Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/53/22. The contractual start date was in June 2011. The draft report began editorial review in June 2020 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Daniels et al. This work was produced by Daniels et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Daniels et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from McPherson et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Symptomology of uterine fibroids
Uterine fibroids are the most common tumour in women of reproductive age and increase in prevalence as women get older. Around 80% of women will have developed a fibroid by the time that they are 50 years old. Approximately half of women with fibroids experience significant symptoms, which can include heavy menstrual bleeding (HMB), pain on intercourse, abdominal pain and a feeling of pressure,2 all of which can have a significant impact on a woman’s quality of life. 3,4
The symptoms experienced by women with fibroids may vary depending on the position, size and number of fibroids. Intramural fibroids are the most common form of fibroid, but are frequently asymptomatic. Subserosal fibroids, located on the outer surface of the uterus, can become very large and create feelings of bulkiness. Submucosal fibroids project into the uterine cavity and are associated with HMB. As submucosal fibroids may distort the uterus and change the local morphology of the uterine tissue, some clinicians believe that the presence of fibroids may have a negative impact on fertility,5,6 although data are contradictory. 7
Burden of disease
According to Hospital Episode Statistics, there were just under 31,000 finished consultant episodes of women with fibroids of the uterus in 2012 and 2013 in the UK. The majority of these women were aged between 40 and 54 years. 8
Diagnosis of fibroids
Fibroids can occur anywhere in the uterus and vary in size from 1 cm to over 30 cm in diameter. Intramural fibroids, the most common type of fibroid, develop in the myometrium. Subserosal fibroids develop outside the wall of the uterus into the pelvis and can become very large. Submucosal fibroids develop in the myometrium but are visible in the uterine cavity. In some cases, subserosal or submucosal fibroids are attached via narrow stalk of tissue, in which case they are known as pedunculated fibroids. 9
Initial investigations include taking a structured medical history, including menstrual patterns, and a physical examination. Pelvic ultrasound is the first-line diagnostic tool for identifying fibroids and distinguishing them from polyps. Further investigation would involve contrast-enhanced magnetic resonance imaging (CEMRI), which is also extremely helpful in determining suitability for surgical or minimally invasive treatments. 10
Medical treatment for fibroids
Pharmaceutical treatment of HMB is recommended when fibroids are < 3 cm and are causing no distortion of the uterine cavity, with the levonorgestrel-releasing intrauterine system the first-line treatment. Gonadotropin-releasing hormone analogues could be considered if all other treatment options are contraindicated. 10
Ulipristal acetate (UPA) (Esmya; Gedeon Richter plc, Budapest, Hungary) is licensed for preoperative treatment of fibroids and intermittent treatment of fibroid-associated bleeding for a maximum of four cycles of 3 months, with dramatic effects on HMB. 11,12 In March 2020, the European Medicines Agency temporarily suspended UPA treatment for uterine fibroids while a safety review considered the risk of liver injury. A 2018 review concluded that there was a risk of rare but serious liver injury from UPA and introduced measures, including liver function tests, to minimise the risk. 13
With the suspension of UPA, and the previous concerns around endometrial changes arising from use of asoprisnil,14 new progesterone receptor modulators are being investigated. Clinical trials of vilaprisan have shown promise,15 but subsequent studies were halted by the pharmaceutical company16 and it remains to be seen whether or not any further drugs in this class will receive marketing authorisation.
Oral gonadotropin-releasing hormone receptor antagonists are also being investigated for the treatment of fibroid-associated HMB. The leading drug in this class, elagolix (ORILISSA®; AbbVie Inc., Lake Bluff, IL, USA), has a licensed indication for endometriosis-related pain and has been shown in placebo-controlled trials to be effective in reducing HMB when taken with add-back hormonal treatment. 17 Another drug in this class, relugolix (RELUMINA®; Takeda, Tokyo, Japan), has been found to improve fibroid-associated pain in the short term. 18 Finally, linzagolix (Yselty®; ObsEva, Plan-les-Ouates, Switzerland) has been found to rapidly reduce bleeding, but simultaneous add-back treatment was necessary to avoid hot flushes. 19
Women with symptomatic fibroids often respond poorly to drug management or risk unacceptable side effects from hormonal preparations. Therefore, these treatments are often used to relieve symptoms over the short term while awaiting an invasive procedure.
Surgical treatment for fibroids
Surgery, either myomectomy or hysterectomy, has traditionally been the main approach for the management of symptomatic fibroids if medical management is ineffective or for fibroids > 3 cm in size. Myomectomy involves the surgical removal of the fibroid, preserving the uterus, and, although significantly reducing heavy bleeding symptoms, can involve myometrial trauma. Depending on the size and position of the fibroid, myomectomy can be undertaken laparoscopically, hysteroscopically, transvaginally or by a laparotomy.
Non-surgical treatment for uterine fibroids
Uterine artery embolisation (UAE) involves temporary occlusion of the arteries supplying the uterus using biocompatible particles and is usually performed under local anaesthetic. UAE causes ischaemic infarction, from which the uterus usually recovers but the fibroids do not.
The use of UAE in women who may want to conceive is controversial in some sections of the medical community. Some clinicians are concerned by the reduction in blood flow to the ovaries. This reduction in blood flow has been suggested to occur if there are significant communications between the ovarian and the uterine arteries, and this has been proposed to decrease ovarian function. 20 The extent of this decreased function, how long it is maintained for and if it occurs at all are disputed. 21,22
There are other non-surgical minimally invasive interventions that aim to ablate fibroids. According to the National Institute for Health and Care Excellence (NICE), there is adequate evidence of the short-term efficacy of magnetic resonance-guided high-intensity transcutaneous-focused ultrasound (MRgHIFU), but further research, both on long-term outcomes and in women who want to get pregnant, is required. 23 A randomised trial of MRgHIFU compared with myomectomy is under way in Germany (NCT03948789), whereas another trial comparing MRgHIFU with UAE showed a lower reintervention rate and greater improvement in symptoms and quality of life from UAE. 24 NICE considers that the evidence on efficacy of ultrasound-guided high-intensity transcutaneous-focused ultrasound is limited and that the procedure should be used only with special arrangements for consent or within a research setting. 23 Transcervical radiofrequency ablation shows significant clinical improvement up to 2 years post procedure. 25 Microwave ablation is also being evaluated for safety and efficacy. 26
Previous research comparing uterine artery embolisation with surgery
Procedural and symptom relief outcomes
There have been two reported randomised trials27,28 comparing UAE with myomectomy. One single-centre trial from Czechia randomised 121 women with intramural fibroids to be treated by myomectomy or UAE. 27 These authors reported statistically significant reductions in the length of the procedures, hospital stay and recovery period in women who had undergone a UAE. There was a much higher reintervention rate in the UAE group than in the myomectomy group. After a mean follow-up period of about 2 years, 19 out of 58 (33%) women in the UAE group had a subsequent myomectomy, compared with 2 out of 62 (3%) women in the myomectomy group, although myomectomy was recommended if 6 months after the UAE there had been no reduction in the size of the fibroid or there was a fibroid measuring > 5 cm. 28 There was no evidence of a difference in reported symptomatic relief, with both groups demonstrating high rates in both the short and the medium term.
The FUME (Fibroids of the Uterus: Myomectomy versus Embolization) study29 was conducted at St George’s Hospital (London, UK). A total of 160 women with symptomatic uterine fibroids were randomised to UAE or myomectomy. The authors reported that by 1 year there was no difference between groups in the substantial improvement of the quality of life in women. However, the reintervention rate was higher in the UAE group than in those who had undergone a myomectomy (i.e. 9/61 women in the UAE group had a second procedure, compared with 3/73 women in the myomectomy group). 29 Unfortunately, there was substantial attrition in this trial, with no follow-up data on 26% of those randomised.
Five randomised controlled trials (RCTs)30–34 compared UAE with either hysterectomy or a mix of hysterectomy and myomectomy. Of the latter, the REST (Randomised trial of Embolisation versus Surgical Treatment) trial30 randomised a total of 157 women in a 2 : 1 ratio to UAE and either myomectomy or hysterectomy before assessing quality of life at 5 years with the Short Form questionnaire-36 items general health survey. Secondary measures included the frequency of complications and adverse events (AEs) and the need for further intervention. A trial of 127 women in China collected similar outcomes. 31 Meta-analysis of the two studies comparing UAE with hysterectomy or myomectomy showed no difference in the number of repeat interventions [pooled risk ratio (RR) 3.45, 95% confidence interval (CI) 0.18 to 64.35; p = 0.45, I2 = 75%] within 2 years and no evidence of a difference in the proportion of women who after 5 years would recommend their procedure (RR 1.11, 95% CI 0.94 to 1.32; p = 0.16, I2 = 50%). 35
Pregnancy and pregnancy outcomes
Perhaps as a result of the uncertainty of the effect of UAE on fertility, some previous RCTs have not included women wishing to get pregnant,29 and those including hysterectomy in the comparison group uninformative. In a study from Czechia,27,28 there was a significant imbalance in the numbers of women attempting to conceive, with 17 pregnancies reported among 26 women in the UAE group and 32 pregnancies reported among 40 women in the myomectomy group. The authors27,28 also reported that, on average, the women in the myomectomy group had become pregnant sooner postoperatively than the women in the UAE group. Notably, live birth rates were significantly lower among those in the UAE group than among those in the myomectomy group (RR 2.32, 95% CI 1.19 to 4.53; p = 0.01). A small Phase II non-randomised study36 from France enrolled 15 women, of whom nine actively tried to get pregnant in the year following UAE and of whom five conceived and had a live birth. 36
These results and uncertainty around the potential impact of UAE on ovarian and uterine function have resulted in recommendations against UAE for women seeking pregnancy. A recent meta-analysis37 suggested that UAE had no appreciable impact on ovarian reserve, as measured by mean serum concentrations of anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH).
Previous research on the cost and cost-effectiveness of treatments for uterine fibroids
The cost-effectiveness of different interventions for the management of uterine fibroids has been evaluated in nine studies. 30,38–45 These studies utilised data from different sources and one study was based solely on a RCT. 30 Economic studies reporting only cost data were not considered here. The interventions used in these studies consisted of MRgHIFU, hysterectomy, myomectomy, UAE and medical management. Cost-effectiveness was assessed from the perspectives of the payer and the society. Only three studies30,39,40 were specific to the UK setting, the remainder being from Canada, the USA and Hong Kong.
The clinical studies underpinning the economic evaluations sometimes gave inconsistent and conflicting results on the clinical effectiveness and safety. Some of the variation can be explained by small sample size and, particularly in the case of the non-randomised studies, considerable differences in baseline characteristics. For example, those undergoing UAE tended to be older and of higher parity and to have poorer quality of life and larger fibroids. This has a direct impact on the results of the associated economic evaluations.
All studies included UAE as a comparator. Six30,38,39,41–43 of the nine studies also included myomectomy as a comparator. Often, myomectomy was considered to be a second-/third-line treatment43,44 or a treatment option when less-invasive methods failed to improve symptoms. 40 The RCT-based study30 categorised UAE as ‘surgical treatment’, along with hysterectomy.
The results showed UAE to be cost-effective when compared with hysterectomy41,44,45 and myomectomy. 41 However, it was not cost-effective when compared with MRgHIFU. 46 In the study conducted by Wu et al. ,40 UAE was cost-effective compared with hysterectomy over the first year after treatment, but this was no longer the case when adopting a longer time horizon. The RCT-based study supports this result,30 as UAE was associated with a greater resource use and higher costs than the surgical treatments (i.e. hysterectomy and myomectomy) during the 1- to 5-year follow-up. However, within the first year, it was associated with lower costs than the surgical treatments. In another two studies,41,43 myomectomy was found to incur higher costs and provide less health benefit than MRgHIFU and UAE (i.e. myomectomy was dominated by these two procedures). 41,43 By contrast, one study42 found that myomectomy was cost-effective when compared with MRgHIFU if productivity costs were not included. However, even when productivity costs were included, the differences in cost and health benefits between myomectomy and MRgHIFU were small. Similarly, in the same study by Cain-Nielsen et al. ,42 UAE was found to be dominated by myomectomy, but, again, the differences in cost and health benefits were small. A study by You et al. ,38 which directly compared UAE with myomectomy from a Hong Kong society perspective over 5 years, reported similar findings (i.e. myomectomy compared with UAE was cost-effective, but with minimal difference).
National and international guidelines on treatment for fibroids
The Royal College of Obstetricians and Gynaecologists (London, UK) and the Royal College of Radiologists (London, UK) jointly issued clinical practice guidelines during recruitment to FEMME (randomised trial of treating Fibroids with either Embolisation or Myomectomy to Measure the Effect on quality of life). 47 These guidelines47 stated that UAE should be considered alongside surgical treatments, endometrial ablation and medical management for women with symptomatic fibroids. These guidelines47 also highlighted that evidence for fertility and pregnancy outcomes after UAE and myomectomy is limited and that there is no robust comparative evidence. Therefore, the guidelines47 deferred making a recommendation for women with fibroids who might want to become pregnant.
In 2012, the American Association of Gynecologic Laparoscopists (Cypress, CA, USA) published a practice guideline48 solely about submucosal fibroids, stating that UAE and MRgHIFU are not appropriate for women who want to get pregnant, while acknowledging that this recommendation was based on expert opinion alone. The American College of Radiology (Reston, VA, USA) presented a variety of clinical vignettes of women with fibroids and derived appropriateness criteria for each potential treatment strategies for each scenario. 49 The American College of Radiology considers UAE appropriate in all situations and myomectomy in most situations, except where there are multiple submucosal fibroids. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (Melbourne, VIC, Australia) recommendations advise caution and recommends that the routine use of UAE in young women wishing to conceive should be avoided as the effects are uncertain50 again based on expert opinion. We are unaware of the production of any other subsequent guidelines on fibroid management from professional societies in English-speaking countries.
Rationale
Both myomectomy and UAE appear to improve the quality of life of women with symptomatic uterine fibroids, but data derived from high-quality RCTs that would support these very different options are sparse. 51 For symptom control, the choice is currently very uncertain, and indications and clinical preferences for either modality are varied. There is a perception that fertility and pregnancy outcomes are poorer following UAE, but there are few RCT data to support this. 52,53 Given that the options are so different, with very different impact on clinical, patient-reported and economic outcomes, a fair and comprehensive comparison is both overdue and necessary.
Specific objectives
Primary objective
The aim of the FEMME trial was to examine the clinical effectiveness of UAE in comparison with myomectomy in the treatment of symptomatic fibroids. This was achieved by conducting a large, multicentre, open RCT with quality of life as the primary outcome.
Secondary objectives
-
Conduct an economic evaluation to determine the relative cost-effectiveness of these two interventions, at 2 and 4 years after randomisation, from the perspective of the NHS.
-
Explore the relative effectiveness of the interventions on HMB symptoms.
-
Explore differences in pregnancy rates and outcomes in women seeking pregnancy.
-
Compare AEs and complications of the two interventions.
-
Compare reintervention rates.
-
Investigate whether or not presenting characteristics have an impact on the comparative clinical effectiveness of the interventions.
-
Measure hormones associated with ovarian reserve following treatment and compare these between the women in each study group.
Chapter 2 Methods for the randomised controlled trial
Parts of this chapter have been reproduced with permission from McPherson et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Design
The FEMME trial was a randomised, open, parallel, multicentre trial of UAE and myomectomy in women with symptomatic uterine fibroids wishing to avoid a hysterectomy. The trial had a favourable ethics opinion from National Research Ethics Service Committee Coventry and Warwickshire (reference 11/WM/0149).
Trial oversight
Trial oversight and monitoring were provided by a Trial Steering Committee (TSC) and by an independent Data Monitoring Committee.
The TSC provided independent supervision for the trial, providing advice to the chief investigator, co-investigators and the sponsor on all aspects of the trial throughout the trial. The Data Monitoring Committee adopted the DAMOCLES charter54 to define its terms of reference and operation in relation to oversight of the FEMME trial. Both the TSC and Data Monitoring Committee met on an approximately annual basis during the period of recruitment and follow-up.
Participants
The participants in the FEMME trial were recruited in gynaecology and interventional radiology clinics in a diverse range of NHS hospitals located across the UK. Hospitals could participate if their patient pathway allowed eligible women to undergo either myomectomy or UAE, whether at the same hospital, in another hospital within the same trust/board or under an arrangement with another NHS trust/board.
Screening of potential participants
A full gynaecological and general history was taken by the gynaecologist alongside a general and pelvic examination. The initial diagnosis of fibroids was informed by the woman’s medical history and symptoms, and was confirmed by transabdominal or transvaginal ultrasound scan (TVUS) or hysteroscopy.
If fibroids were visible and considered to be the likely cause of symptoms, and the felt that the women could respond equally well to UAE or myomectomy, then the clinician made the initial approach to the woman to discuss treatment options and participation in the FEMME trial. Women who were referred to a recruiting gynaecologist with a diagnosis then received written information about the FEMME trial before their appointment. If a woman expressed an interest in taking part in the FEMME trial, an approved member of the local research team discussed the trial with them and provided written information. Women who consented to participate in the FEMME trial were provided with a baseline questionnaire and a diary to estimate their menstrual blood loss and were asked to use this to record when they started their next menstrual period (prior to randomisation). Prior to treatment, most women underwent magnetic resonance imaging (MRI), CEMRI ideally, to enable the gynaecologist to accurately visualise the uterus and locate any fibroids.
Eligibility criteria
Women were eligible for randomisation if they met all inclusion criteria and no exclusion criteria.
Inclusion criteria
-
Women with symptomatic fibroids who did not wish to have a hysterectomy, but who were prepared to accept one in an emergency.
-
Women suitable for, and accepting of, either treatment (i.e. myomectomy or UAE).
-
Women for whom the clinical team were uncertain as to which treatment was indicated.
-
Women who provided written informed consent.
Exclusion criteria
-
Women who refused a hysterectomy, even if an intraoperative complication made this an advisable procedure.
-
Women with recent or ongoing pelvic inflammatory disease.
-
Women with significant adenomyosis, as identified by TVUS or CEMRI. (Women with concurrent adenomyosis were eligible if fibroids were believed to be the predominant cause of their symptoms.)
-
Women with a positive pregnancy test just before consent.
-
Women who were postmenopausal, as defined as > 1 year since previous menstrual period.
-
Women with suspected malignancy.
-
Women aged < 18 years.
-
Women who were unable to provide informed consent because of incapacity [as defined by the Mental Capacity Act 200555 or the Adults with Incapacity (Scotland) Act 200056].
-
Non-English-speaking women for whom translation or interpretation facilities were insufficient to guarantee informed consent.
-
Women who had previously undergone myomectomy via a laparotomy or had previously undergone embolisation.
Recruitment
In the majority of cases, CEMRI was performed to confirm the presence of fibroids, to determine whether or not the fibroids were amenable to treatment by either myomectomy or UAE and to rule out any other pathologies. Consent was obtained in writing from confirmed eligible women who agreed to participate in the trial. The recruitment pathway is summarised in Figure 1.
FIGURE 1.
Eligibility pathway to recruitment and randomisation. BCTU, Birmingham Clinical Trials Unit; GnRH, gonadotropin-releasing hormone; PIS, participant information sheet; TAUS, transabdominal ultrasound.
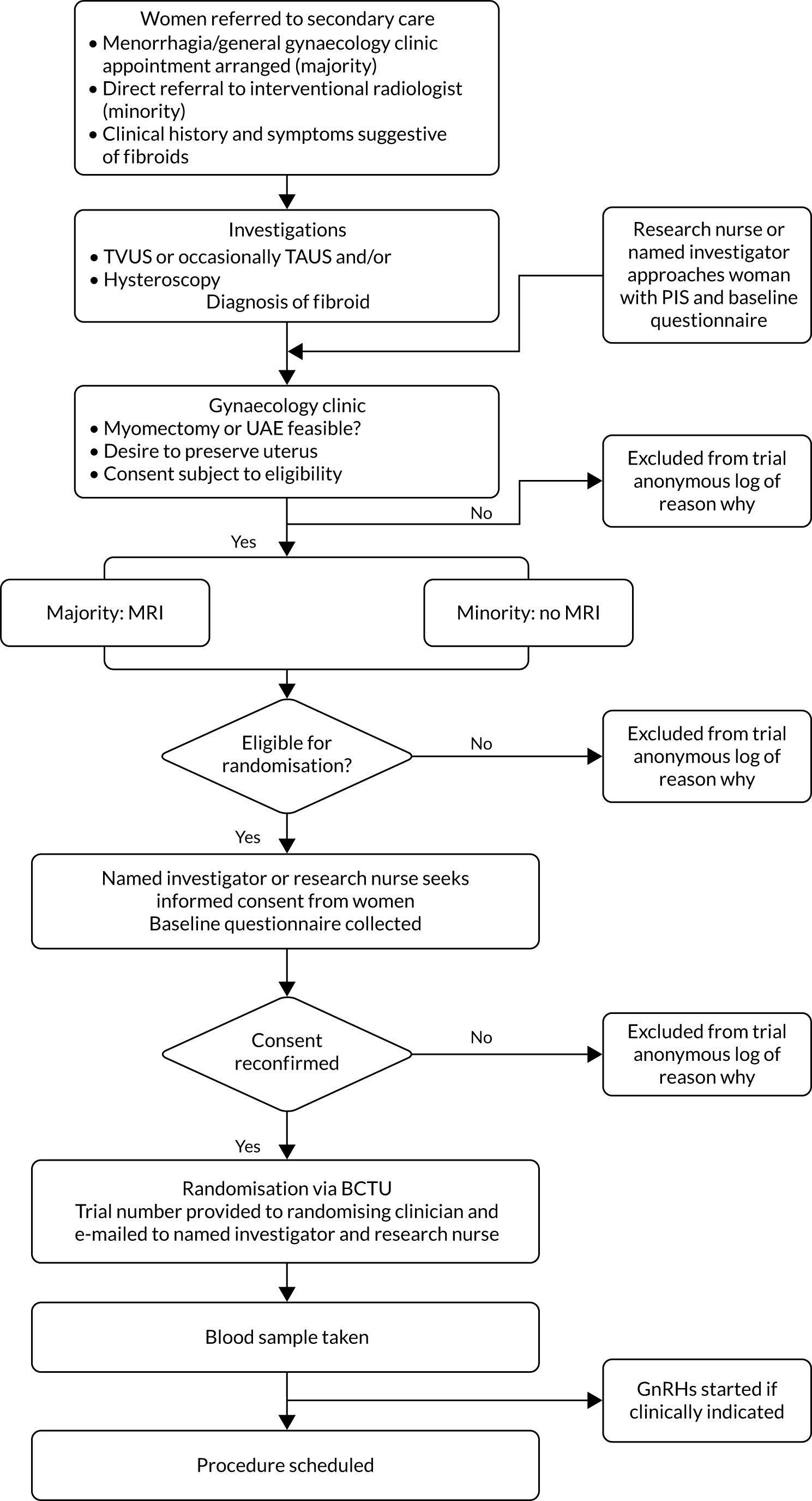
Participating sites were asked to record the number of women who were initially eligible but later excluded or women who were eligible but who declined consent, alongside the reason for non-inclusion.
Randomisation
Allocation concealment
Participants were randomised via a secure, centralised, online randomisation system provided by the University of Birmingham Clinical Trials Unit. No allocation could be given until all participant entry criteria were confirmed by the local study team.
Sequence generation and minimisation
Participants were randomised to undergo myomectomy or UAE in a 1 : 1 ratio. A minimisation procedure using a computer-based algorithm was used to avoid chance imbalances in important stratification variables. The stratification variables used for minimisation were:
-
longest dimension of largest fibroid (≤ 7 cm or > 7 cm)
-
number of fibroids present (1–3, 4–10 or > 10)
-
women desires pregnancy (yes or no).
The choice of subgroups was informed by data from the UK Fibroid Registry, which indicated that 45% of women have fewer than four fibroids and in 50% of women the dominant fibroid is ≤ 7 cm at the time of intervention.
