Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/104/01. The contractual start date was in April 2017. The draft report began editorial review in December 2020 and was accepted for publication in May 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
Copyright © 2022 Blackwood et al. This work was produced by Blackwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Blackwood et al.
Chapter 1 Introduction
Epidemiology of mechanical ventilation
Internationally, the percentage of children who require mechanical ventilation in intensive care units (ICUs) varies between 30% and 64%, depending on country and resources. 1 In the UK and Ireland, 20,000 children are admitted to paediatric ICUs (PICUs) in the NHS each year and 60% receive invasive mechanical ventilation (IMV). The main reasons for ICU admission are cardiovascular (28%), respiratory (28%) and neurological (11%) conditions. 2 In general, 25% of children are discharged from an ICU within 24 hours, 33% remain in the ICU for 1 up to 3 days, 23% from 3 up to 7 days and 19% for > 7 days. 3 Although IMV improves survival, it can lead to complications; therefore, weaning should be carried out as soon as the patient is able to maintain spontaneous breathing.
Mechanisms for weaning
The process of weaning from mechanical ventilation involves gradually transferring the work of breathing from the ventilator to the patient, with the aim of liberating the patient from mechanical ventilation. Strategies to optimise this weaning process need to find a balance between withdrawing ventilator support too early and unnecessarily prolonging ventilation. Premature withdrawal runs the risk of reintubation, which is associated with prolonged hospital stay, increased costs and increased mortality. 4 By contrast, delayed weaning is associated with increased adverse effects, such as ventilator-associated pneumonia, upper airway damage, respiratory muscle weakness, iatrogenic sedation and opiate withdrawal, and increased mortality. 5,6 The requirement for ongoing analgesic and sedative drugs during mechanical ventilation may further contribute to delirium, immobility and generalised muscle weakness. 7 Several studies suggest that most patients weaned successfully could have tolerated the weaning attempts earlier and that such data emphasise the need for the early use of screening tests. 8 There is strong evidence that mechanically ventilated patients should have their ‘readiness to wean’ assessed at least daily, and weaning should be initiated based on objective clinical criteria, rather than the clinician’s subjective impression. 9 Weaning generally involves either allowing a period of spontaneous breathing (on the ventilator) or a gradual reduction in the amount of ventilator support. The spontaneous breathing trial (SBT) was developed to identify patients who are ready to discontinue ventilation. 9 The test aims to monitor signs of respiratory muscle fatigue while the patient is still intubated. Adult studies have shown that most patients do not need gradual weaning; when assessed with a daily evaluation and SBT, approximately 75% of patients were ready to be extubated. 10 Some paediatric studies have shown similar results. 11,12 However, although the introduction of weaning protocols has resulted in decreased ventilation times in adult patients,13 there is limited evidence that similar protocols can benefit the paediatric population. 14
Weaning from ventilation is a complex process that involves recognition of readiness, reducing ventilator support and extubation. Early recognition of a patient’s readiness and starting the process with minimal delay would make a valuable contribution to reducing ventilation duration and its associated risks. Such a process could be enhanced by engaging the wider clinical team in a co-ordinated process. Our pre-trial data on paediatric usual practice showed wide variations in both sedation and ventilator weaning practices, with minimal involvement of junior medical, nursing and physiotherapy staff in the process. 15 Thus, there is opportunity to broaden participation.
Interprofessional co-ordination in weaning
There is strong evidence that co-ordinated team-based care improves quality and saves money in health care, depending on the approach used, how well it is implemented and on the particular environment. 16 Within an ICU, the dynamic, complex and time-pressured environment necessitates a team approach to care delivery that requires effective communication and collaboration. 17 Various studies in adult ICUs have reported inverse associations between rates of high interprofessional collaboration and patient mortality,18,19 as well as between improved clinician-to-clinician communication and reductions in ICU length of stay. 20 A team-based approach that maximises engagement of all relevant clinical staff in the early recognition of readiness and preparation for weaning ventilation could potentially reduce durations of IMV and PICU length of stays, and relieve pressures for beds. In the UK, 67% of nurses employed in PICUs are band 5 (relatively junior) nurses; their engagement, along with other allied health professionals, would greatly enhance the weaning process. 3 Qualitative research indicates that interprofessional collaboration and communication are major factors that influence weaning and the adoption of weaning protocols in PICUs and adult ICUs. 21
Existing knowledge
Protocolised weaning is widely used in adult ICUs to reduce the duration of mechanical ventilation. A systematic review13 of protocolised weaning trials (n = 17 trials, 2434 adults) reported a reduction in the duration of mechanical ventilation of 26% (n = 14 trials) [95% confidence interval (CI) 13% to 37%; p = 0.0002], in weaning duration of 70% (n = 8 trials) (95% CI 27% to 88%; p = 0.009) and in ICU length of stay of 11% (n = 9 trials) (95% CI 3% to 19%; p = 0.01). Although there was statistical heterogeneity in the treatment effects, such that some trials showed a larger effect than others did, the effect was consistent in as much as many trials showed a reduction in these outcomes. Specifically, there was no evidence of any increased risk of harm (mortality or reintubation). Taken together, in adult ICU patients there is moderate certainty of evidence that protocolised weaning reduces the duration of mechanical ventilation, weaning and ICU stay.
We completed a Cochrane review14 of weaning protocols in mechanically ventilated children (n = 3 RCTs). A trial22 that evaluated a daily screening and a SBT intervention (n = 2600) reported a 32-hour (95% CI 8 to 56 hours) significant reduction in IMV duration without harms. 22 The remaining two pilot studies23,24 evaluated computer-driven protocols and reported significant reductions in weaning duration (106 hours, 95% CI 28 to 184 hours; and 21 hours, 95% CI 9 to 32 hours). Although this limited evidence suggested that weaning protocols may reduce the duration of mechanical ventilation, it was inadequate to show whether the achievement of shorter ventilation by protocolised weaning caused children benefit or harm. Furthermore, within these trials relatively few people delivered the intervention in a controlled manner; thus, the findings may not directly translate to wider clinical practice.
Sedation weaning
Almost all mechanically ventilated children require sedative therapy to reduce stress, distress, anxiety and agitation that may lead to endotracheal tube and intravascular catheter dislodgement and cause harm. However, oversedation can result in protracted weaning time. A National Institute for Health Research (NIHR) study of sedatives in UK PICUs (SLEEP trial25) reported that only about one-third of children were adequately sedated and that almost 18% were oversedated. Our feasibility study of site visits reported limited guidance on target sedation scores, and nurses reported that they more often increased than decreased sedatives to ensure patient comfort and safety. 15 Only two PICUs utilised a sedation protocol that guided sedative dose adjustment to the child’s sedation score. Although there is some evidence of an association between the use of sedation protocols and reduced PICU length of stay, there is a paucity of high-quality evidence to guide this practice. 26
Strategies to improve sedation management include guidelines, algorithms and protocols, but there is weak evidence to support their effectiveness in children. 7 In sedation weaning, a Cochrane systematic review27 of two single-centre adult trials (n = 633) and a large multicentre paediatric trial (n = 2449)28 found no clear evidence that protocol-directed sedation was more effective than non-protocolised care. However, a systematic review26 of evidence from six observational studies including 2011 children reported a beneficial association between the use of sedation guidelines and reduced PICU length of stay, the frequency of unplanned extubation, the prevalence of patients experiencing drug withdrawal, the total doses delivered and the duration of sedation.
Need for a trial
Sedation and ventilation weaning are inextricably linked and the clinical co-ordination of this process is an important priority to optimise clinical outcomes. Therefore, it makes sense to package them together in a way that is not overly complicated. We proposed an intervention that included (1) daily assessment of ventilation and readiness for a SBT, (2) conducting a SBT and proceeding to extubation if required, (3) a sedation assessment with a strategy to reduce sedatives to a target sedation level, and (4) maximising the involvement and engagement of all relevant clinical staff. Although sedation and weaning interventions have been evaluated separately in studies, evidence of their efficacy is limited by low quality and they had not been combined and evaluated in a multicentre randomised controlled trial (RCT).
A simple and widely practicable intervention that maximises clinician participation in optimising sedation alongside early screening for readiness for liberation from mechanical ventilation has the potential to reduce the duration of ventilation in the PICU; reduce exposure to the risks of mechanical ventilation and oversedation; and reduce days in the ICU, which is an expensive resource. Our feasibility work found very few policies that specifically addressed sedation and weaning guidelines, and staff interviews confirmed that a strategy for weaning sedation and ventilation was a priority in most PICUs and one that was largely dependent on the consultant on duty at the time. 15 Staff also disclosed continuing uncertainty about readiness to wean, the benefits of a SBT and its potential impact on the duration of ventilation in the UK. Importantly, the overwhelming majority of PICUs (83%) were willing to take part in a cluster randomised clinical trial.
Hypothesis
The extent to which a treatment effect observed in the adult ICU setting might reasonably be generalised to the PICU setting is a scientific judgement based largely on consideration of whether or not the same mechanism of action is likely to apply in both populations. The most important criterion for this generalisation is that the treatment effect is valid and precise. The Cochrane systematic review13 of protocolised weaning in adult ICU patients provided evidence of a treatment effect that was apparently valid, in as much as it was obtained from well-concealed randomised clinical trials and reasonably precise. In addition, the review of protocolised weaning in children also showed a treatment effect, albeit cautiously because the evidence was largely based on one large trial. It is possible, therefore, that a ventilator liberation intervention might also reduce the duration of mechanical ventilation in children. Therefore, the hypothesis for this study was that children weaned with a sedation and ventilation liberation intervention will have a reduced duration of mechanical ventilation compared with children who were weaned with usual care.
Chapter 2 Description of the SANDWICH intervention
In the description of this intervention, we have incorporated all elements of the Template for Intervention Description and Replication checklist to fully describe the intervention and aid replicability of the intervention and its delivery in practice. 29
Name of the intervention
The name of the intervention is ‘sedation and weaning in children, a co-ordinated care protocol’, which is more informally known as the SANDWICH intervention.
Objectives
-
To standardise the proposed SANDWICH intervention.
-
To develop an education package to train staff to deliver the SANDWICH intervention.
-
To develop support tools and materials for staff.
Oversight
Our feasibility work was based on Durlak and DuPre’s30 implementation strategy. We engaged a large group of multidisciplinary paediatric intensive care clinicians (nurses, doctors and physiotherapists) from UK PICUs, parents and young people, and a PICU survivor in discussions about the design, acceptability and outcomes of the intervention components. Paediatric intensive care research team members oversaw the development of the SANDWICH education package and support tools.
Key elements of the SANDWICH intervention
The SANDWICH intervention incorporated co-ordinated multidisciplinary care in sedation and ventilation weaning, regular assessment of sedation and ventilation, and a SBT. It comprised four key components.
1. Ward round clinical assessment
The multidisciplinary clinical ward round was designed to facilitate greater collaboration. Ward rounds provided the clinical team with the opportunity to review patients’ sedation management, including the assessment of sedation levels using the validated COMFORT or COMFORT-B scales,31,32 the sedative regimen/dose and the setting of desired sedation level/targets. In addition, regular clinical review (minimum twice daily) of the child’s ventilation status was undertaken and ventilation goals were set. Sedation and ventilation plans were fed back to the bedside nurse.
2. Sedation assessment
Minimum 6-hourly assessment of sedation using COMFORT or COMFORT-B was undertaken. The original COMFORT scale has eight indicators: alertness, calmness/agitation, respiratory response, physical movement, blood pressure, heart rate, muscle tone and facial tension. Each indicator is scored between 1 and 5 based on the behaviours exhibited by the child. The total score is derived by adding the scores of each indicator; total scores range from 8 to 40.
During the regular ward round, the child’s sedation level was reviewed and the target range within which the COMFORT/COMFORT-B score should lie was agreed according to the child’s progress.
3. Readiness for a spontaneous breathing trial
This component recommended a twice-daily screen (at a time most suitable for the local PICU staff) of five clinical parameters to ascertain the patient’s readiness for a SBT. The screen was conducted largely by the bedside nurse and included the following:
-
fraction of inspired oxygen (FiO2) ≤ 0.45
-
saturation of oxygen in peripheral blood (SpO2) ≥ 95% (or as appropriate for the child’s underlying condition)
-
positive end-expiratory pressure (PEEP) ≤ 8 cm H2O
-
peak inspiratory pressure (PIP) ≤ 22 cm H2O
-
cough present.
When criteria were met, the nurse stopped or reduced sedation (as per ward round) and informed a senior member of staff (medical or nursing) that the child was potentially ready to undertake a 2-hour SBT. The decision to proceed was taken by senior staff. If the child met the criteria but the decision was not to proceed, the reason was recorded on a checklist on the bedside form:
-
neuromuscular weakness
-
low consciousness – sedation or neurological
-
airway protection reasons – secretions, oedema
-
high haemodynamic support
-
expected return to theatre/procedure requiring anaesthetic/external procedure
-
limited staff resources
-
too late in the evening
-
other (please specify).
4. The spontaneous breathing trial
If the SBT proceeded, ventilator support was reduced to a spontaneous breathing ventilator mode with a PEEP of 5 cm H2O and a pressure support (Psupp) of 5 cm H2O (above PEEP) for up to a maximum of 2 hours. During the SBT, the child was continuously monitored for signs of respiratory distress by the bedside nurse, as indicated by:
-
increased heart or respiratory rate by 20% (above pre-SBT rates)
-
signs of increased work of breathing (use of accessory muscles and asynchronous breathing)
-
SpO2 of < 92% (or lower than that expected/allowed for their condition) or an increase in oxygen requirements.
If the SBT was successful, extubation was discussed with, and managed by, the senior medical team. If extubation did not occur, the reason was recorded on a checklist on the bedside form.
The SANDWICH education package
Creating an education package to train critical care staff to deliver each element of the intervention was a major focus of the SANDWICH study. The education package was created by the implementation manager and clinical research team, with specialist support from an established NHS online education provider [LearnPro NHS; www.learnpro.co.uk (accessed 9 February 2022)] and a medical filmmaker from Temple St Hospital, Dublin, Ireland.
The online course
The online course included in-built assessment of learning against objectives. It enabled tracking of staff training completion at the PICU level: this facilitated monitoring and feedback to trainers/researchers during the training and intervention periods. This approach was used successfully by co-applicant Timothy S Walsh in the recent DESIST trial33 in adult ICUs to achieve > 80% training completion within 2–3 months by nursing staff for a sedation–analgesia education package. The course consisted of seven modules, of which five addressed the components of the intervention and two provided background education on the evidence underpinning protocolised weaning, optimum sedation management, and pharmacology of the sedative and analgesic drugs commonly used in PICUs. The topics covered included:
-
Why get sedation right?
-
The pharmacology of commonly used sedative drugs.
-
COMFORT-B.
-
COMFORT original.
-
Multidisciplinary ward round.
-
Bedside screen for SBT readiness.
-
SBTs.
Note that the e-learning module had two pathways to facilitate use of either COMFORT version.
The staff completed an assessment at the end of each of the four essential component modules. A score of > 80% was required in each module to certify training.
The SANDWICH manual
A detailed education manual was compiled to complement the online training; the online training included a PowerPoint slide set (Microsoft Corporation, Redmond, WA, USA) and training folder. The materials were designed using the same palette of fonts and colours, and included photographs, graphics and colourful diagrams. The manual comprised 136 pages, with sections on instructions for accessing the online e-learning course; face-to-face teaching resources, including alternative teaching formats; standard operating procedures for training and assessment; and training logs. The manual was given to the site’s principal investigator (PI) and team champion on the first day of on-site training delivered by the implementation manager.
Other materials used in the intervention
To aid implementation delivery and compliance during the implementation phase, several materials to aid delivery were provided to each unit. During the champion training sessions conducted by the implementation manager, each champion received a training pack to assist training roll-out to the wider ICU team. In addition, each site received ‘quick reference’ resources designed for use at a bedside or during a ward round; these included laminated bedside packs, branded lanyards, pull-out banner pens with the core bundle components attached and documentation checklists (for ward rounds, screening tools, COMFORT and COMFORT-B). Each site received a pack of A3 double-sided laminated teaching posters for face-to-face teaching at the bedside. The variety of teaching resources that was provided allowed each site to individualise its training approach. Further resources included refresher posters, lanyard reminder cards, flyers and screensavers.
A SANDWICH website was designed to contain all of the training resources developed. Owing to the stepped-wedge design of the trial, the website did not go live until all sites crossed over to the intervention arm of the trial [URL: www.qub.ac.uk/sites/sandwich (accessed 27 March 2020)].
Tailoring of the intervention
The core intervention components could not be adapted. However, each PICU could schedule its own times for ward rounds, screening for readiness for SBT and sedation assessment to suit its individual working practices.
Education delivery methods
The SANDWICH education package was delivered using a multifaceted approach that included both online and face-to-face engagement (both individually and in groups). Online education was delivered using an established NHS online education provider (LearnPro NHS; www.learnpro.co.uk). The online module provided training in the protocol and the underpinning clinical evidence supporting protocolised weaning. The module included an in-built assessment of learning against objectives and enabled training completion at the PICU level to be tracked and fed back to local educators/researchers during the training and intervention periods. Face-to-face training was delivered by the implementation manager, PICU trainers, champions and the SANDWICH research nurses. Training was undertaken at the bedside, in training rooms within the PICU or in staff offices, and on designated staff training days.
Staff training
An implementation manager was specifically employed to support and manage the intervention training for local trainers over the course of the trial. The manager was a senior paediatric critical care-registered nurse with 10 years of PICU experience. She holds a Bachelor of Science (BSc) in Nursing, Master of Philosophy (MPhil) in Biomedical Sciences and Postgraduate Higher Diploma in Paediatric Critical Care Nursing. This was important to ensure credibility with local sites around the practical issues of training in a PICU. This strategy has been used in other successful PICU trials (CHiP) in the UK. 34
Prior to the training period, each site identified a number of multidisciplinary SANDWICH champions. Posters were distributed to sites to advertise and aid champion recruitment. The selection of champions was undertaken by individual PICUs, whose staff were encouraged to include multidisciplinary staff at various grades who were willing to train their peers. When site staff were informed of their site’s crossover date, the chief investigator and implementation manager arranged a teleconference with the site research team, the SANDWICH champions and key members of staff to discuss staff engagement and training preparations, and to address questions.
At the beginning of each training period, the implementation manager visited each site for up to 4 days to train the local trainers. The trainers and champions were local PICU staff and included clinical nurse educators, critical care nurses (all grades), critical care doctors (senior house officer to consultant level), advanced nurse practitioners (ANPs), physiotherapists, research nurses and pharmacists. Trainers received full training from the implementation manager and had the responsibility of rolling out the full training to the remaining clinical staff. Each site was encouraged to have at least 80% of eligible staff trained and assessed by the end of the 8-week training phase.
The SANDWICH intervention providers
All clinical staff who were involved in either ventilation or sedation within the PICU were the intervention providers. The ward round had multidisciplinary involvement of doctors, nurses and other relevant disciplines. Decisions regarding ventilation and sedation targets were undertaken collaboratively. If the ward round was conducted at the bedside, bedside nurses were included; if it was conducted in a separate room, a senior nurse communicated information from, and fed back to, the bedside nurse. Bedside nurses undertook sedation assessment, screened for readiness for a SBT and conducted a SBT. Decisions to proceed to a SBT or to extubate were generally made by senior medical staff or ANPs. Extubation was undertaken following standard unit procedure and was not prescribed in the SANDWICH protocol.
Assessment of adherence to the intervention and training
The study target was 80% adherence. Adherence to the following five components of the intervention was measured:
-
minimum of two COMFORT assessments per day
-
minimum of one SBT readiness screen
-
daily ventilation target set
-
daily sedation target set
-
SBT performed when criteria were met.
The minimum achievements were recorded to accommodate patients who may have been admitted halfway through the day. Research nurses collected the data on the daily data collection form during the intervention period. During the intervention period, the sum total percentage for adherence to individual components was reported for all sites. Additionally, for individual sites, their adherence performance was fed back to staff via the SANDWICH research nurses on two occasions. The adherence to training completion was measured at 8 and 12 weeks after the training period. The data were collected by the LearnPro programme team and the number of staff trained was reported to the implementation manager. Training rates were fed back to local unit trainers, the local PI and the research nurses at 8 and 12 weeks.
Conclusion
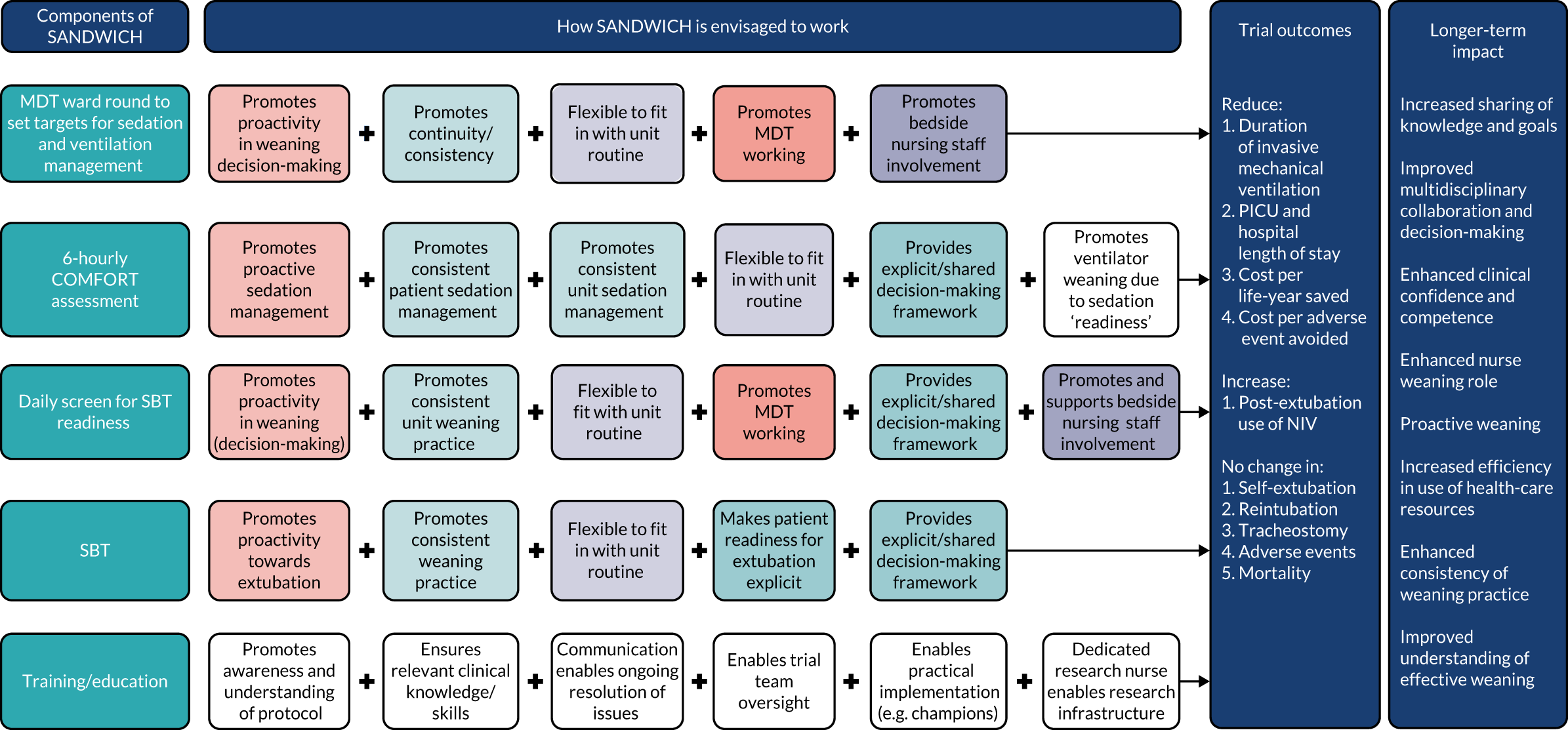
Building on previous work by this team, the proposed intervention was standardised to allow consistent delivery across different hospitals but to allow site staff to ‘adapt’ this intervention to their unit processes to facilitate uptake and adherence. To facilitate this, an education package and associated support tools were developed. The key assumptions and theory underpinning the SANDWICH intervention are diagrammatically represented in Figure 1.
FIGURE 1.
The SANDWICH logic model. The left-hand side of the model sets out the four main components of the intervention, including training and implementation support. Reading across from left to right, the series of linked boxes represent the core features and processes of the SANDWICH theory, indicating how the components are hypothesised to work together to produce the trial outcomes. Each box identifies a constituent theoretical concept: concepts are colour-coded. The ‘plus’ signs and arrows, which move horizontally, capture the dynamic relationship of the intervention components, and signify their fundamental inter-relatedness in terms of producing trial outcomes and the proposed longer-term impact of the SANDWICH intervention. MDT, multidisciplinary team; NIV, non-invasive ventilation.
Chapter 3 Clinical trial methods
Aims and objectives
This study aimed to evaluate whether or not a ventilation liberation intervention incorporating co-ordinated care with greater nursing involvement in managing sedation and weaning ventilation reduced the duration of IMV compared with usual care in children on PICUs.
The primary objective was to determine the impact of the intervention on the duration of IMV in children anticipated to have prolonged IMV. The secondary objectives were to determine the impact of the intervention on (1) all children receiving IMV regardless of their anticipated duration of ventilation; (2) length of PICU and hospital stay; (3) harm, as assessed through reviews of adverse events (AEs) and respiratory complications; (4) cost-effectiveness; and (5) sustainability and acceptability to staff delivering care.
Anticipated prolonged IMV was defined a priori. Using historical data from the Paediatric Intensive Care Audit Network (PICANet) national registry of PICU admissions,2 diagnostic codes associated with a duration of IMV of ≤ 24 hours were identified and categorised as ‘short’. Admissions that did not include a short diagnostic code were categorised as ‘prolonged’ (see Appendix 1).
Trial design
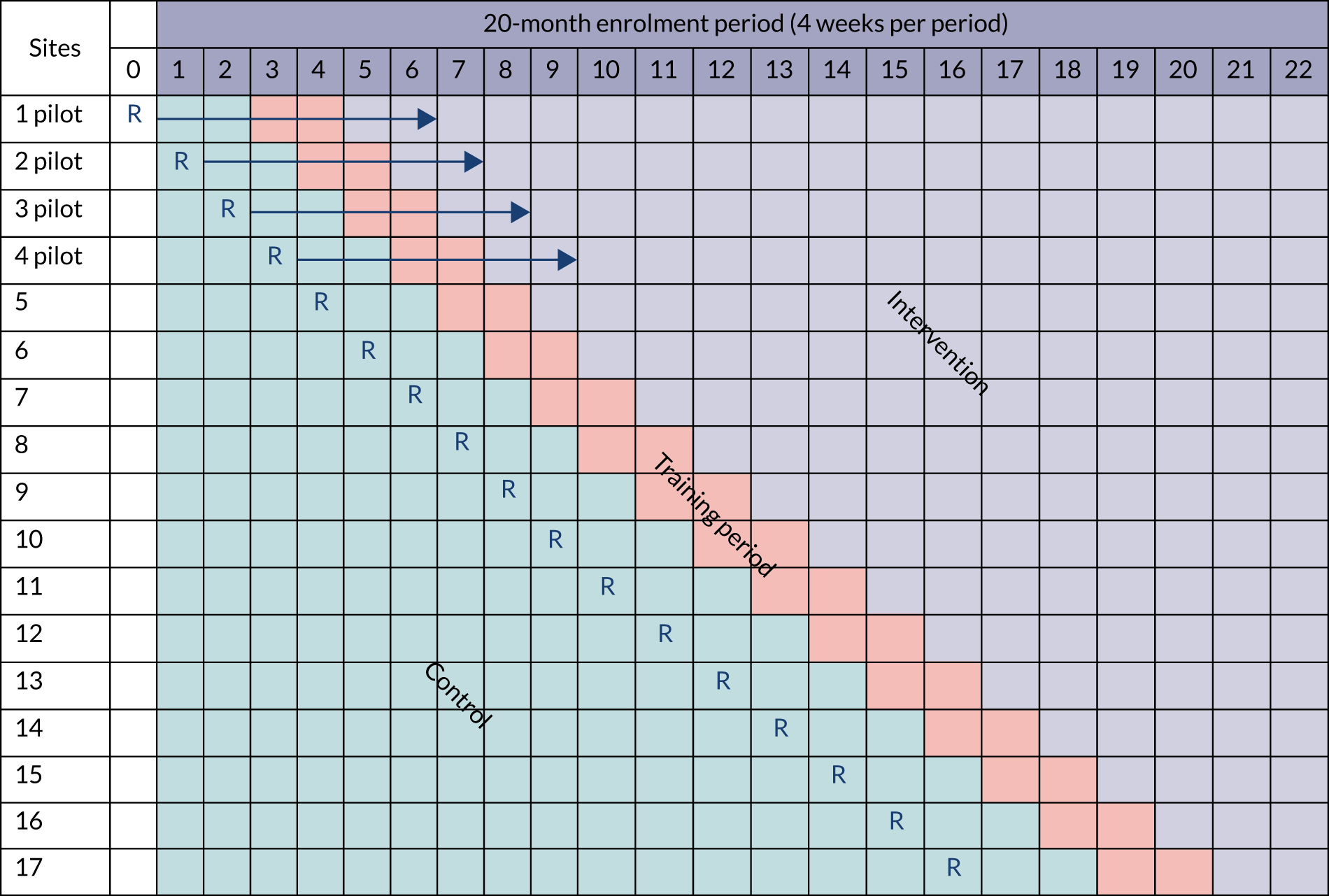
This was a pragmatic,35 stepped-wedge cluster randomised trial (SW-CRT) and cost-effectiveness trial, with an internal pilot phase and a process evaluation. The trial protocol has been published. 36 The rationale for choosing cluster randomisation was that the intervention was delivered at the level of the cluster (the hospital site) and intervention delivery would have been susceptible to contamination if patients had been individually randomised. The SW-CRT design was chosen over the conventional parallel-cluster design for a number of reasons. First, there was a limited number of clusters available to allow detection of the important clinical effect at 90% power. Second, unit staff said that they were more likely to participate in the trial if it was guaranteed that the unit would at some point receive the intervention. Third, it would have been infeasible and more costly to deliver the intervention simultaneously to units randomised to the intervention in a parallel design. Finally, knowledge translation would generally be easier because PICUs participating could potentially continue after the trial, maximising the benefits of any effects to the NHS and patients.
The SW-CRT spanned 20 months and involved 22 time periods that each lasted 4 weeks. All hospital sites started data collection simultaneously in the control period; thereafter, one site crossed over to the intervention period at each step, with the order of crossover randomly determined, and remained exposed to the intervention for the remainder of the study. The intervention period was preceded by an 8-week intensive training period. During the training period, data from existing patients were censored and no new patients were enrolled into the trial. Randomisation was computer generated and restricted to ensure that the trial was balanced across control and intervention periods with respect to size of the site (large/small based on annual PICU admissions published by PICANet). 37 An internal pilot was conducted in the first four clusters. Pilot data on recruitment, opt-out, training targets, adherence to intervention components and feasibility of data collection procedures were collected over the two periods before, during and after training. These data informed study progression, which was agreed by the Trial Steering Committee (TSC) and the NIHR HTA programme. The pilot continued into the trial without interruption.
The study schematic is shown in Figure 2.
FIGURE 2.
Stepped-wedge cluster randomised trial schematic. R, randomisation.
Paediatric intensive care unit and patient eligibility
The trial was conducted in PICUs in the UK with a case mix typical of UK critical care practice. PICUs were ineligible if they did not mainly provide IMV for children or could not apply the opt-out consent process.
All children (aged < 16 years) receiving IMV were eligible for recruitment. Children were excluded if on admission they had a tracheostomy in situ, were not immediately expected to survive or were expected to require treatment withdrawal. Children who were pregnant, as documented in their medical notes, were also excluded. All invasively mechanically ventilated children were screened for eligibility for inclusion. Eligibility was confirmed by authorised nursing/medical staff on the delegation log. A screening log was maintained at each unit that included details of the number of, and reason for, participants excluded.
Informed consent
A favourable ethics opinion was granted by the National Research Ethics Committee East Midlands (reference 17/EM/0301) on 12 September 2017. An opt-out consent approach was used. Leaflets were provided for patients’ parents or legal guardians informing them that the PICU was involved in the trial and that anonymised patient-level information would be collected. The opt-out approach applied to data collection only.
Patient withdrawal
Children could be withdrawn from outcome data collection on the request of parents or legal representatives who declined participation in the research. If parents opted out before data were collected, this was recorded on the PICU screening log. If opt-out occurred after data were collected, the PICU informed the Northern Ireland Clinical Trials Unit (NICTU) and noted the withdrawal in the patient record, on PICANet and on the study database; data collected up to the point of withdrawal were not included in data analysis.
Interventions
The SANDWICH intervention incorporated co-ordinated multidisciplinary care, patient-relevant sedation plans linked to regular assessment using a COMFORT scale, regular assessment of ventilation parameters with a higher than usual trigger for undertaking an extubation readiness test and a SBT on low levels of respiratory support to test extubation readiness. A full description of the protocol-based intervention was provided in the study manual. The manual was available to unit staff when they entered the 8-week training period. Usual care typically involved a slow reduction in ventilator support to very low levels of support before extubation. No participating sites assessed readiness for extubation on higher levels of support using a SBT. Bedside nurses were typically not engaged in the weaning process. 15
Assignment of the intervention
Each participating site was classified as large or small based on the number of children receiving IMV in each site. These data were obtained from the 2017 PICANet Annual Report. 37 Sites were ranked from smallest to largest and were split at the median into two groups. A restricted block randomisation process was used to ensure that the study was balanced with respect to site size across the control and intervention phases. Randomisation was undertaken in blocks of four, with two large and two small sites randomised in each block. The NICTU statistician (CMcD) generated the randomisation schedule before the trial commenced and held this in a restricted folder in the statistics section of the Trial Master File. Every 4 weeks, the trial statistician informed the Trial Management Group which site was next to cross over. Units were notified by trial management 12 weeks prior to moving into the training period in accordance with the randomisation sequence.
Outcomes
The primary outcome was the duration of IMV, which was measured, in hours, from the initiation of IMV to the first successful extubation (success was defined as still breathing spontaneously 48 hours following extubation). If a child was already intubated on admission to a PICU, the initiation of IMV was measured from admission.
The secondary outcomes were:
-
incidence of successful extubation
-
number of unplanned extubations
-
number of reintubations
-
total duration of IMV
-
incidence and duration of post-extubation use of non-invasive ventilation (NIV)
-
tracheostomy insertion
-
post-extubation stridor
-
any AEs
-
length of stay – PICU and hospital
-
mortality – PICU and hospital.
The outcomes were measured from patient admission to 90 days or discharge (whichever was earlier). At the end of the 20-month enrolment period, data collection continued for a maximum of 28 days. Outcomes were reported for the prolonged IMV cohort and for all children.
Data collection and management
The trial collaborated with PICANet to make best use of the established data collection infrastructure that exists in all UK PICUs. Participating PICUs routinely submit clinical data to the PICANet registry. PICUs have full access to, and ownership of, the data that are validated on entry and centrally on the PICANet server. PICANet produced a download facility that allowed participating units to extract data required for the trial, thus reducing the burden of data collection for unit staff.
When registering individual patient data to PICANet, unit staff indicated eligible patients and added a unique trial number. PICANet produced a pseudo-anonymised data set for the SANDWICH trial, which was downloaded by unit staff at required intervals during the study. The PICANet data required for the trial were transmitted from sites to the NICTU electronically, using a secure method. Other non-identifiable patient data required for the trial were collected and recorded on an electronic case report form (CRF) by the PI or designee at each unit.
Data collection was restricted to variables that were required to define patient characteristics at enrolment, monitor the intervention received and AEs, and determine health-care resources. Data collection included the variables detailed below (‘*’ denotes data collected through PICANet).
Baseline data (both observation periods)
-
Inclusion/exclusion criteria and eligibility screen.
-
Patient number [event identification number (ID) generated in PICANet; patient number generated in the CRF*].
-
Sex.*
-
Age on admission (in months).*
-
Gestational age at delivery (if the patient was < 2 years of age).*
-
Date/time of admission.
-
Previous ICU admission (during current hospital stay).*
-
Location at which the child was admitted.*
-
Paediatric Index of Mortality 3 (PIM3) score38 (including breakdown of reason for this admission).*
-
Primary diagnosis for this admission.*
-
Date/time of intubation.
Daily data collection (both observation periods, during invasive mechanical ventilation)
-
Daily (8.00 a.m.), the mode of IMV, FiO2, PEEP, PIP, ventilator rate, tidal volume and the level of Psupp above PEEP (depending on the mode of ventilation).
-
Adverse events.
-
Paediatric critical care minimum data set (for obtaining the health-care resource group for each PICU admission). 39
Additional data (intervention period, during invasive mechanical ventilation)
-
COMFORT scores, ward round sedation and ventilation target set.
-
Readiness-to-wean criteria.
-
Date/time of start/end of SBT and outcome (if applicable).
-
Mode of IMV, FiO2, PEEP, PIP, ventilator rate, tidal volume, and the level of Psupp above PEEP and COMFORT score (prior to SBT) (if applicable).
Additional data (control period, during invasive mechanical ventilation)
-
Mode of IMV, FiO2, PEEP, PIP, ventilator rate, tidal volume, and the level of Psupp above PEEP (2 hours prior to extubation).
-
COMFORT score (2 hours prior to extubation or score recorded closest to this time point prior to extubation).
Outcome data collection
-
Successful extubation.
-
Unplanned extubation.
-
Reintubation (including date and time).
