Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR127550. The contractual start date was in October 2019. The draft report began editorial review in June 2022 and was accepted for publication in May 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Sahota et al. This work was produced by Sahota et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Sahota et al.
Chapter 1 Background and introduction
Response to the commissioned call
This report presents the findings from a National Institute for Health and Care Research (NIHR)-funded study that was conducted between May 2019 and February 2022. The study was developed in response to a Health Technology Assessment (HTA) programme call in 2018, inviting applications for a mixed-methods study to explore and ascertain the evidence for effectiveness and cost-effectiveness of the different bisphosphonate (BP) regimens compared to oral alendronate (ALN), as well as capturing the experience and opinion of these communities.
The commissioned research question was:
What is the clinical and cost-effectiveness of alternative regimens of BP use in comparison to the standard regimen of ALN in preventing osteoporotic fracture in adults?
-
Intervention: Alternative BP regimens for the prevention of osteoporotic fracture in adults.
-
Patient group: Adults diagnosed with osteoporosis and/or fragility fracture, where current guidelines recommend prophylactic treatment with BP.
-
Setting: Primary care and any other suitable setting.
-
Comparator: Current standard ALN regimen (as per guidelines).
-
Study design: (1) A systematic review and cost-effectiveness analysis of different BP regimens. (2) A qualitative study to explore patient and clinician views and experiences of current regimens (including, but not limited to, individuals who are engaged in research) and to identify the most important research questions for these communities in relation to BP use.
-
Outcomes/outputs: Findings of the systematic review and cost-effectiveness, patient and clinician experience and opinion; recommendations for future research, including research questions acceptable to patients, clinicians and researchers.
Bisphosphonate and adherence in context
Osteoporosis is a condition that is characterised by low bone mass and structural deterioration of bone tissue, resulting in bone fragility and susceptibility to fracture – ‘fragility fracture’. 1 The condition is age-related and particularly common in postmenopausal women. Current National Institute for Health and Care Excellence (NICE) guidelines recommend that people with osteoporosis and fragility fracture or with osteoporosis and risk factors indicating high risk of future fracture should be offered BP treatment. 2 This treatment has been shown to increase bone density and reduce the risk of fragility fracture by 20–70%, depending on the site of fracture. 3–5
Alendronate is recommended as the first-line BP treatment in adults in England and Wales;2 however, complex dosing instructions are required to support drug absorption and reduce side effects. This medication is taken orally, once a week, at least 30 minutes before food or other medicines, with a minimum of 200 ml of plain water, and patients are recommended to remain upright while taking it and for at least 30 minutes after. 6 Taking ALN correctly (treatment compliance) is challenging for some patients, in particular, older patients on multiple medications and those with underlying cognitive impairment. 7,8 Long-term treatment persistence (defined as the cumulative time duration from initiation to discontinuation of therapy) is also poor with ALN. The reasons for this are multifactorial and include scepticism over benefits and safety, lack of understanding of the consequences of non-treatment and risk of or experienced side effects. 9–14 In everyday clinical practice, long-term treatment adherence (encompassing both compliance and persistence)15 with ALN is poor, ranging from 16% to 42% over 2 years. 16,17 Alternative BP regimens to ALN are available and vary in frequency of use and/or route of administration. These include monthly oral ibandronate (IBN), 3-monthly intravenous (IV) IBN and yearly IV zoledronate (ZOL). These alternative BP regimens have been shown to improve long-term adherence;18–21 however, the most clinically and cost-effective regimen remains unclear. Furthermore, clinicians should also take into account patient understanding, preferences and characteristics around medication. What is most cost-effective in clinical trials may not be the most cost-effective or acceptable in everyday clinical practice. Therefore, in keeping with the commissioning brief, a mixed-methods research study was undertaken to explore and ascertain the evidence for effectiveness and cost-effectiveness of the different BP regimens compared to ALN, as well as capturing the experience and opinions of clinicians and patients.
Importance in terms of improving the health of patients
Osteoporosis is a common clinical condition, affecting over 3 million people in the UK. This leads to weakening of the bones, making them fragile and more likely to fracture. In the UK, there are approximately 536,000 new fragility fractures each year, comprising 79,000 hip fractures, 66,000 clinically diagnosed vertebral fractures, 69,000 forearm fractures and 322,000 other fractures (i.e. fractures of the pelvis, rib and other long bones). 22 The healthcare costs are enormous, estimated at £4.4 billion per year and are expected to rise by 25% over the next 5 years,23 due to an ageing society. Fragility fractures are a life-changing experience with consequent loss of mobility and independence, social isolation, depression and increased mortality. 24,25 Any fragility fracture approximately doubles the risk of another fracture. 22 A key priority of the NHS and NIHR (in its current themed call – complex health needs) is to promote healthy ageing and prevent unplanned hospital admissions. Hip fractures alone account for 85,000 unplanned admissions and 1.8 million bed-days in the UK per year. 26 Effective fracture prevention is therefore an important strategy in meeting this aim and would impact favourably on several outcomes that are of importance to patients, including the ability to live independently, pain, disability and death. Improving long-term adherence alone with fracture prevention treatments from 60% to 80% would result in a saving to the NHS of £4.3 million over 5 years for secondary prevention. 27,28
There is relatively little qualitative literature regarding patient experiences or preferences for BP regimens; for example, a systematic review and meta-ethnography of patient experiences of living with osteoporosis reported patient uncertainty about the purpose of medication but no findings relating to experiences of taking BP. 29,30 Hiligsmann et al. reviewed existing quantitative preference studies in 2016 and concluded that patients generally preferred less frequent dosing regimens but noted variation in preferences. 31,32 One important limitation of these discrete preference studies is that patients were asked to choose between hypothetical treatments and not real ones and were limited to four attributes (efficacy, side effects, route and frequency of administration). In real life, other practical attributes, such as how and how often the drug is administered, will also be important to patients. In a more recent qualitative study exploring the reasons for non-adherence, upper gastrointestinal (GI) side effects with ALN were graphically described, although anticipation of side effects was as much a deterrent to adherence as was actually experiencing the side effects. 33 For those able to tolerate oral BP, various strategies have been proposed to try and improve long-term adherence, which include the use of reminders, patient education and treatment monitoring. 34–37 A Cochrane review of strategies to improve treatment adherence highlighted the importance of more frequent patient interactions and regular discussion over compliance issues. 38 The International Bone Working Group on treatment adherence recently recommended the routine use of bone turnover markers to aid treatment compliance. 39 A preferred alternative to oral BP, whether daily, weekly or monthly is an annual, IV infusion of BP (ZOL). Patients have reported increased satisfaction with ZOL compared with weekly ALN and higher long-term adherence. 40,41 Administering BP intravenously is an obvious strategy to improve compliance, and ZOL is inexpensive; however, needle phobia, infusion centre costs, side effects, scheduling reminders and the treatment burden of attending hospital for the infusion are potential barriers to long-term persistence. Across Nottinghamshire, to address some of these challenges, IV ZOL is now administered as first-line treatment to older patients with fragility fractures directly in their own home,42 with high patient preference and high satisfaction when compared to the same drug being administered during attendance at a hospital-based infusion centre. 43 Within central Nottingham, IV ZOL is administered as part of the community osteoporosis service, thereby addressing not only issues around drug administration but also issues around patient education, benefits of treatment and long-term persistence. 44 The recent HTA systematic review and economic evaluation of BP for the prevention of fragility fractures, led by our co-applicants SD and NG45 and which informed the NICE TA464 guideline,2 concluded that BP are effective in preventing fragility fractures; however, the benefit-to-risk ratio in the lowest-risk patients may be debatable given the low absolute quality-adjusted life-year (QALY) gains and the potential for adverse events. While the model was structured to allow direct comparisons between different BP, several simplifying assumptions were made that limited the accuracy of the comparisons between the different BP treatments. For example, the model assumed equivalent treatment persistence and adverse effects for all oral BP, whether they are given daily, weekly or monthly. One situation in which this may be problematic is when considering the frequency of GI adverse events, which relates to oral administration. Similarly, the adverse events and treatment persistence for quarterly IV IBN were assumed to be the same as those for yearly IV ZOL.
Chapter 2 Systematic review of bisphosphonate acceptability amongst patients, clinicians and managers
Some text, tables and figures in this chapter have been reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634. https://doi.org/10.1136/bmjopen-2020-040634. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text, tables and figures below include minor additions and formatting changes to the original text.
Introduction
A recent network meta-analysis (NMA) demonstrated that BP treatment reduces the risk of fragility fracture (depending on site) by 33–54%. 2 Oesophageal or GI-related side effects are the most common adverse effects of oral BP use. To counter these, patients taking oral BP are required to remain upright and fast for half an hour after ingestion. Rare side effects of BP include osteonecrosis of the jaw and atypical femur fractures, both of which have received significant media attention. Such media reports are temporally related to declining BP use. 46 Due to the GI side effects and special instructions for taking oral treatment, it has been suggested that alternative BP regimens, for example, IV ZOL, may promote long-term adherence. Studies to date which have examined patient preferences for osteoporosis treatment suggest that patients prefer injections given less frequently;47–49 however, research in other chronic diseases shows that although adherence is improved with less frequent medications and that patients prefer oral to injection treatment. 50 In osteoporosis, the majority of studies that explore patient preferences employ quantitative methods, for example, discrete choice experiments where patients are asked to choose between hypothetical treatments in regard to various attributes (e.g. efficacy, side effects, route and frequency of administration). Such studies cannot provide comprehensive insight into patient views, experiences or the explanations for these preferences. In order to fully understand the osteoporosis treatment gap, and ultimately improve adherence, it is important to understand the perspectives of all relevant stakeholders: patients, healthcare professionals (HCPs), managers, payers and academics. 51,52 This can be achieved using the lens of ‘acceptability’, defined as ‘a multi-faceted construct that reflects the extent to which people delivering, or, receiving a healthcare intervention consider it to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention’. 53,54 In the context of a research programme designed to determine the research agenda for optimising BP treatment, the primary aim of this chapter was to explore the acceptability of different BP regimens among patients, clinicians and managers.
Methods
We conducted a systematic review and framework synthesis of qualitative studies exploring patient and clinician views and experiences of BP. The conduct and reporting of this review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol for this chapter was registered in PROSPERO (CRD42019143526).
Eligibility
To be eligible for inclusion, studies needed to report on patients’, clinicians’, academics’ and/or manager/payers’ experiences and preferences regarding BP regimes for adults (≥ 18 years) with osteoporosis. BP needed to be mentioned by name, or there needed to be sufficient information that was specific to BP (e.g. reference to the special instructions for use of oral BP) to deduce that study findings related to BP, as agreed by two clinically experienced authors independently. Papers describing experiences of osteoporosis more generally were included if there were findings relating to BP treatment in the study abstract. Studies were only included if they were qualitative in design or mixed methods with a qualitative component, relevant to a developed country setting and written in English. Studies were excluded that involved paediatric patients, patients and clinicians receiving/recommending other treatments for osteoporosis and studies in which BP were being used for other indications (e.g. malignancy or Paget’s disease).
Search methods
Systematic searches were conducted in seven bibliographic databases {MEDLINE, EMBASE, AMED, CINAHLPlus, PsycINFO, ASSIA and Web of Science [Social Science Citation Index (SSCI) and Conference Proceedings Citation Index-Social Science and Humanities (CPCI-SSH)]} from inception to 15 July 2019. The search strategy utilised database subject headings and text word searching in title, abstract or keywords, combining terms for: (1) BP; (2) experiences and preferences and (3) qualitative research, based on DeJean et al.’s search filter. Search terms were adapted as appropriate for each database platform.
In addition, grey literature was searched [DART Europe, Open Grey and National Digital Library of Theses and Dissertations (NDLTD)]; the reference lists of all included studies and relevant systematic reviews identified were checked, and key studies were citation tracked.
Study selection
Two-stage screening of articles against eligibility criteria was undertaken. Firstly, titles and abstracts were screened, then full texts. At both stages, screening was conducted independently by sets of two reviewers (co-applicants: NC, EC, ZP), and articles were excluded by agreement. Disagreements were resolved through discussion or by third-reviewer adjudication.
Data extraction
For each paper, data extraction was completed independently by two researchers (co-applicants: ZP and JW, or EC and FM). Key findings from the results sections of papers relating to BP were extracted; a ‘key finding’ was defined as any sentence or statement relating to views or experiences of BP from the results section of the paper or abstract. Wherever possible, the key finding was extracted as written by the author, with minimal edits only for clarification, description of context or for consistency across papers. For each paper, two authors extracted key findings independently and subsequently agreed on a final list of key findings for each paper. Data were also extracted on participant numbers and demographics, data collection technique, setting and country. Additionally, if available for patients, information was extracted on their BP use, including type of drug and current status (adherent, non-adherent, decliner).
Quality appraisal
The quality of each study was assessed using the Critical Appraisal Skills Programme (CASP) qualitative tool. This tool consists of 10 items split into 3 sections (qualitative suitability, data analysis and overall quality). The first two sections consist of items related to qualitative suitability and data analysis, which were evaluated as ‘yes’, ‘no’, ‘unclear’ or ‘partial’. The final question was an assessment based on the overall quality of the paper; this was informed by responses to the previous items (indicating methodological quality) and by the relevance of the study to the review objectives and was rated as ‘high’, ‘moderate’ or ‘low’. All papers were quality-appraised by three researchers independently (FM, SB, JW). Disagreements were resolved through discussion with a fourth reviewer (ZP).
Synthesis
We used a framework synthesis approach informed by the ‘best fit’ model described by Carroll et al. 55 The ‘best fit’ method offered a means to test, reinforce and build on an existing published model, conceived for a different but relevant purpose. This approach was chosen as a published theory identified from the literature that conceptualised acceptability – the theoretical framework of acceptability (TFA). The TFA is a relatively new framework which was developed to inform the understanding of acceptability of complex interventions and consists of seven constructs: affective attitudes – the emotions elicited by an intervention; intervention coherence – the extent to which an intervention makes sense; perceived effectiveness – the perceived extent to which intervention will achieve purpose; burden – the amount of effort required to participate in an intervention; self-efficacy – individual’s confidence that they can perform the behaviour(s) required to participate in the intervention; opportunity costs – the extent to which benefits, profits or values must be given up to engage in an intervention; and ethicality – the extent to which an intervention has a good fit with an individual’s values. The framework also incorporates temporal perspectives on anticipated and experienced acceptability at three time points: before (prospective), during (experienced) and after (retrospective) experience of an intervention.
The TFA has not previously been used to evaluate drug acceptability. We anticipated the seven constructs of the TFA would be relevant to engagement with drug treatment; for example, burden could relate to treatment burden associated with administering the drug or side effects. However, one aspect which did not appear to be explicitly conceptualised within the framework was patient beliefs about medicines. Studies across a range of long-term conditions, healthcare systems and cultures have consistently shown that engagement with treatment is influenced by patients’ personal evaluation of the medicine in question. 56 Particularly important is how they judge their personal need for treatment relative to their concerns about it. For this reason, we therefore included the Necessity-Concerns Framework (NCF) to further explore the TFA domain relating to intervention coherence.
The first author initially conducted inductive open coding on the extracted data, before mapping the codes to a draft framework derived from a priori themes (the domains of the TFA). Authors then met to first discuss the themes and compare findings for each study and the ‘fit’ to the draft framework. A preliminary synthesis was achieved using tabulation of studies, organising the studies into groups relating to temporal perspectives and research questions and exploring relationships between studies and between groups.
A final coding framework was agreed at a second meeting of authors. A second author (FM) recoded the original key findings, where necessary, to the new framework to ensure all findings were represented. Finally, relationships between themes and TFA and NCF domains were explored by further group discussion. We used the Grades of Recommendation, Assessment, Development, and Evaluation Confidence in the Evidence from Qualitative Reviews (GRADE-CERQual) approach to determine confidence in our synthesised findings. 57
Results
The literature search identified 2040 unique articles, of which 25 met eligibility criteria (Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram. Reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634.
A summary of the studies is shown in Table 1. The included studies were categorised into three groups: perceptions of osteoporosis generally,58–64 healthcare service delivery issues unrelated to osteoporosis (de-prescribing),65 interprofessional communication in primary care66 and studies specific to osteoporosis treatments. The latter group was further subdivided into: those examining treatment barriers,51,67–71 adherence,72–74 decision-making75–79 or BP-related side effects. 80,81 Only one study examining adherence and one examining decision-making had research questions which specifically related to BP. 73,78
| First author and year | Studies in Group 1: Views of osteoporosis | ||||||
|---|---|---|---|---|---|---|---|
| Participants | Participant No. (male : female) | BP use and adherence | Data collection methods | Qualitative approach or analysis method | Recruitment setting | Country | |
| First author and year | Studies in Group 2: Views of osteoporosis treatment (treatment barriers) | ||||||
| Participants | Participant No. (male : female) | BP use and adherence | Data collection methods | Qualitative approach or analysis method | Recruitment setting | Country | |
| First author and year | Studies in Group 2: Views of osteoporosis treatment (adherence) | ||||||
| Participants | Participant No. (male : female) | BP use and adherence | Data collection methods | Qualitative approach or analysis method | Recruitment setting | Country | |
| First author and year | Studies in Group 2: Views of osteoporosis treatment (BP side effects) | ||||||
| Participants | Participant No. (male : female) | BP use and adherence | Data collection methods | Qualitative approach or analysis method | Recruitment setting | Country | |
| Besser 201258 | Pts | 14 (0 : 14) | AOD unspecified | Interview | Framework analysis | One hospital | UK |
| Jaglal 200364 | HCPs Family physicians (n = 32) |
32 (12 : 20) | N/A | Focus group | Constant comparison | Primary care | Canada |
| Otmar 201263 | HCPs GP (n = 14), practice nurse (n = 2) |
16 (11 : 5) | N/A | Focus group | Analytic comparison Constant comparison |
Primary care | Australia |
| Sale 201559 | Pts | 28 (2 : 26) | 19/28 pts on AOD Adherent (n = 19) Declined (n = 4) |
Interview | Phenomenological study | National osteoporosis patient group | Canada |
| Sale 201060 | Pts | 24 (6 : 18) | 9/24 pts on AOD RIS (n = 8) Etidronate (n = 1) |
Focus group | Descriptive qualitative study | Fracture clinic | Canada |
| Weston 201161 | Pts | 10 (0 : 10) | AOD unspecified | Interview | Interpretative phenomenological analysis | Primary care | UK |
| Hansen 201762 | Pts | 15 (0 : 15) | AOD unspecified Adherent (n = 12) Declined/stopped AOD (n = 3) |
Interview | Phenomenological hermeneutic approach | Women attending DXA at 2 hospitals | Denmark |
| Alami 201670 | Mixed | Pts: 37 (0 : 37) HCPs: 18 (8 : 10) |
23/47 pts on AOD Adherent (n = 19) Declined/stopped AOD (n = 18) |
Focus group | Grounded theory | Hospital/community over 5 regions | France |
| Drew 201669 | HCPs Nurse (n = 14), GP (n = 2) Specialists (n =17), orthopaedic surgeon (n = 4) Managers (n = 5), DXA technician (n = 1) |
43 (not given) | N/A | Interview | Thematic approach | 11 hospitals in 1 region | UK |
| Feldstein 200851 | Mixed | Pts: 10 (0 : 10) HCPs: 57 (not given) |
AOD unspecified | Interview and focus group | Content analysis | Primary and secondary care | USA |
| Guzman-Clark 200771 | Mixed | 100 (94 : 6) | 24/100 pts on AOD | Focus group | Thematic content analysis | Urban Academic Medical Centre | USA |
| Merle 201967 | HCPs (GP) | 16 (11 : 5) | N/A | Interview | Descriptive thematic analysis | Primary care | France |
| Merle 201968 | Pts | 98 (53 : 45) | AOD unspecified | Focus group | Inductive thematic analysis | Recruited from two existing research studies and community (medical insurance company) | France |
| Iversen 201174 | Mixed | Pts: 32 (2 : 30) HCPs: 12 (5 : 7) |
AOD unspecified | Focus group | Open coding (thematic analysis) | Secondary care | USA |
| Lau 200872 | Pts | 37 (0 : 37) | 33/37 pts on AOD ALN (n = 9), etidronate (n = 5) RIS (n = 19) |
Focus group | Mixed phenomenological design | Primary care, secondary care and community pharmacies | Canada |
| Salter 201473 | Pts | 30 (0 : 30) | 20/30 pts on AOD Adherent (n = 19) Declined (n = 1) Stopped AOD (n = 10) |
Interview | Framework analysis | Primary care | UK |
| Studies in Group 2: Views of osteoporosis treatment (decision-making) | |||||||
| Mazor 201075 | Pts | 36 (0 : 36) | 15/36 pts on AOD Adherent (n = 15) Declined (n = 10) Stopped (n = 11) |
Telephone Interview | (Thematic analysis) | Primary care | USA |
| Sale 201179 | Pts | 24 (6 : 15) | 14/21 pts on AOD | Telephone Interview | Phenomenological study | Hospital-based fracture screening programme | Canada |
| Swart 201877 | Mixed | Pts: 26 (4 : 22) HCPs: 13 (not given) |
10/26 pts on AOD Adherent (n = 10) Declined (n = 16) |
Interview | Thematic analysis with elements of grounded theory | Recruited from a fracture prevention study | Netherlands |
| Scoville 201178 | Mixed | Pt: 18 (0 : 18) HCP: 19 (12 : 7) |
N/A | Videographic | (Deductive checklist and descriptive) | Primary care (osteoporosis choice trial) | USA |
| Wozniak 201776 | Pts | 12 (3 : 9) | 7/12 pts on AOD Adherent (n = 7) Stopped (n = 5) |
Interview | Grounded theory | Recruited from a fracture prevention trial nested in secondary care | Canada |
| Sturrock 201981 | Mixed | 24 (4 : 19) | 13/23 pts on AOD | Interview | Grounded theory | Three regions including from secondary care | UK |
| Sturrock 201780 | Pts | 17 (7 : 10) | N/A | Interview | Grounded theory | Primary care | UK |
| Studies in Group 3: Non-specific osteoporosis issues | |||||||
| Ailabouni 201665 | HCPs | 10 GPs | N/A | Interview | Constant comparison | Primary care | New Zealand |
| Sippli 201766 | HCPs | 28 (6 : 22) | N/A | Interview | Content analysis | Primary care | Germany |
The majority of studies were conducted in North America or Europe. Eight studies explored patient views,58–65 seven explored HCPs’ views,63–69 seven had a mixed sample51,70,71,74,77,78,81 and two studies interviewed managers. 51,69 No studies included academic or payer participants. Of the 18 papers that included patients, 10 studies described how many of the patients were on anti-osteoporotic medication; however, only 2 reported the specific type of medication. Only one study reported patient experience of receiving IV BP. 62
The findings related to quality appraisal are summarised in Table 2. The most common limitations of the included studies were lack of description of author reflexivity, lack of depth of analysis, use of normative statements and relatively small samples or studies conducted in a single site which may limit transferability of the findings. Furthermore, although the characteristics of the sample were generally reasonably described, in order to address our research question, we required information about medication use of participants which was frequently not described.
| First author and year | CASP tool question | Comments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Group1: Views of osteoporosis | |||||||||||
| Besser 201258 | ✓ | ✓ | ✓ | p | ✓ | ✓ | p | ✓ | Moderate | Small sample, no mention of data saturation, limited to ‘psychological’ factors affecting adherence (discounting other factors by omission) and some use of normative statements | |
| Jaglal 200364 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | Moderate | Few findings relevant to our research question |
| Otmar 201263 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | Well-conducted study, but limited findings relating to BP | |
| Sale 201559 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Sale 201060 | ✓ | ✓ | ✓ | p | ✓ | u | ✓ | p | ✓ | Moderate | Small single-site study, although data saturation was reached. Language does not always appear to match approach (e.g. reporting patient’s ‘inability’ to link fractures to osteoporosis suggests prior normative assumptions) |
| Weston 201161 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | |
| Group 2: Views of osteoporosis treatment | |||||||||||
| Alami 201670 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | ||
| Drew 201669 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Feldstein 200851 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Guzman-Clark 200771 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | u | ✓ | Moderate | Only partially relevant for our review given the focus on a specific population (glucocorticoid-induced osteoporosis) |
| Merle 201967 | ✓ | ✓ | ✓ | p | ✓ | u | ✓ | u | ✓ | Moderate | Small sample (although data saturation reached) without attempt to structure to population and analysis lacks depth to answer our objective relating to BP acceptability |
| Merle 201968 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | Moderate | Limited information relevant to our research question in view of general focus on osteoporosis |
| Iversen 201174 | ✓ | ✓ | ✓ | p | ✓ | ✓ | p | ✓ | Moderate | Single-centre study, although data saturation reached, limited information on coding/analysis and no discussion of findings with relevance to wider literature | |
| Lau 200872 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | ||
| Salter 201473 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | ||
| Hansen 201762 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | |
| Mazor 201075 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | u | ✓ | Moderate | Good relevance, single site. Descriptive approach without critical reflexivity or discussion of prior assumptions |
| Sale 201179 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Swart 201877 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | High | |
| Scoville 201178 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | Moderate | Well-conducted videographic study, but data coded against deductive categories of reasons to reject treatment, so limited potential to inform our objective about acceptability |
| Wozniak 201776 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Sturrock 201981 | ✓ | ✓ | ✓ | ✓ | ✓ | u | ✓ | ✓ | ✓ | High | |
| Sturrock 201780 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | Aim only partially relevant to study question | |
| Group 3: Non-specific osteoporosis issues | |||||||||||
| Ailabouni 201665 | ✓ | ✓ | ✓ | p | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | Relatively small (10 respondents) study, although data saturation reached. Only partially relevant for current review with brief coverage of GP’s views on discontinuing BP in light of multimorbidities |
| Sippli 201766 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Moderate | Limited findings related to our research question | |
Using the CASP tool, 12 (48%) studies were scored as high value and the remaining 13 (52%) studies as moderate value. For 5/13 (38%) studies scored as moderate in value, this was due to methodological issues, and for 8/13 (62%) studies this was because the focus of the paper was less relevant to our research question.
Fifteen individual subthemes were identified, which mapped to the seven domains of the TFA. Key findings relating to ethicality related to conflict between BP and participants’ values and were usually discussed as part of sense-making. For this reason, issues relating to ‘ethicality’ were considered as part of ‘intervention coherence’, leaving six main themes, as shown schematically in Figure 2.
FIGURE 2.
Identified themes and subthemes mapped to the TFA. Reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634. https://doi.org/10.1136/bmjopen-2020-040634.
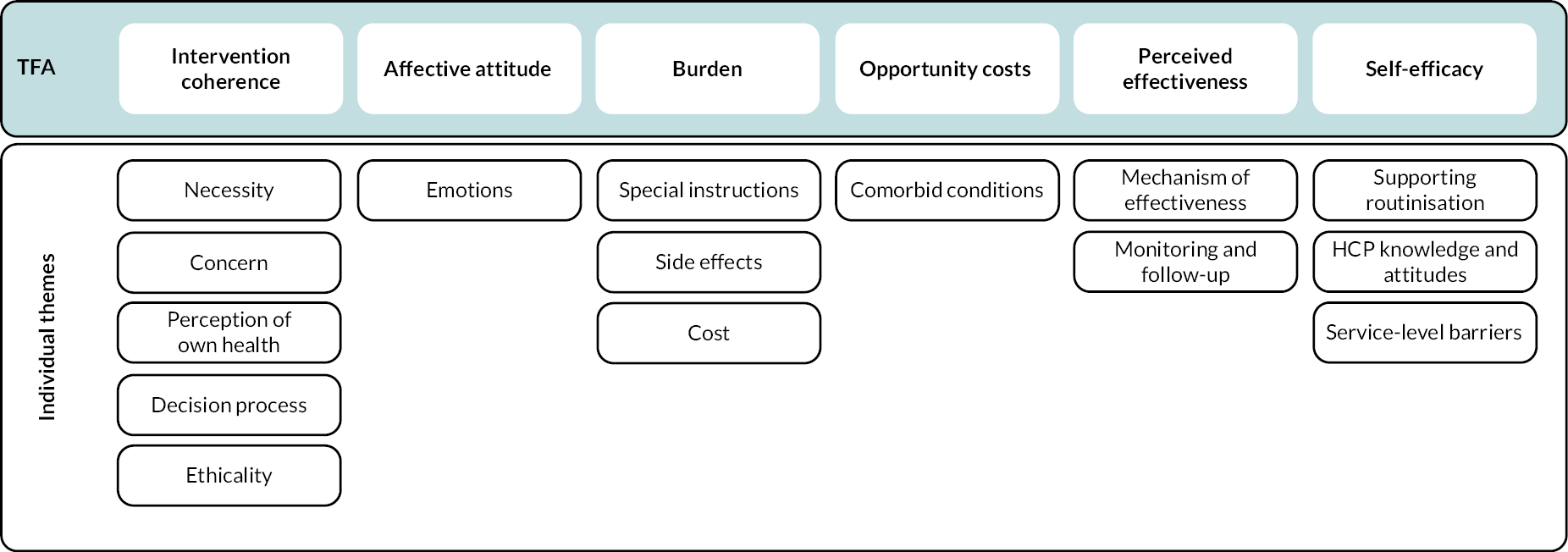
Although it was possible to distinguish between two temporal perspectives related to anticipated and experienced acceptability within most domains (with the exception of self-efficacy), the majority of anticipated acceptability findings related to intervention coherence.
The findings of the review are discussed below with GRADE-CERQual (Confidence in the Evidence from Reviews of Qualitative Research) ratings of confidence in Table 3.
| Review findings (and contributing studies) | Methodological limitations | Coherence | Adequacy | Relevance | CERQual confidence assessment |
|---|---|---|---|---|---|
| Concerns | |||||
| Intervention coherence: Both before starting and during treatment, patients considered the perceived need or necessity for BP based on their views of osteoporosis, including its seriousness and controllability, symptoms and their perception of their own health. Perceived need was weighed up against concerns about medication, including suspicion of drugs in general and specific concerns about BP safety by both patients and HCPs. HCPs sometimes used principles of ethicality to support perceptions of low necessity and their reluctance to prescribe. The decision process of balancing necessity against concerns was influenced by the doctor–patient relationship and wider societal influences, including friends, family and general media. This process influenced whether HCPs reported recommending BP. For patients, the decision process could be explicit or tacit, was revisited over time and influenced both whether they initiated treatment and subsequently adhered. |
Minor 12/22 papers rated moderate value due to sample size, depth of analysis or lack of reflexivity |
None or very minor The finding reflects the complexity and variation of the data, and these influences on sense-making are well supported by details in the underlying studies |
None or very minor 22 papers contributed to this finding, and although some gave little detail, in-depth insights were reported in 10 papers, and information was consistent across studies |
Minor Spread of studies from primary and secondary care and range of countries. Uncertainties remain about sense-making-related patients taking IV BP and influence of gender |
High |
| Perceived effectiveness Both patients and HCPs expressed doubt or uncertainty about the mechanism of effectiveness of BP and expressed a range of treatment expectations, including strengthening bone – improving bone density, preventing worsening of osteoporosis – maintaining bone density and/or total fracture prevention. Patients wanted proof or evidence of effectiveness through more structured monitoring and follow-up and were disincentivised to continue treatment in the absence of evidence of perceived effectiveness. |
Minor 7/15 papers rated moderate value, mostly (4/7) due to limited relevant content. Methodological concerns relate to depth of analysis or lack of reflexivitya |
None or very minor The finding reflects the complexity and variation of the data, and these issues are supported by details in the underlying studies |
None or very minor 15 papers contributed to this finding. Some gave little detail, but in-depth insights were reported in 6 papers, and information was consistent |
Minor Spread of studies from primary and secondary care and range of countries. Uncertainties remain about perceived effectiveness of IV BP |
High |
| Self-efficacy: Measures to help patients integrate medication taking into their daily routines (supporting routinisation), and the provision of information and support, enhanced their feeling of having control over their health and confidence to adhere to BP. Clinicians reported barriers to supporting adherence related to perceptions of their knowledge and attitudes, with several knowledge gaps and uncertainties reported and the perception that osteoporosis was not a priority. Finally, service-level barriers which impaired clinicians’ self-efficacy in recommending and managing patients on BP included uncertainty about professional roles and responsibilities, capacity, access to IV drugs and communication and IT systems. |
Minor 7/15 papers rated moderate value, mostly (4/7) due to limited relevant content. Methodological concerns relate to depth of analysis or sample sizea |
None or very minor The finding reflects the complexity and variation of the data, and these issues are supported by details in the underlying studies |
None or very minor 17 papers contributed to this finding. Some gave little detail, but in-depth insights were reported in 5 papers, and information was consistent |
Minor Spread of studies from primary and secondary care and range of countries. Uncertainties remain about self-efficacy relating to IV BP |
High |
| Affective attitudes: The emotions elicited by BP were closely related to intervention coherence. BP were associated predominantly with negative emotions of fear (of side effects) and annoyance (with special instructions); however, positive emotions of reassurance and hope were noted in two studies, linked to the anticipated protection that BP could incur. |
Minor 2/8 papers rated moderate value due to depth of analysis or lack of reflexivitya |
None or very minor The finding reflects the data, supported by details in the underlying studies |
Moderate Reports of affective attitudes were mostly descriptive with little depth |
Moderate Uncertainties remain about affective attitudes towards injectable BP received in hospital |
Moderate |
| Burden: The burden or effort of oral BP was described mostly relating to the special instructions to take oral BP or experienced side effects, although costs incurred were also a potential source of burden. |
Minor 4/11 papers rated moderate value due to sample size, depth of analysisa |
None or very minor The finding reflects the data, and these aspects of burden are supported by details in the underlying studies |
Moderate Reports mostly descriptive with little depth and a possible focus on presence of burden (side effects) rather than absence |
Moderate Uncertainties remain about burden of indirect costs (travel, dental checks) and burden due to IV BP |
Moderate |
| Opportunity costs: Circumstances where competing priorities challenged adherence or initiation of BP were described relating to comorbid conditions. The presence of comorbid conditions was described as resulting in less time to support discussion about BP in consultations and result in recommendation of, and adherence to, BP being given relative low priority. |
None or very minor 4/11 papers rated moderate value, but this was mostly (n = 3) due to limited relevant content rather than methodological concerns. |
Moderate No discussion of the alternative explanation that having comorbid conditions may facilitate BP acceptability |
Moderate Reports were limited, lacked depth, and three papers contained little content relevant to the research question |
Moderate No information about values, benefits that have to be given up to partake in IV BP, which are likely to be different and likely limited sampling of patients with complex health needs |
Low |
Intervention coherence (high confidence)
Both before starting and during treatment, patients considered the perceived need or necessity for BP based on their views of osteoporosis, including its seriousness and controllability, symptoms and their perception of their own health. Perceived need was weighed up against concerns about medication, including suspicion of drugs in general and specific concerns about BP safety, by both patients and HCPs. HCPs sometimes used principles of ethicality to support perceptions of low necessity and their reluctance to prescribe. The decision process of balancing necessity against concerns was influenced by the doctor–patient relationship and wider societal influences, including friends, family and the general media. This process influenced whether HCPs reported recommending BP. For patients, the decision process could be explicit or tacit, was revisited over time and influenced both whether they initiated treatment and subsequently adhered.
Perceived effectiveness (high confidence)
Both patients and HCPs expressed doubt or uncertainty about the mechanism of effectiveness of BP and expressed a range of treatment expectations, including strengthening bone – improving bone density, preventing worsening of osteoporosis – maintaining bone density and/or total fracture prevention. Patients wanted proof or evidence of effectiveness through more structured monitoring and follow-up and were disincentivised to continue treatment in the absence of evidence of perceived effectiveness.
Self-efficacy (high confidence)
Measures to help patients integrate medication taking into their daily routines (supporting routinisation), and the provision of information and support, enhanced their feeling of having control over their health and confidence to adhere to BP. Clinicians reported barriers to supporting adherence related to perceptions of their knowledge and attitudes, with several knowledge gaps and uncertainties reported and the perception that osteoporosis was not a priority. Finally, service-level barriers which impaired clinicians’ self-efficacy in recommending and managing patients on BP included uncertainty about professional roles and responsibilities, capacity, access to IV drugs and communication and IT systems.
Affective attitudes (moderate confidence)
The emotions elicited by BP were closely related to intervention coherence. BP were associated predominantly with negative emotions of fear (of side effects) and annoyance (with special instructions); however, positive emotions of reassurance and hope were noted in two studies, linked to the anticipated protection that BP could incur.
Burden (moderate confidence)
The burden or effort of oral BP was described mostly relating to the special instructions to take oral BP or experienced side effects, although costs incurred were also a potential source of burden. Only one study included the experience of a patient on an IV BP. This patient described low treatment burden as she only had to go once a year and felt no side effects (62).
Opportunity costs (low confidence)
There were few descriptions of ‘benefits, profits or values’ being given up to take BP. However, circumstances where competing priorities challenged adherence or initiation of BP were described relating to comorbid conditions. The presence of comorbid conditions was described as resulting in less time to support discussion about BP in consultations and resulted in recommendation of, and adherence to, BP being given relative low priority.
Discussion
This systematic review used the lens of acceptability to understand perceptions of BP and the problem of poor adherence. We have identified, with high confidence, how patients and HCPs make sense (coherence) of BP by balancing perceptions of need against concerns, how uncertainty prevails about perceived effectiveness of BP and how a number of individual and service factors have potential to increase self-efficacy in recommending and adhering to BP. We identified with moderate confidence that BP taking induces fear but has the potential to engender reassurance, and that both the side effects and special instructions for taking oral BP can be a source of treatment burden. Finally, we identified with low confidence that multimorbidity plays a role in people’s perception of BP acceptability.
To our knowledge, this is the first use of the TFA, originally developed to evaluate acceptability of complex interventions, to evaluate the acceptability of medication. We explored the utility of the TFA from two perspectives, as an explanatory model for both patient and clinician acceptability and engagement. The TFA was useful for understanding and combining patient and clinician viewpoints; however, there was considerable overlap between domains; perceived efficacy, affective attitudes and self-efficacy beliefs are all likely to impinge on sense-making or intervention coherence. The TFA alone does not provide a comprehensive framework for understanding patient acceptability or engagement with medicines, and of course, it was not intended to do so. The sense-making aspect of the framework appeared pivotal, and the explanatory value of the framework was enhanced by the incorporation of the NCF to operationalise key engagement-related beliefs. In the context of BP, concern and associated fears predominate among patients, and perceived need may be underestimated if the consequences of osteoporosis and fragility fractures are not explained. In our findings, sense-making was dynamic. Patients re-evaluated perceptions of BP over time, expressing uncertainty relating to what represents successful treatment and citing perceived lack of effectiveness as a reason to discontinue. This is likely to be a particular problem for BP, as opposed to other drugs commonly taken for prevention, such as statins and antihypertensive, where measures of feedback and effectiveness are more readily available.
The NICE guidelines for medicine adherence emphasise the need to take into account perceptions (e.g. necessity beliefs and concerns) and practicalities (e.g. capability and resources) that will affect individuals’ motivation and ability to start and continue with treatment. 82 However, interventions designed to improve BP adherence are often designed to ‘educate’ or persuade the patient of importance and are often not targeted to eliciting or addressing health beliefs or informed by underpinning mechanisms of change. There is therefore a need to ensure that any further design of interventions – to promote BP adherence – draws on more comprehensive theoretical models of patient engagement with health conditions and medicines, such as the Extended Common Sense Model. 83 This model situates individuals’ perceptions about drugs and practical issues related to capability, in the context of illness and treatment representations.
Specifically, our findings suggest a need for clinicians to support patients to understand the need for treatment, to allay concerns where possible and to define what constitutes successful BP treatment. Furthermore, clinicians need to support patients in evaluating the advantages and disadvantages over time, given the dynamic nature of these decision processes. It is clear from our findings that clinicians also have necessity–concern dilemmas relating to BP. A number of studies reported clinicians themselves perceiving low patient need, high concerns and perceptions that treatment was not practical. This is perhaps in contrast with a previous quantitative study in asthma which demonstrated that clinicians held stronger positive beliefs about medicines than patients. 84 It is unclear to what extent the perceptions in our findings were generalisations or applied in specific circumstances, or to what extent these views were negotiated on an individual basis in discussion with patients. Problems may arise in the consultation if clinicians assume patients share their views and then maybe less likely to explore patient perceptions of needs or concerns. Furthermore, the limitations of interviewing HCPs are well documented; the accounts presented in an interview may not represent clinicians’ underlying beliefs or behaviours, meaning that observational methods may be more appropriate to fully understand clinical decision-making. 85 Given that the clinician has a pivotal role in sense-making, interventions are also likely needed to address clinician knowledge, attitudes and beliefs. By including the views of clinicians and managers, we have also identified a range of service-level barriers to promoting BP adherence relating to lack of clarity about professional roles, both across primary and secondary care, and within primary care, use of IT systems and access to IV treatments.
Strengths and limitations
A strength of this review is the comprehensive search, use of underpinning theoretical framework, inclusion of clinician views in addition to patients and use of the GRADE-CERQual to give confidence in our findings, which has facilitated a clear identification of where further research is needed. Areas where we have identified moderate or low confidence are in need of further research and specifically relate to the influence of multimorbidity on sense-making, burden and self-efficacy in BP users, the extent to which IV BP may overcome issues related to treatment burden and self-efficacy and the impact of BP on affective attitudes and emotions. Furthermore, we have identified gaps in our understanding of how clinicians make decisions in practice and how views of BP may be influenced by gender. Given that many osteoporosis drugs have a different evidence base and licensing arrangements in men, this is an area in need of further study.
The main limitation of this review relates to the lack of clarity in many of the included studies in the results sections about which osteoporosis treatments or BP were being referred to, meaning that in some cases we may have overinterpreted findings relating to BP that were about other osteoporosis drugs. However, all of our review findings were identified from a comparison of data from several studies, and as BP represent the mainstay of osteoporosis treatment, we consider that overinterpretation is unlikely. As there was frequently little detail about medication participants were taking or referring to, it is also possible that we have missed relevant studies. Only two studies reported the views of managers, but unfortunately neither of these studies distinguished professional roles in the presentation of results, so a further need exists to explore perceptions of this group and perceptions of payors and academics. Finally, although the population from which each study sampled was reasonably well described, it was not always possible to appreciate if the setting was primary or secondary care; the majority of studies appeared to recruit from primary care, which may explain the lack of findings related to IV BP and limit the transferability of our findings to non-primary care settings.
Conclusion
In summary, using the lens of acceptability, we have identified the factors that influence how patients and clinicians make sense of BP, described the experience of BP taking in terms of burden and factors that both facilitate and hinder confidence in taking, and prescribing and monitoring BP. Our findings demonstrate the need for a theoretically informed, whole-system approach to enable clinicians and patients to get the best from BP treatment. Patients need comprehensive support that takes account of the perceptions (e.g. treatment necessity beliefs and concerns) and practicalities (e.g. capability and resources) that influence their motivation and ability to start and continue with treatment. Clinicians need to moderate patient expectations and clarify what constitutes BP treatment success. Further research is needed to explore perspectives of managers, patients receiving IV BP, men receiving BP and the use of BP in the context of multimorbidity.
