing Cross Hospital: Tina Rashid (principal investigator), Roland Morley (principal investigator), Matthias Winkler, Sanela Andrijac, Gillian Hornzee, Fatima Akbar and Daisy Floyd
-
Leeds, St James’s University Hospital – Neil Harris (principal investigator) and Lorraine Wiseman (née Lamb)
-
Leicester, Leicester General Hospital – Jas Rai (principal investigator), Tim Terry, Tina Rashid, Jackie Parker and Robert Radcliffe
-
Liverpool, University Hospital Aintree – Andrew Baird (principal investigator), Jordan Ewing, Melanie Morrison and Marc Lucky
-
Middlesbrough, James Cook Hospital – Simon Fulford (principal investigator), Julie Potts, Mary Gartwaite, Julie McGivern, Clare Proctor, Alycon Walker, Karoline Middleton, Martyn Cain, Matthew Bunting and Kerry Hebbron
-
Newcastle, Freeman Hospital – Chris Harding (principal investigator), Peter Murphy, Wendy Robson, Nicola Brown, Victoria Lavin, Rachel Forrest, Bernadette Kilbane, Paul Hindmarch and Lynn D Langhorne
-
Newport, Royal Gwent Hospital – Alun Thomas (principal investigator), Talal Jabbar (principal investigator), Sarah Scourfield and Coral Seymour
-
Nottingham, Nottingham University Hospital – Richard Parkinson (principal investigator), Andy Jarvis and Bria McAllister
-
Salford, Salford Royal Foundation Trust – Laurence Clarke (principal investigator), Melanie Taylor, Kieran O’Flynn, Zoe Swann and Vicky Thomas
-
Salisbury, Salisbury District Hospital – Melissa Davies, Katie Chadwick, Ruth Fennelly, Caroline Clarke, Alpha Anthony, Eve Parker, Michele Tribbeck and Kate Ames
-
Sheffield, Royal Hallamshire Hospital – Sheilagh Reid (principal investigator), Susannah Hulton, Alison Hyde, Anne Frost, Nadir Osman and Chris Hillary
-
Southampton, Southampton General Hospital – Rowland Rees (principal investigator), McDonald Mupudzi, Elisabeth Harouzet, Miranda Kean, Andrew Guy, Emma Levell, Meirion Ford, Michelle Smith, Caroline Andrews, Robbie Speigal, Abbie Morley, Margarida Salgueiro, Clare Hutchison, Winington Ruiz, Sanchia Triggs and Kimberley Harris
-
Stevenage, Lister Hospital – Charlotte Foley (principal investigator), Linda Fowler, Clare Collins, Louise Peacock, Jemma Gilmore and Faith Wilson
-
St George’s University Hospitals NHS Foundation Trust, St George’s Hospital – Davendra Sharma (principal investigator), Agne Sekmokaite, Ruth Millett, Ella Foncel, Marios Hadjipavlou and Abigail Seward
-
Stockport, Stepping Hill Hospital – Andrew Sinclair (principal investigator), Helen Cochrane, Sarah Scanlon, Lara Smith, Julie Grindey, Nnaemeka Eli, Alissa Kent, Sarah Connolly, Patricia Clitheroe and Patricia Coughlan
-
University College Hospital, Westmoreland Street Hospital – Jeremy Ockrim (principal investigator), Anthony Mundy, Daniella Andrich, Tamsin Greenwell, Rizwan Hamid, Anastasia Frost, Mariya Dragova and Mahreen Pakrad
-
Wakefield, Pinderfields Hospital – Ian Beckley (principal investigator), Victoria Dean, Beverley Taylor, Jim Anderson, Stephen Littler, Julie Ball, Aimee Hayton-Bolt, Asa Ali and Janine Heeley
-
Wrexham, Wrexham Maelor Hospital – Christian Seipp (principal investigator), Stacy Ackerley, Linzi Williams, Kelly Andrews, Lisa Ashley, Rachel Davies, Emma Hall, Natalie Hughes, Paula Conway, Mary Roberts, Jane Stockport, Claire Watkins, Jackie Morris and Rachel Hughes.
Contributions of authors
Lynda Constable (https://orcid.org/0000-0001-9646-3904) (Trial Manager, Triallist) was responsible for the day-to-day management of the trial, conduct of the trial, interpretation of the data and writing/editing of the report.
Paul Abrams (https://orcid.org/0000-0003-2776-2200) (Chief Investigator and Professor) contributed to the conception, design and conduct of the trial, interpretation of the results and writing/editing of the report.
David Cooper (https://orcid.org/0000-0002-9361-4399) (Statistician) conducted the statistical analyses, and contributed to the interpretation of the data and writing/editing of the report.
Mary Kilonzo (https://orcid.org/0000-0002-3450-4536) (Health Economist) contributed to the conception and design of the study, analysis of the health economics data and drafting of Chapter 5.
Nikki Cotterill (https://orcid.org/0000-0001-6921-2712) (Continence Lead) contributed to the conception and design of the study, led the qualitative component and drafted Chapter 6.
Chris Harding (https://orcid.org/0000-0002-9407-382X) (Consultant Urologist and Professor) contributed to the interpretation of the data and conduct of the trial and commented on all chapters.
Marcus J Drake (https://orcid.org/0000-0002-6230-2552) (Consultant Urologist and Professor) contributed to the conception, design and conduct of the trial, interpretation of the results and commenting on all chapters.
Megan N Pardoe (https://orcid.org/0000-0001-7978-9857) (Research Assistant) undertook part of the qualitative component, and completed qualitative interviews, analysis and drafting of the qualitative chapter.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) (Senior Trial Manager) contributed to the conception, design and conduct of the trial and commented on all chapters.
Rebecca Smith (https://orcid.org/0000-0003-4267-8453) (Triallist) contributed to the conception, design and conduct of the trial and commented on chapters.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor, Statistician and Triallist) contributed to the conception, design and conduct of the trial and commented on chapters.
Kirsty McCormack (https://orcid.org/0000-0001-6051-1513) (Triallist) contributed to the conception, design and conduct of the trial and provided comments.
Craig Ramsay (https://orcid.org/0000-0003-4043-7349) (Professor, Statistician, Triallist) contributed to the conception, design and conduct of the trial and commented on chapters.
Alan Uren (https://orcid.org/0000-0001-7486-2322) (Research Associate) conducted interviews for the first two stages of the qualitative component and contributed to the writing/editing of the qualitative chapter.
Tony Mundy (https://orcid.org/0000-0002-1219-2228) (Consultant Urologist and Professor) contributed to the conception, design and conduct of the trial and commented on chapters.
Cathryn Glazener (https://orcid.org/0000-0001-8667-2382) (Emeritus Professor) contributed to the conception, design and conduct of the trial and commented on all chapters.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor, Statistician and Triallist) contributed to the conception, design and conduct of the trial, and the interpretation of the results, oversaw statistical analysis and commented on all chapters.
The Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Publication
Constable L, Cotterill N, Cooper D, Glazener C, Drake MJ, Forrest M, et al. Male synthetic sling versus artificial urinary sphincter trial for men with urodynamic stress incontinence after prostate surgery (MASTER): study protocol for a randomised controlled trial. Trials 2018;19:131.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Constable L, Cotterill N, Cooper D, Glazener C, Drake MJ, Forrest M, et al. Male synthetic sling versus artificial urinary sphincter trial for men with urodynamic stress incontinence after prostate surgery (MASTER): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2501-2.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): two parallel randomised controlled trials. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15240.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011;378:328-37. https://doi.org/10.1016/S0140-6736(11)60751-4.
- Silva LA, Andriolo RB, Atallah ÁN, da Silva EM. Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD008306.pub3.
- National Institute for Health and Care Excellence (NICE) . Lower Urinary Tract Symptoms in Men: Management 2010.
- Goldman H, Averbeck M, Bruschini H, , Abrams P, Cardozo L, et al. Incontinence. Bristol: International Continence Society; 2017.
- Burkhard FC, Bosch JLHR, Cruz F, Lemack GE, Nambiar AK, Thiruchelvam N, et al. Urinary Incontinence n.d. https://uroweb.org/guideline/urinary-incontinence/ (accessed 27 October 2020).
- NHS Digital . Hospital Admitted Patient Care Activity n.d. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19 (accessed November 2019).
- British Association of Urological Surgeons . Radical Prostatectomy Outcomes Data: Summary and Timescale of the Data n.d. www.baus.org.uk/patients/surgical_outcomes/radical_prostatectomy/timescales.aspx (accessed 30 October 2020).
- Armstrong N, Vale L, Deverill M, Nabi G, McClinton S, N’Dow J, et al. BPE Study Group . Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. BMJ 2009;338. https://doi.org/10.1136/bmj.b1288.
- Imamoglu MA, Tuygun C, Bakirtas H, Yiğitbasi O, Kiper A. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol 2005;47:209-13. https://doi.org/10.1016/j.eururo.2004.08.019.
- Abrams P, Avery K, Gardener N, Donovan J. ICIQ Advisory Board . The international consultation on incontinence modular questionnaire: www.iciq.net. J Urol 2006;175:1063-6. https://doi.org/10.1016/S0022-5347(05)00348-4.
- ICIQ Group . International Consultation on Incontinence Questionnaire Male Lower Urinary Tract Symptoms Module (ICIQ-MLUTS) n.d. https://iciq.net/iciq-mluts (accessed 27 October 2020).
- ICIQ Group . International Consultation on Incontinence Questionnaire Male Sexual Matters Associated With Lower Urinary Tract Symptoms Module (ICIQ-MLUTSsex) n.d. https://iciq.net/iciq-mlutssex (accessed 27 October 2020).
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- EuroQol Research Foundation . EQ-5D-3L n.d. https://euroqol.org/ (accessed 27 October 2020).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury, University of Kent: Personal Social Services Research Unit; 2019.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 25 January 2020).
- Department of Health and Social Care . NHS Reference Costs 2017–18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 20 January 2020).
- Public Health Scotland . Theatres: Costs and Detailed Tables n.d. www.isdscotland.org/Health-topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 20 November 2019).
- NHS Business Services Authority . NHS Electronic Drug Tariff 2015. www.drugtariff.nhsbsa.nhs.uk/ (accessed 15 September 2019).
- Office for National Statistics . Earnings and Hours Worked, All Employees: ASHE Table 1 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/allemployeesashetable1 (accessed 17 January 2020).
- NHS . NHS Prescription Charges n.d. www.nhs.uk/common-health-questions/nhs-services-and-treatments/how-much-nhs-prescription-charge/ (accessed 16 January 2020).
- Dolan P. Modelling valuations for EuroQoL health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University; 2005.
- Dowie J, Kaltoft MK, Nielsen JB, Salkeld G. Caveat emptor. NICE; 2015.
- Office for National Statistics . National Life Tables – Life Expectancy in the UK: 2017 to 2019 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2017to2019 (accessed 15 May 2020).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Office for National Statistics . Interim Life Tables n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/singleyearlifetablesuk1980to2018 (accessed 12 October 2019).
- Leon P, Chartier-Kastler E, Roupret M, Ambrogi V, Mozer P, Phe V. Artificial urinary sphincter in men. BJU Int 2015;115:951-7. https://doi.org/10.1111/bju.12848.
- Venn SN, Greenwell TJ, Mundy AR. The long-term outcome of artificial urinary sphincters. J Urol 2000;164:702-7. https://doi.org/10.1016/S0022-5347(05)67285-0.
- Fulford SC, Sutton C, Bales G, Hickling M, Stephenson TP. The fate of the ‘modern’ artificial urinary sphincter with a follow-up of more than 10 years. Br J Urol 1997;79:713-16. https://doi.org/10.1046/j.1464-410x.1997.00151.x.
- Al-Busaidi ZQ. Qualitative research and its uses in health care. Sultan Qaboos Univ Med J 2008;8:11-9.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Lewis-Beck MS, Bryman A, Futing Liao T. The SAGE Encyclopedia of Social Science Research Methods. London: SAGE Publications Ltd; 2004.
- Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep 2015;20. https://doi.org/10.46743/2160-3715/2015.2281.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Braun V, Clarke V. Successful Qualitative Research: A Practical Guide for Beginners. London: SAGE Publications Ltd; 2013.
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 1997;104:1374-9. https://doi.org/10.1111/j.1471-0528.1997.tb11006.x.
- Donovan J, Peters T, Abrams P, Brooks S, de la Rosette J, Schafer W. Scoring the short form ICSmale SF questionnaire. J Urol 2000;164:1948-55. https://doi.org/10.1016/S0022-5347(05)66926-1.
- Frankel SJ, Donovan JL, Peters TI, Abrams P, Dabhoiwala NF, Osawa D, et al. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol 1998;51:677-85. https://doi.org/10.1016/S0895-4356(98)00044-4.
- Harris IA, Harris AM, Naylor JM, Adie S, Mittal R, Dao AT. Discordance between patient and surgeon satisfaction after total joint arthroplasty. J Arthroplasty 2013;28:722-7. https://doi.org/10.1016/j.arth.2012.07.044.
- Marschall-Kehrel D, Roberts RG, Brubaker L. Patient-reported outcomes in overactive bladder: the influence of perception of condition and expectation for treatment benefit. Urology 2006;68:29-37. https://doi.org/10.1016/j.urology.2006.02.046.
- Channel Four Television Corporation . Embarrassing Bodies 2007.
- Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery 2006;139:469-83. https://doi.org/10.1016/j.surg.2005.08.014.
- Gousse AE, Madjar S, Lambert MM, Fishman IJ. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol 2001;166:1755-8. https://doi.org/10.1016/S0022-5347(05)65668-6.
- Dugan E, Cohen SJ, Bland DR, Preisser JS, Davis CC, Suggs PK, et al. The association of depressive symptoms and urinary incontinence among older adults. J Am Geriatr Soc 2000;48:413-16. https://doi.org/10.1111/j.1532-5415.2000.tb04699.x.
- National Institute for Health and Care Excellence (NICE) . NICE Guideline [NG 123]: 1.5: Surgical Management of Stress Urinary Incontinence n.d. www.nice.org.uk/guidance/ng123/chapter/Recommendations#surgical-management-of-stress-urinary-incontinence (accessed 27 October 2020).
- Davey M. What does pelvic mesh do and why are women suing over it? – explainer. The Guardian 2017. www.theguardian.com/society/2017/aug/31/vaginal-pelvic-mesh-explainer.
- BBC News . Mesh 'last option' for incontinence. BBC News 2018. www.bbc.co.uk/news/health-45783127.
- BBC News . 'Immediate' halt on use of mesh implants in Scotland. BBC News 2018. www.bbc.co.uk/news/uk-scotland-scotland-politics-45498019.
- British Association of Urological Surgeons (BAUS) Limited . Insertion of a Synthetic Sling for Stress Urinary Incontinence in Men 2021. www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/Synthetic%20sling%20male.pdf (accessed 27 October 2020).
- Haskell H. Cumberlege review expose stubborn and dangerous flaws in healthcare. BMJ 2020;370. https://doi.org/10.1136/bmj.m3099.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29-36. https://doi.org/10.1016/j.jclinepi.2008.02.010.
- Kamyabi N, Nakhaei M, Nasiri A, Akbari E, Sharifzadeh G. Effects of video- and pamphlet-based patient educations on anxiety and satisfaction among candidates for gastroscopy. Mod Car J 2016;13. https://doi.org/10.17795/modernc.10647.
- American Urological Association . Incontinence After Prostate Treatment: AUA SUFU Guideline (2019). n.d. www.auanet.org/guidelines/incontinence-after-prostate-treatment (accessed 26 November 2020).
- Tutolo M, Cornu JN, Bauer RM, Ahyai S, Bozzini G, Heesakkers J, et al. Efficacy and safety of artificial urinary sphincter (AUS): results of a large multi-institutional cohort of patients with mid-term follow-up. Neurourol Urodyn 2019;38:710-18. https://doi.org/10.1002/nau.23901.
- European Association of Urology (EAU) . EAU Guidelines on Urinary Incontinence in Adults 2020. https://d56bochluxqnz.cloudfront.net/media/EAU-Guidelines-on-Urinary-Incontinence-2020.pdf.
- Royal College of Surgeons of England . Future of Surgery n.d. https://futureofsurgery.rcseng.ac.uk/?_ga=2.195177260.1807836087.1586861042-2095287332.1586861042 (accessed 27 October 2020).
