Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/47. The contractual start date was in September 2011. The draft report began editorial review in June 2022 and was accepted for publication in October 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Brunt et al. This work was produced by Brunt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Brunt et al.
Chapter 1 Introduction
Parts of this report are reproduced or adapted with permission from Brunt et al. 2020,1 Brunt et al. 20212 and Brunt et al. 2016. 3 Sections of this report have also been reproduced from the FAST-Forward study protocol document, available from the NIHR Funding and Awards website. 4 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Breast cancer and the multidisciplinary team
Breast cancer is globally the most commonly occurring cancer in women with over 2 million new cases in 2018, including 58,000 in the UK. It is rare in men, with around 300–400 cases per year in the UK. Since the early 1990s, breast cancer incidence rates in females have increased by around a quarter (24%). 5 Despite increased incidence, UK mortality rates from breast cancer have fallen, due to advances in all aspects of breast cancer diagnosis and treatment, including radiotherapy (RT).
Breast cancer usually presents as symptoms such as a lump, nipple changes or a finding of an abnormal mammogram. Patients are generally assessed in a specific Breast clinic where they have a triple assessment involving clinical assessment, imaging (mammography/ultrasound) and a biopsy. A plan for treatment is then made in a multidisciplinary meeting which nearly always involves surgery to resect the cancer. In the modern era this is a wide local excision (breast-conserving therapy), or a mastectomy, along with nodal surgery. The resected tissue is examined by a pathologist to determine the size, grade, type, receptor profile and resection margins of the cancer along with other features. The lymph nodes may be selectively sampled (sentinel node biopsy) or all removed if involved (axillary clearance).
In larger, or more biologically aggressive cancers [triple negative and human epidermal growth factor receptor-2 (HER-2) positive] chemotherapy is now often given prior to surgery to downstage the cancer in the breast and the axilla, and to assess response to therapy, which may well alter subsequent treatment, including surgery, post-operative drug therapy and RT. Similarly in larger hormone receptor-positive HER-2 negative cancers, upfront antihormonal therapy may be given with similar intent. However, surgery is still used after the neoadjuvant therapy to remove any remaining cancer and to assess residual cancer burden.
Benefits and adverse effects of radiotherapy for patients with breast cancer
Radiotherapy uses high-energy X-rays to destroy cancer cells remaining in the breast after the main lump has been removed. Many studies have shown that this treatment substantially reduces the risk of cancer recurring in the breast and or lymph glands and improves overall survival. A meta-analysis from the Early Breast Cancer Trials Collaborative Group (EBCTCG) showed that after breast-conserving surgery, RT reduces relapse and breast cancer death. 6 Increased risk of local relapse is associated with age under 50 years and grade 3 cancers. Other risk factors include cancer size, cancer biology, negative hormonal receptor status, HER-2 positive status, the presence of lymphovascular invasion and axillary node involvement. The absolute benefits of RT are greatest in those with the highest risk factors. The EBCTCG meta-analysis included individual patient data for 10,801 women in 17 randomised trials of RT versus no RT after breast-conserving surgery, 8337 of whom had pathologically confirmed node-negative (pN0) or node-positive (pN+) disease. Overall, RT reduced the 10-year risk of any (i.e. locoregional or distant) first relapse from 35.0% to 19.3% [absolute reduction 15.7%, 95% confidence interval (CI) 13.7 to 17.7; 2p < 0.00001] and reduced the 15-year risk of breast cancer death from 25.2% to 21.4% (absolute reduction 3.8%, 1.6–6.0; 2p = 0.00005). In women with pN0 disease (n = 7287), RT reduced the first relapse risk from 31.0% to 15.6% (absolute relapse reduction 15.4%, 13.2 to 17.6; 2p < 0.00001) and risk of breast cancer death from 20.5% to 17.2% (absolute mortality reduction 3.3%, 0.8 to 5.8; 2p = 0.005), respectively. In these women with pN0 disease, the absolute relapse reduction varied according to age, grade, oestrogen-receptor (ER) status, tamoxifen use and the extent of surgery. These characteristics were used to predict large (≥ 20%), intermediate (10–19%), or lower (< 10%) absolute reductions in the 10-year relapse risk. The absolute reductions in 15-year risk of breast cancer death in these three prediction categories were 7.8% (95% CI 3.1 to 12.5), 1.1% (–2.0 to 4.2) and 0.1% (–7.5 to 7.7), respectively (trend in absolute mortality reduction 2p = 0.03). In the smaller number of women with pN+ disease (n = 1050), RT reduced the 10-year relapse risk from 63.7% to 42.5% (absolute reduction 21.2%, 95% CI 14.5 to 27.9; 2p < 0.00001) and the 15-year risk of breast cancer death from 51.3% to 42.8% (absolute reduction 8.5%, 1.8–15.2; 2p = 0.01). Overall, about one breast cancer death was avoided by year 15 for every four relapses avoided by year 10. The mortality reduction did not differ significantly from this overall relationship in any of the three prediction categories for pN0 disease or for pN+ disease.
Stepwise improvements in many aspects of patient management have led to a steady reduction in the absolute risk of relapse and death for patients over the decades. This reduction is due to many factors such as improved screening, better surgery with closer adherence to guidelines on achieving negative margins and advances in systemic therapy and RT techniques. A local relapse risk of around 2% at 5 years is a reasonable target to aim for in the current era, although the absolute risk will be higher in younger patients with grade 3 tumours and may be lower in elderly patients with grade 1 tumours. Currently patients at extremely low risk of relapse following surgery are commonly recommended no adjuvant RT based on studies such as PRIME. 7
The absolute benefit of RT for each patient is considered by the multidisciplinary team based on the biological and pathological staging of the tumour, type of surgery and patient factors, including age and comorbidities. The main short-term side effects of RT are tiredness, skin colour changes, discomfort and swelling (oedema). Long-term side effects may develop over many years following RT and these are thought to be due to damage to the small blood vessels supplying tissue that has been irradiated. Long-term side effects include the following: (1) skin changes, where the treatment area appears permanently tanned after treatment has finished, although this is not harmful. Later, the skin might appear to have very tiny broken veins in the skin called telangiectasia. (2) Breast shrinkage or distortion; RT can make the breast tissue contract so that the breast gradually gets smaller. This can happen to natural breast tissue or a reconstructed breast. An implant in a reconstructed breast can become hard (capsular contracture) and may need replacing. (3) Breast induration, where the breast feels hard and less stretchy; this is due to a side effect called radiation fibrosis. (4) Cough and breathlessness may occur in some patients who have RT to the chest area although this is not common. The problems are due to changes in the lung tissue called chronic radiation pneumonitis. They might start many months or a few years after treatment. The chances of this happening increase if the volume of lung irradiated increases, or if the patient has pre-existing lung conditions. (5) Irradiation of the ribs or clavicle may lead to radiation osteitis or rarely to osteoradionecrosis which may cause non-healing fractures. (6) Radiotherapy may cause nerve damage in the arm on the treated side, which can develop many years after treatment. Symptoms include tingling, numbness, pain, and weakness, and in some people it may cause some loss of movement in the arm and shoulder. This is extremely rare with modern RT. (7) Radiotherapy treatment may cause another type of cancer in many years’ time. These include lung cancers, especially in smokers, and the rare angiosarcoma.
Partial breast radiotherapy in low relapse risk patient subgroups
Instead of irradiating the whole breast, partial-breast RT restricted to the region of the original tumour in low relapse risk patients reduces the morbidity of RT without compromising its ability to cure the cancer. This technique is based on international reports of reductions in local relapse incidence, and the recognition that the majority of ipsilateral local relapses occur close to the region of the index tumour (the so-called tumour bed). Several studies have assessed partial-breast RT compared with whole breast RT. 8–12 Most of them show comparable rates of local control in patients receiving partial-breast RT compared with whole breast RT. However, most of the studies not only compared partial versus whole breast RT, but also used differing RT techniques and doses in the treatment groups, making toxicity comparisons difficult.
IMPORT LOW was a multicentre, randomised, controlled, phase 3, non-inferiority trial comparing the safety and efficacy of standard whole breast RT (control, whole breast group) with experimental schedules of RT to the whole breast and partial breast (reduced-dose group), and to the partial breast only (partial-breast group). 12 Patients assigned to whole breast RT (control) received 40 Gy in 15 fractions (daily doses) to the whole breast. Those assigned to the reduced-dose group received 36 Gy in 15 fractions to the whole breast and 40 Gy in 15 fractions to the partial breast containing the tumour bed, and those assigned to the partial-breast group received 40 Gy in 15 fractions to the partial breast only. Women who were aged 50 years or older who had breast-conserving surgery for unifocal invasive ductal adenocarcinoma (excluding invasive carcinoma of classical lobular type) of any grade (1–3) were recruited. Other inclusion criteria were pathological tumour size of 3 cm or less (pT1–2), axillary lymph-node negative or one to three positive lymph nodes (pN0–1), and a minimum microscopic margins of non-cancerous tissue of 2 mm or more. A total of 2018 patients were randomly assigned to the three groups, with around 670 patients per group. Patients were clinically assessed yearly for local relapse and toxicity, and around half completed questionnaires included self-assessments of side effects and health-related quality of life. After a median follow-up of 72.2 months, local relapse had been reported for 18 patients, nine (1%) of whom were in the whole breast group, three (< 1%) in the reduced-dose group, and six (1%) in the partial-breast group. The 5-year estimated cumulative incidence of local relapse was 1.1% (95% CI 0.5 to 2.3) in the whole breast group, 0.2% (95% CI 0.02 to 1.2) in the reduced-dose group, and 0.5% (95% CI 0.2 to 1.4) in the partial-breast group. At 5 years patients reported fewer moderate or marked normal tissue effects (NTE) in terms of skin change, change in overall breast appearance, breast being smaller, and breast being harder or firmer to touch in the partial-breast group than in the whole breast group although this reduction was statistically significant for change in breast appearance only (p < 0.0001). At 5 years, change in breast appearance had the highest cumulative incidence of items reported as moderate or marked by patients in all groups. Reports of breast becoming harder or firmer were significantly reduced in both the reduced-dose group (p = 0.002) and partial-breast group (p < 0.0001) compared with the whole breast group. The conclusions from IMPORT LOW were that for patients over 50, with smaller, generally lymph node-negative breast cancer, partial-breast RT was non-inferior to whole breast RT with regards local control and had less toxicity. Reduced-volume breast cancer treatment will also reduce lung and cardiac doses. Follow-up to 10 years is ongoing in the IMPORT LOW trial to collect data on longer-term efficacy and toxicity outcomes.
The control arm in IMPORT LOW was 40 Gy in 15 fractions to whole breast, which has been adopted as the standard of care for partial-breast RT. The control arm for the FAST-Forward Trial was the same. Therefore, if the 40 Gy/15 fractions schedule was found to be isoeffective for either of the test arms, then it was deemed that this would also be the same for partial-breast RT. It would make no biological sense to have different fractionation schedules with reduced-field RT (partial-breast). For FAST-Forward whole breast RT was still the standard-of-care when the study was conceived and recruiting.
Simplifying radiotherapy dose schedules for patients with breast cancer
For many years, and still in some countries, the international standard regimen for whole breast RT delivers a total dose of 50 Gy in 25 fractions over 5 weeks following surgical resection of primary tumour in women with early breast cancer. Attempts to reduce the number of fractions in the 1970s made inadequate downward adjustments to total dose, resulting in unacceptable rates of late complications. 13 These miscalculations inhibited further research in breast RT fractionation for decades, but interest in fewer larger fractions delivered over a shorter overall treatment time has been rekindled by randomised clinical trials based on a better understanding of normal tissue and tumour responses (radiobiology). Fractionation sensitivity describes responses of normal and malignant tissues to RT fraction size and is quantified in terms of the α/β ratio, expressed in Gy. The lower the α/β ratio, the greater the effect on normal and malignant tissues of changes in fraction size.
Four randomised trials involving a total of > 8000 women have compared a lower total dose in fewer larger fractions against 50 Gy in 25 fractions, and all have reported favourable results in terms of local tumour control and late adverse effects (AE). 14–18 The Royal Marsden Hospital/Gloucestershire Oncology Centre (now sometimes referred to as START-P) and Ontario Clinical Oncology Group (OCOG) trials totalling 2644 women with mainly axillary lymph node-negative tumours < 5 cm diameter were the subject of a 2008 Cochrane review of altered RT fractionation in early breast cancer. 19 Radiotherapy fractions larger than 2.0 Gy did not appear to affect: (1) local relapse-free survival (absolute difference 0.4%, 95% CI −1.5% to 2.4%), (2) breast appearance [risk ratio (RR) 1.01, 95% CI 0.88 to 1.17; p = 0.86], (3) survival at 5 years (RR 0.97, 95% CI 0.78 to 1.19; p = 0.75), (4) late skin toxicity at 5 years (RR 0.99, 95% CI 0.44 to 2.22; p = 0.98 or (5) late radiation toxicity in subcutaneous tissue (RR 1.0, 95% CI 0.78 to 1.28; p = 0.99). The review concluded that the use of unconventional fractionation regimens did not affect breast appearance or toxicity, nor appear to affect local cancer relapse. The results of the UK START trials (N = 4451) were published too late to be included in the overview but were consistent with the findings. The START Trials tested the effects of RT schedules using fraction sizes larger than 2.0 Gy. The START-A Trial tested two dose levels of a 13-fraction regimen delivered over 5 weeks in order to measure the sensitivity of normal and malignant tissues to fraction size. Patients in START-A were randomly assigned to either 50 Gy in 25 fractions (control group) or 41.6 Gy in 13 fractions or 39 Gy in 13 fractions over 5 weeks. Patients in the START-B trial were randomly assigned to either 50 Gy in 25 fractions (control group) over 5 weeks or 40 Gy in 15 fractions over 3 weeks. The START-A trial (N = 2236) showed that the estimated absolute differences in 5-year local-regional relapse rates compared with the control schedule of 50 Gy in 2.0 Gy fractions were 0.2% (95% CI −1.3% to 2.6%) after 41.6 Gy and 0.9% (95% CI −0.8% to 3.7%) after 39 Gy. In START A, photographic and patient self-assessments suggested lower rates of late AE after 39 Gy than with 50 Gy, with a hazard ratio (HR) for late change in photographic breast appearance of 0.69 (95% CI 0.52 to 0.91; p = 0.01). In the START-B trial (N = 2215) the estimated absolute difference in 5-year local-regional relapse rates for 40.05 Gy compared with 50 Gy was −0.7% (95% CI −1.7% to 0.9%), and the HR for late change in photographic breast appearance was 0.83 (95% CI 0.66 to 1.04). Therefore, the START trials reported similar local tumour control with some evidence of lower rates of late AE after schedules with fraction sizes larger than 2.0 Gy compared with the international standard 25-fraction regimen. 18
A 15-fraction schedule has been the UK standard-of-care recommended by the National Institute for Health and Care Excellence (NICE) since 2009, but it was thought to be unlikely to represent the useful limits of hypofractionation for whole breast RT. There is a history of prescribing once-weekly fractions of whole breast RT for women too frail or otherwise unable to attend for conventional schedules. In a French series of 115 patients undergoing primary RT without surgery for non-metastatic breast cancer from 1987 to 1999, the whole breast was treated with two tangential fields and received five once-weekly fractions of 6.5 Gy. 20 One hundred and one were given additional tumour bed boost doses, 7 with 1 fraction, 69 with 2 fractions and 25 with 3 once-weekly fractions of 6.5 Gy using electrons. Kaplan–Meier estimates of late effects in the breast were 24% grade 1, 21% grade 2 and 6% grade 3 at 48 months. The 5-year local progression-free rate was 78% (95% CI 66.6 to 88.4). In a separate French series, five once-weekly fractions of 6.5 Gy to the whole breast with no boost were given to 50 women after local tumour excision. 21 Grade 1 or 2 induration was reported in 33% of the patients at a median follow-up of 93 months (range 9–140). The 7-year local relapse-free survival was 91%.
The UK FAST trial (N = 915) tested two dose levels of a 5-fraction regimen delivering one fraction per week against a control schedule of 50 Gy in 25 fractions, defining RT AE as the primary endpoint. 22 The two test dose levels delivered 5 fractions of 5.7 Gy or 6.0 Gy (total dose 28.5 Gy or 30 Gy), estimated to be isoeffective with the control regimen assuming α/β values of 3.0 Gy or 4.0 Gy, respectively. Nine hundred and fifteen patients were recruited from October 2004 to March 2007. The mean age of participants was 62.7 years. Only 17 patients (5.2%) developed moist desquamation (12 after 50 Gy, 3 after 30 Gy, 2 after 28.5 Gy) out of 327 with Radiation Therapy Oncology Group (RTOG) skin toxicity data available. At a median follow-up of 28.3 months [interquartile range (IQR) 24.1–33.6], 729 patients had 2-year photographic assessments available, with mild and marked change in breast appearance in 19.3% and 1.7% after 50 Gy, 26.2% and 9.3% after 30 Gy, and 20.3% and 3.7% after 28.5 Gy. RRs for mild and marked change for 30 Gy versus 50 Gy were 1.48 (95% CI 1.06 to 2.05) and 6.06 (2.14 to 17.20); p < 0.001 for trend, favouring 50 Gy; and for 28.5 Gy versus 50 Gy were 1.07 (0.75 to 1.54) and 2.25 (0.70 to 7.18); p = 0.26 for trend, favouring 50 Gy. Any clinically assessed moderate or marked AE in the breast were increased for 30 Gy compared with 50 Gy (HR 2.19, 95% CI 1.46 to 3.29; p < 0.001), but similar for 28.5 Gy (HR 1.33, 95% CI 0.86 to 2.08; p = 0.19). At a median follow-up of 37.3 months two local tumour relapses had been recorded.
The 10-year results from the FAST trial were subsequently published in 2020,23 confirming that as far as late toxicity is concerned, the 28.5 Gy schedule was isoeffective to the 50 Gy schedule. The 30 Gy schedule had an increased rate of late toxicity compared with 50 Gy. Any moderate or marked physician-assessed NTE in the breast (shrinkage, induration, telangiectasia, oedema) was reported for 92/774 (11.9%) at 5 years and 55/392 (14.0%) at 10 years. The most prevalent individual effect was breast shrinkage. Five-year prevalence of any moderate or marked breast NTE was estimated to be 10% higher (95% CI 5% to 16%) for 30 Gy versus 50 Gy (p < 0.001), with no statistically significant difference between 28.5 Gy and 50 Gy (2%, 95% CI −2% to +7%; p = 0.349). At 5 years, RRs for moderate or marked breast shrinkage versus 50 Gy were 2.03 (95% CI 1.15 to 3.58; p = 0.017) for 30 Gy and 1.20 (95% CI 0.63 to 2.27; p = 0.604) for 28.5 Gy. There were no statistically significant differences between schedules in 5-year prevalence of moderate or marked breast induration, telangiectasia and breast oedema, nor in 10-year prevalence of any moderate/marked effects, with few marked events. At 10 years, the estimated absolute differences in prevalence of any moderate or marked breast NTE compared with 50 Gy were 9% (95% CI 1% to 18%; p = 0.032) for 30 Gy and 5% (95% CI −2% to +13%; p = 0.184) for 28.5 Gy.
Five- and 10-year cumulative incidence rates of moderate or marked NTE in the breast were higher for 30 Gy compared with 50 Gy, with statistically significant differences for any NTE in the breast, breast shrinkage, breast induration, and breast oedema. Cumulative incidence rates of any moderate or marked NTE in the breast and breast induration were significantly higher for 28.5 Gy versus 50 Gy. Modeling all annual physician assessments over follow-up, rates of moderate or marked effects were statistically significantly higher for 30 Gy compared with 50 Gy [odds ratio (OR) for any breast NTE 2.12, 95% CI 1.55 to 2.89; p < 0.001], but with no significant difference between 28.5 Gy and 50 Gy (OR 1.22, 95% CI 0.87 to 1.72; p = 0.248; Statistically significant differences between the test schedules were found for breast shrinkage, telangiectasia, and breast oedema, with higher rates for 30 Gy compared with 28.5 Gy. The prevalence of breast shrinkage and telangiectasia increased over time, with a decline in breast oedema.
By the time of the 10-year analysis of the FAST trial only 11 local relapses had been reported (50 Gy: 3, 30 Gy: 4, 28.5 Gy: 4), hence analysis of tumour control outcomes would be underpowered.
A gain in local tumour control due to shortening treatment time to 1 week from longer 3- to 5-week schedules is theoretically possible. Evidence based on retrospective studies for an influence of treatment time on local tumour control is conflicting with recent systematic reviews drawing different conclusions. 24,25 Even without a gain in tumour control, accelerated RT is likely to be more convenient for patients, and may ease scheduling with other treatment modalities. A pilot study (N = 30) tested 30 Gy in 5 fractions of 6.0 Gy in 15 days to the whole breast in terms of acute AE and late effects at 2 years. 26 In this series, 23/30 (77%) patients scored no change in post-operative photographic breast appearance at 2 years, 7/30 (23%) scored mild change and none scored marked change. The acute skin reactions were mild, with no reaction more severe than grade 2 erythema, scored in 9/30 (27%) patients. In conclusion, it is fair to say that after decades of resistance to evaluating larger RT fraction sizes in breast cancer, expert opinion is responding to an accumulating body of evidence supporting the safety and effectiveness of this approach.
Against this background, the FAST-Forward phase III randomised trial was designed, with the primary aim of testing local tumour control in women with early breast cancer following a 5-fraction schedule of adjuvant RT delivered in 1 week.
Lymph node radiotherapy for breast cancer
This sub-study to the Main Trial tested the safety of 5-fraction regimens in the context of lymphatic RT. The model of breast cancer spread that was dominant in earlier decades envisaged a limited role for regional therapy beyond protection of quality of life, typically secured by surgery. Systematic overviews of RT effects by the Early Breast Cancer Trialists Collaborative Group (EBCTG) provide level 1A evidence that prevention of local-regional relapse has a major impact on breast cancer mortality. 6,27 An important conclusion to be drawn is that even heavily axillary lymph node-positive axillary patients can be cured by effective local-regional treatment, whether this is achieved by surgery, RT or systemic therapy. 27 The traditional model of breast cancer spread still has some adherents. The Z11 trial randomised 891 out of a planned 1900 patients with clinically lymph node-negative, sentinel lymph node-positive disease to axillary clearance versus no further axillary treatment. 28 Twenty-seven per cent allocated axillary clearance had additional positive lymph nodes. The axillary relapse rate at 5 years was 0.5% after axillary clearance and 0.9% after no axillary clearance, and there was no difference in breast cancer mortality. For some, this result reinforces the traditional model of breast cancer spread that discounts a role of nodal metastases in determining cancer spread. This interpretation fails to take account that standard post-operative tangential beam RT includes at least lower axillary lymph nodes and may be needed to eradicate residual disease. The same issue has been raised in discussion of the IBCSG 23-01 trial that compared axillary dissection versus no further axillary surgery in 929 sentinel lymph node-positive patients, 91% of whom were treated by breast conservation followed by whole breast RT. 29 The disease-free survival events, including axillary relapses, were non-inferior in the group spared axillary dissection, where standard whole breast RT will have included at least level I axillary lymph nodes. Although this remains a contested area, there is a wide consensus that control of axillary disease, whether by surgery, systemic therapy or RT, is an important component of curative therapy.
The AMAROS trial is informative in determining the role of surgery or RT for axillary management. 30 The study randomised 1425 patients with positive sentinel lymph nodes to either axillary lymph node dissection in 744 patients or axillary RT including photon beams to axillary apex and medial supraclavicular fossa (SCF) in 681 patients. The axillary relapse rates were low in both groups; 1.19% after RT and 0.43% after surgery, too low to assess non-inferiority. The main difference is that clinically reported arm swelling was less of a problem after RT than after surgery; 13.6% versus 28.0%, respectively, at 5 years; p < 0.0001. The implication is that lymphatic RT may increasingly be used as an alternative to surgery in this context.
Very little internal mammary chain (IMC) RT has been given in the UK in recent decades, but a modest reduction in breast cancer mortality is suggested by the NCIC MA20 trial and confirmed by the European Organisation for Research and Treatment of Cancer (EORTC) 229922 trial. 6,31 Both tested IMC and SCF RT, so it is possible that therapeutic effects are attributable solely to the SCF component. This does not seem likely since, if SCF RT is needed in order to enhance cure of patients with positive IMC nodes, it is reasonable to assume that the latter require managing too. The same argument might apply to the infraclavicular (ICF) lymph nodes (level III axilla), which are usually included in unshielded (rectangular) fields to the SCF. In the EORTC trial, 4004 patients with axillary lymph node-positive disease or had cancers that were centrally or medially situated but axillary lymph node-negative were randomised to receive IMC/medial SCF RT or not. At a median of 10.9 years of follow-up, the primary endpoint of overall survival improved from 80.75% to 82.3% with the addition of IMC/SCF RT (HR 0.87, 95% CI 0.76 to 1.00; p = 0.0556; p = 0.0496 after adjustment). It is not currently clear how these results will impact on practice. In conclusion, there was a need to test the safety of a 5-fraction schedule of lymphatic RT if the FAST-Forward Trial is to remain relevant to the 25% of patients referred for treatment with lymph node-positive disease. Whereas most are currently referred following axillary dissection, international and UK practices are changing, and more patients are likely to be referred in future for RT to axillary, SCF/ICF and perhaps IMC lymph node groups. When hypofractionation for breast cancer was first introduced in the 1960s, inappropriate dose regimens and uncertain dosimetry combined to cause unacceptably high rates of brachial plexopathy in patients with early breast cancer. 32–44 Even with hindsight, it is difficult to be sure how much of the morbidity was related to technical factors, especially beam overlap, and how much to dose-time factors. The only series describing brachial plexopathy after total dose ≤ 50 Gy delivered in 1.8–2.0 Gy fractions to breast and axilla/SCF reported 3/724 (0.6%) affected patients treated between 1968 and 1985, of whom two resolved and one progressed at 6.5 years median follow-up. 44 All patients were treated at the Joint Center, Boston, in a supine treatment position using a three-field technique with hanging block. A review of all published evidence available in 2005, suggested that brachial plexopathy after local-regional RT for early breast cancer is uncommon (< 1%) at doses < 55 Gy in 2.0 Gy equivalents. 45 Dose regimens were normalised using a linear quadratic model, assuming α/β value of 2.0 Gy.
The dose–response relationships are not difficult to reconcile with current practices in head and neck cancer, where total doses of 60 Gy in 30 fractions are standard. One recent example modelled the relationship between total dose in 2.0 Gy fractions and probability of brachial plexopathy in 330 patients systematically screened for evidence of sensorimotor symptoms a median of 56 months (range 6–135) after radical RT for head and neck cancer. Patients treated with definitive RT received a median dose of 70 Gy, and for those treated post-operatively the median dose was 60 Gy. Intensity-modulated RT was used on 62% cases, and 40% had concurrent chemotherapy, usually cisplatin. The brachial plexus was outlined using RTOG criteria on X-ray computerised tomography (CT) scans. 46
Against this background, the FAST-Forward Trial was extended beyond its original target accrual in order to test the safety of hypofractionated lymphatic RT. It is realistic to test only the common dose-limiting AE, including arm swelling and overall arm function. Very uncommon AE, including brachial plexopathy, cannot be formally tested in such a protocol, since a non-inferiority trial wishing to exclude an excess 1% risk with standard statistical power would require tens of thousands of patients.
Aims and objectives
Main Trial: to identify a 5-fraction schedule of curative RT delivered in once-daily fractions, that is at least as effective and safe as the current UK standard 15-fraction regimen after primary surgery for early breast cancer, in terms of local tumour control, AE, patient-reported outcomes (PRO) and health economic (HE) consequences.
Nodal Sub-Study (from Protocol v3.0, 8 July 2015): to show that a 5-fraction (1 week) schedule of adjuvant RT to level I–III axilla and/or level IV axilla (SCF) is non-inferior to a 15-fraction (3 weeks) standard in terms of patient-reported arm swelling and function, and to contribute additional information to the endpoints of the Main Trial.
Chapter 2 Methods
Trial design
FAST-Forward was a multicentre, three-group, non-blind, phase III randomised controlled non-inferiority clinical trial addressing the hypothesis that a 1-week course of curative whole breast RT would be at least as effective and safe as the UK standard 3-week regimen after primary surgery for early breast cancer. Patients were allocated in a 1 : 1 : 1 ratio to either 40.05 Gy in 15 fractions of 2.67 Gy over 3 weeks (Control group; labelled 40 Gy throughout the reminder of this report); 27.0 Gy in 5 fractions of 5.4 Gy over 1 week (Test Group 1) or 26.0 Gy in 5 fractions of 5.2 Gy over 1 week (Test Group 2). Dose prescriptions are summarised in Appendix 2, Table 33.
A sequential tumour bed RT boost following breast conservation surgery (BCS) was allowed, with centres required to state boost intention and dose (10 Gy or 16 Gy in 2.0 Gy fractions, or radiobiological equivalent from Protocol v4.0) prior to randomisation.
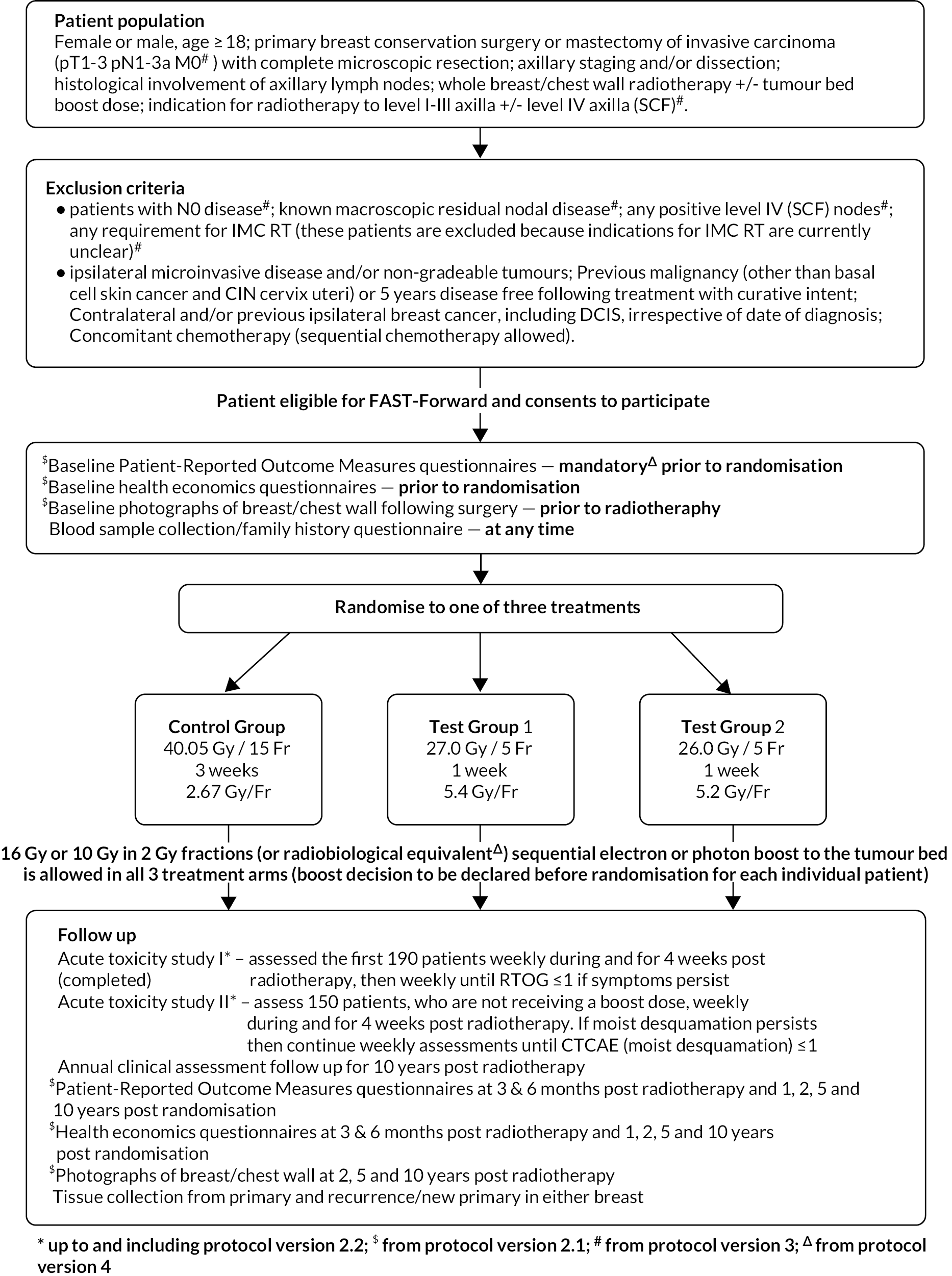
The Nodal Sub-Study (from Protocol v3.0, 8 July 2015) was an extension to the FAST-Forward Main Trial, maintaining the original design as a phase III randomised clinical trial but restricted to patients prescribed RT to level I-III axilla and/or level IV axilla (SCF) in addition to the breast/chest wall area. Trial schemas for the Main Trial and the Nodal Sub-Study are shown in Figures 1 and 2, respectively.
FIGURE 1.
FAST-Forward schema: Main Trial.
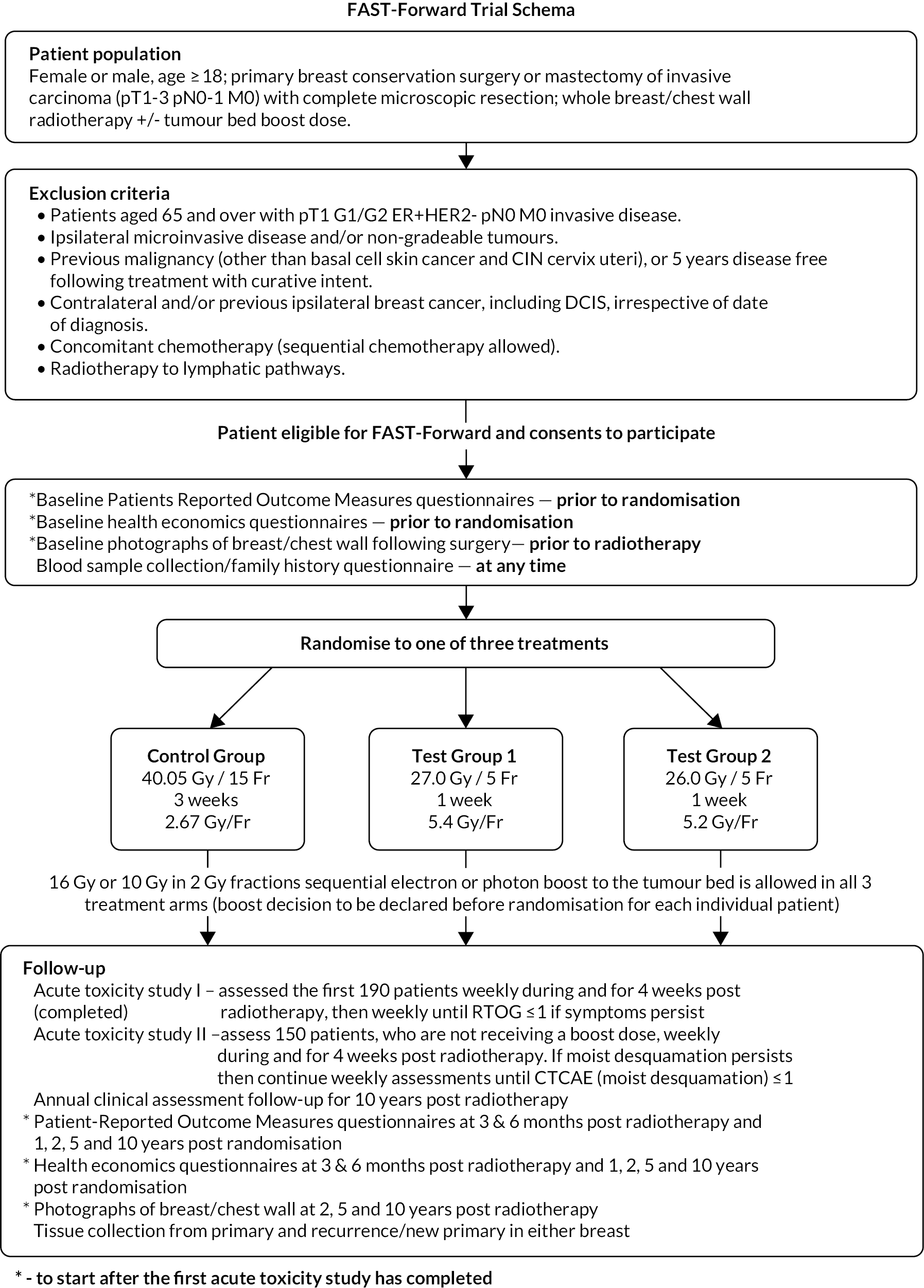
FIGURE 2.
FAST-Forward schema: Nodal Sub-Study.
Test dose levels were informed by α/β estimates describing the fractionation sensitivity of late normal tissues obtained from the START and FAST trials. 17,47 Assuming α/β = 3 Gy and no effect of overall time on outcomes, 27 Gy/5 fractions of 5.4 Gy was predicted to match late NTE of 40 Gy/15 fractions of 2.7 Gy or 46 Gy/23 fractions of 2 Gy. Allowance for a possible effect of treatment time informed the choice of the slightly lower 26 Gy dose level. A 3-group design was used for the trial to allow interpolation between the two test doses in order to estimate the 5-fraction dose equivalent to 40 Gy in 15 fractions in terms of local tumour control, and late NTE.
From Protocol v5.0 (14 December 2017) onwards the trial design of the Nodal Sub-Study was amended to a 2-group trial, with no further randomisation to Test Group 1 (27 Gy in 5 fractions of 5.4 Gy). This amendment was on the advice of the Independent Data Monitoring Committee (IDMC) and Trial Steering Committee (TSC). Further details are presented in the Study Conduct section (see Chapter 2).
Embedded sub-studies
Acute toxicity
Acute skin reactions are more related to the total dose and less sensitive to fraction size than late-reacting normal tissues. Therefore the lower total doses under test in the trial are expected to reduce their severity and duration, despite the shorter overall treatment time. In order to confirm these relationships two acute toxicity sub-studies were undertaken during 2011 and 2013 in patients entered into the Main Trial from a subset of centres. Details of the acute toxicity sub-studies are presented in Chapter 4.
Patient-reported outcomes
There is evidence that RT causes long-term effects on quality of life in terms of altered breast appearance, breast, arm and shoulder symptoms, as well as a possible impact on some general aspects such as fatigue. Results from the START trial highlighted the value of patients’ self-reported post-RT symptoms in discriminating between RT regimens in favour of hypofractionation. 48 The PRO sub-study within FAST-Forward aimed to provide subjective views of key breast symptoms and body image following treatment, to add supportive data in the comparison of a trade-off between local tumour control and AE of treatment. A subset of Main Trial centres participated in the PRO sub-study. For the Nodal Sub-Study PROs were mandatory since the primary outcome was patient-assessed. Details of the PRO methods are included later on in this chapter.
Photographic assessments
Photographic assessments of change in breast appearance following RT as used in other trials including START, IMPORT LOW and HIGH, and FAST, provide an objective assessment of late AE, since they are scored by independent observers blinded to treatment allocation and patient identity. The same centres involved in the PRO sub-study also participated in the photographic assessments. Details of the photographic assessment methods are included later on in this chapter.
Health economics
The HE evaluation aimed to establish the cost-effectiveness of a 5-fraction schedule of curative RT compared with current UK practice using quality of life and health resource data, and health states including cancer relapse. Quality of life and health resource use data were collected on the PRO questionnaires for those patients participating in the PRO sub-study. Details of the HEs evaluation are presented in Chapter 6.
Blood and tissue collection
All patients were asked to consent to donate a single blood sample and complete a family history questionnaire; these were collected at any point during the trial. All patients were also asked to consent to the donation of a tissue sample from their original tumour and to the donation of a tissue sample should a relapse occur. The consent ensured that the samples remain a resource for future use, pending separate funding for translational research.
Participants
Patient selection
Women and men with complete microscopic resection of early invasive breast cancer following BCS or mastectomy prescribed local RT.
Eligibility for Main Trial
Eligibility criteria for the Main Trial from protocol version 2.3 (11 November 2013; last version of Main Trial protocol before addition of Nodal Sub-Study) are as follows:
Main Trial inclusion criteria1
-
Age ≥ 18 years.
-
Female or male.
-
Primary invasive carcinoma of the breast.
-
Breast conservation surgery or mastectomy (reconstruction allowed, providing port of a tissue expander positioned outside the breast).
-
Complete microscopic excision of primary tumour.
-
Axillary staging and/or dissection.
-
pT1-3 pN0-1 M0 disease.
-
Written informed consent.
-
Able to comply with follow-up.
Main Trial exclusion criteria
-
Age ≥ 65 years with pT1 G1/2 ER+ve/HER-2−ve pN0 M0 invasive disease (from protocol v2.0).
-
Ipsilateral microinvasive disease and/or non-gradeable tumours.
-
Past history of malignancy except (1) basal cell skin cancer, (2) cervical intra-epithelial neoplasia (CIN) cervix uteri or (3) non-breast malignancy allowed if treated with curative intent and > 5 years disease-free.
-
Contralateral and/or previous ipsilateral breast cancer, including ductal carcinoma in situ (DCIS), irrespective of date of diagnosis.
-
Concurrent cytotoxic chemotherapy (sequential neoadjuvant or adjuvant cytotoxic therapy allowed with ≥ 2 weeks between therapy and RT).
-
Radiotherapy to any regional lymph node area (excepting lower axilla included in standard tangential fields to breast/chest wall).
Eligibility for Nodal Sub-Study
Protocol Version 3.0 onwards included additional requirement for lymphatic RT. Typical examples of indications for lymphatic RT include (1) Patients with positive lymph nodes removed by axillary clearance who require RT to level I–III axilla and/or level IV axilla (SCF). (2) Patients with sentinel node-positive axillary disease not proceeding to axillary dissection and who require RT to level I–III axilla and/or level IV axilla (SCF). (3) Patients treated by pre-operative systemic therapy who are recommended post-operative RT to level I–III axilla and/or level IV axilla (SCF).
Eligibility criteria for the Nodal Sub-Study from protocol version 4.0 (24 February 2017) onwards are as follows:
Nodal Sub-Study inclusion criteria2
-
Age ≥ 18 years.
-
Female or male.
-
Primary invasive carcinoma of the breast.
-
Breast conservation surgery or mastectomy (reconstruction allowed, providing port of a tissue expander positioned outside the breast).
-
Complete microscopic excision of primary tumour.
-
Axillary staging and/or dissection.
-
pT1-3 pN1-3a M0 disease.
-
Histological involvement of axillary lymph nodes.
-
Indication for RT to level I-III axilla and/or level IV axilla (SCF).
-
Written informed consent.
-
Able to comply with follow-up.
Nodal Sub-Study exclusion criteria
-
Ipsilateral microinvasive disease and/or non-gradeable tumours.
-
Past history of malignancy except (1) basal cell skin cancer, (2) CIN cervix uteri or (3) non-breast malignancy allowed if treated with curative intent and >5 years disease-free.
-
Contralateral and/or previous ipsilateral breast cancer, including DCIS, irrespective of date of diagnosis.
-
Concurrent cytotoxic chemotherapy (sequential neoadjuvant or adjuvant cytotoxic therapy allowed with ≥ 2 weeks between therapy and RT).
-
Patients with N0 disease.
-
Known residual macroscopic nodal disease.
-
Any positive level IV (SCF) nodes.
-
Requirement for IMC RT3.
The eligibility criteria of Fast-Forward Main Trial were amended in versions 2.0 (13 February 2013), 2.2 (2 May 2013) and for the Nodal Sub-Study in versions 3.0 (8 July 2015) and 4.0 (24 February 2017) of the study protocol, as shown in Appendix 2, Table 34.
Eligibility for PRO and photographic assessment sub-studies
For the PRO sub-study all the patients at those centres participating were eligible (and all patients in the Nodal Sub-Study). All patients who had BCS were eligible for the photographic sub-study at participating centres, with the intention that the same group of patients were included in the PRO and photographic studies.
Protocol amendments
Protocol amendments for the FAST-Forward are summarised in Appendix 2, Table 35. The current approved version of the protocol is v5.1 (5 February 2018).
Trial procedures
Participating centres
All hospitals in the UK were invited to participate in the trial and were designated as treating (RT) and referring (non-radiotherapy) centres. All centres had to obtain the necessary regulatory approvals and RT centres completed a rigorous radiotherapy quality assurance (RT QA) approval process (see Chapter 3 for details of RT QA programme). A site initiation visit was performed either in person or by telephone prior to opening to recruitment. Radiotherapy centres identified eligible patients, obtained informed consent, treated patients and performed assessments according to the schedule. Non-radiotherapy hospitals performed the same activities except for the RT and acute toxicity assessments. Centre staff are listed in Table 30.
Patient information and informed consent
Eligible patients (see eligibility criteria) were identified during breast cancer multidisciplinary meetings or from clinic lists at participating sites. Patients were invited to participate in the FAST-Forward Trial during consultations in oncology clinics, where treatment options were discussed. Here the local principal investigator, coinvestigator or other trained healthcare professionals, discussed the trial with the patient and provided them with a copy of the patient information sheet. Patients were given at least 24 hours to consider participation in the trial, to discuss this with their family and friends and to ask questions of the clinical team. Patients who were willing to participate were asked to provide written informed consent to the principal investigator, co-investigator or other trained individual. All patients from the start of the trial were asked to provide consent for the tissue and blood sub-studies. Other optional sub-studies were included in subsequent versions of the consent form.
There were two consent forms available at the start of the trial:
-
For centres participating in the acute toxicity sub-studies (seven centres).
-
For centres participating in the PROMS and photographic sub-studies.
There were two consent forms available from Protocol v2.1:
-
For centres opting to participate in the PRO and photographic assessment sub-studies.
-
For all other centres.
There was only one consent form from Protocol v3.0 (inclusion of Nodal Sub-Study) as the PRO assessments were mandatory, since the primary endpoint of this sub-study was patient-assessed arm/hand swelling.
Randomisation
Patients in the Main Trial and Nodal Sub-Study were allocated to 40.05 Gy in 15 fractions, 27.0 Gy in 5 fractions or 26.0 Gy in 5 fractions in a 1 : 1 : 1 ratio. The 27.0 Gy group in the Nodal Sub-Study was closed to recruitment from Protocol v5.0 (14 December 2017) onwards (as described in Protocol amendments and Study Conduct sections). Treatment allocation was not blinded to patients or clinicians. Randomisation was performed by recruiting centres contacting The Institute of Cancer Research-Clinical Trials and Statistics Unit (ICR-CTSU) by telephone or fax. The randomisation allocation method was computer-generated random permuted blocks [mixed block sizes 6 and 9 (changed to 4 and 6 following the amendment of Nodal Sub-Study to 2-group design) to avoid predictability]. Patients in the Main Trial were stratified according to RT centre and risk group (high: < 50 years or grade 3 vs. low: ≥ 50 years and grade 1 or 2). Patients in the Nodal Sub-Study were stratified according to RT centre and whether or not the patient had a level II/III axillary clearance. A unique trial identifier was assigned to each patient comprising components for centre number, patient number and designated stratification.
Radiotherapy procedures
The whole breast clinical target volume (CTV) including the soft tissues from 5 mm below the skin surface to the deep fascia was either determined retrospectively from field-based tangential fields or volumed prospectively. Post-mastectomy chest wall CTV encompassed post-surgical skin flaps and underlying soft tissues to the deep fascia; both excluded underlying muscle and rib cage. Surgeons were strongly encouraged to mark the tumour cavity walls with titanium clips or gold seeds at the time of BCS in order to aid placement of tangential fields and delineation of tumour bed. A typical margin of 10 mm was added around the breast/chest wall CTV accounting for set-up error, breast swelling and breathing to create a planning target volume (PTV). A full 3D CT set of outlines covering the whole breast and organs at risk was collected with a slice separation up to 5 mm and organs at risk were outlined. A tangential opposing pair beam arrangement encompassed the whole breast/chest wall PTV, minimising the ipsilateral lung and heart exposure.
The lymph node CTV included the axillary chain and/or the supraclavicular nodes (level IV axilla). The axilla could be treated in its entirety, that is levels I–IV, or only the levels specified by the clinician. For the level IV axilla (SCF) PTV a maximum of 5 mm margin was applied medially in order to limit the dose to midline structures.
The treatment plan was optimised with 3D dose compensation to achieve the following PTV dose distribution: > 95% received 95% of prescribed dose, < 5% received ≥ 105%, < 2% received ≥ 107% and global maximum < 110%. Dose constraints for the control group were: volume of ipsilateral lung receiving 12 Gy < 15%, and volume of heart receiving 2 Gy and 10 Gy < 30% and < 5%, respectively. Dose constraints for the 5-fraction schedules were: volume of ipsilateral lung receiving 8 Gy < 15%, and volume of heart receiving 1.5 Gy and 7 Gy < 30% and < 5%, respectively.
X-ray beam energies for treatment were 6 megavoltage (MV) or 10 MV, but a mixture of energies, for example 6 MV and 10–15 MV was allowed for larger patients. Tumour bed boost was delivered via electrons or photons. Verification was carried out using electronic portal imaging using MV or kV X-rays. Control group treatment verification was required for at least three fractions in the first week with correction for any systematic error and then once weekly with a tolerance of 5 mm. The 5-fraction schedules required verification imaging for each fraction with recommendations to correct all measured displacements. A comprehensive quality assurance programme involved every RT centre before trial activation and continued throughout trial accrual (see RT QA Chapter 3).
The RT planning packs for the Main Trial and Nodal Sub-Study are available on the ICR-CTSU FAST-Forward Trial webpage at www.icr.ac.uk/fastforward.
Trial assessments
Table 1 shows the schedule of assessments for all patients participating in the trial.
| Treatment | Follow-up (all taken from date of randomisation except where shown) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Prior to randomisation | Post-randomisation | wk 1 | wk 2 | wk 3 | Weekly for 4 weeks post-RT | mth 3 | mth 6 | yr 1 | yr 2 | yr 3 | yr 4 | yr 5 | yr 6 | yr 7 | yr 8 | yr 9 | yr 10 | |
| Pre-RT | Post-RT | ||||||||||||||||||
| Eligibility checklist | x | ||||||||||||||||||
| Informed consent | x | ||||||||||||||||||
| Randomisation checklist | x | ||||||||||||||||||
| RT QA | Prior to centre initiation and throughout the trial recruitment period | ||||||||||||||||||
| 3D RT planning | x | ||||||||||||||||||
| RT treatment | x | x a | x a | ||||||||||||||||
| RT verification | Up to daily during treatment | ||||||||||||||||||
| Serious adverse event (if applicable) | x | x | x | x | x | ||||||||||||||
| Acute toxicity assessmentsb | Study I (first 190 patients) | x | x | x a | x a | x | |||||||||||||
| Study II (150 patients, no boost) | x | x | x a | x a | x | ||||||||||||||
| Follow-up – annual clinical assessment (all patients) | x | x | x | x | x | x | x | x | x | x | |||||||||
| Sub-studies | |||||||||||||||||||
| PROMSc | x (baseline d ) | x | x | x | x | x | x | ||||||||||||
| Photographic assessmentc | x | x | x | x | |||||||||||||||
| HEs – annual assessment | x (baseline d ) | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Lymphatic RT including PROMSe | x (baseline d ) | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Blood sample collection and family history questionnaire | At any time during the trial, ideally by the end of RT | ||||||||||||||||||
| CT scan if recurrence | At the time of recurrence | ||||||||||||||||||
| Tissue collection 1 tumour recurrence/new 1 tumour |
As requested during the trial | ||||||||||||||||||
Patients were assessed by clinicians for ipsilateral breast tumour relapse (IBTR) and late NTE at annual follow-up visits. Starting 12 months after trial entry, late NTE in ipsilateral breast/chest wall (breast distortion, shrinkage, induration and telangiectasia; breast/chest wall oedema and discomfort) were graded by clinicians on a 4-point ordinal scale (none, a little, quite a bit or very much), interpreted as none, mild, moderate or marked. In addition, patients in the Nodal Sub-Study were assessed annually for the presence of breast lymphoedema, arm lymphoedema and sensorimotor symptoms. Symptomatic rib fracture, symptomatic lung fibrosis and ischaemic heart disease were recorded.
In the PRO sub-study, questionnaires were administered at baseline (pre-randomisation), 3, 6, 12, 24 and 60 months, including the EORTC QLQ-C30 core questionnaire, EORTC QLQ-BR23 breast cancer module, EORTC QLQ-FA13 fatigue module, body image scale (BIS) and protocol-specific questions relating to changes to affected breast following treatment (including breast appearance changed, smaller, harder/firmer, skin appearance changed). Patient assessments used a 4-point ordinal scale (not at all, a little, quite a bit, very much). The EuroQol 5D-5L (EQ-5D-5L) was also included in the PRO questionnaire booklets and healthcare service use questions.
All patients in the Nodal Sub-Study were asked to consent to the PRO sub-study and photographic assessments. The following PRO were included in the Nodal Sub-Study (but not the Main Trial): patient assessments of shoulder stiffness, upper limb pain, sensorimotor symptoms and arm function.
In the photographic sub-study, photographs were taken at baseline, 2 and 5 years after RT. At each visit two frontal views of the chest were taken, one with hands on the hips and the other with hands raised as far as possible above the head; photographs excluded the patient’s head. Change in photographic breast appearance and distortion compared with the post-surgical (pre-radiotherapy) baseline were scored on a 3-point ordinal scale (none, mild or marked) based on changes in breast size and shape relative to the contralateral breast. Patients were ineligible for further photographic assessments following breast reconstruction surgery and further ipsilateral disease. Digital photographs were scored by three observers blind to patient identity and treatment allocation following scoring procedures established in the START trials. 49 Breast size and surgical deficit were assessed from the baseline photographs on a 3-point scale (small, medium, large).
Data management
Data collection
All participating centres were supplied with NCR case report form (CRF) booklets and paper copies of the baseline PRO booklets. Site staff were trained in trial procedures during the site initiation visit which was performed either in person or by telephone. Data were collected according to the schedule in Table 1 and used to populate the CRFs. All CRFs and baseline PRO booklets were returned to the ICR-CTSU where they were logged into a tracking database. The clinical data were entered into a clinical database (MACRO).
Data queries
Visual checks were performed on all CRFs received at ICR-CTSU. Consistency checks of the data were written into the clinical database to identify any discrepancies in the data entered. Data queries were raised for these anomalies, discrepancies and omissions and were sent to centres on a regular basis. Queries were resolved by centres and returned to ICR-CTSU.
Central statistical data monitoring
Details of data monitoring were described in the FAST-Forward Central Statistical Data Monitoring Plan (v1.0 May 2018). Checks of the data stored on the MACRO database were performed regularly and on an ongoing basis, including prior to each analysis for an IDMC report, and queries raised with the study sites. In addition, a formal comparison between FAST-Forward CRFs received (clinical CRFs and PRO booklets) and data entered onto the MACRO database was done for 5% of Main Trial patients for all CRFs from baseline up to and including year 5 follow-up. The error rate found in this data quality control exercise was very low (0.2% of items checked).
Site monitoring
The quality of the data received from sites was assessed on a regular basis by the number of data queries generated per centre. Early intervention with the centre was initiated to discuss the queries and to avoid repeat of the errors. Central statistical monitoring of the data was also performed on a regular basis as described above. No centres were identified as needing an on-site monitoring visit.
Data assumptions/coding
Adverse effects reported under ‘other’ on the annual follow-up forms were reviewed (blind to randomised treatment schedule) by the Chief Investigator and coded according to whether they were likely to be radiotherapy-related. Prior to the Main Trial primary analysis all reported local and regional relapses were reviewed (blind to randomised treatment schedule) along with pathology reports to check coding of the relapses, including location and whether invasive or DCIS.
Endpoints
Efficacy endpoints
The protocol specified that ipsilateral tumour relapse and contralateral primary tumour must be confirmed by cytological/histological assessment. Distant relapse was to be determined by an appropriate combination of clinical, haematological, imaging and pathological assessment, recognising that pathological confirmation was not always possible. Patients remained evaluable for local relapse after distant relapse. Patients without an event were censored at the last follow-up assessment or death. Patients were to have annual clinical assessments for 10 years and annual mammograms for 5 years or until screening age if younger (as per NICE guidelines). 50
Primary endpoint (Main Trial)
-
Ipsilateral local tumour control, where an event was defined as any tumour relapse (invasive or non-invasive) or a new primary tumour in the ipsilateral breast unless confirmed by pathology to represent nodal relapse, in which case classified as regional, see below. Analyses used the date an event was confirmed.
Secondary disease-related and survival endpoints (Main Trial and Nodal Sub-Study)
-
Contralateral breast tumours (invasive and non-invasive).
-
Relapse-free survival, defined as time from randomisation until local relapse, regional relapse (axilla, SCF or other regional) or distant relapse (originating from the primary breast tumour).
-
Disease-free survival, defined as time from randomisation to first confirmed occurrence of local relapse, regional relapse, distant relapse, contralateral breast cancer or death due to breast cancer.
-
Time to distant metastases, defined as time from randomisation to the first confirmed occurrence of any distant metastases originating from the original breast cancer. For patients with second cancers reported and metastases, centres were asked to confirm that the metastases had originated from the primary breast cancer.
-
Overall survival, defined as time from randomisation to death from any cause.
-
Other second primary cancers, reported as incidence of other second primary cancers.
Endpoints of late adverse effects of radiotherapy
Patient questionnaires were to be completed at baseline, 3, 6, 12, 24 and 60 months after randomisation. Clinical assessments of radiotherapy-related late AE were carried out annually from the date of randomisation up to at least 5 years.
Primary endpoint (Nodal Sub-Study)
-
Patient-assessed arm/hand swelling from EORTC QLQ-BR23 breast cancer module (Q18: Did you have a swollen arm or hand?). Scored on a 4-point ordinal scale: ‘not at all’, ‘a little’, ‘quite a bit’, ‘very much’.
Secondary adverse effect endpoints (Main Trial and Nodal Sub-Study)
Patient and clinician assessments of late RT AE were scored on a 4-point scale: ‘not at all’ (none), ‘a little’, ‘quite a bit’, ‘very much’.
-
Patient self-assessments of late AE: These were assessed using the EORTC QLQ-BR23 breast cancer module, BIS and additional protocol-specific items relating to AE in the breast and arm/shoulder/hand. Key AE specific to the Nodal Sub-Study included shoulder stiffness, upper limb pain, sensorimotor symptoms and arm function.
-
Clinician assessments of late AE: The following late AE were assessed on a 4-point ordinal scale: breast distortion, breast shrinkage, breast induration (tumour bed), breast induration (outside tumour bed), telangiectasia, breast/chest wall oedema and breast/chest wall discomfort. A composite endpoint of any clinician-assessed late AE in the breast was defined as the worst grade reported for breast distortion, breast shrinkage, breast induration, telangiectasia and breast oedema. Breast discomfort was not included in the composite endpoint as it was not an external assessment by the clinician (scored by asking the patient), unlike the other assessments. AEs reported under ‘other’ were classified according to whether they were likely to be radiotherapy-related and analysed separately from the other clinician-assessed AE listed above (not included in the composite endpoint as some effects reported under ‘other’ on the CRF were indicated as having resolved prior to the follow-up visit and so were no longer present, unlike the other clinician-assessed effects). Clinical assessments for the Nodal Sub-Study included the following additional AE reported as present/absent (with laterality): breast lymphoedema, arm lymphoedema and sensorimotor symptoms. A separate sensorimotor symptoms CRF was completed if symptoms were noted on the follow-up form.
-
Incidence of symptomatic rib fracture, symptomatic lung fibrosis and ischaemic heart disease.
-
Specialist referral for management of radiotherapy-related AE.
Statistical considerations
Sample size
Main Trial
The target sample size for the Main Trial was 4000 patients, with numbers balanced between randomised groups. This provides 80% power (one-sided α = 0.025 to allow for one-sided hypothesis and a Bonferroni correction accounting for comparisons between each test group and the control group) to exclude an absolute increase of 1.6% in 5-year IBTR incidence for a 5-fraction schedule versus control. A 5-year rate of 2% in the 40 Gy schedule was assumed (using START trial data and allowing for reduction in IBTR due to evolution of systemic therapy and surgical techniques). The 1.6% absolute non-inferiority margin was defined at the trial design stage by the protocol development group including clinicians and patient advocates and was considered to be acceptable. Binary proportions were used for the sample size calculations due to the low expected event rates. Estimates allowed for 10% loss to follow-up or unevaluable (primarily due to development of metastatic disease). A total of 2196 patients (732 per group) was estimated for the photographic and PRO sub-studies to provide 80% power to detect an 8% difference in the 5-year prevalence of late NTE between the 5-fraction schedules (assuming 35% with 5-year mild/marked change in photographic breast appearance from START-B 40 Gy results), allowing for 10% loss to follow-up or unevaluable.
Nodal Sub-Study
The sample size for the Nodal Sub-Study was based on the primary endpoint of patient-assessed arm swelling at 5 years. The original design of the sub-study had three groups that mirrored the Main Trial design but was revised to a 2-group design in Protocol v5.0 (14 December 2017), when accrual into Test Group 1 (27 Gy) was closed. The original and revised sample size justifications for the Nodal Sub-Study are given below.
Original target sample size for Nodal Sub-Study
The target sample size was 627 patients with numbers balanced equally in each of the three randomised groups. This provided 90% power (one-sided α = 0.025 to allow for one-sided hypothesis and multiple testing) to exclude an arm swelling rate at 5 years of 20% in each of the test groups compared to an assumed rate in the control group of 10% (allowing for 10% attrition due to illness or death based on experience from the START trial).
Revised target sample size for Nodal Sub-Study
From Protocol v5.0 onwards the target sample size for the Nodal Sub-Study was reduced to 344 (172 in each of the Control group and Test Group 2). This provided 90% power (one-sided α = 0.05) to exclude an arm swelling rate of 20% in Test Group 2 compared with an assumed rate of 10% in the Control group at 5 years (allowing for 10% attrition). With the numbers recruited into Test Group 1 up until Protocol v5.0, comparison of arm swelling between Test Group 1 and the Control group would have approximately 73% power using the same assumptions as above.
Statistical methods
Baseline characteristics
Baseline patient, clinical and treatment characteristics were described according to randomised group using summary statistics, with no formal comparative tests as these data would be expected to be balanced between the groups due to randomisation.
Primary endpoint (Main Trial)
The number of IBTR primary endpoint events were tabulated by treatment group. Survival analysis methods compared IBTR between treatment groups, censoring patients at date of death or last follow-up. Kaplan Meier methods were used to estimate event rates and graphically display local control rates and cumulative incidence in each treatment group. Kaplan–Meier estimates of 5-year IBTR cumulative incidence were presented along with 95% CI for each group and each Test Group was separately compared with the Control group using the log-rank test.
Crude estimates of treatment effect for each Test Group compared with the Control group were described by HRs (with 95% CI) obtained from an unadjusted Cox regression model. All comparisons were expressed relative to the Control group so that a HR less than one indicates a decreased risk of the event in the Test Group. Absolute treatment differences were calculated (by applying the HRs to the control group’s 5-year event-free estimate) along with both one- and two-sided 95% CI (calculated from the CIs for the HRs and the control group 5-year event-free estimate). As the protocol-defined non-inferiority margin is an absolute excess of 1.6%, the primary assessment of non-inferiority was whether the upper limit of the two-sided 95% CI for the absolute difference in 5-year local tumour control (calculated as above) is < 1.6%.
Additionally, non-inferiority of each Test Group versus Control was tested using the a priori critical HR of 1.8 (ln0.964/ln0.98, derived from the absolute rates specified in the protocol); a p-value of < 0.025 will be deemed statistically significant (the probability of incorrectly accepting an inferior test group treatment). Assessment of non-inferiority using the HR, a measure of relative rather than absolute treatment effect, will aid interpretation of the primary endpoint results particularly if the 5-year rate of ipsilateral disease events in the control group is < 2% assumed in the protocol, in which case relative excess may be deemed important to consider. If non-inferiority in terms of the a priori critical HR of 1.8 was not shown, a sensitivity analysis would be carried out using methods stated above to test the critical HR calculated from the observed (rather than protocol-specified) 5-year event-free rate in the control group and 1.6% absolute excess in the test groups. An exploratory competing risks analysis was done for IBTR, with death from any cause as a competing event in a Fine–Gray competing risks regression model.
Primary endpoint (Nodal Sub-Study)
The primary outcome of patient-assessed arm/hand swelling (‘Did you have a swollen arm or hand?’ Q18 from EORTC QLQ-BR23 breast cancer module) was summarised at each time point from baseline up to 2 years using frequencies and percentages of each response category (‘not at all’, ‘a little’, ‘quite a bit’, ‘very much’) according to treatment group.
Secondary endpoints
Other efficacy outcomes were tabulated in the Main Trial according to treatment group, with deaths presented according to whether or not breast cancer-related or cardiac-related. Analyses of other efficacy outcomes including regional relapse, distant metastases, disease-free survival and overall survival used survival analysis methods as described for IBTR above. Analysis of efficacy outcomes in the Nodal Sub-Study will await 5-year follow-up, when a meta-analysis is conducted with data from the Main Trial.
Clinician and patient assessments of late NTE were tabulated for each treatment group and time point separately for the Main Trial and the Nodal Sub-Study. Descriptive data are shown for the Nodal Sub-Study up to 2 years for PRO and to 3 years for clinical assessments. Formal statistical analysis of primary and secondary endpoints for the Nodal Sub-Study will await 5-year follow-up (as specified in the protocol).
NTE endpoints in the Main Trial were dichotomised as ‘not at all’/’a little’ (none/mild) versus ‘quite a bit’/’very much’ (moderate/marked) and analysed as follows: (1) cross-sectional analyses at key time points compared prevalence of moderate/marked effects versus none/mild between groups using risk differences (95% CI), and Fisher’s exact test; (2) longitudinal regression analyses of moderate/marked effects (vs. none/mild) using generalised estimating equations (GEE), comparing groups across the whole follow-up period including assessments from all timepoints using (ORs, 95% CI) and the Wald test; GEE models included a term representing years of follow-up to assess time trends. RRs are not presented in this report for the 5-year cross-sectional analyses of clinician and patient-assessed NTE since they may be open to over-interpretation given the low absolute event rates for many of the endpoints, as reflected by the wide CIs. Survival analysis methods analysed time to first moderate/marked clinician-assessed NTE; Kaplan–Meier estimates of cumulative incidence were obtained and groups compared using HR (95% CI) from Cox proportional hazards (PH) regression and the pairwise log-rank test. The PH assumption was not met for patient-assessed NTE endpoints, and so time-to-event analyses were not performed.
For the Main Trial photographic assessment scores for change in photographic breast appearance at 2 and 5 years were modelled using GEE. Categories of mild and marked change in photographic breast appearance were combined for analysis as there were very few with marked change. Pairwise comparisons of mild/marked change at 2 and/or 5 years between groups were described by OR (95% CI) obtained from the GEE models and the Wald test.
For the Nodal Sub-Study comparisons between the Control group and Test Group 1 only included Control group patients randomised concurrently (i.e. up to end of 2017 prior to protocol amendment dropping Test Group 1).
For all patient and clinician-assessed NTE endpoints a significance level of 0.005 was used for Main Trial analyses, to allow for multiple testing. No formal testing is done for the Nodal Sub-Study at this stage of follow-up.
Estimates of fractionation sensitivity (α/β values, 95% CI) were obtained for the Main Trial primary endpoint of IBTR and late NTE using methodology from the START and FAST trials. 17,47 The α/β estimate for breast cancer was obtained from a Cox PH regression model of time to first IBTR, and for late NTE from GEE models including all follow-up assessments (separate models for clinician and photographic assessments). Terms for total dose (D) and total dose multiplied by fraction size (Dxd) were included in each model; the α/β ratio was calculated by dividing the two-parameter estimates (D/Dxd), with an estimated 95% CI derived using the covariance of the two estimates (lower confidence limits truncated at zero). Isoeffect equivalent dose in 2 Gy fractions (EQD2) were calculated for both 5-fraction schedules, together with an estimate of the 5-fraction schedule isoeffective in terms of local control and late NTE with 40 Gy in 15 fractions.
There were no pre-planned subgroup analyses for the Main Trial specified in the protocol. Exploratory post hoc subgroup analyses were conducted in the Main Trial for the primary endpoint according to risk factors including age, grade, pathological tumour size, pathological node status, ER/progesterone receptor (PR)/HER-2 status, tumour bed boost and adjuvant chemotherapy. Additionally, exploratory post hoc analyses of NTE in the Main Trial were done according to age, breast size, surgical deficit, tumour bed boost and adjuvant chemotherapy.
There were no formal interim analyses other than for the acute toxicity studies; accumulating data were monitored annually by the IDMC. All analyses were performed on an intention-to-treat basis that included all patients according to their allocated treatment regardless of what was actually received. As the main hypothesis for the Main Trial was non-inferiority the primary endpoint was also tested in the per-protocol population excluding patients for whom a major treatment deviation was reported.
The database snapshot for the Main Trial was taken on 22 November 2019 and Stata® version 15 (StataCorp LP, College Station, TX, USA) used for analyses. For the 3-year descriptive analysis of the Nodal Sub-Study the database snapshot was taken on 1 December 2021; Stata version 17 (StataCorp) was used for the Nodal Sub-Study analyses.
Study conduct
Patient and public involvement
FAST-Forward was part of a broader portfolio of patient-centred ICR-CTSU and National Cancer Research Institute (NCRI) Breast Group trials where patient representatives with lived experience of breast cancer have been partners from concept, formalising their role as members of the protocol working group and Trial Management Group (TMG). In FAST-Forward patient representatives helped shape the trial with active involvement from trial concept, through recruitment, reviewing trial documents, discussing trial results prior to publication and helping to write lay summaries of the results for trial participants. A link to the lay summary of the Main Trial results that was distributed to trial participants following publication of the primary analysis in 2020 can be found on the ICR-CTSU FAST-Forward webpage (www.icr.ac.uk/fastforward). Our patient and public involvement (PPI) partnerships enabled design of an efficient and cost-effective follow-up schedule that truly reflects the patient experience with minimal burden and is patient-centred.
In addition, ongoing links have been forged with local (Royal Marsden) and national (Independent Cancer Patients’ Voice, UK Breast Intergroup, NCRI) patient advocate groups, embedding patient representation in the whole clinical trial lifecycle. PPI work in breast RT trials co-ordinated by ICR-CTSU has included development of alternative patient information formats, focus groups where patients emphasised the need for information on risks/benefits of RT at all stages of treatment and recovery, and future plans to collaborate within a PhD project optimising data capture of AE from trial participants.
The TMG had preferentially at least two patient representatives which enabled mutual support and empowerment. All TMG members were included in any email discussions, encouraging PPI input at all times. A mentoring programme for new TMG members was also initiated which included conversations with the Chief Investigator and PPI lead at ICR-CTSU.
Trial oversight committees
The TMG, TSC and IDMC each worked to a charter issued by ICR-CTSU and met annually until the time of Main Trial publication, with the TMG and TSC continuing to meet thereafter.
Trial Management Group
The FAST-Forward Trial Management Group was responsible for day-to-day trial conduct. Membership included the Chief Investigator, additional Principal Investigators from recruiting sites across the UK, physics and radiographer staff from recruiting sites, members of the NCRI radiotherapy trials quality assurance (RTTQA) group, key ICR-CTSU staff and patient representatives. Members are listed in the Acknowledgements section.
Trial Steering Committee
The ICR-CTSU Breast RT Trials Steering Committee (Chair Professor Malcolm Mason) provided strategic oversight of the study on behalf of the Funder and Sponsor and met annually from the start of the trial. The independent membership comprised four members (listed in Acknowledgements).
Independent Data Monitoring Committee
The IDMC (Chair Professor Matthew Sydes) reviewed emerging trial data on an annual basis. Following each meeting, the IDMC reported their recommendations to the TSC. There were no stopping rules for the Main Trial (only the acute toxicity sub-studies), and no formal interim analyses were done. Following review of the report prepared for the IDMC meeting in October 2016 the IDMC recommended to the TSC that the 27 Gy/5 fractions group be dropped from the Nodal Sub-Study, which was recruiting at the time. The IDMC reviewed emerging results of the Main Trial at a median follow-up of 3 years and recommended presentation or publication of the 3-year NTE data to inform the worldwide evidence base. 51 The IDMC noted that previous breast RT trials (START, FAST and IMPORT LOW) confirmed that normal tissue effect rates at 3 years had been shown to predict 5- and 10-year comparisons. Absolute rates of moderate/marked NTE in the Main Trial were reassuringly low at 3 years, but there was evidence of increased late toxicity for Test Group 1 compared with the control schedule, which although statistically significant was not felt by the TSC and TMG to be clinically significant, as the rate of NTE was comparable to that after 50–52 Gy in 2.0 Gy fractions. Data were shared confidentially with the TSC following further discussions with the IDMC in 2016/2017, and the TMG produced a guidance document for the IDMC to aid interpretation of differences in late NTE between the schedules. It was concluded that Test Group 1 was no longer needed in the Nodal Sub-Study, given that the optimal 5-day schedule was highly unlikely to involve fraction sizes > 5.2 Gy. This allowed simplification of the trial design for the Nodal Sub-Study, allowing a 2-group design to test the safety of 26 Gy in 5 fractions against the Control schedule of 40 Gy in 15 fractions. The design of the Nodal Sub-Study was revised to a 2-group trial in December 2017. IDMC members are listed in the Acknowledgements section.
Approvals, reporting and compliance
FAST-Forward was sponsored by The Institute of Cancer Research and was registered as ISRCTN19906132.
FAST-Forward was approved by the London-Brighton and Sussex Research Ethics Committee (REC) (previously named South East Coast – Kent REC then South East Coast – Brighton and Sussex REC) on 2 September 2011 (11/LO/0958). Local Research and Development department approval was obtained at each participating NHS trust before patients were randomised. The trial was conducted in accordance with the principles and guidelines of Good Clinical Practice, UK legislation, ICR-CTSU Standard Operating Procedures and the REC- and MHRA-approved protocol. All patients provided written informed consent.
The current trial protocol is available online at the ICR-CTSU website www.icr.ac.uk/fastforward.
Chapter 3 Radiotherapy quality assurance
As stated in the Methods (see Chapter 2), all participating centres in the Fast-Forward Trial underwent a comprehensive RT QA programme.
Background
The complex nature of modern RT carries inherent problems both in ensuring reproducibility and accuracy within a RT unit and, more particularly, when carried out on a multicentre basis. Specific issues in the treatment of the breast/chest wall and nodal region arise from the geometry and proximity of the associated treatment volumes, with important radiation-sensitive structures underlying the breast, chest wall and nodal region including the lung, heart and brachial plexus.
Careful localisation, computerised planning, accurate verification of beam position and meticulous attention to alignment and matching during treatment are essential.
A QA programme is ‘a mandatory prerequisite when aiming at high dose, high precision radiotherapy’ and is an integral component of any RT trial as defined by the EORTC guidelines for trial protocols in RT. 52,53
In this multicentre randomised trial, the RT QA programme enabled confirmation that technical guidelines within the protocol were understood and implemented correctly by participants and that the dose prescription was delivered according to protocol, together with appropriate documentation of technique and patient-related data. This ensured that clinical observations in terms of tumour control and normal tissue damage reflect differences in the randomised schedules rather than departures from trial protocol. Techniques used have been documented and these data are available should differences in observed outcomes emerge.
In this way the definition of RT QA as ‘all those planned and systematic actions necessary to provide adequate confidence that a product will satisfy given requirements of quality’54 can be satisfied and the scientific worth of the parent trial be validated.
The RT QA programme for the Fast-Forward Trial was built on the foundations of the START and IMPORT trials. This has provided an element of consensus in RT techniques among RT centres. FAST-Forward helped with the implementation of new technology in some centres where the use of intensity-modulated radiotherapy (IMRT) or image-guided radiotherapy (IGRT) had not been used previously.
Plan of investigation
The RT QA programme followed the guidelines set out by the EORTC53 and the RT QA component of the trial was coordinated by the National RTTQA Group based at Mount Vernon Cancer Centre. 55,56 A total of 47 RT centres took part in the UK over a 3-year period 2011–2014. Protocol Versions 1.0–2.2 of the RT QA programme applied to whole breast/chest wall RT and, from version 3.0 onwards, centres had to gain additional QA approval for nodal RT.
