Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/111/31. The contractual start date was in May 2018. The draft report began editorial review in September 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Watkins et al. This work was produced by Watkins et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Watkins et al.
Chapter 1 Introduction
Parts of this report have been adapted with permission from the trial protocol. 1
Parts of this report have also been adapted with permission from the statistical analysis plan (SAP). 2
Background
This study addressed the management of urinary incontinence (UI) in patients admitted to hospital with acute stroke. UI affects around half of stroke survivors in the acute phase. 3–5 As many as 44% and 38% of stroke survivors remain incontinent at 3 months and 1 year, respectively. 6 UI often presents as a new problem after stroke or, if pre-existing, worsens significantly, adding to the disability and helplessness caused by neurological deficits. 7
The more severe a stroke is, the greater the likelihood of UI. 8,9 Other factors that increase the likelihood of UI include older age and cognitive impairment. 10 Urge incontinence (involuntary leakage immediately following or concurrent with an urgent sensation of needing to void10) is the most common type of UI after stroke11 and is generally the result of detrusor overactivity. 11
It is important to study UI in this population, as the symptoms are more severe and have more of an effect on stroke survivors than on other groups. 7 Furthermore, associated stroke impairments compound bladder control difficulties, with motor, visual or speech problems making the task of accessing toilet facilities a challenge. 12
Urinary incontinence is distressing for individuals and families, and depression is twice as common in stroke survivors who are incontinent. 13 Negative social consequences for survivors and carers cannot be ignored; both may become isolated and marginalised. 14 Continuing incontinence is associated with poor outcomes in both stroke survivors and carers. 4,5,15
Although clinical guidelines state that indwelling urinary catheters (IUCs) should be used to relieve retention only,16 there is an over-reliance on catheterisation as a management strategy for UI in stroke units, especially in the acute phase. 17,18 This puts patients at risk of IUC-associated urinary tract infection (UTI) and its consequences,19–22 including increased morbidity, mortality rate and resource use. 21,23,24 In the Identifying Continence OptioNs after Stroke (ICONS)-I feasibility trial, 48% of patients had been catheterised prior to recruitment in the acute phase. 25 The number of UTIs and extent of antibiotic use are considerably greater in patients with IUCs than in those without, with an increasing risk of infection associated with IUC removal. 25 IUCs should be used not to manage incontinence, but rather to relieve urinary retention. Only by including all patients with IUCs and encouraging a trial without catheter (TWOC) were we able to include all patients with potential incontinence. The ICONS-II trial promoted catheter avoidance and aimed to reduce the number of UTIs and the need for antibiotic treatment.
We expected our intervention to reduce the number of patients with UI by ≥ 5% and improve continence in at least a further 5–10% of patients. If the intervention had been shown to be effective and were to have been adopted across the UK, assuming that 40,000 stroke survivors per annum have UI at 3 months,6 around 2000 patients would have become continent and around 2000–4000 would have had improved continence. Currently, patients with UI after stroke typically receive care focused on containment, using strategies that do not promote continence (e.g. pads) and that are likely to be harmful (e.g. IUCs17,18,26). The ICONS-II trial was designed to provide high-quality evidence regarding the clinical and economic effects of a new approach to assessing, managing and treating UI after stroke.
Rationale for the trial
Stroke is the third-largest cause of death and the largest single cause of severe adult disability,27 with up to 95,000 people per annum surviving a stroke in the UK. The incidence of stroke is unlikely to decline given the ageing population,28 and its prevalence continues to rise. 29,30 Stroke patients with UI have considerably worse outcomes than those without UI: there are clear associations between UI after stroke and death, disability and an increased likelihood of being discharged to residential care. 4,8,31 Addressing UI early and effectively could have a major impact on these outcomes and significantly improve the quality of life (QoL) of patients. 32
Improving the management of UI after stroke has been identified as an urgent priority in successive Sentinel Stroke National Audit Programme (SSNAP) reports,33,34 but there is a lack of evidence that this aspect of care has improved. Consequently, the National Institute for Health and Care Excellence (NICE) has recommended further research into improved continence care after neurological events. 35
To the best of our knowledge, this trial was the first to test the effect of a programme to assess and treat UI after stroke in hospital, building on our feasibility trial. 25,35,36 Although the feasibility trial was not powered to demonstrate effectiveness, there were indications that the intervention may work, particularly for urge incontinence. Staff believed that the programme improved patient outcomes and was sustainable: five out of eight sites reported continuing core aspects of continence promotion after the research was completed.
The update of our Cochrane review37 revealed several new studies,38–43 although the conclusion – that data from the available trials are insufficient to guide continence care – was unlikely to change without a definitive trial. The ICONS-II trial was intended to address this gap in the evidence base.
Chapter 2 Methods
Original and modified trial design
The ICONS-II trial was preceded by the ICONS-I trial, a cluster-randomised feasibility trial of a similar intervention compared with usual care, funded by the National Institute for Health and Care Research (NIHR) Programme Grants for Applied Research (PGfAR) programme. 25 This was implemented as a change in practice in intervention sites, but analysis of recruitment patterns showed differences in participant characteristics between control and intervention sites. Feedback from a rejected NIHR Health Technology Assessment (HTA) application for a cluster-randomised effectiveness trial led to a change in design to individual patient randomisation (IPR) for the ICONS-II trial. It was considered that IPR would reduce the possibility of selection bias, indicated by the difference in participant characteristics between the cluster trial groups. It would also avoid the large inflation of sample size required by clustering (despite an upwards adjustment to the sample size to allow for potential contamination).
As a result, the trial was designed as a pragmatic, multicentre, individual-patient-randomised (1 : 1), parallel-group trial with an internal pilot, with 512 participants in the intervention group undergoing the systematic voiding programme (SVP) and 512 participants in the usual-care group. The original plan was that the results from an internal pilot, with an initial target of recruiting 355 participants and a total of 72 site-months of recruitment, would be used to determine progression to full trial. In May 2019, just before recruitment started, a decision was taken to shorten the duration of the pilot owing to the late opening of the trial. The expected number of site-months in the internal pilot was reduced from 72 to 45, and the number of participants was reduced from 355 to 225–315. A few other minor changes to the trial conduct were introduced, particularly after the first meeting of the ICONS-II Trial Steering Committee (TSC). The list of the minor changes to the ICONS-II trial protocol is provided in Appendix 6. The trial included a process evaluation that investigated fidelity to the intervention and usual care, and an economic evaluation.
Trial summary
Aim and objectives
The aim of the study was to evaluate the clinical and economic effect of a SVP for UI after stroke in secondary care.
The study comprised a trial with an internal pilot. The objective of the internal pilot was to assess the feasibility of participant recruitment and the success of strategies for minimising contamination to the usual-care group.
The objectives of the trial were to:
-
determine if a SVP affects severity of UI compared with usual care at 3 months post randomisation (primary objective)
-
determine if a SVP affects the –
-
number of UTIs
-
number of days IUC in situ
-
urinary symptoms, QoL, functional independence, falls and mortality rate compared with usual care.
-
-
determine if the SVP is cost-effective in terms of quality-adjusted life-years (QALYs) gained compared with usual care at 6 months post randomisation
-
assess fidelity to the intervention and to usual care in a process evaluation.
Participants
The trial was planned to take place in 18 NHS stroke services with stroke units.
The trial population was men and women with stroke and UI, including those with cognitive impairment.
Inclusion criteria
-
Adults with acute stroke. 44
-
UI, defined as ‘involuntary loss of urine’45 within 72 hours of admission to the stroke unit, or presence of IUC at the time of consent.
-
Patient was conscious: ‘alert’ or ‘not alert but arousable’ [National Institutes of Health Stroke Scale (NIHSS) Point 1 A score of 0 or 1] on the NIHSS38,46 within 14 days of admission to the stroke unit. Note that patients had to have been recruited within 72 hours of meeting this criterion whenever possible.
Exclusion criteria
-
Long-term IUC pre stroke.
-
Subdural or subarachnoid haemorrhage.
Identifying and approaching participants
All patients admitted to participating stroke units were screened by Clinical Research Network (CRN) nurses and project-specific research nurses to determine their eligibility. Our aim was to identify all eligible patients, to limit the potential for recruitment bias. To facilitate this, we maintained a screening log in each site. In the case of patients who were ineligible or did not participate, only anonymised data were recorded. To identify eligible patients, CRN nurses and project-specific research nurses checked the case notes and fluid balance charts daily of all patients admitted to the stroke unit to establish whether or not UI was present, apart from those of patients with an IUC, who were eligible for inclusion if they met the other inclusion criteria. Study information was provided to all potentially eligible patients as soon as possible after their admission to the stroke unit (i.e. as soon as they experienced an incontinence episode or had an IUC). Whenever possible, patients meeting the inclusion criteria were recruited within 72 hours of admission to the stroke unit. Some patients were too ill during this period but improved; for these patients, the recruitment window was up to 14 days post admission.
Potential patients who did not speak English were invited to take part in the study if they had a family member or friend who was able to interpret for them.
Informed consent for participation in the study was sought from all patients with the capacity to consent. Our Speakeasy (Bury, UK) Patient, Carer and Public Involvement (PCPI) group developed aphasia-friendly patient information leaflets and consent forms in the ICONS-I feasibility trial. We used these (amended appropriately) in the ICONS-II trial for patients with aphasia. We respected the right of patients to decline to participate, or to withdraw from the study at any time.
Part of our programme, namely prompted voiding, was targeted primarily at patients with cognitive problems. To involve patients who lacked the capacity to consent, we invited someone close to the patient to act as a ‘consultee’ and provide advice (rather than consent). The consultee was someone who knew the patient well and was usually either a friend or a family member. As our intervention was designed to include participants with communication problems and/or cognitive problems, carers could act as:
-
a ‘consultee’, giving assent for their relative/friend to take part in the study
-
an informant on behalf of a participant who was unable to consent or communicate.
If the CRN nurse or project-specific research nurse believed that a patient’s capacity was in question, then they identified this and provided the information to the patient’s personal consultee. If a personal consultee was not available, then a nominated consultee was identified by the study team.
Informed consent
Procedures to seek and gain informed consent from eligible potential participants were agreed with the Research Ethics Committee (REC). NHS Health Research Authority (HRA) approval was received on 3 April 2018 (REC reference 18/WA/0108). The application was submitted to a REC for research involving adults lacking capacity. Ethics approval was also obtained from the University of Central Lancashire Science, Technology, Engineering, Medicine and Health (STEMH) Ethics Committee once NHS ethics approval had been obtained.
Participant recruitment
All recruitment was undertaken by the CRN nurses and project-specific research nurses. If the patient lacked capacity to consent, then a personal or nominated consultee provided advice on what they felt the person’s wishes would be if they had capacity. The consultee signed a declaration form if they believed the patient would choose to agree to participate. Consultees could advise, at any point, that they believed that the person’s wishes about participation had changed and that the person should therefore be withdrawn from study participation.
Participants who fulfilled the eligibility criteria and had consented and completed their baseline questionnaire (and those participants for whom assent had been obtained from a consultee and their baseline questionnaire had been completed) were then randomised to the intervention or to usual care.
Intervention
The SVP comprised assessment, behavioural interventions (bladder training or prompted voiding) and review. Assessment included evaluation of the need for an IUC to minimise inappropriate catheterisation, a protocol for IUC removal (if clinically justifiable), a 3-day bladder diary (to assess the pattern of UI) and an evidence-based continence assessment (to classify the type of UI).
The continence assessment included history taking, urine dipstick examination and (if indicated) a mid-stream urine specimen tested by microscopic examination, culture and sensitivity; a bladder scan to estimate post-void residual urine volume; and identification of the type of incontinence [stress UI: any response other than ‘never’ to the Leicester Urinary Symptom Questionnaire (LUSQ)47 question ‘Do you ever leak when you do any of the following?’; urge UI: the response ‘most of the time’, ‘sometimes’ or ‘occasionally’ to the LUSQ question ‘When you get the urge to pass urine, does any leak before you get to the toilet?’; mixed UI: both stress and urge UI; or ‘functional’ UI: defined as mobility or balance restrictions stopping patients reaching the toilet in time)].
Patients who were catheterised were assessed for a TWOC within 72 hours of admission. Patients who were not catheterised and who were cognitively able received bladder training; patients with cognitive impairment or no control over their bladder received prompted voiding. The ICONS-II trial staff members made this decision (supported by the project-specific research nurse) based on the following criteria:
-
prompted voiding – patients with cognitive impairment at baseline, defined as a score of ≥ 8 on the Six-Item Cognitive Impairment Test (6-CIT);48 or patients who had no control over their bladder, defined as answering ‘all the time’ to the International Consultation on Incontinence Questionnaire – Urinary Incontinence – Short Form (ICIQ-UI-SF) question ‘How often do you leak urine?’
-
bladder training – patients with no cognitive impairment at baseline, defined as a score of 0–7 on the 6-CIT; and some control over their bladder, defined as answering ‘several times a day’, ‘about once a day’, ‘two or three times a week’ or ‘about once a week or less often’ to the ICIQ-UI-SF question ‘How often do you leak urine?’.
For participants catheterised in the acute stage, staff members were asked to conduct a TWOC as early as possible, unless there was a valid clinical reason not to do so, using a modified version of the HOUDINI (Hematuria, Obstruction, Urologic surgery, Decubitus surgery, Intake and outtake, No code, Immobility) protocol. 49 Once the catheter was removed, participants underwent assessment as described above.
Bladder training aimed to help patients regain bladder control and regain continence. 50 It comprised:
-
focused education for patients and carers on lower urinary tract dysfunction and the theory and practice of bladder training
-
individualised voiding regimens to restore regular, normal voiding patterns by progressively lengthening the time between voids
-
urge suppression techniques
-
the completion of a patient-held voiding diary, a cognitive intervention designed to promote self-awareness of voiding habits.
Prompted voiding aimed to improve bladder control and minimise UI episodes using verbal prompts and positive reinforcement from stroke service staff. It comprised:
-
approaching participants according to their individualised regimen (e.g. every 2 hours during waking hours)
-
asking the patient if they were currently dry or wet
-
prompting them to use the toilet
-
offering sensitively constructed feedback for correct reporting of dryness/wetness and successful toileting.
With both regimens, progress was reviewed weekly by clinical staff, with adjustment to the voiding regimen or change from prompted voiding to bladder training if the patient’s cognitive ability or bladder control improved, or from bladder training to prompted voiding if either or both cognitive ability and bladder control deteriorated.
Clinical staff members were encouraged to alert community services (including early supported discharge teams) during the discharge process so that these services could continue the programme post discharge.
Analysis
The analyses were performed in accordance with the SAP. 2 The SAP was signed off on 10 October 2019 and approved by the ICONS-II TSC on 14 October 2019, in advance of the analysis of the internal pilot process evaluation data on intervention delivery and contamination, and before the main effectiveness analysis.
Outcomes
The severity of UI was measured using the ICIQ-UI-SF51 total score. Higher scores indicate greater severity of UI. Participants who were catheterised did not complete the ICIQ-UI-SF.
The primary outcome was severity of UI at 3 months post randomisation, measured using the ICIQ-UI-SF for those not catheterised. Participants who were catheterised were given a maximum ICIQ-UI-SF score of 21.
The secondary outcomes were measured at 3 and 6 months post randomisation. Outcomes 1, 3, 4, 7 and 8 were also measured at discharge from the stroke unit:
-
Severity of UI, measured using the ICIQ-UI-SF (at discharge and 6 months).
-
Urinary symptoms, measured using the LUSQ47 (questions ‘Do you ever leak when you do any of the following?’ and ‘When you get the urge to pass urine, does any leak before you get to the toilet?’ only). The LUSQ was not administered to participants with an IUC.
-
Number of UTIs. A UTI was defined as (1a) symptoms (fever, indicated by temperature of > 37.5 °C, on two occasions, suprapubic tenderness, costovertebral angle pain or dysuria) or (1b) a positive blood/urinary tract pus or tissue culture; and (2) a positive urine culture or, for catheterised patients or if a urine culture was not possible, a dipstick positive for nitrite or white blood cells.
-
Number of days IUC was in situ.
-
Functional independence, measured using the Barthel Index of Activities for Daily Living,52 with lower scores indicating greater disability.
-
QoL, measured using the Incontinence Quality of Life (IQoL) instrument53,54 and transformed to the scale 0–100. Higher IQoL scores indicate better QoL.
-
Number of falls. A fall was defined as ‘any fall requiring a medical/health professional examination such as physical examination, X-ray and/or an intervention such as suturing or surgery’.
-
Death.
-
Cost-effectiveness, based on QALYs gained, estimated from the responses to the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),55,56 using the UK tariff value, and symptom-free days.
In addition, the following baseline characteristics of the patient were recorded following consent:
-
information collected from case notes52 –
-
date of birth (age to be calculated)
-
sex
-
ethnicity
-
date of admission
-
date of stroke onset
-
NIHSS at baseline38,46 (scores closest to the date of baseline questionnaire completion were used)
-
side of body affected by stroke (left, right, neither or both)
-
type of stroke (e.g. cerebral infarct, cerebral haemorrhage)
-
stroke subtype (Oxford Community Stroke Project classification57,58)
-
pre-stroke modified Rankin scale (mRS59)
-
pre-stroke living circumstances
-
pre-stroke UI
-
IUC in situ
-
type of UI (urge, stress, mixed, ‘functional’ or unclear)
-
-
information collected from the participant, consultee or clinical staff –
-
date baseline questionnaire was completed
-
cognitive ability, determined using the 6-CIT,47 with higher cognitive scores indicating greater cognitive impairment; the total cognitive score was also dichotomised,60 with the participant deemed to have cognitive impairment if their cognitive score was ≥ 8
-
ICIQ-UI-SF
-
LUSQ
-
Barthel Index
-
the health-related quality-of-life measure the EQ-5D-5L.
-
Sample size
Based on the 3-month ICIQ-UI-SF total scores, 818 participants were required to provide ≥ 90% power to detect a 1.89-point between-group difference using an independent-samples t-test (alpha = 5%), assuming that ≤ 25% of the true effect would be lost to contamination. It was based on a minimal clinically important difference (MCID) of 2.5261 and a common standard deviation (SD) of 8.32, computed from data collected for the ICONS-I feasibility trial. 26 The target for randomised participants was 1024 to allow for 20% attrition. 26,36
Randomisation and blinding
Randomisation (in a 1 : 1 ratio) was stratified by baseline continence category [catheterised, slight (ICIQ-UI-SF score of 1–5), moderate (ICIQ-UI-SF score of 6–12) or severe/very severe (ICIQ-UI-SF score of 13–21)] using blocks of random length, which we found to be prognostic of outcome in our ICONS-I feasibility trial,26 and by site. The allocation procedure was delivered using the secure remote web-based system provided by Sealed Envelope Ltd (London, UK).
The information required to perform the randomisation was entered into the web-based system by the research nurse who obtained consent; the nurse was required to confirm that they had checked the eligibility criteria prior to the allocation being made. The system provided information on the allocated group to the research nurse in the stroke unit. The research nurse recorded the allocated group on the participant registration form, and informed the stroke unit ICONS-II trial staff, who delivered the intervention to patients randomised to the intervention group.
Blinding of health-care staff and patients was not possible. Data co-ordinators at Lancashire Clinical Trials Unit (CTU) were also not blinded, as they were handling data from the process evaluation in addition to baseline and outcome data. However, the trial statistician in Lancashire CTU, who was responsible for the SAP and the analysis of the effectiveness data, remained blinded to the identity of the group codes until after the effectiveness analysis had been performed. An unblinded statistician independently performed the analysis of the internal pilot data and the process evaluation data at the end of the trial.
Economic evaluation
The aim of the health economics evaluation was to determine the cost-effectiveness of the SVP compared with the usual care provided after stroke in secondary care. The economic analysis was focused on the EQ-5D-5L scores and their changes during follow-up, that is at baseline (discharge from the stroke unit) and at 3 and 6 months post randomisation.
Internal pilot study
An internal pilot examined the feasibility of participant recruitment and the success of strategies for minimising contamination in usual-care patients; it was initially intended that this would run for a total of 72 site-months of recruitment from 18 sites over a 6-month period (project months 3–8), with a staggered start (six sites in project month 3, six sites in project month 5 and six sites in project month 7). The target was approximately five patients per site per month, with an overall target of 355 participants by the end of project month 8. The following progression criteria were used initially:
-
Continue – possible to recruit to time and target – recruitment ≥ 80% of target (minimum of 284 participants). If recruitment was ≥ 80% but < 100% at this stage, we would consider modifications to the recruitment procedure to ensure that our target for the whole trial was met.
-
Continue but modify protocol – it may be possible to recruit to time and target with the implementation of contingency plans (e.g. increased number of sites) – recruitment ≥ 60% but < 80% of target (213–283 participants).
-
Pause trial – it may not be possible to recruit to time and target – recruitment < 60% of target (≤ 212 participants).
Owing to difficulties with sites opening, in June 2019 the internal pilot recruitment targets were changed. The revised schedule for the recruitment criterion was 45 site-months of recruitment, reduced from 72 months. The target was set at five to seven patients per site per month, with an overall target of a minimum of 225 participants during the 4-month recruiting period (4 June to 30 September 2019). The criteria were revised to the following:
-
Continue – possible to recruit to time and target – recruitment ≥ 80% of target (minimum of 180 participants). If recruitment was ≥ 80% but < 100% at this stage, we would consider modifications to the recruitment procedure to ensure that our target for the whole trial was met.
-
Continue but modify protocol – it may be possible to recruit to time and target with the implementation of contingency plans – recruitment ≥ 60% but < 80% of target (135–179 participants).
-
Pause trial – it may not be possible to recruit to time and target – recruitment < 60% of target (≤ 134 participants).
The retention rates, reflecting the completeness of follow-up, were not included in the pilot progression criteria as few primary outcomes were expected to have been received by that time.
However, the trial continued to experience major issues with recruitment and site opening, and, after the Monitoring Hub meeting with the NIHR HTA programme, held on 21 November 2019, the new recruitment targets for the internal pilot were set up by the HTA programme in its decision letter of 9 December 2019. The team was given a further 3 months, with effect from 1 January 2020, to determine if the viability of the trial could be achieved. By 31 March 2020. The seven mature sites (i.e. the seven sites listed as open since July 2018) were required to have achieved a minimum average rate of three participants per site per month, with a total of 63 participants for those sites during that period.
The fact that the majority of stroke patients in the ward were not in the ICONS-II trial should have given a reasonable degree of flexibility of staffing, even with the restriction that the intervention group participants should receive toileting care from the ICONS-II trial-trained (nursing) staff only, and that the usual-care group patients should receive toileting care from (nursing) staff who were not trained on the ICONS-II trial intervention only.
In addition, we aimed to check whether or not the trained staff were providing toileting assistance to usual-care participants to any extent. We felt that this could also be an indicator of a lack of fidelity to usual care. We felt that asking staff to record who performed toileting assistance would deter trained staff from substantial delivering toileting assistance to usual-care participants.
The contamination of usual care was examined as above by looking at (a) the extent to which ICONS-II trial staff provided toileting assistance to usual-care participants and (b) the extent to which usual-care participants received the intervention:
-
Extent to which ICONS-II trial staff provided toileting assistance to usual-care participants –
-
Stroke unit staff were asked to record brief details of toileting assistance (including the signatures of staff providing assistance) on fluid balance charts for patients receiving usual care. All fluid balance charts for eligible participants included in the data extraction were included in the analysis. This enabled us to monitor whether or not and how often ICONS-II trial staff delivered toileting assistance to usual-care patients.
-
Outcomes were set as the following:
-
Continue without modification: ≤ 25% of usual-care participants receive toileting assistance from ICONS-II trial staff on ≥ 50% occasions.
-
Continue with modification: > 25% of usual-care participants receive toileting assistance from ICONS-II trial staff on ≥ 50% occasions.
-
-
-
Extent to which usual-care participants received the intervention –
-
We conducted a review of case notes for all usual-care participants at discharge, focusing on the three key elements of the SVP:
-
– Presence of a strategy for minimising IUC in the acute phase unless IUC is clinically justifiable (to relieve urinary retention or when fluid balance is critical). If IUC is clinically justifiable, presence of a strategy for review and removal (including a TWOC).
-
– Presence of a comprehensive continence assessment [including continence history, diagnosis of UI (urge, stress, mixed or other), pattern of UI and assessment of relevant comorbid conditions].
-
– Presence of a tailored treatment plan including behavioural approaches (specifically, a tailored voiding interval and evidence of review and adjustment).
-
-
Outcomes were set as the following:
-
Continue without modification: ≥ 75% of usual-care participants do not receive all three key elements of the intervention (avoidance of indwelling urinary catheterisation, comprehensive continence assessment and a tailored treatment plan).
-
Pause trial: > 25% of usual-care participants receive all three key elements of the intervention.
-
-
Pre-implementation case-note review patients
To determine baseline continence practice, prior to the start of staff training on the SVP intervention and the subsequent recruitment of trial participants, a retrospective review of the case notes of 40 consecutive patients discharged from each stroke unit during the 3-month pre-implementation period was embedded in the study. A detailed review was conducted for patients identified as incontinent. Data were collected in a format that avoided identification of patients. The case note review focused on the three elements of the SVP. This case note review enabled assessment of whether or not the standard of continence care received by the trial participants randomised to the usual-care group was better than that received by patients immediately prior to commencement of the staff training. Improvement in continence care would be considered an indication of potential contamination of the usual-care group.
Effectiveness analysis
Analysis of baseline characteristics
Baseline patient characteristics were summarised using descriptive statistics that were appropriate for the data type, and these are reported for each trial group. The analysis included summarising the key baseline patient data, including data related to incontinence severity and stroke severity.
Primary outcome analysis
The primary outcome, ICIQ-UI-SF total score, was analysed using a multiple linear regression model to examine the difference in mean ICIQ-UI-SF total score between the trial groups, adjusting for baseline ICIQ-UI-SF total score and for site as a random effect. No other variables were included in the model.
Secondary outcome analysis
The ICIQ-UI-SF total score at 6 months was analysed using a multiple linear regression model to examine the difference in mean ICIQ-UI-SF total score between trial groups, adjusting for baseline ICIQ-UI-SF total score and for site as a random effect. As for the primary outcome, no other variables were included in the model.
The IQoL scores at 3 and 6 months were analysed using multiple linear regression models to examine the difference in mean IQoL score between the trial groups, with site included as a random effect. No other variables were included in these models.
Stress incontinence (yes/no) at 3 and 6 months was analysed using a multiple logistic regression model to examine the odds of the intervention relative to the control (usual care), adjusting for baseline stress incontinence.
Urge incontinence (yes/no) at 3 and 6 months was analysed using a multiple logistic regression model to examine the odds of intervention relative to the control (usual care), adjusting for baseline urge incontinence.
