Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the Evidence Synthesis Programme on behalf of NICE as project number NIHR128897. The protocol was agreed in May 2019. The assessment report began editorial review in November 2019 and was accepted for publication in November 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Brazzelli et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Objectives
The overall objective of this assessment was to summarise the current evidence on the clinical effectiveness and cost-effectiveness of using the NephroCheck® test (Astute Medical, Inc., San Diego, CA, USA), the ARCHITECT® and Alinity i™ urine neutrophil gelatinase-associated lipocalin (NGAL) assays (Abbott Laboratories, Abbott Park, IL, USA), and the BioPorto urine and plasma NGAL tests (BioPorto Diagnostics A/S, Hellerup, Denmark) to help assess the risk of acute kidney injury (AKI) in critically ill hospitalised patients who are considered for admission to critical care. AKI is still a challenging clinical problem for hospitalised patients, especially for those in need of critical care. Earlier detection of kidney injury may facilitate the adoption of strategies to preserve renal function and prevent further progression of kidney disease.
There are several components to this assessment that fall within the scope of the following research questions:
-
Do novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, and BioPorto urine and plasma NGAL tests) accurately detect emerging AKI in critically ill people who are considered for critical care?
-
Do the novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, BioPorto urine and plasma NGAL tests) predict the development of future events [e.g. AKI, mortality, need for long-term renal replacement therapy (RRT)] in critically ill people at risk of developing AKI who are considered for admission to critical care?
-
Does the use of novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, and BioPorto urine and plasma NGAL tests) lead to improvements in clinical outcomes of critically ill people who are considered for admission to critical care (i.e. reduction in events rates, such as mortality and long-term RRT, among patients whose management is guided by the novel biomarkers)?
-
Does routine use of novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, and BioPorto urine and plasma NGAL tests) affect costs to the NHS, length or quality of life [i.e. quality-adjusted life-years (QALYs)], or cost-effectiveness, measured as incremental cost per QALY gained for critically ill people who are considered for admission to critical care?
In brief, the main objectives of this assessment were as follows:
-
to determine the diagnostic accuracy, prognostic accuracy and clinical impact of the use of novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, and BioPorto urine and plasma NGAL tests) for the assessment of AKI in critically ill patients (adults and children) who are being assessed for admission to critical care
-
to develop an economic model to assess the cost-effectiveness of the use of novel biomarkers (i.e. NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, and BioPorto urine and plasma NGAL tests) for the assessment of AKI in critically ill patients (adults and children) who are considered for admission to critical care.
Chapter 2 Background and definition of the decision problem
Health problem
Acute kidney injury is a common and serious complication that typically occurs in the context of an acute critical illness or during a postoperative period. It is associated with prolonged hospital stay, severe morbidity and increased mortality. 1,2 Delayed identification of AKI contributes to worse outcomes. 3
To pre-empt or avoid lasting consequences of AKI, early detection may be beneficial. Traditionally, AKI diagnosis relies on a rise in serum creatinine levels and/or fall in urine output. Despite its widespread use in the monitoring of kidney health and disease, creatinine is an imperfect marker of kidney function because its level in the blood is not solely dependent on kidney function, and changes in creatinine lag behind reduction in kidney function in AKI. 4 When kidney function suddenly falls, even if a reduction in renal excretion occurs instantly, it can take hours or sometimes days for the level of creatinine to rise in the blood sufficiently for AKI to be diagnosed according to current international definitions. 5 Moreover, in response to stress, or even kidney damage, the kidneys have reserve capacity and can compensate so that kidney function is maintained. For this reason, in some clinical settings, significant kidney damage can occur without AKI being apparent from changes in blood creatinine. In other settings, such as during a temporary reduction in blood flow to the kidneys, rises in creatinine and a reduction in urine can occur, even when no significant damage has occurred. These limitations related to the use of creatinine assessment have led to the search for novel biomarkers that may detect kidney damage or stress earlier and more reliably.
Biomarker tests for AKI include the NGAL test, which can be measured using a sample of urine or blood. 6 NGAL is released from neutrophils and is induced by inflammation, indicating tubular injury. 4 One limitation of NGAL is that it is produced throughout the body, making it difficult to distinguish systemic inflammation from localised renal inflammation. 4 Novel NGAL tests include the ARCHITECT and Alinity i urine NGAL assays, the BioPorto NGAL plasma test and the BioPorto NGAL urine test.
Another biomarker for AKI is the NephroCheck test, which is a combination of two urinary biomarkers: the tissue inhibitor of metalloproteinase-2 (TIMP-2) and the insulin-like growth factor-binding protein 7 (IGFBP7). Both TIMP-2 and IGFBP7 are cell-cycle arrest proteins that are released into urine as markers of cellular stress in the early phase of tubular cell injury due to a variety of insults (e.g. toxins, drugs, oxidative stress and inflammation), which leads to AKI. 7 The US Food and Drug Administration has approved these combined biomarkers to assess the risk of AKI in critically ill patients. 4
These novel biomarkers have been developed to detect early damage or stress in the kidneys. If reliable use of these biomarkers can be demonstrated, they may enable earlier identification of AKI, and, therefore, early management of those with a modifiable disease course, with potential for downstream benefits in patients’ clinical outcomes. If demonstrated, the ability of these novel biomarkers for early detection of AKI could have the potential to improve current AKI management by enabling timely measures that could prevent progression to more severe kidney injury, as well as informing decisions about the ‘step-down’ of low-risk patients to a lower level of hospital care, thereby reducing the use of hospital resources.
The purpose of this assessment was to review the current evidence on the diagnostic accuracy, prognostic accuracy, impact on clinical outcomes and cost-effectiveness of novel biomarkers (i.e. the NephroCheck test, ARCHITECT and Alinity i urine NGAL assays, BioPorto NGAL plasma test and BioPorto NGAL urine test) for the assessment of AKI in critically ill patients who are considered for critical care admission.
Aetiology, pathology and prognosis
Acute kidney injury ranges from minor loss of kidney function to complete kidney failure. In current practice, reduced kidney function is identified by elevated serum creatinine levels and/or reduced urine output.
There are many causes of acute kidney injury,8 including the following:
-
pre renal – reduced oxygen delivery to the kidneys, caused by:
-
low blood volume (after bleeding, excessive vomiting or diarrhoea, and severe dehydration)
-
reduced blood flow from the heart (potentially caused by sepsis or heart/liver failure)
-
damage to blood vessels, which can be caused by inflammation or blockages in the kidneys
-
medications that affect blood flow to the kidneys
-
-
intrinsic/renal – damage to the kidney potentially caused by drugs, infections or contrast agents
-
post renal – a blockage preventing drainage from the kidneys (potentially caused by an enlarged prostate, a tumour in the pelvis or kidney stones).
Incidence and/or prevalence
Major surgery is a significant risk factor for the development of AKI. 4 In general, incidence of postoperative AKI depends on the type of surgery. Rates of AKI after cardiac surgery have been reported to range from 8% to 40%, depending on the patient populations. 4 Recent meta-analyses have reported a pooled incidence of AKI in patients admitted to intensive care after abdominal surgery of 13.4% [95% confidence interval (CI) 10.9% to 16.4%],9 and pooled incidences of AKI after major trauma of 24% (95% CI 20% to 29%)8 and 21% (95% CI 16.5% to 24.9%). 10
The incidence of AKI for all major, non-cardiac surgery patients and trauma patients can be as high as 50% (e.g. liver transplant patients). In a retrospective cohort of > 27,000 patients, the incidence of AKI, defined according to the Risk, Injury, Failure, Loss of kidney function and End-stage disease (RIFLE) criteria, was 37%. 11,12
Impact of the health problem
People with AKI have higher mortality and longer hospital stays. 1,2 In addition, AKI is associated with a higher risk of developing chronic kidney disease (CKD) and a need for long-term dialysis. The risk of CKD increases with the increased severity of AKI. More severe AKI has also been associated with increased mortality, length of hospital stay and use of intensive care services, in addition to a reduced chance of renal recovery. 1,2 People with more severe AKI (and a greater loss of renal function) are more likely to need temporary RRT.
Measurement of disease
Several tools are available for determining the stage of AKI. A summary of staging system13 for AKI in adults based on the RIFLE,14 Acute Kidney Injury Network (AKIN)15 and Kidney Disease: Improving Global Outcomes (KDIGO)5 systems is presented in Table 1. A patient’s AKI should be staged by the criteria, and a classification of stage 1 or higher indicates AKI.
| Criteria | Stage | Definition | |
|---|---|---|---|
| Serum creatinine criteria | Urine output | ||
| aKDIGO5 | 1 |
|
< 0.5 ml/kg/hour for 6 hours |
| 2 |
|
< 0.5 ml/kg/hour for 12 hours | |
| 3 |
|
< 0.3 ml/kg/hour for 24 hours or anuria for 12 hours | |
| bRIFLE14 | R |
|
< 0.5 ml/kg/h for 6 hours |
| I |
|
< 0.5 ml/kg/hour for 12 hours | |
| F |
|
< 0.3 ml/kg/hour for 24 hours or anuria for 12 hours | |
| L |
|
||
| E |
|
||
| cAKIN15 | 1 |
|
< 0.5 ml/kg/hour for 6 hours |
| 2 |
|
< 0.5 ml/kg/hour for 12 hours | |
| 3 |
|
< 0.3 ml/kg/hour for 24 hours or anuria for 12 hours | |
Description of the technologies under assessment
The NephroCheck test, the ARCHITECT and Alinity i urine NGAL assays, and the BioPorto urine and plasma NGAL tests may help to assess AKI in critically ill people who are considered for admission to critical care in hospital. These tests may be able to detect kidney injury earlier than the methods currently used for monitoring kidney function.
The NephroCheck test
The NephroCheck test measures urine levels of two biomarkers, TIMP-2 and IGFBP7, to assess the risk of moderate to severe AKI (defined as per KDIGO guidelines) in the subsequent 12 hours. The test result must be used in conjunction with clinical evaluation and the results of other tests, such as serum creatinine levels and urine output.
The concentrations of TIMP-2 and IGFBP7 are used to calculate an AKIRisk® Score (Astute Medical, Inc.) [the concentrations of each (ng/ml) multiplied together and divided by 1000]. A score of ≤ 0.3 indicates a low risk of developing moderate to severe AKI within 12 hours of assessment, whereas a score of > 0.3 indicates a high risk of developing moderate to severe AKI within 12 hours of assessment. 6
When used with the Astute140® Meter (Astute Medical, Inc.), the NephroCheck test system consists of the following components:
-
Astute140 Meter kit (a benchtop analyser)
-
Astute140 Electronic Quality Control device
-
NephroCheck test kit (includes a single-use NephroCheck test cartridge and reagents)
-
NephroCheck Liquid Control kit
-
NephroCheck Calibration Verification kit.
A fresh or thawed urine sample (mixed with reagent) is added to a single-use test cartridge, which is then inserted into an Astute140 Meter for incubation and result calculation. Preparation takes 3–5 minutes and the results of a NephroCheck test are available in ≈ 20 minutes. In the NHS, the Astute140 Meter would be used in a laboratory and not at the point of care.
The test can also be run on the VITROS® 3600 Immunodiagnostic System (Ortho-Clinical Diagnostics Inc., Raritan, NJ, USA) and on the VITROS® 5600 Integrated System (Ortho-Clinical Diagnostics Inc.) clinical chemistry analysers. All systems generate a single numerical result (the AKIRisk Score).
For surgical patients, it is recommended that the NephroCheck test is administered 2–4 hours after surgery. As NephroCheck exhibits a characteristic rise and fall after various exposures, a second administration of the test within the first 24 hours may be considered in patients with an ongoing risk of developing AKI.
In the UK, the NephroCheck test is marketed for people aged > 21 years.
Neutrophil gelatinase-associated lipocalin assays
ARCHITECT and Alinity i urine neutrophil gelatinase-associated lipocalin assays
The ARCHITECT urine NGAL assay is a chemiluminescent microparticle immunoassay for the quantitative determination of NGAL in human urine. NGAL can be used as a marker of kidney injury.
The ARCHITECT urine NGAL assay might be used as follows:
-
for early detection of AKI
-
to provide a measure of the severity of AKI
-
to predict the requirement for RRT
-
to help differentiate AKI from CKD and dehydration.
For diagnostic purposes, the test results should be used in conjunction with clinical assessment and the results of any other testing that has been undertaken (including serum creatinine levels and urine output). In addition, if the NGAL results are inconsistent with clinical assessment and other test results, additional testing can be undertaken to confirm the NGAL results.
The test could be used daily until a diagnosis is made or treatment for AKI is initiated.
The expected range for the assay (for people without kidney injury) is ≤ 131.7 ng/ml, based on the 95th percentile from specimens of non-hospitalised donors, but results from individual laboratories may vary and the manufacturer recommends that each laboratory should determine its own reference range based on the particular locale and population characteristics. The test has no age restrictions in use.
The assay is run on the ARCHITECT system (i1000SR, i2000, i2000SR, ci4100, ci8200 or ci16200) (Abbott Laboratories) in a laboratory. The throughput of the system is up to 200 tests per hour, and the time to first result is 36 minutes.
In addition to the ARCHITECT Urine NGAL Reagent Kit, the following materials are also needed:
-
ARCHITECT Urine NGAL Calibrators
-
ARCHITECT Urine NGAL Controls or other control material
-
ARCHITECT i pre-trigger solution
-
ARCHITECT i trigger solution
-
ARCHITECT i wash buffer
-
ARCHITECT i reaction vessels
-
ARCHITECT i sample cups
-
ARCHITECT i septum
-
ARCHITECT i replacement caps.
The Abbott NGAL assay is also available for use on the Alinity i immunoassay system. The reagents for the Alinity i and ARCHITECT NGAL assays are the same.
The BioPorto neutrophil gelatinase-associated lipocalin test (using urine or plasma)
The BioPorto NGAL test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine, ethylenediaminetetraacetic acid (EDTA) plasma and heparin plasma on automated clinical chemistry analysers. NGAL measurements may be useful in pre-empting the diagnosis of AKI, which may lead to acute renal failure. Urinary NGAL can serve as an early marker of AKI after cardiopulmonary bypass surgery, and both urinary and plasma levels of NGAL provide an early indication of acute renal injury in unselected patients in intensive care.
The NGAL test is intended to be used alongside monitoring of serum creatinine levels and urine output (rather than as a standalone test), and the significance of any raised NGAL level should be interpreted in the light of a patient’s clinical features.
The NGAL test can be administered as a single measurement, but also as a serial measurement, to detect any further development of AKI during hospitalisation or any improvement in the clinical condition. For patients admitted to intensive care, the test can be used to predict stage 2/3 AKI or as a negative predictive marker to rule out the presence of AKI.
To indicate the presence of AKI, the NGAL concentration in an isolated sample of urine and/or EDTA plasma should exceed 250 ng/ml. This threshold has been chosen to minimise the risk of an unacceptably high proportion of false-positive results.
The assay can be run on clinical chemistry analyser systems from F. Hoffman-La Roche Ltd (cobas®, Modular P) (Basel, Switzerland), Siemens Healthineers (ADVIA®) (Erlangen, Germany), Abbott Laboratories (AEROSET®, ARCHITECT) and Beckman Coulter Inc. (Olympus AU) (Brea, CA, USA) in a laboratory. The assay takes 10 minutes to run.
In addition to the NGAL Test Reagent Kit, the following materials are also needed:
-
NGAL Test Calibrator Kit
-
NGAL Test Control Kit
-
0.9% weight by volume (w/v) aqueous sodium chloride solution as zero calibrator
-
analyser-specific reagent containers.
At present, the test has no age restrictions on use.
Identification of important subgroups
The primary scope of this assessment was the optimisation of current secondary care of critically ill patients to decide whether or not the use of novel biomarkers would improve detection of AKI and, consequently, the current care pathway. The relevant population considered in this assessment was critically ill people at risk of developing AKI (i.e. those who are having their serum creatinine levels and urine output monitored) who are being assessed for possible admission to critical care. There is variation in intensive care utilisation across the world; in most studies conducted outside the UK, critically ill participants are usually admitted to critical or intensive care. The following patient subgroups have been identified as particularly relevant for the purpose of this assessment:
-
type of surgery (e.g. major vascular/cardiac surgery, major non-vascular surgery, trauma, solid organ transplant)
-
type of setting [e.g. post-surgery care, cardiac care, intensive or critical care, emergency department (ED)]
-
type of sample medium (i.e. urine, blood plasma)
-
people with a different underlying risk of AKI (e.g. depending on underlying condition: CKD, sepsis, hip fracture, major trauma, chronic liver disease)
-
presence or absence of urinary infection and other inflammatory conditions (tests may perform differently in these populations).
Relevant comparator
Novel biomarkers need to be compared for incremental advantage over standard approaches to measuring kidney function. As discussed previously, AKI diagnosis traditionally relies on a rise in serum creatinine levels and/or fall in urine output. Creatinine has limitations as a biomarker because its concentration depends on the total body muscle mass, which varies between individuals. Some creatinine is also eliminated from the body by mechanisms other than filtering by the kidneys, which can be influenced by a variety of medications, including some commonly used antibiotics. In an illness that causes a sudden fall in kidney function (AKI), there may be a lag ranging from hours to days before creatinine levels in the blood rise to a level sufficient for AKI to be diagnosed according to current international definitions. 5 In addition, in response to stress or even kidney damage, the kidneys have reserve capacity and can compensate so that kidney function is maintained. For this reason, in some clinical settings, significant kidney damage can occur without AKI being apparent from changes in blood creatinine. In other settings, such as during a temporary reduction in blood flow to kidneys, rises in creatinine and a reduction in urine can occur even when no significant damage has occurred.
Care pathway
The NICE clinical guideline on AKI16 recommends measuring serum creatinine and comparing it with the baseline for adults, children and young people with acute illness if risk factors for the condition are likely or present. Risk factors include sepsis, hypovolaemia and deteriorating early warning scores (using a paediatric version for children and young people). NHS England and NHS Improvement have endorsed the National Early Warning Score (NEWS) for use in acute and ambulance settings. 17 An updated version of the score (NEWS2)17 was published in December 2017. The score should not be used with children (aged < 16 years) or pregnant women.
The NICE guideline16 further recommends monitoring serum creatinine regularly in all adults, children and young people with or at risk of AKI. The guideline development group did not wish to define ‘regularly’ because this would vary according to clinical need, but recognised that daily measurement was typical while in hospital.
An AKI algorithm to help with detection and diagnosis of the condition has been endorsed by NHS England. 18 In some hospitals, the algorithm has been integrated into laboratory information management systems to help identify potential cases of AKI from laboratory data in real time.
The KDIGO Clinical Practice Guideline for Acute Kidney Injury19 highlights the importance of screening patients who have had an exposure that may cause AKI (e.g. sepsis or trauma) and recommends that high-risk patients continue to be monitored until the risk subsides. The guideline19 states that the frequency of serum creatinine measurements is a matter of clinical judgement, but suggests as a general rule that high-risk inpatients should have serum creatinine measured at least daily and more frequently after an exposure. Critically ill patients should also have urine output monitored.
For adults who are at risk of AKI, the NICE AKI guideline16 also recommends that systems are put in place to recognise and respond to oliguria (urine output < 0.5 ml/kg/hour).
For children and young people who are at risk of AKI, the guideline16 recommends:
-
measuring urine output
-
recording weight twice daily to determine fluid balance
-
measuring urea, creatinine and electrolytes
-
considering measuring lactate, blood glucose and blood gases.
Further detail on these recommendations and further recommendations on the ongoing assessment of the condition of patients in hospital can be found in section 1.2 of the NICE clinical guideline on AKI. 16
The NICE guideline16 recommends diagnosing AKI in line with the RIFLE14 (or the paediatric-modified RIFLE),20 AKIN15 or KDIGO5 definitions, by using any of the following criteria:
-
a rise in serum creatinine of ≥ 26 µmol/l within 48 hours
-
a ≥ 50% rise in serum creatinine levels known or presumed to have occurred within the previous 7 days
-
a fall in urine output to < 0.5 ml/kg/hour for > 6 hours in adults and for > 8 hours in children and young people
-
a ≥ 25% fall in estimated glomerular filtration rate (eGFR) in children and young people within the previous 7 days.
There are no direct therapies for treating AKI. Care focuses on optimising haemodynamics and fluid status, avoiding nephrotoxic treatments and carrying out investigations to identity and resolve the underlying cause as quickly as possible. In general, the goal of care is to prevent any further kidney injury and to stop the worsening of the underlying illness to prevent mortality or renal progression to such a degree that RRT is needed.
The NICE clinical guideline on AKI16 highlights the importance of identifying the cause(s) of AKI and has recommendations on the use of urinalysis and ultrasound for this purpose.
The KDIGO Clinical Practice Guideline for Acute Kidney Injury19 also recommends prompt evaluation of people with AKI to determine the cause. Identifying possible reversible causes of the condition is highlighted as important in reducing the severity of the condition.
The NICE clinical guideline on AKI16 has recommendations on managing AKI (section 1.5), covering removing urological obstruction, pharmacological management, RRT and referral to nephrology services. The KDIGO Clinical Practice Guideline for Acute Kidney Injury19 recommends staging severity of AKI with serum creatinine and urine output, and to manage the condition according to stage and cause. General management principles for people at high risk of AKI (or with the condition) are to:
-
discontinue nephrotoxic agents if possible
-
monitor volume status and perfusion pressure
-
consider functional haemodynamic monitoring
-
monitor serum creatinine and urine output
-
avoid hyperglycaemia
-
consider alternatives to radiocontrast procedures.
Further actions, such as initiating RRT, should be considered at higher stages of AKI only. Dosages of drugs may also need to be adapted because of reduced kidney function. The KDIGO guideline19 also has more detailed guidance on the prevention and treatment of AKI (section 3). This includes haemodynamic monitoring and support, glycaemic control and nutritional support, the use of diuretics and vasodilator therapy.
In UK clinical practice, the NephroCheck test and NGAL assays are likely to be used for the assessment of AKI among people who are considered for admission to critical care, rather than among patients already in critical care. It is worth pointing out that the NephroCheck test, the ARCHITECT and Alinity i urine NGAL assays and the BioPorto plasma and urine NGAL tests would not replace serum creatinine and urine output monitoring, but would be used alongside current monitoring to facilitate earlier detection of kidney injury and prompt adoption of strategies to prevent further progression of kidney disease.
Chapter 3 Assessment of clinical effectiveness
Systematic review methods
Identification of studies
Comprehensive electronic searches were conducted to identify relevant reports of published studies. Highly sensitive search strategies were developed, including index terms, free-text words, abbreviations and synonyms, to combine biomarkers and AKI. The electronic databases MEDLINE (via Ovid), EMBASE (via Ovid), Web of Science Core Collection, Health Technology Assessment (HTA) Database, Cumulative Index to Nursing and Allied Health Literature, and Cochrane Central Register of Controlled Trials were searched, with no restriction on date or publication type. Full details of the search strategies are presented in Appendix 1. The searches were undertaken between 17 May and 10 June 2019.
In addition, we searched the following sources for ongoing or unpublished studies: ClinicalTrials.gov (www.clinicaltrials.gov/), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (https://apps.who.int/trialsearch/) and the WHO Global Index Medicus (www.who.int/library/about/The_Global_Index_Medicus/en/) (all of these websites were accessed on 10 June 2019). Furthermore, websites of relevant professional organisations and health technology agencies, as well as appropriate clinical experts, were consulted to obtain any additional potentially relevant reports. The reference lists of all included studies were perused to identify further potentially relevant studies. We also considered evidence provided by the manufacturers of the biomarkers included in this assessment (i.e. Astute Medical, Inc.; Abbott Laboratories; and BioPorto Diagnostics A/S).
Inclusion and exclusion criteria
Inclusion and exclusion criteria for each of the clinical effectiveness questions considered in this assessment are summarised in Table 2. Only those studies that fulfilled these criteria were deemed suitable for inclusion.
| Domain | Research question | ||
|---|---|---|---|
| 1. Do novel biomarkers accurately detect emerging AKI in critically ill people who are considered for admission to critical care? | 2. Do the novel biomarkers predict the development of future events in critically ill people at risk of developing AKI who are considered for admission to critical care? | 3. Does the use of novel biomarkers lead to improvements in clinical outcomes of critically ill people who are considered for admission to critical care? | |
| Population and setting |
|
|
|
Excluded:
|
Excluded:
|
Excluded:
|
|
| Biomarkers under investigation |
|
|
AKI care initiated according to the results of the biomarkers under investigation (the NephroCheck test, the ARCHITECT and Alinity i urine NGAL assays, the BioPorto NGAL urine test and the BioPorto NGAL plasma test) |
| The primary time point for biomarker measurement was immediately after surgery or on admission to critical or intensive care. When multiple measurements were reported, we selected that taken at the time closest to the primary time point | The primary time point for biomarker measurement was immediately after surgery or on admission to critical or intensive care. When multiple measurements were reported, we selected that taken at the time closest to the primary time point | ||
Exclusion:
|
Exclusion:
|
||
| Reference standard/comparator | At present, there is no universally accepted reference standard for the diagnosis of AKI. The current methods for detecting or predicting AKI are in line with the RIFLE (or paediatric-modified RIFLE), AKIN and KDIGO classification systems, which are based on the assessment of serum creatinine levels and urine output alongside clinical judgement (see NICE clinical guideline16) | Existing clinical criteria for the monitoring of serum creatinine and urine output used in conjunction with clinical judgement (reference standard) | AKI care initiated according to standard clinical practice (existing clinical criteria without biomarkers) |
| Outcomes | Detection of AKI (using measures of accuracy, i.e. sensitivity and specificity) |
|
|
| Study design |
|
Prospective studies reporting:
|
|
Exclusion:
|
Exclusion:
|
Exclusion:
|
|
Study selection and data extraction
A screening checklist was developed to assist study selection and data extraction (see Appendix 2, Figure 25). The data extraction form is provided in Appendix 3. One reviewer (CR) screened the titles and abstracts identified by the search strategies for inclusion or exclusion. A second reviewer (MI) double checked all non-selected citations. As a lot of relevant information was not available from the titles or abstracts of the reports identified by the literature searches (e.g. information about the immunoassay used and type of analyses), our selection approach was overinclusive. Full-text copies of all potentially relevant reports were retrieved and assessed for inclusion by one reviewer (MAM, MI or CR). A second reviewer (MAM, MI or CR) double checked 20% of the reports. Any disagreement was resolved by discussion or referred to a third reviewer (MB).
One reviewer (MAM, MB, MI, AP or CR) extracted data from each eligible study using a form developed and piloted for the purpose of this assessment. If multiple publications of the same cohort of participants were identified, the publication with the most complete or suitable data set was considered as the primary source of information. Any uncertainty related to the data extraction process was discussed among reviewers and resolved by consensus.
From each study, the following data were extracted:
-
characteristics of studies – first author, year of publication, study centre, country, inclusion and exclusion criteria, method of participant enrolment
-
characteristics of study participants – age, sex, target condition, setting, number of participants enrolled, number of participants analysed, number excluded from analysis, main reasons for exclusion
-
characteristics of the biomarkers (e.g. manufacturer, detection method, threshold, timing of the measurement)
-
characteristics of the reference standard (i.e. creatinine and urine output criteria for AKI)
-
outcome data –
-
data on the diagnostic performance of the biomarkers for detection of AKI [absolute number of true-positive, false-positive, false-negative and true-negative cases; sensitivity and specificity values; area under the curve (AUC) calculated from the receiver operating characteristic (ROC) plot]
-
data on the prediction of development of AKI, worsening of AKI, mortality, need for RRT and CKD, as provided by the study authors [e.g. AUC values, odds ratio (OR) or hazard ratio (HR), duration of follow-up]
-
data on the clinical utility of the biomarkers (impact of the use of the biomarkers on clinical outcomes), as reported by study authors [e.g. number of events and number of participants for each relevant binary outcome; mean, standard deviation (SD) and number of participants for each relevant continuous outcome].
-
Assessment of risk of bias
Validated tools were used to assess the risk of bias of the included studies according to their study design. We used the Quality Assessment of Diagnostic Accuracy Studies, version 2 (QUADAS-2) tool21 to assess the risk of bias of studies assessing the diagnostic and prognostic accuracy of the biomarkers under investigation. The QUADAS-2 tool consists of four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of having a ‘low’, ‘high’ or ‘unclear’ risk of bias, and the first three are also assessed in terms of concern regarding ‘low’, ‘high’ or ‘unclear’ applicability.
We used the Prediction model Risk Of Bias ASsessment Tool (PROBAST),22 which is structured into four domains (participants, predictors, outcome and analysis) to assess the risk of bias and applicability of prediction model studies.
A single reviewer (MAM, MB, MI, AP or CR) assessed the risk of bias of each of the included studies. Any uncertainty was discussed among reviewers and resolved by consensus.
No other types of study design were identified.
Data synthesis and analysis
For each assay, for each study, we calculated sensitivity, specificity and prevalence values from the reported numbers of true-positive, false-positive, false-negative and true-negative cases. If studies did not provide 2 × 2 data, these were derived from the sensitivity and specificity estimates, if given. We entered diagnostic data into Review Manager software (RevMan version 5.3, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to produce forest plots of sensitivity and specificity estimates, together with their 95% CIs.
When appropriate, we performed a meta-analysis of each pair of sensitivity and specificity estimates from each included study for each relevant assay. As reported threshold levels for a positive test differed across studies, we conducted random-effects meta-analyses using the hierarchical summary receiver operating characteristic (SROC) model23,24 implemented in Stata® (metandi command)25 (StataCorp LP, College Station, TX, USA) to estimate summary values for sensitivity and specificity. The model takes into account both of these measures of accuracy and their correlation, assumes that accuracy and thresholds vary between studies, and incorporates both within- and between-study variability. We constructed a SROC plot using the hierarchical model, produced sensitivity and specificity summary estimates, and hence a summary operating point, and calculated the 95% confidence and prediction regions. In accordance with the Stata requirements, we performed meta-analyses when data from four or more studies were available. For studies that reported multiple thresholds, we selected only one threshold to be included in the analysis. We performed separate meta-analyses for each biomarker, clinical setting, mode of sampling (urine or plasma) and type of patient population (adults or children). To inform the economic model, we also performed separate meta-analyses for each biomarker across all clinical settings.
For each biomarker, heterogeneity was assessed by visual inspection of the forest plots of sensitivity and specificity estimates and of the size of the prediction region in the SROC curve plots.
When possible, we performed meta-analyses of AUC values using a random-effects model to measure the performance of each biomarker for the prediction of each relevant outcome (i.e. AKI, mortality, RRT and CKD). We assessed the proportion of between-study variation in the area under ROC curve due to heterogeneity, rather than sample error, using the prediction interval. We considered an AUC of > 0.70 as indicative of a useful risk predictor.
Stata version 15.0 was used for all statistical analyses. Graphs were made using either Stata or RevMan version 5.3.
Patient and public involvement
There was no patient and public involvement in this study. The study was conducted as part of the NICE Diagnostics Assessment Programme (DAP). We did not deem it necessary to involve further patient representatives and laypeople as a range of stakeholders, including members of the public and national groups representing patients and/or their carers, are already involved as participants in the DAP process for each individual assessment.
Results of the assessment of clinical effectiveness
Results of the literature searches
The literature searches identified 6379 records; 86 additional records were identified in either trial registers (i.e. EU Clinical Trials Register, ICTRP, ClinicalTrials.gov) or other literature collections (i.e. HTA Database, WHO Global Index Medicus), for a total of 6465 retrieved records. After de-duplication, 2348 records were screened for relevance. Of these, 1050 were considered to be potentially relevant and were selected for full-text assessment. Four articles could not be obtained. Of the 1046 records retrieved and assessed in depth, 71 met the inclusion criteria. After excluding secondary or multiple publications, we selected 56 studies for inclusion in the systematic review of effectiveness. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram in Figure 1 shows the flow of studies through the selection process. The bibliographic details of the studies retrieved for full-text assessment and subsequently excluded, together with the main reasons for their exclusion, are presented in Appendix 5, Table 26.
FIGURE 1.
The PRISMA flow diagram of selected studies.
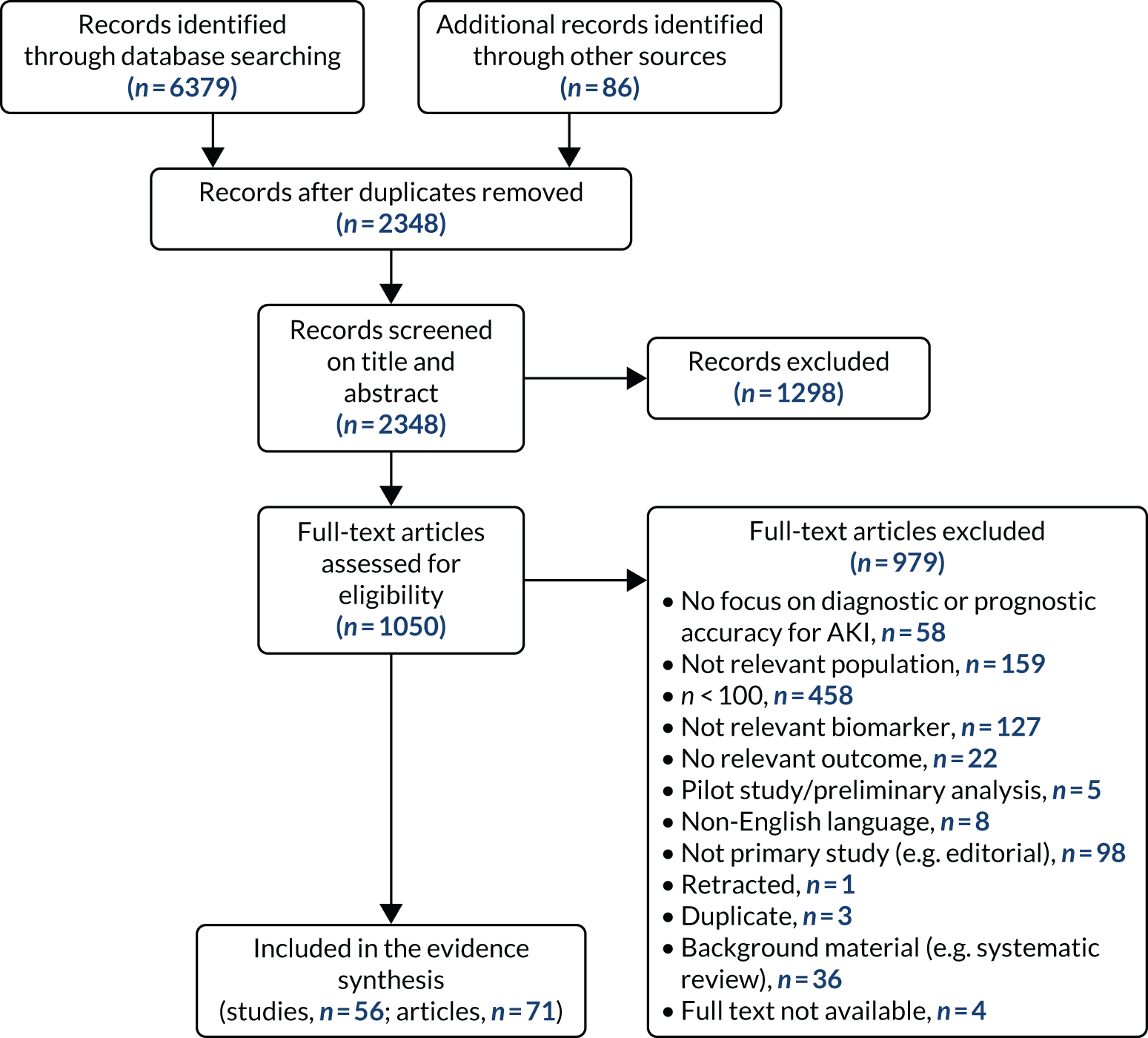
Overview of included studies
A list of all included studies can be found in Appendix 4. General characteristics of the 56 included studies and their associated references are provided in Table 3 for the adult population and in Table 4 for the child population. Further study characteristics are provided in Appendix 6, Table 27. The majority of studies were cohort studies. In 46 studies, data were collected prospectively;17–30,34,37,45–48,50–54,56–59,61–63,65–70,72–75,77–81,83,84,86–91,93 in one study, data were collected prospectively but analysed retrospectively;26 in one study, data were collected retrospectively;82 and in eight studies information on data collection was unclear. 32,33,35,49,55,60,64,92 Fifty-three studies provided suitable data on the use of the biomarkers for detection or prediction of AKI in critically ill patients admitted to hospital,26–30,32–35,37,45–56,58–66,68–70,72–75,77–84,87–93 11 studies provided suitable data for prediction of mortality in critically ill patients at risk of AKI46,49,53,59,62,70,72,75,82,83,87 and four studies provided suitable data for prediction of need for RRT. 46,72,75,87 No studies provided suitable data for prediction of CKD.
| First author, year of publication, country, associated publications | Assay | Target population (setting) | Age (years) | Sample size (n) | AKI events (n) | AKI definition | Time frame for AKI diagnosis |
|---|---|---|---|---|---|---|---|
| Cummings 2019,26 USA | NephroCheck | Cardiac surgery (atorvastatin for AKI cardiac surgery study) | Median 67 (IQR 58–75) | 400 | 14 | KDIGO stage 2/3 | Within 48 hours of surgery |
| Oezkur 2017,27 Germany | NephroCheck | Cardiac surgery (CABG, valve surgery or surgery of the thoracic aorta) |
|
150 | 35 | KDIGO | Within 48 hours of surgery |
| Beitland 2016,28 Norway | NephroCheck | Critical care – mixed population (out-of-hospital cardiac arrest) |
|
195 | 88 | KDIGO | Within 3 days of admission |
| Bihorac 2014,29 USA | NephroCheck | Critical care – mixed population (ICU/ITU) | Mean 63 (SD 17) | 408 | 71 | KDIGO stage 2/3 | Within 12 hours of admission |
| Di Leo 2018,30 Italy | NephroCheck | Critical care – mixed population (ICU/ITU) | Median 68 (IQR 51–78) | 719 | 234 | KDIGO | Within 24 hours of admission |
| Xie 201931 | |||||||
| Gayat 2018,32 France and Belgium | NephroCheck | Critical care – mixed population (ICU, mainly sepsis) | Median 65 (IQR 54–75) | 200 | Unclear | KDIGO | Within 48 hours of admission |
| Hoste 2014,33 USA | NephroCheck | Critical care – mixed population (ICU/ITU) |
|
153 | 27 | KDIGO stage 2/3 | Within 12 hours of admission |
| Kashani 2013,34 North America (21 sites) and Europe (15 sites) | NephroCheck | Critical care – mixed population (ICU/ITU) | Median 64 (IQR 53–73) | 728 | 101 | KDIGO stage 2/3 | Within 12 hours of biomarker measurement (biomarker measurement occurred within 18 hours of ICU admission) |
| Kimmel 2016,35 Germany | NephroCheck, BioPorto urine NGAL, BioPorto plasma NGAL | Critical care – mixed population (ED) | Mean 63 (SD 14) | 298 | 46 | KDIGO (modified version) stage 2/3 | Within 12 hours of sample collection |
| Kimmel 201636 | |||||||
| Parikh 2011,37 North America | ARCHITECT urine NGAL | Cardiac surgery (CABG or valve surgery) | Mean 71 (SD 10) | 1200 | 60 | Acute dialysis or doubling of serum creatinine (consistent with RIFLE stage 1 or AKIN stage 2) | AKI developed at a median of 3 days after surgery (IQR 2–4 days) |
| Parikh 2013,38 Koyner 2015,39 Coca 2014,40 Brown 2019,41 Coca 2016,42 Zhang 201543 and Greenberg 201844 | |||||||
| Albert 2018,45 Germany | ARCHITECT urine NGAL | Cardiac surgery (open-heart surgery with CPB) | Median 70 (IQR 61–77) | 101 | 15 | RIFLE | NR |
| Garcia-Alvarez 2015,46 Spain | ARCHITECT urine NGAL | Cardiac surgery |
|
288 | 104 | KDIGO | Within 7 days of surgery |
| Liebetrau 2013,47 Germany | ARCHITECT urine NGAL | Cardiac surgery (CABG and/or valve replacement with the use of extracorporeal circulation) |
|
141 | 47 | KDIGO stage 2/3 | Within 4 days of surgery |
| Thanakitcharu 2014,48 Thailand | ARCHITECT urine NGAL | Cardiac surgery | Mean 51 (SD 15.6) | 130 | 46 | Increase in serum creatinine of ≥ 0.3 mg/dl within 48 hours | Within 48 hours of surgery |
| Cullen 2014,49 UK | ARCHITECT urine NGAL | Non-cardiac surgery (major abdominal surgery) | Mean 68 (SD 11) | 109 | 16 | AKIN | NR |
| Asada 2016,50 Japan | ARCHITECT urine NGAL | Critical care – mixed population (ICU/ITU) |
|
133 | 31 | KDIGO | Within 7 days of admission |
| Collins 2012,51 USA | ARCHITECT urine NGAL | Critical care – mixed population (acute heart failure) | NR | 399 | 20 | Increase in serum creatinine of ≥ 0.3 mg/dl or RIFLE | Worsening of renal function assessed at 12–24 hours and 72–96 hours |
| Dupont 2012,52 USA | ARCHITECT urine NGAL | Critical care – mixed population (acute decongestive heart failure) | NR | 141 | 35 | Increase in serum creatinine of ≥ 0.3 mg/dl | Within 48 hours of admission |
| Isshiki 2018,53 Japan | ARCHITECT urine NGAL | Critical care – mixed population (ICU/ITU) | Median 62 (IQR 51–73) | 148 | 33 | KDIGO | Within 7 days of admission |
| Kokkoris 2012,54 Greece | ARCHITECT urine NGAL | Critical care – mixed population (ICU/ITU) |
|
100 | 36 | RIFLE | Within 7 days of admission |
| Mårtensson 2015,55 Australia | ARCHITECT urine NGAL | Critical care – mixed population (ICU/ITU) |
|
102 | 28 | RIFLE | NR |
| Nickolas 2012,56 USA and Germany | ARCHITECT urine NGAL | Critical care – mixed population (ED) | Mean 64 (SD 19) | 1635 | 96 | RIFLE | Within 24 hours of admission |
| Park 2017,57 USA | ARCHITECT urine NGAL | Critical care – mixed population (CKD) | Mean 59 (SD 11) | 2466 | NR | Unclear | NR |
| Pipili 2014,58 Greece | ARCHITECT urine NGAL | Critical care – mixed population (mechanically ventilated patients admitted to the ICU) | Mean 64 (SD 18) | 106 | 44 | RIFLE | NR |
| Treeprasertsuk 2015,59 Thailand | ARCHITECT urine NGAL | Critical care – mixed population (cirrhosis) | Mean 57 (SD 15) | 121 | 35 | AKIN | Within 24 hours of admission |
| Haase 2014,60 Germany | ARCHITECT urine NGAL and BioPorto plasma NGAL | Cardiac surgery (open-heart surgery with CPB) | Median 72 (IQR 65–77) | 100 | 23 | RIFLE | NR |
| Albert 201845 | |||||||
| Schley 2015,61 Germany | BioPorto urine NGAL and BioPorto plasma NGAL | Cardiac surgery | Mean 70 (SD 10) | 110 | 37 | AKIN | Within 72 hours of surgery |
| Jaques 2019,62 Switzerland | BioPorto urine NGAL and BioPorto plasma NGAL | Critical care – mixed population (cirrhosis) | Mean 58 (SD 10) | 105 | 55 | AKIN | Within 7 days of admission |
| De Loor 2017,63 Belgium | BioPorto urine NGAL | Cardiac surgery (CPB) | Median 69 (IQR 61–76) | 203 | 95 | KDIGO | NR |
| Tidbury 2019,64 UK | BioPorto urine NGAL | Cardiac surgery |
|
125 | 54 | RIFLE | NR |
| Yang 2017,65 China | BioPorto urine NGAL | Cardiac surgery (atorvastatin for AKI cardiac surgery study) | Mean 46 (SD 15) | 398 | 164 | Acute dialysis or doubling of serum creatinine consistent with KDIGO stage 2 and 3 criteria | NR |
| Cho 2014,66 the Republic of Korea | BioPorto urine NGAL | Non-cardiac surgery (hepatobiliary surgery) | Mean 57 (SD 12) | 131 | 10 | AKIN | Within 5 days of admission |
| Ariza 2016,67 Europe | BioPorto urine NGAL | Critical care – mixed population (liver disease) |
|
716 | NR | NR | NR |
| Barreto 2014,68 Spain | BioPorto urine NGAL | Critical care – mixed population (cirrhosis) | Mean 58 (SD 12) | 132 | 65 | AKIN | An increase in serum creatinine of ≥ 0.3 mg/dl or ≥ 50% over the baseline value obtained in the previous 48–72 hours |
| Cho 2013,69 the Republic of Korea | BioPorto urine NGAL | Critical care – mixed population (ICU medical or surgical) |
|
145 | 54 | AKIN | Within 24 hours of surgery |
| Doi 2014,70 Japan | BioPorto urine NGAL | Critical care – mixed population (ICU/ITU) |
|
339 | 131 | RIFLE | NR |
| Doi 201171 | |||||||
| Hjortrup 2015,72 Denmark | BioPorto urine NGAL and BioPorto plasma NGAL | Critical care – mixed population (ICU/ITU sepsis) | Median 66 (IQR 57–75) | 151 | 91 | KDIGO | Within 48 hours of admission |
| Matsa 2014,73 UK | BioPorto urine NGAL and BioPorto plasma NGAL | Critical care – mixed population (ICU/ITU medical or surgical) | Mean 60 (SD 15) | 194 | 59 | RIFLE | Within 72 hours of admission |
| Nickolas 2008,74 USA | BioPorto urine NGAL | Critical care – mixed population (ED) | Mean 60 (SD 18) | 635 | 30 | RIFLE | NR |
| Nisula 2015,75 Finland | BioPorto urine NGAL | Critical care – mixed population (postoperative) | Median 62 (IQR 50–73) | 855 | 379 | KDIGO | NR |
| Nisula 201476 | |||||||
| Smith 2013,77 UK | BioPorto urine NGAL | Critical care – mixed population (CKD) | Mean 69 (SD 12) | 158 | 40 | KDIGO | NR |
| Tecson 2017,78 USA | BioPorto urine NGAL and BioPorto plasma NGAL | Critical care – mixed population (ICU/ITU) |
|
245 | 33 | KDIGO stage 2/3 | Within 8 days of admission |
| Verna 2012,79 USA | BioPorto urine NGAL | Critical care – mixed population (cirrhosis) | Median 56 (IQR 49–62) | 118 | 52 | Acute elevation in serum creatinine to > 1.5 and 0.3 mg/dl above baseline | NR |
| Zelt 2018,80 USA | BioPorto plasma NGAL | Cardiac surgery (major elective cardiac surgery requiring CPB) | Median 67 (IQR 61–73) | 178 | 35 | AKIN | Within 48 hours of surgery |
| Itenov 2017,81 Denmark | BioPorto plasma NGAL | Critical care – mixed population (ICU/ITU) | Median 67 (IQR 60–76) | 454 | 87 | KDIGO | NR |
| Lee 2018,82 the Republic of Korea | BioPorto plasma NGAL | Critical care – mixed population (comatose cardiac arrest survivors treated with therapeutic hypothermia) | Median 59 (IQR 50–71) | 279 | 111 | KDIGO stage 3 | Within 7 days of return of spontaneous circulation |
| Marino 2015,83 Italy | BioPorto plasma NGAL | Critical care – mixed population (sepsis) | Median 77 (IQR 72–83) | 101 | 49 | RIFLE | Within 7 days of admission |
| First author, year of publication, country, linked publications | Assay | Population (setting) | Age | Sample size (n) | AKI events (n) | AKI definition | Time frame for AKI diagnosis |
|---|---|---|---|---|---|---|---|
| Parikh 2011,84 North America | ARCHITECT urine NGAL | Cardiac surgery (congenital cardiac lesions) | Mean 4 years (SD 5 years) | 311 | 53 | Acute dialysis, or doubling of serum creatinine from baseline | During hospital stay |
| Zappitelli 201585 | |||||||
| Bojan 2014,86 France | ARCHITECT urine NGAL | Cardiac surgery (CPB for surgical correction or palliation of congenital heart lesions) | Mean < 1 year | 100 | NR | AKIN | NR |
| Bennett 2008,87 USA | ARCHITECT urine NGAL | Cardiac surgery (CPB for surgical correction or palliation of congenital heart lesions) | Mean 4 years | 196 | 99 | ≥ 50% increase in serum creatinine from baseline within 72 hours | NR |
| Cantinotti 2012,88 Italy | ARCHITECT urine NGAL | Cardiac surgery (cardiac surgery for correction or palliation of congenital heart defects) | Median 6 months (IQR 1–49 months) | 135 | 52 | Paediatric-modified RIFLE | NR |
| Alcaraz 2014,89 Spain | ARCHITECT urine NGAL | Cardiac surgery (cardiac surgery, mainly CPB, for congenital cardiac lesions) | Median 25 months (IQR 6.0–72.0 months) | 106 | 36 | Paediatric-modified RIFLE | Early AKI defined as renal dysfunction in the first postoperative 72 hours. Late AKI defined as occurring after the fourth postoperative day |
| aSeitz 2013,90 NR | ARCHITECT urine NGAL | Cardiac surgery (CPB for surgical correction of congenital heart disease) | Median 0 years (IQR 0–8 years) | 139 | 76 | Paediatric-modified RIFLE | NR |
| Zwiers 2015,91 the Netherlands | ARCHITECT urine NGAL | Critical care – mixed population (ICU/ITU) | Median 27 days (IQR 1–85 days) | 100 | 35 | RIFLE | Within 48 hours of admission |
| Dong 2017,92 USA | BioPorto urine NGAL | Cardiac surgery |
|
150 | 50 | KDIGO | Within 72 hours of surgery |
| Lagos-Arevalo 2015,93 Canada | BioPorto urine NGAL | Critical care – mixed population (ICU/ITU) |
|
160 | 70 | KDIGO | NR |
| Yang 2017,65 China | BioPorto urine NGAL | Cardiac surgery |
|
|
|
Acute dialysis or doubling of serum creatinine consistent with KDIGO stage 2 and 3 criteria | NR |
No randomised controlled trials (RCTs) or controlled clinical trials were identified; no studies provided data on the incremental value of the use of the biomarkers compared with standard clinical care.
Of the 56 included studies, 36 involved a single centre26–28,30,35,45,46,48–50,53–55,58–61,63,66,68–70,73,74,79,80,82,83,86–93 and 13 involved multiple centres. 29,33,34,37,52,56,57,65,72,77,78,81,84 Seven studies did not provide this information. 32,47,51,62,64,67,75 Twenty-eight studies were conducted in Europe (four in the UK, six in Germany, three in Italy, three in Spain, two in Greece, two in Denmark, one in the Netherlands, one in France, one in Belgium, one in France and Belgium, one in Finland, one in Norway, one in Switzerland and one in several European countries); 15 in North America (12 in the USA, two in the USA and Canada and one in Canada); nine in Asia (three in Japan, three in the Republic of Korea, two in Thailand and one in China); two in North America and Europe; and one in Australia. One study did not provide clear information on the geographical location.
NGAL was the most commonly studied biomarker (41/56 studies; 37 studies used urine NGAL assays and four used plasma NGAL assays). NephroCheck was assessed in eight studies. Seven studies provided data on more than one assay (six studies on urine NGAL and plasma NGAL assays and one study on NephroCheck, urine NGAL and plasma NGAL assays). Among the NGAL studies, 24 used the ARCHITECT urine NGAL platform and 20 used the BioPorto urine NGAL assay. All 11 plasma NGAL studies used the BioPorto Diagnostics assay. No studies used the NGAL Alinity i platform.
Of the 56 included studies, 46 enrolled adults only, eight enrolled children only and two enrolled both adults and children. The total number of participants was 17,967, of whom 16,247 were adults (average age ranged from 49 to 77 years) and 1720 were children (average age ranged from 1 day to 5 years). Of the 46 studies that focused on adults only, 12 assessed patients after cardiac surgery, four assessed patients requiring non-surgical cardiac care, one assessed patients undergoing major abdominal surgery, one assessed patients undergoing hepatobiliary surgery, 16 assessed patients admitted to intensive care units (ICUs), five assessed patients with liver disease (mainly cirrhosis), two assessed patients with sepsis, two assessed patients with CKD and three assessed patients admitted to EDs. Of the eight studies that focused on children, six assessed children (including neonates) undergoing cardiac surgery and two assessed children admitted to a paediatric ICU or neonatal ICU. The two studies that included both adults and children assessed patients undergoing cardiac surgery. For the purpose of the clinical effectiveness and cost-effectiveness analyses, the participants were grouped into three categories according to the clinical setting reported in the included studies: patients undergoing cardiac surgery, patients undergoing major non-cardiac surgery and patients admitted to critical care (mixed patient population). The critical care group includes critically ill patients presenting to the ED and participants admitted to the ICU or considered for critical care for various medical conditions or after surgery (but the studies did not specify which type of surgery or provide separate results for medical and surgical ICU participants).
Study quality
The risk of bias of studies assessing the accuracy of NephroCheck and NGAL assays in identifying people at risk of developing AKI was assessed using the QUADAS-2 tool. The results are summarised in Figure 2 and in Appendix 7, Table 28.
FIGURE 2.
Risk-of-bias assessment of studies assessing the diagnostic performance of the biomarkers using the QUADAS-2 tool.
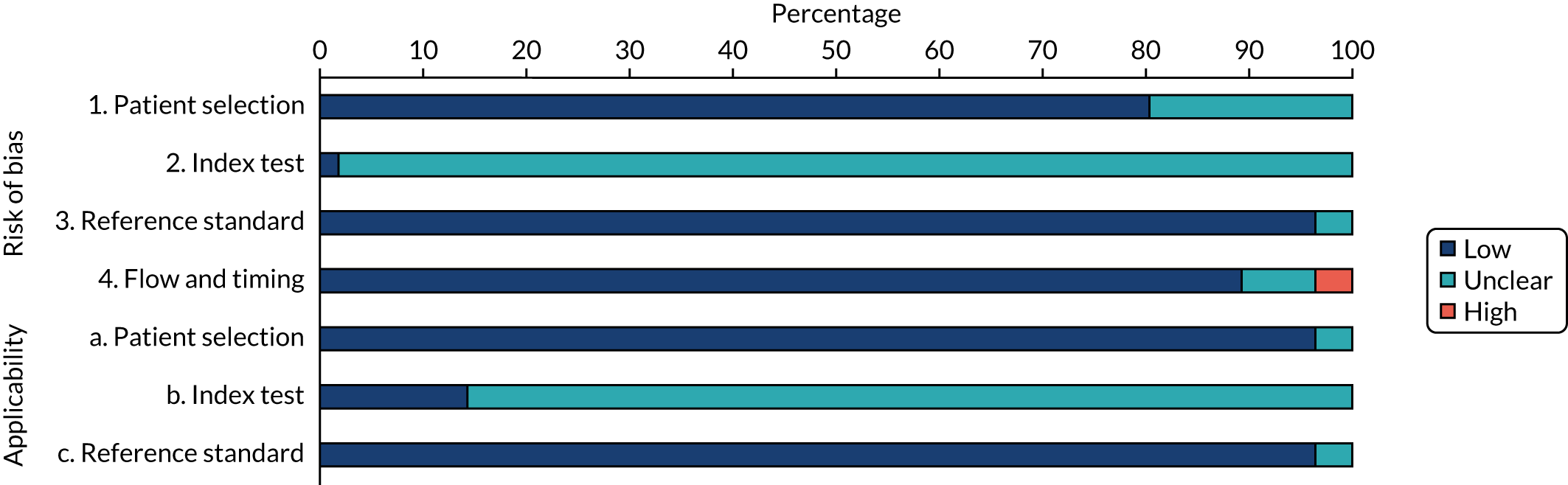
Eleven studies (20%) did not report sufficient information to determine whether or not a selection of patients could have introduced bias; these studies were assessed as having an unclear risk of bias. 32,33,35,49,50,54,55,60,72,82,94 The remaining studies were judged to be at low risk of bias for the patient selection domain.
The main potential source of bias across studies relates to blinding. Most studies (98%) were assessed as having an unclear risk of bias for the conduct and interpretation of the index test because of insufficient information or lack of clarity regarding whether or not the biomarker results were interpreted without knowledge of the reference standard results (see Figure 2). The studies that used NephroCheck were judged to be at low risk of bias with regard to the interpretation of the test because all of them used a common threshold. However, because of the differences in threshold level observed across studies and the lack of a common threshold, the risk of bias for NGAL studies was judged to be unclear. Although some studies alluded to the blinding to patients’ clinical information of personnel performing the biomarker measurements, it was unclear whether or not the personnel were indeed blinded to serum creatinine measurements (reference standard). With regard to whether or not the reference standard, its conduct or its interpretation may have introduced bias, two studies (4%) were judged to be at unclear risk of bias because baseline serum creatinine levels were determined by reviewing records of previous 12-month measurements. 54,56 The remaining studies (96%) were judged to be at low risk of bias for the reference standard domain.
Two studies (4%) were judged to be at high risk of bias in terms of the patient flow (e.g. attrition) because > 50% of the participants were excluded from the analysis62 or because the reporting of the patient selection and flow was poorly detailed. 50 Four studies (7%) were at unclear risk. 54,57,64,92 The remaining studies (89%) were considered to be at low risk of bias regarding the patient flow domain.
Across studies, there was no major concern that the patient population and the conduct and interpretation of reference standard were not applicable to the review question. We observed an expected variation between studies in terms of characteristics of the index tests (biomarker assays) and clinical protocols. In particular, applicability of the index test to the review question was judged to be unclear in many studies, mainly because of the variation with regard to the biomarker thresholds and timing of sample collections.
The risk of bias of studies assessing the role of NephroCheck and NGAL assays for prediction of relevant clinical outcomes (i.e. worsening of AKI, mortality and need for RRT) was assessed using the PROBAST tool. 22 The results are summarised in Table 5.
| Study | Target population (setting) | Assay | Timing of test | Cut-off point (ng/ml2/1000) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Prevalence of AKI |
|---|---|---|---|---|---|---|---|---|
| Oezkur 201727 | Cardiac surgery | NephroCheck | ICU admission | 0.3 | 0.60 | 0.88 | NR | 0.19 |
| Cummings 201926 | Cardiac surgery | NephroCheck | ICU admission | 0.3 | 0.31 (0.09 to 0.61) | 0.78 (0.74 to 0.82) | 0.68 (0.54 to 0.81) | 0.035 |
| Kashani 201334 | Critical care – mixed population (ICU/ITU) | NephroCheck | ICU admission | 0.3 | 0.89 | 0.50 | 0.8 | 0.14 |
| Bihorac 201429 | Critical care – mixed population (ICU/ITU) | NephroCheck | Within 24 hours of admission to ICU | 0.3 | 0.92 (0.85 to 0.98) | 0.46 (0.41 to 0.52) | 0.82 (0.76 to 0.88) | 0.17 |
| Hoste 201433 | Critical care – mixed population (ICU/ITU) | NephroCheck | ICU admission | 0.3 | 0.89 | 0.53 | 0.79 (0.69 to 0.88) | 0.18 |
| Kimmel 201636 | Critical care – mixed population | NephroCheck | Admission to the internal medicine service | 0.3–2.0 | 0.76 (0.63 to 0.87) | 0.53 (0.48 to 0.57) | 0.74 (0.66 to 0.81) | 0.15 |
| Di Leo 201830 | Critical care – mixed population (ICU/ITU) | NephroCheck | ICU admission | 0.3 | 0.64 | 0.56 | 0.63 | 0.34 |
Twelve prediction studies were assessed for risk of bias and applicability. 32,46,49,53,55,59,70,72,75,82,83,87 Three of these studies (25%) reported insufficient information to determine whether or not selection of patients could have introduced bias; these studies were judged to be at unclear risk of bias. 32,55,72 The remaining studies were judged to be at low risk of bias for this domain. No studies were judged to have made predictor assessments without the knowledge of outcome data; therefore, the risk of bias for the predictors domain was judged to be unclear for all studies. The risk of bias in the outcome domain was rated as unclear for all studies, mainly because of inadequate information to assess whether or not outcomes were determined without knowledge of predictor information. The risk of bias for the analysis domain was rated as unclear in 58% of studies and as high in 42% of studies.
The overall risk of bias was considered to be unclear for most studies (70%), mainly because these studies were assessed as being at high risk of bias in the analysis domain. The remaining studies were judged to be at unclear risk of bias.
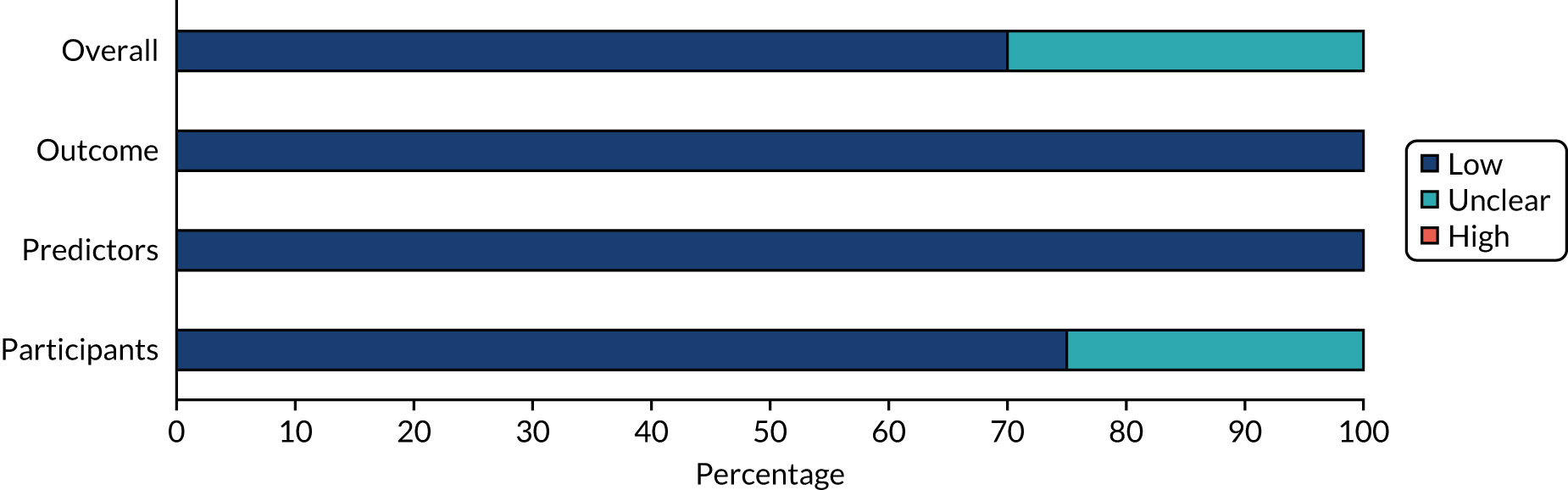
Most studies were judged to be at low risk of bias for applicability to the review question in each of the domain categories. Overall, applicability was judged to be at low risk of bias for 75% of the studies and at unclear risk of bias for the remaining studies. In general, there was no major concern that the studies were not applicable to the research questions of this assessment. Summaries of the results are shown in Figures 3 and 4, and individual study-level results are presented in Appendix 8, Table 29.
FIGURE 3.
Risk-of-bias assessment of studies that assessed the role of biomarkers for prediction of relevant clinical outcomes using the PROBAST tool.
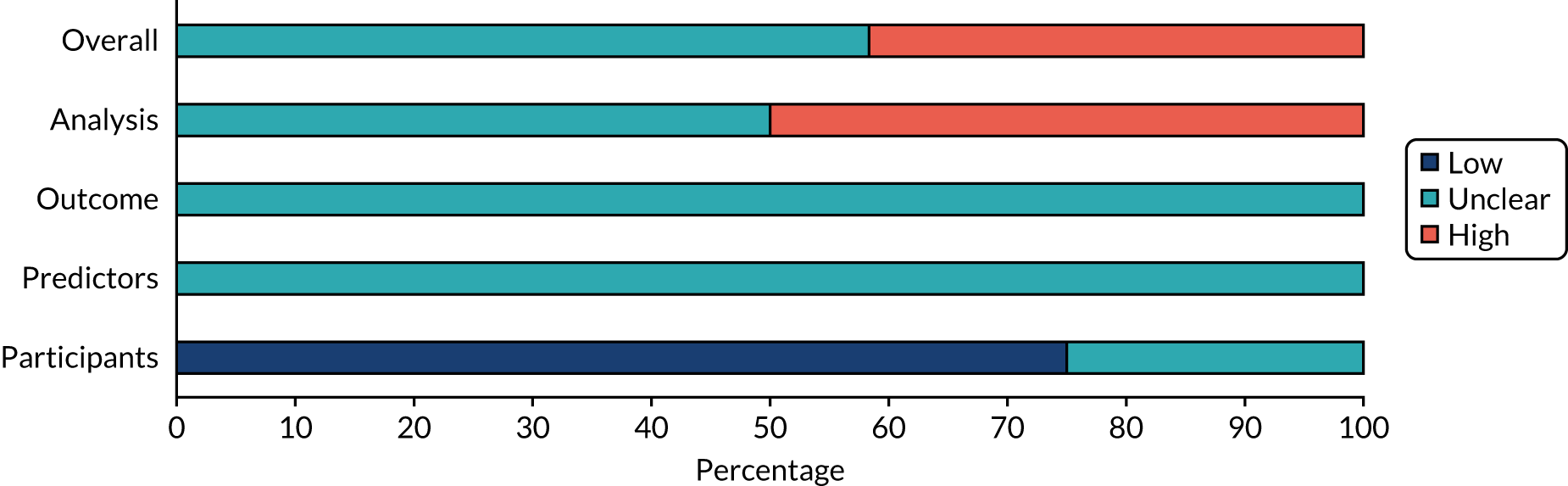
FIGURE 4.
Applicability of prediction studies to the research questions using the PROBAST tool.
Accuracy of the NephroCheck and neutrophil gelatinase-associated lipocalin assays for identifying acute kidney injury
We were able to extract or derive 2 × 2 data from 33 studies that assessed the performance of the NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assays for identifying AKI in critically ill hospitalised patients. These studies are summarised in the following sections.
The summary estimates of accuracy and the SROC plots are provided separately for each assay, clinical setting, mode of sampling and type of patient population (adults and children). We also present analyses across all settings. Studies that could not be combined in a meta-analysis (fewer than four) are summarised narratively.
NephroCheck urine assay: adult population
A summary of the diagnostic data for the seven studies26,27,29,30,33,34,36 that assessed the use of NephroCheck for detection of AKI in adults is presented in Table 5.
Cardiac surgery
Two studies26,27 assessed the use of NephroCheck for detection of AKI in patients after cardiac surgery (data from a total of 500 participants were available for the analyses). Both studies used the same cut-off point (0.3 ng/ml2/1000). The study by Cummings et al. 26 assessed a total of 400 cardiac patients soon after ICU admission. The sensitivity and specificity values were 0.31 (95% CI 0.09 to 0.61) and 0.78 (95% CI 0.74 to 0.82), respectively. The study was consistent with other cardiac surgery cohorts, but showed a low prevalence of AKI (4%). Only 14 participants developed KDIGO stage 2 and 3 AKI. The study by Oezkur et al. 27 analysed 100 patients immediately after cardiac surgery. The reported sensitivity and specificity values were 0.60 (95% CI 0.36 to 0.81) and 0.89 (95% CI 0.80 to 0.95), respectively. The prevalence of AKI was 19%. Table 5 shows a summary of the diagnostic data for the two studies.
No suitable NephroCheck data in other post-surgical settings (major non-cardiac surgery) were available from the included studies.
Critical care: mixed population
Five studies (2279 participants in total) assessed the use of NephroCheck for detection of AKI in hospitalised patients admitted to ICU or critical care for various clinical reasons. The cut-off point used was consistent across studies (0.3 ng/ml2/1000). Table 5 shows a summary of the diagnostic data for the five studies. 29,30,33,34,36 Sensitivity values ranged from 0.64 to 0.92; specificity values ranged from 0.46 to 0.56. The summary estimate of sensitivity was 0.83 (95% CI 0.72 to 0.91) and that of specificity was 0.51 (95% CI 0.48 to 0.54). The SROC plot with 95% confidence region for the summary operating point and 95% prediction region is presented in Appendix 9, Figure 27. The confidence and prediction regions indicate a greater degree of heterogeneity in sensitivity estimates than in specificity estimates between studies. Specificity estimates were low but reasonably homogeneous (see Appendix 9, Figure 26).
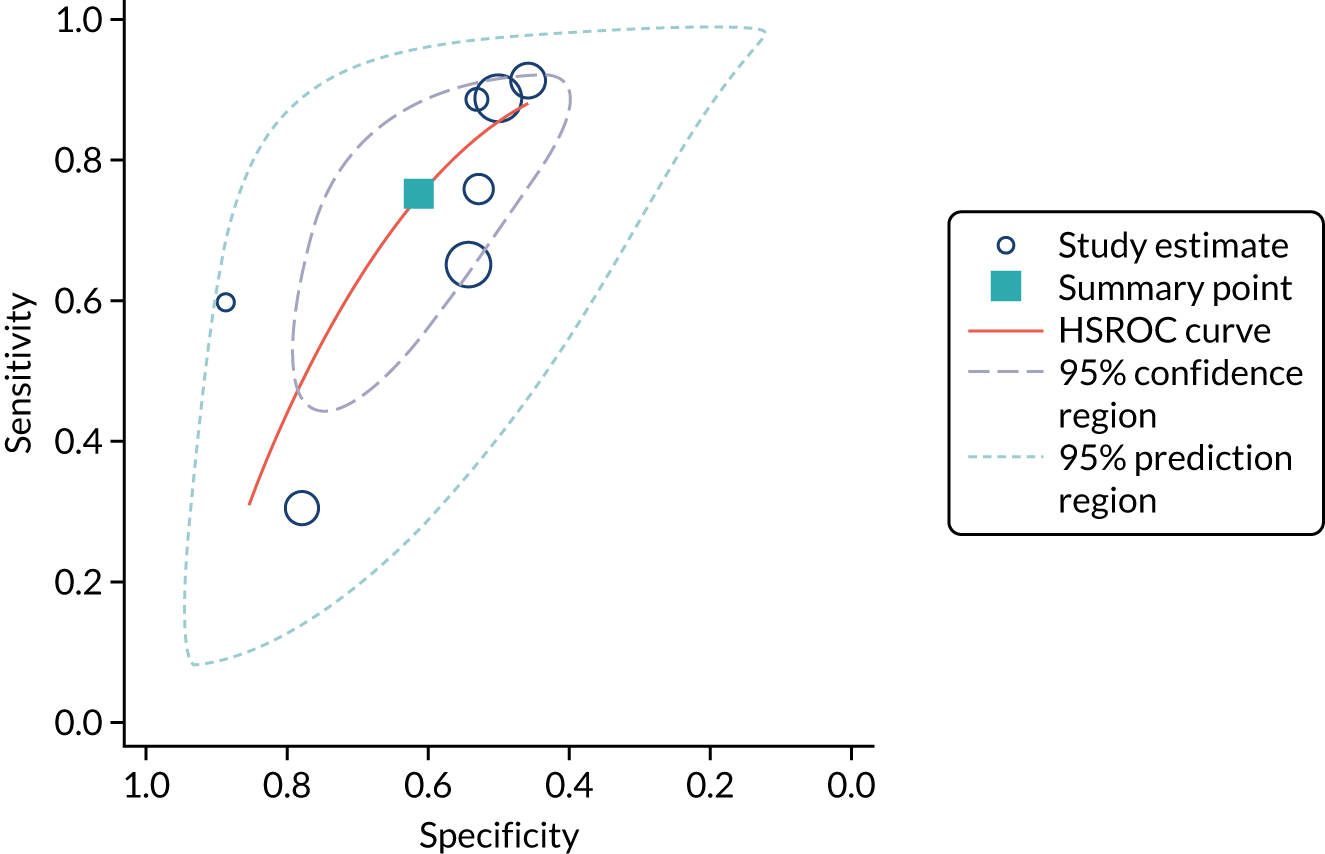
Figure 5 shows the forest plots of sensitivity and specificity estimates for all NephroCheck studies (2778 patients in total) across clinical settings. Sensitivity values ranged from 0.31 to 0.92 and specificity values ranged from 0.46 to 0.89. Summary estimates for sensitivity and specificity were 0.75 (95% CI 0.58 to 0.87) and 0.61 (95% CI 0.49 to 0.72), respectively. Figure 6 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. The confidence and prediction regions are large, indicating considerable heterogeneity between studies. Across studies, estimates of specificity were generally low, apart from two studies that showed higher estimates. Visual inspection of the forest plot and SROC plots shows that the study by Cummings et al. 26 is an outlier, with a trend very different from that of the other studies.
FIGURE 5.
Forest plots of sensitivity and specificity for NephroCheck studies: all clinical settings. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
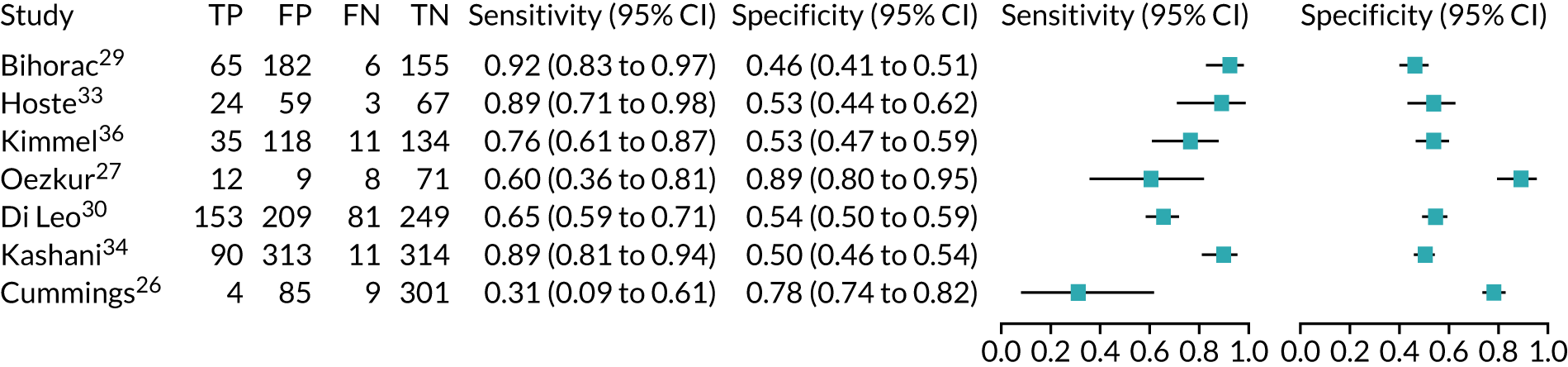
FIGURE 6.
The SROC plot for NephroCheck studies: all clinical settings. HSROC, hierarchical summary receiver operating characteristic.
There were no studies assessing the use of NephroCheck in children, as this biomarker is recommended for adult use only (people aged ≥ 21 years).
ARCHITECT urine neutrophil gelatinase-associated lipocalin assay: adult population
Cardiac surgery
Two studies37,48 provide test accuracy data on the use of the ARCHITECT urine NGAL assay for detection of AKI in patients who underwent cardiac surgery (Table 6). The multicentre cohort study by Parikh et al. 37 assessed a total of 1219 adults after cardiac surgery. The sensitivity and specificity values for the first urine sample collected soon after ICU admission were 0.46 (95% CI 0.33 to 0.59) and 0.81 (95% CI 0.79 to 0.83), respectively. The prevalence of AKI in the study was 5%, similar to that observed previously in the Cummings et al. 26 study, which assessed the role of NephroCheck in 400 participants in the same clinical setting. The single-centre study by Thanakitcharu et al. 48 assessed 130 patients immediately after cardiac surgery. The sensitivity and specificity values for the urine sample collected immediately after surgery were 0.74 (95% CI 0.49 to 0.91) and 0.6 (95% CI 0.51 to 0.70), respectively. The prevalence of AKI in the study was 35%.
| Study | Target population (setting) | Assay | Timing of test | Cut-off point | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Prevalence of AKI |
|---|---|---|---|---|---|---|---|---|
| Parikh 201137 | Cardiac surgery | ARCHITECT urine NGAL | ICU admission | > 102 ng/ml | 0.46 | 0.81 | 0.67 | 0.05 |
| Thanakitcharu 201448 | Cardiac surgery | ARCHITECT urine NGAL | Immediately after surgery | > 11.3 ng/ml | 0.74 | 0.60 | 0.69 (0.52 to 0.72) | 0.35 |
| Dupont 201252 | Critical care – mixed population (acute decongestive heart failure) | ARCHITECT urine NGAL | 48 hours after admission | 32 µg/g of creatinine | 0.63 | 0.58 | 0.61 | 0.25 |
| Kokkoris 201254 | Critical care – mixed population (ICU/ITU) | ARCHITECT urine NGAL | ICU admission | 58.5 ng/ml | 0.78 (0.61 to 0.90) | 0.72 (0.59 to 0.82) | 0.74 (0.64 to 0.82) | 0.36 |
| Nickolas 201256 | Critical care – mixed population (ICU/ITU) | ARCHITECT urine NGAL | Admission to ED | 104 ng/ml | 0.68 | 0.81 | 0.81 (0.76 to 0.86) | 0.059 |
| Treeprasertsuk 201559 | Critical care – mixed population (liver disease) | ARCHITECT urine NGAL | Within 72 hours of admission | 56 ng/ml | 0.77 | 0.73 | 0.83 (0.76 to 0.91) | 0.29 |
No suitable ARCHITECT urine NGAL assay data in other post-surgical settings (major non-cardiac surgery) were available from the included studies.
Critical care: mixed population
Four studies (1998 patients in total) assessed the use of the ARCHITECT urine NGAL assay for the detection of AKI in patients admitted to an ICU or critical care for various clinical reasons. Cut-off values varied across studies (see Table 6). In three studies, urine NGAL levels were reported as ng/ml (per ml of urine), whereas, in one study, urine NGAL levels were normalised by units of urine creatinine (per mg of creatinine). Prevalence of AKI ranged from 6% to 36% across studies. Table 6 shows a summary of the diagnostic data, as reported by the six studies. Sensitivity values ranged from 0.63 to 0.78 and specificity values ranged from 0.58 to 0.81. The summary estimate of sensitivity was 0.70 (95% CI 0.63 to 0.76) and that of specificity was 0.72 (95% CI 0.63 to 0.80). The forest plot of sensitivity and specificity estimates and the SROC plot with 95% confidence region for the summary operating point and 95% prediction region are presented in Appendix 9 (see Figures 28 and 29). The large confidence and prediction regions of the SROC plot indicate considerable heterogeneity in estimates of accuracy across studies, especially for specificity. The analysis appears to be dominated by the Nickolas et al. 56 study, the largest study, which shows a small number of true-positive cases and, subsequently, low sensitivity.
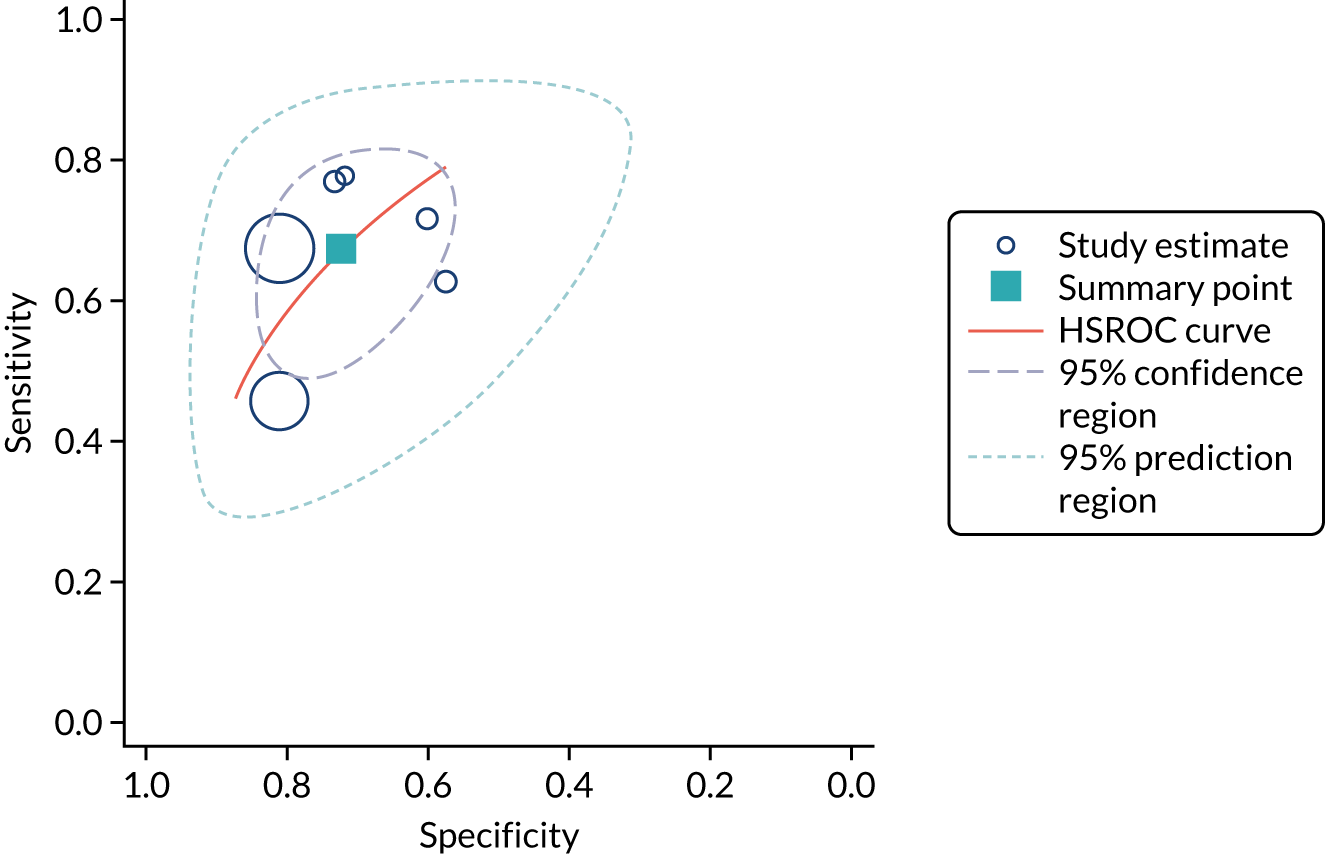
Figure 7 shows the forest plots of sensitivity and specificity estimates for all ARCHITECT urine NGAL studies (3347 patients in total) across all clinical settings. Sensitivity values ranged from 0.46 to 0.78 and specificity values ranged from 0.58 to 0.81. Summary estimates for sensitivity and specificity were 0.67 (95% CI 0.58 to 0.76) and 0.72 (95% CI 0.64 to 0.79), respectively. Figure 8 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. The confidence and prediction regions are large, indicating heterogeneity between studies.
FIGURE 7.
Forest plots of sensitivity and specificity for the ARCHITECT urine NGAL assay for the detection of AKI in adults: all clinical settings. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
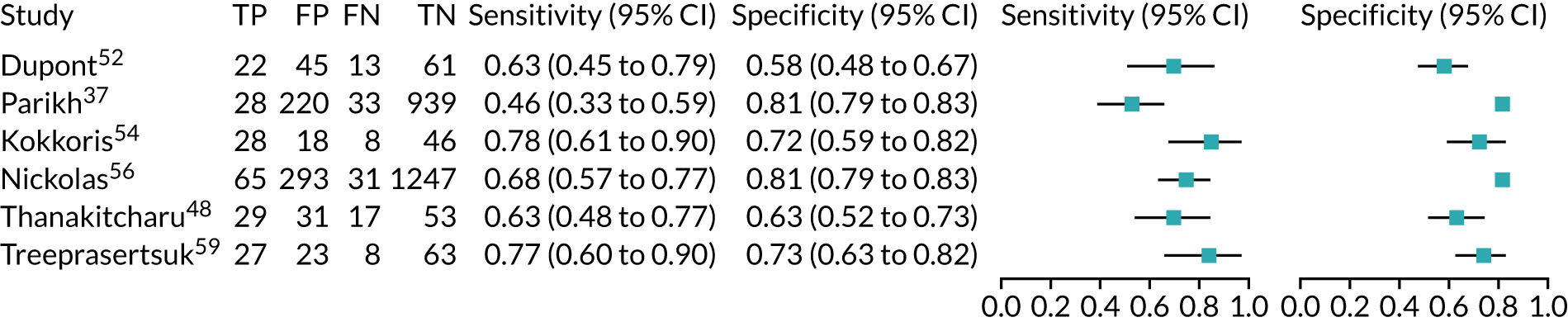
FIGURE 8.
The SROC plot for the ARCHITECT urine NGAL assay studies: all clinical settings (adult population). HSROC, hierarchical summary receiver operating characteristic.
BioPorto urine neutrophil gelatinase-associated lipocalin assay: adult population
Cardiac surgery
One study65 assessed the use of the BioPorto urine NGAL assay for the detection of AKI in a total of 398 patients who had undergone cardiac surgery (Table 7). Urine NGAL levels were normalised by units of urine creatinine (with a cut-off point of 98 µg/g creatinine). The sensitivity and specificity values for the urine sample collected 6 hours after surgery were 0.78 (95% CI 0.71 to 0.84) and 0.48 (95% CI 0.41 to 0.54), respectively. The prevalence of AKI in the study was 41%.
| Study | Target population (setting) | Assay | Timing of test | Cut-off point | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Prevalence of AKI |
|---|---|---|---|---|---|---|---|---|
| Yang, 201765 | Cardiac surgery | BioPorto urine NGAL | 6 hours after surgery | 98 µg/g of creatinine | 0.78 (0.71 to 0.84) | 0.48 (0.41 to 0.54) | 0.72 (0.64 to 0.80) | 41% |
| Cho 201466 | Major non-cardiac surgery | BioPorto urine NGAL | 12 hours after hepatobiliary surgery | 92.85 ng/ml | 0.78 (0.52 to 1.00) | 0.80 (0.73 to 0.87) | 0.78 (0.66 to 0.90) | 8% |
| Nickolas 200874 | Critical care – mixed population (ICU/ITU) | BioPorto urine NGAL | Admission to ED | 130 µg/g of creatinine | 0.90 (0.73 to 0.98) | 1.00 (0.99 to 1.00) | 0.95 (0.88 to 1.00) | 0.047 |
| Cho 201369 | Critical care – mixed population (ICU/ITU) | BioPorto urine NGAL | ICU admission | NR | 0.74 | 0.70 | 0.77 (0.69 to 0.85) | 0.37 |
| Matsa 201473 | Critical care – mixed population (ICU/ITU) | BioPorto urine NGAL | ICU admission | 350 ng/ml | 0.58 (0.44 to 0.70) | 0.84 (0.75 to 0.91) | 0.79 | 0.38 |
| Barreto 201468 | Critical care – mixed population (liver disease) | BioPorto urine NGAL | When the infection was detected | 51 µg/g of creatinine | 0.66 | 0.70 | 0.72 (0.64 to 0.81) | 0.49 |
| Hjortrup 201572 | Critical care – mixed population (ICU/ITU) | BioPorto urine NGAL | ICU admission | 582 ng/ml | 0.75 | 0.77 | 0.71 (0.59 to 0.82) | 0.24 |
| Tecson 201778 | Critical care – mixed population (ICU/ITU) | BioPorto urine NGAL | Within 48 hours of ICU admission | 98 ng/ml | 0.64 (0.45 to 0.80) | 0.81 (0.75 to 0.86) | – | 0.13 |
Non-cardiac surgery
One study66 assessed the use of the BioPorto urine NGAL assay for detection of AKI in 131 patients undergoing hepatobiliary surgery (see Table 7). The urine NGAL cut-off point was 92.85 ng/ml. The sensitivity and specificity values for the urine sample collected 12 hours after surgery were 0.78 (95% CI 0.52 to 1.00) and 0.80 (95% CI 0.73 to 0.87), respectively. The prevalence of AKI in the study was 8%.
Critical care: mixed population
Six studies68,69,72–74,78 (1442 patients in total) assessed the use of the BioPorto urine NGAL assay for the detection of AKI in patients admitted to an ICU or critical care for various clinical reasons (see Table 7). Some studies reported absolute levels of urine NGAL and other levels normalised to urine creatinine. The threshold varied across studies. The prevalence of AKI ranged from 5% to 49% across studies. Sensitivity values ranged from 0.58 to 0.90 and specificity values ranged from 0.70 to 1.00. The summary estimate of sensitivity was 0.72 (95% CI 0.61 to 0.80) and that of specificity was 0.87 (95% CI 0.66 to 0.96). The forest plots of sensitivity and specificity estimates and the SROC plot with 95% confidence region for the summary operating point and 95% prediction region are presented in Appendix 9 (see Figures 30 and 31). The confidence and prediction regions of the SROC plot are large, indicating heterogeneity between studies, especially for specificity.
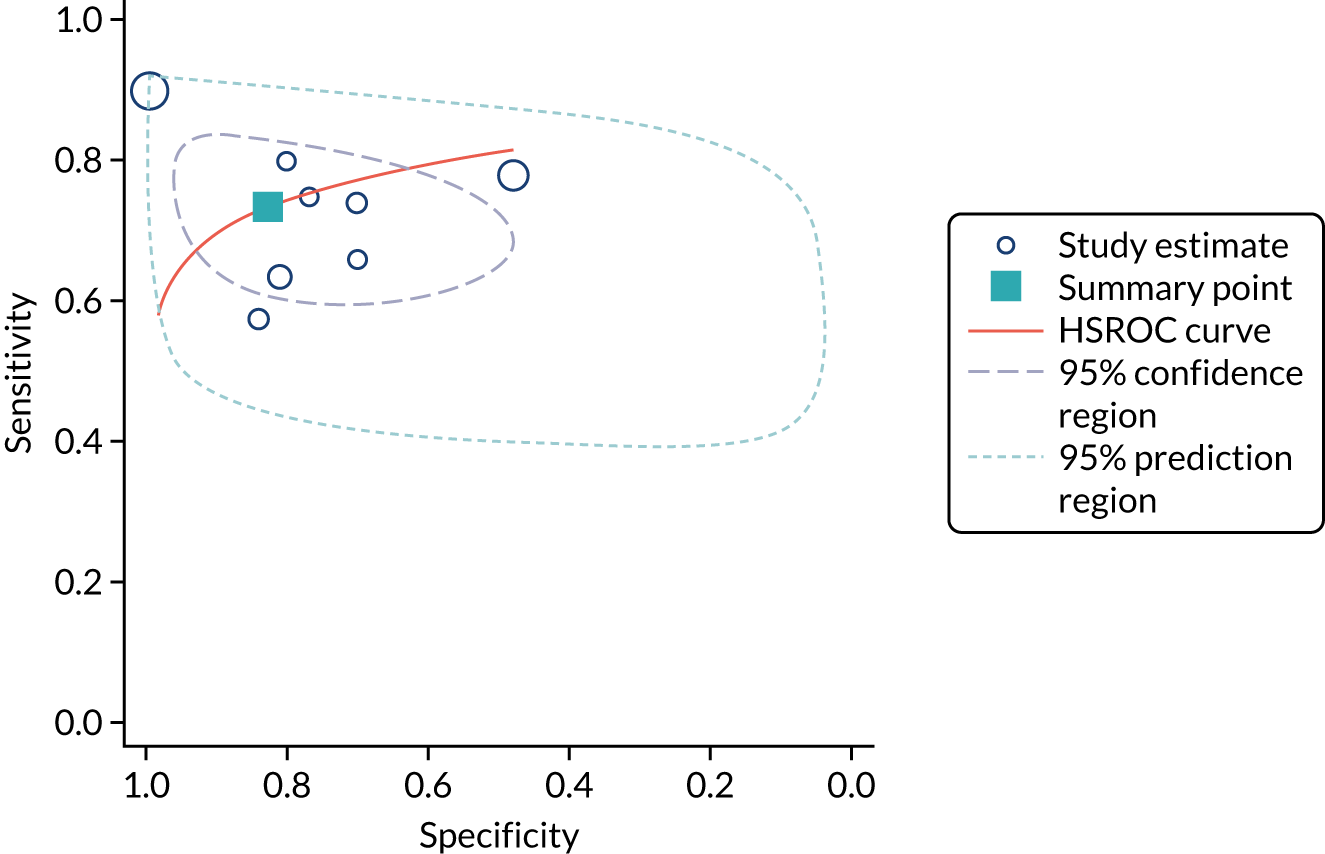
Figure 9 shows the forest plots of sensitivity and specificity estimates for the eight studies (1971 patients in total) assessing the BioPorto urine NGAL assay for the detection of AKI in adults across all clinical settings. Sensitivity values ranged from 0.58 to 0.90 and specificity values ranged from 0.48 to 1.00. Summary estimates for sensitivity and specificity were 0.73 (95% CI 0.65 to 0.80) and 0.83 (95% CI 0.64 to 0.93), respectively. The SROC plot with the 95% confidence region for the summary operating point and the 95% prediction region, is shown in Figure 10. The confidence and prediction regions are large, indicating considerable heterogeneity between studies.
FIGURE 9.
Forest plots of sensitivity and specificity for the BioPorto urine NGAL assay studies for the detection of AKI in adults: all clinical settings. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
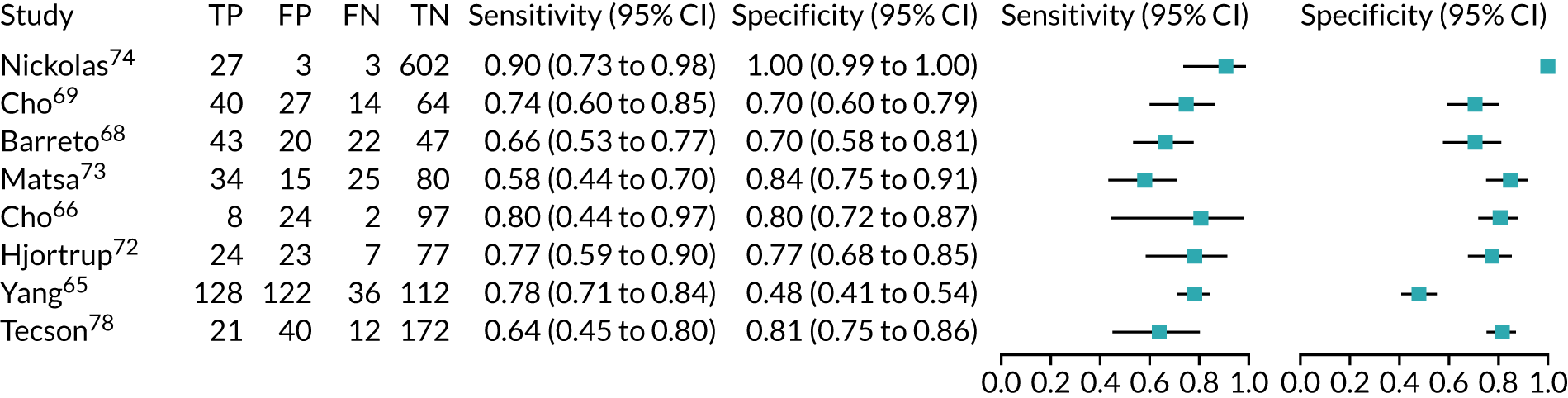
FIGURE 10.
The SROC plot for the BioPorto urine NGAL assay studies for the detection of AKI in adults: all clinical settings. HSROC, hierarchical summary receiver operating characteristic.
Urine neutrophil gelatinase-associated lipocalin assays (ARCHITECT and BioPorto): critical care
Ten studies (3441 patients in total) assessed urine NGAL assays (both ARCHITECT and BioPorto) for the detection of AKI in patients admitted to critical care. Sensitivity values ranged from 0.58 to 0.90 and specificity values ranged from 0.58 to 1.00. Summary estimates for sensitivity and specificity were 0.71 (95% CI 0.64 to 0.77) and 0.82 (95% CI 0.67 to 0.90), respectively. The forest plot of sensitivity and specificity estimates and the SROC plot with 95% confidence region for the summary operating point and 95% prediction region are shown in Appendix 9 (see Figures 32 and 33). The prediction region in the SROC plot is large, especially for specificity, indicating heterogeneity across studies.
Urine neutrophil gelatinase-associated lipocalin assays (ARCHITECT and BioPorto): across all settings
Figure 11 shows the forest plots of sensitivity and specificity estimates for all 14 studies assessing the urine NGAL assays (both ARCHITECT and BioPorto) across all clinical settings (5319 patients in total). Sensitivity values ranged from 0.46 to 0.90 and specificity values ranged from 0.48 to 1.00. Summary estimates for sensitivity and specificity were 0.71 (95% CI 0.64 to 0.76) and 0.78 (95% CI 0.67 to 0.87), respectively. Figure 12 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. The prediction region is large, indicating heterogeneity across studies.
FIGURE 11.