The RT QA programme included both pre-trial and on-trial components. It was completed by all participating centres, unless streamlined through the IMPORT trial, and is detailed below:
Pre-trial QA
-
A facility questionnaire and process document collating details of techniques, equipment and processes to be used within the centre for FAST-Forward patients.
-
A dummy-run (centre-selected sample patient) plan containing target and organ-at-risk (OAR) structures outlined by the principal investigator and used to create a clinical plan, was assessed to ensure adherence to the trial protocol.
-
Once the nodal study was introduced through Protocol v3.0, each centre was required to undergo credentialing for lymphatic nodal outlining and planning through completion of an outlining and a planning benchmark case (RTTQA Group provided cases). Centres were also asked to submit an amended process document detailing additional information in relation to treatment of patients randomised to the nodal study.
A visit by the RTTQA Group was also performed prior to centre participation to validate, independently, the technique used against the information given in the process document. In particular, the following parameters were assessed:
-
Target volume and treatment technique used.
-
Confirmation of plan modulation – implementation, for example IMRT forward planning.
-
Planning of dose distribution across the treatment volume for homogeneity and prescription points.
-
Assessment of routine quality checks (QC) performed by the centre compared with current IPEM guidelines. 57
-
Dose measurements across the treatment volume within a purpose-made phantom, if not performed for the same technique within the last 3 years.
-
Assessment of imaging verification protocol and technique.
On-trial QA
-
A prospective review of the outlines and RT plan was performed for the first breast and first chest wall patients recruited from each centre prior to the patient starting treatment. On introduction of the nodal study a prospective review of the nodal CTV outlines and RT plan was conducted for the first patient recruited at each centre. Feedback was provided to the investigator site within 48 hours of receipt of data. If any major protocol variations were identified, additional reviews were requested.
-
All patients’ RT planning CT, plan file, dose file, structure set and plan assessment forms were anonymised by the centre and submitted electronically to the RTTQA Group.
-
Retrospective outlining and planning reviews were performed to monitor continued protocol compliance. Any protocol variations were reported and discussed with the Chief Investigator (CI) and ICR-CTSU.
Quality control by department for IMRT
Where a centre had an established IMRT programme that had been previously credentialled by the RTTQA Group for an equivalent trial, some aspects of the FAST-Forward RT QA programme were streamlined. Where an established IMRT programme was not set up, additional QA was required such as verification of fluence maps for each field.
Analysis of the RT QA programme
The data from the RT QA programme have been analysed separately from the Main Trial. Any major variations from trial protocol would have been notified to the ICR-CTSU, the CI and participating centres. These included:
-
Discrepancies in documentation, dose prescription and dose recording.
-
Failure to meet upper and lower dose limits for treatment volumes.
-
Systematic errors of technique in any stage of treatment from planning through to implementation.
Detailed analysis of the RT QA data has produced quality information covering the following areas:
-
variations in breast RT practice in participating centres
-
a comparison of methods used for IMRT (multiple static fields, dynamic fields)
-
an assessment of the emerging technologies and their quality control
-
quantification of dose uniformity during the treatment period.
Results
A number of studies were carried out by the RTTQA Group evaluating the data collected within FAST-Forward. These are summarised below:
-
Field-based and volume-based PTVs as plan evaluation structures in the UK FAST-Forward breast trial. 58
Centres were initially given the option of reporting either a field-based or volume-based breast PTV. Analysis of the initial 338 plans from eight centres showed that the majority of centres were using a field-based technique (seven centres). This study also showed that in some patients there were significant areas of high dose (105% of prescription) outside of the reported volume-based PTV. In order to ensure consistency in reporting across all patients and to ensure that all regions of high dose were captured within the reported PTV, field-based PTV reporting was used for all patients within the trial. Field-based PTVs were added by the RTTQA Group for any patients that were submitted with volume-based PTV.
-
Interim analysis of treatment plans in the FAST-Forward hypofractionation breast RT trial. 59
This study analysed the RT treatment plans collected and evaluated their compliance with the trial protocol. An interim analysis was performed when the first 504 plans were available. This demonstrated that 95.6% of these plans complied with the dose requirements of the FAST-Forward protocol, but highlighted differences in OAR doses and PTV coverage related to the treatment technique used. Centres using field-based planning (the majority) included less lung and heart in the field compared to volume-based planning.
-
Results from the RT QA programme for the FAST-Forward breast trial. 60
Additional work was carried out to analyse the RT treatment plans collected in the FAST-Forward Trial to monitor consistency of treatment across all centres and compliance with trial protocol.
The review performed on 3600 plans submitted as part of the FAST-Forward Trial demonstrated that, overall, the quality of the RT treatment plans had been maintained across centres. The majority of plans (97.3%) complied with the dose objectives specified in the protocol, with only 2.7% of all reviewed data not achieving one or more of the dose objectives. On further examination more than half of these variations were either unavoidable or not significant. Unavoidable variations (1.5%) included complex cases where a clinical compromise was deemed necessary, for example eight plans exceeded the V105% and V107% tolerances with higher doses to a non-clinically significant volume. Avoidable variations (0.8% of plans) included misinterpretation of the DVH information at the investigator site and/or inaccurate contouring of evaluation structures.
-
Variability of contouring using the European Society for Radiotherapy and Oncology (ESTRO) guideline for elective breast cancer RT. 61
As part of the FAST-Forward Nodal Sub-Study, interobserver variability of nodal contouring was evaluated against the ESTRO consensus guideline for elective breast cancer RT.
It was concluded that contouring of the regional lymph nodes using the ESTRO consensus guidelines reduced interobserver variability in volumes contoured. 62 Results suggested that experience and/or training is associated with less interobserver variability, promoting the role of RT QA when adopting new outlining guidelines as part of a multicentre trial. In addition, the methodology developed helped to inform evaluation of nodal contouring within the trial as part of prospective and retrospective analyses.
Discussion
Radiotherapy quality assurance plays an important role in ensuring trial protocol compliance and consistency in delivery of RT across multiple centres; establishing best practice and improving RT standards. The benefits of RT QA have been shown by numerous clinical trials in ensuring that clinical outcomes reflect differences in randomisation schedules rather than departures from trial protocol. 63 It is particularly important when introducing novel techniques and frequently centres use clinical trial participation to implement new techniques supported by trial protocol and comprehensive RT QA programmes. The pre-trial and on-trial RT QA programmes implemented for the FAST-Forward Trial monitored the RT planning and delivery with the aim to deliver precise radiation dose to the target, maximising the dose to the tumour and minimising the dose to surrounding organs at risk, in order to improve the therapeutic index. 64 A particular benefit of the detailed QA programme for this trial was that it gave confidence in adopting IGRT in breast cancer RT – which was not common, and often regarded as a low priority. This has benefits for patients both within and outside of the trial.
Findings from the RT QA studies provided reassurance that all FAST-Forward participating centres could comply with the standards required by the trial protocol. The first study examined the use of field-based and volume-based PTV to assess the suitability as breast plan evaluation structures. This informed centres on the difference between the two techniques and enabled centres to make a more informed choice. 58–61 Further studies looked to monitor the consistency of RT treatment across all centres and compliance with the trial protocol. The majority of the plans were protocol compliant and any deviations were explored for future guidance. 60 The variability of contouring using the ESTRO consensus guidelines was reviewed as the FAST-Forward Nodal Sub-Study required delineation of axillary nodal CTV comprising of levels 1–461 and nodal outlining was not routine practice across UK centres. The study helped to identify systematic regions of variance and informed the continued development of the RT planning and delivery guidelines in order to improve conformity. These studies demonstrate the importance of a robust RT QA process, particularly when introducing new techniques, either in clinical studies or in routine care.
Chapter 4 Acute toxicity sub-studies
Background
Acute or short-term effects of RT occur during and shortly after RT. Acute effects are commonly reddening of the skin, usually mild, but occasionally leading to severe redness, or blistering of the skin. They can also include discomfort, swelling and fatigue. The severity of acute side effects is related to total dose given, rather than dose per fraction. In FAST-Forward, the severity of acute side effects was not expected to be worse with the 1-week schedules, but actually to be milder, and shorter-lived due to the lower total dose. This was supported by previous studies such as START and FAST, where in a subset of patients, acute toxicity was evaluated and found to be milder in patients having hypofractionated schedules, compared to the standard 50 Gy in 25 fractions. However, despite this, many clinicians and patients assume that the higher dose per fraction would lead to much more severe reactions, quoting that it would cause severe reactions, or ‘burns’. It was therefore decided to carry out a detailed assessment of acute side effects in the first cohort of patients recruited at a subset of centres participating in the FAST-Forward Main Trial.
Methods
Patients and centres
The acute toxicity sub-studies were conducted in a subset of centres (Royal Marsden Hospital, Royal Cornwall Hospital, University Hospitals of North Midlands, Derriford Hospital Plymouth, Norfolk and Norwich University Hospitals and Addenbrookes Hospital) that had the infrastructure necessary to carry out weekly toxicity assessments. The same centres participated in both sub-studies, with the addition of Torbay Hospital in the second sub-study. Patients receiving a RT tumour bed boost were eligible for the first acute toxicity sub-study but were excluded from the second acute toxicity sub-study since the objective of this sub-study was to quantify the toxicity of 5-fraction schedules relative to control, effects that are independent and additive to those of the boost. At centres participating in the acute toxicity sub-studies, consecutive eligible patients were invited to participate at the time of consent to the Main Trial.
Assessments
Acute reactions of the skin of the treated breast were graded using RTOG criteria for the first sub-study (Protocol version 1.0). Following discussions with both the FAST-Forward IDMC and TSC, it was agreed that this may not be the most appropriate scoring system in this context due to the inclusion of oedema in the RTOG system, since the primary concern was to score moist desquamation. Therefore, it was agreed that a second sub-study would be undertaken using standard common toxicity criteria for adverse effects (CTCAE) criteria (v4.03) (Protocol versions 2.1 and 2.2). Toxicity assessments were made by a healthcare professional at each participating centre using predesigned forms. The assessments were scheduled to be carried out weekly during treatment and for 4 weeks following the end of RT. In the second sub-study, if moist desquamation beyond skin folds or creases (CTCAE grade 3) were seen during this time, weekly assessments were to continue until the reaction resolved to CTCAE grade 1 or less. If any assessment was missed, the centre contacted the patient by telephone to assess and grade acute skin reactions by asking the patient to describe the skin appearance and supplementing this by direct questions. In the first acute toxicity sub-study patient self-assessments of acute AE (breast soreness, reddening, swelling and blistering) were recorded using patient diary cards completed weekly during RT and for at least 4 weeks post-RT. The scores were recorded as ‘none’, ‘a little’, ‘quite a bit’ or ‘very much’. If symptoms persisted, patients were asked to continue scoring their AE on a weekly basis until all scores were graded as ‘none’ or ‘a little’. The submitted patient diaries showed low data completion to the extent that the results were not considered of value to be included in the submitted manuscript.
Acute skin reactions scoring scales
Modified RTOG scale (acute toxicity study I)
-
Grade 0: No visible change.
-
Grade 1: Faint/dull erythema.
-
Grade 2: Tender/bright erythema ± dry desquamation.
-
Grade 3: Patchy moist desquamation, moderate oedema.
-
Grade 4: Confluent moist desquamation, pitting oedema.
CTCAE version 4.03 (acute toxicity study II)
-
Grade 1: Faint erythema or dry desquamation.
-
Grade 2: Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin fold and creases; moderate oedema.
-
Grade 3: Moist desquamation in areas other than skin folds and creases; bleeding induced by minor trauma or abrasion.
-
Grade 4: Life-threatening consequences; skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site; skin graft indicated.
-
Grade 5: Death.
Endpoints
The primary endpoint for each of the sub-studies was the proportion of patients within each treatment group with grade ≥ 3 toxicity (RTOG and CTCAE, respectively) at any time from the start of RT to 4 weeks after completion of RT. The secondary endpoints were clinical assessment of (1) any acute skin toxicity, defined as the worst grade reported from the start of RT to 4 weeks post-RT, (2) acute skin toxicity during RT, defined as the worst grade reported from the start to the end of RT, (3) acute skin toxicity post-RT, defined as the worst grade reported at completion of RT until at least 4 weeks post-RT and (4) adherence to the acute toxicity assessments.
Statistical considerations
Each sub-study aimed to recruit approximately 150 evaluable patients (50 per group) in order to exclude a within-group rate of grade ≥ 3 acute skin reactions of over 11% compared to target rate of under 3% (89% power and one-sided 7.9% significance level). The sample size was informed by data from the FAST pilot trial. 26
Principal analyses were based on the evaluable population, defined as all patients randomised into the study receiving at least one fraction of RT (regardless of whether they were later found to be ineligible or a protocol violator) and with complete data, or, at most, one missing toxicity assessment. For the first sub-study which included some patients receiving a boost, only non-boost assessments were used to define inclusion in the evaluable population. The required number of assessments for a 40 Gy/15 fractions patient was seven (three during and four post-RT) and five for 27 Gy/5 fractions and 26 Gy/5 fractions patients (one during and four post-RT). Given the focus of the sub-studies on safety patients who switched to receive a different trial treatment after randomisation were analysed according to treatment received rather than randomised treatment.
For the primary endpoint, the proportion of patients within each treatment group with grade ≥ 3 RTOG toxicity (first sub-study) or grade ≥ 3 CTCAE toxicity (second sub-study) was estimated with associated upper one-sided 95% CI. Secondary endpoints were estimated as frequencies and percentages, with the prevalence of grades ≥ 1, ≥ 2 and ≥ 3 toxicity at each time point presented graphically. Adherence to toxicity assessments was estimated as frequencies and percentages. Toxicity data were included as reported, and there were no restrictions based on the date of assessment. The acute toxicity sub-study was designed to be non-comparative so no statistical comparisons were made across the treatment groups.
Results
Recruitment to the first acute toxicity sub-study opened in November 2011 and closed in May 2012. Of 194 potentially eligible patients recruited in sub-study centres, 190 patients consented (63, 63 and 64 in the 40 Gy/15 fractions, 27 Gy/5 fractions and 26 Gy/5 fractions groups, respectively). Recruitment was performed at seven treating centres across the UK with the infrastructure to carry out weekly toxicity assessments.
Recruitment to the second acute toxicity sub-study opened in April 2013 and closed in February 2014. Of 269 potentially eligible patients recruited in the 8 sub-study centres, 162 patients consented (55, 44 and 63 in the 40 Gy/15 fractions, 27 Gy/5 fractions and 26 Gy/5 fractions groups, respectively). Two patients subsequently withdrew consent for all data to be used. The number of patients potentially eligible, consented, receiving allocated RT and evaluable for the primary endpoint for each sub-study is given in Figure 3.
FIGURE 3.
Flow diagram of participation in the first and second acute toxicity sub-studies. Reproduced from Brunt et al. (CC BY 4.0). 3 aAll patients recruited at sub-study centres during sub-study period; bAll patients recruiting at sub-study centres during sub-study period for whom a boost was not planned.
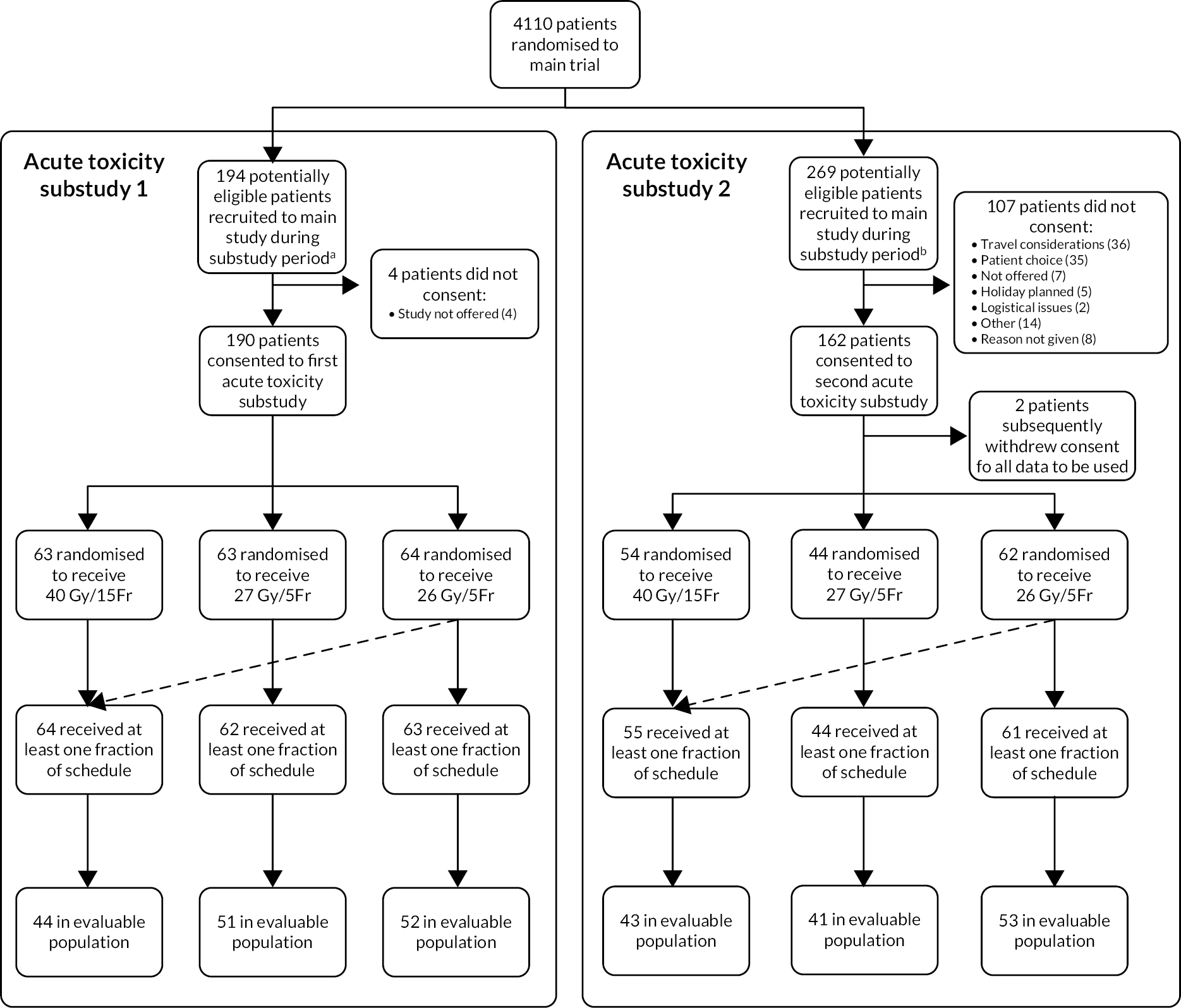
Patients recruited to the sub-studies were broadly comparable with those of the whole trial population. 3 Radiotherapy treatment characteristics (for each sub-study separately) are presented in Appendix 2, Table 36, suggesting no significant imbalances between groups. Compliance to toxicity assessments by treatment group is shown in Appendix 2, Table 37. For the first acute toxicity sub-study, worst RTOG grade experienced by treatment received is reported in Table 2. The proportions of evaluable patients with grade 3 RTOG toxicity during the acute phase were as follows: 40 Gy/15 fractions 6/44 (13.6%); 27 Gy/5 fractions 5/51 (9.8%); 26 Gy/5 fractions 3/52 (5.8%). Twenty-nine patients in the first sub-study received a boost to their treatment, of which 22 were evaluable. No evidence of a higher rate of grade 3 toxicity was observed in this subset of patients [40 Gy/15 fractions 1/9 (11.1%); 27 Gy/5 fractions 0/10; 26 Gy/5 fractions 0/3).
| Worst RTOG grade (on or post-RT) | 40 Gy/15 fractions (N = 44), N (%)a | 27 Gy/5 fractions (N = 51), N (%)a | 26 Gy/5 fractions (N = 52), N (%)a |
|---|---|---|---|
| 0 | 0 | 2 (4) | 3 (6) |
| 1 | 14 (32) | 24 (47) | 32 (62) |
| 2 | 24 (55) | 20 (39) | 14 (27) |
| 3 | 6 (14) | 5 (10) | 3 (6) |
| 4 | 0 | 0 | 0 |
| Percentage of RTOG grade 3+ (upper limit of one-sided 95% CI) | 13.6 (25.2%) | 9.8 (19.5%) | 5.8 (14.2%) |
For the second acute toxicity sub-study, worst CTCAE grade experienced by treatment received is reported in Table 3. The proportions of evaluable patients with a grade 3 CTCAE toxicity during the acute phase were as follows: 40 Gy/15 fractions 0/43; 27 Gy/5 fractions 1/41 (2.4%); 26 Gy/5 fractions 0/53. Grade 2 toxicity was largely due to moderate to brisk erythema, with only three patients with moderate oedema (two 40 Gy/15 fractions and one 26 Gy/5 fractions patient), see Appendix 2, Table 38. CTCAE toxicity score reported at each time point is presented graphically during RT and post-RT (see Figure 4). A single patient in the second cohort randomised to the 27 Gy/5 fractions group after mastectomy, adjuvant cytotoxic chemotherapy and chest wall RT with bolus was assessed as a CTCAE grade 3 toxicity recorded after a missed assessment by telephone appointment 36 days after start of treatment, having been assessed as grade 2 7 days before and assessed with grade 1 erythema 7 days later.
| CTCAE grade | 40 Gy/15 fractions (N = 43), N (%)a | 27 Gy/5 fractions (N = 41), N (%)a | 26 Gy/5 fractions (N = 53), N (%)a |
|---|---|---|---|
| 0 | 0 | 3 (7) | 3 (6) |
| 1 | 21 (49) | 26 (63) | 31 (58) |
| 2 | 22 (51) | 11 (27) | 19 (36) |
| 3 | 0 | 1 (2)b | 0 |
| 4 | 0 | 0 | 0 |
| Proportion grade 3+ (upper limit of one-sided 95% CI) | 0 (6.7%) | 2.4 (11.1%) | 0 (5.5%) |
FIGURE 4.
Acute toxicity sub-study 2 – prevalence of grade 1+, grade 2+ and grade 3+ CTCAE toxicity. Reproduced from Brunt et al. (CC BY 4.0). 3 Source: Grade 3 toxicity reported at 4 weeks post-RT in 27 Gy in 5 fractions patient resolved to grade 1 1 week later.
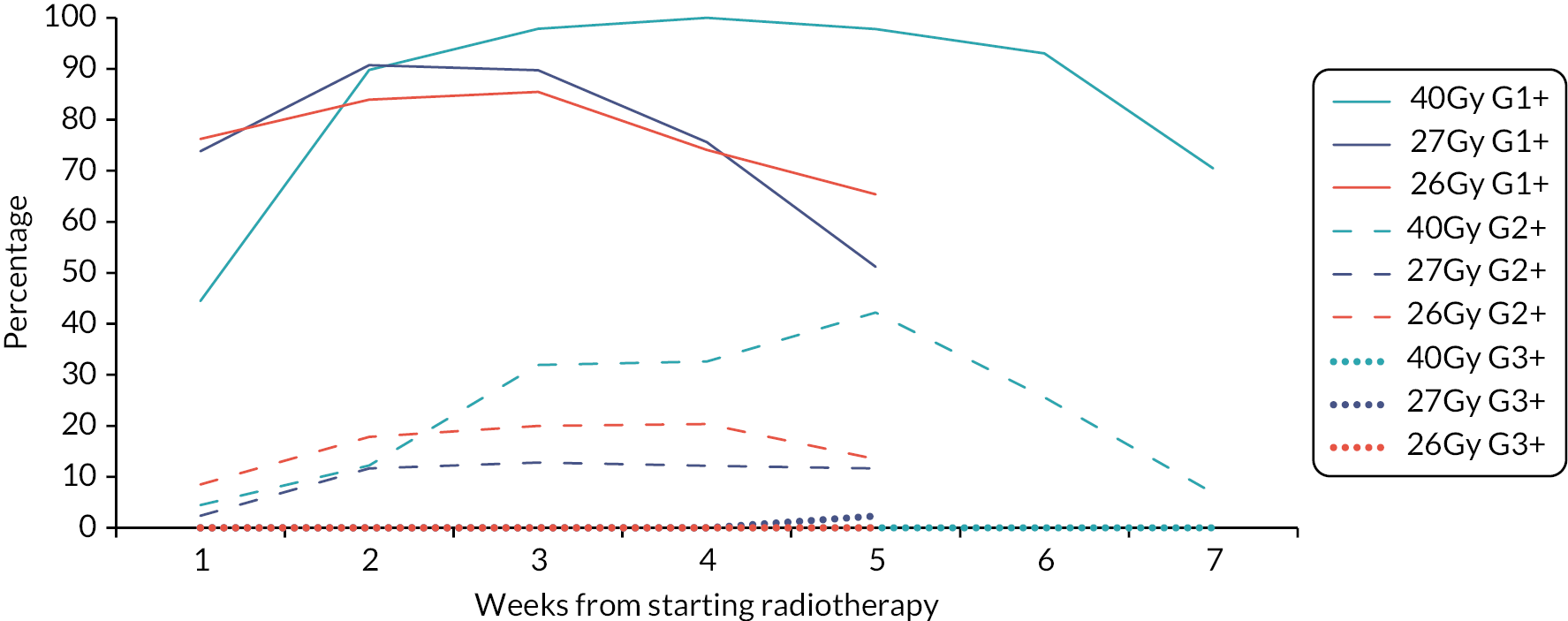
Discussion
The acute skin toxicity sub-studies were not designed to involve statistical hypothesis testing across treatment groups, but to confirm low incidence rates of clinically significant acute skin toxicity associated with each schedule. On this basis, the results raised no concerns that the 5-day schedules led to more severe or longer-lasting acute skin reactions compared with 40 Gy in 15 fractions. The prevalence rates suggested that erythema after the 1-week schedule was less intense and settled about 2 weeks earlier than after the 3-week schedule. An imbalance in numbers in the 27 Gy group was attributed to the play of chance, since consent to participate in the sub-study was obtained prior to allocation of randomised treatment. Overall compliance with the toxicity assessments was high, making it unlikely that patients developing severe skin reactions were under-reported. No suggestion of a dose–response was noted between the two dose levels of the 5-fraction test regimens. The results of the first toxicity sub-study were consistent with those from the second sub-study, despite the unhelpful inclusion of oedema with erythema and desquamation in the RTOG system used for scoring early skin reactions.
The mildness of the acute skin toxicity associated with the 5-fraction regimens was expected. A series of classic studies investigating the dependence of acute skin reactions on total dose, fraction size, interfraction interval and overall treatment time was undertaken in the 1980s and 1990s by Turesson and colleagues, using reflectance spectrophotometry to quantify erythema and clinical grading to score moist desquamation. 65–67 These confirmed the absence of a treatment time effect for the first 4 weeks of RT delivered using fraction sizes of 2.0 Gy and 4.0 Gy delivered using 12 MeV electrons to the IMC daily or twice per week. 65 Using the same experimental system, whereby different fractionation regimens were delivered to the right and left IMCs of the same patient, weak dependence of erythema and desquamation on fraction size was demonstrated, with estimates of α/β ratios between 7.5 Gy and 11.2 Gy. 66 In the context of the FAST-Forward Trial, the reductions in total dose, from 40 Gy to 27 Gy and 26 Gy appear to compensate for increased dose per fraction where acute skin reactions are concerned. In conclusion, the acute skin toxicity sub-studies conducted in patients entered into the FAST-Forward Trial raised no concerns and showed that short-term effects are if anything milder, and shorter lasting.
Chapter 5 Main Trial
Recruitment
Between November 2011 and June 2014, 4110 patients were enrolled in the FAST-Forward Trial from 97 UK centres (47 RT centres and 50 referring hospitals; Appendix 2, Table 31). The trial completed recruitment 17 months ahead of schedule (see Figure 5). One-ninety patients were recruited into acute toxicity study 1, 161 patients into acute toxicity study 2, 1798 patients into the PRO sub-study, 1737 patients into the photographic assessment sub-study, 3878 patients consented to donate a blood sample and 4077 patients consented to donate their primary tissue sample.
FIGURE 5.
Target and actual recruitment for FAST-Forward Main Trial.
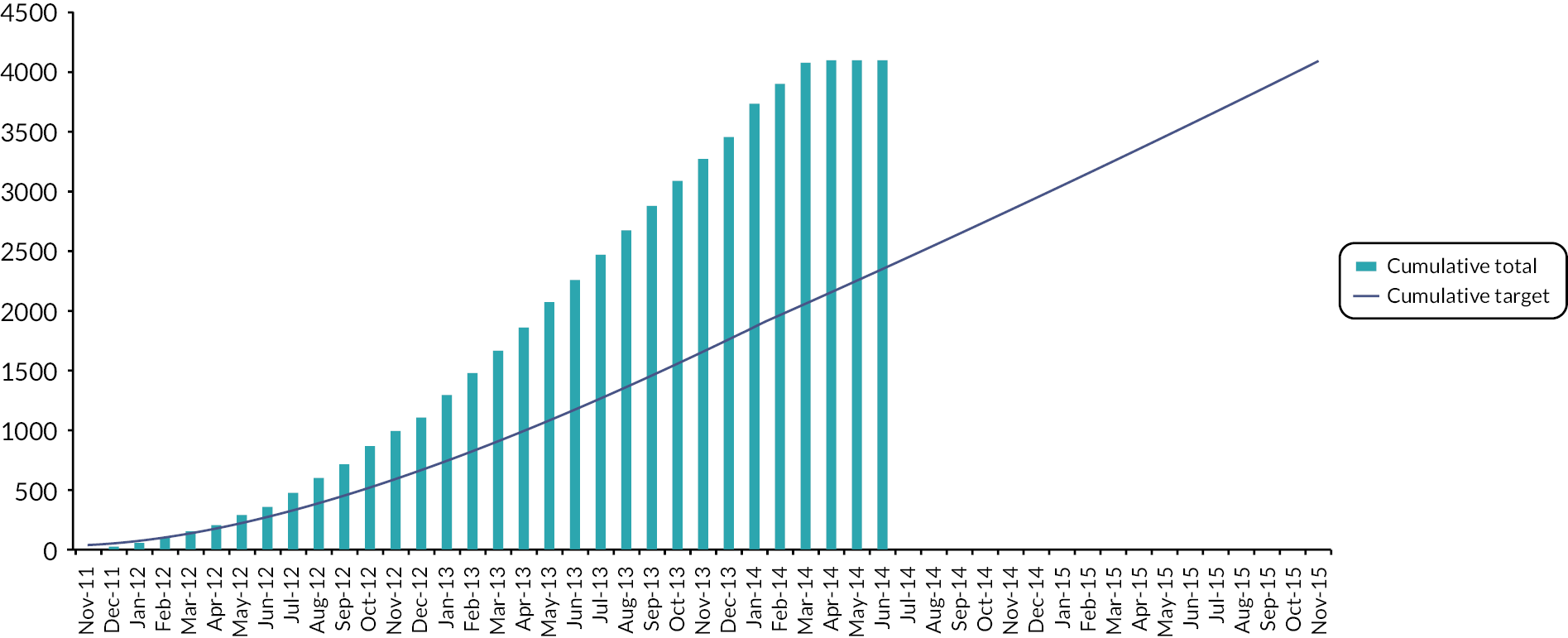
The original target for recruitment was based on five centres opening to recruitment once the trial opened with a further one centre expected to open per month until a total of 25 centres were open to recruitment. Each centre was predicted to recruit four patients per month. Using these figures it was predicted that recruitment would take 54 months to attain. Appendix 3, Figure 20 shows that over 100 centres opened to recruitment which allowed the target to be reached 17 months early. Seventy-two centres recruited at a rate of at least one patient a month.
Deviations and withdrawals
Fourteen patients withdrew consent for use of data and were removed from the intention-to-treat population, therefore results are reported for 4096 consenting participants. A total of 40 patients (7 in 40 Gy, 12 in 27 Gy, 21 in 26 Gy) did not receive the allocated therapy (see Figure 6); compliance with allocated treatment was therefore 99% (4056/4096). These 40 patients included six found to be ineligible after randomisation: two in the 27 Gy group (one with relapse detected after recruitment so RT cancelled, and one with tissue expander in situ) and four in the 26 Gy group (three with metastases detected before start of RT, and one with previous DCIS in contralateral breast).
FIGURE 6.
FAST-Forward Main Trial Profile (CONSORT flow diagram). Reproduced from Brunt et al. (CC BY 4.0). 1 aFourteen patients withdrew consent for any of their data to be used in the analysis (7 × 40 Gy, 3 × 27 Gy, 4 × 26 Gy); bOne no RT given as patient unable to get into stable position for RT; 3 given 40 Gy in 15 fractions: one concern for brachial plexus, one decided on different treatment plan, one constraints of treatment planning; cOne no RT given as patient diagnosed with pemphigoid; Eight given 40 Gy in 15 fractions: one dose constraints not met, one unable to plan within protocol constraints due to tumour bed position, one poor PTV coverage, one technical difficulties in planning, one transferred to direct electron field, one simulator plan as 3D images not possible, one small pericardial effusion found at planning, one reason not given. ITT = intention to treat.

A total of 22 patients withdrew from clinical follow-up before 5 years, and one was lost to follow-up. Of the 1798 patients who consented to participate in the PRO sub-study, 181 had withdrawn from the PRO questionnaires by 5 years.
Data returns
Baseline forms were received for 100% out of those expected. Data return rates of annual follow-up forms were over 90%, with 5-year visit forms available for 3733 (98%) patients out of 3798 still in follow-up (not died, withdrawn or lost). Data returns for individual forms are shown in Appendix 2, Table 39 and are correct up to 1 March 2022.
Baseline data
Demographic and clinical characteristics at baseline were well-balanced between groups (see Table 4). Overall, 2551/4096 (62%) were classified as low-risk (age > 50 and grade 1 or 2); the majority were ER positive/HER-2 negative (3335/4077 with data, 82%), and 407/4077 with data (10%) were HER-2 positive. 3832 (93%) had BCS, 1011 (25%) received a RT boost to tumour bed, 1174 (29%) had neo-adjuvant or adjuvant chemotherapy, 3512/3649 (96%) ER positive patients had endocrine therapy and 311/407 (76%) HER-2 positive patients received trastuzumab. Medical history details at randomisation are presented in Appendix 2, Table 40 and RT details are in Table 5.
| 40 Gy/15 fractions (N = 1361), (%) | 27 Gy/5 fractions (N = 1367), (%) | 26 Gy/5 fractions (N = 1368), (%) | |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 60 (53–66) | 61 (53–67) | 61 (52–66) |
| Range | 29–89 | 25–90 | 25–89 |
| < 40 | 12 (1) | 16 (1) | 28 (2) |
| 40–49 | 186 (14) | 173 (13) | 189 (14) |
| 50–59 | 440 (32) | 423 (31) | 414 (30) |
| 60–69 | 506 (37) | 511 (37) | 524 (38) |
| 70–79 | 175 (13) | 197 (14) | 172 (13) |
| ≥ 80 | 42 (3) | 47 (3) | 41 (3) |
| Sex | |||
| Female | 1355 (99) | 1365 (99) | 1362 (99) |
| Male | 6 (< 1) | 2 (< 1) | 4 (< 1) |
| Not known | 0 | 0 | 2 |
| Tumour grade | |||
| 1 | 315 (23) | 315 (23) | 300 (22) |
| 2 | 660 (48) | 663 (48) | 690 (50) |
| 3 | 386 (28) | 389 (28) | 378 (28) |
| Risk group | |||
| Low (age ≥ 50 and grade 1 or 2) | 843 (62) | 854 (62) | 854 (62) |
| High (age < 50 and/or grade 3) | 518 (38) | 513 (37) | 514 (38) |
| Primary surgery | |||
| BCS | 1270 (93) | 1278 (94) | 1284 (94) |
| BCS with oncoplastic technique | 42 | 33 | 42 |
| Mastectomy | 91 (7) | 89 (6) | 84 (6) |
| Mastectomy with immediate reconstruction | 8 | 11 | 7 |
| Type of reconstruction (mastectomy patients): | |||
| Autologous reconstruction | 5 | 7 | 3 |
| Implant-based reconstruction | 2 | 4 | 4 |
| Reconstruction type not specified | 1 | 0 | 0 |
| Side of primary | |||
| Left | 726 (53) | 674 (49) | 662 (48) |
| Right | 635 (47) | 693 (51) | 704 (51) |
| Not known | 0 | 0 | 2 |
| Maximal extent of axillary staging | |||
| Sentinel node biopsy/guided axillary sampling | 1157 (85) | 1184 (87) | 1164 (85) |
| Axillary clearance | 200 (15) | 181 (13) | 201 (15) |
| Other | 4 (<1) | 2 (<1) | 1 (<1) |
| Not known | 0 | 0 | 2 |
| Pathological node status | |||
| Positive | 257 (19) | 243 (18) | 256 (19) |
| Negative | 1103 (81) | 1124 (82) | 1110 (81) |
| Not known | 1 | 0 | 2 |
| Histological type | |||
| Infiltrating ductal | 1084 (80) | 1096 (80) | 1086 (80) |
| Lobular | 144 (11) | 139 (10) | 127 (9) |
| Mixed | 51 (4) | 63 (5) | 65 (5) |
| Other | 82 (6) | 69 (5) | 87 (6) |
| Not known | 0 | 0 | 3 |
| Pathological tumour size (cm) | |||
| Median (IQR) | 1.6 (1.1–2.2) | 1.6 (1–2.2) | 1.6 (1.1–2.4) |
| pT stage | |||
| T1mi | 4 (< 1) | 5 (< 1) | 6 (< 1) |
| T1a | 69 (5) | 68 (5) | 51 (4) |
| T1b | 258 (19) | 270 (20) | 256 (19) |
| T1c | 612 (45) | 601 (44) | 602 (44) |
| T2 | 394 (29) | 389 (28) | 424 (31) |
| T3 | 21 (1) | 30 (2) | 25 (2) |
| Not known | 3 | 4 | 4 |
| ER/HER-2 status | |||
| ER+/HER-2+ | 103 (8) | 103 (8) | 93 (7) |
| ER+/HER-2− | 1108 (82) | 1130 (83) | 1097 (81) |
| ER−/HER-2+ | 32 (2) | 34 (2) | 42 (3) |
| ER−/HER-2− | 111 (8) | 96 (7) | 128 (9) |
| Not known | 7 | 4 | 8 |
| PgR status | |||
| Positive | 577 (73) | 541 (70) | 566 (70) |
| Negative | 212 (27) | 229 (30) | 245 (30) |
| Not done | 571 | 596 | 555 |
| Missing on form | 1 | 1 | 2 |
| Lymphovascular invasion | |||
| Present | 186 (14) | 178 (14) | 202 (15) |
| Absent | 1085 (83) | 1084 (83) | 1055 (81) |
| Uncertain | 34 (3) | 40 (3) | 51 (4) |
| Not known | 56 | 65 | 60 |
| Neo-adjuvant chemotherapy received | |||
| Yes | 48 (3) | 56 (4) | 43 (3) |
| No | 1312 (97) | 1311 (96) | 1323 (97) |
| Not known | 1 | 0 | 2 |
| Adjuvant therapy received b | |||
| All patients: | |||
| Chemotherapyc | 333/1360 (24) | 324/1367 (24) | 370/1366 (27) |
| HER-2 + patients: | |||
| Trastuzumab | 100/135 (74) | 98/137 (71) | 113/135 (84) |
| Chemotherapy and trastuzumab | 84 | 85 | 100 |
| Trastuzumab, no chemotherapy | 16 | 13 | 13 |
| Chemotherapy, no trastuzumab | 2 | 2 | 0 |
| No chemotherapy, no trastuzumab | 33 | 37 | 22 |
| ER + patients: | |||
| Endocrine therapy | 1169/1216 (96) | 1186/1237 (96) | 1157/1196 (97) |
| Boost given | |||
| Yes | 342 (25) | 337 (25) | 332 (24) |
| No | 1017 (75) | 1027 (75) | 1031 (76) |
| Not known | 2 | 3 | 5 |
| Boost dose | N = 342 | N = 337 | N = 332 |
| 10 Gy/5 fractions | 260 (76) | 273 (81) | 257 (77) |
| 16 Gy/8 fractions | 80 (24) | 64 (19) | 75 (23) |
| Not known | 2 | 0 | 0 |
| RT details | 40 Gy/15 fractions (N = 1360), (%) | 27 Gy/5 fractions (N = 1363), (%) | 26 Gy/5 fractions (N = 1367), (%) |
|---|---|---|---|
| Days from randomisation to starting RT | |||
| N | 1358 | 1362 | 1361 |
| Median (IQR) | 22 (17–29) | 22 (18–29) | 22 (18–29) |
| Range | 3–219 | 3–155 | 3–281 |
| Days of RT with no boost (including weekends and bank holidays) | |||
| N | 1017 | 1025 | 1029 |
| Median (IQR) | 21 (21–22) | 7 (5–7) | 7 (5–7) |
| Range | 23–51 | 5–22 | 13–33 |
| Days of RT with a boost (including weekends and bank holidays) | |||
| N | 341 | 337 | 332 |
| Median (IQR) | 29 (28–31) | 14 (12–15) | 14 (14–16) |
| Range | 14–43 | 5–35 | 5–29 |
| Methods used to localise tumour bed b | |||
| Surgical clips | 1203 (90) | 1184 (88) | 1192 (89) |
| Gold seeds | 2 (< 1) | 2 (< 1) | 0 (0) |
| Other | 114 (8) | 153 (11) | 137 (10) |
| None | 23 (2) | 14 (1) | 15 (1) |
| Not known | 18 | 10 | 23 |
| Volume of breast/chest wall PTV (cc) | |||
| N | 1353 | 1350 | 1343 |
| Median (IQR) | 908 (568–1281) | 942 (584–1352) | 875 (570–1287) |
| Range | 1–9642 | 20–3331 | 3–8908 |
| Bolus used (mastectomy patients only) | N = 91 | N = 89 | N = 84 |
| Yes | 62 (68) | 58 (66) | 55 (66) |
| No | 29 (32) | 30 (34) | 28 (34) |
| Not known | 0 | 1 | 1 |
| Dose homogeneity constraints achieved | |||
| Yes | 1299 (96) | 1311 (96) | 1298 (96) |
| No | 58 (4) | 49 (4) | 60 (4) |
| Not known | 3 | 3 | 9 |
| Organs at risk dose constraints achieved | |||
| Yes | 1332 (98) | 1335 (98) | 1338 (98) |
| No | 24 (2) | 25 (2) | 20 (2) |
| Not known | 4 | 3 | 9 |
| Was patient replanned during RT? | |||
| Yes | 25 (2) | 12 (1) | 10 (1) |
| No | 1333 (98) | 1349 (99) | 1352 (99) |
| Not known | 2 | 2 | 5 |
| Whole breast RT extended by > 3 days | |||
| Yes | 23 (2) | 5 (< 1) | 7 (< 1) |
| No | 1335 (98) | 1357 (99) | 1355 (99) |
| Not known | 2 | 1 | 5 |
| Reasonsc: | |||
| Bank holidays | 15 | 1 | 4 |
| Machine service or breakdown | 4 | 0 | 0 |
| Patient illness | 9 | 2 | 1 |
| Other | 4 | 1 | 1 |
Follow-up
Calculating length of follow-up as the time between randomisation and date last seen (censoring at date of death or withdrawal of consent from follow-up where applicable), median follow-up in Main Trial patients at the time of the primary analysis data snapshot (22 November 2019) was 71.5 months (IQR 71.3–71.7). Five-year visit forms were available for 3733 (98%) patients out of 3798 still in follow-up (not died, withdrawn or lost).
Five-year cancer outcomes in the Main Trial
After a median follow-up of 71.5 months (IQR 71.3–71.7), IBTR was recorded in 79 patients (40 Gy: 31, 27 Gy: 27, 26 Gy: 21) Table 6. Estimated cumulative incidence of IBTR up to 5 years was 2.1% (95% CI 1.4 to 3.1) for 40 Gy (expected incidence 2%), 1.7% (1.2 to 2.6) for 27 Gy and 1.4% (0.9 to 2.2) for 26 Gy (see Table 7, Figures 7 and 8). Estimated absolute differences in IBTR versus 40 Gy were −0.3% (−1.0 to 0.9) for 27 Gy and −0.7% (−1.3 to 0.3) for 26 Gy. Since the upper confidence limits excluded an increase in IBTR of >1.6%, non-inferiority can be claimed for both 5-fraction schedules compared with 40 Gy in 15 fractions. This is confirmed by a test against the critical HR > 1.81, with p = 0.0022 for 27 Gy and p = 0.00019 for 26 Gy compared with 40 Gy. Analyses in the per-protocol population were consistent [estimated absolute difference vs. 40 Gy −0.4%; (−1.0 to 0.8); p = 0.0017 for 27 Gy and −0.6% (−1.2 to 0.4); p = 0.00037 for 26 Gy; full data for per-protocol analyses not shown as 99% treatment compliance]. Comparing the 5-fraction schedules, the estimated absolute difference in IBTR cumulative incidence up to 5 years was −0.4% (−1.0 to 0.6) for 26 Gy versus 27 Gy. The unadjusted α/β estimate for IBTR was 3.7 Gy (0.3, 7.1 Gy), with EQD2 estimates of 44.7 Gy for 40 Gy, 43.1 Gy for 27 Gy and 40.6 Gy for 26 Gy with no correction for treatment time. Adjusting for risk group and ER/HER-2 status made minimal difference (adjusted α/β estimate 3.7 Gy; 0.4, 6.9). HRs obtained from a competing risks analysis of IBTR with death from any cause as a competing event were almost identical to those from the primary analysis reported in Table 19 [HRs from competing risks model: 0.85 (95% CI 0.51 to 1.43) for 27 Gy vs. 40 Gy; 0.67 (0.38 to 1.16) for 26 Gy vs. 40 Gy].
| Event | 40 Gy/15 fractions (N = 1361), (%) | 27 Gy/5 fractions (N = 1367), (%) | 26 Gy/5 fractions (N = 1368), (%) |
|---|---|---|---|
| Local tumour control event (primary endpoint) a , b | 31 (2.3) | 27 (2.0) | 21 (1.5) |
| Local relapse | 23 | 22 | 17 |
| Ipsilateral breast new primary | 6 | 3 | 4 |
| Cannot differentiate | 2 | 2 | 0 |
| Regional relapse | 13 (1.0) | 11 (0.8) | 10 (0.7) |
| Distant relapse | 59 (4.3) | 69 (5.0) | 76 (5.5) |
| Contralateral breast second primary | 23 (1.7) | 20 (1.5) | 23 (1.7) |
| Invasive | 18 | 17 | 20 |
| DCIS | 5 | 3 | 2 |
| Unknown | 0 | 0 | 1 |
| Non-breast second primary | 42 (3.1) | 37 (2.7) | 44 (3.2) |
| Death | 92 (6.8) | 105 (7.7) | 90 (6.6) |
| Breast cancerc | 47 | 51 | 53 |
| Second cancer | 12 | 16 | 10 |
| Cardiac | 10 | 9 | 8 |
| Other cause | 17 | 27 | 16 |
| Unknown | 6 | 2 | 3 |
| Cumulative no. of events/total (%) | Kaplan–Meier estimate (95% CI) of cumulative incidence by 5 years, (%) | HRa (95% CI); p-valueb | Estimated absolute difference vs. 40 Gy at 5 yearsc (95% CI), (%) | |
|---|---|---|---|---|
| IBTR (local)d | ||||
| 40 Gy | 31/1361 (2.3) | 2.1 (1.4 to 3.1) | 1 | |
| 27 Gy | 27/1367 (2.0) | 1.7 (1.2 to 2.6) | 0.86 (0.51 to 1.44); 0.56 | −0.3 (−1.0 to 0.9) |
| 26 Gy | 21/1368 (1.5) | 1.4 (0.9 to 2.2) | 0.67 (0.38 to 1.16); 0.15 | −0.7 (−1.3 to 0.3) |
| Locoregional relapse e | ||||
| 40 Gy | 43/1361 (3.2) | 2.8 (2.0 to 3.9) | 1 | |
| 27 Gy | 35/1367 (2.6) | 2.3 (1.6 to 3.3) | 0.80 (0.51 to 1.25); 0.33 | −0.5 (−1.4 to 0.7) |
| 26 Gy | 29/1368 (2.1) | 1.8 (1.2 to 2.7) | 0.66 (0.41 to 1.06); 0.083 | −0.9 (−1.6 to 0.2) |
| Distant relapse | ||||
| 40 Gy | 59/1361 (4.3) | 3.8 (2.9 to 5.0) | 1 | |
| 27 Gy | 69/1367 (5.0) | 4.7 (3.7 to 6.0) | 1.16 (0.82 to 1.64); 0.41 | 0.6 (−0.7 to 2.3) |
| 26 Gy | 76/1368 (5.6) | 5.1 (4.0 to 6.4) | 1.27 (0.90 to 1.79); 0.17 | 1.0 (−0.4 to 2.9) |
| Any breast cancer-related event f | ||||
| 40 Gy | 119/1361 (8.7) | 7.8 (6.5 to 9.4) | 1 | |
| 27 Gy | 112/1367 (8.2) | 7.2 (5.9 to 8.7) | 0.93 (0.71 to 1.20); 0.56 | −0.6 (−2.2 to 1.5) |
| 26 Gy | 114/1368 (8.3) | 7.5 (6.2 to 9.0) | 0.94 (0.73 to 1.22); 0.65 | −0.4 (−2.1 to 1.6) |
| All-cause mortality | ||||
| 40 Gy | 92/1361 (6.8) | 5.4 (4.3 to 6.8) | 1 | |
| 27 Gy | 105/1367 (7.7) | 6.9 (5.7 to 8.4) | 1.12 (0.85 to 1.48); 0.42 | 0.6 (−0.8 to 2.5) |
| 26 Gy | 90/1368 (6.6) | 5.6 (4.5 to 7.0) | 0.96 (0.72 to 1.28); 0.78 | −0.2 (−1.5 to 1.5) |
FIGURE 7.
Kaplan–Meier plot of IBTR in Main Trial.
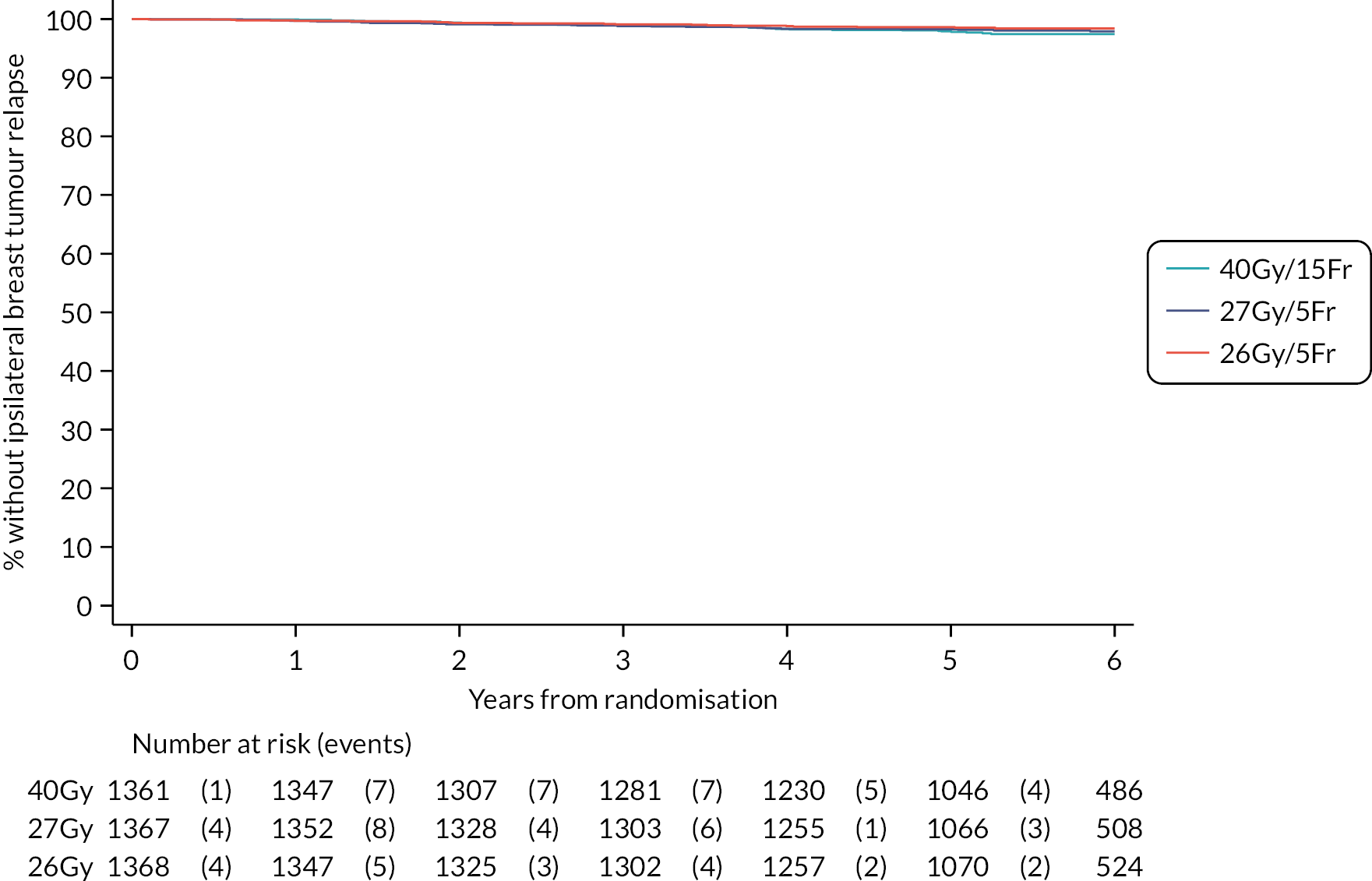
FIGURE 8.
Cumulative risk of IBTR in Main Trial.
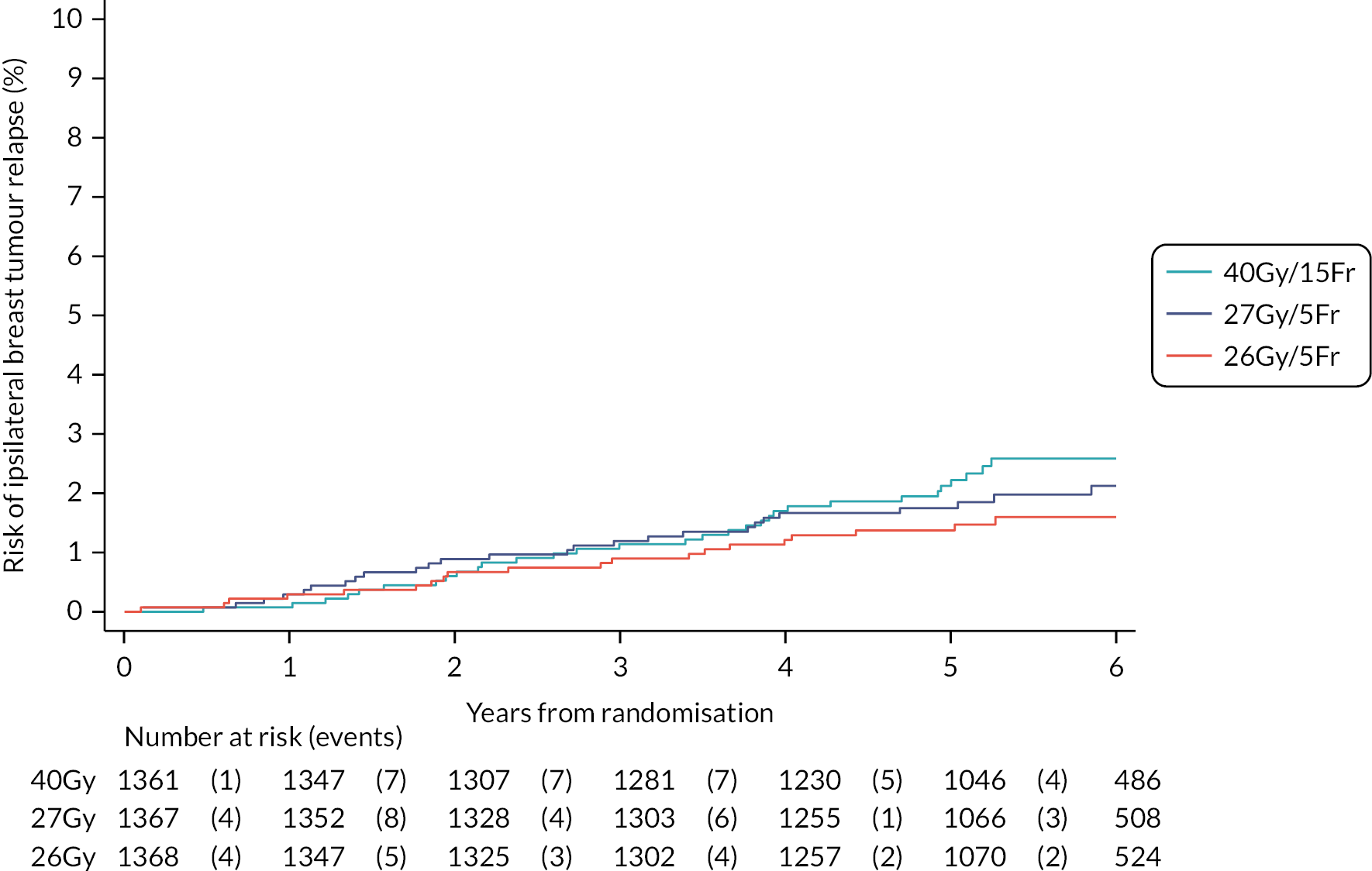
Regional relapses occurred in 34/4096 (1%) patients (40 Gy: 13, 27 Gy: 11, 26 Gy: 10; see Table 6), 6 of which were concurrent with IBTR. Incidence of locoregional relapse, distant relapse, disease-free and overall survival were similar between groups, with no statistically significant differences (see Table 7, Figures 9 and 10, Appendix 3, Figures 21–26). Invasive contralateral breast cancer was reported for 55/4096 (1%) patients (40 Gy: 18, 27 Gy: 17, 26 Gy: 20; see Table 18), and non-breast second primary cancers for 123/4096 (3%) patients (40 Gy: 42, 27 Gy: 37, 26 Gy: 44; see Table 18), the most common being colorectal cancer with 25 cases in total.
FIGURE 9.
Kaplan–Meier plot of disease-free survival in Main Trial.
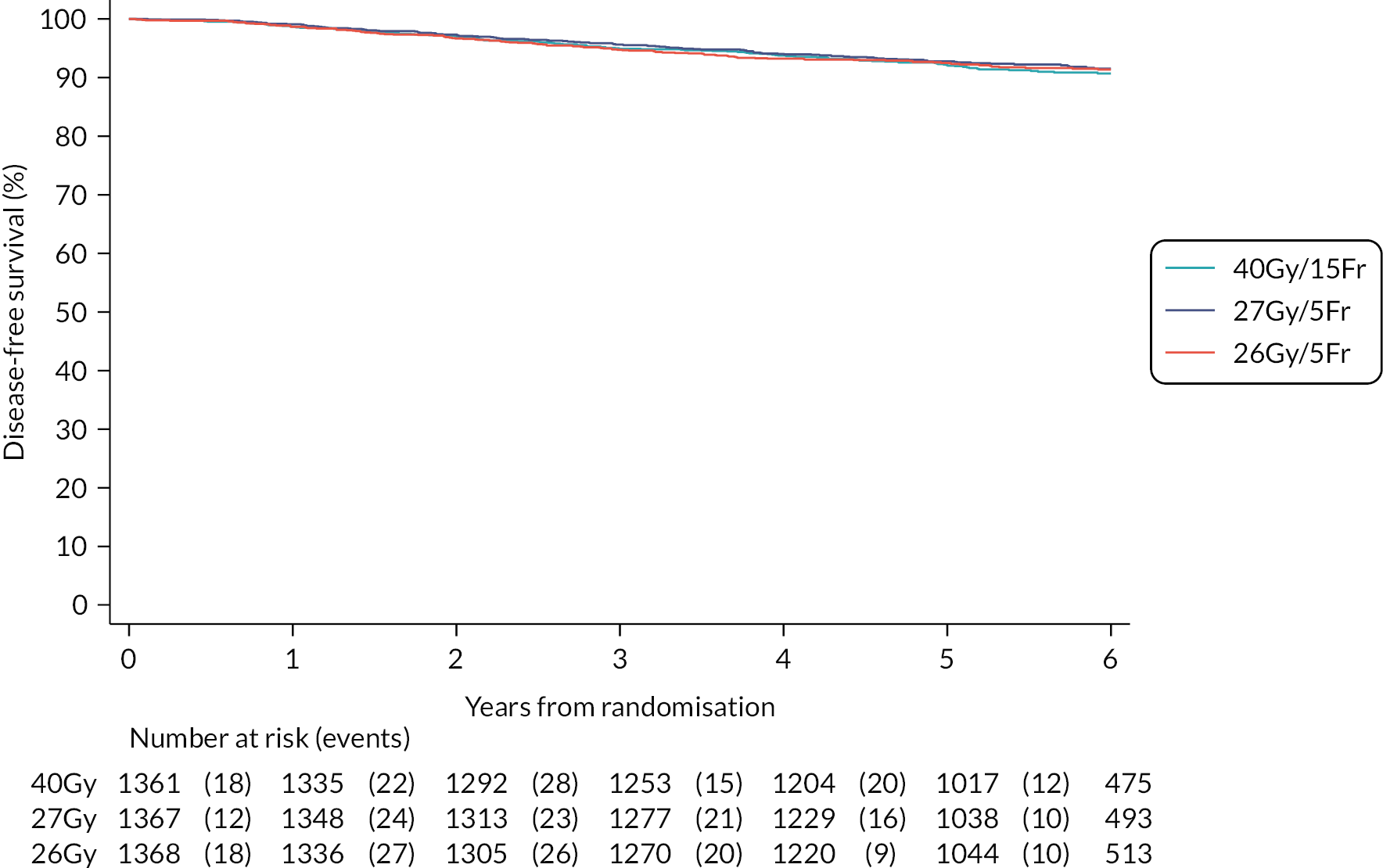
FIGURE 10.
Cumulative risk of any breast cancer-related eventa in Main Trial. aAny breast cancer-related event includes local, regional or distant relapse, breast cancer death and contralateral breast cancer.
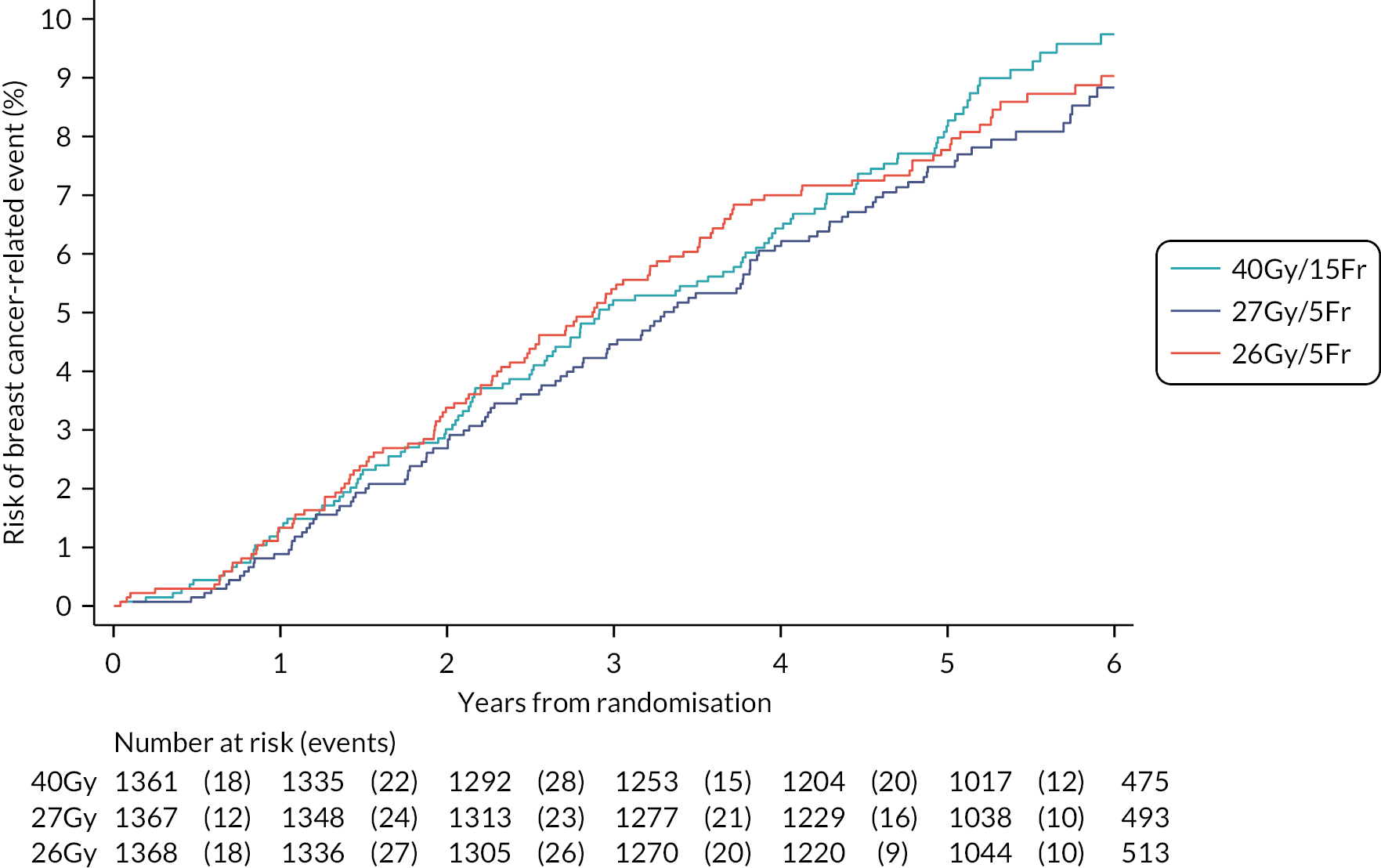
A total of 287/4096 (7%) patients died, 151 (4%) from breast cancer, 125 (3%) from other causes (including 38 (1%) from second cancers and 27 (1%) from cardiac related) and 11 (0.3%) with unknown cause of death and no evidence of disease relapse (see Table 6). Of 27 patients with a cardiac-related death (40 Gy: 10, 27 Gy: 9, 26 Gy: 8), 15 (40 Gy: 7, 27 Gy; 4, 26 Gy: 4) had a history of cardiac disease reported at randomisation or was a current/ex-smoker in past year.
Late normal tissue effects up to 5 years’ follow-up in the Main Trial
Clinician assessments
At least one annual clinical assessment of NTE was available for 3975/4096 (97%) patients. Frequencies of clinician-assessed NTE according to year of follow-up (from 1 to 4 years) and fractionation schedule are shown in Appendix 2, Table 41. Bar charts of clinician-assessed NTE from 1 to 5 years are presented in Appendix 3, Figures 27–33. Cross-sectional analyses of clinician-assessed NTE at 5 years are in Table 8. At 5 years, any moderate/marked clinician-assessed NTE in the breast/chest wall was reported for 98/986 (10%) patients in the 40 Gy group, 155/1005 (15%) for 27 Gy and 121/1020 (12%) for 26 Gy (see Table 8, Figure 11), with a statistically significant difference between 40 Gy and 27 Gy (p = 0.0003) but not between 40 Gy and 26 Gy (p = 0.17). Breast shrinkage was the most prevalent moderate/marked effect at 5 years, reported in 50/916 (6%) for 40 Gy, 78/948 (8%) for 27 Gy and 65/954 (7%) for 26 Gy (see Table 8). Longitudinal analysis of all annual clinical assessments of NTE over follow-up showed a statistically significant increased risk of any moderate/marked effect in the breast/chest wall for the 27 Gy group compared with 40 Gy (OR 1.55, 1.32 to 1.83; p < 0.0001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.12, 0.94 to 1.34; p = 0.20; see Table 9). This pattern was similar for the individual effects of breast distortion, shrinkage, induration and breast/chest wall oedema, with statistically significant higher risk for 27 Gy compared with 40 Gy but not for 26 Gy (see Table 9, Appendix 3, Figures 34–40). Comparing the two 5-fraction schedules, 26 Gy had statistically significantly lower risk of any moderate/marked breast/chest wall NTE (p = 0.0001) and breast shrinkage (p = 0.0018) compared with 27 Gy. Estimates of 5-year cumulative incidence of any moderate/marked clinician-assessed NTE in the breast/chest wall were 26.8% (95% CI 24.4 to 29.4) for 40 Gy, 35.1% (32.4 to 37.9) for 27 Gy and 28.5% (26.0 to 31.1) for 26 Gy (see Table 10). Results for comparison of schedules from the analyses of time to first moderate/marked effect were similar to those from the longitudinal modelling of all annual clinical assessments.
| 40 Gy (N = 1197), (%) | 27 Gy (N = 1220), (%) | 26 Gy (N = 1209), (%) | Moderate/marked vs. None/mild | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 27 Gy vs. 40 Gy | 26 Gy vs. 40 Gy | 27 Gy vs. 26 Gy | |||||||
| Risk difference (95% CI), % | p-valuea | Risk difference (95% CI), % | p-valuea | Risk difference (95% CI), % | p-valuea | ||||
| Any AE in breast/chest wallb | 5.5 (2.6 to 8.4) | 0.0003 | 1.9 (−0.8 to 4.6) | 0.17 | 3.6 (0.6 to 6.5) | 0.020 | |||
| None | 504 (51) | 464 (46) | 535 (52) | ||||||
| Mild | 384 (39) | 386 (38) | 364 (36) | ||||||
| Moderate | 93 (9) | 132 (13) | 105 (10) | ||||||
| Marked | 5 (<1) | 23 (2) | 16 (2) | ||||||
| Not assessed | 211 | 215 | 189 | ||||||
| Breast distortion c | 3.1 (1.2 to 5.1) | 0.0022 | 2.1 (0.2 to 3.9) | 0.035 | 1.0 (−1.0 to 3.2) | 0.34 | |||
| None | 669 (73) | 680 (72) | 717 (75) | ||||||
| Mild | 215 (23) | 206 (22) | 185 (19) | ||||||
| Moderate | 30 (3) | 58 (6) | 45 (5) | ||||||
| Marked | 2 (<1) | 5 (<1) | 8 (<1) | ||||||
| N/A | 67 | 63 | 59 | ||||||
| Not assessed | 214 | 208 | 195 | ||||||
| Breast shrinkage c | 2.8 (0.5 to 5.0) | 0.022 | 1.3 (−0.8 to 3.5) | 0.25 | 1.4 (−0.9 to 3.8) | 0.26 | |||
| None | 645 (70) | 642 (68) | 670 (70) | ||||||
| Mild | 221 (24) | 228 (24) | 219 (23) | ||||||
| Moderate | 48 (5) | 69 (7) | 56 (6) | ||||||
| Marked | 2 (<1) | 9 (1) | 9 (1) | ||||||
| N/A | 67 | 63 | 59 | ||||||
| Not assessed | 214 | 209 | 196 | ||||||
| Breast induration (tumour bed) c | 2.9 (1.1 to 4.8) | 0.0018 | 1.3 (−0.3 to 3.0) | 0.13 | 1.6 (−0.3 to 3.5) | 0.11 | |||
| None | 717 (78) | 712 (75) | 754 (79) | ||||||
| Mild | 172 (19) | 183 (19) | 163 (17) | ||||||
| Moderate | 24 (3) | 49 (5) | 35 (4) | ||||||
| Marked | 1 (< 1) | 5 (< 1) | 4 (< 1) | ||||||
| N/A | 67 | 63 | 59 | ||||||
| Not assessed | 216 | 208 | 194 | ||||||
| Breast induration (outside tumour bed) c | 2.0 (1.1 to 2.9) | < 0.0001 | 2.0 (1.0 to 2.9) | < 0.0001 | 0.0 (−1.3 to 1.3) | >0.99 | |||
| None | 859 (94) | 878 (93) | 879 (92) | ||||||
| Mild | 51 (6) | 50 (5) | 56 (6) | ||||||
| Moderate | 1 (< 1) | 14 (1) | 17 (2) | ||||||
| Marked | 0 | 6 (< 1) | 3 (< 1) | ||||||
| N/A | 67 | 63 | 59 | ||||||
| Not assessed | 219 | 209 | 195 | ||||||
| Telangiectasia | 1.0 (−0.2 to 2.2) | 0.14 | 0.2 (−0.8 to 1.3) | 0.72 | 0.7 (−0.5 to 1.9) | 0.27 | |||
| None | 893 (91) | 878 (87) | 913 (90) | ||||||
| Mild | 78 (8) | 102 (10) | 88 (9) | ||||||
| Moderate | 13 (1) | 18 (2) | 15 (1) | ||||||
| Marked | 1 (< 1) | 6 (< 1) | 2 (< 1) | ||||||
| Not assessed | 212 | 216 | 191 | ||||||
| Breast/chest wall oedema | 1.1 (0.1 to 2.1) | 0.042 | 1.0 (0.0 to 1.9) | 0.063 | 0.1 (−1.0 to 1.3) | 0.87 | |||
| None | 931 (94) | 918 (92) | 950 (93) | ||||||
| Mild | 47 (5) | 66 (6) | 51 (5) | ||||||
| Moderate | 6 (< 1) | 16 (2) | 13 (1) | ||||||
| Marked | 1 (< 1) | 2 (< 1) | 4 (< 1) | ||||||
| Not assessed | 212 | 218 | 191 | ||||||
| Breast/chest wall discomfort | −0.2 (−1.8 to 1.5) | 0.90 | 0.0 (−1.7 to 1.6) | > 0.99 | −0.2 (−1.8 to 1.5) | 0.90 | |||
| None | 816 (83) | 802 (80) | 825 (81) | ||||||
| Mild | 131 (13) | 167 (17) | 153 (15) | ||||||
| Moderate | 29 (3) | 31 (3) | 29 (3) | ||||||
| Marked | 7 (< 1) | 4 (< 1) | 8 (< 1) | ||||||
| Not assessed | 214 | 216 | 194 | ||||||
| Other RT-related | 0.0 (−0.7 to 0.8) | >0.99 | 0.3 (−0.5 to 1.1) | 0.63 | −0.2 (−1.0 to 0.6) | 0.81 | |||
| None | 967 (98) | 984 (98) | 996 (97) | ||||||
| Mild | 12 (1) | 17 (2) | 17 (2) | ||||||
| Moderate | 6 (< 1) | 4 (< 1) | 7 (< 1) | ||||||
| Marked | 1 (< 1) | 4 (< 1) | 3 (< 1) | ||||||
| Not assessed | 211 | 211 | 186 | ||||||
| Normal tissue effect | No. moderate/marked events/total no. of assessments over follow-up (%) | OR for schedulea (95% CI) | Comparison with 40 Gy; p-valueb | Comparison between 27 Gy and 26 Gy; p-valueb | OR for years of follow-up (95% CI); p-valueb |
|---|---|---|---|---|---|
| Any AE in the breast/chest wallc | 0.98 (0.96 to 1.00); 0.055 | ||||
| 40 Gy | 651/6121 (10.6) | 1 | |||
| 27 Gy | 1004/6303 (15.9) | 1.55 (1.32 to 1.83) | <0.0001 | ||
| 26 Gy | 774/6327 (12.2) | 1.12 (0.94 to 1.34) | 0.20 | 0.0001 | |
| Breast distortion d | 0.99 (0.95 to 1.02); 0.38 | ||||
| 40 Gy | 232/5724 (4.0) | 1 | |||
| 27 Gy | 363/5953 (6.1) | 1.51 (1.15 to 1.97) | 0.0028 | ||
| 26 Gy | 299/5945 (5.0) | 1.20 (0.91 to 1.60) | 0.19 | 0.083 | |
| Breast shrinkage d | 1.03 (1.00 to 1.06); 0.023 | ||||
| 40 Gy | 330/5728 (5.8) | 1 | |||
| 27 Gy | 503/5944 (8.5) | 1.50 (1.20 to 1.88) | 0.0004 | ||
| 26 Gy | 369/5943 (6.2) | 1.05 (0.82 to 1.33) | 0.71 | 0.0018 | |
| Breast induration (tumour bed) d | 1.00 (0.96 to 1.04); 0.95 | ||||
| 40 Gy | 185/5713 (3.2) | 1 | |||
| 27 Gy | 304/5948 (5.1) | 1.56 (1.19 to 2.05) | 0.0013 | ||
| 26 Gy | 236/5937 (4.0) | 1.19 (0.90 to 1.59) | 0.23 | 0.047 | |
| Breast induration (outside tumour bed) d | 0.96 (0.90 to 1.02); 0.17 | ||||
| 40 Gy | 45/5712 (0.8) | 1 | |||
| 27 Gy | 137/5943 (2.3) | 2.79 (1.74 to 4.50) | <0.0001 | ||
| 26 Gy | 97/5930 (1.6) | 1.90 (1.15 to 3.14) | 0.013 | 0.059 | |
| Telangiectasia e | 1.21 (1.14 to 1.29); <0.0001 | ||||
| 40 Gy | 63/6087 (1.0) | 1 | |||
| 27 Gy | 100/6272 (1.6) | 1.68 (1.07 to 2.65) | 0.025 | ||
| 26 Gy | 102/6300 (1.6) | 1.53 (0.96 to 2.43) | 0.070 | 0.65 | |
| Breast/chest wall oedema e | 0.73 (0.69 to 0.78); <0.0001 | ||||
| 40 Gy | 89/6097 (1.5) | 1 | |||
| 27 Gy | 217/6287 (3.4) | 2.18 (1.57 to 3.03) | <0.0001 | ||
| 26 Gy | 155/6318 (2.4) | 1.47 (1.03 to 2.09) | 0.032 | 0.0097 | |
| Breast/chest wall discomfort e | 0.93 (0.89 to 0.97); 0.0003 | ||||
| 40 Gy | 234/6086 (3.8) | 1 | |||
| 27 Gy | 269/6285 (4.3) | 1.10 (0.86 to 1.40) | 0.44 | ||
| 26 Gy | 250/6309 (4.0) | 0.98 (0.76 to 1.26) | 0.86 | 0.35 |
| Normal tissue effect | Moderate/marked events/totala (%) | Kaplan–Meier estimate (95% CI) of cumulative incidence (%) of moderate/marked events by 5 yearsb | HR (95% CI) | Comparison with 40 Gy; p-valuec | Comparison between 27 Gy and 26 Gy; p-valuec |
|---|---|---|---|---|---|
| Any NTE in the breast/chest walld | |||||
| 40 Gy | 344/1307 (26.3) | 26.8 (24.4 to 29.4) | 1 | ||
| 27 Gy | 468/1339 (34.9) | 35.1 (32.4 to 37.9) | 1.41 (1.23 to 1.62) | <0.0001 | |
| 26 Gy | 380/1326 (28.7) | 28.5 (26.0 to 31.1) | 1.09 (0.95 to 1.27) | 0.22 | 0.0002 |
| Breast distortion e | |||||
| 40 Gy | 126/1225 (10.3) | 10.8 (9.1 to 12.7) | 1 | ||
| 27 Gy | 190/1265 (15.0) | 15.2 (13.2 to 17.4) | 1.50 (1.20 to 1.88) | 0.0004 | |
| 26 Gy | 159/1249 (12.7) | 12.8 (11.0 to 14.9) | 1.25 (0.99 to 1.57) | 0.066 | 0.083 |
| Breast shrinkage e | |||||
| 40 Gy | 185/1227 (15.1) | 14.9 (12.9 to 17.1) | 1 | ||
| 27 Gy | 247/1265 (19.5) | 19.1 (16.9 to 21.5) | 1.34 (1.11 to 1.62) | 0.0026 | |
| 26 Gy | 189/1249 (15.1) | 146 (12.7 to 16.9) | 0.99 (0.81 to 1.21) | 0.95 | 0.0018 |
| Breast induration (tumour bed) e | |||||
| 40 Gy | 125/1225 (10.2) | 10.3 (8.6 to 12.2) | 1 | ||
| 27 Gy | 178/1266 (14.1) | 14.0 (12.1 to 16.2) | 1.42 (1.13 to 1.78) | 0.0027 | |
| 26 Gy | 133/1249 (10.6) | 9.9 (8.3 to 11.8) | 1.04 (0.81 to 1.32) | 0.78 | 0.0062 |
| Breast induration (outside tumour bed) e | |||||
| 40 Gy | 36/1225 (2.9) | 2.9 (2.1 to 4.1) | 1 | ||
| 27 Gy | 87/1266 (6.9) | 6.7 (5.4 to 8.3) | 2.40 (1.63 to 3.54) | <0.0001 | |
| 26 Gy | 52/1249 (4.2) | 4.3 (3.2 to 5.7) | 1.42 (0.93 to 2.17) | 0.11 | 0.0024 |
| Telangiectasia | |||||
| 40 Gy | 36/1305 (2.8) | 3.0 (2.1 to 4.2) | 1 | ||
| 27 Gy | 59/1337 (4.4) | 4.8 (3.7 to 6.2) | 1.61 (1.06 to 2.44) | 0.023 | |
| 26 Gy | 52/1324 (3.9) | 3.5 (2.6 to 4.8) | 1.41 (0.92 to 2.16) | 0.11 | 0.49 |
| Breast/chest wall oedema | |||||
| 40 Gy | 72/1306 (5.5) | 5.5 (4.3 to 6.9) | 1 | ||
| 27 Gy | 140/1339 (10.5) | 10.5 (8.9 to 12.3) | 1.95 (1.47 to 2.59) | <0.0001 | |
| 26 Gy | 99/1326 (7.5) | 7.5 (6.2 to 9.2) | 1.36 (1.01 to 1.85) | 0.045 | 0.0060 |
| Breast/chest wall discomfort | |||||
| 40 Gy | 156/1306 (11.9) | 12.2 (10.5 to 14.3) | 1 | ||
| 27 Gy | 178/1337 (13.3) | 13.4 (11.6 to 15.5) | 1.12 (0.90 to 1.39) | 0.308 | |
| 26 Gy | 159/1327 (12.0) | 11.8 (10.1 to 13.8) | 0.99 (0.79 to 1.24) | 0.94 | 0.27 |
FIGURE 11.