Chapter 3 Qualitative interview study on the experiences and acceptability of different bisphosphonate regimens
Some text, tables and figures in this chapter have been reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634. https://doi.org/10.1136/bmjopen-2020-040634. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text, tables and figures below include minor additions and formatting changes to the original text.
Some text, tables and figures in this chapter have been reproduced from Narayanasamy M, Bishop S, Sahota O, Paskins Z, Gittoes N, Langley T. Acceptability and engagement amongst patients on oral and intravenous bisphosphonates for the treatment of osteoporosis in older adults. Age Ageing 2022;51(11):afac255. https://doi.org/10.1093/ageing/afac255.
Permission for reuse is in place as per: © The Author(s) 2022. Published by Oxford University Press on behalf of the British Geriatrics Society. All rights reserved. The text, tables and figures below include minor additions and formatting changes to the original text.
Introduction
Our findings from Chapter 2 identified the need for a theoretically informed, whole-system approach to enable clinicians and patients to get the best from BP treatment. Patients need comprehensive support that takes account of the perceptions (e.g. treatment necessity beliefs and concerns) and practicalities (e.g. capability and resources) that influence their motivation and ability to start and continue with treatment. Clinicians need to moderate patient expectations and clarify what constitutes BP treatment success. Further research is needed to explore perspectives of managers, patients receiving IV BP, men receiving BP and the use of BP in the context of multimorbidity.
Therefore, the aim of this qualitative study was to elicit patients’ and clinicians’ experiences of using different BP regimens and understand patients’, clinicians’ and service and research leads’ preferences for alternative BP regimens compared to first-line oral ALN treatment.
Methods
Sampling
The inclusion criteria for patients participating were adults who had taken or received BP for the prevention of fragility fractures within the previous 24 months, and they needed to have the capacity to provide informed consent. The purpose of the interviews was to explore patients’ experiences of BP treatment regimens for the prevention of fragility fractures, focusing on which BP were most acceptable to patients. Originally, we planned to recruit participants via regional primary and secondary care clinicians and the regional Clinical Research Network. Due to COVID-19, recruitment methods were adapted.
With ethical approval, in January 2020 [North West-Preston Research Ethics Committee (REF: 19/NW/0714)], semistructured interviews were conducted between June 2020 and August 2020 and March 2021. A study advertisement in the Spring 2020 edition of the Royal Osteoporosis Society (ROS) newsletter invited individuals to take part in one telephone semistructured interview. Replies were used as part of purposive sampling, thus ensuring that the sample included enough participants who had experience of oral BP, IV BP and those who had experience of both types of treatment. Once major COVID-19 restrictions had been lifted, the research team were able to engage with clinicians across the region, via professional networks, to support the recruitment of patients who were receiving IV BP in the community. Such experiences were sought since community provision of IV BP is not usual practice across the UK.
A total of 78 participants with a mean age of 69.9 years were recruited through the advertisement in the ROS newsletter and through engagement with clinicians via professional networks. Forty-three patients had most recently taken oral BP (for the majority of these participants, the current or most recent oral BP that they had taken was ALN tablets). Thirty-seven participants had most recently received IV ZOL BP infusions in hospital or community settings. Interviews ranged in duration from 20 to 60 + minutes. Table 4 provides an overview of participants’ demographics.
| Participant demographics | N |
|---|---|
| Gender | |
| Female | 73 |
| Male | 5 |
| Age group | |
| Under 50 years | 1 |
| 50–60 years | 7 |
| 61–70 years | 37 |
| 71–79 years | 21 |
| 80 + years | 12 |
| Bisphosphonate treatment history | |
| Oral BP only | 41 |
| IV BP only | 13 |
| Different BP | 24 |
Clinicians, specialist experts and service lead sampling
In order to understand the wider contexts of BP treatments and the service systems surrounding them, general practitioners (GPs), secondary care clinicians, specialist experts (including those involved in research), as well as those providing and leading novel treatments were recruited for qualitative interviews. These groups were purposefully sampled to include those with a good knowledge of the BP regimens in use and involved the following approaches.
First, GPs were contacted through a snowball approach, beginning with the existing professional networks of the study team. Although it was originally planned to focus GP recruitment on the practices in which patient samples were drawn from, due to the COVID-19 changes identified in patient recruitment, existing networks allowed the identification of GPs both with and without specialist/research involvement and commissioning/service leadership for osteoporosis and BP treatment. Study team members identified potential participants, and a research advertisement was also placed in the West Midlands Comprehensive Local Research Network (CLRN) newsletter for research-active GPs. GPs who were interested in taking part were invited to contact the study team and were then sent a formal invitation e-mail/letter and Participant Information Sheet.
Second, we contacted specialist clinicians, including those involved in research and service leadership. These respondents were identified through snowball sampling, beginning with the study team. Eligible participants identified by the study team were sent a Study Information pack, which included an informal invitation e-mail/letter and participant information sheet.
Third, we sampled from two specific areas where different or novel first-line BP regimens are used. This included participants from around the country (Table 5), where first-line ALN is recommended with a programme of blood test monitoring which is not usual practice elsewhere in the UK. Potential participants were sent a formal invitation e-mail/letter and participant information sheet.
| Clinician stakeholder group | Total number of interviewees | Location(s) | Specific services | Specific roles |
|---|---|---|---|---|
| GPs | 9 | West Midlands (n = 5) Northeast England (n = 2) Southeast England (n = 1) East Midlands (n = 1) |
General practice (n = 8) Single Point of Access service (n = 1) |
GP partner (n = 5) Salaried GP (n = 4) Osteoporosis/musculoskeletal specialist roles (n = 2) |
| Secondary care clinicians and service specialists | 10 | East Midlands (n = 7) West Midlands (n = 3) |
Secondary care bone specialist services, for example bone clinics and fracture liaison services (n = 10) | Consultants (n = 7) Specialist nurses (n = 3) |
| Providers of novel treatments | 4 | Midlands (n = 3) Yorkshire and the Humber (n = 1) |
Community nursing service (n = 3) Secondary care bone specialist service |
Nursing lead and nursing team members (n = 3) Consultant (n = 1) |
In total, we recruited 23 clinicians and clinical specialists, including those active in research and service leadership. Interviews ranged in duration from 20 to 60 + minutes, and the background of participants is provided in Table 5.
Data collection
Interview schedules for participants were developed in collaboration with the study team and steering group, which included patient and public involvement and engagement (PPI) representatives and comprised questions about patients’ experiences of the osteoporosis diagnosis, perceptions about their BP treatment regimen(s) and clinician and service factors. The interview schedule was piloted with two PPI representatives and refined as appropriate. All participants provided informed consent. All interviews were conducted over the telephone.
Data analysis
Interviews were digitally recorded, transcribed verbatim and anonymised. The interview transcripts were uploaded to NVivo (version 12) (QSR International, Warrington, UK) and were subjected to intense open coding to identify early ideas and issues (referred to in NVivo as ‘nodes’). Two researchers independently coded the first five transcripts and then compared analyses, allowing interpretations of the data to be critically assessed, refined and agreed. Once first-level nodes had been agreed, the remaining transcripts were coded according to these by two researchers. Newer subnodes were added over time to enable specific and relevant issues to be categorised effectively. These subnodes were developed and agreed by two researchers. Once all the transcripts had been coded, the process of iterative categorisation86 was used to provide a clear and rigorous written trail reflecting the development of themes from initial nodes. This involved identifying key NVivo nodes as particularly pertinent to the research question and exporting the content to Word. Table 6 describes these nodes and what aspects of the data they captured.
| NVivo node | Description |
|---|---|
| Reflections on engagement | Reported reasons why people engaged in treatment regimens and how they engaged |
| Reflections on non-engagement | Reported reasons why people did not engage in treatment regimens and how they disengaged |
| Stopping | Why people stopped engaging in treatment regimens and factors that were causing people to consider stopping |
| Difficulties of use or receiving | Reported difficulties with treatment regimens, for example experiencing side effects |
| Disruptions and inconveniences | Reported factors that made treatment regimens disruptive and inconvenient, for example needing to remain upright for 30 minutes to take oral BP |
| Perceptions of effectiveness | What individuals understood to be a sign of effectiveness and/or indication that the treatment was working, and how they thought this could be measured/assessed |
| Oral vs. other types of treatment | What direct comparisons have individuals made between different treatment types |
Once exported into Word, the data that had been coded to each node were examined and systematically reread. Summary and interpretive notes were added, reflecting on the content of the theme in relation to other themes, research questions and prior literature. The results below first cover the degree to which different forms of treatment were acceptable to patients, with data interpretation considered in relation to our definition of ‘acceptability’ based on Sekhon et al.’s (2017) framework,87 which proposes that it is a multifaceted construct underpinned by seven key domains. These domains are collectively known as the TFA and their descriptive definitions are conveyed in Table 7. The codes were then formally mapped to the TFA as a framework, providing appropriate constructs to capture key dimensions of treatment acceptability and engagement.
| TFA domain | Description |
|---|---|
| Affective attitude | An individual’s feelings about intervention |
| Burden | How much effort is perceived to be necessary for individuals to participate in intervention |
| Ethicality | How well the intervention aligns with individuals’ value systems |
| Intervention coherence | How well the individual understands the intervention and how it works |
| Opportunity costs | The extent to which benefits, profits or values need to be sacrificed for the individual to engage in intervention |
| Perceived effectiveness | Perceptions around the likelihood of an intervention to achieve its purpose |
| Self-efficacy | Individuals’ confidence that they can undertake the necessary behaviour to participate in the intervention |
Results
Acceptability of treatment regimes
Patients’ acceptability and engagement behaviours were captured through the TFA domains. Participant identifiers at the end of each quote indicate their role as patient, clinician, specialist, research expert or service lead. For patient participants, it also indicates the BP treatment they reported experience of. O = oral BP treatment only; IV = intravenous BP treatment only; Dif = experience of different types of BP treatment.
Intervention coherence and perceived effectiveness
Participants' views on ‘intervention coherence’ and ‘perceived effectiveness’ were often closely related, with both requiring participants to make sense of how, and to what extent, the medication could be said to be ‘working’. For the BP medicine, this commonly involved developing both a conception of the future risk posed by reducing bone density and the potential reduction of such risk. Many patients described the treatment as a way of avoiding a negative consequence of ongoing bone deterioration. This is sometimes derived from the personal experience of seeing a family member suffer:
The state of my father really, because mentally he was one hundred percent, and physically he was an absolute wreck. His spine had bent completely.
(BO42_Dif)
The desire to avoid fractures was identified as a key motivation for engaging in treatment, particularly for patients who had already experienced one:
Oh, just because I’d had, I’d fallen and broken my hip and I didn’t want to fall and break something else again.
(BO76_IV)
This motivation had also been observed by secondary care clinicians:
In more, let’s say 70- or 80-year-olds who have had a hip fracture, then they tend not to question it at all, they just go for whichever you tell the best we will take it, that sort of thing.
(B002c)
Moreover, one specialist nurse suggested that certain types of fractures prompted patients to take osteoporosis and the prospect of treatment seriously:
I mean a lot of patients are really scared of like the Dowager’s Hump. The spinal fractures tend to, if you tell somebody they’ve got a fracture in their spine they’re more likely to buy into treatment and understanding because they desperately don’t want to curve over. So that’s a huge thing, but when patients have just like fractured a wrist or a humerus or a hip, yeah, then you do struggle to get them to buy into actually it is quite serious.
(B012c)
As BP do not necessarily address any felt symptoms, the coherence of the medication involved envisioning it as providing a level of protection from either such acute or chronic health problems.
And when they said I would be having it I felt good because it’s protecting me, that’s what I thought you know.
(BO10 IV)
In some instances, patients felt they had sufficient support to understand this protection and were reassured by a good relationship with HCPs.
I felt that everything had been explained and I just thought [treatment] was a way of preventing it getting worse really.
(BO68_Dif)
This was particularly the case where participants felt they had perceived tangible evidence of the treatment working, which drove them to continue taking the medication. This was often prompted by receiving positive results from Dual Energy X-ray Absorptiometry (DEXA) scans denoting improved T-scores. (A T-score is an indication of how close the person’s bone density is to the average peak bone density.)
My hip then improved over three years to -1.1, so OK, it was a slight improvement but as the consultant said, ‘it is an improvement, and it hasn’t got worse’. And I think well, something is working somewhere along the line … That encouraged me then just to keep going with it.
(BO 9_O)
Others disclosed feeling motivated by the fact that they had not sustained fractures since beginning treatment:
I haven’t had any more vertebral fractures and just to take the positives from that, but yeah, and I’m able to walk and do all sorts of things.
(B065p_Dif)
In these cases, patients developed hope that treatment would maintain or improve their bone density. Therefore, working to attain these goals also encouraged patients to engage in treatment:
And if it adds another five years or longer, ten years of me having a more stable life, I want that, I’ll go for it, thank you.
(B055p_O)
However, uncertainties about the effectiveness of BP treatment were also very common, and several questioned the degree to which their efforts to adhere were working. Underpinning this, participants described a wide variability in the level of information, support and/or feedback. This included perceived limitations or contradictions in the way in which the medication was explained.
I was waking up in the middle of the night, thinking I’m not doing it, I’m not going to take it. The side effects aren’t explained to you, well not to me when they were discussing this was the medication that you’re going to get.
(B027p_O)
And my first initial reaction was ‘you’re taking something for osteoporosis that causes a fracture!’ And it didn’t make sense. It was almost too much to take on-board at the time. And from that to say, ‘will you just pop in and collect your prescription and start taking it’- ‘mm-mm, no way’!
(B013p_O)
Further, patients often perceived wide gaps in their ongoing care. For example, a patient spoke about requesting a scan, as opposed to waiting for this to be initiated by her GP, in order to ascertain whether her bone health had improved, which was important for her:
…That’s why I asked for the scan, because I thought, I’m doing all this, I’m doing all this running, I’m doing these weights, I’m doing this diet, I’m taking all the tablets, I’m doing everything that I can do, I needed to know if it was actually making a difference ... I can’t say what was making the difference but all together it was making a difference and that made me feel better sticking with everything.
(B071p_O)
Similarly, others on oral BP described disappointment at the poor follow-up and lack of opportunities to discuss effectiveness, which left them uncertain as to whether the treatment was still appropriate:
I do think that somebody should have carried out a proper, as I said before, a proper review after five years ... and said you know ‘I think you need another DEXA scan to see if that’s the right drug.’
(B031p_O)
The only time I was made aware of the reading from the DEXA scan was when it was the first one in 2010. And so, the subsequent ones, I’ve never had a patient’s copy, or I don’t really know whether there’s been an improvement.
(B019p_O)
Moreover, even when follow-up was planned, the timescale for accessing another DEXA scan after oral BP had been prescribed was sometimes perceived to be too long:
I found that the DEXA scan that I was eventually referred to was quite informative. But I feel the gap between, well 5 years before you can have another one. I feel as if I’m a little bit in the dark about what’s going on and I’ve got an enquiring mind, so I like to know more.
(B038p_O)
The variation in treatment plans and follow-up in primary care was reflected in GPs’ responses. While some had a specialist interest in osteoporosis and had set up systems for periodic checks on patients (e.g. 3 months following treatment initiation), others commented on the lack of scope to check patient adherence or review medication. One GP also mentioned the lack of incentives in the form of quality outcome payments.
Well if we had infinite resources then I would have a sort of annual review in general practice but that’s never going to happen because GPs are just too busy at the moment.
(B006c)
I think we’re as I’ve already discussed, not good at picking up when compliance drops off. Because actually we haven’t got a formal strategy for auditing or reviewing, non-requesting of medication and that’s actually a quite tricky to identify, but perhaps not impossible.
(B016c)
Patients receiving IV treatment generally had fewer concerns over effectiveness and coherence of the medication. This could be seen as due to the fact that this group had more regular scheduled appointments, as well as the way that the administration of the treatment required the presence of a HCP. This enabled more opportunities for patients to interact with clinicians to discuss the reasons for taking the medication and also how to deal with any side effects:
So, she took me through absolutely everything to make sure I didn’t suffer once I’d had the Zoledronate [intravenous treatment]. And I think it was so much easier for her to tell me face to face. I mean she could have done it on the phone, but as opposed to just sending the instructions, which they did too, they did send instructions in the mail as well.
(B050p_Dif)
The care particularly centred on the second infusion was so reassuring … I felt so well looked after. And having it explained to me I think several times in several different ways because people realised that I just wasn’t taking stuff in.
(B066p_IV).
This was further enhanced when IV treatment was provided at home, with the nursing team taking on larger responsibility for organising the appointment, and then being able to focus exclusively on one patient for the duration of the treatment. This included opportunity to discuss effectiveness and the findings or previous scans:
Well, I think they give reassurance, and they give you their time, because I was very impressed with the chap, who came last week, because when I asked about my DEXA scan results he had to go out to the car to get his laptop and he, you know he wasn’t rushing me. He got his laptop, and he sorted out exactly what the data was from the DEXA scan and told me all the data.
(B081p_IV)
Further, patients on IV BP also tended to have well-established time points for follow-up:
Oh, I’m just sent a letter with an appointment date and time. And I can sort of change it, if it’s not convenient … it’s always been alright.
(B080p_IV)
Secondary care consultants confirmed that appointments and follow-up plans were well established for patients on IV BP, underpinned by clear rationales, including scheduling a point at which such treatment could be reviewed:
The nurses follow them up regularly to do the infusion. After three infusions, we will see them, look at the DEXA to see whether they need to remain a bit longer on the bisphosphonates.
(B002c)
This was reflected in the views of the community team delivering IV treatment in community settings who valued the opportunity to provide direct and individual care.
So from, just comparing to when I worked in the hospital and obviously being in their home address, there is only one patient, so your attention is a hundred percent on that person or the people that are in the room with you.
(B022c)
Having clarity around when DEXA scans were going to be scheduled was important for enabling patients to see a potential end point for treatment. One patient pointed out that this is what made IV BP treatment particularly favourable:
And it’s time limited as well, knowing that it’s just for three years and then I will have a bone scan and hopefully it’s better, it’s quite nice as well because you see light at the end of the tunnel with yet one more health condition.
(B066p_IV)
Moreover, a few patients seemed to have higher expectations of IV treatment itself, even before scan results were attained. They associated the format of this type of treatment as facilitating a direct, and seemingly stronger, intervention for the actual problem of osteoporosis. This was reflected in patients’ comparisons with previous oral BP treatment:
I had no sort of medical expertise at all, but to have an infusion would seem to me to get to the heart of the matter more than a pill!
(B051p_Dif)
While this might not have been an explicit message from health professionals, the IV treatment was often seen as a ‘step up’ in the intensity of care for patients who had previously been on oral BP. In this context, a greater efficacy of IV treatment was seen to make sense. Some clinicians providing IV treatments did indeed suggest these would be more effective than oral BP, as captured by a specialist nurse below:
So if we give IV we know there’s no absorption problems, it’s getting straight in, so it will be effective basically, if it’s not going to be effective IV then it’s never going to be effective sort of thing.
(B003c)
In most instances, however, clinicians described IV treatments as recommended due to side effects, non-adherence and/or intolerance to ‘first-line’ oral treatments.
Opportunity costs and burden
When the BP treatments required patients to make sacrifices (i.e. ‘opportunity costs’) and/or the regimens were regarded as burdensome, this contributed to overall negative views, which balanced against whether the treatment was seen as effective and coherent. Although a small portion of patients who were on or who had been on oral BP found the regimen relatively straightforward when weighed against the benefits, a larger portion identified wide-ranging costs and burdens. Most commonly, patients described struggling with the general treatment routine, which was regarded as complex, and the disruption this caused to their morning habits.
I find it a total burden to be honest … I’m an early riser, and I do like to wake up early and have a cup of tea, I know this is all trivial, but just have to swallow a huge amount of water with this tablet, and then sit around for half an hour, trying to occupy myself – it just, it’s just alien.
(B037p_O)
This could be seen to be exacerbated when the patients had other commitments, priorities or health issues:
To take the pill and to sort out the timing of it and everything, I just couldn’t be dealing with that, because I had quite a lot of stresses in other areas of my life, emotional stresses and stuff like that.
(B051p_Dif)
As a point of contrast, others highlighted that their personal circumstances facilitated dealing with the burdens.
It’s a bit of a pain I have to admit, if you want to get on and you’re going out to take it. But it wasn’t – that had nothing to do with the reason why I stopped taking it. I was able to fit that into my daily life quite easily. I can imagine had I been working it might have been a little harder.
(B032p_O)
The common problems for patients on oral BP were the restrictions around not being able to eat straight away and also needing to remain upright. The latter was particularly challenging for patients who had previously suffered from particular fractures or other conditions which affected their posture:
I mean, in fact you can sort of sit bolt upright but I’m not very good at that, so I always seem to hunch a bit and since I had the fractures that’s really not possible.
(B014p_Dif)
Other patients highlighted the practical difficulties of the oral BP treatment regimen which prevented them from taking them properly, such as not being able to swallow the tablets:
I really couldn’t get them down my throat, because I knew that I would start gagging and a good chance of vomiting and I can’t live like this, you know.
(B054p_Dif)
The requirement to follow strict instructions caused high levels of burden for some patients, to the extent that they felt that their mental well-being was compromised:
I try to keep active for an hour – because I’m frightened, you’re frightened taking that particular Alendronic Acid. I don’t like taking it, I find it a real inconvenience.
(B027p_O)
General practitioners in the study also recognised the challenges their patients faced in terms of medication burden.
You’re not meant to take particular drugs at the same time, isn’t it, either, I don’t think, that then messes up timing for other medications, so yes, if it was a pill that you just take at the same time as your anti-hypertensives and your aspirin and your lansoprazole then I think compliance would be much better.
(B010c)
Some patients on oral BP discussed developing strategies to cope with the more challenging aspects of the regimen, thus reducing costs and burdens. This included planning ahead, thinking positively, engaging with the ROS and setting reminders:
I’ve got an alarm on my phone to remind me that it’s Wednesday and I’ve got to take my Alendronic Acid.
(B071p_O)
In some cases, this also included adapting instructions to make the oral BP regimen more bearable:
So, I was really suffering and in the end I just thought I’m going to make my own rules up here. So, I was doing an hour, I was doing a pint [of water], sometimes more than a pint, I was not moving, because if I moved I suffered all day.
(B050p_Dif)
In comparison with the costs and burdens of oral treatment, patients generally identified practical challenges and inconveniences relating to accessing IV BP treatment, as opposed to the treatment per se. This included travelling to hospital appointments, issues around parking and navigating one’s way around the hospital:
I think I had to go to something like atrium three which didn’t happen to be marked and it was all a bit, it was a bit confusing and a bit open, but I found it. I blundered my way towards it. It wasn’t ideal.
(B023p_Dif)
Such issues were removed for patients receiving IV treatment at home. Having home-based IV treatment also enabled some patients to cope better with the regimen since they would have faced difficulties with travelling due to the physical restrictions caused by osteoporosis and/or comorbidities:
I would prefer it at home, because it is a bit of a struggle for me to get to places, you know with the multiple sclerosis.
(B075p_Dif)
Moreover, having IV BP treatment at home was valued since it enabled patients to receive such treatment even more comfortably:
That’s great, because you see, I went to have scans in the hospitals, and I knew what a scan was like and a drip and all that kind of thing. And I was just, well I was amazed to be able to sit in my favourite chair.
(B082p_Dif)
In addition, the fact that the IV treatment regimen required a nurse to administer, it took much of the work out for the patients and did not seem to cause much inconvenience for patients having it at home:
They just come into the dining room, the chair is pulled out, they set their gear up on the table and we’re away, no problem … I mean how could it be better? You just sit down, she puts a needle in, pops it in and then Bob’s your Uncle! No problem!
(B077p_Dif)
Thus, IV treatment seemed to offer patients a regimen that was lower burden, with fewer opportunity costs. Some patients who had direct experience of both oral and IV BP even suggested that side effects from the latter treatment regimen were more favourable:
Basically, it’s a morning and a day of feeling woozy and that’s it for in my case eighteen months, you know, compared with wrestling yet another lot of pills to take every week.
(B014p_Dif)
This was similarly reflected in the experience of those delivering IV treatments for patients at home.
They’re in their own home at the end of the day. If they can’t be comfortable there where can they be comfortable? So we have even done a few infusions in the garden sometimes because the weather is nice.
(B022c)
A small number of patients did comment on difficulties with the administration of IV treatment, for example, having the needle inserted into the vein, and clinicians reported that a number of patients were concerned about needles. There were also some patients who felt happy with the oral BP regimen, and a small number drew on points of comparison with the IV treatment to justify their preference:
I just [worry] about side effects and if you put intravenously, you know, how you counter them. And I thought I’m not going down this road at the moment.
(B013p_O)
Generally, though, there were more positive accounts relating to IV treatment regimens, with patients enthusing about the overall ‘experience’. They recounted the staff approaches that contributed to little burden and opportunity costs on the part of the patients:
It’s wonderful, they even come round and offer you a cup of tea and a sandwich or whatever. Oh, they’re just really friendly, really welcoming and getting you sat down, ask you which arm you want in, making sure you’re comfortable, you’ve got something to put your feet on, you’ve got something to read. No, I couldn’t fault them at all.
(B063p_Dif)
Regardless of treatment type, a regimen which led to no side effects or manageable side effects enabled patients to cope with their treatment regimens, supporting acceptability and engagement. But for patients where side effects were particularly severe, this was enough to cause them to stop engaging in the treatment:
And I just thought the side effects from the Alendronate just weren’t worth it, they were impacting my life.
(B032p_O)
For some patients who did not experience severe side effects, this did not preclude the worry that these could occur. Such concern served to be a burden for some, while living with the risk of such side effects was one of the costs required for engaging in the treatment:
You know, it does worry me that if I have to have a tooth out there could be complications … it’s adding to my decision to stop when I’ve been on it for five years.
(B005p_O)
Mostly, those on IV treatments were less concerned with side effects. While one patient on oral BP did worry more about the potential side effects from the IV treatment, the comparison between the two could also be seen in light of higher levels of support for patients receiving IV treatment, as noted above. Professional support and care may have helped to both reduce side effects as well as reduce the concern of these occurring.
Ethicality
In the context of BP treatment acceptability and engagement, the TFA domain of ‘ethicality’ was linked to the extent to which patients regarded the treatment regimens as aligning with their individual values and whether they perceived the treatment to be fair and suitable for their needs. This often related to the way in which they had come to be prescribed the treatment and the manner in which the treatment had been offered to them.
For some patients who were prescribed ALN oral BP, they felt that their personal wishes had been dismissed, which led to negative attitudes towards the treatment:
I wrote to my doctor and said I’d read all the information, so when she started prescribing, ‘pretty please prescribe the soluble tablet’, which to my way of thinking was the least harmful or least – the one I could tolerate most, of the options. And that got ignored, and I got sent the prescription for the Alendronic tablet, and I refused to take it. I took it once, and I just found it so difficult I felt I’m not taking it – I’d rather not have it.
(B037p_O)
Even for patients who had come to accept any inconveniences of the oral BP treatment regimen and were engaging in the treatment, many still expressed dissatisfaction with the fact that they had not been presented with other treatment options:
I shouldn’t complain really because so many people have so many things, and their lives are really dictated by their meds. But what annoys me is the reluctance to actually discuss any options.
(B044p_O)
One patient described the ramifications of her GP discounting the advice of the osteoporosis nurse to offer the patient a soluble form of ALN to minimise the risk of GI side effects:
So basically, I’ve always had a dodgy tummy. And so, if they give me medication, they give me the stomach liner thing as well. And so [the osteoporosis nurse] said to me ‘Well you might be advised to take the bisphosphonate in a soluble solution form’. So, when I told the GP this, he said ‘No, you can’t have that, you’ve got to have the Alendronate tablet’. And I assume it’s because it was cheaper. So, I started off on the Alendronate. Well, it got to about four months in, or three to four months in and I was, it had made my tummy bad.
(B018p_O)
On the other hand, other patients did not find it a problem that they had not been presented with alternative options, and in fact, this encouraged them to engage in the treatment because of the implications of osteoporosis:
I was, you know I never enjoyed the fact that I had to have my glass of water and potter around the house but well, there was sort of no option, so I was perfectly happy. I knew what, I know what osteoporosis can do.
(B023p_Dif)
Moreover, some patients felt that oral BP were right for them, particularly when they could identify improvements in health which they attributed to the treatment. As such, they did not wish to pursue other options:
I mean when I look back to what it was like when I first had the spinal fractures, you know it was unbelievably painful, and then now as I say, I can mow the grass, pick up weeds, do all these sort of things – clean windows. And so I mean I’m very happy on it really. I can’t see any reason to change it.
(B011p_O)
For some patients on IV BP treatment, they had previously been on oral BP and could make direct comparisons between both regimens. IV BP treatment was generally viewed as easier to engage in with optimal frequency due to being just once a year. This suggested that the IV treatment regimen was a better fit with their lifestyle and therefore more suitable:
I know it’s only little things really but the convenience of having something once a year compared to 52 times a year is amazing.
(B074p_Dif)
While this could be seen as pointing to the generally lower burden of IV treatment, it could also be identified that such comparisons played into overall perceptions of equity; namely, it was more common for those on IV treatment to see themselves as being treated fairly.
Self-efficacy and affective attitude
In the context of accepting and engaging in BP treatments, the balancing of the aforementioned TFA domains could be seen to shape patients’ overall feelings of self-efficacy and affective attitudes towards their regimens. As per Table 1, self-efficacy reflected patients’ confidence in their abilities to undertake the necessary behaviours to engage in the treatment. Affective attitude is related to patients’ feelings towards their treatment.
Positive affective attitudes of patients, including gratitude and hope for the future were apparent when they understood the reasons for the treatment, felt they had made sense of its potential effects and felt that they were well supported by clinicians and the wider service. This often appeared to be facilitated by regular contact with professionals and access to diagnostic tests to track the progress of bone health.
The consultant that diagnosed it, because he looked back at this x-ray and saw it and he just put everything into action and was very clear … so professional … And yes, so that was very positive for me. And the actual treatments, because I’ve always felt quite secure and quite happy with them.
(B060p_IV)
No, but I do appreciate, that you know the trouble, I do appreciate having the treatment that I’ve had. Because, if it helps my bone density then that’s really good.
(B081p_IV)
Many of the patients on IV treatment had previously had difficulties in adhering to regimen for oral BP and were therefore pleased to have ‘arrived at’ a more acceptable form of treatment. Having experience of both types of regimens led to some patients enthusing about IV treatment. Being able to reflect on negative experiences of oral BP led to them conveying positive affective attitudes and feelings about IV treatment.
No, when considering the tablets, I was rather pleased that I was getting this one … I thought I would come off the better actually.
(B076p_IV)
And then of course I hit the jackpot with the infusion and that’s marvellous.
(B014p_Dif)
[Intravenous treatment is] just wonderful … I’ve never been up nor down or anything, and compared to what I was suffering with, fabulous. For me it’s been great.
(B030p_Dif)
Others on IV treatment who had not previously been on oral treatments made speculative comparisons, weighing up the negatives against the positives. The IV treatment was often regarded as the better option, leading to feelings of satisfaction:
I’ve talked to people afterwards who just have the tablet, and they’re surprised that I had the infusion, but I was quite happy to have the infusion.
(B081p_IV)
Equally, some patients on oral BP felt positively towards the tablets and that they were in control of managing them themselves:
I managed the five years without missing them, I think. It was convenient having the tablets because once or twice we were on holiday, to just take them with you and do the same thing.
(B064p_Dif)
Moreover, this patient also highlighted that the COVID-19 pandemic had made the prospect of going to hospital to receive IV treatment more burdensome, thus leading to a more negative affective attitude:
I’m not looking forward to going to the hospital with all these COVID problems.
(B064p_Dif)
Further, despite curiosity about alternative treatments, when the experience of oral BP had been unproblematic, patients described being happy to remain on their current regimen:
Not that I would want to change over, but it was only just something that I read. So no, I wouldn’t want that anyway. I’m quite happy with my tablets.
(B011p_O)
It might be nice to know that they were aware that there are alternatives, but I don’t take a massive amount of notice of what they are because, at the moment, I’m quite happy on the Risedronate.
(B024p_O)
This was also the case for patients who had had a treatment break and needed to resume the oral BP regimen:
I was very happy to go back on Alendronic Acid having had no trouble previously.
(B039p_O)
Regardless of treatment type, in circumstances where opportunity costs and burdens were low, patients expressed high levels of self-efficacy, sharing their confidence in being able to execute the necessary actions to partake in the treatment regimen:
…so I think all in all [the tablet] definitely agrees with me, and it’s so easy to take. You just get into a routine you know, like Friday morning, I know exactly what I’m doing and what times I’m doing it at you know.
(B011p_O)
…they were a good, shaped tablet, you know, they were oblongs – not big round ones. I’ve had all sorts of trouble with big round tablets. But they’re a good shape. I had no trouble in swallowing it with a full glass of water.
(B064p_Dif)
Sometimes, patients discussed the steps that were taken to reduce the burden of undergoing treatment and how such measures made them feel more positive and able to cope with the regimens, thus increasing their self-efficacy. By nature of the oral BP regimen, such strategies had to be instigated by the patients themselves. For example, one patient discussed keeping herself occupied during the period when she needed to remain upright after taking the tablet:
Interviewer: Did you manage to fit it into your weekly routine?
Oh yes, because I got loads of jobs done while I was upright and swallowing loads of water.
(B053p_Dif)
In contrast, for patients receiving IV treatment, it was often the healthcare staff who could facilitate a smooth and comfortable experience for them:
I know what [the staff are] there for and yeah, I just, put your arm out and keep it in a safe place. So, they just, put you in a comfortable position. And then they just do it, so … I don’t really feel anything to be honest.
(B078p_IV)
Also, when the treatment aligned with one’s personal beliefs, this also led to positive affective attitudes and higher levels of self-efficacy. Patients openly expressed their satisfaction when they believed that they were on the most appropriate treatment for osteoporosis:
I need to be on a treatment. I think [intravenous is] the best one for me at the moment but I’m fully aware I am at high fracture risk anyway.
(B023p_Dif)
Some patients described positivity at being on what they perceived to be the right treatment, particularly if their experience of previous treatments had been negative:
I was really sort of rather pleased that I’d finally achieved, you know, some other treatment and I didn’t have to keep taking the wretched [oral] bisphosphonates.
(B014p_Dif)
Therefore, perceived fairness of treatment allocation, including the timing of this allocation, also appeared to be related to the patients’ affective attitudes.
Discussion
We have demonstrated how patients’ acceptability and engagement in BP treatment can be described and explained through the seven TFA domains. Specifically, we have described how the balancing of specific TFA domains impacted on the extent to which patients with osteoporosis accepted and engaged in their treatment, manifesting as self-efficacy and affective attitude. By nature of treatment format, lower regularity of treatment, more established contact points and follow-up with HCPs, IV BP treatment was generally perceived to offer lower opportunity costs, be less burdensome and was often regarded as appropriate treatment by patients. This latter point was the case for some patients who had previous negative experiences with oral BP. Moreover, there were often more opportunities to build up coherence around IV treatment and develop confidence around its effectiveness since such patients were more likely to have frequent contact with HCPs, who could address patients’ queries and explain details around the treatment on more than one occasion. In addition, dual-energy X-ray absorptiometry (DXA) scans (bone density scans used to measure how much bone tissue an individual has undertaken to help assess fracture risk) tended to be implemented at earlier points compared with patients taking oral BP, thus providing a means to measure the success of treatment.
Crucially, TFA domains were found to be interconnected, with patients balancing treatment burden, opportunity costs and ethicality issues against treatment coherence and perceived effectiveness. The outcome of this balancing act ultimately determined patients’ attitudes towards, and engagement in, their treatment regimens, thus informing their affective attitudes and self-efficacy.
This is conveyed in Figure 3.
FIGURE 3.
Relationship between TFA domains. Reproduced from Narayanasamy M, et al. Age Ageing 2022. https://doi.org/10.1093/ageing/afac255.
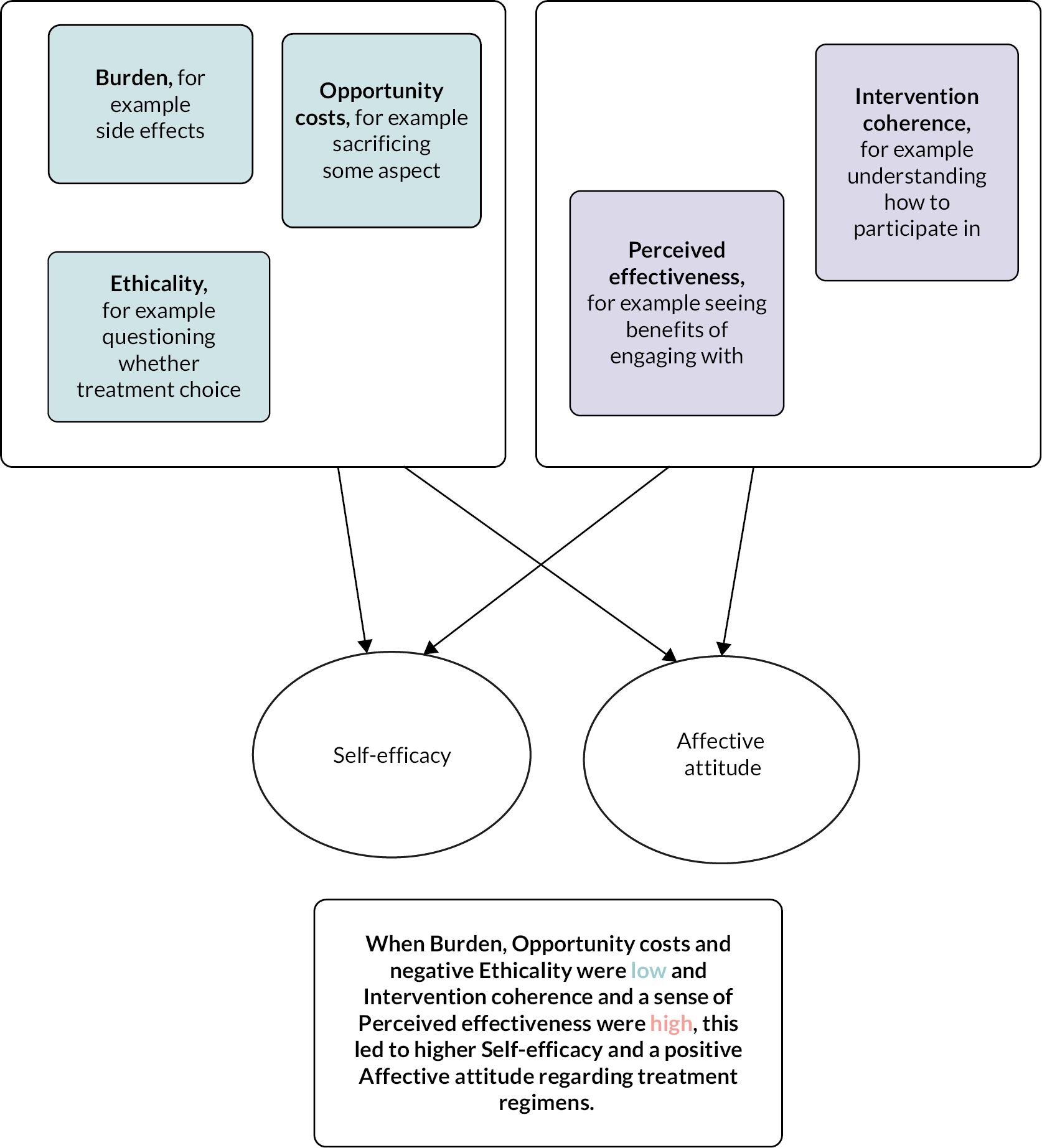
Few studies have previously investigated patient acceptability and engagement for different BP treatment regimens. Roh et al. 88 investigated adherence to BP amongst patients with limited health literacy. The study found that adherence rates were significantly higher amongst patients who were receiving quarterly IV BP compared with those taking weekly oral BP. Moreover, another study found that 65% of newly diagnosed patients with osteoporosis preferred an annual infusion compared with weekly oral BP treatment, and this preference was particularly apparent in patients with a higher perceived risk of future fractures. 89 This may suggest that patients have more confidence that IV BP treatment will be more effective in reducing fracture risk. This certainly reflects the findings of the current study, which highlighted that perceived effectiveness was a key factor in influencing self-efficacy and affective attitude, ultimately impacting treatment acceptability and engagement.
Regarding specific patient groups, two studies90,91 identified that postmenopausal women with osteoporosis preferred treatments that occurred less frequently, citing such regimens as more comfortable, simpler and enabling them to take fewer tablets. This suggests that weekly oral BP regimens are harder to adhere to for certain patient groups due to the frequency and perceived complexity. Furthermore, domiciliary treatment, such as IV drugs at home, may be more beneficial for older patients living with long-term conditions due to the challenges associated with travelling to hospital appointments, including distance, reduced mobility and pain, as highlighted by participants in the study and also in other studies. 92,93 This could be seen as consistent with wider calls to shift aspects of services for chronic conditions away from acute facilities, although this shift remains an ongoing policy and funding challenge. 94
Despite the recognised challenges with taking oral BP, there were clear examples in the current study where participants accepted and engaged with oral BP when opportunity costs and burden levels were low. Similarly, a study investigating BP treatments in women with breast cancer95 confirmed that most participants had been able to accommodate oral BP treatment into their lifestyle and were completely satisfied with the treatment. This suggests that such regimens are not always burdensome for patients and that opportunity costs can be met. However, in the current study, there were patients who continued to engage amid difficult circumstances, such as side effects which were burdensome, and where treatment had a significant impact on lifestyle. In these cases, the TFA cannot account as strongly for such patients’ experiences. Such patients may have prioritised the outcome of treatment (i.e. the hope of improved bone health and fewer fractures) more highly than the treatment experience, thus withstanding the negative aspects of treatment. The complexities of using the TFA in the context of examining treatment acceptability found that there were overlaps between TFA domains, and that it is not always a comprehensive framework for offering understanding into patient acceptability and engagement with medicines. Other frameworks such as the NCF96 may be helpful in understanding and transforming adherence-related beliefs and behaviours. This framework understands patients’ adherence to be the outcome of a cost–benefit analysis whereby adherence is likely to be higher when the perceived need for treatment is prioritised over the risk of negative consequences such as side effects. This lens may be relevant to understanding some patients in our sample who pursued treatment amid high burden levels and significant opportunity costs.
Strengths and limitations
A key strength of this study is the fact that a large sample of participants were recruited to provide in-depth insight into different experiences of BP treatment regimens. The main limitation is that findings were largely drawn from a sample of participants who had membership with the ROS, with a smaller number recruited through NHS services. This may have caused the sample to be biased, for example, it may have largely comprised individuals who had the financial means to fund membership and who were possibly taking a more proactive approach to their health by investing in resources. This may restrict applicability to other patient groups, such as those who are financially disadvantaged and those who are less proactive in their care and treatment. Moreover, our sample of patients receiving IV BP comprised those receiving ZOL only and not 3-monthly IV IBN treatment. However, the paper has been able to effectively demonstrate to some extent the relevance of TFA domains in explaining attitudes and behaviours around acceptability and engagement in BP treatment regimens. Moreover, utilising the TFA domains to explain acceptability and engagement of IV BP treatments is particularly novel. It will be useful to explore whether such findings apply to other patient groups.