Forest plots of sensitivity and specificity for all urine NGAL assays (both ARCHITECT and BioPorto) for the detection of AKI in adults across all clinical settings. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
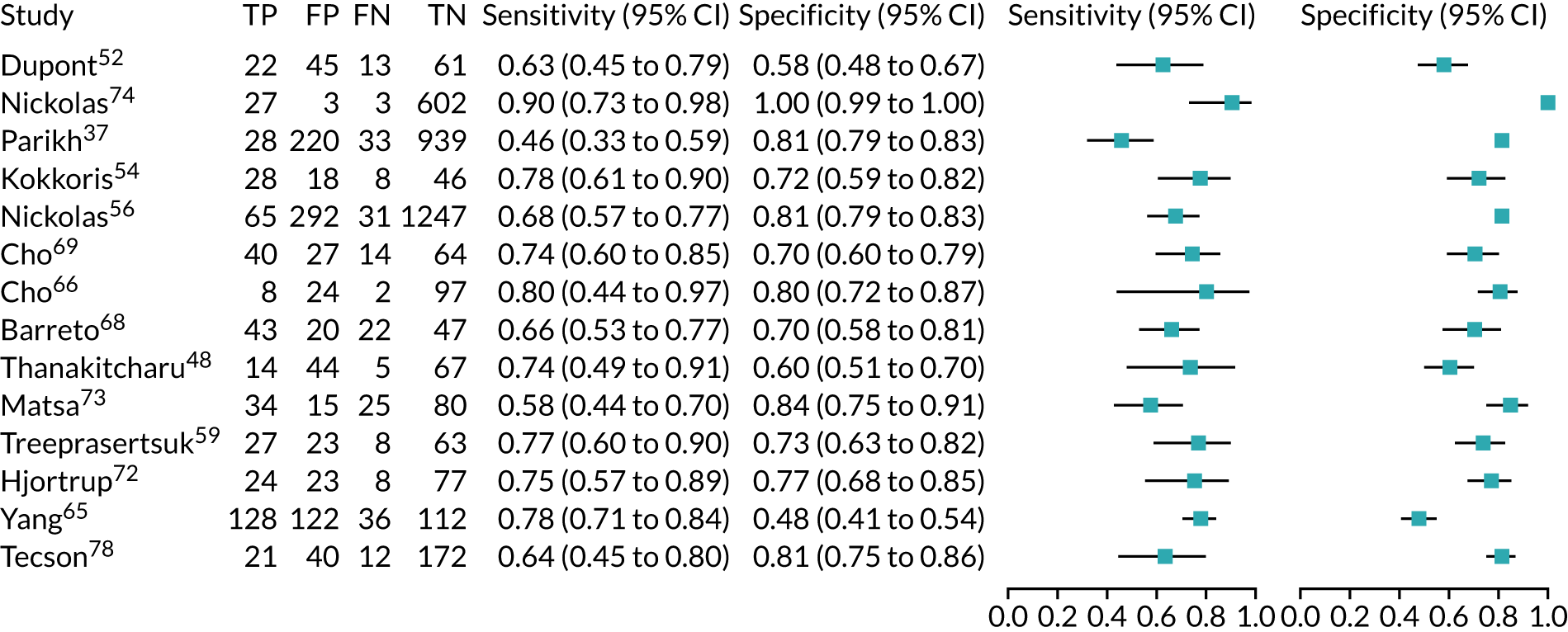
FIGURE 12.
The SROC plot for all urine NGAL assays (both ARCHITECT and BioPorto) for the detection of AKI in adults: all clinical settings. HSROC, hierarchical summary receiver operating characteristic.
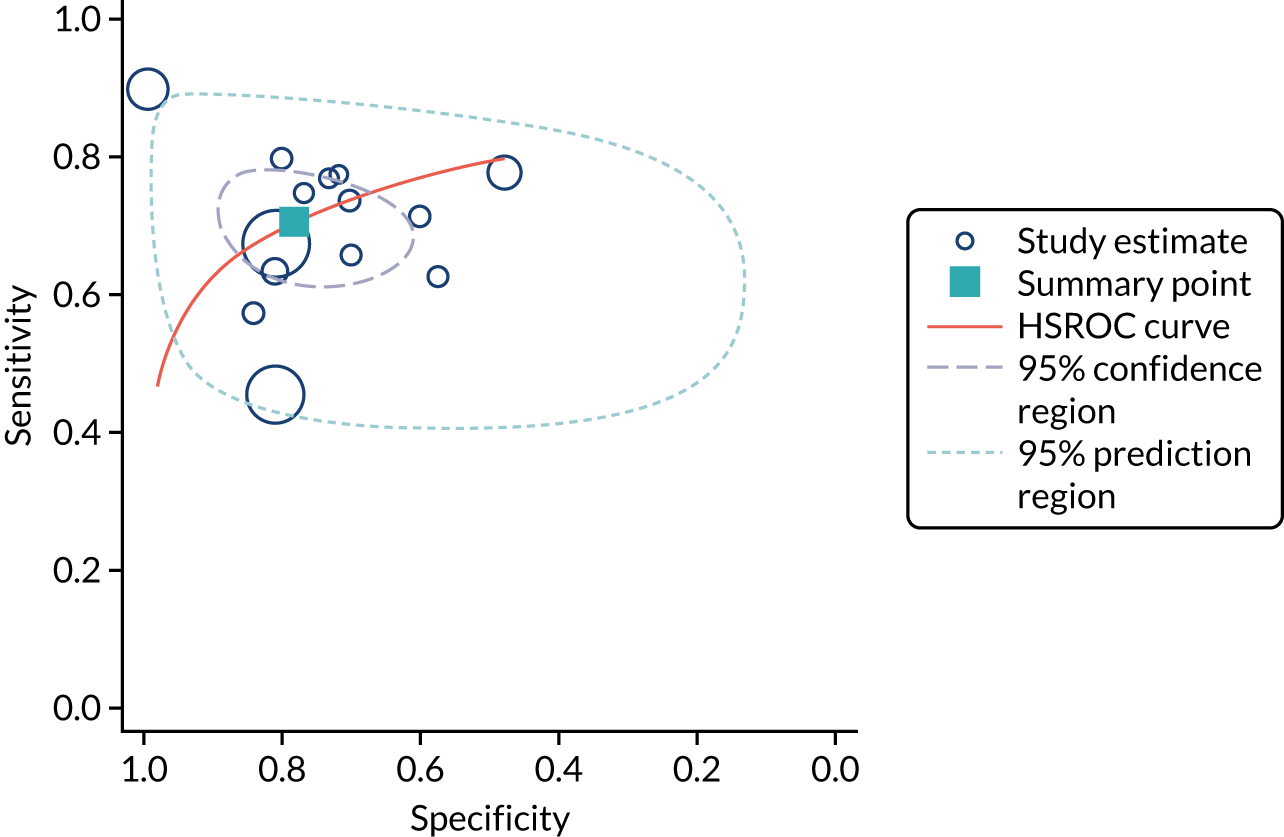
BioPorto plasma neutrophil gelatinase-associated lipocalin assay: adult population
No suitable data in any post-surgical setting (cardiac surgery or major non-cardiac surgery) were available from the included studies.
Critical care: mixed population
Four studies (771 patients in total) assessed the use of the BioPorto plasma NGAL assay for the detection of AKI in patients admitted to ICU or critical care for various clinical reasons (Table 8). Cut-off points varied across studies The prevalence of AKI ranged from 13% to 38% across studies. Figure 13 shows the forest plots of sensitivity and specificity. Sensitivity values ranged from 0.58 to 0.93 and specificity values ranged from 0.23 to 0.85. The summary estimate of sensitivity was 0.76 (95% CI 0.56 to 0.89) and that of specificity was 0.67 (95% CI 0.40 to 0.86). Figure 14 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. Confidence and prediction regions are large and are greater for sensitivity than for specificity. Although this indicates the presence of heterogeneity across studies, it is worth observing that all studies and their confidence and prediction regions are positioned in the left side of the graph, above the diagonal of no effect. It is worth paying attention to the Itenov et al. 81 study, which shows a high sensitivity estimate and a very low specificity estimate.
| Study | Target population (setting) | Assay | Timing of test | Cut-off point (ng/ml) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Prevalence of AKI |
|---|---|---|---|---|---|---|---|---|
| Matsa 201473 | Critical care – mixed population (ICU/ITU) | BioPorto plasma NGAL | ICU admission | 400 | 0.60 (0.47 to 0.73) | 0.85 (0.77 to 0.92) | 0.77 | 0.38 |
| Hjortrup 201572 | Critical care – mixed population (sepsis) | BioPorto plasma NGAL | ICU admission | 558 | 0.58 | 0.76 | 0.66 (0.54 to 0.77) | 0.24 |
| Tecson 201778 | Critical care – mixed population (ICU/ITU) | BioPorto plasma NGAL | Within 48 hours of ICU admission | 142 | 0.79 (0.61 to 0.91) | 0.73 (0.67 to 0.79) | 0.76 (0.64 to 0.87) | 0.13 |
| Itenov 201781 | Critical care – mixed population (ICU/ITU) | BioPorto plasma NGAL | ICU admission | 185 | 0.93 | 0.23 | NR | 0.36 |
FIGURE 13.
Forest plots of sensitivity and specificity for the BioPorto plasma NGAL assay for the detection of AKI in adults: critical care setting. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
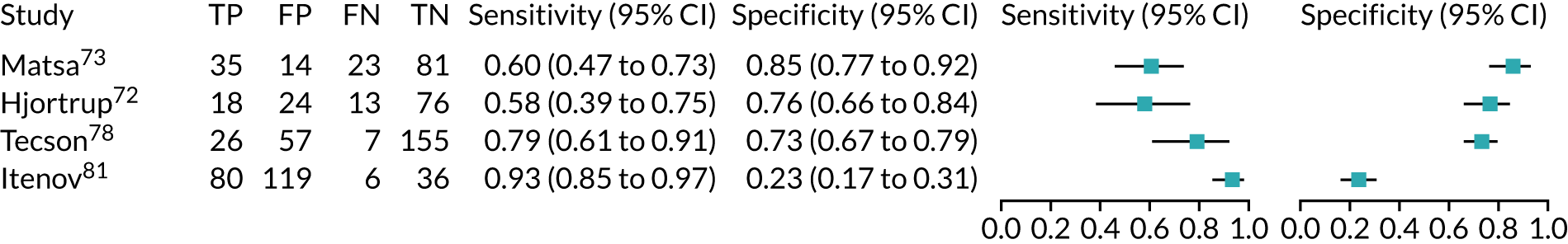
FIGURE 14.
The SROC plot for the BioPorto plasma NGAL assay studies for the detection of AKI in adults: critical care setting. HSROC, hierarchical summary receiver operating characteristic.
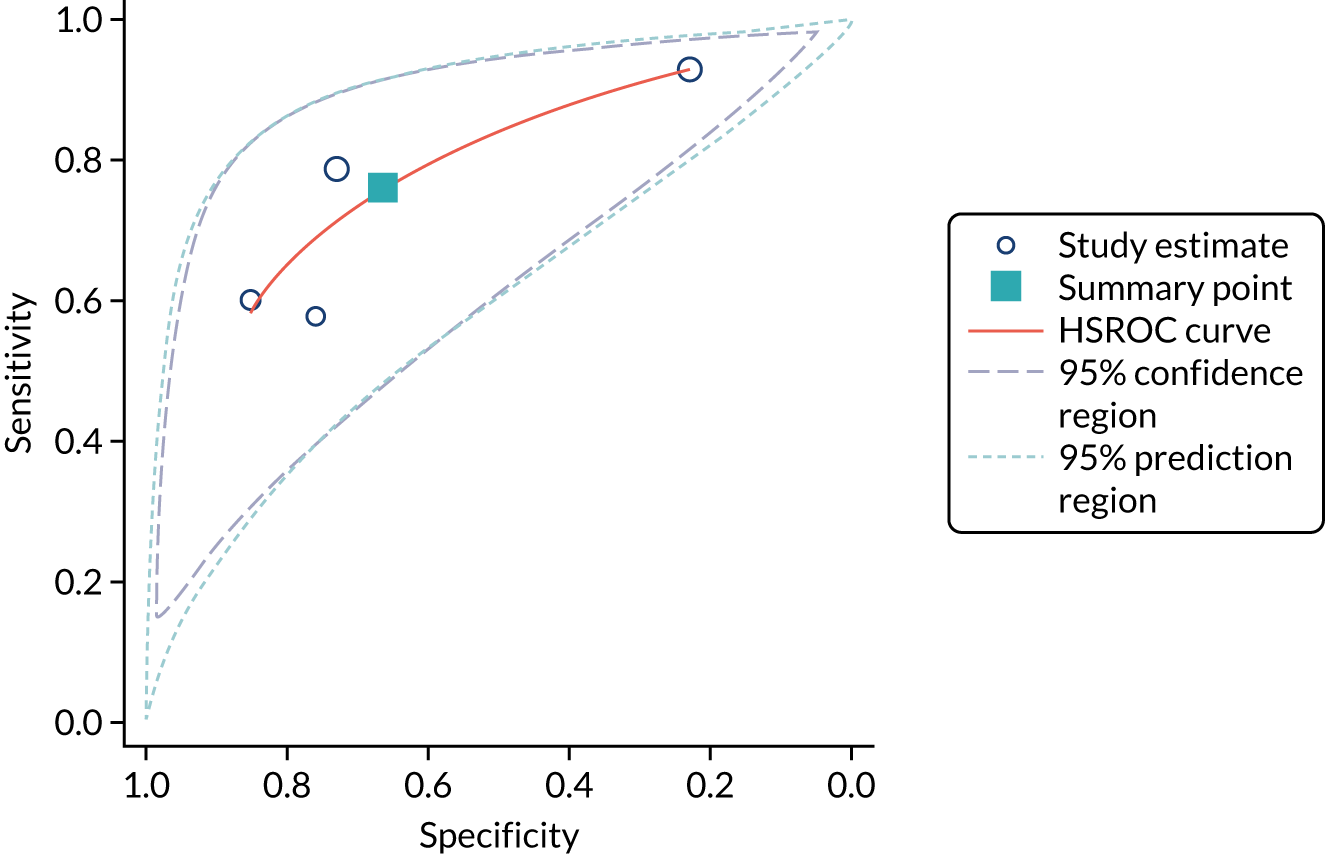
Table 9 presents a summary of the diagnostic data for the seven urine NGAL assay studies that assessed AKI in children. All but one study assessed children who underwent cardiac surgery. Across studies, the age of the paediatric population ranged from 1 day to 8 years.
| Study | Target population (setting) | Assay | Timing of test | Cut-off point | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Prevalence of AKI |
|---|---|---|---|---|---|---|---|---|
| Parikh 201184 | Cardiac surgery | ARCHITECT urine NGAL | ICU admission | > 72 ng/ml | 0.42 | 0.85 | 0.71 | 0.17 |
| Cantinotti 201288 | Cardiac surgery | ARCHITECT urine NGAL | 2 hours after surgery | 49.9 ng/ml | 0.78 | 0.81 | 0.85 (0.77 to 0.91) | 0.27 |
| Bennett 200887 | Cardiac surgery | ARCHITECT urine NGAL | 2 hours after surgery | > 150 ng/ml | 0.79 (0.69 to 0.86) | 0.92 (0.84 to 0.96) | 0.93 | 0.50 |
| Seitz 201390 | Cardiac surgery | ARCHITECT urine NGAL | 2 hours after end of surgery | 27.6 ng/ml | 0.55 | 0.43 | 0.56 | 0.55 |
| Alcaraz 201489 | Cardiac surgery | ARCHITECT urine NGAL | ICU admission | 100 ng/ml | 0.82 | 0.76 | 0.84 (0.76 to 0.92) | 0.34 |
| Yang 201765 | Cardiac surgery | BioPorto urine NGAL | 6 hours after surgery | 186 µg/g of creatinine | 0.77 | 0.47 | 0.72 (0.64 to 0.80) | 0.39 |
| Zwiers 201591 | Critical care – mixed population (ICU/ITU) | ARCHITECT urine NGAL | ICU admission | 126 ng/ml | 0.76 | 0.84 | 0.81 (0.68 to 0.94) | 0.35 |
ARCHITECT urine neutrophil gelatinase-associated lipocalin assay: child population
Cardiac surgery
Five studies (887 children in total) assessed the use of the ARCHITECT urine NGAL assay for the detection of AKI among children who had undergone cardiac surgery (see Table 9). The cut-off point used to define a positive test and the timing of biomarker measurements varied across studies. The prevalence of AKI ranged from 17% to 55% across studies. Figure 15 shows the forest plots of sensitivity and specificity estimates across studies. Sensitivity values ranged from 0.42 to 0.83 and specificity values ranged from 0.43 to 0.92. The summary estimate of sensitivity was 0.68 (95% CI 0.53 to 0.80) and that of specificity was 0.79 (95% CI 0.63 to 0.89). Figure 16 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. The confidence and prediction regions are very large, indicating considerable heterogeneity between studies.
FIGURE 15.
Forest plots of sensitivity and specificity for the ARCHITECT urine NGAL assay for the detection of AKI in children: cardiac surgery setting. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
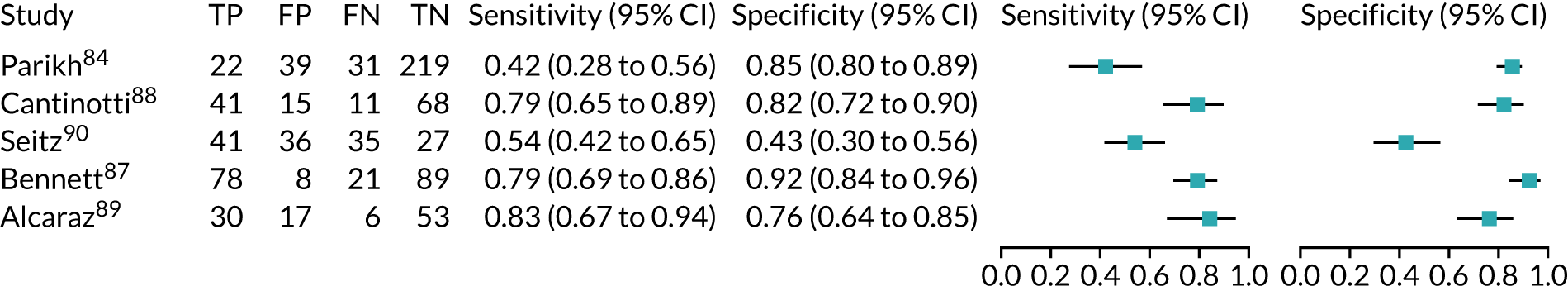
FIGURE 16.
The SROC plot for the ARCHITECT urine NGAL assay studies for the detection of AKI in children: cardiac surgery setting. HSROC, hierarchical summary receiver operating characteristic.
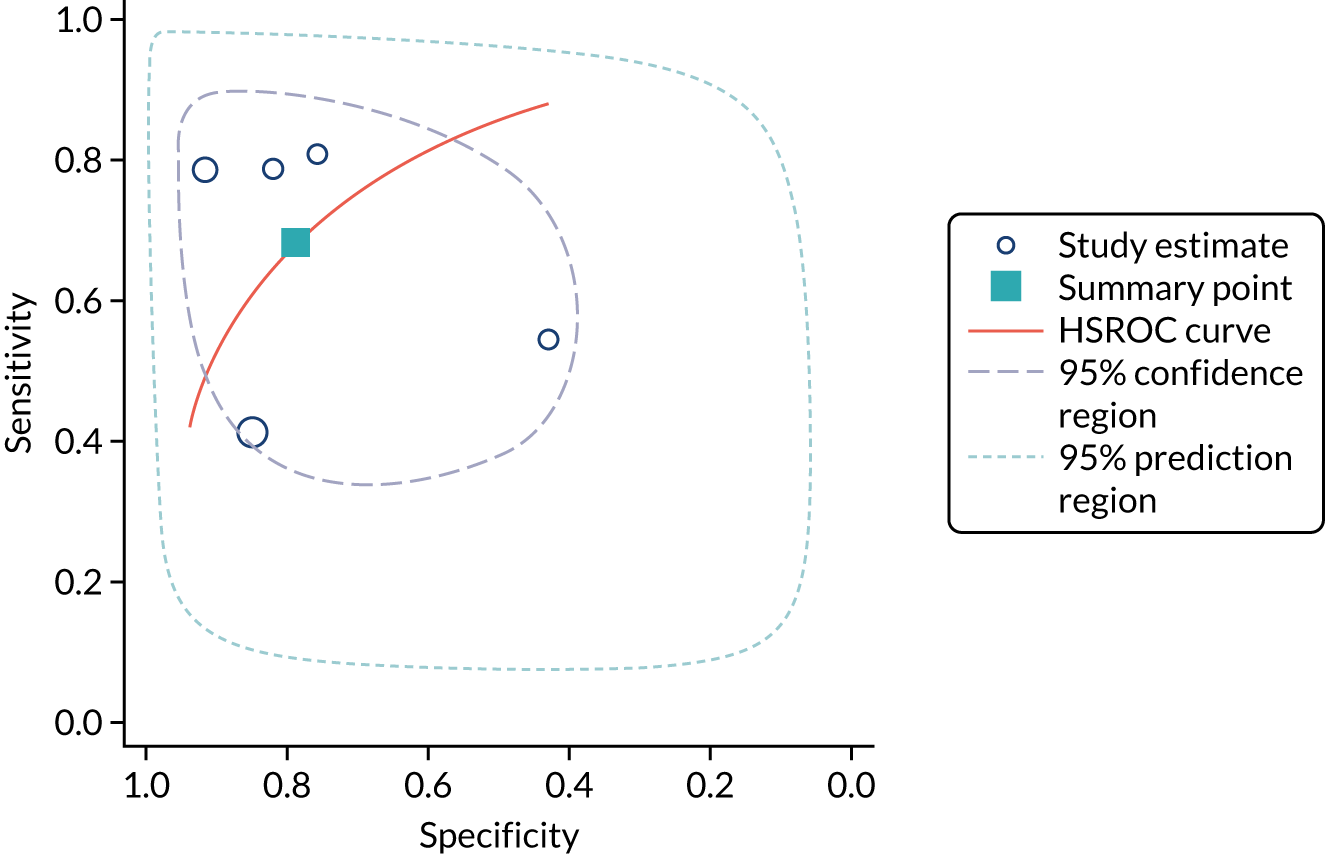
Critical care: mixed population
One study91 assessed the use of the ARCHITECT urine NGAL assay for the detection of AKI in 324 children admitted to ICU or critical care for various clinical reasons (see Table 9). The cut-off point was 126 ng/ml. The prevalence of AKI in the study was 35%. The sensitivity and specificity values for the urine sample collected at ICU admission were 0.77 (95% CI 0.60 to 0.90) and 0.85 (95% CI 0.74 to 0.92), respectively.
BioPorto urine neutrophil gelatinase-associated lipocalin assay: child population
Cardiac surgery
One study65 assessed the use of the BioPorto urine NGAL assay for the detection of AKI in 323 children who underwent cardiac surgery (see Table 9). Urine NGAL was measured using a concentration normalised by units of creatinine. The sensitivity and specificity values for the urine sample collected 6 hours after surgery were 0.77 (95% CI 0.69 to 0.84) and 0.47 (95% CI 0.40 to 0.54), respectively. The prevalence of AKI in the study was 39%.
Urine neutrophil gelatinase-associated lipocalin assays (both ATCHITECT and BioPorto) across all clinical settings: child population
Figure 17 shows the forest plots of sensitivity and specificity estimates for the seven studies (1310 children in total) assessing urine NGAL assays (both ARCHITECT and BioPorto) for the detection of AKI among children across all clinical settings. Sensitivity values ranged from 0.42 to 0.83; specificity values ranged from 0.43 to 0.92. Summary estimates for sensitivity and specificity were 0.71 (95% CI 0.60 to 0.80) and 0.76 (95% CI 0.61 to 0.86), respectively. Figure 18 shows the SROC plot with 95% confidence region for the summary operating point and 95% prediction region. The confidence and prediction regions are very large, indicating considerable heterogeneity between studies.
FIGURE 17.
Forest plots of sensitivity and specificity for all urine NGAL assays (ARCHITECT and BioPorto) for the detection of AKI in children across all clinical settings. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
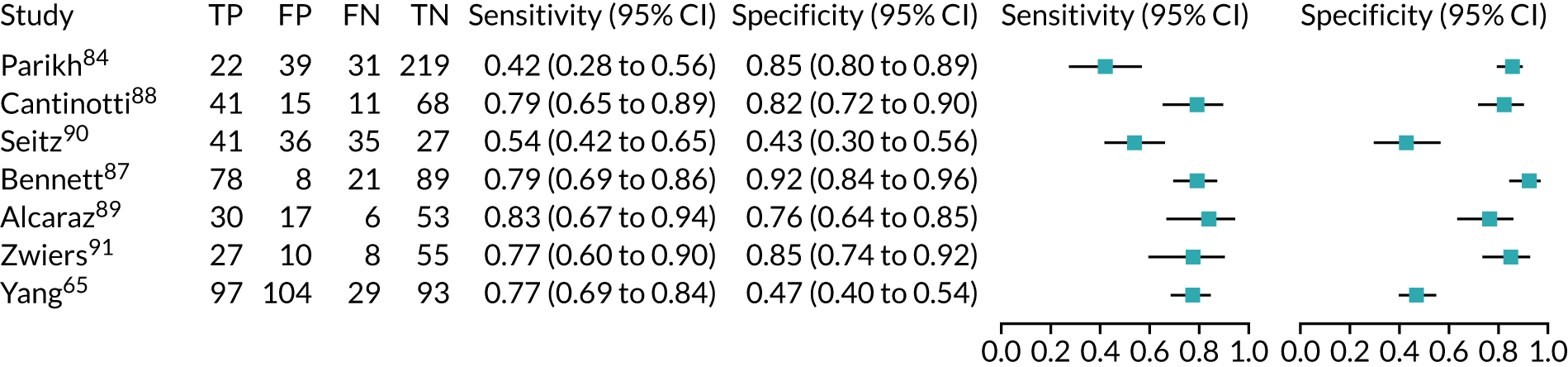
FIGURE 18.
The SROC plot for all urine NGAL assay (ARCHITECT and BioPorto) studies for the detection of AKI in children: all clinical settings. HSROC, hierarchical summary receiver operating characteristic.
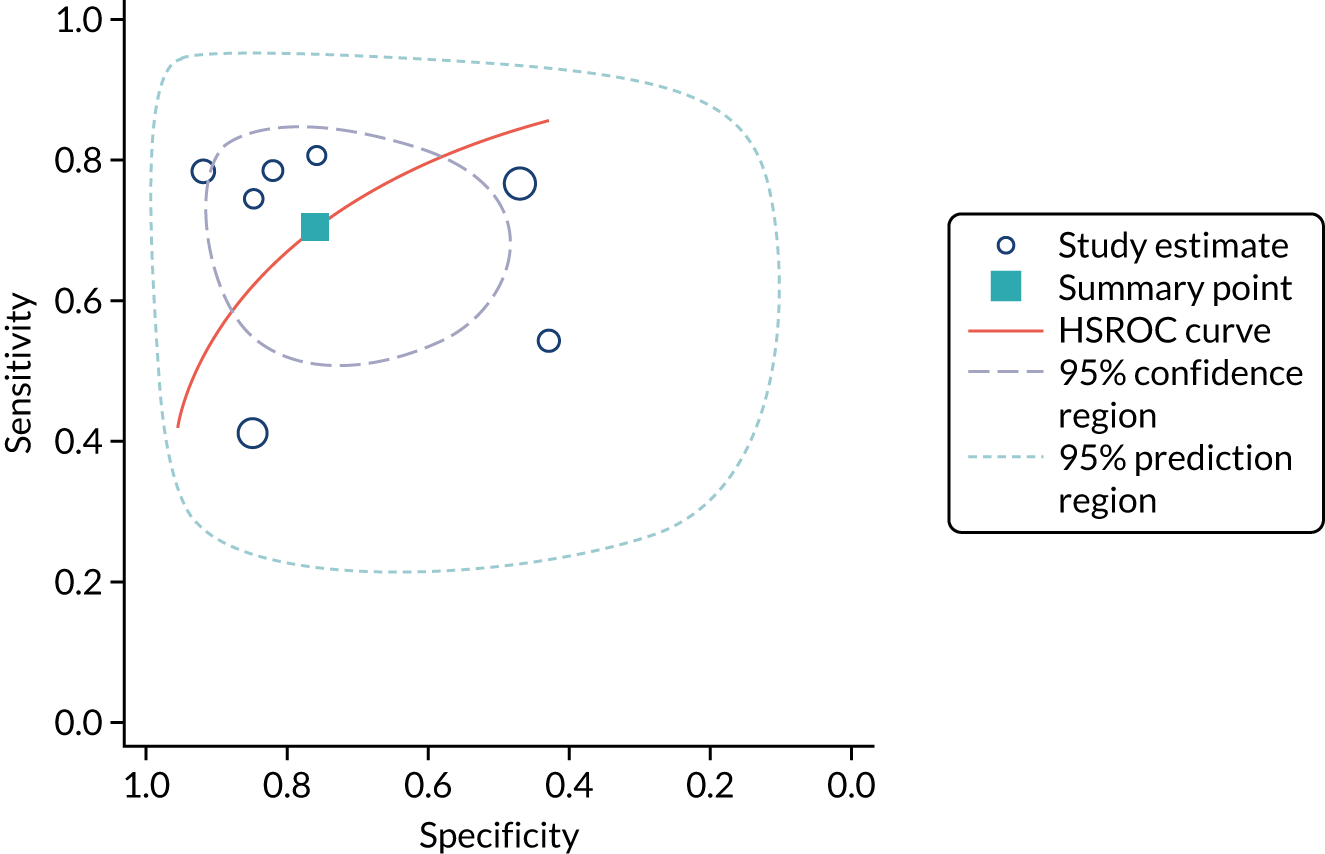
Accuracy of NephroCheck, ARCHITECT neutrophil gelatinase-associated lipocalin and BioPorto neutrophil gelatinase-associated lipocalin assays for the detection of acute kidney injury in critically ill patients
For both adults and children, the accuracy of NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assays for detection of AKI in each clinical setting is shown in Table 10. The table displays either the AUC estimates as reported by individual studies or the AUC summary estimates together with the corresponding prediction intervals when pooling of AUC estimates was feasible. Associated forest plots of the AUC meta-analyses are presented in Appendix 10 (see Figures 34–50). For the adult population, the AUC summary estimates ranged from 0.62 for the BioPorto urine NGAL assay to 0.74 for the BioPorto plasma NGAL assay in the cardiac surgery setting, and from 0.72 for the BioPorto urine and plasma NGAL assays to 0.76 for the ARCHITECT urine NGAL assay in the critical care setting. For the child population in the cardiac surgery setting, the AUC summary estimates ranged from 0.80 for the ARCHITECT urine NGAL assay to 0.88 for the BioPorto urine NGAL assay. All AUC summary estimates had relatively large 95% prediction intervals, indicating heterogeneity between studies. The forest plots in Appendix 10 (see Figures 34–50) show that variation is present both between and within studies.
| Population, biomarker and setting | Studies (n) | AUC (95% CI) | 95% prediction interval | |
|---|---|---|---|---|
| Estimate | Summary | |||
| Adults, NephroCheck, across all settings | 7 | – | 0.76 (0.50 to 0.91) | (0.47 to 0.90) |
| Adults, NephroCheck, cardiac surgery | 1 | 0.68 (0.54 to 0.81) | – | – |
| Adults, NephroCheck, major non-cardiac surgery | – | – | – | – |
| Adults, NephroCheck, critical care | 6 | – | 0.74 (0.67 to 0.81) | (0.44 to 0.91) |
| Adults, ARCHITECT urine NGAL, all settings | 14 | – | 0.73 (0.68 to 0.78) | (0.53 to 0.87) |
| Adults, ARCHITECT urine NGAL, cardiac surgery | 6 | – | 0.70 (0.65 to 0.74) | (0.58 to 0.79) |
| Adults, ARCHITECT urine NGAL, major non-cardiac surgery | 1 | 0.50 (034 to 0.66) | – | – |
| Adults, ARCHITECT urine NGAL, critical care | 7 | – | 0.76 (0.69 to 0.82) | (0.50 to 0.91) |
| Adults, BioPorto urine NGAL, across settings | 15 | – | 0.70 (0.65 to 0.74) | (0.53 to 0.82) |
| Adults, BioPorto urine NGAL, cardiac surgery | 4 | – | 0.62 (0.55 to 0.69) | (0.33 to 0.84) |
| Adults, BioPorto urine NGAL, major non-cardiac surgery | 1 | 0.78 (0.66 to 0.90) | – | – |
| Adults, BioPorto urine NGAL, critical care | 10 | – | 0.72 (0.67 to 0.77) | (0.54 to 0.85) |
| Adults, urine NGAL (ARCHITECT and BioPorto), cardiac surgery | 10 | – | 0.67 (0.62 to 0.78) | (0.53 to 0.78) |
| Adults, urine NGAL (ARCHITECT and BioPorto), major non-cardiac surgery | 2 | – | 0.65 (0.35 to 0.86) | – |
| Adults, urine NGAL (ARCHITECT and BioPorto), critical care | 17 | – | 0.74 (0.70 to 0.78) | (0.56 to 0.86) |
| Adults, urine NGAL (ARCHITECT and BioPorto), all settings | 29 | – | 0.71 (0.68 to 0.74) | (0.55 to 0.84) |
| Adults, BioPorto plasma NGAL, across all settings | 10 | – | 0.72 (0.66 to 0.77) | (0.52 to 0.86) |
| Adults, BioPorto plasma NGAL, cardiac surgery | 3 | – | 0.74 (0.65 to 0.82) | (0.06 to 0.99) |
| Adults, BioPorto plasma NGAL, major non-cardiac surgery | 1 | 0.78 (0.66 to 0.90) | – | – |
| Adults, BioPorto plasma NGAL, critical care | 7 | – | 0.72 (0.65 to 0.78) | (0.47 to 0.88) |
| Children, urine NGAL (ARCHITECT and BioPorto), across settings | 9 | – | 0.81 (0.71 to 0.88) | (0.37 to 0.97) |
| Children, ARCHITECT urine NGAL, cardiac surgery | 5 | – | 0.80 (0.65 to 0.90) | (0.17 to 0.99) |
| Children, BioPorto urine NGAL, cardiac surgery | 2 | – | 0.88 (0.47 to 0.98) | – |
| Children, urine NGAL (ARCHITECT and BioPorto), all cardiac surgery | 7 | – | 0.82 (0.71 to 0.90) | (0.31 to 0.98) |
| Children, ARCHITECT urine NGAL, critical care | 1 | 0.81 (0.69 to 0.94) | – | – |
| Children, BioPorto urine NGAL, critical care | 1 | 0.68 (0.55 to 0.81) | – | – |
| Children, urine NGAL (ARCHITECT and BioPorto), critical care | 2 | – | 0.73 (0.58 to 0.84) | – |
For each biomarker, Table 11 shows the AUC for the detection of AKI compared with that of serum creatinine or conventional clinical assessment, as reported by the individual studies that provided this information. AUC values varied across studies. In the majority of cases, the reported AUC indicated a slightly better performance of the biomarkers than that of serum creatinine or conventional clinical assessment for the detection of AKI. However, in a number of cases, serum creatinine or conventional clinical assessment appeared to perform better than the biomarkers under assessment.
| Study ID, geographical location, patient population | Clinical setting | Biomarker | AUC (95% CI or SEM) | |
|---|---|---|---|---|
| Creatinine or clinical model | Biomarker | |||
| Bihorac 2014,29 USA, adult population | Critical care (mixed population) | NephroCheck | Serum creatinine 0.63 (0.56 to 0.70) | 0.82 (0.76 to 0.88) |
| Kashani 2013,34 North America and Europe, adult population | Critical care (mixed population) | NephroCheck | Serum creatinine 0.75 (0.70 to 0.80) | 0.80 (0.75 to 0.84) |
| Kimmel 2016,36 Germany, adult population | Critical care (mixed population) | NephroCheck | Serum creatinine 0.60 (0.53 to 0.66) | 0.74 (0.66 to 0.81) |
| BioPorto plasma NGAL | Serum creatinine 0.60 (0.53 to 0.66) | 0.55 (0.5 to 0.66) | ||
| BioPorto urine NGAL | Serum creatinine 0.60 (0.53 to 0.66) | 0.66 (0.58 to 0.73) | ||
| Haase 2014,60 Germany, adult population | Cardiac surgery | ARCHITECT urine NGAL | Serum creatinine 0.66 (0.51 to 0.76) | 0.71 (0.6 to 0.83) |
| BioPorto plasma NGAL | Serum creatinine 0.66 (0.51 to 0.76) | 0.71 (0.58 to 0.83) | ||
| Kokkoris 2012,54 Greece, adult population | Critical care (mixed population) | ARCHITECT urine NGAL | Serum creatinine 0.77 (0.67 to 0.84) | 0.74 (0.64 to 0.82) |
| BioPorto plasma NGAL | Serum creatinine 0.77 (0.67 to 0.84) | 0.78 (0.68 to 0.85) | ||
| Liebetrau 2013,47 Germany, adult population | Cardiac surgery | ARCHITECT urine NGAL | Serum creatinine 0.74 (0.58 to 0.91) | 0.90 (0.811 to 0.99) |
| Parikh 2011,37 North America, adult population | Cardiac surgery | ARCHITECT urine NGAL | Clinical model 0.69 (SEM 0.04) | 0.67 (SEM 0.04) |
| Parikh 2011,95 North America, adult population | Cardiac surgery | ARCHITECT urine NGAL | Serum creatinine 0.46 (SEM 0.04) | 0.71 (SEM 0.04) |
| Nickolas 2012,56 USA and Germany, adult population | Critical care (mixed population) | ARCHITECT urine NGAL | Serum creatinine 0.91 (0.87 to 0.94) | 0.81 (NR) |
| Treeprasertsuk 2015,59 Thailand, adult population | Critical care (mixed population) | ARCHITECT urine NGAL | Serum creatinine 0.58 (NR) | 0.83 (0.76 to 0.91) |
| De Loor 2017,63 Belgium, adult population | Cardiac surgery | BioPorto urine NGAL | Serum creatinine 0.78 (0.72 to 0.83) | 0.65 (0.58 to 0.72) |
| Hjortrup 2015,72 Denmark, adult population | Critical care (mixed population) | BioPorto urine NGAL | Plasma creatinine 0.66 (0.56 to 0.77) | 0.71 (0.59 to 0.82) |
| BioPorto plasma NGAL | Plasma creatinine 0.66 (0.56 to 0.77) | 0.66 (0.54 to 0.77) | ||
| Nickolas 2008,74 USA, adult population | Critical care (mixed population) | BioPorto urine NGAL | Serum creatinine 0.92 (0.87 to 0.98) | 0.95 (0.88 to 1.00) |
| Verna 2014,79 USA, adult population | Critical care (mixed population) | BioPorto urine NGAL | Serum creatinine 0.89 | 0.86 (NR) |
| Alcaraz 2014,89 Spain, child population | Cardiac surgery | ARCHITECT urine NGAL | Clinical model 0.85 (0.78 to 0.93) | 0.84 (0.76 to 0.92) |
Role of biomarkers in predicting worsening of acute kidney injury mortality and need for renal replacement therapy
Table 12 displays the AUC and the pooled AUC estimates with corresponding 95% CIs for the NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assay studies for the prediction of worsening of AKI, mortality and RRT in each clinical setting for both adults and children. Only a limited number of studies were available for AUC meta-analyses. Associated forest plots are presented in Appendix 11 (see Figures 51–55). In the critical care setting (adult population), the AUC values reported in individual studies ranged from 0.66 for the BioPorto plasma NGAL assay to 0.71 for the BioPorto urine NGAL assay for worsening of AKI, and from 0.55 for the BioPorto plasma NGAL assay for prediction of 90-day mortality to 0.75 for the ARCHITECT urine NGAL assay for prediction of 30-day mortality. One study reported an AUC of 0.70 for BioPorto plasma NGAL for prediction of need for RRT. The AUC summary estimate (pooling of 2 studies) for worsening of AKI in the critical care setting was 0.65 (95% CI 0.43 to 0.82) for ARCHITECT urine NGAL. AUC summary estimates ranged from 0.62 for BioPorto urine NGAL for 90-day mortality to 0.68 for the BioPorto plasma NGAL assay for in-hospital mortality, with a 95% CI of 0.58 to 0.73. The AUC summary estimate (two studies) for prediction of RRT in critical care for the BioPorto urine NGAL assay was 0.74 (95% CI 0.49 to 0.89). 72,75 In the cardiac surgery setting (adult population), AUC values from individual studies ranged from 0.68 for the ARCHITECT urine NGAL assay to 0.78 for NephroCheck for prediction of need for RRT, with a 95% CI of 0.57 to 0.84.
| Population, biomarker and setting | Follow-up | Studies (n) | AUC (95% CI) | |
|---|---|---|---|---|
| Estimate | Summary estimate | |||
| AKI | ||||
| Adults, ARCHITECT urine NGAL, critical care | During hospital stay | 1 | – | 0.65 (0.43 to 0.82) |
| Adults, BioPorto urine NGAL, critical care | During ICU stay | 1 | 0.71 (0.59 to 0.82) | – |
| Adults, BioPorto plasma NGAL, critical care | During ICU stay | 1 | 0.66 (0.54 to 0.77) | – |
| Mortality | ||||
| Adults, ARCHITECT urine NGAL, cardiac surgery | During hospital stay (11/288 patients died) | 1 | 0.70 (0.56 to 0.84) | – |
| Adults, ARCHITECT urine NGAL, major non-cardiac surgery | 30 days (10/109 patients died) | 1 | 0.65 (0.45 to 0.85) | – |
| Adults, ARCHITECT urine NGAL, critical care | 30 days (17/121 patients died) | 1 | 0.75 (0.66 to 0.85) | – |
| Adults, BioPorto urine NGAL, critical care | 90 days | 2 | – | 0.62 (0.58 to 0.66) |
| Adults, BioPorto plasma NGAL, critical care | In hospital | 2 | – | 0.68 (0.63 to 0.73) |
| Adults, BioPorto plasma NGAL, critical care | 30 days (7/105 patients died) | 1 | 0.72 (0.49 to 0.87) | – |
| Adults, BioPorto plasma NGAL, critical care | 90 days | 1 | 0.55 (0.47 to 0.63) | – |
| Adults, urine NGAL (ARCHITECT and BioPorto) critical care | In hospital | 2 | – | 0.76 (0.64 to 0.85) |
| Children, ARCHITECT urine NGAL, cardiac surgery | In hospital (3/196 patients died) | 1 | 0.91 (0.55 to 0.99) | – |
| Need for RRT | ||||
| Adults, NephroCheck, cardiac surgery | NR | 1 | 0.78 (0.71 to 0.84) | – |
| Adults, ARCHITECT urine NGAL, cardiac surgery | Up to 12 months (22/288 patients received RRT) | 1 | 0.68 (0.57 to 0.79) | – |
| Adults, BioPorto urine NGAL, critical care | – | 2 | – | 0.74 (0.49 to 0.89) |
| Adults, BioPorto plasma NGAL, critical care | During ICU stay (40/222 patients received RRT) | 1 | 0.70 (0.61 to 0.78) | – |
| Children, ARCHITECT urine NGAL, cardiac surgery | During hospital stay (4/196 patients received RRT) | 1 | 0.86 (0.57 to 0.97) | – |
Appendix 12, Table 30, presents the AUC with 95% CI or the OR with 95% CI for the addition of the biomarkers to existing clinical models for the prediction of AKI, mortality and need for RRT. It is worth noting that the statistical models differed between studies and often were not sufficiently detailed. In particular, although most of the adjusting predictors were specified, information on the potential candidate variables was missing. In general, the number of events was small, given the number of predicting variables, even for AKI outcomes. Overall, the addition of biomarkers to the clinical models improved risk prediction of newly developed AKI or worsening of AKI, and mortality. However, only a limited amount of data were available for each biomarker in each clinical setting, thereby restricting any generalisable interpretation.
Interpretation of clinical effectiveness evidence
The results of the meta-analyses of sensitivity and specificity estimates suggest that the biomarkers under investigation (i.e. NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assays) may have a role in the detection of AKI in critically ill patients. However, owing to the considerable clinical and statistical heterogeneity observed across studies and the limited number of studies available for certain clinical settings and/or type of biomarker, these results should be interpreted with caution and require further evidence to substantiate them. Furthermore, the threshold level for NGAL varied considerably across studies. However, as optimal NGAL thresholds for detection of AKI in various clinical settings have yet to be established, we decided to pool results across studies with similar characteristics, despite this obvious limitation. For the adult population, we were able to conduct meta-analyses for studies that assessed patients in the critical care (mixed population) setting and for studies across all clinical settings. There were too few studies assessing patients after cardiac surgery or major non-cardiac surgery. The NephroCheck test had the highest pooled sensitivity (0.83), but the worst pooled specificity (0.51), whereas the ARCHITECT and BioPorto urine NGAL tests had slightly lower pooled sensitivity estimates (0.70 and 0.72, respectively), but better pooled specificity estimates (0.72 and 0.87, respectively). The BioPorto urine NGAL assay pooled sensitivity was similar to that of the BioPorto plasma NGAL assay (0.72 vs. 0.76, respectively), but the pooled specificity was better for the BioPorto urine NGAL assay than for the BioPorto plasma NGAL assay (0.87 vs. 0.67, respectively). The biomarkers had a similar performance across all clinical settings (the NephroCheck test pooled sensitivity and specificity were 0.75 and 0.61, respectively; the ARCHITECT urine NGAL assay pooled sensitivity and specificity were 0.67 and 0.72, respectively; the BioPorto urine NGAL assay pooled sensitivity and specificity were 0.73 and 0.83, respectively; and the BioPorto plasma NGAL assay pooled sensitivity and specificity were 0.76 and 0.67, respectively), with the BioPorto plasma NGAL assay showing the highest sensitivity (0.76) and the BioPorto urine NGAL assay showing the highest specificity (0.83).
With regard to the observed low specificity of the NephroCheck test, we do not know with certainty whether this is because of the relatively poor performance of the biomarker or the fact that serum creatinine is an imperfect reference standard for assessing kidney injury.
We also noted that, when a study had a small number of AKI events (low prevalence), the relationship observed between sensitivity and specificity estimates became quite different from that of studies for which prevalence was higher.
For the child population, we were able to conduct meta-analyses for the five ARCHITECT urine NGAL assay studies that assessed children who underwent cardiac surgery. The pooled sensitivity was 0.68 and the pooled specificity was 0.79. Too few studies were available for the other assays or clinical settings. When we combined all urine NGAL studies (ARCHITECT and BioPorto) across all settings (seven studies), we obtained similar estimates of accuracy (sensitivity 0.71; specificity 0.76). 65,84,87–91
For the prediction of relevant clinical outcomes, only a limited number of studies were available for each biomarker in each clinical setting; this hampered the possibility of performing pooled analyses. Furthermore, the details of the methodology used for the statistical analyses were insufficient, especially for older studies. The more recent studies appeared to use some of the PROBAST22 recommendations and terminology, but they were still far from satisfactory, as demonstrated by the results of the PROBAST assessment (see Figures 3 and 4). Moreover, although information on the adjustment strategies and the process of variables’ selection was provided in individual studies, the original cohort of potential predictors, prior to the multivariable analysis, was never clearly specified, leading to potential risk of data-mining and, hence, methodological bias.
Similarly, although there was an indication that the addition of biomarkers to existing clinical models might improve the prediction of relevant clinical outcomes, studies varied substantially in terms of study characteristics and statistical methods used to assess prediction, thereby limiting any reliable conclusion.
On the whole, we observed considerable clinical and statistical heterogeneity in all analyses, especially with regard to clinical setting, NGAL threshold levels, time of sample collection, definition of AKI, time of AKI diagnosis, number of AKI events and assay platforms. Therefore, we have limited confidence in the validity and reliability of the observed results.
Chapter 4 Assessment of cost-effectiveness
This chapter assesses the cost-effectiveness of alternative biomarkers (NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assays) used in combination with standard clinical assessment (i.e. serum creatinine and urine output), compared with standard clinical assessment alone, for evaluating critically ill people who are at risk of developing AKI and are being considered for possible critical care admission in an NHS hospital setting. The specific objectives were to review the existing cost-effectiveness evidence base for these tests and to develop a de novo economic model to assess cost-effectiveness from an NHS and Personal Social Services perspective.
Systematic review of existing cost-effectiveness evidence
Objective
The aim of the review of economic evaluations was to identify, report and critically appraise existing economic evaluations of NephroCheck, ARCHITECT urine NGAL, and BioPorto urine and plasma NGAL assays for evaluating critically ill people (adults and children) at risk of developing AKI.
Search strategies
Comprehensive electronic searches were conducted to identify economic evaluations of the candidate tests. Highly sensitive search strategies were developed, including index terms, free-text words, abbreviations and synonyms. The following electronic databases were searched: Ovid MEDLINE, Ovid EMBASE, NHS Economic Evaluation Database, HTA Database, Research Papers in Economics, and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Presentations Database, with no restriction on date, language or publication type. The searches were undertaken on 27 May 2019, with additional searches on 11 September 2019.
Inclusion and exclusion criteria
Studies were deemed appropriate for inclusion in the review of economic evaluations if they (1) were full economic evaluations, defined as a comparative assessment of costs and outcomes in the framework of cost–utility, cost-effectiveness, cost–benefit or cost-minimisation analyses; (2) assessed the cost-effectiveness of the candidate tests within the population defined in the NICE scope (i.e. critically ill people, both adults and children, at risk of AKI who are being considered for admission to ICUs); and (3) provided sufficient information to judge the quality of the study and obtain any relevant data (i.e. conference abstracts alone were unlikely to meet this criterion). Economic evaluations conducted alongside single effectiveness studies (e.g. RCTs) and decision-analytic models were all deemed relevant for inclusion. Studies were excluded if they were methodological studies, systematic reviews of cost-effectiveness studies (although these were retained for reference) or cost-of-illness studies. Studies were also excluded if they assessed tests/biomarkers outside the NICE scope (e.g. cystatin C) only or used the candidate tests for a purpose other than determining risk of AKI.
Quality assessment of included studies
Included studies were appraised against the NICE reference case for the assessment of cost-effectiveness of diagnostic tests. 96
Evidence synthesis of cost-effectiveness studies
The main findings are summarised in a narrative review, with key study characteristics and findings tabulated for ease of comparison.
Results
Figure 19 illustrates the PRISMA flow diagram for the review of economic evaluations. The searches identified 125 potentially relevant abstracts. After abstract screening, 99 (79.2%) studies were excluded because they did not meet the inclusion criteria. Full-text articles were sought for the remaining 26 (20.8%) studies for further assessment against the inclusion/exclusion criteria. Of those 26 studies, four studies were ultimately included in the review. 95,97–99 A tabulated summary of the study characteristics and results is provided in Table 13, and a quality assessment against the NICE reference case is provided in Table 14.
FIGURE 19.
The PRISMA flow diagram for the review of cost-effectiveness studies. CA, conference abstract; EE, economic evaluation.
| Characteristic | Study | |||
|---|---|---|---|---|
| Hall et al.97 2018 | Parikh et al.95 2017 | Petrovic et al.98 2015 | Shaw et al.99 2011 | |
| Population | Adults, aged ≥ 18 years | Adults, aged ≥ 18 years, without ESRD or need for RRT | Paediatric, aged ≤ 18 years | Base case: 67-year-old male |
| Setting | Hospital critical care (all-comers) and post-cardiac surgery subgroup | Hospital (ED) setting, data from two sites | Post cardiac surgery, country unclear (assumed Serbia) | Post cardiac surgery |
| Objective(s) |
|
To determine if NGAL can reduce hospital costs | To determine the cost-effectiveness of the candidate tests | To determine the cost-effectiveness of urine NGAL for AKI diagnosis |
| Country | UK | USA | Unclear (assumed Serbia) | UK |
| Intervention(s) | AKI biomarkers plus standard care:
|
Serum creatinine plus NGAL (urine) |
|
NGAL (urine) plus current practice (monitoring of creatinine, blood urea nitrogen, urine output) |
| Comparator(s) | Standard care (serum creatinine and urine output testing) | Serum creatinine alone | Serum creatinine alone | Current practice alone |
| Source of effectiveness/diagnostic accuracy data |
|
N/A (cost only) |
|
|
| Evaluation type (decision-analytic modelling/RCT) | Decision tree (diagnostic pathway) plus Markov cohort model [long-term outcomes, including CKD, ESRD (with or without dialysis), transplant] | Cost simulation | Decision tree (diagnostic pathway) plus Markov cohort model (long-term outcomes including CKD, ESRD, transplant and death) | Decision tree |
| Measure of benefit | QALYs | N/A | QALYs | QALYs |
| Perspective | NHS and PSS | Payer | Third-party payer | NHS perspective (although societal perspective stated) |
| Cost year | 2015 prices | Unclear | Unclear | 2008 |
| Time horizon |
|
Unclear, assume hospital admission duration | Lifetime (maximum age 100 years) | Lifetime |
| Discount rate |
|
NR |
|
NR |
| Sensitivity analyses conducted? | Deterministic sensitivity analyses conducted around time horizon, test costs, AKI incidence, impact of early treatment, costs of AKI intervention, ICU utility, diagnostic accuracy, additional mortality risk for false-positive test results, impact of negative test results | Deterministic sensitivity analysis: varying hospital cost, LOS, proportion with baseline CKD, proportion developing a urinary tract infection, costs of further testing | Deterministic sensitivity analysis: incidence of AKI and associated mortality, sensitivity and specificity | Deterministic sensitivity analysis: mainly different treatment effects, also baseline AKI probability, probability of CKD, effect of early intervention on AKI, change in hospital costs, change in diagnostic accuracy, cost per NGAL test |
|
PSA conducted: cost simulation | PSA conducted: yes | PSA conducted: yes | |
| Base-case results (including summary of incremental analyses) | ICERs vs. standard care:
|
Costs vs. standard care:
|
ICERs vs. standard care:
|
ICERs vs. standard care:
|
| Sensitivity analysis results |
|
|
Significant variation in price was not found to affect overall conclusions | Under all conditions, NGAL in addition to current practice was the most cost-effective strategy when compared with current practice alone, even when the treatment effect was minimal. Results were driven by the impact of early intervention on hospital LOS |
| Attribute | Reference case and technology appraisal methods guidance | Study | |||
|---|---|---|---|---|---|
| Hall et al.97 2018 | Parikh et al.95 2017 | Petrovic et al.98 2015 | Shaw et al.99 2011 | ||
| Comparator(s) | Therapies routinely used in the NHS, including technologies regarded as current best practice | Yes | Yes | Yes | Yes |
| Patient group | As per NICE scope (i.e. critically ill, pre ICU) | No: ICU group of patients outside the NICE scope, which is pre ICU | Partially: the NICE scope includes adults as well as children | Partially; however, the NICE scope is broader than post cardiac surgery only | Partially; however, the NICE scope is broader than post cardiac surgery only |
| Perspective costs | NHS and Personal Social Services | Yes | No | No | Partially |
| Perspective benefits | All health effects on individuals | Yes | No | Yes | Yes |
| Form of economic evaluation | Cost-effectiveness analysis (QALYs) | Yes | No | Yes | Yes |
| Time horizon | Sufficient to capture differences in costs and outcomes | Yes | No | Yes | Unclear |
| Synthesis of evidence on outcomes | Systematic review | Yes | No | No | No |
| Outcome measure | QALYs | Yes | N/A | Yes | Yes |
| Health states for QALY | Described using a standardised and validated instrument (i.e. EQ-5D) | Yes, when possible | N/A | Unclear | Unclear |
| Benefit valuation | Time trade-off or standard gamble | Yes, when possible | N/A | Unclear | Unclear |
| Source of preference data for valuation of changes in health-related quality of life | Representative sample of the public | Yes, when possible | N/A | Unclear | Unclear |
| Discount rate | An annual rate of 3.5% on both costs and health effects | Yes | No | No | No |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes | N/A | Yes | Yes |
| Probabilistic modelling | Probabilistic modelling | Yes | Yes | Yes | Yes |
| Sensitivity analysis | Deterministic sensitivity analyses conducted | Yes | Yes | Yes | Yes |
Relevance of the included studies for the current decision problem
Of the four studies identified in the review, three conducted cost-effectiveness analyses based on decision-analysis modelling. 97–99 Two of these studies included both a decision tree to capture the diagnostic phase of the model and a Markov cohort model to capture the long-term sequelae of diagnosis and possible prevention of AKI. 97,98 Both modelling strategies were similar and appropriate for the current decision problem in that they modelled the progression of AKI to CKD, end-stage renal disease (ESRD), transplantation and death. Although two studies97,99 were conducted in the UK, only one study97 was deemed directly relevant for informing the decision model developed as part of this assessment. Hall et al. 97 was also the only study to assess all of the candidate tests specified in the NICE scope. Although Hall et al. 97 provide a comprehensive and high-quality assessment of the cost-effectiveness of the relevant tests, the setting of the study relates to AKI occurring in people already admitted to ICUs and is therefore outside the scope of this assessment. Therefore, substantial revision of the Hall et al. 97 model is required, particularly for the early acute phase, to generate results that are appropriate for decision-making in critically ill patients who are at risk of AKI and are being considered for possible admission to ICU, but are not yet in the ICU setting.
Additional literature searches
Further searches were conducted to help develop the economic model. Broader searches were carried out to identify existing economic models in the area of AKI in addition to those identified for the candidate biomarker tests. A separate search was also developed for health-state utility data relevant to the health states included in the economic model. As searches for models and parameters were conducted by Hall et al. 97 up to 2016, our searches aimed to identify any relevant studies published after this date. Supplementary searches were carried out in MEDLINE, EMBASE, NHS Economic Evaluation Database, HTA Database, Research Papers in Economics, and ISPOR Scientific Presentations. The searches were undertaken on 11 September 2019, with no language restrictions. The relevant data are discussed in the subsections to follow.
Independent assessment of cost-effectiveness
A two-phase model was developed using TreeAge Pro 2018 (TreeAge Software, Inc., Williamstown, MA, USA) to assess the cost-effectiveness of using biomarker tests to help detect the risk of AKI development and to help initiate early preventative care.
As described in Chapter 3, there was no direct evidence regarding the clinical effectiveness of biomarker-guided preventative care, compared with standard monitoring-guided preventative care, on final health outcomes (e.g. AKI status, mortality, need for RRT). Therefore, a linked-evidence approach was required to determine the potential value of the tests. The model structure was therefore built to reflect hypothesised associative benefits of averting AKI or reducing its severity through biomarker-guided early intervention. The structure was informed by the review of cost-effectiveness studies and was based largely on Hall et al. ,97 who kindly provided access to their model [built in R (The R Foundation for Statistical Computing, Vienna, Austria)] under a Creative Commons licence. The appropriateness of the model structure was validated with the External Assessment Group’s (EAG’s) clinical experts. Data sources to populate the model are described in the sections that follow. The model was built and analysed following the guidelines stipulated in the NICE reference case for diagnostic test evaluation. 101
Methods
Relevant population(s)
The baseline population and prevalence of CKD in hospital for the model were obtained from a Grampian population cohort (described in Model structure: initial decision tree phase). The model base-case analysis is therefore based on a mixed cohort of CKD and non-CKD patients, with an average age of 63 years, and a 54.3% female population.
Diagnostic biomarkers evaluated
The model aims to assess the cost-effectiveness of the NephroCheck test, the ARCHITECT urine NGAL assay and the BioPorto urine and plasma NGAL assays in combination with standard clinical assessment, compared with standard clinical assessment alone (including serum creatinine and urine output), for evaluating critically ill people at risk of developing AKI who are being assessed for possible critical care admission.
Model structure: initial decision tree phase
The systematic review did not identify any randomised trials providing causal evidence for the effect of biomarker-guided care versus standard monitoring (serum creatinine)-guided care on patient-relevant outcomes such as peak AKI severity, admission to ICU, need for RRT, CKD or mortality.
In the absence of such data, the initial decision tree phase of the model used a linked-evidence approach to capture the potential impact of diagnostic test accuracy (sensitivity and specificity) on the probability of averting AKI or reducing its severity through earlier adoption of a KDIGO care bundle triggered by a positive biomarker test result. The model then captured possible effects on changes in health outcomes through associative links between AKI severity and the relevant outcomes [need for ICU care, length of stay (LOS), 90-day mortality, and development of CKD].
These associative links have been built up in the decision tree by reanalysis of observational data from Grampian. 102 The data set includes 17,630 adult patients admitted to hospital in Grampian in 2003 and is the complete population of all patients who had an abnormal kidney function blood test on hospital admission, including all patients who developed AKI. The study methodology is described in detail by Sawhney et al. ,103 but the data derived from the data set used to populate the model are unpublished. These observational, population-level data were used to define the starting age, sex and underlying proportion of prevalent CKD cases in the modelled cohort. The data were also used to populate the model with respect to the distribution of peak AKI severity, as well as LOS in hospital, probability of admission to ICU and 90-day mortality parameters (by KDIGO AKI stage) for the decision tree phase of the model.
In the decision tree, patients who are critically ill in hospital, are at risk of developing AKI and are having their kidney function monitored are divided into two cohorts, those with AKI and those without AKI, depending on the underlying prevalence. 102,103 The underlying prevalence of AKI was calculated directly from a more recent version of the Grampian data set,102 describing all hospital admissions with at least one overnight stay in 2012 (for patients having their kidney function monitored). The base-case prevalence of AKI generated from these data was 9.2%, sampled probabilistically from a beta distribution in the model based on count data. A sensitivity analysis uses prevalence data directly from the systematic review studies used to generate the diagnostic test accuracy parameters.
Acute kidney injury is defined in the model as having, or being destined to develop, AKI while in hospital and is classified based on the peak severity of AKI. There is an assumption in the model that it is possible to avert AKI with early biomarker-guided treatment in people who would otherwise develop it under standard care. However, it should be noted that, in some circumstances, it may not be possible to avoid AKI by earlier detection, as AKI may not always be modifiable. 3 The probability of averting AKI is zero in the standard-care arm. AKI is split into four KDIGO-defined stages (stages 1–3), with stage 3 split by the proportion of patients receiving RRT or not. The initial phase of the model describes the associations between peak AKI classification and probability of admission to ICU, LOS in ICU, LOS in hospital and 90-day mortality. These associative effects are all derived from the Grampian population cohort described previously. At the end of the 90 days, costs and QALY payoffs are assigned based on the decision tree pathway followed, before surviving patients enter the Markov cohort model.
The standard-care cohort is assumed to be perfectly identified as having or not having AKI, based on a combination of serum creatinine levels, other diagnostic workup and clinical expert opinion, which represents clinical practice. The hypothesised advantage of the biomarkers is that they may help to detect AKI earlier, but will not detect additional cases of AKI not detected by current practice. Figure 20 provides an illustration of the initial decision tree pathways for the standard-care arm of the model.
FIGURE 20.
Simplified decision tree structure up to 90 days for the standard-care (serum creatinine) arm of the model. Note that the AKI 3 pathway in the model is replicated for the proportion of the cohort receiving acute RRT and those not receiving acute RRT. M, Markov model. Reproduced with permission from Jacobsen et al. 104 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Participants in the intervention (test) arms of the model are similarly split into those with and without AKI, according to the same prevalence data, but all participants receive additional testing. It is assumed that the background diagnostic workup is similar for all arms of the model (i.e. all patients will continue to have their serum creatinine and urine output monitored). As the diagnostic accuracy test data are primarily based on single use of the test, it is assumed, in the base-case model, that each test will be administered only once. It is assumed that the test is administered as soon as possible after a patient has been determined to be at risk of AKI to enable early detection and preventative measures to be implemented. A sensitivity analysis explores the impact of more frequent multiple-use tests on the results.
The diagnostic accuracy of the candidate tests in addition to serum creatinine, compared with serum creatinine alone, was obtained from the results of the systematic review and meta-analysis described in Chapter 3. Table 15 describes the diagnostic accuracy parameters, namely sensitivity and specificity, used in the modelling. All diagnostic data are incorporated probabilistically in the model, accounting for the joint uncertainty in sensitivity and specificity for each biomarker test. The logit of the sensitivity/specificity for each of the biomarker tests was derived from the meta-analysis of diagnostic accuracy studies. The model specified the correlation between sensitivity and specificity parameters (on the logit scale). These parameters were converted to Cholesky decomposition matrices, with the decomposed data referenced by multinormal distributions, sampling from the mean and standard error (on the logit scale). The probabilistic draws were back-transformed from the logit scale for application in the model. It should be noted that diagnostic accuracy data obtained from the meta-analyses are based on heterogeneous studies with different thresholds; this is particularly true for the NGAL assays. Therefore, the results of the economic model, particularly for comparisons between different NGAL assays, should be interpreted cautiously. Further details have been provided in Chapter 3.
| Test | Parameter | Mean (95% CI) | Mean (logit scale) | Standard error (logit scale) | Correlation for multivariate normal distribution (logit scale) | Source |
|---|---|---|---|---|---|---|
| NephroCheck | Sensitivity | 0.75 (0.58 to 0.87) | 1.1178 | 0.3967 | –0.824 | Meta-analysis (see Chapter 3) |
| Specificity | 0.61 (0.49 to 0.72) | 0.4573 | 0.2567 | |||
| BioPorto plasma NGAL | Sensitivity | 0.76 (0.56 to 0.89) | 1.1563 | 0.4615 | –1.000 | Meta-analysis (see Chapter 3) |
| Specificity | 0.67 (0.40 to 0.86) | 0.6863 | 0.5659 | |||
| ARCHITECT urine NGAL | Sensitivity | 0.67 (0.58 to 0.76) | 0.7273 | 0.2047 | –0.5168 | Meta-analysis (see Chapter 3) |
| Specificity | 0.72 (0.64 to 0.79) | 0.9553 | 0.1909 | |||
| BioPorto urine NGAL | Sensitivity | 0.73 (0.65 to 0.80) | 1.017 | 0.195 | 0.526 | Meta-analysis (see Chapter 3) |
| Specificity | 0.83 (0.64 to 0.93) | 1.562 | 0.511 |
For each biomarker test group, the proportions of true AKI cases that are true positive and false negative are determined by test sensitivity, whereas the proportion of non-AKI cases that are true negatives or false positives are determined by test specificity.
Based on the EAG’s own clinical expert opinion (Simon Sawhney and Callum Kaye, University of Aberdeen, 2019, personal communication), it is assumed in the base case that patients testing negative would not have any adaptions made to their care pathway. This is because it would be unlikely that care would be de-escalated based solely on a negative NephroCheck or NGAL result, as the conservative practitioner would wait to ensure that there was no rise in serum creatinine before concluding that no AKI was present and stepping down the patient’s care.
The model assumes that all patients will receive the KDIGO care bundle once they are defined as AKI positive using current standard practice methods (i.e. monitoring serum creatinine and urine output), regardless of their NephroCheck or NGAL test result. The potential to benefit from use of the biomarkers, therefore, lies in the early adoption of a preventative care bundle. For patients testing positive, the model includes the functionality to reflect uncertainty in clinical decision-making, that is the probability that a positive test would be acted on. This parameter is assumed to take a value of 100%, in accordance with best practice guidance whereby positive biomarker tests should have a preventative KDIGO care bundle implemented, with the associated costs. Although all positive test results will trigger the KDIGO bundle, only those patients whose tests are true positive will accrue any potential benefits of having their AKI averted or having reduced severity (i.e. peak KDIGO stage) AKI. For exploratory scenarios in which a test might not be acted on in practice, the cohort would follow the standard-care pathways according to whether or not they had AKI, as measured using current clinical practice.
There is limited direct evidence to describe the impact of the use of the AKI biomarkers on important health outcomes (such as need for ICU care, length of hospital stay, risk of 90-day mortality or development of new/progression of existing CKD). Therefore, a linked-evidence approach was required, whereby we relied on observational associations to infer how prevention or mitigation of AKI may affect changes in health outcomes. The associative effects are the benefits of averting or mitigating AKI that lead to better health outcomes (i.e. need for ICU care, CKD and mortality).
These associations necessitate causal assumptions, but, although a causal link between AKI and poor outcomes is plausible, the extent of this causal relationship is uncertain and controversial. It cannot necessarily be assumed that, by averting or changing the severity of AKI, a patient would have the exact same risks (associative effects from the Grampian observational data described previously) of ICU and mortality as a patient who was never going to develop AKI in the first place.
As the true causal relationship between AKI and health outcomes is unknown, the model includes the functionality to apply none, all or a proportion of the relative risk (RR) of health outcomes such as need for ICU care, mortality and CKD (AKI vs. none) to the AKI-averted proportion of the cohort. This is achieved while maintaining the observational associations in the standard-care arm of the model.
The base-case analysis assumes that there are no adverse health consequences of a false-positive test on either NephroCheck or NGAL. Clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) indicates that there may be a risk to a patient’s health of inappropriate fluid resuscitation; delay of access to appropriate imaging because of concerns regarding contrast exposure; or removal of the most effective, but potentially nephrotoxic, treatments for a critically ill patient. However, the magnitude of this negative effect is difficult to quantify. Therefore, a sensitivity analysis explores scenarios in which an additional mortality risk is added for false-positive tests.
In summary, the early-stage (up to 90 days) costs and outcomes depend on (1) the diagnostic accuracy of the test, (2) clinical decision-making in the presence of positive or negative test results, (3) the initiation of a KDIGO care bundle to avert AKI and amend the distribution of peak AKI severity and (4) the degree to which the hypothesised associative effects between AKI and final health outcomes, such as length of hospital stay, admission to ICU, need for RRT, 90-day mortality and risk of CKD, can be modified simply by amending the AKI distribution.
Model structure: follow-up Markov model
One potential route to patient benefit is that avoiding AKI or reducing its severity may reduce the risk of later developing CKD. As CKD is defined as a minimum of 3 months of persistent reduced renal function,105,106 progression from AKI to CKD is incorporated into the Markov phase of the model.
Figure 21 illustrates the long-term follow-up model structure.
FIGURE 21.
Markov model structure. Reproduced with permission from Jacobsen et al. 104 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
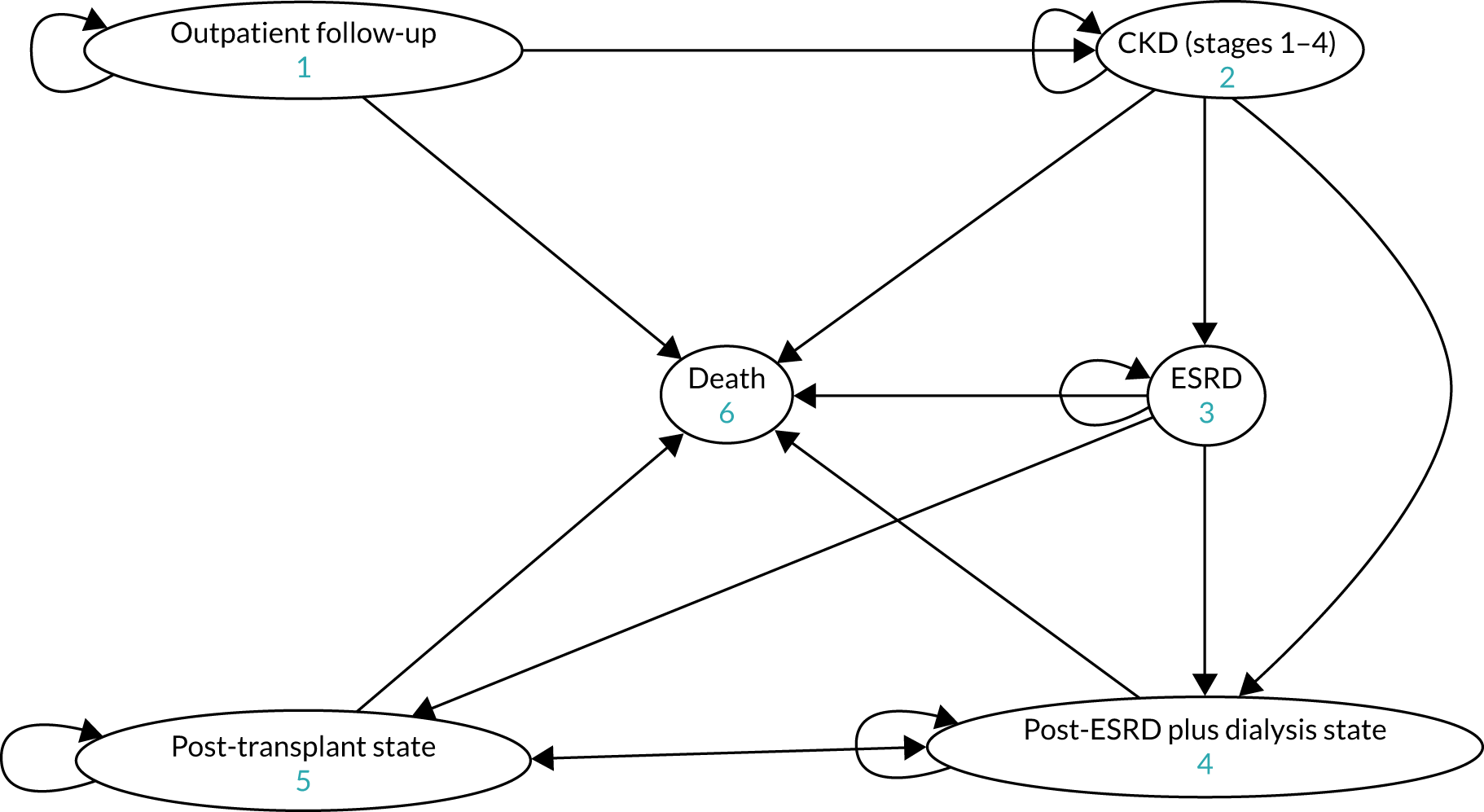
After 90 days, the surviving cohort from each of the decision tree pathways enters a lifetime Markov model. The model follows a similar structure to those of Hall et al. 97 and Parikh et al. ,95 with six mutually exclusive health states: outpatient follow-up, CKD (stages 1–4), ESRD not requiring dialysis, ESRD requiring dialysis, post transplant and death. Members of the cohort enter the model either in the outpatient follow-up state, where they experience an annual baseline risk of developing CKD, or directly in the CKD state, with the proportion starting in the CKD state determined by the underlying CKD prevalence and the severity of AKI from the acute (decision tree) phase of the model. The base-case model assumes that the outpatient cohort have an increased risk of CKD in the first cycle that is dependent on their AKI experience, but, thereafter, the transition between the outpatient follow-up and CKD states is independent of whether or not a patient had AKI in the hospitalisation period. A sensitivity analysis explores the impact of an increased CKD risk applied for the full lifetime time horizon, as per Hall et al. 97
The cohort is then modelled to transition through the disease pathway, starting with CKD stages 1–4 (defined as a single Markov state), to ESRD, with or without the requirement for dialysis, the need for transplant, the success or failure of that transplant, and, ultimately, progression to death, with state-specific mortality probabilities. Those members of the cohort who experience transplant failure are assumed to return to the dialysis health state, in which the probability of a second transplant is the same as the probability of a first transplant. The cohort is exposed to a probability of all-cause mortality from each model state and assigned mortality probabilities based on the higher value of age- and sex-adjusted all-cause mortality or the disease state-specific mortality obtained from the literature.
Model parameters: probabilities and duration of length of stay
Table 16 summarises the probability, LOS and relative effect size parameters used to populate the economic model. Further details and description are provided in the sections that follow.
| Parameter | Mean parameter value | SE | Distribution | Source |
|---|---|---|---|---|
| Incidence of AKIa | ||||
| No AKI | 0.908 | Remainder | Grampian data107 | |
| Any AKI | 0.092 | – | Beta (count) (n = 4314, N = 46,884)b | Grampian data107 |
| AKI 1 (given AKI) | 0.687 | – | Dirichlet | Grampian data107 |
| AKI 2 (given AKI) | 0.194 | – | Dirichlet | Grampian data107 |
| AKI 3 (given AKI) | 0.119 | – | Dirichlet | Grampian data107 |
| Probability of ICU admission | ||||
| No AKI | 0.014 | 0.0038 | Beta | Grampian data102 |
| AKI 1 | 0.100 | 0.0254 | Beta | Grampian data102 |
| RR of ICU admission vs. AKI 1 | ||||
| AKI 2 | 1.423 | 0.1082 | LN vs. AKI 1 | Grampian data102 |
| AKI 3 | 1.930 | 0.1096 | LN vs. AKI 1 | Grampian data102 |
| Probability of 90-day mortality | ||||
| No AKI | 0.049 | 0.0069 | Beta | Grampian data102 |
| AKI 1 | 0.215 | 0.0347 | Beta | Grampian data102 |
| RR of 90-day mortality vs. AKI 1 | ||||
| AKI 2 | 1.602 | 0.0640 | LN vs. AKI 1 | Grampian data102 |
| AKI 3 | 2.151 | 0.0624 | LN vs. AKI 1 | Grampian data102 |
| Probability of requiring RRT | ||||
| No AKI, AKI 1 and 2 | 0 | – | – | Assumption |
| AKI 3 | 0.552 | – | Beta (count) (n = 885, N = 1603) | Truche et al. 2018108 |
| LOS parameters | ||||
| Hospital LOS | Mean | Median | ||
| No AKI | 8.1 | 3 | LN | Grampian data102 |
| AKI 1 | 26.3 | 14 | LN | Grampian data102 |
| AKI 2 | 32.4 | 18 | LN | Grampian data102 |
| AKI 3 | 28.4 | 17 | LN | Grampian data102 |
| ICU LOSc | ||||
| No AKI | 2 | 1 | LN | Bastin et al.109 |
| AKI 1 | 4 | 2 | LN | Bastin et al.109 |
| AKI 2 | 8 | 4 | LN | Bastin et al.109 |
| AKI 3 | 26 | 13 | LN | Bastin et al.109 |
| The effects of early adoption of a KDIGO care bundle (RR)d | ||||
| Mean RRe | SE log RRe | |||
| Any AKI | 0.768 | 0.094 | LN | Meersch et al.110 |
| AKI 1 (given AKI) | 1.232 | 0.180 | LN | Meersch et al.110 |
| AKI 2 (given AKI) | 0.868 | 0.180 | LN | Meersch et al.110 |
| AKI 3 (given AKI) | 0.843 | 0.356 | LN | Meersch et al.110 |
| Parameters linking AKI and CKD | ||||
| Mean | SE | |||
| Prevalence of CKD (starting proportion) | 0.1105 | – | Beta (count) (n = 5935, N = 53,691) | Grampian data102 |
| Baseline incidence of CKD | 0.0044 | 0.0003 | Beta | Rimes-Stigare et al.111 |
| Mean HR | Log SE | |||
| HR of CKD (given AKI 1) | 2.32 | 0.0363 | LN | See et al.112 |
| HR of CKD (given AKI 2) | 4.00 | 0.5656 | LN | See et al.112 |
| HR of CKD (given AKI 3) | 7.98 | 0.9675 | LN | See et al.112 |
| Markov model transition probabilities | ||||
| Mean | SE | |||
| Outpatient to CKD | 0.0044 | 0.0003 | Beta | Rimes-Stigare et al.111 |
| CKD to death | 0.03 | 0.002 | Beta | Kent et al.113 |
| CKD (survivors) to ESRD | 0.01 | 0.001 | Beta | |
| CKD (survivors) to ESRD plus dialysis | 0.04 | 0.002 | Beta | |
| Remain with CKD | Remainder | |||
| ESRD to death | 0.12 | 0.005 | Beta | Kent et al.113 |
| ESRD (survivors) to ESRD plus dialysis | 0.18 | 0.006 | Beta | |
| ESRD (survivors) to transplant | 0.09 | 0.004 | Beta | |
| Remain ESRD, no dialysis | Remainder | |||
| Alpha | Beta | |||
| Outpatient to deathf | No ICU
|
No ICU
|
Beta (count) | Lone et al.114 |
ICU
|
ICU
|
|||
| ESRD plus dialysis to death |
|
|
Beta (count) | UK Renal Registry (table 1.17)115 |
| ESRD plus dialysis to transplant |
|
|
Beta (count) | |
| Remain in ESRD plus dialysis | Remainder | |||
| Transplant to ESRD plus dialysis |
|
|
Beta (count) | UK Renal Registry (table 1.17)115 |
| Transplant to death |
|
|
Beta (count) | |
| Transplant successful | Remainder | |||
Early phase probabilities and length of stay
The potential associative links between AKI and ICU admission, ICU LOS, hospital LOS and 90-day mortality are all sourced from the Grampian data set. 102 For chance nodes in the decision tree with only two possible branches, probabilities are sampled from beta distributions. Where there are three or more branches, probabilities are incorporated using Dirichlet distributions.
The model assumes, based on expert opinion, and consistent with Hall et al. ,97 that RRT is provided in AKI stage 3 only; this is deemed reflective of most current clinical practice. Assuming no RRT for patients who have a peak AKI of stage 1 or 2 might be considered a favourable scenario for biomarker tests that can reduce AKI severity, thereby generating reductions in cost. In the absence of published UK data, the proportion of AKI stage 3 patients requiring RRT is taken from a retrospective analysis of 5242 ICU survivors with AKI across 23 French ICUs. 108 A total of 1603 of these survivors had KDIGO AKI stage 3, of whom 55.2% received RRT. It is assumed that the French ICU setting is broadly transferable to a UK pre-ICU setting for critically ill patients and is therefore appropriate for populating the model. Data reported from Hall et al. 97 are not used because they relate to only a single UK ICU setting with a small sample of patients with AKI 3 patients (n = 18). The EAG’s clinical experts validated these data as relevant to the UK setting and noted that the probability was lower than that applied in Hall et al. ,97 which was consistent with clinical experience outside the ICU setting. Moreover, more detailed data on the need for RRT in England are currently being collected by the UK Renal Registry, but are not yet publicly available.
There are potentially strong associations between AKI status or severity of AKI and the probability of needing ICU care and of dying within 90 days of hospital admission. However, these data should not be interpreted as definitive causative effects and a sensitivity analysis explores the application of different assumptions around these highly uncertain associations.
Data for LOS in hospital are obtained from the Grampian data set,102 but ICU LOS was unavailable by peak AKI status. ICU LOS data were therefore obtained from an alternative source, Bastin et al. ,109 a large cohort study of 1881 adults who had cardiac surgery (and who were, therefore, deemed critically ill and sufficiently matched the scope for this assessment). Bastin et al. 109 reported median LOS in ICU by AKI stage (according to AKIN and KDIGO criteria). Given the likely skewed distribution of LOS data, a log-normal distribution fitted to mean and median days’ duration is used to generate the simulated draws for the probabilistic analysis. As mean LOS in ICU was not available to parameterise the log-normal distribution, it was assumed that the mean was twice the median, reflecting the ratio of mean to median days’ stay as reported in Hall et al. ,97 who obtained the data from the Leeds Teaching Hospitals NHS Trust, AKI registry data, for ICU patients.
As the variable ‘hospital LOS’ also includes the time spent in ICU, the time on a hospital ward is obtained by subtracting the ICU LOS from the total hospital LOS for the application of costs and utilities in the model. As the probabilistic analysis samples independently from these distributions, an additional correction is added to the model to ensure that LOS in an ICU cannot exceed total hospital LOS in any of the sampled draws. The average LOS in hospital/ICU for those with a peak AKI of 3 is applied to both those requiring and those not requiring RRT. The assumption that requirement for RRT would not usually extend the hospital admission for this patient cohort has been validated by the EAG’s clinical experts.
The relative effects of diagnostic biomarkers on acute kidney injury and clinical outcomes: the impact of early adoption of a Kidney Disease: Improving Global Outcomes care bundle
The impact of an early Kidney Disease: Improving Global Outcomes care bundle on acute kidney injury
National-level guidelines16 indicate that, in a patient defined as being at risk of developing AKI through a positive biomarker result, all appropriate efforts should be made to ensure that AKI does not develop, and, if it does, it should be minimised in terms of severity (i.e. providing the maximum support possible for the kidneys). The model therefore assumes that all AKI patients will receive a KDIGO care bundle; the only difference between the testing strategies is the duration for which that bundle is implemented, with earlier implementation assumed to incur additional resource use in terms of fluid management, nephrologist review and pharmacist review of medications, as well as the removal of any potentially nephrotoxic agents when necessary. 5
There are two potential mechanisms by which early adoption of a KDIGO care bundle might lead to patient benefit. These are to (1) avert AKI in people in whom it would otherwise develop and (2) shift the distribution of AKI severity (between KDIGO AKI stages 1–3), if AKI occurs.
Hall et al. 97 conducted a review of the literature to identify studies testing the impact of early preventative intervention for AKI. Their searches identified eight studies relevant to early intervention in the UK setting (excluding early RRT, which was deemed contentious). Four studies explored the impact of early nephrologist involvement, which was deemed to be the most reflective proxy for the non-specific care bundles that a patient may access as part of the KDIGO care bundle recommendations. 5 The largest of these four studies, with a sample of 1096 participants, was used in the Hall et al. 97 economic model, and found that early nephrologist consultation reduced AKI incidence: adjusted odds ratio (early involvement vs. not) 0.71 (95% CI 0.53 to 0.95).
The EAG has conducted a supplementary targeted search of trials for the post-Hall et al. 97 period to identify any further potentially relevant studies exploring the impact of early preventative intervention or application of AKI care bundles on the probability of developing AKI and/or the severity of peak AKI. In brief, 39 additional titles and abstracts were identified from the targeted searches, of which 17 (44%) were full-text assessed. Based on the NICE scope,100 KDIGO care guidelines5 and clinical expert opinion (Simon Sawhney, University of Aberdeen, 2019, personal communication), it was decided that studies testing the impact of a KDIGO care bundle provided the most appropriate source of data to populate the economic model. Three trials110,116,117 (18%) assessed the effect of NephroCheck-guided application of a KDIGO bundle compared with standard care where information about the NephroCheck test result was not available to a patient’s hospital care team. No studies assessed the impact of NGAL-guided treatment.
All three studies reported results in terms of the probability of developing AKI. 110,116,117 However, only one study110 described the impact on both the incidence and severity of AKI. Meersch et al. 110 reported the results of a single-centre trial, with a sample of 276 participants, in a German setting. All of the population had positive NephroCheck test results, using a 0.3 mg/dl threshold, consistent with the sources of diagnostic accuracy data obtained from the systematic review of diagnostic accuracy studies (see Chapter 3). Patients were then randomised to receive a strict implementation of the KDIGO guidelines or standard care. The intervention group included avoidance of nephrotoxic agents, discontinuation of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), close monitoring of urine output, close monitoring of serum creatinine levels, avoidance of hyperglycaemia (for 72 hours), consideration of alternatives to radiocontrast agents, and fluid optimisation. In the control (standard-care) group, Meersch et al. 110 state that the recommendations of the American College of Cardiology Foundation 2011 were followed, including keeping mean arterial pressure at > 65 mmHg and central venous pressure at between 8 and 10 mmHg. ACEIs and ARBs were used only when the haemodynamic situation stabilised and hypertension occurred. It is unclear whether or not knowledge of the NephroCheck test result was revealed to the treating hospital team for patients in the standard-care arm of the study. The primary outcome in Meersch et al. 110 was 72-hour AKI and the trial found an absolute risk reduction of 16.6% (95% CI 5.5% to 27.99%). The Meersch et al. 110 study was supported by the German Research Foundation (Bonn, Germany), the European Society of Intensive Care Medicine (Brussels, Belgium), the Innovative Medizinische Forschung (Münster, Germany) and an unrestricted research grant from Astute Medical, Inc.
A second, smaller study (121 participants),116 also in a German setting, showed that NephroCheck-guided care demonstrated a trend towards a lower probability of AKI, although the results were not statistically significant, with an OR for standard care versus NephroCheck of 1.96 (95% CI 0.93 to 4.10). The study did, however, find significantly greater odds of AKI (defined as stage 2 and 3 combined) in the standard care group than in the NephroCheck group: OR for standard care versus NephroCheck 3.43 (95% CI 1.04 to 11.32). A third study,117 with only 100 participants, compared the effect of a NephroCheck-triggered consultation implementing KDIGO recommendations for AKI with the effect of standard care in an ED in Germany. AKI outcomes were similar in both groups. The probability of AKI stage 2 or 3 at day 1 post admission was 32.1% in the intervention group and 33.3% in the control group; at day 3, it was 38.9% in the intervention group and 39.1% in the control group. Neither the Göcze et al. 116 study nor the Schanz et al. 117 study reports any funding involvement from the test manufacturers.
As the Meersch et al. 110 study has a larger sample, and reports data for both the probability of AKI and the distribution of AKI severity, given that it occurs, these data were used for the model base-case analysis. Although the clinical context of the immediate postoperative period after cardiac surgery from Meersch et al. 110 is likely to be generalisable between the UK and other countries, the nature of the AKI insult (ischaemia/reperfusion, postoperative haemodynamic, oxidative stress, haemolysis, in people with cardiac comorbidity) is specific to this context, as is acknowledged by the authors. Accordingly, this study110 may not be generalisable to AKI in the context of other acute or critical illness circumstances in which biomarker performance and the potential for AKI prevention/mitigation may be different.
The model describes the potential impacts of a biomarker-guided care bundle on (1) the chance that patients may develop AKI and (2) the severity of AKI given that it occurs. The assumption is that early biomarker-guided implementation of the KDIGO care bundle may reduce the proportion of people who develop AKI and help ensure that, if they do develop AKI, it will be of reduced severity. These effects are applied probabilistically as RRs in the model for those with true-positive test results only, using log-normal distributions.
In the absence of any data on the impact of NGAL-guided KDIGO care bundles on the probability of developing AKI or the severity of AKI, the base-case model assumes that the potential to avert AKI is similar for both biomarkers. However, based on clinical expert opinion (Simon Sawhney, personal communication) and the manufacturer-described role of the tests, NGAL measures injury and can be used to define AKI, whereas NephroCheck can identify stress, thereby enabling intervention before AKI develops. Therefore, a sensitivity analysis explores a scenario in which the RR of AKI for NGAL-guided care is equal to 1, while retaining the same effect on the AKI distribution, given that AKI occurs as for NephroCheck. It is acknowledged that these assumptions are uncertain, and the sensitivity analysis may present a bias against NGAL if data were to become available to suggest an effect on AKI prevention.
It was assumed that there are no negative health effects of early intervention for the proportion of each test group with false-positive results, but the additional costs of the bundle were still incurred. The model also includes the functionality to explore the impact of an additional mortality risk, for example because of excessive resuscitation as a result of fluid administration or removal of effective, but nephrotoxic, treatments in patients with a false-positive test result.
Although the model describes the impact of biomarker-guided early intervention on the distribution of AKI, it is unclear whether or not these effects translate into final clinical and patient-relevant health outcomes, such as need for ICU care, need for RRT, mortality or the development of CKD. The limited evidence that exists from Meersch et al. 110 suggests that, although there is a significant reduction in the primary study outcome of AKI within 72 hours for NephroCheck-guided implementation of a care bundle, compared with standard care (OR 0.483, 95% CI 0.293 to 0.796), this ability to avert AKI was not demonstrated to translate into improvements in a range of clinical and patient-relevant outcomes, including need for RRT therapy in hospital (OR 1.618, 95% CI 0.676 to 3.874), 90-day all-cause mortality (OR 1.213, 95% CI 0.486 to 3.028), ICU LOS (median difference 0 days, 95% CI –1 to 0 days) or hospital LOS (median difference 0 days, 95% CI –1 to 1 days). Although the study was not powered to detect differences in these outcomes, there are no trends in the data that are suggestive of an effect size. Furthermore, the uncertainty regarding the link between increased resource use and clinical outcomes is emphasised by Wilson et al. ,118 who demonstrated in their RCT of an electronic alert system for AKI that an early warning system increases resource use (e.g. renal consultation), but with no evidence that this translates into measurable clinical or patient benefit in terms of mortality or LOS. Indeed, for a subgroup on a surgical ward, the mortality rate was significantly higher in the electronic alert group. As these causal links between AKI and changes in health outcomes are highly uncertain and hypothesised based on observational data in the model, extensive sensitivity analyses are conducted to test the impact of a range of plausible assumptions on cost-effectiveness.
Follow-up phase probabilities
Starting proportions applied in Markov cohorts
One plausible route to patient benefit from averting or reducing the severity of AKI is through the prevention of new CKD and the indirectly associated longer-term progression to ESRD and transplant. It should be noted that the model does not assume a direct effect of peak AKI on ESRD at 90 days; therefore, patients can enter the Markov model in either the outpatient follow-up or CKD (stages 1–4) health states only. A reanalysis of 2012 data from the Grampian cohort102 indicated that only a very small proportion [13/4314 (≈ 0.03%)] of patients with AKI, almost all of whom had underlying CKD, progressed directly to ESRD at 90 days. Therefore, we assumed no direct transition from the decision tree to the ESRD state in the Markov model. The starting proportions (after 90 days) for each health state are dependent on the decision tree pathway through which the cohort has come, and the peak AKI severity the cohort experienced. The baseline prevalence of CKD in the UK general population has been estimated from Kerr119 at 6.1%. However, in a group of critically ill, hospitalised patients, this prevalence may be substantially higher. For the base-case analysis, we use the underlying prevalence of CKD in the Grampian data set,102 calculated as the prevalence of CKD in all hospitalised patients having their kidney function monitored. Multiplying through by the sampling fraction for no CKD (20%) and taking the proportion of CKD/full sample gives the baseline prevalence in this group, calculated as (5935/53,691) × 100% = 11.05%.
Health-state transition probabilities
The baseline incidence of new-onset CKD for the Markov model uses the same source as Hall et al. ,97 with an annual probability of progressing from the outpatient state to the CKD state of 0.0044 (95% CI 0.0039 to 0.0049) for patients in the no-AKI cohort. 111 The data are obtained from a large cohort study of 97,782 ICU patients enrolled on the Swedish intensive care register. The parameter value 0.0044 reflects the CKD incidence at 1 year post ICU admission for the proportion of patients with no AKI. The same baseline proportion of CKD was applied for those without AKI and for those modelled to have had AKI averted as a result of early preventative treatment. The proportion of the no-AKI cohort starting in the CKD state at day 90 was calculated as the underlying prevalence plus the new annual incidence, adjusted to the 90-day time horizon of the decision tree component of the model.
Hazard ratios for AKI 1, AKI 2 and AKI 3 on the development of CKD (defined as CKD stage ≥ 3) were obtained from a systematic review by See et al. 112 The review included a total of 82 studies quantifying the association between AKI and longer-term renal outcomes (including CKD) and mortality. However, only three studies reported the impact of each stage of AKI on CKD development. 120–122 One study (104,764 participants) in a US setting generated slightly counterintuitive results, with point estimates of the HR reducing as AKI stage increased. 120 However, two other studies in Asian settings (with 77122 and 1363121 participants) illustrated an increasing HR for more severe AKI stages. The systematic review has meta-analysed these three studies; the summary effects by AKI stage on CKD, defined as CKD stage 3, are used in the base-case analysis. The advantage of these studies is that they allow a demonstration of the impact of adapting the distribution of AKI severity on longer-term development of CKD. However, they are not conducted in a UK setting and may lack relevance. An alternative source, reporting the HR for the association between AKI and CKD that is constant across all AKI stages, is reported by Sawhney et al. 102 for 9004 hospitalised patients with AKI in Grampian. The HR for development of stage 4 CKD (AKI vs. no AKI) was 2.55 (95% CI 1.41 to 4.64). This study has the advantage of relevance to the setting, but does not include risks by AKI severity. However, it should be noted that the definition of CKD is stage 4 in Sawhney et al. ,102 compared with stage 3 in the meta-analysed studies, which may limit the comparability of the reported HRs.
The HRs of CKD by AKI stage are applied to the new incidence over the first 90 days and to the first annual transition in the model. Thereafter, the transition probabilities from outpatient follow-up to CKD follow the baseline 0.0044 per year. This approach is based on expert opinion (Simon Sawhney, personal communication) that any longer-term effect of AKI on CKD development will become attenuated over time, particularly if it has not occurred in the first year following hospital discharge. A sensitivity analysis explores a scenario in which the HR of CKD is applied for the full duration of the model, reflecting the assumption applied in Hall et al. 97
Prevalence of CKD and incidence of new-onset CKD are parameterised in the model using beta distributions, and the HRs for the effect of peak AKI severity on CKD incidence (i.e. transition probabilities to CKD state) are parameterised using log-normal distributions.
Progression from chronic kidney disease
The transition probability from outpatient follow-up to CKD is 0.0044, as described previously. The model cohort can then progress from CKD to ESRD, with or without dialysis, and from ESRD to transplant according to the modelled transition probabilities. It is assumed that AKI can influence only the number of people who get CKD and then has no further direct effect on how fast they progress through the CKD stages to ESRD, dialysis or transplant. The cohort is also exposed to an increasing mortality risk as it progresses through more severe disease states from CKD (stages 1–4) to ESRD without dialysis and ESRD with dialysis. Transitions from CKD (stages 1–4) to ESRD, from ESRD (no dialysis) to ESRD (with dialysis), and from CKD (stages 1–4)/ESRD to death are obtained from Kent et al. ,113 who reported data on progression of kidney disease from the large (7246 participants), international (Europe, North America and Australasia) Study of Heart and Renal Protection (SHARP) RCT. The median study follow-up was 4.9 years, participants had a mean age of 63 years and 64% of participants were male.
For those with ESRD on dialysis, the proportions transitioning to kidney transplant and mortality were obtained from 5-year data published in the 2018 UK Renal Registry report (table 1.17),115 which provided information on transition from incident RRT in 2012 to transplant and mortality 1, 3 and 5 years later. The 3- and 5-year probabilities were annualised; the 3-year probability was applied to years 2 and 3, and the 5-year probability was applied to year 4 onwards. These probabilities were converted to the relevant annual cycle-specific probabilities and applied in the model using tunnel states to track time from entering a given health state. The UK Renal Registry also provided data on the probability of transition back to dialysis for failed transplants and the probability of death over 5 years following transplant. After 5 years post transplant, mortality is assumed to revert to the general population all-cause mortality probability and the annual probability of transplant failure remains at that reported from years 3–5 in the UK Renal Registry. It is further assumed that the proportion of the cohort with a transplant failure return to dialysis, and that their probability of progressing from ESRD on dialysis to a second transplant is the same as the probability of progression to the first transplant.