The number of UTIs at discharge and at 3 and 6 months was presented as a frequency (%) by intervention group. A planned analysis of the number of infections as an outcome with Poisson regression was not feasible because of the small size of the sample collected. The difference in the number of infections between the trial groups was analysed using Wilcoxon rank-sum (Mann–Whitney) tests at each follow-up time point separately.
For patients who had an IUC in situ at any point during the trial, the number of days with an IUC and number of participants with an IUC were presented using descriptive statistics – median [interquartile range (IQR)] and frequencies (%), respectively – by intervention group and for each follow-up time point (at discharge and at 3 and 6 months). The number of catheter-days (number of patients with catheter multiplied by median number of days) was calculated. A planned analysis with Poisson regression, with the total number of days as the outcome, to examine the relative incidence rate of the intervention and usual-care groups was not feasible with the sample collected. The difference between the trial groups in the number of days with a catheter in situ at discharge was tested using Wilcoxon rank-sum (Mann–Whitney) test. For other follow-up time points, the numbers were too small to be tested.
The numbers of falls at discharge and at 3 and 6 months were presented as frequencies (%) by trial group. A planned analysis with Poisson regression, with the number of falls as the outcome, was not feasible because of the small sample size.
The total Barthel scores at 3 and 6 months were analysed using an ordinal regression model, with the Barthel score at the corresponding time point as the outcome, to examine the odds of the intervention relative to usual care, adjusting for the baseline Barthel total score, baseline continence category and site as a random effect.
The numbers of deaths at discharge and at 3 and 6 months were presented as frequencies (%) by trial group. A planned analysis using logistic regression, with a dichotomous variable as the outcome, was not feasible because of the small number of observed deaths.
The EQ-5D-5L scores at 3 and 6 months were analysed using descriptive statistics [frequencies (%) and means (SDs), as appropriate].
Process evaluation methods
The process evaluation was conducted to assess fidelity to (1) the intervention and (2) usual care, or, conversely, the potential level of contamination, repeating the interim analysis performed for the internal pilot on the full data set. As a result, for the assessment of fidelity to usual care, only the results from the final analysis are presented in this report.
Process evaluation: measures of delivery of the intervention
For the participants randomised to the intervention group, we recorded:
-
the number of days on the programme
-
the number of times an IUC was inserted and the reasons for its insertion
-
the number of days with an IUC
-
the number of TWOCs, and the outcome of each one
-
the number of changes of route (and whether from bladder training to prompted voiding or from prompted voiding to bladder training);
-
the number of suspensions (defined as ≥ 1 day off the programme), with reasons
-
the presence of IUC within 72 hours of admission to the stroke unit
-
if catheterised, whether or not there was a TWOC within 72 hours of insertion
-
allocation to correct regime based on criteria for bladder training or prompted voiding
-
for participants receiving bladder training or prompted voiding, percentage of occasions they either self-initiated toileting or were prompted to toilet by ward staff within 30 minutes of the prescribed voiding interval
-
for participants receiving bladder training or prompted voiding, percentage of occasions they were given toileting assistance by ICONS-II trial staff.
However, the focus of the final process evaluation was the possible reasons for the failure of the trial, including the intervention delivery and completion of the documentation regarding intervention delivery. We were also aware of some missing daily log data, but were unable to obtain these data owing to difficulties in later stages of the trial; for the daily logs, we simply focused on the completion of the relevant paperwork, and so we do not report on either (j) or (k) from the list of measures.
Process evaluation: measures of usual care only
For each patient, on each day they were ‘on trial’ during their time in the stroke unit, we recorded the:
-
number of occasions that they were toileted
-
number of occasions that they were toileted by ICONS-II trial staff.
These were summarised as the:
-
proportion of occasions that they were toileted by ICONS-II trial staff.
This was to be used as an indication of potential contamination and deviation from the usual-care protocol.
Process evaluation: measures of intervention and usual-care group participants and of pre-implementation case-note review patients
To summarise the receipt of ‘good-practice’ continence care, which was the basis of the ICONS-II trial intervention, for all three groups (intervention, usual-care and case-note review patients) we recorded the:
-
percentage of patients with –
-
a strategy for minimising IUC insertion in the acute phase unless the IUC was clinically justifiable
-
a comprehensive continence assessment
-
a tailored treatment plan (including behavioural approaches).
-
-
percentage of patients with i, ii and iii.
Data for (o) and (p) were obtained from case-note reviews for usual-care group participants and pre-implementation patients, and from the Log of Ongoing Continence Events, Continence Assessment and Weekly Reviews (as applicable) for intervention group participants.
The process evaluation outcomes were assessed using appropriate variables, derived using algorithms presented in Appendix 1.
Process evaluation: analysis
Process evaluation data were analysed separately for intervention and usual-care group participants, and for pre-implementation case-note review data. Analysis used summary statistics: frequency (%) for dichotomous (presence/absence) or categorical outcomes, and mean (SD) and/or median (IQR) for count and ‘percentage of occasions’ outcomes.
Public and patient involvement
Service user involvement was an important part of the ICONS-II trial. Service users contributed to the development and progression of the trial throughout its course. During the trial, the study team had regular meetings with the PCPI group, with five meetings taking place between January and October 2019. At the meetings, the trial’s progress and difficulties with recruitment were discussed, including recommendations of ways to address the recruitment issues. Members of the PCPI group were engaged in discussion and reviewing the poster to advertise the trial in participating stroke units. The PCPI group also discussed problems or issues that patients might encounter with the SVP, in particular bladder training and prompted voiding. The group emphasised that it is beneficial for patients to be part of a programme.
The group felt that a visit from PCPI members would help increase the motivation of stroke unit staff to progress the trial and recruitment. Unfortunately, the planned site visits were not implemented owing to the trial’s pause and then its early stop.
The study team was involved in active communication and collaboration with the Speakeasy group. The Speakeasy PCPI group developed aphasia-friendly patient information leaflets and consent forms in the ICONS-I feasibility trial; these were used in the ICONS-II trial for patients with aphasia. Members of the PCPI group provided feedback on the study design and participant documents, and attended trial meetings on a regular basis. The TSC included members of the public. Our co-investigators Cliff Panton and, during the early stages of the trial, David Britt took an active part in the trial, including being involved in discussions at the trial development and set-up meetings, in addition to Trial Management Group meetings.
Approaches to maximising retention and patient follow-up
To maximise recruitment and improve retention, a number of approaches were implemented as a response to the low recruitment rate observed during the internal pilot.
The recruitment challenges were as follows:
-
The number of patients identified as having an episode of UI within the required time after admission to the stroke unit was smaller than anticipated.
-
Some eligible patients thought that they were at the end of their life and did not want to consider participating in research.
-
Sites were not recruiting participants classified as palliative.
-
Sites found it difficult to obtain consent/assent within the suggested time window, especially when personal consultees were involved, with additional difficulties in co-ordinating meetings of study staff and potential personal consultees.
-
Sites were unclear about when it was appropriate to use a nominated consultee if no personal consultee was available.
-
Although several intervention training sessions could take place at a site in 1 day, not enough stroke unit staff were trained to enable recruitment to begin immediately after training.
The changes to the methods in response to challenges were as follows:
-
To improve the likelihood of identifying patients as having an episode of UI within the first 72 hours after admission to the stroke unit and to make sure that each eligible patient had the opportunity to take part in the study, stroke unit staff were asked to have a sensitive conversation with patients, including asking them if they were affected by UI and explaining that they may be eligible for the study.
-
End-of-life and palliative care patients were eligible, but they might not have been considering participating in research. To improve the likelihood of participation, it was suggested to sites that, although most patients should be recruited within the 72-hour period, it was acceptable for end-of-life and palliative care patients to be consented after this period to allow them more time to come to terms with their situation.
-
The inclusion criterion regarding level of consciousness was amended to allow patients who improved within the first 14 days after admission to the stroke unit to be considered and approached. These patients were still required to meet the remaining inclusion criteria within 72 hours of admission to the stroke unit.
-
Follow-up was to have been mainly postal, but the protocol1 included the option for this to be conducted over the telephone if the patient preferred this or when postal questionnaires were not returned. It became apparent that most of this patient group fell into the vulnerable category for COVID-19, were shielding and would have difficulty with postal returns. All participants were contacted by CTU staff who gave them the option of completing the questionnaire over the telephone. The order of the questionnaire was also adapted so that core outcome data questions and the 3-month ICIQ-UI-SF, LUSQ, Barthel Index and EQ-5D-5L were asked first when the questionnaire was completed over the telephone.
Chapter 3 Results
Recruitment and participant flow
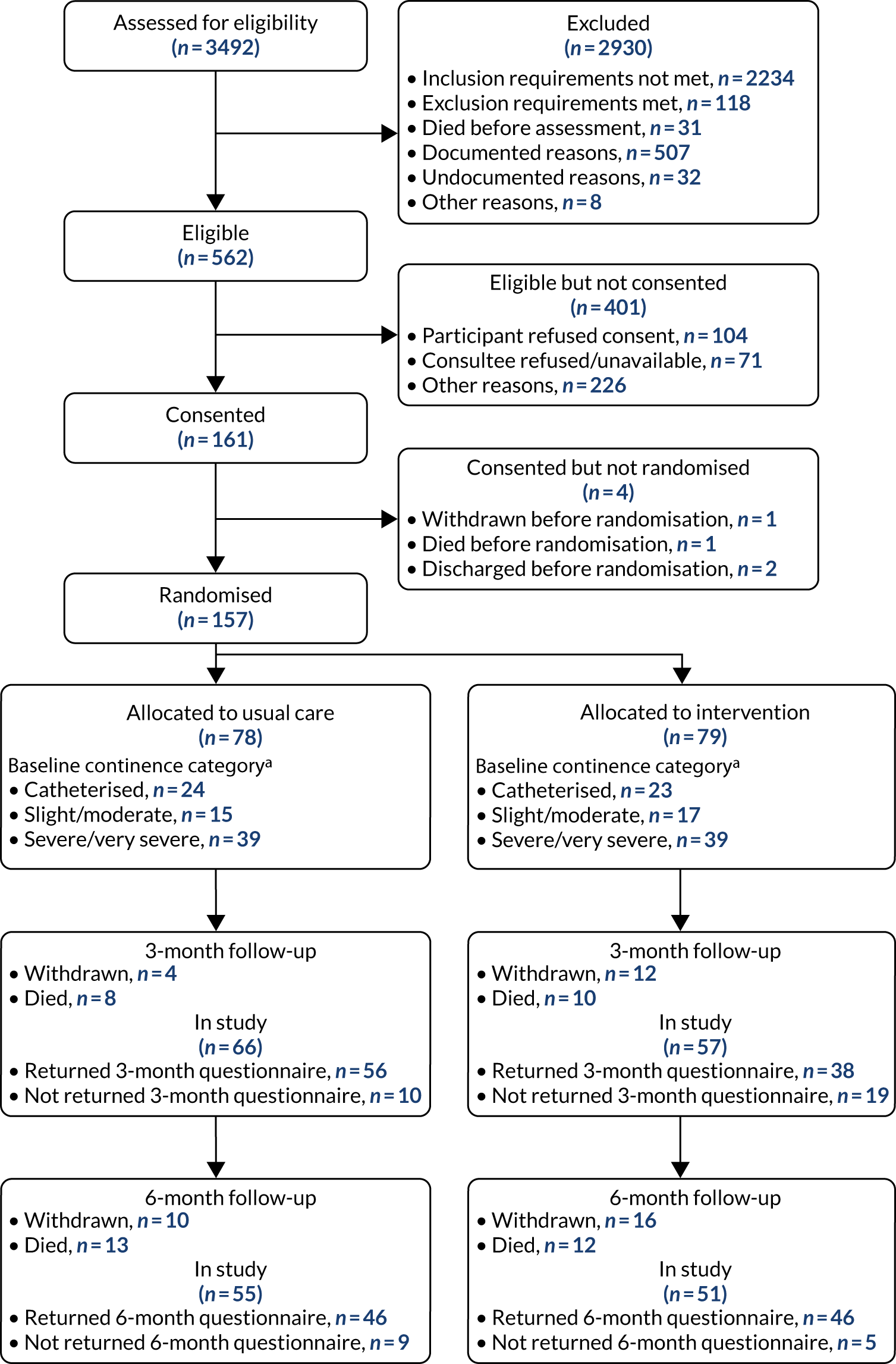
A total of 3492 participants were screened for eligibility across the 17 sites; of those screened, 562 (16%) were deemed eligible. Of those eligible, 161 (29%) were consented and 157 (28%) were randomised, with 78 (50%) of those randomised allocated to usual care and 79 (50%) allocated to the intervention. The details of the patients who were screened but not eligible and of those who were eligible but not consented are given in Table 1.
| Patients screened but not eligible | Patients eligible but not consented | ||
|---|---|---|---|
| Characteristic | n | Characteristic | n |
| Total | 2922 | Total | 401 |
| Does not meet inclusion criteria | 2234 | Patient died before consent | 11 |
| Aged < 18 years | 1 | Refused with reason | 291 |
| Non-stroke | 908 | Patient declined | 43 |
| No UI within 72 hours of admission | 1220 | Personal consultee declined | 23 |
| NIHSS LOC not 0–1 within 72 hours | 105 | Patient unable to commit to trial | 19 |
| Meets exclusion criteria | 118 | Discharged | 6 |
| Long-term IUC pre stroke | 70 | Patient not interested in trial | 2 |
| Subdural/subarachnoid haemorrhage | 48 | Unwell | 18 |
| Non-English speaking | 18 | Patient continent | 2 |
| Out of area | 166 | Out of area | 11 |
| Died before assessment | 31 | No personal consultee available in time | 56 |
| Other exclusion (with reasons) | 323 | Discharged | 40 |
| Discharged | 140 | End of life/poor prognosis/palliative | 21 |
| EOL/poor prognosis/palliative | 63 | Personal consultee declined | 16 |
| Outside 72-hour window | 51 | No longer eligible | 12 |
| Medical reason | 36 | Outside time window | 11 |
| Trial/ward related | 33 | Trial/ward related | 8 |
| No reason given | 32 | ITU | 3 |
| Refused without reason | 99 | ||
For full details, see the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1).
FIGURE 1.
The CONSORT diagram. a, ICIQ-UI-SF score of 1–12 = slight/moderate, and score of 13–21 = severe/very severe.
Details of recruitment by site are given in Table 2, which provides the number of patients screened, eligible, consented and randomised by each site.
| Site number | Screened (n) | Eligible, n (%) | Consented, n (%) | Baseline completed, n (%) | Randomised, n (%) | Assigned intervention, n (%) | Started BT/PV, n (%) | Finished BT/PV, n (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 101 | 37 (37) | 11 (30) | 10 (91) | 10 (91) | 5 (50) | 4 (80) | 4 (100) |
| 2 | 292 | 10 (3) | 1 (10) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| 3 | 45 | 4 (9) | 3 (75) | 3 (100) | 3 (100) | 1 (33) | 0 (0) | 0 (0) |
| 4 | 794 | 53 (7) | 9 (17) | 9 (100) | 9 (100) | 5 (56) | 0 (0) | 0 (0) |
| 5 | 18 | 18 (100) | 18 (100) | 17 (94) | 17 (94) | 9 (53) | 6 (67) | 4 (67) |
| 6 | 2 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 0 (0) |
| 7 | 556 | 86 (15) | 21 (24) | 21 (100) | 21 (100) | 10 (48) | 8 (80) | 5 (63) |
| 9 | 463 | 43 (9) | 17 (40) | 16 (94) | 16 (94) | 8 (50) | 8 (100) | 3 (38) |
| 10 | 337 | 163 (48) | 22 (13) | 22 (100) | 22 (100) | 11 (50) | 8 (73) | 6 (75) |
| 11 | 494 | 72 (15) | 21 (29) | 21 (100) | 21 (100) | 11 (52) | 9 (82) | 7 (78) |
| 13 | 14 | 11 (79) | 11 (100) | 11 (100) | 11 (100) | 5 (45) | 3 (60) | 1 (33) |
| 15 | 35 | 8 (23) | 6 (75) | 6 (100) | 6 (100) | 3 (50) | 3 (100) | 1 (33) |
| 16 | 165 | 39 (24) | 12 (31) | 11 (92) | 11 (92) | 5 (45) | 5 (100) | 3 (60) |
| 17 | 176 | 16 (9) | 7 (44) | 7 (100) | 7 (100) | 5 (71) | 4 (80) | 3 (75) |
| Total | 3492 | 562 (16) | 161 (29) | 157 (98) | 157 (98) | 79 (50) | 59 (75) | 38 (64) |
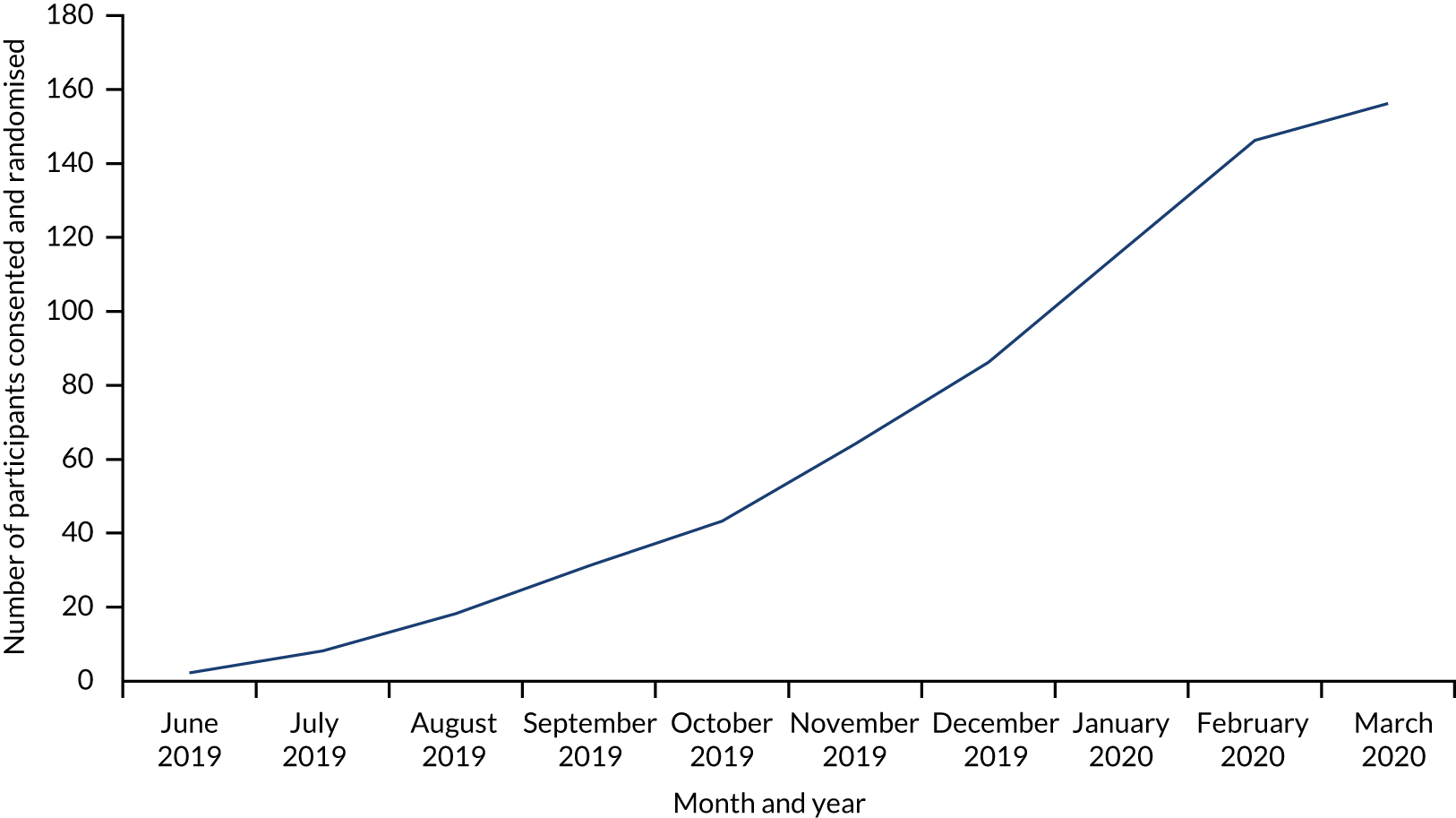
After the internal pilot revealed the difficulties with recruitment, we planned to increase the number of sites to compensate for slow recruitment. However, as the trial was paused because of COVID-19, these plans were not fully implemented. The last patient was recruited on 16 March 2020, the last 3-month outcomes were received on 16 June 2020, and the last 6-month outcomes were received on 22 September 2020. We had 17 sites contributing to the trial. Figure 2 shows the recruitment chart.
FIGURE 2.
Participant recruitment chart.
More details related to the recruitment, the attrition and the implications that recruitment had for the trial results are provided in Brief account of the trial.
Baseline characteristics
The usual-care and intervention group participants were similar in most characteristics and demographics at baseline, as shown in Table 3, with two notable exceptions: (1) the proportion of female patients was larger in the intervention group (54%) than in the usual-care group (42%) and (2) the proportion of participants living alone was larger in the intervention group (39%) than in the usual-care group (29%).
| Characteristic | Trial group | All (N = 157) | |
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | ||
| Age (years), median (IQR) | 76.5 (66–85) | 77 (66–84) | 77 (66–84) |
| Sex, n (%) | |||
| Female | 33 (42.3) | 43 (54.4) | 76 (48.4) |
| Ethnicity, n (%) | |||
| White | 64 (82.1) | 67 (84.8) | 131 (83.4) |
| Asian | 5 (6.4) | 6 (7.6) | 11 (7.0) |
| Black: Caribbean | 2 (2.6) | 2 (2.5) | 4 (2.5) |
| Black: African | 3 (3.8) | 2 (2.5) | 5 (3.2) |
| Mixed | 0 (0.0) | 1 (1.3) | 1 (0.6) |
| Other | 4 (5.1) | 1 (1.3) | 5 (3.2) |
| Living accommodation, n (%) | |||
| House | 49 (62.8) | 47 (59.5) | 96 (61.1) |
| Flat | 15 (19.2) | 12 (15.2) | 27 (17.2) |
| Sheltered housing | 1 (1.3) | 2 (2.5) | 3 (1.9) |
| Residential home | 2 (2.6) | 0 (0.0) | 2 (1.3) |
| Nursing home | 1 (1.3) | 2 (2.5) | 3 (1.9) |
| Other | 10 (12.8) | 16 (20.3) | 26 (16.6) |
| Living situation, n (%) | |||
| Alone | 22 (29.3) | 30 (39.5) | 52 (34.4) |
| Partner | 40 (53.3) | 35 (46.1) | 75 (49.7) |
| Relative/friend | 12 (16.0) | 11 (14.5) | 23 (15.2) |
| Other | 1 (1.3) | 0 (0.0) | 1 (0.7) |
The usual-care group and intervention group participants were similar in most clinical and stroke-related characteristics at baseline, as shown in Table 4, with the notable exception of the median Barthel score, with the intervention group (median score of 6.5) being more functionally independent than the usual-care group (median score of 4).
| Characteristic | Trial group | All (N = 157) | |
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | ||
| Type of stroke, n (%) | |||
| Ischaemic infarct | 62 (80.5) | 65 (82.3) | 127 (81.4) |
| PICH | 12 (15.6) | 12 (15.2) | 24 (15.4) |
| Other | 3 (3.9) | 2 (2.5) | 5 (3.2) |
| Verbal responses at admission, n (%) | |||
| None | 9 (11.5) | 8 (10.1) | 17 (10.8) |
| Incomprehensible | 6 (7.7) | 5 (6.3) | 11 (7.0) |
| Inappropriate | 14 (17.9) | 8 (10.1) | 22 (14.0) |
| Confused | 15 (19.2) | 22 (27.8) | 37 (23.6) |
| Orientated | 29 (37.2) | 31 (39.2) | 60 (38.2) |
| Not recorded | 5 (6.4) | 5 (6.3) | 10 (6.4) |
| OCSP classification reported, n (%) | |||
| TACS | 18 (24.3) | 15 (19.0) | 33 (21.6) |
| PACS | 16 (21.6) | 18 (22.8) | 34 (22.2) |
| POCS | 3 (4.1) | 7 (8.9) | 10 (6.5) |
| LACS | 9 (12.2) | 12 (15.2) | 21 (13.7) |
| Not recorded | 28 (37.8) | 27 (34.2) | 55 (35.9) |
| Side of body, n (%) | |||
| Left | 39 (50.0) | 37 (46.8) | 76 (48.4) |
| Right | 31 (39.7) | 33 (41.8) | 64 (40.8) |
| Both | 5 (6.4) | 3 (3.8) | 8 (5.1) |
| Neither | 3 (3.8) | 6 (7.6) | 9 (5.7) |
| NIHSS score, mean (SD) | 11.0 (7.62) | 9.9 (6.00) | 10.5 (6.86) |
| Comorbidities, n (%) | |||
| None | 5 (6.4) | 3 (3.8) | 8 (5.1) |
| One | 12 (15.4) | 17 (21.5) | 29 (18.5) |
| Two or more | 61 (78.2) | 59 (74.7) | 120 (76.4) |
| Pre-stroke mRS, n (%) | |||
| Fit and well | 34 (43.6) | 30 (38.0) | 64 (40.8) |
| No significant disability | 22 (28.2) | 19 (24.1) | 41 (26.1) |
| Slight disability | 4 (5.1) | 9 (11.4) | 13 (8.3) |
| Moderate disability | 12 (15.4) | 11 (13.9) | 23 (14.6) |
| Moderately severe disability | 5 (6.4) | 9 (11.4) | 14 (8.9) |
| Severe disability | 1 (1.3) | 1 (1.3) | 2 (1.3) |
| Pre-stroke dichotomised mRS, n (%) | |||
| Moderate to severe | 18 (23.1) | 21 (26.6) | 39 (24.8) |
| Barthel score, median (IQR) | 4 (2–9) | 6.5 (2–12) | 5 (2–11) |
| 6-CIT total score, median (IQR) | 7 (2–16) | 7 (2–10) | 7 (2–13) |
| Cognitively impaired based on 6-CIT, n (%) | |||
| Yes | 28 (52.8) | 30 (56.6) | 58 (54.7) |
| Baseline EQ-5D-5L VAS score, mean (SD) | 53.4 (23.49) | 49.6 (24.35) | 51.4 (23.94) |
The usual-care group and intervention group participants were similar in most incontinence characteristics at baseline, as shown in Table 5, apart from pre-stroke use of incontinence pads. A larger proportion of the intervention group (34%) than of the usual-care group (23%) were reliant on incontinence pads pre stroke.