Kaplan–Meier plot of any moderate/marked clinician-assessed NTE in breast/chest wall (Main Trial).
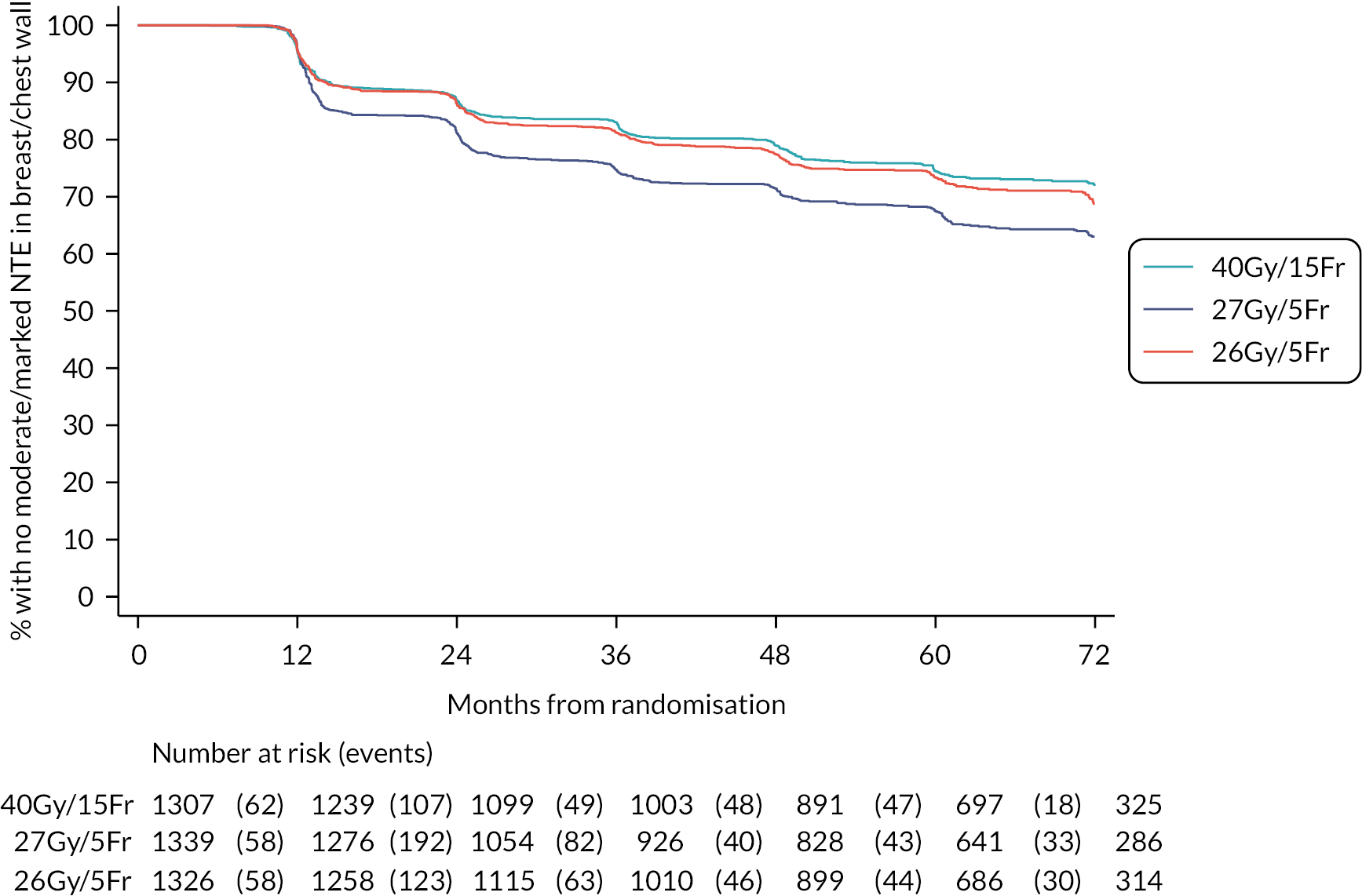
Patient self-assessments
One thousand seven hundred and ninety-six patients consented to the PRO sub-study, of whom 10 withdrew consent immediately after randomisation and eight were not given the baseline booklet. Questionnaires returned from those expected (patients alive and well, not withdrawn) totalled 1771/1778 (99%) at baseline, 1668/1733 (96%) at 3 months, 1622/1722 (94%) at 6 months, 1599/1707 (94%) at 1 year, 1531/1669 (92%) at 2 years and 1334/1589 (84%) at 5 years. Of the 1774 patients with at least one completed questionnaire, 1634 had BCS and 140 mastectomy.
Frequencies of patient-assessed breast/chest wall and arm/shoulder/hand symptoms are shown for each time point from baseline to 2 years and according to fractionation schedule in Appendix 2, Table 41. Cross-sectional analyses of patient-assessed symptoms at 5 years are in Table 11. Bar charts of patient-assessed late AE up to 5 years are presented in Appendix 3, Figures 41–51.
| 40 Gy (N = 444), (%) | 27 Gy (N = 450), (%) | 26 Gy (N = 444), (%) | Moderate/marked vs. none/mild | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 27 Gy vs. 40 Gy | 26 Gy vs. 40 Gy | 27 Gy vs. 26 Gy | |||||||
| Risk difference (95% CI), (%) | p-valuea | Risk difference (95% CI), (%) | p-valuea | Risk difference (95% CI), (%) | p-valuea | ||||
| Protocol-specific items | |||||||||
| Breast appearance changed | 3.5 (−2.8 to 9.8) | 0.28 | −0.7 (−6.9 to 5.5) | 0.83 | 4.2 (−2.1 to 10.5) | 0.20 | |||
| None | 77 (18) | 79 (18) | 86 (20) | ||||||
| Mild | 215 (50) | 203 (46) | 207 (48) | ||||||
| Moderate | 86 (20) | 100 (23) | 79 (18) | ||||||
| Marked | 54 (12) | 58 (13) | 57 (13) | ||||||
| Missing | 12 | 10 | 15 | ||||||
| Breast smaller | −0.7 (−6.7 to 5.3) | 0.88 | −4.5 (−10.4 to 1.4) | 0.14 | 3.9 (−2.0 to 9.6) | 0.21 | |||
| None | 145 (14) | 153 (35) | 166 (39) | ||||||
| Mild | 161 (38) | 161 (37) | 160 (37) | ||||||
| Moderate | 72 (17) | 67 (15) | 52 (12) | ||||||
| Marked | 50 (12) | 54 (12) | 51 (12) | ||||||
| Missing | 16 | 15 | 15 | ||||||
| Breast harder/firmer | 7.0 (2.0 to 12.1) | 0.0075 | 3.2 (−1.7 to 8.0) | 0.22 | 3.9 (−1.4 to 9.2) | 0.17 | |||
| None | 239 (56) | 196 (45) | 209 (49) | ||||||
| Mild | 128 (30) | 144 (33) | 142 (33) | ||||||
| Moderate | 38 (9) | 54 (12) | 47 (11) | ||||||
| Marked | 23 (5) | 38 (9) | 27 (6) | ||||||
| Missing | 16 | 18 | 19 | ||||||
| Skin appearance changed | 1.1 (−3.2 to 5.4) | 0.68 | −1.8 (−5.9 to 2.3) | 0.44 | 2.9 (−1.3 to 7.0) | 0.20 | |||
| None | 251 (58) | 238 (54) | 226 (52) | ||||||
| Mild | 133 (31) | 150 (34) | 163 (38) | ||||||
| Moderate | 32 (7) | 37 (8) | 26 (6) | ||||||
| Marked | 18 (4) | 19 (4) | 16 (4) | ||||||
| Missing | 10 | 6 | 13 | ||||||
| EORTC QLQ-BR23 items | |||||||||
| Breast pain | −1.1 (−5.3 to 3.1) | 0.67 | 0.4 (−3.9 to 4.7) | 0.92 | −1.5 (−5.7 to 2.8) | 0.53 | |||
| None | 198 (45) | 205 (46) | 193 (44) | ||||||
| Mild | 191 (43) | 195 (43) | 189 (43) | ||||||
| Moderate | 35 (8) | 40 (9) | 39 (9) | ||||||
| Marked | 18 (4) | 9 (2) | 15 (3) | ||||||
| Missing | 2 | 1 | 8 | ||||||
| Breast swollen | 3.3 (1.0 to 5.6) | 0.0072 | 2.8 (0.5 to 5.0) | 0.017 | 0.5 (−2.2 to 3.3) | 0.75 | |||
| None | 383 (87) | 362 (81) | 356 (82) | ||||||
| Mild | 50 (11) | 63 (14) | 60 (14) | ||||||
| Moderate | 2 (< 1) | 18 (4) | 17 (4) | ||||||
| Marked | 5 (1) | 4 (1) | 2 (< 1) | ||||||
| Missing | 4 | 3 | 9 | ||||||
| Breast oversensitive | −0.4 (−4.2 to 3.3) | 0.90 | 1.9 (−2.1 to 5.8) | 0.37 | −2.3 (−6.2 to 1.6) | 0.26 | |||
| None | 253 (58) | 252 (56) | 252 (58) | ||||||
| Mild | 144 (33) | 157 (35) | 136 (31) | ||||||
| Moderate | 23 (5) | 24 (5) | 31 (7) | ||||||
| Marked | 16 (4) | 14 (3) | 16 (4) | ||||||
| Missing | 8 | 3 | 9 | ||||||
| Skin problems on breast | 1.5 (−1.4 to 4.4) | 0.36 | 0.5 (−2.2 to 3.3) | 0.75 | 1.0 (−2.0 to 3.9) | 0.55 | |||
| None | 347 (79) | 338 (75) | 351 (81) | ||||||
| Mild | 75 (17) | 84 (19) | 62 (14) | ||||||
| Moderate | 9 (2) | 19 (4) | 18 (4) | ||||||
| Marked | 10 (2) | 7 (2) | 3 (< 1) | ||||||
| Missing | 3 | 2 | 10 | ||||||
| Arm/shoulder pain | −3.0 (−7.6 to 1.6) | 0.21 | 0.4 (−4.5 to 5.2) | 0.93 | −3.4 (−8.0 to 1.3) | 0.18 | |||
| None | 230 (52) | 230 (51) | 236 (54) | ||||||
| Mild | 141 (32) | 162 (36) | 130 (30) | ||||||
| Moderate | 51 (12) | 43 (10) | 50 (11) | ||||||
| Marked | 18 (4) | 14 (3) | 20 (5) | ||||||
| Missing | 4 | 1 | 8 | ||||||
| Arm/hand swollen | −1.2 (−3.9 to 1.5) | 0.42 | 0.0 (−2.8 to 2.9) | >0.99 | −1.2 (−4.0 to 1.5) | 0.41 | |||
| None | 370 (84) | 375 (84) | 365 (84) | ||||||
| Mild | 48 (11) | 54 (12) | 48 (11) | ||||||
| Moderate | 18 (4) | 11 (2) | 16 (4) | ||||||
| Marked | 4 (1) | 6 (1) | 6 (1) | ||||||
| Missing | 4 | 4 | 9 | ||||||
| Difficulty raising arm | −0.6 (−4.1 to 3.0) | 0.80 | −1.3 (−4.8 to 2.2) | 0.52 | 0.7 (−2.7 to 4.1) | 0.70 | |||
| None | 326 (74) | 336 (75) | 321 (74) | ||||||
| Mild | 79 (18) | 77 (17) | 85 (19) | ||||||
| Moderate | 23 (5) | 24 (5) | 21 (5) | ||||||
| Marked | 13 (3) | 10 (2) | 9 (2) | ||||||
| Missing | 3 | 3 | 8 | ||||||
Change in breast appearance had the highest 5-year prevalence, with moderate/marked change reported in 140/432 (32%) for 40 Gy, 158/440 (36%) for 27 Gy and 136/429 (32%) for 26 Gy. There were no statistically significant differences in 5-year prevalence of patient-reported AE between the schedules (see Table 11). There was some evidence of an increase in patient-reported moderate/marked breast hardness/firmness at 5 years for 27 Gy compared with 40 Gy and more breast swelling in both 5-fraction schedules, but these were not statistically significant at the pre-specified cut-off of p = 0.005. Longitudinal analyses of all patient assessments from baseline to 5 years showed a statistically significantly higher risk of moderate/marked breast hardness/firmness for 27 Gy compared with 40 Gy (OR 1.42, 1.17 to 1.72; p = 0.0003), and less change in breast appearance for 26 Gy compared with 27 Gy (p = 0.0018), but no statistically significant differences between schedules for the other NTE (see Table 12).
| Normal tissue effect | No. of patients reporting moderate/marked event at baseline/total (%) | No. of moderate/marked events/total no. of assessments over 3–60 months follow-up (%) | OR for schedulea (95% CI) | Comparison with 40 Gy; p-valueb | Comparison between 27 Gy and 26 Gy; p-valueb | OR for years of follow-up (95% CI); p-valueb |
|---|---|---|---|---|---|---|
| Protocol-specific items | ||||||
| Breast appearance changed | 1.03 (1.01, 1.05); 0.0010 | |||||
| 40 Gy | 170/573 (29.7) | 778/2480 (31.4) | 1 | |||
| 27 Gy | 177/583 (30.4) | 929/2550 (36.4) | 1.22 (1.02 to 1.46) | 0.033 | ||
| 26 Gy | 155/581 (26.7) | 770/2563 (30.0) | 0.91 (0.75 to 1.10) | 0.33 | 0.0018 | |
| Breast smaller | 1.11 (1.09 to 1.13); < 0.0001 | |||||
| 40 Gy | 96/560 (17.1) | 585/2445 (23.9) | 1 | |||
| 27 Gy | 106/576 (18.4) | 606/2520 (24.0) | 1.05 (0.85 to 1.29) | 0.67 | ||
| 26 Gy | 90/574 (15.7) | 515/2542 (20.3) | 0.81 (0.65 to 1.00) | 0.053 | 0.017 | |
| Breast harder/firmer | 0.95 (0.93 to 0.97); < 0.0001 | |||||
| 40 Gy | 94/558 (16.8) | 499/2446 (20.4) | 1 | |||
| 27 Gy | 105/572 (18.4) | 690/2512 (27.5) | 1.42 (1.17 to 1.72) | 0.0003 | ||
| 26 Gy | 95/566 (16.8) | 626/2534 (24.7) | 1.22 (1.00 to 1.48) | 0.048 | 0.1007 | |
| Skin appearance changed | 0.96 (0.93 to 0.99); 0.0080 | |||||
| 40 Gy | 78/577 (13.5) | 345/2505 (13.8) | 1 | |||
| 27 Gy | 61/586 (10.4) | 392/2571 (15.2) | 1.03 (0.83 to 1.28) | 0.77 | ||
| 26 Gy | 67/580 (11.5) | 338/2576 (13.1) | 0.90 (0.72 to 1.13) | 0.37 | 0.23 | |
| EORTC QLQ-BR23 items | ||||||
| Breast pain | 0.96 (0.94 to 0.99); 0.011 | |||||
| 40 Gy | 53/583 (9.1) | 338/2538 (13.3) | 1 | |||
| 27 Gy | 42/590 (7.1) | 428/2601 (16.5) | 1.23 (0.98 to 1.54) | 0.068 | ||
| 26 Gy | 53/588 (9.0) | 417/2597 (16.1) | 1.23 (0.98 to 1.53) | 0.074 | 0.96 | |
| Breast swollen | 0.84 (0.80 to 0.89); < 0.0001 | |||||
| 40 Gy | 56/583 (9.6) | 122/2538 (4.8) | 1 | |||
| 27 Gy | 43/589 (7.3) | 236/2597 (9.1) | 1.46 (1.10 to 1.94) | 0.0080 | ||
| 26 Gy | 47/589 (8.0) | 192/2599 (7.4) | 1.27 (0.95 to 1.69) | 0.11 | 0.22 | |
| Breast oversensitive | 0.96 (0.93 to 0.99); 0.0097 | |||||
| 40 Gy | 57/579 (9.8) | 283/2528 (11.2) | 1 | |||
| 27 Gy | 42/584 (7.2) | 334/2596 (12.9) | 1.10 (0.87 to 1.40) | 0.43 | ||
| 26 Gy | 62/586 (10.6) | 319/2587 (12.3) | 1.11 (0.88 to 1.41) | 0.37 | 0.91 | |
| Skin problems in breast | 0.96 (0.92 to 1.01); | |||||
| 40 Gy | 26/582 (4.5) | 156/2539 (6.1) | 1 | 0.11 | ||
| 27 Gy | 24/290 (4.1) | 209/2596 (8.0) | 1.25 (0.95 to 1.65) | 0.11 | ||
| 26 Gy | 18/590 (3.0) | 164/2592 (6.3) | 0.98 (0.73 to 1.31) | 0.90 | 0.084 | |
| Arm/shoulder pain | 1.00 (0.97 to 1.03); > 0.99 | |||||
| 40 Gy | 66/582 (11.3) | 401/2537 (15.8) | 1 | |||
| 27 Gy | 78/591 (13.2) | 441/2601 (17.0) | 1.12 (0.91 to 1.37) | 0.29 | ||
| 26 Gy | 81/589 (13.7) | 455/2599 (17.5) | 1.14 (0.93 to 1.40) | 0.2006 | 0.83 | |
| Arm/hand swollen | 1.06 (1.00 to 1.11); 0.031 | |||||
| 40 Gy | 24/582 (4.1) | 101/2536 (4.0) | 1 | |||
| 27 Gy | 17/588 (2.9) | 103/2600 (4.0) | 0.95 (0.66 to 1.36) | 0.77 | ||
| 26 Gy | 22/590 (3.7) | 124/2592 (4.8) | 1.14 (0.80 to 1.62) | 0.46 | 0.31 | |
| Difficulty raising arm | 1.04 (0.99 to 1.08); 0.089 | |||||
| 40 Gy | 27/582 (4.6) | 171/2533 (6.7) | 1 | |||
| 27 Gy | 36/589 (6.1) | 209/2599 (8.0) | 1.24 (0.94 to 1.63) | 0.12 | ||
| 26 Gy | 37/587 (6.3) | 188/2596 (7.2) | 1.12 (0.85 to 1.48) | 0.42 | 0.46 | |
Photographic assessments of late adverse effects in the Main Trial
Of the 1737 patients (BCS and post-mastectomy) who consented to the photographic sub-study, baseline photographs were received for 1634 (94%), and 2- and/or 5-year photographs were available for 1385 (80%). The vast majority (1309) were patients who had BCS; for these patients, 2- and 5-year photographs were assessed in 1267 and 875, respectively (see Table 13). A total of 226 patients died or withdrew from the photographic sub-study by year 5, for the remainder the most common reasons for photographs not being taken were appointments not made due to clerical errors at the centres, patients not attending clinic visits, and patients withdrawing consent from the sub-study.
| 2 years | 5 years | OR for mild/ marked change vs 40 Gy (95% CI) | Comparison with 40 Gy; p-valuea | Comparison between 27 Gy and 26 Gy; p-valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | None (%) | Mild (%) | Marked (%) | N | None (%) | Mild (%) | Marked (%) | ||||
| 40 Gy/15 fractions | 411 | 376 (91.5) | 33 (8.0) | 2 (0.5) | 283 | 249 (88.0) | 33 (11.7) | 1 (0.3) | 1 | - | - |
| 27 Gy/5 fractions | 429 | 362 (84.4) | 48 (11.2) | 19 (4.4) | 308 | 225 (73.1) | 70 (22.7) | 13 (4.2) | 2.29 (1.60 to 3.27) | < 0.0001 | - |
| 26 Gy/5 fractions | 427 | 381 (89.2) | 33 (7.7) | 13 (3.0) | 284 | 247 (87.0) | 28 (9.9) | 9 (3.2) | 1.26 (0.85 to 1.86) | 0.24 | 0.0006 |
At 2 years, mild/marked change in photographic breast appearance was reported in 35/411 (8%) for 40 Gy, 67/429 (16%) for 27 Gy and 46/427 (11%) for 26 Gy; corresponding figures at 5 years were 34/283 (12%) for 40 Gy, 83/308 (27%) for 27 Gy and 37/284 (13%) for 26 Gy (see Table 13). Modelling 2- and 5-year photographic assessments together, 27 Gy had a statistically significantly increased risk of mild/marked change in breast appearance compared with 40 Gy (OR 2.29, 1.60 to 3.27; p < 0.0001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.26, 0.85 to 1.86; p = 0.24; see Table 13). Twenty-six Gy had a statistically significantly lower risk of change in photographic breast appearance compared with 27 Gy (p = 0.0006).
At 2 years, mild/marked retraction/distortion was reported in 14/411 (3.4%) for 40 Gy, 27/429 (6.3%) for 27 Gy and 14/427 (3.3%) for 26 Gy; corresponding figures at 5 years were 3/283 (1.1%) for 40 Gy, 25/308 (8.1%) for 27 Gy and 14/284 (4.9%) for 26 Gy (see Table 14). Modelling 2- and 5-year photographic assessments together, 27 Gy had a statistically significantly increased risk of mild/marked change in breast appearance compared with 40 Gy (OR 2.83, 1.50 to 5.34; p = 0.001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.59, 0.79 to 3.18; p = 0.190; see Table 14). There was no statistically significantly difference in risk of retraction/distortion for 26 Gy compared with 27 Gy (p = 0.056).
| 2 years | 5 years | OR for mild/marked change vs 40 Gy (95% CI) | Comparison with 40 Gy; p-valuea | Comparison between 27 Gy and 26 Gy; p-valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | None (%) | Mild (%) | Marked (%) | N | None (%) | Mild (%) | Marked (%) | ||||
| 40 Gy/15 fractions | 411 | 397 (96.6) | 14 (3.4) | 0 | 283 | 280 (98.9) | 3 (1.1) | 0 | 1 | - | - |
| 27 Gy/5 fractions | 429 | 402 (93.7) | 21 (4.9) | 6 (1.4) | 308 | 283 (91.9) | 19 (6.2) | 6 (1.9) | 2.83 (1.50 to 5.34) | 0.001 | - |
| 26 Gy/5 fractions | 427 | 413 (96.7) | 12 (2.8) | 2 (0.5) | 284 | 270 (95.1) | 11 (3.9) | 3 (1.1) | 1.59 (0.79 to 3.18) | 0.190 | 0.056 |
Severe late adverse effects and specialist referrals for radiotherapy-related adverse effects in the Main Trial
The most common specialist referral for radiotherapy-related AE during follow-up was to lymphoedema clinics (see Table 15). Incidence of ischaemic heart disease, symptomatic rib fracture and symptomatic lung fibrosis was very low at this stage of follow-up (see Table 16).
| Specialist referral typea | 40 Gy (N = 1361) (%) | 27 Gy (N = 1367) (%) | 26 Gy (N = 1368) (%) |
|---|---|---|---|
| Lymphoedema | 90 (6.6) | 122 (8.9) | 106 (7.7) |
| Breast surgery/breast surgeon | 13 (0.9) | 13 (0.9) | 17 (1.2) |
| Cardiology | 5 (0.4) | 10 (0.7) | 7 (0.5) |
| Pulmonary/respiratory | 4 (0.3) | 6 (0.4) | 3 (0.2) |
| Dermatology | 4 (0.3) | 2 (0.1) | 3 (0.2) |
| Pain | 6 (0.4) | 5 (0.4) | 2 (0.1) |
| Other | 3 (0.2) | 6 (0.4) | 4 (0.3) |
| 40 Gy (N = 1361 (%) | 27 Gy (N = 1367) (%) | 26 Gy (N = 1368) (%) | |
|---|---|---|---|
| Symptomatic rib fracture | |||
| Reporteda | 14 (1.0) | 25 (1.8) | 20 (1.5) |
| Confirmedb | 6 (0.4) | 13 (1.0) | 12 (0.9) |
| Ipsilateral side | 5 (0.4) | 11 (0.8) | 8 (0.6) |
| Symptomatic lung fibrosis | |||
| Reportedc | 9 (0.7) | 10 (0.7) | 10 (0.7) |
| Confirmedb | 6 (0.4) | 9 (0.7) | 7 (0.5) |
| Ipsilateral side | 4 (0.3) | 8 (0.6) | 5 (0.4) |
| Ischaemic heart disease | |||
| Reportedd | 13 (1.0) | 17 (1.2) | 24 (1.7) |
| Confirmedb | 12 (0.9) | 11 (0.8) | 10 (0.7) |
| Left sided | 6 (0.4) | 8 (0.6) | 3 (0.2) |
Estimation of radiobiology parameters for late adverse effects
The unadjusted α/β estimate for any moderate/marked clinician-assessed NTE in the breast/chest wall was 1.7 Gy (1.2–2.3), giving EQD2 estimates of 47.1 Gy for 40 Gy/15 fractions, 51.6 Gy for 27 Gy/5 fractions and 48.3 Gy for 26 Gy/5 fractions; adjusting for prognostic factors (age, boost, whole breast planning treatment volume as a proxy for breast size) made very little difference. α/β estimated from the photographic endpoint (adjusting for breast size and surgical deficit evaluated from the baseline photographs) was very similar (1.8 Gy; 1.1–2.4). The unadjusted α/β estimate for patient-reported change in breast appearance was 2.3 Gy (1.8–2.9), resulting in EQD2 estimates of 46.1 Gy, 48.2 Gy and 45.2 Gy for the 40 Gy, 27 Gy and 26 Gy schedules, respectively; as above, adjusting for covariates made minimal difference.
Assuming no clinically significant time effect for late AE between 1 and 3 weeks, complete sublethal damage repair between fractions and an α/β of 2.8 Gy for late NTE, the last assumption based on the combined estimates of α/β in START-A and FAST. On this basis, the relative EQD of the FAST-Forward schedules to 50 Gy in 25 fractions are shown in Table 17, where negative values indicate estimated AE rates lower than 50 Gy in 25 fractions.
| Fractionation regimen | EQD2/2.8 (Gy) | ΔAE (%)a |
|---|---|---|
| 50 Gy/25 fractions/5 weeks | 50.0 | Reference |
| 40.05 Gy/15 fractions/3 weeks | 45.6 | −12.3 |
| 27 Gy/5 fractions/1 week (5.4 Gy/fractions) | 46.1 | −11.1 |
| 26 Gy/5 fractions/1 week (5.2 Gy/fractions) | 43.3 | −18.8 |
Five-year subgroup analyses in the Main Trial
As stated in the Statistical Methods section in Chapter 2, the Main Trial protocol did not include pre-specified subgroup analyses, but exploratory post hoc subgroup analyses of the primary endpoint and of NTE were carried out.
Risk group was a stratification factor for the Main Trial at randomisation, with low-risk defined as age ≥ 50 and grade 1 or 2, and high-risk defined as age < 50 and/or grade 3; 1545 (37.8%) patients were in the high-risk category. Retrospective subgroup analyses comparing IBTR in 26 Gy versus 40 Gy provide no evidence of a differential effect according to age, grade, pathological tumour size, nodal status, tumour bed boost, adjuvant chemotherapy, HER-2 status and in triple-negative patients (see Figure 12). CIs for the HRs overlap for the subgroups, although the number of events in these analyses was small (52), hence results should be interpreted with caution as the statistical power is low. Subgroup analysis according to type of primary surgery was not possible as there was only one IBTR event post-mastectomy in a control group patient (out of 91) and none in the 173 patients treated with 5 fractions. Subgroup analyses of IBTR for 27 Gy/5 fractions versus 40 Gy/15 fractions are shown in Figure 13. Table 18 and Appendix 2, Table 43 show the frequencies and number of patients according to subgroups defined by age, grade, primary surgery type and receptor status. No evidence to signal concern was seen for the 5-fraction schedules. The use of boost and dose/fractionation, both declared prior to randomisation, were balanced between the three treatment groups minimising risk of bias in dose intensity between trial groups.
| Subgroup | Event/number | 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions |
|---|---|---|---|---|
| Age under 50 years at randomisation | Events | 3 | 7 | 4 |
| Number at risk | 198 | 189 | 217 | |
| Grade 3 | Events | 20 | 15 | 8 |
| Number at risk | 386 | 389 | 378 | |
| Mastectomy | Events | 1 | 0 | 0 |
| Number at risk | 91 | 89 | 84 | |
| ER negative/HER-2 negativea | Events | 10 | 5 | 3 |
| Number at risk | 111 | 96 | 128 | |
| HER-2 positive | Events | 4 | 7 | 2 |
| Number at risk | 135 | 137 | 135 |
FIGURE 12.
Subgroup analyses of time to IBTR for 26 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0). 2
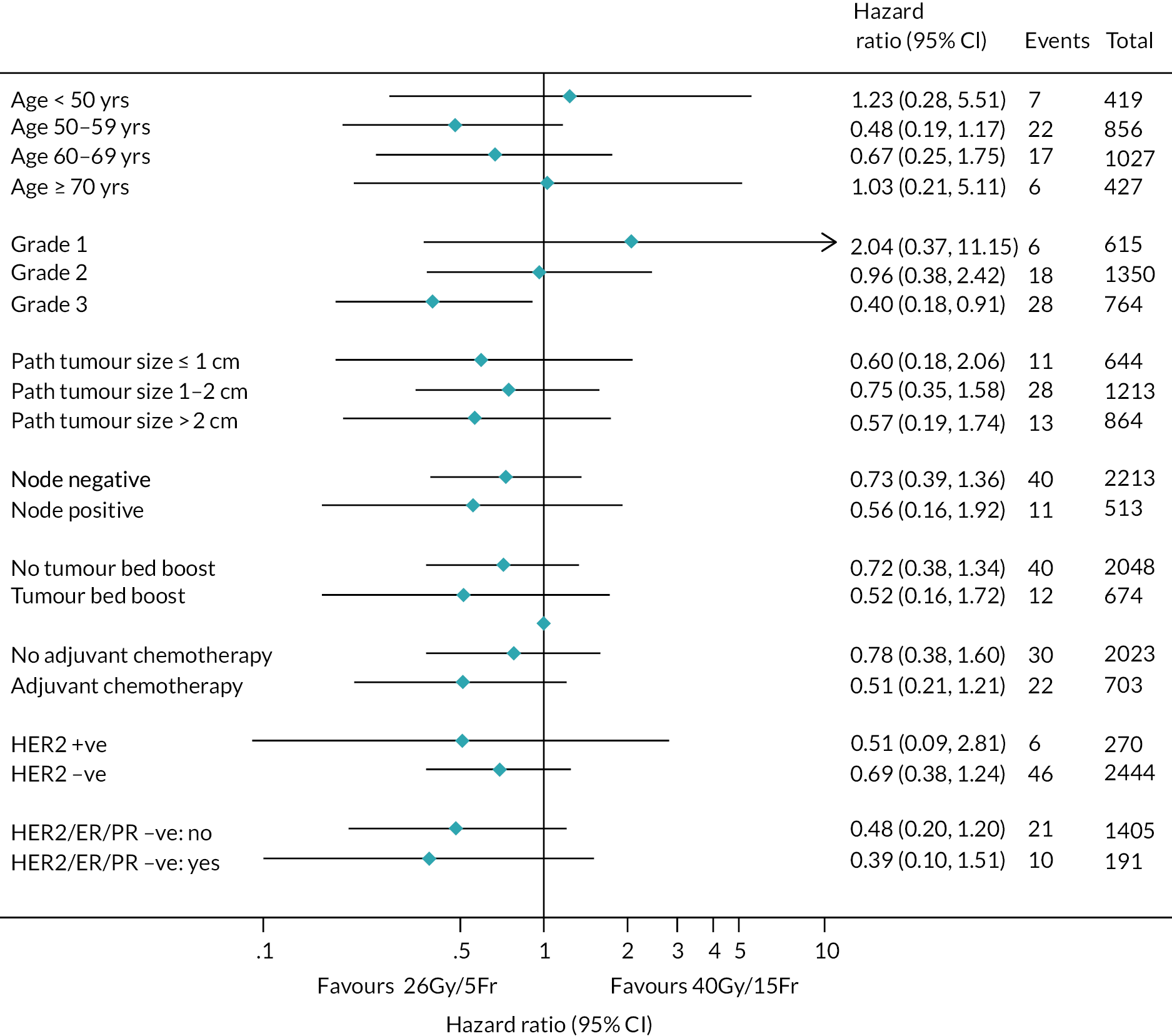
FIGURE 13.
Subgroup analyses of time to IBTR for 27 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0). 2
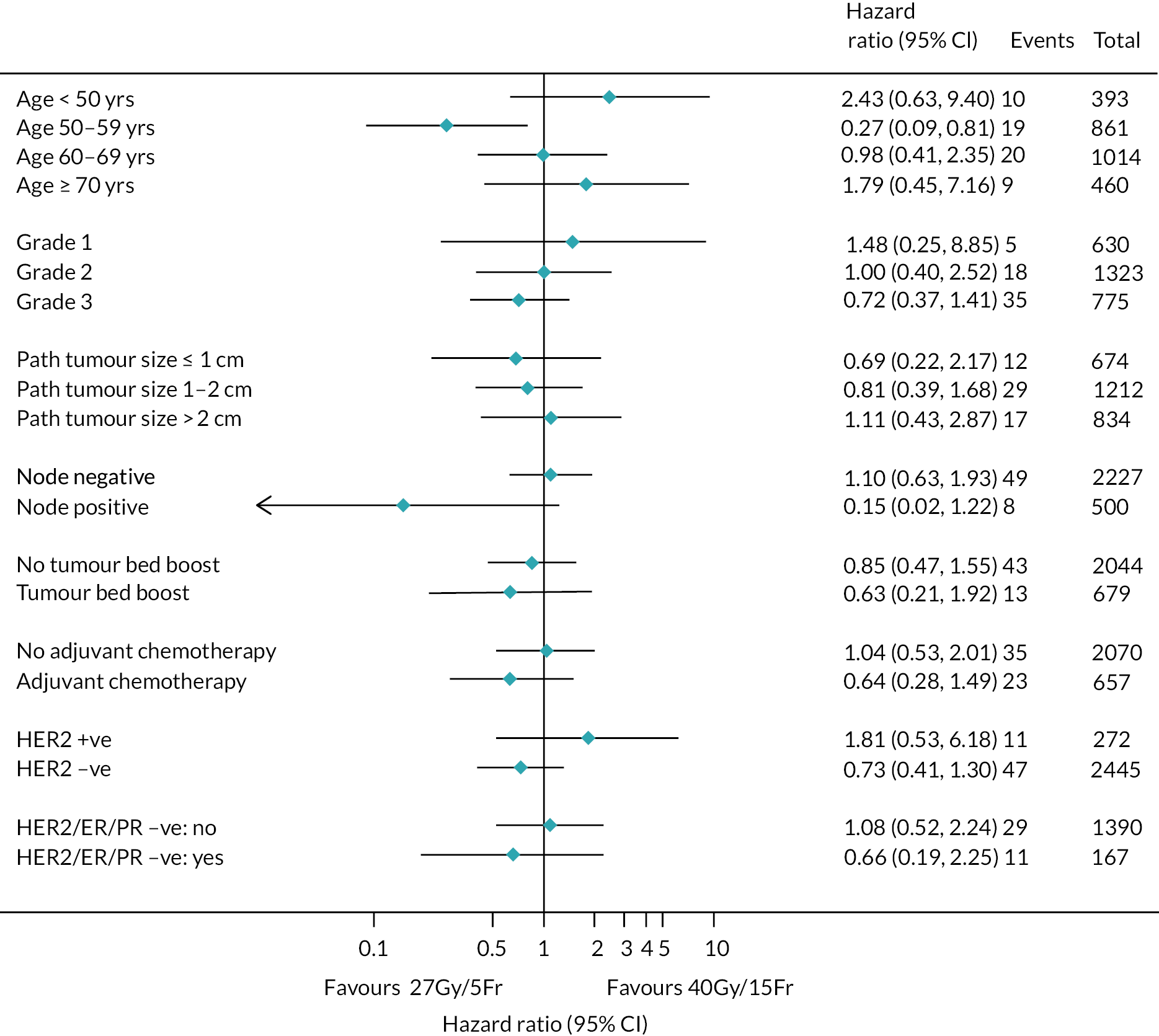
Retrospective subgroup analyses comparing time to first clinician-assessed moderate or marked AE in the breast or chest wall for 26 Gy versus 40 Gy provided no evidence of a differential effect of the 5-fraction schedule according to age, breast size, surgical deficit, tumour bed boost, or adjuvant chemotherapy, as CIs for subgroups overlap, although power for these retrospective subgroup analyses is low (see Figure 14). The corresponding figure for 27 Gy/5 fractions versus 40 Gy/15 fractions is shown in Figure 15.
FIGURE 14.
Subgroup analyses of time to first moderate or marked clinician-assessed adverse event in breast/chest wall for 26 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0). 2
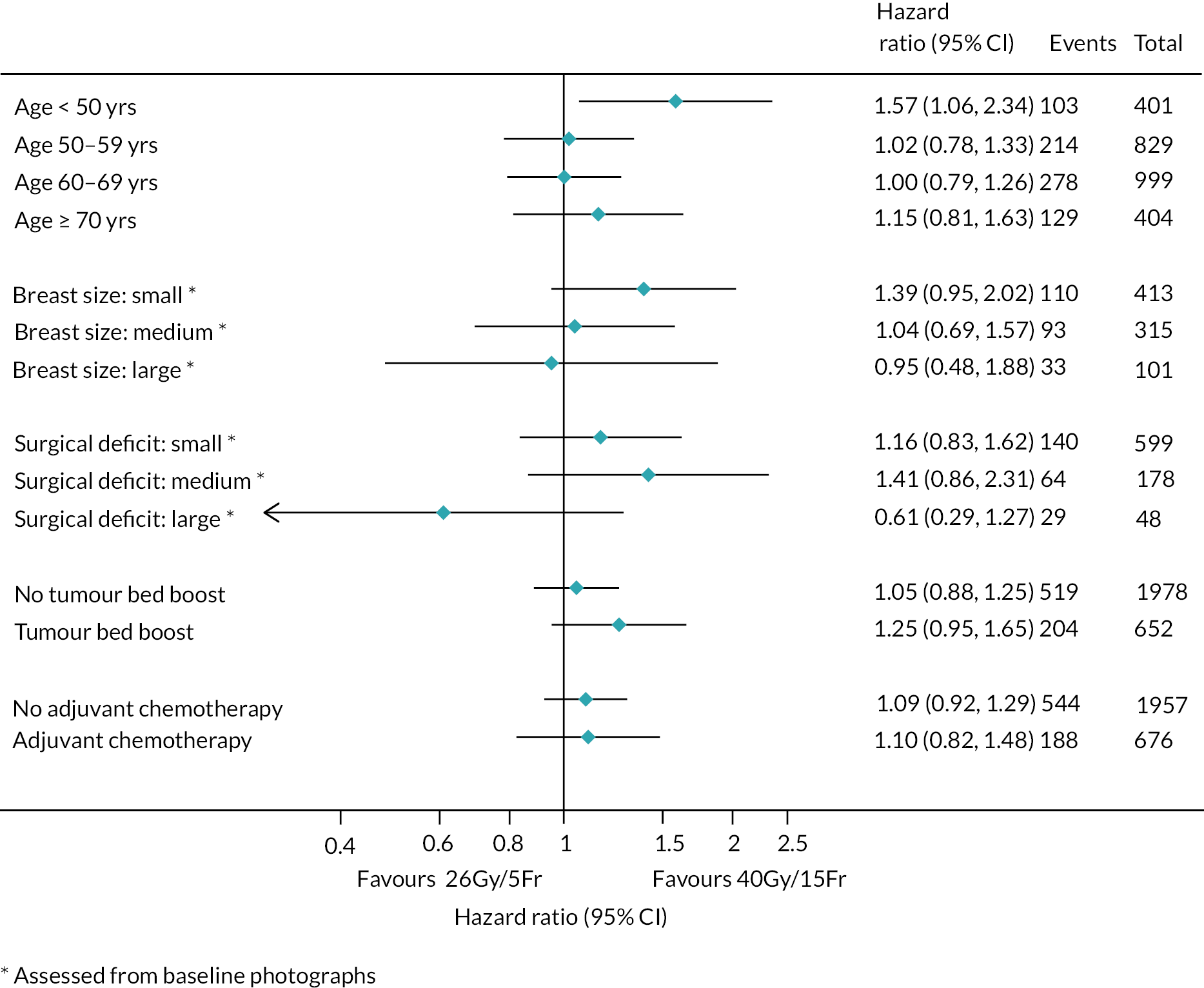
FIGURE 15.
Subgroup analyses of time to first moderate or marked clinician-assessed adverse event in breast/chest wall for 27 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0). 2
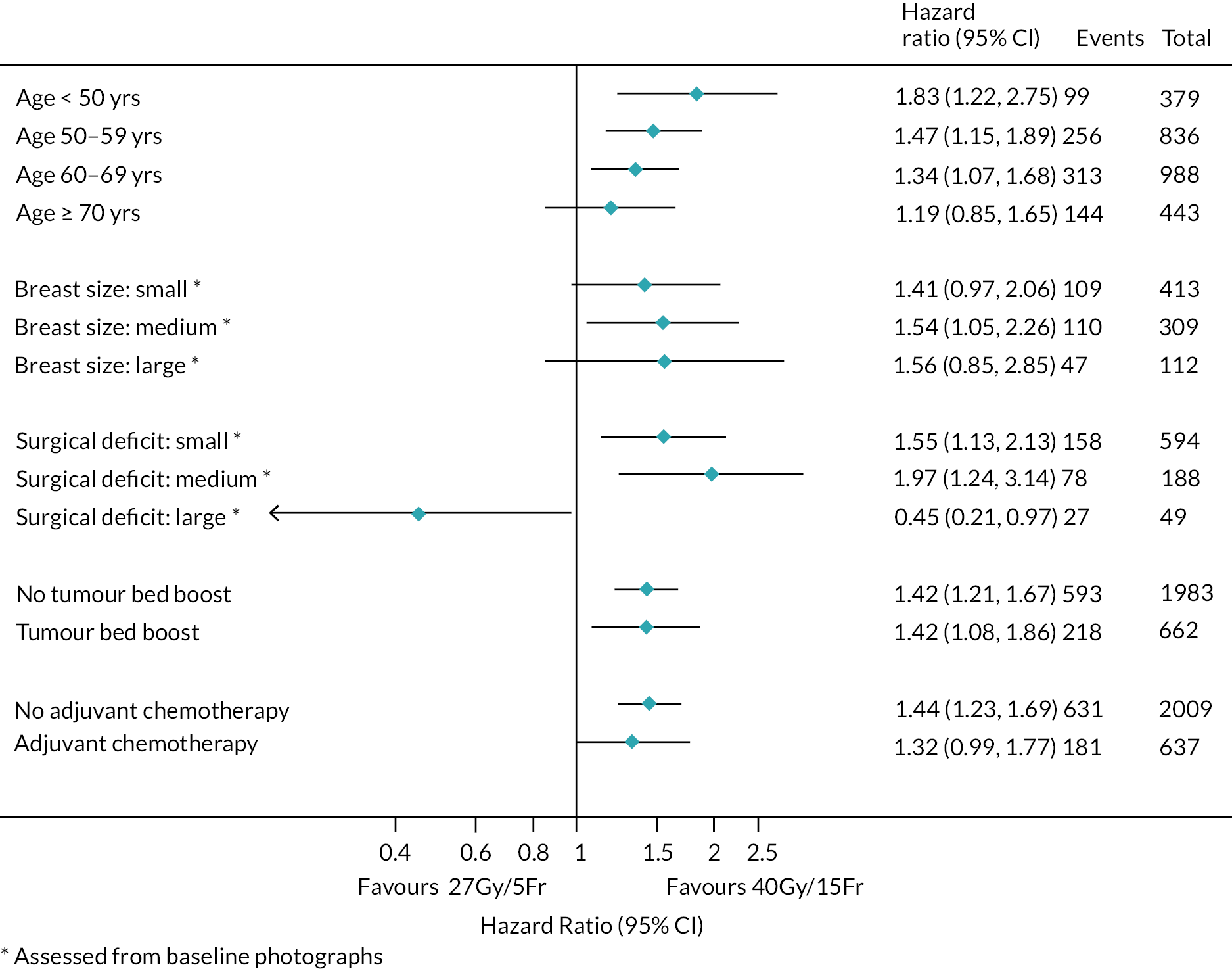
Discussion of Main Trial results is in the main Discussion section (see Chapter 8).
Chapter 6 Main Trial cost-effectiveness analysis
Introduction
An economic evaluation was conducted to assess the cost-effectiveness of whole breast RT with 26 Gy delivered in 5 fractions over 1 week compared to the international standard 3-week schedule of 40 Gy in 15 fractions. The target population was UK adults who have undergone breast-conserving surgery or mastectomy for early breast cancer (stage I/II/IIIa).
A decision analytic model was constructed to estimate the costs and benefits of each option over a lifetime time horizon. The analysis followed the reference case outlined by the NICE in which health effects and costs which fall on the NHS were considered. 68 The economic analysis excludes the trial arm schedule of 27 Gy delivered in 5 fractions over 1 week as this was associated with higher normal tissue effect risk compared to 40 Gy and 26 Gy, and is not considered a relevant comparator for future clinical practice.
Methods
Lifetime costs and health outcomes were estimated for each treatment using a Markov model. A discount rate of 3.5% per year was used for costs and health effects. Costs were set to a base year of 2019, adjusting for inflation. 69 Quality-adjusted life-years (QALYs) were used to capture health impacts.
R Statistical programming language and was used to build the model and coding principle from the DARTH modelling group were followed. 70,71
Model structure and patient population
The target population was UK adults who have undergone breast-conserving surgery or mastectomy for early breast cancer (stage I/II/IIIa). The patient population for the model matched the inclusion criteria for FAST-Forward as described in Chapter 2. This included individuals with tumour grades 1–3, ER positivity and negativity, HER-2 positivity and negativity and those with or without regional lymph node metastasis.
A Markov model was built to estimate the total QALYs and costs for each treatment alternative. The structure of the economic model is illustrated in Figure 16. Circular arrows indicate the possibility of staying in the same state for multiple cycles, and linear arrows represent the possible transitions between states. The model structure was developed in discussion with clinical experts. A single health state was used to capture locoregional relapse, which includes local relapse and regional relapse as these relapses have similar prognostic and cost implications. The Markov model cycle length was 1 year as outcomes occur over a long period. A half-cycle correction was applied.
FIGURE 16.
Markov model structure of early breast cancer.
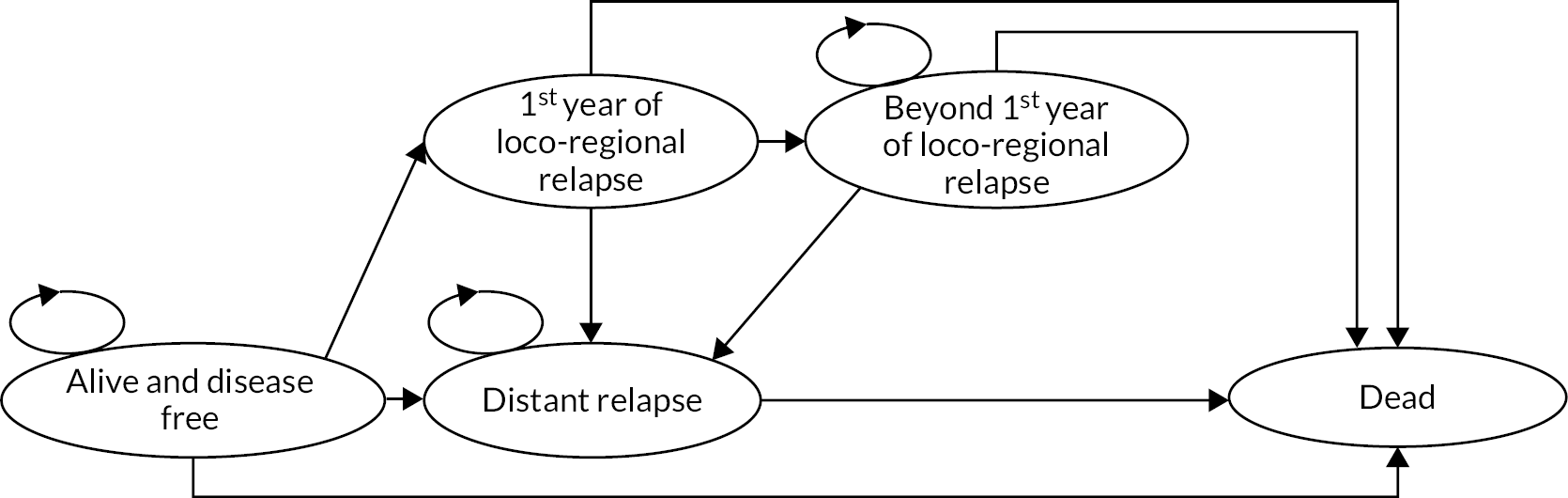
Patients entered the model following local tumour excision and received RT in either 15 fractions or 5 fractions. The RT schedules differed in terms of resource use and the likelihood of inducing acute skin reactions. Following RT, patients began in the ‘alive and disease-free’ health state and over time were at risk of locoregional relapse, distant (metastatic) relapse or death. Radiotherapy modality determined how patients move through states over time.
Radiotherapy treatment period
This section concerns costs of delivering RT and the costs of managing acute side effects. Patients were assumed to receive treatment as specified in the FAST-Forward protocol. 2 Radiotherapy costs were applied at model entry and are calculated as shown in Table 19. As RT delivery resource activity was not recorded in the trials, resource use was informed by expert opinion. A proportion of patients are instructed to follow breath hold techniques while receiving RT in order to protect the heart. This extends the duration of the RT appointment, and we estimated the consequent increase in cost.
| Input | Value | Source |
|---|---|---|
| Radiotherapy delivery | ||
| Radiotherapy planning cost | £315 | 72 |
| Cost of delivering one fraction of RT | £124 | 72 |
| Percentage receiving breath hold with whole breast RT | 25% | Expert opinion |
| Increase in fraction costs associated with breath hold | 30% | Expert opinion,73,74 |
| Treatment | Formula: (unit cost) × (units per patient) × (proportion of patients) | Cost |
| Whole breast 15 fractions (WB15F) | ||
| Planning cost | £315.00 | |
| RT without breath hold | £124 × 15 × 75% | £1395.00 |
| RT with breath hold | (£124 × 1.3) × 15 × 25% | +£604.50 |
| Total | £2314.50 | |
| Whole breast 5 fractions (WB5F) | ||
| Planning cost | £315.00 | |
| RT without breath hold | £124 × 5 × 75% | £465.00 |
| RT with breath hold | (£124 × 1.3) × 5 × 25% | +£201.50 |
| Total | £981.50 | |
We included costs relating to treating acute adverse skin reactions during treatment. These were assumed to be one-off costs which depend on the severity of AE as measured by worst RTOG score observed during treatment. 3 Preference-based measures of health-related quality of life (HRQoL) were collected at baseline and at 3 months, but not during receipt of RT when acute skin reactions would be present. Literature review did not identify any studies that linked HRQoL measures to RTOG scores. Quality of life was assumed to be the same across 5 and 15 fractions during the treatment period due to an absence of preference-based quality of life data for this period. This assumption was viewed to be conservative towards the 26 Gy schedule given the numerically lower number of events compared to 40 Gy.
Post radiotherapy period
Time to locoregional relapse, distant relapse and some maintenance costs and HRQoL parameters were estimated from FAST-Forward individual patient data. Stata 16 was used to carry out all individual patient data analysis. 75 The remaining model parameters were informed by searching the wider literature. All model inputs for this period are presented in Table 20.
| Input | Mean | Standard error | Distribution | Source |
|---|---|---|---|---|
| Population characteristics | ||||
| Average age at beginning of model | ||||
| Subgroup 1 | 63 years | - | - | FF |
| Subgroup 2 | 60 years | - | - | FF |
| Acute side effects during treatment | ||||
| Costs of treating adverse skin reactions for states: RTOG0, RTOG1, RTOG2a, RTOG2b, RTOG3 | £0, £0, £132, £132, £136 | - | - | Expert opinion90 |
| Probability of RTOG states: RTOG0, RTOG1, RTOG2a, RTOG2b, RTOG3 | ||||
| 15 fractions RT | 0%, 32%, 27.5%, 27.5%, 14% | - | Dirichlet | 3 |
| 5 fractions RT | 6%, 62%, 13.5%, 13.5%, 6% | - | Dirichlet | 3 |
| Transition rate between states | ||||
| Alive disease free to locoregional relapse: 15 fractions | ||||
| Subgroup 1 | 0.0038 | 0.0014 | Exponential | FF |
| Subgroup 2 | 0.0073 | 0.0014 | Exponential | FF |
| Alive disease free to distant relapse: 15 fractions | ||||
| Subgroup 1 | 0.0024 | 0.0007 | Exponential | FF |
| Subgroup 2 | 0.0128 | 0.0018 | Exponential | FF |
| Alive and disease free to death | Variable – dependent on age | - | - | 78 |
| Locoregional relapse to distant relapse | 0.0515 | 0.0045 | Exponential | 80 |
| Locoregional relapse to death | Variable – dependent on age | - | - | 78 |
| Distant relapse to death | ||||
| Subgroup 1 | 0.2196 | 0.5102 | Exponential | 79 |
| Subgroup 2 | 0.21 | 0.5102 | ||
| Relative treatment effect estimates | ||||
| Alive disease free to locoregional relapse: 5 fractions vs. 15 fractions | 0.66 | 0.167 | Log-normal | 23 |
| Healthcare costs | ||||
| Alive and disease free (annual) | ||||
| Subgroup 1 | £1216 | £82 | Gamma | FF |
| Subgroup 2 | £1412 | £68 | Gamma | FF |
| Additional costs of 1st year alive and disease free | £402 | £64 | Gamma | FF |
| 1st year of locoregional relapse | ||||
| Treatment costs | £4241 | ±20% | Gamma | 82–84,86 |
| Supportive care costs | £2995 | ±20% | Gamma | 50,86 |
| Beyond 1st year locoregional relapse (annual) | £2139 | ±20% | Gamma | 50,86 |
| Distant relapse (annual) | £13,426 | ±20% | Gamma | 85,86 |
| Health-related quality of life | ||||
| Alive and disease free | ||||
| Subgroup 1 | 0.7880 | 0.0130 | Gamma | FF |
| Subgroup 2 | 0.7730 | 0.0060 | Gamma | FF |
| Locoregional relapse | ||||
| Subgroup 1 | 0.7880 | 0.0130 | Gamma | FF |
| Subgroup 2 | 0.7730 | 0.0060 | Gamma | FF |
| Decrement for distant relapse relative to alive and disease free | 0.3030 | 0.1550 | Gamma | 88 |
| Quality of life decrement with age | Variable – dependent on age | - | - | 89 |
Transition probabilities
Transition probabilities estimated from FAST-Forward
The rate of locoregional relapse and distant relapse outcomes were estimated using separate models. This is the approach used in the clinical analyses in which separate Cox models are fit to locoregional and distant relapse (see Chapter 5). To choose an appropriate statistical model, the algorithm outlined in DSU guidance (2013) was used. 76 It was found that an exponential survival model fit the data best for both locoregional relapse and distant relapse. Data from patients receiving 40 Gy and 26 Gy arm was used to fit the model (n = 2723) with a dummy to indicate the treatment schedule.
In the trial 45% would be considered at lower than average risk of local relapse and eligible for partial breast RT according to Royal College of Radiologists guideline. 77 Our economic model estimates outcomes for the combined FAST-Forward population but allows for a separate analysis of low and high-risk populations. 12,77 A subgroup dummy was included in the survival analysis to reflect differences in baseline risk of local relapse based on patient characteristics. The average ages in subgroups 1 and 2 were 63 and 60, respectively.
Definition of low- and high-risk subgroups
Subgroup 1: Lower risk of local relapse (eligible for partial breast RT under RCR guidelines). All of the following criteria: 50+ years old, and tumour grade 1–2, and tumour size ≤ 3 cm, and ER positive, and HER-2 negative, and with no regional lymph node metastasis (N0).
Subgroup 2: Higher risk of local relapse (not eligible for partial breast RT). Any of the following criteria: < 50 years old, or tumour grade 3, or tumour size > 3 cm or ER negative, or HER-2 positive or with regional lymph node metastasis (N1 or greater).
In the dataset, some individuals were recorded as having a distant relapse and locoregional relapse at the same time point. As a sensitivity analysis, we estimated the time to first event (either distant or locoregional), censoring individuals that experience more than one type of relapse (each participant only contributes their 1st event). For this we used a log-normal survival model which also fit the data well.
Estimated parameters for all survival analyses are reported in Appendix 2, Tables 44–51.
Transition probabilities for the remaining model states
The assumption was made that individuals who have not experienced a distant relapse have the same rate of all-cause mortality as the age- and sex-matched general population. 78 Patients who developed distant relapse were at increased risk of death. This risk was based on a French study of metastatic breast cancer. In the base case, mortality with distant relapse was adjusted for age and based on the hormone receptor-positive and HER-2 negative molecular subtype. This was chosen as it is the most common subtype in FF. The impact of using alternative molecular subtypes was investigated in sensitivity analyses. 79 The assumption was made that increased mortality from breast cancer occurs by first experiencing distant relapse. Therefore, the UK rate of breast cancer mortality was removed from all-cause mortality to avoid double counting. 12
The rate of transition from locoregional to distant relapse was taken from a Dutch study of breast cancer. 80 It was assumed that RT modality did not affect the rate of transition from locoregional to distant relapse or the mortality risk with distant relapse.
Treatment effects
To model the rates of locoregional relapse for 5 fractions relative to 15 fractions, we applied the HR for locoregional relapse estimated in FF to the baseline rate of locoregional relapse estimated in the survival model. For both 5 fractions and 15 fractions, a common rate of transition from alive and disease free to distant relapse was assumed. This assumption was based on the clinical rationale that RT is a local treatment and therefore its causal impact on developing distant relapse (if any) can only occur through reducing locoregional relapses. The impact of this assumption was explored in a sensitivity analysis. For the base case, treatment effects were assumed to persist over time.
Costs
Costs for the alive and disease-free state estimated from FAST-Forward
The resource use questionnaire collected in FAST-Forward covered activities related and unrelated to breast cancer such as general practitioner costs, nursing costs and hospitalisations. To construct per-patient costs, unit costs were applied to resource use, see Appendix 2, Table 52.
A panel of cost data was constructed for each data collection time period. Individuals only contributed data if there were classified as being in the alive and disease-free state. Summary statistics are shown in Appendix 2, Table 53.
A GEE model was fit to the 3-month cost data. 75 This model had an exchangeable within-individual correlation structure. A gamma family distribution was chosen to reflect the non-negativity and skewed distribution of cost data. A log link was chosen given the typical structure of cost data. The GEE model utilises all available observations of the dependent variable. This is equivalent to a complete case analysis (CCA). CCA was chosen as the degree of missing data in the study was low, < 25% at any time point. This implies a missing completely at random assumption (MCAR) that is missingness is independent of both observed and unobserved values. 81 When fitting the models, baseline costs were discarded as it is the post-treatment costs that are to be estimated.
Plotting the data revealed a large drop between costs in the first 6 months after treatment and the costs from 6 months onward. This discontinuity was modelled by defining a binary covariate indicating 6 months pre- and post-treatment. A dummy for high versus low risk was also included in the analysis. Age and treatment were found not to be statistically significant and so were not included in the model.
The following values were estimated after scaling costs up from average costs over 3 months to 12 months:
-
Yearly costs in subgroup 1: low risk = £1216, with SE = £82.
-
Yearly costs in subgroup 2: high risk = £1412, with SE = £68.
-
Additional costs associated with first year after treatment = £402 with SE £64.
Costs for the remaining model states
These costs were sourced from the wider literature as there were insufficient observations to estimate them from FAST-Forward. Locoregional relapse was associated with one-off mastectomy costs in addition to supportive care costs in the first year of the event. 50,82–84 Following this first year supportive care was assumed to consist of one GP visit and one mammogram per year. 50 A UK study was used to estimate supportive care and treatment costs for distant relapse. 85
To make them consistent with the inclusion of related and unrelated costs in the FAST-Forward resource use questionnaire, costs unrelated to breast cancer were added to the breast cancer costs for locoregional and distant relapse health states. 86
Health-related quality of life
Health-related quality of life for the alive and disease-free state estimated from FAST-Forward
Quality of life (QoL) was captured in FAST-Forward by the EuroQol EQ-5D 5 level questionnaire. NICE’s current advice is to map 5L scores to 3L using the method described by van Hout et al. 87
Data was collected in the PRO sub-study (n = 1179) at baseline (before randomisation or RT), 3, 6 months post treatment and 1, 2 and 5 years post randomisation.
A panel of QoL data was constructed for each data collection time period. Individuals only contributed data if there were classified as being in the alive and disease-free state. Summary statistics are shown in Appendix 2, Table 54.
A generalised liner model (GLM) based on the wave of data closest to treatment was used for simplicity as time was found to have no statistical impact on results. A gamma distribution for disutility was used as this fits the range utility data. An identity link was chosen.
A complete case analysis (CCA) was chosen as the degree of missing data in the study was low at < 25% at any time point. This implies a missing completely at random assumption (MCAR) that is missingness is independent of both observed and unobserved values (Faria et al. ). 81
Using the full sample (6143 observations on 1044 patients), we estimated a GLM model. This model estimated disutilities (1 – EQ-5D score). It included a dummy for the alive and disease-free state, for high versus low risk, a time dummy equal to one for all post-treatment time points, all possible interactions between the previous dummies and a dummy for the treatment arm. The average EQ-5D score for patients in the alive and disease-free state in subgroup 1 was 0.788 (SE = 0.013, p-value < 0.001), for subgroup 2 this was 0.773 (SE = 0.006, p-value < 0.001).
Health-related quality of life for the remaining model states
Health-related quality of life post locoregional relapse was assumed to be the same as for the alive and disease-free state. The decrement in HRQoL with distant relapse was taken from a previous RT decision model. 88 A Health Survey for England study was used to model the decline in HRQoL with age. 89
Uncertainty analyses
The distributions described in Table 20 were applied to each of the parameters to represent uncertainty in the model. A probabilistic sensitivity analysis (PSA) was used to reflect the impact of this joint uncertainty on outcomes. This involved taking 10,000 draws from the distributions and calculating model outcomes for each iteration. One-way sensitivity analysis was used to explore the sensitivity of results to one-at-a-time changes in individual inputs and assumptions, see Table 21.
| Scenario | FAST-Forward population | |
|---|---|---|
| Whole breast 15 fractions (%) | Whole breast 5 fractions (%) | |
| ICER (probability treatment ICER < Threshold) | ||
| Base case (£15,000/QALY) | Dominated (0.1) | £0 (99.9) |
| Threshold £20,000/QALY | Dominated (0.2) | £0 (99.8) |
| Threshold £30,000/QALY | Dominated (0.4) | £0 (99.6) |
| Distant relapse HR estimated in FAST-Forward | £0 (66.2) | £6271 (33.8) |
| All treatment effects maintained for 10 years | Dominated (0.1) | £0 (99.9) |
| All treatment effects maintained for 5 years | Dominated (0.1) | £0 (99.9) |
| Mortality rate following distant relapse based on HER-2+ population79 | Dominated (0.1) | £0 (99.9) |
| Mortality rate following distant relapse based on TNBC (HR− and HER-2−) population79 | Dominated (0.1) | £0 (99.9) |
| Distant relapse costs reduced to £8934 per year94 | Dominated (0.1) | £0 (99.9) |
| Distant relapse costs increased to £16,111 per year (20% increase) | Dominated (0.1) | £0 (99.9) |
| Disutility resulting from distant relapse reduced to 0.2695 | Dominated (0.1) | £0 (99.9) |
| Disutility resulting from distant relapse increased to 0.3636 (20% increase) | Dominated (0.1) | £0 (99.9) |
| Use of breath hold assumed to not increase cost to deliver one fraction of RT (£124) | Dominated (0.1) | £0 (99.9) |
| Use of breath hold assumed to double the cost to deliver one fraction of RT (£248) | Dominated (0.1) | £0 (99.9) |
| Rate of adverse skin reactions set equal across all treatment options | Dominated (0.1) | £0 (99.9) |
| Health-related quality of life weight during RT set to zero | Dominated (0.1) | £0 (99.9) |
| Log-normal survival model in which each participant only contributes their 1st event | Dominated (12) | £0 (88) |
Cost-effectiveness analysis
Discounted costs and QALYs were calculated for each treatment option taking account of outcomes over the patient lifetime. Incremental cost-effectiveness ratios (ICERs) were calculated with whole breast 15 fractions as the comparator treatment. The ICER was calculated as the mean additional costs divided by the mean additional health gained for 5 fractions compared to 15 fractions. The ICER was compared to a cost-effectiveness threshold of £15,000/QALY. This was chosen as it is the value used by the Department of Health in the UK to represent the marginal rate at which NHS activities generate QALYs. 91,92 Sensitivity analysis used the threshold values commonly used in NICE decision-making: £20,000–30,000/QALY. 68
Validation
Model validation was carried out using the TECH-VER checklist. 93
Subgroup analyses
FAST-Forward contained a combination of individuals who were both high and low risk. The model allowed for these subgroups to be analysed separately or as a combined population. The primary analysis reports results for the combined population. Secondary analysis reports results for each population separately.
Results
For the base case analysis, mean costs and QALYs for 15 fractions were £31,640 and 11.08 QALYs, respectively. For 5 fractions these were £29,638 and 11.12 QALYs. Therefore, 5 fractions therapy was expected to dominate with expected cost savings of £2002 (95% CI £1245 to £2804) and higher expected QALYs: 0.04 (95% CI −0.01 to 0.09). Across simulations there was a 99.9% chance that 5 fractions either dominated WB15F or had an ICER below £15,000/QALY. The probabilistic results are shown in the incremental cost-effectiveness plane in Figure 17. The dashed line represents the cost-effectiveness threshold of £15,000 per QALY. The points below this line represent simulations in which 5 fractions is cost-effective relative to 15 fractions. This diamond indicates mean cost savings and expected gains in health.
FIGURE 17.
Base case cost-effectiveness plane comparing 5 fractions to 15 fractions.
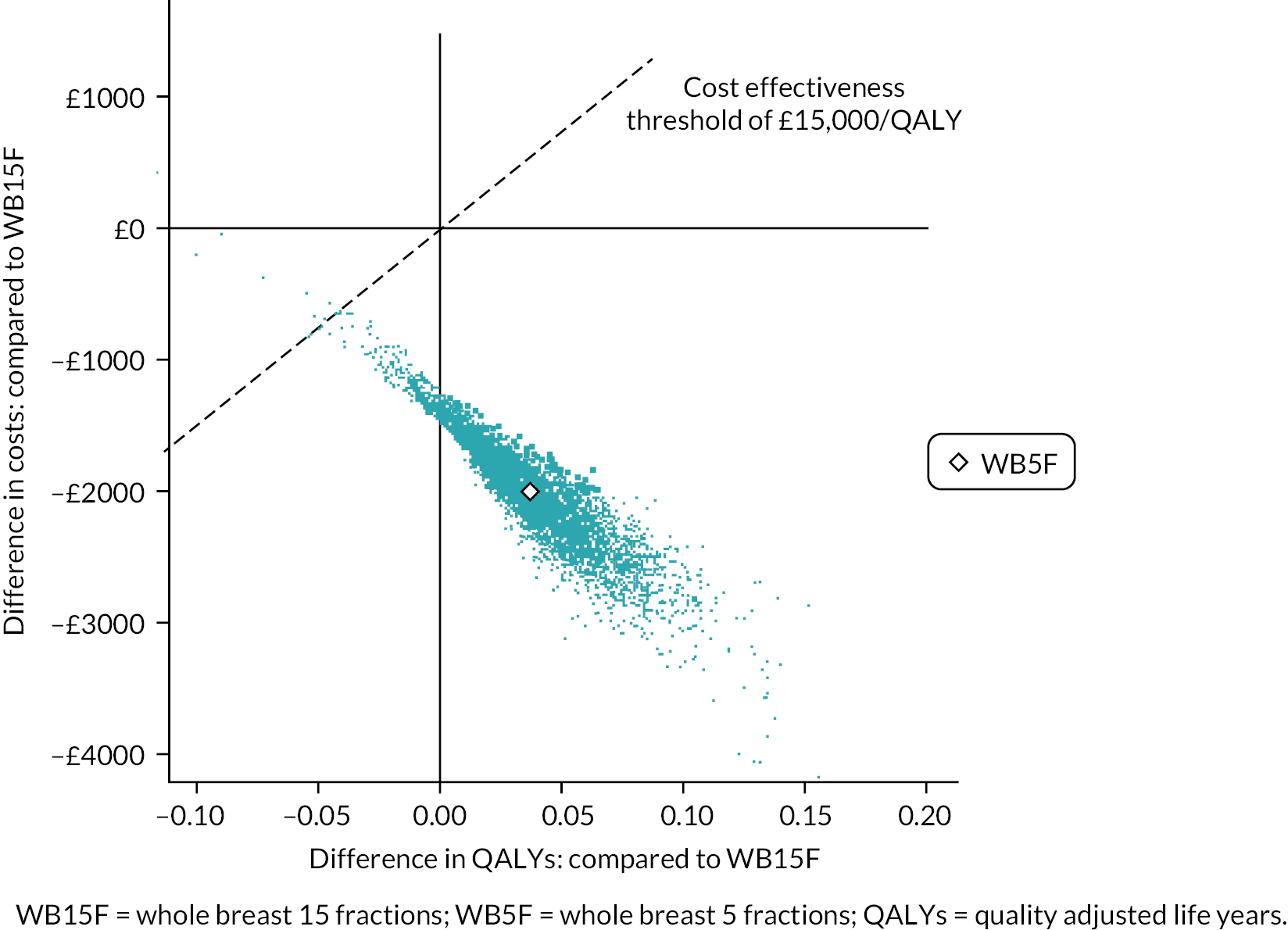
Subgroups
For the low-risk population (subgroup 1), 5 fraction therapy was expected to dominate with expected cost savings of £1881 (95% CI £1252 to £2648) and higher expected QALYs: 0.03 (95% CI −0.01 to 0.07). Across simulations there was a 99.9% chance that 5 fractions either dominated WB15F or had an ICER below £15,000/QALY.