In the first 5 years of the follow-up phase of the model, mortality in all Markov states is modelled as the average mortality risk for patients discharged from hospital and ICU, unless health state-specific (ESRD, dialysis or transplant) mortality is higher, in which case the latter is applied. If, at any point, mortality falls below all-cause mortality, all-cause mortality is applied in the model. The 5-year post-discharge mortality data were based on Lone et al. ,114 a matched UK cohort study (mean age of 60 years) using national registries: the Scottish Intensive Care Society Audit Group, the Scottish Morbidity Record (SMR) of acute hospital admissions (SMR01) and the Scottish death records. The model base case used an average of the ICU and non-ICU cohorts. Beyond 5 years, patients in the outpatient follow-up health state had the age- and sex-adjusted all-cause mortality probability applied,123 and those with CKD, ESRD, chronic dialysis or a transplant would be assigned the health state-specific mortality probability, unless the age- and sex-adjusted all-cause mortality was higher than the health state-specific mortality. A sensitivity analysis explores the impact of assigning long-term mortality risks that are dependent on whether or not the cohort had been admitted to ICU during the index hospitalisation.
Transition probabilities are incorporated into the model probabilistically using beta distributions. As the cycle lengths for the model in Hall et al. 97 are the same as the current assessment (annual), it was not necessary to provide any further adjustment of the published transition probabilities.
Model parameters: costs
The health-care costs included are as follows: (1) the costs of conducting the tests, including equipment and staff resource use; (2) the costs of acute care in the first 90 days post hospital admission, including the additional cost of early application of a KDIGO care bundle, the cost of hospital/ICU LOS, and the cost of acute RRT; and (3) the annual, cycle-specific costs associated with Markov health states (CKD, ESRD, dialysis and transplant) over the longer-term follow-up phase. All costs are included from an NHS perspective and are reported in 2017/18 Great British pound values. When possible, resource use has been costed directly using 2017/18 UK national unit cost sources [the Personal Social Services Research Unit (PSSRU) for staff time,124 NHS reference costs for secondary care procedures125 and the British National Formulary for drugs126]. When this has not been possible, for example if total costs are reported in the literature without enough data regarding the underlying resource use to enable re-costing, these costs are inflated from their base year to 2017–18 values using the Campbell and Cochrane Economics Methods Group online inflation calculation tool. 127 Table 17 details the cost parameters used in the economic model. Full details of the costing approach and associated assumptions are provided in the following sections.
| Parameter | Mean parameter value (£) | Standard error (£) | Distribution | Source |
|---|---|---|---|---|
| Test costs | ||||
| NephroCheck | 92.26 | – | Applied deterministically | See Appendix 13, Table 31 |
| BioPorto (urine and plasma) NGAL | 59.55 | – | Applied deterministically | See Appendix 13, Table 31 |
| ARCHITECT urine NGAL | 66.87 | – | Applied deterministically | See Appendix 13, Table 31 |
| Costs incurred up to 90 days (acute decision tree phase of the model) | ||||
| Three days of KDIGO care bundle | 106.36 | 10.64 (mean × 10%) | Gamma | See Appendix 13, Table 32 |
| Hospital ward setting – daily cost | 313 | 38.27 | Gamma | NHS reference costs 2017/18125 |
| ICU setting – daily cost | 1395 | 251.38 | Gamma | NHS reference costs 2017/18.125 SD calculated using quartiles published from 2015/16 reference costs inflated to 2018 valuesa |
| Excess daily cost of AKIb | 298 | 65.65 | Gamma | NHS reference costs 2017/18.125 SD calculated using quartiles published from 2016/17 reference costs inflated to 2018 valuesa (applied in sensitivity analysis only) |
| Estimated daily cost of RRTc | 197 | Applied deterministically | NHS reference costs 2017/18;125 assumes 48% on intermittent haemodialysis/52% on continuous haemodialysis, from the 2009 National Confidential Enquiry into Patient Outcome and Death report3 | |
| Follow-up costs applied in the Markov model (day 90 +) | ||||
| Annual follow-up costs for the proportion of the cohort that were not admitted to an ICU during the acute phased | ||||
| Year 1 | 3954 | 158 | Gamma | Lone et al.114 |
| Year 2 | 2864 | 139 | Gamma | |
| Year 3 | 2547 | 140 | Gamma | |
| Year 4 | 2277 | 129 | Gamma | |
| Year 5 | 2090 | 125 | Gamma | |
| Year 6 | 1794 | 125 (assumption) | Gamma | Calculation based on Lone et al.114 Costs from year 6 onwards applied in sensitivity analysis only |
| Year 7 | 1618 | 125 (assumption) | Gamma | |
| Year 8 | 1465 | 125 (assumption) | Gamma | |
| Year 9 | 1331 | 125 (assumption) | Gamma | |
| Year 10 | 1210 | 125 (assumption) | Gamma | |
| Years 11 + | 1102 | 125 (assumption) | Gamma | |
| Annual follow-up costs for the proportion of the cohort that were admitted to an ICU during the acute phased | ||||
| Year 1 | 6500 | 198 | Gamma | Lone et al.114 |
| Year 2 | 4183 | 163 | Gamma | |
| Year 3 | 3975 | 176 | Gamma | |
| Year 4 | 3774 | 190 | Gamma | |
| Year 5 | 3315 | 172 | Gamma | |
| Year 6 | 2806 | 172 (assumption) | Gamma | Calculation based on Lone et al.114 Costs from year 6 onwards applied in sensitivity analysis only |
| Year 7 | 2521 | 172 (assumption) | Gamma | |
| Year 8 | 2274 | 172 (assumption) | Gamma | |
| Year 9 | 2056 | 172 (assumption) | Gamma | |
| Year 10 | 1861 | 172 (assumption) | Gamma | |
| Years 11 + | 1685 | 172 (assumption) | Gamma | |
| Health state-specific costs applied in the Markov model | ||||
| CKD stages 1–3 | 453 | 33.53 | Gamma | Kent et al.113 |
| CKD stage 4 | 441 | 14.61 | Gamma | Kent et al.113 |
| Weighted average (CKD stages 1–4) | 446 | – | – | – |
| ESRD (no dialysis)e | 590 | 43.84 | Gamma | Kent et al.113 |
| ESRD year 1 (with dialysis) | 21,328 | 209.77 | Gamma | Kent et al.113 |
| ESRD year 2 onwards (with dialysis) | 26,203 | 54.45 | Gamma | Kent et al.113 |
| Functioning transplant year 1 | 27,636 | 329.84 | Gamma | Kent et al.113 |
| Transplant follow-up | 1290 | 97.43 | Gamma | Kent et al.113 |
| Additional medication costs applied to health states | ||||
| ESRD year 1 (with dialysis) | 2601f | – | Applied deterministically | NICE guidance 2015128 and BNF 2019126 |
| ESRD year 2 onwards (with dialysis) | 2601f | – | Applied deterministically | NICE guidance 2015128 and BNF 2019126 |
| Functioning transplant year 1 | 10,623 | – | Applied deterministically | NICE guidance 2015128 and BNF 2019126 |
| Transplant follow-up | 9063 | – | Applied deterministically | NICE guidance 2015128 and BNF 2019126 |
Diagnostic test costs
NephroCheck testing is usually conducted on an Astute140 Meter, costing £3000, and an additional meter would need to be purchased. This cost was converted to an annuity, assuming that the platform’s lifetime is 5 years and an annual depreciation rate of 3.5%. The test could also be conducted on a VITROS Immunodiagnostic System, although, currently, there is a limited installed base of these in UK hospitals,97 which was confirmed at a NICE scoping workshop. The NGAL tests would not require a new platform for NGAL only, because it would be performed on platforms already available at the hospital laboratories. The capital costs of the laboratory analyser apportioned to each NGAL test are assumed to be negligible. The sensitivity analysis excludes capital and training costs to explore the impact on cost-effectiveness of scenarios in which a hospital might already have the required analyser in place and all staff are fully trained in its use.
The process of taking the sample for analysis, sending samples to the laboratory, processing at the laboratory and interpretation of test results would require the involvement of several members of the hospital team. A urine sample is first collected by a nurse, and then picked up by a porter, who takes it to the laboratory. It is assumed that, because the tests are classified as urgent samples, the porter would generally prioritise single test collection for the laboratory. A biomedical scientist conducts the diagnostic test in the laboratory. After completion of the test, the results from the laboratory would be authorised by a biochemist and released for review on the hospital information management system, where they can be interpreted by a nephrologist, an intensive care specialist or a junior doctor. The base-case analysis assumes an average of the three health-care professional costs for interpretation. Under some criteria (such as very abnormal results), a laboratory team might directly contact the care provider, but we assume that this approach would not be used routinely. For the purposes of test cost calculation, it is assumed that, on average, the role of interpreting the tests is equally split across the three specialist team members. The unit costs per hour for each of the staff resources involved in the testing process were obtained from PSSRU 2018:124 to conduct the test – band 5 nurse (£37.00), porter costed as health-care assistant (£27.26) and band 6 biomedical scientist (£44.00); to interpret the test – medical consultant/nephrologist (£108) and junior doctor foundation year 2 (£32.00). It is assumed that a hospital consultant, nephrologist and junior doctor are all equally likely to be the health-care professional interpreting the test.
The duration of resource use for each member of the team is based on a combination of information provided by the manufacturer and clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) regarding the flow from obtaining the test sample to result interpretation. The staff time to process the test in the laboratory was based on the NICE request for information documents to the different test manufacturers and the final scope (NICE technical team, 2019, personal communication). Estimates of the time taken to prepare the urine sample and interpret the test were based on the EAG’s clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication).
Four test strategies were compared in the economic model: NephroCheck, BioPorto urine NGAL, ARCHITECT urine NGAL and BioPorto plasma NGAL. The NGAL test manufacturer BioPorto has not identified costs separately by sample type (plasma or urine). It is therefore assumed that these tests incur equal costs. The cost of the Alinity i urine NGAL test was not considered in the base-case economic evaluation because the review identified no diagnostic accuracy data for the test. Full costing of each test is provided in Appendix 13, Table 31. Further details of maintenance costs and consumables for each test can be found in Appendix 13, Table 34.
Cost of early treatment
The additional cost of early treatment with the KDIGO care bundle was calculated as £106.36 per patient treated, assuming an additional 3 days’ application of the care bundle in test-positive patients. An additional 3 days of treatment was assumed in line with the primary outcome from Meersch et al. 110 (i.e. AKI at 72 hours) and based on clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) that a care bundle could be implemented for up to an extra 3 days. The care bundle cost is based on the NICE guidelines for preventing AKI,16 which state that measures to prevent AKI are avoidance of nephrotoxic agents, discontinuation of medication (ACEIs and ARBs), close monitoring of serum creatinine and urine output, avoidance of hyperglycaemia, alternatives to radiocontrast and close haemodynamic monitoring. The NICE recommendations for preventing AKI16 include seeking advice from a nephrology team with regard to giving ‘iodinated contrast agent to adults with contraindications to intravenous fluids’ (© NICE 2013 Acute Kidney Injury: Prevention, Detection and Management. Clinical Guideline [CG169]. 16 Available from www.nice.org.uk/Guidance/CG169. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication) and from a pharmacist with regards to medications (ACEIs, ARBs). Therefore, both nephrologist and pharmacist time are included in the cost of the care bundle. Further details of the cost calculation approach can be found in Appendix 13, Table 32. The additional cost of early adoption of the care bundle was applied to the proportion of the cohort with a positive biomarker test result, reflecting an assumption that care would be delivered for an additional 3 days over and above the cohort monitored using serum creatinine alone. The cost was applied using a gamma distribution with a standard deviation of 10% of the mean.
Acute phase costs
The base-case total cost in the acute phase (90 days) of the model depends on the number of days spent in hospital and the ICU. It also depends on the duration of acute RRT delivered to the proportion of AKI stage 3 patients receiving RRT. For the base-case analysis, data from the Adding Insult to Injury report show that 52% of RRT patients receive continuous RRT (daily) and 48% receive intermittent dialysis (an average of three sessions per week). 3 The duration of RRT delivery is obtained from a randomised trial conducted in a US critical care setting129 comparing intensive (6 days per week; n = 563) with less intensive (3 days per week; n = 561) RRT strategies. The mean duration of RRT per patient was similar in both groups: intensive, 13.4 days (SD 9.6 days); n = 563; and less intensive, 12.8 days (SD 9.3 days); n = 561. The base-case model conservatively assumes the less intensive duration for the application of costs in the economic model. To incorporate the uncertainty and to reflect the likely skewed nature of the distribution, the duration of RRT is incorporated probabilistically into the model using a log-normal distribution. Data available from an alternative source,130 as used in NICE guidance for the comparison of early and late RRT, were not considered because median, rather than mean, durations were reported and the data were assessed as being of low quality in the NICE guidance. 16 An additional daily excess cost of AKI was applied in a sensitivity analysis to capture the potential excess cost per day in hospital or an ICU of an AKI patient. This excess cost was not applied in the base-case scenarios because it was assumed that the cost of having AKI is captured in the cost of being in hospital or an ICU. All other acute costs and follow-up costs have a gamma distribution applied.
Long-term follow-up costs
There are four ways in which long-term follow-up costs may be driven by the proportion of the cohort that progress through different pathways from the initial decision tree. These are (1) whether or not long-term follow-up costs depend on whether or not a patient received ICU care in the initial decision tree, (2) whether or not there are additional follow-up costs beyond 5 years’ post index hospitalisation discharge, (3) whether or not an excess long-term cost is applied for the proportion of the cohort coming through AKI arms of the decision tree and (4) health state-specific costs incurred as the cohort progress through CKD stages to dialysis or transplant.
The outpatient follow-up costs in the Markov model post index hospitalisation discharge were obtained from Lone et al. ,114 who reported 5 years of follow-up costs post index ICU and hospital discharge, using a matched cohort obtained from registries in Scotland [Scottish Intensive Care Society Audit Group, the SMR of acute hospital admissions (SMR01) and Scottish mortality data]. The base-case analysis assumes that the average of post-ICU and post-non-ICU admissions is applied in the Markov model. This is because patients in the cohort for this assessment are already deemed to be critically ill and at risk of needing ICU care, so might all be expected to have significant resource use post discharge. A sensitivity analysis allows the application of differential long-term costs that depend on whether or not a patient had received ICU care in the first 90 days.
The annual costs beyond 5 years are unknown. Therefore, the base-case analysis assumes no additional costs beyond year 5. A sensitivity analysis explores the impact of these assumptions by applying further costs between years 6 and 11 that reduce annually following a logarithmic function, with year 11 costs applied for the remaining duration of the model.
The base-case analysis assumes that there are no long-term excess follow-up costs as a result of having had AKI in the initial 90 days post hospitalisation. However, a sensitivity analysis explores a scenario in which patients entering the Markov model having had AKI in hospital incur an additional 15% of the non-AKI cohort costs for the first 5 years. The additional AKI cost factor was based on a proxy using the RR reported in Lone et al. 114 on the number of admissions that patients on RRT had over 5 years, compared with those who were not on RRT. These additional costs are applied in the model as a sensitivity analysis, with a mean ratio of 1.15, log standard error 0.074, sampling from a log-normal distribution.
Annual cycle-specific health-state costs were applied to the proportion of the cohort transitioning through the CKD, ESRD, ESRD on dialysis and transplant health states. Costs were obtained from Kent et al. ,113 using data from the SHARP trial reporting outpatient, day case and inpatient admissions. The CKD (stages 1–4) health-state cost applied in the model was calculated as the weighted average of CKD stages 1–3 and CKD stage 4, as reported in Kent et al. 113 Therefore, the average weighted cost applied was £445.98 per cycle. The cost of medications (immunosuppressants for transplant patients, erythropoiesis-stimulating agents for dialysis patients and blood pressure medications for dialysis patients) were not captured in the study; therefore, these costs were added to the costs observed in Kent et al. 113 The added transplant costs (immunosuppressants) were based on the approach applied in Scotland et al. 131 for calculating the annual cost of immunosuppressants, using 2018 prices. The added costs to dialysis patients arising from blood pressure medications and erythropoiesis-stimulating agents are also based on the approach applied in Scotland et al. ,131 with 2018 prices.
Health measurement and valuation
Table 18 summarises the utilities used throughout the economic model. These are described in more detail in the sections that follow. A full list of studies and utility values considered for population of the economic model can be found in Appendix 13, Tables 33, 37 and 38.
| Parameter | Mean parameter value from source | Standard error | Age-adjusted utility applied in the model | Distribution | Source |
|---|---|---|---|---|---|
| Utilities applied in the acute (decision tree) phase of the model | |||||
| ICUa | –0.402 | 0.02 | –0.402 | Normal | Kind et al.132 (appendix B) |
| Ward | 0.44 | 0.0259 | 0.432 | Beta | Hernández et al.133 |
| Discharge | 0.62 | 0.0268 | 0.608 | Beta | Hernández et al.133 |
| Acute dialysis decrementb | –0.11 | 0.02 | –0.11 | Beta | Wyld et al.134 |
| Death | 0 | – | 0 | – | |
| Utilities applied in the chronic (Markov) phase of the model | |||||
| Post discharge (year 1) | 0.666 | 0.016 | 0.655 | Beta | Cuthbertson et al.135 |
| Post discharge (years 2–4) | 0.701 | 0.016 | 0.689 | Beta | Cuthbertson et al.135 |
| Post discharge (year 5 onwards) | 0.677 | 0.017 | 0.665 | Beta | Cuthbertson et al.135 |
| CKD (stages 1–4)c,d | – | – | 0.575 | Beta | Nguyen et al.136 |
| ESRDd | – | – | 0.396 | Beta | Nguyen et al.136 |
| ESRD: haemodialysise | 0.560 | 0.033 | 0.551 | Beta | Liem et al.;137 Ara and Brazier138 |
| ESRD: peritoneal dialysise | 0.580 | 0.043 | 0.564 | Beta | Liem et al.137; Ara and Brazier138 |
Acute (decision tree) phase of the model
We have updated the searches from Hall et al. 97 to identify studies that report utilities for the initial decision tree phase of the model. Our post-Hall et al. 97 review identified four further potentially relevant studies. However, the only utilities that meet the NICE reference case are those proposed by Hall et al. 97 All other studies identified from the literature review use non-UK value sets, so are not appropriate for UK decision-making. Given that there are no appropriate utility studies for AKI stage, the analysis uses the utilities identified in Hall et al. 97 applied to the model based on LOS in hospital, LOS in ICU and duration discharged prior to 90 days following hospital admission. Owing to a lack of appropriate data and to avoid double-counting the utility impact of time in hospital/ICU, we have not attempted to apply any additional utility decrements by AKI stage (other than those on acute RRT). The application of utilities is consistent with that used by Hall et al. ,97 with utilities age- and sex-adjusted when possible, with normal and beta distributions used to incorporate the data probabilistically in the model. It is difficult to find utility values for patients in an ICU. Two systematic reviews were consulted: one by Dritsaki et al. 139 and one by Gerth et al. 140 Both reviews focused on a population admitted to an ICU; however, no studies identified in the reviews were deemed suitable. Therefore, the utility value of an unconscious patient has been applied for the duration of ICU stay, using data sourced from Kind et al. 132 and following the same approach as Hall et al. 97 As a sensitivity analysis, we consider an alternative approach to calculate ICU utility to explore the substantial uncertainty in this parameter. The alternative value takes the average of the unconscious state (–0.402 from Kind et al. 132) and the average post-ICU discharge from Hernández et al. 133 from the Pragmatic Randomised, Controlled Trial of Intensive Care follow up programmes in improving Longer-term outcomes from critical illness (PRaCTICaL) (0.44), which followed up a cohort of ICU survivors reporting their quality of life using the EuroQol-5 Dimensions (EQ-5D) instrument. The calculated utility value applied in the sensitivity analysis was [(–0.402 + 0.44)/2] = 0.019.
Utility values for the chronic phase of the model
First, the Hall et al. 97 HTA programme assessment and economic model for long-term follow-up post AKI and the Scotland et al. 131 assessment for NICE of multiple-frequency bioimpedance devices to guide fluid management in people with CKD undergoing dialysis were consulted to obtain appropriate health-state utility values for application in the model. Hall et al. 97 conducted a thorough review of the literature prior to 2016 for utility parameters. The authors identified two systematic reviews of utility data that provided data that could be used in the economic model. The first, a systematic review and meta-regression published by Wyld et al. ,134 predicted utility according to treatment (transplant, dialysis, pre treatment, conservative management). This model predicted an EQ-5D utility value of 0.64 for patients on dialysis and an EQ-5D utility value of 0.75 for transplant patients. The utilities from Wyld et al. 134 were used in the Hall et al. 97 model.
However, a limitation of Wyld et al. 134 is that some of the EQ-5D scores were calculated from mapping algorithms and the age to which the mean utility estimates applied was not reported. The earlier systematic review by Liem et al. 137 restricted a meta-analysis to those studies using the EQ-5D index directly for each modality of chronic RRT, and reported the pooled mean age and sex distribution for the corresponding pooled EQ-5D values.
In addition to the two reviews identified by Hall et al. ,97 a further structured literature search was conducted to obtain any more recent utility studies that match the NICE DAP reference case (i.e. studies that included EuroQol-5 Dimensions, three-level version, data for UK patients, valued using UK general population tariffs). A range of databases were searched for English language, full-text publications, published between 2016 (end data of Hall et al. 97 searches) and 2019. Seven publications were identified that were deemed to meet the NICE reference case for the DAP; specifically, they reported EQ-5D-based utilities valued in accordance with the UK general population preference-based value sets. Studies in which the EQ-5D was administered to a non-UK population but the results were valued according to the UK tariff were also included.
The age- and sex-matched EQ-5D UK population norms were calculated using an equation published by Ara and Brazier141 and used to derive age-/sex-adjusted utility multipliers from the raw pooled estimates, based on the age and sex distribution of the source studies. 138 The utility of the proportion of the cohort having a successful transplant is assumed to revert to that of the outpatient follow-up state. All utility data were incorporated into the model probabilistically using beta distributions.
Time horizon and discounting
The model was run over a lifetime time horizon, up to age 100 years (for a cohort with a starting age of 63 years in the model). The lifetime time horizon was chosen to ensure that all of the long-term costs and consequences of AKI-induced CKD were captured, including the long-term health effects of ultimate progression to ESRD, transplant and death. The cycle length for the model was annual, and half-cycle corrections have been applied to costs and utilities. All costs and outcomes accruing beyond the first yearly cycle of the model were discounted at a rate of 3.5% per annum, in line with the NICE reference case. The discount rate was varied between 0% and 6% in deterministic sensitivity analyses.
Analyses
The model calculated the expected costs and expected QALYs over the lifetime of each cohort. This includes the costs and QALYs incurred in the first 90-day acute phase of the model, based on diagnostic test accuracy, preventative action to avert AKI, resultant peak AKI status and requirement for admission to an ICU. It also includes the longer-term extrapolations from the Markov cohort model, simulating the long-term transitions between progressive stages of CKD for those who develop it.
The model is fully probabilistic to simultaneously describe the impact of all parameter uncertainty on the model results. All model parameter estimates are sampled from their assigned distributions, as described in the preceding sections, using 1000 simulations. When it was not possible to derive a distribution, for example when insufficient information existed to determine the SD of the distribution, it was assumed that the SD of a parameter was equal to 10% of its mean, unless otherwise stated.
Results are reported as cost–utility analyses, in terms of incremental cost per QALY, expressed as the incremental cost-effectiveness ratio (ICER). Test strategies are plotted on the cost-effectiveness frontier. Tests are ranked in ascending order of benefit (QALYs), with results reported for all tests incrementally against each other to enable the exclusion of strictly dominated (less beneficial and more costly) alternatives from the ICER calculations. ICERs versus standard care are also reported. Results from the probabilistic analysis simulations are plotted using cost-effectiveness acceptability curves based on the net benefit calculation to identify the optimal diagnostic testing strategy at different threshold values of willingness to pay for a QALY.
Model validation
The economic model was checked for errors using the approach suggested by Tappenden and Chilcott,142 which specified verification tests. Components of the model tested were the estimation of the costs and QALYs, distributions of model parameters and other general tests for accuracy of the implementation of input parameters. No specific issues were identified through the verification tests.
Results
The model was developed and configured to assess the cost-effectiveness of the NephroCheck test, the ARCHITECT urine NGAL assay, the BioPorto urine NGAL test and the BioPorto plasma NGAL test in combination with standard clinical assessment, compared with standard clinical assessment alone.
There is no direct evidence to describe the impact of the use of the AKI biomarkers on important health outcomes (such as need for ICU care, length of hospital stay, risk of 90-day mortality or development of new/progression of existing CKD). Accordingly, the cost-effectiveness results are based on a linked-evidence approach whereby we have relied on observational associations to infer how prevention or mitigation of AKI may affect changes in health outcomes. These associations necessitate causal assumptions, but, although a causal link between AKI and poor outcomes is plausible, the extent of this causal relationship is uncertain and controversial. The cost-effectiveness results are therefore presented for a range of alternative, but potentially plausible, scenario analyses, ranging from a set of optimistic assumptions whereby biomarker-guided care bundles may lead to substantial improvements in health outcomes (need for ICU, CKD, mortality) to a set of more conservative assumptions where change in AKI status has no effect on health outcomes. It is likely that the true estimate of cost-effectiveness lies somewhere between these two extremes.
Furthermore, the model includes the following key assumptions:
-
The model base-case analysis is run for a mixed cohort of CKD and non-CKD patients, average age 63 years, 54.3% female, based on the characteristics of hospitalised patients in Grampian, Scotland, who have at least a one-night hospital stay and are having their kidney function monitored, and so are deemed to be at risk of AKI.
-
It is assumed that NephroCheck and NGAL can rise at similar time points; in the absence of any evidence to suggest otherwise, it is assumed that the time gain, relative to serum creatinine, in terms of early implementation of a KDIGO care bundle is equal for both.
-
The base-case analyses assume that there are no adverse consequences, in terms of health effects, of false-positive or false-negative test results compared with standard care. False-positive results would incur the additional futile application of the care bundle costs, and clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) indicates that false negatives will be monitored until the negative test result is confirmed and would represent current practice without biomarkers. However, there is some concern that a false-positive test may lead to unnecessary fluid resuscitation, especially if encountered by inexperienced clinicians, which could lead to an increased mortality risk, although the magnitude of that risk is unknown. A sensitivity analysis explores this.
-
For the Markov models, it is assumed that a patient can develop CKD linked to the index AKI event for the first cycle of the model only, reflecting a total exposure time to increased CKD risk of 1 year + 90 days. Thereafter, the background risk of developing CKD in the population is applied.
-
It is assumed that the proportion of the cohort that experience graft failure post transplant return to the ‘ESRD on dialysis’ health state, where they are exposed to the same risks of transition to transplant/death as when they first entered the dialysis state.
-
For the proportion of the cohort that do not develop long-term CKD, the base-case models assume that the longer-term follow-up costs and mortality risks are not dependent on events in the acute phase of the model (i.e. AKI severity and associated ICU admission). A sensitivity analysis explores the impact of applying additional costs and mortality risks for those admitted to ICU in the acute phase of the model.
-
The model is run for a lifetime time horizon or 100 years, whichever comes first, with costs and QALYs discounted at an annual rate of 3.5% per annum.
Evidence from Meersch et al. 110 shows that NephroCheck-guided early implementation of a KDIGO care bundle can avert AKI. However, the impact of NGAL-guided implementation of a care bundle is unknown. Therefore, two alternative base-case assumptions are considered. The first assumes that NGAL and NephroCheck have the same potential to avert AKI (based on Meersch et al. 110). The second assumes that NGAL can reduce the severity of AKI (also from Meersch et al. 110), but cannot prevent it from occurring. The rationale for the latter analysis is that NGAL detects injury to the kidneys, whereas NephroCheck can potentially detect stresses on the kidneys and may offer an earlier warning of impending AKI. The two base-case models and a range of scenario analyses conducted around important model assumptions are described in Table 19. A total of 15 scenario analyses are reported on each of these two plausible base-case configurations to illustrate the significant uncertainty in the cost-effectiveness findings. Table 20 reports the results for scenarios in which NGAL can avert AKI, and Table 21 reports results of scenarios in which NGAL cannot avert AKI. The results of additional scenario analyses requested by NICE are provided in Appendix 14 for completeness.
| Parameter/assumptions | Value | Base-case justification/source | Sensitivity/scenario analyses | Scenario analysis reference |
|---|---|---|---|---|
| Alternative base-case assumptions | ||||
| Potential for biomarker tests to avert AKI (vs. standard care) | RR AKI 0.77 | Based on Meersch et al.110 |
|
|
| Scenario analyses applied to base case 1 and base case 2 | ||||
| Proportion of the RR of ICU admission (AKI vs. none) that can be achieved by averting AKI | 0.5 | Based on clinical expert opiniona | Varied between 0 and 1 |
|
| Proportion of the HR of CKD (AKI vs. none) that can be achieved by averting AKI | 1 | Based on clinical expert opiniona/See et al.112 | Varied between 0 and 1 | |
| Proportion of the RR of 90-day mortality (AKI vs. none) that can be achieved by averting AKI | 0 | Based on Meersch et al.,110 who show effects on AKI, but not on mortality. Similar data from Wilson et al.118 | Varied between 0 and 1 | |
| Proportion of the difference in hospital and ICU LOS (AKI vs. none) that can be achieved by averting AKI | 0.5 | Based on clinical expert opiniona | Varied between 0 and 1 | |
| Impact of AKI stage on hospital and ICU LOS | Duration applied by AKI stage | Based on observational data from Grampian102 | Duration assumed not to vary by stage, with same durations applied to all AKI stages based on average from Grampian observational data102 | |
| Impact of AKI stage on the probability of ICU admission | Probability applied by AKI stage | Based on observational data from Grampian102 | Probability assumed not to vary by stage, with same probability applied to all AKI stages based on average from Grampian observational data102 | |
| Impact of AKI stage on the probability of developing CKD | HR applied by AKI stage | Based on systematic review and meta-analysis from See et al.112 | HR assumed not to vary by stage, with same HR applied to all AKI stages, based on Sawhney et al.102 | |
| Impact of AKI stage on the probability of 90-day mortality | Average probability applied for all AKI stages | Based on a lack of evidence that changing AKI severity can affect mortality directly, as per Meersch et al.110 | Probabilities applied by AKI stage to explore uncertainty in this assumption | |
| AKI excess cost per day in hospital/ICU | No excess cost applied | Conservative approach to ensure avoidance of double-counting | Additional hospital excess bed-day cost applied as per Hall et al.97 to all patients | Scenario E: as per scenario D with additional AKI costs |
| The following analyses are applied to the base-case configuration (scenario A) | ||||
| Additional costs per day on RRT | Yes | Based on HRG costs | No additional costs of RRT | Scenario F |
| Impact of AKI on long-term follow-up costs beyond 90 days | None (ratio = 1) | Conservative assumption | All long-term Markov model costs multiplied by 1.15, as per Hall et al.97 | Scenario G: differential long-term outpatient cost and mortality applied according to whether or not patient entered ICU |
| Long-term outpatient follow-up costs, up to 5 years | Average of hospitalised and ICU patients | Based on average of two cohorts from Lone et al.114 | Differential cost streams applied for 5 years according to whether or not cohort was admitted to ICU in first 90 days, based on Lone et al.114 | |
| Long-term outpatient follow-up costs, after 5 years | No additional costs applied | Assumption that patients surviving post ICU to 5 years will incur no further excess costs | Additional annual costs applied for full lifetime, based on extrapolation of Lone et al.114 data, applied separately to those who had an ICU admission and those who had no ICU admission at index hospitalisation | |
| Impact of ICU admission on long-term mortality | Average of hospitalised and ICU patients | Lone et al.114 | Differential mortality applied according to whether or not cohort was admitted to ICU | |
| Duration by which AKI event can affect excess CKD risk | 1 year + 90 days | Assumption | Assume additional risk of CKD development over full lifetime time horizon | Scenario H |
| Discount rate (cost) | 3.5% | NICE methods guide96 | Varied 0–6% |
|
| Discount rate (QALY) | 3.5% | NICE methods guide96 | Varied 0–6% | |
| Source of AKI prevalence data | 9.2% | Grampian data102 for hospitalised patients at risk of AKI | Alternative source: obtained directly from systematic review studies | Scenario K |
| Number of times test is used | 1 | Based on NICE scope | All tests conducted twice | Scenario L |
| RR of 90-day mortality for false-positive test results | 1 | Assumes no additional risk of unnecessary fluid resuscitation | Apply an additional RR of 1.5 to explore impact on results | Scenario M |
| Test capital and training costs in test cost | Included | As per company advice | Exclude in sensitivity analysis, assuming all capital equipment required is available for all tests (including NephroCheck) | Scenario N |
| Source of ICU utility data | –0.402 | Kind et al.132 (unconscious patient) | Average of unconscious patient (–0.402) and utility at discharge from ICU reported in the PRaCTICaL trial (Hernandez et al.133) | Scenario O |
| Long-term outpatient utility | Varies by year | Long-term utility implication of hospitalisation/ICU, based on Hall et al.97 | General population norms, assuming quicker recovery | Scenario P |
| Source of diagnostic accuracy data | All comers | All | Exploratory analysis applying available test accuracy data for children to the adult model | Scenario Q |
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs. standard care | |||||||
| Scenario 1A: preferred base case assuming an associative effect of averting and mitigating AKI | ||||||||
| Test 3 (BioPorto urine NGAL) | 22,887 | – | 6.07332 | – | – | Dominant | 43.5 | 54.6 |
| Test 2 (BioPorto plasma NGAL) | 22,900 | £14 | 6.07332 | 0.00001 | £2,694,918 | Dominant | 11.1 | 47.6 |
| Standard care (serum creatinine) | 22,901 | Dominated | 6.07296 | Dominated | Dominated | – | 45.1 | – |
| Test 4 (ARCHITECT urine NGAL) | 22,912 | Dominated | 6.07328 | Dominated | Dominated | £32,131 | 0.1 | 41.4 |
| Test 1 (NephroCheck) | 22,938 | Dominated | 6.07332 | Dominated | Dominated | £101,456 | 0.2 | 31.9 |
| Scenario 1B: applying the full associative effect on the redistributed cohort only and assuming that the test affects the probability of dying at 90 days | ||||||||
| Standard care (serum creatinine) | 22,829 | – | 6.08377 | – | – | – | 57.5 | – |
| Test 3 (BioPorto urine NGAL) | 22,937 | £108 | 6.08602 | 0.00226 | £47,877 | £47,877 | 30.4 | 42.5 |
| Test 4 (ARCHITECT urine NGAL) | 22,951 | Dominated | 6.08584 | Dominated | Dominated | £58,813 | 0.0 | 37.3 |
| Test 2 (BioPorto plasma NGAL) | 22,951 | £14 | 6.08608 | 0.00006 | £228,616 | £52,816 | 11.9 | 39.5 |
| Test 1 (NephroCheck) | 22,988 | Dominated | 6.08604 | Dominated | Dominated | £70,141 | 0.2 | 31.0 |
| Scenario 1C: no associative effect | ||||||||
| Standard care (serum creatinine) | 23,340 | – | 6.07257 | – | – | – | 100.0 | – |
| Test 3 (BioPorto urine NGAL) | 23,420 | Dominated | 6.07257 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 2 (BioPorto plasma NGAL) | 23,436 | Dominated | 6.07257 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 4 (ARCHITECT urine NGAL) | 23,437 | Dominated | 6.07257 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 1 (NephroCheck) | 23,473 | Dominated | 6.07257 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Scenario 1D: full associative effect | ||||||||
| Standard care (serum creatinine) | 22,959 | – | 6.08383 | – | – | – | 0.7 | – |
| Test 3 (BioPorto urine NGAL) | 23,013 | £54 | 6.11006 | 0.02623 | £2052 | £2052 | 40.7 | 99.3 |
| Test 2 (BioPorto plasma NGAL) | 23,028 | £15 | 6.11091 | 0.00084 | £17,702 | £2538 | 47.5 | 99.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,031 | Dominated | 6.10799 | Dominated | Dominated | £2981 | 1.1 | 98.8 |
| Test 1 (NephroCheck) | 23,065 | Dominated | 6.11064 | Dominated | Dominated | £3955 | 10.0 | 97.7 |
| Scenario 1E: as per scenario 1D, but applying a daily excess AKI cost to patients in hospital/ICU | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,638 | – | 6.11049 | – | – | Dominant | 38.6 | 99.2 |
| Test 2 (BioPorto plasma NGAL) | 23,650 | £12 | 6.11104 | 0.00055 | £21,968 | Dominant | 43.8 | 98.9 |
| Test 4 (ARCHITECT urine NGAL) | 23,664 | Dominated | 6.10823 | Dominated | Dominated | Dominant | 2.0 | 98.9 |
| Standard care (serum creatinine) | 23,681 | Dominated | 6.08377 | Dominated | Dominated | – | 0.8 | – |
| Test 1 (NephroCheck) | 23,687 | Dominated | 6.11102 | Dominated | Dominated | £210 | 14.8 | 98.7 |
| Scenario 1F: exclude RRT cost | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,258 | – | 6.07092 | – | – | Dominant | 39.5 | 49.9 |
| Standard care (serum creatinine) | 23,266 | Dominated | 6.07060 | Dominated | Dominated | – | 49.8 | – |
| Test 2 (BioPorto plasma NGAL) | 23,271 | £14 | 6.07093 | 0.00001 | £1,403,330 | £17,694 | 10.0 | 44.9 |
| Test 4 (ARCHITECT urine NGAL) | 23,282 | Dominated | 6.07089 | Dominated | Dominated | £54,497 | 0.3 | 39.0 |
| Test 1 (NephroCheck) | 23,309 | Dominated | 6.07092 | Dominated | Dominated | £132,748 | 0.4 | 29.5 |
| Scenario 1G: apply the differential long-term follow-up costs and mortality according to whether or not a patient entered an ICU | ||||||||
| Test 3 (BioPorto urine NGAL) | 30,290 | – | 6.56602 | – | – | Dominant | 50.2 | 99.5 |
| Test 2 (BioPorto plasma NGAL) | 30,296 | £7 | 6.56605 | 0.00003 | £227,069 | Dominant | 39.7 | 99.1 |
| Test 1 (NephroCheck) | 30,335 | Dominated | 6.56605 | Dominated | Dominated | Dominant | 8.1 | 97.2 |
| Test 4 (ARCHITECT urine NGAL) | 30,337 | Dominated | 6.56591 | Dominated | Dominated | Dominant | 1.4 | 98.6 |
| Standard care (serum creatinine) | 30,606 | Dominated | 6.56457 | Dominated | Dominated | – | 0.5 | – |
| Scenario 1H: apply an excess CKD risk for those who experienced an AKI event over the full lifetime time horizon | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,201 | – | 6.07247 | – | – | Dominant | 54.8 | 76.5 |
| Test 2 (BioPorto plasma NGAL) | 23,212 | £12 | 6.07251 | 0.00005 | £254,012 | Dominant | 20.3 | 73.0 |
| Test 4 (ARCHITECT urine NGAL) | 23,228 | Dominated | 6.07234 | Dominated | Dominated | Dominant | 0.6 | 68.4 |
| Test 1 (NephroCheck) | 23,251 | Dominated | 6.07250 | Dominated | Dominated | Dominant | 1.0 | 58.2 |
| Standard care (serum creatinine) | 23,254 | Dominated | 6.07086 | Dominated | Dominated | – | 23.3 | – |
| Scenario 1I: 0% discount rate applied to both costs and QALYs | ||||||||
| Test 3 (BioPorto urine NGAL) | 27,644 | – | 8.20147 | – | – | Dominant | 44.3 | 57.9 |
| Test 2 (BioPorto plasma NGAL) | 27,657 | £13 | 8.20149 | 0.00001 | £996,593 | Dominant | 13.5 | 51.4 |
| Standard care (serum creatinine) | 27,664 | Dominated | 8.20095 | Dominated | Dominated | – | 41.6 | – |
| Test 4 (ARCHITECT urine NGAL) | 27,668 | Dominated | 8.20143 | Dominated | Dominated | £9262 | 0.2 | 47.4 |
| Test 1 (NephroCheck) | 27,694 | £37 | 8.20149 | 0.00000 | £48,020,759 | £56,351 | 0.3 | 37.4 |
| Scenario 1J: 6% discount rate applied to both costs and QALYs | ||||||||
| Test 3 (BioPorto urine NGAL) | 20,961 | – | 5.11682 | – | – | Dominant | 39.7 | 49.9 |
| Standard care (serum creatinine) | 20,969 | Dominated | 5.11654 | Dominated | Dominated | – | 49.4 | – |
| Test 2 (BioPorto plasma NGAL) | 20,974 | £13 | 5.11683 | 0.00001 | £1,295,058 | £16,259 | 10.4 | 44.2 |
| Test 4 (ARCHITECT urine NGAL) | 20,984 | Dominated | 5.11680 | Dominated | Dominated | £55,509 | 0.3 | 39.5 |
| Test 1 (NephroCheck) | 21,011 | Dominated | 5.11683 | Dominated | Dominated | £145,369 | 0.1 | 30.6 |
| Scenario 1K: apply alternative source for AKI prevalence (average prevalence of 0.2332 across systematic review studies) | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,050 | – | 5.85835 | – | – | Dominant | 42.3 | 79.0 |
| Test 2 (BioPorto plasma NGAL) | 23,055 | £5 | 5.85837 | 0.00002 | £256,153 | Dominant | 30.7 | 77.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,084 | Dominated | 5.85827 | Dominated | Dominated | Dominant | 1.2 | 75.2 |
| Test 1 (NephroCheck) | 23,093 | £39 | 5.85837 | 0.00000 | £20,956,862 | Dominant | 5.0 | 69.3 |
| Standard care (serum creatinine) | 23,225 | Dominated | 5.85742 | Dominated | Dominated | – | 20.7 | – |
| Scenario 1L: increase the number of times test is conducted to two | ||||||||
| Standard care (serum creatinine) | 22,811 | – | 6.07532 | – | – | – | 70.3 | – |
| Test 3 (BioPorto urine NGAL) | 22,853 | £41 | 6.07567 | 0.00035 | £118,796 | £118,796 | 19.9 | 28.9 |
| Test 2 (BioPorto plasma NGAL) | 22,865 | £13 | 6.07567 | 0.00001 | £2,201,973 | £152,384 | 9.7 | 25.4 |
| Test 4 (ARCHITECT urine NGAL) | 22,884 | Dominated | 6.07564 | Dominated | Dominated | £227,155 | 0.1 | 19.2 |
| Test 1 (NephroCheck) | 22,936 | £71 | 6.07567 | 0.00000 | £69,489,954 | £350,812 | 0.0 | 12.4 |
| Scenario 1M: apply an additional risk of mortality to those with a false-positive test (RR 1.5) | ||||||||
| Test 1 (NephroCheck) | 22,522 | – | 5.93563 | – | – | £3072 | 0.0 | 0.0 |
| Test 2 (BioPorto plasma NGAL) | 22,545 | £22 | 5.95376 | 0.01813 | £1240 | £3344 | 0.0 | 0.0 |
| Test 4 (ARCHITECT urine NGAL) | 22,630 | £86 | 5.97629 | 0.02253 | £3814 | £3238 | 0.0 | 0.0 |
| Test 3 (BioPorto urine NGAL) | 22,718 | £88 | 6.01026 | 0.03397 | £2582 | £3576 | 0.1 | 0.10 |
| Standard care (serum creatinine) | 22,954 | £235 | 6.07608 | 0.06582 | £3576 | – | 99.9 | – |
| Scenario 1N: exclude capital and training costs in test costs | ||||||||
| Test 3 (BioPorto urine NGAL) | 22,952 | – | 6.07161 | – | – | Dominant | 39.6 | 51.7 |
| Standard care (serum creatinine) | 22,964 | Dominated | 6.07126 | Dominated | Dominated | – | 47.9 | – |
| Test 2 (BioPorto plasma NGAL) | 22,965 | £13 | 6.07163 | 0.00001 | £999,957 | £2229 | 12.2 | 45.6 |
| Test 4 (ARCHITECT urine NGAL) | 22,975 | Dominated | 6.07159 | Dominated | Dominated | £35,302 | 0.0 | 40.5 |
| Test 1 (NephroCheck) | 23,002 | Dominated | 6.07162 | Dominated | Dominated | £105,799 | 0.3 | 31.4 |
| Scenario 1O: apply alternative ICU utility value (average of –0.402 and 0.44) | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,020 | – | 6.07328 | – | – | Dominant | 42.4 | 53.9 |
| Standard care (serum creatinine) | 23,032 | Dominated | 6.07296 | Dominated | Dominated | – | 45.9 | – |
| Test 2 (BioPorto plasma NGAL) | 23,033 | £13 | 6.07329 | 0.00001 | £1,565,836 | £1487 | 11.0 | 47.4 |
| Test 4 (ARCHITECT urine NGAL) | 23,044 | Dominated | 6.07326 | Dominated | Dominated | £39,666 | 0.2 | 41.3 |
| Test 1 (NephroCheck) | 23,071 | Dominated | 6.07329 | Dominated | Dominated | £118,201 | 0.5 | 30.2 |
| Scenario 1P: alternative outpatient utility source in the long term (apply general population norms) | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,149 | – | 7.05770 | – | – | Dominant | 41.8 | 53.5 |
| Standard care (serum creatinine) | 23,161 | Dominated | 7.05712 | Dominated | Dominated | – | 45.8 | – |
| Test 2 (BioPorto plasma NGAL) | 23,161 | £12 | 7.05771 | 0.00002 | £779,444 | £1133 | 11.3 | 47.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,172 | Dominated | 7.05765 | Dominated | Dominated | £22,019 | 0.4 | 41.1 |
| Test 1 (NephroCheck) | 23,199 | Dominated | 7.05771 | Dominated | Dominated | £65,271 | 0.5 | 33.6 |
| Scenario 1Q: applying diagnostic test accuracy data for children to the adult AKI model (exploratory only)a | ||||||||
| Standard care (serum creatinine) | 22,952 | 6.07678 | 55.1 | |||||
| Test 4 (ARCHITECT urine NGAL) | 22,957 | £5 | 6.07709 | 0.00031 | £15,835 | £15,835 | 24.2 | 43.3 |
| Test 3 (BioPorto urine NGAL) | 22,968 | £11 | 6.07713 | 0.00004 | £260,525 | £45,510 | 20.6 | 40.4 |
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs.standard care | |||||||
| Scenario 2A: alternative base case assuming that NephroCheck is the only test that can lead to averted AKI | ||||||||
| Standard care (serum creatinine) | 22,978 | – | 6.07277 | – | – | – | 64.5 | – |
| Test 1 (NephroCheck) | 23,016 | £38 | 6.07313 | 0.00036 | £105,965 | £105,965 | 29.7 | 32.0 |
| Test 3 (BioPorto urine NGAL) | 23,049 | Dominated | 6.07290 | Dominated | Dominated | £539,041 | 5.3 | 11.0 |
| Test 2 (BioPorto plasma NGAL) | 23,064 | Dominated | 6.07290 | Dominated | Dominated | £633,846 | 0.3 | 7.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,065 | Dominated | 6.07289 | Dominated | Dominated | £725,061 | 0.0 | 6.3 |
| Scenario 2B: applying the full associative effect on the redistributed cohort only and assuming that the test affects the probability of dying at 90 days | ||||||||
| Standard care (serum creatinine) | 22,947 | – | 6.08411 | – | – | – | 36.8 | – |
| Test 3 (BioPorto urine NGAL) | 23,033 | £87 | 6.08912 | 0.00502 | £17,290 | £17,290 | 34.4 | 53.7 |
| Test 4 (ARCHITECT urine NGAL) | 23,049 | Dominated | 6.08875 | Dominated | Dominated | £22,071 | 0.4 | 43.7 |
| Test 2 (BioPorto plasma NGAL) | 23,050 | £17 | 6.08934 | 0.00022 | £75,026 | £19,717 | 11.1 | 48.9 |
| Test 1 (NephroCheck) | 23,101 | Dominated | 6.08615 | Dominated | Dominated | £75,634 | 17.3 | 31.4 |
| Scenario 2C: no associative effect | ||||||||
| Standard care (serum creatinine) | 23,012 | – | 6.07534 | – | – | – | 100.0 | – |
| Test 3 (BioPorto urine NGAL) | 23,094 | £82 | 6.07534 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 2 (BioPorto plasma NGAL) | 23,110 | £16 | 6.07534 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 4 (ARCHITECT urine NGAL) | 23,110 | Dominated | 6.07534 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Test 1 (NephroCheck) | 23,145 | Dominated | 6.07534 | Dominated | Dominated | Dominated | 0.0 | 0.0 |
| Scenario 2D: full associative effect | ||||||||
| Standard care (serum creatinine) | 23,114 | – | 6.08592 | – | – | – | 0.7 | – |
| Test 3 (BioPorto urine NGAL) | 23,199 | Extendedly dominated | 6.09125 | Extendedly dominated | Extendedly dominated | £15,974 | 0.5 | 55.8 |
| Test 2 (BioPorto plasma NGAL) | 23,214 | Extendedly dominated | 6.09137 | Extendedly dominated | Extendedly dominated | £18,364 | 0.3 | 50.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,215 | Dominated | 6.09080 | Dominated | Dominated | £20,721 | 0.0 | 46.0 |
| Test 1 (NephroCheck) | 23,223 | £109 | 6.11360 | 0.02768 | £3941 | £3941 | 98.5 | 99.1 |
| Scenario 2E: as per scenario 2D, but applying a daily excess AKI cost to patients in hospital/ICU | ||||||||
| Standard care (serum creatinine) | 23,729 | – | 6.08549 | – | – | – | 0.7 | – |
| Test 1 (NephroCheck) | 23,730 | £1 | 6.11261 | 0.02712 | £29 | £29 | 98.8 | 99.1 |
| Test 3 (BioPorto urine NGAL) | 23,815 | Dominated | 6.09063 | Dominated | Dominated | £16,615 | 0.5 | 54.0 |
| Test 4 (ARCHITECT urine NGAL) | 23,830 | Dominated | 6.09020 | Dominated | Dominated | £21,436 | 0.0 | 45.1 |
| Test 2 (BioPorto plasma NGAL) | 23,831 | Dominated | 6.09079 | Dominated | Dominated | £19,153 | 0.0 | 49.9 |
| Scenario 2F: exclude RRT cost | ||||||||
| Standard care (serum creatinine) | 22,779 | – | 6.07846 | – | – | – | 68.1 | – |
| Test 1 (NephroCheck) | 22,823 | £43 | 6.07882 | 0.00036 | £119,317 | £119,317 | 27.7 | 29.6 |
| Test 3 (BioPorto urine NGAL) | 22,850 | Dominated | 6.07859 | Dominated | Dominated | £533,230 | 3.8 | 9.0 |
| Test 2 (BioPorto plasma NGAL) | 22,865 | Dominated | 6.07859 | Dominated | Dominated | £633,002 | 0.4 | 6.8 |
| Test 4 (ARCHITECT urine NGAL) | 22,867 | Dominated | 6.07858 | Dominated | Dominated | £730,093 | 0.0 | 5.6 |
| Scenario 2G: apply the differential long-term follow-up costs and mortality according to whether or not patient entered an ICU | ||||||||
| Test 1 (NephroCheck) | 30,438 | – | 6.55843 | – | – | Dominant | 97.2 | 97.2 |
| Standard care (serum creatinine) | 30,712 | Dominated | 6.55697 | Dominated | Dominated | – | 2.8 | – |
| Test 3 (BioPorto urine NGAL) | 30,776 | Dominated | 6.55733 | Dominated | Dominated | £181,324 | 0.0 | 15.4 |
| Test 2 (BioPorto plasma NGAL) | 30,790 | Dominated | 6.55733 | Dominated | Dominated | £217,350 | 0.0 | 11.8 |
| Test 4 (ARCHITECT urine NGAL) | 30,793 | Dominated | 6.55730 | Dominated | Dominated | £249,264 | 0.0 | 9.3 |
| Scenario 2H: apply an excess CKD risk for those who experienced an AKI event over the full lifetime time horizon | ||||||||
| Test 1 (NephroCheck) | 23,172 | – | 6.07060 | – | – | Dominant | 55.5 | 57.7 |
| Standard care (serum creatinine) | 23,174 | Dominated | 6.06893 | Dominated | Dominated | – | 39.9 | – |
| Test 3 (BioPorto urine NGAL) | 23,231 | Dominated | 6.06947 | Dominated | Dominated | £106,920 | 3.6 | 21.2 |
| Test 2 (BioPorto plasma NGAL) | 23,246 | Dominated | 6.06948 | Dominated | Dominated | £132,282 | 1.0 | 16.6 |
| Test 4 (ARCHITECT urine NGAL) | 23,250 | Dominated | 6.06942 | Dominated | Dominated | £154,900 | 0.0 | 12.7 |
| Scenario 2I: 0% discount rate applied to both costs and QALYs | ||||||||
| Standard care (serum creatinine) | 27,689 | – | 8.20138 | – | – | – | 60.5 | – |
| Test 1 (NephroCheck) | 27,717 | £28 | 8.20191 | 0.00053 | £52,565 | £52,565 | 34.1 | 36.6 |
| Test 3 (BioPorto urine NGAL) | 27,757 | Dominated | 8.20157 | Dominated | Dominated | £371,108 | 4.9 | 12.7 |
| Test 2 (BioPorto plasma NGAL) | 27,771 | Dominated | 8.20157 | Dominated | Dominated | £439,959 | 0.4 | 9.5 |
| Test 4 (ARCHITECT urine NGAL) | 27,774 | Dominated | 8.20155 | Dominated | Dominated | £500,966 | 0.1 | 7.0 |
| Scenario 2J: 6% discount rate applied to both costs and QALYs | ||||||||
| Standard care (serum creatinine) | 21,153 | – | 5.11027 | – | – | – | 67.1 | – |
| Test 1 (NephroCheck) | 21,192 | £40 | 5.11055 | 0.00028 | £140,771 | £140,771 | 27.4 | 30.7 |
| Test 3 (BioPorto urine NGAL) | 21,221 | Dominated | 5.11037 | Dominated | Dominated | £686,941 | 4.7 | 10.8 |
| Test 2 (BioPorto plasma NGAL) | 21,235 | Dominated | 5.11038 | Dominated | Dominated | £808,828 | 0.8 | 8.0 |
| Test 4 (ARCHITECT urine NGAL) | 21,238 | Dominated | 5.11036 | Dominated | Dominated | £937,507 | 0.0 | 6.3 |
| Scenario 2K: apply alternative source for AKI prevalence (average prevalence 0.2332 across systematic review studies) | ||||||||
| Test 1 (NephroCheck) | 23,014 | – | 5.85682 | – | – | Dominant | 63.1 | 67.0 |
| Standard care (serum creatinine) | 23,122 | Dominated | 5.85589 | Dominated | Dominated | – | 28.4 | – |
| Test 3 (BioPorto urine NGAL) | 23,171 | Dominated | 5.85623 | Dominated | Dominated | £142,617 | 6.7 | 33.2 |
| Test 2 (BioPorto plasma NGAL) | 23,183 | Dominated | 5.85624 | Dominated | Dominated | £174,191 | 1.8 | 30.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,188 | Dominated | 5.85620 | Dominated | Dominated | £211,691 | 0.0 | 26.1 |
| Scenario 2L: increase the number of times test is conducted to two | ||||||||
| Standard care (serum creatinine) | 22,746 | – | 6.07904 | – | – | – | 88.8 | – |
| Test 3 (BioPorto urine NGAL) | 22,873 | Extendedly dominated | 6.07916 | Extendedly dominated | Extendedly dominated | £1,053,861 | 1.9 | 2.6 |
| Test 1 (NephroCheck) | 22,875 | £129 | 6.07939 | 0.00035 | £369,737 | £369,737 | 9.0 | 9.4 |
| Test 2 (BioPorto plasma NGAL) | 22,888 | Dominated | 6.07916 | Dominated | Dominated | £1,167,690 | 0.3 | 1.5 |
| Test 4 (ARCHITECT urine NGAL) | 22,898 | Dominated | 6.07915 | Dominated | Dominated | £1,370,281 | 0.0 | 0.7 |
| Scenario 2M: apply an additional risk of mortality to those with a false-positive test (RR 1.5) | ||||||||
| Test 1 (NephroCheck) | 22,533 | – | 5.93052 | 0.00000 | – | £3062 | 0.0 | 0.0 |
| Test 2 (BioPorto plasma NGAL) | 22,632 | £99 | 5.94584 | 0.01532 | £6478 | £2644 | 0.0 | 0.0 |
| Test 4 (ARCHITECT urine NGAL) | 22,715 | £83 | 5.97024 | 0.02440 | £3389 | £2464 | 0.0 | 0.0 |
| Test 3 (BioPorto urine NGAL) | 22,809 | £94 | 6.00383 | 0.03360 | £2801 | £2297 | 0.0 | 0.0 |
| Standard care (serum creatinine) | 22,963 | £155 | 6.07124 | 0.06740 | £2297 | – | 100.0 | – |
| Scenario 2N: exclude capital and training costs in test costs | ||||||||
| Standard care (serum creatinine) | 22,987 | – | 6.08128 | – | – | – | 65.1 | – |
| Test 1 (NephroCheck) | 23,025 | £39 | 6.08162 | 0.00035 | £111,620 | £111,620 | 29.4 | 32.2 |
| Test 3 (BioPorto urine NGAL) | 23,051 | Dominated | 6.08139 | Dominated | Dominated | £546,618 | 4.5 | 12.6 |
| Test 2 (BioPorto plasma NGAL) | 23,066 | Dominated | 6.08140 | Dominated | Dominated | £663,328 | 1.0 | 9.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,069 | Dominated | 6.08138 | Dominated | Dominated | £766,927 | 0.0 | 6.1 |
| Scenario 2O: apply alternative ICU utility value (average of –0.402 and 0.44) | ||||||||
| Standard care (serum creatinine) | 23,234 | – | 6.07749 | – | – | – | 67.2 | – |
| Test 1 (NephroCheck) | 23,274 | £41 | 6.07783 | 0.00034 | £120,580 | £120,580 | 28.0 | 29.9 |
| Test 3 (BioPorto urine NGAL) | 23,302 | Dominated | 6.07761 | Dominated | Dominated | £586,840 | 4.4 | 11.0 |
| Test 2 (BioPorto plasma NGAL) | 23,317 | Dominated | 6.07761 | Dominated | Dominated | £696,184 | 0.4 | 8.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,319 | Dominated | 6.07760 | Dominated | Dominated | £796,431 | 0.0 | 6.2 |
| Scenario 2P: alternative outpatient utility source in the long term (apply general population norms) | ||||||||
| Standard care (serum creatinine) | 22,867 | – | 7.05869 | – | – | – | 63.5 | – |
| Test 1 (NephroCheck) | 22,904 | £36 | 7.05928 | 0.00059 | £61,809 | £61,809 | 32.0 | 34.1 |
| Test 3 (BioPorto urine NGAL) | 22,938 | Dominated | 7.05889 | Dominated | Dominated | £360,613 | 4.3 | 9.9 |
| Test 2 (BioPorto plasma NGAL) | 22,954 | Dominated | 7.05889 | Dominated | Dominated | £431,098 | 0.2 | 7.8 |
| Test 4 (ARCHITECT urine NGAL) | 22,955 | Dominated | 7.05887 | Dominated | Dominated | £483,707 | 0.0 | 5.7 |
| Scenario 2Q: applying diagnostic test accuracy data for children to the adult AKI model (exploratory only)a | ||||||||
| Standard care (serum creatinine) | 23,012 | 6.07121 | 91.0 | |||||
| Test 4 (ARCHITECT urine NGAL) | 23,093 | £80 | 6.07132 | 0.00011 | £713,879 | £713,879 | 6.5 | 8.8 |
| Test 3 (BioPorto urine NGAL) | 23,114 | £21 | 6.07134 | 0.00001 | £1,477,906 | £801,274 | 2.5 | 7.0 |
Scenarios 1A and 2A describe two potential base-case analyses on which all the sensitivity analyses are conducted. These scenarios assume that there is a potential benefit of averting or having less severe AKI, in terms of improved outcomes (need for ICU care, risk of CKD and LOS), but the magnitude of that benefit may be less than that observed in observational data. Given the lack of direct evidence demonstrating the impact of biomarker tests on mortality, the base case assumes that there is no impact on 90-day mortality of averting AKI.
Scenarios B–E illustrate the impact of assumptions around the magnitude of the associative benefits of averting/experiencing less severe AKI on health outcomes. Scenarios F–P explore the impact of applying alternative follow-up costs and mortality, CKD projection, discount rate, alternative source data for AKI prevalence, test costs, excess mortality risk because of a false-positive result, and alternative utility sources.
The results are highly uncertain, with no clear optimal biomarker strategy. The findings are highly sensitive to each of the associative links applied between AKI and health outcomes, namely probability of ICU admission, LOS in hospital, probability of dying at 90 days and the risk of developing CKD.
In scenarios in which NGAL tests are assumed to be equally as effective as NephroCheck at averting AKI, the BioPorto urine NGAL test generally has the greatest probability of cost-effectiveness. This is because the main drivers of the relative cost-effectiveness of each of the biomarker tests against each other are the cost of the test and the diagnostic accuracy. The BioPorto urine NGAL test is slightly cheaper and the meta-analysis shows it as having slightly better diagnostic accuracy in the all-comers cohort. However, these findings should be interpreted cautiously because of the heterogeneity in the diagnostic test accuracy studies, which leads to further uncertainty in the cost-effectiveness results.
Conversely, the NephroCheck and ARCHITECT urine NGAL test are never the most cost-effective strategy when assuming that all tests are equally efficacious in averting AKI, because they are more costly tests, with comparatively poorer diagnostic accuracy. NephroCheck is estimated to have poorer specificity than the NGAL urine tests, thereby generating additional costs of treating false-positive test cases, who unnecessarily receive a KDIGO care bundle. However, under the alternative base-case assumptions, in which the NGAL tests are assumed to have no effect on averting AKI, the probability of NephroCheck being the most cost-effective test rises considerably. In the most optimistic scenario, NephroCheck is 100% cost-effective. In the most pessimistic scenario, standard care is the most cost-effective strategy.
Applying a daily excess cost of AKI in hospital or ICU (i.e. if the cost incurred by patients with AKI is not fully captured in the hospital/ICU daily cost) results in the tests being even more favourable than in the base case because more costs are offset by averting AKI or having less severe AKI in the test arms. This results in the NGAL tests being dominant and NephroCheck being cost-effective (ICER of < £20,000) compared with standard care.
The ARCHITECT urine NGAL test is generally less likely to be cost-effective in all scenarios because of the test accuracy and cost. The ARCHITECT urine NGAL test is estimated to have lower sensitivity and specificity than the other tests, and costs more than the other NGAL tests.
In general, the results are also sensitive to the assumptions on having hospital-/ICU-specific follow-up costs and mortality (instead of an average of the two); increased long-term cost of AKI, including the linked effect between AKI and probability of CKD for the whole duration of the model (instead of for one cycle, as in the base-case); and using an alternative source of AKI prevalence data (with higher prevalence), with all scenarios favouring the test strategies, making them increasingly more cost-effective than standard care. In most of these cases, the BioPorto urine NGAL test is the most cost-effective test strategy; however, in the most optimistic scenario, the BioPorto plasma NGAL test is the most cost-effective choice of test. On the other hand, assuming that a false-positive test result can lead to an increased risk of mortality at 90 days (i.e. RR 1.5) favours standard care, which becomes the strategy with the highest probability of cost-effectiveness.
We have included an exploratory analysis in which the limited available diagnostic accuracy data for children are applied in the adult model. Diagnostic accuracy data were available for only two biomarkers (ARCHITECT urine NGAL and BioPorto urine NGAL). The following diagnostic accuracy estimates were included in this run of the model: BioPorto urine NGAL – sensitivity 0.77 (95% CI 0.70 to 0.84) and specificity 0.47 (95% CI 0.40 to 0.54); ARCHITECT urine NGAL – sensitivity 0.68 (95% CI 0.53 to 0.80) and specificity 0.79 (95% CI 0.63 to 0.89).
This analysis should be considered as speculative only, as to ensure a robust assessment of cost-effectiveness among children would require the reconfiguration of the model for a paediatric cohort, with appropriate care pathways and age-specific risks of transition between health states.
In summary, the results are highly uncertain and it is impossible to ascertain the most likely ICER given the available evidence. The range of ICERs across different plausible sets of assumptions is substantial and the probabilistic analyses indicate substantial uncertainties regarding the optimal test strategy. Any of the scenarios explored might be feasible, so it is important to consider these findings in the light of the substantial uncertainty underlying the impact of the tests on AKI and the causative links between AKI and changes in health outcomes. The substantial heterogeneity in the study populations for the diagnostic accuracy data for the candidate tests raises further concerns about the relative cost-effectiveness of the comparators in the absence of head-to-head trial comparisons across multiple candidate tests.
Cohort traces from the base-case Markov models
Figure 22 shows the Markov traces for the standard-care arm of the model under base case 1 assumptions. In the standard-care arm, at 10 years, the mortality for the cohort aged 63 years was 45% for the no-AKI cohort and 59% for the average of the AKI 1, 2 and 3 cohorts. The mortality for the no-AKI group is consistent with the observed 10-year mortality in the Grampian data. 102 However, the mortality observed for the AKI cohorts at 10 years is lower than in the observational data from Grampian. This is because we did not apply an additional AKI-specific excess mortality risk beyond the first year of follow-up in the model, as to assume that such an additional risk is directly caused by AKI is questionable, based on existing evidence (e.g. Meersch et al. 110).
FIGURE 22.
Markov cohort traces for base-case model configuration. (a) No AKI; (b) AKI 1; (c) AKI 2; and (d) AKI 3.
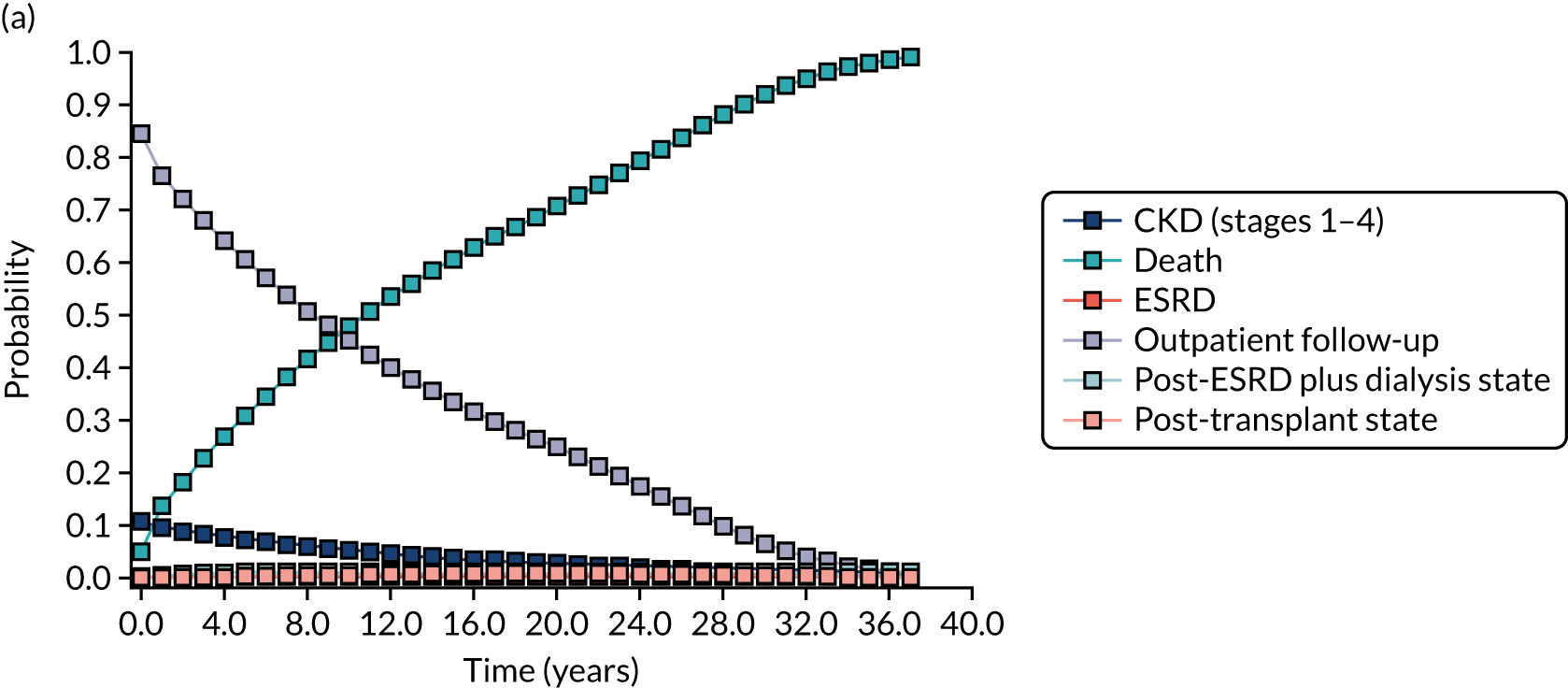
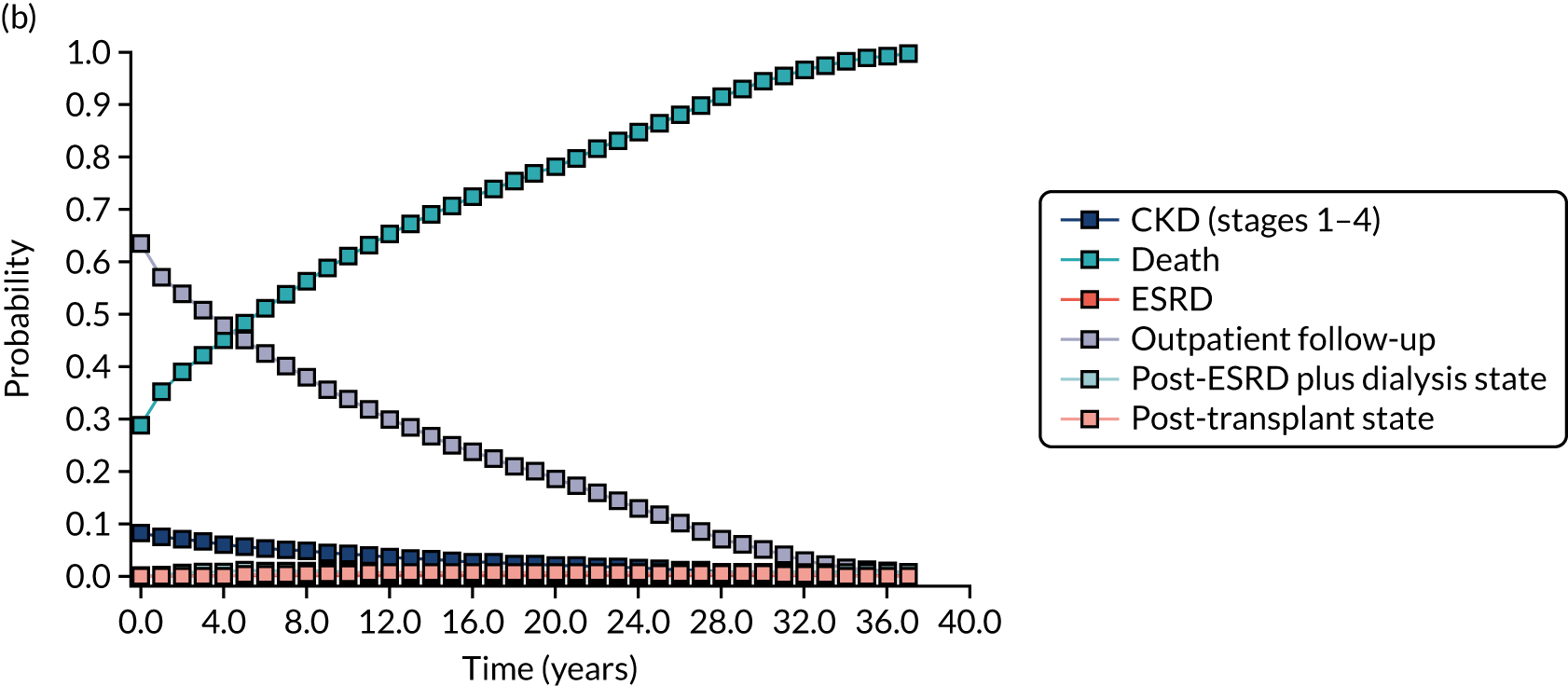
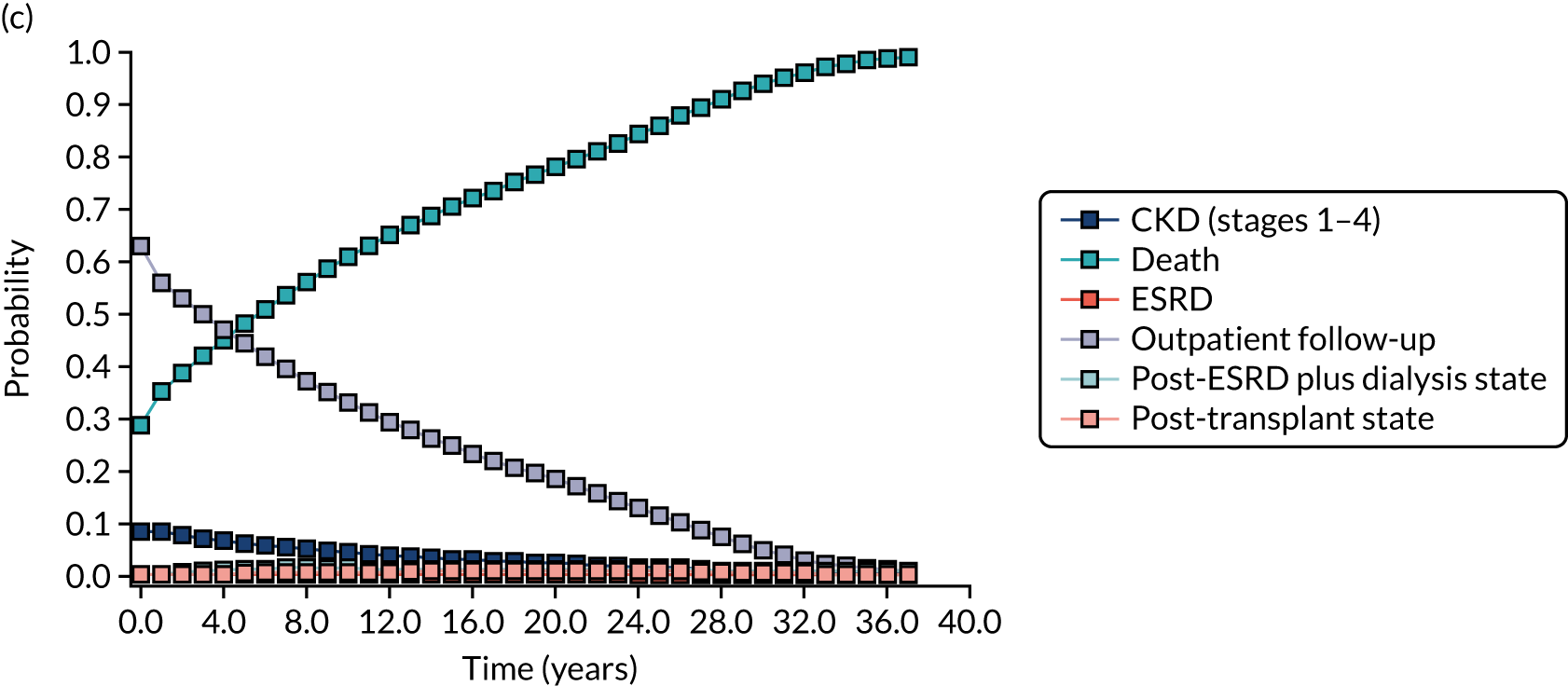
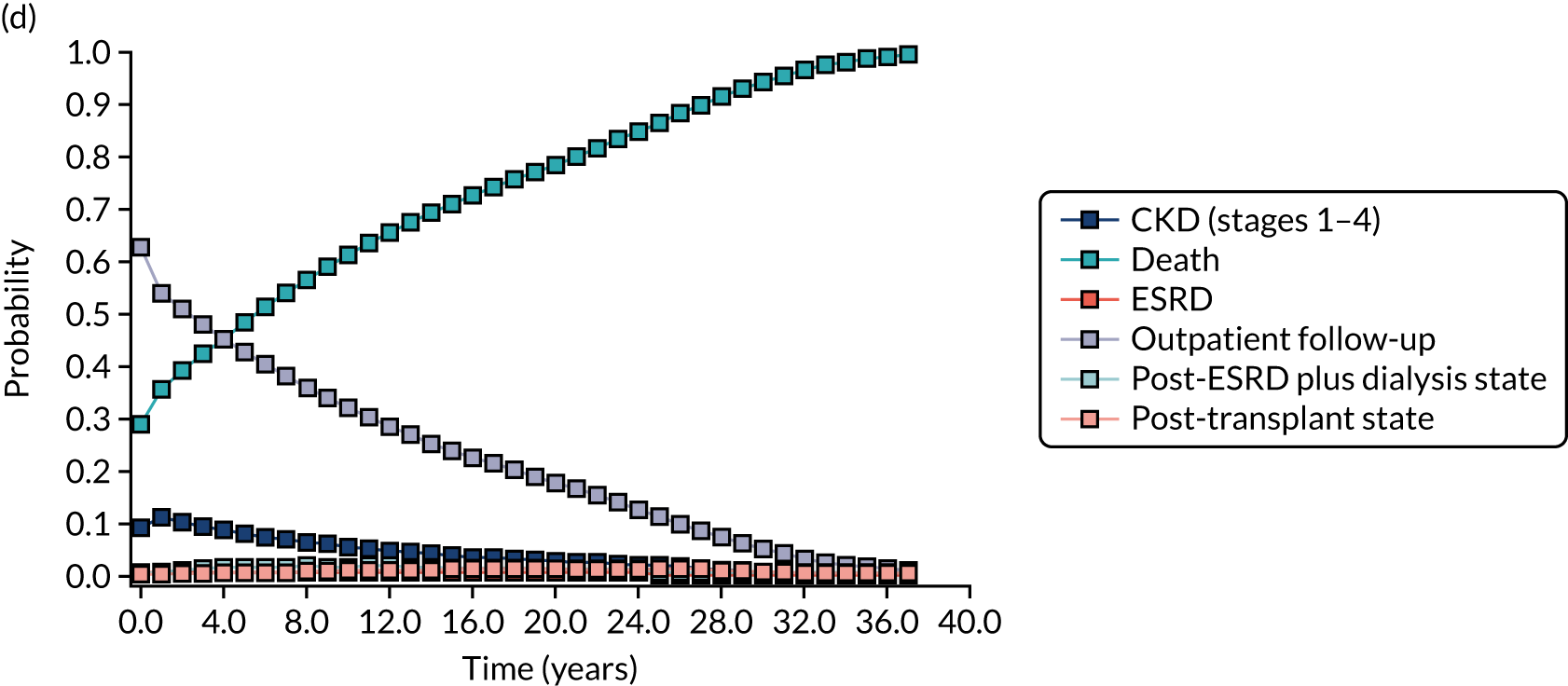
Cost-effectiveness acceptability curves
Figures 23 and 24 report cost-effectiveness acceptability curves for the two potential base-case scenarios.
FIGURE 23.
Cost-effectiveness acceptability curve: base case 1.
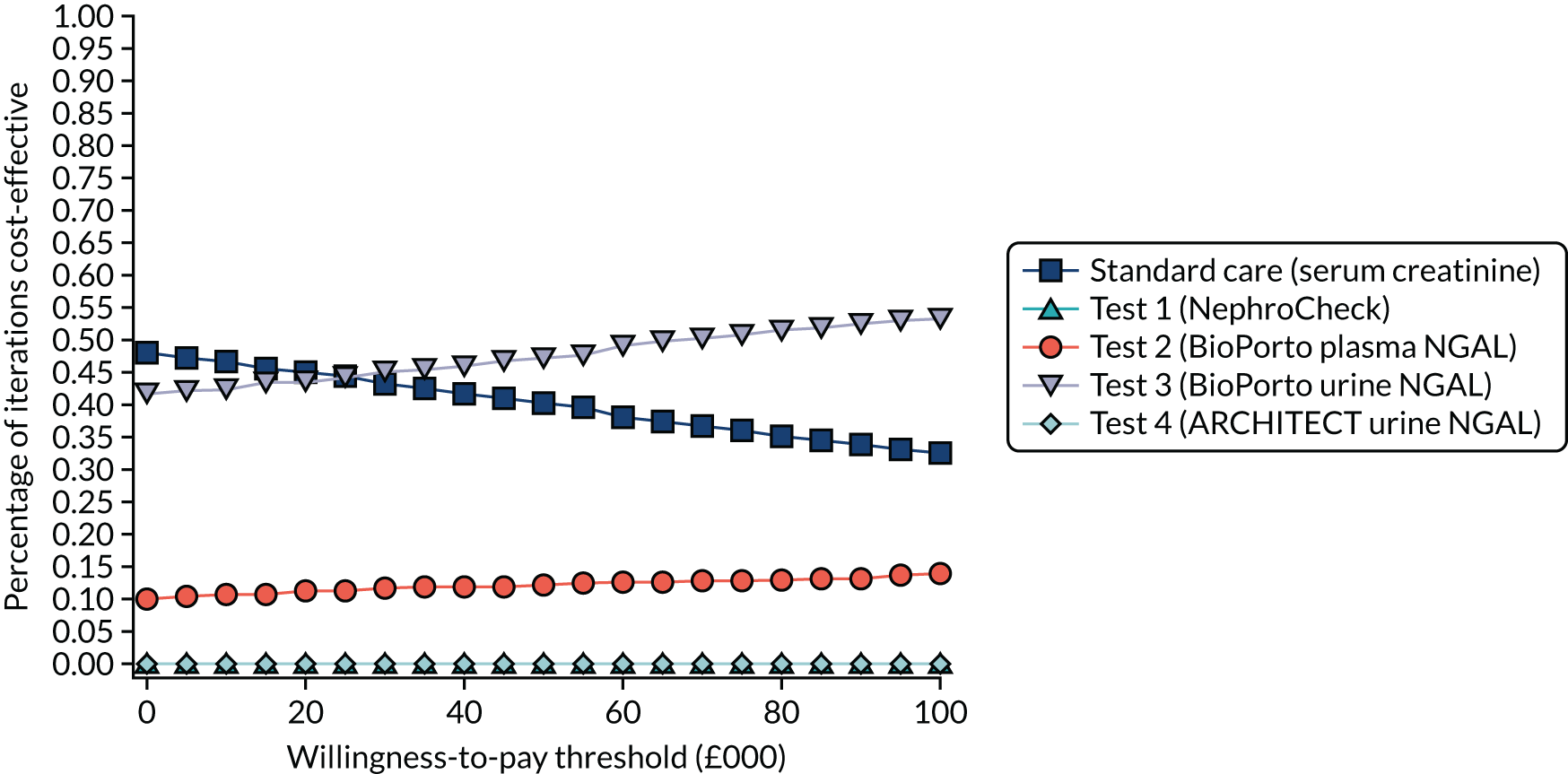
FIGURE 24.
Cost-effectiveness acceptability curve: base case 2 – subgroup analyses.
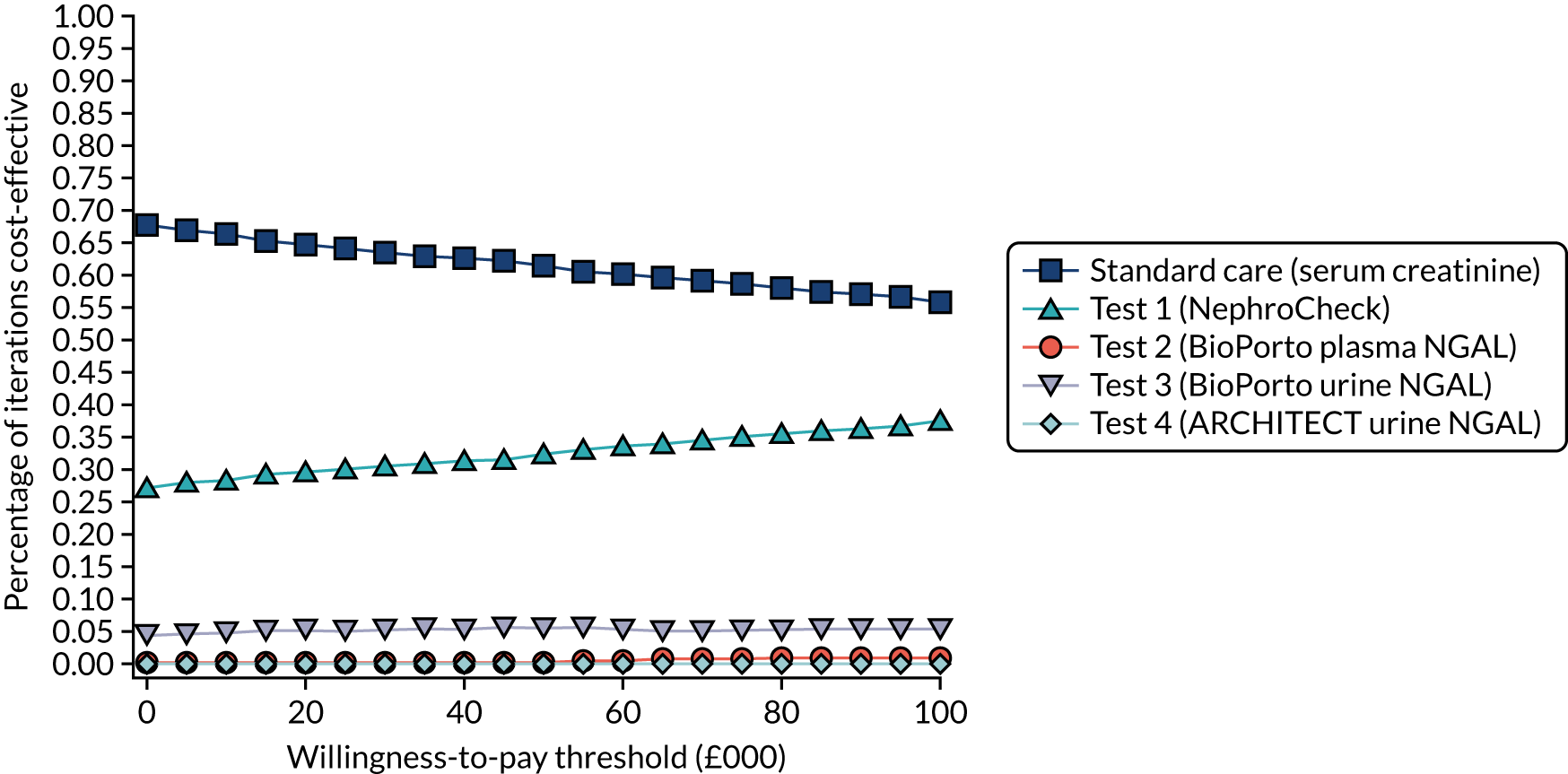
Three subgroup analyses have been carried out on the two EAG-suggested base-case strategies (based on whether or not NGAL is assumed to be capable of averting AKI). The subgroups considered are adult critical care and adult post cardiac surgery. As there was an insufficient amount of data to populate a robust model for a children subgroup, this was considered as an exploratory analysis only (as per Tables 20 and 21).
Critical care subgroup
For the critical care subgroup, the same parameter values as the all-comers are used for the downstream model probabilities, costs and utilities. This subgroup may be useful for decision-making as it could be considered as an alternative, potentially more seriously ill, definition of the population in the NICE scope. Although the group is defined as ‘critical care’, the populations described in the source diagnostic accuracy studies are often more reflective of a seriously ill patient group that would not yet be in ICU in the UK setting. The diagnostic accuracy data used for this subgroup are described in Table 22.
| Test | Parameter | Mean (95% CI) | Mean (logit scale) | SE (logit scale) | Correlation for multivariate normal distribution (logit scale) | Source |
|---|---|---|---|---|---|---|
| NephroCheck | Sensitivity | 0.83 (0.72 to 0.91) | 1.615 | 0.336 | –1.000 | Meta analysis (see Chapter 3) |
| Specificity | 0.51 (0.48 to 0.54) | 0.040 | 0.064 | |||
| BioPorto urine NGAL | Sensitivity | 0.72 (0.61 to 0.80) | 0.926 | 0.247 | 0.905 | Meta analysis (see Chapter 3) |
| Specificity | 0.87 (0.66 to 0.96) | 1.876 | 0.617 | |||
| ARCHITECT urine NGAL | Sensitivity | 0.70 (0.63 to 0.76) | 0.855 | 0.165 | 1.000 | Meta analysis (see Chapter 3) |
| Specificity | 0.72 (0.63 to 0.80) | 0.958 | 0.226 | |||
| BioPorto plasma NGAL | Sensitivity | 0.76 (0.56 to 0.89) | 1.156 | 0.462 | –1.000 | Meta analysis (see Chapter 3) |
| Specificity | 0.67 (0.40 to 0.86) | 0.686 | 0.566 |
The results of the critical care subgroup analysis are provided in Table 23.
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs. standard care | |||||||
| Critical care subgroup, applied to base case 1 | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,008 | – | 6.07439 | – | – | Dominant | 37.0 | 51.5 |
| Test 2 (BioPorto plasma NGAL) | 23,022 | £14 | 6.07440 | 0.00002 | £900,179 | Dominant | 12.1 | 45.7 |
| Standard care (serum creatinine) | 23,024 | Dominated | 6.07406 | Dominated | Dominated | – | 47.9 | – |
| Test 4 (ARCHITECT urine NGAL) | 23,029 | Dominated | 6.07438 | Dominated | Dominated | £15,046 | 1.8 | 42.3 |
| Test 1 (NephroCheck) | 23,057 | £36 | 6.07444 | 0.00004 | £905,334 | £87,368 | 1.2 | 34.2 |
| Critical care subgroup, applied to base case 2 | ||||||||
| Standard care (serum creatinine) | 22,904 | 6.07716 | 65.0 | – | ||||
| Test 1 (NephroCheck) | 22,937 | £32 | 6.07755 | 0.00039 | £82,079 | £82,079 | 31.4 | 32.8 |
| Test 3 (BioPorto urine NGAL) | 22,971 | Dominated | 6.07728 | Dominated | Dominated | £555,173 | 3.0 | 11.1 |
| Test 2 (BioPorto plasma NGAL) | 22,991 | Dominated | 6.07729 | Dominated | Dominated | £676,218 | 0.5 | 8.3 |
| Test 4 (ARCHITECT urine NGAL) | 22,991 | Dominated | 6.07728 | Dominated | Dominated | £732,572 | 0.1 | 7.9 |
Cardiac surgery subgroup
Diagnostic accuracy data were not available from the systematic review for all biomarker strategies for the cardiac surgery group, and were available from only single studies for some tests. When data were not available from the review, we used pooled estimates from Hall et al. ,97 but note that this analysis should be considered with caution as it includes test manufacturers outside the scope of the NICE assessment. The diagnostic accuracy data for the cardiac surgery subgroup are provided in Table 24 and are included probabilistically in the model when possible.
| Test | Measure | Mean (95% CI) | Mean logit | SE logit | Correlation for multivariate normal distributiona | Source |
|---|---|---|---|---|---|---|
| NephroCheck | Sensitivity | 0.31 (0.09 to 0.61) | –0.800 | 0.704 | –0.824 | Cummings et al.26 2019 |
| Specificity | 0.78 (0.74 to 0.82) | 1.266 | 0.120 | |||
| BioPorto urine NGAL | Sensitivity | 0.78 (0.72 to 0.84) | 1.266 | 0.182 | 0.526 | Yang et al.65 2017 |
| Specificity | 0.48 (0.42 to 0.54) | –0.080 | 0.123 | |||
| ARCHITECT urine NGAL | Sensitivity | 0.46 (0.33 to 0.59) | –0.160 | 0.274 | –0.517 | Parikh et al.95 2017 |
| Specificity | 0.81 (0.79 to 0.83) | 1.450 | 0.067 | |||
| BioPorto plasma NGAL | Sensitivity | 0.62 (0.49 to 0.74) | 0.490 | 0.277 | –1.000 | Hall et al.97 2018 |
| Specificity | 0.78 (0.75 to 0.81) | 1.266 | 0.090 |
Again, these results should be interpreted cautiously because of the lack of/limitations with the diagnostic accuracy data, and the questionable relevance of the downstream parameters/model structure for a cohort of post-cardiac patients only.
The results of the post-cardiac surgery subgroup analysis are provided in Table 25.
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs. standard care | |||||||
| Post-cardiac surgery subgroup (applied to scenario 1) | ||||||||
| Standard care (serum creatinine) | 22,912 | 6.07358 | 54.2 | |||||
| Test 2 (BioPorto plasma NGAL) | 22,914 | £2 | 6.07387 | 0.00029 | £7822 | £7822 | 17.8 | 45.5 |
| Test 3 (BioPorto urine NGAL) | 22,922 | £8 | 6.07394 | 0.00007 | £112,645 | £29,127 | 28.0 | 41.9 |
| Test 4 (ARCHITECT urine NGAL) | 22,938 | Dominated | 6.07380 | Dominated | Dominated | £120,552 | 0.0 | 30.1 |
| Test 1 (NephroCheck) | 22,984 | Dominated | 6.07373 | Dominated | Dominated | £484,944 | 0.0 | 9.6 |
| Post cardiac surgery subgroup (applied to scenario 6) | ||||||||
| Standard care (serum creatinine) | 22,983 | 6.07043 | 85.6 | |||||
| Test 2 (BioPorto plasma NGAL) | 23,055 | Extendedly dominated | 6.07054 | Extendedly dominated | Extendedly dominated | £679,042 | 3.8 | 8.4 |
| Test 1 (NephroCheck) | 23,057 | £74 | 6.07059 | 0.00016 | £465,544 | £465,544 | 6.5 | 8.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,062 | Dominated | 6.07051 | Dominated | Dominated | £996,121 | 0.1 | 4.0 |
| Test 3 (BioPorto urine NGAL) | 23,082 | Dominated | 6.07056 | Dominated | Dominated | £737,663 | 4.0 | 7.5 |
Interpretation of the results
Published data show that NephroCheck-guided implementation of a KDIGO care bundle has the potential to avert AKI. However, no such data exist for the NGAL tests. Therefore, two base-case analyses are considered. Base case 1 can be considered an optimistic scenario for the NGAL assays and assumes that all NGAL tests are equally as effective as NephroCheck in terms of the potential to avert AKI. Base case 2 can be considered a more conservative approach, in the absence of evidence, and assumes that only NephroCheck can avert AKI, but that all tests have the potential to reduce AKI severity if AKI occurs.
Fifteen scenario analyses are provided for each potential base case, ranging from a set of optimistic assumptions whereby biomarker-guided care bundles may lead to substantial improvements in health outcomes (need for ICU, hospital LOS, CKD, mortality) to a set of more conservative assumptions whereby changing of AKI status has no effects on health outcomes.
Incremental cost-effectiveness ratios are highly uncertain and subject to wide variation depending on the set of scenarios chosen. The probability of cost-effectiveness at an ICER of < £20,000 per QALY gained for scenarios in which NGAL is assumed to be equally as effective as NephroCheck in preventing AKI ranged from 0% to 15% (NephroCheck), 0% to 55% (BioPorto urine NGAL), 0% to 2% (ARCHITECT urine NGAL) and 0% to 48% (BioPorto plasma NGAL). BioPorto urine NGAL was generally the test associated with the greatest probability of cost-effectiveness, albeit this was highly uncertain, when compared with standard care. This is because BioPorto urine NGAL had slightly better diagnostic test accuracy data and slightly lower test costs than the comparator tests. However, there is substantial uncertainty in the diagnostic test accuracy, driven by study heterogeneity; therefore, results should be interpreted cautiously.
When it is assumed that NGAL tests cannot avert AKI, but can only reduce its severity, the cost-effectiveness case for NephroCheck improves substantially. However, cost-effectiveness remains highly uncertain, with a probability of cost-effectiveness ranging from 0% to 99% across the explored scenarios.
Given the significant uncertainties across the range of scenario analyses undertaken, it is not possible to draw robust conclusions on the cost-effectiveness of the respective biomarkers.
Chapter 5 Discussion
Statement of principal findings
In current clinical practice, identification of patients at risk of developing AKI poses a significant challenge to clinicians. Markers of kidney stress and/or injury are hoped to be a useful adjunct to current clinical care, as they may facilitate patient management and informed decisions about treatment. Nevertheless, pathways of presentation and care for AKI are complex, and the potential for modifiability and clinical benefit is uncertain. This assessment looked at the performance of NephroCheck, ARCHITECT and Alinity i urine NGAL assays and BioPorto urine and plasma NGAL assays to assess the risk of AKI in critically ill patients considered for admission to critical care. We included 56 studies with a total of 17,967 participants.
Clinical effectiveness
The main clinical effectiveness findings suggest that these biomarkers may have a role in AKI risk assessment in patients admitted to critical care. Evidence for other clinical settings (cardiac surgery, major non-cardiac surgery) was limited.
The results of meta-analyses indicate that the use of biomarkers may be useful for identifying AKI. However, because of substantial clinical and statistical heterogeneity between studies, and large 95% confidence and prediction regions, there is considerable uncertainty surrounding the validity and reliability of these findings. Moreover, the overall performance of the biomarkers for the detection of AKI, as seen by the meta-analyses of AUC estimates, appears to be modest, rather than excellent, with large boundaries of uncertainty. For example, for the adult population, the highest AUC value for detection of AKI was 0.76, but prediction intervals ranged from 0.33 to 0.99.
For prediction of relevant clinical outcomes, only a small number of studies were available for each biomarker in each clinical setting; this limited the possibility to perform pooled analyses.
