Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/167/102. The contractual start date was in October 2014. The draft report began editorial review in May 2020 and was accepted for publication in November 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Benger et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health and Care Research, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter are reproduced with permission from Taylor et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy and distribute this work, for non-commercial use, with no derivatives, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background and rationale
In the UK, the reported incidence of out-of-hospital cardiac arrest (OHCA) is 123 cases per 100,000 population per annum. 2 Despite recent improvements, survival rates from cardiac arrest remain poor, with approximately 7–9% of UK patients surviving to hospital discharge, compared with estimates of between 5% and 25% internationally. 3–7 During a cardiac arrest, the brain is exposed to a period of hypoxaemia and ischaemia, which may result in death or cognitive deficits. 8 Optimal cardiopulmonary resuscitation (CPR) and rapid return of spontaneous circulation (ROSC) are key factors associated with avoiding or minimising neurological impairment in survivors of OHCA,9,10 and early effective airway management, which involves techniques and medical procedures to prevent and relieve airway obstruction, is fundamental to this. 1 The scene of an OHCA is often a challenging and unpredictable environment, which can affect these key interventions.
Tracheal intubation (TI) is the placement of a flexible plastic tube into the trachea (windpipe) to keep an airway open. Traditional teaching suggests that TI is the most effective way to manage the airway during OHCA. 11 However, this assumption has not been well tested,12 and pre-hospital intubation attempts by paramedics can cause complications, such as interruptions in chest compressions, unrecognised oesophageal intubation (particularly if waveform capnography is not available) and delays in accessing definitive care. 1,13,14
Supraglottic airway devices (SGAs) are an alternative to intubation. They are quicker and easier to insert and may avoid the complications of TI. 15 SGAs are used safely to manage the airway during routine anaesthesia. 1,16–18 They are also in widespread use in NHS ambulance services. In 2015/16, the London Ambulance Service alone reported 92.4% (3142/3401) successful SGA placements compared with 86.1% (1411/1639) successful TIs for OHCA. 6 However, these data are from a single ambulance service and do not describe the associated clinical outcomes.
Equipoise between the two techniques has led to calls for a large randomised controlled trial (RCT) to compare the two. 19–22 Relatively small gains in survival of 2–3% would be clinically meaningful and worthwhile,23 provided the intervention is cost-effective. This means that large sample sizes are necessary and missing data could substantially undermine the validity of trial results.
The Resuscitation Council UK 2015 guidelines24 state that the optimal airway technique for cardiac arrest is still unknown and is likely to depend on the skills of the operator, the anticipated pre-hospital time and patient-dependent factors. Evidence-based interventions are urgently required to address the currently poor survival rate following OHCA. The AIRWAYS-2 trial was designed to answer important questions about initial advanced airway management (AAM) during OHCA, examining both survival rates and quality of life (QoL) associated with this survival.
To assess the feasibility of recruiting paramedics and enrolling patients to a trial comparing the techniques, we carried out a feasibility study (REVIVE-AIRWAYS)25 between March 2012 and February 2013. This was completed in a single ambulance service and assessed the feasibility of recruiting paramedics and enrolling patients to the trial comparing two SGAs [the i-gel® (Intersurgical Ltd, Wokingham, UK) and the LMA® Supreme™ Airway (Teleflex Medical Europe Ltd, Athlone, Ireland)] with current practice (including TI). 25 REVIVE-AIRWAYS demonstrated that the trial was feasible and informed the detailed design of the AIRWAYS-2 trial.
Aim and objectives
The aim of the AIRWAYS-2 trial was to determine whether or not the i-gel, a second-generation SGA (the trial SGA), is superior to TI in non-traumatic OHCA in adults, in terms of both clinical effectiveness and cost-effectiveness.
The trial objectives were to estimate:
-
The difference in the primary outcome of modified Rankin Scale (mRS) score at hospital discharge (or 30 days post OHCA if the patient was still in hospital) between groups of patients managed by paramedics randomised to use either the i-gel or TI as their initial AAM strategy following OHCA. The mRS is a measure of functional outcome used to measure disability or dependence in the daily activities of people.
-
Differences in secondary outcome measures relating to airway management, hospital stay and recovery at 3 and 6 months between groups of patients managed by paramedics randomised to use either the i-gel or TI.
-
The relative cost-effectiveness of the i-gel compared with TI, including estimation of major in-hospital resource use (e.g. length of stay in intensive and high-dependency care), and associated costs in each group.
Chapter 2 Methods
Parts of this chapter are reproduced with permission from Taylor et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy and distribute this work, for non-commercial use, with no derivatives, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced with permission from Robinson et al. 26 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are reproduced with permission from JAMA 2018;320(8):779–91. 27 Copyright © 2018 American Medical Association. All rights reserved.
Trial design
The AIRWAYS-2 trial was a pragmatic, open, parallel, two-group, multicentre cluster RCT. The trial schema is presented in Figure 1. The trial objectives were addressed by randomising paramedics, rather than patients, to either the i-gel or TI. All enrolled patients were then treated according to the enrolling paramedic’s allocation. This trial is registered as ISRCTN08256118.
FIGURE 1.
Trial schema. HMPS, Her Majesty’s Prison Service. n values missing as this is the methods section and this figure shows the flow of patients through the trial. n values appear in Chapter 3.
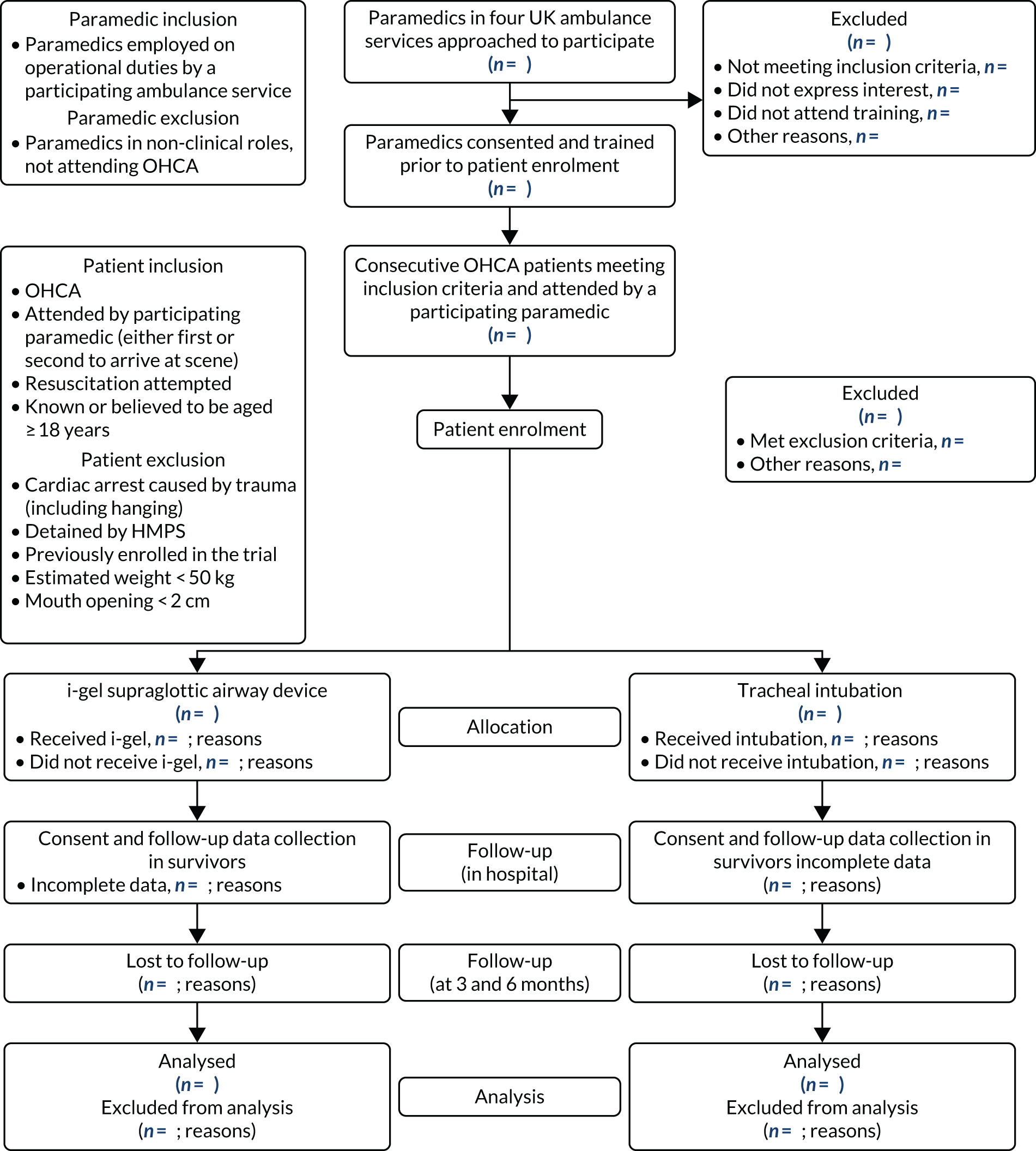
All enrolled patients transferred to the emergency department (ED) required follow-up data to be collected in hospital. Hospitals identified as potential receiving sites for OHCA patients were any of those within or bordering the geographical area served by the four participating ambulance services. It was not possible to predict or influence which hospital an enrolled patient would be taken to and this meant that all 95 hospitals served by the four ambulance services needed to participate in the trial. If a hospital refused to take part or could not provide the necessary approval, the trial could not collect data for enrolled patients taken to that hospital. 26 It was also required that all 95 hospitals started their participation in the trial at the same time, that is as soon as patient enrolment began. Ethics review and approval was provided by South Central – Oxford C Research Ethics Committee (REC) (reference 14/SC/1219). Owing to the immediate and incapacitating nature of OHCA, patients were unable to provide consent at the scene. Every eligible patient attended by a participating paramedic was automatically enrolled in the trial under a waiver of consent provided by the Confidentiality Advisory Group (CAG) (reference 14/CAG/1030). Patients who survived to discharge from the intensive care unit (ICU) were approached to provide consent for ongoing trial follow-up. A consultee could also provide an opinion on the likely views of a patient in instances where the patient was incapacitated.
Changes to trial design after commencement of the trial
During the trial, several amendments were made to the trial protocol. For a more detailed description of these amendments, see Appendix 2. The protocol version in use at the start of the trial was version 2.0. The current full trial protocol can be found on the project web page (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/12167102#/; accessed 3 March 2021).
Throughout the trial, adjustments had to be made to the collection of data for the economic evaluation. We aimed from the outset to make use of routinely collected data from NHS Digital Hospital Episode Statistics (HES)/Office for National Statistics (ONS) data linked to the trial cohort. However, as the trial progressed, it became clear that there was a risk that the HES/ONS routine data would take too long to arrive and might not be available in time for the final analyses. The initial application to NHS Digital for the data was made in July 2016. There were various delays to the application process that were beyond the control of the trial team. In late 2017, it was agreed that the trial team would try to acquire a set of routine data by other means so that data would be available should the HES/ONS data not be available in time for analysis. It was agreed with the sponsor (South Western Ambulance Service NHS Foundation Trust) and REC that a sample of sites could be approached and asked to complete brief data collection forms to be used to estimate resource use by collecting information on admissions, cross-sectional imaging [computerised tomography (CT) and magnetic resonance scans] and interventions received by AIRWAYS-2 participants at their sites. In February 2018, a sample of sites was approached to collect this additional retrospective health economics data. A total of 24 hospitals across the four regions were approached, with 18 hospitals returning completed data collection forms for around 850 patients. However, these data were not used in the final analyses because the HES/ONS data became available.
Some changes were made to the statistical analysis plan (SAP) after the trial had started. For a more detailed description of these changes, see Appendix 3. The initial SAP was finalised in February 2018. In April 2018, version 2.0 of the SAP was signed off.
Participants
Paramedic population
Paramedics were eligible if they were employed by one of the four participating ambulance services (see Settings) and undertook general operational duties and, therefore, could be despatched to attend an OHCA as the first or second paramedic to arrive at the patient’s side. Paramedics had to be registered with the Health and Care Professions Council (HCPC) and be qualified to practise TI in their clinical role. Paramedics were required to undergo trial-specific training prior to providing consent to participate.
Patient eligibility criteria
The trial population was adults who had a non-traumatic OHCA. Patients were treated in accordance with the allocation of the attending paramedic.
The trial inclusion criteria were:
-
patient known or believed to be aged ≥ 18 years
-
patient has had a non-traumatic cardiac arrest outside hospital
-
patient attended by a paramedic who was participating in the trial and was either the first or second paramedic to arrive at the patient’s side*
-
resuscitation was commenced or continued by ambulance staff or responder. †
*The participating paramedic managed the patient’s airway according to their allocation. If both the first and second paramedic were participating in the trial, the patient’s airway was managed in accordance with the allocation of the first paramedic to arrive at the patient’s side (usually designated as the ‘attendant’ in the ambulance service). If the first paramedic to arrive was not an AIRWAYS-2 paramedic (i.e. a paramedic who provided consent and was randomised) but the second paramedic was, the patient was enrolled in the trial unless an advanced airway intervention had already occurred (advanced airway intervention being defined as either a SGA or a tracheal tube being present in the patient’s mouth) at the point that the second paramedic arrived at the patient’s side. If a third or subsequent paramedic arrived at the patient’s side, and the first two paramedics were not participating in the trial and the third or subsequent paramedic was participating in the trial, the patient was excluded (some of these exclusions had to be determined retrospectively).
†Circumstances in which resuscitation should and should not be attempted are described in national guidelines; the Joint Royal Colleges Ambulance Liaison Committee (JRCALC) Recognition of Life Extinct (ROLE) criteria28 are currently used by all ambulance trusts to determine when a resuscitation attempt is inappropriate and these criteria were applied in the trial. These criteria were objectively defined, but the frequency of attempted resuscitation in both groups was examined regularly by the Data Monitoring and Safety Committee (DMSC) to identify any bias in the commencement of resuscitation attempts.
The exclusion criteria were:
-
patient detained by Her Majesty’s Prison Service (HMPS)
-
patient previously enrolled in the trial (determined retrospectively)
-
resuscitation considered inappropriate28
-
advanced airway device inserted by another HCPC-registered paramedic, doctor or nurse already in place when the AIRWAYS-2 paramedic arrived at the patient’s side (when the first paramedic to arrive was not participating in the AIRWAYS-2 trial)
-
known to already be enrolled in another pre-hospital randomised trial
-
mouth opening < 2 cm.
This last exclusion criterion was applied because successful insertion of a SGA requires mouth opening of > 2 cm. There was a risk of post-randomisation bias being introduced by this exclusion criterion, but in our feasibility trial only 2 out of 711 patients (0.3%) were excluded on these grounds. We monitored this exclusion, under the guidance of the DMSC, and had the exclusion rate exceeded 1% we would have taken action to address this through enhanced training and supervision.
Standardised guidelines, based on those produced by the JRCALC, were applied to determine patients for whom a resuscitation attempt was inappropriate. This was the case where there was no chance of survival; where the resuscitation attempt would be futile and distressing for relatives, friends and health-care personnel; and where time and resources would be wasted undertaking such measures.
When any one or more of the following conditions existed, resuscitation and enrolment in the trial would not take place:
-
massive cranial and cerebral destruction
-
hemicorporectomy
-
massive truncal injury incompatible with life (including decapitation)
-
decomposition/putrefaction
-
incineration
-
hypostasis
-
rigor mortis
-
a valid ‘do not attempt resuscitation’ order or an advanced directive (living will) that states the wish of the patient not to undergo attempted resuscitation
-
patient’s death expected owing to terminal illness
-
submersion of adults for > 1 hour
-
efforts would be futile, as defined by the combination of all three of the following being present –
-
> 15 minutes since the onset of collapse
-
no bystander CPR prior to arrival of the ambulance
-
asystole (flat line) for > 30 seconds on the electrocardiogram (ECG) monitor screen (exceptions: drowning and drug overdose/poisoning).
-
Patients were also excluded from the trial if an immediate family member, relative or close friend who was present at the scene of the cardiac arrest indicated to the participating paramedic at the start of the resuscitation attempt that the person had previously expressed an opinion that they would not wish to take part in the AIRWAYS-2 trial. In practice, no patients were excluded for this reason.
Changes to trial eligibility criteria after commencement of the trial
In January 2015, the paramedic exclusion criteria were refined to define routine attendance at OHCA as attending at least two OHCA patients per year in whom resuscitation was attempted. The patient inclusion criterion regarding enrolment by the second trial paramedic on scene (where the first paramedic was not participating in the trial) was refined to state that the second paramedic could enrol the patient unless an AAM intervention had already occurred. Two additional patient exclusion criteria were added: ‘advanced airway device already in place when AIRWAYS-2 paramedic arrives at patient’s side (when the first paramedic to arrive is not participating in the AIRWAYS-2 trial)’ and ‘known to already be enrolled in another pre-hospital randomised trial’.
In April 2015, the paramedic inclusion criteria were updated to state that paramedics soon to be employed by a participating ambulance trust could participate, and that paramedics must be qualified to practise TI in their current clinical role. The patient inclusion criterion ‘must be in non-traumatic cardiac arrest outside hospital’ was changed to ‘patient has had a non-traumatic cardiac arrest outside hospital’, and the patient inclusion criterion ‘resuscitation is attempted or continued by emergency medical services (EMS) staff’ was changed to ‘resuscitation was commenced or continued by ambulance staff or responder’. The patient exclusion criterion ‘advanced airway device already in place when AIRWAYS-2 paramedic arrives at patient’s side (when the first paramedic to arrive is not participating in the AIRWAYS-2 trial)’ was changed to ‘advanced airway device inserted by another HCPC-registered paramedic already in place when AIRWAYS-2 paramedic arrives at patient’s side (when the first paramedic to arrive is not participating in the AIRWAYS-2 trial)’ and the patient exclusion criterion ‘estimated weight < 50 kg’ was removed.
In August 2015, the patient exclusion criterion ‘advanced airway device inserted by another HCPC-registered paramedic already in place when AIRWAYS-2 paramedic arrives at patient’s side (when the first paramedic to arrive is not participating in the AIRWAYS-2 trial)’ was changed to ‘advanced airway device inserted by another HCPC-registered paramedic, doctor or nurse already in place when AIRWAYS-2 paramedic arrives at patient’s side (when the first paramedic to arrive is not participating in the AIRWAYS-2 trial)’. The reason for this minor amendment was to update the protocol so that it reflected clinical practice (i.e. a trial paramedic would not remove an airway device that had already been inserted by a nurse or doctor).
Settings
The trial involved collaboration with four NHS ambulance services – South Western Ambulance Service NHS Foundation Trust (SWAST), East of England Ambulance Service NHS Trust (EEAST), East Midlands Ambulance Service NHS Trust (EMAS) and Yorkshire Ambulance Service NHS Trust (YAS) – and the 95 NHS hospitals served by these ambulance services. The four ambulance services covered 21 million people (40% of England’s population). Each ambulance service employed a research paramedic to work on the trial, liaising with the trial co-ordination team and AIRWAYS-2 paramedics. All eligible patients attended by an AIRWAYS-2 paramedic between June 2015 and June 2016 were automatically enrolled in the trial and treated according to the attending paramedic’s trial allocation. 1
Interventions
Tracheal intubation (control group)
Tracheal intubation, the placement of a cuffed tube in the patient’s trachea to provide oxygen to the lungs and remove carbon dioxide (CO2), has been generally recognised as the ‘gold standard’ of airway management. TI is used universally in comatose survivors of cardiac arrest following their admission to hospital.
i-gel (intervention group)
The intervention was insertion of the i-gel, a second-generation SGA, as an alternative to TI. Because of its speed and ease of insertion, this device is being increasingly used as the SGA of choice during OHCA in England. 29,30
Aspects of airway management common to both groups
A common approach to airway management, from basic to advanced techniques, was agreed by the participating ambulance services. This included the use of bag–mask ventilation (BMV) and simple airway adjuncts prior to AAM.
A standardised airway management algorithm1 (Figure 2) was developed by the four participating ambulance services to guide further actions should the initial approach to airway management prove unsuccessful.
FIGURE 2.
The AIRWAYS-2 trial treatment trial algorithm. (a) i-gel group: AIRWAYS-2 paramedic and at least one other person trained in CPR; (b) i-gel solo: single AIRWAYS-2 paramedic response; (c) intubation group: AIRWAYS-2 paramedic and at least one other person trained in CPR; and (d) intubation solo: single AIRWAYS-2 paramedic response. NP, nasopharyngeal; NPA, nasopharyngeal airway; OP, oropharyngeal; OPA, oropharyngeal airway. This figure is reproduced with permission from Taylor et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy and distribute this work, for non-commercial use, with no derivatives, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
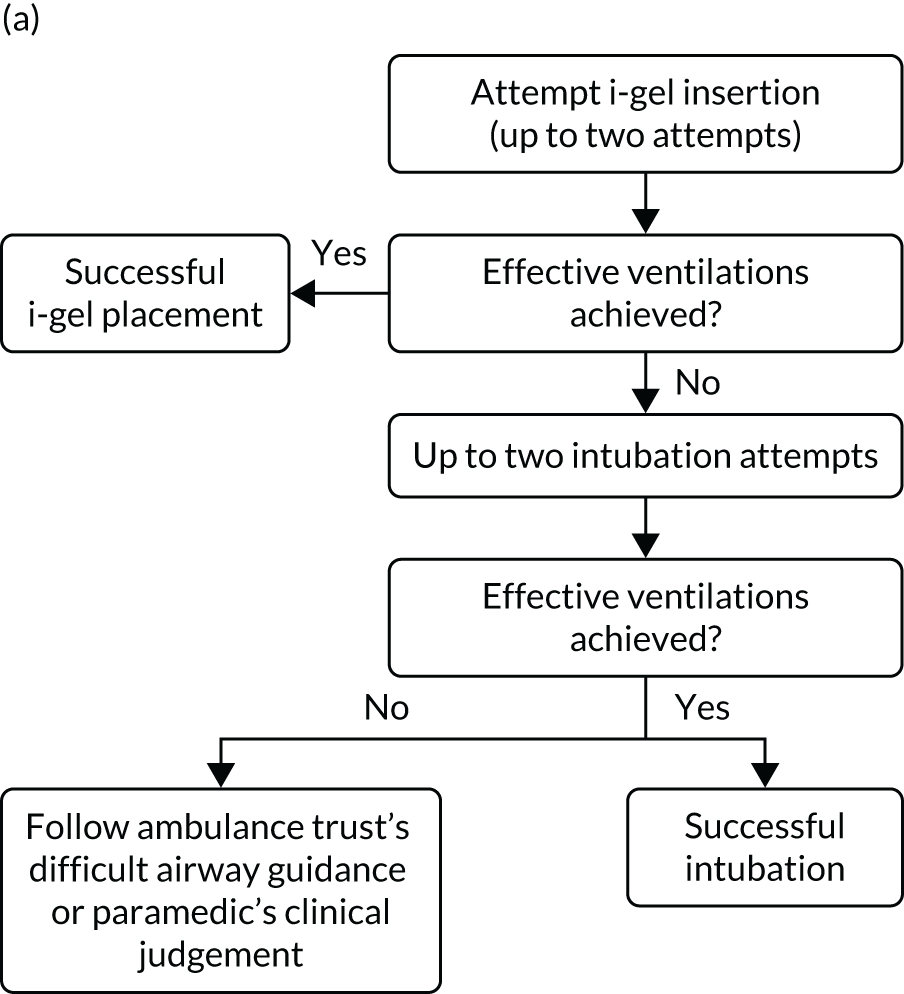
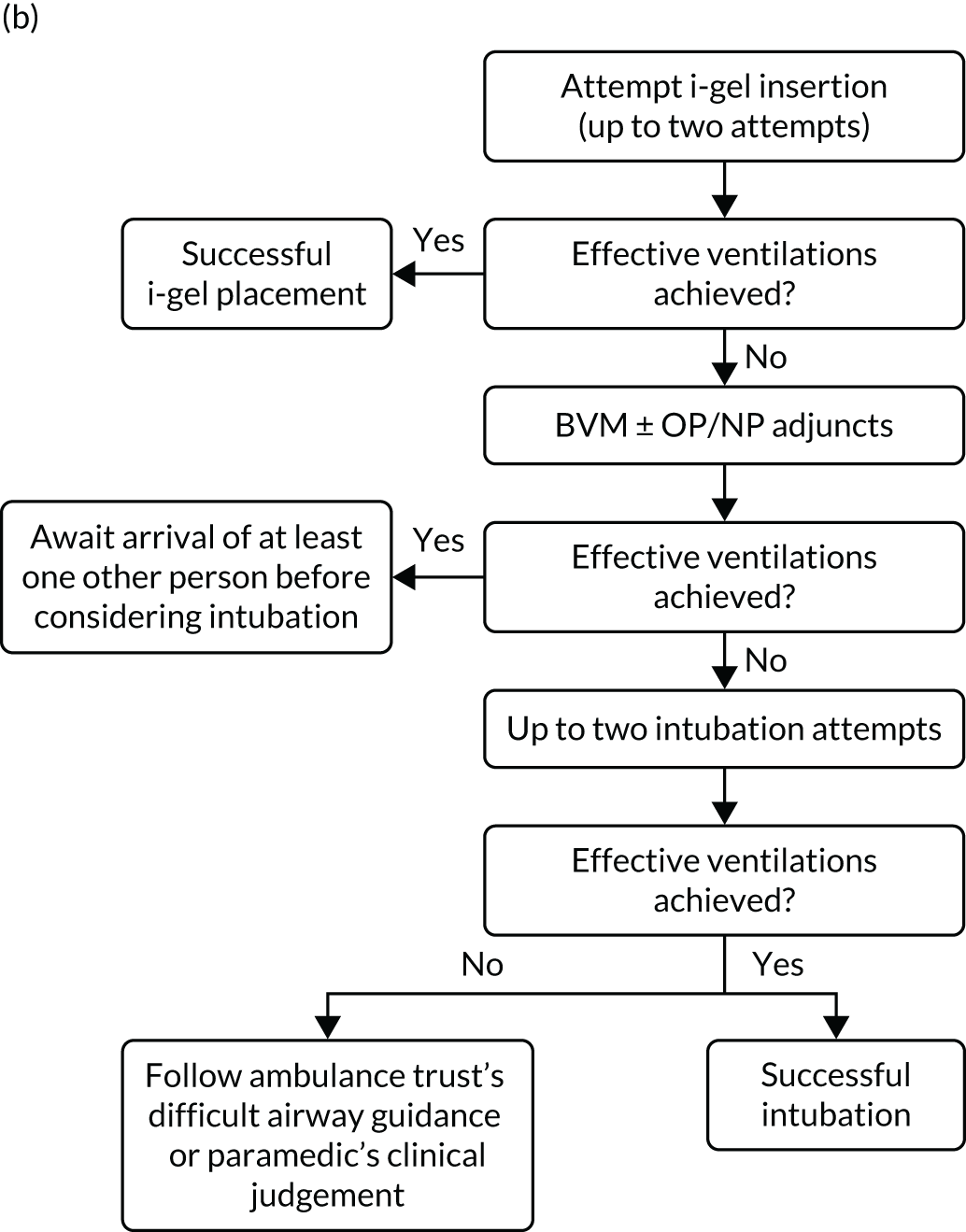
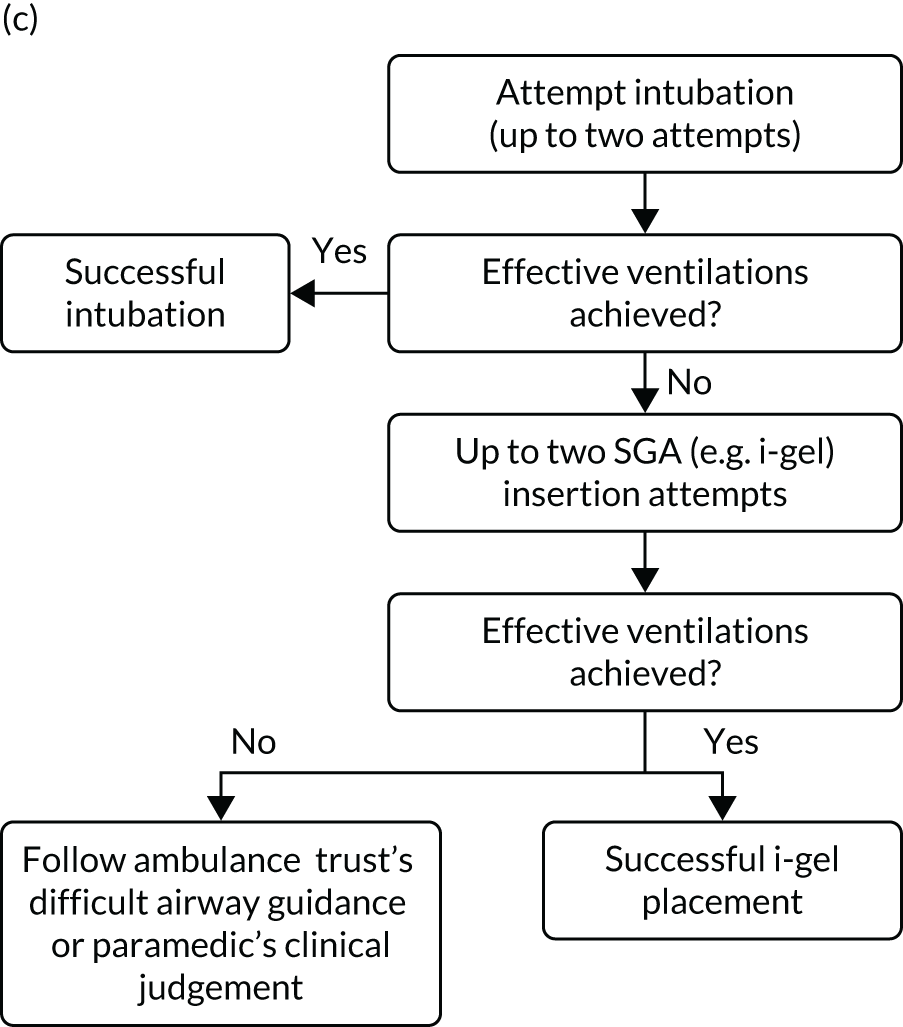
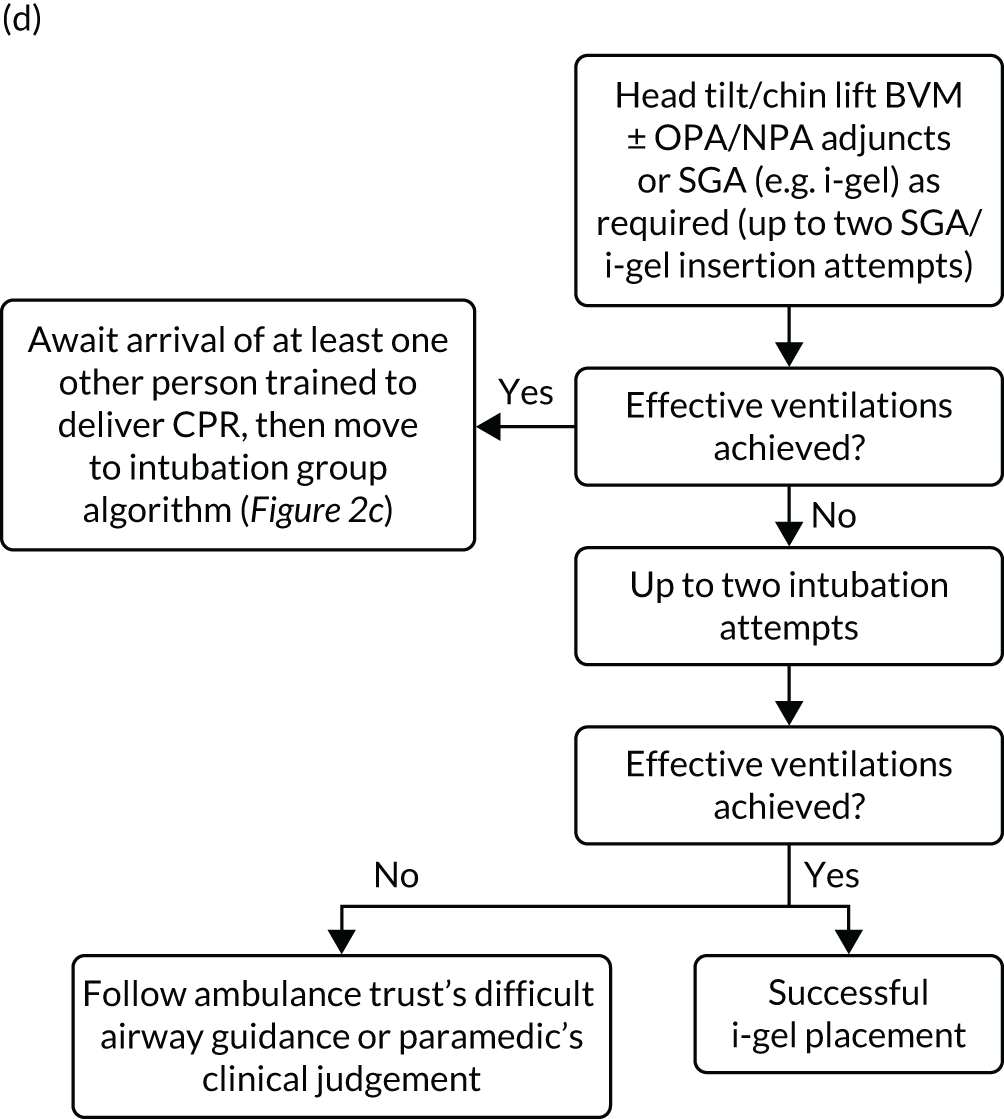
Care proceeded as normal for OHCA patients enrolled in the trial, aside from the initial AAM. All other interventions proceeded in accordance the standard resuscitation guidelines,24 which are disseminated widely in the UK and internationally. Patients who died at the scene were managed in accordance with nationally disseminated protocols. 28 Patients who did not die at scene were transported to hospital and treated using standard post-OHCA care pathways.
Owing to the emergency nature of the trial, we expected deviations from the AIRWAYS-2 trial treatment algorithm (see Figure 2). Protocol deviations could arise because paramedics have both strategies available to them. Usual practice follows a ‘step-wise’ approach from simple to more advanced techniques, but paramedics had clinical discretion to adapt airway management during OHCA to the patient’s anatomy, position and perceived needs. The trial protocol specified two attempts using the allocated strategy before proceeding to the alternative, but paramedics had discretion to deviate from the protocol on clinical grounds. Allowing discretion was necessary to avoid a paramedic feeling obliged to undertake an intervention that they believed to be against the patient’s best interests. This was also necessary to gain REC approval and professional support. 27
True crossover was defined as the patient receiving the incorrect intervention on the first airway management attempt; other deviations could occur during subsequent airway attempts. To try to reduce deviations as much as possible, monthly monitoring was carried out; research paramedics were required to follow up protocol deviations with the relevant AIRWAYS-2 paramedic and reiterate the correct procedures. We estimated that ≥ 80% adherence to the AIRWAYS-2 trial protocol was necessary to maintain the integrity of the trial, with < 10% true crossover. 1
Outcomes
Primary outcome
The primary outcome was mRS score measured at hospital discharge (or 30 days post OHCA if the patient was still in hospital). The mRS is a 7-point scale (0 to 6) widely used in OHCA research. 31,32 Scores are usually dichotomised as good outcome (0–3) or poor outcome/death (4–6; 6 indicates death). The full scale is:
-
0 – no symptoms at all
-
1 – no significant disability despite symptoms; able to carry out all usual duties and activities
-
2 – slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance
-
3 – moderate disability; requiring some help, but able to walk without assistance
-
4 – moderately severe disability; unable to walk and attend to bodily needs without assistance
-
5 – severe disability; bedridden, incontinent and requiring constant nursing care and attention
-
6 – dead.
Patients were conveyed to and followed up in hospital, where mRS scores were collected by assessors blinded to treatment allocation. mRS score was determined by a research nurse, who assessed the patient using a simple flow chart that has been used previously to assess cardiac arrest survivors. 33
With the permission of the Health Research Authority CAG, we were able to collect survival data and mRS score at hospital discharge or 30 days after OHCA for all enrolled patients, regardless of their consent status, thereby ensuring close to 100% ascertainment of the primary outcome.
Secondary outcomes
The trial sought consent from survivors to collect additional data at hospital discharge, 3 months post OHCA and 6 months post OHCA (depending on consent option chosen). The three consent options were:
-
Active follow-up – data were collected from the patient’s medical records and they were invited to complete questionnaires about their ongoing health and well-being at 3 and 6 months post OHCA.
-
Passive follow-up – data were collected from the patient’s medical records, but they were not contacted again or invited to complete follow-up questionnaires.
-
No further involvement – no further information was collected, but it was clearly stated that the information already collected would be retained and included in the data analysis. Anonymity of the participant was assured.
A proportion of participants who experience an OHCA remain incapacitated; therefore, the trial was designed so that a personal consultee (usually a close relative) could provide an opinion on the follow-up option that would probably be preferred by the patient. 26
The following secondary outcomes were collected for all eligible patients, with all but the last two reported by participating paramedics:
-
initial ventilation success, defined as visible chest rise
-
regurgitation (stomach contents visible in the mouth or nose) and aspiration (stomach contents visible below the vocal cords or inside a correctly placed tracheal tube or airway channel of a SGA)
-
loss of a previously established airway (patients with AAM only)
-
sequence of airway interventions delivered (patients with AAM only)
-
ROSC
-
airway management in place when ROSC was achieved, or resuscitation was discontinued
-
chest compression fraction (in a subset of patients in two ambulance services)
-
time to death.
For patients who survived to admission to hospital (estimated to be ≈ 20% of enrolled patients before the trial started), length of intensive care stay and length of hospital stay were also collected. For patients who survived to hospital discharge (estimated to be ≈ 9% of enrolled patients before the trial started), QoL using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), was collected at the time of discharge. For patients who survived beyond hospital discharge, date of death was collected (if applicable), mRS score was collected at 3 and 6 months post OHCA, and QoL (using the EQ-5D-5L) was collected at 3 and 6 months post OHCA.
Chest compression fraction was measured in a subset of patients. Good-quality, continuous CPR is associated with increased survival and improved neurological outcomes following cardiac arrest,19,34 and compression fraction is the standardised way of measuring and expressing this. 35 In this trial, standard resuscitation protocols were agreed with all participating ambulance services. These specified that patients receive continuous chest compressions as soon as an advanced airway device (i-gel or tracheal tube) was placed successfully. Therefore, patients in both groups should receive continuous chest compressions.
The compression fraction is defined as the proportion (or percentage) of resuscitation time without spontaneous circulation during which chest compressions are administered; the higher the compression fraction, the better the quality of CPR and the more likely it is that the patient will survive. 36 Comparison of the compression fraction between the two groups could help to explain the trial findings. Measuring and reporting compression fraction allows heterogeneity between trials to be more consistently described. A suggested mechanism by which SGAs may improve the outcomes of OHCA is a reduction in interruptions to CPR (with an accompanying increase in compression fraction).
Compression fraction is not routinely measured during OHCA in England but it is technically possible. 37 Measurement of compression fraction requires the use of a modified defibrillator, but it was not practical or affordable to measure this in all enrolled patients. Instead, the trial implemented technology that enabled compression fraction to be routinely measured during CPR in a subset of enrolled patients and collected these data alongside the other outcome measures. The technology used was the CPR card (Laerdal Medical AS, Stavanger, Norway), a small disposable device placed in the centre of the patient’s chest during CPR. The specific device we used gives no feedback to the user but records data that can be retrieved subsequently.
Resource use data to be used for the trial cost-effectiveness analysis and longer-term function were also collected (see Economic evaluation).
Adverse events
Serious adverse events (SAEs) and other adverse events (AEs) were recorded and reported in accordance with the Good Clinical Practice guidelines38 and the sponsor (South Western Ambulance Service NHS Foundation Trust)’s Research Related Adverse Event Reporting Policy.
Data on AEs were collected from the start of the intervention for the duration of the participant’s post-operative hospital stay and for the 6-month follow-up period if the patient consented to ongoing data collection. Any elective surgery/intervention/treatment (e.g. planned non-cardiac surgery) during the follow-up period that was planned prior to enrolment to the trial was not reported as an unexpected SAE.
Serious adverse events
Because all patients in this trial were in an immediately life-threatening situation, events related to cardiac arrest resuscitation (including death and hospitalisation) were expected; therefore, we obtained permission from the sponsor (South Western Ambulance Service NHS Foundation Trust) and the REC to report events as SAEs or serious adverse device events (SADEs) only if their cause was clearly unrelated to the cardiac arrest.
Unexpected adverse events
Events were reported as SAE/SADEs only if they were serious, potentially related to trial participation (i.e. may have resulted from trial treatment such as use of the SGA device) and were unexpected (i.e. the event was not an expected occurrence for patients who have had a cardiac arrest).
Examples of events that may have been a SAE/SADEs were the use of a SGA causing a new injury that endangered the patient, malfunction of the device causing injury to ambulance clinicians and malfunction of the device leading to inadequate ventilation.
Changes to trial outcomes after commencement of the trial
In April 2015, the primary outcome measure was amended to state that mRS score could be measured either at hospital discharge or 30 days post OHCA, instead of at hospital discharge only. The reason for this change was that some patients were proving to have very long hospital stays. Indeed, in some cases a patient could remain an inpatient for years, meaning that they would never record a primary outcome measure. It was noted that for patients who survived to hospital discharge (or were still inpatients 30 days after their OHCA) the mRS score would be determined by a research nurse who would assess the patient using a simple flow chart that has been used previously to assess patients who have had a cardiac arrest. 33 Any patient who did not survive to discharge would automatically be assigned a score of 6 (dead).
Sample size
In the REVIVE-AIRWAYS feasibility trial, 9% of enrolled patients survived to hospital discharge. 25,39 This was in line with the prevailing rate of overall survival to discharge reported by EMS in England. 4 No data were available for mRS score. However, death and poor functional outcome after OHCA are closely related because death is the most common outcome. 33 Using survival as a proxy for mRS score, a 2% improvement in the proportion of patients achieving a good neurological outcome (defined as an mRS score of 0–3) would be clinically significant, and similar to the 2.4% difference in survival to discharge between TI and SGAs reported in a retrospective analysis. 13
To identify a difference of 2% (8% vs. 10%, i.e. centred on 9%), we calculated that 4400 patients per group (at the 5% level for statistical significance and 90% power) would be required. However, each OHCA was not an independent observation, as the patients are nested within a limited number of paramedics who participated in the trial. Using data from our feasibility trial of 171 paramedics attending 597 OHCAs, we estimated that the intraclass correlation coefficient (ICC) would be < 0.001. However, when estimating the sample size we assumed a conservative estimate for the ICC of 0.005. Therefore, we required a sample size of 9070 patients (4535 per group).
Paramedic sample size
In our feasibility trial the mean number of patients enrolled per participating paramedic was 3.6 per year. Therefore, to enrol the 9070 patients within the 2-year period of the trial, we estimated that we would need to recruit at least 1300 paramedics. Across the four ambulance services participating in the AIRWAYS-2 trial, there were > 4300 eligible paramedics; therefore, we needed to enrol > 30% of these paramedics.
Interim analyses
A formal 1-year interim analysis of trial data for patients enrolled within the first year of the trial was performed. The purpose of this interim analysis was (1) to determine whether or not there was an unexpected large difference in the primary outcome or in the mortality rates between the two treatment groups that might justify stopping the trial early and (2) to establish whether or not the assumptions underpinning the sample size calculations were still valid. The data and results from this interim analysis were not shared outside the DMSC.
Modified Rankin Scale score and all-cause mortality were the only outcomes that were formally compared. The analyses of mRS score included several subgroup and sensitivity analyses. All other outcomes were described but not formally compared. The criteria for recommending stopping the trial were agreed at the first meeting of the DMSC and documented in the DMSC charter. The agreed threshold for stopping was a p-value ≤ 0.001 for the group comparison in the intention-to-treat (ITT) analysis of the primary outcome. The results of this analysis, along with patient enrolment figures and success rates, were sent to the DMSC in a preliminary report in July 2016. No adjustments to the sample size or statistical significance levels were made.
Randomisation
In the AIRWAYS-2 trial, the potential participants were unconscious and in need of immediate emergency care, and clinical necessity was therefore the overriding priority. For this reason, it was not deemed feasible to randomise individual patients and a cluster randomised design was considered most appropriate. We chose to randomise the paramedics, treating each participating paramedic as a ‘cluster’. This choice meant that the trial had many clusters, with average cluster size being relatively small (the median number of OHCAs attended by a paramedic annually was three in our previous feasibility trial25), minimising the effect of ICC and the risk of chance imbalances between groups.
Paramedics working in SWAST, EMAS, EEAST or YAS who consented to participate in the trial were randomly allocated in a 1 : 1 ratio to one of the two groups: i-gel or intubation (i.e. each paramedic was a randomised cluster). This ensured that the number of paramedics in each group was equal; however, some imbalance in the number of patients enrolled was possible as a result of chance.
The random allocation sequence was generated by the trial statistician using the ralloc command in Stata® version 13 (StataCorp LP, College Station, TX, USA). Blocked randomisation of varying sizes (4, 6, 8) was used and randomisation was stratified by ambulance service, years of paramedic experience (< 5 years’ vs. ≥ 5 years’ full-time operational experience) and urban/rural location of the base ambulance station (≥ 5 miles vs. < 5 miles from the nearest hospital with an ED that receives cardiac arrest patients).
The random allocation sequence was embedded in the database and randomisation was performed by research paramedics using a secure computer system developed by Clinical Trials and Evaluation Unit (CTEU) Bristol, with allocation concealment that could not be changed once allocated. The allocation was not revealed until enough information to identify the paramedic had been entered into the system. 1
To avoid bias caused by paramedics withdrawing from the trial based on their allocation, paramedics were not randomised until halfway through a trial-specific training session; prior to randomisation the trial design and the need for individual equipoise was explained. If the paramedic was willing to treat all OHCA patients they attended during the trial period by either intervention, they gave consent to take part in the trial. The paramedic was then randomised and completed the training session with training that was specific to their allocation. 1 Training comprised theoretical and simulation-based practice over 1 hour, with a brief assessment to confirm competence. For TI, a two-person technique using an intubating bougie was recommended. End-tidal CO2 monitoring was used to confirm correct device placement in all patients.
Blinding
Because of the nature of the intervention, paramedics could not be blinded and were aware of treatment allocations. Therefore, it was necessary to ensure that all eligible patients were enrolled to avoid selection bias. The trial adopted a model whereby every eligible patient attended by a participating paramedic was automatically enrolled in the trial under the waiver of consent provided by the CAG. In this way, the participating paramedics could not influence whether or not a patient was enrolled. However, a disadvantage of this model of automatic enrolment was that the trial protocol might not be followed because the enrolling paramedic could not recall the protocol details (attendance at an OHCA is relatively rare and stressful for paramedics) or the paramedic mistakenly believed the patient to be ineligible.
Ambulance control room personnel were blinded to the allocation of paramedics and followed established protocols when allocating resources to a possible cardiac arrest. This ensured that there was no bias in despatch.
Patients were unaware of their treatment allocation at the time of the intervention and this was likely to be maintained throughout the trial. Research staff assessing outcomes at hospital discharge and at the 3- and 6-month follow-ups were also blinded to treatment group.
Emergency department staff could not be blinded to the treatment group (intubation or i-gel) to which the patient was allocated because the patient would arrive in the ED with either the intubation tube or the i-gel obviously visible in situ. However, we were able to blind clinical staff who cared for the patients beyond the ED to the method of initial airway management used. Therefore, the care of the patient beyond the ED was not affected by knowledge of the intervention used.
Data collection
Data collection included the following elements:
-
a log of all paramedics approached and a record of those who consented to take part in the trial
-
a log of all patients who had an OHCA who were attended by a paramedic within one of the four participating ambulance trusts
-
a log of those attended by an AIRWAYS-2 paramedic (together with details of whether or not resuscitation was attempted)
-
a log of all OHCA patients attended by an AIRWAYS-2 paramedic (where resuscitation was attempted) assessed against the eligibility criteria and, if ineligible, reasons for ineligibility
-
a screening log of all OHCA patients enrolled in the trial who survived to ICU/coronary care unit (CCU) discharge
-
survivors who were approached for consent (including the date that they were given the patient information leaflet) and outcome of the consent process
-
for those who consented to active follow-up, responses to QoL and mRS questionnaires collected at the time of consent and at follow-up at 3 and 6 months
-
key data items from routine data sources for survivors who consented and for those who died prior to discharge from ICU/CCU
-
demographic characteristics of surviving OHCA patients who did not consent and withdrew from the trial.
These data were requested without any direct patient identifiers to maintain anonymity. The following information was sought: NHS number, date of birth, sex and data to characterise socioeconomic status (partial postcode).
Data collection occurred during the out-of-hospital treatment phase, during the inpatient phase of care, at hospital discharge and at 3 and 6 months (± 4 weeks) after the index OHCA (Table 1).
| Data item | Out-of-hospital treatment phase (data collection by paramedics) | Hospital discharge (data collection by hospital staff) | 3 months post OHCA | 6 months post OHCA |
|---|---|---|---|---|
| Eligibility | ✓ | |||
| Airway management | ✓ | |||
| Demography | ✓ | ✓ | ||
| Survival | ✓ | ✓ | ✓ | ✓ |
| Patient movements | ✓ | ✓ | ||
| Approached for consent | ✓ | |||
| mRS score | ✓ | ✓ | ✓ | |
| EQ-5D-5L score | ✓ | ✓ | ✓ | |
| Economic data | ✓ | ✓ | ✓ | ✓ |
| SAEs | ✓ | ✓ | ✓ | ✓ |
| Length of hospital stay/ward movements | ✓ |
Training in data collection and case report form (CRF) (see Appendix 9) completion was provided by the research nurse in each region, co-ordinated and supported by the central trial team at CTEU Bristol. A fixed fee per patient was included in the trial research costs to support the collection of trial-specific outcome data.
To minimise bias, outcome measures were defined as far as possible based on objective criteria. All personnel carrying out an outcome assessment beyond ED care were blinded to help minimise bias.
Identification of patients with out-of-hospital cardiac arrest
All eligible patients attended by a participating paramedic were automatically enrolled in the trial under a waiver of consent. Therefore, it was essential to establish mechanisms that would reliably identify every one of these patients. We achieved this by identifying every OHCA (where resuscitation was attempted) that occurred in the participating ambulance services throughout the trial period, along with the subset of patients eligible for trial inclusion. The process to achieve this is described in the following paragraph. It allowed regular review by the DMSC and supported a complete ITT analysis (see Statistical methods, Sensitivity analyses of the longer-term secondary outcomes).
In April 2011, the Department of Health and Social Care introduced survival from cardiac arrest as part of the Ambulance Service National Quality Indicator set. 40 ROSC and survival to hospital discharge rates are reported for all patients who have resuscitation started or continued by a NHS ambulance service after an OHCA. 41 For this reason, all cardiac arrests are routinely identified by ambulance services in England, with regular data collection and return. This process was being strengthened through the introduction of an electronic patient record and a national OHCA registry, based at the University of Warwick. 42 To ensure near-complete patient identification, we used a triangulation method developed during the feasibility trial. 25 Data were collected on all OHCAs occurring within an ambulance service from three separate sources:
-
Direct paramedic report – participating paramedics were asked to complete a CRF immediately after each eligible OHCA that they attended, and to notify the co-ordinating research paramedic by telephone, text or e-mail.