Baseline information
The following demographic and clinical criteria were requested on each randomised participant:
-
age at randomisation
-
ethnicity, if declared, using standard NHS categories
-
height and weight
-
number of fibroids present (estimated if necessary)
-
location of largest fibroid and its dimensions
-
presence of ovarian pathology
-
uterine dimensions
-
parity and gravidity
-
current use of contraceptives, hormonal treatments or other treatments for HMB
-
previous abdominal surgery.
Baseline blood sample
After randomisation, but before treatment, 5–10 ml of blood was collected into a serum separation Vacutainer tube (Beckton Dickinson UK Ltd, Oxford, UK). This blood sample was sent to the University of Birmingham’s Biobank, where it was transformed into serum on receipt. The serum sample was aliquoted and then stored at –70 °C until assay.
Interventions
Following randomisation, the allocated treatment was scheduled for the woman in accordance with local hospital practice. A negative pregnancy test was required immediately prior to the intervention being performed. Pre-myomectomy treatment of fibroids using gonadotropin-releasing hormone analogues and, later, pre-treatment with UPA could be initiated at the discretion of the gynaecologist, but was not recommended. Immediate pre-procedure use of any medical treatment or hormonal contraceptive to reduce HMB was recorded.
Myomectomy
Myomectomy was performed using the technique preferred by the operating gynaecologist (i.e. open, hysteroscopic or laparoscopic). If thought appropriate, and in consultation with the woman, the gynaecologist could morcellate the fibroid in accordance with local guidelines. The use of morcellation was not recorded.
Uterine artery embolisation
Uterine artery embolisation was performed by an interventional radiologist experienced in the technique. Bilateral selective catheterisation and embolisation of the uterine arteries was performed under fluoroscopic guidance, either in a single procedure or in two-staged unilateral procedures. The angiographic end point of the embolisation procedure was complete or near-complete stasis of blood flow in the uterine artery. The embolic agent used was at the discretion of the interventional radiologist, but was required to carry a Conformitè Europëenne (CE) mark.
Other treatments
The trial did not preclude gynaecologists performing other appropriate procedures (e.g. adhesionolysis) at the time of the allocated procedure. Basic details regarding these additional procedures were recorded.
Participants were free to choose the brand and type of sanitary protection that they used. Participants were free to use any prescribed or over-the-counter pharmacological agents that they required.
Where the woman withdrew her consent to proceed with the allocated treatment, or where immediate preoperative concerns precluded her from proceeding, this was recorded.
Withdrawal from the trial
Participants could voluntarily withdraw their consent to trial participation at any time. It was reiterated to the investigator and participant that non-compliance with the allocated procedure did not mean withdrawal from the trial, and that some procedural data and all participant-reported outcomes would be requested. If a participant did not return for a scheduled visit for follow-up scans or blood samples, numerous attempts by a variety of methods were made to contact her and (where possible) to review compliance and AEs. The reason(s) for self-withdrawal were documented where possible. If participants did not respond to postal questionnaires, attempts were made to contact them by telephone, their general practitioner (GP), their gynaecologist or an approved member of the local research team. Non-response was not considered withdrawal of consent. If a participant explicitly withdrew consent to any further data recording, then this decision was respected and recorded. All communications surrounding the withdrawal were noted in study records and no further data were collected for such participants.
Blinding
Owing to the very different natures of the interventions, it was not possible to blind participants, investigators, research nurses and other attending clinicians to the trial treatment allocation.
Outcomes and assessment
Primary outcome
The primary outcome was the participant-reported health-related quality-of-life (HRQoL) domain of the Uterine Fibroid Symptom Quality of Life (UFS-QOL) tool. 57 The HRQoL domain contains 29 questions over the following six subscales: (1) concern, (2) activities, (3) energy/mood, (4) control, (5) self-consciousness and (6) sexual function. Responses for the HRQoL questions ranged from ‘none of the time’ to ‘all of the time’. Response options for the eight-item severity subscale ranged from ‘not at all’ to ‘a very great deal’. Scores ranged from 0, indicating worst HRQoL, to 100, indicating the best HRQoL. Participants were asked to respond considering their experiences over the previous 3 months. The instrument demonstrates face, construct and discrimination validity and has been demonstrated to be responsive to change. 58,59 The HRQoL score at 2 years of follow-up was the primary outcome. Two years was considered an appropriate medium-term time point, sufficiently distant from the procedure to observe a stable effect, but also able to capture any recurrence of symptoms.
Secondary outcomes
Prespecified secondary outcomes were as follows (see Data collection schedule for assessment times):
-
The HRQoL domain from the UFS-QOL at the other time points (that were not the primary outcome assessment time).
-
The symptom severity domain from the UFS-QOL [scores ranged from 0 (no symptoms) to 100 (worst symptoms)].
-
EuroQol-5 Dimensions, three-level version (EQ-5D-3L), score [on a scale of –0.59 (worst outcome) to 1.0 (best outcome)]. 60
-
EuroQol-5 Dimensions (EQ-5D) visual analogue scale (VAS) [on a scale of 0 (worst outcome) to 100 (best outcome)]. 60
-
Menstrual blood loss using the pictorial bleeding assessment chart (PBAC)61 [on a scale of 0 (no bleeding) as a minimum, but with no fixed upper limit]. This is a validated and well-used assessment of menstrual blood loss in women with uterine fibroids. Further analysis on PBAC scores was carried out by categorising blood loss as amenorrhoea (a score of 0) or light (scores > 0 to ≤ 10), normal (scores > 10 to ≤ 100) or heavy (scores > 100) bleeding. The last cut-off point was chosen because it is known to have good predictive ability for heavy bleeding61 and can also be used to generate rates of amenorrhoea and non-heavy bleeding (defined as a score of < 100).
-
Pregnancy and associated outcomes, specifically the ability to conceive (i.e. overall and in the population who, at the time of randomisation, reported that they wanted to get pregnant) and the subsequent outcome (i.e. live birth, miscarriage, stillbirth and termination). Pregnancy could be reported to the study office either by the women or by a member of the local study team in the first instance.
-
Participant acceptability, as defined by responses to ‘Would you have your operation again?’.
-
Participant acceptability, as defined by responses to ‘Would you recommend operation to a friend?’.
-
Length of hospital stay.
-
Further treatment for fibroids (including hysterectomies) or recurrence of symptoms.
-
Measure of ovarian reserve by assay of FSH, AMH and luteinising hormone (LH). See Ovarian reserve tests for details of the assay.
-
Serious adverse events (SAEs) and procedural complications considered to be related to the study protocol or intervention were collected (see Ovarian reserve tests). SAEs are defined in Serious adverse events.
Ovarian reserve tests
Anti-Müllerian hormone and day 2–4 plasma levels of the gonadotropins FSH and LH were determined. At the time that blood samples were drawn, the woman was asked if she was on day 2, 3 or 4 of her menstrual cycle. Samples were processed as described in Baseline blood sample. The serum aliquots were sent separately in two batches on dry ice to the laboratories of the University of Glasgow (Glasgow, UK) for analysis.
Resource use outcomes
Resource use outcomes are detailed in Chapter 5.
Data collection schedule
The trial addressed outcomes over three time frames, with outcome measures described in Primary outcome and Secondary outcomes. The data collection schedule is summarised in Table 1 and below:
-
Short-term data – immediate impact of UAE and myomectomy on fertility potential up to 1 year, immediate postoperative AEs, resources used in diagnosis and intervention. Operative details and complications will be collected in two time frames: (1) up to discharge from hospital and (2) from discharge to 6 weeks post procedure.
-
Medium-term data – up to 2 years for symptom-specific and generic quality-of-life outcomes and initial cost-effectiveness, fertility potential and pregnancy rates.
-
Long-term data – up to 4 years for pregnancy and further treatment rates, together with quality-of-life outcomes.
| Outcome measure | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Prior to randomisation | Before discharge | 6 weeks | 6 months | 1 year | 2 years | 4 years | |
| UFS-QOL | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| EQ-5D-3L and VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| PBAC | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Pregnancy and associated outcomes | ✗ | ✗ | |||||
| Participant acceptability | ✗ | ✗ | ✗ | ✗ | |||
| Fertility potential (hormonal ovarian reserve) | ✗ | ✗ | ✗ | ✗ | |||
| Resource use (clinical) | ✗ | ✗ | ✗ | ||||
| SAEs | Throughout | ||||||
| Further treatment | ✗ | ✗ | |||||
Sample size
The original sample size was 650 participants. This would have provided 90% power (at a two-sided alpha level of 0.05) to detect a small to moderate difference of 8 points [i.e. 0.29 of a standard deviation (SD)] in the primary outcome, allowing for approximately 20% loss of primary outcome data. There is no validated minimally important difference for the UFS-QOL scale58 and, therefore, this target difference to detect was considered meaningful and plausible based on the results of a similar published study. 29
Following slower than anticipated recruitment to the trial, and with access to individual participant UFS-QOL data from a previous study,12,29 the sample size target was revised to 250 participants in October 2013, when 114 women had been randomised. A re-analysis of these data, using more appropriate regression methods accounting for baseline imbalances, suggested that a larger difference of 12 points was attainable and that the pooled-group SD of UFS-QOL scores was slightly lower than that originally estimated. The revised sample size had 90% power to detect a moderate-sized difference between groups (i.e. 0.55 of a SD). The Data Monitoring Committee and TSC, which remained blind to contemporaneous primary outcome data, approved the changes on the grounds that this revised effect difference was plausible.
Statistical methods
A comprehensive statistical analysis plan was drawn up prior to the 2-year analysis and was provided to the independent TSC for review. A summary of the analytical approaches is described here.
The analysis of the primary outcome was performed in accordance with the intention-to-treat (ITT) principle. Analyses were performed on (1) complete observed data and (2) all randomised participants at all assessment times through imputation of missing responses. Repeated-measures linear regression models, including data at all time points, were used to estimate least-square mean differences (with 95% two-sided CIs) in the primary outcome at 2 years. 57,62 Parameters allowing for participant, treatment group, baseline score, time, time-by-treatment interaction and the minimisation variables were included. For analysis 1, participants were included in the model provided that they had at least one response at any of the three assessment times. For analysis 2, multiple imputation using a Markov chain Monte Carlo method, assuming a joint multivariate normal distribution, was used. The imputation model was consistent with the analysis model in terms of the variables used. All response times were included. 63
For the primary outcome, a p-value was generated through the aforementioned linear regression model. Observed data from secondary continuous outcomes were analysed in a similar fashion to the primary outcome. Reproductive hormone levels were log-transformed and, therefore, for ease of interpretation, are presented as geometric mean ratios. Log-binomial regression was used to estimate relative rates and 95% CIs for binary outcomes, making similar adjustments to the other analyses. The widths of the CIs were not adjusted for multiplicity and, therefore, the intervals should not be used to infer definitive treatment effects.
Several sensitivity analyses for the primary outcome were also performed, including inclusion of time as a continuous linear predictor, assuming no interaction with treatment; addition of a parameter for treating hospital; and a per-protocol analysis, including only those who received the allocated treatment. Some questionnaires were incomplete and, therefore, an additional sensitivity analysis used available subscale scores to generate an overall score.
For 4-year data, continuous outcomes were analysed by adding responses at this time point to the aforementioned regression models. For time to first pregnancy and time to first further procedure for treatment of fibroids, a Cox proportional hazard model was carried out, adjusting for the minimisation variables. Kaplan–Meier plots were produced in which women were censored if they had withdrawn, were lost to follow-up or had undergone a hysterectomy.
We analysed the treatment effect on the primary outcome in prespecified subgroups that matched the minimisation variables. These analyses involved adding the subgroup-by-treatment group interaction parameters to the linear regression model. All analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA) software, version 9.4.
The Data Monitoring Committee reviewed accruing safety data during the period of recruitment.
Serious adverse events
Serious adverse events were defined as AEs that are attributable to the study protocol or study interventions and caused or resulted in any of the following:
-
death
-
life-threatening complications
-
inpatient hospitalisation or prolongation of existing hospitalisation
-
persistent or significant disability or incapacity
-
congenital anomaly/birth defect
-
intervention to prevent disability or incapacity.
For each SAE, the following information was requested:
-
full details in medical terms with a diagnosis, if possible
-
the duration
-
any action taken
-
the outcome
-
causality in the opinion of the investigator and in relation to the study protocol or intervention received and expectedness.
An assessment of causality and expectedness of the SAE in relation to the study protocol or intervention was made by a clinician at the randomising centre. The information was also assessed by one of the clinical lead investigators, although the clinical lead investigator could not contradict any assessment that a SAE had a reasonable causal relationship with the trial intervention.
Participants were asked to report hospitalisations when followed up by the study team. Reported events were followed up in conjunction with the randomising hospital to ascertain if the event constituted a SAE.
The outcome of pregnancies reported to the study was collected by a member of the local research team, although women could also report pregnancies and their outcomes in the participant questionnaires and directly to the FEMME trial office.
Chapter 3 Clinical effectiveness results: postoperative and 2-year follow-up data
Parts of this chapter have been reproduced with permission from Manyonda et al. 64 Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
This chapter reports the clinical results of the FEMME trial, including postoperative and follow-up data to 2 years post randomisation. The chapter starts with a description of the flow of participants through the trial and is followed by demographic information and results of the primary and secondary outcome measures, and postoperative complications.
Recruitment of participants
The randomisation of participants commenced on 6 February 2012 and the last woman was randomised on 21 May 2015. The monthly rate of recruitment of participants into the FEMME trial is shown in Table 2. Participants were recruited from 29 hospitals across England, Scotland and Wales, as shown in Figure 2.
| Recruiting centre | Number (%) of patients randomised |
|---|---|
| St George’s Hospital | 102 (40) |
| Glasgow Royal Infirmary | 36 (14) |
| Royal Infirmary of Edinburgh | 25 (10) |
| Queen’s Hospital, Romford | 14 (6) |
| John Radcliffe Hospital | 10 (4) |
| St Thomas’ Hospital | 10 (4) |
| Birmingham Women’s Hospital | 6 (2) |
| Birmingham Heartlands Hospital | 5 (2) |
| Mayday University Hospital | 5 (2) |
| St Mary’s Hospital | 5 (2) |
| City General Hospital (University Hospital of North Staffordshire) | 3 (1) |
| City Hospital Birmingham | 3 (1) |
| East Surrey Hospital | 3 (1) |
| Leicester General Hospital | 3 (1) |
| The Royal Victoria Infirmary | 3 (1) |
| Royal Blackburn Hospital | 3 (1) |
| Luton and Dunstable Hospital | 2 (1) |
| Neath Port Talbot Hospital | 2 (1) |
| Queen’s Medical Centre | 2 (1) |
| St Helier Hospital | 2 (1) |
| York Hospital | 2 (1) |
| City Hospitals Sunderland | 1 (< 1) |
| Crosshouse Hospital | 1 (< 1) |
| Leeds General Infirmary | 1 (< 1) |
| Ninewells Hospital | 1 (< 1) |
| Norfolk and Norwich University Hospital | 1 (< 1) |
| Royal Free Hospital | 1 (< 1) |
| Royal Hallamshire Hospital | 1 (< 1) |
| St Peter’s Hospital | 1 (< 1) |
| Total | 254 |
FIGURE 2.
Recruitment of participants to the FEMME trial over time.
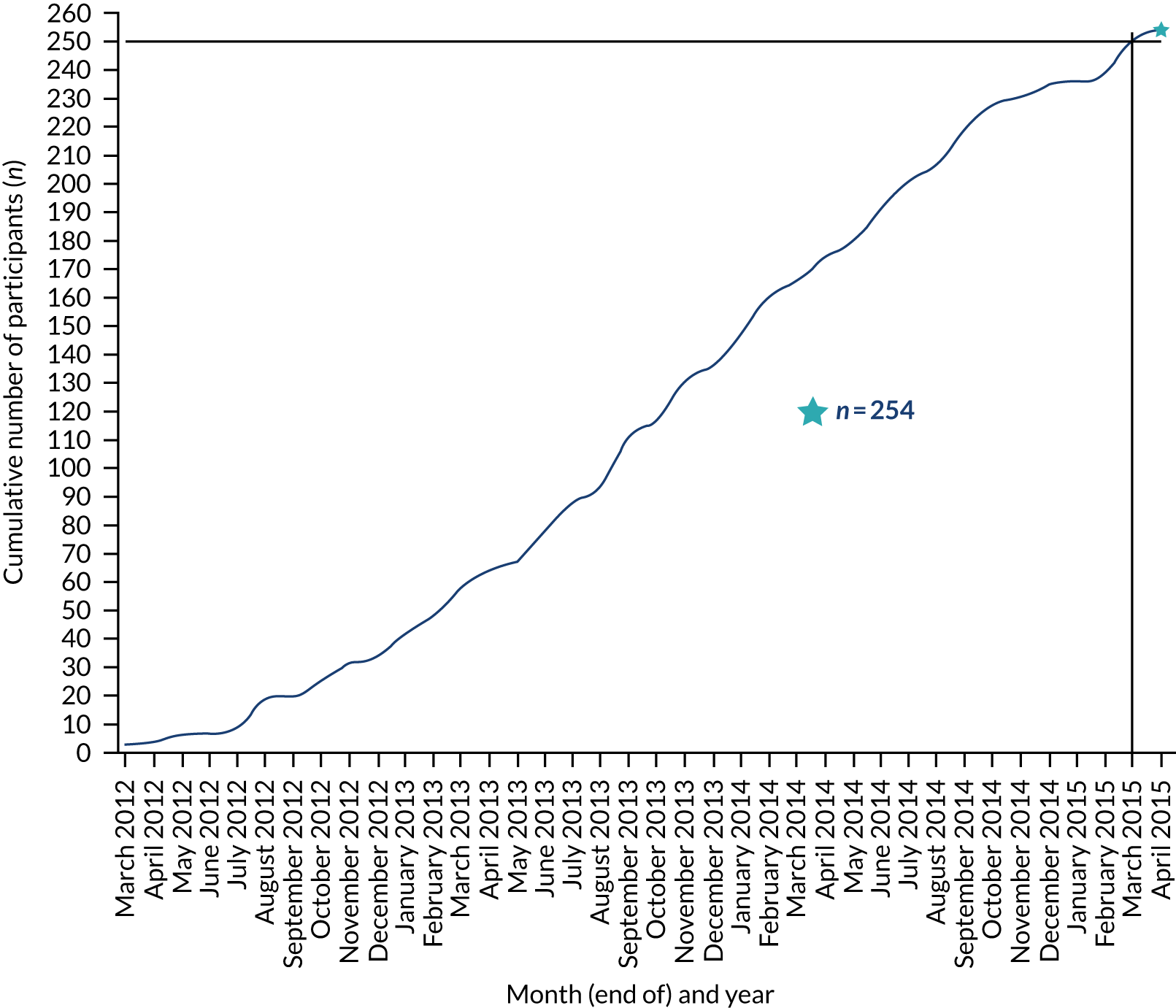
Information was sought for reasons why women declined their invitation to participate in the FEMME trial up to March 2014, at which point 170 women had been randomised. Not every participating hospital provided data on the number of women declining participation and, even among hospitals that did, many did not do so consistently and, therefore, the ratio of women randomised to women declining participation cannot be reliably calculated. Data on 335 women who were assumed to be eligible for randomisation and who declined participation are available. Of these 335 women, 84 (25.1%) requested UAE, 79 (23.6%) requested a myomectomy and 56 (16.7%) requested a hysterectomy. The remaining 116 (34.6%) women gave either another reason or no reason. No accurate information is available on the number of women who were initially approached for participation but were ultimately found to be ineligible for randomisation.
Participant follow-up within 2 years
A total of 127 women were assigned to myomectomy and 127 women were assigned to UAE (Figure 3). The follow-up rate for the primary outcome was 206 out of 254 (81%) women at 2 years (see Figure 3). A total of 227 (89%) women provided scores at least at one assessment time.
FIGURE 3.
Flow of participants through the FEMME trial up to 2 years of follow-up. LTFU, lost to follow-up. Manyonda et al. 64 Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
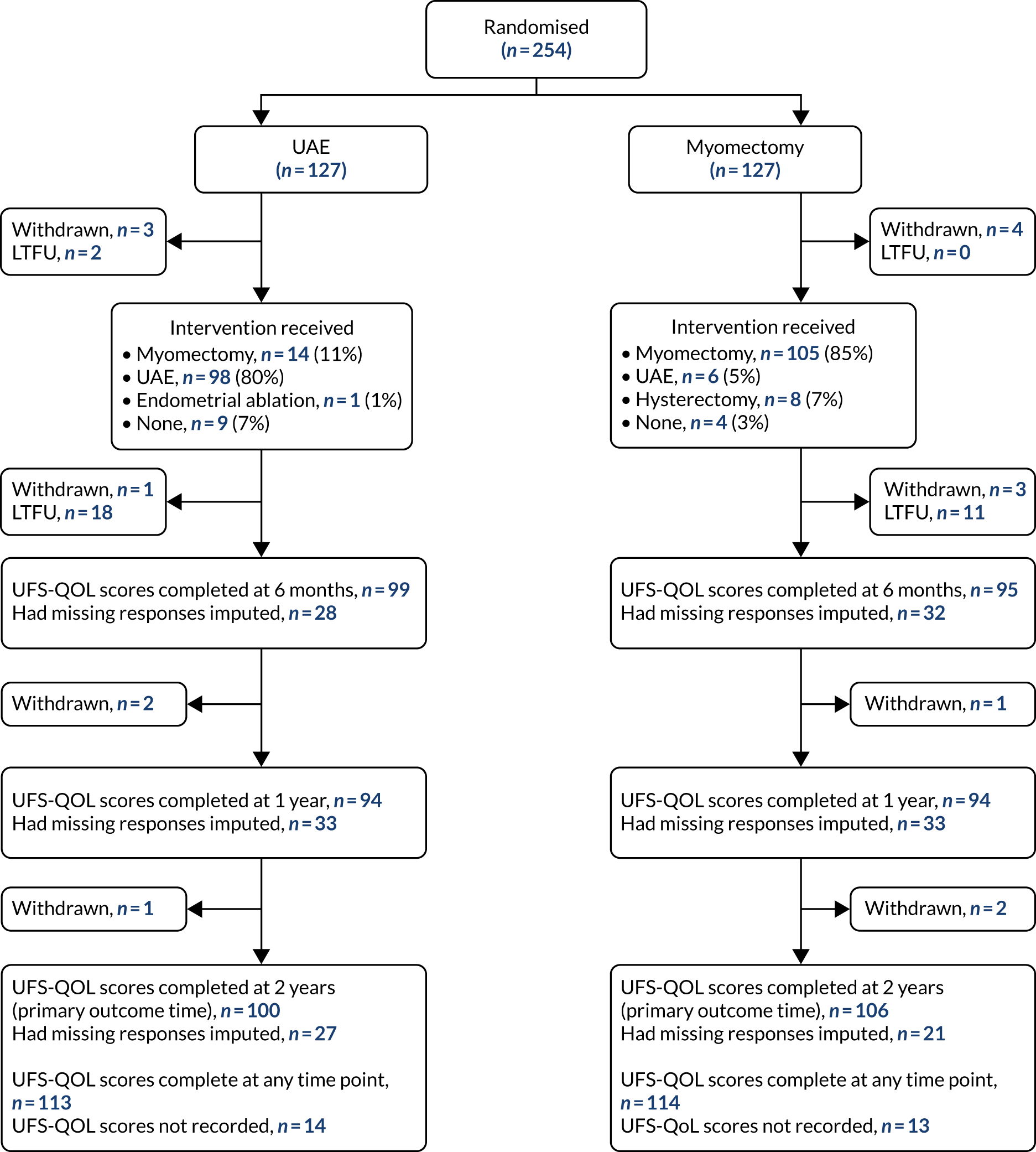
Compliance to treatment allocation
Of the 123 women who were randomised to myomectomy and who did not withdraw from the trial prior to the procedure, 105 (85%) had a myomectomy as their initial operation. Similarly, 98 of the 122 (80%) women in the UAE group underwent UAE. Four women in the myomectomy group and nine women in the UAE group chose not have either procedure initially. The initial procedures following randomisation for each allocation group are shown in Figure 4.