Appendix 1 Urinary symptoms of randomised controlled trial participants at baseline and 6, 12 and 24 months post randomisation
| Randomised group, n (%) | ||
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| Does urine leak when you cough or sneeze? | ||
| Never | 0 | 1 (0.5) |
| Occasionally | 15 (7.9) | 11 (5.8) |
| Sometimes | 24 (12.6) | 22 (11.6) |
| Most of the time | 65 (34.2) | 67 (35.3) |
| All of the time | 78 (41.1) | 73 (38.4) |
| Missing | 8 (4.2) | 16 (8.4) |
| Do you have a sudden need to rush to the toilet to urinate? | ||
| Never | 29 (15.3) | 33 (17.4) |
| Occasionally | 56 (29.5) | 52 (27.4) |
| Sometimes | 75 (39.5) | 64 (33.7) |
| Most of the time | 16 (8.4) | 18 (9.5) |
| All of the time | 6 (3.2) | 5 (2.6) |
| Missing | 8 (4.2) | 18 (9.5) |
| Does urine leak before you can get to the toilet? | ||
| Never | 10 (5.3) | 8 (4.2) |
| Occasionally | 58 (30.5) | 61 (32.1) |
| Sometimes | 55 (28.9) | 48 (25.3) |
| Most of the time | 40 (21.1) | 29 (15.3) |
| All of the time | 18 (9.5) | 28 (14.7) |
| Missing | 9 (4.7) | 16 (8.4) |
| How often have you had a slight wetting of your pants after you have finished urinating and are dressed? | ||
| Never | 25 (13.2) | 18 (9.5) |
| Occasionally | 55 (28.9) | 46 (24.2) |
| Sometimes | 51 (26.8) | 61 (32.1) |
| Most of the time | 35 (18.4) | 30 (15.8) |
| All of the time | 13 (6.8) | 16 (8.4) |
| Missing | 11 (5.8) | 19 (10.0) |
| Do you ever leak for no obvious reason and without feeling that you want to go? | ||
| Never | 9 (4.7) | 3 (1.6) |
| Occasionally | 34 (17.9) | 32 (16.8) |
| Sometimes | 53 (27.9) | 59 (31.1) |
| Most of the time | 56 (29.5) | 45 (23.7) |
| All of the time | 30 (15.8) | 35 (18.4) |
| Missing | 8 (4.2) | 16 (8.4) |
| Do you ever leak urine when you are asleep? | ||
| Never | 45 (23.7) | 60 (31.6) |
| Occasionally | 69 (36.3) | 60 (31.6) |
| Sometimes | 30 (15.8) | 28 (14.7) |
| Most of the time | 22 (11.6) | 18 (9.5) |
| All of the time | 14 (7.4) | 10 (5.3) |
| Missing | 10 (5.3) | 14 (7.4) |
| Is there a delay before you can start to urinate? | ||
| Never | 116 (61.1) | 119 (62.6) |
| Occasionally | 43 (22.6) | 32 (16.8) |
| Sometimes | 15 (7.9) | 13 (6.8) |
| Most of the time | 5 (2.6) | 7 (3.7) |
| All of the time | 0 | 2 (1.1) |
| Missing | 11 (5.8) | 17 (8.9) |
| Do you have to strain to continue urinating? | ||
| Never | 115 (60.5) | 118 (62.1) |
| Occasionally | 44 (23.2) | 31 (16.3) |
| Sometimes | 9 (4.7) | 15 (7.9) |
| Most of the time | 9 (4.7) | 8 (4.2) |
| All of the time | 2 (1.1) | 1 (0.5) |
| Missing | 11 (5.8) | 17 (8.9) |
| Would you say that the strength of your urinary stream is? | ||
| Normal | 100 (52.6) | 107 (56.3) |
| Occasionally reduced | 23 (12.1) | 26 (13.7) |
| Sometimes reduced | 31 (16.3) | 21 (11.1) |
| Reduced most of the time | 21 (11.1) | 14 (7.4) |
| Reduced all of the time | 5 (2.6) | 5 (2.6) |
| Missing | 10 (5.3) | 17 (8.9) |
| Do you stop and start more than once while you urinate? | ||
| Never | 87 (45.8) | 82 (43.2) |
| Occasionally | 56 (29.5) | 61 (32.1) |
| Sometimes | 19 (10.0) | 22 (11.6) |
| Most of the time | 14 (7.4) | 4 (2.1) |
| All of the time | 4 (2.1) | 3 (1.6) |
| Missing | 10 (5.3) | 18 (9.5) |
| How often do you feel that your bladder has not emptied properly after you have urinated? | ||
| Never | 62 (32.6) | 56 (29.5) |
| Occasionally | 64 (33.7) | 73 (38.4) |
| Sometimes | 30 (15.8) | 23 (12.1) |
| Most of the time | 15 (7.9) | 19 (10.0) |
| All of the time | 8 (4.2) | 3 (1.6) |
| Missing | 11 (5.8) | 16 (8.4) |
| Randomised group | ||
|---|---|---|
| Male sling (N = 190) | AUS (N = 190) | |
| How often do you leak urine? n/N (%) | ||
| 6 months | ||
| Never | 20/118 (16.9) | 24/114 (21.1) |
| Once per week or less | 23/118 (19.5) | 23/114 (20.2) |
| Two or three times per week | 13/118 (11.0) | 10/114 (8.8) |
| About once per day | 12/118 (10.2) | 24/114 (21.1) |
| Several times per day | 37/118 (31.4) | 27/114 (23.7) |
| All of the time | 13/118 (11.0) | 6/114 (5.3) |
| Missing | 72/190 (37.9) | 76/190 (40.0) |
| 12 months | ||
| Never | 21/151 (13.9) | 25/154 (16.2) |
| Once per week or less | 31/151 (20.5) | 27/154 (17.5) |
| Two or three times per week | 13/151 (8.6) | 13/154 (8.4) |
| About once per day | 12/151 (7.9) | 20/154 (13.0) |
| Several times per day | 59/151 (39.1) | 60/154 (39.0) |
| All of the time | 15/151 (9.9) | 9/154 (5.8) |
| Missing | 39/190 (20.5) | 36/190 (18.9) |
| 24 months | ||
| Never | 24/132 (18.2) | 26/141 (18.4) |
| Once per week or less | 16/132 (12.1) | 20/141 (14.2) |
| Two or three times per week | 20/132 (15.2) | 18/141 (12.8) |
| About once per day | 13/132 (9.8) | 32/141 (22.7) |
| Several times per day | 48/132 (36.4) | 37/141 (26.2) |
| All of the time | 11/132 (8.3) | 8/141 (5.7) |
| Missing | 58/190 (30.5) | 49/190 (25.8) |
| How much urine do you usually leak? n/N (%) | ||
| 6 months | ||
| None | 21/121 (17.4) | 25/117 (21.4) |
| A small amount | 76/121 (62.8) | 80/117 (68.4) |
| A moderate amount | 12/121 (9.9) | 11/117 (9.4) |
| A large amount | 12/121 (9.9) | 1/117 (0.9) |
| Missing | 69/190 (36.3) | 73/190 (38.4) |
| 12 months | ||
| None | 20/154 (13.0) | 26/158 (16.5) |
| A small amount | 91/154 (59.1) | 103/158 (65.2) |
| A moderate amount | 29/154 (18.8) | 21/158 (13.3) |
| A large amount | 14/154 (9.1) | 8/158 (5.1) |
| Missing | 36/190 (18.9) | 32/190 (16.8) |
| 24 months | ||
| None | 21/140 (15.0) | 21/147 (14.3) |
| A small amount | 83/140 (59.3) | 102/147 (69.4) |
| A moderate amount | 27/140 (19.3) | 17/147 (11.6) |
| A large amount | 9/140 (6.4) | 7/147 (4.8) |
| Missing | 50/190 (26.3) | 43/190 (22.6) |
| Does urine leak when you cough or sneeze? n/N (%) | ||
| 6 months | ||
| Never | 30/115 (26.1) | 26/113 (23.0) |
| Occasionally | 31/115 (27.0) | 38/113 (33.6) |
| Sometimes | 19/115 (16.5) | 29/113 (25.7) |
| Most of the time | 20/115 (17.4) | 14/113 (12.4) |
| All of the time | 15/115 (13.0) | 6/113 (5.3) |
| Missing | 75/190 (39.5) | 77/190 (40.5) |
| 12 months | ||
| Never | 24/147 (16.3) | 30/148 (20.3) |
| Occasionally | 45/147 (30.6) | 51/148 (34.5) |
| Sometimes | 28/147 (19.0) | 27/148 (18.2) |
| Most of the time | 26/147 (17.7) | 30/148 (20.3) |
| All of the time | 24/147 (16.3) | 10/148 (6.8) |
| Missing | 43/190 (22.6) | 42/190 (22.1) |
| 24 months | ||
| Never | 24/130 (18.5) | 25/142 (17.6) |
| Occasionally | 32/130 (24.6) | 45/142 (31.7) |
| Sometimes | 35/130 (26.9) | 37/142 (26.1) |
| Most of the time | 23/130 (17.7) | 26/142 (18.3) |
| All of the time | 16/130 (12.3) | 9/142 (6.3) |
| Missing | 60/190 (31.6) | 48/190 (25.3) |
| Do you have a sudden need to rush to the toilet to urinate? n/N (%) | ||
| 6 months | ||
| Never | 31/115 (27.0) | 38/112 (33.9) |
| Occasionally | 49/115 (42.6) | 40/112 (35.7) |
| Sometimes | 29/115 (25.2) | 29/112 (25.9) |
| Most of the time | 3/115 (2.6) | 4/112 (3.6) |
| All of the time | 3/115 (2.6) | 1/112 (0.9) |
| Missing | 75/190 (39.5) | 78/190 (41.1) |
| 12 months | ||
| Never | 46/147 (31.3) | 49/148 (33.1) |
| Occasionally | 60/147 (40.8) | 55/148 (37.2) |
| Sometimes | 30/147 (20.4) | 36/148 (24.3) |
| Most of the time | 7/147 (4.8) | 7/148 (4.7) |
| All of the time | 4/147 (2.7) | 1/148 (0.7) |
| Missing | 43/190 (22.6) | 42/190 (22.1) |
| 24 months | ||
| Never | 39/130 (30.0) | 41/142 (28.9) |
| Occasionally | 57/130 (43.8) | 66/142 (46.5) |
| Sometimes | 27/130 (20.8) | 31/142 (21.8) |
| Most of the time | 7/130 (5.4) | 4/142 (2.8) |
| All of the time | 0/130 (0.0) | 0/142 (0.0) |
| Missing | 60/190 (31.6) | 48/190 (25.3) |
| Does urine leak before you can get to the toilet? n/N (%) | ||
| 6 months | ||
| Never | 32/117 (27.4) | 47/112 (42.0) |
| Occasionally | 52/117 (44.4) | 44/112 (39.3) |
| Sometimes | 20/117 (17.1) | 18/112 (16.1) |
| Most of the time | 8/117 (6.8) | 1/112 (0.9) |
| All of the time | 5/117 (4.3) | 2/112 (1.8) |
| Missing | 73/190 (38.4) | 78/190 (41.1) |
| 12 months | ||
| Never | 34/146 (23.3) | 57/148 (38.5) |
| Occasionally | 63/146 (43.2) | 62/148 (41.9) |
| Sometimes | 32/146 (21.9) | 16/148 (10.8) |
| Most of the time | 10/146 (6.8) | 8/148 (5.4) |
| All of the time | 7/146 (4.8) | 5/148 (3.4) |
| Missing | 44/190 (23.2) | 42/190 (22.1) |
| 24 months | ||
| Never | 32/130 (24.6) | 55/142 (38.7) |
| Occasionally | 62/130 (47.7) | 57/142 (40.1) |
| Sometimes | 25/130 (19.2) | 23/142 (16.2) |
| Most of the time | 10/130 (7.7) | 4/142 (2.8) |
| All of the time | 1/130 (0.8) | 3/142 (2.1) |
| Missing | 60/190 (31.6) | 48/190 (25.3) |
| How often have you had a slight wetting of your pants after you have finished urinating and are dressed? n/N (%) | ||
| 6 months | ||
| Never | 30/117 (25.6) | 39/112 (34.8) |
| Occasionally | 58/117 (49.6) | 53/112 (47.3) |
| Sometimes | 17/117 (14.5) | 16/112 (14.3) |
| Most of the time | 5/117 (4.3) | 3/112 (2.7) |
| All of the time | 7/117 (6.0) | 1/112 (0.9) |
| Missing | 73/190 (38.4) | 78/190 (41.1) |
| 12 months | ||
| Never | 40/147 (27.2) | 44/148 (29.7) |
| Occasionally | 62/147 (42.2) | 74/148 (50.0) |
| Sometimes | 27/147 (18.4) | 22/148 (14.9) |
| Most of the time | 15/147 (10.2) | 5/148 (3.4) |
| All of the time | 3/147 (2.0) | 3/148 (2.0) |
| Missing | 43/190 (22.6) | 42/190 (22.1) |
| 24 months | ||
| Never | 49/129 (38.0) | 39/143 (27.3) |
| Occasionally | 52/129 (40.3) | 74/143 (51.7) |
| Sometimes | 18/129 (14.0) | 21/143 (14.7) |
| Most of the time | 7/129 (5.4) | 6/143 (4.2) |
| All of the time | 3/129 (2.3) | 3/143 (2.1) |
| Missing | 61/190 (32.1) | 47/190 (24.7) |
| Do you ever leak for no obvious reason and without feeling that you want to go? n/N (%) | ||
| 6 months | ||
| Never | 53/118 (44.9) | 57/112 (50.9) |
| Occasionally | 31/118 (26.3) | 38/112 (33.9) |
| Sometimes | 19/118 (16.1) | 11/112 (9.8) |
| Most of the time | 7/118 (5.9) | 1/112 (0.9) |
| All of the time | 8/118 (6.8) | 5/112 (4.5) |
| Missing | 72/190 (37.9) | 78/190 (41.1) |
| 12 months | ||
| Never | 52/147 (35.4) | 75/149 (50.3) |
| Occasionally | 49/147 (33.3) | 44/149 (29.5) |
| Sometimes | 26/147 (17.7) | 21/149 (14.1) |
| Most of the time | 12/147 (8.2) | 5/149 (3.4) |
| All of the time | 8/147 (5.4) | 4/149 (2.7) |
| Missing | 43/190 (22.6) | 41/190 (21.6) |
| 24 months | ||
| Never | 46/130 (35.4) | 64/142 (45.1) |
| Occasionally | 46/130 (35.4) | 51/142 (35.9) |
| Sometimes | 26/130 (20.0) | 17/142 (12.0) |
| Most of the time | 10/130 (7.7) | 7/142 (4.9) |
| All of the time | 2/130 (1.5) | 3/142 (2.1) |
| Missing | 60/190 (31.6) | 48/190 (25.3) |
| Do you ever leak urine when you are asleep? n/N (%) | ||
| 6 months | ||
| Never | 73/118 (61.9) | 93/112 (83.0) |
| Occasionally | 27/118 (22.9) | 12/112 (10.7) |
| Sometimes | 8/118 (6.8) | 4/112 (3.6) |
| Most of the time | 4/118 (3.4) | 1/112 (0.9) |
| All of the time | 6/118 (5.1) | 2/112 (1.8) |
| Missing | 72/190 (37.9) | 78/190 (41.1) |
| 12 months | ||
| Never | 78/147 (53.1) | 110/149 (73.8) |
| Occasionally | 42/147 (28.6) | 31/149 (20.8) |
| Sometimes | 12/147 (8.2) | 3/149 (2.0) |
| Most of the time | 8/147 (5.4) | 3/149 (2.0) |
| All of the time | 7/147 (4.8) | 2/149 (1.3) |
| Missing | 43/190 (22.6) | 41/190 (21.6) |
| 24 months | ||
| Never | 75/130 (57.7) | 113/141 (80.1) |
| Occasionally | 40/130 (30.8) | 22/141 (15.6) |
| Sometimes | 10/130 (7.7) | 2/141 (1.4) |
| Most of the time | 3/130 (2.3) | 2/141 (1.4) |
| All of the time | 2/130 (1.5) | 2/141 (1.4) |
| Missing | 60/190 (31.6) | 49/190 (25.8) |
| Is there a delay before you can start to urinate? n/N (%) | ||
| 6 months | ||
| Never | 59/117 (50.4) | 83/111 (74.8) |
| Occasionally | 38/117 (32.5) | 18/111 (16.2) |
| Sometimes | 11/117 (9.4) | 8/111 (7.2) |
| Most of the time | 7/117 (6.0) | 2/111 (1.8) |
| All of the time | 2/117 (1.7) | 0 |
| Missing | 73/190 (38.4) | 79/190 (41.6) |
| 12 months | ||
| Never | 72/146 (49.3) | 106/148 (71.6) |
| Occasionally | 48/146 (32.9) | 32/148 (21.6) |
| Sometimes | 18/146 (12.3) | 8/148 (5.4) |
| Most of the time | 5/146 (3.4) | 1/148 (0.7) |
| All of the time | 3/146 (2.1) | 1/148 (0.7) |
| Missing | 44/190 (23.2) | 42/190 (22.1) |
| 24 months | ||
| Never | 65/130 (50.0) | 89/140 (63.6) |
| Occasionally | 42/130 (32.3) | 37/140 (26.4) |
| Sometimes | 15/130 (11.5) | 10/140 (7.1) |
| Most of the time | 6/130 (4.6) | 4/140 (2.9) |
| All of the time | 2/130 (1.5) | |
| Missing | 60/190 (31.6) | 50/190 (26.3) |
| Do you have to strain to continue urinating? n/N (%) | ||
| 6 months | ||
| Never | 69/118 (58.5) | 74/111 (66.7) |
| Occasionally | 30/118 (25.4) | 25/111 (22.5) |
| Sometimes | 13/118 (11.0) | 8/111 (7.2) |
| Most of the time | 5/118 (4.2) | 2/111 (1.8) |
| All of the time | 1/118 (0.8) | 2/111 (1.8) |
| Missing | 72/190 (37.9) | 79/190 (41.6) |
| 12 months | ||
| Never | 85/146 (58.2) | 94/148 (63.5) |
| Occasionally | 43/146 (29.5) | 39/148 (26.4) |
| Sometimes | 12/146 (8.2) | 11/148 (7.4) |
| Most of the time | 3/146 (2.1) | 2/148 (1.4) |
| All of the time | 3/146 (2.1) | 2/148 (1.4) |
| Missing | 44/190 (23.2) | 42/190 (22.1) |
| 24 months | ||
| Never | 73/131 (55.7) | 90/143 (62.9) |
| Occasionally | 36/131 (27.5) | 34/143 (23.8) |
| Sometimes | 16/131 (12.2) | 13/143 (9.1) |
| Most of the time | 3/131 (2.3) | 3/143 (2.1) |
| All of the time | 3/131 (2.3) | 3/143 (2.1) |
| Missing | 59/190 (31.1) | 47/190 (24.7) |
| Would you say that the strength of your urinary stream is: n/N (%) | ||
| 6 months | ||
| Normal | 49/118 (41.5) | 72/111 (64.9) |
| Occasionally reduced | 25/118 (21.2) | 20/111 (18.0) |
| Sometimes reduced | 20/118 (16.9) | 12/111 (10.8) |
| Reduced most of the time | 18/118 (15.3) | 5/111 (4.5) |
| Reduced all of the time | 6/118 (5.1) | 2/111 (1.8) |
| Missing | 72/190 (37.9) | 79/190 (41.6) |
| 12 months | ||
| Normal | 59/145 (40.7) | 96/148 (64.9) |
| Occasionally reduced | 31/145 (21.4) | 25/148 (16.9) |
| Sometimes reduced | 29/145 (20.0) | 15/148 (10.1) |
| Reduced most of the time | 20/145 (13.8) | 9/148 (6.1) |
| Reduced all of the time | 6/145 (4.1) | 3/148 (2.0) |
| Missing | 45/190 (23.7) | 42/190 (22.1) |
| 24 months | ||
| Normal | 56/131 (42.7) | 85/143 (59.4) |
| Occasionally reduced | 28/131 (21.4) | 31/143 (21.7) |
| Sometimes reduced | 24/131 (18.3) | 16/143 (11.2) |
| Reduced most of the time | 20/131 (15.3) | 7/143 (4.9) |
| Reduced all of the time | 3/131 (2.3) | 4/143 (2.8) |
| Missing | 59/190 (31.1) | 47/190 (24.7) |
| Do you stop and start more than once while you urinate? n/N (%) | ||
| 6 months | ||
| Never | 45/116 (38.8) | 50/109 (45.9) |
| Occasionally | 44/116 (37.9) | 42/109 (38.5) |
| Sometimes | 16/116 (13.8) | 10/109 (9.2) |
| Most of the time | 10/116 (8.6) | 6/109 (5.5) |
| All of the time | 1/116 (0.9) | 1/109 (0.9) |
| Missing | 74/190 (38.9) | 81/190 (42.6) |
| 12 months | ||
| Never | 62/146 (42.5) | 75/149 (50.3) |
| Occasionally | 49/146 (33.6) | 53/149 (35.6) |
| Sometimes | 22/146 (15.1) | 13/149 (8.7) |
| Most of the time | 9/146 (6.2) | 6/149 (4.0) |
| All of the time | 4/146 (2.7) | 2/149 (1.3) |
| Missing | 44/190 (23.2) | 41/190 (21.6) |
| 24 months | ||
| Never | 48/131 (36.6) | 74/143 (51.7) |
| Occasionally | 60/131 (45.8) | 42/143 (29.4) |
| Sometimes | 13/131 (9.9) | 18/143 (12.6) |
| Most of the time | 7/131 (5.3) | 7/143 (4.9) |
| All of the time | 3/131 (2.3) | 2/143 (1.4) |
| Missing | 59/190 (31.1) | 47/190 (24.7) |
| How often do you feel that your bladder has not emptied properly after you have urinated? n/N (%) | ||
| 6 months | ||
| Never | 44/117 (37.6) | 49/110 (44.5) |
| Occasionally | 41/117 (35.0) | 47/110 (42.7) |
| Sometimes | 20/117 (17.1) | 9/110 (8.2) |
| Most of the time | 9/117 (7.7) | 3/110 (2.7) |
| All of the time | 3/117 (2.6) | 2/110 (1.8) |
| Missing | 73/190 (38.4) | 80/190 (42.1) |
| 12 months | ||
| Never | 61/145 (42.1) | 73/147 (49.7) |
| Occasionally | 49/145 (33.8) | 57/147 (38.8) |
| Sometimes | 20/145 (13.8) | 11/147 (7.5) |
| Most of the time | 12/145 (8.3) | 3/147 (2.0) |
| All of the time | 3/145 (2.1) | 3/147 (2.0) |
| Missing | 45/190 (23.7) | 43/190 (22.6) |
| 24 months | ||
| Never | 54/131 (41.2) | 61/142 (43.0) |
| Occasionally | 51/131 (38.9) | 59/142 (41.5) |
| Sometimes | 19/131 (14.5) | 17/142 (12.0) |
| Most of the time | 5/131 (3.8) | 4/142 (2.8) |
| All of the time | 2/131 (1.5) | 1/142 (0.7) |
| Missing | 59/190 (31.1) | 48/190 (25.3) |
| Number of times pass urine during the day | ||
| 6 months | ||
| Mean (SD); n | 7.3 (4.8); 114 | 6.8 (2.5); 103 |
| Median (IQR) | 7.0 (5.0–9.0) | 6.0 (5.0–8.0) |
| Minimum, maximum | 0.0, 50.0 | 3.0, 20.0 |
| 12 months | ||
| Mean (SD); n | 7.5 (8.2); 143 | 6.8 (2.8); 141 |
| Median (IQR) | 6.0 (5.0–9.0) | 6.0 (5.0–8.0) |
| Minimum, maximum | 0.0, 99.0 | 3.0, 20.0 |
| 24 months | ||
| Mean (SD); n | 6.8 (2.5); 124 | 6.5 (2.3); 131 |
| Median (IQR) | 6.0 (5.0–8.5) | 6.0 (5.0–8.0) |
| Minimum, maximum | 3.0, 15.0 | 1.0, 15.0 |
| Number of times pass urine during the night | ||
| 6 months | ||
| Mean (SD); n | 1.7 (1.5); 115 | 1.5 (1.2); 109 |
| Median (IQR) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Minimum, maximum | 0.0, 10.0 | 0.0, 8.0 |
| 12 months | ||
| Mean (SD); n | 1.7 (1.3); 146 | 1.5 (1.1); 146 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Minimum, maximum | 0.0–8.0 | 0.0, 8.0 |
| 24 months | ||
| Mean (SD); n | 1.7 (1.4); 129 | 1.4 (1.0); 139 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Minimum, maximum | 0.0, 10.0 | 0.0, 4.0 |
The number of times passing urine during the day and night is presented solely for information. The questions on how often a participant leaks and how much a participant leaks are the components of the primary outcome and are also used in the ICIQ-UI SF score. The other questions are the components of the ICIQ-MLUTS incontinence and voiding scores.