For the high-risk population (subgroup 2), 5 fractions therapy was expected to dominate with expected cost savings of £2102 (95% CI £1230 to £3093) and higher expected QALYs: 0.05 (95% CI −0.01 to 0.11). Across simulations there was a 99.9% chance that 5 fractions either dominated WB15F or had an ICER below £15,000/QALY.
Sensitivity analysis
Based on expected outcomes, 5 fractions dominated all other options except when using the distant relapse HR results estimated in the trial (see Table 21). In this one scenario, 5 fractions compared with 15 fractions was expected to be less expensive with incremental costs of −£908 (95% CI −£2689 to £975) but less effective, with −0.14 incremental QALYs (95% CI −0.43 to 0.12). This placed the expected ICER in the southwest quadrant. With a threshold of £15,000/QALY 5 fractions was expected to have 33.8% chance of being cost-effective in this scenario.
Discussion
Across a range of scenarios, 5 fraction RT were expected to be cost-effective at a threshold of £15,000/QALY. Five fractions was expected to provide more QALYs and have lower costs compared to 15 fractions. The expected cost savings were primarily due to reducing the number of fractions of RT. The improvement in QALYs was driven by the modest expected reduction in locoregional relapses. Figure 17 illustrates how 5 fractions is associated with lower costs and greater QALYs. The figure also illustrates that the spread of points for 5 fractions lies almost completely to the south east of the £15,000/QALY cost-effectiveness threshold. This indicates that the cost-effectiveness of 5 fractions was associated only with minimal parametric uncertainty.
Resource savings from reduced fractionation may enable the same number of patients to be treated with lower linear accelerator capacity therefore freeing capacity to treat breast and other cancers. The increased capacity could also be used to introduce the many improvements in the quality of therapy, such as cardiac-sparing breath hold if not fully implemented. Further, the benefits to patients would immediately be realised in terms of reduced burden of treatment and hospital footfall, and this may have added benefits such as reduced exposure to COVID-19. 96
The sensitivity analyses reported in Table 21 illustrated that these overall conclusions were robust to alternative inputs and assumptions. Across all scenarios the 5 fractions regimens remained the least costly alternative. Using different molecular subtype populations to model mortality following distant relapse did not impact results. 79 This was because in this scenario relative rates of distant relapse were assumed common across arms. In the scenario in which relative rates of distant relapse were calculated using the HR estimated in FAST-Forward, 5 fractions was associated with fewer expected QALYs. With a cost-effectiveness threshold of £15,000/QALY, 5 fractions was not expected to be the cost-effective alternative. The relatively lower QALYs associated with 5 fractions in these scenarios was due to the HR for distant relapse for 5 versus 15 fractions estimated in FAST-Forward. This estimate indicated a higher rate of distant relapse with 5 fractions, HR = 1.27 (0.90 to 1.80) which in absolute terms is equivalent to an increase of 1% (−0.4% to 2.9%). Though this was not statistically significant (p = 0.17) distant relapse had a large impact on both morbidity and mortality in the economic model and so drove differences in expected QALYs between treatment options.
The key assumption in the base case analysis is that there was a common pattern of transition from alive and disease free to distant relapse across treatment options. This assumption was based on the clinical argument that RT is a local treatment and therefore has local effects. One alternative approach to capture treatment effects in the model would be to estimate the rate of any relapse (distant or local or regional). This approach could be used to estimate baseline and relative effects across RT modalities. This alternative approach was not carried out here so that the economic analysis followed the analysis in the published trial. 23 Additionally, estimating the difference in any relapse between arms would imply a common treatment effect on local, distant and regional relapses which may not be clinically plausible. Even if this approach was used, it would remain necessary to separate out the different relapse types as they have different costs and health consequences. A fully comprehensive approach may require a multistate modelling framework and thus a different approach to both data collection and modelling. 97,98 This approach would need to take account of recognised issues with effect identification. 99,100 Further research is required in this area.
Other limitations of the analysis are described here. The HRQoL impact of acute skin reactions was not captured due to lack of EQ-5D data during the treatment period, only treatment costs were included. Including HRQoL impacts into the analysis would likely improve the relative benefits of 5 fraction therapy as this was observed to have fewer severe acute adverse reactions than 15 fractions in Brunt et al. 3
To our knowledge, no other studies have examined the cost-effectiveness of 5 fraction RT in a UK context. For the US system, Deshmukh et al. compared whole breast RT delivered over 5–7 weeks to RT delivered over 3–4 weeks and found that reducing fractionation (and therefore duration of RT) dominated higher fractionation. 101 This is in line with our base case results.
Based on the results presented, 5 fraction RT offers potentially significant benefits to the UK health system compared to 15 fractions.
Chapter 7 Nodal Sub-Study
Recruitment
The original target accrual for the Nodal Sub-Study was 627 patients to be recruited over 2 years. The first patient was recruited into the sub-study on 11 April 2016. Accrual was suspended from 6 December 2017 until 23 January 2018 while the substantial protocol amendment to close accrual into Test Group 1 (27 Gy/5 fractions) for the sub-study was being processed for approval. At this stage a total of 109, 104 and 97 patients had been recruited into the 40 Gy/15 fractions, 27 Gy/5 fractions and 26 Gy/5 fractions groups, respectively. In Protocol v5.0 (14 December 2017) target recruitment for the sub-study was changed to 344 patients (172 in each of the Control Group and Test Group 1). Including the additional 104 patients randomised to Test Group 1 before the protocol amendment the revised overall target sample size was 448. Having reached the revised target accrual into the Nodal Sub-Study, centres were contacted on 29 August 2018 and asked not to approach any new patients regarding the trial. A few additional patients were subsequently randomised who had been previously approached and given information about the trial prior to this date, and the last patient was randomised on 2 October 2018.
The final number of patients entered into the Nodal Sub-Study was 469 from 28 RT centres and 22 referral centres (183 for 40 Gy/15 fractions, 104 for 27 Gy/5 fractions and 182 for 26 Gy/5 fractions). Four hundred and sixteen patients were recruited into the photographic assessment sub-study, 442 patients consented to donate a blood sample and 465 patients consented to donate their primary tissue sample. Recruitment figures according to centre are presented in Appendix 2, Table 32. Cumulative recruitment over time compared with the target recruitment rate is shown in Figure 18.
FIGURE 18.
Target and actual recruitment for Nodal Sub-Study.
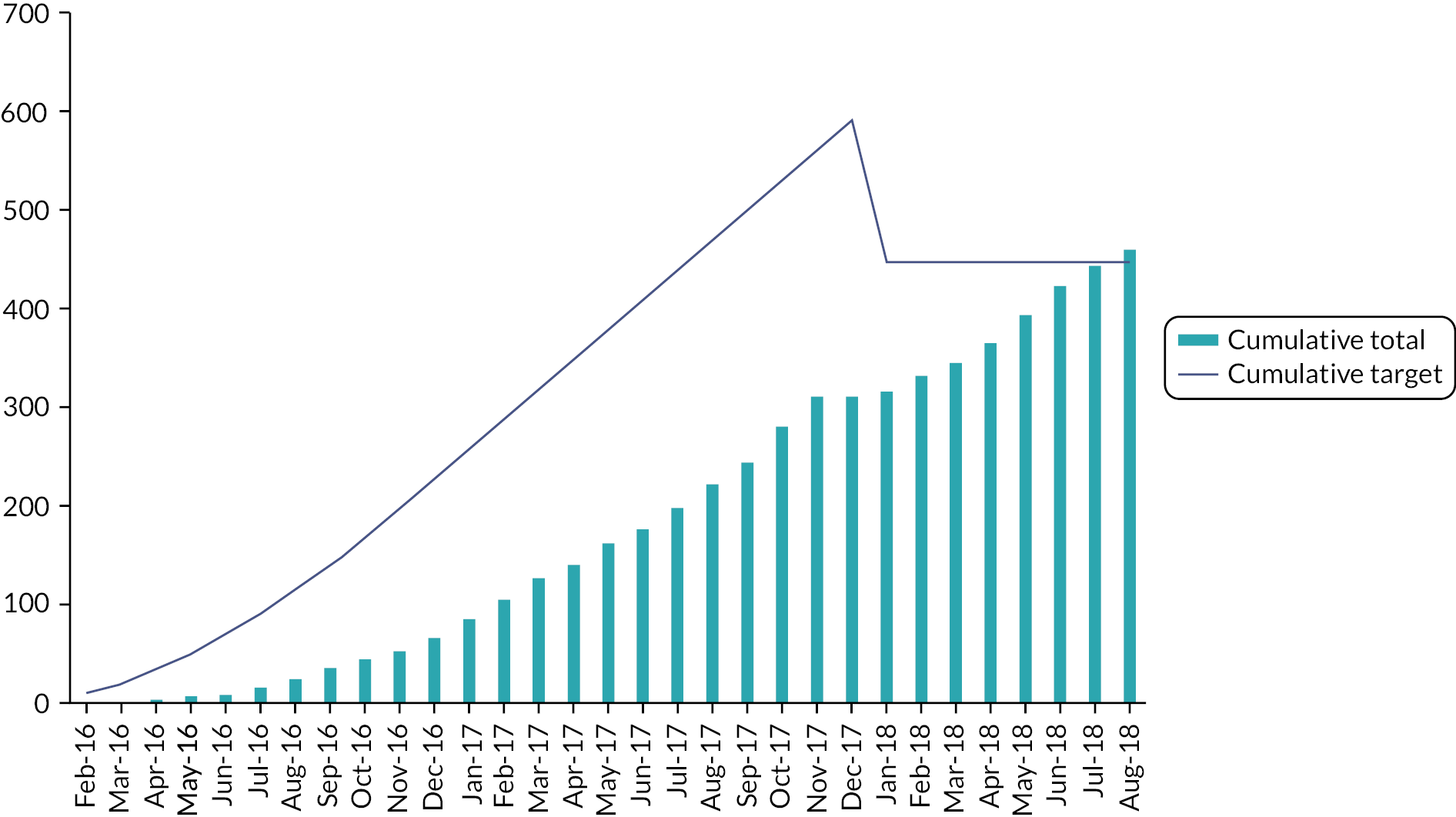
The original target for recruitment was based on five centres opening to recruitment once the Nodal Sub-Study opened with a further two centres expected to open per month until a total of 49 centres were open to recruitment. Each centre was predicted to recruit two patients per month with a maximum of 30 patients per month. Using these figures it was predicted that recruitment would take 24 months to attain. Radiotherapy centres were asked to obtain and additional QA approval procedure and only 32 centres undertook this. Fifty-two centres opened to recruitment to the Nodal Sub-Study with a maximum of 31 patients recruited in any 1 month. Two centres did not recruit any patients.
Deviations and withdrawals
Two patients withdrew consent for use of data and were removed from the intention-to-treat population, therefore Nodal Sub-Study results are reported for 467 consenting patients. A total of 18 patients (8 in 40 Gy, 4 in 27 Gy, 6 in 26 Gy) did not receive the allocated therapy (see Figure 19); compliance with allocated treatment was therefore 96% (449/467). These 18 patients included two found to be ineligible after randomisation: 1 in the 40 Gy group (Non-Hodgkins lymphoma), and 1 in 26 Gy (no axillary staging due to comorbidities).
FIGURE 19.
FAST-Forward Nodal Sub-Study profile (CONSORT diagram).
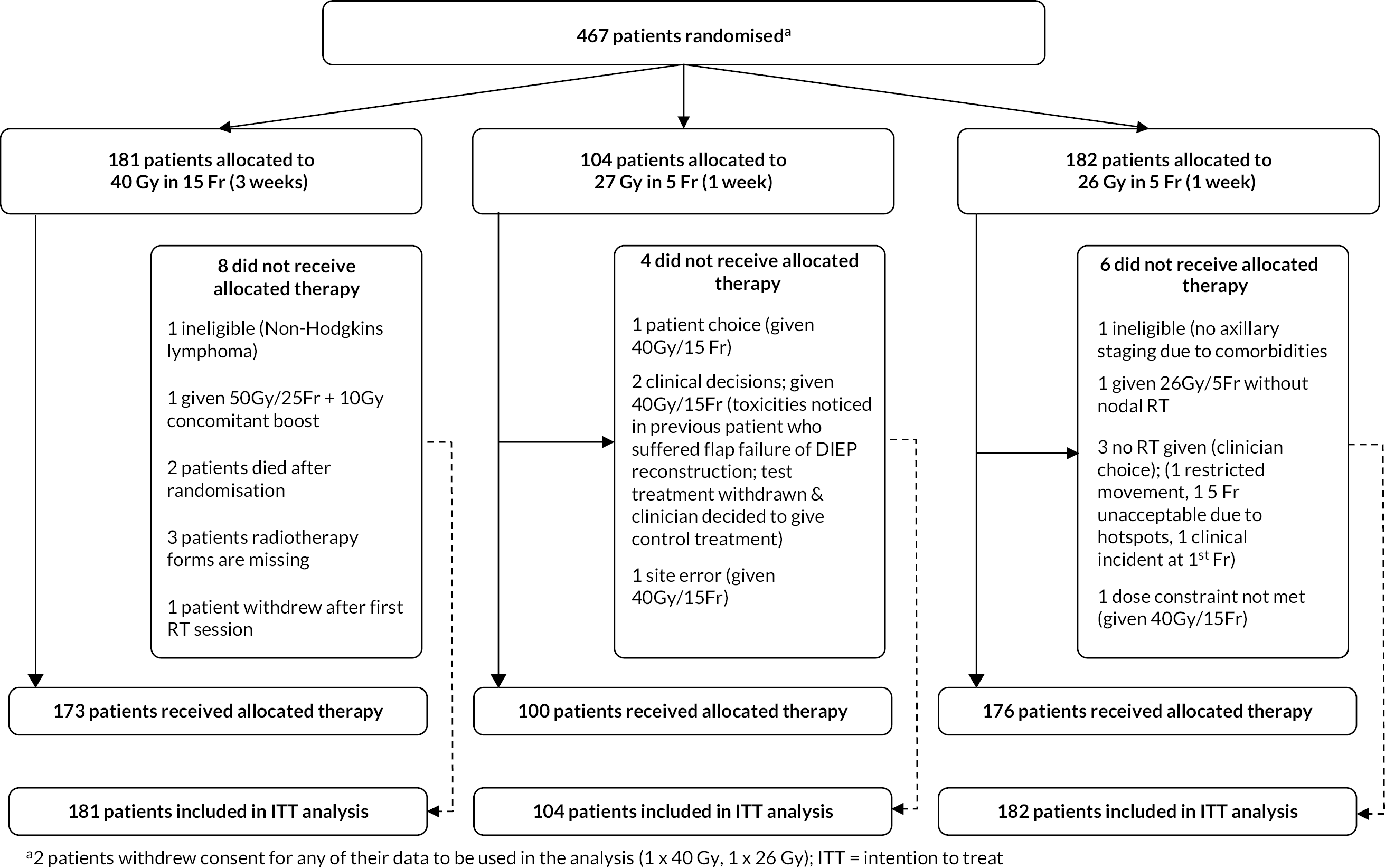
By 3 years’ follow-up 10 patients had withdran from clinical follow-up (9 in 40 Gy and 1 in 26 Gy), and 49 had died (19 in 40 Gy, 10 in 27 Gy, 20 in 26 Gy).
Data returns
Data return rates were calculated as % of CRFs or questionnaires received at ICR-CTSU out of those expected (expected numbers exclude patients who have died or withdrawn). Baseline CRFs were available for 99% of patients. As of 24 January 2022, annual clinical follow-up forms had been returned to the trials office for 98% (441 received out of 451 expected) for year 1, 99% (433/435) for year 2, and 90% (376/420) for year 3. As of the date of this report, all Nodal Sub-Study patients have reached the 3-year time point and further follow-up is ongoing, hence the Consolidated Standards of Reporting Trials diagram and results for the Nodal Sub-Study presented in this report include data up to 3 years. Analysis of follow-up beyond 3 years in the Nodal Sub-Study will be done in the future once data collection is complete.
Return rates for the patient self-completed questionnaires up to 2 years’ follow-up were 98% (460 received out of 467 expected) at baseline, 89% (386/434) at 3 months, 89% (379/427) at 6 months, 87% (359/415) at 1 year and 89% (367/414) at 2 years. As of the date of this report, collection of 5-year questionnaires is underway, with the last one due in October 2023.
Baseline data
Baseline demographic, clinical and treatment characteristics according to treatment group are shown in Table 22, enabling the following comparisons: (1) all patients randomised to 40 Gy versus all those randomised to 26 Gy, and (2) patients randomised to 40 Gy up to end 2017 versus all those randomised to 27 Gy. The median age of all Nodal Sub-Study patients was 60 years (IQR 51–70; overall range 31–89), and it included four men. A total of 250 (53%) were grade 2 and 182 (39%) grade 3. Just over half (255, 55%) had BCS, and 43/212 (20%) mastectomy patients had an immediate breast reconstruction. The majority of patients had axillary clearance (254/467, 54%). Median pathological tumour size was 2.6 cm (IQR 0.9–7.2). The majority of patients were ER positive, HER-2 negative (325, 70%). A total of 113 (24%) received neo-adjuvant chemotherapy, and 216 (46%) received adjuvant chemotherapy; 13 patients received both neo-adjuvant and adjuvant chemotherapy.
| 40 Gy/15 fractions | 26 Gy/5 fractions | 40 Gy/15 fractions | 27 Gy/5 fractions | |
|---|---|---|---|---|
| All randomised (N = 181), (%) | N = 182, (%) | Randomised concurrently with 27 Gy/5 fractions (N = 108), (%) | N = 104, (%) | |
| Age (years) | ||||
| Median (IQR) | 61 (51–72) | 60 (51–70) | 60 (49–72) | 57 (49–67) |
| Range | 34–89 | 34–86 | 36–89 | 30–84 |
| < 40 | 7 (4) | 14 (8) | 6 (6) | 4 (4) |
| 40–49 | 31 (17) | 28 (15) | 22 (20) | 23 (22) |
| 50–59 | 47 (26) | 49 (27) | 26 (24) | 28 (27) |
| 60–69 | 43 (24) | 45 (25) | 23 (21) | 29 (28) |
| 70–79 | 42 (23) | 32 (18) | 25 (23) | 13 (12) |
| ≥ 80 | 11 (6) | 14 (8) | 6 (6) | 7 (7) |
| Sex | ||||
| Female | 180 (99) | 180 (99) | 107 (99) | 103 (99) |
| Male | 1 (1) | 2 (1) | 1 (1) | 1 (1) |
| Tumour grade | ||||
| 1 | 15 (8) | 12 (7) | 13 (12) | 8 (8) |
| 2 | 95 (52) | 97 (53) | 50 (46) | 58 (56) |
| 3 | 71 (39) | 73 (40) | 45 (42) | 38 (36) |
| Primary surgery | ||||
| BCS | 95 (52) | 106 (58) | 60 (56) | 54 (52) |
| BCS with oncoplastic technique | 2 | 2 | 2 | 1 |
| Mastectomy | 86 (48) | 76 (42) | 48 (44) | 50 (48) |
| Mastectomy with immediate reconstruction | 15 | 17 | 8 | 11 |
| Type of reconstruction (mastectomy patients): | ||||
| Autologous reconstruction | 5/15 | 3/17 | 2/8 | 3/11 |
| Implant-based reconstruction | 10/15 | 13/17 | 6/8 | 7/11 |
| Reconstruction type not specified | 0 | 1/17 | 0 | 1/11 |
| Side of primary | ||||
| Left | 87 (48) | 104 (57) | 50 (46) | 55 (53) |
| Right | 94 (52) | 78 (43) | 58 (54) | 49 (47) |
| Maximal extent of axillary staging | ||||
| Sentinel node biopsy/guided axillary sampling | 74 (41) | 90 (50) | 41 (38) | 48 (46) |
| Axillary clearance | 107 (59) | 91 (50) | 67 (62) | 56 (54) |
| Not known | 0 | 1 | 0 | 0 |
| Post-op infection requiring antibiotics | ||||
| Yes | 21 (12) | 21 (12) | 12 (11) | 14 (13) |
| No | 155 (86) | 157 (86) | 94 (87) | 87 (84) |
| Not known | 5 (3) | 4 (2) | 2 (2) | 3 (3) |
| Post-op haematoma requiring surgical evacuation | ||||
| Yes | 1 (< 1) | 1 (< 1) | 0 | 3 (3) |
| No | 174 (96) | 176 (97) | 106 (98) | 97 (93) |
| Not known | 6 (3) | 5 (3) | 2 (2) | 4 (4) |
| Post-op seroma requiring aspiration | ||||
| Yes | 45 (25) | 41 (22) | 30 (28) | 19 (18) |
| No | 132 (73) | 136 (75) | 77 (71) | 80 (77) |
| Not known | 4 (2) | 5 (3) | 1 (1) | 5 (5) |
| Histological type | ||||
| Infiltrating ductal | 136 (75) | 143 (79) | 86 (80) | 85 (82) |
| Lobular | 33 (18) | 25 (14) | 13 (12) | 12 (11) |
| Mixed | 9 (5) | 7 (4) | 7 (6) | 5 (5) |
| Other | 3 (2) | 6 (3) | 2 (2) | 2 (2) |
| Not known | 0 | 1 | 0 | 0 |
| Pathological tumour size (cm) | ||||
| Median (IQR) | 3 (1–7) | 3 (1–8) | 3 (1–7) | 3 (1–7) |
| pT stage | ||||
| T1mi | 2 (1) | 2 (1) | 1 (1) | 1 (1) |
| T1a | 2 (1) | 2 (1) | 2 (2) | |
| T1b | 6 (3) | 7 (4) | 3 (3) | 6 (6) |
| T1c | 49 (27) | 42 (23) | 33 (31) | 31 (30) |
| T2 | 92 (51) | 100 (55) | 54 (50) | 50 (48) |
| T3 | 29 (16) | 27 (15) | 14 (13) | 15 (14) |
| Not known | 1 | 2 | 1 | 1 |
| ER/HER-2 status | ||||
| ER+/HER-2+ | 29 (16) | 27 (15) | 22 (20) | 13 (12) |
| ER+/HER-2− | 124 (68) | 122 (68) | 72 (67) | 79 (76) |
| ER−/HER-2+ | 7 (4) | 6 (3) | 3 (3) | 4 (4) |
| ER−/HER-2− | 21 (12) | 24 (13) | 11 (10) | 8 (8) |
| Not known | 0 | 2 | 0 | 0 |
| PgR status | ||||
| Positive | 80 (44) | 71 (40) | 51 (48) | 48 (47) |
| Negative | 36 (20) | 44 (25) | 19 (18) | 17 (16) |
| Not done | 64 (36) | 63 (35) | 37 (34) | 38 (37) |
| Missing on form | 1 | 3 | 1 | 1 |
| Lymphovascular invasion | ||||
| Present | 75 (41) | 77 (43) | 47 (44) | 48 (46) |
| Absent | 90 (50) | 86 (47) | 48 (44) | 46 (44) |
| Uncertain | 9 (5) | 11 (6) | 8 (7) | 6 (6) |
| Not known | 7 (4) | 7 (4) | 5 (5) | 4 (4) |
| Neo-adjuvant chemotherapy received | ||||
| Yes | 43 (24) | 44 (24) | 27 (25) | 26 (25) |
| No | 137 (76) | 137 (76) | 80 (75) | 78 (75) |
| Adjuvant therapy received 2 | ||||
| All patients: | 169 (94) | 170 (94) | 102 (95) | 99 (95) |
| Chemotherapy3 | 80 (47) | 87 (51) | 48 (47) | 49 (49) |
| HER-2 + patients: | ||||
| Trastuzumab | 26 (72) | 28 (85) | 19 (76) | 16 (94) |
| Chemotherapy and trastuzumab | 14 (82) | 14 (100) | 8 (89) | 9 (100) |
| Trastuzumab, no chemotherapy | 12 (67) | 14 (82) | 11 (69) | 7 (88) |
| Chemotherapy, no trastuzumab | ||||
| No chemotherapy, no trastuzumab | 12 (67) | 14 (82) | 11 (69) | 7 (88) |
| ER + patients: | ||||
| Endocrine therapy | 153 (85) | 151 (83) | 94 (87) | 92 (88) |
Radiotherapy details for the 458 patients who received RT are shown in Table 23. Deviations from allocated treatment are described above. A tumour bed boost was prescribed for 117/458 (25%) of patients, most commonly to a dose of 10 Gy in 5 fractions (58/117, 50%). For the overall trial cohort the median breast/chest wall PTV was 870 cc (IQR 269–1954), and median nodal PTV was 134.6 cc (IQR 41.5–357.2). Bolus was used for 89 of the 209 (43%) mastectomy patients who received RT.
| RT details | 40 Gy/15 fractions | 26 Gy/5 fractions | 40 Gy/15 fractions | 27 Gy/5 fractions |
|---|---|---|---|---|
| All randomised (N = 175), (%) | N = 179, (%) | Randomised concurrently with 27 Gy/5 fractions (N = 107), (%) | N = 104, (%) | |
| Days from randomisation to starting RT | ||||
| N | 174 | 178 | 107 | 104 |
| Median (IQR) | 27 (21–37) | 26 (21–34) | 27 (20–36) | 25.5 (20–33) |
| Range | 7–90 | 8–86 | 7–90 | 6–116 |
| Dose delivered to whole breast/chest wall/nodes | ||||
| 40 Gy/15 fractions | 173 (99) | 1 (< 1) | 107 (100) | 4 (4) |
| 26 Gy/5 fractions | 0 | 176 (99) | 0 | 0 |
| 27 Gy/5 fractions | 0 | 0 | 0 | 100 (96) |
| 2 Gy/1 fractions | 0 | 1 (< 1) | 0 | 0 |
| 50 Gy/25 fractions | 1 (1) | 0 | 0 | 0 |
| Missing on form | 1 | 1 | 0 | 0 |
| Boost given | ||||
| Yes | 46 (26) | 46 (26) | 30 (28) | 25 (24) |
| No | 128 (74) | 132 (74) | 77 (72) | 79 (76) |
| Missing on form | 1 | 1 | 0 | 0 |
| Boost dose received | N = 46 | N = 46 | N = 30 | N = 25 |
| 10 Gy/5 fractions | 19 (42) | 24 (52) | 15 (50) | 15 (60) |
| 16 Gy/8 fractions | 5 (11) | 8 (17) | 5 (17) | 6 (24) |
| 12.5 Gy/5 fractions | 2 (4) | 0 (0) | 2 (7) | 0 |
| 13.35 Gy/5 fractions | 9 (20) | 7 (15) | 6 (20) | 2 (8) |
| 14 Gy/5 fractions | 3 (7) | 2 (4) | 2 (7) | 1 (4) |
| 16 Gy/5 fractions | 0 (0) | 0 (0) | 0 (0) | 1 (4) |
| 12 Gy/4 fractions | 4 (9) | 4 (9) | 0 (0) | 0 (0) |
| 10 Gy/4 fractions | 3 (7) | 0 (0) | 0 (0) | 0 (0) |
| 9 Gy/3 fractions | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Not known | 1 | 0 | 0 | 0 |
| Volume of breast/chest wall PTV (cc) | ||||
| N | 173 | 177 | 105 | 103 |
| Median (IQR) | 850 (566–1114) | 876 (547–1277) | 870 (583–1140) | 915 (552–1368) |
| Range | 64–4911 | 69–7338 | 64–4911 | 119–2794 |
| Nodal areas irradiated | ||||
| Axillary level I | 80 (46) | 91 (51) | 47 (45) | 46 (45) |
| Axillary level II | 89 (51) | 95 (54) | 52 (50) | 51 (50) |
| Axillary level III | 121 (70) | 126 (71) | 76 (72) | 66 (64) |
| Level IV (SCF) | 159 (92) | 163 (92) | 93 (89) | 95 (92) |
| IMC | 0 | 1 (< 1) | 0 | 0 |
| Missing on form | 2 | 2 | 2 | 1 |
| Volume of nodal PTV (cc) | ||||
| N | 172 | 177 | 104 | 103 |
| Median (IQR) | 129 (85–207) | 140 (92–213) | 133 (86–223) | 139 (97–211) |
| Range | 13–1257 | 10–635 | 13–1257 | 14–803 |
| Bolus used (mastectomy patients only) | N = 83 | N = 76 | N = 47 | N = 50 |
| Yes | 33 (41) | 30 (43) | 17 (38) | 26 (54) |
| No | 48 (59) | 40 (57) | 28 (62) | 22 (46) |
| Missing on form | 2 | 6 | 2 | 2 |
| Dose homogeneity constraints achieved | ||||
| Yes | 163 (94) | 162 (91) | 100 (94) | 98 (95) |
| No | 11 (6) | 16 (9) | 6 (6) | 5 (5) |
| Missing on form | 1 | 1 | 1 | 1 |
| Organs at risk dose constraints achieved | ||||
| Yes | 161 (93) | 161 (90) | 96 (91) | 95 (92) |
| No | 13 (7) | 17 (10) | 10 (9) | 8 (8) |
| Missing on form | 1 | 1 | 1 | 1 |
| Was patient replanned during RT? | ||||
| Yes | 5 (3) | 6 (3) | 3 (3) | 0 |
| No | 169 (97) | 172 (97) | 103 (97) | 103 (100) |
| Missing on form | 1 | 1 | 1 | 1 |
| RT extended by > 3 days | ||||
| Yes | 1 (<1) | 2 (1) | 1 (1) | 0 |
| No | 173 (99) | 176 (99) | 105 (99) | 103 (100) |
| Missing on form | 1 | 1 | 1 | 1 |
| Reason: | ||||
| Bank holidays | 0 | 1 | 0 | 0 |
| Patient illness | 1 | 0 | 1 | 0 |
| Other | 0 | 1 | 0 | 0 |
Follow-up
As of the data snapshot on 1 December 2021, median follow-up in each treatment group was 36 months (IQR 31–48) for all 40 Gy/15 fractions patients, 37 months (IQR 31–48) for 26 Gy/5 fractions and 48 months (IQR 37–49) for 27 Gy/5 fractions. Median follow-up in 40 Gy/15 fractions patients randomised prior to the December 2017 protocol amendment (i.e. concurrently with 27 Gy/5 fractions) was 47 months (IQR 34–48). Centres were requested to schedule annual follow-up assessments as close to the due date as possible, which was calculated from date of randomisation. Some leeway was permitted within the trial to accommodate local hospital policy that schedules follow-up based on date of surgery rather than date of trial randomisation. Annual clinical follow-ups occurred within 3 months of the due date in 86% of cases (1054 of 1220 clinical follow-ups).
Annual clinical follow-ups were primarily conducted in person at sites prior to the COVID-10 pandemic. On 1 April 2020 sites were advised that these could be done via telephone by a healthcare professional, and guidance was provided regarding the clinical assessments to be carried out at each visit.
Late normal tissue effects in Nodal Sub-Study
As stated in the Statistical Methods section (see Chapter 2), formal comparisons of NTE in the Nodal Sub-Study will await 5-year follow-up. Descriptive data for PRO up to 2 years and for clinical assessments up to 3 years are included in this report.
Patient-reported outcomes
PRO were available at baseline for 180 patients in the 40 Gy group, 181 in 26 Gy and 103 in 27 Gy, and at 24 months for 127, 135 and 89, respectively.
Symptoms in arm, shoulder or hand
Frequencies of PRO relating to arm/shoulder/hand symptoms from baseline to 24 months are shown in Table 24 for 40 Gy compared with 26 Gy (all randomised patients), and in Table 25 for 40 Gy and 27 Gy patients randomised up to the end of 2017. For all outcomes relating to arm, shoulder or hand, the number of patients reporting marked effects was small at all time points in all treatment groups. Appendix 2, Table 55 shows baseline arm/shoulder/hand symptoms according to maximal extent of axillary surgery and fractionation schedule.
| Arm/shoulder/hand effect | Baseline | 3 months | 6 months | 12 months | 24 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 180), (%) | 26 Gy (N = 181), (%) | 40 Gy (N = 141), (%) | 26 Gy (N = 153), (%) | 40 Gy (N = 137), (%) | 26 Gy (N = 151), (%) | 40 Gy (N = 130), (%) | 26 Gy (N = 142), (%) | 40 Gy (N = 127), (%) | 26 Gy (N = 135), (%) | |
| Swollen arm or hand (EORTC QLQ-BR23) | ||||||||||
| Not at all | 144 (80) | 140 (77) | 100 (71) | 112 (73) | 95 (70) | 104 (69) | 98 (75) | 95 (67) | 93 (73) | 102 (76) |
| A little | 29 (16) | 26 (14) | 29 (20) | 33 (22) | 27 (20) | 37 (25) | 22 (17) | 35 (25) | 21 (17) | 22 (16) |
| Quite a bit | 4 (2) | 8 (5) | 7 (5) | 3 (2) | 9 (7) | 5 (3) | 6 (5) | 8 (6) | 7 (5) | 7 (5) |
| Very much | 3 (2) | 7 (4) | 5 (4) | 5 (3) | 5 (3) | 4 (3) | 4 (3) | 3 (2) | 6 (5) | 3 (2) |
| Missing | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Pain in arm or shoulder (EORTC QLQ-BR23) | ||||||||||
| Not at all | 65 (36) | 72 (40) | 46 (33) | 38 (25) | 40 (30) | 43 (28) | 46 (35) | 47 (33) | 50 (39) | 53 (40) |
| A little | 89 (49) | 68 (38) | 52 (37) | 67 (44) | 57 (42) | 69 (46) | 55 (42) | 59 (42) | 54 (43) | 61 (46) |
| Quite a bit | 20 (12) | 27 (15) | 29 (21) | 34 (22) | 29 (21) | 28 (19) | 21 (16) | 19 (13) | 14 (11) | 15 (11) |
| Very much | 6 (3) | 13 (7) | 13 (9) | 13 (9) | 10 (7) | 11 (7) | 8 (6) | 17 (12) | 9 (7) | 5 (3) |
| Missing | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Difficult to raise arm or move it sideways (EORTC QLQ-BR23) | ||||||||||
| Not at all | 113 (63) | 106 (58) | 76 (54) | 67 (44) | 66 (49) | 65 (43) | 76 (58) | 68 (48) | 78 (61) | 74 (55) |
| A little | 51 (28) | 52 (29) | 47 (33) | 61 (40) | 49 (36) | 68 (45) | 37 (28) | 53 (38) | 34 (27) | 50 (38) |
| Quite a bit | 12 (7) | 16 (9) | 10 (7) | 15 (10) | 12 (9) | 9 (6) | 11 (8) | 8 (6) | 8 (6) | 6 (4) |
| Very much | 3 (2) | 7 (4) | 8 (6) | 10 (6) | 9 (7) | 8 (5) | 6 (5) | 11 (8) | 7 (6) | 4 (3) |
| Missing | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 |
| Shoulder stiffness | ||||||||||
| Not at all | 123 (69) | 115 (64) | 69 (50) | 67 (44) | 61 (45) | 62 (41) | 64 (50) | 61 (44) | 70 (56) | 67 (52) |
| A little | 49 (27) | 49 (27) | 47 (34) | 58 (38) | 50 (37) | 58 (39) | 47 (37) | 56 (40) | 43 (34) | 46 (36) |
| Quite a bit | 6 (3) | 12 (7) | 13 (9) | 17 (11) | 17 (13) | 22 (15) | 9 (7) | 14 (10) | 7 (6) | 12 (9) |
| Very much | 2 (1) | 3 (2) | 8 (6) | 11 (7) | 7 (5) | 7 (5) | 7 (6) | 8 (6) | 5 (4) | 4 (3) |
| Missing | 0 | 2 | 3 | 0 | 2 | 2 | 3 | 3 | 2 | 6 |
| Pins and needles in arm/hand on affected side (protocol-specific item a ) | ||||||||||
| Not at all | 128 (72) | 128 (72) | 83 (60) | 100 (65) | 92 (68) | 89 (59) | 86 (67) | 85 (61) | 82 (66) | 84 (65) |
| A little | 38 (21) | 41 (23) | 39 (28) | 32 (21) | 28 (21) | 40 (27) | 31 (24) | 41 (30) | 31 (25) | 34 (26) |
| Quite a bit | 7 (4) | 6 (3) | 9 (7) | 16 (11) | 8 (6) | 14 (10) | 5 (4) | 10 (7) | 8 (6) | 10 (8) |
| Very much | 6 (3) | 3 (2) | 7 (5) | 5 (3) | 7 (5) | 6 (4) | 6 (5) | 3 (2) | 4 (3) | 1 (1) |
| Missing | 1 | 3 | 3 | 0 | 2 | 2 | 2 | 3 | 2 | 6 |
| Pins and needles in arm/hand on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 152 (85) | 146 (82) | 106 (77) | 119 (78) | 117 (87) | 115 (77) | 105 (82) | 111 (80) | 99 (79) | 106 (82) |
| A little | 18 (10) | 26 (15) | 25 (18) | 20 (13) | 15 (11) | 21 (14) | 17 (13) | 19 (14) | 22 (17) | 17 (13) |
| Quite a bit | 3 (2) | 6 (3) | 4 (3) | 9 (6) | 1 (<1) | 8 (5) | 3 (2) | 6 (4) | 2 (2) | 4 (3) |
| Very much | 6 (3) | 0 | 3 (1) | 5 (3) | 2 (1) | 5 (3) | 3 (2) | 3 (2) | 2 (2) | 3 (2) |
| Missing | 1 | 3 | 2 | 0 | 2 | 2 | 2 | 3 | 2 | 5 |
| Numbness in fingers on affected side (protocol-specific item a ) | ||||||||||
| Not at all | 145 (81) | 137 (77) | 98 (71) | 107 (70) | 97 (72) | 98 (66) | 91 (71) | 92 (66) | 90 (72) | 87 (67) |
| A little | 16 (9) | 33 (19) | 26 (19) | 26 (17) | 28 (21) | 36 (24) | 30 (23) | 39 (28) | 28 (22) | 34 (26) |
| Quite a bit | 10 (6) | 8 (4) | 10 (7) | 15 (10) | 6 (4) | 9 (6) | 2 (2) | 6 (4) | 2 (2) | 8 (6) |
| Very much | 8 (4) | 0 | 4 (3) | 5 (3) | 4 (3) | 6 (4) | 5 (4) | 2 (1) | 5 (4) | 1 (1) |
| Missing | 1 | 3 | 3 | 0 | 2 | 2 | 2 | 3 | 2 | 5 |
| Numbness in fingers on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 146 (82) | 149 (84) | 112 (81) | 124 (81) | 108 (81) | 118 (79) | 103 (81) | 110 (79) | 106 (85) | 111 (85) |
| A little | 14 (7) | 22 (12) | 17 (12) | 17 (11) | 19 (14) | 22 (15) | 19 (15) | 22 (16) | 15 (12) | 13 (10) |
| Quite a bit | 10 (6) | 6 (3) | 6 (4) | 7 (5) | 5 (4) | 3 (2) | 3 (2) | 5 (4) | 2 (2) | 5 (4) |
| Very much | 9 (5) | 1 (1) | 3 (2) | 5 (3) | 2 (1) | 6 (4) | 2 (2) | 2 (1) | 2 (2) | 1 (1) |
| Missing | 1 | 3 | 3 | 0 | 3 | 2 | 3 | 3 | 2 | 5 |
| Weakness in arm/hand on affected side (protocol-specific item a ) | ||||||||||
| Not at all | 112 (63) | 118 (67) | 66 (48) | 74 (48) | 62 (46) | 63 (43) | 63 (49) | 63 (45) | 57 (46) | 69 (53) |
| A little | 51 (28) | 46 (26) | 48 (35) | 52 (34) | 47 (35) | 61 (41) | 45 (35) | 60 (43) | 40 (32) | 48 (37) |
| Quite a bit | 12 (7) | 9 (5) | 15 (11) | 22 (15) | 21 (16) | 17 (12) | 15 (12) | 12 (9) | 18 (14) | 11 (8) |
| Very much | 4 (2) | 4 (2) | 9 (6) | 5 (3) | 4 (3) | 6 (4) | 5 (4) | 4 (3) | 10 (8) | 2 (2) |
| Missing | 1 | 4 | 3 | 0 | 3 | 2 | 2 | 3 | 2 | 5 |
| Weakness in arm/hand on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 159 (89) | 155 (87) | 107 (78) | 120 (78) | 102 (76) | 117 (79) | 106 (83) | 113 (82) | 97 (78) | 102 (78) |
| A little | 14 (8) | 20 (11) | 26 (19) | 24 (16) | 23 (17) | 22 (15) | 16 (13) | 18 (13) | 18 (14) | 23 (18) |
| Quite a bit | 4 (2) | 2 (1) | 3 (2) | 8 (5) | 7 (5) | 5 (3) | 3 (2) | 6 (4) | 5 (4) | 4 (3) |
| Very much | 2 (1) | 1 (<1) | 1 (1) | 1 (1) | 2 (1) | 4 (3) | 3 (2) | 1 (1) | 5 (4) | 1 (1) |
| Missing | 1 | 3 | 3 | 0 | 3 | 3 | 2 | 4 | 2 | 5 |
| Arm/shoulder/hand effect | Baseline | 3 months | 6 months | 12 months | 24 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 108), (%) | 27 Gy (N = 103), (%) | 40 Gy (N = 88), (%) | 27 Gy (N = 94), (%) | 40 Gy (N = 82), (%) | 27 Gy (N = 93), (%) | 40 Gy (N = 79), (%) | 27 Gy (N = 87), (%) | 40 Gy (N = 72), (%) | 27 Gy (N = 89), (%) | |
| Swollen arm or hand (EORTC QLQ-BR23) | ||||||||||
| Not at all | 87 (81) | 87 (84) | 58 (66) | 65 (70) | 59 (72) | 65 (70) | 59 (74) | 57 (65) | 53 (74) | 59 (66) |
| A little | 15 (13) | 13 (13) | 21 (24) | 23 (25) | 14 (17) | 18 (20) | 14 (18) | 23 (26) | 13 (18) | 18 (20) |
| Quite a bit | 3 (3) | 3 (3) | 6 (7) | 4 (4) | 6 (7) | 6 (7) | 3 (4) | 4 (5) | 3 (4) | 8 (9) |
| Very much | 3 (3) | 0 (0) | 3 (3) | 1 (1) | 3 (4) | 3 (3) | 3 (4) | 3 (3) | 3 (4) | 4 (5) |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pain in arm or shoulder (EORTC QLQ-BR23) | ||||||||||
| Not at all | 36 (33) | 36 (33) | 30 (35) | 26 (28) | 25 (30) | 27 (29) | 25 (32) | 23 (26) | 26 (36) | 29 (32) |
| A little | 51 (47) | 51 (47) | 27 (31) | 41 (45) | 28 (34) | 37 (40) | 35 (44) | 39 (45) | 32 (44) | 38 (43) |
| Quite a bit | 17 (16) | 17 (16) | 20 (23) | 20 (22) | 22 (27) | 22 (24) | 12 (15) | 16 (18) | 8 (11) | 15 (17) |
| Very much | 4 (4) | 4 (4) | 10 (11) | 5 (5) | 7 (9) | 7 (7) | 7 (9) | 9 (10) | 6 (8) | 7 (8) |
| Missing | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Difficult to raise arm or move it sideways (EORTC QLQ-BR23) | ||||||||||
| Not at all | 61(57) | 54 (52) | 46 (52) | 48 (52) | 35 (43) | 42 (45) | 46 (58) | 47 (54) | 38 (53) | 51 (57) |
| A little | 37 (34) | 37 (36) | 30 (34) | 30 (32) | 33 (40) | 34 (37) | 22 (28) | 31 (35) | 26 (36) | 25 (28) |
| Quite a bit | 8 (8) | 10 (10) | 7 (8) | 11 (12) | 8 (10) | 14 (15) | 5 (6) | 4 (5) | 5 (7) | 8 (9) |
| Very much | 1 (1) | 2 (2) | 5 (6) | 4 (4) | 6 (7) | 3 (3) | 6 (8) | 5 (6) | 3 (4) | 5 (6) |
| Missing | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shoulder stiffness | ||||||||||
| Not at all | 75 (69) | 59 (57) | 44 (51) | 36 (39) | 35 (43) | 41 (44) | 40 (50) | 41 (47) | 36 (51) | 42 (48) |
| A little | 29 (27) | 34 (33) | 29 (33) | 44 (47) | 31 (38) | 29 (32) | 27 (34) | 34 (39) | 27 (38) | 31 (35) |
| Quite a bit | 3 (3) | 9 (9) | 8 (9) | 10 (11) | 11 (13) | 19 (21) | 6 (8) | 6 (7) | 6 (8) | 10 (11) |
| Very much | 1 (1) | 1 (1) | 6 (7) | 3 (3) | 5 (6) | 3 (3) | 6 (8) | 6 (7) | 2 (3) | 5 (6) |
| Missing | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Pins and needles in arm/hand on affected side (protocol-specific item a ) | ||||||||||
| Not at all | 78 (72) | 74 (72) | 52 (59) | 46 (50) | 53 (65) | 53 (58) | 52 (66) | 53 (61) | 48 (68) | 47 (55) |
| A little | 21 (19) | 26 (25) | 27 (31) | 35 (38) | 18 (22) | 27 (29) | 20 (25) | 26 (30) | 18 (25) | 33 (38) |
| Quite a bit | 3 (3) | 1 (1) | 6 (7) | 6 (7) | 7 (8) | 11 (12) | 2 (3) | 6 (7) | 3 (4) | 3 (3) |
| Very much | 6 (6) | 2 (2) | 3 (3) | 5 (5) | 4 (5) | 1 (1) | 5 (6) | 2 (2) | 2 (3) | 3 (3) |
| Missing | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 3 |
| Pins and needles in arm/hand on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 94 (87) | 88 (85) | 68 (77) | 72 (77) | 71 (87) | 68 (74) | 64 (81) | 68 (78) | 59 (83) | 62 (72) |
| A little | 8 (7) | 13 (13) | 16 (18) | 12 (13) | 10 (12) | 19 (21) | 12 (15) | 14 (16) | 12 (17) | 16 (19) |
| Quite a bit | 1 (1) | 1 (1) | 3 (3) | 7 (8) | 0 (0) | 4 (4) | 1 (1) | 4 (5) | 0 (0) | 5 (6) |
| Very much | 5 (5) | 1 (1) | 1 (1) | 2 (2) | 1 (1) | 1 (1) | 2 (3) | 1 (1) | 0 (0) | 3 (3) |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 3 |
| Numbness in fingers on affected side | ||||||||||
| Not at all | 86 (80) | 72 (70) | 61 (69) | 52 (56) | 60 (72) | 56 (61) | 53 (67) | 60 (69) | 51 (72) | 55 (63) |
| A little | 10 (9) | 24 (23) | 18 (21) | 27 (29) | 16 (20) | 26 (28) | 21 (27) | 21 (24) | 17 (24) | 26 (30) |
| Quite a bit | 7 (6) | 3 (3) | 8 (9) | 8 (9) | 3 (4) | 6 (7) | 1 (1) | 5 (6) | 1 (1) | 3 (3) |
| Very much | 5 (5) | 4 (4) | 1 (1) | 6 (6) | 3 (4) | 4 (4) | 4 (5) | 1 (1) | 2 (3) | 3 (3) |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 |
| Numbness in fingers on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 89 (82) | 79 (77) | 71 (81) | 70 (75) | 68 (84) | 71 (77) | 63 (81) | 68 (78) | 61 (86) | 67 (77) |
| A little | 9 (8) | 20 (19) | 12 (14) | 14 (15) | 10 (12) | 14 (15) | 13 (17) | 16 (18) | 9 (13) | 12 (14) |
| Quite a bit | 5 (5) | 1 (1) | 4 (5) | 6 (7) | 2 (2) | 6 (7) | 0 (0) | 2 (2) | 1 (1) | 6 (7) |
| Very much | 5 (5) | 3 (3) | 0 (0) | 3 (3) | 1 (1) | 1 (1) | 2 (2) | 1 (1) | 0 (0) | 2 (2) |
| Missing | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 |
| Weakness in arm/hand on affected side (protocol-specific item a ) | ||||||||||
| Not at all | 64 (59) | 70 (68) | 41 (47) | 40 (43) | 35 (43) | 35 (38) | 35 (44) | 42 (48) | 33 (47) | 34 (39) |
| A little | 32 (30) | 26 (25) | 29 (33) | 33 (35) | 27 (33) | 36 (39) | 31 (39) | 34 (39) | 22 (31) | 41 (47) |
| Quite a bit | 8 (7) | 5 (5) | 12 (13) | 14 (15) | 16 (20) | 16 (17) | 9 (11) | 6 (7) | 13 (18) | 9 (10) |
| Very much | 4 (4) | 2 (2) | 6 (7) | 6 (7) | 3 (4) | 5 (4) | 4 (5) | 5 (6) | 3 (4) | 3 (4) |
| Missing | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 |
| Weakness in arm/hand on non-affected side (protocol-specific item a ) | ||||||||||
| Not at all | 93 (86) | 91 (88) | 65 (76) | 74 (80) | 59 (73) | 69 (75) | 66 (84) | 71 (82) | 56 (79) | 68 (78) |
| A little | 9 (8) | 8 (8) | 17 (20) | 12 (13) | 16 (20) | 15 (16) | 9 (11) | 13 (15) | 10 (14) | 12 (14) |
| Quite a bit | 4 (4) | 3 (3) | 2 (2) | 4 (4) | 5 (6) | 5 (6) | 1 (1) | 3 (3) | 3 (4) | 5 (6) |
| Very much | 2 (2) | 1 (1) | 2 (2) | 3 (3) | 1 (1) | 3 (3) | 3 (4) | 0 (0) | 2 (3) | 2 (2) |
| Missing | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 |
The majority of patients reported no arm/hand swelling (primary outcome of the Nodal Sub-Study) from baseline up to 24 months, with 80% and 77% in 40 Gy and 26 Gy at baseline and 73% and 76% at 24 months (see Table 24). For the comparison of 40 Gy and 27 Gy, 81% and 84% reported no arm/hand swelling at baseline, and 74% and 66% at 24 months (see Table 25). Most of those reporting arm/hand swelling had mild symptoms (graded as ‘a little’ on the questionnaires). The prevalence of moderate or marked arm/hand swelling at 24 months (corresponding to ‘quite a bit’ or ‘very much’) was 10% versus 7% for 40 Gy compared with 26 Gy, and 8% versus 14% for 40 Gy compared with 27 Gy.
The most frequently reported effect in the arm, shoulder or hand across all time points was pain in the arm or shoulder, with symptoms (any grade) reported at baseline by 64% in 40 Gy, 60% in 26 Gy and 67% in 27 Gy. However, most of these were mild symptoms, with moderate or marked arm or shoulder pain reported at baseline by 15%, 22% and 20% in 40 Gy, 26 Gy and 27 Gy, respectively. At 24 months moderate or marked arm or shoulder pain was reported by 18%, 14% and 25% in 40 Gy, 26 Gy and 27 Gy, respectively.
The prevalence of moderate/marked neuropathic symptoms was low (see Tables 24 and 25). Pins and needles of any grade were reported at baseline by 28% in 40 Gy, 28% in 26 Gy and 28% in 27 Gy on the affected side and by 15% in 40 Gy, 18% in 26 Gy and 15% in 27 Gy on the non-affected side. Pins and needles of any grade were reported at 24 months by 34% in 40 Gy, 35% in 26 Gy and 44% in 27 Gy on the affected side and by 21% in 40 Gy, 18% in 26 Gy and 28% in 27 Gy on the non-affected side. However, most of these were mild symptoms, with moderate or marked pins and needles reported at baseline by 7%, 5% and 3% in 40 Gy, 26 Gy and 27 Gy, respectively on the affected side and by 5%, 3% and 2% in 40 Gy, 26 Gy and 27 Gy, respectively on the non-affected side. At 24 months moderate or marked pins and needles were reported by 9%, 9% and 6% in 40 Gy, 26 Gy and 27 Gy, respectively on the affected side and by 4%, 5% and 9% in 40 Gy, 26 Gy and 27 Gy, respectively on the non-affected side.
Symptoms in breast or chest wall
Frequencies of PRO relating to breast/chest wall symptoms from baseline to 24 months are shown in Table 26 for 40 Gy compared with 26 Gy (all randomised patients), and in Table 27 for 40 Gy and 27 Gy patients randomised up to the end of 2017. For outcomes relating to breast or chest wall symptoms, the number of patients reporting marked effects was generally small at all time points in all treatment groups.
| Breast/chest wall effect | Baseline | 3 months | 6 months | 12 months | 24 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 180), (%) | 26 Gy (N = 181), (%) | 40 Gy (N = 141), (%) | 26 Gy (N = 153), (%) | 40 Gy (N = 137), (%) | 26 Gy (N = 151), (%) | 40 Gy (N = 130), (%) | 26 Gy (N = 142), (%) | 40 Gy (N = 127), (%) | 26 Gy (N = 135), (%) | |
| Pain in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 88 (49) | 77 (42) | 38 (27) | 41 (27) | 47 (35) | 36 (24) | 53 (41) | 56 (39) | 61 (48) | 56 (42) |
| A little | 80 (44) | 87 (48) | 75 (53) | 83 (54) | 66 (48) | 90 (60) | 54 (41) | 67 (47) | 50 (39) | 58 (43) |
| Quite a bit | 9 (5) | 12 (7) | 21 (15) | 22 (14) | 18 (13) | 16 (11) | 17 (13) | 14 (10) | 12 (9) | 14 (10) |
| Very much | 3 (2) | 5 (3) | 7 (5) | 7 (5) | 5 (4) | 8 (5) | 6 (5) | 5 (4) | 4 (3) | 6 (5) |
| Missing | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Swelling in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 123 (69) | 119 (65) | 98 (70) | 92 (60) | 95 (70) | 91 (61) | 100 (77) | 97 (68) | 98 (78) | 103 (77) |
| A little | 42 (24) | 51 (28) | 31 (22) | 37 (24) | 33 (24) | 42 (28) | 22 (17) | 32 (23) | 27 (21) | 23 (17) |
| Quite a bit | 10 (5) | 6 (3) | 10 (7) | 17 (11) | 3 (2) | 13 (9) | 6 (5) | 9 (6) | 1 (1) | 4 (3) |
| Very much | 3 (2) | 5 (3) | 2 (1) | 7 (5) | 5 (4) | 3 (2) | 2 (1) | 4 (3) | 0 | 4 (3) |
| Missing | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 1 |
| Oversensitivity in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 106 (59) | 105 (58) | 71 (51) | 61 (40) | 65 (48) | 63 (42) | 71 (55) | 80 (57) | 82 (65) | 66 (50) |
| A little | 62 (35) | 61 (34) | 48 (34) | 64 (42) | 53 (39) | 64 (43) | 37 (28) | 42 (30) | 35 (28) | 48 (36) |
| Quite a bit | 6 (3) | 9 (5) | 13 (9) | 19 (13) | 14 (10) | 13 (9) | 17 (13) | 12 (8) | 9 (7) | 13 (10) |
| Very much | 5 (3) | 6 (3) | 8 (6) | 8 (5) | 4 (3) | 9 (6) | 5 (4) | 7 (5) | 0 (0) | 5 (4) |
| Missing | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 3 |
| Skin problems on or in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 142 (79) | 137 (76) | 89 (64) | 95 (63) | 97 (72) | 113 (75) | 92 (71) | 107 (75) | 96 (76) | 100 (75) |
| A little | 31 (17) | 36 (20) | 39 (28) | 39 (26) | 26 (20) | 27 (18) | 27 (21) | 31 (22) | 23 (18) | 27 (20) |
| Quite a bit | 0 (0) | 6 (3) | 8 (6) | 8 (5) | 9 (6) | 10 (7) | 8 (6) | 4 (3) | 6 (5) | 6 (5) |
| Very much | 7 (4) | 2 (1) | 4 (3) | 10 (6) | 3 (2) | 1 (<1) | 3 (2) | 0 (0) | 2 (1) | 1 (<1) |
| Missing | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 |
| Change in appearance of skin in area of affected breast (protocol-specific item) | ||||||||||
| Not at all | 97 (55) | 86 (48) | 44 (32) | 52 (35) | 55 (43) | 57 (39) | 56 (45) | 64 (47) | 71 (61) | 74 (59) |
| A little | 51 (29) | 60 (34) | 55 (41) | 56 (38) | 51 (39) | 62 (43) | 42 (34) | 47 (34) | 31 (26) | 40 (32) |
| Quite a bit | 14 (8) | 21 (12) | 20 (15) | 28 (20) | 11 (9) | 17 (12) | 13 (10) | 19 (14) | 9 (8) | 3 (2) |
| Very much | 14 (8) | 10 (6) | 16 (12) | 11 (7) | 12 (9) | 9 (6) | 13 (10) | 7 (5) | 6 (5) | 8 (7) |
| Missing | 4 | 4 | 6 | 5 | 8 | 6 | 6 | 5 | 10 | 10 |
| Change in overall appearance of breast (protocol-specific item) | ||||||||||
| Not at all | 27 (17) | 33 (19) | 17 (13) | 24 (18) | 15 (13) | 16 (12) | 19 (17) | 24 (19) | 20 (18) | 28 (23) |
| A little | 58 (35) | 57 (34) | 49 (39) | 39 (28) | 48 (40) | 48 (37) | 41 (36) | 43 (34) | 38 (35) | 47 (39) |
| Quite a bit | 24 (15) | 40 (24) | 22 (18) | 34 (24) | 20 (17) | 35 (27) | 19 (17) | 30 (24) | 24 (22) | 20 (17) |
| Very much | 55 (33) | 40 (23) | 37 (30) | 41 (30) | 37 (30) | 31 (24) | 34 (30) | 30 (24) | 27 (25) | 25 (21) |
| Missing | 16 | 11 | 16 | 15 | 17 | 21 | 17 | 15 | 18 | 15 |
| Breast smaller (protocol-specific item) | ||||||||||
| Not at all | 55 (35) | 59 (36) | 47 (41) | 56 (42) | 41 (37) | 49 (40) | 41 (40) | 53 (44) | 37 (37) | 48 (43) |
| A little | 37 (24) | 47 (29) | 31 (27) | 35 (26) | 28 (26) | 27 (22) | 29 (29) | 20 (17) | 30 (30) | 28 (25) |
| Quite a bit | 21 (14) | 21 (13) | 14 (12) | 13 (10) | 16 (15) | 24 (20) | 10 (10) | 20 (17) | 16 (16) | 24 (21) |
| Very much | 42 (27) | 37 (25) | 23 (20) | 30 (22) | 24 (22) | 22 (18) | 21 (21) | 26 (22) | 17 (17) | 13 (12) |
| Missing | 25 | 17 | 26 | 19 | 28 | 29 | 29 | 23 | 27 | 22 |
| Breast harder/firmer to touch (protocol-specific item) | ||||||||||
| Not at all | 64 (43) | 79 (48) | 38 (33) | 37 (29) | 40 (37) | 31 (26) | 35 (35) | 28 (24) | 55 (57) | 37 (34) |
| A little | 48 (32) | 35 (21) | 30 (26) | 37 (29) | 31 (28) | 35 (29) | 37 (37) | 38 (33) | 22 (23) | 43 (39) |
| Quite a bit | 19 (13) | 26 (16) | 23 (20) | 23 (18) | 22 (20) | 34 (28) | 13 (13) | 27 (23) | 8 (8) | 20 (18) |
| Very much | 18 (12) | 24 (15) | 24 (21) | 32 (24) | 16 (14) | 20 (17) | 16 (16) | 23 (20) | 12 (12) | 10 (9) |
| Missing | 31 | 17 | 26 | 24 | 28 | 31 | 29 | 26 | 30 | 25 |
| Position of nipple of affected breast different from other side (protocol-specific item) | ||||||||||
| Not at all | 56 (44) | 72 (48) | 39 (39) | 43 (36) | 34 (34) | 44 (41) | 39 (43) | 46 (44) | 34 (40) | 55 (53) |
| A little | 29 (23) | 26 (18) | 25 (25) | 30 (25) | 31 (32) | 25 (23) | 24 (27) | 23 (22) | 30 (35) | 20 (19) |
| Quite a bit | 7 (5) | 9 (6) | 13 (13) | 10 (9) | 7 (7) | 15 (14) | 9 (10) | 14 (13) | 3 (3) | 12 (12) |
| Very much | 36 (28) | 41 (28) | 23 (24) | 35 (30) | 28 (28) | 23 (22) | 18 (20) | 21 (20) | 19 (22) | 16 (16) |
| Missing | 52 | 33 | 41 | 35 | 37 | 44 | 40 | 38 | 36 | 32 |
| Problems getting a bra to fit (protocol-specific item) | ||||||||||
| Not at all | 110 (70) | 113 (68) | 68 (54) | 72 (52) | 63 (51) | 64 (48) | 68 (58) | 74 (57) | 56 (51) | 68 (55) |
| A little | 22 (14) | 26 (16) | 22 (18) | 39 (28) | 35 (28) | 40 (30) | 22 (19) | 29 (23) | 30 (28) | 33 (27) |
| Quite a bit | 10 (6) | 8 (5) | 20 (16) | 7 (5) | 14 (11) | 14 (11) | 12 (10) | 13 (10) | 12 (11) | 13 (11) |
| Very much | 16 (10) | 19 (11) | 15 (12) | 21 (15) | 12 (10) | 15 (11) | 15 (13) | 12 (9) | 11 (10) | 9 (7) |
| Missing | 19 | 15 | 16 | 14 | 13 | 18 | 13 | 14 | 18 | 12 |
| Breast/chest wall effect | Baseline | 3 months | 6 months | 12 months | 24 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 108), (%) | 27 Gy (N = 103), (%) | 40 Gy (N = 88), (%) | 27 Gy (N = 94), (%) | 40 Gy (N = 82), (%) | 27 Gy (N = 93), (%) | 40 Gy (N = 79), (%) | 27 Gy (N = 87), (%) | 40 Gy (N = 72), (%) | 27 Gy (N = 89), (%) | |
| Pain in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 47 (43) | 46 (45) | 22 (25) | 22 (24) | 29 (35) | 20 (22) | 27 (34) | 30 (34) | 29 (40) | 38 (43) |
| A little | 52 (48) | 47 (46) | 48 (55) | 45 (48) | 37 (45) | 54 (58) | 37 (47) | 41 (47) | 34 (47) | 37 (42) |
| Quite a bit | 6 (6) | 9 (8) | 13 (15) | 21 (23) | 13 (16) | 13 (14) | 11 (14) | 10 (12) | 8 (11) | 11 (12) |
| Very much | 3 (3) | 1 (1) | 5 (5) | 5 (5) | 3 (4) | 6 (6) | 4 (5) | 6 (7) | 1 (1) | 3 (3) |
| Missing | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Swelling in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 68 (64) | 68 (67) | 61 (69) | 49 (53) | 59 (72) | 47 (51) | 58 (73) | 55 (63) | 52 (72) | 63 (71) |
| A little | 30 (28) | 22 (21) | 17 (19) | 24 (26) | 17 (20) | 28 (30) | 14 (18) | 18 (21) | 19 (26) | 21 (23) |
| Quite a bit | 6 (5) | 9 (9) | 8 (9) | 16 (17) | 2 (3) | 14 (15) | 5 (6) | 7 (8) | 1 (1) | 5 (6) |
| Very much | 3 (3) | 3 (3) | 2 (2) | 4 (4) | 4 (5) | 4 (4) | 2 (3) | 7 (8) | 0 | 0 |
| Missing | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oversensitivity in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 59 (54) | 63 (62) | 42 (48) | 35 (38) | 42 (51) | 39 (42) | 43 (55) | 43 (50) | 47 (66) | 46 (52) |
| A little | 41 (38) | 29 (28) | 30 (34) | 38 (41) | 26 (32) | 37 (40) | 20 (25) | 32 (37) | 21 (30) | 35 (39) |
| Quite a bit | 4 (4) | 9 (9) | 9 (10) | 14 (15) | 11 (13) | 10 (11) | 12 (15) | 7 (8) | 3 (4) | 7 (8) |
| Very much | 4 (4) | 1 (1) | 7 (8) | 6 (6) | 3 (4) | 6 (7) | 4 (5) | 4 (5) | 0 | 1 (1) |
| Missing | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Skin problems on or in area of affected breast (EORTC QLQ-BR23) | ||||||||||
| Not at all | 81 (75) | 71 (69) | 55 (63) | 48 (52) | 58 (71) | 53 (57) | 54 (68) | 55 (63) | 52 (72) | 66 (74) |
| A little | 21 (19) | 19 (18) | 26 (29) | 24 (26) | 16 (20) | 27 (29) | 15 (19) | 25 (29) | 14 (20) | 15 (16) |
| Quite a bit | 0 (0) | 10 (10) | 3 (3) | 17 (18) | 4 (5) | 8 (9) | 7 (9) | 3 (3) | 6 (8) | 4 (5) |
| Very much | 6 (6) | 3 (3) | 4 (5) | 4 (4) | 3 (4) | 5 (5) | 3 (4) | 4 (5) | 0 | 4 (5) |
| Missing | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Change in appearance of skin in area of affected breast (protocol-specific item) | ||||||||||
| Not at all | 62 (58) | 50 (49) | 23 (27) | 20 (22) | 30 (38) | 23 (26) | 29 (39) | 24 (29) | 40 (60) | 34 (40) |
| A little | 29 (27) | 35 (34) | 34 (41) | 45 (49) | 31 (40) | 39 (44) | 27 (36) | 39 (47) | 16 (24) | 28 (33) |
| Quite a bit | 7 (7) | 9 (9) | 16 (19) | 18 (20) | 8 (10) | 16 (18) | 9 (12) | 13 (16) | 5 (8) | 11 (13) |
| Very much | 8 (8) | 8 (8) | 11 (13) | 8 (9) | 9 (12) | 10 (12) | 10 (13) | 7 (8) | 5 (8) | 11 (13) |
| Missing | 2 | 1 | 4 | 3 | 4 | 5 | 4 | 4 | 6 | 5 |
| Change in overall appearance of breast (protocol-specific item) | ||||||||||
| Not at all | 19 (19) | 16 (17) | 7 (9) | 8 (9) | 8 (11) | 10 (12) | 6 (9) | 10 (13) | 10 (16) | 8 (10) |
| A little | 36 (37) | 30 (31) | 31 (40) | 34 (39) | 32 (43) | 23 (28) | 28 (40) | 21 (28) | 29 (47) | 25 (32) |
| Quite a bit | 15 (15) | 19 (20) | 19 (25) | 13 (15) | 13 (17) | 17 (20) | 11 (16) | 17 (23) | 11 (18) | 16 (20) |
| Very much | 28 (29) | 31 (32) | 20 (26) | 32 (37) | 22 (29) | 33 (40) | 25 (36) | 27 (36) | 12 (19) | 30 (38) |
| Missing | 10 | 7 | 11 | 7 | 7 | 10 | 9 | 12 | 10 | 10 |
| Breast smaller (protocol-specific item) | ||||||||||
| Not at all | 35 (37) | 40 (45) | 31 (42) | 28 (36) | 27 (40) | 29 (38) | 23 (36) | 21 (30) | 20 (36) | 27 (38) |
| A little | 24 (26) | 15 (17) | 18 (25) | 20 (25) | 16 (24) | 14 (18) | 20 (32) | 16 (23) | 21 (38) | 14 (20) |
| Quite a bit | 15 (16) | 7 (8) | 11 (15) | 12 (15) | 8 (12) | 10 (13) | 5 (8) | 11 (16) | 7 (12) | 11 (15) |
| Very much | 20 (21) | 27 (30) | 13 (18) | 19 (24) | 16 (24) | 23 (31) | 15 (24) | 21 (31) | 8 (14) | 19 (27) |
| Missing | 14 | 14 | 15 | 15 | 15 | 17 | 16 | 18 | 16 | 18 |
| Breast harder/firmer to touch (protocol-specific item) | ||||||||||
| Not at all | 37 (40) | 31 (36) | 25 (34) | 18 (23) | 27 (40) | 11 (15) | 20 (32) | 10 (14) | 33 (59) | 20 (29) |
| A little | 32 (35) | 23 (27) | 16 (21) | 23 (30) | 20 (30) | 22 (30) | 24 (38) | 23 (33) | 12 (21) | 22 (31) |
| Quite a bit | 13 (14) | 13 (15) | 18 (24) | 23 (30) | 9 (14) | 18 (25) | 6 (10) | 21 (30) | 7 (13) | 15 (21) |
| Very much | 10 (11) | 19 (22) | 16 (21) | 13 (17) | 11 (16) | 22 (30) | 13 (21) | 16 (23) | 4 (7) | 13 (19) |
| Missing | 16 | 17 | 13 | 17 | 15 | 20 | 16 | 17 | 16 | 19 |
| Position of nipple of affected breast different from other side (protocol-specific item) | ||||||||||
| Not at all | 39 (50) | 39 (51) | 30 (46) | 25 (37) | 21 (35) | 25 (40) | 22 (40) | 20 (34) | 21 (43) | 25 (40) |
| A little | 15 (19) | 13 (17) | 13 (20) | 15 (22) | 18 (30) | 11 (17) | 15 (27) | 12 (20) | 17 (35) | 13 (21) |
| Quite a bit | 3 (4) | 0 (0) | 7 (11) | 5 (8) | 3 (5) | 8 (13) | 4 (7) | 7 (12) | 1 (2) | 6 (10) |
| Very much | 21 (22) | 24 (32) | 15 (23) | 22 (33) | 18 (30) | 19 (30) | 14 (25) | 20 (34) | 10 (20) | 18 (29) |
| Missing | 30 | 27 | 23 | 27 | 22 | 30 | 24 | 28 | 23 | 27 |
| Problems getting a bra to fit (protocol-specific item) | ||||||||||
| Not at all | 67 (70) | 49 (51) | 43 (56) | 43 (47) | 35 (47) | 39 (45) | 39 (54) | 35 (42) | 33 (55) | 30 (36) |
| A little | 12 (13) | 20 (21) | 16 (21) | 22 (24) | 23 (31) | 18 (21) | 14 (19) | 24 (29) | 13 (22) | 26 (32) |
| Quite a bit | 7 (7) | 16 (17) | 10 (13) | 12 (13) | 9 (12) | 14 (16) | 7 (10) | 9 (11) | 8 (13) | 8 (10) |
| Very much | 10 (10) | 11 (11) | 8 (10) | 15 (16) | 8 (11) | 16 (18) | 12 (17) | 15 (18) | 6 (10) | 18 (22) |
| Missing | 12 | 7 | 11 | 2 | 7 | 6 | 7 | 4 | 12 | 7 |
The most frequently reported moderate or marked effect at 24 months was change in overall appearance of breast (as a result of any of the breast cancer treatments), which was reported by 47%, 38% and 58% of 40 Gy, 26 Gy and 27 Gy, respectively. Prevalence of moderate or marked effects were higher for the protocol-specific items than for the breast symptoms from the EORTC QLQ-BR23 breast module, which generally improved over time from 3 to 24 months following RT. Compared with PRO in the Main Trial, prevalence of symptoms from the EORTC QLQ-BR23 breast module for patients in the Nodal Sub-Study were similar, but frequencies of moderate or marked effects on the protocol-specific breast items were higher than reported in the Main Trial (see Chapter 5).