FIGURE 4.
Compliance with treatment allocation, by group. LTFU, lost to follow-up. Manyonda et al. 64 Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
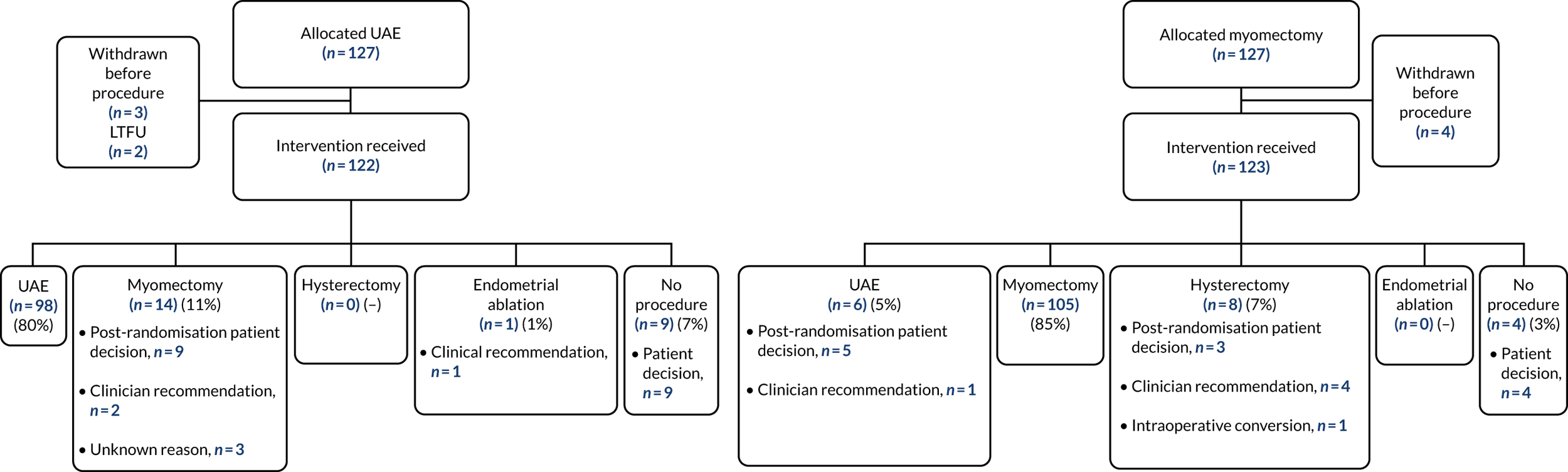
Of those participants who crossed over groups on the basis of a clinical recommendation after randomisation (as opposed to a participant-driven decision), one participant in the myomectomy group was found to have adenomyosis (alongside fibroids) and was offered UAE and two women in the UAE group had a myomectomy (one woman because of heavy bleeding and anaemia and one for an unknown reason).
Four women in the myomectomy group underwent hysterectomy: one because of suspected pelvic infection, one because MRI showed no fibroids but, instead, a large area of adenomyosis, one because she was admitted with acute abdominal pain and one because the surgeon was unable to perform myomectomy. There was one emergency conversion to hysterectomy during a laparoscopic myomectomy following a massive haemorrhage. In the UAE group, one woman underwent a transcervical resection of her fibroids instead.
Baseline characteristics of trial participants
The baseline characteristics of the trial participants are shown in Table 3. Women were, on average, 41 years old and classed as overweight by their body mass index. Although 48% of participants in both groups responded positively to the question ‘at this time, are you seeking to get pregnant?’, about 58% of women were taking treatments, including contraceptives and hormones, to control their HMB or shrink their fibroids.
| Baseline characteristic | UAE group (N = 127) | Myomectomy group (N = 127) |
|---|---|---|
| Demographics and obstetric history | ||
| Age (years), mean (SD), n | 40.2 (6.55), 127 | 42.7 (6.4), 127 |
| Ethnic group, n (%) | ||
| White (British/other) | 59 (46) | 57 (45) |
| Black (Caribbean/African/other) | 48 (38) | 54 (43) |
| South Asian (Indian/Pakistani/Bangladeshi) | 10 (8) | 5 (4) |
| Mixed (white/black/Asian/other) | 6 (5) | 8 (6) |
| Other | 4 (3) | 3 (2) |
| BMI (kg/m2), mean (SD), n | 28.2 (6.2), 119 | 28.1 (5.3), 123 |
| Desiring pregnancy at time of randomisation, n (%)a | 61 (48) | 61 (48) |
| Parity, median (IQR), n | 0 (0–1), 125 | 1 (0–2), 127 |
| Gravidity, median (IQR), n | 1 (0–2), 125 | 2 (0–3), 127 |
| Fibroid assessment | ||
| Imaging modality to diagnose fibroid, n (%)b | ||
| MRI | 89 (70) | 99 (78) |
| Ultrasound | 36 (28) | 27 (21) |
| Not stated | 2 (2) | 1 (1) |
| Location of largest fibroid, n (%) | ||
| Submucosal | 6 (5) | 14 (11) |
| Submucosal (pedunculated) | 1 (1) | 1 (1) |
| Subserosal | 30 (24) | 21 (17) |
| Subserosal (pedunculated) | 6 (5) | 5 (4) |
| Intramural | 74 (58) | 81 (64) |
| Other | 4 (3) | 0 |
| Not stated | 6 (5) | 5 (4) |
| Longest dimension of largest fibroid (cm)a | ||
| ≤ 7, n (%) | 64 (50) | 64 (50) |
| > 7, n (%) | 63 (50) | 63 (50) |
| Mean (SD) | 7.6 (3.2) | 7.7 (4.2) |
| Number of fibroidsa | ||
| 1–3, n (%) | 84 (66) | 84 (66) |
| 4–10, n (%) | 37 (29) | 37 (29) |
| > 10, n (%) | 6 (5) | 6 (5) |
| Median (IQR) | 2 (1–5) | 2 (1–5) |
| Largest fibroid volume (cm3), mean (SD), n | 436 (594), 124 | 446 (548), 126 |
| Uterine volume (cm3), mean (SD), n | 1170 (1280), 118 | 1240 (1120), 118 |
| Medical and surgical history | ||
| Previous abdominal surgery, n (%)c | ||
| Caesarean section | 12 (9) | 19 (15) |
| Laparoscopy | 19 (15) | 15 (12) |
| Endometrial ablation | 3 (2) | 2 (2) |
| Appendectomy | 8 (6) | 7 (6) |
| Sterilisation | 4 (3) | 5 (4) |
| Other | 10 (8) | 15 (12) |
| Taking contraceptive/hormonal treatments to control symptoms at randomisation, n (%) | 75 (59) | 73 (57) |
Procedural details
The details of the initial procedures undertaken are shown in Table 4. Combinations of procedure specifics were possible, according to the clinical situation: one participant from the UAE group had a hysteroscopic myomectomy and then went on to have an open myomectomy; one participant from the myomectomy group had a laparoscopic myomectomy that was converted to an open myomectomy; and six participants from the myomectomy group had a hysteroscopic myomectomy and then went on to have an open myomectomy.
| Procedure | UAE group (N = 113) | Myomectomy group (N = 119) |
|---|---|---|
| Time from randomisation to procedure (weeks), median (IQR), n | 13 (9–20), 113 | 14 (9–20), 119 |
| Length of time of intervention (leaving/entering theatre or suite) (minutes), median (IQR), n | 90 (60–110), 94 | 120 (94–150), 105 |
| Length of hospital stay (days to discharge), median (IQR), n | 2 (2–3), 112 | 4 (3–5), 117 |
| Procedure undertaken: UAE | n = 98 | n = 6 |
| In the opinion of radiologist, successful embolisation of both arteries? n (%) | ||
| Yes | 92 (94) | 5 (83) |
| No | 4 (4) | 0 |
| Missing | 2 (2) | 1 (17) |
| If no, what is the plan? n (%) | ||
| Repeat UAE at a later date | 1 (1) | 0 |
| Unknown | 3 (3) | 0 |
| 6-month post-procedure assessment scan: radiologist opinion of fibroid infarction, n (%) | ||
| Complete (100%) | 32 (40) | 3 (75) |
| Near complete (≥ 90%) | 26 (33) | 0 |
| Incomplete (< 90%) | 22 (28) | 1 (25) |
| Missing | 18 | 2 |
| Procedure undertaken: myomectomy | n = 14 | n = 105 |
| Type of myomectomy, n (%) | ||
| Laparoscopic | 4 (29) | 10 (10) |
| Hysteroscopic | 2 (14) | 9 (9) |
| Open abdominal | 9 (64) | 93 (89) |
| Laparoscopic converted to open abdominal | 0 | 1 (1) |
| Hysteroscopic converted to open abdominal | 1 (7) | 6 (6) |
The vast majority of the UAE procedures were successfully completed in the opinion of the radiologist at the time of procedure. However, among those women who had MRI after 6 months, around one-quarter had fibroids that were considered to be < 90% infarcted.
Primary outcome results
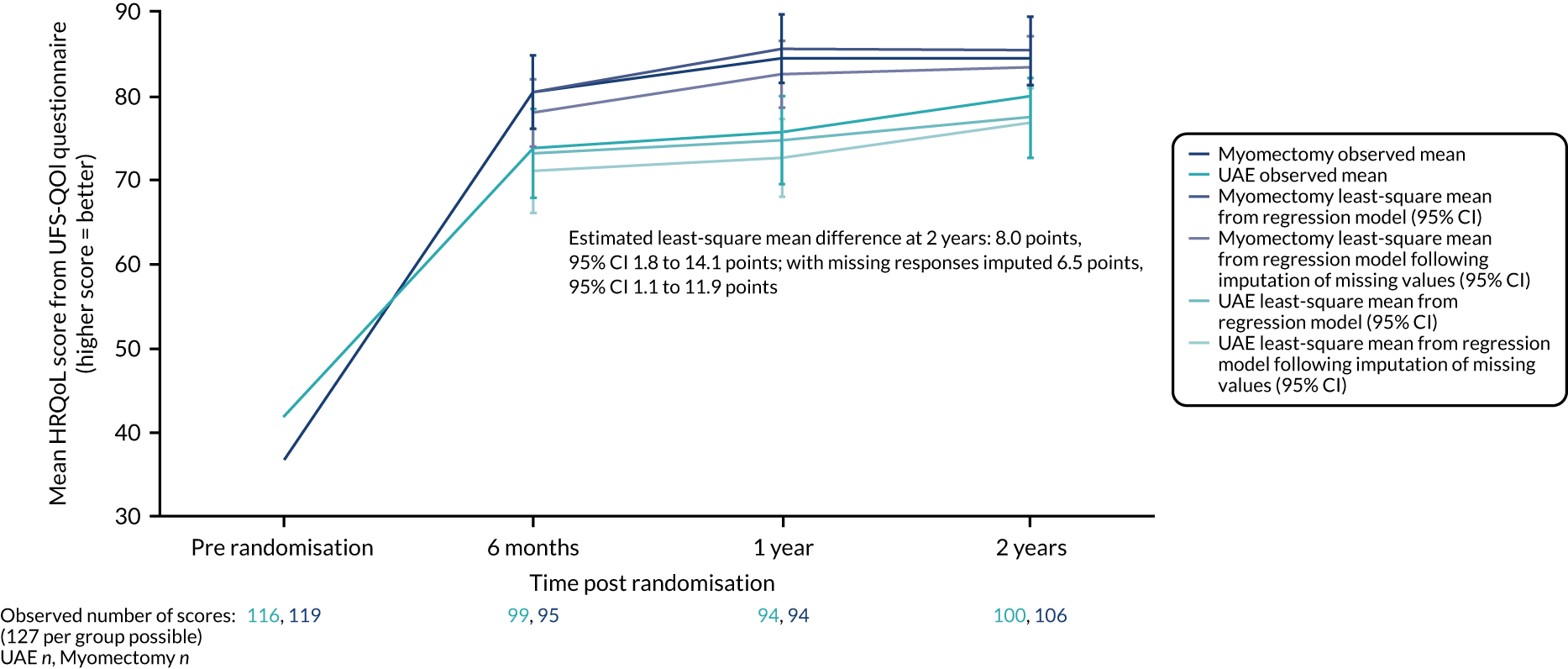
The average HRQoL score at 2 years was substantially improved in both groups, approximately doubling in each group, but these improvements were greater at 2 years in those assigned to the myomectomy group (mean difference from observed data 8.0 points, 95% CI 1.8 to 14.1 points, p = 0.01; mean adjusted difference with missing responses imputed 6.5 points, 95% CI 1.1 to 11.9 points). Significant results were seen at earlier time points, as shown in Table 5 and Figure 5.
| Time point | Mean (SD) score, n | Estimated mean difference from observed data (95% CI)a,b | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| Baseline | 42.1 (26.4), 116 | 37.0 (23.9), 119 | |
| 6 months | 73.9 (26.7), 99 | 80.5 (21.7), 95 | 7.4 (0.5 to 14.2) |
| 1 year | 75.7 (26.1), 94 | 84.7 (22.1), 94 | 10.8 (4.2 to 17.5) |
| 2 years | 80.0 (22.0), 100 | 84.6 (21.5), 106 | 8.0 (1.8 to 14.1) |
FIGURE 5.
Evolution over mean responses over time of UFS-QOL HRQoL scores by group. Manyonda et al. 64 Copyright © 2020 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Sensitivity and subgroup analyses of the primary outcome
Sensitivity analyses
A number of sensitivity analyses were conducted for the primary outcome. These analyses returned estimates of mean differences that were between 6.5 and 8 points, with CIs ranging from 0 to 15 points. The results of all sensitivity analyses are shown in Table 6.
| Sensitivity analysis | Estimated mean difference (95% CI)a |
|---|---|
| Inclusion of time as a continuous linear predictor (over all time points) | 8.6 (3.2 to 14.0) |
| Multiple imputation for missing responses with inclusion of time as a continuous linear predictor (no interaction with treatment; over all time points) | 7.5 (2.8 to 12.2) |
| Multiple imputation for missing responses following use of available subscores to create overall score (where some responses are missing) | 6.6 (1.0 to 12.1) |
| Multiple imputation for missing responses with inclusion of time as a continuous linear predictor (no interaction with treatment) and following use of available subscores to create overall score (where some responses are missing) | 7.3 (2.5 to 12.0) |
| Per-protocol analysis | 8.1 (1.5 to 14.8) |
| Tobit regression model | 7.2 (–0.8 to 15.1) |
| Removing women who have had hysterectomy | 7.4 (1.4 to 13.5) |
| Hospital as fixed | 6.9 (0.7 to 13.2) |
Subgroup analyses
Although the mean differences were higher in the subgroups of women whose largest fibroid measured > 7 cm, with four or more fibroids and not desiring pregnancy at the time of randomisation than in other subgroups, there was no compelling evidence of any differential effect in any of the subgroups in relation to the primary outcome, which is shown by the interaction p-values in Table 7.
| Subgroup | Mean (SD), n | Estimated mean difference (95% CI)a | Interaction p-value | |
|---|---|---|---|---|
| UAE group | Myomectomy group | |||
| Longest dimension (cm) of largest uterine fibroid | ||||
| > 7 | 77.7 (23.3), 52 | 86.5 (18.7), 51 | 12.8 (4.6 to 21.0) | 0.13 |
| ≤ 7 | 82.5 (20.5), 48 | 82.8 (23.8), 55 | 3.1 (–5.9 to 12.0) | |
| Number of fibroids | ||||
| 1–3 | 80.9 (21.8), 64 | 84.0 (22.4), 68 | 6.4 (–1.45 to 14.3) | 0.86 |
| 4–10+ | 78.4 (22.7), 36 | 85.7 (20.1), 38 | 10.4 (0.7 to 20.1) | |
| Currently desiring pregnancy (at time of randomisation) | ||||
| Yes | 78.6 (24.5), 48 | 81.4 (25.2), 48 | 7.1 (–2.8 to 17.1) | 0.41 |
| No | 81.3 (19.7), 52 | 87.2 (17.7), 58 | 8.6 (1.1 to 16.1) | |
Secondary outcomes within 2 years
Symptom severity and quality-of-life questionnaires
The UFS-QOL symptom severity domain scores were higher in the UAE group (Table 8). Small, but consistent, differences in scores on the two EQ-5D instrument domains were observed, in both cases in favour of myomectomy.
| Quality-of-life questionnaire | Mean (SD) score, n | Estimated mean difference (95% CI)a | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| UFS-QOL symptom severity domainb | |||
| Baseline | 58.5 (26.0), 122 | 59.4 (21.0), 125 | |
| 6 months | 27.3 (21.2), 100 | 21.6 (17.1), 97 | –6.1 (–11.4 to –0.9) |
| 1 year | 25.7 (21.5), 95 | 20.4 (19.0), 96 | –5.4 (–11.0 to 0.2) |
| 2 years | 21.9 (20.8), 100 | 19.5 (20.0), 106 | –3.8 (–9.4 to 1.8) |
| EQ-5D-3Lc | |||
| Baseline | 0.62 (0.34), 125 | 0.63 (0.32), 127 | |
| 6 months | 0.77 (0.30), 100 | 0.85 (0.17), 98 | 0.09 (0.03 to 0.14) |
| 1 year | 0.77 (0.30), 98 | 0.85 (0.23), 98 | 0.08 (0.01 to 0.15) |
| 2 years | 0.80 (0.29), 99 | 0.88 (0.20), 106 | 0.07 (0.01 to 0.13) |
| EQ-5D VASd | |||
| Baseline | 62.9 (23.8), 125 | 62.7 (23.2), 127 | |
| 6 months | 74.2 (20.9), 98 | 79.7 (15.7), 100 | 5.7 (1.1 to 10.3) |
| 1 year | 74.4 (21.1), 98 | 81.3 (15.4), 97 | 7.0 (2.1 to 11.9) |
| 2 years | 74.7 (19.4), 101 | 80.8 (14.7), 106 | 6.1 (1.7 to 10.6) |
Menstrual bleeding outcomes within 2 years
There were no apparent differences between groups in terms of menstrual regularity, although no formal comparisons were made. Table 9 shows the women’s reported impression of their menstrual cycle regularity.
| Menstrual cycle regularity | Number of participants (%) | |
|---|---|---|
| UAE group | Myomectomy group | |
| 6 months | ||
| Currently having periods: yes | 88 | 81 |
| Cycle regularity | ||
| Regular | 29 (34) | 15 (19) |
| Fairly regular | 40 (47) | 47 (59) |
| Irregular | 16 (19) | 16 (20) |
| Bleeding on and off | 0 | 2 (3) |
| 1 year | ||
| Currently having periods: yes | 85 | 78 |
| Cycle regularity | ||
| Regular | 20 (25) | 20 (26) |
| Fairly regular | 41 (52) | 42 (55) |
| Irregular | 18 (22) | 12 (16) |
| Bleeding on and off | 2 (2) | 2 (3) |
| 2 years | ||
| Currently having periods: yes | 77 | 73 |
| Cycle regularity | ||
| Regular | 28 (37) | 27 (37) |
| Fairly regular | 27 (36) | 30 (41) |
| Irregular | 16 (21) | 14 (19) |
| Bleeding on and off | 5 (7) | 2 (3) |
There were no apparent and sustained differences between the two groups in the bleeding scores or in the proportions of women reporting amenorrhoea or heavy bleeding over the 2 years of follow-up, as illustrated in Table 10.
| Pictorial blood assessment chart bleeding score | UAE group | Myomectomy group | Estimated mean difference or relative risk (95% CI)a |
|---|---|---|---|
| Baseline | |||
| Sample size, (n) | 102 | 100 | |
| Total score, median (IQR)b | 133 (63–275) | 180 (100–383) | |
| Total score, log-transformed mean (SD)c | 5.0 (1.1) | 5.2 (1.1) | |
| Amenorrhoea, n (%)d | 0 | 1 (1) | |
| Light bleeding, n (%) | 0 | 1 (1) | |
| Normal bleeding, n (%) | 40 (39) | 23 (23) | |
| Heav bleeding y, n (%)e | 62 (62) | 75 (75) | |
| 6 months | |||
| Sample size, (n) | 90 | 93 | |
| Total score, median (IQR)b | 58 (17–126) | 46 (9–83) | |
| Total score, log-transformed mean (SD)c | 3.6 (1.9) | 3.2 (1.8) | –0.5 (–1.05 to –0.06) |
| Amenorrhoea, n (%)d | 14 (16) | 16 (17) | 1.1 (0.6 to 2.1) |
| Light bleeding, n (%) | 5 (6) | 8 (9) | |
| Normal bleeding, n (%) | 45 (50) | 51 (55) | |
| Heavy bleeding, n (%)e | 26 (29) | 18 (19) | 0.7 (0.4 to 1.1)f |
| 1 year | |||
| Sample size, (n) | 81 | 90 | |
| Total score, median (IQR)b | 48 (13–94) | 39 (12–83) | |
| Total score, log-transformed mean (SD)c | 3.4 (1.7) | 3.2 (1.7) | –0.2 (–0.6 to 0.2) |
| Amenorrhoea, n (%)d | 11 (14) | 15 (17) | 0.9 (0.1 to 6.1) |
| Light bleeding, n (%) | 5 (6) | 7 (8) | |
| Normal bleeding, n (%) | 46 (57) | 53 (59) | |
| Heavy bleeding, n (%)e | 19 (23) | 15 (17) | 0.7 (0.4 to 1.3)f |
| 2 years | |||
| Sample size, (n) | 75 | 77 | |
| Total score, median (IQR)b | 32 (0–88) | 41 (11–84) | |
| Total score, log-transformed mean (SD)c | 2.9 (1.9) | 3.3 (1.9) | –0.07 (–0.5 to 0.3) |
| Amenorrhoea, n (%)d | 19 (25) | 14 (18) | 0.7 (0.4 to 1.3)f |
| Light bleeding, n (%) | 5 (7) | 5 (6) | |
| Normal bleeding, n (%) | 36 (48) | 41 (53) | |
| Heavy bleeding, n (%)e | 15 (20) | 17 (22) | 1.0 (0.5 to 1.8)f |
Pregnancy and associated outcomes within 2 years
Fourteen women reported a pregnancy within 2 years of randomisation (nine women in the UAE group and five women in the myomectomy group, accounting for, respectively, 17% and 10% of women who at the time of randomisation expressed a desire to get pregnant). The outcome of preganancy was a live birth for six women in the UAE group and four women in the myomectomy group. There were two miscarriages among women in the UAE group (Table 11).
| Pregnancy outcome | Proportion, n/N (%) | Estimated relative risk (95% CI)a | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| Women reporting pregnancy | 9/112 (8)b | 5/112 (4) | 0.6 (0.2 to 1.7) |
| Pregnancy (in women desiring pregnancy at time of randomisation) | 9/52 (17) | 5/48 (10) | |
| Pregnancy outcome/woman | |||
| Live birth | 6/106 (6) | 4/107 (4) | |
| Miscarriageb | 2/106 (2) | 0/107 | |
| Termination of pregnancy | 1/106 (1) | 1/107 (1) | |
Reproductive hormone levels as markers of ovarian reserve
Hormone assay data are reported as geometrical means, unadjusted and adjusted for baseline scores and age, in Table 12. The adjusted analysis was a deviation from the statistical analysis plan, as there was a 2.5-year age difference between the two groups and hormonal levels are strongly associated with age.
| Reproductive hormone | Geometric mean (95% CI), n | Estimated geometric mean ratio, adjusted for baselinea (95% CI) | Estimated geometric mean ratio, adjusted for baseline and agea (95% CI) | |
|---|---|---|---|---|
| UAE group | Myomectomy group | |||
| AMH (pmol/l) | ||||
| Baseline | 0.70 (0.52 to 0.94), 122 | 0.40 (0.29 to 0.56), 123 | ||
| 6 weeks | 0.45 (0.31 to 0.63), 90 | 0.26 (0.18 to 0.37), 103 | 0.74 (0.54 to 1.01) | 0.82 (0.61 to 1.10) |
| 6 months | 0.49 (0.34 to 0.71), 92 | 0.26 (0.17 to 0.39), 94 | 0.96 (0.72 to 1.29) | 1.08 (0.81 to 1.43) |
| 1 year | 0.43 (0.27 to 0.66), 84 | 0.20 (0.13 to 0.30), 92 | 0.66 (0.49 to 0.89) | 0.75 (0.57 to 0.98) |
| FSH (IU/ml) | ||||
| Baseline | 5.48 (3.90 to 7.71), 41 | 5.65 (4.04 to 7.90), 38 | ||
| 6 weeks | 6.45 (5.31 to 7.82), 35 | 8.27 (6.31 to 10.83), 37 | 1.20 (0.86 to 1.67) | 1.11 (0.79 to 1.54) |
| 6 months | 6.41 (4.85 to 8.46), 34 | 7.37 (4.84 to 11.21), 35 | 1.14 (0.70 to 1.84) | 1.03 (0.64 to 1.65) |
| 1 year | 7.90 (5.66 to 11.04), 36 | 10.80 (6.74 to 17.29), 34 | 1.38 (0.80 to 2.39) | 1.26 (0.74 to 2.13) |
| LH (IU/ml) | ||||
| Baseline | 5.26 (3.70 to 7.46), 41 | 5.09 (3.64 to 7.13), 38 | ||
| 6 weeks | 7.05 (5.38 to 9.23), 35 | 5.91 (3.83 to 9.14), 37 | 0.82 (0.51 to 1.34) | 0.80 (0.49 to 1.29) |
| 6 months | 5.79 (4.45 to 7.53), 34 | 6.90 (4.56 to 10.45), 35 | 1.22 (0.76 to 1.96) | 1.16 (0.73 to 1.86) |
| 1 year | 7.69 (5.43 to 10.90), 36 | 7.42 (4.67 to 11.78), 34 | 0.95 (0.53 to 1.67) | 0.91 (0.52 to 1.59) |
Neither analysis provides any evidence of any material difference between the levels of hormones associated with uterine reserve in each group.