Appendix 2 Non-randomised cohort baseline and clinical outcomes
Non-randomised cohort baseline information
| Time to events | NRC sling (N = 46) | NRC AUS (N = 46) |
|---|---|---|
| Days from consent to 6-month follow-up | ||
| Mean (SD); n | 310 (103); 34 | 324 (116); 36 |
| Median (IQR) | 296 (228–364) | 302 (221–415) |
| Minimum, maximum | 182, 703 | 200, 629 |
| Days from consent to 12-month follow-upa | ||
| Mean (SD); n | 419 (89); 40 | 399 (82); 38 |
| Median (IQR) | 396 (369–435) | 370 (355–420) |
| Minimum, maximum | 336, 822 | 335, 720 |
| Days from consent to 24-month follow-up | ||
| Mean (SD); n | 799 (69); 30 | 798 (115); 35 |
| Median (IQR) | 774 (761–808) | 759 (741–787) |
| Minimum, maximum | 734, 1037 | 730, 1256 |
| Days from surgery to 6-month follow-up | ||
| Mean (SD); n | 211 (51); 34 | 207 (28); 36 |
| Median (IQR) | 197 (191–214) | 197 (188–215) |
| Minimum, maximum | 121, 408 | 162, 297 |
| Days from surgery to 12-month follow-up | ||
| Mean (SD); n | 318 (82); 38 | 277 (143); 37 |
| Median (IQR) | 337 (285–357) | 334 (214–355) |
| Minimum, maximum | 164, 607 | –71, 688 |
| Days from surgery to 24-month follow-up | ||
| Mean (SD); n | 701 (88); 30 | 674 (155); 33 |
| Median (IQR) | 715 (606–779) | 722 (608–753) |
| Minimum, maximum | 577, 888 | 260, 1090 |
| Days from consent to surgery | ||
| Mean (SD); n | 102 (95); 42 | 120 (126); 43 |
| Median (IQR) | 97 (36–159) | 93 (14–194) |
| Minimum, maximum | 0, 519 | 0, 504 |
| NRC sling (N = 46) | NRC AUS (N = 46) | NRC unknown (N = 7) | |
|---|---|---|---|
| Age (years), mean (SD) | 68.3 (5.2) | 67.3 (5.6) | 68.3 (6.1) |
| Previous prostate surgery, n (%) | |||
| Surgery for prostate cancer | 40 (87.0) | 40 (87.0) | 7 (100.0) |
| Surgery for benign prostate obstruction | 2 (4.3) | 0 | 0 |
| Surgery for both | 2 (4.3) | 1 (2.2) | 0 |
| Missing | 2 (4.3) | 5 (10.9) | 0 |
| Type of previous surgery, n (%) | |||
| Radical prostatectomy | 45 (97.8) | 45 (97.8) | 7 (100.0) |
| Channel TURP for obstructing prostate cancer | 0 | 2 (4.3) | 0 |
| Transurethral prostatectomy for benign prostatic obstruction | 3 (6.5) | 0 | 0 |
| Retropubic prostatectomy for benign prostatic obstruction | 0 | 0 | 0 |
| Received radiotherapy for prostatic disease, n (%) | |||
| Yes | 5 (10.9) | 10 (21.7) | 2 (28.6) |
| No | 41 (89.1) | 33 (71.7) | 4 (57.1) |
| Missing | 3 (6.5) | 1 (14.3) | |
| Leaking urine before first prostate operation, n (%) | |||
| Yes | 0 | 0 | 0 |
| No | 46 (100.0) | 44 (95.7) | 6 (85.7) |
| Missing | 0 | 2 (4.3) | 1 (14.3) |
| Previous treatment for urinary/bladder problems, n (%) | |||
| Injectable treatment for SUI | 0 | 0 | 0 |
| Physiotherapy for SUI | 18 (39.1) | 27 (58.7) | 4 (57.1) |
| Drug treatment with duloxetine for SUI | 5 (10.9) | 6 (13.0) | 1 (14.3) |
| Drug treatment for other urinary/bladder problem | 12 (26.1) | 11 (23.9) | 1 (14.3) |
| Any neurological disease | 1 (2.2) | ||
| Do you wear pads or protection because of leaking urine? n (%) | |||
| Yes | 43 (93.5) | 44 (95.7) | 4 (57.1) |
| No | 0 | 1 (2.2) | 0 |
| Missing | 3 (6.5) | 1 (2.2) | 3 (42.9) |
| 24-hour pad test result (weight in g) | |||
| Mean (SD); n | 249.2 (339.6); 32 | 683.2 (536.3); 40 | 430.7 (371.9); 3 |
| Median (IQR) | 113.0 (44.0–272.0) | 522.0 (249.0–1099.5) | 576.0 (8.0–708.0) |
| Pads used on an average day | |||
| Mean (SD); n | 2.6 (1.5); 42 | 5.7 (7.2); 42 | 3.0 (1.4); 4 |
| Health status | NRC sling (N = 46) | NRC AUS (N = 46) | NRC unknown (N = 7) |
|---|---|---|---|
| How often do you leak urine? n (%) | |||
| Once per week or less | 1 (2.2) | 0 | 0 |
| Two or three times per week | 1 (2.2) | 0 | 0 |
| About once per day | 2 (4.3) | 0 | 1 (14.3) |
| Several times per day | 32 (69.6) | 19 (41.3) | 3 (42.9) |
| All of the time | 7 (15.2) | 25 (54.3) | 0 |
| Missing | 3 (6.5) | 2 (4.3) | 0 |
| How much urine do you usually leak? n (%) | |||
| A small amount | 16 (34.8) | 3 (6.5) | 1 (14.3) |
| A moderate amount | 19 (41.3) | 12 (26.1) | 3 (42.9) |
| A large amount | 8 (17.4) | 31 (67.4) | 0 |
| Question missing | 1 (2.2) | 0 | 0 |
| Missing | 2 (4.3) | 0 | 3 (42.9) |
| Score for effect on everyday life, mean (SD); n | 6.7 (2.7); 41 | 8.3 (1.7); 46 | 5.8 (2.2); 4 |
| Number of times pass urine during the daytime, mean (SD); n | 6.7 (2.4); 41 | 6.8 (3.1); 39 | 4.7 (0.6); 3 |
| Number of times get up at night to pass urine, mean (SD); n | 1.8 (1.3); 42 | 2.1 (1.4); 45 | 1.3 (1.0); 4 |
| ICIQ-UI SF score, mean (SD); n | 14.4 (3.9); 40 | 18.1 (2.6); 44 | 13.0 (3.6); 4 |
| ICIQ-MLUTS incontinence score, mean (SD); n | 9.7 (3.9); 41 | 14.0 (3.4); 44 | 10.3 (3.2); 3 |
| ICIQ-MLUTS voiding score, mean (SD); n | 2.8 (2.7); 43 | 3.6 (3.5); 46 | 3.0 (3.6); 4 |
| ICIQ-MLUTSsex, mean (SD); n | 8.2 (1.7); 37 | 8.2 (1.7); 35 | 5.0 (0.0); 2 |
| SF-12 mental score, mean (SD); n | 50.8 (12.1); 40 | 46.6 (12.9); 43 | 47.1 (6.2); 3 |
| SF-12 physical score, mean (SD); n | 49.7 (8.5); 40 | 47.1 (7.9); 43 | 54.0 (4.6); 3 |
| EQ-5D-3L, mean (SD); n | 0.845 (0.270); 42 | 0.767 (0.276); 45 | 0.858 (0.182); 4 |
| NRC sling (N = 46), n (%) | NRC AUS (N = 46), n (%) | NRC unknown (N = 7), n (%) | |
|---|---|---|---|
| Does urine leak when you cough or sneeze? | |||
| Never | 0 | 2 (4.3) | 0 |
| Occasionally | 7 (15.2) | 5 (10.9) | 0 |
| Sometimes | 14 (30.4) | 6 (13.0) | 2 (28.6) |
| Most of the time | 13 (28.3) | 12 (26.1) | 1 (14.3) |
| All of the time | 9 (19.6) | 21 (45.7) | 1 (14.3) |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Do you have a sudden need to rush to the toilet to urinate? | |||
| Never | 9 (19.6) | 14 (30.4) | |
| Occasionally | 15 (32.6) | 14 (30.4) | 2 (28.6) |
| Sometimes | 14 (30.4) | 12 (26.1) | 2 (28.6) |
| Most of the time | 4 (8.7) | 5 (10.9) | 0 |
| All of the time | 0 | 1 (2.2) | 0 |
| Missing | 4 (8.7) | 0 | 3 (42.9) |
| Does urine leak before you can get to the toilet? | |||
| Never | 4 (8.7) | 1 (2.2) | 0 |
| Occasionally | 21 (45.7) | 8 (17.4) | 3 (42.9) |
| Sometimes | 12 (26.1) | 8 (17.4) | 0 |
| Most of the time | 4 (8.7) | 16 (34.8) | 1 (14.3) |
| All of the time | 1 (2.2) | 12 (26.1) | 0 |
| Missing | 4 (8.7) | 1 (2.2) | 3 (42.9) |
| How often have you had a slight wetting of your pants after you have finished urinating and are dressed? | |||
| Never | 6 (13.0) | 3 (6.5) | 0 |
| Occasionally | 16 (34.8) | 7 (15.2) | 2 (28.6) |
| Sometimes | 10 (21.7) | 15 (32.6) | 1 (14.3) |
| Most of the time | 8 (17.4) | 11 (23.9) | 0 |
| All of the time | 1 (2.2) | 8 (17.4) | 0 |
| Missing | 5 (10.9) | 2 (4.3) | 4 (57.1) |
| Do you ever leak for no obvious reason and without feeling that you want to go? | |||
| Never | 4 (8.7) | 0 | 0 |
| Occasionally | 12 (26.1) | 3 (6.5) | 1 (14.3) |
| Sometimes | 12 (26.1) | 7 (15.2) | 1 (14.3) |
| Most of the time | 11 (23.9) | 19 (41.3) | 2 (28.6) |
| All of the time | 4 (8.7) | 17 (37.0) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Do you ever leak urine when you are asleep? | |||
| Never | 18 (39.1) | 9 (19.6) | 2 (28.6) |
| Occasionally | 18 (39.1) | 15 (32.6) | 1 (14.3) |
| Sometimes | 3 (6.5) | 7 (15.2) | 0 |
| Most of the time | 2 (4.3) | 10 (21.7) | 1 (14.3) |
| All of the time | 2 (4.3) | 5 (10.9) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Is there a delay before you can start to urinate? | |||
| Never | 34 (73.9) | 35 (76.1) | 3 (42.9) |
| Occasionally | 8 (17.4) | 7 (15.2) | 1 (14.3) |
| Sometimes | 1 (2.2) | 3 (6.5) | 0 |
| Most of the time | 0 | 0 | 0 |
| All of the time | 0 | 1 (2.2) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Do you have to strain to continue urinating? | |||
| Never | 31 (67.4) | 29 (63.0) | 2 (28.6) |
| Occasionally | 6 (13.0) | 10 (21.7) | 2 (28.6) |
| Sometimes | 3 (6.5) | 6 (13.0) | 0 |
| Most of the time | 3 (6.5) | 0 | 0 |
| All of the time | 0 | 1 (2.2) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Would you say that the strength of your urinary stream is: | |||
| Normal | 27 (58.7) | 28 (60.9) | 3 (42.9) |
| Occasionally reduced | 8 (17.4) | 5 (10.9) | 0 |
| Sometimes reduced | 5 (10.9) | 6 (13.0) | 0 |
| Reduced most of the time | 3 (6.5) | 5 (10.9) | 1 (14.3) |
| Reduced all of the time | 0 | 2 (4.3) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Do you stop and start more than once while you urinate? | |||
| Never | 22 (47.8) | 27 (58.7) | 1 (14.3) |
| Occasionally | 18 (39.1) | 10 (21.7) | 2 (28.6) |
| Sometimes | 3 (6.5) | 7 (15.2) | 1 (14.3) |
| Most of the time | 0 | 2 (4.3) | 0 |
| All of the time | 0 | 0 | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| How often do you feel that your bladder has not emptied properly after you have urinated? | |||
| Never | 16 (34.8) | 19 (41.3) | 2 (28.6) |
| Occasionally | 19 (41.3) | 11 (23.9) | 2 (28.6) |
| Sometimes | 6 (13.0) | 9 (19.6) | 0 |
| Most of the time | 2 (4.3) | 6 (13.0) | 0 |
| All of the time | 0 | 1 (2.2) | 0 |
| Missing | 3 (6.5) | 0 | 3 (42.9) |
| Surgery information | NRC sling (N = 46) | NRC AUS (N = 46) |
|---|---|---|
| Received surgery, n/N (%) | 42/46 (91.3) | 43/46 (93.5) |
| Received sling | 42/42 (100.0) | |
| Received AUS | 43/43 (100.0) | |
| Device replacement/removal (within 24 months), n/N (%) | 6/42 (14.3) | 2/43 (4.7) |
| Replaced by sphincter | 6/6 (100.0) | 2/2 (100.0) |
| Length of surgery (minutes) | ||
| Mean (SD); n | 96.1 (24.9); 42 | 122.2 (22.9); 43 |
| Median (IQR) | 92.0 (79.0–115.0) | 120.0 (105.0–135.0) |
| Minimum, maximum | 30.0, 147.0 | 60.0, 180.0 |
| Length of hospital stay (days) | ||
| Mean (SD); n | 1.5 (0.8); 42 | 1.5 (0.5); 43 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Minimum, maximum | 1.0, 5.0 | 1.0, 2.0 |
| Time to re-admission (days) | ||
| Mean (SD); n | 265.5 (113.8); 6 | 854.0 (79.2); 2 |
| Median (IQR) | 225.0 (196.0–399.0) | 854.0 (798.0–910.0) |
| Minimum, maximum | 135.0, 413.0 | 798.0, 910.0 |
| NRC sling (N = 46) | NRC AUS (N = 46) | |
|---|---|---|
| Total number of complications | 51 | 52 |
| Total number of SAEs | 5 | 1 |
| Received operation, n/N (%) | 42/46 (91.3) | 43/46 (93.5) |
| Participants with complications, n/N (%) | 37/42 (88.1) | 41/43 (95.3) |
| Number of complications per participant, n/N (%) | ||
| 0 | 5/42 (11.9) | 2/43 (4.7) |
| 1 | 26/42 (61.9) | 31/43 (72.1) |
| 2 | 9/42 (21.4) | 9/43 (20.9) |
| 3 | 1/42 (2.4) | 1/43 (2.3) |
| 4 | 1/42 (2.4) | |
| Catheter required for > 24 hours, n/N (%) | 4/42 (9.5) | |
| Postoperative catheter required, n/N (%) | 4/42 (9.5) | 1/43 (2.3) |
| Urinary tract infection | ||
| Pyrexia | 1/42 (2.4) | 2/43 (4.7) |
| Retention requiring surgery | 1/42 (2.4) | |
| New urinary tract symptoms | 1/42 (2.4) | |
| Tape or sling complications | 1/42 (2.4) | 2/43 (4.7) |
| Acute or chronic pain | 1/42 (2.4) | |
| Oral pain relief given | 32/42 (76.2) | 41/43 (95.3) |
| Parenteral pain relief given | 2/42 (4.8) | 4/43 (9.3) |
| Antibiotic treatment for postoperative infection | 2/42 (4.8) | |
Non-randomised cohort outcome data
| NRC sling (N = 46), n/N (%) | NRC AUS (N = 46), n/N (%) | |
|---|---|---|
| Primary outcome | ||
| Continent (12 months) | 8/43 (18.6) | 4/39 (10.3) |
| Continent: less strict definition (12 months) | 20/43 (46.5) | 10/39 (25.6) |
| Other time points | ||
| Continent (6 months) | 6/33 (18.2) | 5/36 (13.9) |
| Continent: less strict definition (6 months) | 15/33 (45.5) | 12/36 (33.3) |
| Continent (24 months) | 5/31 (16.1) | 4/35 (11.4) |
| Continent: less strict definition (24 months) | 15/31 (48.4) | 9/35 (25.7) |
| NRC sling (N = 46) | NRC AUS (N = 46) | |
|---|---|---|
| Wear pads or other protection, n/N (%) | ||
| Baseline | 43/46 (93.5) | 44/46 (95.7) |
| 6 months | 18/35 (51.4) | 13/35 (37.1) |
| 12 months | 20/38 (52.6) | 9/36 (25.0) |
| 24 months | 13/31 (41.9) | 8/33 (24.2) |
| Pads used in an average day, mean (SD); n | ||
| Baseline | 2.6 (1.5); 42 | 5.7 (7.2); 42 |
| 6 months | 0.8 (1.2); 32 | 1.0 (1.2); 33 |
| 12 months | 0.8 (1.2); 36 | 1.6 (1.6); 35 |
| 24 months | 1.0 (1.2); 30 | 1.4 (1.4); 33 |
| Score for effect on everyday life, mean (SD); n | ||
| Baseline | 8.2 (1.7); 37 | 8.2 (1.7); 35 |
| 6 months | 3.1 (3.3); 34 | 2.2 (2.7); 35 |
| 12 months | 2.9 (3.4); 43 | 2.6 (3.2); 38 |
| 24 months | 2.0 (2.4); 30 | 2.8 (3.2); 36 |
| ICIQ-UI SF score, mean (SD); n | ||
| Baseline | 14.4 (3.9); 40 | 18.1 (2.6); 44 |
| 6 months | 7.6 (6.1); 33 | 6.7 (5.0); 34 |
| 12 months | 7.1 (6.1); 39 | 7.9 (5.8); 37 |
| 24 months | 6.2 (4.7); 29 | 7.8 (5.6); 34 |
| ICIQ-MLUTS incontinence score, mean (SD); n | ||
| Baseline | 9.7 (3.9); 41 | 14.0 (3.4); 44 |
| 6 months | 5.8 (3.5); 32 | 5.6 (4.1); 35 |
| 12 months | 5.8 (3.7); 39 | 6.9 (5.1); 36 |
| 24 months | 5.1 (3.8); 31 | 6.3 (4.0); 30 |
| ICIQ-MLUTS voiding score, mean (SD); n | ||
| Baseline | 2.8 (2.7); 43 | 3.6 (3.5); 46 |
| 6 months | 4.3 (3.8); 31 | 3.5 (3.7); 34 |
| 12 months | 3.6 (3.6); 39 | 3.5 (3.8); 36 |
| 24 months | 3.7 (3.6); 30 | 3.2 (3.8); 31 |
| MLUTS sexual function, mean (SD); n | ||
| Baseline | 8.2 (1.7); 37 | 8.2 (1.7); 35 |
| 6 months | 7.3 (1.9); 23 | 8.2 (1.5); 22 |
| 12 months | 7.1 (1.8); 27 | 7.9 (1.7); 21 |
| 24 months | 7.7 (1.7); 20 | 7.8 (1.6); 19 |
| EQ-5D-3L, mean (SD); n | ||
| Baseline | 0.845 (0.270); 42 | 0.767 (0.276); 45 |
| 6 months | 0.872 (0.220); 35 | 0.830 (0.246); 36 |
| 12 months | 0.871 (0.271); 41 | 0.791 (0.311); 38 |
| 24 months | 0.854 (0.237); 30 | 0.774 (0.337); 36 |
| SF-12 physical score, mean (SD); n | ||
| Baseline | 49.7 (8.5); 40 | 47.1 (7.9); 43 |
| 6 months | 49.5 (8.7); 34 | 48.0 (8.4); 35 |
| 12 months | 50.5 (8.2); 41 | 46.9 (8.4); 34 |
| 24 months | 49.0 (8.9); 30 | 48.0 (9.6); 33 |
| SF-12 mental score, mean (SD); n | ||
| Baseline | 50.8 (12.1); 40 | 46.6 (12.9); 43 |
| 6 months | 52.9 (10.9); 34 | 52.4 (7.9); 35 |
| 12 months | 52.9 (10.0); 41 | 51.1 (8.8); 34 |
| 24 months | 51.5 (10.2); 30 | 50.1 (11.2); 33 |
| NRC sling (N = 46) | NRC AUS (N = 46) | |
|---|---|---|
| Urine leakage compared with before surgery, n/N (%) | ||
| Very much better | 25/46 (54.3) | 23/46 (50.0) |
| Much better | 5/46 (10.9) | 5/46 (10.9) |
| A little better | 8/46 (17.4) | 2/46 (4.3) |
| No change | 1/46 (2.2) | 1/46 (2.2) |
| A little worse | 1/46 (2.2) | |
| Much worse | ||
| Very much worse | ||
| Missing | 6/46 (13.0) | 15/46 (32.6) |
| Satisfaction with surgery results, n/N (%) | ||
| Completely satisfied | 21/46 (45.7) | 18/46 (39.1) |
| Fairly satisfied | 10/46 (21.7) | 9/46 (19.6) |
| Fairly dissatisfied | 4/46 (8.7) | 2/46 (4.3) |
| Very dissatisfied | 4/46 (8.7) | 1/46 (2.2) |
| Not sure | 1/46 (2.2) | 1/46 (2.2) |
| Missing | 6/46 (13.0) | 15/46 (32.6) |
| Recommend surgery to a friend, n/N (%) | ||
| Yes | 34/46 (73.9) | 28/46 (60.9) |
| No | 6/46 (13.0) | 2/46 (4.3) |
| Missing | 6/46 (13.0) | 16/46 (34.8) |
| 24-hour pad weight at 12 months (g)a | ||
| Mean (SD); n | 0.0 (0.1); 5 | |
| Median (IQR) | 0.0 (0.0–0.0) | |
| Minimum, maximum | 0, 0.2 | |
| Time until return to normal activities (months) | ||
| Mean (SD); n | 2.1 (1.4); 35 | 2.8 (3.2); 28 |