| Characteristic | Trial group, n (%) | All (N = 157), n (%) | |
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | ||
| Pre-stroke catheter | |||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not known | 4 (5.3) | 2 (2.5) | 6 (3.9) |
| Baseline catheter | |||
| Yes | 25 (33.3) | 23 (29.5) | 48 (31.4) |
| Pre-stroke pad use | |||
| Yes | 18 (23.1) | 27 (34.2) | 45 (28.7) |
| Not known | 7 (9.0) | 3 (3.8) | 10 (6.4) |
| Baseline pad use | |||
| Yes | 54 (70.1) | 55 (72.4) | 109 (71.2) |
| Pre-stroke uridom or similar | |||
| Yes | 1 (1.3) | 2 (2.6) | 3 (1.9) |
| Not known | 5 (6.6) | 2 (2.6) | 7 (4.5) |
| Baseline uridom or similar | |||
| Yes | 7 (9.2) | 5 (6.7) | 12 (7.9) |
| Baseline other incontinence aid | |||
| Yes | 2 (2.8) | 3 (4.3) | 5 (3.6) |
| Type of incontinence | |||
| Stress only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urge only | 20 (25.6) | 23 (29.1) | 43 (27.4) |
| Mixed | 18 (23.1) | 20 (25.3) | 38 (24.2) |
| Unclear | 15 (19.2) | 13 (16.5) | 28 (17.8) |
| Catheterised | 25 (32.1) | 23 (29.1) | 48 (30.6) |
Table 6 presents the number of participants with IUCs at baseline, where they were catheterised and the reason why they were catheterised. It is of note that a larger proportion of participants were catheterised because of retention of urine in the usual-care group (80%) than in the intervention group (64%).
| Characteristic | Trial group, n (%) | All (N = 48), n (%) | |
|---|---|---|---|
| Usual care (N = 25) | Intervention (N = 23) | ||
| Where catheterised | |||
| Accident and emergency | 2 (8.0) | 3 (13.0) | 5 (10.4) |
| Acute admission unit | 2 (8.0) | 2 (8.7) | 4 (8.3) |
| Stroke unit | 13 (52.0) | 13 (56.5) | 26 (56.2) |
| Other | 6 (24.0) | 3 (13.0) | 9 (18.8) |
| Not documented | 2 (8.0) | 2 (8.7) | 4 (8.3) |
| Reason catheteriseda | |||
| Retention of urine | 20 (80.0) | 14 (63.6) | 34 (72.3) |
| Intake/output measurement | 2 (8.0) | 3 (13.6) | 5 (10.6) |
| Other | 1 (4.0) | 2 (9.1) | 3 (6.4) |
| Not documented | 2 (8.0) | 3 (13.6) | 5 (10.6) |
Table 7 shows the ICIQ-UI-SF total scores at each time point they were collected, with the catheterised patients excluded, and then included with the maximum possible score. With catheterised participants excluded from the calculation of ICIQ-SF total, the usual-care and intervention groups were balanced at baseline. The score for each group decreased at each subsequent time point, with the usual-care group performing better than the intervention group at both the 3- and 6-month time points. In both scenarios, with catheterised participants excluded from the calculation of the ICIQ-UI-SF total, and with catheterised patients given the maximum score on the ICIQ-UI-SF total score, the intervention and usual-care groups were balanced at baseline, and the group with the better score switched from the intervention group at 3 months to the usual-care group at 6 months. The most improvement occurred between baseline and discharge, with reductions of > 4 points in all total scores from baseline and the intervention group showing the most improvement during the period.
| Time point | ICIQ-UI-SF total score, mean (SD) [n] | ||
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | All (N = 157) | |
| Catheterised participants excluded | |||
| Baseline | 14.2 (4.77) [53] | 14.3 (4.21) [56] | 14.3 (4.47) [109] |
| Discharge | 8.0 (7.08) [42] | 8.7 (6.40) [50] | 8.4 (6.69) [92] |
| 3 months | 7.2 (6.64) [44] | 7.8 (7.20) [34] | 7.4 (6.85) [78] |
| 6 months | 6.3 (6.12) [40] | 7.5 (7.23) [39] | 6.9 (6.67) [79] |
| Catheterised participants given maximum score | |||
| Baseline | 16.4 (5.04) [78] | 16.3 (4.67) [79] | 16.3 (4.84) [157] |
| Discharge | 11.1 (8.30) [55] | 10.4 (7.31) [58] | 10.8 (7.78) [113] |
| 3 months | 9.1 (7.81) [51] | 8.1 (7.43) [35] | 8.7 (7.62) [86] |
| 6 months | 7.9 (7.41) [45] | 8.5 (7.80) [42] | 8.2 (7.56) [87] |
Internal pilot
During the internal pilot, the target of a minimum of 225 participants recruited was not met, largely because only seven sites, rather than the planned 18, contributed participants during the period and because of difficulties with opening sites. By the end of September 2019, a total of 32 participants had been randomised. Following a monitoring meeting with the HTA programme in November 2019, rather than terminating the trial, the HTA programme allowed recruitment to continue for another 3 months (January–March 2020) to enable a clearer assessment of whether or not recruitment could be improved by implementing of a number of strategies in the seven established sites, as described in Brief account of the trial.
Two of the seven original established sites were closed, one on 4 December 2019 and one on 20 December 2019, because of difficulties identifying suitable participants and being able to deliver the intervention in a systematic way owing to staff shortages. The other five established sites continued to recruit, as did the later-opening sites.
In relation to contamination assessment, the analysis of both the usual-care group participants and the pre-implementation case-note review patients showed similar very low levels of structured voiding but some level of appropriate care (which included TWOCs). There was a strategy for minimising IUC in the acute phase unless clinically justifiable among a substantial percentage of the usual-care group participants (3/6; 50%), but, given the very small numbers, this was not inconsistent with the percentage observed among the pre-implementation case-note review patients (21/65; 32%). However, there was very little evidence of a comprehensive continence assessment being performed on usual-care participants, and it did not progress to a tailored treatment plan in any usual-care participants; this was consistent with the prevalence observed in the pre-implementation case-note review patients.
The conclusion of the internal pilot, therefore, was that there was no evidence of contamination of the usual-care group with the key components of the SVP intervention.
Trial findings: data completeness at follow-up
The results presented in this section and onwards relate to all follow-up data available at 3 or 6 months post randomisation.
At the primary time point of 3 months, of the 157 randomised participants, 94 (59%) returned a follow-up questionnaire, 18 (11%) had died and 16 (10%) had withdrawn from the trial. However, only 86 provided primary outcome data, so the percentage of missing primary outcome data was very high (45%).
When comparing the groups at 3 months, of the 94 returned questionnaires, 56 (60%) were from the usual-care group and 38 (40%) were from the intervention group. Four (5%) participants in the usual-care group and 12 (15%) participants in the intervention group withdrew, and there were 10 (13%) non-responders in the usual-care group and 19 (24%) non-responders in the intervention group at 3 months.
Overall, there was a substantial difference in missing data rates between the usual-care and intervention groups for the primary outcome (ICIQ-UI-SF at 3 months), with primary outcome data for only 35 out of 79 (44%) participants in the intervention group, compared with 51 out of 78 (65%) participants in the usual-care group. There was, therefore, strong evidence of differential percentages for missing primary outcome data in the two groups (p = 0.008; chi-squared test).
At 6 months, 46 (59%) participants from the usual-care group and 46 (58%) participants from the intervention group returned the questionnaire. Although the number of missing primary outcome data was more balanced between the groups at 6 months, it continued to be very large (Table 8).
| Time point | Trial group, n (%) | Test of proportions (chi-squared test) | |
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | ||
| 3 months | 27 (34.6) | 44 (55.7) | p = 0.008 |
| 6 months | 33 (42.3) | 37 (46.8) | p = 0.57 |
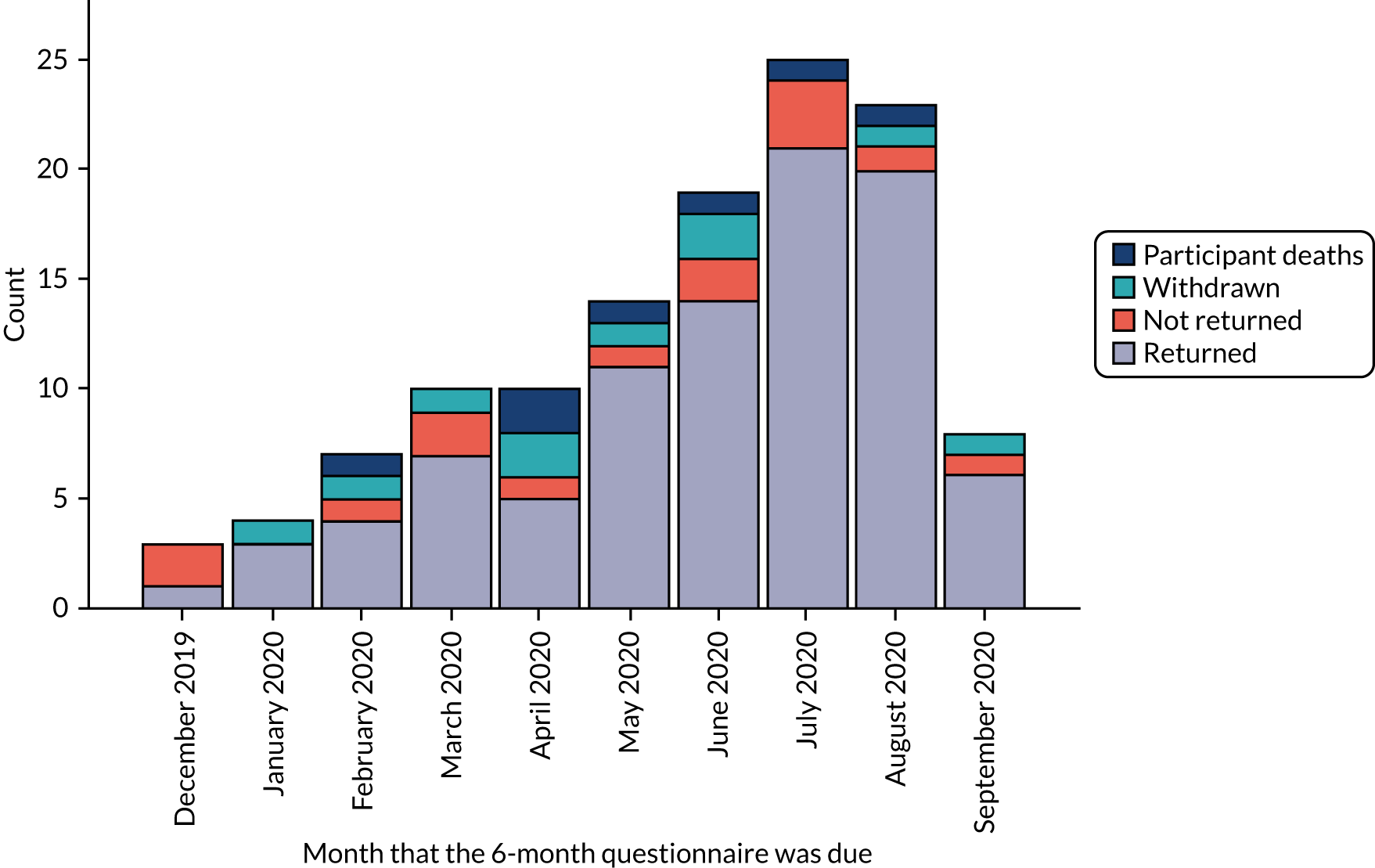
Figures 3 and 4 show the number of participants who returned and the number of participants who did not return their questionnaires by the relevant follow-up time point, and the numbers of deaths and withdrawals from the study. Figure 3 covers the period between September 2019 and June 2020, and Figure 4 covers the period between December 2019 and September 2020. The last 3-month questionnaire was received on 16 June 2020 and the last 6-month questionnaire was received on 22 September 2020. However, in March 2020, new participant recruitment to the trial had to be paused because of the COVID-19 pandemic.
FIGURE 3.
Count in each follow-up category by 3-month questionnaire due date.
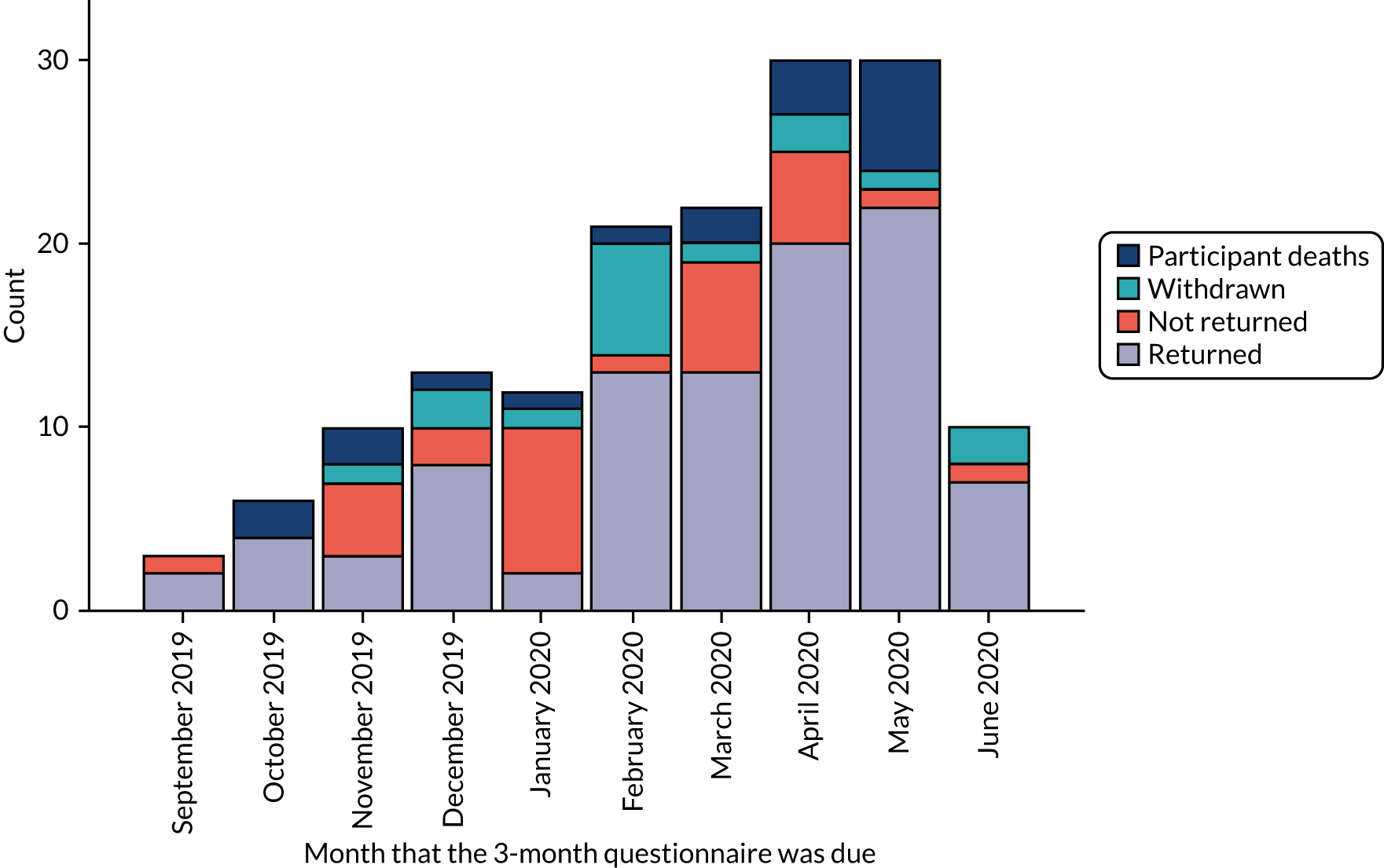
FIGURE 4.
Count in each follow-up category by 6-month questionnaire due date, excluding 3-month participant deaths/withdrawals.
Brief account of the trial
To provide context for the results on recruitment, participant flow and site opening, this section summarises the background of the trial and how it developed.
The initial trial protocol was written on 5 January 2018, and ethics and HRA approvals were granted by early April 2018 in preparation for the initiation of funding in May 2018 and for recruitment to start in July 2018. However, owing to internal delays, although the trial protocol was updated on 4 June 2018, ethics approval for changes was not obtained until late 2018.
Even after HRA and ethics approvals had been obtained, the trial had a difficult start, with aspects of the design being unpopular with prospective sites, which was evident from verbal feedback during site visits and from e-mail correspondence.
The trial opened to recruitment in June 2019, with the first participant recruited on 13 June 2019.
Implementing the trial with IPR required actions aimed at minimising the potential contamination, which was recognised as a risk because participants in both trial groups were treated in the same stroke unit. Specific points of concern and relevant mitigating actions are as follows:
-
The number of patients identified as having an episode of UI within the first 72 hours after admission to the stroke unit was smaller than anticipated.
Actions – sites were informed that patients who were self-caring and mobile often dealt with incontinence in secret because they feel embarrassed. Many of these patients resigned themselves to incontinence, thinking that it was inevitable following a stroke or because of their age. For these patients, it was not possible to detect the presence of UI from fluid balance charts or case notes, and active case finding was required. We asked stroke unit staff to have a sensitive conversation with these patients, including asking them if they were affected by UI.
-
Issues around palliative care.
Some eligible patients thought that they were at the end of their life and did not want to consider participating in research.
Actions – the preference was for most patients to be recruited within the 96-hour period after admission. It was made clear to sites that patients could be consented after this period to give them more time to come to terms with their situation.
Sites were not recruiting participants because they were classed as ‘palliative’.
Actions – sites were advised to consider patients classed as ‘palliative’ very carefully; such patients could live for a substantial time, and improving continence might be a reasonable and achievable goal for these patients, with the potential to improve their QoL.
-
Suggested 96-hour time window for consenting participants.
Sites found it difficult to obtain consent/assent within the suggested 96-hour window, especially when personal consultees were involved.
Actions – the related documentation (ethics application and protocol) was written with some flexibility and notes that ‘informed consent will be made within 96 hours of admission to the stroke unit in the majority of cases’. Sites were informed that there would be occasions when recruitment within 96 hours of admission would not be possible and so patients could be consented after this period.
We amended the inclusion criterion for level of consciousness to allow patients who improved within the first 14 days after admission to the stroke unit to be considered and approached. The revised inclusion criterion (protocol inclusion criteria, section 4.2)1 was changed to ‘conscious: “Alert” or “Not alert but arousable” (NIHSS Point 1A score of 0 or 1) on the NIHSS within 14 days of admission to the stroke unit. Note that patients must be recruited within 72 hours of meeting this criterion wherever possible.’ These patients were still required to meet the remaining inclusion criteria within 72 hours of admission to the stroke unit. This change was submitted for approval on 30 August 2019, and Wales REC 5 approved this amendment on 27 September 2019.
-
Using a personal/nominated consultee.
In some cases, it was difficult to co-ordinate timing so that the study champion or CRN research nurse and potential personal consultees were at the unit at the same time.
Actions – we advised sites to send the personal consultee information leaflet and consultee letter of invitation to potential personal consultees in advance to allow them time to consider these before they visited. If they were willing to provide consultee consent for their relative to take part, they could sign the consent form when they visited.
Sites were unclear when it was appropriate to use a nominated consultee if no personal consultee (family member or friend) was available.
Actions – we had approval from the REC to include a nominated consultee if no personal consultee (family member or friend) was available. Following consultation with the REC and our PCPI groups, we updated our advice to sites, asking them to consider this option if no personal consultee could be identified within 4 days of the patient being admitted to hospital.
-
Staff training.
We anticipated that sites would begin to recruit participants following the ‘intervention training’ session; however, although two or three intervention training sessions could take place at a site in 1 day, this was not enough to enable sufficient numbers of the stroke unit team to be trained to deliver the intervention immediately. We adopted a ‘train-the-trainer’ approach, and sites started to screen/recruit only once the study champion had undertaken further training sessions with stroke unit staff. This often took several weeks.
Actions – we asked sites to ensure that all staff were trained within 4 weeks of the intervention training session.
-
Low staffing levels.
Some sites that initially had staff capacity to take part saw a reduction in staffing levels following the green-light letter. This required the study champion to identify and train further staff to deliver the intervention. In addition, there were low staffing levels in the summer months owing to holiday leave. Nine sites were unable to continue in the trial owing to inadequate staffing levels. This affected the original internal pilot period, but was less of an issue afterwards.
Action – after summer 2019, some of these issues were resolved. Two sites that had particular issues with staff shortages were closed and replaced.
In total, 17 sites participated in the study: 14 sites recruited at least one participant and three sites were in the set-up stage only. Participant recruitment during the extension to the internal pilot progressed much better towards the target of 63 participants in 3 months in the original sites. Capacity and capability approval was obtained from 12 sites, and 11 of these recruited participants. Two of the original seven established sites had difficulties identifying suitable participants and being able to deliver the intervention in a systematic way owing to staff shortages and, in some instances, non-engagement with the delivery. One of sites was closed on 4 December 2019 and the other was closed on 20 December 2019. Recruitment continued in the remaining five established sites and in a further 10 sites that started recruitment later.
During March 2020, recruitment slowed because of the onset of the COVID-19 pandemic. In early March, it became very clear that COVID-19 was dominating resources, and sites started to report that they were unable to support further recruitment to and activities for the ICONS-II trial. Many staff designated as study champions were reallocated to frontline services, and CRN staff (as per HRA guidance) were prioritising recruitment to COVID-19 studies. The final patient was recruited on 16 March 2020. By 26 March 2020, none of the ICONS-II trial sites was conducting study activities. On this date, we contacted all sites with a formal notification of a pause to study activities due to the impingement to safety measures. At this point, 157 participants had been recruited.
In autumn 2020, we considered the possibility of restarting the trial and completed the reanalysis of the data on fidelity to usual care, assessing the degree of contamination. We concluded that the level of contamination of the usual-care group with the key components of the SVP intervention was much lower than the conservative 25% on which the original sample size had been based. Furthermore, we concluded that the impact of contamination of the usual-care group on the treatment effect was unlikely to be > 10%. However, recruiting the required number of participants (≥ 564 further participants, although the number varied depending on the assumptions made) after resuming the trial was unfeasible within the time frame and remaining budget and in the light of the ongoing COVID-19 situation. Moreover, the trial had high and potentially differential rates of missing primary outcome data between the intervention and usual-care groups (56% vs. 35%, respectively). It was also observed that the length of hospital stay in this study was much shorter than that in the cluster-randomised feasibility trial, ICONS-I. The overall median length of stay (IQR) in the stroke unit was 27 (16–45) days in the ICONS-II trial and 47 (30–68) days in the ICONS-I feasibility trial. It was concluded that a purely hospital-based intervention would not provide a patient with a sufficient amount of the SVP to be effective in managing incontinence; a minimum of 6 weeks is recommended for a bladder training programme. 62
Over the course of the study, the trial team identified various barriers that contributed to the difficulties experienced. Several were suspected potential barriers, but these suspicions were not supported by evidence until after recruitment started:
-
Study champions were required at each site for implementation and support. There were delays in identifying and appointing these study champions.
-
Staff were concerned that patients might see a difference in care, with concerns centred around intervention participants getting ‘better treatment’.
-
Staff were concerned about the extra work/paperwork required (in the context of large numbers of vacancies).
-
Managing staff rotation was more complicated when seeking to maintain levels of intervention staff.
-
Staff had difficulty grasping issues of contamination, and the need for toileting of intervention participants to be undertaken by intervention-trained staff.
-
There was poor completion of paperwork.
Trial findings: primary outcome analysis
The study did not recruit a sufficient number of participants, with only 157 (15%) participants recruited out of the target 1024. Only 11% (86 of the target 818) of the target primary outcome measures were available, and they were not balanced between the intervention and usual-care groups.
The results reported are for the intention-to-treat complete-case analysis. For the primary outcome, at 3 months, there were 51 observations in the usual-care group and 35 observations in the intervention group. The results of the primary outcome are shown in Table 9. Participants allocated to the intervention group had a lower ICIQ-UI-SF score than those allocated to the usual-care group at 3 months post randomisation: the mean (SD) ICIQ-UI-SF score at 3 months was 9.1 (7.8) in the usual-care group and 8.1 (7.4) in the intervention group (see Table 9). All comparisons must be interpreted with caution, as they are highly imprecise owing to the small numbers of participants.
| Outcome | Mean (SD) value [n] | Mean difference between intervention and usual-care groups (95% CI) | |
|---|---|---|---|
| Usual-care group | Intervention group | ||
| ICIQ-UI-SF score | |||
| 3 months | 9.1 (7.8) [51] | 8.1 (7.4) [35] | –1.4 (–4.4 to 1.7)a |
| 6 months | 7.9 (7.4) [45] | 8.5 (7.8) [42] | 0.7 (–2.0 to 3.4)a |
| IQoL score | |||
| 3 months | 80.8 (21.9) [36] | 78.4 (27.0) [25] | –2.4 (–14.4 to 9.7) |
| 6 months | 83.9 (18.1) [29] | 82.4 (22.1) [30] | –2.5 (–12.1 to 7.2) |
The assumptions of the model were checked and considered to be reasonably satisfied. The model was also supported by Hausman’s specification test (p = 0.80).
Trial findings: secondary outcome analyses
ICIQ-UI-SF score at 6 months
At the 6-month follow-up, 45 participants in the usual-care group and 42 participants in the intervention group had their ICIQ-UI-SF score measured. The results of the modelling with site as a random effect are shown in Table 9. The number of recruiting sites was 14. At 6 months, participants allocated to the intervention group had a higher ICIQ-UI-SF score than those allocated to the usual-care group, although the difference was smaller than the difference at 3 months: the mean (SD) ICIQ-UI-SF score was 7.9 (7.4) in the usual-care group and 8.5 (7.8) in the intervention group.
IQoL score
The results of linear regression modelling of the mean differences between the intervention and usual-care groups for transformed IQoL score at 3 and 6 months are shown in Table 9. The intervention group had lower IQoL scores than the usual-care group at both follow-up time points, with higher IQoL scores indicating better QoL.