The study has identified several questions for further research, including whether it would be feasible and appropriate to offer specific patients first-line IV ZOL treatment for osteoporosis and how acceptability and engagement can be optimised. In addition, it has also highlighted the possibility of treating long-term conditions in alternative ways, such as in the community, which may be favourable for an ageing population, where hospital travel may be challenging due to comorbidities and the COVID-19 pandemic. These uncertainties will feed into a research priority-setting exercise alongside other questions which have arisen from the wider research study programme.
Conclusion
Intravenous BP, ZOL treatment was generally more acceptable to patients. Such regimens were perceived to be more straightforward to engage in, although a portion of patients taking oral BP were satisfied with their current treatment. The TFA was a useful model in accounting for how patients accept and engage in BP treatments but was limited as a comprehensive framework that could explain all patient experiences. Further research is needed to identify whether findings apply across other patient groups, how acceptability and engagement can be optimised and to identify other frameworks for investigating patient acceptability of, and engagement in, BP treatment regimens.
Chapter 4 Effectiveness of bisphosphonates for the prevention of fragility fractures: an updated systematic review and network meta-analyses
Some text, tables and figures in this chapter have been reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634. https://doi.org/10.1136/bmjopen-2020-040634. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text, tables and figures below include minor additions and formatting changes to the original text.
Some text, tables and figures in this chapter have been reproduced from Bastounis A, et al. JBMR Plus 2022;e10620. https://doi.org/10.1002/jbm4.10620. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text, tables, figures below include minor additions and formatting changes to the original text.
Some text, tables and figures in this chapter have been reproduced from Bastounis A, et al. Osteoporos Int 2022;33:1223–3. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text, tables and figures below include minor additions and formatting changes to the original text.
Introduction
Bisphosphonates, such as ALN, risedronate (RIS), IBN and IV ZOL, have been found to be effective in reducing the risk of osteoporotic fragility fractures. 2 However, there is no conclusive evidence regarding their comparative effectiveness in specific patient groups, such as patients with low bone mineral density (BMD). 97 This can be accounted for by the paucity of comparative trials that would provide insight on how BP works through time in the light of adverse events associated with the use of BP. There is a need, therefore, to undertake a comparative evaluation of BP, testing their effectiveness in reducing the risk of fragility fractures. Adherence to BP is crucial to realise clinical benefits and reduce the risk of fractures; however, recent studies suggest that adherence to oral and parenteral BP is suboptimal and tends to decrease over time. 98,99 Adherence could be conceived as an umbrella term which encompasses the following terms: (1) initiation, (2) implementation and (3) discontinuation of treatment. 100,101 Initiation of treatment refers to the time when people start a prescribed medication (i.e. receive the first dose of a prescribed medication), implementation refers to the level of compliance to the dosing regimen of a prescribed medication from the first to the last dose and discontinuation refers to the time when people stop taking their prescribed medication. 102
The aim of this chapter was to conduct two systematic reviews with NMAs to explore treatment effectiveness and treatment adherence. The treatment effectiveness systematic review was an update of a systematic review that was previously published as part of a NICE HTA report45 but also included an update of the estimates regarding the comparative effectiveness of the BP to inform an economic evaluation regarding BP benefit-to-risk ratio. NMA techniques are particularly well suited in the context of pharmacological interventions when we seek to compare three or more interventions simultaneously, combining both direct and indirect evidence across different networks of studies. 103 NMA can be performed either by adopting a Bayesian approach104 or by a frequentist approach. 105 In these NMAs, a Bayesian approach was followed as it is well suited for informing the cost-effectiveness analysis. The second systematic review sought to provide estimates regarding users’ probability to adherence in BPs’ treatment, exploring patterns of discontinuation, persistence and compliance among people with different clinical profiles.
Methods
Both systematic reviews were registered with PROSPERO (updated review: CRD42020177155; adherence review: CRD42020177166) and reported following the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions checklist. 106
Eligibility criteria
The eligibility criteria of both reviews regarding the population and interventions of interest were the same and have been described elsewhere. 92 Only studies in which the interventions of interest (ALN, IBN-IV, IBN-oral, RIS and ZOL) were assessed within their licensed doses for treating osteoporosis. Studies that reported data for both licensed and unlicensed dose study groups were considered eligible only if data for the licensed groups were separately reported. Studies reporting comparisons among the interventions of interest were considered eligible for inclusion. In the updated review, we targeted the following outcomes: fragility fractures, BMD at the femoral neck, mortality, adverse effects and health-related quality of life (HRQoL), with only randomised controlled trials (RCTs) eligible for inclusion. In the adherence review, the outcomes of interest were persistence and compliance, quantified either as continuous (e.g. absolute numbers or rates) or discrete measures (e.g. absolute number of participants being persistent/compliant based on pre-specified thresholds). In this review, RCTs, non-randomised parallel comparative studies and observational (both prospective and retrospective) studies were considered eligible for inclusion. In the RCTs, persistence was indirectly inferred by assessing the total number of participants who dropped out at 12 and 24 months; in the observational studies, persistence was inferred by assessing the total number of participants who discontinued their treatment based on treatment refill gaps, using data from claim databases or medical records. In the observational studies, compliance was indirectly measured by assessing ‘treatment continuity’ and using percentages/absolute numbers of medication possession ratios (MPR) and number of users with MPRs over a pre-specified threshold. Reports published as abstracts or conference presentations were excluded where insufficient details were reported. RCTs which were judged otherwise eligible but did not report outcome data per treatment arm or reported zero dropouts for both arms were excluded. Studies which reported the outcomes of interest for BP groups collapsed or studies reporting comparisons based solely on the frequency of administration (e.g. daily vs. weekly) were also excluded.
Search strategy and information sources
A set of comprehensive search strategies were undertaken to systematically identify eligible studies in both systematic reviews. The search strategies comprised the following main elements: searching of electronic databases (including unpublished data and trial registries), extensive keyword hand-searching and scrutiny of bibliographies of retrieved papers.
The following databases were searched in both reviews:
-
MEDLINE® In-Process and Other Non-Indexed Citations and MEDLINE (Ovid), including PubMed
-
EMBASE (Ovid)
-
Cochrane Database of Systematic Reviews (Wiley Interscience)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley Interscience)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO)
-
Database of Abstract of Reviews of Effects (Wiley Online Library)
-
HTA Database (CRD Database)
-
NHS Economic Evaluation Database (CRD Database)
-
OpenGrey
-
Science Citation Index (ISI Web of Knowledge)
-
CPCI – Science (Web of Science)
-
ClinicalTrials.gov.
Searches of the updated review covered the period from September 2014 to 1 March 2021. Searches of the adherence review covered the period from January 2000 to 25–26 March 2021. In both reviews, all potentially relevant citations were downloaded to Endnote X8 Reference Manager bibliographic software (version 8.0; Clarivate Analytics, Philadelphia, PA, USA).
Study selection, data collection process and data items
Selected studies were imported into Rayyan online software. 107 Two independent reviewers screened studies for relevance based on titles/abstracts and later full texts (AB and TL) with disagreements resolved through discussion or by consulting a third reviewer (JLB/OS). Two independent reviewers (AB, TL) conducted full-text screening with a high level of agreement observed in both reviews (κ = 0.91 in the update review and κ = 0.84 in the adherence review). A standardised and pilot-tested data extraction form was used to extract relevant data in both reviews. One reviewer (AB) extracted data, with a second reviewer (TL) independently checking at least 50% and 80% of the extracted records in the updated and adherence review, respectively. Data extracted consisted of the following categories: (1) descriptive statistics (e.g. number recruited and randomised, participants’ characteristics); (2) baseline data on outcomes of interest (e.g. comorbidities, fractures at baseline, alcohol use, number of falls); (3) moderators of action [e.g. glucocorticoids (GC) use, patients with osteoporosis, history of fractures/fractures at baseline]; (4) intervention characteristics (e.g. drug type, administration mode, concomitant treatments) and (5) statistics and relevant data on the outcomes of interest expressed either as continuous or binary outcomes [clinical outcomes such as fractures, BMD changes, HRQoL, adverse events mortality, number of participants who dropped out from RCTs, number of users who discontinued with BP treatment, number of users with varying compliance levels based on pre-specified thresholds, mean/range MPR, mean number of infusions/tablet counts, proportion of days covered (PDC) percentage, mean duration of BP treatment]. Authors were contacted when there was lack of data on outcomes of interest and/or further information was needed in order to attest eligibility of relevant studies.
Geometry of networks
Both treatment-placebo (PLB) and treatment-active comparisons were examined, and network plots were created for all outcomes. In both reviews, nodes indicate the different treatments included in the analysis, and thickness of edges connecting the nodes indicates the number of studies informing each comparison (thicker lines indicate more populated comparisons). In the adherence review, node size indicates the number of studies included in each node, and thickness of lines indicates the overall sample size informing each comparison (thicker edges indicate more populated pairwise comparisons).
Risk of bias within individual studies
The methodological quality of the included RCTs was independently assessed at the study level by two reviewers (AB, JLB) using the Cochrane Collaboration risk of bias tool 1.0 (94). The Cochrane Collaboration risk of bias tool 1.0 addresses the following specific domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment and incomplete outcome data and selective outcome reporting. Studies were rated with a low risk of bias in the randomisation sequence if they provided an explicit statement on how they performed the randomisation. Open-label trials were rated as high risk in the ‘blinding’ category, whereas higher than 20% attrition at 12 months’ follow-up resulted in high-risk rating in the ‘incomplete outcome data’ category. The methodological quality of the included observational studies was independently assessed at the study level by one reviewer (AB), with a second reviewer (JLB) independently checking 50% of the included studies. Any disagreements were resolved through discussion. The assessment of methodological quality in observational studies was undertaken using the Cochrane Collaboration Risk Of Bias In Nonrandomised Studies of Interventions tool (ROBINS-I). 108 Risk-of-bias plots were created by using the ‘robvis’ tool. 104
Summary measures and methods of analysis
Updated review
Fractures, mortality and adverse events were reported in a binary form (number of participants experiencing at least one event out of the total number of participants). The data generation process followed a binomial likelihood, assuming an underlying Poisson process for each trial arm. The complementary log–log link function was used to model the NMAs for the binary outcomes. 109 Log hazard ratios (HRs) were estimated from the median and corresponding 95% credibility intervals (CrIs) from the 2.5th and 97.5th centiles of the posterior distribution. Treatment ranking probabilities for all fracture outcomes were reported. Changes in BMD were reported as percentage changes per arm from baseline [mean percentage difference per arm plus standard error (SE) of the mean (SE)]. The data generation process followed a normal likelihood. The identity link function was used to model the NMA for BMD change, including study duration as a trial-level covariate and assuming an equal interaction effect between treatments and reference treatment one. 110 The treatment effects represented the mean difference (MD) between the percentage change in the treatment group and the comparator group. Mean percentage difference plus 95% CrI were estimated from the posterior distribution. Treatment ranking probabilities and surface under the cumulative ranking (SUCRA) were reported for the BMD data. 111
Two different modelling strategies were considered for the treatment effects: (1) a standard, independent random (treatment)-effects model112 was fitted for assessing the comparative effectiveness of BPs in increasing femoral neck BMD and (2) exchangeable treatment-effects models (i.e. effects model where the treatment effects are assumed to arise from a common distribution according to the class of drug)113,114 were fitted for assessing the comparative effectiveness of BPs in preventing fractures, deaths and adverse events, given the relative paucity of data in the aforementioned variables. For BMD changes, the model was completed by using conventional reference prior distributions: (1) trial-specific baseline, μi ~ N(0, 1002); (2) treatment effects relative to reference treatment, d1k ~ N(0, 1002) and (3) between-study standard deviation (SD) of treatment effects, τ ~ U(0, 5). Due to the paucity of data, we used a weakly informative prior distribution for the between-study SD [i.e. τ ~ half-normal HN(0, 0.322)] for the NMAs of hip and wrist fractures and specific-type adverse events (i.e. influenza-like symptoms, myalgia, nasopharyngitis and headache). Based on clinical plausibility, a weakly informative prior distribution for the between-study SD [i.e. τ ~ half-normal HN(0, 0.322)] was used for the NMA of mortality data. All analyses were conducted using OpenBUGS (MRC Biostatistics Unit, Cambridge, UK) and R Studio (R version 4.0.3), using the ‘gemtc’ and ‘rjags’ packages. Convergence to the target posterior distributions was assessed using the Gelman–Rubin statistic for three independent chains with different initial values. For all outcomes, results were based on three independent chains of initial values and 105,000 iterations after a burn-in of 50,000 iterations. Most of NMAs exhibited moderate correlation between successive iterations of the Markov chain, so they were thinned by retaining every 10th sample.
Adherence review
In RCTs, the number of dropouts at 12 and 24 months was reported in a binary form (number of participants who dropped out subtracted from the total number of participants per arm). The data generation process was assumed to follow a binomial likelihood, while NMAs were modelled using the logit function. Log odds ratios (ORs) were estimated from the median and corresponding 95% CrI from the 2.5th and 97.5th centiles of the posterior distribution. In retrospective observational studies, discontinuation was reported in a binary form (number of participants who discontinued the treatment, as this is indicated by pre-specified refill gaps). Given the absence of control conditions in retrospective observational cohorts, ALN was used as the reference treatment. The data generation process was assumed to follow a binomial likelihood. To account for different trial durations, an underlying Poisson process was assumed for each trial arm. The probabilities of any of the aforementioned binary outcomes were considered non-linear functions of event rates, so we modelled the NMAs for the binary outcomes using the complementary log–log link function. Log HRs were estimated from the median and corresponding 95% CrI from the 2.5th and 97.5th centiles of the posterior distribution. Treatment ranking probabilities and SUCRA were also reported. 111 For studies including ZOL users, meaningful (> 12-month) follow-up assessments were selected and included in the NMA. In case, there was a follow-up assessment at 12 months only, ZOL arms were excluded from the NMA. For those observational studies which were not included in the discontinuation NMA, effect sizes of discontinuation or persistence were summarised using the vote-counting synthesis method based on the direction of effects. 115 Similarly, data on compliance drawn from both RCTs and observational studies were summarised based on the vote-counting synthesis method. Findings from both syntheses are presented using cross-study visual displays. 116
Assessment of inconsistency
Consistency of evidence for the NMAs of RCTs was assessed using the node-splitting method117–119 in R Studio (R version 4.0.3). Differences between direct and indirect evidence in all network loops were calculated, with p values < 0.05 indicating the presence of significant inconsistency. In the case of fracture data, inconsistency was assessed for vertebral fractures only. For non-vertebral fractures, no indirect evidence was available. For hip fractures, an assessment of inconsistency was not performed because the direct evidence between ALN and RIS was provided by one small, and unbalanced in terms of sample size, study with zero events in one arm. For wrist fractures, an assessment of inconsistency was not performed because the direct evidence between ALN and RIS was provided by the same small study, and the only direct evidence between ALN and oral IBN-oral was provided by the only three-arm study included in the NMA. For BMD data, the assessment of inconsistency was performed after excluding an outlier study, which was the only study informing the direct relationship between ZOL and ALN, and the three-arm study, which was the only study providing direct evidence for the relationship between RIS and IBN-oral. For the overall adverse event outcome, an assessment of inconsistency was not formally performed because the fit of the model with the data was poor. For myalgia, headache and pyrexia, assessment of inconsistency was not performed because there was no indirect evidence. For influenza-like symptoms, an assessment of inconsistency was not performed because there was only one small study with zero events in the control arm informing the direct relationship between IBN-oral and PLB and three small studies with zero events in control arms informing the direct relationship between ZOL and PLB. Due to the multiple arms reported per study in retrospective observational studies included in the adherence review, a formal assessment of inconsistency in persistence NMA was not performed.
Credibility of the findings
Credibility of findings on persistence was assessed in RCTs only by following the confidence in network meta-analysis (CINeMA) approach,120 where the credibility of findings is accounted for by the assessment of: (1) within-study bias, (2) reporting bias, (3) indirectness, (4) imprecision, (5) heterogeneity and (6) incoherence. In this NMA, HR/OR ranging from 0.8 to 1.25 was used to indicate clinical significance in binary outcomes, while a MD of 2.71 (1/2 SD of baseline control arms) was used to indicate clinical significance in the continuous outcomes (i.e. BMD femoral neck). CINeMA’s freely available web application121 was used to assess credibility of the findings.
Results
Updated review
A PRISMA flow diagram shows the selection of papers for inclusion and exclusion in the updated systematic review (Figure 4). A total of 6623 articles were retrieved, of which 1889 were duplicates. Overall, 4535 studies were excluded following title and abstract screening, and 170 were excluded following full-text screening. Data from 25 newly identified trials obtained from 29 published reports were added to the data obtained from 43 trials identified in the previous review (92) resulting in a total of 68 trials out of 47,007 participants.
FIGURE 4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the selected studies in the updated review. Reproduced from Bastounis A, et al. JBMR Plus 6:e10620. https://doi.org/10.1002/jbm4.10620.
Network structures and geometry
Four networks were created for fracture data. Data for vertebral and hip fractures provided us with one closed loop of evidence. Data for non-vertebral fractures did not provide us with a closed loop of evidence, and the indirect effects were drawn from a single study. Similarly, data for wrist fractures provided us with a single loop after removing the only three-arm study of the network. Data for BMD provided us with five closed loops after removing the single three-arm study, whereas three of the loops were accounted for by single studies. A total of 28,340 (nstudies = 27) participants received BPs (ntreatments = 5) to prevent vertebral fractures. The most commonly studied treatments were ZOL (n = 10) and RIS (n = 10). PLB was used as the comparator arm in 24 studies. The most frequently used comparisons were ZOL versus PLB (n = 9) and RIS versus PLB (n = 8). A total of 26,435 (nstudies = 19) received BPs (ntreatments = 5) for preventing non-vertebral fractures. The drug that was more commonly studied was ZOL (n = 7). PLB was used as the comparator arm in 18 studies. The most commonly studied comparisons were ZOL versus PLB (n = 7) and ALN versus PLB (n = 6). A total of 28,570 (nstudies = 44) participants received BPs (ntreatments = 5), providing us with data for femoral neck BMD. Data were drawn from 43 two-arm studies and 1 three-arm study. The studied medications were more commonly ALN (nstudies = 23) and RIS (nstudies = 16). PLB was used as the comparator arm in 37 studies. The most commonly studied comparisons were ALN versus PLB (n = 17 studies) and RIS versus PLB (n = 11 studies). No trials testing IBN-IV against any of the aforementioned BPs were identified.
Characteristics of studies and risk of bias within individual studies
Of the 25 new trials of 6318 participants identified from 29 published reports, covering the period from 2014 to 2021, 10 studies were conducted in China,122–131 5 studies were conducted in Europe,132–136 3 were conducted in the USA,137–139 3 were conducted in Oceania,140–142 1 in Japan,143 1 in South Korea144 and 2 were conducted internationally. 145,146 Four extensions of original trials147–150 and one ancillary substudy of a main trial137 were available, accounting for the total number of eligible studies identified. In two cases,134,141 trials published before 2014 were deemed eligible for inclusion and included in the updated review after receiving clinicians’ feedback. The sample sizes of the trials identified in the updated review ranged from 30 to 2000 participants.
Overall, 19 trials recruited exclusively female participants. In nine trials, most of the participants had received a diagnosis of osteoporosis before entering the study, participants in nine trials fulfilled the criteria for secondary causes of osteoporosis and participants in four trials received the treatments of interest post operation, whereas the majority of participants had a history of fractures or were recruited on the basis of fractures at baseline in six trials. Overall, 15 trials identified in the updated review provided us with data regarding the occurrence of fractures, whereas 13 trials provided data regarding percentage BMD change at the femoral neck and 3 provided data regarding absolute BMD changes. All but two of the newly identified trials reported prevalence of adverse events. In total, the overall risk of bias was high in 12 trials. Most of the high-risk ratings were observed in the ‘blinding of participants and personnel’ and ‘incomplete outcome data’ domains.
Synthesis of results on the main outcomes (updated review)
Primary outcome: vertebral fractures
Data were available from 27 RCTs. The model fitted the data relatively well [data points: 54; total residual deviance (Dres): 56.34; deviance information criterion (DIC): 298.5]. The between-study SD was estimated to be 0.18 (95% CrI 0.01 to 0.46), whereas the between-treatment SD was estimated to be 0.19 (95% CrI 0.01 to 0.46). All treatments were associated with beneficial treatment effects relative to PLB, and all treatment effects were statistically significant (p < 0.05) (Table 8). ZOL, ALN and RIS were also found to exert clinically significant effects. ZOL was associated with the greatest effect (HR 0.38; 95% CrI 0.28 to 0.49) and was most likely to be the most effective treatment (probability: 0.55).
| ZOL | 3.80 (2.07 to 4.80) | ZOL | 0.33 (0.23 to 0.43) | RIS | - | |||||||||||
| 1.15 (0.24 to 2.08) | ALN | 3.10 (2.40 to 3.80) | 0.88 (0.58 to 1.21) | ALN |
0.43 (0.33 to 0.53) | 0.98 (0.82 to 1.35) | ZOL | - | ||||||||
| 1.31 (−0.08 to 2.73) | 0.15 (−1.00 to 1.32) | IBN-oral | 2.30 (0.21 to 4.30) | 0.87 (0.37 to 1.82) | 0.99 (0.47 to 2.18) | IBN-oral | - | 0.95 (0.50 to 1.33) | 0.98 (0.55 to 1.36) | IBN-oral | - | |||||
| 1.76 (0.82 to 2.74) | 0.6 (−0.09 to 1.31) | 0.45 (−0.80 to 1.72) | RIS | 2.40 (1.05 to 3.30) | 0.76 (0.50 to 1.07) | 0.88 (0.6 to 1.22) | 0.91 (0.37 to 1.82) | RIS | 0.54 (0.39 to 0.69) | 0.92 (0.65 to 1.11) | 0.93 (0.74 to 1.11) | 0.99 (0.63. 1.5) | ALN | - | ||
| 4.02 (3.20 to 4.84) | 2.86 (2.37 to 3.36) | 2.70 (1.56 to 3.86) | 2.25 (1.61 to 2.87) | PLB | 0.38 (0.28 to 0.49) | 0.44 (0.33 to 0.57) | 0.44 (0.02 to 0.94) | 0.50 (0.37 to 0.66) | PLB | 0.70 (0.53 to 0.84) | 0.71 (0.61 to 0.81) | 0.75 (0.51 to 1.26) | 0.77 (0.63 to 0.91) | PLB |
Primary outcome: non-vertebral fractures
Data were available from 19 RCTs. The model fitted the data well (data points: 38; Dres: 28.57; DIC: 224.8). The between-study SD was estimated to be 0.08 (95% CrI 0.06 to 0.24), whereas the between-treatment SD was estimated to be 0.21 (95% CrI 0.005 to 0.99). All treatments were associated with beneficial treatment effects relative to PLB, with RIS, ALN and ZOL being statistically significant (p < 0.05) (see Table 8). RIS was associated with the greatest effect (HR 0.7; 95% CrI 0.53 to 0.84) and was most likely to be the most effective treatment (probability: 0.44). ZOL was found to be comparably effective, showing more precise effects (HR 0.71; 95% CrI 0.61 to 0.81).
Primary outcomes: hip fractures and wrist fractures
Data on the occurrence of hip fractures were available from 14 RCTs. The model fitted the data well (data points: 28; Dres: 22.22; DIC: 144.8). The between-study SD was estimated to be 0.1 (95% CrI 0 to 0.33), whereas the between-treatment SD was estimated to be 0.36 (95% CrI 0 to 1.8). All treatments were associated with beneficial treatment effects relative to PLB, whereas ZOL, ALN and RIS were found to exert statistically significant treatment effects (p < 0.05). ZOL (HR 0.61; 95% CrI 0.47 to 0.79) and ALN (HR 0.61; 95% CrI 0.4 to 0.86) were associated with the greatest effects, with the effects of the former being clinically significant. Data on the occurrence of wrist fractures were available from 10 RCTs, with one RCT comparing three treatments. The model fitted the data well (data points: 21; Dres: 21.83; DIC: 95.26). The between-study SD was estimated to be 0.29 (95% CrI 0 to 0.68), whereas the between-treatment SD was estimated to be 0.44 (95% CrI 0.01 to 1.8). All treatments were associated with beneficial treatment effects relative to PLB, although the treatment effects were not statistically significant (p > 0.05). ZOL was associated with the greatest effect, with HR 0.54 (95% CrI 0.04 to 1.36) and was most likely to be the most effective treatment (probability: 0.47).
Outline of results on the secondary outcomes
Eleven NMAs were conducted on secondary outcomes (Appendix 1, Figure 13). ZOL was found to be significantly worse compared to PLB on overall adverse events (HR 1.52; 95% CrI 1.19 to 1.96), arthralgia (HR 1.95; 95% CrI 1.17 to 3.01), headache (HR 2.76; 95% CrI 2.32 to 3.29), influenza-like symptoms (HR 6.05; 95% CrI 3.07 to 10.86), myalgia (HR 5.21; 95% CrI 4.35 to 6.3) and pyrexia symptoms (HR 9.37; 95% CrI 7.11 to 15.56). The model fit with the data was poor on overall adverse-events outcome (Dres: 91.23; data points: 77), good on arthralgia outcome (Dres: 31.98; data points: 32), moderate on headache outcome (Dres: 25.46; data points: 22), poor on influenza-like symptoms outcome (Dres: 35.93; data points: 24), relatively good on myalgia outcome (Dres: 24.69; data points: 22) and moderate on pyrexia outcome (Dres: 27.27; data points: 24).
Risk of bias across studies and credibility of findings
Risk-of-bias assessment at outcome level was undertaken for all studies conferring data on vertebral fractures and BMD. For vertebral fractures, most of the major concerns were detected in the comparisons of RIS versus PLB (> 70%) and ALN versus RIS (> 40%), with the former being informed by eight direct comparisons and the latter by one direct comparison. From mixed treatment comparisons, findings drawn from two treatment PLB comparisons were rated as highly credible (ALN vs. PLB; ZOL vs. PLB). Findings drawn from RIS versus PLB and RIS versus ZOL comparisons were considered of moderate credibility, with the latter being informed by only one direct pairwise comparison. Findings drawn from ALN versus IBN-oral and ALN versus RIS comparisons were considered of low credibility, with the former comparison being informed by a small study of zero events in the control group. From indirect comparisons, evidence drawn from the treatment–PLB comparison (PLB vs. IBN-oral) and one active comparison (ALN vs. ZOL) were both rated as highly credible, whereas the rest of the indirect comparisons produced evidence of low credibility. For percentage BMD change, most of the major concerns were detected in the active comparison of ALN versus RIS (marginally > 10%), with four studies providing evidence. Proportion of evidence drawn from studies with major concerns was < 10% in the rest of the comparisons. Apart from two active comparisons (ALN vs. ZOL; IBN-oral vs. ZOL), all the comparisons provided us with highly credible findings. With regard to the two comparisons providing us with evidence of low credibility, the direct evidence for the comparison of ALN versus ZOL was drawn from a single, outlier study.
Adherence review
A PRISMA flow diagram (Figure 5) shows the selection of papers for inclusion and exclusion. A total of 10,030 articles were retrieved, of which 1729 were duplicates. Overall, 7976 studies were excluded following title and abstract screening, and 220 were excluded following the full-text screen. Data were extracted from 59 RCTs drawn from 69 published reports and 43 observational studies drawn from 45 published reports, resulting in a total population of 2,656,659 participants. Table 9 lists all the studies (see Appendix 2 list of references).
FIGURE 5.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the selected studies in the adherence review. Reproduced from Bastounis A, et al. Osteoporos Int 2022;33(6):1223–3. https://doi.org/10.1007/s00198-022-06350-w. Epub 21 February 2022. PMID: 35188591; PMCID: PMC9106630.
| Study ID Reference list included as an appendix |
Study design | Country | Na/gender (%)/age (mean, SD)b | % patients with prior incidence of fractures | Drugs | Dose/mode/frequency | % GC users | % patients with (PM) OP | Compliance | Persistence/discontinuation |
|---|---|---|---|---|---|---|---|---|---|---|
| Adachi et al., 2009 | RCT | Canada and Colombia | 438/F (100)/ALN: 65.4 (10.5); PLB: 65.7 (9.9) | P (6.8) | ALN; PLB | 10 mg/oral/daily | NR | NR | NR | Discontinuation (n/N, total), 3 months
ALN: 54/291 PLB: 17/147 Discontinuation due to AEs (n/N, total), 3 months ALN: 39/291 PLB: 14/147 Discontinuation due to other reasons (n/N, total), 3 months ALN: 15/291 PLB: 3/147 |
| Adami et al., 2020 | Retr. | USA | 73,800/F (100)/NR | P (NR) | ALN; RIS; ZOL | NR/oral; IV/NR | P (NR) | P (NR) | NR | Discontinuation (n/N, %), ≈24 months
ALN: 20,455/59,251, 34.5% RIS: 2388/6806, 35.1% ZOL: 3438/7743, 44.4% |
| Bala et al., 2014 | RCT | NR | 163/F (100)/RIS: 62 (6); PLB: 61 (4) | NR | RIS; PLB | 35 mg/oral/weekly | NP | NP | NR | Discontinuation (n/N, total), 12 months
RIS: 9/109 PLB: 2/54 Discontinuation due to AEs (n/N, total), 12 months RIS: 4/109 PLB: 0/54 Discontinuation due to other reasons (n/N, total), 12 months RIS: 5/109 PLB: 2/54 |
| Belhassen et al., 2017 | Retr. | France | 946/NR/NR | NR | ALN; IBN; RIS; ZOL | NR | NR | NR | NR | Persistence (refill gap ≥ 12 months):
12 months: ALN: 328/453 (72.4%); IBN: 148/182 (81.3%); RIS: 236/310 (76.1%); ZOL: 1/1 24 months: ALN: 252/453 (55.6%); IBN: 117/182 (64.3%); RIS: 176/310 (56.8%); ZOL: 0 36 months: ALN: 218/453 (48.1%); IBN: 92/182 (50.5%); RIS: 152/310 (49%); ZOL: 0 48 months: ALN: 196/453 (43.3%); IBN: 76/182 (41.8%); RIS: 131/310 (42.3%); ZOL: 0 60 months; ALN: 159/453 (35.1%); IBN: 0; RIS: 100/310 (32.3%); ZOL: 0 Median discontinuation (years): ALN: 1; IBN: 1.9; RIS: 1.2; ZOL: 1 Discontinuation (without any switch): ALN: 177/453; IBN: 79/182; RIS: 130/310; ZOL: 1 |
| Berecki-Gisolf et al., 2008 | Retr. | Australia | 756/F (100)/80 (5.94) | NR | ALN; RIS | 70 mg (ALN); 35 mg (RIS)/oral/weekly | NR | P (100) | NR | Persistence failure ALN (ref.) vs. RIS (refill gap ≥ half the prescription duration)
HR = 1.19 (95% CI 0.98 to 1.46); p = 0.09 |
| Black et al., 2007 (Ext.)