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Minimum, maximum | 0, 6.0 | 0, 13.0 |
| NRC sling (N = 46) | NRC AUS (N = 46) | |
|---|---|---|
| How often do you leak urine? n/N (%) | ||
| 6 months | ||
| Never | 6/33 (18.2) | 6/36 (16.7) |
| Once per week or less | 9/33 (27.3) | 6/36 (16.7) |
| Two or three times per week | 3/33 (9.1) | 6/36 (16.7) |
| About once per day | 5/33 (15.2) | 6/36 (16.7) |
| Several times per day | 8/33 (24.2) | 11/36 (30.6) |
| All of the time | 2/33 (6.1) | 1/36 (2.8) |
| Missing | 13/46 (28.3) | 10/46 (21.7) |
| 12 months | ||
| Never | 9/39 (23.1) | 3/38 (7.9) |
| Once per week or less | 8/39 (20.5) | 6/38 (15.8) |
| Two or three times per week | 5/39 (12.8) | 6/38 (15.8) |
| About once per day | 5/39 (12.8) | 7/38 (18.4) |
| Several times per day | 10/39 (25.6) | 12/38 (31.6) |
| All of the time | 2/39 (5.1) | 4/38 (10.5) |
| Missing | 7/46 (15.2) | 8/46 (17.4) |
| 24 months | ||
| Never | 7/30 (23.3) | 4/34 (11.8) |
| Once per week or less | 7/30 (23.3) | 4/34 (11.8) |
| Two or three times per week | 4/30 (13.3) | 6/34 (17.6) |
| About once per day | 3/30 (10.0) | 1/34 (2.9) |
| Several times per day | 8/30 (26.7) | 13/34 (38.2) |
| All of the time | 1/30 (3.3) | 6/34 (17.6) |
| Missing | 16/46 (34.8) | 12/46 (26.1) |
| How much urine do you usually leak? n/N (%) | ||
| 6 months | ||
| None | 6/33 (18.2) | 6/35 (17.1) |
| A small amount | 19/33 (57.6) | 24/35 (68.6) |
| A moderate amount | 6/33 (18.2) | 4/35 (11.4) |
| A large amount | 2/33 (6.1) | 1/35 (2.9) |
| Missing | 13/46 (28.3) | 11/46 (23.9) |
| 12 months | ||
| None | 8/43 (18.6) | 4/39 (10.3) |
| A small amount | 27/43 (62.8) | 27/39 (69.2) |
| A moderate amount | 7/43 (16.3) | 4/39 (10.3) |
| A large amount | 1/43 (2.3) | 4/39 (10.3) |
| Missing | 3/46 (6.5) | 7/46 (15.2) |
| 24 months | ||
| None | 5/31 (16.1) | 4/35 (11.4) |
| A small amount | 21/31 (67.7) | 25/35 (71.4) |
| A moderate amount | 5/31 (16.1) | 3/35 (8.6) |
| A large amount | 3/35 (8.6) | |
| Missing | 15/46 (32.6) | 11/46 (23.9) |
| Does urine leak when you cough or sneeze? n/N (%) | ||
| 6 months | ||
| Never | 11/32 (34.4) | 10/35 (28.6) |
| Occasionally | 10/32 (31.3) | 9/35 (25.7) |
| Sometimes | 5/32 (15.6) | 10/35 (28.6) |
| Most of the time | 2/32 (6.3) | 4/35 (11.4) |
| All of the time | 4/32 (12.5) | 2/35 (5.7) |
| Missing | 14/46 (30.4) | 11/46 (23.9) |
| 12 months | ||
| Never | 7/41 (17.1) | 5/36 (13.9) |
| Occasionally | 16/41 (39.0) | 12/36 (33.3) |
| Sometimes | 10/41 (24.4) | 10/36 (27.8) |
| Most of the time | 7/41 (17.1) | 5/36 (13.9) |
| All of the time | 1/41 (2.4) | 4/36 (11.1) |
| Missing | 5/46 (10.9) | 10/46 (21.7) |
| 24 months | ||
| Never | 7/31 (22.6) | 2/30 (6.7) |
| Occasionally | 12/31 (38.7) | 14/30 (46.7) |
| Sometimes | 7/31 (22.6) | 8/30 (26.7) |
| Most of the time | 4/31 (12.9) | 3/30 (10.0) |
| All of the time | 1/31 (3.2) | 3/30 (10.0) |
| Missing | 15/46 (32.6) | 16/46 (34.8) |
| Do you have a sudden need to rush to the toilet to urinate? n/N (%) | ||
| 6 months | ||
| Never | 6/32 (18.8) | 10/35 (28.6) |
| Occasionally | 12/32 (37.5) | 15/35 (42.9) |
| Sometimes | 13/32 (40.6) | 7/35 (20.0) |
| Most of the time | 1/32 (3.1) | 2/35 (5.7) |
| All of the time | 1/35 (2.9) | |
| Missing | 14/46 (30.4) | 11/46 (23.9) |
| 12 months | ||
| Never | 13/40 (32.5) | 9/36 (25.0) |
| Occasionally | 17/40 (42.5) | 15/36 (41.7) |
| Sometimes | 7/40 (17.5) | 6/36 (16.7) |
| Most of the time | 3/40 (7.5) | 6/36 (16.7) |
| All of the time | ||
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 9/31 (29.0) | 7/30 (23.3) |
| Occasionally | 14/31 (45.2) | 15/30 (50.0) |
| Sometimes | 6/31 (19.4) | 6/30 (20.0) |
| Most of the time | 1/31 (3.2) | 2/30 (6.7) |
| All of the time | 1/31 (3.2) | |
| Missing | 15/46 (32.6) | 16/46 (34.8) |
| Does urine leak before you can get to the toilet? n/N (%) | ||
| 6 months | ||
| Never | 8/32 (25.0) | 16/35 (45.7) |
| Occasionally | 18/32 (56.3) | 12/35 (34.3) |
| Sometimes | 5/32 (15.6) | 5/35 (14.3) |
| Most of the time | 1/32 (3.1) | 1/35 (2.9) |
| All of the time | 1/35 (2.9) | |
| Missing | 14/46 (30.4) | 11/46 (23.9) |
| 12 months | ||
| Never | 11/40 (27.5) | 12/36 (33.3) |
| Occasionally | 19/40 (47.5) | 13/36 (36.1) |
| Sometimes | 9/40 (22.5) | 5/36 (13.9) |
| Most of the time | 1/40 (2.5) | 4/36 (11.1) |
| All of the time | 2/36 (5.6) | |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 12/31 (38.7) | 11/31 (35.5) |
| Occasionally | 14/31 (45.2) | 12/31 (38.7) |
| Sometimes | 4/31 (12.9) | 4/31 (12.9) |
| Most of the time | 1/31 (3.2) | 2/31 (6.5) |
| All of the time | 2/31 (6.5) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| How often have you had a slight wetting of your pants after you have finished urinating and are dressed? n/N (%) | ||
| 6 months | ||
| Never | 7/33 (21.2) | 7/35 (20.0) |
| Occasionally | 20/33 (60.6) | 23/35 (65.7) |
| Sometimes | 4/33 (12.1) | 1/35 (2.9) |
| Most of the time | 2/33 (6.1) | 4/35 (11.4) |
| All of the time | ||
| Missing | 13/46 (28.3) | 11/46 (23.9) |
| 12 months | ||
| Never | 14/40 (35.0) | 8/36 (22.2) |
| Occasionally | 19/40 (47.5) | 20/36 (55.6) |
| Sometimes | 4/40 (10.0) | 4/36 (11.1) |
| Most of the time | 3/40 (7.5) | 3/36 (8.3) |
| All of the time | 1/36 (2.8) | |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 12/31 (38.7) | 6/31 (19.4) |
| Occasionally | 14/31 (45.2) | 16/31 (51.6) |
| Sometimes | 3/31 (9.7) | 4/31 (12.9) |
| Most of the time | 2/31 (6.5) | 4/31 (12.9) |
| All of the time | 1/31 (3.2) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| Do you ever leak for no obvious reason and without feeling that you want to go? n/N (%) | ||
| 6 months | ||
| Never | 19/33 (57.6) | 15/35 (42.9) |
| Occasionally | 8/33 (24.2) | 15/35 (42.9) |
| Sometimes | 5/33 (15.2) | 3/35 (8.6) |
| Most of the time | 1/33 (3.0) | 1/35 (2.9) |
| All of the time | 1/35 (2.9) | |
| Missing | 13/46 (28.3) | 11/46 (23.9) |
| 12 months | ||
| Never | 21/41 (51.2) | 16/36 (44.4) |
| Occasionally | 9/41 (22.0) | 10/36 (27.8) |
| Sometimes | 3/41 (7.3) | 4/36 (11.1) |
| Most of the time | 6/41 (14.6) | 3/36 (8.3) |
| All of the time | 2/41 (4.9) | 3/36 (8.3) |
| Missing | 5/46 (10.9) | 10/46 (21.7) |
| 24 months | ||
| Never | 19/31 (61.3) | 13/30 (43.3) |
| Occasionally | 9/31 (29.0) | 10/30 (33.3) |
| Sometimes | 2/31 (6.5) | 5/30 (16.7) |
| Most of the time | 1/30 (3.3) | |
| All of the time | 1/31 (3.2) | 1/30 (3.3) |
| Missing | 15/46 (32.6) | 16/46 (34.8) |
| Do you ever leak urine when you are asleep? n/N (%) | ||
| 6 months | ||
| Never | 23/34 (67.6) | 27/35 (77.1) |
| Occasionally | 6/34 (17.6) | 4/35 (11.4) |
| Sometimes | 3/34 (8.8) | 2/35 (5.7) |
| Most of the time | 1/35 (2.9) | |
| All of the time | 2/34 (5.9) | 1/35 (2.9) |
| Missing | 12/46 (26.1) | 11/46 (23.9) |
| 12 months | ||
| Never | 28/40 (70.0) | 23/36 (63.9) |
| Occasionally | 6/40 (15.0) | 10/36 (27.8) |
| Sometimes | 4/40 (10.0) | 1/36 (2.8) |
| Most of the time | 1/40 (2.5) | 1/36 (2.8) |
| All of the time | 1/40 (2.5) | 1/36 (2.8) |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 21/31 (67.7) | 21/31 (67.7) |
| Occasionally | 8/31 (25.8) | 8/31 (25.8) |
| Sometimes | 1/31 (3.2) | |
| Most of the time | 1/31 (3.2) | |
| All of the time | 1/31 (3.2) | 1/31 (3.2) |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| Is there a delay before you can start to urinate? n/N (%) | ||
| 6 months | ||
| Never | 20/33 (60.6) | 28/34 (82.4) |
| Occasionally | 11/33 (33.3) | 2/34 (5.9) |
| Sometimes | 2/33 (6.1) | 4/34 (11.8) |
| Most of the time | ||
| All of the time | ||
| Missing | 13/46 (28.3) | 12/46 (26.1) |
| 12 months | ||
| Never | 27/40 (67.5) | 26/36 (72.2) |
| Occasionally | 10/40 (25.0) | 6/36 (16.7) |
| Sometimes | 3/40 (7.5) | 3/36 (8.3) |
| Most of the time | 1/36 (2.8) | |
| All of the time | ||
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 20/30 (66.7) | 23/31 (74.2) |
| Occasionally | 10/30 (33.3) | 7/31 (22.6) |
| Sometimes | ||
| Most of the time | 1/31 (3.2) | |
| All of the time | ||
| Missing | 16/46 (34.8) | 15/46 (32.6) |
| Do you have to strain to continue urinating? n/N (%) | ||
| 6 months | ||
| Never | 21/33 (63.6) | 20/34 (58.8) |
| Occasionally | 8/33 (24.2) | 12/34 (35.3) |
| Sometimes | 1/33 (3.0) | |
| Most of the time | 2/33 (6.1) | 1/34 (2.9) |
| All of the time | 1/33 (3.0) | 1/34 (2.9) |
| Missing | 13/46 (28.3) | 12/46 (26.1) |
| 12 months | ||
| Never | 23/40 (57.5) | 23/36 (63.9) |
| Occasionally | 10/40 (25.0) | 9/36 (25.0) |
| Sometimes | 5/40 (12.5) | 2/36 (5.6) |
| Most of the time | 2/40 (5.0) | 1/36 (2.8) |
| All of the time | 1/36 (2.8) | |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 20/31 (64.5) | 19/31 (61.3) |
| Occasionally | 7/31 (22.6) | 9/31 (29.0) |
| Sometimes | 1/31 (3.2) | 1/31 (3.2) |
| Most of the time | 2/31 (6.5) | 2/31 (6.5) |
| All of the time | 1/31 (3.2) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| Would you say that the strength of your urinary stream is, n/N (%) | ||
| 6 months | ||
| Normal | 15/33 (45.5) | 19/34 (55.9) |
| Occasionally reduced | 7/33 (21.2) | 13/34 (38.2) |
| Sometimes reduced | 5/33 (15.2) | |
| Reduced most of the time | 4/33 (12.1) | 2/34 (5.9) |
| Reduced all of the time | 2/33 (6.1) | |
| Missing | 13/46 (28.3) | 12/46 (26.1) |
| 12 months | ||
| Normal | 18/40 (45.0) | 23/36 (63.9) |
| Occasionally reduced | 13/40 (32.5) | 5/36 (13.9) |
| Sometimes reduced | 4/40 (10.0) | 4/36 (11.1) |
| Reduced most of the time | 3/40 (7.5) | 2/36 (5.6) |
| Reduced all of the time | 2/40 (5.0) | 2/36 (5.6) |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Normal | 17/31 (54.8) | 19/31 (61.3) |
| Occasionally reduced | 5/31 (16.1) | 7/31 (22.6) |
| Sometimes reduced | 3/31 (9.7) | 3/31 (9.7) |
| Reduced most of the time | 5/31 (16.1) | 2/31 (6.5) |
| Reduced all of the time | 1/31 (3.2) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| Do you stop and start more than once while you urinate? n/N (%) | ||
| 6 months | ||
| Never | 7/31 (22.6) | 13/34 (38.2) |
| Occasionally | 15/31 (48.4) | 13/34 (38.2) |
| Sometimes | 6/31 (19.4) | 3/34 (8.8) |
| Most of the time | 3/31 (9.7) | 4/34 (11.8) |
| All of the time | 1/34 (2.9) | |
| Missing | 15/46 (32.6) | 12/46 (26.1) |
| 12 months | ||
| Never | 18/40 (45.0) | 14/36 (38.9) |
| Occasionally | 13/40 (32.5) | 14/36 (38.9) |
| Sometimes | 5/40 (12.5) | 5/36 (13.9) |
| Most of the time | 3/40 (7.5) | 2/36 (5.6) |
| All of the time | 1/40 (2.5) | 1/36 (2.8) |
| Missing | 6/46 (13.0) | 10/46 (21.7) |
| 24 months | ||
| Never | 13/31 (41.9) | 15/31 (48.4) |
| Occasionally | 11/31 (35.5) | 10/31 (32.3) |
| Sometimes | 2/31 (6.5) | 3/31 (9.7) |
| Most of the time | 5/31 (16.1) | 2/31 (6.5) |
| All of the time | 1/31 (3.2) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| How often do you feel that your bladder has not emptied properly after you have urinated? n/N (%) | ||
| 6 months | ||
| Never | 11/32 (34.4) | 12/34 (35.3) |
| Occasionally | 13/32 (40.6) | 14/34 (41.2) |
| Sometimes | 6/32 (18.8) | 3/34 (8.8) |
| Most of the time | 2/32 (6.3) | 3/34 (8.8) |
| All of the time | 2/34 (5.9) | |
| Missing | 14/46 (30.4) | 12/46 (26.1) |
| 12 months | ||
| Never | 20/39 (51.3) | 15/36 (41.7) |
| Occasionally | 14/39 (35.9) | 17/36 (47.2) |
| Sometimes | 3/39 (7.7) | |
| Most of the time | 1/39 (2.6) | 2/36 (5.6) |
| All of the time | 1/39 (2.6) | 2/36 (5.6) |
| Missing | 7/46 (15.2) | 10/46 (21.7) |
| 24 months | ||
| Never | 12/31 (38.7) | 14/31 (45.2) |
| Occasionally | 17/31 (54.8) | 10/31 (32.3) |
| Sometimes | 1/31 (3.2) | 5/31 (16.1) |
| Most of the time | 1/31 (3.2) | |
| All of the time | 2/31 (6.5) | |
| Missing | 15/46 (32.6) | 15/46 (32.6) |
| Number of times pass urine during the day | ||
| 6 months | ||
| Mean (SD); n | 6.5 (2.0); 33 | 7.2 (2.9); 33 |
| Median (IQR) | 7.0 (5.0–8.0) | 6.0 (5.0–9.0) |
| Minimum, maximum | 3.0, 13.0 | 3.0, 15.0 |
| 12 months | ||
| Mean (SD); n | 6.8 (2.3); 38 | 6.8 (2.7); 34 |
| Median (IQR) | 6.0 (5.0–8.0) | 6.0 (5.0–8.0) |
| Minimum, maximum | 3.0, 12.0 | 3.0, 15.0 |
| 24 months | ||
| Mean (SD); n | 6.4 (2.5); 28 | 6.6 (2.3); 29 |
| Median (IQR) | 6.0 (4.0–7.5) | 6.0 (5.0–8.0) |
| Minimum, maximum | 3.0, 11.0 | 3.0, 12.0 |
| Number of times pass urine during the night | ||
| 6 months | ||
| Mean (SD); n | 1.6 (1.2); 33 | 1.7 (1.1); 34 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.5 (1.0–2.0) |
| Minimum, maximum | 0.0, 6.0 | 0.0, 5.0 |
| 12 months | ||
| Mean (SD); n | 1.7 (1.2); 38 | 1.8 (1.1); 36 |
| Median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) |
| Minimum, maximum | 0.0, 5.0 | 0.0, 5.0 |
| 24 months | ||
| Mean (SD); n | 1.7 (1.1); 31 | 1.8 (1.2); 31 |
| Median (IQR) | 1.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Minimum, maximum | 0.0, 4.0 | 0.0, 5.0 |
Appendix 3 Health economics
| Drug | Unit price (£) | Unit | Resource use | Cost per average case (£) |
|---|---|---|---|---|
| General anaesthesia | ||||
| Propofol, 1% injection | 20.16 | 20-ml ampoule | One ampoule | 4.03 |
| Fentanyl, 100 µg | 14.33 | 2-ml ampoule (50 µg/ml) | One ampoule | 1.43 |
| Morphine | 10.74 | 1-ml vial | One vial | 1.07 |
| Sevoflurane (volatile agent) | 123 | 250-ml bottle | 25 ml | 12.30 |
| Laryngeal mask | 29.5 | Box of 10 | One mask | 2.95 |
| Total cost | 21.79 | |||
| Spinal anaesthesia | ||||
| Bupivacaine hydrochloride anhydrous injection (1%) | 18.3 | 10-ml ampoule | One ampoule | 0.92 |
| Lidocaine | 7 | 10-ml ampoule | One ampoule | 0.70 |
| Total cost | 1.62 | |||
| Local anaesthesia | ||||
| Lidocaine | 7 | 10-ml ampoule | One ampoule | 0.70 |
| Total cost | 0.70 | |||
| General/spinal | ||||
| Cyclizine lactate injection (50 mg/ml) | 0.65 | 1-ml ampoule | One ampoule | 0.65 |
| Dexamethasone, injection | 4.8 | 2-ml vial | One vial | 4.8 |
| Ondansetron injection (2 mg/ml) | 1 | 2-ml ampoule | One ampoule | 1 |
| Total cost | 6.45 | |||
| Resource22 | Pack size | Packs for 1 year | Unit cost (£) | Total cost (£) |
|---|---|---|---|---|
| Sterile catheterisation insertion pack | 1 | 4 | 1.98 | 7.92 |
| Sterile lubricant for instillation | 1 | 4 | 0.98 | 3.92 |
| Indwelling catheter | 1 | 6 (4 + 2 spares) | 6.55 | 39.30 |
| Leg bags (assumes patients have continuous drainage) | 10 | 3.09 | 31.19 | 96.38 |
| Catheter stabilisation device | 4 | 2 | 8.73 | 17.46 |
| Night drainage bags | 10 | 37 | 3.09 | 114.33 |
| Total | 279.31 | |||
| Cost per week | 5.37 |
| Resource22 | Pack size | Packs for 1 year | Unit cost (£) | Total cost (£) |
|---|---|---|---|---|
| Sheaths | 30 | 37 | 53.65 | 1985.05 |
| Leg bags (assumes patients have continuous drainage) | 10 | 3.09 | 31.19 | 96.38 |
| Catheter stabilisation device | 4 | 2 | 8.73 | 17.46 |
| Night drainage bags | 10 | 37 | 3.09 | 114.33 |
| Total cost | 2213.22 | |||
| Cost per week | 42.56 |
FIGURE 16.
The EQ-VAS scores.
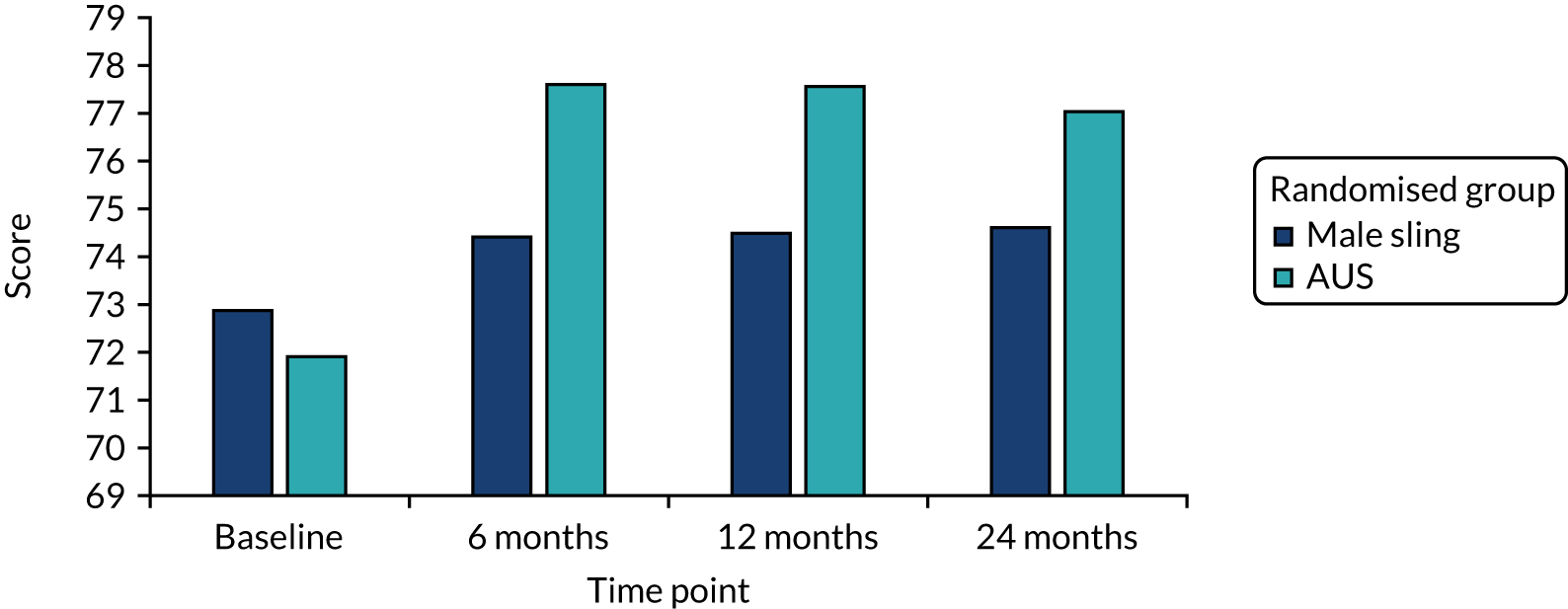
FIGURE 17.