Sensitivity analysis of ICIQ-UI-SF and IQoL scores
A sensitivity analysis was conducted on the ICIQ-UI-SF results because of the change in effect from 3 to 6 months noted in the main analysis, with the intervention group having a lower ICIQ-UI-SF score at 3 months and the usual-care group having a lower score at 6 months. As the cohorts of participants who returned the questionnaire at 6 months and at 3 months were different in the main analysis (16 had an ICIQ-UI-SF at 3 months but not at 6 months, and 17 had an ICIQ-UI-SF at 6 months but not at 3 months), a complete-case analysis was conducted, restricting the analysed participants to those who had a calculable ICIQ-UI-SF at baseline and at 3 and 6 months. A total of 70 participants were analysed (usual care, n = 40; intervention, n = 30) using random-effects modelling adjusted for baseline. There was a mean difference of 0.1 [95% confidence interval (CI) –3.1 to 3.3] and 0.2 (95% CI –3.0 to 3.4) at 3 and 6 months, respectively, showing that the usual-care group had a marginally lower score at both time points. Further details can be found in Tables 10 and 11.
| Outcome | Mean difference between intervention and usual-care groups (95% CI) |
|---|---|
| ICIQ-UI-SF score | |
| 3 months | 0.1 (–3.13 to 3.33)a |
| 6 months | 0.2 (–2.97 to 3.42)a |
| Transformed IQoL score | |
| 3 months | –9.0 (–23.74 to 5.71) |
| 6 months | –5.9 (–17.56 to 5.70) |
| Outcome | Trial group, mean (SD) [n] | All (N = 157), mean (SD) [n] | |
|---|---|---|---|
| Usual care (N = 78) | Intervention (N = 79) | ||
| ICIQ-UI-SF total score, with catheterised participants taken as maximum score | |||
| ICIQ-UI-SF score at baseline | 15.8 (5.85) [40] | 16.6 (4.13) [30] | 16.1 (5.16) [70] |
| ICIQ-UI-SF score at discharge | 9.8 (8.27) [29] | 9.9 (7.69) [22] | 9.8 (7.94) [51] |
| ICIQ-UI score at 3 months | 8.3 (7.69) [40] | 8.4 (7.23) [30] | 8.3 (7.44) [70] |
| ICIQ-UI score at 6 months | 8.0 (7.34) [40] | 8.8 (7.60) [30] | 8.3 (7.41) [70] |
The sensitivity analysis results confirmed that the improvement in ICIQ-UI-SF score at 3 months in the intervention group should be interpreted cautiously and with various factors taken into account, including those that contributed to the very high attrition rate.
Number of days with an indwelling urinary catheter in situ and number of participants with an indwelling urinary catheter
The number of days with an IUC in situ and the number of participants with an IUC at each time point are presented using descriptive statistics [median (IQR) and frequencies (%), respectively] by trial group.
The median (IQR) of the number of days with an IUC in situ, with corresponding number of participants with an IUC at each time point, is shown in Table 12. The table also provides the median number of IUC days, calculated as the number of patients with a catheter multiplied by the median number of days.
| Statistic | Time point | ||
|---|---|---|---|
| During stay in stroke unit | Discharge to 3 months | 3–6 months | |
| Usual care | |||
| Median (IQR) | 15.5 (9–28) | 46 (28–68) | 90 (39–99) |
| Range | 1–70 | 14–78 | 39–99 |
| Number of observations | 34 | 6 | 3 |
| Median number of catheter-days | 527 | 276 | 270 |
| Intervention | |||
| Median (IQR) | 14.5 (10–20) | 7 (4–19) | 45 (3–91) |
| Range | 1–56 | 4–19 | 3–91 |
| Number of observationsa | 24 | 3 | 3 |
| Median number of catheter-days | 348 | 21 | 135 |
The median number of days with an IUC was smaller in the intervention group than in the usual-care group at all follow-up time points. Owing to the small sample size, the number of catheter-days by discharge and by 3 and 6 months are presented as summary statistics only. The median number of IUC days was smaller in the intervention group than in the usual-care group for all three time points.
The number of participants with an IUC was smaller in the intervention group than in the usual-care group at all follow-up time points. The frequencies (percentage of trial group responders at those time points) are presented in Table 13.
| Trial group | Participants, n (%) | ||
|---|---|---|---|
| During stay in stroke unit | Discharge to 3 months | 3–6 months | |
| Usual care | 34 (51) | 11 (20) | 7 (15) |
| Intervention | 25 (36) | 3 (8) | 4 (9) |
In the analysis of differences between the two groups, the odds that an IUC was present during a stay in the stroke unit were < 1 for the intervention group, although the 95% CI includes 1 [odds ratio (OR) 0.55, 95% CI 0.27 to 1.12].
Details of other secondary outcome analyses are given in Appendix 2, Tables 22–30: Table 22 presents results for stress incontinence, Table 23 presents results for urge incontinence, Tables 24–27 present results for the number of UTIs, Table 28 presents results related to the number of falls, Table 29 relates to the total Barthel score and Table 30 relates to the number of deaths. The results of secondary outcomes are summarised in Appendix 2, Table 31.
Process evaluation
Fidelity to the intervention
On admission to the stroke unit, 21 (27%) of the intervention group participants had an IUC inserted within 72 hours, with only one (4%) participant undergoing a TWOC within 72 hours of admission.
Baseline continence (IUC vs. incontinent) was determined prior to participants being randomised into the trial. Of those participants with an IUC on admission, two had a successful TWOC prior to being randomised and were categorised as ‘incontinent’ at baseline. This gave a total of 56 (71%) incontinent participants and 23 (29%) participants with an IUC at baseline.
Among those incontinent at baseline, eight (14%) were not eligible for the SVP following an assessment over a minimum period of 3 days via a continence assessment and 3-day diary (included in ‘n = 12 not eligible for SVP’ in Figure 5); a further four participants’ eligibility for SVP was unclear. Of the remaining 48 intervention group participants eligible or potentially eligible for the SVP, only 39 (87%) commenced a SVP. An additional six participants commenced a SVP despite apparently being ineligible. A total of 45 participants therefore commenced a SVP, of whom 35 were known to be eligible. Of those 35 participants, 21 (60%) were allocated to a SVP and 14 (40%) were allocated to bladder training.
Of the 23 participants with an IUC at baseline, 15 had a successful TWOC, of whom only 7 (47%) subsequently completed a continence assessment and 3-day diary and were found to be incontinent. One participant had an unsuccessful TWOC and three did not have a TWOC prior to discharge (although one was recorded as continent at discharge and so is assumed to have had an undocumented but successful TWOC prior to discharge).
Two of the 56 participants classified as incontinent at baseline were catheterised post randomisation. Neither participant had a documented TWOC; both were discharged with the IUC in situ.
Table 14 summarises the results, along with the number of IUC insertions, for those catheterised at baseline.
| Outcome | n (%) |
|---|---|
| Intervention group participants | |
| Presence of IUC within 72 hours of admission | |
| Yes, ≤ 72 hours | 21 (27) |
| ≥ 72 hours | 4 (5) |
| Yes, unknown | 1 (1) |
| No, IUC | 53 (67) |
| Total | 79 (100) |
| TWOC within 72 hours of admission | |
| ≤ 72 hours | 1 (4) |
| > 72 hours | 20 (80) |
| Missing | 4 (16) |
| Total | 25 (100) |
| Participants catheterised at baseline | |
| TWOC outcome | |
| Number of successful TWOCs | 15 (65) |
| Number of unsuccessful TWOCs | 1 (4) |
| No evidence of a TWOC | 7 (30) |
| Total | 23 (100) |
| Number of IUC insertions | |
| One | 20 (87) |
| Two | 3 (13) |
| Total | 23 (100) |
| Number of days IUC inserted | |
| Number | 21 |
| Range | 3–43 |
| Median (IQR) | 14 (10–17) |
Eligibility criteria for entering a systematic voiding programme
We assumed that all of the participants who were incontinent at baseline, had the continence assessment performed and had the 3-day diary completed were eligible to enter a SVP, unless they were found to be continent.
Participants who were catheterised at baseline became eligible to enter a SVP if they were incontinent after a successful TWOC. Following IUC removal, participants had to complete a 3-day bladder diary to assess the pattern of UI and a continence assessment to determine the type of UI.
Of the 79 participants allocated to the intervention group, 51 (65%) were eligible to enter the SVP. However, only 35 out of 79 (44%) participants were put on a treatment regime (Figure 5).
FIGURE 5.
Flow of intervention group participants through the ICONS-II trial intervention.
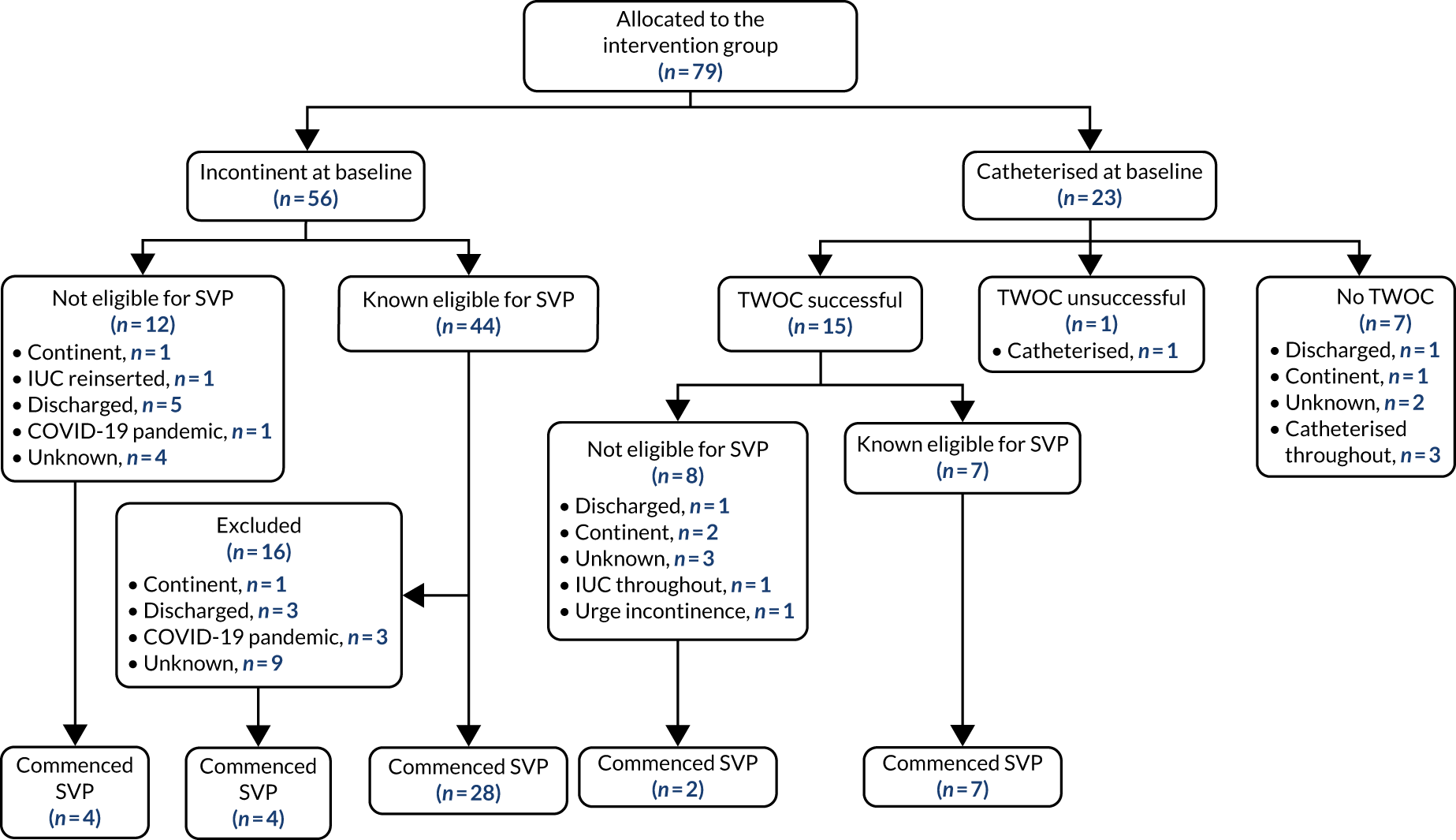
The main documented reasons for ineligibility for a SVP in both the catheterised participants and those incontinent at baseline were participants being continent, catheterised or discharged early. Participants discharged within 4 days of being randomised were not put on a SVP.
A summary of the reasons why participants were ineligible for a SVP is presented in Table 15.
| Participant status | Incontinent participants, n/N (%) | Catheterised participants, n/N (%) | Total, n/N (%) |
|---|---|---|---|
| Baseline status | 56/79 (71) | 23/79 (29) | 79/159 (50) |
| Eligible for SVP | 44/56 (79) | 7/23 (30) | 51/79 (65) |
| Ineligible for SVP | 12/56 (21) | 16/23 (70) | 28/79 (35) |
| Reason ineligible for SVP | |||
| Continent | 1 | 3 | 4 |
| Functional UI | 1 | 1 | |
| Catheterised throughout | 5 | 5 | |
| IUC reinserted | 1 | 1 | |
| Discharged | 5 | 2 | 7 |
| COVID-19 pandemic | 1 | 1 | |
| Unknown | 4 | 5 | 9 |
A total of 35 (44%) of the intervention group participants and 35 (69%) participants eligible for a SVP were put on a treatment regime.
Of the 16 (31%) participants who did not commence a SVP, the majority (n = 15; 94%) had a recommended initial treatment plan documented. However, the reason why a treatment regime was not instituted was recorded for only 7 of these 15 patients. One participant was continent, three were discharged early and three remained in hospital when the trial was paused in March 2020 owing to the COVID-19 pandemic. Further details of these 15 participants can be found in Appendix 3, Table 32.
Just over half of the participants recorded as commencing a SVP were recorded as commencing the correct regime (n = 18; 51%), and only five (14%) participants were recorded as commencing a treatment regime different from that they had been initially recommended (Table 16). Documented reasons why participants changed from prompted voiding to bladder training were participants being aware of the need to void (n = 2), being able to have some control over their bladder (n = 1) and not having the 3-day diary properly completed (n = 1). Reasons why participants changed from bladder training to prompted voiding were not documented.
| Participant regime | n/N (%) |
|---|---|
| Eligible for SVP | 51/79 (65) |
| SVP commenced | 35/51 (69) |
| SVP not commenced | 16/51 (31) |
| Initial treatment plan | 15/16 (94) |
| Unknown | 1/16 (6) |
| Allocated to correct regime | 18/35 (51) |
| PV | 12/18 (67) |
| BT | 6/18 (33) |
| Allocated to incorrect regime | 5/35 (14) |
| PV | 1/5 (20) |
| BT | 4/5 (80) |
| Unknown | 12/35 (34) |
| Missing 6-CIT | 11/12 (92) |
| Started on PV | 8/11 (73) |
| Started on BT | 3/11 (27) |
| Missing frequency of leak | 1/12 (8) |
| Started on BT | 1/12 (8) |
For 12 (34%) participants, it was not possible to determine whether or not the correct regime had been started. Eleven of these participants could not communicate either verbally or in writing and, therefore, the 6-CIT could not be administered at baseline to determine whether or not they were cognitively impaired.
Daily clinical logs and weekly reviews
Forty-five participants randomised to the intervention group started a SVP. The ICONS-II trial clinical staff were asked to complete daily logs to document the participants’ planned and actual toileting activity, and to review, document and revise patients’ individualised treatment plan weekly. Clinical staff also documented any ongoing continence event in a ‘log of ongoing continence events’.
A total of 42 (93%) participants had at least one daily clinical log. One participant’s route was changed from prompted voiding to bladder training because of a significant cognitive and mobility improvement and, therefore, had clinical logs recorded for both regimes. In total, there were 18 logs for bladder training and 25 for prompted voiding.
The majority of the participants known to be eligible for the SVP (n = 32; 91%) and recorded as commencing a treatment regime had at least one daily clinical log recorded. For four (8%) participants, although they were eligible for the SVP, it was not possible to determine whether or not they had commenced a SVP from the continence assessment or log of ongoing continence event forms because of missing data; however, all of these participants had at least one clinical log. Six (21%) of the participants ineligible for the SVP also had some clinical logs recorded. Five (83%) of these participants did not have a 3-day diary, and the other had neither an initial recommended treatment plan nor a completed log of ongoing continence events.
For each participant, we compared the number of daily logs with the number expected from the other documentation. Participants were expected to have a daily log for each day they were on the programme, including, potentially, both the first and the last day. Any documented suspensions from the SVP were subtracted from the calculated duration of the programme. The resulting value was used as an estimate of the upper bound of the actual number of days the participant was in the SVP.
Table 17 shows that a large proportion of the participants (n = 23; 72%) who were put on a treatment regime and had at least one daily clinical log recorded also had the clinical logs reviewed for each full week they spent on the programme. The majority of the nine participants who did not have a weekly review of the clinical logs (n = 7; 78%) were on the programme for < 1 week. Two-thirds of the participants (n = 21; 66%) had at least the expected number of weekly reviews, with five of the remaining participants having one fewer review than expected.
| Actual days on SVP | Number of weekly reviews (N = 42), n (%) | Total, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| < 7 | 10 | 1 | 1 | 12 | ||||
| 7 to < 14 | 1 | 5 | 2 | 8 | ||||
| 14 to < 21 | 1 | 5 | 2 | 8 | ||||
| 21 to < 28 | 1 | 1 | 3 | 5 | ||||
| 28 to < 35 | 1 | 1 | 2 | |||||
| 35 to < 42 | 2 | 1 | 3 | 6 | ||||
| 42 to < 49 | 1 | 1 | ||||||
| 49 to < 56 | 1 | 1 | ||||||
| Total | 12 (28) | 8 (19) | 9 (21) | 8 (19) | 2 (5) | 3 (7) | 1 (2) | 43 (100) |
A summary of the daily clinical logs and weekly reviews is presented in Table 18. The median time participants were in the programme was 14 (IQR 6–26) days, but they typically spent 3 (IQR 1–8) fewer days than they should have on the programme. The median number of weekly reviews was 2 (IQR 1–3).
| Measure | Value |
|---|---|
| Daily clinical logs | |
| Number of logs (n)a | 43 |
| Days participants should have been in SVP | |
| Range | 1–84 |
| Median (IQR) | 25 (11–38) |
| Missing (n) | 2 |
| Actual days participants were in SVP | |
| Range | 1–53 |
| Median (IQR) | 14 (6–26) |
| Missing (n) | 0 |
| Difference | |
| Range | 0–62 |
| Median (IQR) | 3 (1–8) |
| Missing (n) | 2 |
| Weekly reviews | |
| Expected number of weekly reviews | |
| Range | 0–12 |
| Median (IQR) | 2 (1–5) |
| Missing (n) | 8 |
| Actual number of weekly reviews | |
| Range | 1–6 |
| Median (IQR) | 2 (1–3) |
| Missing (n) | 12 |
| Difference | |
| Range | 0–11 |
| Median (IQR) | 1 (0–1) |
| Missing (n) | 17 |
One participant should have been in the SVP for 88 days, but remained on the regime for only 22 days as they were not willing to continue the programme. This increased the range of the variable ‘days participants should have been in SVP’ from 1–68 to 1–84. However, the overall conclusions were not affected.
We did not perform a detailed analysis of the daily clinical logs owing to the early termination of the trial.
Summary results of the key aspects of the systematic voiding programme
In terms of the core measures of fidelity, this section provides a summary of the data for the three key aspects of the SVP:
-
A total of 6 out of 79 (6%) intervention group participants, or 6 out of 21 (29%) intervention group participants known to be catheterised during the acute phase, met the criterion ‘presence of a strategy for minimising indwelling urinary catheterisation in the acute phase unless clinically justifiable (to relieve urinary retention or when fluid balance is critical)’. If clinically justifiable, the presence of a strategy for review and removal (i.e. TWOCs) was required for the patient to meet this criterion.
-
None of the intervention group participants met the criterion ‘presence of a comprehensive continence assessment [including history taking, urine dipstick examination, a mid-stream urine specimen tested by microscopic examination, culture and sensitivity, and identification of the type of UI (urge, stress, mixed or other)]’.
-
A total of 12 out of 79 (15%) of the intervention group participants, or 11 out of 51 (22%) of the intervention group participants known to be eligible to start a SVP, met the criterion ‘presence of a tailored treatment plan including behavioural approaches (prompted voiding or bladder training)’.
None of the intervention group participants met all three key aspects of the SVP (i.e. all of criteria i–iii), nor did any of those never catheterised (who would not be expected to have ‘documentation of a strategy for minimizing IUC in the acute phase unless clinically justifiable’) meet both criterion ii and criterion iii.
There was some evidence of a strategy for minimising IUC in the acute phase unless clinically justifiable and a tailored treatment plan among the intervention group participants. However, there was no evidence that a comprehensive continence assessment had been performed, although we assume that this was undertaken if participants started a SVP. For some of the participants who started a programme, no continence assessment form was received by the CTU or the form had missing data.
Strategies to protect against contamination in the usual-care group
The success of strategies for minimising contamination (defined as providing all three key aspects of the SVP to usual-care participants) was assessed using the data collected on the 78 participants allocated to the usual-care group. In addition, we assessed baseline continence practice by conducting a retrospective review of the case notes of 498 patients discharged from each unit in the 3 months prior to commencement of screening patients for potential recruitment to the trial, that is the ‘pre-implementation period’.
Results: extent to which usual-care participants received the intervention
Extent to which ICONS-II trial staff provided toileting assistance to usual-care participants
Measures of usual care, used as an indication of potential contamination, were described in Process evaluation: measures for usual care only. However, owing to the early termination of the trial, difficulties in obtaining robust data and a lack of concern regarding contamination as a result of looking at outcomes on measures of contamination, we did not perform the analysis of the data collected.
The initial data extracted from the toileting data contained errors in the coding of the staff member who provided toileting assistance to usual-care participants, and, as a result, the analysis is not included in the report. Owing to difficulties with staff completing this information and coding the information based on information entered, it was decided not to pursue the analysis of this, as the data were deemed not sufficiently robust. However, the provision of toileting assistance by ICONS-II trial staff alone is unlikely to have a substantial impact, at least not on the delivery of a SVP, in the absence of evidence that the usual-care participants received the components of the intervention covered in Extent to which usual-care participants received the intervention.
Extent to which usual-care participants received the intervention
The results relating to the key aspects of the SVP were as follows:
-
Of the 78 usual-care group participants, 40 (51%) were recorded as having an IUC at some time between admission to and discharge from the stroke unit. A total of 20 out of 78 (26%) usual-care participants, or 20 out of 40 (50%) usual-care participants known to be catheterised at some time post admission to the stroke unit, met the criterion ‘presence of a strategy for minimising indwelling urinary catheterisation in the acute phase unless clinically justifiable (to relieve urinary retention or when fluid balance is critical)’. If clinically justifiable, presence of a strategy for review and removal (i.e. TWOCs) was required for the patient to be deemed to meet this criterion. The individual items used in the algorithm for assessing the presence of a strategy for minimising IUC in the acute phase (unless clinically justifiable) were the following: for 40 (51%) patients, presence of an IUC was documented at any time between admission to and discharge from the stroke unit; 37 (93%) of these patients had a documented reason, with the reason specified for 32 (86%) patients, of which 26 (70%) specified retention of urine and 6 (16%) specified measuring input and output; the date when the IUC was inserted was available for 38 (95%) participants; the documented evidence of a TWOC was available for 30 (75%) participants, and, for 19 (63%) of those, the trial was successful.
-
Of the 78 usual-care participants, 65 (83%) were documented as having been incontinent at some time between admission to and discharge from the stroke unit. A total of 6 out of 78 (8%) usual-care participants, or 6 out of 65 (9%) of those identified as incontinent at any time post admission to the stroke unit, met the criterion ‘presence of a comprehensive continence assessment [including continence history, diagnosis of UI (urge, stress, mixed or other), pattern of UI and assessment of relevant comorbid conditions]’. The details of the individual items used in the algorithm for the assessment of the presence of a comprehensive continence assessment for usual-care group participants were the following: of the 65 patients with the presence of UI documented at any time between admission to and discharge from the stroke unit, 41 (63%) had documented evidence of any clinical investigations relating to UI, such as urinalysis or bladder scan; however, only 11 (17%) had documented evidence of a current diagnosis of a type of UI (i.e. urge, stress, mixed or other); for 42 (65%), there was documented evidence of the patient’s pattern of UI during this admission (e.g. how often they were incontinent).
-
None of the 78 participants met the criterion for presence of a tailored treatment plan including behavioural approaches (specifically, a tailored voiding interval and evidence of review and adjustment).
None of the usual-care participants met all three key aspects of the SVP (i.e. all of criteria i–iii), and nor did any of those never catheterised (who would not be expected to have ‘documentation of a strategy for minimising IUC in the acute phase unless clinically justifiable’) meet both of criteria ii and iii.
Pre-implementation case-note review patients
Data for 498 pre-implementation case note review patients were collected. One patient had no data (reason unknown), and another patient was identified as continent. Both were excluded from the analysis. The results of the three key aspects of the SVP for the pre-implementation case-note review patients for 496 reviews were as follows:
-
A total of 27 out of 496 (5%) the patients, or 27 out of 102 (26%) of those catheterised at any time post admission to the stroke unit, met the criterion for presence of a strategy for minimising IUC in the acute phase unless clinically justifiable (i.e. to relieve urinary retention or when fluid balance is critical). If clinically justifiable, presence of a strategy for review and removal (i.e. TWOCs) was required for the patient to meet this criterion. The details of the individual items used in the algorithm to assess presence of a strategy for minimising IUC in the acute phase unless clinically justifiable for pre-implementation case-note review patients were the following: 394 out of 496 (79%) did not have IUC documented between admission to and discharge from the stroke unit; of those who had IUC documented, for 77 (75%) there were documented reasons, such as retention of urine (52%) and measurement of input and output (30%); for 53 (52%), there was documented evidence of a TWOC, and, of those, the trial was successful for 38 (72%).
-
Only 5 out of 496 (1%) patients, or 5 out of 150 (3%) patients identified as incontinent at any time post admission to the stroke unit, met the criterion for presence of a comprehensive continence assessment [including continence history, diagnosis of UI (urge, stress, mixed or other), pattern of UI and assessment of relevant comorbid conditions]. The individual items used for the assessment of the presence of a comprehensive continence assessment in pre-implementation case-note review patients were the following: of the 496 pre-implementation patients, 346 (70%) did not have presence of UI documented at any time between admission to and discharge from the stroke unit; of those who did, the date when presence of UI was first documented was available for 147 (98%), there was documented evidence of current clinical investigations relating to UI (e.g. urinalysis, bladder scan) for 62 (41%), there was documented evidence that a current diagnosis of type of UI (i.e. post-stroke urge, stress, mixed or other) was made for 10 (7%) and there was documented evidence of the patient’s pattern of UI during this admission (e.g. how often they were incontinent) for 61 (41%).
-
None of the patients met the criterion for presence of a tailored treatment plan including behavioural approaches (specifically, a tailored voiding interval and evidence of review and adjustment). The details of the individual items used for the assessment of the presence of a tailored treatment plan (pre-implementation case-note review patients) were as follows: 138 (92%) patients had no evidence of starting a voiding programme, and 11 (7%) had documented evidence they had started a voiding programme (e.g. 2-hourly toileting); however, the voiding programme was described as either bladder training or prompted voiding for only four of those 11 (of whom only three had a programme tailored to them, and only two of those three had had their programme reviewed) – only one of those had documented evidence that their programme required adjustment and that it was adjusted.
As per the usual-care participants, none of the pre-implementation case-note review patients met the three key aspects of the SVP (i.e. criteria i–iii), nor did any of those never catheterised (who would not be expected to have ‘documentation of a strategy for minimising IUC in the acute phase unless clinically justifiable’) meet both criteria ii and iii. Twenty-six per cent (27/102) of those catheterised had a strategy for minimising IUC in the acute phase (unless clinically justifiable), compared with 50% (20/40) in the usual-care group.