Participant satisfaction
Participants were asked if they would have their procedure again and if they would recommend the procedure to a friend. In both groups, positive responses were high to the questions of having their procedure again and very high in terms of recommending the operation to a friend in similar circumstances. The latter responses were approximately 10% higher in the myomectomy group than in the UAE at every time point (relative risk 1.1, 95% CI 1.0 to 1.2), as shown in Table 13.
| Participant rating of operation | Proportion, n/N (%) | Estimated relative riska (95% CI) | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| Would you have your operation again? | |||
| 6 months | 70/94 (74) | 67/94 (71) | 1.0 (0.8 to 1.1) |
| 1 year | 68/90 (76) | 68/90 (76) | 1.0 (0.8 to 1.2) |
| 2 years | 70/95 (74) | 73/94 (78) | 1.1 (0.9 to 1.2) |
| Would you recommend operation to a friend? | |||
| 6 months | 78/94 (83) | 88/95 (93) | 1.1 (1.0 to 1.2) |
| 1 year | 77/90 (86) | 87/92 (95) | 1.1 (1.0 to 1.2) |
| 2 years | 79/94 (84) | 87/94 (93) | 1.1 (1.0 to 1.2) |
Hospital stay and further procedures for fibroids
Among the 112 participants in the UAE group for whom length of hospital stay data were available, the median stay was 2 [interquartile range (IQR) 2–3] days, whereas among the 117 women in the myomectomy group for whom data were available, the median stay was 4 (IQR 3–5) days.
More women in the UAE group than in the myomectomy group underwent further fibroid treatment within 2 years (Table 14), with hysterectomy the most common subsequent procedure in both groups.
| Further surgical procedure | UAE group | Myomectomy group | Estimated relative riska (95%CI) |
|---|---|---|---|
| Total number of procedures, n/N (%) | 18/110 (16) | 8/111 (7) | 0.4 (0.2 to 1.0) |
| Myomectomy, n (%) | 5 (5) | 1 (1) | |
| Hysterectomy, n (%) | 8 (7) | 4 (4) | |
| Transcervical resection, n (%) | 5 (5) | 3 (3) |
Procedural complications and adverse events
Perioperative and postoperative complications
Perioperative and postoperative complications occurred in 27 out of 113 (24%) women in the UAE group and 34 out of 118 (29%) women in the myomectomy group, with a relative risk of 1.2 (95% CI 0.8 to 1.9; p = 0.4) (Table 15). Table 16 presents the same complications according to the actual initial procedure undertaken.
| Complication | Number of partipants (%) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Perioperative or postoperative complications | ||
| Sample size | 113 | 118 |
| Access artery occlusion | 1 (1) | 0 |
| Post-embolisation syndrome resulting in a delay in dischargea | 2 (2) | 0 |
| Haematoma | 3 (3) | 0 |
| Major haemorrhage | 2 (2) | 6 (5) |
| Infection | 0 | 5 (4) |
| Otherb | 1 (1) | 3 (3) |
| Post discharge to 6 weeks complicationsc | ||
| Sample size | 109 | 114 |
| Access artery occlusion | 1 (1) | 0 |
| Post-embolisation syndrome resulting in re-admission | 3 (3) | 0 |
| Haematoma | 0 | 2 (2) |
| Infection | 15 (14) | 17 (15) |
| Otherd | 10 (9) | 8 (8) |
| Complication | Number of participants (%) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Perioperative or pre-discharge complications | ||
| Sample size | 98 | 105 |
| Access artery occlusion | 1 (1) | 0 |
| Post-embolisation syndrome delaying dischargea | 2 (2) | 0 |
| Haematoma | 0 | 3 (3) |
| Major haemorrhage | 0 | 4 (4) |
| Blood transfusion | 0 | 11 (10) |
| Infection | 0 | 4 (4) |
| Otherb | 1 (1) | 3 (3) |
| Post-discharge complicationsc | ||
| Sample size | 96 | 103 |
| Access artery occlusion | 1 (1) | 0 |
| Post-embolisation syndrome requiring re-admission | 3 (3) | 0 |
| Haematoma | 0 | 2 (2) |
| Infection | 13 (14) | 17 (17) |
| Otherd | 8 (8) | 6 (6) |
Serious adverse events
There was systematic variation in the way that participating sites reported SAEs, which arised from different interpretations of the definition. This resulted in perioperative and postoperative complications also being reported as SAEs, for example if a wound infection resulted in an overnight re-admission. Repeat procedures were occasionally reported as SAEs. Table 17 shows the numbers of reported SAEs and the number of women experiencing SAEs without disaggregation of procedural complications reported in Tables 15 and 16.
| SAEs reported | Number of participants (%) (N = 127) | p-value | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| Total number of women experiencing a SAE | 38 (30) | 31 (24) | 0.32 |
| Total number of SAEs | 52 (41) | 40 (31) | |
Chapter 4 Clinical effectiveness results: 4-year follow-up data
Parts of this chapter have been reproduced with permission from Daniels et al. 65 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Women were sent UFS-QOL, PBAC and EQ-5D-3L questionnaires to be completed 4 years after undergoing their procedure. Further questions to the participants captured information regarding acceptability, pregnancy and associated outcomes, and further procedures for fibroids, in the same format as at 2 years.
Participant flow within 4 years
Compared with return rates at 2 years, a further 25 women in the myomectomy group and 33 women in the UAE group did not return questionnaires and/or were uncontactable, with 67 (53%) and 81 (64%) women, respectively, returning complete UFS-QOL quality-of-life scores. Interpretation should take this level of missing data into account because it may limit the generalisability of the results.
Participant-reported outcomes within 4 years
Health-related quality of life within 4 years
The estimated mean difference in HRQoL using observed (UFS-QOL) data at the 4-year follow-up time point can be seen in Table 18.
| Outcome | Mean (SD) score, n | Estimated mean difference (95% CI) | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| HRQoLa,b | 86.6 (20.5), 67 | 90.2 (19.7), 81 | 5.0 (–1.4 to 11.5) |
Symptom severity and quality-of-life questionnaire results within 4 years
There was no evidence of any difference between the groups in symptom severity at 4 years. General quality-of-life scores were higher in the myomectomy group than in the UAE group (Table 19).
| Outcome | Mean (SD) score, n | Estimated mean difference (95% CI)a | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| UFS-QOL symptom severity domain scoreb | 18.8 (18.8), 70 | 14.5 (17.5), 80 | –5.0 (–10.8 to 0.8) |
| EQ-5D-3L scorec | 0.79 (0.30), 70 | 0.90 (0.16), 83 | 0.13 (0.06 to 0.20) |
| EQ-5D VAS scored | 75.3 (19.4), 71 | 82.8 (17.5), 82 | 8.7 (3.5 to 13.8) |
Menstrual bleeding outcomes within 4 years
At 4 years, there were fewer women completing the PBAC diary and reporting on their menstrual cycle regularity than at 2 years (Table 20). A larger proportion of women were amenorrhoeic at 4 years than at the 2-year time point (Table 21).
| Menstrual cycle regularity | Number of participants (%) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Currently having periods | 48 | 39 |
| Cycle regularity | ||
| Regular | 13 (27) | 12 (31) |
| Fairly regular | 23 (48) | 18 (46) |
| Irregular | 11 (23) | 9 (23) |
| Bleeding on and off | 1 (2) | 0 |
| PBAC score/category | UAE group | Myomectomy group | Estimated relative risk (95% CI) |
|---|---|---|---|
| Total score, median (IQR) | 28 (0–75) | 29 (0–81) | |
| Total score (log-transformed), mean (SD)a | 2.8 (2.0) | 2.6 (2.0) | –0.01 (–0.4 to 0.4) |
| Amenorrhoea, n (%) | 14 (27) | 15 (35) | 1.3 (0.7 to 2.3)b |
| Light bleeding, n (%) | 3 (6) | 1 (2) | |
| Normal bleeding, n (%) | 26 (51) | 21 (49) | |
| Heavy bleeding, n (%) | 8 (16) | 6 (14) | 0.9 (0.4 to 2.4) |
| Total, n | 51 | 43 |
Pregnancy and associated outcomes within 4 years
At 4 years, pregnancies, and their outcomes, continued to be reported by the participants or members of the local study team. The number of women getting pregnant, reported as cumulative rates, is shown in Table 22. Appropriate denominators cannot be presented here because of the high levels of drop-out between 2 and 4 years. Data are also reported for the per-protocol (i.e. only those who went on to receive the randomised intervention) and treatment-received populations. Figure 6 presents the ITT data as Kaplan–Meier estimates and takes into account the lack of full follow-up for all women.
| Outcome | Number of women (number of events) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Pregnancy by ITT | ||
| Women reporting pregnancya | 12 (15) | 6 (7) |
| Pregnancy (in population desiring pregnancy at time of randomisation) | 12 (15) | 6 (7) |
| Live birth | 7 (9) | 5 (6) |
| Miscarriage | 4 (5) | 0 |
| Termination | 1 | 1 |
| Pregnancy by per protocol | ||
| Women reporting pregnancyb | 7 (8) | 6 (7) |
| Live birth | 4 (5) | 5 (6) |
| Miscarriage | 2 | 0 |
| Termination | 1 | 1 |
| Pregnancy by treatment received | ||
| Women reporting pregnancyc | 7 (8) | 8 (10) |
| Live birth | 4 (5) | 6 (7) |
| Miscarriage | 2 | 1 (2) |
| Termination | 1 | 1 |
FIGURE 6.
Time to pregnancy.
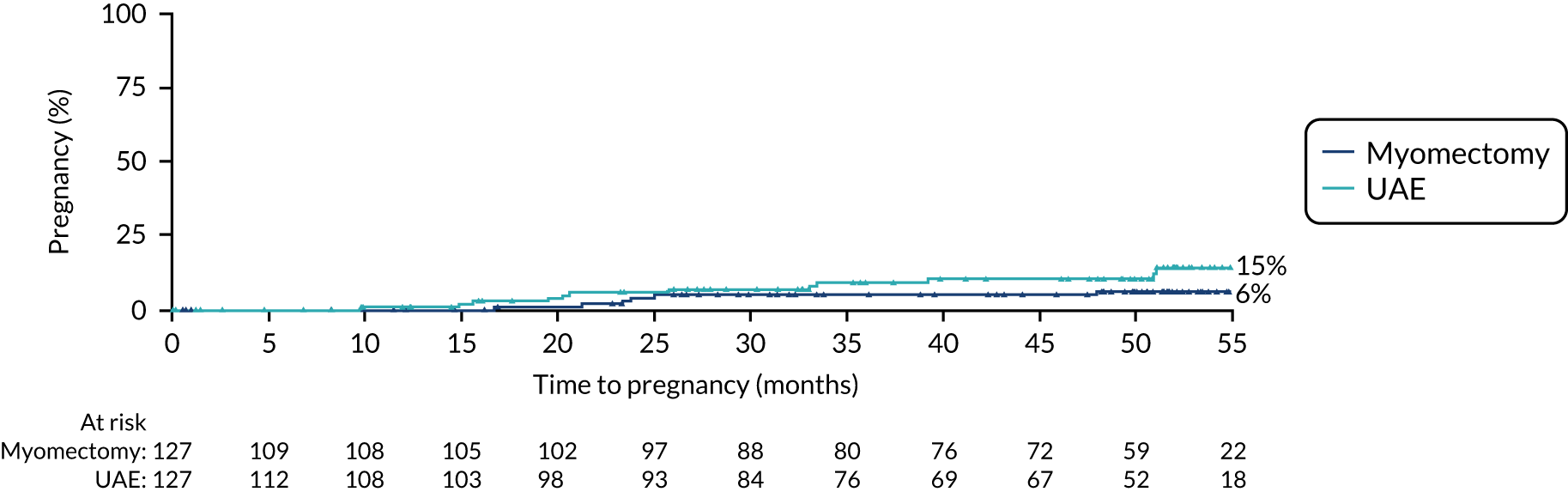
The cumulative pregnancy rate was 15% in the UAE group and 6% in the myomectomy group (hazard ratio from ITT data 0.48, 95% CI 0.18 to 1.28) (see Figure 6).
Participant satisfaction
There were no apparent differences in the participants’ rating of their operation by 4 years, which remained high overall (Table 23).
| Satisfaction question | Proportion, n/N (%) | Estimated risk (95% CI)a | |
|---|---|---|---|
| UAE group | Myomectomy group | ||
| Would you have your operation again? | 50/66 (76) | 59/78 (76) | 1.0 (0.8 to 1.2) |
| Would you recommend operation to a friend? | 51/64 (80) | 69/76 (91) | 1.1 (0.9 to 1.3) |
Further procedures for fibroids within 4 years
The cumulative number of further procedures for treatment of fibroids was 22 in the UAE group and 13 in the myomectomy group (Table 24), which were hysterectomies for 11 and 8 women, respectively. Again, appropriate denominators are not presented here because of the high level of drop out between 2 and 4 years. Figure 7 presents these data as Kaplan–Meier estimates and takes into account the lack of full follow-up for all women.
| Procedure | Number of participants, (n) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Any further procedure | 22 | 13 |
| Method | ||
| Hysterectomy | 11 | 8 |
| Myomectomy | 6 | 2 |
| Transcervical resection | 5 | 3 |
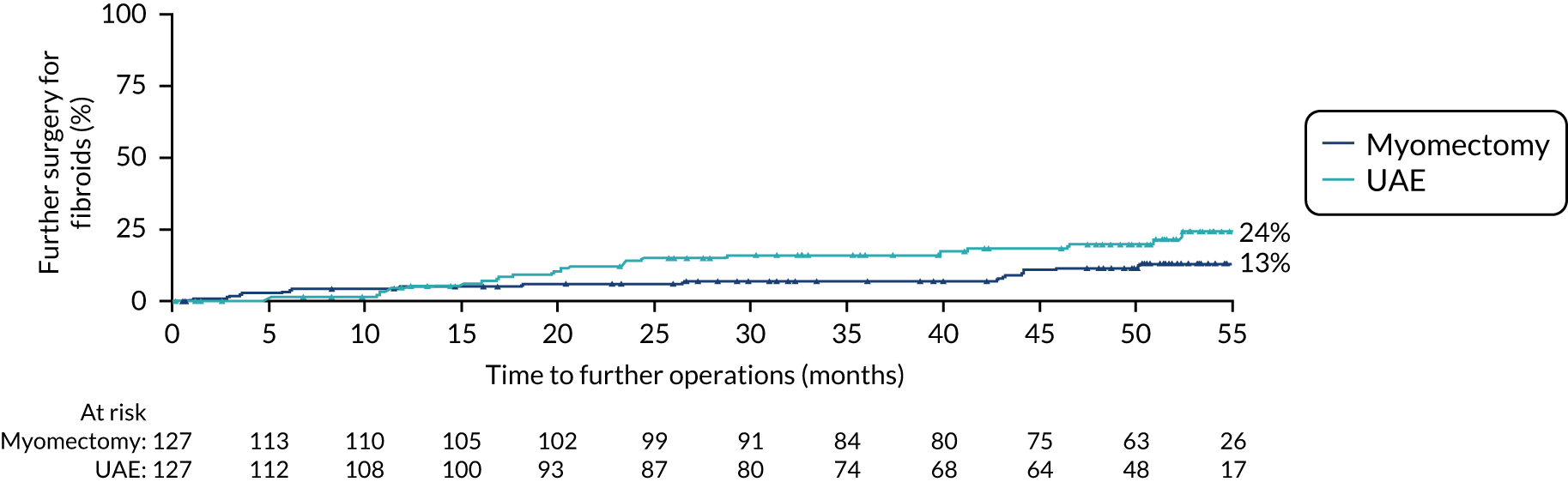
The cumulative repeat procedure rate was 24% in the UAE group and 13% in the myomectomy group (hazard ratio 0.53, 95% CI 0.27 to 1.05).
FIGURE 7.
Time to further procedure for treatment of fibroids. Operation dates were estimated to be half-way between follow-up times if exact dates were not available.
Chapter 5 Methods for economic evaluation
Parts of this chapter have been reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
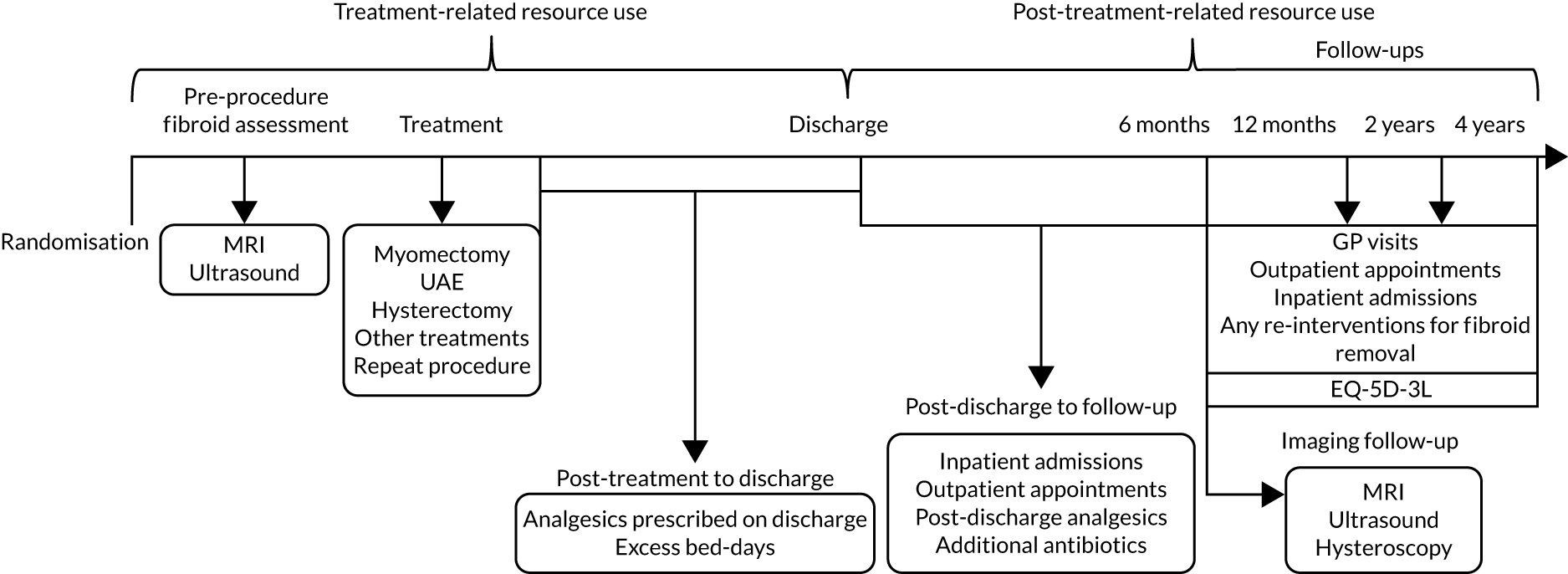
An economic evaluation was conducted from the perspective of the UK NHS over the time horizons of 2 and 4 years. Within-trial analysis was conducted based on individual participant-level data on resource use and HRQoL. The items of resource use and data on HRQoL (EQ-5D-3L) that were collected during the study period are illustrated in Figure 8.
FIGURE 8.
Data on resource use and outcome collected during the study period. Reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
The methodology adopted in this evaluation adhered to good practice guidelines set out by NICE. 67 All costs and outcomes were discounted at the rate of 3.5%.
