Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/137/01. The contractual start date was in March 2013. The draft report began editorial review in March 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Dasgupta et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from McClinton et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
In 2011, the National Institute for Health Research Health Technology Assessment programme called for a randomised controlled trial (RCT) to answer the following question: is extracorporeal shockwave lithotripsy (SWL) a clinically effective and cost-effective treatment for ureteric stones? This report describes the research that was subsequently commissioned and commenced in 2013.
The Therapeutic Interventions for Stones of the Ureter (TISU) trial was a large, pragmatic, multicentre UK-based RCT investigating the clinical effectiveness and cost-effectiveness of SWL as a first-line treatment option, compared with primary ureteroscopic stone treatment (URS), for ureteric stones. Previous studies have suggested that URS is more clinically effective at making patients stone free (albeit with a higher complication rate and longer hospital stay2,3), but SWL is likely to be more cost-effective. However, there was marked uncertainty about which treatment pathway is the more effective and efficient from the perspective of both the UK NHS and patients suffering pain due to a ureteric stone.
The health problem
Urinary tract stone disease, or urolithiasis, is the formation of stones or calculi in the urinary tract. Urinary tract stone disease is very common, with an estimated lifetime prevalence of 13% in the UK,4,5 and it is more common in men than in women. 6 In the UK the prevalence is rising, with the number of interventions for stone disease also increasing. 7 This increasing prevalence has also been observed in other countries, for example in the USA, where the prevalence is expected to continue to rise. 8
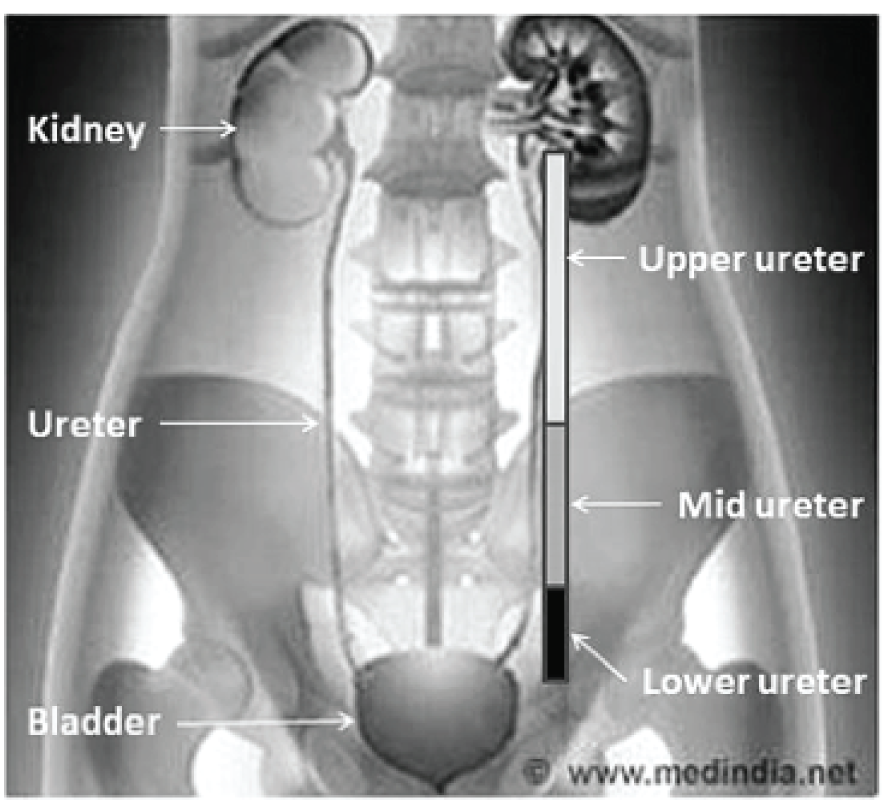
Ureteric stones are crystalline deposits that are originally formed in the collecting part of one or both kidneys that subsequently pass into the ureters. The ureter is the urine drainage tube connecting the kidney to the bladder (Figure 1). Ureteric stones can block the flow of urine and can cause pain (ureteric colic) in the flank, lower abdomen and groin, which is typically severe and recurrent. This sudden severe pain, which is associated with a stone migrating from the kidney into the ureter causing intermittent obstruction, can necessitate urgent attention from a general practitioner (GP) or a hospital emergency department or emergency admission to a urology department.
FIGURE 1.
Anatomy of urinary tract showing definition of ureteric segments. Reproduced with permission from Medindia. 9
Urinary stones often recur and the lifetime recurrence rate is approximately 50%. 10 The interval between recurrences is variable, with approximately 10% recurring within 1 year, 35% within 5 years and 50% within 10 years. 11 The increased incidence of urinary stones in the industrialised world is associated with improved standards of living (mainly due to the high dietary intake of proteins and minerals) and there is also an association with ethnicity and region of residence. 12 Urinary stones affect younger adults (aged 20–55 years) of working age,5 and so have a personal and societal cost owing to working days missed. There is limited evidence on the impact of stone disease on patient quality of life, but patients describe the pain associated with stone disease as one of the worst imaginable. 13
There is an increasing recognition of the rising health burden due to urinary stone disease, with a 63% increase in hospital episodes from 2000 to 2010. 5 There is an increasing trend towards the use of URS as the treatment modality for stone disease. 4 URS is increasingly being used as an emergency intervention, with a 38% increase in emergency URS procedures between 2006 and 2013. 7 This change in clinical practice has occurred despite the lack of evidence of clinical or economic benefit of URS to patients or the NHS.
Treatment options
Most people with a ureteric stone can be expected to pass the stone spontaneously with conservative or supportive care, such as increased fluid intake, pain relief and metabolic expulsive therapy (MET). However, between one-fifth and one-third of cases require an active intervention (i.e. stone removal)14 because of failure to pass the stone, failure of conservative management, continuing pain, infection or obstruction to urine drainage. The two standard active intervention options are SWL and URS. In some cases, a temporising procedure (emergency procedure), such as a ureteric stent or nephrostomy, is needed to treat concurrent infection or obstruction before any active intervention can safely take place.
The size, shape and position of the stone in the ureter (upper, middle or lower; see Figure 1) influences whether or not the person is likely to pass the stone spontaneously and if the person is likely to require an intervention to facilitate stone passage. People with small stones (i.e. < 5 mm) generally do not need an active intervention. 14
The role of MET is unclear given the findings of the Spontaneous Urinary Stone Passage ENabled by Drugs (SUSPEND) trial,15 but current guidelines still advise consideration of alpha-blockers for ureteric stones < 10 mm in size in the lower ureter. The European Association of Urology (EAU’s) urolithiasis 2018 guideline16 advocates the use of either SWL or URS for stones < 10 mm in size, whereas URS is deemed marginally more suitable for stones > 10 mm in size, both in the proximal and in the distal ureters.
Active treatments
Shockwave lithotripsy
Shockwave lithotripsy is a treatment that uses machines (called lithotripters) that generate and focus shock waves of energy that pass through the skin to the stone. The energy is targeted (using ultrasound or X-rays), ensuring that there is minimal impact on surrounding tissues, and breaks the stone into smaller fragments that are passed naturally in the urine in the days following treatment. SWL is usually performed on an outpatient basis within the hospital. The procedure may require pain medication and treatment usually lasts between 30 and 60 minutes. Stone fragmentation is monitored during the procedure, with imaging used post procedure to assess progress, and a second treatment may be required (particularly for larger stones).
Ureteroscopic stone treatment
Ureteroscopic stone treatment involves passing a long, thin telescope called a ureteroscope through the urethra and into the bladder. The ureteroscope is then passed up into the ureter to directly visualise the stone. The surgeon may either try to gently retrieve the stone using specialised instruments, typically for smaller stones, or try to fragment the stone into smaller pieces that can be passed naturally in the urine. The fragmentation is achieved using different energy sources, the most common being a holmium laser, directed onto the stone through the ureteroscope. The progress and degree of success of stone fragmentation and clearance is assessed visually at the time of the procedure, with post-procedure imaging used as needed. URS is normally carried out as a day-case procedure (but may require hospital admission) and almost always requires a general anaesthetic. A ureteric stent may occasionally be inserted as part of the procedure, which is normally removed after a short period of time using a flexible cystoscope under local anaesthesia.
The treatment pathways
The treatment pathway for a patient with a stone that is judged clinically to be unlikely to pass spontaneously, will generally start with either SWL or URS.
If the treatment pathway starts with SWL, after the first SWL session the need for a potential second session is reviewed which will depend on progress with stone fragmentation and passage. If a ureteric stone has not been cleared after two sessions of SWL, urologists would generally advocate changing to ureteroscopic clearance.
If treatment starts with URS, the stone can usually successfully be cleared in a single procedure. This may be by direct removal of the entire stone, fragmentation of the stone with removal of the fragments or fragmentation of the stone with the fragments passed in the day(s) following the procedure. In 10–15% of patients,17 however, it is only possible to insert a stent because the ureter is too tight to reach the stone safely with a ureteroscope (these patients require a subsequent procedure to remove the stone and stent). After URS (with or without stone clearance) the surgeon may also insert a temporary stent to allow safe postoperative drainage of the ureter and reduce the risk of postoperative pain. Stent insertion then requires a further procedure for stent removal (usually carried out under local anaesthesia as a day case).
Some patients presenting with ureteric stones as an emergency may have continuing severe pain or evidence of infection or obstruction, and these patients may require urgent drainage of their renal collecting system either through insertion of a ureteric stent or through a nephrostomy (rather than having primary SWL or URS), with definitive treatment postponed to a later date.
The choice between a non-invasive, outpatient-based treatment (SWL) and the more invasive option of URS (requiring anaesthesia) has implications for the NHS and other health-care systems. The combination of technological advances [miniaturisation of ureteroscopes, effective fragmentation with laser (usually holmium) and improved retrieval devices] and increased availability has been reflected in the global trend of increasing URS cases, with a concomitant decline in SWL procedures. 18 Provision of primary treatment in the NHS setting can be affected by resource availability,19 although the safe clearance of a ureteric stone without the need for a stent should be an achievable target in contemporary stone management in a specialised and fully resourced unit.
Members of the British Association of Urological Surgeons (BAUS) Section of Endourology were surveyed at the association’s annual meeting in 2012. The consensus among UK endourologists was that they would accept up to a 20% inferiority level of SWL compared with URS when discussing treatment options with their patients. This level was also considered acceptable by the BAUS Section of Endourology patient group.
Current evidence base
At the time of funding, a Cochrane review2 suggested that URS was associated with better stone clearance rates but higher complication rates than SWL. A more recent systematic review3 supports these findings. A joint EAU–American Urological Association guideline for ureteric stones,20 current at the time of funding, had similar findings, but the evidence at that time was deemed insufficient to recommend either SWL or URS as the first-line treatment. One of the major conclusions from these publications was to suggest the need for large, multicentre RCTs to compare these modalities.
We describe the TISU trial, which was a large, multicentre RCT. All participants had ureteric stones diagnosed and confirmed by the contemporaneous use of computed tomography scan of the kidneys, ureters and bladder (CTKUB) for stone location and size, and were clinically judged to need active intervention (usually due to failure of the stone to progress, failure of conservative treatment, continuing pain or the size and position of the stone). Only centres with an established fixed-site lithotripter (rather than mobile machines) and with the ability to perform ureteroscopic procedures were able to recruit patients to the study. A key feature of the TISU trial design was that treatment would follow established usual NHS clinical pathways (i.e. both SWL and URS must be available as treatment options to the patient). Assessment of the outcome measure of resolution of the stone episode (stone clearance) was based on the need for any further procedures (i.e. additional to those in the treatment pathway to which the participants were initially randomised) up to 6 months after random allocation. When no further procedures took place, the participant was deemed to be stone free from their definitive initial treatment pathway.
Trial objectives
The TISU trial was a multicentre, non-inferiority RCT of SWL as the initial treatment option (vs. URS) for ureteric stones, in a UK NHS setting.
The aim of the TISU trial was to determine the clinical effectiveness and cost-effectiveness of SWL as the initial treatment option compared with primary URS.
The clinical effectiveness and cost-effectiveness was determined with respect to:
-
resolution of stone episode (stone clearance), defined as no further intervention required to facilitate stone passage
-
incremental cost per quality-adjusted life-year (QALY)
-
participant-reported health outcomes
-
treatment-related harms up to 6 months post randomisation.
Chapter 2 Methods and practical arrangements
Parts of this chapter have been reproduced with permission from McClinton et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
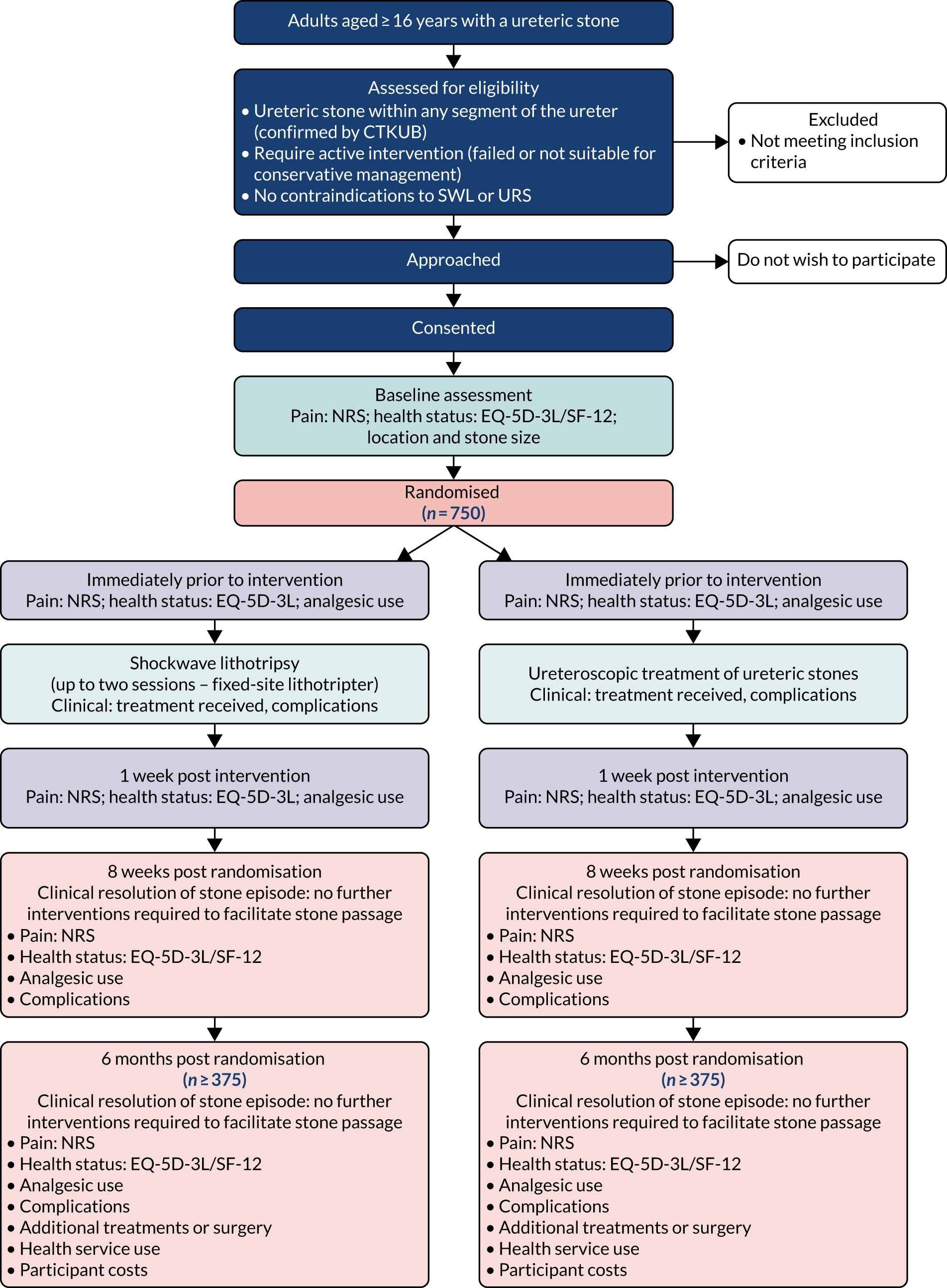
The TISU trial was a multicentre, non-inferiority RCT of SWL as the initial treatment option (compared with URS) for ureteric stones, in a UK NHS setting. Figure 2 summarises the trial design. Details of the trial design can also be found in the study protocol. 1 Neither the participant nor the treating clinicians were masked to the treatment received, as the TISU trial was a pragmatic trial of two very different treatments.
FIGURE 2.
Flow diagram of the TISU trial. EQ-5D-3L, EuroQol-5 Dimensions, three-level version; SF-12, Short Form questionnaire-12 items. This figure has been reproduced with permission from McClinton et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
The main criterion for the selection of UK NHS hospitals was that the site should have a fixed lithotripter. All of the SWL machines used in the TISU trial were ‘fixed-site’ lithotripters (i.e. no mobile lithotripsy services were utilised). This was decided to maximise standardisation of the SWL treatment pathway and because there was some evidence that outcomes from mobile services are inferior to those delivered by fixed-site services. 22
The sites were widely distributed across the UK and are representative of UK urological practice. A total of 25 UK NHS sites took part in the trial (Figure 3). These were Addenbrooke’s Hospital, Cambridge; Bradford Royal Infirmary, Bradford; Broomfield Hospital, Mid Essex; Canterbury Hospital, Canterbury; Charing Cross Hospital, London; Churchill Hospital, Oxford; Darent Valley Hospital, Kent; Freeman Hospital, Newcastle upon Tyne; Guy’s Hospital, London; Hull Royal Infirmary, Hull; Northwick Park Hospital, London; Pinderfields Hospital, Wakefield; Royal Derby Hospital, Derby; Royal Hallamshire Hospital, Sheffield; Salford Royal Hospital, Manchester; Southmead Hospital, Bristol; St George’s Hospital, London; St James’s University Hospital, Leeds; Sunderland Royal Hospital, Sunderland; St Peter’s Hospital, Surrey; The James Cook University Hospital, Middlesbrough; Withenshawe Hospital, Manchester; Western General Hospital, Edinburgh; Whiston Hospital, Prescot; and Wrexham Maelor Hospital, Wrexham.
FIGURE 3.
The location of the 25 TISU trial sites.
Research ethics and research governance approvals
The TISU trial was given a favourable opinion prior to approaching any potential participants by the North of Scotland Research Ethics Committee 1 (reference number 13/NS/0002). It was approved by the sponsors (NHS Grampian and University of Aberdeen) and by the research and development departments of the NHS organisations at each site that took part prior to approaching any potential participant at that site. The trial was conducted in accordance with the principles of Good Clinical Practice, was registered on the UK Clinical Research Network Portfolio (study identification 13979) and was assigned an International Standard Randomised Clinical Trial Number (ISRCTN92289221). A site initiation visit took place at each site prior to starting recruitment. At the site initiation visit, the trial manager detailed and explained trial procedures to the local principal investigator (PI) and clinical research team and provided a trial-specific site file.
Participants
Potential participants were adults presenting with a diagnosis of a unilateral ureteric stone in any segment of the ureter at participating UK NHS hospitals, and were identified according to the inclusion and exclusion specified, as follows.
Inclusion criteria
-
Had a ureteric stone confirmed by CTKUB.
-
Had a ureteric stone requiring surgical intervention (either as a primary intervention or after failed conservative management).
-
Was aged ≥ 16 years.
-
Had a single ureteric stone of any size requiring treatment.
-
Was deemed clinically suitable for either SWL or URS.
-
Was capable of giving written informed consent, which includes adherence with the requirements of the trial.
Exclusion criteria
-
Was pregnant.
-
Had stones not confirmed by CTKUB.
-
Had bilateral ureteric stones.
-
Had abnormal urinary tract anatomy (such as horseshoe kidney or ileal conduit).
-
Was unable to understand or complete trial documentation.
Identifying participants and consent
Local procedures at participating hospitals were different. The timing and mode of approach to patients and the consent process varied to accommodate both the variability at a site and the needs of the patients. Following adequate pain relief and confirmation of their ureteric stone by CTKUB, eligible patients (according to the criteria in Inclusion criteria and Exclusion criteria) were provided with a patient information leaflet (see Report Supplementary Material 1).
Each eligible patient was given the opportunity to discuss the trial with the local clinical team. Eligible patients could decide to participate during a consultation with the local clinical team, during a visit to hospital (e.g. when they attended a clinic appointment or while a patient was in hospital for their initial stone episode) or, alternatively, after consideration of the patient information leaflet at home. Some patients who agreed to be contacted at home may have been called by the local research nurse to discuss any further queries. Patients who decided to participate following telephone consultation sent their completed documents (consent form and baseline questionnaire, see Report Supplementary Material 1) through the post to the local team at their treating hospital or were told to take the documents with them if they returned to hospital for another consultation or treatment.
Signed informed consent forms were obtained from the participants in all centres. Participants who could not give informed consent (e.g. due to incapacity) were not eligible for participation. The participant’s permission was sought to inform their GP that they were taking part in the TISU trial. Patients were randomised to one of the two treatment arms following consent.
Randomisation
Participants were allocated to one of the two intervention arms: SWL or URS.
The randomisation algorithm used trial centre (site), stone size (≤ 10 mm or > 10 mm) and stone location (upper, middle or lower ureter, defined in the EAU urolithiasis guideline16) as minimisation covariates and 1 : 1 allocation was used. A web-based application or a remote telephone interactive voice-response randomisation application, both hosted by the Centre for Healthcare Randomised Trials (CHaRT), Health Services Research Unit (HSRU), at the University of Aberdeen, was used to carried out randomisation.
Trial interventions
We were investigating the care pathways that started with one of the interventions (SWL or URS).
Shockwave lithotripsy involves generation of a shockwave that is focused on the stone and causes it to fragment, with the fragments subsequently passing spontaneously. It is routinely performed in an outpatient setting, with pain relief or sedation as required. Recruitment took place only in established UK centres with fixed-site lithotripters. This allowed some standardisation of pathways on times to treatment and SWL delivery. Up to two sessions of SWL were considered as ‘one intervention’, as per standard practice (usually the second session is delivered within 2–4 weeks of the first). Details of the make and model of lithotripters used and standard site-specific lithotripsy treatment protocols can be found in Appendix 1, Table 16.
Ureteroscopic stone treatment is the use of a small semi-rigid or flexible ureteroscope, in conjunction with intracorporeal lithotripsy devices, such as the holmium laser, to directly visualise and fragment ureteric stones. Smaller stones in the lower ureter can occasionally be removed intact by using basketing devices. It is currently most often performed as a day-case procedure [but may require hospital admission depending on complexity (2014 NHS average = 1.7 days23)] and usually necessitates general anaesthesia.
Outcome measures
Primary outcomes
The TISU trial had a primary clinical outcome and a primary economic outcome.
-
Clinical: the primary clinical outcome measure was the resolution of stone episode or the clearance of ureteric stones, operationally defined as ‘no further intervention required to facilitate stone clearance’, up to 6 months from randomisation.
-
Economic: the primary economic outcome measure was the incremental cost per QALY gained at 6 months from randomisation. The QALYs gained was based on the responses to the EQ-5D-5L questionnaire.
Secondary outcomes
-
Quality of life: the quality of life outcomes were generic health status [EuroQol-5 Dimensions, three-level version (EQ-5D-3L)]; health profile [Short Form questionnaire-12 items (SF-12) version 2] (at 8 weeks and 6 months); and acceptability of the received procedure (at 8 weeks).
-
Pain: the pain outcomes were the severity of pain (Numeric Rating Scale) and use of analgesia.
-
Clinical: the secondary clinical outcomes were further interventions received and serious complications up to 6 months from randomisation.
-
Economic: the secondary economic outcomes were the NHS primary and secondary care use and costs up to 6 months, participant costs and the incremental cost per surgical intervention averted.
Resolution of the stone episode
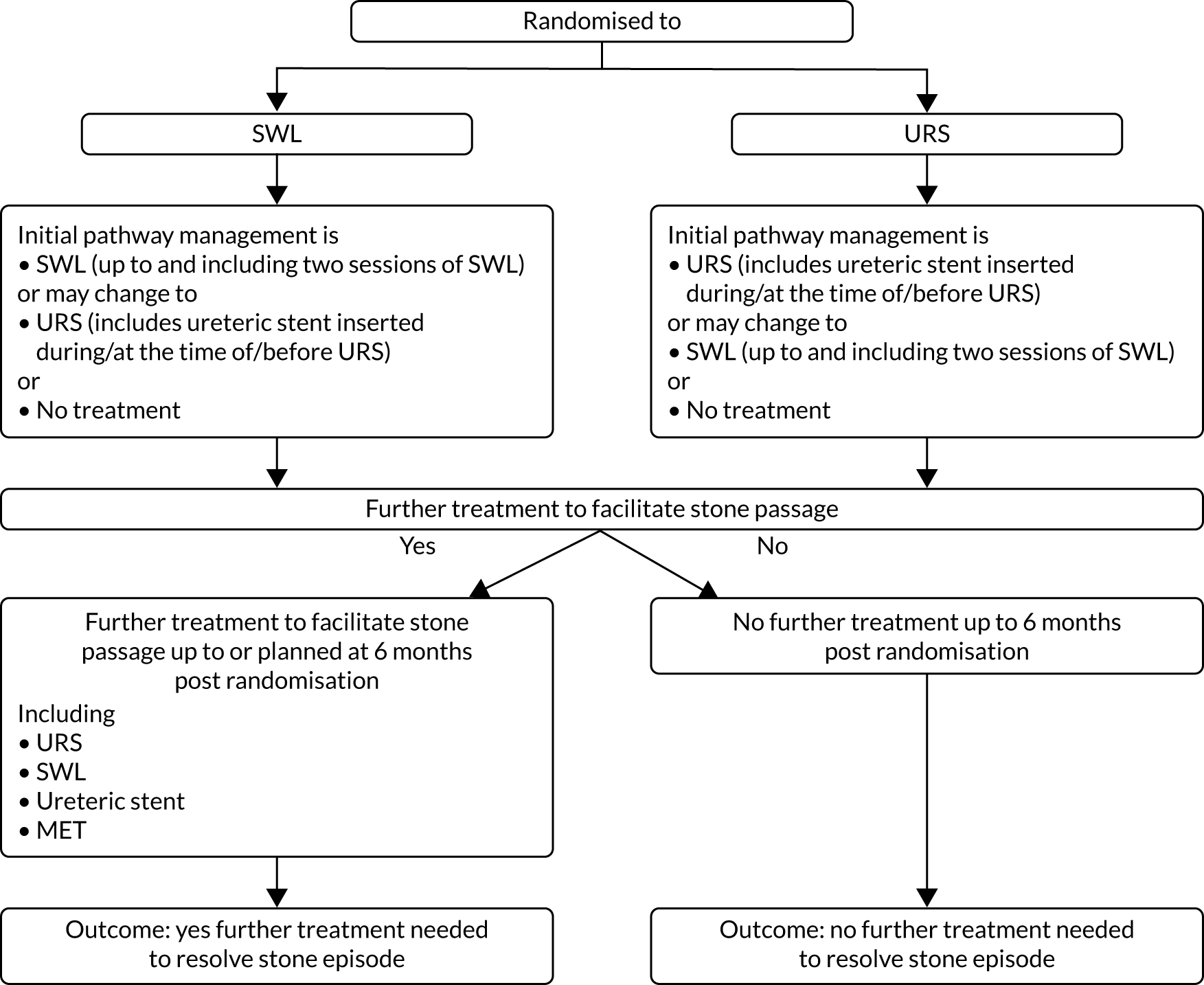
The primary clinical outcome was defined according to the treatment care pathway (Figure 4). The outcome was derived from several fields from case report forms (CRFs) at 8 weeks and 6 months post randomisation (Table 1; see also Data collection). This was checked against treatment CRFs and any supplementary CRFs that were completed between the 8-week and the 6-month CRFs (see Data collection).
FIGURE 4.
Treatment care pathway to define the primary outcome.
| Outcome measure | Source | Timing | ||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Post randomisation | ||||
| Pre | 1 week post | 8 weeks | 6 months | |||
| Interventions received | CRF and PQa | ✓ | ✓ | |||
| Health status: EQ-5D-3L | PQ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Health profile: SF-12 | PQ | ✓ | ✓ | ✓ | ||
| Pain: NRS | PQ | ✓ | ✓ | ✓ | ✓ | |
| Use of analgesics | PQ | ✓ | ✓ | ✓ | ✓ | |
| Complications | CRF | ✓ | ✓ | |||
| NHS primary and secondary health-care use | CRF and PQ | ✓ | ||||
Quality of life
Generic health status was measured using the EQ-5D-3L. 24 The EQ-5D-3L dimensions and scoring are described on the website of the EuroQol Research Foundation. 25 Briefly, the instrument has five dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. Each dimension has three levels: (1) no problems, (2) some problems and (3) extreme problems.
The EuroQol-5 Dimensions visual analogue scale (VAS) records the respondents’ self-rated health on a vertical VAS, with the end points of ‘best imaginable health state’ and ‘worst imaginable health state’.