-
Date/time of start/end of post-extubation use and duration of NIV.
-
Post-extubation stridor.
-
Date and time of tracheostomy.
-
Date and time of extubation.
-
PICU mortality (status on discharge).*
-
PICU length of stay.*
-
Location to which child was discharged from the PICU.*
Data collected after discharge from the paediatric intensive care unit
-
Hospital length of stay (calculated from the date/time of hospital discharge).
-
Destination following hospital discharge.
-
Hospital mortality (status on discharge).
Data management of non-Paediatric Intensive Care Audit Network data
Trial data that were entered onto the electronic CRF of a clinical trial database (MACRO Electronic Data Capture, Version 4.9.1; Elsevier, Amsterdam, the Netherlands) were processed electronically, as per NICTU standard operating procedures and the study-specific data management plan. Data queries were ‘raised’ electronically (MACRO Electronic Data Capture) when clarification from PICU research staff was required for data validations or missing data. Research staff ‘responded’ electronically to data queries, ensuring that amendments, where applicable, were made to the clinical trial database. All essential documentation and trial records were stored securely and access was restricted to authorised personnel. All study documentation, study data and patient medical records were archived as per regulatory requirements, and those responsible for archiving were noted on the sponsor agreement.
Data quality
The chief investigator and the NICTU provided training to unit staff on trial processes and procedures, including CRF completion and data collection. During the trial, adherence to the protocol, trial-specific procedures and good clinical practice was monitored. Within the NICTU, the clinical data management process was governed by standard operating procedures, which ensured standardisation and adherence to International Conference of Harmonisation Good Clinical Practice guidelines40 and regulatory requirements. For data collected in the CRF, data validation was implemented and discrepancy reports were generated following data entry. Data validation checks that were programmed into the clinical trial database identified data that were out of range or inconsistent, or any protocol deviations. Changes to data were recorded and fully auditable. Data errors were documented and corrective actions implemented.
PICANet’s data validation methodology included real-time data validation reporting back to data suppliers using clinical advice on appropriate ranges for clinical data. Comprehensive checking of outcome variables and data used for risk adjustment took place. Missing data, excessive use of exception values and data anomalies were reported and progress chased until resolved. Stringent data quality, logic and range checks were built into the web-based data collection system, which provided real-time data validation reporting. By using a standardised format for data entry and upload, PICANet maintained consistent data quality. In addition, there were validation visits to units by PICANet research nurses, who checked the accuracy of data transcription from clinical notes.
A Data Monitoring Committee (DMC) meeting was convened to carry out reviews of the study data at intervals during the study.
Adverse events
Events and complications associated with the patient’s underlying medical condition were not considered AEs. An AE was defined as any untoward medical occurrence in a study participant. A serious adverse event (SAE) was an AE that fulfilled one or more of the following:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Causality (i.e. relationship to the trial intervention) and expectedness (i.e. expected or unexpected) were assessed by the PI or medically qualified designee as follows:
-
Unrelated if there was no evidence of any causal relationship.
-
Unlikely to be related if there was little evidence to suggest a causal relationship (e.g. the event did not occur within a reasonable time after starting the intervention) or there was another reasonable explanation for the event (e.g. the child’s clinical condition, other concomitant treatment).
-
Possible relationship if there was some evidence to suggest a causal relationship (e.g. the event occurred within a reasonable time after starting the intervention) but the influence of other factors contributed to the event (e.g. the child’s clinical condition, other concomitant treatments).
-
Probable relationship if there was evidence to suggest a causal relationship and the influence of other factors was unlikely.
-
Definitely related if there was clear evidence to suggest that there was a causal relationship and other possible contributing factors were ruled out.
The AEs and SAEs were recorded and reported until the patient was discharged from the PICU or 90 days after admission (whichever was earlier). All reported AEs were recorded in the medical notes. AEs expected within the trial population included:
-
unplanned extubation, with or without reintubation*
-
unplanned removal of vascular lines, with or without reinsertion
-
unplanned removal of any other indwelling line, tube, catheter or drain
-
tracheostomy*
-
post-extubation stridor*
-
need for NIV post extubation*
-
reintubation*
-
bradycardia requiring intervention
-
hypoxia/desaturation requiring intervention
-
need for cardiopulmonary resuscitation.
*These events were collected as outcomes and were, therefore, not reported separately as an AE or a SAE.
Serious adverse event reporting
All SAEs were recorded and reported to the NICTU within 24 hours of the PICU research team becoming aware of the event. Causality and expectedness were confirmed by the CI and medically qualified intensivists from the Trial Management Group (KM or DMcA). No SAEs were deemed to be unexpected and related to the trial.
Statistical methods
Pre-trial power calculation
The original sample size calculation was informed by admission data in the 2011–13 PICANet database from 18 sites that had originally expressed an interest in participating in the SANDWICH trial. The initial sample size was calculated under individual randomisation using the Schoenfeld method and then multiplied by the appropriate design effect, allowing for clustering and the SW-CRT design. 41 We assumed a significance level of 0.05 and allowed for 90% power to detect a hazard ratio between 0.8 and 0.9 (a hazard ratio of 0.8 equates roughly to a reduction of 1 day in the length of stay in the intervention arm). With hindsight, we recognise that this was powered to detect a reduction in hazard when we should have powered to detect an increase in hazard. We considered censoring rates of between 10% and 30% (about 5% of the children were known to die and an additional 10–20% were lost to follow-up or discharged still ventilated). The calculations indicated that the:
-
mean duration of mechanical ventilation was 5.5 [standard deviation (SD) 12] days
-
intracluster correlation coefficient (ICC) was 0.007 (95% CI 0.001 to 0.01)
-
average sample size was 53 patients per site per month.
With the design and the above assumptions, and assuming recruitment of between 13 and 15 PICUs, it was estimated that the total sample size for SW-CRT was 11,024 to 14,310 patients, and this was sufficient to detect, with 90% power, a target effect size of 1 day.
Review of assumptions following the internal pilot
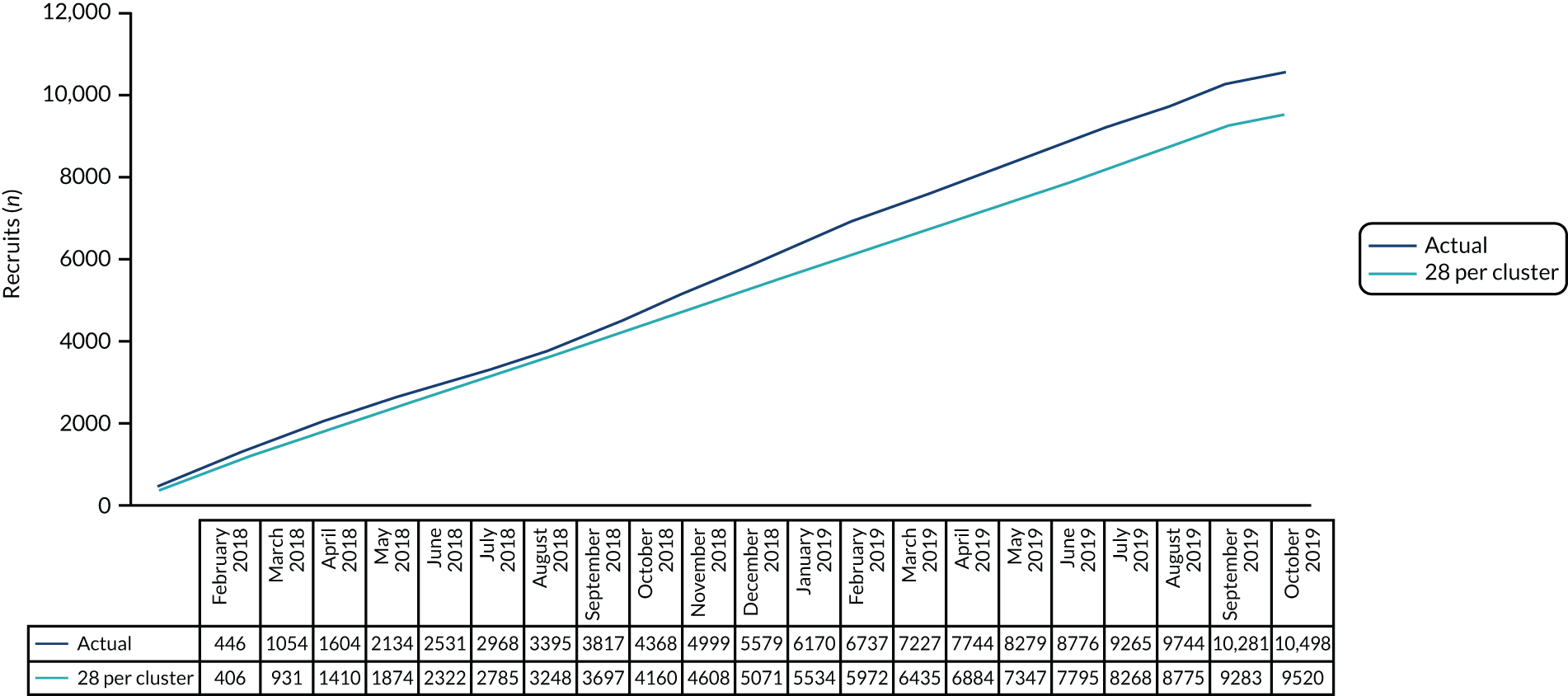
Recruitment during the 9-month internal pilot phase (5 February 2018 to 14 October 2018) for the 17 participating sites was 4025 participants, which represents 62.5% of the original expected target for this time period (6440 recruits), equating to an average of 28 patients per site per 4-week period. In consultation with the TSC and the DMC, a review of assumptions underlying the pre-trial calculation was conducted using the 2014–16 PICANet data set for the PICUs included in the study. Applying censoring criteria to this data set provided a homogeneous population that more accurately reflected the trial population. Although the primary analysis will be a survival analysis, at the interim sample size re-analysis we opted to use more recently developed methodology to determine power in a stepped-wedge trial to allow for more complicated correlation structures than at the time that the original sample size calculation was undertaken. 42 We, therefore, determined the power available assuming a continuous outcome. This is expected to be a conservative approach, meaning that it should have slightly underestimated the power not having allowed for the time to event nature of the data. The cluster sample size application [https://clusterrcts.shinyapps.io/rshinyapp (accessed 1 July 2018)] was used to update the sample size calculation. Revised calculations indicated that the:
-
mean duration of mechanical ventilation was 5.8 (SD 9.6) days
-
ICC was 0.005 (95% CI 0.001 to 0.01).
The smaller SD and ICC indicated that the average of 28 patients per site per 4-week period would provide approximately 80–87% power based on the lower and upper bounds of the 95% CI for the ICC.
Based on the revised calculation, the expected sample size was 9520 patients. The revised sample size was approved following review by the DMC, TSC and NIHR.
Statistical analysis principles
Descriptive statistics, such as proportions, CIs, mean, SD, median and interquartile range (IQR), are reported where appropriate and are summarised in tables. Recruitment and loss to follow-up numbers are provided in a flow diagram. All analyses were conducted by intention to treat and were analysed according to randomised allocation, excluding those with missing outcome data or who were ineligible after randomisation. Data were censored on the date that children moved to another unit prior to extubation, were not weaned before the unit transitioned to the training phase, received a tracheostomy, died or were not weaned by 90 days after admission. Data were reported and summarised in accordance with the Consolidated Standards of Reporting Trials (CONSORT) extension for the SW-CRT. 43 The final analyses were conducted in accordance with the statistical analysis plan published on the NICTU website [www.nictu.hscni.net/sandwich-trial-documents/ (accessed 25 February 2020)] and using Stata®/SE Version 14.2 (StataCorp LP, College Station, TX, USA) and SAS software, version 9.4 (SAS Institute, Marlow, UK).
Primary analysis
There are two requirements to the analysis of SW-CRTs. First, systematically more clusters are observed under the control condition at an early calendar time than under the intervention condition and, second, the study was cluster randomised. The primary estimate of the treatment effect, therefore, was a time- and cluster-adjusted hazard ratio (aHR) along with 95% CIs in children anticipated to have prolonged IMV. For this primary outcome, and other time-to-event secondary outcomes, Cox proportional hazards models adjusting for calendar time to estimate hazard ratio were used. Allowance was made for clustering using a frailty term for each PICU. Calendar time was formulated on the 20-month trial duration that consisted of 22 time periods. To provide an absolute measure of effect, the median of the model-based prediction of survival duration was computed at all 22 time periods for the intervention and control periods, and the difference between the two; the extent of variability was summarised using the IQR over the 22 time periods.
Binary secondary outcomes were analysed using mixed-effects binomial regression with a log-link to estimate the adjusted relative risk (aRR) and a binomial model with identity link to estimate the adjusted risk difference, with estimation using the restricted maximum likelihood approach. All mixed models included cluster as a random effect assuming an exchangeable correlation structure and used the Kenward and Roger small-sample correction44 to correct the potential inflation of the type I error rate owing to the small number of clusters. 45 In the case of non-convergence of binomial linear mixed models to estimate risk differences, marginal estimates of risk differences using generalised estimating equations, assuming an independent correlation structure with a Fay and Graubard small-sample correction46 on standard errors with 95% CIs derived from a z-distribution, were reported. 47 In the case of non-convergence of the binomial model with a log-link, a Poisson model with robust standard errors was fitted. For continuous outcomes, similar models were used with an identity link and assuming a normal distribution, but checking for normality assumptions and making transformations when necessary.
Secondary analysis
A secondary prespecified adjusted analysis of the primary outcome was conducted that included the covariates age, severity of illness (PIM3 score), respiratory compared with other diagnostic grouping, type of admission (planned/unplanned) and reason for admission (surgical/medical).
Subgroup analysis
We conducted a prespecified exploratory subgroup analysis for the duration of IMV using interaction models and 99% CIs for size of unit (large and small, based on annual admissions), adherence to the intervention (tertiles of ranked averages), type of admission to unit (planned and unplanned) and reason for admission (surgical, medical respiratory or medical other).
Sensitivity analyses
To assess sensitivity to assumptions made about the nature of time effects and correlations, we conducted an extensive series of sensitivity analyses for the secondary binary outcomes (see model-based analysis in Appendix 1). Methodology does not yet exist to consider the sensitivity of these assumptions by such a degree for survival outcomes.
The analyses were conducted using Stata®/SE version 16.1 and SAS software, version 9.4. We report variance components and ICCs.
Summary of changes to the study protocol
A summary of key changes is presented in Box 1.
Wording clarification for outcomes:
-
duration of IMV measured in hours from initiation of invasive ventilation until the first successful extubation
-
number of unplanned extubations (instead of accidental self-extubation).
Addition of secondary outcomes:
-
incidence and duration of post-extubation NIV
-
reduces the duration of IMV in all eligible children irrespective of their expected ventilation duration
-
number of reintubations
-
total duration of IMV.
Additional data variables:
-
location from which the child was admitted
-
PICU mortality (status on discharge) at 90 days.
Addition of AEs expected within the trial population:
-
unplanned removal/reinsertion of vascular devices; indwelling line, tube or drain
-
bradycardia requiring intervention
-
hypoxia/desaturation requiring intervention
-
need for cardiopulmonary resuscitation.
Additional exclusion criterion: children who are pregnant, as documented in their medical notes.
Additional baseline data variable: gestational age at delivery.
V4.0, 27 July 2018Description of revised sample size to 9520 participants.
V5.0, 12 March 2019Following sample size recalculation, revised recruitment numbers added to the study timeline. The study sponsor confirmed that this was a minor amendment not requiring ethics approval.
V6.0, 11 September 2019Last study protocol.
Chapter 4 Clinical trial results
Participants: sites
Site selection
Twenty-eight PICUs were assessed for trial eligibility. Three PICUs were not eligible to participate: two PICUs in Scotland were unable to fulfil the requirements for the ability to provide opt-out consent and one small PICU admitted mainly high-dependency patients only. Twenty-five PICUs were eligible for participation: seven (28%) declined, and expressions of interest to participate in the SANDWICH trial were received from 18 PICUs (72%) across England, Wales and Northern Ireland. Two PICUs were based at one hospital site in England and all other sites had one PICU. Site initiation visits were conducted prior to the start of patient screening and recruitment. All sites (15 in England, one in Wales and one in Northern Ireland) obtained local NHS permissions/approvals and opened to recruitment on 5 February 2018, with recruitment continuing until 14 October 2019. Staff at individual sites were informed of their randomised crossover date 12 weeks in advance of the training period to allow for site rota preparation.
Characteristics of sites
The characteristics of the 18 PICUs that participated in the SANDWICH trial compared with all other PICUs in the PICANet (n = 28) are presented in Table 1. PICUs were geographically spread across England, Wales and Northern Ireland. Based on overall UK PICU characteristics, the sites participating in the SANDWICH trial were broadly representative of regions, types of hospitals, bed numbers and annual admissions.
| PICU characteristic | Units in the SANDWICH trial (N = 18) (64.3%), n (%) | Units in the UK (N = 28) (100%), n (%) |
|---|---|---|
| Region | ||
| North | 4 (14.3) | 7 (25.0) |
| Midlands West/East | 3 (10.7) | 6 (21.4) |
| London | 6 (21.4) | 8 (28.6) |
| South West/East/Central | 3 (10.7) | 3 (10.7) |
| Wales | 1 (3.6) | 1 (3.6) |
| Northern Ireland | 1 (3.6) | 1 (3.6) |
| Scotland | 0 (0) | 2 (7.1) |
| Type of hospital | ||
| University | 18 (100) | 28 (100) |
| General | 11 (39.3) | 18 (64.3) |
| General/cardiac mixed | 5 (17.9) | 6 (21.4) |
| Cardiac | 2 (7.1) | 4 (14.3) |
| Size of unit (beds) | ||
| < 8 | 4 (14.3) | 8 (28.6) |
| 8–11 | 5 (17.9) | 7 (25.0) |
| 12–15 | 2 (7.1) | 4 (14.3) |
| ≥ 16 | 7 (25.0) | 9 (32.1) |
| Annual PICU admissions | ||
| < 500 | 4 (14.3) | 10 (35.7) |
| 500–749 | 9 (32.1) | 11 (39.3) |
| 750–999 | 4 (14.3) | 5 (17.9) |
| ≥ 1000 | 1 (3.6) | 2 (7.1) |
Participants: patients
Patient flow
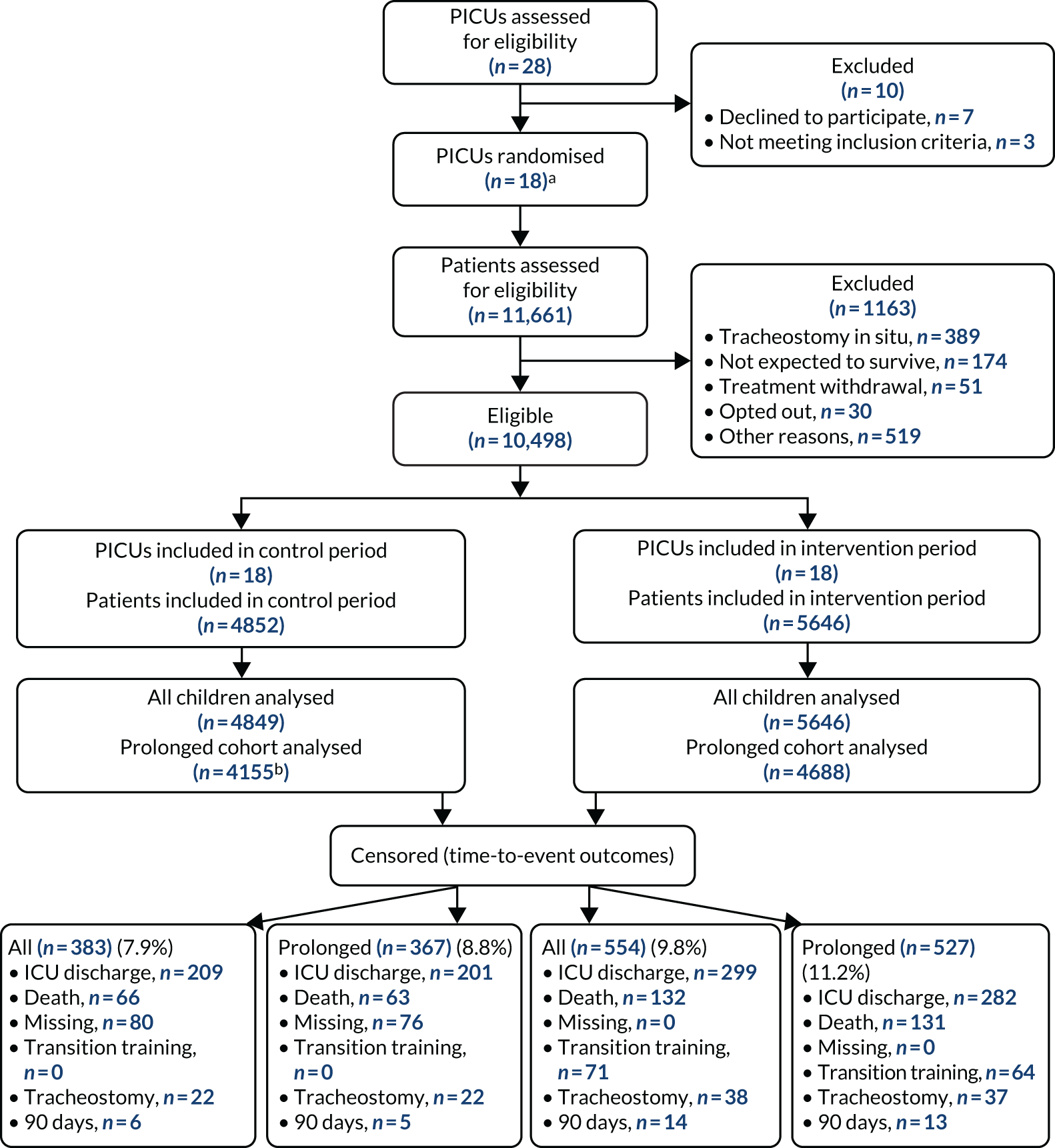
The CONSORT flow diagram in Figure 3 details the flow of patients in both observational periods.
FIGURE 3.
The CONSORT flow diagram. a, Two PICUs from the same site were randomised together to avoid contamination of the intervention; b, three excluded from analysis could not link to PICANet data set.
Recruitment
Patient recruitment took place from 5 February 2018 to 14 October 2019. Of the 12,540 IMV admissions over the study period (numbers obtained via a request to PICANet for data access in June 2020), 11,661 were assessed for eligibility (93%) and 10,498 (90%) of those assessed met the eligibility criteria; three admissions could not be linked for analysis and, therefore, 10,495 admissions were included in the trial. The flow of patients through the trial is shown in Figure 3, and the SW-CRT flow chart of patient admission numbers per step is shown in Appendix 1. Participant exclusion numbers detailed by observation period are provided in Table 2.
| Reason for exclusion | Observation period (n) | |
|---|---|---|
| Control | Intervention | |
| Met exclusion criteria | 615 | 548 |
| Tracheostomy in situ | 171 | 218 |
| Not expected to survive | 76 | 98 |
| Treatment withdrawal | 38 | 13 |
| Pregnant | 0 | 0 |
| Other reasons | 311 | 208 |
| Parent opted out | 19 | 11 |
The number of patient admissions recruited, averaged across all sites for 22 time periods, was 28 admissions per 4-week period. The cumulative patient recruitment against the anticipated pre-trial sample size and the revised minimum target is shown in Figure 4.
FIGURE 4.
Patient recruitment: cumulative recruitment totals.
Patient characteristics
Patient characteristics were broadly similar across the control and intervention periods (Table 3). The median age of patients was 10.5 months (IQR 2–53 months) and a total of 4474 (42.6%) were female and had a similar severity of illness score (PIM3 0.04, SD 0.1). The majority of diagnoses were cardiovascular (n = 3691, 35.2%) and respiratory (n = 2699, 25.7%), a little over half (n = 5952, 56.7%) were unplanned non-surgery, and the majority typically required prolonged ventilation for > 24 hours (n = 8843, 84.3%).
| Characteristic | Observation period | Total | |
|---|---|---|---|
| Control | Intervention | ||
| Total number of participants, n | 4849 | 5646 | 10,495 |
| Sex, n (%) | |||
| Female | 2048 (42.2) | 2426 (43.0) | 4474 (42.6) |
| Male | 2800 (57.7) | 3217 (57.0) | 6017 (57.3) |
| Ambiguous | 1 (0.02) | 3 (0.01) | 4 (0.04) |
| Age at PICU admission (months), median (IQR) | 10.5 (2–52) | 9 (1–54) | 10 (2–53) |
| Age, n (%) | |||
| < 1 month | 802 (16.5) | 1078 (19.1) | 1880 (17.9) |
| 1 to < 24 months | 2245 (46.3) | 2463 (43.6) | 4708 (44.9) |
| 24 to < 72 months | 832 (17.2) | 940 (16.7) | 1772 (16.9) |
| ≥ 72 months | 968 (20.0) | 1165 (20.6) | 2134 (20.3) |
| Previous ICU admission, n (%) | 1176 (24.2) | 1523 (27.0) | 2699 (25.7) |
| PIM 3, mean (SD) | 0.04 (0.1) | 0.04 (0.1) | 0.04 (0.1) |
| Primary diagnostic group, n (%) | |||
| Respiratory | 1289 (26.6) | 1410 (25.0) | 2699 (25.7) |
| Cardiovascular | 1586 (32.7) | 2105 (37.3) | 3691 (35.2) |
| Neurological | 672 (13.9) | 734 (13.0) | 1406 (13.4) |
| Gastroenterology | 294 (6.1) | 316 (5.6) | 610 (5.8) |
| Infection | 309 (6.4) | 255 (4.5) | 564 (5.4) |
| Oncology | 126 (2.6) | 113 (2.0) | 239 (2.3) |
| Other | 573 (11.8) | 713 (12.6) | 1286 (12.2) |
| Type of admission, n (%) | |||
| Planned, following surgery | 1507 (31.1) | 2074 (36.7) | 3581 (34.1) |
| Unplanned, following surgery | 268 (5.5) | 244 (4.3) | 512 (4.9) |
| Planned, other | 167 (3.4) | 283 (5.0) | 450 (4.3) |
| Unplanned, other | 2907 (59.9) | 3045 (53.9) | 5952 (56.7) |
| Anticipated ventilation trajectory, n (%) | |||
| Prolonged | 4155 (85.7) | 4688 (83.0) | 8843 (84.3) |
| Short | 694 (14.3) | 958 (17.0) | 1652 (15.7) |
Primary outcome: duration of invasive mechanical ventilation
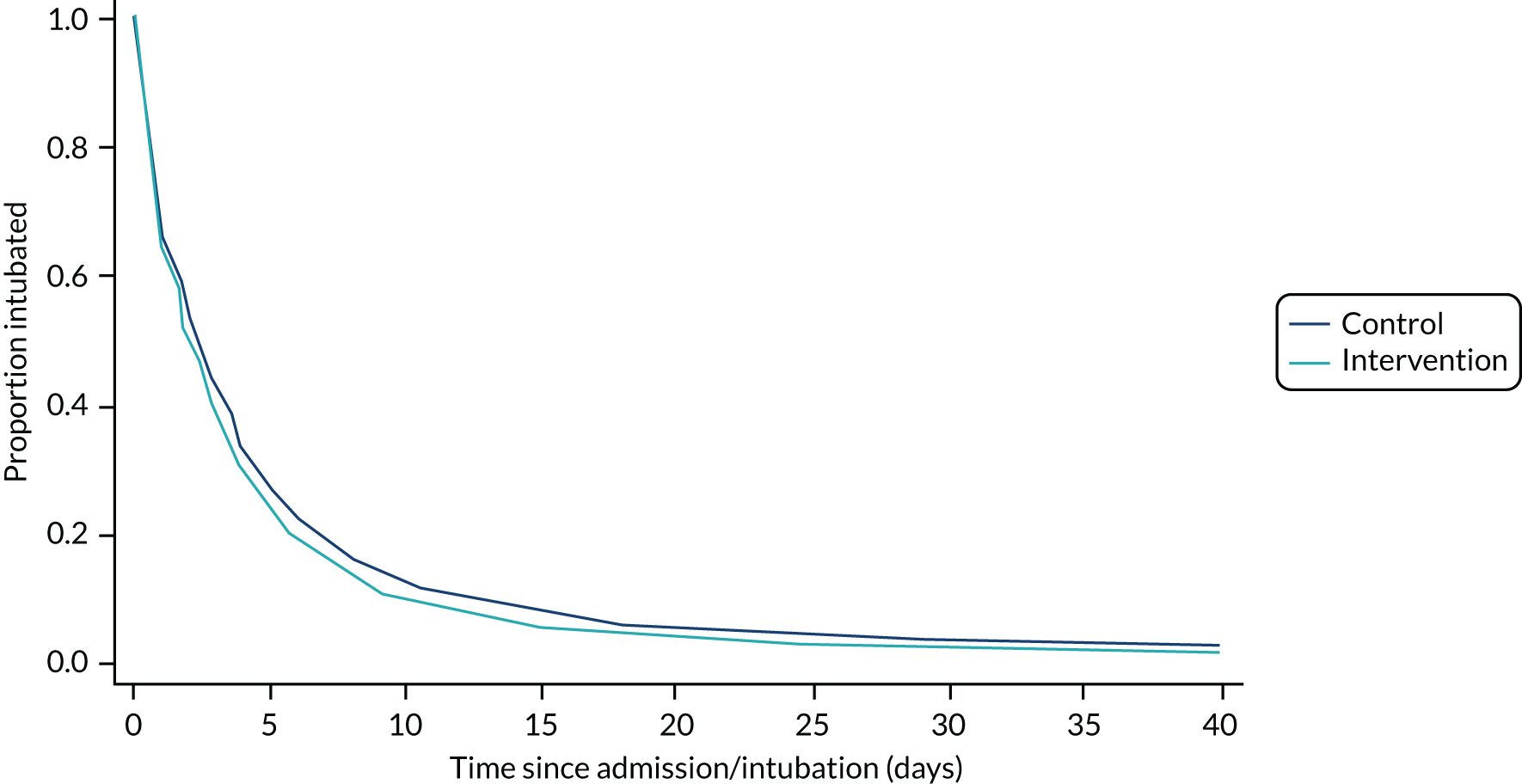
In the prolonged IMV cohort, the SANDWICH intervention resulted in a significantly shorter duration of IMV before successful extubation (aHR for extubation 1.11, 95% CI 1.02 to 1.20; p = 0.02). The median duration was 64.8 hours (IQR 22.1–141.4 hours) in the intervention period compared with 66.2 hours (IQR 21.8–138.0 hours) in the control period, and the adjusted median difference across all time periods was –6.1 hours (IQR –8.2 to –5.3 hours). In all children, a significantly shorter duration of IMV was also observed [intervention median 51.4 hours (IQR 17.0–123.6 hours) vs. control median 55.2 hours (IQR 18.0–123.6 hours); adjusted median difference –7.1 hours (IQR –9.6 to –5.3 hours); aHR 1.11, 95% CI 1.03 to 1.20; p = 0.009] (Figures 5 and 6; Tables 4 and 5).
FIGURE 5.
Probability and time to successful extubation by observation period (prolonged IMV cohort). aHR, 1.1 (95% Cl 1.0 to 1.2); p = 0.2. Median difference –6.1 hours (IQR –8.2 to –5.3 hours).
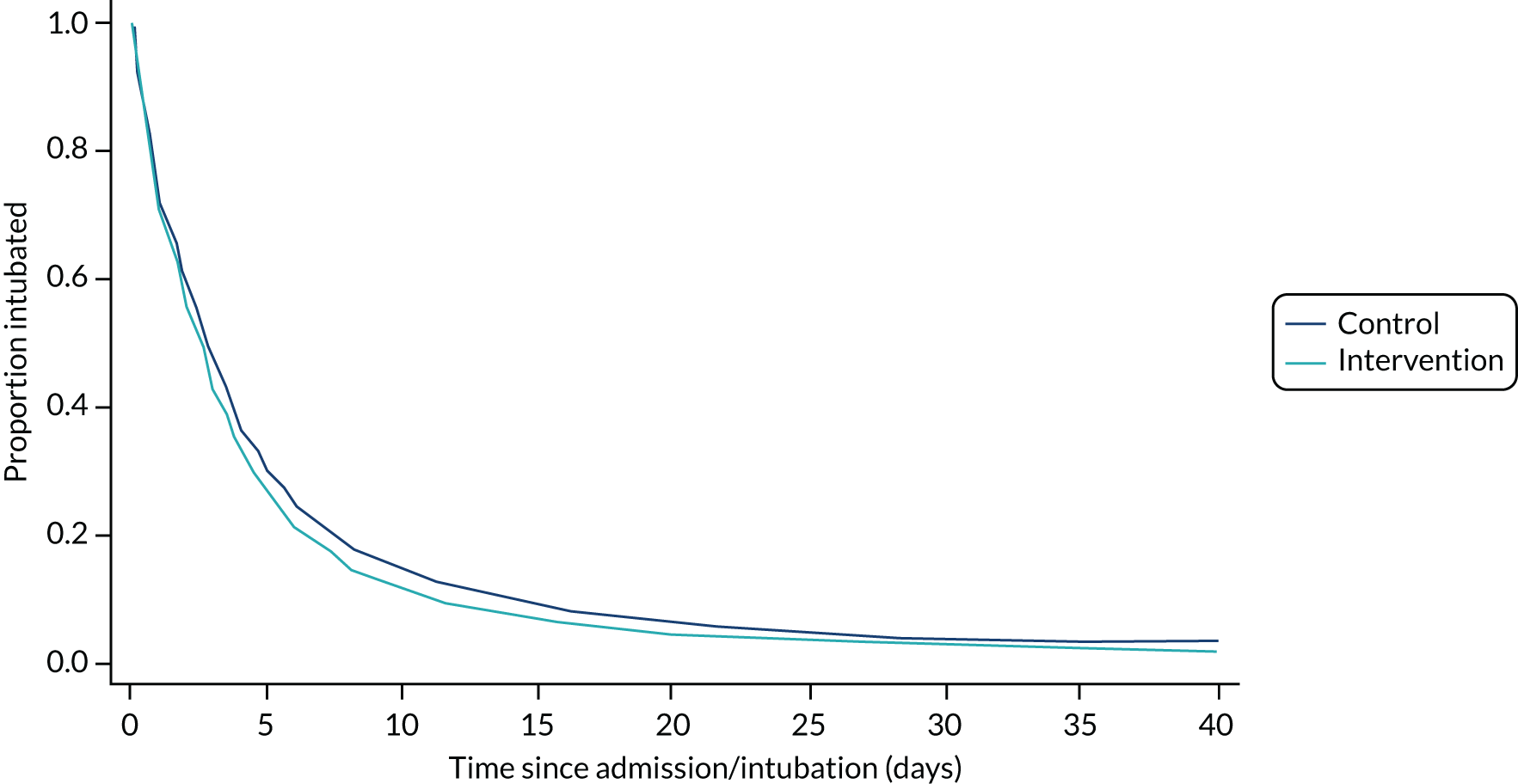
FIGURE 6.