The SF-6D utility scores.
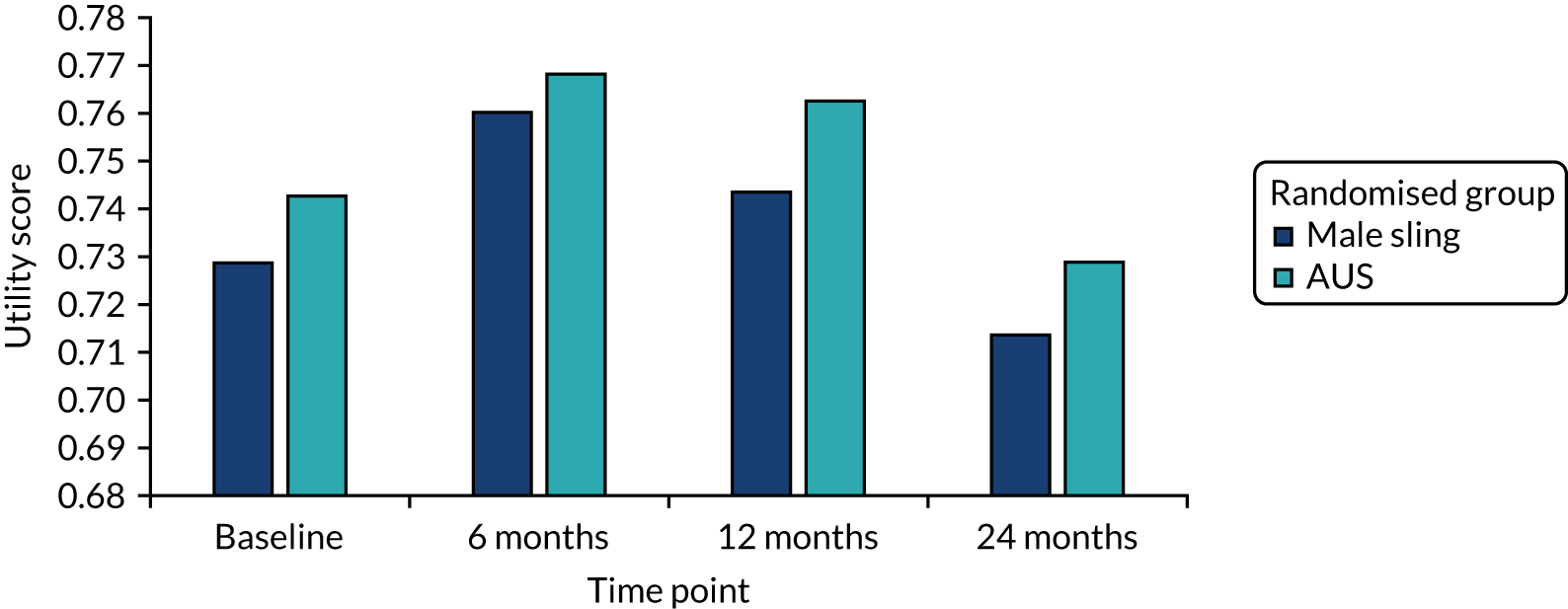
FIGURE 18.
Scatterplot of incremental costs and QALYs for male sling vs. AUS: complete-case data.
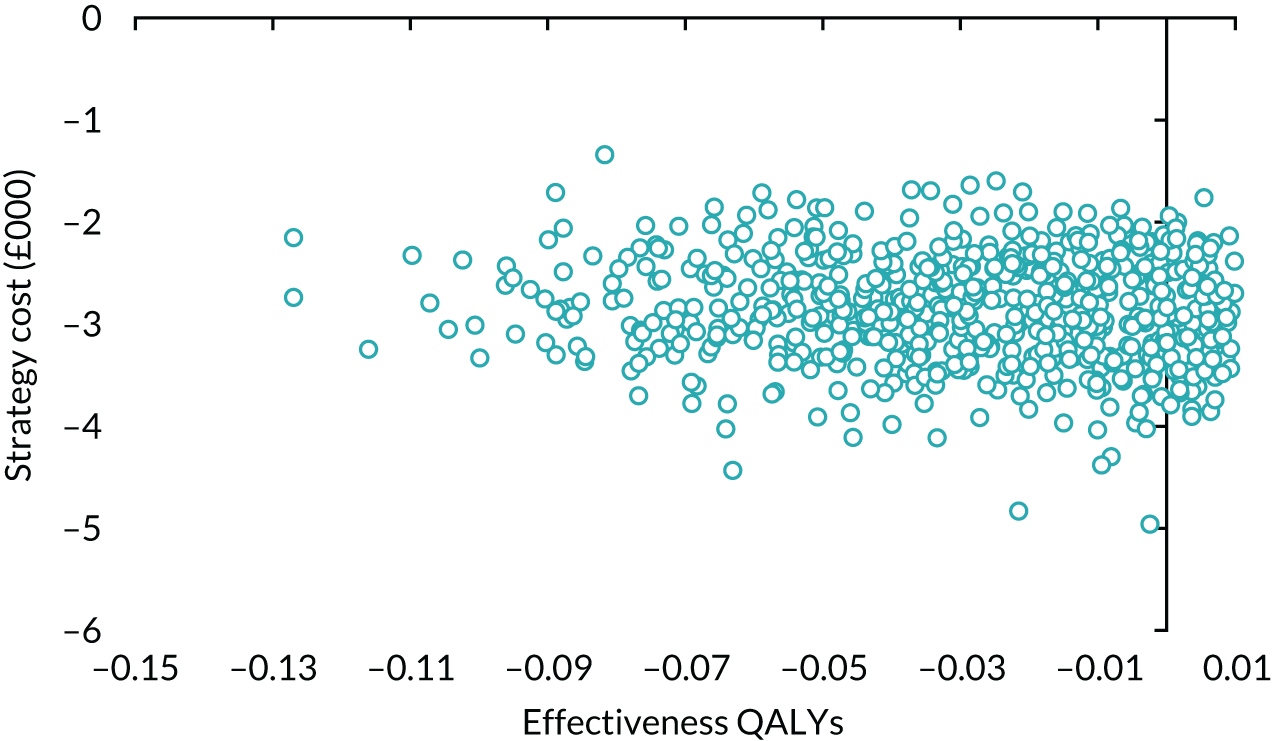
FIGURE 19.
Cost-effectiveness acceptability curve: complete-case data.
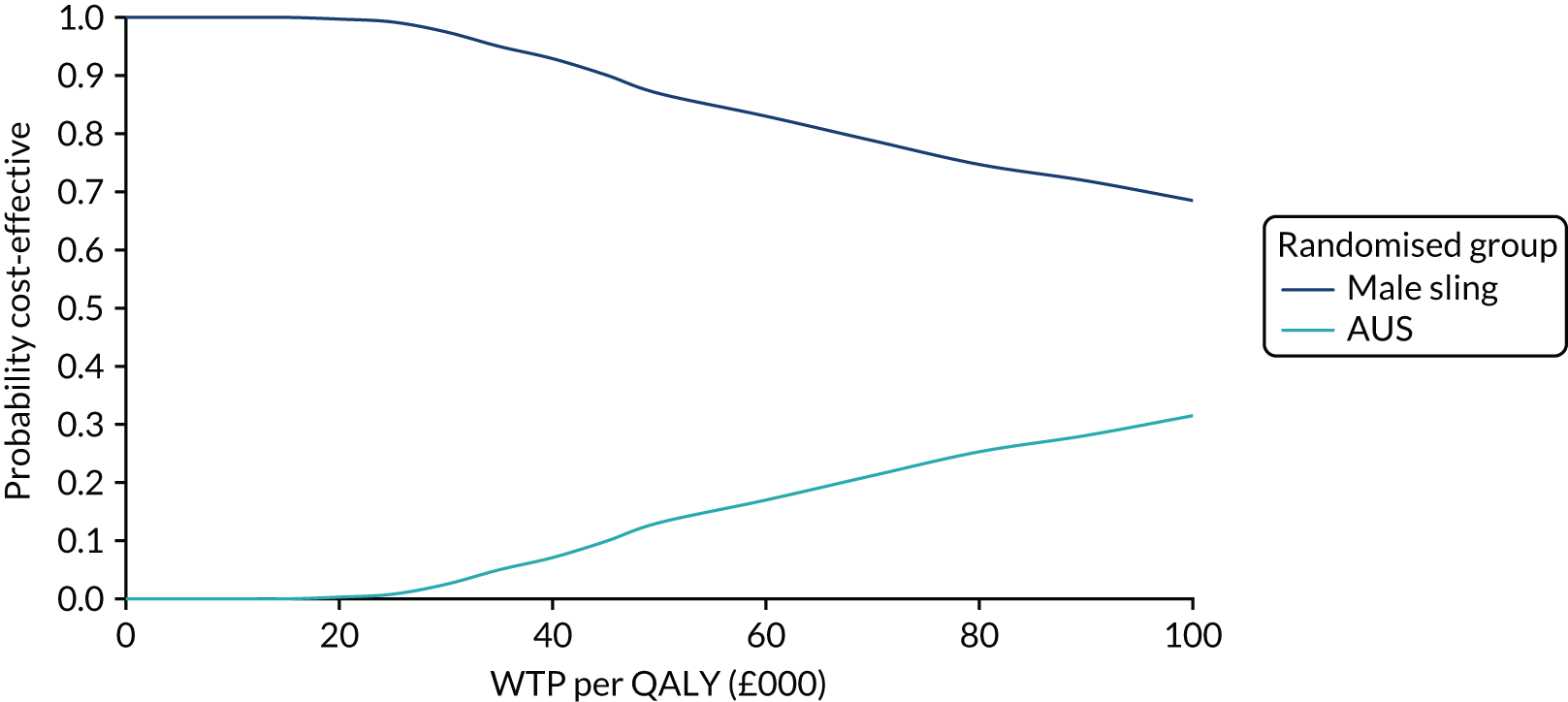
FIGURE 20.
Scatterplot of incremental costs and QALYs for male sling vs. AUS: SF-6D quality-of-life QALY scores.
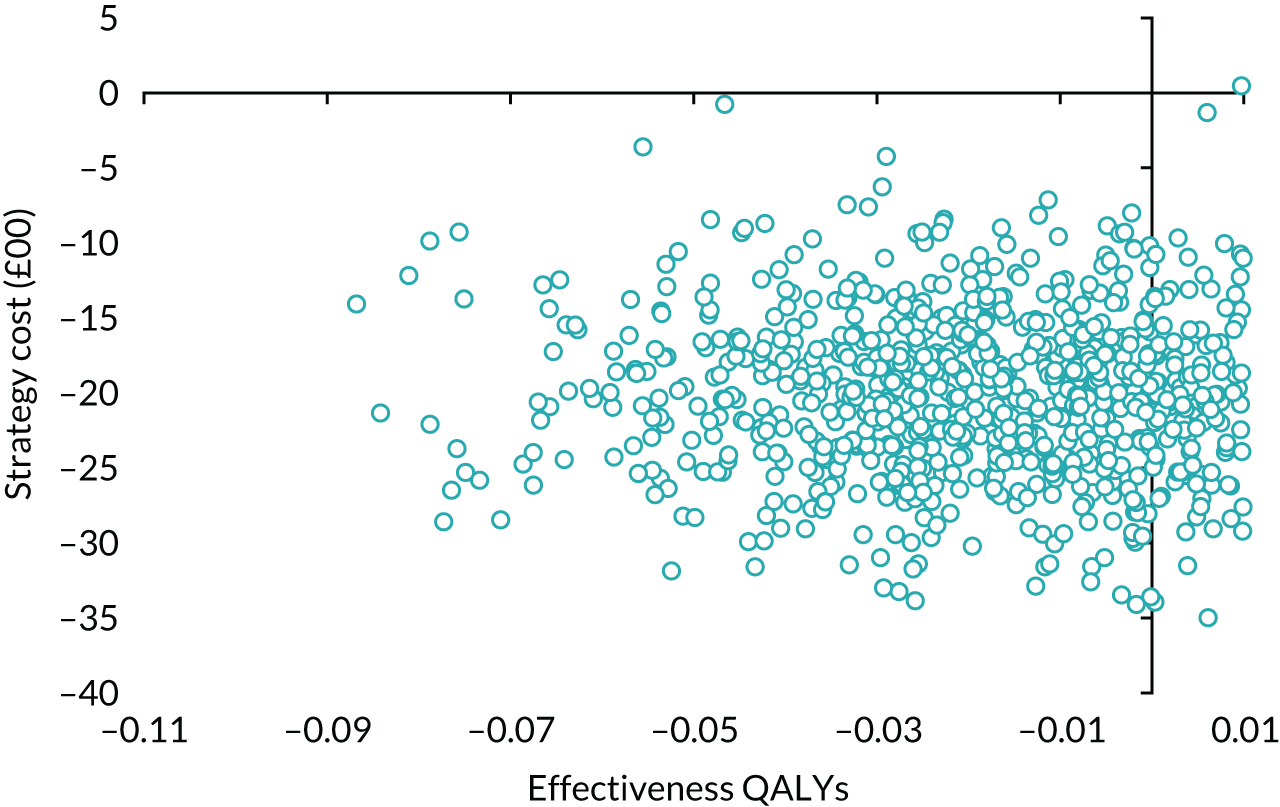
FIGURE 21.
Cost-effectiveness acceptability curve: SF-6D quality-of-life QALY scores.
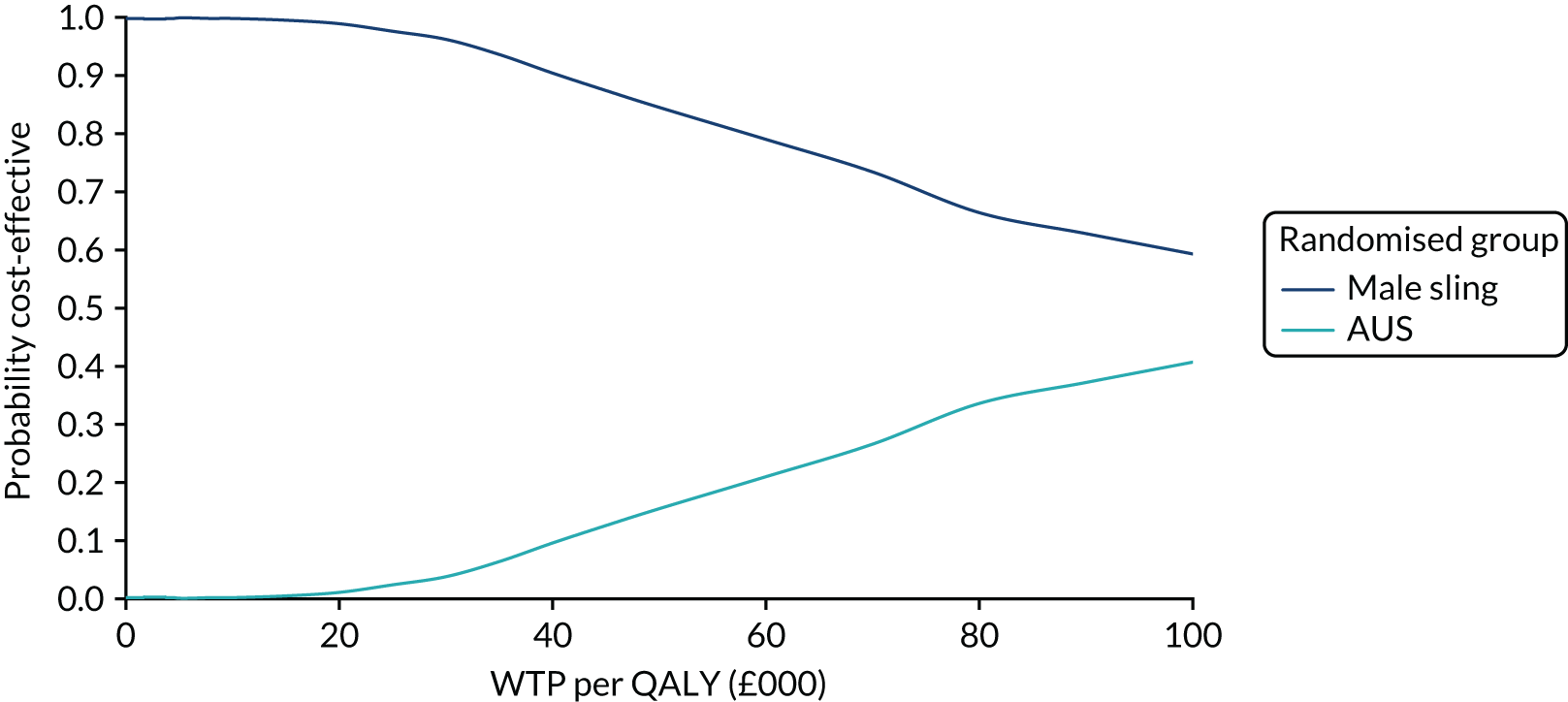
FIGURE 22.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: HRG costs.
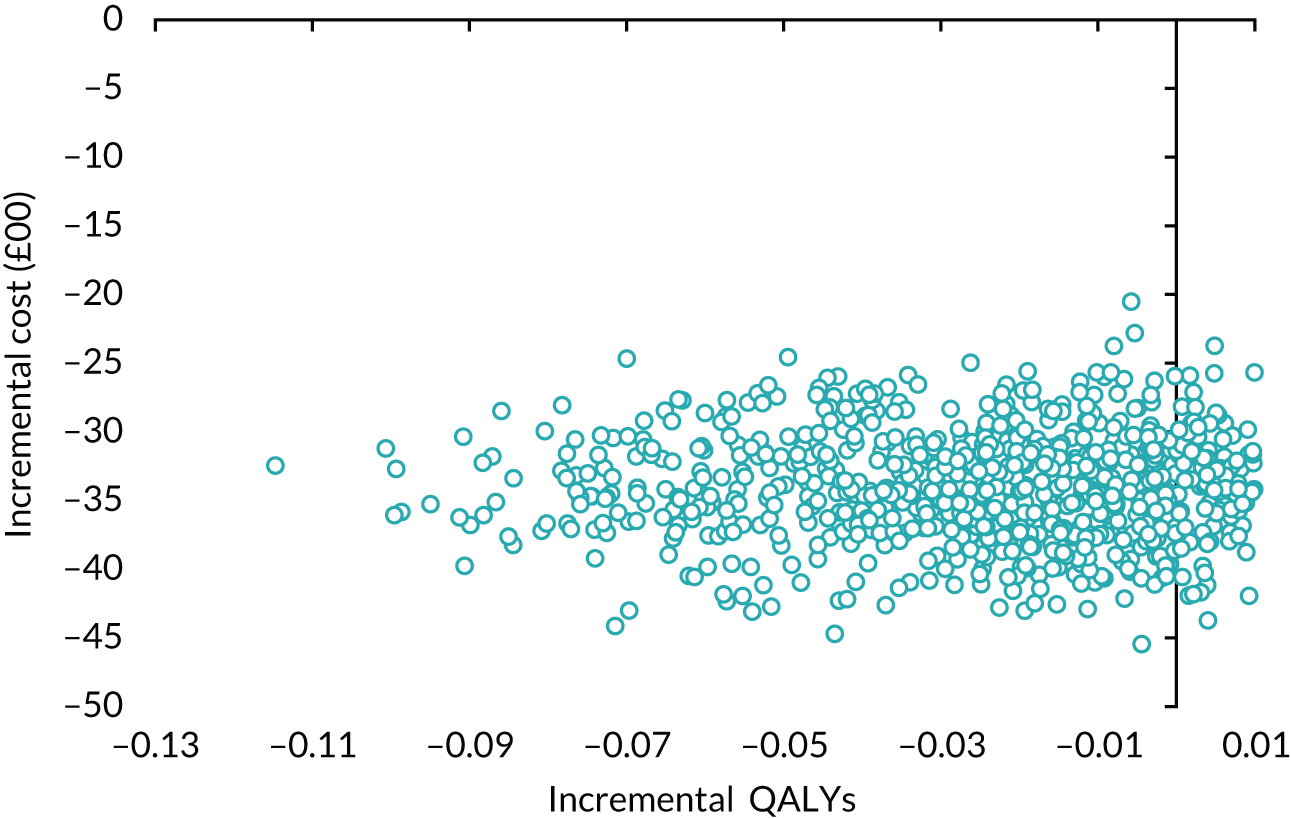
FIGURE 23.
Cost-effectiveness acceptability curve: HRG costs.