Black et al., 2012 Black et al., 2015 (HORIZON) |
RCT | Multicentre (USA; Australia; Argentina; Belgium; Canada; Colombia; Finland; France; Germany; Hong-Kong; Hungary; Italy; New Zealand; Norway; Poland; Sweden; Switzerland; Thailand) | 7765/F (100)/ZOL: 78 (4.71); PLB: 78.1 (4.85) | P (NR) | ZOL; PLB | 5 mg/IV/annually | NP | P (100) | Compliance 100% (n/N, per infusion), 36 months
ZOL: 3086/3875 PLB: 3174/3861 Compliance 66.6% (n/N, per infusion), 36 months ZOL: 344/3875 PLB: 359/3861 Compliance 33.3% (n/N, per infusion), 36 months ZOL: 432/3875 PLB: 319/3861 |
Discontinuation (n/N, total), 36 months
ZOL: 641/3876 PLB: 607/3889 Discontinuation due to AEs (n/N, total), 36 months ZOL: 80/3876 PLB: 70/3889 Discontinuation (n/N, total), 108 months ZOL: 21/95 PLB: 18/95 Discontinuation due to AEs (n/N, total), 108 months ZOL: 2/95 PLB: 1/95 Discontinuation due to other reasons (n/N, total), 108 months ZOL: 19/95 PLB: 17/95 |
| Bone et al., 2000 | RCT | USA | 142/F (100)/ALN: 61 (8); PLB: 62 (9) | NR | ALN; PLB | 10 mg/oral/daily | NR | P (100) | NR | Discontinuation (n/N, total), 24 months
ALN: 24/92 PLB: 16/50 Discontinuation due to AEs (n/N, total), 24 months ALN: 6/92 PLB: 5/50 Discontinuation due to other reasons (n/N, total), 24 months ALN: 18/92 PLB: 11/50 |
| Bonnick et al., 2006
Extension of Rosen et al., 2005 (FACT) |
RCT | USA | 1053/F (100)/64.4 (9.5) | NR | ALN; RIS | 70 mg; 35 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 82/520 RIS: 79/533 Discontinuation due to AEs (n/N, total), 12 months ALN: 33/520 RIS: 33/533 Discontinuation due to other reasons (n/N, total), 12 months ALN: 49/520 RIS: 46/533 Persistence (n/N), 24 months ALN: 375/411 RIS: 375/414 |
| Boonen et al., 2012 | RCT | Europe, South America, Africa, Australia | 1199/F (0)/67 (26) | P (NR) | ZOL; PLB | 5 mg/IV/annually | NP | NA | Number not receiving the 2nd infusion (n/N, %)
ZOL: 52/588 (8.8%) PLB: 53/611 (8.7%) |
Discontinuation (n/N, total), 24 months
ZOL: 58/588 PLB: 71/611 Discontinuation due to AEs (n/N), 24 months ZOL: 11/588 PLB: 11/611 Discontinuation due to other reasons (n/N), 24 months ZOL: 39/588 PLB: 60/611 |
| Boonen et al., 2009 | RCT | Europe, Lebanon, Australia, USA | 284/F (0)/RIS: 60 (11); PLB: 62 (11) | P (NR) | RIS; PLB | 35 mg/oral/weekly | P (100) | NA | Compliance (%)
RIS: 98 PLB: 91 |
Discontinuation (n/N, total), 24 months
RIS: 16/191 PLB: 18/93 Discontinuation due to AEs (n/N, total), 24 months RIS: 7/191 PLB: 9/93 Discontinuation due to other reasons (n/N, total), 24 months RIS: 9/191 PLB: 9/93 |
| Calabria et al., 2016 | Retr. | Italy | 40,003/F (88.25)/71 (10) | NR | ALN; RIS | 70 mg (ALN); 35 mg (RIS)/oral/weekly | P (NR) | NR | MPR ≥ 80% (6 months):
ALN: 8029/15,521 (51.7%) ALN and chol.: 5826/10,485 (55.6%) RIS: 7836/13,997 (56%) MPR ≥ 80% (12 months): ALN: 6855/15,521 (44.2%) ALN and chol.: 5005/10,485 (47.7%) RIS: 6715/13,997 (48%) MPR ≥ 80% (36 months): ALN: 4952/15,521 (31.9%) ALN and chol.: 3540/10,485 (33.8%) RIS: 4880/13,997 (34.9%) |
NR |
| Carbonell-Abella et al., 2015 | Retr. | Spain | 127,722/F (77.5)/66.9 (11) | NR | ALN; RIS; IBN-oral | 70 mg, 10 mg (ALN); 5 mg, 35 mg (RIS); 150 mg (IBN)/oral/daily; weekly; monthly | NR | NR | NR | Discontinuation, SHR (95% CI) (filling gap ≥ 6 months)
ALN (weekly): ref. ALN (daily): HR = 1.64 (95% CI 1.52 to 1.76) RIS (daily): HR = 1.86 (95% CI 1.74 to 1.99) RIS (weekly): HR = 1.12 (95% CI 1.10 to 1.14) IBN (monthly): HR = 1.06 (95% CI 1.04 to 1.08) Discontinuation (filling gap ≥ 12 months): ALN (weekly): ref. |
| ALN (daily): HR = 1.74 (95% CI 1.61 to 1.88)
RIS (daily): HR = 1.98 (95% CI 1.84 to 2.12) RIS (weekly): HR = 1.15 (95% CI 1.13 to 1.17) IBN (monthly): HR = 1.11 (95% CI 1.09 to 1.14) Persistence 12 months: ALN (weekly): 22,012/55,117 ALN (daily): 70/497 IBN (monthly): 4750/13,270 RIS (weekly): 7892/25,312 RIS (daily): 33/430 |
||||||||||
| Cheen et al., 2012 | Retr. | Singapore | 798/F (92)/68.5 (10.8) | P (47.1) | ALN; RIS | NR/oral/daily; weekly | NR | NR | Mean MPR 24 months (%, SD, n)
ALN: 79.3 (27.6%), n = 497, p = 0.67 RIS: 78.3 (27.3%), n = 301 MPR ≥ 80% (24 months) ALN: 66%, n = 497, p = 0.13 RIS: 60.8%, n = 301 |
Mean persistence 12 months (n/N, %, 30-day refill gap)
ALN: 352/497 (70.8%) RIS: 199/301 (66.1%) |
| Cheng et al., 2015 | Retr. | USA | 8495/F (100)/66.2 (11.5) | P (13.6); 13 (ALN); 11.6 (IBN); 12 (RIS) | ALN; IBN; RIS | NR/oral/NR; monthly | NR | P (35.8); 22.2 (ALN); 46.8 (IBN); 35.3 (RIS) | MCR ≥ 80% (12 months)
ALN (n = 5458): 31.3% IBN (n = 1696): 30.4% RIS (n = 1341): 25.6% Weight-adjusted compliance 12 months (women aged ≥ 50 and increased risk of fracture, 60-day refill gap) ALN (n = 2820): 32.8% IBN (n = 1062): 30.1% RIS (n = 716): 25% |
Persistence (12 months, 60-day refill gap)
ALN: 1916/5458 (35.1%) IBN: 573/1696 (33.8%) RIS: 388/1341 (28.9%) Weight-adjusted persistence 12 months (%) ALN: 35.1 IBN: 33.8 RIS: 28.9 Weight-adjusted persistence 12 months (women aged ≥ 50 and increased risk of fracture, 60-day refill gap) ALN (n = 2820): 36% IBN (n = 1062): 33.4% RIS (n = 716): 28% |
| Cheung et al., 2020 | RCT | Australia | 76/F (0)/ZOL: 68.3 (7.77); PLB: 69 (7.01) | P (NR) | ZOL; PLB | 5 mg/IV/annually | NP (95% received ADT) | NR | NR | Discontinuation (n/N), 12 months
ZOL: 4/39 PLB: 11/37 Discontinuation (n/N), 24 months ZOL: 13/39 PLB: 15/37 |
| Cosman et al., 2016 | RCT | USA | 175/F (100)/ALN: 66.9 (7.5); PLB: 67.8 (7.8) | P (NR) | ALN; PLB | 70 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 19/87 PLB: 20/88 Discontinuation due to AEs (n/N), 12 months ALN: 3/87 PLB: 6/88 Discontinuation due to other reasons (n/N), 12 months ALN: 16/87 PLB: 14/88 |
| Cryer et al., 2005 | RCT | USA | 454/F (100)/ALN: 64.6 (10); PLB: 65.8 (9.9) | NP | ALN; PLB | 70 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 6 months
ALN: 31/224 PLB: 31/230 Discontinuation due to AEs (n/N), 6 months ALN: 10/224 PLB: 20/230 Discontinuation due to other reasons (n/N), 6 months ALN: 21/224 PLB: 11/230 |
| Curtis et al., 2012 | Retr. | USA | 1621/F (ZOL: 98.2; IBN1: 94.5; IBN2: 94.2)/NR | 10.2% (ZOL), 9.1% (IBN1), 7.7% (IBN2) | IBN1 and 2 (same drug – different cohorts); ZOL | 5 mg; 3 mg/IV/annually; quarterly | 62.5 (ZOL)
72.4 (IBN1) 46.1 (IBN2) |
P (NR) | Number of infusions 18–27 months
ZOL: 1.7 (SD = 0.5) IBN1: 4.4 (SD = 2.7) IBN2: 5.3 (SD = 2.9) 1 infusion recipients only (%) ZOL: 31.9 IBN1: 18.5 IBN2: 14 |
PDC 18–27 months (mean, SD)
ZOL: 82 (SD = 18), n = 775 IBN1: 58 (SD = 31), n = 275 IBN2: 62 (SD = 31), n = 571 PDC ≥ 80% at 18 months (%, ref.: ZOL) ZOL: 61 IBN1: 43, p < 0.0001 IBN2: 49, p < 0.0001 PDC ≥ 80% at 18–27 months (%) ZOL: 62.8, ref. IBN1: 36, p < 0.0001 IBN2: 33.3, p < 0.0001 |
| Curtis et al., 2006 | Retr. | USA | 1158/F: 77 (ALN); 80 (RIS)/ALN: 53 (13); RIS: 53 (13) | 13% (ALN)
10% (RIS) |
ALN; RIS | 10 mg, 70 mg (ALN); 5 mg, 35 mg (RIS)/oral/daily, weekly | P (100) | NR | MPR (%, SD)
ALN: 72 (26), n = 754 RIS: 74 (26), n = 404, p > 0.05 (NS) |
Discontinuation (≥ 3 months gap, mean duration: 39 months), (HR, 95% CI)
HR (ref.: RIS) = 1.04 (0.86 to 1.25) Computed by graph |
| Downey et al., 2006 | Retr. | USA | 9105/F (100)/64.4 (10.4) | NR | ALN; RIS | ALN: 10 mg, 70 mg (80.7%); RIS: 5 mg, 35 mg (69.2)/oral; weekly | NR | NR | MPR 12 months (% days on drug, SD)
ALN: 60.7 (28.64), n = 6881, p < 0.001 RIS: 58.4 (28.64), n = 2224 |
Discontinuation (mean time), 12 months
ALN: 2.53, n = 6881 RIS: 2.53, n = 2224 Persistence rate (%) 12 months (refill gap ≥ 30 days) ALN: 1466/6881 (21.3%) RIS: 431/2224 (19.4%) |
| Duckworth et al., 2019 | RCT | UK | 421/F (86)/ALN: 63.8 (8.5); PLB: 63 (8.5) | P (100) | ALN; PLB | 70 mg/oral/weekly | NP | NR | Compliance (n/N), 6 months
ALN: 184/215 PLB: 175/206 |
Discontinuation (n/N, total), 6 months
ALN: 17/215 PLB: 24/206 |
| Dugard et al., 2009 | Retr. | UK | 40/F (100)/74.5 (7.1) | P (48.4) | ALN; RIS | NR | P (10.2) | P (100) | MPR ≥ 80, 60 months
ALN: 10/35 RIS: 2/5 |
Persistence 5-year (n/N, refill gap ≥ 12 months)
ALN: 18/35 RIS: 2/5 |
| Durden et al., 2017 | Retr. | USA | 33,435/F (100)/≈64.9 (10.4) | NR/ALN-daily: 9.4; ALN-weekly: 7.4; IBN-monthly: 5.7; IBN-quarterly: 5.5; RIS-daily: 11.3; RIS-weekly: 6.4; ZOL: 8.2 | ALN; IBN; RIS; ZOL | 10 mg, 70 mg; 3 mg; 150 mg; 5 mg, 35 mg; 5 mg/oral; IV/daily; weekly; quarterly; monthly; annual | NR | ALN-daily: 12.1; ALN-weekly: 16.8; IBN-monthly: 19; IBN-quarterly: 49.7; RIS-daily: 22.6; RIS-weekly: 24.3; ZOL: 49.2 | MPR ≥ 80% (%, n), 12 months:
ALN-daily: 19.2 (n = 224) ALN-weekly: 31.3 (n = 19,486) IBN-monthly: 31.7 (n = 5981) IBN-quarterly: 20.6 (n = 165) RIS-daily: 26.4 (n = 53) RIS-weekly: 22.1 (n = 2968) ZOL: 98.5 (n = 4558) MPR ≥ 80% (%, n), 24 months: ALN-daily: 12.5 (n = 224) ALN-weekly: 22.8 (n = 19,486) IBN-monthly: 22.3 (n = 5981) IBN-quarterly: 15.2 (n = 165) RIS-daily: 11.3 (n = 53) RIS-weekly: 15.5 (n = 2968) ZOL: 38.5 (n = 4558) |
Persistence 12-month, n/N (&), 60-day refill gap
ALN-daily: 71/224 (31.7%) ALN-weekly: 7541/19,486 (38.7%) IBN-monthly: 2345/5981 (39.2%) IBN-quarterly: 56/165 (33.9%) RIS-daily: 23/53 (43.4%) RIS-weekly: 1000/2968 (33.7%) ZOL: 4558/4558 (100%) Persistence 24 months, n/N (&), 60-day refill gap ALN-daily: 44/224 (19.6%) ALN-weekly: 4618/19,486 (23.7%) IBN-monthly: 1370/5981 (22.9%) IBN-quarterly: 32/165 (19.4%) RIS-daily: 11/53 (20.8%) RIS-weekly: 606/2968 (20.4%) ZOL: 1545/4558 (33.9%) |
| Dursun et al., 2001 | RCT | Turkey | 101/F (100)/ALN: 60.26 (8.58); Calcium: 60.26 (8.58) | NR | ALN; PLB (calcium) | 10 mg/oral/daily | NR | P (100) | NR | Discontinuation (n/N, total), 24 months
ALN: 13/51 PLB: 15/50 |
| Eastell et al., 2014
Extension Eastell et al., 2011 |
RCT | European countries | 114/F (100)/ALN ≈ 65.7 (4.39); PLB ≈ 65 (4.9) | NR | ALN; PLB | 70 mg/oral/weekly | NR | P (ALN ≈ 82.5; PLB ≈ 82.5) | NR | Discontinuation (n/N, total), 12 months
ALN: 5/57 PLB: 8/57 Discontinuation due to AEs (n/N), 12 months ALN: 3/57 PLB: 2/57 Discontinuation due to other reasons (n/N), 12 months ALN: 2/57 PLB: 6/57 Discontinuation (n/N, total), 24 months ALN: 3/41 PLB: 6/39 |
| Eisenberg et al., 2015 | Retr. | USA | 27,905/F (100)/66.1 (9.7) | NR | ALN; IBN; RIS | NR/oral/NR | NR | P (100) | MPR ≥ 70% 12 months (n/N, %), 13–24 months:
ALN: 6725/15,917, 59.2, p < 0.001 IBN: 1974/5249, 17.4 RIS: 2669/6739, 23.5 Compliance (OR, SE) ALN: ref. IBN: 1.02 (1.07), p = 0.81 RIS: 0.92 (1.06), p = 0.14 Higher OR indicate higher likelihood to be compliant |
Discontinuation 12 months (n/N, refill gap ≥ 60 days)
ALN: 9585/15,917 IBN: 3441/5249 RIS: 4217/6739 |
| Eisman et al., 2004 | RCT | Multicentre (Europe; Americas; Africa; Asia-Pacific) | 449/F (94.2)/ALN: 63.6 (9); PLB: 63.6 (8.5) | NR | ALN; PLB | 70 mg/oral/weekly | NR | NR | NR | Discontinuation (n/N, total), 3 months
ALN: 18/225 PLB: 10/224 Discontinuation due to AEs (n/N), 3 months ALN: 11/225 PLB: 6/224 Discontinuation due to other reasons (n/N), 3 months ALN: 7/225 PLB: 4/224 |
| Fan et al., 2013 | Retr. | USA | 44,635/F (100)/NR | NR | ALN; IBN; RIS | 70 mg; 35 mg; 150 mg/oral/weekly; monthly | NR | NR | MPR (%)
ALN: 55, p < 0.05 IBN: 51 RIS: 52 |
Persistence (45-day refill gap) (n/N, %)
ALN: 8837/25,207, 35.1, p < 0.001 IBN: 225/739, 30.4, p < 0.001 RIS: 6066/18,689, 32.5, p < 0.001 Discontinuation (n/N, %, refill gap ≥ 45-day) ALN: 16,132/25,207, 64, p < 0.001 IBN: 504/739, 68.2, p < 0.001 RIS: 12,418/18,689, 66.4, p < 0.001 Discontinuation (HR, 95% CI) ALN: ref. IBN: 1.3(1.2 to 1.4), p < 0.001 RIS: 1.1 (1.06 to 1.11), p < 0.001 |
| Flodin et al., 2014 | RCT | Sweden | 53/F (70.8)/79 (9) | P (100) | RIS; PLB | 35 mg/oral/weekly | NR | NR | NR | Discontinuation (n/N, total), 12 months
RIS: 3/28 PLB: 1/25 Discontinuation due to other reasons (n/N), 12 months RIS: 3/28 PLB: 1/25 |
| Fogelman et al., 2000 | RCT | Multicentre (France; UK; the Netherlands; Belgium; Germany) | 359/F (100)/RIS: 65 (6.7); PLB: 64 (6.7) | RIS: 32; PLB: 30 | RIS; PLB | 5 mg/oral/daily | NR | P (100) | NR | Discontinuation (n/N, total), 24 months
RIS: 38/177 PLB: 37/180 Discontinuation due to AEs (n/N, total), 24 months RIS: 19/177 PLB: 14/180 Discontinuation due to other reasons (n/N, total), 24 months RIS: 19/177 PLB: 23/180 |
| Gallagher et al., 2008 | Retr. | UK | 44,531/F (81.2)/NR | 11,460 (25.7%) | ALN; RIS | 10 mg, 70 mg; 5 mg, 35 mg/oral/daily, weekly | P (32) | NR | MPR mean FUp ~ 2.3 years, OR (95% CI)
ALN: ref. RIS: 1.30 (95% CI 1.22 to 1.39, more likely to be compliant), p < 0.05 |
Persistence mean FUp ~ 2.3 years, RR (95% CI), (refill gap ≤ 3 months)
ALN: ref. RIS: 1.06 (95% CI 1.02 to 1.09, more likely to continue), p < 0.05 |
| Gold et al., 2006
Ext.: Gold et al., 2009 |
Retr. | USA | 6 months period: 208,762 (compliance); 240,001 (persistence); 12 months period: 232,489 (compliance); 263,383 (persistence)/F (IBN: 94.2; RIS: 93.2)/67.3 (9.4) | NR | IBN; RIS | 150 mg; 35 mg/oral/monthly; weekly | NR | P (100) | MPR (%, SD), 6 months
IBN: 78.5 (20.4), p < 0.0001 RIS: 83.3 (17) MPR (%, SD), 12 months IBN: 74.68 (22.56), p < 0.0001 RIS: 80.15 (18.9) Mean CDA (%, SD%), 6 months IBN: 52.8 (31.5) RIS: 72.7 (24.6), p < 0.0001 Mean CDA (%, SD%), 12 months IBN: 43.38 (32.96) RIS: 64.54 (29.86), p < 0.0001 |
Persistence (days, SD), 6 months (refill gap ≥ 90 days)
IBN: 100.1 (67.4), p < 0.0001 RIS: 144.3 (55.5) Persistence (days, SD), 12 months (refill gap ≥ 90 days), HR (95% CI) IBN: 151.54 (137.24) RIS: 250.04 (132.34) HR = 1.77 (1.73 to 1.82), p < 0.0001 |
| Greenspan, Perrera et al., 2015 | RCT | USA | 181/F (100)/ZOL: 85.4 (5.66); PLB: 85.5 (4.8) | P (ZOL: 52; PLB: 41) | ZOL; PLB | 5 mg/IV/annually | P (NR) | P (ZOL: 48; PLB: 45) | NR | Discontinuation (n/N, total), 24 months
ZOL: 25/89 PLB: 18/92 |
| Greenspan, Vujevich et al., 2015 | RCT | USA | 109/F (100)/RIS: 65 (1); PLB: 64 (1) | NP | RIS; PLB | 35 mg/oral/weekly | NP | NP | Compliance ≥ 80% (%), (range)
RIS: 90–98 PLB: 89–96 |
Discontinuation (n/N, total), 24 months
RIS: 7/55 PLB: 7/54 |
| Greenspan et al., 2003 | RCT | USA | 186/F (100)/ALN: 71 (4); PLB: 72 (5) | P (ALN: 39; PLB: 33) | ALN; PLB | 10 mg/oral/daily | NP | NR | Compliance ≥ 80% medication
ALN: 58/93 PLB: 63/93 |
Discontinuation (n/N, total), 36 months
ALN: 8/93 PLB: 10/93 |
| Grey et al., 2017
Ext. Grey et al., 2014 Grey et al., 2012 |
RCT | New Zealand | 90/F (100)/ZOL: 66 (8); PLB: 63 (8) | NP | ZOL; PLB | 5 mg/IV/annually | NP | NP | NR | Discontinuation (n/N), 12 months
ZOL: 3/45 PLB: 3/45 Discontinuation (n/N), 24 months ZOL: 3/45 PLB: 6/45 Discontinuation (n/N), 60 months ZOL: 5/41 PLB: 9/34 |
| Hadji et al., 2016 | Retr. | Germany | 138,839 (ALN: 90,077; IBN-oral: 6235; IBN-IV: 20,472; RIS: 18,089; ZOL: 3966)/F (100)/NR | NR | ALN; IBN-oral; IBN-IV; RIS; ZOL | 70 mg; 150 mg; 3 mg; 35 mg; 5 mg/oral; IV/weekly; monthly; annually | NR | NR | NR | Persistence in % (≥60-day refill gap), 12 months
ALN: 30.1 IBN-oral: 30.1 IBN-IV: 42.9 RIS: 31.4 ZOL: 33.8 Persistence in % (≥60-day refill gap), 24 months ALN: 17.3 IBN-oral: 16.7 IBN-IV: 24.8 RIS: 17.5 ZOL: 20.9 |
| Persistence in % (≥ 30-day refill gap), 12 months
ALN: 21.5 IBN-oral: 18.4 IBN-IV: 31.1 RIS: 22.6 ZOL: 20.3 Persistence in % (≥ 30-day refill gap), 24 months ALN: 9.7 IBN-oral: 7.3 IBN-IV: 15 RIS: 10.2 ZOL: 6.5 |
||||||||||
| Persistence in % (≥ 90-day refill gap), 12 months
ALN: 35.6 IBN-oral: 35 IBN-IV: 48.2 RIS: 36.9 ZOL: 39.7 Persistence in % (≥ 90-day refill gap), 24 months ALN: 22.5 IBN-oral: 21.2 IBN-IV: 30.2 RIS: 22.6 ZOL: 29.5 |
||||||||||
| Persistence in % (≥ 120-day refill gap), 12 months
ALN: 39.7 IBN-oral: 39.4 IBN-IV: 54.4 RIS: 41.4 ZOL: 43.7 Persistence in % (≥120-day refill gap), 24 months ALN: 26.5 IBN-oral: 25.1 IBN-IV: 36.7 RIS: 26.9 ZOL: 34.1 |
||||||||||
| Hadji et al., 2014 | Prosp. | Germany | 1802/F (100)/
ALN: 71.7 (9.3); IBN: 71.8 (9.3) |
P (NR) | ALN; IBN | 70 mg; 3 mg/oral; IV/weekly; quarterly | P (NR) | P (100) | Discontinuation due to non-compliance (n, %)
IBN: 130/901 (14.4%) ALN: 229/901 (25.4%), p < 0.001 |
Persistence (days) 12 months
IBN: 343, n = 901 ALN: 327, n = 901, p < 0.001 |
| Hadji et al., 2012 | RCT | Germany | 604/F (100)/ALN: 68.1 (7.86); ZOL: 67.6 (8.05) | NP | ALN; ZOL | 70 mg; 5 mg/oral; IV/weekly; annually | NP | P (100) | Compliance rate (%)
ALN: 80.9 ZOL: NR |
Discontinuation (n/N, total), 12 months
ALN: 24/196 ZOL: 19/408 |
| Halpern et al., 2011 | Retr. | USA | 17,284 (ALN: 10,068; IBN: 651; RIS: 6565)/F (100)/commercial: 59.3 (8.6); medicare: 75.7 (6.5) | Commercial: 4; medicare: 6.8 | ALN; IBN; RIS | 5 mg, 10 mg; NR; 5 mg, 35 mg/oral/daily, weekly | Commercial: 17.9; medicare: 16.5 | P (100) | MPR ≤ 50%(%) (commercial) (FUp: 180 days)
ALN: 32 IBN: 30 RIS: 31.4 MPR ≤ 50%(%) (medicare) (FUp: 180 days) ALN: 42.7 IBN: 28.5 RIS: 44 |
NR |
| Hansen et al., 2013;
Hansen et al., 2015 |
Retr. | Denmark | 100,556 (total population)/F (84.5)/70.4 | P (39) | ALN; IBN; RIS | NR | P (24) | NR | NR | Persistence in mean years (mean FUp ~ 5.2 years, 56-day refill gap)
ALN: 4.01 (95% CI = 3.96 to 4.06) IBN: 3.86 (95% CI = 3.5 to 4.22) RIS: 2.79 (95% CI = 2.69 to 2.89) Discontinuation 60 months (n/N, %) ALN: 3504/15,235, 23 IBN: 321/1459, 22 RIS: 91/276, 33 |
| Ho and Kung, 2005 | RCT | Hong Kong | 58/F (100)/ALN: 60.6 (5.5); PLB: 62 (4) | P (37) | ALN; PLB (Calcium) | 70 mg/oral/weekly | NR | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 1/29 PLB: 3/29 |
| Hooper et al., 2005 | RCT | Australia | 254/F (100)/RIS: 52.5 (3.1); PLB: 52.6 (3.3) | P (19.5) | RIS; PLB | 5 mg/oral/daily | NR | NP | NR | Discontinuation (n/N, total), 24 months
RIS: 26/129 PLB: 32/125 Discontinuation due to AEs (n/N, total), 24 months RIS: 7/129 PLB: 8/125 Discontinuation due to other reasons (n/N, total), 24 months RIS: 19/129 PLB: 24/125 |
| Iolascon et al., 2013 | Retr. | Italy | 12,798/F (92.2)/ALN: 68.6 (10.2); ALN + Vit. D: 69 (10.1); IBN: 68.6 (9.9); RIS: 68.4 (10.2) | NR | ALN; ALN + Vit. D; IBN; RIS | NR/oral/NR | NR | NR | NR | Persistence 12 months (n/N, %) (30-day refill gap)
ALN: 292/2317, 12.6 ALN + Vit. D: 707/4501, 15.7 IBN: 341/1581, 21.6 RIS: 695/4399, 15.8 |
| Jones et al., 2008 | Retr. | Canada | 37,032/F (100)/NR | NR | ALN; RIS | 70 mg; 35 mg/oral/weekly | NR | NR | NR | Persistence (n/N, %) 6 months (30-day refill gap)
ALN: 16,752/23,266, 72, p = 0.11 RIS: 9801/13,766, 71.2 Persistence 12 months (n/N, %) (30-day refill gap) ALN: 13,099/23,266, 56.3, p < 0.0001 RIS: 7489/13,766, 54.4 |
| Karimi et al., 2019 | RCT | Iran | 80/F (100)/ALN: 56.5 (6.3); PLB: 55.3 (4.0) | NR | ALN; PLB | 70 mg/oral/weekly | NP | NR | P (100) | Discontinuation (n/N, total), 3 months
ALN: 10/40 PLB: 10/40 |
| Kjellberg et al., 2016 | Retr. | Denmark | 38,234/F (100)/71.7 (9.24) | NR/21.2 | ALN; IBN; RIS | NR/oral/NR | NR | P (33.6) | MPR ≥ 70%, OR (95% CI) (higher ORs indicate higher compliance)
ALN: ref. IBN: 1.49 (1.32 to 1.68), p < 0.001 RIS: 0.84 (0.74 to 0.96), p = 0.011 |
NR |
| Klotz et al., 2013 | RCT | Canada | 186/F (0)/ALN ≈ 73.5 (8.1); PLB ≈ 73.7 (8.6) | P (43) | ALN; PLB | 70 mg/oral/weekly | NP | NA | NR | Discontinuation (n/N, total), 12 months
ALN: 6/84 PLB: 10/102 Discontinuation due to AEs (n/N, total), 12 months ALN: 0/84 PLB: 6/102 Discontinuation due to other reasons (n/N, total), 12 months ALN: 6/84 PLB: 4/102 |
| LaFleur et al., 2015 | Retr. | USA | 35,650/F (100)/65.7 (12.5) | P (21.1) | ALN; IBN; RIS; ZOL | NR/oral; IV/NR | P (11.9) | P (19.7) | NR | Discontinuation (n/N), HR (95% CI), mean FUp ~ 52.8 months (90-day refill gap)
ALN: 26,571/32,971, HR = ref. IBN: 52/78, HR = 0.86 (0.67 to 1.14), p > 0.05(NS) RIS: 1845/2261, HR = 1.07 (1.02 to 1.12), p < 0.05 ZOL: 155/340, HR = 0.58 (0.49 to 0.68), p < 0.05 |
| Landfeldt et al., 2012 | Retr. | Sweden | 54,883/F (ALN: 85.8); (RIS: 87.4)/ALN: 71.1 (10); RIS: 69.7 (10) | P (ALN: 15.4); P (RIS: 15.1) | ALN; RIS | 70 mg; 35 mg/oral/weekly (> 95% in both arms) | P (ALN: 19.6); (RIS: 19.9) | NR | NR | Persistence proportion (%, 95% CI n/N) maximum FUp: 2.33 years (refill gap ≥ 8 weeks)
ALN: 51.7 (51.2 to 52.2), 23,920/46,265 RIS: 50.6 (49.5 to 51.8), 4361/8618 |
| Lewiecki et al., 2008 | Prosp. | USA | 545/F (100)/66 (10.6) | P (32) | IBN-oral/IBN-IV | 150 mg; 3 mg/oral; IV/monthly; quarterly | P (9.8) | P (68.13) | Drug-intake ≥ 75%
IBN-IV: 325/392 IBN-oral: 101/145 |
Discontinuation 12 months (n/N)
IBN-IV: 86/396 IBN-oral: 40/149 Discontinuation due to AEs 12 months (n/N) IBN-IV: 41/396 IBN-oral: 25/149 Discontinuation due to other reasons 12 months (n/N) IBN-IV: 45/396 IBN-oral: 15/149 |
| Lester et al., 2008 | RCT | UK | 50/F (100)/IBN: 66.7 (11.39); PLB: 67.36 (5.81) | NR | IBN; PLB | 150 mg/oral/monthly | NR | NR | NR | Discontinuation (n/N, total), 24 months
IBN: 4/25 PLB: 6/25 Discontinuation due to AEs (n/N, total), 24 months IBN: 2/25 PLB: 1/25 Discontinuation due to other reasons (n/N, total), 24 months IBN: 2/25 PLB: 5/25 |
| Li et al., 2012 | Retr. | UK | 44,526/F (100)/71.4 (11) | P (32.3) | ALN; IBN; RIS | 5 mg, 10 mg; 70 mg; 30 mg, 35 mg; 150 mg/oral/daily; weekly; monthly | P (20.6) | P (58.1) | NR | Persistence (median time in days, 95% CI)
ALN (daily): 56, NR RIS (daily): 98 (86 to 110) ALN (weekly): 221 (211 to 224) RIS (weekly): 223 (210 to 225) IBN (monthly): 300 (270 to 338) Rates of persistence 6 months (%, 95% CI), (n/N), (≥ 30 days refill gap) ALN (daily): 27.0 (26.3 to 27.7), 1379/5107 RIS (daily): 37.8 (36.5 to 39.1), 725/1918 ALN (weekly): 52.8 (52.4 to 53.1), 14,899/28,218 RIS (weekly): 53.1 (52.4 to 53.7), 4598/8660 |
| IBN (monthly): 56.8 (55.1 to 58.5), 354/623
Rates of persistence 12 months (%, 95% CI), (n/N), (≥ 30 days refill gap) ALN (daily): 17.6 (16.9 to 18.2), 899/5107 RIS (daily): 26.4 (25.2 to 27.6), 506/1918 ALN (weekly): 41.3 (40.9 to 41.7), 11,654/28,218 RIS (weekly): 41.0 (40.3 to 41.6), 3551/8660 IBN (monthly): 46.5 (44.7 to 48.3), 290/623 Rates of persistence 36 months (%, 95% CI), (n/N), (≥ 30 days refill gap) ALN (daily): 6.5 (6.0 to 6.9), 332/5107 RIS (daily): 9.6 (8.8 to 10.5), 184/1918 ALN (weekly): 24.6 (24.2 to 25.0), 6942/28,218 RIS (weekly): 19.5 (18.9 to 20.1), 1689/8660 IBN (monthly): 32.1 (29.8 to 34.3), 200/623 Rates of persistence 60 months (%, 95% CI), (n, N), (≥ 30 days refill gap) |
||||||||||
| ALN (daily): 2.8 (2.5 to 3.1), 143/5107
RIS (daily): 4.9 (4.3 to 5.6), 94/1918 ALN (weekly): 16.9 (16.5 to 17.3), 4769/28,218 RIS (weekly): 8.9 (8.2 to 9.6), 771/8660 IBN (monthly): NA |
||||||||||
| Liang et al., 2017 | RCT | China | 285/F (100)/ZOL: 57.22 (2.81); PLB: 57.48 (3.18) | P (NR) | ZOL; PLB | 5 mg/IV/annually | NR | P (100) | NR | Discontinuation (n/N), 6 months
ZOL: 5/175 PLB: 5/110 Discontinuation (n/N), 12 months ZOL: 10/175 PLB: 10/110 Discontinuation (n/N), 24 months ZOL: 20/175 PLB: 15/110 |
| Lima et al., 2019 | Prosp. | Brazil | 158/F (61.66)/RIS: 55.6 (39.54); PLB: 52.66 (32.62) | NR | RIS; PLB | 35 mg/oral/weekly | NP | P (28) | NR | Discontinuation (n/N), 12 months
RIS: 8/73 PLB: 7/85 |
| Liu et al., 2019 | RCT | China | 482/F (ZOL: 47.87, PLB: NR) | P (ZOL: 9.91; PLB: 12.4) | ZOL; PLB | 5 mg/IV/annually | NP | NP | NR | Discontinuation (n/N, total), 12 months
ZOL: 6/353 PLB: 3/129 Discontinuation (n/N, total), 24 months ZOL: 14/353 PLB: 8/129 |
| Livi et al., 2019 | RCT | Italy | 171/F (100)/IBN: 60.6 (9.57); PLB: 60.5 (10.64) | NP | IBN; PLB | 150 mg/oral/monthly | NR | NP | NR | Discontinuation (n/N, total), 60 months
IBN: 17/89 PLB: 10/82 Discontinuation due to AEs (n/N, total), 60 months IBN: 5/89 PLB: 1/82 Discontinuation due to other reasons (n/N, total), 60 months IBN: 12/89 PLB: 9/82 |
| Lyles et al., 2007 | RCT | International | 2127/F (ZOL: 76.7; PLB: 75.5)/ZOL: 74.4 (9.48); PLB: 74.6 (9.86) | P (NR) | ZOL; PLB | 5 mg/IV/annually | NR | P (ZOL: 42.3; PLB: 41.1) | NR | Discontinuation (n/N, total), 36 months
ZOL: 295/1065 PLB: 316/1062 Discontinuation due to AEs (n/N, total), 36 months ZOL: 21/1065 PLB: 18/1062 Discontinuation due to other reasons (n/N, total), 36 months ZOL: 274/1065 PLB: 298/1062 |
| McClung et al., 2014 | RCT | Argentina, Austria, Belgium, Canada, Denmark, Spain and USA | 103/F (100)/ALN: 67.1 (5.8); PLB: 67 (6.5) | NP | ALN; PLB | 70 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 2/51 PLB: 5/52 Discontinuation due to AEs (n/N, total), 12 months ALN: 0/51 PLB: 1/52 Discontinuation due to other reasons (n/N, total), 12 months ALN: 2/51 PLB: 4/52 |
| McClung, Bolognese et al., 2009 | RCT | USA | 160/F (100)/IBN: 53.7 (3.6); PLB: 53.4 (3.8) | NP | IBN; PLB | 150 mg/oral/monthly | NR | NP | NR | Discontinuation (n/N, total), 12 months
IBN: 12/77 PLB: 10/83 |
| McClung, Miller et al., 2009 | RCT | USA | 383/F (100)/ZOL: 59.6 (8.0); PLB: 60.5 (8.0) | NP | ZOL; PLB | 5 mg/oral/annually | NR | NP | NR | Discontinuation (n/N, total), 24 months
ZOL: 27/181 PLB: 14/202 Discontinuation due to AEs (n/N, total), 24 months ZOL: 3/181 PLB: 1/202 Discontinuation due to other reasons (n/N, total), 24 months ZOL: 24/181 PLB: 13/202 |
| McClung et al., 2007 | RCT | USA | 225/F (100)/ZOL: 67.6 (8.3); ALN: 68.0 (7.5) | NR | ZOL; ALN | 5 mg; 70 mg/IV; oral/annually; weekly | NR | P (ZOL: 41.6; ALN: 30.4) | NR | Discontinuation (n/N, total), 24 months
ZOL: 7/113 ALN: 2/112 Discontinuation due to AEs (n/N, total), 24 months ZOL: 4/113 ALN: 1/112 Discontinuation due to other reasons (n/N, total), 24 months ZOL: 3/113 ALN: 1/112 |
| Miller et al., 2008 | RCT | North and Latin America; Europe; South Africa | 1747/F (100)/ALN: 65.6; IBN: 65.6 | P (ALN: 38.2; IBN: 39) | ALN; IBN | 70 mg; 150 mg/oral/weekly; monthly | NR | P (100) | NR | Discontinuation (n/N), 12 months
ALN: 88/873 IBN: 86/874 |
| Modi et al., 2017 | Retr. | USA | 3,361/F (100)/IBN: 71.5 (7.6); ZOL: 72.6 (7.3) | P (IBN: 9.4; ZOL: 12.2) | IBN; ZOL | 3 mg; 5 mg/IV/quarterly; annually | P (IBN: 30; ZOL: 26.4) | P (IBN: 87.1; ZOL: 87.3) | NR | Discontinuation (%), 6 months
IBN (30-day refill gap): 60.5, n = 233 ZOL (30-day refill gap): NA, n = 3128 IBN (60-day refill gap): 54.5 ZOL (60-day refill gap): NA IBN (90-day refill gap): 49.4 ZOL (90-day refill gap): NA Discontinuation (%), 12 months IBN (30-day refill gap): 77.7 ZOL (30-day refill gap): 73.5 |
| IBN (60-day refill gap): 72.1
ZOL (60-day refill gap): 64.5 IBN (90-day refill gap): 69.1 ZOL (90-day refill gap): 59.2 Discontinuation (%), 18 months IBN (30-day refill gap): 87.7 ZOL (30-day refill gap): NA IBN (60-day refill gap): 83.1 ZOL (60-day refill gap): NA IBN (90-day refill gap): 80.9 |
||||||||||
| ZOL (90-day refill gap): NA
Discontinuation (%), 24 months IBN (30-day refill gap): 91.5 ZOL (30-day refill gap): 89.1 IBN (60-day refill gap): 88.1 ZOL (60-day refill gap): 82.8 IBN (90-day refill gap): 87.5 ZOL (90-day refill gap): 79.8 |
||||||||||
| Nakamura et al., 2017 | RCT | Japan | 665/F (ZOL: 93.63; PLB: 94.25)/ZOL: 74 (5.4); PLB: 74.3 (5.4) | P (ZOL: 91.2; PLB: 89.4) | ZOL; PLB | 5 mg/oral/annually | NR | P (ZOL: 62.4; PLB: 72) | NR | Discontinuation (n/N, total), 24 months
ZOL: 75/333 PLB: 48/332 Discontinuation due to AEs (n/N, total), 24 months ZOL: 15/333 PLB: 6/332 Discontinuation due to other reasons (n/N, total), 24 months ZOL: 60/333 PLB: 42/332 |
| Netelenbos et al., 2011 | Retr. | The Netherlands | 7994 (persistence); 100,684 (compliance)/F (80)/NR | NR | ALN; IBN; RIS | 10 mg, 70 mg; 150 mg; 5 mg, 35 mg/oral/daily weekly; monthly; daily, weekly | P (31.1) | P (14.2) | MPR ≥ 80% (%, n), 12-month
ALN (daily): 92.2, n = 3101 ALN (weekly): 91.2, n = 55,195 ALN (weekly) plus vitamin D3: 92.3, n = 8279 IBN: 89, n = 3279 RIS (daily): 91.6, n = 1010 RIS (weekly): 91.5, n = 24,866 RIS (weekly) plus calcium: 93.1, n = 4954 |
Persistence 12-month (n/N, &) (refill gap ≤ 12 months)
ALN (daily): 60/241 (23.2%) ALN (weekly): 1605/3698 (43.4%) ALN (weekly) plus vitamin D3: 509/965 (52.7%) IBN: 205/443 (46.3%) RIS (daily): 33/82 (40.2%) RIS (weekly): 825/1818 (45.4%) RIS (weekly) plus calcium: 317/747 (42.4%) |
| Orwoll, Binkley et al., 2010 | RCT | USA | 135/F (0)/IBN: 63.9 (11.2); PLB: 65 (10.6) | P (IBN: 48; PLB: 32) | IBN; PLB | 150 mg/oral/monthly | NP | P (NR) | Compliance (≥ 80%), (n/N), 12 months
IBN: 69/85 PLB: 40/47 |
Discontinuation (n/N, total), 12 months
IBN: 16/87 PLB: 6/48 Discontinuation due to AEs (n/N, total), 12 months IBN: 4/87 PLB: 3/48 Discontinuation due to other reasons (n/N, total), 12 months IBN: 12/87 PLB: 3/48 |
| Orwoll, Miller, et al., 2010 | RCT | North America; Australia | 302/F (0)/64.0 (10.44) | P (ZOL: 63.6; ALN: 70.3) | ZOL; ALN | 5 mg; 70 mg/IV; oral/annually; weekly | NP | P (ZOL: 42.4; ALN: 35) | Compliance (≥ 80%), (n/N), 24 months
ZOL: 128/154 ALN: 120/148 |
Discontinuation (n/N, total), 24 months
ZOL: 17/154 ALN: 24/148 Discontinuation due to AEs (n/N, total), 24 months ZOL: 6/154 ALN: 12/148 Discontinuation due to other reasons (n/N, total), 24 months ZOL: 11/154 ALN: 12/148 |
| Orwoll et al., 2000 | RCT | USA and 10 other countries | 241/F (0)/ALN: 63 (13); PLB: 63/12 | P (ALN: 49; PLB: 52) | ALN; PLB | 10 mg/oral/daily | NR | P (NR) | NR | Discontinuation (n/N, total), 24 months
ALN: 21/146 PLB: 16/95 |
| Overbeek et al., 2017 | Retr. | The Netherlands | 35,557/F (74–99)/NR | NR | ALN; IBN; RIS; ZOL | 10 mg, 70 mg; 150 mg; 5 mg, 35 mg; 5 mg/oral; IV/daily; weekly; monthly; annually | NR | NR | NR | Persistence (refill gap ≤ 60 days), 12 months
ALN (daily): 140/661 ALN (weekly): 12,417/22,165 IBN-IV: 28/40 IBN-oral: 907/1742 RIS (daily): 69/230 RIS (weekly): 5815/10,483 ZOL: 236/236 Persistence (refill gap ≤ 60 days), 24 months ALN (daily): 78/532 |
| ALN (weekly): 7658/17,645
IBN-IV: 13/29 IBN-oral: 619/1575 RIS (daily): 32/205 RIS (weekly): 3699/8943 ZOL: 106/162 |
||||||||||
| Paggiosi et al., 2014 | RCT | UK | 172/F (100)/IBN: 66.9 (7.2); ALN: 67.8 (7.8); RIS: 66.8 (6.7) | P (IBN: 9; ALN: 23; RIS: 7) | IBN; ALN; RIS | 150 mg; 70 mg; 35 mg/oral/monthly; weekly; weekly | NR | P (NR) | Compliance in % (table count)
IBN: 85.8 ALN: 80.9 RIS: 71.6 |
Discontinuation (n/N, total), 12 months
IBN: 14/57 ALN: 13/57 RIS: 12/58 Discontinuation (n/N, total), 24 months IBN: 25/57 ALN: 26/57 RIS: 27/58 |
| Pazianas et al., 2012 | Retr. | USA | 264,679/F (92.4)/64.9–65.8 | P (≈ 9.65) | ALN; RIS | NR/oral/NR | P (17.7) | NR | MPR ≥ 66.6%, 36 months FUp
ALN: 63,273/167,366 RIS: 38,081/97,313 OR (95% CI) = 0.945 (0.93 to 0.96), p < 0.001 (favouring RIS group) |
NR |
| Penning-van Beest et al., 2006 | Retr. | The Netherlands | 1446/F (100)/71.6 (8.7) | P (2.2) | ALN; RIS | 10 mg, 70 mg; 5 mg/oral/daily; weekly; daily | P (23.49) | NR | NR | Persistence 12-month (≤30 days refill gap), RR (95% CI) (higher RR more likely to persist)
ALN (daily): 327/946, Ref. ALN (weekly): 176/339, RR = 1.56 (1.32 to 1.85) RIS (daily): 68/161, RR = 1.15(0.92 to 1.43) |
| Reid et al., 2018 | RCT | New Zealand | 2000/F (100)/ZOL: 71 (5); PLB: 71 (5.1) | P (ZOL: 23.7; PLB: 23.8) | ZOL; PLB | 5 mg/IV/annually | NR | NP | NR | Discontinuation (n/N, total), 72 months
ZOL: 64/1000 PLB: 75/1000 |
| Reid et al., 2009 | RCT | European countries (12); Australia; Hong Kong; Israel; USA | 833/RIS: 67; ZOL: 68/54.56 (14.26); ZOL: 54.27 (14.55) | P (14) | RIS; ZOL | 5 mg; 5 mg/oral; IV/daily; annually | P (100) | P (NR) | NR | Discontinuation (n/N, total), 12 months
ZOL: 31/416 RIS: 31/417 Discontinuation due to AEs (n/N, total), 12 months ZOL: 9/416 RIS: 6/417 Discontinuation due to other reasons (n/N, total), 12 months ZOL: 22/416 RIS: 25/417 |
| Reid et al., 2006
Extension of Reid et al., 2008 |
RCT | Europe; Middle East; Americas; Asia-Pacific | 936/F (100)/ALN: 64.3; RIS: 63.6 | P (ALN: 35.5; RIS: 31.8) | ALN; RIS | 70 mg; 35 mg/oral/weekly | NR | P (NR) | NR | Discontinuation (n/N, total), 12 months
ALN: 38/468 RIS: 44/468 Discontinuation due to AEs (n/N, total), 12 months ALN: 19/468 RIS: 29/468 Discontinuation due to other reasons (n/N, total), 12 months ALN: 19/468 RIS: 15/468 Discontinuation (n/N, total), 24 months ALN: 366/403 RIS: 350/395 |
| Reid et al., 2000 | RCT | Europe | 196/F (PLB: 62; RIS: 64)/PLB: 59 (12); RIS: 58 (12) | P (PLB: 37; RIS: 35) | RIS; PLB | 5 mg/oral/daily | P (100) | P (PLB: 33; RIS: 34) | NR | Discontinuation (n/N, total), 12 months
RIS: 18/100 PLB: 24/96 Discontinuation due to AEs (n/N, total), 12 months RIS: 16/100 PLB: 17/96 Discontinuation due to other reasons (n/N, total), 12 months RIS: 2/100 PLB: 7/96 |
| Reyes et al., 2017 | Retr. | Spain | 13,035/F (100)/NR | P (23.89) | ALN; IBN; RIS | NR | NR | P (60.37) | NR | Persistence (≤ 90-day refill gap), 12 months
ALN: 5223/10,938 IBN: 450/1115 RIS: 328/982 Persistence (≤ 90-day refill gap), 24 months ALN: 3159/10,938 IBN: 214/1115 RIS: 169/982 12-month IR of discontinuation (95% CI) ALN: 74.17 (72.27 to 76.12) IBN: 90.11 (83.51 to 97.22) |
| RIS: 111.25 (103.05 to 120.12)
24-month IR of discontinuation (95% CI) ALN: 66.3 (64.84 to 67.79) IBN: 86.02 (80.58 to 91.82) RIS: 98.62 (92.06 to 105.63) |
||||||||||
| Ringe et al., 2006
Extension of Ringe et al., 2009 |
RCT | Germany | 316/F (0)/RIS: 55.8 (10.5); PLB: 58 (10.3) | P (RIS: 51.3; PLB: 51.3) | RIS; PLB | 5 mg/oral/daily | NR | P (RIS: 59.5; PLB: 58.2) | NR | Discontinuation (n/N, total), 24 months
RIS: 10/158 PLB: 6/158 |
| Sambrook et al., 2012 | RCT | Australia; Belgium; Czechia; Estonia; Finland; France; Hong Kong; Hungary; Israel; Lithuania; Poland; Romania; Spain; Switzerland; UK; USA | 265/F (0)/56.4 (14.33) | NR | ZOL; RIS | 5 mg; 5 mg/IV; oral/annually; daily | P (100) | NR | NR | Discontinuation (n/N, total), 12 months
ZOL: 14/131 RIS: 14/134 Discontinuation due to AEs (n/N, total), 12 months ZOL: 4/131 RIS: 2/134 Discontinuation due to other reasons (n/N, total), 12 months ZOL: 10/131 RIS: 12/134 |
| Scotti et al., 2014 | Retr. | Italy | 28,558/F (100)/72 (9.6) | NR | ALN; RIS | 10 mg, 70 mg; 5 mg; 35 mg/oral/daily; weekly | P (37.4) | P (100) | NR | PDC ≥ 80% (mean FUp ~ 5.3 years)
ALN: 3153/23,454 RIS: 1022/5104 |
| Sestak et al., 2014
Extension Sestak et al., 2019 |
RCT | International (Europe; Pacific Ocean) | 500/F (100)/60 | NP | RIS; PLB | 35 mg/oral/weekly | NR | NP | NR | Discontinuation (n/N, total), 36 months
RIS: 108/253 PLB: 89/247 Discontinuation (n/N, total), 60 months RIS: 128/253 PLB: 114/247 |
| Sheehy et al., 2009 | Retr. | Canada | 32,804/F: 91.1 (RIS-br.), 89.2 (ALN-br.), 86.6 ALN (gen.)/NR | 3.8 (RIS-br.), 4.1 (ALN-br.), 7.2 ALN (gen.) | ALN; RIS | 70 mg; 35 mg/oral/weekly | NR | NR | NR | Discontinuation (refill gap ≥ 1.5 times the duration of dispensation)
ALN (br.): ref. ALN (gen.): HR = 2.08 (1.89, 2.28) RIS (br.): HR = 1.11 (1.07, 1.15) |
| Shi et al., 2017 | RCT | China | 160/F (100)/ALN: 59.8 (4.7); PLB: 59.4 (4.5) | P (NR) | ALN; PLB | 70 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 6/80 PLB: 9/80 Discontinuation due to AEs (n/N, total), 12 months ALN: 1/80 PLB: 0/80 Discontinuation due to other reasons (n/N, total), 12 months ALN: 5/80 PLB: 9/80 |
| Shilbayeh et al., 2004 | RCT | Jordan | 63/F (100)/57 | NR | ALN; PLB | 10 mg/oral/daily | NR | P (50) | NR | Discontinuation (n/N, total), 12 months
ALN: 7/27 PLB: 18/36 |
| Shin et al., 2017 | RCT | South Korea | 167/F (100)/IBN: 54.5 (9.3); PLB: 55.1 (8.6) | NP | IBN; PLB | 150 mg/oral/monthly | P (100) | NP | NR | Discontinuation (n/N, total), 12 months
IBN: 22/86 PLB: 18/81 Discontinuation due to AEs (n/N, total), 12 months IBN: 1/86 PLB: 0/81 Discontinuation due to other reasons (n/N, total), 12 months IBN: 21/86 PLB: 18/81 |
| Siris et al., 2011 | Retr. | USA | 460,584/F (100)/63.6 (10.9) | P (1.24–2.8) | ALN; IBN; RIS | NR/oral/NR | P (15.8 – 20) | P (32.4 – 36.2) | Mean MPR (%, n/N) (mean FUp: 2.4 years)
ALN: 53.3 IBN: 56 RIS: 53.1 |
NR |
| Smith et al., 2004 | RCT | Australia | 145/F (49)/67 (8.6) | P (NR) | ALN; PLB | 10 mg/oral/daily | P (Inhaled corticosteroids: 88; oral corticosteroids: 17) | P (100) | Compliance (median, IQR)
ALN: 0.97 (0.09) PLB: 0.96 (0.095) |
Discontinuation (n/N, total), 12 months
ALN: 24/65 PLB: 26/79 |
| Reginster et al., 2000
Ext.: Sorensen et al., 2003 |
RCT | Australia and Europe | 814/F (100)/RIS: 71 (7); PLB: 71 (7) | P (100) | RIS; PLB | 5 mg/oral/daily | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
RIS: 74/407 PLB: 77/407 Discontinuation (n/N, total), 36 months RIS: 156/407 PLB: 186/407 Discontinuation due to AEs (n/N, total), 36 months RIS: 65/407 PLB: 83/407 Discontinuation due to other reasons (n/N, total), 36 months |
| RIS: 91/407
PLB: 103/407 Discontinuation (n/N, total), 60 months RIS: 20/135 PLB: 25/130 Discontinuation due to AEs (n/N, total), 60 months RIS: 10/135 PLB: 16/130 Discontinuation due to other reasons (n/N, total), 60 months RIS: 10/135 PLB: 9/130 |
||||||||||
| Stoch et al., 2009 | RCT | USA | 173/F (ALN: 61.4; PLB: 52.5)/ALN: 51.9 (14.4); PLB: 54.6 (14.8) | NP | ALN; PLB | 70 mg/oral/weekly | P (100) | P (100) | Compliance (% actual n of days vs. expected n of days)
ALN: 87.4 PLB: 88.5 |
Discontinuation (n/N, total), 12 months
ALN: 33/114 PLB: 16/59 Discontinuation due to AEs (n/N, total), 12 months ALN: 10/114 PLB: 11/59 Discontinuation due to other reasons (n/N, total), 12 months ALN: 23/114 PLB: 5/59 |
| Stuss et al., 2016 | RCT | Poland | 54/F (100)/IBN: 68. 1(8.1); PLB: 69.2 (8.1) | P (IBN: 28.6; PLB: 6.7) | IBN; PLB | 150 mg/oral/monthly | NP | P (100) | NR | Discontinuation (n/N, total), 6 months
IBN: 5/34 PLB: 5/20 Discontinuation due to AEs (n/N, total), 6 months IBN: 3/34 PLB: 0/20 Discontinuation due to other reasons (n/N, total), 6 months IBN: 2/34 PLB: 5/20 |
| Valimaiki et al., 2007 | RCT | Finland; The Netherlands;
Norway; Spain; Sweden |
171/F (100)/65.9 (6.8) | P (NR) | RIS; PLB | 5 mg/oral/daily | NP | NP | Compliance (mean, SD), 24 months
RIS: 0.938 (0.141) PLB: 0.901 (0.164) Compliance mean (%, SD) RIS: 93.8 (14.14) PLB: 90.1 (16.46) |
Discontinuation (n/N), 12 months
RIS: 16/114 PLB: 14/57 Discontinuation (n/N, total), 24 months RIS: 19/114 PLB: 14/56 Discontinuation due to AEs (n/N, total), 24 months RIS: 10/114 PLB: 9/56 Discontinuation due to other reasons (n/N, total), 24 months RIS: 9/114 PLB: 5/56 Mean persistence in days (SD) RIS: 646.6 (207.9) PLB: 622.9 (230.8) |
| Van Bodegraven et al., 2014 | RCT | The
Netherlands |
131/F (54)/42 (13) | NR | RIS; PLB | 35 mg/oral/weekly | NP | NP | NR | Discontinuation (n/N, total), 24 months
RIS: 8/64 PLB: 5/67 |
| Van Boven et al., 2013 | Retr. | The Netherlands | 8445/F (75.6)/67.5 (13.5) | NR | ALN; RIS; IBN | 10 mg, 70 mg (ALN); 5 mg, 35 mg (RIS); 150 mg (IBN)/oral/daily and weekly (ALN; RIS); monthly (IBN) | P (21.2) | P (NR) | NR | Persistence 12-month (≤ refill 30-day gap)
ALN-daily: 60/137 (43.7%), HR = 1.8 (95% CI 1.41 to 2.3) RIS-daily: 31/71 (44.1%), HR = 1.55 (95% CI 1.12 to 2.16) ALN-weekly (branded): 1145/1931 (59.3%), HR = 1 (95% CI 0.89 to 1.12) ALN-weekly (generic, ref.): 1742/3030 (57.5%) RIS-weekly (branded): 1887/3083 (61.2%), HR = 0.92 (95% CI 0.84 to 1.01) RIS-weekly (generic): 36/73 (48.9%), HR = 1.04 (95% CI 0.75 to 1.45) IBN-monthly: 63/108 (58.5%), HR = 0.93 (95% CI 0.69 to 1.25) |
| Wade et al., 2012 | Retr. | USA | 33,558 (total population)/F (94)/59.5 | P (7.22) | ALN; IBN; RIS | 10 mg, 70 mg; 5 mg, 35 mg; 150 mg, 3 mg/oral; IV/daily; weekly; monthly, quarterly | P (14.53) | P (52.5) | Mean 12-month MPR (SD), n
ALN: 0.58 (0.35), n = 18,328 IBN: 0.57 (0.36), n = 5063 RIS: 0.58 (0.36), n = 10,167 Mean 24-month MPR (SD), n ALN: 0.50 (0.36), n = 11,232 IBN: 0.47 (0.35), n = 2492 RIS: 0.50 (0.36), n = 6398 |
NR |
| Weiss et al., 2007 | Retr. | USA | 165,955/F (100)/ALN: 67.3 (11); IBN: 66.6 (10.6); RIS: 67.1 (11) | NR | ALN; IBN; RIS | 10 mg, 70 mg; 150 mg; 35 mg/oral/weekly; monthly | NR | NR | NR | Persistence in days (SD), (refill gap ≤ 30 days), HR (95% CI); OR (discontinuation)
ALN: 116 (121), HR = ref.; OR = ref. IBN: 98 (113), HR = 1.098, p < 0.0001; OR = 1.383, p < 0.0001 RIS: 113 (120), HR = 1.02, p = 0.0003; OR = 1.065, p < 0.0001 Persistence in days (SD), (refill gap ≤ 45-day) ALN: 129 (128) IBN: 114 (124) RIS: 124 (127) Persistence in days (SD), (refill gap ≤ 60-day) ALN: 136 (131) IBN: 121 (128) RIS: 131 (130) |
| Xu et al., 2013 | Retr. | USA | 36,855/F (≈> 90)/commercial: 58.6 (6.4) l MAPD: 73.6 (7.6) | Commercial: 8.6; MAPD: 15.2 | ALN; IBN; RIS | NR | NR | Commercial: 38.1; MAPD: 55.5 | NR | Discontinuation at 36 months (%, total n) (refill gap ≥ 90-day, commercial database)
ALN (gen.): 55.8, n = 678 ALN (br.): 57.1, n = 2429 IBN: 66.4, n = 1341 RIS: 55.2, n = 1948 Discontinuation at 36 months (%, total n) (refill gap ≥ 90-day, MAPD database) ALN (gen.): 51.6, n = 7692 ALN (br.): 57, n = 11,395 IBN: 67.4, n = 3483 RIS: 64, n = 7889 |
| Yan et al., 2009 | RCT | China | 560/F (100)/ALN: 65.19 (6.47); PLB: 64.66 (5.87) | NP | ALN; PLB | 70 mg/oral/weekly | NP | P (100) | NR | Discontinuation (n/N, total), 12 months
ALN: 53/280 PLB: 42/280 Discontinuation due to AEs (n/N, total), 12 months ALN: 28/280 PLB: 19/280 Discontinuation due to other reasons (n/N, total), 12 months ALN: 25/280 PLB: 23/280 |
| Zambon et al., 2008 | Retr. | Italy | 11,863/F (100)/72 | P (14) | ALN; RIS | 10 mg, 70 mg; 5 mg, 35 mg/oral/daily, weekly | P (19) | P (100) | NR | Discontinuation HR (95% CI) (refill gap ≥ length of time covered by a given dispensation)
ALN: ref. RIS: 0.762 (0.695 to 0.836), p < 0.05 |
| Zhang et al., 2015 | RCT | China | 219/F (100)/ALN: 65.6 (8.0); PLB: 64.8 (7.4) | NP | ALN; PLB (calcitriol) | 70 mg/oral/weekly | NR | P (NR) | NR | Discontinuation (n/N, total), 12 months
ALN: 16/111 PLB: 7/108 Discontinuation due to AEs (n/N, total), 12 months ALN: 8/111 PLB: 1/108 Discontinuation due to other reasons (n/N, total), 12 months ALN: 8/111 PLB: 6/108 |
| Zhou et al., 2020 | RCT | China | 123/F (25.2)/83.54 (2.99) | NR | ALN; PLB | 70 mg/oral/weekly | NP | NP | Compliance (100% use of drug), 18 months
ALN: 3/62 PLB: 8/61 |
Discontinuation (n/N, total), 18 months
ALN: 3/62 PLB: 8/61 |
| Ziller et al., 2012 | Retr. | Germany | 268,568/F ALN-daily: 65.9; ALN-weekly: 85.8; ALN-vit. D: 85.6; IBN-oral: 92; IBN-IV: 86.7; RIS-daily: 85.5; RIS-weekly: 87.4; RIS-calcium: 88.6; ZOL: 55.4/ALN-daily: 73.4; ALN-weekly: 74.7; ALN-vit. D: 74.1; IBN-oral: 72.5; IBN-IV: 72; RIS-daily: 75.4; RIS-weekly: 74.2; RIS-calcium: 74; ZOL: 70.4 | NR | ALN; IBN; RIS; ZOL | 10 mg, 70 mg; 150 mg; 3 mg; 5 mg, 35 mg; 5 mg/oral; IV/daily, weekly, monthly, annually | NR | NR | MPR (mean in %)
ALN (daily): 33 ALN (weekly): 57 ALN + vit. D: 53 IBN-oral: 62 IBN-IV: 70 RIS (daily): 47 RIS (weekly): 53 RIS + cal.: 58 ZOL: NA MPR ≥ 80% ALN (daily): 15.8 ALN (weekly): 37.3 ALN + vit. D: 32.6 IBN-oral: 44.5 IBN-IV: 53 RIS (daily): 28.8 RIS (weekly): 30.2 RIS + cal.: 38.8 |
Persistence in days (mean)
ALN (daily): 141 ALN (weekly): 239.8 ALN + vit. D: 218.7 IBN-oral: 256.4 IBN-IV: 278.6 RIS (daily): 190.9 RIS (weekly): 218.7 RIS + cal.: 238.7 ZOL: 365 Post 12-month persistence (n/N, %), refill gap ≥ 6 months ZOL: 8617/13,132, 65.62% IBN-IV: 7089/12,525, 56.6% IBN-oral: 7326/14,426, 50.78% ALN (weekly): 69,663/155,637, 44.76% ALN (plus vit. D): 5538/14,666, 37.76% ALN (daily): 583/3359, 17.34% RIS (plus calcium): 9010/21,309, 42.28% RIS (weekly): 8481/24,126, 35.15% RIS (daily): 335/1107, 30.26% |
Overall, for persistence, 16,577 participants were included in the NMAs of RCTs, and 985,484 BP users were included in the NMAs of retrospective observational studies.