-
Daily review of the ambulance computer-aided dispatch (CAD) system by a project research paramedic to identify all 999 calls from the previous 24 hours identified as suspected or confirmed cardiac arrest, and follow up with the relevant ambulance staff to determine whether or not OHCA had occurred.
-
Regular review of the OHCA data routinely collected by that ambulance trust and reported as part of the Ambulance Service National Quality Indicator set. 40 This is usually based on the clinical record (paper or electronic) routinely completed by ambulance staff after each case that they attend.
Source 1 was the primary data source for the AIRWAYS-2 trial. However, by triangulating data from all three sources it was possible to reliably identify all, or nearly all, OHCAs where resuscitation was attempted during the trial. Although it was possible for an eligible OHCA to be overlooked by this triangulation process, it would require that a cardiac arrest not be reported to the research team by a participating paramedic, not be identified as an OHCA on the CAD and not be picked up by the ambulance trust’s routine identification and reporting system. We estimated that the chance of this happening was very low, thereby ensuring an exceptionally high rate of eligible patient identification that reduced any bias to an absolute minimum.
Out-of-hospital treatment phase (data collection by paramedics)
After treating an eligible OHCA patient, the participating paramedic responsible for airway management completed a CRF to capture baseline and secondary outcome data. The CRF was completed at the same time as routine ambulance service paperwork: immediately after the patient had been handed over to the receiving hospital team or resuscitation attempts had been discontinued at the scene. The CRF was then returned as soon as possible (preferably within 24 hours) to the co-ordinating research paramedic by a secure method chosen by each ambulance trust (e.g. post, secure fax or e-mail). Occasionally, the participating paramedic would not complete the form immediately, in which case they were contacted by the research paramedic subsequently and encouraged and supported to do so.
Even when this did not occur, relevant data could be extracted from the routine ambulance service record within 48 hours, allowing the patient to be followed up to seek consent and collect primary and secondary outcome data. Ambulance services reliably collect data regarding the individuals attending each patient and the time of staff arrival; therefore, for every eligible patient, the attending ambulance paramedic(s), trial allocation and a range of baseline data could be determined with near-100% accuracy.
Hospital discharge (data collected by hospital staff)
Once a patient had been admitted to hospital, the consent and follow-up process was co-ordinated by a research nurse allocated to each participating ambulance service. This was identified as a separate, hospital-based post to ensure that consent and follow-up was blinded to treatment. The research nurse was usually based in the main ‘heart attack centre’ or major receiving hospital for that region. 43
Each research nurse received regular lists of enrolled patients who had been brought to the receiving hospitals in that ambulance service region. The research nurse co-ordinated the process of identification, consent and follow-up data collection with support from the central team. Although the research nurse undertook this personally where necessary, in most cases the consent and follow-up processes were undertaken by existing research staff at the receiving hospitals.
Statistical methods
Enrolled patients who were subsequently identified as being ineligible remained in the trial and were included in analyses, with the exception of (1) patients who were subsequently found to have been previously enrolled in the trial, (2) patients who were inadvertently enrolled in the trial owing to being treated as a trial participant by a paramedic who arrived later than second at the patient’s side and (3) patients who were subsequently identified as being children (aged < 16 years; individuals aged 16 and 17 years were included in analyses). Analyses were undertaken according to the principle of ITT and reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 44,45
Analysis of the primary outcome, and exploratory analyses of secondary outcomes, were performed according to a pre-specified SAP, which was finalised before data lock and any comparative analysis but after the end of patient enrolment due to staff changes in the statistical team. Some typographical errors were corrected in version 2 and some points were clarified, but no substantive changes were made. No comparative post hoc analyses were performed. 27
Non-adherence to allocated group was documented. The trial was analysed on an ITT basis (i.e. outcomes were analysed in accordance with the treatment allocation of the first trial paramedic on scene, irrespective of future management and events, and every effort was made to include all participants treated by a trial paramedic who met the inclusion criteria). Follow-up for the outcome measures during the participant’s stay in hospital and at the 3-month and 6-month time point should have been complete for all participants who consented to take part in the trial.
All analyses were performed using Stata version 15.1 unless otherwise stated. For hypothesis tests, two-tailed Wald p-values < 0.05 were considered statistically significant. Statistical tests to compare data not listed as outcomes were not performed. All ratio effects are presented as i-gel divided by TI and all difference effects are presented as i-gel minus TI.
Where possible, adjusted differences in proportion of patients (ADPs) experiencing a good outcome were calculated by fitting a model with a binomial family, an identity link and clustered sandwich estimator for paramedic. Risk ratios (RRs) were also calculated, where possible, by fitting a model with a Poisson family, logit link and a clustered sandwich estimator for paramedic.
Data presentation
Continuous variables were summarised using the mean and standard deviation (SD), or the median and interquartile range (IQR) if the distribution was skewed. Categorical variables were summarised as number and percentage.
Adjustment in models
The intention was to adjust all models for paramedic as a random effect and for the three stratification factors included in the randomisation as fixed effects [NHS ambulance trust (YAS, SWAST, EMAS and EEAST), paramedic experience (≥ 5 years and < 5 years) and distance from paramedic’s base ambulance station to the nearest hospital (≥ 5 miles and < 5 miles)]. Where it was not possible to fit paramedic as a random effect, the clustering within paramedic was accounted for using a clustered sandwich estimator, or clustered bootstrap where this was not possible.
Primary and secondary pre-hospital discharge outcome models
The primary outcome of mRS score at discharge or 30 days post OHCA [presented dichotomously as good functional recovery (0–3) or poor functional recovery/death (4–6; 6 indicates death)], and other binary outcomes were analysed using a multilevel logistic regression model. Repeated mRS scores were analysed using multilevel logistic regression at the separate time points owing to convergence issues. The treatment effects for these models were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Compression fraction was transformed owing to skewness and the log of 100 minus the compression fraction was fitted using a multilevel Gaussian model, with the treatment effect presented as a geometric mean ratio (GMR) and 95% CI.
The following secondary outcomes were described but not formally compared: sequence of airway interventions delivered, airway management in place when ROSC was achieved or resuscitation was discontinued, and length of ICU stay. Time to death or last follow-up was formally compared in place of length of ICU and hospital stays. For time to death (up to 72 hours), patients who were known to be alive longer than 72 hours post OHCA were censored at 72 hours (i.e. given a time to death of 72 hours). For time to death or last follow-up, survivors who did not consent to active or passive follow-up were censored at ICU discharge, survivors who consented and provided 6 months’ follow-up data were censored at 6 months post OHCA, survivors who consented and provided 3 months’ but not 6 months’ follow-up data were censored at 3 months post OHCA, and survivors who consented and provided 30 days’/hospital discharge data but not 3 months’ or 6 months’ follow-up data were censored at hospital discharge.
Both time to death or last follow-up and time to death (up to 72 hours) were analysed using Cox proportional hazards models stratified by NHS ambulance trust to allow for varying baseline hazards and adjusted for paramedic experience and distance from base ambulance station. These models were adjusted for clustering of paramedic using a clustered sandwich estimator and presented as hazard ratios (HRs) and 95% CIs.
Sensitivity analyses of the primary outcome
Three pre-specified exploratory sensitivity analyses were performed for the primary outcome. The first extended the trial population to include patients attended by a participating paramedic but who were not resuscitated (i.e. trial patients plus non-resuscitated patients). This was prompted by feedback from a pre-planned, closed interim analysis of half the sample considered by the DMSC. 27 The second and third sensitivity analyses, restricted to the cohort of patients who received AAM (as allocated and treatment received comparisons), were planned from the outset. All three sensitivity analyses were analysed using multilevel logistic regression.
Additional analyses of the primary outcome
In the sensitivity analysis of the primary outcome restricted to the cohort who received AAM, patients who did not receive either trial treatment were excluded. Owing to concerns that this analysis could be prone to bias, one additional analysis was performed to assess the causal effect of the treatment received on the primary outcome. This analysis used two-stage least squares with two instruments: randomisation and an indicator of whether one or two paramedics initially attended the OHCA. In the first stage, the treatment received was regressed on the two instruments and the interaction between the two instruments. Predicted probabilities were obtained from the first-stage model. In the second stage, the mRS score was regressed on these predicted probabilities and the stratification factors used in randomisation. For more information relating to the model fitted, see Appendix 4.
Subgroup analyses of the primary outcome
Two subgroup analyses were planned: (1) Utstein comparator group (OHCA with a likely cardiac cause that was witnessed and had an initial rhythm amenable to defibrillation,46 estimated to make up ≈ 20% of the total) versus non-comparator group and (2) OHCA witnessed by paramedic (estimated to make up 6% of the total) or not. These two subgroup analyses were analysed on an ITT basis.
Because of concerns about ventilation success raised during the trial, an additional subgroup analysis of the primary outcome comparing patients whose i-gel or intubation airway management attempt(s) were or were not ‘successful’ during the first and/or second attempt was also performed. This analysis was performed on an as-treated basis (i.e. according to the first AAM the patient had received). In addition, this third unplanned subgroup analysis included patients who had received at least one AAM using an i-gel and/or TI only.
The treatment effects in subgroups were compared by testing for an interaction between paramedic allocation and the subgroup variable. We described the outcomes in the subgroups and tested for differences in the primary outcome between subgroups by including interaction terms in the models, although we recognised that the power to detect such differences was low as the proportions in the subgroups were unequal.
Longer-term secondary outcomes
The longer-term secondary outcomes were mRS score measured at 3 months and 6 months and QoL [single summary index and visual analogue scale (VAS)] measured at 30 days/hospital discharge, 3 months and 6 months. These longer-term outcomes were obtained from patients who had survived to the follow-up time points and had provided active consent.
The five dimensions of the EuroQol-5 Dimensions (EQ-5D) – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – were described for actively consented survivors. These five dimensions were transformed into a single summary index score using a method that mapped these scores onto the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), value set. 47 A value of 0 was assigned for both VAS and single summary index for patients who had died.
Only patients with outcome data were included in the main analyses of these outcomes (complete-case analysis). The dichotomised mRS scores were analysed using multilevel logistic regression with paramedic fitted as a random effect. Both the single summary index and VAS scores had large spikes at 0 due to the large number of deaths, which meant that a normal or log-normal regression model was inappropriate. Consequently, these two QoL outcomes were analysed using a two-part beta-binomial model. For the purposes of modelling, the QoL scores of survivors were transformed as follows:
where y is the QoL score, a is the lowest possible score (single summary index –0.59, VAS 0), b is the highest possible score (index 1, VAS 100), N is the total number of survivors with data and yn is the transformed score. 48 This transformation was necessary for the purposes of beta regression as it guaranteed that the transformed scores were between 0 and 1 (excluding 0 and 1).
Two estimates were produced from the two-part beta-binomial model. The first, which is the binomial part, is the OR for survival (‘alive vs. dead’). The second estimate, which is the beta part, relates to the QoL of survivors (‘score for survivors’). Thus, these models were able to assess whether or not the use of the i-gel reduces the risk of death and, if the patient survives, assess whether or not it improves the patient’s QoL. An estimate > 1 for ‘alive vs. dead’ means that the odds of survival in the i-gel group is higher than in the TI group. Similarly, an estimate > 1 for ‘score for survivors’ means a better QoL in the i-gel group than in the TI group.
All longer-term outcomes were fitted to each time point separately because convergence issues prevented the fitting of longitudinal models. Convergence issues were also encountered when including paramedic as a random effect in the QoL models. Thus, the CIs were estimated using clustered bootstrapping. 49,50 A total of 1000 cluster bootstrap samples were created by sampling the paramedic clusters with replacement to obtain 1375 paramedic clusters in each bootstrap sample. The ‘alive vs. dead’ and ‘score for survivors’ estimates were obtained from each of the 1000 cluster bootstrap samples by applying the two-part beta-binomial model. The SD of each set of estimates (SDbootstrap) was then used as an approximation of the standard error (SE) and the 95% CIs were estimated using the formula:
Both the clustered bootstrap and two-part beta-binomial models were performed in SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration) and all other analyses were performed in Stata.
Sensitivity analyses of the longer-term secondary outcomes
Two sensitivity analyses were performed on the longer-term secondary outcomes to examine the effect of missing data. The first was the ‘worst-case scenario’, in which the worst possible score for a survivor was assigned to known survivors with missing data. In this scenario, patients whose survival status was unknown were assumed to have died. The ‘imputed case scenario’ was the second sensitivity analysis. In this scenario, multiple imputation (60 imputations) was performed using the ice command in Stata. Estimates were combined using Rubin’s rules.
In the worst-case scenario, multilevel logistic regression was performed for the mRS score at 3 months and 6 months post OHCA. The two-part beta-binomial model was used to analyse the QoL outcomes at 30 days/hospital discharge, 3 months and 6 months post OHCA. For the QoL outcomes, the 95% CIs were adjusted using the clustered bootstrap method described in Longer-term secondary outcomes.
In the ‘multiple imputed case scenario’, the variables included in the multiple imputation model were age; sex; length of ICU stay; treatment group; the randomisation stratification variables; and QoL and mRS scores at 30 days/hospital discharge (whichever was earlier), 3 months and 6 months post OHCA. Predictive mean matching was used for continuous variables. The percentages of missing data were calculated for patients who survived to 30 days/hospital discharge (whichever was earlier) for all QoL and mRS score outcomes and time points in turn. The number of imputations (which was 60) was based on the maximum missingness percentage. For mRS score at 3 months and 6 months post OHCA, multilevel logistic regression with paramedic as a random effect was performed on the multiple imputed data sets and the estimates were pooled using Rubin’s rules. In this scenario, the QoL secondary outcomes were analysed using the two-part beta-binomial model. To obtain the cluster bootstrap-adjusted 95% CIs, the clustered bootstrap was performed to produce the 1000 bootstraps samples. This was followed by multiple imputation (60 imputations) on each of the bootstrap samples as recommended by Schomaker and Heumann. 51 Rubin’s rules were then used to combine the treatment estimates in each of the 1000 bootstrap samples and, as described in Longer-term secondary outcomes, the SDs of these two sets of treatment estimates were used as proxies for the SEs in the calculation of the CIs. These analyses were completed using Stata for the multiple imputation and SAS for the clustered bootstrap and model fitting.
Frequency of analyses
The original intention was for the primary analysis to take place when follow-up was complete for all enrolled participants. Formal interim analysis was planned at the mid-point of patient enrolment (after 12 months) and was presented to the DMSC. Safety data were reported together with any additional analyses the committee requested. In these reports the data were presented by group, but the allocation remained masked.
During the sixth DMSC meeting in September 2017, the DMSC recommended that the primary outcome be analysed prior to completion of patient follow-up and published first. There were three main reasons for early reporting: (1) to realise patient benefit as soon as possible, (2) to inform policy and guidance at a time of increased interest in the future of TI52 and (3) to co-ordinate with publication of the PART, a comparable trial undertaken in North America. 53 The DMSC was happy for this to take place if all relevant data fields could be locked down. This proposal was discussed with and agreed by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme and the Trial Steering Committee (TSC) at their fourth meeting in October 2017.
Economic evaluation
Economic evaluation aims and objectives
The economic evaluation aimed to estimate the incremental cost-effectiveness of the i-gel compared with TI in non-traumatic OHCA adults in line with the AIRWAYS-2 trial.
Economic evaluation overview
The perspective of the evaluation was that of the NHS and Personal Social Services (PSS), as recommended by the National Institute for Health and Care Excellence (NICE). 54 The perspective for outcomes was that of the patients undergoing treatment. The primary outcome measure for the cost-effectiveness analysis was quality-adjusted life-year (QALYs), estimated using the EQ-5D-5L. 55,56 Good practice guidelines on the conduct of economic evaluations were followed. 54,57,58 Table 2 summarises the key aspects of the economic evaluation methods.
| Aspect of methodology | Strategy used in base-case analysis |
|---|---|
| Form of economic evaluation | Cost-effectiveness analysis for comparison between the i-gel and TI |
| Perspective | NHS and PSS |
| Time horizon | A within-trial analysis, taking a 6-month time horizon |
| Data set | All trial patients were included (see Data set) |
| Costs included in analysis | Pre hospital:
|
| Utility measurement | EQ-5D-5L (administered at hospital discharge and at 3 and 6 months post OHCA) |
| QALY calculations | Assume that patients’ utility changes linearly between utility measurements |
| Missing data | Multiple imputation |
Form of analysis, primary outcome and cost-effectiveness decision rules
A cost-effectiveness analysis (specifically a cost–utility analysis) using QALYs as the primary outcome measure was conducted, as advocated by NICE. 54 QALYs combine both quantity of life and QoL into a single measure. Incremental costs (the difference in mean costs between the i-gel and TI groups) were divided by incremental QALYs (the difference in mean QALYs between the groups) and presented as the incremental cost-effectiveness ratio (ICER), which quantifies the incremental cost per QALY gained by switching from using TI to i-gel. The economic evaluation analyses were performed on an ITT basis.
The i-gel was considered cost-effective if the ICER fell below £20,000, which is generally considered to be the threshold that NICE adopts for considering an intervention to be cost-effective. 59
Time horizon
A within-trial analysis, taking a 6-month time horizon, was conducted. It was anticipated that all major resource use would occur within this time frame and, therefore, be captured. Our time horizon began when the first paramedic arrived at the OHCA scene and continued until 6 months later.
Data set
The base-case analysis included all trial patients (see Economic evaluation overview), except seven patients who were transported to hospital but could not be identified and were lost to further follow-up (three randomised to TI and four randomised to i-gel). It was felt that there was insufficient information on these patients to reasonably impute their follow-up data.
Collection of resource use and costs
Resource use data were collected on all significant health service resource inputs for the trial patients to the end of the 6-month follow-up period. Detailed resource use data on the pre-hospital phase in the patient care pathway were collected on the trial CRFs, and inpatient data were largely obtained from HES data sets. CRFs for the pre-hospital phase were completed by the paramedics attending the OHCA patients and by the research paramedics using data from the ambulance CAD system. A small amount of inpatient resource use was captured on the in-hospital CRFs, and primary and community care resource use post hospital discharge was captured on the follow-up questionnaires at 3 and 6 months post OHCA for patients who consented to follow-up. The main resource use categories costed are listed in Table 3, along with details of the sources of unit cost information for each resource category.
| Resource | Source(s) | Source(s) of unit cost information |
|---|---|---|
| Airway devices used and management at the scene (pre hospital) | Trial CRF | NHS Supply Chain Online Catalogue60 |
| Ambulance staff (and vehicles) attending the scene (pre hospital) | Trial CRF | NHS Employers Agenda for Change pay scales 2017/18;61 ambulance trusts |
| Index ED attendance | Trial CRF; HES | NHS Reference Costs 2017/18 62 |
| Admission to a ward, and length of stay by level of care | Trial CRF; HES | NHS Reference Costs 2017/18 62 |
| Operations and procedures (e.g. CT scan, percutaneous coronary intervention) | HES (or trial CRF) | NHS Reference Costs 2017/18 62 |
| Hospital re-admissions | HES (or trial CRF); 3- and 6-month follow-up questionnaires | NHS Reference Costs 2017/18 62 |
| Outpatient and ED attendances | HES | NHS Reference Costs 2017/18 62 |
| Community health-care contacts | 3- and 6-month follow-up questionnaires | Unit Costs of Health and Social Care 2018 63 |
| Long-term care, or stays in nursing/residential homes | Trial CRF (discharge destination); 3- and 6-month follow-up questionnaires | Unit Costs of Health and Social Care 2018 63 |
| Equipment and aids | 3- and 6-month follow-up questionnaires | Unit Costs of Health and Social Care 2018 63 |
Availability of resource use and cost data
Given the large numbers of patients and hospitals involved in the AIRWAYS-2 trial, the trial CRFs asked limited numbers of questions on health-care resource use. The intention was to make use of routinely held data and collect the majority of secondary care resource use from HES. Because we had several issues obtaining data from four HES data sets (ED, inpatients, critical care and outpatients), we made contingency plans early in the trial to mitigate the risk of not receiving all the HES data we would need for the trial cost-effectiveness analysis.
At the outset of the trial, some additional resource use data items were added to the trial data collection forms. Detailed information on trial CRFs captured the date and time of movements between different levels of care during the inpatient admission. An additional question was also added to the 3- and 6-month follow-up questionnaires asking about overnight hospital stays. However, without HES data we would have no information on interventions and procedures patients receive in hospital (notably CT and percutaneous coronary interventions) nor any information after hospital discharge about further contact with secondary care (re-admissions, outpatient appointments, subsequent ED visits) beyond the information described above on the number of additional nights in hospital captured on the follow-up questionnaires.
To further mitigate against a possible lack of HES data as the trial neared completion, we asked the top five hospitals (i.e. the five hospital trusts that had received the most patients) in each of the four ambulance trust regions enrolling patients to the trial for some additional data for up to 50 AIRWAYS-2 patients taken to their hospital. For the index admission, this additional retrospective CRF captured the number of CT and magnetic resonance imaging scans, angiograms performed (and whether or not these included a percutaneous coronary intervention) and any other surgery. For the period from hospital discharge to 6 months post OHCA, the trial CRFs captured information on hospital re-admissions (length of stay and number of days in intensive care) and any other surgery. We sought information on a random sample of 50 patients taken to each hospital (or all patients taken there if this was < 50), sampled in the following ratio: one-third died in the ED, two-thirds admitted to hospital. This extra data collection was made possible by support from the NIHR critical care clinical specialty leads and research nurses.
The intention was for these secondary care resource use data, captured for a sample of AIRWAYS-2 patients on trial CRFs, to be summarised and costed. The mean resource use and associated costs would then be calculated for three subgroups of patients:
-
patients who died in ED
-
patients who survived to hospital admission but died in hospital
-
patients who survived to hospital discharge.
The mean costs of procedures and scans in hospital, and re-admissions, calculated for these subgroups would then be applied to all AIRWAYS-2 patients who were brought to hospital. However, these data were not used, because HES data were obtained and used in our primary analysis. However, having collected these additional data, an extension to this work will compare this resource use and associated costs with the HES data.
Although there was very good case ascertainment for three of the HES data sets received, there were considerably fewer patients in the critical care data set than expected. Based on CRF data, 1450 patients were admitted to intensive care following their OHCA; however, the HES critical care data set contained records for only 314 patients. Given this wide disparity between data sources, we did not use the HES critical care data set; time in intensive care was taken from the CRFs instead. NHS Digital has kindly investigated this issue. The original request for each identified patient was for data from the date of OHCA to 6 months post OHCA. It appears there may be an issue with dates: from a preliminary check, HES critical care records relating to ≈ 1250 patients were identified when data from date of OHCA minus 7 days through to 6 months were extracted, much more in line with expectations. However, it is still unclear why this issue exists. There was a considerable amount of data cleaning required for the resource use figures. For further details, see Appendix 5.
Attaching unit costs to resource use
Unit costs for hospital and community health-care resource use were largely obtained from national sources, for example NHS Reference Costs for ward costs, scans and surgery,62 and Unit Costs of Health and Social Care for community costs. 64,65 Resources were valued in 2017/18 Great British pounds, and any unit costs not in 2017/18 prices have been adjusted to 2017/18 prices using the NHS Cost Inflation Index (NHSCII). 66 For a summary of the sources of unit cost information, see Table 3. For further details on all unit costs and their sources, see Appendix 6.
Measurement of health-related quality of life and quality-adjusted life-years
Measurement of health-related quality of life
The EQ-5D-5L, advocated for use in economic evaluations by NICE,54 was used to measure health-related quality of life (HRQoL). 55,56 The EQ-5D descriptive system is a generic measure of health outcome covering five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Responses to this multiattribute utility scale can be converted to a single index value. The EQ-5D also includes a VAS for patients to rate their overall current health, but that response was not used in the economic evaluation. The EQ-5D-5L was completed by patients at three time points: at hospital discharge (or 30 days if sooner) and at 3 and 6 months post OHCA. Although data were gathered using the EQ-5D-5L (i.e. the five-level version, with five possible responses for each dimension), responses recorded on the instrument were converted into a single index value using the original three-level UK valuation set. 67 Scores were then used to facilitate the calculation of QALYs. Utility values were calculated by mapping the five-level descriptive system to the three-level valuation set using the crosswalk developed by van Hout et al. ,68 in line with NICE recommendations at the time of analysis. 69
Baseline utility
Because OHCA is a medical emergency and patients cannot complete the EQ-5D-5L at (or close to) the time of enrolment, baseline HRQoL data were not available. Although a published review concluded that there is no one clear way of dealing with this problem,70 it did recommend including a constant or imputed baseline value (rather than ignoring such a value), which is in line with our approach. We assumed a baseline EQ-5D value for all patients of –0.402, equivalent to the unconscious heath state for the EQ-5D-3L. An alternative of assuming a zero value (the health state value for death) was explored in a sensitivity analysis.
Calculation of quality-adjusted life-years
The QALY profile for each patient to 6 months post OHCA was estimated based on utility measurements (EQ-5D index values) and their time points and date of death (if applicable). The area under the curve of utility measurements was used to calculate the number of QALYs accrued by each patient. QALYs were calculated assuming that each patient’s utility changes linearly between each of the time points (time of OHCA, hospital discharge and 3 and 6 months post OHCA). For patients who died during the trial, their utility was assumed to change linearly between the preceding time point and the time of death, and to take the value of zero from death onwards.
Missing data
Recommended techniques for handling missing data were used. 71 We first summarised the number of missing data for resource use and outcomes (EQ-5D scores) descriptively. Exploratory analyses were conducted to explore the possible mechanisms and patterns of missing data. 72 Logistic regressions were used to explore associations between missingness and baseline variables, and missingness and previously observed outcomes. If the number of missing data was small (< 1% of cases), then unconditional or conditional mean imputation would be sufficient. However, we anticipated that it would be necessary to use multiple imputation to impute missing values. Multiple imputation is a flexible approach that is valid if data are assumed to be missing at random (the probability that data are missing does not depend on the unobserved values, conditional on the observed data). 72 This assumption was assessed.
Multiple imputation uses regression to predict m values for each missing data cell, and it enables all key variables used in the economic evaluation and demographic data (both complete and incomplete) to be used to predict the values of missing data cells. In accordance with guidelines,72,73 multiple imputation using chained equations was conducted, and the number of imputations set to be at least equal to the percentage of incomplete cases. 73 Multiple imputation was performed separately for each treatment group. Although the data are multilevel in nature (patients at level 1 enrolled by paramedics at level 2), it was not possible to perform multiple multilevel imputation, since the number of patients per cluster (paramedic) was too small. 74–76
Multiple imputation can be conducted, for example, at an aggregated level of total costs, or at a disaggregated level of individual resource use items or EQ-5D domains. Given that imputing large numbers of variables may make the model difficult to estimate, a balance between the two is likely to be required. The patterns of missing data for resource use/costs and outcomes were used to determine the approach to multiple imputation. For example, data collected using a patient follow-up questionnaire may have similar patterns of missing data, in which case the total costs for that follow-up can be imputed, rather than individual resource use items. For each variable with missing data, individual regressions were specified and tailored to the type of data being predicted. Linear regression with prediction mean matching was used because it is particularly flexible.
Once multiple imputation had been conducted, tabulations and summaries of the observed and imputed data were compared to check the validity of the imputations. Rubin’s rule was then used to summarise data across the m data sets. 77 This approach accounts for the variability both within and between imputed data sets and takes uncertainty in the estimated mean into account.
Within-trial statistical analysis of cost-effectiveness results
Analyses were conducted in Stata and Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Initially, descriptive summaries of resource use, costs and HRQoL were performed using means, SDs and SEs around the means using both the central limit theorem and bootstrapping. Cost data are typically positively skewed, but, regardless of this, costs were summarised using the arithmetic mean, because it is this, combined with the total number of patients, that relates to the total budget impact of an intervention.
Given that the AIRWAYS-2 trial is a cluster randomised trial, statistical methods for combining costs and outcomes needed to take account of the correlation between costs and outcomes at both the individual and the cluster level. 78 We used multilevel liner regression modelling to take account of the clusters, since this flexible framework can also accommodate missing data and cost skewness. 79
The ICER was derived from the average costs and QALYs gained in each treatment group, producing an incremental cost per QALY gained of the i-gel compared with TI. Non-parametric bootstrapping of costs and QALYs was used to quantify the degree of uncertainty around the ICER. Results are expressed in terms of a cost-effectiveness acceptability curve (CEAC), which indicates the likelihood that the i-gel is cost-effective for different levels of willingness to pay for health gain. Although the i-gel is considered cost-effective if the ICER falls below £20,000, the ICERs and CEACs presented allow decision-makers to assess cost-effectiveness at a willingness-to-pay threshold of their choice.
The ICER is a ratio of incremental costs (the difference in mean costs between the i-gel and TI groups) divided by incremental QALYs. A negative ICER can be the result of positive incremental costs and negative incremental QALYs, or negative incremental costs and positive incremental QALYs, but has a different meaning depending on the direction of these differences. Uncertainty around the ICER becomes infinite if there is a small difference in QALYs and the denominator of the ICER encompasses zero. If either of these scenarios arise, it is helpful to rearrange the cost-effectiveness decision rule to consider incremental net monetary benefit (NMB) instead, and make comparisons without using ratios.
For a given willingness-to-pay threshold for an additional QALY, incremental NMB is the value of incremental effects on a monetary scale, incremental QALYs multiplied by threshold, minus incremental costs. If incremental NMB is positive for any given threshold, then the i-gel is considered cost-effective compared with TI at that threshold. Because NMB tends to be normally distributed, we can calculate a 95% CI around it; this will be done using the non-parametric bootstrap replicates of costs and QALYs.
Discounting
Costs and effects were not discounted as our time horizon was < 12 months.
Sensitivity analysis
Univariate sensitivity analyses were used to investigate the impact on costs and cost-effectiveness results of variation in key parameters and major cost drivers, and to investigate the impact of uncertainty on the cost-effectiveness results.
Factors examined in the sensitivity analyses for costing were varying the unit costs for paramedics, ED attendance, intensive care stay and inpatient care. The impact of any high-cost patients was also investigated.
Factors examined in the sensitivity analyses for health outcomes were varying the assumed baseline QoL (assuming a baseline utility of 0 rather than –0.402) and considering life-years as an alternative outcome to QALYs.
For details of all sensitivity analyses, see Appendix 7.
Subgroup analysis
No subgroup analyses were pre-planned for the cost-effectiveness analyses.
Chapter 3 Results: trial cohort
Paramedic recruitment and patient enrolment
A total of 2041 paramedics from the four participating NHS ambulance trusts expressed an interest in participating in the trial (ambulance trust 1, n = 697; ambulance trust 2, n = 456; ambulance trust 3, n = 458; ambulance trust 4, n = 430). Altogether, 1523 paramedics were recruited and randomised (764 randomised to TI and 759 randomised to i-gel). The first paramedic randomisation occurred in March 2015.
Of the 1523 randomised paramedics, 28 did not attend any eligible patients and 113 withdrew post randomisation (58 randomised to TI and 55 randomised to i-gel). A total of 98 paramedics out of the 113 who withdrew post randomisation had attended eligible patients before withdrawal and these patients were retained and analysed. The most prevalent reason for withdrawal post randomisation was that the paramedic had left the ambulance trust or service or changed role (70 paramedics). The most common reason for withdrawal between paramedics expressing interest and randomisation of the paramedic was that the paramedic did not book onto training (464 paramedics). The other reasons for withdrawals pre and post randomisation and the number of patients attended by paramedics who withdrew post randomisation are detailed in Figure 3. For details of paramedic recruitment split by ambulance trust, see Appendix 1, Table 27. A total of 1382 paramedic clusters were included in the analysis of the primary outcome.
FIGURE 3.
Flow of paramedics and patients: CONSORT flow diagram. a, A total of 113 paramedics withdrew after randomisation (TI, n = 58; i-gel, n = 55). Reasons given for paramedic withdrawals: 70 (TI, n = 36; i-gel, n = 34) had left the ambulance trust or service or changed role; 13 (TI, n = 7; i-gel, n = 6) did not want to follow algorithm; four (TI, n = 2; i-gel, n = 2) repeatedly failed to follow protocol; six (TI, n = 6; i-gel, n = 0) owing to pregnancy/maternity; three (TI, n = 1; i-gel, n = 2) expressed that there was not enough time; two (TI, n = 0; i-gel, n = 2) were dead; seven (TI, n = 2; i-gel, n = 5) gave no reason; and eight (TI, n = 4; i-gel, n = 4) provided other reasons. Out of these 113 paramedics, 98 (TI, n = 49; i-gel, n = 49) attended an OHCA prior to withdrawal and 83 enrolled at least one eligible patient (TI, n = 40; i-gel, n = 43). These trial patients were retained and analysed. The median number of patients with OHCA attended per withdrawn paramedic for TI was seven (IQR 3–12, range 1–54) and for i-gel was six (IQR 4–11, range 1–31). The median number of trial patients attended per withdrawn paramedic for TI was three (IQR 1.0–5.5, range 1–10) and for i-gel was two (IQR 1–4, range 1–12). b, Patients can have more than one reason for resuscitation not attempted and more than one reason for ineligibility. c, A total of three patients (TI, n = 3; i-gel, n = 0) were admitted to a non-participating hospital and four patients (TI, n = 0; i-gel, n = 4) admitted to a participating hospital could not be identified. d, One patient had consented to active follow-up but died prior to hospital discharge and did not have any data for the longitudinal secondary outcomes. Grouped according to the allocation of the first trial paramedic on scene. Reproduced with permission from JAMA 2018;320(8):779–91. 27 Copyright © 2018 American Medical Association. All rights reserved.
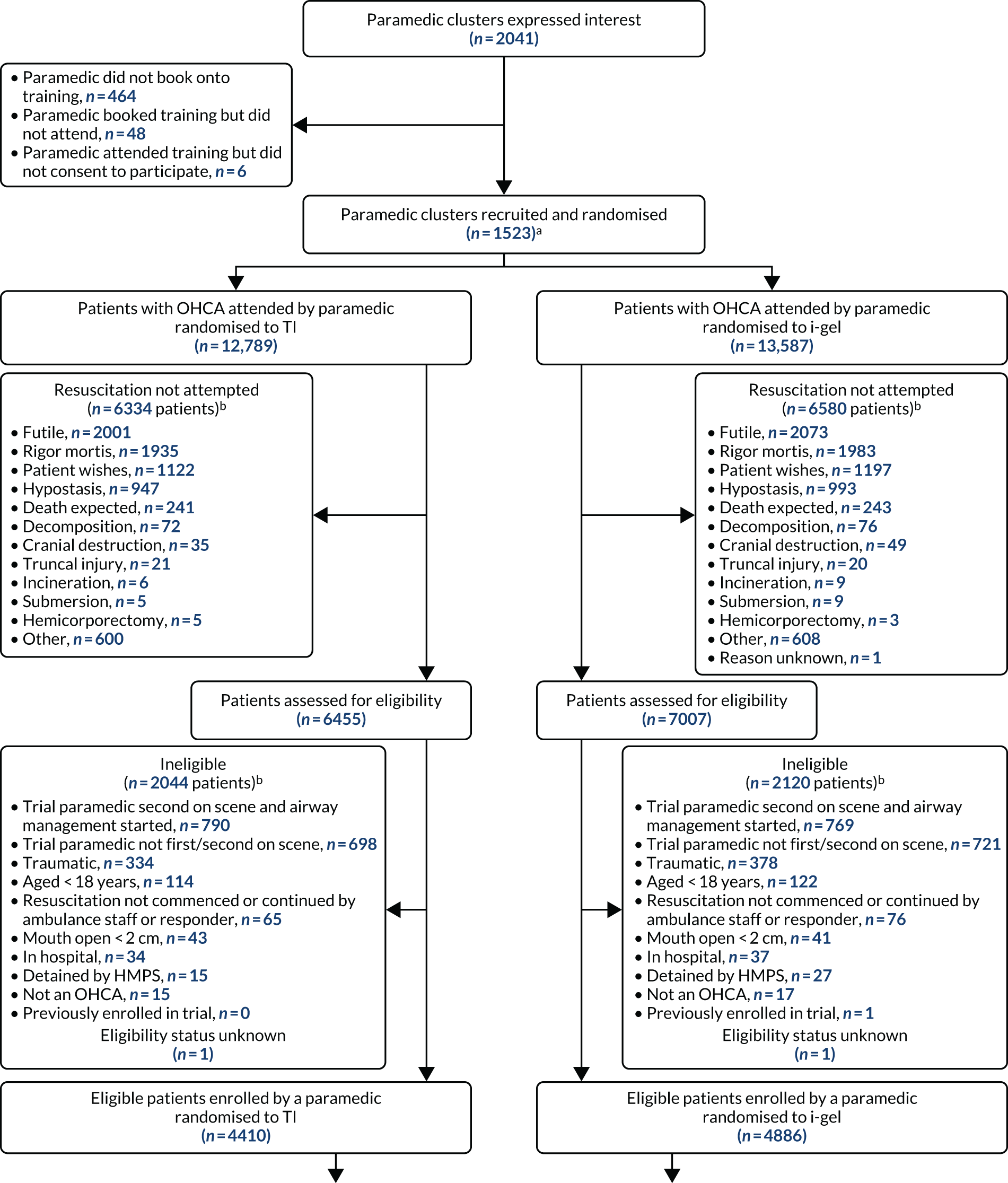
During the trial, 73,893 OHCAs were attended, during which 29,733 (40.2%) patients received resuscitation attempts. Among these patients receiving resuscitation attempts, an AIRWAYS-2 paramedic was first or second to the side of 13,462 (45.3%) of them. A total of 4164 patients were found to be ineligible and two patients had an unknown eligibility status (Figure 4 provides more details). Thus, 9296 eligible patients were attended by 1382 trial paramedics. However, seven patients did not have primary outcome data (four because of an inability to identify the patient in hospital records and three because the patient was admitted to a non-participating hospital). Therefore, only 9289 were included in the analysis of the primary outcome.
FIGURE 4.
Flow of patients. a, Four patients could not be identified and three patients were admitted to hospitals not participating in the AIRWAYS-2 trial. b, This includes patients who bypassed ICU to go straight to a ward (n = 78: TI, n = 35; i-gel, n = 43). c, Additionally, there were four patients in the i-gel group who consented to active follow-up but died prior to hospital discharge; one died prior to providing follow-up, two provided 30 days’ follow-up data at and one provided 3 months’ follow-up data.
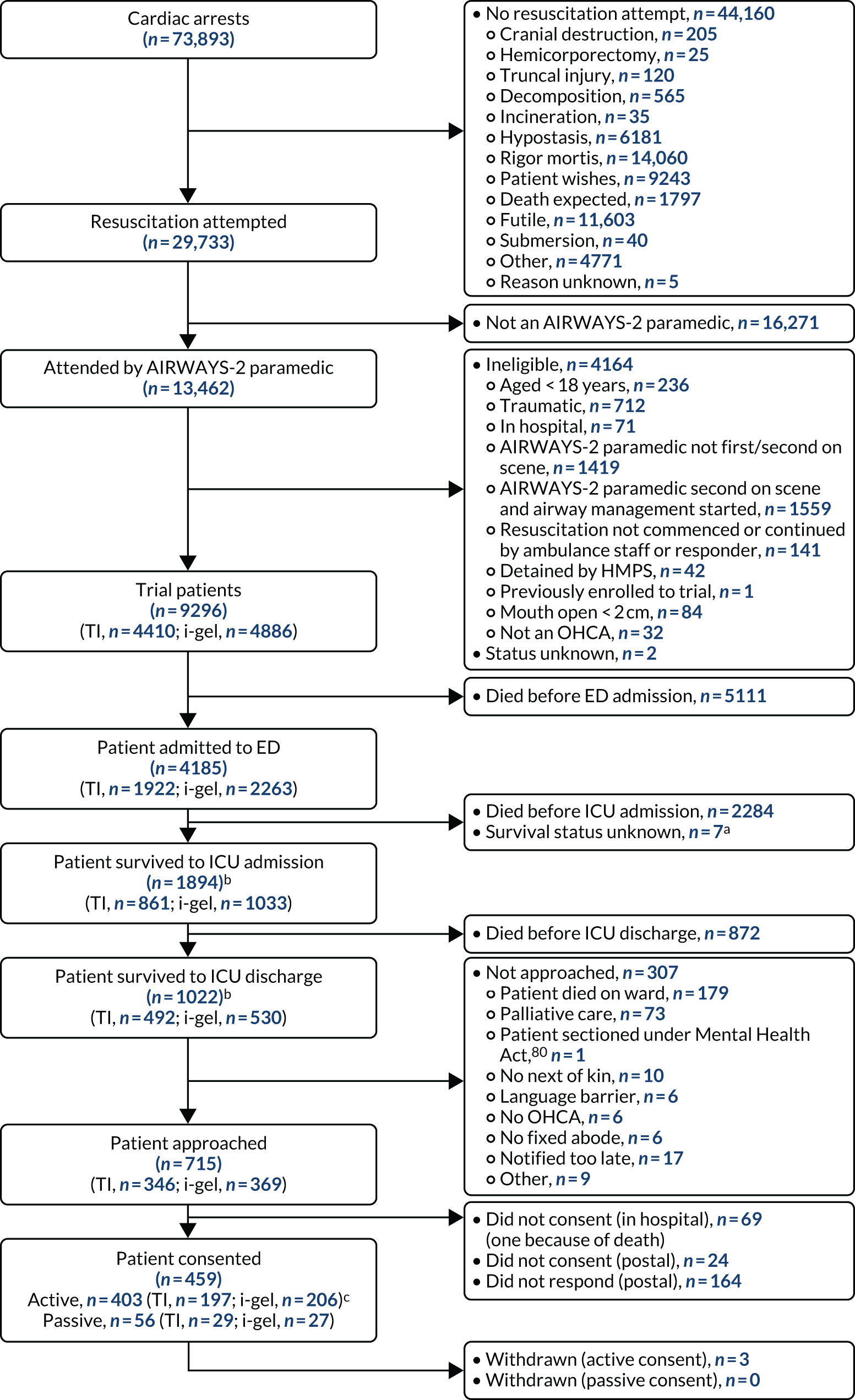
In terms of airway management, 7580 patients received AAM, of whom 2840 received TI first, 4632 received i-gel first and 108 received a non-i-gel SGA first. Figures 3 and 4 provide more details relating to the patients, including reasons for no resuscitation attempt, ineligibility and flow of survival. For patient enrolment details split by ambulance trust, see Appendix 1, Table 28.
Among the 768 patients who survived to 30 days/hospital discharge, 362 (47.1%) were approached by post only, 333 (43.4%) were approached in hospital only, 13 (1.7%) were approached both in hospital and by post and 60 (7.8%) were not approached. With regard to consent, 403 out of 768 (52.5%) patients who survived to 30 days/hospital discharge gave active consent [TI, n = 197/373 (52.8%); i-gel, n = 206/395 (52.2%)]. Among those who were approached by post only, 160 (44.2%) consented to active follow-up. Among those who were approached in hospital only, 239 (71.8%) consented to active follow-up. Among those who were approached by post and in hospital, four (30.8%) consented to active follow-up.
Enrolment rate
Patients were attended between 1 June 2015 and 13 August 2017, with the last patient follow-up completed on 5 April 2018. The trial stopped enrolment of patients on 13 August 2017 because the target number of patients was reached. Based on our feasibility study, the trial was estimated to enrol 167 patients in the first month and 380 patients (95 from each ambulance trust) per month thereafter. These estimates were based on (1) 1300 paramedics participating and each paramedic enrolling seven patients over the 2-year period (i.e. 9100 patients enrolled over the 2-year period), (2) there being 4.3 weeks in a month and (3) ambulance trust staggered enrolment start dates. For a breakdown of enrolment per month by ambulance trust and overall, see Appendix 1, Table 29. Figure 5 displays the overall predicted and actual enrolment per month. For a breakdown by ambulance trust, see Appendix 1, Figure 22. Appendix 1, Table 29, shows that ambulance trust 2 consistently overenrolled, ambulance trusts 1 and 3 remained approximately on target and ambulance trust 4 consistently underenrolled. Appendix 1, Table 29, also shows that in the period between April and October, the total number of patients enrolled was consistently below the estimated total. In the other months of the year, the total number of patients enrolled was consistently above the estimated total.
FIGURE 5.
Predicted and actual enrolment by month. Based on a target enrolment of 95 patients per ambulance trust per month. Calculated based on the estimate of 1300 paramedics enrolling seven patients over the 2-year trial period.
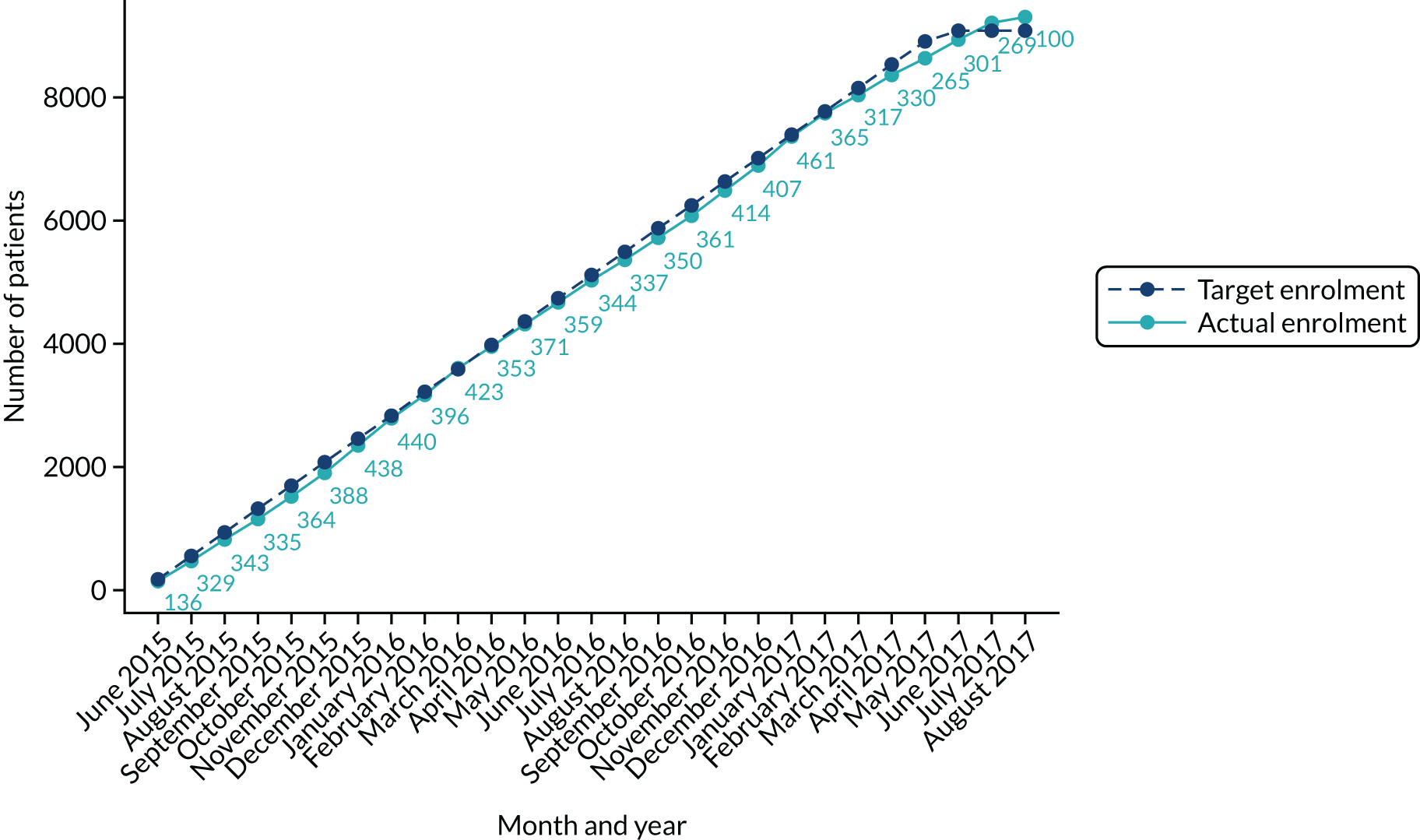
Enrolled patients
Patient demographics and cardiac arrest details for patients for whom (1) resuscitation was attempted and not attended by a trial paramedic, (2) resuscitation was attempted and attended by a trial paramedic and (3) resuscitation was attempted and attended by a trial paramedic and the trial eligibility criteria were met are shown in Table 4. There are no clear differences between these three groups in terms of their age, sex, time from 999 call to first crew arrival and whether or not the event was witnessed. There are slight differences between the groups in the percentage of patients with an asystole- or pulseless electrical activity (PEA)-presenting rhythm, along with the percentage of patients receiving bystander CPR and having their event witnessed by a bystander. However, no formal testing of these characteristics was undertaken.
| Details of all patients in whom resuscitation was attempted by enrolment status | Resuscitation attempted but not attended by a trial paramedic (i.e. excluded patients) (N = 16,271) | Resuscitation attempted and attended by a trial paramedic (N = 13,460)a | Resuscitation attempted and attended by a trial paramedic and patient eligible (i.e. included patients) (N = 9296) |
|---|---|---|---|
| Age (years), median (IQR)b | 72 (59–82) | 71 (57–81) | 73 (62–82) |
| Sex (male), n/N (%) | 10,368/16,266 (63.7) | 8763/13,458 (65.1) | 5923/9296 (63.7) |
| Time (minutes) from 999 call to first crew arrival, median (IQR)c | 8 (5–12) | 8 (5–11) | 8 (5–11) |
| Presenting rhythm, n/N (%) | |||
| Asystole | 8842/15,213 (58.1) | 7220/12,935 (55.8) | 4953/9107 (54.4) |
| VF | 3236/15,213 (21.3) | 2888/12,935 (22.3) | 2073/9107 (22.8) |
| Pulseless VT | 143/15,213 (0.9) | 114/12,935 (0.9) | 83/9107 (0.9) |
| PEA | 2992/15,213 (19.7) | 2713/12,935 (21.0) | 1998/9107 (21.9) |
| Event witnessed, n/N (%) | 9433/16,218 (58.2) | 8168/13,442 (60.8) | 5889/9290 (63.4) |
| By bystander | 7557/9158 (82.5) | 6684/8121 (82.3) | 4724/5888 (80.2) |
| By EMS | 1601/9158 (17.5) | 1437/8121 (17.7) | 1164/5888 (19.8) |
| Bystander CPR, n/N (%) | 9756/16,214 (60.2) | 8625/13,444 (64.2) | 5923/9289 (63.8) |
Withdrawals
Patient and paramedic withdrawals from the trial are summarised in Table 5. In total, 113 paramedics withdrew post randomisation, of whom 98 attended an OHCA prior to withdrawal and 83 enrolled at least one eligible patient. These trial patients were retained and analysed. In total, three patients withdrew after consenting to active or passive follow-up; all three were in the i-gel group and had reported that they were willing for data already collected and data routinely collected about them by the NHS (post consent) to be retained and used in the trial. Two out of the three patients who withdrew had stated that they were unable to complete further questionnaires; the other patient did not provide a reason for withdrawal. One additional patient who had declined consent owing to being overwhelmed by the events had expressed that they were not willing for data already collected or data routinely collected about them by the NHS (post consent) to be used in the trial.
| Withdrawal details | TI group (N = 764) | i-gel group (N = 759) | Overall (N = 1523) |
|---|---|---|---|
| Paramedic withdrawal post randomisation, n (%) | 58 (7.6) | 55 (7.2) | 113 (7.4) |
| Decision taken by, n/N (%) | |||
| Trial team | 2/58 (3.4) | 2/55 (3.6) | 4/113 (3.5) |
| Paramedic | 56/58 (96.6) | 53/55 (96.4) | 109/113 (96.5) |
| Reason for withdrawal, n/N (%) | |||
| Left ambulance trust or service or changed role | 36/58 (62.1) | 34/55 (61.8) | 70/113 (61.9) |
| Did not want to follow algorithm | 7/58 (12.1) | 6/55 (10.9) | 13/113 (11.5) |
| Repeatedly failed to enrol eligible patients | 2/58 (3.4) | 2/55 (3.6) | 4/113 (3.5) |
| Pregnancy/maternity | 6/58 (10.3) | 0/55 (0.0) | 6/113 (5.3) |
| Not enough time | 1/58 (1.7) | 2/55 (3.6) | 3/113 (2.7) |
| Dead | 0/58 (0.0) | 2/55 (3.6) | 2/113 (1.8) |
| No reason given | 2/58 (3.4) | 5/55 (9.1) | 7/113 (6.2) |
| Other | 4/58 (6.9) | 4/55 (7.3) | 8/113 (7.1) |
Protocol deviations
The SAP listed three protocol deviations: (1) the wrong paramedic enrolled the patient (12 patients), (2) the enrolling paramedic did not perform any AAM but another paramedic did (393 patients) and (3) the enrolling paramedic performed an alternative intervention to their allocation on their first AAM attempt (465 patients). Table 6 details these protocol deviations by randomised group and overall. Two other types of deviations were also identified in the SAP: (1) the patient did not meet the trial eligibility criteria but was consciously enrolled in the trial by the attending trial paramedic (141 patients) and (2) the enrolling paramedic made only one attempt at their allocated intervention before swapping to an alternative advanced airway intervention (506 patients). The latter was not considered to be a true protocol deviation for clinical reasons.
| Protocol deviation details | TI group | i-gel group | Overall |
|---|---|---|---|
| All trial patients, n | 4410 | 4886 | 9296 |
| Wrong paramedic enrolled patient, n/N (%) | 4/4403 (0.1) | 8/4881 (0.2) | 12/9284 (0.1) |
| Resulted in randomised allocation crossover, n/N (%) | 3/4 (75.0) | 4/8 (50.0) | 7/12 (58.3) |
| Enrolling paramedic did not perform any AAM but another paramedic did, n/N (%) | 217/4405 (4.9) | 176/4883 (3.6) | 393/9288 (4.2) |
| Trial patients with at least one AAM attempt performed by enrolling paramedic, n | 3419 | 4161 | 7580 |
| Enrolling paramedic did not perform allocated intervention on first advanced airway attempt, n/N (%) | 316/3419 (9.2) | 149/4161 (3.6) | 465/7580 (6.1) |
Patient follow-up
Patients were approached after they were discharged from the ICU. In total, 1022 patients survived to ICU discharge. Among these, one (0.1%) patient had an unknown approach status, 306 (29.9%) patients were not approached, 340 (33.3%) patients were approached in hospital, 362 (35.4%) patients were approached by post and 13 (1.3%) patients were approached both in hospital and by post. The main reason for a patient not being approached was that the patient had died (246 patients). The mRS score at 30 days or hospital discharge was collected for all identified trial patients using CRFs, as well as patient notes for those who had not completed the relevant questionnaire. The mRS at 3 months and 6 months and the QoL outcomes at all three time points were completed by patients who had consented to active follow-up only. Altogether, 403 patients (TI, n = 197; i-gel, n = 206) consented to active follow-up, 56 patients (TI, n = 29; i-gel, n = 27) consented to passive follow-up, 20 patients (TI, n = 10; i-gel, n = 10) declined consent and 543 (TI, n = 256; i-gel, n = 287) did not respond. Overall, 269 (TI, n = 135; i-gel, n = 134), 261 (TI, n = 125; i-gel, n = 126) and 262 (TI, n = 133; i-gel, n = 129) patients provided data for all three time points for the mRS score, single summary index score and EQ-5D VAS score, respectively. For the numbers of patients with data for the follow-up outcomes by randomised group, see Appendix 1, Table 30.