Similarly, although there was an indication that addition of biomarkers to existing clinical models might improve the prediction of relevant clinical outcomes, studies varied considerably in terms of study characteristics and statistical methods used to assess prediction, thereby limiting any reliable conclusion.
Overall, as studies varied considerably in terms of clinical setting, timing of sample collection, optimal threshold level, assay platforms, definition of AKI, number of AKI events, time of AKI diagnosis and inclusion/exclusion criteria, the reliability and generalisability of the observed findings are highly uncertain.
We did not find any study that used the Alinity i urine NGAL test or assessed the performance of the biomarkers for prediction of CKD. Similarly, we did not identify any study that assessed the impact of the routine use of the biomarkers on specific clinical outcomes in critically ill patients compared with current standard care.
Cost-effectiveness
A probabilistic decision tree and Markov model were developed (adapted from the model used by Hall et al. 97) to describe the care pathway for a mixed prevalence cohort of CKD/no-CKD patients in a hospital setting for patients at risk of developing AKI. The decision tree part of the model captured the acute phase, up to the first 90 days, and modelled the risk of AKI and the potential for the use of biomarkers to prevent AKI or reduce its severity. We used a linked-evidence approach to derive hypothesised links between the presence/absence of AKI and AKI severity on changes in health outcomes (need for ICU care, LOS in hospital, need for acute RRT, 90-day mortality and development of CKD). In the absence of robust trial data, we derived these associations from a large, existing observational data set. 102 The Markov model describes the progression of four cohorts (no AKI, AKI 1, AKI 2 and AKI 3) through a set of mutually exclusive health states capturing CKD, ESRD, long-term dialysis, kidney transplant and mortality. Progression through these states depends on an individual’s AKI status in hospital, which influences the starting proportions in the Markov model CKD state.
The model includes health service perspective costs of biomarkers, early application of a KDIGO care bundle, hospitalisation (including ICU and ward costs), acute and long-term dialysis costs, long-term outpatient follow-up costs, and transplant and immunosuppressant costs. Health-state utility values and modelled mortality risk were combined to generate estimates of QALYs gained for each test. The model included the functionality to apply additional follow-up costs and mortality risk over the longer term for patients admitted to the ICU in their index admission.
The cumulative expected value of costs and QALYs were simulated over a lifetime time horizon for each cohort under standard care and each of the biomarker strategies; all results were reported as probabilistic ICERs. We found no trial data that could provide effect estimates for the extent to which biomarkers could both mitigate AKI and improve outcomes. Therefore, the model was built around a series of plausible proportional effects of averting/reducing the severity of AKI on changes in health outcomes. These ranged from optimistic scenarios in which patients who had AKI averted as a result of a biomarker-guided early implementation of a care bundle experienced the same risk of ICU, mortality and CKD as if they were in the no-AKI cohort, to more pessimistic scenarios in which the prevention of AKI or reduction of its severity had no impact on health outcomes.
The costs and QALYs for standard care and for each biomarker test strategy were ranked in ascending order of costs, whereby strategies that were more costly and less effective than an alternative were dominated and excluded from the calculation of the ICERs. In this scenario, the highest ICER under the threshold represents the strategy with the best value for money. All scenarios were also compared directly with standard care. In all cases, the probability of cost-effectiveness from the probabilistic simulation was reported.
Cost-effectiveness results were highly uncertain and ICERs were subject to wide variation depending on the set of scenarios chosen. The probability of cost-effectiveness at an ICER of < £20,000 per QALY gained for scenarios in which NGAL is assumed to be equally as effective as NephroCheck in preventing AKI ranged from 0% to 15% (NephroCheck), 0% to 55% (BioPorto urine NGAL), 0% to 2% (ARCHITECT urine NGAL) and 0% to 48% (BioPorto plasma NGAL). BioPorto urine NGAL was generally the test associated with the greatest probability of cost-effectiveness, albeit this was highly uncertain, when compared with standard care. This is because BioPorto urine NGAL had slightly better diagnostic test accuracy data and slightly lower test costs than the comparator tests. However, there is substantial uncertainty in the diagnostic test accuracy, driven by study heterogeneity; therefore, the results should be interpreted cautiously.
When it is assumed that NGAL tests cannot avert AKI, but can only reduce its severity, the cost-effectiveness case for NephroCheck improves substantially. However, cost-effectiveness remains highly uncertain, with a probability of cost-effectiveness ranging from 0% to 99% across the explored scenarios.
In general, the model results generate a less favourable assessment of cost-effectiveness for the biomarker tests than that of Hall et al. 97 There are five reasons for this. First, the prevalence of AKI in the Hall et al. 97 study was much higher (31.7%) than in our prevalent AKI population (9.2%). The higher prevalence might be explained by AKI being more common in the ICU setting (starting cohort in Hall et al. 97) than in a hospital ward (the starting cohort in our economic model).
Second, the settings are different. Hall et al. 97 evaluated the cost-effectiveness of biomarkers for detecting AKI in a critical care setting, whereas our assessment evaluated the cost-effectiveness of AKI biomarkers in a critically ill hospitalised cohort considered for admission to critical care. The data sources used to populate the acute phase of the model are different. Hall et al. 97 relied on daily transitions between ICU, hospital and discharge up to 90 days, whereas we have relied on a large observational data set to populate the potential link between changes in AKI status and health outcomes. Therefore, the costs and utilities applied in the acute phase of the base-case models differ between the two analyses.
Third, both models produce estimates of cost-effectiveness that are sensitive to the data used for the diagnostic accuracy of the tests. The diagnostic accuracy data applied in Hall et al. 97 are different from those obtained in our meta-analyses, probably because of new studies becoming available since the Hall et al. 97 publication and the wider setting for our model. For example, the sensitivity of NephroCheck was 0.90 in Hall et al. 97 and 0.75 in our meta-analysis. Consequently, NephroCheck identified more true-positive cases, which generated greater QALY gains in Hall et al. 97 than were generated in our model.
Fourth, we take a more conservative approach to the estimation of long-term follow-up costs for the base-case analysis and have not applied excess lifetime costs beyond the 5-year data reported in Lone et al. 114
Fifth, we further assume that there is no impact of AKI on follow-up costs beyond the 90 days, whereas Hall et al. 97 assume excess costs applied for the full lifetime time horizon. We also assume that the causal impact of AKI on CKD development ceases beyond the first cycle of the Markov model (i.e. 1.25 years after the AKI event), whereas Hall et al. 97 assume additional risk of CKD for the full lifetime time horizon of the model.
Overall, both models conclude that there is substantial uncertainty in the results, albeit predicting different base-case ICERs. The results are highly sensitive to key parameters in the model and any combination of the presented scenarios may be plausible.
Strengths and limitations of the assessment
The methods used to conduct this assessment were detailed and thorough. We conducted comprehensive literature searches of major electronic databases and relevant websites and assessed > 1000 full-text studies for eligibility. The large number of screened and extracted articles was necessary because key information (e.g. information on biomarker assays) was not available from the abstracts. This resulted in a need for significant literature screening resources and for considering strict inclusion criteria to ensure that the assessment remained feasible and timely. We restricted inclusion to studies that enrolled at least 100 participants and excluded studies on low-birthweight and preterm babies. It is possible that inclusion of all existing studies, irrespective of the sample size, might produce relevant findings. However, we reached a consensus that small and niche studies would not provide clinically generalisable evidence for pooling and would be underpowered to provide reliable evidence in isolation. Low-birthweight and preterm babies were considered a category of patients with specific care needs that are not generalisable to the population included in this assessment.
The primary weakness of the systematic review of clinical effectiveness evidence was the substantial clinical heterogeneity observed between studies. There was considerable heterogeneity, especially with regard to NGAL threshold levels, time of sample collection, definition of AKI and prevalence of AKI, time of AKI diagnosis, and assay platforms. Consequently, the diagnostic accuracy of individual tests varied considerably and the confidence and prediction regions in the pooled analyses were notably large. Moreover, when the studies had smaller numbers of AKI events (low prevalence), the relationship observed between sensitivity and specificity estimates became quite different from that of studies for which prevalence was higher.
Indeed, the shape and size of the prediction regions in the SROC plots were influenced by studies that showed a different relationship between sensitivity and specificity, compared with other studies. Hence, we do not have much confidence in the pooled estimates.
In particular, the intrinsic complexity of this assessment (multiple research questions, multiple biomarkers and sample media, multiple clinical settings, broad patient population, differences in assay platforms, definition of AKI) means that the findings reported here are also complex, particularly given the absence of robust trial evidence to support economic model development. Although the original scope of this assessment was the assessment of hospitalised patients considered to be at risk of admission to critical care, no studies focused on this specific group of patients (pre admission to critical care). Most studies were conducted outside the UK and assessed patients already admitted to intensive or critical care after different surgical procedures or with different (or multiple) clinical conditions. Furthermore, the provision of intensive care resources across the world are heterogeneous, so many studies will not be representative of how intensive care is utilised in the UK. This means that it is unclear how well the findings of heterogeneous studies which are predominantly based in intensive care and conducted outside the UK can be applied to a UK clinical scenario of people not currently receiving critical care, but at risk of requiring it.
Criteria used for the definition of AKI were consistent with current KDIGO recommendations, but differed slightly across studies with respect to operationalisation. This means that the extent of bidirectional misclassification of AKI and CKD may vary between studies and setting, which may affect biomarker performance. 143 In some studies, it was unclear whether or not the reported associations between biomarkers and AKI were indeed attributed to kidney injury. The current definition of AKI is based on elevations in serum creatinine concentration, which poses the conundrum of using an imperfect standard to assess the performance of biomarkers. Serum creatinine is not always measured at the same frequency as biomarkers are measured, and to ascertain the exact time of creatinine rise is problematic. As a result, the ‘ground truth’ of AKI existence could not be established with a gold-standard reference in any of the studies. In addition, no studies considered alternative methods for early or incipient AKI detection, such as the use of machine learning algorithms. 144
In some studies, we observed a very small number of AKI events, compared with other included studies. Interestingly, two studies conducted in the cardiac surgery setting [a medium-size single-centre study26 with 400 participants and a large multicentre study,38 the Translational Research Investigating Biomarker Endpoints (TRIBE) trial, with 1219 participants)] showed similar prevalence rates (4% and 5%, respectively) and a similar pattern of accuracy (poor sensitivity estimates and good specificity estimates). The number of AKI events are known to vary depending on both AKI definition and clinical setting, which underlies the heterogeneity of existing studies.
An unavoidable limitation of this evaluation is the variation in the use of NGAL tests. Threshold cut-off points to classify patients with and patients without AKI in each clinical setting were not consistent across studies. This means that differences between studies could relate to the chosen threshold, rather than NGAL performance. We selected one threshold per study, according to our inclusion criteria, and estimated the underlying SROC curve using a hierarchical model, which takes into account the within- and between-study variability. NGAL studies also varied with respect to analytic methods of measurement. Some studies used absolute urine concentrations, whereas others used NGAL concentrations normalised for urine creatinine concentrations. There were insufficient data available per type of biomarker and clinical setting to further investigate this source of variability, and determine the extent to which analytic methods influence estimates of diagnostic accuracy and whether or not it was sensible to pool results across studies. Nevertheless, we note that in the multicentre TRIBE prospective study38 assessing 1219 adults undergoing cardiac surgery, the authors repeated the analyses using NGAL urine-creatinine corrected values and did not observe improvements in the AUC values, compared with uncorrected results.
Several studies did not provide sensitivity, specificity and AUC values for the biomarkers for the diagnostic or prognostic accuracy of AKI. In future studies, accuracy measures such as sensitivity and specificity must be considered and defined rigorously at transparent cut-off points for predictive biomarkers, as they may need to vary according to clinical setting. 145
Notwithstanding analytic and threshold heterogeneity, the number of available studies for each type of assay in each clinical setting limited our ability to assess the role of the biomarkers for the prediction of relevant clinical outcomes. Furthermore, the number of events was small in many studies and the duration of follow-up was not consistent across studies, so mortality and RRT could not be reliably assessed at the same time points. Furthermore, details of the methods used for prediction analyses were insufficient in many studies. Although information on adjustment strategies and on the process of variable selection were usually provided, the original cohort of potential predictors, prior to the multivariable analysis, was never clearly specified, leading to potential risk of bias.
Finally, introduction of a biomarker would require evidence not just that it performs well as a predictor of modifiable and intervenable AKI, but also that there is incremental improvement of existing or alternative approaches to clinical care. There was insufficient information to determine with certainty whether or not the biomarkers had an incremental advantage over the traditional marker of serum creatinine and urine output, or had information available for clinical assessment. Only a limited number of studies compared the AUC of the biomarkers under investigation with that of serum creatinine for the detection of AKI, and fewer studies compared the performance of the biomarkers with that of clinical models for prediction of AKI or of relevant patient outcomes.
Uncertainties
Clinical effectiveness evidence
There is considerable uncertainty surrounding the generalisability of the studies to the UK population. Most of the studies were conducted outside the UK and assessed patients already admitted to critical care. Because no studies were identified for inclusion, we were not able to assess the impact that the routine use of these biomarkers may have on clinical outcomes of critically ill people considered for admission to critical care, compared with standard clinical assessment.
At present, in the literature, there is limited information on the benefits of incorporating biomarker results with those of current clinical criteria (serum creatinine and urine output) to improve the clinical management of patients with AKI. Recently, Zarbock et al. ,146 in a RCT of critically ill surgical patients with AKI, assessed the use of early versus delayed RRT. Plasma NGAL of > 150 ng/ml was one of the inclusion criteria, together with the KDIGO criteria. The trial results showed that early RRT, compared with delayed RRT, reduced mortality, duration of RRT and hospital stay, and that the combination of the KDIGO classification system with plasma NGAL was effective in identifying patients with deteriorating AKI. Subsequent negative results from the Artificial Kidney Initiation in Kidney Injury (AKIKI) RCT147 for critically ill medical patients suggest that these findings may apply to targeted circumstances only.
More recently (in 2017), the Zarbock group conducted a biomarker-guided RCT of patients who underwent cardiac surgery. 110 They used a biomarker-based approach (NephroCheck test) to identify high-risk patients and implement a bundle of supportive measures recommended by the KDIGO guidelines to reduce the occurrence of AKI, as well as that of mortality and RRT. Their results showed that implementation of the KDIGO guidelines, compared with standard care, reduced the frequency of AKI in the 72 hours after cardiac surgery. However, the trial did not show a reduction in the need for RRT, an improvement in mortality or a positive effect measure on any hard clinical outcome. The authors concluded that future, adequately powered, multicentre trials are required. Similarly, Göcze et al. ,116 in a study of major non-cardiac surgery patients, showed that the early adoption of a bundle of supportive measures according to the KDIGO guidelines in patients with NephroCheck concentrations of > 0.3 (ng/ml)2/1000 resulted in a reduced occurrence of AKI, decreased hospital and ICU stay, and reduced costs, but, again, there was no evidence of improvement of hard outcomes (need for RRT, mortality, or major kidney events).
Overall, despite some evidence suggesting possible improvement of care processes and health care use when biomarker-guided care bundles are used alongside KDIGO criteria, there is still considerable uncertainty regarding effects on health outcomes, particularly when used in the pre-critical care setting. In addition, the optimal threshold for NGAL and how this changes according to different clinical settings have yet to be established. Future studies should evaluate the targeted use of the biomarkers within specific clinical populations and circumstances in which there is potential for benefit with a plausible and feasible intervention. In particular, they should focus on the assessment of the impact of routine biomarker use on a reduction in mortality, major clinical adverse events, modification of clinical care, and resource use. In other words, future research should evaluate the use of these biomarkers to improve patients’ clinical outcomes and management.
Discrete urine and plasma NGAL cut-off points for differentiating between AKI and non-AKI patients in each clinical setting need to be identified and the timing of collection of biomarker concentrations should be set out more clearly according to each setting. In line with the recommendations from the 10th Acute Dialysis Quality Initiative consensus conference,148 there is also a need to harmonise the methods and platforms for collection, handling and storage of urine and plasma samples. Furthermore, it would be useful to harmonise the reporting of biomarker concentrations (e.g. absolute concentrations, ratio to urine creatinine) and corroborate techniques for normalising urine biomarker concentrations to urine creatinine concentrations.
Finally, it is well recognised that AKI encompasses a range of clinical aetiologies, phenotypes and patterns of renal recovery. In addition, current measures of AKI may be insufficient to disentangle AKI that is predominantly functional without kidney damage from people with incipient subclinical damage, and people with both AKI and kidney damage. In this context, it remains unclear how phenotypic information on people with AKI should most usefully be combined to help target those most likely to benefit from earlier recognition and timely intervention, nor how such an intervention may differ between clinical phenotypes. 148
Cost-effectiveness evidence
There are three key areas of uncertainty in the economic evaluation modelling that limit the robustness of the cost-effectiveness results: (1) the lack of evidence on the impact of the biomarkers on health outcomes, (2) the heterogeneity in the diagnostic accuracy data (including uncertainty in the prevalence of AKI in a broad, poorly defined population) and (3) the uncertainty around the impact of a NGAL-guided implementation of a KDIGO care bundle on the frequency and severity of AKI. Given these uncertainties, the choice of a preferred base-case scenario is challenging and the observed results should be considered cautiously. These are speculative analyses ranging from a set of pessimistic scenarios to a set of optimistic scenarios for the use of the biomarkers under assessment.
Specifically, there is no evidence to describe the impact of the use of the AKI biomarkers on important health outcomes (such as need for ICU care, length of hospital stay, risk of 90-day mortality or development of new/progression of existing CKD). Accordingly, the cost-effectiveness results are based on a linked-evidence approach, whereby we have relied on observational associations to infer how prevention or mitigation of AKI may affect changes in health outcomes. These associations necessitate causal assumptions, but, although a causal link between AKI and poor outcomes is plausible, the extent of this causal relationship is uncertain and controversial. 149,150 The cost-effectiveness results are therefore presented for a range of alternative, but potentially plausible, scenario analyses ranging from a set of optimistic assumptions in which biomarker-guided care bundles may lead to substantial improvements in health outcomes (need for ICU care, CKD, mortality) to a set of more conservative assumptions in which change in AKI status has no effect on health outcomes. It is likely that the true estimate of cost-effectiveness lies somewhere between these two extremes.
Furthermore, the diagnostic accuracy data used in the economic model are obtained from studies that are considerably heterogeneous in terms of baseline AKI prevalence, timing of sample collection, threshold values and definition of AKI. Given the difficulty in defining the population that fits within the scope of this assessment, it is unclear how generalisable the diagnostic accuracy data are to the UK population in which the biomarkers could be used.
Also of note are additional uncertainties in the model that make it difficult to come to conclusions about the relative cost-effectiveness of each biomarker. For example, although there is some evidence in the literature, from Meersch et al. ,110 that early NephroCheck-guided implementation of a KDIGO care bundle may improve AKI status at 72 hours, the potential for similar improvements using NGAL is unknown. Therefore, we have considered two scenarios for the cost-effectiveness analyses. The first assumes, optimistically, that all NGAL tests are equally as effective at preventing AKI or reducing its severity as NephroCheck; the second, based on the available data from Meersch et al. ,110 assumes that NGAL can reduce the severity of AKI once it occurs, but cannot prevent its occurrence.
Because of these uncertainties, the results of the cost-effectiveness modelling are largely speculative and should be interpreted with caution. Although extensive probabilistic analyses are carried out for scenario analyses, these may still not fully capture the uncertainty faced in the implementation of these biomarkers in clinical practice.
In summary, the current evidence base is insufficient to make a full appraisal of the economic value of the biomarkers under investigation to provide cost-effective improvements in clinical outcomes of AKI. Therefore, we have provided a range of scenarios that cannot answer the full remit of this evaluation. We believe that the scenarios illustrate what might be required for the biomarkers to be cost-effective, highlighting, through the assumptions involved, the current gaps where further research is required.
Chapter 6 Conclusions
Implications for clinical practice and future research
We found that novel biomarkers have the ability to predict the presence or onset of AKI, but additional research is required to understand the incremental value of using these biomarkers on top of existing standard care. In addition, research that considers the utility of biomarkers on top of other novel approaches such as machine learning approaches to recognise incipient AKI in different clinical environments would be valuable.
There was limited trial evidence that the course of AKI in critical care circumstances may be modifiable or avoidable with early biomarker-guided care bundle approaches. Future research is needed to understand whether or not this is dependent on a well-performing timely biomarker, a care bundle appropriate for clinical context, or both. Research is also required to further evaluate such approaches outside the critical care setting.
Current literature is inadequate to determine whether or not biomarker-guided intervention can lead to hard clinical and economic outcomes, in addition to amelioration of AKI severity. The specific clinical circumstances in which benefit exists, and whether or not such benefit is dependent on reduction of AKI severity or is mediated through other means, would also be informative for future evaluations.
Uncertainty remains around the process of renal recovery and non-recovery after AKI. Mechanistic work exploring the nature, timing and extent of the recovery process could inform the nature and circumstances in which a biomarker-guided intervention might be effective. Similarly, clinical research on the timing and extent of renal recovery with different AKI phenotypes would enhance the ability to model cost-effectiveness of biomarker-guided therapies in different subsets of AKI.
In brief, we have identified the following research priorities:
-
further research in the form of adequately powered, well-designed RCTs to determine the incremental value of biomarkers on clinical outcomes (such as mortality and development of CKD), quality of life, resource use, costs and cost-effectiveness
-
further research to determine the clinical effectiveness and cost-effectiveness of adopting an early care bundle for the prevention/treatment of AKI, compared with standard care
-
further research to help understand the quality-of-life (utility) implications for patients admitted to ICU care
-
Future research to consider the potential roles for biomarker use in niche clinical areas, such as to assist in the discrimination of high- and low-risk patients who are already receiving critical care immediately after major surgery.
In addition, in the nephrology clinical research community, there is a need for efforts to standardise definitions and methods for studying AKI, kidney disease, progression, operationalisation of biomarkers and their interpretation.
Acknowledgements
The authors are grateful to Shona Methven for her help in developing the research protocol for this assessment and to Lara Kemp for her secretarial support.
Contributions of authors
Miriam Brazzelli (https://orcid.org/0000-0002-7576-6751) (Reader on Research) planned the systematic review of the clinical evidence, contributed to the statistical analyses and interpreted the results. She also oversaw and co-ordinated all aspects of this assessment.
Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Senior Statistician) conducted the statistical analyses.
Magaly Aceves-Martins (https://orcid.org/0000-0002-9441-142X), Clare Robertson (https://orcid.org/0000-0001-6019-6795) and Mari Imamura (https://orcid.org/0000-0003-4871-0354) (Research Fellows) selected relevant papers from the literature, and performed data extraction and risk-of-bias assessments for all included studies.
Elisabet Jacobsen (https://orcid.org/0000-0002-3211-936X) (Research Assistant) reviewed the evidence on the cost-effectiveness of the biomarkers under investigation, and contributed to the acquisition of input data and to the economic evaluation under the supervision of Dwayne Boyers.
Amudha Poobalan (https://orcid.org/0000-0002-6975-3874) (Senior Lecturer in Public Health) contributed to the data extraction and risk-of-bias assessments of included studies.
Paul Manson (https://orcid.org/0000-0002-1405-1795) (Information Specialist) developed and ran the literature searches, retrieved full-text copies of the selected papers and provided information support throughout the project.
Graham Scotland (https://orcid.org/0000-0001-5539-8819) (Reader on Research) provided senior advice and guidance on the development of the economic model.
Callum Kaye (https://orcid.org/0000-0002-6904-7048) (Consultant in Anaesthetics and Intensive Care Medicine) and Simon Sawhney (https://orcid.org/0000-0002-7960-4573) (Clinical Lecturer in Nephrology) provided expert advice and guidance on the clinical aspects of this assessment.
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Health Economist) developed the economic model, conducted cost-effectiveness analyses and interpreted the results.
All authors contributed to the writing of this report.
Publications
Jacobsen E, Sawhney S, Brazzelli M, Aucott L, Scotland G, Aceves-Martins M, et al. Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury. BMC Nephrol 2021;22:399.
Data-sharing statement
All technical data are included in the main text or as appendices to this report. All queries should be submitted to the corresponding author for consideration.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Bedford M, Stevens PE, Wheeler TWK, Farmer CKT. What is the real impact of acute kidney injury?. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-95.
- Selby NM, Crowley L, Fluck RJ, McIntyre CW, Monaghan J, Lawson N, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol 2012;7:533-40. https://doi.org/10.2215/CJN.08970911.
- National Confidential Enquiry into Patient Outcome and Death . Adding Insult to Injury: A Review of the Care of Patients Who Died in Hospital With a Primary Diagnosis of Acute Kidney Injury (Acute Renal Failure). A Report by the National Confidential Enquiry into Patient Outcome and Death 2009.
- Bell S, Lim M. Optimizing peri-operative care to prevent acute kidney injury [published online ahead of print September 11 2018]. Nephrol Dial Transplant 2018. https://doi.org/10.1093/ndt/gfy269.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012;2:1-138.
- National institute for Health and Care Excellence . NephroCheck Test to Help Assess the Risk of Acute Kidney Injury in Critically Ill Patients. Medtech Innovation Briefing [MIB156] 2018. www.nice.org.uk/advice/mib156 (accessed 5 April 2019).
- Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0120863.
- NHS Digital . Acute Kidney Injury n.d. www.nhs.uk/conditions/acute-kidney-injury/ (accessed 18 April 2019).
- O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med 2016;42:521-30. https://doi.org/10.1007/s00134-015-4157-7.
- Haines RW, Fowler AJ, Kirwan CJ, Prowle JR. The incidence and associations of acute kidney injury in trauma patients admitted to critical care: a systematic review and meta-analysis. J Trauma Acute Care Surg 2019;86:141-7. https://doi.org/10.1097/TA.0000000000002085.
- Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg 2010;252:158-65. https://doi.org/10.1097/SLA.0b013e3181deb6bc.
- Bihorac A, Brennan M, Ozrazgat-Baslanti T, Bozorgmehri S, Efron PA, Moore FA, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med 2013;41:2570-83. https://doi.org/10.1097/CCM.0b013e31829860fc.
- National Institute for Health and Care Excellence . Clinical Knowledge Summary: Acute Kidney Injury 2018. https://cks.nice.org.uk/acute-kidney-injury (accessed 5 April 2019).
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup . Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. https://doi.org/10.1186/cc2872.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11. https://doi.org/10.1186/cc5713.
- National Institute for Health and Care Excellence . Acute Kidney Injury: Prevention, Detection and Management. Clinical Guideline [CG169] 2013. www.nice.org.uk/guidance/cg169/chapter/1-Recommendations#assessing-risk-of-acute-kidney-injury (accessed 5 April 2019).
- Royal College of Physicians . National Early Warning Score (NEWS)2 2017. www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (accessed 18 April 2019).
- NHS England . Acute Kidney Injury (AKI) Algorithm 2014. www.england.nhs.uk/akiprogramme/aki-algorithm/ (accessed 18 April 2019).
- Kidney Disease: Improving Global Outcomes . KDIGO Clinical Practice Guideline for Acute Kidney Injury. Online Appendices A–F: March 2012 n.d. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-AKI-Suppl-Appendices-A-F_March2012.pdf (accessed 4 April 2019).
- Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007;71:1028-35. https://doi.org/10.1038/sj.ki.5002231.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51-8. https://doi.org/10.7326/M18-1376.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Harbord RM, Whiting P. metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata J 2009;9:211-29. https://doi.org/10.1177/1536867X0900900203.
- Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FT. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg 2019;157:1545-53.e5. https://doi.org/10.1016/j.jtcvs.2018.08.090.
- Oezkur M, Magyar A, Thomas P, Stork T, Schneider R, Bening C, et al. TIMP-2*IGFBP7 (Nephrocheck®) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hours. Kidney Blood Press Res 2017;42:456-67. https://doi.org/10.1159/000479298.
- Beitland S, Waldum-Grevbo BE, Nakstad ER, Berg JP, Trøseid AS, Brusletto BS, et al. Urine biomarkers give early prediction of acute kidney injury and outcome after out-of-hospital cardiac arrest. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1503-2.
- Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189:932-9. https://doi.org/10.1164/rccm.201401-0077OC.
- Di Leo L, Nalesso F, Garzotto F, Xie Y, Yang B, Virzì GM, et al. Predicting acute kidney injury in intensive care unit patients: the role of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 biomarkers. Blood Purif 2018;45:270-7. https://doi.org/10.1159/000485591.
- Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2) • IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int 2019;95:1486-93. https://doi.org/10.1016/j.kint.2019.01.020.
- Gayat E, Touchard C, Hollinger A, Vieillard-Baron A, Mebazaa A, Legrand M. FROG ICU study investigators . Back-to-back comparison of penKID with NephroCheck® to predict acute kidney injury at admission in intensive care unit: a brief report. Crit Care 2018;22. https://doi.org/10.1186/s13054-018-1945-9.
- Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014;29:2054-61. https://doi.org/10.1093/ndt/gfu292.
- Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17. https://doi.org/10.1186/cc12503.
- Kimmel M, Shi J, Wasser C, Biegger D, Alscher MD, Schanz MB. Urinary [TIMP-2] [IGFBP7] – novel biomarkers to predict acute kidney injury. Am J Nephrol 2016;43:375-82. https://doi.org/10.1159/000446451.
- Kimmel M, Shi J, Latus J, Wasser C, Kitterer D, Braun N, et al. Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol 2016;11:938-46. https://doi.org/10.2215/CJN.10551015.
- Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22:1748-57. https://doi.org/10.1681/ASN.2010121302.
- Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 2013;8:1079-88. https://doi.org/10.2215/CJN.10971012.
- Koyner JL, Coca SG, Thiessen-Philbrook H, Patel UD, Shlipak MG, Garg AX, et al. Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium . Urine biomarkers and perioperative acute kidney injury: the impact of preoperative estimated GFR. Am J Kidney Dis 2015;66:1006-14. https://doi.org/10.1053/j.ajkd.2015.07.027.
- Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 2014;25:1063-71. https://doi.org/10.1681/ASN.2013070742.
- Brown JR, Thiessen-Philbrook H, Goodrich CA, Bohm AR, Alam SS, Coca SG, et al. Are urinary biomarkers better than acute kidney injury duration for predicting readmission?. Ann Thorac Surg 2019;107:1699-705. https://doi.org/10.1016/j.athoracsur.2019.02.005.
- Coca SG, Nadkarni GN, Garg AX, Koyner J, Thiessen-Philbrook H, McArthur E, et al. First Post-operative urinary kidney injury biomarkers and association with the duration of AKI in the TRIBE-AKI cohort. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0161098.
- Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al. Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol 2015;26:3123-32. https://doi.org/10.1681/ASN.2014080764.
- Greenberg JH, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Parikh CR, Zappitelli M. TRIBE-AKI Consortium . Kidney injury biomarkers 5 years after AKI due to pediatric cardiac surgery. Pediatr Nephrol 2018;33:1069-77. https://doi.org/10.1007/s00467-018-3888-4.
- Albert C, Albert A, Bellomo R, Kropf S, Devarajan P, Westphal S, et al. Urinary neutrophil gelatinase-associated lipocalin-guided risk assessment for major adverse kidney events after open-heart surgery. Biomark Med 2018;12:975-85. https://doi.org/10.2217/bmm-2018-0071.
- Garcia-Alvarez M, Glassford NJ, Betbese AJ, Ordoñez J, Baños V, Argilaga M, et al. Urinary neutrophil gelatinase-associated lipocalin as predictor of short- or long-term outcomes in cardiac surgery patients. J Cardiothorac Vasc Anesth 2015;29:1480-8. https://doi.org/10.1053/j.jvca.2015.05.060.
- Liebetrau C, Dörr O, Baumgarten H, Gaede L, Szardien S, Blumenstein J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand J Clin Lab Invest 2013;73:392-9. https://doi.org/10.3109/00365513.2013.787149.
- Thanakitcharu P, Jirajan B. Determination of urinary neutrophil gelatinase-associated lipocalin (NGAL) cut-off level for early detection of acute kidney injury in Thai adult patients undergoing open cardiac surgery. J Med Assoc Thai 2014;97:48-55.
- Cullen MR, Jhanji S, Pearse RM, Fitzgibbon MC. Neutrophil gelatinase-associated lipocalin and albuminuria as predictors of acute kidney injury in patients treated with goal-directed haemodynamic therapy after major abdominal surgery. Ann Clin Biochem 2014;51:392-9. https://doi.org/10.1177/0004563213507438.
- Asada T, Isshiki R, Hayase N, Sumida M, Inokuchi R, Noiri E, et al. Impact of clinical context on acute kidney injury biomarker performances: differences between neutrophil gelatinase-associated lipocalin and L-type fatty acid-binding protein. Sci Rep 2016;6. https://doi.org/10.1038/srep33077.
- Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF, et al. Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail 2012;14:1020-9. https://doi.org/10.1093/eurjhf/hfs087.
- Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail 2012;14:597-604. https://doi.org/10.1093/eurjhf/hfs039.
- Isshiki R, Asada T, Sumida M, Hamasaki Y, Nangaku M, Noiri E, et al. Modest impact of serial measurements of acute kidney injury biomarkers in an adult intensive care unit. Nephron 2018;139:243-53. https://doi.org/10.1159/000488219.
- Kokkoris S, Parisi M, Ioannidou S, Douka E, Pipili C, Kyprianou T, et al. Combination of renal biomarkers predicts acute kidney injury in critically ill adults. Ren Fail 2012;34:1100-8. https://doi.org/10.3109/0886022X.2012.713279.
- Mårtensson J, Glassford NJ, Jones S, Eastwood GM, Young H, Peck L, et al. Urinary neutrophil gelatinase-associated lipocalin to hepcidin ratio as a biomarker of acute kidney injury in intensive care unit patients. Minerva Anestesiol 2015;81:1192-200.
- Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 2012;59:246-55. https://doi.org/10.1016/j.jacc.2011.10.854.
- Park M, Hsu CY, Go AS, Feldman HI, Xie D, Zhang X, et al. Urine kidney injury biomarkers and risks of cardiovascular disease events and all-cause death: the CRIC study. Clin J Am Soc Nephrol 2017;12:761-71. https://doi.org/10.2215/CJN.08560816.
- Pipili C, Ioannidou S, Tripodaki ES, Parisi M, Douka E, Vasileiadis I, et al. Prediction of the renal replacement therapy requirement in mechanically ventilated critically ill patients by combining biomarkers for glomerular filtration and tubular damage. J Crit Care 2014;29:692.e7-13. https://doi.org/10.1016/j.jcrc.2014.02.011.
- Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, Sallapant S, Tiranathanagul K, Komolmit P, et al. Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol 2015;15. https://doi.org/10.1186/s12876-015-0372-5.
- Haase M, Bellomo R, Albert C, Vanpoucke G, Thomas G, Laroy W, et al. The identification of three novel biomarkers of major adverse kidney events. Biomark Med 2014;8:1207-17. https://doi.org/10.2217/bmm.14.90.
- Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, et al. Comparison of plasma and urine biomarker performance in acute kidney injury. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0145042.
- Jaques DA, Spahr L, Berra G, Poffet V, Lescuyer P, Gerstel E, et al. Biomarkers for acute kidney injury in decompensated cirrhosis: a prospective study. Nephrology 2019;24:170-80. https://doi.org/10.1111/nep.13226.
- De Loor J, Herck I, Francois K, Van Wesemael A, Nuytinck L, Meyer E, et al. Diagnosis of cardiac surgery-associated acute kidney injury: differential roles of creatinine, chitinase 3-like protein 1 and neutrophil gelatinase-associated lipocalin: a prospective cohort study. Ann Intensive Care 2017;7. https://doi.org/10.1186/s13613-017-0251-z.
- Tidbury N, Browning N, Shaw M, Morgan M, Kemp I, Matata B. Neutrophil gelatinase-associated lipocalin as a marker of postoperative acute kidney injury following cardiac surgery in patients with preoperative kidney impairment. Cardiovasc Hematol Disord Drug Targets 2019;19:239-48. https://doi.org/10.2174/1871529X19666190415115106.
- Yang X, Chen C, Teng S, Fu X, Zha Y, Liu H, et al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3373-82. https://doi.org/10.1681/ASN.2017020142.
- Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-169.
- Ariza X, Graupera I, Coll M, Solà E, Barreto R, García E, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol 2016;65:57-65. https://doi.org/10.1016/j.jhep.2016.03.002.
- Barreto R, Elia C, Solà E, Moreira R, Ariza X, Rodríguez E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol 2014;61:35-42. https://doi.org/10.1016/j.jhep.2014.02.023.
- Cho E, Yang HN, Jo SK, Cho WY, Kim HK. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J Korean Med Sci 2013;28:100-5. https://doi.org/10.3346/jkms.2013.28.1.100.
- Doi K, Noiri E, Nangaku M, Yahagi N, Jayakumar C, Ramesh G. Repulsive guidance cue semaphorin 3A in urine predicts the progression of acute kidney injury in adult patients from a mixed intensive care unit. Nephrol Dial Transplant 2014;29:73-80. https://doi.org/10.1093/ndt/gft414.
- Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011;39:2464-9. https://doi.org/10.1097/CCM.0b013e318225761a.
- Hjortrup PB, Haase N, Treschow F, Møller MH, Perner A. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand 2015;59:25-34. https://doi.org/10.1111/aas.12427.
- Matsa R, Ashley E, Sharma V, Walden AP, Keating L. Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients. Crit Care 2014;18. https://doi.org/10.1186/cc13958.
- Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 2008;148:810-19. https://doi.org/10.7326/0003-4819-148-11-200806030-00003.
- Nisula S, Yang R, Poukkanen M, Vaara ST, Kaukonen KM, Tallgren M, et al. Predictive value of urine interleukin-18 in the evolution and outcome of acute kidney injury in critically ill adult patients. Br J Anaesth 2015;114:460-8. https://doi.org/10.1093/bja/aeu382.
- Nisula S, Yang R, Kaukonen KM, Vaara ST, Kuitunen A, Tenhunen J, et al. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth Analg 2014;119:95-102. https://doi.org/10.1213/ANE.0000000000000243.
- Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant 2013;28:1569-79. https://doi.org/10.1093/ndt/gfs586.
- Tecson KM, Erhardtsen E, Eriksen PM, Gaber AO, Germain M, Golestaneh L, et al. Optimal cut points of plasma and urine neutrophil gelatinase-associated lipocalin for the prediction of acute kidney injury among critically ill adults: retrospective determination and clinical validation of a prospective multicentre study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016028.
- Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012;57:2362-70. https://doi.org/10.1007/s10620-012-2180-x.
- Zelt JGE, Mielniczuk LM, Liu PP, Dupuis JY, Chih S, Akbari A, et al. Utility of novel cardiorenal biomarkers in the prediction and early detection of congestive kidney injury following cardiac surgery. J Clin Med 2018;7. https://doi.org/10.3390/jcm7120540.
- Itenov TS, Jensen JU, Ostrowski SR, Johansson PI, Thormar KM, Lundgren JD, et al. ‘Procalcitonin And Survival Study’ study group . Endothelial damage signals refractory acute kidney injury in critically ill patients. Shock 2017;47:696-701. https://doi.org/10.1097/SHK.0000000000000804.
- Lee DH, Lee BK, Cho YS, Jung YH, Lee SM, Park JS, et al. Plasma neutrophil gelatinase-associated lipocalin measured immediately after restoration of spontaneous circulation predicts acute kidney injury in cardiac arrest survivors who underwent therapeutic hypothermia. Ther Hypothermia Temp Manag 2018;8:99-107. https://doi.org/10.1089/ther.2017.0039.
- Marino R, Struck J, Hartmann O, Maisel AS, Rehfeldt M, Magrini L, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol 2015;28:717-24. https://doi.org/10.1007/s40620-014-0163-z.
- Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 2011;22:1737-47. https://doi.org/10.1681/ASN.2010111163.
- Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 2015;169:583-91. https://doi.org/10.1001/jamapediatrics.2015.54.
- Bojan M, Vicca S, Lopez-Lopez V, Mogenet A, Pouard P, Falissard B, et al. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol 2014;9:285-94. https://doi.org/10.2215/CJN.04730513.
- Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008;3:665-73. https://doi.org/10.2215/CJN.04010907.
- Cantinotti M, Storti S, Lorenzoni V, Arcieri L, Moschetti R, Murzi B, et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery. Clin Chem Lab Med 2012;50:2009-17. https://doi.org/10.1515/cclm-2012-0125.
- Alcaraz AJ, Gil-Ruiz MA, Castillo A, López J, Romero C, Fernández SN, et al. Postoperative neutrophil gelatinase-associated lipocalin predicts acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med 2014;15:121-30. https://doi.org/10.1097/PCC.0000000000000034.
- Seitz S, Rauh M, Gloeckler M, Cesnjevar R, Dittrich S, Koch AM. Cystatin C and neutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgery. Swiss Med Wkly 2013;143. https://doi.org/10.4414/smw.2013.13744.
- Zwiers AJ, de Wildt SN, van Rosmalen J, de Rijke YB, Buijs EA, Tibboel D, et al. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: a prospective cohort study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0910-0.
- Dong L, Ma Q, Bennett M, Devarajan P. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol 2017;32:2351-60. https://doi.org/10.1007/s00467-017-3748-7.
- Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbiesetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine?. Pediatr Nephrol 2015;30:665-76. https://doi.org/10.1007/s00467-014-2987-0.
- Park JT. Postoperative acute kidney injury. Korean J Anesthesiol 2017;70:258-66. https://doi.org/10.4097/kjae.2017.70.3.258.
- Parikh A, Rizzo JA, Canetta P, Forster C, Sise M, Maarouf O, et al. Does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) & serum creatinine (sCr) for acute kidney injury (AKI) diagnosis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0178091.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] n.d. www.nice.org.uk/process/pmg9/ (accessed 24 October 2019).
- Hall PS, Mitchell ED, Smith AF, Cairns DA, Messenger M, Hutchinson M, et al. The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22320.
- Petrovic S, Bogavac-Stanojevic N, Lakic D, Peco-Antic A, Vulicevic I, Ivanisevic I, et al. Cost-effectiveness analysis of acute kidney injury biomarkers in pediatric cardiac surgery. Biochem Med 2015;25:262-71. https://doi.org/10.11613/BM.2015.027.
- Shaw AD, Chalfin DB, Kleintjens J. The economic impact and cost-effectiveness of urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Clin Ther 2011;33:1713-25. https://doi.org/10.1016/j.clinthera.2011.09.014.
- National Institute for Health and Care Excellence Diagnostics Assessment Programme . The ARCHITECT and Alinity I Urine NGAL Assay, NephroCheck Test and NGAL Test to Help Assess the Risk of Acute Kidney Injury for People Who Are Being Considered for Admission to Critical Care: Final Scope 2019.
- National Institute for Health and Care Excellence . Diagnostics Assessment Programme Manual 2011. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-diagnostics-guidance/Diagnostics-assessment-programme-manual.pdf (accessed 5 April 2019).
- Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18-2. https://doi.org/10.1053/j.ajkd.2016.05.018.
- Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006497.
- Jacobsen E, Sawhney S, Brazzelli M, Aucott L, Scotland G, Aceves-Martins M, et al. Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury. BMC Nephrol 2021;22. https://doi.org/10.1186/s12882-021-02610-9.
- National Institute for Health and Care Excellence . Chronic Kidney Disease in Adults: Assessment and Management. Clinical Guideline [CG182] 2014. www.nice.org.uk/guidance/cg182 (accessed 2 May 2019).
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089-100. https://doi.org/10.1111/j.1523-1755.2005.00365.x.
- Sawhney S, Robinson HA, van der Veer SN, Hounkpatin HO, Scale TM, Chess JA, et al. Acute kidney injury in the UK: a replication cohort study of the variation across three regional populations. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019435.
- Truche AS, Ragey SP, Souweine B, Bailly S, Zafrani L, Bouadma L, et al. ICU survival and need of renal replacement therapy with respect to AKI duration in critically ill patients. Ann Intensive Care 2018;8. https://doi.org/10.1186/s13613-018-0467-6.
- Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013;28:389-96. https://doi.org/10.1016/j.jcrc.2012.12.008.
- Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551-61. https://doi.org/10.1007/s00134-016-4670-3.
- Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling CR, Walther SM, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0920-y.
- See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019;95:160-72. https://doi.org/10.1016/j.kint.2018.08.036.
- Kent S, Schlackow I, Lozano-Kühne J, Reith C, Emberson J, Haynes R, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease?. BMC Nephrol 2015;16. https://doi.org/10.1186/s12882-015-0054-0.
- Lone NI, Gillies MA, Haddow C, Dobbie R, Rowan KM, Wild SH, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 2016;194:198-20. https://doi.org/10.1164/rccm.201511-2234OC.
- UK Renal Registry . UK Renal Registry 21st Annual Report: Data to 31 12 2017 2019. www.renalreg.org/publications-reports/ (accessed 24 October 2019).
- Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery. Ann Surg 2018;267:1013-20. https://doi.org/10.1097/SLA.0000000000002485.
- Schanz M, Wasser C, Allgaeuer S, Schricker S, Dioppon J, Alscher MD, et al. Urinary [TIMP-2].[IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant 2019;34:1902-9. https://doi.org/10.1093/ndt/gfy186.
- Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015;385:1966-74. https://doi.org/10.1016/S0140-6736(15)60266-5.
- Kerr M. Chronic Kidney Disease in England: The Human and Financial Cost 2012. www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Chronic-Kidney-Disease-in-England-The-Human-and-Financial-Cost.pdf (accessed 24 October).
- Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health administration data. Am J Kidney Dis 2016;67:742-52. https://doi.org/10.1053/j.ajkd.2015.10.019.
- Wu B, Ma L, Shao Y, Liu S, Yu X, Zhu Y, et al. Effect of cardiac surgery-associated acute kidney injury on long-term outcomes of Chinese patients: a historical cohort study. Blood Purif 2017;44:227-33. https://doi.org/10.1159/000478967.
- Shimoi T, Ando M, Munakata W, Kobayashi T, Kakihana K, Ohashi K, et al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant 2013;48:80-4. https://doi.org/10.1038/bmt.2012.85.
- Office for National Statistics . National Life Tables: UK 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 31 October 2019).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care 2018 n.d. www.pssru.ac.uk/project-pages/unit-costs/ (accessed 9 September 2019).
- NHS Improvement . Reference Costs 2017. https://improvement.nhs.uk/resources/reference-costs/ (accessed 9 September 2019).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 25 October 2019).
- Campbell and Cochrane Economics Methods Group . CCEMG – EPPI-Centre Cost Converter 2019. https://eppi.ioe.ac.uk/costconversion/ (accessed 24 October 2019).
- National Institute for Health and Care Excellence . Kidney Transplantation (Adults) – Immunosuppressive Therapy (Review of TA 85) [ID456] 2015. www.nice.org.uk/guidance/GID-TAG348/documents/html-content (accessed November 2016).
- Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7-20. https://doi.org/10.1056/NEJMoa0802639.
- Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care 2009;24:129-40. https://doi.org/10.1016/j.jcrc.2007.12.017.
- Scotland G, Cruickshank M, Jacobsen E, Cooper D, Fraser C, Shimonovich M, et al. Multiple-frequency bioimpedance devices for fluid management in people with chronic kidney disease receiving dialysis: a systematic review and economic evaluation. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22010.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Hernández RA, Jenkinson D, Vale L, Cuthbertson BH. Economic evaluation of nurse-led intensive care follow-up programmes compared with standard care: the PRaCTICaL trial. Eur J Health Econ 2014;15:243-52. https://doi.org/10.1007/s10198-013-0470-7.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001307.
- Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14. https://doi.org/10.1186/cc8848.
- Nguyen NTQ, Cockwell P, Maxwell AP, Griffin M, O’Brien T, O’Neill C. Chronic kidney disease, health-related quality of life and their associated economic burden among a nationally representative sample of community dwelling adults in England. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0207960.
- Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11:733-41. https://doi.org/10.1111/j.1524-4733.2007.00308.x.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. PharmacoEconomics 2017;35:501-15. https://doi.org/10.1007/s40273-016-0485-x.
- Gerth AMJ, Hatch RA, Young JD, Watkinson PJ. Changes in health-related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia 2019;74:100-8. https://doi.org/10.1111/anae.14444.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. https://doi.org/10.1016/j.jval.2010.10.029.
- Tappenden P, Chilcott JB. Avoiding and identifying errors and other threats to the credibility of health economic models. PharmacoEconomics 2014;32:967-79. https://doi.org/10.1007/s40273-014-0186-2.
- Sawhney S, Fluck N, Marks A, Prescott G, Simpson W, Tomlinson L, et al. Acute kidney injury – how does automated detection perform?. Nephrol Dial Transplant 2015;30:1853-61. https://doi.org/10.1093/ndt/gfv094.
- Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019;572:116-19. https://doi.org/10.1038/s41586-019-1390-1.
- Janes H, Pepe MS, McShane LM, Sargent DJ, Heagerty PJ. The fundamental difficulty with evaluating the accuracy of biomarkers for guiding treatment. J Natl Cancer Inst 2015;107. https://doi.org/10.1093/jnci/djv157.
- Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190-9. https://doi.org/10.1001/jama.2016.5828.
- Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016;375:122-33. https://doi.org/10.1056/NEJMoa1603017.
- Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014;85:513-21. https://doi.org/10.1038/ki.2013.374.
- Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD?. J Am Soc Nephrol 2012;23:979-84. https://doi.org/10.1681/ASN.2011121185.
- Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol 2012;23:967-9. https://doi.org/10.1681/ASN.2012030222.
- Afiatin KLC, Kristin E, Masytoh LS, Herlinawaty E, Werayingyong P, Nadjib M, et al. Economic evaluation of policy options for dialysis in end-stage renal disease patients under the universal health coverage in Indonesia. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0177436.
- Chang YT, Hwang JS, Hung SY, Tsai MS, Wu JL, Sung JM, et al. Cost-effectiveness of hemodialysis and peritoneal dialysis: a national cohort study with 14 years follow-up and matched for comorbidities and propensity score. Sci Rep 2016;6. https://doi.org/10.1038/srep30266.
- Cho MK, Kim SY, Shim HY. Validity and reliability of the Korean version of the Dialysis Symptom Index for Hemodialysis Patients. J Nurs Res 2018;26:399-410. https://doi.org/10.1097/jnr.0000000000000267.
- Eriksson D, Goldsmith D, Teitsson S, Jackson J, van Nooten F. Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol 2016;17. https://doi.org/10.1186/s12882-016-0312-9.
- El Filali A, Bentata Y, Ada N, Oneib B. Depression and anxiety disorders in chronic hemodialysis patients and their quality of life: a cross-sectional study about 106 cases in the northeast of Morocco. Saudi J Kidney Dis Transpl 2017;28:341-8. https://doi.org/10.4103/1319-2442.202785.
- Hishii S, Miyatake N, Nishi H, Katayama A, Ujike K, Koumoto K, et al. Relationship between sedentary behavior and health-related quality of life in patients on chronic hemodialysis. Acta Med Okayama 2018;72:395-400. https://doi.org/10.18926/AMO/56177.
- Jardine MJ, Zuo L, Gray NA, de Zoysa JR, Chan CT, Gallagher MP, et al. A trial of extending hemodialysis hours and quality of life. J Am Soc Nephrol 2017;28:1898-911. https://doi.org/10.1681/ASN.2015111225.
- Jesky MD, Dutton M, Dasgupta I, Yadav P, Ng KP, Fenton A, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0165675.
- National Institute for Health and Care Excellence . Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. Clinical Guideline [CG73] 2008. http://nice.org.uk/CG73 (accessed 5 April 2019).
- Katayama A, Miyatake N, Nishi H, Ujike K, Hashimoto H, Kurato R, et al. Relationship between changes in physical activity and changes in health-related quality of life in patients on chronic hemodialysis with 1-year follow-up. Acta Med Okayama 2016;70:353-61. https://doi.org/10.18926/AMO/54593.
- Kilshaw L, Sammut H, Asher R, Williams P, Saxena R, Howse M. A study to describe the health trajectory of patients with advanced renal disease who choose not to receive dialysis. Clin Kid J 2016;9:470-5. https://doi.org/10.1093/ckj/sfw005.
- Kularatna S, Senanayake S, Gunawardena N, Graves N. Comparison of the EQ-5D 3L and the SF-6D (SF-36) contemporaneous utility scores in patients with chronic kidney disease in Sri Lanka: a cross-sectional survey. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-024854.
- Lee SJ, Son H. Comparison of health-related quality of life between patients with stage 3 and 4 chronic kidney disease and patients undergoing continuous ambulatory peritoneal dialysis. Jpn J Nurs Sci 2016;13:166-73. https://doi.org/10.1111/jjns.12101.
- Li B, Cairns JA, Draper H, Dudley C, Forsythe JL, Johnson RJ, et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health 2017;20:976-84. https://doi.org/10.1016/j.jval.2017.01.011.
- McNoe B, Schollum JBW, Derrett S, Marshall MP, Henderson A, Samaranayaka A, et al. Recruitment and participant baseline characteristics in the dialysis outcomes in those aged 65 years or older study. BMC Nephrol 2019;20. https://doi.org/10.1186/s12882-019-1328-8.
- Nagasawa H, Sugita I, Tachi T, Esaki H, Yoshida A, Kanematsu Y, et al. The relationship between dialysis patients’ quality of life and caregivers’ quality of life. Front Pharmacol 2018;16. https://doi.org/10.3389/fphar.2018.00770.
- Schlackow I, Kent S, Herrington W, Emberson J, Haynes R, Reith C, et al. A policy model of cardiovascular disease in moderate-to-advanced chronic kidney disease. Heart 2017;103:1880-90. https://doi.org/10.1136/heartjnl-2016-310970.
- Sekercioglu N, Curtis B, Murphy S, Blackhouse G, Barret G. Estimates of health utility scores in chronic kidney disease. Int Urol Nephrol 2017;49:2043-9. https://doi.org/10.1007/s11255-017-1664-1.
- Senanayake S, Mahesh PKB, Gunawardena N, Graves N, Sanjeewa Kularatna. Validity and internal consistency of EQ-5D-3L quality of life tool among pre-dialysis patients with chronic kidney disease in Sri Lanka, a lower middle-income country. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0211604.
- Shah KK, Murtagh FEM, McGeechan K, Crail S, Burns A, Tran AD, et al. Health-related quality of life and well-being in people over 75 years of age with end-stage kidney disease managed with dialysis or comprehensive conservative care: a cross-sectional study in the UK and Australia. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-027776.
- Shimizu U, Aoki H, Sakagami M, Akazawa K. Walking ability, anxiety and depression, significantly decrease EuroQol 5-Dimension 5-Level scores in older hemodialysis patients in Japan. Arch Gerontol Geriatr 2018;78:96-100. https://doi.org/10.1016/j.archger.2018.06.006.
- Snowsill TM, Moore J, Mujica Mota RE, Peters JL, Jones-Hughes TL, Huxley NJ, et al. Immunosuppressive agents in adult kidney transplantation in the National Health Service: a model-based economic evaluation. Nephrol Dial Transplant 2017;32:1251-9. https://doi.org/10.1093/ndt/gfx074.
- Tang CH, Wu YT, Huang SY, Chen HH, Wu MJ, Hsu BG. Economic costs of automated and continuous ambulatory peritoneal dialysis in Taiwan: a combined survey and retrospective cohort analysis. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015067.
- Thaweethamcharoen T, Noparatayaporn P, Sritippayawan S, Aiyasanon N. Comparison of EQ-5D-5L, VAS, and SF-6D in Thai patients on peritoneal dialysis. Value Health Reg Issues 2019;18:59-64. https://doi.org/10.1016/j.vhri.2018.08.005.
- Van Loon IN, Bots ML, Boereboom FTJ, Grooteman MPC, Blankestijn PJ, van den Dorpet MA, et al. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrology 2017;18. https://doi.org/10.1186/s12882-017-0621-7.
- Wee HL, Seng BJ, Lee JJ, Chong KJ, Yyagi P, Vathsala A, et al. Association of anemia and mineral and bone disorder with health-related quality of life in Asian pre-dialysis patients. Health Qual Life Outcomes 2016;14. https://doi.org/10.1186/s12955-016-0477-8.
- Wolfgram DF, Garcia K, Evans G, Zamanian S, Tang R, Wiegmann T, et al. Association of albuminuria and estimated glomerular filtration rate with functional performance measures in older adults with chronic kidney disease. Am J Nephrol 2017;45:172-9. https://doi.org/10.1159/000455388.
- Wong CKH, Chen JY, Fung SKS, Lo WK, Lui SL, Chan TM, et al. Health-related quality of life and health utility of Chinese patients undergoing nocturnal home haemodialysis in comparison with other modes of dialysis. Nephrology (Carlton) 2019;24:630-7. https://doi.org/10.1111/nep.13429.
- Yang F, Wong CKH, Luo N, Piercy J, Moon R, Jackson J. Mapping the kidney disease quality of life 36-item short form survey (KDQOL-36) to the EQ-5D-3L and the EQ-5D-5L in patients undergoing dialysis. Eur J Health Econ 2019;20:1195-206. https://doi.org/10.1007/s10198-019-01088-5.
- Yang F, Luo N, Lau T, Yu ZL, Foo MWY, Griva K. Health-related quality of life in patients treated with continuous ambulatory peritoneal dialysis and automated peritoneal dialysis in Singapore. Pharmacoecon Open 2018;2:203-8. https://doi.org/10.1007/s41669-017-0046-z.
- Zyoud SH, Daraghmeh DN, Mezyed DO, Khdeir RL, Sawafta MN, Ayaseh NH, et al. Factors affecting quality of life in patients on haemodialysis: a cross-sectional study from Palestine. BMC Nephrology 2016;17. https://doi.org/10.1186/s12882-016-0257-z.
- Park JI, Baek H, Jung HH. CKD and health-related quality of life: the Korea National Health and Nutrition Examination Survey. Am J Kidney Dis 2016;67:851-60. https://doi.org/10.1053/j.ajkd.2015.11.005.
- Tan BK, Yu Z, Fang W, Lin A, Ni Z, Qian J. Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int 2016;89:487-97. https://doi.org/10.1038/ki.2015.294.
- Ethgen O, Schneider AG, Bagshaw SM, Bellomo R, Kellum JA. Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients. Nephrol Dial Transplant 2015;30:54-61. https://doi.org/10.1093/ndt/gfu314.
- Kaier K, Gutmann A, Baumbach H, von Zur Mühlen C, Hehn P, Vach W, et al. Quality of life among elderly patients undergoing transcatheter or surgical aortic valve replacement – a model-based longitudinal data analysis. Health Qual Life Outcomes 2016;14. https://doi.org/10.1186/s12955-016-0512-9.
- Oeyen S, De Corte W, Benoit D, Annemans L, Dhondt A, Vanholder R, et al. Long-term quality of life in critically ill patients with acute kidney injury treated with renal replacement therapy: a matched cohort study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-1004-8.
- Soliman IW, Frencken JF, Peelen LM, Slooter AJ, Cremer OL, van Delden JJ, et al. The predictive value of early acute kidney injury for long-term survival and quality of life of critically ill patients. Crit Care 2016;20. https://doi.org/10.1186/s13054-016-1416-0.
Appendix 1 Literature search strategies
NephroCheck/neutrophil gelatinase-associated lipocalin clinical effectiveness search strategies
EMBASE and MEDLINE (via Ovid)
EMBASE
Date range searched: 1974–14 May 2019.
Date searched: 27 May 2019.
MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions
Date range searched: 1946–14 May 2019.
Date searched: 27 May 2019.
Search strategy
-
Acute Disease/and exp Kidney Diseases/use ppezv (8605)
-
exp acute disease/and exp *kidney disease/use oemezd (2443)
-
exp *acute kidney failure/use oemezd (31,083)
-
acute kidney injury/use ppezv (41,584)
-
exp *kidney injury/use oemezd (12,360)
-
kidney tubular necrosis, acute/use ppezv (2352)
-
exp *kidney tubule necrosis/use oemezd (1519)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (50,969)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (58,624)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or “nephrotoxic injur*”).tw. (9425)
-
aki.tw. (27,925)
-
exp *contrast induced nephropathy/use oemezd (2540)
-
“contrast induced nephropathy”.tw. (5028)
-
or/1-13 (159,431)
-
*reperfusion injury/(47,279)
-
reperfusion/use ppezv (4705)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (129,126)
-
exp *Delayed Graft Function/(1612)
-
“delayed graft function*”.tw. (9243)
-
or/15-19 (144,655)
-
(renal or kidney* or nephr* or “tubular necrosis” or aki).tw. (2,045,747)
-
(or/1-7) or 21 (2,056,977)
-
20 and 22 (25,897)
-
14 or 23 [All AKI] (177,838)
-
lipocalins/or lipocalin-2/use ppezv (6282)
-
neutrophil gelatinase associated lipocalin/or lipocalin/use oemezd (12,442)
-
(NGAL or uNGAL or sNGAL).tw,kw. (7409)
-
(“Neutrophil gelatinase-associated lipocalin” or “neutrophil gelatinase lipocalin” or “lipocalin 2” or lcn2 or Oncogene 24p3 or siderocalin).tw,kw,nm. use ppezv (4262)
-
(“Neutrophil gelatinase-associated lipocalin” or “neutrophil gelatinase lipocalin” or “lipocalin 2” or lcn2 or Oncogene 24p3 or siderocalin).tw,kw,tn. use oemezd (5997)
-
or/25-29 [NGAL] (16,697)
-
“Tissue Inhibitor of Metalloproteinase-2”/use ppezv (3445)
-
“tissue inhibitor of metalloproteinase 2”/use oemezd (6871)
-
Metalloproteinase inhibitor 2.tw,nm,kw. use ppezv (15)
-
Metalloproteinase inhibitor 2.tw,kw. use oemezd (28)
-
tissue inhibitor of metalloproteinase-2.tw,nm,kw. use ppezv (3699)
-
tissue inhibitor of metalloproteinase-2.tw,kw. use oemezd (883)
-
TIMP metallopeptidase inhibitor 2.tw,nm,kw. use ppezv (10)
-
TIMP metallopeptidase inhibitor 2.tw,kw. use oemezd (11)
-
(TIMP 2 or TIMP2 or DDC8 or CSC-21K).tw,nm,kw. use ppezv (4818)
-
(TIMP 2 or TIMP2 or DDC8 or CSC-21K).tw,kw. use oemezd (6114)
-
or/31-40 [TIMP2] (14,536)
-
(IGFBP7 or IBP-7 or IGFBP-rP1).tw,nm,kw. use ppezv (410)
-
(IGFBP7 or IBP-7 or IGFBP-rP1).tw,kw. use oemezd (614)
-
IGF-binding protein 7.tw,nm,kw. use ppezv (16)
-
IGF-binding protein 7.tw,kw. use oemezd (23)
-
Insulin-like growth factor-binding protein 7.tw,nm,kw. use ppezv (220)
-
Insulin-like growth factor-binding protein 7.tw,kw. use oemezd (326)
-
MAC25 protein.tw,nm,kw. use ppezv (5)
-
MAC25 protein.tw,kw. use oemezd (5)
-
PGI2-stimulating factor.tw,nm,kw. use ppezv (6)
-
PGI2-stimulating factor.tw,kw. use oemezd (9)
-
“Prostacyclin-stimulating factor”.tw,nm,kw. use ppezv (29)
-
Prostacyclin-stimulating factor.tw,kw. use oemezd (31)
-
#32 or #33 or #34 or #35 or #36 or #37.tw,nm,kw. use ppezv (15)
-
Tumor-derived adhesion factor.tw,kw. use oemezd (7)
-
or/42-55 [IGFBP7] (1273)
-
41 and 56 [TIMP2 AND IGFBP7] (278)
-
nephrocheck.tw,kw. use ppezv (24)
-
nephrocheck.tw,dv,kw. use oemezd (55)
-
58 or 59 (79)
-
30 or 57 or 60 (16,915)
-
24 and 61 (5763)
-
remove duplicates from 62 (4053).
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost; EBSCO Information Services, Ipswich, MA, USA)
Date searched: 17 May 2019.
Search strategy
S1 (MH “Kidney Diseases”) AND (MH “Acute Disease”) (257)
S2 (MM “Kidney Failure, Acute”) (5995)
S3 (MH “Kidney Tubular Necrosis, Acute”) (190)
S4 TX Acute N3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction) (10,442)
S5 TX Acute N3 (renal disease* or renal injury or renal failure or renal dysfunction) (3651)
S6 TX (Acute N3 (Tubular Necrosis or nephrotoxic*)) OR TX “nephrotoxic injur*” (447)
S7 TX aki (3496)
S8 TX “contrast induced nephropathy”. (677)
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 (13,805)
S10 (MM “Reperfusion Injury”) (1816)
S11 (MH “Reperfusion”) (947)
S12 TX “delayed graft function*”. (213)
S13 TX reperfusion N5 (injur* or isch?emi*) (4919)
S14 S10 OR S11 OR S12 OR S13 (5890)
S15 TX renal or kidney* or nephr* or “tubular necrosis” or aki (157,197)
S16 S1 OR S2 OR S3 OR S15 (157,197)
S17 S14 AND S16 (1057)
S18 S9 OR S17 (14,436)
S19 TX (NGAL or uNGAL or sNGAL). (558)
S20 TX “Neutrophil gelatinase-associated lipocalin” or “neutrophil gelatinase lipocalin” or “lipocalin 2” or lcn2 or Oncogene 24p3 or siderocalin (762)
S21 TX “Metalloproteinase inhibitor 2” OR TX “tissue inhibitor of metalloproteinase-2” OR TX “TIMP metallopeptidase inhibitor 2” OR TX (“TIMP 2 or TIMP2 or DDC8 or CSC-21K”) (61)
S22 TX ((IGFBP7 or IBP-7 or IGFBP-rP1)) OR TX “IGF-binding protein 7” OR TX “Insulin-like growth factor-binding protein 7” (63)
S23 TX “MAC25 protein” OR TX “PGI2-stimulating factor” OR TX “Prostacyclin-stimulating factor” (0)
S24 S22 OR S23 (63)
S25 S21 AND S24 (12)
S26 S19 OR S20 OR S25 (853)
S27 S18 AND S26 (473).
Cochrane Central Register of Controlled Trials (via Wiley Online Library)
Date searched: 17 May 2019.
Search strategy
-
MeSH descriptor: [Acute Kidney Injury] explode all trees (1214)
-
MeSH descriptor: [Kidney Tubular Necrosis, Acute] explode all trees (37)
-
(Acute NEAR/3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)):ti,ab,kw (23,064)
-
(Acute NEAR/3 (renal disease* or renal injury or renal failure or renal dysfunction)):ti,ab,kw (23,415)
-
(Acute NEAR/3 (Tubular Necrosis or nephrotoxic*)):ti,ab,kw (312)
-
(“nephrotoxic injur*”):ti,ab,kw (0)
-
(aki):ti,ab,kw (1209)
-
(“contrast induced nephropathy”):ti,ab,kw (822)
-
MeSH descriptor: [Acute Disease] explode all trees (9276)
-
MeSH descriptor: [Kidney Diseases] explode all trees (14,389)
-
#9 and #10 (193)
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #11 (24,407)
-
MeSH descriptor: [Reperfusion Injury] explode all trees (1006)
-
(reperfusion NEAR/5 (injur* or ischemi* or ischaemi*)):ti,ab,kw (2932)
-
MeSH descriptor: [Delayed Graft Function] explode all trees (89)
-
(“delayed graft function*”):ti,ab,kw (597)
-
#13 or #14 or #15 or #16 (3470)
-
(renal or kidney* or nephr* or “tubular necrosis” or aki):ti,ab,kw (79,148)
-
#1 or #2 or #11 or #18 (79,196)
-
#17 and #19 (997)
-
MeSH descriptor: [Lipocalins] explode all trees (199)
-
MeSH descriptor: [Lipocalin-2] explode all trees (93)
-
(NGAL or uNGAL or sNGAL):ti,ab,kw (550)
-
(“Neutrophil gelatinase-associated lipocalin” or “neutrophil gelatinase lipocalin” or “lipocalin 2” or lcn2 or Oncogene 24p3 or siderocalin):ti,ab,kw (533)
-
#21 or #22 or #23 or #24 (816)
-
(“Metalloproteinase inhibitor 2”):ti,ab,kw (0)
-
(“tissue inhibitor of metalloproteinase-2”):ti,ab,kw (70)
-
(“TIMP metallopeptidase inhibitor 2”):ti,ab,kw (0)
-
(TIMP 2 or TIMP2 or DDC8 or CSC-21K):ti,ab,kw (303)
-
MeSH descriptor: [Tissue Inhibitor of Metalloproteinase-2] explode all trees (42)
-
#26 or #27 or #28 or #29 or #30 (315)
-
(IGFBP7 or IBP-7 or IGFBP-rP1):ti,ab,kw (26)
-
(“IGF-binding protein 7”):ti,ab,kw (1)
-
(“Insulin-like growth factor-binding protein 7”):ti,ab,kw (22)
-
(MAC25 protein):ti,ab,kw (0)
-
(“PGI2-stimulating factor”):ti,ab,kw (0)
-
(“Prostacyclin-stimulating factor”):ti,ab,kw (1)
-
(“Tumor-derived adhesion factor”):ti,ab,kw (0)
-
#32 or #33 or #34 or #35 or #36 or #37 (33)
-
#31 and #39 (21)
-
(nephrocheck):ti,ab,kw (4)
-
#25 or #40 or #41 (832)
-
#12 or #20 (25,125)
-
#42 and #43 (292).
Clarivate Analytics Web of Science
Indexes: Science Citation Index Expanded, Conference Proceedings Citation Index – Science, and Conference Proceedings Citation Index – Social Science & Humanities.
Timespan: all years.
Date searched: 22 May 2019.
Search strategy
-
TOPIC: (“acute kidney injury” OR “acute kidney failure”) (24,763)
-
TOPIC: (kidney NEAR/2 necrosis) (715)
-
TOPIC: (Acute NEAR/3 (“kidney disease*” or “kidney injury” or “kidney failure” or “kidney dysfunction”)) (25,532)
-
TOPIC: (Acute NEAR/3 (“renal disease*” or “renal injury” or “renal failure” or “renal dysfunction”)) (32,865)
-
TOPIC: (“contrast induced nephropathy”) (3029)
-
#5 OR #4 OR #3 OR #2 OR #1 (54,254)
-
TOPIC: (NGAL or sNGAL or uNGAL) (3258)
-
TOPIC: (neutrophil NEAR/2 lipocalin) (3078)
-
#8 OR #7 (4099)
-
TOPIC: (Inhibitor NEAR/2 Metalloproteinase) (10,021)
-
TOPIC: (TIMP) (12,633)
-
#11 OR #10 (18,219)
-
TOPIC: (IGFBP7 or IBP-7 or IGFBP-rP1) (470)
-
TOPIC: (“Insulin-like growth factor-binding protein 7”) (242)
-
#14 OR #13 (539)
-
#15 AND #12 (108)
-
TOPIC: (nephrocheck) (29)
-
#17 OR #16 OR #9 (4192)
-
#18 AND #6 (1943)
-
TOPIC: (rat or rats or mouse or mice or murine or dog or dogs or canine or pig or pigs or porcine) (3,841,039)
-
#20 AND #19 (428)
-
#19 not #21 (1543).
Other resources
The following resources were searched using appropriate text terms in combination when allowed by the search interface:
-
HTA Database [www.crd.york.ac.uk/PanHTA/ (accessed 10 June 2019)].
-
WHO’s Global Index Medicus [www.globalhealthlibrary.net/php/index.php?lang=en (accessed 10 June 2019)].
-
EU Clinical Trials Register [www.clinicaltrialsregister.eu/ (accessed 10 June 2019)].
-
ICTRP [www.isrctn.com/ (accessed 10 June 2019)].
-
ClinicalTrials.gov (via the US National Institutes of Health; Advanced Search Interface).
Search terms used:
-
Acute kidney/renal injury.
-
Acute kidney/renal failure.
-
Kidney Tubular Necrosis.
-
contrast induced nephropathy.
-
Nephrocheck.
-
TIMP-2.
-
Metalloproteinase.
-
IGFBP7.
-
Insulin-like growth factor-binding protein 7.
-
NGAL or uNGAL or sNGAL.
-
Neutrophil gelatinase-associated lipocalin.
Results retrieved: 86.
Appendix 2 Screening checklist
FIGURE 25.
Screening checklist. RQ, research question; sCr, serum creatinine.
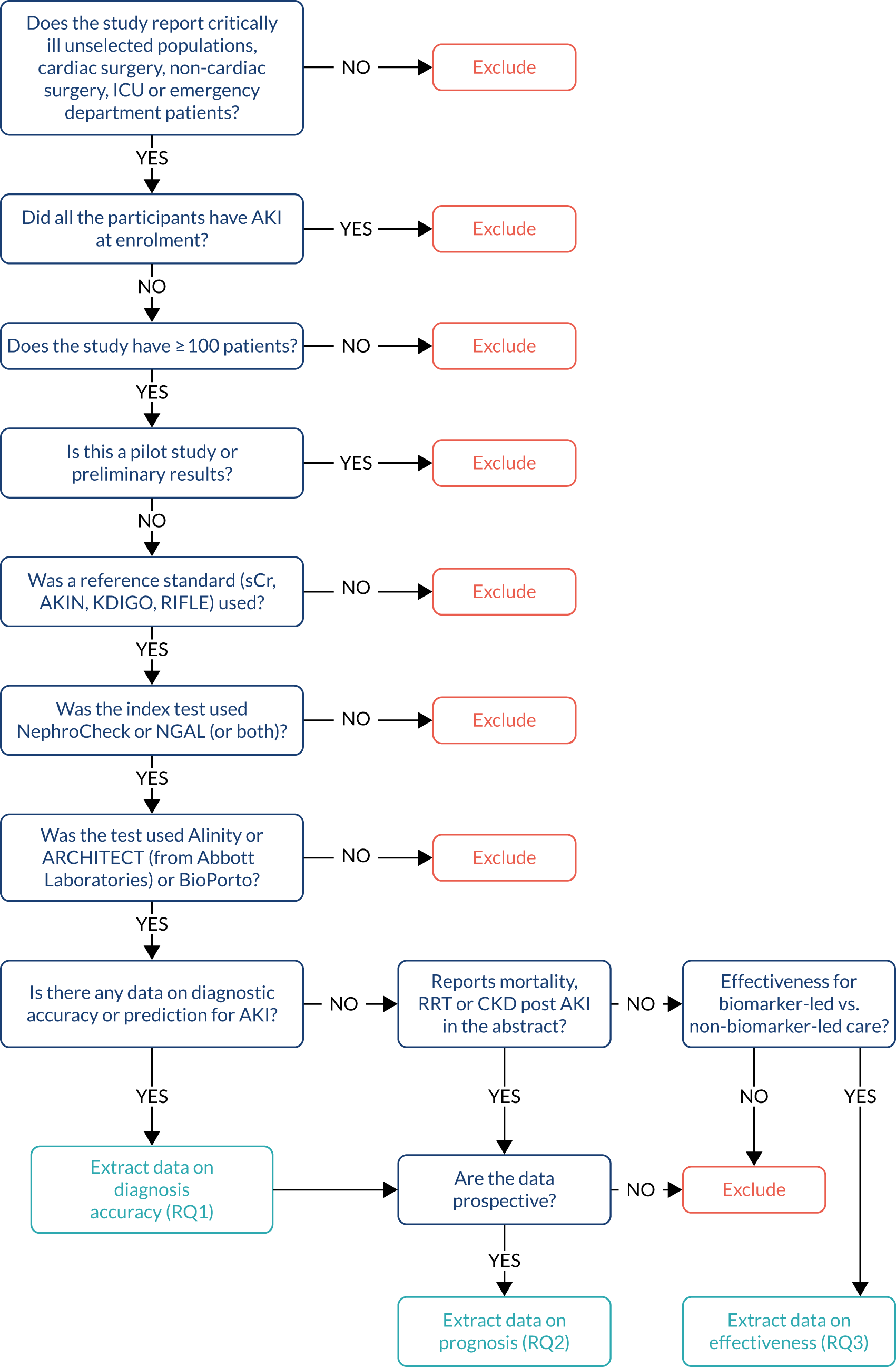
Appendix 3 Data extraction form
-
Reference ID.
-
Study first author.
-
Year.
-
Project study name.
-
-
Reviewer.
Baseline characteristics
-
Population (adults/child/both).
-
Target population.
-
Recruitment period.
-
Study centre (number of centres and names).
-
Country.
-
Funding.
-
Index test 1 (urine NGAL, plasma NGAL or NephroCheck) and index test kit 1 (e.g. ARCHITECT or Alinity i from Abbott, or ELISA BioPorto).
-
Age for whole sample.
-
Sex.
-
Serum creatinine.
-
eGFR.
-
Sequential Organ Failure Assessment score.
-
CKD.
-
Time point of measurement (non-surgical: closest to admission; surgical: immediately after surgery; prognosis studies: various time points).
-
Threshold reported.
-
Report cut-off point for NephroCheck or NGAL.
-
True positive, false negative, false positive, true negative.
-
n with AKI present (true positive plus false negative) confirmed by reference standard.
-
n with AKI absent (false positive plus true negative) confirmed by reference standard.
-
n with AKI present (true positive plus false positive) confirmed by test.
-
n with AKI absent (false negative plus true positive) confirmed by test.
For each outcome (acute kidney injury diagnosis, mortality prognosis, renal replacement therapy prognosis and acute kidney injury prognosis)
-
Sensitivity (lower and upper 95% CI).
-
Specificity (lower and upper 95% CI).
-
AUC (lower and upper 95% CI).
-
Positive predictive value (lower and upper 95% CI).
-
Negative predictive value (lower and upper 95% CI).
-
Positive likelihood ratio (lower and upper 95% CI).
-
Negative likelihood ratio (lower and upper 95% CI).
-
Comment.
Appendix 4 List of included studies
The asterisk (*) denotes a primary reference.
Albert 201845
Albert C, Albert A, Bellomo R, Kropf S, Devarajan P, Westphal S, et al. Urinary neutrophil gelatinase-associated lipocalin-guided risk assessment for major adverse kidney events after open-heart surgery. Biomark Med 2018;12:975–85. https://doi.org/10.2217/bmm-2018-0071
Alcaraz 201489
Alcaraz AJ, Gil-Ruiz MA, Castillo A, López J, Romero C, Fernández SN, Carrillo A. Postoperative neutrophil gelatinase-associated lipocalin predicts acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med 2014;15:121–30. https://doi.org/10.1097/PCC.0000000000000034
Ariza 201667
*Ariza X, Graupera I, Coll M, Solà E, Barreto R, García E, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol 2016;65:57–65.
Markwardt D, Holdt L, Steib C, Benesic A, Bendtsen F, Bernardi M, et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 2017;66:1232–41. https://doi.org/10.1002/hep.29290
Asada 201650
Asada T, Isshiki R, Hayase N, Sumida M, Inokuchi R, Noiri E, et al. Impact of clinical context on acute kidney injury biomarker performances: differences between neutrophil gelatinase-associated lipocalin and L-type fatty acid-binding protein. Sci Rep 2016;6:33077. https://doi.org/10.1038/srep33077
Barreto 201468
Barreto R, Elia C, Solà E, Moreira R, Ariza X, Rodríguez E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol 2014;61:35–42. https://doi.org/10.1016/j.jhep.2014.02.023
Beitland 201628
Beitland S, Waldum-Grevbo BE, Nakstad ER, Berg JP, Trøseid AS, Brusletto BS, et al. Urine biomarkers give early prediction of acute kidney injury and outcome after out-of-hospital cardiac arrest. Crit Care 2016;20:314. https://doi.org/10.1186/s13054-016-1503-2
Bennett 200887
Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008;3:665–73. https://doi.org/10.2215/CJN.04010907
Bihorac 201429
Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189:932–9. https://doi.org/10.1164/rccm.201401-0077OC
Bojan 201486
Bojan M, Vicca S, Lopez-Lopez V, Mogenet A, Pouard P, Falissard B, Journois D. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol 2014;9:285–94. https://doi.org/10.2215/CJN.04730513
Cantinotti 201288
Cantinotti M, Storti S, Lorenzoni V, Arcieri L, Moschetti R, Murzi B, et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery. Clin Chem Lab Med 2012;50:2009–17. https://doi.org/10.1515/cclm-2012-0125
Cho 201369
Cho E, Yang HN, Jo SK, Cho WY, Kim HK. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J Korean Med Sci 2013;28:100–5. https://doi.org/10.3346/jkms.2013.28.1.100
Cho 201466
Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrol 2014;15:169. https://doi.org/10.1186/1471-2369-15-169
Collins 201251
Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF, et al. Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail 2012;14:1020–9. https://doi.org/10.1093/eurjhf/hfs087
Cullen 201449
Cullen MR, Jhanji S, Pearse RM, Fitzgibbon MC. Neutrophil gelatinase-associated lipocalin and albuminuria as predictors of acute kidney injury in patients treated with goal-directed haemodynamic therapy after major abdominal surgery. Ann Clin Biochem 2014;51:392–9.
Cummings 201926
Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FT. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg 2019;157:1545–53.e5.
De Loor 201763
De Loor J, Herck I, Francois K, Van Wesemael A, Nuytinck L, Meyer E, Hoste EAJ. Diagnosis of cardiac surgery-associated acute kidney injury: differential roles of creatinine, chitinase 3-like protein 1 and neutrophil gelatinase-associated lipocalin: a prospective cohort study. Ann Intensive Care 2017;7:24. https://doi.org/10.1186/s13613-017-0251-z
Di Leo 201830
*Di Leo L, Nalesso F, Garzotto F, Xie Y, Yang B, Virzì GM, et al. Predicting acute kidney injury in intensive care unit patients: the role of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 biomarkers. Blood Purif 2018;45:270–7. https://doi.org/10.1159/000485591
Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2) • IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int 2019;95:1486–93.
Doi 201470
*Doi K, Noiri E, Nangaku M, Yahagi N, Jayakumar C, Ramesh G. Repulsive guidance cue semaphorin 3A in urine predicts the progression of acute kidney injury in adult patients from a mixed intensive care unit. Nephrol Dial Transplant 2014;29:73–80. https://doi.org/10.1093/ndt/gft414
Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011;39:2464–9. https://doi.org/10.1097/CCM.0b013e318225761a
Dong 201792
Dong L, Ma Q, Bennett M, Devarajan P. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatr Nephrol 2017;32:2351–60. https://doi.org/10.1007/s00467-017-3748-7
Dupont 201252
Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, et al. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail 2012;14:597–604. https://doi.org/10.1093/eurjhf/hfs039
Garcia-Alvarez 201546
Garcia-Alvarez M, Glassford NJ, Betbese AJ, Ordoñez J, Baños V, Argilaga M, et al. Urinary neutrophil gelatinase-associated lipocalin as predictor of short- or long-term outcomes in cardiac surgery patients. J Cardiothorac Vasc Anesth 2015;29:1480–8. https://doi.org/10.1053/j.jvca.2015.05.060
Gayat 201832
Gayat E, Touchard C, Hollinger A, Vieillard-Baron A, Mebazaa A, Legrand M, FROG ICU study investigators. Back-to-back comparison of penKID with NephroCheck® to predict acute kidney injury at admission in intensive care unit: a brief report. Crit Care 2018;22:24. https://doi.org/10.1186/s13054-018-1945-9
Haase 201460
*Haase M, Bellomo R, Albert C, Vanpoucke G, Thomas G, Laroy W, et al. The identification of three novel biomarkers of major adverse kidney events. Biomark Med 2014;8:1207–17. https://doi.org/10.2217/bmm.14.90
Albert C, Albert A, Kube J, Bellomo R, Wettersten N, Kuppe H, et al. Urinary biomarkers may provide prognostic information for subclinical acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2018;155:2441–52.e13.
Hjortrup 201572
Hjortrup PB, Haase N, Treschow F, Møller MH, Perner A. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand 2015;59:25–34. https://doi.org/10.1111/aas.12427
Hoste 201433
Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014;29:2054–61. https://doi.org/10.1093/ndt/gfu292
Isshiki 201853
Isshiki R, Asada T, Sumida M, Hamasaki Y, Nangaku M, Noiri E, Doi K. Modest impact of serial measurements of acute kidney injury biomarkers in an adult intensive care unit. Nephron 2018;139:243–53. https://doi.org/10.1159/000488219
Itenov 201781
Itenov TS, Jensen JU, Ostrowski SR, Johansson PI, Thormar KM, Lundgren JD, Bestle MH, ‘Procalcitonin And Survival Study’ study group. Endothelial damage signals refractory acute kidney injury in critically ill patients. Shock 2017;47:696–701. https://doi.org/10.1097/SHK.0000000000000804
Jaques 201962
Jaques DA, Spahr L, Berra G, Poffet V, Lescuyer P, Gerstel E, et al. Biomarkers for acute kidney injury in decompensated cirrhosis: a prospective study. Nephrology 2019;24:170–80. https://doi.org/10.1111/nep.13226
Kashani 201334
Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. https://doi.org/10.1186/cc12503
Kimmel 2016
Kimmel M, Shi J, Latus J, Wasser C, Kitterer D, Braun N, Alscher MD. Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol 2016;11:938–46. https://doi.org/10.2215/CJN.10551015
Kimmel M, Shi J, Wasser C, Biegger D, Alscher MD, Schanz MB. Urinary [TIMP-2]·[IGFBP7] – novel biomarkers to predict acute kidney injury. Am J Nephrol 2016;43:375–82. https://doi.org/10.1159/000446451
Kokkoris 201254
Kokkoris S, Parisi M, Ioannidou S, Douka E, Pipili C, Kyprianou T, et al. Combination of renal biomarkers predicts acute kidney injury in critically ill adults. Ren Fail 2012;34:1100–8. https://doi.org/10.3109/0886022X.2012.713279
Lagos-Arevalo 201593
Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbisetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol 2015;30:665–76.
Lee 201882
*Lee DH, Lee BK, Cho YS, Jung YH, Lee SM, Park JS, Jeung KW. Plasma neutrophil gelatinase-associated lipocalin measured immediately after restoration of spontaneous circulation predicts acute kidney injury in cardiac arrest survivors who underwent therapeutic hypothermia. Ther Hypothermia Temp Manag 2018;8:99–107. https://doi.org/10.1089/ther.2017.0039
Cho YS, Lee BK, Lee DH, Jung YH, Lee SM, Park JS, Jeung KW. Association of plasma neutrophil gelatinase-associated lipocalin with acute kidney injury and clinical outcome in cardiac arrest survivors depends on the time of measurement. Biomarkers 2018;23:487–94. https://doi.org/10.1080/1354750X.2018.1452048
Liebetrau 201347
Liebetrau C, Dörr O, Baumgarten H, Gaede L, Szardien S, Blumenstein J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand J Clin Lab Invest 2013;73:392–9. https://doi.org/10.3109/00365513.2013.787149
Marino 201583
Marino R, Struck J, Hartmann O, Maisel AS, Rehfeldt M, Magrini L, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol 2015;28:717–24. https://doi.org/10.1007/s40620-014-0163-z
Mårtensson 201555
Mårtensson J, Glassford NJ, Jones S, Eastwood GM, Young H, Peck L, et al. Urinary neutrophil gelatinase-associated lipocalin to hepcidin ratio as a biomarker of acute kidney injury in intensive care unit patients. Minerva Anestesiol 2015;81:1192–200.
Matsa 201473
Matsa R, Ashley E, Sharma V, Walden AP, Keating L. Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients. Crit Care 2014;18:R137. https://doi.org/10.1186/cc13958
Nickolas 200874
Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 2008;148:810–19.
Nickolas 201256
Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 2012;59:246–55. https://doi.org/10.1016/j.jacc.2011.10.854
Nisula 201575
*Nisula S, Yang R, Poukkanen M, Vaara ST, Kaukonen KM, Tallgren M, et al. Predictive value of urine interleukin-18 in the evolution and outcome of acute kidney injury in critically ill adult patients. Br J Anaesth 2015;114:460–8. https://doi.org/10.1093/bja/aeu382
Nisula S, Yang R, Kaukonen KM, Vaara ST, Kuitunen A, Tenhunen J, et al. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth Analg 2014;119:95–102. https://doi.org/10.1213/ANE.0000000000000243
Oezkur 201727
Oezkur M, Magyar A, Thomas P, Stork T, Schneider R, Bening C, et al. TIMP-2*IGFBP7 (Nephrocheck®) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hours. Kidney Blood Press Res 2017;42:456–67. https://doi.org/10.1159/000479298
Parikh 2011 (TRIBE-Adult)37
*Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22:1748–57. https://doi.org/10.1681/ASN.2010121302
Brown JR, Philbrook HT, Goodrich CA, Bohm AR, Alam SS, Coca SG, et al. Are urinary biomarkers better than acute kidney injury duration for predicting readmission? Ann Thorac Surg 2019;107:1699–1705.
Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 2014;25:1063–71.
Coca SG, Nadkarni GN, Garg AX, Koyner J, Thiessen-Philbrook H, McArthur E, et al. First post-operative urinary kidney injury biomarkers and association with the duration of AKI in the TRIBE-AKI cohort. PLOS ONE 2016;11:e0161098. https://doi.org/10.1371/journal.pone.0161098
Greenberg JH, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Parikh CR, Zappitelli M, TRIBE-AKI Consortium. Kidney injury biomarkers 5 years after AKI due to pediatric cardiac surgery. Pediatr Nephrol 2018;33:1069–77. https://doi.org/10.1007/s00467-018-3888-4
Koyner JL, Coca SG, Thiessen-Philbrook H, Patel UD, Shlipak MG, Garg AX, Parikh CR, Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium. Urine biomarkers and perioperative acute kidney injury: the impact of preoperative estimated GFR. Am J Kidney Dis 2015;66:1006–14. https://doi.org/10.1053/j.ajkd.2015.07.027
Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 2013;8:1079–88. https://doi.org/10.2215/CJN.10971012
Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, et al. Plasma IL-6 and IL-10 concentrations predict aki and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol 2015;26:3123–32. https://doi.org/10.1681/ASN.2014080764
Parikh 2011 (TRIBE-Child)84
*Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 2011;22:1737–47. https://doi.org/10.1681/ASN.2010111163
Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 2015;169:583–91. https://doi.org/10.1001/jamapediatrics.2015.54
Park 201757
Park M, Hsu CY, Go AS, Feldman HI, Xie D, Zhang X, et al. Urine kidney injury biomarkers and risks of cardiovascular disease events and all-cause death: the CRIC study. Clin J Am Soc Nephrol 2017;12:761–71. https://doi.org/10.2215/CJN.08560816
Pipili 201458
Pipili C, Ioannidou S, Tripodaki ES, Parisi M, Douka E, Vasileiadis I, et al. Prediction of the renal replacement therapy requirement in mechanically ventilated critically ill patients by combining biomarkers for glomerular filtration and tubular damage. J Crit Care 2014;29:692.e7–13. https://doi.org/10.1016/j.jcrc.2014.02.011
Schley 201561
Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, et al. Comparison of plasma and urine biomarker performance in acute kidney injury. PLOS ONE 2015;10:e0145042. https://doi.org/10.1371/journal.pone.0145042
Seitz 201390
Seitz S, Rauh M, Gloeckler M, Cesnjevar R, Dittrich S, Koch AM. Cystatin C and neutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgery. Swiss Med Wkly 2013;143:w13744. https://doi.org/10.4414/smw.2013.13744
Smith 201377
Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, Holt SG. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant 2013;28:1569–79. https://doi.org/10.1093/ndt/gfs586
Tecson 201778
Tecson KM, Erhardtsen E, Eriksen PM, Gaber AO, Germain M, Golestaneh L, et al. Optimal cut points of plasma and urine neutrophil gelatinase-associated lipocalin for the prediction of acute kidney injury among critically ill adults: retrospective determination and clinical validation of a prospective multicentre study. BMJ Open 2017;7:e016028. https://doi.org/10.1136/bmjopen-2017-016028
Thanakitcharu 201448
Thanakitcharu P, Jirajan B. Determination of urinary neutrophil gelatinase-associated lipocalin (NGAL) cut-off level for early detection of acute kidney injury in Thai adult patients undergoing open cardiac surgery. J Med Assoc Thai 2014;97(Suppl. 11):48–55.
Tidbury 201964
Tidbury N, Browning N, Shaw M, Morgan M, Kemp I, Matata, B. Neutrophil gelatinase-associated lipocalin as a marker of postoperative acute kidney injury following cardiac surgery in patients with pre-operative kidney impairment. Cardiovasc Hematol Disord Drug Targets 2019;19:239–48.
Treeprasertsuk 201559
Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, Sallapant S, Tiranathanagul K, Komolmit P, Tangkijvanich P. Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol 2015;15:140. https://doi.org/10.1186/s12876-015-0372-5
Verna 201279
Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012;57:2362–70. https://doi.org/10.1007/s10620-012-2180-x
Yang 201765
Yang X, Chen C, Teng S, Fu X, Zha Y, Liu H, et al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3373–82. https://doi.org/10.1681/ASN.2017020142
Zelt 201880
Zelt JGE, Mielniczuk LM, Liu PP, Dupuis JY, Chih S, Akbari A, Sun LY. Utility of novel cardiorenal biomarkers in the prediction and early detection of congestive kidney injury following cardiac surgery. J Clin Med 2018;7:E540.