Summary of process evaluation results for usual-care group
There was a strategy for minimising IUC in the acute phase unless clinically justifiable among a substantial percentage (20/40; 50%) of the catheterised usual-care group participants, which was approximately double the percentage observed in the pre-implementation case-note review patients (27/102; 26%). However, there is very little evidence that a comprehensive continence assessment was performed on usual-care participants. Even for the small number of usual-care participants for whom there was evidence that a comprehensive continence assessment had been performed, there was no evidence that this progressed to a tailored treatment plan of these participants. This is not inconsistent with the corresponding prevalence observed in the pre-implementation case-note review patients.
Approximately half of the usual-care group participants were catheterised at some point during their hospital stay. Half of these participants had a strategy for minimising IUC, which was approximately double the proportion in the pre-implementation phase. There is, therefore, some evidence that this element of the intervention contaminated the usual-care group. There was very little evidence that elements of the SVP were delivered to the usual-care participants, which was similar to the situation in the pre-implementation period.
Chapter 4 Health economic evaluation
The goal was to conduct a patient-level within-trial cost–utility analysis from an NHS and Personal Social Services perspective, producing estimates of cost-effectiveness to generate (within-trial) incremental cost-effectiveness ratio planes and cost-effectiveness acceptability curves (CEACs) using standard methods. For the ‘within-trial’ analysis, changes in both the EQ-5D-5L utility scores and costs would have been estimated for the duration of the trial follow-up, that is 6 months.
Costs included in the analysis were to comprise health-care resource use and the cost of the intervention itself. Costs were to be presented in terms of mean value, SD and mean difference (with 95% CIs) between the groups.
In-hospital resource data were to include recorded costs relating to the management of UI:
-
Staff input in relation to the management of incontinence (grade of staff and time spent on incontinence-related activities).
-
Data were to be recorded regarding total length of hospital stay, toileting and cleaning patients following an episode of incontinence, and use of equipment (bladder scanner, commode, slipper pan and hoist) and consumables (bottles, pads, mattresses and personal items, and catheters).
-
After discharge, resource use data were to be recorded on direct medical and non-medical costs: re-admission to hospital, health service use [e.g. general practitioner (GP) contacts], therapy services, social services, aids, adaptations and consumables in relation to incontinence, and time spent by carers in incontinence-related activities. Unit costs of the resource use items recorded in the patient questionnaire were to be derived from sources relevant to the NHS and social services. Costs were to be expressed in current-year Great British pounds (GBP). The NHS Cost Inflation Index63 was to be used to adjust costs to the current price year, when necessary. Descriptive statistics were to be reported for each resource use item.
It was anticipated that total resource use was to be calculated for each participant in both groups for the duration of the trial.
Participants were provided with questionnaires to complete at baseline and at 3 and 6 months. We were to present descriptive statistics for each resource use item. Health-care resource use results were to be presented for both groups in terms of mean value, SD and mean difference (with 95% CIs) between the groups.
The trial included the use of the EQ-5D-5L questionnaire, which was completed at baseline and at 3 and 6 months. Repeated utility scores over time were to be used to obtain estimates for each patient. The within-trial difference between baseline and week 26 expressed as QALYs was to be estimated using the EQ-5D-5L. A linear change approach was to be used, adjusting for baseline imbalance in mean utility. No discounting would have been applied, taking into consideration the fact that the follow-up period was < 1 year. Descriptive statistics were to be reported for change in EQ-5D-5L utility scores during the intervention between the two groups, along with 95% CIs.
The differences in cost data were to be presented unadjusted and adjusted for covariates (costs and outcomes). QALY data were to be adjusted for baseline EQ-5D-5L utility scores to allow for any differences at baseline between the two groups. Adjustment for covariates that were consistent with those used in the statistical analysis [baseline ICIQ-UI-SF score (with those catheterised imputed as having the worst score) and site, with the latter as a random effect if convergence is achieved] was also to be considered if necessary. Overall mean costs, stratified by NHS and social services personnel, and SDs for both trial groups were to be calculated. Patient-level total costs and QoL data were to be bootstrapped to generate incremental cost-effectiveness ratio planes to estimate average cost-effectiveness and 95% CIs. Uncertainty around cost-effectiveness was to be described using CEACs, which are used to describe the probability of an intervention being cost-effective.
Uncertainty may be introduced into the analysis through the parameters and data sources selected. Aspects of model uncertainty were to be addressed through sensitivity analysis for parameters such as resource use, costs, number of users and effectiveness. Furthermore, a complete-case analysis was also to be performed to understand how the uncertainty due to the imputation methods used would influence the results of the study and the final recommendations.
During the time that the project was running, two resource use questionnaires were developed: one for patients discharged and another for participants discharged into residential homes/care homes. Information collected included re-admissions to hospital, health service use (e.g. GP contacts), therapy services, social services, aids, adaptations, consumables in relation to incontinence and time spent by carers on incontinence-related activities.
The form was sent once per month; however, some of the participants who withdrew from the study said that the reason was the burden of completing the resource use form.
An amendment to the protocol1 was made to consider including a revised form in the 3- and 6-month questionnaires so that participants received only two sets of forms; however, given the circumstances, this did not proceed.
Overall, 508 questionnaires were returned that contained information on resource use: 265 from participants in the intervention group and 243 from participants in the usual-care group (see Appendix 5, Table 35). The small number achieved does not allow for more than the presentation of descriptive statistics for the data collected.
The most used services in both trial groups were GPs and practice nurses (Table 19). Regarding other services, treatment or help from physiotherapy, occupational therapy or district nurses, and home care or home help were the most frequently identified. The number of participants reporting service use was too small for a statistical comparisons of means between groups to be undertaken. An equivalent table showing months 1–6 is provided in Appendix 5 (see Table 36).
| Resource | Usual-care group | Intervention group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | n | SD | Mean | n | SD | Mean | n | SD | |
| Used GP | 1.1 | 39 | 1.44 | 0.8 | 25 | 1.38 | 1.0 | 64 | 1.41 |
| Used practice nurse | 0.9 | 38 | 1.63 | 0.4 | 25 | 0.86 | 0.7 | 63 | 1.40 |
| Admitted to hospital | 0.4 | 38 | 0.95 | 0.1 | 25 | 0.28 | 0.3 | 63 | 0.77 |
| Treated at A&E | 0.2 | 38 | 0.46 | 0.0 | 25 | 0.00 | 0.1 | 63 | 0.36 |
| Received treatment/help from | |||||||||
| Physiotherapy | 7.2 | 20 | 6.68 | 9.6 | 14 | 7.60 | 8.2 | 34 | 7.07 |
| Occupational therapy | 5.1 | 16 | 3.94 | 6.2 | 9 | 2.54 | 5.5 | 25 | 3.49 |
| Chiropodist or podiatrist | 3.6 | 5 | 4.78 | 1.0 | 3 | 0.00 | 2.6 | 8 | 3.85 |
| District nurse | 11.5 | 12 | 25.09 | 5.3 | 3 | 6.66 | 10.3 | 15 | 22.53 |
| Home care/help | 37.9 | 7 | 31.86 | 50.3 | 8 | 35.03 | 44.5 | 15 | 33.01 |
| Continence advisor | 2.0 | 4 | 0.82 | 1.5 | 2 | 0.71 | 1.8 | 6 | 0.75 |
| Stroke Association family support worker | 3.5 | 6 | 3.02 | 1.7 | 3 | 0.58 | 2.9 | 9 | 2.57 |
| Day support (day-care centre) | 1.0 | 1 | 1.0 | 1 | |||||
| Other treatment/help | 3.5 | 2 | 2.12 | 30.0 | 1 | 12.3 | 3 | 15.37 | |
In Table 20, we can observe the average resource use of the participants using the short survey in month 1. As for the participants using the long survey, the most used services are GPs and practice nurses in both trial groups for the overall period. The usual-care group uses more of those services, on average, than the intervention group. Participants using the short survey report using GP services more, although the sample size is too small to allow for any meaningful comparisons. An equivalent table showing months 1–6 is provided in Appendix 5 (see Table 37).
| Resource | Usual-care group | Intervention group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | n | SD | Mean | n | SD | Mean | n | SD | |
| Used GP | 2.9 | 7 | 2.2 | 1.9 | 11 | 2.0 | 2.3 | 18 | 2.1 |
| Used practice nurse | 0.0 | 8 | 0.0 | 0.6 | 11 | 1.5 | 0.3 | 19 | 1.2 |
| Admitted to hospital | 0.0 | 9 | 0.0 | 0.6 | 11 | 0.9 | 0.3 | 20 | 0.7 |
| Treated at A&E | 0.1 | 9 | 0.3 | 0.6 | 11 | 1.0 | 0.4 | 20 | 0.8 |
| Physiotherapy | 0.4 | 9 | 0.5 | 0.5 | 11 | 0.5 | 0.5 | 20 | 0.5 |
| Occupational therapy | 0.3 | 8 | 0.5 | 0.4 | 11 | 0.5 | 0.3 | 19 | 0.5 |
| Chiropodist or podiatric | 0.3 | 8 | 0.5 | 0.3 | 11 | 0.5 | 0.3 | 19 | 0.5 |
| District nurse | 0.0 | 8 | 0.0 | 0.0 | 10 | 0.0 | 0.0 | 18 | 0.0 |
| Continence advisor | 0.0 | 8 | 0.0 | 0.0 | 11 | 0.0 | 0.0 | 19 | 0.0 |
| Stroke Association family support worker | 0.1 | 9 | 0.3 | 0.0 | 11 | 0.0 | 0.1 | 20 | 0.2 |
| Number of times received treatment/help from physiotherapy | 12.0 | 3 | 10.0 | 28.0 | 4 | 28.0 | 21.1 | 7 | 22.3 |
| Number of times received treatment/help from occupational therapy | 11.5 | 2 | 14.8 | 17.3 | 3 | 22.3 | 15.0 | 5 | 17.7 |
| Number of times received treatment/help from chiropodist or podiatrist | 1.0 | 2 | 0.0 | 1.3 | 3 | 0.6 | 1.2 | 5 | 0.4 |
| Number of times received treatment/help from Stroke Association family support worker | 1.0 | 1 | 1.0 | 1 | |||||
The EQ-5D-5L was administered at baseline and at 3 and 6 months to the participants recruited and followed up until the trial was stopped. For participants who were unable to answer the EQ-5D-5L instrument, the EQ-5D-5L, proxy version 1, was used, in which the caregiver (i.e. the proxy) is asked to rate the patient’s health in their (i.e. the proxy’s) opinion. There was some variation in the dimension scores by study group, but the sample size is too small to allow for any statistical comparisons.
The data for all the participants are presented in Table 21. The data are reported separately for the patients’ responses in Appendix 5, Table 38, and proxies’ responses in Appendix 5, Table 39.
| Dimension | Time period, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up 1: 3 months | Follow-up 2: 6 months | ||||
| Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | |
| Mobility | ||||||
| No problems in walking about | 5 (7) | 4 (5) | 6 (11) | 4 (11) | 8 (18) | 8 (17) |
| Slight problems in walking about | 11 (14) | 15 (19) | 7 (13) | 5 (14) | 13 (29) | 5 (11) |
| Moderate problems in walking about | 15 (20) | 13 (16) | 16 (30) | 10 (28) | 13 (29) | 12 (26) |
| Severe problems in walking about | 8 (11) | 10 (13) | 9 (17) | 6 (17) | 6 (13) | 11 (24) |
| Unable to walk about | 37 (49) | 37 (47) | 15 (28) | 11 (31) | 5 (11) | 10 (22) |
| Number | 76 | 79 | 53 | 36 | 45 | 46 |
| Self-care | ||||||
| No problems washing or dressing themselves | 8 (11) | 9 (11) | 18 (34) | 7 (19) | 17 (38) | 14 (30) |
| Slight problems washing or dressing themselves | 8 (11) | 11 (14) | 11 (21) | 3 (8) | 10 (22) | 3 (7) |
| Moderate problems washing or dressing themselves | 16 (21) | 10 (13) | 6 (11) | 7 (19) | 9 (20) | 12 (26) |
| Severe problems washing or dressing themselves | 13 (17) | 18 (23) | 5 (9) | 4 (11) | 5 (11) | 5 (11) |
| Unable to wash or dress themselves | 30 (39) | 31 (39) | 13 (25) | 15 (42) | 4 (9) | 12 (26) |
| Number | 75 | 79 | 53 | 36 | 45 | 46 |
| Usual activities | ||||||
| No problems doing their usual activities | 9 (12) | 6 (8) | 6 (11) | 2 (6) | 8 (18) | 7 (15) |
| Slight problems doing their usual activities | 6 (8) | 8 (10) | 13 (25) | 3 (8) | 11 (24) | 5 (11) |
| Moderate problems doing their usual activities | 10 (13) | 9 (11) | 11 (21) | 8 (22) | 11 (24) | 14 (30) |
| Severe problems doing their usual activities | 10 (13) | 16 (20) | 9 (17) | 6 (17) | 7 (16) | 7 (15) |
| Unable to do their usual activities | 41 (54) | 40 (51) | 14 (26) | 17 (47) | 8 (18) | 13 (28) |
| Number | 76 | 79 | 53 | 36 | 45 | 46 |
| Pain/discomfort | ||||||
| No pain or discomfort | 37 (49) | 34 (43) | 22 (42) | 11 (31) | 15 (33) | 20 (43) |
| Slight pain or discomfort | 24 (32) | 15 (19) | 16 (30) | 6 (17) | 13 (29) | 9 (20) |
| Moderate pain or discomfort | 10 (13) | 16 (20) | 7 (13) | 15 (42) | 11 (24) | 9 (20) |
| Severe pain or discomfort | 4 (5) | 8 (10) | 5 (9) | 2 (6) | 5 (11) | 6 (13) |
| Extreme pain or discomfort | 1 (1) | 4 (5) | 1 (2) | 2 (6) | 0 (0) | 1 (2) |
| Number | 76 | 77 | 51 | 36 | 44 | 45 |
| Anxiety/depression | ||||||
| Not anxious or depressed | 35 (46) | 27 (34) | 20 (38) | 15 (42) | 19 (42) | 18 (39) |
| Slightly anxious or depressed | 24 (32) | 30 (38) | 18 (34) | 10 (28) | 18 (40) | 19 (41) |
| Moderately anxious or depressed | 11 (14) | 16 (20) | 5 (9) | 6 (17) | 7 (16) | 7 (15) |
| Severely anxious or depressed | 3 (4) | 2 (3) | 3 (6) | 1 (3) | 1 (2) | 0 (0) |
| Extremely anxious or depressed | 2 (3) | 1 (1) | 2 (4) | 1 (3) | 0 (0) | 1 (2) |
| Number | 75 | 76 | 48 | 33 | 45 | 45 |
Appendix 5, Table 40, presents the average scores for each EQ-5D-5L dimension by group at baseline and at 3 and 6 months. The average scores presented are slightly better for the participants in the usual-care group than for those in the intervention group.
The average scores computed from the proxies’ answers are higher than those reported by the patients themselves (see Appendix 5, Table 41). Again, the average scores observed in the intervention group are higher than those reported in the usual-care group.
With regard to the observations concerning onsite resource use, owing to recruitment problems and the COVID-19 pandemic, no visits to hospitals took place to collect this information. It had been planned to use these visits to collect information about staff input in relation to the management of incontinence (grade of staff and time spent on incontinence-related activities) and data regarding the use of equipment and consumables, total length of hospital stay and toileting and cleaning patients following an episode of incontinence.
Overall, development work for the economic evaluation took place (see the questionnaires and descriptive statistics included in Appendix 5), but, owing to the recruitment problems and then the pandemic, there were no data for a meaningful economic analysis to be undertaken.
Chapter 5 Discussion
The ICONS-II pragmatic, multicentre, individual-patient-randomised (1 : 1), parallel-group trial with internal pilot aimed to evaluate the clinical effectiveness and cost-effectiveness of a SVP for UI and was informed by the ICONS-I feasibility trial. The ICONS-I feasibility trial was cluster randomised, with usual-care/intervention sites. This facilitated implementation (delivered as change in practice in intervention sites), but there was some evidence of differences in patient selection, which is a known issue in cluster-randomised designs when participant recruitment cannot be completed prior to site randomisation (as in the ICONS-I feasibility trial). Usual-care patients were recruited earlier in their stay and had milder incontinence.
The ICONS-II trial design options were cluster randomisation at site level, with specific criteria for timing of participant selection, a stepped-wedge design with similar criteria, or IPR. Funding application reviewers recommended that IPR be used in the ICONS-II trial, which would remove the bias in allocation to usual-care/intervention groups, and have the added benefit of requiring fewer participants than a clustered trial. Although these reasons were valid, this decision had negative impacts on the practicalities of running the trial in the sites, including necessary actions to minimise the potential for contamination, which was recognised as a risk owing to participants from both trial groups being treated in the same stroke unit. These mitigating actions were described in Brief account of the trial.
The key findings from the study are that it is difficult to find stroke services that can implement and test a SVP in an IPR controlled clinical trial, and that, even in centres that are able to contribute, it is difficult to identify, enrol and retain sufficient numbers of patients. Because of this, and the added difficulties relating to the COVID-19 pandemic, the study could not be completed. A total of 157 out of the planned 1024 participants were recruited. This number was too small to test any of the outcome measures with sufficient power, and the level of attrition, which differed between intervention and usual-care groups at the primary outcome time point, led to a high risk of bias in the estimation of the effectiveness estimate. For the primary outcome, UI severity (ICIQ-UI-SF) at 3 months from baseline, there was a lower ICIQ-UI-SF score in the intervention group than in the usual-care group (8.1 vs. 9.1, respectively). This result was reversed at the 6-month follow-up, with a higher ICIQ-UI-SF score in the intervention group than in the usual-care group (8.5 vs. 7.9, respectively). Interpretation of any observed difference in the primary outcome in terms of effectiveness of the intervention was not appropriate owing to the high and differential attrition, and under-recruitment. This includes interpretation in the context of our specified MCID. We powered the trial based on a MCID of 2.52, adjusted to 1.89 points under the worst-case scenario of 25% contamination (which was not realised, as stated in Brief account of the trial). Although the 95% CI for the primary outcome spanned these values, the point estimate was well below what would have been clinically relevant. However, as we have reduced the amount of result interpretation, reflecting on our specification of MCID would be inappropriate.
Perhaps the most important finding was that a large proportion of patients (31%) were catheterised at baseline, and that this was reduced by 40% in the intervention group at the primary outcome time point of 3 months. There was a large difference in catheter-days between the intervention and usual-care groups at each follow-up time point.
Problems starting and delivering the trial
The trial had a difficult start: verbal feedback during site visits and e-mail correspondence demonstrated that the design was unpopular with prospective sites, in particular in relation to IPR and the differences in care delivered to the two groups.
The intervention required 50% of staff to be trained in mechanisms of incontinence, the prompted voiding/bladder training programme for the intervention group, recording daily toileting logs and weekly assessment of progress. The trial funded site study champions for implementation and support.
The trial team identified various barriers contributing to the difficulties:
-
Delays in identification and appointment of study champions.
-
Staff concerns that patients would see differences in care, with concerns centred around intervention participants receiving ‘better treatment’.
-
Staff concerns about the extra work/paperwork required (exacerbated by large numbers of staff vacancies).
-
Managing staff rotation to maintain levels of intervention staff.
-
Staff difficulty grasping issues of contamination and the need for the toileting of intervention participants to be undertaken by intervention-trained staff and the toileting of usual-care participants to be undertaken by staff who had not been trained in the intervention.
-
Differences in stroke care settings and pathways between the ICONS-I feasibility trial and the ICONS-II trial [e.g. the estimated length of stay in a stroke unit substantially differed between the ICONS-I feasibility trial and the ICONS-II trial, with a median (IQRs) of 47 (30–68) and 27 (16–45) days, respectively]. Further research and work are needed to assess any unfavourable effect of the differences in care setting on the amount of intervention received, effectiveness of the intervention and attrition.
-
Poor adherence and completion of paperwork by staff.
In December 2019, the team conducted an internal review following the monitoring hub meeting with the HTA programme on 22 November 2019. This review shaped radical adjustments to the study: closing underperforming sites, retraining site staff, adapting paperwork to reflect individual site patient records, sourcing new sites, setting performance targets by encouraging sites to provide complete paperwork sets rather than recruitment alone, and moving the focus from recruitment to intervention delivery. To improve site engagement in the trial, we emphasised that consent alone did not qualify for satisfactory participation in the study and that the introduced measures were linked to performance reporting within site research and development departments. Improvement in recruitment was steady until COVID-19 caused study suspension. Sites halted participation without completing data collection, and often before they had transferred outstanding data to the CTU.
From early March 2020, it became very clear that COVID-19 was dominating resources, and sites started to report that they were unable to support further recruitment and activities for the ICONS-II trial. Many staff designated as study champions were being reallocated to frontline services, and CRN staff (as per HRA guidance) were prioritising recruitment to COVID-19 studies. By 26 March 2020, none of the ICONS-II trial sites were conducting study activities. We contacted all sites with a formal notification of a halt to study activities. HRA guidance at this time was that no formal notification needed to be sent to HRA/REC, as this was countrywide.
We had hoped that we would be able to restart the trial, and discussed this with our sites and our funders; however, as time went on, it became apparent that the situation was not improving. We therefore conducted a full feasibility assessment of the study and reviewed the recruitment, attrition, data completeness, contamination, sample size and financial implications of restarting. Regretfully, it became apparent that a restart was not feasible either financially or in the current clinical and research landscape.
We recruited 157 participants, with an expectation of being able to carry out 3- and 6-month follow-up data collection. It was apparent during the COVID-19 pandemic that most of this patient group fell into the vulnerable category and were shielding and so had difficulty completing postal returns. We therefore contacted all participants, offering them the option of completing outcome data collection over the telephone. Although some participants enjoyed the chance to chat, some felt that the process was onerous. Many data collection items involved multiple-choice Likert scales, which were time-consuming to administer, and some participants found it difficult to remember the options. To assist participants, we adapted the order of the questions so that core outcome data questions were asked first.
Many participants reported anxiety and loneliness when contacted, and we experienced a high attrition rate. The questionnaire included questions on anxiety and depression and, in line with the protocol,1 any participant scoring above the protocol threshold (either by postal return or over the telephone) triggered a referral to their general practice. This involved a letter being faxed to their GP, informing them of the participant’s low mood/anxiety, and the requirement for the general practice surgery to acknowledge receipt as a transfer of responsibility (staff from the CTU called the surgery telling them that the fax/e-mail was coming and the general practice surgery faxed/e-mailed confirmation of receipt).
The number of missing data was problematic, as there was a high proportion of missing primary outcome data (attrition rate 46%), making the trial seriously underpowered.
Contamination estimated in the process evaluation analysis was lower than the worst-case scenario of 25% stated in the funding application. However, overall, it remains plausible that contamination of the usual-care group had an impact on the treatment effect. The process evaluation results show that this was limited to the component designed to minimise IUC in the acute phase, so the impact on the overall effect is likely to be far less than the conservative 25% on which the original sample size was based. The analysis assessing the degree of contamination in the usual-care group, together with the fact that the provision of toileting assistance by ICONS-II trial staff alone is unlikely to have had a substantial impact, at least not on the delivery of a SVP, has allowed us to conclude that the level of contamination of the usual-care group with the key components of the SVP intervention was unlikely to be > 10%. This would have allowed an adjustment to the relevant assumption and a reduction in sample size, although the high attrition rate implies that the overall missing data rate would be about 33% by the end of the trial, even with 20% target attrition after reopening, which would have an impact on validity. The trial would require another 564 participants, which would not be feasible within the time frame and remaining budget and in the light of the ongoing COVID-19 situation. All of this led to the conclusion that the ICONS-II trial was not viable.
Difference in attrition rates
There were concerns about the very high and differential rates of missing primary outcome data (ICIQ-UI at 3 months) between the intervention and usual-care groups (56% vs. 35%, respectively), which also suggested that a future trial would need to consider how this attrition might be reduced, otherwise a robust trial would not be possible. There was a substantial, statistically significant difference (p = 0.008) in attrition rate between the groups for the primary outcome, with primary outcome data for only 35 out of 79 (44%) participants in the intervention group and 51 out of 78 (65%) participants in the usual-care group (see Table 8). The attrition rate was more balanced at 6 months. Although the rate continued to be very high, there was an improvement in the intervention group (56% at 3 months vs. 47% at 6 months), with some participants who did not return the 3-month questionnaire returning their questionnaires at 6 months.
Considering the fact that COVID-19 had a much greater effect on the 6-month questionnaire return rate, including restricting access to the post room at University of Central Lancashire, the improvement in attrition rate could, potentially, be attributed to measures that the team applied to boost retention just before COVID-19 emerged. However, it can also be noted that the improvement could be a positive effect of COVID-19 (rather than a positive effect of the CTU measures), as participants may have changed their behaviours because of COVID-19 and introduced changes to their lifestyle.
Differences between the ICONS-I feasibility trial and the ICONS-II trial
The ICONS-II trial was informed by the ICONS-I feasibility trial. In the ICONS-I feasibility trial, 12 sites commenced recruitment between January 2011 and January 2012, with no sites dropping out. Recruitment started on 1 January 2011 and ceased at all sites on 31 July 2012. The parameter estimates obtained from the feasibility study were used in the ICONS-II trial sample size calculation and when planning intervention implementation. However, it became evident that there was a pronounced difference between the trials in the length of hospital stay. In the ICONS-I feasibility trial, the overall median (IQR) length of stay in the stroke unit was 47 (30–68) days; in the ICONS-II trial, it was 27 (16–45) days. This may have had a substantial impact on the amount of intervention patients received in the ICONS-II trial. It became apparent that a purely hospital-based intervention would be unlikely to provide a sufficient amount of the SVP to a patient to be effective in managing incontinence.
Other details of the ICONS-II trial length of stay trend during the recruitment period are presented in Appendix 4, Tables 33 and 34.
Study limitations
The main study limitation was the small sample size, which made the trial underpowered to detect intervention effects. The large number of missing data due to high attrition and problems with intervention fidelity seriously limited the usefulness of the study results. The availability of the data was also limited by poor adherence and completion of paperwork by staff.
A further limitation was that the voiding element of the intervention could not be delivered for as long as had been expected based on data from the ICONS-I feasibility trial. It may be that this part of the intervention, which was based entirely within the hospital setting, was not of sufficient duration to have a reasonable chance of having an impact, given the increasingly short length of stay following a stroke in today’s NHS.