Numbers analysed
In total, 9296 patients (TI, n = 4410; i-gel, n = 4886) were eligible and included in the trial population.
In relation to compression fraction, cards were issued to two of the four ambulance trusts for a subset of patients. A total of 1239 patients (TI, n = 608; i-gel, n = 631) were attended by paramedics with compression fraction cards. A total of 108 cards (TI, n = 49; i-gel, n = 59) were returned and, of these, 25 (TI, n = 8; i-gel, n = 17) were unreadable, 16 (TI, n = 8; i-gel, n = 8) contained no data and one (TI, n = 1; i-gel, n = 0) could not be used owing to a mismatch in dates.
With regard to the longer-term secondary outcomes (QoL outcomes at 30 days, 3 months and 6 months and mRS score at 3 months and 6 months), those who did not survive to hospital discharge were given values of 0 for the EQ-5D and death for the mRS for all time points if mRS score or EQ-5D score were unknown. Patients who consented to active or passive follow-up who were known to have died between hospital discharge and 6 months were also given a score of 0 for the EQ-5D and death for the mRS at the appropriate time point. For further details on the number of patients included in the primary and secondary outcome analyses, see Appendix 1, Table 31.
Baseline data
The summaries of the baseline demographics and cardiac arrest details are shown in Table 7. For summaries of the intervention details not including the secondary outcomes, see Appendix 1, Table 32. These tables are split by randomised treatment group. The age and sex of patients were similar in both groups. A slightly lower median time from 999 call to first crew arrival was observed in the i-gel group than in the TI group. However, the IQRs were the same between the two treatment groups. All other baseline demography and cardiac arrest details are similar in the two treatment groups.
| Baseline details of patients | TI group (N = 4410) | i-gel group (N = 4886) | Overall (N = 9296) |
|---|---|---|---|
| Demography | |||
| Sex (male), n/N (%) | 2791/4410 (63.3) | 3132/4886 (64.1) | 5923/9296 (63.7) |
| Age (years), median (IQR) | 74 (62–83) | 73 (61–82) | 73 (62–82) |
| Initial cardiac arrest details, median (IQR) | |||
| Time (minutes) from 999 call to first crew arrival | 8 (5–11) | 7 (5–11) | 8 (5–11) |
| Time from first crew arrival to trial paramedic arrival (minutes)a | 0 (0–4) | 1 (0–4) | 0 (0–4) |
| Presenting rhythm, n/N (%) | |||
| Asystole | 2356/4316 (54.6) | 2597/4791 (54.2) | 4953/9107 (54.4) |
| VF | 979/4316 (22.7) | 1094/4791 (22.8) | 2073/9107 (22.8) |
| Pulseless VT | 44/4316 (1.0) | 39/4791 (0.8) | 83/9107 (0.9) |
| PEA | 937/4316 (21.7) | 1061/4791 (22.1) | 1998/9107 (21.9) |
| OHCA witnessed, n/N (%) | 2788/4407 (63.3) | 3101/4883 (63.5) | 5889/9290 (63.4) |
| By bystander | 2231/2788 (80.0) | 2493/3100 (80.4) | 4724/5888 (80.2) |
| By EMS | 557/2788 (20.0) | 607/3100 (19.6) | 1164/5888 (19.8) |
| Bystander/responder CPR before response vehicle arrival, n/N (%) | 2774/4406 (63.0) | 3149/4883 (64.5) | 5923/9289 (63.8) |
| Bystander/responder defibrillation before response vehicle arrival,b n/N (%) | 146/4390 (3.3) | 176/4863 (3.6) | 322/9253 (3.5) |
| If yes, ROSC achieved | 20/146 (13.7) | 27/176 (15.3) | 47/322 (14.6) |
| On arrival of trial paramedic, n/N (%) | |||
| Airway management in progress | 1384/4389 (31.5) | 1463/4863 (30.1) | 2847/9252 (30.8) |
| BVM only | 273/1383 (19.7) | 307/1463 (21.0) | 580/2846 (20.4) |
| OPA and BVM | 766/1383 (55.4) | 875/1463 (59.8) | 1641/2846 (57.7) |
| NPA and BVM | 11/1383 (0.8) | 11/1463 (0.8) | 22/2846 (0.8) |
| i-gel | 262/1383 (18.9) | 190/1463 (13.0) | 452/2846 (15.9) |
| Intubation | 3/1383 (0.2) | 3/1463 (0.2) | 6/2846 (0.2) |
| Other SGA | 44/1383 (3.2) | 57/1463 (3.9) | 101/2846 (3.5) |
| Mouth to mouth | 8/1383 (0.6) | 10/1463 (0.7) | 18/2846 (0.6) |
| Face shield/pocket mask | 5/1383 (0.4) | 4/1463 (0.3) | 9/2846 (0.3) |
| Suction | 3/1383 (0.2) | 2/1463 (0.1) | 5/2846 (0.2) |
| Other | 8/1383 (0.6) | 4/1463 (0.3) | 12/2846 (0.4) |
| Successful ventilations ongoing | 1110/1372 (80.9) | 1154/1455 (79.3) | 2264/2827 (80.1) |
| Patient had ROSC on arrival | 300/4393 (6.8) | 328/4862 (6.8) | 628/9255 (6.8) |
There was a slight difference between the two treatment groups in the proportions of patients who received bystander/responder CPR before response vehicle arrival. In the TI group, 63.0% of patients received a bystander/responder CPR, whereas in the i-gel group 64.5% of patients received this. Slight differences were present in terms of the type of airway management device in place on paramedic arrival. There were no clear differences between the two treatment groups in terms of the other patient and event details. However, no formal testing of these differences has taken place.
With regard to the intervention details, a slightly smaller proportion of trial paramedics in the TI group reported at least one AAM attempt and a slightly smaller proportion of patients in the TI group received at least one AAM attempt than in the i-gel group. Similar percentages of patients were reported to have received mechanical CPR during resuscitation in the two treatment groups.
Chapter 4 Results: primary outcome and pre-hospital discharge secondary outcomes
Primary outcome
Primary analysis
A similar proportion of patients in each treatment group had a favourable functional outcome (mRS score) at 30 days/hospital discharge (TI, 6.8%; i-gel, 6.4%).
The results of the logistic regression model showed that the odds of a favourable functional recovery were higher in the TI group than in the i-gel group [OR 0.92 (95% CI 0.77 to 1.09; p = 0.33), ADP experiencing a good outcome –0.6% (95% CI –1.6% to 0.4%; p = 0.24), RR 0.92 (95% CI 0.79 to 1.08; p = 0.32)]. Table 8 and Figure 6 contain the details of the primary outcome.
| Primary outcome details | TI group (N = 4410) | i-gel group (N = 4886) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| mRS score,a n/N (%) | 300/4407 (6.8) | 311/4882 (6.4) | 0.92 (0.77 to 1.09) | 0.33 | 0.05 | –0.6 (–1.6 to 0.4) | 0.24 |
| 0 (no symptoms) | 124/4407 (2.8) | 117/4882 (2.4) | |||||
| 1 | 48/4407 (1.1) | 41/4882 (0.8) | |||||
| 2 | 50/4407 (1.1) | 58/4882 (1.2) | |||||
| 3 | 78/4407 (1.8) | 95/4882 (1.9) | |||||
| 4 | 46/4407 (1.0) | 45/4882 (0.9) | |||||
| 5 | 27/4407 (0.6) | 39/4882 (0.8) | |||||
| 6 (dead) | 4034/4407 (91.5) | 4487/4882 (91.9) | |||||
| Time (days) from OHCA to mRS assessment, median (IQR)b | 25 (10–68) | 28 (10–74) |
FIGURE 6.
Primary outcome analysis results. Reproduced with permission from JAMA 2018;320(8):779–91. 27 Copyright © 2018 American Medical Association. All rights reserved.
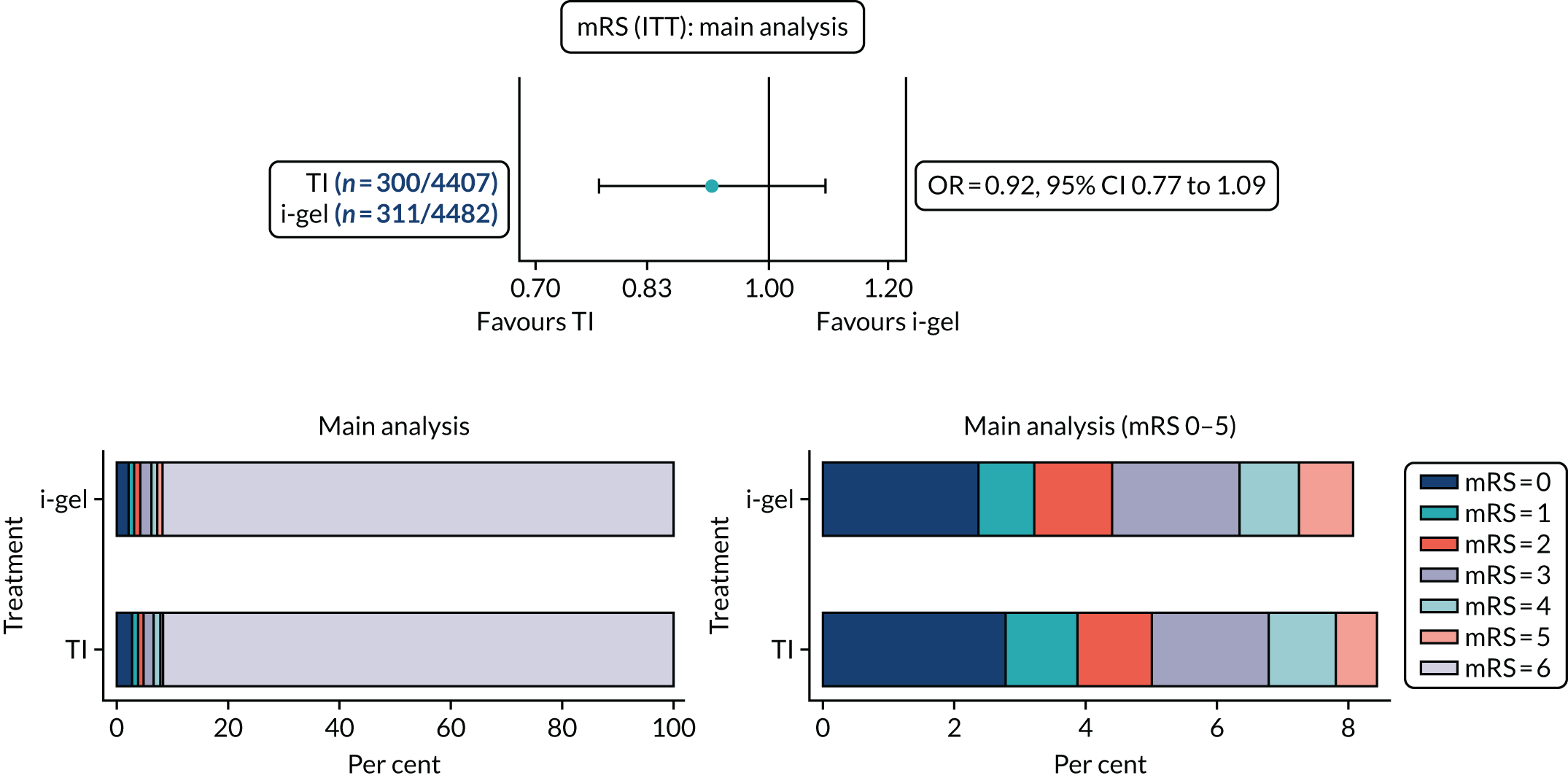
Figure 7 and Appendix 1, Figure 23, contain information about ROSC on arrival, AAM and ROSC during/after AAM, and the breakdown of mRS scores in each of these groups. The median time from OHCA to mRS assessment was slightly lower in the TI group (25 days) than in the i-gel group (28 days). Crossover was more common among patients randomised to TI than among those randomised to i-gel (see Table 6, Figure 7 and Appendix 1, Figure 23).
FIGURE 7.
Interventions received and patient outcome by trial allocation. a, A total of 72 patients in the TI group (2.1%) received a non-trial SGA (i.e. not the i-gel) only, all of whom had an mRS score of 4–6 (71 deaths). Among these 72 patients, two had ROSC on arrival. b, A total of 36 patients (0.9%) in the i-gel group received a non-trial SGA (i.e. not the i-gel) only, all of whom had an mRS score of 4–6 (35 deaths). Among these 36 patients, none had ROSC on arrival. Note: there were 41 patients (TI, n = 17; i-gel, n = 24) with missing ROSC on arrival of trial EMS clinician. Among these 41 patients, 39 (TI, n/N = 15/17; i-gel, n/N = 24/24) had an mRS score of 4–6 (37 deaths: TI, n/N = 14/15; i-gel, n/N = 23/24).
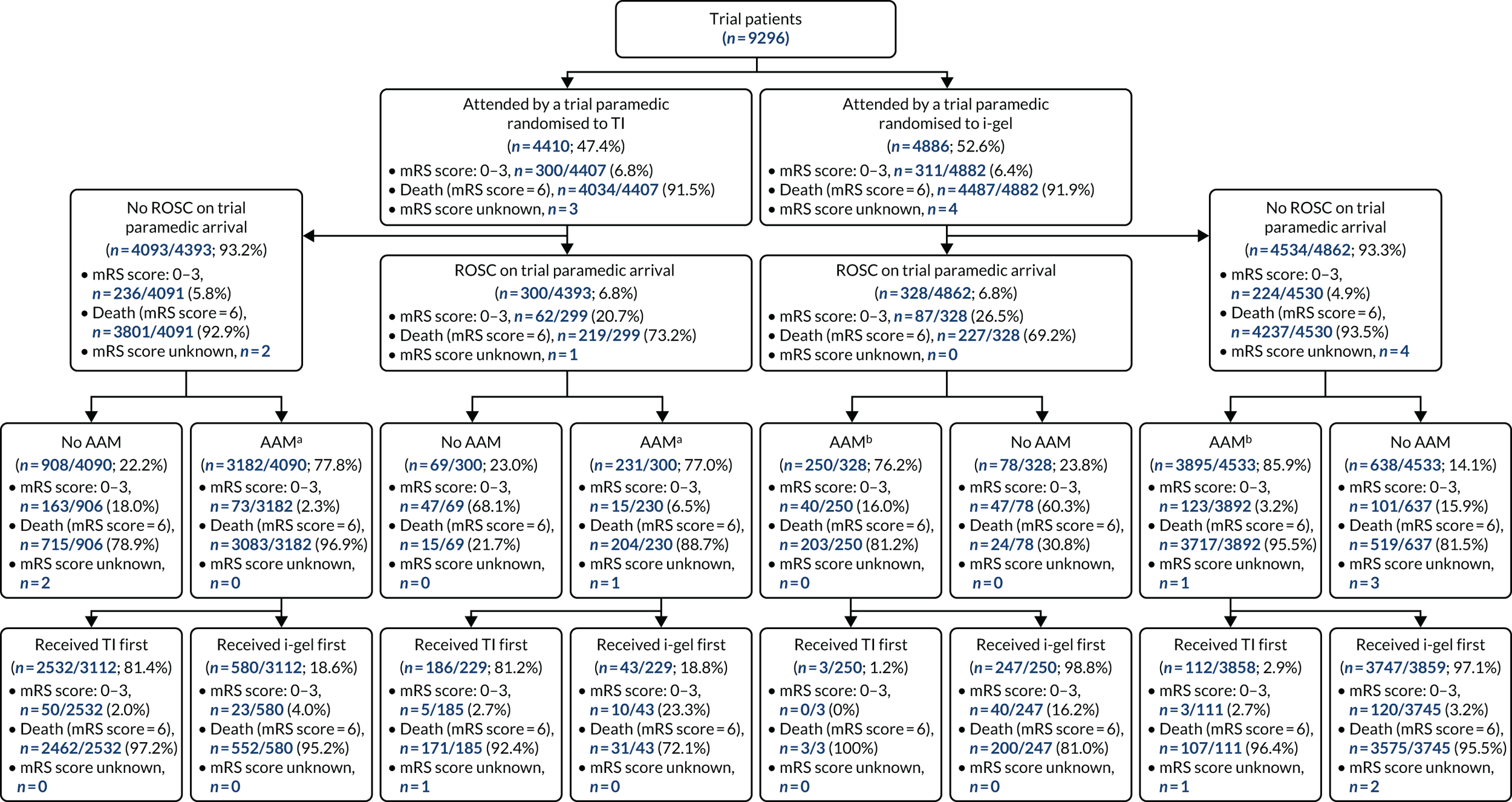
Sensitivity analyses
The first analysis was fitted on trial patients plus patients who were attended by a trial paramedic but not resuscitated, and this analysis was performed on an ITT basis. There were higher odds of a favourable functional recovery in the TI group than in the i-gel group [TI, 2.8%; i-gel, 2.7%; OR 0.96 (95% CI 0.81 to 1.14; p = 0.63), ADP –0.2% (95% CI –0.6% to 0.3%; p = 0.45), RR 0.96 (95% CI 0.81 to 1.13; p = 0.64)] (Figure 8; see also Appendix 1, Table 35).
FIGURE 8.
Sensitivity analyses of the primary outcome. a, Complete-case analysis of the primary outcome. Data missing for seven patients (TI, n = 3; i-gel, n = 4). b, Trial patients plus patients who were attended by a trial paramedic but not resuscitated. Data missing for seven patients (TI, n = 3; i-gel, n = 4). c, Trial patients who received at least one AAM attempt. Data missing for four patients (TI, n = 1; i-gel, n = 3). d, Trial patients who received at least one AAM attempt. Data missing for four patients (TI, n = 2; i-gel, n = 2).
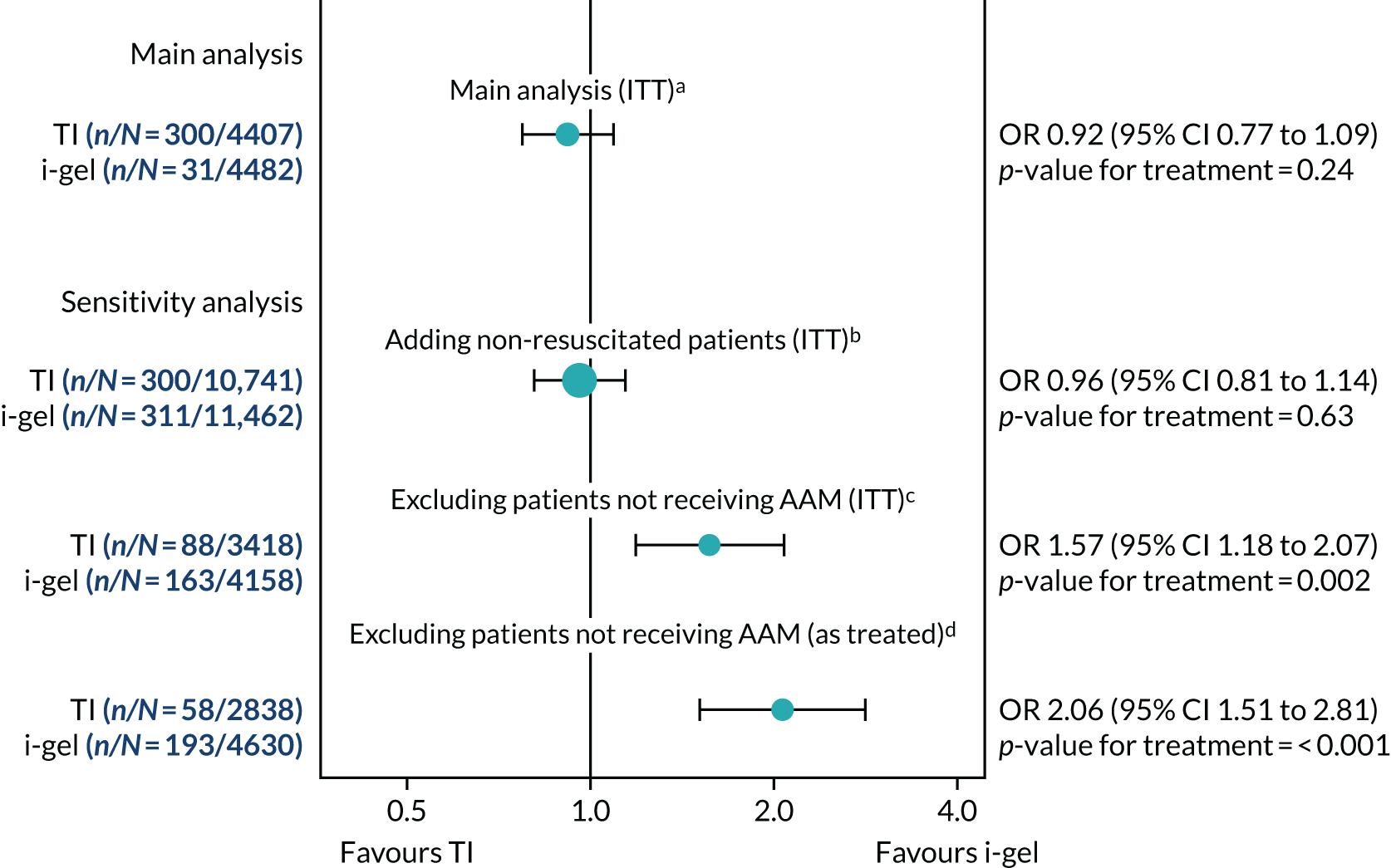
The second sensitivity analysis consisted of analysing trial patients who received at least one AAM attempt on an ITT basis. There were no clear differences (not formally tested) in the baseline characteristics of patients in the trial population, the population that received AAM and the population that did not receive AAM (see Appendix 1, Table 33). A larger proportion of patients had a good functional recovery in the i-gel group (3.9%) than in the TI group (2.6%) [OR 1.57 (95% CI 1.18 to 2.07; p = 0.002), ADP 1.4% (95% CI 0.5% to 2.2%; p = 0.001), RR 1.54 (95% CI 1.18 to 2.01; p = 0.002)] (see Figure 8 and Appendix 1, Table 35).
For the third sensitivity analysis, trial patients who received at least one AAM attempt were compared according to the treatment they had received first. No clear differences (not formally tested) in the baseline characteristics between these two groups were observed (see Appendix 1, Table 34). A larger proportion of patients with a good functional recovery was observed in the i-gel-first group: 2.0% in the TI-first group and 4.2% in the i-gel-first group [OR 2.06 (95% CI 1.51 to 2.81; p < 0.001), ADP 2.1% (95% CI 1.2% to 2.9%; p < 0.001), RR 2.00 (95% CI 1.48 to 2.70; p < 0.001)] (see Figure 8 and Appendix 1, Table 35).
Additional analysis
There was a differential adherence proportion in the two randomised treatment groups: 61.9% of patients in the TI group received TI as their first AAM whereas 82.1% of patients in the i-gel group received i-gel as their first AAM. There were nine patients (TI, n = 6; i-gel, n = 3) in whom it was unknown whether or not any AAM had been performed, and there were 1707 patients (TI, n = 985; i-gel, n = 722) who received no AAM. A total of 7580 patients (TI, n = 3419; i-gel, n = 4161) had received an AAM, 108 (TI, n = 72; i-gel, n = 36) of whom received a non-trial SGA. There were seven patients (TI, n = 3; i-gel, n = 4) who could not be identified and, thus, were missing the primary outcome. Therefore, 9280 patients (TI, n = 4401; i-gel, n = 3879) were included in the instrumental variables analysis. Baseline characteristics were similar between the four groups (split by randomised group and further split by adherence) (see Appendix 1, Table 36).
A causal ADP of –0.49% (95% CI –5.38% to 4.40%; p = 0.84) was obtained from this analysis (Figure 9). This result showed that a strategy of TI first had a better chance of a good functional recovery than a strategy of i-gel first. The CI is wider than that obtained in the primary analysis and includes differences more than the pre-specified clinically important difference of 2% in both directions. See Figure 9 for an illustration of the differences in the results of the ITT, as-treated and causal analyses.
FIGURE 9.
Intention-to-treat, as-treated and causal analyses of the primary outcome. a, Data missing for seven patients (TI, n = 3; i-gel, n = 4). b, Data missing for four patients (TI, n = 2; i-gel, n = 2). c, Data missing for seven patients (TI, n = 3; i-gel, n = 4).
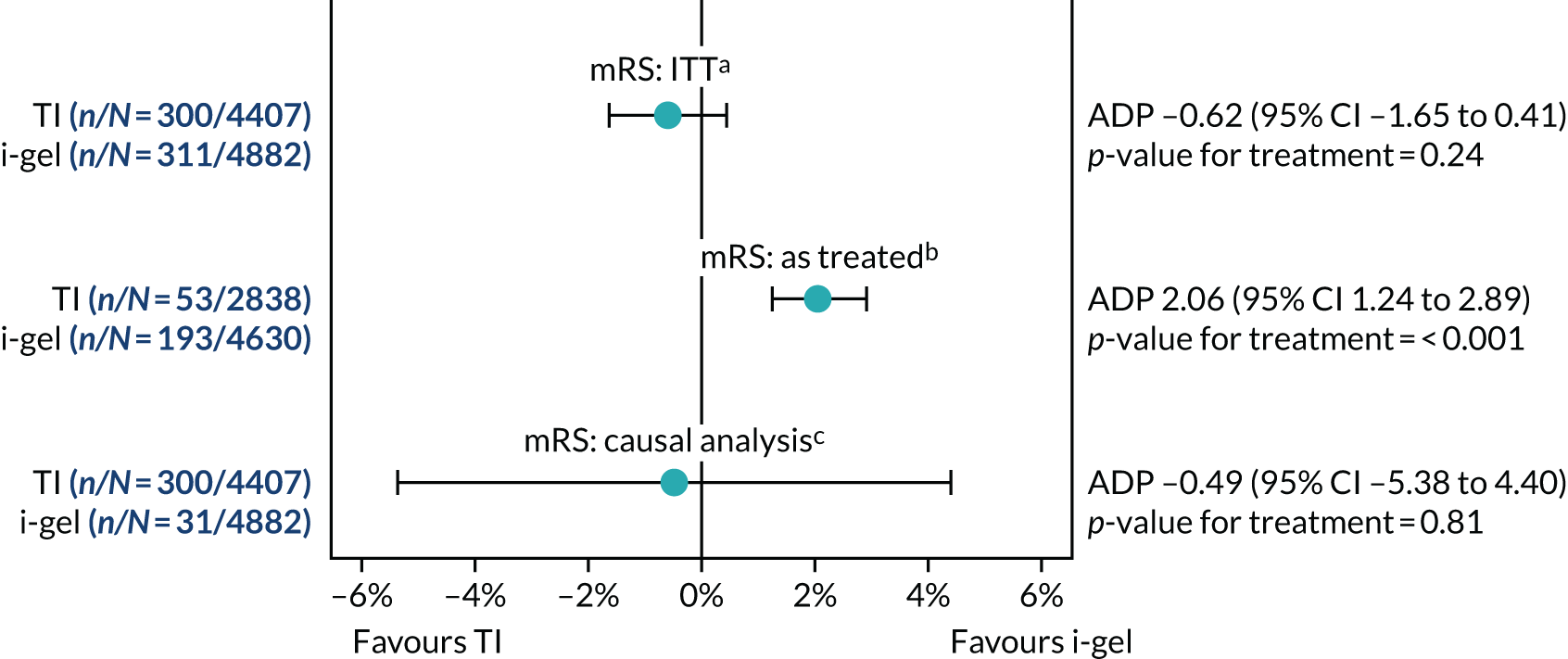
Subgroup analyses
The results of the two pre-specified subgroup analyses and one additional subgroup analysis explored at the request of the DMSC are presented in Figure 10. The first tested for any difference in the primary outcome between the two randomised groups for the Utstein comparator versus non-Utstein comparator groups. No significant interaction was found between these two subgroups (p = 0.07). The second subgroup analysis tested for any difference in the primary outcome between the OHCA witnessed by ambulance staff and OHCA not witnessed by ambulance staff groups. No significant interaction was found between these two subgroups (p = 0.37). The third analysis was performed on the as-treated groups and compared the subgroup of patients who had initial ventilation success with patients who had no initial ventilation success. A significant interaction between these two groups was found (p = 0.001).
FIGURE 10.
Subgroup analyses. Note that the Utstein comparator group includes patients with an OHCA with a likely cardiac cause that is witnessed and has an initial rhythm amenable to defibrillation. For the Utstein comparator vs. non-Utstein comparator analyses, there were missing data for 103 patients (TI, n = 52; i-gel, n = 51). The not witnessed by ambulance staff group includes all cardiac arrests not witnessed by a trial paramedic. For the witnessed vs. not witnessed analyses, there were missing data for seven patients (TI, n = 3; i-gel, n = 4). For the initial ventilation success vs. no initial ventilation success analyses, there were missing data for 26 patients (TI, n = 3; i-gel, n = 23).
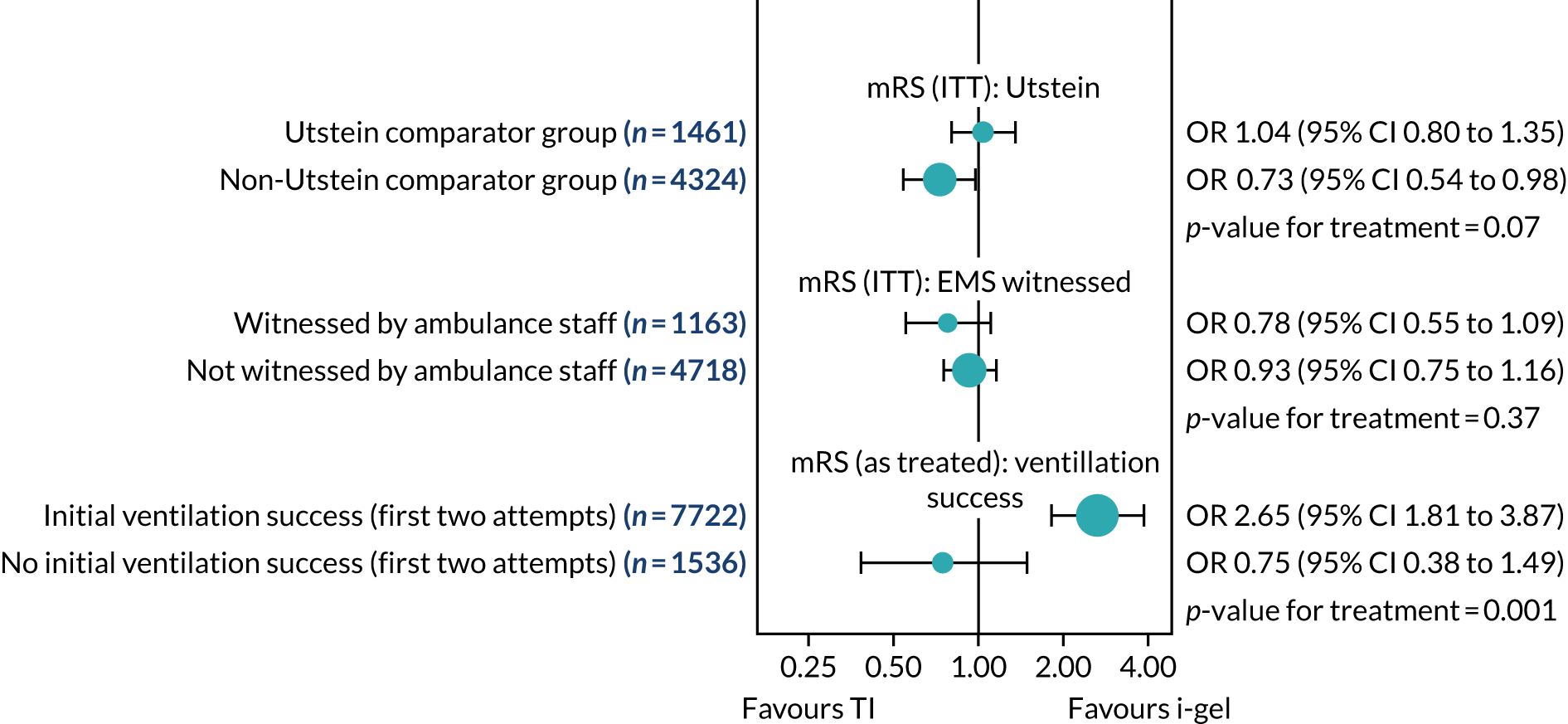
Initial ventilation success
The proportion of patients with initial ventilation success was larger in the i-gel group than in the TI group [TI, 79.0%; i-gel, 87.4%; OR 1.92 (95% CI 1.66 to 2.22; p < 0.001), ADP 8.3% (95% CI 6.3% to 10.2%; p < 0.001), RR 1.11 (95% CI 1.08 to 1.13; p < 0.001)] (Table 9). The proportion of any ventilation success with AAM was smaller in the TI group (92.8%) than in the i-gel group (95.1%). This was not formally tested.
| Initial ventilation success details | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Initial ventilation success (up to two attempts at AAM) | 3473/4397 (79.0) | 4255/4868 (87.4) | 1.92 (1.66 to 2.22) | < 0.001 | 0.12 | 8.3 (6.3 to 10.2) | < 0.001 |
| TI | 1891/2723 (69.4) | 92/116 (79.3) | |||||
| i-gel | 542/617 (87.8) | 3412/3994 (85.4) | |||||
| Other SGA | 55/72 (76.4) | 29/36 (80.6) | |||||
| Any ventilation success with AAM | 4086/4401 (92.8) | 4634/4874 (95.1) | |||||
| TI | 2163/3050 (70.9) | 573/753 (76.1) | |||||
| i-gel | 990/1112 (89.0) | 3465/4011 (86.4) | |||||
| Other SGA | 171/216 (79.2) | 33/44 (75.0) |
Regurgitation and aspiration
The overall regurgitation and aspiration rates, as well as the breakdown for ‘before initial AAM attempt’ and ‘during/after initial AAM attempt’, are shown in Table 10. The odds of patients regurgitating at any time was 1.08 times higher in the i-gel group than in the TI group [TI, 24.5%; i-gel, 26.1%; OR 1.08 (95% CI 0.96 to 1.20; p = 0.21), ADP 1.4% (95% CI –0.6% to 3.4%; p = 0.17), RR 1.06 (95% CI 0.98 to 1.15; p = 0.15)]. There were slightly higher odds of aspiration in the i-gel group than in the TI group [TI, 14.9%; i-gel, 15.1%; OR 1.01 (95% CI 0.88 to 1.16; p = 0.84), ADP 0.1% (95% CI –1.5% to 1.8%; p = 0.86), RR 1.01 (95% CI 0.90 to 1.13; p = 0.85)] (see Table 10).
| Regurgitation and aspiration details | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Regurgitation at any time | 1072/4372 (24.5) | 1268/4865 (26.1) | 1.08 (0.96 to 1.20) | 0.21 | 0.06 | 1.4 (–0.6 to 3.4) | 0.17 |
| Aspiration at any time | 647/4337 (14.9) | 729/4824 (15.1) | 1.01 (0.88 to 1.16) | 0.84 | 0.08 | 0.1 (–1.5 to 1.8) | 0.86 |
| Regurgitation before initial i-gel/TI attempt | 923/4379 (21.1) | 846/4869 (17.4) | |||||
| Aspiration before initial i-gel/TI attempt | 589/4355 (13.5) | 532/4840 (11.0) | |||||
| Regurgitation during or after initial i-gel/TI attempt | 543/4361 (12.5) | 875/4857 (18.0) | |||||
| Aspiration during or after initial i-gel/TI attempt | 304/4344 (7.0) | 473/4829 (9.8) |
The proportion of patients experiencing regurgitation and aspiration before an AAM attempt was larger in the TI group than in the i-gel group. The opposite was observed in terms of regurgitation and aspiration during/after the AAM attempt. These differences were not formally tested (see Table 10).
Loss of a previously established airway
The proportion of patients with loss of a previously established airway was larger in the i-gel group (10.6%) than in the TI group (5.0%) [OR 2.29 (95% CI 1.86 to 2.82; p < 0.001), ADP 5.9% (95% CI 4.6% to 7.2%; p < 0.001), RR 2.17 (95% CI 1.79 to 2.63; p < 0.001)] (Table 11).
| Any loss of a previously established airway details | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Any loss of a previously established airwaya | 153/3081 (5.0) | 412/3900 (10.6) | 2.29 (1.86 to 2.82) | < 0.001 | 0.07 | 5.9 (4.6 to 7.2) | < 0.001 |
| TI | 70/2149 (3.3) | 33/570 (5.8) | |||||
| i-gel | 84/981 (8.6) | 389/3455 (11.3) | |||||
| Other SGA | 5/171 (2.9) | 3/33 (9.1) |
Sequence of airway interventions delivered
Details of the sequence of airway interventions delivered for the first two AAM attempts are shown in Table 12. This secondary outcome was not formally compared. In the TI group, the two most common sequences of airway interventions delivered were (1) oropharyngeal airway (OPA) then TI (30.3%) and (2) TI (one attempt) (29.6%). In the i-gel group, the two most common sequences of airway interventions delivered were (1) i-gel (one attempt) (55.3%) and (2) OPA then i-gel (19.3%).
| Sequence of airway interventions delivered details | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) |
|---|---|---|
| Actual sequence of airway interventions delivered (first two management attempts) | ||
| OPA (one attempt) | 248/3686 (6.7) | 150/4321 (3.5) |
| OPA (two attempts) | 2/3686 (0.1) | 2/4321 (0.1) |
| OPA then NPA | 17/3686 (0.5) | 6/4321 (0.1) |
| OPA then i-gel | 145/3686 (3.9) | 833/4321 (19.3) |
| OPA then TI | 1115/3686 (30.3) | 35/4321 (0.8) |
| OPA then other SGA | 42/3686 (1.1) | 21/4321 (0.5) |
| NPA (one attempt) | 10/3686 (0.3) | 8/4321 (0.2) |
| NPA then OPA | 2/3686 (0.1) | 2/4321 (0.1) |
| NPA then i-gel | 4/3686 (0.1) | 19/4321 (0.4) |
| NPA then TI | 7/3686 (0.2) | 0/4321 (0.0) |
| NPA then other SGA | 1/3686 (0.0) | 0/4321 (0.0) |
| i-gel (one attempt) | 213/3686 (5.8) | 2388/4321 (55.3) |
| i-gel then OPA | 12/3686 (0.3) | 28/4321 (0.7) |
| i-gel then NPA | 0/3686 (0.0) | 4/4321 (0.1) |
| i-gel (two attempts) | 19/3686 (0.5) | 505/4321 (11.7) |
| i-gel then TI | 227/3686 (6.2) | 223/4321 (5.2) |
| i-gel then other SGA | 0/3686 (0.0) | 1/4321 (0.0) |
| TI (one attempt) | 1092/3686 (29.6) | 64/4321 (1.5) |
| TI then OPA | 7/3686 (0.2) | 0/4321 (0.0) |
| TI then NPA | 1/3686 (0.0) | 0/4321 (0.0) |
| TI then i-gel | 79/3686 (2.1) | 9/4321 (0.2) |
| TI (two attempts) | 392/3686 (10.6) | 8/4321 (0.2) |
| TI then other SGA | 24/3686 (0.7) | 0/4321 (0.0) |
| Other SGA (one attempt) | 20/3686 (0.5) | 14/4321 (0.3) |
| Other SGA then TI | 4/3686 (0.1) | 1/4321 (0.0) |
| Other SGA (two attempts) | 3/3686 (0.1) | 0/4321 (0.0) |
Chest compression fraction
Compression fraction cards were used in two ambulance trusts (SWAST and EMAS) for a subset of the patients. The start date for SWAST was 25 July 2017 and the start date for EMAS was 2 December 2016.
A total of 1239 (TI, n = 608; i-gel, n = 631) eligible patients were attended by trial paramedics with compression fraction cards. For these patients, 108 cards (TI, n = 49; i-gel, n = 59) were returned. Among these 108 returned cards, 25 (TI, n = 8; i-gel, n = 17) were unreadable, 16 (TI, n = 8; i-gel, n = 8) contained no data and one (TI) could not be used because of a mismatch in dates. Thus, this analysis was performed on only 66 observations in total. The median compression fraction was larger in the i-gel group than in the TI group [TI, 83%; i-gel, 86%; GMR 0.82 (95% CI 0.62 to 1.07; p = 0.14)] (see Appendix 1, Table 37).
Return of spontaneous circulation
The proportion of any ROSC during/after AAM by trial paramedic was larger in the i-gel group than in the TI group [TI, 29.0%; i-gel, 31.2%; OR 1.13 (95% CI 1.01 to 1.27; p = 0.03), ADP 2.5% (95% CI 0.1% to 4.8%; p = 0.04), RR 1.08 (95% CI 1.00 to 1.17; p = 0.04)].
The rate of ROSC on ED/hospital arrival was also higher in the i-gel group than in the TI group [TI, 28.4%; i-gel, 30.6%; OR 1.12 (95% CI 1.02 to 1.23; p = 0.02), ADP 2.2% (95% CI 0.3% to 4.2%; p = 0.03), RR 1.08 (95% CI 1.01 to 1.16; p = 0.02)]. There were larger proportions of patients with any ROSC during/after AAM by trial paramedic, patients admitted to ED/hospital, and patients surviving to ED discharge in the i-gel group than in the TI group. These differences were not formally compared (see Appendix 1, Table 38).
Advanced airway management in place when return of spontaneous circulation was achieved or the resuscitation discontinued
The secondary outcomes of airway management in place when ROSC was achieved or the resuscitation was discontinued were not formally compared (see Appendix 1, Table 39). The most common AAM in place when a patient first had ROSC, the most common final airway management in place in those who died at the scene and the most common final airway management in place in those who were admitted to ED was TI in the TI group and i-gel in the i-gel group. For these three outcomes, the proportion of patients receiving i-gel in the TI group was consistently larger than the proportion of patients receiving TI in the i-gel group.
Length of intensive care stay, hospital stay and time to death or last follow-up
For the results for time to death or last follow-up (up to 6 months) and time to death (up to 72 hours), 72-hour survival (as a proportion) and other survival details, see Appendix 1, Table 40. No clear differences were observed between the two treatment groups in terms of the survival status categories. The median duration of initial ICU stay among patients who survived to ICU discharge was longer in the i-gel group (100.5 hours) than in the TI group (96.6 hours). The median duration of hospital stay among patients who survived to hospital discharge was longer in the i-gel group (14.0 days) than in the TI group (12.3 days). These differences were not formally compared.
There was a higher hazard rate of death [time to death/last follow-up (up to 6 months)] for patients in the i-gel group than for patients in the TI group [TI, median 63 minutes (IQR 41–267 minutes); i-gel, median 67 minutes (IQR 41–216 minutes); HR 0.97 (95% CI 0.93 to 1.02; p = 0.22)]. Similarly, there was a higher hazard rate of death [time to death (up to 72 hours)] in the i-gel group than in the TI group [TI, median 63 minutes (IQR 41–246 minutes); i-gel, median 67 minutes (IQR 41–205 minutes); HR 0.96 (95% CI 0.92 to 1.00; p = 0.07)]. The odds of surviving to 72 hours were 1.04 times higher in the i-gel group than in the TI group [TI, 13.1%; i-gel, 13.6%; OR 1.04 (95% CI 0.92 to 1.18; p = 0.54); ADP 0.4% (95% CI –1.0% to 1.9%; p = 0.54); RR 1.04 (95% CI 0.93 to 1.15; p = 0.53)].
Adverse events
There were no unexpected SAEs.
Chapter 5 Results: longer-term secondary outcomes
The mRS at 3 months post OHCA and 6 months post OHCA and the QoL outcomes were completed by patients who had survived to these time points and consented to active follow-up only. Some patients were identified as eligible patients between the 3- and 6-month time points and were therefore missing 3-month follow-up data.
Modified Rankin Scale score at 3 and 6 months
Figure 11 presents the results of the complete-case, worst-case and imputed-case analyses of mRS score at 3 and 6 months.
FIGURE 11.
Longer-term mRS scores.