Probability and time to successful extubation by observation period (all children). aHR, 1.1 (95% Cl 1.0 to 1.2); p = 0.01. Median difference –7.1 hours (IQR –9.6 to –5.3 hours).
| Outcome | Observation period | Adjusted analyses | |||||
|---|---|---|---|---|---|---|---|
| Control (N = 4155) | Intervention (N = 4688) | Absolute scale | Relative scale | ICC (95% CI) | |||
| Primary outcome | Median difference (IQR) | p-value | Hazard ratio (95% CI) | p-value | |||
| Duration of IMV until first successful extubation (hours),a median (IQR); n | 66.2 (21.8–138.0); 4144 | 64.8 (22.1–141.4); 4684 | –6.1 (–8.2 to –5.3) | 0.02 | 1.11 (1.02 to 1.20) | 0.02 | |
| Secondary outcome | |||||||
| Total duration of IMV (days),a median (IQR); n | 2.8 (0.9–5.9); 4144 | 2.7 (0.9–6.3); 4684 | –0.20 (–0.25 to –0.18) | 0.06 | 1.09 (1.00 to 1.18) | 0.06 | |
| Duration post-extubation NIV (days),a median (IQR); n | 2.1 (0.7–6.6); 556 | 1.8 (0.7–6.8); 805 | 0.22 (0.18–0.29) | 0.43 | 0.91 (0.72 to 1.15) | 0.43 | |
| Paediatric ICU length of stay (days), median (IQR); n | 5.0 (3.0–9.0); 4155) | 5.0 (3.0–10.0); 4688) | 0.00 (0.00–0.00) | 0.53 | 0.97 (0.90 to 1.06) | 0.53 | |
| Hospital length of stay (days), median (IQR); n | 9.1 (5.0–18.9); 3581) | 9.6 (5.0–19.8); 4010) | 0.91 (0.84–0.97) | 0.01 | 0.89 (0.81 to 0.97) | 0.01 | |
| Point difference (%) (95% CI) | p-value | Relative risk (95% CI) | p-value | ||||
| Successful extubation, n/N (%)b | 3788/3849 (98.4) | 4161/4222 (98.6) | 0.95 (–0.07 to 1.97) | 0.07 | 1.01 (1.00 to 1.02) | 0.03 | 0.001 (0.0001 to 0.013) |
| Unplanned extubation, n/N (%) | 107/4155 (2.6) | 142/4688 (3.0) | 0.98 (–0.32 to 2.27) | 0.14 | 1.62 (1.05 to 2.51) | 0.03 | 0.003 (0.001 to 0.008) |
| Reintubation, n/N (%)c | 507/4155 (12.2) | 544/4688 (11.6) | 0.83 (–1.70 to 3.37) | 0.52 | 1.10 (0.89 to 1.36) | 0.38 | 0.017 (0.008 to 0.038) |
| Post-extubation NIV, n/N (%) | 558/3886 (14.4) | 810/4285 (18.9) | 9.42 (4.30 to 14.54) | < 0.001 | 1.22 (1.01 to 1.49) | 0.04 | 0.050 (0.026 to 0.096) |
| Tracheostomy, n/N (%)d,e | 33/4155 (0.8) | 46/4688 (1.0) | –0.03 (–0.49 to 0.43) | 0.89 | 0.88 (0.36 to 2.17) | 0.79 | 0.004 (0.001 to 0.012) |
| Post-extubation stridor, n/N (%) | 356/4155 (8.6) | 419/4688 (8.9) | 3.05 (–1.71 to 7.80) | 0.21 | 0.94 (0.73 to 1.22) | 0.66 | 0.042 (0.021 to 0.082) |
| Paediatric ICU mortality, n/N (%) | 173/4154 (4.2) | 220/4682 (4.7) | 0.25 (–1.98 to 2.49) | 0.82 | 1.06 (0.73 to 1.54) | 0.75 | 0.007 (0.003 to 0.016) |
| Hospital mortality, n/N (%) | 200/3785 (5.3) | 268/4278 (6.3) | 0.82 (–1.96 to 3.61) | 0.56 | 1.15 (0.82 to 1.63) | 0.41 | 0.009 (0.004 to 0.020) |
| Outcome | Observation period | Adjusted analyses | |||||
|---|---|---|---|---|---|---|---|
| Control (N = 4849) | Intervention (N = 5646) | Absolute scale | Relative scale | ICC (95% CI) | |||
| Primary outcome | Median difference (IQR) | p-value | Hazard ratio (95% CI) | p-value | |||
| Duration of IMV until first successful extubation (hours),a median (IQR); n | 55.2 (18.0–123.6); 4837 | 51.4 (17.0–123.6); 5640 | –7.1 (–9.6 to –5.3) | 0.01 | 1.11 (1.03 to 1.20) | 0.01 | |
| Secondary outcomes | |||||||
| Total duration of IMV (days),a median (IQR); n | 2.4 (0.8–5.5); 4837 | 2.2 (0.7–5.5); 5640 | –0.28 (–0.33 to –0.20) | 0.03 | 1.09 (1.01 to 1.18) | 0.03 | |
| Duration post-extubation NIV (days),a median (IQR); n | 2.0 (0.7–6.3); 613 | 1.8 (0.7–6.5); 911 | 0.12 (0.10–0.16) | 0.67 | 0.95 (0.75 to 1.19) | 0.67 | |
| Paediatric ICU length of stay (days), median (IQR); n | 5.0 (3.0–9.0); 4849 | 5.0 (3.0–9.0); 5646 | 0.00 (0.00–0.00) | 0.83 | 0.99 (0.92 to 1.07) | 0.83 | |
| Hospital length of stay (days), median (IQR); n | 8.4 (4.9–17.6); 4236 | 8.4 (4.5–17.9); 4922 | 0.59 (0.41–0.79) | 0.02 | 0.91 (0.84 to 0.99) | 0.02 | |
| Point difference (%) (95% CI) | p-value | Relative risk (95% CI) | p-value | ICC (95% CI) | |||
| Successful extubation, n/N (%)b | 4466/4530 (98.6) | 5092/5163 (98.6) | 0.87 (–0.14 to 1.89) | 0.09 | 1.01 (1.00 to 1.02) | 0.07 | 0.001 (0.0002 to 0.007) |
| Unplanned extubation, n/N (%) | 123/4849 (2.5) | 167/5646 (3.0) | 0.85 (–0.36 to 2.07) | 0.17 | 1.58 (1.05 to 2.37) | 0.03 | 0.002 (0.001 to 0.007) |
| Reintubation, n/N (%)c | 551/4849 (11.4) | 600/5646 (10.6) | –0.11 (–3.16 to 2.94) | 0.95 | 1.09 (0.89 to 1.33) | 0.42 | 0.011 (0.005 to 0.026) |
| Post-extubation NIV, n/N (%) | 616/4570 (13.5) | 916/5226 (17.5) | 8.19 (3.53 to 12.84) | 0.001 | 1.22 (1.01 to 1.49) | 0.04 | 0.040 (0.021 to 0.078) |
| Tracheostomy, n/N (%)d,e | 34/4849 (0.7) | 48/5646 (0.9) | 0.17 (–0.21 to 0.54) | 0.38 | 0.84 (0.34 to 2.07) | 0.71 | 0.004 (0.001 to 0.011) |
| Post-extubation stridor, n/N (%) | 423/4849 (8.7) | 512/5646 (9.1) | 2.88 (–2.21 to 7.97) | 0.27 | 0.91 (0.72 to 1.16) | 0.45 | 0.045 (0.023 to 0.085) |
| Paediatric ICU mortality, n/N (%) | 186/4848 (3.8) | 230/5639 (4.1) | 0.00 (–2.16 to 2.16) | 1.00 | 1.01 (0.70 to 1.46) | 0.94 | 0.007 (0.003 to 0.015) |
| Hospital mortality, n/N (%) | 213/4454 (4.8) | 282/5204 (5.4) | 0.44 (–2.38 to 3.25) | 0.76 | 1.13 (0.80 to 1.58) | 0.49 | 0.009 (0.004 to 0.020) |
A secondary analysis adjusting for prespecified covariates was broadly supportive of the primary result (prolonged IMV cohort: aHR 1.07, 95% CI 0.98 to 1.16, p = 0.13; all children: aHR 1.06, 95% CI 0.98 to 1.14; p = 0.17).
Secondary outcomes
In the prolonged IMV cohort, the incidence of successful extubation was higher in the intervention period (aRR 1.01, 95% CI 1.00 to 1.02; p = 0.03) than in the control period and the total duration of IMV was shorter [intervention median 2.7 hours (IQR 0.9–6.3 hours) vs. control median 2.8 hours (IQR 0.9–5.9 hours); adjusted median difference –0.20 hours (IQR –0.25 to –0.18 hours); aHR 1.09, 95% CI 1.00 to 1.18; p = 0.06]. The post-extubation incidence of NIV was higher in the intervention period than in the control period (aRR 1.22, 95% CI 1.01 to 1.49; p = 0.04), with no significant difference in the duration of NIV [intervention median 1.8 hours (IQR 0.7–6.8 hours) vs. control median 2.1 hours (IQR 0.7–6.6 hours); adjusted median difference 0.22 hours (IQR 0.18–0.29 hours); aHR 0.91, 95% CI 0.72 to 1.15; p = 0.43]. The PICU length of stay was not significantly different [both periods median 5.0 days (IQR 3.0–9.0 days); aHR 0.97, 95% CI 0.90 to 1.06; p = 0.53], but there was a longer hospital length of stay in the intervention period [intervention median 9.6 days (IQR 5.0–19.8 days)] than the control period [control median 9.1 days (IQR 5.0–18.9 days)] [adjusted median difference 0.91 days (IQR 0.84–0.97 days); aHR 0.89, 95% CI 0.81 to 0.97; p = 0.01). The SANDWICH intervention resulted in a higher incidence of unplanned extubation (aRR 1.62, 95% CI 1.05 to 2.51; p = 0.03), but there was no evidence of a difference in reintubation (aRR 1.10, 95% CI 0.89 to 1.36; p = 0.38) (see Tables 4 and 5).
In all children, there was no evidence of a difference in the incidence of successful extubation between observation periods (aRR 1.01, 95% CI 1.00 to 1.02; p = 0.07), but the total duration of IMV was significantly shorter in the intervention period than in the control period [intervention median 2.2 hours (IQR 0.7–5.5 hours) vs. control median 2.4 hours (IQR 0.8–5.5 hours); adjusted median difference –0.28 hours (IQR –0.33 to –0.20 hours); aHR 1.09, 95% CI 1.01 to 1.18; p = 0.03]. There was a significant increase in the post-extubation incidence of NIV (aRR 1.22, 95% CI 1.01 to 1.49; p = 0.04), but no statistically significant difference in the duration of NIV [intervention median 1.8 hours (IQR 0.7–6.5 hours) vs. control median 2.0 hours (IQR 0.7–6.3 hours); adjusted median difference 0.12 hours (IQR 0.10–0.16 hours); aHR 0.95, 95% CI 0.75 to 1.19; p = 0.67]. The PICU length of stay was not significantly different [both periods median 5.0 days (IQR 3.0–9.0 days); aHR 0.99, 95% CI 0.92 to 1.07; p = 0.83], but hospital length of stay was significantly longer in the intervention period [median 8.4 days (IQR 4.5–17.9 days)] than in the control period [median 8.4 days (IQR 4.9–17.6 days)] [adjusted median difference 0.59 days (IQR 0.41–0.79 days); aHR 0.91, 95% CI 0.84 to 0.99; p = 0.02]. The incidence of unplanned extubation was higher in the intervention period than in the control period (aRR 1.58, 95% CI 1.05 to 2.37; p = 0.03), but there was no significant difference in the risk of reintubation (aRR 1.09, 95% CI 0.89 to 1.33; p = 0.42) (see Tables 4 and 5).
In relation to other patient safety outcomes, there were no statistically significant differences between observation periods in the risk of tracheostomy insertions, post-extubation stridor or mortality in PICU or hospital for both patient cohorts. There were also no significant differences in AEs or SAEs (Table 6).
| Event causality assessment and category | Number of events, n (%) | Number of patients, n (%) | RR (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Total, n | Observation period | Total | Observation period | |||||
| Control | Intervention | Control | Intervention | |||||
| Serious adverse events | ||||||||
| Total | 47 | 26 (55.3) | 21 (44.7) | 44 (0.4) | 24 (0.5) | 20 (0.3) | ||
| Relateda | 3 | 0 (0) | 3 (100) | 3 (0.03) | 0 (0) | 3 (0.1) | ||
| Category | ||||||||
| Cardiovascular | 5 | 2 (40.0) | 3 (60.0) | 5 (0.05) | 2 (0.04) | 3 (0.1) | ||
| Dislodgement (non-vascular) | 11 | 7 (63.6) | 4 (36.4) | 8 (0.1) | 5 (0.1) | 3 (0.1) | ||
| Dislodgement (vascular) | 4 | 3 (75.0) | 1 (25.0) | 4 (0.04) | 3 (0.1) | 1 (0.02) | ||
| Other | 1 | 0 (0) | 1 (100) | 1 (0.01) | 0 (0) | 1 (0.02) | ||
| Respiratoryb | 21 | 11 (52.4) | 10 (47.6) | 21 (0.2) | 11 (0.2) | 10 (0.2) | ||
| Thromboembolic | 5 | 3 (60.0) | 2 (40.0) | 5 (0.1) | 3 (0.1) | 2 (0.04) | ||
| Adverse events | ||||||||
| Total | 305 | 177 (58.0) | 128 (42.0) | 242 (2.3) | 146 (3.0) | 96 (1.7) | 1.6 (0.6 to 4.2) | 0.330 |
| Related | 18 | 2 (11.1) | 16 (88.9) | 16 (0.1) | 2 (0.04) | 14 (0.2) | ||
| Related and unexpected | 1 | 0 (0) | 1 (100) | 1 (0.01) | 0 (0) | 1 (0.02) | ||
| Category | ||||||||
| Allergy | 2 | 2 (100) | 0 (0) | 2 (0.02) | 2 (0.04) | 0 (0) | ||
| Cardiovascular | 12 | 8 (66.7) | 4 (33.3) | 12 (0.1) | 8 (0.2) | 4 (0.1) | ||
| Dislodgement (non-vascular) | 75 | 41 (54.7) | 34 (45.3) | 57 (0.5) | 31 (0.6) | 26 (0.5) | 3.2 (0.4 to 29.6) | 0.290 |
| Dislodgement (vascular) | 114 | 52 (45.6) | 62 (54.4) | 99 (0.9) | 51 (1.0) | 48 (0.8) | 2.13 (0.5 to 9.5) | 0.320 |
| Infection | 6 | 6 (100) | 0 (0) | 6 (0.1) | 6 (0.1) | 0 (0) | ||
| Metabolic | 5 | 5 (100) | 0 (0) | 5 (0.05) | 5 (0.1) | 0 (0) | ||
| Neurological | 6 | 6 (100) | 0 (0) | 6 (0.1) | 6 (0.1) | 0 (0) | ||
| Other | 3 | 2 (66.7) | 1 (33.3) | 3 (0.03) | 2 (0.04) | 1 (0.02) | ||
| Respiratoryb | 71 | 50 (70.4) | 21 (29.6) | 64 (0.6) | 43 (0.9) | 21 (0.4) | 0.6 (0.1 to 2.3) | 0.453 |
| Skin/mucus membranes | 8 | 2 (25.0) | 6 (75.0) | 8 (0.1) | 2 (0.04) | 6 (0.1) | ||
| Thromboembolic | 5 | 3 (60.0) | 2 (40.0) | 5 (0.1) | 3 (0.1) | 2 (0.04) | ||
Adverse events
There were 305 AEs recorded in 242 admissions (2.3% of all admissions), of which 47 (15.4%) were SAEs involving 44 admissions (0.4% of all admissions). Owing to the low event rates, most models would not converge to enable calculation of the relative risks and 95% CIs. The models converged only for total AEs, non-vascular and vascular dislodgements, and respiratory AEs. There were no statistically significant differences in the AE incidence rates (see Table 6).
Subgroup analyses of the primary outcome
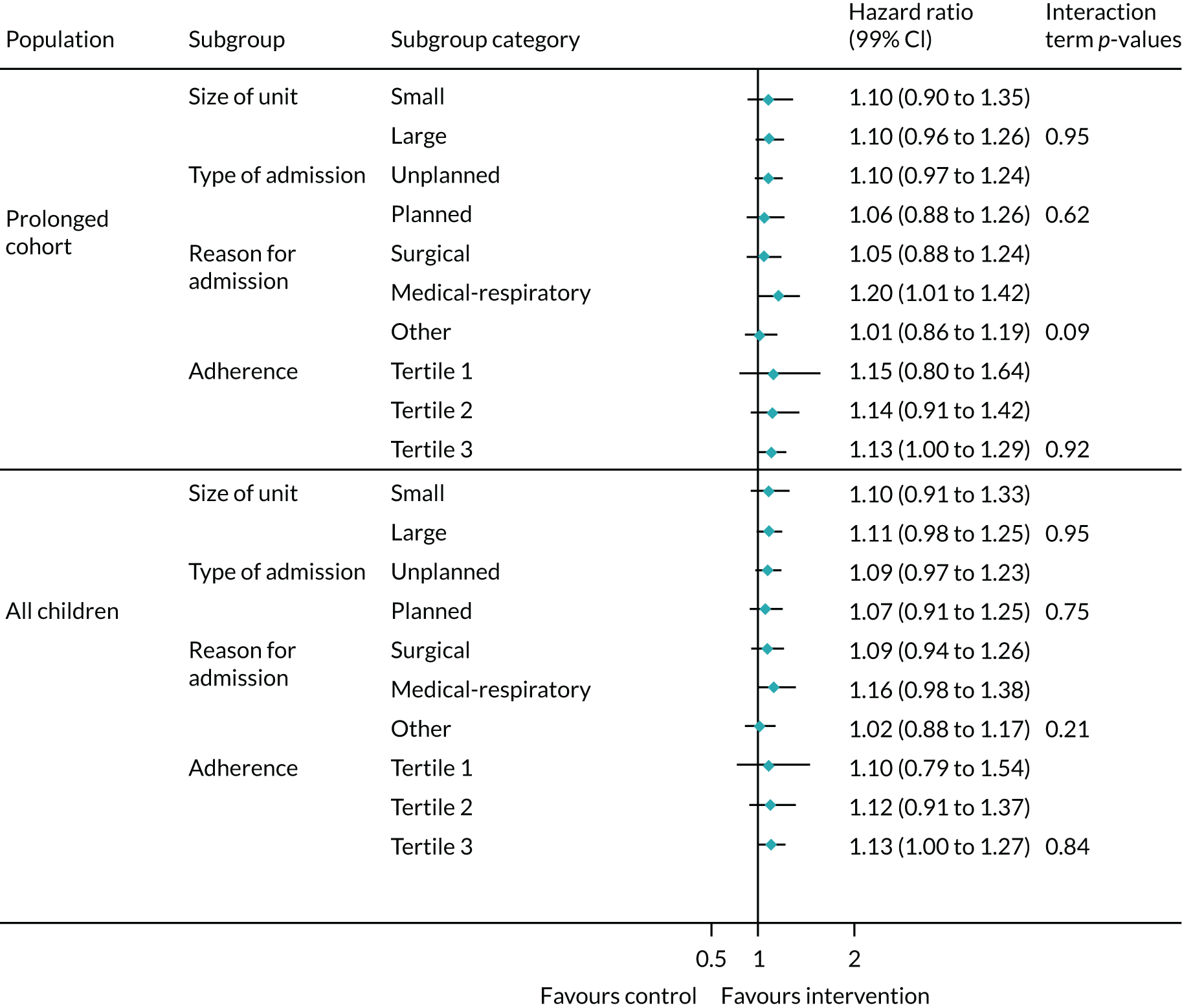
Exploratory subgroup analyses for the duration of IMV until successful extubation showed no significant interactions in the prespecified subgroups based on size of unit, type of admission, reason for admission and adherence to the intervention (Figure 7).
FIGURE 7.
Subgroup analysis for the primary outcome.
Sensitivity analyses
The impact of the model-based assumptions on the estimates for the binary secondary outcomes was explored (see Appendix 1). These binary models were fitted using proc glimmix in SAS because Stata does not accommodate small-sample corrections for binary outcomes and does not allow for correlation structures other than exchangeable. This sensitivity analysis showed very little difference between the more complex correlation structures and the exchangeable correlation structure, indicating that the sensitivity analyses broadly matched the original analyses. These results are available in Report Supplementary Material 1.
Clinical outcomes
The baseline ventilation parameters during the control and intervention periods were similar (Table 7).
| Ventilation parameter | Observation period, mean (SD); n | |
|---|---|---|
| Control | Intervention | |
| Ventilator rate | ||
| Prolonged IMV cohort | 25.5 (8.2); 3265 | 26.5 (8.6); 3425 |
| All children | 25.1 (8.2); 3637 | 26.0 (8.6); 3882 |
| FiO2 | ||
| Prolonged IMV cohort | 0.4 (0.2); 3525 | 0.37 (0.2); 4028 |
| All children | 0.4 (0.2); 3932 | 0.36 (0.2); 4591 |
| PIP | ||
| Prolonged IMV cohort | 19.1 (4.7); 3290 | 19.1 (4.8); 3719 |
| All children | 18.9 (4.6); 3680 | 18.8 (4.8); 4250 |
| PEEP | ||
| Prolonged IMV cohort | 6.0 (1.5); 3435 | 5.9 (1.4); 3858 |
| All children | 6.0 (1.5); 3840 | 5.8 (1.4); 4414 |
| Tidal volume | ||
| Prolonged IMV cohort | 96.4 (107.7); 1056 | 95.0 (106.3); 880 |
| All children | 99.0 (108.5); 1160 | 98.6 (108.1); 984 |
| Level of pressure support above PEEP | ||
| Prolonged IMV cohort | 11.6 (3.4); 2780 | 11.22 (4.1); 3478 |
| All children | 11.5 (3.3); 3086 | 11.06 (4.1); 3983 |
The ventilation parameters immediately before the SBT in the intervention group and 2 hours before extubation in the control period showed no clinically important differences in FiO2, ventilator rate, tidal volume, PIP, PEEP or Psupp (Table 8).
| Ventilation parameter prior to extubation | Observation period, mean (SD); n | Mean difference (95% CI); p-value | |
|---|---|---|---|
| Control | Intervention | ||
| Ventilator rate | |||
| Prolonged IMV cohort | 16.8 (7.9); 2500 | 16.4 (7.7); 1897 | 0.29 (–0.54 to 1.11); 0.5 |
| All children | 16.9 (7.8); 2981 | 16.5 (7.5); 2250 | 0.29 (–0.47 to 1.04); 0.5 |
| FiO2 | |||
| Prolonged IMV cohort | 0.3 (0.1); 4315 | 0.3 (0.1); 2747 | –0.01 (–0.02 to –0.003); 0.01 |
| All children | 0.3 (0.1); 5042 | 0.3 (0.1); 3230 | –0.01 (–0.02 to –0.01); 0.001 |
| PIP | |||
| Prolonged IMV cohort | 14.7 (3.7); 3485 | 14.8 (3.2); 2528 | 0.05 (–0.28 to 0.38); 0.76 |
| All children | 14.7 (3.7); 4120 | 14.8 (3.2); 2974 | 0.03 (–0.28 to 0.33); 0.86 |
| PEEP | |||
| Prolonged IMV cohort | 5.5 (1.0); 4256 | 5.4 (1.0); 2715 | –0.11 (–0.20 to –0.02); 0.01 |
| All children | 5.5 (1.0); 4975 | 5.4 (1.0); 3184 | –0.07 (–0.15 to 0.01); 0.08 |
| Tidal volume | |||
| Prolonged IMV cohort | 105.5 (113.9); 634 | 87.9 (104.1); 333 | 7.71 (–21.38 to 36.80); 0.60 |
| All children | 110.8 (113.9); 767 | 91.1 (105.0); 380 | 2.83 (–24.61 to 30.26); 0.84 |
| Pressure support above PEEP | |||
| Prolonged IMV cohort | 8.2 (2.8); 3383 | 8.0 (2.7); 2581 | –0.09 (–0.35 to 0.18); 0.52 |
| All children | 8.3 (2.8); 3916 | 8.1 (2.8); 3029 | –0.15 (–0.41 to 0.10); 0.23 |
Chapter 5 Process evaluation: methods and results
Introduction
The SANDWICH intervention was complex in that it included a number of inter-related and interdependent components, all of which were dependent on the uptake and adherence of unit-wide staff. Adding to that complexity, the intervention was tested in 18 PICUs with variable characteristics, such as unit size, patient population, staff skill set and routines of working. Such contextual factors had the potential to affect how the intervention was implemented, received and delivered across the PICUs. 50 In such circumstances, it can be difficult to differentiate between intervention failure and implementation failure. To understand how the context into which the intervention was introduced influenced its delivery, we undertook a process evaluation. The insights gained were subsequently used to help explain trial outcome data, focusing on barriers to and facilitators of intervention effectiveness.
Aims and objectives
The aim of the process evaluation was to explore the processes involved in delivering the intervention to identify factors and the mechanisms of their interaction likely to have an impact on trial outcomes. The objectives were to:
-
establish the extent to which the intervention was implemented as intended (implementation fidelity) over time and across different PICUs
-
ascertain how participants received (i.e. understood and responded to) the intervention over time and across different PICUs
-
explore the context over time and across different PICUs and determine factors (including managerial support, economic, organisational and work level) that affected implementation.
Process evaluation methods
We used the Medical Research Council expert guidance on the conduct of process evaluations51 to plan, design, conduct, analyse and report the process evaluation. The guidance outlines the functions of each of these stages, highlights core issues to be addressed and makes key recommendations in respect of both. To maintain impartiality, all aspects of the process evaluation were conducted by a researcher (JJ) who was not a member of the clinical trial team. Their independence was made clear to unit staff when undertaking data collection at each of the sites to provide reassurance and promote transparency.
Logic model
We developed a logic model for the SANDWICH intervention, which made explicit, in diagrammatical form, the theory (i.e. causal assumptions) underpinning the intervention. It set out the main components of the intervention, how the components were envisaged to work together to achieve trial outcomes and longer-term impact, and the resources made available to promote successful implementation and delivery. The assumptions set out in the logic model were subsequently investigated in interviews with unit staff as a means of evaluating their validity (see Figure 1).
Design
A mixed-methods evaluation was conducted throughout the trial, with quantitative and qualitative data collected at baseline, during the intervention period and at the end of the trial.
Quantitative data collection methods
Quantitative data were collected using several data collection documents. During the control period, immediately prior to the PICU staff entering the training period, a structured questionnaire was used to capture unit characteristics and usual organisational routines. Following the training period, training completion data were collected from the LearnPro database at 8 and 12 weeks. During the intervention period, data were collected from the CRF, recruitment logs and PICANet database. These data were used to determine the fidelity (the degree to which the intervention components were implemented as planned), dose (how much of the intended intervention was delivered) and reach (what proportion of eligible patients actually received the intervention) of the SANDWICH intervention.
Qualitative data collection methods
Qualitative data were collected by semistructured interviews; these were face-to-face individual or focus group interviews and informal telephone discussions. Face-to-face interviews lasted between 30 and 90 minutes and took place in a quiet room near the PICU. All interviews were audio-recorded and subsequently transcribed. Telephone calls lasted between 10 and 50 minutes; comprehensive notes were made of all discussions. The audio files, transcripts and telephone notes were stored on password-protected computer folders, as well as on the Queen’s University Belfast password-protected server. Participants’ identities were protected by assigning participant IDs and removing personal identifiers from transcripts and notes.
At baseline
Baseline interviews focused on usual unit sedation and weaning practice and other relevant issues. The data collection preceded the training period to avoid potential contamination. Informed by the logic model, the interviews explored:
-
current unit policy and practice regarding sedation management (e.g. staff roles and responsibilities, consistency of practice and role of ward round discussion)
-
current unit policy and practice regarding ventilator weaning
-
unit and hospital culture regarding change management.
These data provided important insights into local-level contextual factors that had the potential to influence trial implementation and delivery (see Appendix 2, Baseline visits interview guide).
During the intervention period
Regular informal (approximately every 6–8 weeks) telephone contact was maintained throughout the intervention phase with the unit research teams (research nurses and/or PIs) concerning the trial progress and issues arising. Conversations were guided, but participants were encouraged to raise any issues that they considered relevant. The data from these telephone discussions provided valuable stand-alone information about ongoing trial delivery, including adherence to the protocol. They were also used to identify unit-specific issues for follow-up during subsequent conversations and end-of-trial interviews with clinical staff.
End of the trial
On-site semistructured individual interviews and focus groups were held to discuss staff understanding and experience of trial implementation and delivery. Informed by the logic model, the interviews explored:
-
staff receipt of the intervention in relation to both the protocol and the supporting resources, such as the training provided
-
fidelity of intervention delivery
-
impact of contextual factors on trial implementation and delivery (barriers to and facilitators of, such as extent/nature of multidisciplinary working on the unit, workload pressures and unit routines of working)
-
wider impact of trial participation.
The participants were encouraged to consider both positive and negative aspects of trial implementation and delivery, including the nature of the intervention. To avoid potential contamination, all data collection preceded knowledge of trial outcomes (see Appendix 2, End of trial interview guide).
Participants
Participants were the participating PICU staff. They included the PI, the dedicated SANDWICH research nurse, other relevant research nurse support, the SANDWICH champions and a range of professional grades for medical, nursing and physiotherapy staff. Staff were excluded if they had not worked clinically to experience using the SANDWICH intervention.
Recruitment and consent
The selection and recruitment of participants was organised within the PICU in consultation with the SANDWICH nurse or PI. Participants were purposively selected from a convenience sample of staff who were available on the days that the researcher was visiting the unit. Recruitment entailed an initial e-mail from the researcher, comprising an introduction and the participant information sheet outlining the purpose of the interview, information on likely areas of discussion and details of participation (see Appendix 2). This was distributed to staff who were on rota on the days of the visits. Prior to the interview, participants were given an opportunity to read the participant information sheet again and ask questions. Once satisfied that informed consent had been completed, participants were asked to sign the consent form (see Appendix 2).
Data analysis
A framework-based approach was used to analyse the quantitative and qualitative data. This included a strategic focus on the four key components of the intervention and the resources, particularly the training, that were provided to support its delivery. Subsequently, patterns and trends (including exceptions) characterising the factors and processes that affected the receipt, delivery and impact of the intervention components and resources were identified. These two requirements meant that the analysis included both deductive and inductive components.
Deductively, we used the a priori identified categories (key intervention components). Adherence to these components was measured by (1) the proportion of the four intervention components performed, captured daily in the study CRF; (2) the proportion of staff trained by 8 weeks; and (3) the intervention reach, measured by the proportion of admissions screened (recruits and exclusions) divided by IMV admissions over the trial period. The average adherence proportions for each PICU were ranked and divided into tertiles and a prespecified subgroup analysis investigated the impact of adherence on the primary outcome.
Inductively, we used Braun and Clarke’s52 thematic content analysis to generate a set of themes that cross-cut the entirety of the qualitative data set. 52 (Box 2).
Repeated reading of transcripts enabled detailed familiarisation with their content.
Generating initial codesBased on this reading, specific portions of text were assigned a code, which reflected ‘semantic content’, that is the explicit/overt meaning of participants’ responses.
Search for themesThe relationship (similarities and differences) between these initial codes was considered during subsequent rounds of analysis, during which codes could be lost or amended, or new ones created, as their content and meaning were compared in relation to one another and to the data set in its entirety. This extended process brought different codes together to form a ‘candidate’ thematic framework.
Reviewing themesThe candidate framework was refined, involving a moving back and forth between the codes and the themes in which they were embedded and the themes themselves. At this stage, embryonic themes were amended as a final set was developed. A concluding re-reading of the entire data set ensured that these themes adequately accounted for all data.
Defining and naming themesEach of the identified themes was appropriately labelled and explained. Labels reflected the essential meaning and were the basis of a narrative, which made explicit the theme that was addressed/captured, including through the use of relevant quotations.
Producing the reportThe analysis acted as the basis of empirically informed arguments concerning the implications of the findings for understanding the factors and processes involved in trial implementation and delivery as these had an impact on trial outcomes.
Individual and focus group interview data were treated as comparable, an approach regularly adopted in empirical research. 53,54 The primary responsibility for qualitative analysis lay with Joanne Jordan. To ensure confirmability and trustworthiness, a 15% sample of the data was analysed by an independent qualitative researcher (Dr Claire Kydonaki, Edinburgh Napier University) to identify key differences and similarities in pursuit of an agreed final analysis. 55
Results
Delivery of the intervention
In total, 1865 out of 2247 eligible PICU clinical staff members completed the online training within 8 weeks. The median percentage completion across PICUs was 85% (IQR 80–90%), with 15 out of 18 (83.3%) PICUs achieving the 80% prespecified minimum target. By 12 weeks, 1955 out of 2247 staff members were trained (median 88%, IQR 80–90%) and all but one PICU achieved the minimum target (Table 9).
| Centre ID | 8 weeks, n (%) | 12 weeks, n (%) | ||
|---|---|---|---|---|
| Total | Trained | Total | Trained | |
| S08/09 | 369 | 346 (94) | 372 | 335 (90) |
| S03 | 309 | 183 (59) | 282 | 223 (79) |
| S01 | 213 | 191 (90) | 213 | 192 (90) |
| S04 | 142 | 129 (91) | 142 | 129 (91) |
| S14 | 132 | 114 (86) | 132 | 114 (86) |
| S13 | 115 | 97 (84) | 115 | 103 (90) |
| S18 | 108 | 92 (85) | 111 | 98 (88) |
| S11 | 116 | 96 (83) | 116 | 97 (84) |
| S05 | 108 | 87 (81) | 108 | 87 (81) |
| S02 | 88 | 70 (80) | 88 | 70 (80) |
| S12 | 96 | 83 (86) | 96 | 83 (86) |
| S15 | 82 | 62 (76) | 80 | 70 (88) |
| S06 | 84 | 76 (90) | 96 | 92 (96) |
| S16 | 83 | 68 (82) | 94 | 84 (89) |
| S10 | 78 | 61 (78) | 78 | 68 (87) |
| S07 | 71 | 57 (80) | 71 | 57 (80) |
| S17 | 53 | 53 (100) | 53 | 53 (100) |
| Total | 2247 | 1865 (83) | 2247 | 1955 (87) |
Across PICUs, the intervention reached a high proportion of patients (median 82%, IQR 77–89%). The range of percentage adherence to the intervention components across the PICUs was high for sedation assessment (median 83%, IQR 82–91%), setting targets at ward round for sedation level (median 85%, IQR 63–89%) and ventilation support (median 90%, IQR 81–96%). Adherence was moderate for daily screening of readiness for a SBT (median 74%, IQR 66–83%) and lower for undertaking a SBT when criteria were met (median 40%, IQR 31–51%) (Table 10).
| PICU ID | COMFORT assessed | COMFORT target | Ventilation target | SBT criteria daily | SBT when criteria met | Trained at 8 weeks | Reach | Average | Rank |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 84.1 | 89.1 | 91.6 | 66.0 | 48.4 | 80.0 | 92.5 | 78.8 | 1 |
| 9 | 86.6 | 87.4 | 92.7 | 56.8 | 40.6 | 94.0 | 92.5 | 78.7 | 1 |
| 15 | 96.9 | 96.5 | 98.4 | 63.3 | 51.9 | 76.0 | 76.7 | 80.0 | 1 |
| 16 | 93.5 | 98.3 | 99.1 | 81.5 | 54.8 | 82.0 | 88.6 | 85.4 | 1 |
| 17 | 88.0 | 92.0 | 96.0 | 70.8 | 60.0 | 100.0 | 82.3 | 84.2 | 1 |
| 18 | 90.9 | 93.5 | 95.9 | 84.2 | 53.0 | 85.0 | 74.5 | 82.4 | 1 |
| 1 | 82.0 | 87.4 | 87.3 | 89.9 | 21.6 | 90.0 | 88.3 | 78.1 | 2 |
| 5 | 90.7 | 87.1 | 81.4 | 54.0 | 32.0 | 81.0 | 100 | 75.2 | 2 |
| 6 | 69.8 | 65.2 | 95.0 | 81.0 | 50.6 | 90.0 | 82.3 | 76.3 | 2 |
| 8 | 82.8 | 75.6 | 87.2 | 68.4 | 37.8 | 94.0 | 80.7 | 75.2 | 2 |
| 12 | 83.1 | 82.4 | 82.6 | 72.5 | 33.1 | 86.0 | 78.0 | 74.0 | 2 |
| 13 | 81.6 | 89.3 | 91.8 | 74.5 | 38.5 | 84.0 | 86.8 | 78.1 | 2 |
| 3 | 81.8 | 53.7 | 56.8 | 83.4 | 15.0 | 59.0 | 85.3 | 62.1 | 3 |
| 4 | 79.3 | 62.5 | 88.0 | 61.3 | 45.4 | 91.0 | 89.5 | 73.9 | 3 |
| 7 | 77.5 | 63.2 | 96.3 | 76.1 | 19.8 | 80.0 | 75.3 | 69.7 | 3 |
| 10 | 94.0 | 34.8 | 34.8 | 92.5 | 44.7 | 78.0 | 72.6 | 64.5 | 3 |
| 11 | 75.8 | 77.5 | 73.6 | 86.8 | 29.5 | 83.0 | 71.9 | 71.2 | 3 |
| 14 | 83.2 | 39.1 | 28.1 | 66.8 | 30.8 | 86.0 | 80.1 | 59.2 | 3 |
Documented reasons for not progressing to undertake a SBT were mainly airway protection (24.5%), low consciousness (14.7%), expected to return to theatre (13.9%), high haemodynamic support (9.9%) and non-adherence (9.7%) (Table 11).
| Reason | Total (N = 11,114), n (%) |
|---|---|
| Airway protection reasons: secretion, oedema | 2717 (24.5) |
| Expected return to theatre | 1545 (13.9) |
| Low consciousness: sedation or neurological | 1631 (14.7) |
| High haemodynamic support | 1100 (9.9) |
| Neuromuscular weakness | 432 (3.9) |
| Too late in the evening | 351 (3.2) |
| Limited staff resources | 210 (1.9) |
| Other reasons | |
| Non-adherence | 1072 (9.7) |
| Child’s condition | 650 (5.9) |
| Awaiting external specialist review | 106 (1.0) |
| Prioritising weight gain | 106 (1.0) |
| Awaiting hospital transfer | 56 (0.5) |
| LTV or palliative care | 47 (0.4) |
| Self-extubated prior to planned SBT | 39 (0.4) |
| Awaiting further investigation | 36 (0.3) |
| No reason provided | 941 (8.5) |
| Unobtainable | 75 (0.7) |
Documented reasons for not progressing to extubation following a successful SBT were mainly airway protection (23.7%), low consciousness (17.5%) and limited staff resources (10.6%) (Table 12).
| Reason | Total (N = 1437), n (%) |
|---|---|
| Airway protection reasons: secretion, oedema | 341 (23.7) |
| Low consciousness: sedation or neurological | 251 (17.5) |
| Expected return to theatre | 177 (12.3) |
| Limited staff resource | 153 (10.7) |
| Neuromuscular weakness | 45 (3.1) |
| Too late in the evening | 137 (9.5) |
| High haemodynamic support | 27 (1.9) |
| Other reasons | |
| Non-adherence | 90 (6.3) |
| Child’s condition | 87 (6.1) |
| Awaiting external specialist review | 25 (1.7) |
| Awaiting further tests | 10 (0.7) |
| Incomplete fasting period | 7 (0.5) |
| Awaiting hospital transfer | 6 (0.4) |
| Palliative care | 3 (0.2) |
| Self-extubation | 3 (0.2) |
| No reason provided | 75 (5.2) |
Baseline questionnaires
Baseline data on participating PICUs were recorded to provide contextual information on PICU organisation and usual practices that might influence intervention implementation. The types of PICU were mainly general (n = 10, 56%), a mix of general and cardiac (n = 5, 28%), cardiac (n = 1), cardiac with respiratory (n = 1) and general with neonatal (n = 1). The median number of funded beds was 11.5 (IQR 8–17), with a range from 6 to 29.5. Sedation assessment was undertaken in the majority of PICUs (n = 15, 83.3%): assessment periods varied. In all PICUs, the PICU consultant was primarily responsible for ventilator weaning decisions. Typically, weaning involved a slow reduction in ventilator support to very low levels of support prior to extubation. Very few PICUs (n = 3, 16.7%) used a weaning protocol or a sedation protocol (n = 3, 16.7%); no PICUs used both. No formal criteria or SBTs were used to assess readiness for ventilator liberation. Bedside nurses had no formal role in the weaning process. Table 13 details baseline contextual data relevant to the intervention.
| Intervention context factors | No, n (%) | Yes, n (%) | Yes, with considerations, n (%) |
|---|---|---|---|
| Ward round checklist | 9 (50.0) | 9 (50) | – |
| Sedation protocol | 15 (83.3) | 3 (16.7) | – |
| COMFORT sedation tool | 3 (16.7) | 13 (72) | 2 (11.1) non-validated tool |
| Ventilation weaning protocol | 15 (83.3) | 3 (16.7) | – |
| Electronic health record system | 4 (22.2) | 10 (55.6) | 4 (22.2) combination |
| Advanced nurse practitioners | 7 (38.9) | 10 (55.6) | 1 (5.6) in training |
Participants
Research teams made determined efforts to recruit relevant staff to reflect a variety of professional groups and experience. At times this proved difficult because of (changing) staff availability on the day(s) when the interviews were scheduled, resulting in considerable variation in the number and range of staff interviewed across units at baseline and at the end of the trial. In total, there were 378 participants involved in interviews at baseline and towards the end of the trial recruitment period. Interviews were conducted with 187 participants at baseline and 193 participants towards the end of the trial. Participants may have been interviewed on one or both occasions. Figures 8 and 9 show the numbers and disciplines of interviewees.