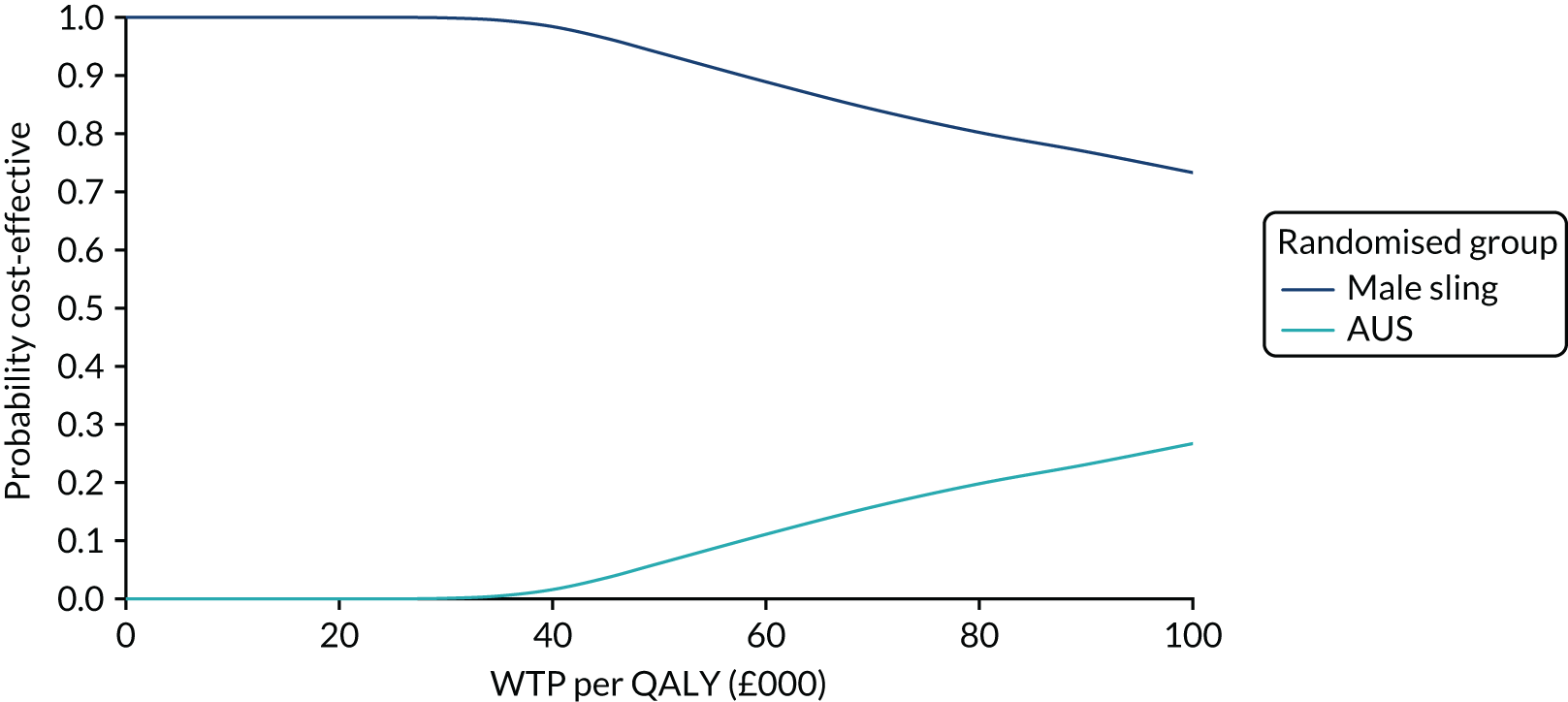
FIGURE 24.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: societal costs.
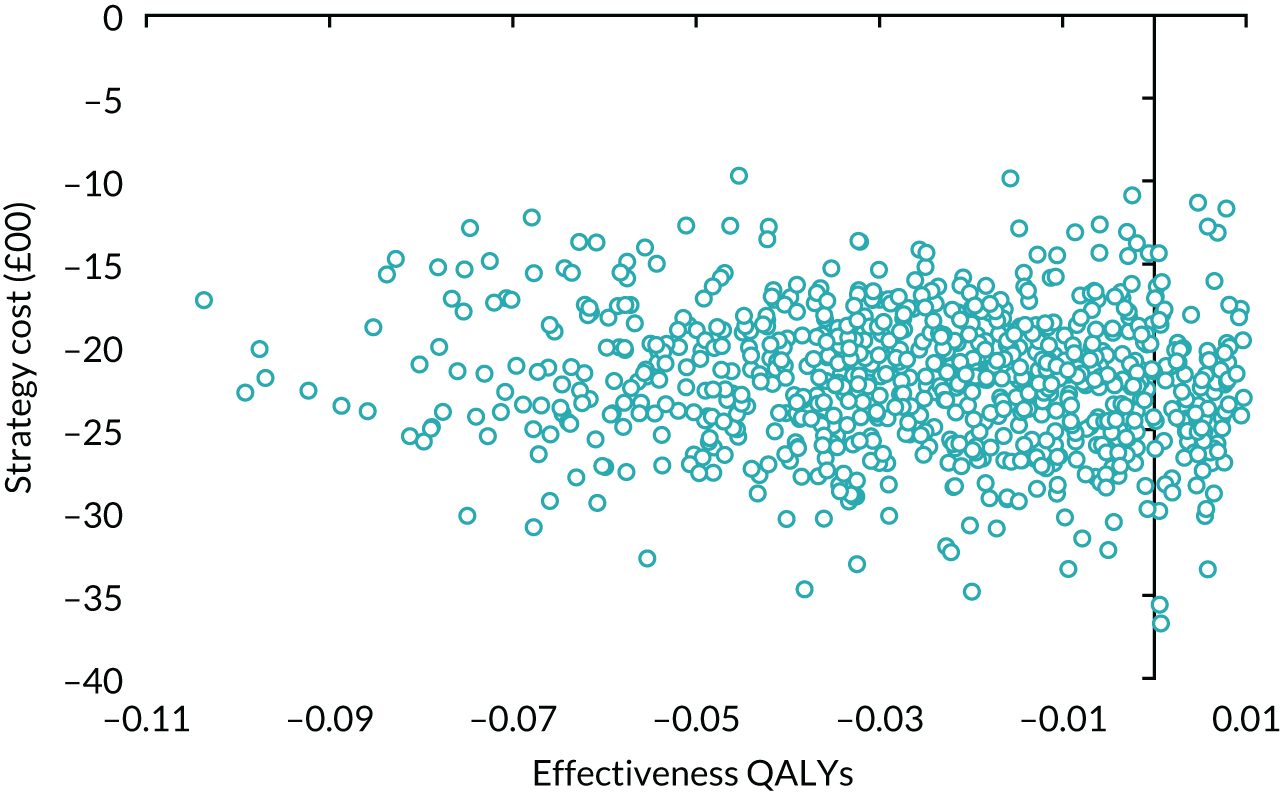
FIGURE 25.
Cost-effectiveness acceptability curve: societal costs.
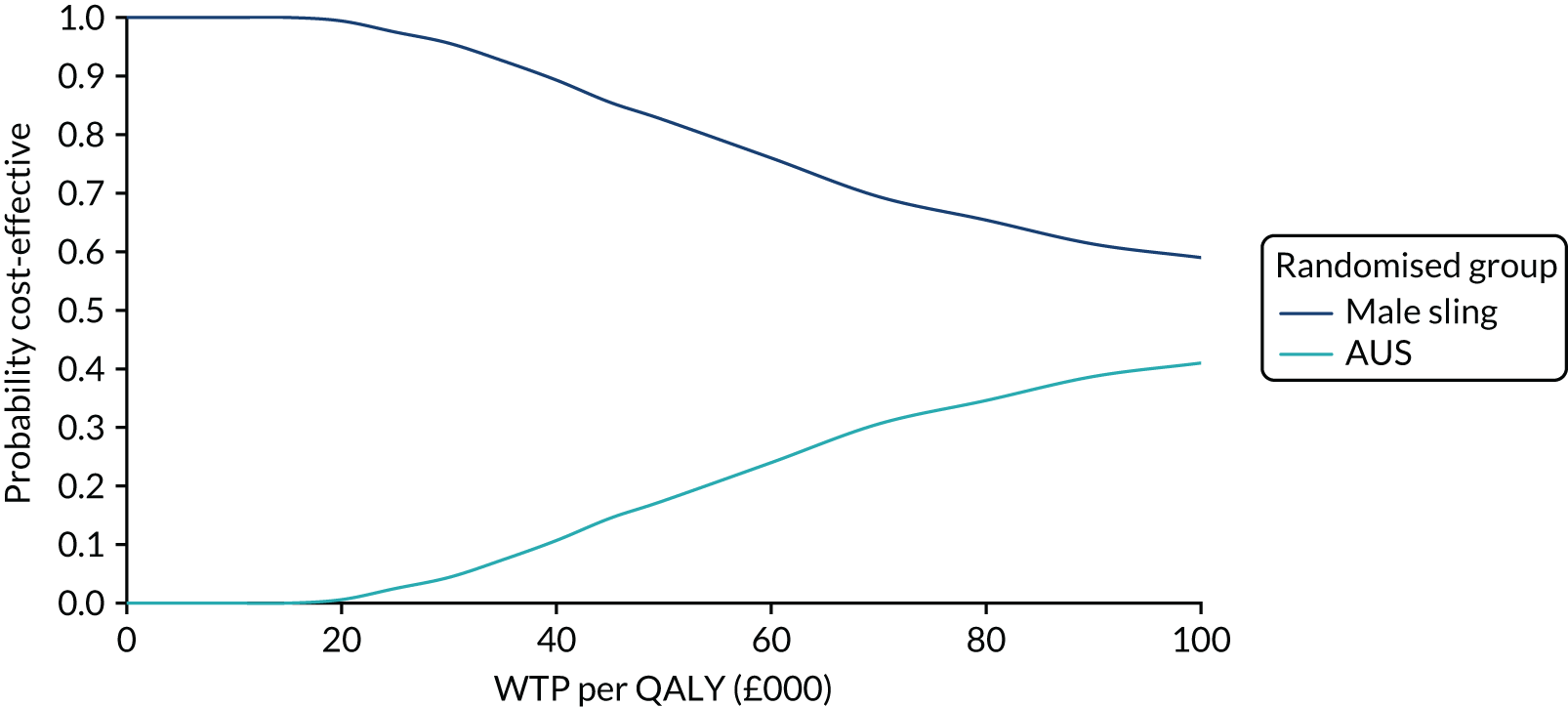
FIGURE 26.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: lowest AUS costs.
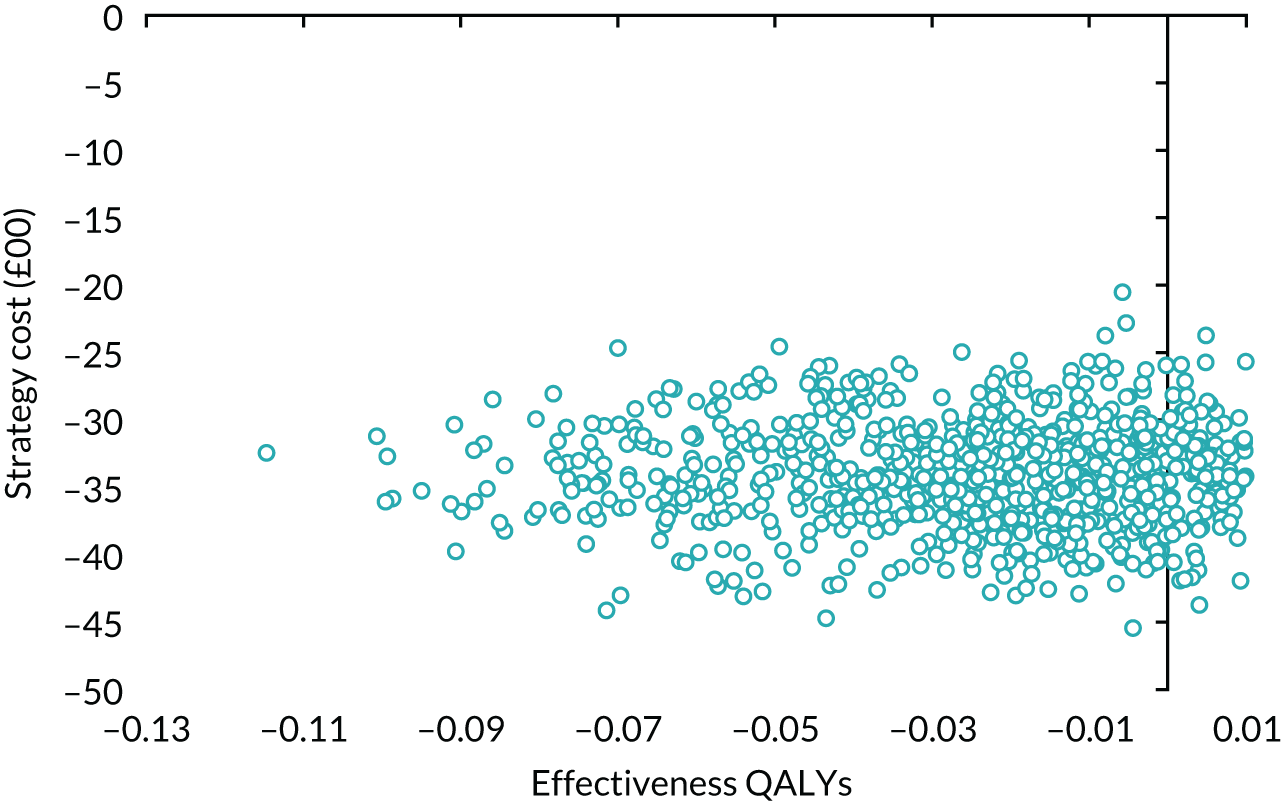
FIGURE 27.
Cost-effectiveness acceptability curve: lowest AUS costs.
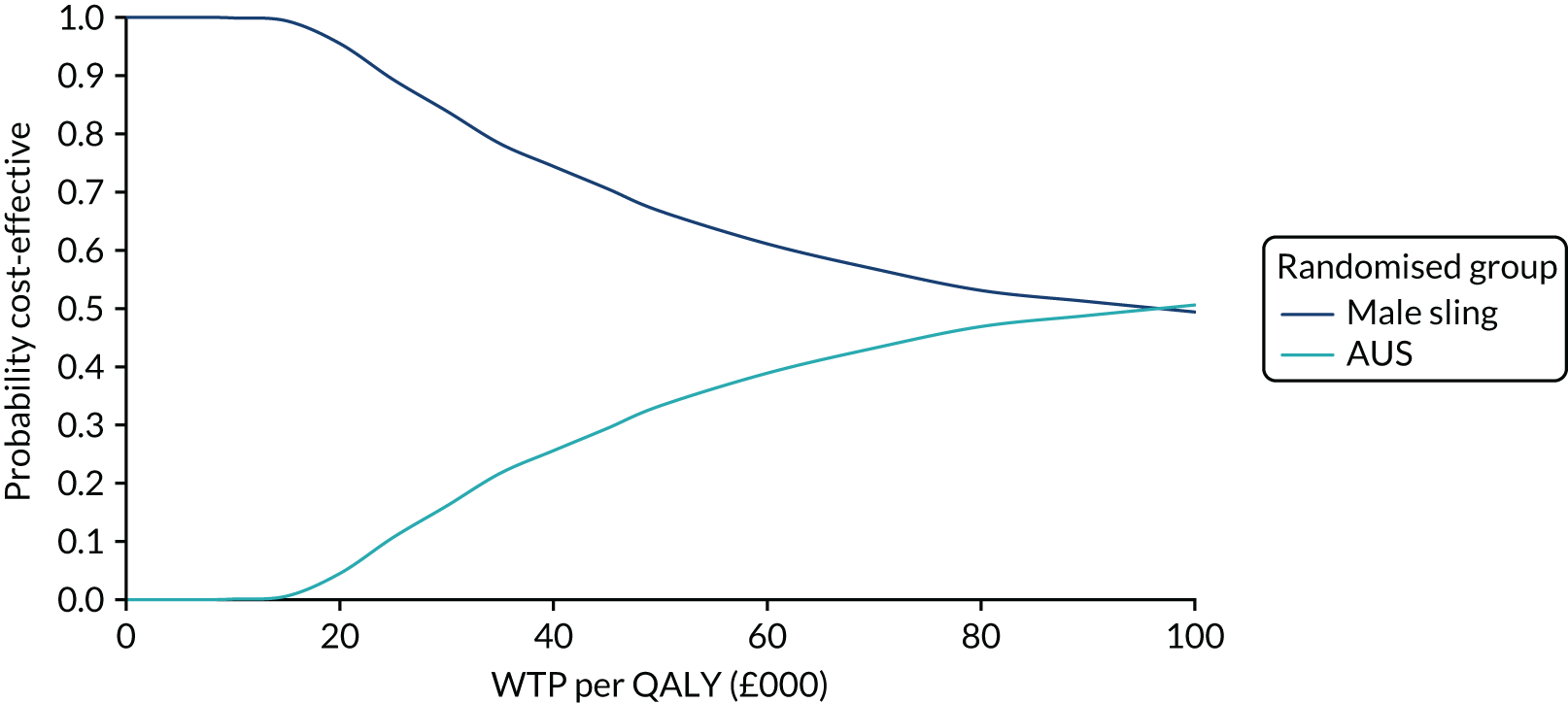
FIGURE 28.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: undiscounted second-year data.
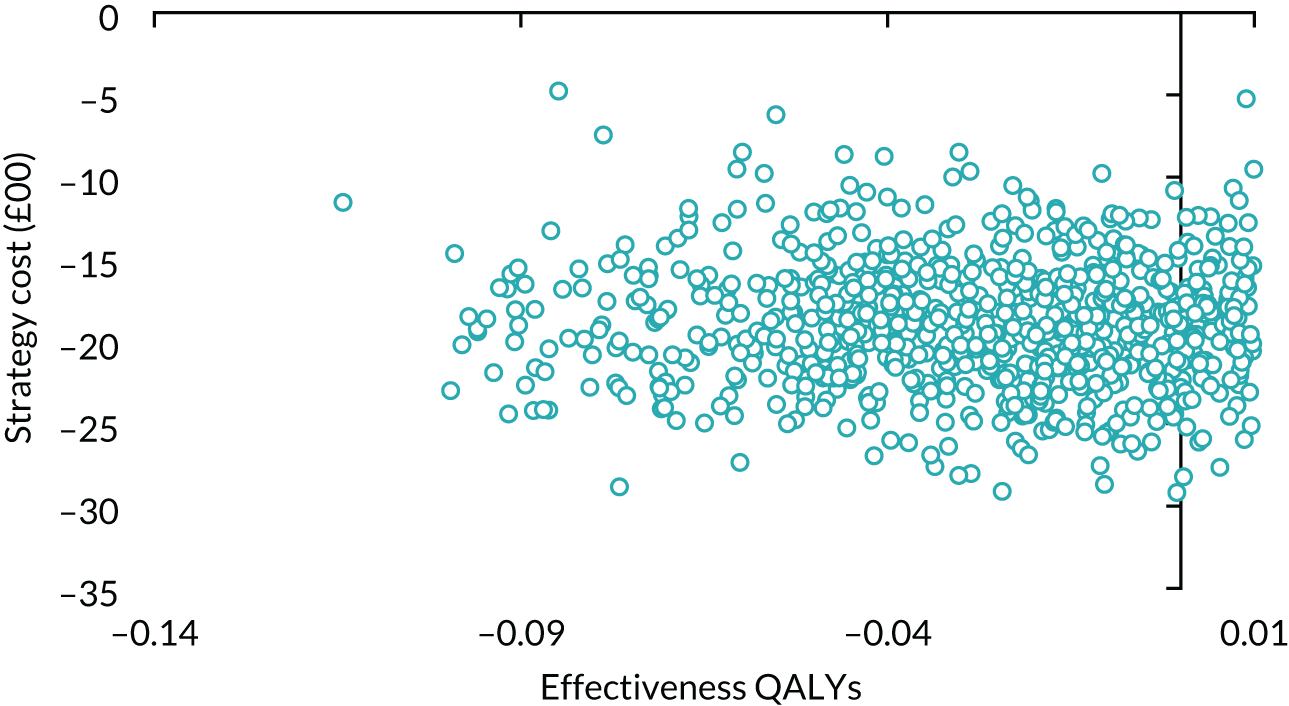
FIGURE 29.
Cost-effectiveness acceptability curve: undiscounted second-year data.
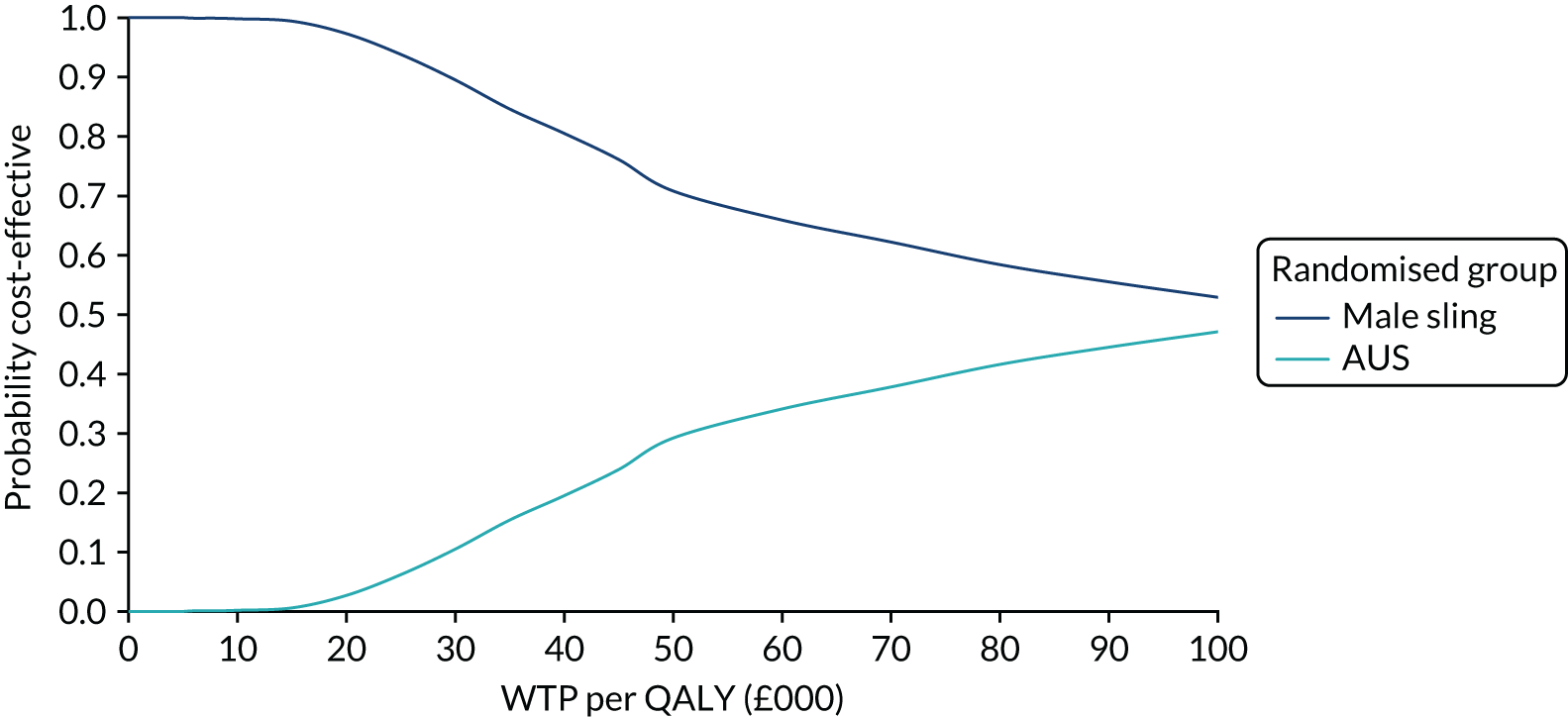
FIGURE 30.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: 6% discount.