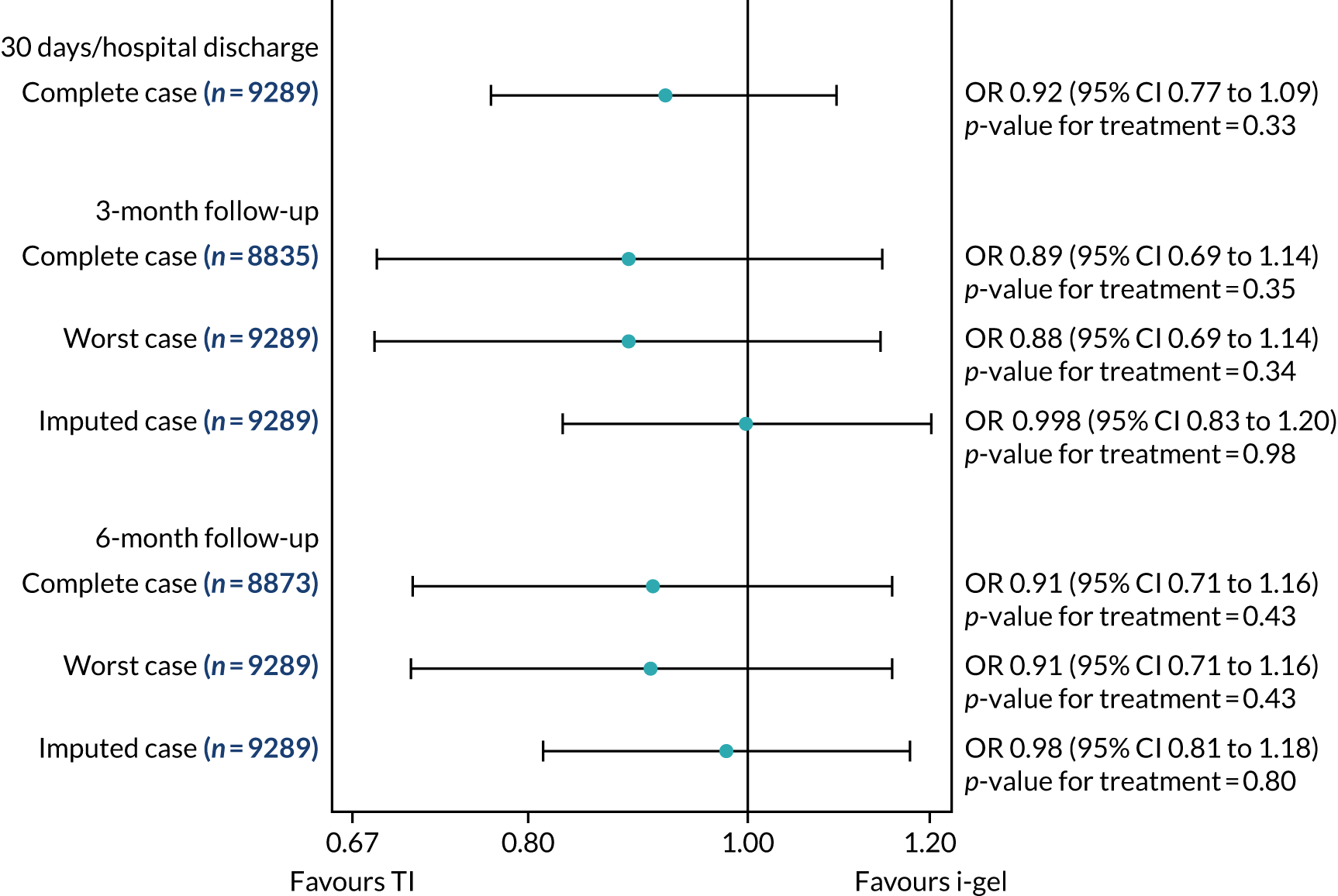
Complete-case analyses
Table 13 contains the details of mRS scores at 30 days/hospital discharge and longer-term mRS scores at 3- and 6-month follow-up (see also Appendix 1, Figure 24). At 3 and 6 months post OHCA, there were higher odds of a better functional recovery in the TI group than in the i-gel group [3 months: TI, 123/4199 patients with data (2.9%), vs. i-gel, 121/4636 patients with data (2.6%), OR 0.89 (95% CI 0.69 to 1.14; p = 0.35), ADP –0.51% (95% CI –1.18% to 0.16%; p = 0.14); 6 months: TI, 134/4212 patients with data (3.2%), vs. i-gel, 136/4661 patients with data (2.9%), OR 0.91 (95% CI 0.71 to 1.16; p = 0.43), ADP –0.39% (95% CI –1.08% to 0.30%; p = 0.27)] (see Table 13 and Figure 11).
| Complete-case mRS scorea | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Discharge/30 days | 300/4407 (6.8) | 311/4882 (6.4) | 0.92 (0.77 to 1.09) | 0.33 | 0.05 | –0.62 (–1.65 to 0.41) | 0.24 |
| 0 (no symptoms) | 124/4407 (2.8) | 117/4882 (2.4) | |||||
| 1 | 48/4407 (1.1) | 41/4882 (0.8) | |||||
| 2 | 50/4407 (1.1) | 58/4882 (1.2) | |||||
| 3 | 78/4407 (1.8) | 95/4882 (1.9) | |||||
| 4 | 46/4407 (1.0) | 45/4882 (0.9) | |||||
| 5 | 27/4407 (0.6) | 39/4882 (0.8) | |||||
| 6 (dead) | 4034/4407 (91.5) | 4487/4882 (91.9) | |||||
| 3 months | 123/4199 (2.9) | 121/4636 (2.6) | 0.89 (0.69 to 1.14) | 0.35 | < 0.001 | –0.51 (–1.18 to 0.16) | 0.14 |
| 0 (no symptoms) | 52/4199 (1.2) | 55/4636 (1.2) | |||||
| 1 | 6/4199 (0.1) | 4/4636 (0.1) | |||||
| 2 | 30/4199 (0.7) | 35/4636 (0.8) | |||||
| 3 | 35/4199 (0.8) | 27/4636 (0.6) | |||||
| 4 | 22/4199 (0.5) | 17/4636 (0.4) | |||||
| 5 | 5/4199 (0.1) | 4/4636 (0.1) | |||||
| 6 (dead) | 4049/4199 (96.4) | 4494/4636 (96.9) | |||||
| Missing mRS score at 3 monthsb | 208/4407 (4.7) | 246/4882 (5.0) | |||||
| 6 months | 134/4212 (3.2) | 136/4661 (2.9) | 0.91 (0.71 to 1.16) | 0.43 | < 0.001 | –0.39 (–1.08 to 0.30) | 0.27 |
| 0 (no symptoms) | 59/4212 (1.4) | 66/4661 (1.4) | |||||
| 1 | 4/4212 (0.1) | 5/4661 (0.1) | |||||
| 2 | 42/4212 (1.0) | 41/4661 (0.9) | |||||
| 3 | 29/4212 (0.7) | 24/4661 (0.5) | |||||
| 4 | 18/4212 (0.4) | 18/4661 (0.4) | |||||
| 5 | 2/4212 (0.1) | 3/4661 (0.1) | |||||
| 6 (dead) | 4058/4212 (96.3) | 4504/4661 (96.6) | |||||
| Missing mRS score at 6 monthsb | 195/4407 (4.4) | 221/4882 (4.5) |
Worst-case scenario sensitivity analyses
Summaries for worst-case longer-term mRS scores are presented in Appendix 1, Figure 25. The trends at 3 and 6 months in this scenario were the same as those in the complete-case analyses. There were higher odds of better functional recovery in the TI group than in the i-gel group [3 months: TI, 2.8%, vs. i-gel, 2.5%, OR 0.88 (95% CI 0.69 to 1.14; p= 0.34), ADP –0.49% (95% CI –1.13% to 0.15%; p = 0.13); 6 months: TI, 3.0%, vs. i-gel, 2.8%, OR 0.91 (95% CI 0.71 to 1.16; p = 0.43), ADP –0.37% (95% CI –1.03% to 0.29%; p = 0.27)] (Table 14; see Figure 11).
| Worst-case mRS scorea | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| 3-month follow-upb | 123/4407 (2.8) | 121/4882 (2.5) | 0.88 (0.69 to 1.14) | 0.34 | < 0.001 | –0.49 (–1.13 to 0.15) | 0.13 |
| 0 (no symptoms) | 52/4407 (1.2) | 55/4882 (1.1) | |||||
| 1 | 6/4407 (0.1) | 4/4882 (0.1) | |||||
| 2 | 30/4407 (0.7) | 35/4882 (0.7) | |||||
| 3 | 35/4407 (0.8) | 27/4882 (0.6) | |||||
| 4 | 22/4407 (0.5) | 17/4882 (0.4) | |||||
| 5 | 174/4407 (4.7) | 243/4882 (5.0) | |||||
| 6 (dead) | 4055/4407 (92.0) | 4501/4882 (92.1) | |||||
| 6-month follow-upb | 134/4407 (3.0) | 136/4882 (2.8) | 0.91 (0.71 to 1.16) | 0.43 | < 0.001 | –0.37 (–1.03 to 0.29) | 0.27 |
| 0 (no symptoms) | 59/4407 (1.3) | 66/4882 (1.4) | |||||
| 1 | 4/4407 (0.1) | 5/4882 (0.1) | |||||
| 2 | 42/4407 (1.0) | 41/4882 (0.8) | |||||
| 3 | 29/4407 (0.7) | 24/4882 (0.5) | |||||
| 4 | 18/4407 (0.4) | 18/4882 (0.4) | |||||
| 5 | 197/4407 (4.5) | 224/4882 (4.6) | |||||
| 6 (dead) | 4058/4407 (92.1) | 4504/4882 (92.3) |
Imputed-case scenario sensitivity analysis
Multiple imputation of mRS score (3 and 6 months), single summary index (30 days/hospital discharge and 3 and 6 months) and EQ-5D VAS score (30 days/hospital discharge and 3 and 6 months) was performed. A total of 60 imputed data sets were created because the highest percentage of missing data was 60% of those who survived to 30 days/hospital discharge.
Summaries for imputed-case mRS score summaries are presented in Appendix 1, Figure 26. There were similar odds of a good functional recovery in the TI and i-gel groups at both 3 and 6 months [3 months: OR 1.00 (95% CI 0.83 to 1.20; p = 0.98), APD –0.13% (95% CI –1.20% to 0.94%; p = 0.81); 6 months: OR 0.98 (95% CI 0.81 to 1.18; p = 0.80), APD –0.26% (95% CI –1.33% to 0.82%; p = 0.64)] (Table 15; see Figure 11). These results support the results found in the complete-case and worst-case scenarios for mRS score.
| Imputed-case mRS scorea | TI group (N = 264,600), n/N (%) | i-gel group (N = 293,160), n/N (%) | OR estimate (95% CI) | p-value | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|
| 3-month follow-up | 16,607/264,420 (6.3) | 18,563/292,920 (6.3) | 1.00 (0.83 to 1.20) | 0.98 | –0.13 (–1.20 to 0.94) | 0.81 |
| 0 (no symptoms) | 6703/264,420 (2.5) | 7947/292,920 (2.7) | ||||
| 1 | 706/264,420 (0.3) | 662/292,920 (0.2) | ||||
| 2 | 4110/264,420 (1.6) | 4896/292,920 (1.7) | ||||
| 3 | 5088/264,420 (1.9) | 5058/292,920 (1.7) | ||||
| 4 | 4059/264,420 (1.5) | 4047/292,920 (1.4) | ||||
| 5 | 814/264,420 (0.3) | 670/292,920 (0.2) | ||||
| 6 (dead) | 242,940/264,420 (91.9) | 269,640/292,920 (92.1) | ||||
| 6-month follow-up | 17,038/292,920 (6.4) | 18,717/292,920 (6.4) | 0.98 (0.81 to 1.18) | 0.80 | –0.26 (–1.33 to 0.82) | 0.64 |
| 0 (no symptoms) | 6719/264,420 (2.5) | 8106/292,920 (2.8) | ||||
| 1 | 514/264,420 (0.2) | 611/292,920 (0.2) | ||||
| 2 | 5497/264,420 (2.1) | 5723/292,920 (2.0) | ||||
| 3 | 4308/264,420 (1.6) | 4277/292,920 (1.5) | ||||
| 4 | 3581/264,420 (1.4) | 3600/292,920 (1.2) | ||||
| 5 | 321/264,420 (0.1) | 363/292,920 (0.1) | ||||
| 6 (dead) | 243,480/264,420 (92.1) | 270,240/292,920 (92.3) |
Quality-of-life outcomes
Complete-case analysis
Crude responses to the five EQ-5D dimension questions showed that there were no clear differences between the two treatment groups considering the small number of patients who completed these forms (see Appendix 1, Table 41). The single summary index and VAS scores at 30 days/hospital discharge and 3 and 6 months post OHCA were completed by patients who had survived to these time points and consented to active follow-up only. The complete-case single summary index score and VAS score summaries at all three time points are presented in Appendix 1, Figures 27 and 28. The results are shown in Figure 12.
FIGURE 12.
Complete-case single summary EQ-5D index scores.
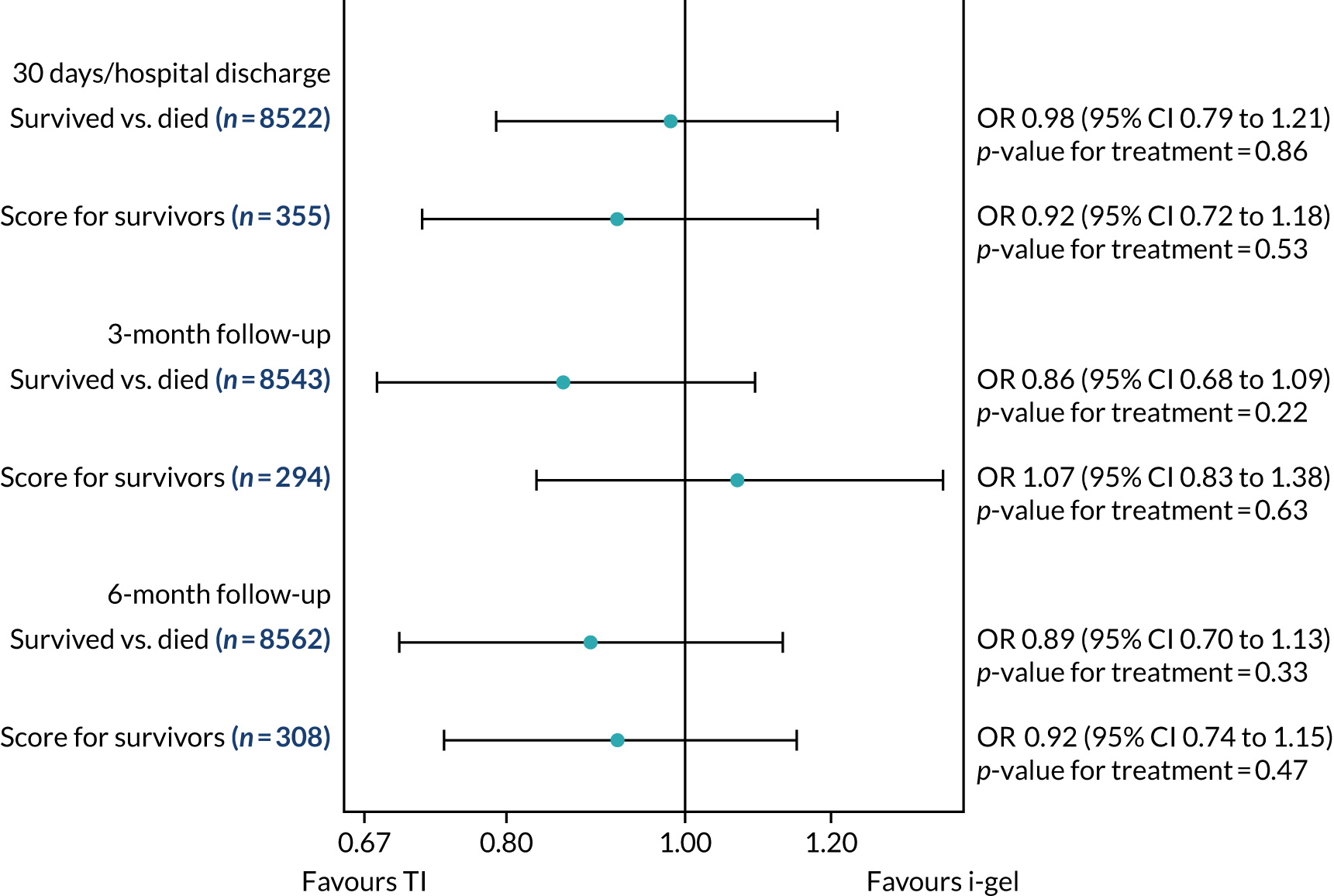
Table 16 contains the details of the single summary index and VAS scores at 30 days/hospital discharge and 3 and 6 months. There were higher median index and VAS scores at 30 days/hospital discharge in the TI group than in the i-gel group and there were similar median scores at the 3- and 6-month time points in both groups. There was a trend towards a higher odds of survival in the TI group than in the i-gel group at all three time points. For the QoL part of the model (‘score for survivors’), there was less consistency across the time points (Figure 13; see Table 16 and Figure 16).
FIGURE 13.
Complete-case VAS scores.
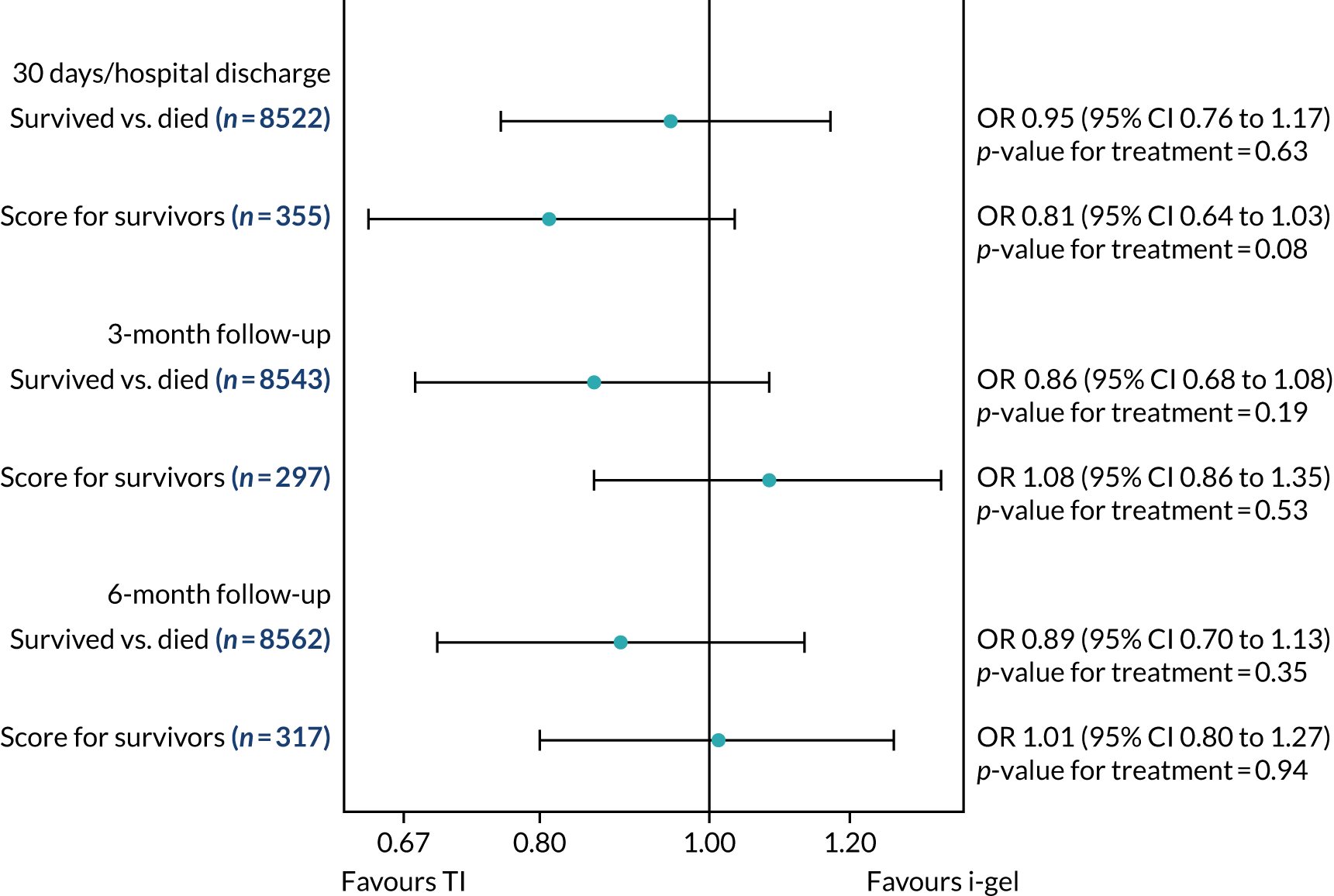
| Complete-case EQ-5D composite outcome | TI group (N = 4410) | i-gel group (N = 4886) | ‘Alive vs. dead’ model | ‘Score for survivors’ model | ||||
|---|---|---|---|---|---|---|---|---|
| Survived, n/N (%) | Median (IQR) | Survived, n/N (%) | Median (IQR) | ORa (95% CI) | p-value | ORb (95% CI) | p-value | |
| Single summary index | ||||||||
| 30 days/hospital dischargec | 170/4205 (4.0) | 0.76 (0.50–0.84) | 185/4672 (4.0) | 0.71 (0.40–0.84) | 0.98 (0.79 to 1.21) | 0.86 | 0.92 (0.72 to 1.18) | 0.53 |
| 3 monthsd | 150/4199 (3.6) | 0.80 (0.67–0.91) | 144/4638 (3.1) | 0.81 (0.68–1.0) | 0.86 (0.68 to 1.09) | 0.22 | 1.07 (0.83 to 1.38) | 0.63 |
| 6 monthse | 155/4213 (3.7) | 0.84 (0.70–1.0) | 153/4657 (3.3) | 0.84 (0.67–1.0) | 0.89 (0.70 to 1.13) | 0.33 | 0.92 (0.74 to 1.15) | 0.47 |
| VAS | ||||||||
| 30 days/hospital dischargef | 173/4208 (4.1) | 70 (50–80) | 182/4669 (3.9) | 65 (45–80) | 0.95 (0.76 to 1.17) | 0.63 | 0.81 (0.64 to 1.03) | 0.08 |
| 3 monthsg | 152/4201 (3.6) | 80 (60–90) | 145/4639 (3.1) | 80 (65–90) | 0.86 (0.68 to 1.08) | 0.19 | 1.08 (0.86 to 1.35) | 0.53 |
| 6 monthsh | 159/4217 (3.8) | 80 (65–90) | 158/4662 (3.4) | 80 (65–90) | 0.89 (0.70 to 1.13) | 0.35 | 1.01 (0.80 to 1.27) | 0.94 |
Worst-case scenario sensitivity analyses
Table 17 contains the results for the worst-case scenario single summary index and VAS scores at 30 days/hospital discharge and 3 and 6 months. The worst-case scenario summaries for single summary index and VAS scores are presented in Appendix 1, Figures 29 and Figure 30. The results of the formal statistical comparisons of worst-case single summary index and VAS scores are shown in Figures 14 and 15.
| Worst-case EQ-5D composite outcome | TI group (N = 4410) | i-gel group (N = 4886) | ‘Alive vs. dead’ model | ‘Score for survivors’ model | ||||
|---|---|---|---|---|---|---|---|---|
| Survived, n/N (%) | Median (IQR) | Survived, n/N (%) | Median (IQR) | ORa (95% CI) | p-value | ORb (95% CI) | p-value | |
| Single summary index | ||||||||
| 30 days/hospital dischargec | 372/4407 (8.4) | –0.59 (–0.59 to 0.72) | 395/4882 (8.1) | –0.59 (–0.59 to 0.68) | 0.94 (0.81 to 1.10) | 0.48 | 1.01 (0.88 to 1.17) | 0.86 |
| 3 monthsc | 352/4407 (8.0) | –0.59 (–0.59 to 0.74) | 381/4882 (7.8) | –0.59 (–0.59 to 0.72) | 0.97 (0.82 to 1.14) | 0.69 | 0.93 (0.81 to 1.08) | 0.36 |
| 6 monthsc | 349/4407 (7.9) | –0.59 (–0.59 to 0.81) | 378/4882 (7.7) | –0.59 (–0.59 to 0.75) | 0.97 (0.82 to 1.14) | 0.69 | 0.92 (0.79 to 1.07) | 0.28 |
| VAS | ||||||||
| 30 days/hospital dischargec | 372/4407 (8.4) | 0 (0 to 70) | 395/4882 (8.1) | 0 (0 to 65) | 0.94 (0.81 to 1.10) | 0.48 | 0.97 (0.85 to 1.10) | 0.60 |
| 3 monthsc | 352/4407 (8.0) | 0 (0 to 75) | 381/4882 (7.8) | 0 (0 to 70) | 0.97 (0.82 to 1.14) | 0.68 | 0.93 (0.81 to 1.06) | 0.26 |
| 6 monthsc | 349/4407 (7.9) | 0 (0 to 80) | 378/4882 (7.7) | 0 (0 to 75) | 0.97 (0.82 to 1.14) | 0.69 | 0.94 (0.81 to 1.09) | 0.42 |
FIGURE 14.
Worst-case single summary index scores.
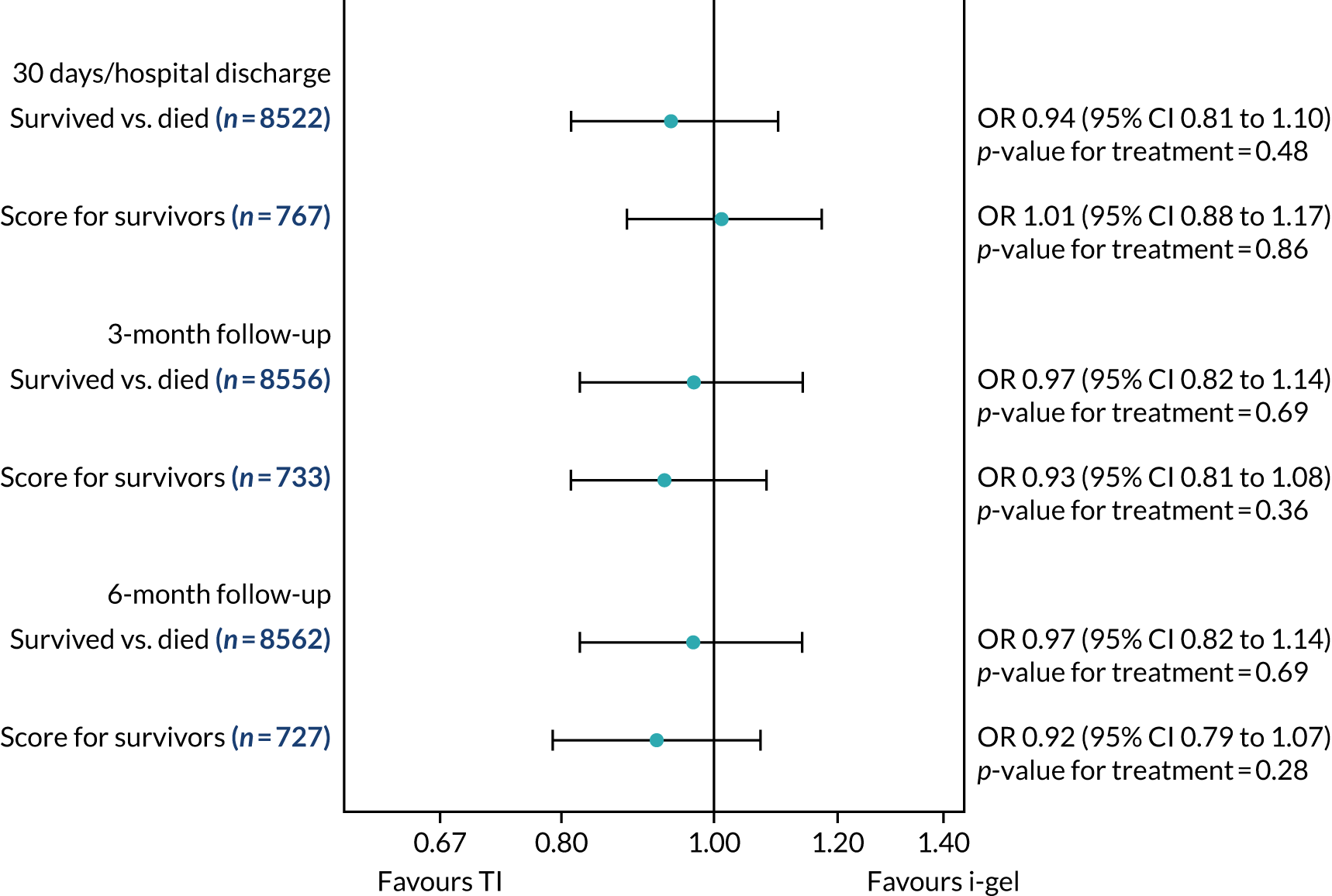
FIGURE 15.
Worst-case VAS scores.
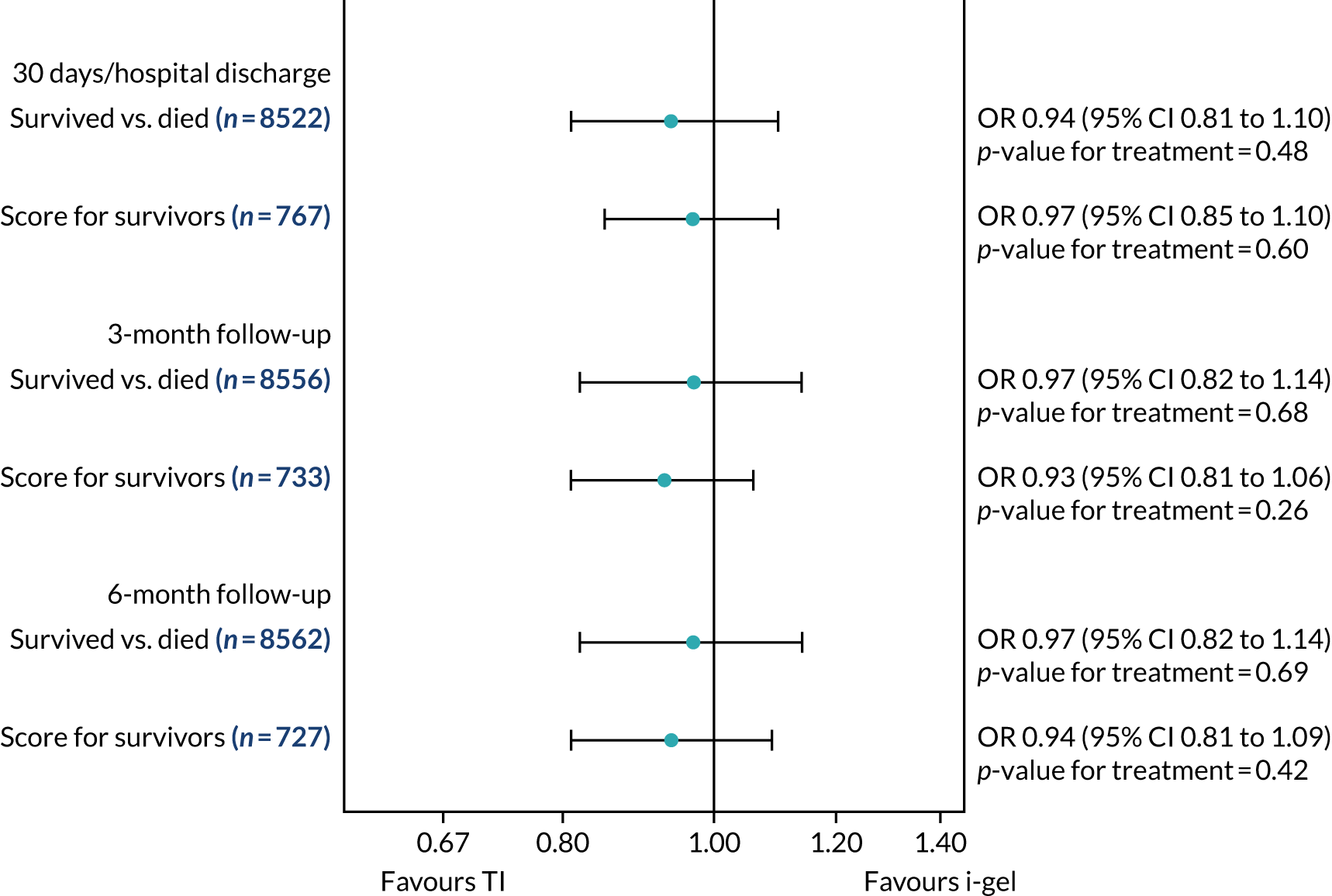
As in the results of the complete-case analysis, for both single summary index and VAS scores, there was a trend towards a higher odds of survival in the TI group than in the i-gel group at all three time points. The ‘score for survivors’ part of the model showed less consistent trends (see Table 17 and Figures 9 and 14).
Imputed-case scenario sensitivity analyses
The results of the imputed-case scenario sensitivity analyses of the QoL outcomes are shown in Table 18. Appendix 1, Figures 31 and 32, shows the worst-case scenario summaries for single summary index and VAS scores. Figures 16 and 17 show the formal statistical comparisons of imputed-case single summary index and VAS scores.
| Imputed-case EQ-5D composite outcome | TI group (N = 264,600) | i-gel group (N = 293,160) | ‘Survived vs. died’ model | ‘Score for survivors’ model | ||||
|---|---|---|---|---|---|---|---|---|
| Survived, n/N (%) | Median (IQR) | Survived, n/N (%) | Median (IQR) | ORa (95% CI) | p-value | ORb (95% CI) | p-value | |
| Single summary index | ||||||||
| 30 days/hospital dischargec | 22,320/264,420 (8.4) | 0.71 (0.41–0.84) | 23,700/292,920 (8.1) | 0.69 (0.39–0.84) | 0.94 (0.80 to 1.11) | 0.450 | 0.95 (0.74 to 1.21) | 0.68 |
| 3 monthsc | 21,480/264,420 (8.1) | 0.75 (0.56–0.88) | 23,280/292,920 (8.0) | 0.75 (0.59–0.88) | 0.87 (0.74 to 1.02) | 0.09 | 1.01 (0.79 to 1.28) | 0.96 |
| 6 monthsc | 20,940/264,420 (7.9) | 0.81 (0.65–1.0) | 22,680/292,920 (7.7) | 0.80 (0.62–0.91) | 0.88 (0.75 to 1.03) | 0.11 | 0.77 (0.61 to 0.96) | 0.02 |
| VAS | ||||||||
| 30 days/hospital dischargec | 22,320/264,420 (8.4) | 70 (50–80) | 23,700/292,920 (8.1) | 65 (45–80) | 0.86 (0.73 to 1.01) | 0.06 | 0.93 (0.74 to 1.16) | 0.52 |
| 3 monthsc | 21,480/264,420 (8.1) | 75 (55–90) | 23,280/292,920 (8.0) | 75 (55–90) | 0.87 (0.74 to 1.02) | 0.09 | 0.97 (0.79 to 1.19) | 0.79 |
| 6 monthsc | 20,940/264,420 (7.9) | 80 (60–90) | 22,680/292,920 (7.7) | 75 (60–90) | 0.88 (0.75 to 1.03) | 0.11 | 0.93 (0.74 to 1.16) | 0.52 |
FIGURE 16.
Imputed-case single summary index scores.
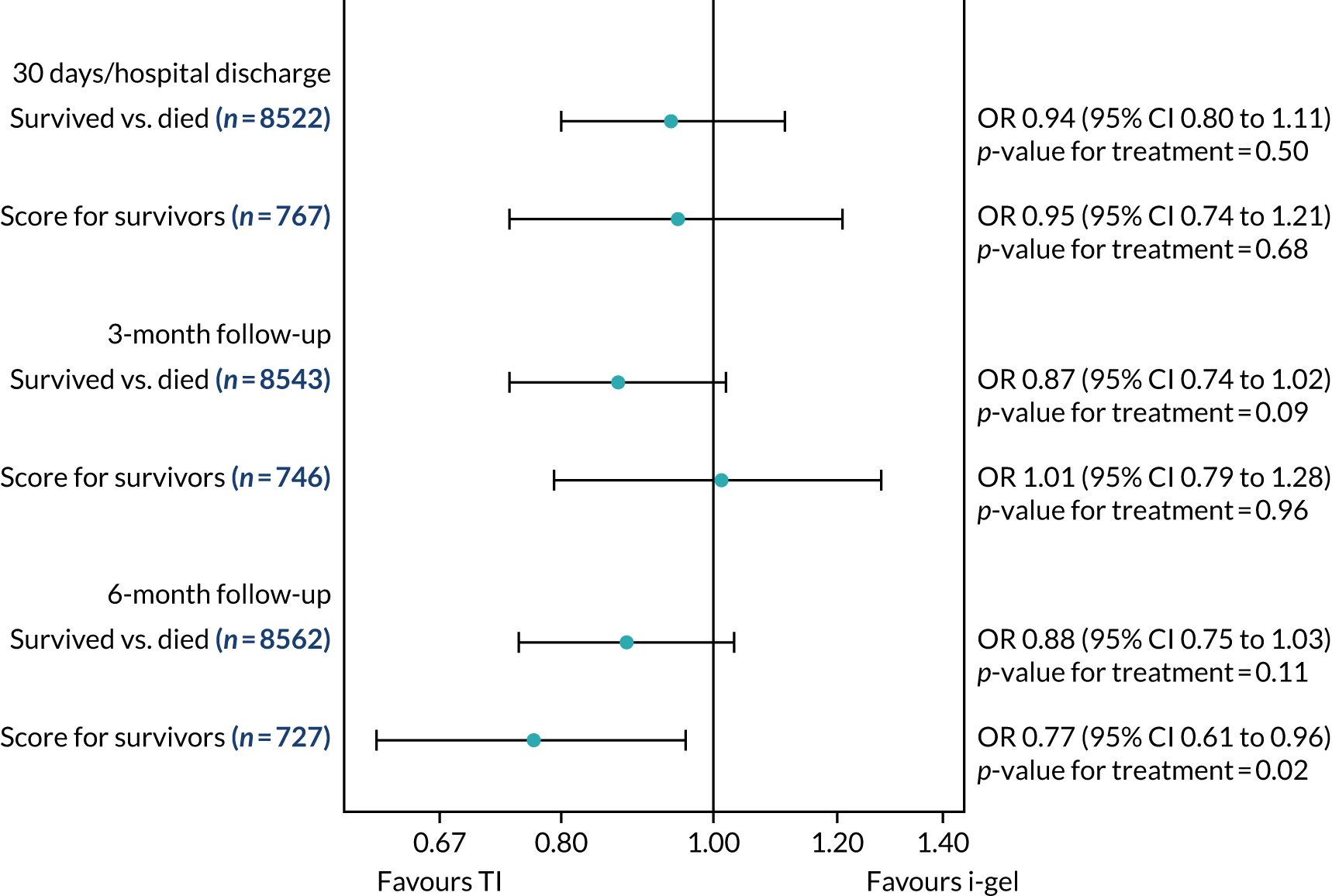
FIGURE 17.
Imputed-case VAS scores.
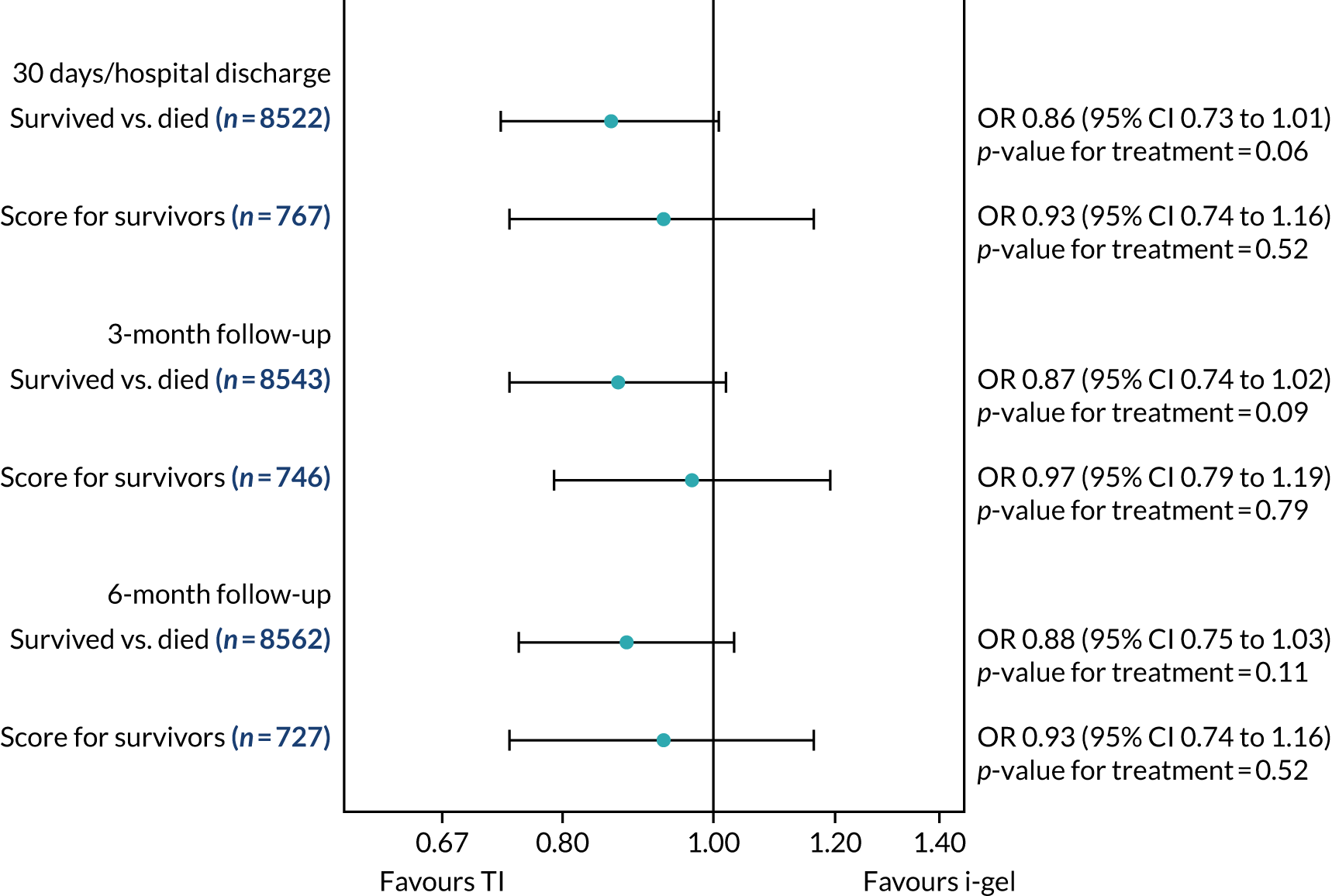
The imputed-case scenario analyses results showed a trend towards a better odds of survival in the TI group than in the i-gel group for both the single summary index and VAS scores at all three follow-up time points. The results of the QoL part of the model (‘score for survivors’) showed less consistent trends (see Table 18 and Figures 16 and 17).
Chapter 6 Results: economic evaluation
Missing data
The number of missing data for resource use/costs and outcomes (individual EQ-5D scores and QALYs) for patients in each treatment group is summarised in Table 19. A total of 21% of patients had some missing resource use/cost data (TI, 24%; i-gel, 19%) and a total of 6% of patients in both groups had one or more missing EQ-5D score across the three time points. Although it was challenging to keep track of patients across so many hospitals, these small numbers of missing data partly reflect that many patients were in the trial for a short duration. Missing data were non-monotonic, for example because individuals with missing costs for the ED may have had complete costs for the index admission that follows, or because patients with missing EQ-5D data at 3 months may have had complete data at 6 months. Multiple imputation can handle non-monotonic missing data.
| Category | TI group (N = 4407), n (%) | i-gel group (N = 4882), n (%) |
|---|---|---|
| Costs | ||
| Pre hospital | 3890 (88) | 4586 (94) |
| ED | 4170 (95) | 4617 (95) |
| Index admission | 4116 (93) | 4556 (93) |
| Follow-up: secondary care | 4293 (97) | 4773 (98) |
| Follow-up: community | 4094 (93) | 4544 (93) |
| All | 3347 (76) | 3961 (81) |
| Outcomes | ||
| EQ-5D at hospital discharge (or 30 days) | 4200 (95) | 4662 (95) |
| EQ-5D at 3 months | 4195 (95) | 4636 (95) |
| EQ-5D at 6 months | 4214 (96) | 4661 (95) |
| QALYs | 4153 (94) | 4601 (94) |
| All costs and QALYs | 3344 (76) | 3955 (81) |
When associations between missing total costs and QALY data and key baseline variables (age, sex, hospital trust and treatment group) were assessed, all were found to be significant predictors of missing costs, and all except treatment group were significant predictors of missing QALYs, at a 5% significance level. This suggests that data were not missing completely at random. Baseline variables were all significant predictors of costs, QALYs or both. Age, sex and treatment group predicted costs; age, sex and hospital trust predicted QALYs. Associations were also found between missingness and previously observed costs and EQ-5D outcomes, which suggests that missing data are dependent on more than just observed baseline covariates.
Overall, these findings support a missing-at-random assumption, and multiple imputation is a flexible and appropriate method for handling the missing data. When conducting the multiple imputation, baseline variables (age, sex and hospital trust) were included in the regression models, because missingness may depend on them. Because 21% of cases were incomplete, multiple imputation with m = 25 was conducted. For further details of the multiple imputation, see Appendix 5.
Quality-adjusted life-years
A summary of the mean EQ-5D scores for patients alive at each of the follow-up time points is shown in Figure 18. The scores are similar between the groups for all time points; scores for the TI group are initially slightly higher than those for the i-gel group, less than those for the i-gel group at 3 months and slightly higher than those for the i-gel group at 6 months. These differences are small and do not suggest a difference between the two groups.
FIGURE 18.
Mean observed EQ-5D scores for patients alive at each time point. Lines represent 95% CIs.
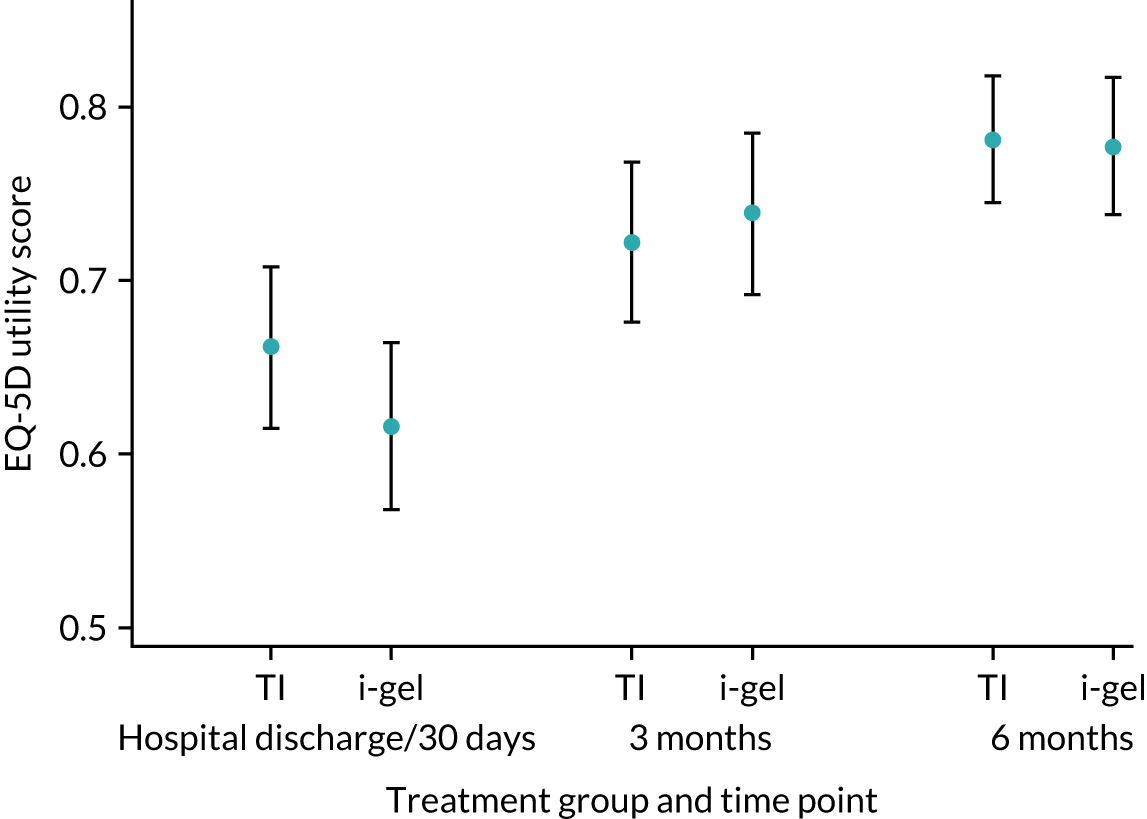
Table 20 reports the mean observed EQ-5D scores at each of the time points for all patients and the observed QALYs gained in each group. There is very little difference in EQ-5D score between the groups at any of the three time points, resulting in a very small (non-significant) difference in QALYs between the groups. The QALYs gained in each group are small, influenced by the large proportion of patients who died during the early stages of the trial. These patients have a tiny negative gain in QALYs because QoL for their brief time alive during the trial is on average –0.201 (an average of –0.402, the value assumed for baseline, and 0 the value assigned to death).
| Outcome | TI group (N = 4407) | i-gel group (N = 4882) | i-gel vs. TI | ||
|---|---|---|---|---|---|
| n (%) | Mean (SE) | n (%) | Mean (SE) | Mean difference (95% CI) | |
| EQ-5D scorea | |||||
| Hospital discharge (or 30 days if sooner) | 4200 (95) | 0.027 (0.002) | 4662 (95) | 0.024 (0.002) | –0.002 (–0.008 to 0.003) |
| 3 months | 4195 (95) | 0.026 (0.002) | 4636 (95) | 0.023 (0.002) | –0.003 (–0.009 to 0.003) |
| 6 months | 4214 (95) | 0.029 (0.002) | 4661 (95) | 0.026 (0.002) | –0.003 (–0.009 to 0.003) |
| QALYs to 6 months | 4153 (94) | 0.0100 (0.0010) | 4601 (94) | 0.0088 (0.0009) | –0.0012 (–0.0037 to 0.0013) |
Table 21 presents EQ-5D scores at each of the time points and QALYs for all patients, with missing data imputed. For further details of the multiple imputation, see Appendix 5. The ratio of QALYs in the i-gel group and in the TI group was similar for the observed and imputed data. The differences in EQ-5D scores between groups for each of the time points are similar to the observed data in Table 20. Because patients with missing EQ-5D scores and QALYs are by definition alive at those time points, then, as long as they have a QoL of greater than zero, these patients will contribute positively to the overall QALYs. Hence, the number of QALYs in each group is greater than in the observed data, but the difference between the groups remains similarly small. Indeed, the difference between the two groups in QALYs to 6 months is < 14 hours.
| Outcome | TI group (N = 4407), mean (SE) | i-gel group (N = 4882), mean (SE) | i-gel vs. TI, mean difference (95% CI) |
|---|---|---|---|
| EQ-5D time pointa | |||
| Hospital discharge (or 30 days if sooner) | 0.056 (0.003) | 0.051 (0.003) | –0.006 (–0.015 to 0.004) |
| 3 months | 0.060 (0.004) | 0.057 (0.003) | –0.003 (–0.013 to 0.007) |
| 6 months | 0.062 (0.004) | 0.059 (0.003) | –0.002 (–0.012 to 0.007) |
| QALYs to 6 months | 0.0274 (0.0016) | 0.0259 (0.0015) | –0.0015 (–0.0059 to 0.0028) |
Resource use and costs
Tables 22 and 23 report information on the main resource use items for the treatment groups to 6 months. These resource items include the pre-hospital phase (initial airway management, paramedics and vehicles attending, and subsequent arrival in the ED for those who survived to hospital), time in hospital and other primary and secondary care resource use during the 6-month follow-up period. Table 22 presents a summary of resource use for all patients, because this reflects total costs. However, because many patients died at the scene or in the ED the mean resource use for time points following conveyance to hospital is small, because large numbers of patients who have died are included. Table 23 shows resource use data for patients known to be alive at each stage.
| Resource use | TI group (N = 4407) | i-gel group (N = 4882) | i-gel vs. TI, mean difference (95% CI) |
|---|---|---|---|
| Pre hospital | |||
| AAM devices used by AIRWAYS-2 paramedic, n (%); mean n (SE) | |||
| TI | 4138 (94); 0.94 (0.01) | 4662 (96); 0.19 (0.01) | –0.75 (–0.78 to –0.72) |
| i-gel | 4138 (94); 0.33 (0.01) | 4662 (96); 1.03 (0.01) | 0.69 (0.66 to 0.73) |
| Other (OPA, NPA, LMA) | 4138 (94); 0.47 (0.01) | 4662 (96); 0.28 (0.01) | –0.19 (–0.23 to –0.16) |
| Ambulance staff at scene (time in hours), n (%); mean (SE) | |||
| Band 6+ | 4402 (100); 0.78 (0.03) | 4873 (100); 0.82 (0.03) | 0.04 (–0.04 to 0.13) |
| Band 5 | 4402 (100); 1.07 (0.03) | 4873 (100); 1.07 (0.03) | 0.00 (–0.07 to 0.08) |
| Band 4 | 4402 (100); 0.28 (0.01) | 4873 (100); 0.26 (0.01) | –0.02 (–0.06 to 0.02) |
| Band 2 or 3 | 4402 (100); 0.61 (0.01) | 4873 (100); 0.63 (0.01) | 0.02 (–0.02 to 0.06) |
| Total time | 4402 (100); 2.73 (0.03) | 4873 (100); 2.76 (0.03) | 0.03 (–0.06 to 0.12) |
| Vehicles, n (%); mean number attending (SE) | |||
| Rapid response vehicle | 4404 (100); 1.13 (0.02) | 4878 (100); 1.14 (0.02) | 0.01 (–0.05 to 0.06) |
| Ambulance | 4404 (100); 1.23 (0.01) | 4878 (100); 1.23 (0.01) | 0.00 (–0.03 to 0.02) |
| Air ambulance | 4404 (100); 0.10 (0.01) | 4878 (100); 0.11 (0.01) | 0.01 (–0.01 to 0.03) |
| Other | 4404 (100); 0.13 (0.01) | 4878 (100); 0.11 (0.01) | –0.01 (–0.04 to 0.01) |
| Total | 4404 (100); 2.59 (0.02) | 4878 (100); 2.60 (0.02) | 0.00 (–0.04 to 0.05) |
| Taken to hospital, n (%); mean (SE) | |||
| ED attendance | 4407 (100); 0.43 (0.01) | 4882 (100); 0.46 (0.01) | 0.03 (0.00 to 0.05) |
| Admitted to hospital, n (%); mean (SE) | |||
| Initial days in ICUa | 4407 (100); 0.76 (0.06) | 4882 (100); 0.90 (0.05) | 0.14 (–0.2 to 0.3) |
| Further days in ICUa | 4158 (94); 0.08 (0.02) | 4605 (94); 0.03 (0.01) | –0.04 (–0.08 to 0.00) |
| Total days in hospital | 4362 (99); 2.26 (0.16) | 4836 (99); 2.49 (0.19) | 0.23 (–0.26 to 0.73) |
| Post hospital discharge (or 30 days if sooner), n (%); mean (SE) | |||
| Further inpatient days | 4382 (99); 0.20 (0.04) | 4861 (100); 0.16 (0.03) | –0.04 (–0.14 to 0.06) |
| Further ED attendances | 4333 (98); 0.04 (0.01) | 4810 (99); 0.04 (0.00) | 0.00 (–0.01 to 0.02) |
| Outpatient appointments | 4352 (99); 0.41 (0.04) | 4831 (99); 0.42 (0.03) | 0.01 (–0.09 to 0.11) |
| GP contacts | 4136 (94); 0.11 (0.01) | 4583 (94); 0.08 (0.01) | –0.03 (–0.07 to 0.00) |
| Nurse contacts | 4138 (94); 0.05 (0.01) | 4578 (94); 0.04 (0.01) | –0.01 (–0.03 to 0.02) |
| Resource use | TI group (N = 4407) | i-gel group (N = 4882) | i-gel vs. TI, mean difference (95% CI) |
|---|---|---|---|
| Pre hospital,a n (%) | 4407 (100) | 4882 (100) | |
| Taken to hospital, n (%) | 1919 (44) | 2259 (46) | |
| ED attendance,b n (%); cost (£), mean (SE) | 1919 (100); 0.99 (0.00) | 2259 (100); 0.99 (0.01) | 0.00 (0.00 to 0.01) |
| Admitted to hospital, n (%) | 861 (20) | 1033 (21) | |
| Initial days in ICU, n (%); cost (£), mean (SE) | 861 (100); 3.87 (0.29) | 1033 (100); 4.25 (0.22) | 0.38 (–0.34 to 1.09) |
| Further days in ICU, n (%); cost (£), mean (SE) | 612 (71); 0.52 (0.11) | 756 (73); 0.21 (0.06) | –0.31 (–0.56 to –0.06) |
| Total days in hospital, n (%); cost (£), mean (SE) | 816 (95); 12.22 (0.84) | 987 (96); 12.19 (0.86) | –0.03 (–2.38 to 2.32) |
| Post-hospital discharge (or 30 days if sooner), n (%) | 377 (9) | 404 (8) | |
| Further inpatient days, n (%); cost (£), mean (SE) | 352 (99); 2.46 (0.49) | 383 (100); 2.01 (0.35) | –0.46 (–1.64 to 0.73) |
| Further ED attendances, n (%); cost (£), mean (SE) | 303 (98); 0.61 (0.08) | 332 (99); 0.63 (0.06) | 0.02 (–0.17 to 0.22) |
| Outpatient appointments, n (%); cost (£), mean (SE) | 322 (99); 5.63 (0.49) | 353 (99); 5.84 (0.35) | 0.20 (–0.99 to 1.40) |
| GP contacts, n (%); cost (£), mean (SE) | 106 (94); 4.21 (0.37) | 105 (94); 3.34 (0.39) | –0.87 (–1.92 to 0.18) |
| Nurse contacts, n (%); cost (£), mean (SE) | 108 (94); 1.92 (0.26) | 100 (94); 2.05 (0.49) | 0.13 (–0.96 to 1.22) |
Resource use at the scene was very similar between the two groups, in terms of both the numbers of vehicles attending and the total time staff spent with the patient. On average, 2.7 hours of paramedic time were spent per OHCA, with two or three vehicles attending. A slightly larger proportion of patients in the i-gel group were taken to hospital (46%, compared with 44% in the TI group; see Table 23). The majority of patients who survived to hospital admission were admitted to intensive care. Overall, patients in the i-gel group spent slightly longer as inpatients and slightly longer in intensive care than patients in the TI group, but these differences were non-significant. Resource use for patients who survived to hospital discharge was similar between groups.