FIGURE 8.
Number and disciplines of participants interviewed at the baseline interviews.
FIGURE 9.
Number and disciplines of participants interviewed at the end-of-trial interviews.
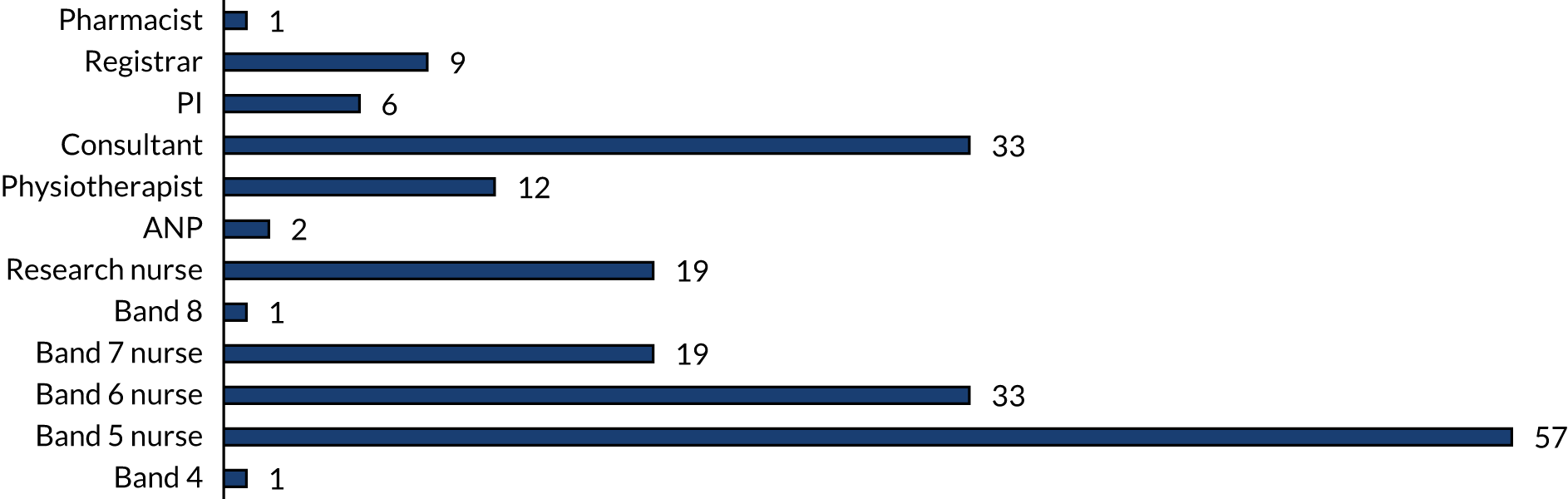
Final interview findings
The information obtained during the baseline interviews informed the end-of-trial interviews and is reflected in the final interview findings; for this reason, these findings are not presented separately. This section provides the overall qualitative results sectioned by the process evaluation objectives.
Objective 1: to establish the extent to which the intervention was implemented as intended (implementation fidelity) over time and across different paediatric intensive care units
Sedation management using COMFORT scoring
Although a few participants talked about limited adherence to COMFORT scoring, descriptions suggested at least reasonable, sometimes good, adherence. Overall, compliance was considered to have improved over time as bedside nurses became increasingly familiar with the use of COMFORT scoring.
Overall, bedside nurses described being able to easily incorporate COMFORT scoring into their routine patient assessments; it tended to become part and parcel of standard practice. Any lack of adherence was associated with two main issues: first, a vulnerability to the prioritisation of other caring responsibilities, so that COMFORT scoring was simply overlooked; and, second, a more fundamental disinclination, so that, even when a bedside nurse was looking after a relatively well patient, COMFORT scoring was not carried out and/or acted on. At times, such failure was associated with a preference for patients to be kept relatively heavily sedated as a means of avoiding risk:
For me, it wasn’t an issue. However, there may have been times on very busy days that I . . . maybe didn’t do it as frequent as I ought to, but as the time went by I think I made a bigger effort to try and fulfil the requirements . . .
Bedside nurse, S01
At times, a perceived lack of practical application undermined the adherence of bedside nurses to COMFORT scoring. Some nurse participants talked about lacking motivation because their efforts failed to have any direct impact on patient care, whereas others could see that they were directly relevant to immediate/ongoing clinical decision-making, including decisions regarding ward round discussions or a SBT screen/SBT. In this context, the effectiveness of the COMFORT scoring component of the protocol could be highlighted, as this aided timely extubation:
It wasn’t pointless necessarily, but we didn’t do anything with it either. We had to write a lot to explain one of the three numbers you’d chosen, and then no one would ever look at it again.
Bedside nurse, S15
I think when the doctors started asking for it on ward round . . . that was the big thing. I think the nurses before were . . . writing the number down and nobody actually cared what this number is for. I think for doing it for the study and the doctors being involved and everyone discussing it basically gave it a sense of worth and actually the nurses then bought in . . .
Senior nurse, S11
Although most of the participants considered COMFORT scoring to have facilitated weaning and extubation, the traffic light colour-coding scheme was, at times, identified as problematic. For example, the ‘green’ category ranged between 12 and 17, but allowed for varying levels of alertness. The lower end of the range (a score of 12) was, on occasion, described as problematic in that patients could still be overly sedated, thus increasing the possibility of failing a SBT or not being suitable for extubation. In this context, several participants commented on what they considered to be a ‘disconnect’ between the COMFORT score and the SBT screening components of the protocol:
. . . I came across a few cases where the child . . . may have been in the green zone but was still sedated, and then nurses were doing SBTs and then saying that they’d failed the SBT, but actually I wouldn’t have actually done an SBT on the amount of sedation the child was on.
Consultant, S07
Ward round weaning discussion/planning
Overall, descriptions of ward round discussions and the associated development of patient sedation and weaning plans highlighted inconsistent adherence within and between units. Three main factors emerged. First, whether or not individual members of staff, particularly those leading the ward round, ensured the discussion of sedation and ventilator plans. Second, the clinical status of patients, in that a discussion was more likely to occur if a patient was considered to be approaching readiness for extubation. Third, the pressure of time, in that large amounts of information had to be shared within very tight time frames. At times, no specific reasons could be identified for the absence of discussion:
The ward round checklist, when it worked, it worked really well, and that was the part that was missing all these years, but that was the part that was probably the hardest to actually implement because there wasn’t consistency . . . but I don’t feel like that that was mirrored from a consultant point of view . . . in their ownership . . . because if they didn’t properly own it then the registrars didn’t get on board.
Senior nurse, S10
And especially on ward round . . . SANDWICH and COMFORT was always mentioned, it always came up and you always had to report back on it. What’s your SBT and what’s the COMFORT scores and what about SANDWICH and the documentation was something that was paid close attention to, it was something that was religiously done.
Bedside nurse, S02
Spontaneous breathing trial screen
A consensus among nursing staff participants across all units was that the SBT screening criteria were easy to understand and the actual screen was simple and quick to complete. Ease of use was considered an important factor in promoting adherence over time:
It doesn’t take very long . . . it’s just a quick tick-box. So I don’t think it was a burden . . . So yeah, I think it was good.
Bedside nurse, S09
Participant accounts showed that all units had set times for SBT screens to be completed. Some units timetabled twice-daily SBT screens (typically one in the early morning and one in the afternoon/evening) and some had three- or four-daily SBT screens scheduled over a 24-hour period. Across all units, the stated rationale underpinning the selected times for SBT screens was the provision of relevant information to fit with the unit and wider hospital routines of patient care, assessment, decision-making and extubation.
Varying levels of adherence to the performance of SBT screens were described. Overall, the highest compliance was associated with the early morning SBT screen, which was typically undertaken towards the end of the night shift. Two reasons were identified. First, night staff were considered to have the requisite time available. Second, more materially, it ensured that information was available to inform the morning ward round discussion, either stand alone or in combination with that provided by a follow-up SBT. The morning ward round was regularly described as the primary forum of important decision-making regarding plans for individual patient care, including in terms of ventilator weaning and extubation:
. . . the 5 o’clock [a.m.] one . . . so by the time it’s dayshift, the nightshift have got them ready to extubate and the dayshift don’t have to do anything apart from wait for . . . usually around ward round. So at 10 o’clock [a.m.] is generally when they’d start to think about it, because ward round is done, they’ve been starved (stopped feeds) for 4 hours or so . . .
Bedside nurse, S13
The afternoon/evening SBT screens were described as less regularly undertaken. Several reasons were identified. First, because the patient may have failed a morning SBT screen or subsequent SBT, and nursing staff were instructed not to repeat either procedure until the following morning. Second, because of the need to prioritise other aspects of patient care. More fundamentally, accounts suggested a diminished impetus to undertake afternoon/evening SBT screens, on the grounds that they were much less likely to lead to SBTs/extubation. Thus, the function of the SBT screens performed later in the day/evening was seen more in terms of providing staff with insight into patient progress towards extubation and, when appropriate, allowing for the planning of such. For example, they could provide useful guidance on how likely the patient was to wean from the ventilator the following day, so that sedation could be appropriately managed overnight:
The 4 p.m., we’d always know right, OK, so overnight we’ll wean for a planned extubation tomorrow.
Bedside nurse, S06
. . . but probably less so if it’s a bit of a surprise that the patient passes the SBT screen in the afternoon. I think a lot of people are then more likely to say well, we’ll just give them another 12 hours and do it in the morning.
Research nurse, S18
More generally, not carrying out SBT screening was associated with staff awareness of specific patients being clinically inappropriate for extubation. This scenario represents one of the most frequently identified departures from the protocol, namely the non-performance of tasks for which unit staff could see no obvious benefit. An awareness of the adverse impact of such behaviour on trial delivery was often accompanied by an acknowledgement that selective performance of the SBT screens was a perfectly rationale response in the circumstances, especially when an already heavy workload was taken into account. In one unit, the subsequent measures taken by the research team to reduce such ‘unnecessary’ workload were welcomed as making the trial a more practical and relevant endeavour:
So that [SBT screen] was a bit that was less well performed. Sometimes the assessments would get forgotten, particularly if we knew that we weren’t thinking of extubating them, so they were being neuro protected or they were oscillated or whatever, they would miss out the assessment. And you’d say you’ve still got to do the assessment and say they’re not ready for an SBT. So that would tend to be less well done.
Consultant, S16
To start off with, there was some kids who were on long-term weaning plans who weren’t being planned to extubate . . . we were doing it four times a day, but then . . . the research team came with stickers and every patient who was identified as a slow wean, we only had to do it once a shift, and I think doing it once a shift wasn’t an issue.
Bedside nurse, S03
Spontaneous breathing trials
Overall, from the descriptions given by participants, the performance of SBTs and extubations following a successful SBT was inconsistent across and within units. Although some units appeared to fare better than others, of all components of the protocol it was SBTs that were talked about most often as not being undertaken, or undertaken without strict adherence to the protocol. Typically, SBTs were meant to follow as soon as possible after a successful SBT screen. As per descriptions of the vulnerability of scheduled afternoon/evening SBT screens, participants highlighted the same for SBTs. Several reasons were identified. First, participants described non-performance of scheduled SBTs owing to lack of fit with unit routines and priorities of care. Established practice led to the majority of extubations being carried out in the early part of the day. Consequently, there was a reduced impetus to carry out afternoon/evening SBTs because they were seen as less likely to have an immediate impact on patient care. Second, hospital-wide routine practice means that afternoons are customarily given over to other aspects/priorities of care, such as off-unit tests and investigations. Consequently, patients and/or unit staff could be engaged in these other care processes. Third, staff accounts emphasised a drive to make the most efficient use of staff time and other resources, so that when a SBT was thought to be superfluous it could be missed:
. . . because we go around at night and I actually did then find myself saying ‘and can I say that there’s no point in this child having an SBT tonight’, but it had taken me a few experiences to realise that I had to say it.
Consultant, S07
. . . but it almost felt a bit pointless if they had failed just a few hours ago and not much else has changed in the interim.
Bedside nurse, S15
Consequently, and again in line with SBT screens, participant accounts across all units confirmed that SBTs scheduled for the (early) morning were both more consistently performed and, when performed, more likely to lead to patient extubation. The reasons identified for this all emphasised the importance of the fit of the morning SBT with unit resources (primarily staffing) and established routines of patient care. This is not to say that SBTs were not carried out later in the day. For example, participants talked about SBTs/extubation being performed in the afternoon following morning SBTs at which patients had been assessed as not quite ready for extubation, on the understanding that by the afternoon they would pass the SBT and be extubated. More generally, participants also talked about the importance of the afternoon SBT in promoting extubation:
I don’t think it influences the 9 o’clock decision very much, but what it does do is it allows us to think, ‘hang on a minute, we’re not ready for 9 o’clock, let’s try the 1 o’clock one,’ instead of saying ‘well let’s see what happens’ and then you leave it and then it comes 5 o’clock and you think ‘oh, 9 o’clock/10 o’clock tomorrow’. So I think definitely the ones that you may not have pushed to get extubated between 4 o’clock or so, the 1 o’clock assessment thing is a difference.
PI, S17
A second factor adversely affecting adherence to SBT performance concerned a straightforward impulse to follow preferred clinical practice. For the most part, this issue was discussed in relation to medical staff, but, on occasions, bedside nurses were implicated. Three main forms of non-adherence were described: non-performance of a SBT following a successful SBT screen, performance of a SBT but in ways that deviated from the protocol and non-performance of an extubation following a successful SBT:
. . . well some of the consultants come round first thing in the morning, but they’d just change the settings, they wouldn’t necessarily have looked at that chart [for SBT screen result] . . . So they went round and put their rate down or put them on to CPAP [continuous positive airway pressure] anyway, regardless of what the score was . . .
Bedside nurse, S02
. . . I think our overall position, as a unit and the nursing staff, was they felt more comfortable waiting until the patient was on less than that before going for a spontaneous extubation.
Consultant, S04
Regarding the performance of a SBT in ways that deviated from the protocol, the main manifestation concerned the use of settings other than ‘5 and 5’ (PEEP 5 cm H2O, Psupp 5 cm 2O above PEEP). Several contributory factors were identified. First, patient clinical status and/or other characteristics (typically age and size) were identified as contributory factors. One of the most frequently cited cases was neonates, for whom these settings were considered inappropriate. Second, another factor identified was medical staff avoiding the use of the ‘5 and 5’ settings when this involved a significant drop from the ventilator support that patients had been on during their successful SBT screen. Participants also talked about, and articulated support for, the use of higher settings for patients who, for clinical reasons, were planned to be weaned onto non-invasive support. Finally, another factor identified was the persistence of medical staff in the use of settings other than ‘5 and 5’ for which no obvious reason was apparent:
A lot of people wouldn’t [even] do the 10 on 5 either. They wanted higher CPAP [continuous positive airway pressure]. I didn’t really understand why we wouldn’t at least try the 10 on 5 and then if we fail then we could maybe try 12 on 6 or whatever. There was no even attempt at that with some of them.
Senior nurse, S18
We did implement a lot of modified SBTs . . . 7 on 7 or, you know, and that was considered an SBT, do you know what I mean, where it wasn’t your exact criteria.
Registrar, S02
For me, it was just conceptual, so I didn’t really mind if it was 5 or 6. If someone left it on 6 I think I even allowed it a few times and just said ‘well, effectively we did it’.
Consultant, S03
A second form of deviation concerned the performance of SBTs that were ended prematurely. Essentially, the reported issue was the reluctance of staff to allow a patient time to overcome any initial difficulties that could have potentially settled. Frequently, this reluctance was traced back to inexperience on the part of staff involved:
. . . sometimes just the adjustment change from going from BIPAP [bilevel positive airway pressure] to CPAP [continuous positive airway pressure], the baby is going to have to get used to that. So you might get an increase work in breathing initially and then it will calm down, but some of the juniors would be like oh, my God, their rate has gone up to 80, and they would stop it . . . they’re [the bedside nurse] scared that they’re going to fail and they don’t know how to cope with it because they’re new . . .
Senior nurse, S16
I think the biggest problem we have here with mode of ventilation is that people leave the apnoea alarms very low on spontaneous mode, particularly the babies, they would get 15 seconds before they’re having an apnoea, and then they’re failing, when actually, if you put it to a realistic amount, you can allow them to pass.
Physiotherapist, S18
Non-performance or delayed performance of an extubation following a successful SBT was highlighted by participants across all units. Doctors were sometimes described as failing to articulate their reasoning in any detail, which caused the nurses additional workload and some frustration. In such cases, none of the reasons (codes) on the trial documentation was relevant, leaving the bedside nurse with no option but to use the ‘other’ code. The accounts of some participants also highlighted non-performance of, or delays to, extubation following a successful SBT in cases in which trial exclusion criteria did not apply. In addition, on a limited number of occasions, consultant participants associated delayed or non-performance of SBTs with a failure on the part of bedside nurses to inform them that a patient had passed the SBT screen. One consultant stressed the responsibility of nursing staff to remind medical colleagues of the need to undertake trial-related activity:
And sometimes doctors are like ‘oh, they’re not ready’, and you’re like ‘give us a better reason’ and then you’ve got to put down ‘doctors have said not ready’. That’s not a proper reason.
Bedside nurse, S15
Although in my experience the nurse doesn’t tell you the child has passed the SBT . . . No, you have to ask them . . . They’re supposed to do it night and day, night and day, so they know . . . Still we have to remind them.
Consultants, S05
Finally, although rarely acknowledged explicitly, some participant accounts suggested that SBTs could be performed in circumstances in which a decision to extubate a patient had already been made. In many cases, this decision would be based on a process of weaning the patient down to ‘extubated-able’ settings and then carrying out a SBT as a final check or justification. This meant that, rather than being used to potentially expedite extubation, the SBT was being ‘shoehorned’ into existing unit practice:
And, to be honest, most of the times we would have decided overnight whether we’ll be extubating or not, so we would just go ahead and do an SBT and that would be sort of extra information, or it would give us more confidence that child has passed an SBT and child is ready for extubation. As I said, the decision would be done the night before . . . So most of the decision will be done on the night before and it would be just sort of an extra precautionary exercise.
Registrar, S06
It’s used as an extubation predictor. So when we think the child is ready to extubate, we do the SBT, not when we’re going, ‘I’m sure this kid could be weaned a bit more, can we do an SBT?’.
Consultant, S08
The option of running a SBT for up to 2 hours was discussed as problematic on three main fronts. First, a lack of explicit direction regarding the action to be taken at the end of a 2-hour SBT meant that staff could be left confused, including in respect of how/when to record the ‘ending’ of the SBT. In the absence of extubation, a number of scenarios were described: typically a patient remained on the SBT settings (sometimes for extended periods), was returned to the pre-SBT settings or returned to reduced support. Second, it allowed the focus of attention to move away from the patient’s weaning progress as other priorities of patient care took precedence, or staff simply forgot about the SBT:
. . . but I think we had lots of kids that were on for far more than the 30 minutes and they weren’t extubated at 2 hours . . . I think it was an acceptance of OK, we can extubate this child, we’ll do it whenever we’re ready to do it, as opposed to actually they’ve passed the 30 minutes and we should try and take the tube out by 2 hours or within the next few hours. That wasn’t the case. It was ‘we know they’ve met it; we know we can take that tube out; we’ll take the tube out when it suits us’.
Physiotherapist, S03
Third, on a few occasions, although participants acknowledged the need for the protocol to be sufficiently flexible to take account of unit routines and (changing) priorities, they also highlighted its failure to stipulate a requirement for extubation to be performed immediately following a successful SBT:
. . . there was no closed loop feedback back to the medic or the ANP to say ‘you know we’ve been SBT 5 on 5 for the last 2 hours, why are we not extubating this child?’ So it’s . . . like ‘oh, I forgot’ . . .
PI, S03
Objective 2: to ascertain how participants received (e.g. understood and responded to) the intervention over time and across different paediatric intensive care units
Enhanced (bedside) nurse understanding of weaning
Across all units, there was widespread endorsement of the trial as enhancing nurse, particularly bedside nurse, understanding of the nature and process of ventilator weaning. As part of this process, bedside nurses saw themselves as having an improved awareness of relevant clinical indicators and what they mean, and what the appropriate response/management should be. Enhanced nurse understanding was echoed by other members of unit staff. More experienced staff tended to associate the learning achieved by less experienced nurses with several positive outcomes. These included the fact that this learning was an important dimension of continuing professional development and that it meant a greater likelihood of bedside nurses involving themselves, and being involved by others, in the weaning process:
It’s definitely for the junior ones, that now they know which criteria you use to decide . . . when you extubate . . . when we started SANDWICH, they didn’t have [that] knowledge . . . they [can] go and escalate that to the doctor and say ‘oh my patient is actually on setting . . .’
Research nurse, S15
Enhanced nursing confidence/autonomy
In line with an awareness of enhanced nurse understanding of the weaning process, the trial was widely associated with improved confidence and autonomy. Bedside nurses were described by colleagues and by themselves as more confident to pursue autonomous practice and to engage in meaningful discussion about this practice with others, especially senior colleagues. Throughout, the crucial factor identified was the shared understanding provided by the protocol. Enhanced confidence was seen as particularly beneficial for more junior/inexperienced nurses, who were considered more likely to be reluctant to raise issues with medical staff. In some accounts, a ‘positive loop’ was identified, whereby increased confidence provided by the trial documentation encouraged junior nurses to initiate discussion with medical staff. When these discussions were positive, subsequent discussion was encouraged:
But I can honestly say that I don’t find any downside to SANDWICH, because I think of the positive things about how we work, the nurses’ mindset is now a part of the working narrative. I like the way they escalate their concerns.
Ward manager, S16
And I think it does. It has empowered the junior staff, it helps them in the ward round, they can talk about the COMFORT score, they can explain where they’re at on the screening tool and I think it has really helped with the junior staff, feeling a bit of self-worth.
Senior nurse, S11
. . . it gave you a bit more backup than just saying I don’t think they’re adequately . . . It’s made it easier to have that objective overview between various members of the team.
Bedside nurse, S13
In most units, the SBT screen gave bedside nurses a designated role in ventilator weaning for the first time. The descriptions of enhanced confidence focused on the SBT screening criteria providing bedside nurses with ‘objective’ evidence regarding a patient’s clinical status and readiness to be weaned, which bedside nurses could use to support multidisciplinary discussions. In addition, the fact that the protocol required the medical team to make their reasoning explicit with regard to the non-execution of a SBT and/or extubation gave bedside nurses the authority to seek explanations:
I don’t know if it’s just now that I’m more experienced, whereas I was quite new before it started, but I definitely would think more about whether they could be extubated, whereas before it was just the doctor’s job and I didn’t really think about it at all, whereas now it makes me think I might bring up a conversation whereas I never would have started a conversation.
Bedside nurse, S05
A similar, but more understated, enhanced confidence was apparent among more senior nursing staff, according to participant accounts. In one unit, senior nurses highlighted the potential of SANDWICH to formalise/cement their greater involvement in patient weaning as part of standard unit practice. In this context, they described themselves as the frequent ‘drivers’ of weaning, but not the technical implementers. Based on the role that they were assigned as part of SANDWICH, namely that they could formally commence a patient on a SBT, there was at least the potential for this to continue. Such a role was seen as representing an important advance in terms of nurse involvement in weaning:
We aren’t the ones that fiddle with the buttons, but I do think we’re quite often the drivers of weaning, but we may not be the ones that are able to actually alter the ventilator . . . For this [SANDWICH] we were able to do an SBT.
Senior nurse, S06
In general, medical staff participants acknowledged that the trial had, to a greater or lesser degree, enhanced nursing confidence and autonomy of practice. This was regarded as a positive development. That said, reluctance on the part of nursing staff, particularly less experienced nurses, to assume greater responsibility was noted as an important obstacle. More widely, some participants described nurses, particularly junior nurses, as inherently reserved in their interactions with senior, typically medical, colleagues. Several bedside nurses also commented on their own reticence. Failure on the part of nurses to question medical colleagues was seen as an impediment to trial implementation, in that it could result in the perpetuation of deviations from the protocol:
. . . involve them in the decision process and to make them proactive in the raising that children were ready, and this has not happened . . . Not really, because I just keep on asking them ‘have you done the . . .?’. So you had to prompt them, so if I was to go and say ‘have you done the bedside checklist?’, and they’re like ‘oh no, I’ll do it now. Passed! Great, what shall we do now?’. So it had to be very led by us.
PI, S04
Enhanced multidisciplinary communication/collaboration
There was widespread awareness of trial participation having improved multidisciplinary communication and collaboration among unit staff. The crux of this improvement centred on the requirement to discuss weaning plans and the shared language provided by the trial to aid these discussions. Nursing staff talked about how they could use this language to articulate their understanding of individual patient sedation weaning progress and/or requirements. They described enhanced communication from doctors regarding their intentions and actions concerning patients. For some participants, a key forum in which enhanced multidisciplinary communication played out was the daily handover/ward round:
I do like [that] they listen when we say they passed it or they failed it, they do listen to that decision. They don’t usually go ‘oh, well, let’s take the tube out anyway’.
Bedside nurse, S15
. . . especially in handover when the doctors are talking about weaning the pressures, whereas sometimes they wouldn’t even tell you, they would just come and wean without you knowing sometimes, I think. They’ve changed yes. So I think sometimes having that conversation if they have failed the SBT . . .
Bedside nurse, S18
Other members of staff tended to endorse the experiences and understandings of nursing staff outlined above. For example, physiotherapists talked about the trial as having facilitated a greater role for their own team because they learnt more about how to maintain appropriate sedation and/or were able to directly influence patient weaning plans based on the use of trial criteria and language. Medical staff, typically consultant participants, were also aware that SANDWICH had provided a shared language of weaning and, through this, enhanced staff communication and collaboration. At times, their reflections were couched in a strategic context of their vision for unit multidisciplinary working. At other times, accounts focused on an awareness that multidisciplinary communication had been improved precisely because staff were required to reflect on their clinical decision-making and to account for it to others:
. . . because I thought the SANDWICH trial was absolutely brilliant, it really helped inform us of what was going on, we felt we were more part of the team as well . . . I think it was just the fact that we could immediately see, because we learnt more about the sedation scoring and what have you . . . but suddenly we had that information, we’d learnt about it a little bit . . .
Physiotherapist, S06
I used to have 10 conversations in a day about the sedation, it used to do my head in . . . and those conversations just went away, people just did the score . . . But what I found when we were doing the trial is the nurses were doing it because they had to, and then instead of coming to me and saying to me ‘can I do this, can I go up, can I go down’, whatever, they would just do it, and that made a massive difference . . .
Consultant, S11
So having common words that we can use, SBT readiness, is really helpful and it makes communication more effective, efficient. So, I found it positive.
Registrar, S13
Enhanced sedation management
One of the most frequently identified positive outcomes of the trial was the increased interest in, and attention paid to, patient sedation. From their diverse roles and responsibilities, unit staff talked about this process in different ways. Underlying all of their accounts was an acknowledgement that the use of COMFORT scores and related target setting in ward round discussion had, to a greater or lesser degree, improved unit practice. This does not mean to say that problems were not identified with both the COMFORT scoring tool itself and the implementation of the component of which it was a part.
Nurses’ (typically bedside and research) accounts tended to emphasise that the ‘point’ of COMFORT scoring, including its application in sedation management, had been made much clearer through participation in the trial. Implicit in some accounts was the idea that, prior to SANDWICH, COMFORT scoring was being carried out in the absence of a full comprehension of its value and purpose. One aspect of the COMFORT scoring system that garnered widespread endorsement was the ability to assess pain. This was seen as enabling a more comprehensive consideration of all potential factors having an impact on how settled or comfortable a patient could appear:
I think the COMFORT scoring itself was fantastic, and we’d do pain scoring at the same time, so we could then compare it to see whether the patient was adequately sedated or whether they needed any analgesics.
Research nurse, S14
In terms of unit practice around sedation management, COMFORT scoring was regularly described as having made several material improvements. First, it had encouraged a greater focus on and proactivity concerning patient sedation. Second, it had encouraged clarity and consistency by providing a shared multidisciplinary language (e.g. COMFORT scores and associated target setting) with which to communicate and/or act in relation to sedation management. For both of these reasons, although problems continued to be identified in all units to a greater or lesser extent, a sense of moving away from keeping patients ‘overly’ sedated was evident:
On the sedation part, I thought that was quite helpful in focusing the whole team’s approach to sedation. I thought that the groupings, if you like, and the colour coding and being able to discuss if they were in the green, for example . . . it did improve the focus everybody, but particularly the bedside nurse on sedation, and give them some additional empowerment to change things.
Consultant, S07
Enhanced weaning practice and related patient care
To a greater or lesser degree, participants in all units talked about the implementation of the trial as having contributed to enhanced weaning knowledge, practice and related patient care. Several relevant processes were identified. First, staff were repeatedly prompted to consider and/or pursue weaning, which encouraged an increased pace that would otherwise be missing. Typically, the core issue was one of having encouraged a move away from a gradual ‘step-down’ approach to one in which patient could be moved forward more dynamically. Even in cases in which, for whatever reason, patients failed a SBT, weaning was understood to have been progressed in other ways:
But what we found was if they failed the SBT, instead of going back to the pressures they were on, we went back halfway up, so even that helped. OK, maybe they’re not ready to go down from 20 to 5, but OK, they want that back, take it up to 16.
PI, S18
Overall, I think it’s been really, really good. It’s changed the mindsets, it’s changed the language, it’s allowed people to positively challenge the way we work in a ‘let’s try something new approach’.
Yes, because it made people question and think, as opposed to just going along.
PICU senior nurses, S03
A second process raised was the enhanced consistency and clarity of weaning practice. The protocol was described as promoting a more structured, systematic and standardised approach, which was based on shared goals and understanding how these goals should be reached. This standardisation was discussed as allowing members of staff to advance weaning according to their roles and responsibilities. This was particularly the case for bedside nursing staff, who described themselves, and were described by colleagues, as proactively advancing weaning. Systemisation was also considered to have helped remove at least some of the inconsistency introduced into the weaning process by the different approaches and preferences of individual members of staff (particularly consultants). In this context, some consultant participants talked about the protocol as removing some of the uncertainty of weaning decision-making. The more structured approach was further associated with improved patient weaning, in that it encouraged staff to plan ahead. Participants talked about being prompted to consider, and prepare for, what might come next. They also talked about a similar planning ahead by their colleagues:
But I think SANDWICH is actually good in that way for extubation, because before that it was all consultant based, if this is the consultant that was on, you would know whether the child is going to or not going to, but SANDWICH has made that more consistent.
Bedside nurse, S02
. . . because it gave everybody a structure on what to do when you come to extubation, isn’t it? Instead of waiting for the consultant to sort of say the magic word, it was all sort of lined up to be done.
PI, S17
And also being a bit more prepared as well. If you know that your patient has passed the SBT and we’re most likely going to extubate, then I might think OK, let me go and get a non-invasive ventilator or some oxygen or whatever so you’re prepared . . .
Bedside nurse, S15
At times, participants linked the protocol with improved patient care beyond the immediate context of ventilator weaning. For example, time spent unnecessarily on ‘nil-by-mouth’ was seen as having been reduced, in that this could be stopped as soon as a patient failed a SBT, rather than having to wait until extubation was attempted. From a physiotherapist perspective, participants talked about improved rehabilitation care. For example, the fact that the trial had encouraged a more ‘light touch’ approach to sedation meant that physiotherapy interventions, such as chest rehabilitation, could not only be initiated earlier, but also were more likely to be effective:
I like that our patients are much lighter on sedation . . . it means, as physios, we have to show that education on how we can still treat patients even if they are awake, we just treat them slightly differently.
Physiotherapist, S16
Contribution to (the development of a) unit research culture
When discussed, most participants considered that participation in SANDWICH had made a positive contribution to the unit’s research culture and capacity. This could include, for example, the development of an appropriate infrastructure, growing a (national) research profile, increasing research interest among unit staff and/or promoting the skills and/or team identity of the research team both within the unit and more widely. All were discussed as having promoted the unit’s practical ability to engage in research. At times, an underlying interest in/support of research among senior members of staff was considered to have helped trial implementation, including in terms of its encouragement of other members of staff.
In one such unit, building on SANDWICH, the unit team had been able to secure additional funding from the hospital trust and local clinical research network to continue to fund research nurses and, thus, to participate in other major studies. In another unit, involvement in SANDWICH had helped the research team in their discussions with hospital management concerning the need for the team to be based on substantive posts, rather than the current fixed-term contracts, which were considered unconducive to strategic research planning and implementation:
And people valuing research as well, like understanding that’s a way of life now and we have to sort of get on board with it. We’ve been doing a lot of projects, which has been good as well.
Senior nurse, S11
So this is another thing that we’ve had a discussion with, with senior hospital management, just about the lack of substantive contracts . . . you’ve got research staff . . . they want to stay but the funding’s not there for them to stay or they don’t actually have a substantive post . . . and then you go back . . . Based on how heavy research can be for us . . . it’s part of the vision of the hospital. Paediatric intensive care has a lot of research going through it, so if that’s the case why don’t we have more permanent [staff].
PICU research nurse, S03
Objective 3: to explore the context over time and across different paediatric intensive care units and determine factors (including managerial support, economic, organisational and work level) that affected implementation
This objective deals with the barriers to and facilitators of trial implementation. ‘Implementation’ is treated in its widest sense, encompassing the immediate delivery of the intervention and the wider context in which this delivery is situated.
Support from the study research team
Overall, the SANDWICH research team was described as having provided effective support throughout the trial. Of perceived central importance were the benefits derived from ongoing communication between the trial and the unit research teams. Here, the trial implementation manager was consistently acknowledged to have delivered not only well-organised, comprehensive training, but also prompt, well-informed answers to queries arising throughout the entirety of the trial. As a single individual with responsibility for managing a very large study, a substantial workload burden was recognised. In addition, the monthly PI teleconferences, attended by unit PIs and research staff, were valued for allowing unit-specific problems and/or queries to be addressed, and unit-to-unit learning to take place. In this context, a research nurse from one unit balanced the perceived disadvantage of being randomised to the intervention phase relatively late in the trial with the learning gained from the experiences of others during the PI teleconferences, which helped pre-empt and/or manage issues arising. Finally, the material resources provided, including, for example, the COMFORT scoring chart, were frequently described as being of considerable practical utility, and the sundry items, such as SANDWICH pens and lanyards, were understood to have encouraged a positive response to the trial:
I think the availability and the visibility, [name of implementation manager] being there, was useful certainly, it raised the profile, and also having the little notepads and the pens and the posters, sort of like a publicity drive, it put that on people’s agenda . . . So I think all those things were helpful.
PI, S18
I can certainly compare it to a few other national studies, the support is so much better in SANDWICH, I’ll definitely say.
Consultant, S15
Intervention training
Overall, the training was described as relevant and comprehensive and, for this reason, to have been important in enabling staff to understand the purpose and content of the protocol and to discharge their roles and responsibilities. The vast majority of participants considered that, in the absence of the training, trial implementation would have been severely compromised. A limited number of participants considered the training not to have been entirely necessary to trial implementation. Significantly, they tended to be more experienced/senior nurses. In a number of units, participants talked about either using or hoping to be able to use components of the training as part of future in-house unit training and/or current guidance. Notwithstanding an acknowledgement of the need for and benefits of the training, it was frequently discussed as intensive and time-consuming. The online component in particular attracted some criticism over the length of time it took to complete:
I didn’t find it too bad, personally. I know a lot of people were saying it was long winded but I didn’t find it too bad.
Bedside nurse, S17
I think we were all very well informed about SANDWICH and how it would work, I thought the training package was very good, so I felt very well informed.