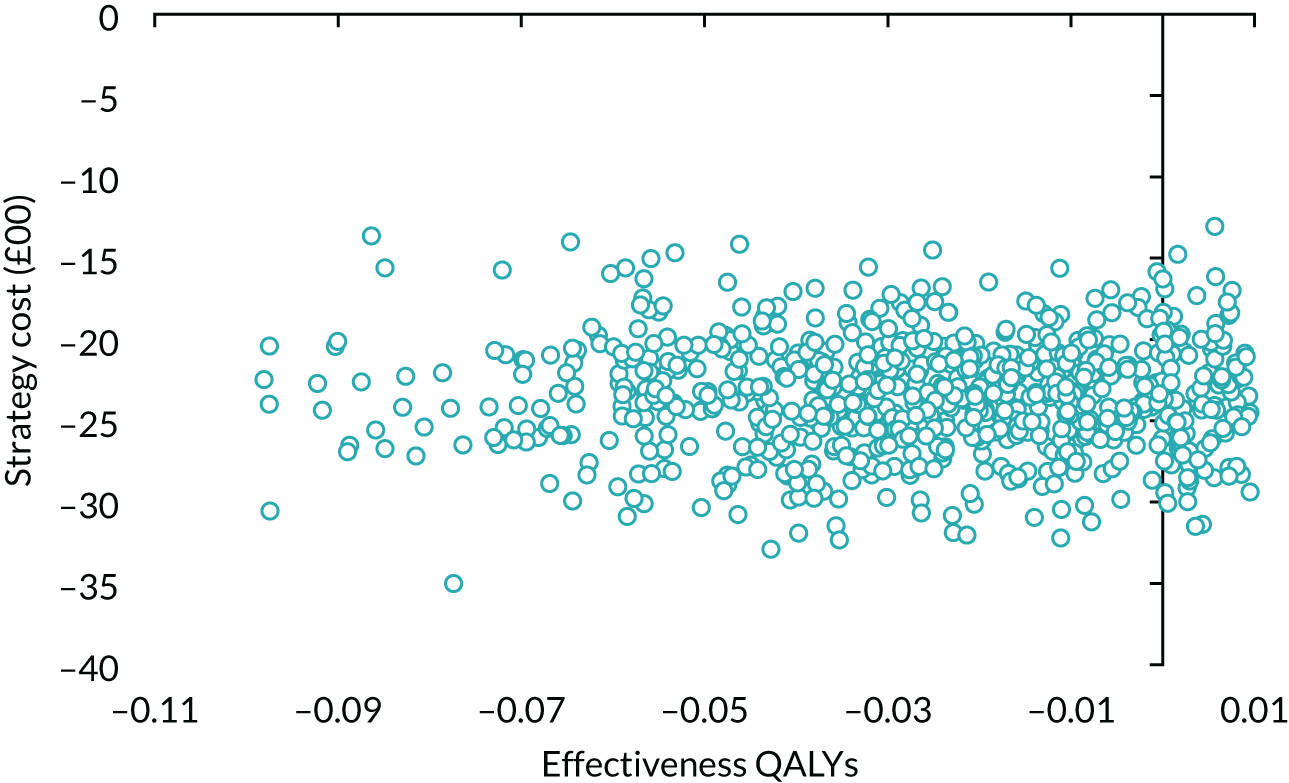
FIGURE 31.
Cost-effectiveness acceptability curve: 6% discount.
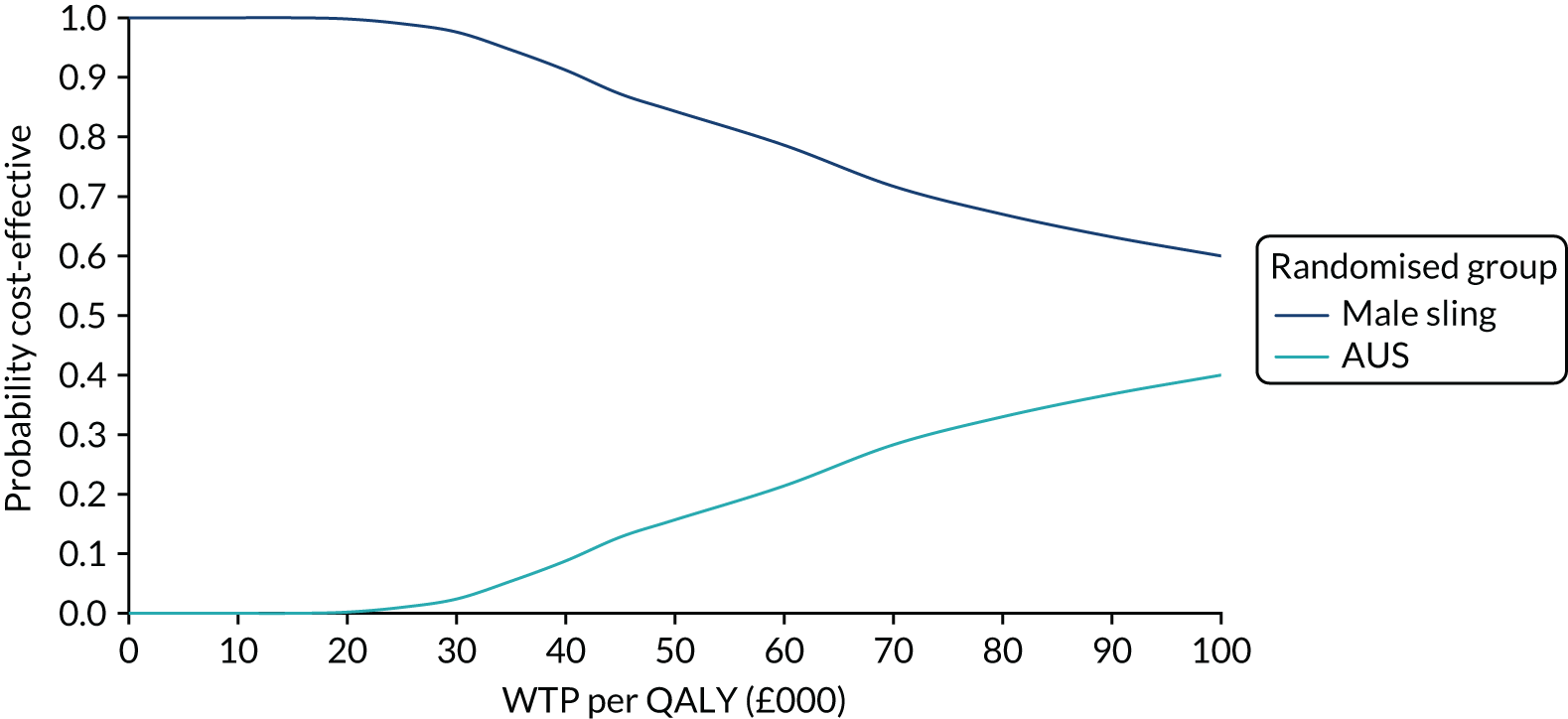
FIGURE 32.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: subgroup analysis had TURP.
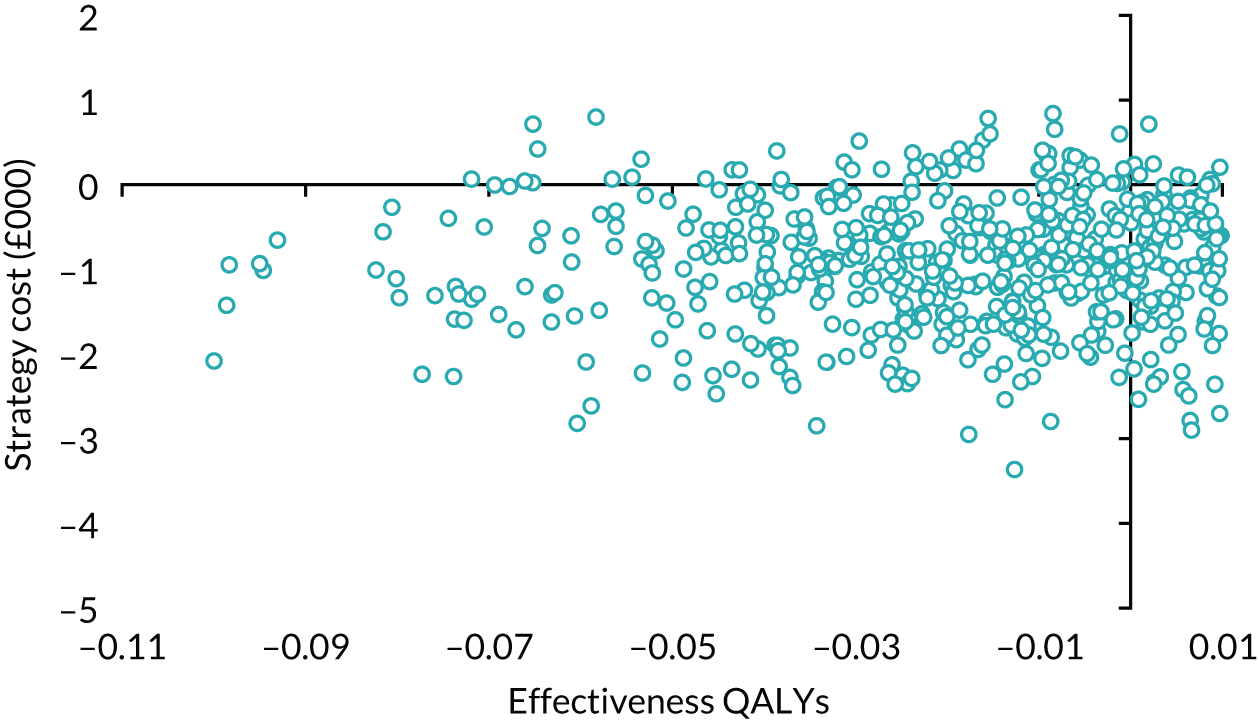
FIGURE 33.
Cost-effectiveness acceptability curve: subgroup analysis had TURP.
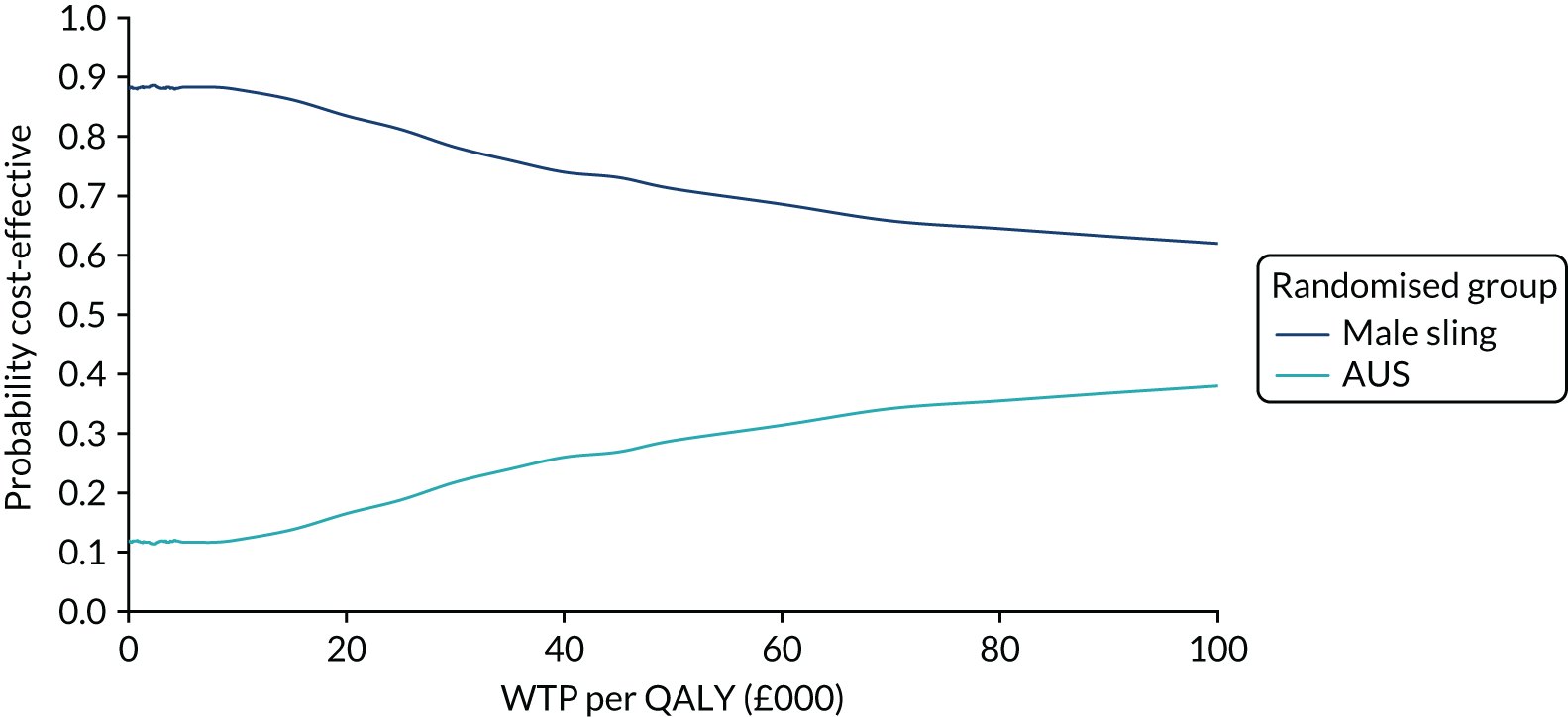
FIGURE 34.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: subgroup analysis had pad weight of < 250 g.
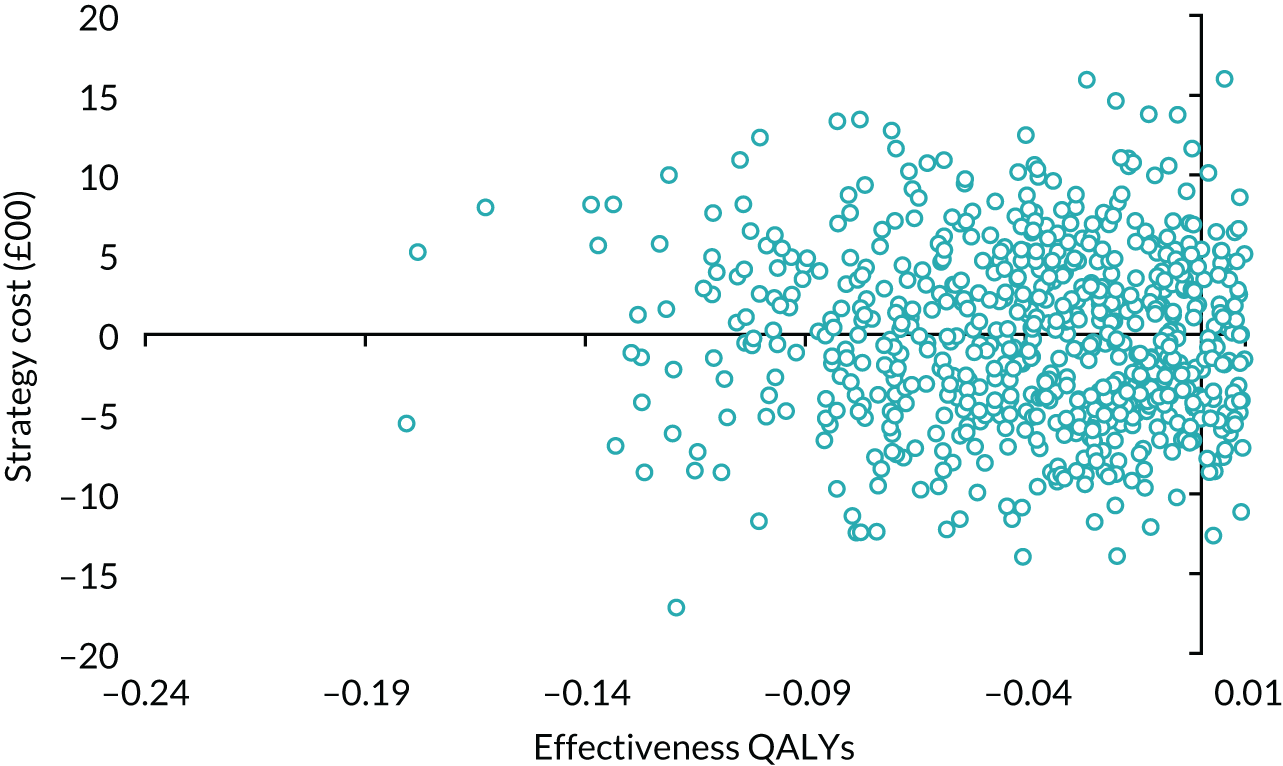
FIGURE 35.
Cost-effectiveness acceptability curve: subgroup analysis had pad weight of < 250 g.
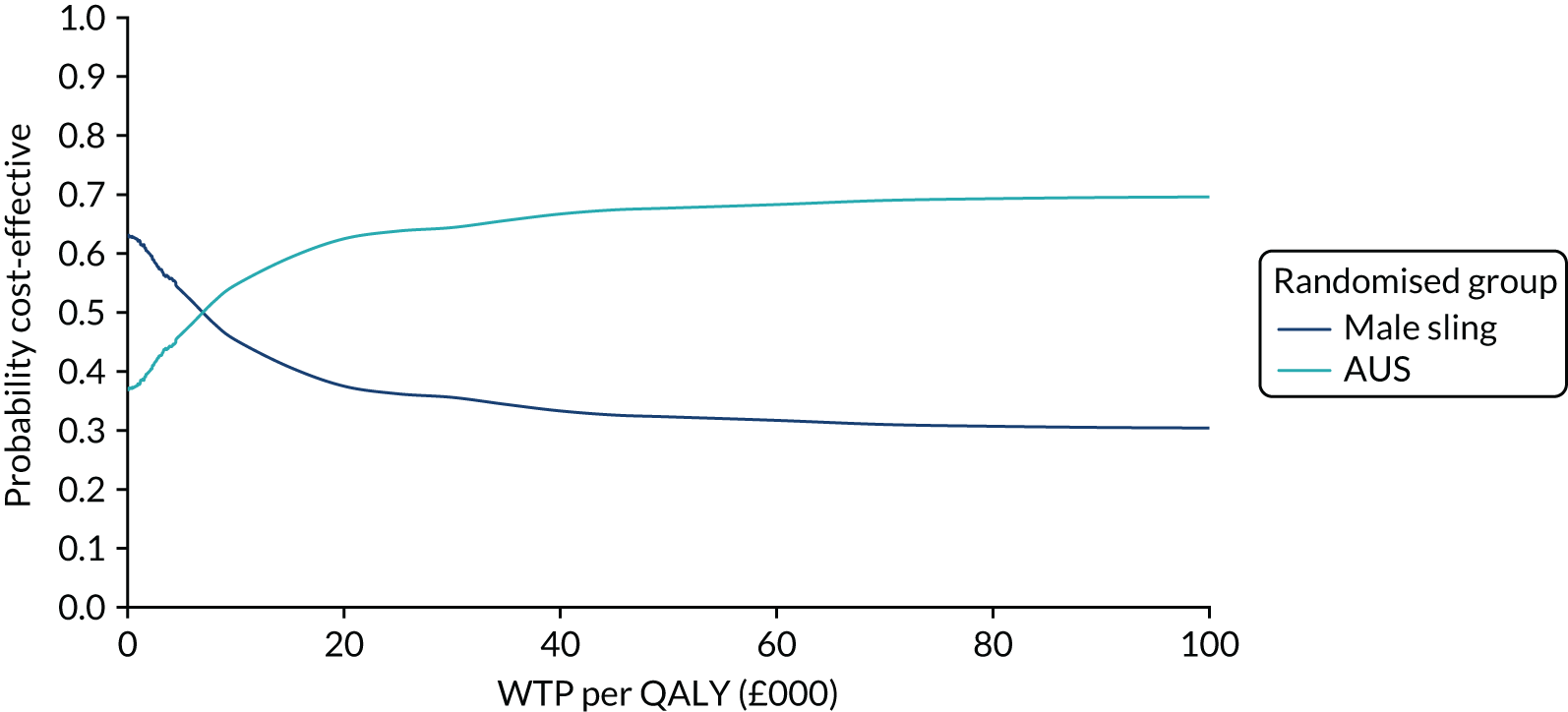
FIGURE 36.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: subgroup analysis had pad weight of ≥ 250 g.