Zwiers 201591
Zwiers AJ, de Wildt SN, van Rosmalen J, de Rijke YB, Buijs EA, Tibboel D, Cransberg K. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: a prospective cohort study. Crit Care 2015;19:181. https://doi.org/10.1186/s13054-015-0910-0
Appendix 5 Excluded studies
| First author | Year of publication | Reason for exclusion | Reference |
|---|---|---|---|
| Abassi | 2013 | < 100 participants | Abassi Z, Shalabi A, Sohotnik R, Nativ O, Awad H, Bishara B, et al. Urinary NGAL and KIM-1: biomarkers for assessment of acute ischemic kidney injury following nephron sparing surgery. J Urol 2013;189:1559–66. https://doi.org/10.1016/j.juro.2012.10.029 |
| Abdelsalam | 2018 | Not a relevant type of population | Abdelsalam M, Elmorsy E, Abdelwahab H, Algohary O, Naguib M, El Wahab AA, et al. Urinary biomarkers for early detection of platinum based drugs induced nephrotoxicity. BMC Nephrol 2018;19:219 |
| Aberg | 2014 | Not a relevant biomarker assay or test | Aberg F, Lempinen M, Hollmén M, Nordin A, Mäkisalo H, Isoniemi H. Neutrophil gelatinase-associated lipocalin associated with irreversibility of pre-liver transplant kidney dysfunction. Clin Transplant 2014;28:869–76. https://doi.org/10.1111/ctr.12394 |
| Adams | 2019 | < 100 participants | Adams PS, Vargas D, Baust T, Saenz L, Koh W, Blasiole B, et al. Associations of perioperative renal oximetry via near-infrared spectroscopy, urinary biomarkers, and postoperative acute kidney injury in infants after congenital heart surgery: should creatinine continue to be the gold standard? Pediatr Crit Care Med 2019;20:27–37. https://doi.org/10.1097/PCC.0000000000001767 |
| Adler | 2018 | < 100 participants | Adler C, Heller T, Schregel F, Hagmann H, Hellmich M, Adler J, Reuter H. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Crit Care 2018;22:126. https://doi.org/10.1186/s13054-018-2042-9 |
| Afify | 2016 | < 100 participants | Afify MFM, Maher SE, Ibrahim NM, El-Hamied WMA. Serum neutrophil gelatinase-associated lipocalin in infants and children with sepsis-related conditions with or without acute renal dysfunction. Clin Med Insights Pediatr 2016;10:85–9 |
| Afzal | 2018 | Not a primary study | Afzal A, Vallabhan RC, McCullough PA. Acute kidney injury in cardiogenic shock: in search of early detection and clinical certainty. Eur J Heart Fail 2018;20:582–4. https://doi.org/10.1002/ejhf.1032 |
| Aghel | 2010 | < 100 participants | Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 2010;16:49–54. https://doi.org/10.1016/j.cardfail.2009.07.003 |
| Ahmad | 2015 | No focus on DTA for AKI | Ahmad T, Wang T, O’Brien EC, Samsky MD, Pura JA, Lokhnygina Y, et al. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail 2015;3:30–9 |
| Ahmad | 2018 | Not a relevant biomarker assay or test | Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 2018;137:2016–28 |
| Ahmed | 2012 | No focus on DTA for AKI | Ahmed MS, Lim R, Selvaratnam V, James A, Kelly PO, Abraham KA, Wong CF. Survival akin to injury, hospitalized patients with acute kidney injury based on the AKIN classification. Clin Nephrol 2012;78:370–5. https://doi.org/10.5414/CN106948 |
| Ahmed | 2014 | Retracted study | Ahmed QA, El Sayed FS, Emad H, Mohamed E, Ahmed B, Heba P. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. Med Arch 2014;68:132–6 |
| Ahn | 2016 | Not a relevant type of population | Ahn JY, Lee MJ, Seo JS, Choi D, Park JB. Plasma neutrophil gelatinase-associated lipocalin as a predictive biomarker for the detection of acute kidney injury in adult poisoning. Clin Toxicol 2016;54:127–33. https://doi.org/10.3109/15563650.2015.1118487 |
| Ejaz | 2012 | Not a relevant biomarker assay or test | Ejaz AA, Kambhampati G, Ejaz NI, Dass B, Lapsia V, Arif AA, et al. Post-operative serum uric acid and acute kidney injury. J Nephrol 2012;25:497–505. https://doi.org/10.5301/jn.5000173 |
| Akcay | 2012 | Not a relevant type of population | Akcay AB, Ozlu MF, Sen N, Cay S, Ozturk OH, Yalcn F, et al. Prognostic significance of neutrophil gelatinase-associated lipocalin in ST-segment elevation myocardial infarction. J Investig Med 2012;60:508–13. https://doi.org/10.2310/JIM.0b013e31823e9d86 |
| Akrawinthawong | 2013 | < 100 participants | Akrawinthawong K, Shaw MK, Kachner J, Apostolov EO, Basnakian AG, Shah S, et al. Urine catalytic iron and neutrophil gelatinase-associated lipocalin as companion early markers of acute kidney injury after cardiac surgery: a prospective pilot study. Cardiorenal Med 2013;3:7–16. https://doi.org/10.1159/000346815 |
| Akrawinthawong | 2015 | < 100 participants | Akrawinthawong K, Ricci J, Cannon L, Dixon S, Kupfer K, Stivers D, et al. Subclinical and clinical contrast-induced acute kidney injury: data from a novel blood marker for determining the risk of developing contrast-induced nephropathy (ENCINO), a prospective study. Ren Fail 2015;37:187–91. https://doi.org/10.3109/0886022X.2014.991994 |
| Al-Afify | 2013 | < 100 participants | Al-Afify AA. Prognostic value of neutrophil gelatinase-associated lipocalin in predicting in-hospital complications in patients with ST-segment elevation myocardial infarction. Res J Cardiol 2013;6:10–18 |
| Albeladi | 2017 | < 100 participants | Albeladi FI, Algethamy HM. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury, severe kidney injury, and the need for renal replacement therapy in the intensive care unit. Nephron Extra 2017;7:62–77. https://doi.org/10.1159/000477469 |
| Albert | 2014 | No focus on DTA for AKI | Albert C, Kube J, Haase-Fielitz A, Dittrich A, Schanze D, Zenker M, et al. Pilot study of association of catechol-O-methyl transferase rs4680 genotypes with acute kidney injury and tubular stress after open heart surgery. Biomark Med 2014;8:1227–38. https://doi.org/10.2217/bmm.14.85 |
| Albuquerque | 2019 | < 100 participants | Albuquerque PLMM, da Silva Jr GB, Meneses GC, Martins AMC, Lima DB, Raubenheimer J, Fathima S, et al. Acute kidney injury induced by bothrops venom: insights into the pathogenic mechanisms. Toxins (Basel) 2019;11:148 |
| Algethamy | 2017 | < 100 participants | Algethamy HM, Albeladi FI. Urinary neutrophil gelatinase-associated lipocalin is an excellent predictor of mortality in intensive care unit patients. Saudi Med J 2017;38:706–14. https://doi.org/10.15537/smj.2017.7.18181 |
| Alharazy | 2014 | Not a relevant type of population | Alharazy SM, Kong N, Saidin R, Gafor AH, Maskon O, Mohd M, Zakaria SZ. Serum neutrophil gelatinase-associated lipocalin and cystatin C are early biomarkers of contrast-induced nephropathy after coronary angiography in patients with chronic kidney disease. Angiology 2014;65:436–42. https://doi.org/10.1177/0003319713483918 |
| Alharazy | 2014 | Not a relevant type of population | Alharazy SM, Kong N, Saidin R, Gafor AHA, Maskon O, Mohd M, Zakaria SZS. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after coronary angiography. Angiol 2014;65:216–23 |
| Aljumah | 2018 | < 100 participants | Aljumah AA, Tamim H, Saeed M, Tamimi W, Alfawaz H, Al Qurashi S, et al. The role of urinary neutrophil gelatinase-associated lipocalin in predicting acute kidney dysfunction in patients with liver cirrhosis. J Clin Med Res 2018;10:419–28. https://doi.org/10.14740/jocmr3366w |
| Allavena | 2013 | < 100 participants | Allavena C, Bach-Ngohou K, Billaud E, Secher S, Dejoie T, Reliquet V, et al. Neutrophil gelatinase-associated lipocalin, a marker of tubular dysfunction, is not increased in long-term virologically controlled patients receiving a tenofovir/emtricitabine + nevirapine regimen. J Antimicrob Chemother 2013;68:2866–70 |
| Almalky | 2015 | < 100 participants | Almalky MA, Hasan SA, Hassan TH, Shahbah DA, Arafa MA, Khalifa NA, Ibrahim RE. Detection of early renal injury in children with solid tumors undergoing chemotherapy by urinary neutrophil gelatinase-associated lipocalin. Mol Clin Oncol 2015;3:1341–6. https://doi.org/10.3892/mco.2015.631 |
| Al-Shamma | 2017 | < 100 participants | Al-Shamma ZAA, Alklyali NG, Alani IY. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a predictive biomarker of kidney injury in renal transplanted patients and chronic kidney disease. Int J Pharm Pharm Sci 2017;9:59–63 |
| Alvelos | 2011 | Not a relevant biomarker assay or test | Alvelos M, Pimentel R, Pinho E, Gomes A, Lourenço P, Teles MJ, et al. Neutrophil gelatinase-associated lipocalin in the diagnosis of type 1 cardio-renal syndrome in the general ward. Clin J Am Soc Nephrol 2011;6:476–81. https://doi.org/10.2215/CJN.06140710 |
| Alvelos | 2013 | Not a relevant biomarker assay or test | Alvelos M, Lourenço P, Dias C, Amorim M, Rema J, Leite AB, et al. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol 2013;165:51–5. https://doi.org/10.1016/j.ijcard.2011.07.080 |
| Anagnostopoulos | 2016 | Not a primary study | Anagnostopoulos PV. Prediction of severe acute kidney injury after pediatric cardiac surgery with the use of novel biomarkers: a new trend in clinical research and risk stratification. J Thorac Cardiovasc Surg 2016;152:187–8. https://doi.org/10.1016/j.jtcvs.2016.04.033 |
| Angeletti | 2016 | < 100 participants | Angeletti S, Fogolari M, Morolla D, Capone F, Costantino S, Spoto S, et al. Role of neutrophil gelatinase-associated lipocalin in the diagnosis and early treatment of acute kidney injury in a case series of patients with acute decompensated heart failure: a case series. Cardiol Res Pract 2016;2016:3708210. https://doi.org/10.1155/2016/3708210 |
| Anonymous | 2008 | Not a primary study | Anonymous. A single measurement of urinary NGAL can identify acute kidney injury. Nat Clin Pract Nephrol 2008;4:466 |
| Anonymous | 2010 | Non-English-language publication | Anonymous. Early biomarker for AKI. Jpn J Nephrol 2010;52:566–571 |
| Antonelli | 2020 | Systematic review – retained as background material | Antonelli A, Allinovi M, Cocci A, Russo GI, Schiavina R, Rocco B, et al. The predictive role of biomarkers for the detection of acute kidney injury after partial or radical nephrectomy: a systematic review of the literature. Eur Urol Focus 2020;6:344–53 |
| Antonopoulos | 2011 | < 100 participants | Antonopoulos CN, Kalkanis A, Georgakopoulos G, Sergentanis TN, Rigopoulos DN. Neutrophil gelatinase-associated lipocalin in dehydrated patients: a preliminary report. BMC Res Notes 2011;4:435. https://doi.org/10.1186/1756-0500-4-435 |
| Anusha | 2015 | < 100 participants | Anusha R, Silambanan S, Veerasamy M. Plasma neutrophil gelatinase associated lipocalin in the early detection of acute kidney injury in patients undergoing cardiac surgery. Int J Pharm Biol Sci 2015;6:B64–B71 |
| Arambašić | 2016 | Not a relevant type of population | Arambašić J, Mandić S, Debeljak Ž, Mandić D, Horvat V, Šerić V. Differentiation of acute pyelonephritis from other febrile states in children using urinary neutrophil gelatinase-associated lipocalin (uNGAL). Clin Chem Lab Med 2016;54:55–61. https://doi.org/10.1515/cclm-2015-0377 |
| Arampatzis | 2017 | Not a relevant biomarker assay or test | Arampatzis S, Chalikias G, Devetzis V, Konstantinides S, Huynh-Do U, Tziakas D. C-terminal fragment of agrin (CAF) levels predict acute kidney injury after acute myocardial infarction. BMC Nephrol 2017;18:202. https://doi.org/10.1186/s12882-017-0611-9 |
| Aregger | 2014 | < 100 participants | Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, Jörres A. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int 2014;85:909–19. https://doi.org/10.1038/ki.2013.363 |
| Arena | 2010 | < 100 participants | Arena A, Stassi G, Iannello D, Gazzara D, Calapai M, Bisignano C, et al. Both IL-1 beta and TNF-alpha regulate NGAL expression in polymorphonuclear granulocytes of chronic hemodialysis patients. Mediators Inflamm 2010;2010:613937 |
| Ariza | 2015 | < 100 participants | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLOS ONE 2015;10:e0128145. https://doi.org/10.1371/journal.pone.0128145 |
| Arora | 2016 | Not a primary study | Arora RC, Rigatto C, Singal RK. Neutrophil gelatinase-associated lipocalin to predict cardiac surgery-associated acute kidney injury: a holy grail or just another fancy cup? J Thorac Cardiovasc Surg 2016;151:1482–3. https://doi.org/10.1016/j.jtcvs.2016.02.042 |
| Arora | 2017 | Not a primary study | Arora RC, Singal RK. Is routine use of renal injury biomarkers in cardiac surgery patients putting the cart before the horse? J Thorac Cardiovasc Surg 2017;154:938–9 |
| Arsalan | 2018 | < 100 participants | Arsalan M, Ungchusri E, Farkas R, Johnson M, Kim RJ, Filardo G, et al. Novel renal biomarker evaluation for early detection of acute kidney injury after transcatheter aortic valve implantation. Proc 2018;31:171–6. https://doi.org/10.1080/08998280.2017.1416235 |
| Arthur | 2014 | < 100 participants | Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 2014;85:431–8. https://doi.org/10.1038/ki.2013.333 |
| Arun | 2015 | < 100 participants | Arun O, Celik G, Oc B, Unlu A, Celik JB, Oc M, Duman A. Renal effects of coronary artery bypass graft surgery in diabetic and non-diabetic patients: a study with urinary neutrophil gelatinase-associated lipocalin and serum cystatin C. Kidney Blood Press Res 2015;40:141–52. https://doi.org/10.1159/000368490 |
| Ascher | 2018 | Not a relevant type of population | Ascher SB, Scherzer R, Estrella MM, Zhang WR, Muiru AN, Jotwani V, et al. Association of urinary biomarkers of kidney injury with estimated GFR decline in HIV-infected individuals following tenofovir disoproxil fumarate initiation. Clin J Am Soc Nephrol 2018;13:1321–9. https://doi.org/10.2215/CJN.01700218 |
| Ashalatha | 2017 | No relevant outcome | Ashalatha VL, Bitla AR, Kumar VS, Rajasekhar D, Suchitra MM, Lakshmi AY, Rao PV. Biomarker response to contrast administration in diabetic and nondiabetic patients following coronary angiography. Indian J Nephrol 2017;27:20–7. https://doi.org/10.4103/0971-4065.179335 |
| Askenazi | 2011 | Not a relevant type of population | Askenazi DJ, Montesanti A, Hundley H, Koralkar R, Pawar P, Shuaib F, et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr 2011;159:907–12.e1 |
| Askenazi | 2011 | Not a relevant type of population | Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 2011;70:302–6 |
| Askenazi | 2012 | < 100 participants | Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N. Urine biomarkers predict acute kidney injury in newborns. J Pediatr 2012;161:270–5.e1 |
| Askenazi | 2016 | Not a relevant type of population | Askenazi DJ, Koralkar R, Patil N, Halloran B, Ambalavanan N, Griffin R. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin J Am Soc Nephrol 2016;11:1527–35 |
| Assadi | 2019 | < 100 participants | Assadi F, Sharbaf FG. Urine KIM-1 as a potential biomarker of acute renal injury after circulatory collapse in children. Pediatr Emerg Care 2019;35:104–7. https://doi.org/10.1097/PEC.0000000000000886 |
| Ataei | 2015 | < 100 participants | Ataei S, Hadjibabaie M, Moslehi A, Taghizadeh-Ghehi M, Ashouri A, Amini E, et al. A double-blind, randomized, controlled trial on N-acetylcysteine for the prevention of acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Hematol Oncol 2015;33:67–74 |
| Ataei | 2018 | < 100 participants | Ataei N, Ameli S, Yousefifard M, Oraei A, Ataei F, Bazargani B, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C in early detection of pediatric acute kidney injury; a diagnostic accuracy study. Emerg 2018;6:e2 |
| Au | 2016 | Not a relevant biomarker assay or test | Au V, Feit J, Barasch J, Sladen RN, Wagener G. Urinary neutrophil gelatinase-associated lipocalin (NGAL) distinguishes sustained from transient acute kidney injury after general surgery. Kidney Int Rep 2016;1:3–9 |
| Audard | 2014 | < 100 participants | Audard V, Moutereau S, Vandemelebrouck G, Habibi A, Khellaf M, Grimbert P, et al. First evidence of subclinical renal tubular injury during sickle-cell crisis. Orphanet J Rare Dis 2014;9:67 |
| Axelrod | 2016 | No focus on DTA for AKI | Axelrod DM, Sutherland SM, Anglemyer A, Grimm PC, Roth SJ. A double-blinded, randomized, placebo-controlled clinical trial of aminophylline to prevent acute kidney injury in children following congenital heart surgery with cardiopulmonary bypass. Pediatr Crit Care Med 2016;17:135–43 |
| Aydin | 2014 | < 100 participants | Aydin SA, Pozam S, Ozdemir F, Ozkan ML, Koksal O. The role of neutrophil gelatinase-associated lipocalin in identifying contrast induced nephropathy development in the emergency department. J Pak Med Assoc 2014;64:1109–13 |
| Aydoğdu | 2013 | Not a relevant biomarker assay or test | Aydoğdu M, Gürsel G, Sancak B, Yeni S, Sarı G, Taşyürek S, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers 2013;34:237–46 |
| Azzalini | 2017 | Not a primary study | Azzalini L, Garcia-Moll X. On contrast-induced acute kidney injury, risk prediction, and the future of predictive model development. Can J Cardiol 2017;33:711–13 |
| Bachorzewska-Gajewska | 2006 | < 100 participants | Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol 2006;26:287–92 |
| Bachorzewska-Gajewska | 2007 | Not a relevant type of population | Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Pawlak K, Mysliwiec M, et al. Could neutrophil-gelatinase-associated lipocalin and cystatin C predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press Res 2007;30:408–15 |
| Bachorzewska-Gajewska | 2009 | < 100 participants | Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S. NGAL (neutrophil gelatinase-associated lipocalin) and L-FABP after percutaneous coronary interventions due to unstable angina in patients with normal serum creatinine. Adv Med Sci 2009;54:221–4 |
| Bachorzewska-Gajewska | 2009 | Not a relevant type of population | Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S. NGAL (neutrophil gelatinase-associated lipocalin) and L-FABP after percutaneous coronary interventions due to unstable angina in patients with normal serum creatinine. Adv Med Sci 2009;54:221–4 |
| Bachorzewska-Gajewska | 2008 | Not a relevant type of population | Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Poniatowski B, Pawlak K, Dobrzycki S. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol 2008;127:290–1 |
| Bachorzewska-Gajewska | 2013 | Not a relevant type of population | Bachorzewska-Gajewska H, Tomaszuk-Kazberuk A, Jarocka I, Mlodawska E, Lopatowska P, Zalewska-Adamiec M, et al. Does neutrophil gelatinase-asociated lipocalin have prognostic value in patients with stable angina undergoing elective PCI? A 3-year follow-up study. Kidney Blood Press Res 2013;37:280–5 |
| Baek | 2019 | Not a relevant type of population | Baek SD, Kang JY, Shin S, Park HS, Kim MS, Kim SM, et al. Predictive factors of duration of continuous renal replacement therapy in acute kidney injury survivors. Shock 2019;52:598–603 |
| Bagheri | 2018 | < 100 participants | Bagheri S, Einollahi N, Goodarzi MT, Tatari H, Moradi-Sardareh H, Sheikh N. Neutrophil gelatinase-associated lipocalin, cystatin C and matrix metalloproteinase-9 as possible biomarkers in early detection of acute kidney injury after cardiac surgery. J Clin Diagnostic Res 2018;12:BC05–9 |
| Bagshaw | 2011 | Not a primary study | Bagshaw SM. Subclinical acute kidney injury: a novel biomarker-defined syndrome. Crit Care Resusc 2011;13:201–3 |
| Bagshaw | 2013 | < 100 participants | Bagshaw SM, Bennett M, Devarajan P, Bellomo R. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care 2013;28:371–8 |
| Balkanay | 2015 | < 100 participants | Balkanay OO, Goksedef D, Omeroglu SN, Ipek G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg 2015;20:209–14 |
| Balkanay | 2018 | < 100 participants | Balkanay OO, Göksedef D, Ömeroğlu SN, İpek G. The reliability of the use of serum neutrophil gelatinase-associated lipocalin levels in the assessment of renal functions after coronary artery bypass grafting. Cardiol Res Pract 2018;2018:7291254 |
| Barbarash | 2017 | No focus on DTA for AKI | Barbarash OL, Bykova IS, Kashtalap VV, Zykov MV, Hryachkova ON, Kalaeva VV, et al. Serum neutrophil gelatinase-associated lipocalin has an advantage over serum cystatin C and glomerular filtration rate in prediction of adverse cardiovascular outcome in patients with ST-segment elevation myocardial infarction. BMC Cardiovasc Disord 2017;17:81 |
| Baron-Stefaniak | 2017 | < 100 participants | Baron-Stefaniak J, Schiefer J, Miller EJ, Berlakovich GA, Baron DM, Faybik P. Comparison of macrophage migration inhibitory factor and neutrophil gelatinase-associated lipocalin-2 to predict acute kidney injury after liver transplantation: an observational pilot study. PLOS ONE 2017;12:e0183162 |
| Bassareo | 2013 | Not a relevant type of population | Bassareo PP, Fanos V, Mussap M, Flore G, Noto A, Puddu M, et al. Urinary NGAL and hematic ADMA levels: an early sign of cardio-renal syndrome in young adults born preterm? J Matern Fetal Neonatal Med 2013;26(Suppl. 2):80–3 |
| Basturk | 2017 | < 100 participants | Basturk T, Sari O, Koc Y, Eren N, Isleem M, Kara E, et al. Prognostic significance of NGAL in early stage chronic kidney disease. Minerva Urol Nefrol 2017;69:307–12 |
| Basu | 2014 | Not a relevant biomarker assay or test | Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 2014;9:654–62 |
| Basu | 2014 | Not a relevant biomarker assay or test | Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014;64:2753–62 |
| Bataille | 2017 | No relevant outcome | Bataille A, Tiepolo A, Robert T, Boutten A, Longrois D, Dehoux M, Provenchère S. Reference change values of plasma and urine NGAL in cardiac surgery with cardiopulmonary bypass. Clin Biochem 2017;50:1098–103 |
| Baumert | 2017 | < 100 participants | Baumert M, Surmiak P, Więcek A, Walencka Z. Serum NGAL and copeptin levels as predictors of acute kidney injury in asphyxiated neonates. Clin Exp Nephrol 2017;21:658–64 |
| Bayram | 2014 | < 100 participants | Bayram A, Ulgey A, Baykan A, Narin N, Narin F, Esmaoglu A, Boyaci A. The effects of dexmedetomidine on early stage renal functions in pediatric patients undergoing cardiac angiography using non-ionic contrast media: a double-blind, randomized clinical trial. Paediatr Anaesth 2014;24:426–32 |
| Bayram | 2014 | < 100 participants | Bayram M, Ezelsoy M, Usta E, Oral K, Saraçoğlu A, Bayramoğlu Z, Yıldırım Ö. Rapid detection of acute kidney injury by urinary neutrophil gelatinase-associated lipocalin in patients undergoing cardiopulmonary bypass. Turk J Anaesthesiol Reanim 2014;42:239–44 |
| Bedford | 2016 | < 100 participants | Bedford M, Stevens P, Coulton S, Billings J, Farr M, Wheeler T, et al. Development of risk models for the prediction of new or worsening acute kidney injury on or during hospital admission: a cohort and nested study. Health Serv Deliv Res 2016;4(6) |
| Beitland | 2018 | Not a primary study | Beitland S, Joannidis M. Biomarkers of acute kidney injury – a mission impossible? Acta Anaesthesiol Scand 2018;62:2–5 |
| Belcher | 2014 | < 100 participants | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014;60:622–32 |
| Belcher | 2015 | Not a relevant type of population | Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 2015;9:1857–67 |
| Bell | 2015 | < 100 participants | Bell M, Larsson A, Venge P, Bellomo R, Mårtensson J. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers 2015;2015:158658 |
| Bellos | 2018 | Meta-analysis – retained as background material | Bellos I, Fitrou G, Daskalakis G, Perrea DN, Pergialiotis V. Neutrophil gelatinase-associated lipocalin as predictor of acute kidney injury in neonates with perinatal asphyxia: a systematic review and meta-analysis. Eur J Pediatr 2018;177:1425–34 |
| Benli | 2017 | < 100 participants | Benli E, Ayyildiz SN, Cirrik S, Noyan T, Ayyildiz A, Cirakoglu A. Early term effect of ureterorenoscopy (URS) on the Kidney: research measuring NGAL, KIM-1, FABP and CYS C levels in urine. Int Braz J Urol 2017;43:887–95 |
| Benoit | 2019 | < 100 participants | Benoit SW, Dixon BP, Goldstein SL, Bennett MR, Lane A, Lounder DT, et al. A novel strategy for identifying early acute kidney injury in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant 2019;54:1453–61 |
| Benzer | 2016 | < 100 participants | Benzer M, Alpay H, Baykan Ö, Erdem A, Demir IH. Serum NGAL, cystatin C and urinary NAG measurements for early diagnosis of contrast-induced nephropathy in children. Ren Fail 2016;38:27–34 |
| Berghaus | 2012 | Not a primary study | Berghaus TM, Schwaiblmair M, von Scheidt W. Renal biomarkers and prognosis in acute pulmonary embolism. Heart 2012;98:1185–6 |
| Bhavsar | 2012 | Not a relevant type of population | Bhavsar NA, Köttgen A, Coresh J, Astor BC. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2012;60:233–40 |
| Biernawska | 2017 | < 100 participants | Biernawska J, Bober J, Kotfis K, Bogacka A, Barnik E, Żukowski M. Cardiac surgery related cardio-renal syndrome assessed by conventional and novel biomarkers – under or overestimated diagnosis? Arch Med Sci 2017;13:1111–20 |
| Biernawska | 2018 | < 100 participants | Biernawska J, Bober J, Kotfis K, Noceń I, Bogacka A, Barnik E, et al. Iron excretion in urine in patients with acute kidney injury after cardiac surgery. Adv Clin Exp Med 2018;27:1671–6 |
| Bignami | 2015 | < 100 participants | Bignami E, Frati E, Meroni R, Simonini M, Di Prima AL, Manunta P, Zangrillo A. Urinary neutrophil gelatinase-associated lipocalin time course during cardiac surgery. Ann Card Anaesth 2015;18:39–44 |
| Bojan | 2016 | < 100 participants | Bojan M, Basto Duarte MC, Ermak N, Lopez-Lopez V, Mogenet A, Froissart M. Structural equation modelling exploration of the key pathophysiological processes involved in cardiac surgery-related acute kidney injury in infants. Crit Care 2016;20:171 |
| Bojan | 2018 | < 100 participants | Bojan M, Basto Duarte MC, Lopez V, Tourneur L, Vicca S, Froissart M. Low perfusion pressure is associated with renal tubular injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a secondary analysis of an observational study. Eur J Anaesthesiol 2018;35:581–7 |
| Bojic | 2015 | Not a relevant biomarker assay or test | Bojic S, Kotur-Stevuljevic J, Kalezic N, Stevanovic P, Jelic-Ivanovic Z, Bilanovic D, et al. Diagnostic value of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in sepsis-associated acute kidney injury. Tohoku J Exp Med 2015;237:103–9 |
| Bolignano | 2008 | < 100 participants | Bolignano D, Lacquaniti A, Coppolino G, Campo S, Arena A, Buemi M. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res 2008;31:255–8 |
| Bolignano | 2009 | < 100 participants | Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 2009;4:337–44 |
| Bolignano | 2009 | Not a primary study | Bolignano D, Coppolino G, Lombardi L, Buemi M. NGAL: a new missing link between inflammation and uremic anemia? Ren Fail 2009;31:622–3 |
| Bolignano | 2009 | Not a primary study | Bolignano D, Coppolino G, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin in the intensive care unit: time to look beyond a single, threshold-based measurement? Crit Care Med 2009;37:2864 |
| Bolignano | 2012 | Not a primary study | Bolignano D. Serum creatinine and the search for new biomarkers of acute kidney injury (AKI): the story continues. Clin Chem Lab Med 2012;50:1495–9 |
| Bolignano | 2013 | < 100 participants | Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 2013;4:337–44 |
| Bolliger | 2018 | Not a primary study | Bolliger D, Siegemund M. The more, the merrier? – urinary biomarkers for prediction of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:2201–2 |
| Bonventre | 2008 | No focus on DTA for AKI | Bonventre JV. Urine neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury in critically ill children. Nat Clin Pract Nephrol 2008;4:78–9 |
| Bouchard | 2015 | No focus on DTA for AKI | Bouchard J, Malhotra R, Shah S, Kao YT, Vaida F, Gupta A, et al. Levels of protein C and soluble thrombomodulin in critically ill patients with acute kidney injury: a multicenter prospective observational study. PLOS ONE 2015;10:e0120770 |
| Bramham | 2016 | No relevant outcome | Bramham K, Seed PT, Lightstone L, Nelson-Piercy C, Gill C, Webster P, et al. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int 2016;89:874–85 |
| Breidthardt | 2012 | Not a relevant biomarker assay or test | Breidthardt T, Socrates T, Drexler B, Noveanu M, Heinisch C, Arenja N, et al. Plasma neutrophil gelatinase-associated lipocalin for the prediction of acute kidney injury in acute heart failure. Crit Care 2012;16:R2 |
| Breidthardt | 2012 | Not a relevant type of population | Breidthardt T, Christ-Crain M, Stolz D, Bingisser R, Drexler B, Klima T, et al. A combined cardiorenal assessment for the prediction of acute kidney injury in lower respiratory tract infections. Am J Med 2012;125:168–75 |
| Brinkman | 2015 | < 100 participants | Brinkman R, HayGlass KT, Mutch WA, Funk DJ. Acute kidney injury in patients undergoing open abdominal aortic aneurysm repair: a pilot observational trial. J Cardiothorac Vasc Anesth 2015;29:1212–19 |
| Brulotte | 2013 | Not a relevant type of population | Brulotte V, Leblond FA, Elkouri S, Thérasse E, Pichette V, Beaulieu P. Bicarbonates for the prevention of postoperative renal failure in endovascular aortic aneurysm repair: a randomized pilot trial. Anesthesiol Res Pract 2013;2013:467326 |
| Brunner | 2006 | < 100 participants | Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 2006;54:2577–84 |
| Bruno | 2016 | < 100 participants | Bruno N, ter Maaten JM, Ovchinnikova ES, Vegter EL, Valente MA, van der Meer P, et al. MicroRNAs relate to early worsening of renal function in patients with acute heart failure. Int J Cardiol 2016;203:564–9 |
| Buelow | 2012 | < 100 participants | Buelow MW, Dall A, Regner K, Weinberg C, Bartz PJ, Sowinski J, et al. Urinary interleukin-18 and urinary neutrophil gelatinase-associated lipocalin predict acute kidney injury following pulmonary valve replacement prior to serum creatinine. Congenit Heart Dis 2012;7:441–7 |
| Buğra | 2014 | Not a relevant type of population | Buğra O, Baysal A, Fedakar A, Erdem K, Sunar H, Dağlar B. Does serum neutrophil gelatinase-associated lipocalin biomarker detect the early deterioration in renal functions in patients with insulin-dependent diabetes mellitus undergoing coronary artery bypass graft surgery? Turk J Thorac Cardiovasc Surg 2014;22:63–70 |
| Bulluck | 2018 | Not a relevant biomarker assay or test | Bulluck H, Maiti R, Chakraborty B, Candilio L, Clayton T, Evans R, et al. Neutrophil gelatinase-associated lipocalin prior to cardiac surgery predicts acute kidney injury and mortality. Heart 2018;104:313–17 |
| Bunchman | 2012 | Not a primary study | Bunchman TE. Biomarkers for acute kidney injury: is the serum creatinine worthless? Pediatr Crit Care Med 2012;13:119–20 |
| Bunel | 2017 | < 100 participants | Bunel V, Tournay Y, Baudoux T, De Prez E, Marchand M, Mekinda Z, et al. Early detection of acute cisplatin nephrotoxicity: interest of urinary monitoring of proximal tubular biomarkers. Clin Kidney J 2017;10:639–47 |
| Bunz | 2015 | < 100 participants | Bunz H, Weyrich P, Peter A, Baumann D, Tschritter O, Guthoff M, et al. Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) and proteinuria predict severity of acute kidney injury in Puumala virus infection. BMC Infect Dis 2015;15:464 |
| Burke-Gaffney | 2014 | < 100 participants | Burke-Gaffney A, Svermova T, Mumby S, Finney SJ, Evans TW. Raised plasma Robo4 and cardiac surgery-associated acute kidney injury. PLOS ONE 2014;9:e111459 |
| Cai | 2009 | < 100 participants | Cai L, Borowiec J, Xu S, Han W, Venge P. Assays of urine levels of HNL/NGAL in patients undergoing cardiac surgery and the impact of antibody configuration on their clinical performances. Clin Chim Acta 2009;403:121–5 |
| Camou | 2013 | < 100 participants | Camou F, Oger S, Paroissin C, Guilhon E, Guisset O, Mourissoux G, et al. [Plasma neutrophil gelatinase-associated lipocalin (NGAL) predicts acute kidney injury in septic shock at ICU admission.] Ann Fr Anesth Reanim 2013;32:157–64 |
| Canakci | 2018 | < 100 participants | Canakci E, Karatas A, Noyan T, Sertacayhan B. Can acute kidney injury be diagnosed using biomarkers in intensive care patients? Acta Medica Mediterr 2018;34:2023–9 |
| Cangemi | 2013 | Not a relevant type of population | Cangemi G, Storti S, Cantinotti M, Fortunato A, Emdin M, Bruschettini M, et al. Reference values for urinary neutrophil gelatinase-associated lipocalin (NGAL) in pediatric age measured with a fully automated chemiluminescent platform. Clin Chem Lab Med 2013;51:1101–5 |
| Capuano | 2009 | < 100 participants | Capuano F, Goracci M, Luciani R, Gentile G, Roscitano A, Benedetto U, Sinatra R. Neutrophil gelatinase-associated lipocalin levels after use of mini-cardiopulmonary bypass system. Interact Cardiovasc Thorac Surg 2009;9:797–801 |
| Carey | 2018 | No focus on DTA for AKI | Carey I, Byrne R, Childs K, Horner M, Bruce M, Wang B, et al. Serum NGAL can act as an early renal safety biomarker during long-term nucleos(t)ide analogue antiviral therapy in chronic hepatitis B. J Viral Hepat 2018;25:1139–50 |
| Carrillo-Esper | 2014 | < 100 participants | Carrillo-Esper R, Perez-Calatayud AA, Pena-Perez CA, Diaz-Carrillo MA, Nava-Lopez JA, De Los Monteros-Estrada IE, Zepeda-Mendoza AD. Urinary sediment microscopic score as diagnostic marker of acute kidney lesion in sepsis. Med Interna Mex 2014;30:602–6 |
| Carter | 2014 | No focus on DTA for AKI | Carter JL, Lamb EJ. Evaluating new biomarkers for acute kidney injury: putting the horse before the cart. Am J Kidney Dis 2014;63:543–6 |
| Carter | 2016 | < 100 participants | Carter JL, Parker CT, Stevens PE, Eaglestone G, Knight S, Farmer CK, Lamb EJ. Biological variation of plasma and urinary markers of acute kidney injury in patients with chronic kidney disease. Clin Chem 2016;62:876–83 |
| Cecchi | 2017 | < 100 participants | Cecchi E, Avveduto G, D’Alfonso MG, Terreni A, Gelera E, Caldini A, Giglioli C. Cystatin C, but not urinary or serum NGAL, may be associated with contrast induced nephropathy after percutaneous coronary invasive procedures: a single center experience on a limited number of patients. Acta Medica Academica 2017;46:34–43 |
| Çelik | 2013 | < 100 participants | Çelik T, Altekin E, İşgüder R, Kenesari Y, Duman M, Arslan N. Evaluation of neutrophil gelatinase-associated lipocalin in pediatric patients with acute rotavirus gastroenteritis and dehydration. Ital J Pediatr 2013;39:52 |
| Cemil | 2014 | < 100 participants | Cemil K, Elif C, Serkan YM, Fevzi Y, Deniz AE, Tamer D, Polat D. The value of serum NGAL in determination of dialysis indication. J Pak Med Assoc 2014;64:739–42 |
| Cervellin | 2012 | Not a primary study | Cervellin G, Di Somma S. Neutrophil gelatinase-associated lipocalin (NGAL): the clinician’s perspective. Clin Chem Lab Med 2012;50:1489–93 |
| Chae | 2015 | Not a relevant type of population | Chae H, Ryu H, Cha K, Kim M, Kim Y, Min CK. Neutrophil gelatinase-associated lipocalin as a biomarker of renal impairment in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2015;15:35–40 |
| Chagan-Yasutan | 2016 | No focus on DTA for AKI | Chagan-Yasutan H, Chen Y, Lacuesta TL, Leano PSA, Iwasaki H, Hanan F, et al. Urine levels of defensin alpha1 reflect kidney injury in leptospirosis patients. Int J Mol Sci 2016;17:1637 |
| Chang | 2009 | < 100 participants | Chang CK, Chuter TA, Niemann CU, Shlipak MG, Cohen MJ, Reilly LM, Hiramoto JS. Systemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:1140–6 |
| Chang | 2009 | < 100 participants | Chang CK, Chuter TA, Niemann CU, Shlipak MG, Cohen MJ, Reilly LM, Hiramoto JS. Systemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:1140–6 |
| Chang | 2015 | Not a relevant biomarker assay or test | Chang CH, Yang CH, Yang HY, Chen TH, Lin CY, Chang SW, et al. Urinary biomarkers improve the diagnosis of intrinsic acute kidney injury in coronary care units. Medicine 2015;94:e1703 |
| Chang | 2017 | No focus on DTA for AKI | Chang C, Hu Y, Hogan SL, Mercke N, Gomez M, O’Bryant C, et al. Pharmacogenomic variants may influence the urinary excretion of novel kidney injury biomarkers in patients receiving cisplatin. Int J Mol Sci 2017;18:1333 |
| Chang | 2018 | Not a relevant biomarker assay or test | Chang W, Zhu S, Pan C, Xie JF, Liu SQ, Qiu HB, Yang Y. Predictive utilities of neutrophil gelatinase-associated lipocalin (NGAL) in severe sepsis. Clin Chim Acta 2018;481:200–6 |
| Channanayaka | 2016 | < 100 participants | Channanayaka C, Venkatkrishnan A. Clinical utility of serum neutrophil gelatinase associated lipocalin (NGAL) as an early marker of acute kidney injury in asphyxiated neonates. J Nepal Paediatr Soc 2016;36:121–5 |
| Che | 2010 | < 100 participants | Che M, Xie B, Xue S, Dai H, Qian J, Ni Z, et al. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron Clin Pract 2010;115:c66–72 |
| Chen | 2014 | < 100 participants | Chen T, Lu YH, Wang WJ, Bian CY, Cheng XY, Su Y, Zhou PM. Elevated urinary levels of cystatin C and neutrophil gelatinase-associated lipocalin in Henoch–Schonlein purpura patients with renal involvement. PLOS ONE 2014;9:e101026 |
| Chen | 2019 | Not a relevant type of population | Chen X, Chen Z, Wei T, Li P, Zhang L, Fu P. The effect of serum neutrophil gelatinase-associated lipocalin on the discontinuation of continuous renal replacement therapy in critically ill patients with acute kidney injury. Blood Purif 2019;48:10–17 |
| Chen | 2012 | Not a relevant biomarker assay or test | Chen TH, Chang CH, Lin CY, Jenq CC, Chang MY, Tian YC, et al. Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLOS ONE 2012;7:e32328 |
| Chen | 2016 | Not a relevant type of population | Chen C, Yang X, Lei Y, Zha Y, Liu H, Ma C, et al. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol 2016;11:1536–44 |
| Chen | 2016 | Not a primary study | Chen CF, Lin CC. Neutrophil gelatinase-associated lipocalin: still a good predictive marker of acute kidney injury in severe septic patients? J Chin Med Assoc 2016;79:411–12 |
| Cheng | 2012 | < 100 participants | Cheng CW, Chen YC, Chang CH, Yu HP, Lin CC, Yang MW, et al. The ratio of plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Transplant Proc 2012;44:776–9 |
| Vermi | 2014 | < 100 participants | Vermi AC, Costopoulos C, Latib A, Piraino D, Maisano F, Naim C, et al. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury after transcatheter aortic valve implantation. Hellenic J Cardiol 2014;55:77–9 |
| Chindarkar | 2015 | No relevant outcome | Chindarkar NS, Chawla LS, Straseski JA, Jortani SA, Uettwiller-Geiger D, Orr RR, et al. Demographic data for urinary Acute Kidney Injury (AKI) marker [IGFBP7]·[TIMP2] reference range determinations. Data Brief 2015;5:888–92 |
| Chindarkar | 2016 | Not a relevant type of population | Chindarkar NS, Chawla LS, Straseski JA, Jortani SA, Uettwiller-Geiger D, Orr RR, et al. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]·[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta 2016;452:32–7 |
| Cho | 2014 | < 100 participants | Cho E, Lee JH, Lim HJ, Oh SW, Jo SK, Cho WY, et al. Soluble CD25 is increased in patients with sepsis-induced acute kidney injury. Nephrology 2014;19:318–24 |
| Cho | 2018 | Not a primary study | Cho SY, Hur M. Neutrophil gelatinase-associated lipocalin as a promising novel biomarker for early detection of kidney injury. Ann Lab Med 2018;38:393–4 |
| Choi | 2013 | Not a relevant type of population | Choi HM, Park KT, Lee JW, Cho E, Jo SK, Cho WY, Kim HK. Urine neutrophil gelatinase-associated lipocalin predicts graft outcome up to 1 year after kidney transplantation. Transplant Proc 2013;45:122–8 |
| Choi | 2015 | Not a relevant type of population | Choi JW, Fujii T, Fujii N. Corrected neutrophil gelatinase-associated lipocalin (NGAL) level adjusted by the scoring system of an inflammation index for screening renal dysfunction in patients with systemic inflammation. Ann Clin Lab Sci 2015;45:248–55 |
| Choi | 2017 | Not a relevant type of population | Choi JW, Fujii T, Fujii N. Diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin (NGAL) as an inflammatory biomarker for low-grade inflammation. Biomed Res India 2017;28:6406–11 |
| Chou | 2013 | Not a relevant type of population | Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLOS ONE 2013;8:e54863 |
| Choudhry | 2018 | Not a relevant type of population | Choudhry N, Ihsan A, Mahmood S, Haq FU, Gondal AJ. Neutrophil gelatinase associated lipocalin, an early biomarker for diagnosis of acute kidney injury after percutaneous coronary intervention. Turk J Biochem 2018;43:15–21 |
| Chun | 2018 | < 100 participants | Chun W, Kim Y, Yoon J, Lee S, Yim H, Cho YS, et al. Assessment of plasma neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury and prediction of mortality in severely burned patients. J Burn Care Res 2018;39:387–93 |
| Civiletti | 2019 | < 100 participants | Civiletti F, Assenzio B, Mazzeo AT, Medica D, Giaretta F, Deambrosis I, et al. Acute tubular injury is associated with severe traumatic brain injury: in vitro study on human tubular epithelial cells. Sci Rep 2019;9:6090 |
| Coca | 2013 | Retained as background material | Coca SG, Garg AX, Swaminathan M, Garwood S, Hong K, Thiessen-Philbrook H, et al. Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant 2013;28:2787–99 |
| Coca | 2008 | Systematic review – retained as background material | Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 2008;73:1008–16 |
| Codorniu | 2018 | Meta-analysis – retained as background material | Codorniu A, Lemasle L, Legrand M, Blet A, Mebazaa A, Gayat E. Methods used to assess the performance of biomarkers for the diagnosis of acute kidney injury: a systematic review and meta-analysis. Biomarkers 2018;23:766–72 |
| Codsi | 2017 | Not a relevant type of population | Codsi E, Garovic VD, Gonzalez-Suarez ML, Milic N, Borowski KS, Rose CH, et al. Longitudinal characterization of renal proximal tubular markers in normotensive and preeclamptic pregnancies. Am J Physiol Regul Integr Comp Physiol 2017;312:R773–R778 |
| Connolly | 2018 | Not a relevant type of population | Connolly M, Kinnin M, McEneaney D, Menown I, Kurth M, Lamont J, et al. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. QJM 2018;111:103–10 |
| Constantin | 2010 | < 100 participants | Constantin JM, Futier E, Perbet S, Roszyk L, Lautrette A, Gillart T, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care 2010;25:176 |
| Corbacıoglu | 2017 | < 100 participants | Corbacıoglu SK, Cevik Y, Akinci E, Uzunosmanoglu H, Dagar S, Safak T, et al. Value of plasma neutrophil gelatinase-associated lipocalin (NGAL) in distinguishing between acute kidney injury (AKI) and chronic kidney disease (CKD). Turk J Emerg Med 2017;17:85–8 |
| Córdova-Sánchez | 2019 | < 100 participants | Córdova-Sánchez BM, Ruiz-García EB, López-Yañez A, Barragan-Dessavre M, Bautista-Ocampo AR, Meneses-García A, et al. Plasma neutrophil gelatinase-associated lipocalin and factors related to acute kidney injury and mortality in critically ill cancer patients. ecancermedicalscience 2019;13:903 |
| Coupes | 2015 | < 100 participants | Coupes B, de Freitas DG, Roberts SA, Read I, Riad H, Brenchley PE, Picton ML. rhErythropoietin-b as a tissue protective agent in kidney transplantation: a pilot randomized controlled trial. BMC Res Notes 2015;8:21 |
| Cruz | 2009 | Not a primary study | Cruz DN, Soni S, Ronco C. NGAL and cardiac surgery-associated acute kidney injury. Am J Kidney Dis 2009;53:565–6 |
| Cruz | 2012 | Systematic review – retained as background material | Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med 2012;50:1533–45 |
| Cruz | 2010 | Not a relevant biomarker assay or test | Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 2010;36:444–51 |
| Cruz | 2016 | < 100 participants | Cruz DN, Virzì GM, Brocca A, Ronco C, Giavarina D. A comparison of three commercial platforms for urinary NGAL in critically ill adults. Clin Chem Lab Med 2016;54:353–62 |
| Cuartero | 2017 | < 100 participants | Cuartero M, Ballús J, Sabater J, Pérez X, Nin N, Ordonez-Llanos J, Betbesé AJ. Cell-cycle arrest biomarkers in urine to predict acute kidney injury in septic and non-septic critically ill patients. Ann Intensive Care 2017;7:92 |
| Cullaro | 2017 | Not a relevant biomarker assay or test | Cullaro G, Kim G, Pereira MR, Brown RS, Verna EC. Ascites neutrophil gelatinase-associated lipocalin identifies spontaneous bacterial peritonitis and predicts mortality in hospitalized patients with cirrhosis. Dig Dis Sci 2017;62:3487–94 |
| Cullaro | 2018 | < 100 participants | Cullaro G, Pisa JF, Brown RS, Wagener G, Verna EC. Early postoperative neutrophil gelatinase-associated lipocalin predicts the development of chronic kidney disease after liver transplantation. Transplantation 2018;102:809–15 |
| da Rocha | 2018 | < 100 participants | da Rocha EP, Yokota LG, Sampaio BM, Cardoso Eid KZ, Dias DB, de Freitas FM, et al. Urinary neutrophil gelatinase-associated lipocalin is excellent predictor of acute kidney injury in septic elderly patients. Aging Dis 2018;9:182–91 |
| Daggülli | 2016 | < 100 participants | Daggülli M, Utangaç MM, Dede O, Bodakci MN, Hatipoglu NK, Penbegül N, et al. Potential biomarkers for the early detection of acute kidney injury after percutaneous nephrolithotripsy. Ren Fail 2016;38:151–6 |
| Dahlén | 2001 | < 100 participants | Dahlén I, Janson C, Björnsson E, Stålenheim G, Peterson CG, Venge P. Changes in inflammatory markers following treatment of acute exacerbations of obstructive pulmonary disease. Respir Med 2001;95:891–7 |
| Dai | 2015 | Not a relevant biomarker assay or test | Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care 2015;19:223 |
| Dai | 2016 | < 100 participants | Dai X, Li T, Zeng Z, Fu C, Wang S, Cai Y, Chen Z. The effect of continuous venovenous hemofiltration on neutrophil gelatinase-associated lipocalin plasma levels in patients with septic acute kidney injury. BMC Nephrol 2016;17:154 |
| Damman | 2017 | Not a relevant biomarker assay or test | Damman K, Valente MAE, van Veldhuisen DJ, Cleland JGF, O’Connor CM, Metra M, et al. Plasma neutrophil gelatinase-associated lipocalin and predicting clinically relevant worsening renal function in acute heart failure. Int J Mol Sci 2017;18:1470 |
| Daniels | 2012 | Not a relevant type of population | Daniels LB, Barrett-Connor E, Clopton P, Laughlin GA, Ix JH, Maisel AS. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: the Rancho Bernardo study. J Am Coll Cardiol 2012;59:1101–9 |
| Daniels | 2012 | Not a primary study | Daniels RC, Bunchman TE. Is it the neutrophil gelatinase-associated lipocalin or the pediatricRIFLE? Pediatr Crit Care Med 2012;13:698 |
| Dankova | 2016 | < 100 participants | Dankova M, Pazmanova T, Hricak V, Gergel J, Svobodova V, Zitny B, et al. Urinary NGAL as a predictor of acute kidney injury in patients with acute heart failure. Cardiol Lett 2016;25:9–15 |
| Dardashti | 2014 | < 100 participants | Dardashti A, Ederoth P, Algotsson L, Bronden B, Grins E, Larsson M, et al. Erythropoietin and protection of renal function in cardiac surgery (the EPRICS trial). Anesthesiology 2014;121:582–90 |
| Darmon | 2011 | Not a primary study | Darmon M, Gonzalez F, Vincent F. Limits of neutrophil gelatinase-associated lipocalin at intensive care unit admission for prediction of acute kidney injury. Am J Respir Crit Care Med 2011;184:142–3 |
| Darmon | 2017 | Not a primary study | Darmon M, Ostermann M, Joannidis M. Predictions are difficult . . . especially about AKI. Intensive Care Med 2017;43:932–4 |
| Datzmann | 2018 | < 100 participants | Datzmann T, Hoenicka M, Reinelt H, Liebold A, Gorki H. Influence of 6% hydroxyethyl starch 130/0.4 versus crystalloid solution on structural renal damage markers after coronary artery bypass grafting: a post hoc subgroup analysis of a prospective trial. J Cardiothorac Vasc Anesth 2018;32:205–11 |
| Daubin | 2017 | No focus on DTA for AKI | Daubin D, Cristol JP, Dupuy AM, Kuster N, Besnard N, Platon L, et al. Urinary Biomarkers IGFBP7 and TIMP-2 for the diagnostic assessment of transient and persistent acute kidney injury in critically ill patients. PLOS ONE 2017;12:e0169674 |
| De Berardinis | 2015 | Not a relevant biomarker assay or test | De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, Femia A, et al. Comparison between admission natriuretic peptides, NGAL and sST2 testing for the prediction of worsening renal function in patients with acutely decompensated heart failure. Clin Chem Lab Med 2015;53:613–21 |
| de Geus | 2010 | Not a primary study | de Geus HR, Betjes MG, Bakker J. Neutrophil gelatinase-associated lipocalin clearance during veno-venous continuous renal replacement therapy in critically ill patients. Intensive Care Med 2010;36:2156–7 |
| de Geus | 2011 | Not a relevant biomarker assay or test | de Geus HR, Bakker J, Lesaffre EM, le Noble JL. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011;183:907–14 |
| de Geus | 2011 | Not a relevant biomarker assay or test | de Geus HR, Woo JG, Wang Y, Devarajan P, Betjes MG, le Noble JL, Bakker J. Urinary neutrophil gelatinase-associated lipocalin measured on admission to the intensive care unit accurately discriminates between sustained and transient acute kidney injury in adult critically ill patients. Nephron Extra 2011;1:9–2 |
| de Geus | 2013 | Not a relevant biomarker assay or test | de Geus HR, Fortrie G, Betjes MG, van Schaik RH, Groeneveld AB. Time of injury affects urinary biomarker predictive values for acute kidney injury in critically ill, non-septic patients. BMC Nephrol 2013;14:273 |
| de Geus | 2013 | Not a relevant biomarker assay or test | de Geus HR, Betjes MG, Schaick Rv, Groeneveld JA. Plasma NGAL similarly predicts acute kidney injury in sepsis and nonsepsis. Biomark Med 2013;7:415–21 |
| de Geus | 2017 | Not a primary study | de Geus HR, Haase M, Jacob L. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin score for postoperative acute kidney injury: does subclinical acute kidney injury matter? J Thorac Cardiovasc Surg 2017;154:939–40 |
| de Grooth | 2018 | Not a primary study | de Grooth HJ, Parienti JJ, Schetz M. AKI biomarkers are poor discriminants for subsequent need for renal replacement therapy, but do not disqualify them yet. Intensive Care Med 2018;44:1156–8 |
| De Loor | 2016 | Pilot study or preliminary analysis only | De Loor J, Decruyenaere J, Demeyere K, Nuytinck L, Hoste EA, Meyer E. Urinary chitinase 3-like protein 1 for early diagnosis of acute kidney injury: a prospective cohort study in adult critically ill patients. Crit Care 2016;20:38 |
| Dede | 2015 | < 100 participants | Dede O, Dağguli M, Utanğaç M, Yuksel H, Bodakcı MN, Hatipoğlu NK, et al. Urinary expression of acute kidney injury biomarkers in patients after RIRS: it is a prospective, controlled study. Int J Clin Exp Med 2015;8:8147–52 |
| Dedeoglu | 2013 | < 100 participants | Dedeoglu B, de Geus HR, Fortrie G, Betjes MG. Novel biomarkers for the prediction of acute kidney injury in patients undergoing liver transplantation. Biomark Med 2013;7:947–57 |
| Deger | 2020 | < 100 participants | Deger SM, Erten Y, Suyani E, Aki SZ, Ulusal Okyay G, Pasaoglu OT, et al. Early diagnostic markers for detection of acute kidney injury in allogeneic hematopoietic stem cell transplant recipients. Exp Clin Transplant 2020;18:98–105 |
| Deininger | 2016 | No relevant outcome | Deininger S, Hoenicka M, Müller-Eising K, Rupp P, Liebold A, Koenig W, Gorki H. Renal function and urinary biomarkers in cardiac bypass surgery: a prospective randomized trial comparing three surgical techniques. Thorac Cardiovasc Surg 2016;64:561–8 |
| Dekker | 2019 | No focus on DTA for AKI | Dekker SEI, Ruhaak LR, Romijn FPHTM, Meijer E, Cobbaert CM, de Fijter JW, Soonawala D. Urinary tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7 do not correlate with disease severity in ADPKD patients. Kidney Int Rep 2019;4:833–41 |
| Delcroix | 2013 | < 100 participants | Delcroix G, Gillain N, Moonen M, Radermacher L, Damas F, Minon JM, Fraipont V. NGAL Usefulness in the intensive care unit three hours after cardiac surgery. ISRN Nephrol 2013;2013:865164 |
| Delfino Duarte | 2015 | < 100 participants | Delfino Duarte PA, Fumagalli AC, Wandeur V, Becker D. Urinary neutrophil gelatinase-associated lipocalin in critically ill surgical cancer patients. Indian J Crit Care Med 2015;19:251–6 |
| Demirtas | 2013 | < 100 participants | Demirtas S, Caliskan A, Karahan O, Yavuz C, Guclu O, Cayir MC, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting. Exp Clin Cardiol 2013;18:107–9 |
| Dent | 2007 | Not a relevant biomarker assay or test | Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 2007;11:R127 |
| Dépret | 2018 | < 100 participants | Dépret F, Boutin L, Jarkovský J, Chaussard M, Soussi S, Bataille A, et al. Prediction of major adverse kidney events in critically ill burn patients. Burns 2018;44:1887–94 |
| Derhaschnig | 2014 | < 100 participants | Derhaschnig U, Testori C, Riedmueller E, Hobl EL, Mayr FB, Jilma B. Decreased renal function in hypertensive emergencies. J Hum Hypertens 2014;28:427–31 |
| Devarajan | 2008 | Meta-analysis – retained as background material | Devarajan P. Emerging urinary biomarkers in the diagnosis of acute kidney injury. Expert Opin Med Diagn 2008;2:387–98 |
| Devarajan | 2008 | Not a primary study | Devarajan P. Neutrophil gelatinase-associated lipocalin – an emerging troponin for kidney injury. Nephrol Dial Transplant 2008;23:3737–43 |
| Devarajan | 2009 | Not a primary study | Devarajan P. (2009) Neutrophil Gelatinase-associated Lipocalin: An Emerging Biomarker for Angina Renalis. In Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer; 2009. pp. 620–6 |
| Devarajan | 2014 | Not a primary study | Devarajan P. NGAL for the detection of acute kidney injury in the emergency room. Biomark Med 2014;8:217–19 |
| Dewey | 2013 | Not a primary study | Dewey M, Schonenberger E. Increase in creatinine for the prediction of contrast-induced nephropathy. Radiology 2013;269:623–4 |
| Dewitte | 2015 | < 100 participants | Dewitte A, Joannes-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P, et al. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol 2015;10:1900–10 |
| Di Nardo | 2013 | < 100 participants | Di Nardo M, Ficarella A, Ricci Z, Luciano R, Stoppa F, Picardo S, et al. Impact of severe sepsis on serum and urinary biomarkers of acute kidney injury in critically ill children: an observational study. Blood Purif 2013;35:172–6 |
| Díaz de León-Martínez | 2019 | < 100 participants | Díaz de León-Martínez L, Díaz-Barriga F, Barbier O, Ortíz DLG, Ortega-Romero M, Pérez-Vázquez F, Flores-Ramírez R. Evaluation of emerging biomarkers of renal damage and exposure to aflatoxin-B1 in Mexican indigenous women: a pilot study. Environ Sci Pollut Res Int 2019;26:12205–216 |
| Doi | 2013 | Not a relevant biomarker assay or test | Doi K, Urata M, Katagiri D, Inamori M, Murata S, Hisagi M, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013;17:R270 |
| Donadio | 2014 | Not a relevant biomarker assay or test | Donadio C. Effect of glomerular filtration rate impairment on diagnostic performance of neutrophil gelatinase-associated lipocalin and B-type natriuretic peptide as markers of acute cardiac and renal failure in chronic kidney disease patients. Crit Care 2014;18:R39 |
| Downes | 2017 | < 100 participants | Downes KJ, Dong M, Fukuda T, Clancy JP, Haffner C, Bennett MR, et al. Urinary kidney injury biomarkers and tobramycin clearance among children and young adults with cystic fibrosis: a population pharmacokinetic analysis. J Antimicrob Chemother 2017;72:254–60 |
| Du | 2011 | Not a relevant biomarker assay or test | Du Y, Zappitelli M, Mian A, Bennett M, Ma Q, Devarajan P, et al. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol 2011;26:267–74 |
| Du | 2014 | < 100 participants | Du Y, Hou L, Guo J, Sun T, Wang X, Wu Y. Renal neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 expression in children with acute kidney injury and Henoch–Schönlein purpura nephritis. Exp Ther Med 2014;7:1130–4 |
| Du | 2017 | < 100 participants | Du W, Shen T, Li H, Liu Y, He L, Tan L, Hu M. Urinary NGAL for the diagnosis of the renal injury from multiple myeloma. Cancer Biomark 2017;18:41–6 |
| Dubin | 2018 | Not a relevant type of population | Dubin RF, Judd S, Scherzer R, Shlipak M, Warnock DG, Cushman M, et al. Urinary tubular injury biomarkers are associated with ESRD and death in the REGARDS study. Kidney Int Rep 2018;3:1183–92 |
| Dusse | 2016 | < 100 participants | Dusse F, Edayadiyil-Dudásova M, Thielmann M, Wendt D, Kahlert P, Demircioglu E, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol 2016;16:76 |
| Dwipa | 2012 | < 100 participants | Dwipa L, Soelaeman R, Roesli RM, Martanto E, Adhiarta IG. Cardiometabolic risk factors and acute kidney injury based on urinary neutrophil gelatinase associated lipocalin (NGALu) in acute coronary syndrome patients. Acta Med Indones 2012;44:3–9 |
| Egal | 2016 | Not a relevant biomarker assay or test | Egal M, de Geus HR, Groeneveld AB. Neutrophil gelatinase-associated lipocalin as a diagnostic marker for acute kidney injury in oliguric critically ill patients: a post-hoc analysis. Nephron 2016;134:81–8 |
| Eilenberg | 2016 | < 100 participants | Eilenberg W, Stojkovic S, Piechota-Polanczyk A, Kaun C, Rauscher S, Gröger M, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg 2016;51:623–31 |
| Eirin | 2012 | < 100 participants | Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, et al. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 2012;27:4153–61 |
| Eisenhart | 2010 | Not a relevant type of population | Eisenhart E, Benson S, Lacombe P, Himmelfarb J, Zimmerman R, Schimelman B, Parker MG. Safety of low volume iodinated contrast administration for arteriovenous fistula intervention in chronic kidney disease stage 4 or 5 utilizing a bicarbonate prophylaxis strategy. Semin Dial 2010;23:638–42 |
| Ejaz | 2015 | < 100 participants | Ejaz AA, Alquadan KF, Dass B, Shimada M, Kanbay M, Johnson RJ. Effects of serum uric acid on estimated GFR in cardiac surgery patients: a pilot study. Am J Nephrol 2015;42:402–9 |
| Raggal | 2013 | < 100 participants | Raggal NE, Khafagy SM, Mahmoud NH, Beltagy SE. Serum neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury in asphyxiated neonates. Indian Pediatr 2013;50:459–62 |
| El Shahawy | 2018 | < 100 participants | El Shahawy MS, Hemida MH, Abdel-Hafez HA, El-Baz TZ, Lotfy AWM, Emran TM. Urinary neutrophil gelatinase-associated lipocalin as a marker for disease activity in lupus nephritis. Scand J Clin Lab Invest 2018;78:264–8 |
| El-Akabawy | 2017 | < 100 participants | El-Akabawy H, Shafee M, Roshdy AM, Abd Al Salam A. Urinary neutrophil gelatinase associated lipocalin as an early marker of acute kidney injury in the recipient after liver transplantation. Egypt J Crit Care Med 2017;5:49–55 |
| El-Farghali | 2012 | < 100 participants | El-Farghali OG, El-Raggal NM, Mahmoud NH, Zaina GA. Serum neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury in critically-ill neonates. Pak J Biol Sci 2012;15:231–7 |
| Elia | 2015 | < 100 participants | Elia C, Graupera I, Barreto R, Solà E, Moreira R, Huelin P, et al. Severe acute kidney injury associated with non-steroidal anti-inflammatory drugs in cirrhosis: a case-control study. J Hepatol 2015;63:593–600 |
| Elmas | 2017 | < 100 participants | Elmas AT, Karadag A, Tabel Y, Ozdemir R, Otlu G. Analysis of urine biomarkers for early determination of acute kidney injury in non-septic and non-asphyxiated critically ill preterm neonates. J Matern Fetal Neonatal Med 2017;30:302–8 |
| Elmedany | 2017 | < 100 participants | Elmedany SM, Naga SS, Elsharkawy R, Mahrous RS, Elnaggar AI. Novel urinary biomarkers and the early detection of acute kidney injury after open cardiac surgeries. J Crit Care 2017;40:171–7 |
| Elmer | 2016 | < 100 participants | Elmer J, Jeong K, Abebe KZ, Guyette FX, Murugan R, Callaway CW, Rittenberger JC, Pittsburgh post-cardiac arrest service. serum neutrophil gelatinase-associated lipocalin predicts survival after resuscitation from cardiac arrest. Crit Care Med 2016;44:111–19 |
| Elsharawy | 2016 | < 100 participants | Elsharawy S, Raslan L, Morsy S, Hassan B, Khalifa N. Plasma neutrophil gelatinase-associated lipocalin as a marker for the prediction of worsening renal function in children hospitalized for acute heart failure. Saudi J Kidney Dis Transpl 2016;27:49–54 |
| Emlet | 2017 | Not a relevant type of population | Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 2017;312:F284–F296 |
| Endre | 2011 | Not a relevant biomarker assay or test | Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int 2011;79:1119–30 |
| Endre | 2014 | No focus on DTA for AKI | Endre ZH. Novel biomarkers of acute kidney injury: time for implementation? Biomark Med 2014;8:1185–8 |
| Endre | 2014 | Not a primary study | Endre ZH, and Pickering JW. Acute kidney injury: late-onset acute kidney injury-subacute or more of the same? Nat Rev Nephrol 2014;10:133–4 |
| Endre | 2014 | Not a primary study | Endre ZH, Pickering JW. Acute kidney injury: cell cycle arrest biomarkers win race for AKI diagnosis. Nat Rev Nephrol 2014;10:683–5 |
| Erturk | 2015 | < 100 participants | Erturk A, Cure E, Parlak E, Cure MC, Sahin SB, Yuce S. Clinical significance of neutrophil gelatinase-associated lipocalin in Crimean-Congo hemorrhagic fever. Biomed Res Int 2015;2015:374010 |
| Espinosa-Sevilla | 2013 | Not a primary study | Espinosa-Sevilla A, Amezcua-Macias AI, Ruiz-Palacios PC, Rodriguez-Weber F, Diaz-Greene E. New markers of acute kidney injury in critically ill patients. Med Interna Mex 2013;29:513–17 |
| Essajee | 2015 | Not a relevant biomarker assay or test | Essajee F, Were F, Admani B. Urine neutrophil gelatinase-associated lipocalin in asphyxiated neonates: a prospective cohort study. Pediatr Nephrol 2015;30:1189–96 |
| Essajee | 2015 | Not a relevant type of population | Essajee F, Were F, Admani B. Urine neutrophil gelatinase-associated lipocalin in asphyxiated neonates: a prospective cohort study. Pediatr Nephrol 2015;30:1189–96 |
| Cho | 2013 | Duplicate of a study that had already been assessed | Cho E, Yang HN, Jo SK, Cho WY, Kim HK. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J Korean Med Sci 2013;28:100–5 |
| Ezenwaka | 2016 | Not a relevant biomarker assay or test | Ezenwaka CE, Idris S, Davis G, Roberts L. Measurement of neutrophil gelatinase-associated lipocalin (NGAL) in patients with non-communicable diseases: any additional benefit? Arch Physiol Biochem 2016;122:70–4 |
| Fadel | 2012 | < 100 participants | Fadel FI, Abdel Rahman AM, Mohamed MF, Habib SA, Ibrahim MH, Sleem ZS, et al. Plasma neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury after cardio-pulmonary bypass in pediatric cardiac surgery. Arch Med Sci 2012;8:250–5 |
| Fagundes | 2012 | No focus on DTA for AKI | C. Fagundes, M. N. Pepin, M. Guevara, R. Barreto, G. Casals, E. Sola, G. Pereira, E. Rodriguez, E. Garcia, V. Prado, E. Poch, W. Jimenez, J. Fernandez, V. Arroyo and P. Gines. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 2012;57:267–73 |
| Fan | 2018 | Not a relevant biomarker assay or test | Fan H, Zhao Y, Sun M, Zhu JH. Urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, N-acetyl-beta-D-glucosaminidase levels and mortality risk in septic patients with acute kidney injury. Arch Med Sci 2018;14:1381–6 |
| Fan | 2014 | Not a relevant biomarker assay or test | Fan H, Zhao Y, Zhu JH, Song FC. Urine neutrophil gelatinase-associated lipocalin in septic patients with and without acute kidney injury. Renal Fail 2014;36:1399–1403 |
| Fanning | 2016 | < 100 participants | Fanning N, Galvin S, Parke R, Gilroy J, Bellomo R, McGuinness S. A prospective study of the timing and accuracy of neutrophil gelatinase-associated lipocalin levels in predicting acute kidney injury in high-risk cardiac surgery patients. J Cardiothorac Vasc Anesth 2016;30:76–81 |
| Fathimah | 2012 | < 100 participants | Fathimah M, Alicezah MK, Thevarajah M. Neutrophil gelatinase-associated lipocalin (NGAL): an early marker for diabetic nephropathy. Int J Diabetes Dev Ctries 2012;32:19–24 |
| Feldkamp | 2011 | Not a primary study | Feldkamp T, Bienholz A, Kribben A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) for the detection of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant 2011;26:1456–8 |
| Feng | 2016 | No relevant outcome | Feng YG, Liang B, Liu J, Jiang MD, Liu HJ, Huang YQ, Xiao L. Correlation study of podocyte injury and kidney function in patients with acute kidney injury. J Acute Dis 2016;5:493–6 |
| Ferguson | 2010 | < 100 participants | Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int 2010;77:708–14 |
| Fernandes | 2014 | < 100 participants | Fernandes A, Ettinger J, Amaral F, Ramalho MJ, Alves R, Modolo NS. General anesthesia type does not influence serum levels of neutrophil gelatinase-associated lipocalin during the perioperative period in video laparoscopic bariatric surgery. Clinics 2014;69:655–9 |
| Filho | 2017 | Systematic review – retained as background material | Filho LT, Grande AJ, Colonetti T, Della ÉSP, da Rosa MI. Accuracy of neutrophil gelatinase-associated lipocalin for acute kidney injury diagnosis in children: systematic review and meta-analysis. Pediatr Nephrol 2017;32:1979–88 |
| Filiopoulos | 2013 | Not a relevant type of population | Filiopoulos V, Biblaki D, Lazarou D, Chrisis D, Fatourou M, Lafoyianni S, Vlassopoulos D. Plasma neutrophil gelatinase-associated lipocalin (NGAL) as an early predictive marker of contrast-induced nephropathy in hospitalized patients undergoing computed tomography. Clin Kidney J 2013;6:578–83 |
| Filiopoulos | 2014 | No focus on DTA for AKI | Filiopoulos V, Biblaki D, Vlassopoulos D. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker of contrast-induced nephropathy after computed tomography. Ren Fail 2014;36:979–86 |
| Finge | 2017 | < 100 participants | Finge T, Bertran S, Roger C, Candela D, Pereira B, Scott C, et al. Interest of urinary [TIMP-2] × [IGFBP-7] for predicting the occurrence of acute kidney injury after cardiac surgery: a gray zone approach. Anesth Analg 2017;125:762–9 |
| Fiorentino | 2019 | < 100 participants | Fiorentino M, Tohme FA, Murugan R, Kellum JA. Plasma biomarkers in predicting renal recovery from acute kidney injury in critically ill patients. Blood Purif 2019;48:253–61 |
| Flechet | 2017 | Not a relevant biomarker assay or test | Flechet M, Güiza F, Schetz M, Wouters P, Vanhorebeek I, Derese I, et al. AKI predictor, an online prognostic calculator for acute kidney injury in adult critically ill patients: development, validation and comparison to serum neutrophil gelatinase-associated lipocalin. Intensive Care Med 2017;43:764–73 |
| Foroughi | 2014 | No focus on DTA for AKI | Foroughi M, Argani H, Hassntash SA, Hekmat M, Majidi M, Beheshti M, et al. Lack of renal protection of ultrafiltration during cardiac surgery: a randomized clinical trial. J Cardiovasc Surg 2014;55:407–13 |
| Forster | 2019 | < 100 participants | Forster CS, Goldstein S, Pohl H, Jackson E. Association between urodynamic parameters and urine neutrophil gelatinase-associated lipocalin concentrations in children with neuropathic bladders. J Pediatr Urol 2019;15:155 |
| Fortova | 2011 | < 100 participants | Fortova M, Lejsek J, Pechova M, Prusa R. Examination of urine neutrophil gelatinase-associated lipocalin following cardiac surgery in adults. Aktual v Nefrol 2011;17:136–141 |
| Fouad | 2019 | < 100 participants | Fouad TR, Abdelsameea E, Elsabaawy M, Ashraf Eljaky M, Zaki El-shenawy S, Omar N. Urinary neutrophil gelatinase-associated lipocalin for diagnosis of spontaneous bacterial peritonitis. Trop Doct 2019;49:189–92 |
| Fouda | 2013 | < 100 participants | Fouda M, Sherif HM, Shehata M, Ibrahim A. Early expression of urinary neutrophil gelatinase-associated lipocalin biomarker predicts acute kidney injury complicating circulatory shock. Egypt J Crit Care Med 2013;1:79–86 |
| Fox | 2018 | Not a relevant type of population | Fox E, Levin K, Zhu Y, Segers B, Balamuth N, Womer R, et al. Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin-related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist 2018;23:762–e79 |
| Francoz | 2014 | Not a primary study | Francoz C, Durand F. Type-1 hepatorenal syndrome in patients with cirrhosis and infection vs. sepsis-induced acute kidney injury: what matters? J Hepatol 2014;60:907–9 |
| Friedrich | 2017 | < 100 participants | Friedrich MG, Bougioukas I, Kolle J, Bireta C, Jebran FA, Placzek M, Tirilomis T. NGAL expression during cardiopulmonary bypass does not predict severity of postoperative acute kidney injury. BMC Nephrol 2017;18:1–7 |
| Fuernau | 2015 | Not a relevant biomarker assay or test | Fuernau G, Poenisch C, Eitel I, Denks D, de Waha S, Pöss J, et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP-SHOCK II-trial. Int J Cardiol 2015;191:159–66 |
| Gaipov | 2015 | < 100 participants | Gaipov A, Solak Y, Turkmen K, Toker A, Baysal AN, Cicekler H, et al. Serum uric acid may predict development of progressive acute kidney injury after open heart surgery. Ren Fail 2015;37:96–102 |
| Gallagher | 2015 | < 100 participants | Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, et al. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int 2015;87:473–81 |
| Gan | 2018 | Not a primary study | Gan J, Zhou X. Comparison of urine neutrophil gelatinase-associated lipocalin and interleukin-18 in prediction of acute kidney injury in adults. Medicine 2018;97:e12570 |
| Garg | 2017 | Not a primary study | Garg N, Gupta R. Can serum neutrophil gelatinase-associated lipocalin be precisely used as a diagnostic marker of sepsis in pediatric cases? Pediatr Crit Care Med 2017;18:1191–2 |
| Gaspari | 2010 | Not a relevant type of population | Gaspari F, Cravedi P, Mandalà M, Perico N, de Leon FR, Stucchi N, et al. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract 2010;115:c154–60 |
| Gerbes | 2011 | Not a primary study | Gerbes AL, Benesic A, Vogeser M, Krag A, Bendtsen F, Møller S. Serum neutrophil gelatinase-associated lipocalin – a sensitive novel marker of renal impairment in liver cirrhosis? Digestion 2011;84:82–3 |
| Ghonemy | 2014 | < 100 participants | Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl 2014;25:582–8 |
| Gil | 2009 | Not a relevant type of population | Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol 2009;47:870–5 |
| Gilquin | 2017 | Not a relevant type of population | Gilquin B, Louwagie M, Jaquinod M, Cez A, Picard G, El Kholy L, et al. Multiplex and accurate quantification of acute kidney injury biomarker candidates in urine using Protein Standard Absolute Quantification (PSAQ) and targeted proteomics. Talanta 2017;164:77–84 |
| Gist | 2017 | < 100 participants | Gist KM, Goldstein SL, Wrona J, Alten JA, Basu RK, Cooper DS, et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol 2017;32:1611–19 |
| Glassford | 2013 | Not a relevant type of population | Glassford NJ, Schneider AG, Xu S, Eastwood GM, Young H, Peck L, et al. The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med 2013;39:1714–24 |
| Gocze | 2015 | Not a relevant type of population | Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLOS ONE 2015;10:e0120863 |
| Göcze | 2017 | No focus on DTA for AKI | Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery. Ann Surg 2017;267:1013–20 |
| Goknar | 2015 | No focus on DTA for AKI | Goknar N, Oktem F, Ozgen IT, Torun E, Kuçukkoc M, Demir AD, Cesur Y. Determination of early urinary renal injury markers in obese children. Pediatr Nephrol 2015;30:139–44 |
| Goksuluk | 2019 | Not a relevant type of population | Goksuluk H, Esenboga K, Kerimli N, Atmaca Y. The effect of renin–angiotensin system blocking agents on the risk of contrast-induced nephropathy and early detection with neutrophil gelatinase-associated lipocalin in diabetic patients undergoing coronary procedures. Acta Medica Mediterr 2019;35:187–92 |
| Goldstein | 2018 | < 100 participants | Goldstein BH, Goldstein SL, Devarajan P, Zafar F, Kwiatkowski DM, Marino BS, et al. First-stage palliation strategy for univentricular heart disease may impact risk for acute kidney injury. Cardiol Young 2018;28:93–100 |
| Gomaa | 2019 | < 100 participants | Gomaa SH, Shamseya MM, Madkour MA. Clinical utility of urinary neutrophil gelatinase-associated lipocalin and serum cystatin C in a cohort of liver cirrhosis patients with renal dysfunction: a challenge in the diagnosis of hepatorenal syndrome. Eur J Gastroenterol Hepatol 2019;31:692–702 |
| Gombert | 2018 | < 100 participants | Gombert A, Prior I, Martin L, Grommes J, Barbati ME, Foldenauer AC, et al. Urine neutrophil gelatinase-associated lipocalin predicts outcome and renal failure in open and endovascular thoracic abdominal aortic aneurysm surgery. Sci Rep 2018;8:12676 |
| Gombert | 2019 | < 100 participants | Gombert A, Martin L, Foldenauer AC, Krajewski C, Greiner A, Kotelis D, et al. Comparison of urine and serum neutrophil gelatinase-associated lipocalin after open and endovascular thoraco-abdominal aortic surgery and their meaning as indicators of acute kidney injury. Vasa 2019;48:79–87 |
| Gong | 2015 | Non-English-language publication | Gong M, Yang Y, Zhang S. [Value of acute renal injury associated biomarkers for patients in intensive care unit.] Zhong Nan Da Xue Xue Bao Yi Xue Ban 2015;40:1083–8 |
| Gordillo | 2016 | < 100 participants | Gordillo R, Ahluwalia T, Woroniecki R. Hyperglycemia and acute kidney injury in critically ill children. Int J Nephrol Renov Dis 2016;9:201–4 |
| Greenberg | 2018 | No relevant outcome | Greenberg JH, Zappitelli M, Jia Y, Thiessen-Philbrook HR, De Fontnouvelle CA, Wilson FP, et al. Biomarkers of AKI progression after pediatric cardiac surgery. J Am Soc Nephrol 2018;29:1549–56 |
| Grosman-Rimon | 2019 | < 100 participants | Grosman-Rimon L, Hui SG, Freedman D, Elbaz-Greener G, Cherney D, Rao V. Biomarkers of inflammation, fibrosis, and acute kidney injury in patients with heart failure with and without left ventricular assist device implantation. Cardiorenal Med 2019;9:108–16 |
| Gubhaju | 2014 | Not a relevant type of population | Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol 2014;307:F149–58 |
| Guerci | 2018 | < 100 participants | Guerci P, Claudot JL, Novy E, Settembre N, Lalot JM, Losser MR. Immediate postoperative plasma neutrophil gelatinase-associated lipocalin to predict acute kidney injury after major open abdominal aortic surgery: a prospective observational study. Anaesth Crit Care Pain Med 2018;37:327–34 |
| Guerrero-Orriach | 2016 | < 100 participants | Guerrero-Orriach JL, Ariza-Villanueva D, Florez-Vela A, Garrido-Sánchez L, Moreno-Cortés MI, Galán-Ortega M, et al. Cardiac, renal, and neurological benefits of preoperative levosimendan administration in patients with right ventricular dysfunction and pulmonary hypertension undergoing cardiac surgery: evaluation with two biomarkers neutrophil gelatinase-associated lipocalin and neuronal enolase. Ther Clin Risk Manag 2016;12:623–30 |
| Güneş | 2016 | < 100 participants | Güneş A, Ece A, Akça H, Aktar F, Mete Ş, Samanci S, et al. Urinary kidney injury molecules in children with febrile seizures. Ren Fail 2016;38:1377–82 |
| Gungor | 2014 | < 100 participants | Gungor G, Ataseven H, Demir A, Solak Y, Gaipov A, Biyik M, et al. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int 2014;34:49–57 |
| Gunnerson | 2016 | No relevant outcome. Subgroup analysis of already included study | Gunnerson KJ, Shaw AD, Chawla LS, Bihorac A, Al-Khafaji A, Kashani K, et al. TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg 2016;80:243–9 |
| Haase | 2009 | Meta-analysis – retained as background material | Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012–24 |
| Haase | 2011 | Not a primary study | Haase M, Bellomo R, Haase-Fielitz A. Neutrophil gelatinase-associated lipocalin: a superior biomarker for detection of subclinical acute kidney injury and poor prognosis. Biomark Med 2011;5:415–17 |
| Haase | 2009 | Not a relevant biomarker assay or test | Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 2009;88:124–30 |
| Haase-Fielitz | 2009 | Not a relevant biomarker assay or test | Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery – a prospective cohort study. Crit Care Med 2009;37:553–60 |
| Haase-Fielitz | 2009 | Not a relevant biomarker assay or test | Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 2009;24:3349–54 |
| Haase-Fielitz | 2014 | Systematic review – retained as background material | Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem 2014;51:335–51 |
| Hahn | 2017 | Not a primary study | Hahn RG, Zdolsek J. Nephrocheck results should be corrected for dilution. Acta Anaesthesiol Scand 2017;61:261–2 |
| Hall | 2012 | Not a relevant type of population | Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR. Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 2012;7:1224–33 |
| Hall | 2018 | Systematic review – retained as background material | Hall PS, Mitchell ED, Smith AF, Cairns DA, Messenger M, Hutchinson M, et al. The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation. Health Technol Assess 2018;22(32) |
| Hamdy | 2018 | < 100 participants | Hamdy HS, El-Ray A, Salaheldin M, Lasheen M, Aboul-Ezz M, Abdel-Moaty AS, Abdel-Rahim A. Urinary neutrophil gelatinase-associated lipocalin in cirrhotic patients with acute kidney injury. Ann Hepatol 2018;17:624–30 |
| Hamishehkar | 2017 | Not a relevant type of population | Hamishehkar H, Sanaie S, Fattahi V, Mesgari M, Mahmoodpoor A. The effect of furosemide on the level of neutrophil gelatinase-associated lipocalin in critically hospitalized patients with acute kidney injury. Indian J Crit Care Med 2017;21:442–7 |
| Han | 2009 | < 100 participants | Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2009;4:873–82 |
| Hang | 2017 | Not a relevant biomarker assay or test | Hang CC, Yang J, Wang S, Li CS, Tang ZR. Evaluation of serum neutrophil gelatinase-associated lipocalin in predicting acute kidney injury in critically ill patients. J Int Med Res 2017;45:1231–44 |
| Hanna | 2016 | < 100 participants | Hanna M, Brophy PD, Giannone PJ, Joshi MS, Bauer JA, RamachandraRao S. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr Res 2016;80:218–23 |
| Hassan | 2017 | < 100 participants | Hassan RH, Kandil SM, Zeid MS, Zaki ME, Fouda AE. Kidney injury in infants and children with iron-deficiency anemia before and after iron treatment. Hematology 2017;22:565–70 |
| Hayashi | 2017 | < 100 participants | Hayashi H, Sato W, Kosugi T, Nishimura K, Sugiyama D, Asano N, et al. Efficacy of urinary midkine as a biomarker in patients with acute kidney injury. Clin Exp Nephrol 2017;21:597–607 |
| Hazle | 2013 | < 100 participants | Hazle MA, Gajarski RJ, Aiyagari R, Yu S, Abraham A, Donohue J, Blatt NB. Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than 6 months of age. J Thorac Cardiovasc Surg 2013;146:861–7.e1 |
| Heise | 2011 | < 100 participants | Heise D, Rentsch K, Braeuer A, Friedrich M, Quintel M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and alpha1-microglobulin for early detection of acute renal injury after cardiac surgery. Eur J Cardiothorac Surg 2011;39:38–43 |
| Helanova | 2015 | Not a relevant type of population | Helanova K, Littnerova S, Kubena P, Ganovska E, Pavlusova M, Kubkova L, et al. Prognostic impact of neutrophil gelatinase-associated lipocalin and B-type natriuretic in patients with ST-elevation myocardial infarction treated by primary PCI: a prospective observational cohort study. BMJ Open 2015;5:e006872 |
| Herbert | 2015 | No focus on DTA for AKI | Herbert C, Patel M, Nugent A, Dimas VV, Guleserian KJ, Quigley R, Modem V. Serum cystatin C as an early marker of neutrophil gelatinase-associated lipocalin-positive acute kidney injury resulting from cardiopulmonary bypass in infants with congenital heart disease. Congenit Heart Dis 2015;10:E180–8 |
| Heung | 2016 | No relevant outcome. Subgroup analysis of already included study | Heung M, Ortega LM, Chawla LS, Wunderink RG, Self WH, Koyner JL, et al. Common chronic conditions do not affect performance of cell cycle arrest biomarkers for risk stratification of acute kidney injury. Nephrol Dial Transplant 2016;31:1633–40 |
| Heydari | 2017 | < 100 participants | Heydari B, Khalili H, Beigmohammadi MT, Abdollahi A, Karimzadeh I. Effects of atorvastatin on biomarkers of acute kidney injury in amikacin recipients: a pilot, randomized, placebo-controlled, clinical trial. J Res Med Sci 2017;22:39 |
| Hinck | 2018 | < 100 participants | Hinck BD, Miyaoka R, Lingeman JE, Assimos DG, Matlaga BR, Pramanik R, et al. Urine kidney injury markers do not increase following gastric bypass: a multi-center cross-sectional study. Can J Urol 2018;25:9199–204 |
| Hirsch | 2007 | Not a relevant type of population | Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007;22:2089–95 |
| Hjortrup | 2013 | Systematic review – retained as background material | Hjortrup PB, Haase N, Wetterslev M, Perner A. Clinical review: predictive value of neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care patients. Crit Care 2013;17:211 |
| Ho | 2009 | < 100 participants | Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis 2009;53:584–95 |
| Ho | 2015 | Meta-analysis – retained as background material | Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015;66:993–1005 |
| Hodgson | 2019 | < 100 participants | Hodgson LE, Venn RM, Short S, Roderick PJ, Hargreaves D, Selby N, Forni LG. Improving clinical prediction rules in acute kidney injury with the use of biomarkers of cell cycle arrest: a pilot study. Biomarkers 2019;24:23–8 |
| Hoffman | 2013 | < 100 participants | Hoffman SB, Massaro AN, Soler-García AA, Perazzo S, Ray PE. A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: a pilot study. Pediatr Nephrol 2013;28:2179–88 |
| Holderied | 2018 | Not a relevant type of population | Holderied A. IGFBP7/TIMP-2 based prevention of acute kidney injury: does ‘time is nephron’ apply in AKI? Nephrologe 2018;13:192–4 |
| Hollmen | 2011 | Not a primary study | Hollmen M. Diagnostic test for early detection of acute kidney injury. Expert Rev Mol Diagn 2011;11:553–5 |
| Holzscheiter | 2014 | Not a relevant type of population | Holzscheiter L, Beck C, Rutz S, Manuilova E, Domke I, Guder WG, Hofmann W. NGAL, L-FABP, and KIM-1 in comparison to established markers of renal dysfunction. Clin Chem Lab Med 2014;52:537–46 |
| Hong | 2013 | < 100 participants | Hong DY, Lee JH, Park SO, Baek KJ, Lee KR. Plasma neutrophil gelatinase-associated lipocalin as early biomarker for acute kidney injury in burn patients. J Burn Care Res 2013;34:e326–32 |
| Honore | 2016 | No relevant outcome. Subgroup analysis of already included study | Honore PM, Nguyen HB, Gong M, Chawla LS, Bagshaw SM, Artigas A, et al. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med 2016;44:1851–60 |
| Honore | 2016 | Not a primary study | Honore PM, Spapen HD. Neutrophil gelatinase-associated lipocalin elimination by renal replacement therapy: minding the membrane! Crit Care 2016;20:87 |
| Hoskova | 2013 | < 100 participants | Hoskova L, Franekova J, Malek I, Secnik P Jr, Pirk J, Kautzner J, et al. Relationship of cardiorenal biomarkers for prediction of renal dysfunction in patients after heart transplantation. Cor Vasa 2013;55:E364–9 |
| Hoskova | 2016 | No relevant outcome | Hoskova L, Franekova J, Malek I, Kautzner J, Szarszoi O, Jabor A, et al. Comparison of cystatin C and NGAL in early diagnosis of acute kidney injury after heart transplantation. Ann Transplant 2016;21:239–45 |
| Hosohata | 2016 | < 100 participants | Hosohata K, Washino S, Kubo T, Natsui S, Fujisaki A, Kurokawa S, et al. Early prediction of cisplatin-induced nephrotoxicity by urinary vanin-1 in patients with urothelial carcinoma. Toxicology 2016;359-360:71–5 |
| Hoste | 2018 | Not a primary study | Hoste EA, Vandenberghe W. Plasma neutrophil gelatinase-associated lipocalin (NGAL) for timing of initiation of renal replacement therapy for acute kidney injury? J Thorac Dis 2018;10(Suppl. 33):S3989–93 |
| Howell | 2015 | < 100 participants | Howell E, Sen S, Palmieri T, Godwin Z, Bockhold J, Greenhalgh D, Tran NK. Point-of-care B-type natriuretic peptide and neutrophil gelatinase-associated lipocalin measurements for acute resuscitation: a pilot study. J Burn Care Res 2015;36:e26–33 |
| Hryniewiecka | 2014 | No focus on DTA for AKI | Hryniewiecka E, Gala K, Krawczyk M, Pączek L. Is neutrophil gelatinase-associated lipocalin an optimal marker of renal function and injury in liver transplant recipients? Transplant Proc 2014;46:2782–5 |
| Hsiao | 2012 | < 100 participants | Hsiao PG, Hsieh CA, Yeh CF, Wu HH, Shiu TF, Chen YC, Chu PG. Early prediction of acute kidney injury in patients with acute myocardial injury. J Crit Care 2012;27:525 |
| Hsu | 2012 | Not a primary study | Hsu RK, Hsu CY. We can diagnose AKI ‘early’. Clin J Am Soc Nephrol 2012;7:1741–2 |
| Huang | 2016 | < 100 participants | Huang CY, Shih CC, Chung K, Kao KC, Wu HP. Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute renal failure in patients with severe sepsis. J Chin Med Assoc 2016;79:428–34 |
| Huelin | 2019 | Not a relevant type of population | Huelin P, Solà E, Elia C, Solé C, Risso A, Moreira R, et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology 2019;70:319–33 |
| Hui-Miao | 2017 | Meta-analysis – retained as background material | Hui-Miao J, Li-Feng H, Zheng Y, Wen-Xiong L, Jia HM, Huang LF, et al. Diagnostic value of urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 for acute kidney injury: a meta-analysis. Crit Care 2017;21:1–11 |
| Hunsicker | 2017 | < 100 participants | Hunsicker O, Feldheiser A, Weimann A, Liehre D, Sehouli J, Wernecke KD, Spies C. Diagnostic value of plasma NGAL and intraoperative diuresis for AKI after major gynecological surgery in patients treated within an intraoperative goal-directed hemodynamic algorithm. Medicine 2017;96:e7357 |
| Hur | 2014 | Not a relevant biomarker assay or test | Hur M, Kim H, Lee S, Cristofano F, Magrini L, Marino R, et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect Dis 2014;14:224 |
| Hurry | 2017 | < 100 participants | Hurry PK, Poulsen JH, Bendtsen F, Møller S. Neutrophil gelatinase-associated lipocalin and cystatin C in cirrhosis and portal hypertension: Relations to organ extraction and dysfunction. J Gastroenterol Hepatol 2017;32:473–81 |
| Hwang | 2014 | < 100 participants | Hwang YJ, Hyun MC, Choi BS, Chun SY, Cho MH. Acute kidney injury after using contrast during cardiac catheterization in children with heart disease. J Korean Med Sci 2014;29:1102–7 |
| Ibrahim | 2019 | < 100 participants | Ibrahim ME, Chang C, Hu Y, Hogan SL, Mercke N, Gomez M, et al. Pharmacokinetic determinants of cisplatin-induced subclinical kidney injury in oncology patients. Eur J Clin Pharmacol 2019;75:51–7 |
| Iguchi | 2012 | < 100 participants | Iguchi N, Uchiyama A, Hosotsubo K, Fujino Y. Plasma neutrophil gelatinase-associated lipocalin clearance during venovenous hemodiafiltration. Clin Exp Nephrol 2012;16:356–7 |
| Iguchi | 2015 | < 100 participants | Iguchi N, Uchiyama A, Ueta K, Sawa Y, Fujino Y. Neutrophil gelatinase-associated lipocalin and liver-type fatty acid-binding protein as biomarkers for acute kidney injury after organ transplantation. J Anesth 2015;29:249–55 |
| In | 2014 | Not a relevant type of population | In JW, Kim JE, Jeong JS, Song SH, Kim HK. Diagnostic and prognostic significance of neutrophil gelatinase-associated lipocalin in disseminated intravascular coagulation. Clin Chim Acta 2014;430:145–9 |
| Innami | 2014 | < 100 participants | Innami Y, Katori N, Mori K, Kosugi S, Suzuki T, Sakurai N, et al. Increased prothrombotic property as a risk factor of acute kidney injury after surgical repair of abdominal aortic aneurysm: a prospective observational study. J Intensive Care 2014;2:46 |