Physiotherapist, S11
Of the two delivery formats, most participants favoured face-to-face training as more enjoyable and effective in terms of delivering knowledge. Clinical and research nurses talked about the training provided at the bedside and/or in small groups as particularly helpful owing to its practical nature and focus, as well as allowing more meaningful engagement with what was being taught, especially because participants were able to ask questions and resolve emerging issues. By contrast, the online component could be described as lacking an immediate ‘real-world’ relevance. The same preference for/appreciation of the practical benefits of training is reflected in participants’ comments regarding the value of the training becoming apparent after the trial was fully implemented. A few participants considered the online training to be more beneficial than that provided face to face. Again, such individuals tended to be more senior/experienced nurses:
. . . definitely face to face was far more successful in terms of how people understood the process.
Bedside nurse, S13
Not particularly useful or necessary [online training] . . . I think probably perhaps just from a very personal point of view, I’m more of a doer. So I have to keep doing it to understand it.
Bedside nurse, S04
In some units, ensuring that the requisite numbers of staff were trained in the time provided was described as difficult, sometimes extremely so. This tended to be the case in larger units. At times, special measures were necessary to ensure completion. These included research nurses relieving bedside nurses so that they could complete the computer-based component during their shift. Where the research nurses had the support of key colleagues, for example unit managers who facilitated the completion of online training during working hours, meeting the required staff training quotas in the allotted time was considered to have been much easier:
It just got ‘we need to get you all done! I’ll have your patient, just go and do it’.
Research nurse, S07
. . . it was made easier, because the matron was lovely . . . we were able to persuade her and she agreed to add the SANDWICH training into the induction for this ward, which was mandatory and which she assigned staff hours to, which meant that we had groups of 10 to 15 people that were sitting in a room and that for half an hour, 45 minutes we could teach SANDWICH to.
Research nurse, S09
Overall, the medical participants (typically consultants) who discussed the training materials considered them to have been well structured and informative, and to have enabled a necessary awareness and understanding of the intervention, especially given its complexity and spread. At times, some queries were raised in relation to two issues, namely the length of time taken to complete the (especially online) training and some of the relevance of the content in the context of participants’ medical training and knowledge:
I think, like with everything, it’s very hard to make it specific for certain people, and there were bits of it that I didn’t need but I understood why it was as it was, because without going into everybody’s background how do you know what they do and don’t understand.
Consultant, S02
Trial ‘champions’
Overall, the appointment of designated champions was thought to have facilitated trial implementation. That said, some difference of opinion emerged concerning the perceived extent of their contribution. These differences were associated with three main issues: the commitment of the particular individuals appointed; the availability of the individuals on the unit; and, irrespective of their individual commitment and/or availability, other unit pressures. For the research nurses, the support of champions in getting the requisite numbers of unit staff trained in the time available was of considerable importance, particularly in the larger units. For clinical staff, especially bedside nurses, the value of champions was associated primarily with their routine presence on the unit, meaning that relevant support and advice were available as and when required. In this context, they were seen as particularly effective at times when research nurses were unavailable. To a lesser degree, the value of the champions was located in their physical presence acting as an automatic prompt to consider the SANDWICH intervention.
In terms of individual commitment, some champions were described as expending considerable time and effort to ensure that staff understood and adhered to the protocol. Others were described as playing a ‘token’ role only. At times, the importance of the role played by champions in the early stages of the trial was highlighted, as they were able to answer initial queries and correct misinterpretations, often immediately. Precisely because of the need for availability, a few participants suggested that in the absence of a fixed presence of champions or research nurses on the unit floor, deficits in unit staff knowledge of, and adherence to, the protocol were more likely to persist:
My champions were great, they did so many of them [training] . . . they really picked up the slack, and so did [name of PI] and all our medics. The training, I don’t think really was much of a problem.
Research nurse, S10
I think after a lot of support by its champions, who wanted to see it done properly, we got into good practice, we got into good routines.
Bedside nurse, S06
. . . that didn’t work . . . it’s around consistency; there needs to be someone consistently, every day, every shift, to support them, otherwise it just doesn’t work.
PI, S01
Nature of the intervention
Overall, the intervention was considered well designed, the written materials user-friendly and the supporting resources (e.g. lanyards and pens) visually appealing and extremely useful. The pens, in which a COMFORT score chart was embedded, were the focus of particular praise. For all of these reasons, the design and content of the intervention (in its entirety) were widely endorsed as having promoted implementation. In the context of acknowledged difficulties involved in any practice change, participants identified two core properties as having been particularly valuable. First, the intervention was relatively straightforward to use. Second, the intervention was flexible, so that it allowed for independent clinical decision-making.
In terms of ease of use, several related features were discussed. First, the tasks to be performed by bedside nursing staff, namely the COMFORT score assessments and SBT screens, were routinely described as relatively undemanding. Participants talked about both as not only making sense to them, but also having the relevant clinical indicators as unambiguous and simple to assess. Second, trial paperwork was routinely described as user-friendly, so that completing the COMFORT scoring and SBT screening sheets was not experienced as overly burdensome. The fact that each component of the protocol was clearly set out in the bedside documentation, outlining what was to be undertaken, when and how, meant that bedside nurses could more easily accommodate it within their working practice:
To be honest, none of it took very long, it wasn’t a huge workload . . .
Senior nurse, S15
So in terms of the bundle, it was a lot of information but I think it was handled well . . . and the way that it was presented was really helpful, the colours and the tables and just the way that the bundle, it just made it easy to use, very user-friendly.
Senior nurse, S03
Some deviation from the above understandings was apparent. A few bedside nurse participants talked about the trial having added to an already heavy workload. In the context of having other, more critical, patient charts to complete, the need to complete additional paperwork was unwelcome. That said, accounts never suggested anything more than a degree of annoyance and that the paperwork was intentionally not completed.
In terms of protocol flexibility, where discussed, this was identified as particularly important for buy in from unit consultants, as it guaranteed clinical independence. In this context, consultant participants expressed no sense of being constrained in their clinical decision-making or having been aware of any such feelings on the part of colleagues. On much rarer occasions, the importance of the same independence was highlighted among nursing staff. In addition, participants pointed to the fact that units had not been constrained in terms of other weaning-related initiatives that were either ongoing at the time or planned for introduction during the lifetime of the trial. The fact that the protocol had been developed by specialists in the field was considered to have further promoted its acceptance by the medical team, as had the extended and inclusive process of development, which enhanced the feeling of ownership of the intervention:
I think if you hadn’t been flexible you wouldn’t have gotten so many people on board, so many units on board. We were particularly reassured by the fact that when you said, ‘OK, if you already planned to introduce your nurse weaning, whatever, it’s OK, continue, we won’t stop you doing that’ . . . that flexibility helped.
PI, S18
. . . some of our colleagues didn’t like putting children under pressure support . . . but when this came in and they looked at it as that it is being reviewed by many experienced intensivists, they did buy into it and there wasn’t any resistance.
Consultant, S15
Although the protocol was acknowledged to have been appropriately flexible, on a more fundamental level it was discussed as inherently unable to deal with the wide variation in patient clinical status, complexities and requirements. The consensus that emerged from participant accounts was that the more complicated a patient’s clinical status, for example in cases of comorbidity and/or underlying fragility, the less appropriate (the use of) a protocol. There were simply too many clinical variables, typically nuanced and often conflicting, that needed to be taken into consideration and which protocols were unable to accommodate:
The problem with the small baby, you know, when you put them on 5 on 5 it doesn’t work for them. So we always fail because they’re going to cough with this sort of tiny tube and this 5. So we couldn’t modify that as an SBT to put a pressure point of 8 or 10. So that wasn’t age appropriate.
Senior nurse, S16
. . . they were having to . . . say why we’re not doing this on patients who it’s not eligible for, and that increased resentment . . . there would still be a discussion about their weanability because of the paperwork, even though it was ridiculous . . . why would we even start the discussion of ‘are they eligible for an SBT?’, their chest is open . . .
Senior nurse, S01
Unit research team
The vast majority of units possessed a dedicated research team comprising nurses trained in paediatric intensive care. The presence of this team was discussed by its members and by unit staff generally as crucially important to trial implementation in several respects. First, a dedicated team was able to maintain a physical presence in the unit. Research nurses talked about themselves, and were described by their colleagues, as regularly visiting the unit floor to undertake various trial-related activities, including reminding staff of the need to complete relevant tasks. Furthermore, their mere presence was considered to act as a visible prompt:
But what I think worked well was . . . even just seeing us on the floor, like ‘oh we have to do COMFORT scores today, they’re in’.
Research nurse, S07
Second, the team could tailor their work pattern in ways that allowed them to monitor adherence, particularly regarding recording data, and undertake activity to make good any identified deficits. This could include, for example, regular ‘spot checks’ on adherence and ‘chasing up’ missing information. Probably more than any other aspect of their work, the ‘chasing’ of information, whether physical or electronic, was considered particularly time-consuming and frustrating. Deficits in record-keeping meant that research teams had persistent difficulties in capturing essential trial data:
The research nurses had to do so much more, the delivery was really difficult, because of the poor compliance with the paperwork we were always chasing stuff, we were always having to go to the wards to track down the SBT . . . we would spend days looking for charts.
Research nurse, S01
Third, across all units, the majority of research team members were nurses who worked part time clinically. These nurses regularly talked about actively progressing the trial when working on the unit floor by, for example, reminding colleagues of the need to perform trial tasks, providing practical advice and/or actually undertaking tasks when colleagues were busy with other aspects of patient care. The fact that they were themselves responsible for the bedside care of patients prompted several to suggest that their efforts to promote adherence were inherently mindful of the context in which bedside nurses worked:
It was good that we got to do it as a unit, as opposed to research nurses from obviously the research department coming down, because they wouldn’t understand all the ventilation.
Research nurse, S17
. . . and also as research nurses, us also having clinical involvement and being the ones who go and speak to the bedside nurses as something to implement, but hearing it from people who do their job, so we understand, I guess, what they’re doing naturally and how you can adapt that in or fit it in in a way that makes sense to them where they don’t feel overwhelmed with additional work to do. So I think that helped.
Research nurse, S03
Overall, the research teams considered SANDWICH to have been a labour-intensive initiative; for some, the time required was more, sometimes much more, than they had originally anticipated. Their articulation of this issue tended to be less in terms of an overt complaint and more in terms of an awareness that, however welcome the trial’s funding of research nurse time, more time was required. This was particularly the case in larger units and/or those that were research active, as in both the teams (often understaffed) were under considerable pressure to support numerous studies. In this context, several nurses talked about being able to undertake core SANDWICH activity only (primarily data collection), with limited time available for ‘extra’ supportive work, such as spending time on the unit encouraging adherence to the protocol:
. . . like the resources taken to implement this have been so much more than what I think I ever thought this study was going to be, and as soon as you step back to give other studies a fair chance, you pretty immediately see that it goes down.
Research nurse, S08
Across all units, participants endorsed the efforts of the research teams to ensure successful trial implementation. Not only were they acknowledged to have extended, sometimes very considerable, efforts, but also their physical presence on the unit was discussed as meaning that they could provide invaluable hands-on, face-to-face advice and guidance. This presence was regularly noted as sufficient to remind staff to complete trial activity that might otherwise have been missed. In addition, the targeted measures taken to improve and support compliance were regularly validated:
I think it’s worked better than I thought it would. I think the main reason it’s worked better than I thought it would, would be the commitment of the research nursing team.
PI, S18
I think if your research nurses are not good then this project is not going to be successful . . . I think that we were very lucky to have a very committed set of nurses.
Consultant, S15
Fit with established hospital and unit organisational and patient care routines
In different ways, participants from all units talked about established hospital and PICU organisational and patient care routines as having an impact on trial implementation. Here, descriptions tended to be couched in an awareness that it would be extremely difficult for any protocol to compensate for, or over-ride, these routines.
Routine extubation practice
Across all units, staff outlined the same general pattern to the timing of extubations, namely that they tended to be performed earlier in the day and were not performed in the evenings/overnight and at weekends. If performed at such times, they were most likely to involve clinically uncomplicated patients. Even when staff confirmed that their unit operated with a formal ‘24/7’ extubation policy, they acknowledged that its implementation was not inclusive of all patients. Across all units, the likelihood of extubation diminished as the day progressed. Although extubations could and did take place into the late afternoon, evening and overnight, they became progressively smaller in number over the course of a day:
We don’t [routinely extubate overnight], but again it’s depending on the consultants as well and the grade of intubation. So if the grade has been more than one then probably they will wait until morning time . . .
Research nurse, S05
So, again, they wouldn’t extubate unless there was a consultant around or whatever, so that would never happen during the night.
Bedside nurse, S02
In terms of the impact on trial activity, this routine meant that if a patient passed a late afternoon, evening or night-time SBT, extubation was likely to be delayed until the following day. Ventilator pressures were often reduced, but the patient remained intubated. That said, SBTs carried out later in the day and evening/overnight were often described as being of value in that the results could be used to plan patient weaning for the following day, including in terms of extubation:
. . . but if it’s after that time [6 p.m.] we’ll wait until tomorrow and let’s just gear him up tomorrow . . . we’ll wean . . . we’ll stop sedation at 4 [a.m.], we are nil-by-mouth anyway at 4/6, and we’ll get going, first thing.
Research nurse, S07
. . . a lot of people would screen at 10 o’clock [p.m.] and do the SBT, but then be like ‘we’re not extubating tonight, we’ll do it in the morning’.
Research nurse, S14
Availability of appropriately skilled staff
Closely related to the preceding section, the impact of medical, especially consultant, availability on trial implementation was a consistent feature of participant accounts. As members of unit staff who were the most relied on to carry out extubations, when they were not available extubations were more likely not to be performed. Although all units were described as operating with a consultant ‘on-call’ system (with many reporting that on-call consultants remained off site), standard practice was that they would be called in emergency situations only and certainly not to perform routine/planned extubations. On occasion, when a consultant considered that a patient’s clinical status allowed safe extubation, instructions would be left for other qualified staff, typically registrars or ANPs, to do so. Otherwise, even when a patient had passed a SBT, they would remain intubated. This issue was sometimes discussed as something that would be particularly difficult to address given the entrenched nature of the NHS-wide system of ‘9–5’ working for senior medics, with only limited senior cover outside these hours. The tailoring of trial activity to take account of consultant availability to maximise the possibilities of timely extubation following successful SBTs was a regular feature of participant accounts. For example, in several units participants highlighted that SBTs scheduled for later in the day and at night-time were dropped because it had become clear that they only rarely led to extubations. In essence, it was seen as a waste of staff time:
. . . but sometimes they [a consultant on overnight call] may say if they feel the doctor’s [registrar] been here a long time and they’re competent and it’s going to be a straightforward extubation . . . and if they don’t need a paediatric or anaesthetist or anything like that, it would be safe.
Research nurse, S17
During periods of consultant absence, the lead responsibility for patient care fell to registrars across all units. Participant accounts highlighted limitations in weaning progression and/or extubations during such times, primarily based on a lack confidence among registrars. On occasion, an underlying lack of interest or disinclination on their part to become fully involved in weaning, including in the context of the SANDWICH intervention, was highlighted. In addition, the fact that registrars rotated through PICUs so quickly/frequently was associated with difficulties in ensuring that all were adequately trained in initiatives such as the SANDWICH intervention. Finally, a lack of confidence in a registrar’s skill set on the part of consultants and senior nursing staff could lead them to circumvent his or her involvement in extubations. The commonly described collective upshot was that patient weaning progress would be slowed, even halted, during periods when registrars were in charge of a unit. This could even include the performance of SBTs, with some participants describing situations in which registrars had refused to undertake a SBT until consultants were available. Although most often associated with overnight shifts, this scenario could also be outlined for day shifts, during which time consultants could be absent from the ward for extended periods:
I think sometimes some of the medics were unsure and would go, ‘well, we’d want to wait’. . . . so the junior doctors, the regs [registrars] would want to have consultant overview on it.
Senior nurse, S04
Registrar reluctance to take responsibility for progressing patient weaning was often discussed in the context of the NHS-wide system of medical trainee rotation. Although participants did not question the actual system, they did point out that it often left the unit reliant on staff who, through no fault of their own, possessed limited specialist knowledge and skills. Not only were registrars in receipt of large amounts of information in an unknown and challenging environment, but also a majority of registrars would not have a personal interest in pursuing a career as intensivists. For all of these reasons, registrars were described as frequently disinclined to take responsibility for anything other than what was necessary:
We have very few trainees who actually want to enter paediatrics.
Consultant, S07
Some units employed ANPs who were specialists trained in patient weaning/extubation and who were rostered on the same basis as registrars. At times, participants described the ANPs as central to the ability of the unit to progress weaning/extubation in the absence of consultant cover. In units where ANPs were employed but were considered not to use their weaning/extubations skills in everyday practice, a missed opportunity for trial implementation was highlighted:
The other thing is I think we have a fairly constant group of advanced nurse practitioners around who’ve been very supportive. So on the medical rota, there may be a registrar who’s not done ICU before and there’s an advanced nurse practitioner.
PI, S18
Or the ANPs would extubate them in the middle of the night but then the trainees wouldn’t.
Consultant, S11
A further relevant issue concerned the extent of physiotherapy involvement in weaning. In the vast majority of units, this involvement encompassed patient rehabilitation and the provision of advice on readiness to wean and/or extubate (e.g. in terms of secretion load). Members of the physiotherapy team were able to wean and extubate in only a limited number of participating units. In those units, physiotherapist participants highlighted the advantages of their involvement in terms of the availability of staff to execute SBTs and/or extubate. Conversely, in other units, physiotherapist participants were aware of the prescribed nature of their role, sometimes contrasting this with that pertaining in adult ICUs, where physiotherapists are routinely responsible for extubation:
I think the reason we probably were interested in the SANDWICH stuff was because we do do quite a bit of weaning on the ventilators, me and my other colleague who’s in a band below me. It’s taken quite a while to get our consultant colleagues to agree to that. But we do do quite a bit of weaning. Perhaps we’ll wean more readily than the trainee medics do, because we’re here all the time, a bit like the ANPs in that respect.
Physiotherapist, S18
The ‘ebb and flow’ of unit activity
Across all units, descriptions of the daily routine of unit activity consistently highlighted its impact on trial implementation. One such routine concerned the location of the main morning ‘handover’. In many units, the system involved initial off-unit discussions (in nearby rooms) involving the medical team and the senior nurse in charge/team leader for the day. Typically, the latter is responsible for collecting information on patient status prior to these discussions and communicating any plans that have been made regarding patient care back to relevant unit staff. Participants described this system as militating against patient weaning progress for several reasons. First, major decisions were taken without the input of the full multidisciplinary team, in particular bedside nursing staff. The need for fully informed decision-making, especially given that this promoted optimum patient weaning, was regularly highlighted. Second, the onus on the nurse in charge/team leader to carry information back and forth between the unit and the handover team represented a potential weak link in the chain of communication because patient information might not be collected, discussed and/or fed back. Notwithstanding, participant accounts made clear that off-unit handovers did not, in themselves, prevent adequate discussion and planning of patient weaning:
. . . that’s when decisions are made, in that handover around extubations, that kind of stuff. So that was where it needed to be discussed . . . But also, I think because we do the handovers away from the bedside. If that handover was done at the bedside then the nurse at the bedside might have more opportunity to be involved . . . The team leaders . . . So they were the ones to provide an update. I think it was challenging . . . to remind the team leaders to come with the correct information and to actually volunteer that information without having to keep being reminded.
Senior nurse, S01
In terms of other unit routines, a need to postpone weaning or extubation was discussed as an inevitable consequence of having to attend to other priorities of patient care. For example, a patient could have completed a successful SBT by the beginning of the day shift but not be extubated for several hours until the morning handover, ward round and/or medical assessment of patients had been completed. Alternatively, a patient could pass either a SBT screen or an actual SBT and be considered appropriate for extubation but remain intubated because of other care requirements, such as further investigations or clinical procedures. In other cases, the straightforward vagaries of unit activity were described as interfering in timely extubation, no matter how well plans had been made. Here, the need for medical staff to attend to other priorities was most often cited, with other factors, such as interruptions caused by staff lunch or breaks, also reported as likely to cause delays to (planned) activity:
. . . hopefully you’ll get extubated before 11 [a.m.], before ward round, but if they’ve done it at 5 [a.m.], that’s like 6 hours sometimes a gap, like in between if they have to do . . . there is a routine inside the unit and sometimes you don’t break the routine, because either it’s unsafe or people are not ready or things like that.
Research nurse, S15
Unit culture and staff customary working
The preceding section addressed the impact of existing unit routines on trial implementation. This section continues that general theme, focusing on participant understanding of underlying culture and customary ways of working because these were thought to both facilitate and impede trial implementation. ‘Culture’ is broadly conceived, encompassing the values, attitudes, beliefs and other ‘ways’ of thinking and doing, all of which were associated with unit staff’s response to the trial and the subsequent delivery of the trial.
With regard to deviation from the protocol in relation to sedation management, a number of factors were discussed. First, in terms of deviation, some participants talked about a preference among bedside nurses, especially the more inexperienced, to keep their patients ‘flat’. Some bedside nurses themselves acknowledged such a preference based on the greater patient safety it afforded. At times, a need to keep patients well sedated because of clinical need was highlighted, such as patients with neurological injuries. In all such cases, although the COMFORT scoring component of the protocol was considered to have improved sedation management, including in terms of encouraging a more ‘light touch’ approach to sedation and/or addressing patient pain rather than automatically increasing sedation, an impetus to oversedate was still identified. On occasions, oversedation was explicitly linked to patient failure of a SBT screen or SBT, with or without the appropriate use of trial exclusion criteria:
I still think they’re oversedated, because, there’s a terrible fright that they accidentally extubate, which, without any doubt, is dangerous, and there is literally a phobia in using other ways of making sure that patients don’t pull their tube out, like muffling . . .
Consultant, S16
I do personally like a well-sedated child. People say because it’s easier, not just because of that. I think as a bedside nurse, it can be really hard when you’ve got an awake child and sometimes that line is very fine of getting sedated enough that they’re awake to a point of being able to extubate but being settled and sedate . . .
Bedside nurse, S17
. . . because everyone likes a still patient . . . they would just put they’re low conscious level . . . so they’d still kind of use one of the SANDWICH kind of get out clauses without really thinking like ‘oh, it’s because they’re too sedated’, . . . but it would be on ward round, mainly when [name of PI] was doing it, if I’m honest. It would be like ‘right, they failed because they’re too sleepy, let’s start waking these people up’.
Research nurse, S10
With regard to potential deviation from the protocol in relation to ventilator weaning, again a range of factors were discussed. In terms of deviation, one such was a preference for weaning patients onto progressively lower ventilator settings before (being considered for) extubation and/or routinely ‘bagging’ a patient in preparation for extubation. Both approaches were talked about as established practice within the unit (to a greater or lesser degree) and, therefore, difficult to change, at least in the short to medium term. Another preference was a unit-wide preference to move a patient onto NIV as quickly as possible, which was considered likely to encourage deviation from the trial ‘5 and 5’ settings, because staff would automatically use higher settings even in situations in which a patient was being fully extubated. Finally, in the context of actions taken following a successful SBT in which extubation could not be performed, participants could highlight a consultant preference for patients to be returned to full ventilator support, given their aversion to leaving patients on Psupp for any length of time:
Yes, because people have worked here for 25 years and that’s always what they’ve done. So they’d be like ‘what’s the point in doing this, because that’s what we’re going to do anyway’.
Bedside nurse, S14
. . . I think, probably for most of us we were too careful . . . I don’t know if it’s because it’s cardiac or it’s the culture or whatever . . . a lot of people would think no, I have to slowly wean this child, gradually come down and then make sure that the child is OK by gradually coming down . . . No one will go from 22 over 8 to 5 over 5, and I think this intervention has proven us that we are probably too cautious with the extubation and the assessment if it’s standardised.
Consultant, S05
Another aspect of unit practice that was understood to facilitate or hinder the implementation of ventilator weaning in the SANDWICH intervention concerned feeding policy. In some units, participants described the routine practice of stopping feeds in the early morning (typically for a period of 4 hours) as having contributed to timely extubation when the early-morning SBT was successful. By contrast, in other units participants talked about how a lack of planning had, on occasion, meant that successful SBTs could not be followed up by extubation because the 4-hour feed withholding period had not been commenced in advance of/in line with the timing of the SBT:
For a few years now what we do is we stop all feeds at 6 o’clock for every child. So we only feed them over 20 hours anyway. So by the time you’ve done the ward round at 9.00, 9.30, you’ve made the decision, you’ve got half an hour and they’ve had 4 hours of starvation.
PI, S17
Well, we usually have to wait 4 hours from stopping feeding, so we usually then, if they managed on CPAP [continuous positive airway pressure] then we’d prepare them for extubation, but then you’d have to wait 4 hours, you have to stop feeding them.
Bedside nurse, S13
Participants could also talk about more ubiquitous features of unit culture given that these affected trial implementation and outcomes. Thus, in the context of discussing how likely the trial was to achieve a reduction in the overall length of time that patients spent on a ventilator, participants discussed a pre-existing culture of proactive practice, encompassing nursing and/or medical staff. The underlying message was that, irrespective of how well the unit adhered to the protocol, an existing culture of weaning patients quickly to the point of extubation and/or being prepared to extubate on relatively high ventilator settings would probably reduce impact in terms of trial outcomes:
The other thing is, I think, by personality, I think our consultant group are not a very cautious group in the sense that . . . they will just get on and wean and extubate. They’re not the kind who are nervous about doing procedures or nervous about extubating. Of course, there’s a spectrum, but as a group we are more towards the early extubators than the later extubators.
PI, S18
. . . we probably already had a culture of not giving it a go recklessly but actually being fairly aggressive in terms of our extubation policy.
Consultant, S07
In a different context, participants could associate perceived limitations in unit multidisciplinary collaboration with deficits in trial implementation. This could involve, for example, consultant acceptance or rejection of information and/or advice given by bedside nurses based on the perceived knowledge and expertise of the particular nurse involved, and/or senior nurses’ ‘interference’ in the process of communication between bedside nurses and medical staff. More broadly, a culture of bedside nurses not being involved in any aspect of ventilator weaning (except titrating oxygen levels) could also mean that they could be limited, or even excluded, by others and limit or exclude themselves from discharging in their trial role:
. . . I think there’s a slightly challenging culture here for hierarchy . . . We’re quite fortunate, the consultants are very approachable, but there is sometimes a bit of a chain of command . . . some of the other team leaders are not necessarily approachable . . . if they didn’t buy into that [SANDWICH], then they would say [to a bedside nurse] ‘oh, I wouldn’t bother talking to your doctor about that. I wouldn’t take that any further’, and that’s then a break in the chain of communication and so then they would be less likely to . . . I think that’s more of a culture here that’s the problem . . .
Senior nurse, S01
But also, if a junior nurse will say something at one time and get shot down, they will never speak about it again, and I think consultants need to recognise that if a tool that the unit is supposed to have adopted is being spoken about, then they should honour it.
Research nurse, S08
By contrast, participants talked about an existing culture of robust multidisciplinary collaboration as facilitating trial implementation. Various manifestations of such working were described: first, sharing clinical roles and responsibilities, including capitalising on the involvement of nursing staff in patient care, and, second, a shared predisposition among staff to work in support of one another. For example, in several units participants talked about senior managers being extremely supportive of allowing training to be undertaken in working hours and/or ‘rewarding’ staff for their participation by giving hours in lieu. In another unit, senior staff members talked about a sustained effort on their and their colleagues’ part in supporting the trial team, including undertaking regular spot checks on COMFORT scoring and SBT screens. Supportive working could even extend to adherence to the protocol among staff who were personally opposed to its use, but who did not want to undermine the unit as a whole. Elsewhere, participants described a positive ‘circle’ of behaviour whereby the observation of colleagues’ commitment encouraged the same commitment on the part of other staff. Thus, bedside nurse participants described their motivation at seeing senior members of staff proactively championing the trial. Typically, the senior staff in question were unit consultants, but other senior nursing staff were also mentioned:
We have a very good working relationship with our consultants, it’s very much if they did say something on ward round, I could completely disagree and it will either be a discussion . . . we’ll come to a compromise. I’ve had it multiple times where a consultant said ‘OK, let’s try it your way first and then we’ll see’ . . . and all of our consultants are like that, we are very lucky.
Research nurse, S10
You have to want to do it, don’t you? You have to have a willingness, and I think what was also very important is that the whole MDT [multidisciplinary team] were engaged in it and supporting it. So it wasn’t just like us nurses and we had resistance from other teams or anything, you know, the doctors wanted to do it, the nurses wanted to do it, the allied health professionals, the physios were aware of it, you know, everyone was aware of the terminology that we had to use, didn’t they?
Senior nurse, S11
Final authority rests with consultants
As foreshadowed elsewhere in this chapter, to a greater or lesser degree, participant accounts across all units stressed the final authority of consultants regarding patient care, and consequent vulnerability of the trial to their willingness to adhere to the protocol and to support the same adherence by other members of staff. In some units, the medical team was described as greatly facilitating unit-wide adherence by demonstrating manifest interest in, and support of, the trial and/or proactively encouraging adherence among colleagues. In other units, unit doctors were variously described as uninterested or even obstructive. In both contexts, as members of staff in positions of final authority, the approach of individual consultants and/or of a unit’s consultant body in general was understood to have played an important role in either promoting or impeding a unit’s adherence to the protocol:
The consultants were really on board with the study and really drove us to try and do it, and also the doctors would come round and they’d say, ‘well, we have to at least see if they’d like to try’. So yes, I think the consultants were really on board, and the research team would come round and double check.
PICU bedside nurse, S13
I think people sort of didn’t change, and I really wished the doctors had pushed it a bit more. I felt like it had to come from them a bit more, because I kept saying like it’s a really safe way, like you know if you’re really not sure then put them on this, and you know, if it doesn’t work then you’ve still got the tube in.
PICU research nurse, S14
Well, one of the consultants was pretty anti it, to speak frankly, and didn’t believe in it, and I think that then sowed seeds for other medics who think ‘well, if they think it’s ridiculous well why would I bother?’. And then that permeates down to other people as well.
PICU senior nurse, S01
Potential consultant deviation from the protocol was identified in respect of three trial-related activities: failure to adequately discuss/set plans for patient weaning during ward rounds, failure to perform a SBT and failure to extubate following a successful SBT. A number of contributory factors were discussed. Participants could describe what they understood to be straightforward consultant lack of interest in research generally. Participants also reported that some consultants' clinical care preferences led them to deviate from the protocol and/or ensure timely extubation. A wide range of examples emerged from participant accounts. These included insisting that they personally assess all patients before progressing, as per the protocol; instructing that a patient not be put on a SBT when, according to the protocol, a SBT should be performed; ‘skipping’ a SBT and moving straight to extubation; and/or refusing to authorise/perform an extubation when, according to the protocol, this was recommended. At times, some frustration was expressed in that the nurses could be left feeling that they had wasted valuable time:
. . . as far as I’m concerned, when I take care of my patients on the unit, it [SANDWICH] hasn’t made any difference, because my criteria for weaning and extubation are my own and I go around patients and turn the knobs and I do it all myself; and SANDWICH or no SANDWICH, I just turn the knobs and I extubate on the basis of criteria that are not just about how the ventilation is weaned but also how the patient behaves and is awake and all these things . . .
PICU consultant, S16
I think the ward round part of it, I had very differing experiences, depending the consultant that was on leading the ward round . . . I think the consultants had varying levels of interest. I mean, if there’s people interested in research then they were fully throwing themselves into it, other ones, you’d really have to encourage them to even use it.
PICU senior nurse, S03
Some participants focused on a different aspect of consultant final authority. They suggested that, although the protocol had promoted confidence and autonomy of practice among bedside nursing staff, especially the less experienced nurses, nurses’ role in weaning was ultimately dependent on that sanctioned by the unit consultants. If the unit consultants were trusting of an individual nurse, their opinion was more likely to be accepted and acted on:
I think it in a way it did give more autonomy and more independence to the nurse to say . . . I’ve done my COMFORT-B, you know, they’re adequately sedated or . . . my SBT is failing because they’re oversedated or something like that, but I still don’t think that maybe the medics would trust . . .
PICU bedside nurse (experienced), S07
Staffing policy and recruitment
In several units, participants associated ongoing staff recruitment issues with limitations in trial implementation. A lack of a full complement of consultants and/or frequent changes to the medical team were discussed as adversely affecting the unit’s adherence to the protocol for two main reasons: first, because existing consultants were under considerable pressures, and, second, because locum consultants were inevitably less versed in unit activity, such as SANDWICH, and more likely to bring greater diversity in terms of individual practice. The same issues could be identified in relation to the nursing workforce. Participants frequently talked about the unit skill mix as compromised because of a dependency on junior nurses, many of whom had been (relatively) recently recruited. Elsewhere, a dependency on bank and/or agency nurses was discussed as problematic:
So we’ve had a lot of locum consultants recently. I don’t think that probably helped, because they always have different ideas, regardless. I think our main consultants mostly sing from a similar hymn sheet, mostly.
Bedside nurse, S17
Yes, we’ve got quite a junior skill mix in our unit at the moment and we’ve had quite a lot of new starters over the last 12 months, and I think sometimes with the more junior nurses, it was the same as anything that’s kind of new to practice, it was, for them, having it on their time management radar, if that makes sense.
Senior nurse, S04
Summary conclusion
Pre- and post-trial interviews with 378 staff identified perceived barriers to and facilitators of protocol implementation and use that provided a narrative explanation of the acceptability and potential effectiveness of the intervention, including over time. Generally, adherence to sedation assessment and to daily screening for readiness for a SBT was high because those processes fitted within existing routines of care. In addition, they were the responsibility of bedside nursing staff, who could undertake these tasks independently. The adherence to setting targets on ward rounds was lower because of existing ward round practice and time pressures, as well as input from senior, particularly medical, staff. Performance of SBTs and, when appropriate, progression to extubation worked best in the early part of the day, again because of the fit with the norms of unit organisation and long-established practices of patient care, particularly with regard to the availability of senior medics. Other factors driving implementation were the support provided by the trial research team, the work of unit research nurses, buy-in from managers and senior staff, and a positive culture of working together among unit staff.
Across units, the intervention enhanced nurses’ understanding, confidence and autonomy of practice in relation to ventilator weaning. Conducting the daily screen for the breathing trial gave bedside nurses a designated role in ventilator weaning for the first time. There was widespread awareness of the intervention having improved multidisciplinary communication and collaboration. The crux of this improvement centred on the requirement to discuss weaning plans and the shared language provided by the trial to aid these discussions.
Chapter 6 Economic evaluation: methods and results
Overview of the economic evaluation
The primary objective of this within-trial economic evaluation was to measure the cost-effectiveness of the SANDWICH intervention compared with usual care in children anticipated to be ventilated for a prolonged period. A secondary objective was to determine whether or not the intervention caused additional harm; therefore, a cost-effectiveness analysis was performed with respiratory complications avoided as the health outcome of interest. The incremental cost-effectiveness ratio (ICER) for the analysis was the cost per respiratory complication avoided at 28 days from the date of recruitment. Although the National Institute for Health and Care Excellence’s preferred type of economic evaluation is a cost–utility analysis [estimating a cost per quality-adjusted life-year (QALY)], a meaningful cost–utility analysis could not be incorporated into this trial design. To obtain a QALY, health state utility data on individual participants would be required, measured using a generic preference-based instrument. This was not possible given that the study was approved by the research ethics committee for opt-out consent because of its low risk and the major challenges in obtaining written informed consent from parents with such a large recruitment target.
Considering that the study had a relatively short follow-up period, a hospital perspective was selected because this would be where the majority of the costs arise within the proposed period. Only hospital costs were included in the analysis. Costs and outcomes were evaluated over a 28-day time horizon starting from the date of the primary/index ICU admission. This period was considered appropriate because it allowed all participants to be followed up for the same length of time and it was expected that the majority of hospital costs (including re-admissions) would be captured during this time period, as observed in another trial in a similar paediatric population. 34 Given that the time horizon for the health economic analysis was shorter than 12 months, discounting costs and outcomes was not required, as recommended by the National Institute for Health and Care Excellence.
Health outcome
The health outcome of interest for the cost-effectiveness analysis was respiratory complications. The occurrence of the following respiratory complications at 28 days from PICU admission was measured: reintubation, unplanned extubation, tracheostomy, post-extubation NIV and post-extubation stridor. These data were collected in the CRF.
Measurement of resource use and costs
Patient-level resource use
Resource use data were collected prospectively using the CRF and from the participating site data downloads obtained directly from the PICANet. The PICANet data were transmitted from the sites to the NICTU electronically via e-mail. Data on the level of care for PICU bed-days and the corresponding Healthcare Resource Group (HRG) were generated by PICANet through the routine collection of the paediatric critical care minimum data sets by sites and were obtained for the purpose of the economic evaluation from the PICANet downloads. Given that a HRG is generated when a patient occupies a bed for ≥ 4 hours on any calendar day, the PICU length of stay was calculated in whole days only. General hospital ward length of stay was calculated using the PICU and hospital discharge dates recorded on the CRF. We were unable to calculate general ward length of stay for 71 participants who died in hospital after PICU discharge because the date of death, as an identifiable variable, could not be recorded. For the purpose of the analysis, the ward length of stay in these cases was assumed to be zero.
For participants who were discharged from the PICU and/or hospital prior to 28 days, data on PICU re-admissions within 28 days were obtained from the PICANet downloads using the patient ID variable to link participants. In addition, we presented the rates of emergency re-admissions within 48 hours for each group because this is a known quality indicator;56 this is defined as any unplanned admission to the same PICU or another PICU within 48 hours of the patient’s last discharge from the PICU. PICU re-admission data were available only for those participants who were re-admitted and eligible for the SANDWICH trial again. This was due to technical PICANet requirements. The PICUs had permission to download data from PICANet for the trial only if the child met the eligibility criteria (as indicated by a ‘yes’ in the registry’s download menu). Any record related to the patient that did not meet this criterion could not be included in the download. Data on re-admissions directly to general hospital wards within this time were not collected. This was expected to lead to only minimal data loss, given that the re-admission rate within 30 days in a similar paediatric population was observed to be low (5%), with a mean hospital length of stay of < 1 day. 34 Furthermore, these hospital re-admissions were unlikely to be related to any respiratory complications that patients experienced during their index PICU admission.