Networks’ structure and geometry
Two network plots comparing BP effects on the absolute numbers of dropouts were created. Data on dropouts at 12 months provided six closed loops of evidence (see Appendix 3, Figure 14). Overall, 30 two-arm and 1 three-arm studies were included in the analysis, resulting in a total of 10,419 participants. The most studied treatment was ALN (nstudies = 16), while PLB was used as a comparator in 24 studies. Data on dropouts at 24 months provided four closed loops of evidence (see Appendix 4, Figure 15). Overall, 21 two-arm and 1 three-arm studies were included in the analysis, resulting in a total of 6158 participants. The most studied treatment was ZOL (nstudies = 10), while PLB was used as a comparator in 19 studies. One network was created for discontinuation data drawn from observational studies (see Appendix 5, Figure 16). Overall, 8 two-arm, 12 three-arm and 4 five-arm studies were included in the analysis. The most common treatments were ALN and RIS, with each contributing 23 arms in the analysis. Studies’ characteristics and risk of bias within individual studies are shown in Table 9.
Of the included trials, 31 were conducted in North America or in multiple countries. Overall, 38 trials exclusively targeted female participants, while in 21 trials, most of the participants fulfilled the criteria of osteoporosis. In total, the overall risk of bias was high in 18 trials. The majority of observational studies adopted a retrospective design, while three of them adopted a prospective comparative design. Twenty observational studies were conducted in Europe, and 18 studies were conducted in the USA. In 14 studies, the majority of participants fulfilled the criteria of osteoporosis. Overall, three studies received a moderate risk-of-bias rating, two received a critical risk-of-bias rating and the rest received a serious risk-of-bias rating.
Synthesis of results on persistence: measured using dropouts in randomised controlled trials
A NMA was used to compare the effects of ALN, RIS, ZOL and IBN-oral relative to PLB on the total number of dropouts at 12 months. Overall, data were available from 30 two-arm and 1 three-arm RCTs. The network provided nine direct treatment comparisons. Each of the direct comparisons between ALN versus ZOL and RIS versus IBN-oral was informed by one small study. Eight contrasts were checked for inconsistency between direct and indirect evidence. None of the comparisons showed significant evidence of inconsistency, as assessed using Bayesian p values (p > 0.05). The model fitted the data relatively well (difference < 3), with a Dres of 64.8 being close to the number of data points included in the analysis, which was 63 (DIC = 369). The between-study SD was estimated to be 0.16 (95% CrI 0.009 to 0.41), implying mild heterogeneity in treatment effects between RCTs. Users of ZOL and RIS were less likely to drop out compared to PLB users, with none of these effects being statistically significant. The lowest likelihood of dropping out was detected in ZOL users OR = 0.73 (95% CrI 0.51 to 1.05; probability: 0.88; SUCRA: 0.95).
A NMA was used to compare the effects of ALN, RIS, ZOL and IBN-oral relative to PLB on the total number of dropouts at 24 months. Overall, data were available from 21 two-arm and 1 three-arm RCTs. The network provided eight direct treatment comparisons. Each of the direct comparisons between ALN versus IBN, ALN versus RIS and RIS versus IBN-oral were informed by one small study. Three contrasts were checked for inconsistency between direct and indirect evidence. None of the comparisons showed significant evidence of inconsistency, as assessed using Bayesian p values (p > 0.05). The model fitted the data well, with a Dres of 45.51 being close to the number of data points included in the analysis, which was 45 (DIC = 276.7). The between-study SD was estimated to be 0.34 (95% CrI 0.09 to 0.64), implying mild to moderate heterogeneity in treatment effects between RCTs but with reasonable uncertainty. Users of ALN, RIS and IBN-oral were less likely to drop out compared to PLB users, with none of these effects being statistically significant. The lowest likelihood of dropping out was detected in IBN-oral users OR = 0.72 (95% CrI 0.31 to 1.66; probability: 0.54; SUCRA: 0.72).
Synthesis of results on persistence: measured using discontinuation of treatment data from observational studies
A NMA was used to compare the effects of RIS, ZOL, IBN-oral and IBN-IV relative to ALN on the absolute number of people who discontinued their BP treatments. Overall, data were available from 24 retrospective observational studies. The model fitted the data well, with a Dres of 73.27 being close to the number of data points included in the analysis, which was 73 (DIC = 762.5). The between-study SD was estimated to be 0.24 (95% CrI 0.19 to 0.31), implying mild heterogeneity in treatment effects between observational studies with reasonable uncertainty. Users of ZOL and IBN-IV were less likely to discontinue compared to ALN, with the effects of the former being statistically significant (Table 10). The lowest likelihood for discontinuation was detected in ZOL users HR = 0.73 (95% CrI 0.61 to 0.88; probability: 0.88; SUCRA: 0.97). Heterogeneity of effects was explored by undertaking a post hoc meta-regression on the absolute number of discontinuers using refill-gap as a moderator variable. Although slightly decreased in magnitude, the direction of effects remained the same. The model fit remained almost the same, with a Dres of 72.63 (DIC: 755.9). The between-study SD was estimated to be 0.29 (95% CrI 0.20 to 0.42), implying mild heterogeneity in treatment effects between RCTs with reasonable uncertainty. Higher medication effects on discontinuation were detected in participants with longer refill gap thresholds, although the results were not statistically significant β = −0.23 [95% confidence interval (CI) −0.72 to 0.21].
| ZOL | 0.81 (0.47 to 1.39) |
IBN-oral | - | ZOL | - | |||||||||||
| 0.76 (0.53 to 1.11) |
RIS | 0.97 (0.66 to 1.5) |
0.86 (0.37 to 2.05) | RIS | 0.84 (0.55 to 1.30) |
0.86 (0.68 to 1.09) |
IBN-IV | - | ||||||||
| 0.73 (0.51 to 1.05) |
0.95 (0.72 to 1.26) |
PLB | 0.84 (0.36 to 2.00) | 0.97 (0.56 to 1.74) | ALN | 0.78 (0.41 to 1.52) |
0.73 (0.61 to 0.88) |
0.84 (0.68 to 1.06) |
ALN | |||||||
| 0.69 (0.49 to 1.02) |
0.90 (0.69 to 1.20) |
0.94 (0.75 to 1.20) |
ALN | 0.72 (0.33 to 1.66) | 0.84 (0.57 to 1.24) | 0.86 (0.53 to 1.37) | PLB | 0.73 (0.6 to 0.88) |
0.84 (0.67 to 1.06) |
0.99 (0.87 to 1.13) |
IBN-oral | |||||
| 0.64 (0.39 to 1.02) | 0.83 (0.54 to 1.23) | 0.87 (0.60 to 1.24) |
0.92 (0.63 to 1.27) |
IBN-oral | 0.67 (0.28 to 1.68) | 0.78 (0.47 to 1.31) |
0.80 (0.47 to 1.33) | 0.92 (0.66 to 1.32) | ZOL | 0.67 (0.56 to 0.80) |
0.77 (0.62 to 0.97) |
0.91 (0.82 to 1.01) |
0.91 (0.80 to 1.04) |
RIS |
Discussion
Updated review
Overall, 44 trials provided data for femoral neck BMD, whereas 27 and 19 trials provided data for vertebral and non-vertebral fractures, respectively. Only 14 and 10 trials provided data for hip and wrist fractures, respectively. ZOL was found to be the most effective treatment in preventing the occurrence of vertebral fractures and increasing femoral neck BMD. ZOL was also found to be comparably effective to RIS and ALN in preventing non-vertebral fractures and hip fractures, respectively. ZOL’s effects in preventing hip and vertebral fractures and increasing femoral neck BMD were found to be clinically significant. In addition, treatment effects in preventing vertebral fractures were found to be stronger in people with osteoporosis compared to PLB. Uptake of ZOL was also found to be accompanied by more frequently reported adverse events; however, these events are likely to be short-lived. Based on these updated estimates, ZOL could be considered as the first-line treatment for people who experience or are at increased risk of fragility fractures.
These findings arguably have important implications for clinical decision-making in terms of the preferred therapeutic approach for people with varying fracture risk. It has recently been suggested that anabolic treatments should be preferred as the first-line treatment for people who are at high risk for developing osteoporotic fractures. 151 Although recent evidence has shown that anabolic treatment is more effective than BP in reducing fracture risk in females who are at high risk of developing fractures,152,153 its effectiveness has only been tested against oral BP. There is therefore an urgent need for future comparative studies to test the effectiveness of anabolic treatments versus ZOL in reducing the fracture risk in high-risk populations. This becomes more apparent when the imminent fracture risk and the need to expedite clinical decision-making154,155 are taken into account. Based on our findings, ZOL seems to be a promising treatment that could decrease the imminent fracture risk for high-risk populations within 24 months after administration. Future studies should investigate whether ZOL or anabolic treatments are more effective in reducing imminent fracture risk in high-risk populations.
Adherence review
For persistence, results from the NMA from RCTs showed that ZOL users may be less likely to drop out from trials at 12 months, although these effects were marginally non-significant. Results from the NMA using data from the observational studies showed that ZOL and IBN-IV users were less likely to discontinue their treatment over time, with ZOL users being statistically significantly more persistent compared to oral BP users. Data drawn from the vote-counting synthesis were in line with the results of NMAs, where ZOL and IBN-IV users were more likely to persist with their treatment, with ZOL users being more persistent compared to their IBN-IV counterparts. Users of ALN and RIS showed comparable persistence rates; however, when we restricted our analysis to weekly administration, ALN users were found to be more likely to persist to treatment over time. Due to the paucity of data and the heterogeneity in reporting compliance data, we were unable to perform NMAs, but synthesis based on vote counting found that compliance to ZOL was greater within 24 months after the initiation of their treatment. Users of IBN-IV were found to be more compliant compared to IBN-oral users. Users of ALN were found to be more compliant than RIS users, while mixed evidence was observed in the comparison between ALN and IBN-oral users.
These findings have important implications for clinical practice and future research. In general, persistence to BP treatment was found to decrease after 12 months, stressing the need to address adherence barriers according to BP treatment and people with different clinical profiles. Nevertheless, ZOL users were found to be less likely to discontinue their treatment over time, and they showed higher compliance rates. These findings are partly in line with the results from the dropout NMA at 12 months. Without ignoring the interplay of individual and contextual factors, which affect participation and adherence in clinical trials,156,157 we can assume that most ZOL users are likely to receive at least two infusions before discontinuing their treatment. The use of ZOL has been generally recommended for at least 3 years5 and, although reduced adherence rates have been observed in ZOL users after the first year,158 simpler drug regimens can improve adherence rates. 159 Results of vote-counting synthesis on oral BPs were partly in line with the NMA results. ALN and RIS users showed comparable persistence rates; however, ALN users were found to be more compliant than their RIS counterparts. When we restricted our synthesis to weekly administration of both BP, weekly ALN users were found to be more persistent to treatment compared to RIS weekly users. Given that ALN is the most widely prescribed medication, clinical decision-making should consider, alongside its clinical effectiveness, ways in which ALN users could be assisted to receive medication properly and remain on treatment long-term.
Strengths and limitations
Updated review
These NMAs provide updated estimates regarding the BP effect in preventing the occurrence of fractures. This updated systematic review has several strengths. First, this review includes a robust search strategy with clearly demarcated eligibility criteria, covering a wide range of databases, trial registries and grey literature. Second, this review employed gold-standard methods in analysing, reporting and assessing the quality of findings, which in turn facilitates clinical decision-making. Inevitably, this review also has some limitations. First, treatment networks for hip and wrist fractures were sparse, something that might limit the generalisation of our conclusions regarding BPs’ effects on those outcomes. Second, none of the included studies had tested IBN-IV against any other BP or PLB, preventing the provision of updated estimates regarding IBN-IV effectiveness. Third, there was scarcity of data regarding BPs’ effects on male populations and populations with exposure to GC. Fourth, there were a large number of studies with an overall high-risk rating in the risk-of-bias assessment.
Adherence review
This systematic review has several strengths. First, this review includes a robust search strategy, covering a wide range of databases, trial registries and grey literature. Second, this systematic review employed gold-standard methods in conducting, reporting and assessing the credibility of findings. Third, this systematic review included both RCTs and observational studies, adopting a combined approach to synthesise data. Inevitably, this review also has some limitations. First, participants’ persistence on BP treatments in RCTs was assessed by using the total number of dropouts as a proxy measure. Given that this was the only way to capture discontinuers in RCTs, dropout NMA findings should be interpreted under this limitation. Second, persistence in the NMA of observational studies was assessed as the absolute number of discontinuers per BP treatment based on varying refill gaps and without accounting for the censored follow-up time. Third, due to the scarcity of data on males, a subgroup analysis of persistence rates between males and females was not conducted. Fourth, compliance was indirectly assessed by measuring treatment continuity based on different measures. Although a NMA would be more informative, vote-counting synthesis is well suited in the presence of incomplete and highly heterogeneous data in both observational studies and RCTs. Fifth, this review did not assess the comparative effectiveness of BP against monoclonal antibody and anabolic medications. Sixth, the paucity of data precluded the subgroup analysis between participants receiving BP for primary prevention and those receiving BP for secondary prevention purposes.
Conclusion
Zoledronate was found to be the most effective BP compared to ALN, RIS and IBN-oral for reducing the risk of fragility fracture. Adherence was higher in intravenously administered BP users. Clinical decision-making could be facilitated by taking into account adherence patterns in BP users who are at increased risk of fractures. Depending on its cost-effectiveness, ZOL could be considered as a first-line option for people at increased risk of subsequent fractures.
Chapter 5 Economic evaluation of bisphosphonates for the treatment of osteoporosis
Some text in this chapter has been reproduced from Mattia L, et al. Bone 2022;158:116347. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text, where applicable, below includes minor additions and formatting changes to the original text.
Parts of Table 11 are reproduced from Davis S., et al. Health Technol Assess 2016;20(78). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The tables include minor additions and formatting changes to the original text.
| Model feature | Description |
|---|---|
| Decision problem | To assess the cost-effectiveness of alternative BP compared to oral ALN and the value of further research into the relative benefits of alternative BP compared to oral ALN |
| Type of economic evaluation | Cost-effectiveness analysis (benefits expressed as QALYs) and EVPI analysis |
| Population/subgroups | The model simulates the heterogeneous patient population eligible for risk assessment under CG146. The population is stratified into 10 risk categories, and results are presented for each risk category. The EVPI analysis focuses on those risk categories where ALN is currently recommended |
| Interventions | RIS Oral IBN IV IBN IV ZOL |
| Comparators | Oral ALN (No treatment is also modelled in order to estimate outcomes for the BP treatments) |
| Perspective | NHS and PSS |
| Model type | DES with heterogeneous patient population |
| Model eventsa | Clinical events are fracture, death (all-cause mortality and fracture-related mortality) and nursing home admission. There are four possible fracture events (hip, wrist, vertebral and proximal humerus) with fractures at other sites included by increasing the incidence of these events. Dummy events are used to update attributes 1 year after fracture and to update fracture risks once treatment finishes |
| Time horizon | Lifetime (up to the age of 100) |
| Duration of treatment | Persistence with treatment for ALN and ZOL was taken from observational studies. Data from reviews on HRs for treatment discontinuation compared to ALN were used to estimate treatment persistence for RIS and oral IBN. Data from reviews on HRs for treatment discontinuation compared to ZOL were used to estimate treatment persistence for IV IBN |
| Natural historya | Time to fracture is based on the estimate of absolute fracture risk for major osteoporotic fractures (hip, wrist, proximal humerus and vertebral) provided by Qfracture or which is uplifted to include fractures at additional sites.b The distribution of fractures across different sites is based on incidence data from Sweden. The increased risks of fracture following incident fracture are based on a published systematic review |
| Effectiveness | The HRs from the systematic review and NMA (see Chapter 4) are applied for the duration of treatment. Some effectiveness is assumed to persist beyond treatment during the ‘offset period’. A linear decline in treatment effect is assumed during this time |
| Adverse events | Upper GI side effects for oral BP and flu-like symptoms for IV BP are included by applying one-off cost and QALY deductions in the first month of treatment. Relative risk of GI side effects for oral BP informed by systematic review (see www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2020to2022#:~:text=We%20have%20also%20published%20single,females%2C%20respectively%2C%20in%202021) |
| Mortalitya | All-cause mortality is based on UK life tables. Fracture-related mortality is based on estimates of excess mortality attributable to hip and vertebrae from a case-control study using routine data from UK general practice |
| Utility data | Utility decrements based on EQ-5D scores pre and post fracture were obtained from a systematic review. Utility decrement for nursing home admission was based on a single study identified from the literature that used EQ-5D. Variation in baseline utility by age and gender was based on UK EQ-5D population estimates |
| Resource use and unit costs | The analysis includes drug, administration and monitoring costs for interventions and costs of fracture, including those falling on primary care, secondary care and PSS. Postfracture costs were based on a case-control study which used routine data from UK general practice. Nursing home admission following hip fracture was based on a UK observational study of discharge destinations. Unit costs are taken from NHS reference costs, PSSRU unit costs, the primary care National Drug Tariff and the eMIT database of generic drug costs in secondary care. Costs are reported in Great British pounds (£) Cost year is 2021 |
| Discounting | 3.5% per annum for both costs and QALYs |
| Sensitivity analysis | Probabilistic sensitivity analysis was undertaken for the base-case scenario to estimate the mean costs and benefits when taking into account parameter uncertainty. Structural uncertainty was assessed through scenario analysis, where parameters were set to their mid-point values |
Introduction
The aims of the economic evaluation were to assess the cost-effectiveness of alternative BP regimens compared to oral ALN. The economic evaluation was based on the model developed by ScHARR to inform the NICE technology appraisal of BP (TA464). 45 The key areas updated relate to the following parameters, informed from the data available from Chapter 4:
-
treatment efficacy
-
treatment persistence
-
adverse effects.
In addition, we have also updated the costs to reflect current prices. Updates to the model made during the NICE appraisal of non-BP to incorporate new HRQoL data and include DXA have also been retained. 160 Drug and administration costs for BP treatment have also been updated. All other model inputs and assumptions not discussed below are unchanged from the model used in TA464. A summary of the key features of the model is provided in Table 11.
Methods
Brief overview the of model structure
The model used a discrete event simulation (DES) framework to simulate lifetime costs and QALYs for a cohort of patients treated using each of the alternative BP treatment regimens (ALN, RIS, IBN-oral, IBN-or, ZOL). A schematic of the model structure is shown in Figure 6. The key clinical events modelled are fractures at the hip, vertebrae, wrist or proximal humerus, all-cause mortality and fracture-related mortality, with the latter only possible following hip or vertebral fractures. Fractures are associated with an acute cost in the year of fracture and an ongoing cost in subsequent years. Costs in the model are estimated from a NHS and Personal Social Services (PSS) perspective, including costs incurred in primary and secondary care and social care provided in the home. In addition, hip fractures are also associated with an increased risk of new admission to a residential care home, with an associated cost for a proportion of patients whose residential care is not self-funded. Fractures are associated with a reduction in HRQoL, with separate decrements applied in the first and subsequent years after fracture, with a further decrement applied to patients admitted to a nursing home following fracture. The prevention of fractures therefore results in QALY gains through the avoidance of these HRQoL decrements, in addition to the QALY gains achieved by preventing fracture-related mortality. Future costs and benefits are discounted at 3.5% per annum.
FIGURE 6.
Clinical events that can occur during a patient’s lifetime in the DES.
Patient cohort
The model is a patient-level simulation that takes into account the heterogeneous patient characteristics present within the cohort simulated. The cohort simulated all patients eligible for fracture risk assessment within CG146. It therefore includes both men and women, patients with and without a prior fracture, those with steroid-induced osteoporosis, those with secondary osteoporosis and those with other risk factors for fragility fractures. In addition, a proportion of the cohort is assumed to reside in a care home at the start of simulation. The characteristics of each individual within the population are simulated. Life expectancy, body mass index (BMI), steroid use, prior fracture and residential status (care home or community dwelling) are sampled dependent on age and sex, whereas the remaining Qfracture risk factors are sampled based on their prevalence within the Qfracture cohort. The Qfracture algorithm estimates the risk of fracture for each simulated individual. As cost-effectiveness varies with baseline risk of fracture, results were presented for fracture risk categories defined according to deciles of absolute risk. This allowed identification of the optimal strategy for patients according to their fracture risk. As the primary aim of the analysis was to compare alternative BP to ALN, the presentation of the expected value of parameter information (EVPI) analysis focused on those groups where ALN was recommended. The NICE quality standard (QS149) provides a table of treatment thresholds that vary by age, with the intervention threshold in the lowest age group being a 10-year absolute fracture risk of 5.9% (NICE 2017). Based on this, we have presented EVPI results for the 8th, 9th and 10th risk categories, as the average risk in the 8th risk category is 5.5% (range of 4.6–6.6%).
Treatment duration
The intended treatment duration was assumed to be 5 years for oral BP and 3 years for IV ZOL. This was based on the national osteoporosis guideline group (NOGG) guideline that states that ‘treatment review should be performed after 3 years of IV ZOL therapy and 5 years of oral BP’. 161 Many patients do not persist with treatment for the full intended treatment period. The review and NMA of treatment persistence based on observational studies (see Chapter 4) found that patients treated with IV ZOL had a statistically significantly lower risk of stopping treatment early compared to those being treated with ALN. Those being treated with IBN-IV had a lower risk of stopping early than those treated with ALN and those treated with RIS had a higher risk, but in both cases, the difference was not statistically significantly different. The risk of stopping treatment with IBN-oral and ALN was very similar.
The duration of treatment persistence for ALN was estimated based on a study by Morley et al. 162 which used routine data from the UK’s Clinical Practice Research Datalink (CPRD), which is a longitudinal database of primary care records. This study was selected as it is based on UK data and provided more up-to-date estimates than the study of CPRD data, which was used previously. The data provided by Morley et al. were pooled across all forms of oral BP, but the estimates obtained are considered to be reasonably representative of the expected treatment persistence for ALN as it comprises 89% of prescriptions for oral BP (Prescription Cost Analysis March 2021). The mean treatment persistence for ALN was calculated by estimating the area under the time-to-discontinuation Kaplan–Meier curve up to 60 months for patients taking oral BP. The estimates presented by Morley et al. for patients not previously treated for osteoporosis were considered most appropriate for informing the choice of BP as BP are generally the first treatment offered. We reconstructed the synthetic patient-level data from the Kaplan–Meier data presented by Morley et al. using the methods described by Guyot et al. 163 These were used to estimate a restricted mean survival (assuming no one is treated beyond 60 months) of 1.92 years (95% CI 1.89 to 1.94). For RIS and IBN-oral, the HRs for treatment persistence relative to ALN from the NMA of observational studies were applied to the Kaplan–Meier curve for ALN to estimate equivalent Kaplan–Meier curves for the other two oral BPs. The areas under these curves were then calculated to provide an estimate of mean treatment persistence when incorporating the uncertainty in the risk ratios (RRs) for treatment persistence. The estimates of mean treatment persistence were 1.81 (95% CI 1.68 to 1.94) and 1.91 (95% CI 1.66 to 2.14) for RIS and IBN-oral, respectively.
For IV ZOL, the CPRD was not considered to be a suitable source of data as Morley et al. state that treatments such as IV ZOL, which are primarily administered in secondary care, may be inadequately captured in the CPRD. No UK source of data on treatment persistence for IV ZOL in secondary care was identified in the review of adherence studies. However, a Swedish study by Spångeus et al. 164 reporting persistence for IV ZOL up to 3 years was identified during the review process (it was not included in the review as it did not compare adherence across two or more treatments). The Swedish system is similar to the UK system in that it is a tax-financed system in which patients do not pay for treatment at the time of need, and it is likely to be more reflective of persistence in the UK NHS than estimates obtained from insurance-based healthcare systems. An average duration of treatment persistence for IV ZOL of 2.59 years (95% CI 2.51 to 2.66) was estimated from the Swedish study. The CI was estimated by using beta-distributions to sample the proportion discontinuing at 1 and 2 years.
No suitable estimate of treatment duration was identified for IBN-IV from the adherence review, Chapter 4. Therefore, the HR for treatment discontinuation for IBN-IV relative to ZOL from the review and NMA of observational studies, Chapter 4, was applied to estimate the expected proportion of patients who would be persisting on treatment at 1, 2 and 3 years for IBN-IV, and a linear reduction between these points was assumed. The mean treatment duration based on the area under the curve was estimated to be 2.30 years (95% CI 2.06 to 2.55), where the CI incorporates the uncertainty in the HR for treatment discontinuation for IBN-IV compared to IV ZOL.
Treatment costs
Drug costs for oral BP were estimated using the NHS Drug Tariff165 NHS Business Services Authority 2021. Where multiple preparations are available, it is assumed that the lowest cost preparation will be prescribed. For IV BP, which is administered in secondary care, drug costs were based on the electronic market information tool (eMIT) database of generic drug costs in secondary care (eMIT 2021). For IV ZOL, the cost of the 5 mg/100 ml preparation licensed for use in osteoporosis is significantly higher now than it was in previous years (£67.84 in 2021 vs. £13.24 in 2018). This price increase is believed to be due to temporary supply issues for the generic formulation, and it was noted by clinical experts that it has become routine clinical practice in some areas to make up the 5 mg dose using two 4 mg vials, which are marketed for another indication and which have a significantly lower price (£3.54 per 4 mg). We applied the price for the 5 mg preparation in our base-case analysis and have explored the impact of assuming that two 4 mg vials are used to make up the 5 mg dose in a scenario analysis.
Administration costs were assumed to be zero for oral BP. Administration of IV BP was assumed to occur as a day-case procedure for both ZOL and IBN-IV. As no suitable NHS reference cost could be identified for day-case administration of IV BP, the approach used in TA464 was to assume that the costs for the day-case procedure would be similar to the administration of simple parenteral chemotherapy (SB12Z). This assumption has been maintained in the current analysis. Administration costs, which are based on 2019/2020 NHS reference costs, are summarised in Table 12. We have assumed that patients would receive a DXA scan on completion of treatment (3 years for IV BP and 5 years for oral BP) as part of the assessment of whether treatment should be continued. This is in keeping with the recommendation in the NOGG guideline and the approach taken when the model was updated for the appraisal of non-BP. The cost of DXA was applied in the model by including the annualised cost of DXA within the drug cost, such that lower DXA costs are incurred when patients are assumed to stop treatment early. The cost for DXA was based on the NHS reference cost for direct access DXA (£84.59 for RD50Z). The monitoring costs, based on 2019/2020 NHS reference costs, are summarised in Table 11.
| ALN | RIS | IBN oral | IBN IV | IV ZOL | |
|---|---|---|---|---|---|
| Intended treatment duration (years) | 5 | 5 | 5 | 3 | 3 |
| Mean persistence (years) | 1.92 | 1.81 | 1.91 | 2.30 | 2.59 |
| Offset period (years) | 1.92 | 1 | 1 | 1 | 6.04 |
| Drug acquisition costs | |||||
| Dosing unit | 70 mg | 35 mg | 150 mg | 3 mg in 3 ml | 5 mg in 100 ml |
| Dosing frequency | Weekly | Weekly | Monthly | Quarterly | Annual |
| Unit cost (£) | 0.87 per 4 | 2.06 per 4 | 2.29 per 1 | 10.89 per 1 | 67.84 per 1 |
| Total drug cost/year (£) | 11.34 | 26.85 | 27.48 | 43.58 | 67.84 |
| Administration costs | |||||
| Route of administration | Oral | Oral | Oral | IV | IV |
| Resource use for administrations | N/A | N/A | N/A | Outpatient | Day case |
| Cost per administration | N/A | N/A | N/A | £295.92 | £295.92 |
| No. administrations/year | N/A | N/A | N/A | 4 | 1 |
| Total admin costs/year (£) | 0.00 | 0.00 | 0.00 | 1183.68 | 295.92 |
| Monitoring costs | |||||
| Years between DXA | 5 | 5 | 5 | 3 | 3 |
| Annualised BMD measurement costs (£) | 16.92 | 16.92 | 16.92 | 28.20 | 28.20 |
| Total annual costs (£) | 28.26 | 43.77 | 44.40 | 1255.44 | 391.95 |
| Total (undiscounted) cost over duration of treatment persistence (£) | 54.22 | 79.12 | 84.62 | 2887.90 | 1013.87 |
Treatment offset
For all BP, it was assumed in the model that the treatment effect persists for some time after treatment is stopped, as the effect of the treatment on BMD does not reverse immediately at the point that treatment is discontinued. The time taken for the treatment effect to reduce to zero is described as the offset period in the model. In keeping with the assumptions made in previous cost-effectiveness analyses, we assumed that the offset period for ALN was equivalent to the treatment duration. This was based on evidence that it took 5 years for hip BMD to return to pre-treatment levels when treatment with ALN was discontinued after 5 years in the FLEX study. 166,167
For RIS, two studies reported findings that gains in hip BMD were lost in the year following treatment discontinuation. 160,168 For IBN-oral data were limited, but one study which used a daily dose of IBN found that hip BMD returned to pre-treatment levels 1 year after completing 1 year of treatment. 169 Based on this, we have assumed that RIS and IBN-oral have a 1-year offset period in the base-case analysis but have explored an offset period equal to treatment duration in a sensitivity analysis. IV ZOL is thought to have a longer offset period than ALN, as femoral neck BMD was found not to have returned to baseline after 3 years of IV ZOL, followed by 3 years of PLB in the HORIZON-PFT extension study. 168 Based on this, we have applied the assumption used in previous models, which is that 3 years of treatment with IV ZOL is expected to provide up to 10 years of effect. We have therefore assumed in the base-case analysis that the offset period for IV ZOL is 2.3 times the treatment duration [i.e. (10 − 3)/3 = 2.3]. We have explored setting the offset period equal to treatment duration for IV ZOL in a sensitivity analysis. For IBN-IV, we have made the same assumption as previous models,170 which have assumed that the offset time for IBN-IV is equivalent to that for IBN-oral as there is a lack of studies reporting treatment efficacy following discontinuation for IBN-IV.
Treatment efficacy
The model uses the patient’s characteristics to estimate their risk of fracture when receiving no antifracture medication based on the Qfracture algorithm. The risks of fracture for patients on each treatment pathway were estimated by adjusting the fracture risks estimated by the Qfracture algorithm to take into account the efficacy of treatment. The model applied the HRs for fracture estimated from the updated review and NMA described in Chapter 4. The same efficacy estimates have been applied to both men and women, as a previous network analysis found that there was no evidence of differential treatment effect by sex. These HRs were applied for the duration of treatment persistence. Treatment efficacy was assumed to wane linearly over the offset period such that no further treatment effect is assumed beyond the end of the offset period.
For hip, vertebral and wrist fractures, the HRs from the relevant NMA were applied, but for proximal humerus fractures, data from the non-vertebral fracture NMA were applied. No data were included in the networks for IBN-IV. We assumed that the treatment efficacy of IBN-IV was equivalent to that of IBN-oral. This was based on the fact that both the 150 mg monthly oral dose and the quarterly 3 mg IV dose were licensed on the basis that they were non-inferior to the 2.5 mg daily dose on BMD outcomes. 171–173
Adverse effects
The review of RCTs described in Chapter 4 identified that arthralgia, headache, myalgia, pyrexia and influenza-like symptoms were all statistically significantly more common for patients having IV ZOL than for patients having PLB. No other BP treatment was found to have a statistically significant higher risk of these adverse effects when compared to PLB, although no data were available for IBN-IV. The cluster of symptoms associated with IV ZOL appears to occur within the same time period (within 3 days of infusion) and is sometimes referred to as an ‘acute-phase reaction’. 174 They are also more common at the time of the first infusion. We have therefore assumed that they occur together as a cluster of symptoms and have used the data reported for influenza-like symptoms to estimate the risk of patients experiencing an acute-phase reaction, which could include arthralgia, headache, myalgia, pyrexia or other influenza-like symptoms. The NMA for influenza-like symptoms was used to estimate the absolute difference in these symptoms between patients treated with IV ZOL and those treated with PLB. This gave an increased risk of influenza-like symptoms of 6.8% (95% CI 3.3% to 12.7%) attributable to treatment with IV ZOL. An identical risk was applied to IBN-IV as there were no comparative data and it was assumed that the acute-phase reaction would be similar for other IV BP. Symptoms were assumed to last 3 days and occur only on the first dose (HRQoL data applied during this period are discussed below). For patients experiencing flu-like symptoms following administration of IV BP, a QALY loss of 0.005 was applied based on the approach used in TA464 in which a utility multiplier of 0.35 was applied for a period of 3 days. This was based on a study reporting a utility value of 0.34 in patients with flu-like symptoms relative to a baseline value of 0.97 prior to flu-like symptoms. 175
The model developed to inform TA464 included a risk of 3% for GI adverse effects for oral BP based on a review of observational studies by Lloyd et al. 45 In that analysis, the risk of experiencing a GI adverse effect was estimated from a study reporting adverse effects for ALN, and it was assumed that an equal event rate would apply to all three oral BPs. However, in this analysis, we are interested in capturing any uncertainty regarding the difference in side effects between the different oral BPs. Therefore, in this analysis, we have applied the RRs versus ALB obtained from the NMA, to the absolute risk of 3%, to estimate the risk of GI adverse effects for RIS and IBN-oral. This gave risks of 3% (95% CI 2% to 5%) and 4% (95% CI 2% to 8%), respectively, for RIS and IBN-oral. We did not include any GI adverse effects for IV BP, as although they are reported, they were considered to be part of the ‘acute-phase reaction’, which has already been incorporated within the risk of influenza-like symptoms. For patients experiencing GI adverse effects, we have applied a QALY loss of 0.0075 and a cost of £39 for a GP appointment176 at the start of treatment.