| Introcaso | 2018 | < 100 participants | Introcaso G, Nafi M, Bonomi A, L’Acqua C, Salvi L, Ceriani R, et al. Improvement of neutrophil gelatinase-associated lipocalin sensitivity and specificity by two plasma measurements in predicting acute kidney injury after cardiac surgery. Biochemia Medica 2018;28:030701 |
| Işıkkent | 2018 | < 100 participants | Işıkkent A, Yılmaz S, Özturan IU, Doğan NO, Yaka E, Gültekin H, et al. Utility of neutrophil celatinase-associated lipocalin in the management of acute kidney injury: a prospective, observational study. Hong Kong J Emerg Med 2018;27:8–14 |
| Isler | 2018 | < 100 participants | Isler Y, Ozdinc S, Kaya H. Can NGAL be used as an early marker of contrast-induced nephropathy in emergency department? Acta Medica Mediterr 2018;34:1889–94 |
| Ismail | 2012 | < 100 participants | Ismail G, Bobeica R, Ioanitescu S, Jurubita R. Association of serum and urinary neutrophil gelatinase-associated lipocalin (NGAL) levels with disease severity in patients with early-stage autosomal dominant polycystic kidney disease. Rev Roman Med Lab 2012;20:109–16 |
| Isshiki | 2016 | No relevant outcome | Isshiki R, Asada T, Sato D, Sumida M, Hamasaki Y, Inokuchi R, et al. Association of urinary neutrophil gelatinase-associated lipocalin with long-term renal outcomes in ICU survivors: a retrospective observational cohort study. Shock 2016;46:44–51 |
| Itenov | 2014 | No focus on DTA for AKI | Itenov TS, Bangert K, Christensen PH, Jensen JU, Bestle MH, Jakobsen ML, et al. Serum and plasma neutrophil gelatinase associated lipocalin (NGAL) levels are not equivalent in patients admitted to intensive care. J Clin Lab Anal 2014;28:163–7 |
| Izadi | 2016 | Systematic review – retained as background material | Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Razaz JM, Ataei N, Hosseini M. Value of plasma/serum neutrophil gelatinase-associated lipocalin in detection of pediatric acute kidney injury; a systematic review and meta-analysis. Int J Pediatr 2016;4:3815–36 |
| Jafari | 2018 | < 100 participants | Jafari M, Ala S, Haddadi K, Alipour A, Mojtahedzadeh M, Ehteshami S, et al. Cotreatment with furosemide and hypertonic saline decreases serum neutrophil gelatinase-associated lipocalin (NGAL) and serum creatinine concentrations in traumatic brain injury: a randomized, single-blind clinical trial. Iran J Pharm Res 2018;17:1130–40 |
| Jahnukainen | 2018 | < 100 participants | Jahnukainen T, Keski-Nisula J, Tainio J, Valkonen H, Patila T, Jalanko H, Suominen P. Efficacy of corticosteroids in prevention of acute kidney injury in neonates undergoing cardiac surgery – a randomized controlled trial. Acta Anaesthesiol Scand 2018;62:1072–9 |
| Jain | 2016 | < 100 participants | Jain V, Mehta Y, Gupta A, Sharma R, Raizada A, Trehan N. The role of neutrophil gelatinase-associated lipocalin in predicting acute kidney injury in patients undergoing off-pump coronary artery bypass graft: a pilot study. Ann Card Anaesth 2016;19:225–30 |
| Jayaraman | 2014 | Not a relevant biomarker assay or test | Jayaraman R, Sunder S, Sathi S, Gupta VK, Sharma N, Kanchi P, et al. Post cardiac surgery acute kidney injury: a woebegone status rejuvenated by the novel biomarkers. Nephrourol Mon 2014;6:e19598 |
| Jelinek | 2018 | Not a relevant type of population | Jelinek MJ, Lee SM, Wyche Okpareke A, Wing C, Koyner JL, Murray PT, et al. Predicting acute renal injury in cancer patients receiving cisplatin using urinary neutrophil gelatinase-associated lipocalin and cystatin C. Clin Transl Sci 2018;11:420–7 |
| Jeong | 2012 | < 100 participants | Jeong TD, Kim S, Lee W, Song GW, Kim YK, Chun S, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of acute kidney injury in liver transplantation. Clin Transplant 2012;26:775–81 |
| Je-Yeob | 2013 | Not a relevant biomarker assay or test | Je-Yeob LEE, Jin-Young KIM, Sang OP, Kyeong-Ryong LEE, Kwang-Je B, Dae-Young H. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury. J Korean Soc Emerg Med 2013;2:157–163 |
| Jia | 2017 | Meta-analysis – retained as background material | Jia HM, Huang LF, Zheng Y, Li WX. Diagnostic value of urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 for acute kidney injury: a meta-analysis. Crit Care 2017;21:77 |
| Jia | 2017 | Meta-analysis – retained as background material | Jia HM, Huang LF, Zheng Y, Li WX. Prognostic value of cell cycle arrest biomarkers in patients at high risk for acute kidney injury: a systematic review and meta-analysis. Nephrology 2017;22:831–7 |
| Jiang | 2015 | Not a relevant type of population | Jiang L, Cui H. Could blood neutrophil gelatinase-associated lipocalin (NGAL) be a diagnostic marker for acute kidney injury in neonates? A systemic review and meta-analysis. Clin Lab 2015;61:1815–20 |
| Jiang | 2018 | No relevant outcome | Jiang QQ, Han MF, Ma K, Chen G, Wan XY, Kilonzo SB, et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol 2018;24:2300–10 |
| Joannes-Boyau | 2012 | Not a primary study | Joannes-Boyau O, Fichet J. NGAL and sepsis. Ann Fr Anesth Reanim 2012;31:8–9 |
| Jobs | 2014 | < 100 participants | Jobs K, Straż-Żebrowska E, Placzyńska M, Zdanowski R, Kalicki B, Lewicki S, Jung A. Interleukin-18 and NGAL in assessment of ESWL treatment safety in children with urolithiasis. Cent Eur J Immunol 2014;39:384–91 |
| Jochmans | 2017 | < 100 participants | Jochmans I, Meurisse N, Neyrinck A, Verhaegen M, Monbaliu D, Pirenne J. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transpl 2017;23:634–44 |
| Journois | 2013 | Not a primary study | Journois D, Jacob L. [NGAL more or less than a biomarker?] Ann Fr Anesth Reanim 2013;3:134–5 |
| Jungbauer | 2011 | No focus on DTA for AKI | Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, Von Bary C, et al. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 2011;13:1104–10 |
| Kaddourah | 2016 | < 100 participants | Kaddourah A, Goldstein SL, Basu R, Nehus EJ, Terrell TC, Brunner L, et al. Novel urinary tubular injury markers reveal an evidence of underlying kidney injury in children with reduced left ventricular systolic function: a pilot study. Pediatr Nephrol 2016;31:1637–45 |
| Kafkas | 2012 | Not a relevant type of population | Kafkas N, Demponeras C, Zoubouloglou F, Spanou L, Babalis D, Makris K. Serum levels of gelatinase associated lipocalin as indicator of the inflammatory status in coronary artery disease. Int J Inflam 2012;2012:189797 |
| Kafkas | 2016 | Not a relevant type of population | Kafkas N, Liakos C, Zoubouloglou F, Dagadaki O, Dragasis S, Makris K. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after elective invasive cardiac procedures. Clin Cardiol 2016;39:464–70 |
| Kahli | 2014 | < 100 participants | Kahli A, Guenancia C, Zeller M, Grosjean S, Stamboul K, Rochette L, et al. Growth differentiation factor-15 (GDF-15) levels are associated with cardiac and renal injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. PLOS ONE 2014;9:e105759 |
| Kališnik | 2017 | < 100 participants | Kališnik JM, Hrovat E, Hrastovec A, Žibert J, Jerin A, Skitek M, et al. Creatinine, neutrophil gelatinase-associated lipocalin, and cystatin C in determining acute kidney injury after heart operations using cardiopulmonary bypass. Artif Organs 2017;41:481–9 |
| Kalisnik | 2017 | Not a primary study | Kalisnik JM, Fischlein T, Santarpino G. Cardiac surgery-associated neutrophil gelatinase-associated lipocalin score for postoperative acute kidney injury: what is the clinical implication? J Thorac Cardiovasc Surg 2017;154:938 |
| Kambhampat | 2013 | Not a relevant biomarker assay or test | Kambhampati G, Ejaz NI, Asmar A, Aiyer RK, Aiyer R, Arif AA, et al. Fluid balance and conventional and novel biomarkers of acute kidney injury in cardiovascular surgery. J Cardiovasc Surg 2013;54:639–46 |
| Kamis | 2016 | Not a relevant biomarker assay or test | Kamis F, Yegenaga I, Musul M, Baydemir C, Bek S, Kalender B, Baykara N. Neutrophil gelatinase-associated lipocalin levels during the first 48 hours of intensive care may indicate upcoming acute kidney injury. J Crit Care 2016;34:89–94 |
| Kanchi | 2017 | < 100 participants | Kanchi M, Manjunath R, Massen J, Vincent L, Belani K. Neutrophil gelatinase-associated lipocalin as a biomarker for predicting acute kidney injury during off-pump coronary artery bypass grafting. Ann Card Anaesth 2017;20:297–302 |
| Kandil | 2017 | < 100 participants | Kandil MA, Abouelenain KM, Alsebaey A, Rashed HS, Afifi MH, Mahmoud MA, Yassen KA. Impact of terlipressin infusion during and after live donor liver transplantation on incidence of acute kidney injury and neutrophil gelatinase-associated lipocalin serum levels: a randomized controlled trial. Clin Transplant 2017;31:e13019 |
| Kandur | 2016 | < 100 participants | Kandur Y, Gonen S, Fidan K, Soylemezoglu O. Evaluation of urinary KIM-1, NGAL, and IL-18 levels in determining early renal injury in pediatric cases with hypercalciuria and/or renal calculi. Clin Nephrol 2016;86:62–9 |
| Karademir | 2016 | < 100 participants | Karademir LD, Dogruel F, Kocyigit I, Yazici C, Unal A, Sipahioglu MH, et al. The efficacy of theophylline in preventing cisplatin-related nephrotoxicity in patients with cancer. Ren Fail 2016;38:806–14 |
| Savran Karadeniz | 2019 | < 100 participants | Savran Karadeniz M, Alp Enişte I, Şentürk Çiftçi H, Usta S, Tefik T, Şanlı Ö, et al. Neutrophil gelatinase-associated lipocalin significantly correlates with ischemic damage in patients undergoing laparoscopic partial nephrectomy Balkan Med J 2019;36:121–8 |
| Karaolanis | 2015 | < 100 participants | Karaolanis G, Katsaros A, Palla VV, Lionaki S, Moris D, Karanikola E, et al. Urine NGAL as a biomarker of kidney damage after on- and off-pump coronary artery bypass graft surgery: a prospective pilot study. Hellenic J Cardiol 2015;56:160–8 |
| Kardakos | 2014 | < 100 participants | Kardakos IS, Volanis DI, Kalikaki A, Tzortzis VP, Serafetinides EN, Melekos MD, Delakas DS. Evaluation of neutrophil gelatinase-associated lipocalin, interleukin-18, and cystatin C as molecular markers before and after unilateral shock wave lithotripsy. Urology 2014;84:783–8 |
| Kari | 2018 | < 100 participants | Kari JA, Shalaby MA, Sofyani K, Sanad AS, Ossra AF, Halabi RS, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) and serum cystatin C measurements for early diagnosis of acute kidney injury in children admitted to PICU. World J Pediatr 2018;14:134–42 |
| Karimzadeh | 2017 | < 100 participants | Karimzadeh I, Heydari M, Ramzi M, Sagheb MM, Zomorodian K. Urinary Neutrophil gelatinase-associated lipocalin as a biomarker of kidney injury in hematologic-oncologic patients receiving amphotericin B. Iran J Kidney Dis 2017;11:201–8 |
| Katagiri | 2012 | < 100 participants | Katagiri D, Doi K, Honda K, Negishi K, Fujita T, Hisagi M, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg 2012;93:577–83 |
| Katagiri | 2013 | Not a relevant biomarker assay or test | Katagiri D, Doi K, Matsubara T, Negishi K, Hamasaki Y, Nakamura K, et al. New biomarker panel of plasma neutrophil gelatinase-associated lipocalin and endotoxin activity assay for detecting sepsis in acute kidney injury. J Crit Care 2013;28:564–70 |
| Katagiri | 2016 | No relevant outcome | Katagiri M, Takahashi M, Doi K, Myojo M, Kiyosue A, Ando J, et al. Serum neutrophil gelatinase-associated lipocalin concentration reflects severity of coronary artery disease in patients without heart failure and chronic kidney disease. Heart Vessels 2016;31:1595–602 |
| Kesik | 2015 | < 100 participants | Kesik V, Demirkaya E, Buyukpamukçu M. Urinary neutrophil gelatinase associated lipocalin as a biomarker in ifosfamide induced chronic renal failure. Eur Rev Med Pharmacol Sci 2015;19:4851–7 |
| Khan | 2014 | No relevant outcome | Khan UA, Coca SG, Hong K, Koyner JL, Garg AX, Passik CS, et al. Blood transfusions are associated with urinary biomarkers of kidney injury in cardiac surgery. J Thorac Cardiovasc Surg 2014;148:726–32 |
| Khan | 2014 | < 100 participants | Khan M, Choudhry N, Haq MFU, Shahjahan, Mahmood S, Sarmad S, Yasmin R. Evaluation of neutrophil gelatinase associated lipocalin, as a biomarker of renal injury in type 2 diabetic patients. Pakistan J Medical Health Sci 2014;8:612–15 |
| Khatami | 2015 | Not a relevant type of population | Khatami MR, Sabbagh MR, Nikravan N, Khazaeipour Z, Boroumand MA, Sadeghian S, Davoudi B. The role of neutrophil-gelatinase-associated lipocalin in early diagnosis of contrast nephropathy. Indian J Nephrol 2015;25:292–6 |
| Khawaja | 2019 | < 100 participants | Khawaja S, Jafri L, Siddiqui I, Hashmi M, Ghani F. The utility of neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury (AKI) in critically ill patients. Biomark Res 2019;7:4 |
| Khosravi | 2013 | < 100 participants | Khosravi MB, Milani S, Kakaei F. Serum Neutrophil gelatinase-associated lipocalin versus serum creatinine for the prediction of acute kidney injury after liver transplantation. Int J Organ Transplant Med 2013;4:102–9 |
| Kidher | 2014 | < 100 participants | Kidher E, Harling L, Ashrafian H, Naase H, Chukwuemeka A, Anderson J, et al. Pulse wave velocity and neutrophil gelatinase-associated lipocalin as predictors of acute kidney injury following aortic valve replacement. J Cardiothorac Surg 2014;9:89 |
| Kift | 2013 | < 100 participants | Kift RL, Messenger MP, Wind TC, Hepburn S, Wilson M, Thompson D, et al. A comparison of the analytical performance of five commercially available assays for neutrophil gelatinase-associated lipocalin using urine. Ann Clin Biochem 2013;50:236–44 |
| Kil | 2018 | < 100 participants | Kil HK, Kim JY, Choi YD, Lee HS, Kim TK, Kim JE. Effect of combined treatment of ketorolac and remote ischemic preconditioning on renal ischemia-reperfusion injury in patients undergoing partial nephrectomy: pilot study. J Clin Med 2018;7:470 |
| Kim | 2011 | < 100 participants | Kim T, Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Klodell CT, et al. Early blood biomarkers predict organ injury and resource utilization following complex cardiac surgery. J Surg Res 2011;168:168–72 |
| Kim | 2013 | Not a relevant biomarker assay or test | Kim H, Hur M, Cruz DN, Moon HW, Yun YM. Plasma neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury in critically ill patients with suspected sepsis. Clin Biochem 2013;46:1414–18 |
| Kim | 2013 | Not a relevant type of population | Kim SM, Park JS, Norwitz ER, Jung HJ, Kim BJ, Park CW, Jun JK. Circulating levels of neutrophil gelatinase-associated lipocalin (NGAL) correlate with the presence and severity of preeclampsia. Reprod Sci 2013;20:1083–9 |
| Kim | 2014 | No focus on DTA for AKI | Kim BH, Yu N, Kim HR, Yun KW, Lim IS, Kim TH, Lee MK. Evaluation of the optimal neutrophil gelatinase-associated lipocalin value as a screening biomarker for urinary tract infections in children. Ann Lab Med 2014;34:354–9 |
| Kim | 2014 | < 100 participants | Kim JD, Chee HK, Shin JK, Kim JS, Lee SA, Kim YH, et al. Novel early predictor of acute kidney injury after open heart surgery under cardiopulmonary bypass using plasma neutrophil gelatinase-associated lipocalin. Korean J Thorac Cardiovasc Surg 2014;47:240–8 |
| Kim | 2016 | Meta-analysis – retained as background material | Kim S, Kim HJ, Ahn HS, Song JY, Um TH, Cho CR, et al. Is plasma neutrophil gelatinase-associated lipocalin a predictive biomarker for acute kidney injury in sepsis patients? A systematic review and meta-analysis. J Crit Care 2016;33:213–23 |
| Kim | 2016 | Not a primary study | Kim JE, Song SW, Kim JY, Lee HJ, Chung KH, Shim YH. Effect of a single bolus of erythropoietin on renoprotection in patients undergoing thoracic aortic surgery with moderate hypothermic circulatory arrest. Ann Thorac Surg 2016;101:690–6 |
| Kim | 2017 | Not a relevant biomarker assay or test | Kim H, Hur M, Lee S, Marino R, Magrini L, Cardelli P, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med 2017;37:388–97 |
| Kim | 2018 | < 100 participants | Kim Y, Cho YS, Kym D, Yoon J, Yim H, Hur J, Chun W. Diagnostic performance of plasma and urine neutrophil gelatinase-associated lipocalin, cystatin C, and creatinine for acute kidney injury in burn patients: a prospective cohort study. PLOS ONE 2018;13:e0199600 |
| Kim | 2019 | Not a primary study | Kim HY, Kim CS, Bae EH, Kim SW, Ma SK. Clinical significance of the interval change of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury and acute kidney injury superimposed on chronic kidney disease. Chonnam Med J 2019;55:68–9 |
| Kipnis | 2016 | Not a primary study | Kipnis E. Predicting acute kidney injury after hip-fracture surgery: join the (renal) resistance! Anaesth Crit Care Pain Med 2016;35:369–70 |
| Kirbiš | 2015 | < 100 participants | Kirbiš S, Gorenjak M, Sinkovič A. The role of urine neutrophil gelatinase – associated lipocalin (NGAL) in acute heart failure in patients with ST – elevation myocardial infarction. BMC Cardiovasc Disord 2015;15:49 |
| Kiseli | 2017 | < 100 participants | Kiseli M, Caglar GS, Yilmaz H, Gursoy AY, Candar T, Pabuccu EG, et al. Neutrophil gelatinase-associated lipocalin levels during pneumoperitoneum. JSLS 2017;21:e2016.00091 |
| Kisoon | 2015 | < 100 participants | Kisoon RYU, Jae-Yun AHN, Mi-Jin LEE, Woo-Young NHO, Seong-Hun KIM. Early detection and staging of acute kidney injury in non-traumatic rhabdomyolysis in emergency department. J Korean Soc Emerg Med 2015;5:370–8 |
| Kit | 2015 | < 100 participants | Kit OI, Frantsiyants EM, Dimitriadi SN, Kaplieva IV, Trepitaki LK, Cheryarina ND, Pogorelova YA. Role of markers for acute kidney injury in surgical management of patients with renal cancer. Onkourologiya 2015;11:34–9 |
| Kit | 2017 | < 100 participants | Kit OI, Frantsiyants EM, Rozenko DA, Ushakova ND, Dimitriadi SN, Pogorelova YA, et al. Dynamics of markers of markers of acute kidney injury when using epidural block during resection under warm ischemia. Onkourologiya 2017;13:25–33 |
| Kitao | 2015 | < 100 participants | Kitao T, Kimata T, Yamanouchi S, Kato S, Tsuji S, Kaneko K. Urinary biomarkers for screening for renal scarring in children with febrile urinary tract infection: pilot study. J Urol 2015;194:766–71 |
| Klein | 2018 | Meta-analysis – retained as background material | Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, Joannidis M. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med 2018;44:323–36 |
| Knafl | 2017 | < 100 participants | Knafl D, Muller M, Pajenda S, Genc Z, Hecking M, Wagner L. The urine biomarker panel [IGFBP7] × [TIMP-2] (NephroCheck® parameter) does not correlate with IGFBP7 and TIMP-2 gene expression in urinary sediment. PLOS ONE 2017;12:e0188316 |
| Ko | 2018 | Not a relevant biomarker assay or test | Ko SW, Chi NH, Wu CH, Huang TM, Chueh SJ, Wang CH, et al. Hemojuvelin predicts acute kidney injury and poor outcomes following cardiac surgery. Sci Rep 2018;8:1938 |
| Koch | 2011 | Not a relevant biomarker assay or test | Koch AM, Dittrich S, Cesnjevar R, Rüffer A, Breuer C, Glöckler M. Plasma neutrophil gelatinase-associated lipocalin measured in consecutive patients after congenital heart surgery using point-of-care technology. Interact Cardiovasc Thorac Surg 2011;13:133–6 |
| Kohagura | 2012 | Not a primary study | Kohagura K, Ohya Y. Early detection and prediction by biomarkers of acute kidney injury after cardiac surgery. Circ J 2012;76:53–4 |
| Kokot | 2012 | < 100 participants | Kokot M, Biolik G, Ziaja D, Fojt T, Cisak K, Antoniak K, et al. Acute kidney injury after abdominal aortic aneurysm surgery: detailed assessment of early effects using novel markers. Pol Arch Med Wewn 2012;122:353–60 |
| Konvalinka | 2014 | Not a primary study | Konvalinka A. Urine proteomics for acute kidney injury prognosis: another player and the long road ahead. Kidney Int 2014;85:735–8 |
| Koo | 2015 | No focus on DTA for AKI | Koo KC, Hong JH, Lee HS, Jeh SU, Choi YD, Rha KH, Ham WS. Accuracy of urinary neutrophil gelatinase-associated lipocalin in quantifying acute kidney injury after partial nephrectomy in patients with normal contralateral kidney. PLOS ONE 2015;10:e0133675 |
| Kooiman | 2015 | Not a relevant type of population | Kooiman J, van de Peppel WR, Sijpkens YW, Brulez HF, de Vries PM, Nicolaie MA, et al. No increase in kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin excretion following intravenous contrast enhanced-CT. Eur Radiol 2015;25:1926–34 |
| Kos | 2013 | < 100 participants | Kos FT, Sendur MAN, Aksoy S, Celik HT, Sezer S, Civelek B, et al. Evaluation of renal function using the level of neutrophil gelatinase-associated lipocalin is not predictive of nephrotoxicity associated with cisplatin-based chemotherapy. Asian Pac J Cancer Prev 2013;14:1111–4 |
| Kostic | 2019 | < 100 participants | Kostic D, Dos Santos Beozzo GPN, do Couto SB, Kato AHT, Lima L, Palmeira P, et al. First-year profile of biomarkers for early detection of renal injury in infants with congenital urinary tract obstruction. Pediatr Nephrol 2019;34:1117–28 |
| Kostrubiec | 2012 | Not a relevant type of population | Kostrubiec M, Łabyk A, Pedowska-Włoszek J, Dzikowska-Diduch O, Wojciechowski A, Garlińska M, et al. Neutrophil gelatinase-associated lipocalin, cystatin C and eGFR indicate acute kidney injury and predict prognosis of patients with acute pulmonary embolism. Heart 2012;98:1221–8 |
| Koukoulaki | 2013 | < 100 participants | Koukoulaki M, Spyropoulos C, Hondrogiannis P, Papachristou E, Mitsi E, Kalfarentzos F, Goumenos DS. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury in patients with morbid obesity who underwent bariatric surgery. Nephron Extra 2013;3:101–5 |
| Koyner | 2010 | Not a relevant biomarker assay or test | Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 2010;5:2154–65 |
| Koyner | 2008 | < 100 participants | Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 2008;74:1059–69 |
| Koyner | 2014 | < 100 participants | Koyner JL, Garg AX, Thiessen-Philbrook H, Coca SG, Cantley LG, Peixoto A, et al. Adjudication of etiology of acute kidney injury: experience from the TRIBE-AKI multi-center study. BMC Nephrol 2014;15:105 |
| Krawczeski | 2011 | Not a relevant biomarker assay or test | Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 2011;58:2301–9 |
| Krawczeski | 2011 | Not a relevant biomarker assay or test | Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr 2011;158:1009–15.e1 |
| Kumar | 2011 | Not a primary study | Kumar AB, Suneja M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology 2011;114:964–70 |
| Kunutsor | 2018 | Not a relevant type of population | Kunutsor SK, Flores-Guerrero JL, Kieneker LM, Nilsen T, Hidden C, Sundrehagen E, et al. Plasma neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease: findings from the PREVEND prospective cohort study. Clin Chim Acta 2018;486:66–75 |
| Kuribayashi | 2016 | Not a relevant type of population | Kuribayashi R, Suzumura H, Sairenchi T, Watabe Y, Tsuboi Y, Imataka G, et al. Urinary neutrophil gelatinase-associated lipocalin is an early predictor of acute kidney injury in premature infants. Exp Ther Med 2016;12:3706–10 |
| Lábr | 2018 | Not a relevant biomarker assay or test | Lábr K, Špinar J, Pařenica J, Špinarová L, Málek F, Špinarová M, et al. Renal functions and prognosis stratification in chronic heart failure patients and the importance of neutrophil gelatinase-associated lipocalin. Kidney Blood Press Res 2018;43:1865–77 |
| Lacquaniti | 2013 | Not a relevant type of population | Lacquaniti A, Buemi F, Lupica R, Giardina C, Murè G, Arena A, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology 2013;267:86–93 |
| Lacquaniti | 2013 | < 100 participants | Lacquaniti A, Giardina M, Lucisano S, Messina R, Buemi A, Risitano CD, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and endothelial progenitor cells (EPCs) evaluation in aortic aneurysm repair. Curr Vasc Pharmacol 2013;11:1001–10 |
| Lahoud | 2015 | < 100 participants | Lahoud Y, Hussein O, Shalabi A, Nativ O, Awad H, Khamaisi M, et al. Effects of phosphodiesterase-5 inhibitor on ischemic kidney injury during nephron sparing surgery: quantitative assessment by NGAL and KIM-1. World J Urol 2015;33:2053–62 |
| Lane | 2020 | < 100 participants | Lane BR, Babitz SK, Vlasakova K, Wong A, Noyes SL, Boshoven W, P et al. Evaluation of urinary renal biomarkers for early prediction of acute kidney injury following partial nephrectomy: a feasibility study. Eur Urol Focus 2020;6:1240–7 |
| Lavery | 2008 | < 100 participants | Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, Schibler KR. Urinary NGAL in premature infants. Pediatr Res 2008;64:423–8 |
| Lee | 2015 | < 100 participants | Lee HE, Kim DK, Kang HK, Park K. The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr Nephrol 2015;30:123–30 |
| Lee | 2016 | < 100 participants | Lee SK, Lanaspa MA, Sánchez-Lozada LG, Johnson RJ. Hyponatremia with persistent elevated urinary fractional uric acid excretion: evidence for proximal tubular injury? Kidney Blood Press Res 2016;41:535–44 |
| Lee | 2018 | Not a relevant biomarker assay or test | Lee CC, Chang CH, Chen SW, Fan PC, Chang SW, Chen YT, et al. Preoperative risk assessment improves biomarker detection for predicting acute kidney injury after cardiac surgery. PLOS ONE 2018;13:e0203447 |
| Lee | 2018 | < 100 participants | Lee CW, Kou HW, Chou HS, Chou HH, Huang SF, Chang CH, et al. A combination of SOFA score and biomarkers gives a better prediction of septic AKI and in-hospital mortality in critically ill surgical patients: a pilot study. World J Emerg Surg 2018;13:41 |
| Lee | 2019 | < 100 participants | Lee NM, Deriy L, Petersen TR, Shah VO, Hutchens MP, Gerstein NS. Impact of isolyte versus 0.9% saline on postoperative event of acute kidney injury assayed by urinary [TIMP-2] × [IGFBP7] in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2019;33:348–56 |
| Legrand | 2014 | < 100 participants | Legrand M, De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, et al. Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLOS ONE 2014;9:e112313 |
| Legrand | 2013 | < 100 participants | Legrand M, Collet C, Gayat E, Henao J, Giraudeaux V, Mateo J, et al. Accuracy of urine NGAL commercial assays in critically ill patients. Intensive Care Med 2013;39:541–2 |
| Legrand | 2013 | Not a primary study | Legrand M, Darmon M, Joannidis M. NGAL and AKI: the end of a myth? Intensive Care Med 2013;39:1861–3 |
| Legrand | 2015 | Not a relevant type of population | Legrand M, Jacquemod A, Gayat E, Collet C, Giraudeaux V, Launay JM, Payen D. Failure of renal biomarkers to predict worsening renal function in high-risk patients presenting with oliguria. Intensive Care Med 2015;41:68–76 |
| Lei | 2018 | Not a relevant biomarker assay or test | Lei L, Li LP, Zeng Z, Mu JX, Yang X, Zhou C, et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep 2018;8:7962 |
| Lentini | 2012 | < 100 participants | Lentini P, de Cal M, Clementi A, D’Angelo A, Ronco C. Sepsis and AKI in ICU patients: the role of plasma biomarkers. Crit Care Res Pract 2012;2012:856401 |
| Leoncini | 2011 | Not a relevant type of population | Leoncini G, Mussap M, Viazzi F, Fravega M, Degrandi R, Bezante GP, et al. Combined use of urinary neutrophil gelatinase-associated lipocalin (uNGAL) and albumin as markers of early cardiac damage in primary hypertension. Clin Chim Acta 2011;412:1951–6 |
| Leung | 2009 | < 100 participants | Leung JC, Lam MF, Tang SC, Chan LY, Tam KY, Yip TP, Lai KN. Roles of neutrophil gelatinase-associated lipocalin in continuous ambulatory peritoneal dialysis-related peritonitis. J Clin Immunol 2009;29:365–78 |
| Levante | 2017 | Pilot study or preliminary analysis only | Levante C, Ferrari F, Manenti C, Husain-Syed F, Scarpa M, Hinna Danesi T, et al. Routine adoption of TIMP2 and IGFBP7 biomarkers in cardiac surgery for early identification of acute kidney injury. Int J Artif Organs 2017;40:714–18 |
| Levitsky | 2014 | < 100 participants | Levitsky J, Baker TB, Jie C, Ahya S, Levin M, Friedewald J, et al. Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology 2014;60:2017–26 |
| Lewandowska | 2014 | < 100 participants | Lewandowska L, Matuszkiewicz-Rowińska J, Jayakumar C, Oldakowska-Jedynak U, Looney S, Galas M, et al. Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLOS ONE 2014;9:e107898 |
| Li | 2012 | < 100 participants | Li Y, Zhu M, Xia Q, Wang S, Qian J, Lu R, et al. Urinary neutrophil gelatinase-associated lipocalin and L-type fatty acid binding protein as diagnostic markers of early acute kidney injury after liver transplantation. Biomarkers 2012;17:336–42 |
| Li | 2014 | Non-English-language publication | Li J, Zhang H, Shang Y, Cao S. [The study of early diagnosis and prognostic effect using detection of NGAL in community acquired pneumonia with acute kidney injury.] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014;26:269–71 |
| Li | 2018 | Not a relevant type of population | Li H, Yu Z, Gan L, Peng L, Zhou Q. Serum NGAL and FGF23 may have certain value in early diagnosis of CIN. Ren Fail 2018;40:547–53 |
| Liangos | 2009 | Not a relevant biomarker assay or test | Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 2009;14:423–31 |
| Liangos | 2009 | Pilot study or preliminary analysis only | Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 2009;14:423–31 |
| Liao | 2019 | Not a relevant type of population | Liao B, Nian W, Xi A, Zheng M. Evaluation of a diagnostic test of serum neutrophil gelatinase-associated lipocalin (NGAL) and urine KIM-1 in contrast-induced nephropathy (CIN). Med Sci Monit 2019;25:565–70 |
| Libório | 2015 | < 100 participants | Libório AB, Braz MB, Seguro AC, Meneses GC, Neves FM, Pedrosa DC, et al. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am J Trop Med Hyg 2015;92:611–16 |
| Lichosik | 2015 | < 100 participants | Lichosik M, Jung A, Jobs K, Mierzejewska A, Zdanowski R, Kalicki B. Interleukin 18 and neutrophil-gelatinase associated lipocalin in assessment of the risk of contrast-induced nephropathy in children. Cent Eur J Immunol 2015;40:447–53 |
| Lim | 2017 | Not a relevant biomarker assay or test | Lim YM, Moon JY, Min D, Kim SH, Yang WI, Kim WJ, et al. Serial measurements of neutrophil gelatinase-associated lipocalin: prognostic value in patients with ST-segment elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Dis 2017;28:690–6 |
| Lin | 2013 | Not a relevant type of population | Lin HYH, Lee SC, Lin SF, Hsiao HH, Liu YC, Yang WC, et al. Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J Medical Sci 2013;29:304–11 |
| Lin | 2018 | Not a primary study | Lin Q, Mao JH. Early prediction of acute kidney injury in children: known biomarkers but novel combination. World J Pediatr 2018;14:617–20 |
| Lindberg | 2016 | Not a relevant type of population | Lindberg S, Jensen JS, Hoffmann S, Iversen AZ, Pedersen SH, Biering-Sørensen T, et al. Plasma neutrophil gelatinase-associated lipocalin reflects both inflammation and kidney function in patients with myocardial infarction. Cardiorenal Med 2016;6:180–90 |
| Lindberg | 2012 | Not a relevant type of population | Lindberg S, Pedersen SH, Mogelvang R, Jensen JS, Flyvbjerg A, Galatius S, Magnusson NE. Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2012;60:339–45 |
| Lindsey-Yoojin | 2017 | Not a relevant biomarker assay or test | Lindsey-Yoojin C, Won-Sik C, Eui-Kyung C, Jeonghee S, Hyung-Eun YIM, Byung-Min C. Clinical utility of rapid plasma neutrophil gelatinase-associated lipocalin assays for diagnosing acute kidney injury in critically ill newborn infants. Neonatal Med 2017;4:164–70 |
| Ling | 2008 | Not a relevant type of population | Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 2008;108:c176–81 |
| Ling | 2008 | Not a relevant type of population | Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 2008;108:c176–81 [3990] |
| Linko | 2013 | Not a relevant biomarker assay or test | Linko R, Pettilä V, Kuitunen A, Korhonen AM, Nisula S, Alila S, et al. Plasma neutrophil gelatinase-associated lipocalin and adverse outcome in critically ill patients with ventilatory support. Acta Anaesthesiol Scand 2013;57:855–62 |
| Lipcsey | 2014 | No focus on DTA for AKI | Lipcsey M, Hayward P, Haase M, Haase-Fielitz A, Eastwood G, Peck L, Matalanis G, Bellomo R. Neutrophil gelatinase-associated lipocalin after off pump versus on pump coronary artery surgery. Biomarkers 2014;19:22–8 |
| Lipinski | 2015 | Not a relevant type of population | Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, Rydzewska G. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015;44:448–52 |
| Lippi | 2012 | Not a primary study | Lippi G, Cervellin G. Neutrophil gelatinase-associated lipocalin: a more specific assay is needed for diagnosing renal injury. Clin Chim Acta 2012;413:1160–1 |
| Liu | 2013 | Not a relevant biomarker assay or test | Liu S, Che M, Xue S, Xie B, Zhu M, Lu R, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers 2013;18:95–101 |
| Liu | 2016 | Compares clinical adjudication between NephroCheck and KDIGO – retained as background material | Liu KD, Vijayan A, Rosner MH, Shi J, Chawla LS, Kellum JA. Clinical adjudication in acute kidney injury studies: findings from the pivotal TIMP-2*IGFBP7 biomarker study. Nephrol Dial Transplant 2016;31:1641–6 |
| Liu | 2017 | Systematic review – retained as background material | Liu C, Lu X, Mao Z, Kang H, Liu H, Pan L, et al. The diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for acute kidney injury in adults. Medicine 2017;96:e7484 |
| Lu | 2019 | < 100 participants | Lu J, Lin L, Ye C, Tao Q, Cui M, Zheng S, et al. Serum NGAL Is superior to cystatin C in predicting the prognosis of acute-on-chronic liver failure. Ann Hepatol 2019;18:155–64 |
| Lubell | 2017 | No focus on DTA for AKI | Lubell TR, Barasch JM, Xu K, Ieni M, Cabrera KI, Dayan PS. Urinary neutrophil gelatinase-associated lipocalin for the diagnosis of urinary tract infections. Pediatrics 2017;140:e20171090 |
| Luka | 2013 | < 100 participants | Luk CC, Chow KM, Kwok JS, Kwan BC, Chan MH, Lai KB, et al. Urinary biomarkers for the prediction of reversibility in acute-on-chronic renal failure. Dis Markers 2013;34:179–85 |
| Lukasz | 2014 | < 100 participants | Lukasz A, Beneke J, Menne J, Vetter F, Schmidt BM, Schiffer M, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) in patients with Shiga toxin mediated haemolytic uraemic syndrome (STEC-HUS). Thromb Haemost 2014;111:365–72 |
| Luo | 2013 | Not a relevant type of population | Luo Q, Zhou F, Dong H, Wu L, Chai L, Lan K, Wu M. Implication of combined urinary biomarkers in early diagnosis of acute kidney injury following percutaneous coronary intervention. Clin Nephrol 2013;79:85–92 |
| Luthra | 2019 | Not a primary study | Luthra A, Tyagi A. [TIMP-2]*[IGFBP7] for predicting early AKI. Anaesth Crit Care Pain Med 2019;38:677 |
| MacDonald | 2012 | < 100 participants | Macdonald S, Arendts G, Nagree Y, Xu XF. Neutrophil Gelatinase-Associated Lipocalin (NGAL) predicts renal injury in acute decompensated cardiac failure: a prospective observational study. BMC Cardiovasc Disord 2012;12:8 |
| Macedo | 2013 | Not a primary study | Macedo E, Mehta RL. Biomarkers for acute kidney injury: combining the new silver with the old gold. Nephrol Dial Transplant 2013;28:1064–7 |
| Madsen | 2012 | < 100 participants | Madsen MG, Norregaard R, Palmfeldt J, Olsen LH, Frokiaer J, Jorgensen TM. Urinary NGAL, cystatin C, beta 2-microglobulin, and osteopontin significance in hydronephrotic children. Pediatr Nephrol 2012;27:2099–106 |
| Maeda | 2017 | < 100 participants | Maeda A, Ando H, Ura T, Muro K, Aoki M, Saito K, et al. Differences in Urinary Renal Failure Biomarkers in Cancer Patients Initially Treated with Cisplatin. Anticancer Res 2017;37:5235–9 |
| Mahmoodpoor | 2018 | < 100 participants | Mahmoodpoor A, Hamishehkar H, Fattahi V, Sanaie S, Arora P, Nader ND. Urinary versus plasma neutrophil gelatinase-associated lipocalin (NGAL) as a predictor of mortality for acute kidney injury in intensive care unit patients. J Clin Anesth 2018;44:12–17 |
| Maisel | 2011 | Not a relevant biomarker assay or test | Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL evaLuation Along with B-type NaTriuretic peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail 2011;13:846–51 |
| Maisel | 2016 | Not a relevant biomarker assay or test | Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKINESIS study. J Am Coll Cardiol 2016;68:1420–31 |
| Maizel | 2019 | Not a relevant type of population | Maizel J, Daubin D, Vong LV, Titeca-Beauport D, Wetzstein M, Kontar L, et al. Urinary TIMP2 and IGFBP7 identifies high risk patients of short-term progression from mild and moderate to severe acute kidney injury during septic shock: a prospective cohort study. Dis Markers 2019;2019:3471215 |
| Makris | 2009 | < 100 participants | Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med 2009;47:79–82 |
| Malyszko | 2009 | Not a relevant type of population | Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail 2009;31:910–19 |
| Malyszko | 2015 | Not a relevant type of population | Malyszko J, Bachorzewska-Gajewska H, Koc-Zorawska E, Malyszko JS, Kobus G, Dobrzycki S. Midkine: a novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed Res Int 2015;2015:879509 |
| Malyszko | 2019 | Not a relevant type of population | Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Koc-Zorawska E, Matuszkiewicz-Rowinska J, Dobrzycki S. Hepcidin – potential biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Adv Med Sci 2019;64:211–15 |
| Mamikonian | 2014 | < 100 participants | Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med 2014;15:e111–9 |
| Mandei | 2015 | < 100 participants | Mandei J, Iskandar E, Umboh A, Lestari H. Relationship between serum cystatin-C and urinary neutrophil gelatinase-associated lipocalin in septic children. Paediatr Indones 2015;55:83–6 |
| Marcelino | 2014 | < 100 participants | Marcelino P, Tavares I, Carvalho D, Marques C, Silvestre MJ, Perdigoto R, Barroso E. Is urinary gamma-glutamyl transpeptidase superior to urinary neutrophil gelatinase-associated lipocalin for early prediction of acute kidney injury after liver transplantation? Transplant Proc 2014;46:1812–18 |
| Mårtensson | 2013 | Not relevant biomarker assay or test | Mårtensson J, Bell M, Xu S, Bottai M, Ravn B, Venge P, Martling CR. Association of plasma neutrophil gelatinase-associated lipocalin (NGAL) with sepsis and acute kidney dysfunction. Biomarkers 2013;18:349–56 |
| Mårtensson | 2016 | < 100 participants | Mårtensson J, Jonsson N, Glassford NJ, Bell M, Martling CR, Bellomo R, Larsson A. Plasma endostatin may improve acute kidney injury risk prediction in critically ill patients. Ann Intensive Care 2016;6:6 |
| Mårtensson | 2010 | < 100 participants | Mårtensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med 2010;36:1333–40 |
| Martin-Moreno | 2015 | Not a relevant type of population | Martin-Moreno PL, Varo N, Martínez-Ansó E, Martin-Calvo N, Sayón-Orea C, Bilbao JI, Garcia-Fernandez N. Comparison of intravenous and oral hydration in the prevention of contrast-induced acute kidney injury in low-risk patients: a randomized trial. Nephron 2015;131:51–8 |
| Martino | 2012 | Pilot study or preliminary analysis only | Martino FK, Filippi I, Giavarina D, Kaushik M, Rodighiero MP, Crepaldi C, et al. Neutrophil gelatinase-associated lipocalin in the early diagnosis of peritonitis: the case of neutrophil gelatinase-associated lipocalin. Contrib Nephrol 2012;178:258–63 |
| Mathew | 2008 | Not a relevant biomarker assay or test | Mathew A, Garg AX. A single measure of urinary neutrophil gelatinase-associated lipocalin was accurate for diagnosing acute kidney injury. ACP J Club 2008;149:13 |
| Matsuura | 2018 | < 100 participants | Matsuura R, Komaru Y, Miyamoto Y, Yoshida T, Yoshimoto K, Isshiki R, et al. Response to different furosemide doses predicts AKI progression in ICU patients with elevated plasma NGAL levels. Ann Intensive Care 2018;8:8 |
| Matys | 2013 | Not a relevant type of population | Matys U, Bachorzewska-Gajewska H, Malyszko J, Dobrzycki S. Assessment of kidney function in diabetic patients. Is there a role for new biomarkers NGAL, cystatin C and KIM-1? Adv Med Sci 2013;58:353–61 |
| Mawad | 2016 | < 100 participants | Mawad H, Laurin LP, Naud JF, Leblond FA, Henley N, Vallée M, et al. Changes in urinary and serum levels of novel biomarkers after administration of gadolinium-based contrast agents. Biomark Insights 2016;11:91–4 |
| Mayer | 2017 | Pilot study or preliminary analysis only | Mayer T, Bolliger D, Scholz M, Reuthebuch O, Gregor M, Meier P, et al. Urine biomarkers of tubular renal cell damage for the prediction of acute kidney injury after cardiac surgery–a pilot study. J Cardiothorac Vasc Anesth 2017;31:2072–9 |
| Mazar | 2014 | No focus on DTA for AKI | Mazar M, Ivancan V, Segotic I, Colak Z, Gabelica R, Rajsman G, et al. A diagnosis of a renal injury by early biomarkers in patients exposed to cardiopulmonary bypass during cardiac surgery. Signa Vitae 2014;9(Suppl. 1):45–8 |
| Mazzeffi | 2016 | < 100 participants | Mazzeffi MA, Stafford P, Wallace K, Bernstein W, Deshpande S, Odonkor P, et al. Intra-abdominal hypertension and postoperative kidney dysfunction in cardiac surgery patients. J Cardiothorac Vasc Anesth 2016;30:1571–7 |
| McCaffrey | 2015 | < 100 participants | McCaffrey J, Coupes B, Chaloner C, Webb NJ, Barber R, Lennon R. Towards a biomarker panel for the assessment of AKI in children receiving intensive care. Pediatr Nephrol 2015;30:1861–71 |
| McCullough | 2011 | Not a primary study | McCullough PA, El-Ghoroury M, Yamasaki H. Early detection of acute kidney injury with neutrophil gelatinase-associated lipocalin. J Am Coll Cardiol 2011;57:1762–4 |
| McCullough | 2012 | Not a relevant type of population | McCullough PA, Williams FJ, Stivers DN, Cannon L, Dixon S, Alexander P, et al. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol 2012;35:509–14 |
| McIlroy | 2010 | Not a relevant biomarker assay or test | McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol 2010;5:211–19 |
| McIlroy | 2010 | Not a primary study | McIlroy DR, Wagener G, Lee HT. Biomarkers of acute kidney injury: an evolving domain. Anesthesiology 2010;112:998–1004 |
| McIlroy | 2015 | Not a relevant biomarker assay or test | McIlroy DR, Farkas D, Matto M, Lee HT. Neutrophil gelatinase-associated lipocalin combined with delta serum creatinine provides early risk stratification for adverse outcomes after cardiac surgery: a prospective observational study. Crit Care Med 2015;43:1043–52 |
| McWilliam | 2012 | < 100 participants | McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, et al. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLOS ONE 2012;7:e43809 |
| McWilliam | 2018 | Not a relevant type of population | McWilliam SJ, Antoine DJ, Jorgensen AL, Smyth RL, Pirmohamed M. Urinary Biomarkers of aminoglycoside-induced nephrotoxicity in cystic fibrosis: kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin. Sci Rep 2018;8:5094 |
| Md Ralib | 2017 | Not a relevant type of population | Md Ralib A, Mat Nor MB, Pickering JW. Plasma Neutrophil gelatinase-associated lipocalin diagnosed acute kidney injury in patients with systemic inflammatory disease and sepsis. Nephrology 2017;22:412–19 |
| Meersch | 2018 | Not a primary study | Meersch M, Zarbock A, Küllmar M. Renal biomarkers for the initiation of renal replacement therapy – is this the future? J Thorac Dis 2018;10:S3229–S3232 |
| Meersh | 2014 | < 100 participants | Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLOS ONE 2014;9:e93460 |
| Meersh | 2017 | No focus on DTA for AKI | Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43:1551–61 |
| Meersh | 2014 | < 100 participants | Meersch M, Schmidt C, Van Aken H, Rossaint J, Görlich D, Stege D, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLOS ONE 2014;9:e110865 |
| Meisner | 2018 | Not a relevant biomarker assay or test | Meisner A, Kerr KF, Thiessen-Philbrook H, Wilson FP, Garg AX, Shlipak MG, et al. Development of biomarker combinations for postoperative acute kidney injury via Bayesian model selection in a multicenter cohort study. Biomark Res 2018;6:3 |
| Mellor | 2012 | < 100 participants | Mellor AJ, Woods D. Serum neutrophil gelatinase-associated lipocalin in ballistic injuries: a comparison between blast injuries and gunshot wounds. J Crit Care 2012;27:419.e1–5 |
| Meneses | 2018 | < 100 participants | Meneses GC, De Francesco Daher E, da Silva Junior GB, Bezerra GF, da Rocha TP, de Azevedo IEP, et al. Visceral leishmaniasis-associated nephropathy in hospitalised Brazilian patients: new insights based on kidney injury biomarkers. Trop Med Int Health 2018;23:1046–57 |
| Menon | 2016 | Not a relevant biomarker assay or test | Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant 2016;31:586–94 |
| Merrikhi | 2014 | < 100 participants | Merrikhi A, Gheissari A, Mousazadeh H. Urine and serum neutrophil gelatinase-associated lipocalin cut-off point for the prediction of acute kidney injury. Adv Biomed Res 2014;3:66 |
| Mertoglu | 2018 | < 100 participants | Mertoglu C, Gunay M, Gurel A, Gungor M. Myo-inositol oxygenase as a novel marker in the diagnosis of acute kidney injury. J Med Biochem 2018;37:1–6 |
| Metzger | 2010 | < 100 participants | Metzger J, Kirsch T, Schiffer E, Ulger P, Mentes E, Brand K, et al. Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int 2010;78:1252–62 |
| Metzger | 2016 | Not a relevant biomarker assay or test | Metzger J, Mullen W, Husi H, Stalmach A, Herget-Rosenthal S, Groesdonk HV, et al. Acute kidney injury prediction in cardiac surgery patients by a urinary peptide pattern: a case-control validation study. Crit Care 2016;20:157 |
| Miah | 2018 | No focus on DTA for AKI | Miah OF, Dowel FA, Latif A, Hai AN, Mahmud MA, Razzak MA, Ahammod T. NGAL (neutrophil gelatinase-associated lipocalin) is an early predictor of acute kidney injury after cardiac surgery and variation of ngal values in homogenous study subject. Mymensingh Med J 2018;27:212–15 |
| Miah | 2018 | < 100 participants | Miah OF, Roy DK, Chowdhury AA, Alam KS, Alam MB, Anwar MR, et al. Plasma Neutrophil gelatinase associated lipocalin (pNGAL) level to identify aki early in patients undergoing cardiac valve surgery. Mymensingh Med J 2018;27:263–9 |
| Mironova | 2019 | Non-English-language publication | Mironova SA, Yudina YS, Ionov MV, Avdonina NG, Emelyanov IV, Vasilyeva EY, et al. Novel biomarkers of kidney injury and fibrosis in patients with different severity of hypertension: relation to vascular reactivity and stiffness. Russ J Cardiol 2019;24:44–51 |
| Mishra | 2013 | < 100 participants | Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2013;365:1231–8 |
| Mishra | 2017 | < 100 participants | Mishra OP, Rai AK, Srivastava P, Pandey K, Abhinay A, Prasad R, et al. Predictive ability of urinary biomarkers for outcome in children with acute kidney injury. Pediatr Nephrol 2017;32:521–7 |
| Mitsnefes | 2007 | < 100 participants | Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 2007;22:101–8 |
| MohamadiSichani | 2017 | < 100 participants | MohamadiSichani M, Tolou Ghamari Z. Investigation of urinary neutrophil gelatinase associated lipocalin (NGAL) for early diagnosis of acute kidney injury after percutaneous nephrolithotomy. Afr J Urol 2017;23:214–18 |
| Mohamed | 2015 | < 100 participants | Mohamed F, Buckley NA, Jayamanne S, Pickering JW, Peake P, Palangasinghe C, et al. Kidney damage biomarkers detect acute kidney injury but only functional markers predict mortality after paraquat ingestion. Toxicol Lett 2015;237:140–50 |
| Mohamed | 2016 | < 100 participants | Mohamed F, Endre ZH, Pickering JW, Jayamanne S, Palangasinghe C, Shahmy S, et al. Mechanism-specific injury biomarkers predict nephrotoxicity early following glyphosate surfactant herbicide (GPSH) poisoning. Toxicol Lett 2016;258:1–10 |
| Mohtat | 2011 | < 100 participants | Mohtat D, Thomas R, Du Z, Boakye Y, Moulton T, Driscoll C, Woroniecki R. Urinary transforming growth factor beta-1 as a marker of renal dysfunction in sickle cell disease. Pediatr Nephrol 2011;26:275–80 |
| Mohtat | 2011 | Not a relevant type of population | Mohtat D, Thomas R, Du Z, Boakye Y, Moulton T, Driscoll C, Woroniecki R. Urinary transforming growth factor beta-1 as a marker of renal dysfunction in sickle cell disease. Pediatr Nephrol 2011;26:275–80 |
| Moledina | 2015 | Not a relevant biomarker assay or test | Moledina DG, Parikh CR, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, et al. Association of perioperative plasma neutrophil gelatinase-associated lipocalin levels with 3-year mortality after cardiac surgery: a prospective observational cohort study. PLOS ONE 2015;10:e0129619 |
| Moledina | 2017 | No focus on DTA for AKI | Moledina DG, Hall IE, Thiessen-Philbrook H, Reese PP, Weng FL, Schröppel B, et al. Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis 2017;70:807–16 |
| Moon | 2019 | Not a relevant type of population | Moon JM, Chun BJ, Shin MH, Cho YS. Predictive value of plasma neutrophil gelatinase-associated lipocalin in acute charcoal-burning carbon monoxide poisoning. Hum Exp Toxicol 2019;38:877–87 |
| Morales-Buenrostro | 2014 | < 100 participants | Morales-Buenrostro LE, Salas-Nolasco OI, Barrera-Chimal J, Casas-Aparicio G, Irizar-Santana S, Pérez-Villalva R, Bobadilla NA. Hsp72 is a novel biomarker to predict acute kidney injury in critically ill patients. PLOS ONE 2014;9:e109407 |
| Moriyama | 2016 | < 100 participants | Moriyama T, Hagihara S, Shiramomo T, Nagaoka M, Iwakawa S, Kanmura Y. Comparison of three early biomarkers for acute kidney injury after cardiac surgery under cardiopulmonary bypass. J Intensive Care 2016;4:41 |
| Moriyama | 2017 | < 100 participants | Moriyama T, Hagihara S, Shiramomo T, Nagaoka M, Iwakawa S, Kanmura Y. The protective effect of human atrial natriuretic peptide on renal damage during cardiac surgery. J Anesth 2017;31:163–9 |
| Mortara | 2013 | < 100 participants | Mortara A, Bonadies M, Mazzetti S, Fracchioni I, Delfino P, Chioffi M, et al. Neutrophil gelatinase-associated lipocalin predicts worsening of renal function in acute heart failure: methodological and clinical issues. J Cardiovasc Med 2013;14:629–34 |
| Mosa | 2018 | Not a relevant biomarker assay or test | Mosa OF. Prognostic significance of serum NGAL and troponin I against acute kidney injury in Egyptian ICU patients after open heart surgery: a pilot study. Kidney Dis 2018;4:246–54 |
| Moyake | 2016 | Not a relevant type of population | Moyake N, Buchmann E, Crowther NJ. Neutrophil gelatinase-associated lipocalin as a diagnostic marker of acute kidney injury in pre-eclampsia. J Obstet Gynaecol Res 2016;42:1483–8 |
| Muhammad Usman | 2013 | < 100 participants | Muhammad Usman M, Dilshad Ahmed K, Farooq Ahmad K, Syed Muhammad Shahab N. Comparison of urine with plasma neutrophil gelatinase-associated lipocalin in detecting acute kidney injury after cardiopulmonary bypass surgery. Pak Armed Forces Med J 2013;63:179–83 |
| Munir | 2013 | < 100 participants | Munir MU, Khan DA, Khan FA, Shahab Naqvi SM. Rapid detection of acute kidney injury by urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass surgery. J Coll Physicians Surg Pak 2013;23:103–6 |
| Munshi | 2014 | Not a primary study | Munshi R, Zimmerman JJ. Neutrophil gelatinase-associated lipocalin-can it predict the future? Pediatr Crit Care Med 2014;15:173–4 |
| Muratoglu | 2016 | Not a relevant type of population | Muratoglu M, Kavalci C, Kilicli E, Findik M, Kayipmaz AE, Durukan P. Serum neutrophil gelatinase-associated lipocalin levels in early detection of contrast-induced nephropathy. Clin Invest Med 2016;39:E88–94 |
| Murphy | 2014 | < 100 participants | Murphy N, Vijayan A, Frohlich S, O’Farrell F, Barry M, Sheehan S, et al. Remote ischemic preconditioning does not affect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 2104;28:1285–92 |
| Musiol | 2016 | < 100 participants | Musiol K, Sobol-Milejska G, Nowotka Ł, Torba K, Kniażewska M, Wos H. Renal function in children treated for central nervous system malignancies. Childs Nerv Syst 2016;32:1431–40 |
| Nadkarni | 2017 | No focus on DTA for AKI | Nadkarni GN, Coca SG, Meisner A, Patel S, Kerr KF, Patel UD, et al. Urinalysis findings and urinary kidney injury biomarker concentrations. BMC Nephrol 2017;18:218 |
| Nam | 2015 | Meta-analysis – retained as background material | Nam MJ, Lim CH, Kim HJ, Kim YH, Choi H, Son HS, et al. A meta-analysis of renal function after adult cardiac surgery with pulsatile perfusion. Artif Organs 2015;39:788–94 |
| Nasonova | 2019 | Non-English-language publication | Nasonova SN, Zhirov IV, Ledyakhova MV, Sharf TV, Bosykh EG, Masenko VP, Tereshchenko SN. Early diagnosis of acute renal injury in patients with acute decompensation of chronic heart failure. Ter Arkh 2019;91:67–73 |
| Nayak | 2016 | Not a relevant biomarker assay or test | Nayak NM, Madhumitha S, Annigeri RA, Venkataraman R, Balasubramaian S, Seshadri R, et al. Clinical utility of urine neutrophil gelatinase-associated lipocalin measured at admission to predict outcomes in heterogeneous population of critically ill patients. Indian J Nephrol 2016;26:119–24 |
| Negrin | 2018 | < 100 participants | Negrin LL, Hahn R, Heinz T, Hajdu S. Diagnostic utility of serum neutrophil gelatinase-associated lipocalin in polytraumatized patients suffering acute kidney injury: a prospective study. Biomed Res Int 2018;2018:2687584 |
| Nehus | 2017 | No focus on DTA for AKI | Nehus E, Kaddourah A, Bennett M, Pyles O, Devarajan P. Subclinical kidney injury in children receiving nonsteroidal anti-inflammatory drugs after cardiac surgery. J Pediatr 2017;189:175–80 |
| Nejat | 2012 | No relevant outcome | Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 2012;81:1254–62 |
| Nguyen | 2019 | Not a relevant type of population | Nguyen LS, Spagnoli V, Kerneis M, Hauguel-Moreau M, Barthélémy O, Collet JP, et al. Evaluation of neutrophil gelatinase-associated lipocalin and cystatin C as biomarkers of acute kidney injury after ST-segment elevation myocardial infarction treated by percutaneous coronary intervention. Arch Cardiovasc Dis 2019;112:180–6 |
| Nickavar | 2016 | < 100 participants | Nickavar A, Safaeian B, Valavi E, Moradpour F. Validity of neutrophil gelatinase associated lipocaline as a biomarker for diagnosis of children with acute pyelonephritis. Urol J 2016;13:2860–3 |
| Niemann | 2009 | < 100 participants | Niemann CU, Walia A, Waldman J, Davio M, Roberts JP, Hirose R, Feiner J. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl 2009;15:1852–60 |
| Ning | 2018 | Not a relevant type of population | Ning L, Li Z, Wei D, Chen H, Yang C, Wu D, et al. Urinary semaphorin 3A as an early biomarker to predict contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Braz J Med Biol Res 2018;51:e6487 |
| Nishida | 2010 | < 100 participants | Nishida M, Kawakatsu H, Okumura Y, Hamaoka K. Serum and urinary neutrophil gelatinase-associated lipocalin levels in children with chronic renal diseases. Pediatr Int 2010;52:563–8 |
| Noto | 2019 | < 100 participants | Noto A, Cortegiani A, David A. Nephrocheck: should we consider urine osmolality? Crit Care 2019;23:23 |
| Noyan | 2015 | < 100 participants | Noyan A, Parmaksiz G, Dursun H, Ezer SS, Anarat R, Cengiz N. Urinary NGAL, KIM-1 and L-FABP concentrations in antenatal hydronephrosis. J Pediatr Urol 2015;11:249.e1–249.e6 |
| Nusca | 2018 | Not a relevant type of population | Nusca A, Miglionico M, Proscia C, Ragni L, Carassiti M, Pepe FL, Sciascio GD. Early prediction of contrast-induced acute kidney injury by a ‘bedside’ assessment of neutrophil gelatinase-associated lipocalin during elective percutaneous coronary interventions. PLOS ONE 2018;13:e0197833 |
| Nymo | 2012 | Not a relevant biomarker assay or test | Nymo SH, Ueland T, Askevold ET, Flo TH, Kjekshus J, Hulthe J, et al. The association between neutrophil gelatinase-associated lipocalin and clinical outcome in chronic heart failure: results from CORONA. J Intern Med 2012;271:436–43 |
| Odum | 2014 | < 100 participants | Odum L, Andersen AS, Hviid TVF. Urinary neutrophil gelatinase-associated lipocalin (NGAL) excretion increases in normal pregnancy but not in preeclampsia. Clin Chem Lab Med 2014;52:221–5 |
| Oh | 2012 | < 100 participants | Oh SW, Chin HJ, Chae DW, Na KY. Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting. J Korean Med Sci 2012;27:506–11 |
| Olvera-Posada | 2017 | < 100 participants | Olvera-Posada D, Dayarathna T, Dion M, Alenezi H, Sener A, Denstedt JD, et al. KIM-1 Is a potential urinary biomarker of obstruction: results from a prospective cohort study. J Endourol 2017;31:111–18 |
| Omerika | 2014 | No focus on DTA for AKI | Omerika L, Rasić S, Serdarević N. Importance of determination of urine neutrophile gelatinase associated lipocalin in early detection of acute kidney injury. Coll Antropol 2014;38:161–6 |
| Oncel | 2016 | < 100 participants | Oncel MY, Canpolat FE, Arayici S, Alyamac Dizdar E, Uras N, Oguz SS. Urinary markers of acute kidney injury in newborns with perinatal asphyxia. Ren Fail 2016;38:882–8 |
| Onk | 2016 | Not a relevant biomarker assay or test | Onk OA, Onk D, Ozcelik F, Gunay M, Turkmen K. Risk factors for acute kidney injury after coronary artery bypass surgery and its detection using neutrophil gelatinase-associated lipocalin. Cardiorenal Med 2016;6:216–29 |
| Opotowsky | 2017 | No focus on DTA for AKI | Opotowsky AR, Baraona FR, Mc Causland FR, Loukas B, Landzberg E, Landzberg MJ, et al. Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart 2017;103:434–42 |
| Ordooei Javan | 2017 | < 100 participants | Ordooei Javan A, Salamzadeh J, Shokouhi S, Sahraei Z. Evaluation of renal toxicity of colistin therapy with neutrophil gelatinase-associated lipocalin: a biomarker of renal tubular damage. Iran J Kidney Dis 2017;11:447–55 |
| Orsolya | 2015 | No focus on DTA for AKI | Orsolya M, Attila-Zoltan M, Gherman V, Zaharie F, Bolboaca S, Chira C, et al. The effect of anaesthetic management on neutrophil gelatinase associated lipocalin (NGAL) levels after robotic surgical oncology. J BUON 2015;20:317–24 |
| Ostermann | 2015 | Not a primary study | Ostermann M, Joannidis M. Biomarkers for AKI improve clinical practice: no. Intensive Care Med 2015;41:618–22 |
| Ostermann | 2018 | Substudy measuring associations in NephroCheck levels and exposure to renal insult – retained as background material | Ostermann M, McCullough PA, Forni LG, Bagshaw SM, Joannidis M, Shi J, et al. Kinetics of urinary cell cycle arrest markers for acute kidney injury following exposure to potential renal insults. Crit Care Med 2018;46:375–83 |
| Owens | 2011 | < 100 participants | Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol 2011;32:183–8 |
| Oz | 2016 | Not a relevant biomarker assay or test | Oz K, Gode S, Basgoze S, Koser M, Oz A, Goksel OS, et al. Cystatin C and NGAL as biomarkers for early detection of acute kidney injury in geriatrics. Int Surg 2016;101:390–8 |
| Ozdemir | 2014 | Not a relevant type of population | Ozdemir O, Oguz AD, Eren A, Sanli C, Sylemezoglu HO, Cayci AB. Cystatin C as biomarker of contrast-induced nephropathy in pediatric cardiac angiography. Turk J Med Sci 2014;44:178–85 |
| Ozkan | 2014 | < 100 participants | Ozkan S, Durukan P, Kavalci C, Duman A, Sayhan MB, Salt O, Ipekci A. Importance of neutrophil gelatinase-associated lipocalin in differential diagnosis of acute and chronic renal failure. Iran Red Crescent Med J 2014;16:e14133 |
| Paapstel | 2016 | < 100 participants | Paapstel K, Zilmer M, Eha J, Tootsi K, Piir A, Kals J. Early biomarkers of renal damage in relation to arterial stiffness and inflammation in male coronary artery disease patients. Kidney Blood Press Res 2016;41:488–97 |
| Paarmann | 2013 | Not a relevant biomarker assay or test | Paarmann H, Charitos EI, Beilharz A, Heinze H, Schon J, Berggreen A, Heringlake M. Duration of cardiopulmonary bypass is an important confounder when using biomarkers for early diagnosis of acute kidney injury in cardiac surgical patients. Appl Cardiopulm Pathophysiol 2013;17:284–97 |
| Padhy | 2014 | Not a relevant type of population | Padhy M, Kaushik S, Girish MP, Mohapatra S, Shah S, Koner BC. Serum neutrophil gelatinase associated lipocalin (NGAL) and cystatin C as early predictors of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Clin Chim Acta 2014;435:48–52 |
| Pajenda | 2015 | < 100 participants | Pajenda S, Ilhan-Mutlu A, Preusser M, Roka S, Druml W, Wagner L. NephroCheck data compared to serum creatinine in various clinical settings. BMC Nephrol 2015;16:0203–5 |
| Palazzuoli | 2014 | Not a relevant biomarker assay or test | Palazzuoli A, Ruocco G, Beltrami M, Franci B, Pellegrini M, Lucani B, et al. Admission plasma neutrophil gelatinase associated lipocalin (NGAL) predicts worsening renal function during hospitalization and post discharge outcome in patients with acute heart failure. Acute Card Care 2014;16:93–101 |
| Palazzuoli | 2014 | Not a relevant biomarker assay or test | Palazzuoli A, Ruocco G, Pellegrini M, Martini S, Del Castillo G, Beltrami M, et al. Patients with cardiorenal syndrome revealed increased neurohormonal activity, tubular and myocardial damage compared to heart failure patients with preserved renal function. Cardiorenal Med 2014;4:257–68 |
| Palazzuoli | 2015 | Not a relevant biomarker assay or test | Palazzuoli A, Ruocco G, Pellegrini M, De Gori C, Del Castillo G, Franci B, et al. Comparison of neutrophil gelatinase-associated lipocalin versus B-type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol 2015;116:104–11 |
| Palermo | 2017 | < 100 participants | Palermo J, Dart AB, De Mello A, Devarajan P, Gottesman R, Garcia Guerra G, et al. Biomarkers for early acute kidney injury diagnosis and severity prediction: a pilot multicenter Canadian study of children admitted to the ICU. Pediatr Crit Care Med 2017;18:e235–e244 |
| Pan | 2018 | < 100 participants | Pan JJ, Sun ZY, Zhou XY, Hu YH, Cheng R, Chen XQ, Yang Y. Is neutrophil gelatinase-associated lipocalin a good diagnostic marker for renal injury in asphyxiated preterm infants? J Res Med Sci 2018;23:90 |
| Pang | 2016 | Not a relevant type of population | Pang Y, Tan Y, Li Y, Zhang J, Guo Y, Guo Z, et al. Pentraxin 3 is closely associated with tubulointerstitial injury in lupus nephritis: a large multicenter cross-sectional study. Medicine 2016;95:e2520 |
| Pang | 2017 | < 100 participants | Pang HM, Qin XL, Liu TT, Wei WX, Cheng DH, Lu H, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: a prospective study. Eur Rev Med Pharmacol Sci 2017;21:4203–13 |
| Papadopoulou-Marketou | 2015 | < 100 participants | Papadopoulou-Marketou N, Skevaki C, Kosteria I, Peppa M, Chrousos GP, Papassotiriou I, Kanaka-Gantenbein C. NGAL and cystatin C: two possible early markers of diabetic nephropathy in young patients with type 1 diabetes mellitus: one year follow up. Hormones 2015;14:232–40 |
| Papassotiriou | 2016 | < 100 participants | Papassotiriou GP, Kastritis E, Gkotzamanidou M, Christoulas D, Eleutherakis-Papaiakovou E, Migkou M, et al. Neutrophil gelatinase-associated lipocalin and cystatin C are sensitive markers of renal injury in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2016;16:29–35 |
| Parekh | 2013 | < 100 participants | Parekh DJ, Weinberg JM, Ercole B, Torkko KC, Hilton W, Bennett M, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 2013;24:506–17 |
| Parikh | 2012 | Not a primary study | Parikh A, Shaw A. The economics of renal failure and kidney disease in critically ill patients. Crit Care Clin 2012;28:99–111 |
| Parikh | 2013 | < 100 participants | Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 2013;70:199–203 |
| Parikh | 2013 | Not a primary study | Parikh CR, Han G. Variation in performance of kidney injury biomarkers due to cause of acute kidney injury. Am J Kidney Dis 2013;62:1023–6 |
| Parikh | 2016 | Uses simulated data from the TRIBE AKI study – retained as background material | Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int 2016;89:1372–9 |
| Parikh | 2017 | No relevant outcome | Parikh CR, Puthumana J, Shlipak MG, Koyner JL, Thiessen-Philbrook H, McArthur E, et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3699–707 |
| Parikh | 2017 | Not a primary study | Parikh A, Rizzo JA, Canetta P, Forster C, Sise M, Maarouf O, et al. Correction: does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) & serum creatinine (sCr) for acute kidney injury (AKI) diagnosis. PLOS ONE 2017;12:e0185772 |
| Parikh | 2017 | Not a primary study | Parikh A, Rizzo JA, Canetta P, Forster C, Sise M, Maarouf O, et al. Does NGAL reduce costs? A cost analysis of urine NGAL (uNGAL) & serum creatinine (sCr) for acute kidney injury (AKI) diagnosis. PLOS ONE 2017;12:e0178091 |
| Park | 2012 | < 100 participants | Park HD, Seo JY, Lee SY. The relationship between serum neutrophil gelatinase-associated lipocalin and renal function in patients with vancomycin treatment. Ann Clin Lab Sci 2012;42:7–13 |
| Park | 2015 | < 100 participants | Park GY, Yu CH, Kim JS, Kang YJ, Kwon O, Choi JY, et al. Plasma neutrophil gelatinase-associated lipocalin as a potential predictor of adverse renal outcomes in immunoglobulin A nephropathy. Korean J Intern Med 2015;30:345–53 |
| Park | 2016 | < 100 participants | Park SO, Ahn JY, Lee YH, Kim YJ, Min YH, Ahn HC, et al. Plasma neutrophil gelatinase-associated lipocalin as an early predicting biomarker of acute kidney injury and clinical outcomes after recovery of spontaneous circulation in out-of-hospital cardiac arrest patients. Resuscitation 2016;101:84–90 |
| Park | 2018 | < 100 participants | Park YR, Oh JS, Jeong H, Park J, Oh YM, Choi S, Choi KH. Predicting long-term outcomes after cardiac arrest by using serum neutrophil gelatinase-associated lipocalin. Am J Emerg Med 2018;36:660–4 |
| Park | 2018 | Not a relevant type of population | Park SY, Eom JS, Lee JS, Ju YS, Park JY. Neutrophil Gelatinase-associated lipocalin as a predictor of acute kidney injury in patients during treatment with colistimethate sodium. Infect Chemother 2018;50:128–37 |
| Park | 2015 | Not a relevant biomarker assay or test | Park CM, Kim JS, Moon HW, Park S, Kim H, Ji M, et al. Usefulness of plasma neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury after cardiopulmonary bypass in Korean cardiac patients: a prospective observational study. Clin Biochem 2015;48:44–9 |
| Parr | 2015 | Not a relevant type of population | Parr SK, Clark AJ, Bian A, Shintani AK, Wickersham NE, Ware LB, et al. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int 2015;87:640–8 |
| Parravicini | 2010 | < 100 participants | Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, et al. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res 2010;67:636–40 |
| Parravicini | 2016 | < 100 participants | Parravicini E, Locatelli C, Lorenz JM, Nemerofsky SL, Bateman DA. Is urinary neutrophil gelatinase-associated lipocalin able to predict acute kidney injury episodes in very low birth weight infants in clinical settings? Pediatr Res 2016;80:663–7 |
| Passov | 2019 | Not a relevant biomarker assay or test | Passov A, Petäjä L, Pihlajoki M, Salminen US, Suojaranta R, Vento A, et al. The origin of plasma neutrophil gelatinase-associated lipocalin in cardiac surgery. BMC Nephrol 2019;20:182 |
| Patel | 2013 | Not a relevant type of population | Patel M, Sachan R, Gangwar R, Sachan P, Natu S. Correlation of serum neutrophil gelatinase-associated lipocalin with acute kidney injury in hypertensive disorders of pregnancy. Int J Nephrol Renovasc Dis 2013;6:181–6 |
| Patel | 2016 | Not a relevant biomarker assay or test | Patel ML, Sachan R, Shyam R, Kumar S, Kamal R, Misra A. Diagnostic accuracy of urinary neutrophil gelatinase-associated lipocalin in patients with septic acute kidney injury. Int J Nephrol Renovasc Dis 2016;9:161–9 |
| Patschan | 2014 | < 100 participants | Patschan D, Heeg M, Brier M, Brandhorst G, Schneider S, Müller GA, Koziolek MJ. CD4+ lymphocyte adenosine triphosphate – a new marker in sepsis with acute kidney injury? BMC Nephrol 2014;15:203 |
| Peco-Antić | 2013 | Not a relevant biomarker assay or test | Peco-Antić A, Ivanišević I, Vulićević I, Kotur-Stevuljević J, Ilić S, Ivanišević J, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem 2013;46:1244–51 |
| Pedersen | 2010 | < 100 participants | Pedersen KR, Ravn HB, Hjortdal VE, Nørregaard R, Povlsen JV. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Invest 2010;70:374–82 |
| Pejović | 2015 | Not a relevant type of population | Pejović B, Erić-Marinković J, Pejović M, Kotur-Stevuljević J, Peco-Antić A. Detection of acute kidney injury in premature asphyxiated neonates by serum neutrophil gelatinase-associated lipocalin (sNGAL) – sensitivity and specificity of a potential new biomarker. Biochem Med 2015;25:450–9 |
| Peralta | 2014 | Not a relevant type of population | Peralta CA, Scherzer R, Grunfeld C, Abraham A, Tien PC, Devarajan P, et al. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med 2014;15:291–300 |
| Peres | 2014 | < 100 participants | Peres LA, da Cunha AD, Assumpção RA, Schäfer A, da Silva AL, Gaspar AD, et al. Evaluation of the cisplatin nephrotoxicity using the urinary neutrophil gelatinase-associated lipocalin (NGAL) in patients with head and neck cancer. J Bras Nefrol 2014;36:280–8 |
| Perrin | 2013 | Not a relevant type of population | Perrin T, Descombes E, Magnin JL, Gachet M, Hemett OM, Hayoz D, et al. Urinary neutrophil gelatinase-associated lipocalin (uNGAL) and contrast-induced acute kidney injury after coronary angiogram. Swiss Med Wkly 2013;143:13853 |
| Perrotti | 2015 | Not a relevant biomarker assay or test | Perrotti A, Miltgen G, Chevet-Noel A, Durst C, Vernerey D, Bardonnet K, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015;99:864–9 |
| Perry | 2010 | Not a relevant biomarker assay or test | Perry TE, Muehlschlegel JD, Liu KY, Fox AA, Collard CD, Shernan SK, Body SC, CABG Genomics Investigators. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth Analg 2010;110:1541–7 |
| Pesonen | 2016 | < 100 participants | Pesonen EJ, Suominen PK, Keski-Nisula J, Mattila IP, Rautiainen P, Jahnukainen T. The effect of methylprednisolone on plasma concentrations of neutrophil gelatinase-associated lipocalin in pediatric heart surgery. Pediatr Crit Care Med 2016;17:121–7 |
| Petrovic | 2013 | < 100 participants | Petrovic S, Bogavac-Stanojevic N, Peco-Antic A, Ivanisevic I, Kotur-Stevuljevic J, Paripovic D, et al. Clinical application neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 as indicators of inflammation persistence and acute kidney injury in children with urinary tract infection. Biomed Res Int 2013;2013:947157 |
| Pezeshgi | 2018 | < 100 participants | Pezeshgi A, Ghodrati S, Kiafar M, Kamali K, Asadi-Khiavi M. Study of neutrophil gelatinase-associated lipocalin in patients with cardiovascular shock. J Ren Inj Prev 2018;7:144–7 |
| Piccoli | 2012 | Not a primary study | Piccoli GB, Ferraresi M, Aroasio E, Gonella S, De Pascale A, Veltri A. The search for perfect biomarkers in acute kidney damage: the case of NGAL, from AKI to acute pyelonephritis: back to the clinic? Nephrol Dial Transplant 2012;27:3665–6 |
| Pickering | 2013 | < 100 participants | Pickering JW, Ralib AM, Endre ZH. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care 2013;17:R7 |
| Pickering | 2013 | Not a relevant biomarker assay or test | Pickering JW, Endre ZH. Linking injury to outcome in acute kidney injury: a matter of sensitivity. PLOS ONE 2013;8:e62691 |
| Pickering | 2013 | Not a relevant biomarker assay or test | Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif 2013;35:295–302 |
| Piirainen | 2018 | < 100 participants | Piirainen A, Huopio J, Kokki H, Holopainen A, Pajunen T, Pulkki K, Kokki M. Novel renal markers for the assessment of renal integrity in patients undergoing knee arthroplasty – a pilot study. J Exp Orthop 2018;5:40 |
| Pilarczyk | 2015 | < 100 participants | Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, et al. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care 2015;5:1–11 |
| Pisano | 2018 | Not a primary study | Pisano DV, Joyce MF. Plasma neutrophil gelatinase-associated lipocalin: biomarker of the future or just another test? J Clin Anesth 2018;45:37–8 |
| Plebani | 2017 | Not a primary study | Plebani M. Biomarkers of acute kidney injury: a step forward. Clin Chem Lab Med 2017;55:1071–3 |
| Plewes | 2014 | Not a relevant type of population | Plewes K, Royakkers AA, Hanson J, Hasan MM, Alam S, Ghose A, et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J 2014;13:91 |
| Polat | 2013 | < 100 participants | Polat M, Fidan K, Derinöz O, Gönen S, Söylemezoglu O. Neutrophil gelatinase-associated lipocalin as a follow-up marker in critically ill pediatric patients with established acute kidney injury. Ren Fail 2013;35:352–6 |
| Poorshahbaz | 2015 | < 100 participants | Poorshahbaz F, Karami A, Jozpanahi M, Pezeshki A, Fagihzadeh S, Esmailzadeh A, et al. Comparison of changes in serum creatinine and PNGAL in predicting renal damage in brucellosis patients receiving gentamycin. Crescent J Medical Biol Sci 2015;2:116–20 |
| Portal | 2010 | < 100 participants | Portal AJ, McPhail MJ, Bruce M, Coltart I, Slack A, Sherwood R, et al. Neutrophil gelatinase – associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl 2010;16:1257–66 |
| Prabhu | 2010 | < 100 participants | Prabhu A, Sujatha DI, Ninan B, Vijayalakshmi MA. Neutrophil gelatinase associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg 2010;24:525–31 |
| Prasad | 2014 | Not a primary study | Prasad A. Acute kidney injury following contrast administration in pediatric congenital heart disease patients: time to move beyond the serum creatinine. Catheter Cardiovasc Interv 2014;84:620–1 |
| Prowle | 2014 | Not a primary study | Prowle JR, Kirwan CJ. Acute kidney injury after cardiac surgery: the injury that keeps on hurting? Crit Care Med 2014;42:2142–3 |
| Prowle | 2015 | Not a primary study | Prowle JR. Measurement of AKI biomarkers in the ICU: still striving for appropriate clinical indications. Intensive Care Med 2015;41:541–3 |
| Prowle | 2015 | < 100 participants | Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Renal Fail 2015;37:408–16 |
| Przybylowski | 2010 | No focus on DTA for AKI | Przybylowski P, Malyszko J, Malyszko JS. Serum neutrophil gelatinase-associated lipocalin correlates with kidney function in heart allograft recipients. Transplant Proc 2010;42:1797–802 |
| Przybylowski | 2011 | Not a relevant type of population | Przybylowski P, Koc-Zorawska E, Malyszko JS, Kozlowska S, Mysliwiec M, Malyszko J. Liver fatty-acid-binding protein in heart and kidney allograft recipients in relation to kidney function. Transplant Proc 2011;43:3064–7 |
| Puiac | 2017 | < 100 participants | Puiac C, Szederjesi J, Lazăr A, Bad C, Puşcaşiu L. Neutrophil gelatinase-associated lipocalin as a marker for renal dysfunction detection in critically ill patients with increased intraabdominal pressure. J Crit Care Med 2017;3:24–8 |
| Puthumana | 2017 | Meta-analysis – retained as background material | Puthumana J, Ariza X, Belcher JM, Graupera I, Ginès P, Parikh CR. Urine interleukin 18 and lipocalin 2 are biomarkers of acute tubular necrosis in patients with cirrhosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:1003–13.e3 |
| Pynn | 2015 | No focus on DTA for AKI | Pynn JM, Parravicini E, Saiman L, Bateman DA, Barasch JM, Lorenz JM. Urinary neutrophil gelatinase-associated lipocalin: potential biomarker for late-onset sepsis. Pediatr Res 2015;78:76–81 |
| Qasem | 2014 | Not a relevant biomarker assay or test | Qasem AA, Farag SE, Hamed E, Emara M, Bihery A, Pasha H. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. ISRN Nephrol 2014;2014:376795 |
| Qiao | 2015 | Not a relevant type of population | Qiao B, Deng J, Li Y, Wang X, Han Y. Rosuvastatin attenuated contrast-induced nephropathy in diabetes patients with renal dysfunction. Int J Clin Exp Med 2015;8:2342–9 |
| Quartin | 2013 | Not a primary study | Quartin A, Schein R, Cely C. Accuracy of plasma neutrophil gelatinase-associated lipocalin in the early diagnosis of contrast-induced acute kidney injury in critical illness. Intensive Care Med 2013;39:1670 |
| Quintavalle | 2015 | Not a relevant type of population | Quintavalle C, Anselmi CV, De Micco F, Roscigno G, Visconti G, Golia B, et al. Neutrophil gelatinase-associated lipocalin and contrast-induced acute kidney injury. Circ Cardiovasc Interv 2015;8:e002673 |
| Radovic | 2019 | No relevant outcome | Radovic M, Bojic S, Kotur-Stevuljevic J, Lezaic V, Milicic B, Velinovic M, et al. Serum lactate as reliable biomarker of acute kidney injury in low-risk cardiac surgery patients. J Med Biochem 2019;38:118–25 |
| Rafiei | 2015 | < 100 participants | Rafiei A, Mohammadjafari H, Bazi S, Mirabi AM. Urinary neutrophil gelatinase-associated lipocalin (NGAL) might be an independent marker for anticipating scar formation in children with acute pyelonephritis. J Renal Inj Prev 2015;4:39–44 |
| Raggal | 2013 | < 100 participants | Raggal NE, Khafagy SM, Mahmoud NH, Beltagy SE. Serum neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury in asphyxiated neonates. Indian Pediatr 2013;50:459–62 |
| Rakkolainen | 2016 | < 100 participants | Rakkolainen I, Vuola J. Plasma NGAL predicts early acute kidney injury no earlier than s-creatinine or cystatin C in severely burned patients. Burns 2016;42:322–8 |
| Ralib | 2011 | Not a primary study | Ralib AM, Pickering JW, Endre ZH. Predictor of early diagnosis, diagnosis, or progression of acute kidney injury. Ann Emerg Med 2011;57:75–6 |
| Ralib | 2012 | Not a relevant biomarker assay or test | Ralib AM, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, Endre ZH. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol 2012;23:322–33 |
| Ralib | 2014 | < 100 participants | Ralib AM, Pickering JW, Shaw GM, Than MP, George PM, Endre ZH. The clinical utility window for acute kidney injury biomarkers in the critically ill. Crit Care 2014;18:601 |
| Ralib | 2017 | Not a relevant type of population | Ralib AM, Nanyan S, Mat Nor MB. Dynamic changes of plasma neutrophil gelatinase-associated lipocalin predicted mortality in critically ill patients with systemic inflammatory response syndrome. Indian J Crit Care Med 2017;21:23–9 |
| Ramchandran | 2013 | Not a primary study | Ramachandran B. Acute kidney injury in critically ill children: More than just urine output. Indian J Crit Care Med 2013;17:203–4 |
| Rampoldi | 2018 | < 100 participants | Rampoldi B, Tessarolo S, Giubbilini P, Gaia P, Corino SD, Mazza S, et al. Neutrophil gelatinase-associated lipocalin and acute kidney injury in endovascular aneurysm repair or open aortic repair: a pilot study. Biochemia Medica 2018;28:010904 |
| Rauen | 2011 | Not a primary study | Rauen T, Weiskirchen R, Floege J. In search of early events in the development of chronic kidney disease: the emerging role for lipocalin-2/NGAL. Nephrol Dial Transplant 2011;26:445–7 |
| Redding | 2013 | Not a primary study | Redding S. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Ann Clin Biochem 2013;50:625–6 |
| Reiter | 2018 | < 100 participants | Reiter K, Balling G, Bonelli V, Pabst Von Ohain J, Braun SL, Ewert P, Ruf B. Neutrophil gelatinase-associated lipocalin reflects inflammation and is not a reliable renal biomarker in neonates and infants after cardiopulmonary bypass: a prospective case-control study. Cardiol Young 2018;28:243–51 |
| Renhua | 2014 | Not a relevant type of population | Renhua L, Miaolin C, Junlin W, Qingwei W, Xiaoping X, Huili D, et al. The level of the biomarkers at the time of nephrology consultation might predict the prognosis of acute kidney injury in hospitalized patients. Blood Purif 2014;38:89–95 |
| Rewa | 2015 | Not a relevant biomarker assay or test | Rewa O, Wald R, Adhikari NK, Hladunewich M, Lapinsky S, Muscedere J, et al. Whole-blood neutrophil gelatinase-associated lipocalin to predict adverse events in acute kidney injury: a prospective observational cohort study. J Crit Care 2015;30:1359–64 |
| Ribitsch | 2011 | Not a primary study | Ribitsch W, Rosenkranz AR. Biomarkers in acute kidney injury: a never ending story? Crit Care Med 2011;39:2570–1 |
| Ribitsch | 2017 | Not a relevant type of population | Ribitsch W, Schilcher G, Quehenberger F, Pilz S, Portugaller RH, Truschnig-Wilders M, et al. Neutrophil gelatinase-associated lipocalin (NGAL) fails as an early predictor of contrast induced nephropathy in chronic kidney disease (ANTI-CI-AKI study). Sci Rep 2017;7:41300 |
| Ricci | 2012 | Not a relevant biomarker assay or test | Ricci Z, Netto R, Garisto C, Iacoella C, Favia I, Cogo P. Whole blood assessment of neutrophil gelatinase-associated lipocalin versus pediatricRIFLE for acute kidney injury diagnosis and prognosis after pediatric cardiac surgery: cross-sectional study. Pediatr Crit Care Med 2012;13:667–70 |
| Ricci | 2015 | No focus on DTA for AKI | Ricci Z, Haiberger R, Pezzella C, Garisto C, Favia I, Cogo P. Furosemide versus ethacrynic acid in pediatric patients undergoing cardiac surgery: a randomized controlled trial. Crit Care 2015;19:2 |
| Roberts | 2011 | Not a relevant type of population | Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett 2011;202:69–74 |
| Robertson | 2019 | < 100 participants | Robertson FP, Yeung AC, Male V, Rahman S, Mallett S, Fuller BJ, Davidson BR. Urinary neutrophil gelatinase associated lipocalins (NGALs) predict acute kidney injury post liver transplant. HPB 2019;21:473–81 |
| Rocha | 2015 | < 100 participants | Rocha PN, Macedo MN, Kobayashi CD, Moreno L, Guimarães LH, Machado PR, et al. Role of urine neutrophil gelatinase-associated lipocalin in the early diagnosis of amphotericin B-induced acute kidney injury. Antimicrob Agents Chemother 2015;59:6913–21 |
| Ronco | 2008 | Not a primary study | Ronco C. NGAL: an emerging biomarker of acute kidney injury. Int J Artif Organs 2008;31:199–200 |
| Ronco | 2013 | Not a primary study | Ronco C. Kidney attack: overdiagnosis of acute kidney injury or comprehensive definition of acute kidney syndromes? Blood Purif 2013;36:65–8 |
| Ronco | 2017 | Not a primary study | Ronco C, Rizo-Topete L, Serrano-Soto M, Kashani K. Pro: Prevention of acute kidney injury: time for teamwork and new biomarkers. Nephrol Dial Transplant 2017;32:408–13 |
| Rostami | 2010 | Not a primary study | Rostami Z, Lessan-Pezeshki M. Role of NGAL for the early detection of acute kidney injury. Int J Nephrol Urol 2010;2:387–9 |
| Roudkenar | 2008 | < 100 participants | Roudkenar MH, Halabian R, Oodi A, Roushandeh AM, Yaghmai P, Najar MR, et al. Upregulation of neutrophil gelatinase-associated lipocalin, NGAL/Lcn2, in beta-thalassemia patients. Arch Med Res 2008;39:402–7 |
| Roudkenar | 2008 | No focus on DTA for AKI | Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, et al. Neutrophil gelatinase-associated lipocalin acts as a protective factor against H2O2 toxicity. Arch Med Res 2008;39:560–6 |
| Rouve | 2018 | < 100 participants | Rouve E, Lakhal K, Salmon GandonnièreC, Jouan Y, Bodet-Contentin L, Ehrmann S. Lack of impact of iodinated contrast media on kidney cell-cycle arrest biomarkers in critically ill patients. BMC Nephrol 2018;19:308 |
| Royakkers | 2012 | Not a relevant biomarker assay or test | Royakkers AA, Bouman CS, Stassen PM, Korevaar JC, Binnekade JM, van de Hoek W, et al. Systemic and urinary neutrophil gelatinase-associated lipocalins are poor predictors of acute kidney injury in unselected critically ill patients. Crit Care Res Pract 2012;2012:712695 |
| Ruf | 2015 | < 100 participants | Ruf B, Bonelli V, Balling G, Hörer J, Nagdyman N, Braun SL, et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit Care 2015;19:27 |
| Saad | 2016 | < 100 participants | Saad A, Wang W, Herrmann SM, Glockner JF, Mckusick MA, Misra S, et al. Atherosclerotic renal artery stenosis is associated with elevated cell cycle arrest markers related to reduced renal blood flow and postcontrast hypoxia. Nephrol Dial Transplant 2016;31:1855–63 |
| Sagatov | 2019 | No focus on DTA for AKI | Sagatov IY, Medeubekov US. Dynamics of urine neutrophil gelatinase-associated lipocalin in cardiac surgery patients in the near term after surgery. Chirurgia 2019;32:59–61 |
| Saleena | 2015 | Not a relevant type of population | Saleena UV, Nalini K, Gopalakrishna K, Prabhu R, Vadhiraja BM, Athiyamaan MS, et al. Early prediction of cisplatin nephrotoxicity in head and neck cancer patients – an evaluation with urinary biomarkers. Int J Pharm Sci Res 2015;6:2893–901 |
| Saleh | 2017 | Not a relevant biomarker assay or test | Saleh NY, Abo El Fotoh WMM, El-Hawy MA. Serum Neutrophil gelatinase-associated lipocalin: a diagnostic marker in pediatric sepsis. Pediatr Crit Care Med 2017;18:e245–e252 |
| Sarafidis | 2012 | < 100 participants | Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti E, Taparkou A, Soubasi V, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol 2012;27:1575–82 |
| Sarafidis | 2014 | < 100 participants | Sarafidis K, Tsepkentzi E, Diamanti E, Agakidou E, Taparkou A, Soubasi V, et al. Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr Nephrol 2014;29:305–10 |
| Sargentini | 2012 | < 100 participants | Sargentini V, Mariani P, D’Alessandro M, Pistolesi V, Lauretta MP, Pacini F, et al. Assessment of NGAL as an early biomarker of acute kidney injury in adult cardiac surgery patients. J Biol Regul Homeost Agents 2012;26:485–93 |
| Sarlak | 2014 | Not a primary study | Sarlak H, Dinc M, Balta S, Cakar M, Arslan E, Demirbas S. Early detection of urinary NGAL and plasma CysC may prevent progression to overt acute renal failure. Swiss Med Wkly 2014;144:w13949 |
| Sarnak | 2014 | Not a relevant type of population | Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, et al. Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol 2014;25:1545–53 |
| Satirapoj | 2016 | Not a relevant type of population | Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel tubular biomarkers predict renal progression in type 2 diabetes mellitus: a prospective cohort study. J Diabetes Res 2016;2016:3102962 |
| Savran Karadeniz | 2019 | < 100 participants | Savran Karadeniz M, Alp Enişte I, Şentürk Çiftçi H, Usta S, Tefik T, Şanlı Ö, et al. Neutrophil gelatinase-associated lipocalin significantly correlates with ischemic damage in patients undergoing laparoscopic partial nephrectomy. Balkan Med J 2019;36:121–8 |
| Saydam | 2018 | < 100 participants | Saydam O, Türkmen E, Portakal O, Arıcı M, Doğan R, Demircin M, et al. Emerging biomarker for predicting acute kidney injury after cardiac surgery: cystatin C. Turk J Med Sci 2018;48:1096–103 |
| Sayed | 2015 | < 100 participants | Sayed S, Idriss NK, Blann A, Sayyed HG, Raafat DM, Fouad D, Tawfeek MS. The number of GT(n) repeats in the hemeoxygenase-1 gene promoter is increased in pediatric heart failure but is unrelated to renal, antioxidant and anti-inflammatory markers. Pediatr Cardiol 2015;36:1204–11 |
| Scazzochio | 2014 | < 100 participants | Scazzochio E, Munmany M, Garcia L, Meler E, Crispi F, Gratacos E, Figueras F. Prognostic role of maternal neutrophil gelatinase-associated lipocalin in women with severe early-onset preeclampsia. Fetal Diagn Ther 2014;35:127–32 |
| Schanz | 2017 | < 100 participants | Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017;40:485–91 |
| Schanz | 2018 | Not a relevant type of population | Schanz M, Wasser C, Allgaeuer S, Schricker S, Dippon J, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant 2018;34:1902–9 |
| Schanz | 2017 | < 100 participants | Schanz M, Hoferer A, Shi J, Alscher MD, Kimmel M. Urinary TIMP2.IGFBP7 for the prediction of platinum-induced acute renal injury. Int J Nephrol Renovasc Dis 2017;10:175–81 |