Of note, for the clinical trial analyses, re-admitted participants were treated as independent events (i.e. they were treated as new participants to the trial). Thus, the sample size reported for the two analyses differed.
Intervention-related resource use
SANDWICH was a multidisciplinary, multicomponent intervention that aimed to standardise the process of weaning and involved weaning sedation and liberation from ventilation. Many of the components incorporated in the intervention were already embedded in usual practice, albeit not formalised (assessing and titrating sedation, assessing and weaning ventilation, and the ward round). As a result, measuring the time involved in delivering the intervention in this complex environment would have been difficult and may have influenced staff behaviour, thus threatening internal validity. Consequently, the economic evaluation focused on estimating the resource use and costs associated with intervention training delivered during the 8-week training phase and the materials necessary to support the training if the intervention was to be implemented in another PICU in the future.
Thus, intervention-related resource use was collected prospectively by the implementation manager over the study period. In keeping with economic evaluations of other behavioural interventions,57–59 resource use was categorised according to the stage that it was used in the research process: planning and preparation for delivery (stage 1) and intervention delivery (stage 2). Pre-start-up resources associated with the development of the SANDWICH intervention and the design of the training materials were not included in the analysis because they would not be incurred should the intervention be adopted into clinical practice in the future. The breakdown of intervention resources is shown in Table 14.
| Item | Description |
|---|---|
| Stage 1: planning and preparation for delivery | |
| Initial training of the champions by implementation manager | Total number of hours spent training the site staff |
| Number of training sessions per site | |
| Duration of each session per site | |
| Number of champions trained, job titles and grade | |
| Training of site staff by champions | Number of staff trained, job titles and grade |
| Duration of training sessions | |
| Venue/facility hire | Hire and management of facilities to conduct training |
| Materials | Training manual |
|
|
| Other: hosting the e-learning module from LearnPro | |
| Stage 2: delivery | |
| Delivery materials | Champion packs (lanyards, name badges), bedside packs |
| Staff resource | Positive or negative effects on staff time (open comments) |
Costs were obtained directly from the implementation manager and/or the wider research team when possible (e.g. printing costs). The costs associated with training site staff were estimated by attaching the appropriate rate per hour to each staff member’s time input using the most up-to-date unit costs (see Appendix 3). The training programme for SANDWICH involved local champions being appointed at each site to act as facilitators within sites and assist in the roll-out and endorsement of the intervention. Given that all site staff had to be trained in preparation for switching from the control to the intervention period, a conservative approach to estimating the cost per patient was adopted in the first instance. The total cost for SANDWICH at each site was calculated and then divided by the total number of unique patients recruited at the site (over both the intervention and the control periods) because this would better reflect the actual numbers of patients who would be affected by the intervention over the same time period in a non-research scenario. A sensitivity analysis was performed to calculate the cost per patient at each site by dividing the total intervention cost by the number of intervention patients recruited at each site.
Unit costs
Patient-level hospital data were combined with unit costs to estimate the costs for each participant (see Appendix 3). The price year was set at 2018/19. To cost PICU and hospital ward stay, we used the NHS Reference Costs. 60 PICANet provided HRG codes for each PICU day and we attached the appropriate unit cost from the paediatric intensive care section. For ward-days, we calculated a weighted average bed-day cost using the average length of stay and cost of non-specific neonatal/paediatric long stays. For staff costs associated with the intervention, we used the unit costs of health and social care from the Personal Social Services Research Unit. 61 The price year was set at 2018/19.
Statistical analysis of costs and outcomes
Analyses were undertaken in Stata 15.1. Descriptive statistics were used to summarise hospital service use (length of PICU stay, level of care and general hospital ward length of stay), costs and respiratory complications. Given that no data were collected by the sites during the 8-week training period, data on respiratory complications and hospital resource use were censored from the point that the sites transitioned from the control to the training period. Given that the health economic analysis linked patients’ re-admissions within 28 days of their primary/index admission using the patient ID variable provided by PICANet, some patients may have been re-admitted to the PICU. Patients were analysed according to their original arm. Multilevel mixed-effects regression modelling was used for total costs and respiratory complications, adjusting for calendar time and clustering.
Cost-effectiveness analysis
Outputs from the multilevel mixed-effects regression modelling were used to estimate incremental (differential) costs, incremental respiratory complications and the ICER. This is a measure of the additional cost per additional unit of effect produced by one intervention compared with another, and was calculated as the ratio of the difference in means costs divided by the difference in mean effects between the intervention period and the control period. For the purpose of the cost-effectiveness analysis, respiratory complications were reported in terms of cases avoided; therefore, a positive difference in respiratory complications avoided indicated a smaller number of respiratory complications in patients in who received the SANDWICH intervention. A negative cost difference indicated a cost saving in favour of the intervention period. The ICER for this study was, therefore, the cost per respiratory complication avoided. Given that negative ICERs are not meaningful, if this occurred we stated whether or not the intervention was dominant (i.e. more effective and less costly than the control) or was dominated (less effective and more costly than the control).
Uncertainty in the within-trial cost-effectiveness estimates was explored by bootstrapping the incremental costs and effects to generate 1000 replications of the ICER, plotting them on the cost-effectiveness plane and constructing a cost-effectiveness acceptability curve (CEAC). This involved a series of lines being placed on the plane representing the different willingness-to-pay (WTP) thresholds per respiratory complication avoided that a decision-maker may have. The proportion of ICER replicates falling below each WTP threshold equates to the probability of the intervention being cost-effective at that threshold. Given that there is no commonly agreed threshold value for cost per respiratory complication avoided, a range of plausible thresholds will be explored. The CEAC was also derived using a net-benefit regression framework. The net monetary benefit (NMB) is a summary statistic representing the value of an intervention in monetary terms when a WTP threshold for a unit of benefit is known. A positive NMB indicates that the intervention is cost-effective and, therefore, can aid interpretation of results when ICERs cannot be interpreted because they are negative. Multilevel mixed-effects modelling was performed using the NMB as the dependent variable at various WTP values. 62,63 We used the p-values from the net benefit regression to calculate the probability of cost-effectiveness following the method described by Hoch et al. 64 to generate a CEAC. 63
Sensitivity analysis
The robustness of the results from the cost-effectiveness analysis was explored via the following one-way sensitivity analyses.
Planned
-
All patients.
-
Adjusting for the covariates age, severity of illness (PIM3 score), respiratory compared with other diagnostic grouping, type of admission (planned/unplanned) and reason for admission (surgical/medical).
Post hoc
-
Change in the calculation of intervention costs: the intervention cost per patient was estimated by dividing total intervention costs by the number of unique patients in the intervention arm only.
-
Change in the calculation of total respiratory complications: all post-extubation NIVs were excluded and unplanned extubations were excluded if they were successful/well tolerated (defined as not requiring reintubation within 48 hours).
Results
For the health economic analysis, we linked patients’ re-admissions within 28 days of their primary/index admission using the patient ID variable provided by PICANet. A count of this variable indicated that 8755 individual patients were responsible for the 10,495 admissions included in the trial. However, on examination of their clinical pathways (i.e. re-admission and discharge dates), it was observed that for 53 patients (53/8755; 0.6%) there were some instances of overlap in their re-admission and discharge dates. Raising queries with sites and PICANet was not possible at the point of discovery; therefore, a decision was made to move the ‘overlaps’ to a new patient ID so that they could remain in the analysis. This resulted in a total of 8808 (intervention, n = 4608; control, n = 4200) unique patients for the health economic analysis; 7318 patients were in the prolonged ventilation population.
Respiratory complications
The respiratory complications for the anticipated prolonged population are presented in Table 15. A larger mean number of all complications except post-extubation stridor was observed in the intervention arm, although only unplanned extubations and post-extubation use of NIV were significantly different. As part of the post hoc sensitivity analysis concerning which AEs were considered to be respiratory complications, we also calculated the number of unplanned reintubations that were followed by a reintubation within 48 hours in this population.
| Complication | Intervention period (N = 3758) | Control period (N = 3560) | Mean difference in complications (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean (95% CI) | n (%) | Mean (95% CI) | ||
| Reintubation | 432 (11.5) | 0.15 (0.13 to 0.18) | 401 (11.3) | 0.13 (0.10 to 0.16) | 0.03 (–0.01 to 0.06) |
| Unplanned extubation | 121 (3.2) | 0.04 (0.03 to 0.05) | 93 (2.6) | 0.02 (0.01 to 0.03) | 0.02 (0.00 to 0.03) |
| Reintubation within 48 hours | 46 (1.2) | 0.02 (0.01 to 0.02) | 48 (1.4) | 0.01 (0.01 to 0.02) | 0.00 (–0.00 to 0.01) |
| Tracheostomy | 27 (0.7) | 0.01 (0.00 to 0.01) | 17 (0.5) | 0.01 (0.00 to 0.01) | 0.00 (–0.00 to 0.01) |
| Post-extubation NIV | 611 (16.3) | 0.18 (0.15 to 0.22) | 468 (13.2) | 0.13 (0.09 to 0.17) | 0.05 (0.02 to 0.08) |
| Post-extubation stridor | 342 (9.1) | 0.12 (0.08 to 0.15) | 319 (9.0) | 0.12 (0.09 to 0.16) | –0.01 (–0.03 to 0.02) |
| Total number of complications | – | 0.50 (0.43 to 0.57) | – | 0.41 (0.33 to 0.48) | 0.10 (0.03 to 0.16) |
Participant-level hospital resource use
Patients’ use of hospital resources (Table 16) and the associated costs up to 28 days (Table 17) for the anticipated prolonged patients showed a larger total number of PICU days, general ward-days and overall hospital days in the intervention period than in the control period, but the overall differences between the mean days in each period were small and not statistically significant. In keeping with the significantly higher rates of NIV observed in the intervention period than in the control period, there was a statistically significantly larger number of days spent at a high-dependency advanced level (XB06Z HRG level) that would include NIV. The larger number of days resulted in the higher total hospital costs observed in the intervention period than in the control period (mean difference of £894 per patient), but the difference in costs was not statistically significant between the observation periods.
| Hospital resource | Observation period | Mean difference (95% CI) | |
|---|---|---|---|
| Intervention (n = 3758) | Control (n = 3560) | ||
| Primary (index) admission, mean (95% CI) | |||
| PICU days | 7.38 (6.86 to 7.90) | 7.24 (6.71 to 7.76) | 0.14 (–0.42 to 0.69) |
| General ward-days | 5.57 (4.67 to 6.46) | 5.43 (4.53 to 6.32) | 0.14 (–0.46 to 0.74) |
| Hospital length of stay | 12.95 (12.04 to 13.86) | 12.70 (11.79 to 13.61) | 0.25 (–0.51 to 1.00) |
| Re-admissions | |||
| Total, n (%) | 232 (6.2) | 201 (5.7) | – |
| Of which within 48 hours, n (%) | 104 (2.8) | 72 (2.0) | – |
| Total (n) re-admissions per patient, mean (95% CI) | 0.08 (0.06 to 0.10) | 0.05 (0.03 to 0.07) | 0.03 (0.01 to 0.05) |
| PICU days | 0.59 (0.44 to 0.74) | 0.39 (0.23 to 0.54) | 0.21 (0.01 to 0.41) |
| General ward-days | 0.21 (0.10 to 0.31) | 0.14 (0.04 to 0.25) | 0.06 (–0.09 to 0.22) |
| Total PICU days (n) (primary and re-admissions), mean (95% CI) | 7.98 (7.44 to 8.52) | 7.61 (7.07 to 8.16) | 0.36 (–0.22 to 0.95) |
| Intensive care: ECMO/ECLS (XB01Z) | 0.16 (0.04 to 0.28) | 0.12 (–0.01 to 0.24) | 0.04 (–0.09 to 0.18) |
| Intensive care: advanced enhanced (XB02Z) | 0.08 (0.03 to 0.13) | 0.11 (0.06 to 0.16) | –0.03 (–0.07 to 0.01) |
| Intensive care: advanced (XB03Z) | 0.50 (0.36 to 0.64) | 0.46 (0.32 to 0.60) | 0.04 (–0.13 to 0.21) |
| Intensive care: basic enhanced (XB04Z) | 1.48 (1.09 to 1.88) | 1.54 (1.14 to 1.94) | –0.06 (–0.34 to 0.23) |
| Intensive care: basic (XB05Z) | 3.36 (2.86 to 3.86) | 3.50 (3.00 to 4.01) | –0.14 (–0.50 to 0.22) |
| High dependency: advanced (XB06Z) | 1.22 (0.96 to 1.47) | 0.88 (0.62 to 1.13) | 0.34 (0.15 to 0.53) |
| High dependency (XB07Z) | 0.52 (0.39 to 0.64) | 0.46 (0.34 to 0.59) | 0.05 (–0.05 to 0.16) |
| Enhanced care (XB09Z) | 0.62 (0.44 to 0.80) | 0.52 (0.34 to 0.70) | 0.10 (0.00 to 0.20) |
| Ungrouped | 0.05 (0.00 to 0.09) | 0.07 (0.02 to 0.11) | –0.02 (–0.09 to 0.05) |
| Total general ward-days (n), mean (95% CI) | 5.82 (4.92 to 6.71) | 5.57 (4.67 to 6.46) | 0.25 (–0.36 to 0.85) |
| Total hospital days (n), mean (95% CI) | 13.80 (12.88 to 14.72) | 13.22 (12.30 to 14.14) | 0.58 (–0.20 to 1.36) |
| Healthcare Resource Group level | Mean cost (£) (95% CI) | Mean difference in cost (£) (95% CI) | |
|---|---|---|---|
| Intervention period (n = 3758) | Control period (n = 3560) | ||
| Level of care | |||
| Intensive care: ECMO/ECLS (XB01Z) | 717.24 (177.21 to 1257.27) | 517.79 (–25.02 to 1060.61) | 199.45 (–400.38 to 799.27) |
| Intensive care: advanced enhanced (XB02Z) | 296.22 (99.41 to 493.03) | 411.22 (214.10 to 608.34) | –115.00 (–269.83 to 39.83) |
| Intensive care: advanced (XB03Z) | 1416.64 (1010.10 to 1823.18) | 1307.82 (898.30 to 1717.33) | 108.82 (–382.94 to 600.58) |
| Intensive care: basic enhanced (XB04Z) | 3969.84 (2902.69 to 5037.00) | 4119.45 (3051.06 to 5787.83) | –149.62 (–901.60 to 602.39) |
| Intensive care: basic (XB05Z) | 7480.18 (6364.02 to 8596.35) | 7793.86 (6676.37 to 8911.35) | –313.67 (–1109.14 to 481.79) |
| High dependency: advanced (XB06Z) | 2271.75 (1795.33 to 2748.17) | 1634.62 (1157.54 to 2111.70) | 637.13 (278.57 to 995.68) |
| High dependency (XB07Z) | 816.74 (621.05 to 1012.43) | 730.50 (534.45 to 926.56) | 86.24 (–76.08 to 248.56) |
| Enhanced care (XB09Z) | 637.19 (454.29 to 820.09) | 533.20 (350.18 to 716.21) | 103.99 (0.03 to 207.95) |
| Ungrouped | 93.44 (–0.04 to 186.92) | 139.37 (43.47 to 235.27) | –45.92 (–191.39 to 99.54) |
| Total PICU cost | 17,738.76 (16,464.99 to 19,012.53) | 17,091.56 (15,810.42 to 18,372.70) | 647.20 (–809.22 to 2103.63) |
| Total ward-day cost | 6177.09 (5229.10 to 7125.07) | 5907.16 (4958.10 to 6856.21) | 269.93 (–394.80 to 934.67) |
| Total hospital costs | 23,925.58 (22,521.83 to 25,329.33) | 23,031.26 (21,620.81 to 24,441.71) | 894.32 (–634.33 to 2422.97) |
For all patients, respiratory complications, hospital resources and costs showed similar results to those in the prolonged cohort. Results for all patients are reported in Appendix 3.
Intervention costs
A full breakdown of the resources and associated costs used in the planning and preparation and the delivery of the intervention for all sites is presented in Table 18. In the planning and preparation stage, the implementation manager delivered face-to-face training at all sites to the designated champions. These sessions lasted, on average, 4 hours and have been broken down by staff level, which included doctors, nurses and allied health professionals. An e-learning module was created on the learning management system LearnPro for all site staff, which included both learning and assessment sections and took approximately 2 hours to complete. Given that the online training was included in the champions’ training, the champions were not required to complete it. Training was broken down by staff level for each site. One site did not provide this detail, so we made the assumption that the proportion of different staff levels completing the training at this site was similar to that of the other sites. The amount of time that staff spent in training was given a monetary value by using hourly rates published by Personal Social Services Research Unit,61 and LearnPro incurred a one-off cost of £11,850.
| Resource use | Unit cost (£) | Number of units | Number of hours | Total cost (£) |
|---|---|---|---|---|
| Planning and preparation for delivery (stage 1) | ||||
| Intervention materials/equipment | ||||
| Site training manual (64 pages) | 5.20 | 18 | NA | 94 |
| Champion packs (64 pages) | 5.20 | 322 | NA | 1674 |
| Poster pack (34 per pack) | 11.18 | 36 | NA | 402 |
| Bedside pack (34 pages) | 2.11 | 264 | NA | 557 |
| LearnPro set-up and maintenance | 11,850.00 | 1 | NA | 11,850 |
| Intervention training | ||||
| Intervention implementation manager (salary £50,900 over 17 months) | 76,350.00 | 1 | NA | 76,350.00 |
| Site champion: medicine | ||||
| NCHD | 47.00 | 21 | 4 | 3948 |
| Consultant | 109.00 | 36 | 4 | 15,696 |
| Site champion: nursing | ||||
| Band 5 nurse | 38.00 | 78 | 4 | 11,856 |
| Band 6 nurse | 47.00 | 94 | 4 | 17,672 |
| Band 7 nurse | 55.00 | 55 | 4 | 12,100 |
| Band 8a nurse | 65.00 | 3 | 4 | 780 |
| ANP | 55.00 | 14 | 4 | 3080 |
| Site champion: AHP | ||||
| Band 4 | 32.00 | 2 | 4 | 256 |
| Band 5 | 35.00 | 4 | 4 | 560 |
| Band 6 | 47.00 | 7 | 4 | 1316 |
| Band 7 | 57.00 | 5 | 4 | 1140 |
| Non-champion staff | ||||
| Non-consultant hospital doctor | 47.00 | 158 | 2 | 14,852 |
| Consultant | 109.00 | 96 | 2 | 20,928 |
| Band 4 nurse | 28.00 | 5 | 2 | 280 |
| Band 5 nurse | 38.00 | 841 | 2 | 63,916 |
| Band 6 nurse | 47.00 | 281 | 2 | 26,414 |
| Band 7 nurse | 55.00 | 120 | 2 | 13,200 |
| ANP | 55.00 | 22 | 2 | 2420 |
| Band 6 AHP | 47.00 | 23 | 2 | 2162 |
| Implementation (stage 2) | ||||
| Materials | ||||
| Lanyard card (champions) | 0.60 | 319 | NA | 191 |
| Name badge (champions) | 0.40 | 319 | NA | 128 |
| Pens (all staff) | 0.50 | 1865 | NA | 933 |
| Core bundle lanyard card (all staff) | 0.60 | 1865 | NA | 1119 |
| Total | 305,874 | |||
| n | 8808 | |||
| Mean cost per patient (£) | 34.73 | |||
The implementation manager’s salary for 17 months was included in the overall intervention cost because their time during this period was solely dedicated to the day-to-day activities related to education in the trial, such as answering queries, site engagement and refresher training. They were also contactable via telephone, text message and e-mail all in the name of correctly delivering the intervention. If SANDWICH was to be fully implemented in clinical practice, this role would be taken up by a clinical nurse educator. For the purposes of calculating site costs, it was assumed that the time allocated to each site by the implementation manager was proportionate to the number of staff working on the study there.
Materials used in the delivery of the intervention included site intervention training manuals, packs of training posters, bedside packs for each physical bed space and laminated lanyard cards containing core aspects of the intervention for all staff. Champions were also provided with separate lanyards and name badges. Pre-start-up costs associated with the development of the training materials and e-learning module were not included in the analysis because these were non-recurring costs. Given that all training was conducted on site at hospitals, there were no venue hire costs.
In total, 1865 members of staff across 17 sites were trained within the initial 8-week training period. The total cost of delivering the intervention was approximately £305,874. This is an estimated £34.73 per patient based on the number of individual patients in the trial (n = 8808). Although recognising that not all patients received the intervention, this is more representative of a real-world cost should it be implemented because it provides an estimated throughput of patients through each PICU. For the purposes of the sensitivity analysis, the total intervention cost was divided only by the total number of unique patients recruited to the intervention arm (n = 4608), adjusting the estimated cost per patient to £66.38.
Cost-effectiveness analyses results
The results of the cost-effectiveness analysis and the sensitivity analyses are shown in Table 19. The cost per patient for the intervention (£34.73) was added to the hospital costs for each patient in the intervention period to generate the total costs. On average, total costs were higher (£929) for the intervention patients than for the control patients and a larger number of respiratory complications were experienced by the intervention patients (mean difference/patient 0.10) than the control patients. For all analyses (primary and sensitivity), the difference in total costs was never statistically significant; however, the difference in complications was significant for all analyses except for the post hoc sensitivity analyses that excluded all post-extubation NIVs and excluded the unplanned extubations that were not followed by a reintubation within 48 hours. The control period, therefore, dominated the intervention period because it was associated with lower total costs and fewer respiratory complications, on average, in all of the analyses.
| Analyses | Mean difference in costs (£) (95% CI) | Mean difference in number of respiratory complications avoideda (95% CI) | ICERb |
|---|---|---|---|
| Primary (base-case) analysis | |||
| Prolonged IMV cohort | 929.05 (–516.54 to 2374.64) | –0.10 (–0.16 to –0.03) | Control dominant |
| Sensitivity analyses | |||
| All patients | 714.48 (–618.86 to 2047.83) | –0.09 (–0.15 to –0.03) | Control dominant |
| Adjusted for baseline characteristics | 993.77 (–484.92 to 2472.46) | –0.10 (–0.17 to –0.04) | Control dominant |
| Change in calculation of intervention costc | 960.70 (–484.89 to 2406.29) | –0.10 (–0.16 to –0.03) | Control dominant |
| Change in calculation of complicationsd | 929.05 (–516.54 to 2374.64) | –0.03 (–0.08 to 0.02) | Control dominant |
Uncertainty surrounding these estimates of total costs and outcomes is represented by the bootstrapped ICERs on the cost-effectiveness plane for the primary cost-effectiveness analysis (Figure 10). The majority of points lie in the north-west quadrant, reflecting the fact that patients receiving the SANDWICH intervention were likely to have higher costs and more respiratory complications than the control patients, although there is some variability about the cost estimates.
FIGURE 10.
Cost-effectiveness plane for the primary cost-effectiveness analysis. The 28-day post-PICU admission shows bootstrapped replications of mean incremental costs and respiratory complications avoided; north-east (NE), SANDWICH is more costly and associated with fewer respiratory complications than control; south-east (SE), SANDWICH less costly and associated with fewer respiratory complications than control; north-west (NW), SANDWICH is more costly and associated with more respiratory complications than control; south-west (SW), SANDWICH is less costly and associated with more respiratory complications than control.
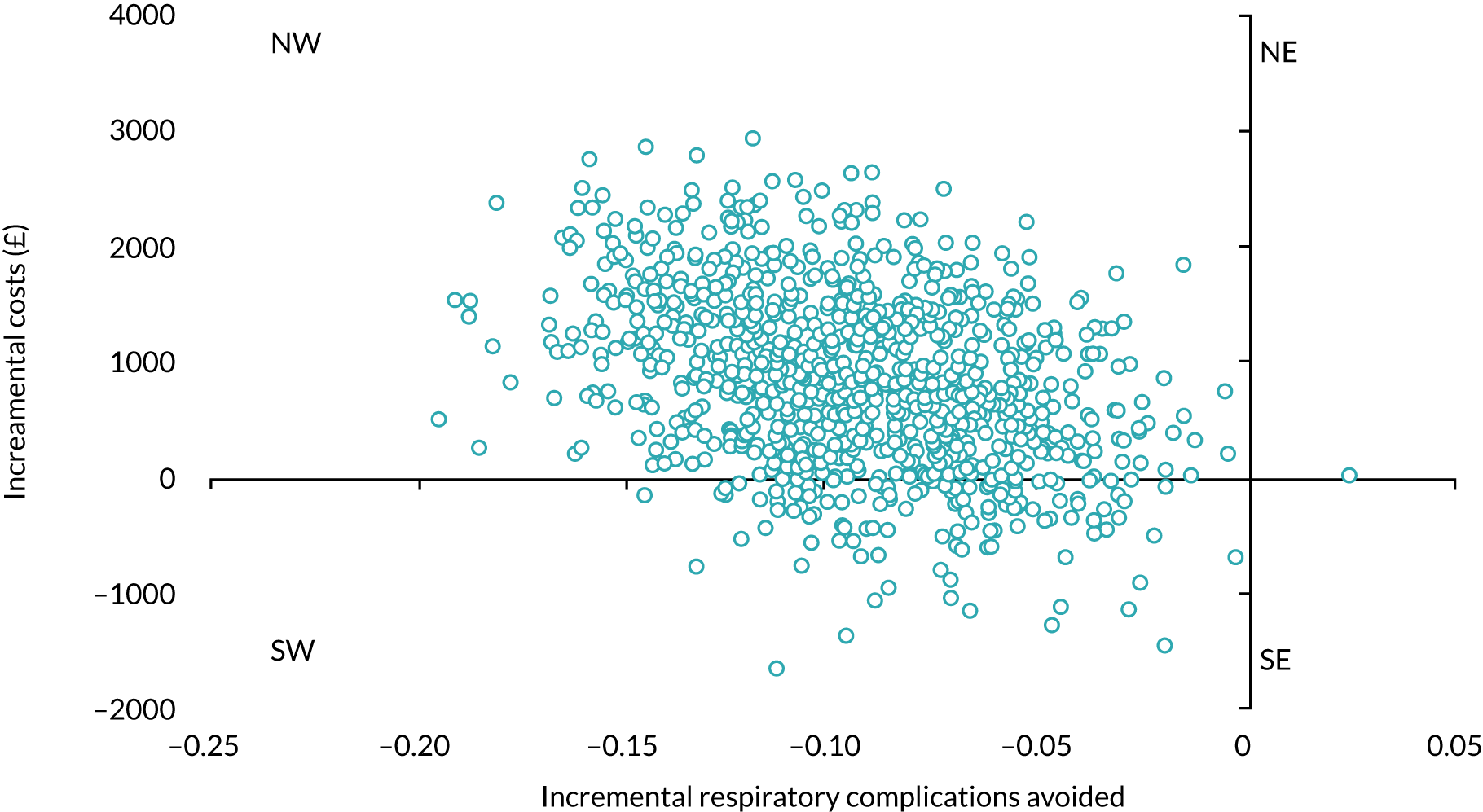
The CEAC for the primary cost-effectiveness analysis is shown in Figure 11. Given that there is no commonly agreed WTP threshold value for the cost per respiratory complication avoided, we considered thresholds ranging from £0 to £2500. It can be seen that the probability of the intervention being cost-effective compared with the control is only 12% at a WTP threshold of £0 and then it drops to zero. This reflects that, although there may be some occasions when the intervention involved cost savings, in virtually all occasions (bar one bootstrapped ICER) the intervention was associated with a larger number of respiratory complications. The sensitivity analyses yielded similar results.
FIGURE 11.
Cost-effectiveness acceptability curve (prolonged cohort). This shows the probability of SANDWICH being cost-effective compared with control at various values of WTP per respiratory complication avoided.
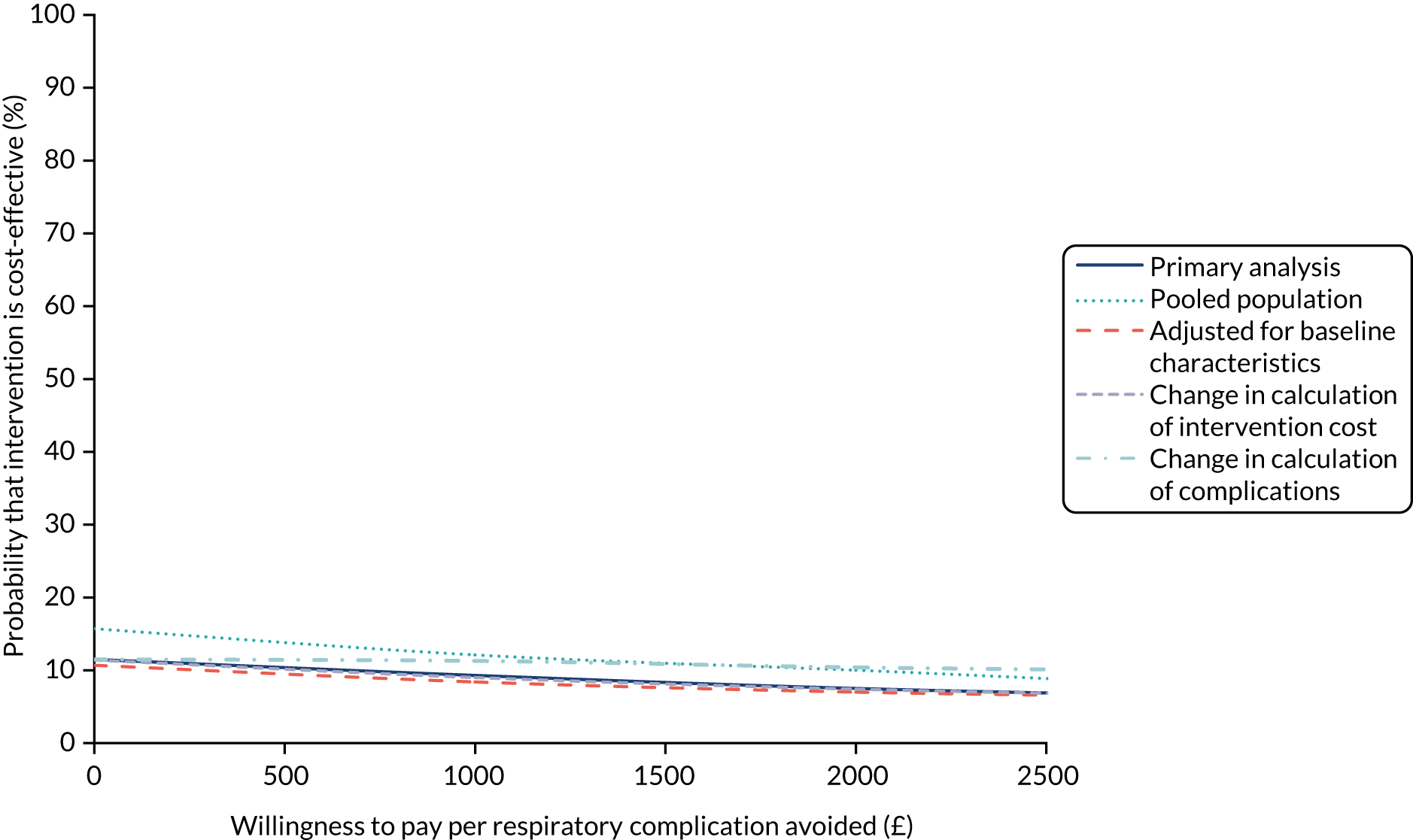
Incremental net benefit framework
The results of the incremental net benefit regression analysis are presented in Table 20. The incremental net benefit was negative for all values of WTP per complication avoided, which indicates that the intervention is not cost-effective compared with the control. The resulting CEAC in Figure 12 is similar to the CEAC for the primary cost-effectiveness analysis generated using bootstrapping (see Figure 11).
| WTP (£) | Incremental net benefit (95% CI) |
|---|---|
| 0 | –714.48 (–2087.25 to 658.28) |
| 500 | –767.71 (–2150.25 to 614.83) |
| 1000 | –821.61 (–2214.36 to 571.14) |
| 1500 | –876.10 (–2279.50 to 527.30) |
| 2000 | –931.24 (–2345.70 to 483.23) |
| 2500 | –986.99 (–2412.93 to 438.94) |
FIGURE 12.
Cost-effectiveness acceptability curve for the incremental net benefit framework. This shows the probability of SANDWICH being cost-effective compared with control at various values of WTP per respiratory complication avoided.
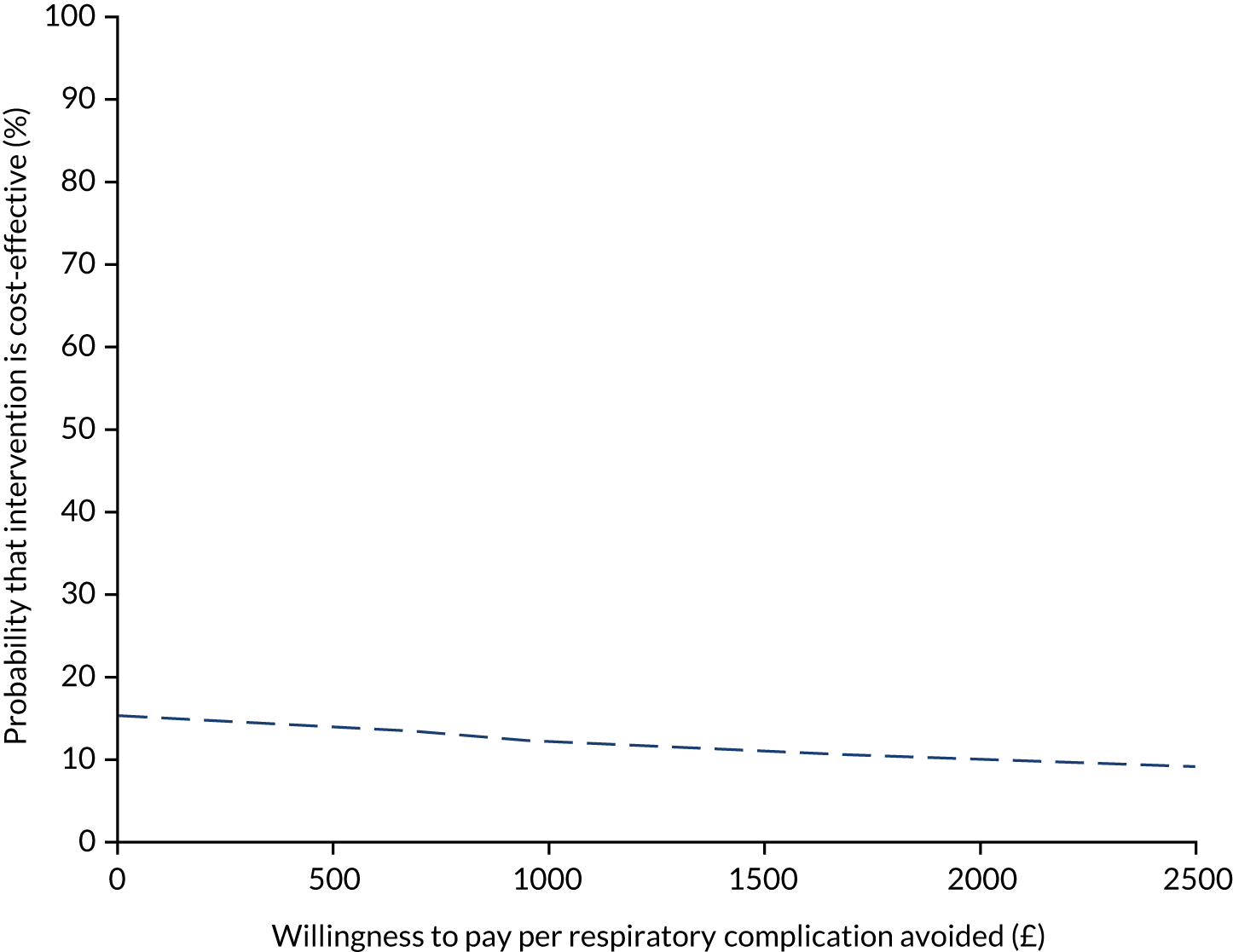
Summary of the health economic analysis
The total cost to deliver the SANDWICH intervention was approximately £305,574 and involved training 1865 staff across 17 sites. At £34.73 per patient, SANDWICH is arguably a relatively low-cost intervention. The outcome of interest for the economic evaluation was the number of respiratory complications, and our results indicated that more complications were observed, on average, in patients who received the SANDWICH intervention than in patients in the control period. Although the mean difference was small (0.10) and statistically significant, it was associated with higher mean hospital costs (£894); however, this cost difference was not statistically significant. The same pattern of results was observed in all patients. The results of the cost-effectiveness analysis indicated that the probability of the intervention being cost-effective compared with the control was low at all levels of WTP to avoid a respiratory complication, never going above 12%.
The difference in the number of complications was largely a result of the significantly higher incidence of unplanned extubation and post-extubation NIV in the intervention arm. There were no differences in the incidence of tracheostomy insertion, post-extubation stridor or reintubation. Unplanned extubation, when followed by reintubation, has been associated with a higher risk of mortality. 65,66 However, we found no difference between groups in the number of unplanned extubations followed by reintubation within 48 hours, and the clinical trial reported no difference in either PICU or hospital mortality.