Generic health profile was measured using the SF-12. 26 The SF-12 is a shorter version of the Short Form questionnaire-36 items, version 2. It uses 12 questions to measure functional health and well-being over the previous 4 weeks. These 12 questions summarise the physical component scores (PCSs) and mental component scores (MCSs) on a scale of 0 to 100, with 100 being best health. The SF-12 is standardised to have a mean of 50 and a standard deviation (SD) of 10.
Pain
Pain intensity and severity were self-rated on a Numeric Rating Scale,27 using the question ‘please rate the level of pain that you are experiencing today?’. Participants were also asked ‘During the last 7 days have you had pain related to your ureteric stone? (yes or no)’.
Analgesic use
Analgesic use was measured with the question ‘How many days out of the last seven have you taken any pain relief medication?’.
Acceptability of the procedure
The acceptability of the procedure was measured using the question ‘Would you recommend the treatment to a friend?’.
Complications
Complications were recorded from randomisation to 6 months post randomisation. Research staff recorded complications on the 8-week and 6-month post randomisation CRFs (see Table 1 and Data collection). If participants attended a hospital outside these time points for any reason, staff were encouraged to record any complication on the supplementary CRFs. A list of common stone treatment-related complications was provided on the CRFs. Serious adverse event (SAE) forms were completed only if the patient suffered a medically significant serious complication.
Data collection
Clinical outcomes measures
Clinical outcome data were collected throughout the trial, from consent to 6 months following randomisation. See Table 1 for the source and timing of outcome measures. Research nurses entered locally collected data in the centres. Staff at the trial office worked closely with the research nurses to ensure that data were as complete and accurate as possible.
Patient-reported outcome measures
Participant-reported outcomes were assessed by a self-completed questionnaire at recruitment (baseline) pre intervention, 1 week post intervention and 8 weeks and 6 month post randomisation (see Report Supplementary Material 1). The baseline questionnaire was completed in hospital prior to randomisation and the pre-intervention questionnaire was, when possible, completed in hospital just prior to treatment delivery. The 1-week post intervention questionnaire was completed 1 week after treatment at the patient’s home. Follow-up questionnaires were sent to participants, at 8 weeks and 6 months post randomisation, from the study office in Aberdeen and returned to the same address. Patients were also given the option to complete follow-up questionnaires online. Patients were sent two reminders to complete postal questionnaires and were sent a postal questionnaire if they failed to complete the questionnaires online. Questionnaire return rates were monitored throughout the trial and it was noted that the 6-month questionnaire response rate was particularly poor. Patients were given a small token of appreciation (£10 high-street shopping voucher) with the 6-month questionnaire to encourage completion and return.
Safety reporting
The TISU trial involved procedures for treating ureteric stones that are well established in clinical practice. Adverse events may occur during or after any type of surgery and were well defined for both procedures in the trial.
Shockwave lithotripsy
Adverse events for SWL were:
-
bleeding on passing urine
-
pain
-
urinary tract infection
-
bruising of abdomen or loin skin
-
stone fragments stuck between kidney and bladder
-
infection
-
kidney damage
-
persistence of stones.
Ureteroscopic stone treatment
Adverse events for URS were:
-
burning or bleeding on passing urine
-
temporary insertion of a bladder catheter
-
insertion of stent and further procedure to remove it
-
pain
-
inability to retrieve stone
-
movement of stone into the kidney
-
kidney damage or infection
-
failure to pass the telescope
-
recurrence of stones
-
damage to ureter
-
scarring of ureter.
The incidence of these non-serious events has been well reported and occurrences of such events were not collected or reported as part of the TISU trial. Planned hospital visits for conditions other than those associated with the ureteric stone were not collected or reported. Hospital visits (planned or unplanned) associated with further interventions to facilitate ureteric stone clearance were recorded as an outcome, but were not reported as SAEs.
Within the TISU trial, ‘relatedness’ was defined as an event that occurred as a result of a procedure that was required by the protocol, whether or not this procedure was the specific intervention under investigation and whether or not it would have been administered outside the trial as normal care.
Any SAEs that were related to the participants’ ureteric stone treatment that were not further interventions to facilitate stone clearance (e.g. if a participant was admitted to hospital for treatment of infection) were recorded on the SAE form. In addition, all deaths for any cause (related or otherwise) were recorded on the SAE form.
A delegated person at the TISU trial centre completed and uploaded the trial SAE form onto the trial website as soon as they were made aware that a SAE had occurred. This automatically notified the trial office team. If, in the opinion of the local PI and the chief investigator, the event was confirmed as being serious, related and unexpected, the chief investigator or the trial manager notified the sponsor within 24 hours of receiving the SAE notification. The sponsor provided assessment of the SAE. The chief investigator (or trial manager) reported any related and unexpected SAEs to the main Ethics Committee and the Data Monitoring Committee (DMC) within 15 days of the chief investigator becoming aware of it. All related SAEs were summarised and reported to the Ethics Committee, the funder and the Trial Steering Committee (TSC) in their regular progress reports.
Original sample size
The original sample size calculations reflect that the TISU trial was a non-inferiority design. Published literature2 suggested that the proportion of participants who were stone free without further intervention up to 6 months would be about 0.75 in the URS arm (P1) and about 0.65 in the SWL arm (P2). A survey of members of the BAUS Section of Endourology was carried out at the association’s annual meeting in 2012. The consensus among UK endourologists was that, when discussing treatment options with their patients, they would continue to recommend SWL as long as its inferiority level compared with URS was no more than 20%. This level was also agreed by the BAUS Section of Endourology patient group as being acceptable to them. The margin of inferiority deemed acceptable was, therefore, set at 0.20, so that P2 – P1 > –0.20. The sample size was estimated using simulations. The power of a non-inferiority trial can be considered as the probability that the lower bound of the estimated confidence interval (CI) around the difference between trial proportions excludes the margin of non-inferiority. Simulating thousands of trials of fixed sizes with the parameters P1 and P2, as above, indicated that a trial of 450 participants per arm was required for the lower bound of the estimated 95% CI to exclude –20%, with 90% power. Adjustment for potential of 10% dropout inflated the number of participants needed to 1000 in total. A trial of this size has 90% power to test superiority on secondary outcomes of an effect size of one-quarter of 1 SD.
Sample size reassessment
Following slower than planned recruitment, our funders requested a reassessment of the assumptions of sample calculation. We did this by looking at trial-aggregated primary outcome from 267 participants and in discussion with our independent DMC, and subsequently the funders. The sample size was amended downwards from 1000 to 750. The amendment was ratified by the trial oversight committees, the sponsor and the funder. Recruitment projections showed us that the original sample size of 1000 participants was unachievable in a realistic time frame, despite measures implemented to improve recruitment. We agreed with the funder that an extension of 18 months would be required to reach a revised sample size of 750 and that this was an achievable target. Our original sample size of 1000 included a 15% uplift from 850 to enable the primary analysis to be a suitably defined per-protocol (PP) analysis, as this approach, in the special context of a non-inferiority design, is often seen as more conservative than the more conventional intention-to-treat (ITT) approach. However, the view of the Health Technology Assessment Board, and which was confirmed by the TISU trial DMC, was that the ITT approach should be preferred over the PP approach as the former would better reflect the TISU trial’s pragmatic effectiveness focus, that is, the fact that its aim was to evaluate the policy of initiating one or other of these treatment options rather than to compare their relative performance. The PP analysis was used as a supporting analysis. Based on the 267 participants with mature outcome data (as of 16 February 2016), the DMC observed that all of the assumptions behind the power calculation remained plausible. Given that the PP analysis was deemed of secondary importance (and under the original design of assuming that the proportion of participants who were stone free would be 65% in the SWL arm and 75% in the URS arm), the achievable sample size of 750 gave 85% power.
Statistical analysis
General methods
Treatment arms were described at baseline and follow-up using means (with SDs), medians [with interquartile ranges (IQRs)] and numbers (with percentages), when relevant. We analysed the primary outcome, which is binary, using a generalised linear model (GLM), essentially a modified Poisson regression with a log-link function and robust error variance to estimate covariate-adjusted relative risks and to derive risk difference. 28 Models were adjusted for design covariates: trial centre (random effect), stone size (≤ 10 mm, > 10 mm), stone location (upper, middle or lower ureter), age and sex. Our main approach to analyse the primary outcome was ITT, given the pragmatic nature of the TISU trial evaluating two care pathways in the NHS setting. We have labelled the results from this analysis ITT-1 (ITT, including all participants). This includes all participants who were ‘randomised’ and those passing their stone before their intervention (this reflects waiting times for both SWL and URS in the NHS). A second analysis, labelled ITT-2 (ITT, excluding those who passed their stone prior to any intervention), repeated this. An ITT approach can be conservative for a non-inferiority trial, so we prespecified PP analyses also. Results labelled PP-1 (PP analysis, including those who passed their stone before treatment) and PP-2 (PP analysis, excluding those who passed their stone before treatment) mirror the ITT analyses above, but included only participants who were treated in line with the care pathway that they were allocated to (i.e. excluding crossovers). The primary outcome reflects the number of participants who required further intervention. Thus, more participants is actually a worse outcome. Consequently, to avoid double negatives, we used the upper bound of the CI around the absolute risk difference (ARD) (estimated from our models), ruling out the prespecified non-inferiority margin of 20% to conclude non-inferiority. We made no adjustment for missing data because we had complete outcome data on all participants who gave consent for their clinical data to be used.
Secondary outcomes were compared in a similar way using GLM that was appropriate for the distributional form of the outcome being analysed, but in a superiority framework. We used linear mixed models for repeated-measures quality-of-life data, estimating treatment effects by including a time-by-treatment interaction for fixed (nominal) time points of 8 weeks and 6 months from randomisation. We used a multiple imputation approach to deal with missing SF-12 outcome data. We generated 50 imputation sets for each arm of the trial separately. Our imputation model used treatment received, stone size, stone location (upper, middle and lower ureter), gender, age, centre and primary outcome status to predict missing SF-12 scores. These data sets were combined using the ‘mi estimate’ command in Stata® (Stata 15, StataCorp LP, College Station, TX, USA), which applies Rubin’s rules to combine estimates from multiple imputed data sets to account for variation both within and between data sets, using linear mixed models for repeated measures. We provide descriptive summaries only for the pre- and post-intervention quality-of-life data. We do not report on EQ-5D-3L outcome data in the clinical effectiveness results chapter; rather, this is reported in the health economic chapter, to reduce repetition (see Chapter 4 for details).
We explored the moderating effect of three a priori subgroup variables on the primary outcome, by including subgroup-by-treatment interactions in our primary outcome model. These were (1) stone size (≤ 10 mm, > 10 mm), (2) stone location (upper, middle or lower ureter) and (3) sex. We used forest plots to summarise the within-subgroup treatment estimates using 99% CIs. We used Stata for all our statistical analyses.
Timings and frequency of analysis
We carried out a single principal analysis at the end of the TISU trial when the last participants had reached their final follow-up time point of 6 months.
Economic evaluation
Economic evaluation was an integral part of the TISU trial. The evaluation considered the costs of the care pathways that patients had received. Resource data collected included the costs of the interventions, SWL and URS, and simultaneous and consequent use of primary and secondary NHS services (including additional interventions received) by participants. See Chapter 4 for a detailed description of the methods used.
Management of the study
The Trial Management Team was centralised, the study office was based within CHaRT, University of Aberdeen, and it provided real-time support for the recruiting centres. Recruiting centres were led by local PIs (urologists) responsible for all aspects of the trial, including recruitment and consent of participants, delivery of interventions and notification of SAEs and breaches. PIs were supported by local research nurses.
The trial was supervised by the Project Management Group (PMG). This group consisted of grant holders and representatives from the study office.
The trial was overseen by a TSC comprising four independent members and by an independent DMC. Both committees met on a yearly basis, and the DMC always met before the TSC and would provide any recommendations to the committee. The DMC did request additional meetings to discuss slow recruitment and to discuss interim analysis conducted during the trial.
Patient and public involvement
Pre-funding application and design of the research
Prior to the TISU trial starting recruitment, we sought support from the stone disease patient advisory section of the BAUS. The purpose of this group is to elicit patients’ views and advice on the needs and requirements for information about stone disease and research priorities, and to provide input into trial design, management and service design, and improved facilities for treating stone disease. A member of the group was a co-applicant on the grant, and gave input into the application and continued to advise the TISU PMG until January 2019.
Oversight of the study
One of the independent members of the TSC was a patient representative. The TSC met throughout the study and reviewed all of the study documentation, including patient-facing documents and questionnaires that were sent to potential and recruited participants in the TISU trial.
Report writing, academic paper preparation and dissemination
The patient and public involvement partner on the TSC has been actively involved in discussions of the trial results with the TSC and supportive of the study in report preparation.
Chapter 3 Results
Parts of this chapter have been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Participant baseline characteristics
Trial recruitment
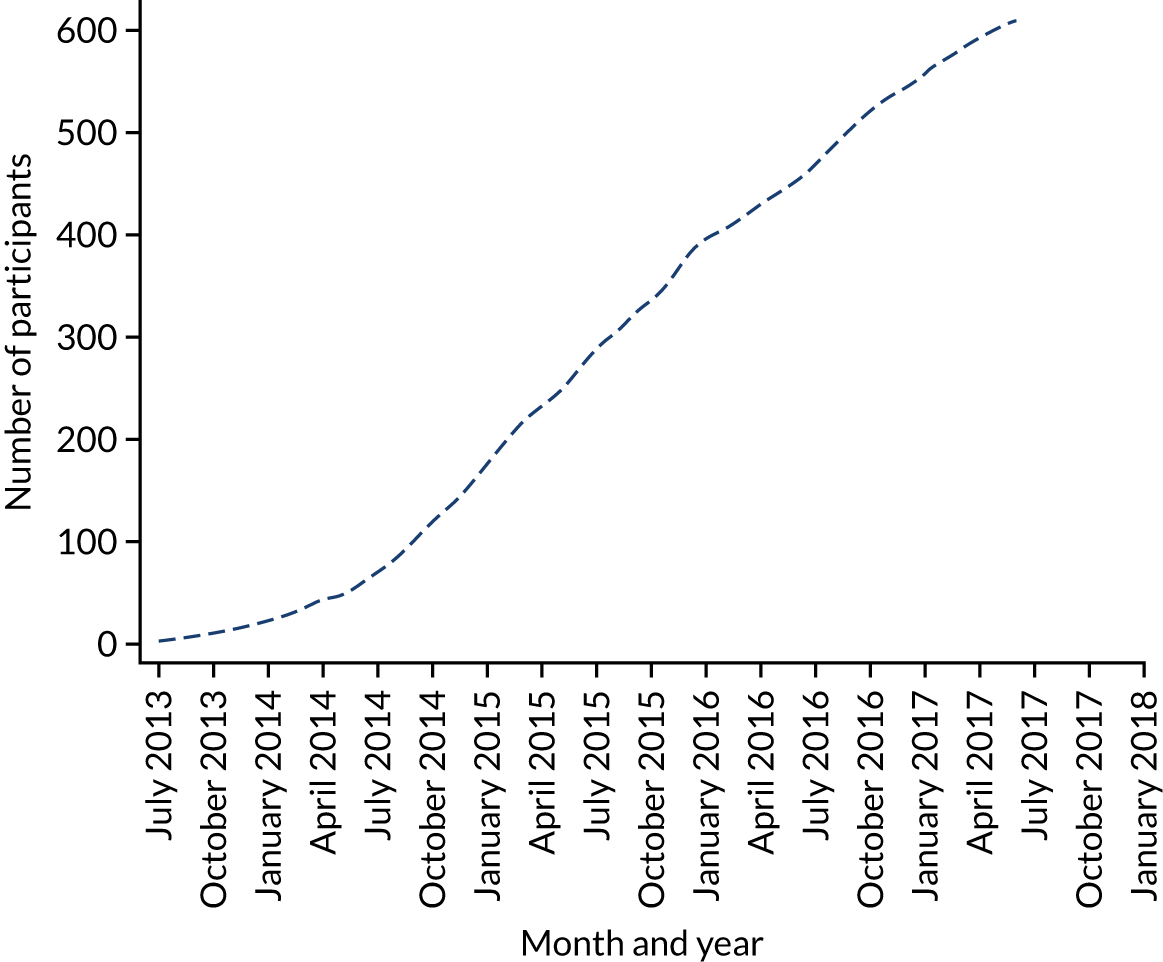
In total, 613 (out of 1291 eligible) participants were recruited from 25 centres and the median number of participants per centre was 21 (IQR 16–27). We randomised 306 participants to the SWL care pathway arm and 307 participants to the URS care pathway arm. Participants were recruited between July 2013 and June 2017, and final follow-up was to December 2017 (Figure 5).
FIGURE 5.
Recruitment graph.
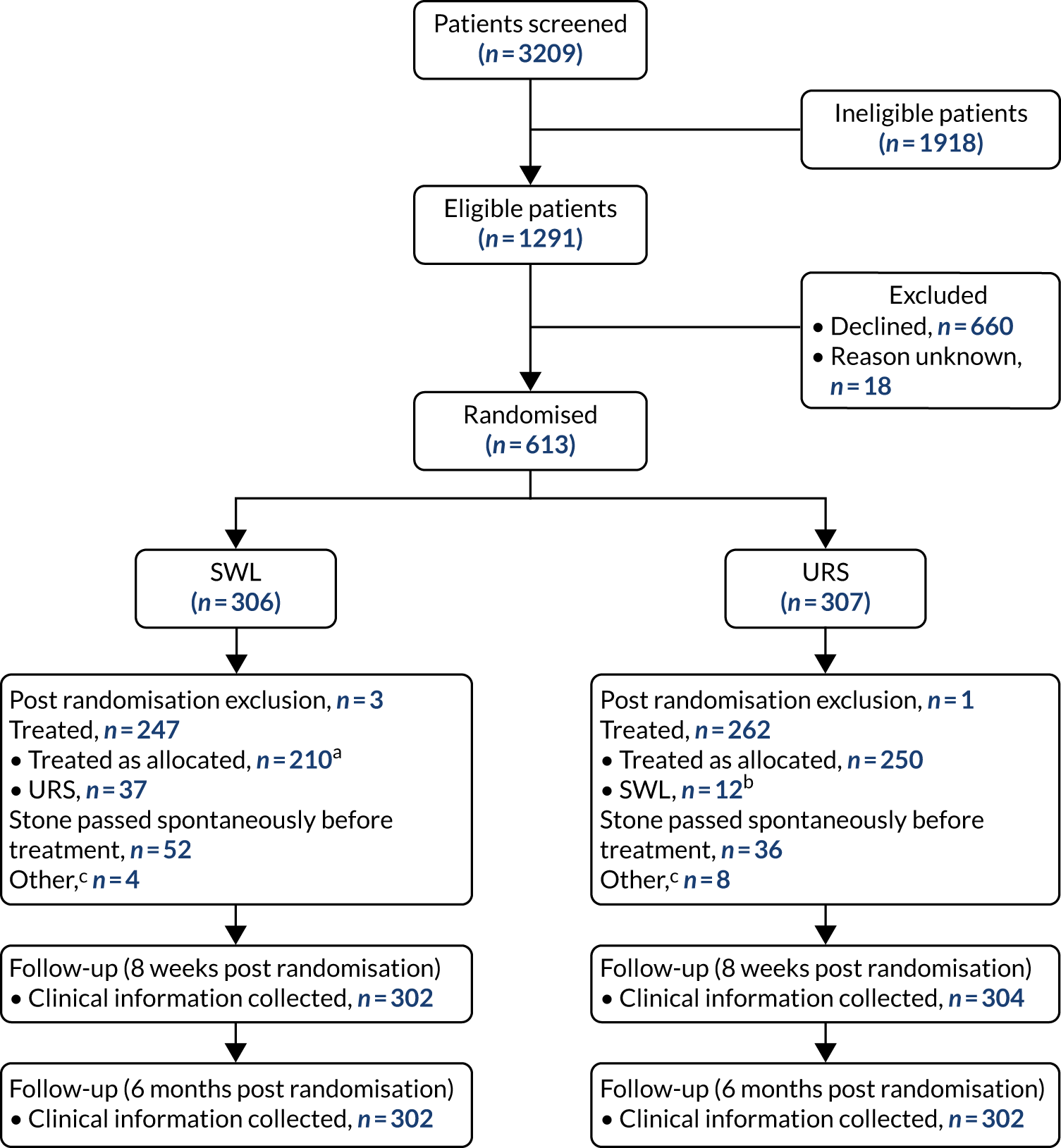
Participant flow
Figure 6 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the TISU trial. We screened 3209 potentially eligible patients, of whom 1918 (60%) were ineligible. The main reasons for ineligibility were that the patient was not suitable for either SWL or URS and the patient presented with abnormal urinary tract anatomy or bilateral stones. We randomised 613 out of 1291 (47.5%) eligible participants. The reasons for not being randomised were that the patient had a preference for one treatment and did not want to be randomised, clinician had a preference for a treatment and, in some cases, patients declined to give a reason. Full details of the reasons for patients being ineligible or declining are tabulated in Appendix 2, Table 17. There were four post-randomisation exclusions [three patients in the SWL arm (one patient on warfarin, one patient not fit for treatment and one patient with bilateral stones) and one patient in the URS arm (patient on cardiac medication)], leaving 303 and 306 participants, respectively.
FIGURE 6.
The CONSORT flow diagram. a, 86 had two sessions of SWL; b, 3 had two sessions of SWL; c, other = unknown as not treated within the NHS, did not attend, unable to treat. This figure has been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Baseline characteristics
The two arms were well balanced at baseline (Table 2). The majority of participants were men. The age distribution was similar in both study arms, with mean ages in the early fifties. Over 95% of participants had a stone size ≤ 10 mm: 45% of stones were in the upper ureter and 38% in the lower ureter. Most participants had experienced pain related to their ureteric stone in the previous 7 days and had taken pain medication.
| Variable | SWL (N = 303) | URS (N = 306) |
|---|---|---|
| Age (years), mean (SD) | 51.5 (14.1) | 50.1 (14.3) |
| Male, n (%) | 241 (79.5) | 234 (76.5) |
| Ureteric stone size (mm), mean (SD) | 6.7 (2.1) | 6.6 (2.4) |
| Ureteric stone size ≤ 10 mm, n (%) | 288 (95.0) | 292 (95.4) |
| Stone location, n (%) | ||
| Upper ureter | 138 (45.5) | 139 (45.4) |
| Middle ureter | 47 (15.5) | 50 (16.3) |
| Lower ureter | 118 (38.9) | 117 (38.2) |
| Currently taking analgesic medications, n (%) | ||
| Yes | 220 (72.6) | 193 (63.1) |
| No | 64 (21.1) | 96 (31.4) |
| Missing | 19 (6.3) | 17 (5.6) |
| Level of pain today, n | 301 | 303 |
| Median (IQR) | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) |
| Pain related to ureteric stone during the last 7 days, n (%) | ||
| Had pain | 236 (77.9) | 232 (75.8) |
| No pain | 63 (20.8) | 69 (22.5) |
| Missing | 4 (1.3) | 3 (1.0) |
| Number of days during last 7 days that the participant has taken pain medication | 298 | 300 |
| Median (IQR) | 3 (1–6) | 2 (0–5) |
| EQ-5D-3L, n | 298 | 297 |
| Mean (SD) | 0.737 (0.263) | 0.729 (0.303) |
| EQ-5D VAS,a n | 283 | 284 |
| Mean (SD) | 67.7 (24.5) | 67.5 (26.5) |
| SF-12 PCS,b n | 290 | 289 |
| Mean (SD) | 43.5 (9.5) | 44.9 (9.7) |
| SF-12 MCS,b n | 290 | 289 |
| Mean (SD) | 48.5 (11.1) | 50.4 (9.6) |
Care pathway and treatment received
The care pathways are outlined in Chapter 2 (see Figure 4). Table 3 describes the allocated care pathway compared with actual treatment received in the TISU trial. There were 210 (69.3%) participants in the SWL pathway and 250 (81.7%) participants in the URS pathway who were treated as allocated. There were 37 (12%) participants who were allocated to the SWL care pathway but received URS as their treatment. The reasons provided were medical (n = 5), participant choice (n = 3) or stone not visible on pre-SWL imaging (n = 10) or no reason was given (n = 18). In the URS care pathway, 12 (4%) participants received SWL as their treatment. The reasons provided were medical (n = 2) or participant choice (n = 6), or no reason was given (n = 4). In 52 (17%) SWL arm participants, no stone was visible on pre-treatment imaging and a decision was made not to proceed with the SWL because of evidence that the stone had passed (no visible stone and cessation of symptoms). This was also the case for 36 (12%) participants in the URS arm. Overall, seven participants (three SWL participants and four URS participants) did not attend for treatment, and surgeons were unable to treat one participant in the SWL arm owing to the position of their stone. Four participants had their treatment outside the TISU trial and we have not been able to establish what treatments they received. Waiting times are described in Table 4. More than 90% of participants in the SWL care pathway received treatment within 8 weeks, with a slightly lower proportion (86%) of participants in the URS care pathway being treated within 8 weeks (excluding those who passed their stone before treatment in both pathways).
| Variable | SWL (N = 303), n (%) | URS (N = 306), n (%) |
|---|---|---|
| SWL | 210 (69.3) | 12 (3.9) |
| URS | 37 (12.2) | 250 (81.7) |
| No evidence of stone presenta | 52 (17.2) | 36 (11.8) |
| Treatment unknown | 1 (0.3) | 3 (1.0) |
| Did not attend | 3 (1.0) | 4 (1.3) |
| Unable to treatb | 1 (0.3) |
| Treatment care pathway | n | Median (IQR) | Range |
|---|---|---|---|
| SWL (N = 303) | |||
| SWL pathway, any treatment | 247 | 8 (2–18) | 0–415 |
| Treated as allocated (SWL) | 210 | 7 (2–15) | 0–79 |
| Treatment with URS | 37 | 25 (2–70) | 0–415 |
| Treated within 8 weeks (56 days), n/N (%) | 229/247 (92.7) | ||
| URS (N = 306) | |||
| URS pathway, any treatmenta | 261 | 25 (9–44) | 0–269 |
| Treated as randomised (URS) | 250 | 25 (9–44) | 0–269 |
| Treatment with SWL | 12 | 22 (2–47) | 0–84 |
| Treated within 8 weeks (56 days), n/N (%) | 225/261 (86.2) | ||
Prior to treatment, participants attended a pre-intervention appointment and were asked to complete questionnaires again, to assess pain and quality of life (Table 5).
| Variable | SWL | URS |
|---|---|---|
| Level of pain today, n | 253 | 218 |
| Median (IQR) | 2 (0–5) | 1 (0–4) |
| Pain related to ureteric stone during last 7 days, n (%) | ||
| Had pain | 181 (63.1) | 147 (53.5) |
| No pain | 71 (24.7) | 71 (25.8) |
| Missing | 2 (0.8) | 1 (0.5) |
| Number of days during last 7 days that the participants has taken pain medication | 253 | 213 |
| Median (IQR) | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) |
| EQ-5D-3L, n | 253 | 211 |
| Mean (SD) | 0.735 (0.260) | 0.758 (0.272) |
| EQ-5D VAS, n | 235 | 198 |
| Mean (SD) | 69.2 (24.7) | 73.8 (22.4) |
Primary outcome
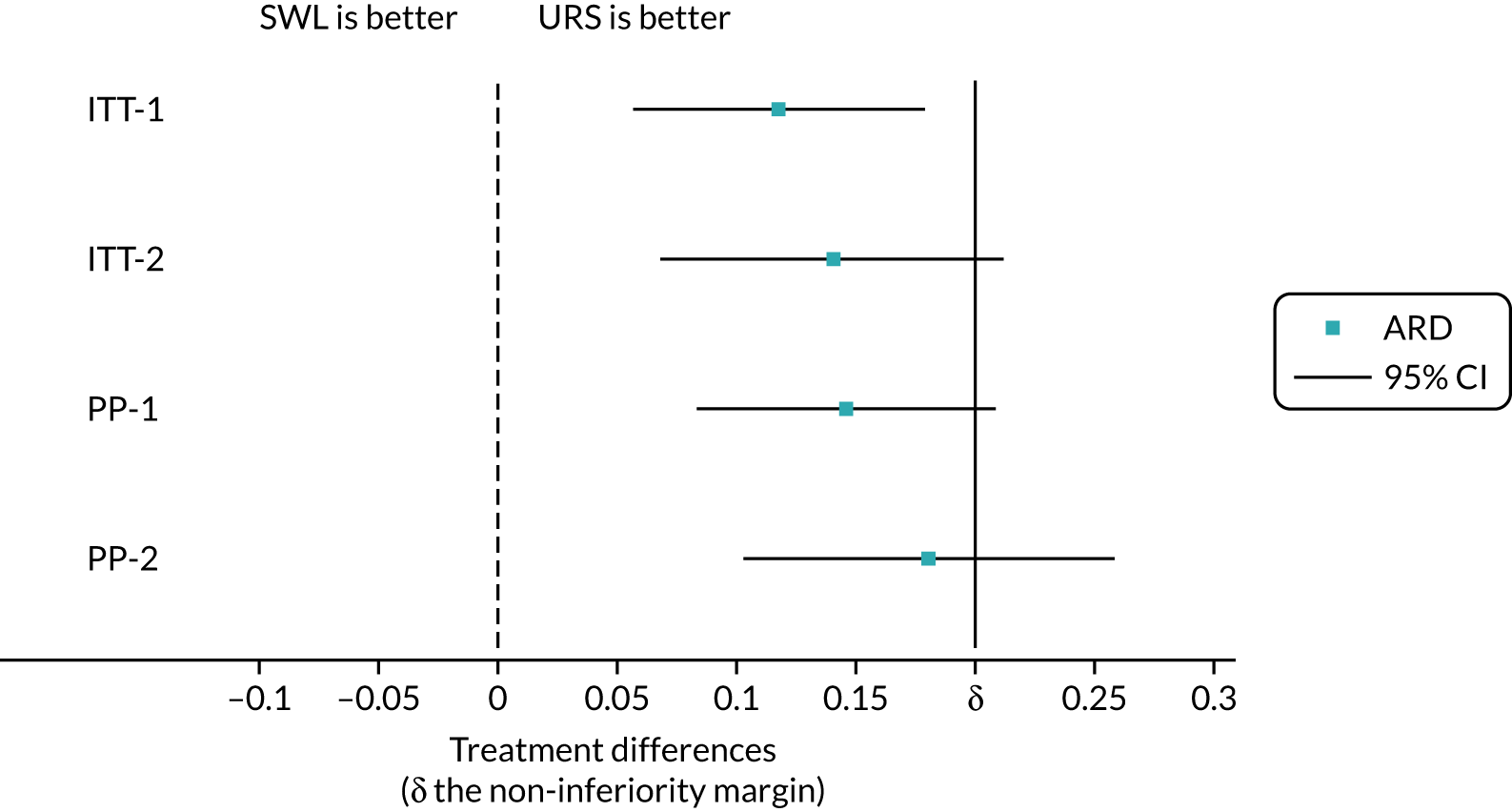
We analysed the primary outcome initially for all participants using an ITT approach (that is analysing participants as they were randomised, regardless of whether or not they passed their stone before intervention or of the intervention received) (Table 6). In the SWL arm, 67 out of 302 (22.2%) participants needed further treatment. In the URS arm, 31 out of 302 (10.3%) participants needed further treatment. The ARD was 11.4% (95% CI 5.0% to 17.8%); the upper bound of the 95% CI ruled out the prespecified margin of non-inferiority (which was 20%) (Figure 7).
| Population | SWL | URS | ARDa,b | 95% CI | Non-inferiority p-valuec | RR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| ITT-1 | 67/302 | 22.2 | 31/302 | 10.3 | 0.114 | 0.050 to 0.178 | 0.004 | 2.089 | 1.333 to 3.274 |
| ITT-2 | 65/250 | 26.0 | 31/266 | 11.7 | 0.137 | 0.063 to 0.211 | 0.051 | 2.155 | 1.389 to 3.345 |
| PP-1 | 64/262 | 24.4 | 27/283 | 9.5 | 0.144 | 0.078 to 0.209 | 0.046 | 2.485 | 1.577 to 3.915 |
| PP-2 | 62/210 | 29.5 | 27/247 | 10.9 | 0.179 | 0.098 to 0.259 | 0.314 | 2.607 | 1.653 to 4.111 |
FIGURE 7.