Frequencies of clinician assessments of radiation-related AE in the breast/chest wall and arm from 1 to 3 years following RT are shown in Table 28 for 40 Gy compared with 26 Gy (all randomised patients), and in Table 29 for 40 Gy and 27 Gy patients randomised up to the end of 2017.
| Year 1 | Year 2 | Year 3 | ||||
|---|---|---|---|---|---|---|
| 40 Gy (N = 166), (%) | 26 Gy (N = 169), (%) | 40 Gy (N = 166), (%) | 26 Gy (N = 159), (%) | 40 Gy (N = 135), (%) | 26 Gy (N = 134), (%) | |
| Arm lymphoedema | ||||||
| No | 149 (93) | 147 (89) | 147 (92) | 138 (89) | 119 (92) | 108 (88) |
| Yes | 11 (7) | 19 (11) | 13 (8) | 17 (11) | 11 (8) | 15 (12) |
| Ipsilateral side | 11 | 19 | 13 | 17 | 11 | 15 |
| Missing | 6 | 3 | 6 | 4 | 5 | 11 |
| Breast lymphoedema | ||||||
| No | 147 (92) | 141 (85) | 153 (96) | 137 (89) | 126 (96) | 113 (90) |
| Yes | 12 (8) | 24 (15) | 7 (4) | 17 (11) | 5 (4) | 12 (10) |
| Ipsilateral side | 12 | 24 | 7 | 16 | 5 | 11 (1 NK) |
| Missing | 7 | 4 | 6 | 5 | 4 | 9 |
| Breast distortion a | ||||||
| None | 58 (70) | 62 (64) | 51 (66) | 39 (53) | 35 (57) | 36 (59) |
| A little | 15 (18) | 23 (24) | 16 (21) | 26 (35) | 14 (23) | 20 (33) |
| Quite a bit | 10 (12) | 10 (10) | 8 (10) | 6 (8) | 11 (18) | 4 (6) |
| Very much | 0 (0) | 2 (2) | 2 (3) | 3 (4) | 1 (2) | 1 (2) |
| N/Ab | 50 | 45 | 45 | 36 | 27 | 26 |
| Not assessed | 33 | 27 | 44 | 49 | 47 | 47 |
| Breast shrinkage a | ||||||
| None | 57 (69) | 61 (62) | 48 (63) | 43 (59) | 38 (62) | 34 (56) |
| A little | 20 (24) | 24 (25) | 18 (23) | 19 (26) | 13 (22) | 17 (28) |
| Quite a bit | 6 (7) | 12 (12) | 11 (14) | 10 (14) | 10 (16) | 9 (15) |
| Very much | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (2) | 1 (1) |
| N/Ab | 50 | 45 | 45 | 36 | 27 | 26 |
| Not assessed | 33 | 26 | 44 | 50 | 46 | 47 |
| Breast induration (tumour bed) a | ||||||
| None | 60 (72) | 66 (68) | 50 (65) | 41 (56) | 38 (66) | 39 (64) |
| A little | 13 (16) | 19 (20) | 17 (22) | 25 (34) | 13 (22) | 17 (28) |
| Quite a bit | 9 (11) | 9 (9) | 8 (10) | 6 (8) | 5 (9) | 5 (8) |
| Very much | 1 (1) | 3 (3) | 2 (3) | 1 (1) | 2 (3) | 0 |
| N/Ab | 50 | 45 | 45 | 36 | 27 | 26 |
| Not assessed | 23 | 27 | 44 | 50 | 50 | 47 |
| Breast induration (outside tumour bed) a | ||||||
| None | 68 (82) | 75 (77) | 64 (83) | 62 (85) | 47 (80) | 54 (89) |
| A little | 8 (10) | 17 (18) | 8 (10) | 8 (11) | 7 (12) | 5 (8) |
| Quite a bit | 6 (7) | 4 (4) | 3 (4) | 2 (3) | 4 (6) | 2 (3) |
| Very much | 1 (1) | 1 (1) | 2 (3) | 1 (1) | 1 (2) | 0 (0) |
| N/Ab | 50 | 45 | 45 | 36 | 27 | 26 |
| Not assessed | 33 | 27 | 44 | 50 | 49 | 47 |
| Telangiectasia | ||||||
| None | 126 (96) | 137 (97) | 108 (88) | 102 (93) | 78 (92) | 78 (87) |
| A little | 5 (4) | 4 (3) | 11 (9) | 5 (5) | 7 (8) | 6 (7) |
| Quite a bit | 0 (0) | 0 (0) | 4 (3) | 3 (3) | 0 (0) | 5 (6) |
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not assessed | 35 | 28 | 43 | 49 | 50 | 45 |
| Breast/chest wall oedema | ||||||
| None | 108 (82) | 111 (79) | 107 (86) | 93 (84) | 78 (91) | 76 (85) |
| A little | 21 (16) | 22 (16) | 14 (11) | 13 (12) | 8 (9) | 9 (10) |
| Quite a bit | 2 (2) | 7 (5) | 2 (2) | 5 (4) | 0 (0) | 3 (3) |
| Very much | 1 (<1) | 1 (<1) | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Not assessed | 34 | 28 | 42 | 48 | 49 | 45 |
| Breast/chest wall discomfort | ||||||
| None | 101 (75) | 100 (69) | 90 (73) | 83 (74) | 61 (69) | 72 (79) |
| A little | 24 (18) | 36 (25) | 27 (22) | 27 (24) | 20 (23) | 15 (17) |
| Quite a bit | 9 (7) | 6 (4) | 7 (6) | 3 (2) | 5 (6) | 3 (3) |
| Very much | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 2 (2) | 1 (1) |
| Not assessed | 32 | 25 | 42 | 46 | 47 | 43 |
| Year 1 | Year 2 | Year 3 | ||||
|---|---|---|---|---|---|---|
| 40 Gy (N = 99), (%) | 27 Gy (N = 103), (%) | 40 Gy (N = 97), (%) | 27 Gy (N = 101), (%) | 40 Gy (N = 85), (%) | 27 Gy (N = 90), (%) | |
| Arm lymphoedema | ||||||
| No | 87 (92) | 84 (85) | 83 (91) | 86 (87) | 73 (91) | 76 (89) |
| Yes | 8 (8) | 15 (15) | 8 (8) | 13 (13) | 7 (9) | 9 (11) |
| Ipsilateral side | 8 | 13 | 8 | 13 | 7 | 9 |
| Missing | 4 | 4 | 6 | 2 | 5 | 5 |
| Breast lymphoedema | ||||||
| No | 87 (92) | 79 (80) | 86 (95) | 90 (90) | 78 (96) | 81 (95) |
| Yes | 8 (8) | 20 (20) | 5 (5) | 9 (9) | 3 (4) | 4 (5) |
| Ipsilateral side | 8 | 18 | 5 | 9 | 3 | 4 |
| Missing | 4 | 4 | 6 | 2 | 4 | 5 |
| Breast distortion a | ||||||
| None | 32 (64) | 40 (68) | 29 (63) | 30 (58) | 21 (58) | 21 (50) |
| A little | 11 (22) | 14 (24) | 10 (22) | 17 (33) | 8 (22) | 15 (36) |
| Quite a bit | 7 (14) | 3 (5) | 7 (15) | 4 (8) | 7 (20) | 4 (9) |
| Very much | 0 (0) | 2 (3) | 0 (0) | 1 (2) | 0 (0) | 2 (5) |
| N/Ab | 26 | 32 | 27 | 32 | 12 | 21 |
| Not assessed | 23 | 12 | 24 | 17 | 37 | 27 |
| Breast shrinkage a | ||||||
| None | 32 (64) | 35 (59) | 29 (63) | 37 (70) | 23 (64) | 20 (47) |
| A little | 16 (32) | 18 (31) | 10 (22) | 11 (21) | 6 (17) | 14 (32) |
| Quite a bit | 2 (4) | 5 (8) | 7 (15) | 4 (8) | 7 (19) | 6 (14) |
| Very much | 0 (0) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 3 (7) |
| N/Ab | 26 | 32 | 27 | 32 | 12 | 21 |
| Not assessed | 23 | 12 | 24 | 16 | 37 | 26 |
| Breast induration (tumour bed) a | ||||||
| None | 37 (74) | 43 (73) | 27 (59) | 37 (70) | 25 (72) | 27 (66) |
| A little | 8 (16) | 12 (20) | 14 (30) | 11 (21) | 6 (17) | 10 (24) |
| Quite a bit | 5 (10) | 3 (5) | 5 (11) | 4 (8) | 4 (11) | 2 (5) |
| Very much | 0 (0) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 2 (5) |
| N/Ab | 26 | 32 | 27 | 32 | 12 | 21 |
| Not assessed | 23 | 12 | 24 | 16 | 38 | 28 |
| Breast induration (outside tumour bed) a | ||||||
| None | 39 (78) | 51 (86) | 36 (78) | 45 (87) | 29 (83) | 32 (78) |
| A little | 7 (14) | 6 (10) | 7 (15) | 5 (10) | 5 (14) | 7 (17) |
| Quite a bit | 4 (8) | 2 (3) | 3 (7) | 2 (4) | 1 (3) | 2 (5) |
| Very much | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| N/Ab | 26 | 32 | 27 | 32 | 12 | 21 |
| Not assessed | 23 | 12 | 24 | 17 | 38 | 28 |
| Telangiectasia | ||||||
| None | 76 (97) | 84 (92) | 67 (89) | 79 (92) | 43 (93) | 58 (92) |
| A little | 2 (3) | 7 (8) | 7 (9) | 5 (6) | 3 (7) | 5 (8) |
| Quite a bit | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Very much | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Not assessed | 21 | 12 | 22 | 16 | 39 | 27 |
| Breast/chest wall oedema | ||||||
| None | 63 (80) | 62 (68) | 62 (82) | 72 (85) | 42 (91) | 59 (92) |
| A little | 13 (16) | 19 (21) | 12 (16) | 13 (15) | 4 (9) | 5 (8) |
| Quite a bit | 2 (3) | 8 (9) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Very much | 1 (1) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Not assessed | 20 | 12 | 21 | 16 | 39 | 26 |
| Breast/chest wall discomfort | ||||||
| None | 61 (77) | 57 (63) | 56 (74) | 60 (68) | 34 (71) | 37 (58) |
| A little | 12 (15) | 25 (17) | 16 (21) | 22 (25) | 12 (25) | 19 (30) |
| Quite a bit | 6 (8) | 8 (9) | 4 (5) | 4 (5) | 2 (4) | 5 (7) |
| Very much | 0 (0) | 1 (1) | 0 (0) | 2 (2) | 0 (0) | 3 (5) |
| Not assessed | 20 | 12 | 21 | 13 | 37 | 26 |
The prevalence of clinician-reported arm lymphoedema remained relatively stable from 1 to 3 years following RT, with 8%, 12% and 11% in 40 Gy, 26 Gy and 27 Gy, respectively at year 3. Clinician-reported breast lymphoedema declined from 1 to 3 years, with 4%, 10% and 5% in 40 Gy, 26 Gy and 27 Gy, respectively at year 3. Frequencies of marked clinician-assessed AE in the breast or chest wall (graded as ‘very much’) were low across 1–3 years’ follow-up. The most frequently reported AE graded as moderate or marked by clinicians was breast distortion, with 20%, 8% and 14% in 40 Gy, 26 Gy and 27 Gy, respectively at 3 years.
Discussion of Nodal Sub-Study results is in the main Discussion section (see Chapter 8).
Chapter 8 Discussion
Opening statements
Previous trials, particularly the UK START trials, have confirmed the feasibility and benefits of hypofractionation such that 40 Gy in 15 fractions was adopted as the UK standard schedule in 200950 and subsequently internationally102 but there is no reason why the concept of hypofractionation should be limited to 15 fractions. The UK FAST trial103 was planned in the early 2000s when 50 Gy in 25 fractions over 5 weeks was considered standard. The FAST trial compared 28.5 Gy in 5 fractions of 5.7 Gy or 30 Gy in 5 fractions of 6 Gy with 50 Gy in 25 fractions, with the 5-fraction regimens delivered once weekly over 5 weeks to reduce overall time as a variable. The primary endpoint was photographic assessment at 2 and 5 years. A low-risk patient population was deliberately chosen, excluding patients requiring a breast boost or systemic chemotherapy for instance. Two test groups were used, as in the START-pilot and START-A trials, to allow interpolation to estimate an isoeffective dose to control if necessary. The FAST trial identified a 5-fraction schedule estimated to be radiobiologically equivalent to the 25-fraction standard in terms of late NTE.
The 5-year results from the START trials,17,18 with the change in standard UK fractionation to 40 Gy/15 fractions (NICE 2009), the first results of the FAST trial,103 plus the acute toxicity and 2-year AE from a pilot study delivering 30 Gy in 5 fractions over 15 days26 gave impetus to the investigation of 1-week 5-fraction schedules in a phase III study. With these results the FAST-Forward Trial was developed to test two 5-fraction schedules over a week against the standard of 40 Gy in 15 fractions over 3 weeks, specifically to be non-inferior for local relapse and without an increase in late AE.
FAST-Forward local relapse
FAST-Forward is a non-inferiority trial with IBTR or local relapse as the primary endpoint. Based on START trial data18 and incorporating subsequent improvements in surgical technique and systemic therapy, it was anticipated that 5-year IBTR rate in the FAST-Forward control group given 40 Gy in 15 fractions would be 2%. Applying a non-inferiority margin of 1.6% at 5 years required a sample size of 4000 patients. This provided 80% power (one-sided α of 0·025 allowing for non-inferiority hypothesis and a simple Bonferroni correction taking into account comparisons between each test schedule and the control group) to exclude an absolute increase of 1·6% in 5-year IBTR incidence for a 5-fraction schedule compared with control. Estimated 5-year IBTR after 40 Gy in 15 fractions was 2.1% (95% CI 1.4 to 3.1), after 27 Gy was 1.7% (1.2 to 2.6) and after 26 Gy was 1.4% (0.2 to 2.2). The upper confidence limits (CI) excluded an increase in IBTR of > 1.6% after both 5-fraction schedules with p = 0.0022 and p = 0.00019 for non-inferiority of 27 Gy and 26 Gy schedules, respectively compared with 40 Gy in 15 fractions.
Therefore, it can be said emphatically that non-inferiority in terms of IBTR of 5-fraction schedules compared with 40 Gy in 15 fractions was demonstrated at 5 years’ follow-up for patients with early breast cancer, the majority of whom were treated by local tumour excision and sentinel node biopsy for node-negative disease. The lack of a detectable dose–response for local tumour control between 26 Gy and 27 Gy in 5 fractions is a potential limit to precision, but this feature reflects the shallowness of the dose–response curve for subclinical breast cancer around the 98% control level, so the −0.4% estimated difference in absolute levels of IBTR between 27 Gy and 26 Gy likely reflects random sampling variability in the IBTR rate and/or chance imbalances in unmeasured prognostic factors between test groups.
Longer follow-up is well recognised as having added value for RT trials. IBTR continues to present beyond 5 years, but trials of hypofractionation have so far reported almost identical HRs for IBTR between test and control groups at 5 and 10 years, the relevant metric for comparisons of effect. For example, START-B18 incidence rates of IBTR at 5/10 years after 50 Gy in 25 fractions versus 40 Gy in 15 fractions were 3.3%/5.2% and 1.9%/3.8%, respectively, reflecting crude HR of 0.72 (95% CI 0.43 to 1.21) and 0.70 (95% CI 0.46 to 1.07) at the respective time points that is barely changed between 5 and 10 years. The OCOG reported IBTR rates at 5/10 years after 50 Gy in 25 fractions versus 42.5 Gy in 16 fractions of 3.2%/6.7% and 2.8%/6.2%, respectively, giving an absolute difference of 0.4% (95% CI −1.5% to 2.4%) and 0.5% (95% CI −2.5% to 3.5%), respectively. 104 This is reassuring but the 10-year IBTR rates will be reported when the data matures to address any remaining concerns over efficacy of five daily fractions.
Application of FAST-Forward to partial breast radiotherapy
IMPORT LOW tested 40 Gy/15 fractions over 3 weeks PBI compared with 40 Gy/15 fractions over 3 weeks to whole breast (control). 12 This is the only PBI RCT worldwide where irradiated volume is the only variable and all other factors including dose/fractionation are constant. The reduced volume of breast tissue treated with PBI was associated with non-inferior local control and reduced late normal tissue toxicity. Given that we know that reduced volume (PBI) results in reduced late normal tissue toxicity for a constant dose/fractionation as per IMPORT LOW, then it is logical that 26 Gy/5 fractions is also adopted for PBI given that both trials used the same dose/fractionation for control. It would be counter-intuitive to state that 26 Gy/5 fractions can be used for whole breast RT with excellent local control and minimal toxicity, but not for PBI given that IMPORT LOW has shown (1) reduced volume is non-inferior for local control, (2) reduced volume has less toxicity, that is it would be illogical (and possibly unethical) to have a greater treatment burden for the lower risk group of patients eligible for PBI.
Use of hypofractionation in the post-mastectomy setting
Wang et al. 105 reported on 810 patients in a single institution randomised non-inferiority trial of moderate hypofractionated RT post-mastectomy. All patients underwent axillary dissection and were at least four-node positive or T3–4, unless they received neoadjuvant chemotherapy in which case either clinical stage III or pathological axillary node-positive patients were eligible. The hypofractionated schedule was 43.5 Gy in 15 fractions over 3 weeks versus 50 Gy in 25 fractions over 5 weeks as standard. The RT target volume included the chest wall, level 3 axilla and SCF. The 5-year locoregional relapse rate was 8.3% (90% CI 5.8% to 10.7%) with the 15-fraction schedule and 8.1% (90% CI 5.4% to 10.6%) with the 25-fraction schedule. With a p < 0.0001 for non-inferiority they concluded that the hypofractionated regimen was non-inferior to standard.
In the FAST-Forward Main Trial there were 264 patients who had had a mastectomy and only one of these had a local relapse. One hundred and seventy-three patients received chest wall only RT with one of the 5-fraction schedules with no local relapse reported. While this is too few events to make any firm statements the data raise no concerns regarding the efficacy or safety of the 5-fraction schedules. There is no logical reason why a 5-fraction schedule would not be as effective when irradiating the chest wall as opposed to the intact breast.
Breast cancer subgroups and local relapse
Commentators have suggested that hypofractionation may be less applicable in certain patient and pathological subgroups. The initial 2011 American Society for Radiation Oncology (ASTRO) evidence-based guidelines106 were unable to confirm agreement on hypofractionation in patients < 50 years. The small number of patients and the increased risk of IBTR at young age were cited, but no evidence of an adverse outcome by age after hypofractionation has been reported. A post hoc analysis of tumour grade in the OCOG trial104 suggested an interaction of grade and randomisation group, but subsequent central analysis of tumour blocks reported no trend for patients with high-grade tumours to be disadvantaged after 42.5 Gy in 16 fractions104 as compared to 50 Gy in 25 fractions. OCOG also found that tumour grade and molecular subtype did not predict response to hypofractionation. A subgroup meta-analysis of locoregional relapse was performed of the START-P, START-A and START-B trials in 5861 patients reporting 10-year results. 107 Treatment effects of hypofractionation were not significantly different from 50 Gy in 25 fractions when examined by age, type of primary surgery, axillary node status, tumour grade, use of adjuvant chemotherapy or boost RT. The HR for locoregional control and the patient numbers for the combined hypofractionation schedules against 50 Gy in 25 fractions were 0.79 (95% CI 0.47 to 1.34) in 343 patients aged under 40 years, 0.88 (95% CI 0.60 to 1.28) in 1046 patients aged 40–49 years, 0.86 (95% CI 0.59 to 1.25) in 1272 patients with grade 3 tumours, 0.91 (95% CI 0.59 to 1.25) in 513 patients following mastectomy and 0.81 (95% CI 0.46 to 1.81) in 1480 patients after adjuvant chemotherapy. The 2018 ASTRO evidence-based guidelines subsequently approved hypofractionated breast RT with 40/42.5 Gy in 15/16 fractions over 3 weeks irrespective of age, tumour grade or receptor status. 102
FAST-Forward reported non-inferior IBTR for both 5-fraction schedules and given the preceding arguments inferiority in any subgroups would not be expected. One thousand and five forty-five (37.8%) patients were in the high-risk category as defined by age < 50 years (604 patients), grade 3 tumour (1153 patients) or both, and this was a stratification factor at randomisation. Formal subgroup analysis was not performed due to the low number of IBTR events but descriptive data according to age, grade, and receptor status showed no signals of concern for the 5-fraction schedules. The use of boost and dose/fractionation, both declared prior to randomisation, were balanced between the three treatment groups so there is no risk of bias in dose intensity between trial groups.
Disease-free survival and overall survival
The EBCTCG meta-analysis reports 10-year relapse and 15-year mortality effect of RT after breast-conserving surgery. 6 After breast-conserving surgery the results from 10,801 women showed that 15-year all-cause mortality was 3.0% (95% CI 0.6% to 5.4%; 2p = 0.03) better following RT; it was only 0.8% after 5 years. The trials included in the meta-analysis came from a different era when local relapse rates and risk of metastases were much higher than is currently the case. Thus, one would not anticipate any differences according to RT schedule after 5 years in FAST-Forward. Incidence of distant relapse, disease-free survival and overall survival were similar between the fractionation schedules, with no evidence of statistically significant differences between groups.
Normal tissue effects
For late NTE the dose–response curve is much steeper than for IBTR, enabling detection of clinically and statistically significant differences in event rates between 26 Gy and 27 Gy in 5 fractions. The 5-fraction schedule isotoxic for late NTE with 40 Gy in 15 fractions allows direct estimation of α/β for late NTE, which is consistent with values generated from our other trials. The α/β value of 3.7 Gy (0.3–7.1) for tumour control in FAST-Forward is similar to 3.5 Gy (1.2–5.7) estimated from the START pilot and START-A trials. 108 Point estimates of α/β (assuming no effect of time) for late NTE in FAST-Forward scored by clinicians, patients and photographic assessments are closer to 2 Gy than the 3 Gy estimated in the earlier START and FAST trials, but 95% CI overlap for each endpoint in all trials. In FAST, 915 women were randomised after BCS for node-negative disease to 50 Gy in 25 fractions versus two dose levels of a 5-fraction regimen delivered once weekly, thereby ensuring complete repair between fractions and controlling for overall treatment time. The α/β value for change in photographic breast appearance in FAST was 2.6 Gy (1.4–3.7). There is uncertainty about biological processes, such as possible influence of a time factor in FAST-Forward. However, this does not interfere with clinical evaluation and thus decisions on implementation of FAST-Forward results in comparable patient groups.
In FAST-Forward, late NTE assessed by clinicians, patients and photographs were key secondary endpoints. On the basis of the similarity between NTEs for the 26 Gy in five daily fractions schedule and for the 40 Gy in 15 fractions schedule, 26 Gy in 5 fractions is the regimen recommended for clinical implementation. By ‘similar’ we mean that NTE were not statistically or clinically significantly different from 40 Gy in 15 fractions with respect to clinician- or patient-assessed NTE including external photographic assessments conducted blind to treatment allocation. In contrast, the 27 Gy 5-fraction schedule was significantly different to the 40 Gy standard for many late NTE and also to the 26 Gy schedule, confirming the sensitivity of trial outcome measures to a difference in dose intensity corresponding to 3 Gy in 2 Gy equivalents assuming α/β = 2 Gy (see below). The 27 Gy 5-fraction regimen exhibited late NTE rates of comparable magnitude to 50 Gy in 25 daily fractions, defended for so long as standard and still in use by many practitioners outside the UK. To provide some perspective for the late NTE after 5-fraction regimens, 40 Gy in 15 fractions is equivalent to about 46 Gy in 2 Gy fractions in terms of late NTE compared to 50 Gy in 25 fractions according to START trial outcomes. 108 In FAST-Forward, the 26 Gy regimen is comparable to 47 Gy in 2 Gy equivalents in terms of late NTE.
Early NTE are much less responsive to fraction size than late NTE, the contribution of total dose to early NTE being relatively more important. FAST-Forward offers a good example in that breast erythema was less intense and also settled a fortnight earlier after 5-fraction than 15-fraction schedules. 3 In this context, the milder erythema was due to 26 and 27 Gy total dose levels much more than to fraction sizes of 5.2 Gy and 5.4 Gy. Acute reactions were also milder in both 5-fraction schedules (total doses 27 and 30 Gy) of the FAST trial than the 50 Gy schedule. 26
Induration is a key late NTE that is expected to increase with the passage of time irrespective of radiation schedule. Other factors contributing to breast appearance include fat necrosis and oedema, particularly in the early years. Appendix 2, Table 56 reproduces results already presented in Chapter 5, but shows clinical and patient assessments together, for the longitudinal FAST-Forward moderate or marked assessments that equate to fibrosis (including clinician-assessed distortion, shrinkage and induration outside the tumour bed) as well as patient-assessed breast appearance change (smaller and harder or firmer). The ORs do not tell the whole story, the absolute level of events also being very important. For example, for breast shrinkage, the most frequent of any clinician-assessed effects, the rate at 5 years was 5.1% in the 40 Gy and 6.3% in the 26 Gy schedule. Also, it should be noted that, for all clinician-assessed events documented in the moderate/marked categories, most were moderate rather than marked in severity. For the patient assessments, in which there was a baseline post-surgery but pre-radiotherapy assessment, the number of longitudinal events is generally not much higher than that post-surgery baseline. This is likely to be the same event rather than a distinct event. These late effect results are very important in terms of giving patients realistic expectations. With regard to increasing late NTE with time, stability of the HR at later time points is clinically relevant as discussed earlier for IBTR. In START-B the breast shrinkage HR for test to control arm was 0.83 (95% CI 0.66 to 1.04) at 5 years and 0.80 (95% CI 0.67 to 0.96) at 10 years. For 5 fractions we are now able to investigate the FAST 5- and 10-year results noting the same principle but with confidence limits which are wider given the smaller number of patients compared to START. 23 For breast shrinkage the test to control arm HR at 5 and 10 years, respectively was 2.03 (95% CI 1.15 to 3.58) and 1.83 (95% CI 0.88 to 3.81) for the 30 Gy schedule and 1.20 (95% CI 0.63 to 2.27) and 1.83 (95% CI 0.88 to 3.81) for 28.5 Gy schedule. For ‘any NTE’ the test to control arm at 5 and 10 years, respectively was 2.40 (95% CI 1.45 to 3.97) and 2.03 (95% CI 1.06 to 3.89) for the 30 Gy schedule and 1.32 (95% CI 0.75 to 2.34) and 1.61 (95% CI 0.81 to 3.18) for 28.5 Gy schedule. The principle of the relative difference between test and control arm changing little with time can therefore be applied to FAST-Forward, again noting the low absolute levels of marked and moderate events.
The Danish-led HYPO trial of 1864 patients was designed to determine whether 40 Gy in 15 fractions would not increase in duration of the breast at 3 years compared to 50 Gy in 25 fractions. Results confirmed no increase in induration with moderate hypofractionation. 109 Breast size was a stratification at randomisation and they reported that induration at 3 years was significantly increased for large compared with small-breasted patients but that at no time point was the hypofractionated schedule worse than control for this NTE. Shaitelman et al. 110 conducted a randomised non-inferiority trial due to low uptake of hypofractionated whole breast RT in the United States, the low uptake being attributed to concerns of safety for patients receiving a tumour bed boost, chemotherapy or having large breast size. The standard arm was 50 Gy in 25 daily fractions with a 10–14 Gy boost in 5–7 fractions and hypofractionation was 42.56 Gy in 16 daily fractions with a 10–12.5 Gy boost in 4–5 fractions. Primarily they investigated the proportion of patients with adverse patient-reported cosmetic outcome at 3 years. One hundred and six patients (36.9%) of the 287 patients were defined as large breast size with a bra cup size of at least D. Adverse patient-reported cosmetic outcome was 5.4% lower (8.2% versus 13.6%, p = 0.002 for inferiority) in the hypofractionated group overall and 18.6% lower (90% upper confidence limit 8% lower) for large breasted patients. They conclude that this offers strong reassurance for hypofractionation not compromising cosmetic outcome based on large breast size.
Tsang et al. 111 looked at dose heterogeneity with regard to the FAST trial and the risk of ‘triple trouble’. 112 Three hundred and ninety full CT-planning data sets were reviewed for patients where there was a baseline and 2-year photographic assessment, the primary endpoint of FAST. The two 5-fraction groups were combined for analysis and there was no significant difference between these and control for breast volume or for patient tumour and treatment characteristics from the whole FAST population. Multiple logistic regression analyses show that after adjusting for breast size (and surgical deficit) there was no evidence of late NTE associated with dose inhomogeneity using various definitions of hotspots. The effect of inhomogeneity was not significantly different for any of the dosimetric parameters between control and 5-fraction schedules. In FAST-Forward the α/β estimate for any clinician-assessed moderate or marked NTE was barely different unadjusted or when adjusted for breast size, using whole breast planning treatment volume as the proxy for breast size. The same lack of difference was found with photographic assessment and breast size. We can conclude that breast size is an established factor for increased NTE following breast RT but that hypofractionation, including 5-fraction schedules, is not an additional concern for larger-breasted patients.
What about other organs at risk? The heart is often mentioned and, given that very long follow-up is required to detect late cardiac events, is cited as a reason to wait for longer-term data before adopting hypofractionation as standard of care. However, there is no specific reason to expect an increased cardiac sensitivity to hypofractionation. Darby et al. have shown that there is no safe dose to the heart and therefore the effort is to reduce or eliminate cardiac dose. 113 At this early stage, after imaging and further investigation, excluding cases confirmed not to be radiotherapy-related, for left-sided RT there are six cases of ischaemic heart disease in the 40 Gy arm and three cases in the 26 Gy arm.
The most frequent specialist referral we have seen is to lymphoedema clinics for breast lymphoedema, 90 (6.6%) following 40 Gy and 106 (7.7%) after 26 Gy. Breast oedema is predominantly an early side effect which we have seen settling such that at 5 years the moderate/marked incidence on clinician-assessment is nine (0.7%) patients after 40 Gy and 17 (1.7%) patients after 26 Gy with none at all in 94% and 93%, respectively. These rates are low and not significantly different. These low rates are also encouraging for patients.
Tumour control and radiobiology
In a review of the linear-quadratic model and implications for practice, Brand and Yarnold present FAST-Forward as an example of a trial evaluating of 5-fraction hypofractionated accelerated RT. 114 To make sense of FAST-Forward in terms of fraction size effects, the START trials offer a good entry point. The START-P/-A trials (1986–2003) each compared 50 Gy in 25 fractions over 5 weeks (control) with two test dose levels of a 13-fraction regimen over 5 weeks (5 fractions per fortnight). By controlling for time-related effects, repopulation being the likeliest mechanism in tumours, and assuming complete repair of sub-lethal damage between fractions in all groups, an unconfounded estimate of sensitivity to fraction size is possible. This simply involves identifying the total dose in 13 fractions matching the IBTR rate in the control group by interpolation between test dose levels if needed. The START-A test dose level of 41.6 Gy in 13 fractions of 3.2 Gy was the closest, from which the α/β estimate of 3.5 Gy (95% CI 1.2 to 5.7) was derived based on a 10-year total of 349 IBTR events in 3646 women. The reduction from 50 Gy to 41.6 Gy (around 8 Gy) total dose needed to match antitumour effects of 13 fractions of 3.2 Gy and 25 fractions of 2.0 Gy is a vivid example of increasing fraction size sensitivity at play.
To our knowledge, START-P/-A generated the only direct clinical estimate of α/β for a cancer, others being based on non-randomised or randomised comparisons that do not control for one or more variables, especially time. START-B is a good example of the latter, testing 50 Gy in 25 fractions over 5 weeks against 40 Gy in 15 fractions of 2.7 Gy over 3 weeks. Applying the α/β = 3.5 generated by START-P/-A, the equivalent total dose in 2.0 Gy fractions (EQD2/3.5) of the 3-week schedule is only 45 Gy, yet based on 95 IBTR events in 2215 patients (4.3%), the test schedule was non-inferior to 50 Gy (HR 0.77, 95% CI 0.51 to 1.16; p = 0.21). In fact, the point estimate 10-year IBTR rate of the 3-week regimen was about 1% superior 50 Gy.
A post hoc analysis asked the question ‘If this difference is real, what would it tell us about the impact of treatment time?’ We know that in laryngeal carcinomas at least 0.5 Gy/day can be ‘wasted’ compensating for accelerated repopulation from the fourth week of treatment onwards, first described by Withers in patients treated with primary RT and confirmed by Overgaard et al. in a randomised clinical trial comparing 60 Gy in 30 fractions delivered five versus six times per week. 115,116 Breast cancers have relatively low mitotic rates at presentation, but they might be in an accelerated phase of repopulation by the time RT starts several weeks or months after primary surgery and/or cytotoxic chemotherapies. In the context of the START-B result, the post hoc analysis estimated 0.6 Gy/day (95% CI 0.1 to 1.8; p = 0.02) ‘wasted’ dose in control group patients during weeks 4 and 5. If true, it implies roughly 14 × 0.6 = 8 Gy of the control regimen (50 Gy) ‘wasted’, leaving an EQD2/3.5 of 42 Gy against 45 Gy for the 3-week schedule. The HYPO trial offers an independent test of START-B regimens in a comparable group of patients, in whom the 9-year risk of locoregional relapse was 3.3% (95% CI 2.0 to 5.0) in the 50 Gy in 25 fractions group compared to 3.0% (95% CI 1.9 to 4.5) in the 40 Gy in 15 fractions group (risk difference 20.3%, 95% CI 22.3 to 1.7),8 a result almost identical to START-B.
What have we seen in FAST-Forward? The trial generated an α/β estimate for IBTR of 3.7 Gy (95% CI 0.3 to 7.1), the CI reflecting very low IBTR rates. The analysis plan did not incorporate a hypothetical time correction, so the α/β estimate of 3.7 Gy necessarily incorporates a putative time effect by contributing to the efficacy of the shorter test regimens. Regardless of whether or not there is a time effect, the clinically effective EQD2/3.7 of 26 Gy in 5 fractions is 41 Gy in 2 Gy fractions, see Table 3. The difference between this EQD and the 45 Gy of 40 Gy in 15 fractions at such high levels of local control may be just too small to detect clinically, but the most important clinical conclusion that can be robustly drawn is that the 5-fraction regimen has demonstrated non-inferiority in relation to the pre-defined ≤ 1.6% excess IBTR boundary set in the protocol. Questions have been raised whether 26 Gy in 5 fractions has any antitumour effect at all. 109 With 5-year IBTR rate of 2.1% (95% CI 1.4% to 3.1%) after 40 Gy in 15 fractions, the rate would be expected to be about 6% at 5 years and perhaps 10% at 10 years without any RT according to systematic overviews of RT effects. 6 The observed 5-year IBTR rates after 26 Gy in 5 fractions are hardly consistent with an absence of effect.
To take the 0.6 Gy/day ‘wasted’ dose further a hypothesis could be generated with regard to 26 Gy in 5 daily fractions. If the ‘wasted dose’ continues down to a week, 40 Gy in 15 fractions is 14 days longer and an EQD2/3.7 of 37 Gy is calculated as opposed to an EQD2/3.7 of 41 Gy for 26 Gy in 5 fractions. The same calculation for 50 Gy in 25 daily fractions gives an EQD2/3.7 of 33 Gy. These are all hypothesis-generating.
Normal tissue effects and radiobiology
Turning to late AE, meticulous data generated in human skin are consistent with minimal measurable effect of time on probability of late NTE; Turesson reported a tiny time effect for telangiectasia associated with complete absence of mitotic figures in capillary endothelium on serial skin biopsies over many weeks of RT, the lack of mitoses excluding repopulation as a mechanism. 66 The effect was thought more likely to represent a very slow component of repair decaying with a T1/2 of around 40 days. 66 The same post hoc analysis of time in START-B described above examined late AE as a negative control, yielding an estimate of 0.14 Gy/day (95% CI −0.09 to 0.34 Gy/day; p = 0.29) for change in photographic breast appearance, consistent with the absence of a time effect for AE between 3 and 5 weeks of breast RT. 117
The reason for going into this amount of detail is that FAST-Forward test dose levels of 27 and 26 Gy assumed firstly no clinically significant time effect for late AE between 1 and 3 weeks, secondly complete sublethal damage repair between fractions and thirdly an α/β of 2.8 Gy for late NTE, the last assumption based on the combined estimates of α/β in START-A and FAST. On this basis, the relative EQD of the FAST-Forward schedules to 50 Gy in 25 fractions were calculated (see Table 31, Chapter 5).
Although the 5-fraction regimen predicted to be isoeffective with 40 Gy in 15 fractions was 27 Gy the observed iso-effect at 5 years was closer to 26 Gy, suggesting a slightly lower α/β value, see Table 3. The α/β point estimates are all around 2 Gy, corresponding to EQD2/2 of 46.8 Gy for 26 Gy in 5 fractions compared to EQD2/2.8 = 45.6 Gy for 40 Gy in 15 fractions, the latter using α/β = 2.8, a combined estimate based on START-A and FAST. The CIs of α/β values generated for a wide range of AE in the FAST-Forward Trial are all consistent with the α/β values of all late AE generated by FAST and START-P/-A. One interpretation, and statistically speaking the most likely, is that they are all internally consistent with each other, as reflected by wide CIs associated with individual point estimates. The implication is that for mean α/β values for specific late AE endpoints are the same across all trials and fraction sizes in the range 2.0–6.0 Gy. A combined estimate based on all trials would likely be somewhere between 2.0 Gy and 2.5 Gy.
In conclusion, the slightly reduced α/β values in FAST and FAST-Forward relative to START-P/-A might be real, in which case, one potential explanation includes a time effect related to a slow component of repair. These somewhat esoteric considerations should not obscure the all-important clinical conclusion that 26 Gy in five daily fractions offers patients comparable AE rates and non-inferior IBT to 40 Gy in 15 fractions.
Resource implications
The economic analysis demonstrated that 5-fraction RT is expected to be cost-effective. When compared to 15 fractions, 5 fraction RT is expected to result in a reduction in direct treatment costs from £2315 to £982 per person treated. Total costs are also expected to reduce from £31,640 to £29,638, where these resource savings are primarily due to reductions in direct treatment costs. The implications of these resource savings for the health system are complex and depend on how the extra capacity is used. The same number of individuals may be treated with lower RT capacity, freeing up these resources for other uses. The additional capacity may also be used to drive quality, for instance increasing the use of cardiac sparing breath hold technique where that is not fully utilised for breast cancer patients. The capacity and technical advances may be utilised for cancers other than breast cancer.
COVID-19
Dissemination of the Main Trial primary results was expedited due to the COVID-19 pandemic. In March 2020 emergency guidelines for RT were published by the NICE118 and the RCR created an online resource of clinical advisory documents containing risk mitigation approaches for RT. 119 The aim was to reduce footfall in RT departments and hospitals to reduce person-to-person contact to minimise the spread of COVID-19. The breast cancer RCR guidance cited FAST-Forward 3-year NTE outcomes, recommending the 26 Gy/5 fractions schedule for adjuvant treatment of early breast cancer, stating that the 5-year local relapse publication was imminent. 51 This schedule was rapidly adopted nationwide, since FAST-Forward involved most UK RT centres which therefore had the required quality assurance measures already in place. From the National Radiotherapy Dataset, 60.6% of breast RT courses used 26 Gy/5 fractions in April 2020 versus only 0.2% in April 2019. 96
Breast consensus/guidelines and moving forward
The UK held a breast fractionation consensus meeting under the auspices of the Royal College of Radiologists and included an in-depth look at the FAST-Forward data. Lewis et al. 120 explain the Royal College of Radiologists consensus statement program and the explicit methodology that is used. There was very strong support to offer 26 Gy in 5 fractions over 1 week for whole breast, chest wall and partial breast RT. Where there had been a mastectomy and breast reconstruction there was strong support to consider 26 Gy in 5 fractions over a week. Using the 10-year FAST data there was very strong support to consider 28.5 Gy in 5 fractions over 5 weeks instead of 26 Gy in 5 fractions over 1 week for patients with significant co-morbidities and/or frailty that make daily RT difficult. For patients requiring a boost there was strong support to offer 26 Gy in 5 fractions whole breast RT plus either a sequential normofractionated boost or a hypofractionated boost (delivered in no more than 5 fractions RCR Consensus Guidance, 2016) or 15 fraction simultaneous integrated boost (SIB), for example 48 Gy to boost volume and 40 Gy to rest of breast all over 3 weeks.
European Society for Radiotherapy and Oncology (ESTRO) Advisory Committee in Radiation Oncology Practice (ACROP) consensus recommendations on patient selection and dose/fractionation for external beam radiation therapy in early breast cancer were developed due to a lack of agreement persisting in the radiation oncology community on radiation dose/fractionation/volume selection in early-stage breast cancer. These were published in 2022. 121 Most of the evidence was on moderate hypofractionation (15–16 fraction regimens) but the FAST-Forward Trial on ‘ultra-(5-fraction-) hypofractionation’ was included. Concentrating on the statements on 26 Gy in 5 fractions in a week there was consensus that whole breast or chest wall irradiation without breast reconstruction can be offered as (1) standard-of-care or (2) within a randomised controlled trial or prospective registration cohort. There was strong consensus that moderate hypofractionation (40 Gy in 15 fractions) and ultra-hypofractionation (26–30 Gy in 5 fractions) represent acceptable schedules for external beam partial breast irradiation.
The St Gallen 2021 International consensus guidelines, produced bi-annually, cover local and systemic therapies for early breast cancer. 122 Regarding 5-fractions, FAST and FAST-Forward schedules, the panel did not endorse these as standard treatment as yet but stated that there was growing interest. 122 The National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines123), probably the most used in the USA, recommend either 25–28 fractions or preferably hypofractionation with 15/16 fractions for whole breast RT. For the chest wall 25–28 fractions are recommended. The current guidelines thus show a mixed picture with the 5-year FAST-Forward results not enough to produce universal change.
Global health
Breast cancer is the commonest cancer worldwide, both in terms of incidence and prevalence and is the leading cause of death from any cancer in women with around 2.3 million cases and 700,000 deaths in 2020. 124 It is predicted that by 2040 there will be around 3 million cases annually with the majority occurring in low- and middle-income countries (LMICs). 125 In addition, 70% of the predicted 1 million deaths will also be in lower-income settings if there is no intervention in the current mortality trends. 125
Radiotherapy is an important component of the multimodality treatment of breast cancer and contributes to improving both local control and survival. 126,127 However, RT infrastructure is severely under resourced in many low- and middle-income countries with some regions having no access to RT at all. 128 Compounding this is the relative lack of uptake of hypofractionated RT in LMICs compared with higher-income countries. 129 Furthermore, these regions also have very high rates of patients who do not initiate or abandon breast cancer treatment for a variety of reasons. 130
Five-fraction RT over 1 week would go some way to helping this disparity. Mature results of the FAST-Forward Nodal Sub-Study will be particularly relevant, given that many people in LMICs present with more advanced disease. This shortened fractionation would help alleviate health system costs and limit the potential financial toxicity for patients who would have received 3–5 weeks of RT. Access to and completion of good quality multimodality treatment, including RT would prevent premature breast cancer deaths currently seen in many LMICs. 130
Other trials and areas of future research and collaboration
FAST-Forward provides the only published randomised controlled trial evidence for 1-week 5 fractions schedules. There are several other studies either in set-up or open to recruitment evaluating the 26 Gy 1-week schedule in early breast cancer; effectively almost a decade behind FAST-Forward. However, it will be many years until any results of these are known, and FAST-Forward has more patients enrolled than all of the other studies combined. The Indian HYPORT-Adjuvant trial opened in 2019 with a recruitment target ~2100 (NCT03788213) and randomises patients requiring adjuvant RT to the breast/chest wall ± regional lymph nodes to 40 Gy/15 fraction over 3 weeks versus 26 Gy/5 fractions in 1 week. The use of a SIB (dose of 8 Gy and 6 Gy, respectively) is allowed in patients who have undergone breast conservation. Another Indian trial HYPART; NCT04472845) is testing 26 Gy/5 fractions in 1 week versus 34 Gy/10 fractions in 2 weeks in high-risk patients; the trial opened in March 2020 with a target sample size of 1018. The Canadian RHEAL trial (NCT04228991) has a similar design to the FAST-Forward Nodal Sub-Study; the target sample size is 588 patients and the estimated trial completion date is end of 2027. There are four other studies involving between 36 and 100 patients investigating the 26 Gy/5 fractions schedule that are currently recruiting and will have no long-term data for many years. The UK is therefore best placed to maximise the knowledge already gained from the testing of 5 fractions schedules.
FAST-Forward will contribute to future collaborations such as the planned EBCTCG meta-analysis breast RT fractionation trials. Another potential collaboration is the CONFLUENCE project. This National Cancer Institute (USA) funded initiative aims to use genome-wide association studies (GWAS) to improve knowledge of breast cancer genetics. This research resource of different races/ethnicities will include at least 300,000 cases of both breast cancer and controls. The confluence of existing GWAS and new genome-wide genotyping data generated through this project will achieve this aim.
Limitations of the trial
The boost policy for each patient (yes or no) and dose prescription (10 Gy in five daily fractions or 16 Gy in eight daily fractions) had to be declared to the CTSU ahead of randomisation. In the planning stage of the trial we debated the sequential normofraction boost against a hypofractionated boost. There was logic in using a hypofractionated boost for a trial of hypofractionation. To limit variables it was decided to continue with the established schedules for boost at the time. Boost is therefore treated as a randomly distributed variable that impacts (randomly) across all groups and contributes, like excision volume, breast size, smoking and genetic factors, to likelihood of adverse events. The reason for retaining sequential boost was to avoid having to commit to synchronous boost dose levels considered most likely to be isoeffective with control before knowing the outcome of the trial. That involves making an assumption about the likely α/β value (testing two values to cover upper and lower estimates would have made things very complex and prone to error). The limitation is that we used standard-of-care 5- or 8-fraction boost schedules in the Main Trial and not hypofractionated boosts. The boost policy was changed via an amendment for the Nodal Sub-Study to allow a hypofractionated boost in no more than five fractions. The trial will not contribute to information on a hypofractionated boost.
Confirmation that the 5 fractions schedule is as safe as 15 fractions for patients also receiving axillary RT is more important than ever as a higher proportion of node-positive patients who require axillary treatment are now having nodal RT rather than surgery than when the FAST-Forward Nodal Sub-Study was conceived. This shift in practice followed publication of the AMAROS study which showed non-inferiority of nodal RT to axillary nodal dissection, with halved lymphoedema rates in the irradiated group. 30 Contemporary practice means there is also increasing use of neoadjuvant chemotherapy in patients with bulky or involved axillary nodes at presentation, especially in the HER-2-positive and triple-negative breast cancer sub-types, where high complete pathological responses are observed. The need for subsequent axillary surgery in these patients is being questioned, and is diminishing, being replaced by axillary RT or no treatment (NIHR ATNEC trial; NCT04109079). Lymphoedema (highlighted in a Living With and Beyond Cancer (LWBC) priority setting workshop) is the focus of the FAST-Forward Nodal Sub-Study which provides the best opportunity to quantify these effects and further funding will supplement the shorter current follow-up currently available since it started midway through the Main Trial.
For some, the long-term results are essential to change practice, long-term meaning at least 10 years. While the START trial’s 5-year outcomes were published in 2008 and 10-year outcomes in 2013, international guidelines were slow to adopt the 15 fractions schedule that has been the UK standard of care since 2009. US guidelines were updated to encompass broader adoption of hypofractionation for breast cancer only in 2018,102 and a number of countries still use the historic standard 25 fractions schedule. Lack of long-term safety data is often cited as a reason for remaining cautious regarding adoption of new standards of RT treatment. To understand this caution in adoption it is worth emphasising what long-term follow-up means for RT trials, and the continued relevance and importance of reporting on outcomes, up to and even beyond 10 years. In the START-B trial of 15 fractions versus 25 fractions moderate/marked breast shrinkage increased from 11.4% at 5 years to 26.2% at 10 years after 40 Gy/15 fractions. Outcomes for local relapse were 1.9% and 3.8%, respectively. Similar increases in prevalence of outcomes after 5 years were seen in other trials. 104,109 Other trials have continued to report outcomes to 15 years. 131,132
There is caution regarding effectiveness of the 5 fractions schedule in higher-risk patients, as mentioned above. 133,134 There are no data to support this concern but the lack of events per biological subgroup at 5 years is the argument used. Again, as stated above there are concerns over late toxicities emerging with longer-term follow-up, such as cardiac and respiratory toxicity, that are not obviously apparent now. Some are concerned that early adoption may lead to long-term irreversible changes for patients and are sticking to their current 15–25 fractions schedules. Particular emphasis has been placed on statistically rather than clinically significant values, such as a 1–2% increase in breast hardening outside the tumour bed with the 1-week schedule. We expect the cumulative effects of long-term toxicity and relapses to go up over time, but do not expect the relative differences between the groups to change. However, we need to demonstrate this.
Long-term follow-up of patients in cancer trials is recognised as essential to understanding recovery from treatment and its long-term effects and limitations as well as developing optimal management strategies for patients. Patients have shown high compliance with long-term follow-up questionnaires, and this will provide important additional data in the planned 10-year outcomes study. This is particularly pertinent for RT where AE continue to occur and progress years after treatment completion. Trial follow-up is essential in order to measure long-term outcomes since in the UK most breast cancer patients are now discharged from routine clinical follow-up within 12 months. Long-term safety data from FAST-Forward, only available with continued trial follow-up, will be invaluable for future patients in order to support fully informed consent and shared decision-making regarding treatment. The UK health data systems do not routinely collect the type of data that is required to assess breast-related radiation toxicity. Even serious toxicity such as lung/heart damage and second malignancies, would be difficult to routinely and accurately attribute in a comprehensive and timely manner. In the earliest studies of breast RT the benefits of reducing relapse were at least counterbalanced if not outweighed by long-term excess of deaths from cardiac causes. Although this is avoided or reduced by heart-sparing techniques in the modern era, we need to be sure that there is no excess of these rare events with the 5 fractions schedule. Although relapses and deaths could be collected from the UK databases, they are not always complete, potentially indistinguishable from new primary breast cancer (contralateral) and are subject to a time lag in availability. With relapse rates so low, it is important to collect as accurately and comprehensively as possible all of these occurrences. National breast RT consent forms developed by the RCR state expected rates of long-term risks after 15 fractions and will need to be updated for 5 fractions in future. 119
Survivorship issues are especially relevant for breast cancer; an estimated 600,000 people are alive in the UK after a diagnosis of breast cancer currently, predicted to rise to 1.2 million by 2030. 135 Given the increasing incidence and low local relapse rates with increasing survival rates in breast cancer the issue of survivorship is magnified. If 50% of patients undergo RT and a side effect was 10% more prevalent then by 2030 60,000 people will be affected. In the recent debate held at the San Antonio breast conference (December 2021), it was argued by some that in the long term, if the 5 fractions regimen led to more rare but expensive complications this could be detrimental. The FAST-Forward Trialists do not believe this will be the case, but it is vital to be able to evidence this with long-term data.
Evaluation of progressive long-term and late side effects occurring many years after cancer treatment are highlighted in the James Lind Alliance Top 10 priorities for LWBC. 136 Data collection beyond 5 years in FAST-Forward is the only available source to provide robust estimates of these effects for 5 fractions schedules contributing significantly to worldwide knowledge in the field of breast RT extreme hypofractionation. Collection of PRO finished at 5 years in our previous UK trials; the planned collection of 10-year data in FAST-Forward will enable a unique comparison of patient and clinician assessments of AE and wider survivorship issues at this time point. Reports published since START-B have illustrated that this is particularly important given that patients and clinicians have different perspectives of symptoms with patients’ ratings of symptomology consistently higher than ratings by clinicians. 137
Future plans
The National Institute for Health and Care Research Health Technology Assessment Programme (NIHR HTA) has approved new funding for follow-up in the Main Trial to 10 years, and to at least 5 years in the Nodal Sub-Study. This proposal contained details for crucial long-term follow-up of breast cancer patients and was developed by the TMG including patient views via a strong PPI partnership. In Q2 2021 hospitals were asked to share a flyer with FAST-Forward patients giving them the chance to work with the trial team to optimise the future plans for the trial. Given that participants were on annual follow-up it was reassuring to have 146 responses within a month. Due to the larger number of people responding than had been anticipated a two-stage consultation was established, firstly to complete an online survey and secondly the establishment of focus groups to gauge participant opinion. In spite of a time lag in sending out the survey due to the need to get appropriate approvals, 82 responses were received (56% response rate). Of these, 39 also attended one of two focus group meetings held in Q4 2021. Survey respondents appeared to be representative for both geographical region and treatment allocation 58/82 (71%) having received hypofractionated treatment (expected 2/3rds).
Participants were unanimous in endorsing the continuation of follow-up to 10 years and largely supportive of the pragmatic approach to reduce burden of data collection at sites during year 8 and 9 while focussing on getting complete data from face-to-face clinical visits at year 10. In the focus groups the emerging themes were the reassurance provided by the annual visits and the recognition that side effects continue to emerge in later years. Some felt that they had signed up for 10 years and that this should be honoured if possible. NHS resource constraints were also noted.
They also confirmed their support for collecting patient views on their health and treatment at 10 years using a PRO questionnaire. Opinions were sought on the aspects of quality of life, toxicity and well-being that should be included. Concerns about continued changes in the treated breast, pain and physical functioning were highlighted but also fear of relapse, psychological wellbeing, fatigue and impact of other life events and comorbidities (previously reported in. 138 The views expressed led the trial team to seek out a variation to the original questionnaires to extend data collection to cover survivorship issues and to offer the PRO assessment to all patients not just those who had previously been in the PRO sub-study.
Forty-six Main Trial sites opted to participate in the PRO sub-study, which was offered to all of their eligible patients (1796 consented to PRO). All Nodal Sub-Study patients were asked to consent to PRO collection since the primary endpoint is patient-assessed arm/hand swelling. We propose to offer all Main Trial patients still in follow-up involvement in the PRO study at 10 years, as this would greatly increase the number evaluable for PRO assessment. The trial provided a unique opportunity to capture extremely valuable PRO assessments of the long-term effects of living with and beyond breast cancer and capturing longer-term outcomes of hypofractionated RT. In reality due to timings this assessment would be collected between follow-up years 10 and 11. Given the chronic nature of RT-related effects this is considered a pragmatic solution and is endorsed by our participant involvement groups which had been retained as an ongoing patient representative collaborative resource.
Our participant focus groups have already demonstrated the huge value of engaging with, listening to and actively involving patients with lived experience of breast cancer. The focus groups may assist with other PPI issues that arise during the trial as the focus group members are keen to continue working with us.
Research recommendations
Future research with regard to hypofractionation of 26 Gy/5 fractions in a week would include confirmation of our results in other geographic populations. The HYPORT-adjuvant trial is open to recruitment in India (NCT03788213). This is a two-arm multicentre RCT aiming to recruit 2100 patients. The control arm is 40 Gy/15 fractions over 3 weeks and is being compared to 26 Gy/5 fractions in a week. The inclusion criteria are described as ‘non-restrictive’ to enable the results to be applicable to an Indian population. The OCOG group in Canada is recruiting to the RHEAL trial of locoregional RT also with a control arm of 40 Gy/15 fractions over 3 weeks compared to 26 Gy/5 fractions in a week (ClinicalTrials.gov Identifier: NCT04228991). There are other trials in the planning phase.
The appropriate boost dose, ideally delivered using a SIB, needs to be determined. The HYPORT trial is using either a sequential or SIB boost for both the trial and test arms of the study. This is a topic that would be appropriate for further study to build on both the IMPORT High and FAST-Forward Trials. For instance the 15-fraction SIB dose of 48 Gy/15 fractions from IMPORT High as the standard schedule could be compared with two 5FR SIB doses over a week.
The FAST-Forward Nodal Sub-Study will provide information on axillary RT in 5 fractions. Evidence on the safety of a hypofractionated approach in patients requiring IMC RT also needs to be gathered. There will be some data from centres that used 5 fractions at the height of the COVID-19 pandemic but trial-based evidence is required. Both the HYPORT and RHEAL trials include patients having IMC RT.
Is 5-fraction hypofractionation the limit for external beam RT, might two fractions be an option? We are unaware of any trials examining this question.
Equality, diversity and inclusion for study participants
The study recruited from nearly 100 centres (RT and referring centres) across the whole of the UK. The inclusion criteria were very broad to allow easy access to the study regardless of where the patient lived. The study was attractive to many patients because of the usual reasons to help with research, but also because of the 2/3 chance of a 1-week RT schedule, thus completing treatment more quickly and with less expense and incursion. The trial included all the subtypes of breast cancer, which were broadly represented as per the incidence that they occur in the UK. The age of patients in FAST-Forward ranged from 25 to 90 years with 16.4% aged 70+ years. Men were eligible although being a rare disease in men, only 12 were entered. Ethnicity data has been collected (for those who donated a blood sample for genetic research) and will be reported with the 10-year results.
The study is in the follow-up stage, so no more patients will be recruited. However, extension of the PRO sub-study to all available Main Trial participants will provide a broader, all-encompassing view of patient outcomes at 10 years.
Patient and public involvement
Patient advocate involvement has been integral to ICR-CTSU breast RT trials for over 25 years as demonstrated by the involvement of the RAGE consortium in the START trials. Such involvement is from trial concept to protocol review and membership of the TMG, the patient advocates are a constant guide to ensure that the trialists are acting on patients’ best interests and answering the questions they want answered. Patient advocates have also been integrally involved in discussions at TMG meetings regarding the trade-off between toxicity and efficacy and in advising on content of PRO questionnaires. Other essential discussions with patient advocates have included differentiating between what is statistically significant with what is clinically relevant to patients. Issues such as, does an increase incidence of 1% of breast hardening at 5 years matter to patients, with a trade-off of a week’s treatment with generally low toxicity?
In November 2021 around 80 FAST-Forward Trial participants completed an online questionnaire seeking their feedback on plans for further follow-up in the trial including content of a PRO questionnaire at 10 years. Over 40 of these patients then participated in participant involvement group discussions organised by ICR-CTSU in December 2021 that further explored patients’ views on long-term follow-up in the trial and issues important to them at 10 years following cancer treatment. Outputs from the online survey and the discussion meetings have informed the future plans of FAST-Forward.
Conclusions
Extremely low rates of IBTR and of clinically assessed moderate/marked late NTE can be attributed to improvements in all diagnostic and treatment modalities and to the commitment of patients to early diagnosis and randomised trials. The 26 Gy dose level has been shown to be comparable to 40 Gy in 15 fractions in terms of patient-assessed NTE, clinician-assessed NTE, and NTE photographic change in breast appearance, and to NTE expected after 46–48 Gy in 2 Gy fractions.
Beyond its safety and effectiveness, the 26 Gy FAST-Forward schedule is convenient and less expensive for patients and for health services. The 26 Gy FAST-Forward schedule reduces the estimated health-care fiscal cost of breast RT by over 50%. The 5-fraction regimen reduces the machine time required for breast RT patients, thus improving patient access for other groups of cancer patients within the NHS.
The reduced number of patient attendances in a 5-fraction schedule places less burden on patients and the carers. In some geographically large countries, where travel times to RT centres are prohibitive, fewer women choose breast conservation for early breast cancer. Reducing the travel and personal financial burden may allow more women to choose breast conservation rather than mastectomy.
In response to global warming, it is important that healthcare systems respond in an environmentally conscious manner. Reducing the number of patient visits to only 5 fractions will reduce the carbon footprint for patient travel. Furthermore, the impact of a 5-fraction regimen on improving access times for other cancer patients will lead to more efficient use of linear accelerators and hence reduce carbon footprint for each RT centre.
It is also likely to be safe for patients requiring regional RT, an approach currently under formal evaluation in our randomised FAST-Forward Nodal Sub-Study comparing 40 Gy in 15 fractions and 26 Gy in 5 fractions. Areas of future research with regard to hypofractionation of 5 fractions would include confirmation of our results in other geographic populations, trials are both ongoing and in set-up. The appropriate boost dose, ideally delivered using a SIB, needs to be determined. Evidence on the safety of a hypofractionated approach in patients requiring IMC RT is being trialled. Is 5-fraction hypofractionation the limit for external beam RT, might two fractions be an option? FAST-Forward can and is influencing both practice and future research.
Acknowledgements
We thank the patients who participated in this trial and staff at the participating centres and at ICR-CTSU; we also thank FAST-Forward Trial Management Group members past and present, and the Independent Data Monitoring Committee and Trial Steering Committee for overseeing the trial (listed in Appendix). The FAST-Forward Trial was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment Programme – HTA (UK) (09/01/47), with programme grants from Cancer Research UK to support the work of ICR-CTSU (C1491/A15955; C1491/A25351). We acknowledge NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research (London, UK). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
| Centre no | Centre name | Centre staff |
|---|---|---|
| 01 | Royal Marsden Hospital Sutton | Dr N Somaiaha, Dr A Kirbya, Dr I Locke, Dr D Tait, Professor J Yarnolda, R Colgan, L Gothard, C Lucy |
| 02 | Royal Cornwall | Dr D Wheatleya, Professor D Radstone, Dr A Thomson, n Ashley, S Eloi, A Griffiths, J Kingston, N Simpson |
| 03 | University Hospital of North Midlands | Professor AM Brunta, Dr D Gahir, Dr A Jegannathen, L Contoret, M Evans, K Glover, A Myatt, R Smith |
| 03a/10c | Stafford General Hospital | Dr A Jegannathen, Dr L Pettit, Dr C Brammer, C Harvey, A Myatt, L Verueco |
| 04 | Derriford Hospital, Plymouth | Dr U Panwar, Dr S Dubey, Dr S Kelly (RIP), N Blacker, L Cadmore, H Congdon, I Harvey |
| 05a | Norfolk and Norwich Hospital | Dr D Geropantas, Dr A Bulman, Dr A Harnetta, S Barber, M Bloomfield, E Malone, J Platt |
| 05aa | James Paget Hospital | Dr S Down, Dr A Harnetta, A Brooks, J Harman |
| 05b/09ba | Queen Elizabeth Hospital, Kings Lynn | Dr M Daly, R Lee, H Webb |
| 06a | Bristol Haematology and Oncology | Dr C Comins, Dr A Bahl, Dr S Masson, Dr N Thanvi, H Appleby, A Lowe, H Saldanha, V Lee |
| 06aa | Weston General Hospital | Dr T Wells, Dr M Tomlinson, K Owens, H Lloyd-Jones, G Saunders |
| 07a | Royal Shrewsbury Hospital | Dr L Pettit, Dr R Agrawal, Dr H Abel Gadir, Dr S Khanduri, S Jose, S Potts, A Welsh |
| 08 | Royal Devon + Exeter Hospital | Dr J Forrest, Dr A Goodmana, Dr A Hong, Dr D Hwang, K Baines, A Betts, T Lawless, S Scrutton |
| 08aa | North Devon District Hospital | Dr J Forrest, Dr D Hwang, R Holbrook, S Ley, L Van Koutrik |
| 09a | Addenbrookes Hospital | Professor C Colesa, Dr L Hughes-Davies, Dr C Wilson, A Bates, C Philpott, C Spain, B Stratton, N Twyman, J Wilkinson |
| 09aa | Hinchingbrooke Hospital | Dr S Russell, V Goss, R Kurian, S Miller |
| 09ca | West Suffolk Hospital | Dr M Moody, Dr C Woodward, S Hale |
| 09da | Bedford Hospital | Dr S Smith, V Bastion, G Lubimbi, A Willis, J Valentine |
| 10 | New Cross Hospital | Dr R Allerton, Dr C Brammer, Dr M Churna, Dr L Pettit, Dr P Ramachandran, R Horton, M James-King |
| 10a/50a | Kidderminster Hospital | Dr M Churna, S Stringer, H Tranter |
| 10ba | Russell’s Hall | Dr R Allerton, Dr G Georgiev, Dr P Ramachandra, K Kanyi, K McGarry, A Watts |
| 11a | Southend University Hospital | Dr H Algurafi, Dr W Ella, Dr A Robinson, T Davies, A McPherson, L Romero, S Shibu-Thomas |
| 11aa | Basildon Hospital | Dr H Swinburn, Dr W Ella, C McCormick |
| 12 | Clatterbridge Cancer Centre | Dr S Tolan, Dr I Syndikusa, Dr N Thorp, S Green, K Hughes, H Mayles |
| 12aa | Warrington Hospital | Dr I Syndikusa, L Lee, C Lowthian, R Madew |
| 12ca | St Helens + Whiston | Dr R Sripadam, N Hornby |
| 12d | University Hospital, Aintree | Dr P Robson, L Beresford |
| 12ea | Countess of Chester | Dr A Hall, S Bennett, E Gallimore, M Moffit, J Prince |
| 13 | Velindre Cancer Centre | Dr H Passanta, Dr J Abraham, Professor P Barrett-Lee, Dr A Borley, Dr T Howe, Dr R Stevens, M Jenkins, S Slade, A Weaver, O Woodley |
| 14a | Royal Surrey County Hospital | Dr R Laing, Dr A Franklin, Dr A Neal, Dr S Whittaker, L Adams, M Flavin, B Moloney-Oates, A Tindall |
| 15a | Essex County Hospital | Dr M Mukesh, Dr V Loo, Dr P Murray, K Cooke, C Driscoll |
| 16 | Beatson West of Scotland Cancer Centre | Dr A Alhassoa, Professor P Canney, Dr G Fraser, Dr G Lumsden, Dr M Rizwanullah, A Armstrong, J Fleming, T Ibotoye, A Leonard, M McJury, C Seager, V Withers |
| 16a | Royal Alexandra Hospital | Dr A Alhassoa, P Eaddy, E MacLeod |
| 16b | Forth Valley Hospital | Dr H Marashi, Dr I Rabnawaz, F Johnston, L Prentice, A Scott |
| 16c | Wishaw Hospital | Dr J Hicks, Dr M Rizwanullah, K Douglas |
| 16da | New Victoria ACH | Dr D Ritchie, Dr J Ansari, E Moody |
| 16e | Hairmyres Hospital | Dr J Hicks, Dr G Dunn, L Devlin, L Glass |
| 16fa | Crosshouse Hospital, Kilmarmock | Dr G Lumsden, K Bain, C Burns, P Cannon |
| 17a | Singleton Hospital, Swansea | Dr M Rolles, Dr C Askill, Dr D Pudney, Dr R Taylor, E Brinkworth, H Cheley, S Foyle, E Harris, N Viney, J Williams |
| 18 | Cheltenham General Hospital | Dr J Bowena, Dr K Benstead, Dr R Counsell, Dr S Elyan, R Bakawala, N Bulmer, J Chittock, J Kukielska, A Skelton, M Tan |
| 18a/50 | Worcester Royal Infirmary | Dr M Churna, Dr J Bowena, Dr R Counsell, D Bak, A Holdsworth, J Tyler |
| 18ba | Hereford County Hospital | Dr D Nelmes, Dr S Guglani, J Birch, M Evans, G Horsfield |
| 19 | Royal Preston | Dr M Hogg |
| 19c | Blackpool Victoria Infirmary | Dr F Danwata, Dr A Hindley, Dr S Susnerwala, E Davies, S Lancaster, L Smith |
| 19d | Royal Lancaster Infirmary | Dr D Williamson, Dr G Skailes, C Bartlett, A Fielding |
| 20a | Queens Hospital, Romford | Dr E Sims, Dr C Bridgewater, Dr M Quigley, Dr E Staples, T Mills-Baldock, J Cook |
| 21 | Peterborough Hospital | Dr C Jephcott, Dr C Round, Dr S Treece, K Cavanagh, C Chisenga, M Cowen |
| 22 | Weston Park Hospital, Sheffield | Dr M Hatton, Dr O Din, Dr C Lee, G Brown, J Conway, L Fiorentino, J Swinscoe |
| 23a | Royal Sussex County Hospital | Dr D Bloomfielda, Dr S Mitra, Dr A Nikapota, Dr R Simcock, Dr S Westwell, P Frattaroli, A Hewines, J Tremlett |
| 23aa | Worthing Hospital | Dr SY Sham, Dr A Nikapota, Dr S Mitra, S Funnell, J Gilbert |
| 23ba | Eastbourne Hospital | Dr S Westwell, K Jones-Skipper |
| 24 | Guys Hospital | Professor E Sawyera, Dr L Brazil, Dr S Harris, Professor A Tutt, G Keunzig, C Thomas |
| 25a | Ipswich Hospital | Dr R Venkitaraman, Dr L Sherwin, C Mackenzie, P Ridley, M Riley |
| 26a | Glan Clwyd Hospital | Dr J Bishop, G Davies, S Owen, V Saul |
| 26aa | Wrexham Maelor Hospital | Dr W Soe, Dr J Bishop, Dr N Ghosal, J Stockport |
| 26ba | Ysbyty Gwynedd Hospital | Dr J Bishop, D Thomas, L Williams. |
| 27 | Queen Elizabeth Hospital, Birmingham | Dr A Stevens, Dr MS Anwar, Dr D Spooner, S Manolopoulos, R Shingler |
| 27aa | Sandwell Hospital | Dr D Spooner, D Devonport |
| 27ba | City Hospital. Birmingham | Dr D Spooner, D Devonport, B Gammon |
| 27c | Manor Hospital, Walsall | Dr S Yaha, Dr MS Anwar, J Fletcher |
| 28 | Charing Cross Hospital | Dr S Cleatora, Dr C Lowdell, P Dunn, S McInerney |
| 28aa | Ealing Hospital | Dr O Hatcher, Dr C Lewanski, S Magwaro, D Murati, K Watson |
| 28b | West Middlesex Hospital | Dr P Riddle, Dr R Ahmad, J Swallow |
| 29 | Mount Vernon Hospital | Dr C Westbury, Dr M Ah-See, Dr A Makris, Dr P Ostler, Dr N Shah, Dr N Thanvi, T Chalk, S Hasan, E Windmill |
| 29aa | Luton and Dunstable University Hospital | Dr A Vinayan, Dr M Ah-See, A Rafiq, M Sarte |
| 30a | Royal United Hospital, Bath | Dr M Beresford, Dr A Jenner, Dr S Mancero, Dr S Manson, Dr H Newman, T Allen, C Milsom, T Tylee, S Whittle |
| 31 | Queen Alexandra Hospital, Portsmouth | Dr K Bradley, Dr JD Dubois, Dr A Suovuori, R Baker, K Haselip, N Rivington |
| 32 | Torbay District General Hospital | Dr A Goodmana, Dr P Bliss, M Allison, P Bowen, S Chamberlain, I Koehler |
| 33 | Southampton General Hospital | Dr C Crowley, Dr J Marshall, Dr S Raj, C Britton, E Cooper, K Meeking, K Stevens |
| 33aa | Salisbury Hospital | Dr C Crowley, J Attlee, S Strong-Sheldrake |
| 33ba | Royal Hampshire County Hospital | Dr S Raj, J Conti, V Corner, J Smith |
| 34 | Royal Berkshire | Dr R Davis, Dr J Adams, Dr J Barrett, Dr C Charlton, J Jones, P Pabari, E Vowell |
| 34aa | Wexham Park Hospital | Dr R Davis, Dr J Adams, Dr S Inayat, N Barnes, S Das, D Mciver, J Weerasinghe |
| 35 | St Bartholomew’s Hospital | Dr V Wolstenholme, Dr C Cottrill, Dr N Patel, Dr K Tipples, F Bibi, H Payne, A Pena-Remorin, A Sivajothi, E Tutor |
| 36 | St James’ University Hospital, Leeds | Dr S Kumar, Dr I Chaudhuri, S Hartup, A Henson, J Lilley, P Shuttleworth |
| 36ab | Pinderfields Hospital, Wakefield | Dr S Kumar, J Ball, S Buckley, C Hirst |
| 36bb | Bradford Royal Infirmary | Dr E Thomas, R Benton, K Cockroft, H Inman, H Robertshaw, J Sewell |
| 36cb | Huddersfield Royal Infirmary | Dr N Roberts, M Brady, S Dale, D Melia, S Mellor |
| 37 | University Hospital of Coventry and Warwickshire | Dr D Hrouda, Dr L Fresco, Dr C Irwin, Dr N Walji, Dr J Wordling, S Manolopoulos, K Sanders |
| 37aa | George Eliot Hospital, Nuneaton | Dr S Lupton, Dr L Fresco, J Lake |
| 37ba | Warwick Hospital | Dr N Walji, J Harris, L Maher |
| 37c/50b | Alexandra Hospital, Redditch | Dr M Churna, Dr D Hrouda, Dr C Irwin, H Hodson, A Morgan |
| 38 | Musgrove Park, Taunton | Dr M Varughese, Dr J Graham, S Mahoney |
| 38a | Yeovil District Hospital | Dr U Barthakur, J McCrory, K Rennie |
| 39 | Lincoln County Hospital | Dr A Chaudhuri, Dr E Murray, Dr T Sreenivasan, O Francis, V Longdon, A Sloan |
| 39a | Pilgrim Hospital, Boston | Dr A Chaudhuri, Dr E Murray, A Kirkby |
| 40 | Northampton General Hospital | Dr R Agrawal, Professor H Eldeeb, Dr C Macmillan, A Kempa, M Polnik, R Tighe, N Whilde |
| 41 | James Cook University Hospital | Dr B Sethugavalar, Dr J Hardman, Dr JCM Van der Voet, H Curtis, E Thompson |
| 41a | University Hospital of North Tees | Dr E Thompson, Dr N Storey, Dr A Rathmell, L Poole |
| 42 | Western General Hospital, Edinburgh | Dr C Bedi, Dr T Evans, Professor I Kunkler, Dr F Yuille, R Allan, L Carruthers, L Primrose |
| 42a | Borders General Hospital | Dr C Bedi, M Tolson |
| 42b | Dumfries and Galloway Hospital | Dr A Hennessy, Dr T Evans, J Duignan |
| 42c | Queen Margaret Hospital, Dunfermline | Dr M MacLennana, Dr A Stillie, Dr T Evans, F Adam |
| 42d | St John’s Hospital, Livingstone | Dr F Yuille, R Allen, A Clark |
| 43 | Belfast City Hospital | Dr H McCarty, Dr J Clarke, Dr G Hanna, Dr L Mulholland, D Irvine, O Stewart, G Totton |
| 44a | University College London Hospitals | Dr G Blackman, Dr A Cassoni, Dr M Gaze, Dr M McCormack, Dr J Tobias, R Patel, S Wickers |
| 45a | Poole Hospital | Dr J Brady, Dr A Chakrabarti, Dr P Crellin, N Gurung, D Forster, F Mellor, B Troke, L Varnham |
| 46 | Leicester Royal Infirmary | Dr K Kancherla, C Belcher, J Potterton, S Wright |
| 47a | Churchill Hospital, Oxford | Dr S Oliveros, Dr B Lavery, N Ann, C Hector, M Flavin, G Samkange |
| 48c | Ninewells Hospital, Dundee | Dr D Adamson, A Black, G Georgiev |
| 49c | Nottingham City Hospital | Dr P Lawton, Dr M Griffin, P Dawson, S Dennis, S Fleet |
| 49ac | Kings Mill Hospital | Dr M Griffin, D Nash |
Independent Data Monitoring Committee members: Professor M Sydes (Chair), Professor S Bentzen, Professor J Staffurth (past member: Professor I Turesson)
Independent Trial Steering Committee members: Professor M Mason (Chair), Dr D Gilbert, Dr V Cosgrove, Professor P Poortmans, Professor D Sebag-Montefiore (past members: Dr J Barrett, Professor S Bentzen)
Trial Management Group members: Dr A Alhasso, A Armstrong, Professor J Bliss, Dr D Bloomfield, Dr J Bowen, Professor AM Brunt, Mr C Chan, H Chantler, Dr M Churn, Dr S Cleator, Professor C Coles, Dr E Donovan, H Fleming, Dr D Gahir, Dr D Glynn, Professor S Griffin, J Haviland, Professor P Hopwood, Dr A Kirby, J Kirk, Professor C Kirwan, Dr M MacLennan, Z Nabi, J Patel, Professor E Sawyer, J Sinclair, Dr N Somaiah, M Sydenham, Dr I Syndikus, J Tremlett, Dr K Venables, Dr D Wheatley, Professor J Yarnold (past members: Dr R Agrawal, Dr J Barrett, Professor P Barrett-Lee, Dr P Bliss, Ms L Ciurlionis, Professor J Dewar, Dr P Dyson, Dr S Guglani, Dr H Mayles, D Megias, C Morris, Dr H Passant, C Rawlings, Dr A Robinson, Professor M Sculphur, Dr E Staples, M Wilcox, R Zotova)
Institute of Cancer Research – Clinical Trials and Statistics Unit staff: J Adkins, S Atkins, J Barnett, L Colby, L Courtney, G Dower, M Emson, C Griffin, J Haviland, R Kaggwa, J Kidd, L Lloyd, K Mertens, J Mills, J Morden, A Obabumoye, D Patel, J Patel, J Prince, S Simmons, E Simms, L Stones, G Sumo, M Sydenham, J Bliss
Radiotherapy Quality Assurance team: D Eaton, D Megias, Z Nabi, R Simoes, Y Tsang, K Venables, R Zotova
Contributions of authors
Adrian Murray Brunt (https://orcid.org/0000-0002-4797-5097) (Chief Investigator) contributed to the conception and design of the study, responsible for oversight throughout the trial, interpretation of the results and writing of the report.
John R Yarnold (https://orcid.org/0000-0002-9010-1854) (Chief Investigator) contributed to the conception and design of the study, responsible for oversight throughout the trial, interpretation of the results and writing of the report.
Duncan A Wheatley (https://orcid.org/0000-0002-1919-5114) (Chief Clinical Co-ordinator) contributed to the conception and design of the study, responsible for oversight throughout the trial, interpretation of the results and writing of the report.
Judith M Bliss (https://orcid.org/0000-0001-7957-7424) (Trial Methodology Lead) contributed to the conception and design of the study, provided oversight and guidance for trial management and analyses throughout the trial, interpretation of the results and writing of the report.
Joanne S Haviland (https://orcid.org/0000-0001-5728-3636) (Principal Statistician) contributed to the conception and design of the study, conduct of the trial, responsible for the analysis and interpretation of the results and writing of the report.
Jaymini Patel (https://orcid.org/0000-0001-5690-8630) (Trial Statistician) was responsible for the analysis and interpretation of some of the results and contributed to the writing of the report.
Mark A Sydenham (https://orcid.org/0000-0002-9157-9710) (Trial Manager) contributed to the design of the study, managed the study and data collection at ICR-CTSU, conduct of the trial, interpretation of the results and writing of the report.
David J Bloomfield (https://orcid.org/0000-0003-3639-7065) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Charlie Chan (https://orcid.org/0000-0002-4140-7590) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Suzy Cleator (https://orcid.org/0000-0003-3859-7888) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Charlotte E Coles (https://orcid.org/0000-0003-4473-8552) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Ellen Donovan (https://orcid.org/0000-0003-1692-1587) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Andrew Goodman (https://orcid.org/0000-0002-2463-5129) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Penelope Hopwood (https://orcid.org/0000-0001-8978-0936) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Anna M Kirby (https://orcid.org/0000-0002-5528-1669) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Cliona C Kirwan (https://orcid.org/0000-0002-1725-4790) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Elinor Sawyer (https://orcid.org/0000-0001-8285-4111) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Navita Somaiah (https://orcid.org/0000-0003-4609-0518) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
Isabel Syndikus (https://orcid.org/0000-0001-5781-2067) (Trial Management Group member) contributed to the design of the study, conduct of the trial, interpretation of the results and writing of the report.
David Glynn (https://orcid.org/0000-0002-0989-1984) (Health Economics team) (Health Economics team) contributed to the conception, design and analysis of the health economics study and the writing of the health economics chapter.
Susan Griffin (https://orcid.org/0000-0003-2188-8400) (Health Economics team) contributed to the conception, design and analysis of the health economics study and the writing of the health economics chapter.
Zohal Nabi (https://orcid.org/0000-0003-3564-1177) (RTTQA team) contributed to the design, conduct and analysis of the QA programme and the writing of the QA chapter.
Karen Venables (https://orcid.org/0000-0001-8829-2367) (RTTQA team) contributed to the design, conduct and analysis of the QA programme and the writing of the QA chapter.
Helen Fleming (https://orcid.org/0000-0002-3524-5652) (Patient and Public Involvement) was the patient advocate member of the TMG, and provided guidance for study documentation and reports and writing of the report.
All authors reviewed and approved the final report.
Publications and presentations
Zotova R, Tsang YM, Conibear J, Miles E. Field-based and volume-based PTVs as plan evaluation structures in the UK FAST-Forward breast trial. Poster presentation, ECCO conference, Amsterdam, September 2013. Radiother Oncol 2013;106(S2):S122 (PD-0317).
Brunt AM, Yarnold JR, Wheatley D, Somaiah N, Kelly S, Harnett A, et al. Bliss on behalf of the FAST-Forward Trial Management Group. Acute skin toxicity reported in the FAST-Forward Trial (HTA 09/01/47): a phase III randomised trial of 1-week whole breast radiotherapy compared to standard 3 weeks in patients with early breast cancer. Poster presentation at NCRI conference, Liverpool, 2–5 November 2014.
Zotova R, Yarnold JR, Wheatley D, Griffin C, Brunt M. Results from the radiotherapy quality assurance programme for the FAST-Forward breast trial. Abstract submission for ESTRO 2015 conference. Radiother Oncol 2015;115(S1):S209–S210(PD-0430).
Brunt AM, Wheatley D, Yarnold JR, Somaiah N, Kelly S, Harnett A, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016 Jul;120(1):114–8. Epub 2016 Apr 1.