| Schaub | 2015 | No focus on DTA for AKI | Schaub JA, Garg AX, Coca SG, Testani JM, Shlipak MG, Eikelboom J, et al. Perioperative heart-type fatty acid binding protein is associated with acute kidney injury after cardiac surgery. Kidney Int 2015;88:576–83 |
| Schetz | 2018 | Not a primary study | Schetz M, Prowle J. Focus on acute kidney injury 2017. Intensive Care Med 2018;44:1992–4 |
| Schilcher | 2011 | Not a primary study | Schilcher G, Ribitsch W, Otto R, Portugaller RH, Quehenberger F, Truschnig-Wilders M, et al. Early detection and intervention using neutrophil gelatinase-associated lipocalin (NGAL) may improve renal outcome of acute contrast media induced nephropathy: a randomized controlled trial in patients undergoing intra-arterial angiography (ANTI-CIN Study). BMC Nephrol 2011;12:39 |
| Schinstock | 2013 | No relevant outcome | Schinstock CA, Semret MH, Wagner SJ, Borland TM, Bryant SC, Kashani KB, et al. Urinalysis is more specific and urinary neutrophil gelatinase-associated lipocalin is more sensitive for early detection of acute kidney injury. Nephrol Dial Transplant 2013;28:1175–85 |
| Schley | 2015 | Poster presentation – may be the same study as Schley61 | Schley G, Koberle C, Manuilova E, Rutz S, Kientsch-Engel R, Eckardt KU, Willam C. Comparative analysis of diagnostic and predictive performance of novel renal biomarkers in plasma and urine of acute kidney injury patients. Intensive Care Med Exp 2015;3(Suppl. 1):A258 |
| Schneider | 2013 | Not a primary study | Schneider AG, Bellomo R. Acute kidney injury: new studies. Intensive Care Med 2013;39:569–71 |
| Schneider | 2018 | Not a primary study | Schneider AG, Mongardon N, Muller L. Biomarkers of renal injury, time for a grey-zone approach? Anaesth Crit Care Pain Med 2018;37:307–9 |
| Schutz | 2017 | Not a relevant type of population | Schutz C, Boulware DR, Huppler-Hullsiek K, von Hohenberg M, Rhein J, Taseera K, et al. Acute kidney injury and urinary biomarkers in human immunodeficiency virus-associated cryptococcal meningitis. Open Forum Infect Dis 2017;4:ofx127 |
| Seibert | 2013 | < 100 participants | Seibert FS, Pagonas N, Arndt R, Heller F, Dragun D, Persson P, et al. Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol 2013;207:700–8 |
| Seibert | 2018 | No focus on DTA for AKI | Seibert FS, Sitz M, Passfall J, Haesner M, Laschinski P, Buhl M, et al. Prognostic value of urinary calprotectin, NGAL and KIM-1 in chronic kidney disease. Kidney Blood Press Res 2018;43:1255–62 |
| Se-Jun | 2017 | < 100 participants | Se-Jun P, Hoseok KOO, Kyoung-Jin LEE, Seo-Hyun KIM, Seo-Young YUN, Seunghyup KIM, et al. Usefulness of neutrophil gelatinase-associated lipocalin (NGAL) to confirm subclinical acute kidney injury and renal prognosis in patients following surgery. Kosin Med J 2017;23:212–20 |
| Seker | 2015 | < 100 participants | Seker MM, Deveci K, Seker A, Sancakdar E, Yilmaz A, Turesin AK, et al. Predictive role of neutrophil gelatinase-associated lipocalin in early diagnosis of platin-induced renal injury. Asian Pac J Cancer Prev 2015;16:407–10 |
| Self | 2010 | Not a primary study | Self WH, Barrett TW. Novel biomarkers: help or hindrance to patient care in the emergency department? Ann Emerg Med 2010;56:60–1 |
| Sellmer | 2017 | No focus on DTA for AKI | Sellmer A, Bech BH, Bjerre JV, Schmidt MR, Hjortdal VE, Esberg G, et al. Urinary neutrophil gelatinase-associated lipocalin in the evaluation of patent ductus arteriosus and AKI in very preterm neonates: a cohort study. BMC Pediatr 2017;17:7 |
| Sen | 2015 | < 100 participants | Sen S, Godwin ZR, Palmieri T, Greenhalgh D, Steele AN, Tran NK. Whole blood neutrophil gelatinase-associated lipocalin predicts acute kidney injury in burn patients. J Surg Res 2015;196:382–7 |
| Şen | 2015 | Not a relevant type of population | Şen V, Ece A, Uluca Ü, Söker M, Güneş A, Kaplan İ, et al. Urinary early kidney injury molecules in children with beta-thalassemia major. Ren Fail 2015;37:607–13 |
| Seo | 2014 | < 100 participants | Seo WH, Nam SW, Lee EH, Je BK, Yim HE, Choi BM. A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. Eur J Pediatr 2014;173:229–32 |
| Shahbazi | 2015 | Not a relevant type of population | Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M, Abdollahi A, Ghahremani MH. Effect of silymarin administration on cisplatin nephrotoxicity: report from a pilot, randomized, double-blinded, placebo-controlled clinical trial. Phytother Res 2015;29:1046–53 |
| Shahbazi | 2015 | Not a relevant type of population | Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M. Urine ratio of neutrophil gelatinase-associated lipocalin to creatinine as a marker for early detection of cisplatin-associated nephrotoxicity. Iran J Kidney Dis 2015;9:305–10 |
| Shaker | 2010 | < 100 participants | Shaker OG, El-Shehaby A, El-Khatib M. Early diagnostic markers for contrast nephropathy in patients undergoing coronary angiography. Angiology 2010;61:731–6 |
| Shaker | 2018 | < 100 participants | Shaker AM, El Mohamed E, Samir HH, Elnokeety MM, Sayed HA, Ramzy TA. Fibroblast growth factor-23 as a predictor biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl 2018;29:531–9 |
| Shao | 2017 | Not a relevant type of population | Shao Y, Fan Y, Xie Y, Yin L, Zhang Y, Deng L, et al. Effect of continuous renal replacement therapy on kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin in patients with septic acute kidney injury. Exp Ther Med 2017;13:3594–602 |
| Shapiro | 2010 | Not a relevant biomarker assay or test | Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. The diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med 2010;56:52–9.e1 |
| Sharma | 2017 | Not a relevant type of population | Sharma A, Demissei BG, Tromp J, Hillege HL, Cleland JG, O’Connor CM, et al. A network analysis to compare biomarker profiles in patients with and without diabetes mellitus in acute heart failure. Eur J Heart Fail 2017;19:1310–20 |
| Shavit | 2011 | < 100 participants | Shavit L, Dolgoker I, Ivgi H, Assous M, Slotki I. Neutrophil gelatinase-associated lipocalin as a predictor of complications and mortality in patients undergoing non-cardiac major surgery. Kidney Blood Press Res 2011;34:116–24 |
| Shavit | 2013 | Not a relevant type of population | Shavit L, Manilov R, Wiener-Well Y, Algur N, Slotki I. Urinary neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury in geriatric patients with urinary tract infection treated by colistin. Clin Nephrol 2013;80:405–16 |
| Shaw | 2011 | Cost-effectiveness – retained as background material | Shaw AD, Chalfin DB, Kleintjens J. The economic impact and cost-effectiveness of urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Clin Ther 2011;33:1713–25 |
| Shema-Didi | 2016 | Not a relevant type of population | Shema-Didi L, Kristal B, Eizenberg S, Marzuq N, Sussan M, Feldman-Idov Y, et al. Prevention of contrast-induced nephropathy with single bolus erythropoietin in patients with diabetic kidney disease: a randomized controlled trial. Nephrology 2016;21:295–300 |
| Shen | 2014 | < 100 participants | Shen SJ, Hu ZX, Li QH, Wang SM, Song CJ, Wu DD, et al. Implications of the changes in serum neutrophil gelatinase-associated lipocalin and cystatin C in patients with chronic kidney disease. Nephrology 2014;19:129–35 |
| Shin | 2017 | < 100 participants | Shin SY, Ha JY, Lee SL, Lee WM, Park JH. Increased urinary neutrophil gelatinase-associated lipocalin in very-low-birth-weight infants with oliguria and normal serum creatinine. Pediatr Nephrol 2017;32:1059–65 |
| Shinke | 2015 | Not a relevant type of population | Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, Sato T, et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol 2015;76:989–96 |
| Shirakabe | 2015 | Not a relevant biomarker assay or test | Shirakabe A, Hata N, Kobayashi N, Okazaki H, Shinada T, Tomita K, et al. Serum heart-type fatty acid-binding protein level can be used to detect acute kidney injury on admission and predict an adverse outcome in patients with acute heart failure. Circ J 2015;79:119–28 |
| Shirakabe | 2019 | Not a relevant biomarker assay or test | Shirakabe A, Hata N, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. Worsening renal failure in patients with acute heart failure: the importance of cardiac biomarkers. ESC Heart Fail 2019;6:416–27 |
| Shlipak | 2012 | Not a relevant type of population | Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 2012;61:565–73 |
| Shoaib | 2019 | < 100 participants | Shoaib M, Mahmud SN, Safdar M. Early diagnosis of acute kidney injury by urinary neutrophil gelatinase associated lipocalin in adult critically ill patients. J Ayub Med Coll Abbottabad 2019;31:12–15 |
| Shrestha | 2011 | No relevant outcome | Shrestha K, Borowski AG, Troughton RW, Thomas JD, Klein AL, Tang WH. Renal dysfunction is a stronger determinant of systemic neutrophil gelatinase-associated lipocalin levels than myocardial dysfunction in systolic heart failure. J Card Fail 2011;17:472–8 |
| Shrestha | 2012 | < 100 participants | Shrestha K, Shao Z, Singh D, Dupont M, Tang WH. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol 2012;110:1329–35 |
| Shukla | 2017 | Not a relevant type of population | Shukla A, Rai MK, Prasad N, Agarwal V. Short-term non-steroid anti-inflammatory drug use in spondyloarthritis patients induces subclinical acute kidney injury: biomarkers study. Nephron 2017;135:277–86 |
| Shulkina | 2016 | < 100 participants | Shulkina SG, Schekotov VV, Smirnova EN, Antipova AA. Vascular endothelial growth factor and lipocalin-2 as markers of early nephron damage in patients with hypertension and obesity. Sovrem Tehnologii Med 2016;8:148–51 |
| Shum | 2015 | Not a relevant biomarker assay or test | Shum HP, Leung NY, Chang LL, Tam OY, Kwan AM, Chan KC, et al. Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care unit patients after major non-cardiac surgery. Nephrology 2015;20:375–82 |
| Shyam | 2017 | < 100 participants | Shyam R, Patel ML, Sachan R, Kumar S, Pushkar DK. Role of urinary neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury in patients with circulatory shock. Indian J Crit Care Med 2017;21:740–5 |
| Nga | 2015 | Not a relevant biomarker assay or test | Nga HS, Medeiros P, Menezes P, Bridi R, Balbi A, Ponce D. Sepsis and AKI in clinical emergency room patients: the role of urinary NGAL. Biomed Res Int 2015;2015:413751 |
| Nga | 2015 | Not a relevant biomarker assay or test | Nga HS, Medeiros P, Menezes P, Bridi R, Balbi A, Ponce D. Sepsis and AKI in clinical emergency room patients: the role of urinary NGAL. Biomed Res Int 2015;2015:413751 |
| Siddappa | 2019 | < 100 participants | Siddappa PK, Kochhar R, Sarotra P, Medhi B, Jha V, Gupta V. Neutrophil gelatinase-associated lipocalin: an early biomarker for predicting acute kidney injury and severity in patients with acute pancreatitis. JGH Open 2019;3:105–10 |
| Sidoti | 2014 | < 100 participants | Sidoti A, Giacalone M, Abramo A, Anselmino M, Donadio C, Salvo CD, et al. Early identification of acute kidney injury after bariatric surgery: role of NGAL and cystatin C. Open Obes J 2014;6:50–9 |
| Siew | 2009 | Not a relevant biomarker assay or test | Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 2009;20:1823–32 |
| Siew | 2010 | Not a relevant biomarker assay or test | Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 2010;5:1497–505 |
| Siew | 2013 | Not a relevant biomarker assay or test | Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, et al. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int 2013;84:786–94 |
| Silvetti | 2014 | < 100 participants | Silvetti S, Meroni R, Bignami E, Bove T, Landoni G, Zangrillo A, et al. Preoperative urinary neutrophil gelatinase-associated lipocalin and outcome in high-risk heart failure patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:323–7 |
| Sim | 2015 | Not a relevant type of population | Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatr Res 2015;78:48–55 |
| Simonazzi | 2015 | < 100 participants | Simonazzi G, Capelli I, Curti A, Comai G, Rizzo N, La Manna G. Serum and urinary neutrophil gelatinase-associated lipocalin monitoring in normal pregnancy versus pregnancies complicated by pre-eclampsia. In Vivo 2015;29:117–21 |
| Singal | 2018 | No focus on DTA for AKI | Singal AK, Jackson B, Pereira GB, Russ KB, Fitzmorris PS, Kakati D, et al. Biomarkers of renal injury in cirrhosis: association with acute kidney injury and recovery after liver transplantation. Nephron 2018;138:1–12 |
| Singer | 2016 | Not a relevant type of population | Singer E, Schrezenmeier EV, Elger A, Seelow ER, Krannich A, Luft FC, Schmidt-Ott KM. Urinary NGAL-positive acute kidney injury and poor long-term outcomes in hospitalized patients. Kidney Int Rep 2016;1:114–24 |
| Balbir Singh | 2016 | Not a relevant type of population | Balbir Singh G, Ann SH, Park J, Chung HC, Lee JS, Kim ES, et al. Remote Ischemic Preconditioning for the prevention of contrast-induced acute kidney injury in diabetics receiving elective percutaneous coronary intervention. PLOS ONE 2016;11:e0164256 |
| Singh | 2018 | Not a relevant type of population | Singh A, Kamal R, Tiwari R, Gaur VK, Bihari V, Satyanarayana GNV, et al. Association between PAHs biomarkers and kidney injury biomarkers among kitchen workers with microalbuminuria: a cross-sectional pilot study. Clin Chim Acta 2018;487:349–56 |
| Sinna | 2019 | < 100 participants | Sinna MM, Altaf FM, Mosa OF. Serum and urinary NGAL and cystatin C levels as diagnostic tools for for acute kidney injury and chronic kidney disease: a histobiochemical comparative study. Curr Pharm Des 2019;25:1122–33 |
| Sirisopha | 2016 | < 100 participants | Sirisopha A, Vanavanan S, Chittamma A, Phakdeekitcharoen B, Thakkinstian A, Lertrit A, et al. Effects of therapy on urine neutrophil gelatinase-associated lipocalin in nondiabetic glomerular diseases with proteinuria. Int J Nephrol 2016;2016:4904502 |
| Sirota | 2013 | < 100 participants | Sirota JC, Walcher A, Faubel S, Jani A, McFann K, Devarajan P, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol 2013;14:17 |
| Sise | 2009 | Not a primary study | Sise ME, Barasch J, Devarajan P, Nickolas TL. Elevated urine neutrophil gelatinase-associated lipocalin can diagnose acute kidney injury in patients with chronic kidney diseases. Kidney Int 2009;75:115–16 |
| Slack | 2013 | < 100 participants | Slack AJ, McPhail MJ, Ostermann M, Bruce M, Sherwood R, Musto R, et al. Predicting the development of acute kidney injury in liver cirrhosis – an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther 2013;37:989–97 |
| Smertka | 2014 | < 100 participants | Smertka M, Wroblewska J, Suchojad A, Majcherczyk M, Jadamus-Niebroj D, Owsianka-Podlesny T, et al. Serum and urinary NGAL in septic newborns. BioMed Res Int 2014;2014:717318 |
| Sokolski | 2017 | Not a relevant biomarker assay or test | Sokolski M, Zymliński R, Biegus J, Siwołowski P, Nawrocka-Millward S, Todd J, et al. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur J Heart Fail 2017;19:760–7 |
| Solak | 2015 | Not a relevant biomarker assay or test | Solak Y, Yilmaz MI, Siriopol D, Saglam M, Unal HU, Yaman H, et al. Serum neutrophil gelatinase-associated lipocalin is associated with cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2015;47:1993–2001 |
| Song | 2017 | Meta-analysis – retained as background material | Song Z, Ma Z, Qu K, Liu S, Niu W, Lin T. Diagnostic prediction of urinary [TIMP-2] x [IGFBP7] for acute kidney injury: a meta-analysis exploring detection time and cutoff levels. Oncotarget 2017;8:100631–100639 |
| Song | 2017 | Not a relevant type of population | Song Y, Sun S, Yu Y, Li G, Song J, Zhang H, Yan C. Diagnostic value of neutrophil gelatinase-associated lipocalin for renal injury in asphyxiated preterm infants. Exp Ther Med 2017;13:1245–8 |
| Song | 2019 | No focus on DTA for AKI | Song Y, Kim DH, Kwon TD, Han DW, Baik SH, Jung HH, Kim JY. Effect of intraoperative dexmedetomidine on renal function after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a randomized, placebo-controlled trial. Int J Hyperthermia 2019;36:1–8 |
| Soto | 2013 | Not a relevant biomarker assay or test | Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol 2013;8:2053–63 |
| Soto | 2016 | Not a relevant biomarker assay or test | Soto K, Campos P, Pinto I, Rodrigues B, Frade F, Papoila AL, Devarajan P. The risk of chronic kidney disease and mortality are increased after community-acquired acute kidney injury. Kidney Int 2016;90:1090–9 |
| Souza | 2015 | Not a relevant type of population | Souza DF, Reis SS, Botelho RV, Ferreira-Filho SR. Relative and absolute changes in urinary neutrophil gelatinase-associated lipocalin and correlation with small increases in serum creatinine levels after coronary angiography: an observational study. Nephron 2015;129:84–90 |
| Soyler | 2015 | Not a relevant biomarker assay or test | Soyler C, Tanriover MD, Ascioglu S, Aksu NM, Arici M. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;37:772–6 |
| Spasojević-Dimitrijeva | 2017 | Not a relevant type of population | Spasojević-Dimitrijeva B, Kotur-Stevuljević J, Đukić M, Paripović D, Miloševski-Lomić G, Spasojević-Kalimanovska V, et al. Serum neutrophil gelatinase-associated lipocalin and urinary kidney injury molecule-1 as potential biomarkers of subclinical nephrotoxicity after gadolinium-based and iodinated-based contrast media exposure in pediatric patients with normal kidney function. Med Sci Monit 2017;23:4299–305 |
| Sporek | 2016 | < 100 participants | Sporek M, Gala-Błądzińska A, Dumnicka P, Mazur-Laskowska M, Kielczewski S, Walocha J, et al. Urine NGAL is useful in the clinical evaluation of renal function in the early course of acute pancreatitis. Folia Med Cracov 2016;56:13–25 |
| Sprenkle | 2013 | No focus on DTA for AKI | Sprenkle PC, Wren J, Maschino AC, Feifer A, Power N, Ghoneim T, et al. Urine neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after kidney surgery. J Urol 2013;190:159–64 |
| Srisawat | 2011 | Not a relevant type of population | Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA, Genetic and Inflammatory Markers of Sepsis (GenIMS) Study Investigators. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int 2011;80:545–52 |
| Srisawat | 2015 | Not a relevant type of population | Srisawat N, Praditpornsilpa K, Patarakul K, Techapornrung M, Daraswang T, Sukmark T, et al. Neutrophil gelatinase associated lipocalin (NGAL) in leptospirosis acute kidney injury: a multicenter study in Thailand. PLOS ONE 2015;10:e0143367 |
| Srisawat | 2018 | < 100 participants | Srisawat N, Laoveeravat P, Limphunudom P, Lumlertgul N, Peerapornratana S, Tiranathanagul K, et al. The effect of early renal replacement therapy guided by plasma neutrophil gelatinase associated lipocalin on outcome of acute kidney injury: a feasibility study. J Crit Care 2018;43:36–41 [4627] |
| Srisawat | 2018 | < 100 participants | Srisawat N, Kongwibulwut M, Laoveeravat P, Lumplertgul N, Chatkaew P, Saeyub P, et al. The role of intraoperative parameters on predicting laparoscopic abdominal surgery associated acute kidney injury. BMC Nephrol 2018;19:289 |
| Srisawat | 2018 | < 100 participants | Srisawat N, Laoveeravat P, Limphunudom P, Lumlertgul N, Peerapornratana S, Tiranathanagul K, et al. The effect of early renal replacement therapy guided by plasma neutrophil gelatinase associated lipocalin on outcome of acute kidney injury: a feasibility study. J Crit Care 2018;43:36–41 |
| Srisawat | 2018 | Not a primary study | Srisawat N, Tangvoraphonkchai K, Lumlertgul N, Tungsanga K, Eiam-Ong S. Role of acute kidney injury biomarkers to guide renal replacement therapy initiation, what we learn from EARLY-RRT trial and FST trial? J Thorac Dis 2018;10:E835–E838 |
| Stads | 2019 | < 100 participants | Stads S, Kant KM, de Jong MFC, de Ruijter W, Cobbaert CM, Betjes MGH, et al. Predictors of short-term successful discontinuation of continuous renal replacement therapy: results from a prospective multicentre study. BMC Nephrol 2019;20:129 |
| Sterling | 2017 | < 100 participants | Sterling M, Al-Ismaili Z, McMahon KR, Piccioni M, Pizzi M, Mottes T, et al. Urine biomarkers of acute kidney injury in noncritically ill, hospitalized children treated with chemotherapy. Pediatr Blood Cancer 2017;64:e26538 |
| Stewart | 2015 | < 100 participants | Stewart IJ, Glass KR, Howard JT, Morrow BD, Sosnov JA, Siew ED, et al. The potential utility of urinary biomarkers for risk prediction in combat casualties: a prospective observational cohort study. Crit Care 2015;19:252 |
| Strazzulla | 2016 | < 100 participants | Strazzulla A, Coppolino G, Di Fatta C, Giancotti F, D’Onofrio G, Postorino MC, et al. Is neutrophil gelatinase associated lipocalin useful in hepatitis C virus infection? World J Hepatol 2016;8:815–24 |
| Stypmann | 2015 | No focus on DTA for AKI | Stypmann J, Fobker M, Rosing K, Engelen M, Gunia S, Dell’Aquila AM, Nofer JR. Neutrophil gelatinase-associated lipocalin (NGAL) in heart transplant recipients after conversion to everolimus therapy. J Cardiol 2015;66:347–52 |
| Su | 2017 | Meta-analysis – retained as background material | Su Y, Gong Z, Wu Y, Tian Y, Liao X. Diagnostic value of urine tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for acute kidney injury: a meta-analysis. PLOS ONE 2017;12:e0170214 |
| Su | 2018 | Meta-analysis – retained as background material | Su LJ, Li YM, Kellum JA, Peng ZY. Predictive value of cell cycle arrest biomarkers for cardiac surgery-associated acute kidney injury: a meta-analysis. Br J Anaesth 2018;121:350–7 |
| Suchojad | 2015 | < 100 participants | Suchojad A, Tarko A, Smertka M, Majcherczyk M, Brzozowska A, Wroblewska J, Maruniak-Chudek I. Factors limiting usefulness of serum and urinary NGAL as a marker of acute kidney injury in preterm newborns. Ren Fail 2015;37:439–45 |
| Sueud | 2019 | < 100 participants | Sueud T, Hadi NR, Abdulameer R, Jamil DA, Al-Aubaidy HA. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diabetes Metab Syndr 2019;13:564–8 |
| Sumida | 2014 | < 100 participants | Sumida M, Doi K, Kinoshita O, Kimura M, Ono M, Hamasaki Y, et al. Perioperative plasma neutrophil gelatinase-associated lipocalin measurement in patients who undergo left ventricular assist device implantation surgery. Circ J 2014;78:1891–9 |
| Sun | 2017 | Not a relevant type of population | Sun IO, Shin SH, Cho AY, Yoon HJ, Chang MY, Lee KY. Clinical significance of NGAL and KIM-1 for acute kidney injury in patients with scrub typhus. PLOS ONE 2017;12:e0175890 |
| Surmiak | 2015 | < 100 participants | Surmiak P, Baumert M, Fiala M, Walencka Z, Więcek A. Umbilical neutrophil gelatinase-associated lipocalin level as an early predictor of acute kidney injury in neonates with hypoplastic left heart syndrome. Biomed Res Int 2015;2015:360209 |
| Suzuki | 2008 | < 100 participants | Suzuki M, Wiers KM, Klein-Gitelman MS, Haines KA, Olson J, Onel KB, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol 2008;23:403–12 |
| Sweetman | 2016 | < 100 participants | Sweetman DU, Onwuneme C, Watson WR, O’Neill A, Murphy JF, Molloy EJ. Renal function and novel urinary biomarkers in infants with neonatal encephalopathy. Acta Paediatr 2016;105:e513–e519 |
| Szeto | 2010 | < 100 participants | Szeto CC, Kwan BC, Lai KB, Lai FM, Chow KM, Wang G, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol 2010;5:2329–37 |
| Szewczyk | 2009 | < 100 participants | Szewczyk M, Wielkoszyński T, Zakliczyński M, Zembala M. Plasma neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine, and glomerular filtration rate in patients after heart and lung transplantation. Transplant Proc 2009;41:3242–3 |
| Taghizadeh-Ghehi | 2015 | < 100 participants | Taghizadeh-Ghehi M, Sarayani A, Ashouri A, Ataei S, Moslehi A, Hadjibabaie M. Urine neutrophil gelatinase associated lipocalin as an early marker of acute kidney injury in hematopoietic stem cell transplantation patients. Ren Fail 2015;37:994–8 |
| Tai | 2020 | Meta-analysis – retained as background material | Tai Q, Yi H, Wei X, Xie W, Zeng O, Zheng D, et al. The accuracy of urinary TIMP-2 and IGFBP7 for the diagnosis of cardiac surgery-associated acute kidney injury: a systematic review and meta-analysis. J Intensive Care Med 2020;35:1013–25 |
| Takahashi | 2016 | < 100 participants | Takahashi G, Shibata S, Fukui Y, Okamura Y, Inoue Y. Diagnostic accuracy of procalcitonin and presepsin for infectious disease in patients with acute kidney injury. Diagn Microbiol Infect Dis 2016;86:205–10 |
| Tamimi | 2018 | < 100 participants | Tamimi A, Kord E, Rappaport YH, Cooper A, Abu Hamad R, Efrati S, et al. Salivary neutrophil gelatinase-associated lipocalin sampling feasibility in acute renal colic. J Endourol 2018;32:566–71 |
| Tanigasalam | 2016 | Not a relevant biomarker assay or test | Tanigasalam V, Bhat BV, Adhisivam B, Sridhar MG, Harichandrakumar KT. Predicting severity of acute kidney injury in term neonates with perinatal asphyxia using urinary neutrophil gelatinase associated lipocalin. Indian J Pediatr 2016;83:1374–8 |
| Tanzil | 2016 | < 100 participants | Tanzil WL, Wilar R, Mantik MFJ, Umboh A, Tatura SNN. Comparison of urine neutrophil gelatinase-associated lipocalin to serum creatinine to assess kidney function in neonatal asphyxia. Paediatr Indones 2016;56:356–9 |
| Tasanarong | 2013 | Not a relevant type of population | Tasanarong A, Hutayanon P, Piyayotai D. Urinary neutrophil gelatinase-associated lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol 2013;14:270 |
| Tavakoli | 2018 | Not a primary study | Tavakoli R, Lebreton G. Biomarkers for early detection of cardiac surgery-associated acute kidney injury. J Thorac Dis 2018;10(Suppl. 33):S3914–18 |
| Tawfik | 2015 | < 100 participants | Tawfik Y, Shaat RM, El-Bassiony SR, Hawas S, Effat N. Urinary and serum neutrophil gelatinase-associated lipocalin as a biomarker in Egyptian systemic lupus erythematosus patients: relation to lupus nephritis and disease activity. Egypt Rheumatol 2015;37:S25–S31 |
| ter Maaten | 2016 | No focus on DTA for AKI | ter Maaten JM, Valente MAE, Metra M, Bruno N, O’Connor CM, Ponikowski P, et al. A combined clinical and biomarker approach to predict diuretic response in acute heart failure. Clin Res Cardiol 2016;105:145–53 |
| Torres-Salido | 2014 | Not a relevant type of population | Torres-Salido MT, Cortés-Hernández J, Vidal X, Pedrosa A, Vilardell-Tarres M, Ordi-Ros J. Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis. Nephrol Dial Transplant 2014;29:1740–9 |
| Testani | 2013 | Not a primary study | Testani JM, Tang WH. Biomarkers of acute kidney injury in chronic heart failure: what do the signals mean? JACC Heart Fail 2013;1:425–6 |
| Tiranathanagul | 2013 | < 100 participants | Tiranathanagul K, Amornsuntorn S, Avihingsanon Y, Srisawat N, Susantitaphong P, Praditpornsilpa K, et al. Potential role of neutrophil gelatinase-associated lipocalin in identifying critically ill patients with acute kidney injury stage 2–3 who subsequently require renal replacement therapy. Ther Apher Dial 2013;17:332–8 |
| Tkaczyk | 2018 | < 100 participants | Tkaczyk M, Tomczyk D, Jander A, Góreczny S, Moszura T, Dryżek P, et al. Glomerular filtration decrease after diagnostic cardiac catheterisation in children with congenital cardiac malformation – the role of serum creatinine, cystatin C, neutrophil gelatinase and urine output monitoring. Postepy Kardiol Interwencyjnej 2018;14:67–74 |
| Tomczyk | 2016 | < 100 participants | Tomczyk D, Jander A, Chrul S, Moszura T, Dryzek P, Krajewski W, et al. Contrast-induced acute kidney injury in children with cardiovascular defects – results of a pilot study. Pediatria i Med Rodz 2016;12:436–44 |
| Tong | 2015 | Meta-analysis – retained as background material | Tong J, Li H, Zhang H, Luo Z, Huang Y, Huang J, et al. Neutrophil gelatinase-associated lipocalin in the prediction of contrast-induced nephropathy: a systemic review and meta-analysis. J Cardiovasc Pharmacol 2015;66:239–45 |
| Toprak | 2017 | Not a relevant type of population | Toprak Z, Cebeci E, Helvaci SA, Toprak ID, Kutlu Y, Sakin A, Tukek T. Cisplatin nephrotoxicity is not detected by urinary cell-cycle arrest biomarkers in lung cancer patients. Int Urol Nephrol 2017;49:1041–7 |
| Torregrosa | 2012 | Not a relevant biomarker assay or test | Torregrosa I, Montoliu C, Urios A, Elmlili N, Juan I, Puchades MJ, et al. Early biomarkers of acute kidney failure after heart angiography or heart surgery in patients with acute coronary syndrome or acute heart failure. Nefrologia 2012;32:44–52 |
| Torregrosa | 2015 | Not a relevant type of population | Torregrosa I, Montoliu C, Urios A, Andrés-Costa MJ, Giménez-Garzó C, Juan I, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels 2015;30:703–11 |
| Trachtman | 2006 | < 100 participants | Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, et al. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol 2006;21:989–94 [4024] |
| Tsuchimoto | 2014 | < 100 participants | Tsuchimoto A, Shinke H, Uesugi M, Kikuchi M, Hashimoto E, Sato T, et al. Urinary neutrophil gelatinase-associated lipocalin: a useful biomarker for tacrolimus-induced acute kidney injury in liver transplant patients. PLOS ONE 2014;9:e110527 |
| Tugba Kos | 2013 | < 100 participants | Tugba Kos F, Sendur MAN, Aksoy S, Celik HT, Sezer S, Civelek B, et al. Evaluation of renal function using the level of neutrophil gelatinase-associated lipocalin is not predictive of nephrotoxicity associated with cisplatin-based chemotherapy. Asian Pac J Cancer Prev 2013;14:1111–14 |
| Tuladhar | 2009 | < 100 participants | Tuladhar SM, Püntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol 2009;53:261–6 |
| Tuladhar | 2009 | < 100 participants | Tuladhar SM, Püntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol 2009;53:261–6 |
| Tung | 2015 | Not a relevant type of population | Tung YC, Chang CH, Chen YC, Chu PH. Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLOS ONE 2015;10:e0125282 |
| Tyagi | 2018 | < 100 participants | Tyagi A, Luthra A, Kumar M, Das S. Epidemiology of acute kidney injury and the role of urinary [TIMP-2]·[IGFBP7]: a prospective cohort study in critically ill obstetric patients. Int J Obstet Anesth 2018;36:77–84 |
| Tyagi | 2018 | < 100 participants | Tyagi A, Lahan S, Verma G, Das S, Kumar M. Role of intra-abdominal pressure in early acute kidney injury: a prospective cohort study in critically ill obstetric patients. Indian J Crit Care Med 2018;22:602–7 |
| Tziakas | 2015 | Not a relevant biomarker assay or test | Tziakas D, Chalikias G, Kareli D, Tsigalou C, Risgits A, Kikas P, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol 2015;197:48–55 |
| Uehara | 2009 | Not a relevant type of population | Uehara Y, Makino H, Seiki K, Urade Y, L-PGDS Clinical Research Group of Kidney. Urinary excretions of lipocalin-type prostaglandin D synthase predict renal injury in type-2 diabetes: a cross-sectional and prospective multicentre study. Nephrol Dial Transplant 2009;24:475–82 |
| Ueta | 2014 | < 100 participants | Ueta K, Watanabe M, Iguchi N, Uchiyama A, Shirakawa Y, Kuratani T, et al. Early prediction of acute kidney injury biomarkers after endovascular stent graft repair of aortic aneurysm: a prospective observational study. J Intensive Care 2014;2:45 |
| Uettwiller-Geiger | 2016 | < 100 participants | Uettwiller-Geiger DL, Vijayendran R, Kellum JA, Fitzgerald RL. Analytical characteristics of a biomarker-based risk assessment test for acute kidney injury (AKI). Clin Chim Acta 2016;455:93–8 |
| Urbschat | 2014 | No focus on DTA for AKI | Urbschat A, Gauer S, Paulus P, Reissig M, Weipert C, Ramos-Lopez E, et al. Serum and urinary NGAL but not KIM-1 raises in human postrenal AKI. Eur J Clin Invest 2014;44:652–9 |
| Vaidya | 2008 | Not a relevant type of population | Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 2008;1:200–8 |
| Valero | 2016 | Not a relevant type of population | Valero E, Rodríguez JC, Moyano P, Miñana G, Sanchis J, Núñez J. Role of neutrophil gelatinase-associated lipocalin in the detection of contrast-induced nephropathy in patients undergoing a coronary angiography. Rev Esp Cardiol 2016;69:524–5 |
| Valette | 2013 | < 100 participants | Valette X, Savary B, Nowoczyn M, Daubin C, Pottier V, Terzi N, et al. Accuracy of plasma neutrophil gelatinase-associated lipocalin in the early diagnosis of contrast-induced acute kidney injury in critical illness. Intensive Care Med 2013;39:857–65 |
| Van Biesen | 2012 | Not a primary study | Van Biesen W, Van Massenhove J, Lameire N, Vanholder R. Does urinary neutrophil gelatinase-associated lipocalin really solve the issue of discriminating prerenal from intrinsic acute kidney injury? Kidney Int 2012;81:321 |
| van Deursen | 2014 | No focus on DTA for AKI | van Deursen VM, Damman K, Voors AA, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL. Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ Heart Fail 2014;7:35–42 |
| van Wolfswinkel | 2016 | < 100 participants | van Wolfswinkel ME, Koopmans LC, Hesselink DA, Hoorn EJ, Koelewijn R, van Hellemond JJ, van Genderen PJ. Neutrophil gelatinase-associated lipocalin (NGAL) predicts the occurrence of malaria-induced acute kidney injury. Malar J 2016;15:464 |
| Vandenberghe | 2017 | Review – retained as background material | Vandenberghe W, De Loor J, Hoste EA. Diagnosis of cardiac surgery-associated acute kidney injury from functional to damage biomarkers. Curr Opin Anaesthesiol 2017;30:66–75 |
| Vanmassenhove | 2013 | No relevant outcome | Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W. Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care 2013;17:R234 |
| Vanmassenhove | 2014 | No focus on DTA for AKI | Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W. AKI in early sepsis is a continuum from transient AKI without tubular damage over transient AKI with minor tubular damage to intrinsic AKI with severe tubular damage. Int Urol Nephrol 2014;46:2003–8 |
| Vanmassenhove | 2015 | No focus on DTA for AKI | Vanmassenhove J, Glorieux G, Lameire N, Hoste E, Dhondt A, Vanholder R, Van Biesen W. Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 2015;16:18 |
| Varela | 2015 | < 100 participants | Varela CF, Greloni G, Schreck C, Bratti G, Medina A, Marenchino R, et al. Assessment of fractional excretion of urea for early diagnosis of cardiac surgery associated acute kidney injury. Ren Fail 2015;37:327–31 |
| Varnell | 2017 | Not a primary study | Varnell CD, Goldstein SL, Devarajan P, Basu RK. Impact of near real-time urine neutrophil gelatinase-associated lipocalin assessment on clinical practice. Kidney Int Rep 2017;2:1243–9 |
| Verbrugge | 2013 | < 100 participants | Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, et al. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail 2013;19:621–8 |
| Vermi | 2014 | < 100 participants | Vermi AC, Costopoulos C, Latib A, Piraino D, Maisano F, Naim C, et al. Urinary neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury after transcatheter aortic valve implantation. Hellenic J Cardiol 2014;55:77–9 |
| Vesnina | 2016 | < 100 participants | Vesnina ZV, Lishmanov YB, Alexandrova EA, Nesterov EA. Evaluation of nephroprotective efficacy of hypoxic preconditioning in patients undergoing coronary artery bypass surgery. Cardiorenal Med 2016;6:328–36 |
| Virzì | 2015 | < 100 participants | Virzì GM, de Cal M, Day S, Brocca A, Cruz DN, Castellani C, et al. Pro-apoptotic effects of plasma from patients with cardiorenal syndrome on human tubular cells. Am J Nephrol 2015;41:474–84 |
| Virzì | 2018 | < 100 participants | Virzì GM, Breglia A, Brocca A, de Cal M, Bolin C, Vescovo G, Ronco C. Levels of proinflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without cardiorenal syndrome type 1. Cardiorenal Med 2018;8:321–31 |
| Vives | 2012 | Not a primary study | Vives M, Lockwood G, Punjabi PP, Krahne D. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Anesthesiology 2012;116:490–1 |
| Volovelsky | 2018 | < 100 participants | Volovelsky O, Gist KM, Terrell TC, Bennett MR, Cooper DS, Alten JA, Goldstein SL. Early postoperative measurement of fibroblast growth factor 23 predicts severe acute kidney injury in infants after cardiac surgery. Clin Nephrol 2018;90:165–71 |
| von Jeinsen | 2017 | No relevant outcome | von Jeinsen B, Kraus D, Palapies L, Tzikas S, Zeller T, Schauer A, et al. Urinary neutrophil gelatinase-associated lipocalin and cystatin C compared to the estimated glomerular filtration rate to predict risk in patients with suspected acute myocardial infarction. Int J Cardiol 2017;245:6–12 |
| Wagener | 2008 | Not a relevant biomarker assay or test | Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 2008;52:425–33 |
| Wagener | 2008 | Not a primary study | Wagener G, Lee HT. Aprotinin and urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Anesth Analg 2008;106:1593 |
| Wagener | 2011 | < 100 participants | Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant 2011;26:1717–23 |
| Wagener | 2006 | < 100 participants | Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485–91 |
| Wai | 2013 | < 100 participants | Wai K, Soler-García AA, Perazzo S, Mattison P, Ray PE. A pilot study of urinary fibroblast growth factor-2 and epithelial growth factor as potential biomarkers of acute kidney injury in critically ill children. Pediatr Nephrol 2013;28:2189–98 |
| Waldherr | 2019 | < 100 participants | Waldherr S, Fichtner A, Beedgen B, Bruckner T, Schaefer F, Tönshoff B, et al. Urinary acute kidney injury biomarkers in very low-birth-weight infants on indomethacin for patent ductus arteriosus. Pediatr Res 2019;85:678–86 |
| Wang | 2014 | Not a relevant biomarker assay or test | Wang M, Zhang Q, Zhao X, Dong G, Li C. Diagnostic and prognostic value of neutrophil gelatinase-associated lipocalin, matrix metalloproteinase-9, and tissue inhibitor of matrix metalloproteinases-1 for sepsis in the emergency department: an observational study. Crit Care 2014;18:634 |
| Wang | 2015 | Not a relevant biomarker assay or test | Wang B, Chen G, Zhang J, Xue J, Cao Y, Wu Y. Increased neutrophil gelatinase-associated lipocalin is associated with mortality and multiple organ dysfunction syndrome in severe sepsis and septic shock. Shock 2015;44:234–8 |
| Wang | 2016 | < 100 participants | Wang W, Saad A, Herrmann SM, Eirin Massat A, McKusick MA, Misra S, et al. Changes in inflammatory biomarkers after renal revascularization in atherosclerotic renal artery stenosis. Nephrol Dial Transplant 2016;31:1437–43 |
| Wang | 2016 | No focus on DTA for AKI | Wang Z, Ma S, Zappitelli M, Parikh C, Wang CY, Devarajan P. Penalized count data regression with application to hospital stay after pediatric cardiac surgery. Stat Methods Med Res 2016;25:2685–703 |
| Wang | 2017 | Not a relevant biomarker assay or test | Wang C, Zhang J, Han J, Yang Q, Liu J, Liang B. The level of urinary IL-18 in acute kidney injury after cardiopulmonary bypass. Exp Ther Med 2017;14:6047–51 |
| Wang | 2017 | < 100 participants | Wang Y, Zou Z, Jin J, Teng J, Xu J, Shen B, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol 2017;18:177 |
| Wang | 2017 | Not a relevant type of population | Wang HJ, Wang P, Li N, Wan C, Jiang CM, He JS, et al. Effects of continuous renal replacement therapy on serum cytokines, neutrophil gelatinase-associated lipocalin, and prognosis in patients with severe acute kidney injury after cardiac surgery. Oncotarget 2017;8:10628–36 |
| Wang | 2018 | Not a relevant biomarker assay or test | Wang JJ, Chi NH, Huang TM, Connolly R, Chen LW, Chueh SJ, et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care 2018;22:108 |
| Washino | 2019 | < 100 participants | Washino S, Hosohata K, Oshima M, Okochi T, Konishi T, Nakamura Y, et al. A novel biomarker for acute kidney injury, vanin-1, for obstructive nephropathy: a prospective cohort pilot study. Int J Mol Sci 2019;20:899 |
| Wasilewska | 2010 | < 100 participants | Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Michaluk-Skutnik J. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of cyclosporine nephrotoxicity? Pediatr Nephrol 2010;25:889–97 |
| Watanabe | 2014 | < 100 participants | Watanabe M, Silva GF, Fonseca CD, Vattimo Mde F. Urinary NGAL in patients with and without acute kidney injury in a cardiology intensive care unit. Rev Bras Ter Intensiva 2014;26:347–54 |
| Weber | 2011 | Not a relevant type of population | Weber CL, Bennett M, Er L, Bennett MT, Levin A. Urinary NGAL levels before and after coronary angiography: a complex story. Nephrol Dial Transplant 2011;26:3207–11 |
| Wen | 2017 | Non-English-language publication | Wen Y, Li Z, Chang C, Zhang P, Lyu Y. [Diagnostic significance of urinary neutrophil gelatin enzyme-related lipid delivery protein and kidney injury molecule-1 in acute kidney injury after cardiac operation with cardiopulmonary bypass operation in children.] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017;29:1112–16 |
| Westhoff | 2015 | < 100 participants | Westhoff JH, Tönshoff B, Waldherr S, Pöschl J, Teufel U, Westhoff TH, Fichtner A. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) • insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLOS ONE 2015;10:e0143628 |
| Westhoff | 2016 | < 100 participants | Westhoff JH, Fichtner A, Waldherr S, Pagonas N, Seibert FS, Babel N, et al. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr Nephrol 2016;31:2353–63 |
| Westhoff | 2017 | < 100 participants | Westhoff JH, Seibert FS, Waldherr S, Bauer F, Tönshoff B, Fichtner A, Westhoff TH. Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eur J Pediatr 2017;176:745–55 |
| Wetz | 2015 | < 100 participants | Wetz AJ, Richardt EM, Wand S, Kunze N, Schotola H, Quintel M, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care 2015;19:3 |
| Wheeler | 2008 | Not a relevant biomarker assay or test | Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008;36:1297–303 |
| Wijerathna | 2018 | < 100 participants | Wijerathna TM, Mohamed F, Dissanayaka D, Gawarammana I, Palangasinghe C, Shihana F, et al. Albuminuria and other renal damage biomarkers detect acute kidney injury soon after acute ingestion of oxalic acid and potassium permanganate. Toxicol Lett 2018;299:182–90 |
| Wijerathna | 2019 | Not a relevant type of population | Wijerathna TM, Gawarammana IB, Mohamed F, Dissanayaka DM, Dargan PI, Chathuranga U, et al. Epidemiology, toxicokinetics and biomarkers after self-poisoning with Gloriosa superba. Clin Toxicol (Phila) 2019;57:1080–6 |
| Woitas | 2017 | Not a relevant type of population | Woitas RP, Scharnagl H, Kleber ME, Delgado GE, Grammer TB, Pichler M, et al. Neutrophil gelatinase-associated lipocalin levels are U-shaped in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study-Impact for mortality. PLOS ONE 2017;12:e0171574 |
| Wong | 2014 | Not a primary study | Wong F, Murray P. Kidney damage biomarkers: Novel tools for the diagnostic assessment of acute kidney injury in cirrhosis. Hepatology 2014;60:455–7 |
| Woo | 2012 | Not a relevant type of population | Woo KS, Choi JL, Kim BR, Kim JE, An WS, Han JY. Urinary neutrophil gelatinase-associated lipocalin levels in comparison with glomerular filtration rate for evaluation of renal function in patients with diabetic chronic kidney disease. Diabetes Metab J 2012;36:307–13 |
| Wu | 2010 | < 100 participants | Wu Y, Su T, Yang L, Zhu SN, Li XM. Urinary neutrophil gelatinase-associated lipocalin: a potential biomarker for predicting rapid progression of drug-induced chronic tubulointerstitial nephritis. Am J Med Sci 2010;339:537–42 |
| Wu | 2013 | Not a relevant type of population | Wu J, Ding Y, Zhu C, Shao X, Xie X, Lu K, Wang R. Urinary TNF-alpha and NGAL are correlated with the progression of nephropathy in patients with type 2 diabetes. Exp Ther Med 2013;6:1482–8 |
| Wu | 2018 | Not a relevant type of population | Wu VC, Shiao CC, Chi NH, Wang CH, Chueh SCJ, Liou HH, et al. Outcome prediction of acute kidney injury biomarkers at initiation of dialysis in critical units. J Clin Med 2018;7:202 |
| Xiao | 2013 | < 100 participants | Xiao J, Niu J, Ye X, Yu Q, Gu Y. Combined biomarkers evaluation for diagnosing kidney injury in preeclampsia. Hypertens Pregnancy 2013;32:439–49 |
| Xiao | 2015 | < 100 participants | Xiao N, Devarajan P, Inge TH, Jenkins TM, Bennett M, Mitsnefes MM. Subclinical kidney injury before and 1 year after bariatric surgery among adolescents with severe obesity. Obesity 2015;23:1234–8 |
| Xiao | 2019 | Not a relevant biomarker assay or test | Xiao W, Chen W, Hu H, Huang X, Luo Y. The clinical significance of neutrophil gelatinase-associated lipocalin in ischemic stroke patients with acute kidney injury. J Clin Lab Anal 2019;33:e22907 |
| Xie | 2014 | < 100 participants | Xie Y, Xu W, Wang Q, Shao X, Ni Z, Mou S. Urinary excretion of liver-type FABP as a new clinical marker for the progression of obstructive nephropathy. Biomark Med 2014;8:543–56 |
| Ximenes | 2015 | < 100 participants | Ximenes RO, Farias AQ, Helou CM. Early predictors of acute kidney injury in patients with cirrhosis and bacterial infection: urinary neutrophil gelatinase-associated lipocalin and cardiac output as reliable tools. Kidney Res Clin Pract 2015;34:140–5 |
| Xin | 2013 | < 100 participants | Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Renal Fail 2013;30:904–13 |
| Xue | 2014 | < 100 participants | Xue W, Xie Y, Wang Q, Xu W, Mou S, Ni Z. Diagnostic performance of urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin for acute kidney injury in an obstructive nephropathy patient. Nephrology 2014;19:186–94 |
| Yamanouchi | 2018 | < 100 participants | Yamanouchi S, Kimata T, Kino J, Kitao T, Suruda C, Tsuji S, et al. Urinary C-megalin for screening of renal scarring in children after febrile urinary tract infection. Pediatr Res 2018;83:662–8 |
| Yamashita | 2014 | < 100 participants | Yamashita T, Doi K, Hamasaki Y, Matsubara T, Ishii T, Yahagi N, et al. Evaluation of urinary tissue inhibitor of metalloproteinase-2 in acute kidney injury: a prospective observational study. Crit Care 2014;18:716 |
| Yamashita | 2016 | < 100 participants | Yamashita T, Noiri E, Hamasaki Y, Matsubara T, Ishii T, Yahagi N, et al. Erythropoietin concentration in acute kidney injury is associated with insulin-like growth factor-binding protein-1. Nephrology 2016;21:693–9 |
| Yang | 2009 | Not a relevant type of population | Yang YH, He XJ, Chen SR, Wang L, Li EM, Xu LY. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine 2009;36:45–51 |
| Yang | 2012 | < 100 participants | Yang CC, Hsieh SC, Li KJ, Wu CH, Lu MC, Tsai CY, Yu CL. Urinary neutrophil gelatinase-associated lipocalin is a potential biomarker for renal damage in patients with systemic lupus erythematosus. J Biomed Biotechnol 2012;2012:759313 |
| Yang | 2014 | < 100 participants | Yang HT, Yim H, Cho YS, Kym D, Hur J, Kim JH, et al. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit Care 2014;18:R151 |
| Yang | 2015 | Not a relevant biomarker assay or test | Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, et al. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two-stage study. J Am Soc Nephrol 2015;26:2032–41 |
| Yang | 2016 | Not a relevant biomarker assay or test | Yang CH, Chang CH, Chen TH, Fan PC, Chang SW, Chen CC, et al. Combination of urinary biomarkers improves early detection of acute kidney injury in patients with heart failure. Circ J 2016;80:1017–23 |
| Yang | 2017 | < 100 participants | Yang J, Lim SY, Kim MG, Jung CW, Cho WY, Jo SK. Urinary tissue inhibitor of metalloproteinase and insulin-like growth factor-7 as early biomarkers of delayed graft function after kidney transplantation. Transplant Proc 2017;49:2050–4 |
| Yap | 2017 | < 100 participants | Yap DY, Seto WK, Fung J, Chok SH, Chan SC, Chan GC, et al. Serum and urinary biomarkers that predict hepatorenal syndrome in patients with advanced cirrhosis. Dig Liver Dis 2017;49:202–6 |
| Yavas | 2013 | < 100 participants | Yavas H, Sahin OZ, Ersoy R, Taşlı F, Gibyeli Genek D, Uzum A, Cirit M. Prognostic value of NGAL staining in patients with IgA nephropathy. Ren Fail 2013;35:472–6 |
| Yavuz | 2014 | < 100 participants | Yavuz S, Anarat A, Acartürk S, Dalay AC, Kesiktaş E, Yavuz M, Acartürk TO. Neutrophil gelatinase associated lipocalin as an indicator of acute kidney injury and inflammation in burned children. Burns 2014;40:648–54 |
| Ye | 2018 | Not a relevant type of population | Ye HH, Shen G, Luo Q, Zhou FF, Xie XL, Wang CY, Han LN. Early diagnosis of acute kidney injury in aged patients undergoing percutaneous coronary intervention. J Zhejiang Univ Sci B 2018;19:342–8 |
| Yegenaga | 2018 | Not a relevant biomarker assay or test | Yegenaga I, Kamis F, Baydemir C, Erdem E, Celebi K, Eren N, Baykara N. Neutrophil gelatinase-associated lipocalin is a better biomarker than cystatin C for the prediction of imminent acute kidney injury in critically ill patients. Ann Clin Biochem 2018;55:190–7 |
| Yeh | 2013 | < 100 participants | Yeh YH, Chang JL, Hsiao PC, Tsao SM, Lin CH, Kao SJ, et al. Circulating level of lipocalin 2 as a predictor of severity in patients with community-acquired pneumonia. J Clin Lab Anal 2013;27:253–60 |
| Yeung | 2018 | Systematic review – retained as background material | Yeung ACY, Morozov A, Robertson FP, Fuller BJ, Davidson BR. Neutrophil Gelatinase-Associated Lipocalin (NGAL) in predicting acute kidney injury following orthotopic liver transplantation: a systematic review. Int J Surg 2018;59:48–54 |
| Yilmaz | 2009 | < 100 participants | Yilmaz A, Sevketoglu E, Gedikbasi A, Karyagar S, Kiyak A, Mulazimoglu M, et al. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr Nephrol 2009;24:2387–92 |
| Ylinen | 2014 | < 100 participants | Ylinen E, Jahnukainen K, Saarinen-Pihkala UM, Jahnukainen T. Assessment of renal function during high-dose methotrexate treatment in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2014;61:2199–202 |
| Yndestad | 2009 | Not a relevant biomarker assay or test | Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009;30:1229–36 |
| Yoon | 2018 | < 100 participants | Yoon KC, Lee KW, Oh SC, Kim H, Kim HS, Hong SK, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker for renal injury in liver transplant recipients using calcineurin inhibitors. Transplant Proc 2018;50:3667–72 |
| Young-Min | 2014 | < 100 participants | Young-Min J, Cheul-Min HA, Ki-Cheul NOH, Chang-Hae PYO. The usefulness of plasma neutrophil gelatinase-associated lipocalin in acute pyelonephritis. J Korean Soc Emerg Med 2014;25:137–144 |
| Youssef | 2012 | < 100 participants | Youssef DM, El-Shal AS. Urinary neutrophil gelatinase-associated lipocalin and kidney injury in children with focal segmental glomerulosclerosis. Iran J Kidney Dis 2012;6:355–60 |
| Youssef | 2013 | < 100 participants | Youssef DM, Esh AM, Helmy Hassan E, Ahmed TM. Serum NGAL in critically ill children in ICU from a single center in Egypt. ISRN Nephrol 2013;2013:140905 |
| Yuan | 2014 | Non-English-language publication | Yuan F, Liu H, Wang WX, Dai JJ, Dai LY, Yang XX, Fang WY. Study on early diagnosis of acute decompensated heart failure combined with acute renal injury. J Shanghai Jiaotong Univ 2014;34:1771–4 |
| Zaleska-Kociecka | 2017 | < 100 participants | Zaleska-Kociecka M, Skrobisz A, Wojtkowska I, Grabowski M, Dabrowski M, Kusmierski K, et al. Serum beta-2 microglobulin levels for predicting acute kidney injury complicating aortic valve replacement. Interact Cardiovasc Thorac Surg 2017;25:533–40 |
| Zaouter | 2018 | < 100 participants | Zaouter C, Priem F, Leroux L, Bonnet G, Bats ML, Beauvieux MC, et al. New markers for early detection of acute kidney injury after transcatheter aortic valve implantation. Anaesth Crit Care Pain Med 2018;37:319–26 |
| Zaouter | 2018 | < 100 participants | Zaouter C, Potvin J, Bats ML, Beauvieux MC, Remy A, Ouattara A. A combined approach for the early recognition of acute kidney injury after adult cardiac surgery. Anaesth Crit Care Pain Med 2018;37:335–41 |
| Zappitelli | 2007 | Not a relevant biomarker assay or test | Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 2007;11:R84 |
| Zappitelli | 2007 | Duplicate of a study that had already been assessed | Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 2007;11:R84 |
| Zappitelli | 2012 | No focus on DTA for AKI | Zappitelli M, Coca SG, Garg AX, Krawczeski CD, Thiessen Heather P, Sint K, et al. The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol 2012;7:1761–9 |
| Zarbock | 2015 | No focus on DTA for AKI | Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015;313:2133–41 |
| Zarbock | 2016 | Not a relevant type of population | Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190–9 |
| Zelt | 2018 | Duplicate of a study that had already been assessed | Zelt JGE, Mielniczuk LM, Liu PP, Dupuis JY, Chih S, Akbari A, Sun LY. Utility of novel cardiorenal biomarkers in the prediction and early detection of congestive kidney injury following cardiac surgery. J Clin Med 2018;7:540 |
| Zeng | 2014 | Not a relevant biomarker assay or test | Zeng XF, Li JM, Tan Y, Wang ZF, He Y, Chang J, et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: a cohort study based on major surgeries. Clin Chem Lab Med 2014;52:671–8 |
| Zhang | 2015 | Not a relevant type of population | Zhang M, Zhao X, Deng Y, Tang B, Sun Q, Zhang Q, et al. Neutrophil gelatinase associated lipocalin is an independent predictor of poor prognosis in cases of papillary renal cell carcinoma. J Urol 2015;194:647–52 |
| Zhang | 2015 | Not a primary study | Zhang Z. Biomarkers, diagnosis and management of sepsis-induced acute kidney injury: a narrative review. Heart Lung Vessel 2015;7:64–73 |
| Zhang | 2016 | Meta-analysis – retained as background material | Zhang A, Cai Y, Wang PF, Qu JN, Luo ZC, Chen XD, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care 2016;20:41 |
| Zhang | 2017 | No focus on DTA for AKI | Zhang Y, Yu Y, Jia J, Yu W, Xu R, Geng L, Wei Y. Administration of HES in elderly patients undergoing hip arthroplasty under spinal anesthesia is not associated with an increase in renal injury. BMC Anesthesiol 2017;17:29 |
| Zhang | 2017 | < 100 participants | Zhang J, Han J, Liu J, Liang B, Wang X, Wang C. Clinical significance of novel biomarker NGAL in early diagnosis of acute renal injury. Exp Ther Med 2017;14:5017–21 |
| Zhang | 2018 | Not a relevant type of population | Zhang Y, Li J, Li F, Qi X, Zhang J. Neutrophil gelatinase-associated lipocalin accurately predicts renal tubular injury in patients with chronic hepatitis B treated with nucleos(t)ide analogs. Hepatol Res 2018;48:144–52 |
| Zhang | 2018 | < 100 participants | Zhang D, Han QX, Wu MH, Shen WJ, Yang XL, Guo J, et al. Diagnostic value of sensitive biomarkers for early kidney damage in diabetic patients with normoalbuminuria. Chin Med J 2018;131:2891–2 |
| Zhang | 2018 | Not a relevant type of population | Zhang J, Lin X, Tian B, Liu C. Evaluation of the efficacy of ischemic post-conditioning for the improvement of contrast induced nephropathy on patients with acute coronary syndrome. Int J Clin Exp Med 2018;11:4663–9 |
| Zhang | 2018 | Not a relevant type of population | Zhang WR, Craven TE, Malhotra R, Cheung AK, Chonchol M, Drawz P, et al. Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: a case-control study. Ann Intern Med 2018;169:610–18 |
| Zheng | 2013 | < 100 participants | Zheng J, Xiao Y, Yao Y, Xu G, Li C, Zhang Q, et al. Comparison of urinary biomarkers for early detection of acute kidney injury after cardiopulmonary bypass surgery in infants and young children. Pediatr Cardiol 2013;34:880–6 |
| Zhou | 2016 | Not a relevant biomarker assay or test | Zhou LZ, Yang XB, Guan Y, Xu X, Tan MT, Hou FF, Chen PY. Development and validation of a risk score for prediction of acute kidney injury in patients with acute decompensated heart failure: a prospective cohort study in China. J Am Heart Assoc 2016;5:e004035 |
| Zhou | 2016 | Meta-analysis – retained as background material | Zhou F, Luo Q, Wang L, Han L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg 2016;49:746–55 |
| Zhou | 2018 | Not a relevant type of population | Zhou F, Song W, Wang Z, Yin L, Yang S, Yang F, et al. Effects of remote ischemic preconditioning on contrast induced nephropathy after percutaneous coronary intervention in patients with acute coronary syndrome. Medicine 2018;97:9579 |
| Zhu | 2014 | < 100 participants | Zhu W, Liu M, Wang GC, Che JP, Xu YF, Peng B, Zheng JH. Urinary neutrophil gelatinase-associated lipocalin, a biomarker for systemic inflammatory response syndrome in patients with nephrolithiasis. J Surg Res 2014;187:237–43 |
| Zhu | 2016 | Non-English-language publication | Zhu L, Shi D. [Early diagnostic value of neutrophil gelatinase-associated lipocalin and interleukin-18 in patients with sepsis-induced acute kidney injury.] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28:718–22 |
| Zughaier | 2013 | < 100 participants | Zughaier SM, Tangpricha V, Leong T, Stecenko AA, McCarty NA. Peripheral monocytes derived from patients with cystic fibrosis and healthy donors secrete NGAL in response to Pseudomonas aeruginosa infection. J Investig Med 2013;61:1018–25 |
| Zwaag | 2019 | No focus on DTA for AKI | Zwaag J, Beunders R, Warle MC, Kellum JA, Riksen NP, Pickkers P, Kox M. Remote ischaemic preconditioning does not modulate the systemic inflammatory response or renal tubular stress biomarkers after endotoxaemia in healthy human volunteers: a single-centre, mechanistic, randomised controlled trial. Br J Anaesth 2019;123:177–85 |
| Zwiers | 2015 | < 100 participants | Zwiers AJ, Cransberg K, de Rijke YB, van Rosmalen J, Tibboel D, de Wildt SN. Urinary Neutrophil gelatinase-associated lipocalin predicts renal injury following extracorporeal membrane oxygenation. Pediatr Crit Care Med 2015;16:663–70 |
Appendix 6 Characteristics of included studies
| Study, country | Test | Age (years) (range or SD) | Sample size (n) | AKI events (n) | AKI definition | Male sex (%) | Serum creatinine | eGFR | SOFA mean score | CKD (%) | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cummings et al.26 2019, USA | NephroCheck | Mean 67 (58–75) | 400 | 14 | KDIGO | 67 | NR | NR | NR | 6 | Patients who were originally enrolled in the AKI Cardiac Surgery RCT | Acute coronary syndrome, liver dysfunction, use of ciclosporin, current RRT, history of kidney transplant, pregnancy |
| Oezkur et al.27 2017, Germany | NephroCheck |
|
150 | 35 | KDIGO | 72 | Median 0.89 (IQR 0.75–1.02) | NR | NR | NR | Adult patients were eligible if they were undergoing elective cardiac surgery (CABG with or without mammary artery bypass, valve surgery with or without removal of the atrial auricle, combined CABG and valve surgery, or surgery of the thoracic aorta) involving CPB | Patients with advanced stages of CKD; signs of active infection; on medication with COMT inhibitors, MAO inhibitors or with immunosuppressive therapy, and women during pregnancy and lactation |
| Beitland et al.28 2016, Norway | NephroCheck | 60 (13) | 195 | 88 | KDIGO |
|
NR | NR | NR |
|
Adult (≥ 18 years) comatose out-of-hospital cardiac arrest patients with return of spontaneous circulation | Patients with known CKD or who died within 24 hours of ICU stay, or who, for some reason, did not receive active treatment, were excluded |
| Kashani et al.34 2013, 21 sites in North America, 15 sites in Europe | NephroCheck | 64 (53–73) | 728 | 101 | KDIGO | 62 | NR | NR | NR | NR | Critically ill patients who were at least aged 21 years, admitted to the ICU within 24 hours of enrolment, and expected to remain in the ICU with a urinary catheter for at least 48 hours | Patients with known existing moderate or severe AKI |
| Bihorac et al.11 2010, USA | NephroCheck | 63 (17) | 408 | 71 | KDIGO | 54 | NR | NR | NR | NR | All enrolled patients were considered critically ill because of significant respiratory or cardiovascular dysfunction. The presence of an indwelling urinary catheter was also a prerequisite for inclusion | Patients with documented moderate to severe AKI (KDIGO stages 2–3) at the time of enrolment |
| Hoste et al.33 2014, USA | NephroCheck |
|
153 | 27 | KDIGO |
|
NR | NR | NR | 20 | Patients at least aged 21 years, admitted to ICU within 24 hours of enrolment and expected to remain in the ICU with a urinary catheter for at least 48 hours after enrolment | NR |
| Di Leo et al.30 2018, Italy | NephroCheck | 68 (51–78) | 719 | 234 | KDIGO |
|
NR | NR | NR | AKI stage 2/3: 33 | All patients aged ≥ 18 years were included in the study | Patients on chronic dialysis and with a life expectancy of < 24 hours were excluded |
| Kimmel et al.36 2016, Germany | NephroCheck, BioPorto urine and plasma NGAL tests | 63 (14) | 298 | 46 | KDIGO (modified version) | 72 | NR | NR | NR | NR | Aged ≥ 18 years, willingness to sign an informed consent form, admission to the internal medicine service of the hospital, and haemoglobin level of ≥ 9.5 g/dl (women) or ≥ 10.5 g/dl (men) | Dialysis requirement, pregnancy, or failure to meet any of the inclusion criteria |
| Gayat et al.32 2018, France | NephroCheck | 65 (54–75) | 200 | Unclear | KDIGO | 78 | NR | NR | NR | NR | NR | NR |
| Zelt et al.80 2018, USA | BioPorto plasma NGAL | 67 (61–73) | 178 | 35 | AKIN | NR | NR | NR | NR | NR | All patients having elective cardiac surgery requiring CPB | ESRD; renal transplantation; solitary kidney, emergent operative status, off-pump procedures, procedures involving circulatory arrest, heart transplantation and left ventricular assist divide implantation |
| Lee et al.82 2018, the Republic of Korea | BioPorto plasma NGAL | 59 (50–71) | 279 | 111 | KDIGO | 66 | NR | NR | NR | 25 | Non-traumatic cardiac arrest survivors aged > 18 years who were treated with therapeutic hypothermia and obtained plasma NGAL level results were enrolled | Transferred to another facility or died during therapeutic hypothermia, they had a pre-arrest cognitive impairment on the Cerebral Performance Categories scale of > 3, they had pre-arrest ESRD with RRT, they had cardiac arrest as a result of AKI, extracorporeal membrane oxygenation was applied during post-cardiac arrest care, or data were missing regarding their NGAL level |
| Itenov et al.81 2017, Denmark | BioPorto plasma NGAL | 67 (60–76) | 454 | 87 | KDIGO or Modification of Diets in Renal Disease study (in patients without serum creatinine samples before admission) | 60 | NR | NR | NR | 21 | Patients (aged ≥ 18 years) enrolled within 24 hours of ICU admission and expected to stay in ICU for at least 24 hours. For the present cohort study, the authors included patients without CKD who survived > 24 hours after admission and with plasma samples from admission available for biomarker analysis | Patients with high plasma concentrations of bilirubin (40 mg/dl) and/or triglycerides (1000 mg/dl) or patients at an increased risk from blood sampling were ineligible |
| Marino et al.83 2015, Italy | BioPorto plasma NGAL | 77 (72–83) | 101 | 49 | RIFLE | 60 | NR | NR | NR | NR | Patients arriving in the ED with the diagnosis of sepsis, severe sepsis or septic shock between December 2011 and April 2012 | Exclusion criteria were patients aged < 18 years and a patient’s inability to give informed consent |
| Parikh et al.37 2011, North America | ARCHITECT urine NGAL | 71 (10) | 1200 | 60 | Acute dialysis or doubling of serum creatinine at a median of 3 days after surgery (IQR 2–4) | 68 | Median 1.0 (IQR 0.9–1.20) | NR | NR | 20 | High risk for AKI was defined by the presence of one or more of the following: emergency surgery, preoperative serum creatinine level of > 2 mg/dl (> 177 µmol/l), ejection fraction of < 35% or grade 3 or 4 left ventricular dysfunction, aged > 70 years, diabetes mellitus, concomitant CABG and valve surgery, or repeat revascularisation surgery | Patients with evidence of AKI before surgery, prior kidney transplantation, preoperative serum creatinine level of > 4.5 mg/dl (> 398 µmol/l), or ESRD. Participants with multiple surgeries could be enrolled in the study only once |
| Albert et al.45 2018, Germany | ARCHITECT urine NGAL | 70 (61–77) | 101 | 15 | RIFLE | 72 | NR | NR | NR | NR | Non-emergency open-heart surgery with CPB | Emergency operation or off-pump surgery, CKD or kidney transplant; patients aged < 18 years and patients on immunosuppression therapy |
| Haase et al.60 2014, Germany | ARCHITECT urine NGAL, BioPorto plasma NGAL | 72 (65–77) | 100 | 23 | RIFLE | 75 | NR | NR | NR | NR | Aged > 70 years, pre-existing renal impairment (preoperative creatinine level of > 120 µmol/l, left ventricular ejection fraction of < 35%, insulin-dependent type 2 diabetes, valvular surgery or valvular and coronary artery bypass surgery, redo cardiac surgery | Patients with chronic renal impairment (preoperative creatinine level of > 300 µmol/l), those undergoing an emergency cardiac surgery procedure, patients on immunosuppression therapy, and those enrolled in a conflicting research study |
| De Loor et al.63 2017, Belgium | BioPorto urine NGAL | 69 (61–76) | 203 | 95 | KDIGO | 66 | NR | NR | NR | NR | Elective cardiac surgery | AKI stage ≥ 1, CKD stage 5; recent kidney transplant; surgery on Saturdays and Sundays |
| Garcia-Alvarez et al.46 2015, Spain | ARCHITECT urine NGAL |
|
288 | 104 | Serum creatinine of ≥ 200% or eGFR of < 50% from baseline |
|
NR | NR | NR | NR | All patients admitted to ICU after cardiac surgery and who provided informed consent | If patients required preoperative chronic or acute haemodialysis, had previously undergone renal transplant or had coronary angiography in the 7 days before surgery |
| Thanakitcharu and Jirajan48 2014, Thailand | ARCHITECT urine NGAL | 51 (15.6) | 130 | 46 | Serum creatinine levels of ≥ 0.3 mg/dl within 48 hours | 59 | Mean 1.0 mg/dl (SD 0.3) | 74.1 (25.9) | NR | NR | All patients who underwent cardiac surgery with CPB | Pre-existing renal dysfunction with baseline serum creatinine level of > 3mg/dl; kidney transplant patients; history of using nephrotoxic agents such as aminoglycoside, NSAIDs, radiocontrast agent in the 2 weeks before surgery; patients with sepsis; patients undergoing emergency operation < 24 hours after admission |
| Tidbury et al.64 2019, UK | BioPorto urine NGAL |
|
125 | 54 | RIFLE |
|
NR | NR | NR | NR | High-risk patients undergoing elective surgery for on-pump such as valve replacement, CABG or combined valve and CABG. All had impaired renal function pre operation, established by an eGFR of < 60 ml/minute/1.73 m2 | Excluded if they were scheduled to undergo surgery with anticipated CPB time of < 60 minutes; undergoing surgery on great vessels such as aortic surgery; had impaired liver function; renal failure or were on dialysis; malignancy; being pregnant |
| Schley et al.61 2015, Germany | BioPorto urine and plasma NGAL tests | 70 (10) | 110 | 37 | AKIN | 76 | Mean 1.2 mg/dl (SD 0.5) | NR | NR | NR | All patients undergoing cardiac surgery using CPB | Pre-existing haemodialysis-dependent ESRD, previous kidney transplantation, immunosuppressive medication and pregnancy |
| Collins et al.51 2012, USA | ARCHITECT urine NGAL | NR | 399 | 20 | Serum creatinine levels of ≥ 0.3 mg/dl or RIFLE | 65 | NR | NR | NR | NR | Modified Framingham criteria for acute heart failure; enrolled within 3 hours of first physician contact; received vasodilators or diuretics in the ED for treatment of acute heart failure | NR |
| Dupont et al.52 2012, USA | ARCHITECT urine NGAL | NR | 141 | 35 | Serum creatinine increase of ≥ 0.3 mg/dl | 58 | NR | NR | NR | NR | Aged > 18 years, clinical evidence of congestion, planned strategy for treatment with intravenous furosemide | Acute coronary syndrome, ESRD or RRT, exposure to nephrotoxic agents, planned surgery at the time of enrolment, haemoglobin level of < 9 mg/dl or active bleeding |
| Cullen et al.49 2014, UK | ARCHITECT urine NGAL | 68 (11) | 109 | 16 | AKIN | NR | NR | NR | NR | NR | Patients admitted to critical care following major abdominal surgery | Refusal of consent, concurrent lithium therapy, acute myocardial ischaemia, acute arrhythmias, pregnancy, patients receiving palliative treatment only and weight of < 40 kg |
| Nisula et al.76 2014, Finland | BioPorto urine NGAL | 62 (50–73) | 855 | 379 | KDIGO | 64 | NR | NR | NR | NR | Emergency ICU admissions and post-operative patients admitted for > 24 hours | Patients aged < 18 years, re-admitted patients who received RRT during their previous admission, patients electively admitted with an ICU LOS of < 24 hours if discharged alive, patients on chronic dialysis, organ donors, patients without permanent residency in Finland or without sufficient language skills, patients transferred between study ICUs if included in the study for 5 days already, and patients receiving intermediate care |
| Doi et al.71 2011, Japan | BioPorto urine NGAL |
|
339 | 131 | RIFLE |
|
NR | NR | NR | NR | Patients aged > 20 years who had been admitted to the mixed ICU | Patients with ESRD or renal transplant were excluded |
| Cho et al.69 2013, the Republic of Korea | BioPorto urine NGAL |
|
145 | 54 | AKIN |
|
NR | NR | NR | NR | Adult patients aged > 18 years who were admitted to the medical or surgical ICU | ESRD or kidney transplantation and those with life expectancy of < 48 hours |
| Pipili et al.58 2014, Greece | ARCHITECT urine NGAL | 64 (18) | 106 | 44 | RIFLE | 64 | 1.0 mg/dl (SD 1.3) | NR | 9 (3) | NR | All consecutive, mechanically ventilated patients admitted to the ICU were considered eligible for inclusion | Aged < 18 years, BMI of > 35 kg/m2, ESRD on chronic haemodialysis, pregnancy, brain death, metastatic cancer and re-admission to ICU or missing baseline creatinine in the 6 months before admission |
| Mårtensson et al.55 2015, Australia | ARCHITECT urine NGAL |
|
102 | 28 | RIFLE |
|
NR | NR | NR | NR | Aged > 18 years, the presence of two or more systemic inflammatory response criteria, the presence of oliguria for ≥ 2 consecutive hours and/or a 25-µmol/l increase in creatinine from baseline | NR |
| Isshiki et al.53 2018, Japan | ARCHITECT urine NGAL | 62 (51–73) | 148 | 33 | KDIGO | 60 | NR | NR | NR | NR | Aged > 18 years who were admitted to the ICU | Anuria patients at ICU admission, those deceased within 24 hours of ICU admission and those with ESRD |
| Tecson et al.78 2017, USA | BioPorto urine and plasma NGAL tests |
|
245 | 33 | KDIGO |
|
NR | NR | NR | NR | NR | NR |
| Matsa et al.73 2014, UK | BioPorto urine and plasma NGAL tests | 60 (15) | 194 | 59 | RIFLE | 66 | 80.8 mol/l (SD 29.1) | NR | NR | NR | Consecutive adult (aged > 18 years) patients admitted to the ICU were screened for inclusion | Refused consent, ESRD, previous renal transplant, patients already on RRT, patients referred to the ICU for RRT and patients with AKI as defined by RIFLE criteria for risk, injury or failure |
| Kokkoris et al.54 2012, Greece | ARCHITECT urine NGAL |
|
100 | 36 | RIFLE | 57 | NR | NR | NR | 0 | All consecutive patients admitted to the ICU were screened for eligibility | ESRD; CKD or nephrectomy or renal transplantation; expected ICU stay of or imminent death in < 48 hours; transfer from another ICU or high-dependency unit; brain death; aged < 18 years; inability to draw blood or urine (anuria) |
| Asada et al.50 2016, Japan | ARCHITECT urine NGAL |
|
133 | 31 | KDIGO |
|
NR | NR | NR | 0 | Patients aged ≥ 18 years who were admitted to the ICU | Presence of ESRD |
| Nickolas et al.56 2012, USA and Germany | ARCHITECT urine NGAL | 64 (19) | 1635 | 96 | RIFLE | 52 | 0.9 (0.4) mg/dl | 70.5 (SD 33.2) | NR | 0 | Patients aged > 18 years, irrespective of their condition, who were in the process of admission to the hospital from the ED | Patients who had 24 hours of follow-up or were on long-term RRT |
| Hjortrup et al.72 2015, Denmark | BioPorto urine and plasma NGAL tests | 66 (57–75) | 151 | 91 | KDIGO | 57 | NR | NR | NR | 0 | Need of fluid resuscitation in the ICU, the fulfilment of severe sepsis criteria in the previous 24 hours and consent from patient or proxy | Aged < 18 years; allergy to hydroxyethyl starch or malic acid; any form of RRT; acute burn injury on > 10% of body surface area; severe hyperkalaemia within the previous 6 hours; liver or kidney transplantation or intracranial bleeding during current hospital admission; enrolment in another ICU trial of drugs with potential action on circulation, renal function or coagulation |
| Park et al.57 2017, USA | ARCHITECT urine NGAL | 59 (11) | 2466 | NR | Serum creatinine (criteria not clearly defined) | 54 | NR | 43 (18) | NR | 0 | Adults with an eGFR of 20–70 ml/minute/1.73 m2 were enrolled | Polycystic kidney disease, multiple myeloma, or glomerulonephritis on active immunosuppression |
| Smith et al.77 2013, UK | BioPorto urine NGAL | 69 (12) | 158 | 40 | KDIGO | 75 | NR | 31 (11) | NR | 0 | NR | NR |
| Ariza et al.67 2016, Europe | BioPorto urine NGAL |
|
716 | NR | Serum creatinine levels of between ≥ 1.5 and < 2 mg/dl |
|
NR | NR | NR | 0 | NR | People with a urinary tract infection at the time of urine collection were excluded because the urine levels of NGAL may be increased as a result of high leucocyte concentration in urine |
| Treeprasertsuk et al.59 2015, Thailand | ARCHITECT urine NGAL | 57 (15) | 121 | 35 | AKIN | 62 | NR | NR | NR | 0 | Cirrhotic patients who were admitted with AKI-prone conditions. All patients had normal baseline serum creatinine in the 3 months prior to admission with cirrhosis, aged > 18 years | Exclusion criteria were CKD, or previous liver or kidney transplantation. The diagnosis of cirrhosis was based on a combination of clinical, biochemical and imaging assessments or liver biopsy |
| Barreto et al.68 2014, Spain | BioPorto urine NGAL | 58 (12) | 132 | 65 | AKIN | 70 | 1.5 (1.0) mg/dl | NR | NR | 0 | Cirrhotic patients with a bacterial infection | Chronic haemodialysis before admission, previous liver and/or kidney transplantation, hepatocellular carcinoma outside the Milan criteria or any other advanced malignancy, lack of informed consent, and patients with urinary tract infection (these patients were excluded because urine NGAL levels are increased in these patients and therefore may not reflect any impairment of kidney function) |
| Jaques et al.62 2019, Switzerland | BioPorto urine and plasma NGAL tests | 58 (10) | 105 | 55 | AKIN | 71 | NR | NR | NR | 0 | Inclusion criteria were patients aged ≥ 18 years and known or suspected cirrhosis with ascites confirmed by ultrasonography | Exclusion criteria were proven multifocal hepatocellular carcinoma, known CKD stage 5 or dialysis before admission, prior kidney or liver transplantation, recent upper gastrointestinal bleeding, or a delay of > 24 hours between the admission and inclusion. Informed consent was sought from all eligible patients, or from a surrogate decision-maker if the patient was unable to provide consent |
| Cho et al.66 2014, the Republic of Korea | BioPorto urine NGAL | 57 (12) | 135 | 54 | AKIN | 63 | NR | NR | NR | 0 | Patients who planned to undergo elective hepatobiliary surgery | Patients aged < 18 years, with baseline eGFR of < 60 ml/minute/1.73 m2, on maintenance RRT, developed AKI preoperatively |
| Nickolas et al.74 2008, USA | BioPorto urine NGAL | 60 (18) | 635 | 30 | RIFLE | 51 | 1.4 (1.8) mg/dl | NR | NR | 0 | Aged > 18 years, admitted to ED | Patients who were receiving haemodialysis and patients without subsequent creatinine measurements |
| Verna et al.79 2012, USA | BioPorto urine NGAL | 56 (49–62) | 118 | 52 | Serum creatinine to > 1.5 and 0.3 mg/dl above baseline, not responding with 48 hours of volume resuscitation and not meeting the criteria for hepatorenal syndrome | 61 | NR | NR | NR | 0 | Adults with cirrhosis | Patients on chronic haemodialysis, anuria for the first 24 hours, urinary tract infection, proteinuria of > 500 mg per day, or urinary obstruction |
| Liebetrau et al.47 2013, Germany | ARCHITECT urine NGAL |
|
141 | 47 | KDIGO |
|
NR | NR | NR | 0 | Consecutive patients scheduled to undergo elective major cardiac surgery (CABG and/or valve replacement) with the use of extracorporeal circulation | Patients with a preoperative eGFR of < 30 ml/minute/1.73 m2 body surface |
| Parikh et al.84 2011, North America | ARCHITECT urine NGAL | 4 (5) years | 311 | 53 | Receipt of acute dialysis or doubling of serum creatinine levels at a median of 3 days after surgery (consistent with RIFLE stage 1 or AKIN stage 2) | 55 | NR | 90 (26) | NR | 0 | All paediatric patients aged 1 month–18 years undergoing CPB | Prior renal transplantation or dialysis |
| Dong et al.92 2017, USA | BioPorto urine NGAL |
|
150 | 50 | KDIGO |
|
NR | NR | NR | 0 | All patients receiving CPB as long as the baseline serum creatinine level is normal for age | Pre-existing CKD |
| Bojan et al.86 2014, France | ARCHITECT urine NGAL | < 1 year | 100 | NR | AKIN | NR | NR | NR | NR | 0 | Surgery with CPB | NR |
| Bennett et al.87 2008, USA | ARCHITECT urine NGAL | 4 years | 196 | 99 | Increase of ≥ 50% in serum creatinine level from baseline within 72 hours | 54 | NR | NR | NR | 0 | Elective CPB surgery | Pre-existing renal insufficiency, diabetes mellitus, peripheral vascular disease and use of nephrotoxic drugs before and during the study |
| Cantinotti et al.88 2012, Italy | ARCHITECT urine NGAL | 6 (1–49) months | 135 | 52 | RIFLE | 58 | NR | NR | NR | 0 | All patients undergoing cardiac surgery for correction/palliation of congenital heart defects | History of prior renal transplantation or dialysis requirements |
| Alcaraz et al.89 2014, Spain | ARCHITECT urine NGAL | 25 (6.0–72.0) months | 106 | 36 | Paediatric RIFLE criteria | 59 | NR | NR | NR | 0 | Cardiac surgery for congenital lesions | Pre-existing renal dysfunction and heart transplantation |
| Lagos-Arevalo et al.93 2015, Canada | BioPorto urine NGAL |
|
160 | 70 | KDIGO | NR | NR | NR | NR | 0 | Children aged between 1 month and 18 years who were not immediately admitted to PICU after cardiac surgery | Known ESRD, having received a renal transplant, a high likelihood of death in the subsequent 48 hours (determined by the PICU attending staff) and presence of < 25% of PICU days with both a cystatin C and a serum creatinine value available (determined by dividing number of available daily values by PICU admission days) |
| Zwiers et al.91 2015, the Netherlands | ARCHITECT urine NGAL | 27 (1–85) days | 100 | 35 | RIFLE | 66 | N | NR | NR | 0 | Children (born at > 37 weeks of gestational age) between the ages of 1 day and 1 year admitted to the ICU and requiring endotracheal intubation and mechanical ventilation | Congenital abnormalities of the kidney or urinary tract, death anticipated within 24 hours or they received mechanical ventilation for other reasons. Patients were excluded when treatment with extracorporeal membrane oxygenation was required during the study period |
| Yang et al.65 2017, China | BioPorto urine NGAL |
|
|
|
Acute dialysis or doubling of serum creatinine levels consistent with KDIGO stages 2 and 3 criteria |
|
|
|
NR | 0 | Patients receiving elective cardiac surgery (CPB) | Exposure to nephrotoxin in the 4 weeks before surgery, pre-existing advanced and urinary tract infection or obstruction |
| Seitz et al.90 2013, NR | ARCHITECT urine NGAL | 0 (0–8) years | 139 | 76 | RIFLE | 55 | Mean 0.38 mg/dl (SD NR) | NR | NR | 0 | Patients undergoing CPB for surgical correction or palliation of congenital heart disease | Patients with pre-existing renal insufficiency, patients with history of nephrotoxin use during pre-operative days |
Appendix 7 The QUADAS-2 risk-of-bias and applicability assessment
| Study | Test | Risk of bias | Applicability | |||||
|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||
| Albert 201845 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Alcaraz 201489 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Ariza 201667 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Asada 201650 | ARCHITECT urine NGAL | Unclear | Unclear | Low | High | Unclear | Unclear | Low |
| Barreto 201468 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Beitland 201628 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| Bennett 200887 | Urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Bihorac 201429 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| Bojan 201486 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cantinotti 201288 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cho 201369 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cho 201466 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Collins 201251 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cullen 201449 | ARCHITECT urine NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Cummings 201926 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| De Loor 201763 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Di Leo 201830 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| Doi 201470 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Dong 201792 | BioPorto urine NGAL | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| Dupont 201252 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Garcia-Alvarez 201546 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Gayat 201832 | NephroCheck | Unclear | Unclear | Low | Low | Low | Low | Low |
| Haase 201460 | ARCHITECT urine NGAL, plasma NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Hjortrup 201572 | BioPorto urine NGAL, plasma NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Hoste 201433 | NephroCheck | Unclear | Unclear | Low | Low | Low | Low | Low |
| Isshiki 201853 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Itenov 201781 | Plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Jaques 201962 | BioPorto urine NGAL, plasma NGAL | Low | Unclear | Low | High | Unclear | Unclear | Low |
| Kashani 201334 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| Kimmel 201636 | NephroCheck, BioPorto urine NGAL, plasma NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Kokkoris 201254 | ARCHITECT urine NGAL, plasma NGAL | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Lagos-Arevalo 201593 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Lee 201882 | Plasma NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Liebetrau 201347 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Marino 201583 | Plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Mårtensson 201555 | ARCHITECT urine NGAL | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Matsa 201473 | BioPorto urine NGAL, plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Nickolas 200874 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Nickolas 201256 | ARCHITECT urine NGAL | Low | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Nisula 201575 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Oezkur 201727 | NephroCheck | Low | Unclear | Low | Low | Low | Low | Low |
| Parikh 201137 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Parikh 201184 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Park 201757 | ARCHITECT urine NGAL | Unclear | Unclear | Low | Unclear | Low | Unclear | Low |
| Pipili 201458 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Schley 201561 | BioPorto urine NGAL, plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Seitz 201390 | ARCHITECT urine NGAL | Low | Low | Low | Low | Low | Unclear | Low |
| Smith 201377 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Tecson 201778 | BioPorto urine NGAL, plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Thanakitcharu 201448 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Tidbury 201964 | BioPorto urine NGAL | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| Treeprasertsuk 201559 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Verna 201279 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Yang 201765 | BioPorto urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Zelt 201880 | Plasma NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Zwiers 201591 | ARCHITECT urine NGAL | Low | Unclear | Low | Low | Low | Unclear | Low |
| Risk of bias/applicability, n (%) | ||||||||
| Low | 45 (80) | 1 (2) | 54 (96) | 50 (89) | 54 (96) | 8 (14) | 54 (96) | |
| Unclear | 11 (20) | 55 (98) | 2 (4) | 4 (7) | 2 (4) | 48 (86) | 2 (4) | |
| High | 0 (0) | 0 (0) | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | |
Appendix 8 The PROBAST risk-of-bias and applicability assessment
| Study | Test | Risk of bias | Applicability | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants | Predictors | Outcome | Analysis | Overall judgement | Participants | Predictors | Outcome | Overall judgement | ||
| Garcia-Alvarez 201546 | Urine NGAL | Low | Unclear | Unclear | High | High | Low | Low | Low | Low |
| Bennett 200887 | Urine NGAL | Low | Unclear | Unclear | High | High | Low | Low | Low | Low |
| Cullen 201449 | Urine NGAL | Low | Unclear | Unclear | High | High | Low | Low | Low | Low |
| Doi 201470 | Urine NGAL | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Nisula 201575 | Urine NGAL | Low | Unclear | Unclear | High | High | Low | Low | Low | Low |
| Marino 201583 | Plasma NGAL | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Hjortrup 201572 | Urine NGAL, plasma NGAL | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Treeprasertsuk 201559 | Urine NGAL | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Gayat 201832 | NephroCheck | Unclear | Unclear | Unclear | High | High | Unclear | Low | Low | Unclear |
| Mårtensson 201555 | Urine NGAL | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Isshiki 201853 | Urine NGAL | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Lee 201882 | Plasma NGAL | Low | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Low |
Appendix 9 Forest plots of sensitivity and specificity estimates and summary receiver operating characteristic plots
FIGURE 26.
Forest plots of sensitivity and specificity for NephroCheck for detection of AKI in adults: critical care setting. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
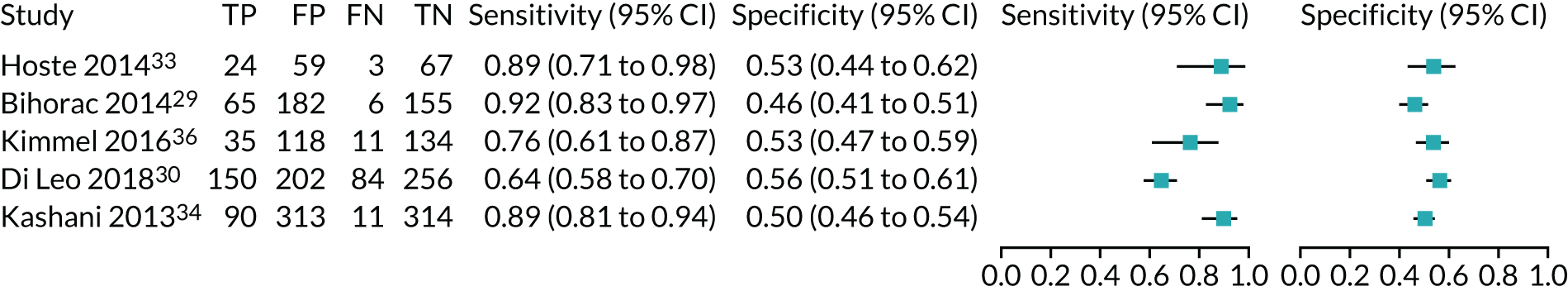
FIGURE 27.
The SROC plot for NephroCheck studies: critical care setting. HSROC, hierarchical summary receiver operating characteristic.
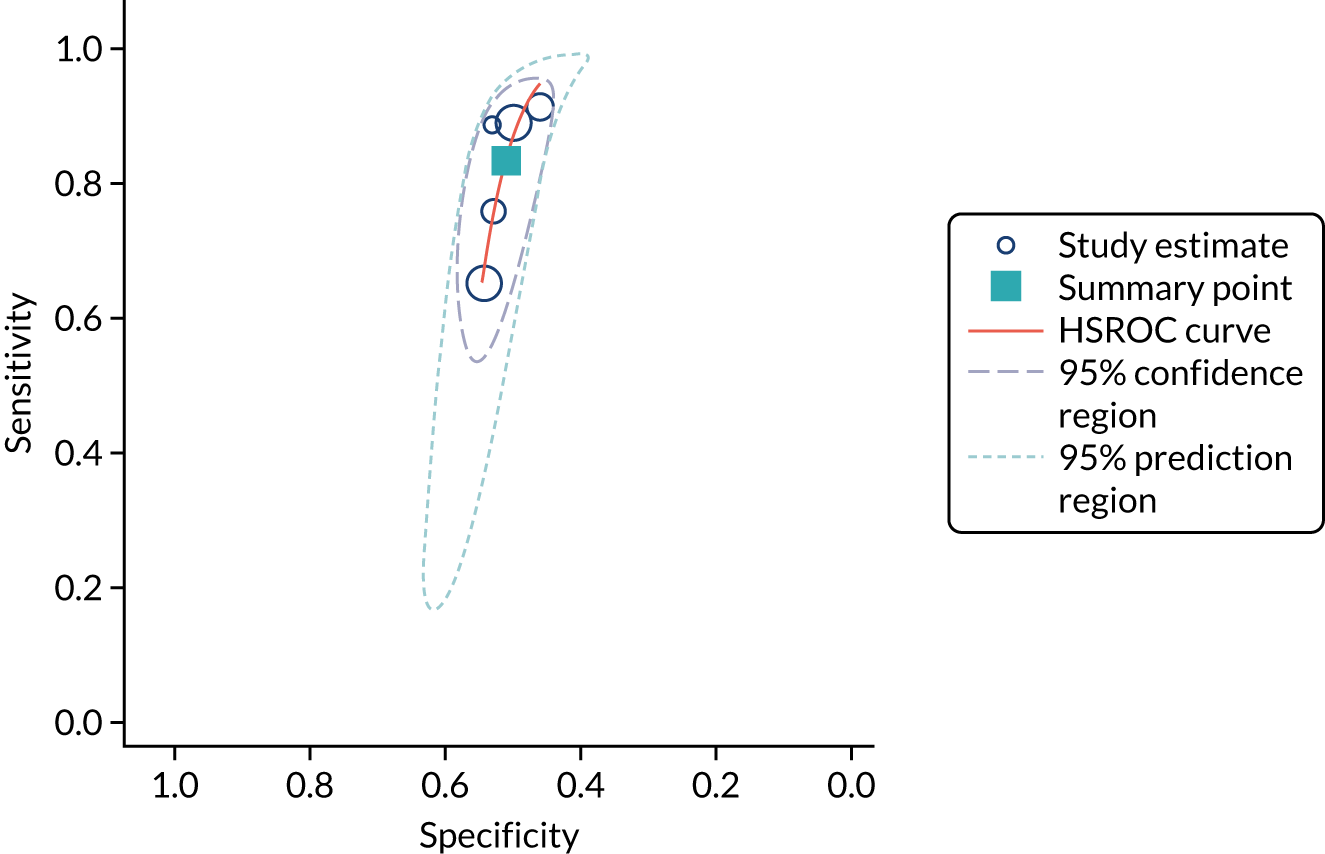
FIGURE 28.
Forest plots of sensitivity and specificity for ARCHITECT urine NGAL for detection of AKI in adults: critical care setting. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
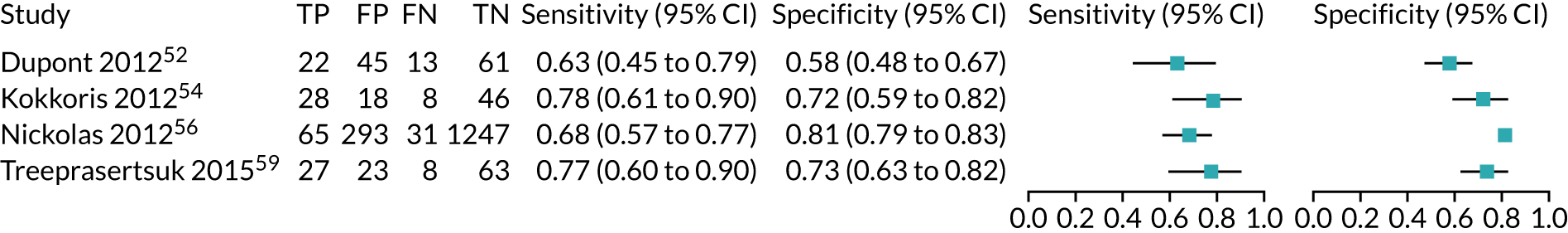
FIGURE 29.
The SROC plot for ARCHITECT urine NGAL studies: clinical care setting (adult population). HSROC, hierarchical summary receiver operating characteristic.

FIGURE 30.
Forest plots of sensitivity and specificity for BioPorto urine NGAL for detection of AKI in adults: critical care setting. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
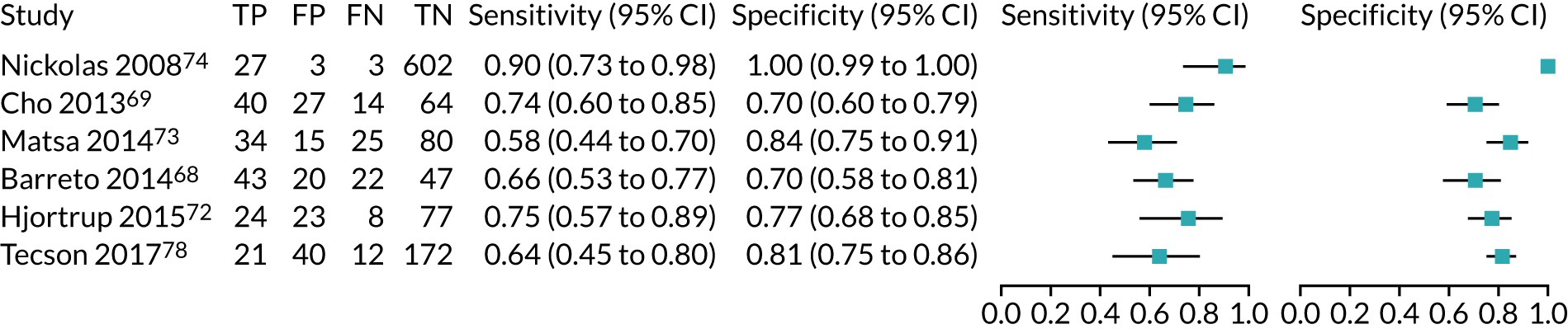
FIGURE 31.
The SROC plot for BioPorto urine NGAL studies: critical care setting. HSROC, hierarchical summary receiver operating characteristic.
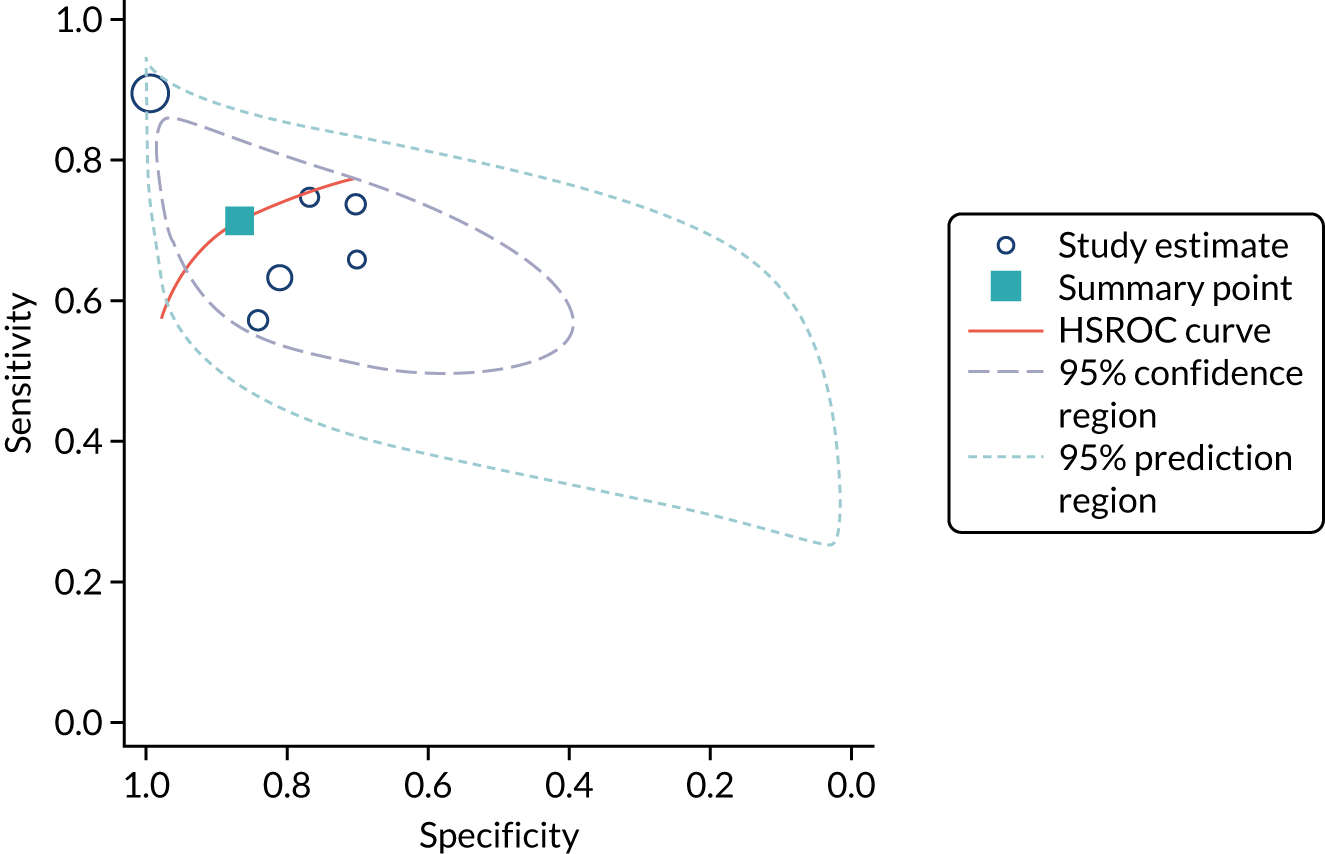
FIGURE 32.
Forest plots of sensitivity and specificity for all urine NGAL assays (ARCHITECT and BioPorto) for detection of AKI in adults admitted to critical care. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
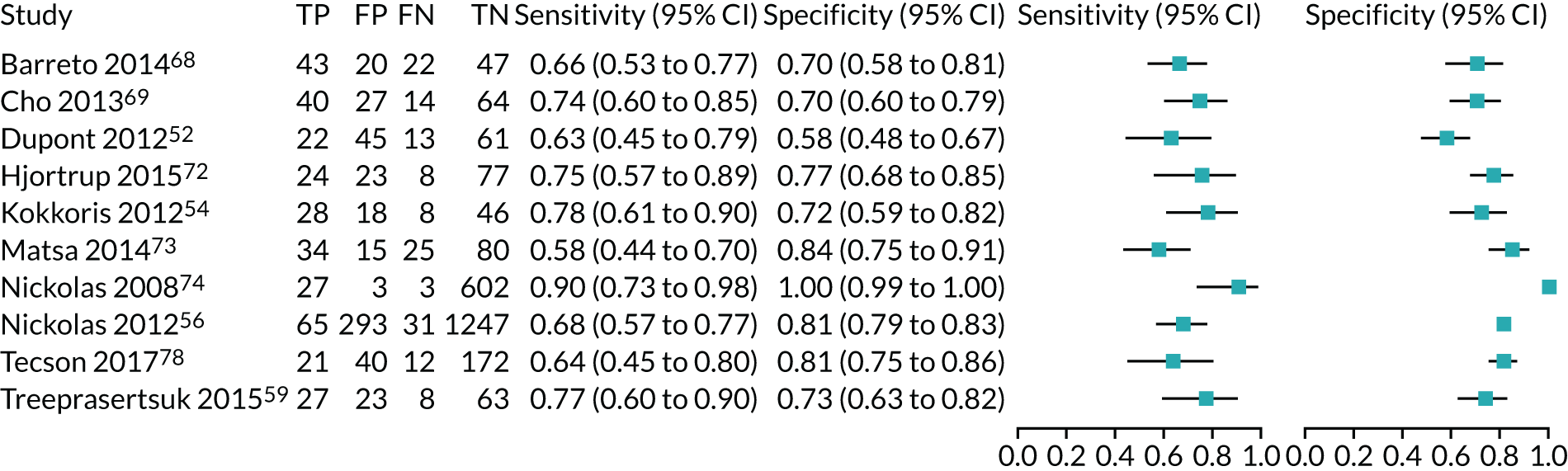
FIGURE 33.
The SROC plot for all urine NGAL assays (ARCHITECT and BioPorto) for detection of AKI in adults: critical care setting. HSROC, hierarchical summary receiver operating characteristic.
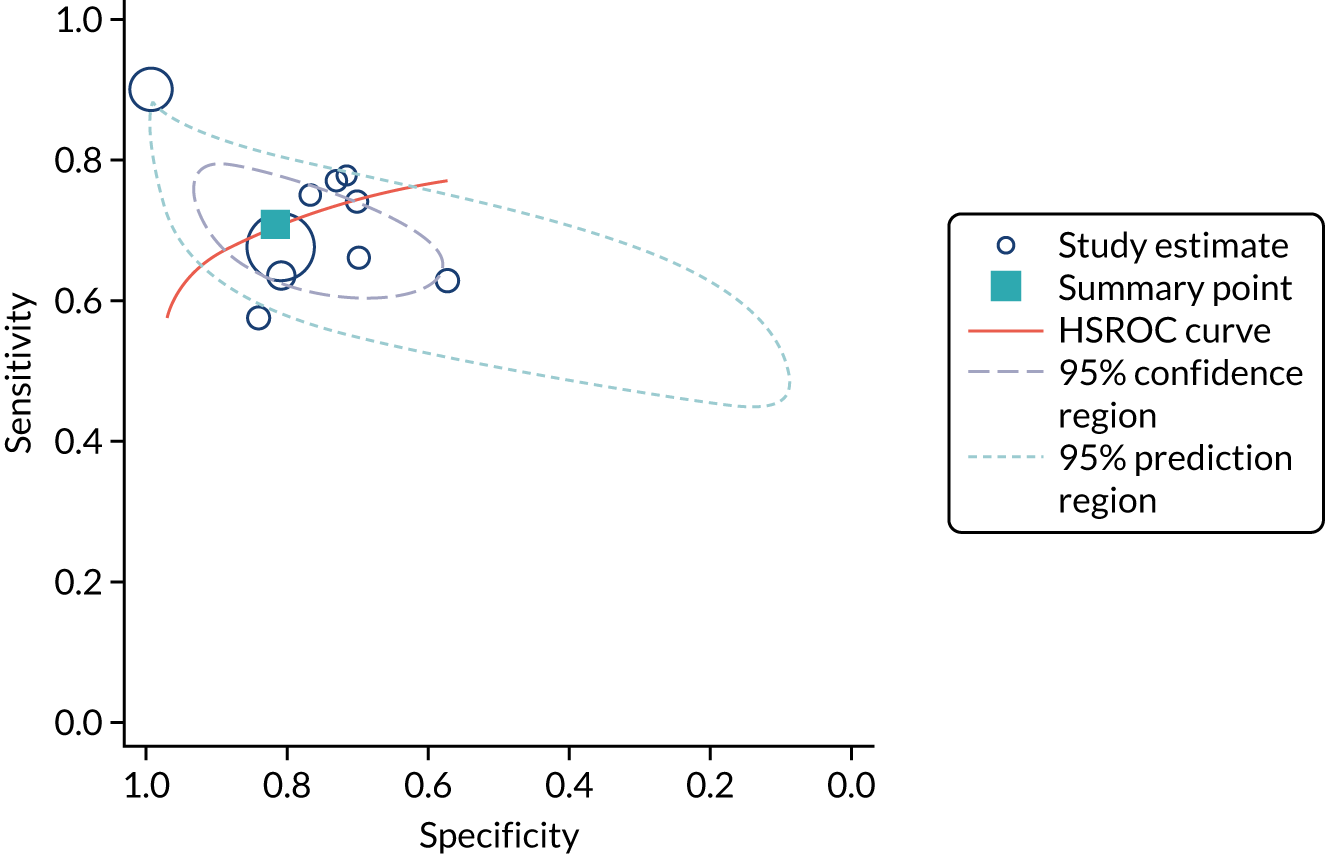
Appendix 10 Forest plots of area under the curve meta-analyses for detection of acute kidney injury
FIGURE 34.
NephroCheck: all settings (adult population).