Resource use and costs
Direct health-care resource use data collected during the treatment and follow-up periods of the trial include items related to diagnostic procedures, interventions, medication, GP visits, outpatient attendance and inpatient admissions (Table 25). The resource use items were categorised into two parts: (1) treatment-related resource use items and (2) post-treatment resource use items. Treatment-related resource use items referred to items that were recorded from the time of pre-procedure fibroid assessment to the time that participants were discharged following the initial treatment. This period included any imaging performed to assess fibroids, actual interventions received, immediate repeat procedures, excess bed-days and medications that were prescribed on discharge. Post-treatment resource use items were recorded during the period from post discharge from initial treatment to the first follow-up at 6 months and, subsequently, to follow-ups at 1, 2 and 4 years.
| Resource use item | Resource use measurement |
|---|---|
| Treatment-related items | |
| Pre-procedure fibroid assessment | Type and number of imaging procedures |
| Myomectomy | Number of patients receiving the intervention |
| UAE | Number of patients receiving the intervention (including repeat procedures) |
| Any other intervention | Number of patients receiving the intervention |
| Excess bed stay | Number of extra days in ward |
| Medications | Number of medicines prescribed during the period, including frequency, dose and duration |
| Post-treatment-related items | |
| Fibroid assessment | Type and number of imaging procedures |
| Reinterventiona | Number of patients who had repeat or additional interventions |
| Medicationsa | Number of medicines prescribed during the period, including frequency, dose and duration |
| GP visitsa | Number of visits to the GP |
| Outpatient visitsa | Number of outpatient clinic attendance |
| Inpatient admissionsa | Number of re-admissions to the hospital as an inpatient |
All unit costs were collected from routine sources, including the NHS reference costs,68 the Personal Social Services Research Unit69 and the British National Formulary (BNF). 70 All costs were expressed in GBP (£) for the price year 2018/19 (Table 26). Costs associated with all resource use were summed as the total cost for each participant.
| Resource use item | Unit | Cost (£) per unit applieda | Source and additional details |
|---|---|---|---|
| Imaging | |||
| MRI | Per procedure | 345.00 | NHS reference costs;68 average of HRG codes RD01A and RD03Z |
| Ultrasound | Per procedure | 229.00 | NHS reference costs;68 average of HRG codes MA36Z, RD40Z and RD41Z |
| CEMRI | Per procedure | 204.00 | NHS reference costs;68 HRG code RD03Z |
| Non-CEMRI | Per procedure | 141.00 | NHS reference costs;68 HRG code RD01A |
| TVUS | Per procedure | 169.00 | NHS reference costs;68 HRG code MA36Z |
| Transabdominal ultrasound | Per procedure | 60.00 | NHS reference costs;68 average of HRG codes RD40Z and RD41Z |
| Hysteroscopy | Per procedure | 405.00 | NHS reference costs;68 HRG codes RD41Z and MA31Z |
| Myomectomy | |||
| Elective inpatient | Per procedure | 3023.00 | NHS reference costs;68 average of unit costs for HRG codes MA09A and MA09B |
| Per excess bed-day | 244.00 | NHS reference costs;68 average of elective inpatient excess bed-day cost | |
| UAE | |||
| Elective inpatient | Per procedure | 2037.00 | NHS reference costs;68 HRG code YR55Z |
| Per excess bed-day | 584.00 | NHS reference costs;68 elective inpatient excess bed-day | |
| Hysterectomy | |||
| Elective inpatient | Per procedure | 3962.00 | NHS reference costs;68 average of unit costs for HRG codes MA08A and MA08B |
| Per excess bed-day | 530.00 | NHS reference costs;68 average of elective inpatient excess bed-day | |
| Other treatment | |||
| Endometrial ablation | Per procedure | 1876.00 | NHS reference costs;68 average of elective inpatient for HRG code MA12Z |
| Transcervical resection of fibroids | Per procedure | 1876.00 | NHS reference costs;68 elective inpatient cost for HRG code MA12Z |
| Other | Per procedure | 2917.00 | NHS reference costs;68 weighted average of all the treatments |
| Per excess bed-day | 530.00 | NHS reference costs;68 average of elective inpatient excess bed-day | |
| Re-admission as inpatient | |||
| Long stay (> 2 days) | Per admission | 3189.00 | NHS reference costs;68 average of overall HRG non-elective short stay and long stay |
| Short stay (≤ 2 days) | Per admission | 630.00 | NHS reference costs;68 average of overall HRG non-elective short stay and long stay |
| Inpatient admissions for follow-ups | Per admission | 1909.00 | NHS reference costs;68 average of overall HRG non-elective short stay and long stay |
| Outpatient appointment | Per appointment | 217.00 | NHS reference costs;68 average of non-admitted face-to-face attendances with HRG codes WF01A and WF01B for gynaecology |
| GP visits | Per visit | 28.00 | PSSRU;69 GP per surgery consultation lasting 9.22 minutes |
| Analgesics prescribed on discharge | |||
| Paracetamol-based analgesics | Per dose | 0.05 | bBNF;70 cost of paracetamol (500 mg) |
| Non-steroidal anti-inflammatories | Per dose | 0.38 | bBNF;70 cost of codeine phosphate (30 mg) |
| Opiates | Per dose | 0.83 | bBNF;70 cost of diclofenac sodium (150 mg) |
| Additional antibioticsb | |||
| Amoxicillin | Per dose | 0.06 | bBNF;70 500 mg |
| Cefalexin | Per dose | 0.13 | bBNF;70 500 mg |
| Cefuroxime | Per dose | 1.27 | bBNF;70 250 mg |
| Ciprofloxacin | Per dose | 0.08 | bBNF;70 750 mg |
| Clarithromycin | Per dose | 0.11 | bBNF;70 500 mg |
| Clavulanic acid | Per dose | 0.06 | bBNF;70 500 mg |
| Clindamycin | Per dose | 0.31 | bBNF;70 600 mg |
| Dalteparin sodium | Per dose | 5.12 | bBNF;70 120 units/kg |
| Erythromycin | Per dose | 0.36 | bBNF;70 500 mg |
| Flucloxacillin | Per dose | 0.38 | bBNF;70 500 mg |
| Gentamicin | Per dose | 0.28 | bBNF;70 7 mg |
| Metronidazole | Per dose | 1.87 | bBNF;70 400 mg |
| Nitrofurantoin | Per dose | 0.30 | bBNF;70 100 mg |
| Penicillin | Per dose | 0.38 | bBNF;70 250 mg |
| Piperacillin | Per dose | 0.38 | bBNF;70 250 mg |
| Tetracycline | Per dose | 0.36 | bBNF;70 250 mg |
| Trimethoprim | Per dose | 0.03 | bBNF;70 200 mg |
A number of assumptions related to resource use and unit costs were made. In the case of treatment-related resource use, all interventions were assigned unit costs according to the Healthcare Resource Group (HRG). All participants were assumed to be admitted as elective inpatients. Repeat interventions were assumed to incur the same resource use and cost as the initial intervention. Length of stay (LOS) was calculated as the difference between admission date and discharge date. In the case of LOS exceeding the ‘trim points’ (i.e. the expected LOS for each HRG), an additional per-diem cost was assigned to estimate costs associated with these excess bed-days. 72 Medications used during the procedure or during the time in a ward were assumed to be included in the HRG episode cost. Any additional medications prescribed on discharge were costed separately using unit costs from the BNF.
In the case of post-treatment resource use, all re-admissions from post discharge to first follow-up were assigned an average cost of non-elective short stay and long stay. Stays of ≤ 2 days were considered to be short stays and stays of > 2 days were considered to be long stays. All participants who did not report hospital re-admissions but had complications, infections or medications during the follow-up period were assumed to attend outpatient clinics. It was assumed that all reinterventions for fibroid removal during the follow-up period were performed on an elective inpatient basis. Resource use data captured in hospital records from post discharge to follow-up and data separately recorded by the participants at their first follow-up (i.e. 6 months) were cross-checked to avoid double-counting.
Health outcomes
Patient-reported HRQoL was measured using the EQ-5D-3L. The EQ-5D-3L was completed by the participants at baseline, 6 months, 1 year, 2 years and 4 years. The EQ-5D-3L describes health status of a patient in five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and there are three levels of response for each dimension. 73 Responses to the five health domains were used to calculate health utilities using a standard UK value set. The area under the curve method was used to estimate quality-adjusted life-years (QALYs) for each participant by adjusting the life-years gained by the quality of life experienced over the study period. 74 The method considers the utilities obtained at different time points because it assumes a linear relationship between these utilities. Subsequently, QALYs from baseline to 2 years and from baseline to 4 years were estimated.
Handling missing data
For the purpose of analysis, the following assumptions were made:
-
With regard to medication, the median duration of treatment was assumed where data were missing and standard BNF doses were assumed where data on dosages were missing.
-
In the case of participants with initial fibroid assessment scans who did not undergo any procedure, but remained in the trial and had cost/utility follow-up data (nine participants in the UAE group and four in the myomectomy group).
-
We did not make assumptions on additional resource use for participants with no resource use data throughout the study period (six in total); participants who had no other resource use data apart from those collected during the follow-up (three in the UAE group); nor participants who had additional fibroid imaging but no fibroid removal reintervention during the follow-up (two in the myomectomy group).
-
We assumed that all participants who had reintervention, but no other imaging related resource use, received fibroid imaging (two in UAE group).
-
The association between missingness, baseline variables and observed outcomes was also explored. A binomial logistic regression was carried out to investigate which variables and outcomes were associated with probability of missingness. We assumed that the data were missing at random. 75 Multiple imputation was performed using multiple imputation by chained equations. 76 Ten imputation data sets were generated with predictive mean matching (the mean of five nearest values). Imputation was performed for total costs at a subaggregate level of treatment and non-treatment costs, whereas QALYs were imputed at aggregate level of the total QALYs level and not on a health utilities level.
Cost-effectiveness analysis
The base-case analysis followed an ITT strategy and was conducted post multiple imputation. Generalised linear models were used to analyse cost and QALY data because of their non-normal distribution, which would otherwise render the calculation of a simple mean as inappropriate. 77 Generalised linear models accommodate this non-normal distributions of cost and QALY data by allowing specification of a more appropriate distributional family and link function through the modified Park test and other tests.
The cost model was analysed using a gamma family and log-link. It was adjusted for minimisation variables used in the clinical analysis (i.e. the participant’s desire to be pregnant at the time of randomisation, the longest dimension of largest fibroid and the number of fibroids). The QALY model was analysed with Gaussian family and identity link. It was adjusted for minimisation variables, as well as the statistically significant predictors of QALYs (i.e. baseline utilities and body mass index). Baseline utilities are usually highly correlated with the patient’s QALY gain, and any imbalance of baseline utility level between treatment groups may lead to misleading cost-effectiveness estimates. 74 The models were then used to predict the marginal mean costs and mean QALYs, which were then used to calculate their cost and QALY differences.
Cost-effectiveness was expressed as incremental cost per QALY gained [incremental cost-effectiveness ratio (ICER) = ΔC/ΔQ)], where appropriate. Incremental mean cost (CUAE – CMyomectomy = ΔC) is the difference in mean costs between the two treatment groups, and incremental QALY (QUAE – QMyomectomy = ΔQ) is the difference in QALYs gained between the two treatment groups. The ICER provides the additional cost of achieving 1 perfect year of health and can be compared against the NICE willingness-to-pay (WTP) threshold of £20,000. 67 In addition, incremental net monetary benefit (NMB), a measure of the health benefit expressed in monetary terms, was also estimated. The NMB was calculated using the following formula:
where ΔQ is the incremental QALY, λ is the WTP threshold (i.e. £20,000 in the UK) and ΔC is the incremental cost. 77 An intervention is generally considered cost-effective compared with the alternative if the incremental NMB is positive.
To quantify the uncertainty around incremental costs and QALYs, a 1000-iteration bootstrap was performed. The results were presented on cost-effectiveness planes. Cost-effectiveness acceptability curves were used to present uncertainty in the decision regarding cost-effectiveness over a range of WTP thresholds. 78 All analysis was carried out using Stata (StataCorp LP, College Station, TX, USA) version 16.0.
Sensitivity analyses
The second scenario involved varying the unit cost applied to the procedures during initial intervention and any reinterventions for fibroid removal. In the base-case analysis, costs were estimated using a top-down approach, which emphasises national average costs. The English NHS reference costs incorporate costing on the basis of HRG, which is a measure of case mix, demonstrating groups of clinically similar treatments that utilise a common set of health-care resources. 72 Therefore, the HRG tariffs are a reflection of NHS average costs and may not capture the difference in practice across different FEMME trial sites. Therefore, we performed a sensitivity analysis to test the robustness of our results according to variations in unit costs that may have resulted from difference in practices. A 20% cost increment and decrement were applied to cost of initial intervention, as well as to the cost of reinterventions for fibroid removal that happened during the study period.
Chapter 6 Results for economic evaluation
Parts of this chapter have been reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Health-care resource use and costs
Overall, 127 women were randomised to each treatment group (Table 27). The majority of women underwent pre-procedure imaging prior to UAE and myomectomy. Across both groups, the majority of women received MRI (UAE group, 71%; myomectomy group, 79%). In the UAE group, 98 women received UAE, 14 received myomectomy and one received endometrial ablation (in addition, 14 women did not receive treatment or withdrew from the study). In the myomectomy group, 105 women received myomectomy, six received UAE and eight received hysterectomy (in addition, eight women did not receive treatment or withdrew from the study). UAE was associated with a median LOS of 2 (IQR 2–3) days, compared with 4 (IQR 3–5) days for myomectomy. Across both groups women were prescribed analgesics on discharge (UAE group, 87%; myomectomy group, 97%).
| Resource use | UAE group (N = 127) | Myomectomy group (N = 127) |
|---|---|---|
| Pre-procedure fibroid assessment | ||
| Sample size, (n) | 126 | 126 |
| MRI | ||
| Total, n (%) | 89 (70.63) | 99 (78.57) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Ultrasound | ||
| Total, n (%) | 36 (28.57) | 27 (21.43) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Not mentioned, n (%) | 1 (0.79) | 0 (0.00) |
| LOS (days) during treatment | ||
| Sample size, (n) | 112 | 117 |
| Mean (SD) | 2.65 (1.34) | 4.24 (1.53) |
| Median (IQR) | 2 (2–3) | 4 (3–5) |
| Medications | ||
| Sample size, (n) | 118 | 118 |
| Total number of analgesics prescribed on discharge | ||
| Total, n (%) | 103 (87.29) | 114 (96.61) |
| Missing, n (%) | 2 (1.69) | 1 (0.85) |
Post-treatment-related resource use is shown in Table 28. During the period from discharge to first follow-up (at 6 months), UAE was associated with a slightly higher proportion (16%) of women with re-admissions than myomectomy (11%). Similar patterns were observed for the mean number of re-admissions from discharge to 2 and 4 years. Resource use associated with outpatient appointments and medications prescribed was similar between the UAE and the myomectomy treatment groups and this pattern was consistent at 2 and 4 years. A larger number of women in UAE group (n = 18) than in the myomectomy group (n = 8) received reintervention within the first 2 years. At the end of 4 years, 22 women from the UAE group had reintervention compared with 13 women in the myomectomy group.
| Resource use | UAE group (N = 127) | Myomectomy group (N = 127) |
|---|---|---|
| Re-admission as inpatient after discharge to first follow-up | ||
| Sample size, (n) | 109 | 114 |
| Number (%) of inpatient re-admissions | 17 (15.60) | 13 (11.40) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Re-admission as inpatient after discharge to 2 and 4 years | ||
| Mean ± SD number of re-admissions at 2 years | 5.87 ± 6.41 | 4.96 ± 7.16 |
| Median (IQR) number of re-admissions at 2 years | 3 (1–8) | 3 (2–5) |
| Mean ± SD number of re-admissions at 4 years | 5.69 ± 6.30 | 5.24 ± 6.67 |
| Median (IQR) number of re-admissions at 4 years | 3 (2–8) | 4 (2–5) |
| Outpatient appointment after discharge to first follow-up | ||
| Sample size, (n) | 109 | 114 |
| Number (%) of outpatient appointments | 92 (84.40) | 101 (88.60) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Outpatient appointments as inpatient after discharge to 2 and 4 years | ||
| Mean ± SD number of outpatient appointments at 2 years | 5.73 ± 13.29 | 5.22 ± 13.85 |
| Median (IQR) number outpatient appointments at 2 years | 3 (1–6) | 2 (1–4) |
| Mean ± SD number of outpatient appointments at 4 years | 6.12 ± 13.30 | 5.51 ± 12.73 |
| Median (IQR) number of outpatient appointments at 4 years | 3 (1–6) | 3 (1–6) |
| Medicines prescribed after discharge to first follow-up | ||
| Sample size, (n) | 92 | 101 |
| Total number (%) of analgesics prescribed on discharge | 26 (28.26) | 23 (22.77) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Number (%) of patients prescribed antibiotics | 11 (11.96) | 15 (14.85) |
| Missing, n (%) | 0 (0.00) | 0 (0.00) |
| Imaging follow-up | ||
| Sample size, (n) | 105 | 94 |
| CEMRI, n (%) | 76 (72.38) | 72 (76.60) |
| Non-CEMRI, n (%) | 17 (16.19) | 13 (13.83) |
| TVUS, n (%) | 4 (3.81) | 6 (6.38) |
| Transabdominal ultrasound, n (%) | 2 (1.90) | 1 (1.06) |
| Hysteroscopy, n (%) | 0 (0.00) | 0 (0.00) |
| Missing, n (%) | 7 (6.67) | 3 (3.19) |
| GP visits | ||
| Mean ± SD number of GP visits at 2 years | 6.24 ± 12.51 | 4.91 ± 4.72 |
| Median (IQR) number GP visits at 2 years | 3 (2–6) | 4 (2–6) |
| Mean ± SD number of GP visits at 4 years | 6.86 ± 12.72 | 7.40 ± 12.47 |
| Median (IQR) number of GP visits at 4 years | 4 (2–7) | 4 (2–8) |
Table 29 provides a breakdown of average costs according to resource use categories. In the case of most resource use categories, the costs incurred are very similar in both treatment arms. The minimal difference in pre-procedure fibroid assessment can be attributed to the type of scans that these women received at the beginning. Most women in the myomectomy group received MRI rather than ultrasound, and MRI is slightly more expensive. The same explanation is applicable for the cost of imaging follow-up for fibroid reassessment. The average cost of treatment is higher in the myomectomy group, given that myomectomy is a more expensive procedure and requires a longer stay in the hospital. By contrast, costs incurred during the period from post treatment to discharge were higher in the UAE group. This reflects the unit cost applied for UAE excess bed-days (see Table 26), which was almost double the excess bed-days unit cost for myomectomy. Mean costs during post discharge to 6-month follow-up period were higher in the UAE group than in the myomectomy group. Women in the UAE group, again, incurred higher mean costs during the follow-up, which included the cost of GP visits, outpatient appointments and inpatient admissions.
| Resource use category | Average cost (£) (SE) | |
|---|---|---|
| UAE group | Myomectomy group | |
| Pre-procedure fibroid assessment | 312 (53) | 320 (48) |
| Treatment | 2176 (373) | 3036 (330) |
| Post-treatment to discharge | 811 (563) | 636 (433) |
| Cost for patients with excess bed-days | 849 (544) | 682 (409) |
| Post-discharge analgesics cost | 7 (7) | 6 (5) |
| Post-discharge to first follow-up (i.e. 6 months) | 518 (859) | 424 (716) |
| Inpatient re-admissions | 2135 (1298) | 2008 (1328) |
| Outpatient appointments | 217 (0) | 217 (0) |
| Post-discharge analgesics | 6 (5) | 4 (4) |
| Additional antibiotics | 7 (7) | 12 (17) |
| Follow-up over 6 months post discharge | 3225 (6064) | 1768 (3014) |
| Follow-up over 1 year post discharge | 4011 (9003) | 3419 (8999) |
| Follow-up over 2 years post discharge | 4889 (9817) | 4020 (9418) |
| Follow-up over 4 years post discharge | 5403 (10,569) | 4830 (9630) |
| GP visits | ||
| Over 6 months | 150 (452) | 75 (76) |
| Over 1 year | 137 (357) | 110 (110) |
| Over 2 years | 175 (350) | 137 (132) |
| Over 4 years | 192 (356) | 207 (349) |
| Outpatient appointments | ||
| Over 6 months | 1722 (5046) | 506 (423) |
| Over 1 year | 1122 (3383) | 1301 (3663) |
| Over 2 years | 1243 (2882) | 1131 (3003) |
| Over 4 years | 1327 (2894) | 1195 (2761) |
| Inpatient admissions | ||
| Over 6 months | 7128 (6058) | 5728 (2887) |
| Over 1 year | 10,328 (11,144) | 9069 (14,097) |
| Over 2 years | 11,210 (12,233) | 9479 (13,665) |
| Over 4 years | 10,873 (12,022) | 10,010 (12,728) |
| Imaging follow-up for fibroid assessment | 191 (34) | 195 (29) |
| Fibroid removal | ||
| Over 6 months | 3649 (542) | 3128 (1142) |
| Over 1 year | 3088 (865) | 3083 (937) |
| Over 2 years | 3092 (824) | 3046 (967) |
| Over 4 years | 3194 (806) | 3306 (872) |
Health outcomes
The proportion of responding participants and their corresponding responses to five health domains at baseline, 6 months, 1 year, 2 years and 4 years are presented in Table 30. Across the two treatment groups, at baseline, only a minority of women reported any problems with mobility (UAE, 16%; myomectomy, 17%) and self-care (UAE, 4%; myomectomy, 2%). Twenty-nine per cent of women in the UAE group and 33% of women in the myomectomy group reported some problems with usual activity. However, in both groups, 79% of women reported any problems with pain/discomfort, and 50% of women in the UAE group and 55% in the myomectomy group reported any problems with anxiety/depression.
| EQ-5D-3L domain | Number of participants (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UAE group | Myomectomy group | |||||||||
| Baseline | 6 months | 1 year | 2 years | 4 years | Baseline | 6 months | 1 year | 2 years | 4 years | |
| Mobility | ||||||||||
| Level 1 | 105 (84.00) | 87 (86.14) | 86 (87.76) | 88 (88.00) | 63 (88.73) | 105 (82.68) | 93 (94.90) | 89 (90.82) | 102 (96.23) | 77 (92.77) |
| Level 2 | 20 (16.00) | 14 (13.86) | 12 (12.24) | 12 (12.00) | 8 (11.27) | 22 (17.32) | 5 (5.10) | 8 (8.16) | 4 (3.77) | 6 (7.23) |
| Level 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.02) | 0 | 0 |
| Total | 125 | 101 | 98 | 100 | 71 | 127 | 98 | 98 | 106 | 83 |
| Self-care | ||||||||||
| Level 1 | 120 (96.00) | 97 (96.04) | 95 (96.94) | 92 (92.93) | 65 (91.55) | 125 (98.43) | 98 (100.00) | 96 (97.96) | 105 (99.06) | 83 (100.00) |
| Level 2 | 5 (4.00) | 4 (3.96) | 3 (3.06) | 6 (6.06) | 6 (8.45) | 2 (1.57) | 0 | 2 (2.04) | 1 (0.94) | 0 |
| Level 3 | 0 | 0 | 0 | 1 (1.01) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 125 | 101 | 98 | 100 | 71 | 127 | 98 | 98 | 106 | 83 |
| Usual activities | ||||||||||
| Level 1 | 84 (67.20) | 82 (82.00) | 76 (77.55) | 84 (84.00) | 58 (81.69) | 79 (62.20) | 85 (85.86) | 88 (89.80) | 94 (88.68) | 74 (89.16) |
| Level 2 | 36 (28.80) | 12 (12.00) | 20 (20.41) | 13 (13.00) | 11 (15.49) | 42 (33.07) | 14 (14.14) | 9 (9.18) | 12 (11.32) | 9 (10.84) |
| Level 3 | 5 (4.00) | 6 (6.00) | 2 (2.04) | 3 (3.00) | 2 (2.82) | 6 (4.72) | 0 | 1 (1.02) | 0 | 0 |
| Total | 125 | 100 | 98 | 100 | 71 | 127 | 99 | 98 | 106 | 83 |
| Pain/discomfort | ||||||||||
| Level 1 | 27 (21.60) | 45 (44.55) | 46 (46.94) | 50 (50.51) | 40 (57.14) | 27 (21.26) | 53 (53.54) | 65 (66.33) | 76 (71.70) | 64 (77.11) |
| Level 2 | 72 (57.60) | 47 (46.53) | 42 (42.86) | 43 (43.43) | 25 (35.71) | 73 (57.48) | 44 (44.44) | 28 (28.57) | 26 (24.53) | 18 (21.69) |
| Level 3 | 26 (20.80) | 9 (8.91) | 10 (10.20) | 6 (6.06) | 5 (7.14) | 27 (21.26) | 2 (2.02) | 5 (5.10) | 4 (3.77) | 1 (1.20) |
| Total | 125 | 101 | 98 | 99 | 70 | 127 | 99 | 98 | 106 | 83 |
| Anxiety/depression | ||||||||||
| Level 1 | 63 (50.40) | 65 (64.36) | 62 (63.27) | 68 (68.00) | 46 (64.79) | 58 (45.67) | 69 (70.41) | 71 (72.45) | 76 (71.70) | 64 (77.11) |
| Level 2 | 45 (36.00) | 30 (29.70) | 30 (30.61) | 26 (26.00) | 19 (26.76) | 60 (47.24) | 28 (28.57) | 26 (26.53) | 28 (26.42) | 18 (21.69) |
| Level 3 | 17 (13.60) | 6 (5.94) | 6 (6.12) | 6 (6.00) | 6 (8.45) | 9 (7.09) | 1 (1.02) | 1 (1.02) | 2 (1.89) | 1 (1.20) |
| Total | 125 | 101 | 98 | 100 | 71 | 127 | 98 | 98 | 106 | 83 |
In the UAE group, the proportions of women reporting any problems with mobility and self-care were consistent over time. There were substantial improvements in the usual activities and pain/discomfort domains, and a modest improvement in the anxiety/depression domain. In the myomectomy group, there were modest improvements in the mobility and self-care domains over time, and substantial improvements in the remaining three domains. In particular, the improvement in the pain/discomfort and anxiety/depression domains was greater than that observed in the UAE group.