As summarised in Problems starting and delivering the trial, a number of difficulties were related to hospital staff involvement in the trial that contributed to limitations affecting the fidelity of the intervention. These included staff concerns about visible differences in care between intervention and usual-care participants, concerns about extra work and excessive paperwork, problems with managing staff rotation to maintain levels of intervention staff and staff’s difficulty understanding issues of contamination between the two ICONS-II trial groups. All of these issues were reported ad hoc to the CTU team, and reflect the limitations of the trial process evaluation, which did not include this aspect.
Another limitation was the influence of COVID-19 on the study. The pandemic affected many aspects of the ICONS-II trial, as discussed in Chapter 3, Brief account of the trial including building staff pressures and public concern about going into hospital for several months, which interfered with the sites’ ability to participate in the study. It was not, however, possible to completely measure the impact of COVID-19 on the trial, which introduced a further limitation to the analysis performed.
Recommendations, lessons learnt and future study planning
Future studies should focus on interventions that are likely to be deliverable in the NHS. The intervention needs to fit with current practices and challenges in the NHS. The approach to stroke care has changed since the ICONS-I feasibility trial, with a more intense hyperacute phase, a considerably greater need for monitoring of vital signs and shorter patient stays. This is reflected in the substantially shorter length of stay in the ICONS-II trial than in the ICONS-I feasibility trial. This affected the opportunity for delivery and, hence, the efficiency of the SVP for UI. One of the main barriers to study start-up and completion was the time taken to implement the continence intervention in an already highly stressed NHS.
In planning a future study, we would recommend that testing a programme of catheter avoidance and removal of catheters is the most promising intervention to take forward. As there were fewer catheterised patients at all three time points in the intervention group in the ICONS-II trial, we have shown that the intervention is feasible, and observational data from other studies64,65 suggest that the presence of catheters increases mortality. A programme of catheter avoidance and catheter removal takes considerably less nurse time to implement than a continence programme, and is, therefore, more likely to be testable in a clinical study and more likely to be implemented once shown to be effective. This approach was also supported by the ICONS-I feasibility trial’s findings, which demonstrated some improvements in the intervention group compared with the usual-care group in terms of catheter removal (median 13 days, IQR 5–35 days, compared with median 20 days, IQR 8.75–35.25 days, respectively) and numbers of participants catheterised at discharge (intervention, n = 19; 15.2%; usual care, n = 35; 21.3%). 25
However, incontinence remains a prevalent and distressing problem, and future studies should not be avoided just because they are difficult. Experience from the ICONS-II trial suggests that such a complex and time-consuming intervention is not feasible in the hyperacute stroke environment. An alternative approach would be to consider a randomised controlled trial in which patients are assessed and recruited in hospital or early after discharge into the community, but the majority of the delivery of the intervention would be patient and carer led and take place in the community. Future studies should consider using a more appropriate intervention design and exploring the potential to reframe the research as an implementation study/trial, with a suitable design to evaluate effectiveness in parallel with the implementation. This would require more feasibility work. Both the cluster-randomised design and the individual-patient-randomised design proved to be difficult for clinical areas to implement.
As part of a future implementation trial, an embedded qualitative study, including an exploration of the views of patients, carers and community staff, would be useful for improving the quality of the evidence needed to fully understand the facilitators of and barriers to successful implementation of the intervention in this trial.
The use of study champions was a critical element of staff training in and staff engagement with the programme. The late appointment of some study champions had a large impact on trial recruitment. As COVID-19 arrived and NHS staff, including study champions, were diverted to COVID-19 duties, recruitment dropped rapidly. A future trial design should include study champions to support delivery and embedding of the intervention for maximum effectiveness. These study champions would not only help deliver the study, but also be instrumental for ensuring implementation of the findings.
Chapter 6 Conclusions
There were difficulties delivering the intervention because of IPR, and there were difficulties recruiting sites. There was some evidence that the intervention was unpopular with sites owing to visible differences between usual care and intervention care, which resulted from IPR; questions were raised about the acceptability of the intervention within the proposed design.
The changes to the recruitment introduced by the team (tailored work with sites, changes to the data collection, etc.) resulted in noticeable improvements in trial recruitment. However, these gains were offset by the arrival of COVID-19, which had a substantial impact on the trial, ultimately making a restart after the pause for COVID-19 not viable.
The trial had major issues with attrition and missing outcome data. The intervention implementation, data collection and follow-up in any trial require very careful planning. This is evidenced by the very large, and largely unexplained, differences in the rates of missing outcome data in the usual-care and intervention groups at 3 months. Differences in attrition between groups were much smaller at 6 months after the additional retention measures were applied; these required considerable effort from the trial team and resulted in additional challenges for the participants, although some welcomed the opportunity for telephone follow-up.
It became apparent that the typical length of hospital stay had become much shorter between the ICONS-I feasibility trial and the start of this trial, indicating that a purely hospital-based intervention would not provide the patient with a sufficient amount of the SVP to be effective in promoting continence. A future study of an appropriate design should consider an intervention that starts in hospital or early after the patient’s transfer to the community, and is mainly or wholly delivered within the community.
The trial outcomes led to the conclusion that the proposed intervention is not feasible and does not merit further evaluation in its current form, as it cannot be delivered to all patients at a sufficient ‘dose’ for it to be likely to be effective; further development of the intervention is needed so that it can continue into the community.
Acknowledgements
We would like to thank the following:
-
all trial participants
-
all sites participating in the study
-
TSC and Data Monitoring and Ethics Committee members: Professor Kate Seers, Professor Pip Logan, Professor Christopher Burton, Dr Christopher Price, Mrs Denise Button, Dr Jonathan Hewitt, Ms Alison Allam, Dr Manuel Gomes, Dr Marta Soares, Dr Philip Bell, Professor Martin Dennis, Professor Peter Langhorne and Dr Munya Dimairo
-
Lancashire CTU staff (past and present) who are not listed among the co-authors for their support of the trial.
We would like to acknowledge Professor Christopher Chapple (Sheffield Teaching Hospitals NHS Foundation Trust; Sheffield University; Sheffield Hallam University), who contributed to the beginning of the trial and attended the first trial meeting.
Dedication
Dr David Britt, a member of the ICONS-II trial Patient and Public Involvement (PPI) group, died during the trial. He made a large contribution to this trial and the ICONS-I feasibility trial, and is greatly missed.
Contributions of authors
Caroline Watkins (https://orcid.org/0000-0002-9403-3772) (Professor of Stroke and Older People’s Care) co-led the original grant application, co-designed the trial, interpreted the study findings and co-led the drafting of the report. She became chief investigator when Lois Thomas retired.
Svetlana Tishkovskaya (https://orcid.org/0000-0003-3087-6380) (Senior Lecturer in Health Statistics, Statistician) co-wrote the SAP, conducted the data analysis, prepared the results for publication, interpreted the study findings and led the drafting of the report.
Chris Brown (https://orcid.org/0000-0002-0162-4988) (Senior Research Assistant, Statistics) co-wrote the SAP, analysed the study data, prepared the results for publication, interpreted the study findings and co-led the drafting of the report.
Chris Sutton (https://orcid.org/0000-0002-6406-1318) (Senior Lecturer in Clinical Trial Statistics) co-led the original grant application, co-designed the trial, co-wrote the SAP, provided statistical oversight, interpreted the study findings and assisted with the drafting of the report.
Yvonne Sylvestre Garcia (https://orcid.org/0000-0002-3288-9006) (Research Fellow in Clinical Trials Statistics) analysed the study data and assisted with the drafting of the report.
Denise Forshaw (https://orcid.org/0000-0001-5725-3736) (Principal Clinical Trials Manager) led on trial data management, contributed to data collection and assisted with the drafting of the report.
Gordon Prescott (https://orcid.org/0000-0002-9156-2361) (Reader in Medical Statistics) provided statistical oversight and co-led on the drafting of the report.
Lois Thomas (https://orcid.org/0000-0001-7177-0393) (Professor of Health Services Research) led on the original grant application, co-designed the trial, and contributed to data collection and drafting of the report.
Christine Roffe (https://orcid.org/0000-0002-5259-6649) (Professor of Stroke Medicine) interpreted the study findings and assisted with the drafting of the report.
Joanne Booth (https://orcid.org/0000-0002-7870-6391) (Professor of Rehabilitation Nursing) interpreted the study findings and assisted with the drafting of the report.
Kina Bennett (https://orcid.org/0000-0002-1427-688X) (Research Operations Manager) was part of the study management group via the TMG, provided advice and guidance as lead NHS site representative on research governance process and local NHS set-up processes, assisted with data collection, substantially contributed to the design of the work and approved the version to be published.
Brenda Roe (https://orcid.org/0000-0001-8227-0116) (Professor Emerita of Health Research) interpreted the study findings and assisted with the drafting of the report.
Bruce Hollingsworth (https://orcid.org/0000-0002-4314-6523) (Professor of Health Economics) contributed to the design of the health economics analysis, interpretation of the data and revision the work, and approved the version to be published.
Ceu Mateus (https://orcid.org/0000-0001-6219-219X) (Professor of Health Economics) conducted the health economics analysis and assisted with the drafting of the report.
David Britt (PPI member) contributed as a PPI representative for the trial.
Cliff Panton (PPI member) contributed as a PPI representative for the trial.
Publication
Thomas L, Roffe C, Booth J, Chapple C, Watkins C, Roe B, et al. ICONS II: Identifying Continence OptioNs after Stroke randomised controlled trial. Br J Neurosci Nurs 2018;14(Suppl. 2):S24–5.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Thomas L, Roffe C, Booth J, Bennett K, Watkins C, Roe B, et al. ICONS II: Identifying Continence OptioNs After Stroke. Protocol 2019. https://www.fundingawards.nihr.ac.uk/award/16/111/31 (accessed 26 May 2022).
- Tishkovskaya S, Sutton C, France A. ICONS II: Identifying Continence OptioNs After Stroke. Statistical Analysis Plan (SAP) 2019. https://www.fundingawards.nihr.ac.uk/award/16/111/31.
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.STR.20.7.864.
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001;32:1279-84. https://doi.org/10.1161/01.str.32.6.1279.
- Nakayama H, Jørgensen HS, Pedersen PM, Raaschou HO, Olsen TS. Prevalence and risk factors of incontinence after stroke. The Copenhagen Stroke Study. Stroke 1997;28:58-62. https://doi.org/10.1161/01.str.28.1.58.
- Kolominsky-Rabas PL, Hilz MJ, Neundoerfer B, Heuschmann PU. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn 2003;22:322-7. https://doi.org/10.1002/nau.10114.
- Williams MP, Srikanth V, Bird M, Thrift AG. Urinary symptoms and natural history of urinary continence after first-ever stroke – a longitudinal population-based study. Age Ageing 2012;41:371-6. https://doi.org/10.1093/ageing/afs009.
- Brittain KR, Perry SI, Peet SM, Shaw C, Dallosso H, Assassa RP, et al. Prevalence and impact of urinary symptoms among community-dwelling stroke survivors. Stroke 2000;31:886-91. https://doi.org/10.1161/01.str.31.4.886.
- Brittain KR, Peet SM, Castleden CM. Stroke and incontinence. Stroke 1998;29:524-8. https://doi.org/10.1161/01.str.29.2.524.
- Burney TL, Senapati M, Desai S, Choudhary ST, Badlani GH. Acute cerebrovascular accident and lower urinary tract dysfunction: a prospective correlation of the site of brain injury with urodynamic findings. J Urol 1996;156:1748-50. https://doi.org/10.1016/S0022-5347(01)65498-3.
- Pettersen R, Stien R, Wyller TB. Post-stroke urinary incontinence with impaired awareness of the need to void: clinical and urodynamic features. BJU Int 2007;99:1073-7. https://doi.org/10.1111/j.1464-410X.2007.06754.x.
- Badlani G, Paulson DF. Problems in Urology, Volume 7. Philadelphia, PA: JB Lippincott; 1993.
- Brittain KR, Peet SM, Potter JF, Castleden CM. Prevalence and management of urinary incontinence in stroke survivors. Age Ageing 1999;28:509-11. https://doi.org/10.1093/ageing/28.6.509.
- Brittain KR. for MRC Continence Programme and R&D Stroke and Incontinence Study . Urinary symptoms and depression in stroke survivors. Age Ageing 1998;27:P72-P73. https://doi.org/10.1093/ageing/27.suppl_2.52-b.
- Brittain KR, Shaw C. The social consequences of living with and dealing with incontinence – a carers perspective. Soc Sci Med 2007;65:1274-83. https://doi.org/10.1016/j.socscimed.2007.04.002.
- Pettersen R, Wyller TB. Prognostic significance of micturition disturbances after acute stroke. J Am Geriatr Soc 2006;54:1878-84. https://doi.org/10.1111/j.1532-5415.2006.00984.x.
- Cowey E, Smith LN, Booth J, Weir CJ. Urinary catheterization in acute stroke: clinical realities. A mixed methods study. Clin Rehabil 2012;26:470-9. https://doi.org/10.1177/0269215511426160.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2016.
- Booth J, Kumlien S, Zang Y, Gustafsson B, Tolson D. Rehabilitation nurses practices in relation to urinary incontinence following stroke: a cross-cultural comparison. J Clin Nurs 2009;18:1049-58. https://doi.org/10.1111/j.1365-2702.2008.02688.x.
- Kwan J, Hand P. Infection after acute stroke is associated with poor short-term outcome. Acta Neurol Scand 2007;115:331-8. https://doi.org/10.1111/j.1600-0404.2006.00783.x.
- Poisson SN, Johnston SC, Josephson SA. Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke 2010;41:e180-4. https://doi.org/10.1161/STROKEAHA.109.576413.
- Roth EJ, Lovell L, Harvey RL, Heinemann AW, Semik P, Diaz S. Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke 2001;32:523-9. https://doi.org/10.1161/01.str.32.2.523.
- Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. GAIN International Steering Committee and Investigators . Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol 2004;11:49-53. https://doi.org/10.1046/j.1468-1331.2003.00749.x.
- Stott DJ, Falconer A, Miller H, Tilston JC, Langhorne P. Urinary tract infection after stroke. QJM 2009;102:243-9. https://doi.org/10.1093/qjmed/hcp012.
- Thomas LH, French B, Sutton CJ, Forshaw D, Leathley MJ, Burton CR, et al. ICONS: Identifying Continence OptioNs after Stroke: an evidence synthesis, case study and exploratory cluster randomised controlled trial of the introduction of a systematic voiding programme for patients with urinary incontinence after stroke in secondary care. Programme Grants Appl Res 2015;3. https://doi.org/10.3310/pgfar03010.
- Roth EJ, Lovell L, Harvey RL, Bode RK, Heinemann AW. Stroke rehabilitation: indwelling urinary catheters, enteral feeding tubes, and tracheostomies are associated with resource use and functional outcomes. Stroke 2002;33:1845-50. https://doi.org/10.1161/01.str.0000020122.30516.ff.
- Griffiths R, Fernandez R. Strategies for the removal of short-term indwelling urethral catheters in adults. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD004011.pub3.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245-54. https://doi.org/10.1016/S0140-6736(13)61953-4.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009;374:1196-208. https://doi.org/10.1016/S0140-6736(09)61460-4.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1. https://doi.org/10.1136/bmjopen-2011-000269.
- Wolfe C, Rudd A, McKevitt C. Modelling, evaluating and implementing cost effective services to reduce the impact of stroke. Programme Grants Appl Res 2014;2. https://doi.org/10.3310/pgfar02020.
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 2000;31:2080-6. https://doi.org/10.1161/01.str.31.9.2080.
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP) Clinical Audit January–March 2016 Public Report 2016.
- Intercollegiate Stroke Working Party . National Sentinel Stroke Audit 2010 2011.
- National Institute for Health and Care Excellence . Urinary Incontinence in Neurological Disease: Management of Lower Urinary Tract Dysfunction in Neurological Disease. Clinical Guideline 148: Methods, Evidence and Recommendations 2012.
- Thomas LH, Watkins CL, French B, Sutton C, Forshaw D, Cheater F, et al. Study protocol: ICONS: identifying continence options after stroke: a randomised trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-131.
- Thomas LH, Coupe J, Cross LD, Tan AL, Watkins CL. Interventions for treating urinary incontinence after stroke in adults. Cochrane Database Syst Rev 2019;2. https://doi.org/10.1002/14651858.CD004462.pub4.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26. https://doi.org/10.1067/mob.2002.125704.
- Guo ZF, Liu Y, Hu GH, Liu H, Xu YF. Transcutaneous electrical nerve stimulation in the treatment of patients with poststroke urinary incontinence. Clin Interv Aging 2014;9:851-6. https://doi.org/10.2147/CIA.S61084.
- Liu Y, Liu L, Wang X. Electroacupuncture at points Baliao and Huiyang (BL35) for post-stroke detrusor overactivity. Neural Regen Res 2013;8:1663-72. https://doi.org/10.3969/j.issn.1673-5374.2013.18.004.
- Shin DC, Shin SH, Lee MM, Lee KJ, Song CH. Pelvic floor muscle training for urinary incontinence in female stroke patients: a randomized, controlled and blinded trial. Clin Rehabil 2016;30:259-67. https://doi.org/10.1177/0269215515578695.
- Thomas LH, Watkins CL, Sutton CJ, Forshaw D, Leathley MJ, French B, et al. Identifying continence options after stroke (ICONS): a cluster randomised controlled feasibility trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-509.
- Tibaek S, Gard G, Dehlendorff C, Iversen HK, Biering-Soerensen F, Jensen R. Is pelvic floor muscle training effective for men with poststroke lower urinary tract symptoms? A single-blinded randomized, controlled trial. Am J Mens Health 2017;11:1460-71. https://doi.org/10.1177/1557988315610816.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-1.
- Goldstein M, Barnett H, Orgogozo JM, Sartorius N, Symon L, Vereshchagin NV. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and Other Cerebrovascular Disorders. Stroke 1989;20:1407-31. https://doi.org/10.1161/01.STR.20.10.1407.
- Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126-31. https://doi.org/10.1212/wnl.53.1.126.
- Shaw C, Matthews RJ, Perry SI, Williams K, Spiers N, Assassa RP, et al. Validity and reliability of a questionnaire to measure the impact of lower urinary tract symptoms on quality of life: the Leicester Impact Scale. Neurourol Urodyn 2004;23:229-36. https://doi.org/10.1002/nau.20017.
- Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 1999;14:936-40. https://doi.org/10.1002/(SICI)1099-1166(199911)14:11<936::AID-GPS39>3.0.CO;2-1.
- Adams D, Bucior H, Day G, Rimmer J. HOUDINI: make that urinary catheter disappear. J Infect Prev 2012;13:44-6. https://doi.org/10.1177/1757177412436818.
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2004;1. https://doi.org/10.1002/14651858.CD001308.pub2.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. https://doi.org/10.1002/nau.20041.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. https://doi.org/10.3109/09638288809164103.
- Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, Buesching DP. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL). Urology 1999;53:71-6. https://doi.org/10.1016/S0090-4295(98)00454-3.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996;47:67-71. https://doi.org/10.1016/S0090-4295(99)80384-7.
- EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Bamford J, Sandercock P, Dennis M, Warlow C, Jones L, McPherson K, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project 1981–86. 1. Methodology, demography and incident cases of first-ever stroke. J Neurol Neurosurg Psychiatry 1988;51:1373-80. https://doi.org/10.1136/jnnp.51.11.1373.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.str.19.5.604.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002;100:72-8. https://doi.org/10.1097/00006250-200207000-00012.
- Nyström E, Sjöström M, Stenlund H, Samuelsson E. ICIQ symptom and quality of life instruments measure clinically relevant improvements in women with stress urinary incontinence. Neurourol Urodyn 2015;34:747-51. https://doi.org/10.1002/nau.22657.
- Booth JBD. Consensus statement on bladder training and bowel training. Neurolurol Urodyn 2020;39:1234-54. https://doi.org/10.1002/nau.24345.
- Personal Social Services Research Unit . NHS Cost Inflation Index n.d. www.pssru.ac.uk/pub/uc/uc2019/NHS-Cost-Inflation-Index.docx (accessed 26 May 2022).
- Ouyang M, Billot L, Song L, Wang X, Roffe C, Arima H, et al. Prognostic significance of early urinary catheterization after acute stroke: secondary analyses of the international HeadPoST trial. Int J Stroke 2021;16:200-6. https://doi.org/10.1177/1747493020908140.
- John G, Primmaz S, Crichton S, Wolfe C. Urinary incontinence and indwelling urinary catheters as predictors of death after new-onset stroke: a report of the South London Stroke Register. J Stroke Cerebrovasc Dis 2018;27:118-24. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.018.
Appendix 1 Algorithms for process evaluation derived variables
Intervention group only.
(a)
Number of days SVP suspended = 0 + SUM((Date programme resumed n – Date programme suspended n) IF (Date programme resumed n) ∼ = MISSING)) + (Date of discharge – Date programme suspended n) IF (Date programme resumed n = MISSING)
Number of days on programme = ((Date programme terminated – Date started on regime) – Number of days SV programme suspended) IF (Date programme terminated ∼ = MISSING) OR = ((Date of discharge – Date started on regime) – Number of days SV programme suspended) IF (Date programme terminated = MISSING)
(b)
Total number of indwelling catheter insertions = SUM(Date of insertion ∼ = MISSING)
Total number of indwelling catheter insertions for retention of urine = Sum of ‘Reasons for insertion = Retention of urine’
Total number of indwelling catheter insertions for input and output measurement = Sum of ‘Reasons for insertion = Input and output measurement’
Total number of indwelling catheter insertions for decubitus ulcer present = Sum of ‘Reasons for insertion = Decubitus ulcer present’
Total number of indwelling catheter insertions for Prevention of decubitus ulcer = Sum of ‘Reasons for insertion = Prevention of decubitus ulcer’
Total number of indwelling catheter insertions for other reasons = Sum of ‘Reasons for insertion = Other reasons’
Note: If it is deemed that any individual ‘other reason’ has a sufficient number (≥ 5), then an additional set of variables for such reasons was created and a ‘Total number’ for that reason was calculated in an analogous manner.
(c)
Number of days with an indwelling catheter (prior to discharge) = 0 + SUM (((Date removed n) – Date of insertion n)) IF (Date removed n ∼ = MISSING) + (Date of discharge – Date of insertion n) IF (Date removed n = MISSING)
(d)
Total number of successful TWOC = Sum of (‘TWOC successful: patient voiding’ OR ‘Date intermittent catheterisation ended = ∼ MISSING)
Total number of unsuccessful TWOC = Sum of (Date reinserted ∼ = MISSING)
(e)
Total number of changes of route = Sum of (Date route changed ∼ = MISSING)
Total number of changes of route BT to PV) = Sum of ((Date route changed ∼ = MISSING) AND (Route changed from = ‘Bladder training to prompted voiding’))
Total number of changes of route PV to BT) = Sum of ((Date route changed ∼ = MISSING) AND (Route changed from = ‘Prompted voiding to bladder training’))
(f)
Total number of suspensions = SUM (‘Date programme suspended n ∼ = MISSING)
There were no coded reasons for suspensions, so the derived variable could not be coded at this stage. However, common reasons for suspension were coded manually with support from the Trial Management Team to perform the analysis required to address the corresponding objective.
(g)
Presence of urinary catheter within 72 hours of admission to the stroke unit = 0 + ((Date of insertion 1 – Date of admission to the stroke unit) < = 3) AND (Date of insertion 1 ∼ = MISSING)
(h)
TWOC within 72 hours = 0 IF ((Presence of urinary catheter within 72 hours of admission to the stroke unit) AND ((Date removed 1 – Date of insertion 1) > 3) OR (Date removed 1 = MISSING)) AND (Presence of urinary catheter within 72 hours of admission to the stroke unit = 1)
= 1 IF ((Presence of urinary catheter within 72 hours of admission to the stroke unit AND ((Date removed 1 – Date of insertion 1) < = 3) AND (Date removed 1 ∼ = MISSING)) AND (Presence of urinary catheter within 72 hours of admission to the stroke unit = 1)
= MISSING (Not applicable) IF (Presence of urinary catheter within 72 hours of admission to the stroke unit = 0)
(i)
Allocated to correct regime = 1 IF (((Frequency of leakage at baseline < = 4) AND (Cognitively (Value label 1: Yes, started on Impaired = 0) AND (Date started on [initial programme] regime correct regime) ∼ = MISSING) AND (Regime = Bladder Training)) OR ((Frequency of leakage at baseline = 5) AND (Date started on [initial programme] regime ∼ = MISSING) AND (Regime = Prompted voiding)) OR ((Cognitively Impaired = 1) AND (Date started on [initial programme] regime ∼ = MISSING) AND (Regime = Prompted voiding)))
(Value label 2: Never started = 2 IF (Date started on [initial programme] regime = MISSING) on regime) THEN Allocated to correct regime
(Value label 0: Started on = 0 OTHERWISE
incorrect regime
(j)
Total times scheduled for toileting = SUM(Proposed time toileted)
Total times toileted within 30 mins of scheduled time = SUM(ABS(Proposed time toileted – Time toileted) < 30))
Percentage of times toileted within 30 minutes of prescribed interval = (Total times toileted within 30 mins of scheduled time/Total times scheduled for toileting) × 100
Percent toileted GT 60 = (Percentage of times toileted within 30 minutes of prescribed interval ≥ 60)
(k)
Total times toileted by ICONS-II staff = SUM (Person providing [toileting] care is ICONS-II staff member) for a participant
Total times toileted = SUM (Time toileted ∼ = MISSING) for a participant
Percentage of occasions an intervention participant was given toileting assistance from ICONS-II staff = (Total times toileted by ICONS-II staff/Total times toileted) × 100
Percent ICONS-II toileted GT 60 = (Percentage of occasions an intervention group participant was given toileting assistance from ICONS-II staff ≥ 60).
Usual-care group only.
(l), (m) and (n)
These were the same variables as created under k. above and were created using a similar algorithm.
Total times toileted by ICONS-II staff = SUM (Person providing [toileting] care is ICONS-II staff member) for a participant
Total times toileted = SUM (Person providing [toileting] care is ICONS-II staff member) + SUM (Person providing [toileting] care is NOT an ICONS-II staff member) for a participant
Percentage of occasions an intervention participant was given toileting assistance from ICONS-II staff = (Total times toileted by ICONS-II staff/Total times toileted) × 100
For usual-care group participants, the criterion-based variables differ, and were computed as:
Percent ICONS-II toileted GT 50 = (Percentage of occasions a usual-care group participant was given toileting assistance from ICONS-II staff ≥ 50).
Usual-care group and intervention group participants, and pre-implementation case-note review patients.
(o)
i.
Usual-care group participants and pre-implementation case-note review patients.
Presence of a strategy for minimising indwelling urinary catheterisation in the acute phase unless clinically justifiable = (Did the patient have indwelling catheter = 1) AND (Documented reason = 1) AND ∼ ((Reason = NOT APPLICABLE) OR (Reason = MISSING) OR (Reason = Other)) AND (Date catheter inserted ∼ = MISSING) AND (Date catheter removed ∼ = MISSING) Documented TWOC = 1))
Intervention group participants.