Table 24 presents the observed costs for patients known to be alive at each stage. Costs are very similar between the groups at each stage for patients who are alive. The airway devices are inexpensive and costs associated with them are small. For completeness, see Appendix 8, which reports the observed costs for patients regardless of survival status, and the observed costs for patients with complete total costs. The longer patients survive, the more cost categories are potentially non-zero and the greater the opportunity for missing cost data. Therefore, observed costs for patients with complete total costs should be interpreted with caution; these subgroups of patients exclude many patients with high levels of resource use who are missing total costs. Total costs for patients with complete data do not accurately represent all patients in each group (costs for these subgroups are much lower than for the whole cohort).
| Cost category | TI group (N = 4407) | i-gel group (N = 4882) | i-gel vs. TI, mean difference (95% CI) |
|---|---|---|---|
| Pre hospital, n (%) | 4407 (100) | 4882 (100) | |
| Initial airway management pre AIRWAYS-2 paramedic, n (%); cost (£), mean (SE) | 4386 (100); 1 (0) | 4859 (100); 1 (0) | 0 (0 to 0) |
| AAM devices used by AIRWAYS-2 paramedic, n (%); cost (£), mean (SE) | |||
| TI | 3901 (89); 11 (0) | 4607 (94); 2 (0) | –9 (–9 to –8) |
| i-gel | 4138 (94); 2 (0) | 4662 (95); 5 (0) | 3 (3 to 4) |
| Other (OPA, NPA, LMA) | 4138 (94); 1 (0) | 4662 (95); 0 (0) | –1 (–1 to –1) |
| Total | 3901 (89); 13 (0) | 4607 (94); 7 (0) | –6 (–7 to –6) |
| Ambulance staff at scene, n (%); cost (£), mean (SE) | |||
| Band 6+ | 4402 (100); 22 (1) | 4873 (100); 23 (1) | 1 (–1 to 4) |
| Band 5 | 4402 (100); 25 (1) | 4873 (100); 25 (1) | 0 (–2 to 2) |
| Band 4 | 4402 (100); 5 (0) | 4873 (100); 5 (0) | 0 (–1 to 0) |
| Band 2 or 3 | 4402 (100); 10 (0) | 4873 (100); 10 (0) | 0 (0 to 1) |
| Total | 4402 (100); 61 (1) | 4873 (100); 62 (1) | 1 (–1 to 3) |
| Vehicles | 4404 (100); 146 (1) | 4878 (100); 147 (1) | 1 (–2 to 3) |
| Pre-hospital total, n (%); cost (£), mean (SE) | 3890 (88); 221 (2) | 4586 (94); 216 (2) | –4 (–9 to 1) |
| Taken to hospital, n (%) | 1919 (44) | 2259 (46) | |
| ED attendance, n (%); cost (£), mean (SE) | 1682 (88); 330 (3) | 1994 (88); 327 (3) | –3 (–11 to 6) |
| Admitted to hospital, n (%) | 861 (20) | 1033 (21) | |
| Index inpatient care, n (%); cost (£), mean (SE) | 802 (93); 6802 (296) | 974 (94); 6469 (269) | –333 (–1118 to 452) |
| ICU days, n (%); cost (£), mean (SE) | 612 (71); 7031 (538) | 756 (73); 6931 (317) | –99 (–1323 to 1124) |
| Post hospital discharge (or 30 days if sooner), n (%) | 377 (9) | 404 (8) | |
| Further inpatient days, n (%); cost (£), mean (SE) | 352 (99); 2082 (324) | 383 (100); 1705 (207) | –378 (–1132 to 377) |
| Further ED attendances, n (%); cost (£), mean (SE) | 303 (98); 132 (16) | 332 (99); 135 (13) | 3 (–37 to 43) |
| Outpatient appointments, n (%); cost (£), mean (SE) | 322 (99); 748 (54) | 353 (99); 840 (60) | 92 (–67 to 251) |
| GP contacts, n (%); cost (£), mean (SE) | 106 (94); 111 (11) | 105 (94); 86 (10) | –26 (–55 to 3) |
| Nurse contacts, n (%); cost (£), mean (SE) | 108 (94); 34 (6) | 100 (94); 41 (14) | 7 (–23 to 37) |
The results for all patients based on the imputed data have been presented. Appendix 5 describes the imputation model and compares the distributions of observed and imputed total costs. The ratio of costs in the i-gel group and the TI group was similar for the observed and imputed data. A breakdown of total costs is provided in Figure 19. The error bars represent the 95% CI around total costs in each treatment group. Mean total costs per patient were £3570 (SE £152) and £3413 (SE £162) in the i-gel and TI groups, respectively (mean difference £157, 95% CI –£278 to £592). Despite only 20% of patients being admitted to hospital, the key cost drivers were the inpatient stay and time in intensive care. As patients in the i-gel group spent slightly longer in hospital and in intensive care than the TI group, these costs were slightly higher in the i-gel group. However, overall, costs were very similar between the groups.
FIGURE 19.
Total costs to 6 months for all patients. Lines represent 95% CIs.
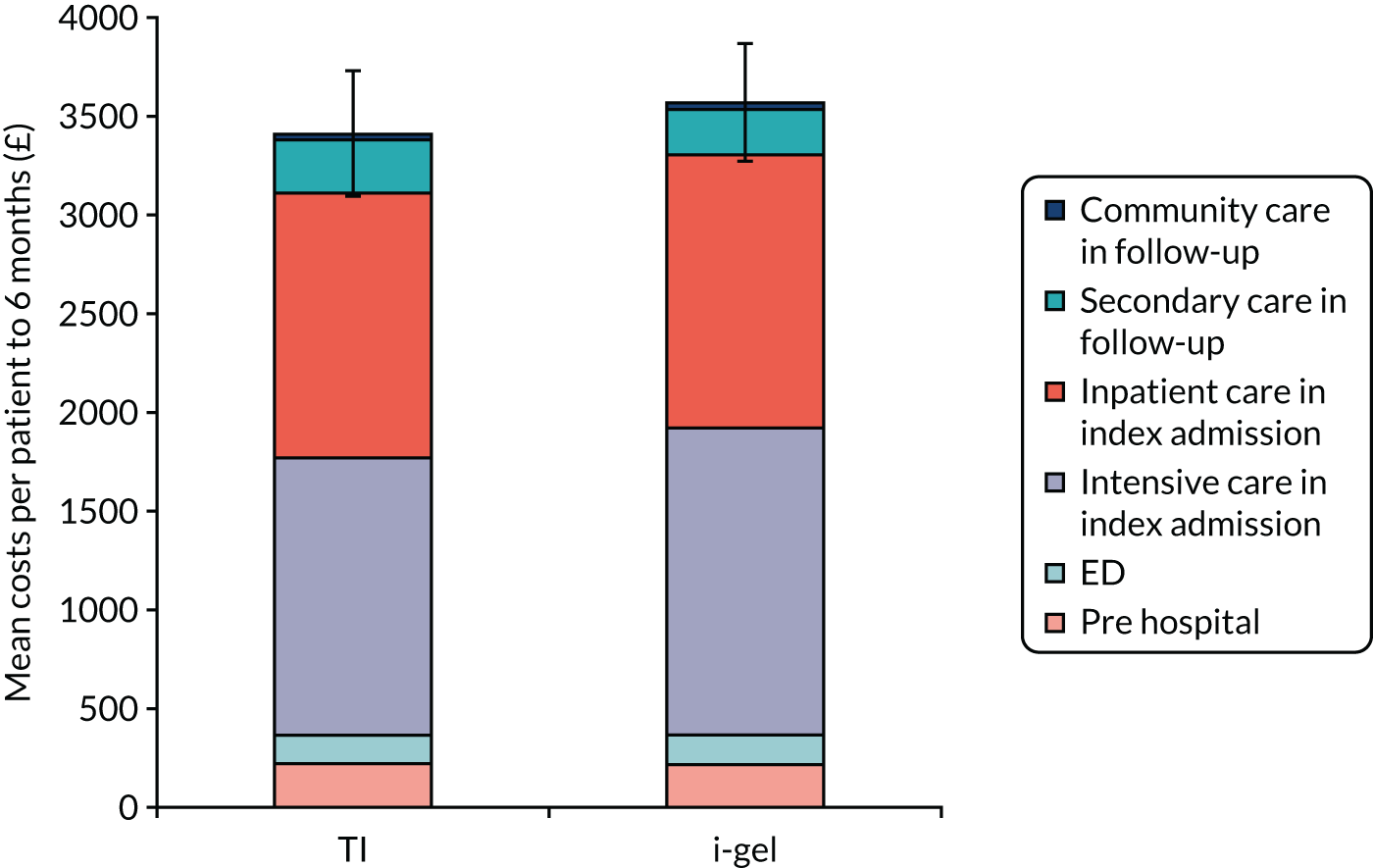
Base-case cost-effectiveness results
Table 25 presents the results of the costs and effects combined: the cost-effectiveness. The differences in costs and QALYs between the groups are small and neither difference is statistically significant. The difference between the groups for QALYs, which is the denominator for the ICER, is especially small. Dividing the difference in costs by a tiny number close to zero results in a very large ICER. Based on the point estimate of the ICER only (–£102,362), TI is dominant over i-gel because it is both more effective and less costly. However, there is great uncertainty around this result, as shown on the cost-effectiveness plane in Figure 20, where the bootstrap replicates of the cost and QALY differences cover three quadrants of the cost-effectiveness plane. The dark-blue dot is the point estimate of the cost and QALY difference, and is close to the origin. The small differences and the large number of points over three quadrants suggest that there is no evidence of a difference in cost-effectiveness between the two groups.
| Cost-effectiveness element | TI group (n = 4407) | i-gel group (n = 4882) | i-gel vs. TI, difference |
|---|---|---|---|
| Total costs (£) (95% CI)a | 3413 (3112 to 3714) | 3570 (3279 to 3860) | 157 (–270 to 583) |
| QALYs (95% CI)a | 0.0274 (0.0243 to 0.0305) | 0.0259 (0.0230 to 0.0287) | –0.0015 (–0.0059 to 0.0028) |
| ICER (cost/QALY) | TI dominant (–102,362) |
FIGURE 20.
Cost-effectiveness plane. The dark-blue dot is the point estimate of the cost and QALY difference.
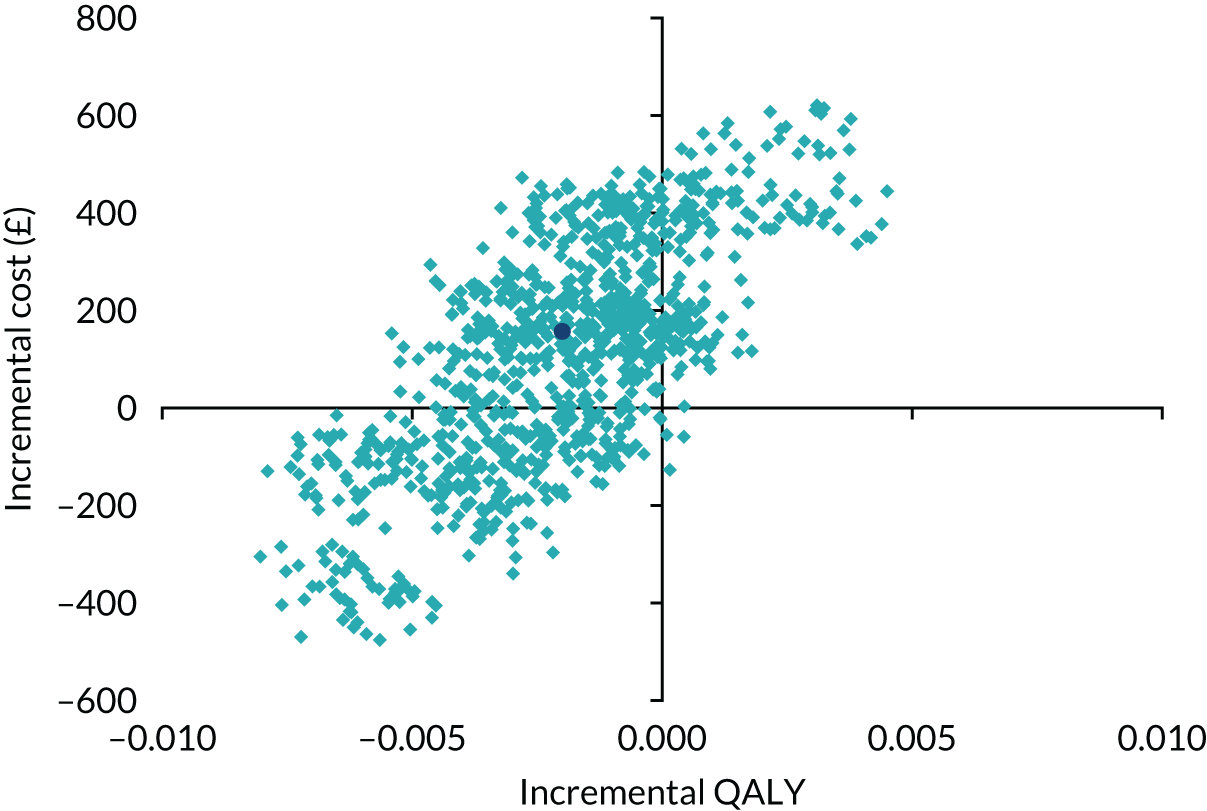
The CEAC in Figure 21 shows the probability that the i-gel is cost-effective for a range of willingness-to-pay thresholds. If a decision-maker is willing to pay £20,000 for an additional QALY, then the probability of the i-gel being cost-effective is 18%. The probability that the i-gel is cost-effective is low across numerous willingness-to-pay thresholds, and gradually reduces as the threshold is increased. Although the i-gel is unlikely to be cost-effective, there is much uncertainty around this. The dotted lines at 0.1 and 0.9 indicate the 80% confidence limits for the probability that the i-gel is cost-effective. The upper limit does not exist because the horizontal line does not cut the curve at any point; the lower 80% confidence limit on cost-effectiveness is approximately £60,000.
FIGURE 21.
Cost-effectiveness acceptability curve.
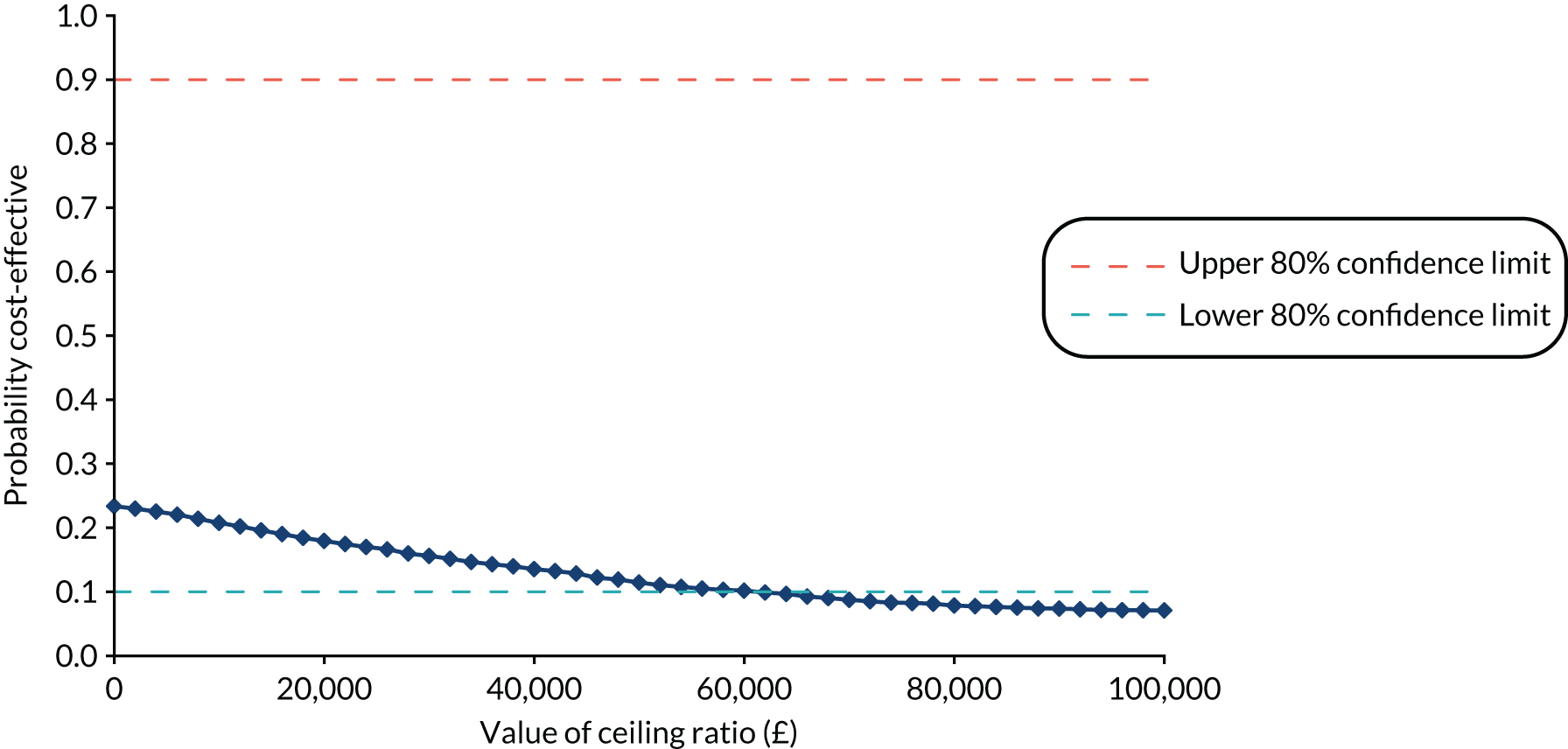
Given the small difference in QALYs, and the large number of negative ICERs generated when costs and QALYs were bootstrapped, we considered incremental NMB so that we could present 95% CIs around a cost-effectiveness summary statistic.
For willingness-to-pay thresholds from £0 to £50,000 per QALY, Table 26 reports the incremental NMB and the probability that the i-gel is cost-effective at each threshold. Given that incremental NMB is calculated as incremental effects × threshold – incremental costs, and incremental effects are negative and incremental costs are positive, there is no value of the threshold at which NMB is positive and no value at which the i-gel is considered cost-effective. In each case, the majority of the 95% CI is below zero. This is reflected in the low probabilities of the i-gel being cost-effective in the final column of the table (which mirrors the CEAC; see Figure 21).
| Willingness-to-pay threshold (£) for a QALY | Incremental NMB (95% CI) | Probability that i-gel is cost-effective |
|---|---|---|
| 0 | –157 (–516 to 349) | 0.24 |
| 10,000 | –172 (–513 to 299) | 0.21 |
| 20,000 | –187 (–520 to 256) | 0.19 |
| 30,000 | –202 (–526 to 213) | 0.16 |
| 40,000 | –217 (–534 to 181) | 0.15 |
| 50,000 | –232 (–559 to 152) | 0.12 |
Sensitivity analyses
For full results of the sensitivity analyses conducted around costs and outcomes, and a summary of key findings, see Appendix 7. None of the sensitivity analyses varying unit costs had a great impact on the cost difference between the groups and findings reinforced how similar the resource use was between groups. Sensitivity analyses around high-cost patients identified nine patients with total costs exceeding £100,000, including one patient in the TI group with total costs of £271,014. Although these patients have a significant impact on the cost results, they do not alter conclusions.
Sensitivity analyses around alternative assumptions for outcomes (assuming a baseline utility of 0 rather than –0.402, and considering life-years rather than QALYs) did not affect the differences between the groups.
Summary
There was very little difference between the groups in either costs or effects, and great uncertainty around the cost-effectiveness results. Mean QALYs to 6 months were 0.03 in both groups and there was a tiny difference between the i-gel and TI groups (mean difference –0.0015, 95% CI –0.0059 to 0.0028). The total costs of care from OHCA to 6 months were £3570 in the i-gel group and £3413 in the TI group, creating a small mean difference of £157 (95% CI –£270 to £583). The point estimate of cost-effectiveness suggested that TI was more effective (a very slightly greater QALY gain) and less costly than the i-gel (i.e. dominant) and, therefore, cost-effective. However, given the extreme uncertainty around this result, the point estimate is less informative and should be interpreted with caution. Uncertainty exists around the CEAC, but this suggests that the i-gel has a low probability of being cost-effective, regardless of the willingness-to-pay threshold. Overall, there is no evidence of a difference in cost-effectiveness between the groups.
Chapter 7 Discussion
Parts of this chapter are reproduced with permission from JAMA 2018;320(8):779–91. 27 Copyright © 2018 American Medical Association. All rights reserved.
Main findings
Trial conduct
The design and implementation of the AIRWAYS-2 trial was informed by a growing, but still limited, body of evidence from previous out-of-hospital RCTs35,81 and a substantial feasibility trial. 25 AIRWAYS-2 was a large and complex trial that aimed to guide future paramedics’ airway management in patients with OHCA. Research in the emergency setting is challenging, but it is essential if we are to improve future clinical practice. We identified a widespread lack of familiarity with pre-hospital research. Many hospitals had no experience of participating in paramedic studies or of approaching patients for consent after they had been enrolled in a trial outside hospital. 26
Blinding of paramedics was not possible; this presented a challenge because it required a trial design that ensured inclusion of all eligible patients attended by a participating paramedic. The only way to achieve this was to enrol eligible patients automatically. To enable this, the investigators gained approval for the collection and retention of a minimal data set without consent. Legal provisions enable research in an emergency setting, providing all proposed activities have approval from an appropriate REC. 82 The ethics committee demonstrated a high degree of understanding of the nature of pre-hospital research. This may have been promoted by recent increases in research activity by EMS and previous work completed by our team. 25 Crucially, our trial design and ethics application was supported by an active and well-informed patient and public research advisory group comprising OHCA survivors, their families and other members of the public.
In most clinical trials, research activities are performed by clinicians who have research-specific training and experience. Most of the paramedics who participated in the AIRWAYS-2 trial had little or no previous exposure to clinical trials. They were nevertheless required to conduct several research-related activities without compromising patient care following a relatively short period of face-to-face and online training. 83 The scene of an out-of-hospital resuscitation attempt for a patient in cardiac arrest is an uncontrolled and challenging environment in terms of patient, scene and team management. The difficulties of recognising eligibility, conducting interventions and reporting patients during and after such events are likely to have had an impact on overall trial performance. However, the successes of the research are a testament to the commitment of the participating paramedics who nonetheless delivered the trial.
The AIRWAYS-2 trial used a cluster randomised design, which has strengths and weaknesses. At the start of the trial, we considered alternative approaches to randomisation. Airway management is a very early step in the management of OHCA by paramedics and occurs in unpredictable, high-stress environments. We were concerned that any attempt to randomise patients individually (for example, using sealed opaque envelopes, or a telephone or web-based randomisation service), although technically possible, would carry an unacceptable risk of treatment delay because any randomisation process would distract paramedics from direct patient care. For a significant proportion of patients enrolled in the AIRWAYS-2 trial (1164/5888; 19.8%), the cardiac arrest occurred in the presence of ambulance staff (EMS witnessed) and randomisation procedures would be particularly problematic for this group because the attending paramedic would have no time to prepare.
Cluster randomisation at the level of the paramedic was supported by our patient and public research advisory group, which was focused on the need for paramedics to deliver high-quality care in an emergency rather than engage in research procedures. This led us to a cluster randomised design, and we elected to continue the approach that we had used successfully in our REVIVE-AIRWAYS feasibility study39 by randomising individual paramedics. We considered using larger randomisation clusters (for example, by ambulance station); however, this would have increased the intra-cluster correlation and require an inflation of an already large sample size with associated costs.
The Pragmatic Airway Resuscitation Trial (PART)53 used a crossover design, which we considered; however, it was deemed impractical to retrain all paramedic participants during the study, with the associated risk of undermining protocol compliance and trial delivery. The PARAMEDIC-2 trial84 was able to randomise patients individually; however, this was facilitated by the fact that PARAMEDIC-2 was a double-blinded drug trial in which paramedics were required to open a pre-prepared pack and give the drug it contained; furthermore, randomisation in PARAMEDIC-2 occurred later in the OHCA, providing more opportunity for the paramedic to prepare (physically and cognitively) for enrolment. Finally, the CAAM trial85 of BMV versus TI in OHCA was able to achieve individual patient randomisation; however, this also occurred later in the OHCA and was undertaken by doctors who usually arrived after earlier EMS responders had undertaken initial airway management; indeed, one of the criticisms of the CAAM trial is that relatively late enrolment reduced the likelihood that the intervention would influence outcome.
However, the use of a cluster randomised design in the AIRWAYS-2 trial had considerable drawbacks. Foremost among these was the lack of allocation concealment, which created a substantial risk of selection bias. To avoid paramedics selectively enrolling patients, we therefore opted to automatically enrol, under a waiver of consent, every eligible patient. The processes that were put in place to achieve this proved to be very labour intensive but successful. 26 However, enrolling all eligible patients had the additional effect of including a significant number of patients who did not receive the trial intervention or crossed over to the alternative AAM strategy, with resulting protocol non-adherence. This was further exacerbated by the requirement to allow paramedics an element of clinical freedom to adapt their approach to prevailing circumstances to assure patient safety. The analysis and interpretation of the trial results became more complex as a result. We believe that the findings of our whole-group ITT and instrumental variable analyses are robust; however, in these circumstances any per-protocol approach should be treated with extreme caution.
The number of paramedic clusters in each group was relatively equal (TI, n = 696; i-gel, n = 686). However, the number of patients randomised by the paramedic clusters in those groups was not equal (TI, n = 4410; i-gel, n = 4886). This can be partly explained by a variation in the median number of patients enrolled per paramedic (TI, n = 5; i-gel, n = 6). There was also a small number of paramedics in the i-gel group who enrolled a disproportionately large number of patients, with a maximum of 56 patients enrolled, compared with a maximum of 48 patients in the TI group. These ‘super-enroller’ paramedics were small in number. It is likely that the different patient group sizes are because of a chance imbalance in the allocation of the ‘super-enrollers’ that favoured the i-gel.
Paramedics randomised to use TI were less likely to use any form of AAM than paramedics randomised to use the i-gel. TI is a more complex skill than SGA insertion and requires two practitioners, additional equipment and good access to the patient’s airway. 86 However, OHCA often occurs in locations where patient access is challenging. It seems likely that the increased complexity of TI influenced the paramedics’ behaviour so that they were less inclined to proceed with AAM if they had been randomised to the TI group than to the i-gel group. This is a pragmatic reflection of actual clinical practice, which resulted in an imbalance in the proportion of patients who received any form of AAM between the two groups. Interestingly, despite the fact that patients allocated to TI were less likely to receive AAM, this did not translate into worse outcomes, suggesting that AAM in any form, provided early in OHCA, may not improve outcomes in cardiac arrest. This is consistent with a recent European RCT85 that did not detect a difference in outcome between patients allocated to TI or basic airway management during OHCA.
We believe that, for trials of non-drug interventions delivered very early in OHCA, cluster randomisation will continue to be required to ensure that randomisation procedures do not delay essential clinical treatment. However, this should be reviewed in the light of ongoing digital and technological developments that may facilitate near-instantaneous patient randomisation in the future. Where cluster randomisation is used and concealment is impossible, strenuous efforts to avoid selection bias, combined with whole-population ITT and/or instrumental variable analyses, should be employed.
Adherence to the trial protocol differed between the two treatment groups, with higher adherence in the i-gel group than in the TI group (82.1% compared with 61.9%) (see Chapter 4, Additional analysis). The higher adherence to the i-gel may reflect paramedic preferences for this device, and ease of use. For an illustration of interventions received by patients, see Figure 7. Rates of AAM use in patients who did not have ROSC on paramedic arrival are also different between the two treatment groups.
Despite considerable effort by the research teams, only 52.4% of survivors consented to active follow-up (see Figure 4), which is lower than observed in some other OHCA trials. 84,85 There are several potential explanations for this. Owing to the trial design and waiver of consent, patients could not be asked to consent to participate in the trial (since the intervention had already occurred). Instead, they were asked to consent to either active or passive follow-up. The lower consent rate could be the result of a lack of perceived benefit to the individual as a result of participating. 87 It may also be related to the patient’s condition, cognitive impairment post OHCA or a feeling of being overwhelmed by other clinical activities required at that time. Several strategies were proposed and adopted to look to increase the active follow-up consent rate. The trial’s patient and public research advisory group advised that we review and refine the timing of the approach for consent. It was agreed that patients should be informed of the trial and approached for consent shortly after they were discharged from the ICU or CCU, but before hospital discharge. It was recommended that all participants receive an in-person approach and invitation to consent prior to discharge from hospital, wherever possible. Seeking consent at this time and in this manner was complex and required the local research teams to carefully monitor the location and capacity of patients recovering from OHCA. This resulted in some patients being discharged from hospital before being approached. When this happened, we requested written consent by post, but this method of requesting consent resulted in a low rate of consent to active follow-up (44.2%). Other recommendations to improve the consent rate included providing a range of contact options for patients, including telephone, e-mail and postal contact details for the local research team and trial co-ordinating centre. The trial team also worked to ensure that consent and active follow-up processes were as clear and streamlined as possible.
Because interruptions in chest compressions during TI have been identified as potentially harmful in OHCA patients,13 we aimed to measure chest compression fraction as a measure of CPR quality. However, use of the compression fraction cards was low, with only a few readable cards returned (see Chapter 4, Chest compression fraction). The cards returned did not show any significant difference in chest compression fraction between the two treatment groups. However, the small numbers mean that there is considerable uncertainty in this result. Encouragingly, the chest compression fraction was comparatively high in the AIRWAYS-2 trial, indicating that good-quality basic life support was delivered by ambulance staff. Chest compression fraction is not routinely measured in OHCA practice or research in the UK, but has been recognised internationally for more than a decade. 36 In retrospect, the use of an additional and separate device may have been impractical for paramedics already participating in the AIRWAYS-2 trial, and more automated approaches to the measurement of chest compression fraction (for example through defibrillator electrodes) should be considered in future trials.
Longer-term secondary trial outcomes and the health economics analyses for this trial use data collected routinely and obtained through NHS Digital. Using routinely collected data enables the trial to undertake more substantial analyses in an efficient way without placing a large burden of direct data collection and entry on local hospital teams, and has enabled the AIRWAYS-2 trial to be one of the largest health economics analyses of OHCA patients completed to date. However, the application process to obtain these data took almost 3 years from initial submission to receipt of data. The application was complicated by several issues, including NHS Digital system challenges and staff changes, the introduction of both the General Data Protection Regulation88 and Information Governance Toolkit,89 protracted contract sign-off periods and the requirement for an amendment to the trial’s CAG approval. The delays to the application process meant that the trial team were unable to obtain an extract of routine data part-way through the trial and ultimately had to apply for extensions to the funder’s final report deadline to include the full secondary outcomes and health economics analyses. When data were received, some problems were identified in one data set that could not be resolved before submission of this report (see Chapter 2, Availability of resource use and cost data).
Trial results
In this pragmatic cluster RCT comparing a strategy of initial AAM with the i-gel versus TI, there was no significant difference in the primary outcome of favourable functional outcome after OHCA. Owing to the large sample size and randomised design, these findings are likely to be generalisable to the wider population of adult, non-traumatic OHCA when attended by NHS paramedics in England.
Paramedics are less likely to provide AAM (TI, SGA or some other method) to patients with a short duration of cardiac arrest or who receive bystander resuscitation and/or defibrillation. These patients are also considerably more likely to survive. 90 This relationship, causing confounding by indication, is an important limitation of many large observational studies that show an association between AAM and poor outcome in OHCA. 91 The phenomenon of resuscitation time bias has been well described. 90 In our trial, 21.1% (360/1704) of patients who received no AAM achieved a good outcome, compared with 3.3% (251/7576) of patients who received AAM.
At the outset, it was expected that most patients with a favourable outcome would not receive AAM and that some crossover would occur. For these reasons, two pre-specified exploratory sensitivity analyses (ITT and as treated) were undertaken in only those patients who received AAM, even though these analyses are susceptible to bias. 27,92 Patients who received AAM were similar in the two groups (see Appendix 1, Tables 33 and 34), and a strategy of i-gel first was associated with better outcomes whenever AAM was undertaken by a trial paramedic (see Appendix 1, Table 35). However, the difference between groups was less than the pre-specified clinically important difference of 2% and less than the minimum important difference of ≈ 3% reported by others. 23 The i-gel-first strategy also achieved initial ventilation success more often, with no increase in the overall rate of reported regurgitation and aspiration, although the i-gel was significantly more likely to dislodge after successful placement than a tracheal tube. 27
As described, these two exploratory analyses were subject to bias because patients who did not receive AAM were excluded and there was differential crossover in the two groups. In the light of this, a causal analysis was undertaken. The causal analysis included all trial participants and took account of the treatment received, the randomised allocations and the fact that a single paramedic could deliver the i-gel intervention but two paramedics were required for TI. The standard airway management strategy meant that paramedics had to wait for another paramedic before they could deliver TI. This meant that the number of paramedics on scene could determine a certain amount of the crossover present. Thus, the causal analysis was not subject to the same biases as the exploratory sensitivity analyses. Moreover, given that this analysis takes into account the differential adherence in the two groups, it better reflects the true level of uncertainty in the difference in outcome between the two treatment groups than the primary ITT analysis. The result of this causal analysis favoured a strategy of TI first, but with a wide 95% CI (–5.38% to 4.40%) for the treatment effect. However, while the 95% CI for ITT excluded the pre-specified clinically important 2% difference, the causal analysis does not rule out the possibility of a difference of this magnitude or higher.
A recent RCT of OHCA patients in France and Belgium tested the non-inferiority of BMV compared with early TI, with both interventions delivered by physicians as part of a pre-hospital team. The non-inferiority margin was 1% mortality. The trial was inconclusive in that it failed to establish the non-inferiority of BMV compared with TI. 85 To our knowledge, no RCT has compared BMV with a SGA in patients with OHCA. Reported rates of ventilation and TI success have been higher in previous studies,85,93,94 but these have been based on selected populations and practitioners with greater training and experience, including physicians. This trial reflects both the pragmatic reality of current paramedic practice in England and the challenges of airway management in a patient group where regurgitation and poor airway access are common. 27
The recent cluster crossover PART53 of North American patients with OHCA randomised 27 EMS to an initial strategy of using a different SGA (the laryngeal tube) or a strategy of using initial TI. The trial showed that a strategy of initial SGA insertion had significantly greater 72-hour survival rates than an initial strategy of TI. A key difference in the design of PART compared with the AIRWAYS-2 trial is the exclusion of patients judged not to require AAM. The AIRWAYS-2 trial included all patients with OHCA, irrespective of the perceived need for AAM, to avoid the risk of bias due to paramedics making different judgements about the need for AAM conditional on knowing the method of AAM to which they were allocated. In PART, it is not clear how the researchers avoided the risk of this bias, although there was little evidence that patients in the SGA and TI groups differed.
As in the AIRWAYS-2 trial, an initial strategy of SGA first also achieved higher initial ventilation success in PART. However, rates of regurgitation and aspiration during or after AAM were not reported. Following a recent systematic review of AAM in cardiac arrest,95 the International Liaison Committee on Resuscitation (ILCOR) published treatment recommendations on this topic. 96 In settings with a low TI success rate (the intubation success rate of 70% documented in this trial would be deemed low), ILCOR suggests using a SGA instead of TI for OHCA.
Loss of a previously established airway occurred twice as frequently in the i-gel group as it did in the TI group. There are some cardiac arrest patients in whom effective ventilation cannot be achieved with basic airway management techniques or a SGA and in whom TI may be the only way of achieving effective ventilation. The exact role of different AAM techniques in adults with OHCA, and the associated implications for skill acquisition and maintenance, remain to be determined. 27
The 3- and 6-month outcomes from the trial were very similar to the primary outcome measured at hospital discharge (or 30 days if sooner). 27 There was no significant difference in either the primary outcome of mRS score or the EQ-5D measure of HRQoL between the two groups at 3 and 6 months. The worst-case and imputed-case sensitivity analyses, designed to determine the potential effect of missing data, did not alter our findings.
Most clinical trials in OHCA have reported short-term outcomes only, and even the most contemporary international advisory statement describing a core outcome set for clinical trials in OHCA patients does not recommend data collection beyond 90 days, mainly because of the substantial resources required and the risk of attrition bias. 97 As a result, the natural history of survivor recovery following OHCA has been documented by only a few investigators,98–101 and there remains a need to examine the longer-term impacts of OHCA on functional status, cognition and QoL. 102,103
Several studies have documented improvements in the functional status of OHCA survivors for at least the first 3 months and up to 6 months after cardiac arrest. 101,102 Our data support this: we have shown a substantial shift in the distribution of mRS scores consistent with improving functional status between hospital discharge and 3 months, and a smaller shift in the same direction between 3 and 6 months. The number of patients with a mRS score of 5 (severe disability) shows a substantial decrease between hospital discharge and 3 months, which likely represents a combination of some patients dying (mRS score of 6) and others improving their functional status. 104
Although PART53 documented a significantly higher rate of favourable neurological outcomes among patients randomised to a strategy of initial laryngeal tube compared with TI, longer-term outcomes have not been reported, so it is unknown if this difference would have been sustained at 3 and 6 months.
Health economics
The main findings from the economic evaluation are that there is very little difference between the groups in either costs or effects and great uncertainty around the cost-effectiveness results. There was very little difference in total costs per patient between the two groups. Patients in the TI group had costs, on average, £157 less than those in the i-gel group. This difference largely relates to slightly lower intensive care costs in the TI group. Varying unit costs in sensitivity analyses had very little impact on mean cost differences, reinforcing the finding that resource use was similar between groups. Nine patients had total costs exceeding £100,000, including one patient in the TI group with total costs of £271,014; these patients exerted a modest impact on cost results but did not alter conclusions. Although only 20% of patients survived to hospital admission, inpatient and intensive care costs were the key drivers of total costs; therefore, it is disappointing that the HES critical care data set could not be used to cost time in intensive care.
The QALYs gained in each group are small, influenced by the large proportion of patients who died at an early stage in the trial and who contribute a tiny negative gain to QALYs. Sensitivity analyses around alternative assumptions for outcomes (assuming a baseline utility of zero rather than a negative value, and considering life-years rather than QALYs) did not affect the differences between the groups. The difference between the groups for QALYs is particularly small, creating a very small denominator for the ICER. Dividing the difference in costs by a tiny number, close to zero, resulted in a very large ICER (–£102,362). The point estimate in the base-case analysis suggests that TI is dominant over the i-gel because it is both more effective (very slightly greater QALY gain) and less costly and, therefore, cost-effective. The CEAC suggests that the i-gel is unlikely to be cost-effective. However, there is a great deal of uncertainty around these results. The point estimate is close to the origin and the bootstrap replicates of the cost and QALY differences cover three quadrants of the cost-effectiveness plane; in reality there is no evidence to suggest any difference between the groups.
Patient and public involvement
Patients and the public were fully engaged in the initial funding application for this trial and also during the initial feasibility trial (which this application followed). 25 The application was developed with members of an OHCA patient and public research advisory group, who contributed fully to discussions relating to information given to patients and relatives, and the consent process, and who submitted a written statement to accompany the trial’s ethics committee application. Two members of this group were members of the TSC throughout the trial.
The full patient and public research advisory group met 12 times during the trial. At each meeting, the group was provided with an update on trial progress, with feedback relating to participants’ resources, patient and public information and trial conduct. The group also contributed to discussion on how data should be analysed and presented to the public, including advice on the dissemination of the research and its findings to a wider public audience. The group remains fully active and engaged with the ongoing dissemination process.
Strengths and limitations
Strengths
To our knowledge, the AIRWAYS-2 trial is the largest RCT of airway management in OHCA to date, assessing a primary outcome of key importance to patients, clinicians and health services, adequately powered for a clinically important target difference, accompanied by a cost-effectiveness analysis and including features to minimise the risk of bias. The latter included (1) automatic enrolment, allowing us to include all eligible patients while assessing whether or not paramedics adopted a different threshold for resuscitation conditional on knowledge of their allocation and (2) blinded assessment of outcome beyond the ED. We obtained the primary outcome for 99.9% of enrolled patients. These two factors combined mean that the primary result has high validity.
Trial limitations
This trial has several limitations. First, there was an imbalance in the number of patients in the two groups, probably because of unequal distribution of a small number of ‘super-enroller’ paramedics in the two groups; it was not possible to stratify for this because ‘super-enroller’ paramedics could not be identified in advance. Second, there was crossover between groups, which was inevitable on practical and ethics grounds. Third, although other elements of care (e.g. initial basic airway management and subsequent on-scene and in-hospital care, such as targeted temperature management and access to angiography) followed established guidelines, differences in these factors between groups could have influenced the findings. Fourth, the participating paramedics were volunteers, and their airway skills may not be representative of those who chose not to take part. Fifth, the findings are applicable to use of the i-gel in countries with similar EMS provision to England, where paramedics attend most OHCAs. The findings may not be applicable in countries with physician-led EMS provision or to other SGAs, which may have different characteristics. However, the principles underpinning the insertion and function of all SGAs are similar. 27 A further limitation was that the degree of clustering was much greater than anticipated. An intracluster correlation of 0.05 (all other sample size inputs remaining unchanged) meant that we had 83% power rather than 90% to detect a difference of 2%.
In keeping with similar studies, our trial had relatively few survivors from whom to gather longer-term outcomes. Furthermore, we were reliant on both active patient consent and co-operation at 3 and 6 months to collect the required mRS and EQ-5D data. Despite considerable effort by the research teams, only 52.4% of survivors consented to active follow-up. As a result, our analyses are undermined by missing data, with limited power and the risk of attrition bias. However, the proportion of missing data was very similar in the two groups, and there was no evidence that the availability of follow-up data was influenced by patient allocation. Furthermore, the sensitivity analyses did not alter our findings to any significant degree.
Lessons for the future
We have described findings relating to trial conduct above (see Main findings, Trial conduct). We believe that these findings lead to the following lessons to be considered for future similar trials:
-
Training of paramedics should be planned carefully and include specific training aiming to reduce crossover. This trial saw differential crossover rates between the treatment groups. The impact of crossover on the interpretation of trial results should be included in paramedic training. However, this needs to be carefully balanced with an understanding that the clinical care of patients during OHCA must always remain a priority.
-
There should be consideration of the sources of outcome data, especially when considering the use of routine data sources such as HES. This trial encountered numerous difficulties in obtaining HES data, which delayed the data analysis phase of the trial. The burden of data collection by paramedics and hospital staff using CRFs should be balanced with the challenges associated with obtaining data from routine sources. The AIRWAYS-2 trial team plans to undertake a piece of work comparing data collected using CRFs with HES data to examine their accuracy.
-
The primary outcome should be carefully considered. This trial chose to use mRS score as the primary outcome instead of survival, because it was felt that survival alone was insufficient to describe the full benefits of any improvements in care. Functional status and QoL following OHCA are recognised as key outcome measures for resuscitation success. 105,106 However, having survival as a primary outcome may make the results more comparable with other trials and is less subjective than mRS score. Any relevant core outcome sets should also be considered and included where possible.
-
The rate of consent to active follow-up was lower than expected. Future trials should consider strategies to increase this consent rate, because the large number of missing data reduced the power of the follow-up analyses. The low consent rate also limited the applicability of descriptive findings about the natural history of recovery after OHCA.
-
Future trials may wish to consider including a measure of CPR quality in their design. It was not possible to collect enough CPR quality data in this trial to effectively compare the two groups, and the inability to report this is a limitation of the trial.
-
The AIRWAYS-2 trial team have published an article looking at the challenges of delivering a large-scale trial in ambulance services in England and their receiving hospitals. 26 This article should be referred to if a similar trial were to be run in the future. Challenges can be seen across all aspects of the trial. The trial design must be carefully considered, given the inevitability of enrolling patients who lack capacity to consent. This trial adopted a cluster randomised design in which each paramedic is randomised to administer one trial device to all eligible patients for the duration of the trial. Other cardiac arrest trials have randomised individual patients,107 but this has associated challenges in device studies. A cluster crossover design, in which paramedics switch their trial allocation half-way through the trial,53 can also be considered. However, in this trial, we observed relatively large numbers of paramedics joining and leaving the ambulance service throughout, which could undermine this approach. A cluster randomisation approach has advantages but, as observed in this trial, even a few ‘super-enroller’ clusters in one group can have a substantial impact on the balance of patients between the two treatment groups. Stratification based on paramedic role or a similar approach may be required. Blinding paramedics to AAM strategy used is not possible, so efforts must be made to avoid both performance and detection bias. Trial management processes can become complex when working with many participating hospitals, and we would encourage the use of electronic trial management systems where possible. This trial used electronic delegation logs and site files to minimise the use of paper, reduce duplication and increase the ease of central monitoring. Electronic data capture is also recommended where possible.
-
A measure of end-tidal CO2 and ventilation should be considered and embedded in any future trial. End-tidal CO2 was measured in this trial (see Appendix 1, Table 32) but was not reported as an outcome.
-
Owing to a lack of previous RCTs looking at OHCA, a substantial feasibility trial was undertaken prior to this main trial. 25 This was important in informing the design of the AIRWAYS-2 trial, in particular the feasibility of using cluster randomisation, and can be a useful approach to guide sample size calculations for large-scale trials when parameter estimates for a sample size calculation are uncertain. However, it is of note that the patient enrolment and survival rates seen in the REVIVE-AIRWAYS feasibility trial were not replicated in the main trial. This is thought to be have been because of local variations across health-care services and should be considered when completing sample size calculations.
-
The use of additional analyses should be considered in any future trial, especially given the richness of data sets obtained during large-scale RCTs. When writing formal SAPs, the inclusion of Bayesian and instrumental variable analyses may be considered.
Future research
The Pragmatic Airway Resuscitation Trial,53 published alongside the findings of this trial, compared another SGA (the laryngeal tube) with TI and reported 72-hour survival as the primary outcome. Given that we have collected 72-hour survival in the AIRWAYS-2 trial, we are collaborating to undertake an individual patient meta-analysis. Further research comparing SGAs with TI in OHCA are not required at this time; however, they may be required in different patient populations or settings.
We recommend the following questions for future research (in priority order):
-
A comparison of SGAs with basic airway techniques (e.g. BMV) during OHCA.
-
A comparison of SGAs with TI for cardiac arrests occurring in hospital.
-
Direct comparison of different types of SGA during cardiac arrest.
-
Comparisons of SGAs with TI during cardiac arrest in other patient groups. Although the AIRWAYS-2 trial focused on the most common cardiac arrest population (adults without trauma), there are theoretical differences that may favour different airway management techniques in children and when cardiac arrest occurs because of trauma.
As well as joint work with the PART team, we intend to compare HES data with data collected at a local level using research CRFs, report the qualitative experiences of paramedics who participated in the AIRWAYS-2 trial and complete a more detailed analysis of the AAM undertaken by paramedics attending OHCA.