Cost-effectiveness analysis
Participants who withdrew, were lost to follow-up, had missing resource use at the main time points or had any missing health utilities data were considered to be missing. EQ-5D-3L data were missing for 32% and 45% of participants at the 2- and 4-year follow-up, respectively. The proportion of participants with missing resource use data was low at both time points (4% and 8%, respectively).
The cost-effectiveness results for the FEMME trial are presented in Table 31. Predicted total mean costs were estimated separately based on resource use from baseline to 2 years and from baseline to 4 years. Each of these costs constituted cost components (i.e. treatment cost, as well as post-treatment costs up to 2 and 4 years).
| Predicted mean cost | UAE group, point estimate (95% CI) | Myomectomy group, point estimate (95% CI) | ||
|---|---|---|---|---|
| Cost (£) | 95% CI | Cost (£) | 95% CI | |
| Treatment costa | 3064 | 2906 to 3222 | 3862 | 3667 to 4056 |
| Post-treatment cost over 2 yearsa | 4918 | 3076 to 6759 | 3431 | 2191 to 4671 |
| Post-treatment cost over 4 yearsa | 5288 | 3445 to 7131 | 4151 | 2745 to 5557 |
| 2 years | ||||
| Mean total cost (£) (95% CI) | 7958 (6304 to 9612) | 7314 (5854 to 8773) | ||
| Mean total QALY (95% CI) | 0.74 (0.70 to 0.78) | 0.83 (0.79 to 0.87) | ||
| Incremental cost (ΔC) (95% CI) | 645 (–1381 to 2580) | |||
| Incremental QALYs (ΔQ) (95% CI) | –0.09 (–0.11 to –0.04) | |||
| ICERb (ΔC/ΔQ) (95% CI) | –7167 (–39,597 to 19,764) | |||
| NMBb (ΔQ × λ) – ΔC, λ = £20,000 (95% CI) | 2445 (–4319 to 15) | |||
| 4 years | ||||
| Mean total cost (£) (95% CI) | 8362 (6640 to 10,083) | 8010 (6422 to 9598) | ||
| Mean total QALY (95% CI) | 0.73 (0.69 to 0.76) | 0.82 (0.79 to 0.87) | ||
| Incremental cost (ΔC) (95% CI) | 352 (–1825 to 2528) | |||
| Incremental QALYs (ΔQ) (95% CI) | –0.09 (–0.12 to –0.05) | |||
| ICERb (ΔC/ΔQ) (95% CI) | –3911 (–31,357 to 23,566) | |||
| NMBb (ΔQ × λ) – ΔC, λ = £20,000 (95% CI) | –2152 (–4350 to 221) | |||
The mean treatment costs were lower in the UAE group than in the myomectomy group (£3064 vs. £3862). Conversely, the UAE group was associated with a higher post-treatment cost than the myomectomy group over 2 years’ follow-up (£4918 vs. £3431) and over 4 years’ follow-up (£5288 vs. £4151). The total mean cost incurred over 2 years was £7958 in the UAE group compared with £7314 in the myomectomy group. The total mean cost over 4 years was £8362 in the UAE group compared with £8010 in the myomectomy group.
Over the period of 2 years, the QALY gain was 0.74 (95% CI 0.70 to 0.78) in the UAE group compared with 0.83 (95% CI 0.79 to 0.87) in the myomectomy group. UAE was associated with higher costs (i.e. a £645 difference in cost, but this was not statistically significant) and lower QALYs (i.e. a –0.09 difference in QALYs) than myomectomy. UAE is dominated by myomectomy.
Similar results were observed over the 4-year time horizon. The QALY gain was 0.73 in the UAE group (95% CI 0.69 to 0.76) compared with 0.82 (95% CI 0.79 to 0.87) in the myomectomy group. UAE was associated with higher costs (i.e. a £352 difference in cost, but this was not statistically significant) and lower QALYs (i.e. a –0.09 difference in QALYs). Again, UAE is dominated by myomectomy. Compared with the 2-year follow-up, the cost difference between the UAE and the myomectomy group at 4 years’ follow-up was slightly lower, while the QALY gain remained the same.
The cost-effectiveness planes for 2 and 4 years are presented in Figure 9. The lines represent the WTP thresholds (i.e. £20,000 per additional QALY gained). The bootstrapped replications were displayed in the north- and south-west quadrants. Most replications were concentrated in the north-west quadrant. Regardless of the time horizons, there is little difference in costs between UAE and myomectomy. The deterministic estimate is close to zero. There is little uncertainty that UAE has lower health benefits than myomectomy. There is some uncertainty relating to the magnitude of the difference in costs between the two groups at 4 years.
FIGURE 9.
Cost-effectiveness plane for (a) 2 years and (b) 4 years. Reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
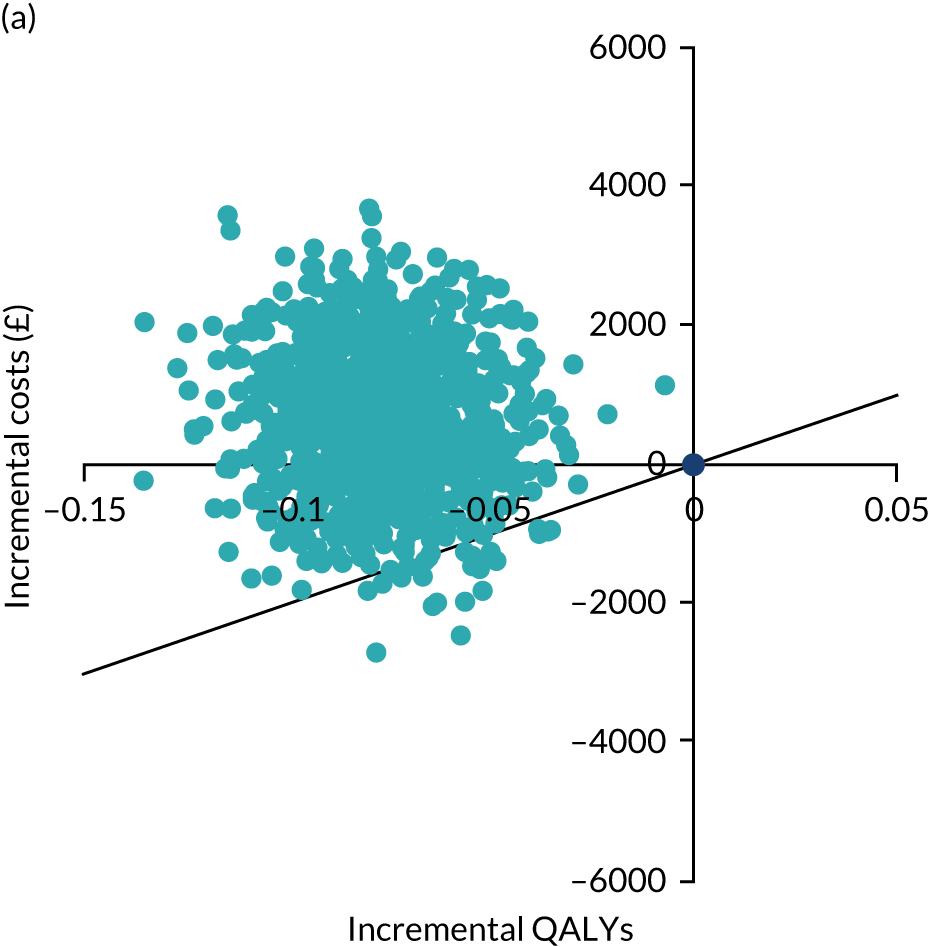
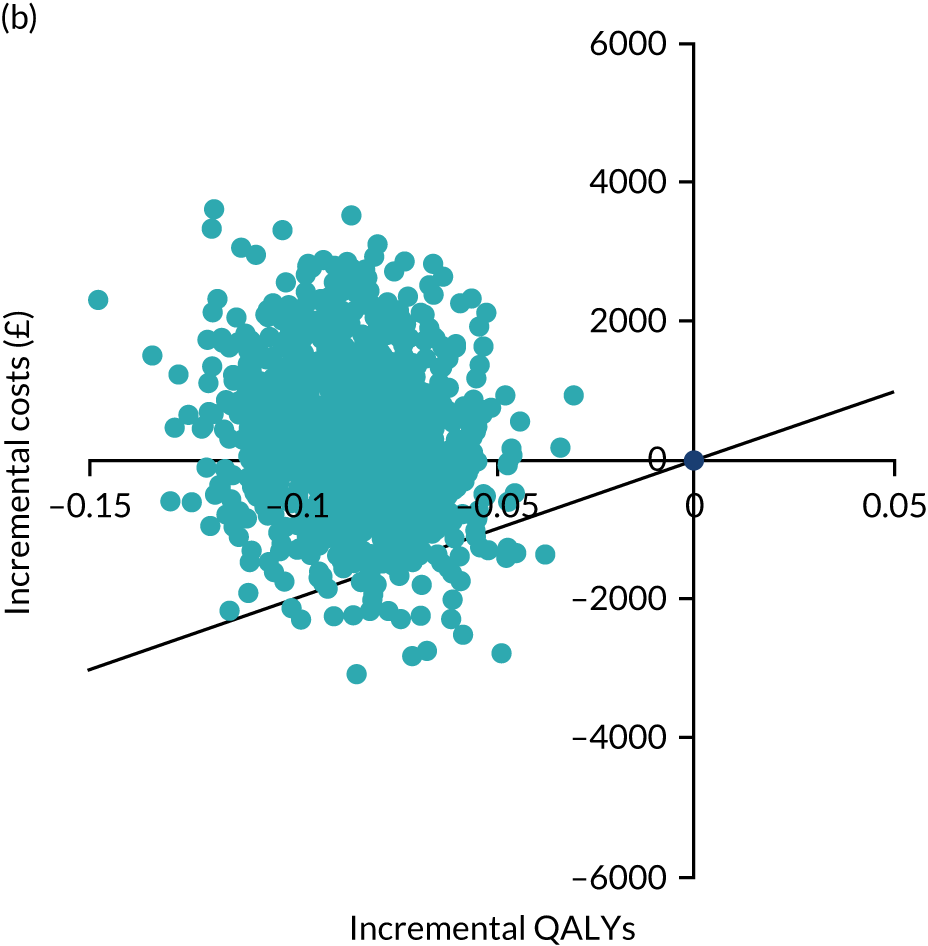
Both of the cost-effectiveness acceptability curve figures (Figure 10) showed that myomectomy had a higher probability of being cost-effective than UAE at WTP thresholds of ≥ £20,000. At a £20,000 WTP threshold, the probability of myomectomy being cost-effective is 98% at 2 years and 96% at 4 years.
FIGURE 10.
Cost-effectiveness acceptability curves for (a) 2 years and (b) 4 years. Reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
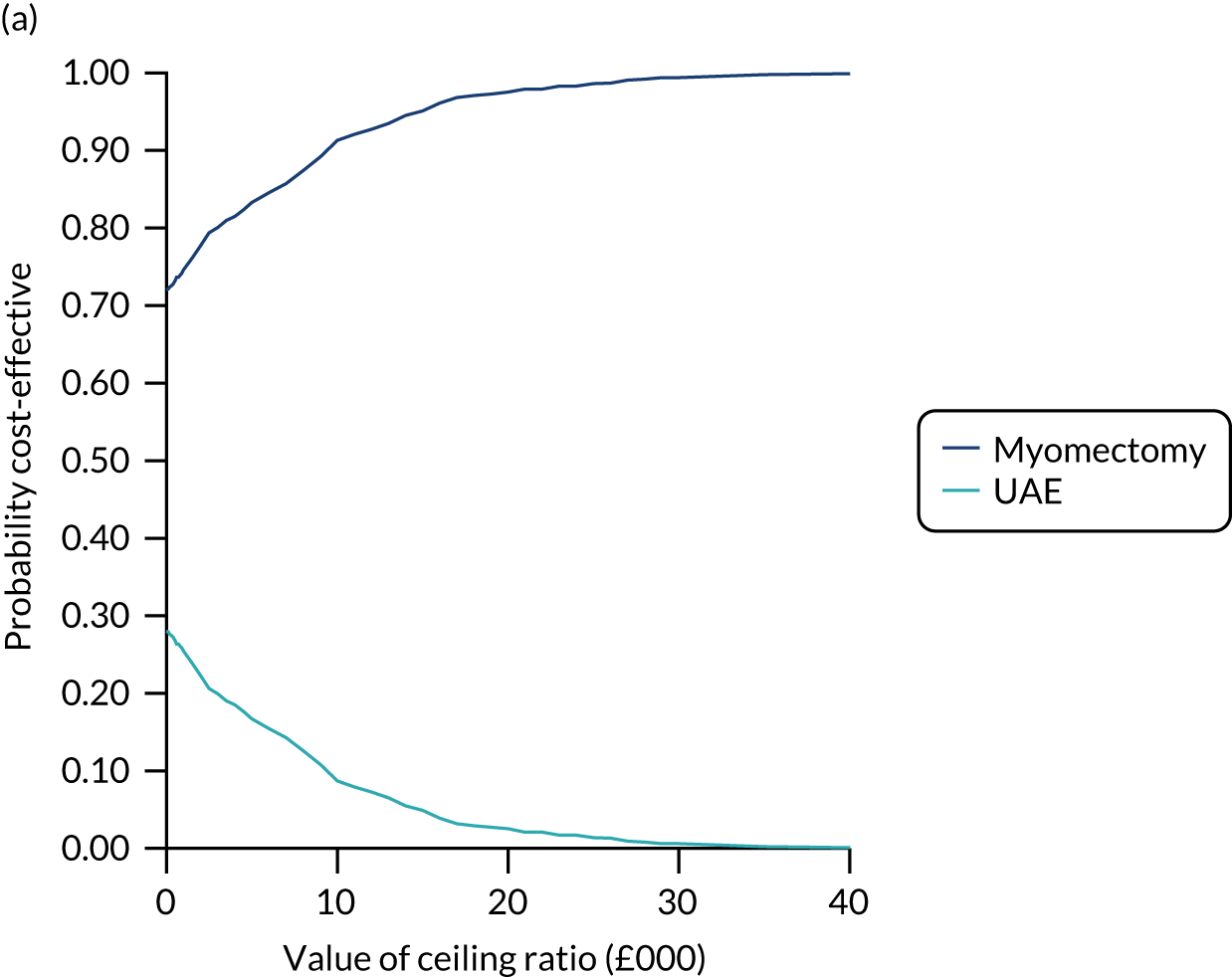
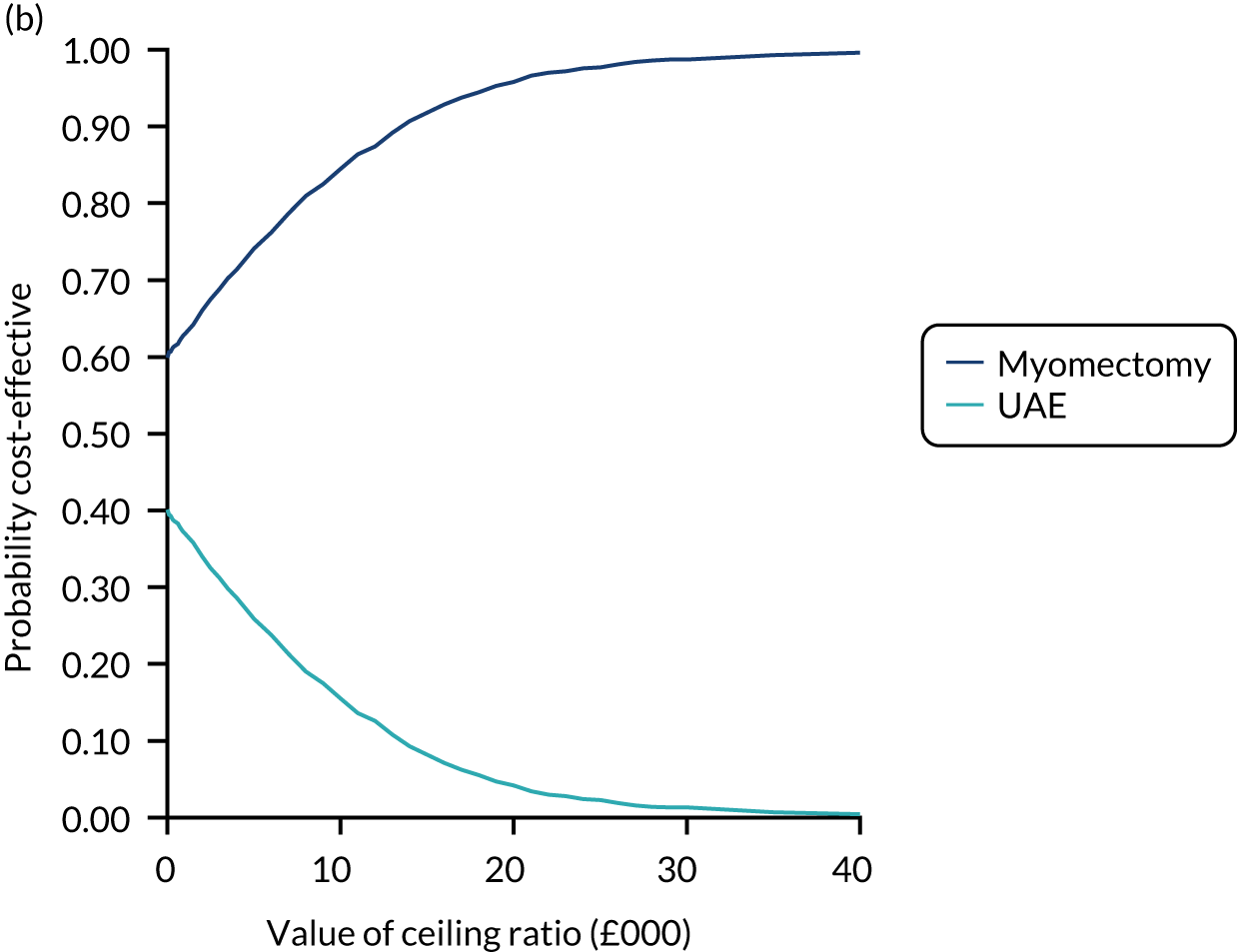
Sensitivity analysis
The results from the scenario in which we performed a complete-case analysis are presented in Table 32. Similar to the base-case analysis, analysis of cost components showed that women in the UAE group had lower treatment costs than women in the myomectomy group (£3073 vs. £3870, respectively). Women in the UAE group also incurred higher post-treatment costs over 2 years than women in the myomectomy group (£4663 vs. £3384, respectively) and 4 years (£5057 vs. £4127, respectively). UAE was associated with higher costs (i.e. a £456 difference in cost, but this was not statistically significant) and a lower QALY gain (difference of –0.06 ) over a time horizon of 2 years. Similar results were observed over a 4-year time horizon. The cost difference was slightly lower than at the 2-year follow-up, but the QALY difference remained the same. A 6% decrease in ICER was observed when we compared the 2-year follow-up ICERs obtained from the base-case analysis with those obtained from the sensitivity analysis. Relatedly, an increase in ICER of 20% was observed for the 4-year follow-up. It is imperative to note that ICERs are not normally calculated when an intervention is dominated by the comparator.
| Predicted mean cost | UAE group, point estimate (95% CI) | Myomectomy group, point estimate (95% CI) | ||
|---|---|---|---|---|
| Cost (£) | 95% CI | Cost (£) | 95% CI | |
| Treatment costa | 3073 | 2920 to 3227 | 3870 | 3678 to 4063 |
| Post-treatment cost over 2 yearsa | 4663 | 2889 to 6438 | 3384 | 2112 to 4657 |
| Post-treatment cost over 4 yearsa | 5057 | 3280 to 6835 | 4127 | 2678 to 5576 |
| 2 years | ||||
| Mean total cost (£) (95% CI) | 7665 (6068 to 9262) | 7209 (5714 to 8704) | ||
| Mean total QALY (95% CI) | 0.76 (0.72 to 0.79) | 0.82 (0.79 to 0.85) | ||
| Incremental cost (ΔC) (95% CI) | 456 (–1823 to 3164) | |||
| Incremental QALYs (ΔQ) (95% CI) | –0.06 (–0.11 to –0.02) | |||
| ICERb (ΔC/ΔQ) (95% CI) | –7600 (–68,356 to 45,346) | |||
| NMBb (ΔQ × λ) – ΔC, λ = £20,000 (95% CI) | –1656 (–4695 to 856) | |||
| 4 years | ||||
| Mean total cost (£) (95% CI) | 7990 (6323 to 9658) | 7802 (6178 to 9426) | ||
| Mean total QALY (95% CI) | 0.76 (0.73 to 0.80) | 0.83 (0.79 to 0.86) | ||
| Incremental cost (ΔC) (95% CI) | 188 (–3435 to 3290) | |||
| Incremental QALYs (ΔQ) (95% CI) | –0.06 (–0.11 to –0.01) | |||
| ICERb (ΔC/ΔQ) (95% CI) | –3133 (–52,975 to 101,463) | |||
| NMBb (ΔQ × λ) – ΔC, λ = £20,000 (95% CI) | –1388 (–5045 to 2610) | |||
The results of the sensitivity analysis on the impact of varying unit cost of procedures on mean total costs show that costs were higher in UAE group than in the myomectomy group (Table 33). Overall, varying cost of interventions had an overall effect on the magnitude of the ICERs (Figure 11). However, the overall results remained consistent with the base-case analysis. UAE was associated with higher costs and lower QALYs gained when compared with myomectomy across the two time horizons. Therefore, myomectomy dominated UAE.
| Treatment | 20% decrement | 20% increment | ||
|---|---|---|---|---|
| Mean total cost (£) (SD) | 95% CI (£) | Mean total cost (£) (SD) | 95% CI (£) | |
| 2 years | ||||
| UAE | 7238 (840) | 5590 to 8885 | 8221 (833) | 6587 to 9855 |
| Myomectomy | 6531 (730) | 5101 to 7961 | 7816 (773) | 6302 to 9330 |
| 4 years | ||||
| UAE | 7536 (863) | 5843 to 9228 | 8546 (873) | 6834 to 10,259 |
| Myomectomy | 7123 (796) | 5562 to 8683 | 8387 (832) | 6757 to 10,017 |
FIGURE 11.