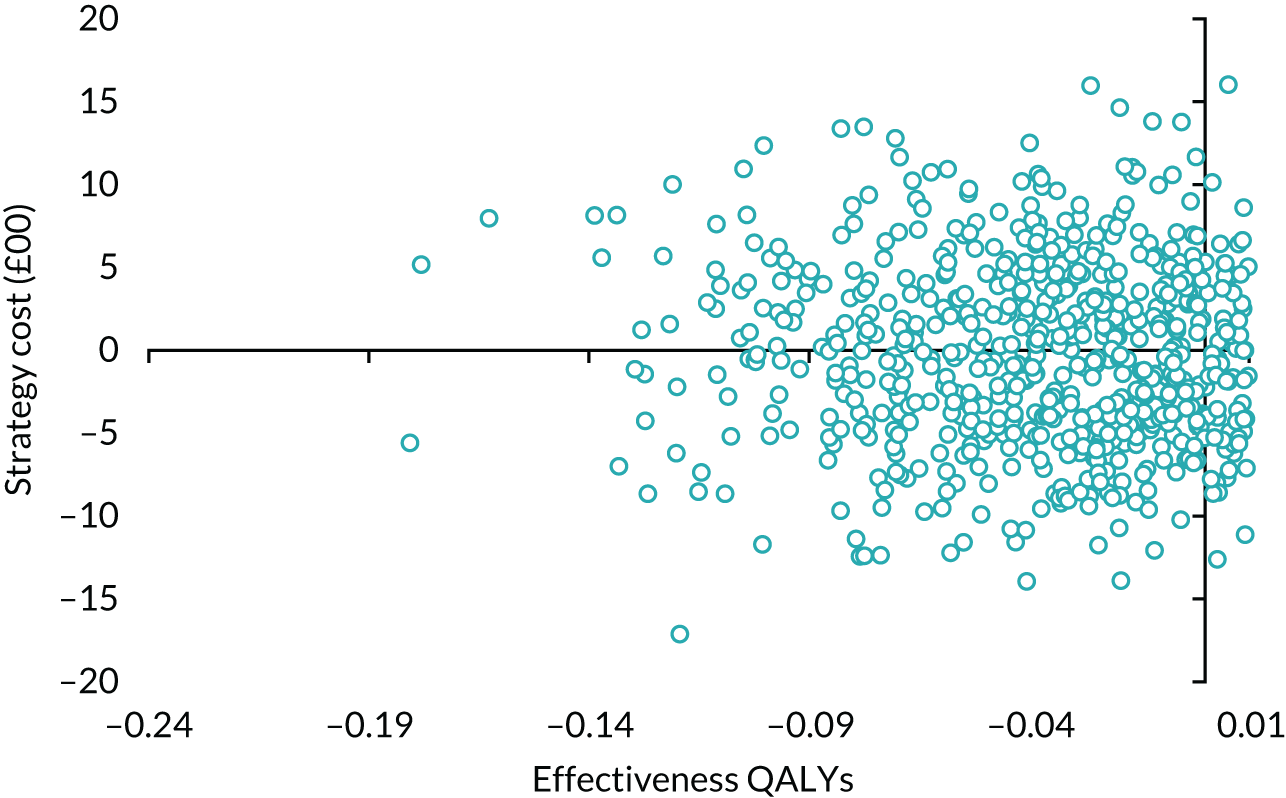
FIGURE 37.
Cost-effectiveness acceptability curve: subgroup analysis had pad weight of ≥ 250 g.
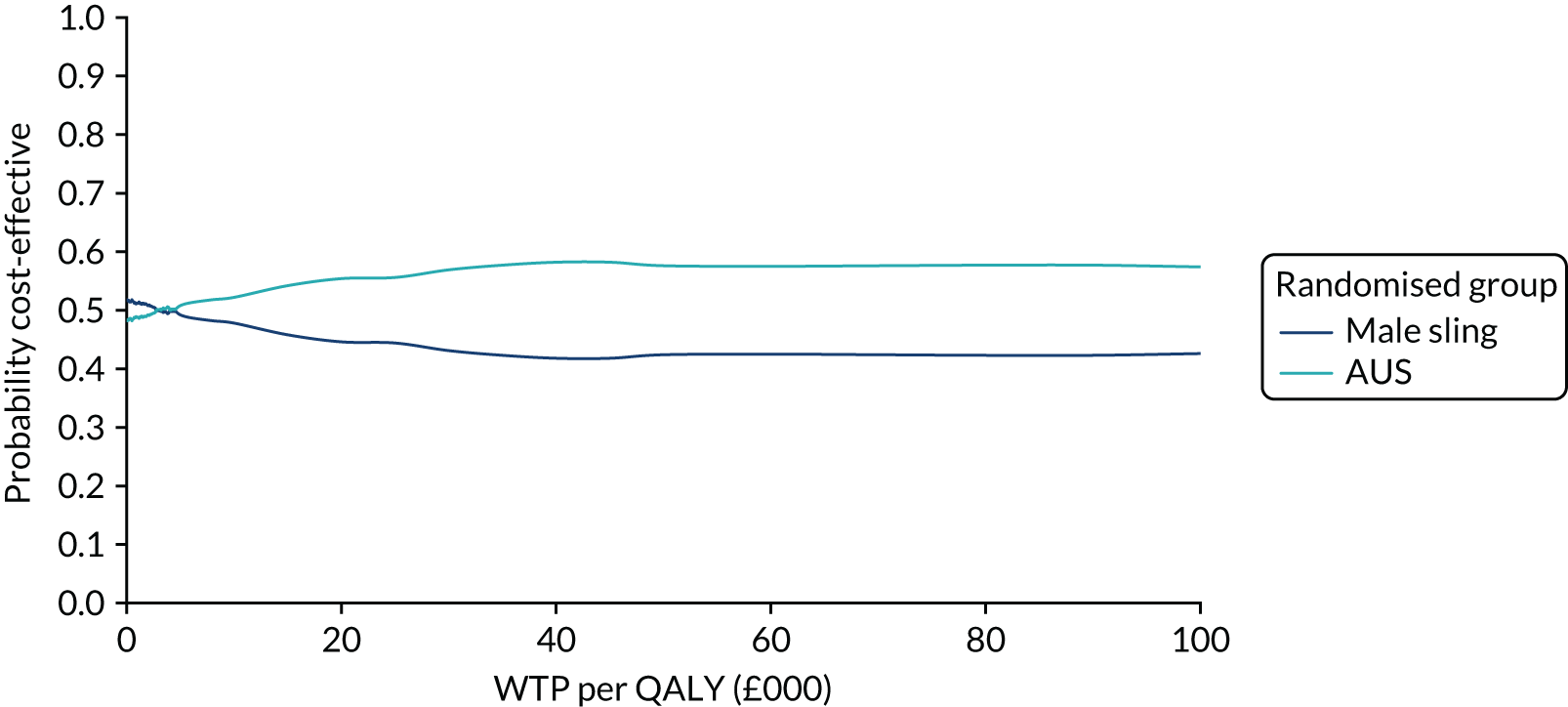
Model-based analysis
FIGURE 38.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: 10-year follow-up.
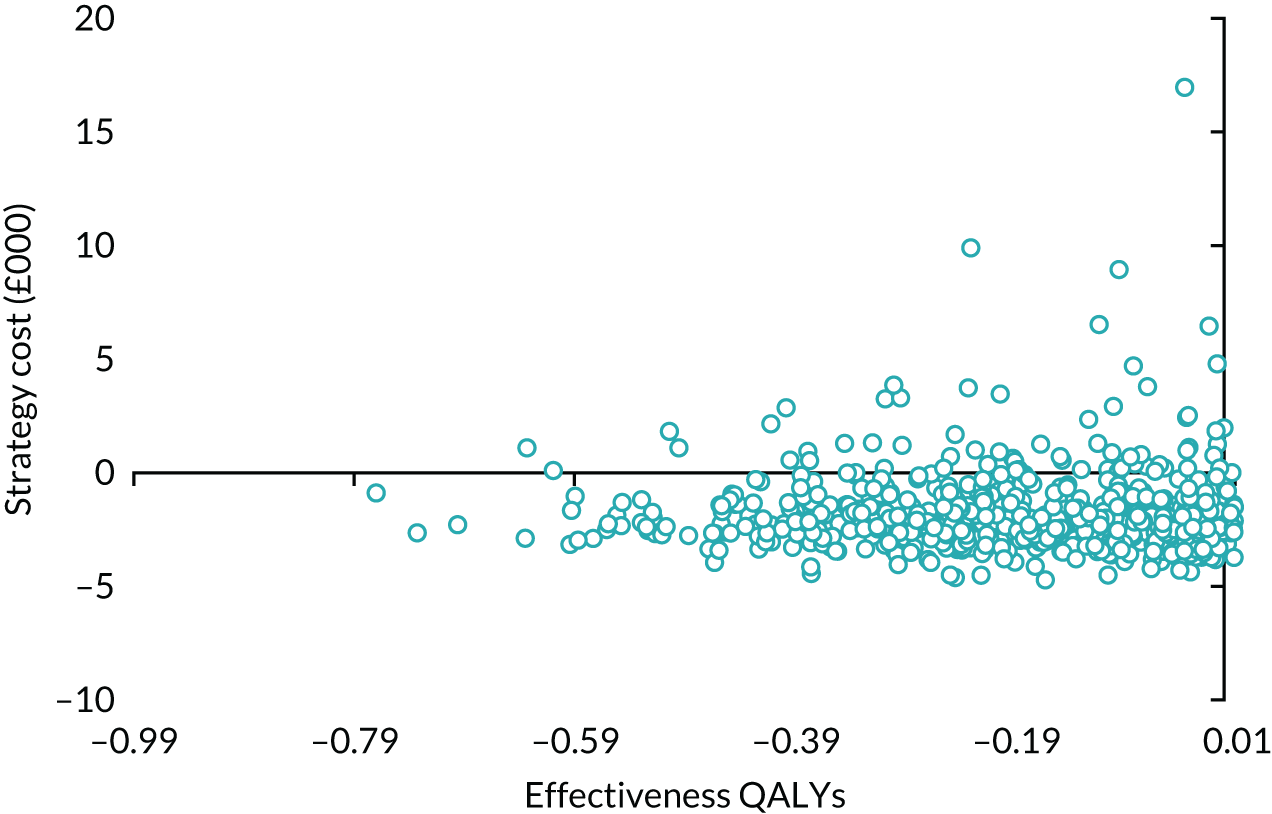
FIGURE 39.
Cost-effectiveness acceptability curve: 10-year follow-up.
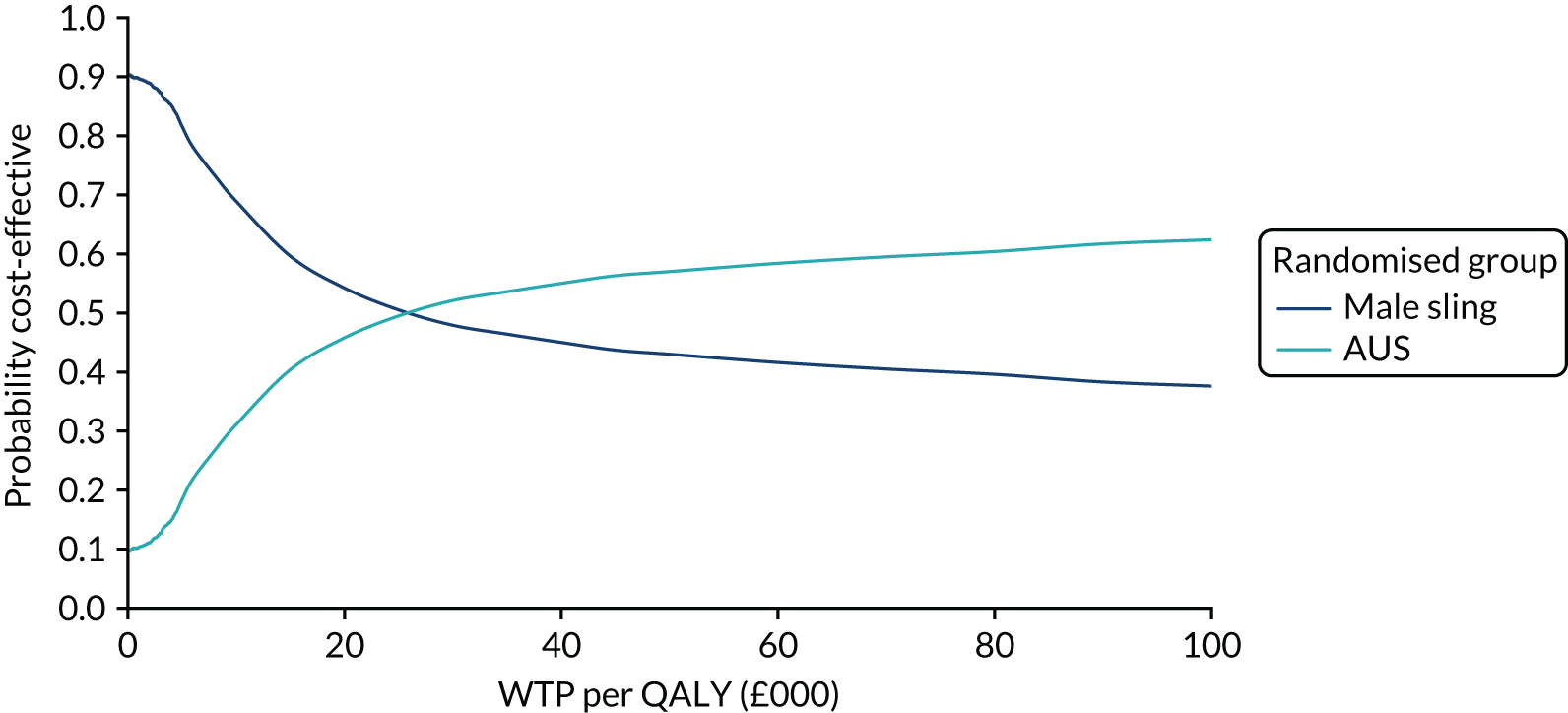
FIGURE 40.
Scatterplot of incremental costs and incremental QALYs for male sling vs. AUS: 5-year follow-up.
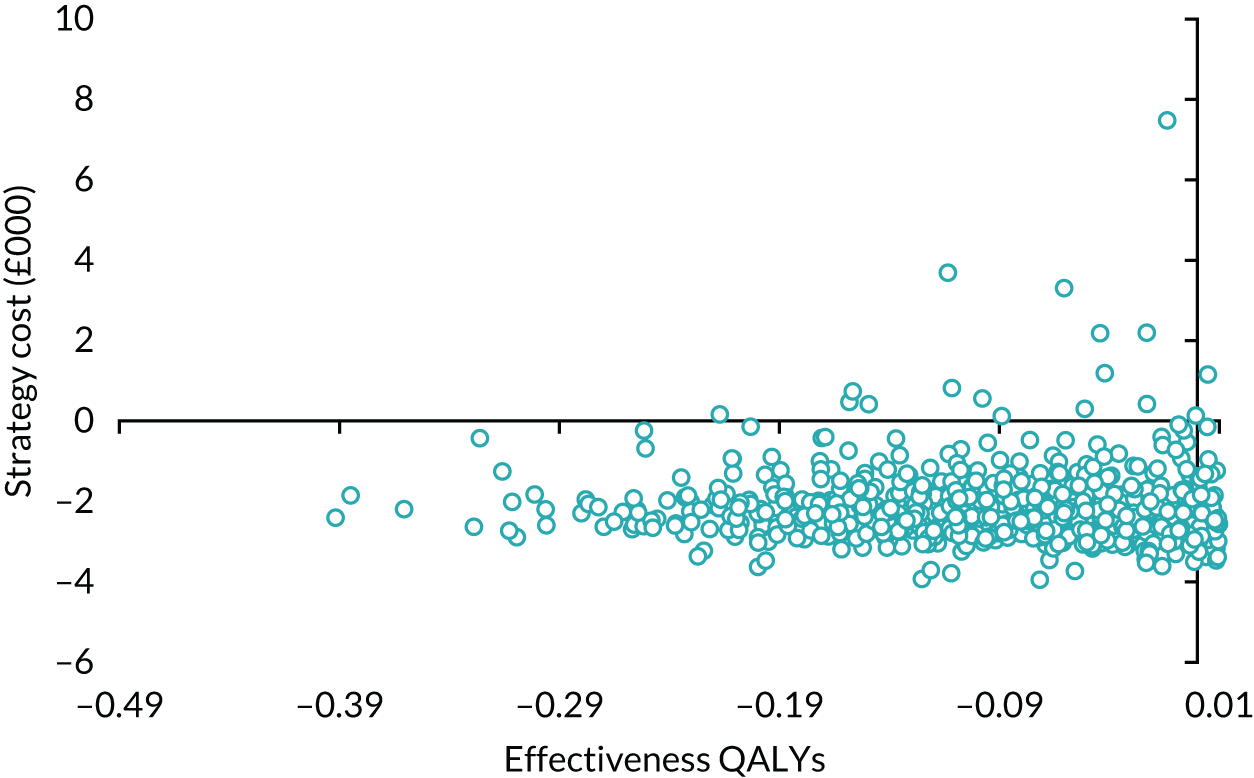
FIGURE 41.
Cost-effectiveness acceptability curves: 5-year follow-up.
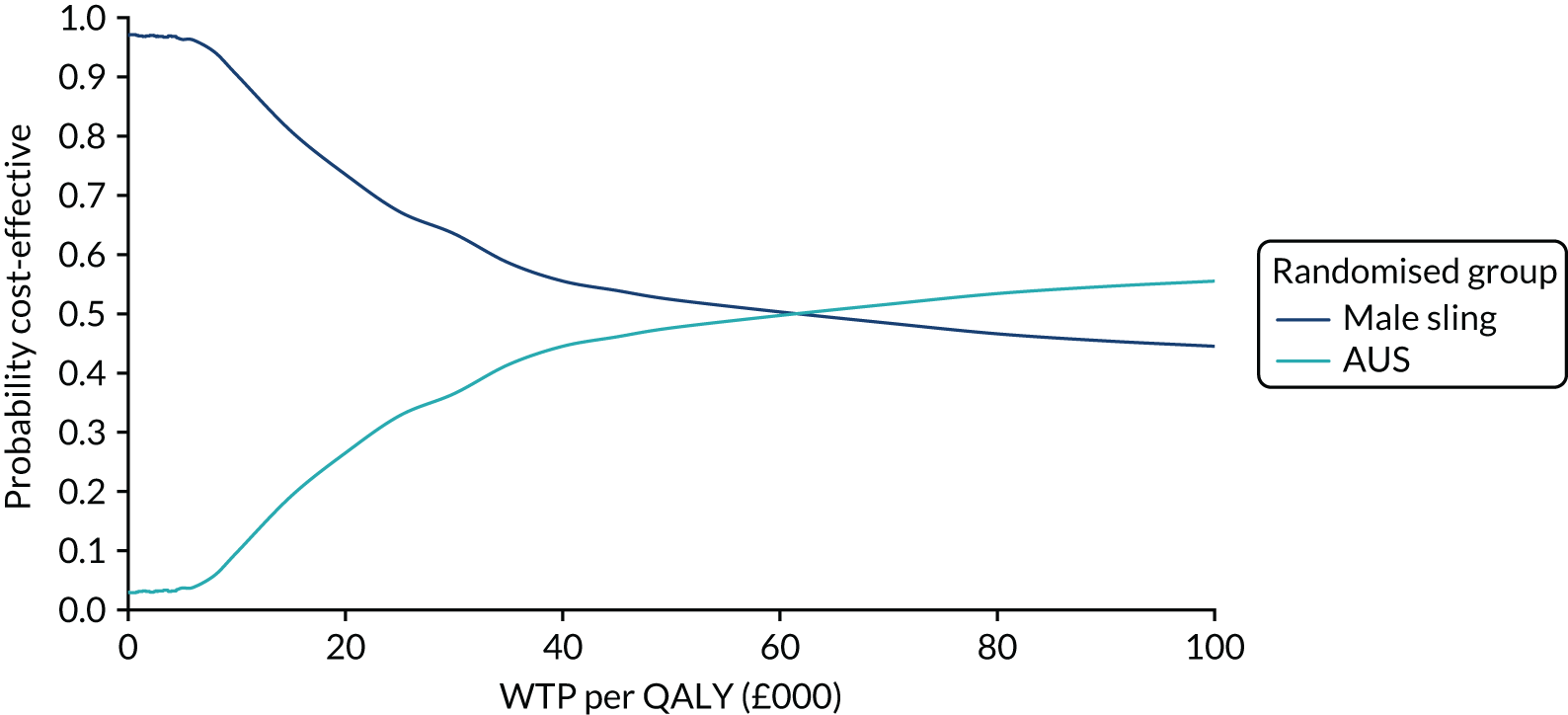
List of abbreviations
- AE
- adverse event
- AUS
- artificial urinary sphincter
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-VAS
- EuroQol-Visual Analogue Scale
- GLM
- generalised linear model
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ICIQ-LUTSqol
- International Consultation on Incontinence Questionnaire Lower Urinary Tract Symptoms Quality of Life
- ICIQ-MLUTS
- International Consultation on Incontinence Questionnaire-Urinary Incontinence – Male Lower Urinary Tract Symptoms
- ICIQ-MLUTSsex
- International Consultation on Incontinence Questionnaire Male Sexual Matters Associated with Lower Urinary Tract Symptoms
- ICIQ-UI SF
- International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form
- ITT
- intention to treat
- LUTS
- lower urinary tract symptom
- MAPS
- Men After Prostate Surgery
- MASTER
- Male synthetic sling versus Artificial urinary Sphincter Trial: Evaluation by Randomised controlled trial
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NRC
- non-randomised cohort
- OPCS
- Office of Population Censuses and Surveys
- OR
- odds ratio
- PGI-I
- Patient Global Impression of Improvement
- PPI
- post prostatectomy incontinence
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SUI
- stress urinary incontinence
- TSC
- Trial Steering Committee
- TURP
- transurethral resection of prostate
- UI
- urinary incontinence
- USI
- urodynamic stress incontinence