Plot of treatment effects for primary outcome. This figure has been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
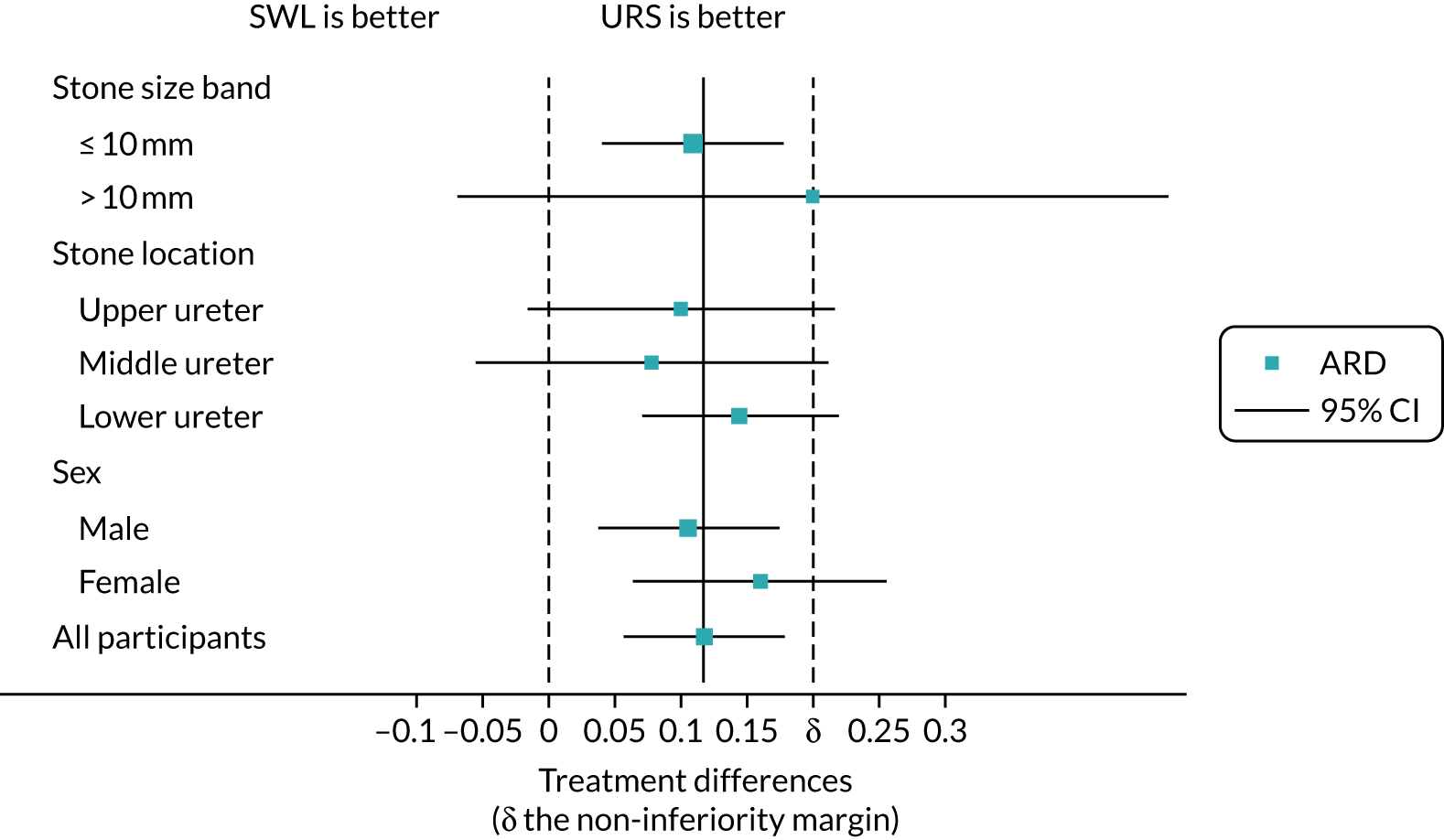
We explored the moderating treatment effect using subgroup analyses of stone size, stone location and gender. Within-subgroup treatment effects are summarised in Figure 8 and were fairly homogeneous across all strata; there was no evidence that subgroup moderated treatment effects. The full subgroup models are summarised (see Appendix 3, Tables 18–20, for details).
FIGURE 8.
Forest plot of subgroup treatment effects. This figure has been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Secondary outcomes
Clinical outcomes
The number of treatment-related complications was similar in both care pathways. There were slightly fewer complications (in participants who received any treatment) in the URS pathway than in the SWL pathway, but with so few events there was some uncertainty around the treatment differences (Table 7). The complications in the SWL pathway were mainly pain or infection necessitating hospital admission. The complications in the URS pathway were often stent related, with postoperative pain and infection requiring hospital admission.
| Participants with treatment-related complication | SWLa | URSb | ARDc,d | 95% CI | RRc,d | 95% CI |
|---|---|---|---|---|---|---|
| n (%) | 9/247 (3.6%) | 7/261 (2.7%) | 0.009 | –0.024 to 0.042 | 1.35 | 0.048 to 3.78 |
| Received SWL | Received URS | |||||
| n (%) | 7/221 (3.2%) | 9/283 (3.2%) | –0.001 | 0.036 to 0.034 | 0.97 | 0.36 to 2.90 |
Patient-reported outcomes
For both the pain measures and the acceptability measure we found a similar pattern between care pathways. At 8 weeks, self-reported pain was low in both arms (Table 8). The participants in the SWL arm reported taking pain relief more frequently, but the number of days reported as requiring pain relief was low in both arms; the median number of days reported was zero. Of those who responded, > 80% in each arm stated that they would recommend their treatment to a friend with ureteric stones, and there was no evidence that this differed between arms.
| Patient-reported measurement | SWL | URS | Effect sizea | 95% CI |
|---|---|---|---|---|
| Pain today | ||||
| n | 183 | 184 | ||
| Median (IQR) | 0 (0–2) | 0 (0–1) | ||
| Mean (SD) | 1.3 (2.4) | 0.97 (2.04) | 0.3b | –0.2 to 0.9 |
| Days with pain relief over the last 7 days | ||||
| n | 178 | 181 | ||
| Median (IQR) | 0 (0–2) | 0 (0–1) | ||
| Mean (SD) | 1.5 (2.5) | 1.0 (1.9) | 1.42c | 0.96 to 2.11 |
| Recommend to a friend n/N (%) | 148/171 (86.6) | 142/171 (83.0) | 1.04d | 0.97 to 1.13 |
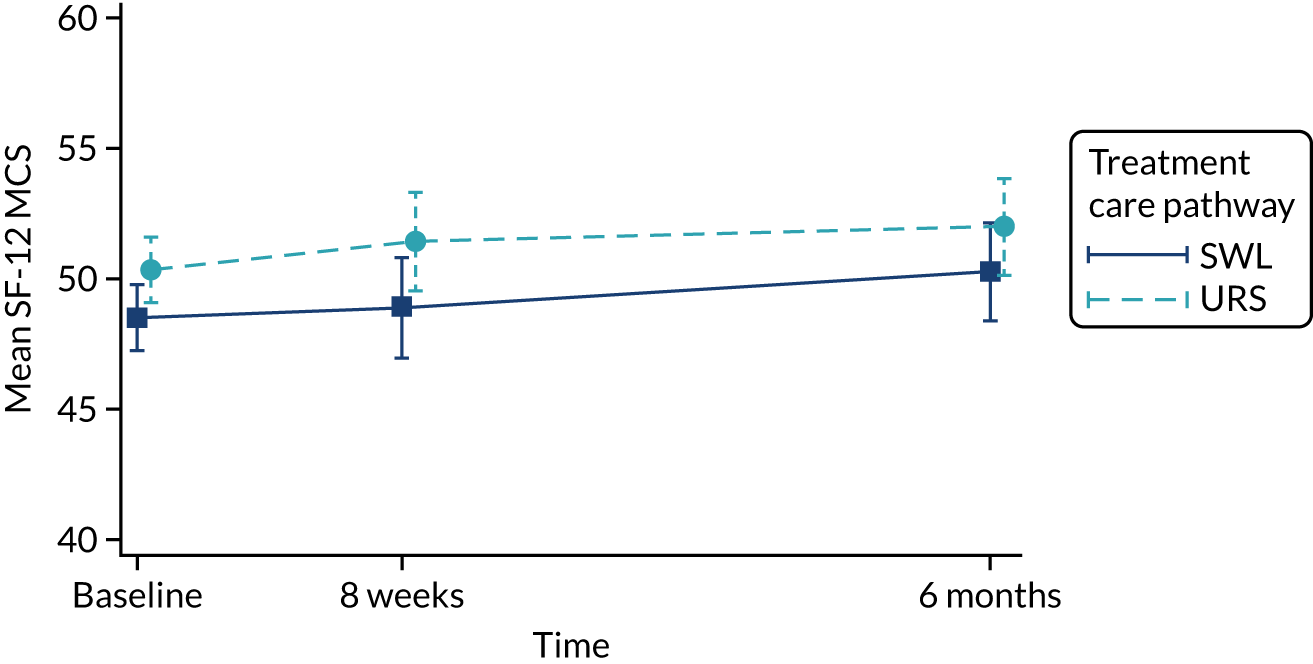
Quality of life, as measured by the SF-12 PCS and MCS components, is reported in Table 9 and in Figures 9 and 10. In both arms of the trial, and on both measures, quality of life improved over the duration of the trial, from baseline to 6 months. When we used observed data only, there were small but consistent effects favouring SWL for both SF-12 PCS and MCS. However, these effects were attenuated when we used multiple imputation models.
| Variable | SWL (N = 303) | URS (N = 306) | Estimatea,b (95% CI); p-value | Imputed estimatea,b,c (95% CI); p-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| SF-12 PCS | ||||||
| Baseline | 290 | 43.5 (9.5) | 289 | 44.8 (9.7) | ||
| 8 weeks | 150 | 47.0 (10.1) | 156 | 47.9 (9.2) | –0.1 (–2.0 to 1.8); 0.95 | –0.82 (–2.70 to 1.05); 0.35 |
| 6 months | 137 | 48.0 (10.5) | 146 | 50.9 (8.8) | –1.7 (–3.7 to 0.2); 0.080 | –0.90 (–2.70 to 1.05); 0.35 |
| SF-12 MCS | ||||||
| Baseline | 290 | 48.5 (11.1) | 289 | 50.4 (9.6) | ||
| 8 weeks | 150 | 48.9 (12.4) | 156 | 51.4 (9.9) | –2.2 (–4.4 to –0.01); 0.056 | –1.84 (–3.93 to 0.26); 0.09 |
| 6 months | 137 | 50.3 (11.6) | 146 | 52.0 (10.4) | –1.1 (–3.4 to 1.2); 0.33 | –1.68 (–3.78 to 0.42); 0.12 |
FIGURE 9.
Short Form questionnaire-12 items PCS over time. This figure has been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
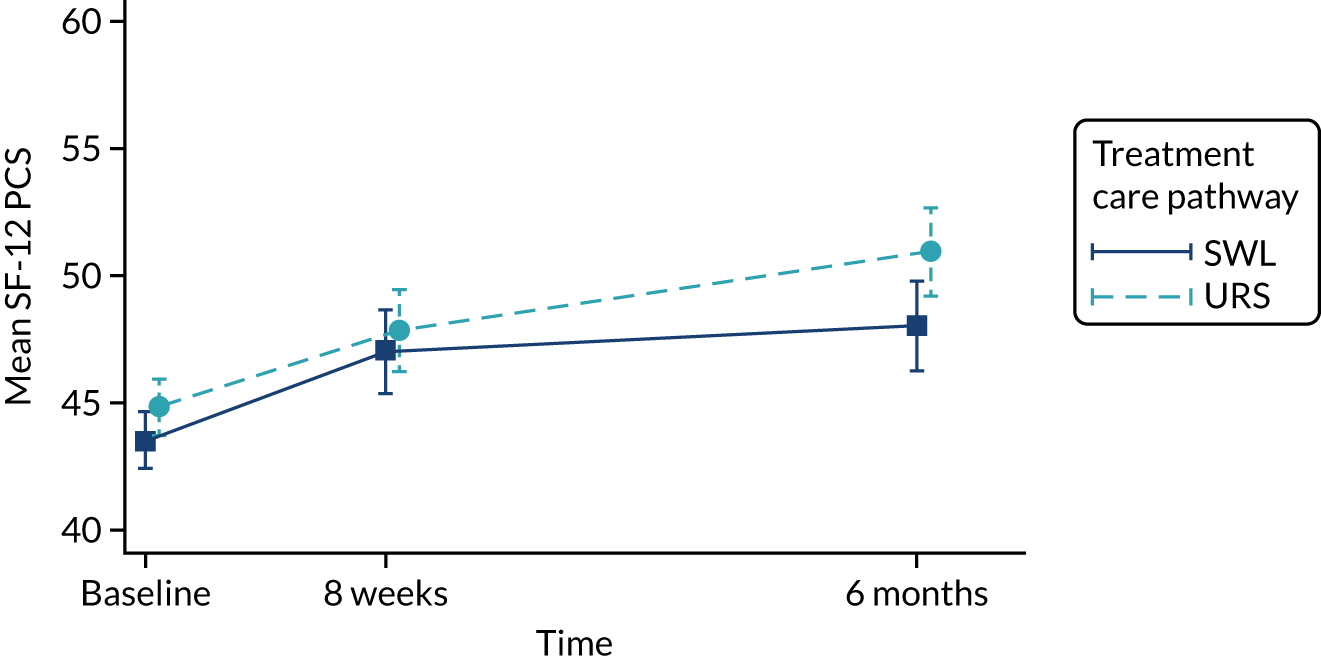
FIGURE 10.
Short Form questionnaire-12 items MCS over time. This figure has been reproduced with permission from Dasgupta et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Chapter 4 Economic evaluation: within-trial analysis
Economics methods
We estimated resource use and costs for each participant, and our evaluation considered the costs of the care pathways that patients received. Data collected on resource use included the intervention, SWL or URS, and simultaneous and consequent use of primary and secondary NHS services (including additional interventions received) by participants. The personal costs that were collected were purchase of medications, particularly analgesics, and visits to private health-care professionals.
The economic evaluation followed the reference case of the National Institute for Health and Care Excellence’s (NICE’s)29 recommendations for economic evaluations alongside a clinical trial. 30 The study adopted the perspective of the NHS, although some personal resource data were collected from the participants. We did not use any discounting because participants were followed up for only 6 months. The cost year utilised was 2017/18 and the currency used was Great British pounds (GBP).
Data collection
The interventions that were used to treat the participants’ stone and the subsequent resource use data were collected via the CRFs (see Report Supplementary Material 1). The CRFs for each participant were completed by research nurses at sites at the time of treatment and at hospital visits, and at 8 weeks and 6 months post randomisation. Information was collected on the treatments received by participants:
-
SWL
-
urgent/elective URS with stone fragmentation
-
endoscopic insertion (or removal) of a stent in the ureter
-
percutaneous insertion of nephrostomy
-
antegrade insertion of a ureteric stent through a nephrostomy.
Other resource use data included the use of imaging, such as plain X-rays, ultrasound, CTKUB and intravenous urography. Information on additional secondary care resource use was also collected from participant-completed questionnaires at 6 months post randomisation. Participants were asked for details on any other treatment that they may have had to treat their ureteric stones and the length of any associated hospital stay. Data on primary care resource use, such as visits to the GP and prescriptions, were collected through the participant questionnaires that were administered at 6 months post randomisation. Information on participant self-purchased health care, such as over-the-counter medications (particularly analgesics), was also collected by questionnaire at 6 months post randomisation.
Costs
The costs of resources that were used from the time of randomisation to 6 months post randomisation were included to reflect the care pathway of patients presenting with ureteric stones. Unit costs (Table 10) were based on published sources, namely the British National Formulary,31 NHS Reference Costs32 and the Personal Social Services Research Unit unit costs of primary care. 33 Imaging costs were based on a weighted average cost for each type and were derived from the diagnostic imaging schedule in the NHS Reference Costs. 32 The costs of the initial treatment, either SWL or URS, were based on the weighted average of the reported Healthcare Resource Group (HRG) activity, which excluded excess bed-days. The unit cost of SWL was based on the weighted average of HRG LB36Z. The unit cost of URS was the weighted average of HRG LB65C-E (ureteric stents were not costed separately, as the URS procedure cost included stenting) (see Appendix 4, Table 21). Each care pathway cost also included any inpatient stay that the participants required for complications from the treatment of their ureteric stone. The trim point (expected length of stay) for inpatient stay for the SWL intervention is 1 day (and 2 days for the URS intervention). The cost of any inpatient stay that was greater than this number of days was based on the elective excess bed-days cost of URS (as there is no excess bed-days cost for SWL). Inpatient cost for participants who received no intervention was based on the URS HRG cost of non-elective inpatient excess days. The cost of the other interventions, such as insertion and removal of stents, was based on the average cost of intermediate endoscopic ureteric procedures for adults. Outpatient hospital visit cost was the weighted average costs of a consultant and non-consultant urology outpatient department visit. The unit cost of GP visits was obtained from the Personal Social Services Research Unit unit costs of primary care. 33
| Resource | Unit cost (£) | Notes (source) |
|---|---|---|
| MET | 5 | Based on a 2-week dose (BNF31) |
| General practice: GP consultation | 31 | Per surgery consultation lasting 9.22 minutes33 |
| General practice: nurse consultation | 11 | Per surgery consultation lasting 15.5 minutes33 |
| X-ray | 31 | Direct-access plain film32 |
| CT | 97 | Weighted average cost of imaging: outpatient CT scans RD20AZ-RD28Z32 |
| Ultrasound scan | 58 | Weighted average cost of imaging: outpatient ultrasound scans RD40AZ-RD46Z32 |
| Contrast fluoroscopy | 155 | Weighted average cost of imaging: outpatient contrast procedures RD30AZ-RD35Z32 |
| Night in hospital | 370 | Weighted average cost of elective inpatient excess days for LB65 C-E32 |
| 386 | Weighted average cost of non-elective inpatient excess days for LB65 C-E32 | |
| Percutaneous insertion of nephrostomy tube M13 | 1027 | Average cost of unilateral, percutaneous insertion of ureteric stent or nephrostomy YL11Z32 |
| Antegrade insertion of stent into ureter M33 | 1054 | Average cost of intermediate endoscopic ureter procedures, aged ≥ 19 years, LB09D32 |
| Therapeutic ureteroscopic operations M27 | 2123 | Weighted average cost of major endoscopic ureter procedures kidney or ureter procedures, aged ≥ 19 years, LB65C-E32 |
| Insertion/removal of stent into ureter M29 | 1054 | Average cost of intermediate endoscopic ureter procedures, aged ≥ 19 years, LB09D32 |
| SWL M31 | 491 | Average cost of day-case SWL procedures (LB36Z)32 |
| Outpatient visit | 110 | Average cost of an outpatient visits to urology department (weighted consultant and non-consultant led), service code 10132 |
Participant costs
Participant costs were self-reported, such as prescription costs (for participants who pay prescription charges), over-the-counter medications and visits to non-NHS health-care providers.
Calculation of total costs
Estimates of resource utilisation were multiplied by unit costs to derive total costs for each item of resource use and each participant. These costs were summed to produce a total cost for each participant and an average total cost per participant in each care pathway arm.
Quality of life
The EQ-5D-3L24 and the SF-1226 were used to measure generic health-related quality of life (HRQoL) and health status. Participants were asked to complete the EQ-5D-3L at baseline (after informed consent but before randomisation), directly prior to treatment (pre intervention), 1 week after intervention/treatment, and at 8 weeks and 6 months post randomisation. The EQ–5D-3L divides health status into five dimensions with three levels of severity. EQ-5D-3L questionnaire responses were transformed into utility values using general population time trade-off-generated preference weights. 34
Quality-adjusted life-years were calculated by multiplying quality (utility) and length of life, assuming linear extrapolation between measurement time points. For each patient, the area under the curve (AUC) was used to estimate QALYs gained (quality of life multiplied by duration of the trial). Calculation of the AUC took into account the length of time that the patient waited for treatment. Information on the time between randomisation and treatment was incorporated into the QALY calculation, when it was available. Calculation of QALYs gained in the case of those for whom a treatment date was missing was based on the post-randomisation time points.
Responses from the SF-12 questionnaire collected at baseline and at 8 weeks and 6 months post randomisation were also used to estimate QALYs. They were mapped onto the existing Short Form questionnaire-6 Dimensions (SF-6D) measure, using the algorithm by Brazier et al.,35 to allow utility values to be estimated for each time point. These utility scores were transformed into QALYs using the methods described above, to provide an alternative measure of QALYs gained for each participant.
Data analysis
The economic analysis was based on the ITT principle. All components of costs were described with the appropriate descriptive statistics: mean and SD for continuous and count outcomes, and numbers and percentages for dichotomous and categorical outcomes (e.g. numbers reporting problems on EQ-5D-3L). All analyses were conducted using Stata.
We investigated skewed cost data (due to a small proportion of participants incurring very high costs), using GLMs to test alternative model specifications for appropriate fit to the data. These GLMs allow for heteroscedasticity by specifying a distributional family that reflects the relationship between mean and variance. 36 We used a modified Park’s test, which identified a Gaussian family as most appropriate (this allows skewness and assumes that the variance is proportional to the square of the mean). We identified a log-link function as the best model to specify the relationship between the set of regressors and the conditional mean. Our selection was based on a combination of results from the Pearson correlation, Pregibon link and modified Hosmer–Lemeshow tests (see Appendix 4, Table 22). We, therefore, analysed the base-case cost analysis data using a Gaussian family and a log-link function. The mean incremental QALYs were estimated using ordinary least squares adjusted for minimisation variables [stone size (≤ 10 mm or > 10 mm), stone location (upper, middle or lower ureter)] and baseline EQ-5D-3L score. Analysis models were run to estimate the incremental effect of treatment arm on costs and QALYs. The coefficient for treatment in each model was taken as the estimate of incremental costs for use in the economic evaluation. 36,37
Missing data
A well-known issue in cost-effectiveness analysis, especially within a RCT setting, is the presence of large proportions of missing data in either or both outcome variables (i.e. the cost and the utility measures). 38 We adapted a decision rule on imputation that if > 10% of complete cost or QALY data were missing, then imputation would be considered for the base-case analysis. Multivariate imputation by chained equations was used to impute values for missing data. Missing data were assumed to be missing at random. The data sets were combined using the ‘mi estimate’ command in Stata, which applies Rubin’s rules when combining estimates from multiple imputed data sets to account for variation both within and between data sets. All imputation models included variables for indicators, such as treatment allocation and patient characteristics [stone size, stone location (upper, middle and lower ureter), gender and age]. For quality of life, the index score was imputed (rather than each domain) and baseline EQ-5D-3L was also included in the imputation model. We, therefore, employed an imputation method for missing values using all available information following multivariate imputation by chained equations39 for missing EQ-5D-3L index scores that were used in the QALY analysis. We chose this multiple imputation approach as it has attractive theoretical and methodological properties and is a more powerful and flexible tool when the level of missingness is between 10% and 60%. Missing EQ-5D-3L and SF-6D data were imputed using predictive mean matching (the mean of five nearest values). Missing cost data were imputed at the category level (imaging, intervention, outpatient, hospitalisation and other treatment costs), using the predictive mean matching approach. Imputations were completed separately for each trial arm.
Incremental cost-effectiveness
Our base-case analysis was based on models that used imputed data and the sensitivity analysis was performed on the complete-case data (cases with both complete cost data and complete QALY data). The overall results of the cost–utility analysis are reported as the incremental cost per QALY gained for the care pathway starting with SWL compared with the care pathway starting with URS. The results are presented as point estimates of mean costs, QALYs and incremental cost per QALY of each treatment care pathway. We used non-parametric bootstrapping of the imputed regression models to consider the impact of sampling uncertainty and generate a probability of cost-effectiveness at several threshold values of decision-makers’ willingness to pay (WTP) for a QALY gain. Non-parametric boot-strapping methods were used to estimate 95% CIs for treatment effects on costs and QALYs, using 1000 replications, to summarise the uncertainty surrounding the incremental cost-effectiveness ratio (ICER). Incremental cost-effectiveness results are presented in terms of cost-effectiveness acceptability curves (CEACs). The bootstrap replications of the models were further used to illustrate sampling uncertainty by plotting the 1000 replications of the bootstrapped estimates of the differences in costs and QALYs on the cost-effectiveness plane. This presentation allows for a visual representation of the joint uncertainty in the effect sizes for cost and QALY estimates, illustrating the probability of a specified intervention (in this case SWL) falling into each quadrant of the cost-effectiveness plane and being (1) less costly and more effective, (2) more costly and less effective, (3) less costly and less effective or (4) more costly and more effective.
The CEACs were generated using these 1000 estimates, using the net monetary benefit (NMB) approach. The NMB associated with a given treatment option is given by the formula:
where effects are measured in QALYs and Rc is the ceiling ratio of WTP per QALY.
Using this formula, the strategy with greatest NMB is identified for each of the 1000 bootstrapped replicates of the analysis, for different ceiling ratios of WTP per QALY. Plotting the proportion of bootstrap iterations favouring each treatment option (in terms of the NMB) against increasing WTP per QALY produces the CEAC for each treatment option. These curves graphically present the probability of each treatment strategy being considered optimal at different levels of WTP per QALY gained. For the purposes of the base-case analysis, Rc was set at £30,000, the upper end of the commonly accepted range of ICERs considered to offer good value for money by NICE. A number of alternative threshold values presented at £0, £10,000, £20,000, £30,000 and £50,000 were explored and are presented numerically within the tables and visually using the CEACs (see Appendix 4).