Sensitivity analysis on cost of interventions. (a) ICERs at 2 years and (b) ICERs at 4 years. ICERs are normally not presented when an intervention is dominated by its comparator. However, we present them for completeness. The mean incremental QALY for the ICER was –0.09, which is consistent with the base case, as we only varied the unit cost of procedures in sensitivity analysis. Reproduced with permission from Rana et al. 66 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
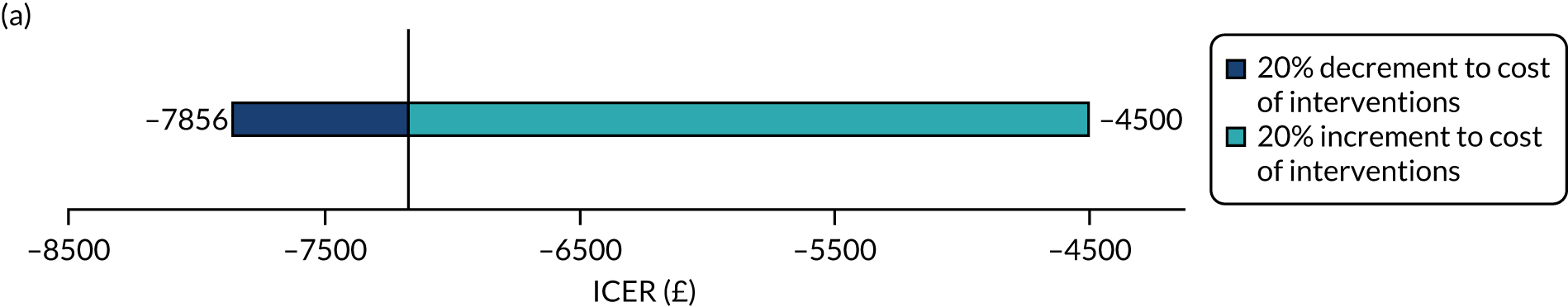
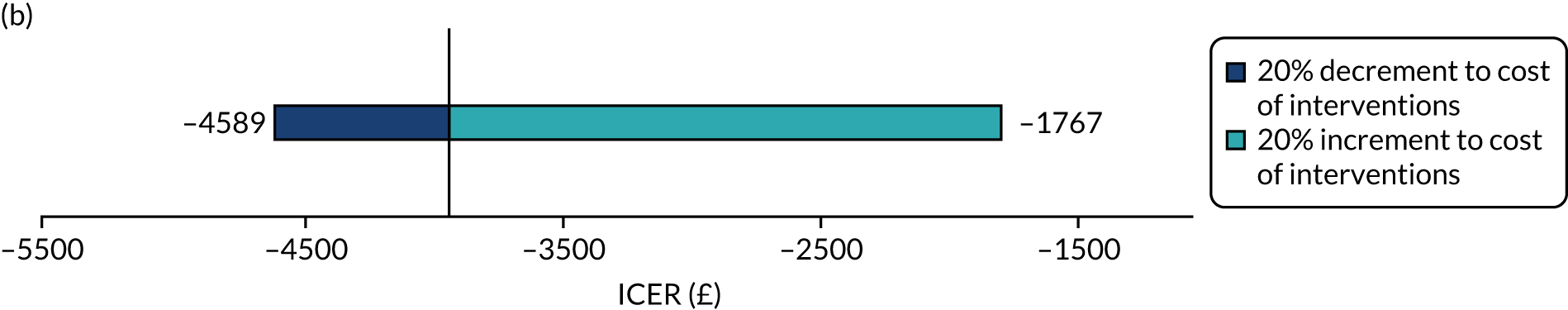
Chapter 7 Discussion
Principal findings
Although both myomectomy and UAE improved participant-reported HRQoL scores, women assigned to the myomectomy group reported higher scores than those assigned to the UAE group. Menstrual bleeding scores appeared similar in both groups. Overall complication rates from all initial procedures occurred in a similar proportion of women in both groups. The need for additional treatments was higher and hospital stay was shorter in the UAE group than in the myomectomy group. There were no consistent differences between groups in biomarkers of ovarian reserve. In total, there were 15 pregnancies in the UAE group and seven pregnancies in the myomectomy group, but these numbers were too small draw a conclusion on the effect of the procedures on fertility. The economic evaluation showed that UAE was associated with higher costs and lower QALYs than myomectomy. At a £20,000 WTP threshold, the probability of myomectomy being cost-effective is 98% at 2 years and 96% at 4 years.
Interpretation
Doubling of the UFS-QOL quality-of-life score from baseline to each time point shows that, on average, both treatments are effective, with an additional 8-point benefit accrued from myomectomy, equating to a small to moderate standardised treatment benefit at 2 years. 79 In a review of the use of EQ-5D in 11 varied populations, a mean minimally important difference of 0.07 was derived. 80 This is consistent with the difference in EQ-5D score observed in this study, corroborating the between-group difference observed for UFS-QOL.
There were substantially more surgical reinterventions in the UAE group in the first 2 years of follow-up, possibly reflecting the higher residual impact on quality of life observed in the UAE group and the marginal patient-reported preference for myomectomy. However, the number of hysterectomies performed as the initial procedure was higher in the myomectomy group because of either patient preference or clinical decision.
Our results show that UAE is associated with higher costs and lower QALYs than myomectomy (£645 and £352 over the 2- and 4-year time horizons, respectively). This incremental cost should be interpreted with caution, as the difference was small and not statistically significant. The difference in QALYs over both the 2- and the 4-year time horizons was 0.09, and this is equivalent to 33 days of perfect health. The cost drivers were GP visits, outpatient appointments and inpatient admissions during the follow-ups and the associated reinterventions for fibroid removal. The key driver for this QALY difference was the substantial improvements associated with pain/discomfort and anxiety/depression observed with the myomectomy group.
Findings in the context of the existing effectiveness literature
The only comparable randomised trial using UFS-QOL29 suggested a between-group difference of 12.1 (95% CI 4.0 to 20.2) points in the HRQoL domain at 1 year, following our reanalysis of their individual patient data, which is consistent with our observation of a 10.8-point difference (95% CI 4.2 to 17.5 points) at the same time point. The changes in score from baseline to 1 year are substantial: an improvement of at least 33 points (maximum score for domain is 100 points) in both the FUME29 and the FEMME trial and following either treatment. Similar large improvements in UFS-QOL scores have been reported following various fibroid treatments. 81,82
In a study from Czechia,27,28 the rate of reintervention within 2 years was 33% in the UAE group and 3% in the myomectomy group, but these rates are not directly comparable with the FEMME results, as the protocol of the former study recommended myomectomy if improvements were not seen 6 months after UAE. Only one participant had a subsequent hysterectomy following myomectomy in the other RCT,29 although the reintervention rate was 15% (i.e. nine participants) more than 1 year after an initial UAE.
There were substantially more pregnancies in the study from Czechia,27,28 with 13 out of 26 women who attempted conception becoming pregnant in the UAE group and 30 out of 40 women in the myomectomy group achieving pregnancy within a mean follow-up period of around 2 years. Half of those women who became pregnant in the UAE group had a subsequent myomectomy. Comparing their pregnancy and pregnancy outcome data with data from the FEMME trial is difficult because of the different proportions of women intending to get pregnant and differences in the age profile of participants.
Previous randomised trials that measured FSH, and used varying thresholds for ovarian failure, also found no evidence of harm from UAE over short and longer time frames. 35 To the best of our knowledge, no other randomised studies have assessed AMH.
Findings in the context of the existing economic literature
To identify the main driver of total mean costs, the costs were categorised according to treatment-related cost and post-treatment cost. The treatment cost of UAE was lower than that of myomectomy. This can be attributed to the fact that the observed LOS was longer for myomectomy than for UAE (i.e. a median of 4 days vs. a median of 2 days), and this is consistent with the literature. 81,83 Women in the UAE group incurred greater post-treatment costs than women in the myomectomy group. The main driver of post-treatment costs was utilisation of health-care resources (e.g. GP visits, outpatient appointments and inpatient admissions), as recorded during the follow-ups associated with reinterventions for fibroid removal. Over the 4 years’ follow-up, the rate of reintervention was higher in the UAE group than in the myomectomy group (hazard ratio 0.53, 95% CI 0.27 to 1.05). Our findings are in line with existing studies that reported on UAE continuously accruing costs in the long term40 and higher reintervention cost than myomectomy. 38 In our study, the majority of the post-treatment costs were accrued within 2 years and only a small amount of additional post-treatment costs incurred between the 2-year and the 4-year follow-up.
Strengths and limitations of the randomised trial
To the best of our knowledge, this study is the largest ever randomised clinical trial to report on treatment of symptomatic fibroids by UAE and myomectomy. The robust study design ensures internal validity, enabling the results to be interpreted with confidence. Randomisation via computer-generated allocation sequence was effective in achieving balanced groups with respect to important prognostic factors.
In the absence of a minimally important difference for between-group comparisons on the UFS-QOL scale,58 the size of the study was driven by a small to moderate standardised effect. This was derived from the published results of the FUME study. 29 Later, we reanalysed the individual participant UFS-QOL data of the FUME study using more appropriate regression methods, accounting for baseline imbalances. This reanalysis generated a larger potential difference and a smaller pooled-group SD. This enabled us to reduce the FEMME trial’s sample size from the original projection, which we were then able to achieve. However, this cannot be considered data driven, as the recalculation was performed before any interim analysis and ratified by the TSC and Data Monitoring Committee, which were blind to any contemporary outcome data.
Our trial design offered a number of other strengths with respect to data collection and analysis. The primary outcome measure and many secondary outcome measures focused on the participants’ report of their symptoms and quality of life. In addition, the primary outcome was fibroid specific and co-developed with women with fibroid symptoms. This ensured that the outcomes reflect the aspects of the condition of most importance to women.
The techniques used for both myomectomy and UAE were determined by the fibroid presentation and the preference of the operating clinician. This enabled a diverse range of fibroid diagnoses to be included in the study, but limits the interpretation of the results to a class comparison of myomectomy with UAE (i.e. the trial did not analyse operative subgroups, e.g. laparoscopic myomectomy vs. UAE, and we did not balance the randomisation by intended myomectomy approach).
We consider the trial design and conduct to have been methodologically robust, but there are some limitations of our study that should be considered. Nineteen per cent of participants failed to return the UFS-QOL questionnaire at 2 years, despite our attempts to retrieve this information. Our analytical approach involved imputation of missing responses using a recognised method, but assumed that data were missing at random and that the reason for non-response was not related to these participants’ quality of life. Any deviation from this assumption could give rise to inconclusive results, given that the lower end of our CIs around effect estimates were close to zero.
A substantial number of women were not recruited because of their preference for a particular treatment option and expectations of treatment benefit were not captured pre randomisation. As with all surgical or interventional procedure trials, not every participant ultimately undergoes the allocated procedure, either for clinical reasons or because of patient preference The proportion of women who did not undergo their allocated procedure differed between groups (UAE group, 20%; myomectomy group, 15%). The numbers undergoing different types of alternative treatment also varied; for example, the number of women undergoing no immediate procedure was nine in the UAE group and four in the myomectomy group. All participants were analysed in the groups to which they were allocated and the per-protocol analysis gave a treatment effect very similar to ITT population analysis, suggesting little impact of non-adherence to the allocation.
The two procedures have considerably different recovery periods, which may be reflected in the first outcome measures reported by participants at 6 months. The duration from randomisation to the procedure averaged around 13 weeks in both groups, and the primary outcome was at 2 years post procedure, as this is a realistic time point at which to compare quality of life.
Some blood samples were unable to be analysed for FSH and LH, as they were not obtained within 5 days of the start of the last menstrual period, reducing the data available to corroborate the AMH results. Despite randomisation, there was a small difference in participant age between the two groups, prompting a post hoc-adjusted analysis of the ovarian reserve markers.
Our statistical analysis plan did not include correction for multiplicity of statistical testing. We have not carried out multiplicity correction for the secondary outcomes in terms of size of CI (p-values were not reported).
Strengths and limitations of the economic evaluation
The economic evaluation is based on robust data collected alongside, to the best of our knowledge, the largest RCT that evaluated this comparison and adhered to good practice guidelines set out by NICE. 67 In accordance with the guidance,67 we took into account relevant resource use and estimated costs, and we estimated health benefits based on the EQ-5D. Robust methods were used in the analysis to explore uncertainties.
A limitation of the analyses is the number of missing health utility data. However, incomplete participant data is an expected feature of trials with long follow-up periods and is attributable to participants being withdrawn or lost to follow-up or non-compliance. We performed sensitivity analysis to examine the impact of missing data. Another limitation is that the health outcomes were assessed using the EQ-5D-3L, rather than the EuroQol-5 Dimensions, five-level version, which is considered to be a more sensitive and precise measure of health status. The former has a tendency to underestimate health utilities by overestimating health problems. 84 Moreover, one inherent limitation of this approach is that it does not take into account patient preference, despite that the literature that suggests that there is no statistically significant difference between UAE and surgery (hysterectomy or myomectomy) in terms of health-related quality of life. 81 Furthermore, this suggestion is supported by the substantial number of women who were not recruited into the trial because of their preference for a particular treatment option. A larger number of participants with similar resource use and outcomes would have decreased uncertainty in our results. Based on the cost–utility framework, the potential trade-off between the additional QALYs gained associated with myomectomy and the potential benefits of avoiding a surgical procedure associated with UAE is not known.
Generalisability
Unlike previous comparisons27–29 of myomectomy and UAE, the FEMME trial was a multicentre clinical trial and did not include or exclude women based on their pregnancy intentions. The generalisability of the findings is increased by the inclusion of multiple centres, gynaecological surgeons and interventional radiologists, allowing both interventions’ impact to be evaluated without confounding by individual variance in clinical practice and skill, although nearly two-thirds of participants were recruited from just three hospitals. A substantial number of participants were of African-Caribbean ethnicity and presented with a wide range of fibroid diagnoses.
Patient and public involvement
We have been supported throughout the project by the Fibroid Network, in particular, and also FEmISA and the British Fibroid Trust. Public and patient involvement was crucial in improving the acceptability of the FEMME trial, in providing authenticity for the trial among women and in promoting recruitment. We engaged with the Fibroid Network founder throughout, developing an appreciation of the lack of choice often presented to women around the treatment of fibroids and uncertainty surrounding UAE and myomectomy among women, as well as the opinions of clinicians and barriers to accessing UAE that the chairperson had encountered. This prompted us to work harder to engage with clinicians to reiterate the uncertainty around both treatments, in particular for women desiring pregnancy.
We will engage with the Fibroid Network and British Fibroid Trust regarding the dissemination of our findings (FEmISA has ceased activity), providing a plain English summary of the findings and the uncertainties around the evidence we have discussed here. This will be distributed via their respective websites and via Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com). Any future research groups taking forward the research recommendations from this project would benefit from engaging with these groups and women directly via social media.
Chapter 8 Conclusions
In conclusion, both UAE and myomectomy are effective treatments for improving the quality of life of women with symptomatic uterine fibroids. UAE is cheaper than myomectomy, but it generates lower health benefits. At a £20,000 WTP threshold, the probability of myomectomy being cost-effective is 98% at 2 years and 96% at 4 years. Based on a cost–utility assessment, UAE is dominated by myomectomy and would not be considered a cost-effective alternative to displace myomectomy. However, this does not take into account any potential preference for a less invasive procedure. In this context, given the small difference in costs between the two procedures, fully informed patient preference should be taken into account and women should have the option to choose between the two procedures.
Implications for health care
Myomectomy and UAE are both established procedures within the repertoire of many gynaecologists and interventional radiologists, respectively, and training of junior doctors should continue to include these procedures. Services should continue to offer both procedures to women where both are potential options. Women, including those desiring a future pregnancy, should be provided with the evidence generated by the FEMME trial to enable them to make a fully informed decision regarding their fibroid treatment.
Research recommendations
The FEMME trial provides high-quality evidence on the clinical effectiveness and cost-effectiveness of UAE compared with myomectomy and, in our opinion, further clinical trials addressing this pragmatic question around quality of life are not required. An individual patient data meta-analysis is under way (PROSPERO CRD42018098676), which aims to identify which patients have the highest risk of unchanged or worsened quality of life and reintervention by 1 year.
Recommendations for further research encompass fertility concerns, long-term outcomes and technical aspects of the interventions. These may be prioritised as follows:
-
The impact of myomectomy, compared with UAE, on live birth rates is the most urgent unanswered question. A comparable trial36 to the FEMME trial, specifically for women with fibroids and no other infertility factors, commenced in France (NCT02577055); however, this trial struggled to recruit in the face of the same concerns regarding the impact of UAE on ovarian function and was terminated after 15 participants were enrolled. 36 The lack of compelling evidence for adverse effects of myomectomy and UAE from the FEMME trial and other sources37 should reduce the barriers to a new randomised trial in women seeking to get pregnant naturally or undergoing assisted reproduction treatment. The effectiveness of hysteroscopic myomectomy of submucosal fibroids in women seeking treatment for infertility or who have had recurrent miscarriages, compared with deferred surgery, is being addressed in a UK randomised trial (URL: https://fundingawards.nihr.ac.uk/award/NIHR128969). The extent of uterine cavity distortion from submusocal fibroids and its impact on the success of fertility following myomectomy may also be revealed from this trial.
-
Long-term follow-up of women who have undergone myomectomy or UAE using routine data to capture further reinterventions in the FEMME cohort would be of benefit.
-
If and when the emerging drug classes of progesterone receptor modulators and oral gonadotropin-releasing hormone receptor antagonists gain marketing authorisation for use by women with uterine fibroids, then these drugs should be evaluated both against standard medical treatments, such the levonorgestrel-releasing intrauterine-releasing system, and against myomectomy or UAE.
-
Adenomyosis is another common menstrual disorder, with symptoms that overlap with those of fibroids, that can often co-exist with fibroids, but is relatively overlooked. A randomised trial of UAE compared with hysterectomy is under way in the Netherlands (URL: www.trialregister.nl/trial/5471; accessed 15 December 2021). Many of the drugs that potentially provide benefit for fibroids or endometriosis could also help with the symptoms of adenomyosis and should also be evaluated in RCTs.
-
Comparison of the surgical approaches to myomectomy may be considered, but the number, size and location of the fibroids are key determinants to the decision to operate hysteroscopically, laparoscopically or by open laparotomy. Outcome data, such as those collected in the FEMME trial and fibroid registries, may help to define and revise the criteria for deciding which types of fibroids require open myomectomy and which can be removed laparoscopically.
-
There are opportunities for further research into some of the technical aspects of myomectomy (e.g. the minimisation of blood loss through the use of tranexamic acid or vasopressin85) and in pain control. Morcellation of fibroid tissue remains a controversial issue and, although modifications to the technique, such as in-bag morcellation, have been compared with uncontained power morcellation,86 the theoretical risk of dissemination of leiomyosarcoma will remain a barrier to further investigation of the value of morcellation.
-
The choice of embolic agent for UAE has generally been at the discretion of the interventional radiologists, although randomised comparisons have been undertaken. 87,88 Further trials could help to refine the endovascular techniques and determine the impact of embolic agent not only on radiological features, such as the extent of infarction, but also on symptom and patient-reported outcomes.
-
Post-embolisation syndrome is a common AE following UAE, particularly associated with larger fibroids, and, although self-limiting, can delay discharge and may mask infection. Identification of risk factors and optimisation of antipyretic and antiemetic therapy would improve the patients’ experience.
-
There are few data on how widely thermal and microwave ablation, and ultrasound-guided high-intensity transcutaneous-focused ultrasound and MRgHIFU techniques, are being used in the UK, as routine data sources do not distinguish between modalities. Anecdotally, these techniques do not appear to be gaining traction in the UK, but if any become more mainstream then their clinical effectiveness and cost-effectiveness should be established against both myomectomy and UAE.
There is also scope for methodological research around the evaluation of fibroid treatments. A core outcome set of the most important outcomes to be measured and reported in clinical trials of fibroid treatment is under development. 89 This initiative will reduce research waste, but will define only the main domains not the actual outcome measures. The UFS-QOL is validated, but was developed from responses from women who had completed their family and, therefore, does not refer to the fertility and pregnancy concerns that women may have. Evaluation of the validity of generic fertility quality-of-life or anxiety questionnaires specifically among women with fibroids desiring pregnancy would be valuable and, if necessary, a new outcome measure for this population could be developed.
Cost–utility analyses using health utilities valued using a generic measure of HRQoL do not take into account patient preference. It is conceivable that some women may place additional value on a non-surgical procedure over a surgical alternative. Further research on how such fully informed patient preference may be quantified and incorporated into subsequent economic analyses of medical, surgical and non-surgical interventions for fibroids would be worthwhile. For example, patient preferences may be elicited through discrete choice experiments as a supplement to QALY measurement.
Acknowledgements
FEMME Collaborative Group
Trial Management and Writing Group
Jane Daniels, Lee Middleton, William McKinnon, Fusun Sirkeci, Isaac Manyonda, Anna-Maria Belli, Mary Ann Lumsden, Jonathan Moss, Olivia Wu and Klim McPherson.
Trial Co-ordinating Team
Trial management: William McKinnon.
Data management: Leanne Beason, Benjamin Ellwood, Jordan Evans and Lisa Leighton.
Database development: Mark Hunter, Adrian Wilcockson and Nicholas Hilken.
Trial Steering Committee
Professor Siladitya Bhattacharya (chairperson), Professor Hilary Critchley, Professor Tony Nicholson and Alison Hurst.
Data Monitoring Committee
Professor Doug Altman (chairperson), Professor Jim Thornton and Dr John Reidy.
Joint Oversight Group
The Joint Oversight Group replaced the TSC and Data Monitoring Committee once recruitment to the trial was completed: Dr John Reidy (chairperson), Mr Nick Amin, Professor Duncan Ettles, Professor Dr Jim Reekers and Dr David Cooper.
Patient and public involvement contributors
Bridgette York (Fibroid Network Support Group), Ginette Camps-Walsh (FEmISA) and Nicki On (British Fibroid Trust).
Investigator group
St George’s Hospital
Dr Lakshmi Ratnam, Dr Leto Mailli, Ms Jocelyn Nocerino, Ms Tabindah Khan, Ms Elizabeth Cruddas, Dr Raj Das and Dr Seyed Renani.
Glasgow Royal Infirmary
Dr Sivanathan Chandramohan, Dr Iain Robertson, Ms Karen Duffy, Dr Grant Urquhart and Dr Ganesh Ananthakrishnan.
Gartnavel General Hospital
Ms Lorraine McGregor, Dr Ibraheem Hamoodi, Ms Isobel Crawford, Ms Lorna McKay and Ms Cecilia Lyons.
Royal Infirmary of Edinburgh
Mr Brian Brady, Mrs Jennifer Devlin, Dr Christine West and Dr Susan Ingram.
University of Edinburgh
Professor Hilary Critchleya and Professor Andrew Horne.
Barking and Havering Hospitals
Mr Leye Thompson, Dr Nissanka Madipola, Ms Annemarie McGregor and Ms Rebecca Murray.
Guy’s and St Thomas’ Hospital
Mr Kumar Kunde, Ms Julia Kopeika, Mr Yakoub Khalaf, Professor Janice Rymer, Mr Rajesh Varma, Dr Irfan Ahmed, Mr Joseph Mechery, Ms Guida Kurzer and Dr Tarun Sabharwal.
John Radcliffe Hospital
Mr Christian Becker, Dr Mark Bratby, Dr Raman Uberoi, Mr Vikram S Rai, Dr Susan Anthony, Mr Stephen Kennedy, Ms Nicola Higgins and Ms Miriam Wilmott-Powell.
Birmingham Women’s Hospital
Professor Justin Clark, Professor Janesh Gupta, Professor Arri Coomarasamy, Ms Shanteela McCooty, Dr Manjeet Shehmar, Miss Diane Mellers and Ms Heather Barrows.
City Hospital Birmingham
Mr Joe Kabukoba, Ms Fiona Kinney, Dr Shagaf Bakour, Dr Ket Tai, Miss Sadia Malick, Miss Abha Sinha and Mr Yousri MM Afifi.
Heartlands Hospital
Ms Shirin Irani, Dr Paul M Crowe, Dr Sarah Manney and Ms Sylvia Turner.
Queen Elizabeth Hospital
Dr Andrew Willis and Dr Robert Jones.
Manchester Royal Infirmary
Dr R Kristina A Naidoo, Mr Leroy Edozien, Dr Nick Chalmers, Ms Mandy Hockey, Dr Anantha-Krishnan Ganapathy, Dr Finn Farquharson, Ms Rebecca Leech, Ms Hannah Phillips, Mrs Lucy Dwyer, Dr Nicola Smith, Dr Gerry Murphy, Miss Linda Green, Miss Julie Melville, Miss Stephanie Bateman and Ms Sharon Kerrison.
Mayday Hospital
Miss Ranee Thakar, Mr Abdul Sultan, Miss Bini Ajay, Mr Michael Chang, Dr David Sarma, Ms Rebecca Byrne and Mr Ajeet Kumar.
Royal Blackburn Hospital
Mr Mark Willett, Sujatha Anand and Ms Susan Gardiner.