Megias D, Sydenham M, Wheatley D, Maclennan M, Spezi E, Brunt AM. Variability of contouring using the ESTRO guideline for elective breast cancer radiotherapy. Oral presentation at ESTRO conference, Vienna, May 2017. Radiother Oncol 2017;123(S1):S136–S137(OC-0265).
Brunt AM, Haviland JS, Sydenham MA, Alhasso A, Bloomfield D, Chan C, et al. OC-0595: FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy: 3-year normal tissue effects. Radiother Oncol 2018 April;127(S1):S311–S312. https://doi.org/10.1016/S0167-8140(18)30905-8
FAST-Forward phase III randomised controlled trial of 1-week hypofractionated breast radiotherapy: 3-year normal tissue effects. Oral presentation at ESTRO 37 conference, Barcelona, 23 April 2018. Late Breaking Abstract OC-595.
FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy: 3-year normal tissue effects. Oral presentation at NCRI conference, Glasgow, UK, 5 November 2018.
Hypofractionation for whole breast irradiation in 5 days (Fast Forward Trial). Oral presentation at ASTRO conference, USA, 22 October 2018.
Hypofractionated Whole Breast Radiation Regimens. Oral presentation at ASTRO conference, USA, 18 September 2019.
Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield D, et al. on behalf of the FAST-Forward Trial Management Group. FAST-Forward phase III multicentre non-inferiority randomised controlled trial of 1- versus 3-week hypofractionated breast radiotherapy: 5-year results for efficacy and late normal tissue effects. Lancet 2020;395:1613–26. https://doi.org/10.1016/S0140-6736(20)30932-6
Brunt AM, Haviland JS, Wheatley DA, Yarnold JR. Breast cancer radiation therapy – Authors’ reply. Lancet 2020;396:1559–60. https://doi.org/10.1016/S0140-6736(20)32320-5
Paradigm shift: the role of hypofractionated breast radiotherapy. Oral presentation at UK Association of Breast Surgeons hypofractionation webinar, UK, 26 May 2020.
Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward). Oral presentation at Virtual Oncology Global webinar Conference organised by Memorial Sloan Kettering, USA, 28 May 2020.
FAST-Forward and beyond. Oral presentation at the CONNECT SENATURK meeting on ‘Hypofractionated radiotherapy in breast cancer: now even shorter’ on 13 June 2020.
NCRI Virtual Webinar titled – Radiotherapy research in the UK: What’s new? Oral presentation of FAST-Forward Trial results, 16 July 2020.
Update on FAST-Forward Trial: Oral presentation at UK Association of Breast Surgeons Webinar Series titled ‘Interesting perspective on patient management since COVID-19 and update on radiotherapy changes’ on 22 September 2020.
FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy: 5-year results for efficacy and late normal tissue effects. Oral presentation at UK Royal College of Radiologists consensus meeting, 15 October 2020.
FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy: 5-year results. Invited speaker presentation by Murray Brunt in the ‘Cancer Breakthough’ session at ASTRO 2020 on 25 October 2020. Selected from best of ESTRO 2020.
Who should have the FAST-FORWARD 5 Fraction Radiotherapy Schedule? Oral presentation at virtual 8th UKBCG annual meeting, 20 November 2020.
FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy: 5-year results for efficacy and late normal tissue effects. Oral presentation at virtual ESTRO 39 conference, 30 November 2020. Abstract number E20-2292/OC-0610.
Shortening breast radiotherapy to 1 week, FAST-Forward to implementation. Oral presentation at Edinburgh Breast Cancer Special Symposium, 27 February 2021.
Oral presentation of FAST-Forward Trial results at annual international BIR Annual Radiotherapy and Oncology Meeting 2021, 26 March 2021.
Oral presentation of FAST-Forward Trial results in the Breast Cancer Session at the Virtual UK Oncology Forum 2021, 21 October 2021.
Brunt AM, Haviland JS, Kirby AM, Somaiah N, Wheatley D, Bliss JM, Yarnold JR. 5-fraction radiotherapy for breast cancer: FAST-Forward to implementation. Clin Oncol (R Coll Radiol) 2021;33(7):430–9. https://doi.org/10.1016/j.clon.2021.04.016
Wheatley D, Haviland J, Patel J, Sydenham M, Alhasso A, Chan C, et al. on behalf of the FAST-Forward Trial Management Group. First results of FAST-Forward phase 3 RCT Nodal Sub-Study: 3-year normal tissue effects. Oral presentation at ESTRO conference, Copenhagen, 7 May 2022. Abstract E22-2401.
Ethics statement
FAST-Forward was approved by the London-Brighton and Sussex Research Ethics Committee (REC) (previously named South East Coast – Kent REC then South East Coast – Brighton and Sussex REC) on 2 September 2011 (11/LO/0958).
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Trial documentation including the protocol are available at www.icr.ac.uk/fastforward (last accessed 20 May 2022).
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613-26. https://doi.org/10.1016/s0140-6736(20)30932-6.
- Brunt AM, Haviland JS, Kirby AM, Somaiah N, Wheatley DA, Bliss JM, et al. Five-fraction radiotherapy for breast cancer: FAST-Forward to Implementation. Clin Oncol (R Coll Radiol) 2021;33:430-9. https://doi.org/10.1016/j.clon.2021.04.016.
- Brunt AM, Wheatley D, Yarnold J, Somaiah N, Kelly S, Harnett A, et al. FAST-Forward Trial Management Group . Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016;120:114-8. https://doi.org/10.1016/j.radonc.2016.02.027.
- Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield D, et al. NIHR National Institute for Health and Care Research Funding & Awards . Fast-Forward: A Randomised Clinical Trial Testing a 1-Week Course of Curative Whole Breast Radiotherapy Against a Standard 3-Week Schedule in Terms of Local Cancer Control and Late Adverse Effects in Women With Early Breast Cancer (Award ID 09 01 47) n.d. https://fundingawards.nihr.ac.uk/award/09/01/47 (accessed 8 September 2022).
- Cancer Research UK . Breast Cancer Statistics 2022. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero (accessed 27 May 2022).
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Early Breast Cancer Trialists’ Collaborative G . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. https://doi.org/10.1016/S0140-6736(11)61629-2.
- Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess 2007;11:1-149, iii. https://doi.org/10.3310/hta11310.
- Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) . 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. https://doi.org/10.1016/s0140-6736(15)00471-7.
- Polgár C, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) . Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:259-68. https://doi.org/10.1016/s1470-2045(17)30011-6.
- Schäfer R, Strnad V, Polgár C, Uter W, Hildebrandt G, Ott OJ, et al. Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO) . Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol 2018;19:834-44. https://doi.org/10.1016/s1470-2045(18)30195-5.
- Livi L, Meattini I, Marrazzo L, Simontacchi G, Pallotta S, Saieva C, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451-63. https://doi.org/10.1016/j.ejca.2014.12.013.
- Coles CE, Griffin CL, Kirby AM, Titley J, Agrawal RK, Alhasso A, et al. IMPORT Trialists . Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048-60. https://doi.org/10.1016/S0140-6736(17)31145-5.
- Bates TD. The 10-year results of a prospective trial of post-operative radiotherapy delivered in 3 fractions per week versus 2 fractions per week in breast carcinoma. Br J Radiol 1988;61:625-30. https://doi.org/10.1259/0007-1285-61-727-625.
- Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. https://doi.org/10.1093/jnci/94.15.1143.
- Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol 2005;75:9-17. https://doi.org/10.1016/j.radonc.2005.01.005.
- Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467-71. https://doi.org/10.1016/S1470-2045(06)70699-4.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. START Trialists’ Group . The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. https://doi.org/10.1016/S1470-2045(08)70077-9.
- Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. https://doi.org/10.1016/S0140-6736(08)60348-7.
- James ML, Lehman M, Hider PN, Jeffery M, Francis DP, Hickey BE. Fraction size in radiation treatment for breast conservation in early breast cancer. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD003860.pub2.
- Courdi A, Ortholan C, Hannoun-Levi JM, Ferrero JM, Largillier R, Balu-Maestro C, et al. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiother Oncol 2006;79:156-61. https://doi.org/10.1016/j.radonc.2006.04.005.
- Kirova YM, Campana F, Savignoni A, Laki F, Muresan M, Dendale R, et al. Institut Curie Breast Cancer Study Group . Breast-conserving treatment in the elderly: long-term results of adjuvant hypofractionated and normofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:76-81. https://doi.org/10.1016/j.ijrobp.2008.11.005.
- Brunt AM, Sydenham M, Bliss J, Coles C, Gothard L, Harnett A, et al. A 5-fraction regimen of adjuvant radiotherapy for women with early breast cancer: first analysis of the randomised UK FAST trial (ISRCTN62488883, CRUKE/04/015. EJC Suppl 2009;7. https://doi.org/10.1016/S1359-6349(09)72026-9.
- Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, Alhasso A, et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole breast radiotherapy for early breast cancer. J Clin Oncol 2020;38:3261-72. https://doi.org/10.1200/JCO.19.02750.
- Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003;21:555-63.
- Hebert-Croteau N, Freeman CR, Latreille J, Brisson J. Delay in adjuvant radiation treatment and outcomes of breast cancer – a review. Breast Cancer Res Treat 2002;74:77-94.
- Martin S, Mannino M, Rostom A, Tait D, Donovan E, Eagle S, et al. Acute toxicity and 2-year adverse effects of 30 Gy in five fractions over 15 days to whole breast after local excision of early breast cancer. Clin Oncol (R Coll Radiol) 2008;20:502-5. https://doi.org/10.1016/j.clon.2008.04.020.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. https://doi.org/10.1016/S0140-6736(05)67887-7.
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 2011;305:569-75. https://doi.org/10.1001/jama.2011.90.
- Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. International Breast Cancer Study Group Trial 23-01 investigators . Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. https://doi.org/10.1016/s1470-2045(13)70035-4.
- Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. https://doi.org/10.1016/S1470-2045(14)70460-7.
- Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. EORTC Radiation Oncology and Breast Cancer Groups . Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015;373:317-27. https://doi.org/10.1056/NEJMoa1415369.
- Stoll BA, Andrews JT. Radiation-induced peripheral neuropathy. Br Med J 1966;1:834-7. https://doi.org/10.1136/bmj.1.5491.834.
- Barr LC, Kissin MW. Radiation-induced brachial plexus neuropathy following breast conservation and radical radiotherapy. Br J Surg 1987;74:855-6. https://doi.org/10.1002/bjs.1800740935.
- Basso-Ricci S, della Costa C, Viganotti G, Ventafridda V, Zanolla R. Report on 42 cases of postirradiation lesions of the brachial plexus and their treatment. Tumori 1980;66:117-22.
- Johansson S, Svensson H, Denekamp J. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiat Oncol Biol Phys 2000;48:745-50. https://doi.org/10.1016/s0360-3016(00)00674-x.
- Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol 1990;18:213-20. https://doi.org/10.1016/0167-8140(90)90057-4.
- Hoeller U, Bonacker M, Bajrovic A, Alberti W, Adam G. Radiation-induced plexopathy and fibrosis. Is magnetic resonance imaging the adequate diagnostic tool?. Strahlenther Onkol 2004;180:650-4. https://doi.org/10.1007/s00066-004-1240-3.
- Fairchild AM, Weir LM, Mates D, Olivotto IA. Locoregional radiation for high-risk breast cancer-results of short fractionation. Breast Cancer Res Treat 2000;64.
- Livsey JE, Magee B, Stewart AL, Swindell R. Axillary recurrence following conservative surgery and radiotherapy in early breast cancer. Clin Oncol (R Coll Radiol) 2000;12:309-14. https://doi.org/10.1053/clon.2000.9181.
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956-62. https://doi.org/10.1056/NEJM199710023371402.
- Olsen NK, Pfeiffer P, Johannsen L, Schroder H, Rose C. Radiation-induced brachial plexopathy: neurological follow-up in 161 recurrence-free breast cancer patients. Int J Radiat Oncol Biol Phys 1993;26:43-9. https://doi.org/10.1016/0360-3016(93)90171-q.
- Delouche G, Bachelot F, Premont M, Kurtz JM. Conservation treatment of early breast cancer: long term results and complications. Int J Radiat Oncol Biol Phys 1987;13:29-34. https://doi.org/10.1016/0360-3016(87)90256-2.
- Fowble BL, Solin LJ, Schultz DJ, Goodman RL. Ten year results of conservative surgery and irradiation for stage I and II breast cancer. Int J Radiat Oncol Biol Phys 1991;21:269-77. https://doi.org/10.1016/0360-3016(91)90771-u.
- Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys 1992;23:915-23. https://doi.org/10.1016/0360-3016(92)90895-o.
- Galecki J, Hicer-Grzenkowicz J, Grudzien-Kowalska M, Michalska T, Zalucki W. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer – a review. Acta Oncol 2006;45:280-4. https://doi.org/10.1080/02841860500371907.
- Chen AM, Hall WH, Li BQ, Guiou M, Wright C, Mathai M, et al. Intensity-modulated radiotherapy increases dose to the brachial plexus compared with conventional radiotherapy for head and neck cancer. Br J Radiol 2011;84:58-63. https://doi.org/10.1259/bjr/62332495.
- The FAST Trialists Group, JM) mB . First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93-100. https://doi.org/10.1016/j.radonc.2011.06.026.
- Weis J, Arraras JI, Conroy T, Efficace F, Fleissner C, Gorog A, et al. Development of an EORTC quality of life phase III module measuring cancer-related fatigue (EORTC QLQ-FA13). Psychooncology 2012;22:1002-7. https://doi.org/10.1002/pon.3092.
- Haviland JS, Ashton A, Broad B, Gothard L, Owen JR, Tait D, et al. Evaluation of a method for grading late photographic change in breast appearance after radiotherapy for early breast cancer. Clin Oncol (R Coll Radiol) 2008;20:497-501. https://doi.org/10.1016/j.clon.2008.03.017.
- National Institute for Health and Care Excellence (NICE) . Early and Locally Advanced Breast Cancer: Diagnosis and Management 2018.
- Brunt AM, Haviland J, Sydenham M, Al-hasso A, Bloomfield D, Chan C, et al. OC-0595: FAST-Forward phase 3 RCT of 1-week hypofractionated breast radiotherapy:3-year normal tissue effects. Radiother Oncol 2018;127:S311-2. https://doi.org/10.1016/S0167-8140(18)30905-8.
- Horiot JC, van der Schueren E, Johansson KA, Bernier J, Bartelink H. The programme of quality assurance of the EORTC radiotherapy group. A historical overview. Radiother Oncol 1993;29:81-4.
- Bolla M, Bartelink H, Garavaglia G, Gonzalez D, Horiot JC, Johansson KA, et al. EORTC guidelines for writing protocols for clinical trials of radiotherapy. Radiother Oncol 1995;36:1-8. https://doi.org/10.1016/0167-8140(95)01573-y.
- Bleehen NM (Chair) . Quality Assurance in Radiotherapy; Report of a Working Party 1991. https://depositedpapers.parliament.uk/depositedpaper/2212040/details.
- Aird EG, Williams C, Mott GT, Dische S, Saunders MI. Quality assurance in the CHART clinical trial. Radiother Oncol 1995;36:235-44. https://doi.org/10.1016/0167-8140(95)01598-b.
- Venables K, Winfield E, Deighton A, Aird E, Hoskin P. START Trial management group . The START trial-measurements in semi-anatomical breast and chest wall phantoms. Phys Med Biol 2001;46:1937-48.
- IPEM . 81 Physics Aspects of Quality Control in Radiotherapy. IPEM Report 1999.
- Zotova R, Conibear J, Tsang Y. PD-0317: Field-based and volume-based PTV as plan evaluation structures in the UK FAST-Forward breast trial. Radiother Oncol 2013;106. https://doi.org/10.1016/S0167-8140(15)32623-2.
- Zotova R, Tsang Y.M., Miles E., Yarnold J.R. Interim Analysis of treatment plans in the FAST – Forward hypofractionation breast radiotherapy trial n.d.
- Zotova R, Yarnold J, Wheatley D, Griffin C, Murray B. PD-0430: Results from the radiotherapy quality assurance programme for the FAST-Forward breast trial. Radiother Oncol 2015;115:S209-10. https://doi.org/10.1016/S0167-8140(15)40426-8.
- Megias D, Sydenham M, Wheatley D, Maclennan M, Spezi E, Brunt AM. OC-0265: Evaluating variability of contouring using ESTRO guidelines for elective breast cancer radiotherapy. Radiother Oncol 2017;123:S136-7. https://doi.org/10.1016/S0167-8140(17)30708-9.
- Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015;114:3-10. https://doi.org/10.1016/j.radonc.2014.11.030.
- Miles E, Venables K. Radiotherapy quality assurance: facilitation of radiotherapy research and implementation of technology. Clin Oncol (R Coll Radiol) 2012;24:710-2. https://doi.org/10.1016/j.clon.2012.06.006.
- Van Gestel D, Dragan T, Gregoire V, Evans M, Budach V. Radiotherapy Quality Assurance for Head and Neck Squamous Cell Carcinoma. Front Oncol 2020;10. https://doi.org/10.3389/fonc.2020.00282.
- Turesson I, Notter G, Wickstrom I, Johansson KA, Eklund S. The influence of irradiation time per treatment session on acute and late skin reactions: a study on human skin. Radiother Oncol 1984;2:235-45. https://doi.org/10.1016/s0167-8140(84)80064-x.
- Turesson I, Thames HD. Repair capacity and kinetics of human skin during fractionated radiotherapy: erythema, desquamation, and telangiectasia after 3 and 5 year’s follow-up. Radiother Oncol 1989;15:169-88. https://doi.org/10.1016/0167-8140(89)90131-x.
- Nyman J, Turesson I. Does the interval between fractions matter in the range of 4–8 h in radiotherapy? A study of acute and late human skin reactions. Radiother Oncol 1995;34:171-8. https://doi.org/10.1016/0167-8140(95)01525-l.
- National Institute of Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: University of Kent; 2019.
- Alarid-Escudero F, Krijkamp EM, Enns EA, Hunink M, Pechlivanoglou P, Jalal H. Cohort state-transition models in R: From conceptualization to implementation 2020. https://doi.org/10.48550/arXiv.2001.07824.
- Team RC . R: A Language and Environment for Statistical Computing 2020.
- England N. 2018/2019 National Cost Collection data. London: NHS England and NHS Improvement; 2020.
- Bartlett FR, Colgan RM, Carr K, Donovan EM, McNair HA, Locke I, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol 2013;108:242-7. https://doi.org/10.1016/j.radonc.2013.04.021.
- Bartlett FR, Donovan EM, McNair HA, Corsini LA, Colgan RM, Evans PM, et al. The UK HeartSpare Study (Stage II): Multicentre Evaluation of a Voluntary Breath-hold Technique in Patients Receiving Breast Radiotherapy. Clin Oncol (R Coll Radiol) 2017;29:e51-6. https://doi.org/10.1016/j.clon.2016.11.005.
- StataCorp . Stata Statistical Software: Release 16 2019.
- Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials-Extrapolation with Patient-level Data. University of Sheffield, UK: School of Health and Related Research; 2011.
- Royal College of Radiologists (RCR) . Postoperative Radiotherapy for Breast Cancer: UK Consensus Statements 2016.
- Office for National Statistics (ONS) . National Life Tables: UK 2019.
- Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer 2020;129:60-7. https://doi.org/10.1016/j.ejca.2020.01.016.
- de Bock GH, Putter H, Bonnema J, van der Hage JA, Bartelink H, van de Velde CJ. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat 2009;117:401-8. https://doi.org/10.1007/s10549-008-0300-2.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- England N. 2012/2013 National Cost Collection Data. London: NHS England and NHS Improvement; 2014.
- Picot J, Copley V, Colquitt JL, Kalita N, Hartwell D, Bryant J. The INTRABEAM® Photon Radiotherapy System for the adjuvant treatment of early breast cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19:1-190. https://doi.org/10.3310/hta19690.
- NHS Digital . National Mastectomy and Breast Reconstruction Audit, Fourth Annual Report 2011.
- Thomas RJ, Williams M, Marshall C, Glen J, Callam M. The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer 2009;100:598-600. https://doi.org/10.1038/sj.bjc.6604911.
- Perry-Duxbury M, Asaria M, Lomas J, van Baal P. Cured today, ill tomorrow: A method for including future unrelated medical costs in economic evaluation in England and Wales. Value Health 2020;23:1027-33. https://doi.org/10.1016/j.jval.2020.05.006.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Campbell HE, Epstein D, Bloomfield D, Griffin S, Manca A, Yarnold J, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: A comparison of no chemotherapy and first, second, and third generation regimens for patients with differing prognoses. Eur J Cancer 2011;47:2517-30. https://doi.org/10.1016/j.ejca.2011.06.019.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- NHS Quality Improvement Scotland . Best Practice Statement: Skincare of Patients Receiving Radiotherapy 2010.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19:1-503, v. https://doi.org/10.3310/hta19140.
- Lomas J, Martin S, Claxton K. Estimating the marginal productivity of the English National Health Service from 2003 to 2012. Value Health 2019;22:995-1002. https://doi.org/10.1016/j.jval.2019.04.1926.
- Buyukkaramikli NC, Rutten-van Molken M, Severens JL, Al M. TECH-VER: A verification checklist to reduce errors in models and improve their credibility. PharmacoEcon 2019;37:1391-408. https://doi.org/10.1007/s40273-019-00844-y.
- Karnon J, Kerr GR, Jack W, Papo NL, Cameron DA. Health care costs for the treatment of breast cancer recurrent events: estimates from a UK-based patient-level analysis. Br J Cancer 2007;97:479-85. https://doi.org/10.1038/sj.bjc.6603887.
- Rautalin M, Färkkilä N, Sintonen H, Saarto T, Taari K, Jahkola T, et al. Health-related quality of life in different states of breast cancer – comparing different instruments. Acta Oncol 2018;57:622-8. https://doi.org/10.1080/0284186X.2017.1400683.
- Spencer K, Jones CM, Girdler R, Roe C, Sharpe M, Lawton S, et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol 2021;22:309-20. https://doi.org/10.1016/S1470-2045(20)30743-9.
- Williams C, Lewsey JD, Briggs AH, Mackay DF. Cost-effectiveness analysis in R using a multi-state modeling survival analysis framework: A tutorial. Med Decis Making 2017;37:340-52. https://doi.org/10.1177/0272989x16651869.
- Crowther MJ, Lambert PC. Parametric multistate survival models: Flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat Med 2017;36:4719-42. https://doi.org/10.1002/sim.7448.
- Moeschberger ML, Klein JP. Statistical methods for dependent competing risks. Lifetime Data Anal 1995;1:195-204. https://doi.org/10.1007/BF00985770.
- Gelman R, Gelber R, Henderson IC, Coleman CN, Harris JR. Improved methodology for analyzing local and distant recurrence. J Clin Oncol 1990;8:548-55. https://doi.org/10.1200/JCO.1990.8.3.548.
- Deshmukh AA, Shirvani SM, Lal L, Swint JM, Cantor SB, Smith BD, et al. Cost-effectiveness analysis comparing conventional, hypofractionated, and intraoperative radiotherapy for early-stage breast cancer. J Natl Cancer Inst 2017;109. https://doi.org/10.1093/jnci/djx068.
- Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-52. https://doi.org/10.1016/j.prro.2018.01.012.
- Bloomfield D, Sydenham M, Haviland J, Morden J, Bliss J, Yarnold J, et al. A 5-Fraction Regimen of Adjuvant Radiotherapy for Women With Early Breast Cancer: First Results of the Randomised UK FAST Trial (ISRCTN62488883, CRUK 04 015) n.d.
- Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. https://doi.org/10.1056/NEJMoa0906260.
- Wang SL, Fang H, Song YW, Wang WH, Hu C, Liu YP, et al. Hypofractionated versus conventional fractionated post-mastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2019;20:352-60. https://doi.org/10.1016/s1470-2045(18)30813-1.
- Smith BD, Bentzen SM, Correa CR, Hahn CA, Hardenbergh PH, Ibbott GS, et al. Fractionation for whole breast irradiation: An American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Int J Radiat Oncol Biol Phys 2011;81:59-68. https://doi.org/10.1016/j.ijrobp.2010.04.042.
- Haviland JS, Yarnold JR, Bentzen SM. Hypofractionated radiotherapy for breast cancer. N Engl J Med 2010;362.
- Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. https://doi.org/10.1016/S1470-2045(13)70386-3.
- Offersen BV, Alsner J, Nielsen HM, Jakobsen EH, Nielsen MH, Krause M, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: The DBCG HYPO Trial. J Clin Oncol 2020;38:3615-25. https://doi.org/10.1200/jco.20.01363.
- Shaitelman SF, Lei X, Thompson A, Schlembach P, Bloom ES, Arzu IY, et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: Results of a randomized, noninferiority clinical trial. J Clin Oncol 2018;36:3495-503. https://doi.org/10.1200/jco.18.00317.
- Tsang YM, Venables K, Sydenham M. on behalf of the FAST Working Party . Interim Quality Assurance (QA) Analysis of Treatment Plans in the ‘Faster Radiotherpay for Breast Cancer patients’ (FAST) Trial n.d.
- Withers HR. Principles and Practice of Radiation Oncology. Philadelphia: J.B. Lippincott; 1992.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. https://doi.org/10.1056/NEJMoa1209825.
- Brand DH, Yarnold JR. The linear–quadratic model and implications for fractionation. Clin Oncol – UK 2019;31:673-7. https://doi.org/10.1016/j.clon.2019.06.007.
- Withers HR, Taylor JMG, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 1988;27:131-46. https://doi.org/10.3109/02841868809090333.
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6&7 randomised controlled trial. Lancet 2003;362:933-40. https://doi.org/10.1016/S0140-6736(03)14361-9.
- Haviland JS, Bentzen SM, Bliss JM, Yarnold JR. START Trial Management Group . Prolongation of overall treatment time as a cause of treatment failure in early breast cancer: An analysis of the UK START (Standardisation of Breast Radiotherapy) trials of radiotherapy fractionation. Radiother Oncol 2016;121:420-3. https://doi.org/10.1016/j.radonc.2016.08.027.
- National Institute of Health and Care Excellence . COVID-19 Rapid Guideline: Delivery of Systemic Anticancer Treatments 2020.
- Royal College of Radiologists (RCR) . Radiotherapy Consent Form for Breast Cancer 2021.
- Lewis P, Brunt AM, Coles C, Griffin S, Locke I, Roques T. Breast Radiotherapy Consensus Working Group . Moving forward fast with FAST-Forward. Clin Oncol (R Coll Radiol) 2021;33:427-9. https://doi.org/10.1016/j.clon.2021.04.007.
- Meattini I, Becherini C, Boersma L, Kaidar-Person O, Marta GN, Montero A, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol 2022;23:e21-31. https://doi.org/10.1016/s1470-2045(21)00539-8.
- Thomssen C, Balic M, Harbeck N, Gnant MS. Gallen/Vienna 2021: A brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care 2021;16:135-43. https://doi.org/10.1159/000516114.
- Salerno KE. NCCN Guidelines Update: Evolving radiation therapy recommendations for breast cancer. J Natl Compr Canc Netw 2017;15:682-4. https://doi.org/10.6004/jnccn.2017.0072.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-42. https://doi.org/10.3322/caac.21492.
- Anderson BO, Ilbawi AM, Fidarova E, Weiderpass E, Stevens L, Abdel-Wahab M, et al. The Global Breast Cancer Initiative: a strategic collaboration to strengthen health care for non-communicable diseases. Lancet Oncol 2021;22:578-81. https://doi.org/10.1016/s1470-2045(21)00071-1.
- Early Breast Cancer Trialists Collaborative Group . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011;378:1707-16. https://doi.org/10.1016/S0140-6736(11)61629-2.
- Early Breast Cancer Trialists Collaborative Group . Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. https://doi.org/10.1016/S0140-6736(14)60488-8.
- Abdel-Wahab M, Gondhowiardjo SS, Rosa AA, Lievens Y, El-Haj N, Rubio JAP, et al. Global Radiotherapy: Current Status and Future Directions – White Paper. JCO Glob Oncol 2021;7:827-42. https://doi.org/10.1200/go.21.00029.
- Rodin D, Tawk B, Mohamad O, Grover S, Moraes FY, Yap ML, et al. Hypofractionated radiotherapy in the real-world setting: An international ESTRO-GIRO survey. Radiother Oncol 2021;157:32-9. https://doi.org/10.1016/j.radonc.2021.01.003.
- Foerster M, McCormack V, Anderson BO, Boucheron P, Zietsman A, Cubasch H, et al. Treatment guideline concordance, initiation, and abandonment in patients with non-metastatic breast cancer from the African Breast Cancer-Disparities in Outcomes (ABC-DO) cohort in sub-Saharan Africa: a prospective cohort study. Lancet Oncol 2022;23:729-38. https://doi.org/10.1016/S1470-2045(22)00198-X.
- Poortmans PM, Weltens C, Fortpied C, Kirkove C, Peignaux-Casasnovas K, Budach V, et al. European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups . Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol 2020;21:1602-10. https://doi.org/10.1016/s1470-2045(20)30472-1.
- Vrieling C, van Werkhoven E, Maingon P, Poortmans P, Weltens C, Fourquet A, et al. European Organisation for Research and Treatment of Cancer, Radiation Oncology and Breast Cancer Groups . Prognostic factors for local control in breast cancer after long-term follow-up in the EORTC Boost vs No Boost Trial: A randomized clinical trial. JAMA Oncology 2017;3:42-8. https://doi.org/10.1001/jamaoncol.2016.3031.
- Levy A, Rivera S. 1-week hypofractionated adjuvant whole-breast radiotherapy: towards a new standard?. Lancet 2020;395:1588-9. https://doi.org/10.1016/S0140-6736(20)30978-8.
- Boersma LJ, Murrer LHP. Three large trials on radiotherapy for early breast cancer: What did we learn?. Radiother Oncol 2021;156:239-43. https://doi.org/10.1016/j.radonc.2020.12.018.
- Breast Cancer Now . Facts and Statistics 2021 2021.
- James Lind Alliance . Priorities for Living With and Beyond Cancer 2018.
- Bhattacharya IS, Haviland JS, Hopwood P, Coles CE, Yarnold JR, Bliss JM, et al. IMPORT Trialists . Can patient-reported outcomes be used instead of clinician-reported outcomes and photographs as primary endpoints of late normal tissue effects in breast radiotherapy trials? Results from the IMPORT LOW trial. Radiother Oncol 2019;134:220-30.
- Mills J, Haviland JS, Moynihan C, Bliss JM, Hopwood P. Women’s free-text comments on their quality of life: An exploratory analysis from the UK Standardisation of Breast Radiotherapy (START) Trials for early breast cancer. Clin Oncol (R Coll Radiol) 2018;30:433-41. https://doi.org/10.1016/j.clon.2018.03.007.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2010.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2018.
- Macmillan Cancer Support 2019.
Appendix 1 Centre recruitment to Main Trial and Nodal Sub-Study
| Centre no | Centre | Principal investigator(s)a | Date site opened | Date first patient | Total |
|---|---|---|---|---|---|
| 1 | RMH Sutton | Dr N Somaiah, Professor J Yarnold | 25 November 2011 | 9 December 2011 | 185 |
| 2 | Royal Cornwall | Dr D Wheatley | 9 January 2012 | 10 January 2012 | 248 |
| 3 | University Hospital of North Staffordshire | Professor AM Brunt | 23 November 2011 | 24 November 2011 | 298 |
| 3a | Stafford General Hospital | Dr A Jegannathen | 16 January 2012 | 25 January 2012 | 87 |
| 4 | Derriford Hospital, Plymouth | Dr U Panwar, Dr S Kelly | 17 January 2012 | 24 January 2012 | 144 |
| 5 | Norfolk and Norwich Hospital | Dr D Geropantas, Dr A Harnett | 2 January 2012 | 10 January 2012 | 100 |
| 5a | James Paget Hospital | Dr S Down, Dr A Harnett | 2 January 2012 | 4 May 2012 | 33 |
| 6 | Bristol Haematology and Oncology | Dr C Comins, Dr A Bahl | 7 February 2012 | 14 March 2012 | 47 |
| 6a | Weston General Hospital | Dr T Wells, Dr M Tomlinson | 30 April 2012 | 1 May 2012 | 22 |
| 7 | Royal Shrewsbury Hospital | Dr L Pettit, Dr R Agrawal, Dr S Khanduri | 28 February 2012 | 9 March 2012 | 126 |
| 8 | Royal Devon + Exeter Hospital | Dr J Forrest, Dr A Hong | 2 May 2012 | 16 May 2012 | 70 |
| 8a | North Devon District Hospital | Dr J Forrest, Dr D Hwang | 10 May 2012 | 17 May 2012 | 46 |
| 9 | Addenbrookes Hospital | Professor C Coles | 7 March 2012 | 20 March 2012 | 99 |
| 9a | Hinchingbrooke Hospital | Dr S Russell | 7 September 2012 | 9 October 2012 | 17 |
| 9b | Queen Elizabeth Hospital, Kings Lynn | Dr M Daly | 26 October 2012 | 10 December 2012 | 5 |
| 9c | West Suffolk Hospital | Dr M Moody | 28 November 2012 | 7 February 2013 | 10 |
| 9d | Bedford Hospital | Dr S Smith | 29 May 2013 | 5 July 2013 | 2 |
| 10 | New Cross Hospital | Dr R Allerton, Dr M Churn | 9 March 2012 | 4 April 2012 | 67 |
| 10a | Kidderminster Hospital | Dr M Churn | 12 March 2012 | 10 May 2012 | 47 |
| 10b | Russell’s Hall | Dr R Allerton | 25 May 2012 | 25 May 2012 | 21 |
| 11 | Southend University Hospital | Dr H Algurafi, Dr A Robinson | 27 April 2012 | 15 May 2012 | 37 |
| 11a | Basildon Hospital | Dr H Swinburn, Dr W Ella | 18 July 2012 | 25 July 2012 | 25 |
| 12 | Clatterbridge Cancer Centre | Dr S Tolan, Dr I Syndikus | 18 May 2012 | 26 July 2012 | 22 |
| 12a | Warrington Hospital | Dr I Syndikus | 18 May 2012 | 7 June 2012 | 27 |
| 12c | St Helens + Whiston | Dr R Sripadam | 21 May 2012 | 28 September 2012 | 4 |
| 12d | University Hospital, Aintree | Dr P Robson | 28 June 2012 | 15 May 2013 | 8 |
| 12e | Countess of Chester | Dr A Hall | 25 July 2012 | 15 August 2012 | 12 |
| 13 | Velindre Cancer Centre | Dr H Passant, Professor P Barrett-Lee | 20 June 2012 | 25 June 2012 | 167 |
| 14 | Royal Surrey County Hospital | Dr R Laing | 8 May 2012 | 2 August 2012 | 40 |
| 15 | Essex County Hospital | Dr M Mukesh, Dr P Murray | 14 May 2012 | 22 August 2012 | 19 |
| 16 | Beatson West of Scotland Cancer Centre | Dr A Al-Hasso | 25 May 2012 | 25 July 2012 | 24 |
| 16a | Royal Alexandra Hospital | Dr A Al-Hasso | 25 May 2012 | 31 May 2012 | 41 |
| 16b | Forth Valley Hospital | Dr H Marashi | 24 July 2013 | 19 November 2013 | 13 |
| 16c | Wishaw Hospital | Dr J Hicks, Dr M Rizwanullah | 28 June 2012 | 14 August 2012 | 25 |
| 16d | New Victoria ACH | Dr D Ritchie | 17 July 2012 | 15 August 2012 | 7 |
| 16e | Hairmyres Hospital | Dr J Hicks, Dr G Dunn | 25 February 2014 | 13 March 2014 | 1 |
| 16f | Crosshouse Hospital, Kilmarmock | Dr G Lumsden | 6 August 2012 | 7 August 2012 | 30 |
| 17 | Singleton Hospital, Swansea | Dr M Rolles, Dr D Pudney | 28 May 2012 | 29 June 2012 | 72 |
| 18 | Cheltenham General Hospital | Dr J Bowen | 27 June 2012 | 9 July 2012 | 48 |
| 18a | Worcester Royal Infirmary | Dr M Churn, Dr J Bowen | 25 July 2012 | 12 October 2012 | 19 |
| 18b | Hereford County Hospital | Dr D Nelmes, Dr S Guglani | 27 June 2012 | 19 November 2012 | 18 |
| 19c | Blackpool Victoria Infirmary | Dr F Danwata, Dr A Hindley | 12 September 2012 | 21 September 2012 | 38 |
| 19d | Royal Lancaster Infirmary | Dr D Williamson, Dr G Skailes | 16 July 2012 | 25 July 2012 | 92 |
| 20 | Queens Hospital, Romford | Dr E Sims, Dr M Quigley | 27 June 2012 | 8 October 2012 | 52 |
| 21 | Peterborough Hospital | Dr C Jephcott | 13 July 2012 | 12 September 2012 | 36 |
| 22 | Weston Park Hospital, Sheffield | Dr M Hatton | 2 July 2012 | 20 August 2012 | 86 |
| 23 | Royal Sussex County Hospital | Dr D Bloomfield | 5 July 2012 | 25 July 2012 | 116 |
| 23a | Worthing Hospital | Dr SY Sham, Dr A Nikapota, Dr S Mitra | 14 January 2013 | 23 January 2013 | 59 |
| 23b | Eastbourne Hospital | Dr S Westwell | 3 December 2012 | 12 December 2012 | 35 |
| 24 | Guys Hospital | Professor E Sawyer | 7 September 2012 | 3 December 2012 | 25 |
| 25 | Ipswich Hospital | Dr R Venkitaraman, Dr L Sherwin | 25 July 2012 | 24 October 2012 | 18 |
| 26 | Glan Clwyd Hospital | Dr J Bishop | 10 January 2013 | 28 January 2013 | 31 |
| 26a | Wrexham Maelor Hospital | Dr W Soe | 10 January 2013 | 11 February 2013 | 24 |
| 26b | Ysbyty Gwynedd Hospital | Dr J Bishop | 23 January 2013 | 12 June 2013 | 10 |
| 27 | Queen Elizabeth Hospital, Birmingham | Dr A Stevens, Dr MS Anwar | 3 August 2012 | 21 August 2012 | 66 |
| 27a | Sandwell Hospital | Dr D Spooner | 26 October 2012 | 23 November 2012 | 4 |
| 27b | City Hospital, Birmingham | Dr D Spooner | 26 October 2012 | 14 November 2012 | 31 |
| 27c | Manor Hospital, Walsall | Dr S Yaha, Dr MS Anwar | 23 May 2013 | 2 August 2013 | 6 |
| 28 | Charing Cross Hospital | Dr S Cleator, Dr C Lowdell | 13 September 2012 | 19 September 2012 | 62 |
| 28a | Ealing Hospital | Dr O Hatcher, Dr C Lewanski | 24 June 2013 | 11 October 2013 | 7 |
| 28b | West Middlesex Hospital | Dr P Riddle | 7 February 2013 | 26 February 2013 | 17 |
| 29 | Mount Vernon Hospital | Dr C Westbury, Dr M Ah-See | 21 September 2012 | 24 September 2012 | 108 |
| 29a | Luton and Dunstable University Hospital | Dr A Vinayan, Dr M Ah-See | 10 May 2013 | 25 September 2013 | 23 |
| 30 | Royal United Hospital, Bath | Dr M Beresford | 14 December 2012 | 3 January 2013 | 45 |
| 31 | Queen Alexandra Hospital, Portsmouth | Dr K Bradley | 14 November 2012 | 29 November 2012 | 92 |
| 32 | Torbay District General Hospital | Dr A Goodman | 14 November 2012 | 27 November 2012 | 62 |
| 33 | Southampton General Hospital | Dr C Crowley | 11 December 2012 | 19 December 2012 | 50 |
| 33a | Salisbury Hospital | Dr C Crowley | 21 December 2012 | 4 March 2013 | 21 |
| 33b | Royal Hampshire County Hospital | Dr S Raj | 10 May 2013 | 1 July 2013 | 10 |
| 34 | Royal Berkshire | Dr R Davis, Dr J Barrett | 10 January 2013 | 21 January 2013 | 91 |
| 34a | Wexham Park Hospital | Dr R Davis, Dr J Adams | 31 May 2013 | 6 August 2013 | 12 |
| 35 | St Bartholomew’s Hospital | Dr V Wolstenholme | 14 January 2013 | 25 January 2013 | 55 |
| 36 | St James’ University Hospital, Leeds | Dr S Kumar | 29 January 2013 | 12 February 2013 | 24 |
| 37 | University Hospital of Coventry and Warwickshire | Dr D Hrouda, Dr N Walji | 11 February 2013 | 10 April 2013 | 40 |
| 37a | George Eliot Hospital, Nuneaton | Dr S Lupton | 11 February 2013 | 26 February 2013 | 35 |
| 37b | Warwick Hospital | Dr N Walji | 11 February 2013 | 6 March 2013 | 56 |
| 37c | Alexandra Hospital, Redditch | Dr M Churn | 11 February 2013 | 3 June 2013 | 12 |
| 38 | Musgrove Park, Taunton | Dr M Varughese, Dr J Graham | 3 May 2013 | 29 May 2013 | 39 |
| 38a | Yeovil District Hospital | Dr U Barthakur | 24 May 2013 | 22 August 2013 | 8 |
| 39 | Lincoln County Hospital | Dr A Chaudhuri, Dr E Murray | 17 May 2013 | 29 May 2013 | 50 |
| 39a | Pilgrim Hospital, Boston | Dr A Chaudhuri, Dr E Murray | 17 May 2013 | 20 June 2013 | 9 |
| 40 | Northampton General Hospital | Dr R Agrawal, Dr C Macmillan | 3 July 2013 | 13 August 2013 | 19 |
| 41 | James Cook University Hospital | Dr B Sethugavalar, Dr J Hardman | 1 August 2013 | 1 October 2013 | 22 |
| 41a | University Hospital of North Tees | Dr E Thompson, Dr N Storey | 18 October 2013 | 21 October 2013 | 8 |
| 42 | Western General Hospital, Edinburgh | Dr C Bedi | 23 September 2013 | 21 January 2014 | 13 |
| 42a | Borders General Hospital | Dr C Bedi | 27 September 2013 | 26 November 2013 | 5 |
| 42b | Dumfries and Galloway Hospital | Dr A Hennessy, Dr T Evans | 24 December 2013 | 12 February 2014 | 3 |
| 42c | Queen Margaret Hospital, Dunfermline | Dr M MacLennan, Dr T Evans | 21 January 2014 | 4 February 2014 | 4 |
| 42d | St John’s Hospital, Livingstone | Dr F Yuille | 27 September 2013 | 19 December 2013 | 7 |
| 43 | Belfast City Hospital | Dr H McCarty, Dr G Hanna | 8 January 2014 | 20 January 2014 | 9 |
| 44 | University College London Hospitals | Dr G Blackman, Dr M McCormack | 7 August 2013 | 28 August 2013 | 16 |
| 45 | Poole Hospital | Dr J Brady | 24 December 2013 | 3 February 2014 | 8 |
| 46 | Leicester Royal Infirmary | Dr K Kancherla | 14 January 2014 | 24 January 2014 | 5 |
| 47 | Churchill Hospital, Oxford | Dr S Oliveros, Dr B Lavery | 4 February 2014 | 13 March 2014 | 1 |
| Total | 4110 | ||||
| Centre no | Centre | Principal investigator(s)a | Date site opened | Date first patient | Total |
|---|---|---|---|---|---|
| 01 | RMH Sutton | Dr N Somaiah, Professor J Yarnold | 21 October 2016 | 2 December 2016 | 37 |
| 02 | Royal Cornwall | Dr D Wheatley | 8 April 2016 | 26 July 2016 | 36 |
| 03 | University Hospital of North Midlands | Dr D Gahir, Professor AM Brunt | 16 February 2016 | 8 April 2016 | 63 |
| 03a | Stafford General Hospital | Dr A Jegannathen | 23 May 2016 | 1 September 2017 | 1 |
| 04 | Derriford Hospital, Plymouth | Dr U Panwar, Dr S Kelly | 20 February 2018 | 1 May 2018 | 17 |
| 08 | Royal Devon + Exeter Hospital | Dr J Forrest, Dr A Hong | 11 April 2016 | 27 April 2016 | 10 |
| 09 | Addenbrookes Hospital | Professor C Coles | 15 April 2016 | Did not recruit | 0 |
| 10 | New Cross Hospital | Dr R Allerton, Dr M Churn | 12 August 2016 | 4 January 2017 | 5 |
| 12 | Clatterbridge Cancer Centre | Dr S Tolan, Dr I Syndikus | 5 June 2017 | Did not recruit | 0 |
| 12d | University Hospital, Aintree | Dr P Robson | 5 June 2017 | 28 March 2018 | 1 |
| 13 | Velindre Cancer Centre | Dr H Passant, Professor P Barrett-Lee | 18 August 2017 | 11 October 2017 | 1 |
| 16 | Beatson West of Scotland Cancer Centre | Dr A Al-Hasso | 5 December 2016 | 18 January 2017 | 12 |
| 16a | Royal Alexandra Hospital | Dr A Al-Hasso | 5 December 2016 | 12 December 2016 | 21 |
| 16b | Forth Valley Hospital | Dr H Marashi | 5 December 2016 | 22 May 2018 | 2 |
| 16c | Wishaw Hospital | Dr J Hicks, Dr M Rizwanullah | 6 February 2017 | 12 May 2017 | 5 |
| 16e | Hairmyres Hospital | Dr J Hicks, Dr G Dunn | 6 February 2017 | 12 May 2017 | 5 |
| 18 | Cheltenham General Hospital | Dr J Bowen | 13 July 2016 | 6 October 2016 | 10 |
| 18b | Hereford County Hospital | Dr D Nelmes, Dr S Guglani | 7 December 2016 | 6 September 2017 | 2 |
| 19 | Royal Preston | Dr M Hogg | 19 May 2017 | Did not recruit | 0 |
| 19c | Blackpool Victoria Infirmary | Dr F Danwata, Dr A Hindley | 19 May 2017 | 23 April 2018 | 2 |
| 19d | Royal Lancaster Infirmary | Dr D Williamson, Dr G Skailes | 13 November 2017 | 9 March 2018 | 7 |
| 21 | Peterborough Hospital | Dr C Jephcott | 14 June 2016 | 8 September 2016 | 2 |
| 22 | Weston Park Hospital, Sheffield | Dr M Hatton | 24 May 2016 | 5 October 2016 | 8 |
| 24 | Guys Hospital | Professor E Sawyer | 6 September 2016 | 6 October 2016 | 2 |
| 27 | Queen Elizabeth Hospital, Birmingham | Dr A Stevens, Dr MS Anwar | 14 December 2016 | 10 March 2017 | 7 |
| 27c | Manor Hospital, Walsall | Dr S Yaha, Dr MS Anwar | 6 June 2017 | 1 February 2018 | 1 |
| 28 | Charing Cross Hospital | Dr S Cleator, Dr C Lowdell | 8 July 2016 | 1 August 2016 | 17 |
| 28b | West Middlesex Hospital | Dr P Riddle | 11 July 2017 | 11 July 2017 | 7 |
| 31 | Queen Alexandra Hospital, Portsmouth | Dr K Bradley | 10 August 2016 | 10 February 2017 | 2 |
| 32 | Torbay District General Hospital | Dr A Goodman | 2 November 2016 | 11 January 2017 | 13 |
| 33 | Southampton General Hospital | Dr C Crowley | 8 July 2016 | 1 August 2017 | 7 |
| 34 | Royal Berkshire | Dr R Davis, Dr J Barrett | 9 February 2017 | 1 August 2017 | 14 |
| 35 | St Bartholomew’s Hospital | Dr V Wolstenholme | 21 July 2016 | 22 September 2016 | 3 |
| 36 | St James’s University Hospital, Leeds | Dr S Kumar | 20 December 2016 | 9 February 2017 | 15 |
| 36a | Pinderfields Hospital, Wakefield | Dr S Kumar | 21 June 2017 | 9 August 2017 | 4 |
| 36b | Bradford Royal Infirmary | Dr E Thomas | 21 November 2017 | 29 November 2017 | 4 |
| 36c | Huddersfield Royal Infirmary | Dr N Roberts | 25 January 2018 | 23 February 2018 | 3 |
| 37 | University Hospital of Coventry and Warwickshire | Dr D Hrouda, Dr N Walji | 27 March 2017 | 31 October 2017 | 2 |
| 38 | Musgrove Park, Taunton | Dr M Varughese, Dr J Graham | 11 February 2017 | 24 January 2017 | 14 |
| 38a | Yeovil District Hospital | Dr U Barthakur | 22 March 2017 | 7 June 2017 | 9 |
| 39 | Lincoln County Hospital | Dr A Chaudhuri, Dr E Murray | 8 June 2017 | 10 July 2017 | 18 |
| 39a | Pilgrim Hospital, Boston | Dr A Chaudhuri, Dr E Murray | 28 June 2017 | 9 August 2017 | 5 |
| 40 | Northampton General Hospital | Dr R Agrawal, Dr C Macmillan | 20 July 2016 | Did not recruit | 0 |
| 41 | James Cook University Hospital | Dr B Sethugavalar, Dr J Hardman | 15 November 2016 | 23 March 2017 | 15 |
| 41a | University Hospital of North Tees | Dr E Thompson, Dr N Storey | 19 December 2016 | 10 May 2017 | 3 |
| 42 | Western General Hospital, Edinburgh | Dr C Bedi | 13 April 2016 | 5 July 2016 | 11 |
| 42a | Borders General Hospital | Dr C Bedi | 13 April 2016 | 23 December 2016 | 7 |
| 42b | Dumfries and Galloway Hospital | Dr A Hennessy, Dr T Evans | 25 May 2016 | 6 September 2016 | 2 |
| 42c | Queen Margaret Hospital, Dunfermline | Dr M MacLennan, Dr T Evans | 13 April 2016 | 7 March 2017 | 7 |
| 42d | St John’s Hospital, Livingstone | Dr F Yuille | 13 April 2016 | Did not recruit | 0 |
| 43 | Belfast City Hospital | Dr H McCarty, Dr G Hanna | 5 June 2017 | 4 October 2017 | 4 |
| 46 | Leicester Royal Infirmary | Dr K Kancherla | 8 March 2017 | 24 March 2017 | 7 |
| 50 | Worcester Royal Infirmary | Dr M Churn | 17 August 2016 | 3 February 2017 | 5 |
| 50a | Kidderminster Hospital | Dr M Churn | 17 August 2016 | 24 August 2016 | 9 |
| 50b | Alexandra Hospital, Redditch | Dr M Churn | 17 August 2016 | 23 March 2017 | 5 |
| Total | 469 | ||||
Appendix 2 Additional tables
Methods
| Trial group | Total dose (Gy) | Dose per fraction (Gy) | Number of fractions | Fractions per week | Treatment time (weeks) |
|---|---|---|---|---|---|
| Control group | 40.05 | 2.67 | 15 | 5 | 3 |
| Test Group 1 | 27.0 | 5.4 | 5 | 5 | 1 |
| Test Group 2 | 26.0 | 5.2 | 5 | 5 | 1 |
| Eligibility criteria | ||||||
|---|---|---|---|---|---|---|
| Exclusion criteria | Version 1 (27 May 2011) | Version 2 (13 February 2013) | Version 2.2 (2 May 2013) | Version 3 [8 July 2015 (Nodal Sub-Study] | Version 4 [24 February 2017 (Nodal Sub-Study)] | |
| 1 | Past history of malignancy except (1) basal cell skin cancer and CIN cervix uteri or (2) non-breast malignancy allowed if treated with curative intent and at least 5 years disease free | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2 | Contralateral breast cancer, including DCIS, irrespective of date of diagnosis (superseded) | ✓ | N/A | N/A | N/A | N/A |
| 2 | Ipsilateral and contralateral breast cancer, including DCIS, irrespective of date of diagnosis (superseded) | N/A | ✓ | N/A | N/A | N/A |
| 2 | Contralateral and/or previous ipsilateral breast cancer, including DCIS, irrespective of date of diagnosis | N/A | N/A | ✓ | ✓ | ✓ |
| 3 | Breast reconstruction using implants | ✓ | N/A | N/A | N/A | N/A |
| 4 | Concurrent cytotoxic chemotherapy (sequential neoadjuvant or adjuvant cytotoxic therapy allowed as long as there is ≥2 weeks between therapy and RT) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5 | Radiotherapy to any regional lymph node areas (excepting lower axilla included in standard tangential fields to breast/chest wall) | ✓ | ✓ | ✓ | N/A | N/A |
| 6 | Age ≥ 65 years and pT1G1/G2 ER + HER-2−pN0 M0 invasive disease | N/A | ✓ | ✓ | N/A | N/A |
| 7 | Ipsilateral microinvasive disease and/or non-gradeable tumours | N/A | ✓ | ✓ | ✓ | ✓ |
| 8 | Any patient with N0 disease | N/A | N/A | N/A | ✓ | ✓ |
| 9 | Known residual macroscopic nodal disease | N/A | N/A | N/A | ✓ | ✓ |
| 10 | ≥10 positive axillary nodes | N/A | N/A | N/A | ✓ | N/A |
| 11 | Any positive level IV (SCF) nodes | N/A | N/A | N/A | ✓ | ✓ |
| 12 | Any requirement for IMC RT because indications for IMC RT are currently unclear | N/A | N/A | N/A | ✓ | ✓ |
| Protocol version | Summary of changes |
|---|---|
| Version 2.0 (13 February 2013) | Change to eligibility criteria to exclude patients with very low risk of relapse from the trial (defined as age ≥ 65 years and pT1G1/G2 ER + HER-2−pN0 M0 invasive disease). Inclusion of the HEs sub-study. Inclusion and renaming of the quality of life sub-study to PRO measures study. Addition of the second short-term side effects study. Updates to the PIS and consent forms due to reflect the opening of the sub-studies. |
| Version 2.1 (13 March 2013) | Minor formatting changes to main protocol and minor changes to PIS. |
| Version 2.2 (2 May 2013) | Correction to the fourth exclusion criteria – insertion of the word ‘previous’. Update to the PIS and consent form to removing text stating ‘breast conservation patients only’, so that mastectomy patients as well will be eligible for the photographic sub-study. |
| Version 3.0 (8 July 2015) | Substantial amendment to include the Nodal Sub-Study. |
| Version 3.1 (15 June 2016) | Minor wording change to one exclusion criterion – removal of word ‘no’ so exclusion reads ‘known residual macroscopic nodal disease’. |
| Version 4.0 (24 February 2017) | Inclusion of patients with > 9 positive nodes provided the treatment intention is adjuvant (curative intent). Hypofractionated boost is now permitted and needs to be declared prior to randomisation. |
| Version 5.0 (14 December 2017) | Change of design of Nodal Sub-Study to a 2-group randomisation (removal of Test Group 1). Revised target sample size for the Nodal Sub-Study. |
| Version 5.1 (5 February 2018) | Minor amendment to include statement regarding the presentation and publication of the analysis of normal tissue effect data up to 3 years’ follow-up. |
Acute toxicity sub-studies
| RT details | Acute toxicity sub-study 1 | Acute toxicity sub-study 2 | ||||
|---|---|---|---|---|---|---|
| 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions | 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions | |
| (N = 64)a, N(%) | (N = 62), N(%) | (N = 63), N(%) | (N = 55)b, N(%) | (N = 44), N(%) | (N = 61), N(%) | |
| RT received | ||||||
| Yes | 64 (100) | 62 (100) | 63 (100) | 54 (98) | 44 (100) | 61 (100) |
| No | 0 | 1 (2)c | 0 | 1 (2)d | 0 | 0 |
| Boost received | ||||||
| Yes | 11 (17) | 11 (18) | 7 (11) | 0 | 0 | 0 |
| No | 53 (83) | 51 (82) | 56 (89) | 54 (100) | 44 (100) | 61 (100) |
| Treatment duration in patients receiving no boost (including weekends and bank holidays) e | ||||||
| Median (IQR) | 22 (21–23) | 5 (5–7) | 7 (5–7) | 21 (20–22) | 6 (5–7) | 6 (5–7) |
| Range | 15–25 | 5–11 | 5–9 | 15–23 | 5–8 | 5–8 |
| Treatment duration in patients receiving boost (including weekends and bank holidays) | ||||||
| Median (IQR) | 30 (26–33) | 18 (12–20) | 14 (12–17) | - | - | - |
| Range | 22–35 | 7–20 | 7–21 | - | - | - |
| Whole breast – total fractions | ||||||
| 5 | 0 | 62 (100) | 63 (100) | 0 | 44 (100) | 61 (100) |
| 15 | 63 (98) | 0 | 0 | 54 (100) | 0 | 0 |
| Otherf | 1 (2) | 0 | 0 | 0 | 0 | 0 |
| Whole breast – total dose (Gy) | ||||||
| 26 | 0 | 0 | 63 (100) | 0 | 0 | 61 (100) |
| 27 | 0 | 62 (100) | 0 | 0 | 44 (100) | 0 |
| 40 | 63 (98) | 0 | 0 | 54 (100) | 0 | 0 |
| Otherf | 1 (2) | 0 | 0 | 0 | 0 | 0 |
| Bolus used (mastectomy patients only) | ||||||
| Yes | 1 (100) | 1 (33) | 3 (50) | 2 (40) | 1 (50) | 5 (100) |
| No | 0 | 2 (67) | 3 (50) | 3 (60) | 1 (50) | 0 |
| Dose homogeneity constraints achieved | ||||||
| Yes | 62 (97) | 61 (98) | 60 (95) | 53 (98) | 44 (100) | 60 (98) |
| No | 2 (3) | 1 (2) | 3 (5) | 1 (2) | 0 | 1 (2) |
| Organs at risk dose constraints achieved | ||||||
| Yes | 64 (100) | 61 (98) | 62 (98) | 53 (98) | 44 (100) | 61 (100) |
| No | 0 | 1 (2) | 1 (2) | 1 (2) | 0 | 0 |
| Any deviations from RTg | ||||||
| Yes | 1 (2) | 0 | 0 | 1 (2) | 0 | 0 |
| No | 63 (98) | 62 (100) | 63 (100) | 53 (98) | 44 (100) | 61 (100) |
| Whole breast RT extended by > 3 days | ||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 |
| No | 64 (100) | 62 (100) | 63 (100) | 54 (100) | 44 (100) | 61 (100) |
| Total number of acute toxicity assessments during and post-treatment | Acute toxicity sub-study 1 | Acute toxicity sub-study 2 | ||||
|---|---|---|---|---|---|---|
| 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions | 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions | |
| (N = 64), N(%) | (N = 62), N(%) | (N = 63), N(%) | (N = 55), N(%) | (N = 44), N(%) | (N = 61), N(%) | |
| Expected number of assessments | 7 | 5 | 5 | 7 | 5 | 5 |
| Number of completed assessments a | ||||||
| 0 | 2 (3) | 0 | 1 (2) | 6 (11) | 0 | 1 (2) |
| 1 | 3 (5) | 3 (5) | 4 (6) | 0 | 0 | 1 (2) |
| 2 | 2 (3) | 3 (5) | 0 | 0 | 1 (2) | 1 (2) |
| 3 | 5 (8) | 5 (8) | 6 (10) | 1 (2) | 2 (5) | 5 (8) |
| 4 | 1 (2) | 16 (26) | 14 (22) | 1 (2) | 5 (11) | 7 (11) |
| 5 | 7 (11) | 35 (56) | 38 (60) | 4 (7) | 36 (82) | 46 (75) |
| 6 | 14 (22) | - | - | 9 (16) | - | - |
| 7b | 30 (47) | - | - | 34 (62) | - | - |
| Total number evaluable | 44 | 51 | 52 | 43 | 41 | 53 |
| CTCAE grade 1 and 2 symptoms | 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions |
|---|---|---|---|
| N (%)a | N (%)a | N (%)a | |
| Total evaluable | 43 | 41 | 53 |
| Grade 1 | |||
| Faint erythema | 42 (98) | 38 (93) | 47 (89) |
| Dry desquamation | 7 (16) | 1 (2) | 8 (15) |
| Grade 2 | |||
| Moderate to brisk erythema | 20 (47) | 11 (27) | 16 (30) |
| Patchy moist desquamation confined to skin folds/creases | 8 (19) | 2 (5) | 6 (11) |
| Moderate oedema | 2 (5) | 0 | 1 (2) |
Main Trial
| Form | Expected | Received | Percentage received |
|---|---|---|---|
| Randomisation checklist | 4107 | 4107 | 100 |
| Medical history | 4104 | 4104 | 100 |
| Baseline pathology | 4104 | 4104 | 100 |
| Primary surgery | 4104 | 4104 | 100 |
| Planned systemic treatment | 4103 | 4103 | 100 |
| RT | 4099 | 4099 | 100 |
| Year 1 follow-up | 4051 | 4045 | 99.8 |
| Year 2 follow-up | 3995 | 3984 | 99.7 |
| Year 3 follow-up | 3937 | 3850 | 99.2 |
| Year 4 follow-up | 3901 | 3723 | 98.7 |
| Year 5 follow-up | 3798 | 3733 | 98.0 |
| Year 6 follow-up | 3661 | 3486 | 95.2 |
| Year 7 follow-up | 3570 | 3273 | 91.7 |
| Medical history | 40 Gy/15 fractions | 27 Gy/5 fractions | 26 Gy/5 fractions |
|---|---|---|---|
| (N = 1361), (%) | (N = 1367), (%) | (N = 1368), (%) | |
| Current smoker | |||
| Yes | 201 (15) | 206 (15) | 203 (15) |
| No | 1159 (85) | 1161 (85) | 1162 (85) |
| Not known | 1 | 0 | 3 |
| Previous smoker (in past year) | N = 1159 | N = 1161 | N = 1162 |
| Yes | 83 (7) | 81 (7) | 69 (6) |
| No | 1073 (93) | 1077 (93) | 1086 (94) |
| Not known | 3 | 3 | 7 |
| History of cardiovascular disease | |||
| Yes | 94 (7) | 123 (9) | 87 (6) |
| No | 1267 (93) | 1244 (91) | 1279 (94) |
| Not known | 0 | 0 | 2 |
| Diabetes | |||
| Yes | 88 (6) | 95 (7) | 99 (7) |
| No | 1273 (94) | 1272 (93) | 1267 (93) |
| Not known | 0 | 0 | 2 |
| Hypertension | |||
| Yes | 360 (26) | 404 (29) | 407 (30) |
| No | 1000 (74) | 963 (71) | 959 (70) |
| Not known | 1 | 0 | 2 |
| Statin use | |||
| Yes | 220 (16) | 250 (18) | 259 (19) |
| No | 1140 (84) | 1117 (82) | 1106 (81) |
| Not known | 1 | 0 | 3 |
| ACE inhibitor use | |||
| Yes | 147 (11) | 162 (12) | 172 (13) |
| No | 1214 (89) | 1204 (88) | 1193 (87) |
| Not known | 0 | 1 | 3 |
| NTE | Year 1 | Year 2 | Year 3 | Year 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 1341), (%) | 27 Gy (N = 1350), (%) | 26 Gy (N = 1351), (%) | 40 Gy (N = 1319), (%) | 27 Gy (N = 1336), (%) | 26 Gy (N = 1327), (%) | 40 Gy (N = 1285), (%) | 27 Gy (N = 1300), (%) | 26 Gy (N = 1309), (%) | 40 Gy (N = 1243), (%) | 27 Gy (N = 1260), (%) | 26 Gy (N = 1261), (%) | |
| Any AE in breast/chest walla | ||||||||||||
| None | 592 (51) | 510 (43) | 587 (49) | 618 (54) | 547 (46) | 586 (50) | 587 (54) | 548 (49) | 581 (52) | 569 (54) | 497 (46) | 554 (51) |
| Mild | 421 (36) | 479 (40) | 465 (39) | 411 (36) | 445 (37) | 436 (37) | 383 (35) | 414 (37) | 405 (36) | 368 (35) | 412 (38) | 414 (38) |
| Moderate | 135 (12) | 175 (14) | 133 (11) | 114 (10) | 178 (15) | 115 (10) | 104 (10) | 140 (12) | 108 (10) | 101 (10) | 134 (13) | 108 (10) |
| Marked | 12 (1) | 34 (3) | 18 (1) | 8 (<1) | 29 (2) | 26 (2) | 7 (1) | 25 (2) | 24 (2) | 6 (1) | 28 (3) | 18 (2) |
| Not assessed | 181 | 152 | 148 | 168 | 137 | 164 | 204 | 173 | 191 | 199 | 189 | 167 |
| Breast distortion b | ||||||||||||
| None | 834 (77) | 837 (74) | 874 (77) | 818 (76) | 811 (72) | 816 (74) | 772 (76) | 780 (73) | 808 (77) | 723 (74) | 729 (72) | 776 (75) |
| Mild | 205 (19) | 218 (19) | 196 (17) | 203 (19) | 246 (22) | 225 (21) | 197 (19) | 228 (22) | 198 (19) | 210 (22) | 222 (22) | 208 (20) |
| Moderate | 38 (3) | 67 (6) | 54 (5) | 54 (5) | 63 (6) | 46 (4) | 43 (4) | 46 (4) | 35 (3) | 36 (4) | 46 (5) | 38 (4) |
| Marked | 4 (<1) | 9 (1) | 6 (<1) | 3 (<1) | 8 (<1) | 10 (1) | 2 (<1) | 9 (1) | 11 (1) | 3 (<1) | 12 (1) | 8 (1) |
| N/A | 79 | 72 | 73 | 75 | 71 | 70 | 71 | 69 | 68 | 71 | 65 | 64 |
| Not assessed | 181 | 147 | 148 | 166 | 137 | 160 | 200 | 168 | 189 | 200 | 186 | 167 |
| Breast shrinkage b | ||||||||||||
| None | 802 (74) | 795 (70) | 835 (74) | 776 (72) | 783 (69) | 801 (73) | 717 (71) | 765 (72) | 767 (73) | 682 (70) | 691 (69) | 721 (70) |
| Mild | 218 (20) | 230 (20) | 239 (21) | 241 (22) | 248 (22) | 228 (21) | 238 (23) | 207 (20) | 218 (21) | 234 (24) | 233 (23) | 250 (24) |
| Moderate | 56 (5) | 87 (8) | 49 (4) | 59 (5) | 81 (7) | 59 (5) | 56 (5) | 79 (7) | 58 (5) | 54 (6) | 70 (7) | 53 (5) |
| Marked | 5 (<1) | 17 (2) | 8 (1) | 3 (<1) | 15 (1) | 7 (<1) | 4 (<1) | 9 (1) | 9 (1) | 2 (<1) | 13 (1) | 6 (<1) |
| N/A | 79 | 72 | 73 | 75 | 71 | 70 | 71 | 69 | 68 | 71 | 65 | 64 |
| Not assessed | 181 | 149 | 147 | 165 | 138 | 162 | 199 | 171 | 189 | 199 | 188 | 167 |
| Breast induration (tumour bed) b | ||||||||||||
| None | 828 (77) | 824 (73) | 875 (78) | 857 (80) | 856 (76) | 858 (79) | 822 (82) | 828 (78) | 852 (81) | 795 (82) | 783 (78) | 816 (79) |
| Mild | 211 (19) | 242 (21) | 210 (19) | 184 (17) | 215 (19) | 185 (17) | 150 (15) | 187 (18) | 164 (16) | 150 (15) | 180 (18) | 177 (17) |
| Moderate | 40 (4) | 59 (5) | 34 (3) | 32 (3) | 47 (4) | 39 (4) | 33 (3) | 36 (3) | 27 (3) | 27 (3) | 39 (4) | 33 (3) |
| Marked | 2 (<1) | 4 (<1) | 8 (<1) | 2 (<1) | 11 (1) | 10 (<1) | 3 (<1) | 8 (1) | 9 (<1) | 1 (<1) | 7 (<1) | 3 (<1) |
| N/A | 79 | 72 | 73 | 75 | 71 | 70 | 71 | 69 | 68 | 71 | 65 | 64 |
| Not assessed | 181 | 149 | 151 | 169 | 136 | 165 | 206 | 172 | 189 | 199 | 186 | 168 |
| Breast induration (outside tumour bed) b | ||||||||||||
| None | 986 (91) | 990 (88) | 1005 (90) | 1012 (94) | 1031 (91) | 1014 (93) | 943 (93) | 976 (92) | 969 (92) | 909 (93) | 922 (92) | 935 (91) |
| Mild | 84 (8) | 103 (9) | 96 (9) | 52 (5) | 73 (7) | 66 (6) | 53 (5) | 64 (6) | 68 (6) | 59 (6) | 62 (6) | 74 (7) |
| Moderate | 10 (1) | 30 (3) | 16 (1) | 11 (1) | 16 (1) | 10 (1) | 11 (1) | 14 (1) | 8 (1) | 5 (<1) | 18 (2) | 16 (2) |
| Marked | 1 (<1) | 5 (<1) | 5 (<1) | 0 (0) | 9 (1) | 3 (<1) | 2 (<1) | 4 (<1) | 6 (1) | 1 (<1) | 5 (<1) | 3 (<1) |
| N/A | 79 | 72 | 73 | 75 | 71 | 70 | 71 | 69 | 68 | 71 | 65 | 64 |
| Not assessed | 181 | 150 | 156 | 169 | 136 | 164 | 205 | 173 | 190 | 198 | 188 | 169 |
| Telangiectasia | ||||||||||||
| None | 1107 (96) | 1130 (95) | 1143 (96) | 1090 (95) | 1092 (92) | 1074 (93) | 990 (93) | 1005 (90) | 1026 (92) | 963 (93) | 951 (89) | 1000 (92) |
| Mild | 38 (3) | 53 (4) | 48 (4) | 50 (4) | 80 (7) | 68 (6) | 71 (7) | 94 (8) | 61 (5) | 57 (5) | 93 (9) | 67 (6) |
| Moderate | 5 (<1) | 6 (<1) | 5 (<1) | 6 (<1) | 17 (1) | 13 (1) | 8 (1) | 17 (2) | 22 (2) | 16 (2) | 23 (2) | 17 (2) |
| Marked | 0 (0) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) | 3 (<1) | 0 (0) | 1 (<1) | 4 (<1) | 3 (<1) | 2 (<1) | 3 (<1) |
| Not assessed | 191 | 161 | 155 | 172 | 146 | 169 | 216 | 183 | 196 | 204 | 191 | 174 |
| Breast/chest wall oedema | ||||||||||||
| None | 969 (84) | 900 (75) | 970 (81) | 1030 (90) | 994 (83) | 1005 (86) | 995 (93) | 962 (86) | 1001 (89) | 973 (93) | 938 (88) | 996 (91) |
| Mild | 146 (13) | 217 (18) | 174 (15) | 96 (8) | 155 (13) | 130 (11) | 67 (6) | 127 (11) | 97 (9) | 60 (6) | 103 (10) | 74 (7) |
| Moderate | 38 (3) | 70 (6) | 50 (4) | 20 (2) | 41 (3) | 24 (2) | 8 (1) | 27 (2) | 16 (1) | 9 (1) | 22 (2) | 16 (1) |
| Marked | 4 (<1) | 9 (1) | 8 (<1) | 0 (0) | 7 (1) | 4 (<1) | 0 (0) | 6 (1) | 4 (<1) | 0 (0) | 5 (<1) | 2 (<1) |
| Not assessed | 184 | 154 | 149 | 173 | 139 | 164 | 215 | 178 | 191 | 201 | 192 | 173 |
| Breast/chest wall discomfort | ||||||||||||
| None | 837 (73) | 832 (70) | 869 (73) | 888 (77) | 890 (74) | 895 (77) | 857 (80) | 854 (76) | 881 (79) | 833 (80) | 830 (78) | 864 (79) |
| Mild | 251 (22) | 286 (24) | 267 (22) | 207 (18) | 255 (21) | 224 (19) | 185 (17) | 225 (20) | 202 (18) | 172 (17) | 195 (18) | 186 (17) |
| Moderate | 52 (4) | 65 (5) | 48 (4) | 41 (4) | 42 (3) | 39 (3) | 26 (2) | 44 (4) | 32 (3) | 26 (2) | 43 (4) | 36 (3) |
| Marked | 11 (1) | 3 (<1) | 10 (1) | 10 (1) | 8 (1) | 6 (1) | 6 (1) | 4 (<1) | 5 (<1) | 5 (<1) | 2 (<1) | 3 (<1) |
| Not assessed | 190 | 164 | 157 | 173 | 141 | 163 | 211 | 173 | 189 | 207 | 190 | 172 |
| Other RT-related | ||||||||||||
| None | 1116 (95) | 1147 (95) | 1156 (95) | 1107 (96) | 1151 (96) | 1137 (97) | 1049 (96) | 1103 (97) | 1098 (97) | 1015 (97) | 1049 (97) | 1058 (97) |
| Mild | 39 (3) | 46 (4) | 40 (3) | 37 (3) | 35 (3) | 23 (2) | 25 (2) | 15 (1) | 17 (1) | 20 (2) | 18 (2) | 25 (2) |
| Moderate | 14 (1) | 11 (1) | 13 (1) | 8 (1) | 8 (1) | 7 (<1) | 8 (1) | 10 (1) | 7 (1) | 10 (1) | 6 (1) | 9 (1) |
| Marked | 3 (<1) | 7 (<1) | 3 (<1) | 4 (<1) | 7 (<1) | 2 (<1) | 5 (<1) | 5 (<1) | 4 (<1) | 4 (<1) | 3 (<1) | 3 (<1) |
| Not assessed | 169 | 139 | 139 | 163 | 135 | 158 | 198 | 167 | 183 | 194 | 184 | 166 |
| Baseline | 3 months | 6 months | 12 months | 24 months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 584) | 27 Gy (N = 594) | 26 Gy (N = 591) | 40 Gy (N = 541) | 27 Gy (N = 563) | 26 Gy (N = 562) | 40 Gy (N = 530) | 27 Gy (N = 542) | 26 Gy (N = 552) | 40 Gy (N = 527) | 27 Gy (N = 532) | 26 Gy (N = 540) | 40 Gy (N = 506) | 27 Gy (N = 521) | 26 Gy (N = 514) | |
| Protocol-specific items | |||||||||||||||
| Breast appearance changed | |||||||||||||||
| None | 96 (17) | 133 (23) | 133 (23) | 78 (15) | 85 (15) | 95 (17) | 73 (14) | 60 (11) | 94 (17) | 62 (12) | 55 (11) | 86 (16) | 75 (15) | 83 (16) | 105 (21) |
| Mild | 307 (54) | 273 (47) | 293 (50) | 287 (54) | 276 (50) | 291 (53) | 278 (54) | 274 (52) | 277 (51) | 293 (57) | 266 (51) | 291 (55) | 264 (54) | 240 (47) | 261 (52) |
| Moderate | 104 (18) | 104 (18) | 86 (15) | 107 (20) | 120 (22) | 107 (19) | 94 (18) | 110 (21) | 117 (21) | 95 (19) | 121 (23) | 105 (20) | 99 (20) | 108 (21) | 83 (16) |
| Marked | 66 (11) | 73 (12) | 69 (12) | 57 (11) | 71 (13) | 61 (11) | 70 (14) | 86 (16) | 57 (11) | 63 (12) | 77 (15) | 47 (9) | 53 (11) | 78 (15) | 56 (11) |
| Missing | 11 | 11 | 10 | 12 | 11 | 8 | 15 | 12 | 7 | 14 | 13 | 11 | 15 | 12 | 8 |
| Breast smaller | |||||||||||||||
| None | 249 (44) | 264 (46) | 290 (50) | 211 (41) | 272 (50) | 276 (50) | 201 (40) | 239 (46) | 255 (47) | 179 (35) | 210 (41) | 234 (45) | 171 (35) | 202 (40) | 225 (45) |
| Mild | 215 (38) | 206 (36) | 194 (34) | 206 (40) | 172 (32) | 182 (33) | 182 (36) | 165 (31) | 178 (33) | 204 (40) | 175 (34) | 180 (34) | 200 (41) | 165 (33) | 171 (34) |
| Moderate | 49 (9) | 64 (11) | 41 (7) | 58 (11) | 57 (10) | 51 (9) | 76 (15) | 64 (12) | 62 (12) | 65 (13) | 72 (14) | 65 (12) | 65 (13) | 77 (15) | 54 (11) |
| Marked | 47 (8) | 42 (7) | 49 (9) | 43 (8) | 43 (8) | 40 (7) | 48 (9) | 57 (11) | 44 (8) | 57 (11) | 56 (11) | 45 (9) | 51 (11) | 59 (12) | 51 (10) |
| Missing | 24 | 28 | 17 | 23 | 19 | 13 | 23 | 17 | 13 | 22 | 19 | 16 | 19 | 18 | 13 |
| Breast harder/firmer | |||||||||||||||
| None | 246 (44) | 255 (44) | 249 (44) | 168 (32) | 176 (32) | 178 (33) | 170 (33) | 135 (26) | 160 (30) | 188 (37) | 141 (27) | 174 (33) | 217 (45) | 194 (39) | 223 (44) |
| Mild | 218 (39) | 212 (37) | 222 (39) | 227 (44) | 220 (41) | 233 (43) | 219 (43) | 209 (40) | 209 (39) | 205 (41) | 209 (41) | 202 (39) | 186 (39) | 198 (39) | 178 (35) |
| Moderate | 70 (13) | 78 (14) | 64 (11) | 86 (17) | 89 (16) | 79 (14) | 78 (15) | 106 (20) | 108 (20) | 73 (15) | 107 (21) | 97 (19) | 55 (11) | 65 (13) | 68 (13) |
| Marked | 24 (4) | 27 (5) | 31 (5) | 39 (7) | 57 (11) | 56 (10) | 43 (8) | 70 (13) | 61 (11) | 39 (8) | 58 (11) | 48 (9) | 25 (5) | 46 (9) | 35 (7) |
| Missing | 26 | 22 | 25 | 21 | 21 | 16 | 20 | 22 | 14 | 22 | 17 | 19 | 23 | 18 | 10 |
| Skin appearance changed | |||||||||||||||
| None | 246 (43) | 284 (48) | 270 (46) | 203 (38) | 204 (37) | 211 (38) | 201 (38) | 200 (38) | 218 (40) | 214 (41) | 217 (41) | 245 (46) | 263 (53) | 253 (49) | 271 (54) |
| Mild | 253 (44) | 241 (41) | 243 (42) | 237 (44) | 265 (48) | 257 (46) | 236 (45) | 244 (46) | 239 (44) | 234 (45) | 220 (42) | 222 (42) | 188 (38) | 188 (37) | 186 (37) |
| Moderate | 62 (11) | 46 (8) | 50 (9) | 67 (13) | 59 (11) | 59 (11) | 55 (11) | 60 (11) | 63 (11) | 48 (9) | 66 (13) | 53 (10) | 31 (6) | 52 (10) | 32 (6) |
| Marked | 16 (3) | 15 (3) | 17 (3) | 28 (5) | 28 (5) | 31 (5) | 31 (6) | 29 (5) | 26 (5) | 22 (4) | 23 (4) | 14 (3) | 13 (3) | 19 (4) | 18 (4) |
| Missing | 7 | 8 | 11 | 6 | 7 | 4 | 7 | 9 | 6 | 9 | 6 | 6 | 11 | 9 | 7 |
| EORTC QLQ-BR23 items | |||||||||||||||
| Breast pain | |||||||||||||||
| None | 223 (38) | 250 (42) | 220 (37) | 130 (24) | 124 (22) | 130 (23) | 154 (29) | 137 (26) | 142 (26) | 182 (35) | 176 (33) | 189 (35) | 205 (41) | 198 (38) | 193 (38) |
| Mild | 307 (53) | 298 (51) | 315 (54) | 318 (59) | 315 (56) | 313 (56) | 300 (57) | 287 (53) | 302 (55) | 281 (53) | 279 (53) | 269 (50) | 241 (48) | 257 (50) | 260 (51) |
| Moderate | 44 (7) | 36 (6) | 45 (8) | 73 (14) | 100 (18) | 93 (17) | 59 (11) | 91 (17) | 88 (16) | 51 (10) | 61 (11) | 65 (12) | 47 (9) | 46 (9) | 46 (9) |
| Marked | 9 (2) | 6 (1) | 8 (1) | 17 (3) | 23 (4) | 24 (4) | 14 (3) | 27 (5) | 20 (4) | 12 (2) | 15 (3) | 15 (3) | 12 (2) | 16 (3) | 12 (2) |
| Missing | 1 | 4 | 3 | 3 | 1 | 2 | 3 | 0 | 0 | 1 | 1 | 2 | 1 | 4 | 3 |
| Breast swollen | |||||||||||||||
| None | 360 (62) | 375 (64) | 360 (61) | 375 (70) | 344 (61) | 359 (64) | 369 (70) | 319 (59) | 352 (64) | 418 (79) | 362 (68) | 388 (72) | 428 (85) | 379 (74) | 406 (79) |
| Mild | 167 (29) | 171 (29) | 182 (31) | 120 (22) | 151 (27) | 138 (25) | 125 (24) | 156 (29) | 138 (25) | 83 (16) | 120 (23) | 127 (24) | 65 (13) | 105 (20) | 83 (16) |
| Moderate | 40 (7) | 35 (6) | 42 (7) | 31 (6) | 51 (9) | 39 (7) | 19 (4) | 50 (9) | 49 (9) | 14 (3) | 34 (6) | 19 (3) | 9 (2) | 23 (4) | 18 (4) |
| Marked | 16 (3) | 8 (1) | 5 (1) | 12 (2) | 16 (3) | 24 (4) | 14 (3) | 17 (3) | 13 (2) | 12 (2) | 14 (3) | 5 (1) | 4 (1) | 9 (2) | 6 (1) |
| Missing | 1 | 5 | 2 | 3 | 1 | 2 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 5 | 1 |
| Breast oversensitive | |||||||||||||||
| None | 307 (53) | 312 (53) | 283 (48) | 238 (44) | 213 (38) | 214 (38) | 238 (45) | 197 (37) | 224 (41) | 268 (51) | 247 (47) | 253 (47) | 265 (53) | 255 (49) | 268 (52) |
| Mild | 215 (37) | 230 (39) | 241 (41) | 216 (40) | 262 (47) | 256 (46) | 225 (43) | 257 (48) | 244 (44) | 209 (40) | 216 (41) | 224 (42) | 189 (38) | 206 (40) | 197 (39) |
| Moderate | 41 (7) | 29 (5) | 53 (9) | 67 (13) | 61 (11) | 61 (11) | 50 (9) | 62 (11) | 59 (11) | 34 (6) | 55 (10) | 42 (8) | 32 (6) | 40 (8) | 34 (7) |
| Marked | 16 (3) | 13 (2) | 9 (2) | 15 (3) | 26 (5) | 27 (5) | 15 (3) | 23 (4) | 21 (4) | 14 (3) | 13 (2) | 17 (3) | 17 (3) | 16 (3) | 11 (2) |
| Missing | 5 | 10 | 5 | 5 | 1 | 4 | 2 | 3 | 4 | 2 | 1 | 4 | 3 | 4 | 4 |
| Skin problems on breast | |||||||||||||||
| None | 457 (78) | 471 (80) | 462 (78) | 360 (67) | 374 (66) | 373 (67) | 369 (70) | 379 (70) | 396 (72) | 387 (73) | 369 (70) | 408 (76) | 403 (80) | 389 (75) | 417 (82) |
| Mild | 99 (17) | 95 (16) | 110 (19) | 134 (25) | 140 (25) | 142 (25) | 121 (23) | 106 (20) | 112 (20) | 109 (21) | 118 (22) | 96 (18) | 78 (15) | 90 (17) | 71 (14) |
| Moderate | 21 (4) | 18 (3) | 11 (2) | 30 (6) | 36 (6) | 31 (6) | 22 (4) | 38 (7) | 30 (5) | 20 (4) | 29 (5) | 23 (4) | 14 (3) | 22 (4) | 16 (3) |
| Marked | 5 (1) | 6 (1) | 7 (1) | 15 (3) | 13 (2) | 13 (2) | 16 (3) | 16 (3) | 14 (3) | 11 (2) | 14 (3) | 9 (2) | 9 (2) | 15 (3) | 7 (1) |
| Missing | 2 | 4 | 1 | 2 | 0 | 3 | 2 | 3 | 0 | 0 | 2 | 4 | 2 | 5 | 3 |
| Arm/shoulder pain | |||||||||||||||
| None | 279 (48) | 296 (50) | 290 (49) | 224 (42) | 217 (39) | 218 (39) | 229 (43) | 212 (39) | 212 (38) | 250 (48) | 253 (48) | 262 (49) | 254 (50) | 261 (50) | 255 (50) |
| Mild | 237 (41) | 217 (37) | 218 (37) | 213 (40) | 233 (42) | 239 (43) | 212 (40) | 221 (41) | 241 (44) | 206 (39) | 206 (39) | 185 (34) | 177 (35) | 165 (32) | 166 (32) |
| Moderate | 43 (7) | 63 (11) | 63 (11) | 78 (14) | 83 (15) | 81 (14) | 65 (12) | 80 (15) | 75 (14) | 52 (10) | 60 (11) | 64 (12) | 55 (11) | 63 (12) | 68 (13) |
| Marked | 23 (4) | 15 (2) | 18 (3) | 24 (4) | 27 (5) | 23 (4) | 20 (4) | 29 (5) | 24 (4) | 18 (3) | 12 (2) | 26 (5) | 20 (4) | 30 (6) | 24 (5) |
| Missing | 2 | 3 | 2 | 2 | 3 | 1 | 4 | 0 | 0 | 1 | 1 | 3 | 0 | 2 | 1 |
| Arm/hand swollen | |||||||||||||||
| None | 501 (86) | 516 (88) | 507 (86) | 451 (84) | 473 (84) | 477 (85) | 447 (85) | 446 (82) | 464 (84) | 448 (85) | 449 (85) | 445 (83) | 428 (85) | 430 (83) | 431 (84) |
| Mild | 57 (10) | 55 (9) | 61 (10) | 68 (13) | 67 (12) | 58 (10) | 61 (11) | 72 (13) | 61 (11) | 61 (12) | 65 (12) | 64 (12) | 53 (11) | 66 (13) | 55 (11) |
| Moderate | 14 (2) | 11 (2) | 19 (3) | 15 (3) | 19 (3) | 20 (4) | 12 (2) | 19 (4) | 16 (3) | 6 (1) | 12 (2) | 16 (3) | 15 (3) | 18 (3) | 17 (3) |
| Marked | 10 (2) | 6 (1) | 3 (1) | 5 (1) | 3 (1) | 4 (1) | 9 (2) | 4 (1) | 9 (2) | 10 (2) | 5 (1) | 11 (2) | 7 (1) | 6 (1) | 9 (2) |
| Missing | 2 | 6 | 1 | 2 | 1 | 3 | 1 | 1 | 2 | 2 | 1 | 4 | 3 | 1 | 2 |
| Difficulty raising arm | |||||||||||||||
| None | 429 (74) | 443 (75) | 422 (72) | 354 (66) | 361 (64) | 379 (68) | 345 (65) | 313 (58) | 347 (63) | 381 (73) | 376 (71) | 383 (71) | 379 (75) | 363 (70) | 375 (74) |
| Mild | 126 (22) | 110 (19) | 128 (22) | 143 (27) | 155 (28) | 137 (24) | 142 (27) | 171 (32) | 160 (29) | 118 (22) | 125 (24) | 117 (22) | 95 (19) | 113 (22) | 104 (20) |
| Moderate | 15 (3) | 25 (4) | 28 (5) | 29 (5) | 36 (6) | 37 (7) | 28 (5) | 40 (7) | 38 (7) | 17 (3) | 27 (5) | 29 (5) | 22 (4) | 28 (5) | 21 (4) |
| Marked | 12 (2) | 11 (2) | 9 (1) | 9 (2) | 8 (1) | 7 (1) | 13 (3) | 17 (3) | 7 (1) | 9 (2) | 3 (1) | 9 (2) | 8 (2) | 16 (3) | 10 (2) |
| Missing | 2 | 5 | 4 | 6 | 3 | 2 | 2 | 1 | 0 | 2 | 1 | 2 | 2 | 1 | 4 |
| Subgroup | IBTR | Regional relapse | Distant relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 Gy/15 fractions (N = 31) | 27 Gy/5 fractions (N = 27) | 26 Gy/5 fractions (N = 21) | 40 Gy/15 fractions (N = 13) | 27 Gy/5 fractions (N = 11) | 26 Gy/5 fractions (N = 10) | 40 Gy/15 fractions (N = 59) | 27 Gy/5 fractions (N = 69) | 26 Gy/5 fractions (N = 76) | |
| Age at randomisation (years) | |||||||||
| < 50 | 3 | 7 | 4 | 6 | 4 | 2 | 8 | 20 | 18 |
| ≥ 50 | 28 | 20 | 17 | 7 | 7 | 8 | 51 | 49 | 58 |
| Grade | |||||||||
| 1 | 2 | 3 | 4 | 1 | 0 | 0 | 2 | 2 | 2 |
| 2 | 9 | 9 | 9 | 7 | 3 | 4 | 20 | 24 | 30 |
| 3 | 20 | 15 | 8 | 5 | 8 | 6 | 37 | 43 | 44 |
| ER/HER-2 status | |||||||||
| ER+/HER-2+ | 3 | 4 | 1 | 0 | 2 | 1 | 6 | 7 | 9 |
| ER+/HER-2− | 17 | 15 | 16 | 9 | 3 | 4 | 36 | 44 | 42 |
| ER−/HER-2+ | 1 | 3 | 1 | 0 | 3 | 0 | 0 | 5 | 5 |
| ER−/HER-2− | 10 | 5 | 3 | 3 | 3 | 5 | 16 | 13 | 18 |
| Unknown | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
Denominators representing numbers at risk for subgroups are in Table 4.