Chapter 8 Conclusion
The AIRWAYS-2 trial conducted successful and ethical research in severely ill patients who lacked capacity and required immediate life-saving treatment.
Among patients with OHCA, randomisation to a strategy of AAM with an i-gel SGA compared with TI resulted in no difference in favourable functional outcome between the two groups at hospital discharge or 30 days, whichever occurred sooner.
Longer-term follow-up was consistent with the results of the primary analysis. There were no significant differences in functional outcome or QoL between the i-gel and TI groups 3 and 6 months post OHCA.
In the economic component of the trial, we concluded that there is no evidence to suggest a difference between the two groups.
Acknowledgements
The funding organisation had no role in the design and conduct of the trial; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This trial was designed and delivered in collaboration with the CTEU Bristol, a UK Clinical Research Collaboration registered clinical trials unit which, as part of the Bristol Trials Centre, is in receipt of NIHR Clinical Trials Unit support funding.
Contributions of others
Megan Rhys (SWAST, Exeter, UK; lead research paramedic in the feasibility trial) supported paramedic engagement and provided expertise when developing the protocol.
Rachel Brophy (CTEU, Bristol Medical School, University of Bristol, Bristol, UK; assistant clinical trial co-ordinator) managed patient follow-up.
Jenny Lamb (CTEU, Bristol Medical School, University of Bristol, Bristol, UK; assistant clinical trial co-ordinator) managed patient follow-up.
Abby O’Connell (CTEU, Bristol Medical School, University of Bristol, Bristol, UK; assistant clinical trial co-ordinator) managed patient follow-up.
Adam Wallis (SWAST, Exeter, UK; people analytics and development business partner) provided financial management.
Tom Hill (SWAST, Exeter, UK; management accountant) provided financial management.
Tony West (SWAST, Exeter, UK; income assistant) provided financial management.
Jonathan Green (SWAST, Exeter, UK; lead research paramedic for SWAST) delivered regional paramedic recruitment and training, managed patient screening and data collection; and was funded by this NIHR grant.
Helen Hall (EEAST, Melbourn, UK; research paramedic for EEAST) delivered regional paramedic recruitment and training, managed patient screening and data collection; and was funded by this NIHR grant.
Richard Pilbery (YAS, Wakefield, UK; research paramedic for YAS) delivered regional paramedic recruitment and training, managed patient screening and data collection; and was funded by this NIHR grant.
Gregory Adam Whitley (EMAS, Nottingham, UK; research paramedic for EMAS) delivered regional paramedic recruitment and training, managed patient screening and data collection; and was funded by this NIHR grant.
Theresa Foster (EEAST, Melbourn, UK, Research Manager for EEAST) provided research support and governance; no compensation was received for contribution to the trial.
Jane Shewan (YAS, Wakefield, UK; Head of Research for YAS) provided research support and governance.
Anne Spaight (EMAS, Nottingham, UK; Head of Clinical Governance, Audit and Research for EMAS) provided research support and governance.
Marcus Bailey (EEAST, Melbourn, UK; consultant paramedic for EEAST) acted as EEAST Principal Investigator.
Steven Dykes (YAS, Wakefield, UK; Deputy Medical Director for YAS) acted as YAS Principal Investigator.
A Niroshan Siriwardena (EMAS, Nottingham, UK; Research Lead at EMAS) acted as EMAS Principal Investigator.
Lisa Grimmer (University Hospitals Bristol NHS Foundation Trust, Bristol, UK; Regional Research Nurse in South Western Ambulance Service NHS Foundation Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Katie Sweet (University Hospitals Bristol NHS Foundation Trust, Bristol, UK; Regional Research Nurse in South Western Ambulance Service NHS Foundation Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Rosalyn Squire (University Hospitals Plymouth NHS Trust, Plymouth, UK; Regional Research Nurse in South Western Ambulance Service NHS Foundation Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Prematie Andreou (University Hospitals of Leicester NHS Trust, Leicester, UK; Regional Research Nurse in East Midlands Ambulance Service NHS Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Lucy Ryan (Nottingham University Hospitals NHS Trust, Nottingham, UK; Regional Research Nurse in East Midlands Ambulance Service NHS Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Sara Jones (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Regional Research Nurse in East of England Ambulance Service NHS Trust), co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Helen Foot (Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK; Regional Research Nurse in Yorkshire Ambulance Service NHS Trust) co-ordinated and supported data collection in receiving hospitals; funded by this NIHR grant.
Simon Gate (University of Birmingham, Birmingham, UK; chairperson and independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Charles Deakin (University of Southampton, Southampton, UK; independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Keith Douglas (patient representative and independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Margaret Douglas (patient representative and independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Gavin D Perkins (University of Warwick, Coventry, UK; independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Jasmeet Soar (Southmead Hospital, North Bristol NHS Trust, Bristol, UK; independent member of TSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Gordon Taylor (University of Exeter Medical School, Exeter, UK; chairperson and independent member of DMSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Richard Lyon (NHS Lothian, Edinburgh, UK; independent member of DMSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Andrew Newton (College of Paramedics, Bridgwater, UK; independent member of DMSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Tom Quinn (Kingston University, London, and St George’s, University of London, London, UK; independent member of DMSC) received reimbursement of travel expenses incurred during attendance at oversight meetings.
Helen Snooks (Swansea University, Swansea, UK; independent member of DMSC) received reimbursement of travel expenses incurred during attendance at oversight meetings and is a member of the NIHR HTA and Efficacy and Mechanism Evaluation editorial board.
Contributions of authors
Professor Jonathan R Benger (https://orcid.org/0000-0001-6131-0916) (Professor of Emergency Care and Consultant in Emergency Medicine) had full access to all data in the trial, took responsibility for the integrity of the data and the accuracy of the data analysis, was involved in the trial concept and design, interpreted the data and drafted and critically revised this manuscript.
Ms Kim Kirby (https://orcid.org/0000-0002-8092-7978) (NIHR Clinical Doctoral Research Trainee and Lead Research Paramedic) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Ms Sarah Black (https://orcid.org/0000-0001-6678-7502) (Research and Audit Manager) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Dr Stephen J Brett (https://orcid.org/0000-0003-4545-8413) (Head of Research for the Directorate of Anaesthetics and Critical Care) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Ms Madeleine Clout (https://orcid.org/0000-0001-7645-1199) (Clinical Trial Manager) interpreted the data, drafted and critically revised the manuscript and provided technical and project support.
Ms Michelle J Lazaroo (https://orcid.org/0000-0002-8893-0522) (Medical Statistician) interpreted the data, critically revised the manuscript and undertook statistical analysis.
Dr Jerry P Nolan (https://orcid.org/0000-0003-3141-3812) (Consultant in Anaesthesia and Intensive Care Medicine) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Professor Barnaby C Reeves (https://orcid.org/0000-0002-5101-9487) (Professor in Health Services Research) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Ms Maria Robinson (https://orcid.org/0000-0002-6978-8575) (Research Manager) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Ms Lauren J Scott (https://orcid.org/0000-0003-3129-5123) (Research Associate in Medical Statistics) was involved in the trial concept and design, interpreted the data, critically revised the manuscript and undertook statistical analysis.
Dr Helena Smartt (https://orcid.org/0000-0001-5285-2262) (Medical Statistician/Data Manager) interpreted the data, critically revised the manuscript and undertook statistical analysis.
Mr Adrian South (https://orcid.org/0000-0003-2121-8829) (Deputy Clinical Director) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Dr Elizabeth A Stokes (https://orcid.org/0000-0002-4179-1369) (Senior Researcher) was involved in the trial concept and design, interpreted the data, critically revised the manuscript and undertook the health economic analysis.
Dr Jodi Taylor (https://orcid.org/0000-0001-7171-8923) (Research Associate in Clinical Trials Management) was involved in the trial concept and design, interpreted the data, drafted and critically revised the manuscript and provided technical and project support.
Dr Matthew Thomas (https://orcid.org/0000-0002-3299-3495) (Consultant in Anaesthesia and Intensive Care) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Dr Sarah Voss (https://orcid.org/0000-0001-5044-5145) (Senior Research Fellow in Emergency Care) was involved in the trial conception and design, interpreted the data and critically revised the manuscript.
Professor Sarah Wordsworth (https://orcid.org/0000-0002-2361-3040) (Professor of Health Economics) was involved in the trial concept and design, interpreted the data and critically revised the manuscript.
Professor Chris A Rogers (https://orcid.org/0000-0002-9624-2615) (Reader in Medical Statistics and Consultant Statistician) had full access to the data in the trial, took responsibility for the integrity of the data and the accuracy of the data analysis, was involved in the trial concept and design, interpreted the data, critically revised the manuscript and undertook statistical analysis.
Publications
Taylor J, Black S, J Brett S, Kirby K, Nolan JP, Reeves BC, et al. Design and implementation of the AIRWAYS-2 trial: a multi-centre cluster randomised controlled trial of the clinical and cost effectiveness of the i-gel supraglottic airway device versus tracheal intubation in the initial airway management of out of hospital cardiac arrest. Resuscitation 2016;109:25–32.
Benger JR, Kirby K, Black S, Brett SJ, Clout M, Lazaroo MJ, et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the airways-2 randomized clinical trial. JAMA 2018;320:779–91.
Robinson MJ, Taylor J, Brett SJ, Nolan JP, Thomas M, Reeves BC, et al. Design and implementation of a large and complex trial in emergency medical services. Trials 2019;20:108.
Benger JR, Lazaroo MJ, Clout M, Voss S, Black S, Brett SJ, et al. Randomized trial of the i-gel supraglottic airway device versus tracheal intubation during out of hospital cardiac arrest (AIRWAYS-2): patient outcomes at three and six months. Resuscitation 2020;157:74–82.
Green J, Robinson M, Pilbery R, Whitley G, Hall H, Clout M, et al. Research paramedics’ observations regarding the challenges and strategies employed in the implementation of a large-scale out-of-hospital randomised trial. Br Paramed J 2020;5:26–31.
Kirby K, Brandling J, Robinson M, Thomas, M, Voss S and Benger J. The experiences of EMS providers taking part in a large randomised trial of airway management during out of hospital cardiac arrest, and the impact on their views and practice. Results of a survey and telephone interviews. Resuscitation 2020;149:1–9.
Stokes EA, Lazaroo MJ, Clout M, Brett SJ, Black S, Kirby K, et al. Cost-effectiveness of the i-gel supraglottic airway device compared to tracheal intubation during out-of-hospital cardiac arrest: findings from the AIRWAYS-2 randomised controlled trial [published online ahead of print June 10 2021]. Resuscitation 2021.
Data-sharing statement
All data enquiries and requests should be submitted to the corresponding author for consideration in the first instance. Data requests should include a pre-specified protocol describing the purpose, methods and analysis of the planned research and analysis (e.g. a protocol for a Cochrane systematic review). Access to anonymised data may be granted following review and assurances being in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Taylor J, Black S, J Brett S, Kirby K, Nolan JP, Reeves BC, et al. Design and implementation of the AIRWAYS-2 trial: a multi-centre cluster randomised controlled trial of the clinical and cost effectiveness of the i-gel supraglottic airway device versus tracheal intubation in the initial airway management of out of hospital cardiac arrest. Resuscitation 2016;109:25-32. https://doi.org/10.1016/j.resuscitation.2016.09.016.
- Woollard M. Public access defibrillation: a shocking idea?. J Public Health Med 2001;23:98-102. https://doi.org/10.1093/pubmed/23.2.98.
- Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479-87. https://doi.org/10.1016/j.resuscitation.2010.08.006.
- NHS England . Ambulance Quality Indicators Data 2015–16 n.d. www.england.nhs.uk/statistics/statistical-work-areas/ambulance-quality-indicators/ambulance-quality-indicators-data-2015-16/ (accessed 1 August 2018).
- Department of Health and Social Care . Ambulance Quality Indicators Data Downloads n.d. http://webarchive.nationalarchives.gov.uk/20130402162554/http://transparency.dh.gov.uk/2012/06/19/ambqidownloads/ (accessed 1 August 2018).
- London Ambulance Service NHS Trust . Cardiac Arrest Annual Report 2015 16 n.d. www.emergency-live.com/wp-content/uploads/2017/03/Cardiac-Arrest-Annual-Report-2015-16-Final.pdf (accessed 5 June 2021).
- Gräsner JT, Lefering R, Koster RW, Masterson S, Böttiger BW, Herlitz J, et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation 2016;105:188-95. https://doi.org/10.1016/j.resuscitation.2016.06.004.
- Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. The neurological and cognitive sequelae of cardiac arrest. Neurology 2004;63:1774-8. https://doi.org/10.1212/01.WNL.0000144189.83077.8E.
- van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J 2004;148:416-21. https://doi.org/10.1016/j.ahj.2004.01.031.
- Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation 2011;124:2158-77. https://doi.org/10.1161/CIR.0b013e3182340239.
- Wang HE, Kupas DF, Paris PM, Bates RR, Yealy DM. Preliminary experience with a prospective, multi-centered evaluation of out-of-hospital endotracheal intubation. Resuscitation 2003;58:49-58. https://doi.org/10.1016/S0300-9572(03)00058-3.
- Nolan JP, Soar J. Airway techniques and ventilation strategies. Curr Opin Crit Care 2008;14:279-86. https://doi.org/10.1097/MCC.0b013e3282f85bc8.
- Wang HE, Simeone SJ, Weaver MD, Callaway CW. Interruptions in cardiopulmonary resuscitation from paramedic endotracheal intubation. Ann Emerg Med 2009;54:645-52.e1. https://doi.org/10.1016/j.annemergmed.2009.05.024.
- Lyon RM, Ferris JD, Young DM, McKeown DW, Oglesby AJ, Robertson C. Field intubation of cardiac arrest patients: a dying art?. Emerg Med J 2010;27:321-3. https://doi.org/10.1136/emj.2009.076737.
- Kurola J, Harve H, Kettunen T, Laakso JP, Gorski J, Paakkonen H, et al. Airway management in cardiac arrest – comparison of the laryngeal tube, tracheal intubation and bag-valve mask ventilation in emergency medical training. Resuscitation 2004;61:149-53. https://doi.org/10.1016/j.resuscitation.2004.01.014.
- Verghese C, Brimacombe JR. Survey of laryngeal mask airway usage in 11,910 patients: safety and efficacy for conventional and nonconventional usage. Anesth Analg 1996;82:129-33. https://doi.org/10.1097/00000539-199601000-00023.
- Cook T, Howes B. Supraglottic airway devices: recent advances. Cont Ed Anaesth Crit Care Pain 2011;11:56-61. https://doi.org/10.1093/bjaceaccp/mkq058.
- Nicholson A, Cook TM, Smith AF, Lewis SR, Reed SS. Supraglottic airway devices versus tracheal intubation for airway management during general anaesthesia in obese patients. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD010105.pub2.
- Soar J, Nolan JP. Airway management in cardiopulmonary resuscitation. Curr Opin Crit Care 2013;19:181-7. https://doi.org/10.1097/MCC.0b013e328360ac5e.
- Wang HE, Yealy DM. Managing the airway during cardiac arrest. JAMA 2013;309:285-6. https://doi.org/10.1001/jama.2012.216998.
- Gazmuri RJ, Nolan JP, Nadkarni VM, Arntz HR, Billi JE, Bossaert L, et al. Scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care identified during the 2005 International Consensus Conference on ECC and CPR Science with Treatment Recommendations. A consensus statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Stroke Council; and the Cardiovascular Nursing Council. Resuscitation 2007;75:400-11. https://doi.org/10.1016/j.resuscitation.2007.09.008.
- Kleinman ME, Perkins GD, Bhanji F, Billi JE, Bray JE, Callaway CW, et al. ILCOR scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care: a consensus statement. Resuscitation 2018;127:132-46. https://doi.org/10.1016/j.resuscitation.2018.03.021.
- Nichol G, Brown SP, Perkins GD, Kim F, Sterz F, Broeckel Elrod JA, et al. What change in outcomes after cardiac arrest is necessary to change practice? Results of an international survey. Resuscitation 2016;107:115-20. https://doi.org/10.1016/j.resuscitation.2016.08.004.
- Resuscitation Council UK. Resuscitation Guidelines n.d. www.resus.org.uk/resuscitation-guidelines/ (accessed 1 August 2018).
- Benger J, Coates D, Davies S, Greenwood R, Nolan J, Rhys M, et al. Randomised comparison of the effectiveness of the laryngeal mask airway supreme, i-gel and current practice in the initial airway management of out of hospital cardiac arrest: a feasibility study. Br J Anaesth 2016;116:262-8. https://doi.org/10.1093/bja/aev477.
- Robinson MJ, Taylor J, Brett SJ, Nolan JP, Thomas M, Reeves BC, et al. Design and implementation of a large and complex trial in emergency medical services. Trials 2019;20. https://doi.org/10.1186/s13063-019-3203-0.
- Benger JR, Kirby K, Black S, Brett SJ, Clout M, Lazaroo MJ, et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. JAMA 2018;320:779-91. https://doi.org/10.1001/jama.2018.11597.
- Association of Ambulance Chief Executives and Joint Royal Colleges Ambulance Liaison Committee . JRCALC Clinical Guidelines 2019 2019.
- Duckett J, Fell P, Han K, Kimber C, Taylor C. Introduction of the I-gel supraglottic airway device for prehospital airway management in a UK ambulance service. Emerg Med J 2014;31:505-7. https://doi.org/10.1136/emermed-2012-202126.
- Häske D, Schempf B, Gaier G, Niederberger C. Performance of the i-gel™ during pre-hospital cardiopulmonary resuscitation. Resuscitation 2013;84:1229-32. https://doi.org/10.1016/j.resuscitation.2013.04.025.
- Wang HE, Szydlo D, Stouffer JA, Lin S, Carlson JN, Vaillancourt C, et al. Endotracheal intubation versus supraglottic airway insertion in out-of-hospital cardiac arrest. Resuscitation 2012;83:1061-6. https://doi.org/10.1016/j.resuscitation.2012.05.018.
- Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation 2011;82:247-56. https://doi.org/10.1016/j.resuscitation.2010.10.030.
- Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation 2011;82:1036-40. https://doi.org/10.1016/j.resuscitation.2011.03.034.
- Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA 2013;310:1377-84. https://doi.org/10.1001/jama.2013.278483.
- Hostler D, Everson-Stewart S, Rea TD, Stiell IG, Callaway CW, Kudenchuk PJ, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. BMJ 2011;342. https://doi.org/10.1136/bmj.d512.
- Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation 2009;120:1241-7. https://doi.org/10.1161/CIRCULATIONAHA.109.852202.
- Lyon RM, Clarke S, Gowens P, Egan G, Clegg GR. Resuscitation quality assurance for out-of-hospital cardiac arrest – setting-up an ambulance defibrillator telemetry network. Resusc 2010;81:1726-8. https://doi.org/10.1016/j.resuscitation.2010.09.007.
- European Medicines Agency (EMA) . Guideline for Good Clinical Practice E6(R2) 2016. https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/ema-gcp-guidance.pdf (accessed 22 April 2021).
- Benger JR, Voss S, Coates D, Greenwood R, Nolan J, Rawstorne S, et al. Randomised comparison of the effectiveness of the laryngeal mask airway supreme, i-gel and current practice in the initial airway management of prehospital cardiac arrest (REVIVE-Airways): a feasibility study research protocol. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002467.
- Association of Ambulance Chief Executives . Measuring Patient Outcomes: Clinical Quality Indicators n.d. https://aace.org.uk/uk-ambulance-service/national-performance/ (accessed 24 July 2021).
- Department of Health and Social Care . Technical Guidance for the 2011 12 Operating Framework for the NHS 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216304/dh_123660.pdf (accessed 10 May 2021).
- Warwick Clinical Trials Unit . OHCAO: Project Overview n.d. https://warwick.ac.uk/fac/sci/med/research/ctu/trials/ohcao/overview/ (accessed 24 June 2018).
- Tagami T, Hirata K, Takeshige T, Matsui J, Takinami M, Satake M, et al. Implementation of the fifth link of the chain of survival concept for out-of-hospital cardiac arrest. Circulation 2012;126:589-97. https://doi.org/10.1161/CIRCULATIONAHA.111.086173.
- Taljaard M, Weijer C, Grimshaw JM, Eccles MP. Ottawa Ethics of Cluster Randomised Trials Consensus Group . The Ottawa Statement on the ethical design and conduct of cluster randomised trials: precis for researchers and research ethics committees. BMJ 2013;346. https://doi.org/10.1136/bmj.f2838.
- Campbell MK, Elbourne DR, Altman DG. CONSORT group . CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. https://doi.org/10.1136/bmj.328.7441.702.
- Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328-40. https://doi.org/10.1016/j.resuscitation.2014.11.002.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods 2006;11:54-71. https://doi.org/10.1037/1082-989X.11.1.54.
- Cameron AC, Gelbach JB, Miller DL. Bootstrap-based improvements for inference with clustered errors. Rev Econ Stat 2008;90:414-27. https://doi.org/10.1162/rest.90.3.414.
- Hyslop T. SAS Macros for Bootstrap Samples With Stratification and Multiple Observations Per Subject n.d. www.lexjansen.com/nesug/nesug95/NESUG95148.pdf (accessed 10 May 2021).
- Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252-66. https://doi.org/10.1002/sim.7654.
- College of Paramedics Working Group . Consensus Statement 2018. www.collegeofparamedics.co.uk/COP/Professional_development/Intubation_Consensus_Statement_/COP/ProfessionalDevelopment/Intubation_Consensus_Statement_.aspx?hkey=5c999b6b-274b-42d3-8dbc-651c367c0493 (accessed 10 May 2021).
- Wang HE, Schmicker RH, Daya MR, Stephens SW, Idris AH, Carlson JN, et al. Effect of a strategy of initial laryngeal tube insertion vs. endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest: a randomized clinical trial. JAMA 2018;320:769-78. https://doi.org/10.1001/jama.2018.7044.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2015.
- National Institute for Health and Care Excellence (NICE) . Social Value Judgments: Principles for the Development of NICE Guidance 2008.
- NHS Supply Chain . National Catalogue n.d. https://my.supplychain.nhs.uk/Catalogue/browse/0/browsecatalogue (accessed 5 June 2021).
- NHS Employers . Agenda for Change Pay Scales and Points Poster 2017 18 n.d. www.nhsemployers.org/case-studies-and-resources/2017/03/agenda-for-change-pay-scales-and-points-april-2017 (accessed 3 April 2018).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017 18 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2016 17 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 3 April 2020).
- Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. PharmacoEconomics 2017;35:501-15. https://doi.org/10.1007/s40273-016-0485-x.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Carpenter JR, Goldstein H, Kenward MG. REALCOM-IMPUTE software for multilevel multiple imputation with mixed response types. J Stat Software 2011;45. https://doi.org/10.18637/jss.v045.i05.
- Díaz-Ordaz K, Kenward MG, Grieve R. Handling missing values in cost effectiveness analyses that use data from cluster randomized trials. J Royal Stat Soc: Series A 2014;177:457-74. https://doi.org/10.1111/rssa.12016.
- Gomes M, Díaz-Ordaz K, Grieve R, Kenward MG. Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies: an application to cluster randomized trials. Med Decis Making 2013;33:1051-63. https://doi.org/10.1177/0272989X13492203.
- Rubin DB. Multiple Imputation for Non-response in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987.
- Gomes M, Ng ES, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decision Making 2012;32:350-61. https://doi.org/10.1177/0272989X11418372.
- Ng ES, Diaz-Ordaz K, Grieve R, Nixon RM, Thompson SG, Carpenter JR. Multilevel models for cost-effectiveness analyses that use cluster randomised trial data: an approach to model choice. Stat Methods Med Res 2016;25:2036-52. https://doi.org/10.1177/0962280213511719.
- Her Majesty’s Government of the United Kingdom of Great Britain and Northern Ireland . Mental Health Act 2007 n.d. www.legislation.gov.uk/ukpga/2007/12/contents (accessed 24 July 2021).
- Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. https://doi.org/10.1016/S0140-6736(14)61886-9.
- Great Britain . The Medicines for Human Use (Clinical Trials) Amendment (No.2) Regulations 2006 2006.
- Lerner EB, Weik T, Edgerton EA. Research in prehospital care: overcoming the barriers to success. Prehosp Emerg Care 2016;20:448-53. https://doi.org/10.3109/10903127.2014.980480.
- Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 2018;379:711-21. https://doi.org/10.1056/NEJMoa1806842.
- Jabre P, Penaloza A, Pinero D, Duchateau FX, Borron SW, Javaudin F, et al. Effect of bag-mask ventilation vs endotracheal intubation during cardiopulmonary resuscitation on neurological outcome after out-of-hospital cardiorespiratory arrest: a randomized clinical trial. JAMA 2018;319:779-87. https://doi.org/10.1001/jama.2018.0156.
- Higgs A, McGrath BA, Goddard C, Rangasami J, Suntharalingam G, Gale R, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth 2018;120:323-52. https://doi.org/10.1016/j.bja.2017.10.021.
- Moorcraft SY, Marriott C, Peckitt C, Cunningham D, Chau I, Starling N, et al. Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey. Trials 2016;17. https://doi.org/10.1186/s13063-015-1105-3.
- The European Parliament and the Council of the European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) n.d. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679 (accessed 24 July 2021).
- NHS Digital . Data Security and Protection Toolkit n.d. https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit (accessed 24 July 2021).
- Andersen LW, Grossestreuer AV, Donnino MW. ‘Resuscitation time bias’ – a unique challenge for observational cardiac arrest research. Resuscitation 2018;125:79-82. https://doi.org/10.1016/j.resuscitation.2018.02.006.
- Carlson JN, Reynolds JC. Does advanced airway management improve outcomes in adult out-of-hospital cardiac arrest?. Ann Emerg Med 2014;64:163-4. https://doi.org/10.1016/j.annemergmed.2013.12.003.
- Swanson SA, Robins JM, Miller M, Hernán MA. Selecting on treatment: a pervasive form of bias in instrumental variable analyses. Am J Epidemiol 2015;181:191-7. https://doi.org/10.1093/aje/kwu284.
- Hubble MW, Brown L, Wilfong DA, Hertelendy A, Benner RW, Richards ME. A meta-analysis of prehospital airway control techniques part I: orotracheal and nasotracheal intubation success rates. Prehosp Emerg Care 2010;14:377-401. https://doi.org/10.3109/10903121003790173.
- Dyson K, Bray JE, Smith K, Bernard S, Straney L, Nair R, et al. Paramedic intubation experience is associated with successful tube placement but not cardiac arrest survival. Ann Emerg Med 2017;70:382-90.e1. https://doi.org/10.1016/j.annemergmed.2017.02.002.
- Granfeldt A, Avis SR, Nicholson TC, Holmberg MJ, Moskowitz A, Coker A, et al. Advanced airway management during adult cardiac arrest: a systematic review. Resuscitation 2019;139:133-43. https://doi.org/10.1016/j.resuscitation.2019.04.003.
- Soar J, Maconochie I, Wyckoff MH, Olasveengen TM, Singletary EM, Greif R, et al. 2019 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation 2019;145:95-150. https://doi.org/10.1016/j.resuscitation.2019.10.016.
- Haywood K, Whitehead L, Nadkarni VM, Achana F, Beesems S, Böttiger BW, et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Circulation 2018;137:e783-e801. https://doi.org/10.1161/CIR.0000000000000562.
- Smith K, Andrew E, Lijovic M, Nehme Z, Bernard S. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation 2015;131:174-81. https://doi.org/10.1161/CIRCULATIONAHA.114.011200.
- Andrew E, Nehme Z, Wolfe R, Bernard S, Smith K. Long-term survival following out-of-hospital cardiac arrest. Heart 2017;103:1104-10. https://doi.org/10.1136/heartjnl-2016-310485.
- Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33°C versus 36°C. Circulation 2015;131:1340-9. https://doi.org/10.1161/CIRCULATIONAHA.114.014414.
- Steinbusch CVM, van Heugten CM, Rasquin SMC, Verbunt JA, Moulaert VRM. Cognitive impairments and subjective cognitive complaints after survival of cardiac arrest: a prospective longitudinal cohort study. Resuscitation 2017;120:132-7. https://doi.org/10.1016/j.resuscitation.2017.08.007.
- Tong JT, Eyngorn I, Mlynash M, Albers GW, Hirsch KG. Functional neurologic outcomes change over the first 6 months after cardiac arrest. Crit Care Med 2016;44:e1202-e1207. https://doi.org/10.1097/CCM.0000000000001963.
- Lim C, Verfaellie M, Schnyer D, Lafleche G, Alexander MP. Recovery, long-term cognitive outcome and quality of life following out-of-hospital cardiac arrest. J Rehabil Med 2014;46:691-7. https://doi.org/10.2340/16501977-1816.
- Arrich J, Zeiner A, Sterz F, Janata A, Uray T, Richling N, et al. Factors associated with a change in functional outcome between one month and six months after cardiac arrest: a retrospective cohort study. Resuscitation 2009;80:876-80. https://doi.org/10.1016/j.resuscitation.2009.04.045.
- Stiell I, Nichol G, Wells G, De Maio V, Nesbitt L, Blackburn J, et al. Health-related quality of life is better for cardiac arrest survivors who received citizen cardiopulmonary resuscitation. Circulation 2003;108:1939-44. https://doi.org/10.1161/01.CIR.0000095028.95929.B0.
- Stiell IG, Nesbitt LP, Nichol G, Maloney J, Dreyer J, Beaudoin T, et al. Comparison of the Cerebral Performance Category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med 2009;53:241-8. https://doi.org/10.1016/j.annemergmed.2008.03.018.
- Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther AM, et al. Pre-hospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug administration In Cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation 2016;108:75-81. https://doi.org/10.1016/j.resuscitation.2016.08.029.
- NHS Digital . HRG4+ 2017 18 Reference Costs Grouper n.d. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing-hrg4-2017-18-reference-costs-grouper (accessed 24 July 2021).
- South Western Ambulance Service NHS Foundation Trust . Annual Report and Accounts 1 April 2018–31 March 2019 2019. www.england.nhs.uk/wp-content/uploads/2019/10/South_Western_Ambulance_Service_NHS_FT_Annual_Report_and_Accounts_2018-19.pdf (accessed 10 May 2021).
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life. London: Nuffield Trust; 2014.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
Appendix 1 Additional tables and figures
| Paramedic recruitment details | Ambulance trust | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Paramedic expressed interest, n | 697 | 456 | 458 | 430 |
| Paramedic did not book onto training, n/N (%) | 147/697 (21.0) | 95/456 (20.8) | 111/458 (24.2) | 111/430 (25.8) |
| Paramedic did not attend training, n/N (%) | 33/697 (4.7) | 0/456 (0.0) | 10/458 (2.2) | 5/430 (1.2) |
| Paramedic did not consent to participate, n/N (%) | 6/697 (0.9) | 0/456 (0.0) | 0/458 (0.0) | 0/430 (0.0) |
| Paramedic clusters recruited and randomised, n/N (%) | 511/697 (73.3) | 361/456 (79.2) | 337/458 (73.6) | 314/430 (73.0) |
| Paramedic withdrew post randomisation, n/N (%) | 39/511 (7.6) | 15/361 (4.2) | 35/337 (10.4) | 24/314 (7.6) |
| Left ambulance trust, n/N (%) | 18/39 (46.2) | 9/15 (60.0) | 27/35 (77.1) | 16/24 (66.7) |
| Did not want to follow algorithm, n/N (%) | 7/39 (17.9) | 2/15 (13.3) | 1/35 (2.9) | 3/24 (12.5) |
| Repeatedly failed to enrol eligible patients, n/N (%) | 2/39 (5.1) | 1/15 (6.7) | 1/35 (2.9) | 0/24 (0.0) |
| Pregnancy, n/N (%) | 4/39 (10.3) | 0/15 (0.0) | 0/35 (0.0) | 2/24 (8.3) |
| Not enough time, n/N (%) | 2/39 (5.1) | 0/15 (0.0) | 1/35 (2.9) | 0/24 (0.0) |
| Dead, n/N (%) | 1/39 (2.6) | 1/15 (6.7) | 0/35 (0.0) | 0/24 (0.0) |
| Other, n/N (%) | 3/39 (7.7) | 0/15 (0.0) | 2/35 (5.7) | 2/24 (8.3) |
| Patient enrolment details | Ambulance trust | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| No reason given, n/N (%) | 2/39 (5.1) | 2/15 (13.3) | 3/35 (8.6) | 1/24 (4.2) |
| Number of patients with OHCA attended by a randomised paramedic | 7219 | 7662 | 6226 | 5269 |
| Resuscitation attempted, n/N (%) | 3900/7219 (54.0) | 3950/7662 (51.6) | 3075/6226 (49.4) | 2537/5269 (48.1) |
| Resuscitation not attempted, n/N (%) | 3319/7219 (46.0) | 3712/7662 (48.4) | 3151/6226 (50.6) | 2732/5269 (51.9) |
| Futile, n | 125 | 1334 | 2317 | 298 |
| Rigor mortis, n | 1078 | 1153 | 285 | 1402 |
| Patient wishes, n | 570 | 683 | 471 | 595 |
| Hypostasis, n | 955 | 697 | 30 | 258 |
| Death expected, n | 160 | 111 | 87 | 126 |
| Decomposition, n | 31 | 52 | 12 | 53 |
| Cranial destruction, n | 39 | 25 | 2 | 18 |
| Truncal injury, n | 14 | 5 | 1 | 21 |
| Incineration, n | 7 | 3 | 1 | 4 |
| Submersion, n | 7 | 2 | 1 | 4 |
| Hemicorporectomy, n | 1 | 1 | 1 | 5 |
| Other reason,a n | 1169 | 1 | 2 | 36 |
| Reason unknown, n | 0 | 1 | 0 | 0 |
| Eligible, n/N (%) | 2491/3900 (63.9) | 2918/3950 (73.9) | 2219/3075 (72.2) | 1668/2537 (65.7) |
| Ineligible, n/N (%) | 1409/3900 (36.1) | 1032/3950 (26.1) | 854/3075 (27.8) | 869/2537 (34.3) |
| Eligibility status unknown, n/N (%) | 0/3900 (0.0) | 0/3950 (0.0) | 2/3075 (0.1) | 0/2537 (0.0) |
| Trial paramedic second on scene and airway management started, n | 614 | 236 | 349 | 360 |
| Trial paramedic not first or second on scene, n | 416 | 462 | 228 | 313 |
| Traumatic OHCA, n | 219 | 220 | 151 | 122 |
| Aged < 18 years, n | 73 | 25 | 76 | 62 |
| Resuscitation not commenced or continued by ambulance staff or responder, n | 62 | 38 | 32 | 9 |
| Mouth open < 2 cm, n | 31 | 23 | 15 | 15 |
| In-hospital cardiac arrest, n | 32 | 24 | 11 | 4 |
| Detained by HMPS, n | 13 | 11 | 8 | 10 |
| Not an OHCA, n | 17 | 0 | 14 | 1 |
| Previously enrolled to trial, n | 1 | 0 | 0 | 0 |
| Previously expressed wish not to participate, n | 0 | 0 | 0 | 0 |
| Number of patients with primary outcome data, n/N (%) | 2490/2491 (100.0) | 2916/2918 (99.9) | 2216/2219 (99.9) | 1667/1668 (99.9) |
| Consented to active follow-up, n | 143 | 105 | 79 | 76 |
| Consented to passive follow-up, n | 20 | 17 | 10 | 9 |
| Ambulance trust | Month and year of randomisation, n | Total | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||||||||||||||||||||||||||
| June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | ||
| 1 | 70 | 62 | 68 | 89 | 75 | 88 | 105 | 120 | 108 | 111 | 95 | 106 | 104 | 79 | 92 | 96 | 92 | 121 | 96 | 119 | 81 | 86 | 104 | 88 | 92 | 105 | 39 | 2491 |
| 2 | 26 | 112 | 107 | 100 | 98 | 123 | 127 | 132 | 139 | 114 | 108 | 126 | 105 | 112 | 102 | 125 | 105 | 118 | 147 | 166 | 145 | 116 | 90 | 80 | 102 | 70 | 23 | 2918 |
| 3 | 40 | 90 | 88 | 88 | 102 | 107 | 107 | 123 | 92 | 116 | 76 | 88 | 88 | 79 | 59 | 64 | 86 | 98 | 100 | 97 | 77 | 66 | 76 | 67 | 66 | 59 | 20 | 2219 |
| 4 | 0 | 65 | 80 | 58 | 89 | 70 | 99 | 65 | 57 | 82 | 74 | 51 | 62 | 74 | 84 | 65 | 78 | 77 | 64 | 79 | 62 | 49 | 60 | 30 | 41 | 35 | 18 | 1668 |
| Estimated total | 166 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 380 | 10,046 |
| Actual total | 136 | 329 | 343 | 335 | 364 | 388 | 438 | 440 | 396 | 423 | 353 | 371 | 359 | 344 | 337 | 350 | 361 | 414 | 407 | 461 | 365 | 317 | 330 | 265 | 301 | 269 | 100 | 9296 |
FIGURE 22.
Predicted and actual enrolment by time point and ambulance trust. Based on a target enrolment of 95 patients per ambulance trust per month. This was calculated based on the estimate of 1300 paramedics enrolling seven patients over the 2-year period of the trial.
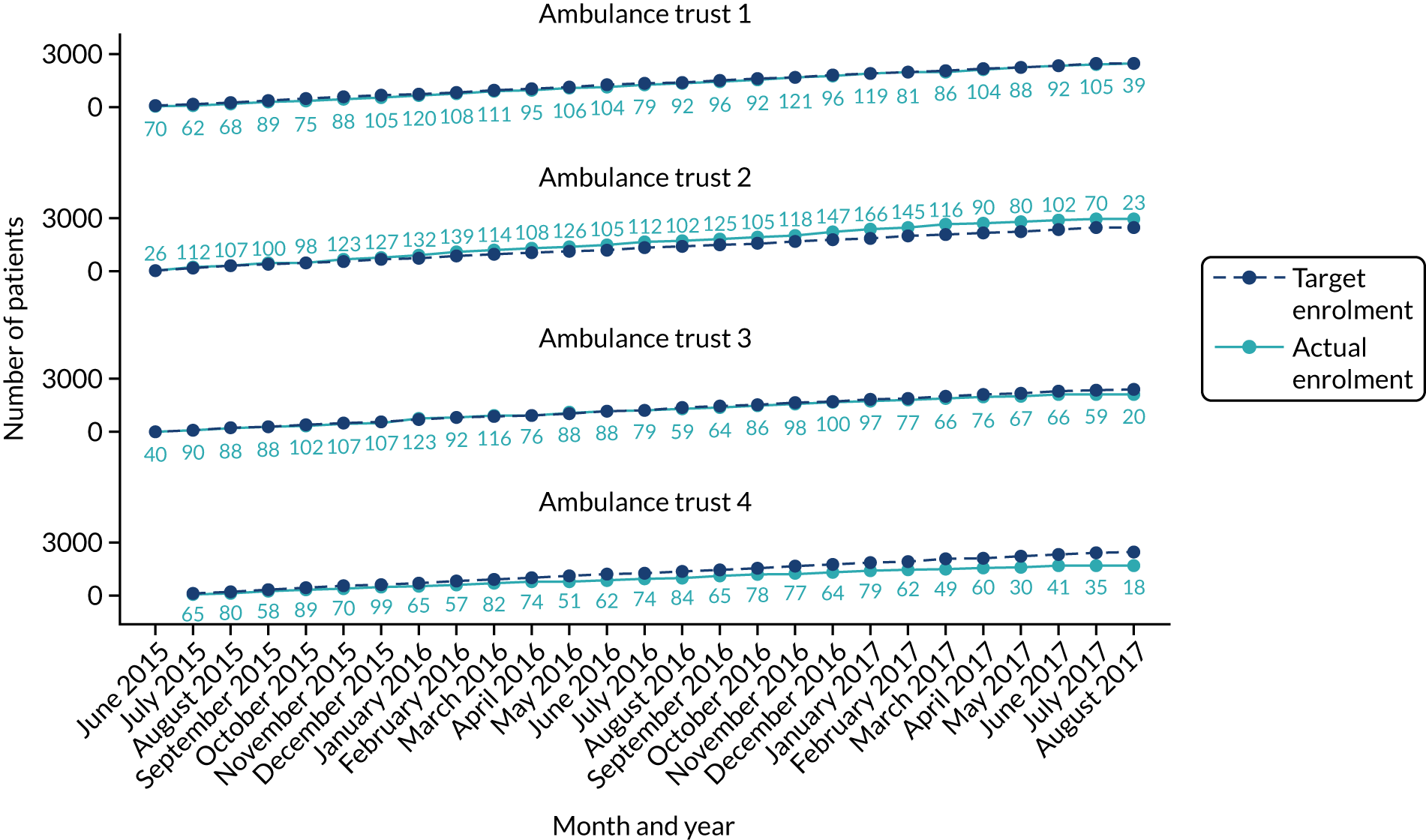
| Number of patients with data at the respective time points | TI, n/N (%) | i-gel, n/N (%) | Overall, n/N (%) |
|---|---|---|---|
| mRS score | |||
| 30 days post cardiac arrest/hospital discharge | 4407/4410 (99.9) | 4882/4886 (99.9) | 9289/9296 (99.9) |
| 3 months post cardiac arrest | 4199/4410 (95.2) | 4636/4886 (94.9) | 8835/9296 (95.0) |
| 6 months post cardiac arrest | 4212/4410 (95.5) | 4661/4886 (95.4) | 8873/9296 (95.4) |
| Single summary index | |||
| 30 days post cardiac arrest/hospital discharge | 4205/4410 (95.4) | 4672/4886 (95.6) | 8877/8924 (95.5) |
| 3 months post cardiac arrest | 4199/4410 (95.2) | 4638/4886 (94.9) | 8837/8924 (95.1) |
| 6 months post cardiac arrest | 4213/4410 (95.5) | 4657/4886 (95.3) | 8870/8924 (95.4) |
| EQ-5D VAS score | |||
| 30 days post cardiac arrest/hospital discharge | 4208/4410 (95.4) | 4669/4886 (95.6) | 8877/8924 (95.5) |
| 3 months post cardiac arrest | 4201/4410 (95.3) | 4639/4886 (94.9) | 8840/8924 (95.1) |
| 6 months post cardiac arrest | 4217/4410 (95.6) | 4662/4886 (95.4) | 8879/8924 (95.5) |
| Patients included in analyses | Was this outcome formally compared? | Number of patients for whom data were available, n/N (%) | ||
|---|---|---|---|---|
| TI group | i-gel group | Overall | ||
| Primary outcome | ||||
| mRS score at 30 days/hospital discharge | Yes, formally compared | 4407/4410 (99.9) | 4882/4886 (99.9) | 9289/9296 (99.9) |
| Secondary outcome | ||||
| Initial ventilation success | Yes, formally compared | 4397/4410 (99.7) | 4868/4886 (99.6) | 9265/9296 (99.7) |
| Regurgitation at any time | Yes, formally compared | 4372/4410 (99.1) | 4865/4886 (99.6) | 9237/9296 (99.4) |
| Aspiration at any time | Yes, formally compared | 4337/4410 (98.3) | 4824/4886 (98.7) | 9161/9296 (98.5) |
| Any loss of a previously established airwaya | Yes, formally compared | 3081/3419 (90.1) | 3900/4161 (93.7) | 6981/7580 (92.1) |
| Actual sequence of airway interventions delivered | Descriptive only | 3686/4410 (83.6) | 4321/4886 (88.4) | 8007/9296 (86.1) |
| Chest compression fraction | Yes, formally compared | 32/608 (5.3) | 34/631 (5.4) | 66/1239 (5.3) |
| ROSC during/after AAMa | Yes, formally compared | 3416/3419 (99.9) | 4155/4161 (99.9) | 7571/7580 (99.9) |
| ROSC on ED arrival | Yes, formally compared | 4404/4410 (99.9) | 4880/4886 (99.9) | 9284/9296 (99.9) |
| AAM in place when first ROSC was achieveda | Descriptive only | 1029/3419 (30.1) | 1323/4161 (31.8) | 2352/7580 (31.0) |
| Duration of ICU stay in patients who survived to ICU discharge | Descriptive only. However, time to death (0–72 hours) and 72-hour survival were formally compared in place of this outcome | 321/321 (100) | 366/366 (100) | 687/687 (100) |
| Duration of ICU stay in patients who died in ICU | Descriptive only. However, time to death (0–72 hours) and 72-hour survival were formally compared in place of this outcome | 369/369 (100) | 503/503 (100) | 872/872 (100) |
| Duration of hospital stay in patients who survived to discharge | Descriptive only. However, time to death (0–72 hours) and 72-hour survival were formally compared in place of this outcome | 225/372 (60.5) | 229/392 (58.4) | 454/764 (59.4) |
| Duration of hospital stay in patients who died prior to discharge | Descriptive only. However, time to death (0–72 hours) and 72-hour survival were formally compared in place of this outcome | 1531/1547 (99.0) | 1852/1867 (99.2) | 3383/3414 (99.1) |
| Time to death (0–72 hours) | Yes, formally compared in place of duration of ICU stay and length of hospital stay | 4400/4410 (99.8) | 4871/4886 (99.7) | 9271/9296 (99.7) |
| 72-hour survival | Yes, formally compared in place of duration of ICU stay and length of hospital stay | 4395/4410 (99.7) | 4872/4886 (99.7) | 9267/9296 (99.7) |
| Time to death or last follow-up | Yes, formally compared | 4400/4410 (99.8) | 4871/4886 (99.7) | 9271/9296 (99.7) |
| EQ-5D single summary index at 30 days/hospital discharge | Yes, formally compared | 4205/4410 (95.4) | 4672/4886 (95.6) | 8877/9296 (95.5) |
| EQ-5D VAS score at 30 days/hospital discharge | Yes, formally compared | 4208/4410 (95.4) | 4669/4886 (95.6) | 8877/9296 (95.5) |
| mRS score at 3 months’ follow-up | Yes, formally compared | 4199/4410 (95.2) | 4636/4886 (94.9) | 8835/9296 (95.0) |
| EQ-5D single summary index score at 3 months’ follow-up | Yes, formally compared | 4199/4410 (95.2) | 4638/4886 (94.9) | 8837/9296 (95.1) |
| EQ-5D VAS score at 3 months’ follow-up | Yes, formally compared | 4201/4410 (95.3) | 4639/4886 (94.9) | 8840/9296 (95.1) |
| mRS score at 6 months’ follow-up | Yes, formally compared | 4212/4410 (95.5) | 4661/4886 (95.4) | 8873/9296 (95.5) |
| EQ-5D single summary index score at 6 months’ follow-up | Yes, formally compared | 4213/4410 (95.5) | 4657/4886 (95.3) | 8870/9296 (95.4) |
| EQ-5D VAS score at 6 months’ follow-up | Yes, formally compared | 4217/4410 (95.6) | 4662/4886 (95.4) | 8879/9296 (95.5) |
| Intervention details (excluding secondary outcomes) | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | Overall (N = 9296), n/N (%) |
|---|---|---|---|
| Airway management details | |||
| At least one airway management attempt reported by trial paramedic | 3687/4405 (83.7) | 4321/4883 (88.5) | 8008/9288 (86.2) |
| Reasons for not reporting any airway management | |||
| Resuscitation successful/ceased | 334/718 (46.5) | 241/562 (42.9) | 575/1280 (44.9) |
| No AAM | 89/718 (12.4) | 86/562 (15.3) | 175/1280 (13.7) |
| Not managed by enrolling trial paramedic | 245/718 (34.1) | 198/562 (35.2) | 443/1280 (34.6) |
| Patient had a tracheostomy | 5/718 (0.7) | 5/562 (0.9) | 10/1280 (0.8) |
| Other | 31/718 (4.3) | 14/562 (2.5) | 45/1280 (3.5) |
| Airway management details unknown | 14/718 (1.9) | 18/562 (3.2) | 32/1280 (2.5) |
| Patient received at least one AAM attempt by a trial paramedic | 3419/4404 (77.6) | 4161/4883 (85.2) | 7580/9287 (81.6) |
| TI | 3051/3419 (89.2) | 753/4161 (18.1) | 3804/7580 (50.2) |
| i-gel | 1118/3419 (32.7) | 4026/4161 (96.8) | 5144/7580 (67.9) |
| Other SGA | 216/3419 (6.3) | 44/4161 (1.1) | 260/7580 (3.4) |
| CO2 monitoring/capnography used | 3356/4379 (76.6) | 3748/4852 (77.2) | 7104/9231 (77.0) |
| If not used, reason:a | |||
| Unavailable | 136/962 (14.1) | 333/983 (33.9) | 469/1945 (24.1) |
| Faulty equipment | 35/962 (3.6) | 41/983 (4.2) | 76/1945 (3.9) |
| N/A – no AAM | 791/962 (82.2) | 609/983 (62.0) | 1400/1945 (72.0) |
| If used, type of CO2 monitoring/capnography: | |||
| Colour only | 368/3356 (11.0) | 371/3748 (9.9) | 739/7104 (10.4) |
| Capnometry (number only) | 1360/3356 (40.5) | 1687/3748 (45.0) | 3047/7104 (42.9) |
| Capnography (waveform) | 2262/3356 (67.4) | 2339/3748 (62.4) | 4601/7104 (64.8) |
| Mechanical CPR used during resuscitation | 1066/4395 (24.3) | 1084/4869 (22.3) | 2150/9264 (23.2) |
| Airway management handed over during pre-clinical care | 979/4386 (22.3) | 1281/4862 (26.3) | 2260/9248 (24.4) |
| If yes, to: | |||
| Doctor | 222/973 (22.8) | 330/1277 (25.8) | 552/2250 (24.5) |
| Nurse | 1/973 (0.1) | 2/1277 (0.2) | 3/2250 (0.1) |
| Paramedic | 750/973 (77.1) | 945/1277 (74.0) | 1695/2250 (75.3) |
FIGURE 23.
Return of spontaneous circulation and patient outcome by trial allocation and treatment received. a, A total of 72 patients in the TI group (2.1%) received a non-trial SGA only, all of whom had an mRS score of 4–6 (71 deaths). Among these 72 patients, 18 had ROSC during/after AAM. b, A total of 36 patients in the i-gel group (0.9%) received a non-trial SGA only, all of whom had an mRS score of 4–6 (35 deaths). Among these 36 patients, 12 had ROSC during/after AAM. c, ROSC here represents ROSC during or after AAM. A total of 41 patients (TI, n = 17; i-gel, n = 24) were missing data on ROSC on arrival, 39 of whom (TI, n = 15; i-gel, n = 24) had an mRS score of 4–6 (deaths: TI, n = 14; i-gel, n = 23). There were four patients (TI, n = 3; i-gel, n = 1) missing AAM data, all of whom died. There were eight patients (TI, n = 3; i-gel, n = 5) who were missing data on ROSC during/after AAM, seven of whom (TI, n = 2; i-gel, n = 5) had an mRS score of 4–6 (deaths: TI, n = 2; i-gel, n = 4).