Presence of a strategy for minimising indwelling urinary catheterisation in the acute phase unless clinically justifiable = (TWOC within 72 hours ∼ = 0) AND ((Total number of indwelling catheter insertions – (Total number of indwelling catheter insertions for retention of urine + Total number of indwelling catheter insertions for input and output measurement + Total number of indwelling catheter insertions for decubitus ulcer present + Total number of indwelling catheter insertions for Prevention of decubitus ulcer) ≤ 1)
ii.
Usual-care group participants and pre-implementation case-note review patients.
Presence of a comprehensive continence assessment = IF ((Documented evidence of presence of UI) AND (Recorded date of presence of UI) AND (Documented evidence of current clinical investigations relating to UI) AND (Documented evidence of a current diagnosis of type of UI) AND (Documented evidence of patient’s pattern of UI))
Intervention group participants.
Presence of a comprehensive continence assessment = IF ((How often do you leak urine? ∼ = ‘Never’) AND (∼ MISSING(Date of this assessment) AND (∼ MISSING(How often do you leak urine?)) AND ((Sex = ‘Male’) AND (Has the patient had a rectal examination? = 1) AND (Was the prostate examined? = 1) OR ((Sex = ‘Female’) AND (Has the patient had a rectal examination? = 1) AND (Has the patient had a vaginal examination?)) AND (Has a dipstick urinalysis been performed = 1) AND (Has an MSSU been performed? = 1) AND (Has a bladder scan been performed?)) AND ((Stress incontinence = 1) OR (Urge incontinence = 1) OR (Mixed incontinence = 1) OR (Physical/Functional Incontinence = 1)) AND (∼ MISSING(How often do you leak urine?))
iii.
Usual-care group participants and pre-implementation case-note review patients.
Presence of a tailored treatment plan including behavioural approaches =
IF ((Documented Evidence patient put onto a voiding programme) AND (Voiding programme described as either bladder training or prompted voiding) AND (Programme was tailored to patient’s pattern of UI) AND ((Date of discharge – Randomisation date < 11) OR (Evidence that patient’s programme was reviewed) AND ((Evidence that patient’s programme required adjustment AND Evidence that patient’s programme was adjusted) OR (No evidence that patient’s programme required adjustment))
Intervention group participants.
Presence of a tailored treatment plan including behavioural approaches =
IF (((∼ MISSING(Date started on regime) AND ((Regime = ‘Bladder Training’) OR Regime = ‘Prompted Voiding’)) AND (Allocated to correct regime = 1) AND ((Number of days on programme < 8) OR (∼ MISSING(Weekly Review Date 1)) AND (((Does the regime need changing 1 = 1) AND ((What regime has the patient been on in the last week? 1) ∼ = (What regime has the patient been on in the last week? 2)) OR (Does the regime need changing 1 = 0)) AND ((Does the regime need changing 2 = 1) AND ((What regime has the patient been on in the last week? 2 ∼ = (What regime has the patient been on in the last week? 3)) OR . . . OR (Does the regime need changing 2 n = 1)))
Contamination of usual care.
The primary measure of contamination of usual care was created as:
Presence of a strategy for minimising indwelling urinary catheterisation in the acute phase unless clinically justifiable (i) AND Presence of a comprehensive continence assessment (ii) AND Presence of a tailored treatment plan (including behavioural approaches) (ii).
A separate measure of contamination of usual care amongst those not catheterised throughout was also created as follows:
Presence of a comprehensive continence assessment (ii) AND Presence of a tailored treatment plan (including behavioural approaches) (iii)
It was assessed amongst usual-care participants, excluding those catheterised throughout their inpatient stay (i.e. those with Catheterised throughout inpatient stay = 1) using the following definition:
Catheterised throughout inpatient stay = 0
Catheterised throughout inpatient stay = 1 IF ((Did the patient have indwelling catheter = 1) AND (Date catheter removed = MISSING) AND (Catheterised at baseline = 1) AND (Continence status at discharge = ‘Catheterised’))
Measures of the individual ‘items’ within each of ii. and iii. above were also presented to aid with decision-making and monitoring of contamination.
Appendix 2 Analysis of the secondary outcomes
Stress incontinence
Table 22 shows the numbers of participants with stress incontinence at 3 and 6 months. The estimated odds of stress incontinence, adjusted for baseline stress incontinence type, were lower in the intervention group than in the usual-care group at both follow-up time points (3 months: OR 0.80, 95% CI 0.28 to 2.31; 6 months: OR 0.60, 95% CI 0.20 to 1.81).
| Time point | Classed as stress UI, n (%) | ||
|---|---|---|---|
| Usual-care group | Intervention group | Total | |
| Baseline | 18 (34) | 20 (36) | 38 (35) |
| 3 months | 18 (37) | 11 (30) | 29 (34) |
| 6 months | 14 (34) | 10 (23) | 24 (29) |
Urge incontinence
Table 23 shows the numbers of participants with urge incontinence at 3 and 6 months. The estimated odds of urge incontinence, adjusted for baseline urge incontinence type, were higher in the intervention group than in the usual-care group at both follow-up time points (3 months: OR 1.69, 95% CI 0.37 to 7.78; 6 months: OR 1.83, 95% CI 0.43 to 7.76).
| Time point | Classed as urge UI, n (%) | ||
|---|---|---|---|
| Usual-care group | Intervention group | Total | |
| Baseline | 38 (95) | 43 (96) | 81 (95) |
| 3 months | 30 (68) | 20 (65) | 50 (67) |
| 6 months | 22 (54) | 24 (60) | 46 (57) |
Number of urinary tract infections
For patients who had UTIs at discharge, and at 3 and 6 months, the distribution of the number of infections is presented as frequencies (%) by trial group in Tables 24–27. For example, Table 24 shows the number of UTIs by the time of discharge. In the usual-care group, 17 people experienced a single infection; in the intervention group, 12 people experienced a single infection and one person experienced two infections. Table 25 shows the number of people who experienced between one and five infections between discharge and 3 months. Table 26 shows the number of people who experienced between one and six infections in the previous 3 months; this is completed at 6 months but reported since discharge only.
| Trial group | Infections, n (%) | ||
|---|---|---|---|
| 1 | 2 | Total | |
| Usual care | 17 (59) | 0 (0) | 17 (57) |
| Intervention | 12 (41) | 1 (100) | 13 (43) |
| Total | 29 (100) | 1 (100) | 30 (100) |
| Trial group | Infections, n (%) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | |
| Usual care | 7 (64) | 2 (67) | 1 (100) | 0 (0) | 1 (100) | 11 (65) |
| Intervention | 4 (36) | 1 (33) | 0 (0) | 1 (100) | 0 (0) | 6 (35) |
| Total | 11 (100) | 3 (100) | 1 (100) | 1 (100) | 1 (100) | 17 (100) |
| Trial group | Infections, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| Usual care | 4 (33) | 1 (25) | 1 (100) | 2 (100) | 1 (100) | 0 (0) | 9 (43) |
| Intervention | 8 (67) | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 12 (57) |
| Total | 12 (100) | 4 (100) | 1 (100) | 2 (100) | 1 (100) | 1 (100) | 21 (100) |
| Trial group | Infections, median (IQR) | ||
|---|---|---|---|
| Discharge | 3 months | 6 months | |
| Usual care | 1 (1–1) | 1 (1–2) | 2 (1–4) |
| Intervention | 1 (1–1) | 1 (1–2) | 1 (1–2) |
As the numbers were small and regression analysis was not feasible, the Mann–Whitney U (Wilcoxon rank sum)-test was applied. It did not show a statistically significant difference in the number of UTIs between the two groups at each time point. (For details, see the summary table of the results, Table 31.)
Number of falls
The numbers of people who had experienced at least one fall by discharge and by 3 and 6 months are presented as frequencies by trial group in Table 28.
| Statistic | During stay in stroke unit | Discharge to 3 months | 3–6 months |
|---|---|---|---|
| Usual care | |||
| Median (IQR) | 2 (1–2) | 1 (1–2) | 1 (1–1) |
| Range | 1–3 | 1–8 | 1–4 |
| Number of people who experienced at least one fall | 13 | 11 | 7 |
| Intervention | |||
| Median (IQR) | 1 (1–1) | 2 (2–4) | 2 (1–2) |
| Range | 1–1 | 1–6 | 1–2 |
| Number of people who experienced at least one fall | 4 | 5 | 7 |
There had been fewer falls in the intervention group than in the usual-care group by discharge (4 vs. 13, respectively) and between discharge and 3 months (5 vs. 11, respectively). There were equal numbers of falls in both groups by 6 months (within the previous 3 months).
Total Barthel score
The summary statistics of total Barthel scores at 3 and 6 months, analysed using ordinal logit regression and examining relative cumulative odds between intervention groups, are given in Table 29 (lower scores indicate greater disability). The results are adjusted for the baseline total Barthel score and baseline continence category, with site included as a random effect.
| Time point | Median (IQR) | Mean (SD) |
|---|---|---|
| Usual care | ||
| Baseline | 4 (2–9) | 5.91 (5.39) |
| 3 months | 14 (10–18) | 12.92 (6.00) |
| 6 months | 16 (10–18) | 13.33 (6.08) |
| Intervention | ||
| Baseline | 6.5 (2–12) | 7.82 (6.59) |
| 3 months | 11 (5–17) | 10.82 (6.34) |
| 6 months | 13 (7–18) | 12.29 (6.21) |
For the intervention group, at 3 and 6 months, the adjusted odds of higher Barthel scores (indicating less disability) compared with lower Barthel scores were 0.43 and 0.48 times lower, respectively, than for the usual-care group.
Number of deaths
The numbers of deaths are presented as frequencies (%) by discharge and by 3 and 6 months for each trial group in Table 30.
| Trial group | Deaths, n (%) | ||
|---|---|---|---|
| Discharge (N = 156) | 3 months (N = 122) | 6 months (N = 143) | |
| Usual care | 3 (4) | 8 (12) | 13 (19) |
| Intervention | 4 (5) | 10 (18) | 12 (16) |
Summary table of the results of secondary outcomes
Table 31 shows a summary of the results of the secondary outcomes, presented in this appendix, where inferential analysis was possible.
| Variable | Sample/complete cases (n) | Usual-care group, n (%) | Intervention group, n (%) | Between-group difference: intervention vs. usual care, OR (95% CI) |
|---|---|---|---|---|
| 3 months | ||||
| Stress incontinence: yes | 94/64 | 18 (32.1) | 11 (29.0) | 0.80 (0.28 to 2.31)a |
| Urge incontinence: yes | 75/43 | 30 (68.2) | 20 (64.5) | 1.69 (0.37 to 0.78)a |
| Barthel total score,c median (IQR) | 85 | 14 (8) | 11 (12) | 0.43a (0.18 to 1.04) |
| 6 months | ||||
| Stress incontinence: yes | 92/64 | 14 (30.4) | 10 (21.7) | 0.60 (0.20 to 1.81)a |
| Urge incontinence: yes | 81/48 | 22 (53.66) | 24 (60.0) | 1.83 (0.43 to 7.76)a |
| Barthel total score,c median (IQR) | 90 | 16 (8) | 13 (11) | 0.48a (0.22 to 1.07) |
| Number of UTIs | Sample/complete cases (n) | Median (IQR) | Statisticsb | |
| Usual care | Intervention | |||
| Discharge | 30 | 1 (0) | 1 (0) | –1.14 |
| 3 months | 17 | 1 (1) | 1 (1) | 0.12 |
| 6 months | 21 | 2 (3) | 1 (1) | 1.23 |
The estimated odds for stress incontinence were lower in the intervention group than in the usual-care group at both follow-up time points. The usual-care group had lower estimated odds of urge incontinence than the intervention group at both follow-up time points, and the odds of higher Barthel scores were higher in the usual-care group than in the intervention group.
Appendix 3 Process evaluation additional table
| PID | Length of hospital stay 1a | Length of hospital stay 2b | Reasons for not starting SVP | Comment | Status at discharge | Continence status at discharge |
|---|---|---|---|---|---|---|
| 3003 | 2 | Discharged early | Reason unknown | Alive | Incontinent | |
| 4002 | 8 | Discharged early | Discharged early. Notes: ‘patient was discharged prior to starting on regime’ | Alive | Incontinent | |
| 4003 | 6 | Discharged early | Notes: regime delay as no staff available | Alive | Incontinent | |
| 5006 | 38 | Unknown | No log of ongoing continence events | Alive | ||
| 5007 | 17 | Unknown | No log of ongoing continence events | Alive | ||
| 5010 | 96 | Unknown | No log of ongoing continence events | Alive | Incontinent | |
| 5017 | 21 | Unknown | No log of ongoing continence events | Alive | ||
| 5018 | 34 | Unknown | No log of ongoing continence events | Alive | Incontinent | |
| 7018 | 9 | Unknown | No log of ongoing continence events | Alive | Incontinent | |
| 9010 | 53 | Unknown | No log of ongoing continence events | Alive | Incontinent | |
| 9011 | 11 | Unknown | No log of ongoing continence events | Alive | Continent | |
| 10020 | 32 | Unknown | No log of ongoing continence events | Alive | ||
| 15004 | 66 | 71 | Unknown | No log of ongoing continence events | Deceased | Incontinent |
| 17004 | 15 | Continent | Participant remained continent during 3-day bladder diary. This participant did not have a recommended initial treatment | Alive | ||
| 17007 | 37 | Unknown | No log of ongoing continence events | Alive | Incontinent |
Appendix 4 Length of stay additional information
| Month randomised | Randomised (n) | Length of stay (days), median (IQR) | 3-month ICIQ-UI-SF calculable, n (%) | 6-month ICIQ-UI-SF calculable, n (%) |
|---|---|---|---|---|
| All | 157 | 27 (16–45) | 86 (54.8) | 87 (55.4) |
| June 2019 | 3 | 13 (8–18) | 2 (66.7) | 1 (33.3) |
| July 2019 | 6 | 28.5 (8–42) | 4 (66.7) | 3 (50.0) |
| August 2019 | 10 | 18 (10–38) | 3 (30.0) | 3 (30.0) |
| September 2019 | 13 | 21 (17–41) | 7 (53.8) | 7 (53.8) |
| October 2019 | 12 | 25.5 (17.5–41.5) | 2 (16.7) | 5 (41.7) |
| November 2019 | 21 | 21.5 (13–26.5) | 13 (61.9) | 11 (52.4) |
| December 2019 | 22 | 30.5 (13–61) | 12 (54.5) | 13 (59.1) |
| January 2020 | 30 | 43 (24–57) | 17 (56.7) | 20 (66.7) |
| February 2020 | 30 | 30.5 (17–58) | 19 (63.3) | 19 (63.3) |
| March 2020 | 10 | 30 (17–45) | 7 (70.0) | 5 (50.0) |
| Month randomised | Length of stay up to and including (days), median (IQR) [n] | Length of stay post (days), median (IQR) [n] |
|---|---|---|
| June 2019 | 13 (8–18) [3] | 27 (16–46) [152] |
| July 2019 | 18 (8–31) [9] | 27 (16–48) [146] |
| August 2019 | 18 (8–38) [19] | 27 (17–48) [136] |
| September 2019 | 21 (13–39.5) [32] | 27 (17–48) [123] |
| October 2019 | 21.5 (13–41) [44] | 27 (17–51) [111] |
| November 2019 | 21.5 (13–34) [64] | 35 (17–57) [91] |
| December 2019 | 23.5 (13–38) [86] | 36 (21–57) [69] |
| January 2020 | 26 (16–44) [115] | 30.5 (17–52) [40] |
| February 2020 | 26 (16–45) [145] | 30 (17–45) [10] |
Appendix 5 Health economics evaluation additional tables
| Month | Usual-care group (n) | Intervention group (n) | Total (n) | |||
|---|---|---|---|---|---|---|
| Long version | Short version | Long version | Short version | Long version | Short version | |
| 1 | 41 | 10 | 34 | 16 | 75 | 26 |
| 2 | 35 | 8 | 32 | 15 | 67 | 23 |
| 3 | 30 | 9 | 27 | 12 | 57 | 21 |
| 4 | 30 | 12 | 33 | 13 | 63 | 25 |
| 5 | 30 | 11 | 32 | 13 | 62 | 24 |
| 6 | 21 | 6 | 27 | 11 | 48 | 17 |
| All | 187 | 56 | 185 | 80 | 372 | 136 |
| Resource | Usual-care group | Intervention group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | n | SD | Mean | n | SD | Mean | n | SD | |
| Month 1: number of times | |||||||||
| Used GP | 1.13 | 39 | 1.436 | 0.80 | 25 | 1.384 | 1.00 | 64 | 1.414 |
| Used practice nurse | 0.92 | 38 | 1.634 | 0.36 | 25 | 0.860 | 0.70 | 63 | 1.399 |
| Admitted to hospital | 0.39 | 38 | 0.946 | 0.08 | 25 | 0.277 | 0.27 | 63 | 0.766 |
| Treated at A&E | 0.18 | 38 | 0.457 | 0.00 | 25 | 0.000 | 0.11 | 63 | 0.364 |
| Received treatment/help from | |||||||||
| Physiotherapy | 7.15 | 20 | 6.683 | 9.57 | 14 | 7.603 | 8.15 | 34 | 7.067 |
| Occupational therapy | 5.06 | 16 | 3.941 | 6.22 | 9 | 2.539 | 5.48 | 25 | 3.490 |
| Chiropodist or podiatrist | 3.60 | 5 | 4.775 | 1.00 | 3 | 0.000 | 2.63 | 8 | 3.852 |
| District nurse | 11.50 | 12 | 25.094 | 5.33 | 3 | 6.658 | 10.27 | 15 | 22.531 |
| Home care/help | 37.86 | 7 | 31.861 | 50.25 | 8 | 35.030 | 44.47 | 15 | 33.008 |
| Continence advisor | 2.00 | 4 | 0.816 | 1.50 | 2 | 0.707 | 1.83 | 6 | 0.753 |
| Stroke Association family support worker | 3.50 | 6 | 3.017 | 1.67 | 3 | 0.577 | 2.89 | 9 | 2.571 |
| Day support (day-care) centre | 1.00 | 1 | 1.00 | 1 | |||||
| Other treatment/help | 3.50 | 2 | 2.121 | 30.00 | 1 | 12.33 | 3 | 15.373 | |
| Month 2: number of times | |||||||||
| Used GP | 0.82 | 33 | 1.261 | 0.90 | 21 | 1.446 | 0.85 | 54 | 1.323 |
| Used practice nurse | 0.79 | 33 | 1.516 | 0.33 | 21 | 0.913 | 0.61 | 54 | 1.323 |
| Admitted to hospital | 0.09 | 33 | 0.384 | 0.05 | 21 | 0.218 | 0.07 | 54 | 0.328 |
| Treated at A&E | 0.12 | 33 | 0.331 | 0.00 | 21 | 0.000 | 0.07 | 54 | 0.264 |
| Received treatment/help from | |||||||||
| Physiotherapy | 6.00 | 14 | 4.690 | 9.54 | 13 | 7.546 | 7.70 | 27 | 6.366 |
| Occupational therapy | 5.00 | 11 | 3.975 | 8.33 | 6 | 3.077 | 6.18 | 17 | 3.941 |
| Chiropodist or podiatrist | 2.83 | 6 | 4.491 | 1.00 | 2 | 0.000 | 2.38 | 8 | 3.889 |
| District nurse | 19.83 | 6 | 34.747 | 7.50 | 2 | 7.778 | 16.75 | 8 | 30.061 |
| Home care/help | 47.75 | 4 | 41.153 | 57.67 | 6 | 33.957 | 53.70 | 10 | 35.091 |
| Continence advisor | 1.25 | 4 | 0.500 | 2.00 | 1 | 1.40 | 5 | 0.548 | |
| Stroke Association family support worker | 2.75 | 4 | 3.500 | 1.20 | 5 | 0.447 | 1.89 | 9 | 2.315 |
| Day support (day-care) centre | 1.00 | 1 | 1.00 | 1 | |||||
| Other treatment/help | 3.00 | 2 | 2.828 | 30.00 | 1 | 12.00 | 3 | 15.716 | |
| Month 3: number of times | |||||||||
| Used GP | 0.93 | 27 | 1.269 | 0.88 | 16 | 1.408 | 0.91 | 43 | 1.306 |
| Used practice nurse | 0.85 | 27 | 1.610 | 0.50 | 16 | 0.894 | 0.72 | 43 | 1.386 |
| Admitted to hospital | 0.00 | 27 | 0.000 | 0.00 | 16 | 0.000 | 0.00 | 43 | 0.000 |
| Treated at A&E | 0.26 | 27 | 0.859 | 0.00 | 16 | 0.000 | 0.16 | 43 | 0.688 |
| Received treatment/help from | |||||||||
| Physiotherapy | 6.83 | 12 | 6.191 | 7.18 | 11 | 5.344 | 7.00 | 23 | 5.673 |
| Occupational therapy | 5.64 | 11 | 5.784 | 7.50 | 6 | 4.135 | 6.29 | 17 | 5.205 |
| Chiropodist or podiatrist | 1.00 | 4 | 0.000 | 1.00 | 2 | 0.000 | 1.00 | 6 | 0.000 |
| District nurse | 21.20 | 5 | 38.739 | 4.25 | 4 | 5.852 | 13.67 | 9 | 29.034 |
| Home care/help | 4.00 | 1 | 57.17 | 6 | 38.665 | 49.57 | 7 | 40.616 | |
| Continence advisor | 1.33 | 3 | 0.577 | 2.00 | 1 | 1.50 | 4 | 0.577 | |
| Stroke Association family support worker | 3.33 | 3 | 4.041 | 2.00 | 1 | 3.00 | 4 | 3.367 | |
| Day support (day-care) centre | 1.00 | 1 | 1.00 | 1 | |||||
| Other treatment/help | 11.50 | 4 | 9.950 | 30.00 | 1 | 15.20 | 5 | 11.946 | |
| Month 4: number of times | |||||||||
| Used GP | 1.00 | 28 | 1.414 | 0.67 | 27 | 1.144 | 0.84 | 55 | 1.288 |
| Used practice nurse | 0.71 | 28 | 1.436 | 0.78 | 27 | 1.528 | 0.75 | 55 | 1.468 |
| Admitted to hospital | 0.29 | 28 | 0.713 | 0.11 | 28 | 0.315 | 0.20 | 56 | 0.553 |
| Treated at A&E | 0.14 | 28 | 0.356 | 0.00 | 27 | 0.000 | 0.07 | 55 | 0.262 |
| Received treatment/help from | |||||||||
| Physiotherapy | 4.63 | 8 | 4.658 | 10.29 | 7 | 14.625 | 7.27 | 15 | 10.539 |
| Occupational therapy | 4.50 | 4 | 5.066 | 10.33 | 6 | 16.158 | 8.00 | 10 | 12.754 |
| Chiropodist or podiatrist | 1.20 | 5 | 0.447 | 1.00 | 1 | 1.17 | 6 | 0.408 | |
| District nurse | 2.00 | 2 | 1.414 | 2.50 | 4 | 1.915 | 2.33 | 6 | 1.633 |
| Home care/help | 94.50 | 2 | 6.364 | 67.00 | 3 | 47.843 | 78.00 | 5 | 37.169 |
| Stroke Association family support worker | 1.25 | 4 | 0.500 | 1.25 | 4 | 0.500 | |||
| Other treatment/help | 1.00 | 1 | 27.00 | 2 | 21.213 | 18.33 | 3 | 21.221 | |
| Month 5: number of times | |||||||||
| Used GP | 0.96 | 28 | 1.427 | 0.60 | 25 | 1.155 | 0.79 | 53 | 1.306 |
| Used practice nurse | 0.89 | 28 | 1.641 | 0.84 | 25 | 1.573 | 0.87 | 53 | 1.594 |
| Admitted to hospital | 0.29 | 28 | 0.713 | 0.12 | 26 | 0.326 | 0.20 | 54 | 0.562 |
| Treated at A&E | 0.18 | 28 | 0.390 | 0.00 | 25 | 0.000 | 0.09 | 53 | 0.295 |
| Received treatment/help from | |||||||||
| Physiotherapy | 5.00 | 7 | 4.899 | 11.75 | 4 | 20.189 | 7.45 | 11 | 12.177 |
| Occupational therapy | 4.00 | 5 | 4.528 | 9.80 | 5 | 18.006 | 6.90 | 10 | 12.749 |
| Chiropodist or podiatrist | 1.20 | 5 | 0.447 | 1.00 | 1 | 1.17 | 6 | 0.408 | |
| District nurse | 2.33 | 3 | 1.155 | 2.50 | 4 | 1.915 | 2.43 | 7 | 1.512 |
| Home care/help | 83.00 | 3 | 20.421 | 94.50 | 2 | 6.364 | 87.60 | 5 | 16.072 |
| Stroke Association family support worker | 1.25 | 4 | 0.500 | 1.25 | 4 | 0.500 | |||
| Day support (day-care) centre | 4.00 | 1 | 4.00 | 1 | |||||
| Other treatment/help | 1.50 | 2 | 0.707 | 18.67 | 3 | 20.817 | 11.80 | 5 | 17.470 |
| Month 6: number of times | |||||||||
| Used GP | 1.00 | 19 | 1.599 | 0.35 | 20 | 0.933 | 0.67 | 39 | 1.325 |
| Used practice nurse | 1.00 | 19 | 1.915 | 0.55 | 20 | 1.234 | 0.77 | 39 | 1.597 |
| Admitted to hospital | 0.26 | 19 | 0.562 | 0.10 | 21 | 0.301 | 0.18 | 40 | 0.446 |
| Treated at A&E | 0.16 | 19 | 0.375 | 0.00 | 20 | 0.000 | 0.08 | 39 | 0.270 |
| Received treatment/help from | |||||||||
| Physiotherapy | 4.50 | 4 | 5.066 | 1.00 | 2 | 0.000 | 3.33 | 6 | 4.320 |
| Occupational therapy | 5.67 | 3 | 5.508 | 1.50 | 4 | 0.577 | 3.29 | 7 | 3.904 |
| Chiropodist or podiatrist | 1.25 | 4 | 0.500 | 1.00 | 2 | 0.000 | 1.17 | 6 | 0.408 |
| District nurse | 2.00 | 2 | 1.414 | 1.00 | 2 | 0.000 | 1.50 | 4 | 1.000 |
| Home care/help | 83.00 | 3 | 20.421 | 69.67 | 3 | 43.247 | 76.33 | 6 | 31.117 |
| Stroke Association family support worker | 1.50 | 2 | 0.707 | 1.50 | 2 | 0.707 | |||
| Day support (day-care) centre | 4.00 | 1 | 4.00 | 1 | |||||
| Other treatment/help | 1.00 | 1 | 7.00 | 2 | 7.071 | 5.00 | 3 | 6.083 | |
In Table 37, we can observe the average resource use of the participants using the short survey between months 1 and 6. As is the case for participants using the long survey (see Table 36), the most used services are GPs and practice nurses in both trial groups for the overall period. The usual-care group used more of those services than the intervention group, on average. Participants using the short survey report more use of GP services; however, the sample size is too small to allow for any meaningful comparisons.