Sensitivity analysis
Deterministic sensitivity analysis was used to explore the impact of important choices surrounding assumptions and analysis models on the cost-effectiveness findings. The results of the sensitivity analyses were also be presented as CEACs. Various sensitivity analyses were conducted to explore the importance of such uncertainties. A sensitivity analysis using the complete case (for participants with both cost and QALY data) was also performed to assess the impact of missing data on the results. There is some uncertainty as to whether or not the dimensions in the EQ-5D-3L are sensitive enough to capture the loss in quality of life, particularly in reference to acute pain. Therefore, SF-12 responses were mapped on the SF-6D measure, using the algorithm by Brazier et al. 35 to facilitate the estimation of utility values for each time point. These scores were used in the same way as the EQ-5D-3L to provide an alternative measure of QALYs for each patient. Analyses were undertaken on both imputed and complete-case SF-6D data.
NHS Reference Cost data32 were used to estimate the cost of the interventions that were used in this study. The HRG unit cost of SWL is almost one-quarter of the cost of URS. Several studies outside an NHS setting18 have indicated that SWL costs more than URS. Therefore, sensitivity analyses were undertaken, using the elective inpatient tariff of SWL. Several scenarios were considered depending on the proportion of patients treated as inpatients: 25%, 50%, 75% and 100%. Analyses were undertaken on imputed data.
Economics results
Data completeness
Details of missing resource use and EQ-5D-3L data are reported in Table 11. There were very few missing data for secondary care resource use, as this information was collected using CRFs. Thirty-eight per cent of the data were missing from the patient-reported outcomes because questionnaires were not returned or were incomplete. The proportion of missing resource use data was the same in both arms. The number of participants with complete quality-of-life data was different at each time point. The percentage of missing data was highest at 6 months (55%). The proportion of missing data for utility outcomes based on the EQ-5D-5L was the same in both treatment arms (apart from the pre-intervention scores, which were higher in the URS arm). Complete utility data at each specified time point were available for only 23% of SWL participants and 25% of URS participants. The proportion of missing data was even greater in the case of SF-6D utility scores. Complete QALY data based on the SF-6D utility scores were available for only 15% of SWL participants and 17% of URS participants. A summary of missing data for each time period and total QALYs is presented in Table 11.
| SWL (N = 303) | URS (N = 306) | Total (N = 609) | ||||
|---|---|---|---|---|---|---|
| Missing, n | % | Missing, n | % | Missing, n | % | |
| NHS resource use | ||||||
| General practice: GP consultation | 114 | 38 | 115 | 38 | 229 | 38 |
| General practice: nurse consultation | 112 | 37 | 114 | 37 | 226 | 37 |
| MET | 112 | 37 | 115 | 38 | 227 | 37 |
| Outpatient hospital visits | 0 | 0 | 4 | 1 | 4 | 1 |
| X-ray | 0 | 0 | 3 | 1 | 3 | 0 |
| Ultrasound | 0 | 0 | 3 | 1 | 3 | 0 |
| CT | 0 | 0 | 3 | 1 | 3 | 0 |
| IVU | 0 | 0 | 3 | 1 | 3 | 0 |
| Nephrostomy tube | 0 | 0 | 3 | 1 | 3 | 0 |
| Antegrade stent insert/removal | 0 | 0 | 3 | 1 | 3 | 0 |
| URS | 0 | 0 | 3 | 1 | 3 | 0 |
| Ureteric stent insertion | 0 | 0 | 0 | 0 | 0 | 0 |
| Ureteric stent removal | 0 | 0 | 3 | 1 | 3 | 0 |
| SWL | 0 | 0 | 3 | 1 | 3 | 0 |
| Inpatient stay | 5 | 2 | 16 | 5 | 21 | 3 |
| Patient personal resource | ||||||
| Over-the-counter medicine | 137 | 45 | 139 | 45 | 276 | 45 |
| Private provider visit | 136 | 45 | 136 | 44 | 272 | 45 |
| Quality of life | ||||||
| EQ-5D-3L | ||||||
| Baseline | 5 | 2 | 9 | 3 | 14 | 2 |
| Pre treatment | 51 | 17 | 95 | 31 | 146 | 24 |
| 1 week post treatment | 117 | 39 | 131 | 43 | 248 | 41 |
| 8 weeks | 154 | 51 | 154 | 50 | 308 | 51 |
| 6 months | 173 | 57 | 163 | 53 | 336 | 55 |
| QALY | 233 | 77 | 229 | 75 | 462 | 75 |
| SF-6D | ||||||
| Baseline | 108 | 36 | 113 | 37 | 221 | 36 |
| 8 weeks | 197 | 65 | 199 | 65 | 396 | 65 |
| 6 months | 199 | 66 | 197 | 64 | 396 | 65 |
| QALY | 258 | 85 | 255 | 83 | 513 | 84 |
Resource use
Table 12 details the mean resource use for the interventions and the subsequent use of health services over the 6-month period. SWL participants made more outpatient hospital visits than URS patients, received more imaging of all types apart from intravenous urography (IVU) and, unsurprisingly, were more likely to receive SWL. It is common for a stent to be inserted during the URS procedure. However, stent insertion was not counted as additional resource use if it took place at the time of URS, but stent removal was considered as resource use and incurred a cost. Resource use for URS and stent removal was higher in the URS arm.
| Resource (NHS) | SWL (N = 303) | URS (N = 306) | Difference SWL vs. URSa | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean | 95% CI | |
| MET | 191 | 0.24 | 0.43 | 191 | 0.23 | 0.42 | 0.02 | –0.06 to 0.10 |
| General practice: GP consultation | 191 | 0.17 | 1.00 | 192 | 0.08 | 0.40 | 0.02 | –0.10 to 0.15 |
| General practice: nurse consultation | 189 | 0.22 | 0.67 | 191 | 0.21 | 0.80 | 0.06 | –0.03 to 0.16 |
| Outpatient hospital visits | 303 | 1.59 | 0.95 | 302 | 0.84 | 0.88 | 0.74 | 0.53 to 0.95 |
| X-ray | 303 | 1.62 | 1.19 | 303 | 0.72 | 0.88 | 0.88 | 0.69 to 1.07 |
| Ultrasound | 303 | 0.39 | 0.82 | 303 | 0.08 | 0.34 | 0.32 | –0.05 to 0.69 |
| CT | 303 | 0.26 | 0.52 | 303 | 0.18 | 0.46 | 0.08 | 0.02 to 0.15 |
| IVU | 303 | 0.01 | 0.08 | 303 | 0.00 | 0.06 | 0.00 | –0.01 to 0.02 |
| Nephrostomy tube | 303 | 0.01 | 0.08 | 303 | 0.00 | 0.06 | 0.00 | –0.01 to 0.02 |
| Antegrade stent insert/removal | 303 | 0.02 | 0.17 | 303 | 0.01 | 0.08 | 0.02 | –0.01 to 0.04 |
| URS | 303 | 0.29 | 0.48 | 303 | 0.88 | 0.46 | 0.59 | –0.68 to –0.51 |
| Ureteric stent insertion | 303 | 0.01 | 0.10 | 303 | 0.00 | 0.06 | 0.01 | –0.01 to 0.02 |
| Ureteric stent removal | 303 | 0.16 | 0.43 | 303 | 0.32 | 0.51 | 0.17 | –0.25 to –0.09 |
| SWL | 303 | 1.12 | 0.88 | 303 | 0.11 | 0.44 | 1.01 | 0.82 to 1.21 |
| Inpatient stay (days) | 298 | 0.53 | 1.49 | 290 | 0.46 | 1.45 | 0.01 | –0.16 to 0.19 |
Participant resource use
In total, 32 participants reported purchasing over-the-counter medicine (12 participants in the SWL arm and 20 participants in the URS arm) and only two participants reported that they saw a private health-care provider over the 6-month follow-up period (one in each arm).
Costs results
Table 13 provides information about the mean cost per participant by the different categories of resource use. Similar to resource use, costs were higher in the SWL arm for hospital visits, all imaging (apart from IVU), endoscopic stent insertion and SWL. Endoscopic ureteric stent insertion costs were higher for the SWL arm as they were not costed separately for the URS group, which is because they are included in the overall cost of URS (unless they were not inserted during the URS procedure); the difference in costs was minimal. Costs in the URS arm were higher for URS and stent removal. The total complete-case analysis costs were higher in the URS arm, mainly driven by the cost of URS.
| Resource | SWL | URS | Difference SWL vs. URSa | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | Mean (£) | 95% CI | |
| MET | 191 | 1.18 | 2.10 | 191 | 1.13 | 2.06 | 0.02 | –0.23 to 0.28 |
| General practice: GP consultation | 189 | 8.22 | 24.83 | 191 | 7.75 | 29.60 | 0.86 | –3.65 to 5.38 |
| General practice: nurse consultation | 191 | 1.84 | 11.02 | 192 | 0.92 | 4.41 | 0.69 | –0.36 to 1.74 |
| Outpatient hospital visits | 303 | 173.67 | 104.10 | 302 | 92.01 | 96.12 | 80.94 | 57.71 to 104.17 |
| X-ray | 303 | 48.16 | 35.35 | 303 | 21.52 | 26.25 | 26.21 | 20.41 to 31.99 |
| Ultrasound | 303 | 21.88 | 45.83 | 303 | 4.60 | 18.95 | 17.74 | –2.83 to 38.31 |
| CT | 303 | 26.03 | 51.50 | 303 | 18.45 | 45.83 | 8.20 | 1.57 to 14.83 |
| IVU | 303 | 0.20 | 2.42 | 303 | 0.10 | 1.71 | 0.12 | –0.31 to 0.55 |
| Nephrostomy tube | 303 | 6.43 | 78.97 | 303 | 3.21 | 55.93 | 3.47 | –11.42 to 18.37 |
| Antegrade stent insert/removal | 303 | 24.10 | 178.46 | 303 | 6.89 | 84.61 | 17.48 | –8.21 to 43.17 |
| URS | 303 | 633.89 | 1030.48 | 303 | 1894.54 | 997.19 | –1282.18 | –1468.92 to –1095.43 |
| Ureteric stent insert | 303 | 10.33 | 103.45 | 306 | 3.41 | 59.63 | –6.50 | –10.40 to 23.41 |
| Ureteric stent removal | 303 | 165.25 | 450.79 | 303 | 333.95 | 536.72 | –173.63 | –256.59 to –90.66 |
| SWL | 303 | 506.77 | 399.64 | 303 | 49.19 | 200.30 | 458.24 | 371.92 to 544.56 |
| Inpatient stay (days) | 298 | 160.69 | 453.39 | 290 | 138.83 | 442.36 | 4.46 | –50.23 to 59.15 |
| Total cost | 182 | 1549.53 | 1586.10 | 179 | 2498.33 | 1436.43 | –808.20 | –1044.24 to –571.00 |
Participant resource costs
The SWL arm spent, on average, £2, and the URS arm spent, on average, £3, on over-the-counter medicine. The mean cost spent on private care was £24 in the SWL arm and £2 in the URS arm.
Quality-adjusted life-years
Table 14 shows the EQ-5D-3L, VAS and SF-6D utility scores for each care pathway at different time points. The baseline utility scores were all similar. The EQ-5D-3L utility scores pre treatment and at 8 weeks and 6 months post randomisation were higher for URS than for SWL. The mean estimated QALYs gained were 0.411 (SD 0.112) for the SWL pathway and 0.439 (SD 0.070) for the URS pathway. The adjusted mean QALY difference for the SWL care pathway was –0.029 (95% CI –0.062 to 0.005). The VAS scores were higher in the URS arm at each time point, but the differences were small. The mean estimated QALYs for SF-6D utility scores were 0.393 (SD 0.075) for the SWL arm and 0.400 (SD 0.064) for the URS arm. The adjusted mean QALY difference (SWL vs. URS) was –0.009 (95% CI –0.036 to 0.018). The QALY results should be interpreted with caution, taking into account the fact that the proportion of missing data was high.
| Measure | SWL | URS | Difference SWL vs. URSa | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean | 95% CI | |
| EQ-5D-3L | ||||||||
| Baseline | 298 | 0.737 | 0.263 | 297 | 0.729 | 0.303 | ||
| Pre treatment | 252 | 0.735 | 0.260 | 211 | 0.758 | 0.272 | –0.041 | –0.085 to 0.002 |
| 1 week post treatment | 186 | 0.756 | 0.267 | 175 | 0.757 | 0.263 | –0.007 | –0.068 to 0.055 |
| 8 weeks post randomisation | 149 | 0.797 | 0.293 | 152 | 0.874 | 0.207 | –0.081 | –0.152 to –0.009 |
| 6 months post randomisation | 130 | 0.837 | 0.289 | 143 | 0.912 | 0.182 | –0.081 | –0.146 to –0.016 |
| QALYb | 70 | 0.407 | 0.116 | 74 | 0.436 | 0.070 | –0.029 | –0.062 to 0.005 |
| EQ-5D VAS | ||||||||
| Baseline | 282 | 68 | 24 | 283 | 67 | 27 | ||
| Pre treatment | 235 | 69 | 25 | 198 | 74 | 22 | –4 | –7 to –1 |
| 1 week post treatment | 180 | 74 | 22 | 172 | 74 | 20 | –1 | –7 to 5 |
| 8 weeks post randomisation | 150 | 77 | 21 | 153 | 79 | 21 | –4 | –9 to 1 |
| 6 months post randomisation | 131 | 78 | 21 | 143 | 81 | 18 | –3 | –9 to 3 |
| SF-6D | ||||||||
| Baseline | 195 | 0.699 | 0.168 | 193 | 0.737 | 0.175 | ||
| 8 weeks post randomisation | 106 | 0.762 | 0.169 | 107 | 0.782 | 0.151 | –0.003 | –0.047 to 0.040 |
| 6 months post randomisation | 104 | 0.789 | 0.173 | 109 | 0.837 | 0.139 | –0.069 | –0.123 to –0.015 |
| QALYb | 45 | 0.393 | 0.075 | 51 | 0.400 | 0.064 | –0.009 | –0.036 to 0.018 |
There was an increase in all (EQ-5D-3L, VAS and SF-6D) scores over time, as illustrated by Figures 11–13.
FIGURE 11.
EuroQol-5 Dimensions, three-level version scores at each time point by arm. EQ-5D, EuroQol-5 Dimensions.
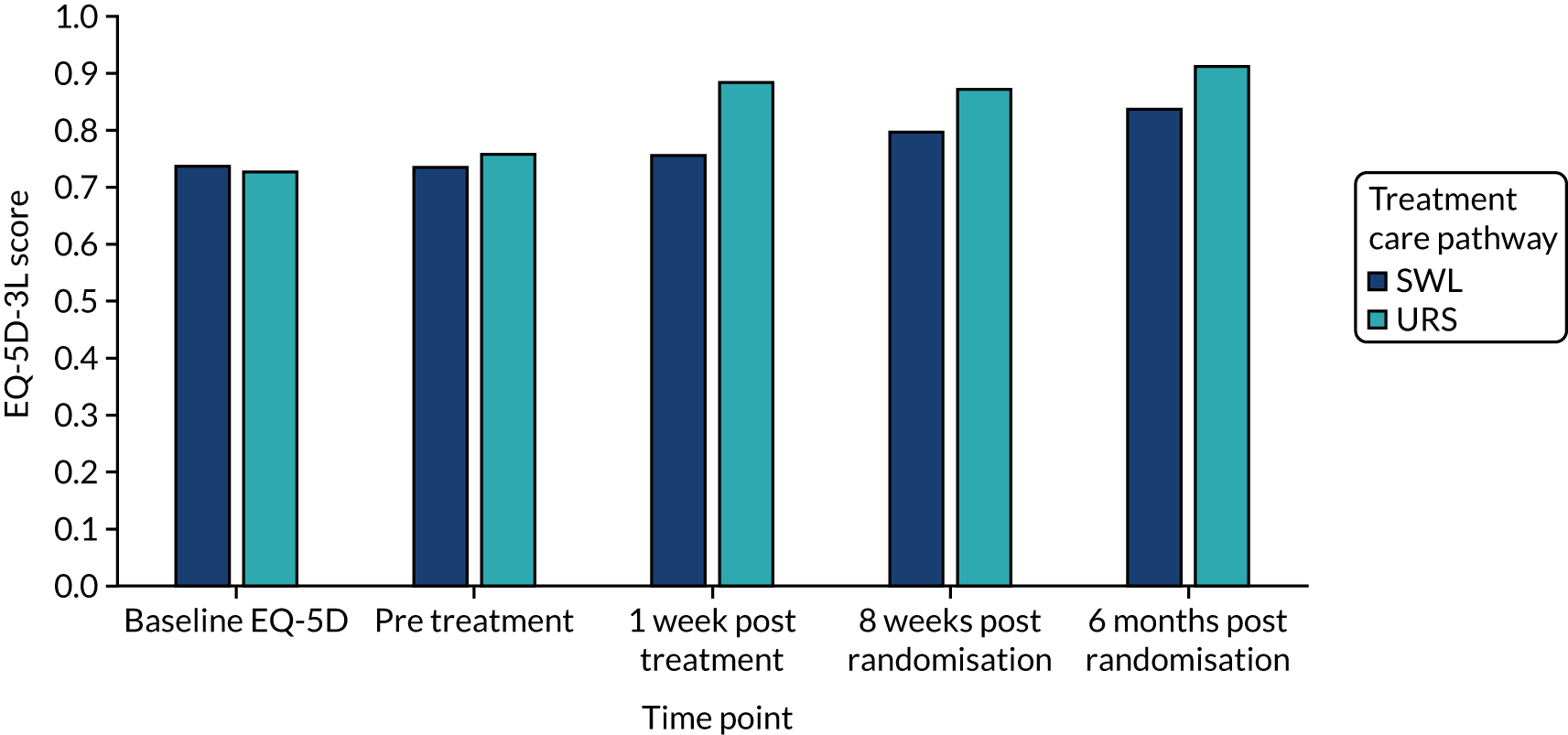
FIGURE 12.
EuroQol-5 Dimensions VAS scores at each time point by arm. EQ-5D, EuroQol-5 Dimensions.
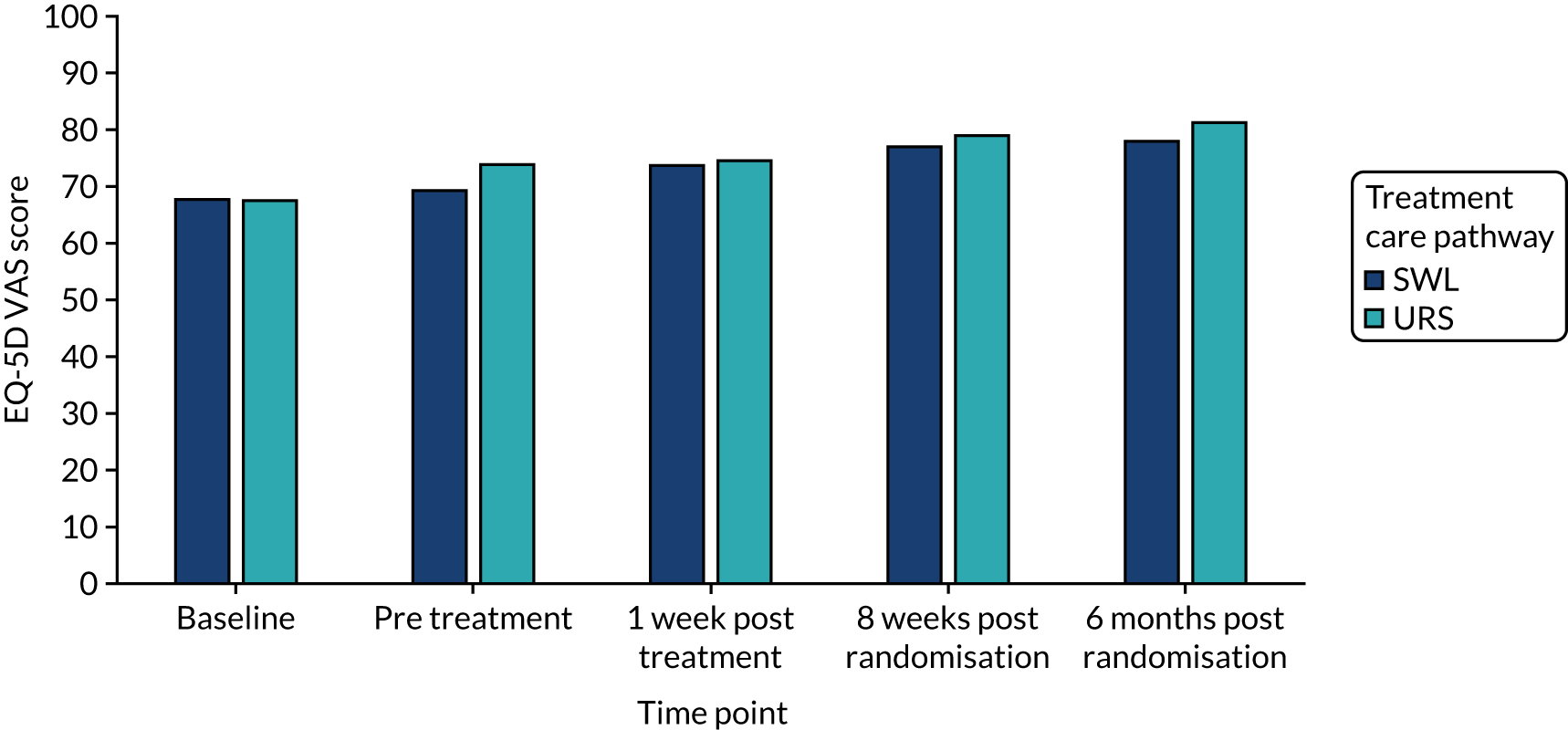
FIGURE 13.
Short Form questionnaire-6 Dimensions scores at each time point by arm.
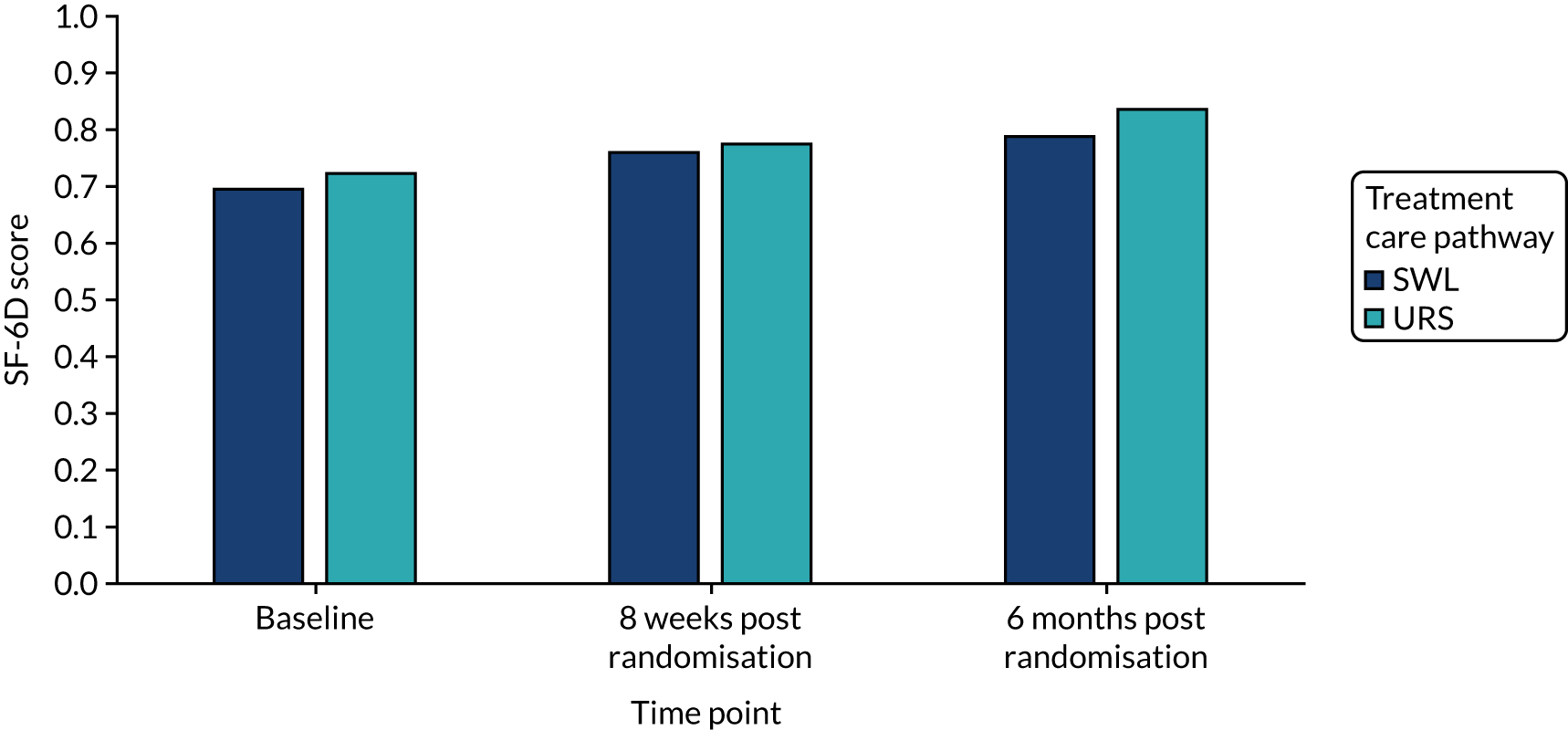
Cost-effectiveness analysis
The results of the base-case analysis are reported in Table 15. The base-case analysis (using multiple imputation) showed that the mean cost for participants on the SWL care pathway was £809 less than that for those on the URS care pathway, but resulted in a QALY gain 0.021 lower than the URS care pathway. The point estimate of the incremental cost per QALY is a cost saving of £39,118 per QALY lost and the uncertainty around this estimate is illustrated in Figures 14 and 15. This means that a decision-maker would save £39,118 for each lost QALY, with 79% probability that SWL would be considered to be cost-effective at society’s WTP £30,000 for a QALY. The CEAC is derived from the joint distribution of incremental costs and incremental effects. Most of the results fall in the south-west quadrant of the cost effectiveness plane as SWL always costs less but is less effective.
| Intervention | Cost (£) | Differencea (£) | QALYs | Differencea | ICER £/QALY | Probability of being cost-effective at different WTP thresholds (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Base-case analysis imputed data analysis | ||||||||||
| SWL | 1790 | 0.403 | 1 | 1 | 0.98 | 0.79 | 0.25 | |||
| URS | 2599 | 809b | 0.424 | 0.021b | 39,118 | 0 | 0 | 0.02 | 0.21 | 0.75 |
| Complete-case analysis | ||||||||||
| SWL | 1584 | 0.407 | 1 | 1 | 0.96 | 0.80 | 0.46 | |||
| URS | 2932 | 1348 | 0.436 | 0.029 | 46,297c | 0 | 0 | 0.04 | 0.20 | 0.52 |
| Using SF-6D utility scores imputed data | ||||||||||
| SWL | 1790 | 0.385 | 1 | 1 | 1 | 1 | 1 | |||
| URS | 2599 | 809 | 0.387 | 0.002 | 432,432c | 0 | 0 | 0 | 0 | 0 |
| Complete case using SF-6D utility scores | ||||||||||
| SWL | 2102 | 0.388 | 0.81 | 0.76 | 0.70 | 0.64 | 0.53 | |||
| URS | 2502 | 500 | 0.398 | 0.010 | 52,313c | 0.20 | 0.24 | 0.30 | 0.36 | 0.47 |
| Assuming all patients with missing EQ-5D-3L 6-month scores are in full health at 6 months as stones have passed | ||||||||||
| SWL | 1790 | 0.423 | 1 | 1 | 1 | 0.95 | 0.65 | |||
| URS | 2599 | 795 | 0.437 | 0.014 | 57,889 | 0 | 0 | 0 | 0.05 | 0.35 |
| Higher cost of SWL assuming 25% of patients are inpatient | ||||||||||
| SWL | 1952 | 0.403 | 1 | 1 | 0.90 | 0.58 | 0.13 | |||
| URS | 2614 | 663 | 0.424 | 0.021 | 32,034 | 0 | 0 | 0.10 | 0.42 | 0.87 |
| Higher cost of SWL assuming 50% of patients are inpatient | ||||||||||
| SWL | 2073 | 0.403 | 1 | 0.98 | 0.77 | 0.42 | 0.08 | |||
| URS | 2627 | 555 | 0.424 | 0.021 | 26,820 | 0 | 0.02 | 0.23 | 0.58 | 0.92 |
| Higher cost of SWL assuming 75% of patients are inpatient | ||||||||||
| SWL | 2190 | 0.403 | 1 | 0.94 | 0.60 | 0.23 | 0.04 | |||
| URS | 2642 | 453 | 0.424 | 0.021 | 21,888 | 0 | 0.06 | 0.40 | 0.77 | 0.96 |
| Higher cost of SWL assuming 100% of patients are inpatient | ||||||||||
| SWL | 2306 | 0.403 | 0.99 | 0.81 | 0.38 | 0.13 | 0.02 | |||
| URS | 2652 | 346 | 0.424 | 0.021 | 16,710 | 0.01 | 0.19 | 0.62 | 0.87 | 0.98 |
FIGURE 14.
Cost-effectiveness acceptability curve: SWL vs. URS – base-case analysis based on multiple imputation data.