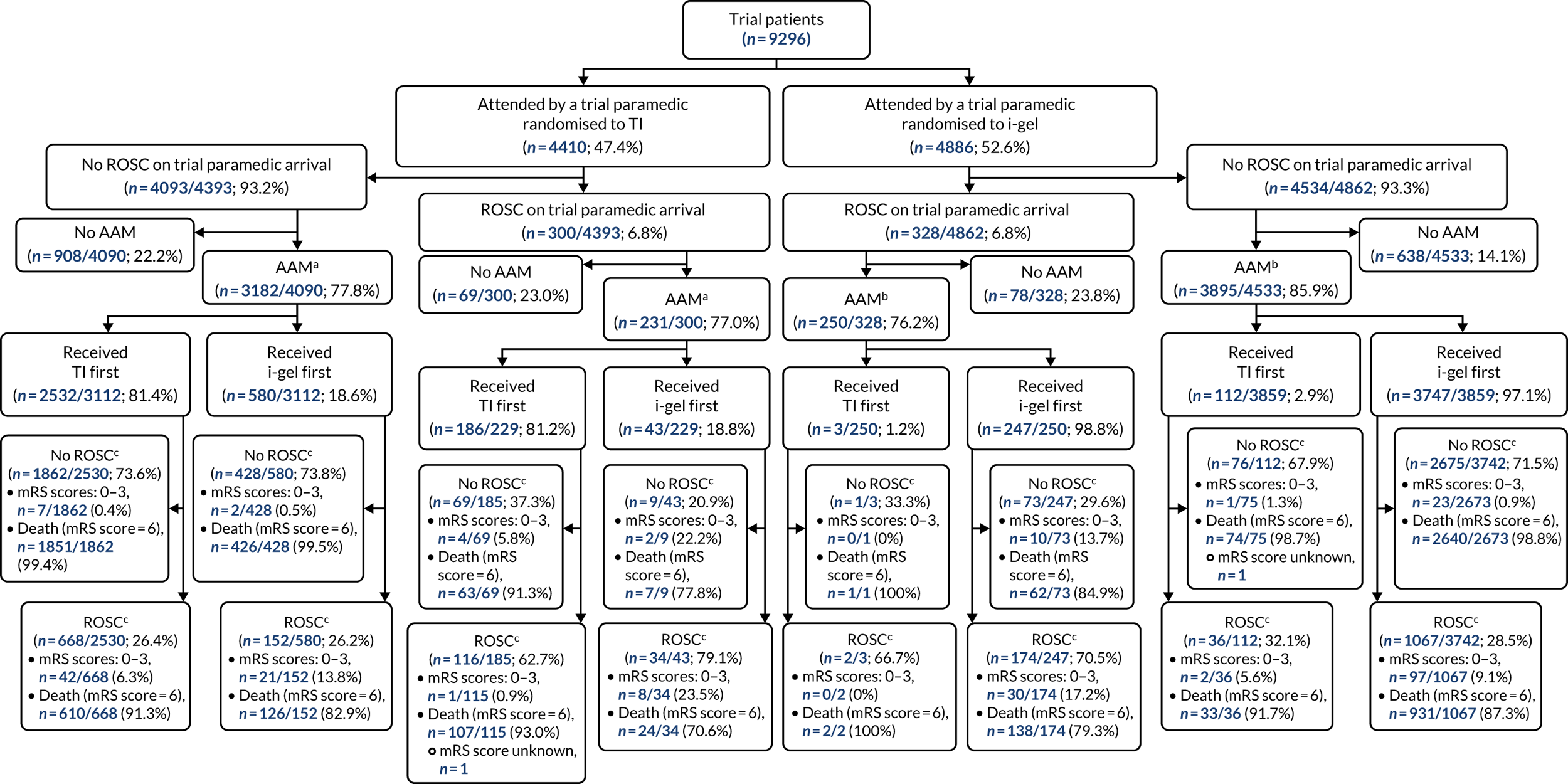
| Patient details by allocated intervention and use of airway management for all trial patients | Trial population | No AAMa | AAMa | |||
|---|---|---|---|---|---|---|
| TI group (N = 4410) | i-gel group (N = 4886) | TI group (N = 985) | i-gel group (N = 722) | TI group (N = 3419) | i-gel group (N = 4161) | |
| Age (years), median (IQR) | 74 (62–83) | 73 (61–82) | 73 (60–83) | 71 (60–82) | 74 (63–83) | 73 (62–82) |
| Sex (male), n/N (%) | 2791/4410 (63.3) | 3132/4886 (64.1) | 615/985 (62.4) | 472/722 (65.4) | 2174/3419 (63.6) | 2658/4161 (63.9) |
| Time (minutes) from 999 call to first crew arrival, median (IQR) | 8 (5–11) | 7 (5–11) | 8 (5–11) | 7 (5–11) | 8 (5–11) | 7 (5–11) |
| Presenting rhythm, n/N (%) | ||||||
| Asystole | 2356/4316 (54.6) | 2597/4791 (54.2) | 453/937 (48.3) | 348/681 (51.1) | 1901/3375 (56.3) | 2248/4108 (54.7) |
| VF | 979/4316 (22.7) | 1094/4791 (22.8) | 297/937 (31.7) | 226/681 (33.2) | 681/3375 (20.2) | 868/4108 (21.1) |
| Pulseless VT | 44/4316 (1.0) | 39/4791 (0.8) | 18/937 (1.9) | 11/681 (1.6) | 26/3375 (0.8) | 28/4108 (0.7) |
| PEA | 937/4316 (21.7) | 1061/4791 (22.1) | 169/937 (18.0) | 96/681 (14.1) | 767/3375 (22.7) | 964/4108 (23.5) |
| Event witnessed, n/N (%) | 2788/4407 (63.3) | 3101/4883 (63.5) | 641/983 (65.2) | 490/719 (68.2) | 2144/3419 (62.7) | 2608/4161 (62.7) |
| By bystander | 2231/2788 (80.0) | 2493/3100 (80.4) | 472/641 (73.6) | 355/489 (72.6) | 1757/2144 (81.9) | 2135/2608 (81.9) |
| By EMS | 557/2788 (20.0) | 607/3100 (19.6) | 169/641 (26.4) | 134/489 (27.4) | 387/2144 (18.1) | 473/2608 (18.1) |
| Bystander CPR, n/N (%) | 2774/4406 (63.0) | 3149/4883 (64.5) | 567/983 (57.7) | 437/720 (60.7) | 2204/3418 (64.5) | 2709/4160 (65.1) |
| Patient details by first intervention received for trial patients with at least one AAM attempt | Received TI first (n/N = 2840/9296; 30.6%) | Received i-gel first (n/N = 4632/9296; 49.8%) |
|---|---|---|
| Age (years), median (IQR) | 74 (63–83) | 73 (61–82) |
| Sex (male), n/N (%) | 1780/2840 (62.7) | 2975/4632 (64.2) |
| Time (minutes) from 999 call to first crew arrival, median (IQR) | 8 (5–11) | 7 (5–11) |
| Presenting rhythm, n/N (%) | ||
| Asystole | 1594/2806 (56.8) | 2509/4571 (54.9) |
| VF | 555/2806 (19.8) | 966/4571 (21.1) |
| Pulseless VT | 19/2806 (0.7) | 34/4571 (0.7) |
| PEA | 638/2806 (22.7) | 1062/4571 (23.2) |
| Event witnessed, n/N (%) | 1782/2840 (62.7) | 2904/4632 (62.7) |
| By bystander | 1483/1782 (83.2) | 2353/2904 (81.0) |
| By EMS | 299/1782 (16.8) | 551/2904 (19.0) |
| Bystander CPR, n/N (%) | 1836/2839 (64.7) | 2998/4631 (64.7) |
| Sensitivity analysis | TI group | i-gel group | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| 1: trial patients plus patients attended by a trial paramedic but not resuscitateda | |||||||
| Total, N | 10,744 | 11,466 | |||||
| mRS score (0 to 3; good functional recovery), n/N (%) | 300/10,741 (2.8) | 311/11,462 (2.7) | 0.96 (0.81 to 1.14) | 0.63 | 0.06 | –0.2 (–0.6 to 0.3) | 0.45 |
| 2: trial patients who received at least one AAMa | |||||||
| Total, N | 4410 | 4886 | |||||
| mRS score (0 to 3; good functional recovery), n/N (%) | 88/3418 (2.6) | 163/4158 (3.9) | 1.57 (1.18 to 2.07) | 0.002 | 0.10 | 1.4 (0.5 to 2.2) | 0.001 |
| 3: trial patients who received at least one AAMb | |||||||
| Total, N | 2840c | 4632d | |||||
| mRS score (0 to 3; good functional recovery), n/N (%) | 58/2838 (2.0) | 193/4630 (4.2) | 2.06 (1.51 to 2.81) | < 0.001 | 0.10 | 2.1 (1.2 to 2.9) | < 0.001 |
| Patient details | Enrolled by a paramedic randomised to TI group (n = 4410) | Enrolled by a paramedic randomised to i-gel group (n = 4886) | ||
|---|---|---|---|---|
| Did not receive TI as first AAM treatment (n = 1680; 38.2%) | Received TI as first AAM treatment (n = 2724; 61.9%) | Did not receive i-gel as first AAM treatment (n = 874; 17.9%) | Received i-gel as first AAM treatment (n = 4009; 82.1%) | |
| Age (years), median (IQR)b | 73 (60–83) | 74 (63–83) | 71 (60–82) | 73 (62–82) |
| Sex (male), n/N (%) | 1075/1680 (64.0) | 1714/2724 (62.9) | 562/874 (64.3) | 2568/4009 (64.1) |
| Time (minutes) from 999 call to first crew arrival, median (IQR)c | 8 (5–12) | 8 (5–11) | 7 (5–11) | 8 (5–11) |
| Presenting rhythm, n/N (%) | ||||
| Asystole | 831/1621 (51.3) | 1523/2691 (56.6) | 432/831 (52.0) | 2164/3958 (54.7) |
| VF | 444/1621 (27.4) | 534/2691 (19.8) | 259/831 (31.2) | 835/3958 (21.1) |
| Pulseless VT | 25/1624 (1.5) | 19/2691 (0.7) | 11/831 (1.3) | 28/3958 (0.7) |
| PEA | 321/1624 (19.8) | 615/2691 (22.9) | 129/831 (15.5) | 931/3958 (23.5) |
| Event witnessed, n/N (%) | 1078/1678 (64.2) | 1707/2724 (62.7) | 590/871 (67.7) | 2508/4009 (62.6) |
| By bystander | 814/1078 (75.5) | 1415/1707 (82.9) | 444/589 (75.4) | 2046/2508 (81.6) |
| By EMS | 264/1078 (24.5) | 292/1707 (17.1) | 145/589 (24.6) | 462/2508 (18.4) |
| Bystander CPR, n/N (%) | 1020/1678 (60.8) | 1751/2723 (64.3) | 548/872 (62.8) | 2598/4008 (64.8) |
| Compression fraction details (for trial patients enrolled during the period that compression fraction data were collected) | TI group (N = 32), median (IQR) | i-gel group (N = 34), median (IQR) | GMR estimate (95% CI) | p-value |
|---|---|---|---|---|
| Compression fraction | 83 (74–89) | 86 (81–91) | 0.82a (0.62 to 1.07) | 0.14 |
| ROSC details | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| All trial patients | |||||||
| Any ROSC during/after AAM by trial paramedica | 992/3416 (29.0) | 1295/4155 (31.2) | 1.13 (1.01 to 1.27) | 0.03 | 0.04 | 2.5 (0.1 to 4.8) | 0.04 |
| Any ROSC during/after airway management by trial paramedica | 1139/3685 (30.9) | 1379/4318 (31.9) | |||||
| Admitted to ED/hospital | 1922/4410 (43.6) | 2263/4886 (46.3) | |||||
| ROSC on ED/hospital arrival | 1249/4404 (28.4) | 1495/4880 (30.6) | 1.12 (1.02 to 1.23) | 0.02 | 0.01 | 2.2 (0.3 to 4.2) | 0.03 |
| Survived to ED discharge | 861/1919 (44.9) | 1033/2259 (45.7) | |||||
| Airway management details when ROSC was achieved or resuscitation discontinued | TI group (N = 4410), n/N (%) | i-gel group (N = 4886), n/N (%) |
|---|---|---|
| AAM in place when patient first had ROSCa | ||
| TI | 689/1029 (67.0) | 165/1323 (12.5) |
| i-gel | 241/1029 (23.4) | 1092/1323 (82.5) |
| Other SGA | 29/1029 (2.8) | 12/1323 (0.9) |
| Other | 70/1029 (6.8) | 54/1323 (4.1) |
| Final airway management in place in those who died on sceneb | ||
| TI | 1322/1990 (66.4) | 364/2261 (16.1) |
| i-gel | 501/1990 (25.2) | 1829/2261 (80.9) |
| Other SGA | 114/1990 (5.7) | 17/2261 (0.8) |
| Other | 53/1990 (2.7) | 51/2261 (2.3) |
| Final airway management in place in those who were admitted to EDc | ||
| TI | 1008/1429 (70.5) | 272/1899 (14.3) |
| i-gel | 316/1429 (22.1) | 1558/1899 (82.0) |
| Other SGA | 72/1429 (5.0) | 18/1899 (0.9) |
| Other | 33/1429 (2.3) | 51/1899 (2.7) |
| Survival details | TI group (N = 4410) | i-gel group (N = 4886) | OR estimate (95% CI) | p-value | ICC | ADP estimate, % (95%CI) | p-value |
|---|---|---|---|---|---|---|---|
| Survival status, n/N (%) | |||||||
| Died at scene | 2488/4407 (56.5) | 2623/4882 (53.7) | |||||
| Died prior to ICU admission | 1058/4407 (24.0) | 1226/4882 (25.1) | |||||
| Died prior to ICU discharge | 369/4407 (8.4) | 503/4882 (10.3) | |||||
| Died prior to hospital discharge | 120/4407 (2.7) | 138/4882 (2.8) | |||||
| Survived to 30 days/hospital discharge | 372/4407 (8.4) | 392/4882 (8.0) | |||||
| Extended survival status, n/N (%) | |||||||
| Died on scene | 2488/4410 (56.4) | 2623/4886 (53.7) | |||||
| Died prior to ICU admission | 1058/4410 (24.0) | 1226/4886 (25.1) | |||||
| Died prior to ICU discharge | 369/4410 (8.4) | 503/4886 (10.3) | |||||
| Died prior to hospital discharge; no active consent | 119/4410 (2.7) | 135/4886 (2.8) | |||||
| Died prior to hospital discharge; active consent, n/N (%) | |||||||
| No EQ-5D or mRS forms | 1/4410 (0.0) | 0/4886 (0.0) | |||||
| EQ-5D and/or mRS forms at 30 days | 0/4410 (0.0) | 2/4886 (0.0) | |||||
| EQ-5D and/or mRS forms at 30 days and 3 months’ follow-up | 0/4410 (0.0) | 1/4886 (0.0) | |||||
| Survived to hospital discharge; no active consent, n/N (%) | |||||||
| Died within 3 months of OHCA | 12/4410 (0.3) | 3/4886 (0.1) | |||||
| Died between 3 and 6 months of OHCA | 6/4410 (0.1) | 6/4886 (0.1) | |||||
| Survived to 6 months’ follow-up | 124/4410 (2.8) | 141/4886 (2.9) | |||||
| Unknown survival status post hospital discharge | 34/4410 (0.8) | 39/4886 (0.8) | |||||
| Survived to 3 months post OHCA and unknown survival status at 6 months post OHCA | 0/4410 (0.0) | 0/4886 (0.0) | |||||
| Survived to hospital discharge; active consent, n/N (%) | |||||||
| Died within 3 months of OHCA | 2/4410 (0.1) | 2/4886 (0.0) | |||||
| Died between 3 and 6 months of OHCA | 3/4410 (0.1) | 3/4886 (0.1) | |||||
| Survived to 6 months’ follow-up | 190/4410 (4.3) | 198/4886 (4.1) | |||||
| Unknown survival status post hospital discharge | 0/4410 (0.0) | 0/4886 (0.0) | |||||
| Survived to 3 months post OHCA and unknown survival status at 6 months post OHCA | 1/4410 (0.0) | 0/4886 (0.0) | |||||
| Patients unable to be identified owing to being admitted or transferred to a non-participating hospital | 3/4410 (0.1) | 4/4886 (0.1) | |||||
| ICU stay (patients survived to ED discharge only), n | 861 | 1033 | |||||
| Admitted to ICU from ED, n/N (%) | 690/860 (80.2) | 869/1031 (84.3) | |||||
| Survived to ICU discharge, n/N (%) | 321/690 (46.5) | 366/869 (42.1) | |||||
| Duration (hours) of initial ICU stay in patients who survived to ICU discharge, median (IQR) | 96.6 (45.7–169.6) | 100.5 (50.3–197.5) | |||||
| Duration (hours) of ICU stay in patients who died in ICU, median (IQR) | 47.4 (18.0–98.0) | 47.0 (17.2–97.9) | |||||
| Hospital stay (patients admitted to ED only), n | 1922 | 2263 | |||||
| Survived to hospital discharge, n/N (%) | 372/1919 (19.4) | 392/2259 (17.4) | |||||
| Duration (days) of hospital stay in patients who survived to discharge, median (IQR)a | 12.3 (6.9–20.3) | 14.0 (8.0–23.8) | |||||
| Duration (hours) of hospital stay in patients who died before discharge, median (IQR)b | 1.7 (0.3–20.4) | 2.0 (0.4–26.8) | |||||
| Time (minutes) to death, median (IQR; n)c | 63 (41–216; 4400) | 67 (41–267; 4871) | HR 0.97 (0.93 to 1.02) | 0.22 | |||
| Time (minutes) to death up to 72 hours post OHCA, median (IQR; n)c | 63 (41–205; 4400) | 67 (41–246; 4871) | HR 0.96 (0.92 to 1.00) | 0.07 | |||
| 72-hour survival, n/N (%) | 575/4395 (13.1) | 664/4872 (13.6) | OR 1.04 (0.92 to 1.18) | 0.54 | 0.02 | 0.4 (–1.0 to 1.9) | 0.54 |
FIGURE 24.
Summaries of complete-case mRS scores at all three time points: (a) 30 days/hospital discharge; (b) 3-month follow-up; and (c) 6-month follow-up. This figure displays the percentages of each of the categories of the mRS for the complete-case analyses.
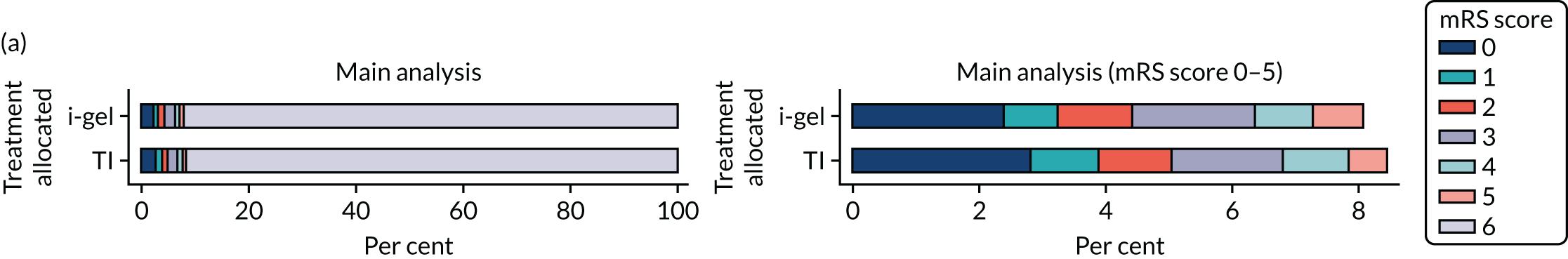
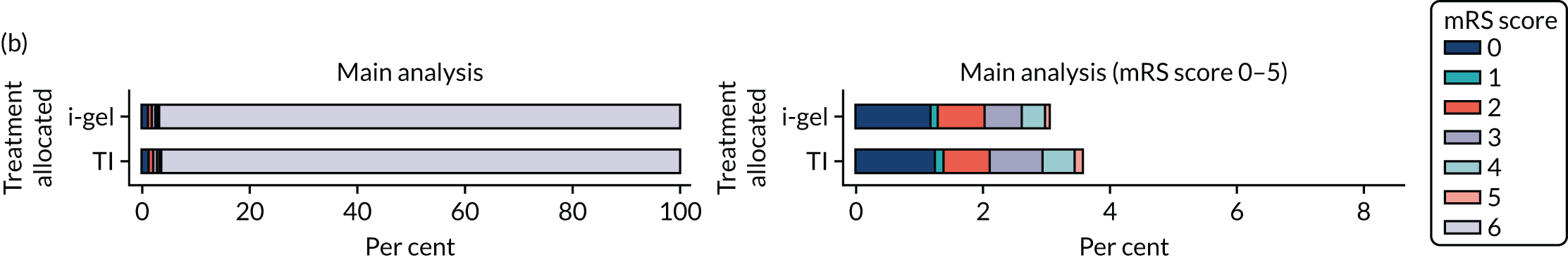
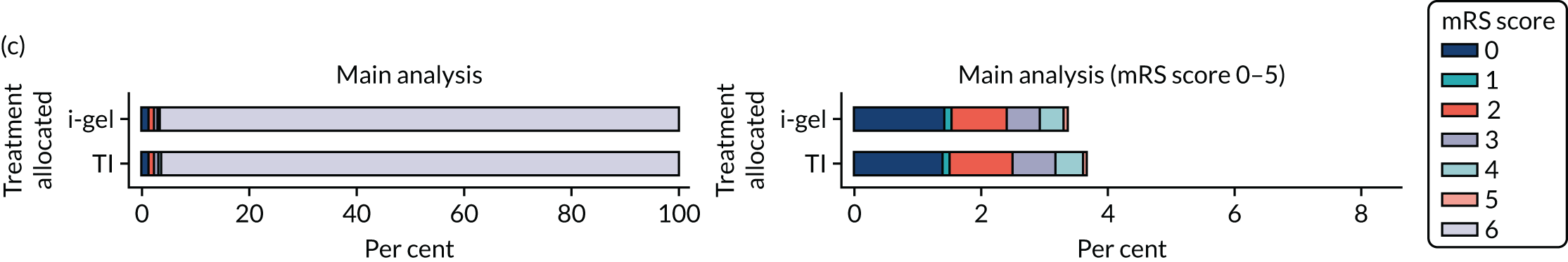
FIGURE 25.
Summaries of worst-case longer-term mRS scores: (a) at 3-month follow-up; and (b) at 6-month follow-up. This figure displays the percentages of each of the categories of the mRS for the worst-case analyses.
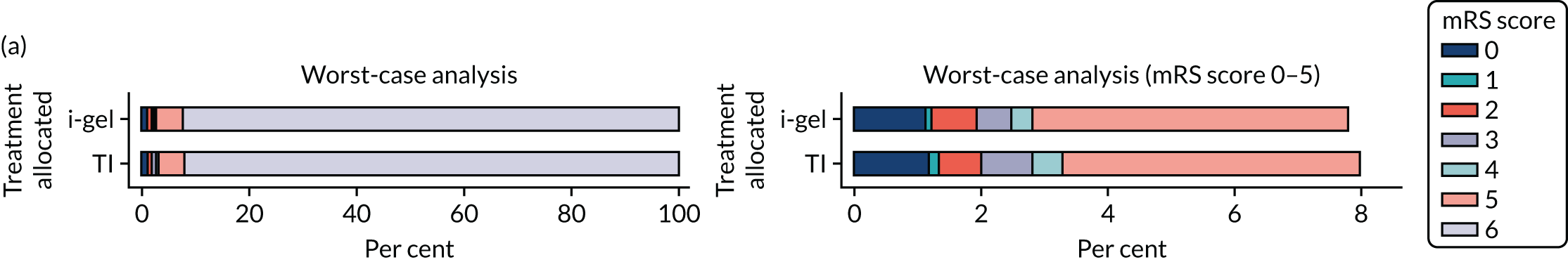
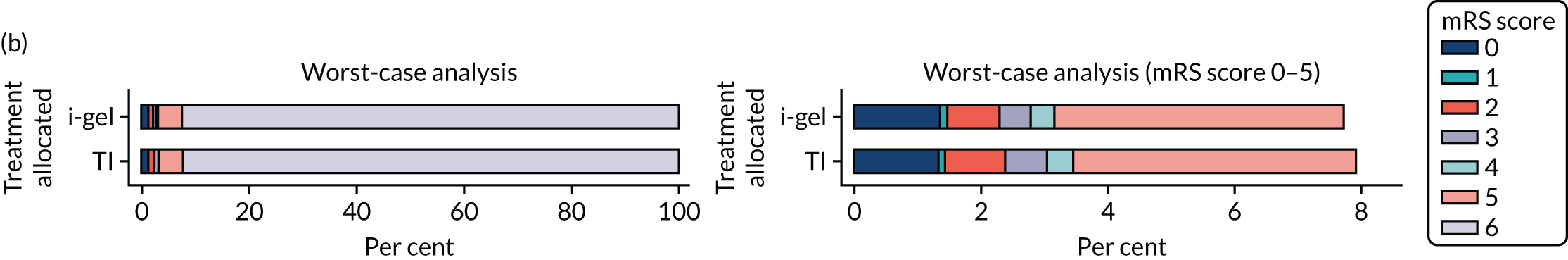
FIGURE 26.
Summaries of imputed-case longer-term mRS scores: (a) at 3-month follow-up; and (b) at 6-month follow-up. This figure displays the percentages of each of the categories of the mRS for the imputed-case analyses.
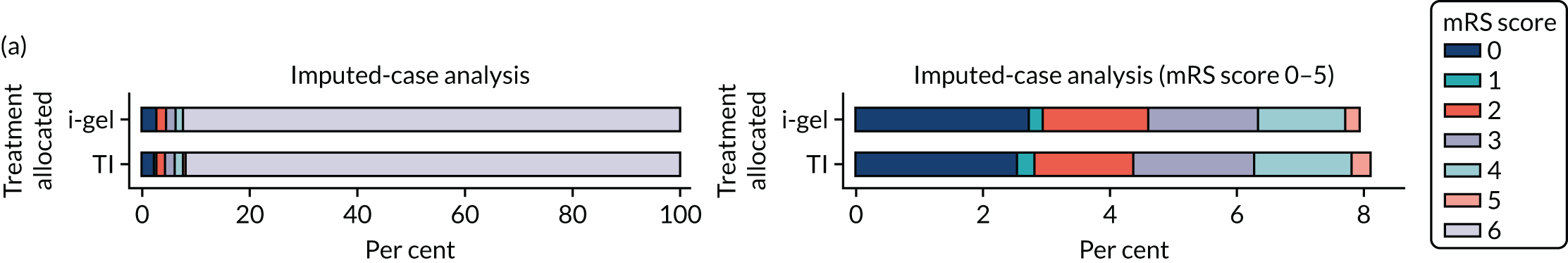
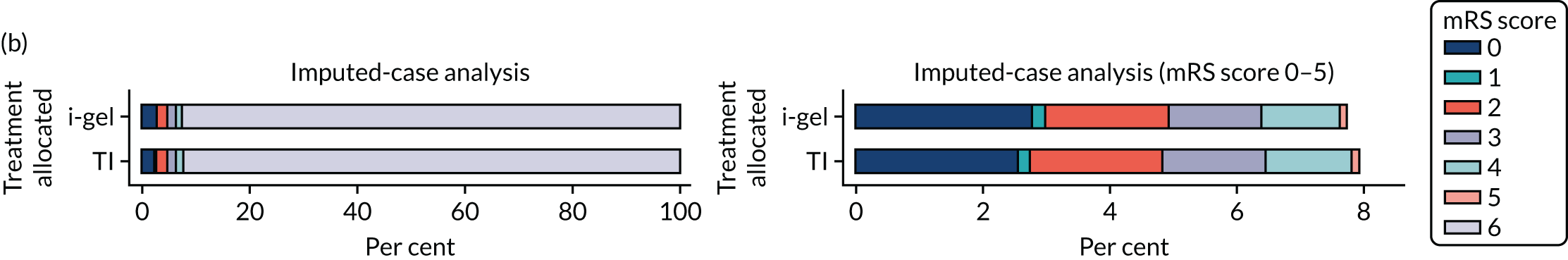
| EQ-5D question details | TI group (N = 197), n/N (%) | i-gel group (N = 206), n/N (%) | Overall (N = 403), n/N (%) | |
|---|---|---|---|---|
| Mobility | ||||
| Hospital discharge/30 days | No problems walking about | 98/174 (56.3) | 84/186 (45.2) | 182/360 (50.6) |
| Slight problems walking about | 38/174 (21.8) | 50/186 (26.9) | 88/360 (24.4) | |
| Moderate problems walking about | 23/174 (13.2) | 24/186 (12.9) | 47/360 (13.1) | |
| Severe problems walking about | 8/174 (4.6) | 14/186 (7.5) | 22/360 (6.1) | |
| Unable to walk about | 7/174 (4.0) | 14/186 (7.5) | 21/360 (5.8) | |
| 3 months | No problems walking about | 89/152 (58.6) | 85/146 (58.2) | 174/298 (58.4) |
| Slight problems walking about | 24/152 (15.8) | 30/146 (20.5) | 54/298 (18.1) | |
| Moderate problems walking about | 24/152 (15.8) | 21/146 (14.4) | 45/298 (15.1) | |
| Severe problems walking about | 9/152 (5.9) | 5/146 (3.4) | 14/298 (4.7) | |
| Unable to walk about | 6/152 (3.9) | 5/146 (3.4) | 11/298 (3.7) | |
| 6 months | No problems walking about | 105/158 (66.5) | 94/158 (59.5) | 199/316 (63.0) |
| Slight problems walking about | 23/158 (14.6) | 29/158 (18.4) | 52/316 (16.5) | |
| Moderate problems walking about | 21/158 (13.3) | 26/158 (16.5) | 47/316 (14.9) | |
| Severe problems walking about | 8/158 (5.1) | 4/158 (2.5) | 12/316 (3.8) | |
| Unable to walk about | 1/158 (0.6) | 5/158 (3.2) | 6/316 (1.9) | |
| Self-care | ||||
| Hospital discharge/30 days | No problems with washing or dressing | 113/174 (64.9) | 112/186 (60.2) | 225/360 (62.5) |
| Slight problems washing or dressing | 30/174 (17.2) | 28/186 (15.1) | 58/360 (16.1) | |
| Moderate problems washing or dressing | 15/174 (8.6) | 25/186 (13.4) | 40/360 (11.1) | |
| Severe problems washing or dressing | 8/174 (4.6) | 6/186 (3.2) | 14/360 (3.9) | |
| Unable to wash or dress | 8/174 (4.6) | 15/186 (8.1) | 23/360 (6.4) | |
| 3 months | No problems with washing or dressing | 121/152 (79.6) | 120/146 (82.2) | 241/298 (80.9) |
| Slight problems washing or dressing | 11/152 (7.2) | 13/146 (8.9) | 24/298 (8.1) | |
| Moderate problems washing or dressing | 11/152 (7.2) | 5/146 (3.4) | 16/298 (5.4) | |
| Severe problems washing or dressing | 3/152 (2.0) | 2/146 (1.4) | 5/298 (1.7) | |
| Unable to wash or dress | 6/152 (3.9) | 6/146 (4.1) | 12/298 (4.0) | |
| 6 months | No problems with washing or dressing | 130/158 (82.3) | 130/156 (83.3) | 260/314 (82.8) |
| Slight problems washing or dressing | 14/158 (8.9) | 13/156 (8.3) | 27/314 (8.6) | |
| Moderate problems washing or dressing | 9/158 (5.7) | 6/156 (3.8) | 15/314 (4.8) | |
| Severe problems washing or dressing | 2/158 (1.3) | 2/156 (1.3) | 4/314 (1.3) | |
| Unable to wash or dress | 3/158 (1.9) | 5/156 (3.2) | 8/314 (2.5) | |
| Usual activities | ||||
| Hospital discharge/30 days | No problems with usual activities | 56/171 (32.7) | 50/186 (26.9) | 106/357 (29.7) |
| Slight problems with usual activities | 51/171 (29.8) | 43/186 (23.1) | 94/357 (26.3) | |
| Moderate problems with usual activities | 27/171 (15.8) | 39/186 (21.0) | 66/357 (18.5) | |
| Severe problems with usual activities | 13/171 (7.6) | 16/186 (8.6) | 29/357 (8.1) | |
| Unable to perform usual activities | 24/171 (14.0) | 38/186 (20.4) | 62/357 (17.4) | |
| 3 months | No problems with usual activities | 65/151 (43.0) | 73/146 (50.0) | 138/297 (46.5) |
| Slight problems with usual activities | 42/151 (27.8) | 35/146 (24.0) | 77/297 (25.9) | |
| Moderate problems with usual activities | 19/151 (12.6) | 22/146 (15.1) | 41/297 (13.8) | |
| Severe problems with usual activities | 10/151 (6.6) | 3/146 (2.1) | 13/297 (4.4) | |
| Unable to perform usual activities | 15/151 (9.9) | 13/146 (8.9) | 28/297 (9.4) | |
| 6 months | No problems with usual activities | 82/158 (51.9) | 80/157 (51.0) | 162/315 (51.4) |
| Slight problems with usual activities | 39/158 (24.7) | 37/157 (23.6) | 76/315 (24.1) | |
| Moderate problems with usual activities | 25/158 (15.8) | 26/157 (16.6) | 51/315 (16.2) | |
| Severe problems with usual activities | 5/158 (3.2) | 8/157 (5.1) | 13/315 (4.1) | |
| Unable to perform usual activities | 7/158 (4.4) | 6/157 (3.8) | 13/315 (4.1) | |
| Pain/discomfort | ||||
| Hospital discharge/30 days | No pain or discomfort | 68/174 (39.1) | 75/185 (40.5) | 143/359 (39.8) |
| Slight pain or discomfort | 59/174 (33.9) | 65/185 (35.1) | 124/359 (34.5) | |
| Moderate pain or discomfort | 36/174 (20.7) | 34/185 (18.4) | 70/359 (19.5) | |
| Severe pain or discomfort | 9/174 (5.2) | 7/185 (3.8) | 16/359 (4.5) | |
| Extreme pain or discomfort | 2/174 (1.1) | 4/185 (2.2) | 6/359 (1.7) | |
| 3 months | No pain or discomfort | 78/152 (51.3) | 71/146 (48.6) | 149/298 (50.0) |
| Slight pain or discomfort | 49/152 (32.2) | 52/146 (35.6) | 101/298 (33.9) | |
| Moderate pain or discomfort | 14/152 (9.2) | 18/146 (12.3) | 32/298 (10.7) | |
| Severe pain or discomfort | 9/152 (5.9) | 4/146 (2.7) | 13/298 (4.4) | |
| Extreme pain or discomfort | 2/152 (1.3) | 1/146 (0.7) | 3/298 (1.0) | |
| 6 months | No pain or discomfort | 90/157 (57.3) | 84/155 (54.2) | 174/312 (55.8) |
| Slight pain or discomfort | 40/157 (25.5) | 45/155 (29.0) | 85/312 (27.2) | |
| Moderate pain or discomfort | 18/157 (11.5) | 18/155 (11.6) | 36/312 (11.5) | |
| Severe pain or discomfort | 9/157 (5.7) | 7/155 (4.5) | 16/312 (5.1) | |
| Extreme pain or discomfort | 0/157 (0.0) | 1/155 (0.6) | 1/312 (0.3) | |
| Anxiety/depression | ||||
| Hospital discharge/30 days | Not anxious or depressed | 99/173 (57.2) | 100/185 (54.1) | 199/358 (55.6) |
| Slightly anxious or depressed | 44/173 (25.4) | 45/185 (24.3) | 89/358 (24.9) | |
| Moderately anxious or depressed | 20/173 (11.6) | 30/185 (16.2) | 50/358 (14.0) | |
| Severely anxious or depressed | 6/173 (3.5) | 7/185 (3.8) | 13/358 (3.6) | |
| Extremely anxious or depressed | 4/173 (2.3) | 3/185 (1.6) | 7/358 (2.0) | |
| 3 months | Not anxious or depressed | 84/151 (55.6) | 81/144 (56.3) | 165/295 (55.9) |
| Slightly anxious or depressed | 43/151 (28.5) | 46/144 (31.9) | 89/295 (30.2) | |
| Moderately anxious or depressed | 16/151 (10.6) | 12/144 (8.3) | 28/295 (9.5) | |
| Severely anxious or depressed | 5/151 (3.3) | 4/144 (2.8) | 9/295 (3.1) | |
| Extremely anxious or depressed | 3/151 (2.0) | 1/144 (0.7) | 4/295 (1.4) | |
| 6 months | Not anxious or depressed | 99/158 (62.7) | 94/156 (60.3) | 193/314 (61.5) |
| Slightly anxious or depressed | 38/158 (24.1) | 39/156 (25.0) | 77/314 (24.5) | |
| Moderately anxious or depressed | 14/158 (8.9) | 20/156 (12.8) | 34/314 (10.8) | |
| Severely anxious or depressed | 5/158 (3.2) | 1/156 (0.6) | 6/314 (1.9) | |
| Extremely anxious or depressed | 2/158 (1.3) | 2/156 (1.3) | 4/314 (1.3) | |
FIGURE 27.
Summaries of complete-case single summary index scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median single summary index score, IQR and range (complete case).
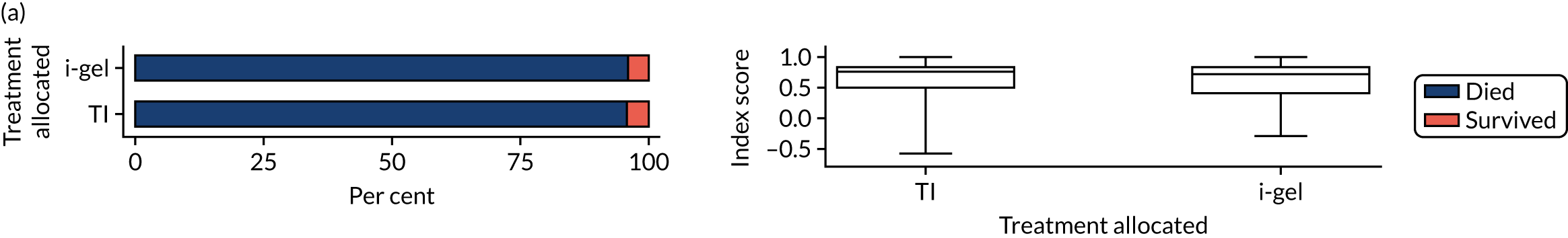
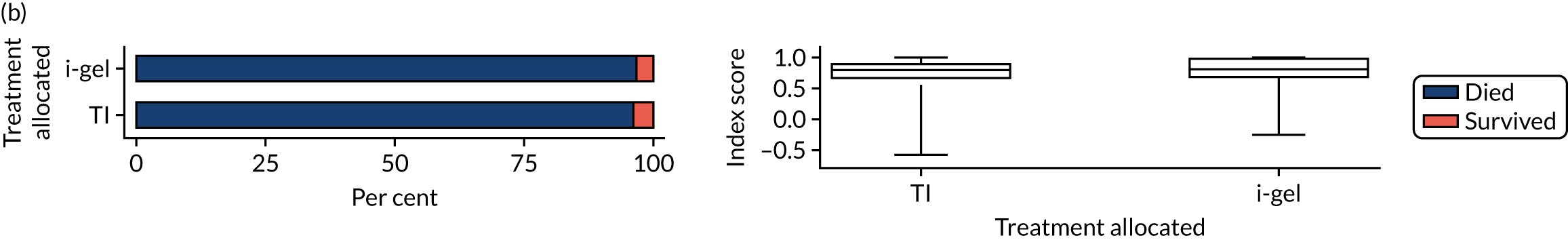
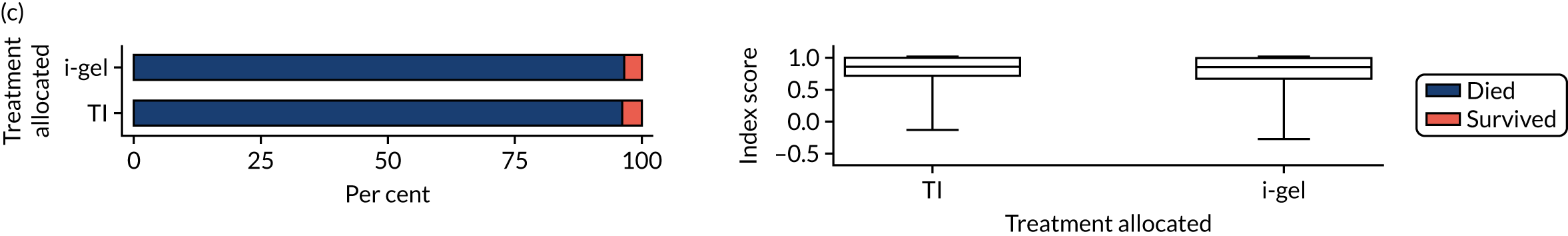
FIGURE 28.
Summaries of complete-case VAS scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median VAS score, IQR and range (complete case).
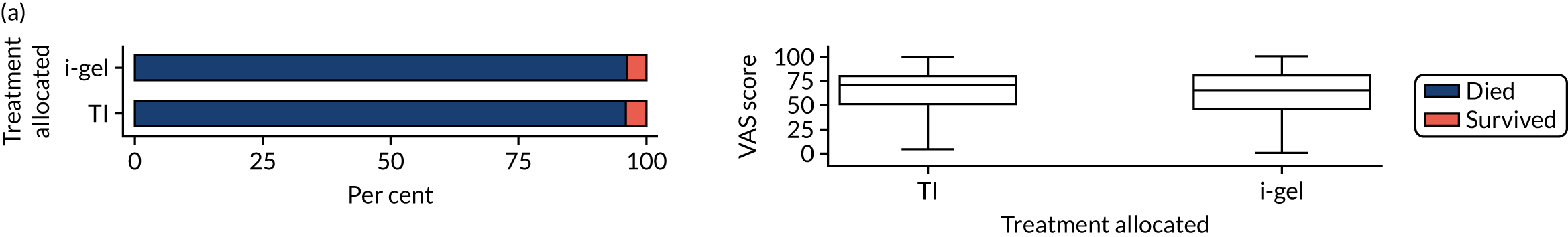
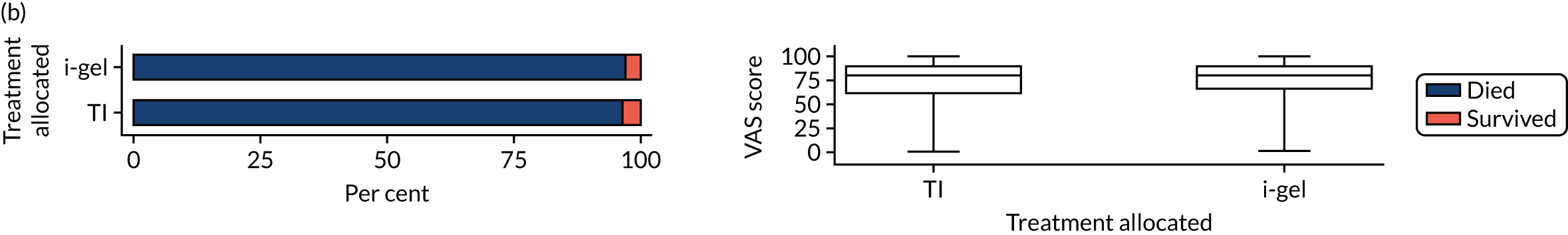
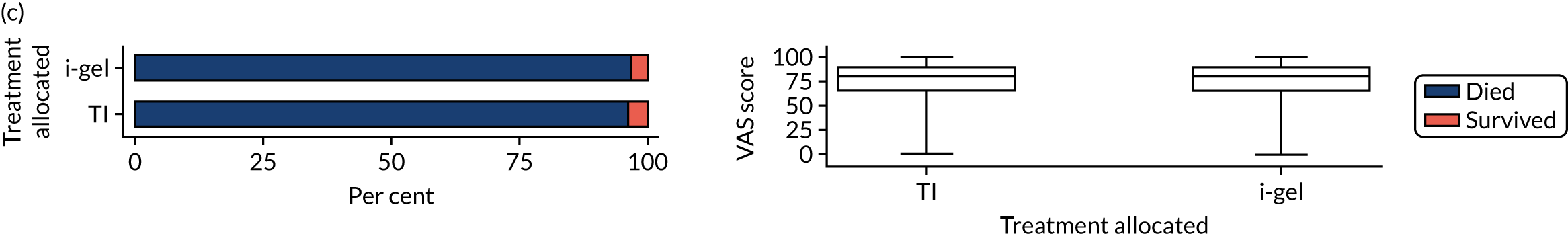
FIGURE 29.
Summaries of worst-case single summary index scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median single summary index score, IQR and range (worst case).
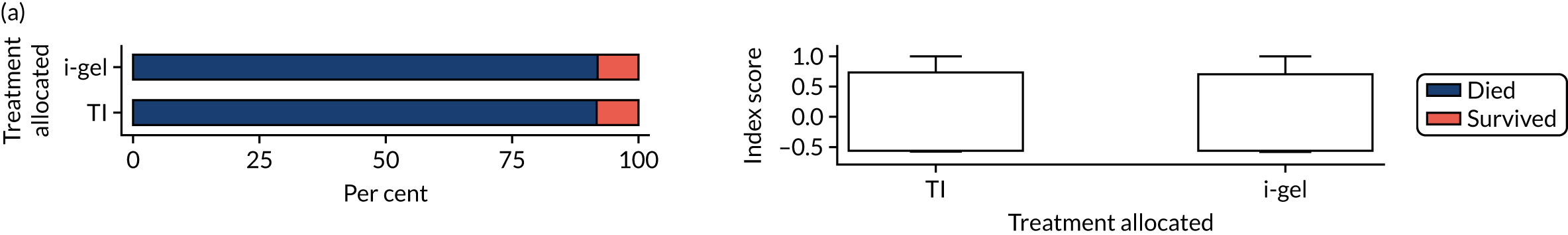
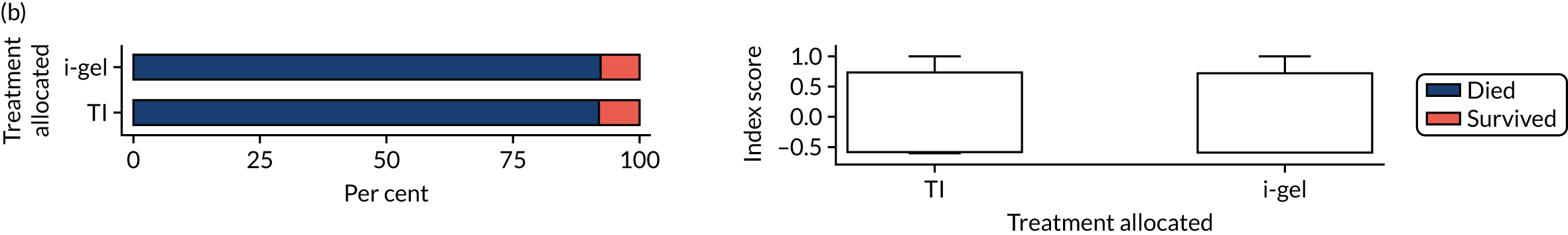
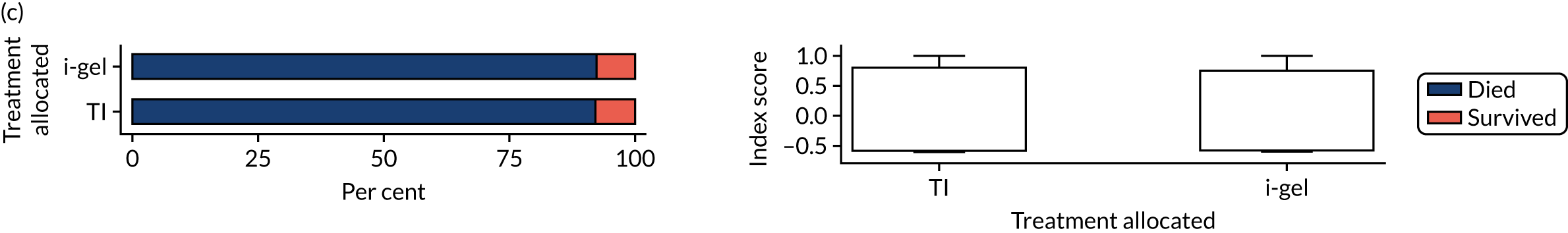
FIGURE 30.
Summaries of worst-case VAS scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median single summary index score, IQR and range (worst case).
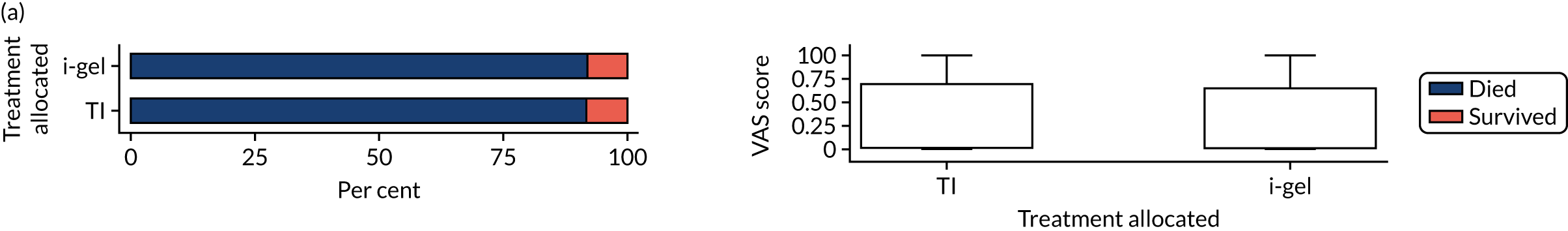
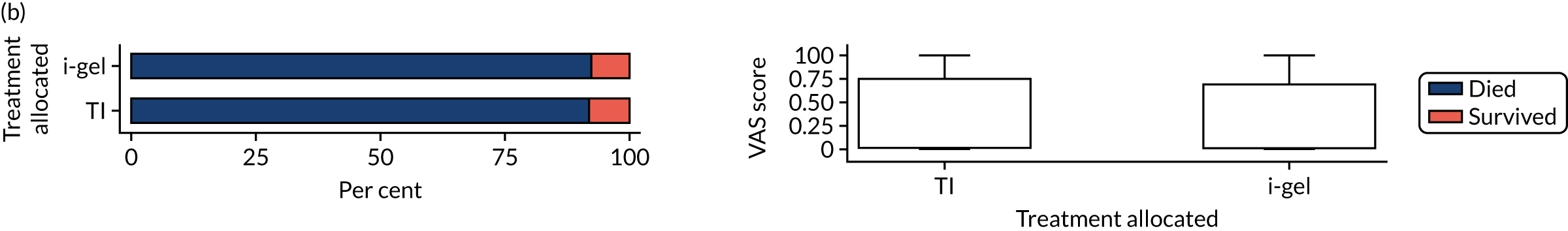
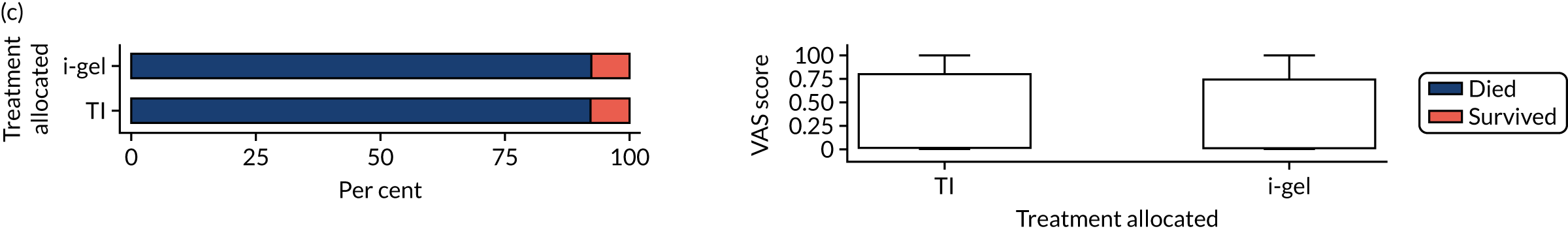
FIGURE 31.