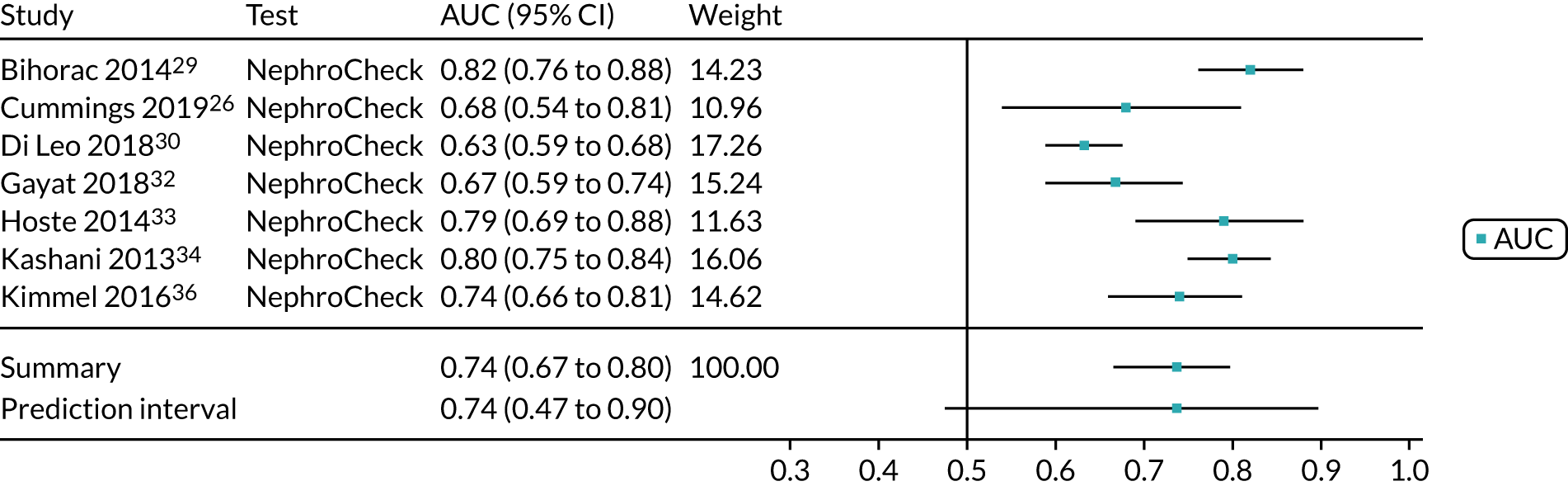
FIGURE 35.
NephroCheck: critical care (adult population).
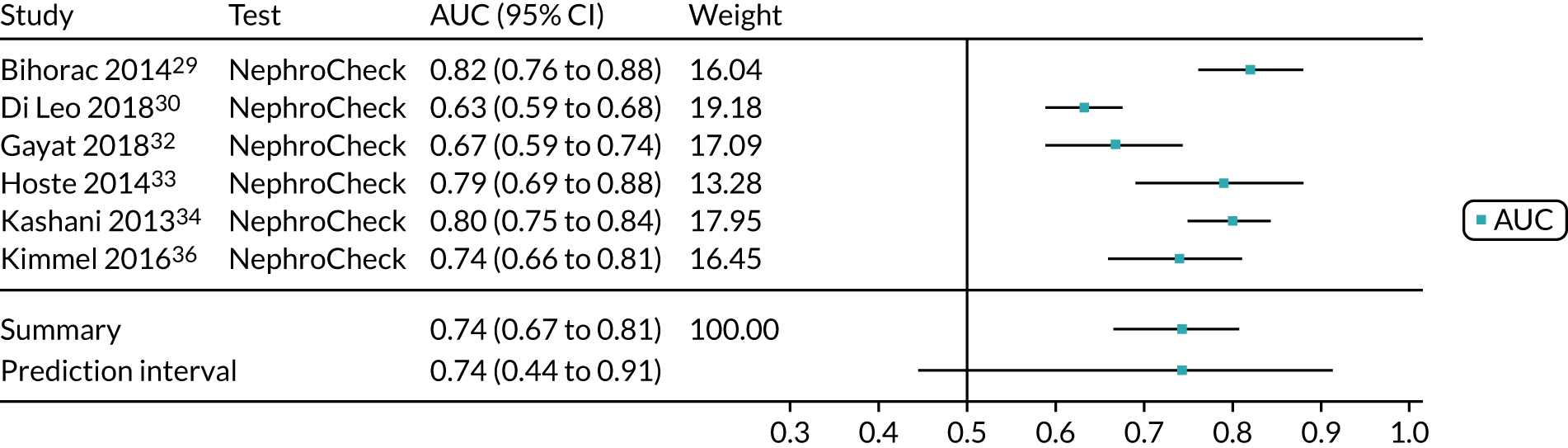
FIGURE 36.
ARCHITECT urine NGAL: all settings (adult population). uNGAL, urine NGAL.
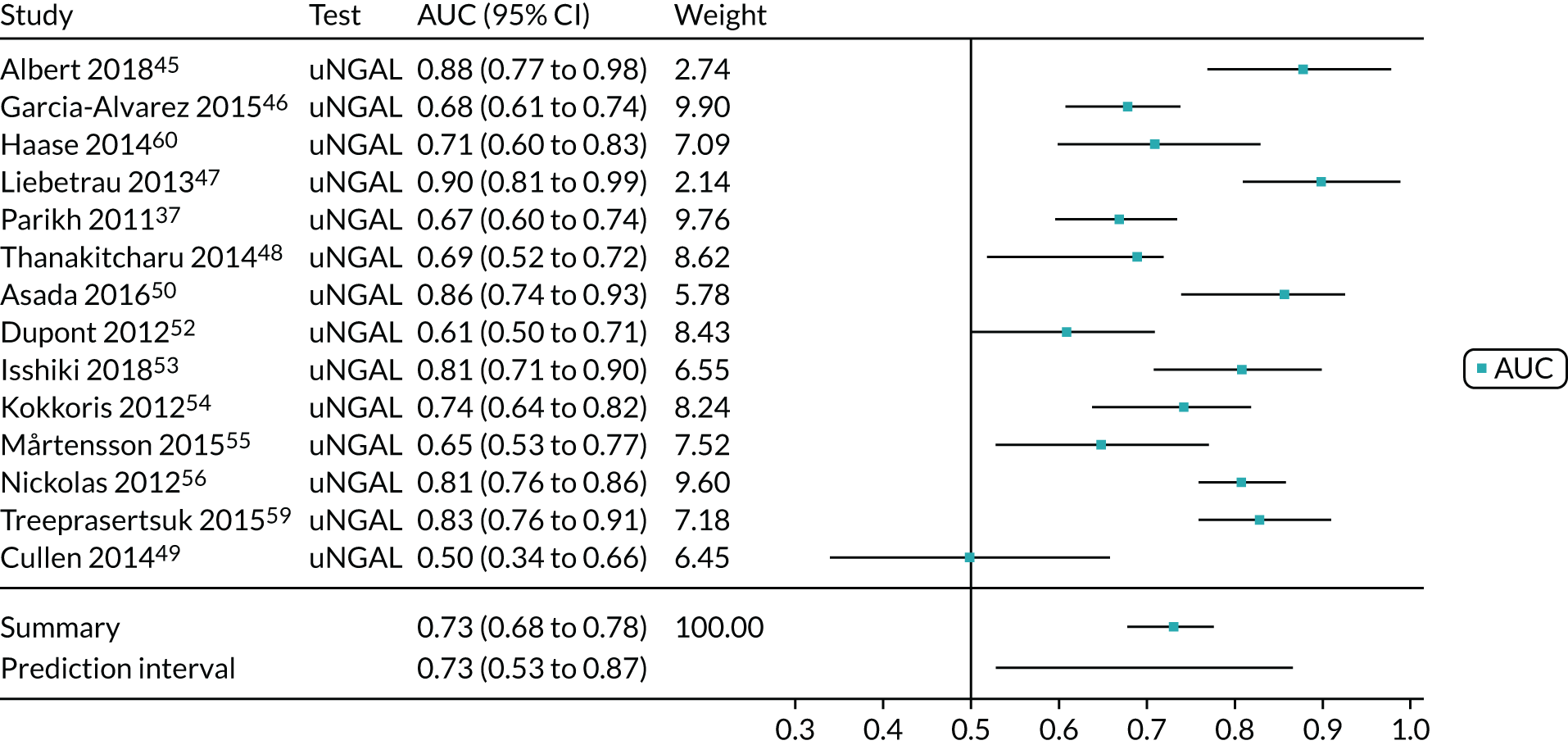
FIGURE 37.
ARCHITECT urine NGAL: cardiac surgery (adult population). uNGAL, urine NGAL.
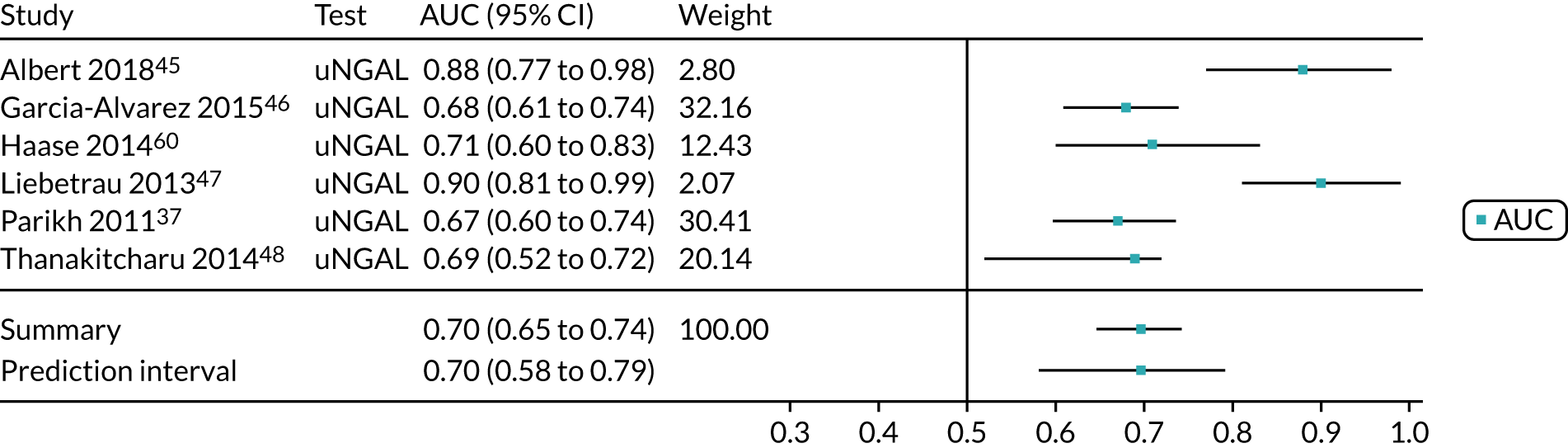
FIGURE 38.
ARCHITECT urine NGAL: critical care (adult population). uNGAL, urine NGAL.
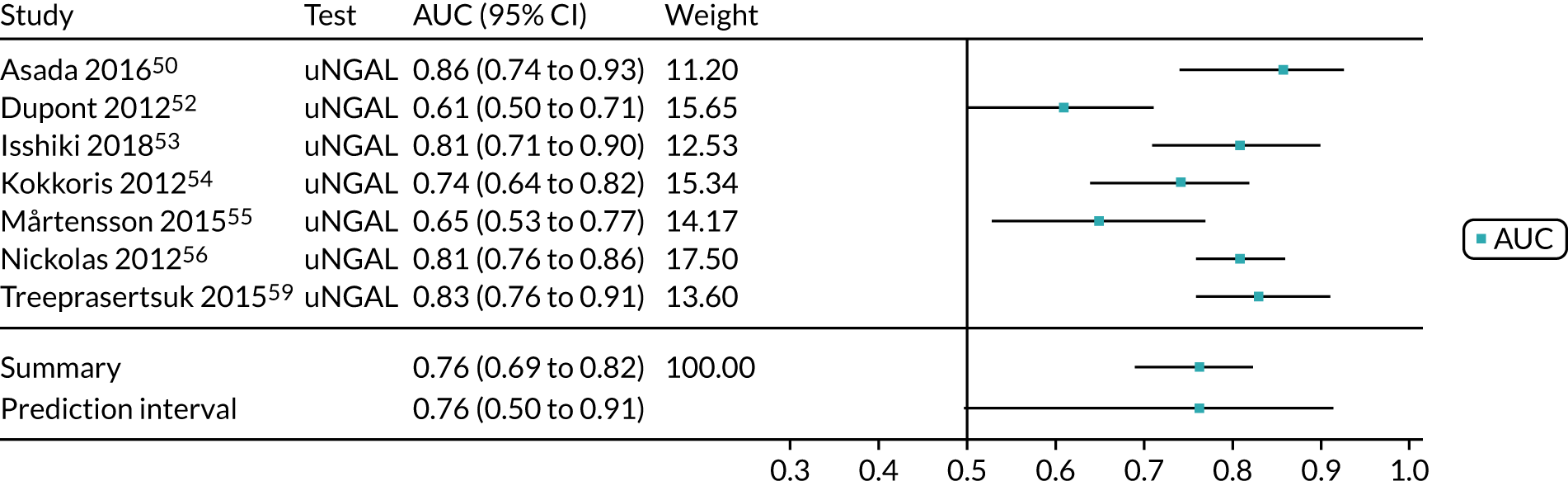
FIGURE 39.
BioPorto urine NGAL: all settings (adult population). uNGAL, urine NGAL.
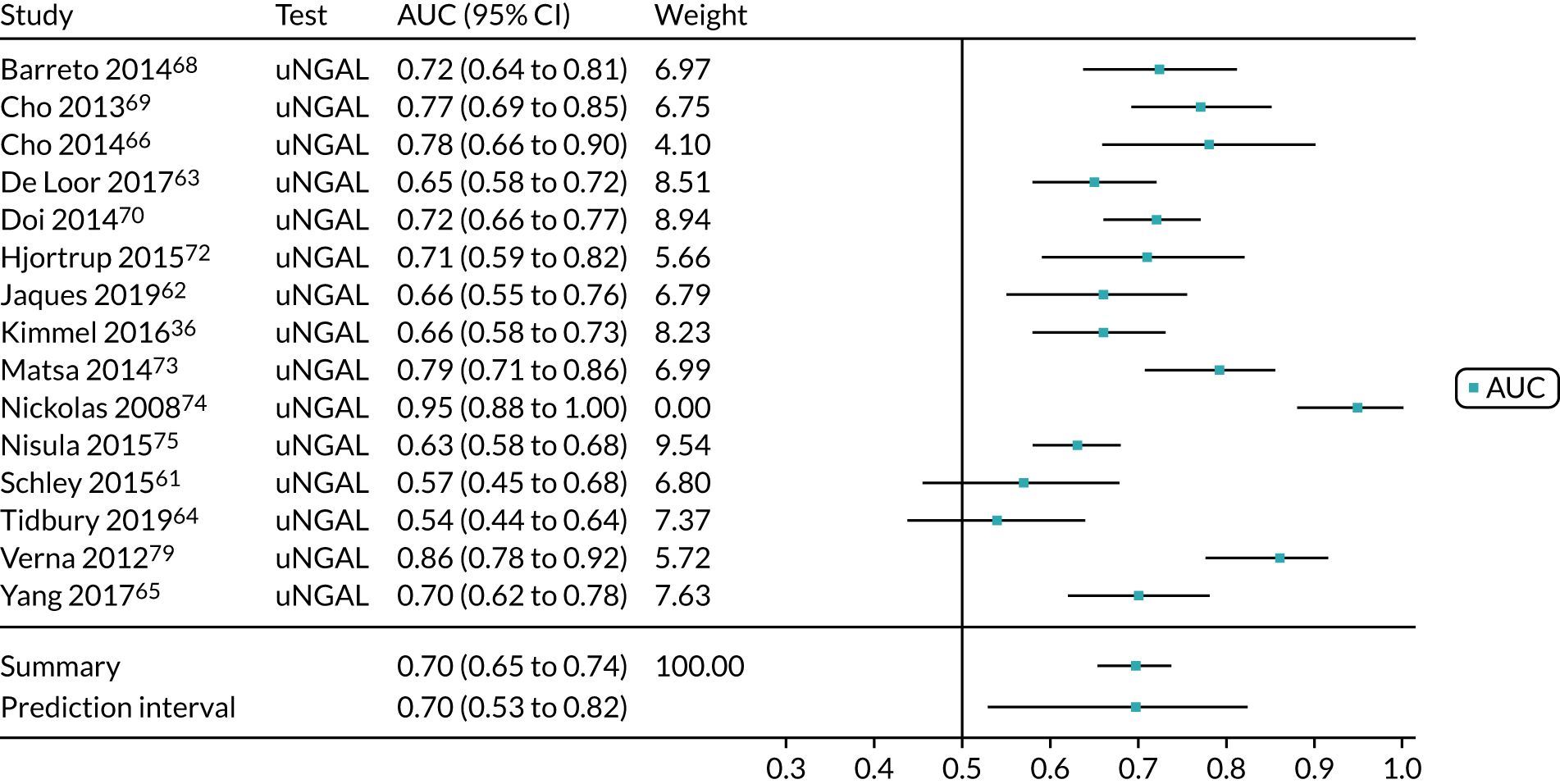
FIGURE 40.
BioPorto urine NGAL: cardiac surgery (adult population). uNGAL, urine NGAL.
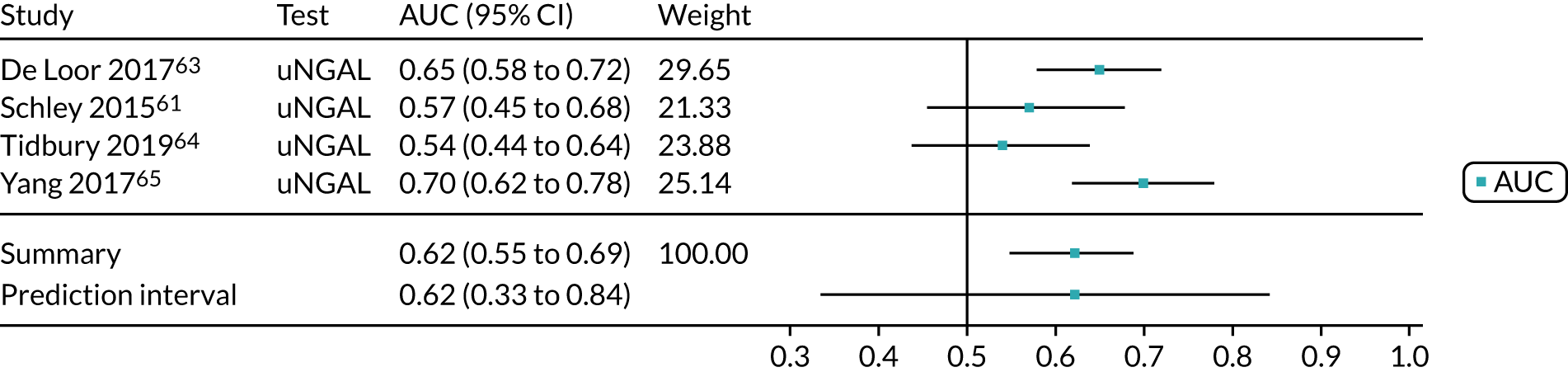
FIGURE 41.
BioPorto urine NGAL: critical care (adult population). uNGAL, urine NGAL.
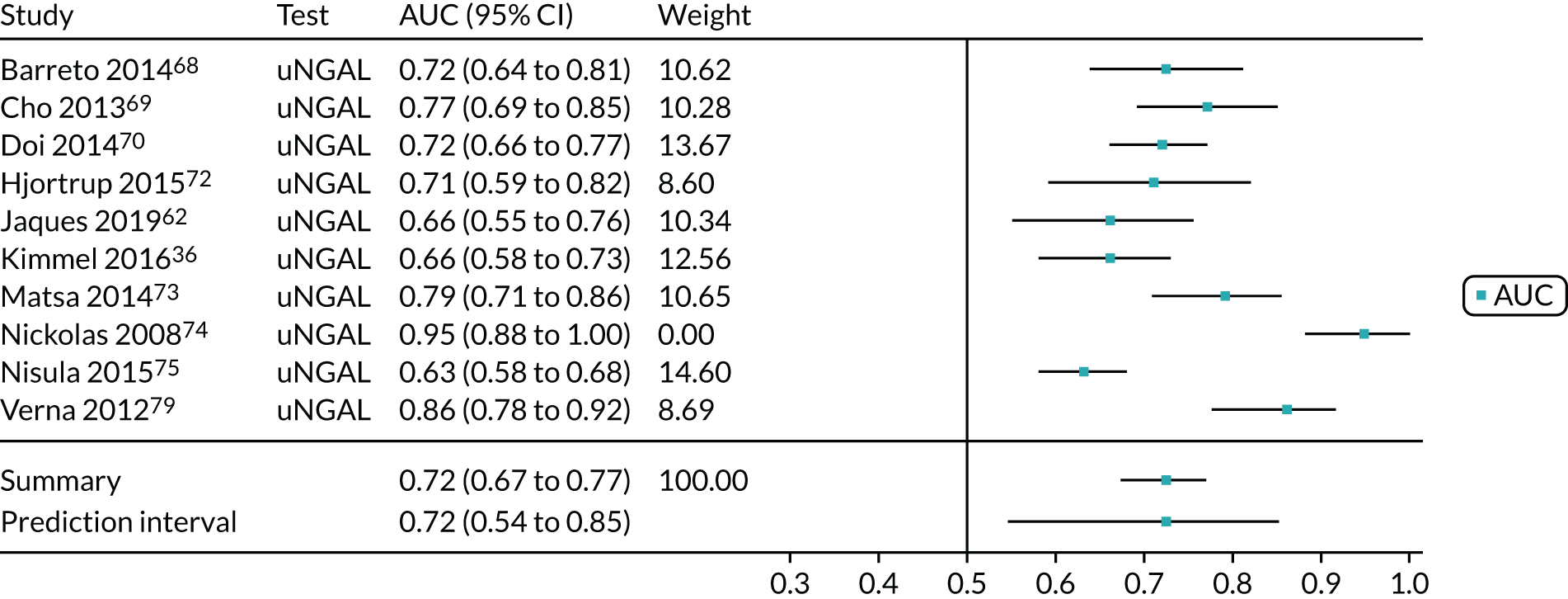
FIGURE 42.
Urine NGAL (ARCHITECT and BioPorto): cardiac surgery (adult population). uNGAL, urine NGAL.
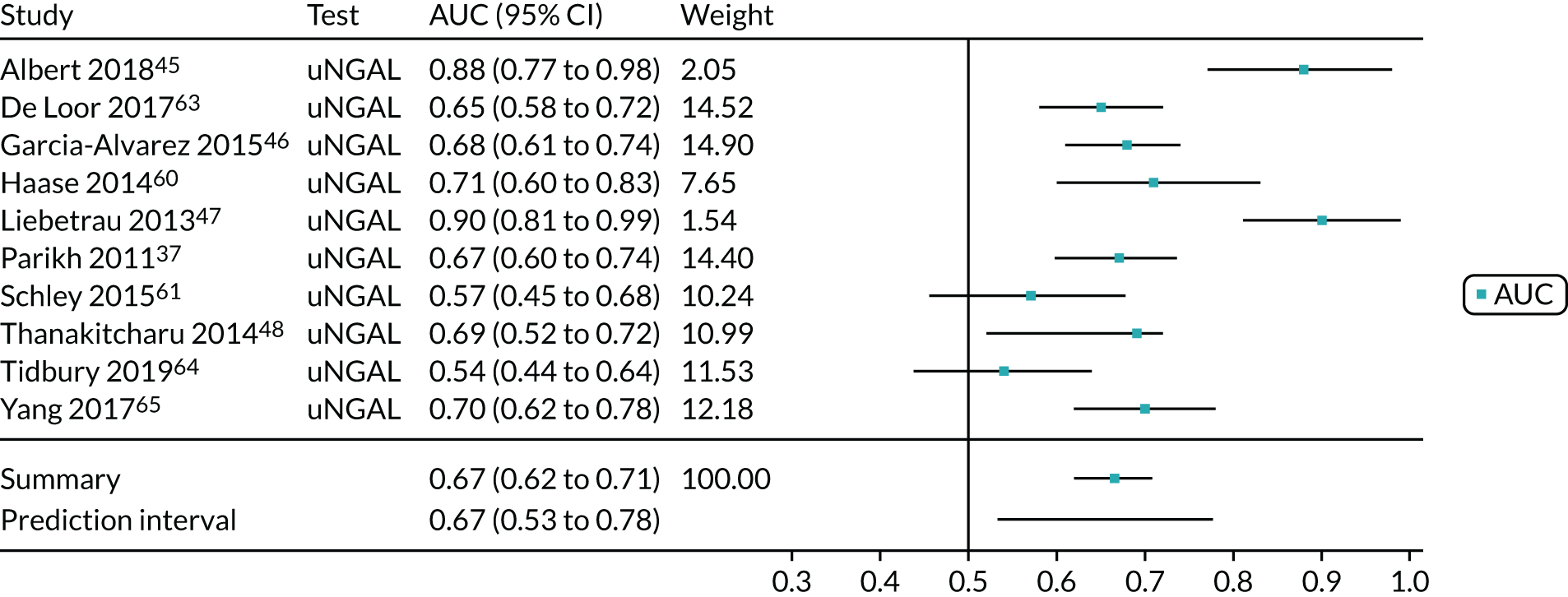
FIGURE 43.
Urine NGAL (ARCHITECT and BioPorto): critical care (adult population). uNGAL, urine NGAL.
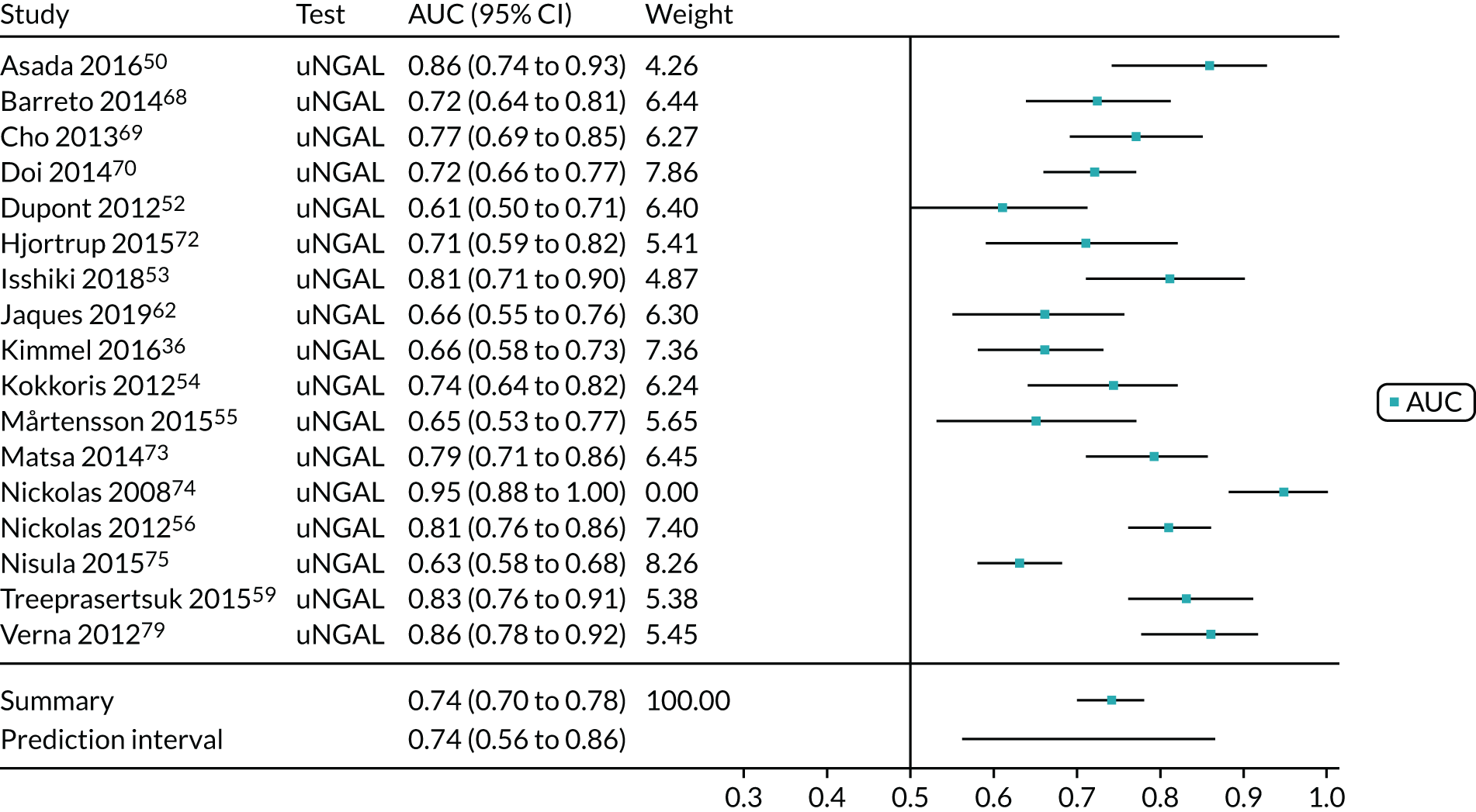
FIGURE 44.
Urine NGAL (ARCHITECT and BioPorto): all settings (adult population). uNGAL, urine NGAL.
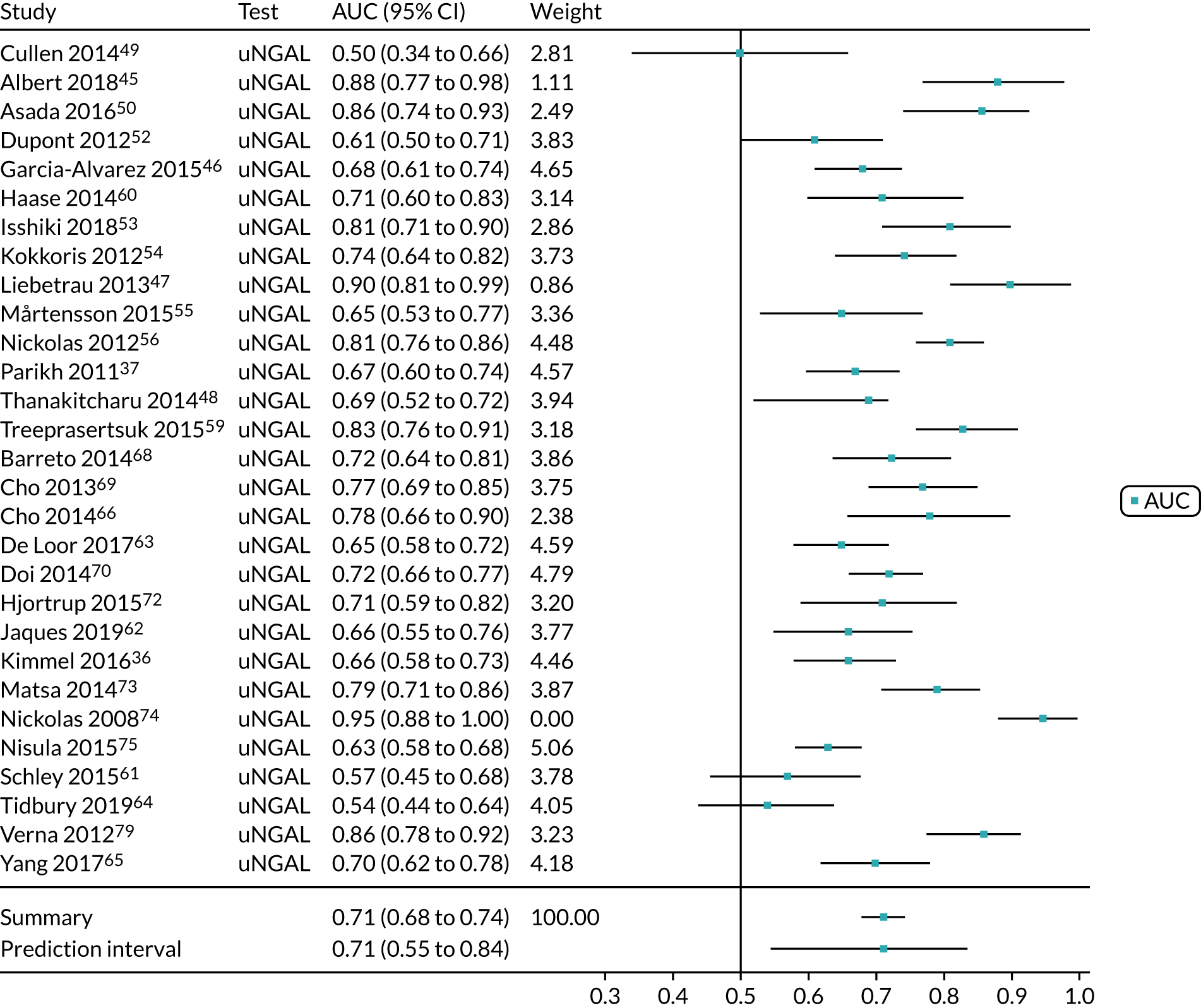
FIGURE 45.
BioPorto plasma NGAL: all settings (adult population). pNGAL, plasma NGAL.
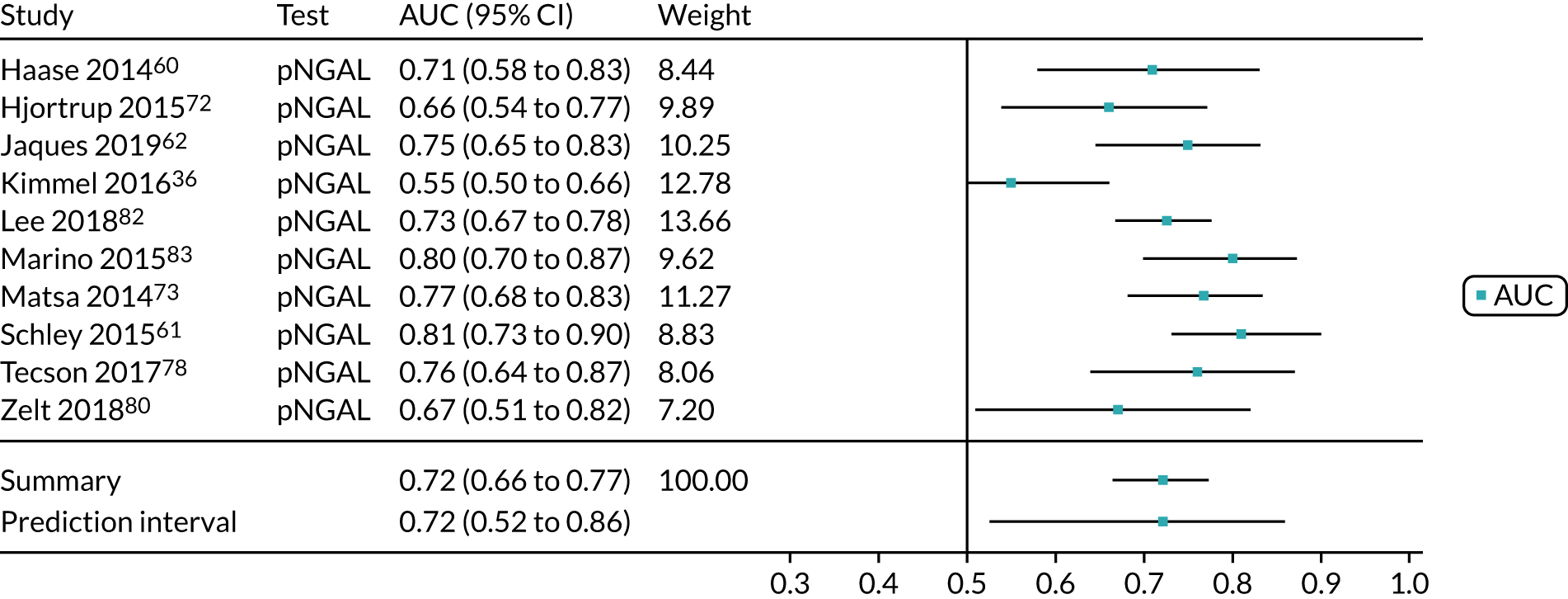
FIGURE 46.
BioPorto plasma NGAL: cardiac surgery (adult population). pNGAL, plasma NGAL.
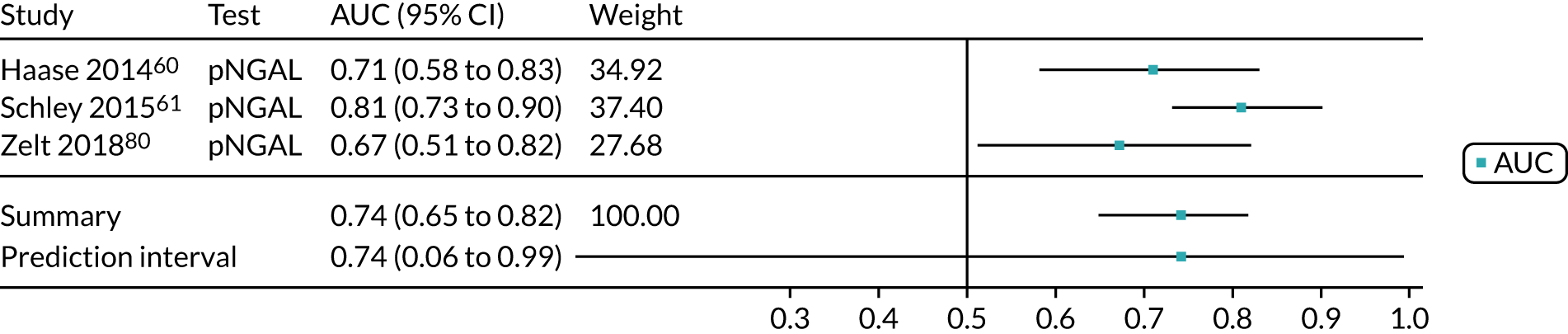
FIGURE 47.
BioPorto plasma NGAL: critical care (adult population). pNGAL, plasma NGAL.
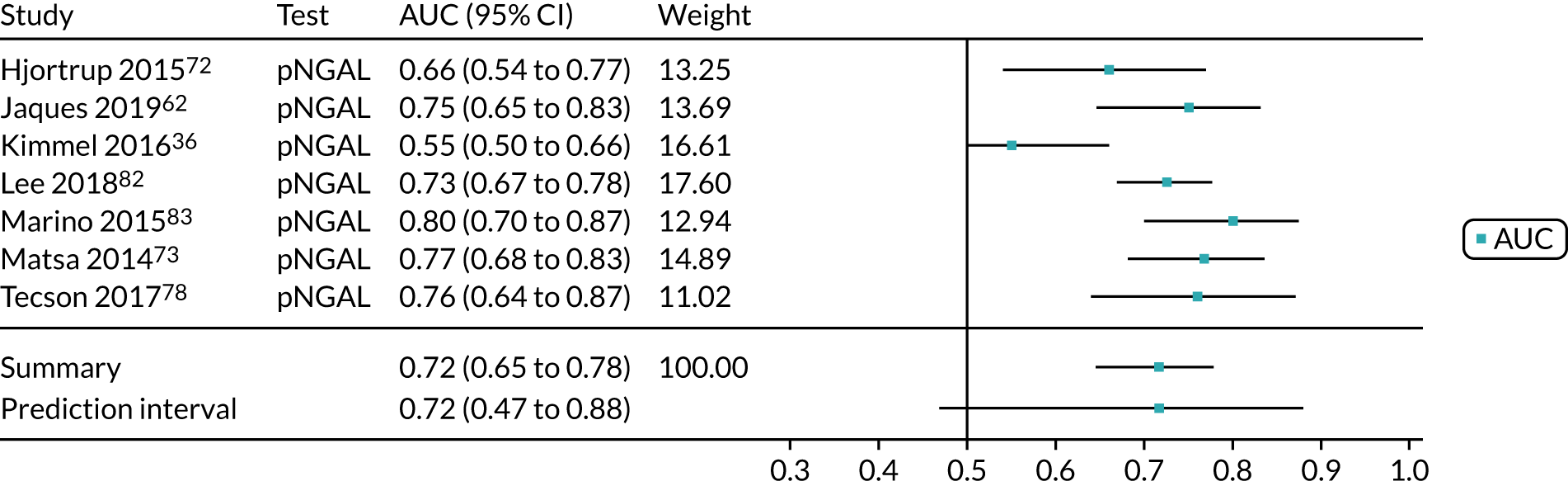
FIGURE 48.
Urine NGAL (ARCHITECT and BioPorto): all settings (child population). uNGAL, urine NGAL.
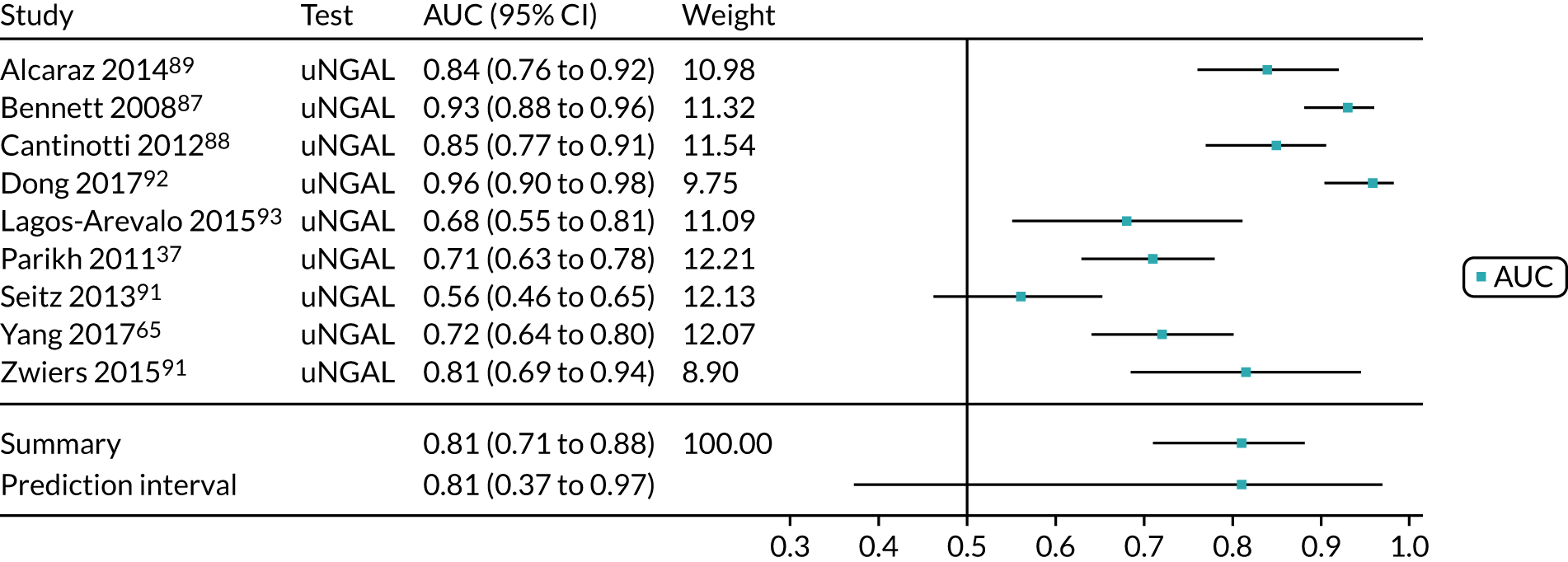
FIGURE 49.
ARCHITECT urine NGAL: cardiac surgery (child population). uNGAL, urine NGAL.

FIGURE 50.
Urine NGAL (ARCHITECT and BioPorto): all cardiac surgery (child population). uNGAL, urine NGAL.
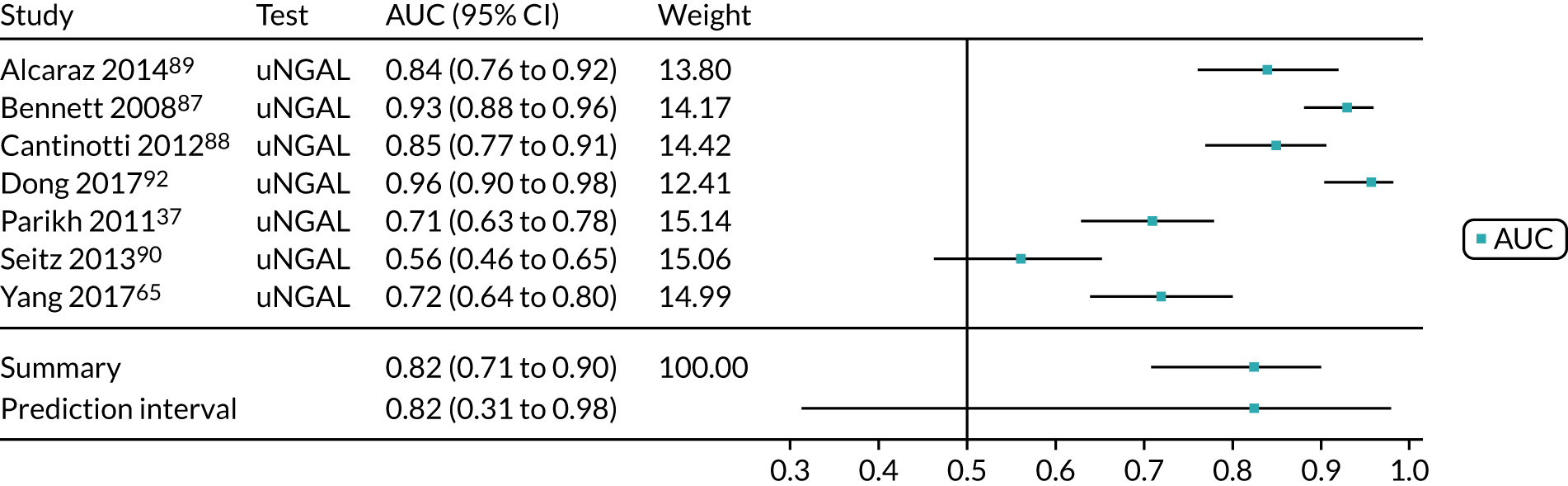
Appendix 11 Forest plots of area under the curve meta-analyses for prediction of worsening of acute kidney injury, mortality and need for renal replacement therapy
FIGURE 51.
Prediction of AKI among adults: ARCHITECT urine NGAL – critical care setting. uNGAL, urine NGAL.

FIGURE 52.
Prediction of mortality among adults: BioPorto urine NGAL – critical care setting. uNGAL, urine NGAL.
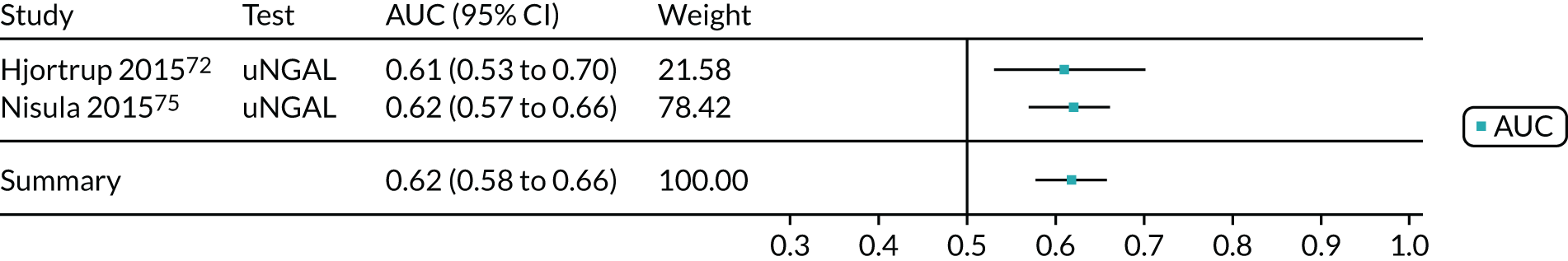
FIGURE 53.
Prediction of mortality among adults: BioPorto plasma NGAL – critical care setting. pNGAL, plasma NGAL.
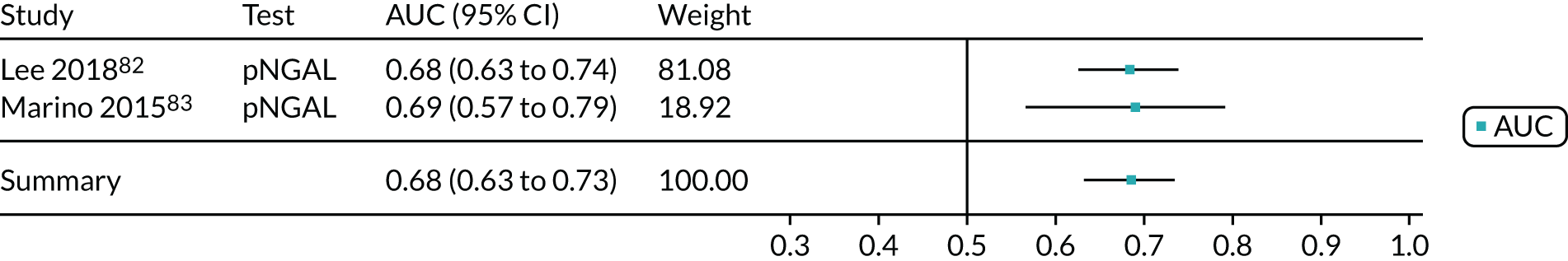
FIGURE 54.
Prediction of mortality among adults: urine NGAL (ARCHITECT and BioPorto) – critical care setting. uNGAL, urine NGAL.
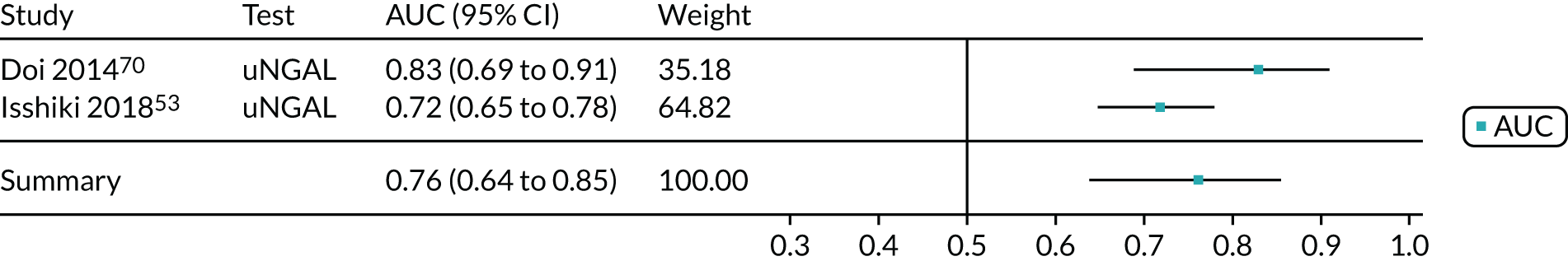
FIGURE 55.
Prediction of RRT among adults: BioPorto urine NGAL – critical care setting. uNGAL, urine NGAL.
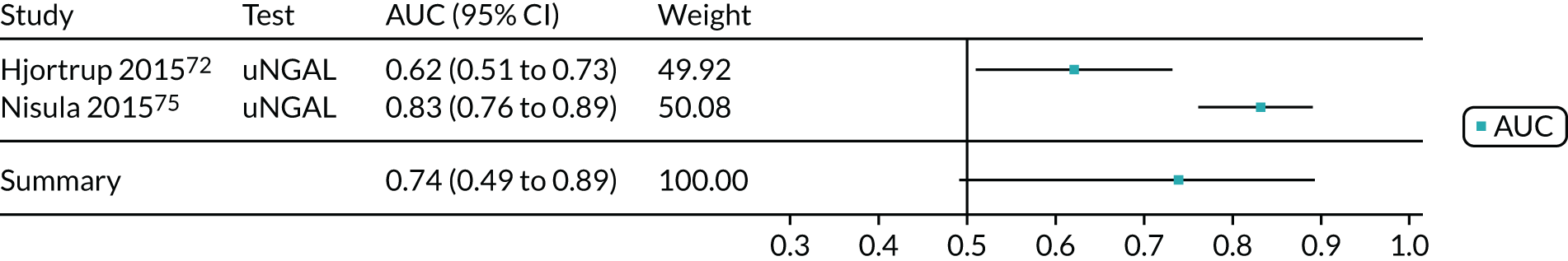
Appendix 12 Addition of biomarkers to existing clinical models
| Study, geographical location, biomarker, setting | Diagnosis or prediction | AUC (95% CI or SEM) | OR (95% CI) | Adjustment of the model | ||||
|---|---|---|---|---|---|---|---|---|
| Clinical model | Biomarker | Biomarker plus clinical model | Clinical model | Biomarker | Biomarker plus clinical model | |||
| AKI | ||||||||
| Kashani 2013,34 North America and Europe, NephroCheck, critical care – mixed population |
Diagnosis of AKI within 12 hours Events: 101 |
0.81 (0.76 to 0.85)a | 0.80 (0.75–0.84)a | 0.87 (0.84 to 0.90)a | NR | NR | NR | Age, serum creatinine level, APACHE III score, hypertension, nephrotoxic diagnosis, liver disease, diabetes mellitus and CKD |
| Bihorac 2014,29 USA, NephroCheck, critical care – mixed population |
Diagnosis of AKI within 12 hours Events: 71 |
0.70 (0.63 to 0.76); p < 0.001 | NR | 0.86 (0.80 to 0.90); p < 0.001 | NR | NR | NR | Included clinical variables for which a univariate association with AKI at p < 0.1 was found. Also included serum creatinine and the KDIGO criteria. They used a univariate significance level of < 0.1. Final model seems to include enrolment serum creatinine level, APACHE III score (non-renal), BMI |
| Parikh 2011,37 North America, ARCHITECT urine NGAL, cardiac surgery |
Diagnosis of AKI within 72 hours Events: 60 |
0.69 (0.04) | 0.67 (0.04) | 0.73 (0.04); p = 0.12 | NR | NR | NR | Variables included in the clinical model were age, sex, white ethnicity, CPB time of > 120 minutes, non-elective surgery, pre-operative eGFR, diabetes, and hypertension. The improvement of risk prediction with the addition of biomarkers to the clinical model was determined using the NRI index and the IDI |
| Schley 2015,61 Germany, BioPorto urine and plasma NGAL, cardiac surgery |
Diagnosis of AKI within 72 hours of surgery Events: 37 |
0.76; p < 0.001 |
|
|
NR | NR | NR | The clinical model was based on the European System for Cardiac Operative Risk Evaluation (EuroSCORE). A multivariable analysis was conducted to analyse the combination of biomarkers and clinical scores |
| Kokkoris 2012,54 Greece, ARCHITECT urine NGAL, critical care – mixed population |
AKI detection within 7 days Events: 36 |
0.76 (0.66 to 0.83) | 0.78 (0.68 to 0.85) | 0.85 (NR); p = 0.03 | NR | NR | NR | The most efficient reference clinical model for AKI prediction included the SAPS III and INR. Addition of plasma NGAL to the clinical model improved the AUC. However, the combination of plasma NGAL and serum creatinine showed the best AUC (0.86; p = 0.04) |
| Isshiki 2017,53 Japan, ARCHITECT urine NGAL, critical care – mixed population |
Worsening kidney function within 7 days Events: 58 |
0.85 (0.77 to 0.92) | 0.74 (0.65 to 0.84) | 0.85 (0.77 to 0.92) | NR | NR | NR | Variables included in the clinical model for the prediction of newly developed AKI were age, sex, APACHE II score, sepsis, baseline eGFR, serum creatinine level at ICU admission |
| Lee 2018,82 the Republic of Korea, BioPorto plasma NGAL, critical care – mixed population |
Development of AKI Events: 111 |
NR | NR | NR | 5.31 (0.67 to 11) | 0.6 (0.2 to 1.7); p = 0.314 | 1.004 (1.002 to 1.006); p = 0.001 | Adjusting for potential confounders as determined by the univariate analyses. The adjusted model includes age, CHF, diabetes mellitus, adrenaline dosage, time to ROSC, Glasgow Coma Scale score, lactate, PaO2 and PaCO2 after ROSC, initial creatinine level, SOFA score, CVI and NGAL level. Of these, only SOFA renal, NGAL and CVI were significant, but a final model was not selected |
| Alcaraz 2014,89 Spain, ARCHITECT urine NGAL, cardiac surgery – child population |
Prediction of AKI Events: 36 |
0.85 (0.78 to 0.93) | 0.84 (0.76 to 0.92) | 0.91 (0.84 to 0.97); p = 0.057 | NR | NR | NR | A multivariable logistic regression analysis was used to assess the predictors of AKI and the performance of the model. The clinical model (age, CPB time, total circulatory arrest use, and RACHS-1 score) was determined using backward elimination |
| Mortality | ||||||||
| Isshiki 2017,53 Japan, ARCHITECT urine NGAL, critical care – mixed population | In-hospital mortality (38 patients died) | 0.79 (0.71 to 0.86) | 0.72 (0.65 to 0.78) | 0.79 (0.71 to 0.86) | NR | NR | NR | Variables included in the clinical model for the prediction of mortality were age, sex, APACHE II score, sepsis. Variables were derived from univariate logistic regression analysis |
| Verna 2012,79 USA, BioPorto urine NGAL, critical care – mixed population (cirrhosis) | In-hospital mortality (15 patients died) | NR | NR | NR | 2.95 (1.68 to 5.61) | 2.00 (1.36 to 2.94) | 6.05 (1.35 to 27.2) | Adjusted for age, serum creatinine level, MELD score of > 17, hepatorenal syndrome |
Appendix 13 Assessment of cost-effectiveness: additional tables
| Cost element | NephroCheck | BioPorto urine and plasma NGAL | ARCHITECT urine NGAL | Alinity ia urine NGAL |
|---|---|---|---|---|
| Platform | ||||
| Astute140 Meter (NephroCheck only) | ||||
| Cost (£) | 3000.00 | – | – | – |
| Expected service life (years) | 5.00 | – | – | – |
| Equivalent annual cost (£) | 664.44b | – | – | – |
| Subtotal: platform (cost per test) (£) | 0.53c | – | – | – |
| Subtotal: equipment (cost per test) (£) | 49.80d | 20.00e | 25.71f | 28.29g |
| Subtotal: maintenance/consumables (cost per test)h (£) | 4.23 | 1.90 | 3.51 | 3.51 |
| Staff resource use | ||||
| Time to conduct test (sample preparation plus time to get result) (minutes) | 20 | 20 | 20 | 20 |
| Time to interpret test (minutes) | 5 | 5 | 5 | 5 |
| Prepare urine sample: band 5 nurse (minutes) | 15 | 15 | 15 | 15 |
| Bring urine sample to laboratory: porter (minutes) | 15 | 15 | 15 | 15 |
| Cost of staff time for testing (per test) (£) | 14.67 | 14.67 | 14.67 | 14.67 |
| Cost of staff time for interpreting (per test) (£) | 6.89 | 6.89 | 6.89 | 6.89 |
| Cost of staff time to prepare urine sample (per test) (£) | 9.25 | 9.25 | 9.25 | 9.25 |
| Cost of delivery to laboratory (per test) (£) | 6.82 | 6.82 | 6.82 | 6.82 |
| Subtotal: staff costs (per test) (£) | 37.62 | 37.62 | 37.62 | 37.62 |
| Staff trainingi | ||||
| Assumed average turnover (years) | 5 | 5 | 5 | 5 |
| Time for training (minutes) | 90 | 30 | 30 | 30 |
| Total training costs (£) | 438.00 | 146.00 | 146.00 | 146.00 |
| Equivalent annual cost of total training (£) | 97.01 | 32.34 | 32.34 | 32.34 |
| Equivalent annual cost of total training per test (£) | 0.08 | 0.03 | 0.03 | 0.03 |
| Total cost (£) | 92.26 | 59.55 | 66.87 | 69.44 |
| Resource use | Assumptions | Care bundle cost (£) | Source |
|---|---|---|---|
| Intravenous fluids | |||
| Intravenous sodium chloride, 0.9% infusion, 2-l bags (Terumo BCT Ltd, Larne, UK) | 1 l per hour for 3 hours, thereafter 2 l per day for 3 days (five 2-l bags total) | 22.14 | Clinical expert opinion,a BNF 2019126 |
| Band 6 nurse | Initial fluid: 10 minutes | 5.33 | Clinical expert opinion,a PSSRU 2018124 |
| Band 6 nurse | Fluid replacement: 5 minutes | 10.67 | Clinical expert opinion,a PSSRU 2018124 |
| Nephrologist review | |||
| Hospital-based doctor, medical consultant | 30 minutes | 54.00 | Clinical expert opinion,a PSSRU 2018124 |
| Pharmacist review | |||
| Pharmacist, band 6 (AfC) | 20 minutes | 15.00 | Clinical expert opinion,a PSSRU 2018124 |
| Stop blood pressure medication | |||
| N/A | Stop blood pressure medication for 3 days | –0.78 | Clinical expert opinion,a BNF 2019.126 Based on the annual cost of blood pressure medication (see Table 36), and calculated over 3 days |
| Total cost for 3 additional days of the KDIGO care bundle | 106.36 | ||
| First author | Year | Population | Country | Utility measure | Valuation set | Sample size (n) | Age (years)a | Male (%) | Utility values reported | Mean | Median | SE | SD | 95% CI | IQR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afiatin151 | 2017 | ESRD with PD and HD | Indonesia | EQ-5D-3L | Thailand | 68 | ≥ 18 | 55.9 | PD (no comp) | 0.82 | 0.03 | ||||
| HD (no comp) | 0.70 | 0.04 | |||||||||||||
| PD (+comp) | 0.31 | 0.09 | |||||||||||||
| HD (+comp) | 0.37 | 0.11 | |||||||||||||
| Chang152 | 2016 | ESRD with PD and HD | Taiwan | EQ-5D-3L | UK | Total: 1687 | Total: NR | ||||||||
| HD: 1403 | HD: mean 57.1 (SD 13.6) | HD: 49.9 | HD | 0.83 | 0.19 | ||||||||||
| PD: 284 | PD: mean 46.7 (SD 13.2) | PD: 51.1 | PD | 0.90 | 0.16 | ||||||||||
| Cho153 | 2018 | CKD requiring dialysis | The Republic of Korea | EQ-5D-3L | The Republic of Korea | 50 | NR | NR | CKD, undergoing dialysis | 0.63 | 0.04 | ||||
| Eriksson154 | 2016 | CKD patients (anaemic and non-anaemic) with/without dialysis | France, Germany, Italy, Spain, UK | EQ-5D-3L | Unclear, presume UK | Total: 1177
|
Mean 63.7 (SD 15.1) | 60 | Non-anaemic: (27%) | Non-anaemic: (27%) | Non-anaemic: (27%) | ||||
| CKD stage 3 | 0.85 | 0.21 | |||||||||||||
| CKD stage 4 | 0.81 | 0.22 | |||||||||||||
| Dialysis | 0.74 | 0.29 | |||||||||||||
| Total | 0.83 | 0.23 | |||||||||||||
| Anaemic: (73%) | Anaemic: (73%) | Anaemic: (73%) | |||||||||||||
| CKD stage 3 | 0.78 | 0.29 | |||||||||||||
| CKD stage 4 | 0.71 | 0.28 | |||||||||||||
| Dialysis | 0.70 | 0.32 | |||||||||||||
| Total | 0.72 | 0.31 | |||||||||||||
| El Filali155 | 2017 | Chronic HD patients | Morocco | EQ-5D-3L | Unclear | 103 | Mean 49.7 (SD 14.7) | 45.60 | HD | 0.41 | 0.36 | ||||
| Hishii156 | 2018 | Chronic HD patients | Japan | EQ-5D-3L | Unclear | 60 | Mean 71.1 (SD 12) | 51.67 | HD | 0.688 | 0.233 | ||||
| Jardine157 | 2017 | Maintenance HD | Australia (28%), Canada (6%), China (62%), New Zealand (4%) | EQ-5D-3L | Unclear | 200 | Mean 51.8 (SD 12.1) | 69.50 | HD | 0.78 | 0.24 | ||||
| Jesky158 | 2016 | Pre-dialysis CKD (as per NICE guidance159) | UK | EQ-5D-3L | UK |
|
[Median (IQR) values]
|
|
All CKD | 0.74 | 0.66–0.88 | ||||
| G1/2 | 0.85 | 0.70–1 | |||||||||||||
| G3a | 0.80 | 0.69–1 | |||||||||||||
| G3b | 0.80 | 0.68–1 | |||||||||||||
| G4 | 0.74 | 0.62–0.85 | |||||||||||||
| G5 | 0.73 | 0.62–1 | |||||||||||||
| Katayama160 | 2016 | Chronic HD patients | Japan | EQ-5D-3L | Unclear | Baseline: 71 | Mean 70.9 (SD 10.6) | 58 | HD (baseline) | 0.720 | 0.224 | ||||
| 1-year follow-up: 43 | Mean 69.1 (SD 10.8) | 60 | HD (1 year) | 0.790 | 0.181 | ||||||||||
| Kilshaw161 | 2016 | ESRD (CM) | UK | EQ-5D-5L | None | 41 | Mean 82.7 (SD 5.7) | 56 | NR | NR | NR | NR | NR | NR | NR |
| Kularatna162 | 2019 | CKD | Sri Lanka | EQ-5D-3L | UK | Early stage: 254 | Median: ≈ 41 | 56.10 | Early | 0.588 | 0.30 | ||||
| Stage 4: 614 | Stage 4 | 0.566 | 0.42 | ||||||||||||
| Stage 5: 151 | Stage 5 | 0.467 | 0.42 | ||||||||||||
| Dialysis: 38 | Dialysis | 0.126 | 0.39 | ||||||||||||
| Lee163 | 2016 | Early- to mid-stage CKD | The Republic of Korea | EQ-5D-3L | The Republic of Korea | CKD stage 3/4: 75 | Mean 61.4 (SD 9.9) | 41 | CKD stage 3/4 | 0.87 | 0.19 | ||||
| CAPD: 75 | Mean 59.1 (SD 12.9) | 41 | CAPD | 0.90 | 0.15 | ||||||||||
| Li164 | 2017 | Kidney transplant recipients and waiting list | UK | EQ-5D-5L | UK value set | Transplant recipients: 512 | Median: ≈ 50 | 60 | Waiting list | 0.773 | 0.005 | ||||
| Waiting list: 1704 | Median: ≈ 50 | 58 | Transplant (inc) | 0.054 | 0.011 | ||||||||||
| McNoe165 | 2019 | ESRD with or without dialysis | New Zealand | EQ-5D-3L | VAS only | No dialysis: 56 | ≥ 65 | 66.1 | No dialysis | 70 | 50–80 | ||||
| HD: 109 | 57.8 | HD | 70 | 60–80 | |||||||||||
| PD: 60 | 73.3 | PD | 67.5 | 70–80 | |||||||||||
| Nagasawa166 | 2018 | Patients receiving dialysis | Japan | EQ-5D-3L | Japan | 51 | Mean 67.7 (SD 12.1) | 70.60 | Dialysis patients with CKD or ESRD | 0.779 | 0.193 | ||||
| Nguyen136 | 2018 | CKD and ESRD | UK | EQ-5D-3L | UK | CKD stage 1: 56 | Mean 44.6 (SD 18.2) | 33.9 | CKD stage 1 | Base NR | Base NR | ||||
| CKD stage 2: 106 | Mean 60 (SD 17.4) | 50.0 | CKD stage 2 | –0.112 | –0.189 to –0.034 | ||||||||||
| CKD stage 3a: 155 | Mean 65.3 (SD 14.8) | 46.5 | CKD stage 3a | –0.062 | –0.128 to 0.005 | ||||||||||
| CKD stage 3b: 35 | Mean 74.1 (SD 13.4) | 60.0 | CKD stage 3b | –0.185 | –0.299 to –0.071 | ||||||||||
| CKD stage 4/5: 5 | Mean 72.2 (SD 10.3) | 40.0 | CKD stage 4/5 | –0.284 | –0.408 to –0.160 | ||||||||||
| Schlackow167 | 2017 | Moderate to advanced CKD | UK | EQ-5D-3L | UK | 6356 | Mean 62 (SD 12) | 63 | Regression | ||||||
| Mean (intercept) | 0.86 | 0.84 to 0.88 | |||||||||||||
| Male | 0.06 | 0.05 to 0.07 | |||||||||||||
| Age + 10 years | –0.05 | –0.05 to –0.04 | |||||||||||||
| PFKT | –0.07 | –0.11 to –0.03 | |||||||||||||
| Dialysis | –0.06 | –0.07 to –0.04 | |||||||||||||
| Sekercioglu168 | 2017 | CKD | Canada | SF-6D | Canada | All: 303 | Mean 62.7 (SD 14.5) | 58.8 | All CKD | 0.720 | 0.110 | ||||
| Dialysis: 101 | Mean 60.6 (SD 14.4) | 57.0 | Dialysis | 0.670 | 0.110 | ||||||||||
| Non-dialysis: 202 | Mean 63.8 (SD 14.4) | 61.0 | No dialysis | 0.740 | 0.100 | ||||||||||
| Senanayake169 | 2019 | Pre-dialysis patients | Sri Lanka | EQ-5D-3L | Sri Lanka | 1036 | Median: ≈ 60 | 62.40 | Pre-dialysis CKD | 0.52 | 0.33 | ||||
| Shah170 | 2019 | ESRD (dialysis or CM) | UK and Australia | SF-6D | UK | Total: 129 | ≥ 75 | 69 | Total | 0.62 | 0.14 | ||||
| Dialysis: 83 | 59 | Dialysis | 0.61 | 0.13 | |||||||||||
| CM: 46 | 65 | CM | 0.65 | 0.15 | |||||||||||
| Shimizu171 | 2018 | HD patients | Japan | EQ-5D-5L | Japan | All: 717 | Mean 72.9 (SD 6.5) | 62.50 | All HD | 0.738 | 0.207 | ||||
| Aged 60–69 years: 278 | Aged 60–69 years | 0.784 | 0.179 | ||||||||||||
| Aged 70–79 years: 311 | Aged 70–79 years | 0.744 | 0.202 | ||||||||||||
| Aged ≥ 80 years: 118 | Aged ≥ 80 years | 0.616 | 0.231 | ||||||||||||
| Snowsill172 | 2017 | Kidney transplant recipients | UK | EQ-5D-3L | UK | N/A | N/A | N/A | Regression | NR | |||||
| Mean (intercept) | 0.968 | ||||||||||||||
| Age | –0.002 | ||||||||||||||
| Age sq | –0.000 | ||||||||||||||
| Male | 0.023 | ||||||||||||||
| FG | –0.053 | ||||||||||||||
| HD | –0.277 | ||||||||||||||
| PD | –0.264 | ||||||||||||||
| PTDM | –0.060 | ||||||||||||||
| Tang173 | 2017 | ESRD | Taiwan | EQ-5D-5L | Japan | APD: 117 | NR | NR | APD | 0.82 | 0.19 | ||||
| CAPD: 129 | CAPD | 0.82 | 0.21 | ||||||||||||
| Thaweethamcharoen174 | 2019 | Patients receiving PD | Thailand | EQ-5D-5L | Thailand | 64 | Mean 63.44 (SD 16.57) | 68.75 | PD | 0.801 | 0.228 | ||||
| Van Loon175 | 2019 | ESRD | The Netherlands | EQ-5D-3L | Dutch | CM: 89 | Mean 82 (SD 6) | NR | CM | 0.77 | 0.21 | ||||
| Dialysis (23% PD): 192 | Mean 75 (SD 7) | Dialysis | 0.82 | 0.18 | |||||||||||
| Wee176 | 2016 | Pre dialysis, CKD stages 3–5 | Singapore | EQ-5D-3L | USA | 309 | Mean 62.6 (SD 11.06) | 58.20 | CKD stages 3–5, pre dialysis | 0.8 | 0.24 | ||||
| Wolfgram177 | 2017 | Hypertensive CKD and non-CKD patients | USA | EQ-5D-3L | USA | All: 2620 | Mean 79.85 (SD 3.99) | 62.1 | All | 0.85 | 0.00 | 0.13 | |||
| Non-CKD: 1459 | Mean 79.39 (SD 3.73) | 62 | Non-CKD | 0.85 | 0.01 | 0.13 | |||||||||
| CKD: 1161 | Mean 80.42 (SD 4.23) | 62.3 | CKD | 0.84 | 0.01 | 0.13 | |||||||||
| eGFR of ≥ 60 ml/minute/1.73 m2 (CKD stage 2 or better): 372 | NR | NR | eGFR of ≥ 60 ml/minute/1.73 m2 | 0.85 | |||||||||||
| eGFR of 44–60 ml/minute/1.73 m2 (CKD stage 3a): 781 | NR | NR | eGFR of 44–60 ml/minute/1.73 m2 | 0.85 | |||||||||||
| eGFR of < 44 ml/minute/1.73 m2 (CKD stage 3b or worse): 1449 | NR | NR | eGFR of < 44 ml/minute/1.73 m2 | 0.82 | |||||||||||
| Wong178 | 2019 | ESRD on dialysis | China | SF-6D | China/Hong Kong | All: 397 | Mean 57.3 (SD 12.7) | 61.9 | All dialysis | 0.766 | 0.111 | ||||
| PD: 103 | Mean 63.1 (SD 12.7) | 61.2 | PD | 0.778 | 0.110 | ||||||||||
| Hospital HD: 135 | Mean 56.4 (SD 12.6) | 57.0 | Hospital HD | 0.731 | 0.114 | ||||||||||
| Home HD: 41 | Mean 47.9 (SD 8.5) | 67.4 | Home HD | 0.778 | 0.091 | ||||||||||
| Community HD: 118 | Mean 56.8 (SD 11.6) | 66.1 | Community HD | 0.790 | 0.107 | ||||||||||
| Yang179 | 2019 | ESRD on dialysis | Singapore | SF-12 mapped to EQ-5D-3L | Unclear | Total: 266 | Mean 59.3 (SD 12.5) | 45.5 | Total | 0.59 | 0.21 | ||||
| CAPD: 145 | Mean 60.8 (SD 11.4) | 45.5 | CAPD | 0.58 | 0.21 | ||||||||||
| APD: 121 | Mean 57.4 (SD 13.6) | 45.5 | APD | 0.60 | 0.22 | ||||||||||
| Yang180 | 2018 | Dialysis | France, Germany, Italy, Spain Singapore | EQ-5D-3L, EQ-5D-5L | Country-specific value sets | France: 299 | Mean 66.6 (SD 14.1) | 62.5 | France | 0.622 | 0.383 | ||||
| Germany: 413 | Mean 61.8 (SD 14.4) | 57.1 | Germany | 0.796 | 0.224 | ||||||||||
| Italy: 278 | Mean 60.8 (SD 13.4) | 54.7 | Italy | 0.864 | 0.185 | ||||||||||
| Spain: 225 | Mean 60.6 (SD 16.4) | 60.0 | Spain | 0.746 | 0.292 | ||||||||||
| Singapore (EQ-5D-5L): 163 | Mean 60.5 (SD 11.5) | 52.2 | Singapore | 0.621 | 0.447 | ||||||||||
| Zyoud181 | 2016 | ESRD on HD | Palestine | EQ-5D-5L | Unclear | Aged ≥ 60: 97 | Mean NR | 52.1 | ESRD | 0.17 | 0.4 | ||||
| Park182 | 2016 | CKD | The Republic of Korea | EQ-5D-3L | The Republic of Korea | All: 46,676 | 45.4 (SE 0.1) | 49.5 | All | 0.943 | 0.001 | ||||
| No CKD: 44,108 | 44.6 (SE 0.2) | 49.9 | No CKD | 0.946 | 0.001 | ||||||||||
| CKD stage 1: 793 | 38.7 (SE 2.8) | 42.8 | Stage 1 | 0.955 | 0.011 | ||||||||||
| CKD stage 2: 444 | 54.9 (SE 3.3) | 56.6 | Stage 2 | 0.901 | 0.017 | ||||||||||
| CKD stage 3a: 1030 | 72.8 (SE 0.5) | 42.6 | Stage 3a | 0.826 | 0.005 | ||||||||||
| CKD stage 3b: 211 | 73.2 (SE 1.1) | 44.9 | Stage 3b | 0.787 | 0.011 | ||||||||||
| CKD stage 4/5 (ESRD): 90 | 64.0 (SE 1.8) | 44 | Stage 4/5 | 0.793 | 0.018 |
| Maintenance/consumables | Price (£) | Cost per test (£) | Formula |
|---|---|---|---|
| NephroCheck | |||
| Paper roll | 2.50 | 0.10 | £2.50/number of tests in kit ( = 25 tests) |
| Liquid quality control (one per kit) | 100.00 | 4.00 | £100/number of tests in kit ( = 25 tests) |
| Electronic quality control (every 6 months) | 80.00 | 0.13 | £80 × 2/number of tests performed per year in hospital laboratory ( = 1253, in St. James’s University Hospital, Leeds (source: Hall et al.97) |
| BioPorto | |||
| NGAL calibrator | 385.00 | 1.28 | £385/number of tests in kit ( = 300) |
| NGAL control kit | 185.00 | 0.62 | £185/number of tests in kit ( = 300) |
| ARCHITECT | |||
| ARCHITECT urine calibrator kit | 165.00 | 2.06 | £165/number of tests a kit can produce ( = 80) |
| ARCHITECT urine control kit | 115.00 | 1.44 | £115/number of tests a kit can produce ( = 80) |
| Reaction vessels and bulk solutions | 0.01 | Manufacturer estimation (sourced from NICE’s request for information document) | |
| Alinity i | |||
| Alinity i urine calibrator kit | 165.00 | 2.06 | £165/number of tests a kit can produce ( = 80) |
| Alinity i urine control kit | 115.00 | 1.44 | £115/number of tests a kit can produce ( = 80) |
| Reaction vessels and bulk solutions | 0.01 | Manufacturer estimation (sourced from NICE’s request for information document) | |
| Cost element | NeoRecormon® (epoetin beta; F. Hoffman-La Roche Ltd, Basel, Switzerland) | Aranesp® (darbepoetin alba; Amgen Inc., Thousand Oaks, CA, USA) | Source |
|---|---|---|---|
| Price per IU | £0.007 | £0.007 | BNF 2019126 |
| Haemodialysis | Peritoneal dialysis | ||
| Proportion taking erythropoiesis-stimulating agents | 92.6% | 78.6% | UK Renal Registry (2019)115 |
| Dose per week | 8000 IU | 4000 IU | UK Renal Registry (2019)115 |
| Cost per year | £2765 | £1174 | |
| Proportion on haemodialysis | 87.5% | 12.5% | |
| Total cost per year (based on the proportion on haemodialysis and peritoneal dialysis) | £2566 | ||
| Medication | Unit cost per year (£) | Proportion of patients on each type of medication | Total average cost (£) | Source |
|---|---|---|---|---|
| ACEIs | 35.74 | 0.211 | 7.54 | Tan et al. 2016,183 BNF 2019126 |
| ARBs | 43.04 | 0.156 | 6.70 | Tan et al. 2016,183 BNF 2019126 |
| Calcium-channel blockers | 28.80 | 0.219 | 6.31 | Tan et al. 2016,183 BNF 2019126 |
| Diuretics | 21.92 | 0.487 | 10.66 | Tan et al. 2016,183 BNF 2019126 |
| Beta blockers | 15.52 | 0.248 | 3.85 | Tan et al. 2016,183 BNF 2019126 |
| Alpha blockers | 7.83 | 0.172 | 1.35 | Tan et al. 2016,183 BNF 2019126 |
| Total average cost per year | 36.41 | Tan et al. 2016,183 BNF 2019126 |
| First author | Year | Population | Country | Utility measure | Valuation set | Sample size (n) | Age (years) | Male (%) | Utility values reported | Mean | Median | SD | IQR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethgen184 | 2015 | AKI, intensive care | USA | Decision-analytic modelling: unclear (sourced from literature) | Unclear | NR | NR | NR | CRRT (ICU) | 0.13 | NR | ||
| CRRT (DI) | 0.84 | ||||||||||||
| CRRT (DD) | 0.62 | ||||||||||||
| IRRT (ICU) | 0.13 | ||||||||||||
| IRRT (DI) | 0.84 | ||||||||||||
| IRRT (DD) | 0.62 | ||||||||||||
| Hall97 | 2018 | AKI, intensive care | UK | Mix | Various | Mix | ICU | –0.402 | 0.20 | ||||
| Ward (post ICU) | 0.44 | 0.31 | |||||||||||
| Discharged (post ICU) | 0.62 | 0.32 | |||||||||||
| DD decrement | 0.11 | 0.02 | |||||||||||
| Kaier185 | 2016 | Surgical aortic valve replacement | Germany | EQ-5D-3L | German |
|
82.15 (5.16) | NR | Baseline | 0.78 | 0.23 | ||
| Follow-up | 0.77 | 0.25 | |||||||||||
| AKIN 1 | 0.0659 | NR | |||||||||||
| AKIN 2 | –0.158 | NR | |||||||||||
| AKIN 3 | –0.177 | NR | |||||||||||
| Oeyen186 | 2015 | Critically ill after AKI, need RRT | Belgium | EQ-5D-3L | None | 141 | 57 | 66 | None | NR | NR | ||
| Soliman187 | 2016 | AKI patients in a mixed ICU population | The Netherlands | EQ-5D-3L | Dutch | [Median (IQR) values] | |||||||
| All: 2420 | 59 (47–69) | 58.7 | All | 0.806 | 0.590–0.940 | ||||||||
| No AKI: 1588 | 59 (47–69) | 58.3 | No AKI | 0.810 | 0.640–1.000 | ||||||||
| Risk: 456 | 59 (47–69) | 57.0 | Risk | 0.778 | 0.570–0.890 | ||||||||
| Injury: 253 | 59 (47–69) | 61.7 | Injury | 0.772 | 0.470–0.870 | ||||||||
| Failure: 123 | 59 (47–69) | 63.4 | Failure | 0.666 | 0.370–0.850 |
| First author | Year | Population | Country | Utility measure | Valuation set | n | Age (years) | Proportion male (%) | Utility values reported | Mean | Median | SE | SD | 95% CI | IQR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang152 | 2016 | ESRD with PD and HD | Taiwan | EQ-5D-3L | UK | Total: 1687 | Total: NR | ||||||||
| HD: 1403 | HD: mean 57.1 (SD 13.6) | HD: 49.9 | HD | 0.83 | 0.19 | ||||||||||
| PD: 284 | PD: mean 46.7 (SD 13.2) | PD: 51.1 | PD | 0.90 | 0.16 | ||||||||||
| Jesky158 | 2016 | Pre-dialysis CKD (as per NICE guidance)159 | UK | EQ-5D-3L | UK | All CKD: 745 | All CKD: median 64 (IQR 50–76) | All CKD: 60.80 | All CKD | 0.74 | 0.66–0.88 | ||||
| G1/2: 29 | G1/2: median 41 (IQR 34.5–55.5) | G1/2: 65.52 | G1/2 | 0.85 | 0.70–1 | ||||||||||
| G3a: 45 | G3a: median 55 (IQR 45–66.5) | G3a: 71.11 | G3a | 0.80 | 0.69–1 | ||||||||||
| G3b: 173 | G3b: median 61.5 (IQR 48.3–73.8) | G3b: 66.86 | G3b | 0.80 | 0.68–1 | ||||||||||
| G4: 423 | G4: median 69 (IQR 54–75.5) | G4: 59.00 | G4 | 0.74 | 0.62–0.85 | ||||||||||
| G5: 75 | G5: median 64 (IQR 53.5–75.5) | G5: 49.35 | G5 | 0.73 | 0.62–1 | ||||||||||
| Kularatna162 | 2019 | CKD | Sri Lanka | EQ-5D-3L | UK | Early stage: 254 | Median approximate age 41 | 56.10 | Early stage | 0.588 | 0.30 | ||||
| Stage 4: 614 | Stage 4 | 0.566 | 0.42 | ||||||||||||
| Stage 5: 151 | Stage 5 | 0.467 | 0.42 | ||||||||||||
| Dialysis: 38 | Dialysis | 0.126 | 0.39 | ||||||||||||
| Li164 | 2017 | Kidney transplant recipients and waiting list | UK | EQ-5D-5L | UK | Waiting list: 1704 | Median ≈ 50 | 58 | Waiting list | 0.773 | 0.005 | ||||
| Transplant recipients: 512 | Median ≈ 50 | 60 | Transplant (inc) | 0.054 | 0.011 | ||||||||||
| Nguyen136 | 2018 | CKD and ESRD | UK | EQ-5D-3L | UK | CKD stage 1: 56 | Mean 44.6 (SD 18.2) | 33.9 | CKD stage 1 | Base NR | Base NR | ||||
| CKD stage 2: 106 | Mean 60 (SD 17.4) | 50.0 | CKD stage 2 | –0.112 | –0.189 to –0.034 | ||||||||||
| CKD stage 3a: 155 | Mean 65.3 (SD 14.8) | 46.5 | CKD stage 3a | –0.062 | –0.128 to 0.005 | ||||||||||
| CKD stage 3b: 35 | Mean 74.1 (SD 13.4) | 60.0 | CKD stage 3b | –0.185 | –0.299 to –0.071 | ||||||||||
| CKD stage 4/5: 5 | Mean 72.2 (SD 10.3) | 40.0 | CKD stage 4/5 | –0.284 | –0.408 to –0.160 | ||||||||||
| Schlackow167 | 2017 | Moderate to advanced CKD | UK | EQ-5D-3L | UK | 6356 | Mean 62 (SD 12) | 63 | Regression | ||||||
| Mean (intercept) | 0.86 | 0.84 to 0.88 | |||||||||||||
| Male | 0.06 | 0.05 to 0.07 | |||||||||||||
| Age + 10 years | –0.05 | –0.05 to –0.04 | |||||||||||||
| PFKT | –0.07 | –0.11 to –0.03 | |||||||||||||
| Dialysis | –0.06 | –0.07 to –0.04 | |||||||||||||
| Snowsill172 | 2017 | Kidney transplant recipients | UK | EQ-5D-3L | UK | N/A | N/A | N/A | Regression | NR | |||||
| Mean (intercept) | 0.968 | ||||||||||||||
| Age | –0.002 | ||||||||||||||
| Age sq | –0.000 | ||||||||||||||
| Male | 0.023 | ||||||||||||||
| FG | –0.053 | ||||||||||||||
| HD | –0.277 | ||||||||||||||
| PD | –0.264 | ||||||||||||||
| PTDM | –0.060 |
Appendix 14 Addendum to the External Assessment Group report
This addendum was prepared by the EAG in response to the consultation comments for the assessment, whereby several comments were made in relation to the test costs applied for NephroCheck and NGAL (BioPorto), the cost applied for fluids in the KDIGO preventative care bundle, and the RR parameters applied in the model for averting and reducing the severity of AKI. With respect to this last issue, the economic model used RR estimates derived from Meersch et al. ,110 when another study by Göcze et al. 116 was also available. Therefore, in this addendum we present three further scenario analyses that explore (1) alternative test costs for NephroCheck and NGAL (BioPorto), (2) alternative costs of fluids in those with a positive test who receive the care bundle and (3) alternative RRs for the aversion and redistribution of AKI in the cohort that receives the care bundle.
Additional cost-effectiveness results
The three additional scenario analyses are conducted on base case 1 (Table 39) and base case 2 (Table 40). The scenarios are labelled as 1R to 1T, and 2R to 2T.
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs. standard care | |||||||
| Base case 1 | ||||||||
| Test 3 (BioPorto urine NGAL) | 22,887 | – | 6.07332 | – | – | Dominant | 43.5 | 54.6 |
| Test 2 (BioPorto plasma NGAL) | 22,900 | £14 | 6.07332 | 0.00001 | £2,694,918 | Dominant | 11.1 | 47.6 |
| Standard care (serum creatinine) | 22,901 | Dominated | 6.07296 | Dominated | Dominated | – | 45.1 | – |
| Test 4 (ARCHITECT urine NGAL) | 22,912 | Dominated | 6.07328 | Dominated | Dominated | £32,131 | 0.1 | 41.4 |
| Test 1 (NephroCheck) | 22,938 | Dominated | 6.07332 | Dominated | Dominated | £101,456 | 0.2 | 31.9 |
| 1R: Alternative test costs for NephroCheck and NGAL (BioPorto) | ||||||||
| Test 3 (BioPorto urine NGAL) | 22,746 | – | 6.074431 | – | – | Dominant | 39.6 | 52.5 |
| Test 2 (BioPorto plasma NGAL) | 22,758 | £12 | 6.074439 | 0.000008 | £1,621,578 | Dominant | 12.2 | 45.7 |
| Standard care (serum creatinine) | 22,760 | Dominated | 6.074090 | Dominated | Dominated | – | 46.8 | – |
| Test 4 (ARCHITECT urine NGAL) | 22,766 | Dominated | 6.074404 | Dominated | Dominated | £16,592 | 0.9 | 42.6 |
| Test 1 (NephroCheck) | 22,789 | Dominated | 6.074438 | Dominated | Dominated | £80,747 | 0.4 | 34.3 |
| 1S: Alternative solution for fluid assistance (Hartmann’s solution) | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,121 | – | 6.071715 | – | – | Dominant | 39.3 | 51.5 |
| Standard care (serum creatinine) | 23,132 | Dominated | 6.071353 | Dominated | Dominated | – | 47.8 | – |
| Test 2 (BioPorto plasma NGAL) | 23,135 | £14 | 6.071729 | 0.00001 | £1,015,368 | £9202 | 12.3 | 46.7 |
| Test 4 (ARCHITECT urine NGAL) | 23,146 | Dominated | 6.071686 | Dominated | Dominated | £41,624 | 0.3 | 40.7 |
| Test 1 (NephroCheck) | 23,173 | Dominated | 6.071724 | Dominated | Dominated | £112,505 | 0.3 | 31.4 |
| 1T: Alternative RR parameters (Göcze et al.116) | ||||||||
| Test 3 (BioPorto urine NGAL) | 23,079 | – | 6.082680 | – | – | Dominant | 49.3 | 67.1 |
| Test 2 (BioPorto plasma NGAL) | 23,091 | £12 | 6.082690 | 0.000010 | £1,158,117 | Dominant | 16.8 | 62.2 |
| Test 4 (ARCHITECT urine NGAL) | 23,107 | Dominated | 6.082632 | Dominated | Dominated | Dominant | 0.3 | 57.5 |
| Test 1 (NephroCheck) | 23,129 | Dominated | 6.082688 | Dominated | Dominated | Dominant | 0.9 | 47.9 |
| Standard care (serum creatinine) | 23,135 | Dominated | 6.082137 | Dominated | Dominated | – | 32.7 | – |
| Scenario | Cost (£) | Incremental cost | QALY | Incremental QALY | ICER (incremental) | ICER vs. standard care | Probability (%) of being cost-effective at | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £20,000 vs. standard care | |||||||
| Base case 2 | ||||||||
| Standard care (serum creatinine) | 22,978 | – | 6.07277 | – | – | – | 64.5 | – |
| Test 1 (NephroCheck) | 23,016 | £38 | 6.07313 | 0.00036 | £105,965 | £105,965 | 29.7 | 32.0 |
| Test 3 (BioPorto urine NGAL) | 23,049 | Dominated | 6.07290 | Dominated | Dominated | £539,041 | 5.3 | 11.0 |
| Test 2 (BioPorto plasma NGAL) | 23,064 | Dominated | 6.07290 | Dominated | Dominated | £633,846 | 0.3 | 7.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,065 | Dominated | 6.07289 | Dominated | Dominated | £725,061 | 0.0 | 6.3 |
| 2R: Alternative test costs for NephroCheck and NGAL (BioPorto) | ||||||||
| Standard care (serum creatinine) | 22,865 | – | 6.07020 | – | – | – | 65.0 | – |
| Test 1 (NephroCheck) | 22,899 | £34 | 6.07055 | 0.00035 | £97,745 | £97,771 | 31.2 | 33.0 |
| Test 3 (BioPorto urine NGAL) | 22,937 | Dominated | 6.07033 | Dominated | Dominated | £581,613 | 3.1 | 9.6 |
| Test 4 (ARCHITECT urine NGAL) | 22,951 | Dominated | 6.07032 | Dominated | Dominated | £751,404 | 0.0 | 6.1 |
| Test 2 (BioPorto plasma NGAL) | 22,952 | Dominated | 6.07033 | Dominated | Dominated | £686,614 | 0.7 | 7.2 |
| 2S: Alternative solution for fluid assistance (Hartmann’s solution) | ||||||||
| Standard care (serum creatinine) | 22,934 | – | 6.07636 | – | – | – | 65.5 | – |
| Test 1 (NephroCheck) | 22,977 | £42 | 6.07671 | 0.00035 | £119,969 | £119,969 | 29.1 | 31.1 |
| Test 3 (BioPorto urine NGAL) | 23,002 | Dominated | 6.07648 | Dominated | Dominated | £545,923 | 4.6 | 11.3 |
| Test 2 (BioPorto plasma NGAL) | 23,017 | Dominated | 6.07648 | Dominated | Dominated | £650,943 | 0.8 | 8.3 |
| Test 4 (ARCHITECT urine NGAL) | 23,019 | Dominated | 6.07647 | Dominated | Dominated | £751,697 | 0.0 | 6.3 |
| 2T: Alternative RR parameters (Göcze et al.116) | ||||||||
| Test 1 (NephroCheck) | 23,048 | – | 6.06367 | – | – | Dominant | 38.9 | 46.9 |
| Standard care (serum creatinine) | 23,051 | Dominated | 6.06314 | Dominated | Dominated | – | 45.6 | – |
| Test 3 (BioPorto urine NGAL) | 23,099 | Dominated | 6.06341 | Dominated | Dominated | £175,838 | 12.6 | 29.5 |
| Test 2 (BioPorto plasma NGAL) | 23,115 | Dominated | 6.06342 | Dominated | Dominated | £227,728 | 2.9 | 25.1 |
| Test 4 (ARCHITECT urine NGAL) | 23,118 | Dominated | 6.06339 | Dominated | Dominated | £268,527 | 0.0 | 20.9 |
In the first scenario analysis (1R and 2R), the alternative testing costs for NephroCheck and NGAL (BioPorto) were explored to address the company’s (bioMérieux SA, Marcy-l'Étoile, France) concerns about the costing assumptions. The following assumptions were made in this alternative scenario, as suggested by bioMérieux:
-
Excluding all capital cost (on the basis that the company provide the capital equipment without charge).
-
Assuming the liquid quality control for NephroCheck is conducted monthly. The monthly test throughput is assumed to be ≈ 104 (= 1253/12), in which the test throughput of 1253 is based on throughput at an ICU department in a hospital in Leeds (St James’s University Hospital) (Hall et al. 97). The liquid quality control cost is therefore slightly cheaper at £1.91 {= [£100 + (2 × £49.8)]/(1253/12)}.
-
Assuming wastage may occur for NGAL (BioPorto) owing to the 4-week shelf-life of the calibrator and test control kit once opened. This results in a slightly more expensive test maintenance cost of £5.46 [= (385 + 185)/(1253/12)] for NGAL (BioPorto).
The impact on the cost-effectiveness results is very limited and does not change the cost-effectiveness conclusions.
In the second scenario (1S and 2S), we apply a more expensive buffered solution for fluids given as part of the KDIGO care bundle. The more expensive solution was assumed to be Hartmann’s solution, at a list price of £3.25 per litre [Baxter International Inc. (Deerfield, IL, USA), 2019, personal communication]. This resulted in a slightly greater care bundle cost (£7.11 greater care bundle cost overall) applied to those with a positive biomarker test result. However, the slightly greater care bundle cost had very little impact on the cost-effectiveness results.
In the third scenario (1T and 2T), the RR applied to the averted and redistributed cohort was equal to that reported in Göcze et al. 116 The RRs of having AKI versus no AKI, having AKI 1 given AKI, and having AKI 2/3 given AKI were 0.666, 1.347 and 0.509, respectively. Therefore, the effect sizes are larger than those reported in the Meersch et al. study,110 which was used in the base case. Consequently, all the tests accrued greater QALYs and greater ICU cost savings in these scenarios, with all tests being dominant compared with standard care in base case 1. In base case 2, NephroCheck was the only dominant strategy.
List of abbreviations
- ACEI
- angiotensin-converting enzyme inhibitor
- AKI
- acute kidney injury
- AKIN
- Acute Kidney Injury Network
- ARB
- angiotensin receptor blocker
- AUC
- area under the curve
- CI
- confidence interval
- CKD
- chronic kidney disease
- DAP
- Diagnostics Assessment Programme
- EAG
- External Assessment Group
- ED
- emergency department
- EDTA
- ethylenediaminetetraacetic acid
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- EuroQol-5 Dimensions
- ESRD
- end-stage renal disease
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- ICU
- intensive care unit
- IGFBP7
- insulin-like growth factor-binding protein 7
- ISPOR
- International Society for Pharmacoeconomics and Outcomes Research
- KDIGO
- Kidney Disease: Improving Global Outcomes
- LOS
- length of stay
- NEWS
- National Early Warning Score
- NGAL
- neutrophil gelatinase-associated lipocalin
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PRaCTICaL
- Pragmatic Randomised, Controlled Trial of Intensive Care follow up programmes in improving Longer-term outcomes from critical illness
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST
- Prediction model Risk Of Bias ASsessment Tool
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies, version 2
- RCT
- randomised controlled trial
- RIFLE
- Risk, Injury, Failure, Loss of kidney function, and End-stage disease
- ROC
- receiver operating characteristic
- RR
- relative risk
- RRT
- renal replacement therapy
- SD
- standard deviation
- SHARP
- Study of Heart and Renal Protection
- SMR
- Scottish Morbidity Record
- SROC
- summary receiver operating characteristic
- TIMP-2
- tissue inhibitor of metalloproteinase-2
- TRIBE
- Translational Research Investigating Biomarker Endpoints
- WHO
- World Health Organization