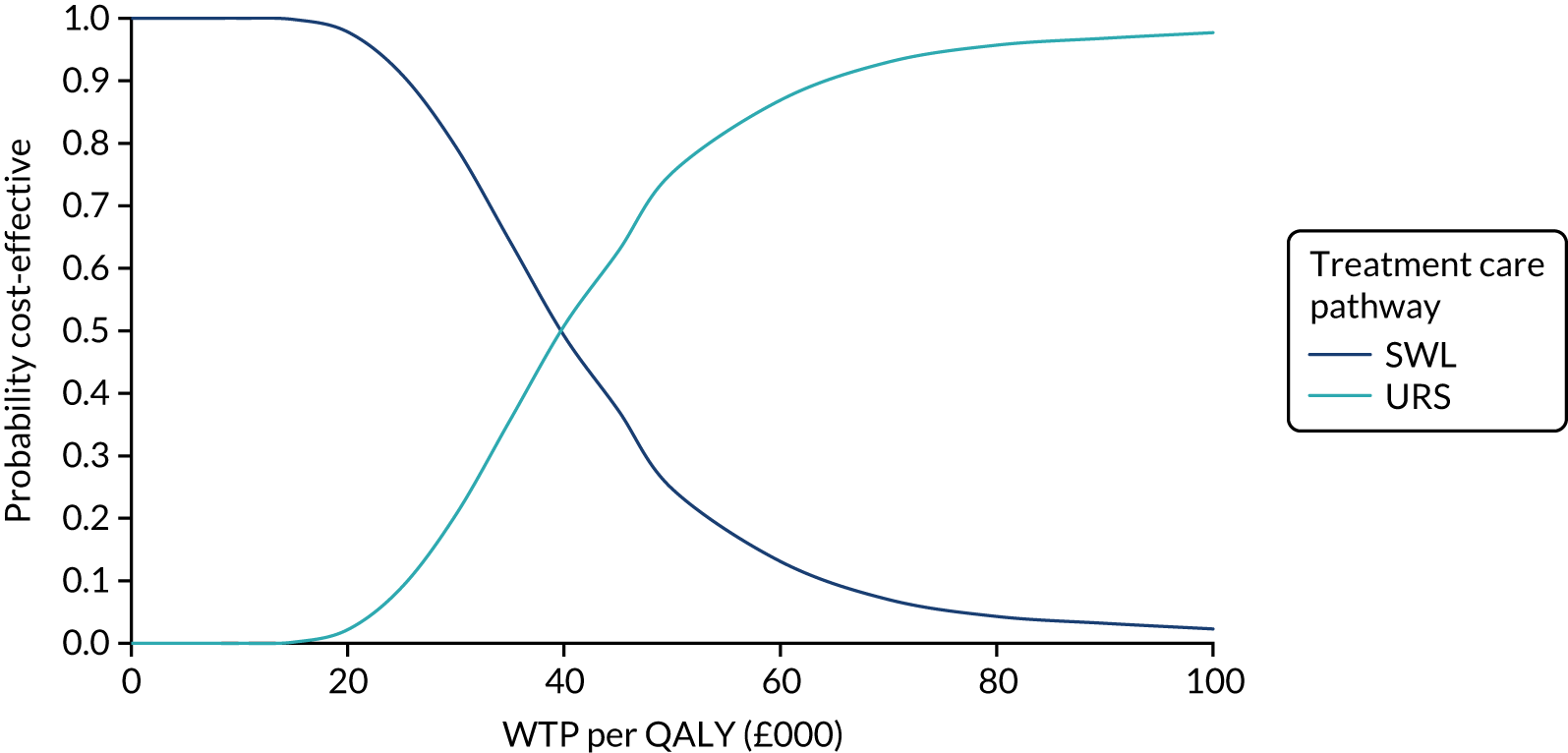
FIGURE 15.
Scatterplot of incremental cost and incremental QALYs (imputed data): SWL vs. URS.
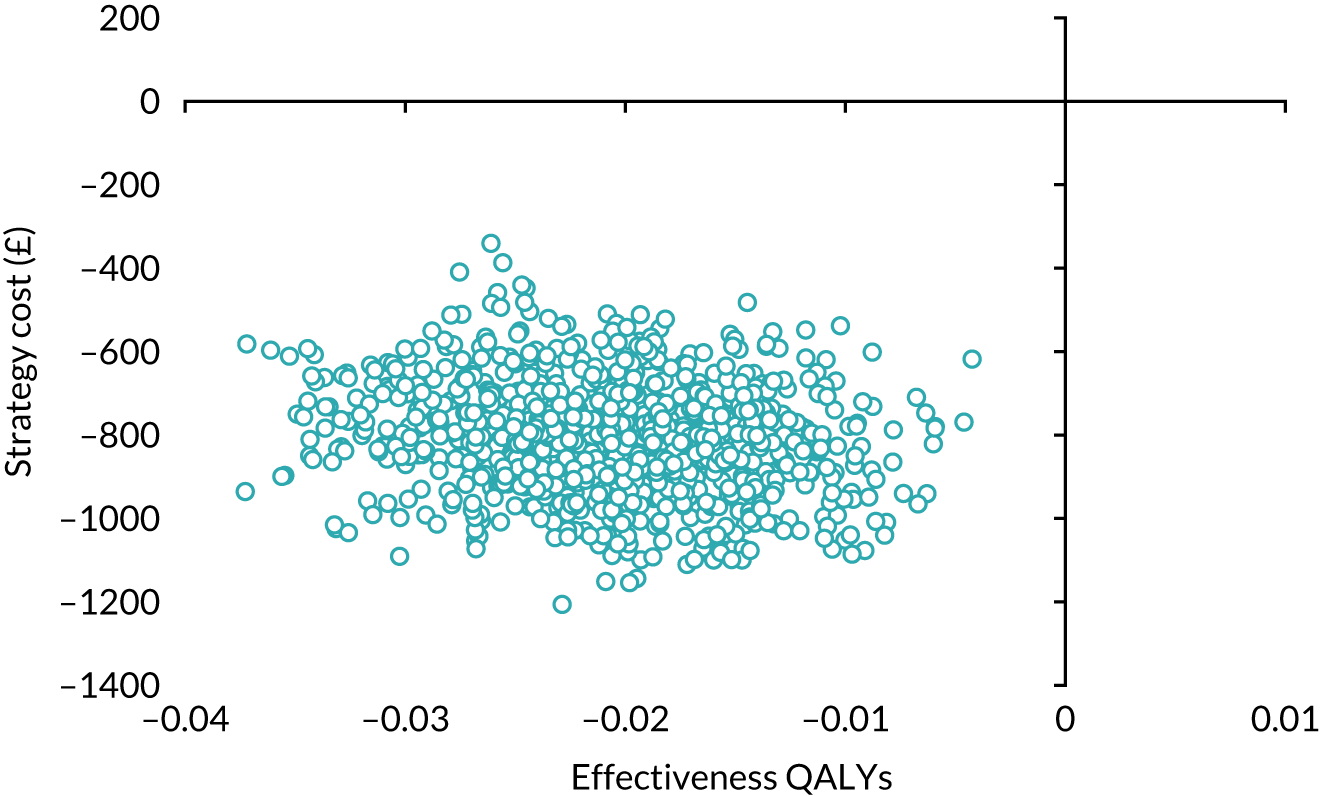
Figure 15 shows that a large number of bootstrapped iterations lie to the left of the vertical axis and below the horizontal axis, indicating that the SWL is less costly than URS, with marginally fewer QALYs achieved throughout the intervention.
Sensitivity analysis
Complete-case analysis
The results of the complete-case analysis are reported in Table 15 and Figures 16 and 17. The cost results were in the same direction as in the base-case analysis: SWL cost less but the cost difference was higher [£1348 (complete case) vs. £809 (imputed data)]. The QALY difference was similar (0.029). The ICER is higher (£46,297) than the base-case analysis and the probability that SWL is cost-effective is 80% at a threshold of society’s WTP for a QALY of £30,000.
FIGURE 16.
Cost-effectiveness acceptability curve for sensitivity analysis based on complete-case data: SWL vs. URS.
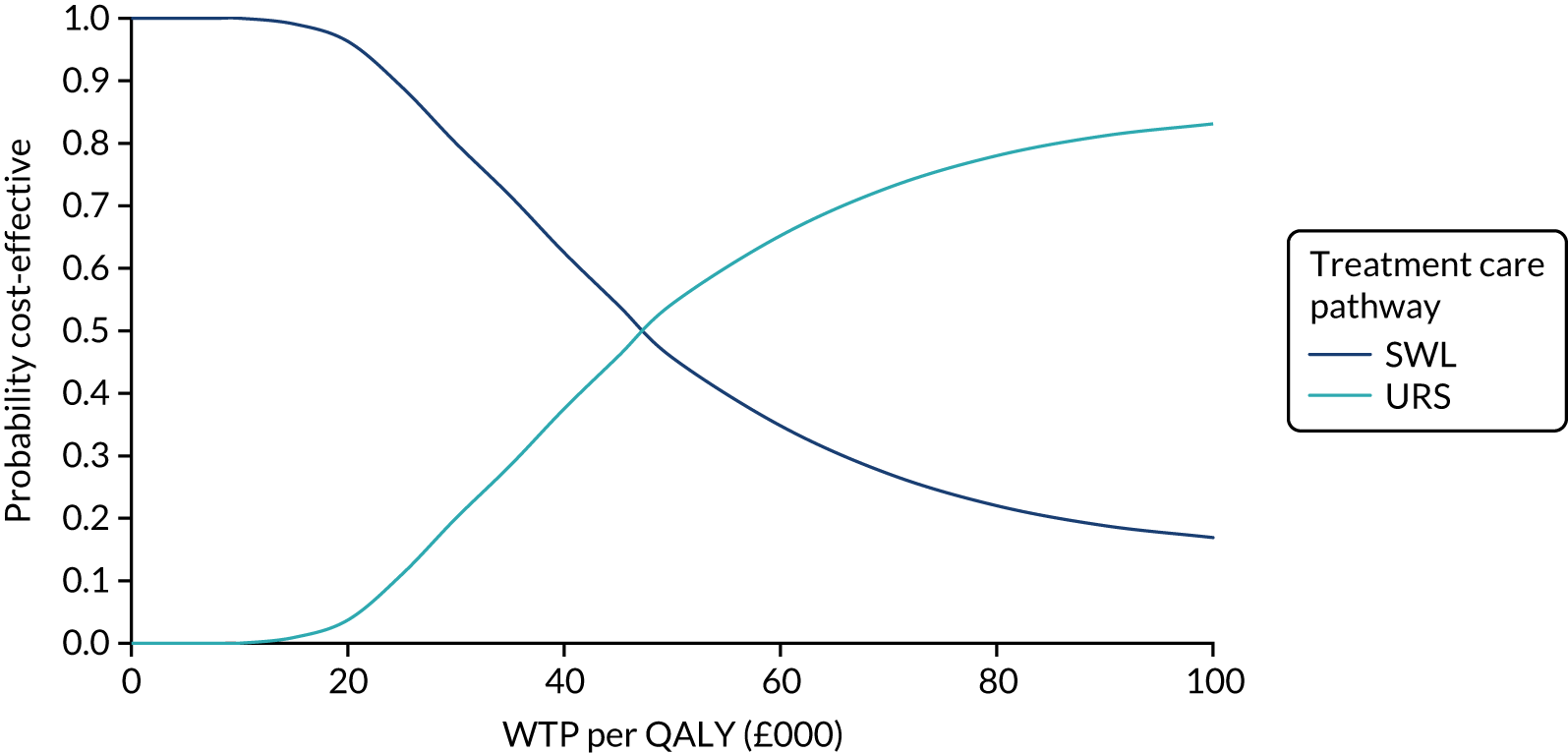
FIGURE 17.
Scatterplot of incremental costs and QALYs based on complete-case data: SWL vs. URS.
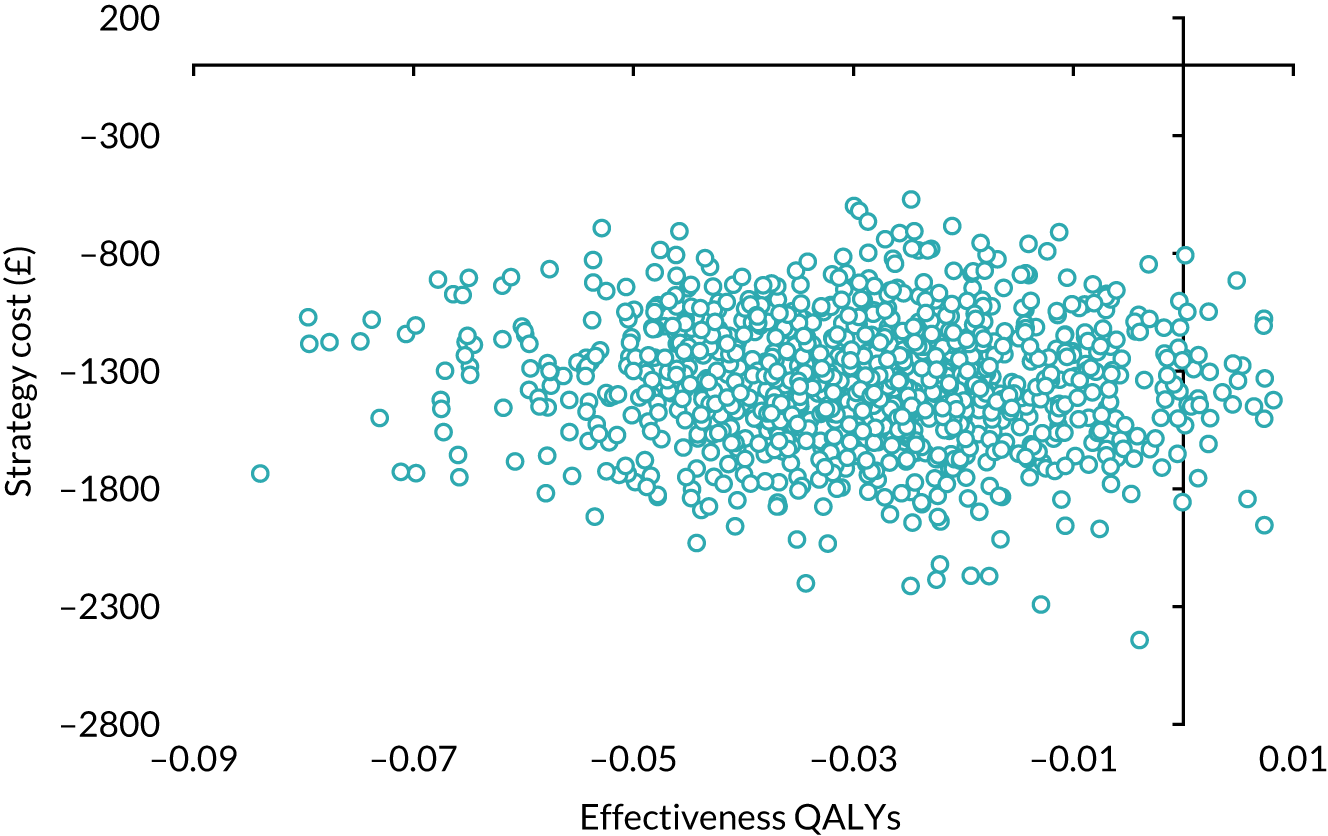
Imputation analysis using Short Form questionnaire-6 Dimensions data
The results of the sensitivity analysis using imputed SF-6D data are reported in Table 15 and Figures 18 and 19. The direction of the difference in cost result remained the same as in the base-case analysis: SWL cost less (£809) than URS (£1790 vs. £2599). The direction of the QALY difference was the same as in the base case (lower), but the magnitude changed. The lower difference in quality-of-life estimates (0.002) meant that the ICER increased to £432,432. The probability that society will be willing to pay for a QALY loss was 100% if the threshold of society’s WTP for a QALY is £30,000.
FIGURE 18.
Cost-effectiveness acceptability curve for sensitivity analysis based on SF-6D QALY multiple imputation data: SWL vs. URS.
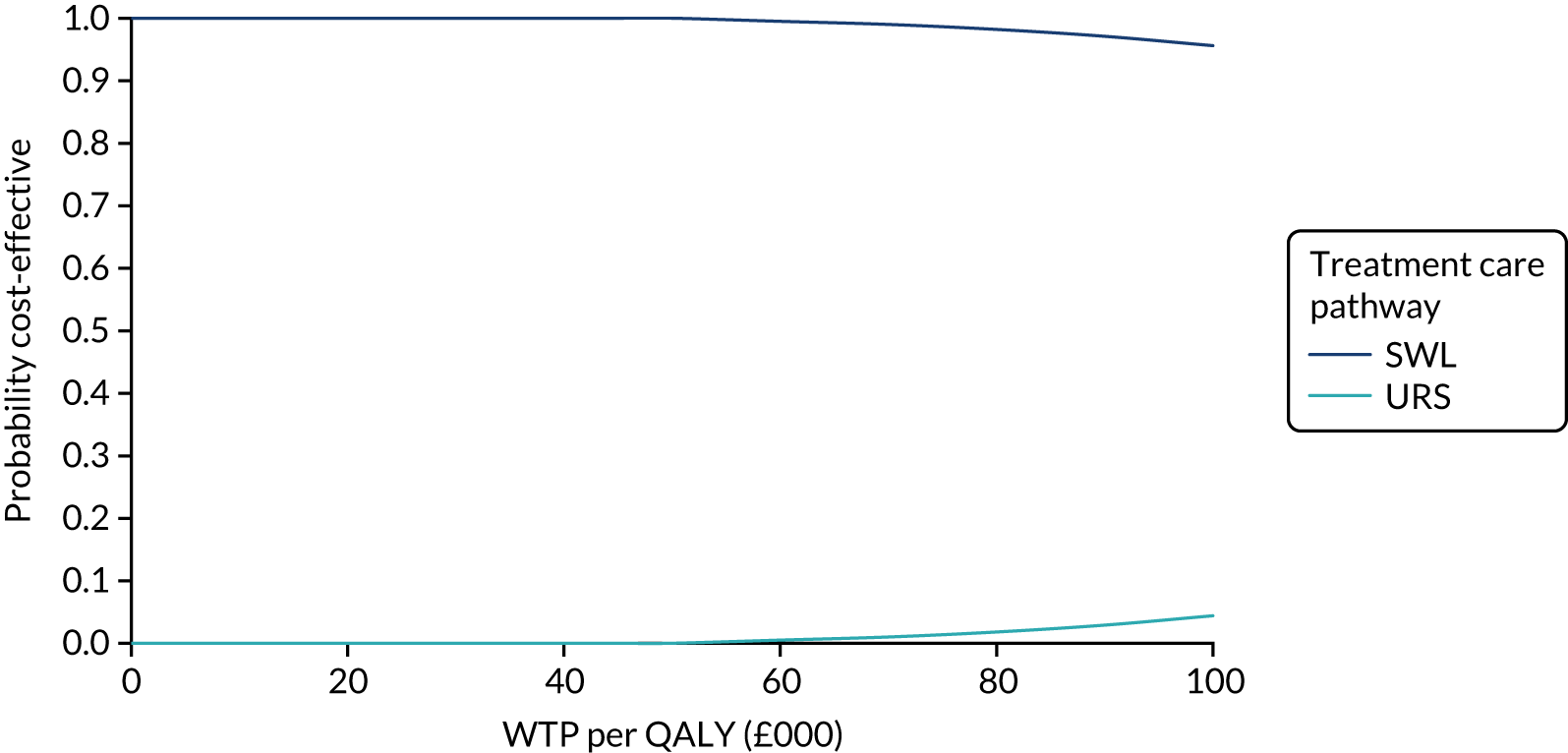
FIGURE 19.
Scatterplot of incremental costs and incremental QALYs based on imputation data: SWL vs. URS.
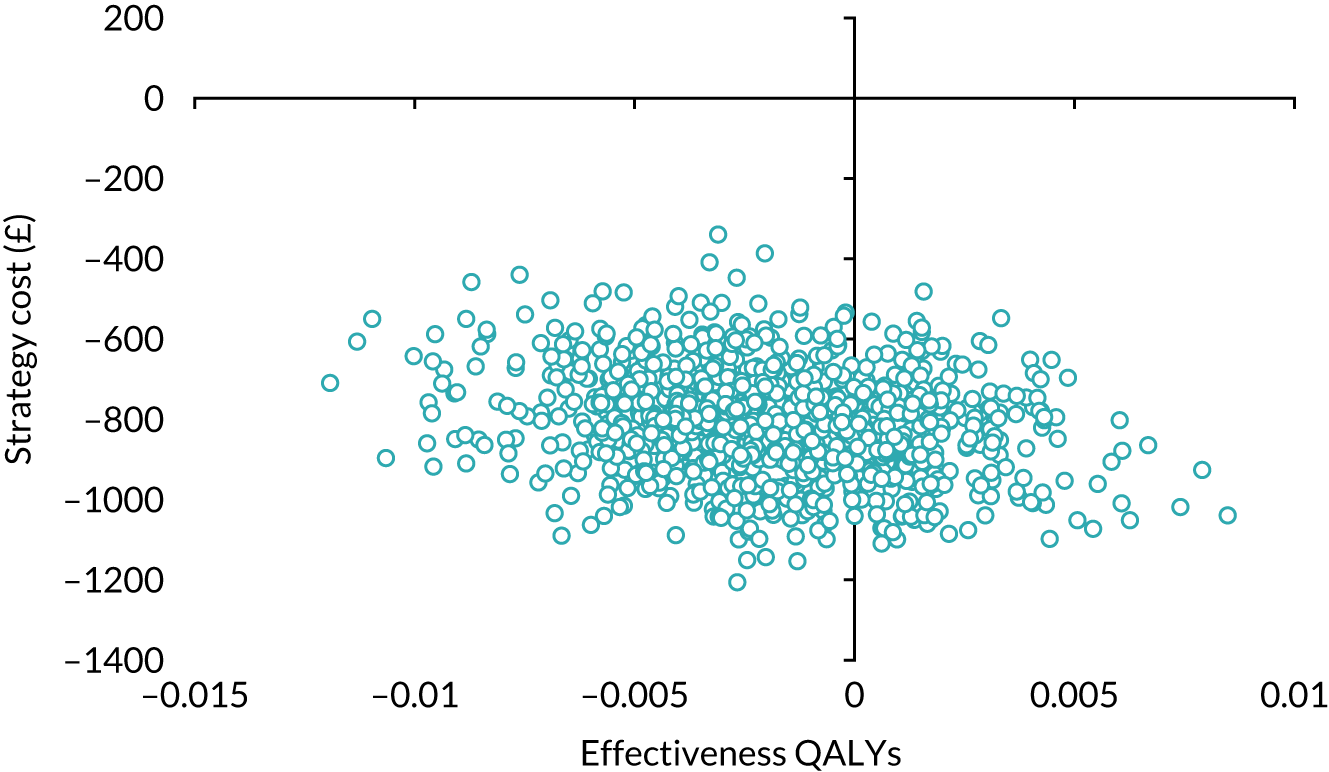
The results for the complete-case analysis using complete-case SF-6D data are reported in Table 15 and Figures 20 and 21. These results should be interpreted cautiously, as the number of patients with complete data (costs and SF-6D QALYs) was very low [138/609 (23%)]. The cost difference was lower than in the base case (£500 vs. £809) and the QALY difference was also lower than in the base-case analysis (0.010 vs. 0.021), and this increased the ICER to £52,313. The probability that SWL would be considered cost-effective was 64% at a threshold of society’s WTP of £30,000. The scatterplot (see Figure 21) illustrates the uncertainty, as the estimates are distributed over all quadrants of the cost-effectiveness plane.
FIGURE 20.
Cost-effectiveness acceptability curve for SF-6D QALYs complete-case data: SWL vs. URS.
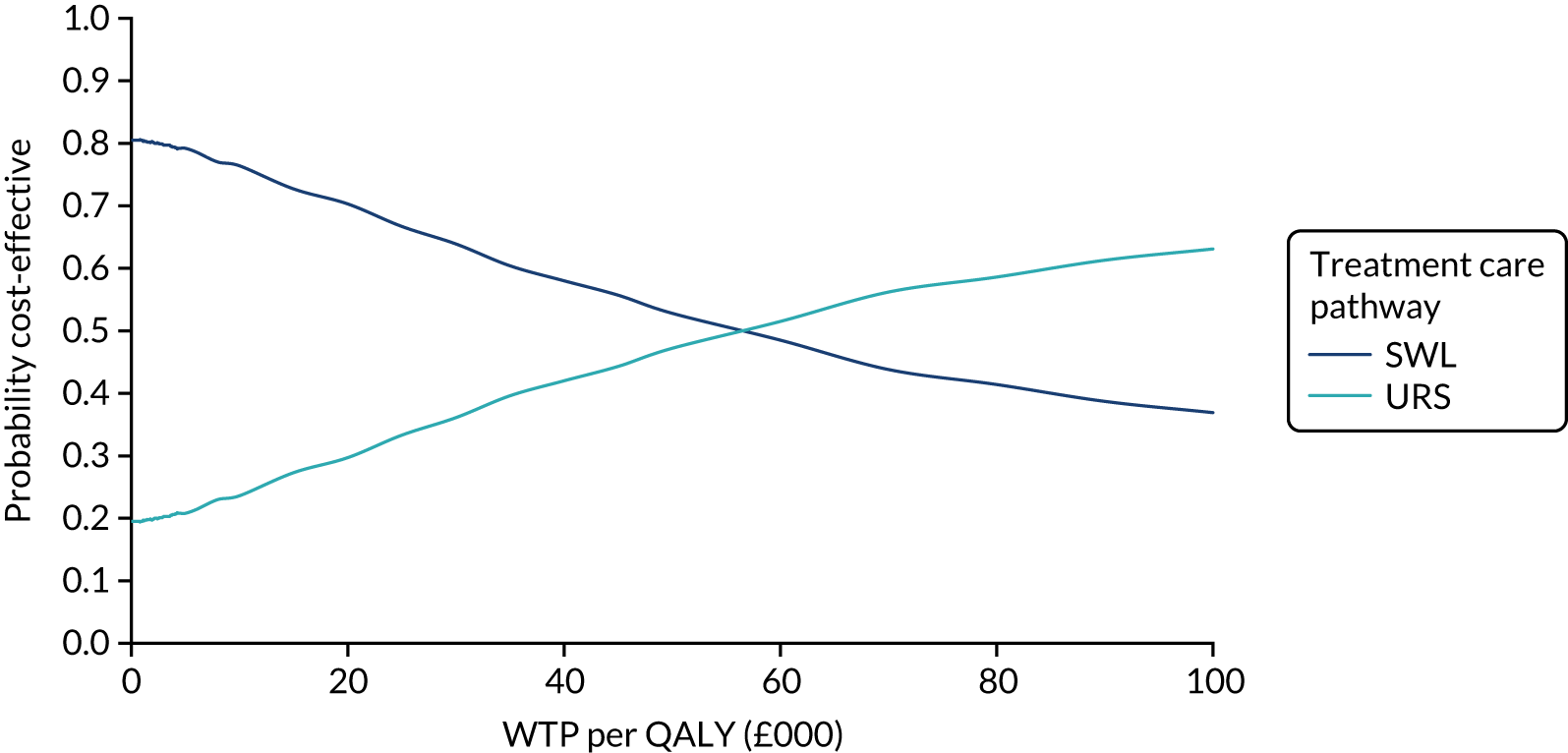
FIGURE 21.
Scatterplot of incremental costs and QALYs based on SF-6D QALYs complete-case data: SWL vs. URS.