Royal Bolton Hospital
Dr James Park Yuen Lay.
Royal Victoria Infirmary
Dr Mark Roberts, Dr David Richardson, Mr Tony Chalhoub, Ms Jemma Fenwick, Ms Victoria Murtha, Miss Fiona Campbell, Ms Fiona Yelnoorkar, Ms Judith Ormonde, Ms Kayleigh Lennox, Ms Alison Kimber and Mrs Patricia Wake.
Sunderland Royal Infirmary
Mr Kim Hinshaw, Mr Menem Yossry, Mr Jonathan Chamberlain, Ms Denise Milford, Ms Eileen Walton, Mrs Gill Campbell, Ms Karen Armstrong and Ms Eileen Walton.
County Durham and Darlington
Dr Partha Sengupta and Ms Jean Dent.
Epsom and St Hellier Hospital
Ms Krithiga Ilangovan, Miss Sadia Malick, Dr Saikat Banerjee, Mr Khaled Abaoub and Dr Kashif Burney.
East Surry Hospital
Dr Karen Jermy, Miss Mahalakshmi Gorti and Mr Ajay Pankhania.
Frimley Park Hospital
Dr Andrew Hatrick.
St Peter’s Hospital
Mr Shaheen Khazali, Ms Linda Bailey, Miss Claire Atkinson, Ms Martha Wrigley, Ms Michelle Davies, Mr Michael Katesmark and Miss Shree Datta.
Royal Stoke Hospital
Mr Jason Cooper, Ms Amanda Redford, Dr David Wells, Ms Suzanne Jerreat, Ms Angela Rooney, Mr Gourab Misra, Dr Zeiad El-Gizawy, Mr Bruce Jarvest, Miss Joanne Lakin and Professor Patrick Shaughn O’Brien.
Leicester General Hospital
Dr Douglas Tincello, Mr Ian Scudamore, Miss CJ Elson, Mr Marwan Habiba, Dr Tanu Singhal, Dr Amman Bolia, Dr Adebimpe Matiluko, Dr Farook Al-Azzawi, Dr Michael Glasby, Ms Carla Christie and Mrs Katie Warwick.
York Hospital
Mr David Pring, Mr Olujimi Jibodu, Miss Nicola Dean, Dr Anthony Bowker, Mr Andrew Gibson, Ms Zoe Coleman, Mr Thomas Smith and Mr Chris Rhymes.
Neath, Port Talbot and Swansea Hospitals
Dr Lavinia Margarit, Mr Richard Penketh, Ms Catherine Lloyd-Bennett, Ms Helen Goldring, Dr Tudor Young and Dr Gareth Tudor.
Queens Medical Centre
Mr Martin C Powell, Mr Nicholas Raine-Fenning, Ms Anna Molnar, Ms Gill Kirkwood and Ms Lucinda Wilson.
Luton and Dunstable Hospital
Miss Shahnaz Akbar, Miss Preeti Patil and Ms Pauline Barrett.
Royal Hallamshire Hospital
Mr Hany Lashen, Dr Trevor Cleveland, Ms Clare Pye, Sister Sheila Duffy, Dr Douglas Turner, Dr Steven Thomas, Mr Christopher Wragg, Mr Andrew J Baxter and Ms Julie Metherall.
Norwich and Norfolk Hospital
Mr Edward P Morris, Dr Simon Girling, Ms Helene Woodhouse, Mr Paul Simpson and Dr Thilina Palihawadana.
Ninewells Hospital
Dr John Graeme Houston, Ms Vanessa Kay, Ms Wendy McMullen, Mrs Audrey Lyall, Mrs Rachael Banks, Ms Helen Cumming and Ms Pamel Duthie.
St James’ Hospital
Mr Richard Hutson, Dr Tony Nicholson, Mr Jaime Canbell, Dr Chris Hammond, Dr David Kessel, Dr Simon J McPherson, Miss Jacqueline Tay, Dr Jai Patel, Dr Sapna Puppala, Mr Jonathan Pearce, Ms Marie Home, Ms Viv Dolby, Ms Cathy Cocking and Ms Sharon Nettleton.
Royal Free Hospital
Dr Neil Davies, Dr Eleni Mavrides, Mr Adam Magos, Mr Ioannis Tsimpanakos and Ms Mina Karamshi.
Contributions of authors
Jane Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials) was involved in the conceptualisation, writing (original draft, except Chapters 5 and 6, review and editing), project administration, investigation and funding acquisition.
Lee J Middleton (https://orcid.org/0000-0003-4621-1922) (Lead Medical Statistician) was involved in the statistical analysis plan, data curation, formal analysis and writing (review and editing).
Versha Cheed (https://orcid.org/0000-0002-6713-0913) (Medical Statistician) was involved in data curation, formal analysis and writing (review and editing).
William McKinnon (https://orcid.org/0000-0001-8734-2932) (Trial Management Team Leader) was involved in writing (review and editing), investigation and project administration.
Dikshyanta Rana (https://orcid.org/0000-0001-9133-3094) (Research Assistant, Health Economics) was involved in data curation, formal analysis (economic evaluation) and writing (original draft, including Chapters 5 and 6, review and editing).
Fusun Sirkeci (https://orcid.org/0000-0002-8221-5763) (Clinical Research Fellow, Gynaecology) was involved in investigation and writing (review and editing).
Isaac Manyonda (https://orcid.org/0000-0003-3080-2357) (Professor of Obstetrics and Gynaecology) was involved in the conceptualisation, writing (review and editing), investigation, funding acquisition and was a site principal investigator.
Anna-Maria Belli (https://orcid.org/0000-0002-3703-9596) (Professor of Interventional Radiology) was involved in the conceptualisation, writing (review and editing), investigation, funding acquisition and was a site principal investigator.
Mary Ann Lumsden (https://orcid.org/0000-0003-2998-9185) (Professor of Obstetrics and Gynaecology) was involved in the conceptualisation, writing (review and editing), investigation, funding acquisition and was a site principal investigator.
Jonathan Moss (https://orcid.org/0000-0002-4589-9174) (Professor of Interventional Radiology) was involved in the conceptualisation, writing (review and editing), investigation, funding acquisition and was a site principal investigator.
Olivia Wu (https://orcid.org/0000-0002-0570-6016) (Professor of Health Economics) was involved in the conceptualisation, formal analysis (economic evaluation), writing (review and editing) and funding acquisition.
Klim McPherson (https://orcid.org/0000-0002-5461-2569) (Emeritus Professor of Public Health) was involved in the conceptualisation, statistical analysis plan, writing (review and editing) and funding acquisition.
Publications
McPherson K, Manyonda I, Lumsden MA, Belli AM, Moss J, Wu O, et al. A randomised trial of treating fibroids with either embolisation or myomectomy to measure the effect on quality of life among women wishing to avoid hysterectomy (the FEMME study): study protocol for a randomised controlled trial. Trials 2014;15:468.
Manyonda I, Belli A, Lumsden MA, Moss J, McKinnon W, Middleton L, et al. Uterine artery embolisation or myomectomy for uterine fibroids. N Engl J Med 2020;383:440–51.
Rana D, Wu O, Cheed V, Middleton LJ, Moss J, Lumsden M-A, et al. Uterine artery embolisation or myomectomy for women with uterine fibroids wishing to avoid hysterectomy: a cost–utility analysis of the FEMME trial. BJOG 2021;128:1793–802.
Daniels J, Middleton LJ, Cheed V, McKinnon W, Sirkeci F, Manyonda I, et al. Uterine artery embolization or myomectomy for women with uterine fibroids: four-year follow-up of a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol 2021;13:100139.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- McPherson K, Manyonda I, Lumsden MA, Belli AM, Moss J, Wu O, et al. A randomised trial of treating fibroids with either embolisation or myomectomy to measure the effect on quality of life among women wishing to avoid hysterectomy (the FEMME study): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-468.
- Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet 2015;131:117-22. https://doi.org/10.1016/j.ijgo.2015.04.051.
- Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, Maroulis C, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol 2010;152:96-102. https://doi.org/10.1016/j.ejogrb.2010.05.012.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1-319.e20. https://doi.org/10.1016/j.ajog.2013.07.017.
- Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update 2007;13:465-76. https://doi.org/10.1093/humupd/dmm013.
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009;91:1215-23. https://doi.org/10.1016/j.fertnstert.2008.01.051.
- Sundermann AC, Velez Edwards DR, Bray MJ, Jones SH, Latham SM, Hartmann KE. Leiomyomas in pregnancy and spontaneous abortion: a systematic review and meta-analysis. Obstet Gynecol 2017;130:1065-72. https://doi.org/10.1097/AOG.0000000000002313.
- NHS Digital . Hospital Episode Statistics, Admitted Patient Care, England – 2012–2013: Diagnosis 2013.
- Munro MG, Critchley HOD, Fraser IS. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet 2018;143:393-408. https://doi.org/10.1002/ijgo.12666.
- National Collaborating Centre for Women’s and Children’s Health . Heavy Menstrual Bleeding. Clinical Guideline CG044 2007.
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421-32. https://doi.org/10.1056/NEJMoa1103180.
- Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, Zatik J, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165-73.e4. https://doi.org/10.1016/j.fertnstert.2015.09.032.
- DTB Team . Ulipristal acetate (Esmya): restrictions on use. Drug Ther Bull 2018;56.
- Diamond MP, Stewart EA, Williams ARW, Carr BR, Myers ER, Feldman RA, et al. A 12-month extension study to evaluate the safety and efficacy of asoprisnil in women with heavy menstrual bleeding and uterine fibroids. Hum Reprod Open 2019;2019. https://doi.org/10.1093/hropen/hoz027.
- Ciebiera M, Vitale SG, Ferrero S, Vilos GA, Barra F, Caruso S, et al. Vilaprisan, a new selective progesterone receptor modulator in uterine fibroid pharmacotherapy-will it really be a breakthrough?. Curr Pharm Des 2020;26:300-9. https://doi.org/10.2174/1381612826666200127092208.
- LaCroix AZ, Freeman EW, Larson J, Carpenter JS, Joffe H, Reed SD, et al. Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial. Maturitas 2012;73:361-8. https://doi.org/10.1016/j.maturitas.2012.09.006.
- Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med 2020;382:328-40. https://doi.org/10.1056/NEJMoa1904351.
- Osuga Y, Enya K, Kudou K, Hoshiai H. Relugolix, a novel oral gonadotropin-releasing hormone antagonist, in the treatment of pain symptoms associated with uterine fibroids: a randomized, placebo-controlled, phase 3 study in Japanese women. Fertil Steril 2019;112:922-9.e2. https://doi.org/10.1016/j.fertnstert.2019.07.013.
- Pohl O, Marchand L, Bell D, Gotteland JP. Effects of combined GnRH receptor antagonist linzagolix and hormonal add-back therapy on vaginal bleeding-delayed add-back onset does not improve bleeding pattern. Reprod Sci 2020;27:988-95. https://doi.org/10.1007/s43032-020-00172-z.
- Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod 2007;22:1996-2005. https://doi.org/10.1093/humrep/dem105.
- Rashid S, Khaund A, Murray LS, Moss JG, Cooper K, Lyons D, et al. The effects of uterine artery embolisation and surgical treatment on ovarian function in women with uterine fibroids. BJOG 2010;117:985-9. https://doi.org/10.1111/j.1471-0528.2010.02579.x.
- Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. J Vasc Interv Radiol 2013;24:459-67. https://doi.org/10.1016/j.jvir.2012.12.002.
- National Institute for Health and Care Excellence (NICE) . Magnetic Resonance Image-Guided Transcutaneous Focused Ultrasound for Uterine Fibroids. Interventional Procedures Guidance [IPG413] 2011.
- Laughlin-Tommaso S, Barnard EP, AbdElmagied AM, Vaughan LE, Weaver AL, Hesley GK, et al. FIRSTT study: randomized controlled trial of uterine artery embolization vs focused ultrasound surgery. Am J Obstet Gynecol 2019;220:174.e1-174.e13. https://doi.org/10.1016/j.ajog.2018.10.032.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the SONATA pivotal trial. J Gynecol Surg 2019;35:345-9. https://doi.org/10.1089/gyn.2019.0012.
- Ierardi AM, Savasi V, Angileri SA, Petrillo M, Sbaraini S, Pinto A, et al. Percutaneous high frequency microwave ablation of uterine fibroids: systematic review. Biomed Res Int 2018;2018. https://doi.org/10.1155/2018/2360107.
- Mara M, Fucikova Z, Maskova J, Kuzel D, Haakova L. Uterine fibroid embolization versus myomectomy in women wishing to preserve fertility: preliminary results of a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2006;126:226-33. https://doi.org/10.1016/j.ejogrb.2005.10.008.
- Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol 2008;31:73-85. https://doi.org/10.1007/s00270-007-9195-2.
- Manyonda IT, Bratby M, Horst JS, Banu N, Gorti M, Belli AM. Uterine artery embolization versus myomectomy: impact on quality of life – results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) trial. Cardiovasc Intervent Radiol 2012;35:530-6. https://doi.org/10.1007/s00270-011-0228-5.
- Moss JG, Cooper KG, Khaund A, Murray LS, Murray GD, Wu O, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG 2011;118:936-44. https://doi.org/10.1111/j.1471-0528.2011.02952.x.
- Jun F, Yamin L, Xinli X, Zhe L, Min Z, Bo Z, et al. Uterine artery embolization versus surgery for symptomatic uterine fibroids: a randomized controlled trial and a meta-analysis of the literature. Arch Gynecol Obstet 2012;285:1407-13. https://doi.org/10.1007/s00404-011-2065-9.
- Ruuskanen A, Hippeläinen M, Sipola P, Manninen H. Uterine artery embolisation versus hysterectomy for leiomyomas: primary and 2-year follow-up results of a randomised prospective clinical trial. Eur Radiol 2010;20:2524-32. https://doi.org/10.1007/s00330-010-1829-0.
- Hehenkamp WJ, Volkers NA, Donderwinkel PF, de Blok S, Birnie E, Ankum WM, et al. Uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids (EMMY trial): peri- and postprocedural results from a randomized controlled trial. Am J Obstet Gynecol 2005;193:1618-29. https://doi.org/10.1016/j.ajog.2005.05.017.
- Pinto I, Chimeno P, Romo A, Paúl L, Haya J, de la Cal MA, et al. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment – a prospective, randomized, and controlled clinical trial. Radiology 2003;226:425-31. https://doi.org/10.1148/radiol.2262011716.
- Fonseca MCM, Castro R, Machado M, Conte T, Girao MJBC. Uterine artery embolization and surgical methods for the treatment of symptomatic uterine leiomyomas: a systemic review and meta-analysis followed by indirect treatment comparison. Clin Ther 2017;39:1438-55.e2. https://doi.org/10.1016/j.clinthera.2017.05.346.
- Torre A, Fauconnier A, Kahn V, Limot O, Bussierres L, Pelage JP. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol 2017;27:2850-9. https://doi.org/10.1007/s00330-016-4681-z.
- El Shamy T, Amer SAK, Mohamed AA, James C, Jayaprakasan K. The impact of uterine artery embolization on ovarian reserve: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2020;99:16-23. https://doi.org/10.1111/aogs.13698.
- You JH, Sahota DS, Yuen PM. Uterine artery embolization, hysterectomy, or myomectomy for symptomatic uterine fibroids: a cost–utility analysis. Fertil Steril 2009;91:580-8. https://doi.org/10.1016/j.fertnstert.2007.11.078.
- Zowall H, Cairns JA, Brewer C, Lamping DL, Gedroyc WM, Regan L. Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG 2008;115:653-62. https://doi.org/10.1111/j.1471-0528.2007.01657.x.
- Wu O, Briggs A, Dutton S, Hirst A, Maresh M, Nicholson A, et al. Uterine artery embolisation or hysterectomy for the treatment of symptomatic uterine fibroids: a cost-utility analysis of the HOPEFUL study. BJOG 2007;114:1352-62. https://doi.org/10.1111/j.1471-0528.2007.01525.x.
- Babashov V, Palimaka S, Blackhouse G, O’Reilly D. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) for treatment of symptomatic uterine fibroids: an economic analysis. Ont Health Technol Assess Ser 2015;15:1-61.
- Cain-Nielsen AH, Moriarty JP, Stewart EA, Borah BJ. Cost-effectiveness of uterine-preserving procedures for the treatment of uterine fibroid symptoms in the USA. J Comp Eff Res 2014;3:503-14. https://doi.org/10.2217/cer.14.32.
- O’Sullivan AK, Thompson D, Chu P, Lee DW, Stewart EA, Weinstein MC. Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care 2009;25:14-25. https://doi.org/10.1017/S0266462309090035.
- Kong CY, Meng L, Omer ZB, Swan JS, Srouji S, Gazelle GS, et al. MRI-guided focused ultrasound surgery for uterine fibroid treatment: a cost-effectiveness analysis. Am J Roentgenol 2014;203:361-71. https://doi.org/10.2214/AJR.13.11446.
- Beinfeld MT, Bosch JL, Isaacson KB, Gazelle GS. Cost-effectiveness of uterine artery embolization and hysterectomy for uterine fibroids. Radiology 2004;230:207-13. https://doi.org/10.1148/radiol.2301021482.
- Canadian Agency for Drugs and Technologies in Health . Uterine-Preserving Interventions for the Management of Symptomatic Uterine Fibroids: A Systematic Review of Clinical and Cost-Effectiveness 2016. www.cadth.ca/interventions-for-the-management-of-symptomatic-uterine-fibroids (accessed 2 June 2020).
- Royal College of Radiologists . Clinical Recommendations on the Use of Uterine Artery Embolisation in the Management of Fibroids 2009.
- American Association of Gynecologic Laparoscopists . AAGL practice report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol 2012;19:152-71. https://doi.org/10.1016/j.jmig.2011.09.005.
- Knuttinen MG, Stark G, Hohenwalter EJ, Bradley LD, Braun AR, Gipson MG, et al. ACR Appropriateness Criteria® radiologic management of uterine leiomyomas. J Am Coll Radiol 2018;15:S160-70. https://doi.org/10.1016/j.jacr.2018.03.010.
- Royal Australian and New Zealand College of Obstetricians and Gynaecologists . Uterine Artery Embolisation for the Treatment of Uterine Fibroids 2020. https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical%20-%20Gynaecology/Uterine-Artery-Embolisation-(C-Gyn-23)_2.pdf?ext=.pdf (accessed 2 June 2020).
- Sandberg EM, Tummers FHMP, Cohen SL, van den Haak L, Dekkers OM, Jansen FW. Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and meta-analysis. Fertil Steril 2018;109:698-707.e1. https://doi.org/10.1016/j.fertnstert.2017.11.033.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online 2020;40:429-44. https://doi.org/10.1016/j.rbmo.2020.01.003.
- Homer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril 2010;94:324-30. https://doi.org/10.1016/j.fertnstert.2009.02.069.
- DAMOCLES Study Group . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Great Britain . Mental Capacity Act 2005 2005.
- Great Britain . Adults With Incapacity (Scotland) Act 2000 2000.
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290-30. https://doi.org/10.1097/00006250-200202000-00021.
- Harding G, Coyne KS, Thompson CL, Spies JB. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-99.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health 2012;15:135-42. https://doi.org/10.1016/j.jval.2011.07.007.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734-9. https://doi.org/10.1111/j.1471-0528.1990.tb16249.x.
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. New York, NY: John Wiley & Sons, Inc.; 2002.
- Manyonda I, Belli A, Lumsden MA, Moss J, McKinnon W, Middleton L, et al. Uterine artery embolisation or myomectomy for uterine fibroids. New Engl J Med 2020;383:440-51. https://doi.org/10.1056/NEJMoa1914735.
- Daniels J, Middleton LJ, Cheed V, McKinnon W, Sirkeci F, Manyonda I, et al. Uterine artery embolization or myomectomy for women with uterine fibroids: four-year follow-up of a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol 2021;13.
- Rana D, Wu O, Cheed V, Middleton LJ, Moss J, Lumsden M-A, et al. Uterine artery embolisation or myomectomy for women with uterine fibroids wishing to avoid hysterectomy: a cost–utility analysis of the FEMME trial. BJOG 2021;128:1793-802. https://doi.org/10.1111/1471-0528.16781.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Department of Health and Social Care . Reference Costs 2017–2018 2018. www.england.nhs.uk/national-cost-collection/ (accessed 2 June 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. 2018. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2018 (accessed 2 June 2020).
- The Royal Pharmaceutical Society . British National Formulary 2020. www.medicinescomplete.com (accessed 2 June 2020).
- NHS Cost Inflation Index 2018 19 2019. www.google.com/url?sa=t%26rct=j%26q=%26esrc=s%26source=web%26cd=%26ved=2ahUKEwjZve78juP0AhVbilwKHRsZB-8QFnoECBAQAQ%26url=https%3A%2F%2Fwww.pssru.ac.uk%2Fpub%2Fuc%2Fuc2019%2FNHS-Inflation-Index-version-1.9A.xlsx%26usg=AOvVaw1N3WPzF18cF5tFAsG3djog (accessed 14 December 2021).
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. https://doi.org/10.1002/hec.1785.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521-33. https://doi.org/10.1111/j.1524-4733.2005.00045.x.
- Glick H, Doshhi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2011.
- Cohen J. Statistical Power Analysis for Behavioral Sciences: Revised. New York, NY: Academic Press; 1977.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Gupta JK, Sinha AS, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD005073.pub2.
- Narayan A, Lee AS, Kuo GP, Powe N, Kim HS. Uterine artery embolization versus abdominal myomectomy: a long-term clinical outcome comparison. J Vasc Interv Radiol 2010;21:1011-17. https://doi.org/10.1016/j.jvir.2010.03.012.
- Goodwin SC, Bradley LD, Lipman JC, Stewart EA, Nosher JL, Sterling KM, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril 2006;85:14-21. https://doi.org/10.1016/j.fertnstert.2005.05.074.
- Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. PharmacoEconomics 2018;36:675-97. https://doi.org/10.1007/s40273-018-0623-8.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev 2014;8. https://doi.org/10.1002/14651858.CD005355.pub5.
- Zullo F, Venturella R, Raffone A, Saccone G. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy. Cochrane Database Syst Rev 2020;5. https://doi.org/10.1002/14651858.CD013352.pub2.
- Yadavali R, Ananthakrishnan G, Sim M, Monaghan K, McNaught G, Hamoodi I, et al. Randomised trial of two embolic agents for uterine artery embolisation for fibroids: gelfoam versus embospheres (RAGE trial). CVIR Endovasc 2019;2. https://doi.org/10.1186/s42155-018-0044-y.
- Das R, Champaneria R, Daniels JP, Belli AM. Comparison of embolic agents used in uterine artery embolisation: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2014;37:1179-90. https://doi.org/10.1007/s00270-013-0790-0.
- COMET Initiative . CoreUF: Developing a Core Outcome Set for Symptomatic Uterine Fibroids 2020. www.comet-initiative.org/studies/details/1361 (accessed 2 June 2020).
List of abbreviations
- AE
- adverse event
- AMH
- anti-Müllerian hormone
- BNF
- British National Formulary
- CEMRI
- contrast-enhanced magnetic resonance imaging
- CI
- confidence interval
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FSH
- follicle-stimulating hormone
- FUME
- Fibroids of the Uterus: Myomectomy versus Embolization
- GP
- general practitioner
- HMB
- heavy menstrual bleeding
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- LH
- luteinising hormone
- LOS
- length of stay
- MRgHIFU
- magnetic resonance-guided high-intensity transcutaneous-focused ultrasound
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- PBAC
- pictorial bleeding assessment chart
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- TSC
- Trial Steering Committee
- TVUS
- transvaginal ultrasound scan
- UAE
- uterine artery embolisation
- UFS-QOL
- Uterine Fibroid Symptom Quality of Life
- UPA
- ulipristal acetate
- VAS
- visual analogue scale
- WTP
- willingness to pay