Disease costs
NHS resource following fracture in TA464 was based on two UK resource use studies which reported costs for hip and non-hip fractures and included activity in both primary care and secondary care. 177,178 These were combined with NHS reference costs to estimate NHS costs in the year following fracture and in subsequent years for each fracture type (hip, vertebral, wrist and proximal humerus). These fracture costs were retained in the current analysis, but they were uplifted to reflect current prices using PSSRU inflation indices. 177 Similarly, costs for home help and residential/nursing home admission incorporated in the model for TA464 were retained but were similarly uplifted to reflect current prices. Costs following fracture are summarised in Table 13.
| Parameter | Hip | Vertebrae | Proximal humerus | Wrist | New admission to residential care |
|---|---|---|---|---|---|
| Costs in year of fracturea (£) | 9063 | 4594 | 1437 | 947 | 25,938 |
| Costs in subsequent yearsa (£) | 117 | 365 | 77 | 77 | 25,938 |
| Utility multiplier in year of fracture | 0.55b | 0.68b | 0.78c | 0.83b | 0.625d |
| Utility in subsequent years | 0.86b | 0.85b | 1.00c | 0.99b | 0.625d |
Health-related quality of life
In the model developed to inform TA464, the impact of treatment on HRQoL was incorporated through the impact of treatment on fragility fractures and adverse events. Although HRQoL was included as an outcome in the safety and efficacy review, it was only reported in two studies, and neither of these reported using a preference-based measure of HRQoL that would be suitable for incorporation in the economic model. Therefore, there was insufficient evidence to incorporate a difference in HRQoL measured directly between BP regimens, and the previous approach to model HRQoL through its impact on fragility fractures and adverse events was maintained.
The main source of utility data in the model for TA464 was the Swedish KOFOR study,179 which was later expanded into the ICUROS international study. 180 At the time the model was developed for TA464, only short-term data (4 months post fracture) were available from the ICUROS study, so the KOFOR study, which had longer follow-up (12 months), was preferred. However, an updated review of the impact of fragility fractures on HRQoL was conducted during the NICE appraisal of non-BP. 171 In that review, a set of more recent publications were identified describing longer-term utility values from the ICUROS study. 180,181 Based on that review, the model had been updated to include these more recent estimates. These publications provide a source of utility values that is based on the EQ-5D, using a UK time trade-off valuation set, which is NICE’s preferred method for estimating utility values. 182 They provide estimates of utility pre-fracture (based on recall shortly after fracture) and at 4, 12 and 18 months post fracture, allowing an average utility value to be estimated in both the year of fracture and in subsequent years. Utility values were provided for all four fracture sites, providing consistency in the methods used, although only three out of the four fracture sites (hip, wrist and vertebral) were reported from the whole international cohort. 181 Therefore, the estimate for proximal humerus fracture was taken from the Australian arm of the ICUROS study. 182 These updated utility estimates were retained for the current analysis. The utility value for patients admitted to residential care following fracture previously applied in TA464 was also retained. Utility values applied following fracture or nursing home admission (see Table 13).
Approach to sensitivity analysis
A probabilistic sensitivity analysis (PSA) was conducted to estimate the mean costs and QALYs gained when taking into account the uncertainty in the parameter values used in the model. The sampling of parameters was consistent with the methods previously reported by Davis et al. 45 The exceptions were the parameters capturing efficacy, treatment persistence and adverse events, which have all been updated in this analysis. For the estimates of treatment efficacy, we used the convergence diagnosis and output analysis (CODA) samples from the NMA described in Chapter 4. We assumed that the mean treatment persistence for ALN was normally distributed using the 95% CI calculated from the Kaplan–Meier data from Guyot et al. 163 We used beta distributions to sample the proportion discontinuing IV ZOL at 1 and 2 years165 and generated sampled estimates of mean treatment persistence for IV ZOL. For the remaining oral and IV treatments, we applied the CODA samples from the HRs for treatment persistence relative to the baseline treatment persistence curves for ALN and IV ZOL, respectively. For the adverse effects of GI symptoms on oral BP, we applied the CODA samples for the relative risk of GI symptoms in patients having RIS and IBN-oral compared to ALN. This was applied to a fixed risk of 3% for ALN. For the adverse effect of flu-like symptoms in patients having IV ZOL, we used the CODA samples from the NMA described in Chapter 4 to estimate the absolute risk of flu-like symptoms for IV ZOL and applied this identically to both IV BP.
For the base-case cost-effectiveness estimates, we ran the model for 2 million patients with a single set of parameter samples per patient. As cost-effectiveness is dependent on absolute fracture risk, results were presented by risk category, with each category representing a decile of absolute fracture risk and therefore informed by approximately 200,000 patients.
Structural sensitivity analyses were conducted to explore whether the results were sensitive to different model assumptions. To reduce model computation time, the structural sensitivity analyses were conducted using mid-point parameter inputs rather than using the full PSA version of the model.
Value of information analysis
Expected value of parameter information provides an estimate of how much more net monetary benefit could be achieved if every parameter informing the model was known precisely, and this allowed the decision-maker to refine their choice of treatment accordingly. The value of knowing every model parameter precisely is known as the overall EVPI. In addition, partial EVPI can be estimated for single parameters or groups of parameters, and this can tell the decision-maker how much more net benefit could be achieved if perfect information was known just about those particular parameters. 183 EVPI is useful in determining which model parameters are associated with the greatest decision uncertainty and would therefore be priorities for further research. It should be noted that EVPI does not address whether a particular study to obtain further research data would be worthwhile, as this would also depend on the ease of obtaining further data and how much this is likely to reduce the current uncertainty.
The EVPI was estimated for the higher risk categories (8–10), where there is overlap between the risk level in those categories and the risk levels at which patients qualify for treatment according to the treatment thresholds in the NICE clinical standard. The no-treatment option was excluded for the EVPI calculation as we are interested in which parameters drive decision uncertainty regarding whether alternative BP should be used instead of ALN.
In addition to estimating the overall EVPI, partial EVPI was estimated for parameters that are specific to the individual BP: treatment persistence, adverse event rates and HRs for fracture risk reduction. The EVPI was calculated by running a cohort of 50,000 patients repeatedly for 1000 different sets of parameter inputs. Then the SAVI tool was used to estimate the partial EVPI using non-parametric regression for groups of parameters184
Results
Base-case results
The adverse clinical outcomes avoided for each BP treatment compared to no treatment are summarised in Table 14. These are based on average outputs from the probabilistic model (run for a cohort of 2 million patients, resampling parameter inputs for each patient). It can be seen that the fractures avoided by IV ZOL treatment are higher than for the other BP treatments. This is largely driven by the longer duration of treatment persistence and the longer offset period. Of the remaining treatments, ALN prevents the most hip and vertebral fractures, which translates into fewer fatal fractures and fewer new admissions to nursing/residential care. Although IBN-IV prevents slightly more fractures overall than ALN, this is driven by it preventing more wrist fractures, which have a smaller impact on costs and utilities than hip and vertebral fractures.
| Adverse clinical outcomes avoided per 100,000 patients treated when compared to no treatment | Total LYS gained per patient vs. no treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Total fractures | Hip fracture | Vertebral fracture | Proximal humerus fracture | Wrist fracture | Nursing home/residential care admission | Fatal fracture | ||
| ALN | 424 | 126 | 113 | 53 | 132 | 23 | 20 | 0.00151 |
| RIS | 404 | 86 | 81 | 59 | 179 | 16 | 14 | 0.00100 |
| IBN (oral) | 365 | 44 | 89 | 47 | 184 | 9 | 8 | 0.00060 |
| IBN (IV) | 428 | 53 | 104 | 56 | 214 | 11 | 10 | 0.00068 |
| IV ZOL | 1239 | 265 | 259 | 148 | 567 | 52 | 41 | 0.00319 |
The base-case cost-effectiveness results from the probabilistic model are summarised in Table 15, which shows the incremental cost-effectiveness ratio (ICER) for each treatment relative to no treatment within each risk category. It can be seen that ALN has an ICER under £20,000 per QALY relative to no treatment from the sixth risk category, RIS from the eighth risk category and IBN-oral from the ninth risk category (10-year average risks of 2.7%, 5.5% and 8.4%, respectively). Table 16 shows the ICERs for each of the alternative BP relative to ALN. It can be seen that all of the alternative BPs, except IV ZOL, are dominated by ALN in that they have a higher cost and lower QALY gain. The ICERs for IV ZOL compared to ALN are greater than £30,000 per QALY across all risk categories. While ICERs are useful when determining which treatment is cost-effective compared to no treatment, it can be more helpful to use incremental net monetary benefit (INMB) to determine the most cost-effective intervention. Treatments which are cost-effective compared to no treatment have an INMB relative to no treatment that is greater than zero, and the optimal treatment has the highest INMB. Figure 7 shows the variation in INMB (when valuing a QALY at £20,000) with absolute risk. Results for risk levels above 40% were not plotted, as the estimates are unstable at high levels as they are based on very small numbers of patients.
| Risk category | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Qfracture score (%) | 0.5 | 0.7 | 1.0 | 1.4 | 2.0 | 2.7 | 3.9 | 5.5 | 8.4 | 16.0 |
| ALN (£) | 147,158 | 65,238 | 53,581 | 32,471 | 23,002 | 7487 | 3674 | Dominates | Dominates | Dominates |
| RIS (£) | 324,992 | 155,655 | 119,906 | 104,826 | 65,443 | 27,846 | 24,390 | 12,317 | Dominates | Dominates |
| IBN (oral) (£) | 361,368 | 151,306 | 190,001 | 97,999 | 114,879 | 57,259 | 45,647 | 21,066 | 4719 | 172 |
| IBN (IV) (£) | 15,180,545 | 5,011,285 | 5,214,323 | 3,787,207 | 4,375,584 | 2,153,472 | 1,517,354 | 1,007,064 | 849,143 | 591,036 |
| IV ZOL (£) | 984,029 | 529,580 | 429,053 | 332,504 | 252,373 | 171,363 | 120,270 | 87,487 | 59,632 | 40,542 |
| Max NMB | ||||||||||
| at £20K | NT | NT | NT | NT | NT | ALN | ALN | ALN | ALN | ALN |
| at £30K | NT | NT | NT | NT | ALN | ALN | ALN | ALN | ALN | ALN |
| Risk category | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Qfracture score (%) | 0.5 | 0.7 | 1.0 | 1.4 | 2.0 | 2.7 | 3.9 | 5.5 | 8.4 | 16.0 |
| RIS | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| IBN (oral) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| IBN (IV) | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated | Dominated |
| IV ZOL (£) | 1,437,173 | 839,103 | 676,889 | 551,636 | 403,936 | 318,228 | 206,192 | 155,529 | 122,559 | 105,722 |
FIGURE 7.
Incremental net monetary benefit for BP compared to usual care plotted against absolute risk.
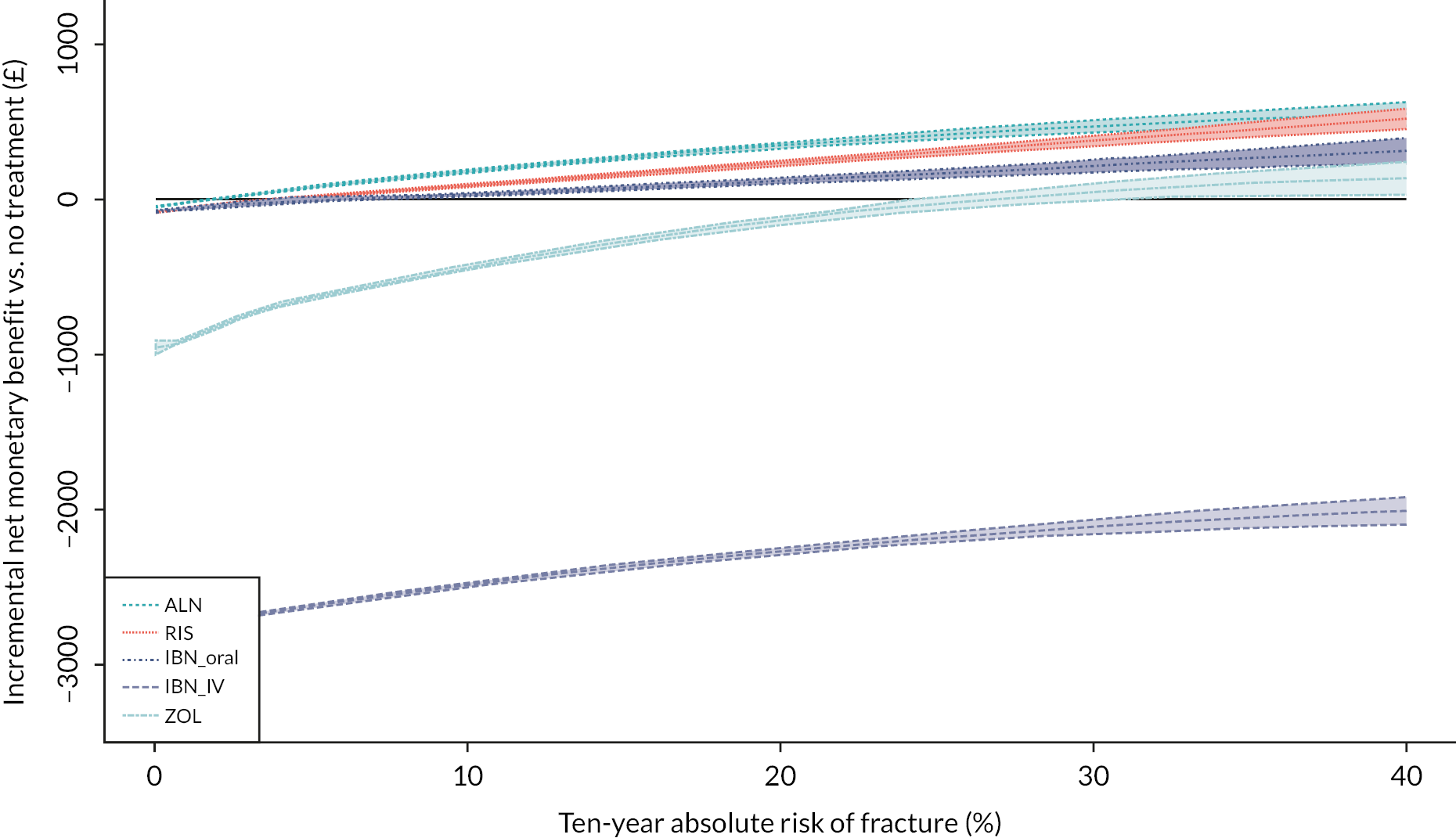
From Figure 7 it can be seen that each of the BP, with the exception of IBN-IV, achieves an ICER under £20,000, relative to no treatment, once a certain level of absolute risk is reached because their INMB becomes positive. The risk levels required for the BP to achieve an ICER under £20,000 compared to no treatment are 1.9%, 4.0%, 6.7% and 26.9% for ALN, RIS, IBN-oral and IV ZOL. However, even at higher levels of absolute risk, ALN is the most cost-effective intervention, as it has the highest INMB. Figure 7 also shows the CIs around the estimates of INMB. These widen at higher levels of absolute risk, but there is no crossing of the CIs until the risk is above 30%. The CIs for INMB for ALN and RIS do overlap at higher levels of absolute risk, and they overlap substantially when the risk reaches 40%. This suggests there may be some uncertainty regarding the most cost-effective treatment, but only at very high levels of absolute risk.
Scenario analysis results
A scenario analysis was conducted to explore the impact of assuming that all treatments have an offset time equal to treatment duration, which is consistent with what was assumed for ALN. This scenario analysis was run using a cohort of 2 million patients but with parameters fixed at their mid-point values. Figure 8 shows the base-case results when running the model in this manner, and Figure 9 shows the scenario analysis assuming an offset time equal to treatment duration for all drugs. It can be seen by comparing Figures 8 and 9 that this had the impact of bringing the INMBs estimates for RIS and IBN-oral closer to those for ALN. Therefore, both RIS and IBN-oral have an ICER under £20,000 compared to no treatment in the 7th risk category in this scenario. It also reduced the INMBs for IV ZOL and increased the INMBs for IBN-IV. However, the overall conclusion that the INMB was highest for ALN across all 10 risk categories was unchanged.
FIGURE 8.
Incremental net monetary benefit compared to usual care for 10 risk categories when using mid-point parameter inputs – base case.
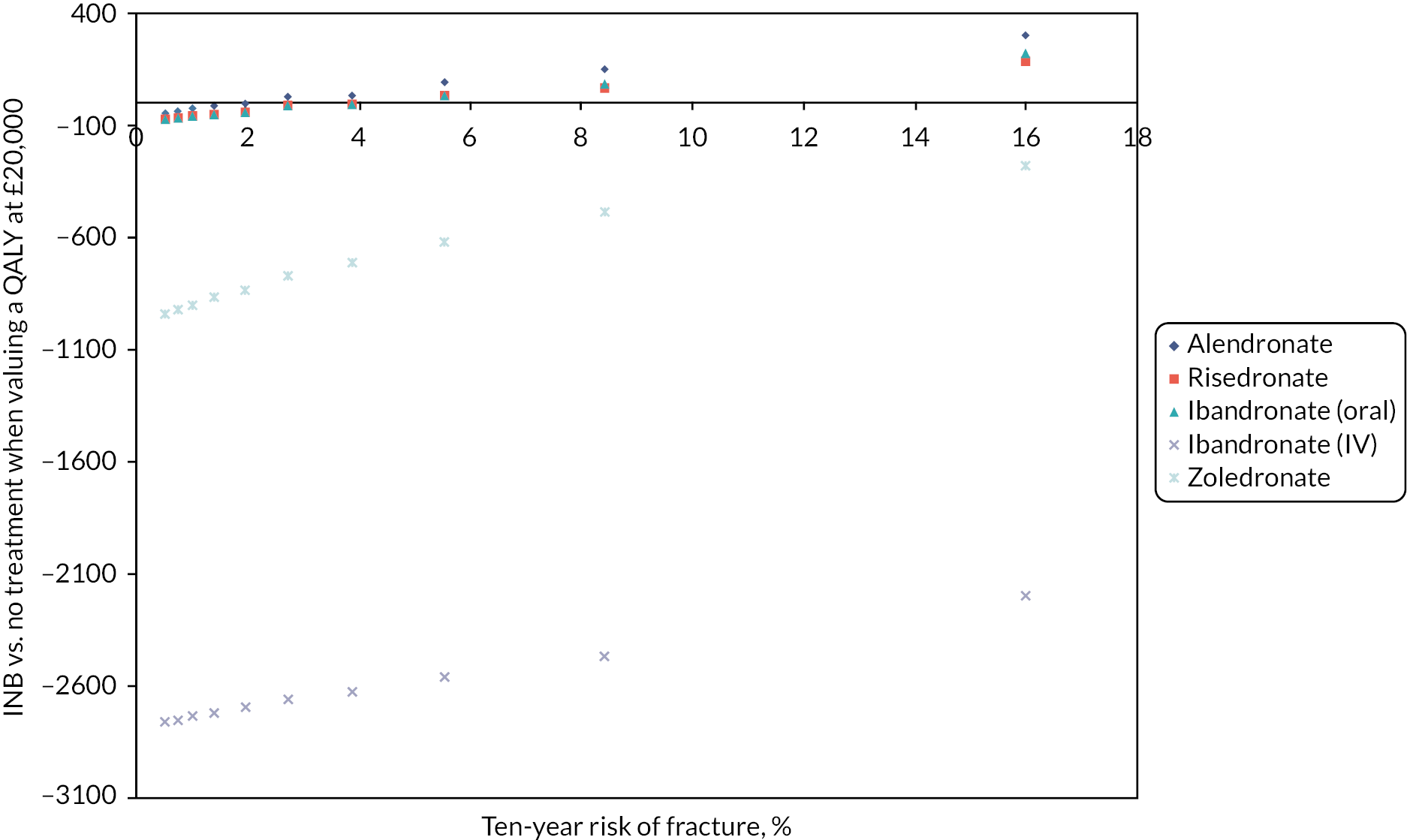
FIGURE 9.
Incremental net monetary benefit compared to usual care for 10 risk categories when using mid-point parameter inputs – assuming offset equal to treatment duration.
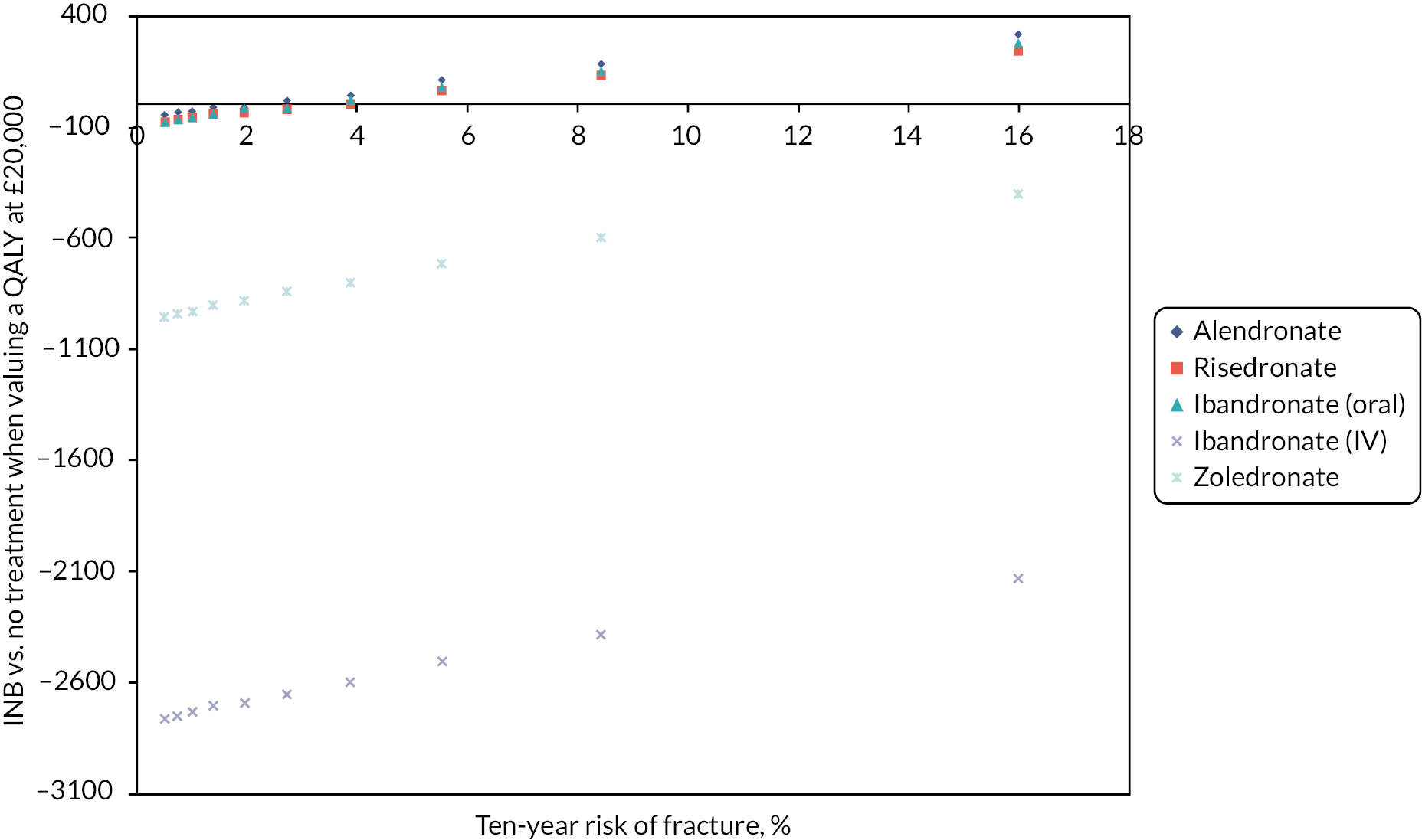
A scenario analysis was conducted exploring the impact of assuming that clinicians use two 4 mg vials to make up the 5 mg dose of IV ZOL. This reduced the risk level at which IV ZOL achieved an ICER under £20,000 versus usual care from 26.9% to 19.9%. However, it did not alter the conclusion that ALN was more cost-effective than IV ZOL at all levels of absolute risk. A scenario analysis was also conducted to explore increasing the annual cost of prescribing oral BP to reflect prescribing time and dispensing fees for monthly prescriptions in addition to drug costs. A cost analysis exploring the differences in costs between monthly and quarterly prescribing of repeat prescriptions from a UK NHS perspective assumed that GPs take just under a minute (49 seconds) to process a repeat prescription. 184 Applying the unit cost per hour of GP activity (£156 per hour) and the cost of pharmacy dispensing fees (£1.29 per item) would result in an additional cost of £40.96 per annum for oral BP or £78.64 across the average duration of treatment persistence. Under these assumptions, IV ZOL would still not be the optimal treatment, even at higher levels of absolute risk. However, the risk level at which ALN would achieve an ICER under £20,000 compared to no treatment would increase to 5.0%. This would have no impact on current recommendations as the lowest intervention threshold, according to the NICE Quality Standard, is a 10-year absolute fracture risk of 5.9%.
Expected value of parameter information results
Figure 10 shows the partial EVPI indexed to overall EVPI for risk categories 8–10 for various groups of parameters. It can be seen that the HRs for hip fracture account for a high proportion of the overall EVPI, and this is fairly consistent across risk categories 8–10 (denoted D8–D10 on the plot). It can also be seen that the HRs for any type of fracture for IBN-oral are also quite important and that both of these are being driven by a high EVPI for the HR of hip fracture in IBN-oral as a single parameter. The decision uncertainty associated with adverse events and treatment persistence is minimal in comparison. This is because there is a great degree of uncertainty around the HR for hip fracture for IBN-oral. Consequently, the probability that IBN-oral is the most cost-effective BP is 22%, 21% and 10% in risk categories 8, 9 and 10, respectively (when valuing a QALY at £20,000), whereas the probability that RIS is the most cost-effective BP is less than 5% in each of these risk categories, and the probability that either of the IV BP is the most cost-effective BP is < 0.1%.
FIGURE 10.
Expected value of perfect information for groups of parameters in risk categories 8–10 indexed to total EVPI.
Discussion
The previous economic evaluations conducted to inform the NICE appraisal of BP made several simplifying assumptions that limited the accuracy of the comparisons between the different BP treatments. For example, long-term persistence with treatment and the incidence of adverse events were assumed to be the same for all oral BPs. The key strength of this analysis is that it has taken the model used previously to inform the NICE appraisal of BP and has incorporated updated systematic reviews to better quantify the relative advantages of alternative BP regimens in terms of treatment persistence and adverse effects. It has also included an EVPI analysis to identify the key areas of decision uncertainty when selecting the optimum BP treatment regimen.
The economic evaluation has identified that when selecting the most cost-effective BP in those with a risk level sufficient to be currently eligible to receive ALN, the most important area of decision uncertainty relates to the relative efficacy of IBN-oral compared to ALN in preventing hip fractures. This is due to the wide CI for the RR of hip fracture for IBN-oral relative to PLB, which is based on a single RCT. This factor is more important than uncertainty surrounding adverse effects or treatment persistence. The higher administration costs for IV BP mean that there is minimal uncertainty relating to whether IV BP is more cost-effective. This is despite the fact that IV ZOL is predicted to result in fewer fractures than ALN due to its higher treatment persistence and a longer offset period. Whether alternatives to hospital administration, such as IV ZOL delivered in a home care setting, are more cost-effective requires further evaluation.
Strengths and limitations
Limitations of this analysis include the minimal comparative data available for IBN-IV, which meant that it was necessary to assume that IBN-IV had efficacy similar to IBN-oral and adverse events similar to ZOL. Therefore, the results for IBN-IV should be treated with caution. In the NMA conducted to inform the NICE appraisal of BP, studies that reported outcomes for the daily 2.5 mg IBN-oral were included in the analysis, despite the fact that this was not a licensed dose of IBN-oral. However, in the previous analysis, efficacy estimates from this unlicensed dose were applied in the economic model for the licensed doses of IBN-oral (i.e. 150 mg monthly IBN-oral and the quarterly 3 mg IBN-IV), where data specifically from these licensed doses were lacking in the NMA. The approach taken in our review and economic evaluation was to limit the data to trials reporting outcomes for licensed doses of IBN, which better reflects the evidence gap for IBN-oral and IBN-IV. This is preferable given that one of the aims of this project was to identify where further research to reduce uncertainties in the current evidence base would be useful. Finally, our analysis was conducted from a UK NHS and PSS perspective, and the conclusions are not expected to apply to other countries or healthcare settings.
Chapter 6 Research priorities regarding the use of bisphosphonates for osteoporosis: a United Kingdom priority-setting exercise
Some text in this chapter has been reproduced from Bastounis A, et al. Osteoporos Int 2022;33:1223–3. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
In order to enhance adherence to BP, and thus contribute to addressing the osteoporosis care gap, it is important to understand perspectives of all relevant stakeholders in using these drugs. There are many possible research agendas to pursue and, traditionally, researchers have identified health research priorities. However, PPI in research, including the prioritisation of research agendas, is now well established. 185–191 Involving patients and the public ensures that research is grounded in patient relevance, research questions are meaningful and important research topics are identified that researchers may not have previously considered. 192 Over the last decade, a number of initiatives, such as INVOLVE, part of the NIHR, have been established to facilitate and promote active public involvement in all aspects of research, including priority setting. The James Lind Alliance (JLA) was formed in 2004 and aimed to bring patients and clinicians together in a new way to identify and address important uncertainties about the effects of care and treatments. 193
Despite the apparent revolution in patient engagement, evidence suggests the mismatch between the research that is conducted and the research patients want still persists. A previous report commissioned by the JLA established that the majority of charitable funders in the UK funded research in a responsive mode, with only a minority funding research that met pre-identified priorities. 194 With respect to BP as a treatment for osteoporosis, no studies have investigated the research priorities of stakeholders. Paskins et al. (2017) conducted the first national study of public and patient research priorities in osteoporosis and fracture. 195 Participants were asked to indicate their top priority for research across 40 different research items. Understanding the safety and benefit of osteoporosis drug treatments was identified as the second priority research area. However, a need was identified for more refinement to translate this research focus into specific research questions. This paper aims to address this gap by conducting a research prioritisation exercise to understand priorities relating to BP treatment regimens for prevention of osteoporotic fractures in adults.
Methods
We used a three-step approach based on the JLA methodology for identification and prioritisation of research questions. 196 An overview of the methods is shown in Figure 11. This prioritisation study did not require ethics approval as per the JLA guidance.
FIGURE 11.
Overview of methods.

Step 1: gathering uncertainties
Uncertainties were gathered from (1) Chapters 2–5 and (2) existing published research recommendations. Over a series of four group meetings, the group study team reviewed and discussed the findings from (1) Chapters 2–5 and generated a list of potential arising uncertainties. A final meeting involved a patient advisory group (PAG) to further inform the process. Separately, a systematic search of relevant electronic databases and websites of professional organisations was conducted to identify (2) research recommendations highlighted within recent clinical guidelines. Databases searched included Epistemonikos, NICE, SIGN, Guidelines international network, Guidelines.co.uk and TRIP database. Inclusion criteria were (1) international guidelines from non-low- or middle-income country (LMIC), (2) about osteoporosis (including glucocorticoid osteoporosis), (3) published since 2016 and (4) developed on behalf of a professional organisation. Exclusion criteria were: (1) guidelines from LMIC, (2) about osteoporosis only in the context of another specific health condition, (3) published before 2016 and (4) written by individuals not representing a broader organisation. Attempts were made to translate guidelines that were not in the English language. Relevant sections on recommendations for research were extracted, and a list of research recommendations was produced. Subsequently, research recommendations were considered as in- or out-of-scope initially by two members of the study team (ZP, NC) and then approved by the whole team, with in-scope recommendations defined as relating to the use of BP. This generated a list of research recommendations.
Step 2: processing and refining uncertainties
Using stakeholder input, we refined the list of uncertainties from (1) and research recommendations (2) into research questions. One 3-hour stakeholder meeting was convened with patients and carers, clinicians (medical and non-medical) and academics to include representatives from primary and secondary care. Potential participants were invited from the ROS Effectiveness Working Group of the Bone Research Academy, Nottingham osteoporosis patient support group and clinical networks of the study team. We recorded the professional role and sex of attendees, but we did not collect data on age or ethnicity. The list of uncertainties and list of research recommendations [outputs from (1) and (2)] were circulated to attendees before the meeting. In the meeting, within small groups, the list of uncertainties were discussed and refined, with some uncertainties combined as appropriate. Attendees and study team members had the opportunity to suggest additional uncertainties during this process. The uncertainties were also categorised into groups. Each uncertainty was then refined into a research question with particular attention to defining the population and setting, intervention, comparison and outcomes of interest. 197 These were then combined with (2) forming a final list of research questions (3).
In order to validate that the research questions (3) were true research questions and not already answered, a search was subsequently conducted of the Cochrane Database of Systematic Reviews, PubMed and references of NICE guidelines, SIGN clinical guidelines, NOGG guidelines and ROS guidance for any relevant systematic reviews. If no systematic review was found to exist, the research question proceeded to Step 3.
Step 3: prioritisation
A full-day online workshop was convened in February 2022, aiming for between 12 and 30 participants to include a mix of patients, carers and primary and secondary care clinicians. Potential participants were invited as per the Step 2 workshop; in addition, the workshop was advertised on Twitter and via the Keele research User Group to particularly target lay, non-medical and primary care representatives. People were allocated on a first-come, first-served basis with the aim of achieving a balance of attendees across professional and lay groups. Study team members attended and acted as facilitators but did not vote or discuss ranking. Information on participant interests and disclosures was collected and reviewed to ensure balance across the group. Participants were sent the research questions in advance and asked to rank their top twenty questions before the workshop. Participants were permitted to send in pre-ranking if interested but unable to attend the workshop. In the workshop, an adapted nominal group technique was used. As per updated JLA guidance for online workshops, a four-step approach was used (removing a fifth plenary step, which has been difficult to operationalise online). 198 The workshop started with a plenary session to introduce the task and explain the background. Thereafter, four small groups compared and discussed their initial pre-workshop rankings. After a break, the same groups then produced their own combined ranking of at least the top 20 questions. The ranking of the four small groups was then combined and shared with the group in a plenary session. Finally, a second round of group prioritisation took place, to revise the shared ranking, in new small groups. These small group rankings were combined, reviewed and agreed as the final prioritised list.
Patient and public involvement
Members of the Nottingham ROS (NotROS) Support Group were involved in a series of meetings to discuss the design of the BLAST-OFF research programme and confirmed that understanding acceptability of BP from a range of perspectives was important. A PAG helped the study team identify the research uncertainties emerging from BLAST-OFF and public contributors were involved in both stakeholder groups (Steps 2 and 3).
Results
Step 1: gathering uncertainties
The study team and PAG identified 22 uncertainties. Eleven uncertainties were informed by Chapter 2, 9 by Chapter 3, 11 by Chapter 4 and 7 by Chapter 5. The PAG talked about the importance of outcomes other than fracture, for example, meeting people’s information needs. They discussed and particularly informed uncertainties relating to how patients could be supported to make decisions, how treatment could be made easier and how effectiveness could be monitored.
Sixty-nine potential clinical guidelines were identified, of which 17 included relevant research recommendations (Figure 12).
FIGURE 12.
Search for clinical guidelines results.
Sixteen research recommendations were informed from the clinical guidelines; six of these overlapped with uncertainties from our study. In addition, the clinical guideline research recommendations highlighted populations in need of specific study, including men, people without BMD-defined osteoporosis, frail older adults, those with cognitive impairment and those with glucocorticoid-induced osteoporosis.
Step 2: processing and refining uncertainties
Eleven people attended Workshop 1. Characteristics of those attending are listed in Table 17.
| Characteristics of participants | Workshop 1 N (%) |
Workshop 2 N (%) |
|---|---|---|
| HCPs | 5 (42) | 8 (40) |
| Female | 2 (40) | 5 (63) |
| Secondary care doctor | 5 (100) | 4 (50) |
| Nurse/allied health professional | 0 | 2 (25) |
| Primary care clinicians | 0 | 2 (25) |
| Public contributor | 7 (58) | 12 (60) |
| Female | 5 (71) | 10 (83) |
| Patient representative | 6 (86) | 12 (100) |
| Carer | 1 (14) | 0 |
| Total number | 12 | 20 |
The group was asked to consider the specific populations highlighted in the research recommendations when rewording and refining all the research uncertainties; younger adults emerged as a further group from discussion where further research was needed. Following the workshop, the uncertainties and research recommendations were finalised into 33 distinct research questions.
Step 3: prioritisation
Thirty-three questions went forward for prioritisation, organised into five categories relating to patient factors and patient support; clinical support and policy; safety; effectiveness and delivery. Twenty people attended Workshop 2, with a further individual (a GP) submitting individual rankings for consideration in the first small group work without attending. Characteristics of attendees were similar to those shown in Table 17.
The final top 10 priorities are shown in Box 1. Research questions 11–20 were also ranked, with the remainder unranked (attached in Appendices 6 and 7, Boxes 2–3).
Discussion
This chapter reports, for the first time, topics of importance to stakeholders in the research of BP treatment regimens for the prevention of osteoporotic fracture in adults, refining previously identified priority areas into specific questions. We identified a number of previously undescribed priority areas relating to BP regimens for people with osteoporosis, including research into the best regimen for people aged under 50 and research comparing the safety, clinical and cost-effectiveness of IV treatment given in peoples’ homes versus hospitals. Furthermore, there was also a particular call to research patient factors influencing treatment selection and effectiveness, highlighting the importance of this research being underpinned by the ethos of personalised care.
-
Which people with osteoporosis should be offered IV BP first line to optimise medicine effectiveness?
-
What is the optimal duration of treatment with BP for people with osteoporosis?
-
What is the role of bone turnover markers in determining the duration of treatment breaks in people with osteoporosis?
-
What healthcare support do people with osteoporosis receiving BP need for medicine optimisation?
-
How can primary care practitioners be supported to make decisions about BP with people with osteoporosis?
-
What is the comparable safety, clinical and cost-effectiveness of ZOL administered in community (homes or GP surgeries) versus in hospital for people with osteoporosis?
-
How do we ensure quality standards are met for people with osteoporosis receiving BP?
-
What is the long-term model of care for people taking oral BP in primary care?
-
What is the best BP choice and frequency for people aged under 50 with osteoporosis?
-
How can people with osteoporosis be supported to make decisions about taking BP?
The top research priority ‘Which people with osteoporosis should be offered IV BP first line to optimise medicine effectiveness?’ could be influenced by a range of different patient factors, which, in turn, would influence treatment selection and effectiveness. Patients are typically not given a choice between oral or IV BP. While clinicians may choose to offer IN BP on the basis of tolerability and safety issues, more empirical evidence is needed which specifically investigates which patients would benefit most from first-line IV treatment. Published research recommendations and previous prioritisation exercises largely focus on safety and optimal duration of drug treatment,199–201 both of which were included within the top 10 research priorities identified in this study. However, the top 10 list also highlights the importance of developing a long-term model of care, providing more support for ongoing medicine optimisation and researching the role of monitoring (bone turnover markers). These areas have been highlighted in a recent rapid realist review exploring the effective characteristics of interventions to support medicine optimisation in osteoporosis, which identified a need for a person-centred model of long-term care for osteoporosis;202 interestingly, this review also highlighted the need and role of providing primary care practitioners with decisional support to improve patient outcomes – also highlighted in our top 10. The question relating to ensuring quality standards are met highlights the importance of knowledge mobilisation and applied health services research, which addresses barriers to implementation of clinical guidelines.
The previous prioritisation exercise in this area identified that ‘having easy access to advice and information from health professionals’ was the highest rating research priority. This top 10 includes the more specific question ‘supporting people with osteoporosis to make decisions about taking BP’. Our preceding qualitative research identified that people reported the benefits of BP to be ambiguous; previous research studies have investigated the role of decision support in osteoporosis, and ongoing development work and trials will hopefully provide further evidence to support this area over the coming years. 203,204
Our findings highlight the importance of conducting priority-setting exercises which involve all stakeholders and to not solely focus on guideline recommendations. Of the top 10 identified research priorities in this study, only 3 were derived from guideline recommendations (research priorities 3, 4 and 8). Particularly novel questions relate to the use of ZOL in the community and the best BP regimen for young adults. Research has shown that the majority of guidelines do not include the views of public and patients205 and, when mentioned, their views were only conceptualised as preferences for one medication over another.
Strengths and limitations
While the study provided some important insights, it is subject to some limitations. Patient and caregiver responses within the workshops may have been influenced by the presence of HCPs. Furthermore, the stakeholders involved might not be entirely representative of the wider population. The study may not have adequately represented underserved populations, and stakeholders’ ethnicity data were not collected; this may have affected the final questions prioritised. Employing survey methods may have identified a more representative sample of stakeholders; however, qualitative research to inform priority setting is well-established and useful. The strengths of the study included the comprehensive guideline search, which ensured existing, relevant and published research recommendations were included and discussed when gathering uncertainties to discuss within the workshops. The depth of research in the BLAST-OFF study was also a strength, particularly the qualitative interview study, which included in-depth, rich descriptions from 78 patients receiving BP regimens.
Conclusions
In summary, this prioritisation exercise highlights the importance of including stakeholders when setting research priorities and provides a more in-depth understanding of the priorities of stakeholders in BP regimens. While some research priorities, such as supporting people with osteoporosis to make decisions about their treatment, are being addressed, the findings illustrate a need for further research to address the issues relating to patient factors influencing treatment selection and effectiveness and how to optimise long-term care. In addition, these findings have implications for research into implementation to address the care gap and education of HCPs.
Chapter 7 Patient and public engagement and involvement
We have worked closely with ROS UK – the only UK-wide charity dedicated to improving the care of people with osteoporosis and the NotROS Patient Support Group. NotROS has 250 members in Nottingham, is actively involved in fund-raising events, holds local awareness campaigns and educational update meetings and is closely aligned to ROS. Supporting research is featured highly within the NotROS group, and a number of patients are currently involved as research lay members.
Following the commissioned call, we undertook two focus groups, one with the ROS (n = 3) and one with NotROS (n = 7), who influenced the design of this application, choice of study outcomes and agreed to be involved throughout the study. In particular, the NotROS group was keen that we include the views of patients offered a range of treatments, who have received treatments both in the hospital and the community and the views of older as well as younger patients. ROS felt it would be important to include the views of commissioners since often their views were in conflict with those of the patients due to competing healthcare priorities and cost-efficiency savings. In terms of dissemination, ROS were keen we include webinars, which they have found to be effective and wide-reaching and happy to support stakeholder and dissemination events given their established networks. Two members of NotROS agreed to be co-applicants, both with previous research experience, and ROS agreed to be a co-investigator to support the delivery on the study.