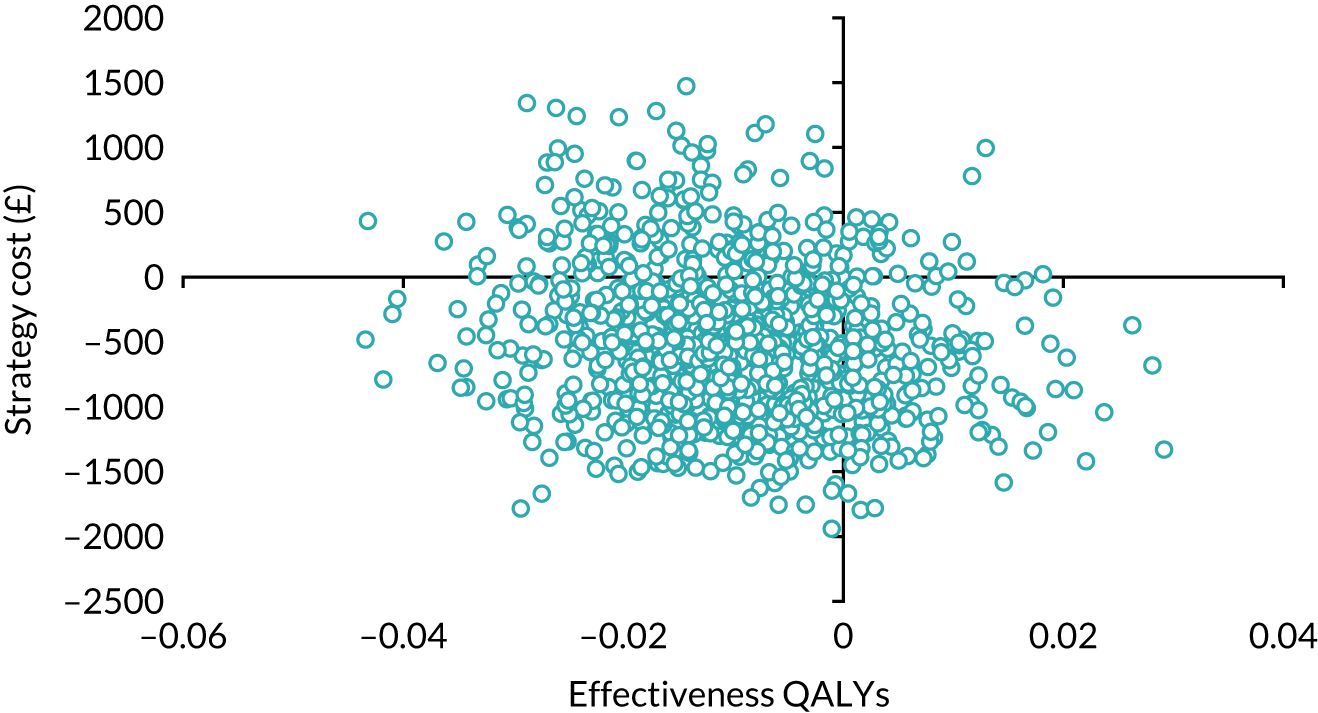
Best-case scenario for missing EQ-5D-3L 6-month data (assuming that all the participants have perfect health)
These results varied from the base-case analysis, as the QALY difference between the two arms reduced from 0.021 to 0.014 (base-case analysis) (see Table 15). The cost difference remained the same as in the base-case analysis (£795). The smaller QALY difference led to an increase in the ICER to £57,899. The probability that SWL would be considered cost-effective at the £30,000 WTP threshold was 95% (Figures 22 and 23).
FIGURE 22.
Cost-effectiveness acceptability curve assuming all missing EQ-5D-3L 6-month scores are full health: SWL vs. URS.
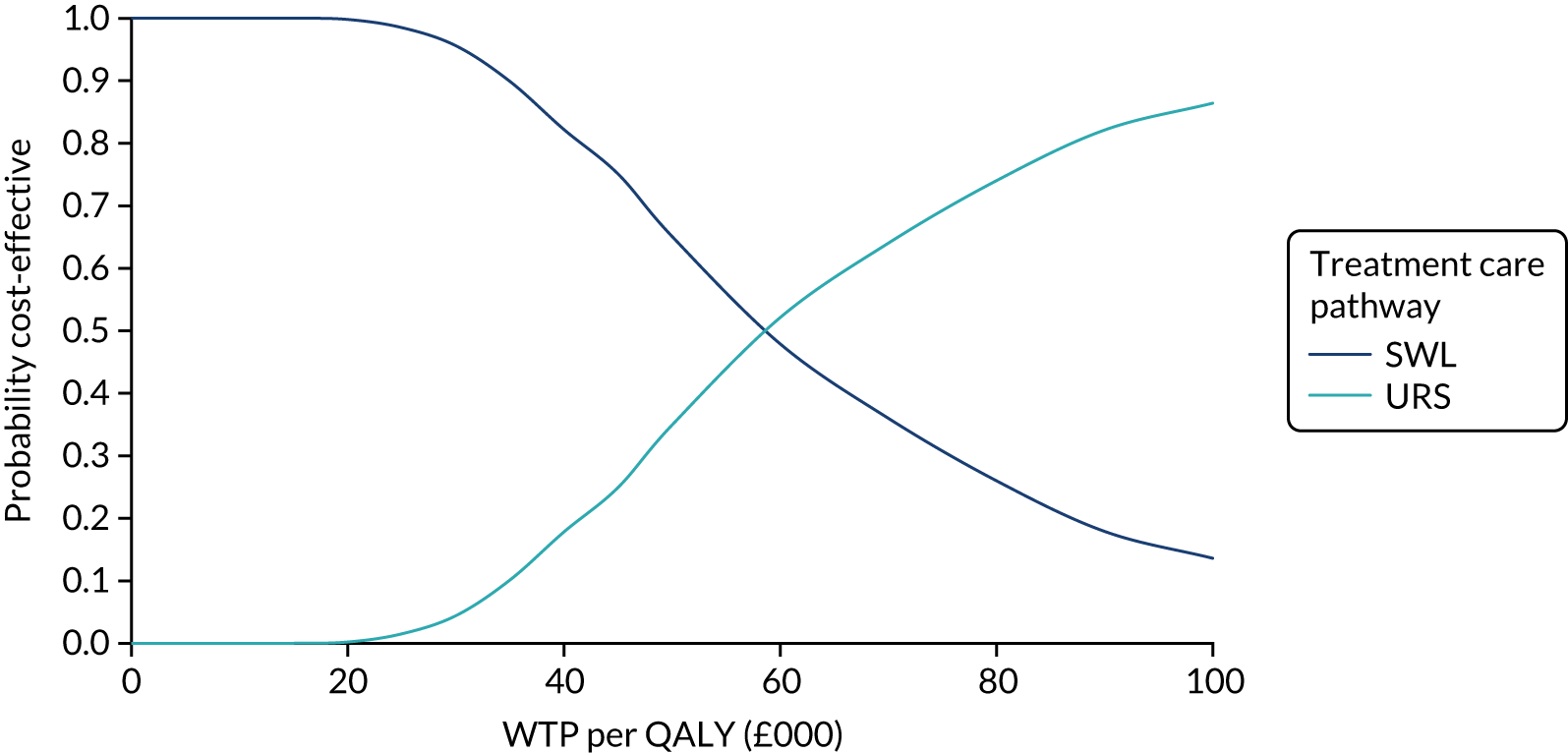
FIGURE 23.
Scatterplot of incremental costs and QALYs assuming all missing EQ-5D-3L 6-month scores are full health: SWL vs. URS.
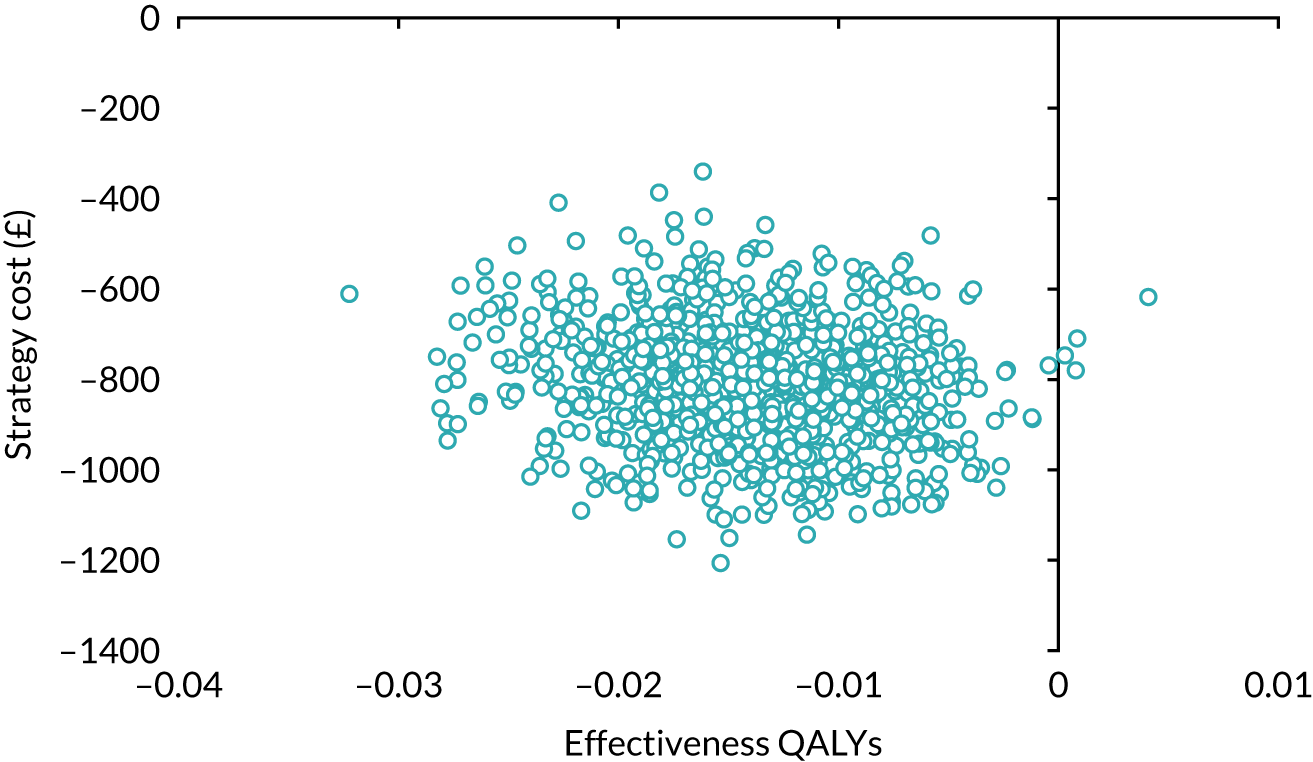
Four scenarios of higher cost of shockwave lithotripsy imputed data
The cost-effectiveness of SWL when different proportions of patients undergo the procedure as an elective inpatient is reported in Table 15 (see also Figures 24, 25 and 28–33). Figures 24 and 25 present the results of the worst-case scenario, that is when 100% of patients receive SWL as an elective inpatient procedure (further results are in Appendix 4). The cost difference was smaller than that in the base-case analysis (£346 vs. £809), whereas the QALY difference was the same, 0.021. The ICER was £16,710. The smaller cost difference leads to a reduction in the probability that SWL would be considered cost-effective to 13%, if society’s WTP for a QALY is £30,000. The scenario ICERs range from £17,000 to £32,000.
FIGURE 24.
Cost-effectiveness acceptability curve for higher cost of SWL using EQ-5D-3L and imputed data: SWL vs. URS.
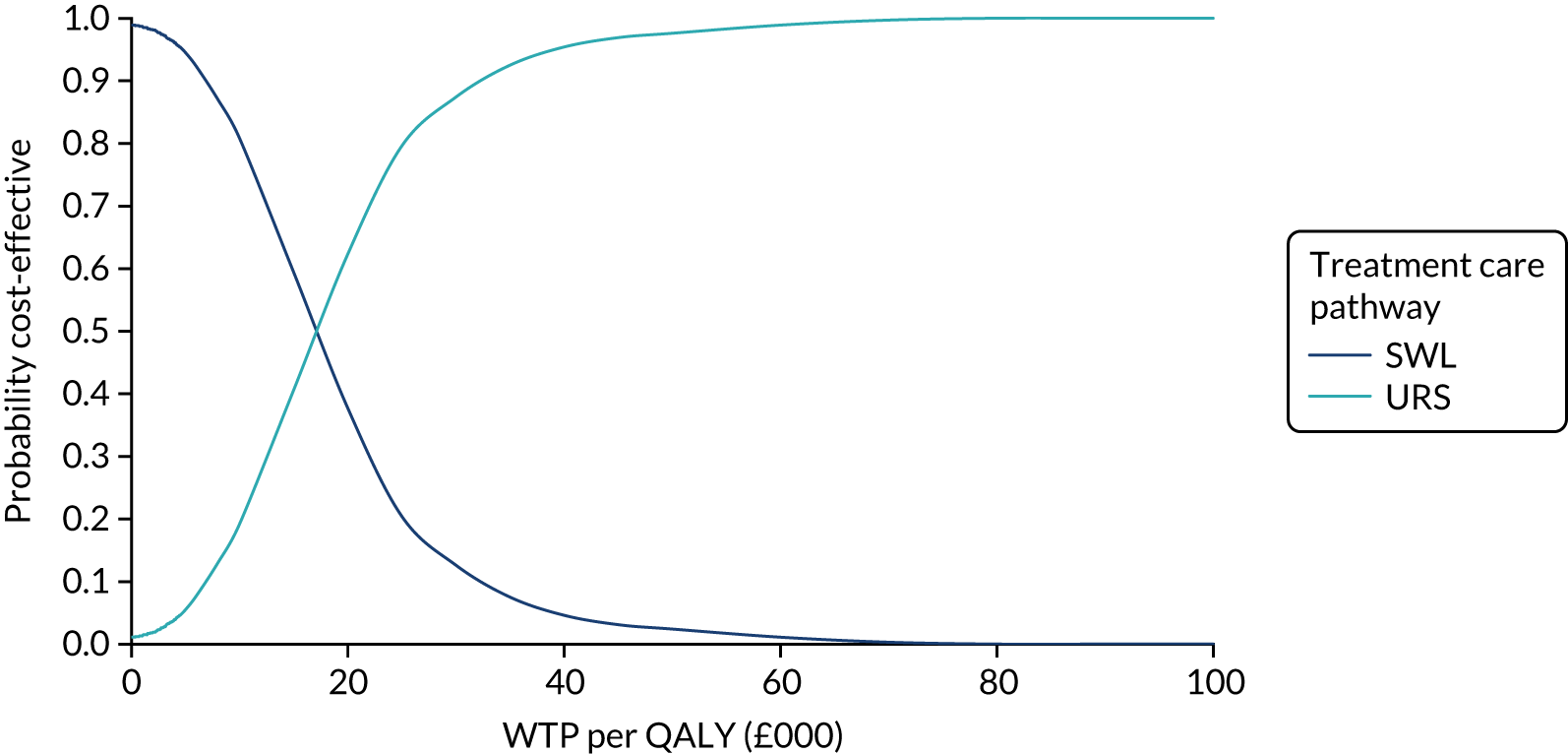
FIGURE 25.
Scatterplot of incremental costs and QALYs for higher cost of SWL: SWL vs. URS.
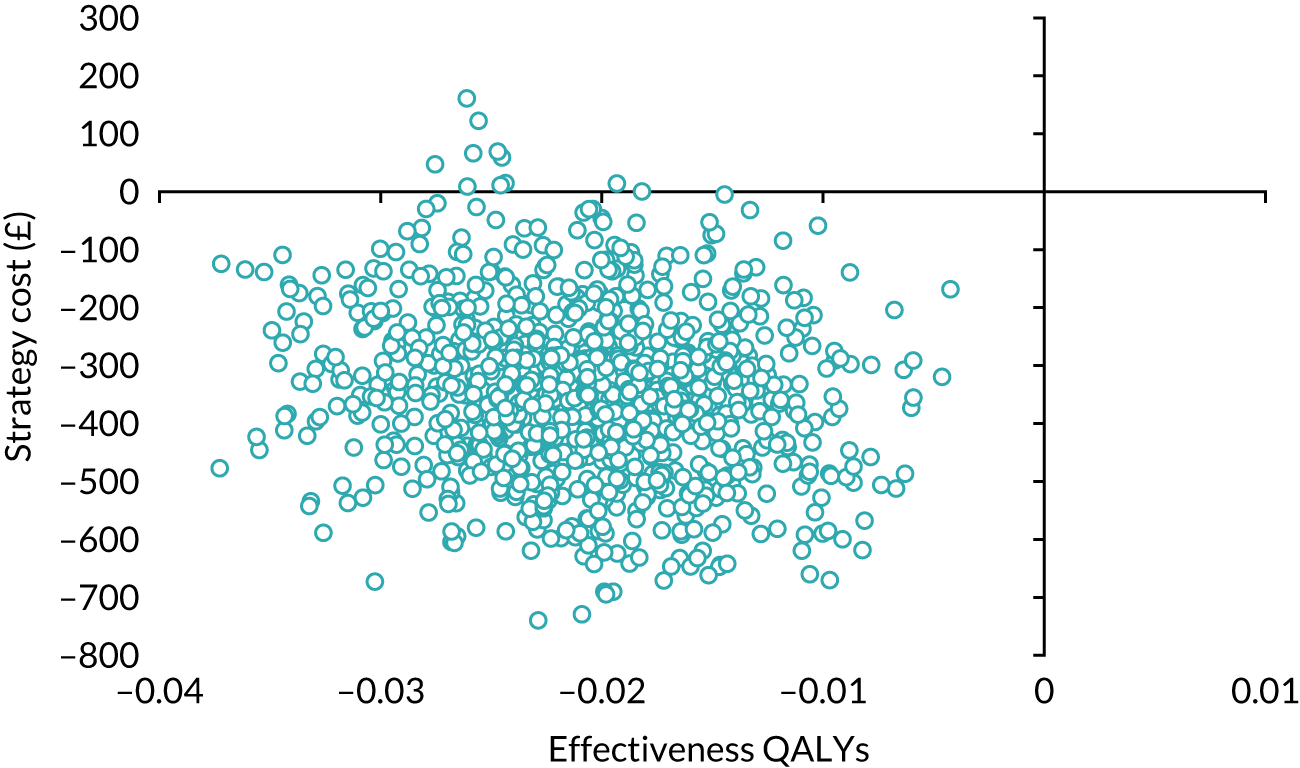
Higher cost shockwave lithotripsy complete-case analysis
The results of the complete-case analysis of higher costs for SWL (see Table 15 and Figures 26 and 27) were in the same direction as in the base-case analysis: SWL cost less and resulted in a lower QALY gain; however, the magnitude of the cost difference was higher [£818 vs. £809 (base case)]. This resulted in an ICER of £29,434 and a probability of 51% that SWL would be considered cost-effective at society’s threshold of WTP for a QALY of £30,000.
FIGURE 26.
Cost-effectiveness acceptability curve for higher cost of SWL using EQ-5D-3L QALYs and complete-case data: SWL vs. URS.
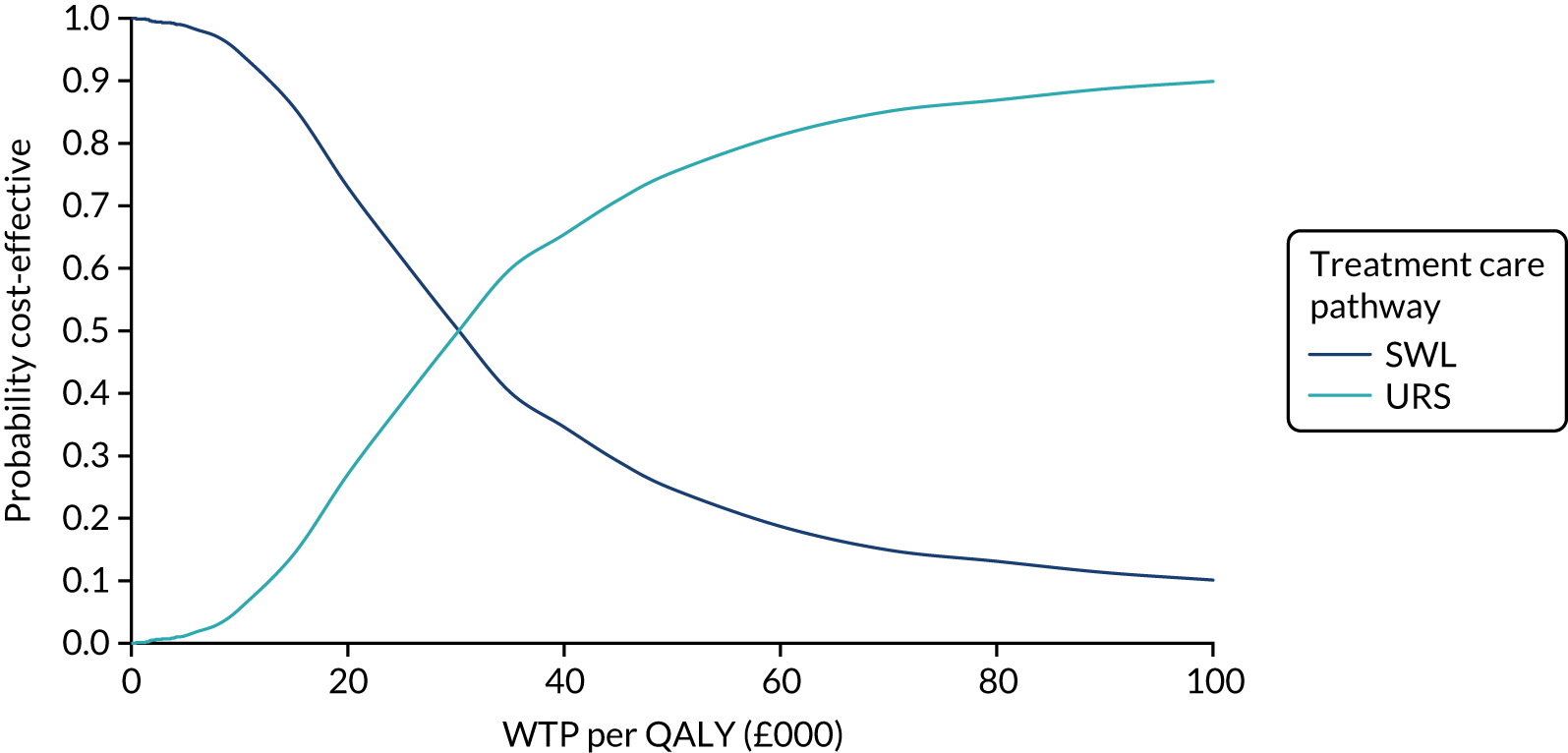
FIGURE 27.
Scatterplot of incremental costs and QALYs for higher cost of SWL: SWL vs. URS.
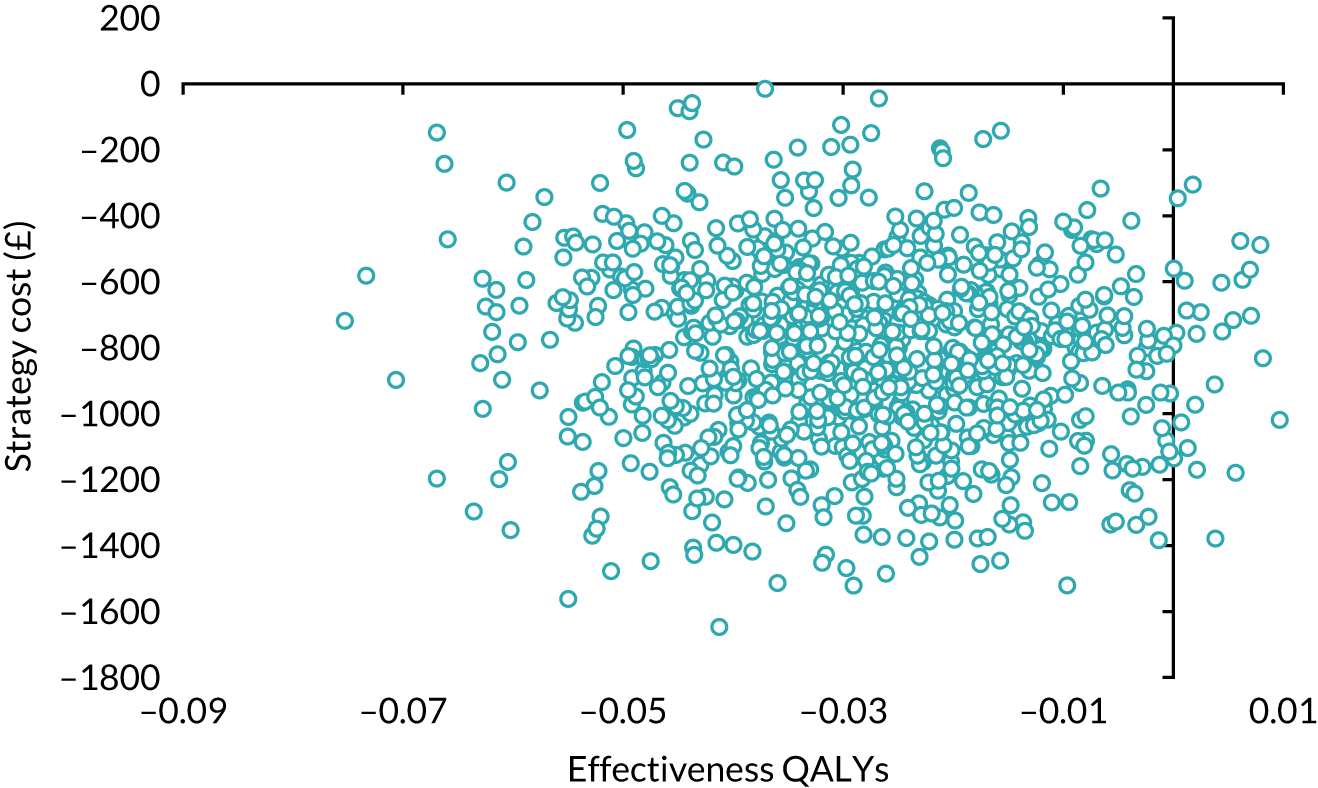
Economics discussion
To our knowledge, this is the first economic evaluation that performs a direct comparison of clinical pathways that start with SWL and URS in a RCT setting. The results suggest that SWL, on average, costs less, but results in a lower QALY gain, than URS. The difference in cost is mainly driven by the unit intervention cost of URS, which is, on average, four times higher than the unit cost of SWL. Additionally, more participants who received URS had a stent inserted, leading to more stent removal procedures in the URS arm. The unit cost of stent insertion and removal is twice that of SWL. The initial cost of endoscopic stent insertion during the URS procedure was not costed separately, as it was included in the overall cost of URS. Although, on average, participants who followed the pathway starting with SWL had greater subsequent resource use, the cost was not high enough to offset the initial high cost of URS and the stent removal costs that URS care pathway participants incur.
The direction of difference in utility scores derived from both the EQ-5D-3L and the SF-6D was the same; however, the magnitude of the difference was higher for the EQ-5D-3L scores than for the SF-6D scores. On average, SWL resulted in fewer additional QALYs than URS; however, although the QALY difference based on the EQ-5D-3L scores was statistically significant, the QALY difference based on SF-6D scores was not. When interpreting results, it should be noted that there were more missing SF-12 data than missing EQ-5D-3L data. Overall, participants’ utility scores increased over time.
One of the limitations of the study was the low return and low completion rates of the participant questionnaires. This was addressed by imputing the missing data. The overall conclusions were sensitive to the data that were used and the assumptions that were made. On average, SWL cost less than URS and resulted in a smaller QALY gain than URS. The base-case analysis results indicated that SWL had a 79% chance of being considered cost-effective at a WTP threshold of £30,000, whereas the complete-case analysis indicated that SWL had an 80% chance of being considered cost-effective at the same threshold. Similar results were noted in the SF-6D sensitivity analysis, which explored the effect of the higher cost of SWL. However, the results of the assumption surrounding the cost of SWL suggested that the higher the cost of SWL (the more people who received SWL as an elective inpatient procedure), the lower the probability that SWL would be considered cost-effective at the different WTP thresholds. The ICERs of the different analyses ranged from £16,710 to £432,432.
The cost results of the TISU trial are similar to those which have been published in a similar setting. 40,41 In these studies40,41 SWL was the primary treatment for stones between 10 mm and 20 mm in size. SWL was undertaken in an outpatient setting and patients did not receive sedation or anaesthesia, but had analgesia according to their tolerance. Flexible ureteroscopy was performed under general anaesthesia. The authors of both studies concluded that SWL, on average, is cheaper than URS. A recent systematic review18 reported that URS was more cost-effective than SWL for stone treatment. However, the measurement of cost varied across studies and the cost of procedures also varied between health-care systems. The systematic review was based on retrospective case series and the authors reported that the evidence base was poor and suggested that there is a need for large RCTs. The Lotan et al. 42 study performed cost-effectiveness analyses using a decision tree model and reported that URS was the most cost-effective treatment strategy for ureteral stones at all locations after observation failed. The Lotan et al. 42 study cited the high cost of purchasing and maintaining a lithotriptor as the driver of the higher SWL cost in their study. Pearle et al. 43 also reported that SWL was slightly more costly than URS. Similarly, Cone et al. 44 reported that URS had superior clinical effectiveness and cost-effectiveness to SWL.
One of the strengths of this study is that it is the first study that we know of to measure and report quality of life in patients following these two care pathways using generic tools. 18 Over time, quality of life in increased in both groups, and at both the 8-week and the 6-month follow-ups the QALY gains in the URS arm, based on EQ-5D-3L scores and calculated from the AUC, were statistically significant. On average, QALY gain based on EQ-5D-3L scores was significantly lower in the SWL arm than in the URS arm, and this difference translated into approximately 10 more healthy days over the 6-month period for patients in the URS care pathway. In the case of utility scores based on the SF-12 questionnaire, the results were in the same direction in both arms. Scores in the URS arm were higher, on average than in the SWL group, and were statistically significantly higher at 6 months. However, the difference in SF-6D QALY score between the two groups over the 6-month period was not statistically significant.
Chapter 5 Discussion
When patients present with pain due to a ureteric stone, a decision about the most appropriate treatment pathway has to be made by the patient and by the clinicians involved in their care. This normally occurs after a discussion of the pros and cons of the various treatment pathways. If the decision to pursue active intervention is made, two main pathways are available: treatment that starts with SWL and treatment that starts with URS. In clinical practice it is not always possible to deliver the treatment choice that was originally planned, or the need to change to a different treatment may arise at some point during the treatment pathway. The treatment pathways in this pragmatic study reflected this and the primary outcome was measured taking this into account. Current guidelines45 support the use of either treatment modality, as there is uncertainty about the balance between clinical effectiveness and economic effectiveness when comparing the two treatments. This uncertainty reflects the differences in delivery of the two treatments, the clinical outcomes achieved and the costs associated with each. It also reflects the fact that, for patients, there are major differences between the treatments in terms of invasiveness, need for general anaesthesia, time in hospital and time to full recovery.