It is important to highlight that different approaches were used in the health economic and main statistical analysis. In the main statistical analysis, each admission was treated individually, which resulted in a sample size of 10,495, and the relative risk of each complication was calculated. By contrast, the health economic analysis linked patients’ re-admissions up to 28 days with their primary/index admission, which resulted in a sample size of 8808, and we calculated the total numbers of each complication. Despite this, the findings were compatible.
Strengths of the economic evaluation include the high level of data quality and completeness as a result of utilising PICANet’s routinely collected data. This enabled the accurate estimation of PICU costs for every patient in the study. In addition, we worked closely with the SANDWICH implementation manager throughout the study to ensure the accurate and prospective collection of the key resources used in the preparation and delivery of the intervention. This shows the high level of resource input required to implement a complex intervention on such a large scale.
A small limitation of the analysis was that the duration of ward stay could not be calculated for those patients who died in hospital after PICU discharge. Dates of death were not recorded because we did not have permission to collect this information. For these patients we imputed their ward length of stay as zero, which may have led to an underestimation of their hospital costs; however, given that this affected only 71 children, the impact on the overall means costs would have been minimal. We also did not collect data on re-admissions directly to the ward, but these were unlikely to be related to any respiratory complications experienced during the index PICU admission. We had data on re-admissions to the PICU only for those patients who were re-admitted and eligible again for the trial; this may have led to some data loss. Furthermore, some patients may have been discharged from the PICU to their local hospital for rehabilitation and, therefore, we would not have data in these cases either. We acknowledge that linking to other routine data sets (e.g. Hospital Episode Statistics) would have given us a more complete data set of patients’ overall hospital resource use. Another limitation of the analysis relates to the choice of outcome for the CEAC. Respiratory complications were selected to align with a secondary objective of the trial: to assess whether or not the intervention caused additional harm. The selection of respiratory complications was made at the protocol design stage informed by the literature and the clinical opinion of co-investigators. However, despite a weak evidence base,67,68 the use of post-extubation NIV in the paediatric setting has increased because it is thought to protect the very immature lung. Given that post-extubation NIV may not have been viewed as an AE at sites, we performed a post hoc sensitivity analysis to explore the impact of excluding all post-extubation NIV events. We also excluded those unplanned extubations that were not followed by reintubation within 48 hours from the total complication count. Excluding these complications changed the effect in that it was no longer statistically significant; however, the change did not meaningfully affect the probability of the intervention’s cost-effectiveness owing to the higher costs associated with intervention patients.
The findings from the economic evaluation indicate that the SANDWICH intervention is associated with higher hospital costs and this probably reflects the larger number of respiratory complications observed in the intervention arm. The intervention had a low probability of being cost-effective in reducing respiratory complications, and the sensitivity analysis showed this outcome to be robust to changes in different parameters.
Chapter 7 Discussion and conclusion
In this large, multicentre, pragmatic trial, the SANDWICH intervention led to a significant, albeit small, reduction in time to successful extubation, of 6 and 7 hours (prolonged cohort and all children, respectively). In the prolonged IMV cohort, the intervention was associated with a significantly higher incidence of successful extubation and the use of NIV post extubation. We found no evidence that the reduction in time to successful extubation in the intervention period resulted in a shorter PICU length of stay and, indeed, hospital stay was longer. From a safety perspective, there was an increased risk of unplanned extubation in the intervention period without evidence of a difference in reintubation rates, mortality, tracheostomy insertion, post-extubation stridor or AEs. Sensitivity analyses were broadly supportive of these interpretations.
We propose a number of possible considerations for the effect on the time to successful extubation. First, we recruited all children with various conditions, except those who would not meet the primary end point. Consequently, variability in the treatment effect may have reduced the overall effect, resulting in the small effect size. As a result, we cannot ascertain if the intervention would have been more beneficial in children with specific conditions. Second, there was a high rate of completion of medical, nursing and allied health professional staff training, and, consequently, an enhanced shared understanding of and engagement in the intervention. Given bedside nurses’ historical lack of involvement in ventilator weaning,15 engaging them in SBT screening granted bedside nurses a designated role in ventilator weaning for the first time. Providing feedback from screening to the medical team triggered earlier consideration of readiness for discontinuation, resulting in a shortening of time to successful extubation. Third, despite feedback signalling that screening criteria were satisfied, there was lower adherence to the protocol for progressing to a SBT. Enabling nurses to perform the SBT when a screen was positive and inform medical staff of the outcome may have resulted in a greater effect. This was a key factor in one of the earliest landmark studies, which showed that daily screening followed by SBT by respiratory therapists who subsequently informed the physician of the patient’s readiness for liberation resulted in earlier discontinuation. 69 The reluctance of medical staff may have been influenced by the limitation of the five screening criteria to capture all parameters that would indicate progression. There were understandable clinical reasons recorded for non-progression, but non-adherence and providing no reason accounted for 18% of explanations for not undertaking a SBT. Plausibly, reluctance and non-adherence may be a sign of the difficulties clinicians experience in changing long-standing practices. 70 Furthermore, as the process evaluation indicated, in many instances, non-progression to SBT and extubation was often couched by established hospital, unit and patient care routines. Often, successful screening in the afternoon or evening did not lead to a SBT until early morning, when there was more chance of conducting a safe extubation, although there may have been further weaning in ventilator support. This may be a plausible explanation for the lack of difference between the PIP recorded immediately before a SBT in the intervention and that recorded 2 hours before extubation in the control period. Together, these factors may have mitigated the beneficial effect shown in smaller paediatric explanatory trials evaluating a SBT as a weaning intervention. 22,71,72
Given the small number of paediatric trials that have evaluated SBT as a weaning strategy, it is no surprise that there are discordant results reporting duration of IMV across studies. In a two-centre RCT recruiting mainly medical patients, Foronda et al. 22 reported a reduction in the duration of IMV of > 24 hours in the SBT group (n = 294, median 3.5 vs. 4.7 days; p = 0.01). Daily screening and SBT were performed by physicians (fellows) specifically trained in this procedure. Ferreira et al. ’s72 single-site RCT of cardiac surgical patients (n = 110) reported a significant reduction in extubation success in the SBT group compared with a usual-care group (83% vs. 68%; p = 0.02), but not duration of IMV (median 29.4 vs. 21.5 hours; p = 0.29), when daily screening and the SBT were undertaken by a physician and respiratory therapist in the study team. In both trials, relatively few people delivered the intervention in a controlled manner and not necessarily staff by the bedside; thus, the findings may not be directly transferable to wider clinical UK practice. By contrast, in a larger 31-site cluster RCT recruiting mainly medical patients, Curley et al. 28 evaluated a protocol involving targeted sedation, arousal assessments, extubation readiness testing, 8-hourly sedation adjustment and sedation weaning. Delivery of the protocol involved training and involvement of each site’s multidisciplinary team. They reported no significant differences in duration of IMV between groups (n = 2449, both groups median 6.5 days), but showed reduced variation in sedation management with multidisciplinary involvement. The median duration of IMV reported in our study in the control period was < 3 days, which is much shorter than that reported in other studies. It is also lower than our pre-trial estimations, which were based on available PICANet data. Thus, it is possible that, with a normally short IMV duration, the intervention had a reduced effect.
The incidence of successful extubation, although significantly higher in the prolonged IMV cohort in the intervention period, differed by only 1 percentage point. A small proportion of extubations were unplanned (2.5% control and 3% intervention periods lower than the 4–8% reported in other studies),28 and did not result in a difference in reintubation rates. Furthermore, the proportion of patients who experienced an unplanned extubation and required reintubation was 15% lower in the intervention period. The higher unplanned extubation rate in the intervention period may have contributed to the greater use of NIV after extubation. Greater use of NIV may reflect clinician discomfort with a more accelerated weaning and extubation approach and a perceived need for additional support. Used in this way, NIV could prolong the period of ventilator support unnecessarily, particularly if it led to continuation of NIV longer than would have occurred with invasive ventilation alone. Furthermore, earlier extubation followed by NIV may be beneficial in that the requirement for sedation for patients receiving NIV is usually significantly less than on invasive ventilation,73 although sedative use was not measured in our study.
The 6–7 hours beneficial effect in reducing duration of IMV did not influence the duration of PICU length of stay. Indeed, hospital length of stay was significantly longer, by approximately 1 day. This paradoxical finding cannot be readily explained. Care after PICU and whether or not specific populations contributed to this effect were not explored in this study. Further research into the longer-term impact of reduced ventilation time and more controlled weaning of sedation in specific populations may be required.
The SANDWICH trial has several strengths. Cluster randomisation was chosen over individual patient randomisation to overcome the risk of clinicians using the intervention in the control period. Furthermore, by using the SW-CRT design we were able to maximise power to detect an effect, facilitate intervention training and increase PICU participation by guaranteeing that each PICU would at some point receive the intervention. The design may also facilitate knowledge translation because participating PICUs could potentially continue using the intervention after the trial, maximising potential benefits for the health service and patients. 74
The study had some limitations. First, owing to the nature of the trial, the assignment of the intervention was unblinded and, as a result, this may have led to performance and/or detection bias. Second, in this cluster trial, the hospitals were the unit of randomisation and the children enrolled represent a heterogeneous case mix of infants and children with a variety of respiratory, cardiac and other impairments. The ability of the intervention to perform differently in a more homogeneous group remains to be determined. Third, the intervention included several components, and adherence to components was not uniform, particularly progressing to undertake a SBT when screening criteria were satisfied. It is possible that this component influenced the observed effect. Variable adherence may have been influenced by established usual practice that is challenging to change. Fourth, we did not obtain a link to other routine data sets (e.g. Hospital Episode Statistics), which would have given us a more complete data set of patients’ overall hospital resource use. Finally, we did not measure sedative use or sedation levels, which may have provided additional insight to understanding the intervention impact.
Conclusions
The intervention led to a significant reduction in the average time to successful extubation of 6–7 hours; thus, the clinical importance of the effect size is uncertain. It also led to a significantly higher, albeit small, incidence of unplanned extubation. Unplanned extubation did not lead to a greater risk of reintubation, although there was a higher incidence of NIV after extubation. There was no difference in PICU length of stay between periods, but hospital length of stay was longer and hospital costs were higher in the intervention period.
Implications for health care
The beneficial effect in time to successful extubation may have been influenced by engaging bedside nurses in daily screening that promoted earlier identification of readiness for liberation. It may also have been moderated by established practices of conducting extubations in the mornings owing to limited senior medical cover at night. The intervention led to a significantly higher, albeit small, incidence of unplanned extubation. Unplanned extubation did not lead to a greater risk of reintubation, although there was a higher incidence of NIV after extubation. There was no difference in PICU length of stay between periods, but hospital length of stay was longer in the intervention period.
The intervention was associated with higher hospital costs, most likely a result of the longer hospital stay. This may reflect the larger number of unplanned extubations and use of post-extubation NIV. The intervention, therefore, had a low probability of being cost-effective. Overall, the intervention was well received by staff. In particular, it enhanced nurses’ understanding, confidence and autonomy in ventilator weaning practice and improved multidisciplinary communication through the shared language provided by the training.
Recommendations for research
Several recommended research questions are suggested for future potential work:
-
Would the intervention exert a greater effect on a more homogeneous population, such as the cardiac surgery population or children with respiratory medical conditions?
-
What is the association between unplanned extubation and the use of NIV, in terms of whether NIV is used as a rescue therapy following post extubation deterioration or a safety net?
-
What are the longer-term impacts of reducing sedation and ventilation time on children?
-
To what extent has this health technology been embedded and integrated into UK PICU practice?
Patient and public involvement
Aim
The aim of the patient and public involvement (PPI) work in the SANDWICH trial was to engage young people and parents throughout the study to inform its development, management and dissemination.
Methods
-
We undertook interviews with a children and young people group and parents to obtain their views on the proposed study design and consent process.
-
We worked with two PPI representatives to design appropriate study documentation.
-
We recruited PPI representatives to the TSC to provide strategic direction and lay interpretation of the study.
Results
-
The interviews with the children and young people group and parents contributed to pre-funding preparation, particularly with regard to identifying the trial’s primary outcome and informing the approach for an opt-out consent process (leaflets and posters).
-
Two PPI representatives (father and son, Lewis and Archie Veale – Archie had been a patient in a PICU for several months) assisted us in designing the posters and leaflets to explain the trial and the opt-out process for parents.
-
Subsequently, Lewis and Archie Veale joined the TSC and actively contributed their views on the study direction. They provided a lay insight into the study results, interpretation and wrote the Plain English summary for the report.
Discussion
The positive effect of the PPI engagement was their integration at the pre-funding stage that informed the study design. This was acknowledged positively by the Research Ethics Committee. The PPI representatives’ input into providing clear and easy-to-read study documentation and the summary of results was found to be highly beneficial by the research team. We found no negative effects of PPI engagement.
Reflections
Engaging PPI representatives in the study has been important to the research and very insightful. It was challenging to the team to ensure that study materials and discussions, particularly at meetings, were clear and concise to ensure lay understanding. A limitation in gaining PPI engagement was the difficulty in finding sufficient numbers of PPI representatives with experience of the PICU. This proved very challenging, particularly because there are no voluntary PICU support groups. Further work needs to establish whether or not forums exist within individual hospitals for parents who have had a child in a PICU and to develop a viable PPI forum for future PICU research.
Acknowledgements
We wish to thank the NIHR HTA programme for funding the trial. We acknowledge the contribution of Dr Claire Kydonaki to the process evaluation.
Patient and public involvement
We would like to thank the PPI representatives of the TSC, Archie and Lewis Veale, for providing a patient and public perspective on the design, findings and interpretation of the study. In particular, we acknowledge their input into the design of the information leaflets, posters and for writing the Plain English summary of this report.
Importantly, we gratefully acknowledge all the children and infants who were part of the trial, and the parents/guardians who enabled their participation.
Research team and champions at participating sites
The SANDWICH study was a substantial collaborative effort across many PICUs. We acknowledge that all staff at participating sites have made a valuable contribution to this study, both in delivering the intervention and in participating in interviews, and we thank them sincerely. It is impossible to list everyone; however, we would like to acknowledge the following research teams and SANDWICH champions who worked tirelessly to train staff in implementing the SANDWICH intervention.
Addenbrooke’s Hospital, Cambridge
Nazima Pathan, Deborah White, Esther Daubney, Zoe Stone, Rosalie Campbell, Naomi Rowel, Liz Nash, Phil Castle, Mark Harvey, Clare King, Sarah Hardwick and James Nicholas.
Alder Hey Children’s Hospital, Liverpool
Ben Lakin, Laura Rad, Dawn Jones, Laura O’Malley, Sean Cuddihy, Alex Taylor, Jaspreet Sodhi, Katie Bailey, Emily McDonald, Tom Collins, Adam McNeill, Bron Robinson, Amanda Bull, Helen Cosgrove, Becky Garrett, Sarah Hindley, Lindsey Kenworthy, Louise Foster, Vicky Llewellyn, Liz Mansfield, Andrea Rodriguez, Lorraine Abbott, Shirley George, Roberto Iavarrone, Paul Ritson and Katie Kaye.
Birmingham Children’s Hospital
Kevin Morris, Katie Price, Rachel Loughead, Sarah Fox, Samantha Owen, Roxanne Williams, Carly Tooke, Lauren Dowd, Kate Penny-Thomas, Sophie Dance, Helen Winmill, Julie Menzies, Alison Jones and Rakesh Sheinmar.
Bristol Royal Hospital for Children
Mireia Garcia Cusco, Sarah Mogan, Kate Baptiste, Helen Marley, Hope Lacy, Laura Dodge, Reanate Reisinger, Christina Linton, Sophie Coles, Kimberley Hamilton, Jen Bond, Emily Madge, Kelly-Marie Brock, Peter Davis, Sarah Goodwin and Dora Wood.
Great Ormond Street Hospital, London
Suzan Kakat, Lauran O’Neill, Holly Belfield, Ana Luisa Tomas, Francesca Standing, Yvonne Leonard, Helen Vander-Johnson, Samiran Ray, Olugbenga Akinkugbe, Gareth Jones, Eugenia Abaleke, Hamza Meghari, Adela Mattatore, Tom Brick, Yael Feinstein, Sarah Caley, Grace Banks, Joanne Bowley, Katy Maguire, Lorna O’Rourke, Deborah Lees, Caitriona Morrisey, Emma Hart, Ann Maguire, Nicola Pearson, Joanne Broadhurst, Clare Paley, Alison Drew, Carmen Kurtzner, Harriet McCauley, Katie Smith, Isabella Wright, Eleni Tamvaki, Lisa Cooke, Grace Banks, Sarah Napier, Annabelle Linger, Renee Barrett, Jo Rendle, Daryl Herring, Lolinda Mago, Joanna Goniak, Zaina Ahmed, Charlotte Hambly, Fiona O’Mahony, Rosemary Jamieson, Katrina Capey, Charlotte Donovan, Nicola Barker and Helen Mercer.
Great North Children’s Hospital, Newcastle upon Tyne
Chris MacKerness, Rachel Agbecko, Angela Woodhall, Lindsay Cooper, Dawn Metcalfe, Ashleigh Robson, Amanda Soulsby, Kate Teeley, Anna Stancombe, Kirsty Mulgrew, Louisa Hunter, Shelley Sweeney, Ashley Marley, Julie Allen, Erin Bonney, Gemma Conroy, Joanne Cowley, Alison Crozier, Julie Dodds, Angela Doherty, Deborah Ehala, Gillian Green, Melanie Haughan, Nicola Mears, Jo Mulholland, Janine Palmer, Elaine Pantry, Claire Riddell, Kirstine Stait, Katherine Brunton and Anna Yearham.
John Radcliffe Hospital, Oxford
Deirdre O’Shea, Kirsten Beadon, Nicola Howell, Teresa Liu, Hannah Sparkes, Rachel McMinnis, Jackie Fulton, Sarah Addison, Katie Mogg, Rebecca Harmer, Rachel Lynch, Joanna Bartlett, Rosie Priddy and Janet McCluskey.
King’s College Hospital, London
Pam D’Silva, Sam Archer, Stacey Bedford, Lauren Jameson and Asha Hylton.
Leeds General Infirmary, Leeds
Jo Lumsden, Louise Turner, Heather Rostron, Donna Ellis, Sarah Hanson, Emily Scriven, Sian Cooper, Mark Wareing, Sharron Frost, Beverley Robinson, Tim Haywood, Andrew McNulty, Tammy Jaques, Rachael Funk, Sharon Coulson, Sharon Beanland, Helen Townson, Sarah Mawer, Katie Hill, Kathryn Reeves, Jennifer Carter, Marie Webster, Darren Hewett and Adrian Watson.
Noah’s Ark Children’s Hospital for Wales, Cardiff
Julie Armstrong, Siva Oruganti, Iona Buchanan, Claire Speirs, Jade Smallman, Emma Smith, Rhiannydd Poynter, Rebecca Thomas, Emily Stacey-Cox and Sarah Stacey.
Royal Belfast Hospital for Sick Children, Belfast
Julie Richardson, Caroline McCluskey, Becky Simpson, Carolyn Green, Rachel Anderson, Jeremy Lyons, Mohammed Babiker, Ben Kennedy, Roisin McDonald, Philip Ross, Rory Sweeney, Fiona Wallace, Pauline Blair, Niamh McPeake, Paul Magowan, Deborah Black, Sinead McAteer, Andrea Burrell, Sharon McAuley, Maria McCreight, Cealaigh Quinn, Carol McCormick, Heather Tough, Stewart Reid, Mark Terris, Ann Maguire and Lucy Simms.
Royal Brompton Hospital, London
Angela Aramburo, Helena Sampaio, Laura Alcantara, Laura Tous, Katie Goodliffe, Stephanie Gleissner, Melisa Ollivier, Joana Gracio, Diana Freitas, Chelsea Nichola Nilsson, Tessa Shewan, Stephen Tugwell, Matt Smith, Jennifer Armstrong, Mary Anton, Patricia Hernandez, Dan Blacke, Alicia Arias, Shabnam Gabriel, Zarine Wessels, Esmee Stirrup, Marta Fernandez, Ana Pedro, Varsha Depala, Silvia Fernandez Velasco, Justin Wang, Visitacion Jimenez, Laura Diaz, Florence O’Connor and Jess Robinson.
Royal Stoke University Hospital, Stoke-on-Trent
John Alexander, Penny Percica, Claire Sidley, Jo Tomlinson, Vicky Riches, Claire Boissery, Emma Cooke, Abbie Cliffe, Mark Bebbington, Kathryn Lea and James Chapman.
Sheffield Children’s Hospital, Sheffield
Rum Thomas, Samantha Burns, Jade Bryant, Amy Pickard, Anton Mayer, Lara Jackman, Nicholas Roe, Pauline Athwal, Alison Widdas, Alison Donolan, Anna Collister, Alex Howlett, Nat Colley, Jenny Nolan, Nicola McAdam, Linzi Boreham, Suzie Birkitt, Charlotte Clark, Charlotte Hussey, Kate Conolley, Emily Cleveland, Olivia Hudson, Lauren Williams, Stuart Conquer, Simon Steel, Ranjana Dhar, Megan Burrill, Nick Mills, Helen Cook, Jenny Longden, Erica Miccoli, Malik Hai, Bethan Stone, Michelle Lee, Michelle Gilley, Ceri Jack, Rachael Saxby and Louise McCarthy.
Southampton Children’s Hospital, Southampton
John Pappachan, Christie Mellish, Nicki Etherington, Jenny Pond, Cat Postlethwaite, Amber Cook, Anna Hardy, Lorena Caruana, Sophie Bullyment, James Hardwick, Lisa Gosby, Katy Morton, Donna Austin, Angela Ledgham, Emily Tracey, Dr Oliver Ross, Ahmed Osman, Michael Griksaitis and Kelly Field.
St George’s Hospital, London
Soumendu Manna, Elena Maccacari, Joana Queiroz, Sian Butler, Buvana Dwarakanathan, Nicholas Prince, Julie Geevarghese, Usha Chandran, Sharmaine Monrose, Josephine Rhodes, Juliemol Thomas, Kirsty Felstead, Laura Poletti, Lindsey Burnham and Hannah Downing.
St Mary’s Hospital, London
David Inwald, Thomas Bycroft, Sarah Darnell, Ladan Ali, Suzanne Laing, Naomi Storkes, Wendy Dadson, Tanya Lincoln, Anne Dowson, Michelle Pash, Kelly Wood, Carey Corrigan, Debbie Lee, Karen Downer and Katy Bridges.
Trial Management Group
Professor Bronagh Blackwood (chairperson), Dr Ashley Agus, Ms Roisin Boyle, Ms Pauline Bradley, Dr Joanne Jordan, Ms Lisa McIlmurray, Professor Kevin Morris, Professor Danny McAuley, Ms Cliona McDowell, Dr Margaret Murray and Dr Lyvonne Tume.
Trial Steering Committee
-
Chairperson: Professor Katherine Fielding (Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine).
-
Dr Joseph Manning (Clinical-Academic Senior Research Fellow, University of Nottingham).
-
Dr Jillian McFadzean (Clinical Lead for Paediatric Intensive Care, Royal Hospital for Sick Children Edinburgh).
-
Dr Tsz-Yan Milly Lo (Consultant Paediatric Intensivist, Royal Hospital for Sick Children Edinburgh).
-
Dr Stephen Playfor (Consultant Paediatric Intensivist, Royal Manchester Children’s Hospital).
-
Mr Archie Veale (Patient and Public Involvement Representative).
-
Mr Lewis Veale (Patient and Public Involvement Representative).
Data Monitoring Committee
-
Chairperson: Professor Gavin Perkins (Professor of Critical Care Medicine, Director of Warwick CTU, University of Warwick).
-
Professor Sarah Walker (Professor of Medical Statistics and Epidemiology, MRC Clinical Trials Unit at University College London).
-
Dr Shane Tibby (Consultant Paediatric Intensivist, Evelina London Children’s Hospital).
Sponsor representative
Dr Paula Tighe (Research Governance, Ethics and Integrity Manager, Queen’s University Belfast).
Northern Ireland Clinical Trials Unit
-
Trial co-ordinator: Margaret Murray.
-
Trial managers: Roisin Boyle and Gavin Kennedy.
-
Data managers: Pauline Bradley and Gerard O’Hanlon.
-
Health economists: Ashley Agus and Glenn Phair.
-
Statisticians: Cliona McDowell and Sorcha Toase.
-
Administrators: Ruth Holman and Kevin Devlin.
Contributions of authors
Bronagh Blackwood (https://orcid.org/0000-0002-4583-5381) (Professor of Critical Care, Queen’s University Belfast) was the chief investigator; led the team of co-applicants, researchers and trial support staff named below who conceived and designed the trial; and contributed to data collection, analysis and interpretation of the trial findings. She led drafting this report and revised it critically for important intellectual content.
Kevin P Morris (https://orcid.org/0000-0001-8911-6306) (Consultant Paediatric Intensivist, Birmingham Children’s Hospital) was involved in the feasibility work before the study; was involved in the conception and design of the study, contributed to the acquisition and interpretation of the data, drafted the work and revised the work critically for important intellectual content.
Joanne Jordan (https://orcid.org/0000-0003-3308-9104) (Senior Research Fellow, Queen’s University Belfast) was involved in the conception, design and conduct of the process evaluation; led the acquisition, analysis and interpretation of the qualitative data; and drafted and critically reviewed the manuscript.
Lisa McIlmurray (https://orcid.org/0000-0001-6376-468X) (SANDWICH Implementation Manager, Queen’s University Belfast) designed the SANDWICH training programme, trained the PICU trainers and champions, contributed to the acquisition and interpretation of the data, drafted the work and critically reviewed the manuscript.
Ashley Agus (https://orcid.org/0000-0001-9839-6282) (Senior Health Economist, NICTU) contributed to the design of the economic research and provided oversight of all aspects of the economic evaluation, including its design, conduct, analysis and reporting.
Roisin Boyle (https://orcid.org/0000-0002-1189-5722) (Trial Manager, NICTU) provided training and support to sites, co-ordinated and managed the trial, was involved in the management of the study and critically reviewed the manuscript.
Mike Clarke (https://orcid.org/0000-0002-2926-7257) (Professor, Director of the All Ireland Hub for Trials Methodology Research, Queen’s University Belfast) was involved in the conception and design of the study, contributed to the interpretation of the data, drafted the work and revised the work critically for important intellectual content.
Christina Easter (https://orcid.org/0000-0002-7028-1421) (Statistician, University of Birmingham) performed the SAS statistical analyses, assisted with interpretation of data, drafted the work and revised it, and critically reviewed the manuscript.
Richard G Feltbower (https://orcid.org/0000-0002-1728-9408) (Reader, University of Leeds) contributed to the acquisition and interpretation of the data and critically reviewed the manuscript.
Karla Hemming (https://orcid.org/0000-0002-2226-6550) (Professor of Biostatistics, University of Birmingham) contributed to the conception and design of the trial, provided oversight for the stepped wedge analysis, assisted with interpretation of data, drafted the work and revised it critically for important intellectual content.
Duncan Macrae (https://orcid.org/0000-0002-7158-6585) (Consultant Paediatric Intensivist, Royal Brompton, London) was involved in the design of the study, contributed to the acquisition and interpretation of the data, and critically reviewed the manuscript.
Clíona McDowell (https://orcid.org/0000-0002-7644-7197) (Senior Statistician, NICTU) contributed to the design of the work, performed the statistical analyses reported, assisted with interpretation of data, and drafted the work and revised it critically for important intellectual content.
Margaret Murray (https://orcid.org/0000-0002-5118-6007) (Trial Co-ordinator, NICTU) provided training and support to site staff, co-ordinated the trial on a daily basis, was involved in the acquisition of data and drafting of trial reports, and critically reviewed the manuscript.
Roger Parslow (https://orcid.org/0000-0002-3945-5294) (Senior Lecturer, University of Leeds) was involved in the conception and design of the study, contributed to the acquisition and interpretation of the data, and critically reviewed the manuscript.
Mark J Peters (https://orcid.org/0000-0003-3653-4808) (Professor of Paediatric Intensive Care) was involved in the feasibility work before the study, the conception and design of the study, contributed to the acquisition and interpretation of the data, drafted the work and revised it critically for important intellectual content.
Glenn Phair (https://orcid.org/0000-0001-8137-0959) (Health Economist, NICTU) analysed and contributed to the economic evaluation report and critically reviewed the manuscript.
Lyvonne N Tume (https://orcid.org/0000-0002-2547-8209) (Associate Professor of Nursing, Salford University) was involved in the feasibility work before the study and the conception and design of the trial, contributed to the interpretation of the data, drafted the work and revised it critically for important intellectual content.
Timothy S Walsh (https://orcid.org/0000-0002-3590-8540) (Professor of Intensive Care Medicine, University of Edinburgh) was involved in the conception and design of the SANDWICH training package, contributed to the interpretation of the data and revised the work critically for important intellectual content.
Daniel F McAuley (https://orcid.org/0000-0002-3283-1947) (Professor of Critical Care Medicine, Queen’s University Belfast) was involved in the conception and design of the study, contributed to the interpretation of the data, drafted the work and revised it critically for important intellectual content.
Publications
Blackwood B, Agus A, Boyle R, Clarke M, Hemming K, Jordan J, et al. Sedation AND Weaning In CHildren (SANDWICH): protocol for a cluster randomised stepped wedge trial. BMJ Open 2019;9:e031630.
Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, et al. Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA 2021;326:401–10.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data or trial materials may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Valenzuela J, Araneda P, Cruces P. Weaning from mechanical ventilation in paediatrics. State of the art. Arch Bronconeumol 2014;50:105-12. https://doi.org/10.1016/j.arbres.2013.02.003.
- PICANet . Paediatric Intensive Care Audit Network (PICANet) 2019 Annual Report 2019. www.picanet.org.uk/annual-reporting-and-publications/ (accessed 16 July 2020).
- PICANet . Paediatric Intensive Care Audit Network (PICANet) 2015 Annual Report 2015. www.picanet.org.uk/wp-content/uploads/sites/25/2018/05/PICANet_2015_Annual_Report_Summary.pdf (accessed 27 March 2021).
- Choi AY, Kim M, Park E, Son MH, Ryu JA, Cho J. Outcomes of mechanical ventilation according to WIND classification in pediatric patients. Ann Intensive Care 2019;9. https://doi.org/10.1186/s13613-019-0547-2.
- Principi T, Fraser DD, Morrison GC, Farsi SA, Carrelas JF, Maurice EA, et al. Complications of mechanical ventilation in the pediatric population. Pediatr Pulmonol 2011;46:452-7. https://doi.org/10.1002/ppul.21389.
- Medjo B, Vunjak N, Atanaskovic-Markovic M, Rsovac S, Nikolic D, Kalanj J, et al. Indications and complications of mechanical ventilation in pediatric intensive care unit patients. Arch Dis Child 2008;93.
- Vet NJ, Kleiber N, Ista E, de Hoog M, de Wildt SN. Sedation in critically ill children with respiratory failure. Front Pediatr 2016;4. https://doi.org/10.3389/fped.2016.00089.
- Mhanna MJ, Anderson IM, Iyer NP, Baumann A. The use of extubation readiness parameters: a survey of pediatric critical care physicians. Respir Care 2014;59:334-9. https://doi.org/10.4187/respcare.02469.
- Hess DR, MacIntyre NR. Ventilator discontinuation: why are we still weaning?. Am J Respir Crit Care Med 2011;184:392-4. https://doi.org/10.1164/rccm.201105-0894ED.
- Frutos-Vivar F, Esteban A. Our paper 20 years later: how has withdrawal from mechanical ventilation changed?. Intensive Care Med 2014;40:1449-59. https://doi.org/10.1007/s00134-014-3362-0.
- Farias JA, Alía I, Esteban A, Golubicki AN, Olazarri FA. Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med 1998;24:1070-5. https://doi.org/10.1007/s001340050718.
- Farias JA, Retta A, Alía I, Olazarri F, Esteban A, Golubicki A, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med 2001;27:1649-54. https://doi.org/10.1007/s001340101035.
- Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD006904.pub3.
- Blackwood B, Murray M, Chisakuta A, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of invasive mechanical ventilation in critically ill paediatric patients. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD009082.pub2.
- Blackwood B, Tume L. The implausibility of ‘usual care’ in an open system: sedation and weaning practices in Paediatric Intensive Care Units (PICUs) in the United Kingdom (UK). Trials 2015;16. https://doi.org/10.1186/s13063-015-0846-3.
- Øvretveit J. Does Clinical Coordination Improve Quality and Save Money? 2011. www.health.org.uk/publications/does-clinical-coordination-improve-quality-and-save-money (accessed 16 July 2020).
- Rose L. Interprofessional collaboration in the ICU: how to define?. Nurs Crit Care 2011;16:5-10. https://doi.org/10.1111/j.1478-5153.2010.00398.x.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. An evaluation of outcome from intensive care in major medical centers. Ann Intern Med 1986;104:410-18. https://doi.org/10.7326/0003-4819-104-3-410.
- Wheelan SA, Burchill CN, Tilin F. The link between teamwork and patients’ outcomes in intensive care units. Am J Crit Care 2003;12:527-34. https://doi.org/10.4037/ajcc2003.12.6.527.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725-32. https://doi.org/10.1056/NEJMoa061115.
- Jordan J, Rose L, Dainty KN, Noyes J, Blackwood B. Factors that impact on the use of mechanical ventilation weaning protocols in critically ill adults and children: a qualitative evidence-synthesis. Cochrane Database Syst Rev 2016;10. https://doi.org/10.1002/14651858.CD011812.pub2.
- Foronda FK, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med 2011;39:2526-33. https://doi.org/10.1097/CCM.0b013e3182257520.
- Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med 2013;39:919-25. https://doi.org/10.1007/s00134-013-2837-8.
- Maloney C. Computerized Weaning of Childhood Respiratory Failure 2007.
- Wolf A, McKay A, Spowart C, Granville H, Boland A, Petrou S, et al. Prospective multicentre randomised, double-blind, equivalence study comparing clonidine and midazolam as intravenous sedative agents in critically ill children: the SLEEPS (Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation) study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18710.
- Poh YN, Poh PF, Buang SN, Lee JH. Sedation guidelines, protocols, and algorithms in PICUs: a systematic review. Pediatr Crit Care Med 2014;15:885-92. https://doi.org/10.1097/PCC.0000000000000255.
- Aitken LM, Bucknall T, Kent B, Mitchell M, Burmeister E, Keogh SJ. Protocol-directed sedation versus non-protocol-directed sedation to reduce duration of mechanical ventilation in mechanically ventilated intensive care patients. Cochrane Database Syst Rev 2015;1. https://doi.org/10.1002/14651858.CD009771.pub2.
- Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. Protocolized sedation vs. usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015;313:379-89. https://doi.org/10.1001/jama.2014.18399.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol 2008;41:327-50. https://doi.org/10.1007/s10464-008-9165-0.
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol 1992;17:95-109. https://doi.org/10.1093/jpepsy/17.1.95.
- Boerlage AA, Ista E, Duivenvoorden HJ, de Wildt SN, Tibboel D, van Dijk M. The COMFORT behaviour scale detects clinically meaningful effects of analgesic and sedative treatment. Eur J Pain 2015;19:473-9. https://doi.org/10.1002/ejp.569.
- Walsh TS, Kydonaki K, Antonelli J, Stephen J, Lee RJ, Everingham K, et al. Staff education, regular sedation and analgesia quality feedback, and a sedation monitoring technology for improving sedation and analgesia quality for critically ill, mechanically ventilated patients: a cluster randomised trial. Lancet Respir Med 2016;4:807-17. https://doi.org/10.1016/S2213-2600(16)30178-3.
- Macrae D, Grieve R, Allen E, Sadique Z, Betts H, Morris K, et al. A clinical and economic evaluation of Control of Hyperglycaemia in Paediatric intensive care (CHiP): a randomised controlled trial. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18260.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Blackwood B, Agus A, Boyle R, Clarke M, Hemming K, Jordan J, et al. Sedation AND Weaning In CHildren (SANDWICH): protocol for a cluster randomised stepped wedge trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-031630.
- PICANet . Paediatric Intensive Care Audit Network (PICANet) 2017 Annual Report 2017. www.picanet.org.uk/wp-content/uploads/sites/25/2019/01/PICANet_2017_Annual_Report_Tables_and_Figures_FINAL_v2.0-compressed.pdf (accessed 1 November 2016).
- Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, et al. Paediatric Index of Mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med 2013;14:673-81. https://doi.org/10.1097/PCC.0b013e31829760cf.
- NHS . NHS Data Model and Dictionary Service 2020. https://datadictionary.nhs.uk/data_sets/supporting_data_sets/paediatric_critical_care_minimum_data_set.html (accessed 20 November 2020).
- Dixon JR. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 1998;6. https://doi.org/10.1080/105294199277860.
- Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 2007;28:182-91. https://doi.org/10.1016/j.cct.2006.05.007.
- Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol 2020;49:979-95. https://doi.org/10.1093/ije/dyz237.
- Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ 2018;363. https://doi.org/10.1136/bmj.k1614.
- McNeish D. Small sample methods for multilevel modeling: a colloquial elucidation of REML and the Kenward-Roger correction. Multivariate Behav Res 2017;52:661-70. https://doi.org/10.1080/00273171.2017.1344538.
- Leyrat C, Morgan KE, Leurent B, Kahan BC. Cluster randomized trials with a small number of clusters: which analyses should be used?. Int J Epidemiol 2018;47. https://doi.org/10.1093/ije/dyy057.
- Fay MP, Graubard BI. Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics 2001;57:1198-206. https://doi.org/10.1111/j.0006-341X.2001.01198.x.
- Thompson JA, Hemming K, Forbes A, Fielding K, Hayes R. Comparison of small-sample standard-error corrections for generalised estimating equations in stepped wedge cluster randomised trials with a binary outcome: a simulation study. Stat Methods Med Res 2020;30:425-39. https://doi.org/10.1177/0962280220958735.