In addition, both of our co-applicants from NotROS have had a range of alternative BP treatments over the last 10 years, from daily ALN, weekly ALN and monthly IBN to more recently IV ZOL (both as a day case attendee in hospital and community IV ZOL service at home). Thus, they were able to present their own experiences as well as those of service users.
Our PPI groups were closely involved in the further management of the research, supporting the regulatory approvals, developing participant information resources, contributing to the reporting of the research and dissemination of research findings. More specifically, ROS UK took a leading role in convening and ensuring a nationally representative sample of multidisciplinary stakeholders at the stakeholder/consensus events (Stage 2) and will support a wider national dissemination programme. The proposed dissemination programme will include outputs to policy-makers, commissioners, operational managers and change agents, health professionals, patients and the public. ROS have a successful record of organising and delivering both national and regional multistakeholder events, and costs have been included for the stakeholder events (three bespoke regional dissemination meetings and three dissemination webinars).
AB and MH are named co-applicants for the NotROS group and worked with the Study Management Group throughout the project. Their experiences as patients suffering with osteoporosis and access to services (as described above) were found to be invaluable in better understanding the patient journey. AB and MH were also able to draw on the wider views of the NotROS 250 patient membership support group at regular intervals throughout the study. Our PPI members from the NotROS group were further supported by the hospital PPI team.
Chapter 8 Overall discussion and conclusion
Some text in this chapter has been reproduced from Paskins Z, et al. BMJ Open 2020;10:e040634. https://doi.org/10.1136/bmjopen-2020-040634. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Some text in this chapter has been reproduced from Narayanasamy M, Bishop S, Sahota O, Paskins Z, Gittoes N, Langley T. Acceptability and engagement amongst patients on oral and intravenous bisphosphonates for the treatment of osteoporosis in older adults. Age Ageing 2022;51(11):afac255. https://doi.org/10.1093/ageing/afac255. Permission for reuse is in place as per: © The Author(s) 2022. Published by Oxford University Press on behalf of the British Geriatrics Society. All rights reserved. The text below includes minor additions and formatting changes to the original text.
Some text, tables and figures in this chapter have been reproduced from Bastounis A, et al. Osteoporos Int 2022;33:1223–3. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See https://creativecommons.org/licenses/by/4.0/. The text, tables and figures below include minor additions and formatting changes to the original text.
Discussion
We have identified, through a systematic review of previous studies on patient and clinician experiences of BP treatment, how patients and HCPs make sense (coherence) of BP by balancing perceptions of need against concerns, how uncertainty prevails about BP perceived effectiveness and a number of individual and service factors that have potential to increase self-efficacy in recommending and adhering to BP. We identified with moderate confidence that BP taking induces fear, but has the potential to engender reassurance, and that both the side effects and special instructions for taking oral BP can be a source of treatment burden. The use of TFA was originally developed to evaluate acceptability of complex interventions. We explored the utility of the TFA from two perspectives, as an explanatory model for both patient and clinician acceptability and engagement. The TFA was useful for understanding and combining patient and clinician viewpoints; however, there was considerable overlap between domains; perceived efficacy, affective attitudes and self-efficacy beliefs are all likely to impinge on sense-making or intervention coherence. The TFA alone does not provide a comprehensive framework for understanding patient acceptability or engagement with medicines. The sense-making aspect of the framework appeared pivotal, and the explanatory value of the framework was enhanced by the incorporation of the NCF to operationalise key engagement-related beliefs.
The systematic review of previous studies was followed by prospective qualitative interviews, which was designed to examine the experiences of alternate BP regimens through the seven TFA domains. This showed that IV BP treatment with ZOL was generally more acceptable to patients than oral BP treatment. Such regimens were perceived to be more straightforward to engage in, although a portion of patients taking oral BP ALN were satisfied with their current treatment. Balancing of the specific TFA domains impacted the extent to which patients with osteoporosis accepted and engaged in their treatment, manifesting as self-efficacy and affective attitude. Crucially, the TFA domains were found to be interconnected, with patients balancing treatment burden, opportunity costs and ethicality issues against treatment coherence and perceived effectiveness. The outcome of this balancing act ultimately determined patients’ attitudes towards, and engagement in, their treatment regimens, thus informing their affective attitudes and self-efficacy. The complexities of using the TFA in the context of examining treatment acceptability found that there were overlaps between TFA domains and that it is not always a comprehensive framework for offering understanding into patient acceptability and engagement with medicines. Other frameworks, such as the NCF, may be helpful in understanding and transforming adherence-related beliefs and behaviours. This framework understands patients’ adherence to be the outcome of a cost–benefit analysis whereby adherence is likely to be higher when the perceived need for treatment is prioritised over the risk of negative consequences such as side effects.
Conducted in parallel to systematic review of previous studies and prospective qualitative interviews, our systematic review and NMA of previous studies of effectiveness found that IV ZOL was the most effective BP compared to ALN, RIS and oral IBN in preventing the occurrence of vertebral fractures and increasing femoral neck BMD. IV ZOL was also found to be comparably effective to RIS and ALN in preventing non-vertebral fractures and hip fractures, respectively. Uptake of IV ZOL was also found to be accompanied by more frequently reported adverse events; however, these events were short-lived. For persistence, results from the NMA from RCTs showed that IV ZOL users may be less likely to drop out from trials at 12 months, although these effects were marginally non-significant. Results from the NMA using data from the observational studies showed that IV ZOL and IBN-IV users were less likely to discontinue their treatment over time, with IV ZOL users being statistically significantly more persistent compared to oral BP users. Data drawn from the vote-counting synthesis were in line with the results of NMAs, where IV ZOL and IBN-IV users were more likely to persist with their treatment, with IV ZOL users being more persistent compared to their IBN-IV counterparts. Due to the paucity of data and the heterogeneity in reporting compliance data, we were unable to perform NMAs, but synthesis based on vote counting found that compliance to IV ZOL was greater within 24 months after the initiation of their treatment. Users of IBN-IV were found to be more compliant compared to IBAN-oral users. Users of ALN were found to be more compliant than RIS users, while mixed evidence were observed in the comparison between ALN and IBN-oral users.
Using data from the systematic review and NMA on effectiveness and NMA on persistence, we updated the previous economic evaluations conducted to inform the NICE appraisal of BP. The previous NICE model made several simplifying assumptions that limited the accuracy of the comparisons between the different BP treatments. For example, long-term persistence with treatment and the incidence of adverse events were assumed to be the same for all oral BP. The key strength of our analysis was that we took a model used previously to inform the NICE appraisal of BP and incorporated our systematic review and NMA on effectiveness and NMA on persistence to better quantify the relative advantages of alternative BP regimens in terms of treatment persistence and adverse effects. It has also included an expected value of perfect information analysis to identify the key areas of decision uncertainty when selecting the optimum BP treatment regimen. Our economic evaluation identified that higher hospital administration costs for IV ZOL meant that there was minimal uncertainty relating to whether IV ZOL was more cost-effective, despite the fact that IV ZOL was predicted to result in fewer fractures than ALN due to having higher treatment persistence and a longer offset period. Whether alternatives to hospital administration, such as IV ZOL delivered in a home care setting, are more cost-effective requires further evaluation.
Our concluding, final prioritisation exercise highlighted topics of importance to stakeholders in the research of BP regimens for the prevention of osteoporotic fracture in adults, refining previously identified priority areas into specific questions. Our top research priority identified was ‘Which people with osteoporosis should be offered IV ZOL first line to optimise medicine effectiveness?’ This could be influenced by a range of different patient factors, which, in turn, would influence treatment selection and effectiveness. Patients are typically not given a choice between oral or IV ZOL. Whilse clinicians may choose to offer IV ZOL on the basis of tolerability and safety issues, more empirical evidence is needed which specifically investigates which patients would benefit most from first-line IV ZOL. Published research recommendations and previous prioritisation exercises largely focus on safety and optimal duration of drug treatment, both of which were included within the top 10 research priorities. However, our top 10 list also highlighted the importance of developing a long-term model of care, providing more support for ongoing medicine optimisation and researching the role of monitoring (bone turnover markers). Overall, of the top 10 identified research priorities in this study, only 3 were derived from guideline recommendations. Particularly novel questions relate to the use of IV ZOL in the community and the best BP regimen for young adults.
Equality, diversity and inclusion
In our qualitative study, our sample population was largely drawn from a sample of participants who had membership with the ROS, with a smaller number recruited through NHS services. The majority of the participants were white. This may have caused the sample to be biased, for example, it may have largely comprised individuals who had the financial means to fund membership and who were possibly taking a more proactive approach to their health by investing in resources. This may restrict its applicability to other patient groups, such as those who are financially disadvantaged and those who are less proactive in their care and treatment.
Future studies may consider recruiting through GP practices. This method may also address some issues related to ethnicity since the majority of our recruited population was white.
In the stakeholder meetings, the stakeholders involved might not be entirely representative of the wider population. The study may not have adequately represented underserved populations, and stakeholders’ ethnicity data were not collected, which may have affected the final questions prioritised. Furthermore, patient and caregiver responses within the workshops may have been influenced by the presence of HCPs.
Future studies may give greater consideration to ethnicity mix in the stakeholder group.
The research team itself was widely representative, with male and female members, members from the BAME community, and experience and expertise across the research team. PPI members were representative of the disease and ongoing treatment modalities. Two junior researchers were supported directly by the senior members with supervised development opportunities.
Conclusions
We have identified the factors that influence how patients and clinicians make sense of BP, describe the experience of BP taking in terms of burden and identified factors that both facilitate and hinder confidence in taking, and prescribing and monitoring BP. Our findings demonstrate the need for a theoretically informed, whole-system approach to enable clinicians and patients to get the best from BP treatment. Patients need comprehensive support that takes account of the perceptions (e.g. treatment necessity beliefs and concerns) and practicalities (e.g. capability and resources) that influence their motivation and ability to start and continue with treatment. IV ZOL treatment was generally more acceptable to patients. IV ZOL was found to be the most effective BP and with greater adherence; however, there was uncertainty relating to whether IV ZOL was more cost-effective due to the high hospital administration costs. The prioritisation exercise highlighted the importance of including stakeholders when setting research priorities and provided a more in-depth understanding of the priorities of stakeholders in BP regimens. While some research priorities, such as supporting people with osteoporosis to make decisions about their treatment are being addressed, the findings illustrate a need to address the issues relating to patient factors influencing treatment selection and effectiveness and how to optimise long-term care.
Further research is needed to explore perspectives of managers, patients receiving IV BP, men receiving BP and the use of BP in the context of multimorbidity. Research is also needed to support people to make decisions influencing treatment selection and effectiveness, and establish how to optimise long-term care, using frameworks for investigating patient acceptability of and engagement in treatment. In addition, research is needed to explore the clinical and cost-effectiveness of IV ZOL delivered in alternate settings, such as the community, compared to ALN treatment.
Acknowledgements
We would first like to acknowledge the intervention participants for taking part in this study and sharing their views and experiences on being involved.
We would like to thank our external trial steering committee members, led Dr Nicola Peel (Chair), Professor Karen Barker, Dr Alex Thompson and Ms Lindsey Wallis (Nottingham Osteoporosis Society patient support group), members of the Royal Osteoporosis Society Francesca Thompson (Clinical and Operations Director), Jill Griffin (Professional Development Lead, ROS) and Belinda Thompson (Research Manager, ROS). Finally, a thank you to Rachael Taylor (Research Nurse/Study Coordinator, Nottingham University Hospital) and Maribel Cameron (Research Practitioner, Nottingham University Hospital).
Elizabeth Cottrell (NIHR Academic Clinical Lecturer in Primary Care) was the Co-Applicant for Stakeholder/consensus methodology (Stage 2) and was involved in conduct, analysis and dissemination. Dawn van Berkel (Research Fellow) supported the ethics submissions for Stage 1A.
We had a strong ethnic and gender split within the research team, with a commitment to empowerment and improving male and female workforce productivity.
Two of our research fellows were junior members, supported and guided by the more senior members within the team.
Contributions of the authors
Opinder Sahota (https://orcid.org/0000-0003-0055-7637) (Chief Investigator) was responsible for the overall conception, design, data acquisition and analysis and interpretation of findings at each phase of this research. He is responsible for the overall content of this report and supervision of Dawn van Berkel.
Melanie Narayanasamy (https://orcid.org/0000-0003-3483-0777) (Research Fellow) was the recruited Research Fellow for the study and oversaw the day-to-day management of stage 1A. She led the recruitment of participants, conducted or oversaw the data collection and analysis and supported Simon Bishop.
Anastasios Bastounis (https://orcid.org/0000-0001-5861-9373) (Research Fellow) was the recruited Research Fellow for the study and oversaw the day-to-day management of stage 1B of the study. He led the reviews, conducted and oversaw the data collection and analysis, supported by Tessa Langley and Jo Leonardi-Bee.
Zoe Paskins (https://orcid.org/0000-0002-7783-2986) (Senior Reader and Honorary Consultant Rheumatologist) was the Co-Applicant Lead for Stage 2 and was involved in trial design, conduct, analysis and dissemination.
Simon Bishop (https://orcid.org/0000-0001-8527-7081) (Associate Professor in Organisational Behaviour) was the Co-Applicant Lead for Stage 1A and was involved in trial design, conduct, analysis and dissemination and supervision of Melanie Narayanasamy.
Tessa Langley (https://orcid.org/0000-0001-9560-1148) (Associate Professor in Health Economics) was the Co-Applicant Lead for Stage 1B and was involved in trial design, conduct, analysis and dissemination and supervision of Anastasios Bastounis.
Neil Gittoes (https://orcid.org/0000-0001-5963-214X) (Honorary Professor and Consultant Endocrinologist) was a Co-Applicant and was involved in overall trial design, conduct and analysis.
Sarah Davis (https://orcid.org/0000-0002-6609-4287) (Senior Lecturer in Health Economics) was a Co-Applicant for Health Economic Modelling (Stage 1B) and was involved in analysis and dissemination.
Ann Baily (Lay Member) was a Co-Applicant and Chair of PPI for Nottingham Osteoporosis Society Patient Support group.
Moira Holmes (Lay Member) was a Co-Applicant and a PPI member of Nottingham Osteoporosis Society Patient Support group.
Jo Leonardi-Bee (https://orcid.org/0000-0003-0893-6068) (Professor and Head of the Systematic Review Research group) was a Co-Applicant for Systematic review (Stage 1A) and was involved in trial conduct and analysis.
Publications
Bastounis A, Langley T, Davis S, Paskins Z, Gittoes N, Leonardi-Bee J, Sahota O. Assessing the effectiveness of bisphosphonates for the prevention of fragility fractures: an updated systematic review and network meta-analyses. JBMR Plus 2022;6(5):e10620. https://doi.org/10.1002/jbm4.10620. eCollection 2022 May.
Paskins Z, Bullock L, Manning F, Bishop S, Campbell P, Cottrell E, et al. Acceptability of, and preferences for, remote consulting during COVID-19 among older patients with two common long-term musculoskeletal conditions: findings from three qualitative studies and recommendations for practice. BMC Musculoskelet Disord 2022;23(1):312. https://doi.org/10.1186/s12891-022-05273-1
Paskins Z, Crawford-Manning F, Cottrell E, Corp N, Wright J, Jinks C, et al. Acceptability of bisphosphonates among patients, clinicians and managers: a systematic review and framework synthesis. BMJ Open 2020;10(11):e040634. https://doi.org/10.1136/bmjopen-2020-040634
Data-sharing statement
Owing to the sample size, known geographical locations and personal and organisational sensitivities, the qualitative and quantitative data sets will not be available for sharing. All requests for data should be sent to the corresponding author.
Ethics statement
Ethics approval required and achieved for Chapter 3: Jan 2020, North West – Preston Research Ethics Committee, REF: 19/NW/0714/.
Information governance statement
Nottingham University Hospitals NHS Trust (NUH) is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation NUH is the Data Processor; the Department for Health and Social Care (DHSC) is the Data Controller, and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for DHSC’s Data Protection Officer here (https://www.nuh.nhs.uk/data-requests-your-privacy).
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- International Osteoporosis Foundation . What Is Osteoporosis? n.d. www.iofbonehealth.org/what-is-osteoporosis (accessed 1 May 2019).
- National Institute for Health and Care Excellence . Bisphosphonates for Treating Osteoporosis 2017. www.nice.org.uk/guidance/ta464 (accessed June 2022).
- Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 2013;24:209-17.
- Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC. Effectiveness of antiosteoporotic drugs to prevent secondary fragility fractures: systematic review and metaanalysis. Osteoporos Int 2017;28:3289-300.
- Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43-9.
- Electronic Medicines Compendium n.d. https://medicines.org.uk/emc/ (accessed 1 August 2021).
- Hawley S, Javaid MK, Rubin KH, Judge A, Arden NK, Vestergaard P, et al. Incidence and predictors of multiple fractures despite high adherence to oral bisphosphonates: a binational population-based Cohort study. J Bone Miner Res 2016;31:234-44.
- Dugard MN, Jones TJ, Davie MW. Uptake of treatment for osteoporosis and compliance after bone density measurement in the community. J Epidemiol Community Health 2010;64:518-22.
- Silverman SL, Schousboe JT, Gold DT. Oral bisphosphonate compliance and persistence: a matter of choice?. Osteoporos Int 2011;22:21-6.
- International Osteoporosis Foundation . The Adherence Gap: Why Osteoporosis Patients Don’t Continue With Treatment 2005. www.iofbonehealth.org/sites/default/files/PDFs/adherence_gap_report_2005.pdf (accessed 14 May 2018).
- Siris ES, Gehlbach S, Adachi JD, Boonen S, Chapurlat RD, Compston JE, et al. Failure to perceive increased risk of fracture in women 55 years and older: the Global Longitudinal Study of Osteoporosis in Women (GLOW). Osteoporos Int 2011;22:27-35.
- Roh YH, Koh YD, Noh JH, Gong HS, Baek GH. Effect of health literacy on adherence to osteoporosis treatment among patients with distal radius fracture. Arch Osteoporos 2012;12:42-8.
- Modi A, Fan CS, Tang J, Weaver JP, Sajjan S. Association of gastrointestinal events with osteoporosis treatment initiation and treatment compliance in Germany: an observational study. Bone Rep 2016;5:208-13.
- Raybould G, Babatunde O, Evans AL, Jordan JL, Paskins Z. Expressed information needs of patients with osteoporosis and/or fragility fractures: a systematic review. Arch Osteoporos 2018;13:14-9.
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44-7.
- Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Strom O. Persistence with denosumab and persistence with oral bisphosphonates for the treatment of postmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int 2015;26:2401-11.
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007;18:1023-31.
- Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 2013;121:1291-9.
- Akarırmak U, Koçyiğit H, Eskiyurt N, Esmaeilzadeh S, Kuru O, Yalçinkaya EY, et al. INSTRUCT Study Group . Influence of patient training on persistence, compliance, and tolerability of different dosing frequency regimens of bisphosphonate therapy: an observational study in Turkish patients with postmenopausal osteoporosis. Acta Orthop Traumatol Turc 2016;50:415-23.
- Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos 2015;10:231-9.
- Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 2017;12:22-9.
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, et al. EU Review Panel of IOF . The EU review panel of the IOF Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 2013;8:137-42.
- Williamson S, Landeiro F, McConnell T, Fulford-Smith L, Javaid MK, Judge A, et al. Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporos Int 2017;28:2791-800.
- Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 2006;17:637-50.
- Tarride JE, Burke N, Leslie WD, Morin SN, Adachi JD, Papaioannou A, et al. Loss of health related quality of life following low-trauma fractures in the elderly. BMC Geriatr 2016;16:84-91.
- Royal College of Physicians . National Hip Fracture Database (NHFD): Annual Report 2017 n.d. www.nhfd.co.uk/files/2017ReportFiles/NHFD-AnnualReport2017.pdf (accessed 1 May 2020).
- Ross S, Samuels E, Gairy K, Iqbal S, Badamgarav E, Siris E. A meta-analysis of osteoporotic fracture risk with medication nonadherence. Value Health 2011;14:571-81.
- Shalev V, Sharman Moser S, Goldshtein I, Yu J, Weil C, Ish-Shalom S, et al. Adherence With bisphosphonates and long-term risk of hip fractures: a nested case-control study using real-world data. Ann Pharmacother 2017;51:757-67.
- Barker KL, Toye F, Minns Lowe CJ. A Qualitative Systematic Review of Patients’ Experience of Osteoporosis Using Meta-Ethnography 2016. https://link.springer.com/article/10.1007/s11657-016-0286-z (accessed 1 August 2020).
- Raybould G, Babatunde O, Evans AL, Jordan JL, Paskins Z. Expressed Information Needs of Patients With Osteoporosis and Or Fragility Fractures: A Systematic Review 2018. https://link.springer.com/article/10.1007/s11657-018-0470-4 (accessed 1 August 2020).
- Hiligsmann M, Bours SPG, Boonen A. A Review of Patient Preferences for Osteoporosis Drug Treatment 2015. https://link.springer.com/content/pdf/10.1007%2Fs11926-015-0533-0.pdf (accessed 1 August 2020).
- Hiligsmann M, Dellaert BG, Dirksen CD, Watson V, Bours S, Goemaere S, et al. Patients’ preferences for anti-osteoporosis drug treatment: a cross-European discrete choice experiment. Rheumatology 2017;56:1167-76. https://academic.oup.com/rheumatology/article/56/7/1167/3111548#90614539 (accessed 1 August 2020).
- Salter C, McDaid L, Bhattacharya D, Holland R, Marshall T, Howe A. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PLOS ONE n.d. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0083552 (accessed 1 August 2020).
- Shu AD, Stedman MR, Polinski JM, Jan SA, Patel M, Truppo C, et al. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care 2009;15:417-24.
- Nho JH, Lee YK, Ha YC, Kim CH, Suh YS, Koo KH. Can alarming improve compliance with weekly bisphosphonate in patients with osteoporosis?. J Bone Metab 2016;23:51-4.
- Seuffert P, Sagebien CA, McDonnell M, O’Hara DA. Evaluation of osteoporosis risk and initiation of a nurse practitioner intervention program in an orthopedic practice. Arch Osteoporos 2016;11:10-8.
- Tuncel T, Hasselberg J, Peters KM. Compliance of osteoporosis patients with additional specific osteoporosis-training course. Orthopade 2017;46:256-62.
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;2014.
- Diez-Perez A, Naylor KE, Abrahamsen B, Agnusdei D, Brandi ML, Cooper C, et al. Adherence Working Group of the International Osteoporosis Foundation and the European Calcified Tissue Society . Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 2017;28:767-74.
- Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int 2011;22:741-53.
- Tremblay E, Perreault S, Dorais M. Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 2016;11:30-8.
- Wong SM, Pacey S, Sahota O. Setting up a homecare service for zoledronic acid treatment of osteoporosis. Eur J Hosp Pharm 2016;23:364-5.
- Marshall L, Wong SM, Sahota O. Innovative intravenous osteoporosis at home service. Age Ageing 2014;43:i10-1.
- n.d. www.bma.org.uk/news/2016/august/vanguards-of-an-integrated-care-revolution (accessed 1 August 2020).
- Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess 2016;20:1-406.
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015;30:2179-87.
- Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care 2009;15:22-33.
- Hiligsmann M, Bours SPG, Boonen A. A review of patient preferences for osteoporosis drug treatment. Curr Rheumatol Rep 2015;17:61-6.
- de Bekker-Grob EW, Essink-Bot ML, Meerding WJ, Pols HA, Koes BW, Steyerberg EW. Patients’ preferences for osteoporosis drug treatment: a discrete choice experiment. Osteoporos Int 2008;19:1029-37.
- Alten R, Krüger K, Rellecke J, Schiffner-Rohe J, Behmer O, Schiffhorst G, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence 2016;10:2217-28.
- Feldstein AC, Schneider J, Smith DH, Vollmer WM, Rix M, Glauber H, et al. Harnessing stakeholder perspectives to improve the care of osteoporosis after a fracture. Osteoporos Int 2008;19:1527-40.
- Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporos Int 2006;17:1309-17.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:88-95.
- Dejean D, Giacomini M, Simeonov D, Smith A. Finding qualitative research evidence for Health Technology Assessment. Qual Health Res 2016;26:1307-17.
- Carroll C, Booth A, Cooper K. A worked example of ‘best fit’ framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol 2011;11.
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related Beliefs about Medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLOS ONE 2013;8.
- Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci 2018;13:34-29.
- Besser SJ, Anderson JE, Weinman J. How do osteoporosis patients perceive their illness and treatment? Implications for clinical practice. Arch Osteoporos 2012;7:115-24.
- Sale JEM, Beaton D, Sujic R, Bogoch ER. ‘If it was osteoporosis, I would have really hurt myself.’ Ambiguity about osteoporosis and osteoporosis care despite a screening programme to educate fragility fracture patients. J Eval Clin Pract 2010;16:590-6.
- Sale JEM, Hawker G, Cameron C, Bogoch E, Jain R, Beaton D, et al. Perceived messages about bone health after a fracture are not consistent across healthcare providers. Rheumatol Int 2015;35:97-103.
- Weston JM, Norris EV, Clark EM. The invisible disease: making sense of an osteoporosis diagnosis in older age. Qual Health Res 2011;21:1692-704.
- Hansen CA, Abrahamsen B, Konradsen H, Pedersen BD. Women’s lived experiences of learning to live with osteoporosis: a longitudinal qualitative study. BMC Womens Health 2017;17:17-26.
- Otmar R, Reventlow SD, Nicholson GC, Kotowicz MA, Pasco JA. General medical practitioners’ knowledge and beliefs about osteoporosis and its investigation and management. Arch Osteoporos 2012;7:107-14.
- Jaglal SB, Carroll J, Hawker G, McIsaac WJ, Jaakkimainen L, Cadarette SM, et al. How are family physicians managing osteoporosis?: qualitative study of their experiences and educational needs. Can Fam Physician 2003;49:462-8.
- Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. General practitioners’ insight into deprescribing for the multimorbid older individual: a qualitative study. Int J Clin Pract 2016;70:261-76.
- Sippli K, Rieger MA, Huettig F. GPs’ and dentists’ experiences and expectations of interprofessional collaboration: findings from a qualitative study in Germany. BMC Health Serv Res 2017;17.
- Merle B, Haesebaert J, Bedouet A, Barraud L, Flori M, Schott AM, et al. Osteoporosis prevention: where are the barriers to improvement in French general practitioners? A qualitative study. PLOS ONE 2019;14.
- Merle B, Dupraz C, Haesebaert J, Barraud L, Aussedat M, Motteau C, et al. Osteoporosis prevention: where are the barriers to improvement in a French general population? A qualitative study. Osteoporos Int 2019;30:177-85.
- Drew S, Judge A, Cooper C, Javaid MK, Farmer A, Gooberman-Hill R. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteoporos Int 2016;27:1719-27.
- Alami S, Hervouet L, Poiraudeau S, Briot K, Roux C. Barriers to effective postmenopausal osteoporosis treatment: a qualitative study of patients’ and practitioners’ views. PLOS ONE 2016;11.
- Guzman-Clark JRS, Fang MA, Sehl ME, Traylor L, Hahn TJ. Barriers in the management of glucocorticoid-induced osteoporosis. Arthritis Care Res 2007;57:140-6.
- Lau E, Papaioannou A, Dolovich L, Adachi J, Sawka AM, Burns S, et al. Patients’ adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician 2008;54:394-402.
- Salter C, McDaid L, Bhattacharya D, Holland R, Marshall T, Howe A. Abandoned acid? Understanding adherence to bisphosphonate medications for the prevention of osteoporosis among older women: a qualitative longitudinal study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0083552.
- Iversen MD, Vora RR, Servi A, Solomon DH. Factors affecting adherence to osteoporosis medications: a focus group approach examining viewpoints of patients and providers. J Geriatr Phys Ther 2011;34:72-81.
- Mazor KM, Velten S, Andrade SE, Yood RA. Older women’s views about prescription osteoporosis medication: a cross-sectional, qualitative study. Drugs Aging 2010;27:999-1008.
- Wozniak LA, Johnson JA, McAlister FA, Beaupre LA, Bellerose D, Rowe BH, et al. Understanding fragility fracture patients’ decision-making process regarding bisphosphonate treatment. Osteoporos Int 2017;28:219-29.
- Swart KMA, Van Vilsteren M, Van Hout W, Draak E, van der Zwaard BC, van der Horst HE, et al. Factors related to intentional non-initiation of bisphosphonate treatment in patients with a high fracture risk in primary care: a qualitative study. BMC Fam Pract 2018;19:141-9.
- Scoville EA, de Leon Lovaton PP, Shah ND, Pencille LJ, Montori VM. Why do women reject bisphosphonates for osteoporosis? A videographic study. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0018468.
- Sale JEM, Gignac MA, Hawker G, Frankel L, Beaton D, Bogoch E, et al. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: a qualitative study. BMC Musculoskelet Disord 2011;12. https://doi.org/10.1186/1471-2474-12-92.
- Sturrock A, Preshaw P, Hayes C, Wilkes S. Attitudes and perceptions of GPs and community pharmacists towards their role in the prevention of bisphosphonate-related osteonecrosis of the jaw: a qualitative study in the North East of England. BMJ Open 2017;7.
- Sturrock A, Preshaw PM, Hayes C, Wilkes S. Perceptions and attitudes of patients towards medication-related osteonecrosis of the jaw (MRONJ): a qualitative study in England. BMJ Open 2019;9.
- Nunes V, Neilson J, O’flynn N. Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence (CG76). NICE Guidance; 2009.
- Horne R, Cooper V, Wileman V, Parham R, Freemantle N, Forbes A, et al. Supporting adherence to medicines for long-term conditions. Eur Psychol 2019;24:82-96. https://doi.org/10.1027/1016-9040/a000353.
- Driesenaar JA, De Smet PA, van Hulten R, Horne R, Zwikker H, van den Bemt B, et al. Beliefs about inhaled corticosteroids: comparison of community pharmacists, pharmacy technicians and patients with asthma. J Asthma 2016;53:1051-8.
- Checkland K, Harrison S, Marshall M. Is the metaphor of ‘barriers to change’ useful in understanding implementation? Evidence from general medical practice. J Heal Serv Res Policy 2007;12:95-00.
- Neale J. Iterative categorization (IC): a systematic technique for analysing qualitative data. Addiction 2016;111:1096-106.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17:1-13.
- Roh YH, Noh JH, Gong HS, Baek GH. Comparative adherence to weekly oral and quarterly intravenous bisphosphonates among patients with limited heath literacy who sustained distal radius fractures. J Bone Miner Metab 2018;36:589-95.
- Fraenkel L, Gulanski B, Wittink D. Patient treatment preferences for osteoporosis. Arthritis Care Res 2006;55:729-35.
- Kendler DL, Bessette L, Hill CD, Gold DT, Horne R, Varon SF, et al. Preference and satisfaction with a 6-month subcutaneous injection versus a weekly tablet for treatment of low bone mass. Osteoporos Int 2010;21:837-46.
- Payer J, Cierny D, Killinger Z, Sulková I, Behuliak M, Celec P. Preferences of patients with post-menopausal osteoporosis treated with bisphosphonates – the VIVA II study. J Int Med Res 2009;37:1225-9.
- Fjose M, Eilertsen G, Kirkevold M, Grov EK. ‘Non-palliative care’ – a qualitative study of older cancer patients’ and their family members’ experiences with the health care system. BMC Health Serv Res 2018;18.
- Selman LE, Bristowe K, Higginson IJ, Murtagh FEM. The views and experiences of older people with conservatively managed renal failure: a qualitative study of communication, information and decision-making. BMC Nephrol 2019;20:1-12.
- HSJ . Daily Insight: Long-Term Plan, Short-Sighted Move? 2021. www.hsj.co.uk/daily-insight/daily-insight-long-term-plan-short-sighted-move/7032261.article (accessed 25 May 2023).
- Fallowfield L, Stebbing J, Braybrooke J, Langridge C, Jenkins V. The preferences and experiences of different bisphosphonate treatments in women with breast cancer. Psycho-Oncology 2011;20:755-61.
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLOS ONE 2013;8.
- Qaseem A, Forciea MA, McLean RM, Denberg TD, Barry MJ, Cooke M, et al. Clinical Guidelines Committee of the American College of Physicians . Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 2017;166:818-39.
- Fatoye F, Smith P, Gebrye T, Yeowell G. Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 2019;9.
- Koller G, Goetz V, Vandermeer B, Homik J, McAlister FA, KendlerYe DC. Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int 2020;31:2093-102.
- Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691-705.
- De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, et al. ESPACOMP medication adherence reporting guideline (EMERGE). Ann Intern Med 2018;169:30-5.
- Hiligsmann M, Cornelissen D, Vrijens B, Abrahamsen B, Al- Daghri N, Biver E, et al. Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF). Osteoporos Int 2019;30:2155-65.
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G, Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken: John Wiley & Sons; 2019.
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021;12:55-61.
- White IR. Network meta-analysis. Stata J 2015;15:951-85.
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84.
- Higgins JT, Altman D. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: The Cochrane Collaboration and John Wiley & Sons Ltd; 2011.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:23-9.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. (Technical Support Document in Evidence Synthesis; No. TSD2). London: National Institute for Health and Clinical Excellence; 2012.
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity – subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618-40.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71.
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Chapter 4. Generalised Linear Models. Network Meta-Analysis for Decision-Making. Oxford, UK: John Wiley & Sons Ltd.; 2018.
- Dakin HA, Welton NJ, Ades AE, Collins S, Orme M, Kelly S. Mixed treatment comparison of repeated measurements of a continuous endpoint: an example using topical treatments for primary open-angle glaucoma and ocular hypertension. Stat Med 2011;30:2511-35.
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Chapter 8. Meta-Regression for Relative Treatment Effects. Network Meta-Analysis for Decision-Making. Oxford, UK: John Wiley & Sons Ltd.; 2018.
- McKenzie JE, Brennan SE. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
- Thomson HJ, Thomas S. The effect direction plot: visual display of non-standardised effects across multiple outcome domains. Res Synth Methods 2013;4:95-101.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44.
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Chapter 7. Checking for Inconsistency. Network Meta-Analysis for Decision-Making. Oxford, UK: John Wiley & Sons Ltd.; 2018.
- van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80-93.
- Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLOS Med 2020;17.
- Papakonstantinou T, Nikolakopoulou A, Higgins JP, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell System Rev 2020;16.
- Tan W, Sun J, Zhou L, Li Y, Wu X. Randomized trial comparing efficacies of zoledronate and alendronate for improving bone mineral density and inhibiting bone remodelling in women with postmenopausal osteoporosis. J Clin Pharm Ther 2016;41:519-23.
- Hu W, Wang H, Shi X, Song Y, Zhang G, Xing S, et al. Effect of preoperative zoledronic acid administration on pain intensity after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Pain Res Manag 2020;2020:1-8.
- Li H, Li C, Yi X, Liu H, Wang Y. Effects of sodium alendronate on osteoporosis and apoptosisrelated factors Cyt C, Apaf-1 and caspase-9. Biomed Res 2018;29:121-9.
- Li Y, Zhao WB, Wang DL, He Q, Li Q, Pei FX, et al. Treatment of osteoporotic intertrochanteric fractures by zoledronic acid injection combined with proximal femoral nail antirotation. Chin J Traumatol 2016;19:259-63.
- Liang BC, Shi ZY, Wang B, Wu P, Kong LC, Yao JL, et al. Intravenous zoledronic acid 5 mg on bone turnover markers and bone mineral density in East China subjects with newly diagnosed osteoporosis: a 24-month Clinical Study. Orthop Surg 2017;9:103-9.
- Liu Z, Li CW, Mao YF, Liu K, Liang BC, Wu LG, et al. Study on zoledronic acid reducing acute bone loss and fracture rates in elderly postoperative patients with intertrochanteric fractures. Orthop Surg 2019;11:380-5.
- Shi ZY, Zhang XG, Li CW, Liu K, Liang BC, Shi XL. Effect of traditional Chinese medicine product, QiangGuYin, on bone mineral density and bone turnover in Chinese postmenopausal osteoporosis. Evid Based Complementary Altern Med 2017;2017:1-8.
- Zhang J, Zhang T, Xu X, Cai Q, Zhao D. Zoledronic acid combined with percutaneous kyphoplasty in the treatment of osteoporotic compression fracture in a single T12 or L1 vertebral body in postmenopausal women. Osteoporos Int 2019;30:1475-80.
- Zhang ZL, Liao EY, Xia WB, Lin H, Cheng Q, Wang L, et al. Alendronate sodium/vitamin D3 combination tablet versus calcitriol for osteoporosis in Chinese postmenopausal women: a 6-month, randomized, open-label, active-comparator-controlled study with a 6-month extension. Osteoporos Int 2015;26:2719-20.
- Zhou J, Liu B, Qin MZ, Liu JP. Fall prevention and anti-osteoporosis in osteopenia patients of 80 years of age and older: a randomized controlled study. Orthop Surg 2020;12:890-9.
- Paggiosi MA, Peel N, McCloskey E, Walsh JS, Eastell R. Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 2014;25:2729-41.
- Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S, et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res 2011;26:1303-12.
- Cesareo R, Di Stasio E, Vescini F, Campagna G, Cianni R, Pasqualini V, et al. Effects of alendronate and vitamin D in patients with normocalcemic primary hyperparathyroidism. Osteoporos Int 2015;26:1295-302.
- Livi L, Scotti V, Desideri I, Saieva C, Cecchini S, Francolini G, et al. Phase 2 placebo-controlled, single-blind trial to evaluate the impact of oral ibandronate on bone mineral density in osteopenic breast cancer patients receiving adjuvant aromatase inhibitors: 5-year results of the single-centre BONADIUV trial. Eur J Cancer 2019;108:100-10.
- Popp AW, Buffat H, Cavelti A, Windolf M, Perrelet R, Senn C, et al. Cortical bone loss at the tibia in postmenopausal women with osteoporosis is associated with incident non-vertebral fractures: results of a randomized controlled ancillary study of HORIZON. Maturitas 2014;77:287-93.
- Cosman F, Gilchrist N, McClung M, Foldes J, de Villiers T, Santora A, et al. A phase 2 study of MK-5442, a calcium-sensing receptor antagonist, in postmenopausal women with osteoporosis after long-term use of oral bisphosphonates. Osteoporos Int 2016;27:377-86.
- Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med 2015;175:913-21.
- Greenspan SL, Vujevich KT, Brufsky A, Lembersky BC, van Londen GJ, Jankowitz RC, et al. Prevention of bone loss with risedronate in breast cancer survivors: a randomized, controlled clinical trial. Osteoporos Int 2015;26:1857-64.
- Grey A, Bolland M, Wong S, Horne A, Gamble G, Reid IR. Low-dose zoledronate in osteopenic postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 2012;97:286-92.
- Cheung AS, Hoermann R, Ghasem-Zadeh A, Tinson AJ, Ly V, Milevski SV, et al. Differing effects of zoledronic acid on bone microarchitecture and bone mineral density in men receiving androgen deprivation therapy: a randomized controlled trial. J Bone Miner Res 2020;35:1871-80.
- Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 2018;379:2407-16.
- Nakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos Int 2017;28:389-98.
- Shin K, Park SH, Park W, Baek HJ, Lee YJ, Kang SW, et al. Monthly oral ibandronate reduces bone loss in Korean women with rheumatoid arthritis and osteopenia receiving long-term glucocorticoids: a 48-week double-blinded randomized placebo-controlled investigator-initiated trial. Clin Ther 2017;39:268-78.
- McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412-20.
- Sestak I, Singh S, Cuzick J, Blake GM, Patel R, Gossiel F, et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2014;15:1460-8.
- Black DM, Reid IR, Cauley JA, Cosman F, Leung PC, Lakatos P, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2015;30:934-44.
- Grey A, Bolland M, Mihov B, Wong S, Horne A, Gamble G, et al. Duration of antiresorptive effects of low-dose zoledronate in osteopenic postmenopausal women: a randomized, placebo-controlled trial. J Bone Miner Res 2014;29:166-72.
- Grey A, Bolland MJ, Horne A, Mihov B, Gamble G, Reid IR. Duration of antiresorptive activity of zoledronate in postmenopausal women with osteopenia: a randomized, controlled multidose trial. CMAJ 2017;189:E1130-6.
- Eastell R, Nagase S, Small M, Boonen S, Spector T, Ohyama M, et al. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J Bone Miner Res 2014;29:458-66.
- Kanis JA, Harvey NC, McCloskey E, Bruyère O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 2020;31:1-2.
- Kendler DL, Marin F, Zerbini CA, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018;391:230-40.
- Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017;377:1417-27.
- Banefelt J, Åkesson KE, Spångeus A, Ljunggren O, Karlsson L, Ström O, et al. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int 2019;30:601-9.
- Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 2019;30:79-92.
- Noirmain C, Gil-Wey B, Pichon I, Brindel P, Haller G. Factors associated with patient willingness to participate in anaesthesia clinical trials: a vignette-based cross-sectional study. BMC Med Res Methodol 2020;20.
- Avis NE, Smith KW, Link CL, Hortobagyi GN, Rivera E. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol 2006;24:1860-7.
- Curtis JR, Yun H, Matthews R, Saag KG, Delzell E. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res 2012;64:1054-60.
- Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318-g3318.
- Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 2008;19:365-72.
- National Osteoporosis Guideline Group . NOGG 2017: Clinical Guideline for the Prevention and Treatment of Osteoporosis 2017. https://sheffield.ac.uk/NOGG/NOGG%20Guideline%202017.pdf (accessed 10 October 2021).
- Morley J, Moayyeri A, Ali L, Taylor A, Feudjo-Tepie M, Hamilton L, et al. Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int 2020;31:533-45. https://doi.org/10.1007/s00198-019-05228-8.
- Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Spångeus A, Johansson S, Woisetschläger M. Adherence to and persistence with zoledronic acid treatment for osteoporosis – reasons for early discontinuation. Arch Osteoporos 2020;15. https://doi.org/10.1007/s11657-020-00733-4.
- NHS Business Services Authority . Prescription Cost Analysis – England 2021. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysis-pca-data (accessed 10 October 2021).
- Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, et al. FLEX Research Group . Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010;25:976-82.
- Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27:243-54.
- Eastell R, Hannon RA, Wenderoth D, Rodriguez-Moreno J, Sawicki A. Effect of stopping risedronate after long-term treatment on bone turnover. J Clin Endocrinol Metab 2011;96:3367-73.
- Ravn P, Christensen JO, Baumann M, Clemmesen B. Changes in biochemical markers and bone mass after withdrawal of ibandronate treatment: prediction of bone mass changes during treatment. Bone 1998;22:559-64.
- Davis S, Simpson E, Hamilton J, Martyn-St James M, Rawdin A, Wong R, et al. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation. Health Technol 2020;24:1-314.
- Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 2006;65:654-61. http://doi.org/10.1136/ard.2005.044958.
- Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 2006;54:1838-46.
- Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008;35:488-97.
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010;95:4380-7.
- van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLOS ONE 2011;6. http://doi.org/10.1371/journal.pone.0017030.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
- Gutiérrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, et al. Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ 2011;14:99-107.
- Gutiérrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, et al. Clinical burden and incremental cost of fractures in postmenopausal women in the United Kingdom. Bone 2012;51:324-31.