| Resource | Usual-care group | Intervention group | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | n | SD | Mean | n | SD | Mean | n | SD | |
| Month 1: number of times | |||||||||
| Used GP | 2.86 | 7 | 2.193 | 1.91 | 11 | 2.023 | 2.28 | 18 | 2.081 |
| Used practice nurse | 0.00 | 8 | 0.000 | 0.55 | 11 | 1.508 | 0.32 | 19 | 1.157 |
| Admitted to hospital | 0.00 | 9 | 0.000 | 0.55 | 11 | 0.934 | 0.30 | 20 | 0.733 |
| Treated at A&E | 0.11 | 9 | 0.333 | 0.55 | 11 | 1.036 | 0.35 | 20 | 0.813 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.44 | 9 | 0.527 | 0.45 | 11 | 0.522 | 0.45 | 20 | 0.510 |
| Occupational therapy | 0.25 | 8 | 0.463 | 0.36 | 11 | 0.505 | 0.32 | 19 | 0.478 |
| Chiropodist or podiatric | 0.25 | 8 | 0.463 | 0.27 | 11 | 0.467 | 0.26 | 19 | 0.452 |
| District nurse | 0.00 | 8 | 0.000 | 0.00 | 10 | 0.000 | 0.00 | 18 | 0.000 |
| Continence advisor | 0.00 | 8 | 0.000 | 0.00 | 11 | 0.000 | 0.00 | 19 | 0.000 |
| Stroke Association family support worker | 0.11 | 9 | 0.333 | 0.00 | 11 | 0.000 | 0.05 | 20 | 0.224 |
| Number of times received treatment/help from physiotherapy | 12.00 | 3 | 10.000 | 28.00 | 4 | 28.036 | 21.14 | 7 | 22.349 |
| Number of times received treatment/help from occupational therapy | 11.50 | 2 | 14.849 | 17.33 | 3 | 22.279 | 15.00 | 5 | 17.706 |
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 2 | 0.000 | 1.33 | 3 | 0.577 | 1.20 | 5 | 0.447 |
| Number of times received treatment/help from Stroke Association family support worker | 1.00 | 1 | 1.00 | 1 | |||||
| Month 2: number of times | |||||||||
| Used GP | 3.80 | 5 | 1.789 | 1.60 | 10 | 2.066 | 2.33 | 15 | 2.193 |
| Used practice nurse | 0.00 | 5 | 0.000 | 0.70 | 10 | 1.567 | 0.47 | 15 | 1.302 |
| Admitted to hospital | 0.00 | 6 | 0.000 | 0.70 | 10 | 0.949 | 0.44 | 16 | 0.814 |
| Treated at A&E | 0.17 | 6 | 0.408 | 0.60 | 10 | 1.075 | 0.44 | 16 | 0.892 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.17 | 6 | 0.408 | 0.70 | 10 | 0.483 | 0.50 | 16 | 0.516 |
| Occupational therapy | 0.17 | 6 | 0.408 | 0.40 | 10 | 0.516 | 0.31 | 16 | 0.479 |
| Chiropodist or podiatrist | 0.17 | 6 | 0.408 | 0.20 | 10 | 0.422 | 0.19 | 16 | 0.403 |
| District nurse | 0.00 | 6 | 0.000 | 0.11 | 9 | 0.333 | 0.07 | 15 | 0.258 |
| Continence advisor | 0.00 | 6 | 0.000 | 0.00 | 10 | 0.000 | 0.00 | 16 | 0.000 |
| Stroke Association family support worker | 0.17 | 6 | 0.408 | 0.00 | 10 | 0.000 | 0.06 | 16 | 0.250 |
| Number of times received treatment/help from physiotherapy | 12.00 | 1 | 20.67 | 6 | 24.712 | 19.43 | 7 | 22.795 | |
| Number of times received treatment/help from occupational therapy | 1.00 | 1 | 17.33 | 3 | 22.279 | 13.25 | 4 | 19.939 | |
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 1 | 1.50 | 2 | 0.707 | 1.33 | 3 | 0.577 | |
| Number of times received treatment/help from district nurse | 1.00 | 1 | 1.00 | 1 | |||||
| Number of times received treatment/help from Stroke Association family support worker | 1.00 | 1 | 1.00 | 1 | |||||
| Month 3: number of times | |||||||||
| Used GP | 2.00 | 7 | 2.000 | 1.57 | 7 | 1.902 | 1.79 | 14 | 1.888 |
| Used practice nurse | 0.86 | 7 | 1.864 | 1.00 | 7 | 1.826 | 0.93 | 14 | 1.774 |
| Admitted to hospital | 0.00 | 7 | 0.000 | 0.86 | 7 | 1.069 | 0.43 | 14 | 0.852 |
| Treated at A&E | 0.14 | 7 | 0.378 | 0.86 | 7 | 1.215 | 0.50 | 14 | 0.941 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.43 | 7 | 0.535 | 0.57 | 7 | 0.535 | 0.50 | 14 | 0.519 |
| Occupational therapy | 0.43 | 7 | 0.535 | 0.29 | 7 | 0.488 | 0.36 | 14 | 0.497 |
| Chiropodist or podiatric | 0.43 | 7 | 0.535 | 0.29 | 7 | 0.488 | 0.36 | 14 | 0.497 |
| District nurse | 0.00 | 7 | 0.000 | 0.00 | 6 | 0.000 | 0.00 | 13 | 0.000 |
| Continence advisor | 0.00 | 7 | 0.000 | 0.00 | 7 | 0.000 | 0.00 | 14 | 0.000 |
| Stroke Association family support worker | 0.14 | 7 | 0.378 | 0.00 | 7 | 0.000 | 0.07 | 14 | 0.267 |
| Number of times received treatment/help from physiotherapy | 5.33 | 3 | 5.774 | 20.00 | 4 | 26.870 | 13.71 | 7 | 20.822 |
| Number of times received treatment/help from occupational therapy | 1.33 | 3 | 0.577 | 4.50 | 2 | 2.121 | 2.60 | 5 | 2.074 |
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 2 | 0.000 | 1.50 | 2 | 0.707 | 1.25 | 4 | 0.500 |
| Number of times received treatment/help from Stroke Association family support worker | 1.00 | 1 | 1.00 | 1 | |||||
| Month 4: number of times | |||||||||
| Used GP | 2.17 | 12 | 1.899 | 1.17 | 2 | 1.528 | 1.67 | 24 | 1.761 |
| Used practice nurse | 1.00 | 12 | 1.758 | 0.17 | 12 | 0.389 | 0.58 | 24 | 1.316 |
| Admitted to hospital | 0.58 | 12 | 1.443 | 0.33 | 12 | 0.492 | 0.46 | 24 | 1.062 |
| Treated at A&E | 0.17 | 12 | 0.389 | 0.17 | 12 | 0.389 | 0.17 | 24 | 0.381 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.42 | 12 | 0.515 | 0.25 | 12 | 0.452 | 0.33 | 24 | 0.482 |
| Occupational therapy | 0.25 | 12 | 0.452 | 0.08 | 12 | 0.289 | 0.17 | 24 | 0.381 |
| Chiropodist or podiatrist | 0.33 | 12 | 0.492 | 0.00 | 12 | 0.000 | 0.17 | 24 | 0.381 |
| District nurse | 0.00 | 12 | 0.000 | 0.00 | 11 | 0.000 | 0.00 | 23 | 0.000 |
| Continence advisor | 0.00 | 12 | 0.000 | 0.17 | 12 | 0.389 | 0.08 | 24 | 0.282 |
| Stroke Association family support worker | 0.00 | 11 | 0.000 | 0.00 | 12 | 0.000 | 0.00 | 23 | 0.000 |
| Number of times received treatment/help from physiotherapy | 4.50 | 4 | 5.000 | 21.33 | 3 | 25.146 | 11.71 | 7 | 17.442 |
| Number of times received treatment/help from occupational therapy | 7.67 | 3 | 5.859 | 3.00 | 1 | 6.50 | 4 | 5.323 | |
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 3 | 0.000 | 1.00 | 3 | 0.000 | |||
| Number of times received treatment/help from continence advisor | 1.00 | 2 | 0.000 | 1.00 | 2 | 0.000 | |||
| Month 5: number of times | |||||||||
| Used GP | 2.27 | 11 | 1.954 | 1.25 | 12 | 1.485 | 1.74 | 23 | 1.764 |
| Used practice nurse | 1.09 | 11 | 1.814 | 0.17 | 12 | 0.389 | 0.61 | 23 | 1.340 |
| Admitted to hospital | 0.64 | 11 | 1.502 | 0.33 | 12 | 0.492 | 0.48 | 23 | 1.082 |
| Treated at A&E | 0.18 | 11 | 0.405 | 0.17 | 12 | 0.389 | 0.17 | 23 | 0.388 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.36 | 11 | 0.505 | 0.25 | 12 | 0.452 | 0.30 | 23 | 0.470 |
| Occupational therapy | 0.18 | 11 | 0.405 | 0.17 | 12 | 0.389 | 0.17 | 23 | 0.388 |
| Chiropodist or podiatrist | 0.18 | 11 | 0.405 | 0.00 | 12 | 0.000 | 0.09 | 23 | 0.288 |
| District nurse | 0.00 | 11 | 0.000 | 0.00 | 11 | 0.000 | 0.00 | 22 | 0.000 |
| Continence advisor | 0.00 | 11 | 0.000 | 0.17 | 12 | 0.389 | 0.09 | 23 | 0.288 |
| Stroke Association family support worker | 0.00 | 10 | 0.000 | 0.00 | 12 | 0.000 | 0.00 | 22 | 0.000 |
| Number of times received treatment/help from physiotherapy | 5.33 | 3 | 5.774 | 20.33 | 3 | 25.813 | 12.83 | 6 | 18.638 |
| Number of times received treatment/help from occupational therapy | 12.00 | 2 | 0.000 | 2.50 | 2 | 0.707 | 7.25 | 4 | 5.500 |
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 2 | 0.000 | 1.00 | 2 | 0.000 | |||
| Number of times received treatment/help from continence advisor | 1.00 | 2 | 0.000 | 1.00 | 2 | 0.000 | |||
| Month 6: number of times | |||||||||
| Used GP | 2.17 | 6 | 2.041 | 1.20 | 10 | 1.619 | 1.56 | 16 | 1.788 |
| Used practice nurse | 1.00 | 6 | 1.673 | 0.10 | 10 | 0.316 | 0.44 | 16 | 1.094 |
| Admitted to hospital | 1.00 | 6 | 2.000 | 0.20 | 10 | 0.422 | 0.50 | 16 | 1.265 |
| Treated at A&E | 0.17 | 6 | 0.408 | 0.20 | 10 | 0.422 | 0.19 | 16 | 0.403 |
| Received treatment/help from | |||||||||
| Physiotherapy | 0.50 | 6 | 0.548 | 0.20 | 10 | 0.422 | 0.31 | 16 | 0.479 |
| Occupational therapy | 0.17 | 6 | 0.408 | 0.10 | 10 | 0.316 | 0.13 | 16 | 0.342 |
| Chiropodist or podiatrist | 0.17 | 6 | 0.408 | 0.00 | 10 | 0.000 | 0.06 | 16 | 0.250 |
| District nurse | 0.00 | 6 | 0.000 | 0.00 | 9 | 0.000 | 0.00 | 15 | 0.000 |
| Continence advisor | 0.00 | 6 | 0.000 | 0.20 | 10 | 0.422 | 0.13 | 16 | 0.342 |
| Stroke Association family support worker | 0.00 | 6 | 0.000 | 0.00 | 10 | 0.000 | 0.00 | 16 | 0.000 |
| Number of times received treatment/help from physiotherapy | 7.00 | 2 | 7.071 | 26.50 | 2 | 33.234 | 16.75 | 4 | 22.618 |
| Number of times received treatment/help from occupational therapy | 12.00 | 1 | 3.00 | 1 | 7.50 | 2 | 6.364 | ||
| Number of times received treatment/help from chiropodist or podiatrist | 1.00 | 1 | 1.00 | 1 | |||||
| Number of times received treatment/help from continence advisor | 1.00 | 2 | 0.000 | 1.00 | 2 | 0.000 | |||
The EQ-5D-5L was administered at baseline and at 3 and 6 months to the participants recruited and followed up until the cancellation of the trial. The data are presented in Table 38. There was some variation in the dimension scores by study group, but the sample size was too small for statistical comparisons to be undertaken. For participants who were unable to answer the EQ-5D-5L instrument, the proxy version was used. Results can be found in Table 39.
| Dimension | Time period, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 1–3 months’ follow-up | 2–6 months’ follow-up | ||||
| Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | |
| Mobility | ||||||
| I have no problems in walking about | 3 (7) | 2 (4) | 5 (17) | 3 (14) | 5 (21) | 6 (23) |
| I have slight problems in walking about | 7 (15) | 12 (24) | 3 (10) | 4 (19) | 7 (29) | 2 (8) |
| I have moderate problems in walking about | 10 (22) | 8 (16) | 9 (31) | 8 (38) | 7 (29) | 8 (31) |
| I have severe problems in walking about | 6 (13) | 6 (12) | 6 (21) | 2 (10) | 5 (21) | 7 (27) |
| I am unable to walk about | 20 (43) | 22 (44) | 6 (21) | 4 (19) | 0 (0) | 3 (12) |
| Number | 46 | 50 | 29 | 21 | 24 | 26 |
| Self-care | ||||||
| I have no problems washing or dressing myself | 7 (15) | 6 (12) | 13 (45) | 7 (33) | 11 (46) | 10 (38) |
| I have slight problems washing or dressing myself | 6 (13) | 11 (22) | 7 (24) | 1 (5) | 5 (21) | 2 (8) |
| I have moderate problems washing or dressing myself | 9 (20) | 7 (14) | 3 (10) | 6 (29) | 6 (25) | 9 (35) |
| I have severe problems washing or dressing myself | 9 (20) | 12 (24) | 3 (10) | 3 (14) | 2 (8) | 4 (15) |
| I am unable to wash or dress myself | 14 (30) | 14 (28) | 3 (10) | 4 (19) | 0 (0) | 1 (4) |
| Number | 45 | 50 | 29 | 21 | 24 | 26 |
| Usual activities | ||||||
| I have no problems doing my usual activities | 8 (17) | 4 (8) | 4 (14) | 2 (10) | 5 (21) | 5 (19) |
| I have slight problems doing my usual activities | 5 (11) | 7 (14) | 8 (28) | 2 (10) | 6 (25) | 2 (8) |
| I have moderate problems doing my usual activities | 5 (11) | 8 (16) | 7 (24) | 6 (29) | 7 (29) | 9 (35) |
| I have severe problems doing my usual activities | 6 (13) | 11 (22) | 5 (17) | 4 (19) | 5 (21) | 5 (19) |
| I am unable to do my usual activities | 22 (48) | 20 (40) | 5 (17) | 7 (33) | 1 (4) | 5 (19) |
| Number | 46 | 50 | 29 | 21 | 24 | 26 |
| Pain/discomfort | ||||||
| I have no pain or discomfort | 24 (52) | 21 (42) | 15 (52) | 7 (33) | 9 (38) | 12 (46) |
| I have slight pain or discomfort | 12 (26) | 8 (16) | 9 (31) | 5 (24) | 7 (29) | 4 (15) |
| I have moderate pain or discomfort | 8 (17) | 9 (18) | 2 (7) | 8 (38) | 7 (29) | 6 (23) |
| I have severe pain or discomfort | 2 (4) | 7 (14) | 2 (7) | 0 (0) | 0 (0) | 4 (15) |
| I have extreme pain or discomfort | 0 (0) | 4 (8) | 1 (3) | 1 (5) | 0 (0) | 0 (0) |
| Number | 46 | 49 | 29 | 21 | 23 | 26 |
| Anxiety/depression | ||||||
| I am not anxious or depressed | 20 (43) | 19 (38) | 13 (45) | 9 (43) | 12 (50) | 13 (50) |
| I am slightly anxious or depressed | 17 (37) | 19 (38) | 12 (41) | 6 (29) | 8 (33) | 9 (35) |
| I am moderately anxious or depressed | 6 (13) | 10 (20) | 3 (10) | 3 (14) | 4 (17) | 2 (8) |
| I am severely anxious or depressed | 1 (2) | 1 (2) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| I am extremely anxious or depressed | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (4) |
| Number | 45 | 49 | 29 | 19 | 24 | 25 |
| Dimension | Time period, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 1–3 months’ follow-up | 2–6 months’ follow-up | ||||
| Usual-care group | Intervention group | Usual-care group | Intervention group | Usual-care group | Intervention group | |
| Mobility | ||||||
| No problems in walking about | 2 (7) | 2 (7) | 1 (4) | 1 (7) | 3 (14) | 2 (10) |
| Slight problems in walking about | 4 (13) | 3 (10) | 4 (17) | 1 (7) | 6 (29) | 3 (15) |
| Moderate problems in walking about | 5 (17) | 5 (17) | 7 (29) | 2 (13) | 6 (29) | 4 (20) |
| Severe problems in walking about | 2 (7) | 4 (14) | 3 (13) | 4 (27) | 1 (5) | 4 (20) |
| Unable to walk about | 17 (57) | 15 (52) | 9 (38) | 7 (47) | 5 (24) | 7 (35) |
| Number | 30 | 29 | 24 | 15 | 21 | 20 |
| Self-care | ||||||
| No problems washing or dressing themselves | 1 (3) | 3 (10) | 5 (21) | 0 (0) | 6 (29) | 4 (20) |
| Slight problems washing or dressing themselves | 2 (7) | 0 (0) | 4 (17) | 2 (13) | 5 (24) | 1 (5) |
| Moderate problems washing or dressing themselves | 7 (23) | 3 (10) | 3 (13) | 1 (7) | 3 (14) | 3 (15) |
| Severe problems washing or dressing themselves | 4 (13) | 6 (21) | 2 (8) | 1 (7) | 3 (14) | 1 (5) |
| Unable to wash or dress themselves | 16 (53) | 17 (59) | 10 (42) | 11 (73) | 4 (19) | 11 (55) |
| Number | 30 | 29 | 24 | 15 | 21 | 20 |
| Usual activities | ||||||
| No problems doing their usual activities | 1 (3) | 2 (7) | 2 (8) | 0 (0) | 3 (14) | 2 (10) |
| Slight problems doing their usual activities | 1 (3) | 1 (3) | 5 (21) | 1 (7) | 5 (24) | 3 (15) |
| Moderate problems doing their usual activities | 5 (17) | 1 (3) | 4 (17) | 2 (13) | 4 (19) | 5 (25) |
| Severe problems doing their usual activities | 4 (13) | 5 (17) | 4 (17) | 2 (13) | 2 (10) | 2 (10) |
| Unable to do their usual activities | 19 (63) | 20 (69) | 9 (38) | 10 (67) | 7 (33) | 8 (40) |
| Number | 30 | 29 | 24 | 15 | 21 | 20 |
| Pain/discomfort | ||||||
| No pain or discomfort | 13 ( 43) | 13 (45) | 7 (29) | 4 (27) | 6 (29) | 8 (40) |
| Slight pain or discomfort | 12 (40) | 7 (24) | 7 (29) | 1 (7) | 6 (29) | 5 (25) |
| Moderate pain or discomfort | 2 (7) | 7 (24) | 5 (21) | 7 (47) | 4 (19) | 3 (15) |
| Severe pain or discomfort | 2 (7) | 1 (3) | 3 (13) | 2 (13) | 5 (24) | 2 (10) |
| Extreme pain or discomfort | 1 (3) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 1 (5) |
| Number | 30 | 28 | 22 | 15 | 21 | 19 |
| Anxiety/depression | ||||||
| Not anxious or depressed | 15 (50) | 8 (28) | 7 (29) | 6 (40) | 7 (33) | 5 (25) |
| Slightly anxious or depressed | 7 (23) | 11 (38) | 6 (25) | 4 (27) | 10 (48) | 10 (50) |
| Moderately anxious or depressed | 5 (17) | 6 (21) | 2 (8) | 3 (20) | 3 (14) | 5 (25) |
| Severely anxious or depressed | 2 (7) | 1 (3) | 3 (13) | 0 (0) | 1 (5) | 0 (0) |
| Extremely anxious or depressed | 1 (3) | 1 (3) | 1 (4) | 1 (7) | 0 (0) | 0 (0) |
| Number | 30 | 27 | 19 | 14 | 21 | 20 |
| Dimension | Trial group | |||||
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| Mean | n | SD | Mean | n | SD | |
| Baseline | ||||||
| Mobility | 3.7 | 46 | 1.34 | 3.7 | 50 | 1.36 |
| Self-care | 3.4 | 45 | 1.45 | 3.3 | 50 | 1.41 |
| Usual activities | 3.6 | 46 | 1.58 | 3.7 | 50 | 1.34 |
| Pain/discomfort | 1.7 | 46 | 0.91 | 2.3 | 49 | 1.37 |
| Anxiety/depression | 1.8 | 45 | 0.92 | 1.9 | 49 | 0.82 |
| 3 months | ||||||
| Mobility | 3.2 | 29 | 1.37 | 3.0 | 21 | 1.30 |
| Self-care | 2.2 | 29 | 1.39 | 2.8 | 21 | 1.54 |
| Usual activities | 3.0 | 29 | 1.32 | 3.6 | 21 | 1.33 |
| Pain/discomfort | 1.8 | 29 | 1.08 | 2.2 | 21 | 1.08 |
| Anxiety/depression | 1.8 | 29 | 0.91 | 1.8 | 19 | 0.92 |
| 6 months | ||||||
| Mobility | 2.5 | 24 | 1.06 | 3.0 | 26 | 1.34 |
| Self-care | 2.0 | 24 | 1.04 | 2.4 | 26 | 1.27 |
| Usual activities | 2.6 | 24 | 1.17 | 3.1 | 26 | 1.37 |
| Pain/discomfort | 1.9 | 23 | 0.85 | 2.1 | 26 | 1.16 |
| Anxiety/depression | 1.7 | 24 | 0.76 | 1.7 | 25 | 0.95 |
| Dimension | Trial group | |||||
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| Mean | n | SD | Mean | n | SD | |
| Baseline | ||||||
| Mobility | 3.9 | 30 | 1.39 | 3.9 | 29 | 1.33 |
| Self-care | 4.1 | 30 | 1.17 | 4.2 | 29 | 1.28 |
| Usual activities | 4.3 | 30 | 1.09 | 4.4 | 29 | 1.18 |
| Pain/discomfort | 1.9 | 30 | 1.04 | 1.9 | 28 | 0.93 |
| Anxiety/depression | 1.9 | 30 | 1.13 | 2.1 | 27 | 1.01 |
| 3 months | ||||||
| Mobility | 3.6 | 24 | 1.28 | 4.0 | 15 | 1.25 |
| Self-care | 3.3 | 24 | 1.66 | 4.4 | 15 | 1.12 |
| Usual activities | 3.5 | 24 | 1.41 | 4.4 | 15 | 0.99 |
| Pain/discomfort | 2.2 | 22 | 1.05 | 2.7 | 15 | 1.23 |
| Anxiety/depression | 2.2 | 19 | 1.27 | 2.0 | 14 | 1.18 |
| 6 months | ||||||
| Mobility | 3.0 | 21 | 1.40 | 3.6 | 20 | 1.40 |
| Self-care | 2.7 | 21 | 1.52 | 3.7 | 20 | 1.66 |
| Usual activities | 3.2 | 21 | 1.51 | 3.6 | 20 | 1.43 |
| Pain/discomfort | 2.4 | 21 | 1.16 | 2.1 | 19 | 1.24 |
| Anxiety/depression | 1.9 | 21 | 0.83 | 2.0 | 20 | 0.73 |
Appendix 6 Protocol amendment list
Following the first meeting of our ICONS-II TSC, we made a few minor changes to the ICONS-II trial protocol.
Inclusion criteria
We revised the inclusion criterion for level of consciousness to:
-
conscious: ‘Alert’ or ‘Not alert but arousable’ (NIHSS Point 1A score of 0 or 1) on the NIHSS3,46 within 72 hours of stroke onset.
The TSC suggested that people with an NIHSS Point 1A score of 2 would not be likely to benefit from the intervention.
We revised the inclusion criterion for UI to:
-
UI, defined as ‘involuntary loss of urine’,38 within 72 hours of stroke onset or presence of IUC at the time of consent.
This brings the time period in line with that of the level of consciousness inclusion criterion.
Contamination monitoring: extent to which usual-care participants receive the intervention
We initially planned to conduct a retrospective review of case notes of 50% of patients admitted to each unit in the 3-month pre-intervention period to determine continence practice. However, as many of the sites that expressed an interest in participating were large (between 400 and 1300 admissions with stroke per annum), we believed that using this approach would generate too many data and waste research nurse time and resources. Our revised plan was as follows:
-
To determine baseline continence practice, we will conduct a retrospective audit of case notes of 40 patients admitted to each unit in the 3-month pre-intervention period. Detailed review will be conducted of patients identified as incontinent. Audit data will be collected by the project-specific research nurse in each site.
Internal pilot trial continuation criteria
The TSC recommended changing the words ‘stop trial’ to ‘pause trial’ to allow the opportunity for rectifying causes of recruitment and contamination issues, should these arise (protocol section 3.4: internal pilot study).
Outcome measurement
We added an additional data point for the primary outcome, ICIQ-UI-SF: discharge from the stroke unit.
We added ‘number of urinary tract infections’ and ‘number of days indwelling urethral catheter in situ’ to our secondary outcomes. We will collect these at discharge from the stroke unit and at 3 and 6 months post randomisation.
List of abbreviations
- 6-CIT
- Six-Item Cognitive Impairment Test
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- CTU
- Clinical Trials Unit
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- ICIQ-UI-SF
- International Consultation on Incontinence Questionnaire – Urinary Incontinence – Short Form
- ICONS
- Identifying Continence OptioNs after Stroke
- IPR
- individual patient randomisation
- IQoL
- Incontinence Quality of Life
- IQR
- interquartile range
- IUC
- indwelling urinary catheter
- LUSQ
- Leicester Urinary Symptom Questionnaire
- MCID
- minimal clinically important difference
- mRS
- modified Rankin scale
- NIHR
- National Institute for Health and Care Research
- NIHSS
- National Institutes of Health Stroke Scale
- OR
- odds ratio
- PCPI
- Patient, Carer and Public Involvement
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- REC
- Research Ethics Committee
- SAP
- statistical analysis plan
- SD
- standard deviation
- SVP
- systematic voiding programme
- TSC
- Trial Steering Committee
- TWOC
- trial without catheter
- UI
- urinary incontinence
- UTI
- urinary tract infection