- Healthcare Quality Improvement Partnership . Paediatric Intensive Care Audit Network. Annual Report 2018. Summary Report 2018. www.picanet.org.uk/wp-content/uploads/sites/25/2018/11/PICANet-2018-annual-report-summary-v1.1.pdf (accessed 27 March 2021).
- Healthcare Quality Improvement Partnership . Paediatric Intensive Care Audit Network. Annual Report 2018. Appendices 2018. www.picanet.org.uk/wp-content/uploads/sites/25/2018/11/PICANet-Annual-Report-Appendices-2018_v1.0.pdf (accessed 27 March 2021).
- Sheard L, O’Hara J, Armitage G, Wright J, Cocks K, McEachan R, et al. Evaluating the PRASE patient safety intervention – a multi-centre, cluster trial with a qualitative process evaluation: study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-420.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psych 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Guest G, Namey E, Taylor J, Eley N, McKenna K. Comparing focus groups and individual interviews: findings from a randomized study. Int J Soc Res Methodol 2017;20:693-708. https://doi.org/10.1080/13645579.2017.1281601.
- Hunt A, Coad J, West E, Hex N, Staniszewska S, Hacking S, et al. The Big Study for Life-Limited Children and Their Families – Final Research Report 2013. www.togetherforshortlives.org.uk/resource/the-big-study/ (accessed 1 November 2020).
- Silverman D. Interpreting Qualitative Data: Methods for Analysing Talk, Text and Interaction. London: SAGE Publications Ltd; 2015.
- Angus DC. Grappling with intensive care unit quality – does the readmission rate tell us anything?. Crit Care Med 1998;26:1779-80. https://doi.org/10.1097/00003246-199811000-00008.
- Agus A, McKay M, Cole J, Doherty P, Foxcroft D, Harvey S, et al. Cost-effectiveness of a combined classroom curriculum and parental intervention: economic evaluation of data from the Steps Towards Alcohol Misuse Prevention Programme cluster randomised controlled trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-027951.
- Tully MA, Cunningham C, Wright A, McMullan I, Doherty J, Collins D, et al. Peer-led walking programme to increase physical activity in inactive 60- to 70-year-olds: Walk with Me pilot RCT. Public Health Res 2019;7. https://doi.org/10.3310/phr07100.
- Lohan M, Aventin Á, Maguire L, Curran R, McDowell C, Agus A, et al. Increasing boys’ and girls’ intentions to avoid teenage pregnancy: a cluster randomised controlled feasibility trial of an interactive video drama-based intervention in post-primary schools in Northern Ireland. Public Health Res 2017;5. https://doi.org/10.3310/phr05010.
- Department of Health and Social Care, NHS Improvement . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed 13 December 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018 2018. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018/ (accessed 20 October 2020).
- Isaranuwatchai W, Alam F, Hoch J, Boet S. A cost-effectiveness analysis of self-debriefing versus instructor debriefing for simulated crises in perioperative medicine in Canada. J Educ Eval Health Prof 2017;13. https://doi.org/10.3352/jeehp.2016.13.44.
- Hoch JS, Hay A, Isaranuwatchai W, Thavorn K, Leighl NB, Tu D, et al. Advantages of the net benefit regression framework for trial-based economic evaluations of cancer treatments: an example from the Canadian Cancer Trials Group CO.17 trial. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-5779-x.
- Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of ‘community acquired’ syncope. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-68.
- Peñuelas Ó, Frutos-Vivar F, Esteban A. Unplanned extubation in the ICU: a marker of quality assurance of mechanical ventilation. Crit Care 2011;15. https://doi.org/10.1186/cc10049.
- da Silva PSL, Fonseca MCM. Factors associated with unplanned extubation in children: a case-control study. J Intensive Care Med 2020;35:74-81. https://doi.org/10.1177/0885066617731274.
- Hull J. The value of non-invasive ventilation. Arch Dis Child 2014;99:1050-4. https://doi.org/10.1136/archdischild-2013-305322.
- Doyle LW, Carse E, Adams AM, Ranganathan S, Opie G, Cheong JLY. Victorian Infant Collaborative Study Group. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med 2017;377:329-37. https://doi.org/10.1056/NEJMoa1700827.
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996;335:1864-9. https://doi.org/10.1056/NEJM199612193352502.
- Gupta DM, Boland RJ, Aron DC. The physician’s experience of changing clinical practice: a struggle to unlearn. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0555-2.
- Ely EW, Baker AM, Evans GW, Haponik EF. The prognostic significance of passing a daily screen of weaning parameters. Intensive Care Med 1999;25:581-7. https://doi.org/10.1007/s001340050906.
- Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti APCP. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med 2019;20:940-6. https://doi.org/10.1097/PCC.0000000000002006.
- Mayordomo-Colunga J, Medina A, Rey C, Concha A, Menéndez S, Los Arcos M, et al. Non invasive ventilation after extubation in paediatric patients: a preliminary study. BMC Pediatr 2010;10. https://doi.org/10.1186/1471-2431-10-29.
- Li F, Hughes JP, Hemming K, Taljaard M, Melnick ER, Heagerty PJ. Mixed-effects models for the design and analysis of stepped wedge cluster randomized trials: an overview. Stat Methods Med Res 2020;30:612-39. https://doi.org/10.1177/0962280220932962.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ (accessed 1 March 2020).
- NHS Improvement . National Schedule of NHS Costs 2018 19 2021. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 11 February 2022).
Appendix 1 Additional statistical analysis material
Categorisation of short and prolonged ventilation groups
Anticipated prolonged invasive mechanical ventilation was defined a priori. Using historical PICANet data (accessed 30 October 2018), diagnostic codes associated with a short duration of IMV (≤ 24 hours) were identified and categorised as ‘short’. Admissions that did not include a short diagnostic code were categorised as ‘prolonged’.
In total, there were 35,105 codes associated with a short ventilation time. They were classified into 11 categories:
-
allergic reactions
-
atrial septal defect
-
atrial surgery/mitral valve surgery
-
aortic coarctation
-
epilepsy
-
fracture
-
musculoskeletal surgery
-
poisoning/drug overdose
-
pulmonary vein abnormality
-
scoliosis
-
ventricular septal defect (isolated repair).
Model-based analysis plan for binary and secondary outcomes, including detailed sensitivity analysis
There are a number of requirements for the analysis model for this SW-CRT. First, this is a clustered trial and all analysis will take clustering into account. Second, the trial has 17 clusters and the model will allow for a correction owing to the small number of clusters. Third, the design is a stepped-wedge study and we will adjust for temporal confounding. Full details on how each of these will be undertaken, with justification and detailed sensitivity analysis to all underlying assumptions, is provided in the following sections for all binary and continuous outcomes. 74
Binary outcomes
A mixed-effects binomial regression with a log-link will be used to estimate the relative risk, and a binomial model with identity link will be used to estimate the risk difference, with estimation using restricted maximum likelihood. In the case of non-convergence of the binomial model with a log-link, a Poisson model with robust standard errors will be fitted. If the binomial model with the identity link does not converge, only a relative risk will be reported. If neither the log-link nor the identity link converge, we will use the logistic link and report odds ratios. We will include fixed effects for period and a fixed effect for intervention exposure. The primary analysis will allow for clustering as a random effect assuming an exchangeable correlation structure. To correct the potential inflation of the type I error rate owing to a small number of clusters, the Kenward and Roger small-sample correction will be used. In cases in which there was non-convergence of binomial linear mixed models to estimate risk differences, we have reported marginal estimates of risk differences using generalised estimating equations, assuming an independent correlation structure, with a Fay and Graubard small-sample correction on standard errors, with 95% CIs derived from a z-distribution. 47
Continuous outcomes
For continuous outcomes, we will report mean differences estimated from a mixed-effects linear regression with an identity link. All continuous outcomes will be checked for normality and appropriate transformations used. All analyses other than choice of link function will take the same form above, including small-sample corrections.
Additional sensitivity analyses
In a sensitivity analysis, we will explore if models with more complicated correlation structures are a better fit to the data. These models are not being used as our primary analysis models because there is limited understanding as to when such models will converge and how to choose between the various different correlation structures that might be plausible. To this end, we will also fit generalised linear mixed models (with same link functions and fixed effects as described above) to include the following correlation structures: a block exchangeable correlation structure to include a random cluster and random cluster by period effect, and a discrete time decay correlation structure including a random cluster effect with auto-regressive structure [AR(1)]. We will report Akaike information criteria and log-likelihoods from all models so we can make an informal comparison of goodness of fit. Although there are currently no recommended models to formally compare goodness of fit between different correlation structures, any large differences in goodness of fit between these models should be evident from conventional goodness of fit statistics. Should there be large differences and differences between results (point estimates of treatment effects and CIs, results will be interpreted cautiously).
In addition, to explore whether or not the categorical effect for time (i.e. fixed period effect) is both parsimonious and adequate to represent the extent of the secular trend, we will model the time effect using a spline function. The number of knots used here will be taken as the default. Again, for verification of results, this model will also be fitted in Stata under the exchangeable correlation structure and without a small-sample correction. Models will be extended to include random cluster by intervention effects (with a non-zero covariance term) to examine whether or not results are sensitive to the assumption of no intervention by cluster interaction. Models will also be extended to include an interaction between treatment and number of periods since first treated, to examine whether or not there is any indication of a relationship between duration of exposure to the intervention and outcomes.
Estimation and reporting of within-cluster correlations
We will report time-adjusted within-cluster correlations for all outcomes. We will report correlations from the different assumed correlation structures (so we will report ICCs, within- and between-period correlations, and within-period correlations and exponential decay). As well as reporting correlations, we will also report all variance components. For all outcomes (continuous and binary), we will report correlations on the latent scale (i.e. proportions scale for binary outcomes) as is appropriate to inform future sample size calculations. To this end, to estimate the intracluster correlations, a linear mixed-effects regression model with an identity link will be fitted, with a random cluster effect, fixed period effect and fixed intervention effect. To report the estimated within-period ICC, between-period ICC assuming a block-exchangeable correlation structure we will fit a linear mixed-effects regression model with an identity link, with a random cluster and random cluster by period effect, and fixed period effect and fixed intervention effect. To report the within-period ICC and the rate of exponential decay under the discrete time decay correlation structure, we will fit a linear mixed-effects regression model with an identity link, with a random cluster and auto-regressive structure [AR(1)], and fixed period effect and fixed intervention effect. No small-sample corrections will be made when fitting models for intracluster correlation estimates because interest here is in the variance components and not the treatment effect.
Implementation
These binary models will be fitted in SAS using proc glimmix because Stata accommodates neither small-sample corrections for binary outcomes nor correlation structures other than the exchangeable one. However, binary outcomes will be analysed in Stata without the small-sample correction and under the exchangeable correlation structure as a means of verification of results.
FIGURE 13.
The SW-CRT CONSORT flow diagram.
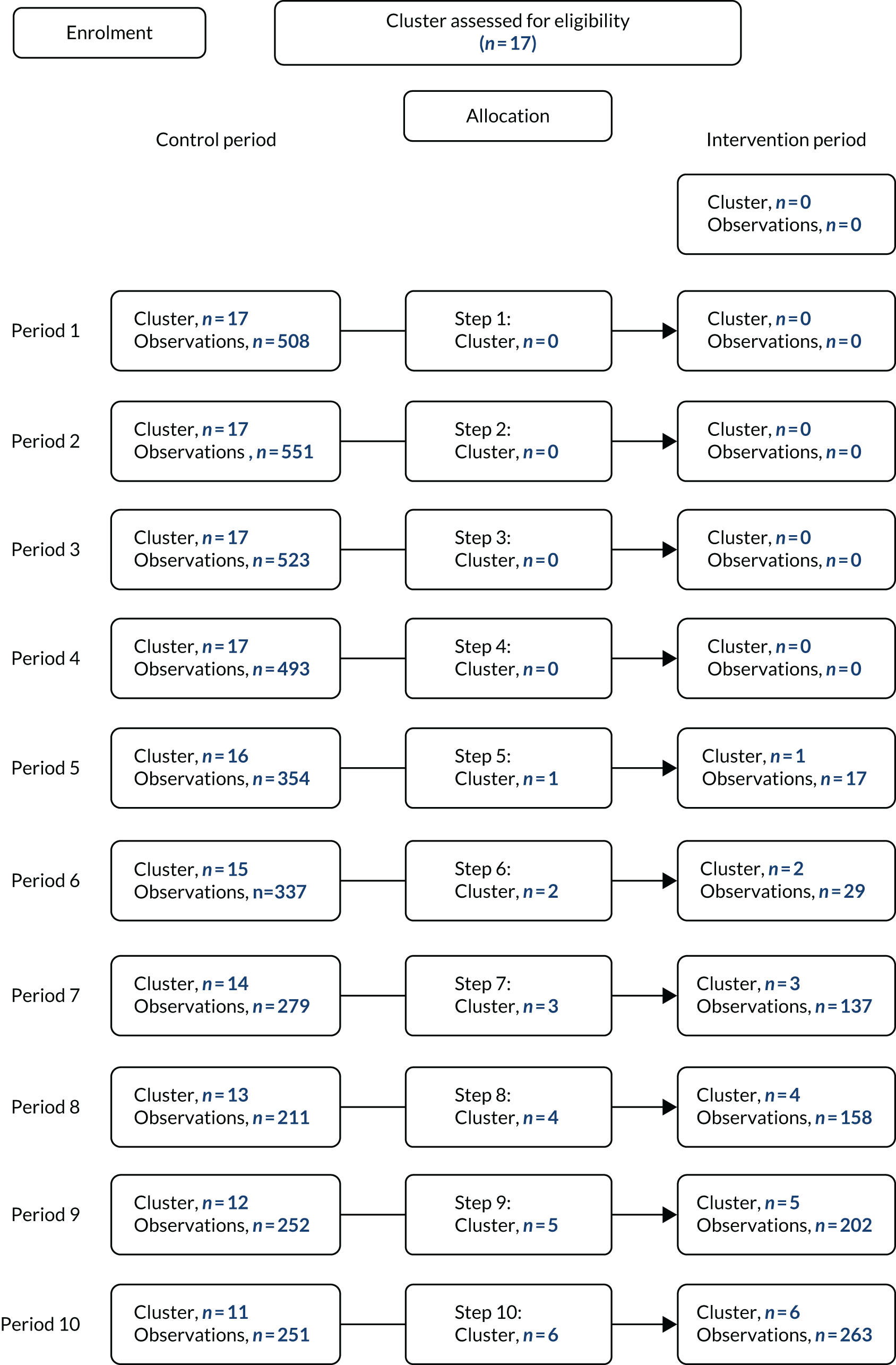
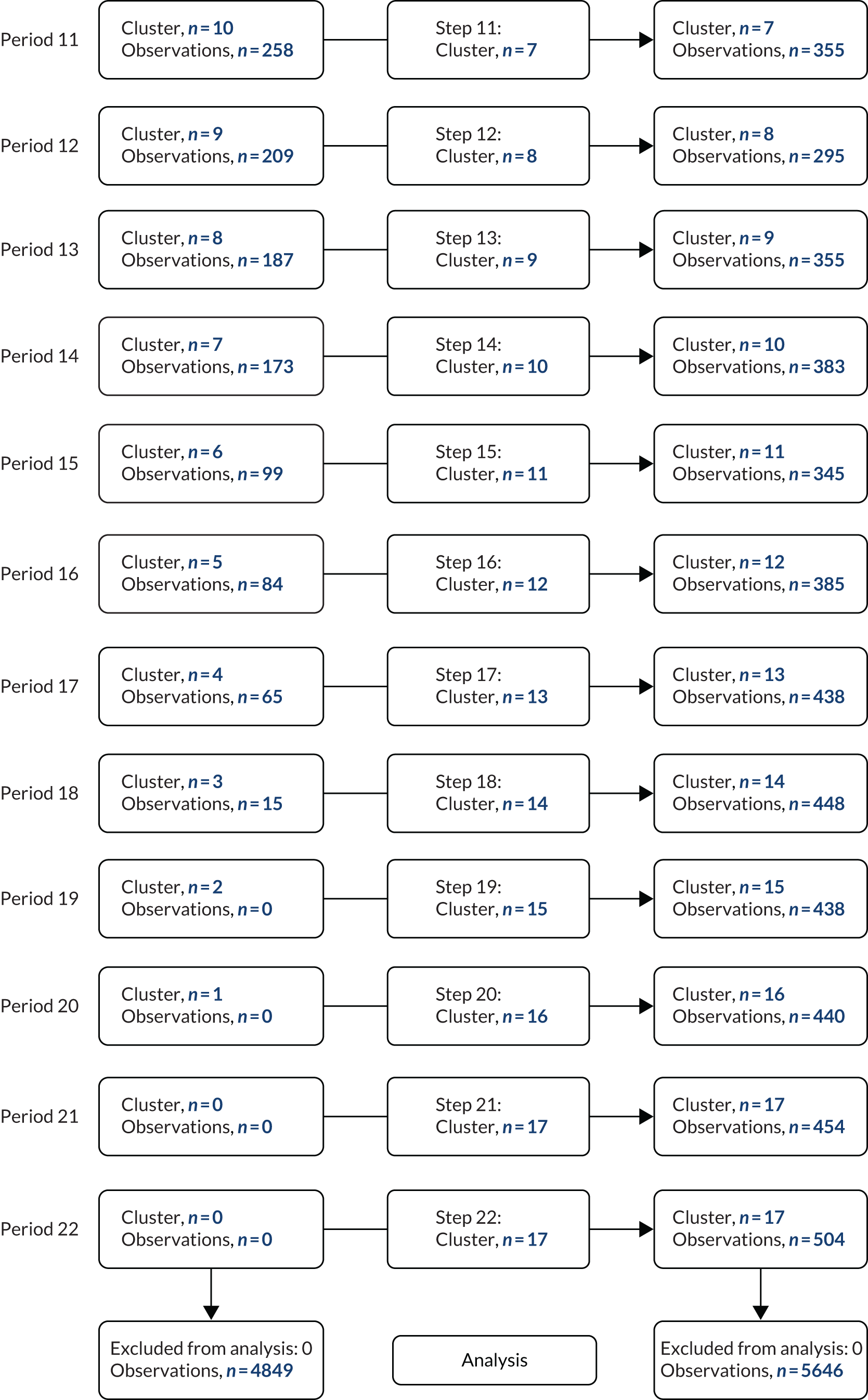
The CONSORT flow diagram follows the guidelines for presenting for participant flow in a SW-CRT. 43 A cluster represents one hospital site. Each cluster began in the control period and eventually crossed over to the intervention period, with the order of crossover randomly determined. The trial consisted of 22 calendar time periods, each of 4-week duration. At time periods 1 to 4, all clusters were in the control period (hence zero observations in the intervention period); thereafter, one cluster crossed over to the intervention at each subsequent period. Each step shows the cumulative number of clusters that transitioned across, and at each time period the number of control and intervention observations (patient admission numbers) are shown. At time periods 21 and 22, all clusters had crossed over to the intervention (hence zero observations in the control period). The summary boxes show the number of patient admissions in each treatment period.
Appendix 2 Additional process evaluation materials
Baseline visits interview guide version 1.0, 14 July 2017
Introduction
Brief explanation of the process evaluation, including:
-
its purpose and how it fits with the trial
-
the way that the process evaluation will be undertaken throughout the life of the trial
-
the important role to be played by unit staff in terms of sharing their knowledge and experience of how the protocol has been implemented and used throughout the trial and, through this, in helping us to understand trial outcomes
-
the specific purpose of the ‘baseline’ focus groups (as an opportunity for us to gain an insight into how the unit is currently organised and operates, including in terms of weaning) – through this we can understand the different contexts in which the trial is being introduced.
Summary of how the focus group will operate in terms of:
-
there are no ‘right’ and ‘wrong’ answers; all knowledge and experience is valid and important
-
confidentiality in terms of a ‘safe’ space in which to share knowledge and experience
-
‘housekeeping’, for example not all talking at once.
Guiding questions
-
Can you describe the unit in terms of, for example:
-
The different types of staff who work in it, how many there are and what their different roles are?
-
The types of children you care for, the main illnesses/problems they experience?
-
What you think the main strengths of the unit are?
-
Are there any problems or challenges faced by the unit? How have these problems or challenges arisen? How can they be addressed?
-
-
How well do you think the unit is properly resourced and otherwise supported? Probe for:
-
staffing – numbers and skill mix
-
other resources, for example equipment
-
staff training and professional development
-
hospital management and policy.
-
-
What is current unit practice in relation to weaning a child off ventilation? Probe for:
-
The ‘priority’ given to weaning.
-
Who is involved?
-
How are decisions taken on weaning and by whom?
-
How are these decisions communicated to other staff, for example from consultant to nurse?
-
Have protocols been developed, either formally or informally?
-
How (well) are these protocols used?
-
Differences in how different staff approach weaning, for example by profession/at the level of the individual? How do these differences affect how (well) weaning is undertaken?
-
-
What do you think are the main strengths of current weaning practice? Why are these so important?
-
What do you think are the main problems or challenges faced in relation to weaning in terms of:
-
the unit in general?
-
your own role in particular?
How have these problems or challenges come about? How can they be addressed?
-
-
Can you tell me what you know about the SANDWICH study? Probe for:
-
Levels of knowledge.
-
How this knowledge has been gained.
-
Perceived adequacy of knowledge.
-
Perceptions regarding is the study worthwhile? Why/why not?
-
-
From what you know about SANDWICH, how well do you think it will work in the unit? What are the reasons? Probe for:
-
acceptability to themselves and to colleagues/underpinning reasons
-
differences in acceptability in terms of grade and profession
-
hospital ‘buy-in’, for example in terms of research culture/management support
-
fit with existing unit culture in terms of practice, for example multidisciplinary working/use of protocols/professional control
-
necessary resources, for example availability of staff
-
fit with current weaning practice
-
fit with existing unit routines, for example in terms of ward rounds, patient visiting.
-
-
Thinking about all that we have discussed, what do you think will be the most important factors in determining whether or not the SANDWICH study succeeds in what it hopes to achieve?
End of trial interview guide version 1.0, 11 March 2018
Introduction
Brief explanation of interview, including:
-
its purpose and how it fits with the trial
-
the specific purpose of the ‘end of trial’ focus groups – the important role played by unit staff in terms of sharing their knowledge and experience of how the study generally, and adherence to the protocol specifically, has worked throughout the trial and, through this, in helping us to understand trial outcomes.
Summary of how the focus group will operate in terms of:
-
there are no ‘right’ and ‘wrong’ answers; all knowledge and experience is valid and important
-
confidentiality in terms of a ‘safe’ space in which to share knowledge and experience
-
‘housekeeping’, for example not all talking at once.
Guiding questions
-
Training received for SANDWICH.
-
How effective/successful was the SANDWICH training? Probe for:
-
the very fact that training was included as part of the intervention
-
means of delivery – dual face to face and computer based
-
thoroughness – did it include everything it needed to
-
length of time available to complete initial training
-
need to provide ongoing to new staff – how was this training provided (embedded in unit inductions or always discrete training)?
-
-
How did unit staff respond to the training? Probe for:
-
Did they find it useful/actively engage with it or was it more of a ‘tick-box’ exercise?
-
Did the training have an impact beyond SANDWICH?
-
-
-
Recruitment of patients to SANDWICH.
-
Were all eligible patients entered into the trial? Why/why not?
-
-
The SANDWICH protocol – issues affecting adherence.
-
What was it about the protocol itself that had an impact on adherence? Probe for:
-
pre-existing ‘fit’ or adaptable to fit with existing unit practice and culture
-
visibility of protocol
-
complexity of protocol
-
acceptability to staff.
-
-
Which features/components of the SANDWICH bundle were the ‘easiest’ to adopt/adhere to? Why?
-
Which features/components were the most difficult to adopt/adhere to? Why?
-
Probe for particular issues:
-
Regular sedation assessment using the COMFORT original/COMFORT B score –
-
for example, ‘regular’ assessment varied/promoted better sedation management/professional benefits (patient ‘easier’ to manage)/provided justification for decision-making/scores taken but failure to record them.
-
-
Twice-daily assessment of readiness for a SBT –
-
for example, assumption that patient will fail a SBT so ‘no point’ in undertaking assessment/assessments not undertaken at most appropriate times/assessment undertaken but failure to record the results.
-
-
Conducting a SBT if criteria are met –
-
for example, other patient priorities that prevented SBT, such as patient going for further tests/medical reticence regarding SBT parameters/consultants unavailable and lack of confidence to proceed in absence of consultant ‘go-ahead’/SBTs not undertaken according to protocol (e.g. 2-hour observation).
-
-
Multidisciplinary ward round –
-
for example, ward round time pressurised/bedside nurse not part of discussion.
-
-
Other issues impacting on SANDWICH.
-
Staff characteristics. Probe for:
-
staff clinical knowledge and skills
-
staff confidence
-
staff awareness of a need for change in weaning practice.
-
-
Extent/nature of multidisciplinary working on the unit.
-
Extent/nature of communication among staff on the unit.
-
Workload pressures/availability of staff.
-
Support/‘buy-in’ from management. Probe for:
-
provision of time for training/other resources
-
provision of other incentives, for example ‘treats’/SANDWICH mandated as policy (must be adopted)
-
staff response (extra work/unnecessary/intrusion; worthwhile/helpful)
-
any difference in response by grade/profession
-
research in general, received how – SANDWICH different from other studies?
-
-
-
Promoting adherence to SANDWICH.
-
How helpful were the measures included within SANDWICH to promote adherence (e.g. appointment of champions/the SANDWICH ward round checklist/SBT screen bedside record sheet)?
-
Were other measures taken, for example by the research nurses/other staff? If so, what were they/why those particular measures? How successful/useful were they?
-
-
Adherence to SANDWICH over time.
-
In what ways did adherence to SANDWICH change over time?
-
Why did it (not) change? Probe for:
-
patient benefits becoming clear over time
-
professional benefits becoming clear over time (e.g. patient ‘easier’ to manage/nurses increasingly comfortable with role/growing awareness of protocol as a basis of effective interprofessional communication)
-
growing commitment to the trial
-
trial ‘fatigue’.
-
-
-
Wider impact of SANDWICH on the unit:
-
To what extent have the sedation and weaning processes the unit adopted for the SANDWICH trial become ‘part and parcel’ of unit practice?
-
Have all the components/process become embedded? Which ones and why these and not the others?
-
How can you tell they have become adopted/embedded?
-
At what point/how long into the trial did this ‘embeddedness’ happen? And why then?
-
Will they continue to be part and parcel of unit practice after the trial has come to an end? Why/why not?
-
Even if the specific components/processes of SANDWICH have not/will not become adopted, has it encouraged a more proactive approach to weaning? How?
-
Other impact? For example, on staff clinical knowledge/on confidence/on multidisciplinary working?
-
Appendix 3 Health economics additional material
| Role | Unit cost (hourly unless stated) (£) | Source |
|---|---|---|
| Nurse | ||
| Band 4 | 28.00 | PSSRU 2019 p. 14775 |
| Band 5 | 38.00 | PSSRU 2019 p. 14775 |
| Band 6 | 47.00 | PSSRU 2019 p. 14775 |
| Band 7 | 55.00 | PSSRU 2019 p. 14775 |
| Band 7 salary | 55,102.00 | PSSRU 2019 p. 14775 |
| Band 8a | 65.00 | PSSRU 2019 p. 14775 |
| ANP: band 7 | 55.00 | PSSRU 2019 p. 14775 |
| Physiotherapist | ||
| Band 6 | 47.00 | PSSRU 2019 p. 14375 |
| Band 7 | 57.00 | PSSRU 2019 p. 14375 |
| Administrator: band 4 | 32.00 | PSSRU 2019 p. 14375 |
| Hospital professional staff: band 5 | 35.00 | PSSRU 2019 p. 14375 |
| Consultant | 109.00 | PSSRU 2019 p. 15075 |
| Non-consultant hospital doctor | 47.00 | PSSRU 2019 p. 15075 |
| PICANet definition | HRG code | Cost (£) | Source |
|---|---|---|---|
| Intensive care: ECMO/ECLS | XB01Z | 4492 | Paediatric Critical Care, Advanced Critical Care 5, NHS Reference Costs 2018–1976 |
| Intensive care: advanced enhanced | XB02Z | 3808 | Paediatric Critical Care, Advanced Critical Care 4, NHS Reference Costs 2018–1976 |
| Intensive care: advanced | XB03Z | 2845 | Paediatric Critical Care, Advanced Critical Care 3, NHS Reference Costs 2018–1976 |
| Intensive care: basic enhanced | XB04Z | 2674 | Paediatric Critical Care, Advanced Critical Care 2, NHS Reference Costs 2018–1976 |
| Intensive care: basic | XB05Z | 2225 | Paediatric Critical Care, Advanced Critical Care 1, NHS Reference Costs 2018–1976 |
| High dependency: advanced | XB06Z | 1868 | Paediatric Critical Care, Intermediate Critical Care, NHS Reference Costs 2018–1976 |
| High dependency | XB07Z | 1573 | Paediatric Critical Care, Basic Critical Care, NHS Reference Costs 2018–1976 |
| Enhanced care | XB09Z | 1023 | Paediatric Critical Care, Enhanced Care, NHS Reference Costs 2018–1976 |
| Ungrouped | – | 2072 | Paediatric Critical Care, NHS Reference Costs 2018–1976 weighted average |
| Ward-day (non-elective) | – | 713 | Based on average length of stay and cost of non-elective non-specific neonatal/paediatric long stay |
| Ward-day (elective) | – | 1262 | Based on average length of stay and cost of elective non-specific neonatal/paediatric long stay |
| Complication | Intervention (N = 4608) | Control (N = 4200) | Mean difference in complications (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean (95% CI) | n (%) | Mean (95% CI) | ||
| Reintubation | 479 (10.4) | 0.14 (0.12 to 0.17) | 441 (10.5) | 0.12 (0.09 to 0.14) | 0.02 (–0.01 to 0.05) |
| Unplanned extubation | 143 (3.1) | 0.04 (0.03 to 0.04) | 109 (2.6) | 0.02 (0.01 to 0.03) | 0.01 (0.00 to 0.03) |
| Tracheostomy | 28 (0.6) | 0.01 (0.00 to 0.01) | 18 (0.4) | 0.00 (0.00 to 0.01) | 0.00 (–0.00 to 0.01) |
| Post-extubation NIV | 698 (15.2) | 0.17 (0.14 to 0.20) | 524 (12.5) | 0.12 (0.09 to 0.15) | 0.05 (0.02 to 0.08) |
| Post-extubation stridor | 429 (9.3) | 0.12 (0.08 to 0.15) | 380 (9.1) | 0.12 (0.09 to 0.16) | –0.00 (–0.03 to 0.02) |
| Total number of complications | – | 0.47 (0.40 to 0.54) | – | 0.38 (0.32 to 0.45) | 0.09 (0.03 to 0.15) |
| Hospital resource | Observation period, mean (95% CI) | Mean difference (95% CI) | |
|---|---|---|---|
| Intervention (N = 4608) | Control (N = 4200) | ||
| Primary (index) admission (days) | |||
| PICU | 6.85 (6.40 to 7.31) | 6.72 (6.26 to 7.18) | 0.14 (–0.35 to 0.63) |
| General ward | 5.31 (4.44 to 6.17) | 5.29 (4.42 to 6.16) | 0.02 (–0.52 to 0.55) |
| Hospital length of stay | 12.17 (11.30 to 13.05) | 12.03 (11.15 to 12.91) | 0.14 (–0.55 to 0.83) |
| Re-admissions | |||
| Total re-admissions, n (%) | 259 (5.6) | 233 (5.6) | – |
| Total re-admissions within 48 hours, n (%) | 119 (2.6) | 87 (2.1) | – |
| Number of re-admissions | 0.07 (0.06 to 0.09) | 0.05 (0.03 to 0.07) | 0.02 (0.00 to 0.04) |
| PICU days | 0.55 (0.41 to 0.68) | 0.36 (0.22 to 0.50) | 0.19 (0.01 to 0.36) |
| General ward-days | 0.19 (0.10 to 0.29) | 0.17 (0.07 to 0.26) | 0.02 (–0.12 to 0.17) |
| Total PICU days | 7.41 (6.93 to 7.88) | 7.07 (6.60 to 7.55) | 0.33 (–0.18 to 0.85) |
| Intensive care: ECMO/ECLS (XB01Z) | 0.15 (0.05 to 0.24) | 0.08 (–0.01 to 0.18) | 0.06 (–0.05 to 0.17) |
| Intensive care: advanced enhanced (XB02Z) | 0.07 (0.02 to 0.12) | 0.10 (0.5 to 0.15) | –0.03 (–0.06 to 0.01) |
| Intensive care: advanced (XB03Z) | 0.44 (0.32 to 0.57) | 0.39 (0.27 to 0.52) | 0.05 (–0.10 to 0.20) |
| Intensive care: basic enhanced (XB04Z) | 1.36 (0.99 to 1.72) | 1.46 (1.09 to 1.83) | –0.10 (–0.35 to 0.15) |
| Intensive care: basic (XB05Z) | 3.14 (2.66 to 3.62) | 3.23 (2.75 to 3.71) | –0.08 (–0.40 to 0.23) |
| High dependency: advanced (XB06Z) | 1.13 (0.90 to 1.37) | 0.84 (0.60 to 1.07) | 0.29 (0.13 to 0.46) |
| High dependency (XB07Z) | 0.48 (0.37 to 0.59) | 0.42 (0.31 to 0.53) | 0.05 (–0.03 to 0.14) |
| Enhanced care (XB09Z) | 0.62 (0.44 to 0.80) | 0.51 (0.33 to 0.69) | 0.11 (0.02 to 0.20) |
| Ungrouped | 0.04 (–0.00 to 0.08) | 0.07 (0.02 to 0.11) | –0.03 (–0.09 to 0.03) |
| Total ward-days | 5.54 (4.67 to 6.41) | 5.44 (4.57 to 6.32) | 0.09 (–0.44 to 0.63) |
| Total hospital days | 12.96 (12.07 to 13.85) | 12.54 (11.65 to 13.44) | 0.42 (–0.29 to 1.12) |
| Level of care | Observation period, mean cost (£) (95% CI) | Mean cost difference (£) (95% CI) | |
|---|---|---|---|
| Intervention (N = 4608) | Control (N = 4200) | ||
| Intensive care: ECMO/ECLS (XB01Z) | 652.62 (221.67 to 1083.56) | 380.14 (–57.22 to 817.49) | 272.48 (–231.21 to 776.17) |
| Intensive care: advanced enhanced (XB02Z) | 272.46 (88.58 to 456.34) | 368.35 (183.72 to 552.98) | –95.89 (–226.57 to 34.79) |
| Intensive care: advanced (XB03Z) | 1257.91 (897.75 to 1618.07) | 1123.67 (758.04 to 1489.31) | 134.24 (–289.56 to 558.03) |
| Intensive care: basic enhanced (XB04Z) | 3627.49 (2643.34 to 4611.64) | 3896.25 (2908.63 to 4883.87) | –268.76 (–925.84 to 388.33) |
| Intensive care: basic (XB05Z) | 6994.13 (5927.40 to 8060.86) | 7182.33 (6112.01 to 8252.65) | –188.20 (–886.01 to 509.60) |
| High-dependency: advanced (XB06Z) | 2116.02 (1677.59 to 2554.45) | 1565.90 (1125.69 to 2006.11) | 550.12 (238.96 to 861.29) |
| High dependency (XB07Z) | 750.28 (578.75 to 921.80) | 663.99 (491.49 to 836.49) | 86.28 (–53.96 to 226.53) |
| Enhanced care (XB09Z) | 636.81 (454.67 to 818.95) | 523.35 (340.87 to 705.83) | 113.45 (22.24 to 204.67) |
| Ungrouped | 78.51 (–7.20 to 164.22) | 140.51 (51.45 to 229.57) | –62.00 (–192.19 to 68.18) |
| Total PICU cost | 16,396.53 (15,315.39 to 17,477.67) | 15,783.36 (14,685.55 to 16,881.17) | 613.17 (–664.76 to 1891.10) |
| Total ward-day cost | 5818.93 (4906.30 to 6731.55) | 5724.38 (4808.79 to 6639.98) | 94.54 (–493.26 to 682.34) |
| Total hospital cost | 22,213.23 (20,908.02 to 23,518.44) | 21,533.48 (20,214.03 to 22,852.93) | 679.75 (–693.01 to 2052.52) |
List of abbreviations
- AE
- adverse event
- aHR
- adjusted hazard ratio
- ANP
- advanced nurse practitioner
- aRR
- adjusted relative risk
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- FiO2
- fraction of inspired oxygen
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- ID
- identification number
- IMV
- invasive mechanical ventilation
- IQR
- interquartile range
- NICTU
- Northern Ireland Clinical Trials Unit
- NIHR
- National Institute for Health Research
- NIV
- non-invasive ventilation
- NMB
- net monetary benefit
- PEEP
- positive end-expiratory pressure
- PI
- principal investigator
- PICANet
- Paediatric Intensive Care Audit Network
- PICU
- paediatric intensive care unit
- PIM3
- Paediatric Index of Mortality 3
- PIP
- peak inspiratory pressure
- PPI
- patient and public involvement
- Psupp
- pressure support
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SANDWICH
- Sedation AND Weaning In CHildren
- SBT
- spontaneous breathing trial
- SD
- standard deviation
- SpO2
- saturation of oxygen in peripheral blood
- SW-CRT
- stepped-wedge cluster randomised trial
- TSC
- Trial Steering Committee
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/TCFX3817).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