Summaries of imputed-case single summary index scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median single summary index score, IQR and range (imputed case).
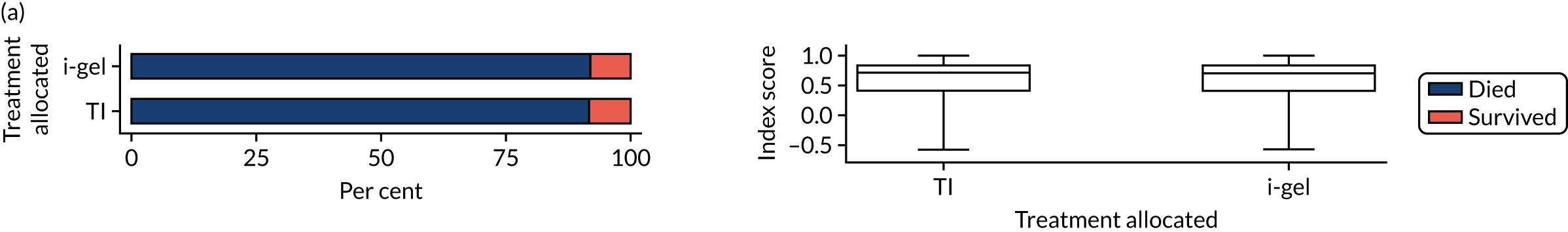
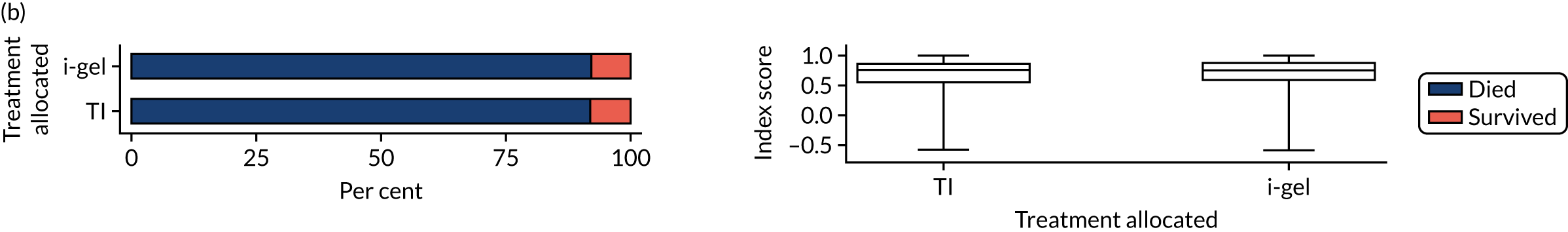
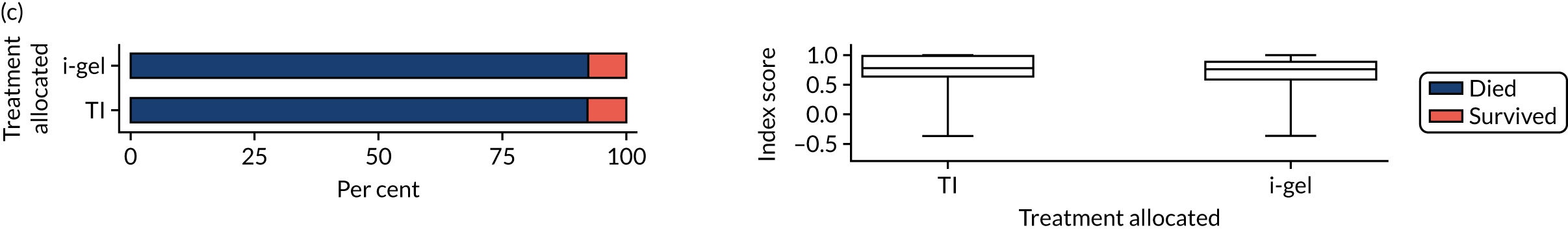
FIGURE 32.
Summaries of imputed-case VAS scores: (a) at 30 days/hospital discharge; (b) at 3-month follow-up; and (c) at 6-month follow-up. This figure displays the percentages of patients who died and survived, as well as the median single summary index score, IQR and range (imputed case).
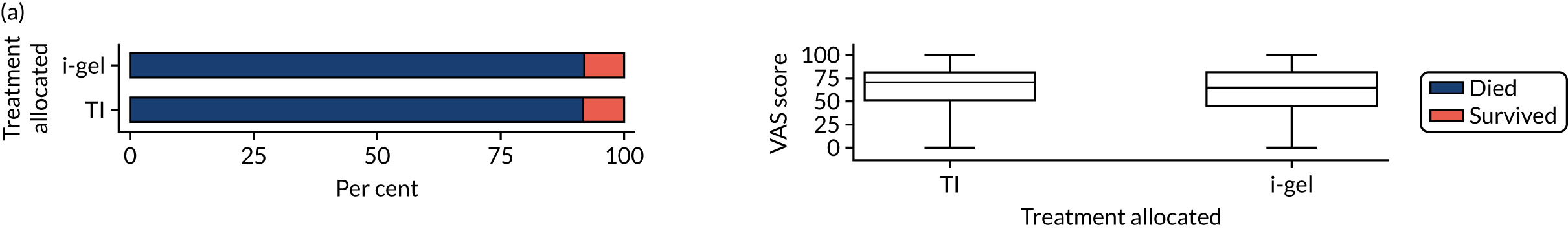
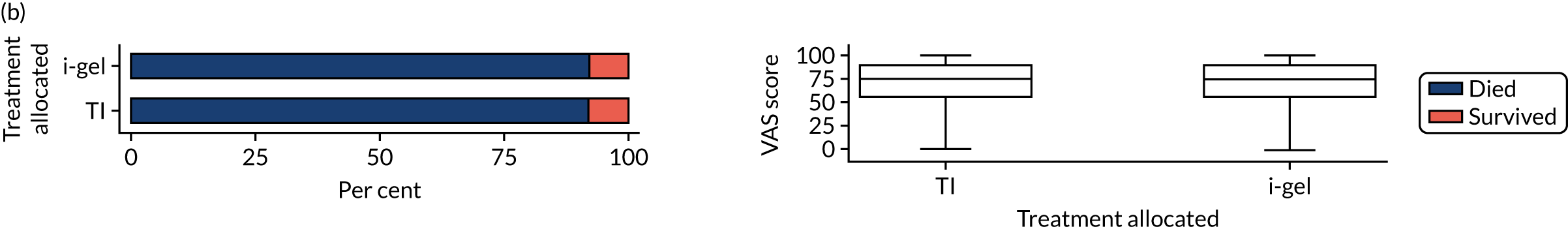
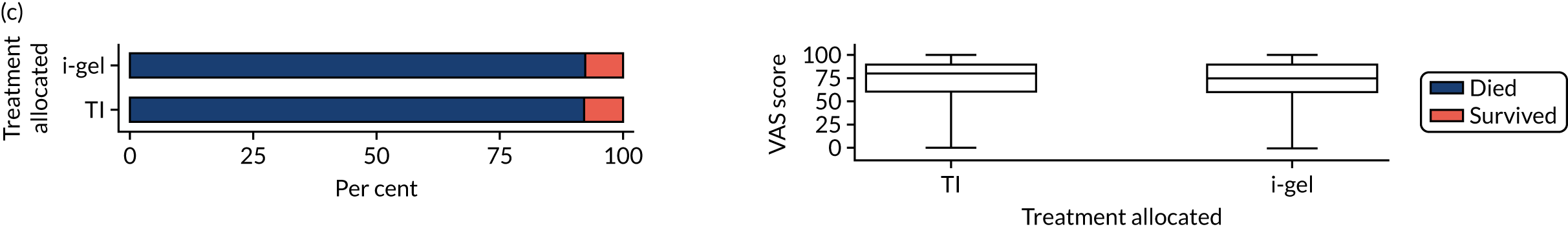
Appendix 2 Summary of substantial amendments to the protocol
Amendments to version 2.0
The randomisation of paramedics was further stratified by ambulance trust and paramedic clinical experience. This change was implemented to ensure that patients had an equal chance of being treated in each of the two treatment groups regardless of the location of their OHCA or the experience of the attending paramedic. The definition of the end of the trial for a participant was clarified depending on which consent option the patient selected. Information relating to data collection was updated to show that a log would be kept of all patients attended by an AIRWAYS-2 paramedic, and that NHS number and date of birth would be collected for all patients regardless of consent status. Information relating to source data was updated to state that the source data for the health resource outcomes would be HES data or, if this was not available, the patients’ medical records. Information relating to the follow-up data collection was amended to state that this would be carried out by telephone or post from the co-ordinating centre at the CTEU Bristol, rather than by physical follow-up by a research nurse. Information relating to data storage was updated to state that personal identifiers for participants would be held until the database had been locked, data validated and results published to ensure that all data linkage took place successfully and all data queries were fully resolved.
Amendments to version 3.0
The economic evaluation section of the protocol was amended to remove reference to an analysis of comparative costs for provision of paramedic pre-registration training because this was considered unlikely to be a key cost driver in the overall cost-effectiveness analysis comparing the two techniques.
Amendments to version 4.0
The analysis plan was amended to state that enrolled patients subsequently identified as being children (aged < 16 years) would be removed from the analysis population. It was expected that this would occur in very few cases where a trial paramedic had inadvertently enrolled a child into the trial.
Amendments to version 5.0
The economic evaluation section of the protocol was updated to include information on linkage of trial data to the HES data set, undertaken with prior permission of the CAG, to allow HES data to be obtained for all patients regardless of consent status. This change was required by the CAG as part of a CAG amendment to progress the application for HES/ONS data.
Appendix 3 Summary of amendments to the statistical analysis plan
The changes from SAP version 1.0 to version 2.0 were as follows:
-
clarified definition of enrolling paramedic to match the protocol
-
changed wording around timing from incident to first crew arrival at the suggestion of the DMSC and TSC
-
corrected inconsistent naming of survival status
-
added new variable at the suggestion of the DMSC and TSC
-
added clarification of what would be reported in the primary outcome paper
-
added detail to the EQ-5D analysis
-
labelling of tables was clarified
-
some figures and tables were amended to improve readability at the suggestion of the DMSC and TSC
-
one protocol deviation removed.
In July 2018, version 3.0 of the SAP was signed off. The changes from SAP version 2.0 to version 3.0 were as follows:
-
clarification around EQ-5D score for consented patients known to have died post discharge
-
extended analysis of EQ-5D scores
-
revised derivation of time to death (0–72 hours)
-
added derivation of event witnessed by ambulance staff
-
revised derivation of the Utstein comparator
-
revised derivation of initial ventilation success
-
regurgitation and aspiration derivations combined
-
derivation of ROSC on ED admission was added
-
binary outcomes brought in line with CONSORT recommendations
-
time-to-event analysis clarified
-
‘per protocol’ changed to ‘as treated’ throughout
-
sensitivity analysis for longitudinal analysis of EQ-5D and mRS scores added.
In August 2019, version 4.0 of the SAP was signed off. The changes from SAP version 3.0 to version 4.0 were as follows:
-
revised the derivation of complete-case and worst-case scenario outcomes using HES
-
extended analysis of EQ-5D scores
-
abbreviations were managed throughout.
Appendix 4 Causal analysis model fitted
The causal analysis model was fitted in two stages. Two instruments were used in this analysis: (1) randomised treatment (R) and (2) whether or not the paramedic was first to the patient’s side (S). The latter was included as an instrument to take into account the guidelines for airway management (i.e. if a paramedic was the first and only person on scene, they would have had to wait for another CPR trained person to arrive before being able to attempt TI).
Patients were classified as having received TI first if the first AAM attempt was TI. Similarly, patients were classified as having received i-gel first if the first AAM attempt was i-gel. Thus, patients could be (1) attended by a paramedic randomised to TI and receive TI, (2) attended by a paramedic randomised to TI and receive i-gel, (3) attended by a paramedic randomised to TI and receive neither i-gel nor TI, (4) attended by a paramedic randomised to i-gel and receive TI, (5) attended by a paramedic randomised to i-gel and receive i-gel or (6) attended by a paramedic randomised to i-gel and receive neither i-gel nor TI. These classifications did not consider whether or not these AAM attempts were successfully established.
The three usual assumptions of the instrumental variable analysis were (1) the relevance assumption [i.e. that randomisation predicted the AAM treatment a patient received (to a certain degree)], (2) the exchangeability assumption (i.e. that randomisation was independent of the confounding factors that could contribute to the outcome) and (3) the exclusion restriction (i.e. that randomisation affected the outcome through influencing the treatment received only). In addition to these, the following three assumptions were made: (4) S does not directly influence the effect of TI or i-gel, (5) S differentially predicts adherence across the two treatments and (6) S does not affect the outcome, except through treatment received. Assumptions 1–3 were likely to be valid owing to the nature of RCTs. Additionally, assumptions 4–6 were likely to be valid because S was not deemed to be a confounder in the original ITT or as-treated analyses; in addition, the algorithms in place meant that paramedics could not administer TI if they were the only trained person on scene.
First, the treatments received were regressed on the two instruments and the interaction between the two instruments to obtain the predicted values X1⌢ and X2⌢:
where X1 = 1 if the patient received TI as first AAM and X1 = 0 if the patient received a first AAM treatment that was not TI, X2 = 1 if the patient received i-gel first as AAM and X2 = 0 if the patient received a first AAM treatment that was not i-gel, R1 = 1 if the paramedic who enrolled the patient was randomised to TI and R1 = 0 otherwise, R2 = 1 if the paramedic who enrolled the patient was randomised to i-gel and R2 = 0 otherwise, S = 1 if the paramedic attended on their own and was the first to the patient’s side and S = 0 otherwise, and α0,. . .,α7 are the parameters of the variables in these two models.
Second, the primary outcome variable (dichotomised mRS score) was regressed on the estimates X1⌢ and X2⌢ and the randomisation stratification variables to obtain the ADP, β^ (i-gel – TI):
where paramedic experience had two levels (< 5 years and ≥ 5 years); distance from base ambulance station had two levels [< 5 miles (urban) and ≥ 5 miles (rural)]; ambulance trusts 2, 3 and 4 were dummy variables, each of which had two levels (patient from relevant ambulance trust and patient not from relevant ambulance trust); and β0,. . . β7 are the parameters of the variables in this model.
The models in both stage 1 and stage 2 were fitted with a binomial error family and an identity link to obtain the ADP. They were also fitted with a clustered sandwich estimator for paramedic to adjust for the fact that paramedics were randomised rather than patients. Finally, the estimate of the ADP, β^ (i-gel – TI), between the two treatment groups was obtained by taking the difference between β2 : β1:β^=β2−β1.
Appendix 5 Data cleaning for the economic evaluation and handling of missing data
Data cleaning for the economic evaluation
This appendix provides further details on how data were cleaned and analysed, which was undertaken without reference to treatment group to minimise bias.
Pre hospital
In the pre-hospital phase, a series of dates and times were collected: time each ambulance arrived at the scene, time of death or time the patient left the scene for hospital, and time of arrival at hospital. These data were used to calculate the duration spent by ambulance staff with patients. For each paramedic attending an AIRWAYS-2 patient, the time from arrival at the scene of the OHCA to the time they took the patient into hospital (or the time the patient left the scene if transported in another vehicle), or until time of death if the patient died at the scene, was calculated. Where this generated implausible durations with the patient, such as negative durations of ≥ 10 minutes, details of all staff and vehicles attending the patient were reviewed manually to identify typos in the series of dates and times. Negative durations of < 10 minutes were assumed to be paramedics arriving too late to assist, unless the paramedic was arriving > 1 hour after the first paramedic on scene, in which case details were reviewed manually. The series of dates and times were reviewed manually for durations > 3 hours to correct typos and/or check that this was plausible against the other times recorded. Time at the scene after a patient’s death (awaiting police, for example) or after a patient had left for hospital in another vehicle was not costed.
Intensive care unit stays
Dates and times of initial admission to ICU, discharge from ICU or death in ICU were captured on the trial CRFs. Dates were complete for all patients; mean imputation was used to handle five missing times. Additional time in ICU for patients subsequently re-admitted to ICU later in their index admission was available for patients who had consented to the trial only.
Hospital Episde Statistics data sets: emergency medicine, admitted inpatient care and non-admitted consultations
The three HES data sets for ED attendance, inpatient care and outpatients visits were cleaned and inputted into the HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108). This produced Healthcare Resource Group (HRG)/service codes for each activity, which were then costed using NHS Reference Costs 2017/18. 62
For admitted inpatient care, one of the inputs the grouper requires is the number of days spent in ICU for that episode of care. ICU care is costed separately, but days in ICU need to be excluded from the length of stay for an episode to correctly calculate any additional bed-days to be costed beyond a standard length of stay for that HRG code (known as excess bed-days). The initial ICU length of stay from the trial CRFs was merged into this HES data set and the days split through one or more episodes of care for the initial inpatient stay. Given the complexity of the data set, and missing data for patients who did not consent, it was not possible to merge time in ICU beyond the initial stay into the admitted inpatient care data set.
For each of the data sets, duplicate records were dropped. Non-identical records with the same date for ED attendance and the same date and speciality for outpatients appointments were assumed to be duplicates, and one was dropped. Only those outpatient appointments that the patients attended were costed.
Handling of missing data
As approximately 100 resource use variables were used in the analyses, patterns of missing data were explored for subsections of the data, by time point (pre hospital, in hospital, post discharge) and by data source (various CRFs, HES and patient follow-up questionnaires) to determine the level of aggregation for imputation.
To make best use of available data on key cost drivers in cost components, mean imputation was used for a few low-cost items. In the pre-hospital phase, staff and vehicles were the key cost drivers and were complete for all but 14 patients. Mean imputation was used to complete the costs of airway devices. Similarly, in the patient follow-up questionnaires, mean imputation was used to complete partially missing data, for example where patients had responded ‘yes’ to having seen a health-care professional but did not record the number of visits.
Multiple imputation
The following cost components were imputed for initial care: pre hospital, ED attendance, inpatient care, subsequent intensive care days. The following cost components were imputed for the follow-up period to 6 months: ED attendance, inpatient care, outpatients visits, community care up to 3 months and community care from 3 to 6 months. These cost components were imputed together with the three EQ-5D scores; the complete variables age, sex and ambulance trust; initial ICU length of stay; indicator variables for whether or not the patient survived to the ED and for alive at 6 months; and a time alive variable, separately by treatment group. The time alive variable was either days to death or 183 days for patients who survived to 6 months. Each variable was imputed conditional on being alive at that stage. Prediction mean matching with 10 nearest neighbours was used (so, based on the variables included, the 10 most similar patients were identified and the costs for one randomly selected patient assigned to the patient with missing data).
Survival status to 6 months was unknown for 11 patients in the trial: 10 patients who survived to hospital discharge (five in each group) and one patient who survived to 3 months. To accommodate these patients in the imputation, they were assigned a weighted average of time in follow-up/time to death of patients who survived to hospital discharge and 3 months.
Figures 33 and 34 compare the observed total costs for patients with complete data and total costs for all patients, respectively, based on the imputed data. Given the skewed nature of the distributions, the figures are split into two so that the shape of the distribution can be seen more clearly. The outer figures show the total costs for patients with costs ≤ £20,000; the inset figures show the patients with costs > £20,000. The figures of imputed data reflect what is known of patients with missing costs; the larger number of patients with low costs reflects those who died early on in the trial who were missing the cost of airway devices only, and the heavier positive tail reflects the many patients with high resource use who were missing total costs.
FIGURE 33.
Observed total costs. The outer figure shows the total costs for patients with costs ≤ £20,000; the inset figure shows the total costs for patients with costs > £20,000.
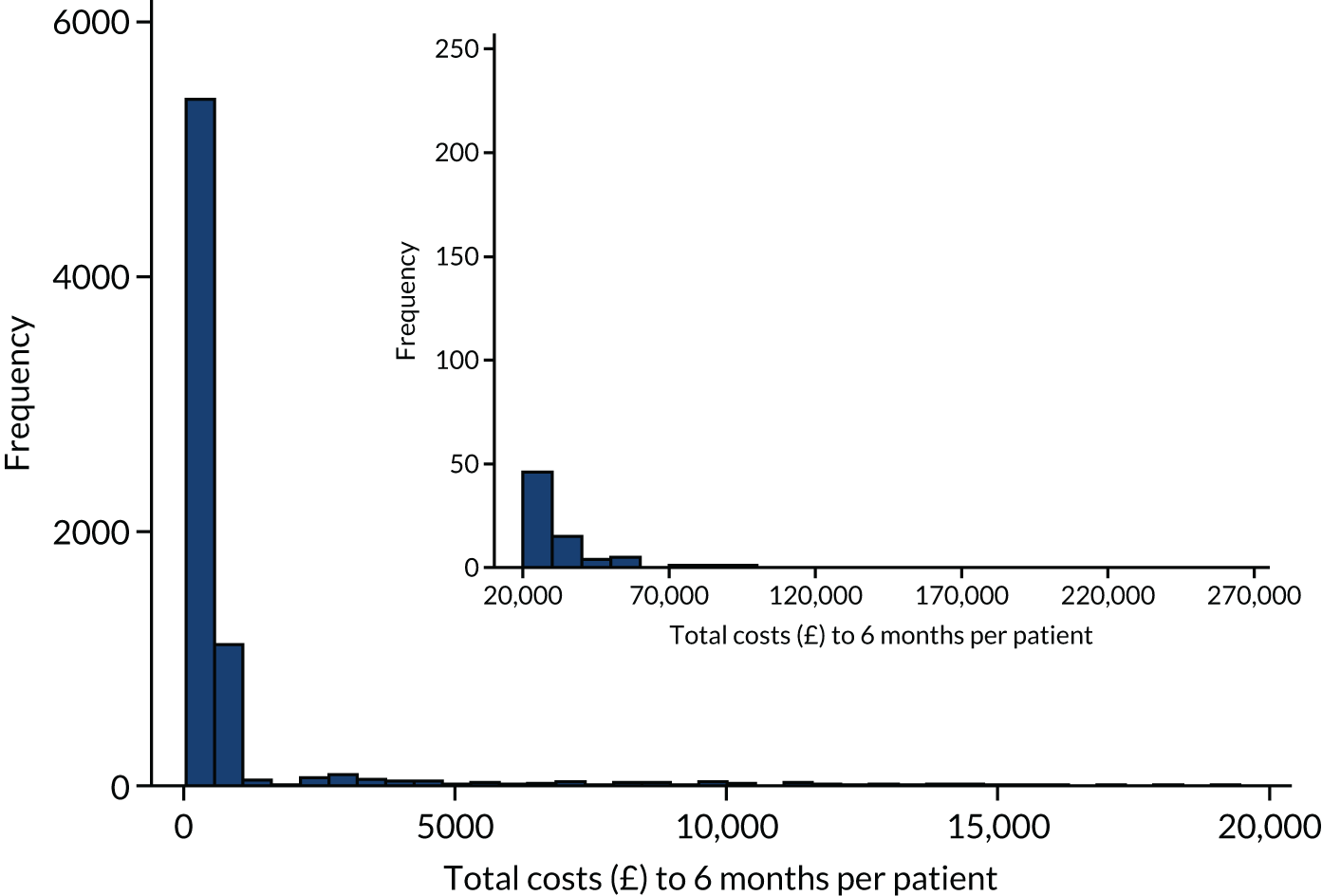
FIGURE 34.
Total costs for all patients (with missing data imputed). The outer figures show the total costs for patients with costs ≤ £20,000; the inset figures show the patients with costs > £20,000.
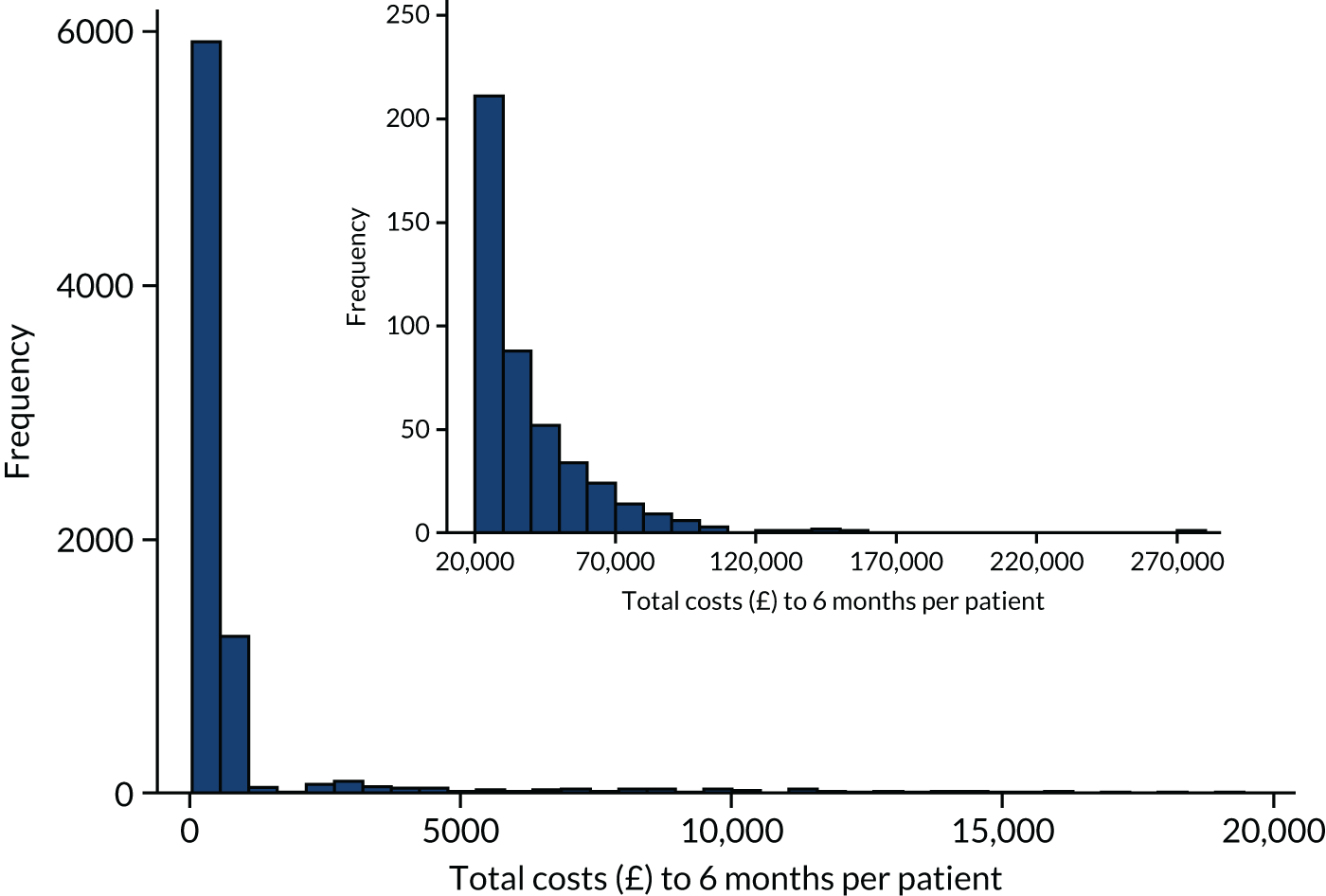
Appendix 6 Unit costs used in the economics evaluation
Note that for unit costs not in 2017/2018, prices have been adjusted to 2017/2018 prices using the NHSCII. 66
| Resource | Unit cost (£) | Reference |
|---|---|---|
| Airway management | ||
| i-gel | 4.88 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 |
| Endotracheal tube | 0.78 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 (range £0.61–0.98) |
| Disposable laryngoscope handles | 2.71 | Supplied by SWAST on 18 May 2018 from PROACT Medical Ltd (Motherwell, UK) |
| Disposable laryngoscope blades | 2.78 | Supplied by SWAST on 18 May 2018 from PROACT Medical Ltd |
| OPA | 0.19 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 |
| NPA | 0.52 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 |
| Laryngeal mask airway | 15.52 | Supplied by SWAST on 18 May 2018 from MedTree (Telford, UK) |
| Bag valve mask: adult | 4.79 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 |
| Bougie | 10.86 | Supplied by SWAST on 18 May 2018 from NHS Supply Chain60 |
| Pocket mask | 1.72 | NHS Supply Chain.60 FDD1672. CPR personal pocket mask |
| Face shield | 0.86 | NHS Supply Chain.60 FDG1623. Mouth-to-mouth resuscitation face shield |
| Ambulance staff (per hour) | ||
| Band 6+ | 28.01 | NHS Employers.61 Assumed average of top and bottom salary for pay band; added 20% for national insurance and superannuation; assumed 42.6 weeks worked per year and 37.5 hours per week; added 20% for overheads |
| Band 5 | 22.93 | |
| Band 4 | 18.97 | |
| Band 2 or 3 | 15.89 | |
| Vehicles | ||
| Vehicle attending patient at the scene but does not convey patient to hospital | 52.00 | NHS Reference Costs 2017/18.62 Ambulance. See and treat or refer. Assumed the proportion of this cost relating to vehicles was 27.1% (staff costs made up 72.9% of SWAST operating expenditure in 2017/18)109 |
| Vehicle attending patient at the scene and conveys patient to hospital | 68.00 | NHS Reference Costs 2017/18.62 Ambulance. See and treat and convey. Assumed the proportion of this cost relating to vehicles was 27.1% (staff costs made up 72.9% of SWAST operating expenditure in 2017/18)109 |
| In hospital | ||
| ED attendance | Various | NHS Reference Costs 2017/18.62 Currency codes generated by HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108), costed using the same currency codes on emergency medicine sheet |
| Inpatient care | Various | NHS Reference Costs 2017/18.62 HRG codes generated by HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108), costed using the elective inpatients, elective inpatients’ excess bed-days, non-elective inpatients, non-elective inpatients’ excess bed-days, non-elective short stay, day case, regular day or night admissions, chemotherapy, diagnostic imaging, nuclear medicine, rehabilitation, specialist palliative care and renal dialysis sheets. Five costs that were unavailable were sourced from the high-cost drugs sheet in NHS Reference Costs 2016/1765 |
| ICU bed-day | 1467.00 | NHS Reference Costs 2017/18.62 Critical care. Non-specific, general adult critical-care patients predominate [weighted average of XC01Z–XC07Z; 0–6 (or more) organs supported] |
| Resource | Unit cost (£) | Reference |
|---|---|---|
| Secondary care | ||
| Inpatient care | Various | NHS Reference Costs 2017/18.62 HRG codes generated by HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108), costed using the elective inpatients, elective inpatients’ excess bed-days, non-elective inpatients, non-elective inpatients’ excess bed-days, non-elective short stay, day case, regular day or night admissions sheets |
| Outpatient appointments | Various | NHS Reference Costs 2017/18.62 Currency codes generated by HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108), costed using the same currency codes on consultant-led, non-consultant-led and outpatient procedures sheets |
| ED attendance | Various | NHS Reference Costs 2017/18.62 Currency codes generated by HRG4+ 2017/18 Reference Costs Grouper (NHS Digital108), costed using the same currency codes on the Emergency Medicine Sheet |
| Stays away from home | ||
| Nursing home (1 week) | 793.00 | Unit Costs of Health and Social Care 2018.63 1.1. Private sector nursing homes for older people (aged ≥ 65 years). Mean per-person weekly PSS and NHS contributions to nursing home care |
| Residential home (1 week) | 591.00 | Unit Costs of Health and Social Care 2018.63 1.2. Private sector residential care for older people (aged ≥ 65 years). Mean per-person weekly PSS contributions to residential care |
| Hospice (per day) | 141.00 | Cost per day in hospice and estimate of proportion paid for by government (one-third) sourced from Georghiou and Bardsley110 |
| Equipment and aids | ||
| Wheelchair | 101.00 | Unit Costs of Health and Social Care 2018.63 7.2. Cost per self- or attendant-propelled chair per year |
| Shower chair | 35.50 | NHS Supply Chain.60 GTB256. Shower chair commode |
| Handrails | 91.20 | Unit Costs of Health and Social Care 2018.63 7.3. Assumed two internal rails (materials and fitting) |
| Walking stick | 3.36 | NHS Supply Chain.60 GVK019. Walking stick, aluminium |
| Zimmer frame | 13.40 | NHS Supply Chain.60 GTF7609. Walking frame without wheels |
| Other | ||
| Air cushion | 117.96 | NHS Supply Chain.60 TLC898. High-pressure relieving air cushion |
| Air mattress | 282.86 | NHS Supply Chain.60 FYC930. Dynamic air mattress |
| Bath seat | 21.36 | NHS Supply Chain.60 GKC012. Bath seat |
| Bath lift | 206.14 | NHS Supply Chain.60 TRK001. Bath lift |
| Bed rail | 13.15 | NHS Supply Chain.60 GKZ132. Bed support rail |
| Bed prop | 20.84 | NHS Supply Chain.60 GTB1208. Bed mattress and pillow raiser bed wedge |
| Commode | 22.54 | NHS Supply Chain.60 FYB724. Commode |
| Crutches | 8.93 | NHS Supply Chain.60 GTB111. Crutch, double adjustable (pair) |
| Food trolley | 35.31 | Sheba trolley (Complete Care, Coalville, UK) |
| Incontinence pads | 31.73 | NHS Supply Chain.60 CFP1975. Belt product for moderate to heavy incontinence. Pack of 75 |
| Kitchen seat | 27.48 | NHS Supply Chain.60 GTB1117. Perching stool |
| Memory assist monitor | 159.37 | Memrabel 2i daily memory prompting aid (Medpage Ltd, Corby, UK) |
| Panic alarm | 160.21 | Age UK. Personal alarm (PPP Taking Care Ltd, London, UK). Cost for 6 months |
| Rollator | 43.52 | NHS Supply Chain.60 GTF470. Four-wheel walker rollator |
| Toilet seat and frame | 25.77 | NHS Supply Chain.60 FYB953. Toilet seat and frame |
| Toilet frame | 20.61 | NHS Supply Chain.60 GTB1567. Toilet frame |
| Raised armchair seat | 22.90 | NHS Supply Chain.60 FER11207. Static seating cushion |
| Sensor/alarm in bedroom | 27.68 | PIR motion sensor and voice alert alarm system (Living Made Easy Ltd, Oldbury, UK) |
| Telephone door release | 443.77 | Radio frequency door latch release system (Living Made Easy Ltd) |
| Wedge cushion | 12.14 | NHS Supply Chain.60 FTN180. Wedge cushion |
| Resource | Unit cost (£) | Reference |
|---|---|---|
| GP at surgery | 28.00 | Unit Costs of Health and Social Care 2018.63 10.3b. GP – unit costs. Per surgery consultation lasting 9.22 minutes. Excluding qualification costs and direct care staff costs |
| GP at home | 50.00 | Unit Costs of Health and Social Care 2018.63 10.3b. GP – unit costs. £110 per hour of General Medical Services activity. Excluding qualification costs and direct care staff costs. Assume home visit same duration/cost as surgery visit, and 12 minutes of travel time111 |
| GP telephone call | 12.00 | Unit Costs of Health and Social Care 2018.63 10.3b. GP – unit costs. £3 per minute of patient contact. Excluding qualification costs and direct care staff costs. From 10.5. Telephone triage – GP led and nurse led, average time per intervention 4 minutes |
| Out-of-hours GP | 28.00 | As for GP at surgery |
| Walk-in centre | 28.00 | As for GP at surgery |
| GP nurse | 12.09 | Unit Costs of Health and Social Care 2018.63 10.2. Nurse (GP practice). £36 per hour, excluding qualification costs. Ratio of direct-to-indirect time on face-to-face contacts is 1 : 0.30, and average contact time is 15.5 minutes111 |
| District nurse | 38.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N02AF, District nurse, adult, face to face |
| Other NHS or social services | ||
| Cardiac nurse/cardiac rehabilitation nurse/heart failure nurse | 86.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N11AF, specialist nursing – cardiac nursing/liaison, adult, face to face |
| Cardiac rehabilitation/exercise class | 43.00 | NHS Reference Costs 2017/18.62 Rehabilitation. Non-specialist rehabilitation services level 3, other, rehabilitation for acute myocardial infarction or other cardiac disorders |
| Dentist | 92.00 | NHS Reference Costs 2017/18.62 Community health services – medical and dental, M01B, general dental service, attendance |
| Diabetic nurse | 67.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N15AF, specialist nursing – diabetic nursing/liaison, adult, face to face |
| Mental health counsellor/anxiety and depression service/counselling/psychotherapy treatment | 95.00 | Unit Costs of Health and Social Care 2018.63 2.1. NHS reference costs for mental health services; mental health specialist teams (per care contact); improving access to psychological therapies, adult and elderly |
| Occupational therapist | 81.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A06A1, occupational therapist, adult, one to one |
| Palliative care nurse | 104.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N21AF, specialist nursing, palliative/respite care, adult, face to face |
| Physiotherapist | 57.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A08A1, physiotherapist, adult, one to one |
| Podiatrist | 41.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A09A, podiatrist, tier 1, general podiatry |
| Social worker | 61.00 | Unit Costs of Health and Social Care 2018.63 11.1. Social worker (adult services). £61 per hour of client-related work, excluding qualification costs. Assume 1 hour for direct contact and case-related work |
| Other NHS or social services at home | ||
| Cardiac nurse/cardiac rehabiliation nurse/heart failure nurse | 86.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N11AF, specialist nursing – cardiac nursing/liaison, adult, face to face |
| Cardiac rehabilitation | 43.00 | NHS Reference Costs 2017/18.62 Rehabilitation. Non-specialist rehabilitation services level 3, other, rehabilitation for acute myocardial infarction or other cardiac disorders |
| Carer | 13.50 | Unit Costs of Health and Social Care 2018.63 11.5. Home care worker. Assume 30-minute visit |
| Clinical health psychologist | 95.00 | Unit Costs of Health and Social Care 2018.63 2.1. NHS reference costs for mental health services; mental health specialist teams (per care contact); IAPT, adult and elderly |
| Community stroke team | 91.00 | NHS Reference Costs 2017/18.62 Community health services – community rehabilitation teams, stroke community rehabilitation teams |
| Diabetic nurse | 67.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N15AF, specialist nursing – diabetic nursing/liaison, adult, face to face |
| Dietitian | 86.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A03 – dietitian |
| Emergency response team nurse/quick response | 104.00 | NHS Reference Costs 2017/18.62 Community health services – intermediate care, IC01, intermediate care, crisis response and early discharge services |
| Health visitor | 53.00 | NHS Reference Costs 2017/18.62 Community health services – N03F, health visitor, other clinical intervention |
| Home hospital | 82.00 | NHS Reference Costs 2017/18.62 Community health services – intermediate care, ic03, intermediate care home based services |
| Occupational therapist | 81.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A06A1, occupational therapist, adult, one to one |
| Paramedics | 192.00 | NHS Reference Costs 2017/18.62 Ambulance. ASS01 – see and treat or refer |
| Pharmacist technician | 29.00 | Unit Costs of Health and Social Care 2018.63 Scientific and professional staff. Band 4. Cost per working hour £29. Assume 1 hour for direct and indirect patient care and travel |
| Physiotherapist | 57.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A08A1, physiotherapist, adult, one to one |
| Podiatrist | 41.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A09 A, Podiatrist, Tier 1, General podiatry |
| Nurse specialist (used for neurology/psychiatric/urology and continence care) | 79.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N29AF, other specialist nursing, adult, face to face |
| Respiratory nurse | 85.00 | NHS Reference Costs 2017/18.62 Community health services – nursing, N08AF, specialist nursing – asthma and respiratory nursing/liaison, adult, face to face |
| Social worker | 76.00 | Unit Costs of Health and Social Care 2018.63 11.1. Social worker (adult services). £61 per hour of client-related work, excluding qualification costs. Assume 1.25 hours for direct contact, travel and case-related work |
| Speech therapist | 96.00 | NHS Reference Costs 2017/18.62 Community health services – allied health professionals, A13A1, speech and language therapist, adult, one to one |
Appendix 7 Sensitivity analyses for the economic evaluation
Sensitivity analyses for costing were conducted to investigate varying a number of unit costs and the impact of any high-cost patients. Sensitivity analyses around outcomes explored the impact of an assumed baseline utility of 0 rather than –0.402, and considered life-years rather than QALYs. Each of these sensitivity analyses is considered in turn.
Sensitivity analyses around unit costs
Table 45 describes the unit costs around paramedic time, ED attendance, intensive care and inpatient care that were varied in sensitivity analyses. Given concerns among the team that NHS reference costs for ED attendance underestimate the resources required for these very ill patients, sensitivity analyses were used to explore higher unit costs for this activity (sensitivity analysis 2); Table 46 reports the results. In line with inpatient and intensive care costs being key drivers of total costs, varying these costs by ± 50% (in sensitivity analyses 3 and 4) had the greatest impact on total costs in each group. However, none of the sensitivity analyses had a great impact on the cost difference between groups. The cost differences across the sensitivity analyses ranged from £90 to £229, bracketing, and all very similar to, the base-case cost difference of £157.
| Sensitivity analysis | Resource | Unit costs (£) used in base-case analysis | Alternative strategy for sensitivity analysis |
|---|---|---|---|
| 1 | Paramedic time | 16, 19, 23, 28 for bands 2/3, 4, 5, 6+, respectively, per hour | ± 50% |
| 2 | Index ED attendance | Various (67–445) | + 50%, + 100% |
| 3 | Intensive care bed-days in index admission | 1467 | ± 50% |
| 4 | Inpatient care in index admission | Various (161–65,406) | ± 50% |
| Sensitivity analysis | Cost (£), mean (SE) | Cost (£) difference, mean (95% CI) | |
|---|---|---|---|
| TI group (n = 4407) | i-gel group (n = 4882) | i-gel vs. TI | |
| Base case | 3413 (162) | 3570 (152) | 157 (–278 to 592) |
| 1 (paramedic staff) | |||
| + 50% | 3451 (163) | 3605 (153) | 154 (–284 to 592) |
| – 50% | 3384 (163) | 3538 (152) | 154 (–284 to 591) |
| 2 (ED) | |||
| + 50% | 3483 (163) | 3644 (152) | 161 (–277 to 599) |
| + 100% | 3554 (164) | 3716 (152) | 161 (–278 to 600) |
| 3 (intensive care) | |||
| + 50% | 4117 (210) | 4345 (191) | 229 (–326 to 784) |
| – 50% | 2708 (120) | 2798 (116) | 90 (–238 to 418) |
| 4 (inpatient care) | |||
| + 50% | 4080 (191) | 4260 (184) | 179 (–341 to 699) |
| – 50% | 2732 (136) | 2875 (121) | 142 (–215 to 500) |
Sensitivity analyses around high-cost patients
The distribution of total costs per patient is positively skewed in both treatment groups. It is possible that a few high-cost outliers are exerting influence over the mean costs in each group and the overall findings; therefore, we investigated the existence of outliers and their effects. There were nine patients with costs > £100,000, of whom three were in the TI group and six were in the i-gel group. These patients all stayed in intensive care for > 30 days and had high inpatient and intensive care costs. There was one extremely high-cost patient in the TI group (who was in intensive care throughout the 6-month time horizon), with total costs of £271,014. There are no grounds for excluding these patients from the analyses; nevertheless, it is instructive to investigate the impact that they have on the cost results, as an imbalance across groups of these outliers could easily have arisen by chance.
Table 47 shows the effects on costs in each treatment group of excluding the highest-cost patient and of excluding the nine highest-cost patients, each with total costs > £100,000. When the highest-cost patient in the TI group is excluded, the mean difference in costs between the groups increases from £157 to £217. If participants with the nine highest costs are excluded, mean costs in each group fall, and the mean cost difference between groups falls to £120. Although these patients exert a significant impact on the cost results, they do not alter conclusions.
| Sensitivity analysis | Cost (£), mean (SE) | Cost (£) difference, mean (95% CI) | |
|---|---|---|---|
| TI group (n = 4407) | i-gel group (n = 4882) | i-gel vs. TI | |
| Base case (all patients) | 3413 (162) | 3570 (152) | 157 (–278 to 592) |
| Exclude highest-cost patient | 3353 (152) | 3570 (152) | 217 (–204 to 638) |
| Exclude nine highest-cost patients | 3299 (144) | 3419 (142) | 120 (–276 to 516) |
Sensitivity analyses around outcomes
Two sensitivity analyses were conducted around outcomes. In the base-case analysis, baseline utility at the time of OHCA was assumed to be –0.402, the value for unconscious. Baseline utility was assumed to be 0 in a sensitivity analysis. Under this assumption, the large proportion of patients who die early on in the trial will contribute nothing, rather than negatively, to QALYs. Given that a large number of patients in the trial died, a sensitivity analysis exploring life-years as an outcome measure rather than QALYs was also conducted. Results are shown in Table 48. Increasing baseline utility to 0 increases QALYs to 6 months slightly in both treatment groups, but the difference between groups is unchanged. Life-years are higher than QALYs in each treatment group, but the difference between groups is small, not statistically significant and similar to the difference in QALYs.
| Sensitivity analysis | TI group (n = 4407), mean (SE) | i-gel group (n = 4882), mean (SE) | i-gel vs. TI, mean difference (95% CI) |
|---|---|---|---|
| QALYs to 6 months | |||
| Base case | 0.0274 (0.0016) | 0.0259 (0.0015) | –0.0015 (–0.0059 to 0.0028) |
| Baseline utility = 0 | 0.0284 (0.0017) | 0.0269 (0.0015) | –0.0015 (–0.0059 to 0.0029) |
| Life-years to 6 months | 0.0426 (0.0022) | 0.0415 (0.0020) | –0.0011 (–0.0070 to 0.0048) |
Appendix 8 Additional cost tables
| Cost category | TI group | i-gel group | i-gel vs. TI | ||
|---|---|---|---|---|---|
| n (%) | Cost (£), mean (SE) | n (%) | Cost (£), mean (SE) | Mean difference (95% CI) | |
| Pre hospital | |||||
| See Table 24 | |||||
| Taken to hospital | |||||
| ED attendance | 4170 (95) | 132 (3) | 4617 (95) | 140 (3) | 8 (–1 to 16) |
| Admitted to hospital | |||||
| Index inpatient care | 4348 (99) | 1260 (66) | 4823 (99) | 1319 (70) | 58 (–130 to 247) |
| ICU days | 4158 (94) | 1035 (88) | 4605 (94) | 1138 (65) | 103 (–110 to 317) |
| Post hospital discharge (or 30 days if sooner) | |||||
| Further inpatient days | 4382 (99) | 167 (28) | 4861 (100) | 134 (18) | –33 (–97 to 31) |
| Further ED attendances | 4333 (98) | 9 (1) | 4810 (99) | 9 (1) | 0 (–3 to 3) |
| Outpatient appointments | 4352 (99) | 54 (5) | 4831 (99) | 60 (5) | 5 (–8 to 19) |
| Community care | 4094 (93) | 4 (1) | 4544 (93) | 4 (1) | 1 (–2 to 3) |
| Total | 3347 (76) | 1175 (71) | 3961 (81) | 1392 (70) | 217 (22 to 412) |
| Cost category | Cost (£), mean (SE) | Mean difference (95% CI) | |
|---|---|---|---|
| TI group (N = 3347) | i-gel group (N = 3961) | i-gel vs. TI | |
| Pre hospital | |||
| Initial airway management pre AIRWAYS-2 paramedic | 1 (0) | 1 (0) | 0 (0 to 0) |
| AAM devices used by AIRWAYS-2 paramedic | |||
| TI | 12 (0) | 2 (0) | –9 (–10 to –9) |
| i-gel | 2 (0) | 5 (0) | 4 (3 to 4) |
| Other (OPA, NPA, LMA) | 1 (0) | 0 (0) | –1 (–1 to –1) |
| Total | 14 (0) | 8 (0) | –7 (–7 to –6) |
| Ambulance staff at scene | |||
| Band 6+ | 21 (1) | 21 (1) | 0 (–2 to 3) |
| Band 5 | 23 (1) | 23 (1) | 1 (–1 to 2) |
| Band 4 | 5 (0) | 5 (0) | 0 (–1 to 0) |
| Band 2 or 3 | 9 (0) | 9 (0) | 0 (0 to 1) |
| Total | 58 (1) | 58 (1) | 0 (–2 to 3) |
| Vehicles | 143 (1) | 143 (1) | 0 (–3 to 3) |
| Pre-hospital total | 215 (2) | 210 (2) | –6 (–11 to –1) |
| Taken to hospital | |||
| ED attendance | 114 (3) | 123 (3) | 8 (–1 to 18) |
| Admitted to hospital | |||
| Index inpatient care | 316 (27) | 404 (32) | 88 (8 to 169) |
| ICU days | 504 (46) | 632 (42) | 129 (7 to 250) |
| Post hospital discharge (or 30 days if sooner) | |||
| Further inpatient days | 14 (5) | 9 (5) | –5 (–20 to 10) |
| Further ED attendances | 1 (0) | 0 (0) | 0 (–1 to 0) |
| Outpatient appointments | 6 (1) | 9 (2) | 3 (–2 to 8) |
| Community care | 2 (1) | 3 (1) | 0 (–1 to 2) |
| Total | 1175 (71) | 1392 (70) | 217 (22 to 412) |
Appendix 9 Trial case report forms
Page redacted for copyright reasons.
Page redacted for copyright reasons.
Page redacted for copyright reasons.
Page redacted for copyright reasons.
Page redacted for copyright reasons.
Page redacted for copyright reasons.
List of abbreviations
- AAM
- advanced airway management
- ADP
- adjusted difference in proportions of patients
- AE
- adverse event
- BMV
- bag–mask ventilation
- CAD
- computer-aided dispatch
- CAG
- Confidentiality Advisory Group
- CCU
- coronary care unit
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPR
- cardiopulmonary resuscitation
- CRF
- case report form
- CT
- computerised tomography
- CTEU
- Clinical Trials and Evaluation Unit
- DMSC
- Data Monitoring and Safety Committee
- ECG
- electrocardiogram
- ED
- emergency department
- EEAST
- East of England Ambulance Service NHS Trust
- EMAS
- East Midlands Ambulance Service NHS Trust
- EMS
- emergency medical services
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GMR
- geometric mean ratio
- HCPC
- Health and Care Professions Council
- HES
- Hospital Episode Statistics
- HMPS
- Her Majesty’s Prison Service
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- ILCOR
- International Liaison Committee on Resuscitation
- IQR
- interquartile range
- ITT
- intention to treat
- JRCALC
- Joint Royal Colleges Ambulance Liaison Committee
- mRS
- modified Rankin Scale
- NHSCII
- NHS Cost Inflation Index
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- OHCA
- out-of-hospital cardiac arrest
- ONS
- Office for National Statistics
- OPA
- oropharyngeal airway
- OR
- odds ratio
- PART
- Pragmatic Airway Resuscitation Trial
- PEA
- pulseless electrical activity
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROSC
- return of spontaneous circulation
- RR
- risk ratio
- SADE
- serious adverse device event
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- SGA
- supraglottic airway device
- SWAST
- South Western Ambulance Service NHS Foundation Trust
- TI
- tracheal intubation
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- YAS
- Yorkshire Ambulance Service NHS Trust