Main Trial cost-effectiveness analysis
| Model | Constant | Subgroup 2 | Treatment | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Exponential | −4.9097 | 0.1736 | −0.6557 | 0.2566 | −0.3878 | 0.2414 |
| Exponential variance-covariance matrix | |||
|---|---|---|---|
| Treatment | Subgroup 2 | Constant | |
| Treatment | 0.0582923 | ||
| Subgroup 2 | 0.00001 | 0.065863 | |
| Constant | −0.023813 | 0.020412 | 0.030136 |
| Model | Constant | Subgroup 2 | Treatment | Sigma | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Log-normal | 5.7267 | 0.5110 | 0.7109 | 0.2649 | 0.4814 | 0.2533 | 0.8032 | 0.1141 |
| Log-normal variance-covariance matrix | ||||
|---|---|---|---|---|
| Treatment | Subgroup 2 | Constant | Sigma | |
| Treatment | 0.064143 | - | - | - |
| Subgroup 2 | 0.0038388 | 0.070187 | - | - |
| Constant | 0.0030285 | 0.007884 | 0.2611 | - |
| Sigma | 0.0066437 | 0.007125 | 0.0546 | 0.01302 |
| Model | Constant | Subgroup 2 | Treatment | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Exponential | −4.3506 | 0.1347 | −1.6704 | 0.2475 | 0.2429 | 0.1735 |
| Exponential variance-covariance matrix | |||
|---|---|---|---|
| Treatment | Subgroup 2 | Constant | |
| Treatment | 0.0301071 | ||
| Subgroup 2 | −0.00007 | 0.061252 | |
| Constant | −0.01694 | −0.008583 | 0.018152 |
| Model | Constant | Subgroup 2 | Treatment | Sigma | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Log-normal | 5.0030 | 0.3101 | 1.5875 | 0.2485 | −0.2730 | 0.1862 | 0.7769 | 0.0775 |
| Log-normal variance-covariance matrix | ||||
|---|---|---|---|---|
| Treatment | Subgroup 2 | Constant | Sigma | |
| Treatment | 0.034668 | - | - | - |
| Subgroup 2 | −0.00332 | 0.061755 | - | - |
| Constant | −0.0242 | 0.019531 | 0.096166 | - |
| Sigma | −0.00162 | 0.008413 | 0.021288 | 0.006011 |
| Resource use questionnaire | |||
|---|---|---|---|
| Question | Unit cost | Explanation | Source |
| How many times have you been visited by your GP for any reason? | £167.99 | GP visit unit cost is £134.89 which includes travel time. Curtis et al., 2010 (PSSRU) used as not reported in more recent publications. Add to this average prescription cost per consultation £33.10 (Curtis et al., 2019). | 139 p167 (inflated),69 p119 |
| How many times have you visited your GP for any reason? | £72.10 | GP surgery consultation unit cost £39 plus average prescription costs per visit £33.10. | 69 p119 |
| How many times have you been visited by a district nurse? | £32.50 | District Nurse cost £44.22 per hour according to Curtis et al., 2018 (PSSRU) (inflated), Curtis et al., 2010 (PSSRU) reports face to face home visit for community nurse (includes district nurse) takes 20 minutes. Ratio of indirect to direct time for home visits is reported to be 1 : 1.21 resulting in total time 44.2 mins and cost £30.76 (this includes travel time). Curtis et al., 2010 (PSSRU) also reported travel costs of £1.74 (inflated) per visit. | 140 p18;139 p159 |
| How many times have you been visited by a MacMillan nurse? | £23.10 | Nurses cost £29 per hour according to MacMillan (2019) costing document. Curtis et al., 2010 (PSSRU) report face-to-face home visit for community nurse takes 20 minutes. Ratio of indirect to direct time for home visits is reported to be 1 : 1.21 resulting in total time 44.2 mins and cost £21.36 (this includes travel time). Curtis et al., 2010 (PSSRU) also reported travel costs of £1.74 (inflated) per visit. | 139,141 p159 |
| How many days have you spent in hospital related to your breast cancer? | £378.90 | Activity weighted average of elective and non-elective cost per day for Malignant Breast Disorders with and without interventions reported in 2018/19 reference costs. Includes elective inpatients, Non-elective inpatients, non-elective short stay, day case and regular day or night admissions. | 79 JA12D, JA12E, JA12F, JA12G, JA12H, JA12J, JA12K, JA12L |
| How many days have you spent in hospital for other reasons? | £568.88 | Activity weighted average of elective and non-elective inpatient costs per day reported in 2018/19 reference costs. | 72 |
| How many hospital outpatient visits have you had related to breast cancer? | £94.93 | Activity weighted average of outpatient procedure costs in clinical oncology (previously RT) reported in 2018/19 reference costs. | 72 |
| How many hospital outpatient visits have you had for other reasons? | £148.00 | Weighted average of cost for all outpatient attendances reported in 2018/19 reference costs. | 72 |
| Variable | Alive and disease free | ||||
|---|---|---|---|---|---|
| Obs | Mean | SE | Min | Max | |
| Three-month total costs | |||||
| Month 6 | 1035 | £556 | £1293 | £0 | £24,255 |
| Year 1 | 1030 | £340 | £445 | £0 | £4682 |
| Year 2 | 972 | £329 | £638 | £0 | £13,720 |
| Year 5 | 821 | £295 | £533 | £0 | £7991 |
| Variable | Alive and disease free | ||||
|---|---|---|---|---|---|
| Obs | Mean | SE | Min | Max | |
| EQ-5D-3L utility score (cross-walked) | |||||
| Baseline | 1146 | 0.800 | 0.174 | −0.209 | 1 |
| Month 3 | 1044 | 0.778 | 0.180 | −0.181 | 1 |
| Month 6 | 1052 | 0.770 | 0.186 | −0.594 | 1 |
| Year 1 | 1050 | 0.788 | 0.191 | −0.594 | 1 |
| Year 2 | 974 | 0.777 | 0.196 | −0.161 | 1 |
| Year 5 | 811 | 0.782 | 0.215 | −0.594 | 1 |
Nodal Sub-Study
| Arm/shoulder/hand effect | All randomised patients | Patients randomised up to end 2017 | ||||||
|---|---|---|---|---|---|---|---|---|
| 40 Gy (N = 181) | 26 Gy (N = 182) | 40 Gy (N = 108) | 27 Gy (N = 103) | |||||
| Axillary sampling (N = 74), (%) | Axillary clearance (N = 107), (%) | Axillary sampling (N = 90), (%) | Axillary clearance (N = 91), (%) | Axillary sampling (N = 41), (%) | Axillary clearance (N = 67), (%) | Axillary sampling (N = 48), (%) | Axillary clearance (N = 56), (%) | |
| Swollen arm or hand (EORTC QLQ-BR23) | ||||||||
| Not at all | 61 (84) | 83 (78) | 78 (87) | 61 (68) | 34 (83) | 53 (79) | 39 (83) | 48 (86) |
| A little | 9 (12) | 20 (19) | 6 (7) | 20 (22) | 5 (12) | 10 (152) | 6 (13) | 7 (13) |
| Quite a bit | 1 (1) | 3 (3) | 3 (3) | 5 (6) | 0 (0) | 3 (4) | 2 (4) | 1 (2) |
| Very much | 2 (3) | 1 (<1) | 3 (3) | 4 (4) | 2 (5) | 1 (1) | 0 (0) | 0 (0) |
| Missing | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Pain in arm or shoulder (EORTC QLQ-BR23) | ||||||||
| Not at all | 38 (52) | 27 (25) | 41 (46) | 31 (35) | 21 (52) | 15 (22) | 22 (47) | 16 (29) |
| A little | 27 (37) | 62 (58) | 33 (37) | 34 (38) | 14 (34) | 37 (55) | 15 (32) | 30 (54) |
| Quite a bit | 5 (7) | 15 (14) | 11 (12) | 16 (18) | 4 (10) | 13 (19) | 10 (21) | 7 (13) |
| Very much | 3 (4) | 3 (3) | 5 (6) | 8 (9) | 2 (5) | 2 (3) | 0 (0) | 3 (5) |
| Missing | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Difficult to raise arm or move it sideways (EORTC QLQ-BR23) | ||||||||
| Not at all | 60 (82) | 53 (50) | 60 (67) | 45 (50) | 33 (80) | 28 (42) | 28 (60) | 26 (46) |
| A little | 9 (12) | 42 (40) | 27 (33) | 28 (31) | 6 (15) | 31 (47) | 13 (28) | 24 (43) |
| Quite a bit | 4 (5) | 8 (8) | 4 (4) | 12 (13) | 2 (5) | 6 (9) | 6 (13) | 4 (7) |
| Very much | 0 (0) | 3 (3) | 2 (2) | 5 (6) | 0 (0) | 1 (2) | 0 (0) | 2 (4) |
| Missing | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Shoulder stiffness | ||||||||
| Not at all | 63 (86) | 60 (56) | 62 (70) | 53 (60) | 36 (88) | 39 (58) | 30 (64) | 29 (52) |
| A little | 9 (12) | 40 (37) | 23 (26) | 25 (28) | 5 (12) | 24 (36) | 12 (26) | 22 (39) |
| Quite a bit | 1 (1) | 5 (5) | 4 (4) | 8 (9) | 0 (0) | 3 (4) | 5 (11) | 4 (7) |
| Very much | 0 (0) | 2 (2) | 0 (0) | 3 (3) | 0 (0) | 1 (1) | 0 (0) | 1 (2) |
| Missing | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0 |
| Pins and needles in arm/hand on affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 66 (92) | 62 (58) | 69 (78) | 59 (67) | 36 (88) | 42 (63) | 37 (79) | 37 (66) |
| A little | 3 (4) | 35 (33) | 14 (16) | 26 (30) | 3 (7) | 18 (27) | 9 (19) | 17 (30) |
| Quite a bit | 2 (3) | 5 (5) | 6 (8) | 0 (0) | 1 (2) | 2 (3) | 0 (0) | 1 (2) |
| Very much | 1 (1) | 5 (5) | 0 (0) | 3 (3) | 1 (2) | 5 (7) | 1 (2) | 1 (2) |
| Missing | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0 |
| Pins and needles in arm/hand on non-affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 68 (94) | 84 (79) | 73 (82) | 72 (81) | 38 (93) | 56 (84) | 37 (79) | 51 (91) |
| A little | 1 (1) | 17 (16) | 14 (16) | 12 (14) | 1 (2) | 7 (10) | 8 (17) | 5 (9) |
| Quite a bit | 2 (3) | 1 (< 0) | 2 (2) | 4 (5) | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
| Very much | 1 (1) | 5 | 0 (0) | 0 (0) | 1 (2) | 4 (6) | 1 (2) | 0 (0) |
| Missing | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Numbness in fingers on affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 65 (90) | 80 (75) | 72 (81) | 64 (73) | 37 (90) | 49 (73) | 37 (79) | 35 (63) |
| A little | 3 (4) | 13 (12) | 11 (12) | 22 (25) | 3 (7) | 7 (10) | 8 (17) | 16 (29) |
| Quite a bit | 3 (4) | 7 (7) | 6 (7) | 2 (2) | 1 (2) | 6 (9) | 0 (2) | 3 (5) |
| Very much | 1 (1) | 7 (7) | 0 (0) | 0 (0) | 0 (0) | 5 (7) | 2 (4) | 2 (4) |
| Missing | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0 |
| Numbness in fingers on non-affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 63 (88) | 83 (76) | 73 (82) | 75 (85) | 36 (88) | 53 (79) | 38 (81) | 41 (73) |
| A little | 4 (6) | 10 (9) | 11 (12) | 17 (13) | 4 (10) | 5 (7) | 8 (17) | 12 (21) |
| Quite a bit | 4 (6) | 6 (6) | 5 (6) | 1 (1) | 1 (2) | 4 (6) | 0 (0) | 1 (2) |
| Very much | 1 (1) | 8 (7) | 0 (0) | 1 (1) | 0 (0) | 5 (7) | 1 (2) | 2 (4) |
| Missing | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0 |
| Weakness in arm/hand on affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 58 (81) | 54 (50) | 67 (75) | 51 (59) | 31 (76) | 33 (49) | 32 (68) | 38 (68) |
| A little | 9 (13) | 42 (39) | 18 (20) | 27 (31) | 6 (15) | 26 (39) | 12 (26) | 14 (25) |
| Quite a bit | 5 (6) | 7 (7) | 2 (2) | 7 (8) | 4 (10) | 4 (6) | 2 (4) | 3 (5) |
| Very much | 0 (0) | 4 (4) | 2 (2) | 2 (2) | 0 (0) | 4 (6) | 1 (2) | 1 (2) |
| Missing | 2 | 0 | 1 | 4 | 0 | 0 | 1 | 0 |
| Weakness in arm/hand on non-affected side (protocol-specific item 1 ) | ||||||||
| Not at all | 62 (86) | 97 (91) | 74 (83) | 80 (91) | 35 (85) | 58 (87) | 39 (83) | 52 (93) |
| A little | 6 (8) | 8 (7) | 12 (13) | 8 (9) | 2 (5) | 7 (10) | 6 (13) | 2 (4) |
| Quite a bit | 4 (6) | 0 (0) | 2 (2) | 0 (0) | 4 (10) | 0 (0) | 1 (2) | 2 (4) |
| Very much | 0 (0) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 2 (3) | 1 (2) | 0 (0) |
| Missing | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0 |
Discussion
| Normal tissue effect | Clinician or patient assessed | Moderate or marked events in 40 Gy at 5 years (%) | Moderate or marked events in 26 Gy at 5 years (%) | OR comparison with 40 Gy across follow-upa (95% CI) | p-value comparison with 40 Gyb |
|---|---|---|---|---|---|
| Breast distortion | Clinician | 32/916 (3.5) | 53/955 (5.5) | 1.20 (0.91 to .60) | 0.19 |
| Breast shrinkage | Clinician | 50/916 (5.5) | 65/954 (6.8) | 1.05 (0.82 to 1.33) | 0.71 |
| Breast induration outside tumour bed | Clinician | 1/911 (0.1) | 20/955 (2.1) | 1.90 (1.15 to 3.14) | 0.013 |
| Breast appearance changed | Patient | 140/432 (32.4) | 136/429 (31.7) | 0.91 (0.75 to 1.10) | 0.33 |
| Breast smaller | Patient | 122/428 (28.5) | 103/429 (24.0) | 0.81 (0.65 to 1.00) | 0.053 |
| Breast harder or firmer | Patient | 61/428 (14.2) | 74/425 (17.4) | 1.22 (1.00 to 1.48) | 0.048 |
Appendix 3 Additional figures
Main Trial
FIGURE 20.
Recruitment by centre in FAST-Forward Main Trial.
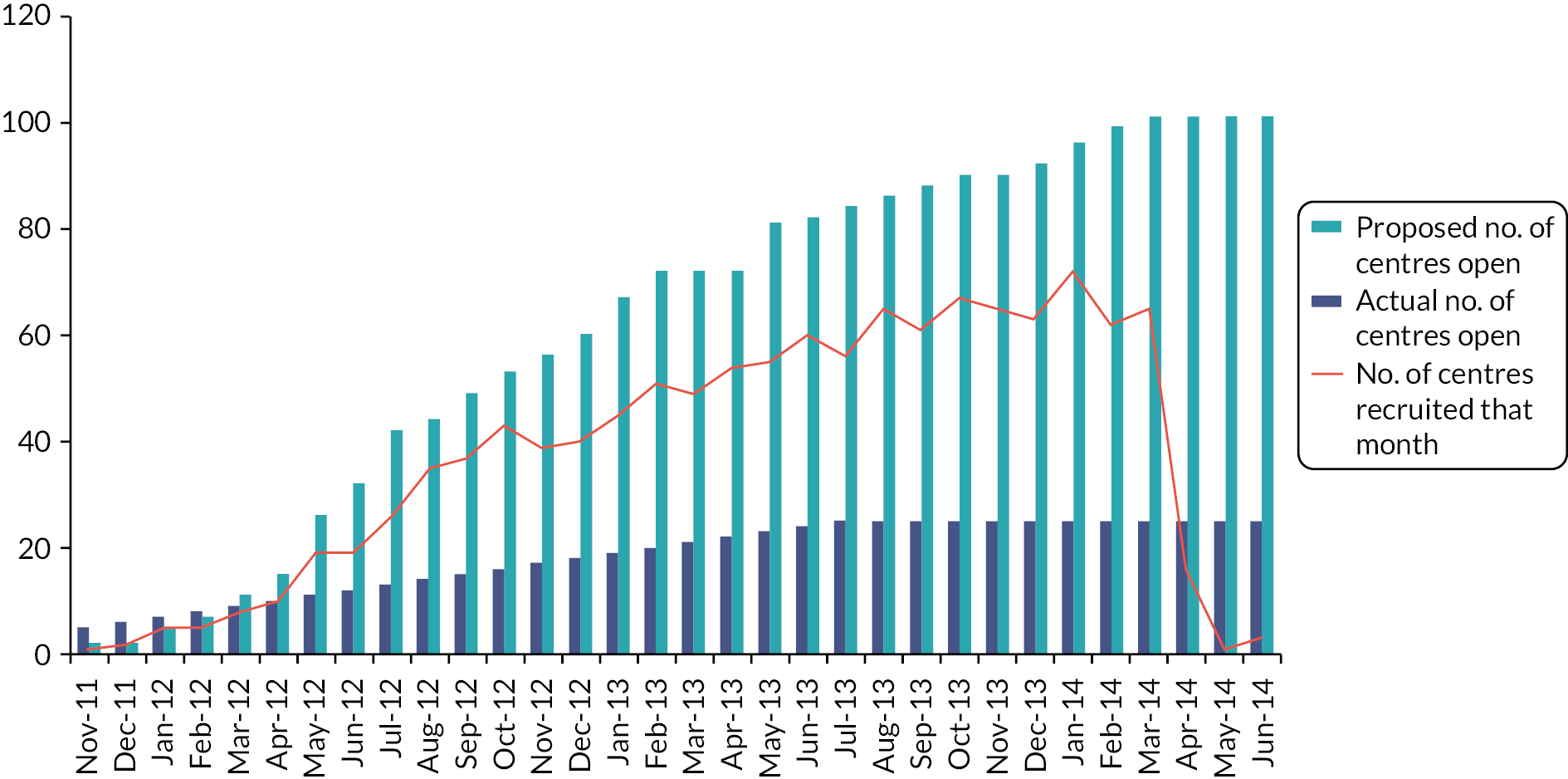
FIGURE 21.
Kaplan–Meier plot of locoregional relapse in Main Trial.
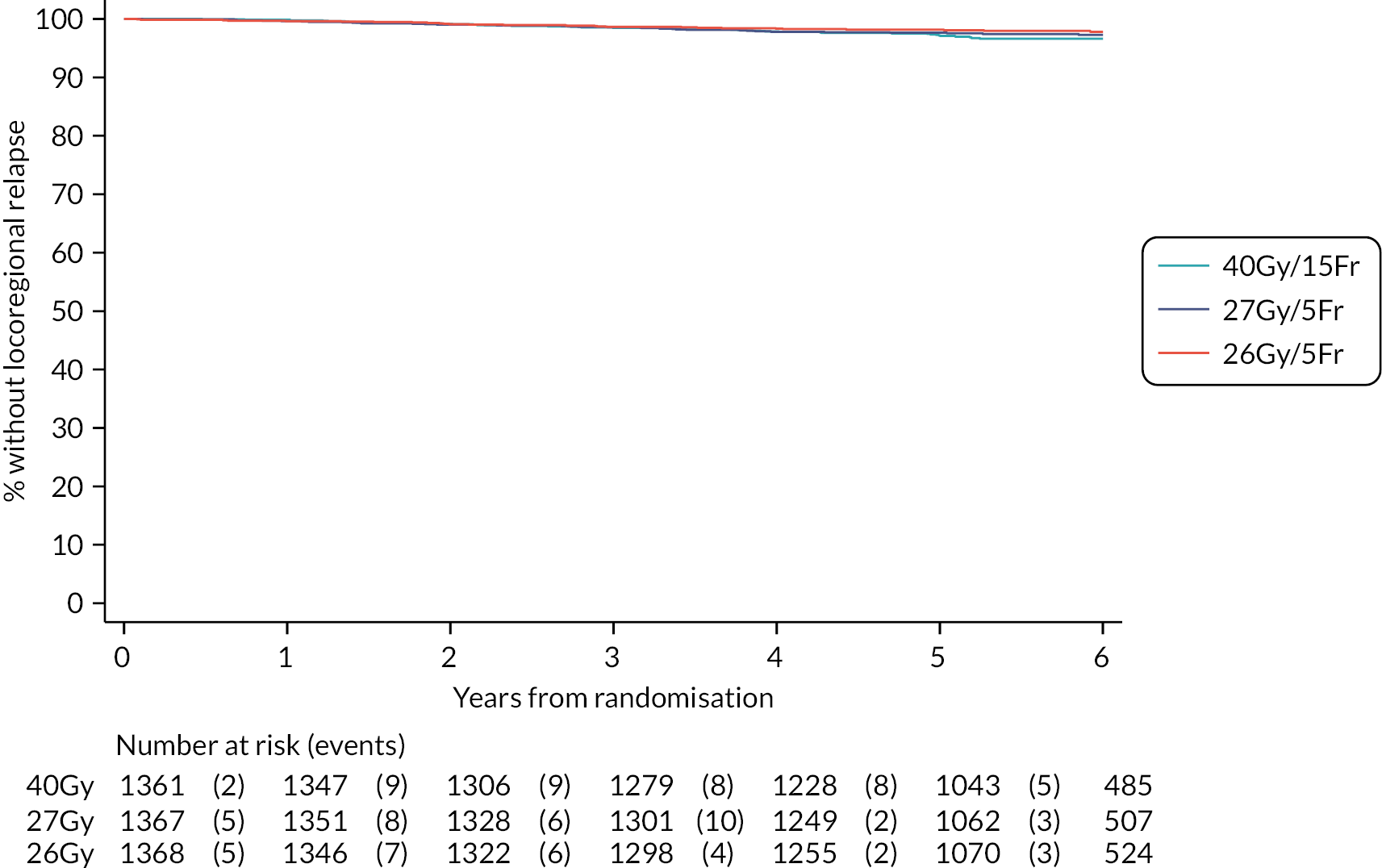
FIGURE 22.
Cumulative risk of locoregional relapse in Main Trial.
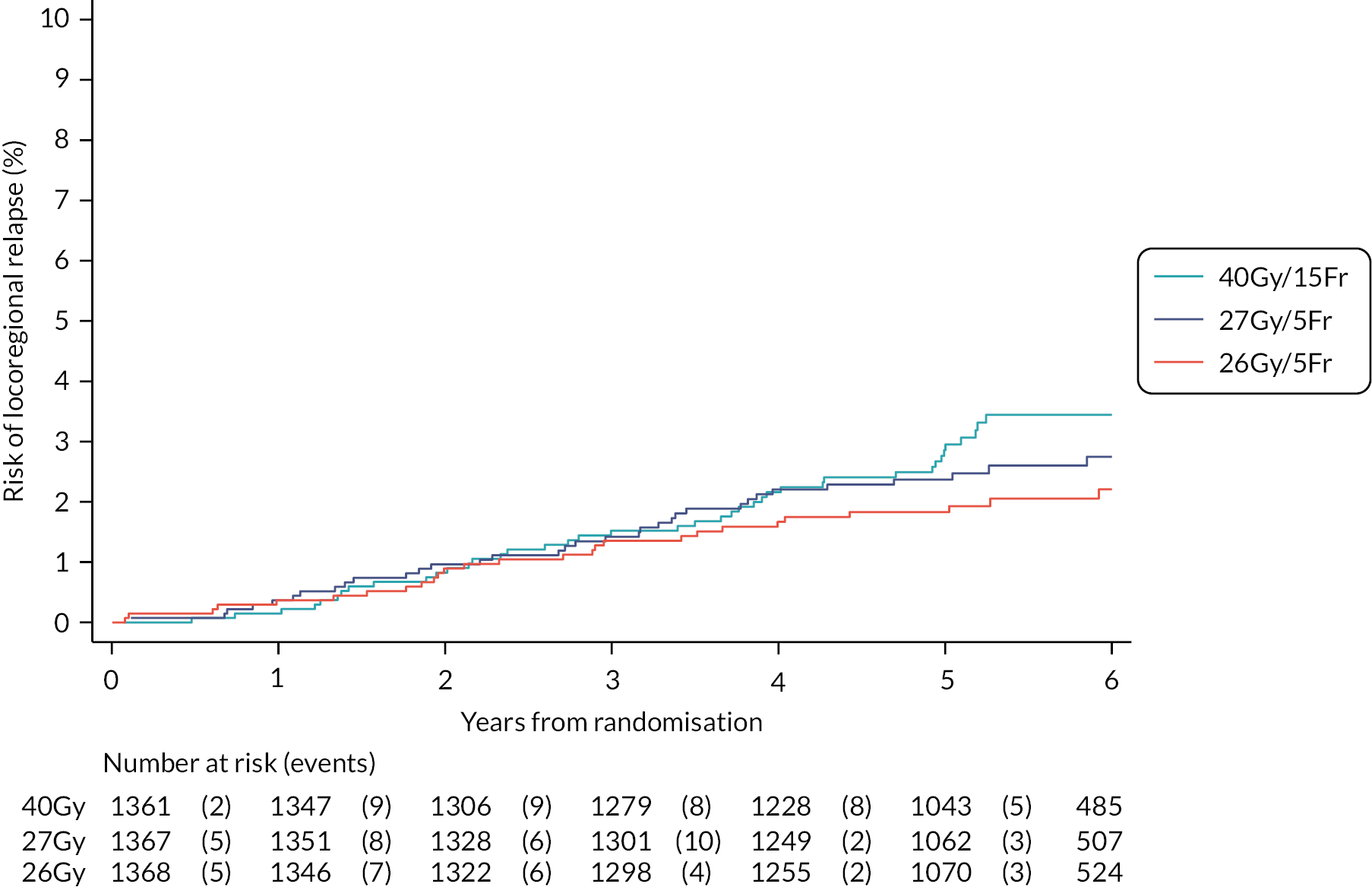
FIGURE 23.
Kaplan–Meier plot of distant relapse in Main Trial.
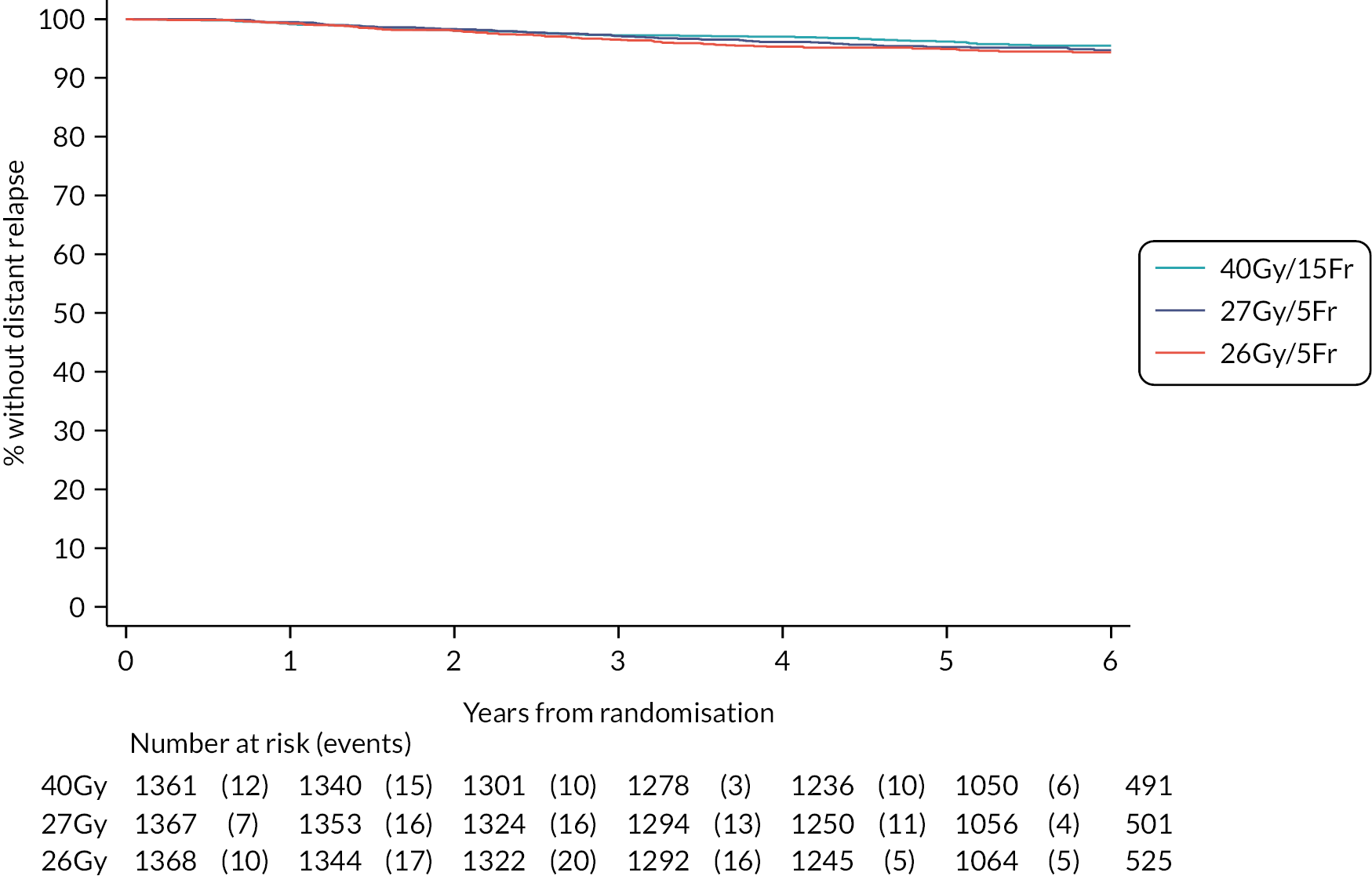
FIGURE 24.
Cumulative risk of distant relapse in Main Trial.
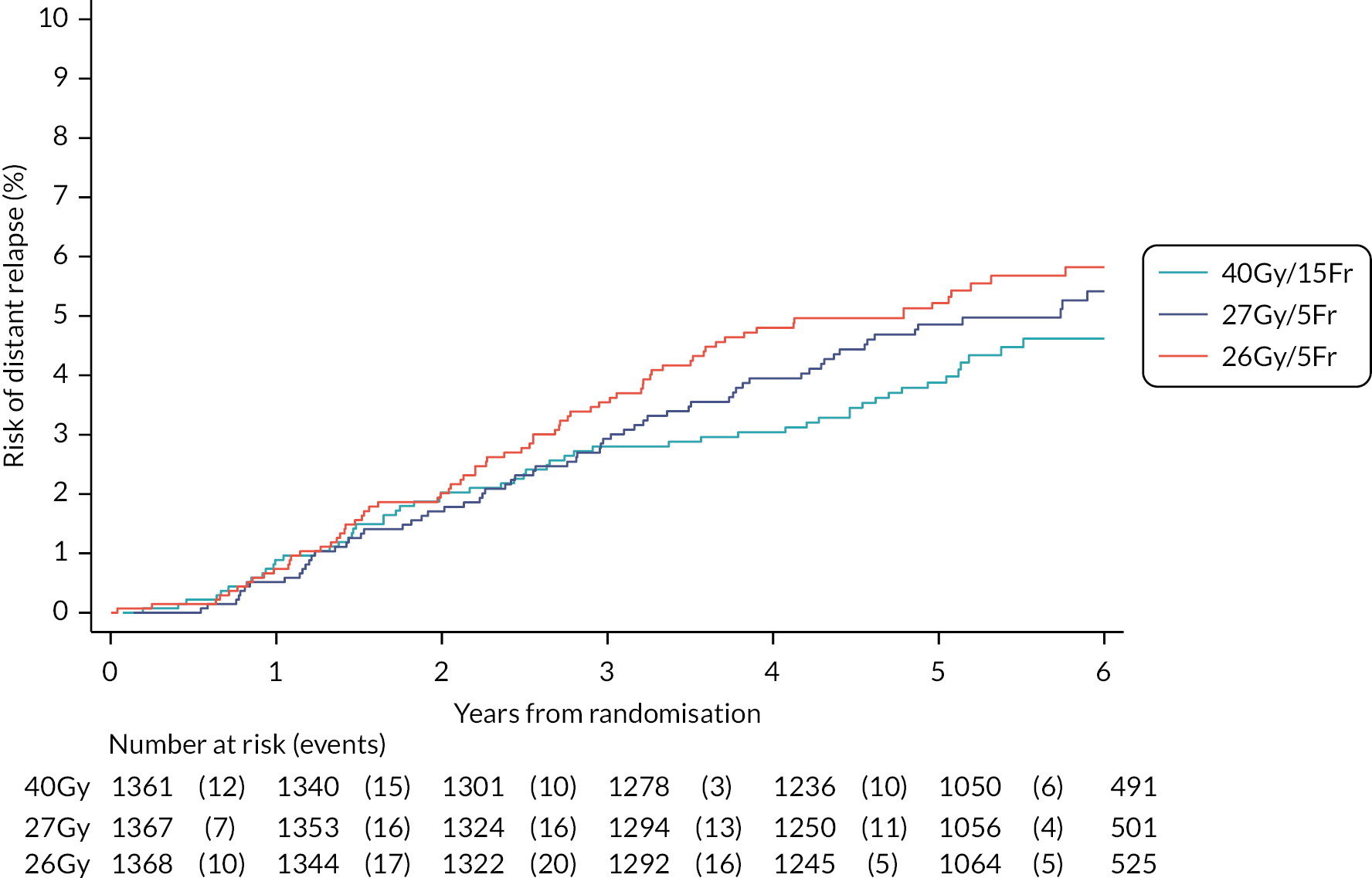
FIGURE 25.
Kaplan–Meier plot of overall survival in Main Trial.
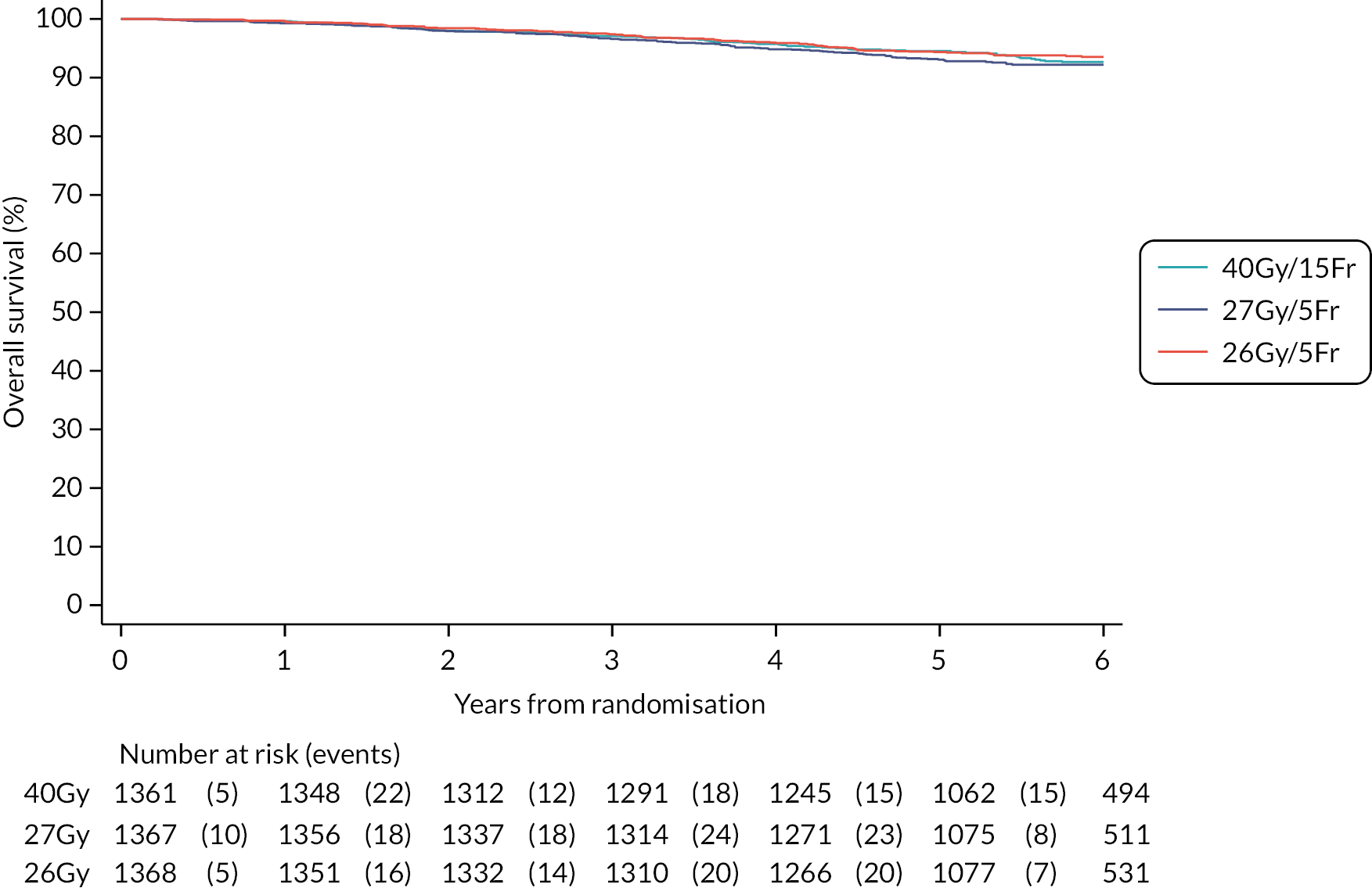
FIGURE 26.
Cumulative risk of death from any cause in Main Trial.
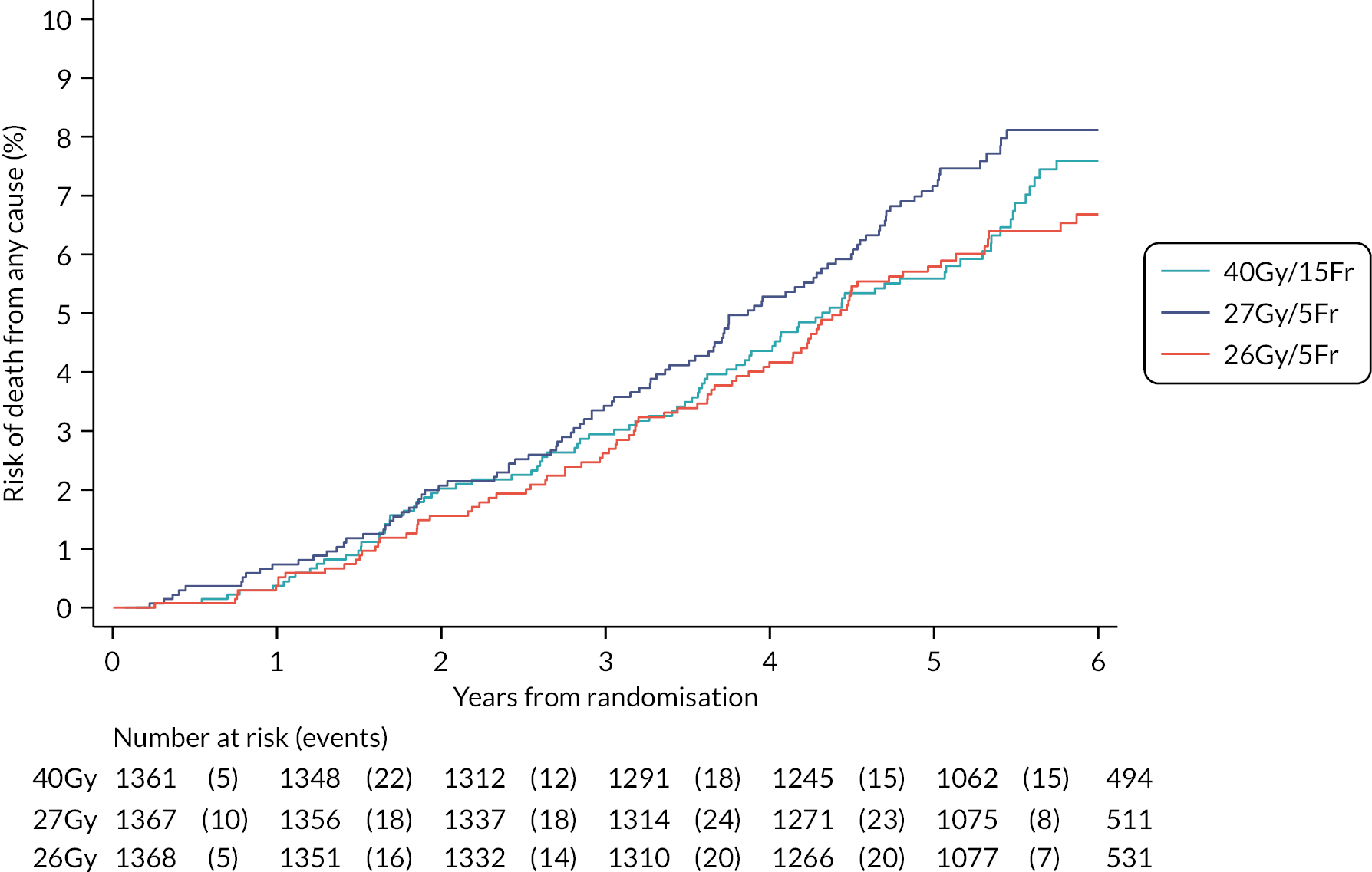
Bar charts of clinical assessments of late normal tissue effects to 5 years
Reproduced from Brunt et al. (CC BY 4.0)1
FIGURE 27.
Clinician-assessed breast distortion from 1 to 5 years in Main Trial.
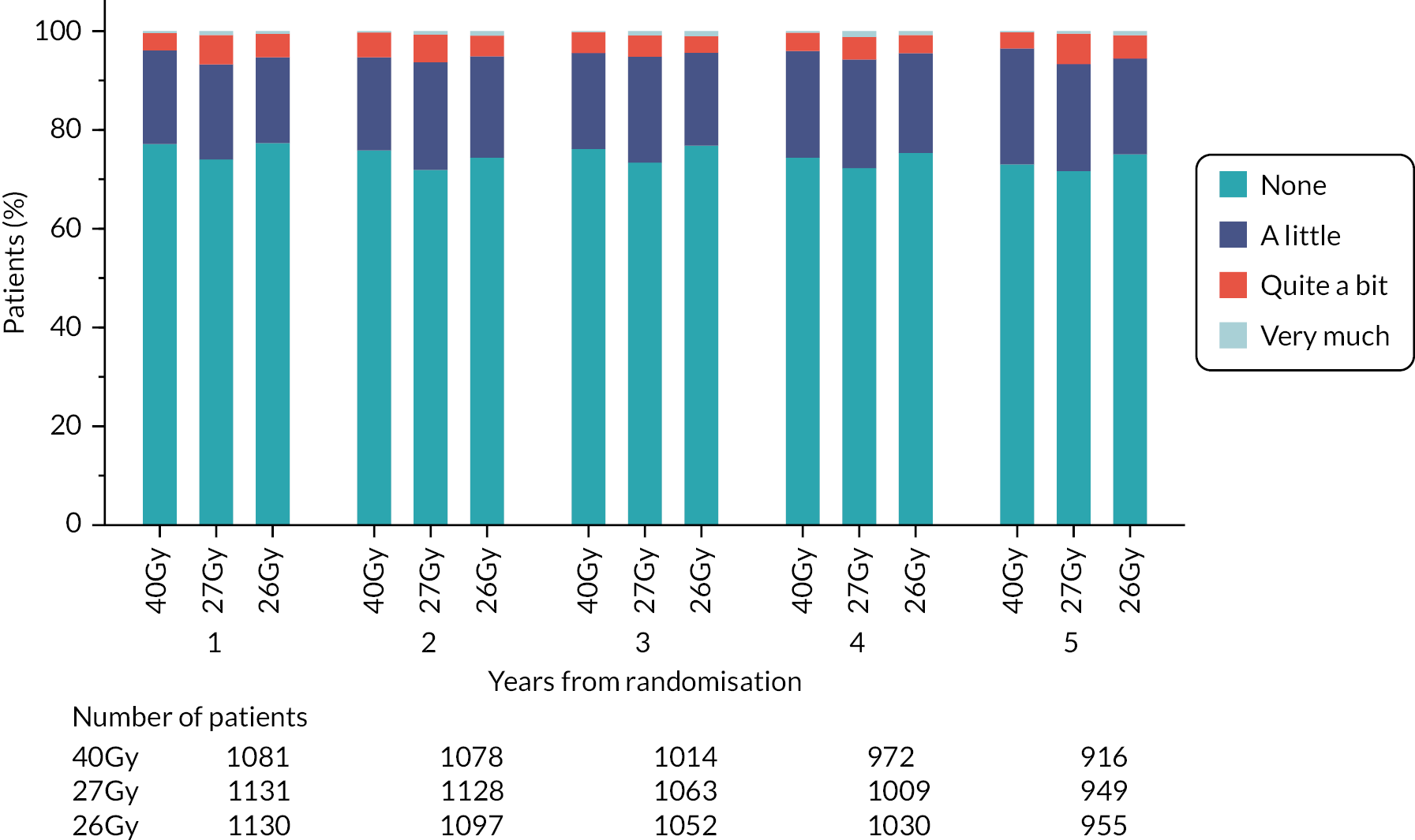
FIGURE 28.
Clinician-assessed breast shrinkage from 1 to 5 years in Main Trial.
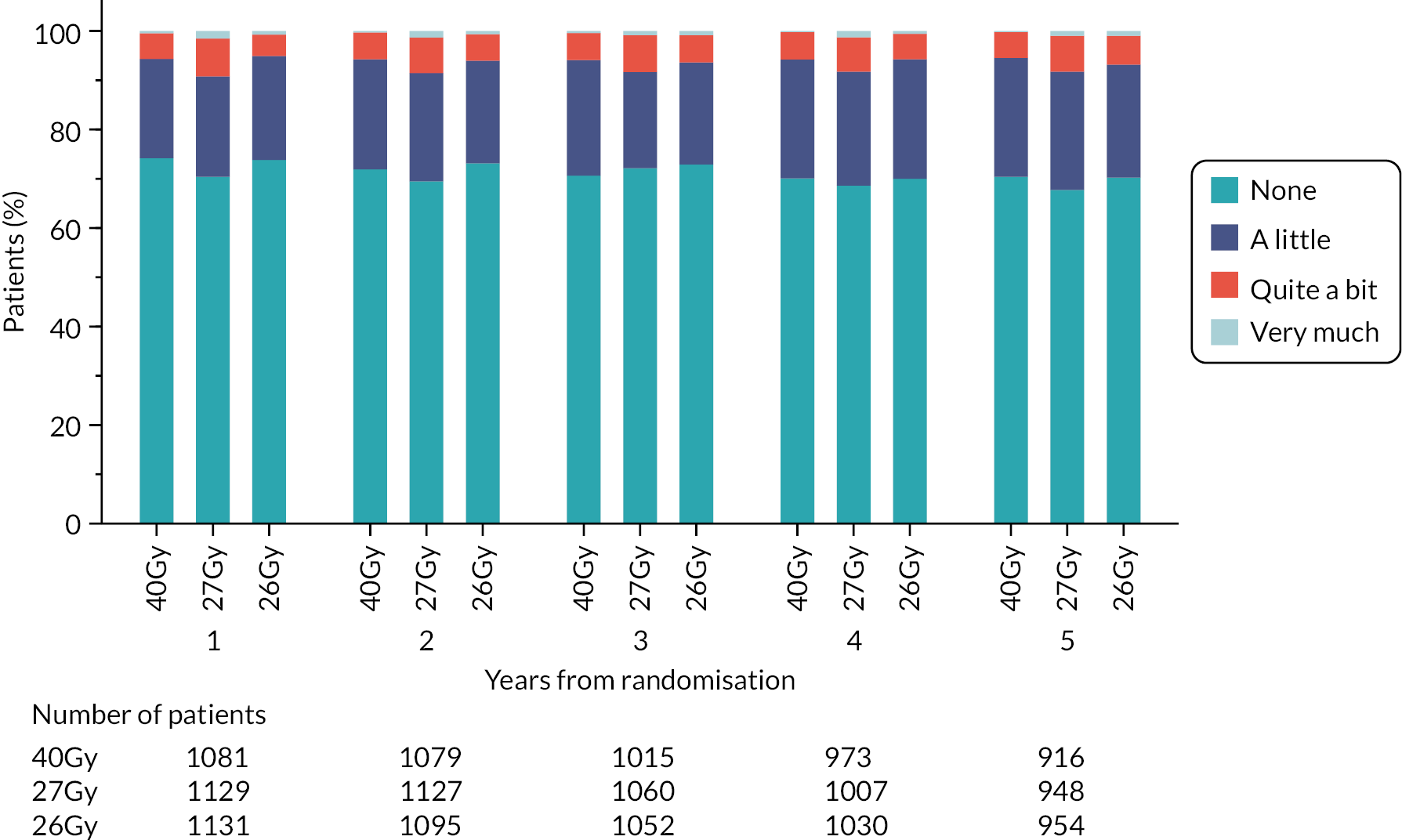
FIGURE 29.
Clinician-assessed breast induration (tumour bed) from 1 to 5 years in Main Trial.
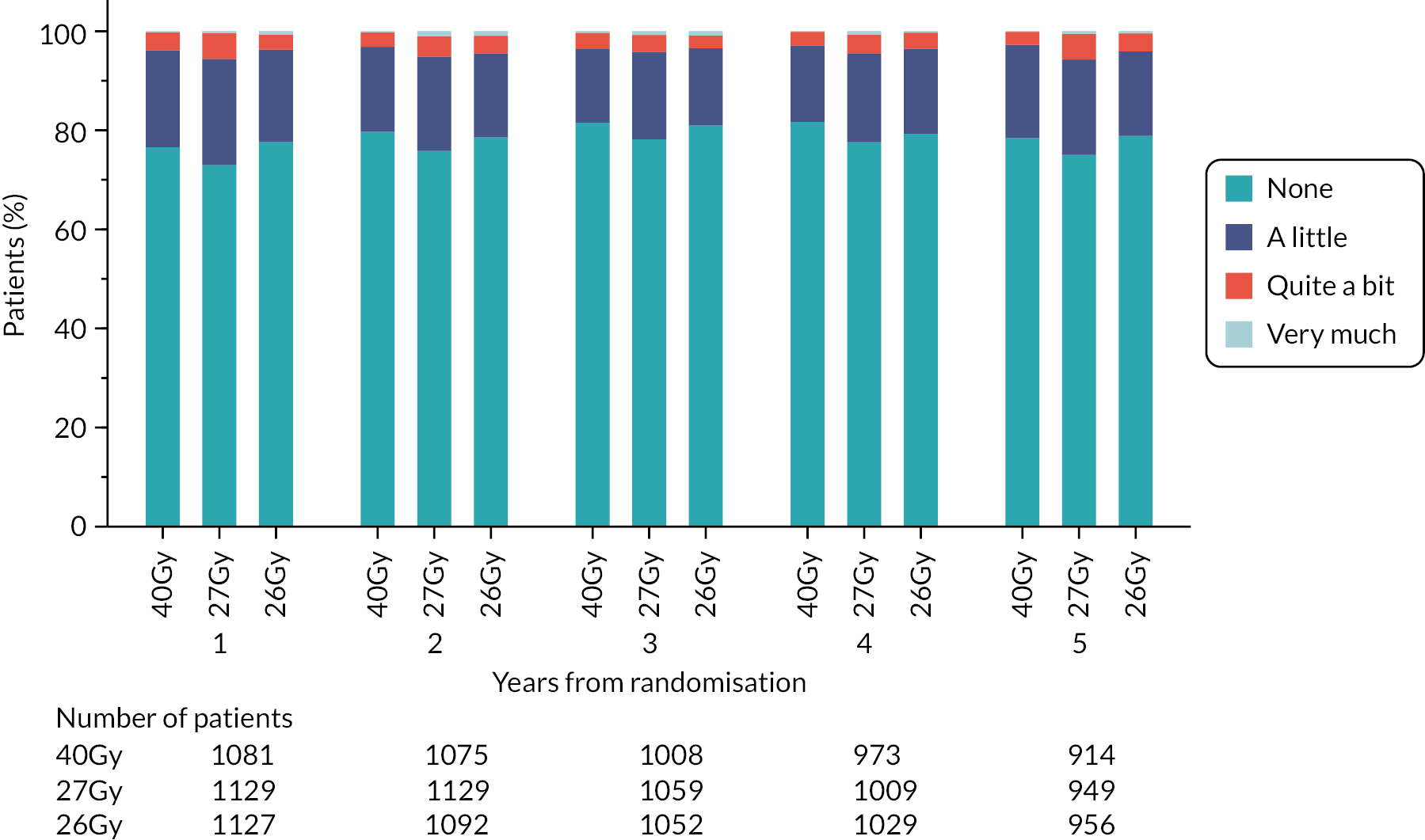
FIGURE 30.
Clinician-assessed breast induration (outside tumour bed) from 1 to 5 years in Main Trial.
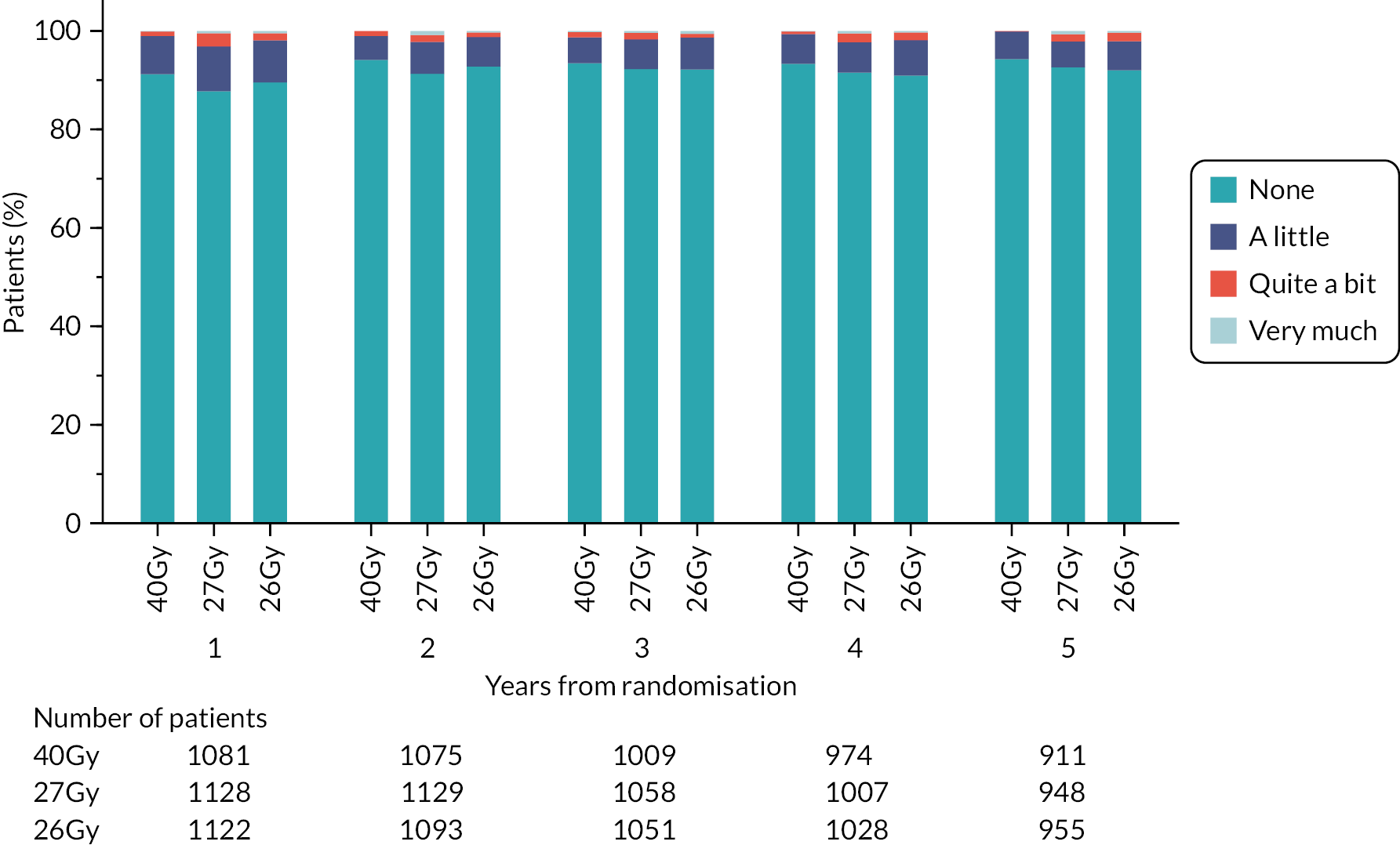
FIGURE 31.
Clinician-assessed telangiectasia from 1 to 5 years in Main Trial.
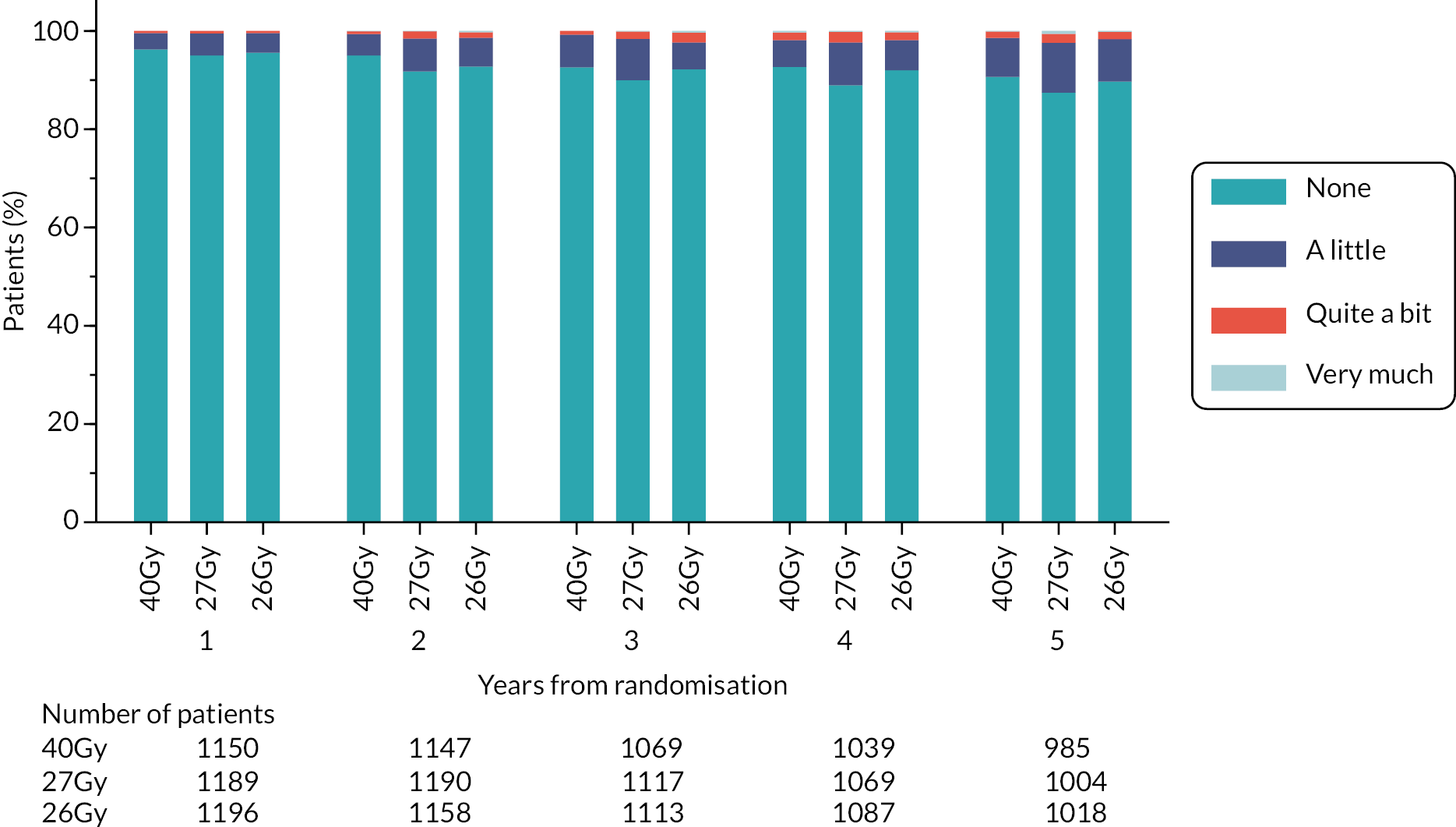
FIGURE 32.
Clinician-assessed breast/chest wall oedema from 1 to 5 years in Main Trial.
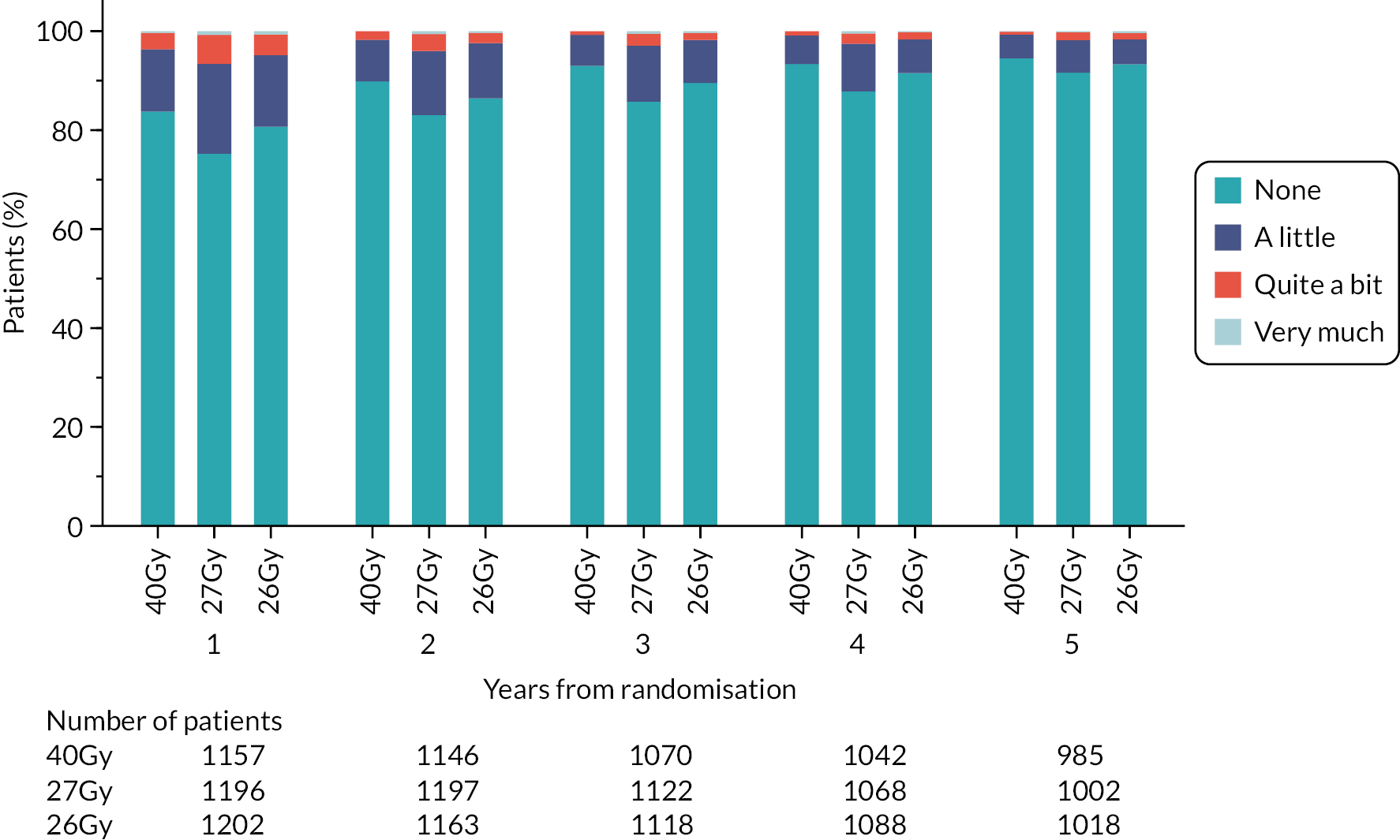
FIGURE 33.