This pragmatic RCT compared outcomes for initial treatment with SWL (allowing two sessions of SWL) with primary URS (see Figure 4).
Primary outcomes
Research to date about the relative merits of URS and SWL has focused on the balance between achieving a stone-free state for the patient and the need for further procedures or reintervention, with consideration also given to complication rates. In the TISU trial we found that the reintervention rate in the SWL arm was 11.4% (95% CI 5.0% to 17.8%) higher than in the URS arm. This is within the 20% limit set at the outset of the study to demonstrate non-inferiority, which would make it acceptable to patients and urologists as the initial treatment pathway. Our results corroborate previous findings,3 demonstrating better stone clearance with URS as the initial treatment pathway, but at a higher cost.
The higher reintervention rate seen in the SWL arm is almost certainly related to the fact that SWL can be limited by certain factors (e.g. skin-to-stone distance, patient tolerability of the shockwave treatment and stone density) that do not affect outcomes in a procedure under general anaesthesia using (typically) laser energy to fragment the stone, as is the case with URS. It has also been shown that effective delivery of SWL requires as much expertise and attention to detail as URS, and this is known to vary from centre to centre. 46
Secondary outcomes
As well as the need for further intervention, a key factor for patient choice and clinical advice is the complication rate associated with each of the two treatment pathways. For the purposes of this study we did not record expected, low-grade complications, such as bleeding or pain on passing urine (see Chapter 2, Safety reporting), but we did collect data on serious complications. The serious complication rate was similarly low in both treatment pathways, with SWL having a marginally higher serious complication rate of 3.6% (compared with 2.7% for URS).
There was only one death, which occurred in the URS arm, and this was unrelated to the treatment; this low number (< 1%) was as expected, as these procedures are generally associated with extremely low mortality. There were two life-threatening complications, both in the URS arm: one was cardiac (in a patient with a previous myocardial infarction) and the other pulmonary (pulmonary embolus in a patient who had also undergone recent orthopaedic surgery). Neither was assessed as being attributable to the trial intervention (or anaesthesia).
Quality of life in patients in this study was measured by the two generic HRQoL measures, as there is no condition-specific tool for stone disease. In both treatment pathways, by 8 weeks there was an overall improvement from baseline, but this was greater in the URS arm (and was maintained up to 6 months). The real extent of this difference is important to patients and the development of condition-specific HRQoL tools for use in urinary stone disease trials in the future would allow a more accurate measurement of quality of life following treatment of ureteric stones.
Pain and the need for analgesia over the treatment period was similar in both pathways, with no indication that one treatment pathway resulted in less pain or need for analgesia than the other.
However, the methods used to assess pain are more suited to chronic pain measurement and we are less certain of the impact of acute pain related to treatments delivered during their pathway. The acceptability of the treatments received by patients was assessed at 8 weeks and showed that both interventions were reassuringly acceptable to a high proportion of patients (SWL 86% and URS 83%).
Economics
On average, the URS care pathway cost more than the SWL care pathway and this cost difference was mainly driven by the unit cost of URS. The estimated unit cost of SWL was less than that of URS. Koo et al. 41 reported the cost in a UK setting of the SWL and flexible ureteroscopic laser lithotripsy procedure. The cost was calculated by the specialty costing department of the hospital and was based on the purchase, maintenance, repair and service costs of the lithotripter machine and flexible ureteroscope, as well as the cost of medical and nursing staff, the overhead cost for administration and the pharmaceutical and utility costs. This was defined as the ‘perceived cost’ and was formulated as the cost per session or procedure of SWL or flexible ureteroscopic laser lithotripsy. 1 The ‘actual cost’ was the perceived cost combined with the cost of additional procedures. The additional procedural cost was formulated according to the cost of instruments used that were specific to each patient (e.g. guidewires, stents, stone retrieval basket and stent removal) and the additional overhead cost was formulated as the cost per day’s stay in the hospital specific to each patient. The total mean costs of the interventions were similar to those used in our study. The measurement of the economic cost of a procedure and of ancillary procedures is clearly difficult to standardise within one health-care system, let alone across different health-care systems. These shortcomings are illustrated in a recent meta-analysis,18 in which the authors attempt to tabulate measurements of URS compared with SWL, in studies from several countries.
Strengths and weaknesses of the trial
Strengths
-
This was a pragmatic trial embedded within current urological practice across the UK; therefore, it delivered outcomes that are relevant to the NHS.
-
Baseline characteristics indicate no selection bias.
-
Baseline characteristics show that the trial population was similar to that in previously published studies.
Weaknesses
-
Treatment received by participants could not be blinded.
-
Response rates to HRQoL questionnaires became lower as the patients recovered from their stone episode.
Access to treatment
When assessing and designing a clinical pathway for a patient with ureteric colic, it is important to note that accessibility to the treatment is likely to be crucially important. For instance, although the planning for a session of SWL is relatively straightforward, particularly in centres with an in-house lithotripter, booking an urgent theatre slot (for URS) requires several hurdles to be overcome. For example, most emergency theatres will have resources for the insertion of a ureteric stent; however, planned laser stone fragmentation is a semi-elective procedure and requires the co-ordination of technology (URS, laser machine) and the appropriate theatre team (e.g. anaesthetist, radiographer and scrub team), not to mention the logistics of adding a case to an already full elective theatre list. Adopting the clinical pathway in which SWL is the preferred initial treatment option would potentially mean that 78% of all patients would not need further intervention with URS and, therefore, remove the need to find theatre time and space. From the NHS perspective, it is important to note that many hospitals dealing with patients presenting with ureteric stones currently do not have an on-site lithotripter and so cannot offer urgent treatment with SWL (a mobile service may be available on a monthly basis only). 47
A further, and more subtle, issue relates to the availability of flexible ureterorenoscopes, as opposed to semi-rigid ureteroscopes. The latter can treat most stones in the distal ureter and some mid-ureteric stones (55% of those in this study), but proximal ureteric stones in a male patient can sometimes be reached only with a flexible ureterorenoscope. Furthermore, a more distal stone is sometimes retropulsed proximally, again requiring clearance using flexible instrumentation. This is significant if the premise is that URS has a 90% chance of resulting in the patient being stone free, regardless of stone location within the ureter. Although all centres in this study had access to this flexible technology (as well as in-house lithotripsy), this is not true of all NHS hospitals and this means that decision-making will continue to depend on local equipment availability.
Waiting time
There was a substantial difference in waiting time to first treatment: 7 days from randomisation to treatment for the SWL arm, compared with 25 days from randomisation to treatment for the URS arm. This is likely to be representative of the challenges of booking semi-urgent cases into busy NHS theatre timetables. Although some NHS centres have trialled the use of dedicated theatre slots for urgent urology cases (as opposed to booking onto an emergency theatre list), this practice is not universally feasible. Although this route has the added attraction of ensuring that the necessary technology (e.g. laser and X-ray) is available, individual centres still face the pressure of having to allocate resources. There is recent evidence to support better outcomes (reduced need for further procedures and reduced hospitalisation) if SWL is delivered within 24 hours,48 just as the recent NICE guidelines have also suggested to aim for treatment within 48 hours. 45 The recommendations in the NICE guidelines were based on evidence of benefit to patients in terms of stone removal, repeated or ancillary procedures, and need for stent insertion.
There are other consequences for patients of waiting > 3 weeks after presentation, as this will inevitably mean that a proportion of patients will reattend the emergency department because of pain. Furthermore, among those in whom a stent has been inserted, a waiting time of several weeks for definitive surgery is known to be associated with an increasing risk of stent-related sepsis.
An exact time to achieve stone-free status from time of presentation is difficult to measure, even if all patients were to undergo post-treatment computerised tomography, and patient-reported passage of stones is unreliable. This pragmatic study based stone-free status on the need for further intervention to achieve stone clearance using standard NHS pathways and imaging techniques (mainly plain X-ray and ultrasound). The time to become stone free can, in the case of the URS arm, be the time to URS (with a proportion needing further time to be fragment free and stent free), whereas in the SWL arm it generally takes a few more days for stone fragments to be passed and, therefore, time to become stone free is much less clear in this arm. An agreed, standardised core outcome set for stone trials would be very helpful, as it could address issues such as time to stone-free measurements and ensure that all future studies use comparable data.
Other limitations
We had initially powered the study for 500 patients in each arm, but reached 303 and 306 participants in the SWL and URS arms, respectively. The CONSORT diagram shows that a large number of patients were screened for the study, but were not randomised. This may be because waiting times for treatment to start were different in the two arms of the study or patients had already received treatments before (i.e. were experiencing stone recurrence) or wanted to avoid a general anaesthetic.
The non-inferiority margin was placed at a figure of 20% based on the views of urologists and patients from the BAUS Section of Endourology; this is what they judged would be an acceptable limit, and is not a validated limit. However, the overall difference in stone-free rates of 11% was in accordance with our knowledge of contemporary URS and is still an acceptable outcome for the vast majority of patients with ureteric colic in this study.
Chapter 6 Conclusion
The TISU trial shows that primary URS for ureteric stones that are clinically deemed to need intervention is more effective than SWL and is associated with less need for further interventions. However, the overall costs of URS are higher than those for SWL, despite the fact that patients who are initially treated with SWL are more likely to need a subsequent intervention. The difference in the primary clinical outcome was at a level that was low enough to suggest that all patients should be initially treated with SWL, with the failures progressing to URS. From an NHS perspective, the cost savings associated with this approach are potentially substantial.
The data presented have implications for advising patients of expected clinical outcomes (i.e. confirming that URS is more effective, as already suggested from earlier studies) from the different treatment pathways available to them. This will be part of the discussion between clinicians and patients about which treatment pathway to adopt in their case. The choice made by patients and clinicians has significant implications for service provision, as the economic results indicate that SWL costs less, but also results in lower QALYs, and the decision-maker needs to determine whether or not the cost saving made in the SWL care pathway justifies the loss of QALYs. A 79% probability that SWL will be considered cost-effective means that SWL has a higher chance that it will be considered cost effective at a £30,000 willingness to pay for a QALY threshold.
Acknowledgements
The authors wish to thank the patients who participated in the TISU trial. We also thank Stanley Coutts (patient representative) and Charles Clark (patient representative and co-applicant) for their contribution to the design of the participant-facing documents (patient information sheet and questionnaires); Sharon Wren for her secretarial support and data management; previous data co-ordinators, Jessica Wood and Margery Heath, for their data and trial management support; the CHaRT programming team led by Gladys McPherson (to 2016) and Mark Forrest (2016–present); other staff within CHaRT and the HSRU for their assistance with the trial (Cynthia Fraser); members of the PMG for their ongoing advice and support of the trial, plus the independent members of the TSC and DMC; and the staff at the recruitment sites who facilitated the recruitment, treatment and follow-up of trial participants (all listed below); and, finally, we would like to thank the National Institute for Health Research and the Health Technology Assessment programme for funding the TISU trial.
Project Management Group
Samuel McClinton (chief investigator), James N’Dow, Graeme MacLennan, Mary Kilonzo, Frank Keeley, Ken Anson, Charles Clark, John Norrie, Rob Pickard, Sara MacLennan, Ruth Thomas, Kath Starr, Neil Burgess and Thomas Lam.
Independent members of the Trial Steering Committee
Roger Kockelbergh (TSC chairperson), John McGrath, Sarah Meredith and Stanley Coutts.
Independent members of the Data Monitoring Committee
Elaine McColl (DMC chairperson), Simon Harrison and Richard Emsley.
Principal investigators
David Thomas, Ken Anson, Joe Philip, Graham Young, Raj Gowda, Tony Browning, Ranjan Thilagarajah, Chris Betts, Ben Turney, Nitin Shrotri, Andrew Myatt, Oliver Wiseman, Benjamin Jenkins, Michael Kimuli, Simon Phipps, Ranan Dasgupta, Jeff Webster, Jake Patterson, Kay Thomas, Seshadri Sriprasad, Iqbal Shergill, James Forster, Giacomo Caddeo, John McCabe and Sachin Agrawal.
Research nurses/fellows/clinical trial assistants
Wendy Robson, Peter Murphy, Bernadette Kilbane, Nicola Brown, Leigh Morrison, Rebecca Ilyas, Susan Walker, Beverley Taylor, Jim Anderson, Fiona McNeela, Lauren Fergey, Tracy Camburn, Vicky Thomas, Emily Grout, Fiona Hammonds, Joshua O’Donnell, Tracey Cosier, Julie Rawlings, Kelly Leonard, Eleanor Dungca, Louise Fairlie, Lorraine Wiseman, Louise Williamson, Katherine Lawrence, Finny Patterson, Geraldine Cummings, Kareen Darnley, Gillian Hornzee, Sanela Andijac, Daisy Floyd, Annamaria Harmathova, Byiravey Pathmanathan, Susannah Hulton, Helen Bowyer, Joanna Peel, Rachel Muir, Golda-Grace Azanu, Rebecca Gare, Matthew Hogben, Kate Ripalda, Sherma Turner, Jnine Travis, Olivia McHugh, Stacy Ackerley, Claire Watkins, Linzi Williams, Hyley Inman, Dawn McNulty, Deborah Morgan, Charlotte Downes, Sharon Dealing, Susan Dowling, Catherine Gray, Maria Croft and Victoria Frost.
Contributions of authors
Ranan Dasgupta (https://orcid.org/0000-0001-8044-8902) (Consultant Urologist) contributed to the interpretation of the data and writing of the final report.
Sarah Cameron (https://orcid.org/0000-0002-4308-1416) (Trial Manager, Trialist) was responsible for the day-to-day management of the trial and contributed to the interpretation of the data and the writing of the report.
Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Senior Statistician) conducted the statistical analyses and contributed to the interpretation of the data and the writing of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor, CHaRT Director, Statistician and Trialist) contributed to the conception and design of the trial, the conduct of the trial and the interpretation of results and made significant contributions to the writing and editing of the report.
Mary M Kilonzo (https://orcid.org/0000-0002-3450-4536) (Health Economist) contributed to the conception and design of the study, the analysis of the health economics data and the drafting of the health economics chapters.
Thomas BL Lam (https://orcid.org/0000-0003-1582-3387) (Honorary Senior Lecturer in Urology and Consultant Urologist) contributed his clinical experience to the design of the study and writing of the final report.
Ruth Thomas (https://orcid.org/0000-0002-8316-2616) (Trialist) contributed to the conception and design of the study and the conduct of the trial and made significant contributions to the interpretation of the data and to the writing of the report.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor of Medical Statistics and Trial Methodology, Director of Edinburgh Clinical Trials Unit) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) (Senior Trial Manager, Trialist) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Ken Anson (https://orcid.org/0000-0001-8111-2802) (Consultant Urological Surgeon and Reader in Urology) contributed to the design of the study, the delivery of the trial and the writing of the final report.
James N’Dow (https://orcid.org/0000-0001-5340-0081) (Professor of Urology) contributed to the design of the study and the writing of the final report.
Neil Burgess (https://orcid.org/0000-0003-1986-0839) (Consultant Urologist) contributed to the design of the study and the writing of the final report.
Charles T Clark (https://orcid.org/0000-0003-1813-9767) (Member of Stone Patient Advisory Group) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Francis X Keeley (https://orcid.org/0000-0001-7826-5664) (Consultant Urological Surgeon) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Sara J MacLennan (https://orcid.org/0000-0003-1405-6964) (Health Psychologist) contributed to the conception and design of the study and made significant contributions to interpretation of the data and the writing of the report.
Kath Starr (https://orcid.org/0000-0003-3356-7751) (Trial Manager, Trialist) contributed to the design of the study, the delivery of the trial and the writing of the final report.
Samuel McClinton (https://orcid.org/0000-0002-0539-9570) (Chief Investigator and Professor of Urology) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of the results and the writing of the report.
Contributions of others
Rob Pickard (Professor of Urology) contributed his clinical expertise to the design of the study, recruitment, interpretation of the trial findings and the writing of final report.
Publications
Skea ZC, Treweek S, Gillies K. ‘It’s trying to manage the work’: a qualitative evaluation of recruitment processes within a UK multi-centre trial. BMJ Open 2017;7:e016475.
Dasgupta R, Cameron S, Aucott L, MacLennan G, Thomas RE, Kilonzo M, et al. Shockwave lithotripsy versus ureteroscopic treatment as therapeutic interventions for stones of the ureter (TISU): a multicentre randomised controlled non-inferiority trial. Eur Urol 2021;80:46–54.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- McClinton S, Cameron S, Starr K, Thomas R, MacLennan G, McDonald A, et al. TISU: extracorporeal shockwave lithotripsy, as first treatment option, compared with direct progression to ureteroscopic treatment, for ureteric stones: study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2652-1.
- Aboumarzouk OM, Kata SG, Keeley FX, McClinton S, Nabi G. Extracorporeal shock wave lithotripsy (ESWL) versus ureteroscopic management for ureteric calculi. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD006029.pub4.
- Drake T, Grivas N, Dabestani S, Knoll T, Lam T, Maclennan S, et al. What are the benefits and harms of ureteroscopy compared with shock-wave lithotripsy in the treatment of upper ureteral stones? A systematic review. Eur Urol 2017;72:772-86. https://doi.org/10.1016/j.eururo.2017.04.016.
- Heers H, Turney BW. Trends in urological stone disease: a 5-year update of hospital episode statistics. BJU Int 2016;118:785-9. https://doi.org/10.1111/bju.13520.
- Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int 2012;109:1082-7. https://doi.org/10.1111/j.1464-410X.2011.10495.x.
- Seitz C, Fajkovic H. Epidemiological gender-specific aspects in urolithiasis. World J Urol 2013;31:1087-92. https://doi.org/10.1007/s00345-013-1140-1.
- Rukin N, Siddiqui Z, Chedgy E, Somani B. Trends in upper tract stone disease in England: evidence from the hospital episodes statistics (HES) database. BJU Int 2016;117:11-2.
- Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol 2014;66:724-9. https://doi.org/10.1016/j.eururo.2014.06.036.
- Medindia . Michigan Hospitals Lead the Way in Preventing Common and Costly Urinary Tract Infections n.d. www.medindia.net/news/michigan-hospitals-led-the-way-in-preventing-common-and-costly-urinary-tract-infections-116433-1.htm (accessed January 2019).
- Bihl G, Meyers A. Recurrent renal stone disease-advances in pathogenesis and clinical management. Lancet 2001;358:651-6. https://doi.org/10.1016/S0140-6736(01)05782-8.
- Wilkinson H. Clinical investigation and management of patients with renal stones. Ann Clin Biochem 2001;38:180-7. https://doi.org/10.1258/0004563011900623.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003;63:1817-23. https://doi.org/10.1046/j.1523-1755.2003.00917.x.
- New F, Somani BK. A complete world literature review of quality of life (QOL) in patients with kidney stone disease (KSD). Curr Urol Rep 2016;17. https://doi.org/10.1007/s11934-016-0647-6.
- Bultitude M, Rees J. Management of renal colic. BMJ 2012;345. https://doi.org/10.1136/bmj.e5499.
- Pickard R, Starr K, MacLennan G, Lam T, Thomas R, Burr J, et al. Medical expulsive therapy in adults with ureteric colic: a multicentre, randomised, placebo-controlled trial. Lancet 2015;386:341-9. https://doi.org/10.1016/S0140-6736(15)60933-3.
- Türk C, Neisius A, Petrick A, Seitz C, Thomas K, Skolarikos A. EAU Guidelines on Urolithiasis 2018 2018. http://uroweb.org/guideline/urolithiasis/ (accessed September 2018).
- Perez Castro E, Osther PJ, Jinga V, Razvi H, Stravodimos KG, Parikh K, et al. Differences in ureteroscopic stone treatment and outcomes for distal, mid-, proximal, or multiple ureteral locations: the Clinical Research Office of the Endourological Society ureteroscopy global study. Eur Urol 2014;66:102-9. https://doi.org/10.1016/j.eururo.2014.01.011.
- Geraghty RM, Jones P, Herrmann TRW, Aboumarzouk O, Somani BK. Ureteroscopy is more cost effective than shock wave lithotripsy for stone treatment: systematic review and meta-analysis. World J Urol 2018;36:1783-93. https://doi.org/10.1007/s00345-018-2320-9.
- Dasgupta R, Hegarty N, Thomas K. Emergency shock wave lithotripsy for ureteric stones. Curr Opin Urol 2009;19:196-9. https://doi.org/10.1097/mou.0b013e32831e4263.
- Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, et al. 2007 guideline for the management of ureteral calculi. Eur Urol 2007;52:1610-31. https://doi.org/10.1016/j.eururo.2007.09.039.
- Dasgupta R, Cameron S, Aucott L, MacLennan G, Thomas RE, Kilonzo M, et al. Shockwave lithotripsy versus ureteroscopic treatment as therapeutic interventions for stones of the ureter (TISU): a multicentre randomised controlled non-inferiority trial. Eur Urol 2021;80:46-54. https://doi.org/10.1016/j.eururo.2021.02.044.
- Nafie S, Dyer JE, Minhas JS, Mills JA, Khan MA. Efficacy of a mobile lithotripsy service: a one-year review of 222 patients. Scand J Urol 2014;48:324-7. https://doi.org/10.3109/21681805.2014.886288.
- Department of Health and Social Care . NHS Reference Costs 2013–14 n.d. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed May 2014).
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- EuroQol Research Foundation . EQ-5D-3L: About 2017. https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/ (accessed August 2019).
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis 1978;37:378-81. https://doi.org/10.1136/ard.37.4.378.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. https://doi.org/10.1093/aje/kwh090.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed August 2019).
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Joint Formulary Committee . British National Formulary 2018. https://about.medicinescomplete.com/publication/british-national-formulary/ (accessed January 2018).
- Department of Health and Social Care . NHS Reference Costs 2017–18 2018. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed January 2018).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Kind P, Hardman G, Macran S. Population Norms for EQ-5D. Centre for Health Economics Discussion Paper 172. York: University of York; 1999.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2007.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Gabrio A, Mason AJ, Baio G. Handling missing data in within-trial cost-effectiveness analysis: a review with future recommendations. Pharmacoecon Open 2017;1:79-97. https://doi.org/10.1007/s41669-017-0015-6.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Chan LH, Good DW, Laing K, Phipps S, Thomas BG, Keanie JY, et al. Primary SWL is an efficient and cost-effective treatment for lower pole renal stones between 10 and 20 mm in size: a large single center study. J Endourol 2017;31:510-16. https://doi.org/10.1089/end.2016.0825.
- Koo V, Young M, Thompson T, Duggan B. Cost-effectiveness and efficiency of shockwave lithotripsy vs flexible ureteroscopic holmium:yttrium-aluminium-garnet laser lithotripsy in the treatment of lower pole renal calculi. BJU Int 2011;108:1913-16. https://doi.org/10.1111/j.1464-410X.2011.10172.x.
- Lotan Y, Gettman MT, Roehrborn CG, Cadeddu JA, Pearle MS. Management of ureteral calculi: a cost comparison and decision making analysis. J Urol 2002;167:1621-9. https://doi.org/10.1016/S0022-5347(05)65166-X.
- Pearle MS, Nadler R, Bercowsky E, Chen C, Dunn M, Figenshau RS, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for management of distal ureteral calculi. J Urol 2001;166:1255-60. https://doi.org/10.1016/S0022-5347(05)65748-5.
- Cone EB, Eisner BH, Ursiny M, Pareek G. Cost-effectiveness comparison of renal calculi treated with ureteroscopic laser lithotripsy versus shockwave lithotripsy. J Endourol 2014;28:639-43. https://doi.org/10.1089/end.2013.0669.
- National Institute for Health and Care Excellence (NICE) . Renal and Ureteric Stones: Assessment and Management (NICE Guideline 118) n.d. www.nice.org.uk/guidance/ng118/evidence (accessed January 2019).
- Chaussy CG, Tiselius H. How can and should we optimize extracorporeal shockwave lithotripsy?. Urolithiasis 2018;46:3-17. https://doi.org/10.1007/s00240-017-1020-z.
- Sharma NL, Alexander CE, Grout E, Turney BW. Shock-wave lithotripsy: variance within UK practice. Urolithiasis 2017;45:193-201. https://doi.org/10.1007/s00240-016-0886-5.
- Bucci S, Umari P, Rizzo M, Pavan N, Liguori G, Barbone F, et al. Emergency extracorporeal shockwave lithotripsy as opposed to delayed shockwave lithotripsy for the treatment of acute renal colic due to obstructive ureteral stone: a prospective randomized trial. Minerva Urol Nefrol 2018;70:526-33. https://doi.org/10.23736/S0393-2249.18.03084-9.