- Ström O, Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, et al. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop 2008;79:269-80.
- Svedbom A, Borgstöm F, Hernlund E, Ström O, Alekna V, Bianchi ML, et al. Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int 2018;29:557-66.
- Abimanyi-Ochom J, Watts JJ, Borgström F, Nicholson GC, Shore-Lorenti C, Stuart AL, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int 2015;26:1781-90.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26.
- Doble B, Payne R, Harshfield A, Wilson ECF. Retrospective, multicohort analysis of the Clinical Practice Research Datalink (CPRD) to determine differences in the cost of medication wastage, dispensing fees and prescriber time of issuing either short (< 60 days) or long (≥ 60 days) prescription lengths in primary care for common, chronic conditions in the UK. BMJ Open 2017;7.
- de Wit MP, Berlo SE, Aanerud GJ, Aletaha D, Bijlsma JW, Croucher L, et al. European league against rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis 2011;70:722-6.
- Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients’, clinicians’ and the research communities’ priorities for treatment research: there is an important mismatch. Res Involv Engagem 2015;1.
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 2017;8:136-45.
- Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med 1997;103:S12-9.
- Cochrane methods Priority Setting . Top Tips for Research Priority Setting 2015. http://methods.cochrane.org/prioritysetting/top-tips-research-priority-setting-cochrane-vienna2015-workshop (accessed 1 August 2021).
- Mahmood W, Jinks C, Jayakumar P, Gwilym S, Paskins Z. 115 public priority setting for research in osteoporosis. Rheumatology 2016;55:109-i110.
- Jinks C, Carter P, Rhodes C, Taylor R, Beech R, Dziedzic K, et al. Patient and public involvement in primary care research-an example of ensuring its sustainability. Res Involv Engagem 2016;2.
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65.
- Partridge N, Scadding J. The James Lind Alliance: patients and clinicians should jointly identify their priorities for clinical trials. Lancet 2014;364:1923-4.
- Staley K, Hanley B. Scoping Research Priority Setting (and the Presence of PPI in Priority Setting) with UK Clinical Research Organisations and Funders. James Lind Alliance Oxford; 2018.
- Paskins Z, Jinks C, Mahmood W, Jayakumar P, Sangan CB, Belcher J, et al. Public priorities for osteoporosis and fracture research: results from a general population survey. Arch Osteoporos 2017;12:1-8.
- Katherine Cowan, Senior Advisor to JLA, Sandy Oliver, Professor of Public Policy at Social Science Research Unit and EPPI-Centre, Institute of Education, University of London; James Lind Alliance (JLA) Guidebook, Chapter 1. Katherine oversaw versions 1 – 5. The JLA team at the School of Healthcare Enterprise and Innovation . University of Southampton, and Katherine Cowan, With Input from the JLA Advisers, Have Updated Versions 6 Onwards n.d. http://jla.nihr.ac.uk/jla-guidebook/downloads/JLA-Guidebook-Version-10-March-2021.pdf (accessed 25 May 2023).
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12-3.
- Katherine Cowan, Senior Advisor to JLA, Sandy Oliver, Professor of Public Policy at Social Science Research Unit and EPPI-Centre, Institute of Education, University of London; James Lind Alliance (JLA) Guidebook, Chapter 7 Report on JLA PSP online priority setting workshop – Caroline Whiting, School of Healthcare Enterprise and Innovation, University of Southampton n.d. www.jla.nihr.ac.uk/jla-guidebook/downloads/JLS-Guidebook-Version-10-2021.pdf (accessed 25 May 2023).
- National Institute for Health and Care Excellence (NICE) . Alendronate, Etidronate, Risedronate, Raloxifene and Strontium Ranelate for the Primary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women 2008. https://nice.org.uk/guidance/ta160/documents/osteoporosis-secondary-prevention-including-strontium-ranelate-overview2 (accessed 1 August 2022).
- National Institute for Health and Care Excellence (NICE) . Alendronate, Etidronate, Risedronate, Raloxifene, Strontium Ranelate and Teriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women 2008. www.nice.org.uk/guidance/ta464 (accessed 1 August 2022).
- Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 2010;62:1515-26.
- Paskins Z, Babatunde O, Sturrock A, Toh LS, Horne R, Maidment I, et al. On behalf of the Effectiveness Working Group of the Royal Osteoporosis Society Osteoporosis and Bone Research Academy . Supporting patients to get the best from their osteoporosis treatment: a rapid realist review. Osteoporos Int 2022;33:2245-57.
- Paskins Z, Bullock L, Crawford-Manning F, Cottrell E, Fleming J, Leyland S, et al. Improving uptake of fracture prevention drug treatments: a protocol for development of a consultation intervention (iFraP-D). BMJ Open 2021;11. http://doi.org/10.1136/bmjopen-2021-048811.
- Cornelissen D, Boonen A, Evers S, van den Bergh JP, Bours S, Wyers CE, et al. Improvement of osteoporosis Care Organized by Nurses: ICON study – protocol of a quasi-experimental study to assess the (cost)-effectiveness of combining a decision aid with motivational interviewing for improving medication persistence in patients with a recent fracture being treated at the fracture liaison service. BMC Musculoskelet Disord 2021;22. http://doi.org/10.1186/s12891-021-04743-2.
- Sale J, Marwah A, Naeem F, Yu W, Meadows L. Evidence of patient beliefs, values, and preferences is not provided in osteoporosis clinical practice guidelines. Osteoporos Int 2019;30:1325-37. https://doi.org/10.1007/s00198-019-04913-y.
Appendix 1 Search strategies
FIGURE 13.
Updated review network plots. Reproduced from Bastounis A. et al. JBMR Plus 6:e10620. https://doi.org/10.1002/jbm4.10620
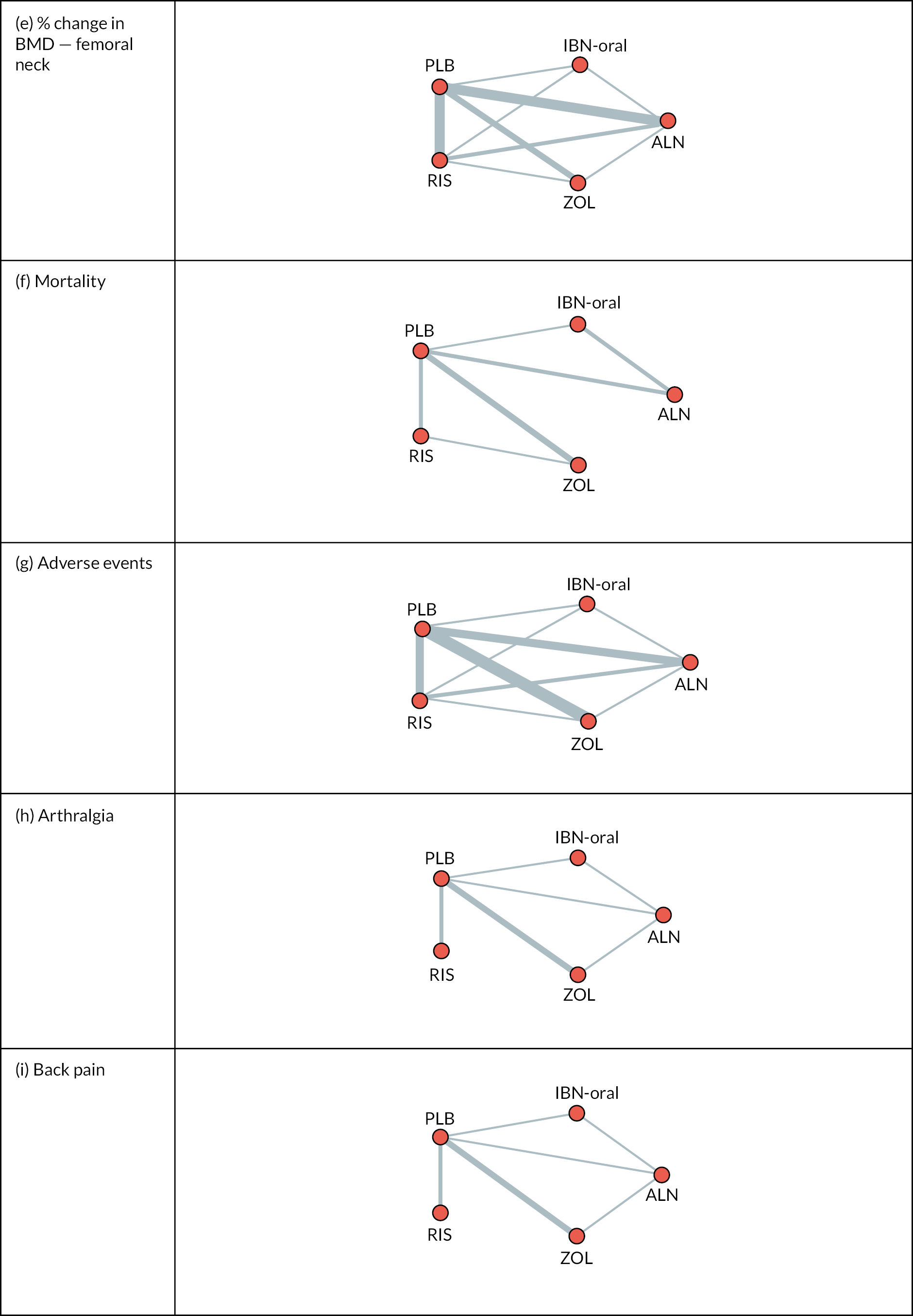
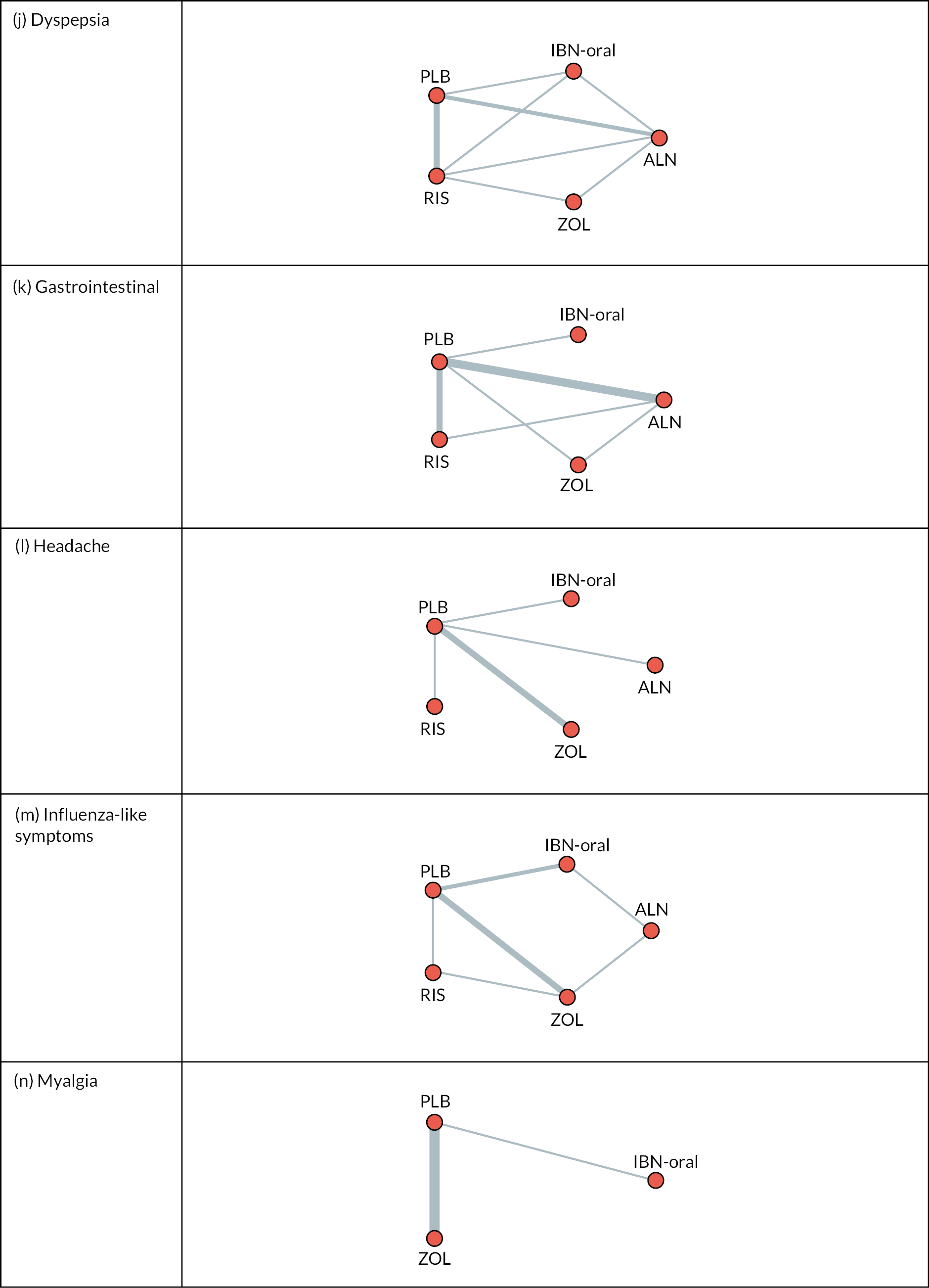
Appendix 2 List of references from Table 9
Reproduced from Bastounis A et al. Osteoporos Int 2022;33(6):1223–3. https://doi.org/10.1007/s00198-022-06350-w. Epub 21 February 2022. PMID: 35188591; PMCID: PMC9106630
-
Adachi JD, Faraawi RY, O’Mahony MF, Nayar A, Massaad R, Evans JK, Yacik C. Upper gastrointestinal tolerability of alendronate sodium monohydrate 10 mg once daily in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled, exploratory study. Clin Ther 2009;31(8):1747–53.
-
Bala Y, Chapurlat R, Cheung AM, Felsenberg D, LaRoche M, Morris E, et al. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Miner Res 2014;29(2):380–8.
-
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356(18):1809–22.
-
Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27(2):243–54.
-
Black DM, Reid IR, Cauley JA, Cosman F, Leung PC, Lakatos P, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2015;30(5):934–44.
-
Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab 2000;85(2):720–6.
-
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SA, et al. Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 2006;91(7):2631–7.
-
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 2005;20(1):141–51.
-
Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012;367(18):1714–23.
-
Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 2009;24(4):719–25.
-
Cheung AS, Hoermann R, Ghasem-Zadeh A, Tinson AJ, Ly V, Milevski SV, et al. Differing effects of Zoledronic acid on bone microarchitecture and bone mineral density in men receiving androgen deprivation therapy: a randomized controlled trial. J Bone Miner Res 2020;35(10):1871–80.
-
Cosman F, Gilchrist N, McClung M, Foldes J, de Villiers T, Santora A, et al. A phase 2 study of MK-5442, a calcium-sensing receptor antagonist, in postmenopausal women with osteoporosis after long-term use of oral bisphosphonates. Osteoporos Int 2016;27(1):377–86.
-
Cryer B, Binkley N, Simonelli C, Lewiecki EM, Lanza F, Chen E, et al. A randomized, placebo-controlled, 6-month study of once-weekly alendronate oral solution for postmenopausal osteoporosis. Am J Geriatr Pharmacother 2005;3(3):127–36.
-
Duckworth AD, McQueen MM, Tuck CE, Tobias JH, Wilkinson JM, Biant LC, et al. Effect of alendronic acid on fracture healing: a multicenter randomized placebo-controlled trial. J Bone Miner Res 2019;34(6):1025–32.
-
Dursun N, Dursun E, Yalcin S. Comparison of alendronate, calcitonin and calcium treatments in postmenopausal osteoporosis. Int J Clin Pract 2001;55(8):505–9.
-
Eastell R, Nagase S, Ohyama M, Small M, Sawyer J, Boonen S, et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res 2011;26(6):1303–12.
-
Eastell R, Nagase S, Small M, Boonen S, Spector T, Ohyama M, et al. Effect of ONO-5334 on bone mineral density and biochemical markers of bone turnover in postmenopausal osteoporosis: 2-year results from the OCEAN study. J Bone Miner Res 2014;9(2):458–66.
-
Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, Melton ME. Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 2004;20(5):699–705.
-
Flodin L, Sääf M, Cederholm T, Al-Ani AN, Ackermann PW, Samnegård E, et al. Additive effects of nutritional supplementation, together with bisphosphonates, on bone mineral density after hip fracture: a 12-month randomized controlled study. Clin Interv Aging 2014;9:1043.
-
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 2000;85(5):1895–900.
-
Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med 2015;175(6):913–21.
-
Greenspan SL, Vujevich KT, Brufsky A, Lembersky BC, van Londen GJ, Jankowitz RC, et al. Prevention of bone loss with risedronate in breast cancer survivors: a randomized, controlled clinical trial. Osteoporos Int 2015;26(6):1857–64.
-
Greenspan SL, Resnick NM, Parker RA. Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA 2003;289(19):2525–33.
-
Grey A, Bolland M, Wong S, Horne A, Gamble G, Reid IR. Low-dose zoledronate in osteopenic postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 2012;97(1):286–92.
-
Grey A, Bolland M, Mihov B, Wong S, Horne A, Gamble G, Reid IR. Duration of antiresorptive effects of low-dose zoledronate in osteopenic postmenopausal women: a randomized, placebo-controlled trial. J Bone Miner Res 2014;29(1):166–72.
-
Grey A, Bolland MJ, Horne A, Mihov B, Gamble G, Reid IR. Duration of antiresorptive activity of zoledronate in postmenopausal women with osteopenia: a randomized, controlled multidose trial. CMAJ 2017;189(36):E1130–6.
-
Hadji P, Gamerdinger D, Spieler W, Kann PH, Loeffler H, Articus K, et al. Rapid Onset and Sustained Efficacy (ROSE) study: results of a randomised, multicentre trial comparing the effect of zoledronic acid or alendronate on bone metabolism in postmenopausal women with low bone mass. Osteoporos Int 2012;23(2):625–33.
-
Ho AY, Kung AW. Efficacy and tolerability of alendronate once weekly in Asian postmenopausal osteoporotic women. Ann Pharmacother 2005;39(9):1428–33.
-
Hooper MJ, Ebeling PR, Roberts AP, Graham JJ, Nicholson GC, D’Emden M, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric 2005;8:251–62.
-
Karimi Fard M, Aminorroaya A, Kachuei A, Salamat MR, Hadi Alijanvand M, Aminorroaya Yamini S, et al. Alendronate improves fasting plasma glucose and insulin sensitivity, and decreases insulin resistance in prediabetic osteopenic postmenopausal women: a randomized triple-blind clinical trial. J Diabetes Investig 2019;10(3):731–7.
-
Klotz LH, McNeill IY, Kebabdjian M, Zhang L, Chin JL; Canadian Urology Research Consortium. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur Urol 2013;63(5):927–35.
-
Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res 2008;14(19):6336–42.
-
Liang BC, Shi ZY, Wang B, Wu P, Kong LC, Yao JL, et al. Intravenous zoledronic acid 5 mg on bone turnover markers and bone mineral density in East China subjects with newly diagnosed osteoporosis: a 24-month clinical study. Orthop Surg 2017;9(1):103–9.
-
Liu Z, Li CW, Mao YF, Liu K, Liang BC, Wu LG, Shi XL. Study on zoledronic acid reducing acute bone loss and fracture rates in elderly postoperative patients with intertrochanteric fractures. Orthop Surg 2019;11(3):380–5.
-
Livi L, Scotti V, Desideri I, Saieva C, Cecchini S, Francolini G, et al. Phase 2 placebo-controlled, single-blind trial to evaluate the impact of oral ibandronate on bone mineral density in osteopenic breast cancer patients receiving adjuvant aromatase inhibitors: 5-year results of the single-centre BONADIUV trial. Eur J Cancer 2019;108:100–10.
-
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357(18):1799–809.
-
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370(5):412–20.
-
McClung MR, Bolognese MA, Sedarati F, Recker RR, Miller PD. Efficacy and safety of monthly oral ibandronate in the prevention of postmenopausal bone loss. Bone 2009;44(3):418–22.
-
McClung M, Miller P, Recknor C, Mesenbrink P, Bucci-Rechtweg C, Benhamou CL. Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet Gynecol 2009;114(5):999–1007.
-
McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 2007;41(1):122–8.
-
Miller PD, Epstein S, Sedarati F, Reginster JY. Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 2008;24(1):207–13.
-
Nakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos Int 2017;28(1):389–98.
-
Orwoll ES, Binkley NC, Lewiecki EM, Gruntmanis U, Fries MA, Dasic G. Efficacy and safety of monthly ibandronate in men with low bone density. Bone 2010;46(4):970–6.
-
Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, et al. Efficacy and safety of a once-yearly iv infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 2010;25(10):2239–50.
-
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000;343(9):604–10.
-
Paggiosi MA, Peel N, McCloskey E, Walsh JS, Eastell R. Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 2014;25(12):2729–41.
-
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 2018.
-
Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009;373(9671):1253–63.
-
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Weryha G, et al. Alendronic acid produces greater effects than risedronic acid on bone density and turnover in postmenopausal women with osteoporosis. Clin Drug Investig 2006;26(2):63–74.
-
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Marques-Neto JF, et al. A comparison of the effect of alendronate and risedronate on bone mineral density in postmenopausal women with osteoporosis: 24-month results from FACTS-International. Int J Clin Pract 2008;62(4):575–84.
-
Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res 2000;15(6):1006–13.
-
Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int 2006;26(5):427–31.
-
Ringe JD, Farahmand P, Faber H, Dorst A. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int 2009;29(3):311–5.
-
Sambrook PN, Roux C, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 2012;50(1):289–95.
-
Sestak I, Singh S, Cuzick J, Blake GM, Patel R, Gossiel F, et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2014;15(13):1460–8.
-
Sestak I, Blake GM, Patel R, Coleman RE, Cuzick J, Eastell R. Comparison of risedronate versus placebo in preventing anastrozole-induced bone loss in women at high risk of developing breast cancer with osteopenia. Bone 2019;124:83–8.
-
Shi ZY, Zhang XG, Li CW, Liu K, Liang BC, Shi XL. Effect of traditional Chinese medicine product, QiangGuYin, on bone mineral density and bone turnover in Chinese postmenopausal osteoporosis. Evid Based Complementary Altern Med 2017;2017:6062707.
-
Shilbayeh S, Hilow HM. The efficacy and safety of calidron tablets for management of Osteoporosis in Jordanian Women: a random clinical trial. Saudi Pharm J 2004;12(2–3):86–95.
-
Shin K, Park SH, Park W, Baek HJ, Lee YJ, Kang SW, et al. Monthly oral ibandronate reduces bone loss in Korean women with rheumatoid arthritis and osteopenia receiving long-term glucocorticoids: a 48-week double-blinded randomized placebo-controlled investigator-initiated trial. Clin Ther 2017;39(2):268–78.
-
Smith BJ, Laslett LL, Pile KD, Phillips PJ, Phillipov G, Evans SM, et al. Randomized controlled trial of alendronate in airways disease and low bone mineral density. Chron Respir Dis 2004;1(3):131–7.
-
Reginster JY, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000;11(1):83–91.
-
Sorensen OH, Crawford GM, Mulder H, Hosking DJ, Gennari C, Mellstrom D, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 2003;32(2):120–6.
-
Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, et al. Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol 2009;36(8):1705–14.
-
Stuss M, Sewerynek E, Król I, Stępień-Kłos W, Jędrzejczyk S. Assessment of OPG, RANKL, bone turnover markers serum levels and BMD after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis. Endokrynologia Polska 2016;67(2):174–84.
-
Välimäiki MJ, Farrerons-Minguella J, Halse J, Kröger H, Maroni M, Mulder H, et al. Effects of risedronate 5 mg/d on bone mineral density and bone turnover markers in late-postmenopausal women with osteopenia: a multinational, 24-month, randomized, double-blind, placebo-controlled, parallel-group, phase III trial. Clin Ther 2007;29(9):1937–49.
-
van Bodegraven AA, Bravenboer N, Witte BI, Dijkstra G, van der Woude CJ, Stokkers PC, et al. Treatment of bone loss in osteopenic patients with Crohn’s disease: a double-blind, randomised trial of oral risedronate 35 mg once weekly or placebo, concomitant with calcium and vitamin D supplementation. Gut 2014;63(9):1424–30.
-
Yan Y, Wang W, Zhu H, Li M, Liu J, Luo B, et al. The efficacy and tolerability of once-weekly alendronate 70 mg on bone mineral density and bone turnover markers in postmenopausal Chinese women with osteoporosis. J Bone Miner Metab 2009;27(4):471–8.
-
Zhang ZL, Liao EY, Xia WB, Lin H, Cheng Q, Wang L, et al. Alendronate sodium/vitamin D 3 combination tablet versus calcitriol for osteoporosis in Chinese postmenopausal women: a 6-month, randomized, open-label, active-comparator-controlled study with a 6-month extension. Osteoporos Int 2015;26(9):2365–74.
-
Zhou J, Liu B, Qin MZ, Liu JP. Fall prevention and anti-osteoporosis in osteopenia patients of 80 years of age and older: a randomized controlled study. Orthop Surg 2020;12(3):890–9.
-
Adami G, Jaleel A, Curtis JR, Delzell E, Chen R, Yun H, et al. Temporal trends and factors associated with bisphosphonate discontinuation and restart. J Bone Miner Res 2020;35(3):478–87.
-
Belhassen M, Confavreux CB, Cortet B, Lamezec L, Ginoux M, Van Ganse E. Anti-osteoporotic treatments in France: initiation, persistence and switches over 6 years of follow-up. Osteoporos Int 2017;28(3):853–62.
-
Berecki-Gisolf J, Hockey R, Dobson A. Adherence to bisphosphonate treatment by elderly women. Menopause 2008;15(5):984–90.
-
Calabria S, Cinconze E, Rossini M, Rossi E, Maggioni AP, Pedrini A, De Rosa M. Adherence to alendronic or risedronic acid treatment, combined or not to calcium and vitamin D, and related determinants in Italian patients with osteoporosis. Patient Preference Adherence 2016;10:523.
-
Carbonell-Abella C, Pages-Castella A, Javaid MK, Nogues X, Farmer AJ, Cooper C, et al. Early (1-year) discontinuation of different anti-osteoporosis medications compared: a population-based cohort study. Calcif Tissue Int 2015;97(6):535–41.
-
Cheen MH, Kong MC, Zhang RF, Tee FM, Chandran M. Adherence to osteoporosis medications amongst Singaporean patients. Osteoporos Int 2012;23(3):1053–60.
-
Cheng LI, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, et al. Persistence and compliance with osteoporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm 2015;21(9):824–33.
-
Curtis JR, Yun H, Matthews R, Saag KG, Delzell E. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res 2012;64(7):1054–60.
-
Curtis JR, Westfall AO, Allison JJ, Freeman A, Saag KG. Channeling and adherence with alendronate and risedronate among chronic glucocorticoid users. Osteoporos Int 2006;17(8):1268–74.
-
Downey TW, Foltz SH, Boccuzzi SJ, Omar MA, Kahler KH. Adherence and persistence associated with the pharmacologic treatment of osteoporosis in a managed care setting. South Med J 2006;99(6):570–6.
-
Dugard MN, Jones TJ, Davie MW. Uptake of treatment for osteoporosis and compliance after bone density measurement in the community. J Epidemiol Community Health 2010;64(6):518–22.
-
Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos 2017;12(1):22.
-
Eisenberg DF, Placzek H, Gu T, Krishna A, Tulsi BB. Cost and consequences of noncompliance to oral bisphosphonate treatment. J Manag Care Spec Pharm 2015;21(1):56–65.
-
Fan T, Zhang Q, Sen SS. Persistence with weekly and monthly bisphosphonates among postmenopausal women: analysis of a US pharmacy claims administrative database. Clinicoecon Outcomes Res 2013;5:589.
-
Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res 2008;23(10):1569–75.
-
Gold DT, Trinh H, Safi W. Weekly versus monthly drug regimens: 1-year compliance and persistence with bisphosphonate therapy. Curr Med Res Opin 2009;25(8):1831–9.
-
Gold DT, Safi W, Trinh H. Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Curr Med Res Opin 2006;22(12):2383–91.
-
Hadji P, Kyvernitakis I, Kann PH, Niedhart C, Hofbauer LC, Schwarz H, et al. GRAND-4: the German retrospective analysis of long-term persistence in women with osteoporosis treated with bisphosphonates or denosumab. Osteoporos Int 2016;27(10):2967–78.
-
Hadji P, Felsenberg D, Amling M, Hofbauer LC, Kandenwein JA, Kurth A. The non-interventional BonViva Intravenous Versus Alendronate (VIVA) study: real-world adherence and persistence to medication, efficacy, and safety, in patients with postmenopausal osteoporosis. Osteoporos Int 2014;25(1):339–47.
-
Halpern R, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm 2011;17(1):25–39.
-
Hansen C, Pedersen BD, Konradsen H, Abrahamsen B. Anti-osteoporotic therapy in Denmark – predictors and demographics of poor refill compliance and poor persistence. Osteoporos Int 2013;24(7):2079–97.
-
Hansen L, Jørgensen AD, Gerstoft F, Modi A, Vestergaard P. Health Care Costs in Patients Who Became Nonadherent During Long-term Oral Bisphosphonate Use. World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO 2015): Poster Abstracts; S71–S380; Milan, Italy. Osteoporosis International; 2015.
-
Iolascon G, Gimigliano F, Orlando V, Capaldo A, Di Somma C, Menditto E. Osteoporosis drugs in real-world clinical practice: an analysis of persistence. Aging Clin Exp Res 2013;25(1):137–41.
-
Jones TJ, Petrella RJ, Crilly R. Determinants of persistence with weekly bisphosphonates in patients with osteoporosis. J Rheumatol 2008;35(9):1865–73.
-
Kjellberg J, Jorgensen AD, Vestergaard P, Ibsen R, Gerstoft F, Modi A. Cost and health care resource use associated with noncompliance with oral bisphosphonate therapy: an analysis using Danish health registries. Osteoporos Int 2016;27(12):3535–41.
-
LaFleur J, DuVall SL, Willson T, Ginter T, Patterson O, Cheng Y, et al. Analysis of osteoporosis treatment patterns with bisphosphonates and outcomes among postmenopausal veterans. Bone 2015;78:174–85.
-
Landfeldt E, Ström O, Robbins S, Borgström F. Adherence to treatment of primary osteoporosis and its association to fractures – the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012;23(2):433–43.
-
Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther 2008;30(4):605–21.
-
Li L, Roddam A, Gitlin M, Taylor A, Shepherd S, Shearer A, Jick S. Persistence with osteoporosis medications among postmenopausal women in the UK General Practice Research Database. Menopause 2012;19(1):33–40.
-
Lima TB, Santos LA, de Carvalho Nunes HR, Silva GF, Caramori CA, Qi X, Romeiro FG. Safety and efficacy of risedronate for patients with esophageal varices and liver cirrhosis: a non-randomized clinical trial. Sci Rep 2019;9(1):1–9.
-
Modi A, Sajjan S, Insinga R, Weaver J, Lewiecki EM, Harris ST. Frequency of discontinuation of injectable osteoporosis therapies in US patients over 2 years. Osteoporos Int 2017;28(4):1355–63.
-
Netelenbos JC, Geusens PP, Ypma G, Buijs SJ. Adherence and profile of non-persistence in patients treated for osteoporosis – a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 2011;22(5):1537–46.
-
Overbeek JA, Intorcia M, Kuiper JG, PenningvanBeest FGA, Worth G. Persistence with Treatments for Osteoporosis: A Real-world Study in the Pharmo Database Network. World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO 2017), Poster Abstracts. S127–S636; Florence, Italy, Osteoporosis International; 2017.
-
Pazianas M, Abrahamsen B, Wang Y, Russell RG. Incidence of fractures of the femur, including subtrochanteric, up to 8 years since initiation of oral bisphosphonate therapy: a register-based cohort study using the US MarketScan claims databases. Osteoporos Int 2012;23(12):2873–84.
-
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 2006;28(2):236–42.
-
Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, Prieto-Alhambra D. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int 2017;28(10):2997–3004.
-
Scotti L, Arfè A, Zambon A, Merlino L, Corrao G. Cost-effectiveness of enhancing adherence with oral bisphosphonates treatment in osteoporotic women: an empirical approach based on healthcare utilisation databases. BMJ Open 2014;4(3):e003758.
-
Sheehy O, Kindundu CM, Barbeau M, LeLorier J. Differences in persistence among different weekly oral bisphosphonate medications. Osteoporos Int 2009;20(8):1369.
-
Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 2011;26(1):3–11.
-
van Boven JF, de Boer PT, Postma MJ, Vegter S. Persistence with osteoporosis medication among newly-treated osteoporotic patients. J Bone Miner Metab 2013;31(5):562–70.
-
Wade SW, Curtis JR, Yu J, White J, Stolshek BS, Merinar C, et al. Medication adherence and fracture risk among patients on bisphosphonate therapy in a large United States health plan. Bone 2012;50(4):870–5.
-
Weiss TW, Henderson SC, McHorney CA, Cramer JA. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Med Res Opin 2007;23(9):2193–203.
-
Xu Y, Viswanathan HN, Ward MA, Clay B, Adams JL, Stolshek BS, et al. Patterns of osteoporosis treatment change and treatment discontinuation among commercial and medicare advantage prescription drug members in a national health plan. J Eval Clin Pract 2013;19(1):50–9.
-
Zambon A, Baio G, Mazzaglia G, Merlino L, Corrao G. Discontinuity and failures of therapy with bisphosphonates: joint assessment of predictors with multi-state models. Pharmacoepidemiol Drug Saf 2008;17(3):260–9.
-
Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P. Persistence and compliance of medications used in the treatment of osteoporosis – analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther 2012;50(5):315.
Appendix 3 Network plots
FIGURE 14.
Network plots of dropout at 12 months NMA. Reproduced from Bastounis A, et al. Osteoporos Int 2022;33(6):1223–3. https://doi.org/10.1007/s00198-022-06350-w. Epub 21 February 2022.
Appendix 4 Treatment ranking probabilities
FIGURE 15.
Network plots of dropout at 24 months NMA. Reproduced from Bastounis A, et al. Osteoporos Int 2022;33(6):1223–3. https://doi.org/10.1007/s00198-022-06350-w. Epub 2022 Feb 21.
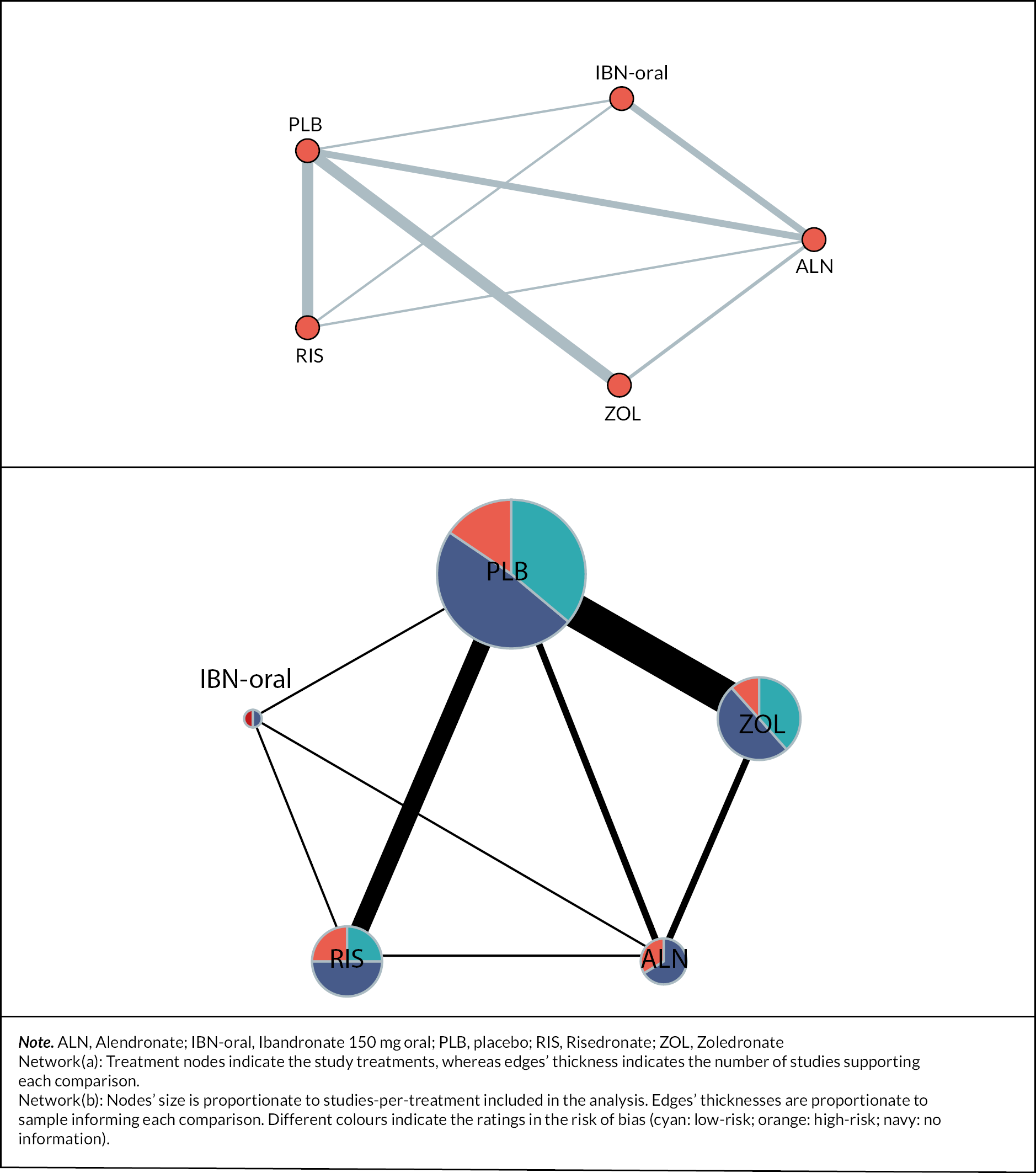
Appendix 5 Additional analyses (sensitivity analyses and meta-regressions)
FIGURE 16.
Network plot for discontinuation NMA. Reproduced from Bastounis A, et al. Osteoporos Int 2022;33(6):1223–3. https://doi.org/10.1007/s00198-022-06350-w. Epub 2022 Feb 21.
Appendix 6 Risk of bias assessment
-
11. How can oral BP effectiveness be defined, explained and monitored to promote medicines optimisation for people with osteoporosis?
-
12. How do we better identify people with osteoporosis who will have difficulty taking or continuing with oral BP?
-
13. What is the optimum frequency to give ZOL to maximise clinical and cost-effectiveness in people with osteoporosis?
-
14. Is lowering the dose of BP an alternative approach to a treatment break for people with osteoporosis?
-
15. What is the incidence and what are the risk factors for BP (prescribed for osteoporosis)-related osteonecrosis of the jaw and atypical femur fracture?
-
16. What is the best BP choice and frequency for people who are unable to manage their medicines specifically due to cognitive impairment?
-
17. Which resources or incentives for primary care would optimise the use of BP for people with osteoporosis?
-
18. How do we define and manage treatment failure in people with osteoporosis taking BP?
-
19. What is the comparable safety, clinical and cost-effectiveness of ZOL versus ALN in people at high risk of fracture?
-
20. What is the best BP choice, dose and frequency for people with low BMI or kidney impairment?
Appendix 7 Quality of evidence
-
What proportion of a population need to adhere to their BP to deliver clinical and cost-effectiveness?
-
What different regimens of ZOL are used in practice for people with osteoporosis and what is patients’ adherence to these regimens?
-
Does dose reduction of BP decrease the risk of atypical femur fractures for people with osteoporosis?
-
What is the role of Fracture Risk Assessment Tool (FRAX) in informing decisions about BP treatment breaks in people with osteoporosis?
-
What is the comparable frequency and duration of adverse events (side effects) of the different BP?
-
What is the effect of BP on fracture healing, in people with fragility and atypical femur fractures?
-
What is the comparable safety, clinical and cost-effectiveness of ZOL versus ALN in people with steroid induced osteoporosis?
-
What is the best BP choice and frequency for people who are unable to manage their medicines?
-
What is the comparable safety, clinical and cost-effectiveness of ZOL versus anabolic agents in people with osteoporosis at very high fracture risk?
-
What is the comparable safety, clinical and cost-effectiveness of ZOL versus anabolic agents in people with steroid-induced osteoporosis?
-
What is the comparable safety, clinical and cost-effectiveness of oral IBN versus ALN in people with osteoporosis?
-
What is the comparable clinical and cost-effectiveness of BP combined with other non-pharmacological approaches versus BP alone in people with osteoporosis?
-
What is the best way to measure renal function when considering BP treatment?
Appendix 8 Assessment of inconsistency
| Amendment no. | Protocol version no. | Date issued | Details of changes made |
|---|---|---|---|
| SA01 | (V 1.1) | 30 April 2020 | Change PIC sites to research sites as interviews are done on site. Remove University Hospitals Birmingham and add Haywood Community Hospital. Add 2 community osteoporosis sites Categorised as non-substantial |
|
NSA01
(COVID-19 Amendment) |
v 1.2 | 15 May 2020 | COVID-19 contingency – additional recruitment from ROS members Protocol v 1.2 30 April 2020 Poster for ROS recruitment v1.0 30 April 2020 Verbal consent form v1.0 30 April 2020 PIS patient open recruitment call ROS v1.0 30 April 2020 Patient reply form v1.0 30 April 2020 |
| SA02 | v 1.3 | 29 July 2020 | To add separate G.P and clinician interview schedules. Add new interview schedules to protocol appendix 10.1 Update SOE to v2.0 to change postage cost from SSC to Research cost. Interview schedule clinicians v1.0 26 February 2020 Interview schedule G.Ps v 1.0 26 February 2020 Schedule of events v2.0 18 March 2020 |
|
NSA 02
(COVID-19 Amendment) |
v 1.4 | To facilitate telephone or web-based patient and clinician interviews rather than face-to face contact under the current COVID-19 restrictions. To justify over recruitment of primary and secondary care patients via open invitation to reach data saturation as an end point. Protocol v 1.4 Verbal consent Script and Confirmation Form patient v1.0 2 October 2020 Verbal Consent Script and Confirmation Form clinician v1.0 2 October 2020 Patient Information Sheet v1.2 2 October 2020 Clinician Information sheet v1.2 2 October 2020 G.P and Health Professional Invitation letter 1.0 10 September 20 Clinic patient invitation letter v1.2 9 September 2020 |
List of abbreviations
- ALN
- alendronate
- BMD
- bone mineral density
- BP
- bisphosphonates
- CINeMA
- confidence in network meta-analysis
- CrI
- credible interval
- DEXA
- Dual Energy X-ray Absorptiometry
- DIC
- deviance information criterion
- Dres
- total residual deviance
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBN
- ibandronate
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NOGG
- national osteoporosis guideline group
- NotROS
- Nottingham ROS
- RIS
- risedronate
- ROS
- Royal Osteoporosis Society
- SD
- standard deviation
- SE
- standard error
- SUCRA
- surface under the cumulative ranking
- ZOL
- zoledronate