Clinician-assessed breast/chest wall discomfort from 1 to 5 years in Main Trial.
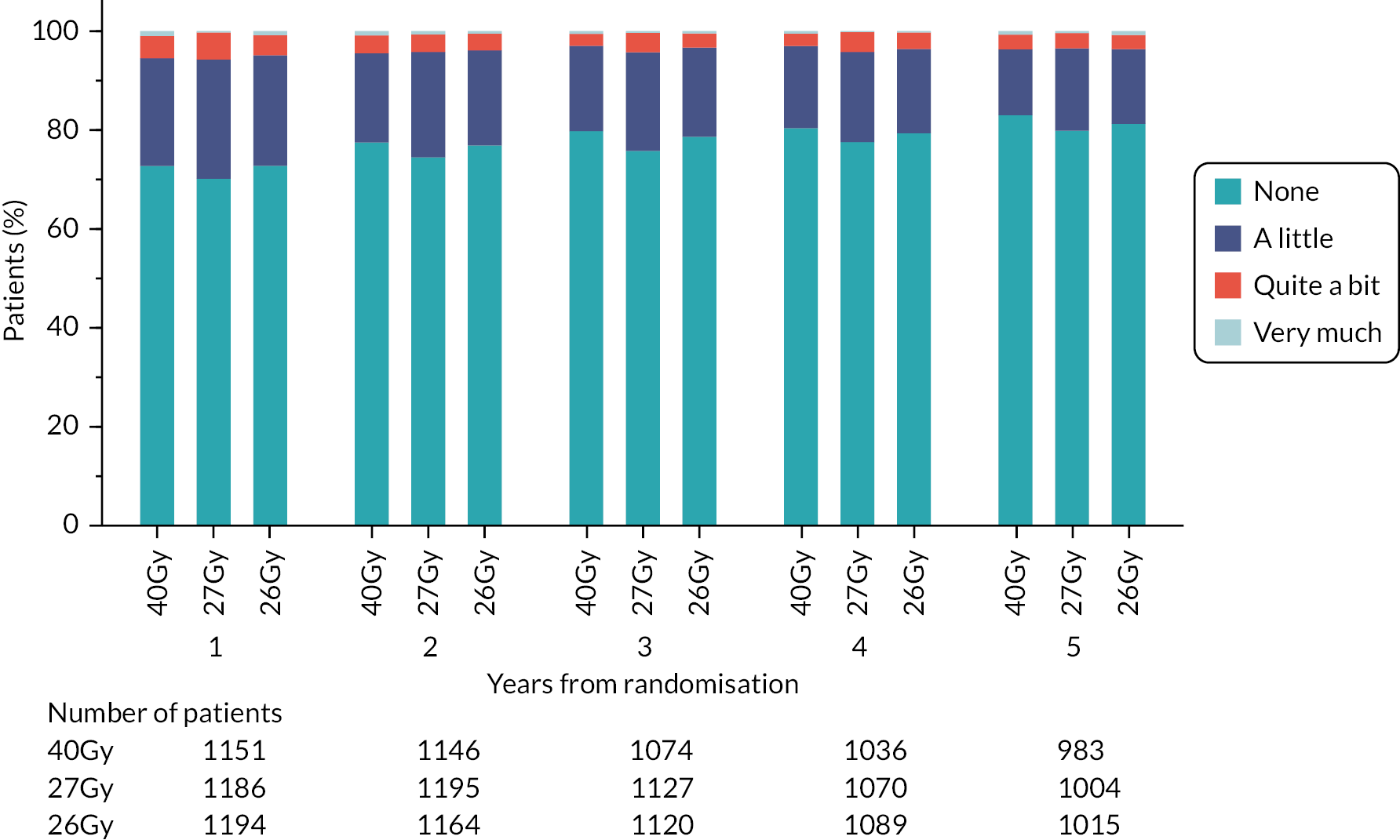
Kaplan–Meier plots for clinician-assessed NTEs in FAST-Forward Main Trial
Reproduced from Brunt et al. (CC BY 4.0)1
FIGURE 34.
Kaplan–Meier plot of clinician-assessed breast distortion (Main Trial).
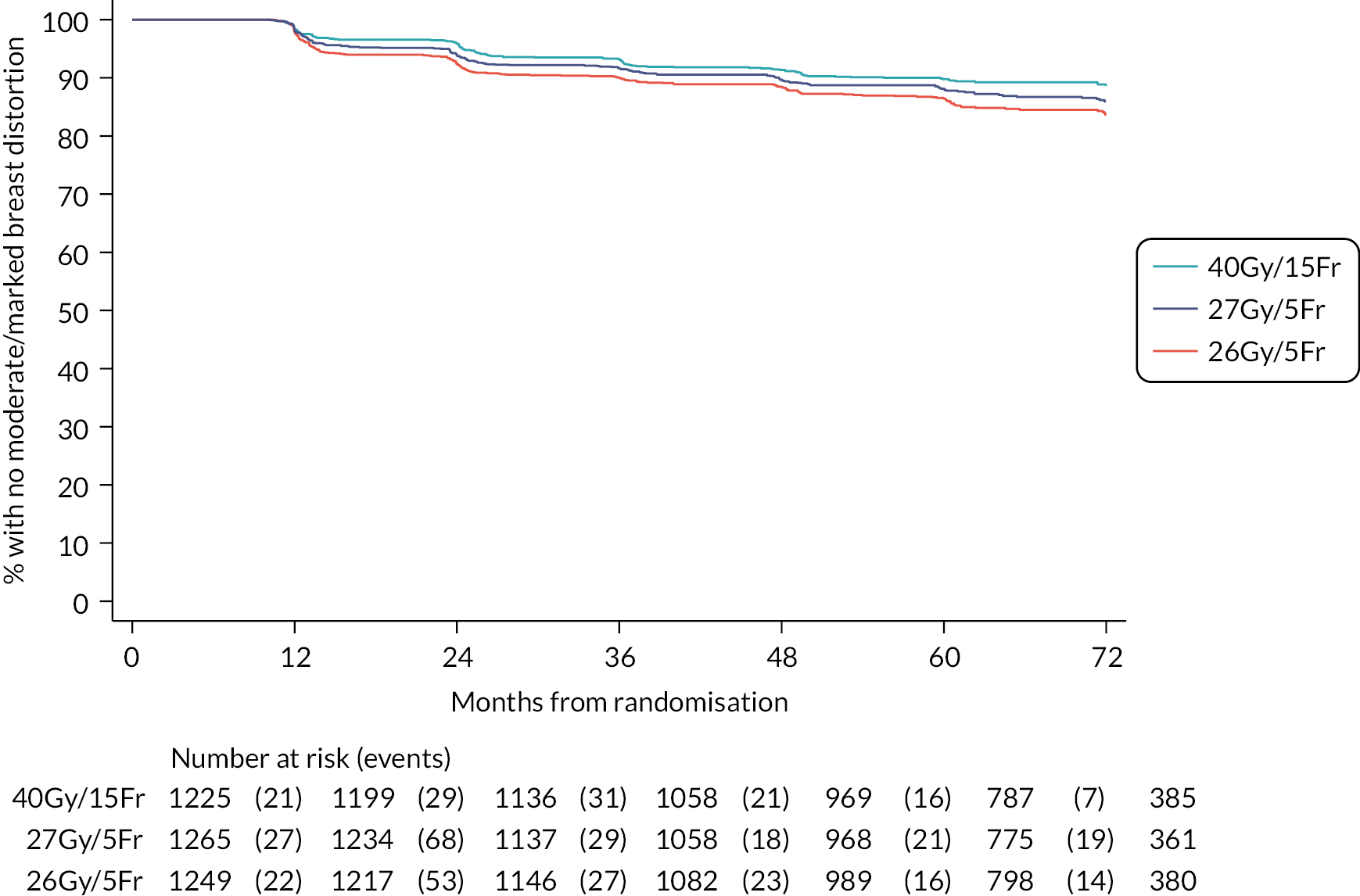
FIGURE 35.
Kaplan–Meier plot of clinician-assessed breast shrinkage (Main Trial).
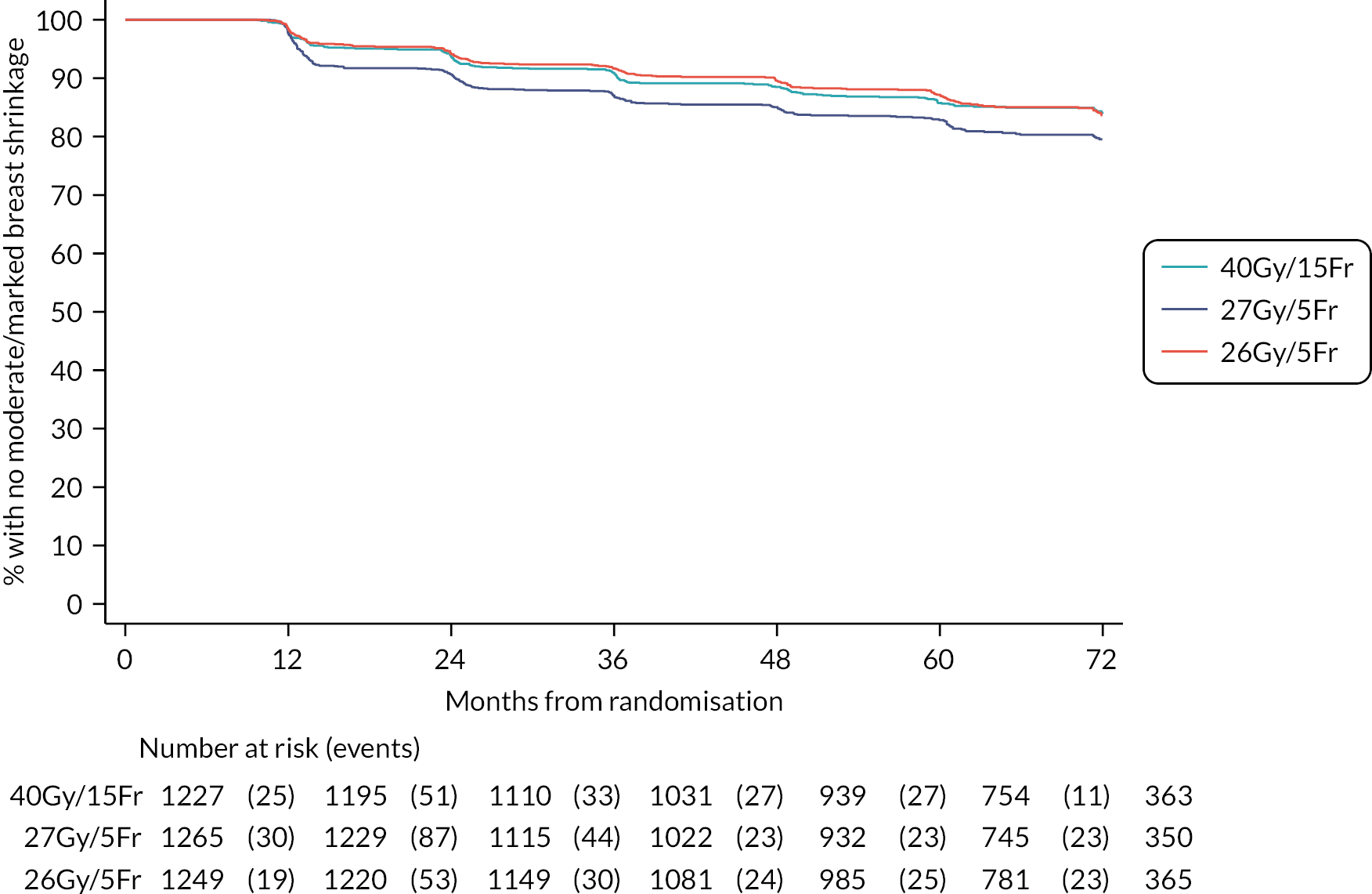
FIGURE 36.
Kaplan–Meier plot of clinician-assessed breast induration (tumour bed) (Main Trial).
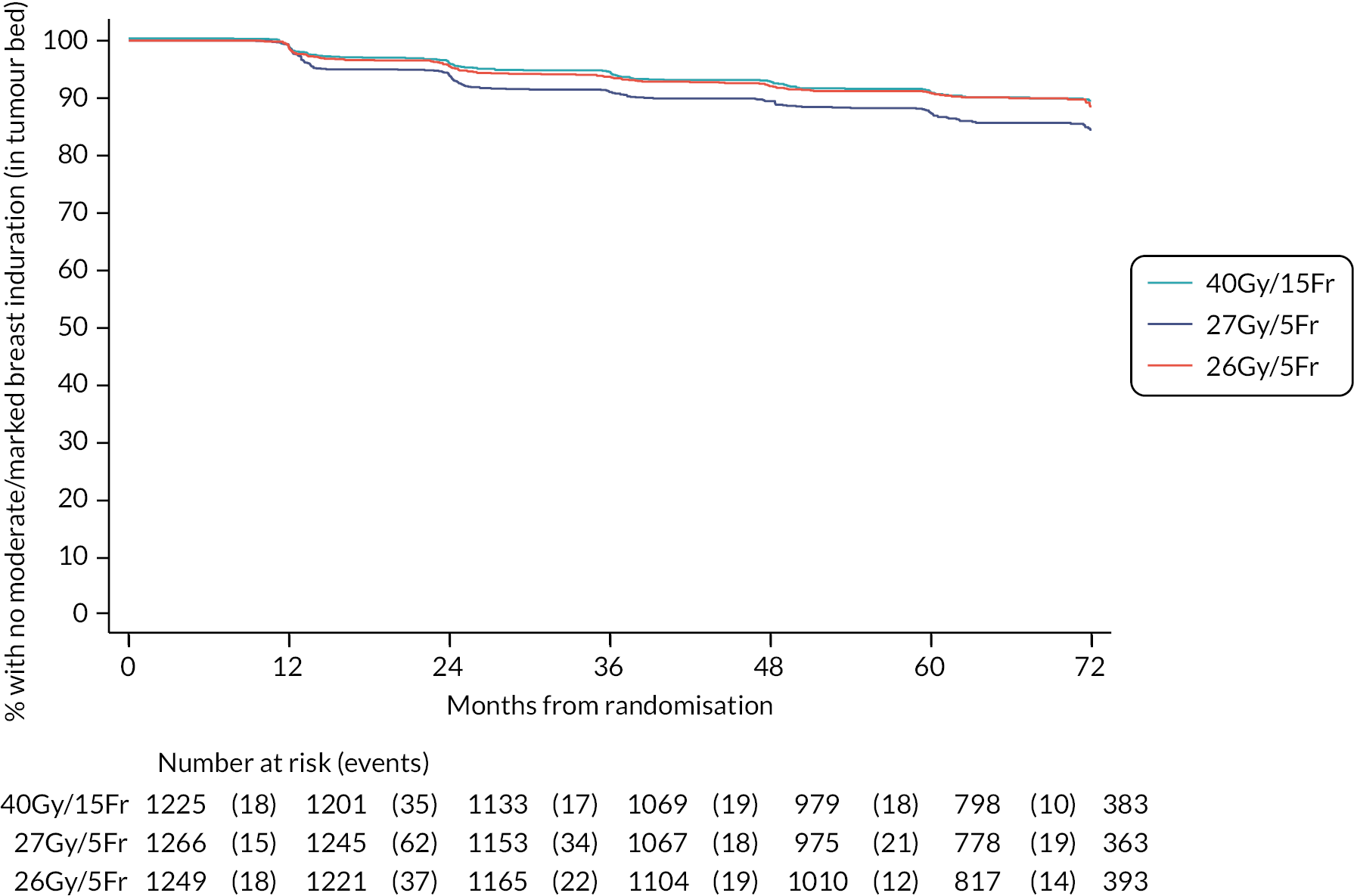
FIGURE 37.
Kaplan–Meier plot of clinician-assessed breast induration (outside tumour bed) (Main Trial).
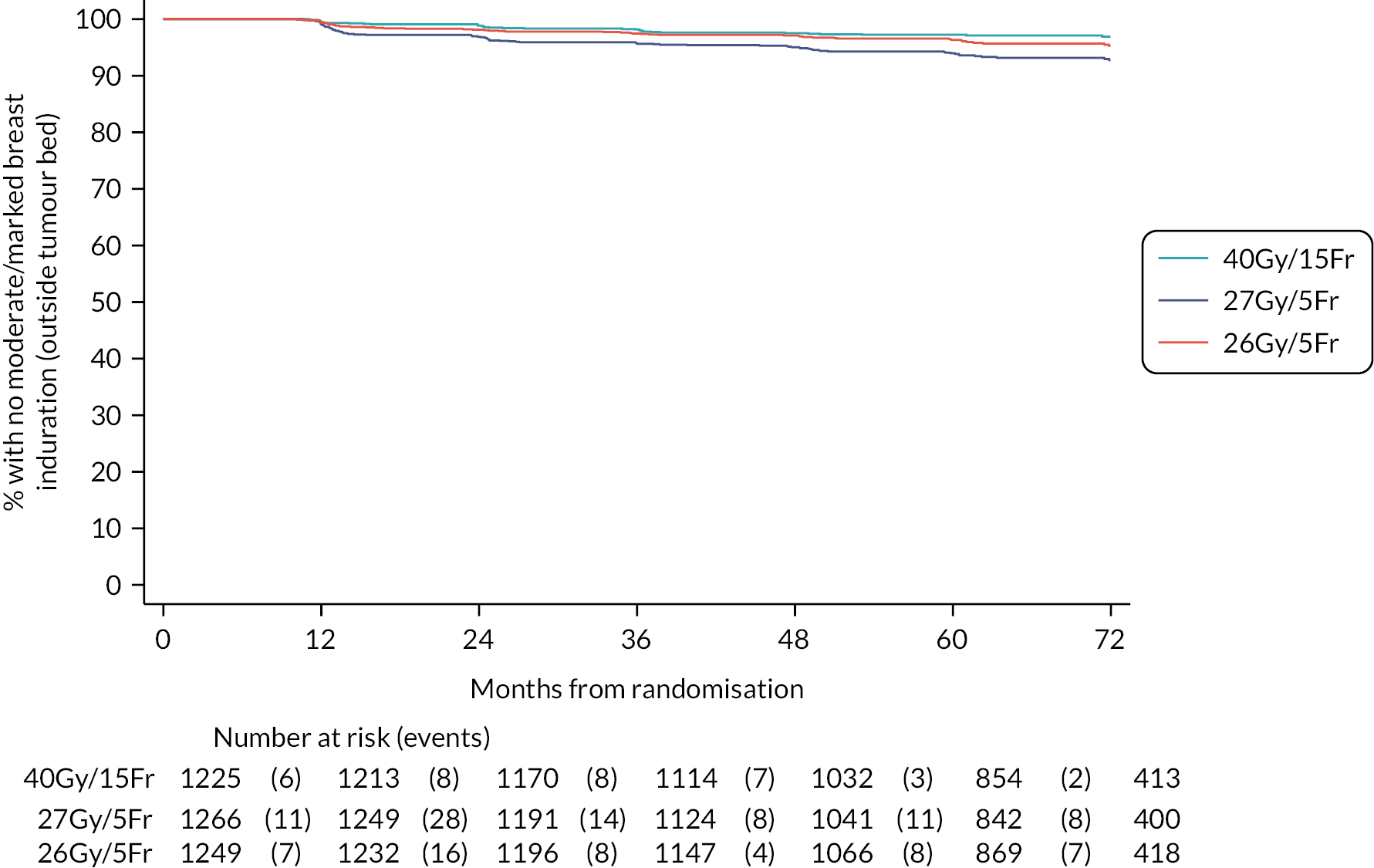
FIGURE 38.
Kaplan–Meier plot of clinician-assessed telangiectasia (Main Trial).
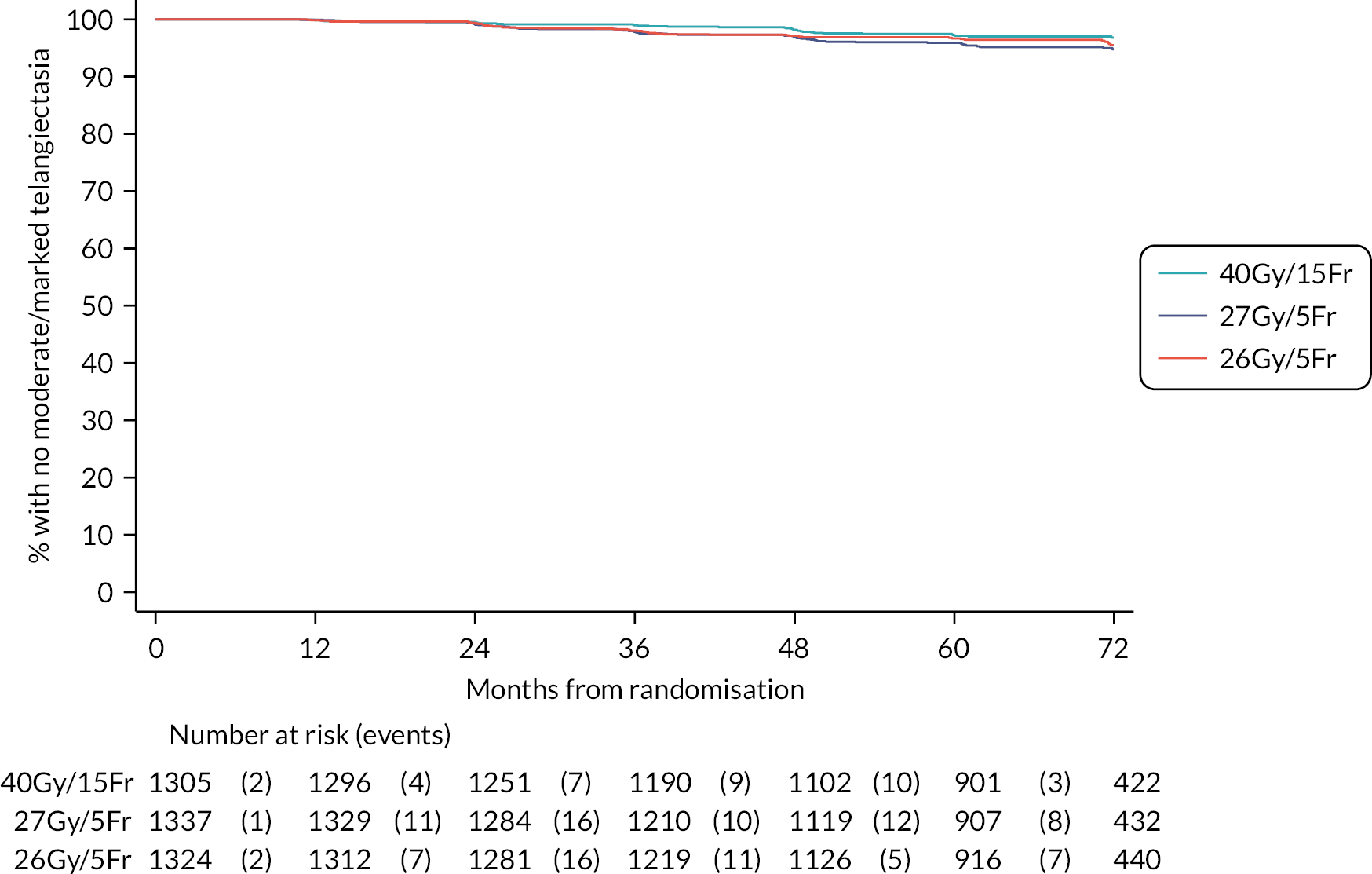
FIGURE 39.
Kaplan–Meier plot of clinician-assessed breast/chest wall oedema (Main Trial).
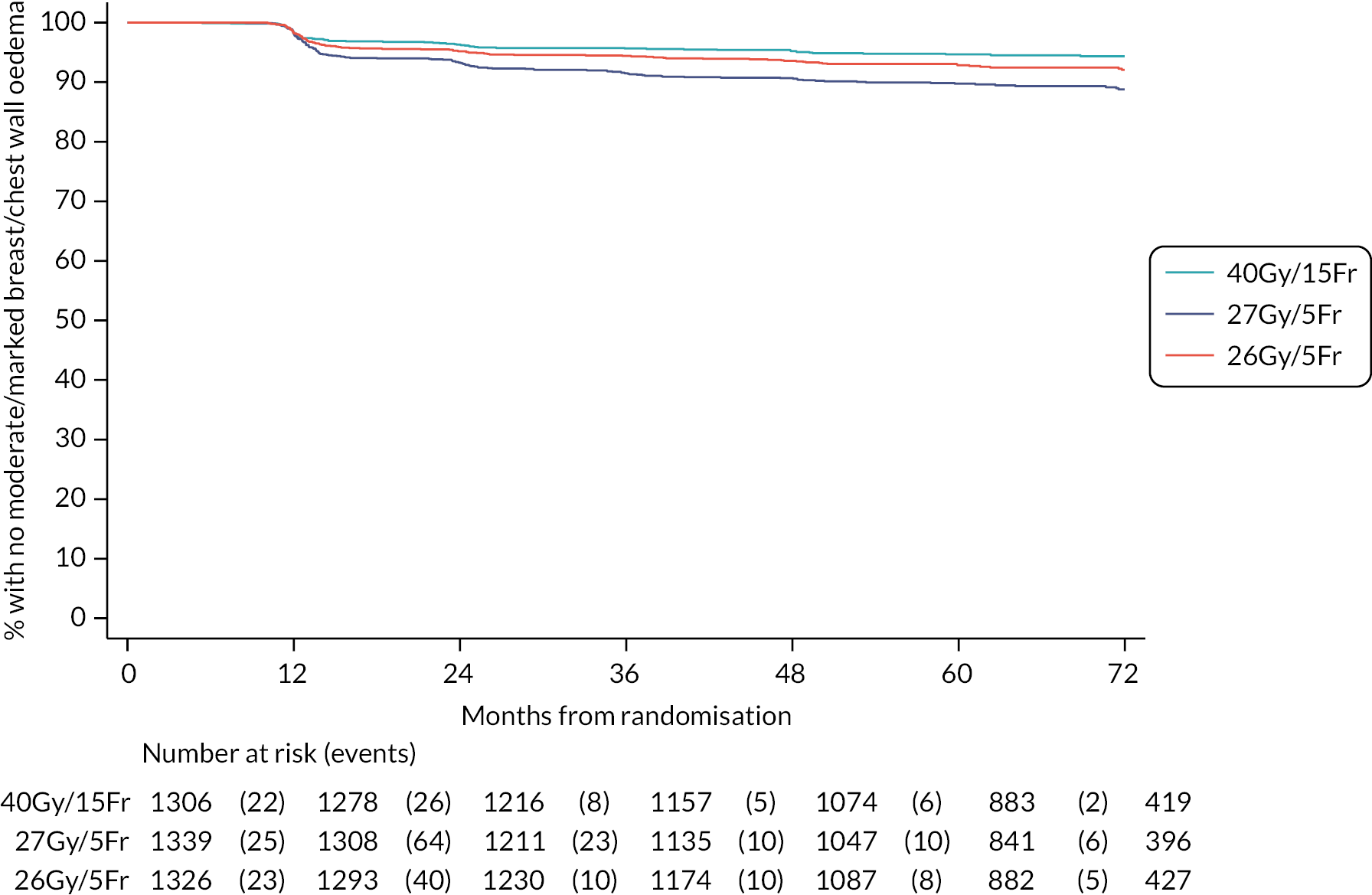
FIGURE 40.
Kaplan–Meier plot of clinician-assessed breast/chest wall discomfort (Main Trial).
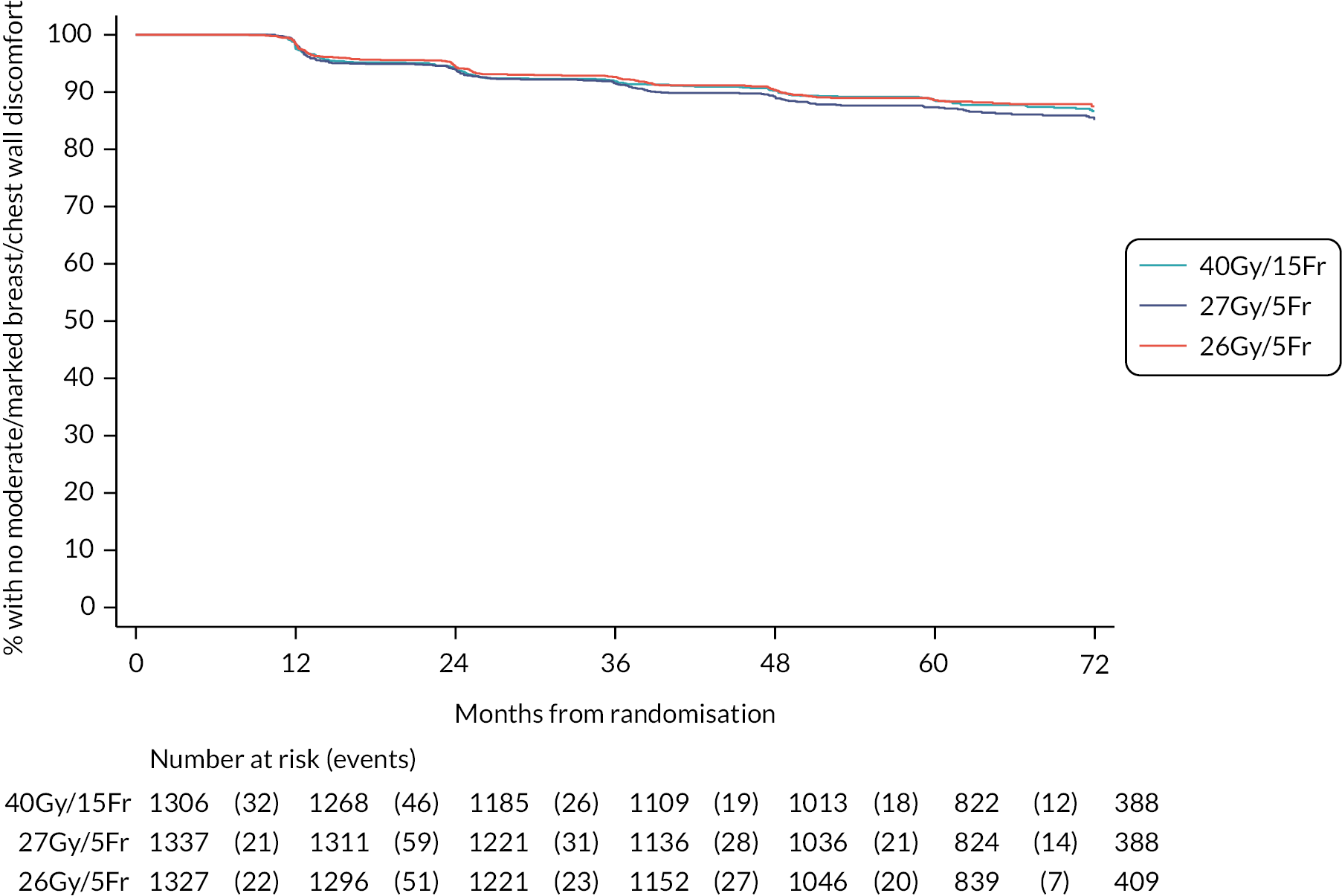
Bar charts for patient-assessed adverse effects to 5 years in the Main Trial
Reproduced from Brunt et al. (CC BY 4.0)1
FIGURE 41.
Patient-assessed pain in arm or shoulder up to 5 years in the Main Trial.
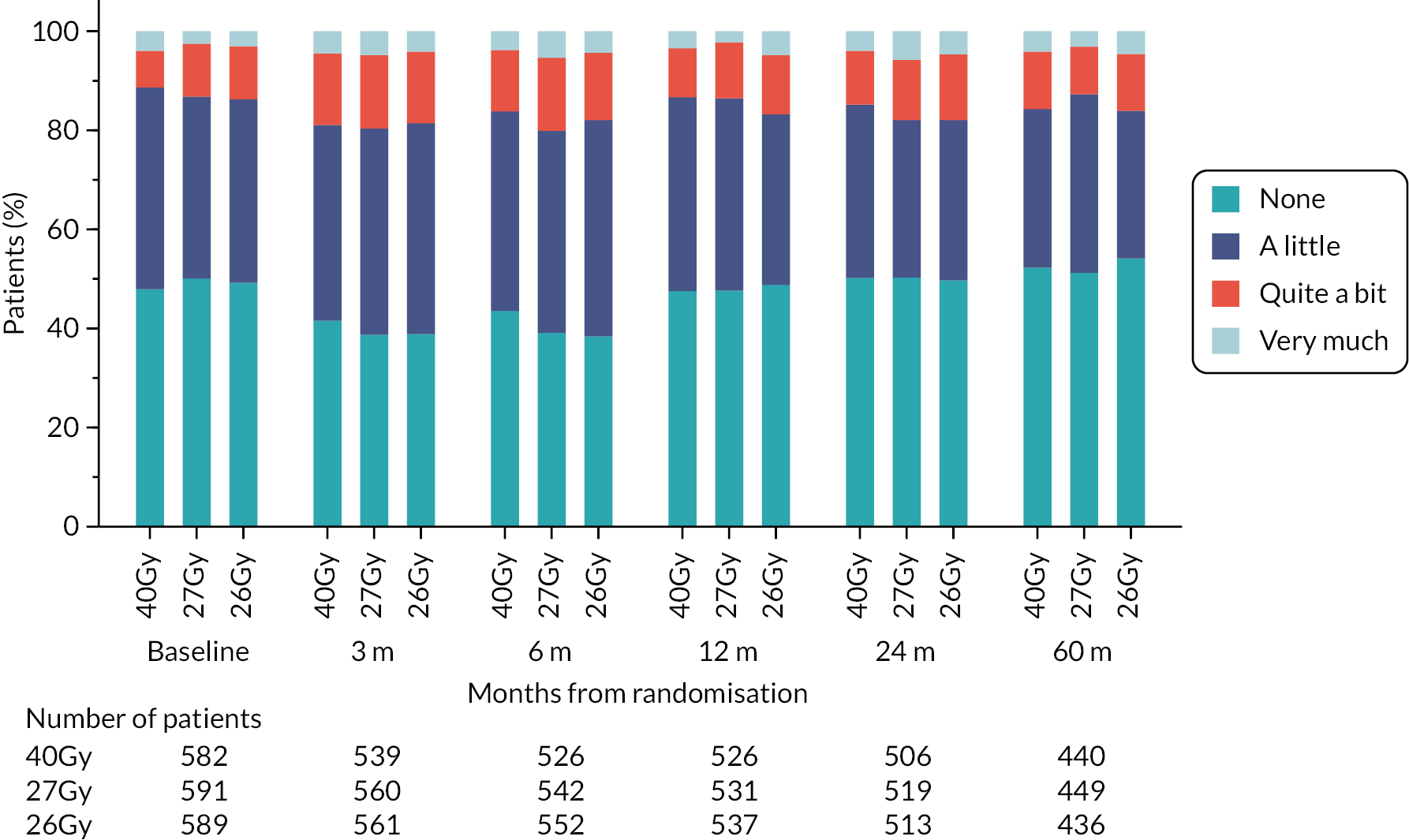
FIGURE 42.
Patient-assessed swollen arm or hand up to 5 years in the Main Trial.
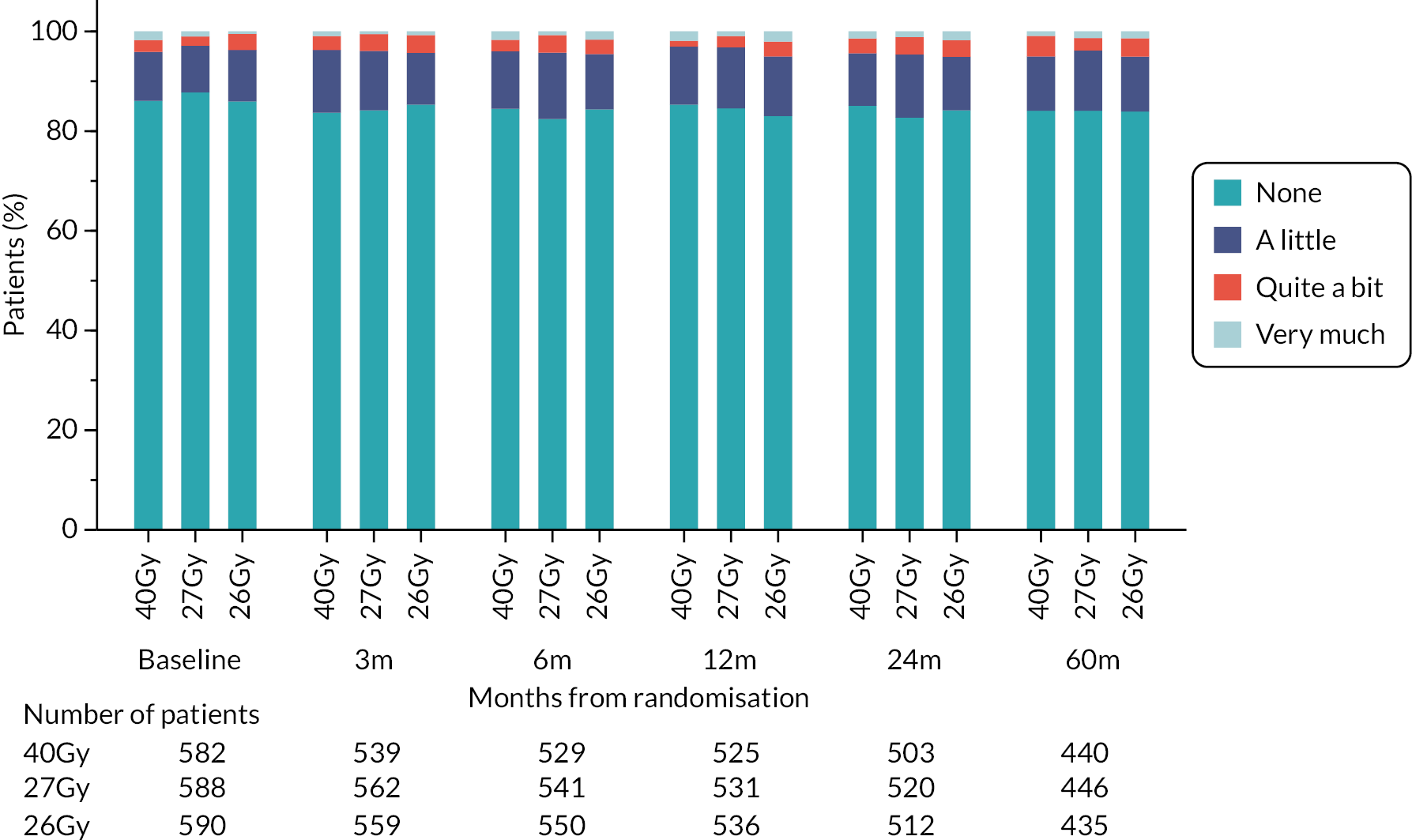
FIGURE 43.
Patient-assessed difficulty raising arm/moving it sideways up to 5 years in the Main Trial.
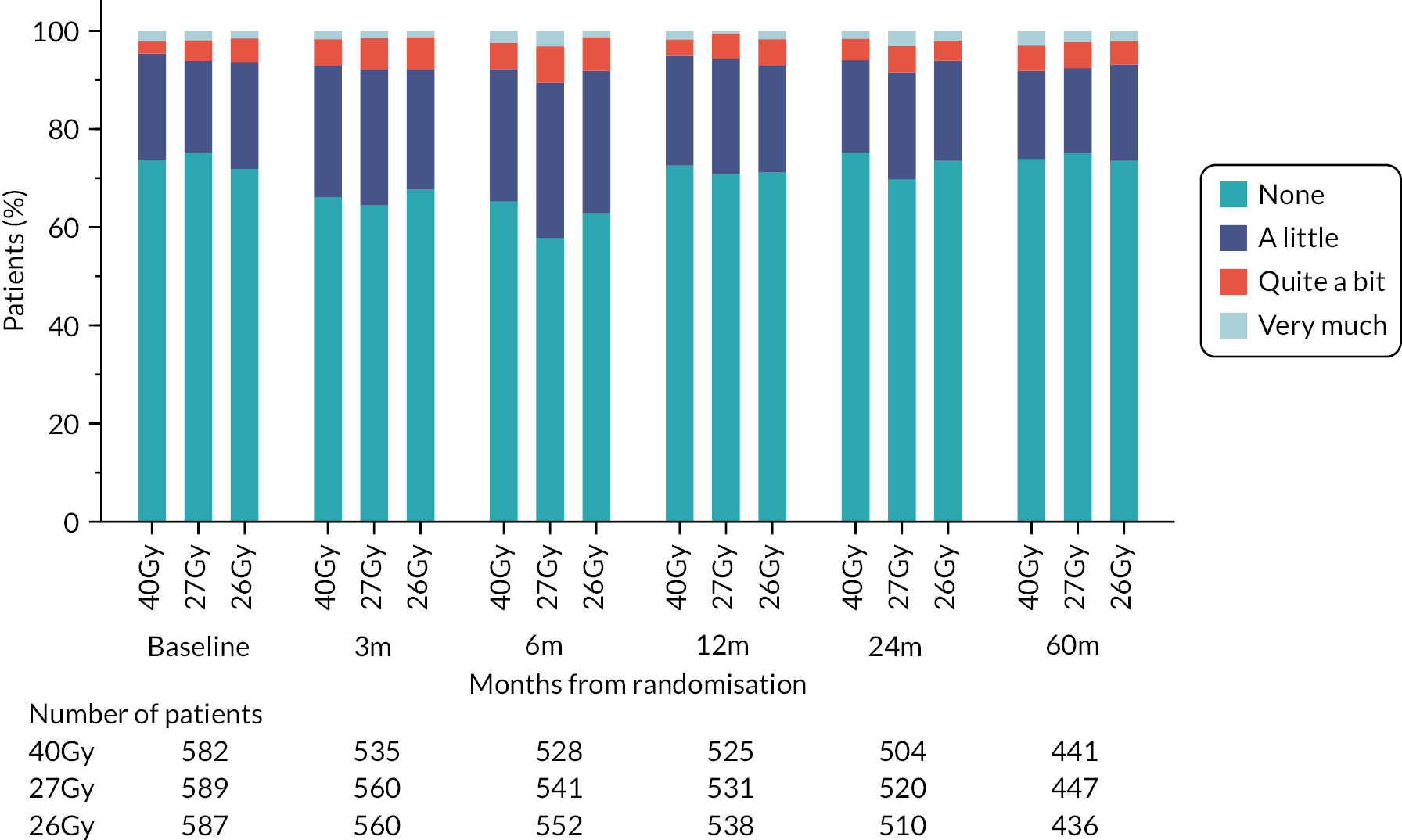
FIGURE 44.
Patient-assessed breast pain up to 5 years in the Main Trial.
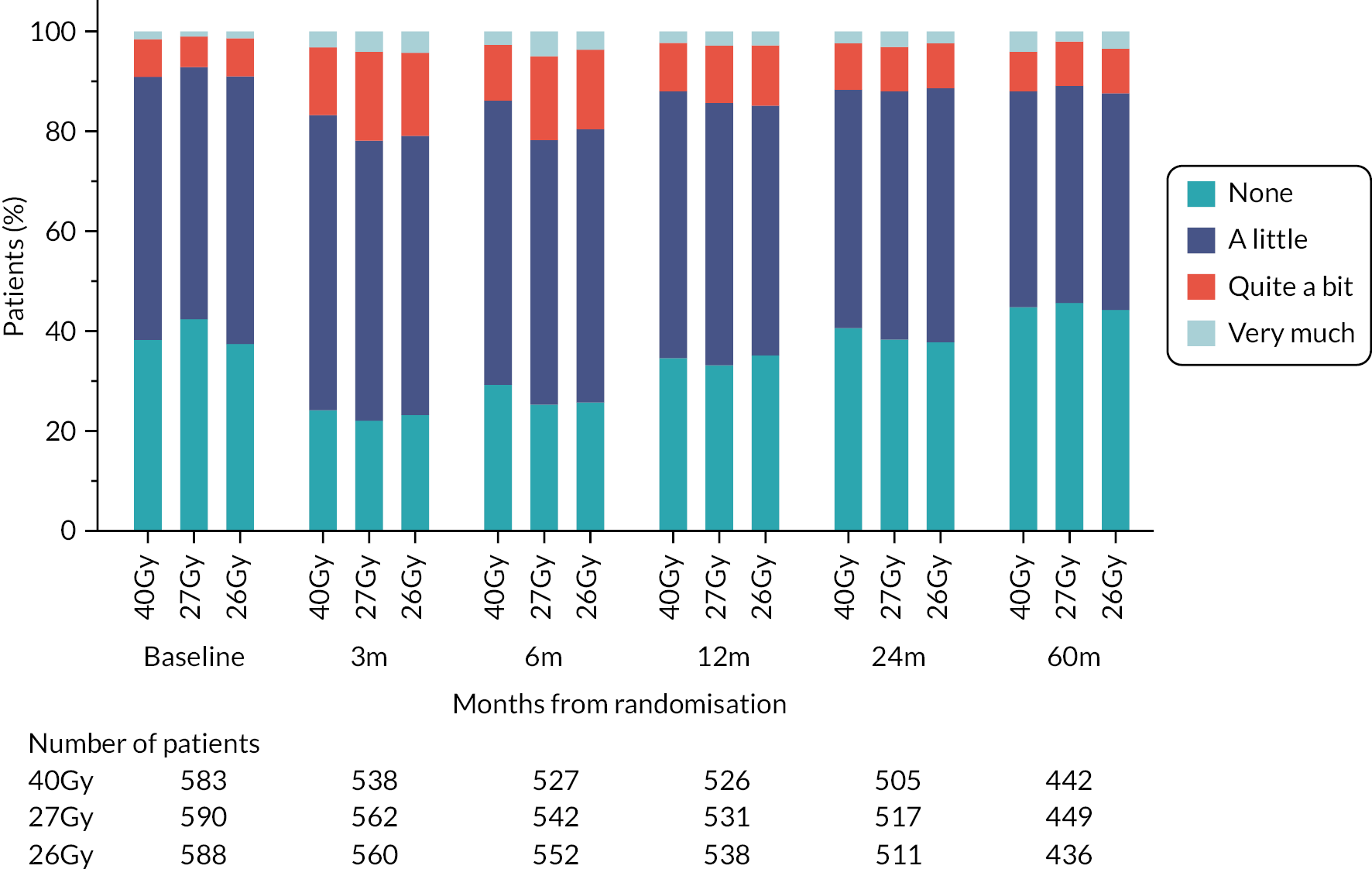
FIGURE 45.
Patient-assessed breast swelling up to 5 years in the Main Trial.
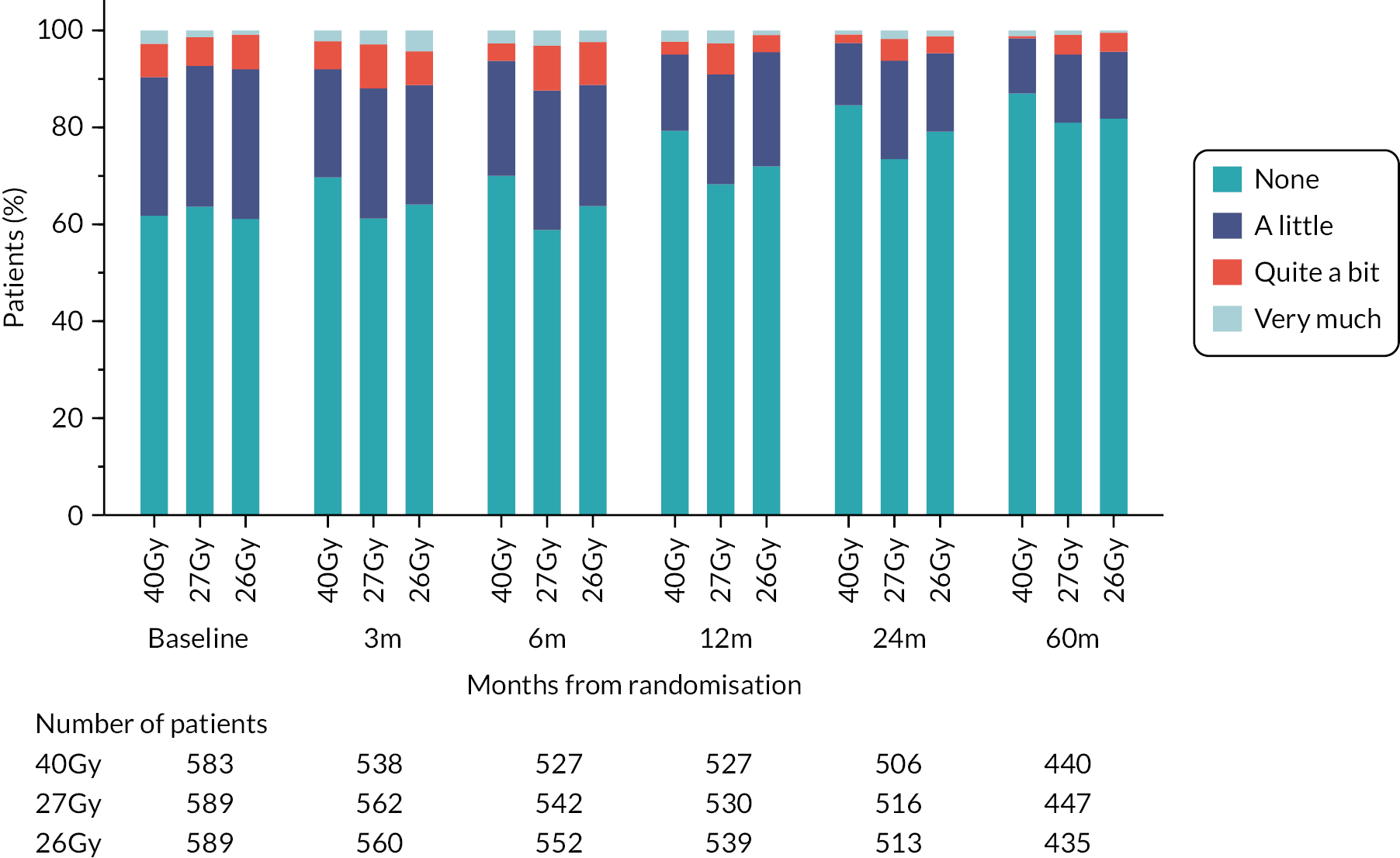
FIGURE 46.
Patient-assessed breast oversensitivity up to 5 years in the Main Trial.
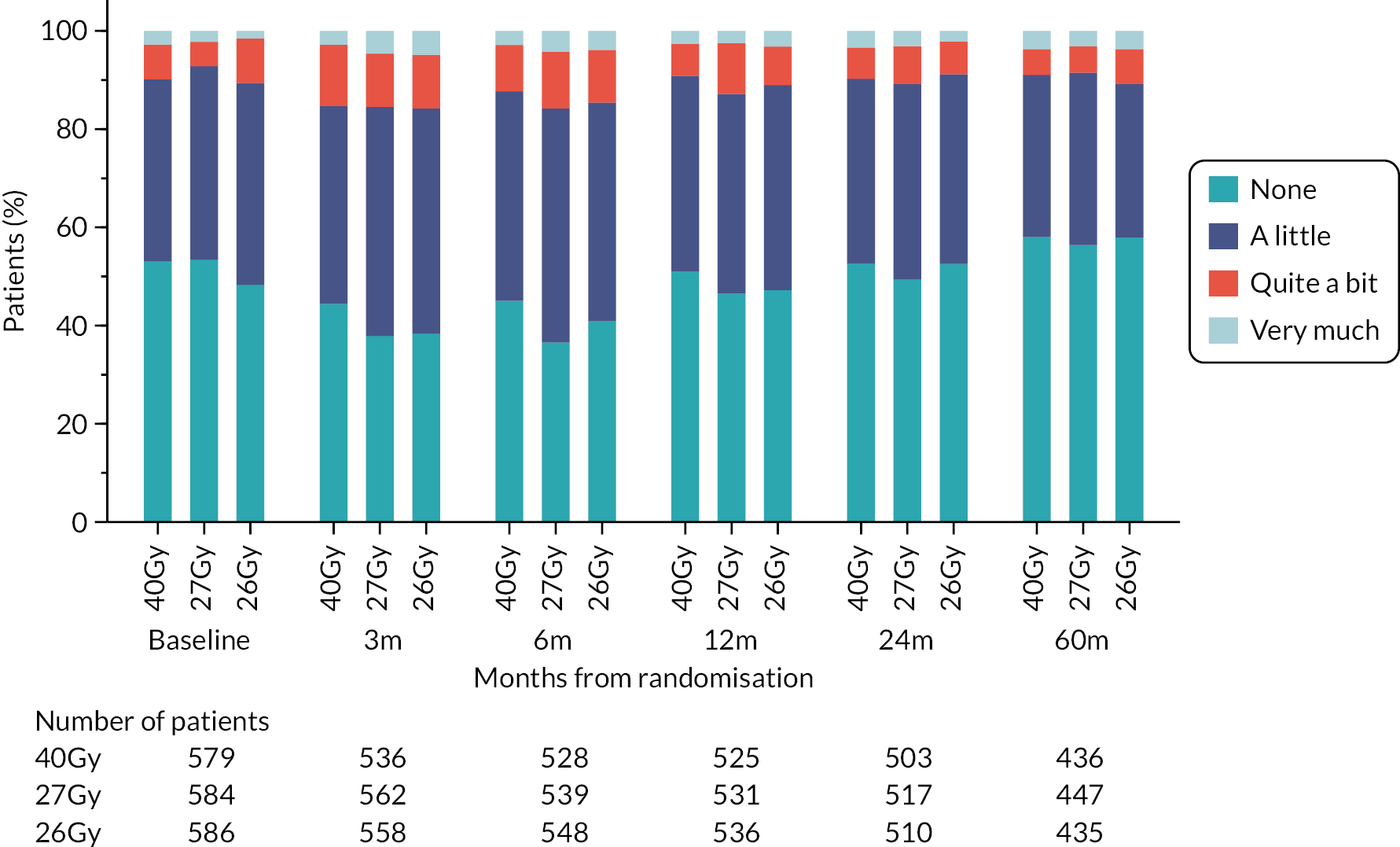
FIGURE 47.
Patient-assessed skin problems on affected breast up to 5 years in the Main Trial.
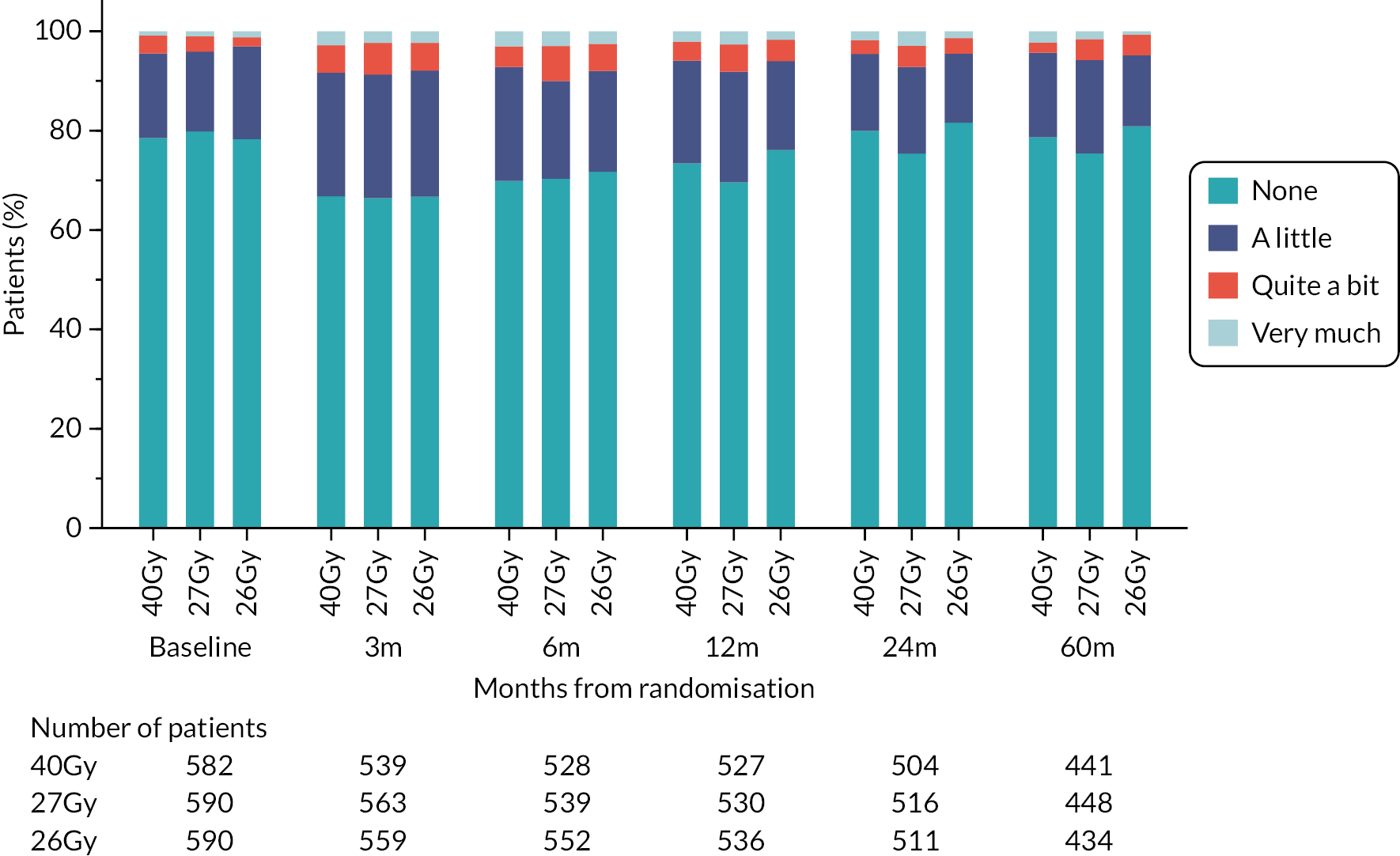
FIGURE 48.
Patient-assessed change in skin appearance on affected breast up to 5 years in the Main Trial.
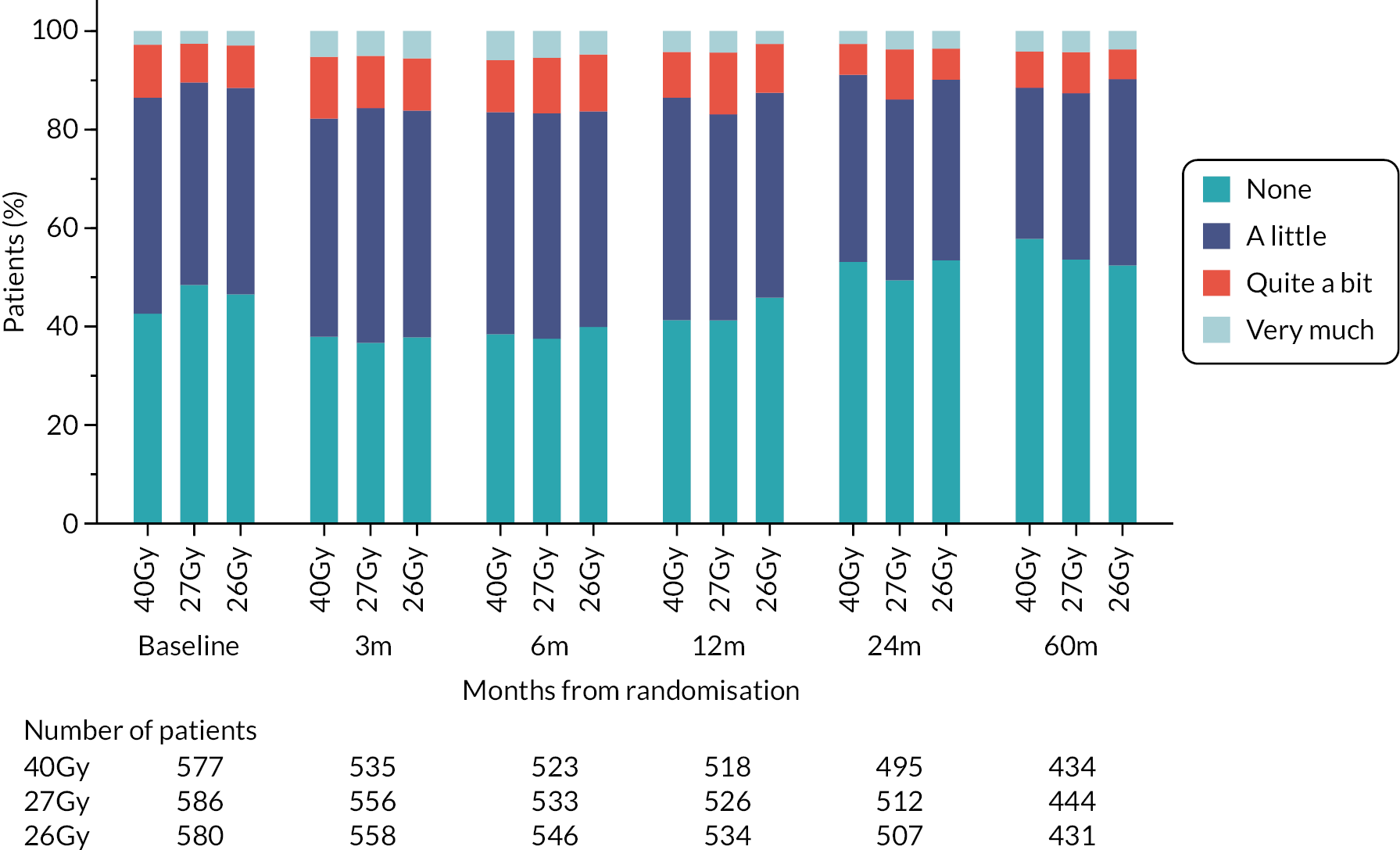
FIGURE 49.
Patient-assessed change in overall breast appearance up to 5 years in the Main Trial.
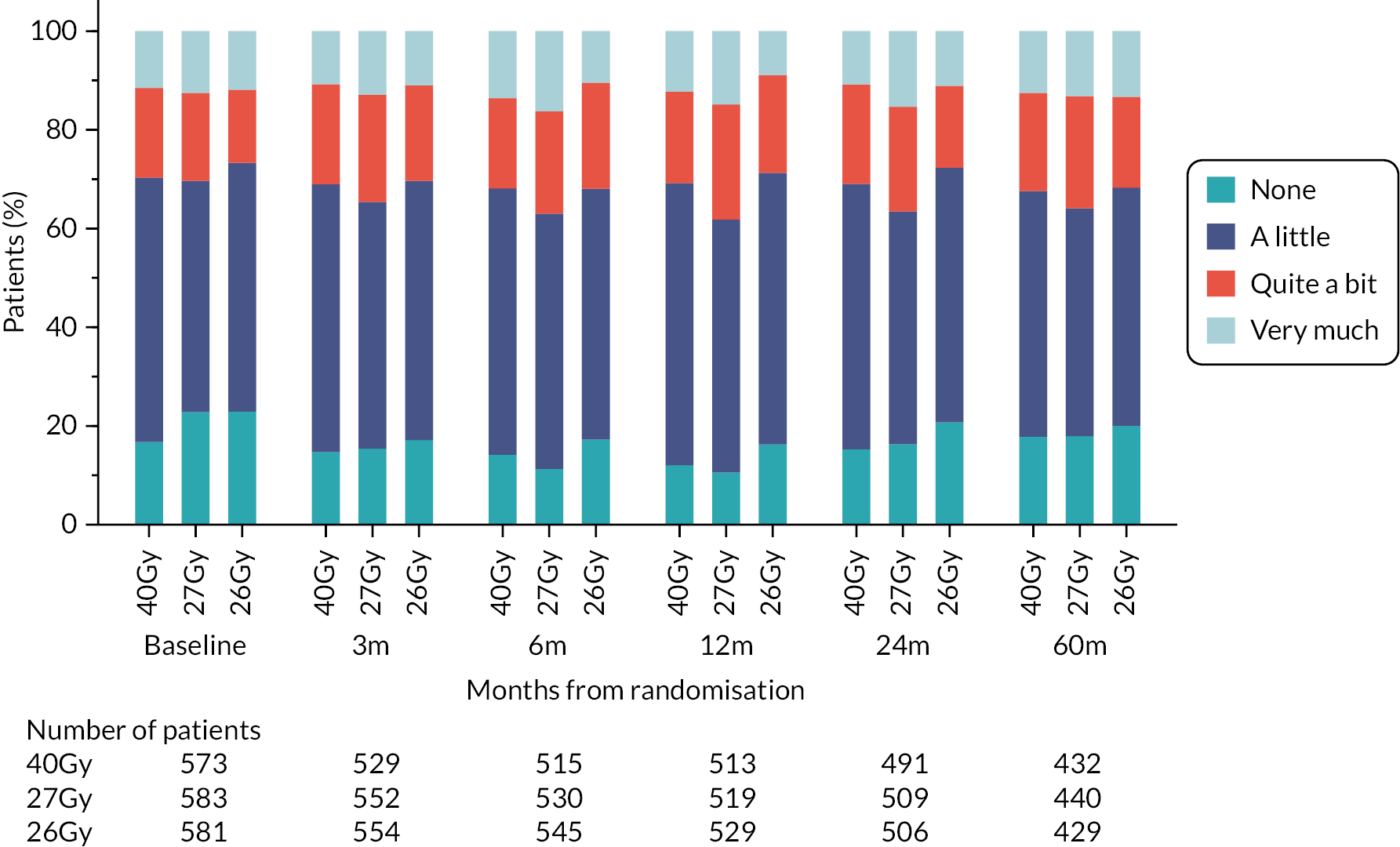
FIGURE 50.
Patient-assessed breast shrinkage up to 5 years in the Main Trial.
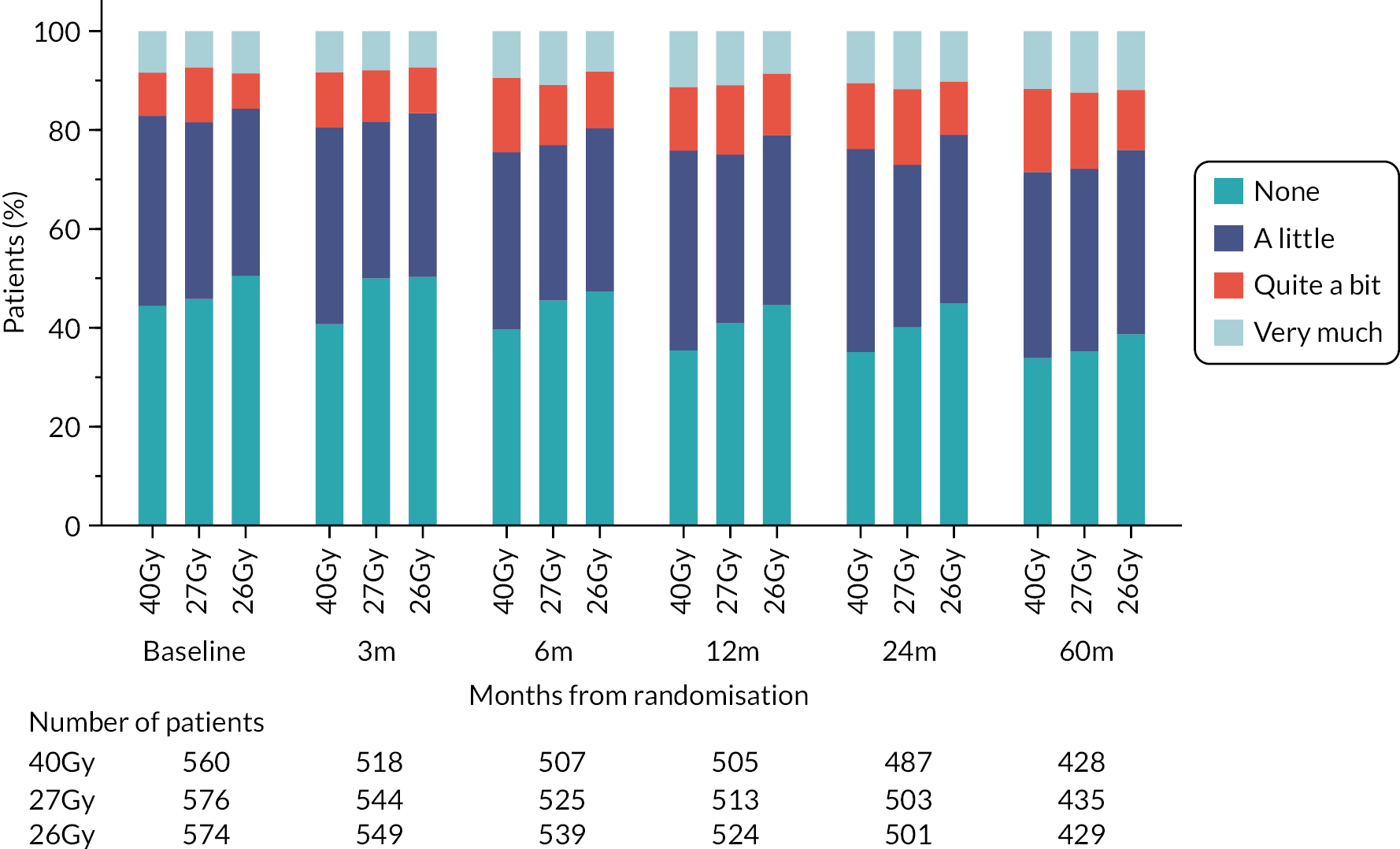
FIGURE 51.
Patient-assessed breast hardness up to 5 years in the Main Trial.
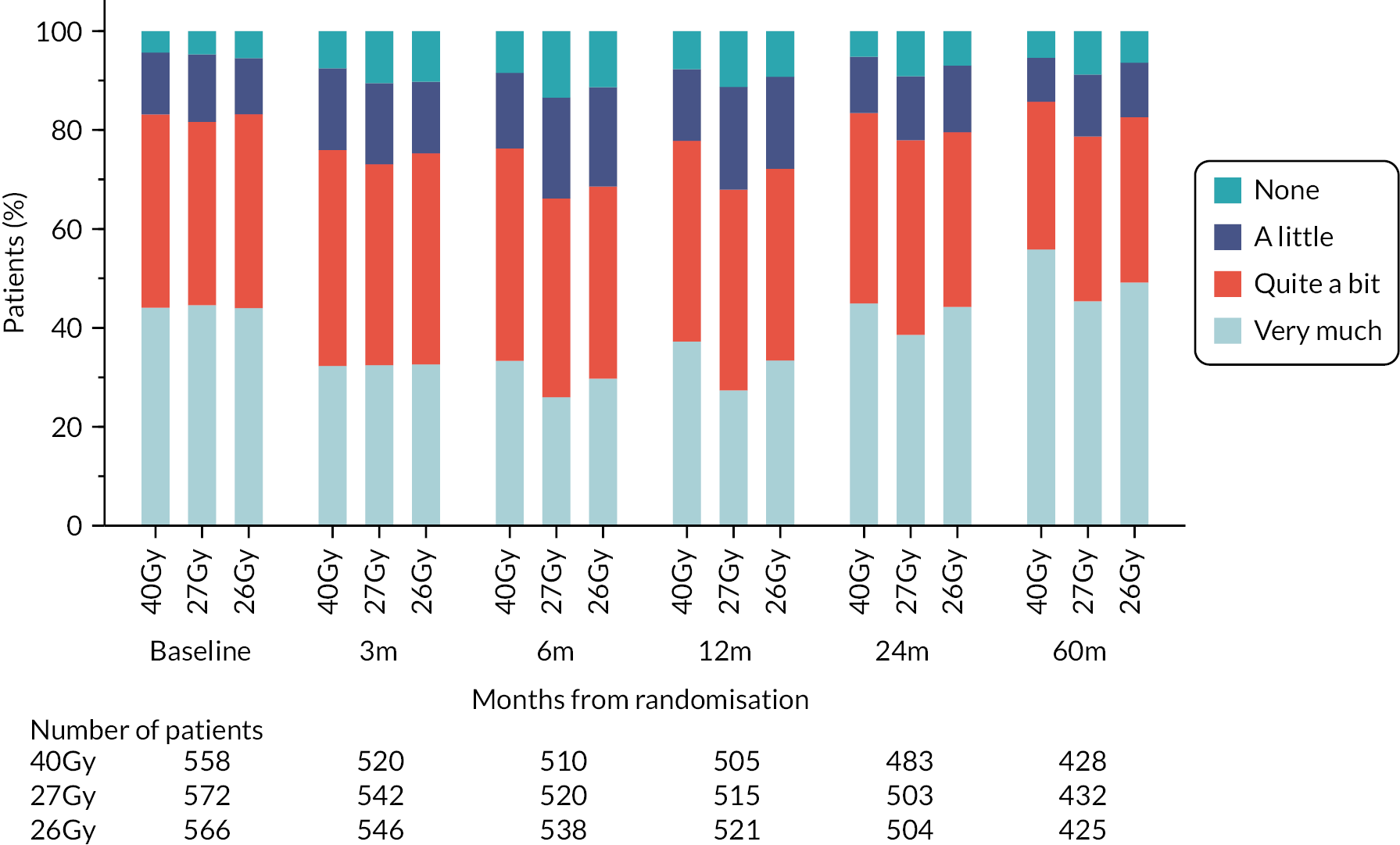
List of abbreviations
- AE
- adverse effects
- ASTRO
- American Society for Radiation Oncology
- BCS
- breast conservation surgery
- BIS
- body image scale
- CI
- confidence interval
- CIN
- cervical intra-epithelial neoplasia
- CRF
- case report form
- CT
- computerised tomography
- CTCAE
- common toxicity criteria for adverse effects
- CTV
- clinical target volume
- DCIS
- ductal carcinoma in situ
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQD2
- equivalent dose in 2 Gy fractions
- ER
- oestrogen receptor
- ESTRO
- European Society for Radiotherapy and Oncology
- GEE
- generalised estimating equations
- HE
- health economic
- HER-2
- human epidermal growth factor receptor-2
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- IBTR
- ipsilateral breast tumour relapse
- ICR-CTSU
- The Institute of Cancer Research-Clinical Trials and Statistics Unit
- IDMC
- Independent Data Monitoring Committee
- IGRT
- image-guided radiotherapy
- IMC
- internal mammary chain
- IMRT
- intensity-modulated radiotherapy
- IQR
- interquartile range
- NCRI
- National Cancer Research Institute
- NICE
- National Institute for Health and Care Excellence
- NTE
- normal tissue effects
- OCOG
- Ontario Clinical Oncology Group
- OR
- odds ratio
- PH
- proportional hazards
- PPI
- patient and public involvement
- PR
- progesterone receptor
- PRO
- patient-reported outcome
- PTV
- planning target volume
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RR
- risk ratio
- RT
- radiotherapy
- RT QA
- radiotherapy quality assurance
- RTOG
- Radiation Therapy Oncology Group
- RTTQA
- radiotherapy trials quality assurance
- SCF
- supraclavicular fossa
- SIB
- simultaneous integrated boost
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