Appendix 1 Site lithotripter model and protocol
| Site name | Lithotripter make and model | Lithotripter protocol |
|---|---|---|
| Addenbrooke’s Hospital | Wolf Piezolith 3000 |
Shocks: up to 4000 Frequency: 2 Hz |
| Bradford Teaching Hospitals | Storz Modulith SLK inline |
Shocks: 4000 Maximum intensity: 70% Maximum frequency: 2 Hz |
| Broomfield Hospital | EDAP Sonolith | Shocks: 3000, two shocks per second |
| Canterbury Hospital | Storz MLK | |
| Charing Cross Hospital | Storz Modulith SLX-F2 |
Shocks: 2000–3000 Frequency: 1–2 Hz |
| Churchill Hospital | Storz Modulith SLX-F2 | Shocks: 4000 at 2 Hz over 35 minutes or 3000 at 1.5 Hz over 35 minutes |
| Darent Valley Hospital | Storz Modulith SLX | Shocks: 3000 over 30–40 minutes |
| Freeman Hospital | Storz Modulith SLX 2 | Shocks: 4000 over 40–45 minutes |
| Guy’s Hospital | Philips Intellivue MP30 | Shocks: 3000 over 30–40 minutes |
| Hull Royal Infirmary | Storz Modulith SLX F2 | Shocks: 4000 over 40 minutes; or 1000–1500 over 20–25 minutes |
| Northwick Park Hospital | Storz Modulith SLX F2 |
Shocks: 3200 over 30 minutes Frequency: 1.5–2 Hz |
| Royal Derby Hospital | Storz Modulith SLK | Shocks: 2400, one shock per second |
| Royal Hallamshire Hospital | Storz Modulith SLX-F2 |
Shocks: 4000 over 35 minutes Frequency: 2 Hz |
| Salford Royal Hospital | Edap Sonolith-isys | Shocks: 3000 over 45 minutes |
| Southmead Hospital | Storz Modulith SLX-F2 | Shocks: 3000 over 30 minutes |
| St George’s Hospital | Storz Lithotripter |
Shocks: 4000 for 45 minutes Frequency: 2 Hz |
| St James’s University Hospital | Storz Modulith SLK | Shocks: 4000 over 30–40 minutes |
| Sunderland Royal Hospital | EDAP sonolith i-sys | Shocks: 4100 over 35–45 minutes |
| The James Cook University Hospital | Dornier Compact Delta II | Shocks: 2000 over 40 minutes |
| Western General Hospital | Sonolith I-sys | Shocks: 4000–4500 over 30–45 minutes |
| Whiston Hospital | Richard Wolf Piezolith P3000 |
Shocks: 4000 over 40–45 minutes Frequency: 2 Hz |
| Wrexham Maelor Hospital | Richard Wolf Piezolith P3000 | Shocks: 4000 over 45 minutes |
| Wythenshawe Hospital | Storz Modulith SLX | Shocks: 4000 over 40–60 minutes |
Appendix 2 Ineligible and declined information
| TISU trial centre | Declined to take part, n | Declined to give reason, n | Pregnant, n | Bilateral ureteric stone(s), n | Abnormal urinary tract anatomy, n | Unable to understand documentation, n | Other reason, n |
|---|---|---|---|---|---|---|---|
| Freeman Hospital | 206 | 4 | 1 | 9 | 8 | 4 | 1160 |
| St George’s Hospital | 4 | ||||||
| Southmead Hospital | 53 | 1 | 2 | 21 | |||
| Wythenshawe Hospital | 1 | ||||||
| The James Cook University Hospital | 27 | 6 | 7 | 4 | 10 | 37 | |
| Pinderfields Hospital | 4 | ||||||
| Broomfield Hospital | 29 | 1 | 8 | 3 | 237 | ||
| Salford Royal Hospital | 3 | 11 | |||||
| Churchill Hospital | 14 | 2 | 1 | 1 | 9 | ||
| Canterbury Hospital | |||||||
| Hull Royal Infirmary | 3 | 2 | 1 | ||||
| Addenbrooke’s Hospital | 29 | 1 | 7 | ||||
| Sunderland Royal Hospital | |||||||
| St James’s University Hospital | 36 | 2 | 5 | 6 | 11 | ||
| Western General Hospital | 88 | 19 | 5 | 13 | |||
| Charing Cross Hospital | 49 | 1 | 1 | 81 | |||
| Northwick Park Hospital | |||||||
| Royal Hallamshire Hospital | 11 | 1 | 1 | 2 | |||
| Guy’s Hospital | 25 | 1 | 2 | 2 | 31 | ||
| Darent Valley Hospital | 11 | 1 | 1 | 1 | 44 | ||
| Wrexham Maelor Hospital | 1 | ||||||
| Bradford Royal Infirmary | 16 | 2 | 1 | 1 | 28 | ||
| Royal Derby Hospital | 6 | ||||||
| Whiston Hospital | 4 | 2 | |||||
| St Peter’s Hospital |
Appendix 3 Statistical subgroup analysis models
| Population | Main treatment effect/interaction effect | ARDa,b | 95% CI | Non-inferiority p-valuec | RRa,b | 95% CI |
|---|---|---|---|---|---|---|
| ITT-1 | SWL | 0.114 | 0.050 to 0.177 | < 0.001 | 2.070 | 1.239 to 3.456 |
| Stone size # SWL | 0.135 | –0.159 to 0.430 | 0.116 | 1.098 | 0.336 to 3.591 | |
| ITT-2 | SWL | 0.137 | 0.063 to 0.211 | < 0.001 | 2.163 | 1.318 to 3.549 |
| Stone size # SWL | 0.101 | –0.202 to 0.404 | 0.116 | 0.967 | 0.306 to 3.060 | |
| PP-1 | SWL | 0.144 | 0.078 to 0.209 | < 0.001 | 2.471 | 1.452 to 4.204 |
| Stone size # SWL | 0.177 | –0.153 to 0.508 | 0.156 | 1.051 | 0.303 to 3.649 | |
| PP-2 | SWL | 0.179 | 0.098 to 0.259 | < 0.001 | 2.643 | 1.568 to 4.453 |
| Stone size # SWL | 0.124 | –0.223 to 0.470 | 0.157 | 0.882 | 0.258 to 3.011 |
| Population | Main treatment effect/interaction effect | ARDa,b | 95% CI | Non-inferiority p-valuec | RRa,b | 95% CI |
|---|---|---|---|---|---|---|
| ITT-1 | SWL | 0.114 | 0.051 to 0.176 | < 0.001 | 1.603 | 0.872 to 2.949 |
| Stone Loc1 # SWL | –0.004 | –0.221 to 0.213 | 0.035 | 1.521 | 0.289 to 8.017 | |
| Stone Loc2 # SWL | 0.052 | –0.074 to 0.177 | 0.001 | 2.290 | 0.847 to 6.197 | |
| ITT-2 | SWL | 0.137 | 0.063 to 0.211 | < 0.001 | 1.694 | 0.937 to 3.062 |
| Stone Loc1 # SWL | –0.009 | –0.257 to 0.239 | 0.056 | 1.496 | 0.275 to 8.144 | |
| Stone Loc2 # SWL | 0.059 | –0.081 to 0.200 | 0.003 | 2.129 | 0.790 to 5.734 | |
| PP-1 | SWL | 0.144 | 0.079 to 0.208 | < 0.001 | 1.839 | 1.020 to 3.317 |
| Stone Loc1 # SWL | –0.010 | –0.242 to 0.222 | 0.044 | 1.817 | 0.296 to 11.165 | |
| Stone Loc2 # SWL | 0.045 | –0.094 to 0.184 | 0.003 | 2.565 | 0.820 to 8.020 | |
| PP-2 | SWL | 0.179 | 0.098 to 0.259 | < 0.001 | 1.962 | 1.103 to 3.490 |
| Stone Loc1 # SWL | –0.011 | –0.281 to 0.259 | 0.072 | 1.829 | 0.288 to 11.596 | |
| Stone Loc2 # SWL | 0.059 | –0.100 to 0.218 | 0.008 | 2.433 | 0.786 to 7.530 |
| Population | Main treatment effect/interaction effect | ARDa,b | 95% CI | Non-inferiority p-valuec | RRa,b | 95% CI |
|---|---|---|---|---|---|---|
| ITT-1 | SWL | 0.114 | 0.048 to 0.180 | < 0.001 | 1.807 | 1.164 to 2.806 |
| Gender # SWL | 0.057 | –0.054 to 0.167 | < 0.001 | 3.490 | 0.554 to 21.974 | |
| SWL | 0.137 | 0.061 to 0.213 | < 0.001 | 1.910 | 1.245 to 2.932 | |
| ITT-2 | Gender # SWL | 0.036 | –0.098 to 0.170 | 0.002 | 3.088 | 0.502 to 19.008 |
| SWL | 0.144 | 0.075 to 0.212 | < 0.001 | 2.141 | 1.387 to 3.303 | |
| Gender # SWL | 0.054 | –0.055 to 0.162 | < 0.001 | 3.237 | 0.537 to 19.512 | |
| PP-2 | SWL | 0.179 | 0.096 to 0.262 | < 0.001 | 2.304 | 1.501 to 3.538 |
| Gender # SWL | 0.027 | –0.111 to 0.165 | 0.002 | 2.864 | 0.492 to 16.652 |
Appendix 4 Economics
| Procedure | Procedure description | Activity | Unit cost (£) |
|---|---|---|---|
| SWL | |||
| LB36Z | Extracorporeal lithotripsy | 20,104 | 491.13 |
| Stent insertion/removal | |||
| LB09D | Intermediate endoscopic ureter procedures, 19 years and over | 29,926 | 1054 |
| URS | |||
| LB65C | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of ≥ 5 | 1321 | 4160 |
| LB65D | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 3–4 | 2060 | 2745 |
| LB65E | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 0–2 | 17,820 | 1900 |
| Weighted average | 2122.55 | ||
| Nephrostomy tube | |||
| YL11Z | Unilateral, percutaneous insertion of ureteric stent or nephrostomy | 6105 | 1027.35 |
| Elective inpatient excess bed-days | |||
| LB65C | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of ≥ 5 | 169 | 440 |
| LB65D | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 3–4 | 103 | 345 |
| LB65E | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 0–2 | 203 | 323 |
| Weighted average | 475 | 370 | |
| Non-elective excess bed-days | |||
| LB65C | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of ≥ 5 | 1101 | 366 |
| LB65D | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 3–4 | 282 | 422 |
| LB65E | Major endoscopic, kidney or ureter procedures, 19 years and over, with a CC score of 0–2 | 634 | 406 |
| Weighted average | 2017 | 386 | |
| Test | Id/Gauss | Log/Gauss | Power.65/Gauss |
|---|---|---|---|
| Pearson’s correlation | 1 | 0.9896 | 0.9834 |
| Pregibon link | 0.6785 | 0.9684 | 0.7864 |
| Modified Hosmer–Lemeshow | 0.5625 | 0.8988 | 0.5835 |
FIGURE 28.
Cost-effectiveness acceptability curve for higher cost of SWL, using 25% inpatient stay scenario for SWL pathway patients: SWL vs. URS.
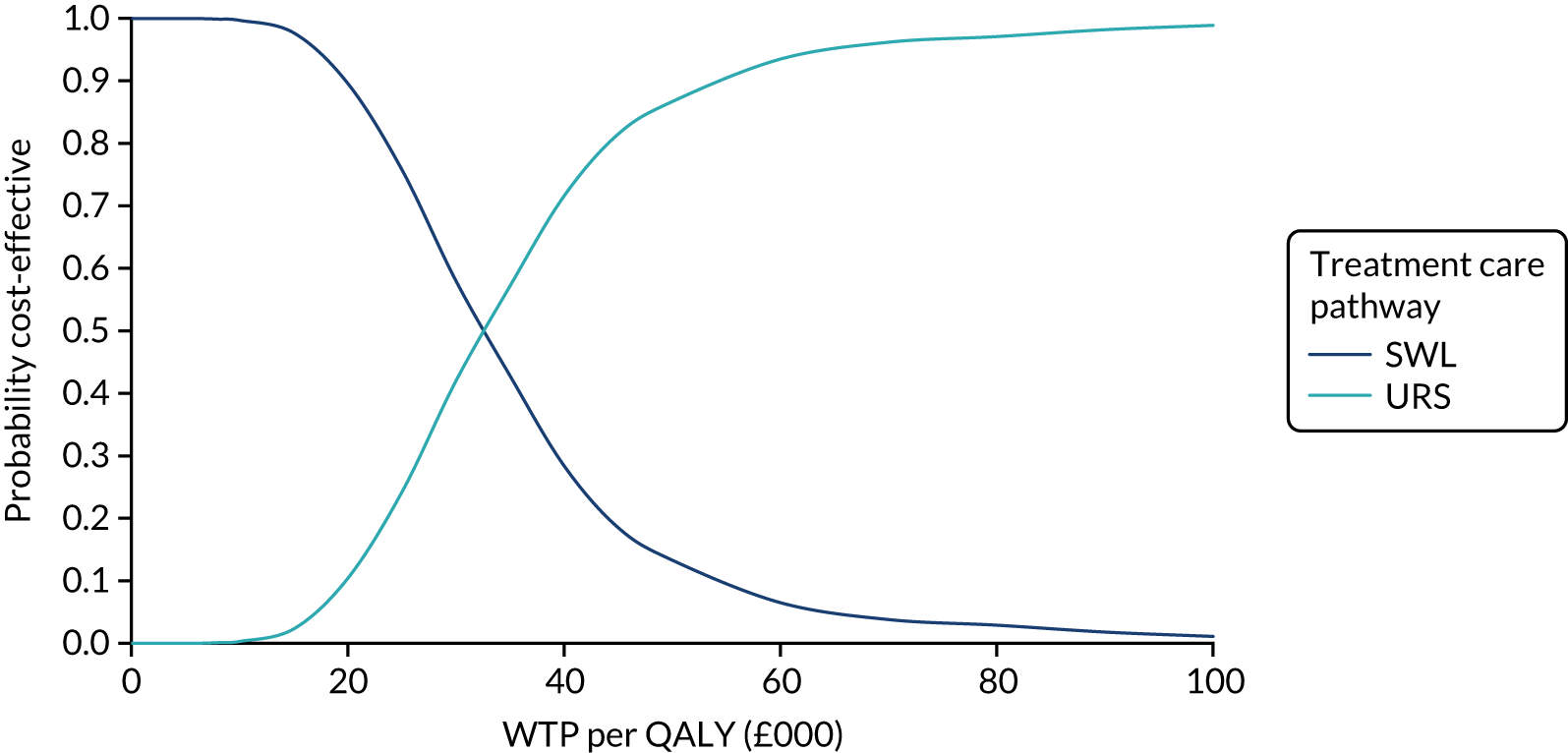
FIGURE 29.
Scatterplot of incremental costs and QALYs (assuming 25% inpatient stay for SWL pathway): SWL vs. URS.
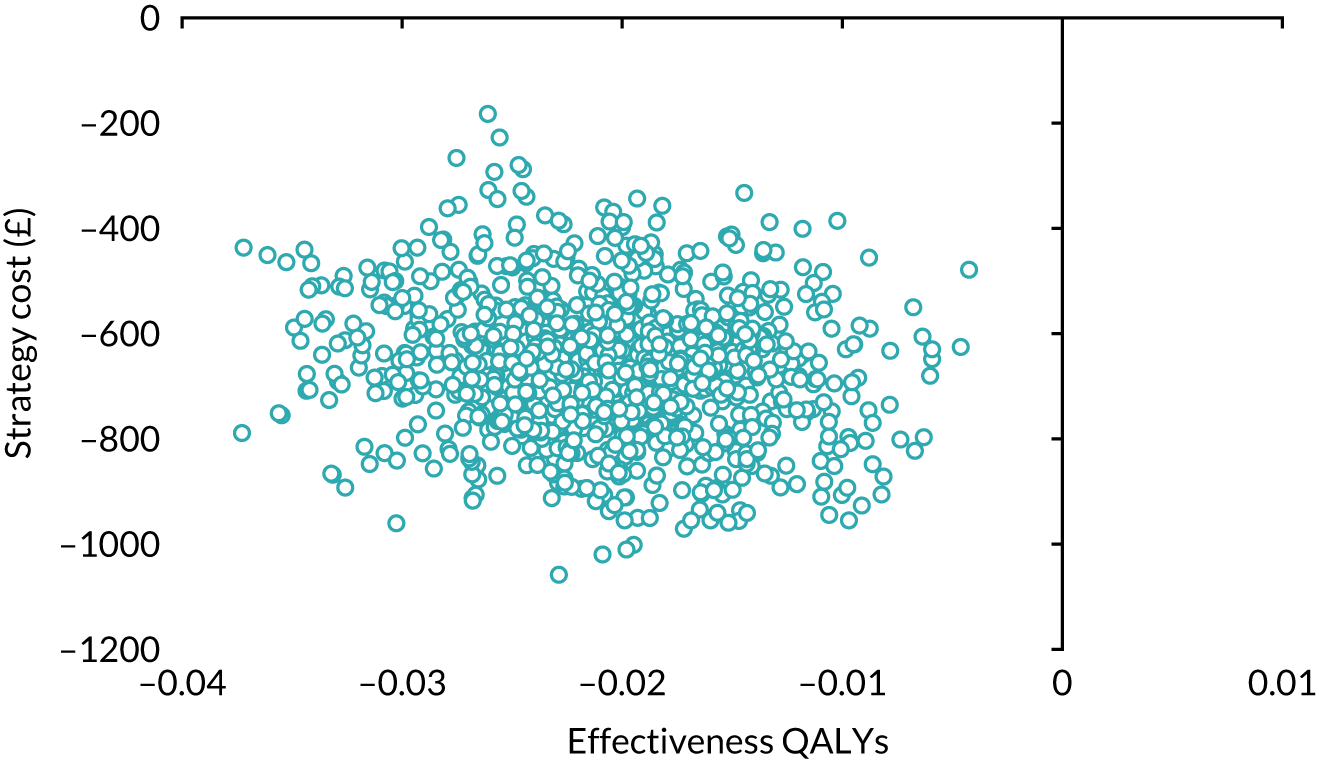
FIGURE 30.
Cost-effectiveness acceptability curve for higher cost of SWL, using 50% inpatient stay scenario for SWL pathway patients: SWL vs. URS.
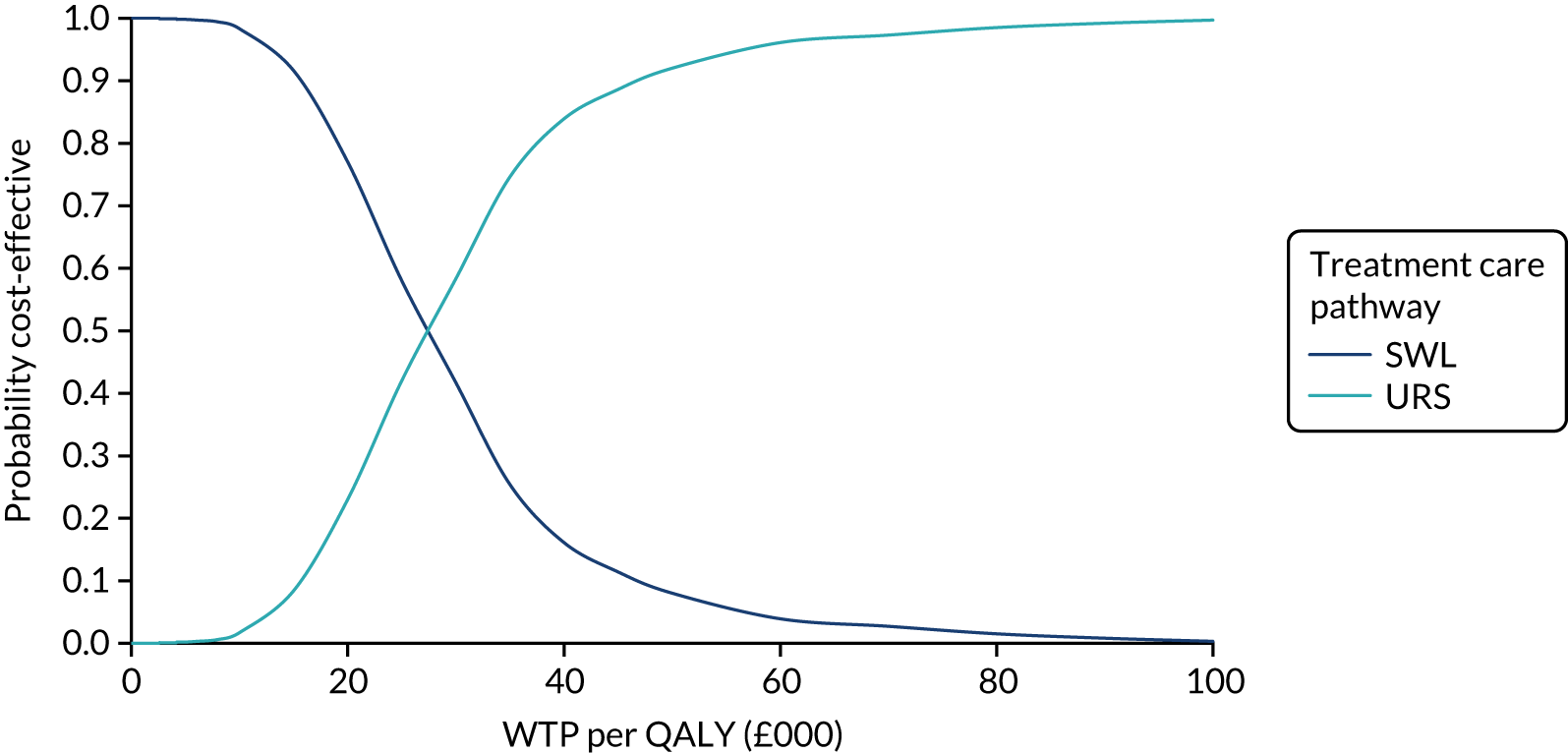
FIGURE 31.
Scatterplot of incremental costs and QALYs (assuming 50% inpatient stay for SWL pathway): SWL vs. URS.
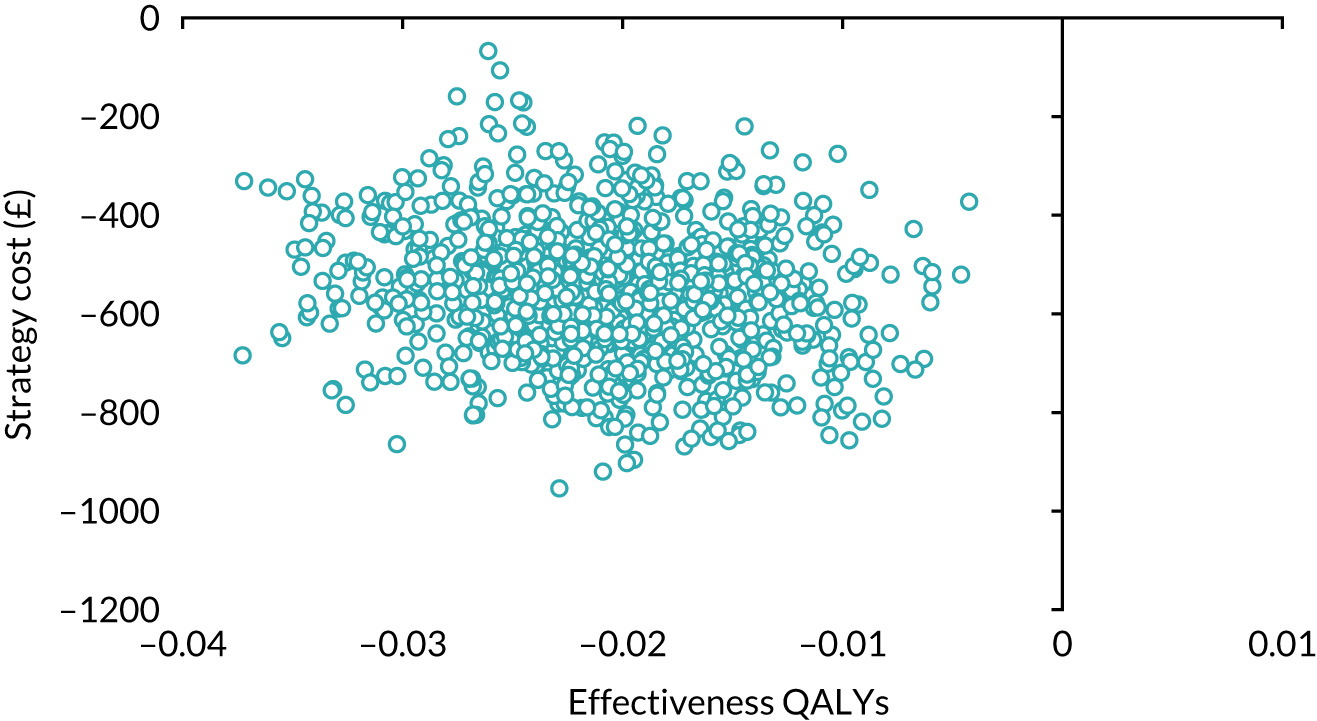
FIGURE 32.
Cost-effectiveness acceptability curve for higher cost of SWL, using 75% inpatient stay scenario for SWL pathway patients: SWL vs. URS.
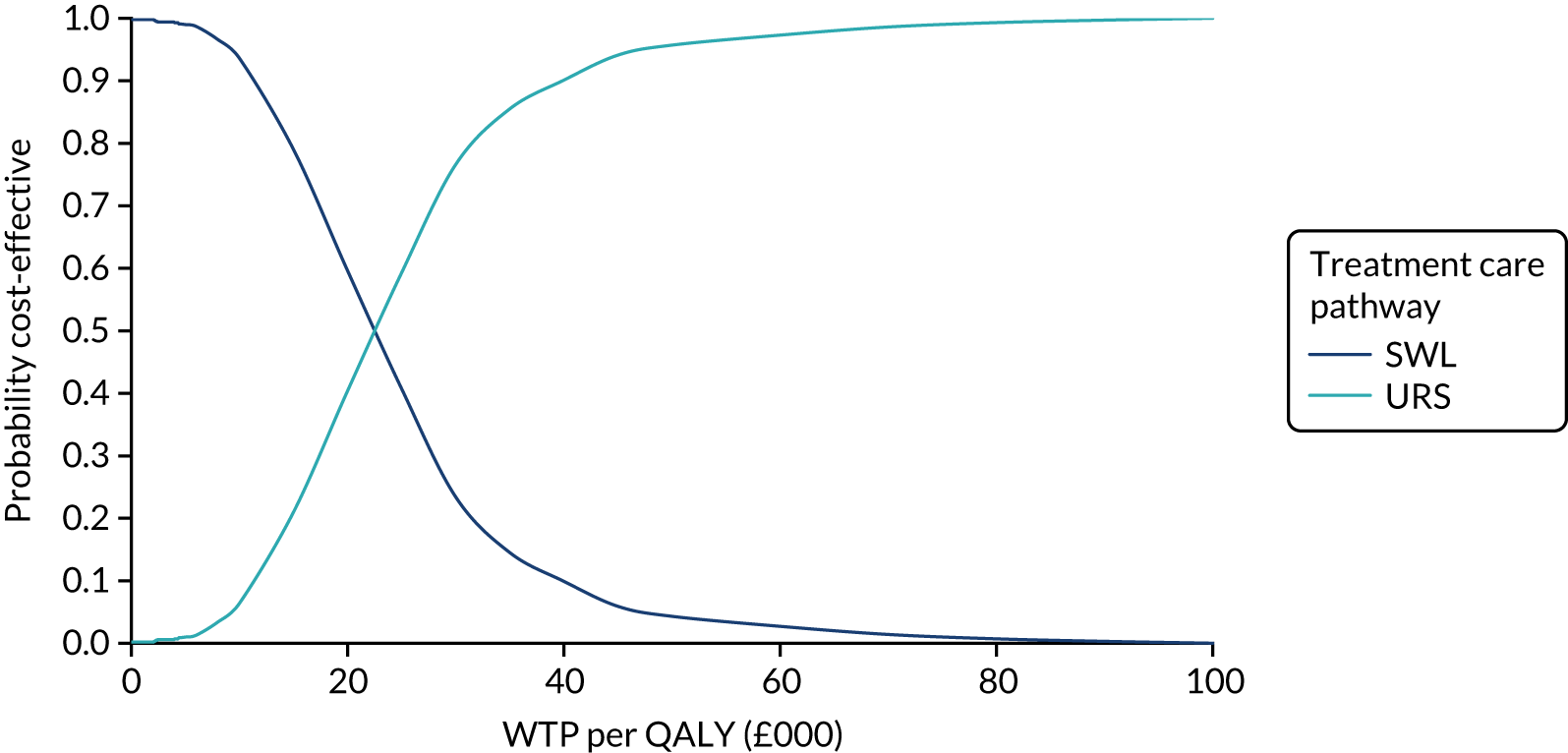
FIGURE 33.
Scatterplot of incremental costs and QALYs (assuming 75% inpatient stay for SWL pathway): SWL vs. URS.
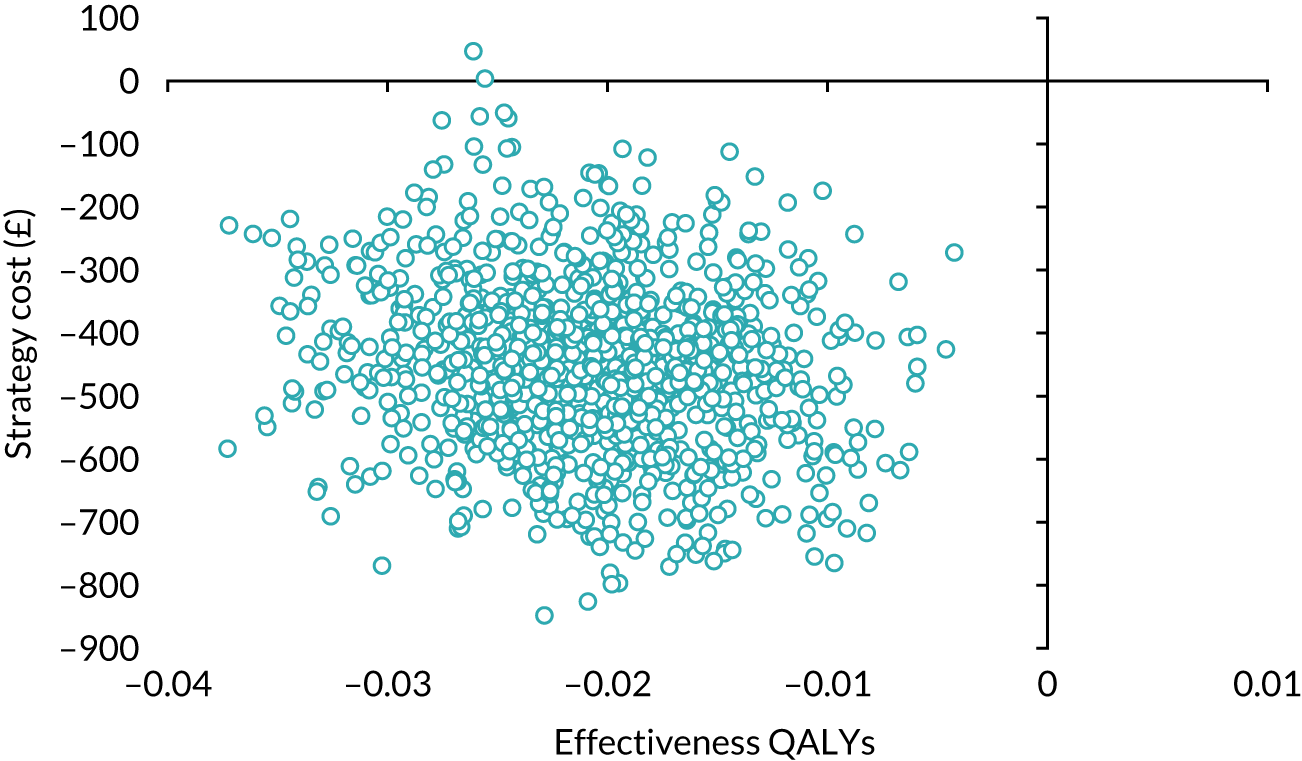
List of abbreviations
- ARD
- absolute risk difference
- AUC
- area under the curve
- BAUS
- British Association of Urological Surgeons
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTKUB
- computed tomography scan of the kidneys, ureters and bladder
- DMC
- Data Monitoring Committee
- EAU
- European Association of Urology
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GBP
- Great British pounds
- GLM
- generalised linear model
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSRU
- Health Services Research Unit
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- ITT-1
- intention to treat, including all participants
- ITT-2
- intention to treat, excluding those who passed their stone prior to any intervention
- IVU
- intravenous urography
- MCS
- mental component score
- MET
- metabolic expulsive therapy
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PCS
- physical component score
- PI
- principal investigator
- PMG
- Project Management Group
- PP
- per protocol
- PP-1
- per-protocol analysis, including those who passed their stone before treatment
- PP-2
- per-protocol analysis, excluding those who passed their stone before treatment
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SWL
- shockwave lithotripsy
- TISU
- Therapeutic Interventions for Stones of the Ureter
- TSC
- Trial Steering Committee
- URS
- ureteroscopic stone treatment
- VAS
- visual analogue scale
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WUZW9042).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
